UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (date of earliest event reported): August 17, 2023
Cadrenal Therapeutics, Inc.
(Exact name of registrant as specified in charter)
| Delaware | 001-41596 | 88-0860746 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) | (IRS Employer Identification No.) |
822 A1A North, Suite 306
Ponte Vedra, Florida 32082
(Address of principal executive offices and zip code)
(904) 300-0701
(Registrant’s telephone number including area code)
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12(b) under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbols | Name of each exchange on which registered | ||
| Common Stock, par value $0.001 per share | CVKD | The Nasdaq Stock Market LLC (Nasdaq Capital Market) |
Indicate by check mark whether the registrant is an emerging growth company as defined in in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by checkmark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure
On August 17, 2023, Cadrenal Therapeutics, Inc. (the “Company”) posted an updated corporate presentation within the Investor Relations section of the Company’s website, which is furnished as Exhibit 99.1 to this Current Report on Form 8-K. Representatives of the Company may use the updated presentation in various meetings with investors from time to time.
The information contained in this Item 7.01 (including Exhibit 99.1) is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section and shall not be deemed to be incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
The investor presentation furnished as Exhibit 99.1 to this Current Report on Form 8-K includes “safe harbor” language pursuant to the Private Securities Litigation Reform Act of 1995, as amended, indicating that certain statements contained therein are “forward-looking” rather than historical.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
The following exhibits are furnished with this Current Report on Form 8-K:
| Exhibit Number |
Exhibit Description | |
| 99.1 | Investor Presentation of Cadrenal Therapeutics, Inc., dated August 17, 2023 | |
| 104 | Cover Page Interactive Data File (the cover page XBRL tags are embedded within in the inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Dated: August 18, 2023 | CADRENAL THERAPEUTICS, INC. | |
| By: | /s/ Quang Pham | |
| Name: | Quang Pham | |
| Title: | Chairman and Chief Executive Officer | |
2
Exhibit 99.1

1 CREATE A COVER SLIDE Cadrenal Therapeutics, Inc. NASDAQ: CVKD August 2023

2 Cautionary Statement Concerning Forward Looking Statements This document contains forward - looking statements. In addition, from time to time, we or our representatives may make forward - looking statements orally or in writing. We base these forward - looking statements on our expectations and projections about future events, which we derive from the information currently available to us. Such forward - looking statements relate to future events or our future performance, including: our financial performance and projections; our growth in revenue and earnings; and our business prospects and opportunities. You can identify forward - looking statements by those that are not historical in nature, particularly those that use terminology such as “may,” “should,” “expects,” “anticipates,” “contemplates,” “estimates,” “believes,” “plans,” “projected,” “predicts,” “potential,” or “hopes” or the negative of these or similar terms. In evaluating these forward - looking statements, you should consider various factors, including: our ability to successfully develop and commercialize product candidates, our ability to raise capital when needed, and the competitive environment of our business. These and other factors may cause our actual results to differ materially from any forward - looking statement, including those risk factors disclosed in our Annual Report on Form 10 - K for the year ended December 31, 2023 filed with the Securities and Exchange Commission on March 30, 2023. Forward - looking statements are only predictions. The forward - looking events discussed in this document and other statements made from time to time by us or our representatives may not occur, and actual events and results may differ materially and are subject to risks, uncertainties, and assumptions about us. We are not obligated to publicly update or revise any forward - looking statement, whether as a result of uncertainties and assumptions, the forward - looking events discussed in this document, and other statements made from time to time by us or our representatives might not occur.

3 Corporate Overview o Developing tecarfarin, a late - stage novel oral and reversible anticoagulant (blood thinner) to prevent heart attacks, strokes an d deaths due to blood clots in patients with certain rare medical conditions where “Legacy” Vitamin K Antagonists ( e.g. warfarin) have failed to achieve sufficiently reliable anticoagulation. These areas include: o End Stage Kidney Disease (ESKD) with Atrial Fibrillation (AFib) o Left Ventricular Assist Devices (LVADs) o Antiphospholipid Syndrome (APS) o Tecarfarin was designed as a Vitamin K Antagonist specifically to solve warfarin's metabolism problem via an alternate pathwa y that is abundant and essentially insaturable, providing a much more reliable pharmacokinetic profile. o Orphan drug and Fast track designations for ESKD with AFib providing for 7 - year marketing exclusivity o Phase III remaining for ESKD with AFib; pursuing development for patients with LVADs and/or APS o Tecarfarin has been evaluated in 11 clinical trials in 1,003 subjects and has generally been well - tolerated in healthy adult sub jects and patients with chronic kidney disease (CKD). o Approximately $90 million invested in clinical and regulatory work to date. o U.S. market potential estimated in excess of $2 billion for three focused rare medical condition indications. 1 Based upon 2019 study, adjusted for inflation. 3 Late - stage Ready Drug with Orphan Drug and Fast track Designations 5 The Problem: Rare Medical Conditions Lacking Effective Anticoagulation o In 1954, warfarin, first developed as a rat poison, was approved to treat and prevent blood clots.

4 TECARFARIN OPPORTUNITY

o Warfarin was widely used until 2010 when Pradaxa was approved. o Sub - optimal anticoagulation for the patients with certain rare medical conditions o Between 2010 - 2015, the FDA approved the first innovations in 50+ years to treat and prevent blood clots. o Direct Oral Anticoagulants (DOACs) - Pradaxa , Xarelto, Eliquis and Savaysa o DOACs are not prescribed for a wide range of indications, including implanted medical devices for heart diseases, such as left ventricular assist devices o No known ongoing clinical trials or future research for rare medical conditions Tecarfarin is targeted for indications where warfarin FAILS to achieve sufficiently stable anticoagulation and DOACs (Eliquis - class drugs) are NOT widely prescribed for these rare medical conditions.

6 6 Tecarfarin Looks to Solve Warfarin’s Major Problems Warfarin CHALLENGING TO CONTROL despite nearly 70 years of experience Metabolism via the cytochrome P450 pathway Significant variability in PK due to genetic variants and competition with other drugs MAJOR PROBLEM for patients with rare medical conditions Variable/Unreliable anticoagulation in patients at high risk for thrombotic events x Tecarfarin SPECIFICALLY DESIGNED to solve the warfarin metabolism problem Metabolized via an alternate pathway that is abundant and essentially insaturable Reliable, stable PK profile STABLE ANTICOAGULATION with proven mechanism of action for patients with rare medical conditions Tecarfarin potentially provides a more stable anticoagulation than warfarin due to its metabolism, thereby decreasing the ris k o f stroke and bleeding 7 Tecarfarin Clinical Development Pipeline Program Prioritized Target Indications Regulatory Strategy/Status Development Phase Discovery Preclinical Phase I Phase II Phase III Tecarfarin End Stage Kidney Disease with AFib FDA Orphan Drug Designation Granted FDA Fast Track Designation Granted EMA Orphan Drug Designation In process Left Ventricular Assist Devices (LVADs) Developing Antiphospholipid Syndrome (APS) Developing Late - stage ready drug with orphan drug and Fast track designations


8 Large Addressable Market Opportunities for Rare Medical Conditions * Based on management’s market analysis studies and expected adoption rates. 8 Approximately $600 Million Annual Market Potential (if approved by the FDA) * Left Ventricular Assist Devices Antiphospholipid Syndrome 14,000 patients 167,000 patients Approximately $1 Billion Annual Market Potential (if approved by the FDA) End Stage Kidney Disease with AFib 150,000 patients Approximately $1 Billion Annual Market Potential (if approved by the FDA) U.S.

market potential estimated in excess of $2 billion for three focused rare medical condition indications 9 Premium Pay for High Value Cardiovascular Orphan Drugs Developed by FoldRx with $88 million in private financing, before FoldRx was subsequently acquired by Pfizer FDA DESIGNATIONS Orphan Drug, Fast Track, Priority Review & Breakthrough Therapy EXPEDITED APPROVAL Based on ATTR - ACT trial (Transthyretin Amyloidosis Cardiomyopathy Clinical Trial) - randomized 441 patients to tafamidis or placebo for 30 months INVESTIGATORS ASSESSED All - cause mortality followed by CV hospitalizations PRICE $225,000 a year ($616/day) - the most expensive CV dru g YEAR 2 SALES FOLLOWING LAUNCH $1.3 billion collectively for Vyndaqel® and Vyndamax® Developed by MyoKardia and the company was subsequently acquired by BMS for $13 billion FDA DESIGNATIONS Orphan Drug, Priority Review EXPEDITED APPROVAL Based on a 251 - patient study called EXPLORER , in which patients randomized to take the drug had significantly better peak oxygen consumption and improved on a widely used measurement of heart failure when compared to those who got a placebo. INVESTIGATORS ASSESSED The primary composite functional endpoint, assessed at 30 weeks, was defined as the proportion of patients who achieved either improvement of peak oxygen consumption (pVO 2 ) by ≥1.5 mL/kg/min plus improvement in NYHA class by at least 1 or improvement of pVO 2 by ≥3.0 mL/kg/min plus no worsening in NYHA class. PRICE $89,500 a year ($245/day) – one of the most expensive CV dru g NOTABLY The approval came with a warning for the risk of heart failure and an FDA - mandated plan to manage that risk. Track record of recent transactions for orphan drugs in cardiovascular space 10 10 Why Tecarfarin Now? 1.

Certain rare medical conditions requiring chronic anticoagulation where warfarin has been unreliable, and DOACs (Eliquis - class drugs) are either contraindicated or not prescribed for these conditions. 2. Tecarfarin provides potentially more stable anticoagulation than warfarin because of its metabolism, thereby de creases the risk of stroke and bleeding . 3. Retrometabolic drug design process targets a different metabolic pathway from the one targeted by warfarin. 4. Provides stable and effective anticoagulation based on studies demonstrating stable INR. 5. Tecarfarin's pharmacokinetics (renal clearance and plasma half - life) were not significantly affected by severe renal impairment. In contrast, warfarin clearance was substantially reduced resulting in a significant increase in half - life based on a head - to - head PK study. 6. Newest class anticoagulants (Factor XIs) are still in development and are not being pursued for these rare medical conditions. Tecarfarin is targeted for indications where warfarin FAILS to achieve sufficiently stable anticoagulation and DOACs (Eliquis - class drugs) are NOT widely prescribed for these rare medical conditions.

11 CLINICAL DATA

12 Vitamin K Antagonism Inhibits Multiple Factors (II, VII, IX, X, Proteins C & S) in the Clotting Cascade vs. Single Targets of Newer Agents ANTI - Fxa Apixaban Edoxaban Rivaroxaban ANTI - FIIa Dabigatran TISSUE FACTOR (TF) PATHWAY CONTACT ACTIVATION PATHWAY COMMON PATHWAY FIXa FXIIa FVIIa TF - FVIIa complex TF FXIa FVIIIa Tenase complex (FIXa + FVllla) Prothrombinase complex ( FIXa + FVllla + FXa) FXa FIIa CLOTTING MULTI - TARGETS VKA MULTI - TARGETS VKA MULTI - TARGETS VKA MULTI - TARGETS VKA clotting SINGLE - TARGET DOACs SINGLE - TARGET DOACs Proven mechanism of action resulting in clinically meaningful anticoagulation in certain conditions where DOACs have failed 13 13 Tecarfarin is Designed to be an Improvement to Warfarin Whitlock RP, Fordyce CB, Midei MG, et al.
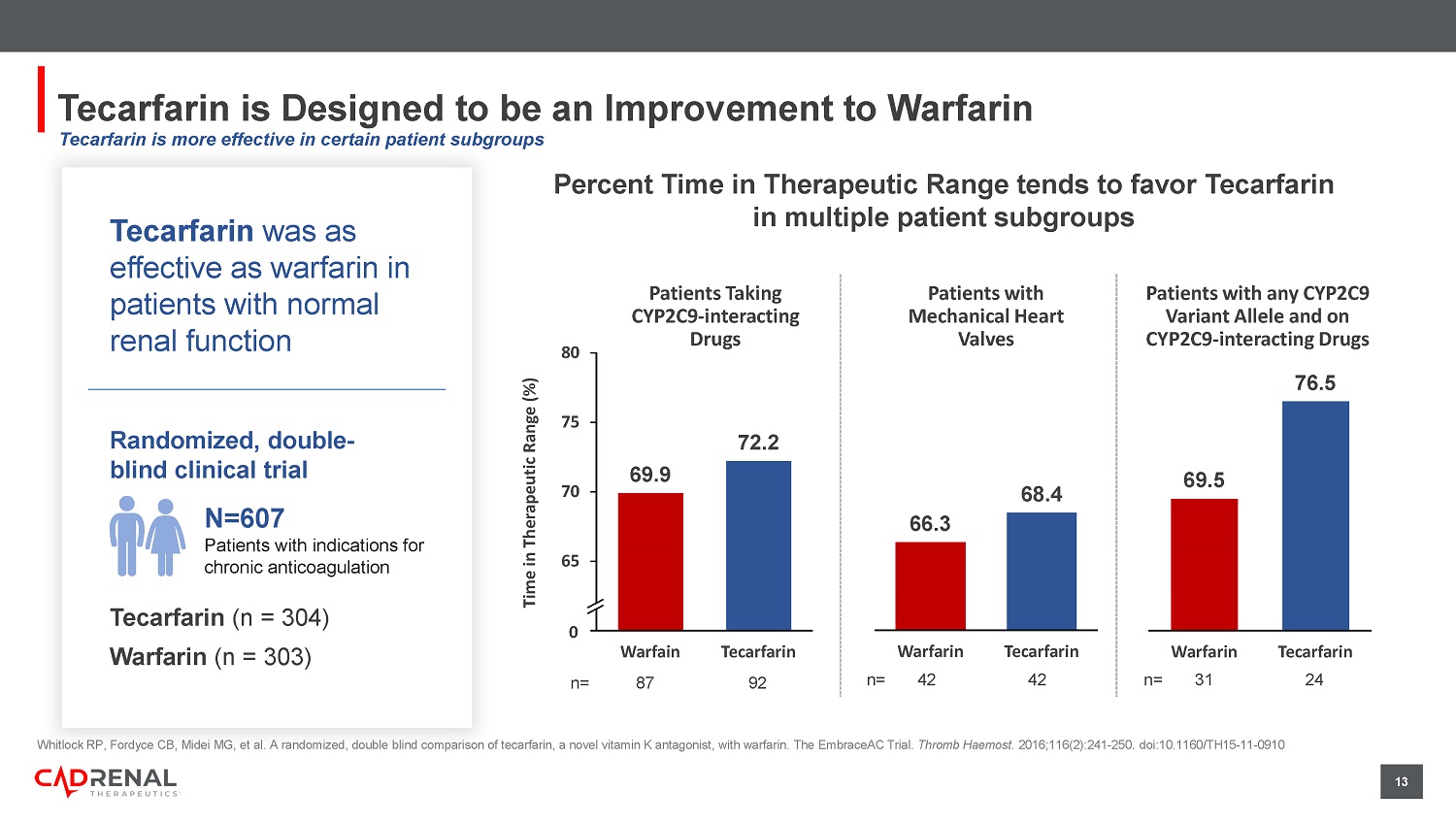
A randomized, double blind comparison of tecarfarin, a novel vitamin K antagonist, wit h warfarin. The EmbraceAC Trial. Thromb Haemost. 2016;116(2):241 - 250. doi:10.1160/TH15 - 11 - 0910 Percent Time in Therapeutic Range tends to favor Tecarfarin in multiple patient subgroups Tecarfarin was as effective as warfarin in patients with normal renal function Randomized, double - blind clinical trial N=607 P atients with indications for chronic anticoagulation Tecarfarin (n = 304) Warfarin (n = 303) 66.3 68.4 Warfarin Tecarfarin Patients with Mechanical Heart Valves n= 42 42 69.5 76.5 Warfarin Tecarfarin Patients with any CYP2C9 Variant Allele and on CYP2C9 - interacting Drugs n= 31 24 Patients Taking CYP2C9 - interacting Drugs n= 87 92 69.9 72.2 60 65 70 75 80 Warfain Tecarfarin Time in Therapeutic Range (%) 0 Tecarfarin is more effective in certain patient subgroups 14 44 15 0 5 10 15 20 25 30 35 40 45 50 Warfarin Tecarfarin Percent Increase 20 - 8 -10 -5 0 5 10 15 20 25 Warfarin Tecarfarin Percent Increase/Decrease AUC t 1/2 Kidney Failure Had a Significant Impact on Warfarin PK vs Tecarfarin Percent Increase in Exposure for Chronic Kidney Disease subjects vs Healthy subjects for Warfarin and Tecarfarin (n =23) Albrecht D, Turakhia MP, Ries D, et al.

Pharmacokinetics of Tecarfarin and Warfarin in Patients with Severe Chronic Kidney Di sea se. Thromb Haemost. 2017;117(11):2026 - 2033. doi:10.1160/TH16 - 10 - 0815 Tecarfarin metabolism not as impacted by kidney failure 15 15 Despite the Significantly Increased Risk of Stroke in ESKD pts with AFib, Most Patients are Not Anticoagulated Due to the Lack of Evidence of Benefit 1.

Yoon CY, Noh J, Jhee JH, et al. Warfarin Use in Patients With Atrial Fibrillation Undergoing Hemodialysis: A Nationwide Popul ati on - Based Study. Stroke . 2017;48(9):2472 - 2479. doi:10.1161/STROKEAHA.117.017114 2. Randhawa MS, Vishwanath R, Rai MP, et al. Association Between Use of Warfarin for Atrial Fibrillation and Outcomes Among Pati ent s With End - Stage Renal Disease: A Systematic Review and Meta - analysis. JAMA Netw Open. 2020;3(4):e202175.
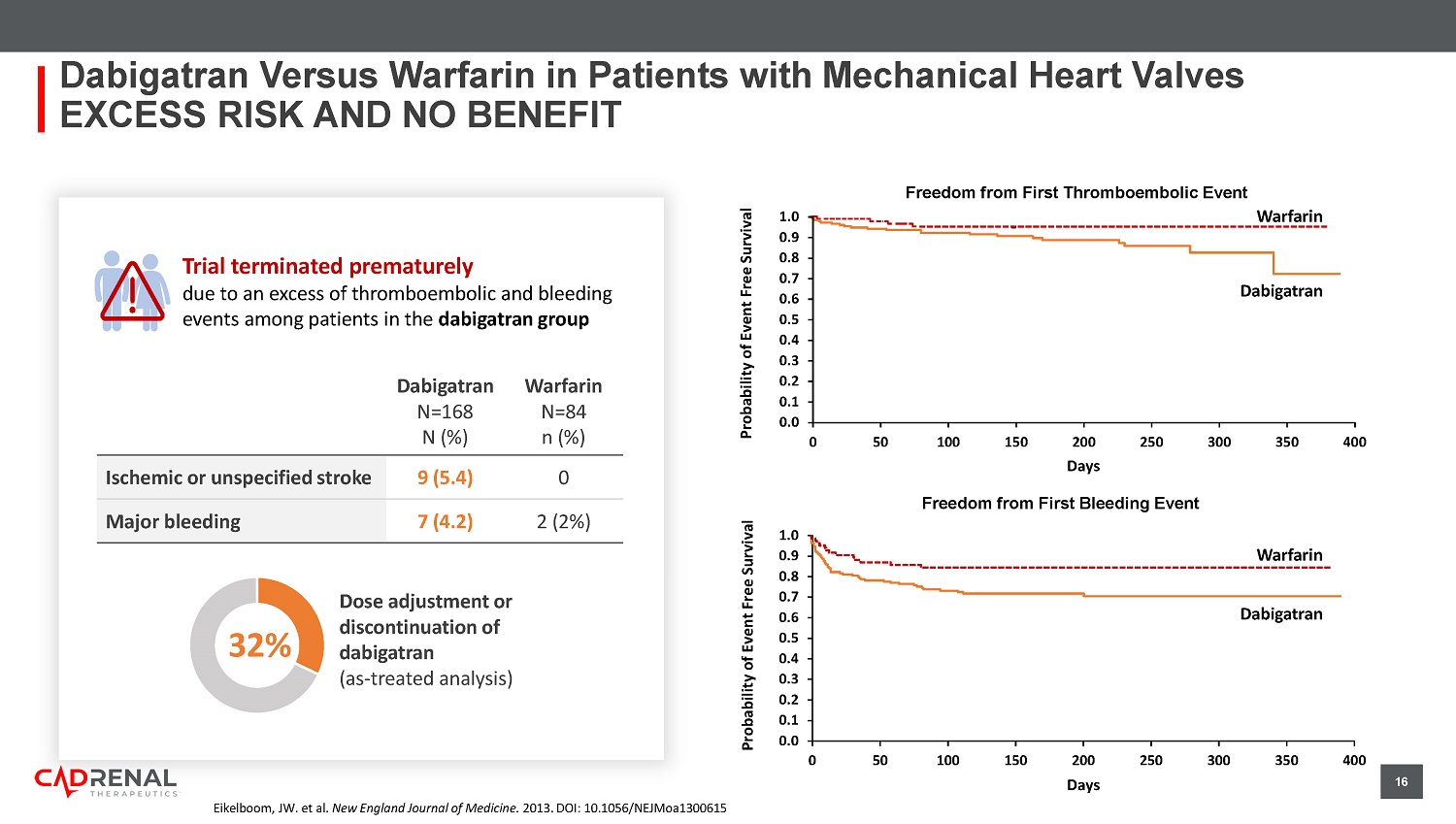
doi:10.1001/jamanetworkopen.2020.2175 70.7% 29.3% 0% 20% 40% 60% 80% 100% Warfarin Non - user Warfarin User (N=9,974) 1 n= 7,053 2,921 78.0% 22.0% Warfarin Non - user Warfarin User (N=47,480) 2 n= 37,035 10,445 Use of warfarin in ES K D + AFib Patients 1 Most patients with ESKD + AFib are not prescribed ANY anticoagulation to reduce their risk of stroke 16 Trial terminated prematurely due to an excess of thromboembolic and bleeding events among patients in the dabigatran group Dabigatran Versus Warfarin in Patients with Mechanical Heart Valves EXCESS RISK AND NO BENEFIT Eikelboom , JW. et al. New England Journal of Medicine. 2013. DOI: 10.1056/NEJMoa1300615 Dabigatran N=168 N (%) Warfarin N=84 n (%) Ischemic or unspecified stroke 9 (5.4) 0 Major bleeding 7 (4.2) 2 (2%) Dose adjustment or discontinuation of dabigatran ( a s - treated analysis) 32% 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 0 50 100 150 200 250 300 350 400 First Bleeding Event Days Warfarin Dabigatran Warfarin Dabigatran Days First Thromboembolic Event 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 0 50 100 150 200 250 300 350 400 Probability of Event Free Survival Probability of Event Free Survival Freedom from First Thromboembolic Event Freedom from First Bleeding Event 17 Composite arterial thrombosis 5.43 (1.87, 15.75) Venous thromboembolism events 1.20 (0.31, 4.55) Composite of arterial or venous thrombosis 4.46 (1.12, 17.84) Stroke 10.74 (2.29, 50.38) Major bleeding 1.02 (0.42, 2.47) All - cause death 1.43 (0.44, 4.62) 0.1 1 10 100 Higher in VKAs Higher in DOACs Khairani CD, Bejjani A, Piazza G, et al.

J Am Coll Cardiol . 2023;81(1):16 - 30. doi:10.1016/j.jacc.2022.10.008 Use of DOACs compared with VKAs was associated with: • Increased odds of arterial thrombotic events, especially stroke • No change in the odds of VTE or major bleeding • Results were consistent within subgroups Vitamin K Antagonists (VKAs) Direct Oral Anticoagulants (DOACs) R Patients with Thrombotic APS N = 474 RAPS UK (N = 116) TRAPS Italy (N = 120) Ordi - Ros, et al.

18 UPCOMING MILESTONES

Spain (N = 190) ASTRO - APS US (N = 48) TRIAL SITES RESULTS Antiphospholipid Syndrome (APS) Patients Randomized to DOACs Have Increased Arterial Thrombosis Risk While VKA is preferred therapy patients with APS treated with Warfarin have high rates of recurrent thrombosis 19 19 Accomplishments, Goals and Future Milestones Accomplishments Completed Initial Public Offering in January 2023 and Follow - On Offering to Raise a Combined $14.5 million in 2023 Formed Scientific Advisory Board in support of the development of tecarfarin Expanded focus for tecarfarin development for patients with implanted medical devices for heart diseases Future Goals and Milestones Trial Design for Left Ventricular Assist Devices (LVADs) Trial Design for Antiphospholipid Syndrome Advance CMC/Manufacturing program Licensing and Partnership Developments Commence a Registration Study H2 2023 H2 2023 Received Orphan Drug Designation for the prevention of systemic thromboembolism of cardiac origin in patients with ESKD and AFib. Granted Fast Track designation for the prevention of systemic thromboembolism of cardiac origin in patients with ESKD and AFib. H2 2023 H1 2024 2024 20 MANAGEMENT, BOARD & SCIENTIFIC ADVISORY BOARD


21 Leadership Team Quang Pham CEO & Founder, Chairman Douglas Losordo, MD Chief Medical Officer Matthew Szot, CPA Chief Financial Officer John R.

Murphy Director Steven Zelenkofske, OD Director Glynn Wilson, PhD Director Robert Lisicki Director 22 Scientific Advisory Board (SAB) Christopher Granger, MD o Professor of Medicine in the Division of Cardiology at Duke University o Member, Duke Clinical Research Institute (DCRI) Sean Pokorney, MD, MBA o Electrophysiologist and Assistant Professor of Medicine Elaine M. Hylek, MD, MPH o Professor of Medicine, Boston University School of Medicine o Director of the Thrombosis and Anticoagulation Service at Boston Medical Center (BMC) C. Michael Gibson, MD o Professor of Medicine, Harvard Medical School o Interventional Cardiologist, Beth Israel Deaconess Medical Center o President & CEO, Baim Institute for Clinical Research Wolfgang C. Winkelmayer, MD, MPH o Chief, Section of Nephrology, Professor of Medicine, Baylor University o Director, Selzman Institute for Kidney Health Richard Whitlock, MD o Cardiac Surgeon and Professor of Surgery, McMaster University Medical Center o Investigator, Population Health Researchtute A. Michael Lincoff, MD o Vice Chairman, Dept. of Cardiovascular Medicine, Cleveland Clinic o Director of Clinical Research, Lerner Research Institute 24 Cap Table (as of 8/10/23) Cash (at 8/10/23) $9.6 million Debt NONE Common Shares Outstanding (excluding pre - funded warrants) 13,022,754 Pre - Funded Warrants 2,985,715 As Adjusted – Common Shares Outstanding 16,008,469 Warrants – Investors (avg.

23 FINANCIALS & HIGHLIGHTS
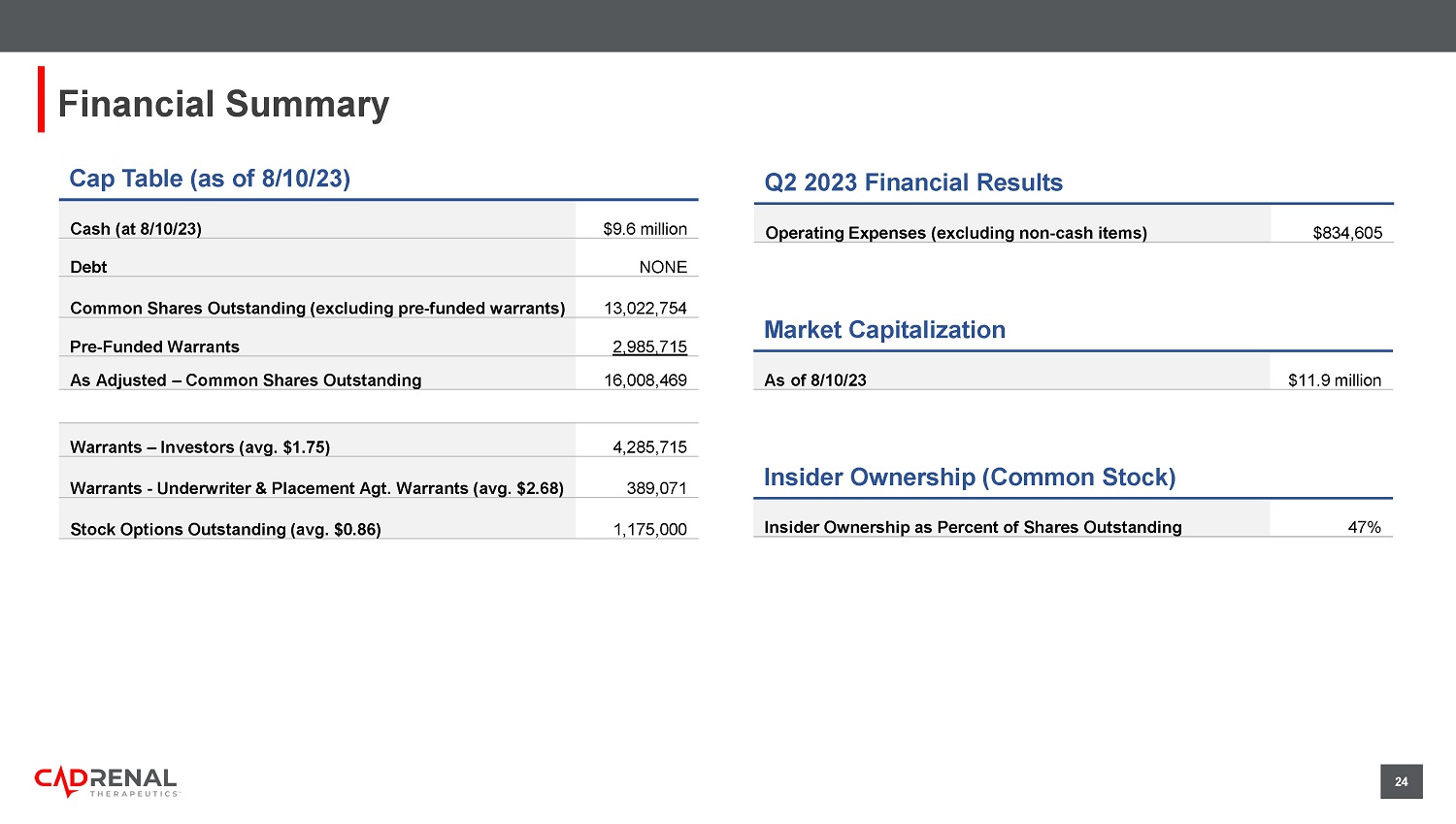
$1.75) 4,285,715 Warrants - Underwriter & Placement Agt. Warrants (avg. $2.68) 389,071 Stock Options Outstanding (avg.

$0.86) 1,175,000 Financial Summary Q2 2023 Financial Results Operating Expenses (excluding non - cash items) $834,605 Market Capitalization As of 8/10/23 $11.9 million Insider Ownership (Common Stock) Insider Ownership as Percent of Shares Outstanding 47% 25 Summary of Highlights PORTFOLIO o Late - stage ready orphan drug candidate with IP rights in North America, Europe, Australia, Asia and Africa o One 7 - year U.S. orphan drug marketing exclusivity potential upon orphan drug approval R&D o Retrometabolic design eliminates or minimizes the CYP450 metabolism in the liver REGULATORY o FDA orphan drug designation of tecarfarin for patients with ESKD + AFib o FDA Fast Track designation o Pursuing possible use for patients with left ventricular assist devices (LVADs) and Antiphospholipid Syndrome (APS) CLINICAL o Expected single pivotal Phase 3 placebo - controlled trial remaining for tecarfarin (N=492), based on latest FDA correspondence o Existing safety database with 1,003 subjects in 11 clinical trials COMMERCIAL o More than $2 billion U.S. annual market potential for tecarfarin drug candidate ADVANTAGE 26 Contact Us Matthew Szot CFO matthew.szot@cadrenal.com Quang Pham CEO & Founder quang.pham@cadrenal.com


27 APPENDIX

28 Warfarin vs Tecarfarin Metabolism Pathways S - Warfarin R - Warfarin CYP2C19 CYP1A2 CYP1A1 CYP3A4 CYP1A1 CYP2C8 CYP1A2 CYP2C18 CYP2C9 CYP2C9 CYP2C9 CYP2C9 6 - hydroxywarfarin 8 - hydroxywarfarin 10 - hydroxywarfarin 7 - hydroxywarfarin 4 - hydroxywarfarin 7 - hydroxywarfarin 6 - hydroxywarfarin dehydroxywarfarin 7 Different CYP450 Isoenzymes involved in Warfarin Metabolism! Warfarin CYP450 Elimination via kidney Elimination via bile ABCB1 Tecarfarin Human CarboxylEsterase 2 (CES2) Tecarfarin CES2 Although genetic variation in CES2 has been identified there have been no reports of clinically significant impact ~30 % of population CYP2C9 genetic variations Genetic variations Drug interactions Elimination via bile Tecarfarin provides potentially a more reliable anticoagulation than warfarin because of its metabolism, thereby decreases th e r isk of stroke and bleeding.

29 Warfarin Metabolism via CYP450 is Complicated by Known Competitors, Inhibitors and Inducers and the Established Impact of Genetic Variants Enzymes Substrates Inhibitors Inducers CYP 3A4 amlodipine, simvastatin, warfarin , amiodarone, sildenafil, midazolam, fluoxetine, haloperidol, codeine, oxycodone, methadone, fentanyl ciprofloxacin, ketoconazole, ritonavir, methylprednisone, imatinib, tamoxifen, cimetidine, grapefruit juice simvastatin, efavirenz, pentobarbital, carbamazepine, phenobarbital, phenytoin, valproic acid, caffeine CYP IA2 alosetron, caffeine, duloxetine, melatonin, ramelteon, tacrine, tizanidine ciprofloxacin, enoxacin, fluvoxamine, oral contraceptives, phenylpropanolamine montelukast, phenytoin, smoking components of cigarettes CYP 2C8 repaglinide, paclitaxel, methadone gemfibrozil, fluvoxamine, ketoconazole, trimethoprim rifampin CYP 2C9 celecoxib, warfarin , phenytoin amiodarone, fluconazole, miconazole, oxandrolone, capecitabine, etravirine, fluvastatin, metronidazole, Sulfinpyrazone, tigecycline carbamazepine, rifampin, aprepitant, bosentan, phenobarbital, St.

John's wort CYP 2D6 lidocaine, metoprolol, haloperidol, fluoxetine, amitriptyline, metoclopramide, codeine, oxycosone, tramadol amiodarone, chlorpromazine, citalopram, bupropion rifampin, dexamethasone 30 Tecarfarin is Metabolized in the Human CarboxylEsterase 2 pathway (CES2 Enzymes Substrates Inhibitors Inducers CYP 3A4 amlodipine, simvastatin, warfarin , amiodarone, sildenafil, midazolam, fluoxetine, haloperidol, codeine, oxycodone, methadone, fentanyl ciprofloxacin, ketoconazole, ritonavir, methylprednisone, imatinib, tamoxifen, cimetidine, grapefruit juice simvastatin, efavirenz, pentobarbital, carbamazepine, phenobarbital, phenytoin, valproic acid, caffeine CYP IA2 alosetron, caffeine, duloxetine, melatonin, ramelteon, tacrine, tizanidine ciprofloxacin, enoxacin, fluvoxamine, oral contraceptives, phenylpropanolamine montelukast, phenytoin, smoking components of cigarettes CYP 2C8 repaglinide, paclitaxel, methadone gemfibrozil, fluvoxamine, ketoconazole, trimethoprim rifampin CYP 2C9 celecoxib, warfarin , phenytoin amiodarone, fluconazole, miconazole, oxandrolone, capecitabine, etravirine, fluvastatin, metronidazole, Sulfinpyrazone, tigecycline carbamazepine, rifampin, aprepitant, bosentan, phenobarbital, St.

John's wort CYP 2D6 lidocaine, metoprolol, haloperidol, fluoxetine, amitriptyline, metoclopramide, codeine, oxycosone, tramadol amiodarone, chlorpromazine, citalopram, bupropion rifampin, dexamethasone Antiplateletes/Anticoagulants • Acetylsalicylic acid • Prasugrel • Dabigatran etexilate Angiotensin receptor blockers • Candesartan cilexetil • Olmesartan medoxomil • Azilsartan medoxomil Antivitral agents • Tenfovir disoproxil • Adefovir dipivoxil • Valacyclovir CNS agents • Cocaine • Heroin • 6 - monoacetylmorphine Immunosuppressive agents • MethyIprednisolone sodium succinate • Deflazacort Oncology agents • Irinotecan • Capecitabine Anesthetic drug • Procaine CES2 Substrate Drugs Limited Substrates Identified Genetic variation exists, but limited evidence of clinical impact 31 Tecarfarin Shows TTR Above Anticoagulation Control Threshold 0% 20% 40% 60% 80% 100% Overall (N=607) On CYP2C9 inhibiting drug (N=179) On CYP2C9 inhibitor with genetic variant (N = 55) 72.3% +0.8% △ Warfarin (p = 0.51) 76.5% +7.0% △ Warfarin (p = 0.09) 72.2% +2.3% △ Warfarin (p = 0.15) Tecarfarin percent time in therapeutic range (TTR) TTR of 70% or greater is generally accepted as the goal for stable anticoagulation with a VKA Source: EmbraceAC Review by Whitlock et al., 2016 o Tecarfarin vs. well - controlled warfarin trial o Randomized, double - blind trial designed to compare the quality of anticoagulation o Average TTR as measured by the International Normalized Ratio (INR) o Dosing managed by a centralized dose control center Phase 2/3 Trial Design Key Tecarfarin Findings: Demonstrated TTR >72% overall and across key subgroups Demonstrated trends suggesting improved TTR control i n key subgroups expected to do poorly with warfarin Demonstrated similar major bleeding as warfarin and no thrombotic events Tecarfarin provides stable anticoagulation 32 607 Patient Randomized Controlled Trial Shows Tecarfarin is Well - Tolerated for Stroke and Thrombus Prevention, with Fewer Hemorrhagic Events 0 0 0 2 2 1 Ischemic Stroke Deep Venous Thrombosis Pulmonary Embolis Tecafarin Warfarin T ecarfarin treated subjects experienced fewer major hemorrhages than the warfarin treated patients Tecarfarin had fewer thrombotic events compared to warfarin Randomized, double - blind clinical trial N=607 P atients with indications for chronic anticoagulation Tecarfarin (n = 304) Warfarin (n = 303) Whitlock RP, Fordyce CB, Midei MG, et al.

A randomised, double blind comparison of tecarfarin, a novel vitamin K antagonist, wit h warfarin. The EmbraceAC Trial. Thromb Haemost. 2016;116(2):241 - 250. doi:10.1160/TH15 - 11 - 0910 33 33 Tecarfarin was Designed to be an Improvement to Warfarin Whitlock RP, Fordyce CB, Midei MG, et al.

A randomized, double blind comparison of tecarfarin, a novel vitamin K antagonist, wit h warfarin. The EmbraceAC Trial. Thromb Haemost. 2016;116(2):241 - 250. doi:10.1160/TH15 - 11 - 0910 71.5 72.3 0 10 20 30 40 50 60 70 80 90 100 Warfarin (N=304) Tecarfarin (N=303) Time in Therapeutic Range (%) = 0.8% Percent Time in Therapeutic Range Based on Interpolated INR Tecarfarin was as effective as warfarin in patients with normal renal function Randomized, double - blind clinical trial N=607 P atients with indications for chronic anticoagulation Tecarfarin (n = 304) Warfarin (n = 303)

34 Summary of Guidelines Recommendations on Anticoagulant Treatment Prescription in Patients with Antiphospholipid Syndrome ESC = European Society of Cardiology ASH = American Society of Hematology EULAR = European Alliance of Associations for Rheumatology BSH = British Society for Haematology ISH = International Society of Hematology VKAs as First Choice (acenocoumarol, warfarin) DOACs NOT r ecommended PATIENTS WITH DEFINITE APS ESC, ASH, EULAR*,BSH, ISTH Arterial thrombotic event (ischemic stroke, myocardial infarction, systemic embolism) with any positivity (single, double or triple) ESC, ASH, ISTH Triple positive APS with both venous or arterial thrombotic event ESC, ASH, EULAR*, BSH, NICE EULAR, BSH, ISTH VKAs as First Choice Triple positive Single/double positive Venous thrombotic event (PE/DVT) * EULAR recommends to not use Rivaroxaban Pastori D et al. Front. Cardiovasc. Med . 2021. 8:715878.

doi: 10.3389/fcvm.2021.715878 35 Summary Table % change between Stage 4 CKD patients vs healthy subjects matched for each drug using a randomized crossover design (n=23) Tecarfarin (% change) (S) - Warfarin (% change) AUC +15% +44% C max +6% +7% t 1/2 - 8% +19% This study suggests that tecarfarin does not require any dose adjustments in renal impaired/CKD patients Tecarfarin Phase 1 PK Trial in Stage 4 CKD Patients Provides Evidence that CKD does not alter Tecarfarin exposure while Warfarin Exposure is Increased Exposure increased 44% in Stage 4 CKD patients Plasma concentration and half - life increased in Stage 4 CKD patients Warfarin Elimination from the body was not affected by severe kidney dysfunction Half - life and the amount of drug in the body were similar in Stage 4 CKD patients and healthy subjects Tecarfarin Result Highlights Tecarfarin does not require any dose adjustments in renal impaired/CKD patients 36 Tecarfarin vs. Placebo in Patients with ESKD and AFib Randomized, Double - Blind, Placebo - Controlled (no active control) Tecarfarin Phase 3 Trial Design for ESKD and AFib Randomization stratified according to VKORC1 status Tecarfarin (n = 246) Placebo (n = 246) 12 - month follow - up for Primary Endpoint of time to combined endpoint of ischemic stroke or systemic embolism (80% power to detect a 25% treatment benefit) R 1:1 ES K D (eGFR < 15 mL/min/1.73 mm 2 ) documented chronic paroxysmal, persistent or permanent AFib Trial Sites: U.S. and Canada, ROW TBD Planned Enrollment N = 492
