UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): October 22, 2025
ARCTURUS THERAPEUTICS HOLDINGS INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-38942 | 32-0595345 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
10628 Science Center Drive, Suite 250
San Diego, California 92121
(Address of principal executive offices)
Registrant’s telephone number, including area code: (858) 900-2660
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
||
| Common stock, par value $0.001 per share | ARCT | The NASDAQ Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01. | Regulation FD Disclosure. |
On October 22, 2025, Arcturus Therapeutics Holdings Inc. (the “Company” or “Arcturus”), published a corporate presentation regarding LUNAR-CF (ARCT-032) Interim Phase 2 results (the “CF Results”) on the Company’s website (the “Presentation”). A copy of the Presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information in this Item 7.01 of this Current Report on Form 8-K, and Exhibit 99.1 attached hereto, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained in this Item 7.01, and in the Presentation attached as Exhibit 99.1 to this Current Report on Form 8-K, shall not be incorporated by reference into any filing with the Securities and Exchange Commission (the “SEC”) made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
| Item 8.01. | Other Events. |
Recent Events
Release of Data
On October 22, 2025, the Company announced interim results from its ongoing Phase 2 clinical trial of ARCT-032, an investigational inhaled mRNA therapy for cystic fibrosis (CF). A copy of the press release (the "Press Release") announcing the interim results is filed as Exhibit 99.2 to this Current Report on Form 8-K and the information therein is incorporated herein by reference.
Legal Proceedings
On September 23, 2025, the Company filed a lawsuit against AbbVie Inc., Capstan Therapeutics, Inc. and other defendants in the United States District Court for the Southern District of California, asserting claims for trade secret misappropriation and breach of contract. The current deadline for the defendants to respond to the complaint is December 1, 2025. The Court has not set a case schedule.
Business Updates
CSL Seqirus MAA
As previously announced, the Company has partnered with Seqirus, Inc. (“CSL Seqirus”), a part of CSL Limited and one of the world’s leading influenza vaccine providers, on the development and commercialization of mRNA vaccines for COVID-19, influenza and certain other infectious diseases. In June 2025, CSL Seqirus submitted a marketing authorization application (“MAA”) to the UK Medicines and Healthcare Products Regulatory Agency (MHRA) for KOSTAIVE® (“KOSTAIVE”), the world’s first approved self-amplifying messenger RNA vaccine for individuals 18 years and older. Although there can be no assurances, approval of the MAA is expected in the first quarter of 2026.
KOSTAIVE Regulatory Updates
On September 5, 2025, the week prior to the planned Biologics License Application (“BLA”) submission related to KOSTAIVE, the U.S. Food and Drug Administration requested that the Company delay its submission of the BLA filing based on the FDA’s expectation of providing additional advice. On October 14, 2025, the FDA informed the Company that, although the FDA had previously agreed that the Company’s proposed data package could support a single-dose indication, upon further consideration it finds that additional data from a clinical endpoint efficacy study will be needed to align with the current COVID-19 vaccine regulatory framework requirements published in The New England Journal of Medicine in May 2025. The Company and CSL Seqirus are evaluating the new requirements.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit No. | Description | |
| 99.1 | Presentation dated October 22, 2025 | |
| 99.2 | Press Release dated October 22, 2025 |
Forward Looking Statements
Each of the Presentation, the Press Release, and this Current Report on Form 8-K contain forward-looking statements. These statements relate to future events and involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future performances or achievements expressed or implied by the forward-looking statements. Each of these statements is based only on current information, assumptions and expectations that are inherently subject to change and involve a number of risks and uncertainties. Forward-looking statements include, but are not limited to, statements about: our strategy, future operations, collaborations, trends of clinical activity observed including reductions in mucus plugs and potential biological activity, the potential of ARCT-032 to address the underlying pathology of cystic fibrosis, the likelihood of success (including safety and efficacy) and promise of ARCT-032 and ARCT-154, the likelihood that clinical study results will be predictive of future clinical results, the likelihood that clinical data will be sufficient for regulatory approval, the ability to continue enrolling in the Phase 2 study of ARCT-032, the continued clinical development of ARCT-032 including the initiation and size of a third cohort in the CF study, plans to initiate a 12-week safety and preliminary efficacy study including the size and timing thereof, the continued determination of ARCT-032 to be generally safe and well tolerated including ongoing review of adverse effects the likelihood that any measure of clinical results will correlate with any other measure of clinical results, the likelihood of and timing for conducting any future clinical study, including a Phase 2b, the future submission of a BLA with the FDA related to ARCT-154, the timing of MAA approval of KOSTAIVE, and the design and scope of any future clinical study, the likelihood that clinical data will be sufficient for regulatory approval or completed in time to submit an application for regulatory approval within a particular timeframe, the anticipated timing for regulatory submissions, the potential administration regimen or dosage, and any statements other than statements of historical fact. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts,” “potential” and similar expressions (including the negative thereof) intended to identify forward looking statements. Arcturus may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in any forward-looking statements such as the foregoing, and you should not place undue reliance on such forward-looking statements. The forward-looking statements contained or implied in this presentation are subject to other risks and uncertainties, including those discussed under the heading "Risk Factors" in the Company’s most recent Annual Report on Form 10-K with the SEC and in other filings that the Company makes with the SEC. Except as otherwise required by law, we disclaim any intention or obligation to update or revise any forward-looking statements, which speak only as of the date they were made, whether as a result of new information, future events or circumstances or otherwise.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: October 22, 2025 | Arcturus Therapeutics Holdings Inc. | |
| By: |
/s/ Joseph E. Payne |
|
| Name: | Joseph E. Payne | |
| Title: | Chief Executive Officer | |



Arcturus Therapeutics Provides Interim Phase 2 Data for Cystic Fibrosis (CF) Program
ARCT-032 generally safe and well tolerated
Meaningful trends of clinical activity observed via high resolution CT scans
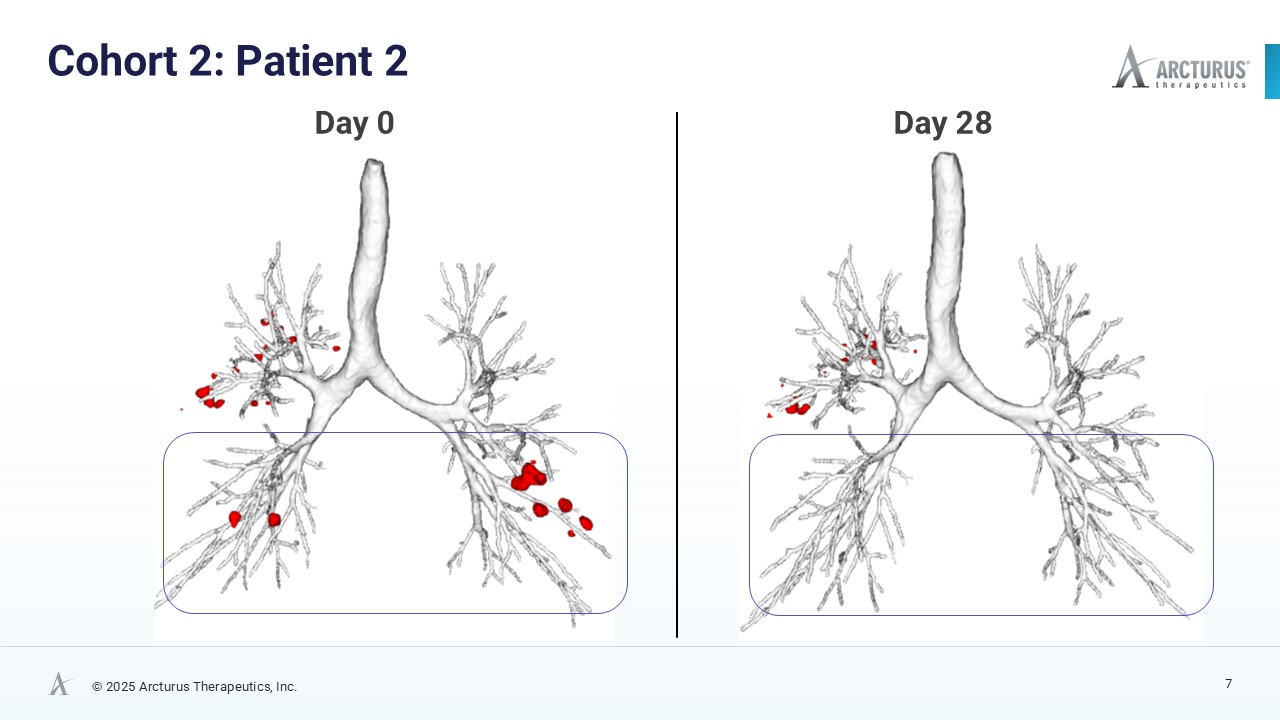
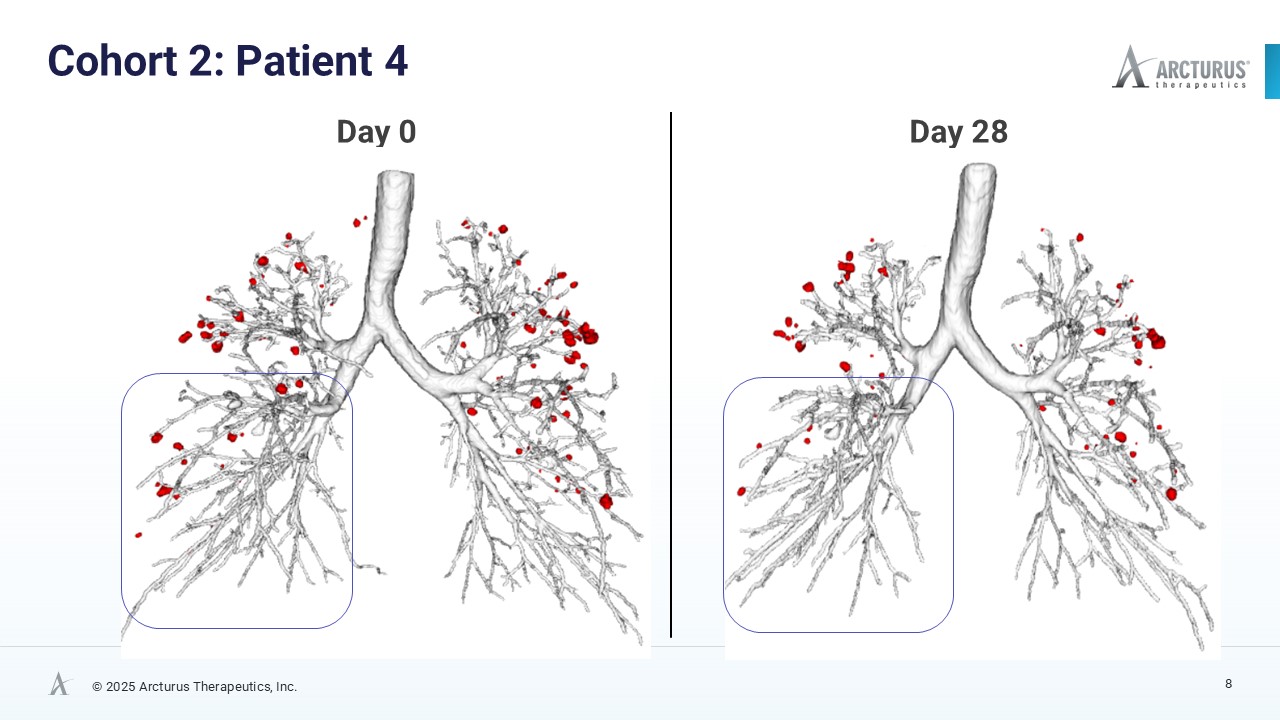
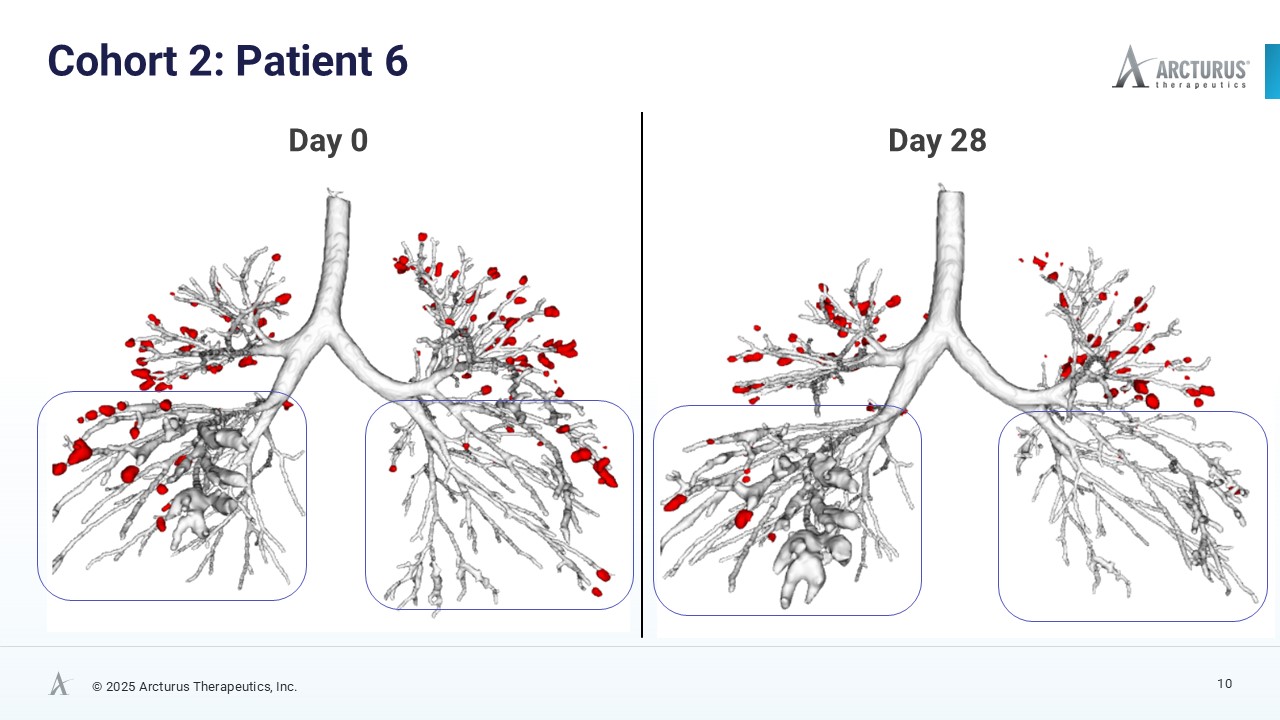
After only 28 days of treatment with ARCT-032 (10 mg), 4 out of 6 Class I CF participants exhibited encouraging reduction of mucus plug number and mucus volume
12-week study enrolling up to 20 CF participants planned to begin first half of 2026
SAN DIEGO--(BUSINESS WIRE)—Oct 21, 2025-- Arcturus Therapeutics Holdings Inc. (the “Company”, “Arcturus”, Nasdaq: ARCT), a commercial messenger RNA medicines company focused on the development of liver and respiratory rare disease therapeutics and infectious disease vaccines, today announced interim results from its ongoing Phase 2 clinical trial of ARCT-032, an investigational inhaled mRNA therapy for people with cystic fibrosis.
In the second cohort of the study, six Class I CF adults received inhaled 10 mg doses of ARCT-032 daily over 28 days. The treatment was generally safe and well tolerated. Treatment related AEs that were identified in the single-dose Phase 1 study were also observed in some participants for the first few doses but ceased with continued dosing. One SAE occurred in a participant well after the end of the dosing period. The Data Monitoring Committee found no convincing evidence that the SAE is related to ARCT-032 and approved the study to proceed. The expanded third cohort is ongoing and aims to enroll up to six subjects to determine if there is a dose escalation response at 15 mg and if ARCT-032 continues to be generally safe and well tolerated. The Company intends to initiate a 12-week safety and preliminary efficacy study in up to 20 CF participants in the first half of 2026.
“We are particularly encouraged by the early signals of mucus plug reduction in Class I CF participants treated with ARCT-032 because Class I CF individuals do not produce CFTR and therefore do not respond to available CFTR modulator therapy,” said Juergen Froehlich, MD, Chief Medical Officer of Arcturus. “The AI-enhanced lung imaging data before and after treatment with ARCT-032, supported by exploratory FEV1 lung function data analysis in this short-term study, warrant further investigation at higher doses and longer treatment durations. We look forward to initiating our 12-week study in the first half of next year for this promising therapeutic intended to address significant unmet medical need.”
“The early imaging data from this trial are encouraging,” said Dr. Harm Tiddens, Professor Emeritus of Pediatric Pulmonology at Erasmus Medical Center, and internationally respected expert in the field of imaging techniques and imaging outcome measures for chronic lung diseases such as cystic fibrosis. “Seeing a trend towards a reduction in mucus plugs and volume after only 28 days of treatment suggests biological activity. Mucus plug reduction can be followed with longer-term lung function improvements over multi-month treatment durations. These findings suggest that ARCT-032 may be addressing the underlying pathology of cystic fibrosis in a meaningful way.”
Exploratory Lung Function Analysis Suggests Potential Therapeutic Activity
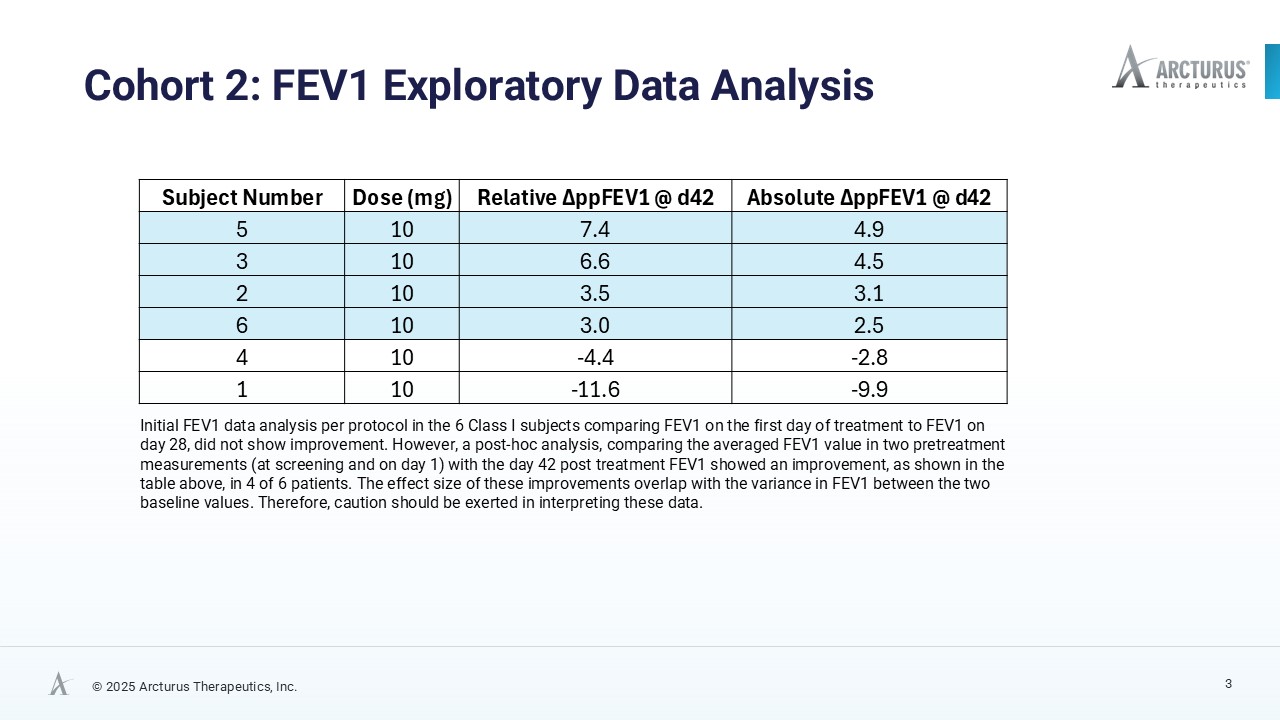
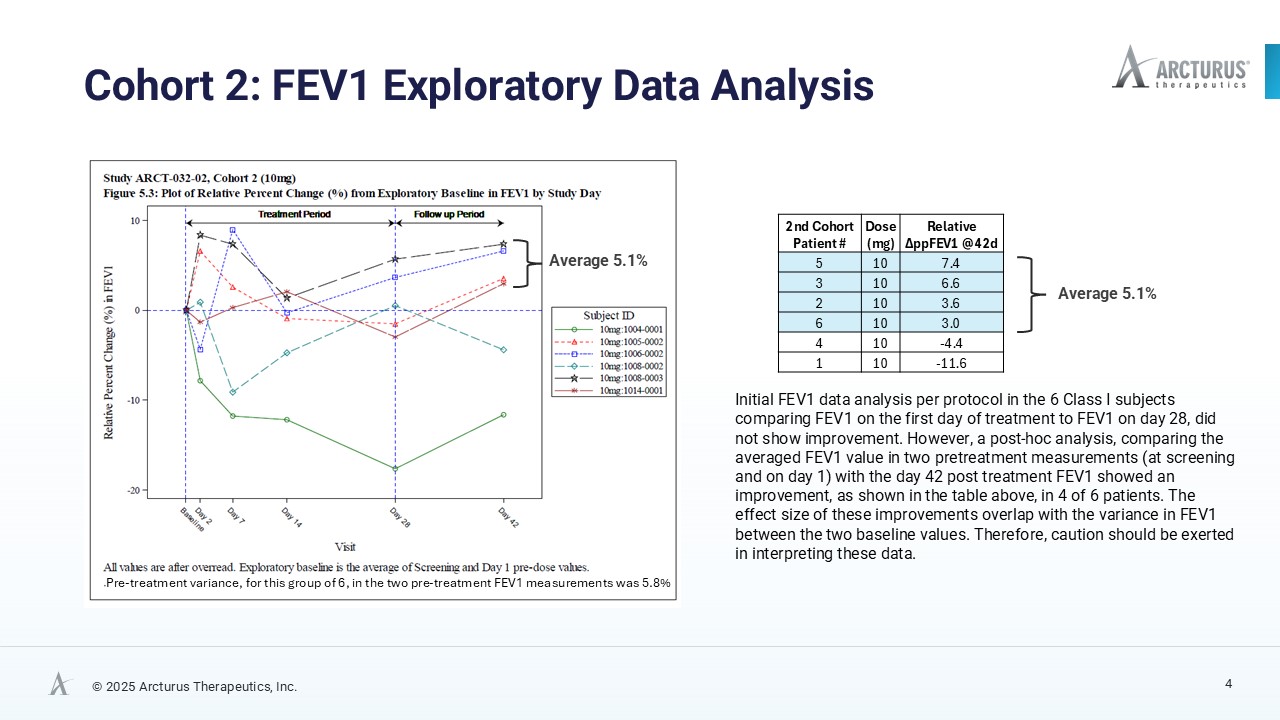
Initial analysis comparing FEV₁ values from Day 1 to Day 28 did not demonstrate meaningful improvement. However, a post hoc exploratory analysis comparing the average of two pre-treatment FEV₁ measurements (screening and Day 1) for the baseline with the Day 42 post-treatment value, assuming an extended activity of functional CFTR protein triggered by daily ARCT-032 administration, suggests improvements in lung function, in four of six Class I CF participants with an average absolute increase of 3.8% and a relative increase of 5.1% in percent predicted FEV₁ (ppFEV₁). While the magnitude of these changes falls within the range of natural variability of FEV1 measurements, such exploratory analyses can provide valuable directional signals, though they warrant cautionary interpretation. Full FEV1 data tables are available on Arcturus’ website.
AI-enhanced High Resolution Computed Tomography (HRCT) Data Show Encouraging Mucus Reduction
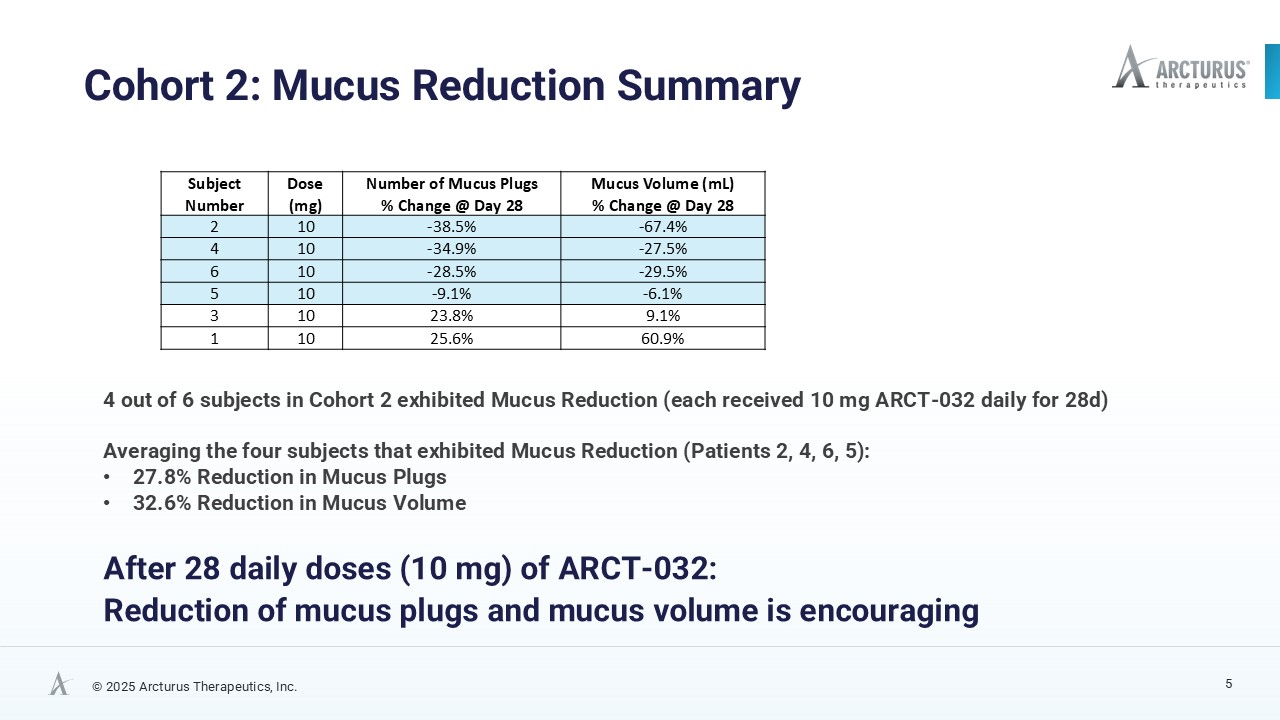
High resolution computed tomography (HRCT) scans, analyzed using FDA 501(k)-cleared AI technology from Thirona, revealed reductions in mucus burden in four of six Class I CF participants. Decrease in mucus plugs and mucus volume in four of six Class I CF participants is a meaningful trend indicative of ARCT-032 therapeutic activity. Before and after HRCT scan images of the four subjects that exhibited mucus plug reduction are available on Arcturus’ website.
| Subject Number | Dose (mg) |
Number of Mucus Plugs % Change @ Day 28 |
Mucus Volume (mL) % Change @ Day 28 |
| 2 | 10 | -38.5% | -67.4% |
| 4 | 10 | -34.9% | -27.5% |
| 6 | 10 | -28.5% | -29.5% |
| 5 | 10 | -9.1% | -6.1% |
| 3 | 10 | 23.8% | 9.1% |
| 1 | 10 | 25.6% | 60.9% |
A larger, longer-duration study is planned to substantiate the clinical relevance of these findings. The HRCT mucus reduction and supporting lung function data from the second cohort (10 mg), combined with additional data collected from the ongoing third cohort (15 mg) will guide dose selection, treatment duration, and endpoint strategy for future studies, including the conduct of a 12-week safety and preliminary efficacy clinical trial that is planned to begin in the first half of 2026.
About Cystic Fibrosis
Cystic fibrosis is a life-shortening disease with a worldwide distribution. Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene result in a reduction or absence of CFTR protein and/or function in the airways, causing insufficient chloride transport to maintain airway surface homeostasis. CF mucus is more difficult to clear, thus clogging the airways and leading to infection, inflammation, respiratory failure, or other life-threatening complications. Currently approved CFTR modulator therapies are designed to increase function of the CFTR channel to help reduce symptoms yet are ineffective in some people with CF because of their underlying mutations.
About ARCT-032
ARCT-032 has received Orphan Medicinal Product Designation from the European Medicines Agency (EMA) and Orphan Drug Designation from the U.S. Food and Drug Administration (FDA) to treat Cystic Fibrosis. ARCT-032 utilizes Arcturus' LUNAR® lipid-mediated aerosolized platform to deliver CFTR messenger RNA to the lungs. Expression of a functional copy of the CFTR mRNA in the lungs of people with CF has the potential to restore CFTR activity and mitigate the downstream effects that cause progressive lung disease. The ARCT-032 program is supported by preclinical data in rodents, ferrets and primates, as well as demonstrating restoration of CFTR expression and function in human bronchial epithelial cells.
About Arcturus
Founded in 2013 and based in San Diego, California, Arcturus Therapeutics Holdings Inc. (Nasdaq: ARCT) is a commercial mRNA medicines and vaccines company with enabling technologies: (i) LUNAR® lipid-mediated delivery, (ii) STARR® mRNA technology (sa-mRNA) and (iii) mRNA drug substance along with drug product manufacturing expertise. Arcturus developed KOSTAIVE®, the first self-amplifying messenger RNA (sa-mRNA) COVID vaccine in the world to be approved. Arcturus has an ongoing global collaboration for innovative mRNA vaccines with CSL Seqirus, and a joint venture in Japan, ARCALIS, focused on the manufacture of mRNA vaccines and therapeutics. Arcturus’ pipeline includes RNA therapeutic candidates to potentially treat OTC deficiency and cystic fibrosis (CF), along with its partnered mRNA vaccine programs for SARS-CoV-2 (COVID-19) and influenza. Arcturus’ versatile RNA therapeutics platforms can be applied toward multiple types of nucleic acid medicines including messenger RNA, small interfering RNA, circular RNA, antisense RNA, self-amplifying RNA, DNA, and gene editing therapeutics. Arcturus' technologies are covered by its extensive patent portfolio (over 500 patents and patent applications in the U.S., Europe, Japan, China, and other countries). For more information, visit www.ArcturusRx.com. In addition, please connect with us on X (formerly Twitter) and LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact included in this press release, are forward-looking statements, including those regarding strategy, future operations, trends of clinical activity observed including reductions in mucus plugs and potential biological activity, the potential of ARCT-032 to address the underlying pathology of cystic fibrosis, the continued clinical development of ARCT-032 including the initiation and size of a third cohort in the CF study, plans to initiate a 12-week safety and preliminary efficacy study including the size and timing therefore, the continued determination of ARCT-032 to be generally safe and well tolerated including ongoing review of adverse events, the likelihood of success (including safety and efficacy) of ARCT-032, and the impact of general business and economic conditions. Arcturus may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in any forward-looking statements such as the foregoing and you should not place undue reliance on such forward-looking statements. These statements are only current predictions or expectations, and are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from those anticipated by the forward-looking statements, including those discussed under the heading "Risk Factors" in Arcturus’ most recent Annual Report on Form 10-K, and in subsequent filings with, or submissions to, the SEC, which are available on the SEC’s website at www.sec.gov. Except as otherwise required by law, Arcturus disclaims any intention or obligation to update or revise any forward-looking statements, which speak only as of the date they were made, whether as a result of new information, future events or circumstances or otherwise.
Arcturus Therapeutics
Public Relations & Investor Relations
Neda Safarzadeh
VP, Head of IR/PR/Marketing
(858) 900-2682
IR@ArcturusRx.com