UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): May 28, 2025
IMMUNIC, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-36201 | 56-2358443 |
| (State or other jurisdiction of incorporation) |
(Commission File Number) | (IRS Employer Identification No.) |
1200 Avenue of the Americas, Suite 200
New York, NY 10036
USA
(Address of principal executive offices)
Registrant’s telephone number, including area code: (332) 255-9818
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of exchange on which registered |
| Common Stock, par value $0.0001 | IMUX | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. Yes ☐ No ☐
| Item 8.01. Other Events. |
Announcement of Public Offering
On May 28, 2025, Immunic, Inc. (the “Company”) issued a press release announcing that it had commenced an underwritten public offering (the “Offering”) of (i) pre-funded warrants to purchase shares of common stock, par value $0.0001 per share (“Common Stock”) of the Company (the “Pre-Funded Warrants”), (ii) series A warrants to purchase shares of Common Stock of the Company (or pre-funded warrants) (the “Series A Warrants”), and (iii) series B warrants to purchase shares of Common Stock of the Company (or pre-funded warrants) (the “Series B Warrants”) pursuant to an effective registration statement on Form S-3 (Registration Statement No. 333-275717) previously filed with the Securities and Exchange Commission (the “SEC”) on November 22, 2023, and declared effective by the SEC on May 31, 2024. The Pre-Funded Warrants, Series A Warrants and Series B Warrants are being sold together but are immediately separable. A copy of the press release is attached hereto as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
In connection with the Offering, the Company filed a preliminary prospectus supplement to the Registration Statement on May 28, 2025 pursuant to Rule 424(b) under the Securities Act of 1933, as amended (the “Securities Act”).
The disclosures on this Current Report on Form 8-K shall not constitute an offer to sell or the solicitation of an offer to buy these securities, nor shall there be any sale of these securities in any state or jurisdiction in which such an offer,
solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. Any offers, solicitations, or offers to buy, or any sales of securities will be made in accordance with the
registration requirements of the Securities Act.
Publishing of Presentation
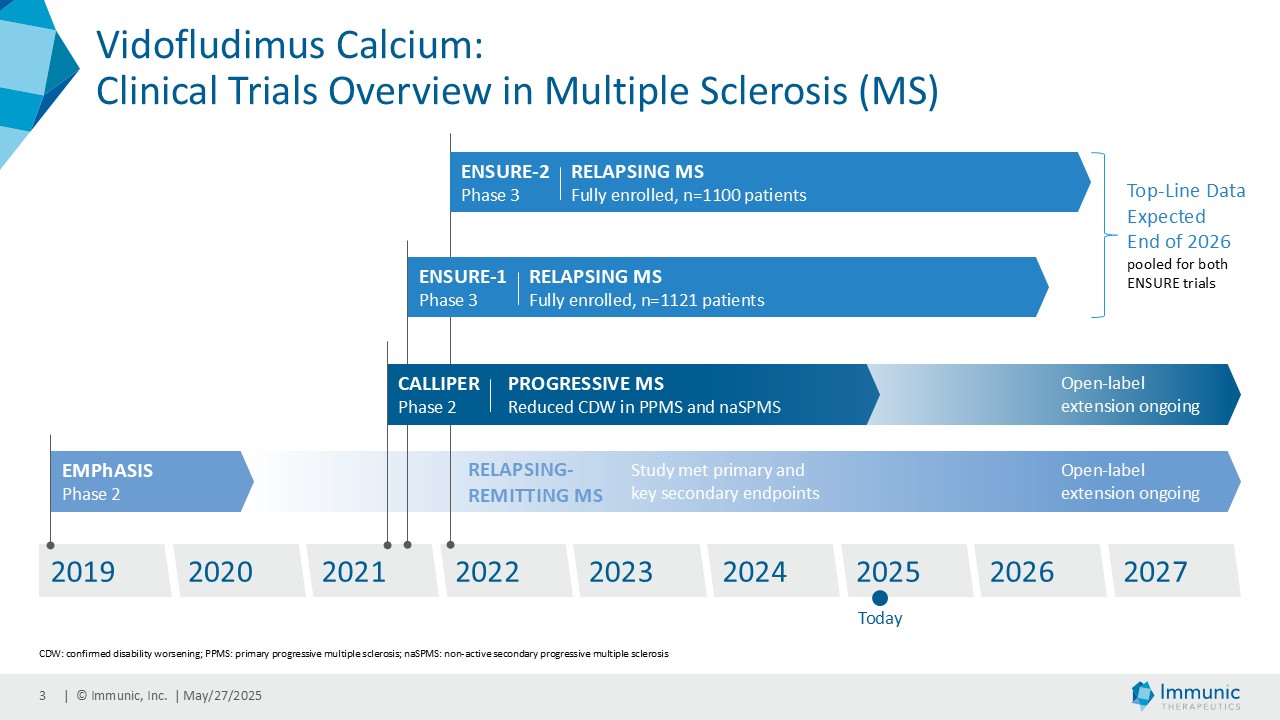
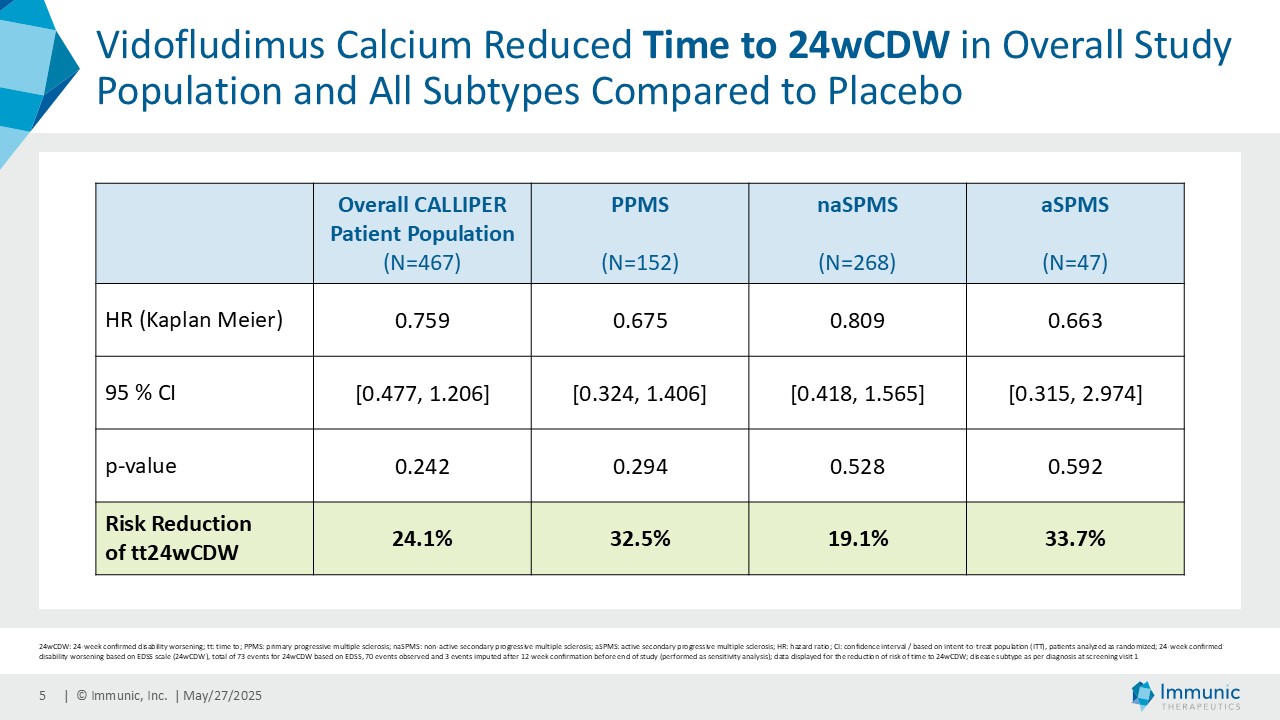
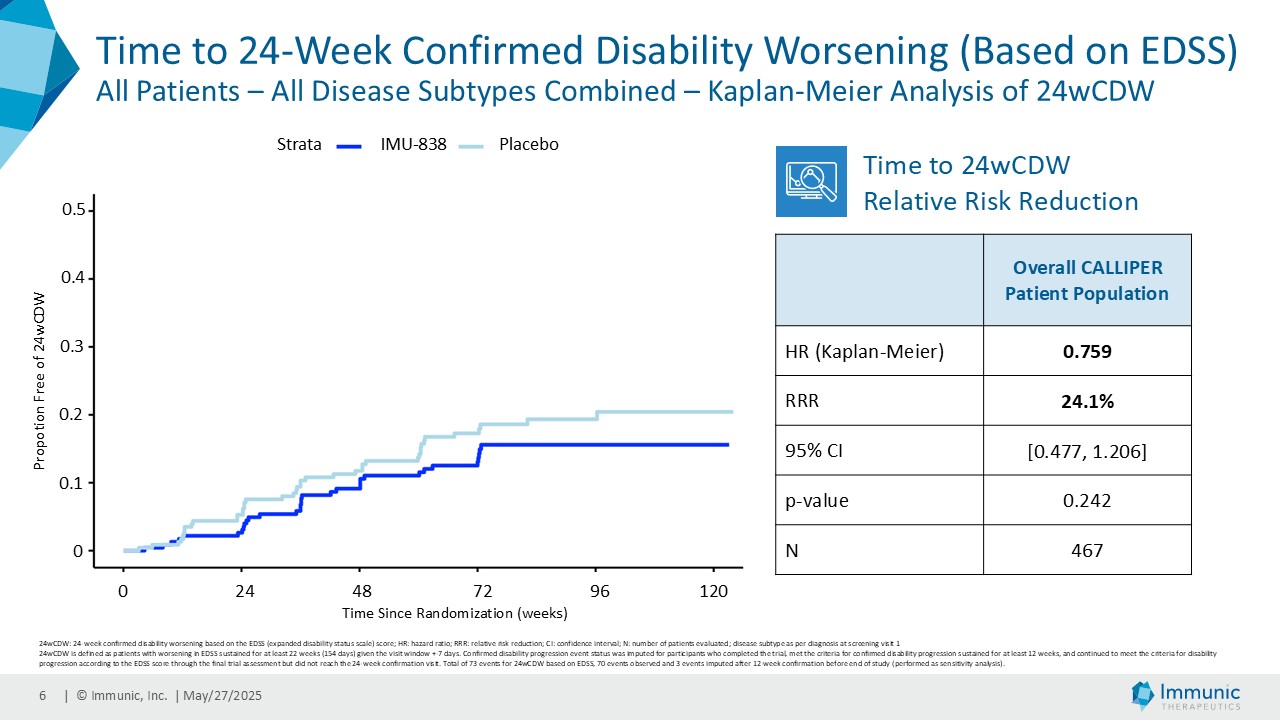
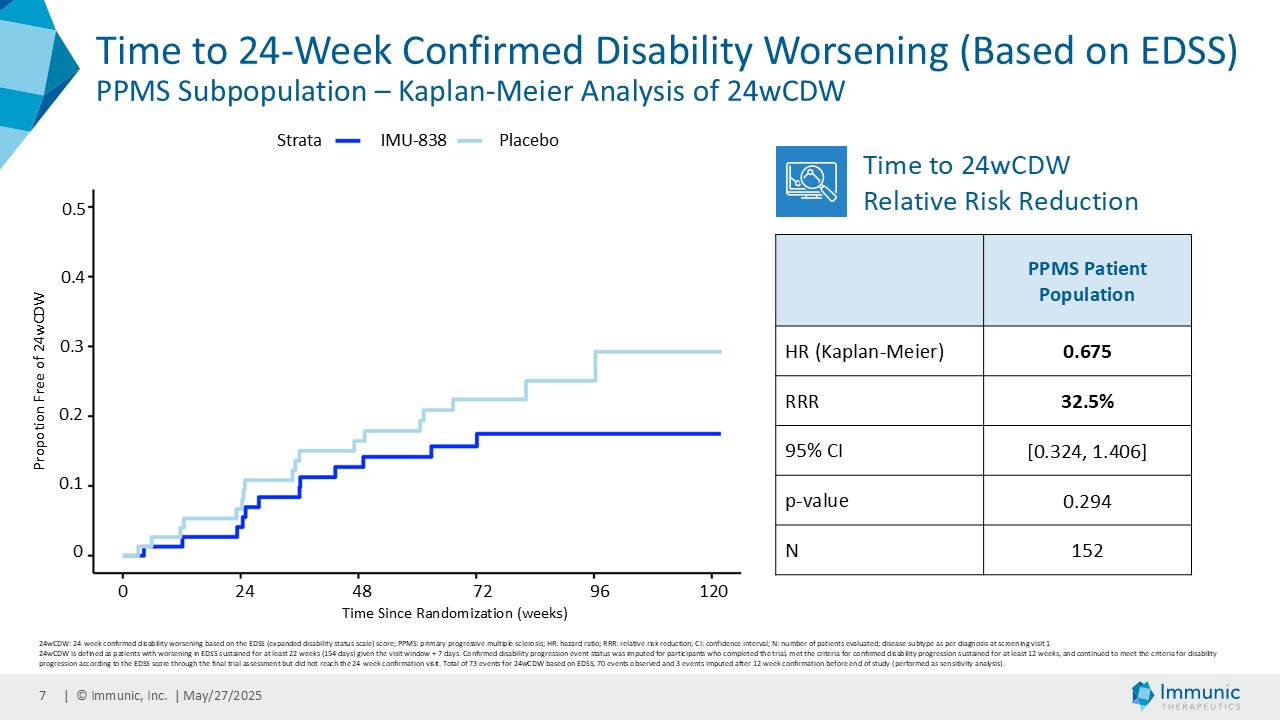
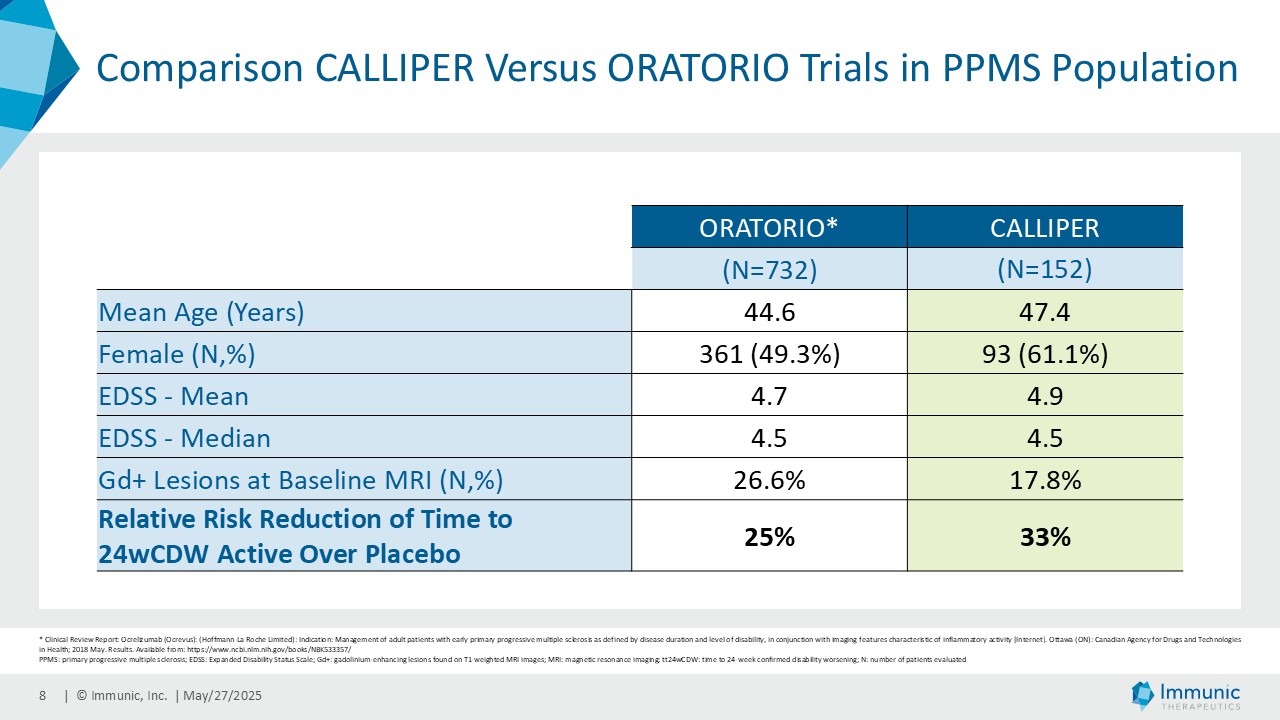
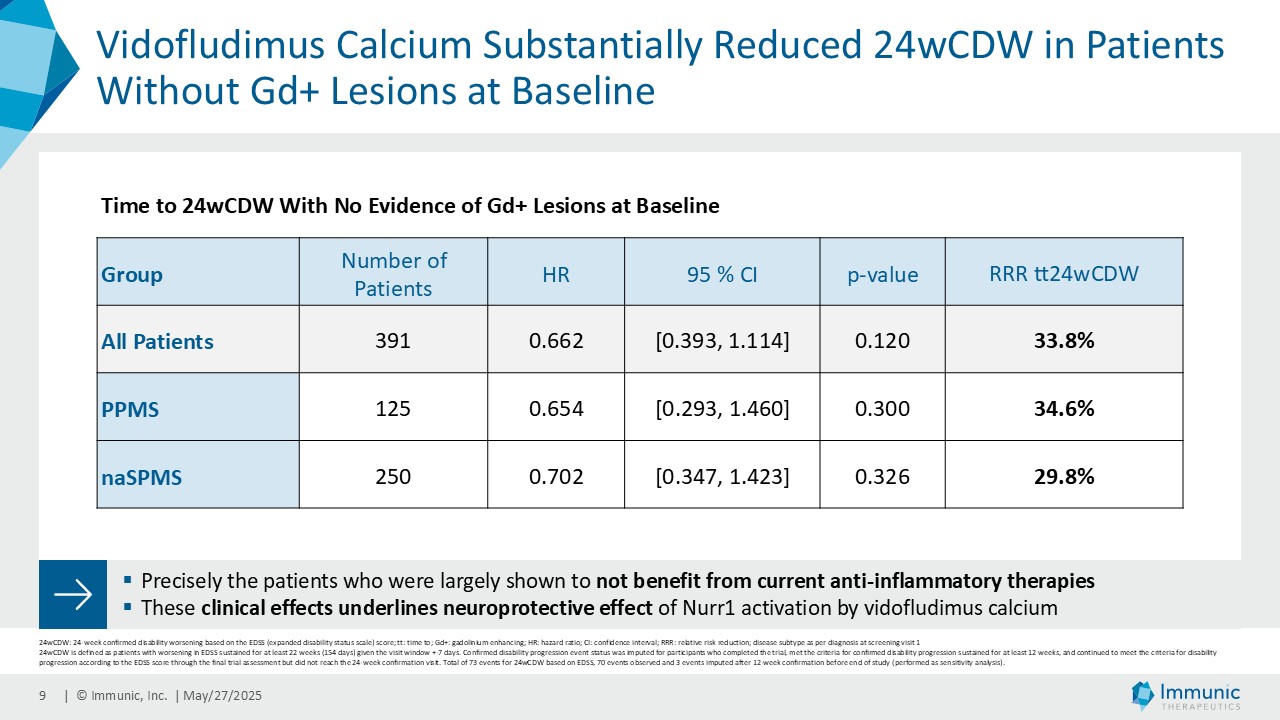
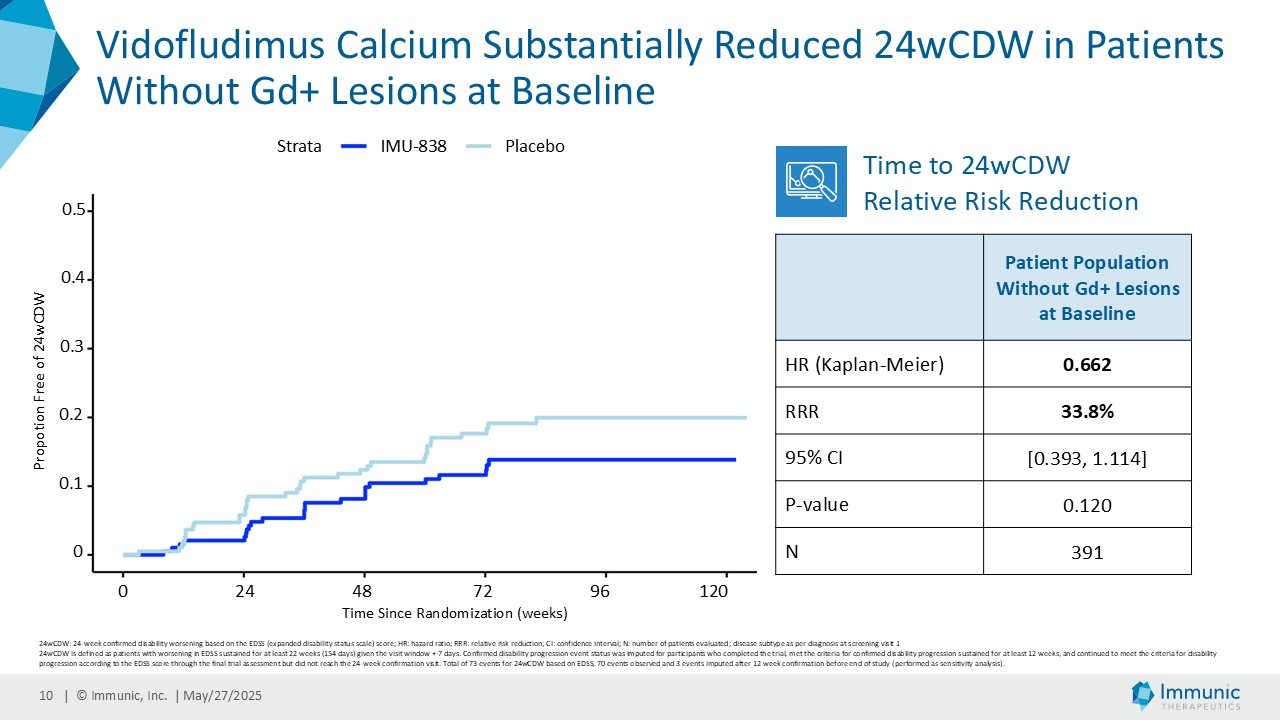
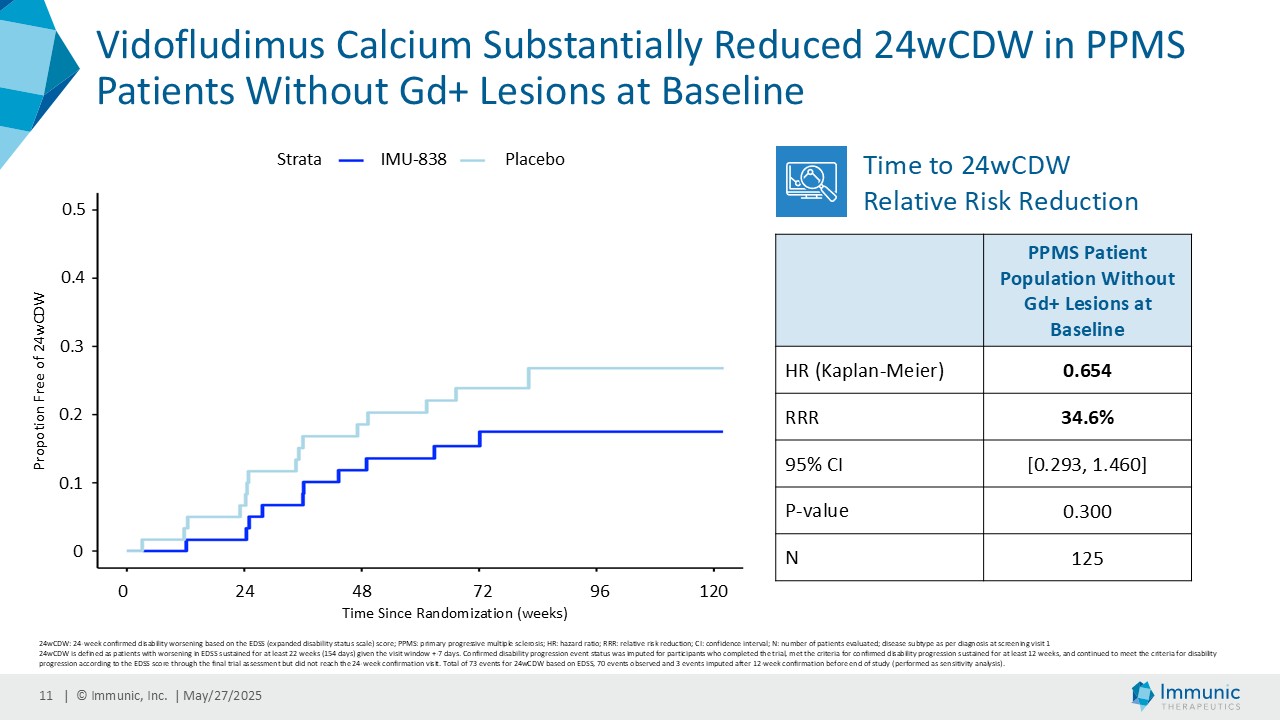
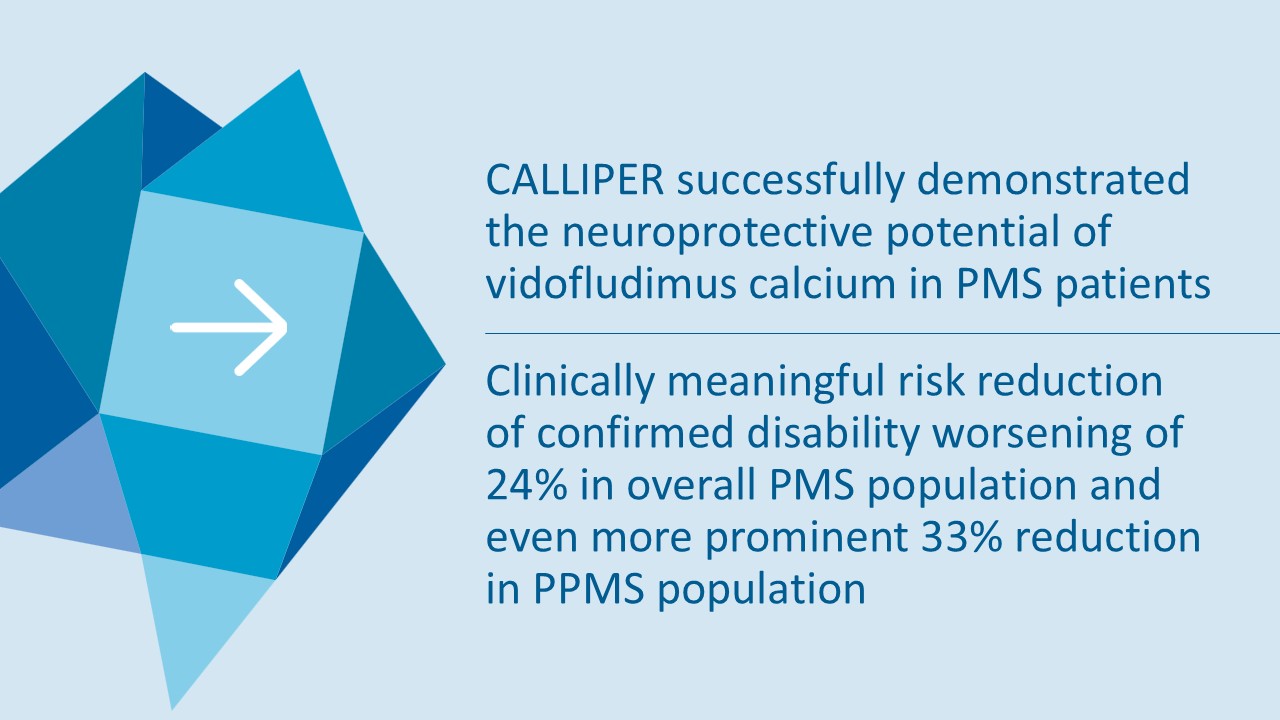
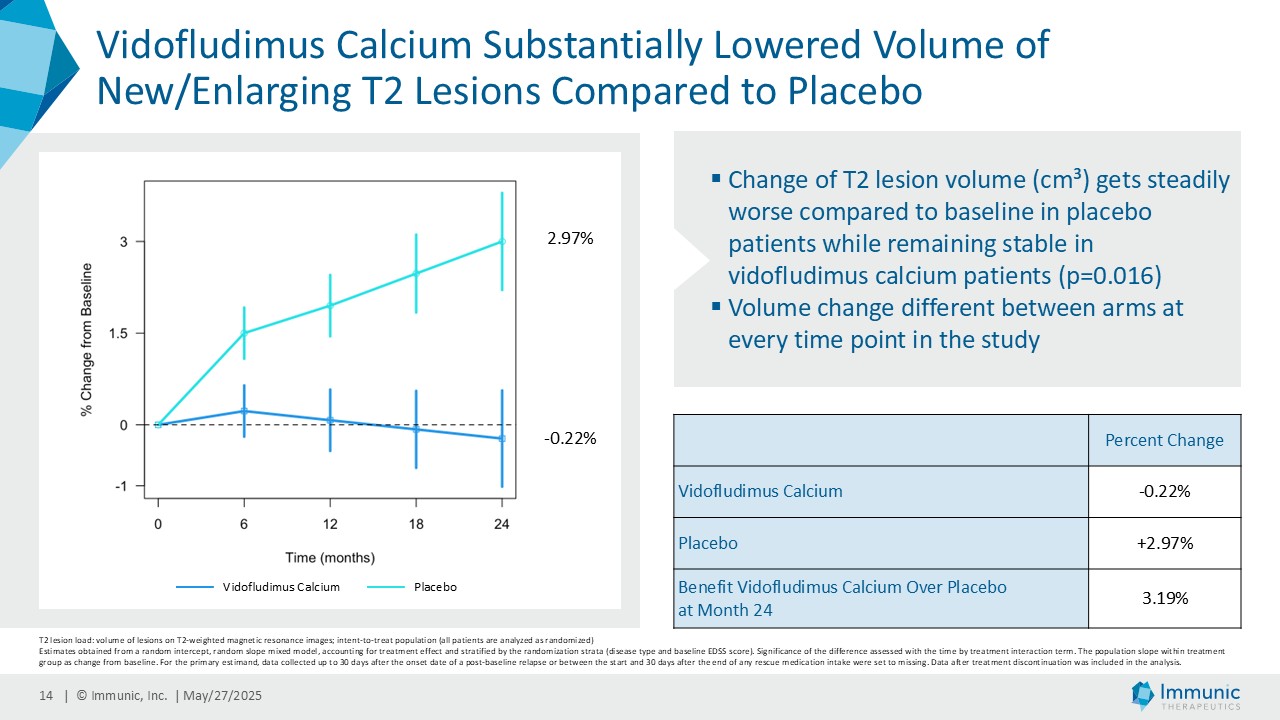
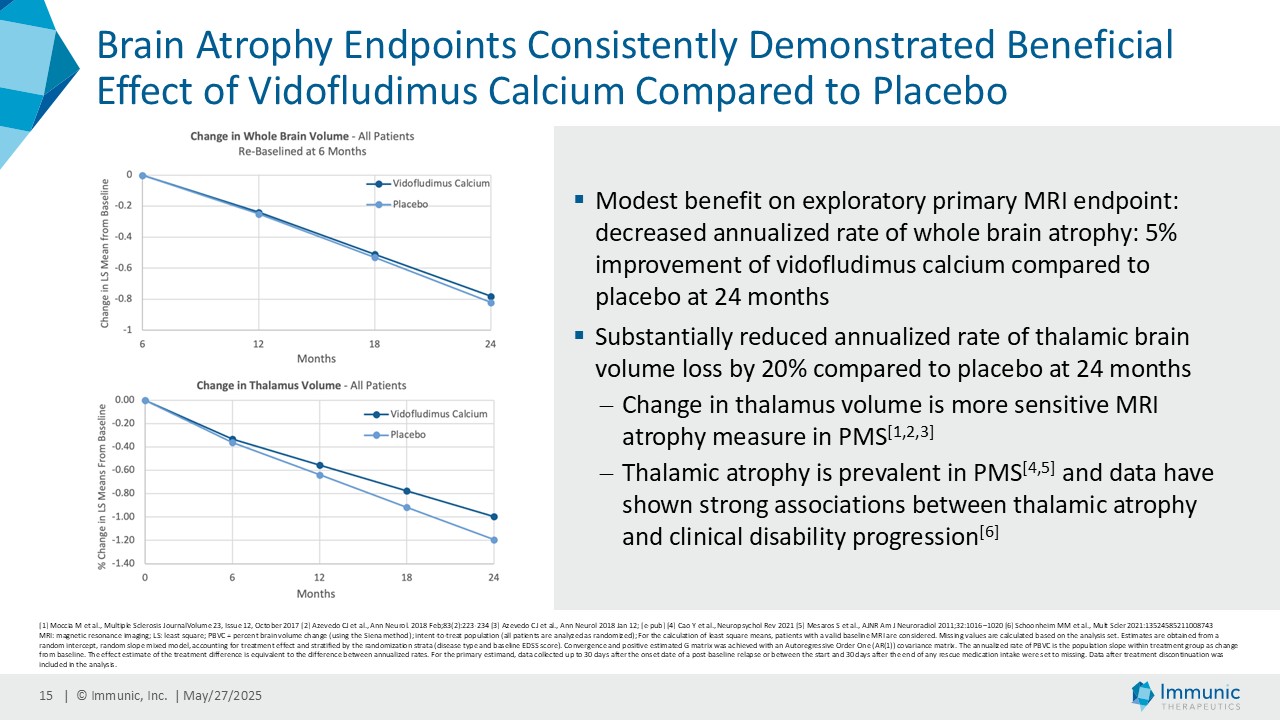
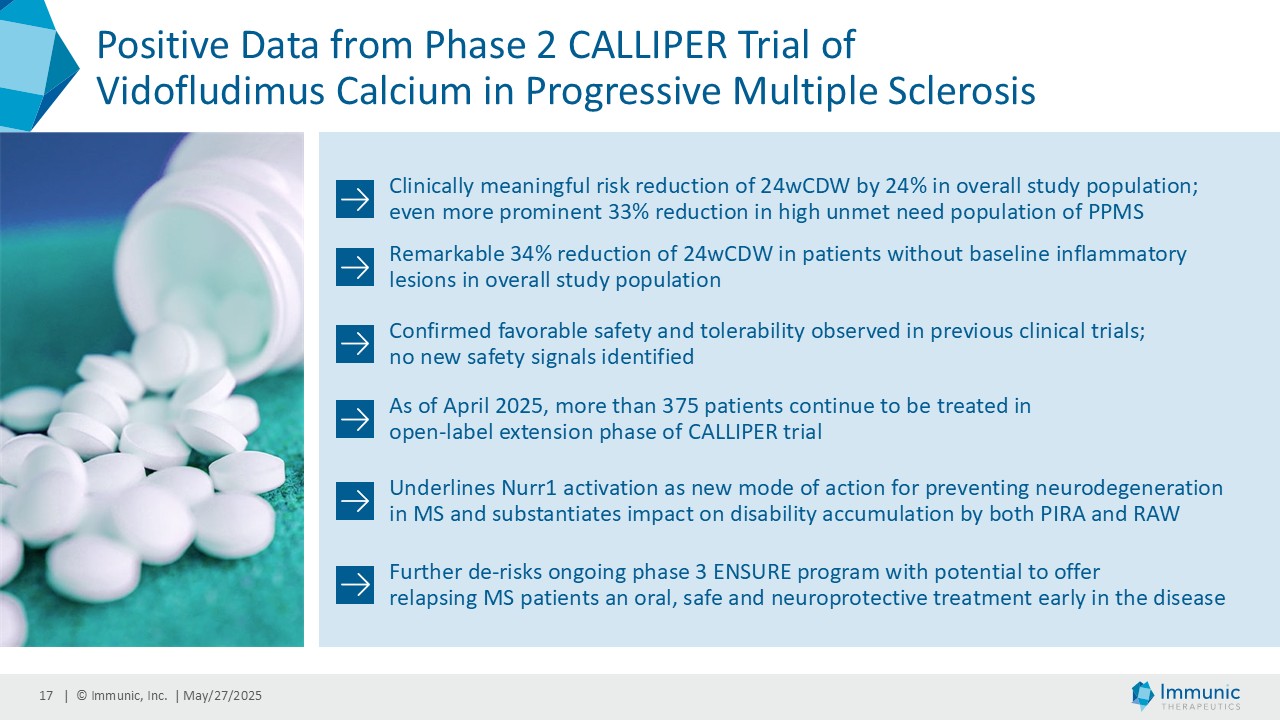
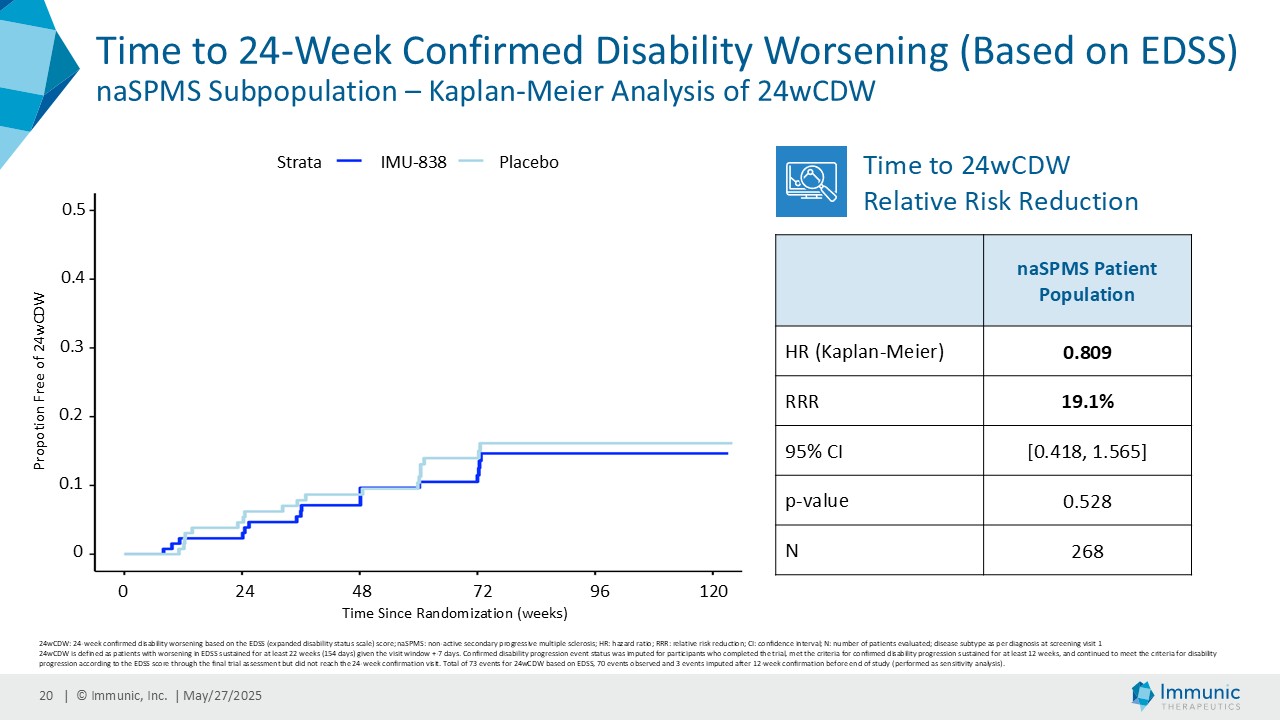
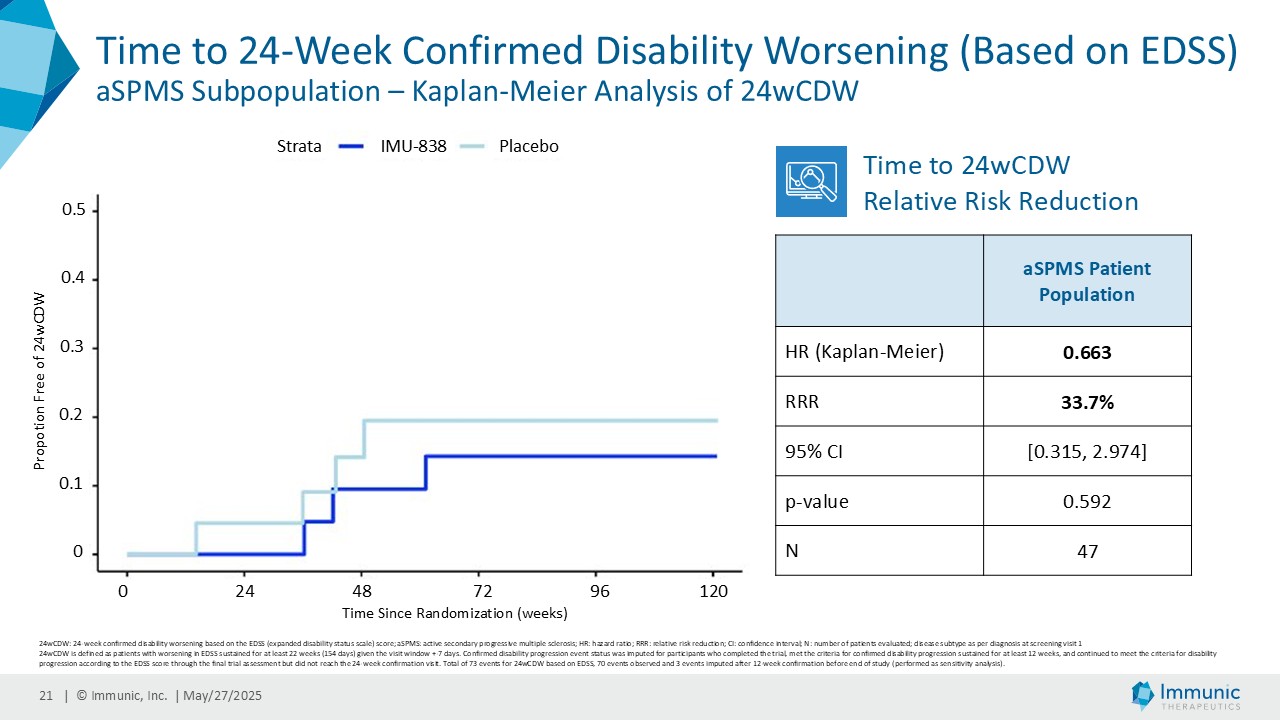
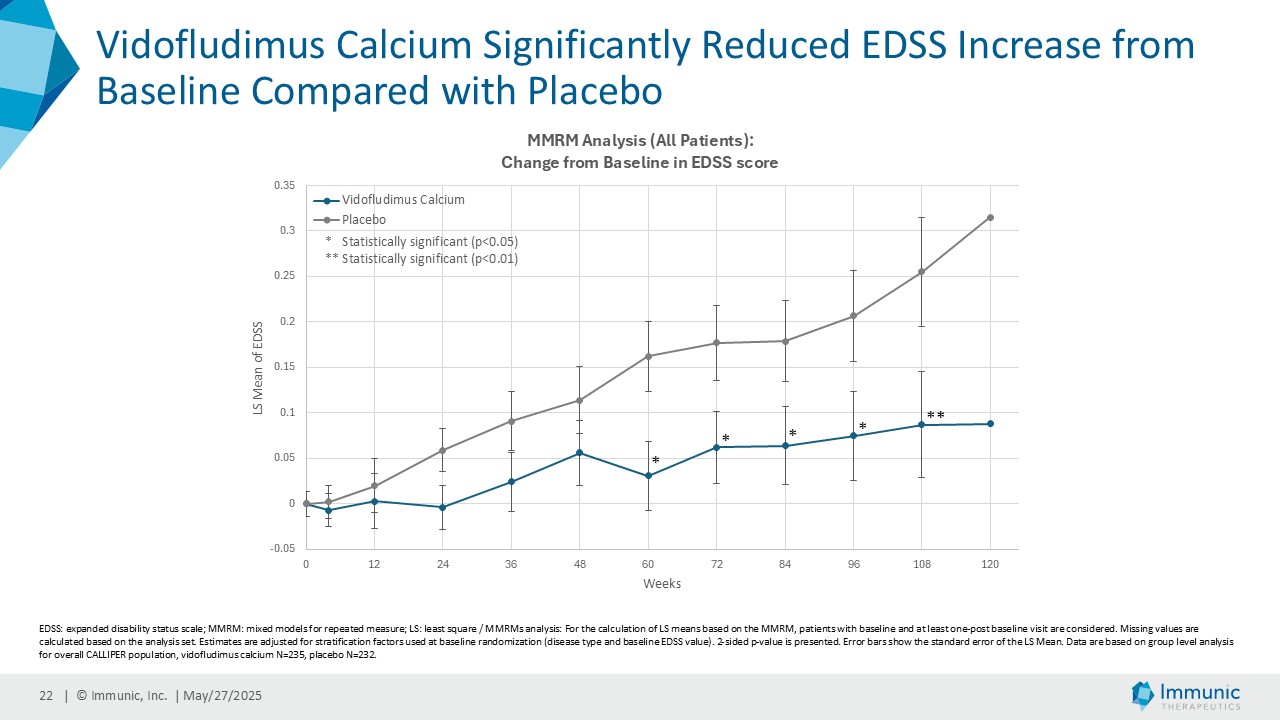
On May 28, 2025, the Company posted an updated corporate presentation on the Company’s website (the “Presentation”) announcing extended data from the Company’s phase 2 CALLIPER trial of vidofludimus calcium in patients with progressive multiple sclerosis. Vidofludimus calcium is an orally administered investigational small molecule drug being developed for chronic inflammatory and autoimmune diseases.
The Presentation is attached as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated herein by reference.
| Item 9.01. Financial Statements and Exhibits. |
(d) Exhibits.
|
Exhibit No. |
Description | |
| 99.1 | Press Release, dated May 28, 2025 | |
| 99.2 | Presentation, dated May 27, 2025 | |
| 104 | Cover Page to this Current Report on Form 8-K in Inline XBRL | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, hereunto duly authorized.
| Dated: May 28, 2025 | Immunic, Inc. | |
| By: | /s/ Daniel Vitt | |
| Daniel Vitt | ||
| Chief Executive Officer | ||
Immunic, Inc. Announces Proposed Public Offering
NEW YORK, May 28, 2025 /PRNewswire/ — Immunic, Inc. (“Immunic” or the “Company”) (Nasdaq: IMUX), a biotechnology company developing a clinical pipeline of orally administered, small molecule therapies for chronic inflammatory and autoimmune diseases, today announced that it has commenced an underwritten public offering of (i) pre-funded warrants to purchase shares of common stock, (ii) Series A warrants to purchase shares of common stock (or pre-funded warrants) expiring on December 31, 2025, (iii) and Series B warrants to purchase shares of common stock (or pre-funded warrants) expiring five years following the issuance date. The Series A and B warrants will immediately expire in proportion to the extent that the corresponding pre-funded warrant offered hereby is exercised on or prior to September 30, 2025, subject to certain exceptions. All of the securities to be sold in the proposed offering will be offered by Immunic. The proposed offering is subject to market and other conditions, and there can be no assurance as to whether or when the offering may be completed, or the actual size or terms of the offering.
The Company intends to use the net proceeds received from the proposed offering to fund its clinical trials and operations and for other general corporate purposes.
Leerink Partners is acting as the sole bookrunner for the offering.
The offering is being made by Immunic pursuant to a shelf registration statement on Form S-3 (File No. 333-275717), as amended, initially filed with the Securities and Exchange Commission (the “SEC”) on November 22, 2023, which became effective on May 31, 2024. The offering is being made only by means of a preliminary prospectus supplement and the accompanying base prospectus that form a part of the registration statement. A preliminary prospectus supplement and the accompanying base prospectus relating to the offering will be filed with the SEC and will be available on the SEC’s website at www.sec.gov. Copies of the preliminary prospectus supplement and the accompanying base prospectus, and when available, copies of the final prospectus supplement and the accompanying base prospectus relating to the offering, may be obtained by contacting Leerink Partners LLC, Syndicate Department, 53 State Street, 40th Floor, Boston, MA 02109, or by telephone at (800) 808-7525 ext. 6105, or by email at syndicate@leerink.com.
This press release shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of, these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of such state or jurisdiction.
About Immunic, Inc.
Immunic, Inc. (Nasdaq: IMUX) is a biotechnology company developing a clinical pipeline of orally administered, small molecule therapies for chronic inflammatory and autoimmune diseases. The Company's lead development program, vidofludimus calcium (IMU-838), is currently in phase 3 clinical trials for the treatment of relapsing multiple sclerosis, for which top-line data is expected to be available end of 2026. It has already shown therapeutic activity in phase 2 clinical trials in patients suffering from relapsing-remitting multiple sclerosis and progressive multiple sclerosis. Vidofludimus calcium combines neuroprotective effects, through its mechanism as a first-in-class nuclear receptor related 1 (Nurr1) activator, with additional anti-inflammatory and anti-viral effects, by selectively inhibiting the enzyme dihydroorotate dehydrogenase (DHODH). IMU-856, which targets the protein Sirtuin 6 (SIRT6), is intended to restore intestinal barrier function and regenerate bowel epithelium, which could potentially be applicable in numerous gastrointestinal diseases, such as celiac disease as well as inflammatory bowel disease, Graft-versus-Host-Disease and weight management. IMU-381, which currently is in preclinical testing, is a next generation molecule being developed to specifically address the needs of gastrointestinal diseases.
Cautionary Note Regarding Forward-Looking Statements
This press release contains "forward-looking statements" that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, included in this press release regarding strategy, future operations, future financial position, future revenue, projected expenses, sufficiency of cash and cash runway, expected timing, development and results of clinical trials, prospects, plans and objectives of management are forward-looking statements. Examples of such statements include, but are not limited to, statements relating to consummation of the proposed offering as well as the timing and size of the proposed offering, Immunic's development programs and the targeted diseases; the potential for vidofludimus calcium to safely and effectively target diseases; preclinical and clinical data for vidofludimus calcium; the feasibility of advancing vidofludimus calcium to a confirmatory phase 3 clinical trial in progressive multiple sclerosis; the timing of current and future clinical trials and anticipated clinical milestones; the nature, strategy and focus of the Company and further updates with respect thereto; and the development and commercial potential of any product candidates of the Company. Immunic may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in the forward-looking statements and you should not place undue reliance on these forward-looking statements. Such statements are based on management's current expectations and involve substantial risks and uncertainties. Actual results and performance could differ materially from those projected in the forward-looking statements as a result of many factors, including, without limitation, increasing inflation, tariffs and macroeconomics trends, impacts of the Ukraine – Russia conflict and the conflict in the Middle East on planned and ongoing clinical trials, risks and uncertainties associated with the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations, the availability of sufficient financial and other resources to meet business objectives and operational requirements, the fact that the results of earlier preclinical studies and clinical trials may not be predictive of future clinical trial results, any changes to the size of the target markets for the Company's products or product candidates, the protection and market exclusivity provided by Immunic's intellectual property, risks related to the drug development and the regulatory approval process and the impact of competitive products and technological changes, the Company’s ability to close the proposed public offering, and the risk that warrants issued in this offering will not be exercised for cash in the future. A further list and descriptions of these risks, uncertainties and other factors can be found in the section captioned "Risk Factors," in the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2024, filed with the SEC on March 31, 2025, and in the Company's subsequent filings with the SEC. Copies of these filings are available online at www.sec.gov or ir.imux.com/sec-filings. Any forward-looking statement made in this release speaks only as of the date of this release. Immunic disclaims any intent or obligation to update these forward-looking statements to reflect events or circumstances that exist after the date on which they were made. Immunic expressly disclaims all liability in respect to actions taken or not taken based on any or all of the contents of this press release.
Contact Information
Immunic, Inc.
Jessica Breu
Vice President Investor Relations and Communications
+49 89 2080 477 09
jessica.breu@imux.com
US IR Contact
Rx Communications Group
Paula Schwartz
+1 917 633 7790
immunic@rxir.com
US Media Contact
KCSA Strategic Communications
Caitlin Kasunich
+1 212 896 1241
ckasunich@kcsa.com