UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 20-F
☐ REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended October 31, 2023
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
☐ SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Date of event requiring this shell company report ____________
For the transition period from ____________ to ____________
Commission File No.: 001-41557
Clearmind Medicine Inc.
(Exact name of registrant as specified in its charter)
Translation of registrant’s name into English: Not applicable
| British Columbia | 101 – 1220 West 6th Avenue Vancouver, British Columbia |
|
| (Jurisdiction of incorporation or organization) | (Address of principal executive offices) |
Dr. Adi Zuloff-Shani
Chief Executive Officer
101 – 1220 West 6th Avenue
Vancouver, British Columbia V6H1A5
Tel: 973.536.1016
Email: invest@clearmindmedicine.com
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
| Title of each class to be registered | Trading Symbol(s) | Name of each exchange on which each class is to be registered |
||
| Common shares, no par | CMND | The Nasdaq Stock Market LLC |
Securities registered or to be registered pursuant to Section 12(g) of the Act: None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: None Number of outstanding shares of each of the issuer’s classes of capital or common stock as of October 31, 2023: 607,337 Common shares.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Exchange Act of 1934. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months. Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company.
| Large accelerated filer ☐ | Accelerated filer ☐ | Non-accelerated filer ☒ | ||||
| Emerging Growth Company ☒ |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
†The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing.
| U.S. GAAP ☐ | International Financial Reporting Standards as issued by the International Accounting Standards Board ☒ | Other ☐ |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow. ☐ Item 17 ☐ Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company. Yes ☐ No ☒
TABLE OF CONTENTS
INTRODUCTION
Unless the context otherwise requires, references in this annual report on Form 20-F to the “Company,” “Clearmind,” “we,” “us,” “our” and other similar designations refer to Clearmind Medicine Inc. All references to “common shares” are to our Common Shares, no par value.
On November 1, 2022, our reporting currency and functional currency in our financial statements is the United States dollar. Amounts denominated in United States Dollars are states as “$” “dollars” or “USD”. Unless otherwise expressly stated or the context otherwise requires, references in this Annual Report to “CAD$” are to Canadian Dollars.
We report under International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board, or the IASB. None of the financial statements were prepared in accordance with generally accepted accounting principles in the United States.
All information in this annual report on Form 20-F relating to shares or price per share reflects the one-for-30 consolidation of our issued and outstanding common shares effected by us on November 28, 2023.
Unless otherwise indicated, or the context otherwise requires, references in this Annual Report to financial and operational data for a particular year refer to the fiscal year of our Company ended October 31 of that year.
EMERGING GROWTH COMPANY STATUS
We qualify as an “emerging growth company,” as defined in the U.S. Jumpstart Our Business Startups Act of 2012, or JOBS Act, and we may take advantage of certain exemptions, including exemptions from various reporting requirements that are otherwise applicable to public traded entities that do not qualify as emerging growth companies. These exemptions include:
| ● | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act; and |
| ● | not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (i.e., an auditor discussion and analysis). |
Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of the extended transition period provided in Section 13(a) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, for complying with new or revised accounting standards. This means that an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. Given that we currently report and expect to continue to report our financial results under IFRS as issued by the IASB, we will not be able to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required by the IASB.
We will remain an emerging growth company until the earliest of: (i) the last day of the first fiscal year in which our annual gross revenues exceed $1.235 billion; (ii) the last day of the fiscal year following the fifth anniversary of the date of our initial public offering; (iii) the date that we become a “large accelerated filer” as defined in Rule 12b-2 under the Exchange Act, which would occur if the aggregate worldwide market value of our common shares, including common shares represented by warrants, held by non-affiliates is at least $700 million as of the last business day of our most recently completed second fiscal quarter; or (iv) the date on which we have issued more than $1.0 billion in non-convertible debt securities during any three-year period.
TRADEMARKS
All trademarks or trade names referred to in this Annual Report on Form 20-F are the property of their respective owners. Solely for convenience, the trademarks and trade names in this Annual Report on Form 20-F are referred to without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Certain information included or incorporated by reference in this Annual Report on Form 20-F may be deemed to be “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 and other securities laws. Forward-looking statements are often characterized by the use of forward-looking terminology such as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “predict,” “potential,” “positioned,” “seek,” “should,” “target,” “will,” “would,” or other similar words, but are not the only way these statements are identified.
These forward-looking statements may include, but are not limited to, statements relating to our objectives, plans and strategies, statements that contain projections of results of operations or of financial condition, expected capital needs and expenses, statements relating to the research, development, completion and use of our products, and all statements (other than statements of historical facts) that address activities, events or developments that we intend, expect, project, believe or anticipate will or may occur in the future.
Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties. We have based these forward-looking statements on assumptions and assessments made by our management in light of their experience and their perception of historical trends, current conditions, expected future developments and other factors they believe to be appropriate.
Important factors that could cause our actual results to differ materially from any future results expressed or implied by the forward-looking statements. Many factors could cause our actual activities or results to differ materially from the activities and results anticipated in forward-looking statements, including, but not limited to, the factors summarized below:
| ● | the ability of our pre-clinical and any future clinical trials to demonstrate safety and efficacy of our future product candidates, and other positive results; |
| ● | the timing and focus of our future preclinical studies and clinical trials, and the reporting of data from those studies and trials; |
| ● | the size of the market opportunity for our future product candidates, including our estimates of the number of patients who suffer from the diseases we are targeting; |
| ● | the success of competing therapies that are or may become available; |
| ● | the beneficial characteristics, safety, efficacy and therapeutic effects of our future product candidates, as well as the potential healthcare costs saved through utilizing our future product candidates; |
| ● | the ability of our future product candidates to address needs not currently addressed by the psychedelic industry; |
| ● | the ability of our future product candidates to address needs not currently addressed by the psychedelic industry; |
| ● | our ability to obtain and maintain regulatory approval of our future product candidates; |
| ● | our plans relating to the further development of our future product candidates, including additional disease states or indications we may pursue; |
| ● | existing regulations and regulatory developments in the United States and other jurisdictions; |
| ● | our plans and ability to obtain or protect intellectual property rights, including extensions of patent terms where available and our ability to avoid infringing the intellectual property rights of others; |
| ● | the ability of our management team to oversee our drug research programs; |
| ● | the need to hire additional personnel and our ability to attract and retain such personnel; |
| ● | our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; |
| ● | our dependence on third parties; |
| ● | our ability to compete with other companies who offer products that address similar issues that our future product candidates will address; |
| ● | our financial performance; |
| ● | the period over which we estimate our existing cash and cash equivalents will be sufficient to fund our future operating expenses and capital expenditure requirements; |
| ● | our ability to generate revenue and profit margin under our anticipated contracts which is subject to certain risks; |
| ● | difficulties in our partners’ ability to recruit and retain qualified physicians and other healthcare professionals, and enforce our non-compete agreements with our physicians; |
| ● | our ability to restructure our operations to comply with future changes in government regulation; |
| ● | security, political and economic instability in the Middle East that could harm our business, including due to the current war between Israel and Hamas; and |
| ● | those factors referred to in “Item 3.D. Risk Factors,” “Item 4. Information on the Company,” and “Item 5. Operating and Financial Review and Prospects,” as well as in this Annual Report on Form 20-F generally. |
These statements are only current predictions and are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance, or achievements to be materially different from those anticipated by the forward-looking statements. We discuss many of these risks in this Annual Report on Form 20-F in greater detail under the heading “Risk Factors” and elsewhere in this Annual Report on Form 20-F. You should not rely upon forward-looking statements as predictions of future events.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, or achievements. Except as required by law, we are under no duty to update or revise any of the forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this Annual Report on Form 20-F.
MARKET, INDUSTRY AND OTHER DATA
Market data and certain industry data and forecasts used throughout this Annual Report on Form 20-F were obtained from sources we believe to be reliable, including market research databases, publicly available information, reports of governmental agencies, and industry publications and surveys. We have relied on certain data from third party sources, including industry forecasts and market research, which we believe to be reliable based on our management’s knowledge of the industry. While we are not aware of any misstatements regarding the industry data presented in this Annual Report on Form 20-F, our estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed under the heading “Risk Factors” and elsewhere in this Annual Report on Form 20-F.
Statements made in this Annual Report on Form 20-F concerning the contents of any agreement, contract or other document are summaries of such agreements, contracts or documents and are not a complete description of all of their terms. If we filed any of these agreements, contracts or documents as exhibits to this Report or to any previous filing with the Securities and Exchange Commission, or SEC, you may read the document itself for a complete understanding of its terms.
PART I
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
Not applicable.
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
Not applicable.
ITEM 3. KEY INFORMATION
A. [Reserved]
B. Capitalization and Indebtedness
Not applicable.
C. Reasons for the Offer and Use of Proceeds
Not applicable.
D. Risk Factors
You should carefully consider the risks described below, together with all of the other information in this Annual Report on Form 20-F. The risks and uncertainties described below are those material risk factors, currently known and specific to us, that we believe are relevant to an investment in our securities. Additional risks and uncertainties not currently known to us or that we now deem immaterial may also harm us. If any of these risks materialize our business, results of operations or financial condition could suffer, and the price of our common shares could decline substantially.
Summary Risk Factors
Our business is subject to numerous risks and uncertainties, including those highlighted in the section titled “Risk Factors” below. These risks include, among others, the following:
Risks Related to Our Financial Condition and Capital Requirements
| ● | We have incurred losses since our inception. We anticipate that we will incur significant losses for the foreseeable future, and we may never achieve or maintain profitability. |
| ● | Our financial statements contain an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern. |
| ● |
We have never generated any revenue from product sales and may never be profitable.
|
|
| ● | We expect that we will need to raise substantial additional funding, which may not be available on acceptable terms, or at all. |
Risks Related to the Clinical Development, Regulatory Review and Approval of our Product Candidates
| ● | Most of our product candidates are in preclinical development. To date, we have not enrolled any patients to our clinical trials |
| ● | We may not receive, or may be delayed in receiving, the necessary approvals for MEAI or future products. |
| ● | Clinical and preclinical development is uncertain. |
| ● | Clinical trials of our product candidates may be delayed, and certain programs may never advance in the clinic or may be more costly to conduct than we anticipate. |
| ● |
Our current product candidates and future therapeutic candidates contain psychedelic substances, and may be subject to controlled substance laws and regulations.
|
|
| ● |
Our product candidates contain potentially controlled substances, the use of which may generate public controversy.
|
|
| ● |
Any clinical trials that we may conduct in the future to demonstrate substantial evidence of the safety and effectiveness of product candidates that we may identify and pursue for their intended uses.
|
|
| ● | Even if we complete the necessary preclinical studies and clinical trials, the marketing approval process is expensive, time consuming and uncertain. | |
| ● | The results of early-stage clinical trials and preclinical studies may not be predictive of future results. | |
| ● | Research and development of drugs targeting the Central Nervous System is particularly difficult. | |
| ● | If we encounter difficulties enrolling patients in clinical trials, our clinical development activities could be delayed or otherwise adversely affected. | |
| ● |
Use of our product candidates could be associated with side effects, adverse events or safety risks.
|
|
| ● |
Even if any of our current or future product candidates receives regulatory approval, it may fail to achieve market acceptance necessary for commercial success.
|
|
| ● |
If we are unable to obtain regulatory approval in one or more jurisdictions for any product candidates that we may identify and develop, our business will be substantially harmed.
|
|
| ● |
Interim, “top-line,” and preliminary data from our clinical trials that we announce or publish from time to time may change, which could result in material changes in the final data.
|
|
| ● |
Certain of the product candidates we are developing are complex and difficult to manufacture. We could experience manufacturing problems that result in delays or otherwise harm our business.
|
|
| ● |
We may not elect or be able to take advantage of any expedited development or regulatory review and approval processes available to drug product candidates.
|
|
| ● | For any approved product, we will be subject to ongoing regulatory obligations and continued regulatory review and we may be subject to penalties if we fail to comply. |
Risks Related to Commercialization
| ● |
If we are unable to establish sales and marketing capabilities or enter into agreements to sell and market any product candidates, we may not be successful in commercializing those product candidates.
|
|
| ● |
The third-party payor coverage and reimbursement status of newly approved products is uncertain. |
| ● |
If we fail to comply with healthcare laws, we could face substantial penalties and our business, financial condition and results of operations could be adversely affected.
|
|
| ● |
We may become subject to U.S. federal and state forfeiture laws which could negatively impact our business operations.
|
|
| ● |
Failure to comply with health and data protection laws and regulations could lead to government enforcement actions, private litigation, and/or adverse publicity.
|
|
| ● | We face significant competition in an environment of rapid technological and scientific change, and there is a possibility that our competitors may achieve regulatory approval before we do or develop therapies that are safer, more advanced or more effective than ours. |
| ● | If the market opportunities for our product candidates are smaller than we believe they are, our revenue may be adversely affected, and our business may suffer. |
Risks Related to Reliance on Third Parties
| ● | We may seek to enter into additional collaborations and other similar arrangements and may not be successful in entering into new arrangements, and even if we are, we may not realize the benefits of such relationships. |
| ● | Collaborative relationships with third parties could cause us to expend significant resources and incur substantial business risk with no assurance of financial return. |
| ● | We rely on third parties to assist in conducting our clinical trials and some aspects of our research and preclinical testing, and those third parties may not perform satisfactorily. |
| ● | Our use of third parties to manufacture and develop our product candidates for preclinical studies and clinical trials may increase the risk that we will not have sufficient quantities of materials. |
| ● | We have no sales, distribution, or marketing experience, and may invest significant financial and management resources to establish these capabilities. |
Risks Related to Our Intellectual Property
| ● | If we are unable to obtain and maintain effective patent rights for our product candidates or any future product candidates, we may not be able to compete effectively in our markets. |
| ● | We may not have sufficient patent lifespan to effectively protect our products and business. |
| ● | Patent policy and rule changes could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of any issued patents. |
| ● | If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected. |
| ● |
If we are unable to maintain effective proprietary rights for our product candidates or any future product candidates, we may not be able to compete effectively in our markets.
|
|
| ● |
Intellectual property rights of third parties could adversely affect our ability to commercialize our product candidates.
|
|
| ● |
Third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts.
|
| ● |
We may not be successful in obtaining or maintaining necessary rights to our product candidates through acquisitions and in-licenses.
|
|
| ● |
We may be subject to claims challenging the inventorship of our intellectual property.
|
|
| ● |
We may not be able to protect our intellectual property rights throughout the world.
|
Risks Related to Our Business Operations
| ● | Our business and operations have been and are likely to further continue to be adversely affected by the evolving and ongoing COVID-19 global pandemic. |
| ● | We will need to expand our organization, and we may experience difficulties in managing this growth, which could disrupt our operations. |
| ● | Due to our limited resources and access to capital, we must, and have in the past decided to, prioritize development of certain product candidates over other potential candidates. |
| ● | We may not be successful in our efforts to identify, discover or license additional product candidates. |
| ● |
European data collection is governed by restrictive regulations governing the collection, use, processing and cross-border transfer of personal information.
|
|
| ● |
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on our business.
|
|
| ● |
Our employees and independent contractors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
|
|
| ● |
International expansion of our business exposes us to business, regulatory, political, operational, financial and economic risks.
|
Risks Related to Ownership of Our Common Shares
| ● | We may be or may become classified as a passive foreign investment company. |
| ● | As a foreign private issuer, we are permitted, and intend, to follow certain home country corporate governance practices and we will not be subject to certain U.S. securities laws. |
| ● | We are an emerging growth company and the reduced disclosure requirements applicable to emerging growth companies may make our Common Shares less attractive to investors. |
| ● | Failure to remediate material weaknesses in internal accounting controls could result in material misstatements in our financial statements. |
Risks Related to our Location in Israel and our International Operations
| ● | Part of our operations are conducted in Israel. Conditions in Israel, including the recent attack by Hamas and other terrorist organizations and Israel’s war against them, may affect our operations. |
| ● | Because we are incorporated in British Columbia and some of our directors and officers are in Canada, it may be difficult for U.S. investors to enforce civil liabilities. Similarly, it may be difficult for Canadian investors to enforce civil liabilities against our directors and officers outside of Canada. |
| ● | Operating outside of the United States presents specific risks to our business. |
Risks Related to Our Financial Condition and Capital Requirements
We have incurred losses since our inception. We anticipate that we will incur significant losses for the foreseeable future, and we may never achieve or maintain profitability.
We are a clinical pharmaceutical company. We have incurred operating losses each year since our inception, including operating losses of $6,303,434, $6,251,431 and $2,693,397 for the years ended October 31, 2023, 2022 and 2021, respectively. As of October 31, 2023, we had an accumulated deficit of $18,768,063. We have devoted substantially all of our financial resources to designing and developing MEAI, including preclinical studies and providing general and administrative support for these operations. We expect that our expenses and operating losses will increase for the foreseeable future as we continue clinical development of MEAI for the treatment of alcohol use disorder, or AUD and obesity and metabolic disorder. Our ability to ultimately achieve revenues and profitability is dependent upon our ability to successfully complete the development of MEAI and any future product candidates, obtain necessary regulatory approvals for and successfully manufacture, market and commercialize our products.
We anticipate that our expenses will increase substantially based on a number of factors, including to the extent that we:
| ● | continue our pre-clinical development and initiate clinical trials of MEAI; | |
| ● | seek regulatory and marketing approvals for any product candidates that successfully complete clinical trials; | |
| ● | advance our preclinical and research and development programs; | |
| ● | identify, assess, acquire, license and/or develop other product candidates; | |
| ● | manufacture current good manufacturing practices, or cGMP, material for future clinical trials or potential commercial sales; | |
| ● | establish a sales, marketing and distribution infrastructure to commercialize any products for which we may obtain marketing approval; | |
| ● | hire personnel and invest in additional infrastructure to support our operations as a public company and expand our product development; | |
| ● | enter into agreements to license intellectual property from third parties; | |
| ● | develop, maintain, protect and expand our intellectual property portfolio; and | |
| ● | experience any delays or encounter issues with respect to any of the above, including, but not limited to, failed trials, complex results, safety issues or other regulatory challenges that require longer follow-up of future clinical trials, additional clinical trials or additional supportive studies in order to pursue marketing approval. |
To date, we have financed our operations primarily through the sale of equity securities. The amount of any future operating losses will depend, in part, on the rate of our future expenditures and our ability to obtain funding through equity or debt financings, strategic collaborations or grants. Even if we obtain regulatory approval to market one or more product candidates, our future revenue will depend upon the size of any markets in which such product candidates receive approval and our ability to achieve sufficient market acceptance, pricing, reimbursement from third-party payors for such product candidates. Further, the operating losses that we incur may fluctuate significantly from quarter to quarter and year to year, such that a period-to-period comparison of our results of operations may not be a good indication of our future performance. Other unanticipated costs may also arise.
Our financial statements contain an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern, which could prevent us from obtaining new financing on reasonable terms or at all.
Our audited consolidated financial statements for the year ended October 31, 2023, contain an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern. We have incurred losses in each year since our inception, including net losses of $8,620,837, $6,894,868 and $2,725,288 for the years ended October 31, 2023, 2022 and 2021, respectively. As of October 31, 2023, we had an accumulated deficit of $18,768,063. These events and conditions, along with other matters, indicate that a material uncertainty exists that raise substantial doubt on our ability to continue as a going concern. The financial statements for 2023 do not include any adjustments that might result from the outcome of this uncertainty. Further financial statements may include an explanatory paragraph with respect to our ability to continue as a going concern. Until we can generate significant recurring revenues, we expect to satisfy our future cash needs through debt or equity financing. We cannot be certain that additional funding will be available to us on acceptable terms, if at all. If funds are not available, we may be required to delay, reduce the scope of, or eliminate research or development plans for, or commercialization efforts with respect to our products. This may raise substantial doubts about our ability to continue as a going concern.
We have never generated any revenue from product sales and may never be profitable.
We are a clinical stage pharmaceutical company, have no products approved for marketing in any jurisdiction and we have never generated any revenue from product sales. Our ability to generate revenue and achieve profitability depends on our ability, alone or with strategic collaboration partners, to successfully complete the development of, and obtain the regulatory and marketing approvals necessary to commercialize, MEAI or any future product candidates. We do not anticipate generating revenue from product sales for at least the next several years. Our ability to generate future revenue from product sales will depend heavily on our ability to:
| ● | complete research and preclinical and clinical development of MEAI and any future product candidates in a timely and successful manner, including MEAI for the treatment of depression and addiction; | |
| ● | obtain regulatory and marketing approval for any product candidates for which we complete clinical trials; | |
| ● | maintain and enhance a commercially viable, sustainable, scalable, reproducible and transferable manufacturing process for MEAI and any future product candidates that is compliant with cGMPs; |
| ● | establish and maintain supply and, if applicable, manufacturing relationships with third parties that can provide, in both amount and quality, adequate products to support pre-clinical and future clinical development and the market demand for MEAI and any future product candidates, if and when approved; | |
| ● | launch and commercialize any product candidates for which we obtain regulatory and marketing approval, either directly by establishing a sales force, marketing and distribution infrastructure, and/or with collaborators or distributors; | |
| ● | expose and educate physicians and other medical professionals to use our products; | |
| ● | obtain market acceptance, if and when approved, of MEAI and any future product candidates from the medical community and third-party payors; | |
| ● | ensure our product candidates are approved for reimbursement from governmental agencies, health care providers and insurers in jurisdictions where they have been approved for marketing; | |
| ● | address any competing technological and market developments that impact MEAI and any future product candidates or their prospective usage by medical professionals; | |
| ● | identify, assess, acquire and/or develop new product candidates; | |
| ● | negotiate favorable terms in any collaboration, licensing or other arrangements into which we may enter and perform our obligations under such collaborations; | |
| ● | maintain, protect and expand our portfolio of intellectual property rights, including patents, patent applications, trade secrets and know-how; | |
| ● | avoid and defend against third-party interference or infringement claims; | |
| ● | attract, hire and retain qualified personnel; and | |
| ● | locate and lease or acquire suitable facilities to support our pre-clinical and future clinical development, manufacturing facilities and commercial expansion. |
Even if MEAI or any future product candidates are approved for marketing and sale, we anticipate incurring significant incremental costs associated with commercializing such product candidates. Our expenses could increase beyond expectations if we are required by the FDA, the European Medicines Agency, or EMA or other regulatory agencies, domestic or foreign, or ethical committees in medical centers, to change our manufacturing processes or assays or to perform clinical, nonclinical or other types of studies in addition to those that we currently anticipate. Even if we are successful in obtaining regulatory approvals to market MEAI or any future product candidates, our revenue earned from such product candidates will be dependent in part upon the breadth of the product label, the size of the markets in the territories for which we gain regulatory approval for such products, the accepted price for such products, our ability to obtain reimbursement for such products at any price, whether we own the commercial rights for that territory in which such products have been approved and the expenses associated with manufacturing and marketing such products for such markets. Therefore, we may not generate significant revenue from the sale of such products, even if approved. Further, if we are not able to generate significant revenue from the sale of our approved products, we may be forced to curtail or cease our operations. Due to the numerous risks and uncertainties involved in product development, it is difficult to predict the timing or amount of increased expenses, or when, or if, we will be able to achieve or maintain profitability.
We expect that we will need to raise substantial additional funding, which may not be available on acceptable terms, or at all. Failure to obtain funding on acceptable terms and on a timely basis may require us to curtail, delay or discontinue our product development efforts or other operations.
We are currently advancing MEAI through pre-clinical and clinical development in multiple indications, in order to obtain regulatory approvals. Developing product candidates is expensive, and we expect our research and development expenses to increase substantially in connection with our ongoing activities, particularly as we advance product candidates through clinical trials and regulatory approvals. Furthermore, we expect to incur additional ongoing costs associated with operating as a public company.
To date, we have financed our operations primarily through the sale of equity securities. As of October 31, 2023, we had cash, cash equivalents, short-term and long-term deposits of $5,427,739. We will require significant additional financing to fund our operations. Our future funding requirements will depend on many factors, including but not limited to:
| ● | the progress, results and costs of our ongoing pre-clinical and anticipated clinical trials of MEAI and any future product candidates; |
| ● | the cost, timing and outcomes of regulatory review of MEAI and any future product candidates; | |
| ● | the scope, progress, results and costs of product development, laboratory testing, manufacturing, preclinical development and clinical trials for any other product candidates that we may develop or otherwise obtain in the future; | |
| ● | the cost of our future activities, including establishing sales, marketing and distribution capabilities for any product candidates in any particular geography where we receive marketing approval for such product candidates; | |
| ● | the terms and timing of any collaborative, licensing and other arrangements that we may establish; | |
| ● | the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims; and | |
| ● | the level of revenue, if any, received from commercial sales of any product candidates for which we receive marketing approval. |
Identifying potential product candidates and conducting preclinical testing and clinical trials is a time-consuming, expensive and uncertain process that takes years to complete, and we may never generate the necessary data or results required to obtain marketing approval and achieve product sales. In addition, our product candidates, if and when approved, may not achieve commercial success. Our product revenues, if any, will be derived from or based on sales of product candidates that may not be commercially available for many years, if at all. Accordingly, we will need to continue to rely on additional financing to achieve our business objectives. Any additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our product candidates.
We cannot guarantee that financing will be available in sufficient amounts or on terms acceptable to us, if at all, and the terms of any financing may adversely affect the interests or rights of our shareholders. Even if we believe that we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations. The issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our shares to decline. Further, our ability to raise additional capital may be adversely impacted by potential worsening global economic conditions and the recent disruptions to and volatility in the credit and financial markets in the United States and worldwide, including from any resurgence of the COVID-19 pandemic.
To the extent that we raise capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of such securities may include liquidation or other preferences that adversely affect your rights as a shareholder. Debt financing, if available, may involve covenants restricting our operations or our ability to incur additional debt. If we raise funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish certain rights to our technologies or our product candidates, or to grant licenses on terms that are not favorable to us.
If we are unable to obtain funding on acceptable terms and on a timely basis, we may be required to significantly curtail, delay or discontinue one or more of our research, development or manufacturing programs or the commercialization of any approved product, or be unable to expand our operations or otherwise capitalize on our business opportunities, as desired, which could materially affect our business, financial condition and results of operations.
Risks Related to the Clinical Development, Regulatory Review and Approval of our Product Candidates
Most of our product candidates are in preclinical development. To date, we have not commenced enrolment to our clinical trial which is a lengthy and expensive process with uncertain outcomes and the potential for substantial delays. We cannot give any assurance that any of our product candidates will receive regulatory approval, which is necessary before they can be commercialized.
We are a clinical stage pharmaceutical company. To date, we have not commenced enrollment to our first clinical trial. Before obtaining marketing approval from regulatory authorities for the sale of our product candidates, we must conduct extensive clinical trials to demonstrate the safety and efficacy of the product candidates in humans. To date, we have focused substantially all of our efforts and financial resources on identifying, acquiring, and developing product candidates, preclinical studies and FIH study providing general and administrative support for these operations. We cannot be certain that any future clinical trials will be conducted. Our inability to successfully complete preclinical and clinical development could result in additional costs to us and negatively impact our ability to generate revenue. Our future success is dependent on our ability to successfully develop, obtain regulatory approval for, and then successfully commercialize product candidates. We currently have no products approved for sale and have not generated any revenue, and we may never be able to develop or successfully commercialize any of our product candidates.
All of our product candidates require additional development, management of preclinical, clinical and manufacturing activities and regulatory approval. In addition, we will need to obtain adequate manufacturing supply, build a commercial organization, commence marketing efforts and obtain reimbursement before they generate any significant revenue from commercial product sales, if ever. In addition, while our new program selection criteria include prior evidence in humans and we believe the product candidates we have selected have the potential for a favorable safety profile based on third party trials and studies, many of our product candidates are in early-stage research phases of development, and the risk of failure for these programs is high. We cannot be certain that any of our product candidates will be successful in clinical trials or receive regulatory approval. Further, our product candidates may not receive regulatory approval even if they are successful in clinical trials. If we do not receive regulatory approvals for our product candidates, we may not be able to continue operations, which may result in dissolution, out-licensing the technology or pursuing an alternative strategy.
We may not receive, or may be delayed in receiving, the necessary approvals for MEAI or future products, and failure to timely obtain necessary approvals for MEAI or future products would adversely affect our ability to grow our business.
Assuming successful initiation and completion of required clinical testing and other requirements, the results of the preclinical studies and clinical trials, together with detailed information relating to the product’s chemistry, manufacture, controls and proposed labeling, among other things are submitted to the FDA as part of an NDA requesting approval to market the product candidate for one or more indications.
The FDA conducts a preliminary review of an NDA within 60 days of its receipt and strives to inform the sponsor by the 74th day after the FDA’s receipt of the submission to determine whether the application is sufficiently complete to permit substantive review. This is known as the filing decision. The FDA may request additional information rather than accept an NDA for filing. In this event, the application must be resubmitted with the additional information. The resubmitted application is also subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. The FDA has agreed to certain performance goals in the review process of NDAs. Most such applications are meant to be reviewed within ten months from the date of filing, and most applications for “priority review” products are meant to be reviewed within six months of filing. A product that has been designated as a breakthrough therapy may also be eligible for review within six months if supported by clinical data at the time of submission of the NDA. The review process may be extended by the FDA for three additional months to consider new information or clarification provided by the applicant to address an outstanding deficiency identified by the FDA following the original submission.
Before approving an NDA, the FDA typically will inspect the facility or facilities where the product is or will be manufactured. These pre-approval inspections may cover all facilities associated with an NDA submission, including drug component manufacturing such as active pharmaceutical ingredients, finished drug product manufacturing, control testing laboratories, as well as packaging and labeling facilities. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP. The applicant of the NDA may also have their records, processes, procedures, training, and other aspects reviewed during an inspection. The FDA must implement a protocol to expedite review of responses to inspection reports pertaining to certain drug applications, including applications for drugs in a shortage or drugs for which approval is dependent on remediation of conditions identified in the inspection report.
In addition, as a condition of approval, the FDA may require an applicant to develop a REMS. REMS use risk minimization strategies beyond the professional labeling to ensure that the benefits of the product outweigh the potential risks.
Finally, the FDA may refer an application for a novel drug to an advisory committee or explain why such referral was not made. Typically, an advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Legislative or regulatory reforms in the United States or the European Union may make it more difficult and costly for us to obtain regulatory clearances or approvals for our products or to manufacture, market or distribute our products after clearance or approval is obtained.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the regulatory clearance or approval, manufacture and marketing of regulated products or the reimbursement thereof. In addition, the FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of our product candidates. We cannot determine what effect changes in regulations, statutes, legal interpretation or policies, when and if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things, require:
| ● | changes to manufacturing methods; | |
| ● | change in protocol design; | |
| ● | additional treatment arm (control); | |
| ● | recall, replacement, or discontinuance of one or more of our products; and | |
| ● | additional recordkeeping. |
In addition, in the United States, there have been a number of legislative and regulatory proposals to change the health care system in ways that could affect our ability to sell our products profitably. The pharmaceutical industry in the United States, as an example, has been affected by the passage of the ACA, which, among other things, imposed new fees on entities that manufacture or import certain branded prescription drugs and expanded pharmaceutical manufacturer obligations to provide discounts and rebates to certain government programs. There have been executive, judicial and Congressional challenges to certain aspects of the ACA. Concurrently, Congress considered legislation to repeal or repeal and replace all or part of the ACA. While Congress has not passed comprehensive repeal legislation, several bills affecting the implementation of certain taxes under the ACA have been enacted. The Tax Cuts and Jobs Act of 2017 includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate”. In addition, the 2020 federal spending package permanently eliminated, effective January 1, 2020, the ACA-mandated “Cadillac” tax on high-cost employer-sponsored health coverage and medical device tax and, effective January 1, 2021, also eliminated the health insurer tax. On December 14, 2018, a Texas U.S. District Court Judge ruled that the ACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress as part of the Tax Act. Additionally, on December 18, 2019, the U.S. Court of Appeals for the 5th Circuit upheld the District Court ruling that the individual mandate was unconstitutional and remanded the case back to the District Court to determine whether the remaining provisions of the ACA are invalid as well. The United States Supreme Court is currently reviewing this case, but it is unknown when a decision will be reached. Although the Supreme Court has not yet ruled on the constitutionality of the ACA, on January 28, 2021, President Biden issued an executive order to initiate a special enrollment period from February 15, 2021 through May 15, 2021 for purposes of obtaining health insurance coverage through the ACA marketplace. The executive order also instructs certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. It is unclear how the Supreme Court ruling, other such litigation and the healthcare reform measures of the Biden administration will impact the ACA and our business.
Other legislative changes have been proposed and adopted in the United States since the ACA was enacted. For example, COVID-19 relief legislation suspended the 2% Medicare sequester from May 1, 2020 through March 31, 2021.
Clinical and preclinical development is uncertain. Our current pre-clinical and clinical programs may experience delays, or our preclinical and clinical programs may never advance to clinical trials, which would adversely affect our ability to obtain regulatory approvals or commercialize these programs on a timely basis or at all, which would have an adverse effect on our business.
Our product candidates are in the pre-clinical and clinical stage, and their risk of failure is high. Before we can commence clinical trials for a product candidate, it must complete extensive preclinical testing and studies that support the planned INDs in the United States or similar applications in other jurisdictions. We cannot be certain of the timely completion or outcome of our preclinical testing and studies and cannot predict if the FDA or other regulatory authorities will accept the proposed clinical programs or if the outcome of preclinical studies will ultimately support the further development of the programs. As a result, we cannot be sure that we will be able to submit INDs or similar applications for our preclinical programs on the timelines we expect, if at all, and we cannot be sure that submission of INDs or similar applications will result in the FDA, the EMA or other regulatory authorities allowing clinical trials to begin.
In addition, clinical trial design for some of our product candidates can be complex given their characteristics. We will need to design our clinical trial to demonstrate efficacy across a range of doses to ensure that we can attain optimal potential efficacy. Our trial design may not demonstrate efficacy as we expect, and this may adversely impact our ability to successfully develop this product candidate.
We also cannot be certain that any of our product candidates will be successful in clinical trials or receive the necessary regulatory approval. Further, our product candidates may not receive regulatory approval even if they are successful in clinical trials. If we do not receive regulatory approvals for our product candidates, we may not be able to continue operations.
In March 2023, we announced that we had submitted an IND application with the FDA, requesting approval to initiate our first-in-human Phase I/IIa clinical trial with CMND-100 in patients suffering from AUD. Subsequently, and after submission of a similar application to the Israeli Ministry of Health, in May 2023 we initiated the CM-CMND-001 clinical trial in both Israel and the United States, including at the Yale School of Medicine’s Department of Psychiatry and Johns Hopkins University School of Medicine. The CM-CMND-001 clinical trial is designed to be a multinational, multi-center, Phase I/IIa single- and multiple-dose tolerability, safety and pharmacokinetic study in healthy volunteers and AUD subjects. Upon completion of the Phase I/IIa studies, if successful, we will be required to conduct additional clinical trials subject to securing additional financing.
Future clinical trials of our product candidates may be delayed, and certain programs may never advance in the clinic or may be more costly to conduct than we anticipate, any of which can affect our ability to fund our operations and would have a material adverse impact on our platform or our business.
Clinical testing is expensive, time consuming and subject to uncertainty. We cannot guarantee that any of our planned clinical trials will be conducted as planned or completed on schedule, if at all. Moreover, even if these trials are initiated or conducted on a timely basis, issues may arise that could result in the suspension or termination of such clinical trials. A failure of one or more clinical trials can occur at any stage of testing, and our clinical trials may not be successful. Events that may prevent successful or timely initiation or completion of clinical trials include:
| ● | inability to generate sufficient preclinical, toxicology, or other in vivo or in vitro data to support the initiation or continuation of clinical trials; |
| ● | delays in confirming target engagement, patient selection or other relevant biomarkers (with respect to certain of our clinical trials) to be utilized in preclinical and clinical product candidate development; |
| ● | delays in reaching a consensus with regulatory agencies as to the design or implementation of our clinical studies; |
| ● | delays in reaching agreement on acceptable terms with prospective contract research organizations, or CROs, and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and clinical trial sites; |
| ● | delays in identifying, recruiting and training suitable clinical investigators; |
| ● | delays in obtaining required Institutional Review Board, or IRB, approval at each clinical trial site; |
| ● | imposition of a temporary or permanent clinical hold by regulatory agencies for a number of reasons, including after review of an IND (including the IND application that we submitted to the FDA in March 2023 requesting approval to initiate our first-in-human Phase I/IIa clinical trial with CMND-100 in patients suffering from AUD) or amendment, clinical trial application, or CTA, or amendment, investigational device exemption, or IDE, or supplement, or equivalent application or amendment; as a result of a new safety finding that presents unreasonable risk to clinical trial participants; or a negative finding from an inspection of our clinical trial operations or study sites; |
| ● | developments in trials for other product candidates with the same targets or related modalities as our product candidates conducted by competitors that raise regulatory or safety concerns about risk to patients of the treatment, or if the FDA finds that the investigational protocol or plan is clearly deficient to meet its stated objectives; |
| ● | difficulties in securing access to materials for the comparator arm of certain of our clinical trials; |
| ● | delays in identifying, recruiting and enrolling suitable patients to participate in clinical trials, and delays caused by patients withdrawing from clinical trials or failing to return for post-treatment follow-up; |
| ● | difficulties in finding a sufficient number of trial sites, or trial sites deviating from trial protocol or dropping out of a trial; |
| ● | difficulty collaborating with patient groups and investigators; |
| ● | failure by CROs, other third parties, or us to adhere to clinical trial requirements; |
| ● | failure to perform in accordance with the FDA’s or any other regulatory authority’s current good clinical practices requirements, or GCPs, or regulatory guidelines in other countries, including deficiencies in the manufacturing process, test procedures and specifications or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; |
| ● | the FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from nonclinical studies or clinical trials; |
| ● | occurrence of adverse events, or AEs, undesirable side effects or other unexpected characteristics associated with the product candidate that are viewed to outweigh its potential benefits; |
| ● | changes in regulatory requirements and guidance that require amending or submitting new clinical protocols; |
| ● | changes in the standard of care on which a clinical development plan was based, which may require new or additional trials; |
| ● | the cost of clinical trials of any product candidates that we may identify and pursue being greater than we anticipate; |
| ● | clinical trials of any product candidates that we may identify and pursue producing negative or inconclusive results, which may result in our deciding, or regulators requiring us, to conduct additional clinical trials or abandon product development programs; |
|
|
● | transfer of manufacturing processes to larger-scale facilities operated by a Contract Manufacturing Organization, or CMO and delays or failures by our CMOs or us to make any necessary changes to such manufacturing process; and |
| ● | delays in manufacturing, testing, releasing, validating or importing/exporting sufficient stable quantities of product candidates that we may identify for use in clinical trials or the inability to do any of the foregoing. |
Any inability to successfully initiate or complete clinical trials could result in additional costs to us or impair our ability to generate revenue. In addition, if we make manufacturing or formulation changes to our product candidates, we may be required to, or we may elect to, conduct additional preclinical studies or clinical trials to bridge data obtained from the modified product candidates to data obtained from preclinical and clinical research conducted using earlier versions. Clinical trial delays could also shorten any periods during which our products have patent protection and may allow our competitors to bring products to market before we do, which could impair our ability to successfully commercialize product candidates and may harm our business and results of operations.
In addition, any disruptions that may be caused by any resurgence of the COVID-19 pandemic may increase the likelihood that we encounter such difficulties or delays in initiating, enrolling, conducting or completing future clinical trials. We could also encounter delays if a clinical trial is suspended or terminated by us, by the data safety monitoring board, or DSMB, or by the FDA, the EMA or other comparable foreign regulatory authorities, or if the IRBs of the institutions in which such trials are being conducted suspend or terminate the participation of their clinical investigators and sites subject to their review. Such authorities may suspend or terminate a clinical trial due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA, the EMA or other comparable foreign regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a product candidate, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial.
Delays in the initiation, conduct or completion of any clinical trial of our product candidates will increase our costs, slow down the product candidate development and approval process and delay or potentially jeopardize our ability to commence product sales and generate revenue. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates. In the event we identify any additional product candidates to pursue, we cannot be sure that submission of an IDE, IND, CTA or equivalent application, as applicable, will result in the FDA, the EMA or comparable foreign regulatory authority allowing clinical trials to begin in a timely manner, if at all. Any of these events could have a material adverse effect on our business, prospects, financial condition and results of operations.
Our current product candidates and future therapeutic candidates contain psychedelic substances, and may be subject to controlled substance laws and regulations in the territories where the product will be marketed, such as the United States and Europe, and failure to comply with these laws and regulations, or the cost of compliance with these laws and regulations, may adversely affect the results of our business operations, both during clinical development and post approval, and our financial condition.
Our product candidates contain psychedelic substances. The psychedelic drug industry is a fairly new treatment industry and we cannot predict the impact of the ever-evolving compliance regime in respect of this industry. Similarly, we cannot predict the time required to secure all appropriate regulatory approvals for future products, or the extent of testing and documentation that may, from time to time, be required by governmental authorities. The impact of compliance regimes, any delays in obtaining, or failure to obtain regulatory approvals may significantly delay or impact the development of markets, its business and products, and sales initiatives and could have a material adverse effect on the business, financial condition and operating results of the Corporation.
Our product candidates may be regulated by the U.S. Drug Enforcement Administration, or DEA, as “Controlled Substances” or scheduled substances, under the Comprehensive Drug Abuse Prevention and Control Act of 1970, also known as the Controlled Substances Act, or the CSA. The DEA regulates compounds as Schedule I, II, III, IV or V substances. Schedule I substances by definition have a high potential for abuse, have no currently “accepted medical use” in the United States, lack accepted safety for use under medical supervision and may not be prescribed, marketed or sold in the United States. Pharmaceutical products approved for use in the United States may be listed as Schedule II, III, IV or V, with Schedule II substances considered to present the highest potential for abuse or dependence and Schedule V substances the lowest relative risk of abuse among such substances. Schedule I and II drugs are subject to the strictest controls under the CSA, including manufacturing and procurement quotas, security requirements and criteria for importation. In addition, dispensing of Schedule II drugs is further restricted. Commercial marketing in the United States will also require scheduling-related legislative or administrative action.
Scheduling determinations by the DEA are dependent on FDA approval of a substance or a specific formulation of a substance. This scheduling determination will be dependent on FDA approval and the FDA’s recommendation as to the appropriate schedule. During the review process, and prior to approval, the FDA may determine that it requires additional data, either from non-clinical or clinical studies, including with respect to whether, or to what extent, the substance has abuse potential. This may introduce a delay into the approval and any potential rescheduling process. That delay would be dependent on the quantity of additional data required by the FDA. This scheduling determination will require the DEA to conduct notice and comment rule making, including issuing an interim final rule. Such action will be subject to public comment and requests for hearing, which could affect the scheduling of these substances. There can be no assurance that the DEA will make a favorable scheduling decision. Even assuming categorization as a Schedule II or lower controlled substance (i.e., Schedule III, IV or V), at the federal level, such substances would also require scheduling determinations under state laws and regulations.
If approved by the FDA, and if any of our product candidates is listed by the DEA as a Schedule II, III, IV or V controlled substance, their manufacture, importation, exportation, domestic distribution, storage, sale and legitimate use will continue to be subject to a significant degree of regulation by the DEA. In addition, the scheduling process may take significantly longer than the 90-day deadline set forth in the CSA, thereby delaying the launch of our product candidates in the United States. Furthermore, the FDA, DEA or any foreign regulatory authority could require us to generate more clinical or other data than we currently anticipate to establish whether or to what extent the substance has an abuse potential, which could increase the cost and/or delay the launch of our product candidates and any future therapeutic candidates containing controlled substances. In addition, therapeutic candidates containing controlled substances are subject to DEA regulations relating to manufacturing, storage, distribution and physician prescription procedures, including:
| ● | DEA registration and inspection of facilities. Facilities conducting research, manufacturing, distributing, importing or exporting, or dispensing controlled substances must be registered (licensed) to perform these activities and have the security, control, recordkeeping, reporting and inventory mechanisms required by the DEA to prevent drug loss and diversion. All these facilities must renew their registrations annually, except dispensing facilities, which must renew every three years. The DEA conducts periodic inspections of certain registered establishments that handle controlled substances. Obtaining and maintaining the necessary registrations may result in delay of the importation, manufacturing or distribution of our product candidates. Furthermore, failure to maintain compliance with the CSA, particularly non-compliance resulting in loss or diversion, can result in regulatory action that could have a material adverse effect on our business, financial condition and results of operations. The DEA may seek civil penalties, refuse to renew necessary registrations or initiate proceedings to restrict, suspend or revoke those registrations. In certain circumstances, violations could lead to criminal proceedings. |
| ● | State-controlled substances laws. Individual U.S. states have also established controlled substance laws and regulations. Though state-controlled substances laws often mirror federal law, because the states are separate jurisdictions, they may separately schedule our product candidates. While some states automatically schedule a drug based on federal action, other states schedule drugs through rule making or a legislative action. State scheduling may delay commercial sale of any product for which we obtain federal regulatory approval, and adverse scheduling could have a material adverse effect on the commercial attractiveness of such product. If any of our product candidates becomes subject to controlled substance laws, we or our collaborators would also be required to obtain separate state registrations, permits or licenses in order to be able to obtain, handle and distribute controlled substances for clinical trials or commercial sale, and failure to meet applicable regulatory requirements could lead to enforcement and sanctions by the states in addition to those from the DEA or otherwise arising under federal law. |
| ● | Clinical trials. Research sites must submit a research protocol to the DEA and obtain and maintain a DEA researcher registration that will allow those sites to handle and dispense our product candidates and to obtain the product from our importer. If the DEA delays or denies the grant of a researcher registration to one or more research sites, the clinical trial could be significantly delayed, and clinical trial sites could be lost. The importer for the clinical trials would also be required to obtain a Schedule I importer registration and an import permit for each import. |
| ● | Importation. If our product candidates are approved and classified as a Schedule II, III or IV substance, an importer can import them for commercial purposes if it obtains an importer registration and files an application for an import permit for each import. The DEA provides annual assessments/estimates to the International Narcotics Control Board, which guides the DEA in the amounts of controlled substances that the DEA authorizes to be imported. The failure to identify an importer or obtain the necessary import authority, including specific quantities, could affect the availability of our product candidates and have a material adverse effect on our business, results of operations and financial condition. In addition, an application for a Schedule II importer registration must be published in the Federal Register, and there is a waiting period for third-party comments to be submitted. It is always possible that adverse comments may delay the grant of an importer registration. If our product candidates are approved and classified as a Schedule II controlled substance, federal law may prohibit the import of the substance for commercial purposes. If our product candidates are listed as a Schedule II substance, we will not be allowed to import the drug for commercial purposes unless the DEA determines that domestic supplies are inadequate or there is inadequate domestic competition among domestic manufacturers for the substance as defined by the DEA. Moreover, Schedule I controlled substances have never been registered with the DEA for importation for commercial purposes, only for scientific and research needs. Therefore, if neither our product candidates nor our drug substances could be imported, the product candidates would have to be wholly manufactured in the United States, and we would need to secure a manufacturer that would be required to obtain and maintain a separate DEA registration for that activity. |
| ● | Manufacture in the United States. If, because of a Schedule II classification or voluntarily, we were to conduct manufacturing or repackaging/relabeling in the United States, our contract manufacturers would be subject to the DEA’s annual manufacturing and procurement quota requirements. The annual quota allocated to us or our contract manufacturers for the active ingredient in our product candidates may not be sufficient to complete clinical trials or meet commercial demand. Consequently, any delay or refusal by the DEA in establishing our, or our contract manufacturers’, procurement and/or production quota for controlled substances could delay or stop our clinical trials or product launches, which could have a material adverse effect on our business, financial position and results of operations. |
| ● | Distribution in the United States. If our product candidates are scheduled as Schedule II, III or IV, we would also need to identify wholesale distributors with the appropriate DEA registrations and authority to distribute our product candidates and any future therapeutic candidates. These distributors would need to obtain Schedule II, III or IV distribution registrations. This limitation in the ability to distribute our product candidates more broadly may limit commercial uptake and could negatively impact our prospects. The failure to obtain, or delay in obtaining, or the loss of any of those registrations could result in increased costs to us. If our product candidates are a Schedule II drug, participants in our supply chain may have to maintain enhanced security with alarms and monitoring systems and they may be required to adhere to recordkeeping and inventory requirements. This may discourage some pharmacies from carrying the product. In addition, our product candidates will likely be determined to have a high potential for abuse and therefore required to be administered at our trial sites, which could limit commercial update. Furthermore, state and federal enforcement actions, regulatory requirements and legislation intended to reduce prescription drug abuse, such as the requirement that physicians consult a state prescription drug monitoring program, may make physicians less willing to prescribe, and pharmacies to dispense, Schedule II products. |
In the event MEAI is evaluated as a controlled substance and is scheduled under Schedule I of the U.S. Schedules of Controlled Substances, it will be prevented from being prescribed, marketed or sold in the United States.
Currently in the United States, MEAI has not been officially evaluated for its status as a controlled substance. It is currently unknown how it will be scheduled, and it is not currently found in Schedule II of the U.S. Schedules of Controlled Substances. However, if the molecule is considered to be similar to amphetamines, then it may fall under this schedule as a stimulant, under which amphetamines are listed. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation which contains any quantity of the following substances having a stimulant effect on the central nervous system are in this schedule: Amphetamine, its salts, optical isomers, and salts of its optical isomers. This molecule also does not exist on the U.S. Toxic Substances Control Act Chemical Substance Inventory List.
In Canada, Health Canada has stated that MEAI is a controlled substance due to its structure being similar to amphetamines, which are on Health Canada’s Controlled Substances List. However, at this point in time, the molecule MEAI itself is not listed in Schedule I of Health Canada’s Controlled Substances List, which includes: amphetamines, their salts, derivatives, isomers and analogues and salts of derivatives, isomers and analogues.
In Israel, the Israeli Ministry of Health has stated that 2 – Aminoindane, including derivatives and isomers, is a controlled substance. Since, MEAI is a derivative of 2 – Aminoindane, it is considered a controlled substance.
Our product candidates contain potentially controlled substances, the use of which may generate public controversy. Adverse publicity or public perception regarding our current or future product candidates may negatively influence the success of these therapies.
Our therapies containing potentially controlled substances may generate public controversy. Political and social pressures and adverse publicity could lead to delays in approval of, and increased expenses for our current product candidates and any future therapeutic candidates we may develop. Opponents of these therapies may seek restrictions on marketing and withdrawal of any regulatory approvals. In addition, these opponents may seek to generate negative publicity in an effort to persuade the medical community to reject these therapies. Adverse publicity from misuse may adversely affect the commercial success or market penetration achievable by our product candidates. Anti-psychedelic protests have historically occurred and may occur in the future and generate media coverage. Political pressures and adverse publicity could lead to delays in, and increased expenses for, and limit or restrict the introduction and marketing of, our product candidates or any future therapeutic candidates.
If our product candidates or any future therapeutic candidates are approved for commercial sale and are deemed to be controlled substances, we will be highly dependent upon consumer perceptions of the safety and quality of our therapies. We may face limited adoption if third-party therapy sites, therapists or patients are unwilling to try such a novel treatment given that some of our therapies are from substances that might be controversial, overlooked or underused. There has been a history of negative media coverage regarding psychedelic substances, including compounds in many of our product candidates, which may affect the public’s perception of our therapies. In addition, compounds in most of our product candidates may elicit intense psychological experiences, and this could deter patients from choosing this course of treatment. Our business could be adversely affected if we were subject to negative publicity or if any of our therapies or any similar therapies distributed by other companies prove to be, or are asserted to be, harmful to patients. Because of our dependence upon consumer perception, any adverse publicity associated with illness or other adverse effects resulting from patients’ use or misuse of our therapies or any similar therapies distributed by other companies could have a material adverse impact on our business, prospects, financial condition and results of operations.
Future adverse events in research into alcohol abuse and binge-drinking, on which we focus our research efforts, or the pharmaceutical industry more generally, could also result in greater governmental regulation, stricter labeling requirements and potential regulatory delays in the testing or approvals of our therapies. Any increased scrutiny could delay or increase the costs of obtaining regulatory approval for our product candidates or any future therapeutic candidates.
Any clinical trials that we may conduct in the future may fail to demonstrate substantial evidence of the safety and effectiveness of product candidates that we may identify and pursue for their intended uses, which would prevent, delay or limit the scope of regulatory approval and potential commercialization.
Before obtaining regulatory approvals for the commercial sale of any of our product candidates, we must demonstrate through lengthy, complex and expensive preclinical studies and clinical trials that the applicable product candidate is both safe and effective for use in each target indication. Each product candidate must demonstrate an adequate risk versus benefit profile in its intended patient population and for its intended use.
Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical development process. Most product candidates that begin clinical trials are never approved by regulatory authorities for commercialization. We have limited experience in designing clinical trials and may be unable to design and execute a clinical trial to support marketing approval.
We cannot be certain that our clinical trials will be successful. Additionally, any safety concerns observed in any one of our clinical trials in our targeted indications could limit the prospects for regulatory approval of our product candidates in those and other indications, which could have a material adverse effect on our business, financial condition and results of operations. In addition, even if such clinical trials are successfully completed, we cannot guarantee that the FDA, the EMA or comparable foreign regulatory authorities will interpret the results as we do, and more trials could be required before we submit our product candidates for approval. Even if regulatory approval is secured for a product candidate, the terms of such approval may limit the scope and use of the specific product candidate, which may also limit its commercial potential.
Additionally, we may utilize an “open-label” trial design for some of our future clinical trials. An open-label trial is one where both the patient and investigator know whether the patient is receiving the test article or either an existing approved drug or placebo. Open-label trials are subject to various limitations that may exaggerate any therapeutic effect as patients in open-label studies are aware that they are receiving treatment. Open-label trials may be subject to a “patient bias” where patients perceive their symptoms to have improved merely due to their awareness of receiving an experimental treatment. Patients selected for early clinical studies often include the most severe sufferers, and their symptoms may have been bound to improve notwithstanding the new treatment. In addition, open-label trials may be subject to an “investigator bias” where those assessing and reviewing the physiological outcomes of the clinical trials are aware of which patients have received treatment and may interpret the information of the treated group more favorably given this knowledge. The opportunity for bias in clinical trials as a result of open-label design may not be adequately handled and may cause any of our trials that utilize such design to fail or to be considered inadequate and additional trials may be necessary to support future marketing applications. Moreover, results acceptable to support approval in one jurisdiction may be deemed inadequate by another regulatory authority to support regulatory approval in that other jurisdiction. To the extent that the results of the trials are not satisfactory to the FDA, the EMA or comparable foreign regulatory authorities for support of a marketing application, we may be required to expend significant resources, which may not be available to us, to conduct additional trials in support of potential approval of our product candidates. Even if regulatory approval is secured for a product candidate, the terms of such approval may limit the scope and use of the specific product candidate, which may also limit its commercial potential.
Even if we complete the necessary preclinical studies and clinical trials, the marketing approval process is expensive, time consuming and uncertain and may prevent us from obtaining approvals for the potential commercialization of our product candidates.
Any product we may develop and the activities associated with their development and potential commercialization, including their design, testing, manufacture, safety, efficacy, recordkeeping, labeling, storage, approval, advertising, promotion, sale and distribution, are subject to comprehensive regulation by the FDA, the EMA and other comparable foreign regulatory authorities. Failure to obtain marketing authorization for a product candidate will prevent us from commercializing the product candidate in a given jurisdiction.
We expect to rely on assistance from third-party CROs or regulatory consultants to assist us in filing and supporting the applications necessary to gain marketing authorizations. Securing regulatory approval requires the submission of extensive preclinical and clinical data and supporting information to the various regulatory authorities for each therapeutic indication to establish the product candidate’s safety, purity, efficacy and potency. Securing regulatory approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the relevant regulatory authority. Any product candidates we develop may not be effective, may be only moderately effective, or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude our obtaining marketing approval or prevent or limit commercial use, if approved.
The process of obtaining marketing authorizations, both in the United States and abroad, is expensive, may take many years if additional clinical trials are required, if approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the product candidates involved.
Changes in marketing authorization policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for each submitted product application, may cause delays in the approval or rejection of an application. The FDA and comparable authorities in other countries have substantial discretion in the approval process and may refuse to accept any application or may decide that our data are insufficient for approval and require additional preclinical, clinical or other studies. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit or prevent marketing approval of a product candidate. Any marketing approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable.
If we experience delays in obtaining approval, or if we fail to obtain approval of any product candidates we may develop, the commercial prospects for those product candidates may be harmed, and our ability to generate revenues will be materially impaired.
The results of early-stage clinical trials and preclinical studies may not be predictive of future results. Initial data in clinical trials may not be indicative of results obtained when these trials are completed or in later stage trials.
The results of preclinical studies may not be predictive of the results of clinical trials, and the results of any early-stage clinical trials we commence may not be predictive of the results of the later-stage clinical trials. The results of preclinical studies and clinical trials in one set of patients or disorder indications, or from preclinical studies or clinical trials that we did not lead, may not be predictive of those obtained in another. In some instances, there can be significant variability in safety or efficacy results between different clinical trials of the same product candidate due to numerous factors, including changes in trial procedures set forth in protocols, differences in the size and type of the patient populations, changes in and adherence to the dosing regimen and other clinical trial protocols and the rate of dropout among clinical trial participants. In addition, preclinical and clinical data are often susceptible to various interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval. A number of companies in the pharmaceutical, biopharmaceutical and biotechnology industries have suffered significant setbacks in clinical development even after achieving promising results in earlier studies, and any such setbacks in our clinical development could have a material adverse effect on our business and operating results. Even if early-stage clinical trials are successful, we may need to conduct additional clinical trials of our product candidates in additional patient populations or under different treatment conditions before we are able to seek approvals from the FDA, the EMA or other comparable foreign regulatory authorities to market and sell these product candidates. Failure to obtain marketing authorization for our product candidates could substantially harm our business, prospects, financial condition and results of operations.
Research and development of drugs targeting the CNS is particularly difficult, which makes it difficult to predict and understand why the drug has a positive effect on some patients but not others.
Discovery and development of new drug candidates designed to target CNS disorders are particularly difficult and time-consuming, evidenced by the higher failure rate for new drugs for CNS disorders compared with most other areas of drug discovery. For example, Pharmos, Roche, Allegran’s NMDA and Merck have all failed to meet their end points. Any such setbacks in our clinical development could have a material adverse effect on our business and operating results. In addition, our later-stage clinical trials may present challenges related to conducting adequate and well-controlled clinical trials, particularly as it regards managing placebo effects.
If we encounter difficulties enrolling patients in clinical trials, our clinical development activities could be delayed or otherwise adversely affected.
Identifying and qualifying trial participants to participate in clinical studies is critical to our success. The timing of our clinical studies depends, among other things, on the speed at which we can recruit trial participants to participate in testing our product candidates and our ability to enroll a sufficient number of patients who remain in the trial until its conclusion. Delays in enrollment and withdrawals from the trial may result in increased costs or may affect the timing or outcome of the planned clinical trials, which could prevent completion of these trials and adversely affect our ability to advance the development of our product candidates. If trial participants are unwilling to participate in our studies because of negative publicity from adverse events in our trials or other trials of similar products, or those related to specific therapeutic area, or for other reasons, including competitive clinical studies for similar patient populations, the timeline for recruiting trial participants, conducting studies, and obtaining regulatory approval of potential products may be delayed. These delays could result in increased costs, delays in advancing our product candidate development, delays in testing the effectiveness of these product candidates, or termination of the clinical studies altogether.
We may not be able to identify, recruit and enroll a sufficient number of trial participants, or those with required or desired characteristics to achieve diversity in a study, to initiate and complete any future clinical studies in a timely manner. Patient and subject enrollment is affected by factors including:
| ● | the size and nature of a patient population; |
| ● | the patient eligibility criteria defined in the applicable clinical trial protocols, which may limit the patient populations eligible for clinical trials to a greater extent than competing clinical trials for the same indication; |
| ● | the size of the study population required for analysis of the trial’s primary endpoints; |
| ● | the severity of the disorder under investigation; |
| ● | the proximity of patients to a trial site; |
| ● | the inclusion and exclusion criteria for the trial in question; |
| ● | the design of the trial protocol; |
| ● | the ability to recruit clinical trial investigators with the appropriate competencies and experience; |
| ● | the approval or concurrent enrollment of clinical trials involving competing product candidates currently under development or competing clinical trials for similar therapies or targeting patient populations meeting our patient eligibility criteria; |
| ● | the availability and efficacy of approved medications or therapies for the disorder or condition under investigation; |
| ● | clinicians’ and patients’ perceptions as to the potential advantages and side effects of the product candidate being studied in relation to other available therapies and product candidates; |
| ● | the ability to obtain and maintain patient consents; and |
| ● | the risk that patients enrolled in clinical trials will not complete such trials, for any reason. |
Additionally, our or our collaborators’ ability to successfully initiate, enroll and conduct a clinical trial outside the United States is subject to numerous additional risks, including:
| ● | difficulty in establishing or managing relationships with CROs and physicians; |
| ● | differing standards for the conduct of clinical trials; |
| ● | differing standards of care for patients with a particular disorder; |
| ● | an inability to locate qualified local consultants, physicians and partners; and |
| ● | the potential burden of complying with a variety of foreign laws, medical standards and regulatory requirements, including the regulation of pharmaceutical and biotechnology products and treatments. |
Further, successful and timely enrollment in clinical trials may be adversely affected by global health factors, including, among other things, pandemics such as COVID-19, such as:
| ● | the diversion of healthcare resources away from the conduct of clinical trial matters to focus on pandemic concerns, including the attention of physicians serving as our clinical trial investigators, hospitals serving as our clinical trial sites and hospital staff supporting the conduct of our clinical trials; |
| ● | the limitation of available participants for our trials and a decrease in enrollment of our trials; |
| ● | the inability of patients, therapists or physicians to come to hospitals and universities to participate in our trials, leading to delays and increased costs; |
| ● | limitations on travel that interrupt key trial activities, such as clinical trial site initiations and monitoring and patient preparation and integration sessions; |
| ● | interruption in global shipping affecting the transport of clinical trial materials, such as investigational drug product and comparator drugs used in our trials; and |
| ● | employee furlough days that delay necessary interactions with local regulators, ethics committees and other important agencies and contractors. |
These and other factors arising from the COVID-19 pandemic could worsen in countries that are already afflicted with the virus or could continue to spread to additional countries, each of which may further adversely impact our clinical trials. The global outbreak of COVID-19 continues to evolve, and the conduct of our trials may continue to be adversely affected.
If we have difficulty enrolling sufficient numbers of patients to conduct clinical trials as planned, we may need to delay or terminate clinical trials, either of which would have an adverse effect on our business.
Use of our product candidates could be associated with side effects, adverse events or other properties or safety risks, which could delay or halt their clinical development, prevent their regulatory approval, cause us to suspend or discontinue clinical trials, abandon a product candidate, limit their commercial potential, if approved, or result in other significant negative consequences that could severely harm our business, prospects, financial condition and results of operations.
As is the case with pharmaceuticals generally, it is likely that there may be unexpected or undesirable side effects, but they can’t be disclosed since no clinical trials have been conducted yet with this compound. Undesirable side effects may be found in the future. The side effects caused by these product candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA, the EMA or other comparable foreign regulatory authorities. The side effects related to the product candidate could affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims. Any of these occurrences may harm our business, financial condition, results of operations and prospects significantly.
Moreover, if our product candidates are associated with undesirable side effects in preclinical studies or clinical trials or have characteristics that are unexpected, we may elect to abandon their development or limit their development to more narrow uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective, which may limit the commercial expectations for the product candidate if approved. We may also be required to modify or terminate our study plans based on findings in our preclinical studies or clinical trials. Many product candidates that initially show promise in early-stage testing may later be found to cause side effects that prevent further development. As we work to advance existing product candidates and to identify new product candidates, we cannot be certain that later testing or trials of product candidates that initially showed promise in early testing will not be found to cause similar or different unacceptable side effects that prevent their further development.
It is possible that as we test our product candidates in larger, longer and more extensive clinical trials, or as the use of these product candidates becomes more widespread if they receive regulatory approval, illnesses, injuries, discomforts and other AEs that were not observed in earlier trials, as well as conditions that did not occur or went undetected in previous trials, may be reported by subjects. If such side effects become known later in development or upon approval, if any, such findings may harm our business, financial condition, results of operations and prospects significantly.
Additionally, adverse developments in clinical trials of pharmaceutical, biopharmaceutical or biotechnology products conducted by others may cause the FDA or other regulatory oversight bodies to suspend or terminate our clinical trials or to change the requirements for approval of any of our product candidates.
In addition to side effects caused by the product candidate, the administration process or related procedures also can cause adverse side effects. If any such AEs occur, our clinical trials could be suspended or terminated. If we are unable to demonstrate that any AEs were caused by the administration process or related procedures, the FDA, the European Commission, the EMA, or other regulatory authorities could order us to cease further development of, or deny approval of, a product candidate for any or all targeted indications. Even if we can demonstrate that all future serious adverse events, or SAEs, are not product-related, such occurrences could affect patient recruitment or the ability of enrolled patients to complete the trial. Moreover, if we elect, or are required, to not initiate, delay, suspend or terminate any future clinical trial of any of our product candidates, the commercial prospects of such product candidates may be harmed and our ability to generate product revenues from any of these product candidates may be delayed or eliminated. Any of these occurrences may harm our ability to develop other product candidates, and may harm our business, financial condition, results of operations and prospects significantly.
Additionally, if any of our product candidates receives marketing authorization, the FDA could impose contraindications or a boxed warning in the labeling of the product. For any of our drug product candidates receiving marketing authorization, the FDA could require us to adopt a risk evaluation and mitigation strategy, or REMS, and could apply elements to assure safe use to ensure that the benefits of the product outweigh its risks, which may include, among other things, a Medication Guide outlining the risks of the product for distribution to patients and a communication plan to health care practitioners. Furthermore, if we or others later identify undesirable side effects caused by our product candidates if approved, several potentially significant negative consequences could result, including:
| ● | regulatory authorities may suspend or withdraw approvals of such product candidate, or seek an injunction against its manufacture or distribution; |
| ● | regulatory authorities may require additional warnings on the label, including “boxed” warnings, or issue safety alerts, Deal Healthcare Provider letters, press releases or other communications containing warnings or other safety information about the product; |
| ● | we may be required by the FDA to implement a REMS; |
| ● | we may be required to change the way a product candidate is administered or conduct additional clinical trials; |
| ● | we may be subject to fines, injunctions or the imposition of civil or criminal penalties; |
| ● | we could be sued and held liable for harm caused to patients; and |
| ● | our reputation may suffer. |
Any of these occurrences could prevent us from achieving or maintaining market acceptance of the particular product candidate, if approved, and may harm our business, financial condition, results of operations and prospects significantly.
Even if any of our current or future product candidate receives regulatory approval, it may fail to achieve the degree of market acceptance by physicians, patients, third-party payors and others in the medical community necessary for commercial success, in which case we may not generate significant revenues or become profitable.
We are a clinical stage company. We have never commercialized a product, and even if any of our current or future product candidate is approved by the appropriate regulatory authorities for marketing and sale, it may nonetheless fail to gain sufficient market acceptance by physicians, patients, third-party payors and others in the medical community. Physicians may be reluctant to take their patients off their current medications and switch their treatment regimen. Further, patients often acclimate to the treatment regime that they are currently taking and do not want to switch unless their physicians recommend switching products or they are required to switch due to lack of coverage and adequate reimbursement. In addition, even if we are able to demonstrate our product candidates’ safety and efficacy to the FDA and other regulators, safety or efficacy concerns in the medical community may hinder market acceptance.
Efforts to educate the medical community and third-party payors on the benefits of our product candidates may require significant resources, including management time and financial resources, and may not be successful. The degree of market acceptance of our product candidates, if approved for commercial sale, will depend on a number of factors, including:
| ● | the efficacy and safety of the product as demonstrated in pivotal clinical trials; |
| ● | the potential and perceived advantages of the product compared to competitive and alternative therapies; |
| ● | the prevalence and severity of any side effects; |
| ● | whether the product is designated under physician treatment guidelines as a first-, second- or third-line therapy; |
| ● | our ability, or the ability of any future collaborators, to offer the product for sale at competitive prices; |
| ● | the product’s convenience and ease of dosing and administration compared to alternative treatments, including the need to have products administered in clinical settings, rather than the home, for patients who are prescribed the products; |
| ● | the willingness of the target patient population to try, and of physicians to prescribe, the product; | |
| ● | limitations or warnings, including distribution or use restrictions contained in the product’s approved labeling; |
| ● | the strength of sales, marketing and distribution support; |
| ● | restrictions on how the product is distributed; |
| ● | the timing of market introduction of competitive products; |
| ● | publicity concerning these products or competing products and treatments; |
| ● | changes in the standard of care for the targeted indications for the product; and |
| ● | availability and adequacy of coverage and reimbursement from government payors, managed care plans and other third-party payors. |
Sales of medical products also depend on the willingness of physicians to prescribe the treatment, which is likely to be based on a determination by these physicians that the products are safe, therapeutically effective and cost effective. In addition, the inclusion or exclusion of products from treatment guidelines established by various physician groups and the viewpoints of influential physicians can affect the willingness of other physicians to prescribe the treatment. We cannot predict whether physicians, physicians’ organizations, hospitals, other healthcare providers, government agencies or private insurers will determine that any of our products is safe, therapeutically effective and cost effective as compared with competing treatments. If any product candidates we develop do not achieve an adequate level of acceptance, they may not generate significant product revenue, and we may not become profitable.
For any of our current or future product candidates that obtains regulatory approval, any failure to achieve market acceptance or commercial success would adversely affect our business prospects. In addition, for any approved product, any negative perception of such product once commercialized, or of a similar product developed by a competitor, may adversely affect our reputation in the marketplace or among industry participants and our business prospects.
We currently, and may in the future continue to, conduct clinical trials for product candidates outside the United States, and the FDA, the EMA and comparable foreign regulatory authorities may not accept data from such trials.
We currently, and may in the future continue to, conduct one or more clinical trials outside the United States, including in Europe. The acceptance of study data from clinical trials conducted outside the United States or another jurisdiction by the FDA, the EMA or any comparable foreign regulatory authority may be subject to certain conditions or may not be accepted at all. In cases where data from foreign clinical trials are intended to serve as the basis for marketing approval in the United States, the FDA will generally not approve the application on the basis of foreign data alone unless (i) the data are applicable to the U.S. population and U.S. medical practice; (ii) the trials were performed by clinical investigators of recognized competence and pursuant to GCP regulations; and (iii) if necessary, the FDA is able to validate the data through an on-site inspection or other appropriate means. Additionally, the FDA’s clinical trial requirements, including sufficient size of patient populations and statistical powering, must be met. Many foreign regulatory authorities have similar approval requirements. There can be no assurance that the FDA, the EMA or any comparable foreign regulatory authority will accept data from trials conducted outside of the United States or the applicable jurisdiction. If the FDA, the EMA or any comparable foreign regulatory authority does not accept such data, it would result in the need for additional trials, which would be costly and time consuming and delay aspects of our business plan, and which may result in product candidates that we may develop not receiving approval or clearance for commercialization in the applicable jurisdiction.
In addition, such foreign trials would be subject to the applicable local laws of the foreign jurisdictions where the trials are conducted. The conduct of clinical trials outside the United States could have a significant impact on us. Risks inherent in conducting international clinical trials include:
| ● | foreign regulatory requirements that could burden or limit our ability to conduct our clinical trials; |
| ● | administrative burdens of conducting clinical trials under multiple foreign regulatory schema; |
| ● | foreign exchange fluctuations; |
| ● | manufacturing, customs, shipment and storage requirements; |
| ● | cultural differences in medical practice and clinical research; and |
| ● | diminished protection of intellectual property in some countries. |
If we are unable to obtain regulatory approval in one or more jurisdictions for any product candidates that we may identify and develop, our business will be substantially harmed.
We cannot commercialize a product until the appropriate regulatory authorities have reviewed and approved the product candidate. Approval by the FDA, the EMA and comparable foreign regulatory authorities is lengthy and unpredictable, and depends upon numerous factors, including substantial discretion of the regulatory authorities. Approval policies, regulations, or the type and amount of preclinical or clinical data necessary to gain approval may change during the course of a product candidate’s development and may vary among jurisdictions, which may cause delays in the approval or the decision not to approve an application. We have not obtained regulatory approval for any of our product candidates, and it is possible that our current product candidates and any other product candidates that we may seek to develop in the future will not ever obtain regulatory approval. We cannot be certain that any of our product candidates will receive regulatory approval or be successfully commercialized, even if they receive regulatory approval.
Obtaining marketing approval is an extensive, lengthy, expensive and inherently uncertain process, and regulatory authorities may delay, limit or deny approval of our product candidates for many reasons, including but not limited to:
| ● | the inability to demonstrate to the satisfaction of the FDA, the EMA or comparable foreign regulatory authorities that the applicable product candidate is safe and effective as a treatment for our targeted indications or otherwise meets the applicable regulatory standards for approval; |
| ● | the FDA, the EMA or comparable foreign regulatory authorities may disagree with the design, endpoints or implementation of our clinical trials; |
| ● | the population studied in the clinical program may not be sufficiently broad or representative to assure safety or efficacy in the full population for which we seek approval; |
| ● | the FDA, the EMA or comparable foreign regulatory authorities may require additional preclinical studies or clinical trials beyond those that we currently anticipate; |
| ● | the FDA, the EMA or comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials; |
| ● | the data collected from clinical trials of product candidates that we may identify and pursue may not be sufficient to support the submission of a New Drug Application, or NDA, or other submission for regulatory approval in the United States or elsewhere; |
| ● | we may be unable to demonstrate to the FDA, the EMA or comparable foreign regulatory authorities that a product candidate’s risk-benefit ratio for its proposed indication is acceptable; |
| ● | the FDA, the EMA or comparable foreign regulatory authorities may identify deficiencies in the manufacturing processes, test procedures and specifications, or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; and |
| ● | the approval policies or regulations of the FDA, the EMA or comparable foreign regulatory authorities may change in a manner that renders the clinical trial design or data insufficient for approval. |
The lengthy approval process, as well as the unpredictability of the results of clinical trials and evolving regulatory requirements, may result in our failure to obtain regulatory approval to market product candidates that we may pursue in the United States or elsewhere, which would significantly harm our business, prospects, financial condition and results of operations.
Furthermore, approval by the FDA in the United States, if obtained, does not ensure approval by regulatory authorities in other countries or jurisdictions. In order to market any products outside of the United States, we must establish and comply with numerous and varying regulatory requirements of other countries regarding safety and effectiveness. Clinical trials conducted in one country may not be accepted by regulatory authorities in other countries, and regulatory approval in one country does not mean that regulatory approval will be obtained in any other country. Approval processes vary among countries and can involve additional product testing and validation and additional or different administrative review periods from those in the United States, including additional preclinical studies or clinical trials, as clinical trials conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the United States, a product candidate must be approved for reimbursement by insurers before it can be approved for sale in that jurisdiction. In some cases, the price that we intend to charge for our products is also subject to approval.
Seeking foreign regulatory approval could result in difficulties and costs for us and require additional preclinical studies or clinical trials which could be costly and time consuming. Regulatory requirements can vary widely from country to country and could delay or prevent the introduction of our products in those countries. The foreign regulatory approval process involves all of the risks associated with FDA approval. We do not have any product candidates approved for sale in international markets. If we fail to comply with regulatory requirements in international markets or to obtain and maintain required approvals, or if regulatory approvals in international markets are delayed, our target market will be reduced and our ability to realize the full market potential of our products will be harmed.
Interim, “top-line,” and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available or as additional analyses are conducted, and as the data are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we may publish interim, “top-line,” or preliminary data from our clinical studies. Interim data from clinical trials are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available. Preliminary or “top-line” data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published. As a result, interim and preliminary data should be viewed with caution until the final data are available. Material adverse changes between preliminary, “top-line,” or interim data and final data could significantly harm our business prospects.
Further, others, including regulatory agencies, may not accept or agree with our assumptions, estimates, calculations, conclusions or analyses or may interpret or weigh the importance of data differently, which could impact the value of the particular program, the approvability or commercialization of the particular product candidate or product and our company in general, and regulatory agencies may request further data from us. In addition, the information we choose to publicly disclose regarding a particular study or clinical trial is based on what is typically extensive information, and you or others may not agree with what we determine is the material or otherwise appropriate information to include in our disclosure. Any information we determine not to disclose may ultimately be deemed significant by you or others with respect to future decisions, conclusions, views, activities or otherwise regarding a particular product candidate or our business. If the top-line data that we report differ from actual results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for, and commercialize any future product candidate, our business, prospects, financial condition and results of operations may be harmed.
Certain of the product candidates we are developing are complex and difficult to manufacture. We could experience manufacturing problems that result in delays in our development or commercialization programs or otherwise harm our business.
The manufacturing processes our CMOs use to produce our product candidates are complex, and materials are challenging to source. Several factors could cause production interruptions, including inability to develop efficient manufacturing processes, equipment malfunctions, facility contamination, raw material shortages or contamination, natural disasters, disruption in utility services, human error or disruptions in the operations of our suppliers, including acquisition of the supplier by a third party or declaration of bankruptcy.
Our CMOs must employ multiple steps to control the manufacturing process to assure that the process is reproducible and the product candidate is made strictly and consistently in compliance with the process. Problems with the manufacturing process, even minor deviations from the normal process, could result in product defects or manufacturing failures that result in lot failures, product recalls, product liability claims or insufficient inventory to conduct clinical trials or supply commercial markets. We may encounter problems achieving adequate quantities and quality of clinical-grade materials that meet the FDA, the EMA or other applicable standards or specifications with consistent and acceptable production yields and costs.
In addition, the FDA, the EMA and other foreign regulatory authorities may require us to submit samples of any lot of any approved product together with the protocols showing the results of applicable tests at any time. Under some circumstances, the FDA, the EMA or other foreign regulatory authorities may require that we do not distribute a lot until the agency authorizes its release. Slight deviations in the manufacturing process, including those affecting quality attributes and stability, may result in unacceptable changes in the product that could result in lot failures or product recalls. Lot failures or product recalls could cause us to delay product launches or clinical trials, which could be costly to us and otherwise harm our business, financial condition, results of operations and prospects.
We or our CMOs also may encounter problems hiring and retaining the experienced scientific, quality assurance, quality-control and manufacturing personnel needed to operate our manufacturing processes, which could result in delays in production or difficulties in maintaining compliance with applicable regulatory requirements.
Any problems in our or our CMOs’ manufacturing process or facilities could result in delays in planned clinical trials and increased costs, and could make us a less attractive collaborator for potential partners, including larger biotechnology companies and academic research institutions, which could limit access to additional attractive development programs. Problems in our or our CMOs’ manufacturing process could restrict our or their ability to meet potential future market demand for products.
We may not elect or be able to take advantage of any expedited development or regulatory review and approval processes available to drug product candidates granted breakthrough therapy or fast track designation by the FDA.
We intend to evaluate and continue ongoing discussions with the FDA on regulatory strategies that could enable us to take advantage of expedited development pathways for certain of our product candidates in the future, although we cannot be certain that our product candidates will qualify for any expedited development pathways or that regulatory authorities will grant, or allow us to maintain, the relevant qualifying designations. Potential expedited development pathways that we could pursue include breakthrough therapy and fast track designation.
Breakthrough therapy designation is intended to expedite the development and review of drug product candidates that are designed to treat serious or life-threatening diseases when preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The designation of a product candidate as a breakthrough therapy provides potential benefits that include more frequent meetings with the FDA to discuss the development plan for the product candidate and ensure collection of appropriate data needed to support approval; more frequent written correspondence from the FDA about such things as the design of the proposed clinical trials and use of biomarkers; intensive guidance on an efficient drug development program, beginning as early as Phase 1; organizational commitment involving senior managers; and eligibility for rolling review and priority review.
Fast track designation is designed for drug product candidates intended for the treatment of a serious or life-threatening disease or disorder, where preclinical or clinical data demonstrate the potential to address an unmet medical need for this disease or disorder. Accordingly, even if we believe a particular product candidate is eligible for breakthrough therapy or fast track designation, we cannot assure you that the FDA would decide to grant it. Breakthrough therapy designation and fast track designation do not change the standards for product approval, and there is no assurance that such designation or eligibility will result in expedited review or approval or that the approved indication will not be narrower than the indication covered by the breakthrough therapy designation or fast track designation. Thus, even if we receive breakthrough therapy or fast track designation, we may not experience a faster development process, review or approval compared to conventional FDA procedures. The FDA may withdraw breakthrough therapy or fast track designation if it believes that the product no longer meets the qualifying criteria. Our business may be harmed if we are unable to avail ourselves of these or any other expedited development and regulatory pathways.
For any approved product, we will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense, and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our product candidates.
If any of our product candidates are approved, they will be subject to ongoing regulatory requirements for manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping, conduct of post-marketing studies, and submission of safety, efficacy and other post-market information, including both federal and state requirements in the United States and requirements of comparable foreign regulatory authorities.
Manufacturers and manufacturers’ facilities are required to comply with extensive requirements imposed by the FDA, the EMA and other comparable foreign regulatory authorities, including ensuring that quality control and manufacturing procedures conform to current good manufacturing practices, or cGMP, regulations. As such, we and our CMOs are subject to continual review and inspections to assess compliance with cGMP and adherence to commitments made in any NDA or marketing authorization application, or MAA, or equivalent application. We and our CMOs are also subject to requirements pertaining to the registration of our and their manufacturing facilities and the listing of our product and product candidates with the FDA; continued complaint, adverse event and malfunction reporting; corrections and removals reporting and labeling and promotional requirements. Accordingly, we and others with whom we work must continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production and quality control.
Any regulatory approvals that we may receive for our product candidates may contain requirements for potentially costly post-marketing testing, such as Phase 4 clinical trials and surveillance to monitor the safety and efficacy of a drug product. We are required to report certain adverse reactions and production problems, if any, to the FDA, the EMA and other comparable foreign regulatory authorities. Any new legislation addressing drug or medical safety issues could result in delays in product development or commercialization or increased costs to assure compliance.
The FDA and other agencies, including the U.S. Department of Justice, and for certain products, the Federal Trade Commission, closely regulate and monitor the post-approval marketing, labeling, advertising and promotion of products to ensure that they are manufactured, marketed and distributed only for the approved indications and in accordance with the provisions of the approved label. We are, and will be, required to comply with requirements concerning advertising and promotion for our product candidates, if approved. For example, promotional communications with respect to prescription drugs and medical devices are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the product’s label or labeling. Accordingly, we may not promote our products for indications or uses for which they do not have approval.
The holder of an approved NDA, MAA or equivalent marketing authorization must submit new or supplemental applications and obtain approval for certain changes to the approved product, product labeling, or manufacturing process. Delays in obtaining required approvals would harm our ability to introduce new or enhanced product in a timely manner, which in turn would harm our or our future growth. Failure to submit a new or supplemental application and to obtain approval for certain changes prior to marketing the modified product may require a recall or to stop selling or distributing the marketed product as modified and may lead to significant enforcement actions.
In the European Economic Area, or the EEA, any medical devices will need to comply with the Essential Requirements set forth in Medical Device Regulation. Compliance with these requirements is a prerequisite to be able to affix the CE mark to a product, without which a product cannot be marketed or sold in the EEA. To demonstrate compliance with the Essential Requirements and obtain the right to affix the CE mark, we must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. The conformity assessment procedure requires the intervention of a Notified Body, which is an organization designated by a competent authority of an EEA country to conduct conformity assessments. The Notified Body issues a CE Certificate of Conformity following successful completion of a conformity assessment procedure and quality management system audit conducted in relation to the medical device and its manufacturer and their conformity with the Essential Requirements. This Certificate entitles the manufacturer to affix the CE mark to its medical products after having prepared and signed a related EC Declaration of Conformity.
We could also be required to conduct post-marketing clinical trials to verify the safety and efficacy of our products in general or in specific patient subsets. If original marketing approval of a drug was obtained via an accelerated approval pathway, we could be required to conduct a successful post-marketing clinical trial to confirm clinical benefit for our products. An unsuccessful post-marketing study or failure to complete such a study could result in the withdrawal of marketing approval.
If a regulatory agency discovers previously unknown problems with a product, such as AEs of unanticipated severity or frequency, or problems with the facility where the product is manufactured, or disagrees with the promotion, marketing or labeling of a product, such regulatory agency may impose restrictions on that product or us, including requiring withdrawal of the product from the market. If we fail to comply with applicable regulatory requirements, a regulatory agency or enforcement authority may, among other things:
| ● | issue warning letters or untitled letters; |
| ● | impose civil or criminal penalties; |
| ● | suspend, withdraw or modify regulatory approvals; |
| ● | suspend or modify any of our ongoing clinical trials; |
| ● | refuse to approve pending applications or supplements to approved applications submitted by us; |
| ● | mandate modifications to promotional materials or require us to provide corrective information to healthcare practitioners, or require other restrictions on the labeling or marketing of such products; |
| ● | impose restrictions on our operations, including closing our programs’ or our or their CMOs’ facilities; |
| ● | seize or detain products, refuse to permit the import or export of products; or |
| ● | require a product recall. |
Any government investigation of alleged violations of law could require us to expend significant time and resources in response, and could generate negative publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our, our ability to commercialize and generate revenue. If regulatory sanctions are applied or if regulatory approval is withdrawn, the value of our Company and our operating results will be adversely affected.
The FDA’s and other regulatory authorities’ policies may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates.
We also cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad.
The FDA and other regulatory agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses.
If any of our product candidates are approved and we are found to have improperly promoted off-label uses of those products, we may become subject to significant liability. The FDA and other regulatory agencies strictly regulate the promotional claims that may be made about prescription products, if approved. In particular, while the FDA permits the dissemination of truthful and non-misleading information about an approved product, a manufacturer may not promote a product for uses that are not approved by the FDA or such other regulatory agencies as reflected in the product’s approved labeling. If we are found to have promoted such off-label uses, we may become subject to significant liability. The federal government has levied large civil and criminal fines against companies for alleged improper promotion of off-label use and has enjoined several companies from engaging in off-label promotion. The government has also required companies to enter into consent decrees, corporate integrity agreements or imposed permanent injunctions under which specified promotional conduct must be changed or curtailed. If we cannot successfully manage the promotion of our product candidates, if approved, we could become subject to significant liability, which would materially adversely affect our business, financial condition and results of operations.
Risks Related to Commercialization
If, in the future, we are unable to establish sales and marketing capabilities or enter into agreements with third parties to sell and market any product candidates we may develop, we may not be successful in commercializing those product candidates if and when they are approved.
We do not have a sales or marketing infrastructure and have little experience in the sale, marketing or distribution of pharmaceutical products. To achieve commercial success for any approved product for which we retain sales and marketing responsibilities, we must either develop a sales and marketing organization or outsource these functions to third parties. In the future, we may choose to build a focused sales, marketing and commercial support infrastructure to market and sell our product candidates, if and when they are approved. We may also elect to enter into collaborations or strategic partnerships with third parties to engage in commercialization activities with respect to selected product candidates, indications or geographic territories, including territories outside the United States, although there is no guarantee we will be able to enter into these arrangements even if the intent is to do so.
There are risks involved with both establishing our own commercial capabilities and entering into arrangements with third parties to perform these services. For example, recruiting and training a sales force or reimbursement specialists is expensive and time consuming and could delay any product launch. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing and other commercialization capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition commercialization personnel.
Factors that may inhibit our efforts to commercialize any approved product on our own include:
| ● | the inability to recruit and retain adequate numbers of effective sales, marketing, reimbursement, customer service, medical affairs, and other support personnel; |
| ● | the inability of sales personnel to obtain access to physicians or persuade adequate numbers of physicians to prescribe any future approved products; |
| ● | the inability of reimbursement professionals to negotiate arrangements for formulary access, reimbursement, and other acceptance by payors; |
| ● | the inability to price products at a sufficient price point to ensure an adequate and attractive level of profitability; |
| ● | restricted or closed distribution channels that make it difficult to distribute our products to segments of the patient population; |
| ● | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and |
| ● | unforeseen costs and expenses associated with creating an independent commercialization organization. |
If we enter into arrangements with third parties to perform sales, marketing, commercial support and distribution services, the profitability of product revenue may be lower than if we were to market and sell any products developed by us. In addition, we may not be successful in entering into arrangements with third parties to commercialize our product candidates or may be unable to do so on terms that are favorable to us or them. We may have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively or may expose us to legal and regulatory risk by not adhering to regulatory requirements and restrictions governing the sale and promotion of prescription drug products, including those restricting off-label promotion. If we do not establish commercialization capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates, if approved.
The third-party payor coverage and reimbursement status of newly approved products is uncertain. Our product candidates may become subject to unfavorable pricing regulations or third-party coverage and reimbursement policies, any of which could harm our business. Failure to obtain or maintain coverage and adequate reimbursement for new or current products could limit our ability to market those products and decrease our ability to generate revenue.
The regulations that govern marketing approvals, pricing, coverage and reimbursement for new drugs and other medical products vary widely from country to country. In the United States, healthcare reform legislation may significantly change the approval requirements in ways that could involve additional costs and cause delays in obtaining approvals. Some countries require approval of the sale price of a product before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we might obtain marketing approval for a product in a particular country but then be subject to price regulations that delay the commercial launch of the product, possibly for lengthy time periods, and negatively impact the revenue we are able to generate from the sale of the product in that country. Adverse pricing limitations may hinder our ability to recoup our investment in one or more of our product candidates, even if any of our product candidates obtain marketing approval.
Our ability to successfully commercialize our product candidates also will depend in part on the extent to which coverage and adequate reimbursement for these products and related treatments will be available from government health administration authorities, private health insurers and other organizations. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. The availability of coverage and extent of reimbursement by governmental and private payors is essential for most patients to be able to afford treatments. Sales of these or other product candidates that we may identify will depend substantially, both domestically and abroad, on the extent to which the costs of our product candidates will be paid by health maintenance, managed care, pharmacy benefit and similar healthcare management organizations, or reimbursed by government health administration authorities, private health coverage insurers and other third-party payors. If coverage and adequate reimbursement is not available, or is available only to limited levels, we may not be able to successfully commercialize our products or product candidates. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us to establish or maintain pricing sufficient to realize a sufficient return on our investment. For products administered under the supervision of a physician, obtaining coverage and adequate reimbursement may be particularly difficult because of the higher prices often associated with such drugs.
A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Government authorities and third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. In many countries, the prices of medical products are subject to varying price control mechanisms as part of national health systems. In general, the prices of medicines under such systems are substantially lower than in the United States. Other countries allow companies to fix their own prices for medicines but monitor and control company profits. Additional foreign price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our product candidates. Accordingly, in markets outside the United States, the reimbursement for products may be reduced compared with the United States and may be insufficient to generate commercially reasonable revenues and profits.
There is also significant uncertainty related to the insurance coverage and reimbursement of newly approved products, and coverage may be more limited than the purposes for which the medicine is approved by the FDA or comparable foreign regulatory authorities. In the United States, no uniform policy of coverage and reimbursement for products exists among third-party payors and coverage and reimbursement levels for products can differ significantly from payor to payor. Commercial payors often rely upon Medicare coverage policy and payment limitations in setting their own policies, but also have their own methods and approval process apart from Medicare determinations. As a result, the coverage determination process is often a time consuming and costly process that may require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance. It is difficult to predict what the Centers for Medicare & Medicaid Services, or CMS, the federal agency responsible for administering the Medicare program, will decide with respect to reimbursement for fundamentally novel products such as ours, as there is no body of established practices and precedents for these new products. Reimbursement agencies in Europe may be more conservative than CMS. For example, a number of cancer drugs have been approved for reimbursement in the United States and have not been approved for reimbursement in certain European countries. Moreover, eligibility for reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim reimbursement levels for new drugs, if applicable, may also not be sufficient to cover our costs and may not be made permanent. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs and may be incorporated into existing payments for other services. Our inability to promptly obtain coverage and profitable payment rates from both government-funded and private payors for any approved products we may develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize product candidates and our overall financial condition.
Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. Our inability to promptly obtain coverage and profitable reimbursement rates third-party payors for any approved products that we develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
Increasingly, third-party payors are requiring that pharmaceutical companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. We cannot be sure that reimbursement will be available for any product candidate that we commercialize and, if reimbursement is available, the level of reimbursement. Reimbursement may impact the demand for, or the price of, any product or product candidate for which we obtain marketing approval. In order to obtain reimbursement, physicians may need to show that patients have superior treatment outcomes with our products compared to standard of care drugs, including lower-priced generic versions of standard of care drugs. We expect to experience pricing pressures in connection with the sale of any of our product candidates, due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative changes. The downward pressure on healthcare costs in general, particularly prescription drugs and surgical procedures and other treatments, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products.
Additionally, we may develop companion diagnostic tests for use with our product candidates. We may be required to obtain coverage and reimbursement for these tests separate and apart from the coverage and reimbursement we seek for our product candidates, once approved. Even if we obtain regulatory approval for such companion diagnostics, there is significant uncertainty regarding our ability to obtain coverage and adequate reimbursement for the same reasons applicable to our product candidates. Medicare reimbursement methodologies, whether under Part A, Part B, or clinical laboratory fee schedule may be amended from time to time, and we cannot predict what effect any change to these methodologies would have on any product candidate or companion diagnostic for which we receive approval.
If we fail to comply with healthcare laws, we could face substantial penalties and our business, financial condition and results of operations could be adversely affected.
Healthcare providers, physicians and third-party payors in the United States and elsewhere play a primary role in the recommendation and prescription of pharmaceutical products. Arrangements with healthcare providers, third-party payors and customers can expose pharmaceutical manufacturers to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which such companies sell, market and distribute pharmaceutical products. In particular, the promotion, sales and marketing of healthcare items and services, as well as certain business arrangements in the healthcare industry, are subject to extensive laws designed to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of ownership, pricing, discounting, marketing and promotion, structuring and commission(s), certain customer incentive programs and other business arrangements generally. Activities subject to these laws also involve the improper use of information obtained in the course of patient recruitment for clinical trials. The applicable federal and state healthcare laws and regulations laws that may affect our ability to operate include, but are not limited to:
| ● | the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe, or rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce, or in return for, either the referral of an individual, or the purchase, lease, order or recommendation of any good, facility, item or service for which payment may be made, in whole or in part, under a federal healthcare program, such as the Medicare and Medicaid programs. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. Violations are subject to civil and criminal fines and penalties for each violation, plus up to three times the remuneration involved, imprisonment of up to ten years, and exclusion from government healthcare programs. The Anti-Kickback Statute has been interpreted to apply to arrangements between pharmaceutical manufacturers, on the one hand, and prescribers, purchasers and formulary managers, on the other; |
| ● | federal civil and criminal false claims laws, including the False Claims Act, or FCA, which impose criminal and civil penalties, including through civil “qui tam” or “whistleblower” actions, against individuals or entities for, among other things, knowingly presenting, or causing to be presented, claims for payment or approval from Medicare, Medicaid, or other federal health care programs that are false or fraudulent; knowingly making or causing a false statement material to a false or fraudulent claim or an obligation to pay money to the federal government; or knowingly concealing or knowingly and improperly avoiding or decreasing such an obligation. Manufacturers can be held liable under the FCA even when they do not submit claims directly to government payors if they are deemed to “cause” the submission of false or fraudulent claims. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the FCA. The FCA also permits a private individual acting as a “whistleblower” to bring actions on behalf of the federal government alleging violations of the FCA and to share in any monetary recovery. When an entity is determined to have violated the federal civil False Claims Act, the government may impose civil fines and penalties for each false claim, plus treble damages, and exclude the entity from participation in Medicare, Medicaid and other federal healthcare programs; |
| ● | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created additional federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private) and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for, healthcare benefits, items or services relating to healthcare matters. Similar to the federal Anti-Kickback Statute, a person or entity can be found guilty of violating HIPAA without actual knowledge of the statute or specific intent to violate it; |
| ● | the federal civil monetary penalties laws, which impose civil fines for, among other things, the offering or transfer or remuneration to a Medicare or state healthcare program beneficiary if the person knows or should know it is likely to influence the beneficiary’s selection of a particular provider, practitioner, or supplier of services reimbursable by Medicare or a state healthcare program, unless an exception applies; |
| ● | the federal Physician Payments Sunshine Act, created under the Affordable Care Act, or the ACA, and its implementing regulations, which require manufacturers of drugs, devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to the U.S. Department of Health and Human Services, or HHS, under the Open Payments Program, information related to payments or other transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. Effective January 1, 2022, these reporting obligations will extend to include transfers of value made to certain non-physician providers such as physician assistants and nurse practitioners; |
| ● | federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; |
| ● | federal price reporting laws, which require manufacturers to calculate and report complex pricing metrics to government programs, where such reported prices may be used in the calculation of reimbursement and/or discounts on approved products; and |
| ● | analogous state and foreign laws and regulations, such as state and foreign anti-kickback, false claims, consumer protection and unfair competition laws which may apply to pharmaceutical business practices, including but not limited to, research, distribution, sales and marketing arrangements as well as submitting claims involving healthcare items or services reimbursed by any third-party payer, including commercial insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government that otherwise restricts payments that may be made to healthcare providers and other potential referral sources; state laws that require drug manufacturers to file reports with states regarding pricing and marketing information, such as the tracking and reporting of gifts, compensations and other remuneration and items of value provided to healthcare professionals and entities; and state and local laws requiring the registration of pharmaceutical sales representatives. |
Because of the breadth of these laws and the narrowness of the statutory exceptions and regulatory safe harbors available, it is possible that some of our business activities could, despite efforts to comply, be subject to challenge under one or more of such laws. Additionally, the FDA or foreign regulators may not agree that we have mitigated any risk of bias in our clinical trials due to payments provided to investigators or institutions which could limit a regulator’s acceptance of those clinical trial data in support of a marketing application. Moreover, efforts to ensure that our business arrangements will comply with applicable healthcare laws may involve substantial costs.
It is possible that governmental and enforcement authorities will conclude that such business practices may not comply with current or future statutes, regulations or case law interpreting applicable fraud and abuse or other healthcare laws and regulations. If any such actions are instituted against us and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant civil, criminal and administrative penalties, damages, disgorgement, monetary fines, exclusion from participation in Medicare, Medicaid and other federal healthcare programs, integrity and oversight agreements to resolve allegations of non-compliance, contractual damages, reputational harm, diminished profits and future earnings, and curtailment or restructuring of our, our operations, any of which could adversely affect our ability to operate our business and our results of operations. Even if we are successful in defending ourselves or asserting our rights, the existence of these actions may adversely affect market prices of our common shares. In addition, the approval and commercialization of any of our product candidates outside the United States will also likely subject us to foreign equivalents of the healthcare laws mentioned above, among other foreign laws.
We may become subject to U.S. federal and state forfeiture laws which could negatively impact our business operations.
Violations of any U.S. federal laws and regulations could result in significant fines, penalties, administrative sanctions, convictions or settlements arising from civil proceedings conducted by either the federal government or private citizens, or criminal charges, including, but not limited to, seizure of assets, disgorgement of profits, cessation of business activities or divestiture. As an entity that conducts business involving scheduled drugs, we are potentially subject to federal and state forfeiture laws (criminal and civil) that permit the government to seize the proceeds of criminal activity. Civil forfeiture laws could provide an alternative for the federal government or any state (or local police force) that wants to discourage residents from conducting transactions with scheduled drugs but believes criminal liability is too difficult to prove beyond a reasonable doubt. Also, an individual can be required to forfeit property considered to be the proceeds of a crime even if the individual is not convicted of the crime, and the standard of proof in a civil forfeiture matter is lower than the standard in a criminal matter. Depending on the applicable law, whether federal or state, rather than having to establish liability beyond a reasonable doubt, the federal government or the state, as applicable, may be required to prove that the money or property at issue is proceeds of a crime only by either clear and convincing evidence or a mere preponderance of the evidence.
Investors located in jurisdictions where any of our product candidates remain illegal may be at risk of prosecution under conspiracy, aiding and abetting, and money laundering statutes, and be at further risk of losing their investments or proceeds under forfeiture statutes. Many jurisdictions remain fully able to take action to prevent the proceeds of such product candidates from entering their state. Our investors and prospective investors should be aware of these potentially relevant laws in considering whether to invest in us.
Failure to comply with health and data protection laws and regulations could lead to government enforcement actions (which could include civil or criminal penalties), private litigation, and/or adverse publicity and could negatively affect our operating results and business.
We and any potential collaborators may be subject to federal, state, and foreign data protection laws and regulations, policies and contractual obligations that apply to the collection, transmission, storage, processing and use of personal information or personal data, which among other things, impose certain requirements relating to the privacy, security and transmission of personal information. The legislative and regulatory landscape for privacy and data protection continues to evolve in jurisdictions worldwide, and there has been an increasing focus on privacy and data protection issues with the potential to affect our business. Complying with such requirements can be difficult, time-consuming, expensive, and could require us to change our business practices and put in place additional compliance mechanisms. Failure to comply with laws, regulations and contractual and other obligations governing personal or other sensitive information could result in enforcement actions against us, including fines, imprisonment of company officials and public censure, processing penalties, claims for damages by affected individuals, damage to our reputation and loss of goodwill. It is possible that new and existing laws may be interpreted and applied in a manner that is inconsistent with our practices and our efforts to comply with the evolving data protection rules may be unsuccessful.
In the United States, numerous federal and state laws and regulations, including federal health information privacy laws, state data breach notification laws, state health information privacy laws, and federal and state consumer protection laws (e.g., Section 5 of the Federal Trade Commission Act), that govern the collection, use, disclosure and protection of health-related and other personal information could apply to our operations or the operations of our programs and their collaborators. In addition, we may obtain health information from third parties (including research institutions from which we obtain clinical trial data) that are subject to privacy and security requirements under HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH. HIPAA establishes privacy and security standards that limit the use and disclosure of individually identifiable health information, or protected health information, and require the implementation of administrative, physical and technological safeguards to protect the privacy of protected health information and ensure the confidentiality, integrity and availability of electronic protected health information. Depending on the facts and circumstances, we could be subject to civil, criminal, and administrative penalties if we knowingly obtain, use, or disclose individually identifiable health information maintained by a HIPAA-covered entity in a manner that is not authorized or permitted by HIPAA.
In addition to HIPAA, additional privacy and data security laws and regulations have been enacted in the United States and additional laws and regulations may be enacted in the near future. For example, the California Consumer Privacy Act, or the CCPA, which became effective on January 1, 2020, requires companies that process information on California residents to make new disclosures to consumers about their data collection, use and sharing practices, provides such individuals with new data privacy rights, including the ability to opt out of certain sales of personal information, imposes new operational requirements for covered businesses, provides a private right of action for data breaches and creates a statutory damages framework. Many other states are considering similar legislation, and a broad range of legislative measures also have been introduced at the federal level. In addition, California voters recently approved a new privacy law, the California Privacy Rights Act, or the CPRA, which significantly modifies the CCPA, including by expanding consumers’ rights with respect to certain personal information and creating a new state agency to oversee implementation and enforcement efforts. Many of the CPRA’s provisions became effective on January 1, 2023. Although there are limited exemptions for clinical trial data under the CCPA, the CCPA and other similar laws could impact our business activities depending on how it is interpreted and exemplifies the vulnerability of our business to the evolving regulatory environment related to personal information.
In the event we decide to conduct clinical trials or continue to enroll subjects in our ongoing or future clinical trials in Europe, we may be subject to additional privacy restrictions. The collection, use and transfer of personal health data in the EEA is governed by the provisions of the General Data Protection Regulation 2016/679, or the GDPR. The GDPR went into effect in May 2018 and establishes a strengthened individual data rights regime and imposes several requirements for controllers and processors of personal data relating to the establishment of a legal basis for processing, the consent of the individuals to whom the personal data relates, notification of data processing obligations to the competent national data protection authorities, the implementation of safeguards to protect the security and confidentiality of the personal data that requires the adoption of administrative, physical and technical safeguards, shortened timelines for data breach notifications to appropriate data protection authorities or data subjects, limitations on retention and secondary use of information, as well as increased requirements pertaining to health data and pseudonymized (i.e., key-coded) data and additional obligations when data controllers contract with third-party processors in connection with the processing of the personal data. The GDPR also imposes strict rules on the transfer of personal data out of the EEA to recipients in countries outside the EEA, such as the United States. The GDPR allows EU and EEA member states to make additional laws and regulations further limiting the processing of genetic, biometric or health data.
Failure to comply with the requirements of the GDPR and the related national data protection laws of the EU and EEA Member States may result in fines of up to €20,000,000 or up to 4% of the total worldwide annual turnover of the preceding financial year, whichever is higher, and other administrative penalties. In addition, the GDPR confers a private right of action on data subjects and consumer associations to lodge complaints with supervisory authorities, seek judicial remedies, and obtain compensation for damages resulting from violations of the GDPR. The GDPR may impose additional responsibility and liability in relation to personal data that we process and we may be required to put in place additional mechanisms ensuring compliance with these and/or new data protection rules. For example, the GDPR increases our obligations with respect to clinical trials conducted in the EEA by expanding the definition of personal data to include coded data and requiring changes to informed consent practices and more detailed notices for clinical trial subjects and investigators. This may be onerous and adversely affect our business, prospects, financial condition and results of operations. The United Kingdom has transposed the GDPR into domestic law, with its version of the GDPR taking effect in January 2021, which could expose us to two parallel regimes, each of which potentially authorizes similar fines for certain violations. Other EU countries have also passed or are considering passing similar laws.
Compliance with U.S. and international data protection laws and regulations, including the GDPR, the CCPA and the CPRA could require us to take on more onerous obligations in our contracts, restrict our ability to collect, use and disclose data, change our business practices and put in place additional compliance mechanisms, which may interrupt or delay our development, regulatory and commercialization activities and increase our cost of doing business or in some cases, impact our ability to operate in certain jurisdictions. Failure to comply with these laws and regulations could result in government enforcement actions (which could include civil, criminal and administrative penalties), regulatory investigations, private litigation, significant fine and remediation costs and/or adverse publicity and could negatively affect our operating results and business. Moreover, clinical trial subjects, employees and other individuals about whom we or our potential collaborators obtain personal information, as well as the providers who share this information with us, may limit our ability to collect, use and disclose the information. Claims that we or our programs have violated individuals’ privacy rights, failed to comply with data protection laws, or breached our contractual obligations, even if we or they are not found liable, could be expensive and time consuming to defend and could result in adverse publicity that could harm our business, financial condition and results of operations.
Healthcare legislative measures aimed at reducing healthcare costs may have a material adverse effect on our business and results of operations.
The United States and many foreign jurisdictions have enacted or proposed legislative and regulatory changes affecting the healthcare system that could prevent or delay marketing approval of our product candidates or any future product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product for which we obtain marketing approval. Changes in regulations, statutes or the interpretation of existing regulations could impact our business in the future by requiring, for example: (i) changes to our manufacturing arrangements; (ii) additions or modifications to product labeling; (iii) the recall or discontinuation of our products or (iv) additional record-keeping requirements. If any such changes were to be imposed, they could adversely affect the operation of our business.
In the United States, there have been and continue to be a number of legislative initiatives and judicial challenges to contain healthcare costs. For example, in March 2010, the ACA was passed, which substantially changed the way healthcare is financed by both governmental and private insurers, and significantly impacted the U.S. pharmaceutical industry. The ACA, among other things, subjects biological products to potential competition by lower-cost biosimilars, addresses a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected, increases the minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program and extends the rebate program to individuals enrolled in Medicaid managed care organizations, establishes annual fees and taxes on manufacturers of certain branded prescription drugs, and creates a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 70 percent (effective as of 2019) point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D.
Payment methodologies may be subject to changes in healthcare legislation and regulatory challenges. For example, in order for a drug product to receive federal reimbursement under the Medicaid or Medicare Part B programs or to be sold directly to U.S. government agencies, the manufacturer must extend discounts to entities eligible to participate in the 340B drug pricing program. For the 2018 and 2019 fiscal years, CMS altered the reimbursement formula from Average Sale Price, or ASP, plus 6 percent to ASP minus 22.5 percent on specified covered outpatient drugs, or SCODs, but did so without issuing a formal notice of proposed rulemaking. On December 27, 2018, the District Court for the District of Columbia invalidated that formula change, ruling the change was not an “adjustment” that was within the Secretary’s discretion to make but was instead a fundamental change in the reimbursement calculation, and such a dramatic change was beyond the scope of the Secretary’s authority. On July 31, 2020, the U.S. Court of Appeals for the District of Columbia reversed the District Court’s decision. Based on the D.C. Circuit’s decision, CMS proposed for calendar year 2021 and subsequent years to pay for drugs acquired under the 340B program at ASP minus 34.7 percent, plus an add-on, for a net payment rate of ASP minus 28.7, or continue to pay ASP minus 22.5 percent. In December 2020, CMS instead finalized its current policy of paying ASP minus 22.5 percent for 340B-acquired drugs, effective January 1, 2021. It is unclear how future changes to the payment methodology may affect pharmaceutical manufacturers and hospitals who purchase their products now and in the future.
There have been a number of significant changes to the ACA and its implementation. The Tax Cuts and Jobs Act of 2017, or the Tax Act, includes a provision that repealed, effective January 1, 2019, the tax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” The U.S. Supreme Court is currently reviewing the constitutionality of the ACA, although it is unclear when a decision will be made or how the Supreme Court will rule. It is also unclear how other efforts to challenge, repeal or replace the ACA will impact the ACA or our business.
In addition, other legislative changes have been proposed and adopted in the United States since the ACA was enacted. In August 2011, the Budget Control Act of 2011, among other things, resulted in aggregate reductions of Medicare payments to providers of 2 percent per fiscal year, which went into effect in 2013, and, due to subsequent legislative amendments, will remain in effect through 2030, with the exception of a temporary suspension from May 1, 2020 through March 31, 2021 unless additional Congressional action is taken. The American Taxpayer Relief Act of 2012 further reduced Medicare payments to several types of providers, including hospitals and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
There has been increasing legislative and enforcement interest in the United States with respect to drug pricing practices. Specifically, there have been several recent U.S. Congressional inquiries and proposed federal and state legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. The likelihood of implementation of any of these reform initiatives is uncertain, particularly in light of the new Presidential administration. The policies and priorities of an incoming administration are unknown and could materially impact the regulation governing our product candidates, if approved.
In addition, on May 30, 2018, the Right to Try Act was signed into law. The law, among other things, provides a federal framework for certain patients to access certain investigational new drug products that have completed a Phase 1 clinical trial and that are undergoing investigation for FDA approval. Under certain circumstances, eligible patients can seek treatment without enrolling in clinical trials and without obtaining FDA permission under the FDA expanded access program. There is no obligation for a drug manufacturer to make its drug products available to eligible patients as a result of the Right to Try Act, but the manufacturer must develop an internal policy and respond to patient requests according to that policy.
At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. Furthermore, there has been increased interest by third-party payors and governmental authorities in reference pricing systems and publication of discounts and list prices.
There have been, and likely will continue to be, legislative and regulatory proposals at the foreign, federal and state levels directed at containing or lowering the cost of healthcare. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our product. Such reforms could have an adverse effect on anticipated revenue from product candidates that we may successfully develop and for which we may obtain regulatory approval and may affect our overall financial condition and ability to develop product candidates. We cannot predict the initiatives that may be adopted in the future. The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of healthcare and/or impose price controls may adversely affect:
| ● | the demand for our product candidates, if approved; |
| ● | our ability to receive or set a price that we believe is fair for our products; |
| ● | our ability to generate revenue and achieve or maintain profitability; | |
| ● | the amount of taxes that we are required to pay; and | |
| ● | the availability of capital. |
We expect that the other healthcare reform measures that may be adopted in the future, may result in additional reductions in Medicare and other healthcare funding, more rigorous coverage criteria, lower reimbursement, and new payment methodologies. This could lower the price that we receive for any approved product. Any denial in coverage or reduction in reimbursement from Medicare or other government-funded programs may result in a similar denial or reduction in payments from private payors, which may prevent us from being able to generate sufficient revenue, attain profitability or commercialize our product candidates, if approved.
Governments outside the United States may impose strict price controls, which may adversely affect our revenues, if any.
In some countries, including Member States of the European Union, the pricing of prescription drugs is subject to governmental control. Additional countries may adopt similar approaches to the pricing of prescription drugs. In such countries, pricing negotiations with governmental authorities can take considerable time after receipt of regulatory approval for a product. In addition, there can be considerable pressure by governments and other stakeholders on prices and reimbursement levels, including as part of cost containment measures. Political, economic and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after coverage and reimbursement have been obtained. Reference pricing used by various countries and parallel distribution, or arbitrage between low-priced and high-priced countries, can further reduce prices. In some countries, we may be required to conduct a clinical study or other studies that compare the cost-effectiveness of any of our product candidates to other available therapies in order to obtain or maintain reimbursement or pricing approval, which is time-consuming and costly. We cannot be sure that such prices and reimbursement will be acceptable to us. Publication of discounts by third-party payors or authorities may lead to further pressure on the prices or reimbursement levels within the country of publication and other countries. If pricing is set at unsatisfactory levels or if reimbursement of our products is unavailable or limited in scope or amount, our revenues from sales by us or our strategic partners and the potential profitability of any of our product candidates in those countries would be negatively affected.
We face significant competition in an environment of rapid technological and scientific change, and there is a possibility that our competitors may achieve regulatory approval before we do or develop therapies that are safer, more advanced or more effective than ours, which may negatively impact our ability to successfully market or commercialize any product candidates we may develop and ultimately harm our financial condition.
The pharmaceutical industry is highly competitive, with new approaches and technologies regularly emerging. We expect to face competition across our current programs and with any future programs we may seek to develop and/or commercialize from major pharmaceutical, biotechnology, specialty pharmaceutical and generic pharmaceutical companies among others. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization. In addition, programs that we currently believe to be complementary may eventually become competitors.
If any of our competitors receives FDA approval before we do, our product candidates would not be the first treatment on the market, and our market share may be limited. In addition to competition from other companies targeting our target indications, any products we may develop may also face competition from other types of therapies.
In addition, psychedelic drugs have recently attracted attention as a potential treatment for various mental illnesses. Leveraging earlier research, a relatively small variety of organizations have taken the medical and pharmaceutical approach to introduce psychedelic drugs in the market, potentially resulting in an entirely new class of drugs and treatment protocols, disrupting legacy therapies. We will face competition from psychedelic companies in general, and also from psychedelic companies that are focusing on alcohol abuse, like Awakn Life Sciences, B.More Inc. and Journey Colab Corp.
Many of our current or potential competitors, either alone or with their strategic partners, may have or develop in the future:
| ● | greater financial, technical, and human resources than we have at every stage of the discovery, development, manufacture, and commercialization of products; |
| ● | more extensive experience in preclinical testing, conducting clinical trials, obtaining regulatory approvals, and in manufacturing, marketing, and selling drug products; |
| ● | products that have been approved or are in late stages of development; and |
| ● | collaborative arrangements in our target markets with leading companies and research institutions. |
Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products we may develop. Furthermore, currently approved products could be discovered to have application for treatment of our targeted disorder indications or similar indications, which could give such products significant regulatory and market timing advantages over our product candidates. Our competitors may also obtain FDA, EMA or other comparable foreign regulatory approval for their products more rapidly than we may obtain approval for ours or may obtain orphan product exclusivity from the FDA for indications that we are targeting, which could result in our competitors establishing a strong market position before we are able to enter the market. Additionally, products or technologies developed by our competitors may render our potential product candidates uneconomical or obsolete, and we may not be successful in marketing any product candidates we may develop against competitors.
In addition, we could face litigation or other proceedings with respect to the scope, ownership, validity and/or enforceability of our programs’ patents relating to our competitors’ products, and our competitors may allege that our products infringe, misappropriate or otherwise violate their intellectual property. The availability of our competitors’ products could limit the demand, and the price we are able to charge, for any products that we may develop and commercialize.
If the market opportunities for our product candidates are smaller than we believe they are, our revenue may be adversely affected, and our business may suffer. Our ability to successfully identify patients and acquire a significant market share will be necessary for us to achieve profitability and growth.
We focus research and product development on treatments for the treatment of alcohol abuse and binge drinking. Our projections of both the number of individuals who are affected by our target disorder indications and have the potential to benefit from treatment with our product candidates, are based on our beliefs and estimates. These estimates have been derived from a variety of sources, including the scientific literature, and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of these disorders. The number of patients may turn out to be lower than expected. The effort to identify patients with these mental health disorders we seek to treat is in early stages, and we cannot accurately predict the number of patients for whom treatment might be possible. Additionally, the potentially addressable patient population for our product candidates that we may identify may be limited or may not be amenable to treatment with our product candidates, and new patients may become increasingly difficult to identify or gain access to, which would adversely affect our results of operations and our business. Further, even if we obtain significant market share for our product candidates, because the potential target populations are small, we may never achieve profitability.
Risks Related to Reliance on Third Parties
We may seek to enter into additional collaborations and other similar arrangements and may not be successful in entering into new arrangements, and even if we are, we may not realize the benefits of such relationships.
After the completion of collaboration agreements with two universities, we expect to enter into additional collaboration agreements as part of our business strategy. The success of our current and any future collaboration arrangements may depend heavily on the efforts and activities of our collaborators. Collaborations are subject to numerous risks, which may include risks that:
| ● | collaborators may have significant discretion in determining the efforts and resources that they will apply to collaborations; |
| ● | collaborators may not pursue development and commercialization of our product candidates or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in their strategic focus due to their acquisition of competitive products or their internal development of competitive products, availability of funding or other external factors, such as a business combination that diverts resources or creates competing priorities; |
| ● | collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial, abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing; |
| ● | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our products or product candidates; |
| ● | a collaborator with marketing, manufacturing and distribution rights to one or more products may not commit sufficient resources to or otherwise not perform satisfactorily in carrying out these activities; |
| ● | we could grant exclusive rights to collaborators that would prevent us from collaborating with others; |
| ● | collaborators may not properly maintain or defend our programs’ intellectual property rights or may use our programs’ intellectual property or proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate such intellectual property or proprietary information or expose us or our programs to potential liability; |
| ● | disputes may arise between us and a collaborator that cause the delay or termination of the research, development or commercialization of our current or future product candidates or that results in costly litigation or arbitration that diverts management attention and resources; |
| ● | collaborations may be terminated, which may result in a need for additional capital to pursue further development or commercialization of the applicable current or future product candidates; |
| ● | collaborators may own or co-own intellectual property covering products that result from our collaboration with them, and in such cases, we would not have the exclusive right to develop or commercialize such intellectual property; |
| ● | disputes may arise with respect to the ownership of any intellectual property developed pursuant to our collaborations; and |
| ● | a collaborator’s sales and marketing activities or other operations may not be in compliance with applicable laws resulting in civil or criminal proceedings. |
Additionally, we may seek to enter into additional collaborations, joint ventures, licenses and other similar arrangements for the development or commercialization of our product candidates, due to capital costs required to develop or commercialize the product candidate or manufacturing constraints. We may not be successful in our efforts to establish such collaborations for our product candidates because our research and development pipeline may be insufficient, our product candidates may be deemed to be at too early of a stage of development for collaborative effort or third parties may not view our product candidates as having the requisite potential to demonstrate safety and efficacy or significant commercial opportunity. In addition, we face significant competition in seeking appropriate strategic partners, and the negotiation process can be time consuming and complex. Further, any future collaboration agreements may restrict us from entering into additional agreements with potential collaborators. We cannot be certain that, following a strategic transaction or license we will achieve an economic benefit that justifies such transaction.
Even if we are successful in our efforts to establish such collaborations, the terms that we agree upon may not be favorable to us and we may not be able to maintain such collaborations if, for example, development or approval of a product candidate is delayed, the safety of a product candidate is questioned or sales of an approved product candidate are unsatisfactory.
In addition, any potential future collaborations may be terminable by our strategic partners, and we may not be able to adequately protect our rights under these agreements. Furthermore, strategic partners may negotiate for certain rights to control decisions regarding the development and commercialization of our product candidates, if approved, and may not conduct those activities in the same manner as we do. Any termination of collaborations we enter into in the future, or any delay in entering into collaborations related to our product candidates, could delay the development and commercialization of our product candidates and reduce their competitiveness if they reach the market, which could have a material adverse effect on our business, financial condition and results of operations.
Collaborative relationships with third parties could cause us to expend significant resources and incur substantial business risk with no assurance of financial return.
We anticipate relying upon strategic collaborations for marketing and commercializing our existing product candidates, if approved, and we may rely even more on strategic collaborations for research and development of other of our product candidates or discoveries. We may sell product offerings through strategic partnerships with pharmaceutical and biotechnology companies. If we are unable to establish or manage such strategic collaborations on terms favorable to us in the future, our research and development efforts and potential to generate revenue may be limited.
If we enter into research and development collaborations during the early phases of product development, success will in part depend on the performance of research collaborators. We will not directly control the amount or timing of resources devoted by research collaborators to activities related to product candidates. Research collaborators may not commit sufficient resources to our research and development programs. If any research collaborator fails to commit sufficient resources, the preclinical or clinical development programs related to the collaboration could be delayed or terminated. Also, collaborators may pursue existing or other development-stage products or alternative technologies in preference to those being developed in collaboration with us. Finally, if we fail to make required milestone or royalty payments to collaborators or to observe other obligations in agreements with them, the collaborators may have the right to terminate or stop performance of those agreements.
Establishing strategic collaborations is difficult and time consuming. Our discussions with potential collaborators may not lead to the establishment of collaborations on favorable terms, if at all. Potential collaborators may reject collaborations based upon their assessment of our financial, regulatory or intellectual property position. In addition, there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential future collaborators. Even if we successfully establish new collaborations, these relationships may never result in the successful development or commercialization of product candidates or the generation of sales revenue. To the extent that we enter into collaborative arrangements, the related product revenues are likely to be lower than if we directly marketed and sold products. Such collaborators may also consider alternative product candidates or technologies for similar indications that may be available to collaborate on and whether such a collaboration could be more attractive than the one with us for any future product candidate.
Management of our relationships with collaborators will require:
| ● | significant time and effort from our management team; | |
| ● | coordination of our marketing and research and development programs with the marketing and research and development priorities of our collaborators; and | |
| ● | effective allocation of our resources to multiple projects. |
We rely on third parties to assist in conducting our clinical trials and some aspects of our research and preclinical testing, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials, research, or testing.
We currently rely and expect to continue to rely on third parties, such as CROs, clinical data management organizations, medical institutions and clinical investigators, to conduct some aspects of research and preclinical testing and clinical trials. Any of these third parties may terminate their engagements with us or be unable to fulfill their contractual obligations. If any of our relationships with these third parties terminate, we may not be able to enter into arrangements with alternative third parties on commercially reasonable terms, or at all. If we need to enter into alternative arrangements, it could delay product development activities.
Further, although our reliance on these third parties for clinical development activities limits our control over these activities, we remain responsible for ensuring that each trial is conducted in accordance with the applicable protocol, legal and regulatory requirements and scientific standards. For example, notwithstanding the obligations of a CRO for a trial of one of our product candidates, we remain responsible for ensuring that each clinical trial is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA requires compliance with requirements, commonly referred to as GCPs for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. The FDA enforces these GCPs through periodic inspections of trial sponsors, principal investigators, clinical trial sites and IRBs. If we or our third-party contractors fail to comply with applicable GCPs, the clinical data generated in their clinical trials may be deemed unreliable, and the FDA may require additional clinical trials before approving our product candidates, which would delay the regulatory approval process. We cannot be certain that, upon inspection, the FDA will determine that any of our clinical trials comply with GCPs. We are also required to register certain clinical trials and post the results of completed clinical trials on a government-sponsored database, ClinicalTrials.gov, within certain timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions.
Furthermore, the third parties conducting clinical trials on our behalf are not our employees, and except for remedies available to us under the agreements with such contractors, we cannot control whether or not such contractors devote sufficient time, skill and resources to their ongoing development programs. These contractors may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical trials or other drug or medical device development activities, which could impede their ability to devote appropriate time to our clinical programs. If these third parties, including clinical investigators, do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we may not be able to obtain, or may be delayed in obtaining, regulatory approvals for our product candidates. If that occurs, we will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates. In such an event, our financial results and the commercial prospects for any product candidates that we seek to develop could be harmed, our costs could increase and our ability to generate revenues could be delayed, impaired or foreclosed.
Our use of third parties to manufacture and develop our product candidates for preclinical studies and clinical trials may increase the risk that we will not have sufficient quantities of our product candidates, products, or necessary quantities of such materials on time or at an acceptable cost.
We do not currently have, nor do we plan to acquire, the infrastructure or capability internally to manufacture drug supplies for our ongoing clinical trials or any future clinical trials that they may conduct, and we lack the resources to manufacture any product candidates on a commercial scale. In May 2023, we announced that we had entered into a clinical supply agreement with IMP for the Phase I/IIa clinical study. Pursuant to the clinical supply agreement, IMP is responsible for the global clinical supply chain of CMND-100 from manufacture to the various clinical sites. Clinical drug supply is a critical factor for trial success so collaboration with best-in-class clinical drug supply vendor forestalls the possibility of it being a bottleneck to successful drug delivery. Moreover, it can also deliver enormous benefits, including better investigator and patient experiences as well as cost savings in clinical drug supply. See “Item 4.B. Business Overview—Strategic Focus—Phase I/IIa Clinical Study.” We rely, and expect to continue to rely, on third-party manufacturers to produce our product candidates or other product candidates that we may identify for clinical trials, as well as for commercial manufacture if any product candidates receive marketing authorization and approval. Although we generally do not begin a clinical trial unless we believe they have a sufficient supply of a product candidate to complete the trial, any significant delay or discontinuity in the supply of a product candidate, or the raw material components thereof, for an ongoing clinical trial due to the need to replace a third-party manufacturer could considerably delay the clinical development and potential regulatory authorization of our product candidates, which could harm our business and results of operations.
We may be unable to identify and appropriately qualify third-party manufacturers or establish agreements with third-party manufacturers or do so on acceptable terms. Even if they are able to establish agreements with third-party manufacturers, reliance on third-party manufacturers entails additional risks, including:
| ● | reliance on the third party for sourcing of raw materials, components, and such other goods as may be required for execution of its manufacturing processes and the oversight by the third party of its suppliers; |
| ● | reliance on the third party for regulatory compliance and quality assurance for the manufacturing activities each performs; |
| ● | the possible breach of the manufacturing agreement by the third party; |
| ● | the possible misappropriation of proprietary information, including trade secrets and know-how; and |
| ● | the possible termination or non-renewal of the agreement by the third party at a time that is costly or inconvenient for us. |
Furthermore, we and our CMOs are engaged with other companies to supply and/or manufacture materials or products for such companies, which exposes our manufacturers to regulatory risks for the production of such materials and products. The facilities used by our contract manufacturers to manufacture their drug or medical device product candidates are subject to review by the FDA pursuant to inspections that will be conducted after we submit an NDA, a biologics license application, or BLA, premarket approval application, or PMA, or other marketing application to the FDA. We do not control the manufacturing process of, and are to some extent dependent on, our contract manufacturing partners for compliance with the regulatory requirements, known as cGMP requirements for manufacture of drug and device products. If our contract manufacturers cannot successfully manufacture material that conforms to our specifications and the strict regulatory requirements of the FDA or others, we will not be able to secure or maintain regulatory authorization for our product candidates manufactured at these manufacturing facilities. In addition, we have no control over the ability of our contract manufacturers to maintain adequate quality control, quality assurance and qualified personnel. If the FDA, the EMA or another comparable foreign regulatory agency does not approve these facilities for the manufacture of our product candidates or if any agency withdraws its approval in the future, we may need to find alternative manufacturing facilities, which would negatively impact our ability to develop, obtain regulatory authorization for or market our product candidates, if approved.
Our product candidates may compete with other product candidates and marketed products for access to manufacturing facilities. Any performance failure on the part of our existing or future manufacturers could delay clinical development, marketing approval or commercialization. Our current and anticipated future dependence upon others for the manufacturing of our product candidates may adversely affect our future profit margins and our ability to commercialize any product candidates that receive marketing approval on a timely and competitive basis.
If the contract manufacturing facilities on which we rely do not continue to meet regulatory requirements or are unable to meet our supply demands, our business will be harmed.
All entities involved in the preparation of product candidates for clinical trials or commercial sale, including our existing CMOs for our product candidates, are subject to extensive regulation. Components of a finished drug or product approved for commercial sale or used in late-stage clinical trials must be manufactured in accordance with cGMP, or similar regulatory requirements outside the United States. These regulations govern manufacturing processes and procedures, including recordkeeping, and the implementation and operation of quality systems to control and assure the quality of investigational products and products approved for sale. Poor control of production processes can lead to the introduction of contaminants or to inadvertent changes in the properties or stability of our product candidates. Our failure, or the failure of third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed on us, including clinical holds, fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, suspension of production, seizures or recalls of product candidates or marketed drugs, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect clinical or commercial supplies of our product candidates.
We and our CMOs must supply all necessary documentation, as applicable, in support of a marketing application, such as an NDA, BLA, PMA or MAA, on a timely basis and must adhere to regulations enforced by the FDA and other regulatory agencies through their facilities inspection program. Some of our CMOs have never produced a commercially approved pharmaceutical product and therefore have not obtained the requisite regulatory authority approvals to do so. The facilities and quality systems of some or all of our third-party contractors must pass a pre-approval inspection for compliance with the applicable regulations as a condition of regulatory approval of our product candidates or any of our other potential products. In addition, the regulatory authorities may, at any time, audit or inspect a manufacturing facility involved with the preparation of our product candidates or our other potential products or the associated quality systems for compliance with the regulations applicable to the activities being conducted. Although we oversee the CMOs, we cannot control the manufacturing process of, and are completely dependent on, our CMO partners for compliance with the regulatory requirements. If these facilities do not pass a pre-approval plant inspection, regulatory approval of the products may not be granted or may be substantially delayed until any violations are corrected to the satisfaction of the regulatory authority, if ever.
The regulatory authorities also may, at any time following approval of a product for sale, audit the manufacturing facilities of our third-party contractors. If any such inspection or audit identifies a failure to comply with applicable regulations or if a violation of product specifications or applicable regulations occurs independent of such an inspection or audit, we or the relevant regulatory authority may require remedial measures that may be costly and/or time consuming for us or a third party to implement, and that may include the temporary or permanent suspension of a clinical study or commercial sales or the temporary or permanent closure of a facility. Any such remedial measures imposed upon us or third parties with whom we contract could materially harm our business.
Additionally, if supply from one approved manufacturer is interrupted, an alternative manufacturer would need to be qualified. For drug products, an NDA or MAA variation, or equivalent foreign regulatory filing is also required, which could result in further delay. Similarly, for a medical device, a new marketing application or supplement may be required. The regulatory agencies may also require additional studies if a new manufacturer is relied upon for commercial production. Switching manufacturers may involve substantial costs and is likely to result in a delay in our desired clinical and commercial timelines.
These factors could cause us to incur higher costs and could cause the delay or termination of clinical trials, regulatory submissions, required approvals, or commercialization of our product candidates. Furthermore, if our suppliers fail to meet contractual requirements and we are unable to secure one or more replacement suppliers capable of production at a substantially equivalent cost, our clinical trials may be delayed, and we could lose potential revenue.
We have no sales, distribution, or marketing experience, and may invest significant financial and management resources to establish these capabilities. If we are unable to establish such capabilities or enter into agreements with third parties to market and sell our future products, if approved, we may be unable to generate any revenues.
Given our stage of development, we have no sales, distribution, or marketing experience. To successfully commercialize any products that may result from our development programs, we will need to develop sales and marketing capabilities in the United States, Europe and other regions, either on our own or with others. We may enter into strategic alliances with other entities to utilize their mature marketing and distribution capabilities, but we may be unable to enter into marketing agreements on favorable terms, if at all. If our future strategic collaborators do not commit sufficient resources to commercialize our future products, if any, and we are unable to develop the necessary marketing capabilities on our own, we may be unable to generate sufficient product revenue to sustain our business. We will be competing with many companies that currently have extensive and well-funded marketing and sales operations. Without a significant internal team or the support of a third party to perform marketing and sales functions, we may be unable to compete successfully against these more established companies.
Risks Related to Our Intellectual Property
If we are unable to obtain and maintain effective patent rights for our product candidates or any future product candidates, we may not be able to compete effectively in our markets. If we are unable to protect the confidentiality of our trade secrets or know-how, such proprietary information may be used by others to compete against us.
We rely upon a combination of patent protection and confidentiality agreements to protect the intellectual property related to our technologies and product candidates. Our success depends in large part on our ability to obtain and maintain patent and other intellectual property protection in the United States and in other countries with respect to our proprietary technology and product candidates.
We have sought to protect our proprietary position by filing patent applications in the United States and in other countries, with respect to our novel technologies and product candidates, which are important to our business. Patent prosecution is expensive and time consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection.
As of October 31, 2023, our portfolio consists of fifteen utility patent families including patents and applications having method of use and composition of matter claims. Thirteen of the patent families are solely owned by the Company and two are jointly owned; one with the Bar Ilan University Research and Development Company BIRAD and the other with Yissum Research Development Company of the Hebrew University of Jerusalem. The fifteen patent families include twenty-seven granted patents and twenty-four pending applications. We cannot offer any assurances about which, if any, patent applications will issue, the breadth of any such patent or whether any issued patents will be found invalid and unenforceable or will be threatened by third parties. Any successful opposition to these patents or any other patents owned by or licensed to us after patent issuance could deprive us of rights necessary for the successful commercialization of any product candidates that we may develop. Further, if we encounter delays in regulatory approvals, the period of time during which we could market a product candidate under patent protection could be reduced.
Further, the patent position of pharmaceutical companies generally is highly uncertain and involves complex legal and factual questions. This renders the patent prosecution process particularly expensive and time-consuming. There is no assurance that all potentially relevant prior art relating to our patent applications has been found, which can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue, and even if such patents cover our product candidates, third parties may challenge their validity, enforceability, or scope, which may result in such patents being narrowed, found unenforceable or invalidated. Furthermore, even if they are unchallenged, our patent applications and any future patents may not adequately protect our intellectual property, provide exclusivity for our product candidates, or prevent others from designing around our claims. Any of these outcomes could impair our ability to prevent competition from third parties, which may have an adverse impact on our business.
If we cannot obtain and maintain effective patent rights for our product candidates, we may not be able to compete effectively, and our business and results of operations would be harmed.
We may not have sufficient patent lifespan to effectively protect our products and business.
Patents have a limited lifespan. In the United States, the natural expiration of a patent is 20 years after its earliest effective filing date. Although various extensions may be available, the life of a patent, and the protection it affords, is limited. Even if any of our patent applications mature into issued patents, if we do not have sufficient patent terms or regulatory exclusivity to protect our products, our business and results of operations will be adversely affected.
Patent policy and rule changes could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of any issued patents.
Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of any patents that may issue from our patent applications or narrow the scope of our patent protection. The laws of foreign countries may not protect our rights to the same extent as the laws of the United States. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. We therefore cannot be certain that we or our licensors were the first to make the invention claimed in our owned and licensed patents or pending applications, or that we or our licensor were the first to file for patent protection of such inventions. Assuming the other requirements for patentability are met, for United States patent applications filed prior to March 15, 2013, the first to conceive a claimed invention is entitled to the patent, while outside the United States, the first to file a patent application is entitled to the patent. After March 15, 2013, under the Leahy-Smith America Invents Act, or the AIA, enacted on September 16, 2011, the United States has moved to a first to file system. The AIA also includes a number of significant changes that affect the way patent applications are prosecuted and may also affect patent litigation. The courts have yet to address many of these provisions and the applicability of the AIA and new regulations on specific patents discussed herein have not been determined and would need to be reviewed. In general, the AIA and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of any issued patents, all of which could have a material adverse effect on our business and financial condition.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our registered or unregistered trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition by potential partners or customers in our markets of interest. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be adversely affected. If other entities use trademarks similar to ours in different jurisdictions, or have senior rights to ours, it could interfere with our use of our current trademarks throughout the world.
If we are unable to maintain effective proprietary rights for our product candidates or any future product candidates, we may not be able to compete effectively in our markets.
In addition to the protection afforded by any patents that have been or may be granted, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent, processes for which patents are difficult to enforce and any other elements of our product candidate discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. However, trade secrets can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors and contractors. We also seek to preserve the integrity and confidentiality of our data, trade secrets and intellectual property by maintaining the physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in these individuals, organizations and systems, agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets and intellectual property may otherwise become known or be independently discovered by competitors.
Although we expect all of our employees and consultants to assign their inventions to us, and all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information, or technology to enter into confidentiality agreements, we cannot provide any assurances that all such agreements have been duly executed or that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Misappropriation or unauthorized disclosure of our trade secrets and intellectual property could impair our competitive position and may have a material adverse effect on our business. Additionally, if the steps taken to maintain our trade secrets and intellectual property are deemed inadequate, we may have insufficient recourse against third parties for misappropriating the trade secret.
Intellectual property rights of third parties could adversely affect our ability to commercialize our product candidates, and we might be required to litigate or obtain licenses from third parties in order to develop or market our product candidate. Such litigation or licenses could be costly or not available on commercially reasonable terms.
It is inherently difficult to conclusively assess our freedom to operate without infringing on third party rights. Our competitive position may suffer if patents issued to third parties or other third-party intellectual property rights cover our product candidates or elements thereof, or our manufacturing or uses relevant to our development plans. In such cases, we may not be in a position to develop or commercialize products or our product candidates unless we successfully pursue litigation to nullify or invalidate the third party intellectual property right concerned, or enter into a license agreement with the intellectual property right holder, if available on commercially reasonable terms. There may also be pending patent applications that if they result in issued patents, could be alleged to be infringed by our product candidates. If such an infringement claim should be brought and be successful, we may be required to pay substantial damages, be forced to abandon our product candidates or seek a license from any patent holders. No assurances can be given that a license will be available on commercially reasonable terms, if at all.
It is also possible that we have failed to identify relevant third-party patents or applications. For example, U.S. applications filed before November 29, 2000 and certain U.S. applications filed after that date that will not be filed outside the U.S. remain confidential until patents issue. Patent applications in the U.S. and elsewhere are published approximately 18 months after the earliest filing to which priority is claimed, with such earliest filing date being commonly referred to as the priority date. Therefore, patent applications covering our product candidates or platform technology could have been filed by others without our knowledge. Additionally, pending patent applications which have been published can, subject to certain limitations, be later amended in a manner that could cover our platform technologies, our product candidates or the use of our product candidates. Third party intellectual property right holders may also actively bring infringement claims against us. We cannot guarantee that we will be able to successfully settle or otherwise resolve such infringement claims. If we are unable to successfully settle future claims on terms acceptable to us, we may be required to engage in or continue costly, unpredictable and time-consuming litigation and may be prevented from or experience substantial delays in pursuing the development of and/or marketing of our product candidates. If we fail in any such dispute, in addition to being forced to pay damages, we may be temporarily or permanently prohibited from commercializing our product candidates that are held to be infringing. We might, if possible, also be forced to redesign our product candidates so that we no longer infringe the third-party intellectual property rights. Any of these events, even if we were ultimately to prevail, could require us to divert substantial financial and management resources that we would otherwise be able to devote to our business.
Third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts.
Our commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties. There have been many lawsuits and other proceedings involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries, including patent infringement lawsuits, interferences, oppositions and reexamination proceedings before the USPTO and corresponding foreign patent offices. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing product candidates. As the pharmaceutical industry expands and more patents are issued, the risk increases that our product candidates may be subject to claims of infringement of the patent rights of third parties.
Third parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture, or methods for treatment related to the use or manufacture of our product candidates. Because patent applications can take many years to issue, there may be currently pending patent applications that may later result in issued patents that our product candidates may infringe. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. If any third-party patents were held by a court of competent jurisdiction to cover the manufacturing process of any of our product candidates, any materials formed during the manufacturing process or any final product itself, the holders of any such patents may be able to block our ability to commercialize such product candidates unless we obtain a license under the applicable patents, or until such patents expire or are finally determined to be invalid or unenforceable.
Similarly, if any third-party patents were held by a court of competent jurisdiction to cover aspects of our formulations, processes for manufacture, or methods of use, the holders of any such patents may be able to block our ability to develop and commercialize the applicable product candidate unless we obtain a license or until such patent expires or is finally determined to be invalid or unenforceable. In either case, such a license may not be available on commercially reasonable terms or at all.
Parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, pay royalties, redesign our infringing products or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure.
We may not be successful in obtaining or maintaining necessary rights to our product candidates through acquisitions and in-licenses.
Because our programs may require the use of proprietary rights held by third parties, the growth of our business will likely depend in part on our ability to acquire, in-license, or use these proprietary rights. In addition, our product candidates may require specific formulations to work effectively and efficiently and the rights to these formulations may be held by others. We may be unable to acquire or in-license any compositions, methods of use, processes, or other third-party intellectual property rights from third parties that we identify as necessary for our product candidates. The licensing and acquisition of third-party intellectual property rights is a competitive area, and a number of more established companies are also pursuing strategies to license or acquire third-party intellectual property rights that we may consider attractive. These established companies may have a competitive advantage over us due to their size, cash resources, and greater clinical development and commercialization capabilities.
For example, we sometimes collaborate with academic institutions to accelerate our preclinical research or development under written agreements with these institutions. Typically, these institutions provide us with an option to negotiate a license to any of the institution’s rights in technology resulting from the collaboration. Regardless of such option, we may be unable to negotiate a license within the specified timeframe or under terms that are acceptable to us. If we are unable to do so, the institution may offer the intellectual property rights to other parties, potentially blocking our ability to pursue our program.
In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment. If we are unable to successfully obtain rights to required third-party intellectual property rights, we may have to abandon development of that program and our business and financial condition could suffer.
We may be involved in lawsuits to protect or enforce our intellectual property, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our intellectual property or that of our licensors that we may acquire in the future. If we or a future licensing partner were to initiate legal proceedings against a third party to enforce a patent covering one of our product candidates, the defendant could counterclaim that the patent covering our product candidate is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. Under the AIA, the validity of U.S. patents may also be challenged in post-grant proceedings before the USPTO. The outcome following legal assertions of invalidity and unenforceability is unpredictable.
Interference proceedings provoked by third parties or brought by us or declared by the USPTO may be necessary to determine the priority of inventions with respect to our patent or patent applications or those of our licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Our defense of litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. In addition, the uncertainties associated with litigation could have a material adverse effect on our ability to raise the funds necessary to continue our clinical trials, continue our research programs, license necessary technology from third parties, or enter into development partnerships that would help us bring our product candidates to market.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions, or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our Common Shares.
We may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
We employ individuals who were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants and independent contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants, or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of any of our employees’ former employers or other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
We may be subject to claims challenging the inventorship of our intellectual property.
We may be subject to claims that former employees, collaborators or other third parties have an interest in or right to compensation with respect to our current patent and patent applications, future patents or other intellectual property as an inventor or co-inventor. For example, we may have inventorship disputes arise from conflicting obligations of consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship or claiming the right to compensation. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees. To the extent that our employees have not effectively waived the right to compensation with respect to inventions that they helped create, they may be able to assert claims for compensation with respect to our future revenue. As a result, we may receive less revenue from future products if such claims are successful which in turn could impact our future profitability.
Changes in United States and international patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
Our success is heavily dependent on intellectual property. Obtaining and enforcing patents in the pharmaceutical industry involves both technological and legal complexity. Therefore, obtaining and enforcing these patents is costly, time consuming and inherently uncertain. In addition, the United States has recently enacted and is currently implementing wide-ranging patent reform legislation. Recent United States Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on future actions by the United States Congress, the federal courts and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain patents or to enforce patents that we might obtain in the future.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States may be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States.
Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own product candidates and may also export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our product candidates. Future patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to biotechnology products or methods of treatment, which could make it difficult for us to stop the marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our future patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Risks Related to Our Business Operations
We will need to expand our organization, and we may experience difficulties in managing this growth, which could disrupt our operations.
Our future financial performance and our ability to commercialize product candidates and compete effectively will depend, in part, on our ability to effectively manage any future growth. As our development and commercialization plans and strategies develop, we expect to need additional managerial, operational, sales, marketing, financial and legal personnel. Our management may need to divert a disproportionate amount of its attention away from our day-to-day activities and devote a substantial amount of time to managing these growth activities. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Our expected growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of additional product candidates. If our management is unable to effectively manage our growth, our expenses may increase more than expected, our ability to generate and/or grow revenue could be reduced, and we may not be able to implement our business strategy.
Due to our limited resources and access to capital, we must, and have in the past decided to, prioritize development of certain product candidates over other potential candidates. These decisions may prove to have been wrong and may adversely affect our revenues.
Because we have limited resources and access to capital to fund our operations, we must decide which product candidates to pursue and the amount of resources to allocate to each. Our decisions concerning the allocation of research, collaboration, management and financial resources toward particular product candidates may not lead to the development of viable commercial products and may divert resources away from better opportunities. Similarly, our decisions to delay, terminate or collaborate with third parties in respect of certain product development programs may also prove not to be optimal and could cause us to miss valuable opportunities. If we make incorrect determinations regarding the market potential of our product candidates or misread trends in the pharmaceutical industry, in particular for our lead product candidate, our business, financial condition and results of operations could be materially adversely affected.
We may not be successful in our efforts to identify, discover or license additional product candidates.
Although a substantial amount of our effort will focus on the continued clinical testing, potential approval and commercialization of MEAI, the success of our business also depends upon our ability to identify, discover or license additional product candidates. Our research programs or licensing efforts may fail to yield additional product candidates for clinical development for a number of reasons, including: lack of financial or personnel resources to acquire or discover additional product candidates; new product candidates may not succeed in preclinical or clinical testing, or may be shown to have harmful side effects or may have other characteristics that may make them unmarketable or unlikely to receive marketing approval; our competitors may develop alternatives that render our product candidates obsolete or less attractive; the market for a product candidate may change during our development program so that such product may become unprofitable to continue to develop; new product candidates may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and new product candidates may not be accepted as safe and effective by patients, the medical community, or third-party payors.
We may be forced to abandon our development efforts for a program or programs that are unsuccessful, or we may not be able to identify, license, or discover additional product candidates, which would have a material adverse effect on our business and could potentially cause us to cease operations. Further, research programs to identify new product candidates require substantial technical, financial and human resources. We may focus our efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful.
European data collection is governed by restrictive regulations governing the collection, use, processing and cross-border transfer of personal information.
We may collect, process, use or transfer personal information from individuals located in the European Union in connection with our business, including in connection with conducting clinical trials in the European Union. Additionally, we intend to commercialize MEAI, and any of our product candidates that receive marketing approval, in the European Union. The collection and use of personal health data in the European Union is governed by the provisions of the General Data Protection Regulation ((EU) 2016/679), or the GDPR, along with other European Union and country-specific laws and regulations. The United Kingdom and Switzerland have also adopted data protection laws and regulations. These legislative acts (together with regulations and guidelines) impose requirements relating to having legal bases for processing personal information relating to identifiable individuals and transferring such information outside of the EEA, including to the United States, providing details to those individuals regarding the processing of their personal information, keeping personal information secure, having data processing agreements with third parties who process personal information, responding to individuals’ requests to exercise their rights in respect of their personal information, reporting security breaches involving personal data to the competent national data protection authority and affected individuals, appointing data protection officers, conducting data protection impact assessments and record-keeping. The GDPR imposes additional responsibilities and liabilities in relation to personal data that we process and we may be required to put in place additional mechanisms ensuring compliance with the new data protection rules. Failure to comply with the requirements of the GDPR and related national data protection laws of the member states of the European Union may result in substantial fines, other administrative penalties and civil claims being brought against us, which could have a material adverse effect on our business, prospects, financial condition and results of operations.
The United Kingdom’s withdrawal from the European Union may adversely impact our ability to obtain regulatory approvals of our product candidates in the United Kingdom, result in restrictions or imposition of taxes and duties for importing our product candidates into the United Kingdom, and may require us to incur additional expenses in order to develop, manufacture and commercialize our product candidates in the United Kingdom.
Following the result of a referendum in 2016, the United Kingdom left the European Union on January 31, 2020, commonly referred to as Brexit. Pursuant to the formal withdrawal arrangements agreed to by the United Kingdom and the European Union, the United Kingdom was subject to a transition period until December 31, 2020, or the Transition Period, during which European Union rules continued to apply. A trade and cooperation agreement that outlines the future and trading relationship between the United Kingdom and the European Union was agreed to in December 2020.
Since a significant proportion of the regulatory framework in the United Kingdom applicable to our business and our product candidates is derived from European Union directives and regulations, Brexit has had, and will continue to have, a material impact on the regulatory regime with respect to the development, manufacture, importation, approval and commercialization of our product candidates in the United Kingdom. For example, Great Britain is no longer covered by the centralized procedures for obtaining European Union-wide marketing authorizations from the EMA, and a separate marketing authorization will be required to market our product candidates, including MEAI in Great Britain. It is currently unclear whether the Medicines & Healthcare products Regulatory Agency in the United Kingdom is sufficiently prepared to handle the increased volume of marketing authorization applications that it is likely to receive. Any delay in obtaining, or an inability to obtain, any marketing approvals, as a result of Brexit or otherwise, would delay or prevent us from commercializing our product candidates in the United Kingdom and limit our ability to generate revenue and achieve and sustain profitability. We could face significant additional expenses to obtain regulatory approval for our products in the United Kingdom.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
Our research, development and manufacturing activities and our third party manufacturers’ and suppliers’ activities involve the controlled storage, use and disposal of hazardous materials, including the components of our product candidates and other hazardous compounds. We and our manufacturers and suppliers are subject to laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. In some cases, these hazardous materials and various wastes resulting from their use are stored at our and our manufacturers’ facilities pending their use and disposal. We cannot eliminate the risk of contamination, which could cause an interruption of our commercialization efforts, research and development efforts and business operations, environmental damage resulting in costly clean-up and liabilities under applicable laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. Although we believe that the safety procedures utilized by our third-party manufacturers for handling and disposing of these materials generally comply with the standards prescribed by these laws and regulations, we cannot guarantee that this is the case or eliminate the risk of accidental contamination or injury from these materials. In such an event, we may be held liable for any resulting damages, such liability could exceed our resources, and state or federal or other applicable authorities may curtail our use of certain materials and/or interrupt our business operations. Furthermore, environmental laws and regulations are complex, change frequently and have tended to become more stringent. We cannot predict the impact of such changes and cannot be certain of our future compliance. We do not currently carry biological or hazardous waste insurance coverage.
Our business may be adversely affected if there is a resurgence of the COVID-19 pandemic.
Public health epidemics or outbreaks could adversely impact our business. In late 2019, a novel strain of COVID-19, also known as coronavirus, was reported in Wuhan, China. Initially the outbreak was largely concentrated in China, but it rapidly spread to countries across the globe, including in Israel and the United States. Many countries around the world, including in Israel and the United States, implemented significant governmental measures to control the spread of the virus, including temporary closure of businesses, severe restrictions on travel and the movement of people, and other material limitations on the conduct of business. In response, for several months in 2020, we implemented remote working and workplace protocols for our employees in accordance Israeli Ministry of Health requirements to ensure employee safety and all employees have been instructed on and encouraged to practice best social distancing behaviors.
If there is a resurgence of COVID-19 its spread may materially affect us economically. While the potential economic impact brought by, and the duration of, any future resurgence of the COVID-19 pandemic may be difficult to assess or predict, it has already caused, and could result in further, significant disruption of global financial markets, reducing our ability to access capital, which could in the future negatively affect our liquidity and financial position. In addition, the trading prices for other companies have been highly volatile as a result of the COVID-19 pandemic. As a result, we may face difficulties raising capital through sales of our common shares or other securities and such sales may be on unfavorable terms. To the extent that future waves of COVID-19 disrupt normal business operations, we may face operational challenges with our services, and we likely will have to adopt remote working and workplace protocols for employees in accordance with government requirements and other measures to minimize such impact.
The extent to which COVID-19 impacts our operations will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration and severity of the outbreak, and the actions that may be required to contain COVID-19 or treat its impact. In particular, the extent to which any resurgence of the COVID-19 pandemic may impact our business and financial performance will depend on future developments, which are highly uncertain and cannot be predicted with confidence including our research and clinical trials and our ability to raise capital, could affect the operations of key governmental agencies and could result in the inability of our suppliers to deliver components or raw materials on a timely basis or at all, each of which in turn could have an adverse impact on our business, financial condition and results of operation.
Our business, operating results and growth rates may be adversely affected by current or future unfavorable economic and market conditions and adverse developments with respect to financial institutions and associated liquidity risk.
Our business depends on the economic health of the global economies. If the conditions in the global economies remain uncertain or continue to be volatile, or if they deteriorate, including as a result of the impact of military conflict, such as the war between Russia and Ukraine, terrorism or other geopolitical events, our business, operating results and financial condition may be materially adversely affected. Economic weakness, inflation and increases in interest rates, limited availability of credit, liquidity shortages and constrained capital spending have at times in the past resulted, and may in the future result, in challenging and delayed sales cycles, slower adoption of new technologies and increased price competition, and could negatively affect our ability to forecast future periods, which could result in an inability to satisfy demand for our products and a loss of market share.
In addition, increases in inflation raise our costs for commodities, labor, materials and services and other costs required to grow and operate our business, and failure to secure these on reasonable terms may adversely impact our financial condition. Additionally, increases in inflation, along with the uncertainties surrounding COVID-19, geopolitical developments and global supply chain disruptions, have caused, and may in the future cause, global economic uncertainty and uncertainty about the interest rate environment, which may make it more difficult, costly or dilutive for us to secure additional financing. A failure to adequately respond to these risks could have a material adverse impact on our financial condition, results of operations or cash flows.
There can be no assurance that future credit and financial market instability and a deterioration in confidence in economic conditions will not occur. Our general business strategy may be adversely affected by any such economic downturn, liquidity shortages, volatile business environment or continued unpredictable and unstable market conditions. If the current equity and credit markets deteriorate, or if adverse developments are experienced by financial institutions, it may cause short-term liquidity risk and also make any necessary debt or equity financing more difficult, more costly, more onerous with respect to financial and operating covenants and more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial performance and stock price and could require us to alter our operating plans. In addition, there is a risk that one or more of our service providers, financial institutions, manufacturers, suppliers and other partners may be adversely affected by the foregoing risks, which could directly affect our ability to attain our operating goals on schedule and on budget.
Environmental, social and corporate governance (ESG) issues, including those related to climate change and sustainability, may have an adverse effect on our business, financial condition and results of operations and damage our reputation.
There is an increasing focus from certain investors, customers, consumers, employees and other stakeholders concerning ESG matters. Additionally, public interest and legislative pressure related to public companies’ ESG practices continue to grow. If our ESG practices fail to meet regulatory requirements or investor, customer, consumer, employee or other shareholders’ evolving expectations and standards for responsible corporate citizenship in areas including environmental stewardship, support for local communities, board of directors and employee diversity, human capital management, employee health and safety practices, product quality, supply chain management, corporate governance and transparency, our reputation, brand and employee retention may be negatively impacted, and our customers and suppliers may be unwilling to continue to do business with us.
Customers, consumers, investors and other shareholders are increasingly focusing on environmental issues, including climate change, energy and water use, plastic waste and other sustainability concerns. Concern over climate change may result in new or increased legal and regulatory requirements to reduce or mitigate impacts to the environment. Changing customer and consumer preferences or increased regulatory requirements may result in increased demands or requirements regarding plastics and packaging materials, including single-use and non-recyclable plastic products and packaging, other components of our products and their environmental impact on sustainability, or increased customer and consumer concerns or perceptions (whether accurate or inaccurate) regarding the effects of substances present in certain of our products. Complying with these demands or requirements could cause us to incur additional manufacturing, operating or product development costs.
If we do not adapt to or comply with new regulations, including the SEC’s published proposed rules that would require companies to provide significantly expanded climate-related disclosures in their periodic reporting, which may require us to incur significant additional costs to comply and impose increased oversight obligations on our management and board of directors, or fail to meet evolving investor, industry or stakeholder expectations and concerns regarding ESG issues, investors may reconsider their capital investment in our Company, we may become subject to penalties, and customers and consumers may choose to stop purchasing our products, if approved for commercialization, which could have a material adverse effect on our reputation, business or financial condition.
Our employees and independent contractors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk of fraud or other misconduct by our employees and independent contractors. Misconduct by these parties could include intentional failures to comply with FDA regulations, provide accurate information to the FDA, comply with manufacturing standards we may establish, comply with federal and state healthcare fraud and abuse laws and regulations, report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee and independent contractor misconduct could also involve the improper use of information obtained in the course of clinical trials, including individually identifiable information, creating fraudulent data in our preclinical studies or clinical trials or illegal misappropriation of product candidates, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter misconduct by employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. Additionally, we are subject to the risk that a person or government could allege such fraud or other misconduct, even if none occurred. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions.
Under applicable employment laws, we may not be able to enforce covenants not to compete and therefore may be unable to prevent our competitors from benefiting from the expertise of some of our former employees.
We generally enter into non-competition agreements with our employees and certain key consultants. These agreements prohibit our employees and certain key consultants, if they cease working for us, from competing directly with us or working for our competitors or clients for a limited period of time. We may be unable to enforce these agreements under the laws of the jurisdictions in which our employees work and it may be difficult for us to restrict our competitors from benefitting from the expertise our former employees or consultants developed while working for us.
International expansion of our business exposes us to business, regulatory, political, operational, financial and economic risks associated with doing business outside of the United States, Canada or Israel.
Other than our headquarters, which are located in Canada, and other operations, which are located in Israel and the United States, we currently have limited international operations, but our business strategy incorporates potentially significant international expansion, particularly in anticipation of approval of our product candidates. We plan to retain sales representatives and third party distributors and conduct physician, specialist, hospital pharmacist and patient association outreach activities, as well as clinical trials, outside of Canada, the United States and Israel. Doing business internationally involves a number of risks, including but not limited to:
| ● | multiple, conflicting and changing laws and regulations such as privacy regulations, tax laws, export and import restrictions, employment laws, regulatory requirements and other governmental approvals, permits, and licenses; | |
| ● | failure by us to obtain regulatory approvals for the use of our product candidates in various countries; | |
| ● | additional potentially relevant third-party patent rights; | |
| ● | complexities and difficulties in obtaining protection and enforcing our intellectual property; | |
| ● | difficulties in staffing and managing foreign operations; | |
| ● | complexities associated with managing multiple payor reimbursement regimes, government payors, prince controls or patient self-pay systems; | |
| ● | limits in our ability to penetrate international markets; | |
| ● | financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our products and exposure to foreign currency exchange rate fluctuations; | |
| ● | an outbreak of a contagious disease, including COVID-19, which may cause us or our distributors, third party vendors and manufacturers and/or customers to temporarily suspend our or their respective operations in the affected city or country; | |
| ● | natural disasters, political and economic instability, including wars, terrorism, and political unrest, boycotts, curtailment of trade, and other business restrictions; | |
| ● | certain expenses including, among others, expenses for travel, translation and insurance; and | |
| ● | regulatory and compliance risks that relate to maintaining accurate information and control over sales and activities that may fall within the purview of the U.S. Foreign Corrupt Practices Act its books and records provisions, or its anti-bribery provisions. |
Any of these factors could significantly harm our future international expansion and operations and, consequently, our results of operations.
Certain of our directors and officers may have interests that compete with ours.
Certain of our directors currently operate, manage or are engaged as officers and/or directors of other entities, which may have similar or different objectives than ours. Such activities could detract from the time these people have to allocate to our affairs. For example, Adi Zuloff-Shani, our Chief Executive Officer, serves as Chief Technology Officer of SciSparc Ltd. (Nasdaq: SPRC), or SciSparc; Amitay Weiss, the Chairman of our Board of Directors, serves as the Chairman of the Board of Directors of SciSparc; and Oz Adler, one of our directors, serves as the Chief Executive Officer and Chief Financial Officer of SciSparc. Further to the agreement signed between us and SciSparc in March 2022, the purposes of which is to explore the technological and business feasibility of creating pharmaceutical solutions for several indications including CNS related indications by combining the respective capabilities and technologies of both parties. In June 2023, we announced that, as part of our ongoing collaboration with SciSparc, we entered into a research agreement with the Hebrew University of Jerusalem to evaluate our and SciSparc’s combination treatment for obesity and metabolic syndrome. Moreover, certain of our directors and officers are affiliated with our current shareholders, and may have different interests than other shareholders. For additional information regarding related party transactions and potential conflicts of interest, see “Item 7.B. Related Party Transactions.” Under the Business Corporations Act (British Columbia) [SBC 2002] c.57, or the BCBCA, each director and officer of must disclose the nature and extent of any interest that he or she has in a material contract or material transaction whether made or proposed with us, if the director or officer is a party to the contract or transaction, is a director or an officer or an individual acting in a similar capacity of a party to the contract or transaction, or has a material interest in a party to the contract or transaction. Subject to certain limited exceptions under the BCBCA, no director may vote on a resolution to approve a material contract or material transaction which is subject to such disclosure requirement. See “Item 6.C. Compensation—Board Practices—Requirement for Directors and Officers to Disclose Interest in a Contract or Transaction.” In addition, we have adopted a code of ethics and conduct that requires our employees, officers and directors to disclose any situation that reasonably would be expected to give rise to a conflict of interest.
Risks Related to Ownership of Our Common Shares
We may be or may become classified as a passive foreign investment company. If we are or become classified as a passive foreign investment company, our U.S. shareholders may suffer adverse tax consequences as a result.
Generally, for any taxable year, if at least 75% of our gross income is passive income, or at least 50% of the value of our assets is attributable to assets that produce passive income or are held for the production of passive income, including cash, we would be characterized as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes. For purposes of these tests, passive income includes dividends, interest gains from commodities and securities transactions, the excess of gains over losses from the disposition of assets which produce passive income (including amounts derived by reason of the temporary investment of funds raised in offerings of our shares) and rents and royalties other than rents and royalties which are received from unrelated parties in connection with the active conduct of a trade or business. If we are characterized as a PFIC, our U.S. shareholders may suffer adverse tax consequences, including having gains realized on the sale of our Common Shares treated as ordinary income, rather than capital gain, the loss of the preferential rate applicable to dividends received on our Common Shares by individuals who are U.S. holders, and having interest charges apply to distributions by us and gains from the sales of our shares.
Our status as a PFIC will depend on the nature and composition of our income and the nature, composition and value of our assets (which, assuming we are not a “controlled foreign corporation,” or a CFC, under Section 957(a) of the Internal Revenue Code of 1986, as amended, or the Code, for the year being tested, may be determined based on the fair market value of each asset, with the value of goodwill and going concern value determined in large part by reference to the market value of our Common Shares, which may be volatile). Based upon the estimated value of our assets, including any goodwill, and the nature and estimated composition of our income and assets, we may be classified as a PFIC for the taxable year ended October 31, 2023 and in future taxable years. In particular, so long as we do not generate revenue from operations for any taxable year and do not receive any research and development grants, or even if we receive a research and development grant, if such grant does not constitute gross income for United States federal income tax purposes, we likely will be classified as a PFIC for such taxable year. Because the determination of whether we are a PFIC for any taxable year is a factual determination made annually after the end of each taxable year, there can be no assurance that we will not be considered a PFIC in any taxable year.
The tax consequences that would apply if we are classified as a PFIC would also be different from those described above if a U.S. shareholder were able to make a valid qualified electing fund, or QEF, election. At this time, we do not expect to provide U.S. shareholders with the information necessary for a U.S. shareholder to make a QEF election. Prospective investors should assume that a QEF election will not be available.
If a United States person is treated as owning at least 10% of our shares, such holder may be subject to adverse U.S. federal income tax consequences.
If a United States person is treated as owning (directly, indirectly or constructively) at least 10% of the value or voting power of our shares, such person may be treated as a “United States shareholder” with respect to each “controlled foreign corporation” in our group (if any). Because our group includes one or more U.S. subsidiaries, we expect that certain of our non-U.S. subsidiaries will be treated as controlled foreign corporations (regardless of whether we are or are not treated as a controlled foreign corporation). A United States shareholder of a controlled foreign corporation may be required to annually report and include in its U.S. taxable income its pro rata share of “Subpart F income,” “global intangible low-taxed income” and investments in U.S. property by controlled foreign corporations, whether or not we make any distributions. An individual that is a United States shareholder with respect to a controlled foreign corporation generally would not be allowed certain tax deductions or foreign tax credits that would be allowed to a United States shareholder that is a U.S. corporation. A failure to comply with these reporting obligations may subject you to significant monetary penalties and may prevent the statute of limitations with respect to your U.S. federal income tax return for the year for which reporting was due from starting. We cannot provide any assurances that we will assist investors in determining whether any of our non-U.S. subsidiaries are treated as a controlled foreign corporation or whether such investor is treated as a United States shareholder with respect to any of such controlled foreign corporations or furnish to any United States shareholders information that may be necessary to comply with the aforementioned reporting and tax paying obligations. A United States investor should consult their own advisors regarding the potential application of these rules to its investment in the shares.
As a foreign private issuer, we are permitted, and intend, to follow certain home country corporate governance practices instead of otherwise applicable Nasdaq requirements, and we will not be subject to certain U.S. securities laws including, but not limited to, U.S. proxy rules and the filing of certain Exchange Act reports.
As a foreign private issuer, we will be permitted, and intend, to follow certain home country corporate governance practices instead of those otherwise required by the Nasdaq Stock Market for domestic U.S. issuers. Following our home country governance practices as opposed to the requirements that would otherwise apply to a U.S. company listed on The Nasdaq Global Market may provide less protection to you than what is accorded to investors under the listing rules of Nasdaq applicable to domestic U.S. issuers.
As a foreign private issuer, we will be exempt from the rules and regulations under the Securities Exchange Act of 1934, or the Exchange Act, related to the furnishing and content of proxy statements, including the applicable compensation disclosure requirements. Nevertheless, pursuant to regulations promulgated under Canadian Securities Administrators National Instrument 51-102 “Continuous Disclosure Obligations”, or the Instrument, we are required to disclose in the context of sending an information circular to shareholders all compensation paid, payable, awarded, granted, given or otherwise provided, directly or indirectly, by the issuer, or a subsidiary of the issuer, to each Named Executive Officer (as such term is defined in the Instrument) and director, in any capacity, including, for greater certainty, all plan and non-plan compensation, direct and indirect pay, remuneration, economic or financial award, reward, benefit, gift or perquisite paid, payable, awarded, granted, given, or otherwise provided to the NEO or director for services provided, directly or indirectly, to the issuer or a subsidiary of the issuer. Such disclosure will not be as extensive as that required of a U.S. domestic issuer. Our officers, directors and principal shareholders will also be exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we will not be required under the Exchange Act to file reports and financial statements with the SEC as frequently or as promptly as U.S. domestic companies whose securities are registered under the Exchange Act and we will be exempt from filing quarterly reports with the SEC under the Exchange Act. Moreover, we will not be required to comply with Regulation FD, which restricts the selective disclosure of material information, although we intend to voluntarily adopt a corporate disclosure policy substantially similar to Regulation FD. These exemptions and leniencies will reduce the frequency and scope of information and protections to which you may otherwise have been eligible in relation to a U.S. domestic issuer.
We would lose our foreign private issuer status if a majority of our shares are owned by U.S. residents and a majority of our directors or executive officers are U.S. citizens or residents or we fail to meet additional requirements necessary to avoid loss of foreign private issuer status. The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic issuer may be significantly higher. If we are not a foreign private issuer, we will be required to file periodic reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive than the forms available to a foreign private issuer. We may also be required to modify certain of our policies to comply with accepted governance practices associated with U.S. domestic issuers. Such conversion and modifications will involve additional costs. In addition, we would lose our ability to rely upon exemptions from certain corporate governance requirements on U.S. stock exchanges that are available to foreign private issuers.
We are an emerging growth company and the reduced disclosure requirements applicable to emerging growth companies may make our Common Shares less attractive to investors.
We are an emerging growth company, as defined in the JOBS Act, and we may take advantage of certain exemptions from various requirements that are applicable to other public companies that are not emerging growth companies.
For as long as we remain an emerging growth company we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not “emerging growth companies.” These exemptions include:
| ● | not being required to comply with the auditor attestation requirements in the assessment of our internal control over financial reporting; | |
| ● | Section 107 of the JOBS Act, which provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, or the Securities Act, for complying with new or revised accounting standards. This means that an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. Given that we currently report and expect to continue to report our financial results under IFRS as issued by IASB, we will not be able to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required by IASB; | |
| ● | not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements; | |
| ● | reduced disclosure obligations regarding executive compensation; and | |
| ● | exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. |
We will remain an emerging growth company until the earliest of: (i) the last day of our fiscal year during which we have total annual gross revenues of at least $1.235 billion; (ii) October 31, 2025; (iii) the date on which we have, during the previous three-year period, issued more than $1.0 billion in non-convertible debt; or (iv) the date on which we are deemed to be a “large accelerated filer” under the Exchange Act. We have opted out of the extended transition period made available to emerging growth companies to comply with newly adopted public company accounting requirements.
When we are no longer deemed to be an emerging growth company, we will not be entitled to the exemptions provided in the JOBS Act discussed above. We cannot predict if investors will find our Common Shares less attractive as a result of our reliance on exemptions under the JOBS Act. If some investors find our Common Shares less attractive as a result, there may be a less active trading market for our Common Shares and our share price may be more volatile.
Failure to remediate material weaknesses in internal accounting controls could result in material misstatements in our financial statements.
Our management has identified material weaknesses in our internal control over financial reporting related to lack of segregation of duties within account processes, and systems, inadequate documentation to evidence the operation of some of our controls, inconsistent procedures and approvals, lack of periodic user access reviews, lack of assessment of controls of financially significant vendors and insufficient written policies and procedures for accounting, IT and financial reporting and record keeping. Our management has concluded that, due to such material weakness, our disclosure controls and procedures were not effective as of October 31, 2023. We have implemented remediation efforts including independent review and approval of transactions and reconciliations in some processes by hiring personnel and segregating duties amongst the team. Management is implementing processes to document and retain evidence to support reviews and reconciliations. Such changes may not, however, be effective in establishing the adequacy of our internal control over financial reporting. If the material weaknesses are not adequately remedied, or if we identify further material weaknesses in our internal controls, our failure to establish and maintain effective disclosure controls and procedures and internal control over financial reporting could result in material misstatements in our financial statements and a failure to meet our reporting and financial obligations, each of which could have a material adverse effect on our financial condition and the trading price of our securities. In addition, investors’ perceptions that our internal control over financial reporting is inadequate or that we are unable to produce accurate financial statements may materially adversely affect the price of our securities.
Risks Related to our Location in Israel and our International Operations
Part of our operations are conducted in Israel. Conditions in Israel, including the recent attack by Hamas and other terrorist organizations and Israel’s war against them, may affect our operations.
Our operations are located in the State of Israel. In addition, certain of our key employees and officers, including our chief executive officer, are residents of Israel. Accordingly, political, economic and military conditions in Israel and the surrounding region may directly affect our business.
On October 7, 2023, an unprecedented attack was launched against Israel by terrorists from the Hamas terrorist organization that infiltrated Israel’s southern border from the Gaza Strip and in other areas within the state of Israel attacking civilians and military targets while simultaneously launching extensive rocket attacks on the Israeli population. These attacks resulted in extensive deaths, injuries and kidnapping of civilians and soldiers. In response, the Security Cabinet of the State of Israel declared war against Hamas and a military campaign against these terrorist organizations commenced in parallel to their continued rocket and terror attacks. To date, the State of Israel continues to be at war with Hamas.
Since the war broke out on October 7, 2023, our operations have not been adversely affected by this situation, and we have not experienced disruptions to our clinical studies, facilities or the manufacturing or supply of our drug candidates. However, at this time, it is not possible to predict the intensity or duration of the war, nor can we predict how this war will ultimately affect Israel’s economy in general and we continue to monitor the situation closely and examine the potential disruptions that could adversely affect our operations.
In connection with the Israeli security cabinet’s declaration of war against Hamas and possible hostilities with other organizations, several hundred thousand Israeli military reservists were drafted to perform immediate military service. While none of our employees in Israel have been called to active military duty, we rely on service providers located in Israel and have entered into certain agreements with Israeli counterparties. Employees of such service providers or contractual counterparties may be called for service in the current or future wars or other armed conflicts with Hamas and such persons may be absent from their positions for a period of time. As of January 28, 2024, we have not been impacted by any absences of personnel at our service providers or counterparties located in Israel. However, military service call ups that result in absences of personnel from us, our service providers or contractual counterparties in Israel may disrupt our operations and absences for an extended period of time may materially and adversely affect our business, prospects, financial condition and results of operations.
Following the attack by Hamas on Israel’s southern border, Hezbollah, a terrorist organization in Lebanon has also launched missile, rocket, and shooting attacks against Israeli military sites, troops, and Israeli towns in northern Israel. In response to these attacks, the Israeli army has carried out a number of targeted strikes on sites belonging to Hezbollah in southern Lebanon. It is possible that other terrorist organizations, including Palestinian military organizations in the West Bank, as well as other hostile countries, such as Iran, will join the hostilities. Such hostilities may include terror and missile attacks. Any hostilities involving Israel or the interruption or curtailment of trade between Israel and its trading partners could adversely affect our operations and results of operations. Our insurance policies do not cover losses that may occur as a result of events associated with war and terrorism. Although the Israeli government currently covers the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, we cannot assure you that this government coverage will be maintained or that it will sufficiently cover our potential damages. Any losses or damages incurred by us could have a material adverse effect on our business. Any armed conflicts or political instability in the region would likely negatively affect business conditions and could harm our results of operations.
Several countries, principally in the Middle East, still restrict doing business with Israel and Israeli companies, and additional countries may impose restrictions on doing business with Israel and Israeli companies, whether as a result of hostilities in the region or otherwise. In addition, there have been increased efforts by activists to cause companies, research institutions and consumers to boycott Israeli goods and cooperation with Israeli-related entities based on Israeli government policies. Such actions, particularly if they become more widespread, may adversely impact our ability to cooperate with research institutions and collaborate with other third parties. Any hostilities involving Israel, any interruption or curtailment of trade or scientific cooperation between Israel and its present partners, or a significant downturn in the economic or financial condition of Israel could adversely affect our business, financial condition and results of operations. We may also be targeted by cyber terrorists specifically because we may be perceived as an Israeli-related company.
Any hostilities involving Israel or the interruption or curtailment of trade between Israel and its present trading partners, or a significant downturn in the economic or financial condition of Israel, could affect adversely our operations. Ongoing and revived hostilities or other Israeli political or economic factors could harm our operations, product development and results of operations.
It may be difficult to enforce a U.S. judgment against us, our officers and directors named in this Annual Report in Israel or the United States, or to assert U.S. securities laws claims in Israel or serve process on our officers and directors.
Not all of our directors or officers are residents of the United States and most of their and our assets are located outside the United States. Service of process upon us or our non-U.S. resident directors and officers may be difficult to obtain within the United States. We have been informed by our legal counsel in Israel that it may be difficult to assert claims under U.S. securities laws in original actions instituted in Israel or obtain a judgment based on the civil liability provisions of U.S. federal securities laws. Israeli courts may refuse to hear a claim based on a violation of U.S. securities laws against us or our non-U.S. officers and directors because Israel may not be the most appropriate forum to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Canadian law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact, which can be a time-consuming and costly process. Certain matters of procedure will also be governed by Canadian law. There is little binding case law in Israel addressing the matters described above. Additionally, Israeli courts might not enforce judgments obtained in the United States against us or our non-U.S. our directors and executive officers, which may make it difficult to collect on judgments rendered against us or our non-U.S. officers and directors.
Moreover, an Israeli court will not enforce a non-Israeli judgment if it was given in a state whose laws do not provide for the enforcement of judgments of Israeli courts (subject to exceptional cases), if its enforcement is likely to prejudice the sovereignty or security of the State of Israel, if it was obtained by fraud or in the absence of due process, if it is at variance with another valid judgment that was given in the same matter between the same parties, or if a suit in the same matter between the same parties was pending before a court or tribunal in Israel at the time the foreign action was brought.
Because we are a corporation incorporated in British Columbia and some of our directors and officers are resident in Canada, it may be difficult for investors in the United States to enforce civil liabilities against us based solely upon the federal securities laws of the United States. Similarly, it may be difficult for Canadian investors to enforce civil liabilities against our directors and officers residing outside of Canada.
We are a corporation incorporated under the laws of British Columbia with our principal place of business in Vancouver, Canada. Some of our directors and officers and the predecessor auditors or other experts named herein are residents of Canada and all or a substantial portion of our assets and those of such persons are located outside the United States. Consequently, it may be difficult for U.S. investors to effect service of process within the United States upon us or our directors or officers or such auditors who are not residents of the United States, or to realize in the United States upon judgments of courts of the United States predicated upon civil liabilities under the Securities Act. Investors should not assume that Canadian courts: (1) would enforce judgments of U.S. courts obtained in actions against us or such persons predicated upon the civil liability provisions of the U.S. federal securities laws or the securities or blue-sky laws of any state within the United States or (2) would enforce, in original actions, liabilities against us or such persons predicated upon the U.S. federal securities laws or any such state securities or blue-sky laws.
Similarly, some of our directors and officers and experts are residents of countries other than Canada and all or a substantial portion of the assets of such persons are located outside Canada. As a result, it may be difficult for Canadian investors to initiate a lawsuit within Canada against these non-Canadian residents. In addition, it may not be possible for Canadian investors to collect from these non-Canadian residents’ judgments obtained in courts in Canada predicated on the civil liability provisions of securities legislation of certain of the provinces and territories of Canada. It may also be difficult for Canadian investors to succeed in a lawsuit in the United States, based solely on violations of Canadian securities laws.
Operating outside of the United States presents specific risks to our business, and we have substantial operations outside of the United States.
Most of our employee base and operations are located outside the United States, primarily in Canada and Israel. Most of our clinical testing, research, development and manufacturing operations are conducted outside the United States.
Risks associated with operations outside the United States include:
| ● | fluctuating foreign currency rates could restrict sales, increase costs of purchasing, and impact collection of receivables outside of the United States; |
| ● | volatility in foreign credit markets may affect the financial well-being of our customers and suppliers; |
| ● | violations of anti-corruption laws, including the Foreign Corrupt Practices Act and the U.K. Bribery Act could result in large fines and penalties; |
| ● | violations of privacy and data security laws could result in large fines and penalties; and |
| ● | tax disputes with foreign taxing authorities, and any resultant taxation in foreign jurisdictions associated with operations in such jurisdictions, including with respect to transfer pricing practices associated with such operations. |
Foreign currency fluctuations may reduce our competitiveness and sales in foreign markets.
The relative change in currency values creates fluctuations in potential product pricing for international customers. These changes in foreign end-customer costs may result in lost orders and reduce the competitiveness of our products in certain foreign markets. These changes may also negatively impact the financial condition of some foreign customers and reduce or eliminate their future orders of our products.
Adverse changes in, or uncertainty of, local business laws or practices, including the following:
| ● | foreign governments may impose burdensome tariffs, quotas, taxes, trade barriers, or capital flow restrictions; |
| ● | restrictions on the export or import of technology may reduce or eliminate the ability to sell in or purchase from certain markets; |
| ● | political and economic instability, including deterioration of political relations between the United States and other countries, may reduce demand for our solutions or put our non-U.S. assets at risk; |
| ● | potentially limited intellectual property protection in certain countries may limit recourse against infringing on our solutions or cause us to refrain from selling in certain geographic territories; |
| ● | staffing may be difficult along with higher turnover at international operations; |
| ● | transportation delays and customs related delays that may affect production and distribution of our products; and |
| ● | integration and enforcement of laws vary significantly among jurisdictions and may change significantly over time. |
Our failure to manage any of these risks successfully could harm our international operations and adversely impact our business, operating results and financial condition.
General Risk Factors
We will incur significant increased costs as a result of the listing of our securities for trading on Nasdaq. By becoming a public company in the United States, our management will be required to devote substantial time to new compliance initiatives as well as compliance with ongoing U.S. requirements.
Upon the listing of securities on Nasdaq, we became a publicly traded company in the United States. As a public company in the United States, we will incur additional significant accounting, legal and other expenses that we did not incur before the initial public offering. We also anticipate that we will incur costs associated with corporate governance requirements of the SEC, as well as requirements under Section 404 and other provisions of the Sarbanes-Oxley Act. We expect these rules and regulations to increase our legal and financial compliance costs, introduce new costs such as investor relations, stock exchange listing fees and shareholder reporting, and to make some activities more time consuming and costly. The implementation and testing of such processes and systems may require us to hire outside consultants and incur other significant costs. Any future changes in the laws and regulations affecting public companies in the United States, including Section 404 and other provisions of the Sarbanes-Oxley Act, and the rules and regulations adopted by the SEC, for so long as they apply to us, will result in increased costs to us as we respond to such changes. These laws, rules and regulations could make it more difficult or more costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our Board of Directors, our Board committees, or as executive officers.
If we are not able to attract and retain highly skilled managerial, scientific, technical and marketing personnel, we may not be able to implement our business model successfully.
Our success depends in part on our continued ability to attract, retain and motivate highly qualified management, clinical and scientific personnel. We are highly dependent upon our senior management as well as other employees, consultants and scientific and medical collaborators. Our management team must be able to act decisively to apply and adapt our business model in the rapidly changing markets in which we will compete. In addition, we will rely upon technical and scientific employees or third-party contractors to effectively establish, manage and grow our business. Consequently, we believe that our future viability will depend largely on our ability to attract and retain highly skilled managerial, sales, scientific and technical personnel. In order to do so, we may need to pay higher compensation or fees to our employees or consultants than currently expected and such higher compensation payments may have a negative effect on our operating results. Competition for experienced, high-quality personnel in the medical device field is intense. We may not be able to hire or retain the necessary personnel to implement our business strategy. Our failure to hire and retain quality personnel on acceptable terms could impair our ability to develop new products and services and manage our business effectively.
Our business and operations might be adversely affected by security breaches, including any cybersecurity incidents.
We depend on the efficient and uninterrupted operation of our computer and communications systems, and those of our consultants, contractors and vendors, which we use for, among other things, sensitive company data, including our intellectual property, financial data and other proprietary business information.
While certain of our operations have business continuity and disaster recovery plans and other security measures intended to prevent and minimize the impact of IT-related interruptions, our IT infrastructure and the IT infrastructure of our consultants, contractors and vendors are vulnerable to damage from cyberattacks, computer viruses, unauthorized access, electrical failures and natural disasters or other catastrophic events. We could experience failures in our information systems and computer servers, which could result in an interruption of our normal business operations and require substantial expenditure of financial and administrative resources to remedy. System failures, accidents or security breaches can cause interruptions in our operations and can result in a material disruption of our targeted phage therapies, product candidates and other business operations. The loss of data from completed or future studies or clinical trials could result in delays in our research, development or regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur regulatory investigations and redresses, penalties and liabilities and the development of our product candidates could be delayed or otherwise adversely affected.
Even though we believe we carry commercially reasonable business interruption and liability insurance, we might suffer losses as a result of business interruptions that exceed the coverage available under our insurance policies or for which we do not have coverage. For example, we are not insured against terrorist attacks or cyberattacks. Any natural disaster or catastrophic event could have a significant negative impact on our operations and financial results. Moreover, any such event could delay the development of our product candidates.
Sales of a significant number of our Common Shares in the public markets or significant short sales of our Common Shares, or the perception that such sales could occur, could depress the market price of our Common Shares and impair our ability to raise capital.
Sales of a substantial number of our Common Shares or other equity-related securities in the public markets, could depress the market price of our Common Shares. If there are significant short sales of our Common Shares, the price decline that could result from this activity may cause the share price to decline more so, which, in turn, may cause long holders of the Common Shares to sell their shares, thereby contributing to sales of Common Shares in the market. Such sales also may impair our ability to raise capital through the sale of additional equity securities in the future at a time and price that our management deems acceptable, if at all.
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they adversely change their recommendations or publish negative reports regarding our business or the Common Shares, our share price and trading volume could decline.
The trading market for the Common Shares will be influenced by the research and reports that industry or securities analysts may publish about us, our business, our market or our competitors. We do not have any control over these analysts and we cannot provide any assurance that analysts will cover us or provide favorable coverage. If any of the analysts who may cover us adversely change their recommendation regarding the Common Shares, or provide more favorable relative recommendations about our competitors, the price of our Common Shares would likely decline. If any analyst who may cover us were to cease coverage of our Company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause the price of our Common Shares or trading volume to decline.
ITEM 4. INFORMATION ON THE COMPANY
A. History and Development of the Company
We were incorporated in British Columbia under the name Cyntar Ventures Inc. on July 18, 2017, pursuant to the provisions of the Business Corporations Act (British Columbia). On March 24, 2021, we changed our name to Clearmind Medicine Inc.
Originally, we operated as a mineral resource exploration operations company. In September 2020, we announced a shift om the focus of our business to the development of innovative psychedelic therapies. This process involved the acquisition of all rights, title and interests in several patent applications for the treatment of alcohol abuse disorder and various other non-controlled binge behaviors. As part of this process, we announced a Change of Business, or COB, listing on the Canadian Securities Exchange, or CSE. The COB became effective in November 2020. In May 2021, we completed all of the requirements of the CSE for a COB listing.
Our principal executive offices are located at 101 – 1220 W. 6th Ave, Vancouver, BC V6H1A5. Our telephone number is 973.536.1016. Our website address is https://www.clearmindmedicine.com/. Information contained on or accessible through our website is not a part of this Annual Report on Form 20-F, and the inclusion of our website address herein is an inactive textual reference only. Puglisi & Associates, or Puglisi, serves as our authorized representative in the United States for certain limited matters. Puglisi’s address is 850 Library Avenue, Newark, Delaware 19711.
Our cash used in investing activities for 2021, 2022 and 2023 amounted to $184,035, $5,563 and $26,228 respectively. This increased amount in 2021 was mainly due to an assignment agreement whereby the Company acquired patents and patent applications to be used in pre-clinical drug research programs and due to a payment related to exploration and evaluation activity.
The SEC maintains an internet site that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC at http://sec.gov. We use our website (http://www.clearmindmedicine.com), LinkedIn (https://www.linkedin.com/company/clearmind-medicine), Instagram (https://www.instagram.com/clearmindmed), Twitter (https://twitter.com/ClearmindCMND) and Facebook (https://www.facebook.com/ClearmindMedicine) as channels of distribution of Company information. The information we post through this channel may be deemed material. Accordingly, investors should monitor our website, in addition to following our press releases, SEC filings and public conference calls and webcasts. The contents of our website are not, however, a part of this Annual Report on Form 20-F.
As a foreign private issuer, we are exempt from certain rules and regulations under the Exchange Act that are applicable to other public companies that are not foreign private issuers. For example, although we intend to report our financial results on a quarterly basis, we will not be required to issue quarterly reports, proxy statements that comply with the requirements applicable to U.S. domestic reporting companies, or individual executive compensation information that is as detailed as that required of U.S. domestic reporting companies. We will also have four months after the end of each fiscal year to file our annual report with the SEC and will not be required to file current reports as frequently or promptly as U.S. domestic reporting companies. Our senior management, directors, and principal shareholders will be exempt from the requirements to report transactions in our equity securities and from the short-swing profit liability provisions contained in Section 16 of the Exchange Act. As a foreign private issuer, we will also not be subject to the requirements of Regulation FD (Fair Disclosure) promulgated under the Exchange Act.
B. Business Overview
Overview
We are a clinical pharmaceutical company approaching phase 1 clinical trials, that develops novel psychedelic medicines to solve widespread, yet under-served, health problems. Our goal is to develop and provide new type of treatments for mental health disorders, including AUD, binge drinking and obesity and metabolic disorder, where there is significant unmet need and lack of innovation. We see psychedelic therapies, which previously may have been overlooked or underused, as the future of treatment for a variety of indications. We believe that our solution for AUD can help solve one of the world’s biggest health problems, which costs the United States alone roughly $250 billion each year.
Our flagship treatment is our proprietary MEAI and the initial indication we are pursuing is Alcohol Use Disorder, which is incredibly common. It varies from mild to excessive and describes a person’s inability to restrict their alcohol consumption, despite negative social, occupational, or health consequences. Alcohol consumption contributes to 3 million deaths each year globally and is the third most common preventable cause of death in the United States. Apart from potentially changing people’s lives, we believe that our treatment could potentially reduce the amount currently being spent on the consequences of AUD in the United States, Europe, India, China and other countries around the world. We also believe that our treatment may address binge drinking, which a recent study published in JAMA Network Open, an medical journal published by the American Medical Association covering all aspects of the biomedical sciences, showed that 20% of U.S. adults between the ages over 20 and 49 died as a result of binge drinking during the four-year period of the study.
We have completed a series of pre-clinical, IND-enabling studies in the United States and China that are required before we can study our compound for the first time in humans. These studies include pharmacokinetic and toxicological studies in rats and dogs in order to assess the safety profile of our compound and characterization of the drug metabolism. We have conducted several metabolism studies designed to better understand the way MEAI is digested in several species. In addition, we have conducted a pre-clinical animal model of AUD to characterize the effect of MEAI on alcohol consumption. This study involved testing the effect of MEAI’s ability to curb alcohol cravings after exposing mice to prolonged alcohol consumption over a short period, mimicking binge alcohol consumption in humans.
In March 2023, we announced that we had submitted an IND application with the FDA, requesting approval to initiate our first-in-human Phase I/IIa clinical trial with CMND-100 in patients suffering from AUD. Subsequently, and after submission of a similar application to the Israeli Ministry of Health, in May 2023 we initiated the CM-CMND-001 clinical trial in both Israel and the United States, including at the Yale School of Medicine’s Department of Psychiatry and Johns Hopkins University School of Medicine.
The CM-CMND-001 clinical trial is designed to be a multinational, multi-center, double blind, Phase I/IIa single- and multiple-dose tolerability, safety and pharmacokinetic study in healthy volunteers and AUD subjects. Upon completion of the Phase I/IIa studies, if successful, we will be required to conduct additional clinical trials subject to securing additional financing.
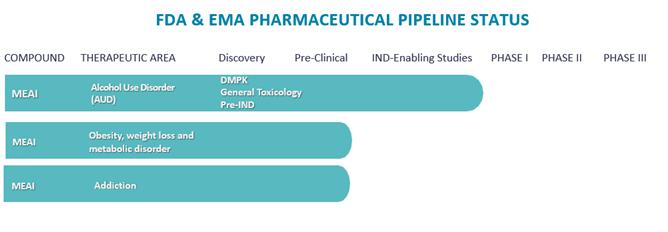
Looking further, we are also on the journey to tackle some of the world’s major mental health problems and disorders. We believe that psychedelic solutions such as our drug candidate may hold the key to providing much needed solutions to various disorders and conditions, including, but not limited to, obesity, weight loss and metabolic disorder, depression, anxiety, and cocaine addiction. Those suffering from these sorts of mental health disorders have higher mortality rates than the general population and often experience decreased quality of life as a result of emotional, behavioral or physical manifestations. Also, globally, the total costs of mental health disorders are significant and expected to increase substantially. In 2020, the total U.S. expenditure for mental health reached $238.4 billion, and this figure includes expenditures on healthcare service providers, retail prescription drugs and insurance administration. Nearly 22% of all U.S. adults received some form of mental health treatment in 2021, up from about 19% just two years earlier. Also, according to Mordor Intelligence, in 2022, Adult Prevalence of Mental Illness statistics show that 19.86% of adults in the United States are experiencing a mental illness, which is equivalent to nearly 50 million Americans, with 4.91% experiencing a severe mental illness.
Common mental disorders in the United States that can be attributed to mental health care costs include anxiety disorders, depression, bipolar disorder, obsessive compulsive disorder (OCD), and dementia. While current treatments, such as selective serotonin reuptake inhibitors, or SSRIs, and serotonin–norepinephrine reuptake inhibitors, or SNRIs, are well established and effective for certain patients.
We believe that the world is transitioning to become a place that is more accepting of psychedelic use, and a revolution in medicine is on the horizon. As we continue to grow our Company, our goal is to continue to invest, develop and produce solutions to help patients that suffer from mental health disorders. We strive to get millions of people out of the cycle of addiction, anxiety, and depression. Not only do we aim to uplift those that are struggling but also their families, communities, and societies.
Our People
Collectively, our team has vast experience across the business and pharmaceutical industries. Our Chief Executive Officer, Adi Zuloff-Shani, PhD, is a biomedical research and development executive with over 25 years of strategic and operational leadership in the healthcare industry, including clinical trials, and a deep understanding of therapeutics development in heavily regulated environments. Our award-winning advisory board includes professionals such as Prof. John Krystal. Prof. Krystal is the Robert L. McNeil, Jr. Professor of Translational Research a Professor of Psychiatry, Neuroscience, and Psychology and Chair of the Department of Psychiatry at the Yale University. He is also Chief of Psychiatry and Behavioral Health at Yale-New Haven Hospital. He is a graduate of the University of Chicago, Yale University School of Medicine, and the Yale Psychiatry Residency Training Program. He has published extensively on the neurobiology and treatment of schizophrenia, alcoholism, PTSD, and depression. Notably, his laboratory discovered the rapid antidepressant effects of ketamine in humans. He is the Director of the NIAAA Center for the Translational Neuroscience of Alcoholism and the Clinical Neuroscience Division of the VA National Center for PTSD. Prof. Krystal is a member of the U.S. National Academy of Medicine and a Fellow of the American Association for the Advancement of Science. Currently, he is co-director of the Neuroscience Forum of the U.S. National Academies of Sciences, Engineering, and Medicine; and editor of Biological Psychiatry (IF=12.1). He has chaired the NIMH Board of Scientific Counselors and served on the national advisory councils for both NIMH and NIAAA. Also, he is past president of the American College of Neuropsychopharmacology (ACNP) and International College of Neuropsychopharmacology (CINP). Prof. Gabriele Fischer, Prof. Christian Schültz, Prof. Wim van den Brink, Prof. Michael Davidson, Prof. Alon Friedman, Prof. Gal Yadid, Prof. Yossi Tam, Dr. Fatima Cody Stanford and Prof. Henry R. Kranzler are all also part of our outstanding advisory board.
About MEAI
MEAI is a synthetic molecule. Its mechanism of action has been studied and published in past scientific papers. It was found to interact with the serotonergic receptors 5-HT1A and 5-HT2B and the adrenergic receptors α2A, α2B and α2C receptors. Studies conducted in animals and humans have demonstrated the role of 5-HT1A receptors in alcohol-drinking behavior. Several 5-HT1A receptor agonists have been tested in animal models to demonstrate the role of this receptor in alcohol dependence. These preclinical studies suggest that 5-HT1A receptor agonists may play a role in reducing alcohol intake. In addition, evidence suggests that α2-adrenergic receptor agonist signaling may play a role in mediating alcohol-drinking behavior in both rodents and humans.
The literature shows that 5-HT1A receptors are associated with controlling craving behaviors across the board. This indicates that MEAI may have a wide range of applications beyond AUD and binge drinking. Until today, only pre-clinical studies have been conducted with MEAI, including in-vitro and in-vivo studies. The studies were conducted in the United States, China, France and Israel.
MEAI is a psychoactive non-hallucinogenic molecule, exerting a euphoric alcohol-like experience, and based on human recreational use testimonials and pre-clinical trials, we believe also reduces the desire to consume alcoholic beverages. While determinations of safety and efficacy are solely within the authority of the FDA and comparable regulatory bodies, in pre-clinical studies, MEAI was well-tolerated by the tested animals. Although MEAI remains in development and is not cleared or approved by the FDA or similar foreign regulatory bodies, we believe that our drug candidate has the potential to change the lives of millions who struggle to drink in moderation.
We believe that MEAI holds the potential to break the vicious binge-drinking cycle at the decision point to drink more alcohol, by potentially innervating neural pathways such as 5-HT1A that lead to “sensible behavior”.
Research Programs
Our IP portfolio is composed of several psychoactive molecules and we successfully conducted research in several programs on different molecules, which were led by our highly skilled, focused team, with deep expertise in their respective fields. Several of our team members have taken products from the discovery phase to clinical trials in the United States in their previous respective roles and are also key members of our scientific advisory board who have participated in numerous clinical trials in the areas of alcoholism and addiction. We are currently focusing our research programs on the uses of MEAI for the treatment of AUD, obesity and metabolic disorders and as an alcohol substitute consumer product.
In our research program aimed at treating depression and treatment resistant depression, or TRD, we studied the effects of administering 2-fluorodeschloroketamine, or 2-FDCK and we investigated 2-FDCK in a pre-clinical proof-of-concept study. Following the completion of the study, while we do not have any immediate plans to further investigate this now, we may in the future further investigate the effect of depression and the mechanisms of action around it.
In our two research programs aimed at finding substances that can be utilized for the same therapeutic purposes as MDMA, we studied 1-(Benzofuran-5-yl)-N-methylpropan-2-amine, or 5-MAPB and 1-Benzofuran-6-yl propan-2-amine, or 6-APB. We believe these treatments may be beneficial for fail-safes for MDMA based on a September 2016 article from Naunyn-Schmiedeberg’s Archives of Pharmacology, which reported the receptor binding profiles of 5-MAPB and 6-APB are different enough from MDMA to effectively perform a substitute role in the therapy while being similar enough so as not to have to change the therapeutic protocol. We currently hold patents with respect to the compounds, but we do not have any immediate research plans.
Strategic Focus
With respect to our AUD programs, we developed MEAI as a new chemical entity (NCE) drug candidate. We intend to seek regulatory approval through the FDA’s 505(b)(1) regulatory path. The FDA’s 505(b)(1) regulatory path is typically used for novel drugs that have not previously been studied or approved, and drug development pursuant to this path requires drug developers to conduct all studies needed to demonstrate the safety and efficacy of the drug. Given its nature, this type of submission requires extensive research, including both clinical and nonclinical studies, to prove the product’s safety and efficacy for the indication being sought.
Phase I/IIa Clinical Study
In March 2023, we announced that we had submitted an IND application with the FDA, requesting approval to initiate our first-in-human Phase I/IIa clinical trial with CMND-100 in subjects suffering from AUD. Subsequently and after submission of a similar application to the Israeli Ministry of Health, in May 2023 we initiated the CM-CMND-001 clinical trial in both Israel and the United States.
The Israeli study is be led by Prof. Mark Weisser, M.D. Our U.S. sites for the CM-CMND-001 clinical trial are Yale School of Medicine’s Department of Psychiatry and Johns Hopkins University School of Medicine. The Yale site is led by Anahita Bassir Nia, MD, a specialist in substance abuse, including alcohol abuse. The Johns Hopkins site will be led by Jennifer Ellis, PhD, Associate Professor of Psychiatry and Behavioral Sciences as the principal investigator, who will be supported by co-investigators Professor Eric Strain, Director, Behavioral Pharmacology Research Unit of the Johns Hopkins University School of Medicine.
The CM-CMND-001 clinical trial is designed to be a double blind, multinational, multi-center, Phase I/IIa single- and multiple-dose tolerability, safety, initial efficacy and pharmacokinetic study in healthy volunteers and AUD subjects. Upon completion of the Phase I/IIa studies, if successful, we will be required to conduct additional clinical trials subject to securing additional financing.
In April 2023, we announced that we formed a Data and Safety Monitoring Board, or DMSB, to oversee the Phase I/IIa clinical study. The DSMB – an independent group of experts– includes a specialist in internal medicine, a specialist in psychiatry, and a biostatistician. The DSMB is a committee of experts responsible for reviewing clinical trial data on an ongoing basis to ensure the safety of study subjects and validity and integrity of the data.
In May 2023, we announced that we had entered into a clinical supply agreement with IMP Clinical Supply Services, or IMP, for the Phase I/IIa clinical study. Pursuant to the clinical supply agreement, IMP is responsible for the global clinical supply chain of CMND-100 from manufacture to the various clinical sites. IMP is known for its comprehensive, tailor-made clinical supply services that address all study requirements. The array of services to be performed are fully compliant with GMP, GCP, and GDP standards. Clinical drug supply is a critical factor for trial success so collaboration with best-in-class clinical drug supply vendor forestalls the possibility of it being a bottleneck to successful drug delivery. Moreover, it can also deliver enormous benefits, including better investigator and patient experiences as well as cost savings in clinical drug supply.
The Phase I part of the Phase I/IIa clinical study is an open label, randomized study to evaluate the safety and pharmacokinetics of single ascending doses of MEAI oral capsules. If approved by the FDA, we will utilize a hybrid model where we study the effects of MEAI in healthy volunteers and AUD patients. Single ascending doses will be distributed in up to 4 cohorts, with 6 subjects per dose. We intend to submit applications to conduct the Phase I/IIa study in Israel, while we await the FDA to make a decision on our IND application. Once the regulatory bodies have provided us their approvals or denials on the matter, we will determine the distribution of the cohorts based, among other things, on the rate in which we can enroll patients at each site.
The Phase IIa part of the Phase I/IIa clinical study is a double-blind, randomized, , placebo-controlled study to assess the safety of multiple doses of MEAI in healthy volunteers and AUD subjects and as a secondary endpoint, the potential effect of MEAI on drinking patterns and cravings in individuals with AUD in accordance with DSM-V criteria. Two cohorts of 18 subjects per cohort will be given the highest tolerated doses and placebo (2:1 ratio). Oral capsules will be given once daily, for several consecutive days. The patients will complete diaries and questionnaires to report on their drinking patterns and craving for alcohol during the clinical trial period.
We will be using a Pharmacokinetics, or PK, assessment in both phases of the Phase I/IIa study. PK is defined by the American Society of Health-System Pharmacists as the study of the time course of a drug absorption, distribution, metabolism, and excretion in animals and human. Typical PK assessments include blood and organs collections at standard time intervals in order to test the drug level in the various tissues as function of time. The pharmacokinetic information as studied in animals has application to the safe and effective therapeutic management of drugs in an individual patient. In our study, our PK assessment will consist of collecting blood from all subjects enrolled in the study at the following timepoints:
| (i) | Phase 1 - before dosing and at multiple time points (at 0.5, 1.0, 2.0, 4.0, 6.0, 8.0, 12.0, 18.0 and 24-hour post drug administration. |
| (ii) | Phase 2 - before the first dosing and at multiple time points (at 0.5, 1.0, 2.0, 4.0, 6.0, 8.0, 12.0, 18.0 and, 24-hour post drug administration and before the last dosing and at multiple time points (at 0.5, 1.0, 2.0, 4.0, 6.0, 8.0, 12.0, 18.0 and, 24-hour post last drug administration |
The primary objective of the study is the evaluation of tolerability, safety and pharmacokinetics of CMND-100 in both healthy and subjects with binge drinking/AUD. Safety will be assessed from randomization of subjects until the last follow-up visit.
The primary safety endpoint will therefore consist of evaluation of incidence of adverse events, changes in vital signs, clinical significant abnormal laboratory data, clinical significant abnormal physical examination and ECG following subject randomization. For assessment of AEs please see Section 11. Additional safety assessments include, among others, abuse liability measured by Visual Analogue Scale (VAS) and suicide risk assessment measured by the Columbia-Suicide Severity Rating Scale (CSSRS).
The primary PK endpoint parameters will be evaluated in all subjects: Tmax, Cmax, AUC0-∞, t1/2, Cl, Vd.
Treating Unmet Mental Health Disorders
We believe that AUD is not being addressed by most of the psychedelic industry. Global spending necessitated by the implications of AUD is sky-rocketing; in 2010 there was an estimated cost of nearly $250 billion in the United States, comprised of costs due to productivity losses, healthcare expenditures, crime, and traffic crashes among other things. A 2020 survey in the United States conducted by the National Survey on Drug Use and Health showed that 28.3 million people aged 12 years and older reported having AUD, with 2.1 million of this group having reported receiving treatment for alcohol use. Despite the pressing need to address AUD, psychedelic industry is mostly focusing on research and development on other areas of health. We believe that these unmet needs exist across efficacy, safety and time to onset of current treatment options.
Tackling a Social Burden to Improve the Lives of Patients and People Surrounding Them
Alcoholism and binge drinking are a huge social burden in terms of costs by direct and indirect implications. These health problems impact a wide range of social cycles surrounding the alcoholic/ binge drinkers life – especially when it is a parent, life partner, sibling or co-worker. According to the National Council on Alcoholism and Drug Dependence:
The time, effort, and resources formerly dedicated to life-sustaining activities, such as working and spending time with the family, are disrupted. Initially, a person may think that abusing alcohol will help them deal with these stressors, but as they continue to drink a lot, over time, this use can turn into dependence on the substance. Once individuals become psychologically addicted, alcohol abuse can become all-consuming. As individuals are often part of social networks, it is easy to understand how alcohol abuse has a ripple effect across a person’s entire network of family, friends, employers, colleagues, and anyone else who depends on the person.
Reducing the problem for the patient will have a rebound effect, reducing the burden for the patient’s social circle as well.
Lack of Therapeutic Innovation and New Treatments
Historically, there has been a lack of innovation in new treatments and medicines for the treatment of alcohol related disorders. There are only three medications that are currently FDA-approved for the treatment of moderate to severe AUD: disulfiram, naltrexone (oral and long-acting injectable formulations), and acamprosate. Both naltrexone and acamprosate were associated with reduction in relapse to drinking. When directly compared with one another, no significant differences were found between acamprosate and naltrexone for controlling alcohol consumption. Disulfiram is used as a second-line treatment, behind acamprosate and naltrexone, for alcohol dependence. Based on results in open-label studies, disulfiram is a safe and efficacious treatment compared to other abstinence supportive pharmacological treatments or to no disulfiram in supervised studies for problems of alcohol abuse or dependence. In a meta-analysis conducted on disulfiram use with alcoholics in comparison to any alcoholic control group, it was concluded that blinded studies with disulfiram were incapable of distinguishing a difference between treatment and control groups. An additional drug, Nalmefene, is approved for AUD treatment in Europe, but is not currently available in the United States. According to a meta-analysis, the drug is able to improve behavioral outcomes in patients with AUD, while others show that it has a limited efficacy in alcohol dependence therapy, and rather, it is more useful as an as-needed, harm-reducing pharmacotherapy.
There are other medications that are used off-label for AUD including gabapentin, topiramate, baclofen, and ondansetron For most medications used off-label, evidence was either insufficient to determine whether they are associated with reduced consumption or evidence suggested that they are not (Jonas et al., 2014).
Most well-controlled trials of disulfiram do not show overall reductions in alcohol consumption. However, psychedelics seems to emerge as a potential new treatment. An increasing validation of preclinical clinical and regulatory studies of new psychedelics pharmacological classes have been approved by the FDA. This combination of market opportunity and recent advances signal an increased acceptance of these novel approaches, and we believe we are well positioned to capitalize on this potential opportunity.
In May 2021, Nature Medicine published findings from a first Phase 3 clinical trial conducted with psychedelic-assisted therapy. The study found that MDMA combined with psychological counseling yielded “marked relief to patients” with severe PTSD. Based on the Phase 3 clinical trial results, MAPS Public Benefit Corp. recently filed an application with the U.S. FDA to approve the psychedelic drug MDMA — also known as Ecstasy — in combination with therapy to treat PTSD. The filing is a milestone in researchers’ quest to move psychedelic drugs from tightly restricted substances into mainstream medical treatments that are widely accessible to patients. This comes after decades of studies have demonstrated the promise of psychedelics to treat mental health disorders.
Esketamine was approved by the FDA on March 5, 2019, for treatment-resistant depression. It is sold under the trade name, Spravato. Esketamine became the first FDA-approved psychedelic treatment for a psychiatric disorder. In addition, recently the FDA extended its approval for esketamine to adults with major depressive disorder with acute suicidal ideation or behavior. In April 2021, the FDA granted psilocybin “breakthrough” therapy designation. In addition, the FDA cleared MAPS (Multidisciplinary Association for Psychedelics Studies) to conduct Phase III trials of MDMA as assisted therapy for PTSD.
COVID-19 Pandemic implications
AUD is a huge problem that costs billions every year. But the problem was only escalated and more people suffer, partially due to the COVID-19 pandemic. Economists as well as healthcare and addiction specialists agree the pandemic and quarantines of 2020 had a significant impact on nationwide alcohol consumption. For example, the National Center for Drug Abuse Statistics has reported that during the pandemic and quarantines of 2020, year-over-year alcohol sales were up 234%, 34.1% of respondents in a survey conducted by Johns Hopkins University reported binge drinking at least once, with 7.0% of respondents reporting extreme binge drinking and 45.7% of respondents reporting increased stress as a reason for their increased drinking. In addition, it has been estimated that depression symptoms increased by a factor of 3 compared to pre-COVID-19 levels, with severe depression symptoms increasing by a factor of 7.5 compared to pre-COVID-19 levels.
Markets Overview and Opportunity
The current indications we are pursuing with our MEAI molecule are focused on two main verticals: (1) AUD and binge drinking (2) obesity and metabolic disorder. Additionally, we have engaged Professor Gal Yadid from the Gonda Multidisciplinary Brain Research Center located at Bar Ilan University (Ramat Gan, Israel) to advance the evaluation of MEAI as a potential treatment for addictions and other binge behaviors. Prof. Yadid, a prominent figure in the research and development of treatments for psychiatric disease, specifically drug addiction, depression, and PTSD, who is also a member of our Scientific Advisory Board, and his team conducted a series of studies to explore the mechanism of action and specific neural targets of MEAI to evaluate its efficacy as a potential therapeutic agent in the treatment of addiction and related behaviors. The results of pre-clinical research studies suggested that MEAI may be effective in the treatment of substance addiction and specifically cocaine without interrupting the natural reward process. According to the United Nations Office on Drugs and Crime’s “2022 World Drug Report”, of the estimated 284 million people who had used drugs in the past year, approximately 13.6%, 38.6 million, are estimated to have suffered from drug use disorders. While some addictions, such as drugs and alcohol, are more concerning than others, addiction and compulsive behavior is not limited to these substances. Compulsive consumption of food, shopping, gambling, sex, television, and technology, many of which increased in prevalence during the recent pandemic, can all be destructive when their use cannot be managed and all can potentially be treated by MEAI.
Alcohol Use Disorder
The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria of AUD are as follows:
| ● | A maladaptive pattern of substance use leading to clinically significant impairment or distress, as manifested by 2 or more of the following, occurring at any time in the same 12-month period: |
| ● | Alcohol is often taken in larger amounts or over a longer period than was intended. |
| ● | There is a persistent desire or unsuccessful efforts to cut down or control alcohol use. |
| ● | A great deal of time is spent in activities necessary to obtain alcohol, use alcohol, or recover from its effects. |
| ● | Craving, or a strong desire or urge to use alcohol. |
| ● | Recurrent alcohol use resulting in a failure to fulfill major role obligations at work, school, or home. |
| ● | Continued alcohol use despite having persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of alcohol. |
| ● | Important social, occupational, or recreational activities are given up or reduced because of alcohol use. |
| ● | Recurrent alcohol use in situations in which it is physically hazardous. |
| ● | Alcohol use is continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by alcohol. |
| ● | Tolerance, as defined by either of the following: |
| o | A need for markedly increased amounts of alcohol to achieve intoxication or desired effect. |
| o | A markedly diminished effect with continued use of the same amount of alcohol. |
Excessive alcohol use is costly, as described by the graphic below:
“Excessive Drinking is Draining the U.S. Economy:”
|
 |
According to the CDC, the cost of excessive alcohol uses in the United States reached $249 billion in 2010, which equates to about $2.05 per drink consumed, and $807 per person.
There are limited pharmacological agents available to treat AUD. Below are the main treatments available today for AUD:
Antabuse (disulfiram) was the first medicine approved by the FDA for the treatment of alcohol use and alcohol dependence. It works by causing a severe adverse reaction when someone taking the medication consumes alcohol. Most people who take it will vomit after a drink of alcohol. This, in turn, is thought to create a deterrent to drinking.
Disulfiram was first developed in the 1920s for use in manufacturing processes. The alcohol-aversive effects of Antabuse were first recorded in the 1930s. Workers in the vulcanized rubber industry who were exposed to tetraethylthiuram disulfide became ill after drinking alcohol.
In 1948, Danish researchers trying to find treatments for parasitic stomach infections discovered the alcohol-related effects of disulfiram when they too became ill after drinking alcohol. The researchers began a new set of studies on using disulfiram to treat alcohol dependence.
Shortly thereafter, the FDA approved disulfiram to treat alcoholism. It was first manufactured by Wyeth-Ayerst Laboratories under the brand name Antabuse.
Initially, disulfiram was given in larger dosages to produce aversion conditioning to alcohol by making the patients very sick if they drank. Later, after many reported severe reactions (including some deaths), Antabuse was administered in smaller dosages to support alcohol abstinence.
In addition to disulfiram, other drugs have been used to try to curb alcoholism, such as naltrexone, naloxone and nalmefene.
Naltrexone is sold under the brand names Revia and Depade. An extended-release, monthly injectable form of naltrexone is marketed under the trade name Vivitrol. It works in the brain by blocking the high that people experience when they drink alcohol or take opioids like heroin.
Naltrexone was first developed in 1963 to treat addiction to opioids. In 1984, it was approved by the FDA for the treatment of use of drugs such as heroin, morphine, and oxycodone. At the time, it was marketed by DuPont under the brand name Trexan.
In the 1980s, animal studies discovered that naltrexone also reduced alcohol consumption. Human clinical trials followed in the late 80s and early 90s. These showed that when combined with psychosocial therapy, naltrexone could reduce alcohol cravings and decrease relapse rates in alcoholics.
The FDA approved the use of naltrexone to treat AUD in 1994. DuPont then renamed the drug Revia.
Campral (acamprosate) is the most recent medication approved for the treatment of alcohol dependence or alcoholism in the U.S. It works by normalizing alcohol related changes in the brain, reducing some of the extended physical distress and emotional discomfort people can experience when they quit drinking (also known as post-acute withdrawal syndrome) that can lead to relapse.
In 1982, the French company Laboratoires Meram developed acamprosate for the treatment of alcohol dependence. It was tested for safety and efficacy from 1982 until 1988 when it was authorized for use by the French government to treat alcoholism. It was first marketed under the name Aotal.
For more than 20 years, acamprosate was widely used throughout Europe for treating people with AUD. It was not approved for use in the United States until July 2004. It was first marketed in the United States in January 2005 under the brand name Campral. Campral is currently marketed in the United States by Forest Pharmaceuticals.
These therapies suffer from a number of limitations, including high relapse rates, inconvenient treatment regimens, difficult access and an inability to maintain abstinence after medically assisted withdrawal.
Buprenorphine, methadone and naltrexone are used as maintenance therapy with the primary goal of preventing relapse while naloxone is used as rescue therapy for opioid overdose. Access to treatments such as buprenorphine and methadone is limited by their treatment regimens and inherent risks of abuse, placing significant requirements and regulations on practitioners. In addition to these limitations, current treatment options are not considered to be highly effective; approximately 75% of patients undergoing Opioids Use Disorder, or OUD, therapy experience relapse within one year of treatment. For abuse of other substances, such as cocaine or methamphetamine, no pharmacological agents have been approved for treatment.
Despite the limitations of current treatment options for AUD, we believe that very few psychedelic treatment candidates are being developed to address this problem.
Weight loss, Obesity, and Metabolic Syndrome
The prevalence of obesity has reached epidemic proportions, with approximately one billion people worldwide projected to be affected by 2030, including approximately 1 in 5 women and 1 in 7 men, according to the recently published World Obesity Atlas 2022 by the World Obesity Federation. Obesity is a chronic disease that has been linked to several conditions, including cardiovascular disease (CVD), type 2 diabetes (T2D), and non-alcoholic fatty liver disease (NAFLD). While multiple metabolic factors have been associated with the development of obesity, the underlying molecular mechanisms are not yet fully understood. Despite the gravity of the situation, the lack of effective anti-obesity treatments poses a significant challenge.
Several factors, including eating habits, fast food consumption, personality traits, depression, and genetics, have been implicated in the etiologies of obesity. Recently, the notion of food addiction has gained attention as a potential explanation for the obesity epidemic, with links observed between food addiction and substance-related and eating disorders (most prominently binge-eating). The similarities between food addiction and drug dependence, including an overactive response in the mesolimbic reward circuits, contribute to an inability to control food intake and maintain weight loss, leading to a cycle of binge eating that is difficult to break. This pattern is similar to what is seen in drug addiction, where the activation of these reward circuits contributes to an inability to control drug use. The similarities between food addiction and drug dependence suggest that similar therapeutic approaches may be effective in treating both obesity and addictive disorders. Psychedelics, including lysergic acid diethylamide (LSD), mescaline, psilocybin, and N,N-dimethyltryptamine (DMT), interact with the serotonin receptors, particularly 5-HT2C, 5-HT2A, and 5-HT1A. These receptors are densely located in the prefrontal cortex and mesolimbic dopamine pathways, areas involved in emotion control, reward, and food intake. Previous studies have shown that stimulation of the serotonergic system induces weight reduction and decreases food intake. Moreover, cerebral 5-HT2A binding was significantly and positively correlated with BMI and provided a predictive value for weight loss after gastric bypass surgery. Importantly, psychedelics may offer a unique approach to addressing both addiction and obesity, by disrupting rigid behavioral patterns, promoting cognitive flexibility, and increasing neuronal plasticity.
Other Application Markets
We are currently in the discovery phase of additional innovative molecules, that will potentially address the following markets
Anxiety Disorder Overview
Although anxiety is considered a common aspect of life, anxiety disorders develop when feelings of apprehension and unease persist over an extended period and potentially worsen over time. Anxiety disorders can present with a range of symptoms and may impact personal health as well as both social and professional interactions. Furthermore, it is common for those suffering with an anxiety disorder to also have co-occurring mental health disorders or physical illness, which can compound symptoms and complicate recovery. For example, it is estimated that half of patients diagnosed with depression also suffer from an anxiety disorder.
There are several types of anxiety disorders, including generalized anxiety disorder, or GAD, social anxiety disorder and panic disorder, which are distinct but share common symptoms. In the aggregate, anxiety disorders are considered to be the most common mental illness in the United States, affecting approximately 40 million adults, or 18% of the population.
Anxiety disorders are generally treated with medication, psychotherapy or both. Treatment often involves the use of antidepressants, including SSRIs, such as paroxetine, sertraline and citalopram. However, SSRIs typically have a slow onset of action and have a number of side effects, such as sexual dysfunction, drowsiness and weight gain. Benzodiazepines are also used to treat anxiety and can offer rapid reduction of symptoms, but their long-term use is associated with the development of tolerance, respiratory depression, drug dependence and sedative side effects. Finally, benzodiazepines have been noted to exacerbate the respiratory depression associated with opioids, thus contributing to the mortality associated with OUD.
We are aware of several biopharmaceutical companies with psychedelic therapies in development for anxiety disorders including MAPS, Cybin, Incannex and MindMed.
Post-Traumatic Stress Disorder Background
PTSD is a psychiatric disorder that affects app approximately 6% of the U.S. population. PTSD symptoms include recurring and intrusive negative thoughts, mood and memories, reduced cognitive abilities, hyperarousal, reactivity and avoidance that persist for longer periods than a month after experiencing a traumatic event. Overall reduction in the quality of life is common in individuals with PTSD leading to disability and the further manifestation of other comorbidities such as obesity, hypertension, concomitant mental health conditions and suicidality.
The current first line treatment for PTSD is the use of trauma-focused psychotherapy, but access to these psychotherapies is typically difficult, and not all with PTSD respond to psychotherapy alone. Given the issues with access to trauma-focused psychotherapy and ineffectiveness of current pharmacotherapy, PTSD is a mental health disorder of high unmet medical need. In fact, it is believed that conventional therapies and medications only help, at best, around 50 percent of patients. Therefore, we believe novel interventions are needed to better treat PTSD.
There are currently two SSRIs, Zoloft and Paxil, approved for the treatment of PTSD, and these two drugs are generic. There are several other generic products on the market and many drugs currently under development for anxiety and trauma-related disorders are also being evaluated for PTSD, which we believe reflects the limitations of the available therapies and an urgent need for better treatment.
We are aware of several biopharmaceutical companies with therapies in development for PTSD, including, Otsuka, Bionomics, Corcept Therapeutics, Aptinyx, Azevan Pharmaceuticals, Bionorica, Seelos Therapeutics and Tonix Pharmaceuticals.
Supporters of using psychedelic drugs to help patients lessen their anxiety are consistently increasing as psychedelic drugs are among the most promising treatments for people with conditions like PTSD and severe depression. Multidisciplinary Association for Psychedelic Studies (MAPS) Public Benefit Corp. recently filed an application with FDA to approve the psychedelic drug MDMA in combination with therapy to treat post-traumatic stress disorder. The filing is a milestone in researchers’ quest to move psychedelic drugs from tightly restricted substances into mainstream medical treatments that are widely accessible to patients. It comes after decades of studies have demonstrated the promise of psychedelics to treat mental health disorders.
Depression and TRD
Multiple therapies for depression exist, including common pharmacological treatments such as anti-depressants and psychosocial interventions such as cognitive based therapy. There are also non-pharmacological, somatic treatments for depression such as electroconvulsive therapy and transcranial magnetic stimulation, among others. However, these current therapies are ineffective or inadequately effective for a significant portion of patients. This treatment-resistant subset of depression is one of the targets for our proprietary 2FDCK drug candidate under our development program. For TRD there are currently only two pharmacological treatments approved in the United States: (i) SPRAVATO (S-ketamine) nasal spray, an NMDA receptor antagonist, approved by the FDA in March 2019 and marketed by Janssen Pharmaceutical Companies of Johnson & Johnson, and (ii) a fixed dose combination of olanzapine and fluoxetine hydrochloride, which are individually available generically. These treatments are typically used alongside antidepressants and other treatments used in earlier lines of therapy for depression. Psychosocial interventions and non-pharmacological, somatic treatments may also be used for patients.
Pre-Clinical Studies
So far, only pre-clinical studies have been conducted with MEAI, including In-vitro and In-vivo studies. Some of these studies were conducted by outside research organizations in the United States France, Israel and China
Volcani Center and Hebrew University Study
In one of these studies, conducted by researchers at the Ministry of Agriculture and Rural Development, Volcani Center and Hebrew University, MEAI’s pharmacokinetic (PK) profile was determined in a rat model. The study included 84 male rats that were administered 0-90 mg/kg body weight dosages, and blood samples were collected at 5 minutes, 10 minutes, 20 minutes, 40 minutes, 60 minutes, 1 hour, 2 hour, 4 hour, 7 hour and 24 hour intervals. MEAI displayed extensive total clearance and a very short plasma and brain half-life. The significance of this study is that a high portion of MEAI reached its target organ – the brain, in a rapid manner. This is important as it demonstrated a substantial dosage of MEAI will reach the brain without “going to waste” during the distribution in the body. In addition, this study demonstrated that a dose of 10 mg/kg in the rat was considered to be the NOAEL (No-observed-adverse-effect level) which corresponds to 1.6 mg/kg body weight in humans. Therefore, it was determined that a single oral dosage of 1 mg/kg/day of MEAI in humans, is not expected to result in clinically acute severe adverse effects. The only mortality observed in the study occurred in the 90 mg/kg dosage group, where one rat was found dead after dosing. Gross pathology showed that the animal had urine hemolysis, swollen kidneys and bleeding in the spleen. As it was the only mortality observed it was considered incidental and not related to MEAI.
Kimron Veterinary Institute Study
In another study conducted by the Kimron Veterinary Institute’s Department of Toxicology, a toxicological evaluation was performed where acute and subacute toxicity was evaluated on rats as well as in-vitro cytotoxic and mutagenic effects. The study involved 48 subjects, with 18 rats testing acute toxicity at three different dosages (10 mg/kg, 100 mg/kg and 1,000 mg/kg per day) and 30 rats testing subacute toxicity for five consecutive days at three different dosages (10 mg/kg, 30 mg/kg and 90 mg/kg per day). Firstly, it was determined that acute, single oral administration of MEAI to rats at a dose level of 10 mg/kg was well tolerated. In the 100 mg/kg MEAI group, all rats displayed transient adverse clinical signs that lasted for one day post dosing. Major adverse clinical signs observed: tremor, straub tail, dyspnea, piloerection, hunched back, decreased motor activity and salivation. No gross pathology abnormalities were detected in the groups treated with 10 mg/kg and 100 mg/kg MEAI at the scheduled termination day. In the 1,000 MEAI mg/kg group, all animals died within 30–120 minutes post dosing. Tremors, Straub tail, dyspnea, piloerection, decreased motor activity and convulsions were the major adverse clinical signs observed in these animals before death. Enlarged lungs, whitish spleen and surface white spots on the liver were the only gross pathology abnormalities observed postmortem. Later, it was determined that sub-acute, five-day oral administration of MEAI to rats at dose levels of 10 mg/kg and 30 mg/kg were well tolerated and did not cause any significant adverse clinical effects. In the 90 mg/kg group, all rats displayed transient adverse clinical signs that lasted for five days during dosing. These adverse clinical signs were resolved spontaneously on the next day. It seems that the adverse clinical signs were more severe after the first dosing and less severe after the third dosing, as if the animals developed some kind of tolerance. The major adverse clinical signs observed included: on day 1, tremors, straub tail, dyspnea, piloerection, decreased motor activity, lacrimation, peri nasal staining and salivation; on days 2 and 3, dyspnea, salivation and piloerection; on day 4, salivation and piloerection; on day 5, piloerection and lacrimation in female rats only. These adverse clinical signs were resolved spontaneously on day 6 and no abnormal clinical signs were observed until study termination. MEAI showed no adverse effects at 10 and 30mg/kg/day in rats, corresponding to the human doses of 1.6 mg/kg/day and 4.8 mg/kg/day, respectively.
In-vitro assessments established that cell viability was not affected in both rat brain striatum primary neurons and human primary hepatocytes using up to 100 mg/L MEAI. In addition, the combination of ethanol and MEAI was characterized and revealed that the combination of 6% or 7.5% ethanol with 100 mg/L MEAI displayed no statistically significant increase in cytotoxic effect. In addition, in the absence of metabolic activation MEAI was considered as not mutagenic.
National Institute on Drug Abuse Study
In another study, conducted by a researcher from the National Institute on Drug Abuse, National Institute of Health in 2019 that was published in the scientific journal of Psychopharmacology, MEAI was screened against 29 targets. In this study the investigators assessed, among other things, the interaction of MEAI, with 29 receptor targets and compared it with the known literature about MDMA. The reason for the comparison between MEAI and MDMA was that the authors opined in their study that MEAI and MDMA have similar mechanisms of action, and as such may induce similar effects when ingested. MEAI was found to have moderate affinities for the 5-HT1a and 5-HT2b receptors, and higher affinities for the α2 subtypes. In addition, high potency at the serotonin transporter was determined, having 6-fold lower potency at the norepinephrine transporter and 20-fold lower potency at the dopamine transporter. This suggests that the serotonin receptors family is involved in the mechanism of action of MEAI. Although MEAI was not tested in human clinical studies with respect to its abuse liability, the authors of this paper were of the opinion that that based on the mechanism of action of MEAI, it is likely to have less abuse liability compared to MDMA, meaning it has less tendency to be used in non-medical situations, even sporadically. The authors opinion about MEAI having less abuse liability stemmed from the fact that based on their research, MEAI was shown to release less dopamine and more serotonin when compared to MDMA, and dopamine releases have been shown in drug abuse literature to create more abuse liability than serotonin releases. Based on the findings of the study that MEAI and MDMA produce similar effects, we believe that if approved for therapeutic use by the FDA and comparable regulatory bodies, MEAI will be able to be used for similar forms of pharmaceutical therapy for mental disorders that MDMA is currently being tested in clinical trials for, such as for treating AUD and PTSD. Furthermore, if the author’s assertion that MEAI has less of a tendency to be abused is accepted by the FDA and comparable regulatory bodies, MEAI may be able to offer a more appealing alternative to MDMA for these pharmaceutical treatments.
Limitations of this study include that it was conducted only on 29 targets and not on full panels of all the central nervous system receptors. In addition, only affinity values for each compound at the specific receptors were measured without measuring functional activity, meaning that the study only examined whether MEAI binds to a specific receptor but did not determine receptor activation due to MEAI binding.
Evaluation of the Efficacy of MEAI in Binge Alcohol Consumption in Mice.
An efficacy study mimicking voluntary alcohol consumption was conducted in female C57BL/6 mice to assess the ability of MEAI to alter ethanol intake. The study was completed by Pharmaseed, Israel’s largest good laboratory certified CRO specializing in translational and regenerative studies. The principle of the study is based on intermittent access to 20% alcohol in a two-bottle choice (IA2BC) model in mice: one bottle contains water, and the other bottle contains a 20% ethanol solution. In total, the mice were exposed to 20% ethanol 24 hours a day, three days a week, for seven-eight weeks (amounting to 21-22 alcohol consumption days). Alcohol consumption was measured after each 24-hour period of alcohol exposure. Following five weeks of intermittent access to 20% alcohol, the mice were allocated to nine groups of nine mice according to their alcohol consumption and were then administered MEAI (20 to 100 mg/kg/day) or vehicle for 13 days. Overall the target screening and the efficacy animal model were performed in order to characterize the mechanism of action of MEAI. The data illustrated that MEAI at doses of 40 mg/kg/day (p<0.001) and above produced a marked and statistically significant reduction in alcohol consumption as compared to controls, although the reduction in alcohol consumption did not directly correlate with an increase of MEAI dosage. P-value is a statistical measure of significance used to predict the probability of outcomes being replicable. In the case of this study, a p-value of “p<0.001” means that the aforementioned study, when replicated using MEAI at doses of 40 mg/kg/day, have less than a one in one thousand chance of being incorrect. There was no effect at 20 mg/kg/day.
There were no clinical abnormalities observed in most of the mice during this study. One rat from the 20mg/kg/day group showed Dyspnea, Decrease Motor Activity during day 38 of the study. Another rat from the 60 mg/kg group showed necrotic tail during day 45 of the study. In addition, mortality was observed in two animals from the high dose groups. However, it is important to take into consideration that these animals were treated with MEAI following a prolonged period of alcohol consumption. Supporting evidence to the harmful effect of alcohol consumption can be found in the gross pathology analysis which exhibited macroscopic findings mostly in the liver, kidneys, heart and spleen, of all groups including the control.
Based on the results from the pre-clinical studies described above, we have conducted and continue to conduct our own pre-clinical studies in the United States and China.
In order to unravel additional modes of action by which MEAI may exert its therapeutic activity it was tested both in in-vitro and in-vivo contexts by characterizing its interaction with receptors, and by assessing its efficacy to mitigate additional disorders, respectively. These studies were conducted under the sponsors responsibility and are not part of the IND-enabling studies that will be discussed later.
Eurofins Binding Studies
A few in-vitro studies were conducted to unravel the molecular targets MEAI interacts with. For this, MEAI binding was evaluated using a panel of CNS receptors and enzymes (87 Safety Screen Panel and 168 GPCR Max Screen Panel). Results showed that MEAI’s main inhibition effect was obtained on 5-HT agonist radioligand, reaching a 76.8% inhibition of 5-HT2b receptor (compared to 100% inhibition with DOI), 62.6% inhibition of 5-HT1A receptor (compared to 100% inhibition with 8-OH-DPAT) and 53.6% inhibition of 5-HT2A receptor (compared to 100% inhibition with DOI). To rule out a possible agonist effect of MEAI on the 5-HT2b receptor, an additional study was conducted, in which the effect of MEAI on calcium flux on the 5-HT2b receptor in agonist format was evaluated in a dose response manner (using 10 concentrations of MEAI ranging between 0.0005 to 10µM). Results showed a maximal response of 18% while serotonin induced 100% maximal response. These results indicate that MEAI is not considered an agonist at the 5-HT2b receptor. Collectively, these results are in line with the previous published reports and further support the safety of MEAI.
Hebrew University Study: MEAI’s Effect on binge-eating, Obesity, and its Metabolic Complications
MEAIs effect on high fat diet induced obesity was evaluated in mice model mimicking binge eating disorder. For this purpose, an in vivo study was conducted in three phases: in the first phase, the effective dose of MEAI in producing positive metabolic changes in an acute mice model was determined. In the second phase, the efficacy of MEAI was evaluated in high fat diet induced obesity following 28 daily doses of MEAI at the effective dose. In the third phase MEAIs effect on anhedonia, or the decreased ability to experience pleasure, (represents one of the core symptoms of depression which is often associated with binge-behavior) was assessed by the sucrose preference test (SPT) (a reward-based test used as in indicator of anhedonia).
In the first phase four groups of adult male mice (n=8 mice per group, total of 32 animals) received a single dose of either vehicle or MEAI by oral gavage (either 40, 60 or 100 mg/kg). Drug tolerability, food and water intake, activity respirometric parameters (respiratory quotient, rate of oxygen consumption, rate of carbon dioxide emission, total energy expenditure, fat oxidation, carbohydrate oxidation) were monitored for up to 48 hours, after which animals were euthanized. MEAI was tolerated with no visible changes in behavior at both the 40, and 60 mg/kg, although an increase in activity profile and changes in respirometric parameters, as well as a dose dependent increase in food and water intake were observed. At the 100 mg/kg dose group, death was observed in 2 out of 8 animals at 1-3 hours after drug administration. Furthermore, a significant inhibition of wheel running was observed at this dose, indicative of increased anxiety and animals’ stress conditions. Based on these results the dose of 40 mg/kg was selected as dose for the next phase.
In the second phase mice (total of 20 animals) were fed high fat diet (HFD) to generate diet-induced obesity for a period of 18 weeks. As control, mice (10 animals) were kept at a standard diet (STD) for the same period. The induced obese mice were then daily treated with either MEAI (40 mg/kg) by gavage injection for a period of 28 days or vehicle (saline)(10 animals/group). Due to a technical error in the administration of MEAI, 5 mice died within 24 h of receiving the compound on Day 1 due to the administration of much higher doses than intended. To supplement for the animal loss, 4 mice on a HFD from a parallel batch were added, and the experiment was reinitiated with the following group distribution: STD = 10 animals, HFD-Veh = 8 animals, and HFD-MEAI = 11 animals. At the intended 40 mg/kg/day dose, no animal deaths occurred throughout the 28 days of testing. During the treatment period mice were monitored for body weight. At the end of the treatment period mice were assessed for food and water consumption and body composition changes, respiratory assessments, locomotor activity and wheel running patterns, glucose tolerance (ipGTT test) and insulin sensitivity (ipITT), blood lipid profile and urine chemistry. Animals were euthanized and the kidneys, brain, liver, and fat pads, were removed and weighed, and samples were either snap-frozen or fixed in buffered 4% formalin. Trunk blood was collected for determining the biochemical parameters. Food and water consumptions remained the same in all animals. MEAI significantly reduced the overweight of the obese mice, as well as reduced adiposity associated with obesity. Moreover, MEAI led to normalization of obese induced hyperglycemia, glucose intolerance and hyperinsulinemia as well as insulin sensitivity, indicating positive effects of MEAI on glucose metabolism. MEAI also had a positive impact on liver steatosis in the obesity induced mice, as seen by reduced liver weight and its ratio to body weight. MEAI did not have an impact on liver enzymes ALT and AST but significantly reduced ALP levels, indicating reduction in liver injury. MEAI also exhibited a positive effect on hepatic lipid accumulation, with reduction in liver triglycerides and cholesterol, as well as blood LDL levels, resulting in reduction in HDL to LDL ratio. MEAI was found also to have an impact on respiratory parameters, with elevations in oxygen consumption, and carbon dioxide production, energy expenditure as well as increase in overall fat oxidation rate. MEAI also led to an increase in locomotive activity as well as in wheel running parameters. Overall, the data provided evidence of the anti-obesity effect of MEAI treatment at a dose of 40 mg/kg.
In the third phase mice (total of 16 animals) were acclimated to the presence of two water bottles in their home cage for 48 h. The water bottles were weighed to obtain a baseline intake. On the test day (Day 1), 2 h prior to the onset of the dark phase, bottles containing fresh water and a 1.5% sucrose solution were added to the home cages, and mice were subsequently treated orally with MEAI (40 mg/kg, N = 8) or isotonic saline (N = 8). Mice were free to consume liquid from either bottle for 24 h, after which the bottles were weighed to measure consumption. The study was repeated for an additional day (Day 2), with the bottles reversed in position (in the cage) to account for side preference. Sucrose and water intake over the study days were averaged. The sucrose preference index was calculated as the average consumed sucrose across the 2-day period divided by the average volume of total consumed liquid (average water plus average sucrose solution). Results indicate that MEAI at 40 mg/kg significantly reduced the preference of mice to the sucrose solution, an effect that was most pronounced during the first 24 hours but appears to also be reduced in the following 24 hours.
In the fourth phase, conducted under the cooperation agreement with SciSparc, we examined the combined treatment of MEAI, and Palmitoylethanolamide (PEA), with the goal to identify the optimal dosage for this combination and to observe its safety and impact on various metabolic and behavioral parameters, including fat oxidation, locomotor activity, and feeding behavior.
Fourteen different treatment groups were created (for a total of 84 animals) receiving single treatment doses ranging from 40, 20, 10, 5, 1, to 0.5 mg/kg of MEAI with or without a constant PEA dose of 25 mg/kg. MEAI administration exhibited a remarkable degree of tolerance, leaving the animals’ viability unaffected across all experimental groups. Similar results were also observed in groups treated by the combination of MEAI and PEA with the most prominent effects being observed when combining MEAI and PEA, particularly at 20 and 10 mg/kg. Results indicated that:
| ● | The administered treatment exhibited a remarkable degree of tolerance, leaving the mices’ viability unaffected across all experimental groups. |
| ● | Combining MEAI and PEA, particularly at 20 and 10 mg/kg, led to increased oxygen consumption and carbon dioxide emission, coupled with elevated energy expenditure and fat oxidation. Oxygen consumption and carbon dioxide emission indicates increase in metabolic process and fat burn. |
| ● | A striking reduction in food consumption (appetite) and meal sizes was also observed, primarily at 40 and 20 mg/kg of MEAI. |
| ● | Slight elevations in carbohydrate oxidation were noted, particularly at 20 and 10 mg/kg. |
| ● | At 40 and 20 mg/kg significant reductions in ambulation was noted, without affecting voluntary activity. |
Collectively, these data provide strong evidence for the metabolism modulating and anti-obesity effects of MEAI treatment, warranting further preclinical testing.
Bar-Ilan University Study: Elucidation of MEAIs anti-reward/addictive-like properties
The purpose of this study was to determine MEAI’s non-rewarding effect on rats and to test whether MEAI is an effective treatment for cocaine preference in rats utilizing the Conditioned Place Preference (CPP) model. Additionally, the study goal was to test the effect of MEAI on substance-use-disorder (SUD) rat model using the cocaine self-administration paradigm. Sprague Dawley rats were maintained on a 12 h light/12 h dark reversed cycle, with food and water available ad libitum. Experiments were conducted during the dark cycle.
Elucidation of MEAI’s Non-Rewarding Effect on Rats (Experiment 1):
MEAI’s effect on reward was tested using doses ranging from 2.5 to 20 mg/kg. The selection of the high dose was based on a previous pharmacokinetic study (Volcani Center and Hebrew University Study). The high dose of 20 mg/kg showed low duration in the non-preferred place (sec) which indicates low reward. However, due to unexpected observed adverse clinical signs that occurred at this dose (low body posture, aggressiveness, diarrhea, tremors, and piloerection), a range of lower doses was tested. The second dose tested was 10 mg/kg. A higher duration in the non-preferred place was observed with this dose which indicates that there was a rewarding effect, yet this was still significantly less than cocaine. Therefore, two additional lower doses were tested, 2.5 and 5 mg/kg. At these two doses, the duration in the non-preferred place was negative showing no-reward effect. Overall, the dose that displayed the most non-rewarding effect was 5 mg/kg, therefore, this dose was selected to be tested for efficacy as a treatment for cocaine preference in rats in the following experiments.
Determination of MEAI as an Effective Treatment for Cocaine Preference in Rats (Experiment 2):
In the second part of this experiment, MEAI’s effectiveness as a treatment for cocaine preference was tested in rats. Based on the previous results, a dose of 5 mg/kg MEAI was selected as the treatment dose, since it had a satisfying non-reward effect. Twelve rats were conditioned with cocaine (15 mg/kg i.p.) and on the day of the test were injected with MEAI (5 mg/kg i.p.), followed by a 10-minute wait in their home cage. The rats were then placed into the middle compartment of the arena for the test. The results were mixed with some animals showing a preference to the non-preferred compartment (negative values) and some animals showing a preference for the preferred compartment (positive values). It was decided to use a K-cluster test to evaluate the data; this test is a method that enables separation of two different sub-groups with statistical significance in a non-biased manner with high integrity. The two groups were divided as values below 100 seconds in a non-preferred compartment (MEAI A) and above 100 seconds (MEAI B). Based on the findings, a higher dose of MEAI (10 mg/kg i.p.) was tested to see if a more effective treatment response could be obtained but eventually it was revealed that 5 mg/kg MEAI gave better results than 10 mg/kg MEAI in decreasing reward associated with cocaine.
The Effect of MEAI on the Substance-Use-Disorder (SUD) Rat Model using Cocaine as the Substance of Abuse (Experiment 3): The raw data from the Self-Administration experiment show that MEAI did not significantly affect cocaine craving. Once again, applying the K-Cluster method, it was observed that rats clustered into two groups: one that decreased craving (MEAI A; 60% of total responders) and one that increased it (MEAI B; 40% of total responders). This pattern of results was similar to those from the previous experiment in which the K-Cluster method was used to partition the MEAI (5 mg/kg) values into two distinct groups.
In conclusion, experiment 1 concluded that there was no intrinsic reward effect from MEAI administration at a dose of 5 mg/kg. This is an important step when looking for a new therapy for drug addiction. According to the results in experiment 2, it was concluded that MEAI at 5 mg/kg had a potential non-reward effect as it decreased the duration in a non-preferred compartment in about 40% of the rats. From the results of experiments 1 and 2, it was postulated that MEAI is a safe compound as MEAI seems to not be rewarding. Hence it is not likely that MEAI will express addiction-like properties. Moreover, its ability to abolish cocaine induced CPP was demonstrated. The data showed a clear separation (all vs no effect) that may result from the suggestive-like nature of MEAI. Finally, the results from experiment 3 showed a significant attenuation in craving during extinction in the MEAI- A subgroup whereas the counterpart group, MEAI- B, showed the opposite apparent effect that may interpreted as an exacerbation of caving.
IND – Enabling Studies
We are developing CMND-100, which consists of gelatin capsules each containing the active ingredient 5-methoxy-2-aminoindane monohydrochloride (MEAI), a new chemical entity. MEAI is being developed for AUD, specifically for the management of selected chronic alcohol patients who want to reduce their alcohol consumption and/or remain in a state of enforced sobriety. The initial clinical trial will be a Phase 1/2 randomized double blind placebo controlled, in healthy and AUD subjects evaluating the tolerability, safety and preliminary efficacy and pharmacokinetics/pharmacodynamics of single and repeated doses of CMND-100. To evaluate potential toxicity risks prior to human studies and to estimate starting doses for clinical trials, we have completed a series of pre-clinical, IND-enabling studies in the United States and China as required by the FDA for an investigational new drug designation before we can study our compound for the first time in humans. All studies were conducted by US based StageBio, a leading provider of GLP-compliant necropsy, histology, pathology, and specimen archiving services for the biopharmaceutical and research industry, and China based Wuxi AppTech, a certified, CRO that specializes in non-clinical drug development, and were organized by the following topics: safety pharmacology studies, pharmacokinetic studies, and toxicological studies. Pharmacokinetic (PK) and toxicokinetic (TK) of MEAI have been established in vitro and in the non-clinical species (mice, rats and/or dogs) used for assessing safety and efficacy. MEAI was found to have relatively rapid absorption and moderate to rapid elimination with no accumulation and no gender differences. The distribution of MEAI in tissues was evaluated in a binding study in vitro and in an in vivo study in mice. Collectively, this data provide a clear picture of the kinetic profile of MEAI in nonclinical species. Several in vitro studies were also conducted to investigate the metabolism of MEAI. Furthermore, drug interaction was studied in a series of in vitro assays. The toxicological effects of MEAI were studied by Clearmind in rats and dogs and included both single and repeat dose toxicity studies, genotoxicity, and special toxicity studies. Overall, a consistent toxicological profile was noted in the nonclinical species with the primary finding being adverse, transient, central nervous system (CNS)-related clinical signs. The type and severity of clinical signs increased in both rats and dogs in a dose-dependent manner. In the pivotal studies, no-observed-adverse-effect-level (NOAEL) doses were determined for both species. Genotoxicity studies conducted indicated that MEAI does not represent a mutagenic or clastogenic risk to humans. Based on these studies, we are planning to conduct a Phase I/II single and multiple dose tolerability, safety, and pharmacokinetic clinical study of CMND-100 in healthy volunteers and AUD subjects.
Competition
The pharmaceutical industry is intensely competitive and subject to rapid and significant technological change. Our potential competitors include large and experienced companies that have significant competitive advantages over us, such as greater financial, research and development, manufacturing, personnel and marketing resources, greater brand recognition, and more experience and expertise in obtaining marketing approvals from the FDA and foreign regulatory authorities. These companies may develop new drugs to treat the indications that we target or seek to have existing drugs approved for use in the indications that we target.
These potential competitors may therefore introduce competing products without our prior knowledge and without our ability to take preemptive measures in anticipation of their commercial launch. Competition may increase further as a result of advances in the commercial applicability of technologies and greater availability of capital for investment in this industry. Our competitors may succeed in developing, acquiring or licensing on an exclusive basis, products that are more effective, easier to administer or less costly than our product candidates.is highly competitive, with new approaches and technologies regularly emerging. We expect to face competition across our current programs and with any future programs we may seek to develop and/or commercialize from major pharmaceutical, biotechnology, specialty pharmaceutical and generic pharmaceutical companies among others. Potential competitors also include academic institutions, governmental agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
Psychedelic Therapy Competitive Landscape for AUD
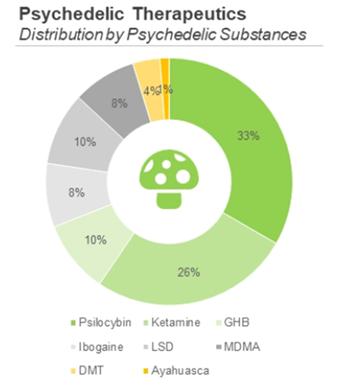
Other than us, more than 45 players from across the world presently claim to be engaged in the development and evaluation of therapeutic candidates based on a number of psychedelic compounds.
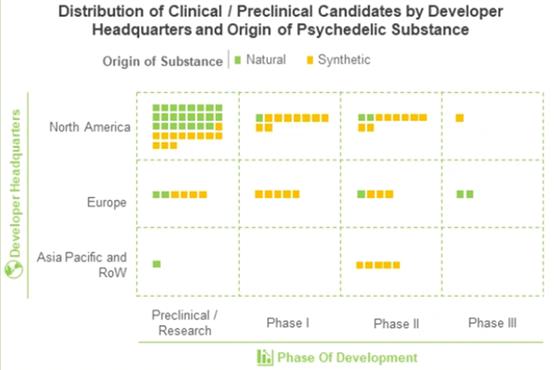
In the mental illnesses segments, we will face competition from psychedelic companies in general, and also from psychedelic companies that are focusing on alcohol abuse, like Awakn Life Sciences, B.More Inc. and Journey Colab Corp.
Intellectual Property
Our success depends in large part on our ability to obtain and maintain protection of intellectual property, particularly patents, in the United States and other countries with respect to product candidates and technology that are important to our business. In addition to patent protection, we also may rely on trade secrets to protect aspects of our business for which we do not consider patent protection appropriate.
The Company’s intellectual portfolio currently consists of fifteen patent families. One, “Binge Behavior Regulators,” has been granted in the U.S., Europe, China and India, with a pending divisional applications in Europe and granted continuing applications in the United States and China. Another, “Alcohol Beverage Substitute,” has been approved for a United States, European and Indian patent, with pending applications in China. As of January 25, 2024, our portfolio consists of fifteen utility patent families, including patents and applications having method of use and composition of matter claims. Thirteen of the patent families are solely owned by the Company, one is jointly owned with BIRAD, the R&D company of Bar-Ilan University and one is jointly owned with Yissum, the Research Development Company of the Hebrew University of Jerusalem. The fifteen patent families include twenty-seven granted patents and twenty-four pending applications:
| ● | Alcoholic Beverage Substitute: 9 patents, 3 pending applications. The 20 year term for the “Alcoholic Beverage Substitutes” family ends on December 9, 2035 |
| ● | Binge Behavior Regulators: 18 patents, 6 pending applications. The “Binge Behavior Regulators” family has a 20-year term that ends December, 9, 2035 |
| ● | Benzofurans as Fail Safes for MDMA Therapy: 1 pending application |
| ● | 2-FDCK for Treatment of Depression, Including Treatment-Resistant Depression: 1 pending application |
| ● | Compositions comprising MEAI and n-acylethanolamines and uses thereof: 1 pending application |
| ● | MEAI for the treatment of Cocaine Addiction: 1 pending application |
| ● | MEAI for the treatment of Metabolic Syndrome: 1 pending application |
| ● | Compositions comprising 3MMC and n-acylethanolamines for the treatment of dyskinesia: 1 pending application |
| ● | Compositions comprising MDMA and n-acylethanolamines uses thereof: 1 pending application |
| ● | Combinations of LSD and n-acylethanolamines: 1 pending application |
| ● | Compositions comprising of ketamine and n-acylethanolamines: 1 pending application |
| ● | Compositions comprising of ibogaine and n-acylethanolamines: 1 pending application |
| ● | Compositions comprising of psilocybin and n-acylethanolamines: 1 pending application |
| ● | Compositions comprising of DMT and n-acylethanolamines: 1 pending application |
| ● | MEAI for the treatment of depression: 1 pending application |
The Company intends to seek additional patents for its compounds whenever warranted and will remain opportunistic regarding the acquisition of additional intellectual property to build its portfolio.
The Alcoholic Beverage Substitute patent family discloses a large genus of compounds suitable for use in alcoholic beverage substitutes comprising a derivative of 2-aminoindan having the characteristics of being intoxicating in a way like alcohol, being physiologically benign (non-harmful), and being devoid of interactions with other drugs. A disclosed species of the genus is MEAI.
The Binge Behavior Regulators patent family discloses compositions comprising a derivative of 2-aminoindan, as described herein, useful as binge regulators or binge mitigation agents that impart a feeling of satisfaction, satiety or contentedness and thereby regulate (e.g., discourage) binge behavior such as binge drinking.
The Benzofurans as Fail Safes for MDMA Therapy patent family is a PCT International Application filed October 6, 2022. The invention disclosed therein is a method for treating subjects who are resistant to MDMA-assisted psychotherapy, or those for whom MDMA has become ineffective, undesirable, or inappropriate.
The 2-FDCK for Treatment of Depression, Including Treatment-Resistant Depression patent family is a PCT International Application filed October 18, 2022. The inventions disclosed therein is directed to methods of administering 2-fluorodeschloroketamine to treat depression, including depression in patients who have developed resistance to previously used treatment therapies, such as ketamine.
Compositions comprising MEAI and n-acylethanolamines and uses thereof patent family is a PCT International Application filed on March 6, 2023, as part of our cooperation agreement with SciSparc. The invention disclosed therein is directed to the compositions and methods for potentiating therapeutic effects and/or reducing side-effects of MEAI. The present invention provides pharmaceutical compositions comprising MEAI and N-acylethanolamines, for example, Palmitoylethanolamide, or PEA, and methods for their use in a variety of indications amenable to treatment with MEAI.
MEAI for the Treatment of Cocaine Addiction patent family is a PCT International Application filed Jointly with BIRAD on May 31, 2023, and as part of our cooperation agreement with SciSparc. The invention disclosed therein is directed to the methods for treating cocaine addiction by administering a therapeutically effective amount of MEAI and the methods of treatment comprise administering MEAI in combination with N-acylethanolamines, for example, PEA.
MEAI for the Treatment of Metabolic Syndrome patent family is a PCT International Application filed jointly with Yissum, on September 20, 2023. The invention disclosed therein is directed to the methods for treating metabolic syndrome by administering a therapeutically effective amount of MEAI and the methods of treatment comprise administering MEAI in combination with N-acylethanolamines, for example, PEA.
Compositions comprising 3MMC and n-acylethanolamines and methods for treating dyskinesias such as tardive dyskinesia is a patent family application filed on July 27, 2023, as part of our cooperation agreement with SciSparc. The invention disclosed therein is directed to the compositions and methods for potentiating therapeutic effects and/or reducing side-effects of 3MMC. The present invention provides pharmaceutical compositions comprising 3MMC and N-acylethanolamines, for example, Palmitoylethanolamide, or PEA, and methods for their use in treating dyskinesias such as tardive dyskinesia.
Compositions comprising 3,4-METHYLENEDIOXYMETHAMPHETAMINE and n-acylethanolamines and uses thereof is a patent family application filed on February 15, 2023, as part of our cooperation agreement with SciSparc. The invention disclosed therein is directed to the compositions and methods for potentiating therapeutic effects and/or reducing side-effects of 3,4-METHYLENEDIOXYMETHAMPHETAMINE. The present disclosure relates to compositions and methods for potentiating therapeutic effects and/or reducing side-effects of 3,4-methylenedioxymethamphetamine (MDMA). The present disclosure provides pharmaceutical compositions or combinations comprising MDMA and N-acylethanolamines, for example, Palmitoylethanolamide (“PEA”) and methods for their use in a variety of indications amenable to treatment, including, but not limited to post-traumatic stress disorder (PTSD), anxiety, and eating disorders.
Compositions comprising LYSERGIC ACID DIETHYLAMIDE and N-acylethanolamines and uses thereof is a patent family application filed on February 10, 2023, as part of our cooperation agreement with SciSparc. The invention disclosed therein is directed to the compositions and methods for potentiating therapeutic effects and/or reducing side-effects of lysergic acid diethylamide (LSD). The patent provides pharmaceutical compositions or combinations comprising LSD and N-acylethanolamines, and methods for their use in a variety of indications amenable to treatment, including, but not limited to depression, anxiety, headaches, alcoholism, and pain.
Compositions comprising KETAMINE and N-acylethanolamines and uses thereof is a patent family application filed on February 15, 2023, as part of our cooperation agreement with SciSparc. The patent application relates to compositions and methods for potentiating therapeutic effects and/or reducing side-effects of ketamine. The patent application disclosure provides pharmaceutical compositions and combinations comprising ketamine and N-acylethanolamines, for example, Palmitoylethanolamide (“PEA”) and methods for their use in a variety of indications amenable to treatment, including, but not limited to depression, suicidal ideation, bipolar disorder, seizures, and pain.
Compositions comprising IBOGAINE and N-acylethanolamines and uses thereof is a patent family application filed on February 15, 2023, as part of our cooperation agreement with SciSparc. The patent application relates to compositions and methods for potentiating therapeutic effects and/or reducing side-effects of ibogaine. The patent application disclosure provides pharmaceutical compositions and combinations comprising ibogaine and N-acylethanolamines for example, Palmitoylethanolamide (“PEA”) and methods for their use in a variety of indications amenable to treatment, including, but not limited to substance addiction and opioid dependence.
Compositions comprising PSILOCYBIN and N-acylethanolamines and uses thereof is a patent family application filed on February 10, 2023, as part of our cooperation agreement with SciSparc. The patent application relates to compositions and methods for potentiating therapeutic effects and/or reducing side-effects of ibogaine. The patent application provides pharmaceutical compositions and combinations comprising psilocybin and N-acylethanolamines, for example, Palmitoylethanolamide (“PEA”) and methods for their use in a variety of indications amenable to treatment, including, but not limited to substance addiction and dependence.
Compositions comprising N,N-DIMETHYLTRYPTAMINE and N-acylethanolamines and uses thereof is a patent family application filed on February 10, 2023, as part of our cooperation agreement with SciSparc. The patent application relates to compositions and methods for potentiating therapeutic effects and/or reducing side-effects of N,Ndimethyltryptamine. The patent application provides pharmaceutical compositions and combinations comprising N,N-dimethyltryptamine and N-acylethanolamines for example, Palmitoylethanolamide (PEA) and methods for their use in a variety of indications amenable to treatment with N,Ndimethyltryptamine, including, but not limited to cerebral ischemia and hypoxia.
Compositions comprising 5-METHOXY-2-AMINOINDAN for treatment of depression is a patent family application filed on March 29, 2023. The patent application relates to compositions and methods for treatment of depression. The patent application disclosure provides pharmaceutical compositions comprising 5-methoxy-2-aminoindan (“MEAI”) and methods for their use in treatment of depression.
With respect to the aforementioned provisional patent applications, we intend to decide whether to file non-provisional patent applications at some time before each application lapses (for each, within one year of their filing date).
We intend to seek additional patents for its compounds whenever warranted and will remain opportunistic regarding the acquisition of additional intellectual property to build its portfolio.
Medigus Product Development Agreement
On February 8, 2022, we entered into a subscription agreement with Medigus Ltd., or Medigus, for a private placement pursuant to which we raised approximately $1.25 million, or the Medigus Agreement. On June 29, 2022, we entered into an amendment to the Medigus Agreement, or the Medigus Amendment. In addition, and pursuant to the Medigus Agreement, we are exploring the possibility of entering into a joint venture with Medigus for the purpose of developing our proprietary MEAI product candidate as a novel food product. A “novel food product” is a product that has never been used as food or that does not have a significant history of consumption or is produced by a method that has not previously been used for food. If we pursue the joint venture, it is contemplated that Medigus will assist us in obtaining regulatory approvals to enable us to sell our products in certain targeted countries in Eastern Europe and the Pacific region of Asia. We have not entered into any binding agreements with Medigus with respect to the joint venture company. However, it is contemplated that all intellectual property conceived or created under the joint venture will remain the property of the joint venture company. In addition, it is contemplated that Medigus will be entitled to 10% of the initial equity under the joint venture, but in the event that additional investors join the joint venture, their equity stake and ours could be diluted. Due to the fact that no binding agreements are in place with Medigus, we cannot be sure that the joint venture will proceed as currently contemplated, or at all. In addition, we cannot be certain that the laws in any of the jurisdictions where our joint venture with Medigus may pursue commercial sales of MEAI will allow the sale of MEAI as a novel food product.
Government Regulation
The FDA, the EMA, U.S. Department of Health and Human Services Office of Inspector General, CMS, DEA, and comparable regulatory authorities in state and local jurisdictions and in other countries impose substantial and burdensome requirements upon companies involved in the clinical development, manufacture, marketing and distribution of drugs such as those we are developing. These agencies and other federal, state and local entities regulate, among other things, the research and development, testing, manufacture, quality control, safety, effectiveness, labeling, storage, record keeping, approval, advertising and promotion, distribution, post-approval monitoring and reporting, sampling and export and import of our product candidates. Any drug candidates that we develop must be approved by the FDA before they may be legally marketed in the United States and by the appropriate foreign regulatory agency before they may be legally marketed in those foreign countries. Generally, our activities in other countries will be subject to regulation that is similar in nature and scope as that imposed in the United States, although there can be important differences. Additionally, some significant aspects of regulation in the European Union are addressed in a centralized way, but country-specific regulation remains essential in many respects.
U.S. Drug Development Process
In the United States, the FDA regulates drugs under FDCA and its implementing regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval may subject an applicant to administrative or judicial sanctions. These sanctions could include the FDA’s refusal to approve pending applications, withdrawal of an approval, a clinical hold, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties.
The process required by the FDA before a drug may be marketed in the United States generally involves the following:
| ● | completion of preclinical laboratory tests, animal studies and formulation studies in accordance with FDA’s good laboratory practice requirements and other applicable regulations; |
| ● | submission to the FDA of an IND protocol which must become effective before human clinical trials may begin; |
| ● | approval by an IRB or ethics committee at each clinical site before each trial may be initiated; |
| ● | performance of adequate and well-controlled human clinical trials in accordance with GCP requirements to establish the safety and efficacy of the proposed drug for its intended use; |
| ● | submission to the FDA of an NDA after completion of all pivotal trials; |
| ● | payment of user fees for the FDA review of the NDA; |
| ● | a determination by the FDA within 60 days of its receipt of an NDA to file the NDA for review; |
| ● | satisfactory completion of an FDA advisory committee review, if applicable; |
| ● | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the drug is produced to assess compliance with cGMP requirements to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity, and of selected clinical investigation sites to assess compliance with GCPs; |
| ● | potential FDA audit of the preclinical and/or clinical trial sites that generated the data in support of the NDA, and |
| ● | FDA review and approval of the NDA to permit commercial marketing of the product for particular indications for use in the United States. |
Prior to beginning the first clinical trial with a product candidate in the United States, we must submit an IND to the FDA. An IND is a request for authorization from the FDA to administer an investigational new drug product to humans. The central focus of an IND submission is on the general investigational plan and the protocol(s) for clinical studies. Some preclinical testing may continue even after the IND is submitted. The IND also includes results of animal and in vitro studies assessing the toxicology, pharmacokinetics, pharmacology, and pharmacodynamic characteristics of the product; chemistry, manufacturing, and controls information; and any available human data or literature to support the use of the investigational product. An IND must become effective before human clinical trials may begin. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises safety concerns or questions about the proposed clinical trial. In such a case, the IND may be placed on clinical hold and the IND sponsor and the FDA must resolve any outstanding concerns or questions before the clinical trial can begin. Submission of an IND therefore may or may not result in FDA authorization to begin a clinical trial.
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators in accordance with GCPs, which include the requirement that all research subjects provide their informed consent for their participation in any clinical study. Clinical trials are conducted under protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. A separate submission to the existing IND must be made for each successive clinical trial conducted during product development and for any subsequent protocol amendments. Furthermore, an independent IRB for each site proposing to conduct the clinical trial must review and approve the plan for any clinical trial and its informed consent form before the clinical trial begins at that site and must monitor the study until completed. An IRB is charged with protecting the welfare and rights of trial participants and considers such items as whether the risks to individuals participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the informed consent form that must be provided to each clinical trial subject or his or her legal representative and must monitor the clinical trial until completed. Regulatory authorities, the IRB or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects are being exposed to an unacceptable health risk or that the trial is unlikely to meet its stated objectives. Some studies also include oversight by an independent group of qualified experts organized by the clinical study sponsor, known as a data safety monitoring board, which provides authorization for whether or not a study may move forward at designated check points based on access to certain data from the study and may halt the clinical trial if it determines that there is an unacceptable safety risk for subjects or other grounds, such as no demonstration of efficacy. There are also requirements governing the reporting of ongoing clinical studies and clinical study results to public registries.
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
| ● | Phase 1: The product candidate is initially introduced into healthy human subjects or patients with the target disease or condition. These studies are designed to test the safety, dosage tolerance, absorption, metabolism and distribution of the investigational product in humans, the side effects associated with increasing doses, and, if possible, to gain early evidence on effectiveness. In the case of some products for severe or life-threatening diseases, such as cancer, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients. |
| ● | Phase 2: The product candidate is administered to a limited patient population with a specified disease or condition to evaluate the preliminary efficacy, optimal dosages, dose tolerance and dosing schedule and to identify possible adverse side effects and safety risks. Multiple Phase 2 clinical trials may be conducted to obtain information prior to beginning larger and more expensive Phase 3 clinical trials. |
| ● | Phase 3: The product candidate is administered to an expanded patient population to further evaluate dosage, to provide statistically significant evidence of clinical efficacy and to further test for safety, generally at multiple geographically dispersed clinical trial sites. These clinical trials are intended to establish the overall risk/benefit ratio of the investigational product and to provide an adequate basis for product approval. Generally, two adequate and well-controlled Phase 3 clinical trials are required by the FDA for approval of an NDA. |
Post-approval trials, sometimes referred to as Phase 4 studies, may be conducted after initial marketing approval. These trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication. In certain instances, such as with accelerated approval drugs, the FDA may mandate the performance of Phase 4 trials. In certain instances, the FDA may mandate the performance of Phase 4 clinical trials as a condition of approval of an NDA.
The FDA or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients.
During the development of a new drug, sponsors are given opportunities to meet with the FDA at certain points. These points may be prior to submission of an IND, at the end of Phase 2, and before an NDA is submitted. Meetings at other times may be requested. These meetings can provide an opportunity for the sponsor to share information about the data gathered to date, for the FDA to provide advice, and for the sponsor and the FDA to reach agreement on the next phase of development. Sponsors typically use the meetings at the end of the Phase 2 trial to discuss Phase 2 clinical results and present plans for the pivotal Phase 3 clinical trials that they believe will support approval of the new drug.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final drug. In addition, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
While the IND is active and before approval, progress reports summarizing the results of the clinical trials and nonclinical studies performed since the last progress report must be submitted at least annually to the FDA, and written IND safety reports must be submitted to the FDA and investigators for serious and unexpected suspected adverse events, findings from other studies suggesting a significant risk to humans exposed to the same or similar drugs, findings from animal or in vitro testing suggesting a significant risk to humans, and any clinically important increased incidence of a serious suspected adverse reaction compared to that listed in the protocol or investigator brochure.
U.S. Review and Approval Process
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, the results of product development, preclinical and other non-clinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug, proposed labeling and other relevant information are submitted to the FDA as part of an NDA requesting approval to market the product. Data may come from company-sponsored clinical trials intended to test the safety and effectiveness of a use of a product, or from a number of alternative sources, including studies initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and effectiveness of the investigational drug product to the satisfaction of the FDA. The submission of an NDA is subject to the payment of substantial user fees; a waiver of such fees may be obtained under certain limited circumstances. Additionally, no user fees are assessed on NDAs for products designated as orphan drugs, unless the product also includes a non-orphan indication.
The FDA reviews an NDA to determine, among other things, whether a product is safe and effective for its intended use and whether its manufacturing is cGMP-compliant to assure and preserve the product’s identity, strength, quality and purity. Under the Prescription Drug User Fee Act, or PDUFA, guidelines that are currently in effect, the FDA has a goal of ten months from the date of “filing” of a standard NDA for a new molecular entity to review and act on the submission. This review typically takes twelve months from the date the NDA is submitted to FDA because the FDA has approximately two months to make a “filing” decision after it the application is submitted. The FDA conducts a preliminary review of all NDAs within the first 60 days after submission, before accepting them for filing, to determine whether they are sufficiently complete to permit substantive review. The FDA may request additional information rather than accept an NDA for filing. In this event, the NDA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing.
The FDA may refer an application for a novel drug to an advisory committee. An advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving an NDA, the FDA will typically inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCPs. If the FDA determines that the application, manufacturing process or manufacturing facilities are not acceptable, it will outline the deficiencies in the submission and often will request additional testing or information. Notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
After the FDA evaluates an NDA, it will issue an approval letter or a Complete Response Letter. An approval letter authorizes commercial marketing of the drug with prescribing information for specific indications. A Complete Response Letter indicates that the review cycle of the application is complete, and the application will not be approved in its present form. A Complete Response Letter usually describes the specific deficiencies in the NDA identified by the FDA and may require additional clinical data, such as an additional pivotal Phase 3 trial or other significant and time-consuming requirements related to clinical trials, nonclinical studies or manufacturing. If a Complete Response Letter is issued, the sponsor must resubmit the NDA, addressing all of the deficiencies identified in the letter, or withdraw the application. Even if such data and information are submitted, the FDA may decide that the NDA does not satisfy the criteria for approval.
If regulatory approval of a product is granted, such approval will be granted for particular indications and may contain limitations on the indicated uses for which such product may be marketed. For example, the FDA may approve the NDA with a Risk Evaluation and Mitigation Strategy, or REMS, to ensure the benefits of the product outweigh its risks. A REMS is a safety strategy to manage a known or potential serious risk associated with a medicine and to enable patients to have continued access to such medicines by managing their safe use, and could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries, and other risk minimization tools. The FDA also may condition approval on, among other things, changes to proposed labeling or the development of adequate controls and specifications. Once approved, the FDA may withdraw the product approval if compliance with pre- and post-marketing requirements is not maintained or if problems occur after the product reaches the marketplace. The FDA may also require one or more Phase 4 post-market studies and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization and may limit further marketing of the product based on the results of these post-marketing studies. In addition, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could impact the timeline for regulatory approval or otherwise impact ongoing development programs.
Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug intended to treat a rare disease or condition, which is a disease or condition that affects fewer than 200,000 individuals in the United States or, if it affects more than 200,000 individuals in the United States, there is no reasonable expectation that the cost of developing and making a drug product available in the United States for this type of disease or condition will be recovered from sales of the product. Orphan designation must be requested before submitting an NDA. After the FDA grants orphan designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same drug or biological product for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity or inability to manufacture the product in sufficient quantities. The designation of such drug also entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages and user-fee waivers. Competitors, however, may receive approval of different products for the indication for which the orphan product has exclusivity or obtain approval for the same product but for a different indication for which the orphan product has exclusivity. Orphan exclusivity also could block the approval of one of our products for seven years if a competitor obtains approval of the same drug as defined by the FDA or if our product candidate is determined to be contained within the competitor’s product for the same indication or disease. If an orphan designated product receives marketing approval for an indication broader than what is designated, it may not be entitled to orphan exclusivity. In addition, exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition.
Expedited Development and Review Programs
The FDA has a number of programs intended to expedite the development or review of products that meet certain criteria. For example, new drugs are eligible for Fast Track designation if they are intended to treat a serious or life-threatening disease or condition and demonstrate the potential to address unmet medical needs for the disease or condition. Fast track designation applies to the combination of the product and the specific indication for which it is being studied. The sponsor of a Fast Track-designated product has opportunities for more frequent interactions with the review team during product development, and the FDA may consider for review sections of the NDA on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the NDA, the FDA agrees to accept sections of the NDA and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the NDA.
Any product submitted to the FDA for approval, including a product with a Fast Track designation, may also be eligible for other types of FDA programs intended to expedite development and review, such as priority review and accelerated approval. A product is eligible for priority review if it has the potential to provide safe and effective therapy where no satisfactory alternative therapy exists or a significant improvement in the treatment, diagnosis or prevention of a disease compared to marketed products. The FDA will attempt to direct additional resources to the evaluation of an application for a new drug designated for priority review in an effort to facilitate the review. The FDA endeavors to review applications with priority review designations within six months of the filing date as compared to ten months for review of new molecular entity NDAs under its current PDUFA review goals.
In addition, a product may be eligible for accelerated approval. Drug products intended to treat serious or life-threatening diseases or conditions may be eligible for accelerated approval upon a determination that the product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. As a condition of approval, the FDA may require that a sponsor of a drug receiving accelerated approval perform adequate and well-controlled post-marketing clinical trials. In addition, the FDA currently requires pre-approval of promotional materials as a condition for accelerated approval, which could adversely impact the timing of the commercial launch of the product.
In addition, the Food and Drug Administration Safety and Innovation Act established a category of drugs referred to as “breakthrough therapies” that may be eligible to receive breakthrough therapy designation. A sponsor may seek FDA designation of a product candidate as a “breakthrough therapy” if the product is intended, alone or in combination with one or more other products, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The designation includes all of the Fast Track program features, as well as more intensive FDA interaction and guidance. The breakthrough therapy designation is a distinct status from both accelerated approval and priority review, which can also be granted to the same drug if relevant criteria are met. If a product is designated as breakthrough therapy, the FDA will work to expedite the development and review of such drug.
Fast Track designation, breakthrough therapy designation, priority review, and accelerated approval do not change the standards for approval, but may expedite the development or approval process. Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or decide that the time period for FDA review or approval will not be shortened. We may explore some of these opportunities for our product candidates as appropriate.
Post-approval Requirements
Any products manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to record-keeping, reporting of adverse experiences, periodic reporting, product sampling and distribution, and advertising and promotion of the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims, are subject to prior FDA review and approval. There also are continuing, annual program fees for any marketed products. Drug manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP, which impose certain procedural and documentation requirements upon us and our third-party manufacturers. Changes to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting requirements upon us and any third-party manufacturers that we may decide to use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance.
The FDA may withdraw approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical studies to assess new safety risks; or imposition of distribution restrictions or other restrictions under a REMS program. Other potential consequences include, among other things:
| ● | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
| ● | fines, warning letters, or untitled letters; |
| ● | clinical holds on clinical studies; |
| ● | refusal of the FDA to approve pending applications or supplements to approved applications, or suspension or revocation of product license approvals; |
| ● | product seizure or detention, or refusal to permit the import or export of products; |
| ● | consent decrees, corporate integrity agreements, debarment or exclusion from federal healthcare programs; |
| ● | mandated modification of promotional materials and labeling and the issuance of corrective information; |
| ● | the issuance of safety alerts, Dear Healthcare Provider letters, press releases and other communications containing warnings or other safety information about the product; or |
| ● | injunctions or the imposition of civil or criminal penalties. |
The FDA also may require post-marketing testing, known as Phase 4 testing, and surveillance to monitor the effects of an approved product. Discovery of previously unknown problems with a product or the failure to comply with applicable FDA requirements can have negative consequences, including adverse publicity, judicial or administrative enforcement, warning letters from the FDA, mandated corrective advertising or communications with doctors, and civil or criminal penalties, among others. Newly discovered or developed safety or effectiveness data may require changes to a product’s approved labeling, including the addition of new warnings and contraindications, and also may require the implementation of other risk management measures.
The FDA closely regulates the marketing, labeling, advertising and promotion of drug products. A company can make only those claims relating to safety and efficacy, purity and potency that are approved by the FDA and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses. Failure to comply with these requirements can result in, among other things, adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties. Physicians may prescribe, in their independent professional medical judgment, legally available products for uses that are not described in the product’s labeling and that differ from those tested by us and approved by the FDA. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, restrict manufacturer’s communications on the subject of off-label use of their products. The federal government has levied large civil and criminal fines against companies for alleged improper promotion of off-label use and has enjoined companies from engaging in off-label promotion. The FDA and other regulatory agencies have also required that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed. However, companies may share truthful and not misleading information that is otherwise consistent with a product’s FDA-approved labeling.
In addition, the distribution of prescription pharmaceutical products is subject to the Prescription Drug Marketing Act, or PDMA, which regulates the distribution of drugs and drug samples at the federal level and sets minimum standards for the registration and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution.
Marketing Exclusivity
Market exclusivity provisions authorized under the Food Drug and Cosmetic Act, or FDCA, can delay the submission or the approval of certain marketing applications. The FDCA provides a five-year period of non-patent marketing exclusivity within the United States to the first applicant to obtain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not approve or even accept for review an ANDA or an NDA submitted under Section 505(b)(2), or 505(b)(2) NDA, submitted by another company for another drug based on the same active moiety, regardless of whether the drug is intended for the same indication as the original innovative drug or for another indication, where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement to one of the patents listed with the FDA by the innovator NDA holder.
The FDCA alternatively provides three years of marketing exclusivity for an NDA, or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example new indications, dosages or strengths of an existing drug. This three-year exclusivity covers only the modification for which the drug received approval on the basis of the new clinical investigations and does not prohibit the FDA from approving ANDAs or 505(b)(2) NDAs for drugs containing the active agent for the original indication or condition of use. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA. However, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to any preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Controlled Substances
MEAI, the active ingredient in our lead product candidates may potentially be Schedule as a controlled substance. The CSA and its implementing regulations establish a “closed system” of regulations for controlled substances. The CSA imposes registration, security, recordkeeping and reporting, storage, manufacturing, distribution, importation and other requirements under the oversight of the U.S. Drug Enforcement Agency, or the DEA. The DEA is the federal agency responsible for regulating controlled substances, and requires those individuals or entities that manufacture, import, export, distribute, research, or dispense controlled substances to comply with the regulatory requirements in order to prevent the diversion of controlled substances to illicit channels of commerce.
The DEA categorizes controlled substances into one of five schedules—Schedule I, II, III, IV or V—with varying qualifications for listing in each schedule. Schedule I substances by definition have a high potential for abuse, have no currently accepted medical use in treatment in the United States and lack accepted safety for use under medical supervision. They may be used only in federally approved research programs and may not be marketed or sold for dispensing to patients in the United States. Pharmaceutical product candidates having a currently accepted medical use that are otherwise approved for marketing may be listed as Schedule II, III, IV or V substances, with Schedule II substances presenting the highest potential for abuse and physical or psychological dependence, and Schedule V substances presenting the lowest relative potential for abuse and dependence. The regulatory requirements are more restrictive for Schedule II substances than Schedule III substances. For example, all Schedule II drug prescriptions must be signed by a physician, physically presented to a pharmacist in most situations and cannot be refilled.
Following NDA approval of a drug containing a Schedule I controlled substance, that substance must be rescheduled as a Schedule II, III, IV or V substance before it can be marketed. On November 17, 2015, H.R. 639, Improving Regulatory Transparency for New Medical Therapies Act, passed through both houses of Congress. On November 25, 2015 this bill was signed into law. The new law removes uncertainty associated with timing of the DEA rescheduling process after NDA approval. Specifically, it requires DEA to issue an “interim final rule,” pursuant to which a manufacturer may market its product candidate within 90 days of FDA approval. The new law also preserves the period of orphan marketing exclusivity for the full seven years such that this period only begins after DEA scheduling. This contrasts with the previous situation whereby the orphan “clock” began to tick upon FDA approval, even though the product candidate could not be marketed until DEA scheduling was complete.
Facilities that manufacture, distribute, import or export any controlled substance must register annually with the DEA. The DEA registration is specific to the particular location, activity(ies) and controlled substance schedule(s). For example, separate registrations are required for importation and manufacturing activities, and each registration authorizes which schedules of controlled substances the registrant may handle. However, certain coincident activities are permitted without obtaining a separate DEA registration, such as distribution of controlled substances by the manufacturer that produces them.
The DEA inspects all manufacturing facilities to review security, recordkeeping, reporting and handling prior to issuing a controlled substance registration. The specific security requirements vary by the type of business activity and the schedule and quantity of controlled substances handled. The most stringent requirements apply to manufacturers of Schedule I and Schedule II substances. Required security measures commonly include background checks on employees and physical control of controlled substances through storage in approved vaults, safes and cages, and through use of alarm systems and surveillance cameras. An application for a manufacturing registration as a bulk manufacturer (not a dosage form manufacturer or a repacker/relabeler) for a Schedule I or II substance must be published in the Federal Register, and is open for 30 days to permit interested persons to submit comments, objections or requests for a hearing. A copy of the notice of the Federal Register publication is forwarded by DEA to all those registered, or applicants for registration, as bulk manufacturers of that substance. Once registered, manufacturing facilities must maintain records documenting the manufacture, receipt and distribution of all controlled substances. Manufacturers must submit periodic reports to the DEA of the distribution of Schedule I and II controlled substances, Schedule III narcotic substances, and other designated substances. Registrants must also report any controlled substance thefts or significant losses, and must obtain authorization to destroy or dispose of controlled substances. As with applications for registration as a bulk manufacturer, an application for an importer registration for a Schedule I or II substance must also be published in the Federal Register, which remains open for 30 days for comments. Imports of Schedule I and II controlled substances for commercial purposes are generally restricted to substances not already available from domestic supplier or where there is not adequate competition among domestic suppliers. In addition to an importer or exporter registration, importers and exporters must obtain a permit for every import or export of a Schedule I and II substance or Schedule III, IV and V narcotic, and submit import or export declarations for Schedule III, IV and V non-narcotics. In some cases, Schedule III non-narcotic substances may be subject to the import/export permit requirement, if necessary to ensure that the United States complies with its obligations under international drug control treaties.
For drugs manufactured in the United States, the DEA establishes annually an aggregate quota for the amount of substances within Schedules I and II that may be manufactured or produced in the United States based on the DEA’s estimate of the quantity needed to meet legitimate medical, scientific, research and industrial needs. This limited aggregate amount of cannabis that the DEA allows to be produced in the United States each year is allocated among individual companies, which, in turn, must annually apply to the DEA for individual manufacturing and procurement quotas. The quotas apply equally to the manufacturing of the API and production of dosage forms. The DEA may adjust aggregate production quotas a few times per year, and individual manufacturing or procurement quotas from time to time during the year, although the DEA has substantial discretion in whether or not to make such adjustments for individual companies.
The states also maintain separate controlled substance laws and regulations, including licensing, recordkeeping, security, distribution, and dispensing requirements. State Authorities, including Boards of Pharmacy, regulate use of controlled substances in each state. Failure to maintain compliance with applicable requirements, particularly as manifested in the loss or diversion of controlled substances, can result in enforcement action that could have a material adverse effect on our business, operations and financial condition. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to revoke those registrations. In certain circumstances, violations could lead to criminal prosecution.
European Government Regulation
Our product candidates will be subject to similar laws and regulations imposed by jurisdictions outside of the United States, and, in particular, Europe, which may include, for instance, applicable post-marketing requirements, including safety surveillance, anti-fraud and abuse laws and implementation of corporate compliance programs and reporting of payments or other transfers of value to healthcare professionals.
In order to market our future product candidates in the EEA (which is comprised of the 28 Member States of the European Union plus Norway, Iceland and Liechtenstein) and many other foreign jurisdictions, we must obtain separate regulatory approvals. More concretely, in the EEA, medicinal product candidates can only be commercialized after obtaining a Marketing Authorization, or MA. There are two types of marketing authorizations:
| ● | the “Community MA,” which is issued by the European Commission through the Centralized Procedure, based on the opinion of the Committee for Medicinal Product candidates for Human Use of the EMA and which is valid throughout the entire territory of the EEA. The Centralized Procedure is mandatory for certain types of product candidates, such as biotechnology medicinal product candidates, orphan medicinal product candidates and medicinal product candidates indicated for the treatment of AIDS, cancer, neurodegenerative disorders, diabetes, auto-immune and viral diseases. The Centralized Procedure is optional for product candidates containing a new active substance not yet authorized in the EEA, or for product candidates that constitute a significant therapeutic, scientific or technical innovation or which are in the interest of public health in the EU; and |
| ● | “National MAs,” which are issued by the competent authorities of the Member States of the EEA and only cover their respective territory, are available for product candidates not falling within the mandatory scope of the Centralized Procedure. Where a product has already been authorized for marketing in a Member State of the EEA, this National MA can be recognized in another Member State through the Mutual Recognition Procedure. If the product has not received a National MA in any Member State at the time of application, it can be approved simultaneously in various Member States through the Decentralized Procedure. |
Under the above-described procedures, before granting the MA, the EMA or the competent authorities of the Member States of the EEA make an assessment of the risk-benefit balance of the product on the basis of scientific criteria concerning its quality, safety and efficacy.
Data and marketing exclusivity. In the EEA, new product candidates authorized for marketing, or reference product candidates, qualify for eight years of data exclusivity and an additional two years of market exclusivity upon marketing authorization. The data exclusivity period prevents generic or biosimilar applicants from relying on the preclinical and clinical trial data contained in the dossier of the reference product when applying for a generic or biosimilar marketing authorization in the European Union during a period of eight years from the date on which the reference product was first authorized in the European Union. The market exclusivity period prevents a successful generic or biosimilar applicant from commercializing its product in the European Union until 10 years have elapsed from the initial authorization of the reference product in the European Union. The 10-year market exclusivity period can be extended to a maximum of eleven years if, during the first eight years of those 10 years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies.
Orphan drug designation. In the EEA, a medicinal product can be designated as an orphan drug if its sponsor can establish that the product is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting not more than five in 10,000 persons in the European Union when the application is made, or that the product is intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition in the European Community and that without incentives it is unlikely that the marketing of the drug in the European Union would generate sufficient return to justify the necessary investment. For either of these conditions, the applicant must demonstrate that there exists no satisfactory method of diagnosis, prevention or treatment of the condition in question that has been authorized in the European Union or, if such method exists, the drug will be of significant benefit to those affected by that condition.
In the EEA, an application for designation as an orphan product can be made any time prior to the filing of an application for approval to market the product. Marketing authorization for an orphan drug leads to a ten-year period of market exclusivity. During this market exclusivity period, the EMA or the competent authorities of the Member States, cannot accept another application for a marketing authorization, or grant a marketing authorization, for a similar medicinal product for the same indication. The period of market exclusivity is extended by two years for orphan medicinal products that have also complied with an agreed pediatric investigation plan.
This period of orphan market exclusivity may, however, be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for which it received orphan drug destination, that is the prevalence of the condition has increased above the threshold or it is judged that the product is sufficiently profitable not to justify maintenance of market exclusivity. Granting of an authorization for another similar orphan medicinal product where another product has market exclusivity can happen only in selected cases, such as, for example, demonstration of “clinical superiority” by a similar medicinal product, inability of a manufacturer to supply sufficient quantities of the first product or where the manufacturer itself gives consent. A company may voluntarily remove a product from the orphan register. Medicinal products or medicinal product candidates designated as orphan are eligible for incentives made available by the European Union and its Member States to support research into, development and availability of orphan medicinal products.
Adaptive pathways. The EMA has an adaptive pathways program which allows for early and progressive patient access to a medicine. The adaptive pathways concept is an approach to medicines approval that aims to improve patients’ access to medicines in cases of high unmet medical need. To achieve this goal, several approaches are envisaged: identifying small populations with severe disease where a medicine’s benefit-risk balance could be favorable; making more use of real-world data where appropriate to support clinical trial data; and involving health technology assessment bodies early in development to increase the chance that medicines will be recommended for payment and ultimately covered by national healthcare systems. The adaptive pathways concept applies primarily to treatments in areas of high medical need where it is difficult to collect data via traditional routes and where large clinical trials would unnecessarily expose patients who are unlikely to benefit from the medicine. The approach builds on regulatory processes already in place within the existing EU legal framework. These include: scientific advice; compassionate use; the conditional approval mechanism (for medicines addressing life-threatening conditions); patient registries and other pharmacovigilance tools that allow collection of real-life data and development of a risk-management plan for each medicine.
The adaptive pathways program does not change the standards for the evaluation of benefits and risks or the requirement to demonstrate a positive benefit-risk balance to obtain marketing authorization.
PRIME scheme. In July 2016, the EMA launched the PRIME scheme. PRIME is a voluntary scheme aimed at enhancing the EMA’s support for the development of medicines that target unmet medical needs. It is based on increased interaction and early dialogue with companies developing promising medicines, to optimize their product development plans and speed up their evaluation to help them reach patients earlier. Product developers that benefit from PRIME designation can expect to be eligible for accelerated assessment, but this is not guaranteed. The benefits of a PRIME designation includes the appointment of a rapporteur from the Committee for Medicinal Product candidates for human use before submission of an MAA, early dialogue and scientific advice at key development milestones, and the potential to qualify product candidates for accelerated review earlier in the application process.
Israeli Government Regulations.
Pursuant to the applicable laws & regulations in Israel, and specifically the Dangerous Drugs Ordinance, 5733 – 1973, 2-aminoindane, including its isomers and structural derivatives (which include MEAI), unless expressly excluded and excluding Rasagiline (R)-N-(prop-2-ynyl)-2,3-dihydro-1H-inden-1-amine ), is considered a “dangerous substance” (i.e. a “controlled substance”) and therefore, its importation to Israel requires a license, by the Israeli Ministry of Health, or the MOH. Once imported, the possession and use of such substance, for research purposes, requires the submission of a separate application to the MOH and a subsequent permit. Such permit will be considered by the MOH, as part of examining the application for a clinical trial to be conducted accordance with the applicable laws & regulations, as more fully described below.
Clinical Trials in Human Subjects in Israel
Clinical trials in Israel are conducted in accordance with the Public Health Regulations (Clinical Trials in Humans) 1980, and the updated MOH Clinical Trials Procedure No. 14.
The MOH Clinical Trials Department is authorized to examine and grant approvals to clinical trials with human participants, in accordance with all the terms& conditions specified in the applicable legislation/regulations and supervise their actual performance.
Any clinical trial (including planning, design, approval, conduct, documentation, recording, and reporting thereof) must be carried out while ensuring strict compliance with the following:
| ● | The principles of the Helsinki Declaration; |
| ● | the updated Public Health Regulations (Clinical Trials in Humans) 1980; |
| ● | the Genetic Information Law 2000; and |
| ● | the provisions of the applicable MOH Procedure and guidelines. |
In addition, every clinical trial must be carried out adhering to the following international guidelines:
| ● | The Harmonised Tripartite Guideline for Good Clinical Practice (ICH-GCP E6); and |
| ● | the International Standard Organisation (ISO); ISO 14155-1; ISO 14155-2: Clinical Investigation of Medical Devices for Human Subjects. |
In the event of an inconsistency between the aforesaid guidelines, the guidelines of the MOH shall prevail. In matters not covered by binding provisions in the guidelines of MOH, the international guidelines should be followed.
Informed Consent in Human Clinical Trials
Apart from exceptional cases, clinical trial participants must sign on an “informed consent” form, pre-approved by the applicable Helsinki Committee.
Human Clinical Trials Pre-Conditions
The following conditions must be met prior to the beginning of any such trial:
| 1. | Insurance – the sponsor of the trial must provide appropriate insurance coverage. |
| 2. | Free supply of the investigational product to the trial participants shall be guaranteed throughout the duration of the trial. A participant must not pay to participate in the trial. |
| 3. | The trial protocol must include provisions for safeguarding participants’ privacy and confidentiality of gathered information. |
Clinical Trial Agreements
Every contractual agreement between a clinical trial sponsor and a principal investigator conducting a clinical trial requires approval by the Committee for Contracts with Commercial Companies and by the Director of the medical institution in which the trial is conducted or designee thereof appointed for this purpose, such as the director of the institutional research fund. Approval by the director of the institution is also required for any contract between a Sponsor or representative thereof and a Principal Investigator or any other Investigator who is taking part in the clinical trial.
Controlled Substance Status in the U.S., Canada and Israel
In the U.S., MEAI has not been officially evaluated for its status as a controlled substance. It is currently unknown how it will be scheduled by the FDA, and it is not currently found in Schedule II of the US Schedules of Controlled Substances. However, if the molecule is considered to be similar to amphetamines or other control substances, then it may fall under this schedule as a stimulant, under which you will find amphetamine or other control substances listed. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation which contains any quantity of the following substances having a stimulant effect on the central nervous system are in this schedule: amphetamine, its salts, optical isomers, and salts of its optical isomers. This molecule also does not exist on the U.S. Toxic Substances Control Act Chemical Substance Inventory List.
In Canada, Health Canada has stated that MEAI is a controlled substance due to its structure being similar to amphetamines, which are on Health Canada’s Controlled Substances List. However, at this point in time, the molecule MEAI itself is not listed in Schedule I of Health Canada’s Controlled Substances List, which includes: amphetamines, their salts, derivatives, isomers and analogues and salts of derivatives, isomers and analogues.
In Israel, the Israeli Ministry of Health has stated that 2 – Aminoindane, including derivatives and isomers, is a controlled substance. Since, MEAI is a derivative of 2 – Aminoindane, it is considered a controlled substance.
Food products containing MEAI may be determined to be illegal. For example, one Canadian food product containing MEAI was determined to be illegal in Canada. However, to the best of our knowledge, this specific product sold in Canada was not manufactured under cGMP conditions and may have contained other substances as well. In addition, to the best of our knowledge, no regulatory process took place and no regulatory dossier was submitted in Canada or any other jurisdiction, in that matter, to ask for approval for commercialization of that product.
Other Healthcare Laws
Pharmaceutical companies are subject to additional healthcare regulation and enforcement by the federal government and by authorities in the states and foreign jurisdictions in which they conduct their business. Such laws include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, data privacy and security and physician sunshine laws and regulations. If their operations are found to be in violation of any of such laws or any other governmental regulations that apply, they may be subject to penalties, including, without limitation, civil and criminal penalties, damages, fines, the curtailment or restructuring of operations, exclusion from participation in federal and state healthcare programs and individual imprisonment.
Coverage and Reimbursement
Sales of any product depend, in part, on the extent to which such product will be covered by third-party payors, such as federal, state, and foreign government healthcare programs, commercial insurance and managed healthcare organizations, and the level of reimbursement for such product by third-party payors. Decisions regarding the extent of coverage and amount of reimbursement to be provided are made on a plan-by-plan basis. These third-party payors are increasingly reducing reimbursements for medical products, drugs and services. For products administered under the supervision of a physician, obtaining coverage and adequate reimbursement may be particularly difficult because of the higher prices often associated with such drugs.
In addition, the U.S. government, state legislatures and foreign governments have continued implementing cost-containment programs, including price controls, restrictions on coverage and reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit sales of any product. Decreases in third-party reimbursement for any product or a decision by a third-party payor not to cover a product could reduce physician usage and patient demand for the product and also have a material adverse effect on sales.
Healthcare Reform
In March 2010, Congress enacted the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, each as amended, collectively known as the ACA, which substantially changed the way healthcare is financed by both governmental and private insurers, and significantly affected the pharmaceutical industry. The ACA contains a number of provisions, including those governing enrollment in federal healthcare programs, reimbursement adjustments and fraud and abuse changes. Additionally, the ACA increased the minimum level of Medicaid rebates payable by manufacturers of brand name drugs from 15.1% to 23.1%; required collection of rebates for drugs paid by Medicaid managed care organizations; required manufacturers to participate in a coverage gap discount program, under which they must agree to offer 70 percent point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; and imposed a non-deductible annual fee on pharmaceutical manufacturers or importers who sell “branded prescription drugs” to specified federal government programs.
Since its enactment, there have been judicial and Congressional challenges to certain aspects of the ACA, and we expect there will be additional challenges and amendments to the ACA in the future. Other legislative changes have been proposed and adopted since the ACA was enacted, including aggregate reductions of Medicare payments to providers of 2% per fiscal year, which was temporarily suspended from May 1, 2020 through March 31, 2021, and reduced payments to several types of Medicare providers. Moreover, there has recently been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries, proposed and enacted legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. The likelihood of implementation of any of these reform initiatives is uncertain, particularly in light of the new presidential administration. Individual states in the United States have also become increasingly active in implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
Environmental, Health and Safety
We are also subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures, the generation, handling, use, storage, treatment, release and disposal of, and exposure to, hazardous materials and wastes and worker health and safety. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials, and produce hazardous waste products and the risk of injury, contamination or non-compliance with environmental, health and safety laws and regulations cannot be eliminated. Environmental, health and safety laws and regulations are complex, change frequently and have tended to become more stringent, and we may incur substantial costs in order to comply with such current or future laws and regulations.
Data Privacy and Security Laws
Numerous state, federal and foreign laws, including consumer protection laws and regulations, govern the collection, dissemination, use, access to, confidentiality and security of personal information, including health-related information. In the United States, federal and state laws and regulations, including data breach notification laws, health information privacy laws, and federal and state consumer protection laws and regulations (e.g., Section 5 of the Federal Trade Commission Act, or the FTC Act), that govern the collection, use, disclosure, and protection of health-related and other personal information could apply to our operations or the operations of our partners. For example, HIPAA, as amended by HITECH imposes privacy, security and breach notification obligations on certain health care providers, health plans, and health care clearinghouses, known as covered entities, as well as their business associates that perform certain services that involve creating, receiving, maintaining or transmitting individually identifiable health information for or on behalf of such covered entities. Entities that are found to be in violation of HIPAA as the result of a breach of unsecured protected health information, a complaint about privacy practices or an audit by the HHS, may be subject to significant civil, criminal and administrative fines and penalties and/or additional reporting and oversight obligations if required to enter into a resolution agreement and corrective action plan with HHS to settle allegations of HIPAA non-compliance. Further, entities that knowingly receive individually identifiable health information from a HIPAA covered entity in a manner that is not authorized or permitted by HIPAA may be subject to criminal penalties.
Even when HIPAA does not apply, according to the Federal Trade Commission, or FTC, violating consumers’ privacy rights or failing to take appropriate steps to keep consumers’ personal information secure may constitute unfair acts or practices in or affecting commerce in violation of Section 5 of the FTC Act. The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities.
In addition, certain federal, state and foreign laws, such as the GDPR, govern the privacy and security of personal data, including health-related data in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. For example, California enacted the CCPA, which went into effect on January 1, 2020. The CCPA, among other things, creates new data privacy obligations for covered companies and provides new privacy rights to California residents, including the right to access and delete their personal information, opt out of certain personal information sharing, and receive detailed information about how their personal information is used. The CCPA also creates a private right of action with statutory damages for certain data breaches, thereby potentially increasing risks associated with a data breach. Further, the CPRA was recently voted into law by California residents. The CPRA significantly amends the CCPA and imposes additional data protection obligations on covered companies doing business in California, including additional consumer rights processes and opt outs for certain uses of sensitive data. It also creates a new California data protection agency specifically tasked to implement and enforce the CCPA and the CPRA, which would likely result in increased regulatory scrutiny of California businesses in the areas of data protection and security. The substantive requirements for businesses subject to the CPRA went into effect on January 1, 2023 and became enforceable on July 1, 2023.
Patent Term Restoration and Extension
Depending upon the timing, duration and specifics of FDA approval of product candidates, some U.S. patents may be eligible for limited patent term extension under the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period generally is one-half the time between the effective date of an IND and the submission date of a BLA less any time the sponsor did not act with due diligence during the period, plus the time between the submission date of a BLA and the approval of that application less any time the sponsor did not act with due diligence during the period. Only one patent applicable to an approved biologic product is eligible for the extension, only those claims covering the approved drug, a method for using it, or a method for manufacturing it may be extended, and the application for the extension must be submitted prior to the expiration of the patent. Moreover, a given patent may only be extended once based on a single product. The USPTO, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. Similar provisions are available in Europe and other foreign jurisdictions to extend the term of a patent that covers an approved drug. In the future, if and when our products receive FDA approval, we expect to apply for patent term extensions on patents covering those products, however there is no guarantee that the applicable authorities, including the FDA in the United States, will agree with our assessment of whether such extensions should be granted, and if granted, the length of such extensions. See “Item 3.D. Risk Factors—Risks Related to Our Intellectual Property.”
Legal proceedings
We are not currently subject to any material legal proceedings.
Employees
As of November 1, 2023, our senior management team is comprised of Adi Zuloff Shani (Chief Executive Officer), Mark Haden (Vice President of Business Development), and Alan Rootenberg (Chief Financial Officer). Beyond our senior management team, we have one additional part-time employee and we engage with various consultants and service provides from time to time, including outsourced research and development providers. All of our employees are located in Israel and Canada. None of our employees are represented by labor unions or covered by collective bargaining agreements. We believe that we maintain good relations with all of our employees. However, in Israel, we are subject to certain Israeli labor laws, regulations and national labor court precedent rulings, as well as certain provisions of collective bargaining agreements applicable to us by virtue of extension orders issued in accordance with relevant labor laws by the Israeli Ministry of Economy and which apply such agreement provisions to our employees even though they are not part of a union that has signed a collective bargaining agreement.
All of our employment and consulting agreements include employees’ and consultants’ undertakings with respect to non-competition and assignment to us of intellectual property rights developed in the course of employment and confidentiality. The enforceability of such provisions is limited by Israeli law.
C. Organizational Structure
We currently have two (2), wholly-owned subsidiaries, Clearmindmed Ltd., a company incorporated in the State of Israel, and Clearmind Labs Corp. (inactive), incorporated in Canada.
D. Property, Plant and Equipment
On July 1, 2021, the we entered into a lease agreement (“2021 Lease”) with Scisparc Ltd, a related party (“Scisparc”) and a third party for a total area of approximately 240m2, of which the Company occupies approximately 120m2 for the Company’s offices, in Tel Aviv, Israel. The Company, Scisparc and the third party had an option to extend the 2021 Lease for an additional three-year period. The Company’s base rent was ILS11,000 per month ($3,080) during the term of the 2021 Lease. The lease liability was discounted using the Company’s estimated incremental borrowing rate of 20%. On December 31, 2021, the third party elected to leave the office space, and a new lease agreement was signed with the Company and Scisparc. As a result, the Company’s base rent was increased to ILS 18,200 per month ($5,094).
The lease expired on June 30, 2023. As of October 31, 2023 the Company and SciSparc were in the process of negotiating the terms of a new lease contract. On December 25, 2023, the rental agreement was renewed for a further 18 months. The Company’s base rent was increased to ILS 23,300 per month ($6,500). See Note 6b.
As of October 31, 2023, the Company has a pledge on its short-term deposit in favor of an Israeli bank in the amount of approximately $33 thousand to secure the Company’s lease commitment.
We consider that our current office space is sufficient to meet our anticipated needs for the foreseeable future and is suitable for the conduct of our business.
ITEM 4A. UNRESOLVED STAFF COMMENTS
Not applicable.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
CLEARMIND MEDICINE INC.
(Expressed in United States Dollars)
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our financial statements and the related notes included elsewhere in this annual report. The discussion below contains forward-looking statements that are based upon our current expectations and are subject to uncertainty and changes in circumstances. Actual results may differ materially from these expectations due to inaccurate assumptions and known or unknown risks and uncertainties, including those identified in “Cautionary Note Regarding Forward-Looking Statements” and under “Risk Factors” elsewhere in the annual report.
Overview
We are a clinical pharmaceutical company that develops novel psychedelic medicines to solve widespread, yet under-served, health problems. Our goal is to develop and provide a new type of treatment for mental health disorders, including AUD, binge drinking and obesity and metabolic disorder, where there is significant unmet need and lack of innovation. We see psychedelic therapies, which previously may have been overlooked or underused, as the future of treatment for a variety of indications. We believe that our solution for AUD can help solve one of the world’s biggest health problems, which costs the United States alone $250 billion each year.
We have experienced net losses in every period since our inception in 2017. We incurred net losses of $8,620,837, $6,894,868 and $2,725,288 for the years ended October 31, 2023, 2022 and 2021, respectively. As of October 31, 2023, we had an accumulated deficit of $18,768,063. We anticipate that we will continue to incur significant losses for the foreseeable future as our operating expenses and capital expenditures increase substantially due to our continued investment in our research and development activities and as we hire additional employees over the coming years. Furthermore, we expect to incur additional expenses associated with operating as a U.S. public company, including significant legal, accounting, investor relations and other expenses.
A. Operating Results
Components of Operating Results
Research and Development Expenses
Research and development activities are our primary focus. We do not believe that it is possible at this time to accurately project total expenses required for us to reach the point at which we will be ready to out-license our technologies. Development timelines, the probability of success and development costs can differ materially from expectations. In addition, we cannot forecast whether and when collaboration arrangements will be entered into, if at all, and to what degree such arrangements would affect our development plans and capital requirements. We expect our research and development expenses to increase over the next several years as our development program progresses. We would also expect to incur increased research and development expenses if we were to identify and develop additional technologies.
Research and development expenses include the following:
| ● | employee-related expenses, such as salaries and share-based compensation; |
| ● | expenses relating to outsourced and contracted services, such as manufacturing, consulting, research and advisory services; |
| ● | supply and development costs; and |
| ● | costs associated with regulatory compliance. |
We recognize research and development expenses as we incur them.
General and Administrative Expenses
General and administrative expenses consist primarily of personnel costs, including share-based compensation related to directors, employees and consultants, facility costs, patent application and maintenance expenses, and external professional service costs, including legal, accounting, audit, finance, business development, investor relations and human resource services, and other consulting fees.
We anticipate that our general and administrative expenses will increase in the future as we increase our administrative headcount and infrastructure to support our continued research and development programs and the potential commercialization of our products. We also anticipate that we will incur increased expenses related to audit, legal, regulatory and tax-related services associated with maintaining compliance with Nasdaq and SEC requirements, director and officer insurance premiums, director compensation, and other costs associated with being a public company.
Income Taxes
We have yet to generate taxable income in Canada. We anticipate that we will continue to generate tax losses for the foreseeable future and that we will be able to carry forward these tax losses indefinitely to future taxable years. Accordingly, we do not expect to pay taxes in Canada until we have taxable income after the full utilization of our carry forward tax losses.
Results of Operations
Our results of operations have varied in the past and can be expected to vary in the future due to numerous factors. We believe that period-to-period comparisons of our operating results are not necessarily meaningful and should not be relied upon as indications of future performance.
Year Ended October 31, 2023 Compared to Year Ended October 31, 2022
Research and development expenses
Research and development expenses for the year ended October 31, 2023, amounted to $1,544,229, representing a decrease of $1,536,762, or 50%, compared to $3,080,991 for the year ended October 31, 2022. The decrease resulted mainly from decrease in in pre-clinical studies expenses related to our new business of researching and developing designer therapeutics of $1,221,795.
We have completed a series of pre-clinical, IND-enabling studies in the United States and China that are required before we can study our compound for the first time in humans. These studies include pharmacokinetic and toxicological studies in rats and dogs in order to assess the safety profile of our compound and characterization of the drug metabolism. We have conducted several metabolism studies designed to better understand the way MEAI is digested in several species. In addition, we have conducted a pre-clinical animal model of AUD to characterize the effect of MEAI on alcohol consumption. This study involved testing the effect of MEAI’s ability to curb alcohol cravings after exposing mice to prolonged alcohol consumption over a short period, mimicking binge alcohol consumption in humans.
In March 2023, we announced that we had submitted an IND application with the FDA, requesting approval to initiate our first-in-human Phase I/IIa clinical trial with CMND-100 in patients suffering from AUD. Subsequently, in May 2023 we initiated the CM-CMND-001 clinical trial in both Israel and the United States, including at the Yale School of Medicine’s Department of Psychiatry and Johns Hopkins University School of Medicine.
The CM-CMND-001 clinical trial is designed to be a double blind, placebo controlled, multinational, multi-center, Phase I/IIa single- and multiple-dose tolerability, safety and pharmacokinetic study in healthy volunteers and AUD subjects. Upon completion of the Phase I/IIa studies, if successful, we will be required to conduct additional clinical trials subject to securing additional financing.
Additionally, the Company entered into research agreements with scientists from several universities in Israel; three Agreements with Yissum Research Development Company of the Hebrew University of Jerusalem Ltd., or Yissum, with two out of them already completed.
In addition, the Company recently completed a serious of research programs, with Professor Joseph Tam from the Hebrew University through Yissum for the research of MEAI on food intake, metabolic and activity profiles. Following the completion of the research program, we and Yissum obtained a jointly owned patent in for which we entered into a license Agreement in December 2022 in connection with the patent for the use of MEAI in methods for methods for treating metabolic syndrome titled “use of 5-METHOXY-2-AMINOINDAN (“MEAI”) in methods for treating metabolic syndrome”.
Also, a research agreement signed with BIRAD, Bar Ilan University Research and Development Company, in Ramat Gan, Israel, for the research of the safety, efficacy and other characteristics of MEAI as completed under the leadership of Professor Gal Yadid was also completed. Following the completion of the research program, we and BIRAD obtained a jointly owned patent in May 2022, for the use of MEAI for the Treatment of Cocaine Addiction titled “Use of 5-METHOXY-2-AMINOINDAN (“MEAI”) in methods for treating cocaine addiction”
In addition, we have expended our IP portfolio that now includes fifteen utility patent families including patents and applications having method of use and composition of matter claims.
General and administrative expenses
General and administrative expenses for the year ended October 31, 2023, amount to $4,759,205, representing an increase of $1,588,765, or 50%, compared to $3,170,440 for the year ended October 31, 2022. The increase resulted primarily from increase in professional fees of $1,116,924, increase in investor relations of $1,092,864, and an increase in directors and officers insurance of $214,522, offset by a decrease in share-based compensation expense of $702,607.
Net loss and comprehensive loss
Net loss for the year ended October 31, 2023, amounted to $8,620,837 representing an increase of $1,725,969, or 25%, compared to $6,894,868 for the year ended October 31, 2022. The increase is as a result of an increase in general and administrative expenses and an increase in the warrant liability.
Year Ended October 31, 2022 Compared to Year Ended October 31, 2021
Research and development expenses
Research and development expenses for the year ended October 31, 2022, amounted to $3,080,991, representing an increase of $2,607,339, or 550%, compared to $473,652 for the year ended October 31, 2021. The increase resulted mainly from increase in share-based compensation expense of $213,299 and an increase in pre-clinical studies expenses related to our new business of researching and developing designer therapeutics.
General and administrative expenses
General and administrative expenses for the year ended October 31, 2022, amount to $3,170,440 representing an increase of $950,695, or 43%, compared to $2,219,745 for the year ended October 31, 2021. The increase resulted from increase in professional and consulting fees of $283,950, increase in share-based compensation expense of $326,385 and increase in investor relations of $358,400.
Net loss and comprehensive loss
Net loss for the year ended October 31, 2022, amounted to $6,894,868 representing an increase of $4,169,580, or 153%, compared to $2,725,288 for the year ended October 31, 2021. The increase resulted mainly from increased research and development expenses and general and administrative expenses as discussed above.
B. Liquidity and Capital Resources
As of October 31, 2023, the Company had cash and cash equivalents on hand of $5,427,739 and a positive working capital of $824,760, compared to $128,777 and negative working capital of $1,497,720 as of October 31, 2022, respectively.
The Company may have capital requirements in excess of its currently available resources. In the event the Company’s plans change, its assumptions change or prove inaccurate, or its capital resources in addition to projected cash flow, if any, prove to be insufficient to fund operations, the Company may be required to seek additional financing. There can be no assurance that the Company will have sufficient financing to meet its future capital requirements or that additional financing will be available on terms acceptable to the Company in the future.
As of October 31, 2023, the Company had accounts payable and accrued liabilities of $617,004. In November 2022, April 2023, September 2023 and January 2024, we completed public offerings of our common shares and warrants to purchase our common shares for gross proceeds of $7.5 million, $3.5 million, $2.25 million and $2.4 million, respectively. These funds will be used to fund the Company in the near term.
We have financed our operations for the last year primarily from our issuance of Common Shares and warrants.
Since the Company announced its Change of Business on May 25, 2021, we raised an aggregate of $19.6 million net of issuance costs. As of October 31, 2023, we had $ 5,427,739 in cash.
The table below shows a summary of our cashflows for the periods indicated:
| Year Ended October 31, |
||||||||||||
| 2023 | 2022 | 2021 | ||||||||||
| USD in thousands | ||||||||||||
| Net cash used in operating activities | (6,301 | ) | (3,724 | ) | (2,077 | ) | ||||||
| Net cash used in investing activities | (26 | ) | (6 | ) | (184 | ) | ||||||
| Net cash provided by financing activities | 11,664 | 512 | 5,389 | |||||||||
| Effect of foreign exchange on cash | (38 | ) | (23 | ) | - | |||||||
| Net increase (decrease) in cash | 5,299 | (3,241 | ) | 3,128 | ||||||||
Year Ended October 31, 2023 Compared to Year Ended October 31, 2022
Net cash used in operating activities
Net cash used in operating activities for the year ended October 31, 2023, amounted to $6,301,472 representing an increase of $2,576,798, or 69%, compared to $3,724,494 for the year ended October 31, 2022. This increase was mainly due to decrease in accounts payable and accrued liabilities.
Net cash used in investing activities
Net cash used in investing activities for the year ended October 31, 2023, amounted to $26,228 representing an increase of $20,665, or 371%, compared to $5,563 for the year ended October 31, 2022. This increase was mainly due to change in restricted cash (office rental security deposit).
Net cash provided by financing activities
Net cash provided by financing activities for the year ended October 31, 2023, amounted to $11,664,396 representing an increase of $11,152,708, or 2,179%, compared to $511,688 for the year ended October 31, 2022. This increase was due to November 2022 Public Offering, April 2023 Public Offering and September 2023 Public Offering.
Year Ended October 31, 2022 Compared to Year Ended October 31, 2021
Net cash used in operating activities
Net cash used in operating activities for the year ended October 31, 2022, amounted to $3,724,494 representing an increase of $1,647,398, or 79%, compared to $2,077,096 for the year ended October 31, 2021. This increase was mainly due to hiring additional employees and engaging consultants in connection with our new business of researching and developing designer therapeutics.
Net cash used in investing activities
Net cash used in investing activities for the year ended October 31, 2022, amounted to $5,563 representing a decrease of $178,472, or 97%, compared to $184,035 for the year ended October 31, 2021. This decrease was mainly due to an assignment agreement whereby the Company acquired patents and patent applications to be used in pre-clinical drug research programs and due to a payment related to exploration and evaluation activity in 2021.
Net cash provided by financing activities
Net cash provided by financing activities for the year ended October 31, 2022, amounted to $511,688 representing a decrease of $4,877,582, or 91%, compared to $5,389,270 for the year ended October 31, 2021. This decrease was due to increase in share capital issuance relating the Company’s change of business in 2021.
We have incurred losses and cash flow deficits from operations since inception, resulting in an accumulated deficit as of October 31, 2023 of $18,768,063. We anticipate that we will continue to incur net losses for the foreseeable future. To meet future capital needs, we would need to raise additional capital through equity or debt financing or other strategic transactions. However, any such financing may not be on favorable terms or even available to us. Our failure to obtain sufficient funds on commercially acceptable terms when needed would have a material adverse effect on our business, results of operations and financial condition. Our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement that involves risks and uncertainties, and the actual amount of our expenses could vary materially and adversely as a result of a number of factors. We have based our estimates on assumptions that may prove to be wrong, and our expenses could prove to be significantly higher than we currently anticipate.
Our future capital requirements will depend on many factors, including, but not limited to:
| ● | the progress and costs of our research and development activities; |
| ● | the costs of development and expansion of our operational infrastructure; |
| ● | our ability, or that of our collaborators, to achieve development milestones and other events or developments under potential future licensing agreements; |
| ● | the amount of revenues and contributions we receive under future licensing, collaboration, development and commercialization arrangements with respect to our technologies; |
| ● | the costs of filing, prosecuting, enforcing and defending patent claims and other intellectual property rights; |
| ● | the costs of contracting with third parties to provide sales and marketing capabilities for us or establishing such capabilities ourselves, once our technologies are developed and ready for commercialization; |
| ● | the costs of acquiring or undertaking development and commercialization efforts for any future products or technology; |
| ● | the magnitude of our general and administrative expenses; and |
| ● | any additional costs that we may incur under future in- and out-licensing arrangements relating to our technologies and futures products. |
Until we can generate significant recurring revenues, we expect to satisfy our future cash needs through capital raising or by out-licensing and/or co-developing applications of one or more of our product candidates. We cannot be certain that additional funding will be available to us on acceptable terms, if at all. If funds are not available on favorable terms, or at all, we may be required to delay, reduce the scope of or eliminate research or development efforts or plans for commercialization with respect to our technologies and make necessary change to our operations to reduce the level of our expenditures in line with available resources.
We are a development-stage technology company and it is not possible for us to predict with any degree of accuracy the outcome of our research and development efforts. As such, it is not possible for us to predict with any degree of accuracy any significant trends, uncertainties, demands, commitments or events that are reasonably likely to have a material effect on our net loss, liquidity or capital resources, or that would cause financial information to not necessarily be indicative of future operating results or financial condition. However, to the extent possible, certain trends, uncertainties, demands, commitments and events are described herein.
C. Research and development, patents and licenses, etc.
For a description of our research and development programs and the amounts that we have incurred over the last two years pursuant to those programs, please see “Item 5. Operating and Financial Review and Prospects— A. Operating Results—Research and Development Expenses” and “Item 5. Operating and Financial Review and Prospects— A. Operating Results— Comparison of the year ended October 31, 2023 to the year ended October 31, 2022— Research and Development Expenses.”
D. Trend Information
The COVID-19 pandemic and its impacts continue to evolve. We cannot predict the duration and severity of any further disruptions as a result of COVID-19 or their impacts on us, but business disruptions for us or any of the third parties with whom we engage, including the manufacturers, suppliers, customers, regulators and other third parties with whom we conduct business could materially and negatively impact our ability to conduct our business in the manner and on the timelines presently planned. Further, we cannot predict impacts, trends and uncertainties involving the pandemic’s effects on economic activity, the size of our labor force, and the extent to which our income, profitability, liquidity, or capital resources may be materially and adversely affected. The global economy, including credit and financial markets, has experienced extreme volatility and disruptions, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates, increases in inflation rates and uncertainty about economic stability. We are unable to determine the extent of the impact of the pandemic on our clinical trials, operations and financial condition going forward. See also “Item 3.D. Risk Factors– Risks Related to Our Business Operations.”
E. Critical Accounting Estimates
Management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with IFRS as issued by the IASB. The preparation of the consolidated financial statements requires management to make estimates and assumptions for the reported amounts of assets, liabilities, expenses and related disclosures. Our estimates are based on our historical experience and on various other factors that management believes are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions and any such differences may be material.
While our significant accounting policies are more fully described in Note 2 to the audited consolidated financial statements for the year ended October 31, 2023 appearing elsewhere in this Annual Report on Form 20-F, management believes the following discussion addresses our most critical accounting policies, which are those that are most important to the financial condition and results of operations and require our most difficult, subjective and complex judgments.
Significant Estimates
Share-based Compensation
Fair values are determined using the Black-Scholes option pricing model.
Estimating fair value requires determining the most appropriate valuation model for a grant of equity instruments, which is dependent on the terms and conditions of the grant. Option-pricing models require the use of highly subjective estimates and assumptions including the expected stock price volatility. Changes in the underlying assumptions can materially affect the fair value estimates and, therefore, existing models do not necessarily provide reliable measurement of the fair value of the Company’s stock options.
We selected the Black-Scholes option-pricing model as a fair value method for our options awards. The option-pricing model requires a number of assumptions:
Expected dividend yield - The expected dividend yield assumption is based on our historical experience and expectation of no future dividend payouts. We have historically not paid cash dividends and have no foreseeable plans to pay cash dividends in the future.
Volatility - The expected volatility is based on similar companies traded on North American exchanges.
Risk free interest rate - The risk free interest rate is based on the government money Canada marketable bonds, in accordance with the option’s contractual term.
Contractual term - An option’s contractual term must at least include the Vesting Period and the employees’ historical exercise and post-vesting employment termination behavior for similar grants. If the amount of past exercise data is limited, that data may not represent a sufficiently large sample on which to base a robust conclusion on expected exercise behavior.
Share price - The share price is determined according to the last known or above closing price of our common shares at the grant date.
Warrant Liability
The Company analyses warrants issued to determine whether they meet the classification as liabilities or equity. Derivative warrant liabilities are adjusted to reflect fair value at each reporting period, with any increase or decrease in the fair value recorded in the results of operations. The Company uses a fair valuation specialist to estimate the value of these instruments using the Black and Scholes and binomial pricing model.
The key assumptions used in the models are the expected future volatility in the price of the Company's shares, the expected life of the warrants and the probability of any future adjustment event.
Significant Judgments
The critical judgments that the Company’s management has made in the process of applying the Company’s accounting policies that have the most significant effect on the amounts recognized in the Company’s consolidated financial statements are as follows:
Going Concern
The application of the going concern assumption which requires management to take into account all available information about the future, which is at least but not limited to, 12 months from the year end of the reporting period. The Company is aware that material uncertainties related to events or conditions raise substantial doubt upon the Company’s ability to continue as a going concern.
Recently Issued Accounting Pronouncements
Accounting Standards Issued But Not Yet Effective
| i) | Amendments to IAS 1 Presentation of Financial Statements—Classification of Liabilities as Current or Non-current |
The IASB issued a narrow-scope amendment to IAS 1, in January 2020, to clarify that liabilities are classified as either current or non-current, depending on the rights that exist at the end of the reporting period. The amendment could affect the classification of liabilities, particularly for entities that previously considered management’s intentions to determine classification and for some liabilities that can be converted into equity. Inter alia, the amendment requires the following:
Liabilities are classified as non-current if the entity has a substantive right to defer settlement for at least 12 months at the end of the reporting period. The amendment no longer refers to unconditional rights. The assessment determines whether a right exists, but it does not consider whether the entity will exercise the right.
’Settlement’ is defined as the extinguishment of a liability with cash, other economic resources or an entity’s own equity instruments. There is an exception for convertible instruments that might be converted into equity, but only for those instruments where the conversion option is classified as an equity instrument as a separate component of a compound financial instrument.
The Company early adopted the narrow-scope amendment to IAS 1 on January 1, 2019, with no effect to the Company’s financial statements.
| ii) | Amendments to IAS 1 Presentation of Financial Statements and IFRS Practice Statement 2 Making Materiality Judgements - Disclosure of Accounting Policies |
The amendments change the requirements in IAS 1 with regard to disclosure of accounting policies. The amendments replace all instances of the term ‘significant accounting policies’ with ‘material accounting policy information’. Accounting policy information is material if, when considered together with other information included in an entity’s financial statements, it can reasonably be expected to influence decisions that the primary users of general-purpose financial statements make on the basis of those financial statements.
The supporting paragraphs in IAS 1 are also amended to clarify that accounting policy information that relates to immaterial transactions, other events or conditions is immaterial and need not be disclosed. Accounting policy information may be material because of the nature of the related transactions, other events or conditions, even if the amounts are immaterial. However, not all accounting policy information relating to material transactions, other events or conditions is itself material.
The IASB has also developed guidance and examples to explain and demonstrate the application of the ‘four-step materiality process’ described in IFRS Practice Statement 2.
The amendments are applied for annual periods beginning on or after January 1, 2023.
| iii) | Amendments to IAS 7 Statement of Cash Flows and IFRS 7 Financial Instruments: Disclosures - Supplier Finance Arrangements |
The amendments add a disclosure objective to IAS 7 stating that an entity is required to disclose information about its supplier finance arrangements that enables users of financial statements to assess the effects of those arrangements on the entity’s liabilities and cash flows. In addition, IFRS 7 was amended to add supplier finance arrangements as an example within the requirements to disclose information about an entity’s exposure to concentration of liquidity risk.
The amendments, which contain specific transition reliefs for the first annual reporting period in which an entity applies the amendments, are applicable for annual reporting periods beginning on or after 1 January 2024. Earlier application is permitted.
Recent Offerings
On November 14, 2022, the Company completed an underwritten public offering of 38,462 common shares at a price to the public of US$195.00 per share, for aggregate gross proceeds of US$7.5 million, prior to deducting underwriting discounts and offering expenses. The offering closed on November 17, 2022.
In addition, the Company granted Aegis Capital Corp., or Aegis, who acted as the underwriters for the deal, a 45-day option to purchase up to 5,769 additional common shares, equal to 15% of the number of common shares sold in the offering solely to cover over-allotments, if any (“Over-Allotment”). The public purchase price per additional common share would have been $195.00 per share. The Over-Allotment was not exercised.
Aegis received 1,923 underwriter warrants, each such warrant entitling the agents to receive one common share upon payment of $243.75 per share, exercisable six months after the commencement of sales of this offering and expiring on a date which is no more than five years after the commencement of sales of the offering. The fair value for underwriter warrants total of $337,579 and have been credited to the Share-based payment reserve.
| a. | On April 4, 2023, we entered into a definitive placement agent agreement, or the April 2023 Placement Agent Agreement, with Aegis, or the Placement Agent, pursuant to which the Placement Agent acted as our placement agent in connection with the issuance and sale in a public offering on a reasonable best efforts basis, or the April 2023 Public Offering, of (i) 103,249 Common Shares, (ii) 46,942 pre-funded warrants to purchase 46,942 Common Shares and (iii) 150,191 warrants to purchase 150,191 Common Shares. The pre-funded warrants are immediately exercisable at an exercise price of $23.40 per Common Share and will not expire until exercised in full. The common warrants have an exercise price of $1.0777 per Common Share (after giving effect to adjustments and subject to further adjustments as set forth therein), are immediately exercisable, and expire five years from the date of issuance. These warrants include a cashless exercise provision and repricing provisions, under certain circumstances. |
The gross proceeds to us from the April 2023 Public Offering were approximately $3.5 million before deducting estimated offering expenses payable by us. As of the date hereof, the pre-funded warrants have been exercised in full and warrants were exercised to purchase 771,954 Common Shares issued in the April 2023 Public Offering, resulting in gross proceeds of $2.7 million to us.
| b. | On September 14, 2023, we entered into a definitive placement agent agreement, or the September 2023 Placement Agent Agreement, with the Placement Agent pursuant to which the Placement Agent acted as our placement agent in connection with the issuance and sale in a public offering on a reasonable best efforts basis, or the September 2023 Public Offering, of (i) 225,833 Common Shares, (ii) 24,166 pre-funded warrants to purchase 24,166 Common Shares and (iii) 250,000 warrants to purchase 250,000 Common Shares. The pre-funded warrants are immediately exercisable at an exercise price of $0.03 per Common Share and will not expire until exercised in full. The common warrants have an exercise price of $1.0777 per Common Share (after giving effect to adjustments and subject to further adjustments as set forth therein), are immediately exercisable, and expire five years from the date of issuance. These warrants include a cashless exercise provision and repricing provisions, under certain circumstances. |
The gross proceeds to us from the September 2023 Public Offering were approximately $2.25 million before deducting estimated offering expenses payable by us. As of the date hereof, the pre-funded warrants have been exercised in full and warrants were exercise to purchase 409,667 Common Shares issued in the September 2023 Public Offering, resulting in gross proceeds of $1.3 million to us.
| c. | On January 11, 2024, we entered into a definitive securities purchase agreement, or the January 2024 Securities Purchase Agreement, with investors in connection with the issuance and sale in a registered direct offering and concurrent private placement, or the January 2024 Offering, of (i) 1,468,000 Common Shares, (ii) 32,000 pre-funded warrants to purchase 32,000 Common Shares and (iii) 1,500,000 unregistered common warrants to purchase 1,500,000 Common Shares. The pre-funded warrants are immediately exercisable at an exercise price of $0.0001 per Common Share and will not expire until exercised in full. The unregistered common warrants have an exercise price of $1.60 per Common Share (after giving effect to adjustments and subject to further adjustments as set forth therein), are immediately exercisable, and expire five years from the date of issuance. These warrants include a cashless exercise provision and repricing provisions, under certain circumstances. |
The gross proceeds to us from the January 2024 Offering were approximately $2.4 million before deducting estimated offering expenses payable by us. As of the date hereof, the pre-funded warrants have been exercised.
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
A. Directors and Senior Management
Executive Officers and Directors
The following table sets forth information regarding our executive officers and directors, including their ages as of October 31, 2023
| Name | Age | Position | ||
| Dr. Adi Zuloff-Shani | 56 | Chief Executive Officer | ||
| Alan Rootenberg | 71 | Chief Financial Officer | ||
| Prof. Mark Haden | 70 | Vice President of Business Development | ||
| Amitay Weiss | 62 | Chairman of the Board of Directors | ||
| Yehonatan Shachar | 33 | Director | ||
| Oz Adler | 38 | Director | ||
| Asaf Itzhaik | 52 | Director |
Dr. Adi Zuloff-Shani, Chief Executive Officer
Dr. Adi Zuloff-Shani has served as our Chief Executive Officer since July 14, 2021. Dr. Zuloff-Shani has more than 20 years of experience as a research and development executive. Dr. Zuloff-Shani has also served as the Chief Technologies Officer of SciSparc Ltd. since February 2016. In addition, Dr. Zuloff-Shani has served as a Member of the Scientific Advisory Board, Save Foods, Inc. (Nasdaq: SVFD) since October 2021, and as Chairman of the board of directors of ORSUS Therapeutics Limited since November 2021. Prior to joining us, and from 2012 to 2016, Dr. Zuloff-Shani served as a vice president development at Macrocure Ltd. (Nasdaq: MCUR) where besides leading all research and development activities, she interacted and was involved with the activities of all departments including clinical, operations, quality assurance, quality control, finance, and regulatory affairs. Dr. Zuloff-Shani holds a Ph.D. in human biology and immunology from Bar-Ilan University, Israel.
Alan Rootenberg, Chief Financial Officer
Mr. Alan Rootenberg has served as our Chief Financial Officer since June 14, 2022. Mr. Rootenberg also served as our Director from April 15, 2020 until December 2022. Mr. Rootenberg has served as the Chief Financial Officer of Alpha Gold North Inc. since August 2021. In addition, Mr. Rootenberg has served as the Chief Financial Officer of BioHarvest Sciences, Inc. (CSE: BHSC) since November 2018 and has served as the Chief Financial Officer of Eco (Atlantic) Oil & Gas Ltd. (TSXV: EOG) since November 2011 and a public company, Anteros Metals Inc. These companies include mineral exploration, mining, technology and nutraceutical products. He also serves as a director of a publicly traded company, A2Z Smart Technologies Corp. (TSXV: AZ). Mr. Rootenberg has a Bachelor of Commerce degree from the University of the Witwatersrand in Johannesburg, South Africa and received his CPA designation in Ontario, Canada.
Mark Haden, Vice President of Business Development
Prof. Mark Haden has served as our Vice President of Business Development since July 2021. Previously, from April 2021 until March 2022, Prof. Haden served as a Director of Clinical Trials at Psygen. From 2017 until May 2021, Prof. Haden served as the Executive Director of Multidisciplinary Association for Psychedelic Studies (MAPS) Canada. Since 2012, Prof. Haden has served as an Adjunct Professor at the University of British Columbia’s Population and Public Health. Prof. Haden holds a Bachelor’s degree in both Social Work and Psychology, as well as a Master’s degree in Social Work from the university of British Columbia.
Amitay Weiss, Director
Mr. Amitay Weiss has served Chairman of our Board of Directors since August 19, 2019. Mr. Weiss has served as chairman of the board of directors of Save Foods Inc. (Nasdaq: SVFD) since August 2020, chairman of the board of directors of ParaZero Technologies Ltd. (Nasdaq: PRZO) since August 2023 and as a member of its board since February 2022, chairman of the board of directors of Infimer Ltd. (TASE:INFR-M) since July 2021 and chairman of the board of directors of Upsellon Brands Holdings Ltd. (previously Chiron Ltd.) (TASE: UPSL) since June 2019. He has also served as a member of the board of directors of Automax Motors Ltd. (TASE: AMX) since March 2021, Gix Internet Ltd. (previously Algomizer Ltd.) (TASE:GIX) since March 2019, Clearmind Medicine Inc. (previously Cyntar Ventures Inc.) (CSE: CMND) since August 2019, Perihelion Capital Ltd (PCL.P:CVE) since June 2021, as an external director of Cofix Group Ltd. (TASE: CFCS) since August 2015 and as a member of the board of directors and chief executive officer of SciSparc Ltd. (previously Therapix Biosciences Ltd.) (OTC:SPRCY) since August 2020. He previously served as chairman of the board of directors of Value Capital One Ltd. (previously P.L.T Financial Services Ltd.) (TASE:VALU) from April 2016 to February 2021, Matomy Media Group Ltd. (LSE:MTMY, TASE:MTMY.TA) from May 2020 to March 2021. In April 2016, Mr. Weiss founded Amitay Weiss Management Ltd., an economic consulting company, and now serves as its chief executive officer. Mr. Weiss holds a B.A in economics from New England College, M.B.A. in business administration and LL.B. from Ono Academic College, Israel.
Yehonatan Shachar, Director
Mr. Yehonatan Shachar has served as our Director since April 15, 2020. Mr. Shachar has served as the Chief Executive Officer of Heroic Media Ltd., a digital marketing agency that works with top Israeli e-commerce brands since February 2020. Before this role, from June 2019 until February 2021, Mr. Shachar served as the CEO of Chiron Refineries, where he lead a merger with Upsellon brands. Mr. Shachar has an LLB in Law and M.B.A in Business from the IDC International University in Herzliya, Israel.
Oz Adler, Director
Mr. Oz Adler has served as our Director since September 2021. Mr. Adler currently serves as the chief executive officer and chief financial officer of SciSparc Ltd. From December 2020 to April 2021, Mr. Adler served as the chief financial officer of Medigus Ltd. Mr. Adler also worked in the audit department of Kost Forer Gabbay & Kasierer, a member of Ernst & Young Global between December 2012 and August 2017. Additionally, Mr. Adler currently serves on the board of directors of numerous private and publicly traded companies, including Rail Vision Ltd (NASDAQ:RVSN), Clearmind Medicine Inc. (CSE: CMND) (OTC: CMNDF) (FSE:CWY), Chairman of Jeffs’ Brands Ltd and various private companies. Mr. Adler is a certified public accountant in Israel and holds a B.A. degree in Accounting and Business Management from The College of Management, Israel.
Asaf Itzhaik, Director
Mr. Asaf Itzhaik has served as our director since December 2022. Mr. Itzhaik has served as the chief executive officer of A.K.A Optics Ltd., a manufacturer of adaptive optics, since 1994 and as a member of the board of directors of A.K.A Optics Ltd. since 1998. Mr. Itzhaik has also served as a member of the board of directors of Jeffs’ Brands Ltd. since August 2022, Tzmicha Ltd. since August 2021 and Gix Internet Ltd. since August 2021. Mr. Itzhaik in a certified optometrist and graduated a program in corporate board leadership in Merkaz Hashilton Hamkomi. Mr. Yitzchaick has completed a continuing education director’s course in Israel.
Family Relationships
There are no family relationships between any members of our executive management and our directors.
Scientific Advisory Board
We have a Scientific Advisory Board of eight researchers in the field(s) of: Psychiatry, Neuroscience, Neuropsychopharmacology, addiction, alcoholism and Clinical& Regulatory Affairs. We consult with the members of our Scientific Advisory Board on a regular basis.
Prof. Michael Davidson, MD has published over 350 articles in peer reviewed prestigious journals in the area of Psychiatry and of Alzheimer’s disease, including on development of novel treatments. Previously he was Department Chairman at the Tel Aviv University and Chief Editor of the European Neuropsychopharmacology journal. He is currently Chairman of the department of psychiatry at the Nicosia University medical school, the President of the Alzheimer Center in Israel, and actively involved in drug development.
Prof. Alon Friedman, MD, PhD is Professor of Neuroscience at both Ben-Gurion University of the Negev (BGU) in Beersheba, Israel, and Dalhousie University, Halifax, Canada. Prof. Friedman, is best known for his discoveries of the link between blood–brain barrier dysfunction (BBBd) and neuropathologies, and the mechanisms underlying BBBd-related diseases. His team developed novel imaging modalities for the diagnosis of BBBd in neuro-psychiatric disorders.
Prof. Gal Yadid, PhD is an expert in neuropsychopharmacology, and is a Lab Chief in the Brain center of the Bar Ilan University. His field research and treatment development for reward-related psychiatric disorders, specifically Addiction, PTSD and depression. His research moved from preclinical (using unique animal models) to clinical applying various methods (behavioral, cognitive, histochemical, neurochemical, genetic / epigenetic, microbiome).
Prof. Christian Schültz, MD, PhD, is an Associate Professor at the Department of Psychiatry in University of British Columbia’s Faculty of Medicine, and focuses his work on clinical interventions and health service in substance use disorders and dual diagnoses (mental and substance use disorders), as well as neurobiological and neurocognitive aspects of impulsive decision-making. His main research focuses on understanding mechanisms of relapse and impulsive decision-making to improve treatment of addiction and concurrent disorders.
Prof. Wim van den Brink MD, PhD, received his medical degree from the Free University in Amsterdam. He received his PhD degree from the State University of Groningen. Since 1992, he has served as a professor of Addiction Psychiatry at the Academic Medical Center of the University of Amsterdam. In 2014 he received the lifetime achievement award for science from the Netherlands Association of Psychiatry and in 2015 he was granted the status of honorable member of the Spanish Society for Dual disorders. In 2017 he received the European Addiction Research Award from the European Federation of Addiction Societies (EUFAS) and in 2020 he became a Doctor et Professor Honoris Causa at the Eötvös Loránd University in Budapest, Hungary. Until recently, he was an associate editor of Drug and Alcohol Dependence and chief editor of European Addiction Research. He has been the chair of the Workgroups that developed the Dutch Treatment Guidelines on Alcohol Use Disorders, Opiate Addiction and Drugs other than opioids. He is one of the founders and president of the International Collaboration of ADHD and Substance Abuse (ICASA).
Prof. Gabriele Fischer MD, PhD, completed her residency in psychiatry at Washington University and at the Medical University Vienna, where she serves at head of the Addiction clinic. For many decades she has worked as a consultant for the United Nations Office on Drugs and Crime, WHO and other international organizations, in addition to her duty as member of the scientific board of European monitoring center for drug & drug addiction. Prof. Fischer’s research is in the field of neuropsychopharmacology, with a focus on human rights and gender issues.
Prof., John Krystal is the Robert L. McNeil, MD, Jr., Professor of Translational Research, as well as a Professor of Psychiatry, Neuroscience, and Psychology and Chair of the Department of Psychiatry at the Yale University. He is also Chief of Psychiatry and Behavioral Health at Yale-New Haven Hospital. He is a graduate of the University of Chicago, Yale University School of Medicine, and the Yale Psychiatry Residency Training Program. He has researched extensively on the neurobiology and treatment of schizophrenia, alcoholism, PTSD, and depression. Notably, his laboratory discovered the rapid antidepressant effects of ketamine in humans. He is the Director of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) Center for the Translational Neuroscience of Alcoholism and the Clinical Neuroscience Division of the VA National Center for PTSD. Dr. Krystal is a member of the U.S. National Academy of Medicine and a Fellow of the American Association for the Advancement of Science. Currently, he is co-director of the Neuroscience Forum of the U.S. National Academies of Sciences, Engineering, and Medicine; and editor of the scientific journal Biological Psychiatry (IF=12.1). He has chaired the National Institute of Mental Health (NIMH) Board of Scientific Counselors and served on the national advisory councils for both NIMH and NIAAA.
Prof. Yossi Tam, DMD, PhD, received his B.Med.Sc., M.Sc., Ph.D. and D.M.D. from the Hebrew University of Jerusalem. He did his postdoctoral training at the National Institutes of Health (NIH), and in 2014 he moved to the Hebrew University to head the Obesity and Metabolism Laboratory at the Institute for Drug Research, where he focuses on targeting the endocannabinoid system for Obesity, Diabetes and the metabolic syndrome. Prof. Tam won major national and international grants (ERC, ISF, JDRF, BARD, GIF, EFSD, FPWR, TOS). He authored over 80 peer-reviewed papers in leading journals (such as Science, Nature Medicine, Cell Metabolism, JCI, PNAS, Hepatology, Gastroenterology, Molecular Metabolism, to name a few), and three book chapters, with 5,412 citations yielding an H-index of 36. In addition, Prof. Tam co-invented 14 patents that were commercialized to different pharmaceutical companies for further clinical development and testing. Prof. Tam also serves as the Director of the Hebrew University’s Multidisciplinary Center for Cannabinoid Research and a Scientific Advisory Board Member of several biotech companies, which develop a portfolio of non-psychoactive cannabinoid-modulating medicines for unmet market needs. Prof. Tam’s research projects over the past twenty years has crossed subjects, disciplines and methodologies, yet the main research interests remain focused on the different pathophysiological aspects of the endocannabinoid system. Having a clinical background with basic science training, he has always been interested in how science can directly improve people’s everyday lives. Thus, he has strived unceasingly to integrate his clinical curiosity and experimental knowledge, in order to deepen the understanding of clinically relevant research questions.
Fatima Cody Stanford, MD, MPH, MPA, is currently associated with Massachusetts General Hospital and is an Associate Professor of Medicine and Pediatrics at Harvard Medical School, where she serves as an educator, researcher, and policy maker. Her extensive academic background includes a Bachelor of Science and Master of Public Health from Emory University, a MD from the Medical College of Georgia School of Medicine, a Masters of Public Administration from the Harvard Kennedy School of Government and a MBA from the Quantic School of Business and Technology. Dr. Stanford completed her Obesity Medicine & Nutrition Fellowship at Massachusetts General Hospital and Harvard Medical School, further enhancing her expertise in the field. With a career dedicated to bridging the gaps between medicine, public health, and policy, Dr. Stanford has become a sought-after expert in obesity medicine, both nationally and internationally.
Prof. Henry R. Kranzler, MD, PhD, is the Benjamin Rush Professor of Psychiatry, and Director of the Center for Studies of Addiction, at the University of Pennsylvania’s Perelman School of Medicine. His research focuses on the genetics and pharmacological treatment of substance dependence, with emphasis on precision addiction medicine. His research has been continuously supported since 1987 by grants from the National Institutes of Health. Professor Kranzler has authored or co-authored more than 600 journal articles, book chapters, and books, is a member of the editorial board of three peer-review journals as well as the editor of Alcohol: Clinical and Experimental Research. His work currently focuses on the molecular genetics of substance dependence and the personalized treatment of alcohol, opioid, and nicotine use disorders using a pharmacogenetic approach.
Arrangements for Election of Directors and Members of Management
There are no arrangements or understandings with major shareholders, customers, suppliers or others pursuant to which any of our executive management or our directors were selected. See “Item 7.B. Related Party Transactions” for additional information.
Diversity of the Board of Directors
Board Diversity Matrix (As of January 29, 2024)
| Country of Principal Executive Offices | Canada | |||
| Foreign Private Issuer | Yes | |||
| Disclosure Prohibited under Home Country Law | No | |||
| Total Number of Directors | 4 |
| Part I: Gender Identity | Female | Male | Non- Binary | Did Not Disclose Gender |
||||||||
| Directors | 0 | 4 | 0 | 0 | ||||||||
| Part II: Demographic Background | ||||||||||||
| Underrepresented Individual in Home Country Jurisdiction | 0 | |||||||||||
| LGBTQ+ | 0 | |||||||||||
| Did Not Disclose Demographic Background | 0 | |||||||||||
B. Compensation
The following table presents in the aggregate all compensation we paid to all of our directors and senior management as a group for the year ended October 31, 2023. The table does not include any amounts we paid to reimburse any of such persons for costs incurred in providing us with services during this period.
All amounts reported in the table below reflect our cost, in United States dollars. Amounts paid in NIS are translated into United States dollars at the rate of NIS 3.6424= USD$1.00 for the year ended October 31, 2023 and NIS 3.2975 = USD$1.00 for the year ended October 31, 2022, based on the average representative rate of exchange between the NIS and the Unites States dollars as reported by the Bank of Israel.
| For the year ended October 31, |
||||||||
| 2023 | 2022 | |||||||
| Compensation of key management personnel of the Company: | ||||||||
| CEO management fees | $ | 284,194 | $ | 190,972 | ||||
| Share-based payment to CEO | 125,695 | 266,624 | ||||||
| CFO management fees | 90,278 | 116,765 | ||||||
| Share-based payment to CFO | 2,824 | 287,017 | ||||||
| Vice President of Business Development consulting fees | 35,594 | 35,167 | ||||||
| Share-based payment to Vice President of Business Development | (3,711 | ) | 5,684 | |||||
| Other related party transactions: | ||||||||
| Directors’ fees | 76,246 | 124,746 | ||||||
| Share-based payment to Directors | 11,038 | 43,485 | ||||||
| Chairman management fees | 106,015 | 17,584 | ||||||
| Share-based payment to Chairman | 110,943 | 204,223 | ||||||
| Other officer | - | 125,564 | ||||||
| Share-based payment to Other officer | - | 9,238 | ||||||
| All directors and officers as a group | $ | 839,116 | $ | 1,427,069 | ||||
For so long as we qualify as a foreign private issuer, we will not be required to comply with the proxy rules applicable to U.S. domestic companies regarding disclosure of the compensation of certain executive officers on an individual basis. Pursuant to applicable Canadian Securities Laws, we are required to disclose the annual compensation of each of the following:
| a) | the Company’s Chief Executive Officer (“CEO”); |
| b) | the Company’s Chief Financial Officer (“CFO”); |
| c) | the Company’s three most highly compensated executive officers, other than the CEO and CFO, who were serving as executive officers as at the applicable year end, and whose total compensation was more than $150,000 for the financial year ended; and |
| d) | each individual who would be a named executive officer under paragraph (c) but for the fact that the individual was neither an executive officer of the Company, of which there are none. |
This disclosure will not be as extensive as that required of a U.S. domestic issuer.
As of October 31, 2023, options to purchase 5,588 Common Shares were granted to our directors and executive officers under our Omnibus Equity Incentive Plan, or the Omnibus Plan. The options have at a weighted average exercise price of $434.81 per share. The following table sets forth information regarding options granted to our executive officers and directors during the year ended October 31, 2023 that are outstanding as of the date of this filing:
| Weighted Average | ||||||||||||
| Options | ||||||||||||
| Name | Grant Date | Stock Options |
Exercise Price (USD) |
Expiration Date | ||||||||
| Adi Zuloff Shani | May - July 2021 | 1,334 | $ | 715.50 | May - July 2026 | |||||||
| February 1, 2022 | 333 | $ | 504.00 | February 1, 2032 | ||||||||
| Amitay Weiss | May 26, 2021 | 67 | $ | 675.00 | May 26, 2026 | |||||||
| February 1, 2022 | 1,044 | $ | 720.00 | February 1, 2032 | ||||||||
| Yonatan Shachar | May 26, 2021 | 67 | $ | 675.00 | May 26, 2026 | |||||||
| Oz Adler | October 22, 2021 | 67 | $ | 702.00 | October 22, 2026 | |||||||
| February 1, 2022 | 67 | $ | 504.00 | February 1, 2032 | ||||||||
| Mark Haden | May 2, 2021 | 66 | $ | 675.00 | May 2, 2026 | |||||||
| February 1, 2022 | 67 | $ | 504.00 | February 1, 2032 | ||||||||
| Alan Rootenberg | May 26, 2021 | 67 | $ | 675.00 | May 26, 2026 | |||||||
Our compensation policies are based on the principles that compensation should, to a significant extent, be reflective of the financial performance of our executive officers and directors, and that a significant portion of executive officers’ and directors’ compensation should provide long-term incentives. The Board and Compensation Committee of the Board, or the Compensation Committee, seeks to have compensation of our directors and executive officers set at levels that are sufficiently competitive so that we may attract, retain and motivate highly qualified directors and executive officers to contribute to our success. In assessing the overall compensation for directors and executive officers, the Board and Compensation Committee considers the Company’s performance, relative stockholder return and industry position, general industry data, and awards given to our executive officers in past years. It is our general compensation philosophy to provide a blend of base salaries/consulting fees, incentive bonuses and equity-based compensation.
Elements of Compensation
Base Salary/Consulting Fees
Each executive officers receives a fee, which constitutes a significant portion of the executive officer’s compensation package. Consulting fees are paid for discharging day-to-day duties and responsibilities and reflects the executive officer’s performance over time, as well as that individual’s particular experience and qualifications. An executive officer’s fee is reviewed by the Compensation Committee from time to time.
Incentive Bonus
Incentive bonuses, in the form of cash payments, are designed to add a variable component of compensation based on corporate and individual performances for each officer and employee. Both individual and corporate performances are also taken into account. No bonuses were paid to our executive officers during the most recently completed financial year.
Equity-Based Compensation
Our directors, officers, employees and consultants are eligible under our Omnibus Plan to receive grants of stock options. The Omnibus Plan is an important part of our long-term incentive strategy for its officers and directors, permitting them to participate in appreciation of the market value of the Common Shares over a stated period of time. The Omnibus Plan is intended to reinforce commitment to long-term growth in profitability and shareholder value.
The Board believes that the Omnibus Plan aligns the interests of our executive officers and the Board with shareholders by linking a component of executive compensation to the longer term performance of the Common Shares.
Compensation Risk
The Board has not formally considered the implications of risks associated with our compensation policies and practices as, in their view, the current structure of our executive compensation arrangements is focused on long-term value and is designed to correlate to the long-term performance of the Company, which includes but is not limited to performance of its share price.
Financial Instruments
Except as may be prohibited by law, our executive officers and directors are not currently prohibited from purchasing financial instruments, such as prepaid variable forward contracts, equity swaps, collars or units of exchange funds, that are designed to hedge or offset a decrease in market value of equity securities granted as compensation or held, directly or indirectly, by an executive officer or director. To our knowledge, none of our executive officers or directors have entered into or purchased such a financial instrument. Our Insider Trading Policy stipulates that our insiders are prohibited from short-selling our securities for the purpose of realizing the short-term profits.
Share-based and option-based Awards
As discussed above, the Option Plan is maintained for our directors, officers, consultants and employees and any of our present and future subsidiaries. The CEO will make initial recommendations to the Compensation Committee on the setting of option grants, taking into account the seniority and contribution of the individuals eligible for the grants and the number of previously granted stock options. The Compensation Committee will then recommend to the Board for approval all incentive compensation for our executives, based on both individual and Company performance in any given year, and will take into consideration the levels of compensation paid to persons in the same or similar management positions at comparable companies, in making such recommendations.
Option-based Awards
Pursuant to the Omnibus Plan, an option exercise price cannot be less than the closing price of the Common Shares on the Exchange on the last trading day preceding the option grant. The purchase price for the Common Shares under each option shall be determined by the Compensation Committee. The maximum term is ten (10) years. There are no specific vesting provisions under the Omnibus Plan. Options are non-assignable and non-transferable other than by will or by the laws of descent and distribution. As at the date hereof, there are 5,521 stock options outstanding under the Omnibus Plan, which represents approximately 0.88% of the Common Shares reserved for issuance under the Omnibus Plan. Please see “Item 6.A. Directors, Senior Management and Employees – Share Ownership – Omnibus Equity Incentive Plan” below, for a summary of the Omnibus Plan.
Compensation Governance
Given the Corporation’s size and stage of operations, it has not appointed a Compensation Committee or formalized any guidelines with respect to compensation at this time. The amounts paid to our executive officers are determined by the independent Board members. The Board determines the appropriate level of compensation reflecting the need to provide incentive and compensation for the time and effort expended by the executives, while taking into account our financial and other resources.
Pension Plan Benefits
No benefits were paid, and no benefits are proposed to be paid to any of our executive officers under any pension or retirement plan.
Termination and Change of Control Benefits
No Termination or Change of control benefits were paid or are proposed to be paid to any of our executive officers.
Directors Compensation
For a description of the terms of our options and option plans, see “Item 6.A. Directors, Senior Management and Employees – Share Ownership –Stock Option Plan” below.
Directors’ Service Contracts
Other than with respect to our directors that are also executive officers, we do not have written agreements with any director providing for benefits upon the termination of his employment with our Company.
Differences between the BCBCA (“Canadian law”) and Nasdaq Requirements
| ● | Distribution of periodic reports to shareholders; proxy solicitation. As opposed to the Nasdaq Rules, or the Nasdaq Rules, which require listed issuers to make such reports available to shareholders in one of a number of specific manners, Canadian law does not require us to distribute periodic reports directly to shareholders, and the generally accepted business practice in Canada is not to distribute such reports to shareholders but to make such reports available through a public website. In addition to making such reports available on a public website, we currently make our audited financial statements available to our shareholders at our offices and will only mail such reports to shareholders upon request. |
| ● | Quorum. Under applicable Canadian law, a company is entitled to determine in its articles of association the number of shareholders and percentage of holdings required for a quorum at a shareholders meeting. Our articles of association provide that a quorum of is at least one person who is, or who represents by proxy, two or more shareholders who, in the aggregate, hold at least 5% of the issued shares entitled to be voted at the meeting, is required for commencement of business at a general meeting. However, the quorum set forth in our articles of association with respect to an adjourned meeting, if no quorum is present within half an hour of the time arranged, consists of at least two shareholder present in person or by proxy. |
| ● | Nomination of our directors. With the exception of directors elected by our Board of Directors, our directors are elected by an annual meeting of our shareholders to hold office until the next annual meeting following one year from his or her election. The nominations for directors, which are presented to our shareholders by our Board of Directors, are generally made by the Board of Directors itself, in accordance with the provisions of our articles of association and the Companies Law. Nominations need not be made by a nominating committee of our Board of Directors consisting solely of independent directors, as required under the Nasdaq Rules. |
| ● | Compensation of officers. Canadian law and our articles of association do not require that the independent members of our Board of Directors (or a Compensation Committee composed solely of independent members of our Board of Directors). Instead, compensation of executive officers is determined and approved by our Compensation Committee and our Board of Directors. |
| ● | Independent directors. Canadian law does not require that a majority of the directors serving on our Board of Directors be “independent,” as defined under Nasdaq Rule 5605(a)(2). |
| ● | We are required, however, to ensure that all members of our Audit Committee are “independent” under the applicable Nasdaq and SEC criteria for independence (as we cannot exempt ourselves from compliance with that SEC independence requirement, despite our status as a foreign private issuer), and we must also ensure that a majority of the members of our Audit Committee are “independent directors” as defined in Canadian Securities Administrators National Instrument 52-110 “Audit Committees”. Furthermore, Canadian law does not require, nor do our independent directors conduct, regularly scheduled meetings at which only they are present, which the Nasdaq Rules otherwise require. |
| ● | Approval of Related Party Transactions. All related party transactions are approved in accordance with the requirements and procedures for approval of interested party acts and transactions as set forth in applicable Canadian Securities Laws, which requires the approval of shareholders, as may be applicable, for specified transactions. |
C. Board Practices
Except as stated below, we intend to comply with the rules generally applicable to U.S. domestic companies listed on the Nasdaq. We may in the future decide to use other foreign private issuer exemptions with respect to some of the other Nasdaq listing requirements. Following our home country governance practices, as opposed to the requirements that would otherwise apply to a company listed on the Nasdaq, may provide less protection than is accorded to investors under the Nasdaq Rules applicable to U.S. domestic issuers.
The Canadian securities regulatory authorities have issued corporate governance guidelines pursuant to National Policy 58-201—Corporate Governance Guidelines, or the Corporate Governance Guidelines, or NP 58-201, together with certain related disclosure requirements pursuant to National Instrument 58-101—Disclosure of Corporate Governance Practices, or NI 58-101. The Corporate Governance Guidelines are recommended as “guidance” for issuers to follow. We recognize that good corporate governance plays an important role in our overall success and in enhancing shareholder value and, accordingly, we have adopted certain corporate governance policies and practices which reflect our consideration of the recommended Corporate Governance Guidelines.
The disclosure set out below includes disclosure required by NI 58-101 describing our approach to corporate governance in relation to the Corporate Governance Guidelines.
Composition of our Board
Under our articles of incorporation, our Board is to consist of three directors and up to that number which was last set by ordinary resolution of the shareholders. We currently have four directors and under the BCBCA, as a reporting issuer, we must have no fewer than three directors. Under the BCBCA, a director may be removed with or without cause, prior to expiration of the director’s term, by a resolution passed by at least two-thirds of the votes cast by shareholders present in person or by proxy at a meeting and who are entitled to vote. The directors are appointed at the annual general meeting of shareholders and the term of office for each of the directors will expire at the time of our next annual shareholders meeting. Our articles of incorporation provide that, between annual general meetings of our shareholders, the directors may appoint one or more additional directors, but the number of additional directors may not at any time exceed one-third of the number of directors who held office at the expiration of the last meeting of our shareholders. Under the BCBCA, there is no minimum number of directors required to be resident Canadians.
Director Term Limits and Other Mechanisms of Board Renewal
Our Board has not adopted director term limits or other automatic mechanisms of board renewal. Rather than adopting formal term limits, mandatory age-related retirement policies and other mechanisms of board renewal, the Nominating and Corporate Governance Committee of our Board will develop a skills and competencies matrix for our Board as a whole and for individual directors. The Nominating and Corporate Governance Committee will also conduct a process for the assessment of our Board, each committee and each director regarding his or her effectiveness and contribution, and will report evaluation results to our Board on a regular basis.
Director Independence under Canadian Regulation
Under NI 58-101, a director is considered to be independent if he or she is independent within the meaning of Section 1.4 of National Instrument 52-110—Audit Committees. Section 1.4 of NI 52-110 generally provides that a director is independent if he or she has no direct or indirect “material relationship” with the issuer which could, in the view of the issuer’s board of directors, be reasonably expected to interfere with the exercise of the director’s independent judgment. Notwithstanding the foregoing, the following are deemed as being in a material relationship: (a) an individual who is, or has been within the last three years, an employee or executive officer of the issuer; (b) an individual whose immediate family member is, or has been within the last three years, an executive officer of the issuer; (c) an individual who: (i) is a partner of a firm that is the issuer’s internal or external auditor, (ii) is an employee of that firm, or (iii) was within the last three years a partner or employee of that firm and personally worked on the issuer’s audit within that time; (d) an individual whose spouse, minor child or stepchild, or child or stepchild who shares a home with the individual: (i) is a partner of a firm that is the issuer’s internal or external auditor, (ii) is an employee of that firm and participates in its audit, assurance or tax compliance (but not tax planning) practice, or (iii) was within the last three years a partner or employee of that firm and personally worked on the issuer’s audit within that time; (e) an individual who, or whose immediate family member, is or has been within the last three years, an executive officer of an entity if any of the issuer’s current executive officers serves or served at that same time on the entity’s compensation committee; and (f) an individual who received, or whose immediate family member who is employed as an executive officer of the issuer received, more than $75,000 in direct compensation from the issuer during any 12 month period within the last three years.
Our Board has undertaken a review of the independence of each director. Based on information provided by each director concerning his or her background, employment and affiliations, our Board has determined that Yehonatan Shachar, Amitay Weiss, and Oz Adler, representing three of the four members of our Board, are “independent” as that term is defined under the Nasdaq Rules and NI 58-101. In making this determination, our Board considered the current and prior relationships that each non-employee director has with our Company and all other facts and circumstances our Board deemed relevant in determining their independence, including the beneficial ownership of our shares by each non-employee director.
Certain members of our Board are also members of the boards of other public companies. Our Board has not adopted a director interlock policy, but is keeping informed of other public directorships held by its members.
Mandate of the Board of Directors
Our Board is responsible for supervising the management of our business and affairs, including providing guidance and strategic oversight to management. Our Board will adopt a formal mandate that will include the following:
| ● | appointing our Chief Executive Officer; |
| ● | developing the corporate goals and objectives that our Chief Executive Officer is responsible for meeting and reviewing the performance of our Chief Executive Officer against such corporate goals and objectives; |
| ● | taking steps to satisfy itself as to the integrity of our Chief Executive Officer and other executive officers and that our Chief Executive Officer and other executive officers create a culture of integrity throughout the organization; |
| ● | reviewing and approving our code of conduct and reviewing and monitoring compliance with the code of conduct and our enterprise risk management processes; |
| ● | reviewing and approving management’s strategic and business plans and our financial objectives, plans and actions, including significant capital allocations and expenditures; and |
| ● | reviewing and approving material transactions not in the ordinary course of business. |
Meetings of Independent Directors
Our Board will hold regularly-scheduled quarterly meetings as well as ad hoc meetings from time to time. The independent members of our Board will also meet, from time to time as may be required, without the non-independent directors and members of management before or after each regularly scheduled Board meeting.
Monitoring Compliance with the Code of Conduct
Our Nominating and Corporate Governance Committee is responsible for reviewing and evaluating the code of conduct at least annually and recommending any necessary or appropriate changes to our Board for consideration. The Nominating and Corporate Governance Committee assists our Board with the monitoring of compliance with the code of conduct, and is responsible for considering any waivers therefrom (other than waivers applicable to members of the Nominating and Corporate Governance Committee, which shall be considered by the Audit Committee, or waivers applicable to our directors or executive officers, which shall be subject to review by our Board as a whole).
Requirement for Directors and Officers to Disclose Interest in a Contract or Transaction
In accordance with the BCBCA, each director and officer must disclose the nature and extent of any interest that he or she has in a material contract or material transaction whether made or proposed with us, if the director or officer is a party to the contract or transaction, is a director or an officer or an individual acting in a similar capacity of a party to the contract or transaction, or has a material interest in a party to the contract or transaction. Subject to certain limited exceptions under the BCBCA, no director may vote on a resolution to approve a material contract or material transaction which is subject to such disclosure requirement.
As of the date hereof, except as otherwise disclosed in this Annual Report on Form 20-F, to the knowledge of the Board or the management of the Company, there are no material interests, whether direct or indirect, of any informed person of the Company, any proposed director of the Company, or any associate or affiliate of any informed person or proposed director, in any transaction since the commencement of the Company’s most recently completed financial year or in any proposed transaction which has materially affected or would materially affect the Company of any of its subsidiaries.
Complaint Reporting
In order to foster a climate of openness and honesty in which any concern or complaint pertaining to a suspected violation of the law, our code of conduct or any of our policies, or any unethical or questionable act or behavior, our code of conduct will require that our employees promptly report the violation or suspected violation. In order to ensure that violations or suspected violations can be reported without fear of retaliation, harassment or an adverse employment consequence, we will adopt a whistleblowing policy which will contain procedures that are aimed to facilitate confidential, anonymous submissions of complaints by our directors, officers, employees and others.
Committees of the Board of Directors
Audit Committee
Our Audit Committee is currently comprised of Oz Adler, Yehonatan Shachar and Amitay Weiss, and is chaired by Oz Adler. Our Board has determined that each of the members of the committee are financially literate and meets the independence requirements for directors, including the heightened independence standards for members of the Audit Committee under Rule 10A-3 under the Exchange Act and NI 52-110. Our Board has determined that Oz Adler is “financially sophisticated” within the meaning of the Nasdaq Rules, “financially literate” within the meaning of NI 52-110, and a “financial expert” as defined by Rule 10A-3 under the Exchange Act. For a description of the education and experience of each member of the Audit Committee, see “Item 6.A. Directors, Senior Management and Employees.”
We have adopted an Audit Committee Charter setting forth the purpose, composition, authority and responsibility of the Audit Committee.
The role of the Audit Committee is to provide oversight of the Company’s financial management and of the design and implementation of an effective system of internal financial controls as well as to review and report to the Board on the integrity of the financial statements of the Company, its subsidiaries and associated companies. This includes helping directors meet their responsibilities, facilitating better communication between directors and the external auditor, enhancing the independence of the external auditor, increasing the credibility and objectivity of financial reports and strengthening the role of the directors by facilitating in-depth discussions among directors, management and the external auditor. Management is responsible for establishing and maintaining those controls, procedures and processes and the Committee is appointed by the Board to review and monitor them. The Company’s external auditor is ultimately accountable to the Board and the Committee as representatives of the Company’s shareholders.
The Committee’s primary duties and responsibilities are to:
| ● | Serve as an independent and objective party to monitor the Company’s financial reporting and internal control system and review Company’s financial statements; |
| ● | Review and appraise the performance of the Company’s external auditors; and |
| ● | Provide an open avenue of communication among the Company’s auditors, financial and senior management and the Board. |
The Audit Committee shall meet at least annually, or more frequently as circumstances dictate. As part of its job to foster open communication, the Audit Committee will meet at least annually with the external auditors.
To fulfill its responsibilities and duties, the Audit Committee shall:
| ● | Review and update the Audit Committee’s charter annually; |
| ● | Review the Company’s financial statements, Management Discussion & Analysis and any annual and interim earnings, press releases before the Company publicly discloses this information and any reports or other financial information (including quarterly financial statements), which are submitted to any governmental body, or to the public, including any certification, report, opinion, or review rendered by the external auditors; |
| ● | Review annually, the performance of the external auditors who shall be ultimately accountable to the Board and the Committee as representatives of the shareholders of the Company; |
| ● | Obtain annually, a formal written statement of external auditors setting forth all relationships between the extern auditors and the Company, consistent with Independence Standards Board Standard I; |
| ● | Review and discuss with the external auditors any disclosed relationships or services that may impact the objectivity and independence of the external auditors; |
| ● | Take, or recommend that the full Board take, appropriate action to oversee the independence of the external auditors; |
| ● | Recommend to the Board the selection and, where applicable, the replacement of the external auditors nominated annually for shareholder approval; |
| ● | Review and approve the Company’s hiring policies regarding partners, employees and former partners and employees of the present and former external auditors of the Company; |
| ● | Review and pre-approve all audit and audit-related services and the fees and other compensation related thereto; |
| ● | In consultation with the external auditors, review with management the integrity of the Company’s financial reporting process, both internal and external; |
| ● | Consider the external auditors’ judgments about the quality and appropriateness of the Company’s accounting principles as applied in its financial reporting; |
| ● | Consider and approve, if appropriate, changes to the Company’s auditing and accounting principles and practices as suggested by the external auditors and management; |
| ● | Review significant judgments made by management in the preparation of the financial statements and the view of the external auditors as to appropriateness of such judgments; |
| ● | Following completion of the annual audit, review separately with management and the external auditors any significant difficulties encountered during the course of the audit, including any restrictions on the scope of work or access to required information; |
| ● | Review any significant disagreement among management and the external auditors in connection with the preparation of the financial statements; |
| ● | Review with the external auditors and management the extent to which changes and improvements in financial or accounting practices have been implemented; |
| ● | Review any complaints or concerns about any questionable accounting, internal accounting controls or auditing matters; |
| ● | Review certification process; and |
| ● | Review any related-party transactions. |
Compensation Committee
Compensation committees are not mandatory in Canada. However, NP 58-201 recommends that a Board appoint a Compensation Committee composed entirely of independent directors with responsibilities for oversight of the compensation payable to senior executives. The members of the Compensation Committee are not required to be independent or to have any particular expertise.
Our Compensation Committee is comprised of Yehonatan Shachar, Amitay Weiss and Oz Adler, and is chaired by Amitay Weiss. The Compensation Committee is appointed by the board to assist in promoting a culture of integrity throughout the Company and to:
| ● | attract and retain skilled and experienced executives and senior managers; |
| ● | review and approve the structure and guidelines for various incentive compensation and benefit plans and recommend for the Board’s approval of said plans; |
| ● | monitor and review the performance of the executive officers of the Company; |
| ● | motivate executives and senior managers to achieve corporate objectives and create shareholder value; and |
| ● | encourage executives and senior managers to link their personal financial interest to those of the shareholders. |
The Board relies on the knowledge and experience of the members of the Compensation Committee to set appropriate levels of compensation for senior officers. Neither the Company nor the Compensation Committee currently has, or has had at any time since incorporation, any contractual arrangement with any executive compensation consultant who has a role in determining or recommending the amount or form of senior officer compensation.
When determining compensation payable, the Compensation Committee may consider both external and internal data. External data includes general markets conditions and well as information regarding compensation paid to directors, CEOs and CFOs of companies of similar size and at a similar stage of development in the industry. Internal data includes annual reviews of the performance of the directors, CEO and CFO in light of the Company’s corporate objectives and considers other factors that may have impacted the Company’s success in achieving its objectives.
Nominating and Corporate Governance Committee
Nominating and Corporate Governance Committees are not mandatory in Canada. However, NP 58-201 recommends that a board appoint a corporate governance committee composed entirely of independent directors with responsibility for overseeing the process for nominating directors for election by shareholders. The members of the corporate governance committee are not required to be independent or to have any particular expertise.
The Nominating and Corporate Governance Committee is appointed by the Board to assist in fulfilling its corporate governance responsibilities under applicable laws. The Nominating and Corporate Governance Committee is responsible for, among other things, developing the Company’s approach to governance issues and establishing sound corporate governance practices that are in the interests of shareholders and that contribute to effective and efficient decision-making, as well as identifying, reviewing and evaluating candidates to serve as directors of the Company.
Our Nominating and Corporate Governance Committee is currently comprised of Yehonatan Shachar, Oz Adler and Amitay Weiss, and is chaired by Yehonatan Shachar.
Exculpation, Insurance and Indemnification of Directors and Officers
Under the BCBCA, a company may indemnify: (i) a current or former director or officer of that company; (ii) a current or former director or officer of another corporation if, at the time such individual held such office, the corporation was an affiliate of the company, or if such individual held such office at the company’s request; or (iii) an individual who, at the request of the company, held, or holds, an equivalent position in another entity, or an indemnifiable person, against all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, reasonably incurred by him or her in respect of any civil, criminal, administrative or other legal proceeding or investigative action (whether current, threatened, pending or completed) in which he or she is involved because of that person’s position as an indemnifiable person, unless: (i) the individual did not act honestly and in good faith with a view to the best interests of such company or the other entity, as the case may be; or (ii) in the case of a proceeding other than a civil proceeding, the individual did not have reasonable grounds for believing that the individual’s conduct was lawful. A company cannot indemnify an indemnifiable person if it is prohibited from doing so under its articles or by applicable law. A company may pay, as they are incurred in advance of the final disposition of an eligible proceeding, the expenses actually and reasonably incurred by an indemnifiable person in respect of that proceeding only if the indemnifiable person has provided an undertaking that, if it is ultimately determined that the payment of expenses was prohibited, the indemnifiable person will repay any amounts advanced. Subject to the aforementioned prohibitions on indemnification, a company must, after the final disposition of an eligible proceeding, pay the expenses actually and reasonably incurred by an indemnifiable person in respect of such eligible proceeding if such indemnifiable person has not been reimbursed for such expenses, and was wholly successful, on the merits or otherwise, in the outcome of such eligible proceeding or was substantially successful on the merits in the outcome of such eligible proceeding. On application from an indemnifiable person, a court may make any order the court considers appropriate in respect of an eligible proceeding, including the indemnification of penalties imposed or expenses incurred in any such proceedings and the enforcement of an indemnification agreement.
We maintain insurance policies relating to certain liabilities that our directors and officers may incur in such capacity.
D. Employees.
See “Item 4.B. Business Overview—Employees.”
E. Share Ownership.
See “Item 7.A. Major Shareholders” below.
Omnibus Equity Incentive Plan
Our Omnibus Equity Incentive Plan, or the Omnibus Plan, was previously approved by the shareholders at our annual and special meeting on November 14, 2023. Pursuant to the Omnibus Plan, we are authorized to grant options or restricted share units (“RSUs” and together with “Options”, “Awards” or “Stock Awards”) to officers, directors, employees and consultants enabling them to acquire up to 20% of our issued and outstanding Common Shares (including Stock Awards under previous plans). The Awards can be granted for a maximum of 10 years and vest as determined by the Board. The Omnibus Plan replaces our Company’s previous plan. All Stock Awards will be issued under the Omnibus Plan.
Pursuant to the policies of the CSE, we are required to obtain shareholder approval of the Option Plan every three years because the Option Plan is a rolling maximum option plan whereby the maximum number of Common Shares that may be reserved for issuance and which can be purchased upon the exercise of all options granted under the Option Plan is fixed at 20% of the outstanding Common Shares from time to time. The next shareholder approval must be obtained prior to November 14, 2026.
Summary of the Omnibus Plan
Pursuant to the Omnibus Plan, the Board may grant Awards to eligible persons as determined by the Omnibus Plan. The aggregate number of Common Shares which may be made available for issuance under the Omnibus Plan will not exceed with respect to the number of Common Shares issuable pursuant to all Awards, 20% of the total number of issued and outstanding Common Shares from time to time.
The purpose of the Omnibus Plan is to advance the interests of the Company and its subsidiaries by (i) promoting a significant alignment between directors, officers, employees and consultants of the Company and its subsidiaries (“Awardees”) and the growth objectives of the Company; (ii) associating a portion of Awardees’ compensation with the performance of the Company over the long term; and (iii) attracting, motivating and retaining the critical Awardees to drive the business success of the Company.
The following is a summary of the principal terms of the Omnibus Plan, which is qualified in its entirety by reference to the text of the Omnibus Plan:
| ● | The aggregate number of Common Shares issuable pursuant to all Awards shall not exceed 20% of the issued and outstanding Common Shares at the time of granting Awards (on a non-diluted basis). |
| ● | Any increase in the issued and outstanding Common Shares will result in an increase in the available number of Common Shares issuable upon issuance of Awards granted under the Omnibus Plan, and any exercises of Options, or settlements of Awards other than Options, will make new grants of Options available under the Omnibus Plan, effectively resulting in a re-loading of the number of Options available to grant under the Omnibus Plan. If any Awards granted expire or terminate for any reason without having been exercised or settled in full, as applicable, the unissued shares subject thereto shall again be available for the purposes of the Omnibus Plan. |
| ● | Subject to the provisions of the Omnibus Plan and rules of the CSE, the Board or its delegate shall have authority to interpret the Omnibus Plan and all Award agreements entered into in connection with the grant of Awards under the Omnibus Plan, to define the terms used in the Omnibus Plan and in all Award agreements entered into thereunder, to prescribe, amend and rescind the terms of the Omnibus Plan and to make all other determinations necessary or advisable for the administration of the Omnibus Plan. |
| ● | The price per share at which any Common Share which is the subject of an Option may be purchased (the “Option Exercise Price”) will be established by the Board or its delegate, subject to the rules of the regulatory authorities having jurisdiction over the securities of the Company, provided that the Option Exercise Price shall not be less than the greater of the closing market prices of the underlying securities on (a) the trading day prior to the date of grant of the stock options, and (b) the date of grant of the stock options (all as more particularly described in the policies of the CSE). The term of each Option will be fixed by the Board or its delegate but may not exceed 10 years from the date of the grant. |
| ● | Options granted pursuant to the Omnibus Plan shall be exercisable at such times and on the occurrence of such events, and be subject to such restrictions and conditions, as the Board or its delegate shall in each instance approve, which need not be the same for each grant or for each Awardee. Without limiting the foregoing, the Board or its delegate may permit the exercise of an Option through either a cashless exercise mechanism or net exercise mechanism pursuant to the terms of the Omnibus Plan and subject to the rules of the CSE. |
| ● | Awards may be granted to Awardees as compensation for employment or consulting services or services as a director or officer and may entitle Awardees to receive, for no additional cash consideration, Common Shares upon specific time or other vesting conditions being met as determined by the Board or its delegate. The value of Awards is influenced by the fair market value of the underlying Common Shares, as determined by the Board or its delegate, pursuant to the terms of the Omnibus Plan. |
| ● | If the expiry date, redemption date, or settlement date, as applicable, of any Award, would otherwise occur in a blackout period, the expiry date shall be extended to the tenth business day following the last day of the blackout period, where “blackout period” means a period of time during which the Company prohibits Awardees from exercising, redeeming or settling their Awards, due to applicable law or policies of the Company. |
| ● | The maximum number of Common Shares which may be issued to any one Awardee, who is a Related Party (as such term is defined in the Omnibus Plan) may not exceed: (a) within any 12-month period under the Omnibus Plan is 5% of the number of Common Shares outstanding (on a fully-diluted basis) from time to time; and (b) at any time under the Omnibus Plan is 5% of the Common Shares outstanding on a fully-diluted basis from time to time, in each case unless shareholder approval is obtained pursuant to the Regulatory Rules (as such term is defined in the Omnibus Plan). |
| ● | The maximum number of Common Shares which may be issuable to all Investor Relations Service Providers (as defined in the Omnibus Plan) within any 12-month period under the Omnibus Plan shall not exceed 2% of the number of Common Shares outstanding. |
| ● | The maximum number of Common Shares which may be issuable to all Related Persons at any time under the Omnibus Plan shall not exceed (a) 15% of the Common Shares outstanding on a fully-diluted basis from time to time; and (b) within any 12 month period under the Omnibus Plan 15% of the number of outstanding Common Shares on a fully-diluted basis from time to time, in each case unless shareholder approval is obtained pursuant to the Regulatory Rules (as such term is defined in the Omnibus Plan). As the aforementioned 15% threshold exceeds 10% then, in accordance with the requirements of the CSE and Canadian Securities Administrators National Instrument 45-106—Prospectus Exemptions, the Company must obtain approval of Shareholders excluding votes attached to Common Shares held by directors, officers or holders of at least ten percent (10%), and their Associates (as such term is defined in the Omnibus Plan) of the issued and outstanding Common Shares (collectively, the “Interested Shareholders”). This means that Interested Shareholders are not eligible to vote their securities in respect of the Omnibus Plan Resolutions. As such, an aggregate of 36,716 Common Shares will not be eligible to vote on the Omnibus Plan Resolutions. |
| ● | No financial assistance or support agreement will be provided to any Awardee by the Company or any related entity of the Company to facilitate the purchase of Awards. |
| ● | In the event of death, or disability, of an Awardee, unless otherwise determined by the Board or its delegate, (i) the executor or administrator of the Awardee’s estate may exercise any vested Options for a period until the earlier of the original expiry date and 12 months after the date of death, and any unvested Options shall terminate and become void on the date of death; and (ii) any unvested RSUs previously credited to the Awardee’s account will be cancelled, and vested RSUs will be paid to the Awardee’s estate, with any settlement or redemption to occur within 12 months following the termination date. |
| ● | Except as may otherwise be provided in an Awardee’s employment agreement or as otherwise determined by the Board or its delegate, if an Awardee’s employment or other relationship with the Company is terminated for any reason other than death or disability, (i) each vested Option held by that Awardee will cease to be exercisable on the earlier of the original expiry date and 90 days after the termination date; and (ii) any RSUs held by the Awardee that have vested before the termination date will remain with the Awardee. In all cases, any unvested Options or RSUs held by the Awardee shall terminate and become void on the date of termination. |
| ● | Unless otherwise determined by the Board or its delegate, where an Awardee is terminated for cause, any unvested Options or RSUs held by the Awardee will be immediately cancelled and forfeited to the Company for no consideration. |
| ● | In the event of a change of control (as defined in the Omnibus Plan), unless otherwise provided in the Omnibus Plan or an Award agreement, the Board or its delegate may deal with any or all outstanding Awards (or any portion thereof) in the manner it deems fair and reasonable in the circumstances of the change of control, including but not limited to cancelling all outstanding awards with or without payment or accelerating vesting and/or expiry of outstanding Awards and/or cause all Awards or portions thereof to become exchanged for stock awards of another Company. |
| ● | Unless restricted by law or CSE policies, the Board or its delegate may alter, amend, modify, suspend or terminate the Omnibus Plan or any Award in whole or in part without notice to, or approval from, Shareholders, including, but not limited to, for the purposes of: |
| ● | making any amendments to the general vesting provisions of any Award; |
| ● | making any amendments to the general term of any Award as permitted by the Omnibus Plan; |
| ● | making any amendments to add covenants or obligations of the Company for the protection of Awardees; |
| ● | making any amendments not inconsistent with the Omnibus Plan as may be necessary or desirable with respect to matters or questions which, in the good faith opinion of the Board, it may be expedient to make, including amendments that are desirable as a result of changes in law or as a “housekeeping” matter; or |
| ● | making such changes or corrections which are required for the purpose of curing or correcting any ambiguity or defect or inconsistent provision or clerical omission or mistake or manifest error. |
| ● | Shareholder approval is required to make the following amendments to the Omnibus Plan: |
| o | a reduction in the Option Exercise Price of a previously granted Option benefitting a Related Person or one of his/her/its Affiliates (unless done pursuant to Section 11.2 of the Omnibus Plan); |
| o | any amendment or modification which would increase the total number of Common Shares available for issuance under the Omnibus Plan; or |
| o | an increase to the limit on the number of Common Shares issued or issuable under the Omnibus Plan to Insiders of the Company. |
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
A. Major Shareholders
The following table sets forth information with respect to the beneficial ownership of our ordinary Common Shares as of October 31, 2023 by:
| ● | each person or entity known by us to own beneficially 5% or more of our outstanding Common Shares; |
| ● | each of our directors and executive officers individually; and |
| ● | all of our directors and executive officers as a group. |
The beneficial ownership of our Common Shares is determined in accordance with the rules of the SEC and generally includes any shares over which a person exercises sole or shared voting or investment power, or the right to receive the economic benefit of ownership. For purposes of the table below, we deem options and RSUs that are currently exercisable or exercisable within 60 days of October 31, 2023 to be outstanding and to be beneficially owned by the person holding the options for the purposes of computing the percentage ownership of that person, but we do not treat them as outstanding for the purpose of computing the percentage ownership of any other person. Percentage of shares beneficially owned is based on 607,337 Common Shares issued and outstanding as of October 31, 2023.
As of January 17, 2024, there were 51 holders of record of our Common Shares, out of which 4 holders of record had a registered address in the United States. We have also set forth below information known to us regarding any significant change in the percentage ownership of our Common Shares by any major shareholders during the past three years. Except where otherwise indicated, we believe, based on information furnished to us by such owners, that the beneficial owners of the Common Shares listed below have sole investment and voting power with respect to such Common Shares.
All of our shareholders, including the shareholders listed below, will have the same voting rights attached to their Common Shares, and neither our principal shareholders nor our directors and executive officers will have different or special voting rights with respect to their Common Shares. A description of any material relationship that our principal shareholders have had with us or any of our predecessors or affiliates within the past three years is included under “Related Party Transactions.”
Unless otherwise noted below, the address of each shareholder, director and executive officer is c/o Clearmind Medicine Inc., 101 – 1220 West 6th Avenue, Vancouver, British Columbia, V6H1A5.
| Name of beneficial owner | Common Shares beneficially owned |
Percentage of Common Shares beneficially owned |
||||||
| 5% or Greater Shareholders | ||||||||
| More Provident Fund (1) | 3,333 | * | % | |||||
| SciSparc Ltd | 7,692 | * | % | |||||
| Directors and Executive Officers | ||||||||
| Yonatan Shachar | 67 | * | % | |||||
| Amitay Weiss | 1,111 | * | % | |||||
| Alan Rootenberg | 67 | * | % | |||||
| Oz Adler | 245 | * | % | |||||
| Adi Zuloff Shani | 1,667 | * | % | |||||
| Mark Haden | 133 | * | % | |||||
| All directors and executive officers as a group (6 persons) | 3,290 | * | % | |||||
| * | Indicates beneficial ownership of less than 1% of the total Common Shares outstanding. |
| (1) | More Provident Fund is an Israeli company for which Lior Greenhouse holds investment and voting control over the entity. The address of More Provident Fund is Ben Gurion Road 2, Ramat Gan, 5257334, Israel. |
Record Holders
As of January 17, 2024, there were 51 holders of record of our Common Shares, out of which 4 holders of record had a registered address in the United States. These numbers are not representative of the number of beneficial holders of our shares nor is it representative of where such beneficial holders reside, since many of these shares were held of record by brokers or other nominees.
The Company is not controlled by another corporation, by any foreign government or by any natural or legal persons except as set forth herein, and there are no arrangements known to the Company which would result in a change in control of the Company as a subsequent date.
B. Related Party Transactions
The following is a description of the material terms of those transactions with related parties to which we, or our subsidiaries, are party.
Private Placements of our Securities
| ● | On April 16, 2021, we announced the closing of a private placement offering, or the April 2021 Offering, with certain investors, including one of our directors and our former Chief Financial Officer, for gross proceeds of CAD$1,500,000. We issued 11,111 Common Shares at a price of CAD$135.00 per Common Share. A finder’s fee equal to 7.5% of the gross proceeds was paid in connection with the April 2021 Offering with net proceeds to us being $1,387,500. The April 2021 Offering did not result in any new holders of 10% or more of the Common Shares. |
| ● | On April 22, 2021, we announced the closing of a private placement warrant offering, or the Warrant Offering, with certain investors, including one of our directors and our former Chief Financial Officer, for gross proceeds of CAD$375,000. We issued 8,333 Warrants at a price of CAD$45.00 per Warrant. Each Warrant is exercisable into one Common Share of the Company at CAD$135.00 per Common Share until April 22, 2024. A finder’s fee equal to 7.9% of the gross proceeds was paid in connection with the Warrant Offering with net proceeds to us of CAD$345,500. The Warrant Offering did not result in any new holders of 10% or more of the Common Shares. |
| ● | On June 22, 2021, we closed a private placement for gross proceeds of CAD$6,225,000, with certain investors, including one of our 5% holders. We issued 9,222 units of the Company at a price of C CAD$675.00 per unit for gross proceeds of CAD$6,225,000. Each unit consisted of one common share and one Common Share purchase warrant, with each such warrant entitling its holder to acquire one additional Common Share at an exercise price of CAD$1,125.00 per Warrant Share until December 22, 2022. We paid CAD$618,000 in finders’ fees relating to this private placement. |
Cooperation Agreement with SciSparc Ltd.
On March 7, 2022, we entered into a cooperation agreement with SciSparc, pursuant to which we will explore with SciSparc the scientific and commercial potential for creating pharmaceutical solutions for several indications including CNS related indications. Under such agreement, the companies are exploring a combined molecule that the parties have created that is undergoing a joint pre-clinical study. To date, the collaboration has resulted in the filing of nine patent applications. To the extent the parties determine to proceed to a commercial cooperation, they will enter into a joint venture where the parties share the economics and rights on a 50%-50% basis. To date, no determination has been made to pursue the joint venture and the development of the molecule remains in a very early stage.
Adi Zuloff-Shani, our Chief Executive Officer, serves as SciSparc’s Chief Technologies Officer; Amitay Weiss, the Chairman of our Board of Directors, serves as the Chairman of the Board of Directors of SciSparc; and Oz Adler, one of our directors, serves as the Chief Executive Officer and Chief Financial Officer of SciSparc.
In June 2023, we announced that, as part of our ongoing collaboration with SciSparc, we entered into a research agreement with the Hebrew University of Jerusalem to evaluate our and SciSparc’s combination treatment for obesity and metabolic syndrome.
Agreements and Arrangements With, and Compensation of, Directors and Executive Officers
We have entered into written employment agreements or service agreements with each of our executive officers. All of these agreements contain customary provisions regarding noncompetition, confidentiality of information and assignment of inventions. See “Item 6.A. Directors and Senior Management—Compensation.”
Indemnification Agreements
Our articles of association provide that we may indemnify any of our directors, former directors, officers or former officers, any other person and his or her heirs and legal personal representatives against all eligible penalties to which such person is or may be liable, and we may, after the final disposition of an eligible proceeding, pay the expenses actually and reasonably incurred by such person in respect of that proceeding. Each of our directors and officers is deemed to have contracted with us on terms of the indemnity contained in our articles of association. In addition, we may indemnify any other person in accordance with the BCBCA.
We also have entered and intend to enter into separate indemnification agreements with our directors and executive officers, in addition to indemnification provided for in our articles of association. These agreements, among other things, provide for indemnification of our directors and executive officers for expenses, judgments, fines and settlement amounts incurred by such persons in any action or proceeding arising out of this person’s services as a director or executive officer or at our request. We believe that these provisions in our articles of association and indemnification agreements are necessary to attract and retain qualified persons as directors and executive officers.
C. Interests of Experts and Counsel
Not applicable.
ITEM 8. FINANCIAL INFORMATION.
A. Consolidated Statements and Other Financial Information.
See “Item 18. Financial Statements.”
Legal Proceedings
See “Item 4.B. Business Overview—Legal Proceedings.”
Dividend Policy
We have never declared or paid any cash dividends to our shareholders of our Common Shares, and we do not anticipate or intend to pay cash dividends in the foreseeable future. Payment of cash dividends, if any, in the future will be at the discretion of our Board, in compliance with applicable legal requirements and will depend on a number of factors, including future earnings, our financial condition, operating results, contractual restrictions, capital requirements, business prospects, our strategic goals and plans to expand our business, applicable law and other factors that our Board may deem relevant.
The BCBCA imposes further restrictions on our ability to declare and pay dividends.
Payment of dividends may be subject to Canadian withholding taxes. See “Item 10.E. Taxation—Material Canadian Federal Income Tax Considerations” for additional information.
B. Significant Changes
Other than as otherwise described in this Annual Report on Form 20-F and as set forth below, no significant change has occurred in our operations since the date of our financial statements included in this Annual Report on Form 20-F.
ITEM 9. THE OFFER AND LISTING
A. Offer and Listing Details
Our Common Shares are currently traded on the Nasdaq Capital Market under the symbols “CMND” and on the Canadian Securities Exchange, or CSE under the symbol “CMND”. Our Common Shares also trade on the Frankfurt Stock Exchange under the symbol “CWY”.
B. Plan of Distribution
Not applicable.
C. Markets
Our common shares are listed on the Nasdaq Capital Market, the Canadian Securities Exchange and the Frankfurt Stock Exchange.
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
F. Expenses of the Issue
Not applicable.
ITEM 10. ADDITIONAL INFORMATION
A. Share Capital
Not applicable.
B. Articles of Association
A copy of our Articles of Association is attached as Exhibit 1.1 to this Annual Report. Other than as disclosed below, the information called for by this Item is set forth in Exhibit 1.1 to this Annual Report and is incorporated by reference into this Annual Report.
C. Material Contracts
We have not entered into any material contracts other than in the ordinary course of business and other than those described in Item 4. “Information on Our Company,” Item 7B “Major Shareholders and Related Party Transactions – Related Party Transactions” or elsewhere in this Annual Report.
D. Exchange Controls
We are not aware of any governmental laws, decrees, regulations or other legislation in Canada that restrict the export or import of capital, including the availability of cash and cash equivalents for use by our affiliated companies, or that affect the remittance of dividends, interest or other payments to non-resident holders of our securities. Any remittances of dividends to residents of the United States and to other non-resident holders are, however, subject to withholding tax.
E. Taxation.
The following description is not intended to constitute a complete analysis of all tax consequences relating to the acquisition, ownership and disposition of our common shares. You should consult your own tax advisor concerning the tax consequences of your particular situation, as well as any tax consequences that may arise under the laws of any state, local, foreign, or other taxing jurisdiction.
MATERIAL CANADIAN FEDERAL INCOME TAX CONSIDERATIONS
The following summary describes, as of the date hereof, the principal Canadian federal income tax considerations under the Income Tax Act (Canada) (the “Tax Act”) generally applicable to a holder who acquires, as beneficial owner, Common Shares, who has not elected to report its Canadian tax results in a currency other than the Canadian currency, and who deals at arm’s length with us and the underwriters for purposes of the Tax Act (a “Holder”).
This summary is based on the provisions of the Tax Act and the regulations thereunder (the “Regulations”) in force as of the date hereof, all specific proposals to amend the Tax Act and the Regulations that have been publicly announced prior to the date hereof (the “Proposed Amendments”), and our understanding of the current published administrative policies and practices of the Canada Revenue Agency. This summary assumes that the Proposed Amendments will be enacted in the form proposed; however, no assurance can be given that the Proposed Amendments will be enacted in the form proposed, if at all. This summary is not exhaustive of all possible Canadian federal income tax considerations and, except for the Proposed Amendments, does not take into account any changes in law, whether by legislative, governmental or judicial action, nor does it take into account provincial, territorial or foreign tax considerations, which may differ from those discussed herein.
This summary is of a general nature only and is not intended to be, nor should it be construed to be, legal or tax advice to any particular Holder, and no representations with respect to the income tax consequences to any Holder are made. Consequently, Holders and prospective holders of Common Shares should consult their own tax advisors for advice with respect to the tax consequences to them of acquiring such shares, having regard to their particular circumstances. This summary does not address any tax considerations applicable to persons other than Holders and such persons should consult their own tax advisors regarding the consequences of acquiring, holding and disposing of Common Shares under the Tax Act and any jurisdiction in which they may be subject to tax.
Foreign Exchange
For purposes of the Tax Act, all amounts expressed in a currency other than Canadian dollars relating to the acquisition, holding or disposition of Common Shares, including dividends, adjusted cost base and proceeds of disposition, must be determined in Canadian dollars using the relevant rate of exchange required under the Tax Act.
Residents of Canada
The following portion of this summary is generally applicable to a Holder who, at all relevant times for purposes of the Tax Act (a) is, or is deemed to be, resident in Canada, (b) holds Common Shares as “capital property”, and (c) is not affiliated with us or the underwriters (a “Resident Holder”). Generally, Common Shares will be considered to be capital property to a Resident Holder unless they are held in the course of carrying on a business or as part of an adventure or concern in the nature of trade. Certain Resident Holders whose Common Shares do not otherwise qualify as capital property may, in certain circumstances, make an irrevocable election in accordance with subsection 39(4) of the Tax Act to have their Common Shares and every other “Canadian security” (as defined in the Tax Act) owned by such holder in the taxation year of the election and in all subsequent taxation years deemed to be capital property. Resident Holders are advised to consult their own tax advisors to determine whether such an election is available and desirable in their particular circumstances.
This summary is not applicable to a Resident Holder: (i) that is a “financial institution” for the purposes of the “mark-to-market” rules contained in the Tax Act; (ii) that is a “specified financial institution”; (iii) an interest in which would be a “tax shelter investment”; or (iv) that enters into a “derivative forward agreement” in respect of Common Shares, as each of those terms is defined in the Tax Act. This summary does not address the possible application of the “foreign affiliate dumping” rules that may be applicable to a Resident Holder that is a corporation resident in Canada (for the purposes of the Tax Act) and is, or becomes, or does not deal at arm’s length with a corporation resident in Canada that is, or that becomes, as part of a transaction or event or series of transactions or events that includes the acquisition of the Common Shares, controlled by a non-resident corporation, individual, trust or a group of any combination of non-resident individuals, trusts, and/or corporations who do not deal with each other at arm’s length for purposes of the rules in section 212.3 of the Tax Act. Any such Resident Holder should consult its own tax advisor with respect to an investment in Common Shares.
Dividends
In the case of a Resident Holder who is an individual (other than certain trusts), dividends received or deemed to be received on the Common Shares will be included in computing the Resident Holder’s income and will be subject to the gross-up and dividend tax credit rules that apply to taxable dividends received from taxable Canadian corporations. Provided that we make the appropriate designations, such dividend will be treated as an “eligible dividend” for the purposes of the Tax Act and a Resident Holder who is an individual will be entitled to an enhanced dividend tax credit in respect of such dividend. There may be limitations on our ability to designate dividends and deemed dividends as eligible dividends.
Dividends received or deemed to be received on the Common Shares by a Resident Holder that is a corporation will be required to be included in computing the corporation’s income for the taxation year in which such dividends are received, but such dividends will generally be deductible in computing the corporation’s taxable income. In certain circumstances, subsection 55(2) of the Tax Act will treat a taxable dividend received by a Resident Holder that is a corporation as proceeds of disposition or a capital gain. Resident Holders that are corporations should consult their own tax advisors having regard to their own circumstances.
A Resident Holder that is a “private corporation” or a “subject corporation” (each as defined in the Tax Act) may be liable under Part IV of the Tax Act to pay a refundable tax on dividends received or deemed to be received on the Common Shares to the extent that such dividends are deductible in computing the Resident Holder’s taxable income for the taxation year.
Dividends received by a Resident Holder who is an individual (including certain trusts) may result in such Resident Holder being liable for alternative minimum tax under the Tax Act. Resident Holders who are individuals should consult their own tax advisors in this regard.
Dispositions of Common Shares
A disposition or deemed disposition of a Common Share by a Resident Holder will generally result in the Resident Holder realizing a capital gain (or capital loss) equal to the amount by which the proceeds of disposition of the Common Share, net of any reasonable costs of disposition, are greater (or less) than the Resident Holder’s adjusted cost base of the Common Shares. Such capital gain (or capital loss) will be subject to the tax treatment described below under “—Taxation of Capital Gains and Capital Losses.”
The adjusted cost base to the Resident Holder of a voting share acquired will, at any particular time, be determined in accordance with certain rules in the Tax Act by averaging the cost of such share with the adjusted cost base of all Common Shares owned by the Resident Holder as capital property at that time, if any.
Taxation of Capital Gains and Capital Losses
Generally, one-half of any capital gain (a “taxable capital gain”) realized by a Resident Holder in a taxation year must be included in computing the Resident Holder’s income for the year, and one-half of any capital loss (an “allowable capital loss”) realized by a Resident Holder in a taxation year must be deducted from taxable capital gains realized by the Resident Holder in that year. Allowable capital losses for a taxation year in excess of taxable capital gains for that year generally may be carried back and deducted in any of the three preceding taxation years or carried forward and deducted in any subsequent taxation year against net taxable capital gains realized in such years, to the extent and under the circumstances described in the Tax Act.
The amount of any capital loss realized by a Resident Holder that is a corporation on the disposition of a Common Share may be reduced by the amount of any dividends received or deemed to have been received on such Common Share (or on a share for which such Common Share has been substituted) to the extent and under the circumstances described in the Tax Act. Analogous rules apply to a partnership or trust of which a corporation, trust or partnership is a member or beneficiary. Resident Holders should consult their own tax advisors in this regard.
Taxable capital gains realized by a Resident Holder who is an individual (including certain trusts) may give rise to liability for alternative minimum tax as calculated under the detailed rules set out in the Tax Act. A Resident Holder that is a “Canadian-controlled private corporation” (as defined in the Tax Act) may be liable to pay an additional refundable tax on certain investment income, including taxable capital gains.
Eligibility for Investment
Following the closing of the offering, the Common Shares become qualified investments under the Tax Act and the regulations thereunder for trusts governed by registered retirement savings plans, registered retirement income funds, registered education savings plans, registered disability savings plans, tax-free savings accounts (collectively “Registered Plans”) and deferred profit sharing plans, or DPSPs, all as defined in the Tax Act.
Notwithstanding the foregoing, the holder of, subscriber or annuitant under, a Registered Plan (the “Controlling Individual”) will be subject to a penalty tax in respect of Common Shares acquired by the Registered Plan if such shares are a prohibited investment for the particular Registered Plan. A Common Share generally will not be a “prohibited investment” for a Registered Plan provided the Controlling Individual deals at arm’s length with the Company for the purposes of the Tax Act and the Controlling Individual does not have a “significant interest” (as defined in subsection 207.01(4) the Tax Act) in the Company.
Prospective investors who intend to hold Common Shares in a Registered Plan or DPSP are advised to consult their personal tax advisors.
Non-Residents of Canada
The following portion of this summary is generally applicable to a Holder who, at all relevant times for purposes of the Tax Act and any applicable tax treaty or convention (a) is not, and is not deemed to be, resident in Canada, and (b) does not use or hold, and is not deemed to use or hold, Common Shares in the course of carrying on a business in Canada (a “Non-Resident Holder”). Special rules which are not discussed in this summary may apply to a Non-Resident Holder that is an insurer which carries on an insurance business in Canada and elsewhere.
Dividends
Dividends paid or credited or deemed to be paid or credited to a Non-Resident Holder by us on Common Shares are subject to Canadian withholding tax at the rate of 25% on the gross amount of the dividend unless such rate is reduced by the terms of an applicable tax treaty. For example, under the Canada – United States Tax Convention (1980), as amended (the “Treaty”), the rate of withholding tax on dividends paid or credited to a Non-Resident Holder who is a resident of the United States for purposes of the Treaty and who is fully entitled to the benefits of the Treaty (a “U.S. Holder”) is generally limited to 15% of the gross amount of the dividend (or 5% in the case of a U.S. Holder that is a company that beneficially owns at least 10% of our Common Shares). Non-Resident Holders should consult their own tax advisors to determine their entitlement to relief under any applicable income tax treaty.
Dispositions of Common Shares
A Non-Resident Holder will not be subject to tax under the Tax Act in respect of a capital gain realized on the disposition or deemed disposition of a voting share unless the voting share constitutes “taxable Canadian property” to the Non-Resident Holder for purposes of the Tax Act and the Non-Resident Holder is not entitled to relief under the terms of an applicable tax treaty between Canada and the Non-Resident Holder’s jurisdiction of residence.
Provided the Common Shares are listed on a “designated stock exchange”, as defined in the Tax Act (which currently includes the TSXV) at the time of disposition, the Common Shares will generally not constitute taxable Canadian property of a Non-Resident Holder at that time unless, at any time during the 60-month period immediately preceding the disposition, the following two conditions are satisfied: (i) (a) the Non-Resident Holder, (b) persons with whom the Non-Resident Holder did not deal at arm’s length for purposes of the Tax Act, (c) partnerships in which the Non-Resident Holder or a person described in (b) holds a membership interest directly or indirectly through one or more partnerships, or (d) any combination of the persons and partnerships described in (a) through (c), owned 25% or more of the issued shares of any class or series of our shares, and (ii) more than 50% of the fair market value of the Common Shares was derived directly or indirectly from one or any combination of: real or immovable property situated in Canada, “Canadian resource properties”, “timber resource properties” (each as defined in the Tax Act), and options in respect of, or interests in or for civil law rights in, such properties, whether or not the property exits. Notwithstanding the foregoing, the shares may also be deemed to be taxable Canadian property to a Non-Resident Holder under other provisions of the Tax Act.
Non-Resident Holders who may hold Common Shares as taxable Canadian property should consult their own tax advisors.
CERTAIN U.S. FEDERAL INCOME TAX CONSIDERATIONS
THE FOLLOWING SUMMARY IS NOT INTENDED TO BE, AND SHOULD NOT BE CONSIDERED TO BE, LEGAL OR TAX ADVICE. EACH U.S. HOLDER SHOULD CONSULT WITH HIS OR HER OWN TAX ADVISOR AS TO THE U.S. FEDERAL INCOME TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND SALE OF COMMON SHARES, INCLUDING THE EFFECTS OF APPLICABLE STATE, LOCAL, FOREIGN OR OTHER TAX LAWS AND POSSIBLE CHANGES IN THE TAX LAWS.
The following discussion summarizes certain U.S. federal income tax consequences to a “U.S. Holder” arising from the purchase, ownership and sale of the Common Shares. For this purpose, a “U.S. Holder” is a holder of Common Shares that is, for U.S. federal income tax purposes: (1) an individual citizen or resident of the United States; (2) a corporation (or entity treated as a corporation for U.S. federal income tax purposes) created or organized under the laws of the United States or the District of Columbia or any political subdivision thereof; (3) an estate, the income of which is includable in gross income for U.S. federal income tax purposes regardless of its source; or (4) a trust if (A) a court within the United States is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have authority to control all substantial decisions of the trust, or (B) the trust has a valid election in effect to be treated as a U.S. person to the extent provided in U.S. Treasury regulations.
This discussion does not purport to be a comprehensive description of all of the U.S. federal income tax considerations that may be relevant to a decision to purchase our Common Shares. This summary generally considers only U.S. Holders that will own our Common Shares as capital assets and does not consider the U.S. federal income tax consequences to a person that is not a U.S. Holder, nor does it describe the rules applicable to determine a taxpayer’s status as a U.S. Holder. This summary is based on the provisions of the Internal Revenue Code of 1986, as amended, or the Code, final, temporary and proposed U.S. Treasury regulations promulgated thereunder, administrative and judicial interpretations thereof, and the income tax treaty currently in force between the United States and Canada, or the Treaty, all as in effect as of the date hereof and all of which are subject to change, possibly on a retroactive basis, and all of which are open to differing interpretations. We will not seek a ruling from the Internal Revenue Service, or the IRS, with regard to the U.S. federal income tax treatment of an investment in our Common Shares by U.S. Holders and, therefore, can provide no assurances that the IRS will agree with the conclusions set forth below.
This discussion does not address all aspects of U.S. federal income taxation that may be relevant to a particular U.S. Holder based on such U.S. Holder’s particular circumstances, such as the alternative minimum tax and the net investment income tax, and does not discuss any estate, gift, generation-skipping transfer, state, local, excise or non-U.S. tax considerations. In addition, this discussion does not address the U.S. federal income tax treatment of a U.S. Holder who is subject to special rules under the Code, including: (1) a bank, insurance company, regulated investment company, or other financial institution; (2) a broker or dealer in securities or foreign currency; (3) a person who acquired our Common Shares in connection with employment or other performance of services; (4) a U.S. Holder that holds our Common Shares as a hedge or as part of a hedging, straddle, conversion or constructive sale transaction or other risk-reduction transaction for U.S. federal income tax purposes; (5) a tax-exempt organization, qualified retirement plan, individual retirement account or other tax-deferred account; (6) real estate investment trusts or grantor trusts; (7) a U.S. expatriate or a former long-term resident of the United States; or (8) a person having a functional currency other than the U.S. dollar. This discussion does not address the U.S. federal income tax treatment of a U.S. Holder that owns, directly, indirectly or constructively, at any time, Common Shares representing 10% or more of the stock of our Company. Additionally, the U.S. federal income tax treatment of partnerships (or other pass-through entities) or persons who hold Common Shares through a partnership or other pass-through entity are not addressed.
EACH PROSPECTIVE INVESTOR IS ADVISED TO CONSULT HIS OR HER OWN TAX ADVISER FOR THE SPECIFIC TAX CONSEQUENCES TO THAT INVESTOR OF PURCHASING, HOLDING OR DISPOSING OF OUR COMMON SHARES, INCLUDING THE EFFECTS OF APPLICABLE STATE, LOCAL, FOREIGN OR OTHER TAX LAWS AND POSSIBLE CHANGES IN THE TAX LAWS.
Taxation of Dividends Paid on Common Shares
We do not intend to pay dividends in the foreseeable future. In the event that we do pay dividends, and subject to the discussion under the heading “Passive Foreign Investment Company Rules” below and the discussion of “qualified dividend income” below, a U.S. Holder will be required to include in gross income as ordinary income the amount of any distribution paid on the Common Shares (including the amount of any Canadian tax withheld on the date of the distribution), to the extent that such distribution does not exceed our current and accumulated earnings and profits, as determined for U.S. federal income tax purposes. The amount of a distribution that exceeds our earnings and profits will be treated first as a non-taxable return of capital, reducing the U.S. Holder’s tax basis for the Common Shares to the extent thereof, and then as capital gain. We do not expect to maintain calculations of our earnings and profits under U.S. federal income tax principles and, therefore, U.S. Holders should expect that the entire amount of any distribution generally will be reported as dividend income.
In general, preferential tax rates for “qualified dividend income” and long-term capital gains are applicable for U.S. Holders that are individuals, estates or trusts. For this purpose, “qualified dividend income” means, inter alia, dividends received from a “qualified foreign corporation,” provided that certain holding-period requirements and other conditions are satisfied. A “qualified foreign corporation” is a corporation that is entitled to the benefits of a comprehensive tax treaty with the United that the Secretary of Treasury of the United States determines is satisfactory for purposes of this provision and which includes an exchange of information program. The IRS has stated that the Treaty satisfies this requirement and we believe we are eligible for the benefits of the Treaty. In addition, our dividends will be qualified dividend income if our Common Shares are readily tradable on Nasdaq or another established securities market in the United States. However, we will not be a qualified foreign corporation if we are a passive foreign investment company, or PFIC, as described below under “Passive Foreign Investment Company Rules,” for the taxable year in which we pay a dividend or for the preceding taxable year.
Cash distributions paid by us in Canadian dollars will be included in the income of U.S. Holders at a U.S. dollar amount based upon the spot rate of exchange in effect on the date the dividend is includible in the income of the U.S. Holder, and U.S. Holders will have a tax basis in such Canadian dollars for U.S. federal income tax purposes equal to such U.S. dollar value. If the U.S. Holder subsequently converts the Canadian dollars into U.S. dollars or otherwise disposes of them, any subsequent gain or loss in respect of such Canadian dollars arising from exchange rate fluctuations will be U.S. source ordinary exchange gain or loss.
Subject to certain conditions and limitations, non-refundable withholding taxes (at a rate not in excess of any applicable tax treaty rate), if any, on dividends paid by us may be treated as foreign taxes eligible for credit against a U.S. Holder’s U.S. federal income tax liability under the U.S. foreign tax credit rules. However, as a result of recent changes to the U.S. foreign tax credit rules, a withholding tax generally will need to satisfy certain additional requirements in order to be considered a creditable tax for a U.S. Holder. We have not determined whether these requirements have been met and, accordingly, no assurance can be given that any withholding tax on dividends paid by us will be creditable. For purposes of calculating the U.S. foreign tax credit, dividends paid with respect to our Common Shares generally will be treated as foreign source income and as passive category income. In lieu of claiming a foreign tax credit, a U.S. Holder may deduct foreign taxes, including any foreign income tax imposed with respect to their Common Shares, in computing their taxable income, subject to generally applicable limitations under U.S. federal income tax law.
The rules relating to the determination of the foreign tax credit are complex, and each U.S. Holder should consult its tax advisors to determine whether and to what extent such U.S. Holder will be entitled to this credit.
Taxation of the Sale, Exchange or other Disposition of Common Shares
Except as provided under the PFIC rules described below under “Passive Foreign Investment Company Rules,” upon the sale, exchange or other disposition of our Common Shares, a U.S. Holder will recognize capital gain or loss in an amount equal to the difference between such U.S. Holder’s tax basis for the Common Shares, determined in U.S. dollars, and the U.S. dollar value of the amount realized on the disposition (or its U.S. dollar equivalent determined by reference to the spot rate of exchange on the date of disposition, if the amount realized is denominated in a foreign currency). The gain or loss realized on the sale, exchange or other disposition of Common Shares will be long-term capital gain or loss if the U.S. Holder has a holding period of more than one year at the time of the disposition. Individuals who recognize long-term capital gains may be taxed on such gains at reduced rates of tax. The deduction of capital losses is subject to various limitations.
Passive Foreign Investment Company Rules
The treatment of U.S. Holders of Common Shares could be materially different from that described above if we are treated as a PFIC for U.S. federal income tax purposes. We will be treated as a PFIC for U.S. federal income tax purposes for any taxable year with respect to which either:
| ● | 75% or more of our gross income constitutes passive income for purposes of the PFIC rules; or |
| ● | 50% or more of our assets (generally determined on the basis of a quarterly average value) are held for the production of, or produce, passive income. |
For this purpose, passive income generally consists of rents, dividends, interest, royalties, gains from the disposition of passive assets and gains from certain commodities and securities transactions. Cash generally is treated as generating passive income. The value of goodwill is an active asset to the extent attributable to activities that produce active income. The determination of whether a foreign corporation is a PFIC is based upon the composition of such foreign corporation’s income and assets (including, among others, its proportionate share of the income and assets of any other corporation in which it owns, directly or indirectly, 25% (by value) of the stock), and the nature of such foreign corporation’s activities. A separate determination must be made after the close of each taxable year as to whether a foreign corporation was a PFIC for that year.
As of the date hereof, we have not made a determination as to our PFIC status for our current taxable year or any other taxable year. The tests for determining PFIC status are applied annually after the close of the taxable year, and therefore our possible status as a PFIC may be subject to change. Further, because the value of our goodwill may be determined based on the market value of the Common Shares, a decrease in the market value of the Common Shares and/or an increase in cash or other passive assets would increase the relative percentage of our passive assets. The application of the PFIC rules is subject to uncertainty in several respects and, therefore, no assurances can be provided that we will not be a PFIC for any taxable year. If we are treated as a PFIC during a U.S. Holder’s holding period for Common Shares, we will, with respect to such U.S. Holder, continue to be treated as a PFIC, regardless of whether we satisfied either of the qualification tests in subsequent years, subject to certain exceptions.
If we currently are or become a PFIC, each U.S. Holder who has not elected to mark the shares to market (as discussed below), would, upon receipt of certain “excess distributions” by us and upon disposition of our Common Shares at a gain: (1) have such excess distribution or gain allocated ratably over the U.S. Holder’s holding period for the Common Shares, as the case may be; (2) the amount allocated to the current taxable year and any period prior to the first day of the first taxable year in which we were a PFIC would be taxed as ordinary income; and (3) the amount allocated to each of the other taxable years would be subject to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed deferral benefit would be imposed with respect to the resulting tax attributable to each such other taxable year. Distributions received by a U.S. Holder in a taxable year that are greater than 125% of the average annual distributions received during the shorter of the three preceding taxable years or the U.S. Holder’s holding period for the Common Shares will be treated as excess distributions. Indirect investments in a PFIC may also be subject to these special U.S. federal income tax rules.
As an alternative to the foregoing rules, a U.S. Holder may make a mark-to-market election with respect to the Common Shares, provided such Common Shares are treated as “marketable stock.” The Common Shares generally will be treated as marketable stock if they are regularly traded on a “qualified exchange or other market,” as defined in applicable U.S. Treasury regulations. The Common Shares will be marketable stock as long as they remain listed on Nasdaq, which is a qualified exchange for this purpose, and are regularly traded.
If a U.S. Holder makes a valid mark-to-market election with respect to the Common Shares, the holder generally will (i) include as ordinary income for each taxable year that we are a PFIC the excess, if any, of the fair market value of Common Shares held at the end of the taxable year over the adjusted tax basis of such Common Shares and (ii) deduct as an ordinary loss in each such taxable year the excess, if any, of the adjusted tax basis of the Common Shares over the fair market value of such Common Shares held at the end of the taxable year, but such deduction will only be allowed to the extent of the net amount previously included in income as a result of the mark-to- market election. The U.S. Holder’s adjusted tax basis in the Common Shares would be adjusted to reflect any income or loss resulting from the mark-to-market election. If a U.S. Holder makes a mark-to-market election in respect of the Common Shares and we cease to be classified as a PFIC, the holder will not be required to take into account the gain or loss described above during any period that we are not classified as a PFIC. If a U.S. Holder makes a mark-to-market election, any gain such U.S. Holder recognizes upon the sale or other disposition of the Common Shares in a year when we are a PFIC will be treated as ordinary income and any loss will be treated as ordinary loss, but such loss will only be treated as ordinary loss to the extent of the net amount previously included in income as a result of the mark-to-market election.
If we are determined to be a PFIC, we do not intend to provide the information necessary for a U.S. Holder to make a qualified electing fund, or QEF, election with respect to the Common Shares which, if available, would result in a tax treatment different from (and generally less adverse than) the general tax treatment for PFICs.
A U.S. Holder that owns (or is deemed to own) shares in a PFIC during any taxable year of the U.S. Holder generally is required to file an IRS Form 8621 with such U.S. Holder’s U.S. federal income tax return and provide such other information as the IRS may require. Failure to file IRS Form 8621 for each applicable taxable year may result in substantial penalties and the statute of limitations on the assessment and collection of U.S. federal income taxes of such U.S. Holder for the related taxable year may not close until three years after the date on which the required information is filed.
The rules dealing with PFICs are very complex and are affected by various factors in addition to those described above. Accordingly, U.S. Holders of Common Shares are urged to consult their tax advisors concerning the application of the PFIC rules to their Common Shares under their particular circumstances.
Additional Reporting Requirements
Certain U.S. Holders holding specified foreign financial assets with an aggregate value in excess of the applicable dollar thresholds are required to report information to the IRS relating to such assets, subject to certain exceptions (including an exception for specified foreign financial assets held in accounts maintained by U.S. financial institutions), by attaching a complete IRS Form 8938 to their tax return, for each year in which they hold such assets. Failure to file IRS Form 8938 for each applicable taxable year may result in substantial penalties and the statute of limitations on the assessment and collection of U.S. federal income taxes of such U.S. Holder for the related taxable year may not close until three years after the date on which the required information is filed. U.S. Holders should consult their tax advisors regarding the effect, if any, of these rules on the ownership and disposition of the Common Shares.
Information Reporting and Backup Withholding
Information reporting requirements may apply to cash received in redemption of the Common Shares, dividends paid on the Common Shares and the proceeds received on the disposition of the Common Shares effected within the United States (and, in certain cases, outside the United States), in each case other than with respect to U.S. Holders that are exempt recipients (such as corporations). Backup withholding may apply to such amounts if the U.S. Holder fails to provide an accurate taxpayer identification number (generally on an IRS Form W-9 provided to the paying agent of the U.S. Holder’s broker) or is otherwise subject to backup withholding. U.S. Holders should consult their tax advisors regarding the application of the U.S. information reporting and backup withholding rules.
F. Dividends and Paying Agents
Not applicable.
G. Statement by Experts
Not applicable.
H. Documents on Display
We are subject to certain information reporting requirements of the Exchange Act, applicable to foreign private issuers and under those requirements will file reports with the SEC. The SEC maintains an internet site at http://www.sec.gov that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. Our filings with the SEC will also be available to the public through the SEC’s website at www.sec.gov.
As a foreign private issuer, we are exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders will be exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file annual, quarterly and current reports and financial statements with the SEC as frequently or as promptly as U.S. domestic companies whose securities are registered under the Exchange Act. However, we will file with the SEC, within 120 days after the end of each fiscal year, or such applicable time as required by the SEC, an annual report on Form 20-F containing financial statements audited by an independent registered public accounting firm, and may submit to the SEC, on a Form 6-K, unaudited quarterly financial information.
I. Subsidiary Information.
Not applicable.
ITEM 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Quantitative and Qualitative Disclosures about Market Risk
Liquidity Risk
Liquidity risk is the risk that we will encounter difficulty in meeting the obligations associated with our financial liabilities that are settled in cash. Cash flow forecasting is performed in our operating entities and aggregated at a consolidated level. We monitor forecasts of our liquidity requirements to ensure we have sufficient cash to meet operational needs. The Company has no long liabilities as of October 31, 2023, however we entered into an office lease agreement in Israel commits us to $6,500 per month until June 30, 2025. We remain reliant on our ability to raise additional investment capital from the issuance of both debt and equity securities to fund our business operating plans and future obligations.
Credit risk
Financial instruments that potentially subject the Company to a concentration of credit risk consist primarily of cash and cash equivalents and receivables. The Company limits its exposure to credit loss by placing its cash with high credit quality financial institutions. The carrying amount of financial assets represents the maximum credit exposure.
Credit risk is the risk of financial loss to us if a debtor or counterparty to a financial instrument fails to meet its contractual obligations, and arises mainly from our receivables.
We restrict exposure to credit risk in the course of our operations by investing only in bank deposits.
Equity price risk
As we do not have material investments in securities riskier than short-term bank deposits, we do not believe that changes in equity prices pose a material risk to our holdings. However, decreases in the market price of our Common Shares could make it more difficult for us to raise additional funds in the future or require us to raise funds at terms unfavorable to us.
Inflation risk
We do not believe that inflation has had a material effect on our business, financial condition or results of operations in the reporting period. If our costs were to become subject to significant inflationary pressures, we may not be able to fully offset such higher costs through hedging transactions. Our inability or failure to do so could harm our business, financial condition and results of operations.
Foreign Currency Exchange Risk
Our results of operations and cash flow are subject to fluctuations due to changes mainly in NIS/USD currency exchange rates. As of October 31, 2023, the majority of our liquid assets are held in USD, and the majority of our expenses is denominated in U.S. dollars or ILS. Changes of both 5% and 10% in the NIS/USD and CAD/USD exchange rate would decrease/increase our loss for 2023 by less than 0.02% and 0.04%, respectively. However, these historical figures may not be indicative of future exposure, as we expect that the percentage of our NIS denominated expenses will materially decrease in the near future, therefore reducing our exposure to exchange rate fluctuations.
We do not hedge our foreign currency exchange risk. In the future, we may enter into formal currency hedging transactions to decrease the risk of financial exposure from fluctuations in the exchange rates of our principal operating currencies. These measures, however, may not adequately protect us from the material adverse effects of such fluctuations.
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
A. Debt Securities.
Not applicable.
B. Warrants and rights.
Not applicable.
C. Other Securities.
Not applicable.
D. American Depositary Shares
Not applicable.
PART II
ITEM 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
None.
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
There are no material modifications to the rights of security holders.
ITEM 15. CONTROLS AND PROCEDURES
(a) Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of October 31, 2023, or the Evaluation Date. Based on such evaluation, those officers have concluded that, as of the Evaluation Date, our disclosure controls and procedures are not effective in recording, processing, summarizing and reporting, on a timely basis, information required to be included in periodic filings under the Exchange Act and that such information is accumulated and communicated to management, including our principal executive and financial officers, as appropriate to allow timely decisions regarding required disclosure. In particular, we identified material weaknesses in internal control over financial reporting, as discussed below.
(b) Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) under the Exchange Act. Our management conducted an assessment of the effectiveness of our internal control over financial reporting as of October 31, 2023 based on the criteria set forth in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework). Based on that assessment, our management concluded that our internal control over financial reporting was not effective as of October 31, 2023, due to ineffective controls over period end financial reporting, which is considered as a “material weakness”.
As defined in Regulation 12b-2 under the Exchange Act, a “material weakness” is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim consolidated financial statements will not be prevented, or detected on a timely basis.
As previously disclosed in our Annual Report on Form 20-F for the year ended October 31, 2022, as of October 31, 2022, our internal control over financial reporting was ineffective due to the following material weaknesses: lack of segregation of duties within account processes, and systems, inadequate documentation to evidence the operation of controls, inconsistent procedures and approvals, lack of periodic user access reviews, lack of assessment of controls of financially significant vendors and insufficient written policies and procedures for accounting, IT and financial reporting and record keeping. In response to these material weaknesses, we implemented a remediation plan.
As part of such remediation plan, during the year ended October 31, 2023, we have implemented remediation efforts including independent review and approval of transactions and reconciliations in some processes by hiring personnel and segregating duties amongst the team. Management is also implementing processes to document and retain evidence to support reviews and reconciliations.
As the Company continues to improve its internal controls over financing, we are in final stages of engaging outside consultants, experts in the controls and procedures over financial reporting, and internally, we continue to review and improve these processes. In light of the remediation occurring, our internal controls are expected to be changed in the future, once the planned changes are finalized.
(c) Attestation Report of the Registered Public Accounting Firm
This Annual Report on Form 20-F does not include an attestation report of our independent registered public accounting firm regarding internal control over financial reporting due to an exemption for emerging growth companies provided in the JOBS Act.
(d) Changes in Internal Control over Financial Reporting
During the year ended October 31, 2023, we have implemented measures to remediate identified material weaknesses, as described above. Other than such measures, there were no changes in our internal control over financial reporting that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
With the completion of our listing in the US, management continues to identify and improve our internal control over financial reporting, in the context of which we have identified material weaknesses in our internal control over financial reporting related to lack of segregation of duties within account processes, and systems, inadequate documentation to evidence the operation of controls, inconsistent procedures and approvals, lack of periodic user access reviews, lack of assessment of controls of financially significant vendors and insufficient written policies and procedures for accounting, IT and financial reporting and record keeping. Our management has concluded that, due to such material weakness, our disclosure controls and procedures were not effective as of October 31, 2023. As the Company continues to improve its internal controls over financing, we are in final stages of engaging outside consultants, experts in the controls and procedures over financial reporting, and internally, we continue to review and improve these processes. In light of the remediation occurring, our internal controls are expected to be changed in the future, once the planned changes are finalized.
ITEM 16. [RESERVED]
Not applicable.
ITEM 16A. AUDIT COMMITTEE FINANCIAL EXPERT
Our Audit Committee is currently comprised of Oz Adler, Yehonatan Shachar and Amitay Weiss, and is chaired by Oz Adler. Our Board has determined that each of the members of the committee are financially literate and meets the independence requirements for directors, including the heightened independence standards for members of the Audit Committee under Rule 10A-3 under the Exchange Act and NI 52-110. Our Board has determined that Oz Adler is “financially sophisticated” within the meaning of the Nasdaq Rules, “financially literate” within the meaning of NI 52-110, and a “financial expert” as defined by Rule 10A-3 under the Exchange Act.
ITEM 16B. CODE OF ETHICS
Our Board has adopted a Code of Business Conduct and Ethics applicable to all of our directors and employees, including our Chief Executive Officer, Chief Financial Officer, controller or principal accounting officer, or other persons performing similar functions, which is a “code of ethics” as defined in Item 16B of Form 20-F promulgated by the SEC. The full text of our code of business conduct and ethics is available under the Corporate Governance section of our website at www.clearmindmedicine.com. In addition, we intend to post on our website all disclosures that are required by law or the Nasdaq Rules concerning any amendments to, or waivers from, any provision of the code. The reference to our website address does not constitute incorporation by reference of the information contained at or available through our website, and you should not consider it to be a part of this Annual Report on Form 20-F.
ITEM 16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Brightman Almagor Zohar & Co., a Firm in the Deloitte Global Network, an independent registered public accounting firm, or Deloitte, has served as our principal independent registered public accounting firm for the year ended October 31, 2023.
The following table provides information regarding fees paid or to be paid by us to Brightman Almagor Zohar & Co., a firm in the Deloitte Global Network, an independent registered public accounting firm, for all services, including audit services, for the years ended October 31, 2023, and 2022:
| Year Ended October 31, |
||||||||
| (USD) | 2023 | 2022 | ||||||
| Audit fees (1) | 180,000 | 80,000 | ||||||
| Audit-related fees | - | - | ||||||
| Tax fees | - | - | ||||||
| All other fees | - | - | ||||||
| Total | 180,000 | 80,000 | ||||||
| (1) | Audit fees consist of professional services provided in connection with the audit of our annual financial statements. |
Pre-Approval of Auditors’ Compensation
Our Audit Committee has a pre-approval policy for the engagement of our independent registered public accounting firm to perform certain audit and non-audit services. Pursuant to this policy, which is designed to assure that such engagements do not impair the independence of our auditors, the Audit Committee pre-approves annually a catalog of specific audit and non-audit services in the categories of audit services, audit-related services and tax services that may be performed by our independent registered public accounting firm. If a type of service, that is to be provided by our auditors, has not received such general pre-approval, it will require specific pre-approval by our Audit Committee. The policy prohibits retention of the independent registered public accounting firm to perform the prohibited non-audit functions defined in applicable SEC rules.
ITEM 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Not applicable.
ITEM 16E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
Not applicable.
ITEM 16F. CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT
Effective on January 18, 2023, Saturna Group resigned as our independent registered public accounting firm. Saturna Group’s report on the financial statements for the fiscal years ended October 31, 2020 and 2021, contained no adverse opinion or disclaimer of opinion, and was not qualified or modified as to uncertainty, audit scope or accounting principle, other than to include an explanatory paragraph relating to the Company’s ability to continue as a going concern.
During the fiscal years ended October 31, 2021 and 2022, and in the subsequent interim periods through January 18, 2023, the date of resignation of Saturna Group, there were no disagreements with Saturna Group on any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedure, which disagreements, if not resolved to the satisfaction, would have caused them to make reference to the subject matter of the disagreements in its report on the financial statements for such year. During the fiscal year ended October 31, 2022, and in the subsequent interim period through January 18, 2023, the date of resignation of Saturna Group, there were no reportable events as defined in Item 304(a)(1)(v) of Regulation S-K. Saturna Group provided us with a letter, dated January 29, 2024, addressed to the Commission stating that it agreed with the above disclosure.
Effective January 18, 2023, Brightman Almagor Zohar & Co., a Firm in the Deloitte Global Network, was appointed as our new independent registered public accounting firm.
During the fiscal years ended October 31, 2021 and 2022, and the subsequent interim period prior to the engagement of Brightman Almagor Zohar & Co., we did not consult Brightman Almagor Zohar & Co. regarding (i) the application of accounting principles to any specified transaction, either completed or proposed; (ii) the type of audit opinion that might be rendered on our financial statements, and either a written report was provided to the registrant or oral advice was provided that the new accountant concluded was an important factor considered by the registrant in reaching a decision as to the accounting, auditing or financial reporting issue; or (iii) any matter that was either the subject of a disagreement (as defined in Item 304(a)(1)(v)) of Regulation S-K or a reportable event (as defined in Item 304(a)(1)(v)) of Regulation S-K.
ITEM 16G. CORPORATE GOVERNANCE
As a foreign private issuer, we will be permitted, and intend, to follow certain home country corporate governance practices instead of those otherwise required by the Nasdaq for domestic U.S. issuers. Following our home country governance practices as opposed to the requirements that would otherwise apply to a U.S. company listed on The Nasdaq Global Market may provide less protection to you than what is accorded to investors under the Nasdaq Rules applicable to domestic U.S. issuers.
Accordingly, we have elected to follow the provisions, rather than the Nasdaq Rules, with respect to the following requirements:
| ● | Distribution of periodic reports to shareholders; proxy solicitation. As opposed to the Nasdaq Rules, which require listed issuers to make such reports available to shareholders in one of a number of specific manners, Israeli law does not require us to distribute periodic reports directly to shareholders, and the generally accepted business practice in Israel is not to distribute such reports to shareholders but to make such reports available through a public website. In addition to making such reports available on a public website, we currently make our audited financial statements available to our shareholders at our offices and will only mail such reports to shareholders upon request. As a foreign private issuer, we are generally exempt from the SEC’s proxy solicitation rules. | |
| ● | Quorum. While the Nasdaq Rules require that the quorum for purposes of any meeting of the holders of a listed Company’s common voting stock, as specified in the Company’s bylaws, be no less than 33 1/3% of the Company’s outstanding issued and outstanding share capital, under Israeli law, a company is entitled to determine in its articles of association the number of shareholders and percentage of holdings required for a quorum at a shareholders meeting. Our amended and restated articles of association provides that a quorum of two or more shareholders holding at least 50% of the voting rights in person or by proxy is required for commencement of business at a general meeting. However, the quorum set forth in our amended and restated articles of association with respect to an adjourned meeting consists of any number of shareholders present in person or by proxy. |
| ● | Nomination of our directors. Our amended and restated articles of association provides that with the exception of directors elected by our Board and external directors, which will be elected by an annual or special meeting of our shareholders and shall hold office until the next annual meeting following one year from his or her election, or three (3) years period in case of external director, shareholders are entitled to appoint a director to the Company’s Board for each 10% of the Company’s outstanding share capital that they own and such appointment shall be for undefined period. Additional Board members are elected at our shareholder’s annual meetings and in such case they shall serve on the Board until the next annual general meeting (except for external directors which will serve for up to three terms, each term of three years’ period). In addition, our amended and restated articles allow our Board to appoint directors to fill vacancies and/or as an addition to the Board (subject to the maximum number of directors) to serve until the next annual general meeting. All of the elected directors, other than external directors, may be re-elected for an unlimited number of terms upon completion of their then-current term of office. The nominations for directors are made in accordance with the provisions of our amended and restated articles of association and the Companies Law. Nominations need not be made by a nominating committee of our Board consisting solely of independent directors, as required under the Nasdaq Rules. |
| ● | Compensation of officers. Israeli law and our amended and restated articles of association does not require that the independent members of our Board (or a Compensation Committee composed solely of independent members of our Board) determine an executive officer’s compensation, as is generally required under the Nasdaq Rules with respect to the chief executive officer and all other executive officers. Instead, compensation of executive officers is determined and approved by our Compensation Committee and our Board, and in certain circumstances by our shareholders, either in consistency with our office holder compensation policy or, in special circumstances in deviation therefrom, taking into account certain considerations stated in the Companies Law. | |
| ● | Independent directors. Israeli law does not require that a majority of the directors serving on our Board be “independent,” as defined under Nasdaq Rule 5605(a)(2), and rather requires we have at least two external directors who meet the requirements of the Companies Law, as described below under “Directors, Senior Management and Employees—Board Practices—External Directors.” The definition of independent director under Nasdaq Rules and external director under the Companies Law overlap to a significant degree such that we would generally expect the directors serving as external directors to satisfy the requirements to be independent under Nasdaq Rules. However, it is possible for a director to qualify as an ’‘external director’’ under the Companies Law without qualifying as an ’‘independent director’’ under the Nasdaq Rules, or vice-versa. Notwithstanding Israeli law, we believe that a majority of our directors are currently “independent” under the Nasdaq Rules. Our Board has determined that Mr. Yossi Daskal, Oz Adler, and Mrs. Inbal Kreiss are “independent” for purposes of the Nasdaq Rules. We are required, however, to ensure that all members of our Audit Committee are “independent” under the applicable Nasdaq and SEC criteria for independence (as we cannot exempt ourselves from compliance with that SEC independence requirement, despite our status as a foreign private issuer), and we must also ensure that a majority of the members of our Audit Committee are “independent directors” as defined in the Companies Law. Furthermore, Israeli law does not require, nor do our independent directors conduct, regularly scheduled meetings at which only they are present, which the Nasdaq Rules otherwise require. | |
| ● | Shareholder approval. We will seek shareholder approval for all corporate actions requiring such approval under the requirements of the Companies Law, rather than seeking approval for corporate actions in accordance with Nasdaq Stock Market Rule 5635. In particular, under this Nasdaq Stock Market rule, shareholder approval is generally required for: (i) an acquisition of shares or assets of another company that involves the issuance of 20% or more of the acquirer’s shares or voting rights or if a director, officer or 5% shareholder has greater than a 5% interest in the target company or the consideration to be received; (ii) the issuance of shares leading to a change of control; (iii) adoption or amendment of equity compensation arrangements (although under the provisions of the Companies Law there is no requirement for shareholder approval for the adoption/amendment of the equity compensation plan); and (iv) issuances of 20% or more of the shares or voting rights (including securities convertible into, or exercisable for, equity) of a listed company via a private placement (and/or via sales by directors, officers or 5% shareholders) if such equity is issued (or sold) at below the greater of the book or market value of shares. By contrast, under the Companies Law, shareholder approval is required for, among other things: (i) transactions with directors concerning the terms of their service or indemnification, exemption and insurance for their service (or for any other position that they may hold at a company), for which approvals of the Compensation Committee, Board and shareholders are all required, (ii) extraordinary transactions with controlling shareholders of publicly held companies, which require the special approval, and (iii) terms of employment or other engagement of the controlling shareholder of us or such controlling shareholder’s relative, which require special approval. In addition, under the Companies Law, a merger requires approval of the shareholders of each of the merging companies. |
| ● | Approval of Related Party Transactions. All related party transactions are approved in accordance with the requirements and procedures for approval of interested party acts and transaction as set forth in the Companies Law, which requires the approval of the Audit Committee, or the Compensation Committee, as the case may be, the Board and shareholders, as may be applicable, for specified transactions, rather than approval by the Audit Committee or other independent body of our Board as required under the Nasdaq Rules. |
ITEM 16H. MINE SAFETY DISCLOSURE
Not applicable.
ITEM 16I. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
ITEM 16J. INSIDER TRADING POLICIES
Pursuant to applicable SEC transition guidance, the disclosure required by Item 16J will only be applicable to the Company from the fiscal year ending on October 31, 2024.
ITEM 16K. CYBERSECURITY
Pursuant to applicable SEC transition guidance, the disclosure required by Item 16K will only be applicable to the Company from the fiscal year ending on October 31, 2024.
PART III
ITEM 17. FINANCIAL STATEMENTS
We have elected to provide financial statements and related information pursuant to Item 18.
ITEM 18. FINANCIAL STATEMENTS
The financial statements and the related notes required by this Item are included in this Annual Report on Form 20-F beginning on page F-1.
ITEM 19. EXHIBITS.
| * | Filed herewith. |
| # | Management contract or compensatory plan. |
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this Annual Report on Form 20-F filed on its behalf.
| CLEARMIND MEDICINE INC. | ||
| Date: January 29, 2024 | By: | /s/ Dr. Adi Zuloff-Shani |
| Dr. Adi Zuloff-Shani | ||
| Chief Executive Officer | ||
CLEARMIND MEDICINE INC.
Consolidated Financial Statements
(Expressed in United States Dollars)
F-
CLEARMIND MEDICINE INC.
Index of Financial Statements
As of October 31, 2023
F-

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the shareholders and the Board of Directors of Clearmind Medicine Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated statements of financial position of Clearmind Medicine Inc. and its subsidiaries (the “Company”) as of October 31, 2023, and 2022, the related consolidated statements of operations and comprehensive loss, changes in shareholders’ equity (deficit) and cash flows for each of the two years in the period ended October 31, 2023, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of October 31, 2023 and 2022, and the results of its operations and its cash flows for each of the two years in the period ended October 31, 2023, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Change in Presentation Currency
As discussed in Note 1d to the financial statements, the Company changed the presentation currency from Canadian dollar to United States dollar and has applied this change retrospectively for all periods presented.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1b to the financial statements, the Company’s accumulated losses and the additional funds needed to maintain its operations raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1b. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Brightman Almagor Zohar & Co.
Certified Public Accountants
A Firm in the Deloitte Global Network
Tel Aviv, Israel
January 29, 2024
We have served as the Company’s auditor since 2023.
F-

INDEPENDENT AUDITOR’S REPORT
To the Shareholders and Board of Directors of Clearmind Medicine Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated statements of operations and comprehensive loss, changes in shareholders’ equity, and cash flows for the year ended October 31, 2021 of Clearmind Medicine Inc. (the “Company”) and related notes (collectively, the “financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the results of their operations and cash flows for the year ended October 31, 2021, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Explanatory Paragraph Regarding Going Concern
Without modifying our opinion, we draw attention to Note 1 in the consolidated financial statements which indicates that there are material uncertainties that cast significant doubt about the going concern assumption. The Company has not generated any revenues, has negative cash flow from operations, and has an accumulated deficit. These conditions, along with other matters as set forth in Note 1, indicate the existence of a material uncertainty that casts significant doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also discussed in Note 1 to the consolidated financial statements. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to fraud or error. The Company is not required to have, nor were we engaged to perform, an audit of its internal controls over financial reporting. As part of our audits, we are required to obtain an understanding of the Company’s internal controls over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal controls over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to fraud or error, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the Board of Directors and that: (i) relate to accounts or disclosures that are material to the financial statements; and (ii) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
| /s/ SATURNA GROUP CHARTERED PROFESSIONAL ACCOUNTANTS LLP |
Saturna Group Chartered Professional Accountants LLP
We served as the Company’s auditor since 2017.
In January 2023, we became the predecessor auditor.
Vancouver, Canada
March 8, 2022, except as to Notes 1(c) and 1(d) which is as of January 26, 2024
F-
CLEARMIND MEDICINE INC.
Consolidated Statements of Financial Position
(Expressed in United States Dollars)
| October 31, | ||||||||
| 2023 | 2022 | |||||||
| Assets | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | $ | 5,427,739 | $ | 128,777 | ||||
| Other receivables | 104,320 | 50,933 | ||||||
| Short-term investment (Note 5) | 86,112 | 193,750 | ||||||
| Prepaid expenses | 40,403 | 14,245 | ||||||
| Related parties (Note 6b) | 136,002 | 46,988 | ||||||
| Total current assets | 5,794,576 | 434,693 | ||||||
| Non-current assets | ||||||||
| Property and equipment | 1,727 | 12,902 | ||||||
| Intangible assets (Note 3) | 119,310 | 130,264 | ||||||
| Restricted cash | 37,675 | 14,653 | ||||||
| Right-of-use asset (Note 4) | 35,730 | |||||||
| Deferred offering costs | 198,173 | |||||||
| Total non-current assets | 158,712 | 391,722 | ||||||
| Total assets | $ | 5,953,288 | $ | 826,415 | ||||
| Liabilities | ||||||||
| Current liabilities | ||||||||
| Accounts payable and accrued liabilities | $ | 617,004 | $ | 1,396,960 | ||||
| Due to related parties (Note 6) | 42,433 | 206,494 | ||||||
| Derivative warrants liabilities (Note 7) | 4,310,379 | |||||||
| Derivative liability (Note 8d (iii)) | 290,569 | |||||||
| Lease liability (Note 4) | 38,390 | |||||||
| Total liabilities | $ | 4,969,816 | $ | 1,932,413 | ||||
| Shareholders’ equity (deficit) | ||||||||
| Share capital and share premium (Note 8) | 17,131,223 | 6,706,644 | ||||||
| Warrants (Note 9) | 459,341 | 459,110 | ||||||
| Share-based payment reserve (Notes 10,11) | 2,182,221 | 1,896,724 | ||||||
| Accumulated other comprehensive loss | (21,250 | ) | (21,250 | ) | ||||
| Accumulated deficit | (18,768,063 | ) | (10,147,226 | ) | ||||
| Total shareholders’ equity (deficit) | 983,472 | (1,105,998 | ) | |||||
| Total liabilities and shareholders’ equity (deficit) | $ | 5,953,288 | $ | 826,415 | ||||
Approved and authorized for issuance on behalf of the Board of Directors on January 29, 2024:
| /s/ “Alan Rootenberg” | /s/ “Adi Zuloff-Shani” | |
| Alan Rootenberg, CFO | Adi Zuloff-Shani, CEO |
(The accompanying notes are an integral part of these consolidated financial statements)
F-
CLEARMIND MEDICINE INC.
Consolidated Statements of Operations and Comprehensive Loss
(Expressed in United States Dollars)
| Year ended October 31, | ||||||||||||
| 2023 | 2022 | 2021 | ||||||||||
| Operating expenses | ||||||||||||
| General and administrative (Note 17) | $ | 4,759,205 | $ | 3,170,440 | $ | 2,219,745 | ||||||
| Research and development, net (Note 18) | 1,544,229 | 3,080,991 | 473,652 | |||||||||
| Total operating expenses | 6,303,434 | 6,251,431 | 2,693,397 | |||||||||
| Impairment of exploration and evaluation assets | – | – | (7,327 | ) | ||||||||
| Finance expenses | ||||||||||||
| Changes in fair value of derivative warrants liabilities (Note 7) | (2,189,986 | ) | ||||||||||
| Unrealized loss on short-term investment (Note 5) | (107,638 | ) | (308,188 | ) | ||||||||
| Foreign exchange loss | (78,136 | ) | (404 | ) | (24,564 | ) | ||||||
| Other finance income (expense), net | 59,174 | (14,417 | ) | |||||||||
| Change in fair value of derivative liability (Note 8d (iii)) | (290,569 | ) | ||||||||||
| Total finance expenses | (2,316,586 | ) | (613,578 | ) | (24,564 | ) | ||||||
| Other income | ||||||||||||
| Dividend received | 16,555 | |||||||||||
| Total other income | 16,555 | |||||||||||
| Loss Before taxes | (8,603,465 | ) | (6,865,009 | ) | (2,725,288 | ) | ||||||
| Tax expenses | (17,372 | ) | (29,859 | ) | ||||||||
| Net Loss | (8,620,837 | ) | (6,894,868 | ) | (2,725,288 | ) | ||||||
| Other comprehensive loss for the year | ||||||||||||
| Items that may be reclassified subsequently to profit or loss: | ||||||||||||
| Foreign exchange differences on translation of foreign operations | (21,250 | ) | ||||||||||
| Comprehensive loss | $ | (8,620,837 | ) | $ | (6,916,118 | ) | $ | (2,725,288 | ) | |||
| $ | (42.58 | ) | $ | (159.11 | ) | $ | (88.16 | ) | ||||
| 202,481 | 43,335 | 30,911 | ||||||||||
| (*) | On September 30, 2022 and on November 28, 2023, the Company effected a 1-for-30 reverse split of its issued and outstanding common shares, pursuant to which holders of the Company’s common shares received 0.0333 of a common share for every one common share. All share amounts have been retroactively restated for all periods presented. |
(The accompanying notes are an integral part of these consolidated financial statements)
F-
CLEARMIND MEDICINE INC.
Statements of Changes in Shareholders’ Equity (Deficit)
(Expressed in United States Dollars)
| Share
capital and share premium |
Share-based | Accumulated other |
Total | |||||||||||||||||||||||||
| Number of shares (*) |
Amount | Warrants | payment reserve |
comprehensive income |
Accumulated deficit |
shareholders’ equity (deficit) |
||||||||||||||||||||||
| Balance, October 31, 2020 | 21,362 | $ | 653,308 | $ | $ | 107,921 | $ | $ | (527,070 | ) | $ | 234,159 | ||||||||||||||||
| Net loss for the year | – | (2,725,288 | ) | (2,725,288 | ) | |||||||||||||||||||||||
| Issuance of common shares in private placement | 20,333 | 5,659,755 | 5,659,755 | |||||||||||||||||||||||||
| Share issuance costs | – | (521,054 | ) | (521,054 | ) | |||||||||||||||||||||||
| Issuance of shares in private placement | – | 274,745 | 274,745 | |||||||||||||||||||||||||
| Share issuance costs | – | (24,178 | ) | (24,178 | ) | |||||||||||||||||||||||
| Share-based compensation | – | 619,939 | 619,939 | |||||||||||||||||||||||||
| Balance, October 31, 2021 | 41,695 | $ | 5,792,009 | $ | 250,567 | $ | 727,860 | $ | $ | (3,252,358 | ) | $ | 3,518,078 | |||||||||||||||
| Net loss for the year | – | (6,894,868 | ) | (6,894,868 | ) | |||||||||||||||||||||||
| Foreign currency translation gain | – | (21,250 | ) | (21,250 | ) | |||||||||||||||||||||||
| Total comprehensive loss for the year | – | (21,250 | ) | (6,894,868 | ) | (6,916,118 | ) | |||||||||||||||||||||
| Common shares issued for services | 89 | 38,684 | 107,222 | 145,906 | ||||||||||||||||||||||||
| Units issued for cash | 1,325 | 559,118 | 139,779 | 698,897 | ||||||||||||||||||||||||
| Issuance costs | – | (55,912 | ) | (13,978 | ) | (69,890 | ) | |||||||||||||||||||||
| Units issued for short-term investment | 883 | 372,745 | 82,742 | 455,487 | ||||||||||||||||||||||||
| Share-based compensation | – | – | 1,061,642 | 1,061,642 | ||||||||||||||||||||||||
| Balance, October 31, 2022 | 43,992 | $ | 6,706,644 | $ | 459,110 | $ | 1,896,724 | $ | (21,250 | ) | $ | (10,147,226 | ) | $ | (1,105,998 | ) | ||||||||||||
| Net loss for the year | – | (8,620,837 | ) | (8,620,837 | ) | |||||||||||||||||||||||
| Issuance of common shares (Note 8c(i)) | 38,462 | 6,026,327 | 337,579 | 6,363,906 | ||||||||||||||||||||||||
| Common shares and warrants issued to Medigus (Note 8c(i)) | 1,494 | 296,845 | 231 | 297,076 | ||||||||||||||||||||||||
| Issuance of common shares, pre-funded warrants and warrants (Note 8c(iii)) | 150,191 | 1,455,832 | 1,455,832 | |||||||||||||||||||||||||
| Issuance of common shares upon vesting of restricted stock units (Note 8c(ii,v,vii)) |
1,391 | 263,408 | (263,408 | ) | ||||||||||||||||||||||||
| Issuance of common shares, pre-funded warrants and warrants (Note 8c(viii)) | 250,000 | 1,069,478 | 1,069,478 | |||||||||||||||||||||||||
| Exercise of warrants (Note 7a) | 119,433 | 1,164,117 | 1,164,117 | |||||||||||||||||||||||||
| Common shares for services | 2,374 | 148,572 | (101,888 | ) | 46,684 | |||||||||||||||||||||||
| Share-based compensation (Notes 10, 11b(i)) | – | 313,214 | 313,214 | |||||||||||||||||||||||||
| Balance, October 31, 2023 | 607,337 | $ | 17,131,223 | $ | 459,341 | $ | 2,182,221 | $ | (21,250 | ) | $ | (18,768,063 | ) | $ | 983,472 | |||||||||||||
| (*) | On September 30, 2022 and on November 28, 2023, the Company effected a 1-for-30 reverse split of its issued and outstanding common shares, pursuant to which holders of the Company’s common shares received 0.0333 of a common share for every one common share. All share amounts have been retroactively restated for all periods presented. |
(The accompanying notes are an integral part of these consolidated financial statements)
F-
CLEARMIND MEDICINE INC.
Consolidated Statements of Cash Flows
(Expressed in United States Dollars)
| Year ended October 31, | ||||||||||||
| 2023 | 2022 | 2021 | ||||||||||
| Operating activities | ||||||||||||
| Net loss | $ | (8,620,837 | ) | $ | (6,894,868 | ) | $ | (2,725,288 | ) | |||
| Adjustments for: | ||||||||||||
| Amortization of intangible assets | 10,954 | 14,830 | – | |||||||||
| Amortization of right-of-use asset | 35,381 | 60,665 | – | |||||||||
| Interest on lease liability | 2,907 | 14,433 | – | |||||||||
| Exchange rate differences | 78,171 | – | – | |||||||||
| Issuance costs allocated to derivate warrants liability | 468,408 | – | – | |||||||||
| Depreciation of property and equipment | 11,175 | 7,572 | 1,884 | |||||||||
| Impairment of exploration and evaluation assets | – | – | 7,327 | |||||||||
| Changes in fair value of derivative liabilities | 2,189,986 | 290,569 | – | |||||||||
| Share-based compensation | 366,405 | 1,207,549 | 619,939 | |||||||||
| Unrealized loss on short-term investment | 107,638 | 308,187 | – | |||||||||
| Tax expenses | 17,372 | 29,859 | – | |||||||||
| Changes in working capital: | ||||||||||||
| Decrease (increase) in other receivable | (154,621 | ) | 19,938 | (115,568 | ) | |||||||
| Decrease (increase) in prepaid expenses | (26,134 | ) | 103,026 | (117,271 | ) | |||||||
| Increase (decrease) in accounts payable and accrued liabilities | (620,421 | ) | 913,303 | 251,738 | ||||||||
| Increase (decrease) in due to / from related parties | (167,856 | ) | 200,443 | 143 | ||||||||
| Net cash used in operating activities | (6,301,472 | ) | (3,724,494 | ) | (2,077,096 | ) | ||||||
| Investing activities | ||||||||||||
| Acquisition of property and equipment | – | (5,563 | ) | (16,961 | ) | |||||||
| Acquisition of intangible assets | – | – | (145,094 | ) | ||||||||
| Exploration and evaluation asset expenditures | – | – | (7,327 | ) | ||||||||
| Changes in restricted cash | (26,228 | ) | – | (14,653 | ) | |||||||
| Net cash used in investing activities | (26,228 | ) | (5,563 | ) | (184,035 | ) | ||||||
| Financing activities | ||||||||||||
| Proceeds from issuance of common shares and warrants, net of issuance costs (Notes 7, 8 c (i,iii,viii)) | 11,115,241 | 582,558 | 5,389,270 | |||||||||
| Proceeds received from exercise of warrants (Note 7) | 590,077 | – | – | |||||||||
| Repayment of lease liabilities | (40,922 | ) | (70,870 | ) | – | |||||||
| Net cash provided by financing activities | 11,664,396 | 511,688 | 5,389,270 | |||||||||
| Effect of foreign exchange rate changes on cash and cash equivalents | (37,734 | ) | (22,652 | ) | – | |||||||
| Net increase (decrease) in cash and cash equivalents | 5,298,962 | (3,241,021 | ) | 3,128,139 | ||||||||
| Cash and cash equivalents at beginning of year | 128,777 | 3,369,798 | 241,659 | |||||||||
| Cash and cash equivalents at end of year | $ | 5,427,739 | $ | 128,777 | $ | 3,369,798 | ||||||
| Supplementary disclosure of cash flow information: | ||||||||||||
| Cash received for interest | $ | 71,733 | $ | 8 | $ | – | ||||||
| Dividend received | 16,555 | |||||||||||
| Cash paid for taxes | 12,678 | – | – | |||||||||
| Non-cash financing and investing activities | ||||||||||||
| Common shares issued to Medigus (Note 8d(iii)) | 296,569 | – | – | |||||||||
| Shares obtained from Medigus in issuance of shares and warrants | $ | – | $ | 455,488 | $ | – | ||||||
| Deferred offering costs included in non-current assets | $ | – | $ | 198,173 | $ | – | ||||||
| Right of use assets obtained in exchange for lease liabilities | $ | – | $ | 92,731 | $ | – | ||||||
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 1. | Nature of Operations and Going Concern |
| a. | Clearmind Medicine Inc. (the “Company”) was incorporated in the province of British Columbia on July 18, 2017. The Company is a clinical pharmaceutical company approaching phase 1 clinical trials, that develops novel psychedelic medicines to solve widespread, yet under-served, health problems. The Company’s head office is located at Suite 101 -1220 West 6th Avenue, Vancouver, BC, V6H 1A5. The Company’s Israeli subsidiary (Clearmindmed Ltd.) provides research and development services to the Company. |
On November 14, 2022, the Company completed a public offering for aggregate gross proceeds of $7.5 million and listing on the Nasdaq Capital Market (“Nasdaq”), see note 8c (i). The Company trades under the symbol CMND on both the Nasdaq and the Canadian Securities Exchange (“CSE”) in Toronto and on the Frankfurt Stock Exchange, or FSE, under the symbol “CWY”.
On April 6, 2023, the Company completed an underwritten public offering for aggregate gross proceeds of $3.5 million and net proceeds of $2.9 million. See note 8c (iii).
On September 18, 2023, the Company completed an underwritten public offering for aggregate gross proceeds of $2.25 million and net proceeds of $1.8 million. See note 8c (viii).
On January 16, 2024, the Company completed a registered direct and private placement for aggregate gross proceeds of $2.40 million. See note 19(d).
| b. | Going concern |
These consolidated financial statements have been prepared on the going concern basis, which assumes that the Company will be able to realize its assets and discharge its liabilities in the normal course of business. For the year ended October 31, 2023, the Company has not generated any revenues and has negative cash flow from operations of $6,301,472. As of October 31, 2023, the Company has an accumulated deficit of $18,768,063. The continued operations of the Company are dependent on its ability to generate future cash flows or obtain additional financing through debt or equity. Management is of the opinion that sufficient working capital will be obtained from external financing to meet the Company’s liabilities and commitments as they become due, although there is a risk that additional financing will not be available on a timely basis or on terms acceptable to the Company. These factors raise substantial doubt on the Company’s ability to continue as a going concern. These consolidated financial statements do not reflect any adjustments that may be necessary if the Company is unable to continue as a going concern.
| c. | Reverse share split |
On September 30, 2022, the Company’s Board of Directors (the “Board”) approved a 1-for-30 reverse split of its issued and outstanding common shares, effective as of September 30, 2022, pursuant to which holders of the Company’s common shares received 0.0333 of a common share for every one common share.
On November 28, 2023, the Company’s Board of Directors (the “Board”) approved a 1-for-30 reverse split of its issued and outstanding common shares, effective as of November 28, 2023, such that each thirty of the Company’s common shares, no par value, were consolidated into one common share, no par value.
All issued and outstanding common shares or instruments convertible into common shares contained in these financial statements have been retroactively adjusted to reflect the reverse share split for all periods presented, unless explicitly stated otherwise.
| d. | Functional Currency and Presentation Currency |
As a result of the Company’s listing on the NASDAQ which was completed in November 2022, the primary economic environment and generation and use of cash flows of the Company became mainly denominated in U.S dollars. Consequently, effective on November 1, 2022, the Company changed both its functional currency and the presentation currency of its consolidated financial statements from the Canadian dollar to the U.S dollar.
Clearmind Medicine Inc. is in its research and development stages and has not generated revenues since inception. In consideration of the indicators in IAS 21, the Company determined that the new and future financing activities of the Company and the currency in which cash and cash equivalents are retained were relevant factors indicating that the Canadian dollar will not be the currency to reflect the principal economic environment in which the Company will generate and expend its cash.
Under IAS 21, a change in an entity’s functional currency is applied prospectively from the date of the change. The Company applied the practical expedient to apply the transaction procedures applicable to the new functional currency prospectively beginning on November 1, 2022. The change in the functional currency was accounted for prospectively from the date of the change by translating all items of the financial statement into the new functional currency using the exchange rate of $0.7327 CAD/USD at the date of the change.
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 1. | Nature of Operations and Going Concern (continued) |
The change in the presentation currency of the consolidated financial statement was accounted for as a change in accounting policy and applied retrospectively, as if the new presentation currency had always been the presentation currency of the consolidation financial statements. Consequently, comparative figures for the years prior to the effective date of November 1, 2022 have been restated to the new presentation currency using the exchange rate in effect on effective date of the change.
Following the change in the Company's functional currency, the Company elected, as an accounting policy, not to reclassify instruments which, had had the change in functional currency occurred before initial recognition of the instrument, would have changed its classification.
| e. | On October 7, 2023, an unprecedented attack was launched against Israel by terrorists from the Hamas terrorist organization that infiltrated Israel’s southern border from the Gaza Strip and in other areas within the State of Israel attacking civilians and military targets while simultaneously launching extensive rocket attacks on the Israeli population, which led to the declaration of the ‘Iron Swords’ War (the “War”). The War is on-going as of the issuance date of these financial statements. The Company’s clinical trials, the laboratory that supports such clinical trials and the Contract Research Organization (CRO) are based in Israel. The extent to which the War may impact the Company’s financial condition, results of operations, or liquidity is uncertain, and as of the date of issuance of these consolidated financial statements, the Company is not aware of any specific event or circumstance that would require an update to its estimates or judgments or an adjustment to the carrying value of the Company’s assets or liabilities as of October 31, 2023. |
| 2. | Significant Accounting Policies |
| a. | Basis of Presentation |
The accompanying consolidated financial statements have been prepared in accordance with International Financial Reporting Standards (“IFRS”), as issued by the International Accounting Standards Board (“IASB”) on a going concern basis.
These consolidated financial statements include the accounts of the Company and its 100% owned subsidiaries, Clearmindmed Ltd. and Clearmind Labs Corp. (inactive). All inter-company balances and transactions have been eliminated on consolidation.
These consolidated financial statements have been prepared on a historical cost basis, except for financial assets and liabilities (including derivatives) which are presented at fair value through profit or loss (“FVTPL”), and are presented in United States dollars, which is the Company’s functional currency as of November 1, 2022.
| b. | Significant Accounting Estimates and Judgments |
The preparation of consolidated financial statements in accordance with IFRS requires management to make judgments, estimates, and assumptions that affect the application of policies and reported amounts of assets, liabilities, income, and expenses. The estimates and associated assumptions are based on historical experience and various other factors that are believed to be reasonable under the circumstances, the results of which form the basis of making the judgments about carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates.
Significant Estimates
Share-based Compensation
Fair values are determined using the Black-Scholes option pricing model. Estimating fair value requires determining the most appropriate valuation model for a grant of equity instruments, which is dependent on the terms and conditions of the grant. Option-pricing models require the use of highly subjective estimates and assumptions including the expected stock price volatility. Changes in the underlying assumptions can materially affect the fair value estimates and, therefore, existing models do not necessarily provide reliable measurement of the fair value of the Company’s stock options.
Warrant Liability
The Company analyses warrants issued to determine whether they meet the classification as liabilities or equity. Derivative warrant liabilities are adjusted to reflect fair value at each reporting period, with any increase or decrease in the fair value recorded in the results of operations. The Company uses a fair valuation specialist to estimate the value of these instruments using the Black and Scholes and binomial pricing model.
The key assumptions used in the models are the expected future volatility in the price of the Company's shares, the expected life of the warrants and the probability of any future adjustment event.
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 2. | Significant Accounting Policies (continued) |
Significant Judgments
The critical judgments that the Company’s management has made in the process of applying the Company’s accounting policies that have the most significant effect on the amounts recognized in the Company’s consolidated financial statements are as follows:
Going Concern
The application of the going concern assumption requires management to take into account all available information about the future, which is at least but not limited to, 12 months from the year end of the reporting period. The Company is aware that material uncertainties related to events or conditions raise substantial doubt upon the Company’s ability to continue as a going concern.
| c. | Cash and Cash Equivalents |
The Company considers all highly liquid instruments with a maturity of three months or less at the time of issuance, which are readily convertible to known amounts of cash, and which are subject to insignificant risk of changes in value to be cash equivalents.
| d. | Provisions |
Provisions are recognized where a legal or constructive obligation has been incurred as a result of past events; it is probable that an outflow of resources embodying economic benefit will be required to settle the obligation; and a reliable estimate of the amount of the obligation can be made. Provisions are measured at the present value of the expenditures expected to be required to settle the obligation.
| e. | Property and Equipment |
Property and equipment are stated at cost less accumulated depreciation and accumulated impairment losses. Cost includes expenditures that are directly attributable to the acquisition of the asset. Subsequent costs are included in the asset’s carrying amount or recognized as a separate asset, as appropriate, only when it is probable that future economic benefits associated with the item will flow to the Company and the cost can be measured reliably. The carrying amount of a replaced asset is derecognized when replaced. Repairs and maintenance costs are charged to the consolidated statements of operations and comprehensive loss during the period in which they are incurred.
Depreciation of property and equipment is provided using the straight-line method at the following rates approximating their estimated useful lives:
| Research and development equipment | 3 years | |
| Computer equipment | 3 years |
| f. | Intangible Assets |
Intangible assets consist of patents and patent applications acquired to be used in drug research programs. Intangible assets are carried at cost less accumulated amortization and impairment losses and are capitalized when the costs can be measured reliably, and it is probable that future economic reliably benefits that are attributable to the asset will flow to the Company. The Company amortizes patents over the term of the patent.
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 2. | Significant Accounting Policies (continued) |
| g. | Impairment of Non-Current Assets |
At each reporting date, the Company reviews the carrying amounts of its non-current assets to determine whether there are any indications of impairment. If any such indication exists, the recoverable amount of the asset is estimated in order to determine the extent of the impairment, if any.
Where the asset does not generate cash flows that are independent from other assets, the Company estimates the recoverable amount of the cash generating unit (“CGU”) to which the asset belongs. The recoverable amount is determined as the higher of fair value less direct costs to sell and the asset’s value in use. In assessing value in use, the estimated future cash flows are discounted to their present value. Estimated future cash flows are calculated using estimated recoverable reserves, estimated future commodity prices and the expected future operating and capital costs. The pre-tax discount rate applied to the estimated future cash flows reflects current market assessments of the time value of money and the risks specific to the asset for which the future cash flow estimates have not been adjusted.
If the carrying amount of an asset or CGU exceeds its recoverable amount, the carrying amount of the asset or CGU is reduced to its recoverable amount through an impairment charge to the consolidated statement of operations and comprehensive loss.
Assets that have been impaired are tested for possible reversal of the impairment whenever events or changes in circumstance indicate that the impairment may have reversed. When an impairment subsequently reverses, the carrying amount of the asset or CGU is increased to the revised estimate of its recoverable amount, but only so that the increased carrying amount does not exceed the carrying amount that would have been determined (net of depreciation, depletion and amortization) had no impairment loss been recognized for the asset or CGU in prior periods. A reversal of impairment is recognized as a gain in the consolidated statement of operations and comprehensive loss.
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 2. | Significant Accounting Policies (continued) |
| h. | Financial Instruments |
Financial assets and financial liabilities are recognized when the Company becomes a party to the contractual provisions of the respective instrument.
Financial assets and financial liabilities are initially measured at fair value. Transaction costs that are directly attributable to the acquisition or issuance of financial assets and financial liabilities (other than financial assets and financial liabilities at FVTPL) are included in the initial carrying value of the related instrument and are amortized using the effective interest method. Transaction costs directly attributable to the acquisition of financial assets or financial liabilities at FVTPL are recognized immediately in the consolidated statement of operations and comprehensive loss.
Financial assets
The classification of financial assets depends on the nature and purpose of the financial assets and is determined at the time of initial recognition.
Financial assets at FVTPL
Financial assets are classified as FVTPL when the financial asset is either held for trading or it is designated as FVTPL. A financial asset is classified as held for trading if:
| ● | it has been acquired principally for the purpose of selling it in the near term; or |
| ● | on initial recognition it is part of a portfolio of identified financial instruments that the Company manages together and has a recent actual pattern of short-term profit-taking. |
Financial assets at amortized cost
Financial assets at amortized cost are non-derivative financial assets which are held within a business model whose objective is to hold assets to collect contractual cash flows and its contractual terms give rise on specified dates to cash flows that are solely payments of principal and interest on the principal amount outstanding. A financial asset (unless it is a trade receivable without a significant financing component that is initially measured at the transaction price) is initially measured at fair value plus, for an item not at FVTPL, transaction costs that are directly attributable to its acquisition. Subsequent to initial recognition, financial assets are measured at amortized cost using the effective interest method, less any impairment.
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 2. | Significant Accounting Policies (continued) |
| h. | Financial Instruments (continued) |
Financial liabilities and equity instruments
Classification as debt or equity
Debt and equity instruments issued by the Company are classified as either financial liabilities or as equity in accordance with the substance of the contractual arrangements and the definitions of a financial liability and an equity instrument.
Equity instruments
An equity instrument is any contract that evidences a residual interest in the assets of an entity after deducting all of its liabilities. Equity instruments issued by the Company are recognized as the proceeds received, net of direct issue costs.
Other financial liabilities
All financial liabilities are measured subsequently at amortized cost using the effective interest method or at FVTPL.
Financial liabilities at FVTPL
Financial liabilities are classified as at FVTPL when the financial liability is (i) contingent consideration of an acquirer in a business combination, (ii) held for trading or (iii) it is designated as at FVTPL.
A financial liability is classified as held for trading if either:
| ● | It has been acquired principally for the purpose of repurchasing it in the near term | |
| ● | On initial recognition it is part of a portfolio of identified financial instruments that the group manages together and has a recent actual pattern of short-term profit-taking | |
| ● | It is a derivative, except for a derivative that is a financial guarantee contract or a designated and effective hedging instrument |
Financial liabilities at FVTPL are measured at fair value, with any gains or losses arising on changes in fair value recognized in profit or loss.
Financial liabilities measured subsequently at amortized cost
Financial liabilities that are not (i) contingent consideration of an acquirer in a business combination, (ii) held-for-trading, or (iii) designated as at FVTPL, are measured subsequently at amortized cost using the effective interest method.
The effective interest method is a method of calculating the amortised cost of a financial liability and of allocating interest expense over the relevant period. The effective interest rate is the rate that exactly discounts estimated future cash payments (including all fees and points paid or received that form an integral part of the effective interest rate, transaction costs and other premiums or discounts) through the expected life of the financial liability, or (where appropriate) a shorter period, to the amortised cost of a financial liability.
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 2. | Significant Accounting Policies (continued) |
| i. | Income Taxes |
Current income tax
The tax currently payable is based on taxable profit for the year. Taxable profit differs from net profit as reported in profit or loss because it excludes items of income or expense that are taxable or deductible in other years and it further excludes items that are never taxable or deductible. The Company’s liability for current tax is calculated using tax rates that have been enacted or substantively enacted by the end of the reporting period.
Deferred income tax
Deferred income tax is provided using the statement of financial position method on temporary differences at the reporting date between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes. The carrying amount of deferred income tax assets is reviewed at the end of each reporting period and recognized only to the extent that it is probable that sufficient taxable income will be available to allow all or part of the deferred income tax asset to be utilized. Deferred income tax assets and liabilities are measured at the tax rates that are expected to apply to the year when the asset is realized or the liability is settled, based on tax rates (and tax laws) that have been enacted or substantively enacted by the end of the reporting period. Deferred income tax assets and deferred income tax liabilities are offset, if a legally enforceable right exists to set off current tax assets against current income tax liabilities and the deferred income taxes relate to the same taxable entity and the same taxation authority.
| j. | Foreign Currency Translation |
The Company’s reporting currency is the United States dollar. The functional currency for the Company and its subsidiary is the currency of the primary economic environment in which the entity operates. As of November 1, 2023, the functional currency of the Company and its Canadian and Israeli subsidiaries is the United States dollar.
Transactions denominated in currencies other than the functional currency are translated using the exchange rate in effect on the transaction date or at the annual average rate. Monetary assets and liabilities denominated in foreign currencies are retranslated at the rate of exchange in effect at the consolidated statement of financial position date. Non-monetary items are translated using the historical rate on the date of the transaction. Foreign exchange gains and losses are included in the consolidated statement of operations and comprehensive loss.
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 2. | Significant Accounting Policies (continued) |
| k. | Share-based Payments |
The grant date fair value of share-based payment awards granted to employees is recognized as stock-based compensation expense, with a corresponding increase in equity, over the period that the employees unconditionally become entitled to the awards. The amount recognized as an expense is adjusted to reflect the number of awards for which the related service and non-market vesting conditions are expected to be met, such that the amount ultimately recognized as an expense is based on the number of awards that do meet the related service and non-market performance conditions at the vesting date. For share-based payment awards with non-vesting conditions, the grant date fair value of the share-based payment is measured to reflect such conditions and there is no true-up for differences between expected and actual outcomes.
Where equity instruments are granted to parties other than employees, they are recorded by reference to the fair value of the services received. If the fair value of the services received cannot be reliably estimated, the Company measures the services received by reference to the fair value of the equity instruments granted, measured at the date the counterparty renders service. The fair value of services received from consultants cannot be reliably measured; therefore, the fair value of the underlying equity instruments was utilized for all periods presented.
All equity-settled share-based payments are reflected in share-based payment reserve, unless exercised. Upon exercise, shares are issued and the amount reflected in share-based payment reserve is credited to share capital, adjusted for any consideration paid.
| l. | Restricted Share Units |
The Company recognizes compensation expense for restricted share units (“RSU’s”) awarded based on the grant-date fair value of the common shares. The grant-date fair value, which is determined by multiplying the Company’s share price by the number of RSU’s granted, is amortized over the vesting period and is included in compensation expense with a corresponding increase in the share-based payment reserve. If RSU’s are for services that have been provided and are non-cancellable, the Company recognizes the full cost of the RSU’s on the date of grant. The amount recognized is adjusted to reflect the number of RSU’s expected to eventually vest.
| m. | Leases |
The Company’s Israeli subsidiary leased an office through to June 30, 2023. At inception of a contract, the Company assesses whether a contract is, or contains, a lease. A contract is, or contains, a lease if the contract conveys the right to control the use of an identified asset for a period of time in exchange for consideration. The Company reassesses whether a contract is, or contains, a lease only if the terms and conditions of the contract are changed.
At the commencement date, the Company measures the lease liability at the present value of the lease payments that are not paid at that date, including, inter alia, the exercise price of a purchase option if the Company is reasonably certain to exercise that option. Simultaneously, the Company recognizes a right-of-use (“ROU”) asset in the amount of the lease liability.
The discount rate applied by the Company is the rate of interest that the Company would have to pay to borrow over a similar term, and with a similar security, the funds necessary to obtain an asset of a similar value to the ROU asset in a similar economic environment. The weighted average of lessee’s incremental annual borrowing rate applied to the lease liabilities as of June 30, 2023 was estimated at 20%.
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 2. | Significant Accounting Policies (continued) |
| m. | Leases (continued) |
The lease term is the non-cancellable period for which the Company has the right to use an underlying asset, together with both, the periods covered by an option to extend the lease if the Company is reasonably certain to exercise that option and periods covered by an option to terminate the lease if the Company is reasonably certain not to exercise that option.
After the commencement date, the Company measures the ROU asset applying the cost model, less any accumulated depreciation and any accumulated impairment losses and adjusted for any remeasurement of the lease liability.
Assets are depreciated by the straight-line method over the estimated useful lives of the ROU asset or the lease period, whichever is shorter.
Interest on the lease liability is recognized in profit or loss in each period during the lease term in an amount that produces a constant periodic rate of interest on the remaining balance of the lease liability.
The Company applied the following practical expedients:
| ● | Non-lease components: practical expedient by class of underlying asset not to separate non-lease components (services) from lease components and, instead, account for each lease component and any associated non lease components as a single lease component. |
| ● | The practical expedient for short-term leases is applied. |
| n. | Employee benefits |
| 1) | Pension and retirement benefit obligations |
The Company operates a number of post-employment defined contribution plans.
A defined contribution plan is a program that benefits an employee after termination of employment, under which the Company regularly makes fixed payments to a separate and independent entity so that the Company has no legal or constructive obligation to pay additional contributions if the fund does not hold sufficient assets to pay all employees the benefits relating to employee service in the current and prior periods. The fund assets are not included in the Company’s statement of financial position.
The Company operates pension and severance compensation plans subject to Section 14 of the Israeli Severance Pay Law. The Option Plans are funded through payments to insurance companies or pension funds administered by trustees. In accordance with its terms, the Option Plans meet the definition of a defined contribution plans as defined above. The expenses in respect of defined contribution plans during the years ended October 31, 2023, 2022 and 2021 were $13,988, $31,157 and $50,661, respectively.
| 2) | Vacation and recreation pay |
Under Israeli law, each employee is entitled to vacation days and recreation pay, both computed on an annual basis. The entitlement is based on the period of employment. The Company records a liability and an expense for vacation and recreation pay, based on the benefit accumulated for each employee.
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 2. | Significant Accounting Policies (continued) |
| o. | Research and Development |
Research costs are charged to operations as incurred. Development activities involve a plan or design for the production of new or substantially improved products and processes. Development expenditures are capitalized only if development costs can be measured reliably, the product is technically and commercially feasible, future economic benefits are probable, and the Company intends to or has sufficient resources to complete development and to use or sell the asset. The expenditure capitalized includes the cost of materials, direct labor and overhead costs that are directly attributable to preparing the asset for its intended use and borrowing costs on qualifying assets. Other development expenditures are recognized in the consolidated statement of operations and comprehensive loss as incurred. The Company has not capitalized any development costs for the years ended October 31, 2023, 2022 and 2021.
| p. | Loss Per Share |
Basic loss per share is computed using the weighted average number of common shares outstanding during the period. The treasury stock method is used for the calculation of diluted loss per share, whereby all “in the money” stock options and share purchase warrants are assumed to have been exercised at the beginning of the period and the proceeds from their exercise are assumed to have been used to purchase common shares at the average market price during the period. When a loss is incurred during the period, basic and diluted loss per share is the same as the exercise of stock options and share purchase warrants is considered to be anti-dilutive. As of October 31, 2023, the Company had 833,810 (2022 – 26,419, 2021- 21,507) potentially dilutive shares outstanding.
| q. | The Company capitalizes certain legal and other third-party fees that are directly related to the Company’s in- process equity financing until such financing is consummated. After consummation of such equity financing, these costs are recorded as a reduction of the respective gross proceeds. Should a planned equity financing be abandoned, terminated or significantly delayed, the deferred offering costs are written off to operating expenses. Where the financing includes both an equity and liability component, pro-rata issuance costs relating to the liability component are charged to the statement of operations. |
| r. | Accounting Standards Issued But Not Yet Effective |
| i) | Amendments to IAS 1 Presentation of Financial Statements—Classification of Liabilities as Current or Non-current |
The IASB issued a narrow-scope amendment to IAS 1, in January 2020, to clarify that liabilities are classified as either current or non-current, depending on the rights that exist at the end of the reporting period. The amendment could affect the classification of liabilities, particularly for entities that previously considered management’s intentions to determine classification and for some liabilities that can be converted into equity. Inter alia, the amendment requires the following:
Liabilities are classified as non-current if the entity has a substantive right to defer settlement for at least 12 months at the end of the reporting period. The amendment no longer refers to unconditional rights. The assessment determines whether a right exists, but it does not consider whether the entity will exercise the right.
’Settlement’ is defined as the extinguishment of a liability with cash, other economic resources or an entity’s own equity instruments. There is an exception for convertible instruments that might be converted into equity, but only for those instruments where the conversion option is classified as an equity instrument as a separate component of a compound financial instrument.
The amendments are applied retrospectively for annual periods beginning on or after 1 January 2024, with early application permitted.
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 2. | Significant Accounting Policies (continued) |
| ii) | Amendments to IAS 1 Presentation of Financial Statements and IFRS Practice Statement 2 Making Materiality Judgements - Disclosure of Accounting Policies |
The amendments change the requirements in IAS 1 with regard to disclosure of accounting policies. The amendments replace all instances of the term ‘significant accounting policies’ with ‘material accounting policy information’. Accounting policy information is material if, when considered together with other information included in an entity’s financial statements, it can reasonably be expected to influence decisions that the primary users of general-purpose financial statements make on the basis of those financial statements.
The supporting paragraphs in IAS 1 are also amended to clarify that accounting policy information that relates to immaterial transactions, other events or conditions is immaterial and need not be disclosed. Accounting policy information may be material because of the nature of the related transactions, other events or conditions, even if the amounts are immaterial. However, not all accounting policy information relating to material transactions, other events or conditions is itself material.
The IASB has also developed guidance and examples to explain and demonstrate the application of the ‘four-step materiality process’ described in IFRS Practice Statement 2.
The amendments are applied for annual periods beginning on or after January 1, 2023.
| 3. | Intangible Assets |
On May 4, 2021, the Company entered into an assignment agreement to acquire certain intellectual property including a patent for “Binge Behavior Regulators,” which has been granted in the U.S., Europe, China and India, with pending divisional applications in Europe and the U.S, and a patent for “Alcohol Beverage Substitute,” which has been approved for a European patent, with pending applications in the U.S., China and India. In consideration for the acquired intellectual property, the Company paid $160,000. The patents are amortized over their estimated remaining life of 14.6 years.
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 4. | Leases |
On July 1, 2021, the Company entered into a lease agreement (“2021 Lease”) with Scisparc Ltd, a related party (“Scisparc”) and a third party for a total area of approximately 240m2, of which the Company occupies approximately 120m2 for the Company’s offices, in Tel Aviv, Israel. The Company, Scisparc and the third party had an option to extend the 2021 Lease for an additional three-year period. The Company’s base rent was ILS11,000 per month ($3,080) during the term of the 2021 Lease. The lease liability was discounted using the Company’s estimated incremental borrowing rate of 20%. On December 31, 2021, the third party elected to leave the office space, and a new lease agreement was signed with the Company and Scisparc. As a result, the Company’s base rent was increased to ILS 18,200 per month ($5,094).
The lease expired on June 30, 2023. As of October 31, 2023 the Company and SciSparc were in the process of negotiating the terms of a new lease contract. On December 25, 2023, the rental agreement was renewed for a further 18 months. The Company’s base rent was increased to ILS 23,300 per month ($6,500). See Note 6b.
As of October 31, 2023, the Company has a pledge on its short-term deposit in favor of an Israeli bank in the amount of approximately $33 thousand to secure the Company’s lease commitment.
| ROU office space |
||||
| Balance, October 31, 2022 | $ | 35,730 | ||
| Addition | ||||
| Accumulated depreciation | (35,381 | ) | ||
| Foreign currency fluctuation | (349 | ) | ||
| Balance, October 31, 2023 | $ | |||
| Lease liability |
||||
| Balance, October 31, 2022 | $ | 38,390 | ||
| Addition | ||||
| Lease payments | (40,922 | ) | ||
| Interest expense | 2,907 | |||
| Foreign currency translation | (375 | ) | ||
| Balance, October 31, 2023 | $ | |||
Amounts recognized in profit or loss
| Year ended | ||||
| October 31, | ||||
| 2023 | ||||
| Depreciation | $ | 35,381 | ||
| Interest | 2,907 | |||
| Foreign currency fluctuation | 722 | |||
| $ | 39,010 | |||
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 5. | Short-term Investment |
Pursuant to the Share Exchange Agreement (as defined in Note 8d(iii)) with Medigus Ltd (“Medigus”), on February 14, 2022, the Company received 27,778 (*) ordinary shares of Medigus (the “Medigus Agreement”).
As of October 31, 2023, the Company holds 27,778 ordinary shares of Medigus (approximately 0.11%) with a total fair value of $86,112. The fair value of ordinary shares held was determined by reference to public price quotations in an active market. See Note 12.
| October 31, 2022 |
Additions | Unrealized loss |
October 31, 2023 |
|||||||||||||
| Medigus Ltd. – Shares | $ | 193,750 | $ | - | $ | (107,638 | ) | $ | 86,112 | |||||||
| October 31, 2021 |
Additions | Unrealized loss |
October 31, 2022 |
|||||||||||||
| Medigus Ltd. – Shares | $ | $ | 501,938 | $ | (308,188 | ) | $ | 193,750 | ||||||||
| * | The share amount is following the Medigus 1:15 share split from November 14, 2022. |
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 6. | Related Party Transactions |
| a. | Compensation to key management personnel |
| (i) | The compensation to key management personnel for employment services they provide to the Company is as follows: |
| Year ended | Year ended | Year ended | ||||||||||
| October 31, | October 31, | October 31, | ||||||||||
| 2023 | 2022 | 2021 | ||||||||||
| Officers: | ||||||||||||
| Payroll and other short-term benefits | $ | $ | 32,533 | $ | 297,188 | |||||||
| Consulting fees | 410,066 | 453,519 | 49,820 | |||||||||
| Share based compensation | 124,808 | 772,786 | 179,996 | |||||||||
| $ | 534,874 | $ | 1,258,838 | $ | 527,004 | |||||||
| Directors: | ||||||||||||
| Directors’ fees | $ | 182,261 | $ | 124,746 | $ | 38,098 | ||||||
| Share based compensation | 121,981 | 43,485 | 17,218 | |||||||||
| $ | 304,242 | $ | 168,231 | $ | 55,316 | |||||||
| (ii) | Balances with related parties |
| October 31, | October 31, | |||||||
| 2023 | 2022 | |||||||
| Amounts owed to officers | $ | 29,666 | $ | 136,149 | ||||
| Amounts owed to directors | 12,767 | 70,345 | ||||||
| $ | 42,433 | $ | 206,494 | |||||
| b. |
On March 7, 2022, the Company signed an agreement with SciSparc Ltd (“SciSparc”), pursuant to which the Company and SciSparc agreed to cooperate in conducting a feasibility study using certain molecules developed by each party (the “Cooperation Agreement”). Certain of the Company’s officers and directors currently operate, manage or are engaged as officers and/or directors of SciSparc.
In June 2023, the Company entered into a research agreement with the Hebrew University of Jerusalem to evaluate our and SciSparc’s combination treatment for obesity and metabolic syndrome.
To date, the collaboration has resulted in the filing of nine patent applications. To the extent the parties determine to proceed to a commercial cooperation, they will enter into a joint venture where the parties share the economics and rights on a 50%-50% basis. To date, no determination has been made to pursue the joint venture and the development of the molecule remains in a very early stage.
For the year ended October 31, 2023, the Company incurred research and development expenses conducted within the framework of the Cooperation Agreement in the amount of $131,409, $194,205 and nil for the years ended October 31, 2023, 2022 and 2021, respectively. As of October 31, 2023, $136,002 is owed to the Company by SciSparc (2022-$46,988). |
| c. | The Company shares office space with SciSparc and participates for paying office expenses– see Note 4. |
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 7. | Derivative warrants liabilities |
| a. | On April 6, 2023, the Company issued 150,191 warrants in connection with its April 2023 Public Offering (“April 2023 Warrants”). The warrant includes a cashless exercise provision and repricing adjustments for offerings at a price lower than the existing exercise price of the warrants, stock splits, reclassifications, subdivisions, and other similar transactions (“April 2023 Warrant Adjustments”) and therefore, these warrants were recorded at their fair value as a derivative liability and the time of the grant and are revalued at the end of each reporting period. |
On September 24, 2023, following the September 2023 Public Offering, which included the offering of common shares at a price lower than the exercise price of the April 2023 Warrants, the exercise price of the April 2023 Warrants was reduced to $5.124, and each April 2023 Warrant became convertible into 4.6 common shares of the Company.
During the period between October 17, 2023 and October 20, 2023, 26,153 April 2023 Warrants with a fair value of $574,040 were exercised into 119,433 common shares. (See note 19(b)).
| b. | On September 18, 2023, the Company issued 250,000 warrants in connection with its September 2023 Public Offering (“September 2023 Warrants”). The warrant includes a cashless exercise provision and repricing adjustments for offerings at a price lower than the existing exercise price of the warrants, stock splits, reclassifications, subdivisions, and other similar transactions (“September 2023 Warrant Adjustments”) and therefore, these warrants were recorded at their fair value as a derivative liability and the time of the grant and are revalued at the end of each reporting period. |
| c. | During the year ended October 31, 2023, the Company recorded a loss on the revaluation of the total derivative warrants liabilities of $2,189,986, in the Consolidated Statements of Operations and Comprehensive Loss. |
| d. | The binomial model was used to measure the derivative warrant liability with the following assumptions: |
| October 31, 2023 |
||||
| Share Price | $ | 3.66-$11.52 | ||
| Exercise Price | $ | 5.12-$23.40 | ||
| Expected life | 4.43-5 years | |||
| Risk-free interest rate | 3.37-4.95 | % | ||
| Dividend yield | 0.00 | % | ||
| Expected volatility | 74.79-157.7 | % | ||
| e. | The following table presents the changes in the warrants liability during the period: |
| Balance as of November 1, 2022 | $ | |||
| Issuance of April 2023 Warrants | 1,771,208 | |||
| Issuance of September 2023 Warrants | 923,225 | |||
| Exercise of warrants | (574,040 | ) | ||
| Changes in fair value of warrants | 2,189,986 | |||
| Balance as of October 31, 2023 | $ | 4,310,379 |
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 8. | Share Capital |
| a. | The Company’s authorized share capital is unlimited common shares without par value share. As of October 31, 2023, the number of common shares issued and outstanding is 607,337 (October 31, 2022 – 43,992). |
| b. | On September 30, 2022 and November 28, 2023, the Company effected a 1-for-30 share consolidation (“Reverse Share Split”) of its issued and outstanding common shares. All share amounts and instruments convertible into common shares prior to the date of the reverse share split have been retroactively restated for all periods presented. |
| c. | Share transactions during the year ended October 31, 2023: |
| (i) | On November 14, 2022, the Company completed an underwritten public offering of 38,462 common shares at a price to the public of US$195.00 per share, for aggregate gross proceeds of US$7.5 million, prior to deducting underwriting discounts and offering expenses. The offering closed on November 17, 2022. Net proceeds from the offering were $6,363,906. |
In addition, the Company granted Aegis Capital Corp. (“Aegis”), who acted as the underwriters for the deal, a 45-day option to purchase up to 5,769 additional common shares, equal to 15% of the number of common shares sold in the offering solely to cover over-allotments, if any (“Over-Allotment”). The public purchase price per additional common share would have been $195.00 per share. The Over-Allotment was not exercised.
Aegis received 1,923 underwriter warrants, each such warrant entitling the agents to receive one common share upon payment of $243.75 per share, exercisable six months after the commencement of sales of this offering and expiring on a date which is no more than five years after the commencement of sales of the offering. The fair value of the underwriter warrants of $337,579 were accounted for as an issuance cost within the share-based payment reserve. The fair value of the underwriters warrants was estimated using the Black-Scholes option pricing model assuming no expected dividends or forfeitures and the following weighted average assumptions:
| Risk-free interest rate | 1.43 | % | ||
| Expected life (in years) | 5 | |||
| Expected volatility | 150 | % |
In connection with the offering, the Company’s common shares were approved for listing on the Nasdaq and began trading on the Nasdaq (in addition to the CSE) under the symbol “CMND” on November 15, 2022.
Following the public offering and pursuant to the Medigus SPA (see note 8d(iii)), Medigus was entitled to receive 1,494 common shares and 75 warrants pursuant to an anti-dilution clause included in the agreement signed between the Company and Medigus on June 29, 2022. The anti-dilution feature was recorded as a derivative liability as of October 31, 2022. On May 23, 2023, the Company issued the 1,494 common shares and 75 warrants to Medigus. The fair value of the issuance ($296,845) was recorded in shareholder equity against the extinguishment of the derivative liability.
| (ii) | On January 16, 2023, 161 common shares were issued in respect of RSU’s that had been fully vested. The RSU’s had a fair value of $39,975 at the time of issuance. |
On February 22, 2023, 400 common shares were issued in respect of fully vested RSU’s that had been fully vested. The RSU’s had a fair value of $110,096 at the time of issuance.
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 8. | Share Capital (continued) |
| (iii) | On April 6, 2023, the Company completed an underwritten public offering of 103,249 common shares at a price to the public of US$23.40 per share and pre-funded warrants to purchase 46,942 common shares at a price to the public of US$23.37 per pre-funded warrant (“Pre-Funded Warrants”), for aggregate gross proceeds of US$3.5 million (the “April 2023 Public Offering”). The Pre-Funded Warrants were exercisable at $0.03 into one common share, and all the Pre-Funded Warrants were exercised by April 30, 2023. In addition, each April 2023 Public Offering shareholder and each Pre-Funded Warrant holder received a common warrant, which was immediately exercisable, will expire five years from the date of issuance and have an exercise price of US$23.40 per common share (“April 2023 Public Offering Warrant”). The April 2023 Public Offering warrants include a cashless exercise provision and repricing provisions under certain circumstances, that also includes a potential change in the number of shares to be issued for each warrant depending on the change in the exercise price of the warrant. On September 24, 2023, as a result of the September 2023 Public Offering, which included the offering of common shares at a price lower than the exercise price of the April 2023 Warrants, the exercise price of these warrants was reduced to $5.124, and each April 2023 Warrant is convertible into 4.6 common shares of the Company (See also note 19(b)). |
Net proceeds from the offering were $2,936,079.
| (iv) | On May 23, 2023, 239 common shares with a fair value of $27,965 were issued to consultants and 1,494 common shares with a fair value of $110,000 were issued to providers of investor services in respect of services. |
| (v) | On June 1, 2023, 245 common shares were issued in respect of RSU’s that had been fully vested. The RSU’s had a fair value of $48,194 at the time of issuance. |
| (vi) | On July 10, 2023, 597 common shares with a fair value of $10,000 were issued to providers of investor services in respect of services. |
| (vii) | On August 28, 2023, 585 common shares were issued in respect of RSU’s that had been fully vested. The RSU’s had a fair value of $65,143 at the time of issuance and 44 common shares with a fair value of $607 were issued to providers of investor services in respect of services. |
| (viii) | On September 18, 2023 the Company completed an underwritten public offering of 225,833 common shares at a price to the public of US$9.00 per share and pre-funded warrants to purchase 24,167 common shares at a price to the public of US$8.97 per pre-funded warrant (“Pre-Funded Warrants”), for aggregate gross proceeds of US$2.25 million (the “September 2023 Public Offering”). The Pre-Funded Warrants were exercisable at $0.03 into one common share, and all the Pre-Funded Warrants were exercised by September 30, 2023. In addition, each September 2023 Public Offering shareholder and each Pre-Funded Warrant holder received a common warrant, which was immediately exercisable, will expire five years from the date of issuance and have an exercise price of US$9.00 per common share (“September 2023 Public Offering Warrant”). The September 2023 Public Offering warrants include a cashless exercise provision and repricing provisions under certain circumstances that also includes a potential change in the number of shares to be issued for each warrant depending on the change in the exercise price of the warrant. Net proceeds from the offering were $1,814,193 (See also note 19(b)). |
| (ix) | During the period between October 17, 2023 and October 20, 2023, 26,153 April 2023 Warrants with a fair value of $574,040 were exercised into 119,433 common shares. |
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 8. | Share Capital (continued) |
| d. | Share transactions during the year ended October 31, 2022: |
| (i) | On November 26, 2021, the Company issued 44 shares of common stock with a fair value of $21,100 to the Chief Science Officer (“CSO”) of the Company. |
| (ii) | On February 14, 2022, the Company issued 45 shares of common stock with a fair value of $17,584 to the CSO. |
| (iii) | On February 14, 2022, the Company completed a share purchase agreement with Medigus, whereby the Company issued a total of 2,208 units to Medigus in consideration for US$750,000 (“Cash Financing”) and 27,778 common shares of Medigus (“Share Exchange”). Each unit is comprised of one common share and one warrant, with each warrant exercisable for a period of 18 months at CAD$60.00 per share (the “Medigus SPA”). The Medigus SPA contains certain anti dilution provisions. |
Pursuant to the Cash Financing, the Company issued 1,325 units at $720.00 per unit for proceeds of $698,897.
In connection with the Cash Financing, the Company incurred finder’s fees of $69,890, which have been charged to the statement of changes in equity.
Pursuant to the Share Exchange, the Company issued 883 units with a fair value of $455,487, consisting of common shares with a fair value of $372,745 and warrants with a fair value of $82,742. The fair value of the warrants was determined using the Black-Scholes option pricing model with the following assumptions: Risk-free rate of 1.43%, expected life of 1.5 years, and volatility of 107.46%.
In connection with the Share Exchange, the Company incurred finder’s fees of $50,000, which were allocated to the base cost of the shares in Medigus.
On June 29, 2022, the Company signed an amendment to the Medigus Agreement (“Medigus Amended Agreement”), pursuant to which, in the event of an initial public offering, Medigus would receive, in respect of the Cash Financing, for no additional consideration, the number of shares necessary to maintain an ownership percentage of the Company’s shares equal to the level of ownership it had at the time of the Medigus Agreement.
As of October 31, 2022, the Company recorded the fair value of this derivative financial liability in the amount of $290,569 as a current liability.
| e. | Share transactions during the year ended October 31, 2021: |
| (i) | On April 9, 2021, the Company issued 11,111 common shares at $98.91 per share for gross proceeds of $1,098,982. In connection with this private placement, the Company paid a finder’s fee of $85,857. |
| (ii) | On June 22, 2021, the Company issued 9,222 units at $494.54 per unit for gross proceeds of $4,560,773. Each unit consisted of one common share and one share purchase warrant exercisable at $824.24 per common share expiring on December 22, 2022. In connection, with this private placement, the Company paid finders’ fees of $435,197. |
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 9. | Share Purchase Warrants |
The following table summarizes the changes in the Company’s share purchase warrants:
| Number of warrants |
Weighted average exercise price |
|||||||
| Balance, October 31, 2021 | 17,555 | $ | 482.55 | |||||
| Issued (i) | 2,208 | 1,325.67 | (*) | |||||
| Balance, October 31, 2022 | 19,763 | 576.66 | ||||||
| Issuance of underwriter warrants (Note 8c (i)) | 1,923 | 243.75 | ||||||
| Issuance of April 2023 warrants (Note 7a) | 150,191 | (**) | 5.124 | |||||
| Issuance of September 2023 warrants (Note 7b) | 250,000 | 9.00 | ||||||
| Issuance of Medigus warrants (Note 8c (i)) | 75 | 1,297.67 | (*) | |||||
| Expiration of warrants | (11,430 | ) | 922.98 | |||||
| Exercise of warrants (Note 7a) | (26,153 | ) | 5.124 | |||||
| Balance, October 31, 2023 | 384,369 | $ | 7.90 | |||||
| Number of shares to be issued from the exercise of warrants | 826,781 | (***) | ||||||
| (*) | Warrants issued with an exercise price of CAD$1,800.00. |
| (**) | These warrants convert into 685,883 shares. |
| (***) | See note 19(b). |
| (i) | On February 14, 2022, the Company completed the Medigus Agreement, whereby the Company completed the Cash Financing and the Share Exchange. Each unit was comprised of one common share and one warrant, with each warrant exercisable at $1,297.67 per common share for a period of 18 months. |
As of October 31, 2023, the following share purchase warrants were outstanding:
| Number of warrants outstanding |
Number of shares
to be issued from the exercise of warrants |
Exercise price | Exercise price (USD) | Expiry date | ||||||||||||
| 8,333 | 8,333 | C$ | 135.00 | $ | 97.33 | April 22, 2024 | ||||||||||
| 1,923 | 1,923 | $ | 243.75 | $ | 243.75 | November 17, 2027 | ||||||||||
| 124,038 | 566,450 | $ | 5.124 | $ | 5.124 | April 6, 2028 | ||||||||||
| 75 | 75 | C$ | 1,800 | $ | 1,297.67 | November 23, 2024 | ||||||||||
| 250,000 | 250,000 | $ | 9.00 | $ | 9.00 | September 17, 2028 | ||||||||||
| 384,369 | 826,781 | (*) | ||||||||||||||
| (*) | See note 19(b). |
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 10. | Stock Options |
| a. | On September 1, 2021, the Company implemented a stock option plan pursuant to which stock options may be granted to directors, officers, employees, and consultants of the Company. The Board is authorized to grant the maximum number of common shares reserved for issuance in any 12-month period to anyone, optionee, other than a consultant may not exceed 5% of the issued and outstanding common shares at the date of the grant. The maximum number of common shares reserved for issuance in any 12-month period to any consultant may not exceed 2% of the issued and outstanding common shares at the date of the grant and the maximum number of common shares reserved for issuance in any 12-month period to all persons engaged in investor relations activities may not exceed 2% of the issued and outstanding number of common shares at the date of the grant. See also note 19(e). |
| b. | The following table summarizes the changes in the Company’s stock options for the years ended October 31, 2023 and October 31, 2022: |
| Number of options |
Weighted (C$) |
Weighted (USD$) |
||||||||||
| Outstanding, October 31, 2021 | 3,729 | C$ | 620.10 | $ | 455.49 | |||||||
| Granted (i) | 2,877 | 620.40 | 455.71 | |||||||||
| Cancelled (ii) | (1,352 | ) | 646.80 | 475.10 | ||||||||
| Outstanding, October 31, 2022 | 5,254 | C$ | 613.5 | $ | 450.64 | |||||||
| Granted (iii, iv, v) | 334 | 442.22 | 318.81 | |||||||||
| Outstanding, October 31, 2023 | 5,588 | C$ | 603.12 | $ | 434.81 | |||||||
| Exercisable, October 31, 2023 | 4,039 | C$ | 606.00 | $ | 436.89 | |||||||
| i) | During the year ended October 31, 2022, the Company granted 2,877 stock options to officers, directors and consultants with weighted average exercise price of $455.71 per Common Share. The options vest over 3 years with expiration dates between 5 to 10 years. |
| ii) | On July 2, 2022, 222 options canceled with a fair value of $17,649. On May 7, 2022, 1,111 options canceled with a fair value of $92,170. On May 1, 2022 ,19 options canceled with a fair value of $3,959. |
| iii) | On May 23, 2023, the Company granted 61 stock options to a consultant of the Company. The options are exercisable at CAD$315.00 (USD$227.09) per share. The options expire on May 23, 2033. |
| iv) | On June 26, 2023, the Company granted 223 stock options to a consultant of the Company. The 156 options are exercisable at CAD$504 (USD$363.35) per share and the 67 options are exercisable at CAD$720.00 (USD$519.07) per share. The options expire on June 26, 2033. |
| v) | On July 6, 2023, the Company granted 50 stock options to a consultant of the Company. The options are exercisable at $23.40 per share. The options expire on July 6, 2033. |
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 10. | Stock Options (continued) |
| c. | Additional information regarding stock options outstanding as of October 31, 2023, is as follows: |
| Outstanding | Exercisable | |||||||||||||||||||||||||
| Number of stock options |
Weighted average remaining contractual life (years) |
Weighted average exercise price (C$) |
Weighted average exercise price (USD$) |
Number of stock options |
Weighted average exercise price (C$) |
Weighted average exercise price (USD$) |
||||||||||||||||||||
| 533 | 2.57 | C$ | 166.50 | $ | 120.03 | 400 | C$ | 166.50 | $ | 120.03 | ||||||||||||||||
| 978 | 8.26 | 504.00 | 363.35 | 667 | 504.00 | 363.35 | ||||||||||||||||||||
| 1,166 | 2.57 | 675.00 | 486.63 | 916 | 675.00 | 486.63 | ||||||||||||||||||||
| 200 | 5.50 | 702.00 | 506.09 | 142 | 702.00 | 506.09 | ||||||||||||||||||||
| 133 | 2.89 | 747.00 | 538.53 | 133 | 747.00 | 538.53 | ||||||||||||||||||||
| 422 | 8.11 | 612.00 | 441.21 | 422 | 612.00 | 441.21 | ||||||||||||||||||||
| 1,044 | 8.26 | 720.00 | 519.07 | 609 | 720.00 | 519.07 | ||||||||||||||||||||
| 667 | 2.67 | 756.00 | 545.02 | 500 | 756.00 | 545.02 | ||||||||||||||||||||
| 111 | 8.11 | 900.00 | 648.84 | 74 | 900.00 | 648.84 | ||||||||||||||||||||
| 61 | 9.57 | 315.00 | 227.09 | 31 | 315.00 | 227.09 | ||||||||||||||||||||
| 67 | 9.66 | 720.00 | 519.07 | 33 | 720.00 | 519.07 | ||||||||||||||||||||
| 156 | 9.66 | 504.00 | 363.35 | 104 | 504.00 | 363.35 | ||||||||||||||||||||
| 50 | 9.69 | 32.46 | 23.40 | 8 | 32.46 | 23.40 | ||||||||||||||||||||
| 5,588 | 5.70 | C$ | 603.12 | $ | 434.81 | 4,039 | C$ | 606.00 | $ | 436.89 | ||||||||||||||||
The fair value of stock options previously granted to certain consultants for ongoing services measured during the period have been estimated using the Black-Scholes option pricing model assuming no expected dividends or forfeitures and the following weighted average assumptions:
| Year ended October 31, | ||||||||
| 2023 | 2022 | |||||||
| Risk-free interest rate | 4.10 | % | 2.05 | % | ||||
| Expected life (in years) | 5.61 | 8.67 | ||||||
| Expected volatility | 140.41-160.81 | % | 94.09 | % | ||||
| d. | The portion of the total fair value of stock options expensed during the year ended October 31, 2023, was $221,625 (2022 - $788,657) which was recorded as share-based compensation expense. |
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 11. | Restricted Share Units |
| a. | On August 4, 2021, the Company approved an RSU plan, which is designed to provide certain directors, officers, employees, and consultants of the Company with the opportunity to acquire RSU’s of the Company. Each unit is equivalent in value to a common share and upon vesting results in the holder thereof being issued, at the discretion of the Board, either (i) a common share, or (ii) an amount of cash equal to the fair market value of a common share. |
| b. | The following table summarizes the changes in RSUs: |
| Number of RSUs |
Weighted average issue price (C$) |
Weighted average issue price (USD$) |
||||||||||
| Balance, October 31, 2021 | $ | $ | ||||||||||
| Granted | 1,177 | 316.50 | 232.48 | |||||||||
| Vested | (1,177 | ) | 316.50 | 232.48 | ||||||||
| Balance, October 31, 2022 | $ | $ | ||||||||||
| Granted (i) | 2,200 | 55.97 | 41.46 | |||||||||
| Vested | (2,200 | ) | 55.97 | 41.46 | ||||||||
| Balance, October 31, 2023 | $ | $ | ||||||||||
| (i) | During the year ended October 31, 2023, the Company issued 2,200 RSU’s with a fair value of $91,589 to consultants (2022 - $272,985). |
| c. | During the year ended October 31, 2023, the Company recognized share-based compensation of $(171,819), being the fair value of the RSU’s that vested during the period. This amount was charged against the share- based payment reserve in the Consolidated Statement of Changes in Shareholders’ Equity (2022 - $380,207). |
| 12. | Financial Instruments and Risk Management |
| a. | Fair Values |
Fair value measurements are classified using a fair value hierarchy that reflects the significance of inputs used in making the measurements. The fair value hierarchy has the following levels:
| ● | Level 1 - valuation based on quoted prices (unadjusted) in active markets for identical assets or liabilities; |
| ● | Level 2 - valuation techniques based on inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly (i.e. as prices) or indirectly (i.e. derived from prices); and |
| ● | Level 3 - valuation techniques using inputs for the asset or liability that are not based on observable market data (unobservable inputs). |
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 12. | Financial Instruments and Risk Management (continued) |
Assets and liabilities measured at fair value on a recurring basis were presented on the Company’s statement of financial position as of October 31, 2023, as follows:
| Fair Value Measurements Using | ||||||||||||||||
| Quoted prices in active markets for identical instruments (Level 1) |
Significant other observable inputs (Level 2) |
Significant unobservable inputs (Level 3) |
Balance October 31, 2023 |
|||||||||||||
| Short-term investment | $ | 86,112 | $ | – | $ | – | $ | 86,112 | ||||||||
| Derivative warrants liability | – | – | 4,310,379 | 4,310,379 | ||||||||||||
Assets and liabilities measured at fair value on a recurring basis were presented on the Company’s statement of financial position as of October 31, 2022, as follows:
| Fair Value Measurements Using | ||||||||||||||||
| Quoted prices in active markets for identical instruments (Level 1) |
Significant other observable inputs (Level 2) |
Significant unobservable inputs (Level 3) |
Balance October 31, 2022 |
|||||||||||||
| Short-term investment | $ | 193,750 | $ | $ | $ | 193,750 | ||||||||||
| Derivative liability | 290,569 | 290,569 | ||||||||||||||
The fair value of other assets and liabilities, which include cash and cash equivalents, amounts receivable, accounts payable and accrued liabilities, and amounts due to / from related parties, approximate their carrying values due to the relatively short-term maturity of these instruments.
| b. | Credit Risk |
Financial instruments that potentially subject the Company to a concentration of credit risk consist primarily of cash and cash equivalents. The Company limits its exposure to credit loss by placing its cash with high credit quality financial institutions. The carrying amount of financial assets represents the maximum credit exposure.
| c. | Foreign Exchange Rate Risk |
Foreign currency risk is the risk that the fair value of future cash flows of a financial instrument will fluctuate due to changes in foreign exchange rates. The Company is exposed to foreign currency risk to the extent that monetary assets and liabilities are denominated in a foreign currency. The Company’s subsidiary operates in Israel and has certain monetary financial instruments denominated in NIS and CAD. The Company has not entered into foreign exchange rate contracts to mitigate this risk.
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 12. | Financial Instruments and Risk Management (continued) |
| c. | Foreign Exchange Rate Risk (continued) |
The following table indicates the impact of foreign currency exchange risk on net working capital as of October 31, 2023. The table below also provides a sensitivity analysis of a 10% strengthening of the foreign currency against functional currencies identified which would have increased (decreased) the Company’s net loss by the amounts shown in the table below. A 10% weakening of the foreign currency against the functional currencies would have had the equal but opposite effect as of October 31, 2023.
| Cash | $ | 78,503 | ||
| Amounts receivable | 104,320 | |||
| Accounts payable and accrued liabilities | (177,226 | ) | ||
| Due to related parties | (42,433 | ) | ||
| Total foreign currency financial assets and liabilities | $ | (36,836 | ) | |
| Impact of a 10% strengthening or weakening of foreign exchange rate | $ | (3,684 | ) |
| d. | Interest Rate Risk |
Interest rate risk is the risk that the fair value of future cash flows of a financial instrument will fluctuate because of changes in market interest rates. The fair value of the warrant liability can fluctuate depending on the fluctuation in the risk free interest rate.
| e. | Liquidity Risk |
Liquidity risk is the risk that the Company will not be able to meet its financial obligations as they fall due. The Company’s objective to managing liquidity risk is to ensure that it has sufficient liquidity available to meet its liabilities when due. The Company relies on raising debt or equity financing in a timely manner.
The following amounts are the contractual maturities of financial liabilities as of October 31, 2023 and 2022:
| October 31, 2023 | Total | Within 1 year |
Within 2-5 years |
|||||||||
| Accounts payable and accrued liabilities | $ | 617,004 | $ | 617,004 | $ | |||||||
| Due to related parties | 42,433 | 42,433 | ||||||||||
| $ | 659,437 | $ | 659,437 | $ | ||||||||
| October 31, 2022 | Total | Within 1 year |
Within 2-5 years |
|||||||||
| Accounts payable and accrued liabilities | $ | 1,396,960 | $ | 1,396,960 | $ | |||||||
| Due to related parties | 206,494 | 206,494 | ||||||||||
| Lease liability | 38,390 | 38,390 | ||||||||||
| $ | 1,641,844 | $ | 1,641,844 | $ | ||||||||
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 13. | Capital Management |
The Company manages its capital to maintain its ability to continue as a going concern and to provide returns to shareholders and benefits to other stakeholders. The capital structure of the Company consists of cash and equity comprised of issued share capital and share premium, warrants and share-based payment reserve.
The Company manages its capital structure and makes adjustments to it in light of economic conditions. The Company, upon approval from its Board, will balance its overall capital structure through new share issuances or by undertaking other activities as deemed appropriate under the specific circumstances.
The Company is not subject to externally imposed capital requirements and the Company’s overall strategy with respect to capital risk management remains unchanged from the year ended October 31, 2022.
| 14. | Commitments |
The Company enters into contracts in the ordinary course of business with contract research organizations for clinical trials and clinical supply manufacturing and with vendors for non-clinical research studies and other services and products for operating purposes, which generally provide for termination upon 30 to 120 days’ notice or less, and therefore are cancelable contracts and not considered as commitment or purchase obligations.
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 15. | Segmented Information |
As of October 31, 2023, the Company has one operating segment, research and development of psychedelic medicine, which takes place primarily in Israel.
| 16. | Income Taxes |
Tax rates applicable to the Company:
The combined Canadian federal and provincial statutory income tax rate is 27% for years ended October 31, 2023, 2022 and 2021 and the Israeli statutory corporate tax rate was 23% in 2023, 2022 and 2021.
The tax effect (computed by applying the Canadian federal and provincial statutory rate) of the significant differences, which comprise deferred income tax assets and liabilities, are as follows:
| 2023 $ |
2022 $ |
2021 $ |
||||||||||
| Canadian statutory income tax rate | 27 | % | 27 | % | 27 | % | ||||||
| Income tax recovery at statutory rate | (2,322,936 | ) | (1,861,476 | ) | (771,373 | ) | ||||||
| Tax effect of: | ||||||||||||
| Permanent differences and other | 204,134 | 335,494 | (3,255 | ) | ||||||||
| Tax rate difference for foreign jurisdiction | 10,715 | 56,175 | 40,185 | |||||||||
| Change in unrecognized deferred income tax assets | 2,125,459 | 1,499,666 | 734,443 | |||||||||
| Income tax provision | 17,372 | 29,859 | - | |||||||||
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 16. | Income Taxes (continued) |
As of October 31, 2023, the Company has Canadian non-capital losses carried forward of $13,176,000, which is available to offset future years’ taxable income in Canada. These losses expire as follows:
| $ | ||||
| 2037 | 4,085 | |||
| 2038 | 75,465 | |||
| 2039 | 73,535 | |||
| 2040 | 175,671 | |||
| 2041 | 1,183,266 | |||
| 2042 | 4,887,566 | |||
| 2043 | 6,775,908 | |||
| 13,176,216 | ||||
As of October 31, 2023, the Company has a non-capital loss carried forward of approximately $2,253,000 which is available to offset future years’ taxable income in Israel.
Deferred tax assets have not been recognized in respect of these items because it is not probable that future taxable profit will be available against which the Company can utilize the benefits therefrom.
| 17. | General and Administrative Expenses |
| Year ended | Year ended | Year ended | ||||||||||
| October 31, | October 31, | October 31, | ||||||||||
| 2023 | 2022 | 2021 | ||||||||||
| Professional fees | $ | 2,210,795 | $ | 1,093,871 | $ | 480,630 | ||||||
| Investor relations | 1,565,970 | 473,106 | 114,706 | |||||||||
| Share-based compensation (Notes 6, 10 and 11) | 243,717 | 946,324 | 619,939 | |||||||||
| Consulting fees (Note 6) | 96,421 | 192,448 | 521,739 | |||||||||
| Insurance | 364,059 | 149,537 | 103,096 | |||||||||
| Transfer agent and regulatory fees | 171,439 | 86,605 | 42,492 | |||||||||
| Depreciation of ROU asset | 35,381 | 60,665 | - | |||||||||
| Salaries and benefits (Note 6) | 37,838 | 92,296 | 297,188 | |||||||||
| Office and miscellaneous | 31,670 | 68,016 | 38,069 | |||||||||
| Depreciation of property and equipment | 1,915 | 7,572 | 1,886 | |||||||||
| Total general and administrative | $ | 4,759,205 | $ | 3,170,440 | $ | 2,219,745 | ||||||
F-
CLEARMIND MEDICINE INC.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 18. | Research and Development, net |
| Year ended | Year ended | Year ended | ||||||||||
| October 31, | October 31, | October 31, | ||||||||||
| 2023 | 2022 | 2021 | ||||||||||
| Research and preclinical studies | $ | 1,159,147 | $ | 2,380,942 | $ | |||||||
| Salaries, regulatory, professional and other expenses | 469,667 | 666,125 | 473,652 | |||||||||
| Share-based compensation (Notes 6, 10 and 11) | 45,870 | 213,299 | ||||||||||
| Reimbursements (Note 6) | (141,409 | ) | (194,205 | ) | ||||||||
| Amortization of intangible assets | 10,954 | 14,830 | ||||||||||
| Total research and development, net | $ | 1,544,229 | $ | 3,080,991 | $ | 473,652 | ||||||
| 19. | Subsequent Events |
| a. | Reverse share split – see note 1(c). |
| b. | Warrant exercises |
As a result of the Reverse Share Split, on November 28, 2023, pursuant to the April 2023 Warrant Adjustments and September 2023 Warrant Adjustments, the exercise price of the April 2023 Warrants and September 2023 Warrants was adjusted to $3.181 and the April 2023 Warrant and September 2023 Warrant is convertible into a total 1,619,768 common shares of the Company.
Between November 30, 2023 and December 4, 2023, April 2023 Warrants and September 2023 Warrants were exercise into 840,511 shares, resulting in gross proceeds of $2,673,665.
On December 5, 2023, the exercise price of the April 2023 Warrants and September 2023 Warrants was further adjusted to $3.6223 such that the April 2023 Warrant and September 2023 Warrant is convertible into a total of 684,315 common shares of the Company.
On December 5, 2023, April 2023 Warrants and September 2023 Warrants were exercised into 221,677 shares, resulting in gross proceeds of $802,981.
On January 21, 2024, following a registered direct and private placement (See below), the April 2023 Warrants and September 2023 Warrant exercise prices were further adjusted to $1.0777 and are convertible into a total of 1,554,991 common shares of the Company.
| c. | New lease agreement – see note 4. |
| d. | Registered direct and private placement |
On January 16, 2024, the Company completed a registered direct and private placement (the “January 2024 Offering”) of 1,468,00 common shares at a price to the public of $1.60 per share and pre-funded warrants to purchase 32,000 common shares at a price to the public of US$1.5999 per pre-funded warrant (“Pre-Funded Warrants”), for aggregate gross proceeds of US$2.40 million. The Pre-Funded Warrants are exercisable at $0.0001 into one common share. As of the date hereof, the pre-funded warrants have been exercised. In addition, each investor in the January 2024 Offering received a common warrant, which is immediately exercisable, expires five years from the date of issuance and has an exercise price of $1.60 per common share (“January 2024 PIPE Warrant”). The January 2024 PIPE Warrants include a cashless exercise provision and repricing provisions under certain circumstances that also includes a potential change in the number of shares to be issued for each January 2024 PIPE Warrant depending on the change in the exercise price of such January 2024 PIPE Warrant. Net proceeds from the January 2024 Offering were approximately $2.0 million.
| e. | Omnibus Equity Inventive Plan |
On November 14, 2023, the shareholders of the Company approved the Omnibus Equity Incentive Plan, or the Omnibus Plan. Pursuant to the Omnibus Plan, the Company is authorized to grant options or restricted share units (“RSUs” and together with “Options”, “Awards” or “Stock Awards”) to officers, directors, employees and consultants enabling them to acquire up to 20% of our issued and outstanding Common Shares. The Awards can be granted for a maximum of 10 years and vest as determined by the Board.
F-36
Exhibit 2.1
DESCRIPTION OF SECURITIES
Description of Common Shares
General
This section summarizes the material rights of shareholders of Clearmind Medicine Inc. (“CMND”) under the Business Corporations Act (British Columbia) (the “BCA”) and the material provisions of the articles of CMND (the “Articles”).
On September 30, 2022, our shareholders approved a one-for-30 reverse split of our issued and outstanding Common Shares (the “Common Shares”). In addition, on November 28, 2023, we effected a one-for-30 consolidation of our issued and outstanding Common Shares. Except where otherwise indicated, all share and per share data in this prospectus have been retroactively restated to reflect the Reverse Split.
Share Capital
The authorized share capital of CMND consists of an unlimited number of Common Shares, no par value.
As of January 28, 2024, there were 3,169,570 Common Shares issued and outstanding.
Except as otherwise provided for by resolution of the board of directors of CMND (the “Board”), the holders of outstanding Common Shares have the exclusive right to vote on all matters requiring shareholder action. On each matter on which holders of Common Shares are entitled to vote, each outstanding share of Common Share is entitled to one vote.
The following descriptions of share capital and provisions of the Articles are summaries and are qualified by reference to the Articles, a copy of which is filed as Exhibit 1.1 to CMND’s Annual Report on Form 20-F.
Dividend Rights
Under the BCA, a company may pay a dividend in money or other property unless there are reasonable grounds for believing that the company is insolvent, or the payment of the dividend would render a company insolvent.
Holders of Common Shares will be entitled to receive dividends as and when declared by CMND’s board of directors at its discretion out of funds properly applicable to the payment of dividends, subject to the rights, if any, of shareholders holding shares with special rights to dividends. The timing, declaration, amount and payment of future dividends will depend on CMND’s financial condition, earnings, capital requirements and debt service obligations, as well as legal requirements, regulatory constraints, industry practice and other factors that CMND’s board of directors deems relevant.
Preemptive Rights
There are no preemptive rights relating to Common Shares.
Redemption and Sinking Fund Provisions
There are neither redemption nor sinking fund provisions relating to Common Shares.
Liquidation Rights
Holders of our Common Shares have equal rights to receive our assets and funds available for distribution to shareholders in the event of any liquidation, dissolution or winding up our affairs, whether voluntary or involuntary.
Amendment of Notice of Articles and Articles and Alteration of Share Capital
Under the BCA, CMND may amend the Articles by (1) the type of resolution specified in the BCA, (2) if the BCA does not specify a type of resolution, then by the type specified in the Articles, or (3) if the Articles do not specify a type of resolution, then by special resolution, which requires two-thirds of the votes cast by shareholders in order to pass. The BCA permits many substantive changes to a corporation’s articles (such as a change in the corporation’s authorized share structure or a change in the special rights or restrictions that may be attached to a certain class or series of shares) to be changed by the resolution specified in that company’s articles. The Articles provide that alterations to CMND’s authorized share structure (other than a subdivision or consolidation of all or any of its shares) and the applicable alteration to its Notice of Articles may be authorized by special resolution. A subdivision or consolidation of all or any of its shares or a change in CMND’s name may be authorized by a resolution of the directors. Furthermore, the Articles state that, if the BCA does not specify the type of resolution required for an alteration, and if the Articles do not specify a type of resolution, CMND may resolve to alter the Articles by ordinary resolution, which requires a majority of shareholder votes cast in order to pass.
Dissent Rights
The BCA provides that shareholders of a company are entitled to exercise dissent rights in respect of certain matters and to be paid the fair value of their shares in connection therewith. The dissent right is applicable where CMND resolves to (i) alter our articles to alter the restrictions on the powers of the company or on the business it is permitted to carry on; (ii) approve certain amalgamations; (iii) approve an arrangement, where the terms of the arrangement or court orders relating thereto permit dissent; (iv) sell, lease or otherwise dispose of all or substantially all of its undertaking; or (v) continue the company into another jurisdiction.
Dissent may also be permitted if authorized by resolution. A court may also make an order permitting a shareholder to dissent in certain circumstances.
Annual Meetings
The Articles provide that, unless an annual general meeting is deferred or waived in accordance with the BCA, CMND must hold its first annual general meeting within 18 months after the date on which it was incorporated or otherwise recognized, and after that must hold an annual general meeting at least once in each calendar year and not more than 15 months after the last annual reference date at such time and place as may be determined by the directors. An annual general meeting may be partially or entirely virtual.
Board and Shareholder Ability to Call Special Meetings
The Articles provide that meetings of the shareholders may be called by the board of directors at any time. In addition, under the BCA, the holders of not less than 5% of the issued shares of a company that carry the right to vote at a general meeting may requisition that the directors call a meeting of shareholders for the purpose of transacting any business that may be transacted at a general meeting. Upon receiving a requisition that complies with the technical requirements set out in the BCA, the directors must, subject to certain limited exceptions, call a meeting of shareholders to be held not more than 4 months after receiving the requisition. If the directors do not call such a meeting within 21 days after receiving the requisition, the requisitioning shareholders or any of them holding in aggregate not less than 2.5% of our issued shares that carry the right to vote at general meetings may call the meeting.
Shareholder Meeting Quorum
The Articles provide that two persons who are, or who represent by proxy, shareholders who, in the aggregate, hold at least 5% of the issued shares of CMND entitled to be voted at the meeting, constitute a quorum at any annual or special meeting of the shareholders.
Voting Rights
Under the BCA, at any meeting of shareholders at which a quorum is present, any action that must or may be taken or authorized by the shareholders, except as otherwise provided under the BCA and Articles, may be taken or authorized by an “ordinary resolution,” which is a simple majority of the votes cast by shareholders voting shares that carry the right to vote at general meetings. The Articles provide that every motion put to a vote at a meeting of shareholders will be decided by a show of hands or the functional equivalent unless a poll is directed by the chair or demanded by any shareholder entitled to vote who is present in person or by proxy. Votes by a show of hands or functional equivalent result in each person having one vote (regardless of the number of shares such person is entitled to vote). If voting is conducted by poll, each holder of Common Shares is entitled to one vote for each Common Share held.
There are no limitations on the right of nonresident or foreign owners to hold or vote CMND securities imposed by Canadian law or by the charter or other constituent document of CMND.
Shareholder Action by Written Consent
Under the BCA, shareholder action without a meeting may be taken by a “consent resolution” of shareholders, which requires that, after being submitted to all shareholders entitled to vote at a general meeting, the resolution is consented to in writing by: (1) in the case of a matter that would normally require an ordinary resolution, shareholders who, in the aggregate, hold shares carrying at least 66 2/3% of the votes entitled to be cast on such consent resolution, or (2) in the case of any other resolution of the shareholders, all of the shareholders entitled to vote on such resolution. A consent resolution of shareholders is deemed to be a proceeding at a meeting of those shareholders and to be as valid and effective as if it had been passed at a meeting of shareholders that satisfies all the requirements of the BCA and its related regulations, and all the requirements of the Articles, relating to meetings of shareholders.
Inspection of Corporation Records
CMND must keep at its records office, or at such other place as the BCA may permit, the documents, copies, registers, minutes and other records which CMND is required by the BCA to keep at such places. CMND must keep adequate accounting records to record properly its financial affairs and condition in compliance with the provisions of the BCA. Under the BCA, any director or shareholder may, without charge, inspect certain of CMND’s records at CMND’s records office or such other place where such records are kept during the corporation’s statutory business hours. Former shareholders and directors may also inspect certain records, free of charge, but only those records pertaining to the times that they were shareholders or directors. Further, a public company must allow all persons to inspect certain records of the company free of charge. As permitted by the BCA, the Articles prohibit shareholders from inspecting any accounting records of CMND, unless the directors determine otherwise.
Election and Appointment of Directors
Under the BCA and the Articles, a vacancy among the directors created by the removal of a director may be filled by the shareholders at the meeting at which the director is removed or, if not filled by the shareholders at such meeting, by the shareholders or by the remaining directors. In the case of a casual vacancy, the remaining directors may fill the vacancy. Under the BCA, directors may increase the size of the board of directors by one third of the number of current directors.
Under the BCA and the Articles, if as a result of one or more vacancies, the number of directors in office falls below the number required for a quorum, the remaining directors may appoint as directors the number of individuals that, when added to the number of remaining directors, will constitute a quorum and/or call a shareholders’ meeting to fill any or all vacancies among directors and to conduct such other business that may be dealt with at that meeting, but must not take any other action until a quorum is obtained.
Removal of Directors
Pursuant to the Articles, the shareholders of CMND may remove any director before the expiration of his or her term of office by special resolution, which requires a special majority requirement of two-thirds of the votes cast in favor of the resolution. In that event, the shareholders may elect, or appoint by ordinary resolution, another individual as director to fill the resulting vacancy. If the shareholders do not appoint a director to fill the vacancy contemporaneously with removal, then either the directors or the shareholders by ordinary resolution may appoint an additional director to fill that vacancy.
The directors of CMND may remove a director before the expiration of his or her period of office if the director is convicted of an indictable offence or otherwise ceases to qualify as a director and the directors may appoint a director to fill the resulting vacancy.
Proceedings of Board of Directors
A resolution of the directors or of any committee of the directors consented to in writing by all of the directors entitled to vote on it is as valid and effective as if it had been passed at a meeting of the directors or of the committee of the directors duly called and held.
Approval of Mergers and Other Corporate Transactions
Under the BCA, certain corporate actions, such as: (1) amalgamations (other than with certain affiliated corporations); (2) continuances; (3) sales, leases or exchanges of all, or substantially all, the undertaking of a corporation other than in the ordinary course of business; (4) reductions of paid-up capital for any purpose, e.g. in connection with the payment of special distributions (subject, in certain cases, to the satisfaction of solvency tests); and
(5) other actions such as liquidations or arrangements, are required to be approved by “special resolution.” A “special resolution” is a resolution passed by not less than two-thirds of the votes cast by the shareholders who voted in respect of the resolution or signed by all shareholders entitled to vote on the resolution.
In certain cases where share rights or special rights may be prejudiced or interfered with, a special separate resolution of shareholders of the affected class or series, including a class or series of shares not otherwise carrying voting rights, to approve the corporate action in question is also required. In specified extraordinary corporate actions, such as approval of plans of arrangement and amalgamations, all shares have a vote, whether or not they generally vote and, in certain cases, have separate class votes.
Limitations on Director Liability
Under the BCA, a director or officer of a company must (i) act honestly and in good faith with a view to the best interests of the company; (ii) exercise the care, diligence and skill that a reasonably prudent individual would exercise in comparable circumstances; (iii) act in accordance with the BCA and the regulations thereunder; and (iv) subject to (i) to (iii), act in accordance with the articles of the company. These statutory duties are in addition to duties under common law and equity.
No provision in a contract or the articles of a company may relieve a director or officer of a company from the above duties. A director is not liable under the BCA for certain acts if the director relied, in good faith, on (1) financial statements of the corporation represented to the director by an officer of the corporation or in a written report of the auditor of the corporation to fairly reflect the financial position of the corporation, (2) a written report of a lawyer, accountant, engineer, appraiser or other person whose profession lends credibility to a statement made by that person, (3) a statement of fact represented to the director by an officer of the corporation to be correct, or (4) any record, information or representation that the court considers provides reasonable grounds for the actions of the director, whether or not the record was forged, fraudulently made or inaccurate or the information or representation was fraudulently made or inaccurate. Further, a director is not liable for certain acts if the director did not know and could not reasonably have known that the act done by the director or authorized by the resolution voted for or consented to by the director was contrary to the BCA.
Derivative Actions and Other Remedies
Under the BCA, a shareholder (including a beneficial shareholder) or director of a company and any person who, in the discretion of the court, is an appropriate person to make an application to court to prosecute or defend an action on behalf of a company (a derivative action) may, with judicial leave: (i) bring an action in the name and on behalf of the company to enforce a right, duty or obligation owed to the company that could be enforced by the company itself or to obtain damages for any breach of such right, duty or obligation or (ii) defend, in the name and on behalf of the company, a legal proceeding brought against the company.
Under the BCA, the court may grant leave if: (i) the complainant has made reasonable efforts to cause the directors of the company to prosecute or defend the action; (ii) notice of the application for leave has been given to the company and any other person that the court may order; (iii) the complainant is acting in good faith; and (iv) it appears to the court to be in the interests of the company for the action to be prosecuted or defended.
Under the BCA, upon the final disposition of a derivative action, the court may make any order it determines to be appropriate. In addition, under the BCBCA, a court may order a company to pay the complainant’s interim costs, including legal fees and disbursements. However, the complainant may be held accountable for the costs on final disposition of the action.
The BCA also contains an oppression remedy. The BCA’s oppression remedy enables a court to make an order (interim or final) to rectify the matters complained of if the court is satisfied upon application by a shareholder (as defined below) that the affairs of the company are being conducted or that the powers of the directors have been exercised in a manner that is oppressive, or that some action of the company or shareholders has been or is threatened to be taken which is unfairly prejudicial, in each case to one or more shareholders. The applicant must be one of the persons being oppressed or prejudiced and the application must be brought in a timely manner. A “shareholder” for the purposes of the oppression remedy includes legal and beneficial owners of shares as well as any other person whom the court considers appropriate.
The oppression remedy provides the court with extremely broad and flexible jurisdiction to intervene in corporate affairs to protect shareholders. While conduct that is in breach of fiduciary duties of directors or that is contrary to the legal right of a complainant would normally be expected to trigger the court’s jurisdiction under the oppression remedy, the exercise of that jurisdiction does not depend on a finding of a breach of such legal and equitable rights.
Transfer Agents and Registrar
The transfer agent and registrar for Common Shares is Computershare Trust Company of Canada. Its address is 100 University Avenue, 8th Floor, Toronto, Ontario, Canada M5J 2Y1.
5
Exhibit 4.7
Clearmind Medicine, Inc.
Executive Officer Clawback Policy
Approved by the Compensation Committee of the Board of Directors on November 13, 2023 (the “Adoption Date”)
| I. | Purpose |
This Executive Officer Clawback Policy describes the circumstances under which Covered Persons of Clearmind Medicine, Inc. and any of its direct or indirect subsidiaries (the “Company”) will be required to repay or return Erroneously-Awarded Compensation to the Company.
This Policy and any terms used in this Policy shall be construed in accordance with any SEC regulations promulgated to comply with Section 954 of the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, including without limitation Rule 10D-1 promulgated under the Securities Exchange Act of 1934, as amended, and the rules adopted by Nasdaq.
Each Covered Person of the Company shall sign an Acknowledgement and Agreement to the Clawback Policy in the form attached hereto as Exhibit A as a condition to his or her participation in any of the Company’s incentive-based compensation programs; provided that this Policy shall apply to each Covered Person irrespective of whether such Covered Person shall have failed, for any reason, to have executed such Acknowledgement and Agreement.
| II. | Definitions |
For purposes of this Policy, the following capitalized terms shall have the respective meanings set forth below:
| (a) | “Accounting Restatement” shall mean an accounting restatement (i) due to the material noncompliance of the Company with any financial reporting requirement under the securities laws, including any required accounting restatement to correct an error in previously issued financial restatements that is material to the previously issued financial statements (a “Big R” restatement), or (ii) that corrects an error that is not material to previously issued financial statements, but would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period (a “little r” restatement). Notwithstanding the foregoing, none of the following changes to the Company’s financial statements represent error corrections and shall not be deemed an Accounting Restatement: (a) retrospective application of a change in accounting principle; (b) retrospective revision to reportable segment information due to a change in the structure of the Company’s internal organization; (c) retrospective reclassification due to a discontinued operation; (d) retrospective application of a change in reporting entity, such as from a reorganization of entities under common control; and (e) retrospective revision for share splits, reverse share splits, share dividends or other changes in capital structure. |
| (b) | “Board” shall mean the Board of Directors of the Company. |
| (c) | “Clawback-Eligible Incentive Compensation” shall mean, in connection with an Accounting Restatement, any Incentive-Based Compensation Received by a Covered Person (regardless of whether such Covered Person was serving at the time that Erroneously-Awarded Compensation is required to be repaid) (i) on or after the Nasdaq Effective Date, (ii) after beginning service as a Covered Person, (iii) while the Company has a class of securities listed on a national securities exchange or national securities association and (iv) during the Clawback Period. |
| (d) | “Clawback Period” shall mean, with respect to any Accounting Restatement, the three completed fiscal years immediately preceding the Restatement Date and any transition period (that results from a change in the Company’s fiscal year) of less than nine months within or immediately following those three completed fiscal years. |
| (e) | “Committee” shall mean the Compensation Committee of the Board. |
| (f) | “Covered Person” shall mean any person who is, or was at any time, during the Clawback Period, an Executive Officer of the Company. For the avoidance of doubt, Covered Person may include a former Executive Officer that left the Company, retired or transitioned to an employee non-Executive Officer role (including after serving as an Executive Officer in an interim capacity) during the Clawback Period, and this Policy applies regardless of whether the Covered Person was at fault for an accounting error or other action that resulted in, or contributed to, the Accounting Restatement. |
| (g) | “Erroneously-Awarded Compensation” shall mean the amount of Clawback-Eligible Incentive Compensation that exceeds the amount of Incentive-Based Compensation that otherwise would have been Received had it been determined based on the restated amounts. This amount must be computed without regard to any taxes paid. |
| (h) | “Executive Officer” shall mean (i) the Company’s president, principal financial officer, principal accounting officer (or if there is no such accounting officer, the controller), any vice-president in charge of a principal business unit, division, or function (such as sales, administration, or finance), any other officer who performs a policy-making function or (ii) any other person (including an officer of the Company’s parent(s) or subsidiaries) who performs similar policy-making functions for the Company. For the sake of clarity, at a minimum, all persons who would be executive officers pursuant to Rule 401(b) under Regulation S-K shall be deemed “Executive Officers”. |
| (i) | “Financial Reporting Measures” shall mean measures that are determined and presented in accordance with the accounting principles used in preparing the Company’s financial statements, and all other measures that are derived wholly or in part from such measures, including, without limitation, measures that are “non-GAAP financial measures” for purposes of Exchange Act Regulation G and Item 10(e) of Regulation S-K, as well other measures, metrics and ratios that are not non- GAAP measures. For purposes of this Policy, Financial Reporting Measures shall include stock price and total shareholder return (and any measures that are derived wholly or in part from stock price or total shareholder return). A Financial Reporting Measure need not be presented within the Company’s financial statements or included in a Company filing with the SEC. |
| (j) | “Incentive-Based Compensation” shall have the meaning set forth in Section III below. |
| (k) | “Nasdaq” shall mean The Nasdaq Stock Market. |
| (l) | “Nasdaq Effective Date” shall mean October 2, 2023. |
| (m) | “Policy” shall mean this Executive Officer Clawback Policy, as the same may be amended and/or restated from time to time. |
| (n) | “Received” shall mean Incentive-Based Compensation received, or deemed to be received, in the Company’s fiscal period during which the Financial Reporting Measure specified in the Incentive-Based Compensation is attained, even if the payment or grant occurs after the fiscal period. |
| (o) | “Repayment Agreement” shall have the meaning set forth in Section V below. |
| (p) | “Restatement Date” shall mean the earlier of (i) the date the Board, a committee of the Board or the officers of the Company authorized to take such action if Board action is not required, concludes, or reasonably should have concluded, that the Company is required to prepare an Accounting Restatement, or (ii) the date that a court, regulator or other legally authorized body directs the Company to prepare an Accounting Restatement. |
| (q) | “SARs” shall mean stock appreciation rights. |
| (r) | “SEC” shall mean the U.S. Securities and Exchange Commission. |
| III. | Incentive-Based Compensation |
“Incentive-Based Compensation” shall mean any compensation that is granted, earned or vested wholly or in part upon the attainment of a Financial Reporting Measure.
For purposes of this Policy, specific examples of Incentive-Based Compensation include, but are not limited to:
| ● | Non-equity incentive plan awards that are earned based, wholly or in part, based on satisfaction of a Financial Reporting Measure performance goal; | |
| ● | Bonuses paid from a “bonus pool,” the size of which is determined, wholly or in part, based on satisfaction of a Financial Reporting Measure performance goal; | |
| ● | Other cash awards based on satisfaction of a Financial Reporting Measure performance goal; | |
| ● | Restricted stock, restricted stock units, performance share units, stock options and SARs that are granted or become vested, wholly or in part, on satisfaction of a Financial Reporting Measure performance goal; and | |
| ● | Proceeds received upon the sale of shares acquired through an incentive plan that were granted or vested based, wholly or in part, on satisfaction of a Financial Reporting Measure performance goal. |
For purposes of this Policy, Incentive-Based Compensation excludes:
| ● | Any base salaries (except with respect to any salary increases earned, wholly or in part, based on satisfaction of a Financial Reporting Measure performance goal); |
| ● | Bonuses paid solely at the discretion of the Committee or Board that are not paid from a “bonus pool” that is determined by satisfying a Financial Reporting Measure performance goal; |
| ● | Bonuses paid solely upon satisfying one or more subjective standards and/or completion of a specified employment period; |
| ● | Non-equity incentive plan awards earned solely upon satisfying one or more strategic measures or operational measures; and |
| ● | Equity awards that vest solely based on the passage of time and/or satisfaction of one or more non-Financial Reporting Measures. |
| IV. | Determination and Calculation of Erroneously-Awarded Compensation |
In the event of an Accounting Restatement, the Committee shall promptly determine the amount of any Erroneously-Awarded Compensation for each Executive Officer in connection with such Accounting Restatement and shall promptly thereafter provide each Executive Officer with a written notice containing the amount of Erroneously-Awarded Compensation and a demand for repayment, forfeiture or return thereof, as applicable.
| (a) | Cash Awards. With respect to cash awards, the Erroneously-Awarded Compensation is the difference between the amount of the cash award (whether payable as a lump sum or over time) that was Received and the amount that should have been Received applying the restated Financial Reporting Measure. |
| (b) | Cash Awards Paid From Bonus Pools. With respect to cash awards paid from bonus pools, the Erroneously-Awarded Compensation is the pro rata portion of any deficiency that results from the aggregate bonus pool that is reduced based on applying the restated Financial Reporting Measure. |
| (c) | Equity Awards. With respect to equity awards, if the shares, options, SARs or other equity awards are still held at the time of recovery, the Erroneously-Awarded Compensation is the number of such securities Received in excess of the number that should have been received applying the restated Financial Reporting Measure (or the value in excess of that number). If the options, SARs or other equity awards have been exercised, vested, settled or otherwise converted into underlying shares, but the underlying shares have not been sold, the Erroneously-Awarded Compensation is the number of shares underlying the excess options or SARs (or the value thereof). If the underlying shares have already been sold, the Erroneously-Awarded Compensation is the higher of the value of the stock upon vesting, exercise or sale. |
| (d) | Compensation Based on Stock Price or Total Shareholder Return. For Incentive-Based Compensation based on (or derived from) stock price or total shareholder return, where the amount of Erroneously-Awarded Compensation is not subject to mathematical recalculation directly from the information in the applicable Accounting Restatement, the amount shall be determined by the Committee based on a reasonable estimate of the effect of the Accounting Restatement on the stock price or total shareholder return upon which the Incentive-Based Compensation was Received (in which case, the Committee shall maintain documentation of such determination of that reasonable estimate and provide such documentation to Nasdaq in accordance with applicable listing standards). |
| V. | Recovery of Erroneously-Awarded Compensation |
Once the Committee has determined the amount of Erroneously-Awarded Compensation recoverable from the applicable Covered Person, the Committee shall take all necessary actions to recover the Erroneously-Awarded Compensation. Unless otherwise determined by the Committee, the Committee shall pursue the recovery of Erroneously-Awarded Compensation in accordance with the below:
| (a) | Cash Awards. With respect to cash awards, the Committee shall either (i) require the Covered Person to repay the Erroneously-Awarded Compensation in a lump sum in cash (or such property as the Committee agrees to accept with a value equal to such Erroneously-Awarded Compensation) reasonably promptly following the Restatement Date or (ii) if approved by the Committee, offer to enter into a Repayment Agreement. If the Covered Person accepts such offer and signs the Repayment Agreement within a reasonable time as determined by the Committee, the Company shall countersign such Repayment Agreement. |
| (b) | Unvested Equity Awards. With respect to those equity awards that have not yet vested, the Committee shall take all necessary action to cancel, or otherwise cause to be forfeited, the awards in the amount of the Erroneously-Awarded Compensation. |
| (c) | Vested Equity Awards. With respect to those equity awards that have vested and the underlying shares have not been sold, the Committee shall take all necessary action to cause the Covered Person to deliver and surrender the underlying shares in the amount of the Erroneously-Awarded Compensation. |
In the event that the Covered Person has sold the underlying shares, the Committee shall either (i) require the Covered Person to repay the Erroneously-Awarded Compensation in a lump sum in cash (or such property as the Committee agrees to accept with a value equal to such Erroneously-Awarded Compensation) reasonably promptly following the Restatement Date or (ii) if approved by the Committee, offer to enter into a Repayment Agreement. If the Covered Person accepts such offer and signs the Repayment Agreement within a reasonable time as determined by the Committee, the Company shall countersign such Repayment Agreement.
| (d) | Repayment Agreement. “Repayment Agreement” shall mean an agreement (in a form reasonably acceptable to the Committee) with the Covered Person for the repayment of the Erroneously-Awarded Compensation as promptly as possible without unreasonable economic hardship to the Covered Person. |
| (e) | Effect of Non-Repayment. To the extent that a Covered Person fails to repay all Erroneously-Awarded Compensation to the Company when due (as determined in accordance with this Policy), the Company shall, or shall cause one or more other members of the Company to, take all actions reasonable and appropriate to recover such Erroneously-Awarded Compensation from the applicable Covered Person. Unless otherwise determined by the Committee in its discretion, the applicable Covered Person shall be required to reimburse the Company for any and all expenses reasonably incurred (including legal fees) by the Company in recovering such Erroneously-Awarded Compensation in accordance with the immediately preceding sentence. |
The Committee shall have broad discretion to determine the appropriate means of recovery of Erroneously-Awarded Compensation based on all applicable facts and circumstances and taking into account the time value of money and the cost to shareholders of delaying recovery. However, in no event may the Company accept an amount that is less than the amount of Erroneously-Awarded Compensation in satisfaction of a Covered Person’s obligations hereunder.
| VI. | Discretionary Recovery |
Notwithstanding anything herein to the contrary, the Company shall not be required to take action to recover Erroneously-Awarded Compensation if any one of the following conditions are met and the Committee determines that recovery would be impracticable:
| (i) | The direct expenses paid to a third party to assist in enforcing this Policy against a Covered Person would exceed the amount to be recovered, after the Company has made a reasonable attempt to recover the applicable Erroneously-Awarded Compensation, documented such attempts and provided such documentation to Nasdaq; |
| (ii) | Recovery would violate home country law where that law was adopted prior to November 28, 2022, provided that, before determining that it would be impracticable to recover any amount of Erroneously-Awarded Compensation based on violation of home country law, the Company has obtained an opinion of home country counsel, acceptable to Nasdaq, that recovery would result in such a violation and a copy of the opinion is provided to Nasdaq; or |
| (iii) | Recovery would likely cause an otherwise tax-qualified retirement plan, under which benefits are broadly available to employees of the Company, to fail to meet the requirements of 26 U.S.C. 401(a)(13) or 26 U.S.C. 411(a) and regulations thereunder. |
| VII. | Reporting and Disclosure Requirements |
The Company shall file all disclosures with respect to this Policy in accordance with the requirements of the federal securities laws, including the disclosure required by the applicable filings required to be made with the SEC.
| VIII. | Effective Date |
This Policy shall apply to any Incentive-Based Compensation Received on or after the Nasdaq Effective Date.
| IX. | No Indemnification |
The Company shall not indemnify any Covered Person against the loss of Erroneously-Awarded Compensation and shall not pay, or reimburse any Covered Persons for premiums, for any insurance policy to fund such Covered Person’s potential recovery obligations.
| X. | Administration |
The Committee has the sole discretion to administer this Policy and ensure compliance with Nasdaq Rules and any other applicable law, regulation, rule or interpretation of the SEC or Nasdaq promulgated or issued in connection therewith. Actions of the Committee pursuant to this Policy shall be taken by the vote of a majority of its members. The Committee shall, subject to the provisions of this Policy, make such determinations and interpretations and take such actions as it deems necessary, appropriate or advisable. All determinations and interpretations made by the Committee shall be final, binding and conclusive.
| XI. | Amendment; Termination |
The Committee may amend this Policy from time to time in its discretion and shall amend this Policy as it deems necessary, including as and when it determines that it is legally required by any federal securities laws, SEC rule or the rules of any national securities exchange or national securities association on which the Company’s securities are then listed. The Committee may terminate this Policy at any time. Notwithstanding anything in this Section XI to the contrary, no amendment or termination of this Policy shall be effective if such amendment or termination would (after taking into account any actions taken by the Company contemporaneously with such amendment or termination) cause the Company to violate any federal securities laws, SEC rule or the rules of any national securities exchange or national securities association on which the Company’s securities are then listed.
| XII. | Other Recoupment Rights; No Additional Payments |
The Committee intends that this Policy will be applied to the fullest extent of the law. The Committee may require that any employment agreement, equity award agreement or any other agreement entered into on or after the Adoption Date shall, as a condition to the grant of any benefit thereunder, require a Covered Person to agree to abide by the terms of this Policy. Any right of recoupment under this Policy is in addition to, and not in lieu of, any other rights under applicable law, regulation or rule or pursuant to any similar policy in any employment agreement, equity plan, compensation policy, equity award agreement or similar arrangement and any other legal remedies available to the Company. However, this Policy shall not provide for recovery of Incentive-Based Compensation that the Company has already recovered pursuant to Section 304 of the Sarbanes-Oxley Act or other recovery obligations.
| XIII. | Successors |
This Policy shall be binding and enforceable against all Covered Persons and their beneficiaries, heirs, executors, administrators or other legal representatives.
Exhibit A
ACKNOWLEDGEMENT AND AGREEMENT
TO THE
EXECUTIVE OFFICER CLAWBACK POLICY
OF
CLEARMIND MEDICINE, INC.
By signing below, the undersigned acknowledges and confirms that the undersigned has received and reviewed a copy of Clearmind Medicine, Inc. Executive Officer Clawback Policy (the “Policy”). Capitalized terms used but not otherwise defined in this Acknowledgement Form (this “Acknowledgement Form”) shall have the meanings ascribed to such terms in the Policy.
By signing this Acknowledgement Form, the undersigned acknowledges and agrees that the undersigned is and will continue to be subject to the Policy and that the Policy will apply both during and after the undersigned’s employment with the Company. Further, by signing below, the undersigned agrees to abide by the terms of the Policy, including, without limitation, by returning any Erroneously-Awarded Compensation (as defined in the Policy) to the Company to the extent required by, and in a manner permitted by, the Policy.
| Signature | |
| Name | |
| Date |
7
Exhibit 12.1
CERTIFICATION PURSUANT TO EXCHANGE ACT RULE 13a-14(a) or 15d-14(a)
I, Dr. Adi Zuloff-Shani, certify that:
| 1. | I have reviewed this annual report on Form 20–F of Clearmind Medicine Inc.; |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report; |
| 4. | The company’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the company and have: |
| a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| c) | Evaluated the effectiveness of the company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| d) | Disclosed in this report any change in the company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company’s internal control over financial reporting. |
| 5. | The company’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company’s auditors and the audit committee of the company’s board of directors: |
| a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company’s ability to record, process, summarize and report financial information; and |
| b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the company’s internal control over financial reporting. |
| Date: January 29, 2024 | /s/ Dr. Adi Zuloff-Shani |
| Dr. Adi Zuloff-Shani | |
| Chief Executive Officer |
Exhibit 12.2
CERTIFICATION PURSUANT TO EXCHANGE ACT RULE 13a-14(a) or 15d-14(a)
I, Alan Rootenberg, certify that:
| 1. | I have reviewed this annual report on Form 20–F of Clearmind Medicine Inc.; |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report; |
| 4. | The company’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the company and have: |
| a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the Company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| c) | Evaluated the effectiveness of the Company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| d) | Disclosed in this report any change in the Company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting; and |
| 5. | The Company’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the Company’s auditors and the audit committee of the Company’s board of directors (or persons performing the equivalent function): |
| a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the Company’s ability to record, process, summarize and report financial information; and |
| b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the Company’s internal control over financial reporting. |
| Date: January 29, 2024 | /s/ Alan Rootenberg |
| Alan Rootenberg | |
| Chief Financial Officer |
Exhibit 13.1
CERTIFICATION PURSUANT TO
18 U.S.C. Section 1350
In connection with the filing of the Annual Report on Form 20-F for the period ended October 31, 2023 (the “Report”) by Clearmind Medicine Inc. (the “Company”), the undersigned, as the Chief Executive Officer of the Company, hereby certifies pursuant to 18 U.S.C. Section 1350, that, to my knowledge:
| (1) | the Report fully complies with the requirements of Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934; and |
| (2) | the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
| Date: January 29, 2024 | /s/ Dr. Adi Zuloff-Shani |
| Dr. Adi Zuloff-Shani | |
| Chief Executive Officer |
Exhibit 13.2
CERTIFICATION PURSUANT TO
18 U.S.C. Section 1350
In connection with the filing of the Annual Report on Form 20-F for the period ended October 31, 2023 (the “Report”) by Clearmind Medicine Inc. (the “Company”), the undersigned, as the Chief Financial Officer of the Company, hereby certifies pursuant to 18 U.S.C. Section 1350, that, to my knowledge:
| (1) | the Report fully complies with the requirements of Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934; and |
| (2) | the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
| Date: January 29, 2024 | /s/ Alan Rootenberg |
| Alan Rootenberg | |
| Chief Financial Officer |
Exhibit 15.1
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We consent to the incorporation by reference in Registration Statements on Form F-3 (No. 333-275991, 333-270859, and 333-273293) of our report dated January 29, 2024, relating to the financial statements of Clearmind Medicine Inc., appearing in this Annual Report on Form 20-F of Clearmind Medicine Inc. for the year ended October 31, 2023.
/s/ Brightman Almagor Zohar & Co.
Certified Public Accountants
A Firm in the Deloitte Global Network
Tel Aviv, Israel
January 29, 2024
Exhibit 15.2

CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We consent to the incorporation by reference therein of our audit report dated March 8, 2022 (except as to Note 1(c) which is as of October 19, 2022), included in its Annual Report on Form 20-F for the year ended October 31, 2023, filed with the Securities and Exchange Commission, in the Registration Statements on Form F-3 (Registration Nos. 333-275991, 333-270859 and 333- 273293) and related Prospectuses of Clearmind Medicine Inc.
/s/ SATURNA GROUP CHARTERED PROFESSIONAL ACCOUNTANTS LLP
SATURNA GROUP CHARTERED PROFESSIONAL ACCOUNTANTS LLP
Vancouver, Canada
January 29, 2024
Exhibit 16.1

Re: Clearmind Medicine Inc. (the “Company”)
This letter will confirm that we reviewed Item 16F of the Company’s Form 20-F, which we understand will be filed with the United States Securities and Exchange Commission, on or about January 29, 2024, captioned “Change in the Registrant’s Certifying Accountant” and that we agree with the statements made therein as they relate to Saturna Group Chartered Professional Accountants LLP.
We hereby consent to the filing of this letter as an exhibit to the foregoing report on Form 20-F.
/s/ SATURNA GROUP CHARTERED PROFESSIONAL ACCOUNTANTS LLP
SATURNA GROUP CHARTERED PROFESSIONAL ACCOUNTANTS LLP
Vancouver, Canada
January 29, 2024