UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): April 30, 2025
IMMUNIC, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-36201 | 56-2358443 |
| (State or other jurisdiction of incorporation) |
(Commission File Number) | (IRS Employer Identification No.) |
1200 Avenue of the Americas, Suite 200
New York, NY 10036
USA
(Address of principal executive offices)
Registrant’s telephone number, including area code: (332) 255-9818
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of exchange on which registered |
| Common Stock, par value $0.0001 | IMUX | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. Yes ☐ No ☐
Item 8.01. Other Events.
On April 30, 2025, Immunic, Inc. (the “Company” or “Immunic”) issued a press release (the “Press Release”) and posted an updated corporate presentation on the Company’s website (the “Presentation”) announcing the data from the Company’s phase 2 CALLIPER trial of nuclear receptor related 1 (Nurr1) activator, vidofludimus calcium (IMU-838), in patients with progressive multiple sclerosis. Vidofludimus calcium is an orally administered investigational small molecule drug being developed for chronic inflammatory and autoimmune diseases.
The Company will discuss the data and the Presentation during a live webinar event at 8:00 a.m. Eastern Time on April 30, 2025.
The Press Release and Presentation are attached as Exhibits 99.1 and 99.2 to this Current Report on Form 8-K, respectively, and are incorporated herein by reference.
Item 9.01. Financial Statements and Exhibits.
| Exhibit | Description |
| 99.1 | Press Release, dated April 30, 2025. |
| 99.2 | Presentation, dated April 30, 2025. |
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL). |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this report to be signed on its behalf by the undersigned, hereunto duly authorized.
| Dated: April 30, 2025 | Immunic, Inc. | |
| By: | /s/ Daniel Vitt | |
| Daniel Vitt | ||
| Chief Executive Officer | ||

Immunic Announces Vidofludimus Calcium Reduced Risk of Disability Worsening by 30% in Primary Progressive Multiple Sclerosis Patients from Phase 2 CALLIPER Trial
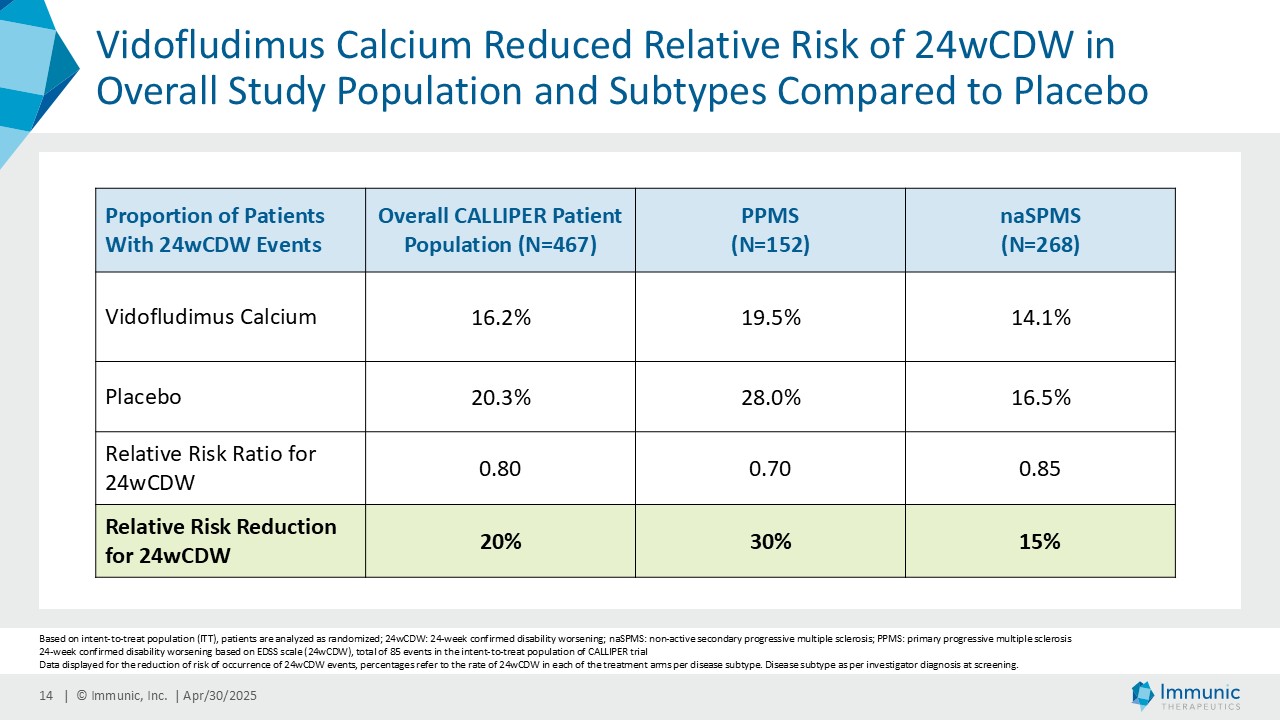
– Reduced Relative Risk of 24-Week Confirmed Disability Worsening Events by 20% in Overall Study Population Compared to Placebo; Even More Prominent 30% Reduction in High Unmet Need Population of Primary Progressive Multiple Sclerosis –
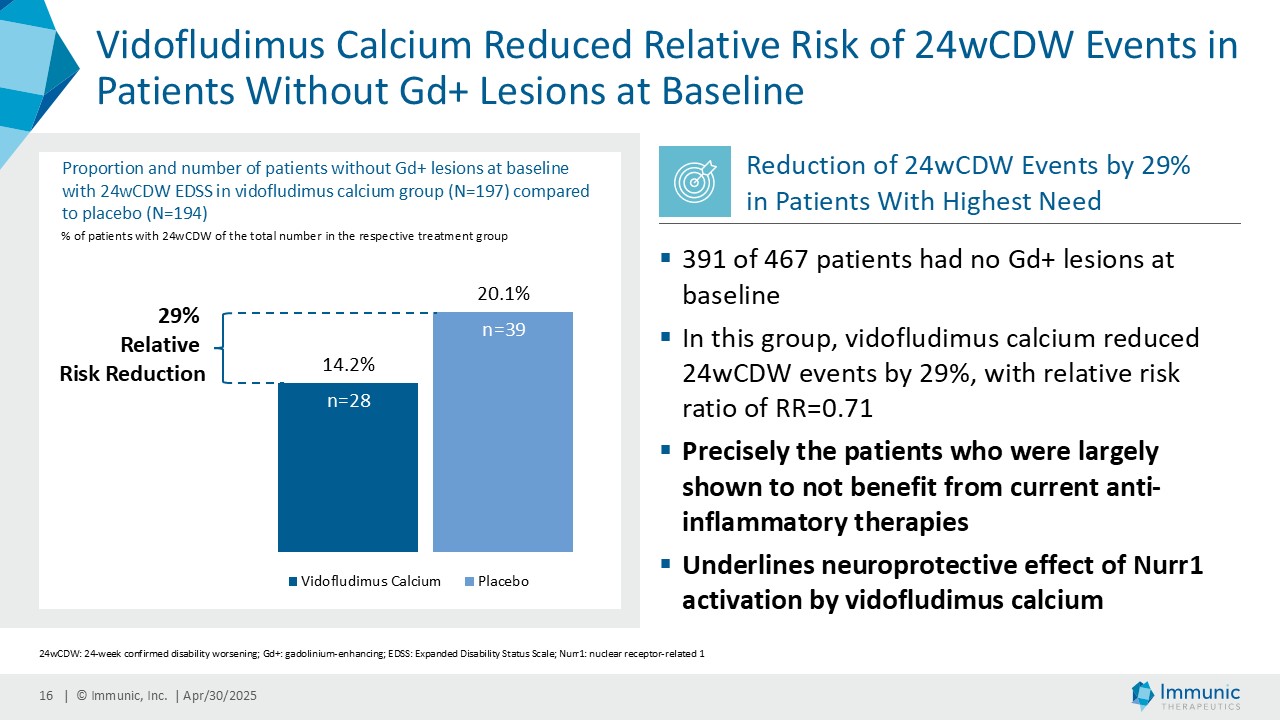
– Showed Consistent Reduction of Disability Worsening in Subpopulations Without Inflammatory Lesions at Baseline in Overall Study Population; Reduced Relative Risk of 24-Week Confirmed Disability Worsening Events in Patients Without Gadolinium-Enhancing Lesions at Baseline by 29% Compared to Placebo –
– Reduced Annualized Rate of Thalamic Brain Volume Loss by 20% Compared to Placebo –
– Confirmed Favorable Safety and Tolerability Observed in Previous Clinical Trials; No New Safety Signals Identified –
– Webcast to be Held Today, April 30, at 8:00 am ET –
NEW YORK, April 30, 2025 – Immunic, Inc. (Nasdaq: IMUX), a biotechnology company developing a clinical pipeline of orally administered, small molecule therapies for chronic inflammatory and autoimmune diseases, today announced positive data from its phase 2 CALLIPER trial of nuclear receptor related 1 (Nurr1) activator, vidofludimus calcium (IMU-838), in patients with progressive multiple sclerosis (PMS).
Clinical Endpoints
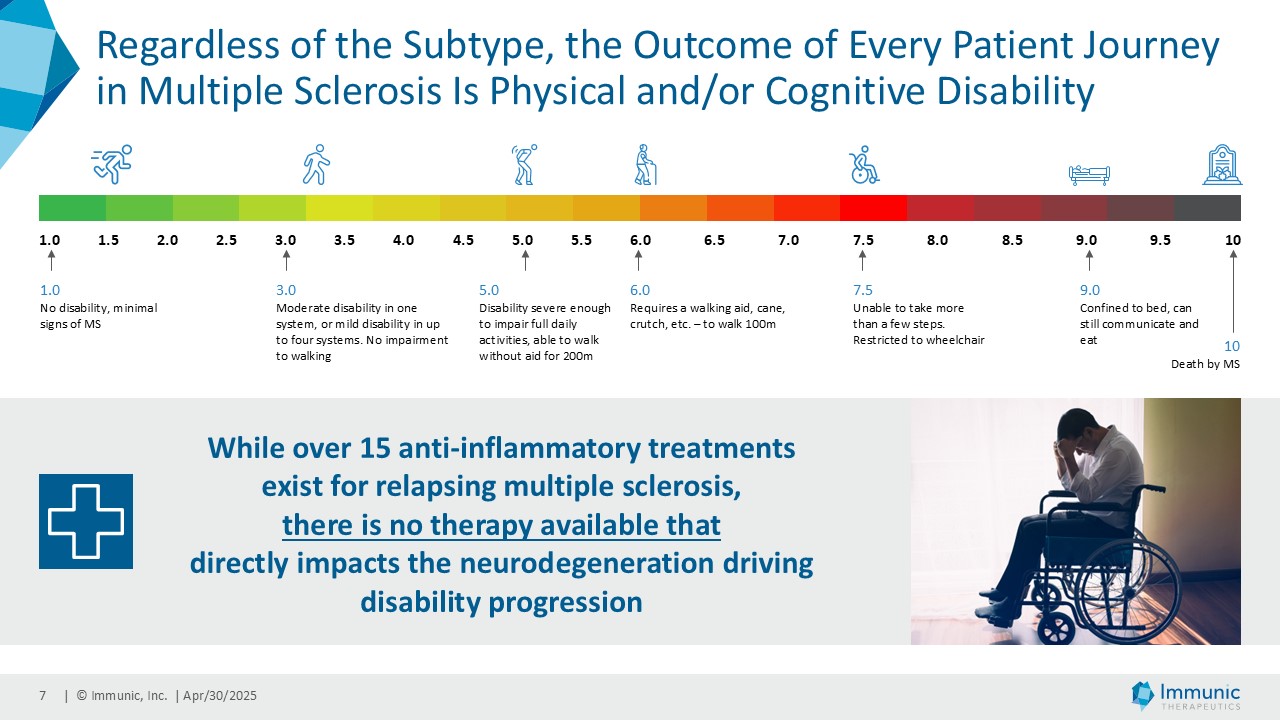
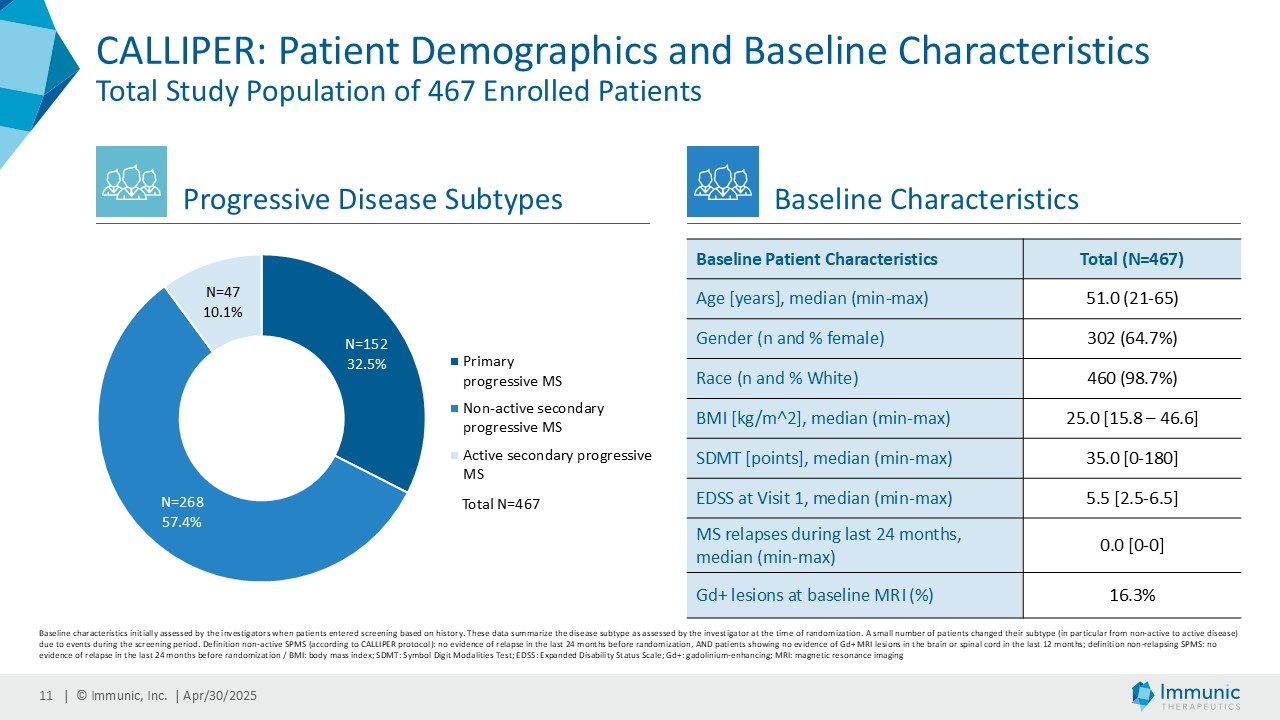
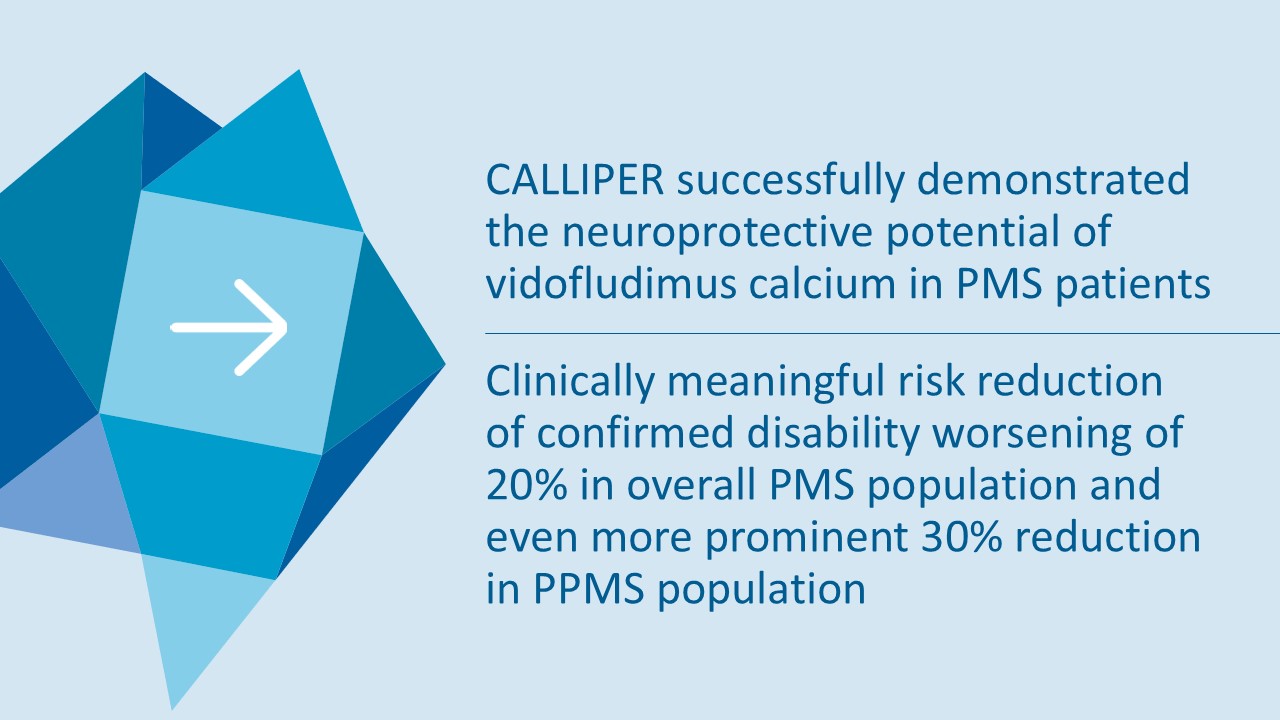
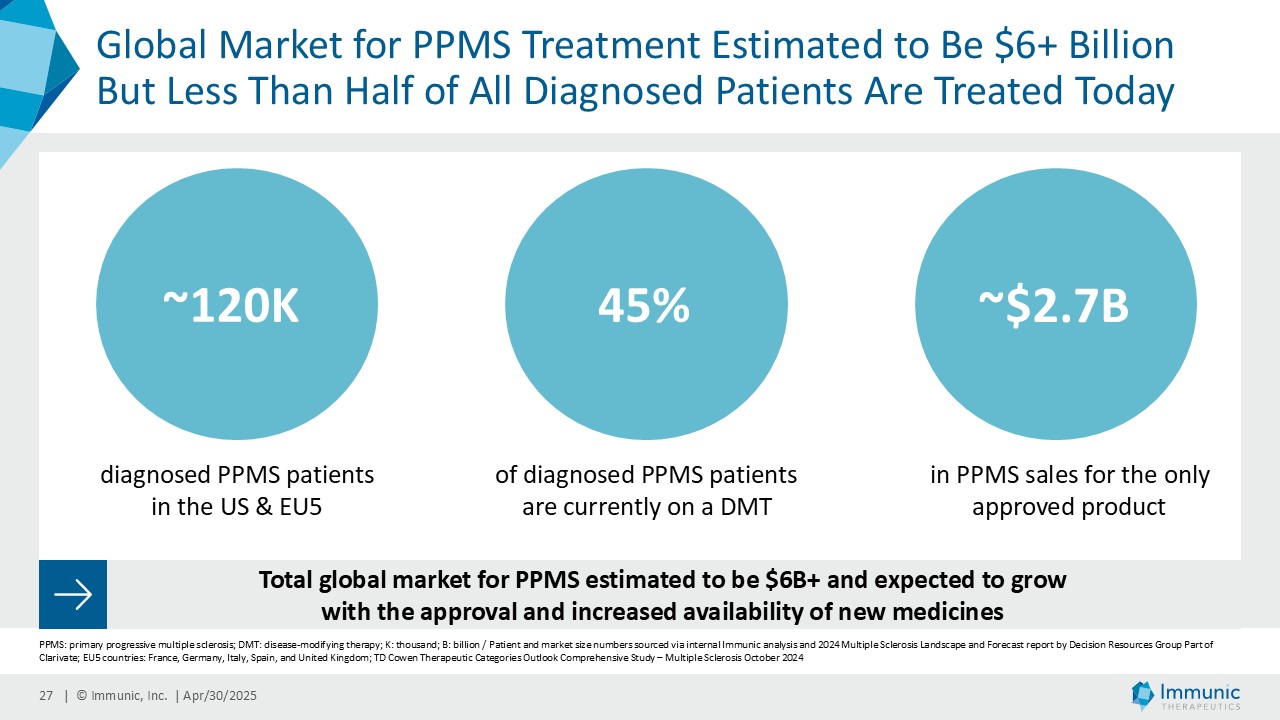
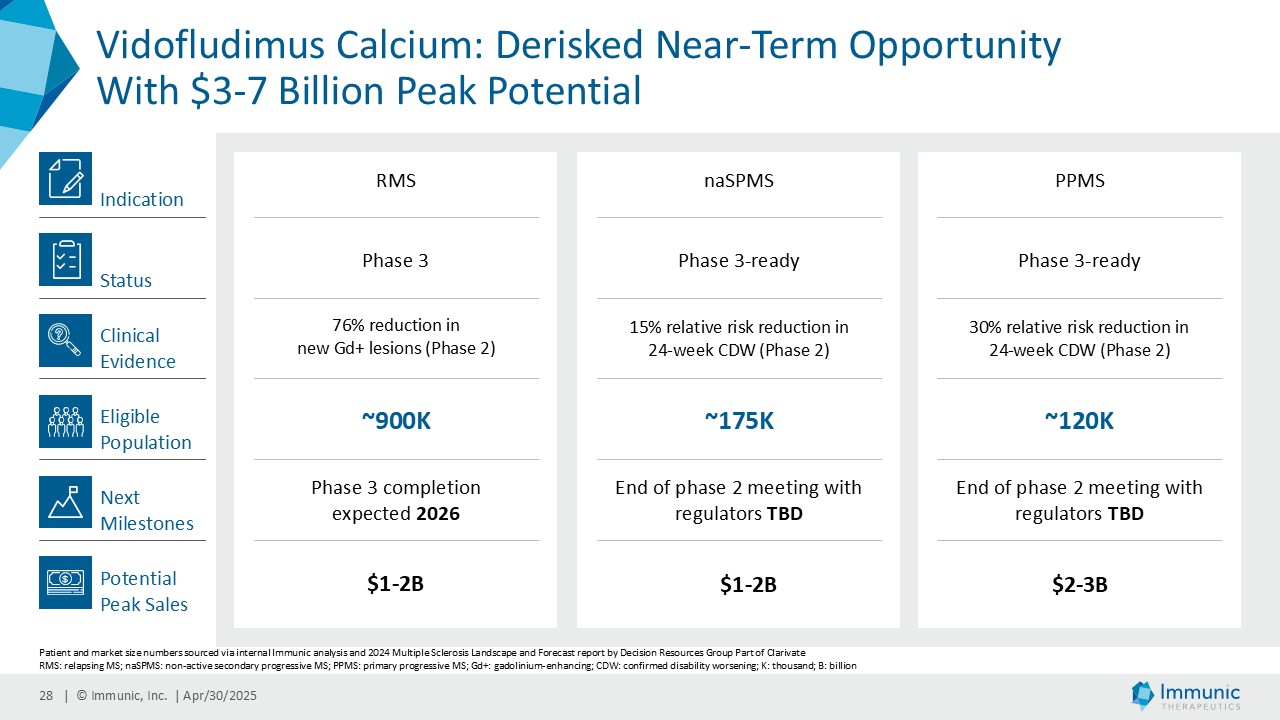
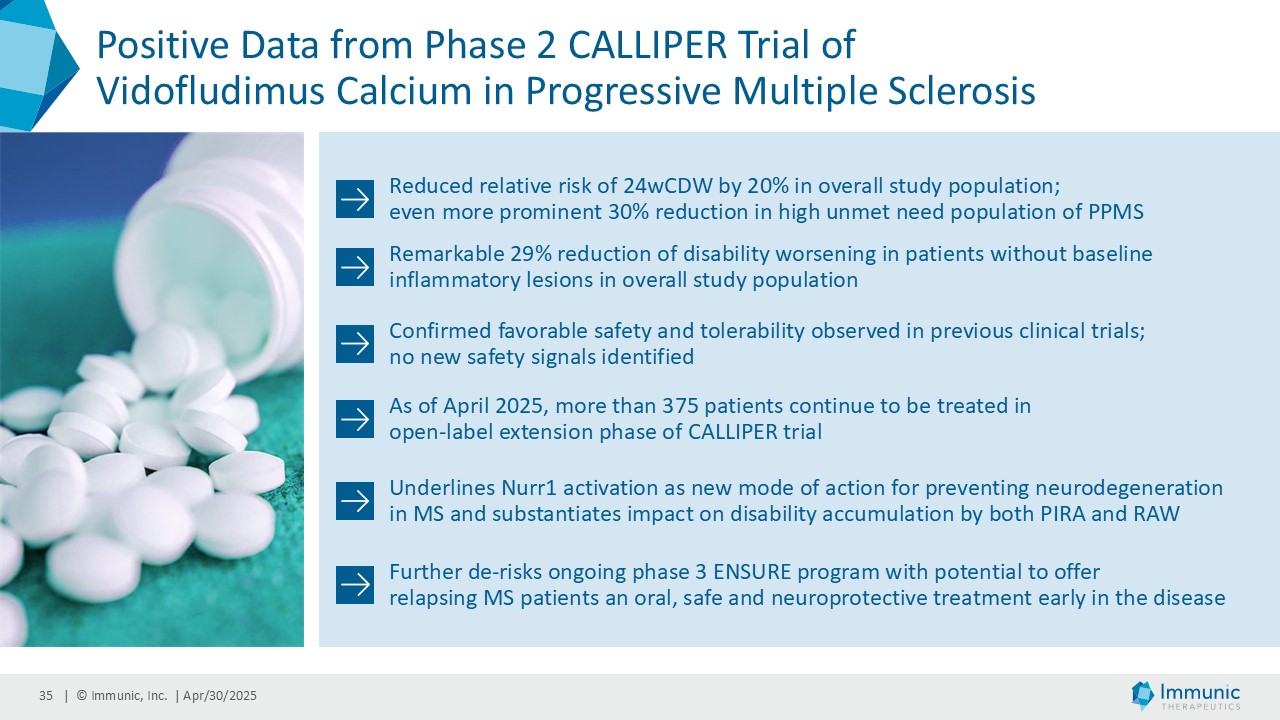
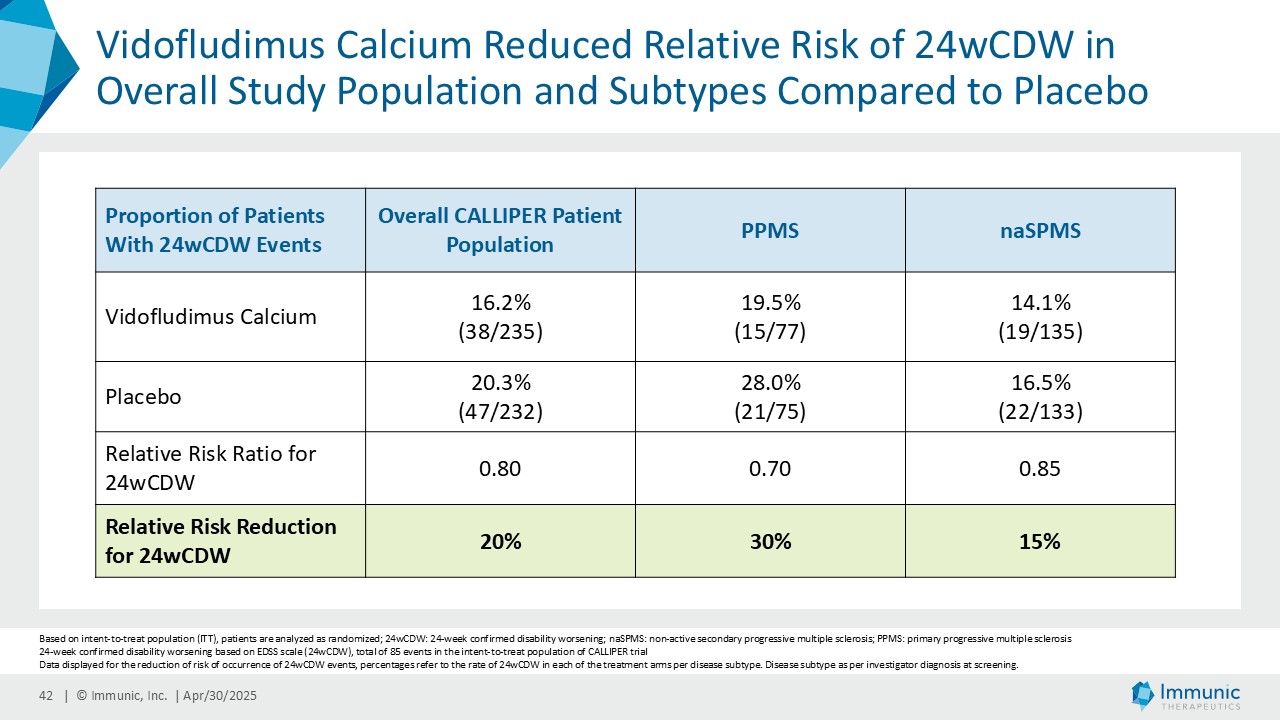
In the overall PMS patient population (n=467), vidofludimus calcium reduced the relative risk of 24-week confirmed disability worsening (24wCDW) events based on changes in the expanded disability status scale (EDSS) by 20% compared to placebo. Further analyses by disease subtype demonstrated that vidofludimus calcium was associated with a 30% reduction in the relative risk of 24wCDW events in the primary progressive multiple sclerosis (PPMS) study population (n=152) compared to placebo and a respective 15% reduction in the non-active secondary progressive multiple sclerosis (naSPMS) study population (n=268). Reduction of confirmed disability worsening is widely considered to be the most recognized regulatory approval endpoint for registrational studies in progressive forms of multiple sclerosis (MS).
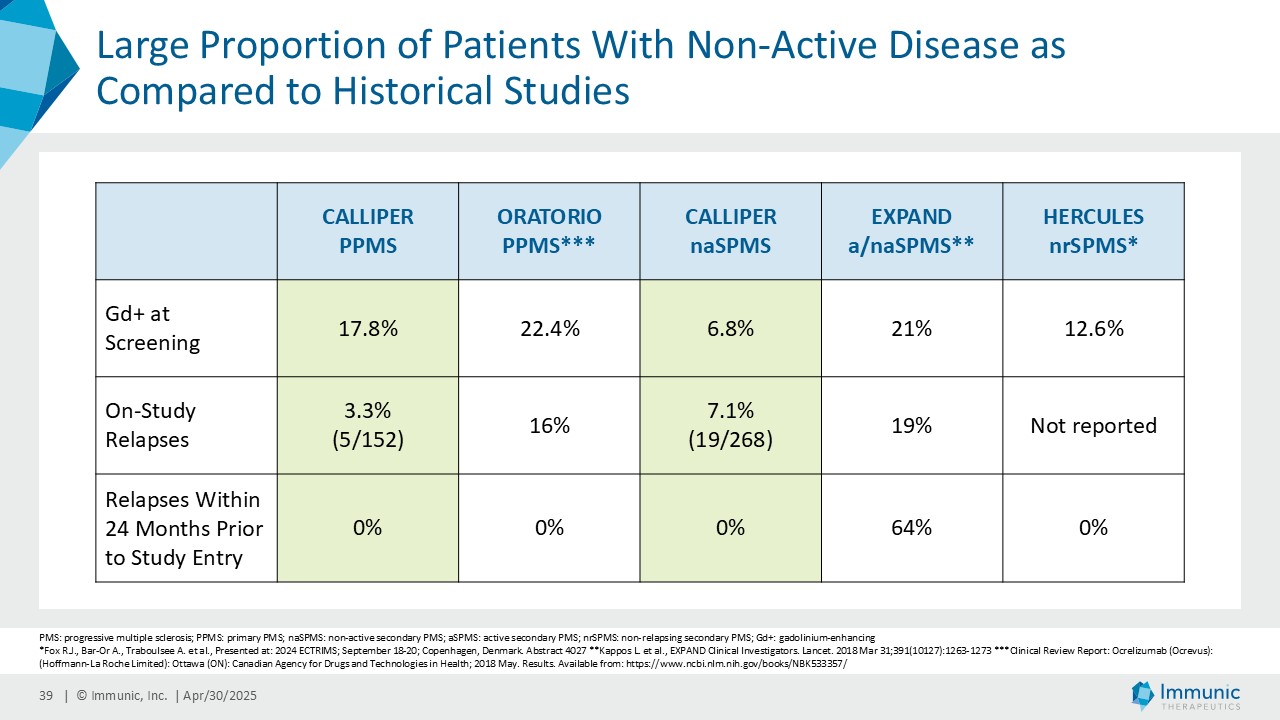
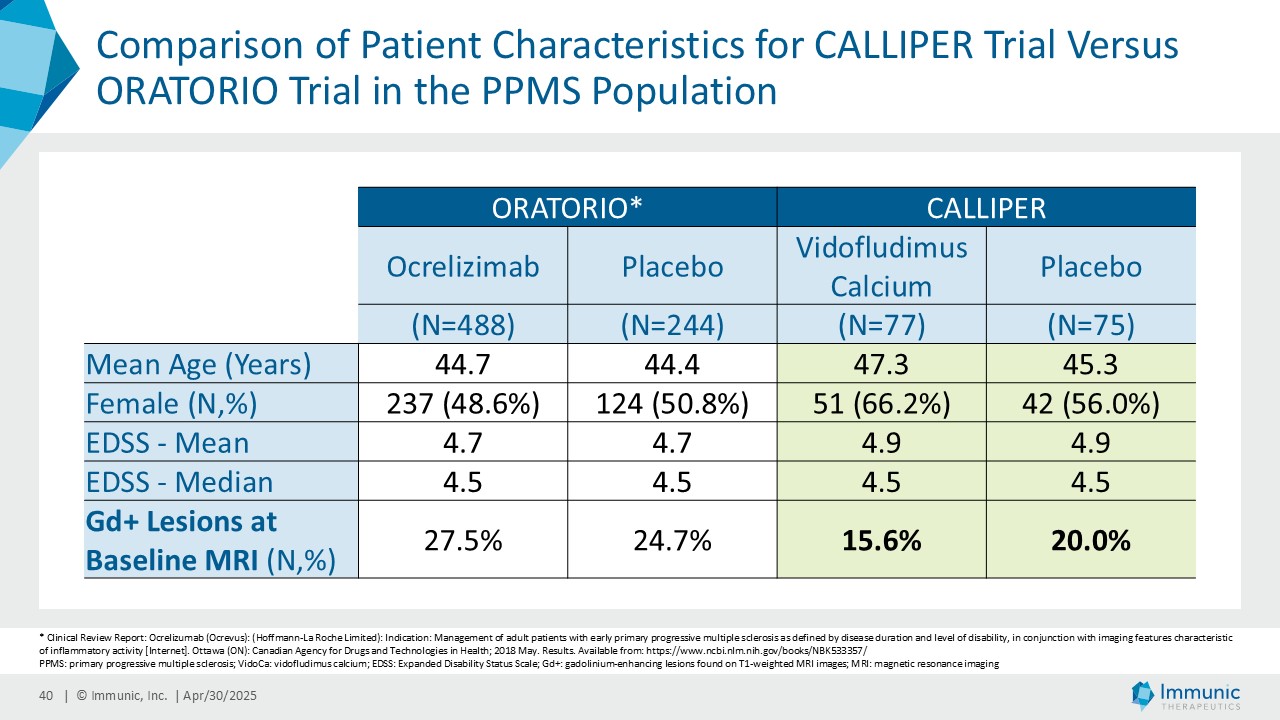
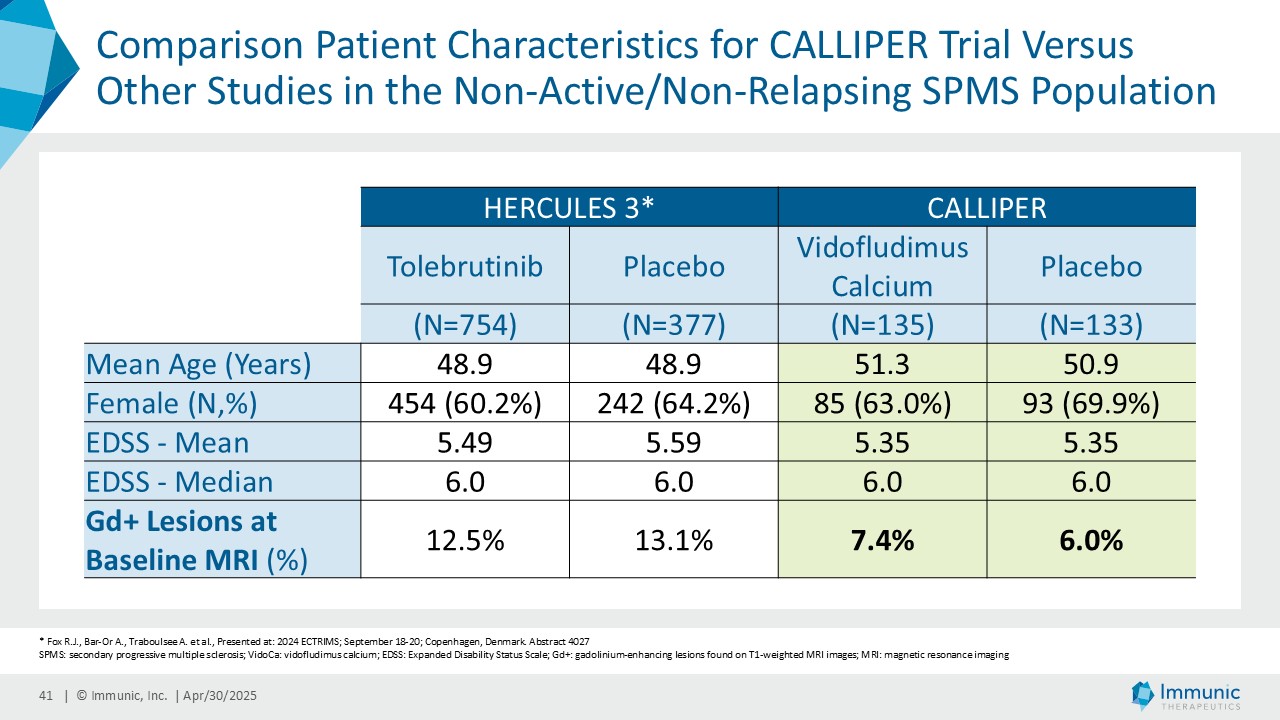
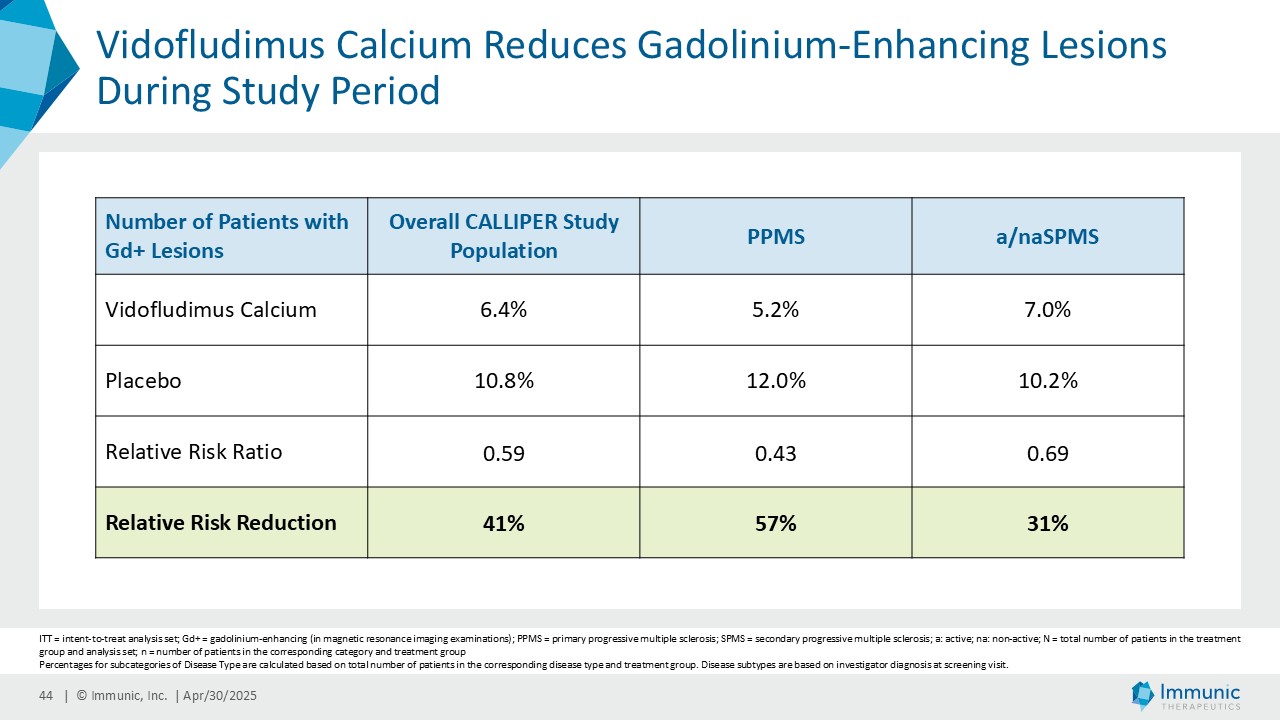
Immunic believes that these reductions in 24wCDW events are remarkable in light of the overwhelming non-activity of the CALLIPER population. In support, the evidence of focal inflammatory disease (gadolinium-enhancing lesions at baseline in 6.8% of naSPMS and 17.8% of PPMS patients) and the number of on-study relapses (5.8% in the overall patient population) are lower than in most historical PMS trials.

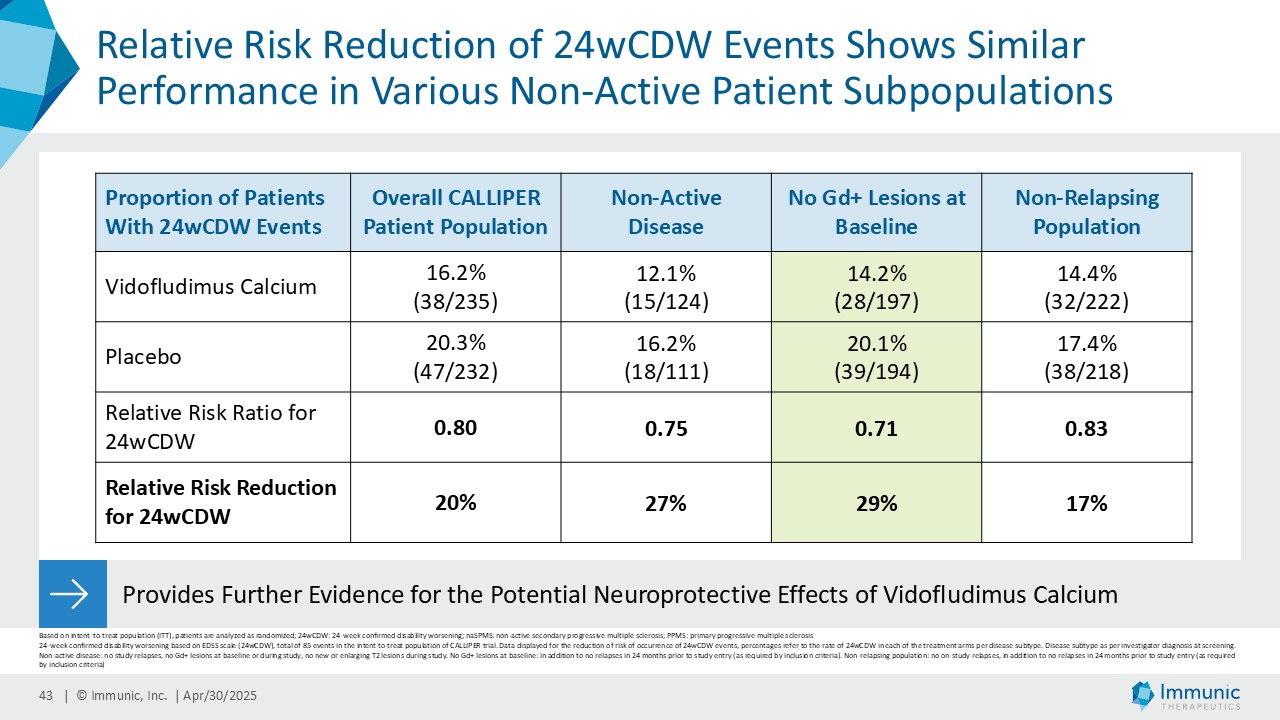
A consistent reduction of disability worsening was observed in the different subpopulations with or without inflammatory gadolinium-enhanced lesion activity at baseline and during the study. Vidofludimus calcium reduced the relative risk of 24wCDW events in patients without gadolinium-enhancing lesions at baseline by 29% compared to placebo. In historical studies, such patients were largely shown to not benefit from current anti-inflammatory therapies. Immunic believes the substantial effect in these patients, therefore, underlines the previously observed neuroprotective effect of Nurr1 activation by vidofludimus calcium.
Magnetic Resonance Imaging (MRI) Endpoints
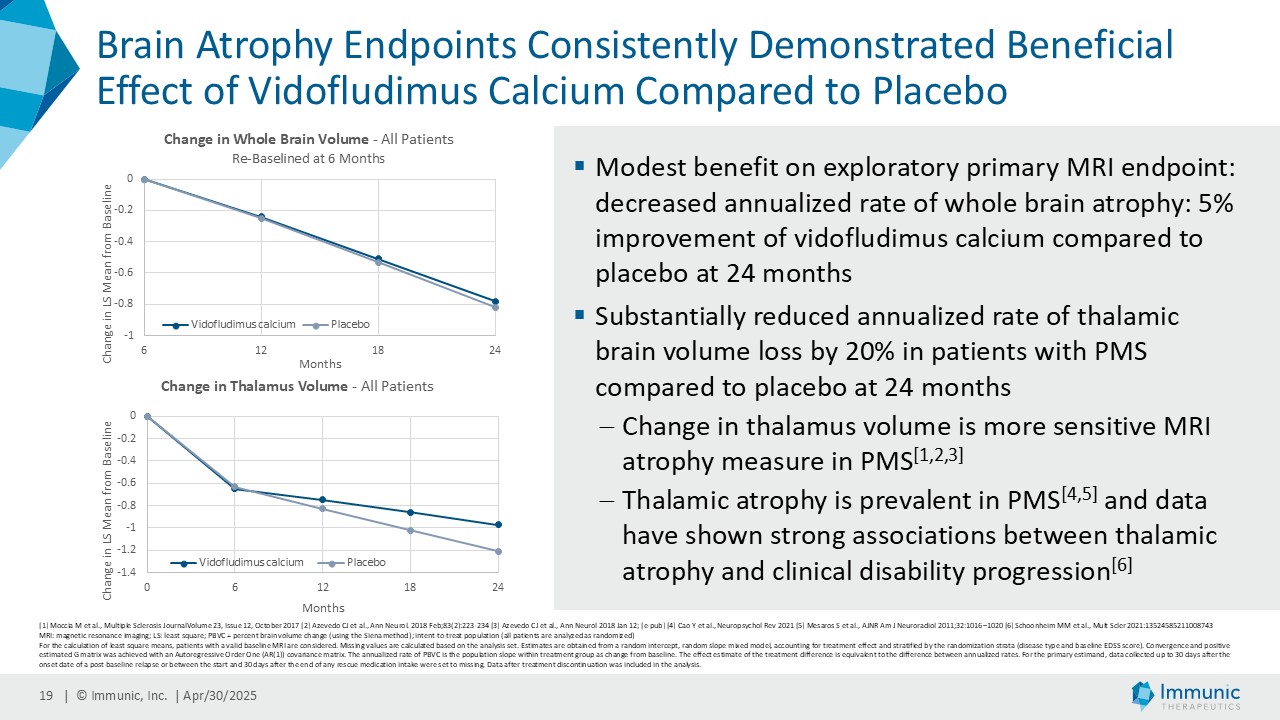
While vidofludimus calcium had a modest benefit on the exploratory primary MRI endpoint (annualized rate of percent brain volume change: 5% improvement compared to placebo), vidofludimus calcium substantially reduced the annualized rate of thalamic brain volume loss by 20% in patients with PMS compared to placebo. Change in thalamic volume is considered a more sensitive MRI atrophy marker. Thalamic atrophy is prevalent in PMS and data has shown strong associations with clinical disability progression.
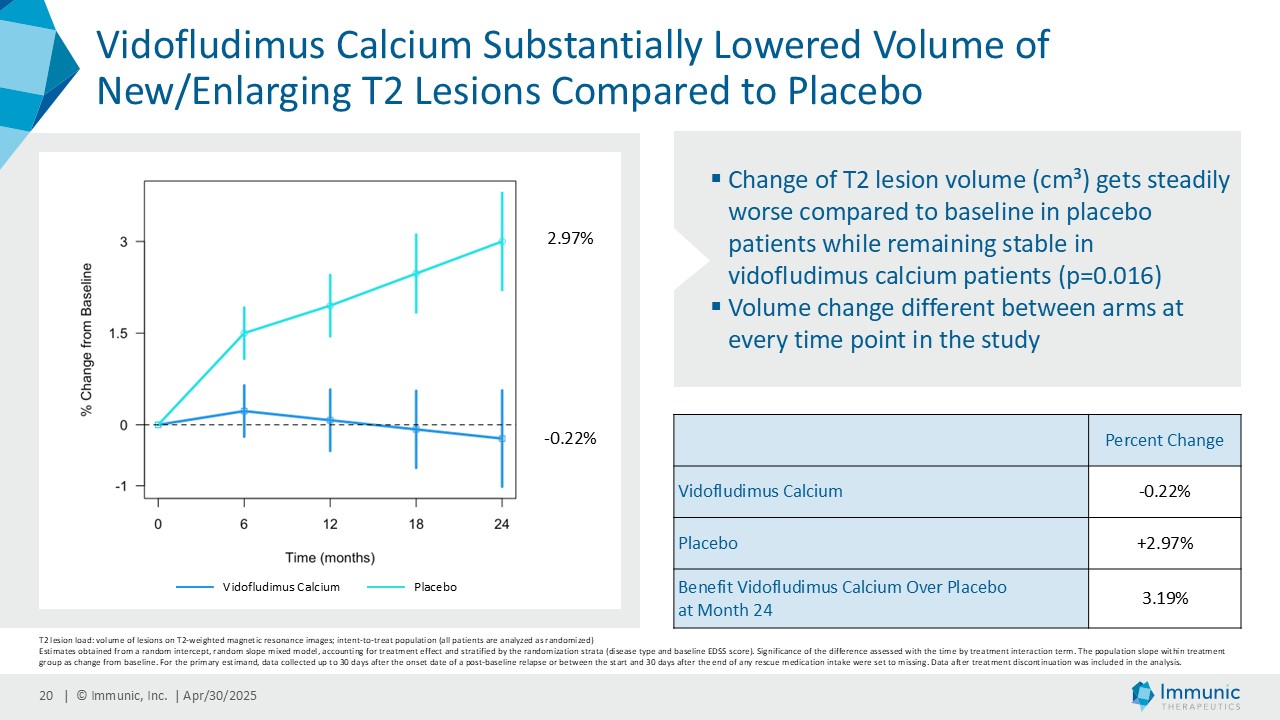
The total volume of new or enlarging T2 lesions showed a substantial difference between vidofludimus calcium and placebo over time, with vidofludimus calcium decreasing and placebo increasing (mean percent change, 3.19% benefit for vidofludimus calcium (-0.22%) over placebo (+2.97%) at month 24).
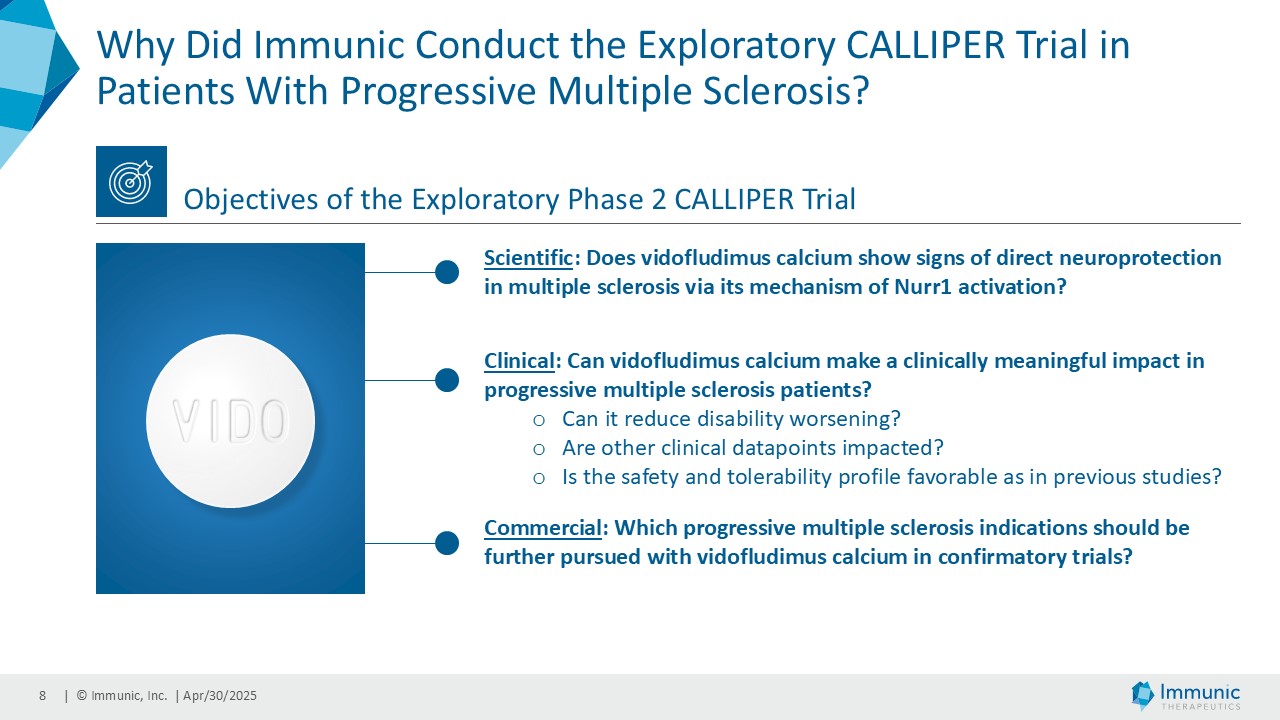
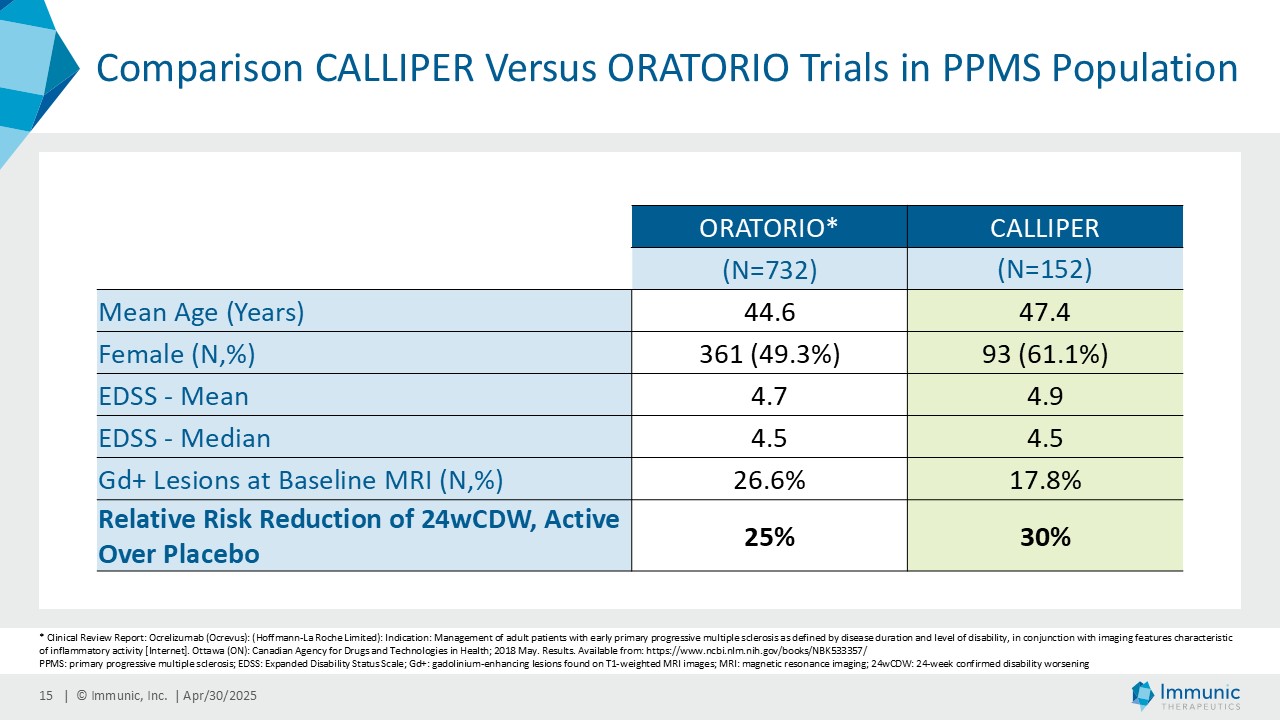
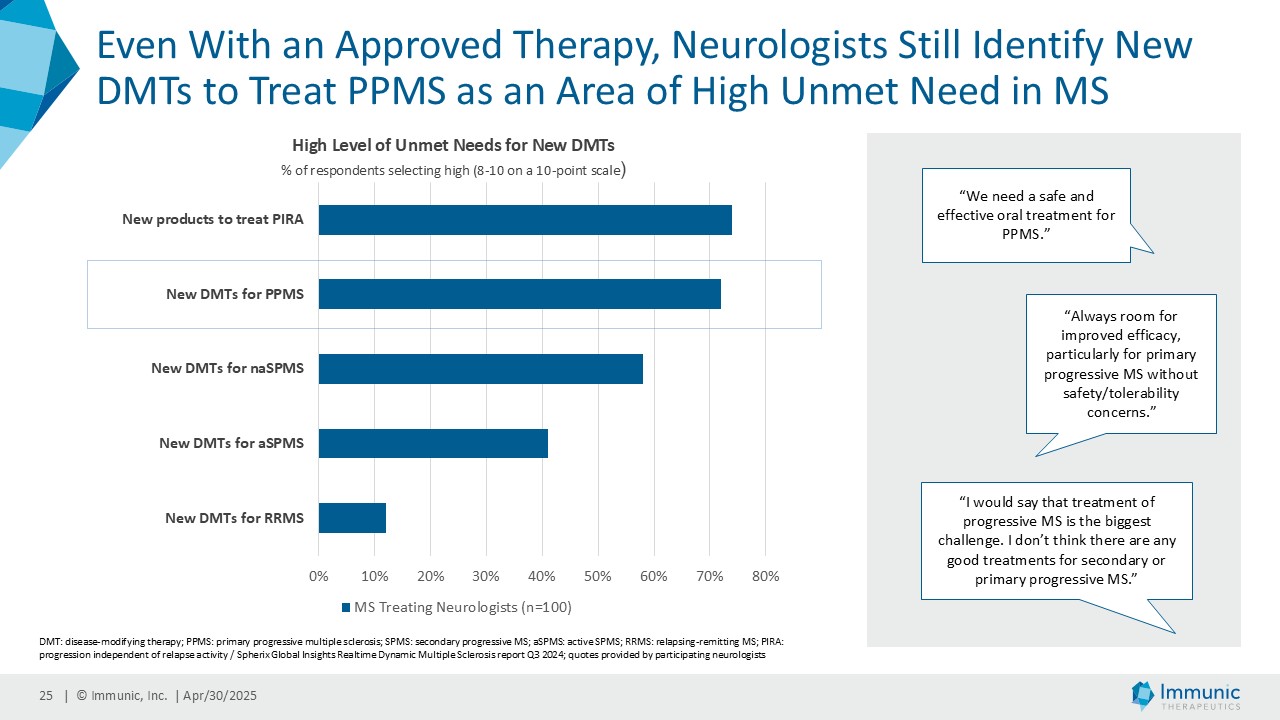
“The tremendous reduction of confirmed disability worsening in PMS patients is a wonderful confirmation of the objectives of this exploratory phase 2 trial. CALLIPER was designed to evaluate the clinical efficacy, safety and tolerability of vidofludimus calcium in a broad set of PMS patients to determine the suitability of advancing to a confirmatory phase 3 program,” said Daniel Vitt, Ph.D., Chief Executive Officer of Immunic. “We are particularly thrilled to see such a clinically meaningful effect in the PPMS population, with a 30% reduction in the relative risk of 24-week confirmed disability worsening events, which would be the endpoint of a future phase 3 registration study, outperforming historic trials in PPMS regarding numerical reduction of disability progression events. We believe that vidofludimus calcium may represent a novel and exciting approach for people living with PMS, where there continues to be a huge unmet medical need given only one approved therapy. We look forward to discussing these results with healthcare authorities to determine appropriate next steps for vidofludimus calcium in PMS.”
“The most significant unmet need in MS continues to be the lack of safe and effective therapies that can slow or halt disease progression, especially in PPMS and non-active SPMS. Vidofludimus calcium is the only medicine in development for MS that has been shown to be a potent activator of Nurr1, which plays a key role in neuroprotection. With this unique mode of action, vidofludimus calcium could become the first real neuroprotective treatment option for patients with progressive forms of MS,” added Andreas Muehler, M.D., M.B.A., Chief Medical Officer of Immunic. “In particular, the 29% reduction of 24-week confirmed disability worsening events for vidofludimus calcium over placebo in the 391 patients without gadolinium-enhancing lesions at baseline underlines the drug’s breakthrough potential in addressing the high unmet need of slowing neurodegeneration in MS patients. We believe today’s exciting results of the phase 2 CALLIPER trial further validate vidofludimus calcium’s scientific rationale, specifically driven by the impressive numerical benefit in reducing disability worsening, which deserves to be further tested in a phase 3 registration trial. Since almost all disability events in a non-active PMS population are known to be progression independent of relapse activity (PIRA), we believe the CALLIPER results also corroborate the benefit on disability progression expected for people suffering from relapsing forms of MS, allowing for a beneficial read-through to our ongoing phase 3 ENSURE trials.”

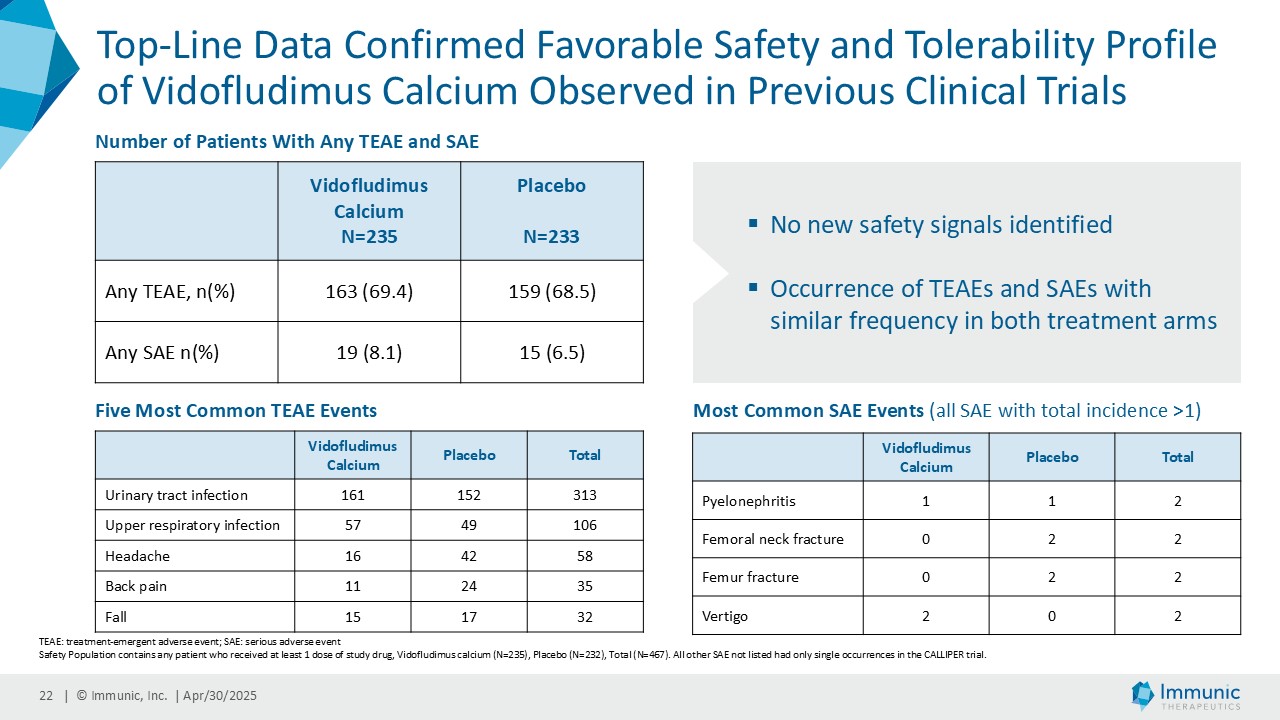
Safety and Tolerability
The top-line CALLIPER data set confirmed the favorable safety and tolerability profile of vidofludimus calcium already observed in previous clinical trials. No new safety signals were identified. The occurrence of treatment-emergent adverse events and serious adverse events showed a similar frequency between both treatment arms. The rate of treatment-emergent adverse events was 69.4% of vidofludimus calcium-treated patients compared with 68.5% of patients on placebo. Likewise, serious adverse events were rare and only observed in 8.1% of vidofludimus calcium-treated patients, and in 6.5% of patients on placebo. Additionally, no Hy’s law range cases regarding elevations of liver enzymes were observed during the study.
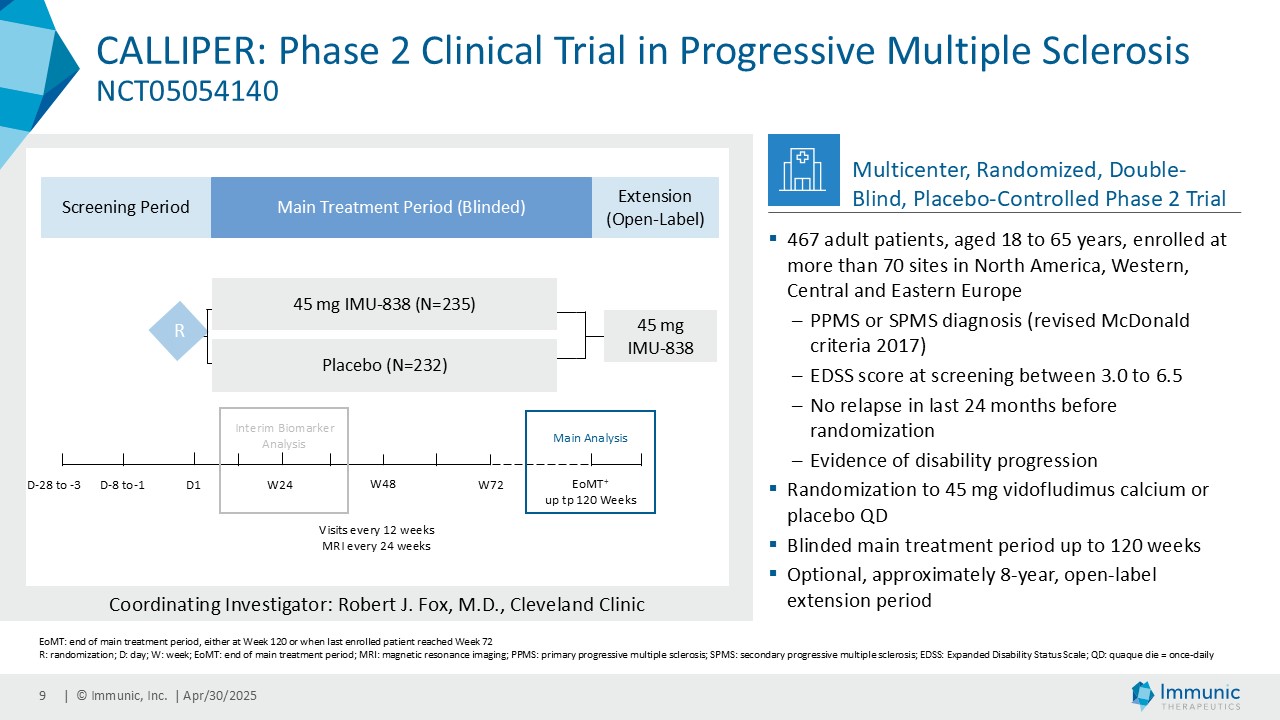
About CALLIPER
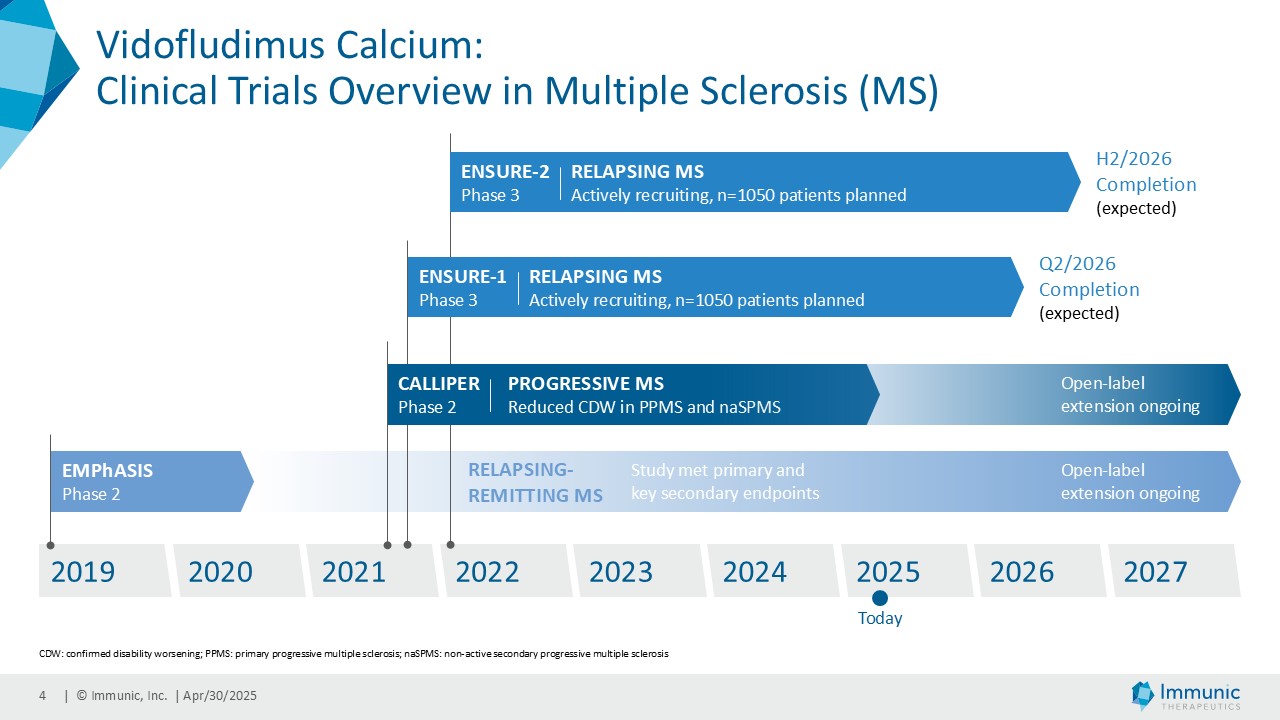
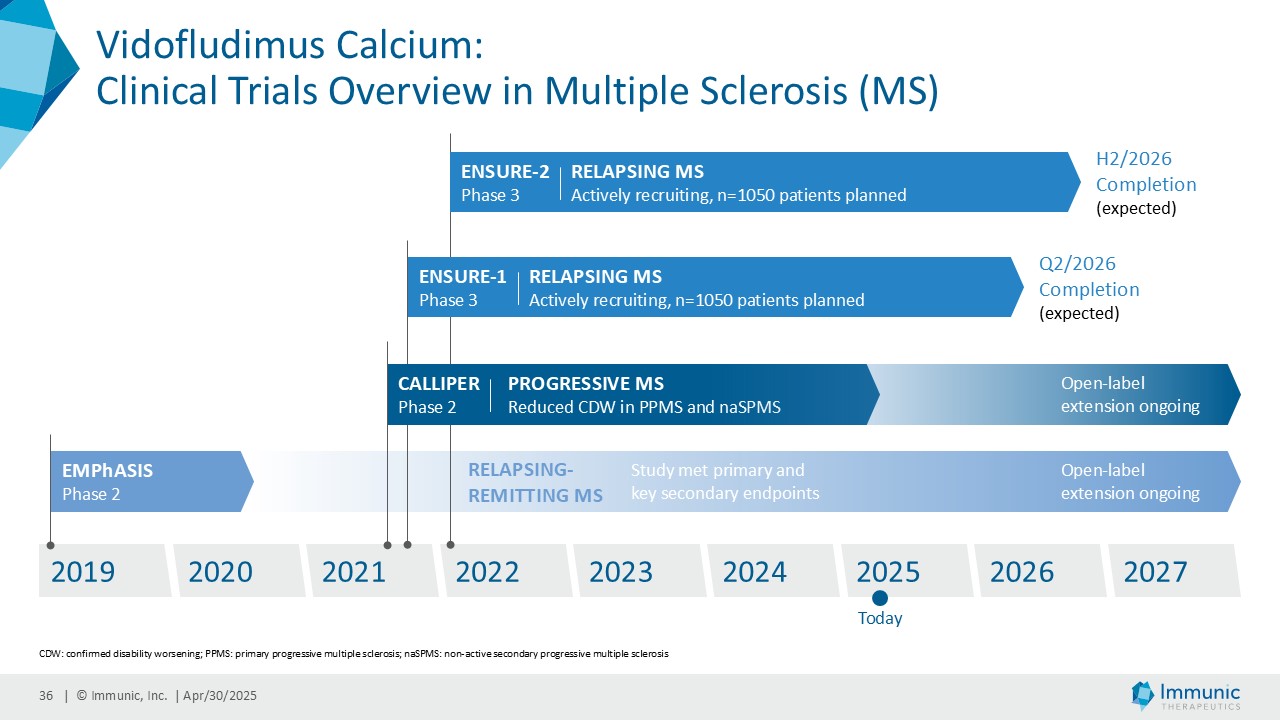
CALLIPER is an international, multicenter, randomized, double-blind, placebo-controlled, exploratory phase 2 trial which enrolled 467 patients at more than 70 sites throughout North America as well as Western, Central and Eastern Europe. Patients, aged 18 to 65 years and without limitation on disease duration, were randomized to either 45 mg daily doses of vidofludimus calcium or placebo and treated for up to 120 weeks. CALLIPER enrolled mainly patients with PPMS (n=152/467) and naSPMS (n=268/467). All patients showed no evidence of relapse in the last 24 months before randomization. For more information, please visit: www.clinicaltrials.gov, NCT05054140.
Analysis of the full CALLIPER data set is ongoing and will be presented at upcoming scientific meetings. The company’s phase 3 clinical trial program of vidofludimus calcium in relapsing multiple sclerosis is ongoing and expected to be completed in 2026.
Webcast Information
Immunic will host a webcast today at 8:00 am ET to discuss these results. To participate in the webcast, please register in advance at: https://imux.zoom.us/webinar/register/WN_tzX8otsFRAawwEmjwgCjeA or on the “Events and Presentations” section of Immunic’s website at: ir.imux.com/events-and-presentations. Registrants will receive a confirmation email containing a link for online participation or a telephone number for dial in access.
An archived replay of the webcast will be available approximately one hour after completion on Immunic’s website at: ir.imux.com/events-and-presentations.
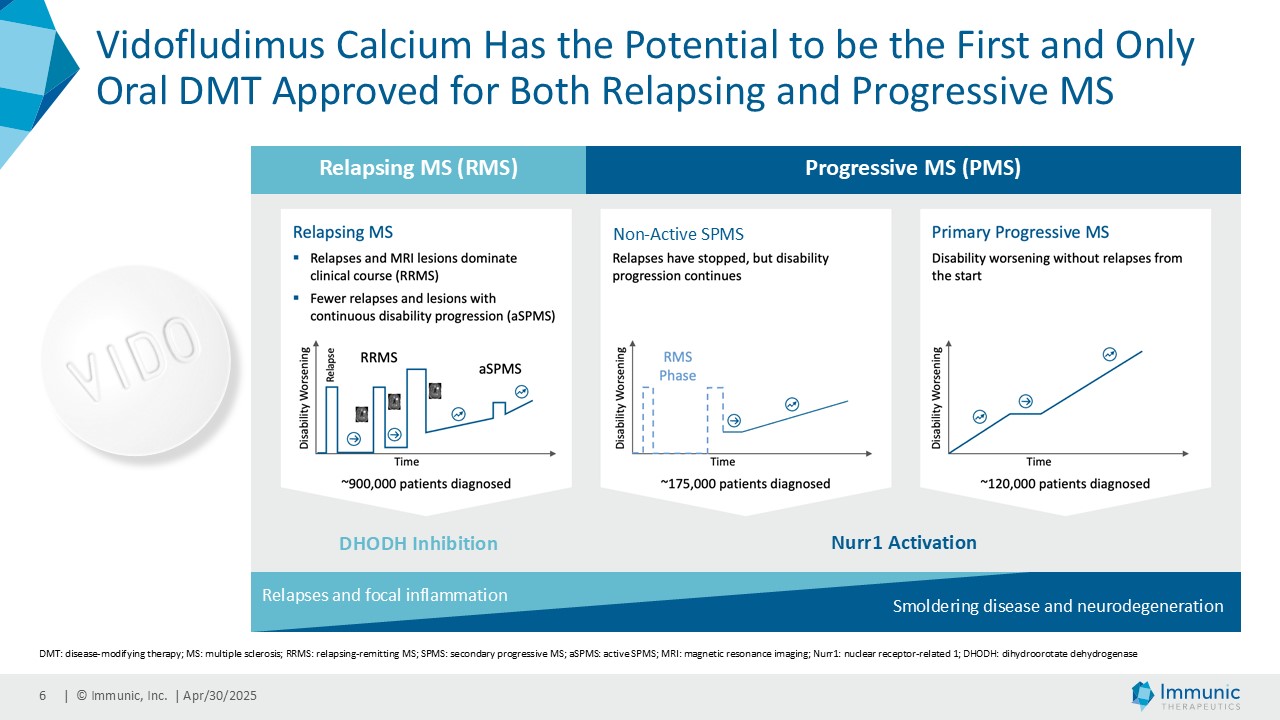
About Progressive Multiple Sclerosis
While multiple sclerosis (MS) in general is an autoimmune disease characterized by immune-mediated demyelination that affects the brain, spinal cord and optic nerve, the progressive phase of the disease seems to be dominated by significant neurodegenerative mechanisms. Progressive multiple sclerosis (PMS) is a clinical form of MS characterized by gradual accrual of disability independent of relapses over time. PMS includes both primary progressive MS (PPMS) and secondary progressive MS (SPMS). PPMS is characterized by steadily worsening neurologic function from the onset of symptoms without initial relapse or remissions. SPMS is identified following an initial relapsing-remitting course, after which the disease becomes more steadily progressive, with (active SPMS) or without (non-active SPMS) MRI lesions and/or relapses present.

About Vidofludimus Calcium (IMU-838)
Vidofludimus calcium is an orally administered investigational small molecule drug being developed for chronic inflammatory and autoimmune diseases, currently in late-stage clinical trials for multiple sclerosis (MS). Uniquely, vidofludimus calcium’s first-in-class, dual mode of action combines neuroprotective, anti-inflammatory and anti-viral effects to target the complex pathophysiology of MS. As a selective immune modulator, it activates the neuroprotective transcription factor, nuclear receptor-related 1 (Nurr1), which provides direct and indirect neuroprotective effects. Additionally, vidofludimus calcium achieves anti-inflammatory and anti-viral effects through highly selective inhibition of the enzyme dihydroorotate dehydrogenase (DHODH). Vidofludimus calcium is currently being evaluated in phase 3 and phase 2 clinical trials for the treatment of relapsing and progressive MS, respectively. In a phase 2 clinical trial, it has shown therapeutic activity in patients suffering from relapsing-remitting MS, significantly reducing MRI lesions and demonstrating encouraging results in reducing confirmed disability worsening. Additionally, vidofludimus calcium has demonstrated clinical benefits in progressive MS patients by showing substantial reductions in the relative risks of 24-week confirmed disability progression events and in thalamic brain volume in a phase 2 clinical trial. To date, vidofludimus calcium has been exposed to approximately 2,700 individuals and has shown an attractive pharmacokinetic, safety and tolerability profile. Vidofludimus calcium is not yet licensed or approved in any country.
About Immunic, Inc.
Immunic, Inc. (Nasdaq: IMUX) is a biotechnology company developing a clinical pipeline of orally administered, small molecule therapies for chronic inflammatory and autoimmune diseases. The company's lead development program, vidofludimus calcium (IMU-838), is currently in phase 3 clinical trials for the treatment of relapsing multiple sclerosis, which are expected to be completed in 2026. It has already shown therapeutic activity in phase 2 clinical trials in patients suffering from relapsing-remitting multiple sclerosis and progressive multiple sclerosis. Vidofludimus calcium combines neuroprotective effects, through its mechanism as a first-in-class nuclear receptor related 1 (Nurr1) activator, with additional anti-inflammatory and anti-viral effects, by selectively inhibiting the enzyme dihydroorotate dehydrogenase (DHODH). IMU-856, which targets the protein Sirtuin 6 (SIRT6), is intended to restore intestinal barrier function and regenerate bowel epithelium, which could potentially be applicable in numerous gastrointestinal diseases, such as celiac disease as well as inflammatory bowel disease, Graft-versus-Host-Disease and weight management. IMU-381, which currently is in preclinical testing, is a next generation molecule being developed to specifically address the needs of gastrointestinal diseases. For further information, please visit: www.imux.com.

Cautionary Statement Regarding Forward-Looking Statements
This press release contains “forward-looking statements” that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, included in this press release regarding strategy, future operations, future financial position, future revenue, projected expenses, sufficiency of cash and cash runway, expected timing, development and results of clinical trials, prospects, plans and objectives of management are forward-looking statements. Examples of such statements include, but are not limited to, statements relating to Immunic’s development programs and the targeted diseases; the potential for vidofludimus calcium to safely and effectively target diseases; preclinical and clinical data for vidofludimus calcium; the feasibility of advancing vidofludimus calcium to a confirmatory phase 3 clinical trial in progressive multiple sclerosis; the timing of current and future clinical trials and anticipated clinical milestones; the nature, strategy and focus of the company and further updates with respect thereto; and the development and commercial potential of any product candidates of the company. Immunic may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in the forward-looking statements and you should not place undue reliance on these forward-looking statements. Such statements are based on management’s current expectations and involve substantial risks and uncertainties. Actual results and performance could differ materially from those projected in the forward-looking statements as a result of many factors, including, without limitation, increasing inflation, tariffs and macroeconomics trends, impacts of the Ukraine – Russia conflict and the conflict in the Middle East on planned and ongoing clinical trials, risks and uncertainties associated with the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations, the availability of sufficient financial and other resources to meet business objectives and operational requirements, the fact that the results of earlier preclinical studies and clinical trials may not be predictive of future clinical trial results, any changes to the size of the target markets for the Company’s products or product candidates, the protection and market exclusivity provided by Immunic’s intellectual property, risks related to the drug development and the regulatory approval process and the impact of competitive products and technological changes. A further list and descriptions of these risks, uncertainties and other factors can be found in the section captioned “Risk Factors,” in the company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2024, filed with the SEC on March 31, 2025, and in the company’s subsequent filings with the SEC. Copies of these filings are available online at www.sec.gov or ir.imux.com/sec-filings. Any forward-looking statement made in this release speaks only as of the date of this release. Immunic disclaims any intent or obligation to update these forward-looking statements to reflect events or circumstances that exist after the date on which they were made. Immunic expressly disclaims all liability in respect to actions taken or not taken based on any or all of the contents of this press release.
Contact Information
Immunic, Inc.
Jessica Breu
Vice President Investor Relations and Communications
+49 89 2080 477 09
jessica.breu@imux.com
US IR Contact
Rx Communications Group
Paula Schwartz
+1 917 633 7790
immunic@rxir.com
US Media Contact
KCSA Strategic Communications
Caitlin Kasunich
+1 212 896 1241
ckasunich@kcsa.com