UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): June 30, 2025
ARCTURUS THERAPEUTICS HOLDINGS INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-38942 | 32-0595345 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
10628 Science Center Drive, Suite 250
San Diego, California 92121
(Address of principal executive offices)
Registrant’s telephone number, including area code: (858) 900-2660
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
||
| Common stock, par value $0.001 per share | ARCT | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01. Regulation FD Disclosure.
On June 30, 2025, Arcturus Therapeutics Holdings Inc. (the “Company” or “Arcturus”) issued a press release, announcing positive Phase 2 interim results in people with OTC deficiency treated with ARCT-810, an mRNA therapeutic candidate designed to replace the OTC enzyme and restore urea cycle activity preventing hyperammonemia crises (the “Release”). The Company also provided a corporate presentation regarding ARCT-810 via webcast, which is available on the Company’s website (the “Presentation”).
A copy of the Release is furnished herewith as Exhibit 99.1 and incorporated into this Item 7.01 by reference. A copy of the Presentation is furnished herewith as Exhibit 99.2 and incorporated into this Item 7.01 by reference.
The information in this Item 7.01 of this Current Report on Form 8-K, and Exhibit 99.1 and 99.2 attached hereto, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained in this Item 7.01, the Release attached as Exhibit 99.1, and in the Presentation attached as Exhibit 99.2 to this Current Report on Form 8-K, shall not be incorporated by reference into any filing with the Securities and Exchange Commission made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
| Exhibit No. | Description of Exhibit |
| 99.1 | Press Release dated June 30, 2025 |
| 99.2 | Presentation dated June 30, 2025 |
| 104 | Cover Page to this Current Report on Form 8-K in Inline XBRL |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Arcturus Therapeutics Holdings Inc. | ||
| Date: June 30, 2025 | ||
| By: | /s/ Joseph E. Payne | |
| Name: | Joseph E. Payne | |
| Title: | Chief Executive Officer | |
Arcturus Therapeutics Announces Positive Interim Phase 2 Multiple Dose Data for Ornithine Transcarbamylase (OTC) Deficiency Program
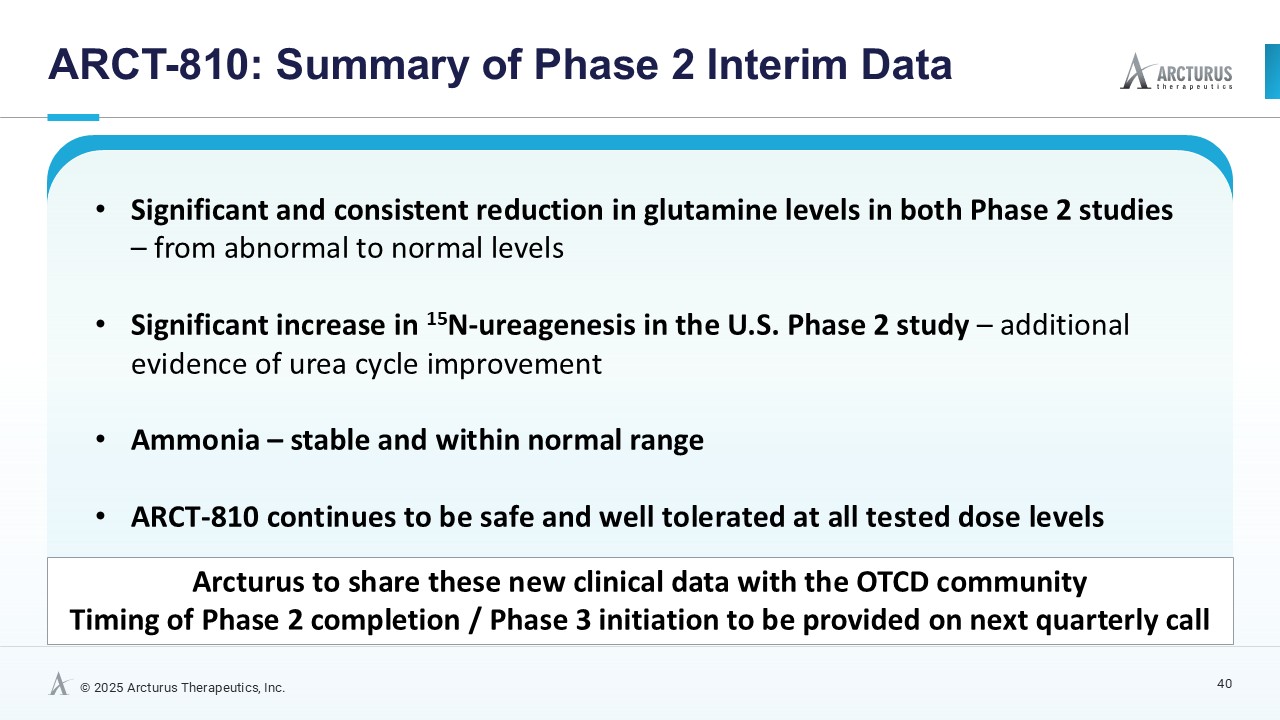
ARCT-810 significantly and consistently reduces biomarker glutamine to levels within normal range
15N-ureagenesis data provide first evidence of an mRNA therapeutic improving urea cycle function
Ammonia remained stable and within normal range
Multiple administrations of ARCT-810 continue to be safe and well tolerated at all tested dose levels
Virtual KOL Presentation at 12:00 p.m. ET Today
SAN DIEGO--(BUSINESS WIRE)--Jun. 30, 2025-- Arcturus Therapeutics Holdings Inc. (the “Company”, “Arcturus”, Nasdaq: ARCT), a commercial messenger RNA medicines company focused on the development of infectious disease vaccines and opportunities within liver and respiratory rare diseases, today announces positive Phase 2 interim results in people with OTC deficiency treated with ARCT-810, an mRNA therapeutic candidate designed to replace the OTC enzyme and restore urea cycle activity preventing hyperammonemia crises.
“We are very pleased with these new ARCT-810 clinical results, where we have achieved strong biological effects in both Phase 2 studies, including significant and consistent reduction and normalization of abnormally elevated glutamine, an important biomarker to monitor urea cycle function,” said Dr. Juergen Froehlich, Chief Medical Officer of Arcturus. “Furthermore, in our ongoing U.S. Phase 2 study, we are excited to report the first significant relative ureagenesis function (RUF) improvements using a new and optimized 15N-ureagenesis assay. Along with the observation of stable ammonia levels in all patients during treatment, these data add a level of robustness to this new interim dataset. I am also very pleased to see that our LUNAR® delivery technology continues to be generally safe and well tolerated. The combined biomarker data is unprecedented for an mRNA rare disease therapeutic and, importantly, provides a potentially accelerated path forward to a multi-biomarker driven pivotal study.”
“It is extremely rewarding to see the first mRNA therapeutic produce such solid clinical results in an area of important unmet medical need,” said Dr. Marshall Summar, CEO of Uncommon Cures. “This is a very positive step for the OTC deficient community as there are currently limited options for symptomatic patients suffering from this devastating disease.”
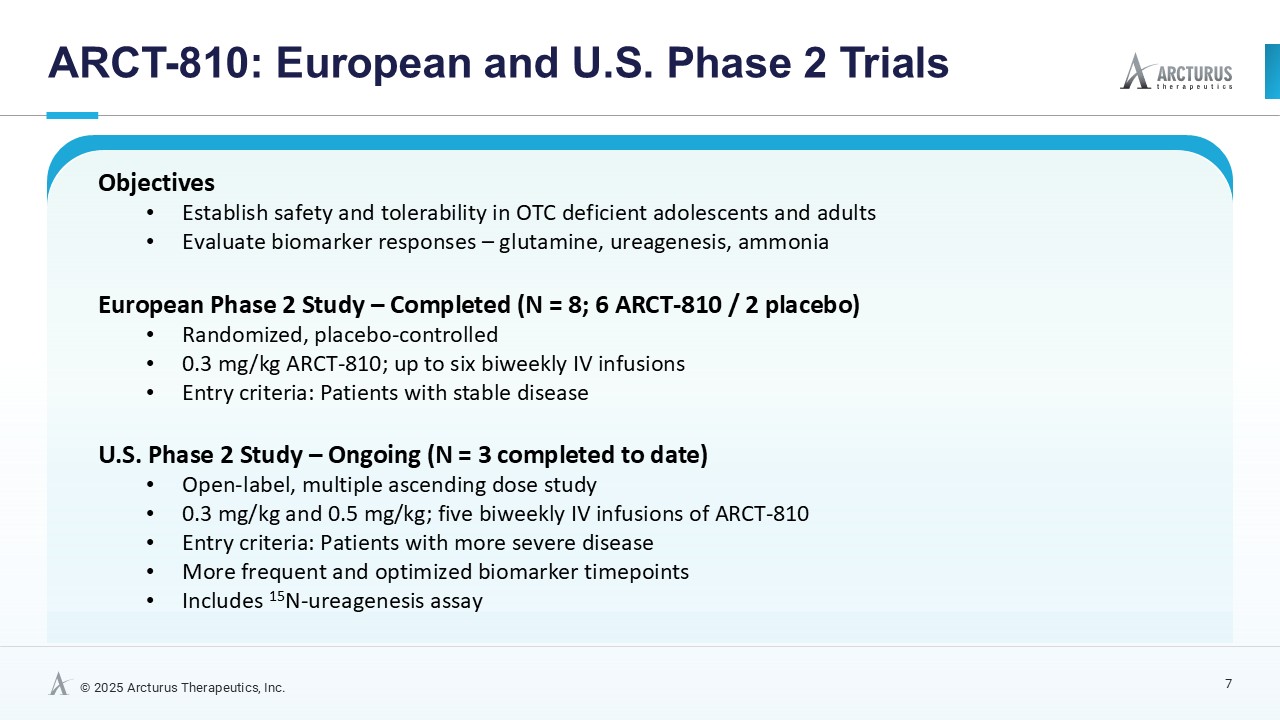
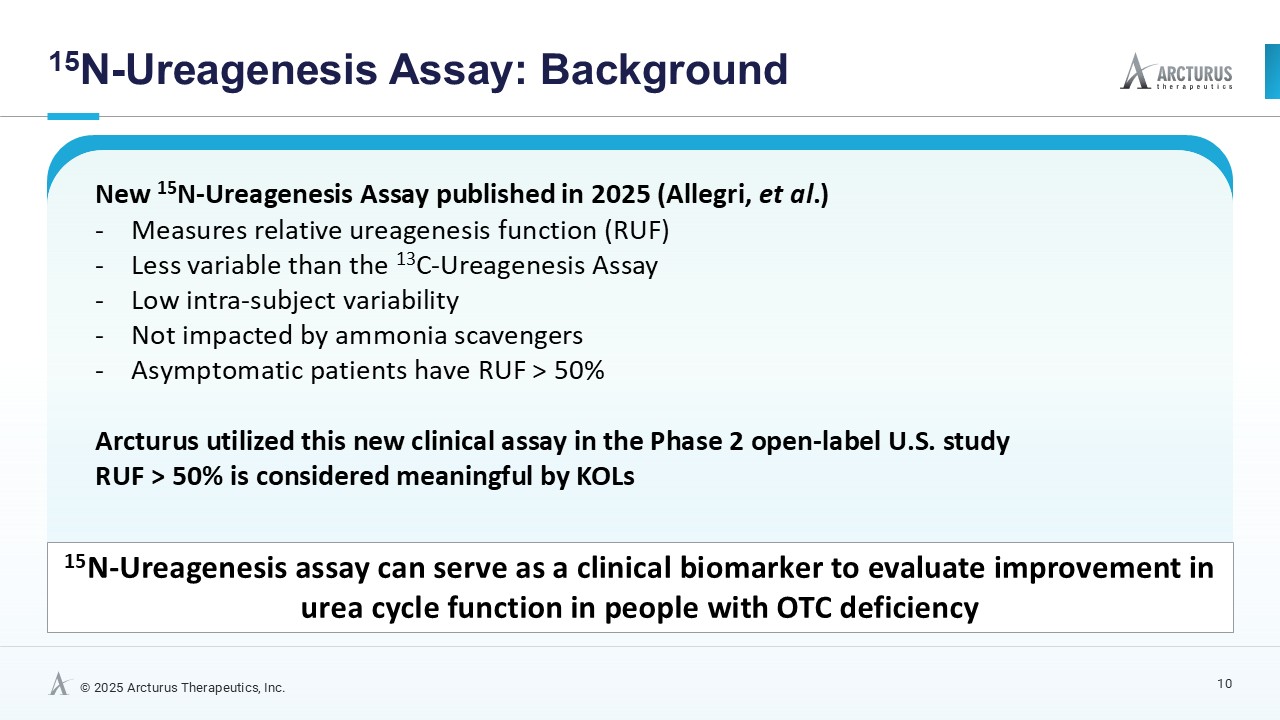
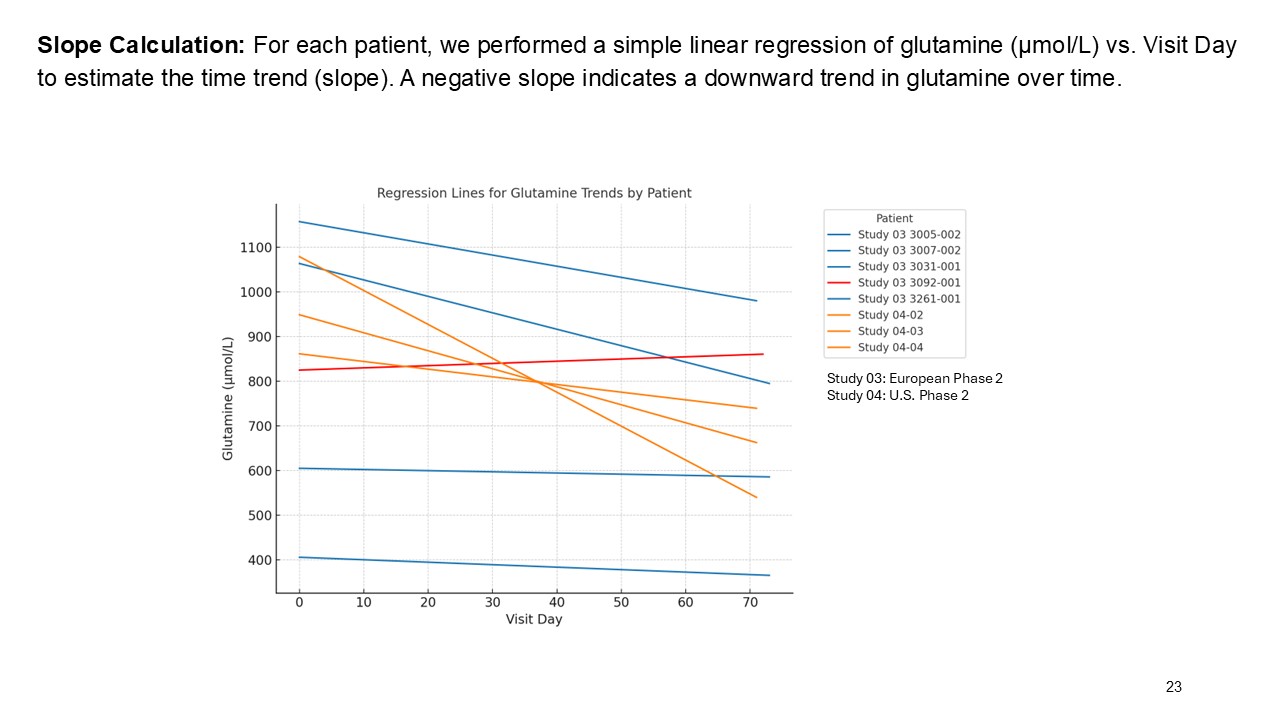
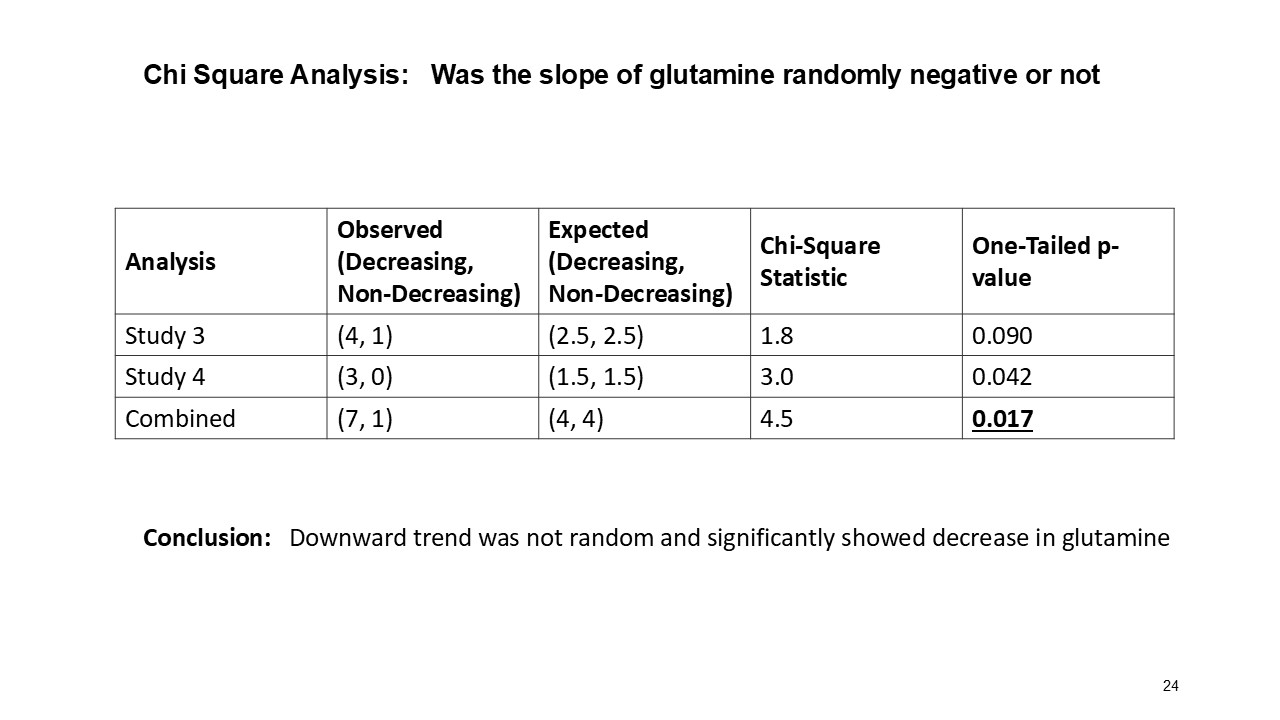
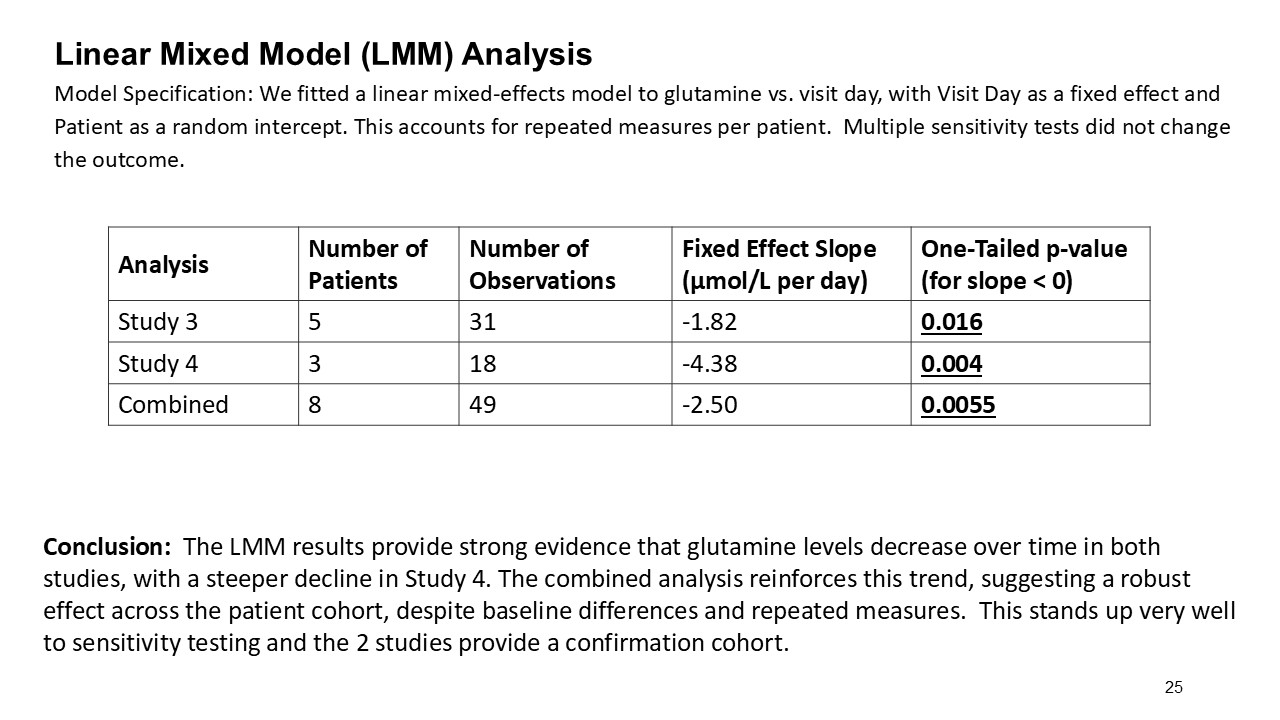
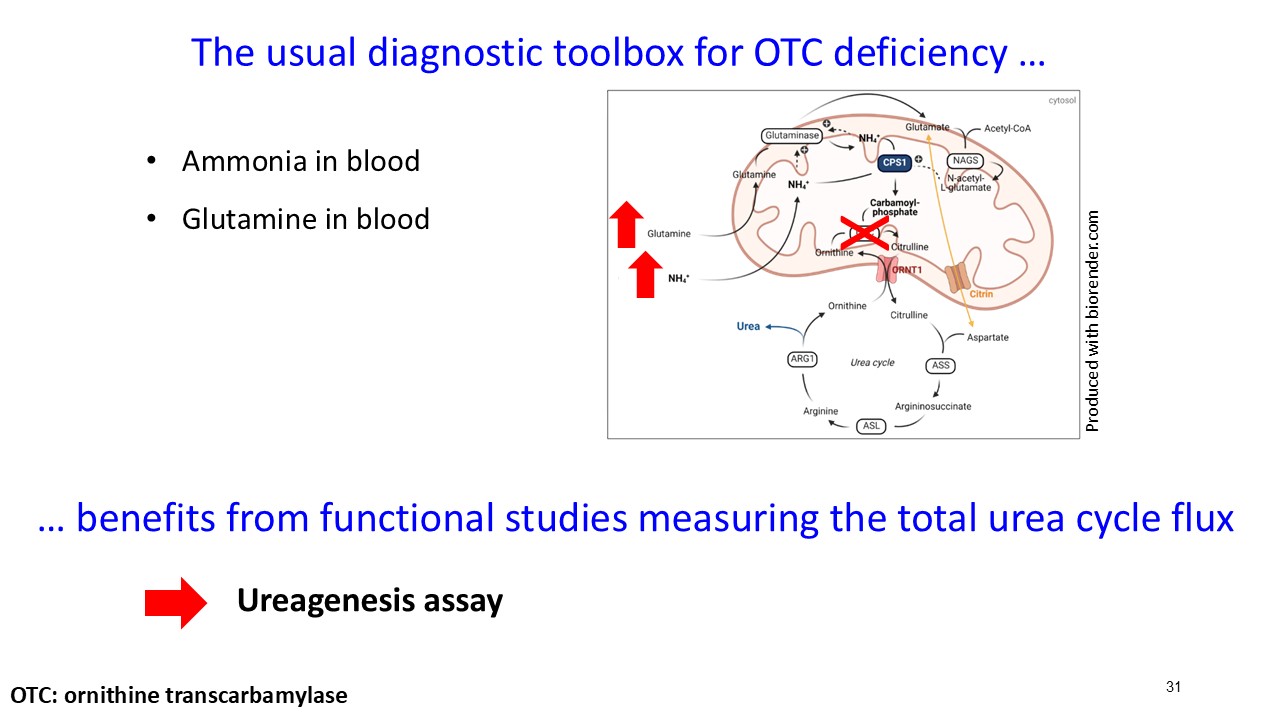
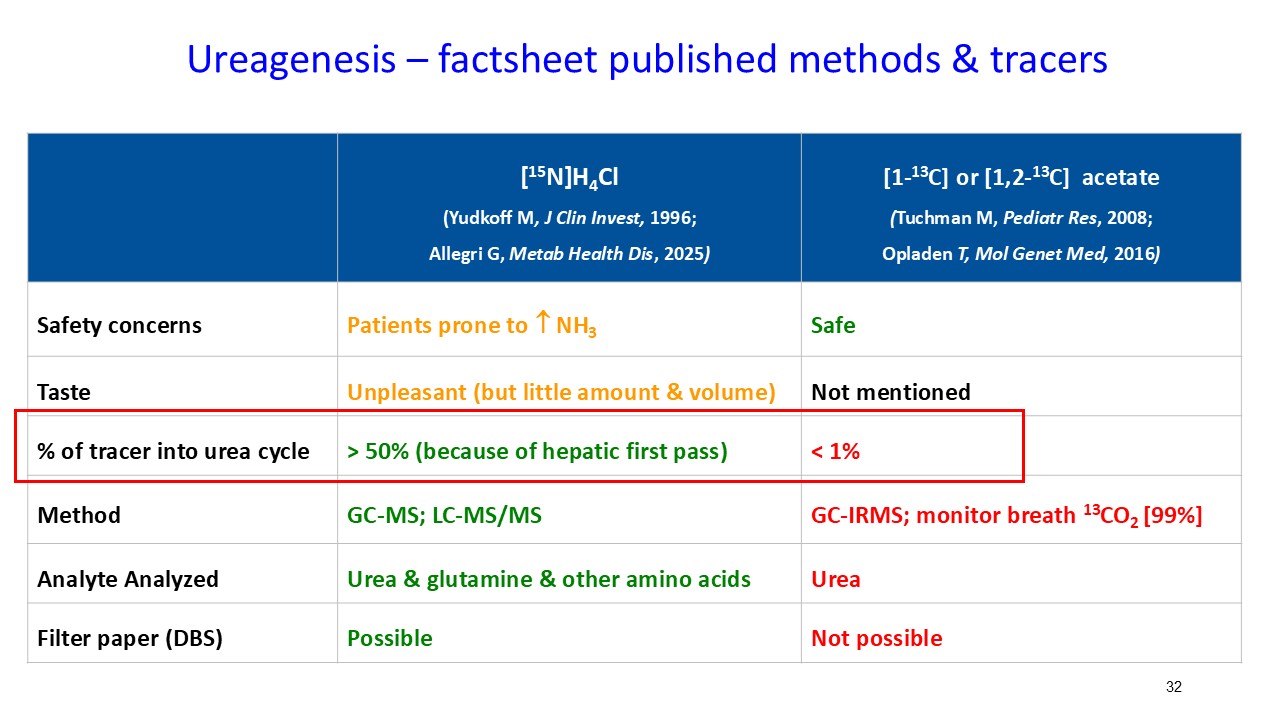
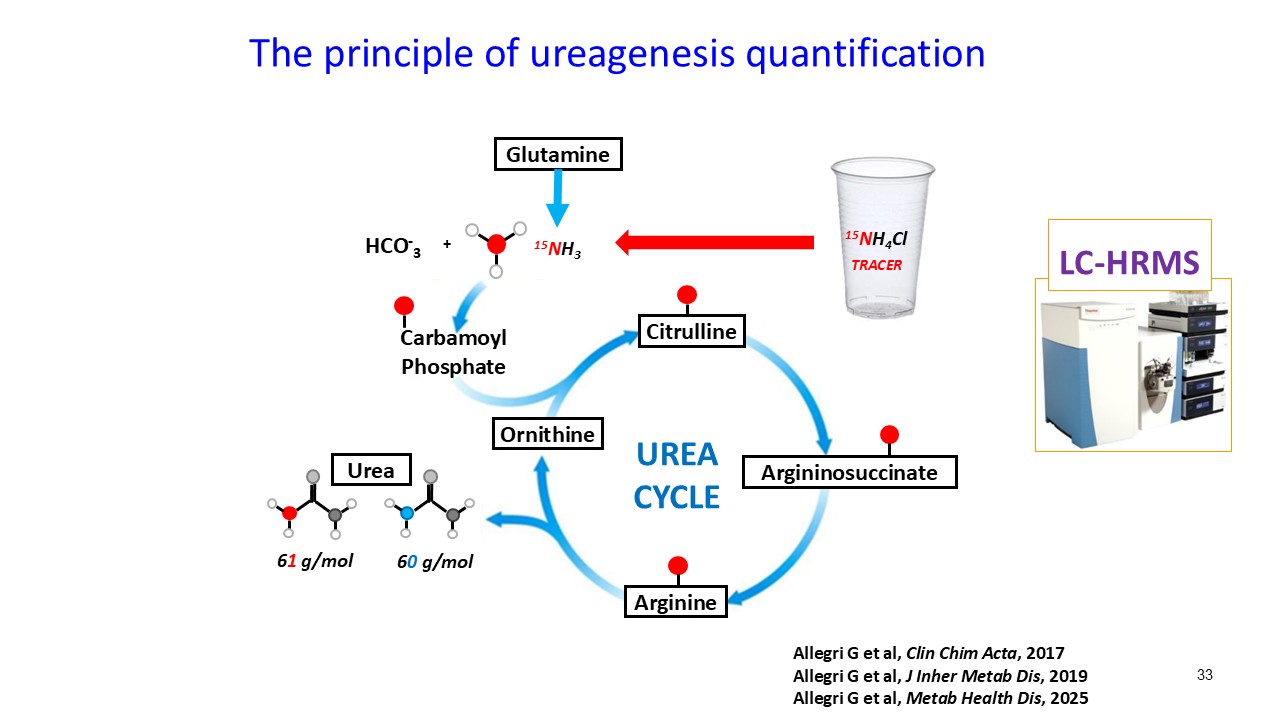
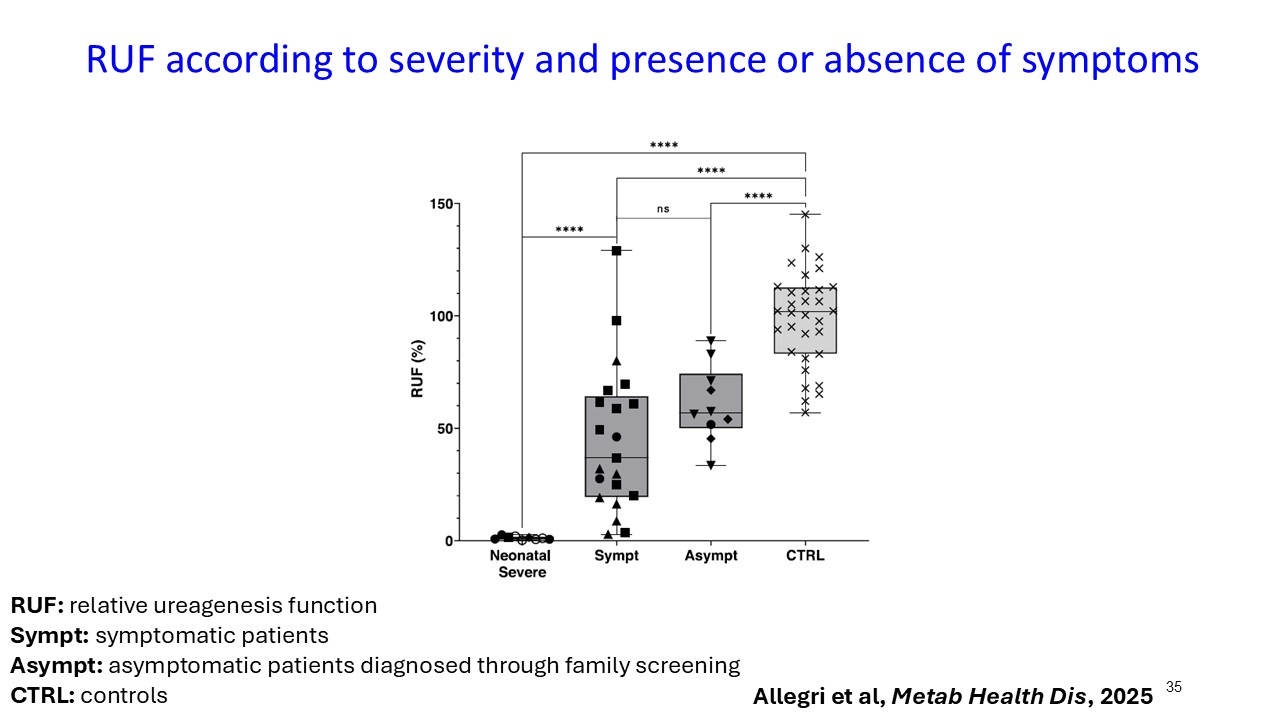
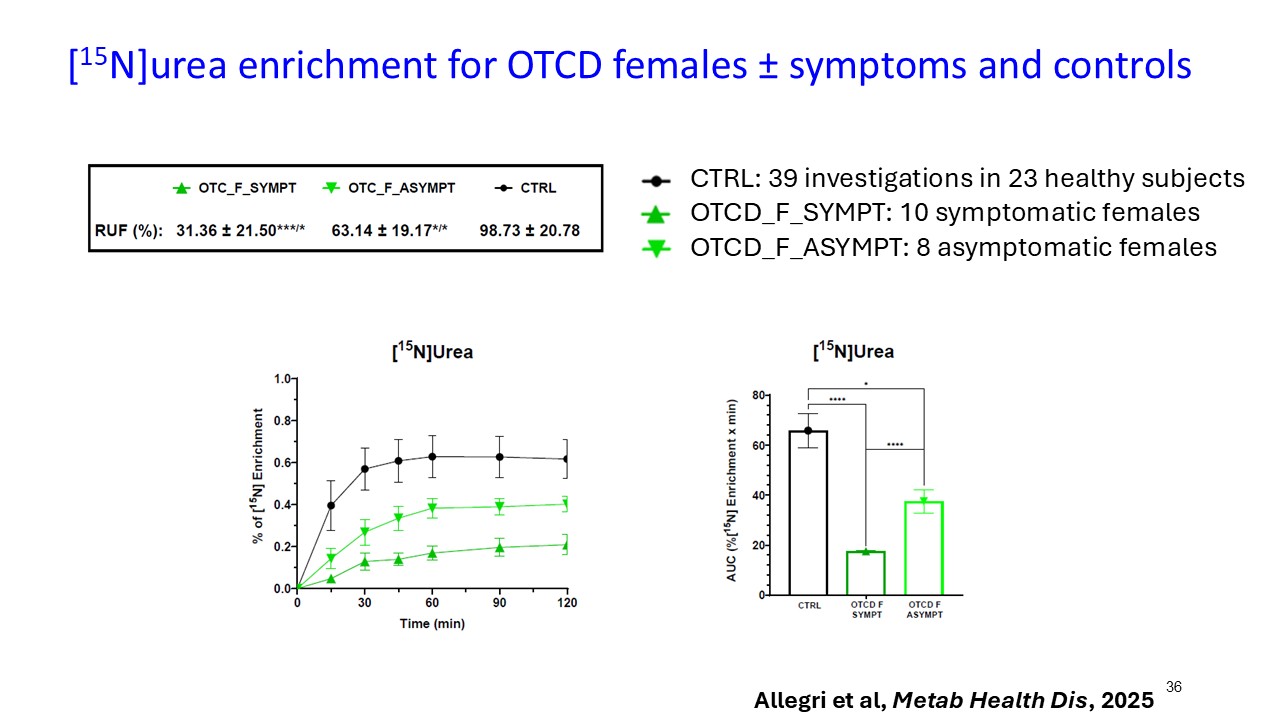
Multiple dosing data are available from two Phase 2 studies; a completed placebo-controlled study in Europe that randomized six participants to ARCT-810 doses and an open-label multiple ascending dose study with interim data from the initial three completed participants. The ongoing U.S. Phase 2 open-label study uses a modified and improved 15N-ureagenesis assay (Allegri et al., 2025). The assay measures relative ureagenesis function (RUF) against a normal range established from healthy controls (N = 29). The assay is not impacted by ammonia scavengers, has low intraindividual variability, and can distinguish between symptomatic and asymptomatic OTC deficient patients. Linear Mixed-Effects Model (LMM) was applied as an exploratory analysis to the Phase 2 glutamine and ureagenesis data. LMM is suitable for small-N analyses, i.e. rare disease trial datasets.
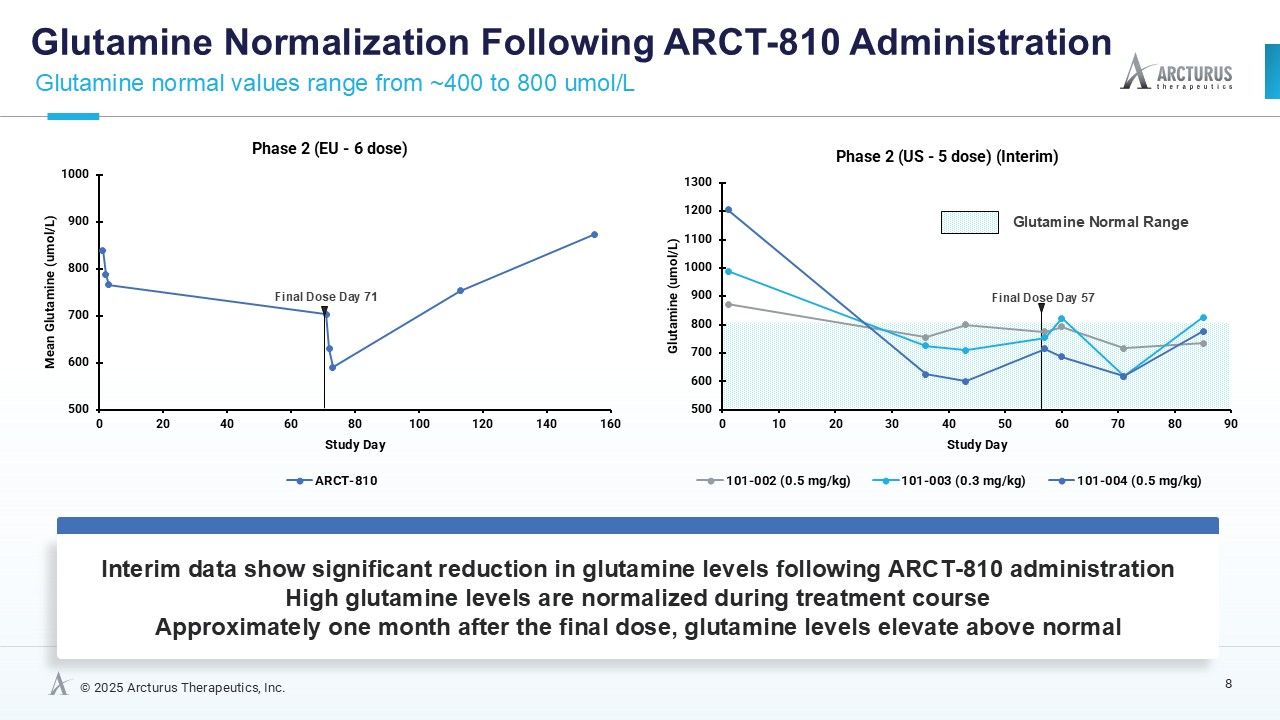
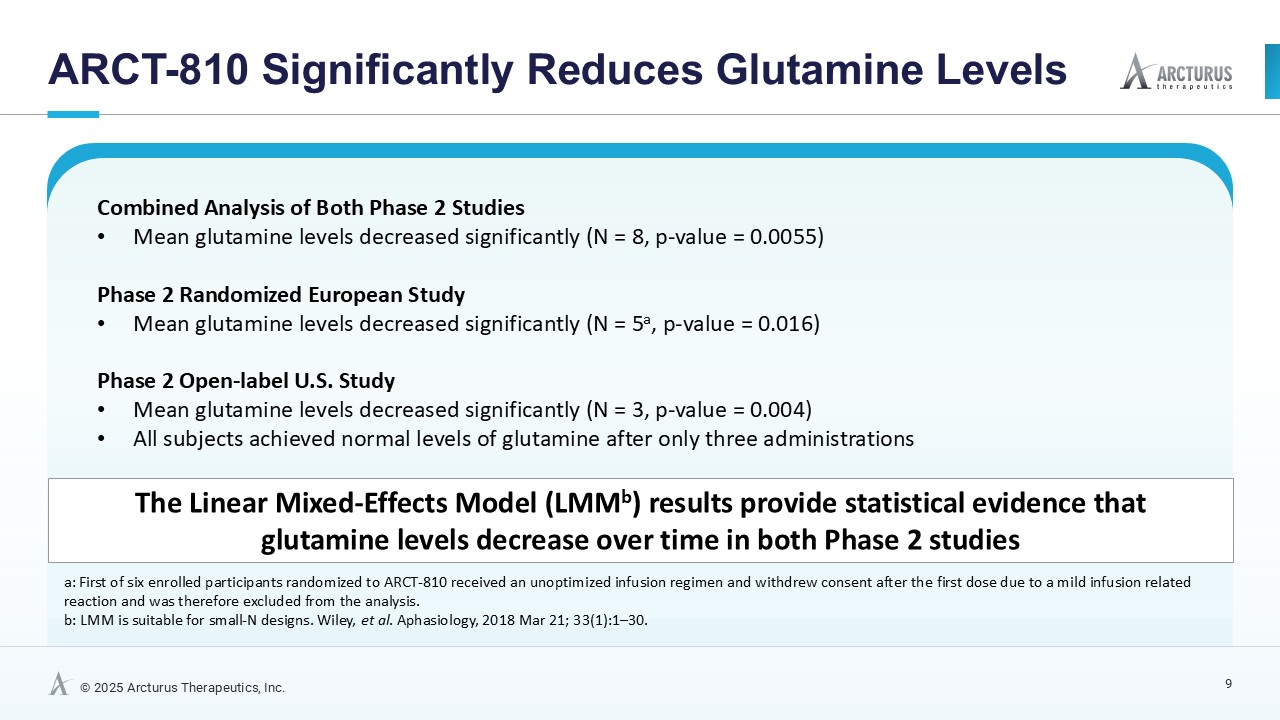
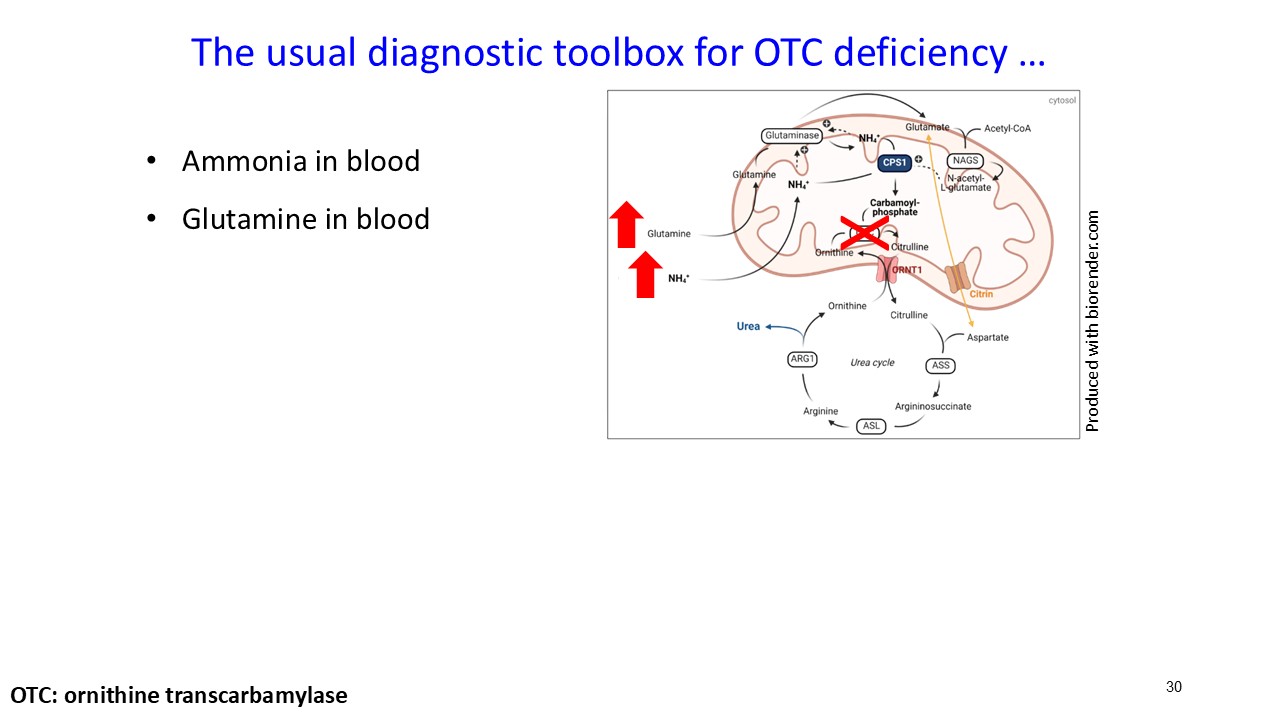
ARCT-810 treatment significantly reduces glutamine levels
In the combined analysis of both Phase 2 studies, significantly (p-value = 0.0055; LMM) decreased glutamine levels were observed following multiple ARCT-810 administrations to patients who remained on their standard of care therapy. These results provide statistical evidence that glutamine levels decrease over time in both Phase 2 studies, suggesting a robust effect across the patient cohorts. In the Phase 2 randomized European study, glutamine levels in patients who received multiple doses of ARCT-810 significantly (p-value = 0.016; LMM) decreased during the dosing period. In the Phase 2 open-label U.S. study, all three participants had a sustained and significant (p-value = 0.004; LMM) decrease in glutamine from baseline reaching normal levels after the first three doses.
| Analysis | Number of Patients | Number of Observations | Fixed Effect Slope (µmol/L/day) | One-Tailed p-value (for slope < 0) |
| European Phase 2 | 5* | 31 | -1.82 | 0.016 |
| U.S. Phase 2 (interim) | 3 | 18 | -4.38 | 0.004 |
| Combined | 8 | 49 | -2.50 | 0.0055 |
| *First of six enrolled participants randomized to ARCT-810 received an unoptimized infusion regimen and withdrew consent after the first dose due to a mild infusion related reaction and was therefore excluded from the analysis | ||||
These results provide strong evidence that glutamine levels decreased in both Phase 2 studies, with a steeper decline in the U.S. Phase 2 study, likely attributed to the two participants with more severe disease. After treatment completion, glutamine levels returned to baseline over a period of several weeks.
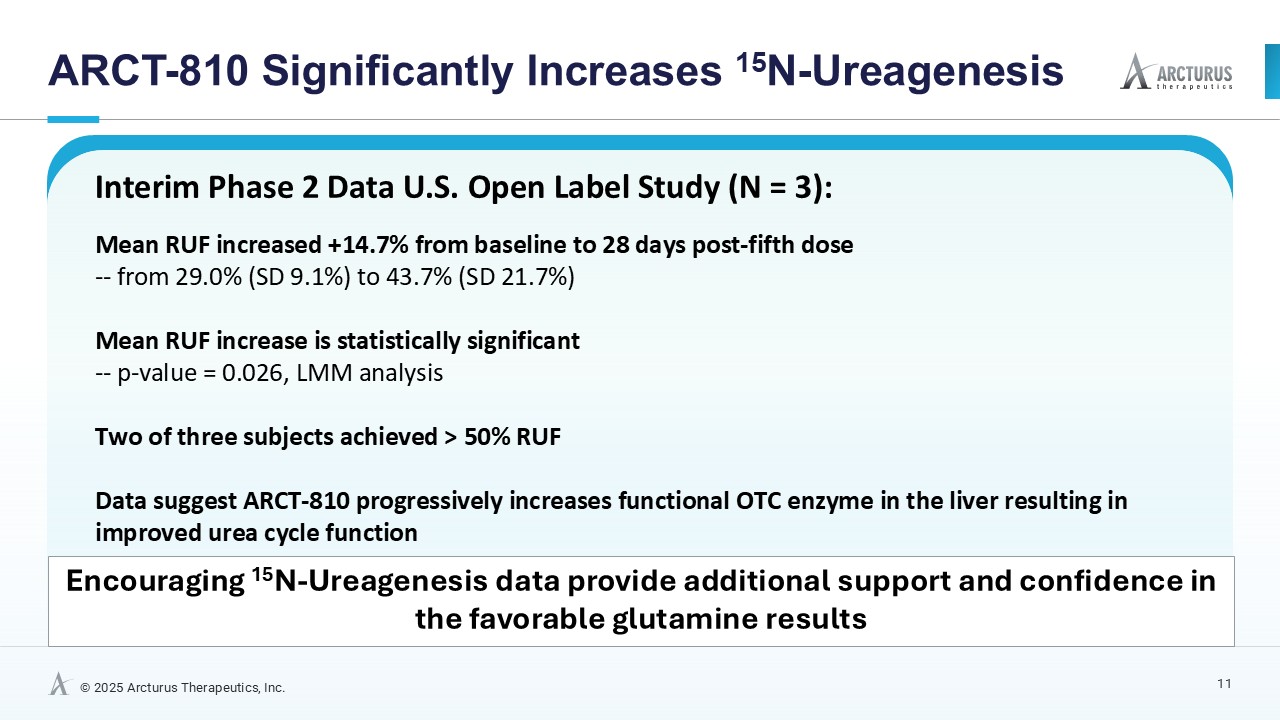
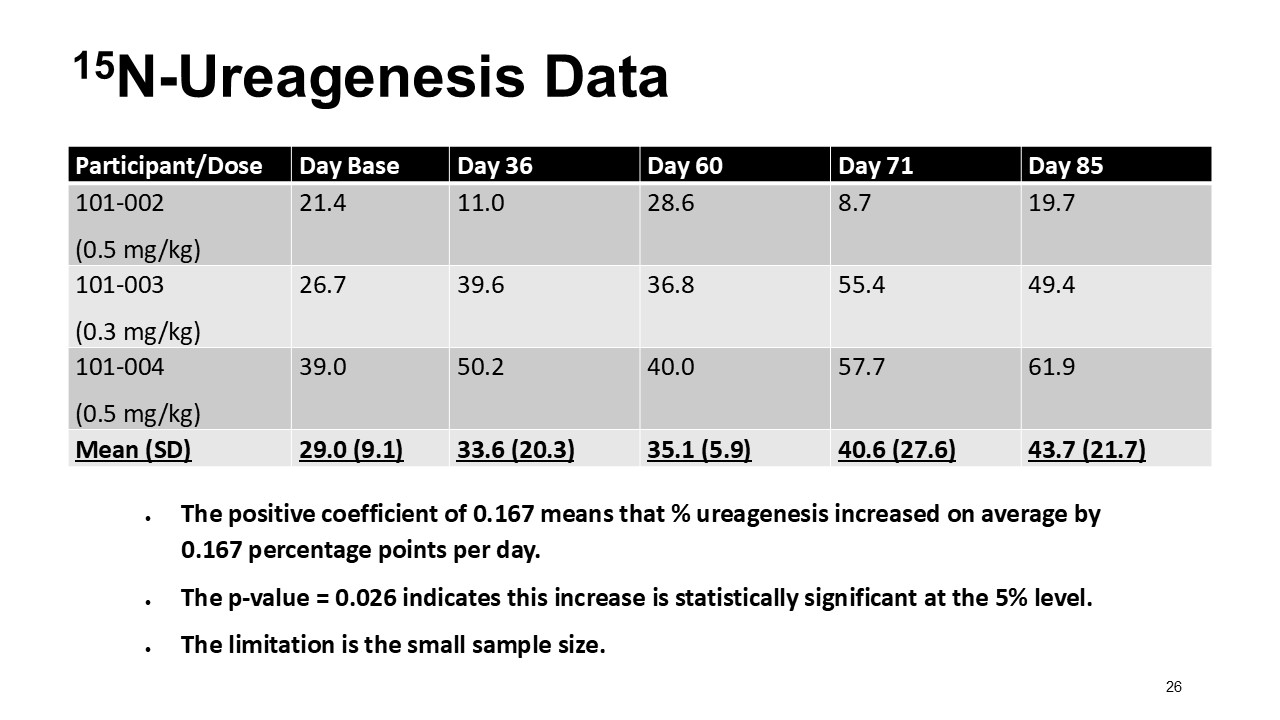
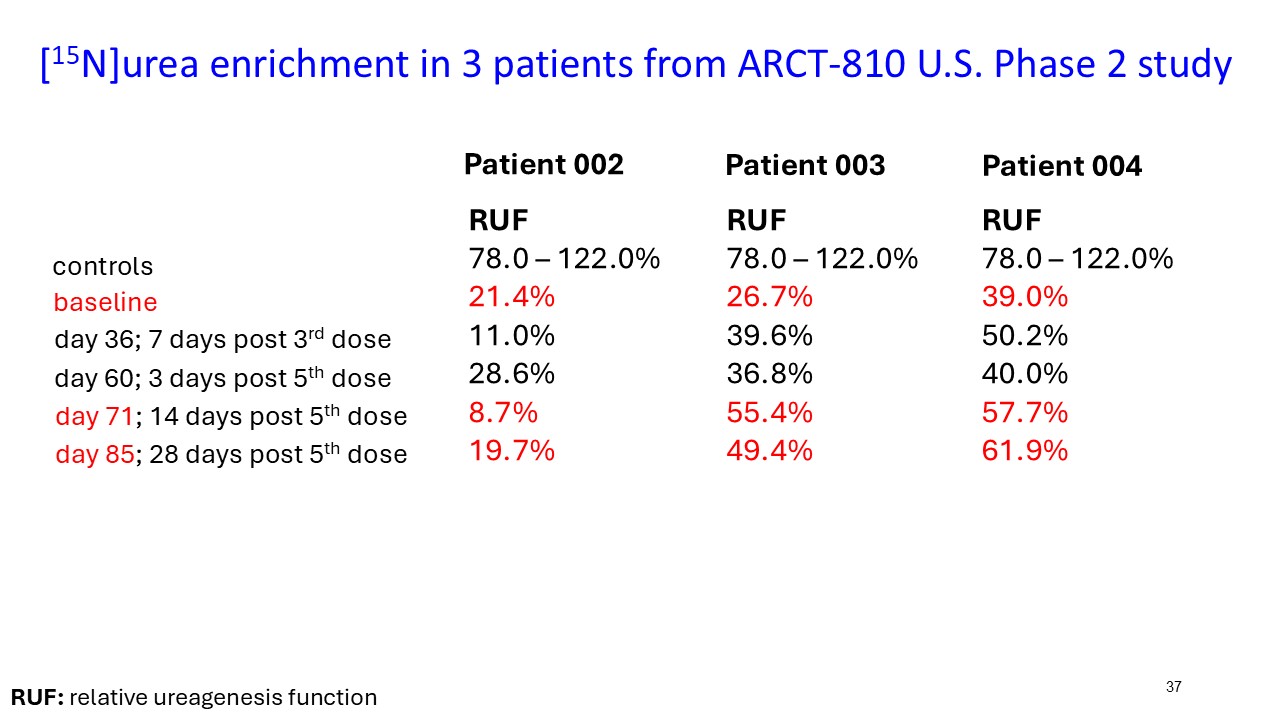
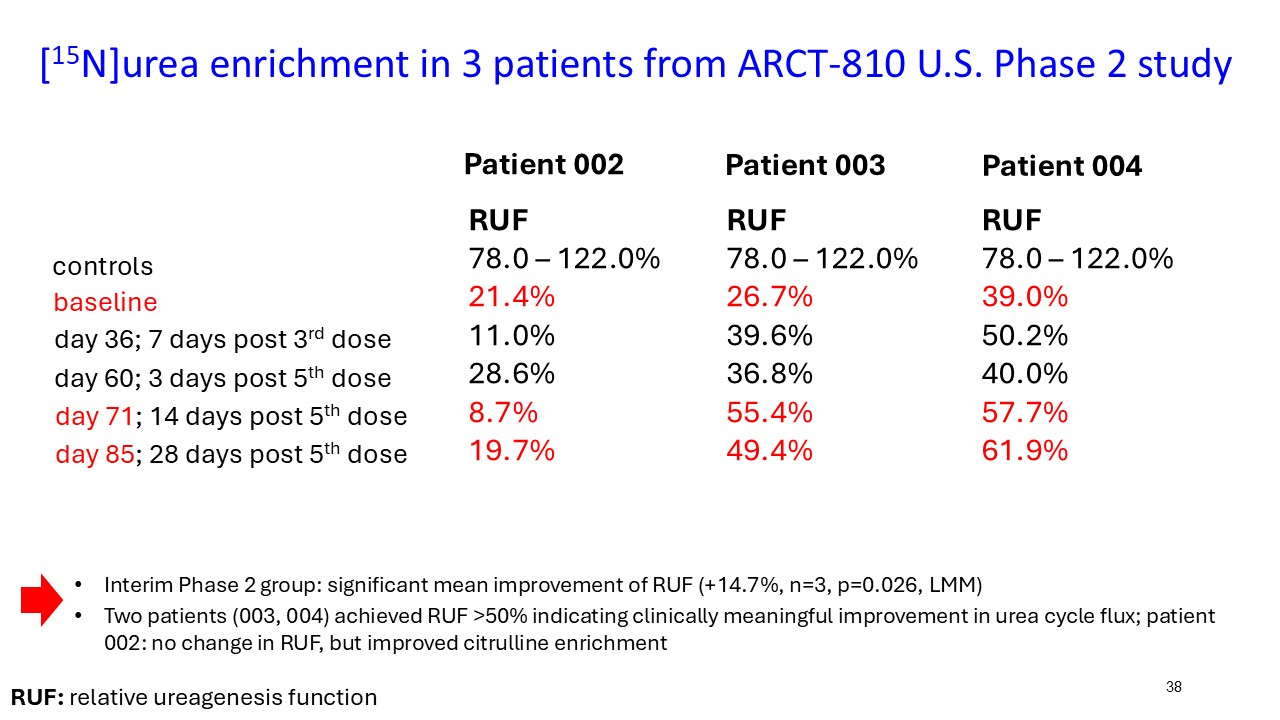
ARCT-810 treatment significantly increases 15N-ureagenesis
In the first three participants in the ongoing Phase 2 open-label study, RUF statistically (p-value = 0.026, LMM) increased at all post treatment evaluations from a baseline of 29.0% (SD; 9.1%) to 43.7% (SD; 21.7%) at 28 days post-fifth dose. These results suggest a progressive increase of functional OTC enzyme in the liver with continued administrations. Two of the three participants achieved RUF > 50% indicating a clinically meaningful improvement in urea cycle flux. These encouraging 15N-Ureagenesis data provide additional support and confidence in the favorable glutamine results.
Ammonia remained stable and within normal range
Further supporting the favorable glutamine and ureagenesis data, patients receiving ARCT-810, in both Phase 2 studies, maintained ammonia levels within the normal range following at least two doses and remained stable for approximately 28 days after completion of dosing. Two participants in the European Phase 2 randomized study (one receiving placebo, one receiving ARCT-810) reported a hyperammonemia event (ammonia ≥ 100 μmol/L). One participant had normal ammonia levels that remained stable during ARCT-810 treatment; four weeks after the last dose, this participant received an oral corticosteroid to treat an asymptomatic transaminase elevation (a laboratory SAE that did not meet Hy’s law criteria). Subsequently, transaminase levels returned to normal range, and the temporary increase of ammonia was considered related to corticosteroid treatment.
ARCT-810 continues to be safe and well tolerated
ARCT-810 was generally safe and well tolerated in single dose Phase 1/1b and multi-dose Phase 2 studies, comprising 40 participants to date, including 20 OTC deficient participants. The early studies enabled the Company to improve the tolerability of the infusion regimen without using corticosteroid pre-treatment. To date, no serious IRRs have been observed using the improved 3-hour IV regimen (N = 8; up to 6 infusions) in the Phase 2 protocols.
Virtual KOL Event: Monday, June 30, 2025 @12:00 p.m. ET
| · | Domestic: 1-877-407-0784 |
| · | International: 1-201-689-8560 |
| · | Conference ID: Arcturus |
| · | Webcast: https://viavid.webcasts.com/starthere.jsp?ei=1723089&tp_key=21003e1b34 |
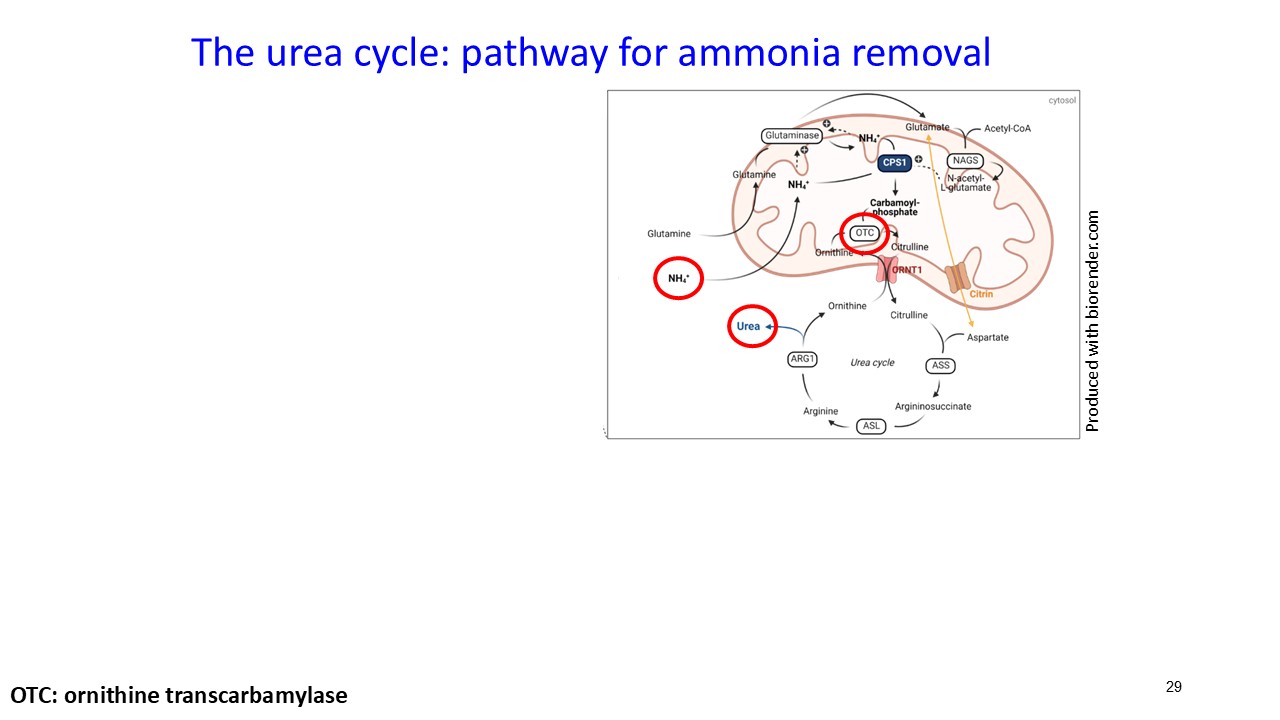
About Ornithine Transcarbamylase Deficiency
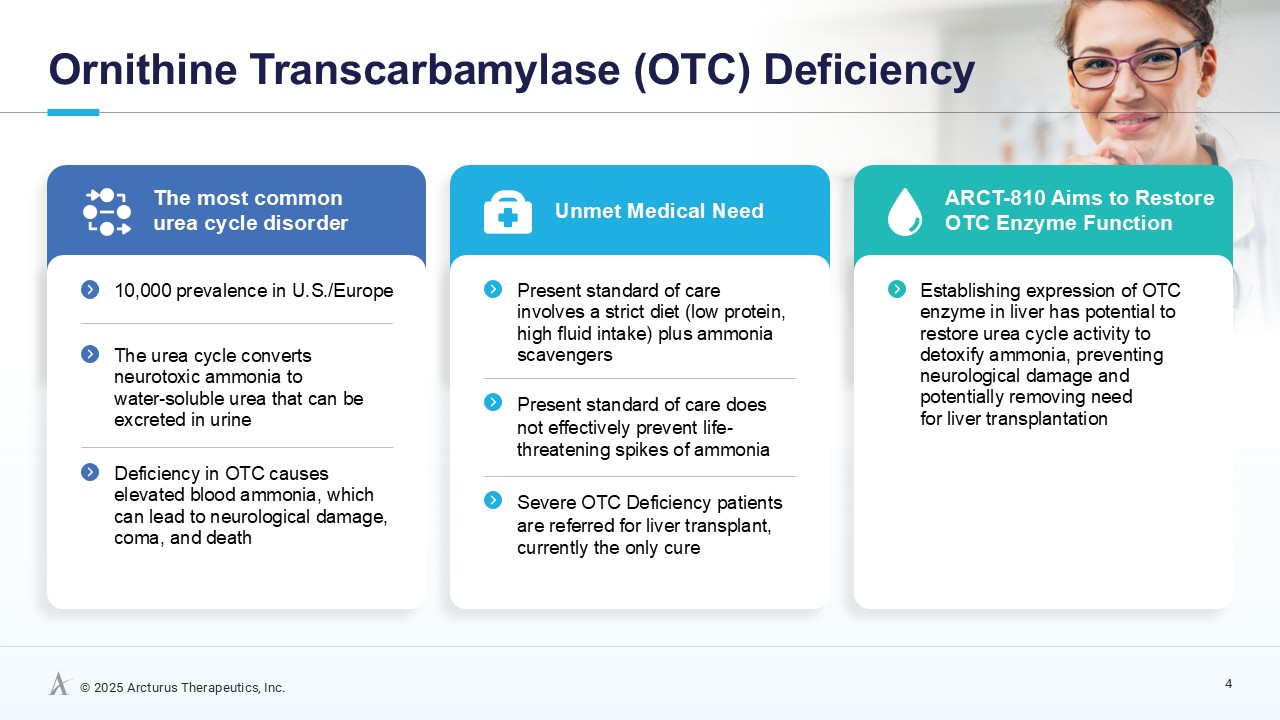
Ornithine transcarbamylase (OTC) deficiency is the most common urea cycle disorder. Urea cycle disorders are a group of inherited metabolic disorders of the liver that make it difficult for affected patients to remove toxic waste products as proteins are digested. OTC deficiency caused by mutations in the X-linked OTC gene, leads to a non-functional or deficient OTC enzyme and usually affects males more severely. OTC is a critical liver enzyme which catalyzes a metabolic process that converts toxic ammonia to urea that is excreted by the kidney. This conversion does not occur properly in patients with OTC deficiency and, aside from the risk of high ammonia levels, leads to increased blood concentrations of glutamine with low to normal levels of citrulline and increases in urine orotic acid. High blood ammonia levels in OTC deficiency may cause health crises with seizures, progressive neurocognitive impairment, coma, and death. Severe cases of OTC deficiency usually present early in life, but patients with less severe symptoms may be diagnosed as adolescents and adults. There is currently no cure for OTC deficiency, apart from liver transplant. However, liver transplantation comes with significant risks of surgical and postsurgical complications such as organ rejection, and recipients must take immunosuppressant drugs for the rest of their lives. The current standard of care for OTC deficiency patients is a well-controlled, but challenging to maintain, low-protein diet, substitution of essential amino acids and treatment with nitrogen scavenging medications that keeps the ammonia from rising to acutely toxic levels but may not prevent chronic neurotoxic effects. These treatments do not address the underlying cause of disease. In Europe and the U.S., approximately 10,000 people have OTC deficiency.
About ARCT-810
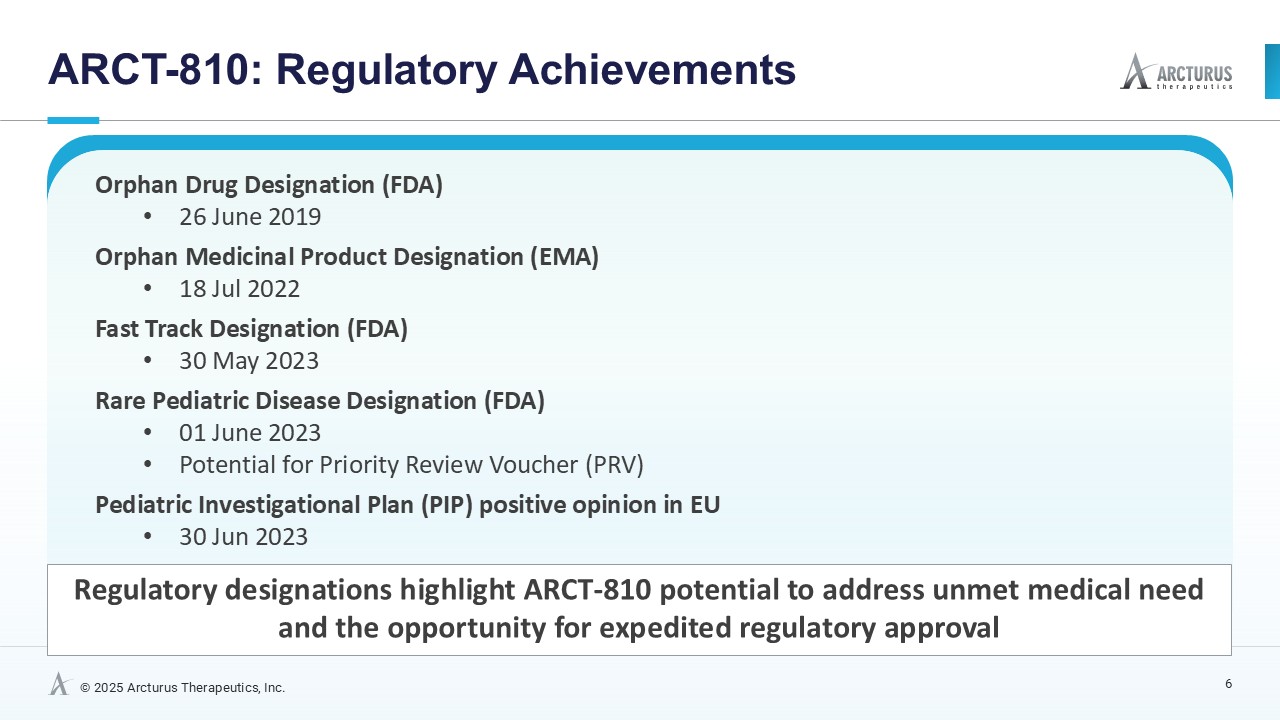
ARCT-810 is an intravenously administered investigational mRNA therapeutic designed to express normal functional OTC enzyme in the liver of individuals with OTC deficiency. ARCT-810 has received Orphan Medicinal Product Designation and an approved pediatric investigation plan (PIP) from the European Medicines Agency (EMA), and Orphan Drug Designation, Fast Track Designation along with Rare Pediatric Disease Designation from the U.S. Food and Drug Administration (FDA) for the treatment of OTC deficiency. OTC is a key enzyme in the urea cycle which converts toxic ammonia into urea. Elevated ammonia can lead to metabolic crises with progressive and irreversible neurocognitive damage. A safe and effective mRNA therapeutic may restore normal functional OTC enzyme in the liver which could improve urea cycle activity, reduce abnormally elevated glutamine, maintain normal ammonia levels and potentially eliminate the risk of future metabolic crises. ARCT-810 is based on Arcturus’ mRNA design construct and proprietary manufacturing process. ARCT-810 also utilizes Arcturus’ extensive and propriety lipid library and employs the Company's LUNAR® delivery platform to deliver OTC mRNA to hepatocytes.
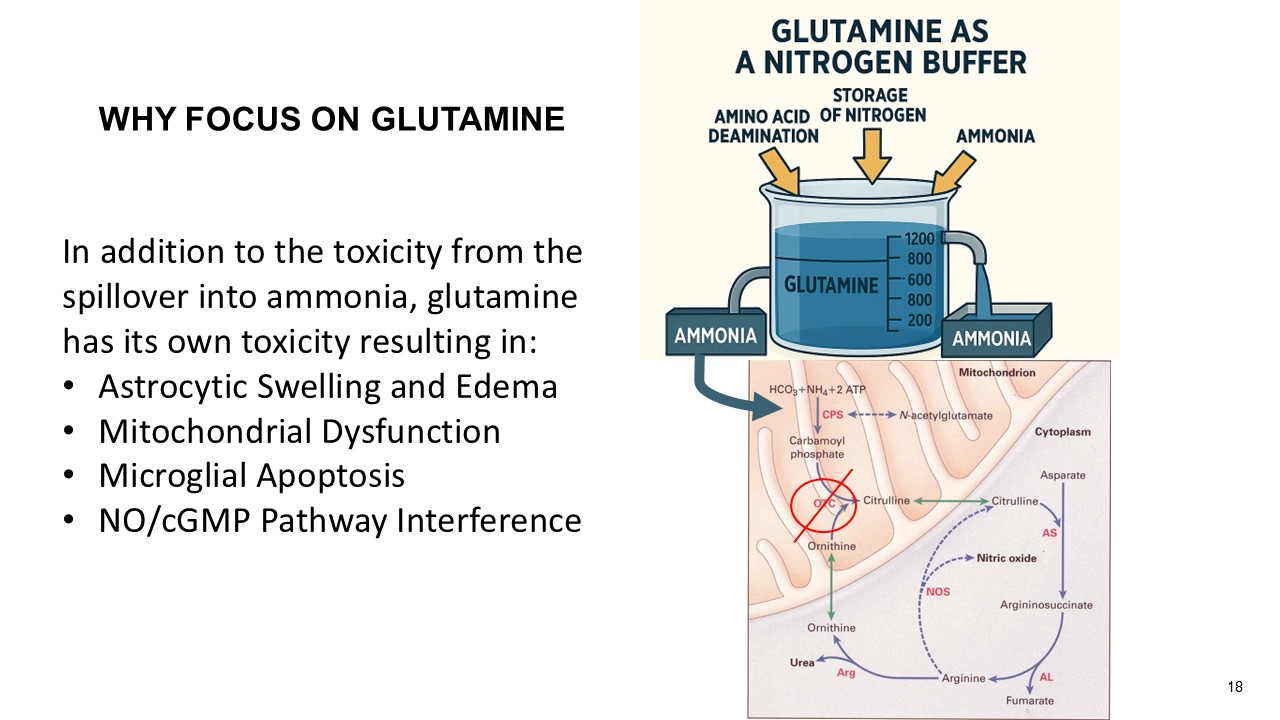
About Glutamine as a Biomarker
Glutamine is used as an important biomarker by clinicians to monitor urea cycle function in OTC deficient patients. Glutamine reflects the body’s nitrogen buffering capacity. In urea cycle disorders, excess nitrogen is initially incorporated into glutamine, allowing glutamine to rise steadily as a compensatory mechanism before ammonia levels begin to spike. This role makes glutamine a more stable and predictive biomarker in patients who are not experiencing hyperammonemia. Glutamine assessments have significantly lower intra-subject variability than ammonia (15% vs. 56%), making it more reliable for monitoring metabolic control in stable conditions (Lichter-Konecki et al., 2016).
About Arcturus
Founded in 2013 and based in San Diego, California, Arcturus Therapeutics Holdings Inc. (Nasdaq: ARCT) is a commercial mRNA medicines and vaccines company with enabling technologies: (i) LUNAR® lipid-mediated delivery, (ii) STARR® mRNA technology (sa-mRNA) and (iii) mRNA drug substance along with drug product manufacturing expertise. Arcturus developed KOSTAIVE®, the first self-amplifying messenger RNA (sa-mRNA) COVID vaccine in the world to be approved. Arcturus has an ongoing global collaboration for innovative mRNA vaccines with CSL Seqirus, and a joint venture in Japan, ARCALIS, focused on the manufacture of mRNA vaccines and therapeutics. Arcturus’ pipeline includes RNA therapeutic candidates to potentially treat OTC deficiency and cystic fibrosis (CF), along with its partnered mRNA vaccine programs for SARS-CoV-2 (COVID-19) and influenza. Arcturus’ versatile RNA therapeutics platforms can be applied toward multiple types of nucleic acid medicines including messenger RNA, small interfering RNA, circular RNA, antisense RNA, self-amplifying RNA, DNA, and gene editing therapeutics. Arcturus' technologies are covered by its extensive patent portfolio (over 500 patents and patent applications in the U.S., Europe, Japan, China, and other countries). For more information, visit www.ArcturusRx.com. In addition, please connect with us on X (formally Twitter) and LinkedIn.
Forward Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact included in this press release, are forward-looking statements, including those regarding strategy, future operations, the likelihood of success of the Company’s pipeline (including ARCT-810), the likelihood that clinical results will be predictive of future clinical results or of potential therapeutic benefit, likelihood of continuation of the OTC program, likelihood of further enrollment in the ongoing ARCT-810 Phase 2 study, the likelihood of a path toward and initiation of a multi-biomarker driven pivotal study, the likelihood that continued ARCT-810 administrations will result in a progressive increase of functional OTC enzyme, the likelihood of any regulatory agency recognizing any biomarker in determinations of regulatory approval including glutamine levels or 15N assay results, and the impact of general business and economic conditions. Arcturus may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in any forward-looking statements such as the foregoing and you should not place undue reliance on such forward-looking statements. These statements are only current predictions or expectations, and are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from those anticipated by the forward-looking statements, including those discussed under the heading "Risk Factors" in Arcturus’ most recent Annual Report on Form 10-K, and in subsequent filings with, or submissions to, the Securities and Exchange Commission (the “SEC”), which are available on the SEC’s website at www.sec.gov. Except as otherwise required by law, Arcturus disclaims any intention or obligation to update or revise any forward-looking statements, which speak only as of the date they were made, whether as a result of new information, future events or circumstances or otherwise.
Arcturus Therapeutics
Public Relations & Investor Relations
Neda Safarzadeh
VP, Head of IR/PR/Marketing
(858) 900-2682
IR@ArcturusRx.com