UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 4, 2024
IMMUNIC, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-36201 | 56-2358443 |
| (State or other jurisdiction of incorporation) |
(Commission File Number) | (IRS Employer Identification No.) |
|
1200 Avenue of the Americas, Suite 200 New York, NY 10036 USA |
||
| (Address of principal executive offices) |
Registrant’s telephone number, including area code: (332) 255-9818
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of exchange on which registered |
| Common Stock, par value $0.0001 | IMUX | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. Yes ☐ No ☐
Item 1.01. Entry into Material Definitive Agreement.
Securities Purchase Agreement
On January 4, 2024, Immunic, Inc. (the “Company”) entered into a Securities Purchase Agreement (the “Securities Purchase Agreement”) with select accredited investors (the “Investors”), pursuant to which the Company agreed to issue and sell to the Investors in a three-tranche private placement (the “Private Placement”) shares of the Company’s common stock, $0.0001 par value per share (the “Common Stock”), or in lieu thereof, pre-funded warrants to purchase shares of Common Stock (the “Pre-Funded Warrants”). The Pre-Funded Warrants are exercisable immediately for $0.0001 per share and until exercised in full.
| · | The first tranche, which is expected to close on January 8, 2024, will result in the purchase by the Investors of an aggregate of $80 million of Common Stock (or Pre-Funded Warrants) from the Company at a price of $1.43 per share; |
| · | The second tranche is a conditional mandatory purchase by the Investors of an additional $80 million of Common Stock (or Pre-Funded Warrants) from the Company at a price of $1.716 per share, equal to 120% of the price paid in the first tranche and is subject to the satisfaction of three conditions: |
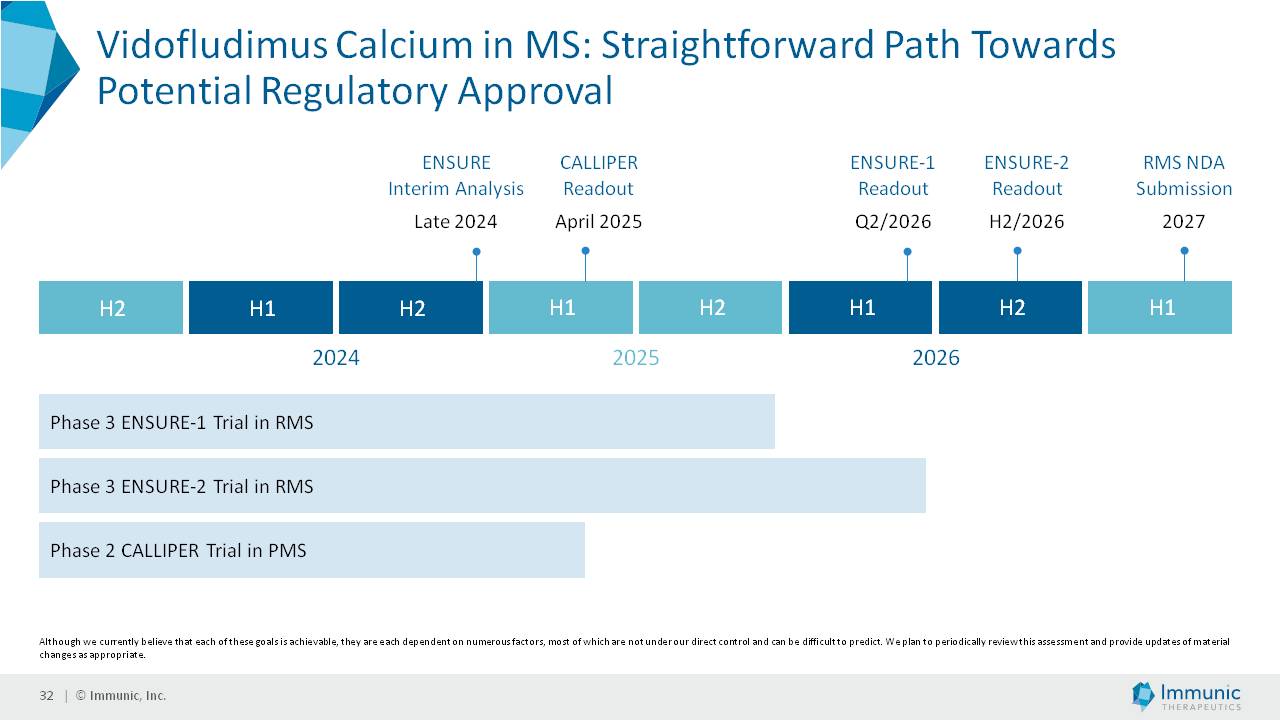
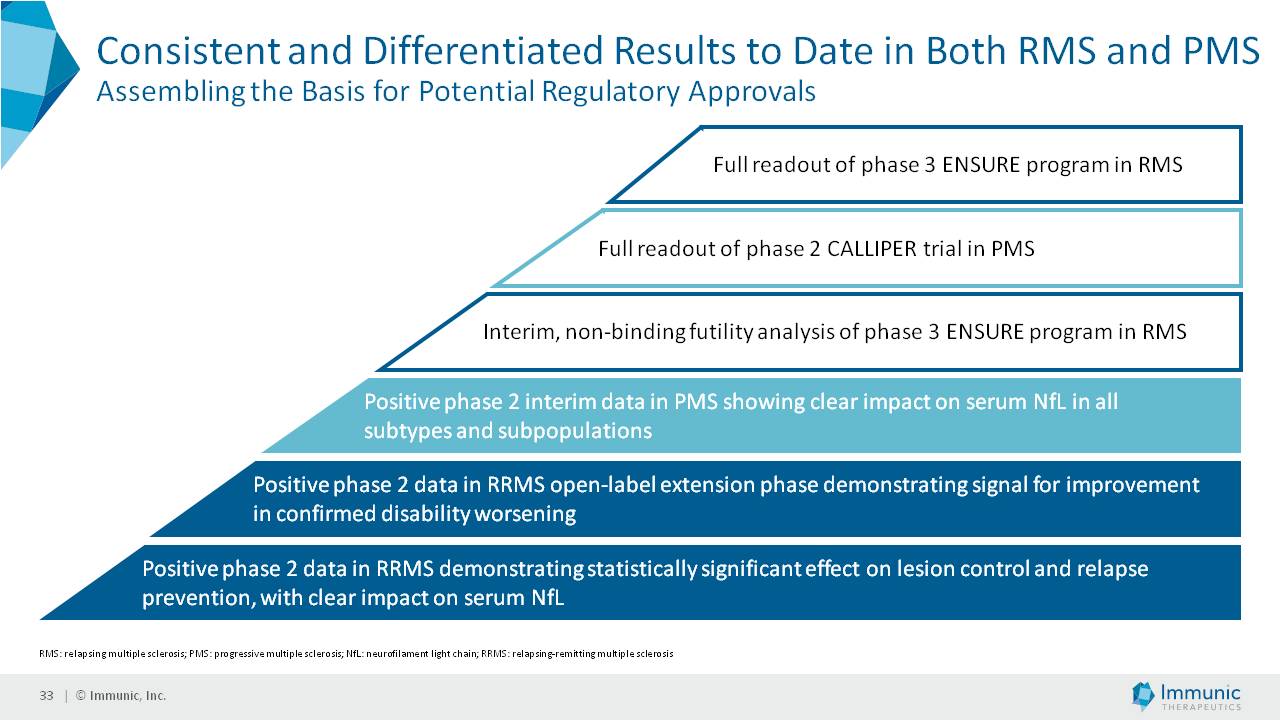
| 1) | release by the Company of topline data from its Phase 2b clinical trial of vidofludimus calcium (IMU-838) in progressive multiple sclerosis, which data is currently expected in or around April 2025; |
| 2) | the 10-day volume-weighted average price of the Common Stock is at least $8.00 per share during the 6 months following the data release; and |
| 3) | aggregate trading volume during the same 10-day period is at least $100 million. |
| · | The third tranche must occur no later than three years after the second tranche and is conditioned on the same volume-weighted average share price and minimum trading volumes as the second tranche. The third tranche provides for the issuance of $80 million of shares of common stock (or pre-funded warrants) at the same price per share as the second tranche, but permits investors to fund their purchase obligations on a “cashless” or net settlement basis, which would reduce the cash proceeds to be raised by the Company in the Private Placement. |
Any of the conditions in the second or third tranches can be waived by holders of a majority of the outstanding securities (including the lead Investor).
The Securities Purchase Agreement contains customary representations and warranties and agreements of the Company and the Investors and customary indemnification rights and obligations of the parties. The initial closing of the Private Placement is expected to occur on January 8, 2024.
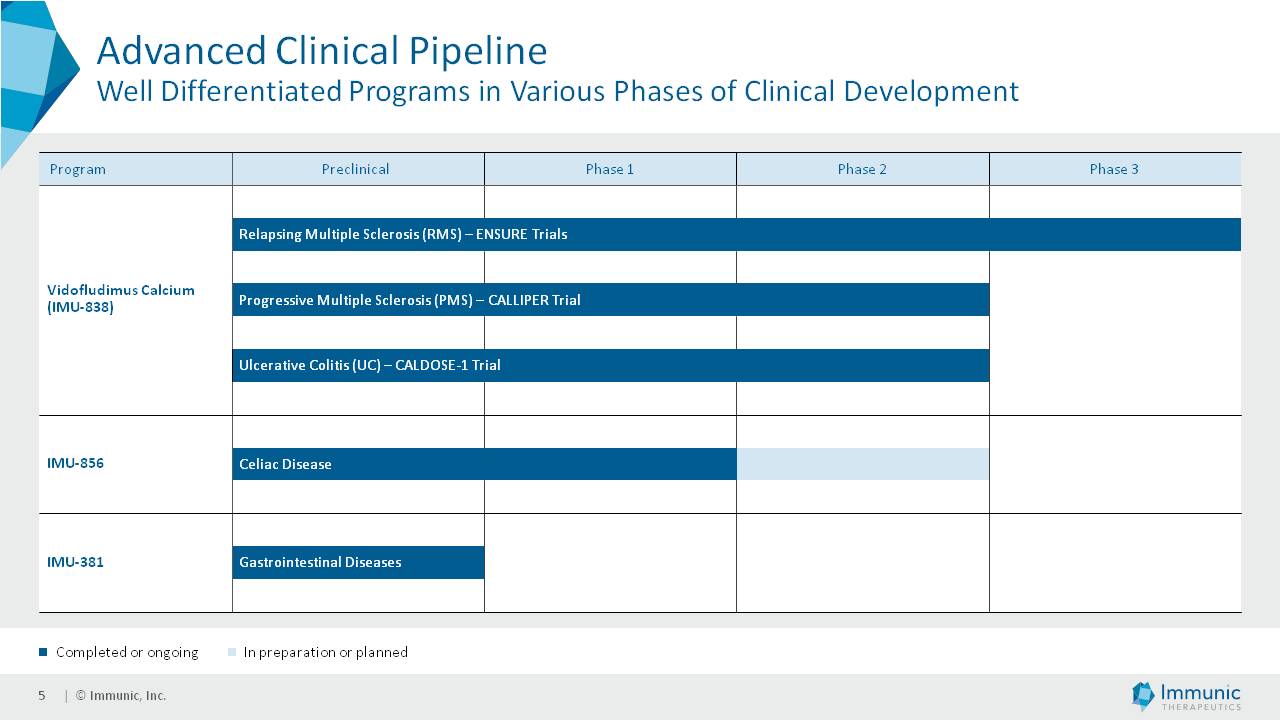
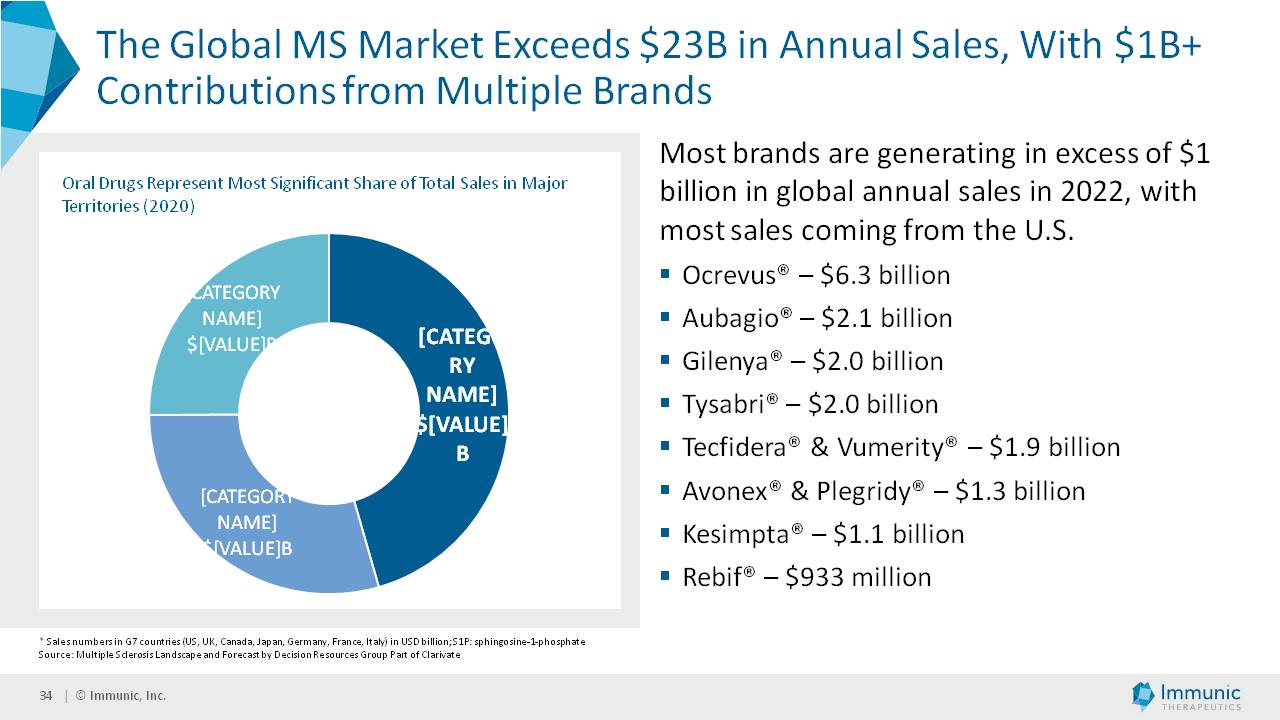
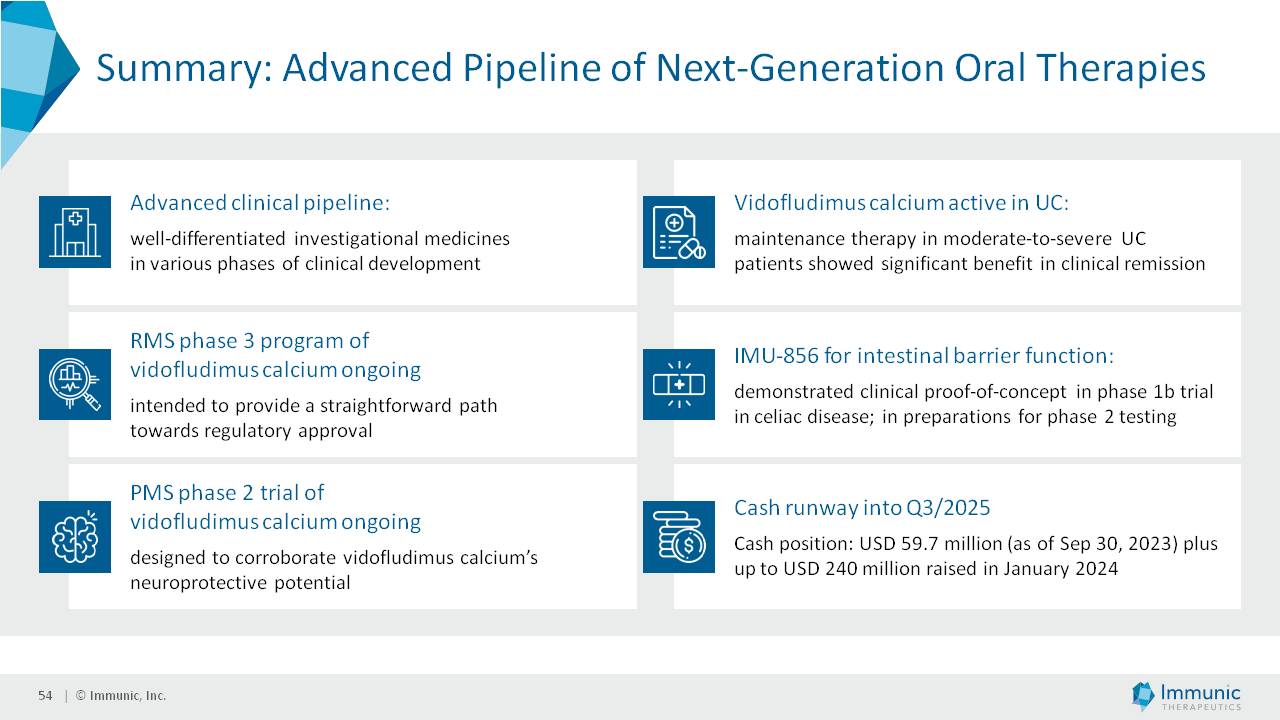
The Private Placement is expected to result in gross proceeds to the Company of approximately $80 million in the first tranche, and an additional $80 million if and when the second tranche occurs. Assuming that the second tranche is completed and conditions for the third tranche are satisfied or waived, the Company could receive up to an additional $80 million in the third tranche. However, the amount of cash received in the third tranche would depend on the extent to which the Investors elect to fund the third tranche through a “cashless” or net settlement basis. Therefore, total gross proceeds from the offering to the Company could actually be between $80 million and $240 million. Gross proceeds to the Company will be reduced by fees paid to the placement agents, capital markets advisors and payments of transaction expenses. The Company intends to use the net proceeds from the Private Placement to fund the ongoing clinical development of its three lead product candidates, vidofludimus calcium (IMU-838), IMU-856 and IMU-381, and for other general corporate purposes.
The Shares of Common Stock, the Pre-Funded Warrants, and the shares of Common Stock issuable upon the exercise of the Pre-Funded Warrants (the “Warrant Shares”), have not been registered under the Securities Act of 1933, as amended (the “Securities Act”), and are offered pursuant to the exemption from registration provided in Section 4(a)(2) under the Securities Act and Rule 506 of Regulation D promulgated thereunder.
The second and third tranches are also conditioned upon the Company obtaining stockholder approval to amend its certificate of incorporation to increase its authorized shares of common stock from 130 million to 500 million. The Company is obligated to file a proxy statement within 21 calendar days of the closing of the first tranche and to convene a special meeting of its stockholders to obtain such approval (the “Special Meeting”).
The Securities Purchase Agreement provides for the registration for resale by the Investors of the Common Stock (including the Warrant Shares) pursuant to a registration statement (the “Registration Statement”) to be filed with the Securities and Exchange Commission (the “SEC”) within 20 calendar days from the filing of the preliminary proxy statement for the Special Meeting (the “Filing Date”). The Company has agreed to use its best efforts to cause the Registration Statement to be declared effective as soon as possible, but in no event later than 75 days after the first closing of the Private Placement (or 120 days in the event of a full review of the Registration Statement by the SEC) (the “Effectiveness Date”). The Registration Statement will need to be amended (or additional Registration Statements will need to be filed) to provide for the resale of Common Stock (and Warrant Shares) issued in the second and third tranches. The Company is obligated to keep all Registration Statements continuously effective from the date on which the SEC declares each Registration Statement to be effective until such date that all Registrable Securities (as such term is defined in the Securities Purchase Agreement) covered by such Registration Statement have been sold pursuant to a registration statement under the Securities Act or under Rule 144 as promulgated by the SEC under the Securities Act, or otherwise shall have ceased to be Registrable Securities.
Leerink Partners is acting as the lead placement agent and Ladenburg Thalmann is acting as a placement agent in connection with the Private Placement. Piper Sandler, B. Riley Securities and Brookline Capital Markets, a division of Arcadia Securities, LLC, are acting as capital markets advisors to the Company.
The foregoing description of the Securities Purchase Agreement and the Pre-Funded Warrants does not purport to be complete and is qualified in its entirety by reference to the complete text of the Securities Purchase Agreement and the form of the Pre-Funded Warrant, which are attached hereto as Exhibits 10.1 and 4.1, respectively, and are hereby incorporated by reference into this Item 1.01.
Item 3.02 Unregistered Sales of Equity Securities.
The information contained above under Item 1.01 is hereby incorporated by reference in response to this Item 3.02 of this Current Report on Form 8-K.
The Company will sell the securities to “accredited investors,” as that term is defined in the Securities Act, in reliance on the exemption from registration afforded by Section 4(a)(2) of the Securities Act and Rule 506 of Regulation D promulgated under the Securities Act and corresponding provisions of state securities or “blue sky” laws. The Investors represented that they are acquiring the Common Stock (or Pre-Funded Warrants) for investment only and not with a view towards the resale or distribution thereof in violation of the Securities Act. Accordingly, the Common Stock, the Pre-Funded Warrants and the Warrant Shares have not been registered under the Securities Act and may not be offered or sold in the United States absent registration or an exemption from registration under the Securities Act and any applicable state securities laws.
Neither this Current Report on Form 8-K, nor any exhibit attached hereto, is an offer to sell or the solicitation of an offer to buy the Securities described herein.
Item 7.01. Regulation FD Disclosure.
On January 5, 2024, the Company issued a press release announcing the Private Placement. The press release is attached as Exhibit 99.1 to this Current Report on Form 8-K and incorporated into this Item 7.01 by reference.
The information in this Item 7.01, including Exhibit 99.1 attached hereto, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act, except as expressly set forth by specific reference in such filing.
Item 8.01. Other Events
On January 5, 2024, the Company issued a press release and presentation highlighting 2023 accomplishments and upcoming milestones and providing a corporate update. The press release and presentation are attached as Exhibits 99.2 and 99.3 to this Current Report on Form 8-K and are incorporated herein by reference.
Forward Looking Statements
This report contains certain forward-looking statements regarding the business of the Company that are not a description of historical facts within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include statements regarding the expected amounts and uses of proceeds of the offering, the satisfaction of conditions and completion of multiple tranches of the offering, future operations, future financial position, future revenue, projected expenses, sufficiency of cash and expected cash runway, expected timing and results of clinical trials, prospects, plans and objectives of management are forward-looking statements. Examples of such statements include, but are not limited to, statements relating to Immunic’s three development programs and the targeted diseases, the potential for Immunic’s development programs to safely and effectively target diseases, interpretation of preclinical and clinical data for Immunic’s development programs and potential effects, the timing of current and future clinical trials and anticipated clinical milestones, the nature, strategy and focus of the Company and further updates with respect thereto, the development and commercial potential of any product candidates of the Company, and the Company’s expected cash runway. Immunic may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in the forward-looking statements and you should not place undue reliance on these forward-looking statements. Such statements are based on management’s current expectations and involve substantial risks and uncertainties. Actual results and performance could differ materially from those projected in the forward-looking statements as a result of many factors, including, without limitation, the COVID-19 pandemic, impacts of the Ukraine – Russia conflict and the conflict in the Middle East on clinical trials, risks and uncertainties associated with the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations, the availability when needed of sufficient financial and other resources on acceptable terms to meet business objectives and operational requirements, the fact that the results of earlier preclinical studies and clinical trials may not be predictive of future clinical trial results, the protection and potential market exclusivity provided by Immunic’s intellectual property, risks related to the drug development and the regulatory approval process and the impact of competitive products and technological changes. A further list and descriptions of these risks, uncertainties and other factors can be found in the section captioned “Risk Factors,” in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed with the SEC on February 23, 2023, and in the Company’s subsequent filings with the SEC. Copies of these filings are available online at www.sec.gov or ir.imux.com/sec-filings. Any forward-looking statement made in this release speaks only as of the date of this report.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
|
Exhibit No. |
Description | |
| 4.1 | Form of Pre-Funded Warrant | |
| 10.1 | Securities Purchase Agreement, dated January 4, 2024, by and among the Company and the Investors | |
| 99.1 | Press release issued by the Company on January 5, 2024, furnished herewith. | |
| 99.2 | Press release issued by the Company on January 5, 2024, furnished herewith. | |
| 99.3 | Presentation, dated January 5, 2024, furnished herewith. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, hereunto duly authorized.
| Dated: January 5, 2024 | Immunic, Inc. | |
| By: | /s/ Daniel Vitt | |
| Daniel Vitt | ||
| Chief Executive Officer | ||
EXHIBIT A
NEITHER THIS SECURITY NOR THE SECURITIES FOR WHICH THIS SECURITY IS EXERCISABLE HAVE BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR THE SECURITIES LAWS OF ANY STATE OF THE UNITED STATES. THE SECURITIES MAY NOT BE SOLD, OFFERED FOR SALE, PLEDGED, HYPOTHECATED, TRANSFERRED OR ASSIGNED IN THE ABSENCE OF AN EFFECTIVE REGISTRATION STATEMENT FOR THE SECURITIES UNDER APPLICABLE SECURITIES LAWS, OR UNLESS OFFERED, SOLD, PLEDGED, HYPOTHECATED OR TRANSFERRED PURSUANT TO AN AVAILABLE EXEMPTION FROM THE REGISTRATION REQUIREMENTS OF THOSE LAWS. THE COMPANY SHALL BE ENTITLED TO REQUIRE AN OPINION OF COUNSEL SATISFACTORY TO THE COMPANY THAT SUCH REGISTRATION IS NOT REQUIRED TO THE EXTENT THAT SUCH OPINION IS REQUIRED PURSUANT TO THAT CERTAIN SECURITIES PURCHASE AGREEMENT UNDER WHICH THE SECURITIES WERE ISSUED.
PRE-FUNDED WARRANT TO PURCHASE COMMON STOCK
IMMUNIC, INC.
Warrant Shares: [●]
Issue Date: January [●], 2024
THIS PRE-FUNDED WARRANT TO PURCHASE COMMON STOCK (the “Warrant”) certifies that, for value received, [●] or its assigns (the “Holder”) is entitled, upon the terms and subject to the limitations on exercise and the conditions hereinafter set forth, at any time on or after the date hereof (the “Initial Exercise Date”) until this Warrant is exercised in full (the “Termination Date”), to subscribe for and purchase from IMMUNIC, INC., a Delaware corporation (the “Company”), up to [●] shares of common stock, par value $0.0001 per share (the “Common Stock”) (as subject to adjustment hereunder, the “Warrant Shares”). The purchase price of one share of Common Stock under this Warrant shall be equal to the Exercise Price, as defined in Section 2(b).
Section 1. Definitions. Capitalized terms used and not otherwise defined herein shall have the meanings set forth in that certain Securities Purchase Agreement (the “Purchase Agreement”), dated January 4, 2024, among the Company and the purchasers signatory thereto.
Section 2. Exercise.
(a) Exercise of Warrant. Exercise of the purchase rights represented by this Warrant may be made, in whole or in part, at any time or times on or after the Initial Exercise Date and on or before the Termination Date by delivery to the Company of a duly executed facsimile copy or PDF copy submitted by e-mail (or e-mail attachment) of the Notice of Exercise in the form annexed hereto (the “Notice of Exercise”). Within the earlier of (i) two (2) Trading Days and (ii) the number of Trading Days comprising the Standard Settlement Period (as defined in Section 2(d)(i) herein) following the date of exercise as aforesaid, the Holder shall deliver the aggregate Exercise Price for the Warrant Shares specified in the applicable Notice of Exercise by wire transfer or cashier’s check drawn on a United States bank unless the cashless exercise procedure specified in Section 2(c) below is specified in the applicable Notice of Exercise. No ink-original Notice of Exercise shall be required, nor shall any medallion guarantee (or other type of guarantee or notarization) of any Notice of Exercise be required. Notwithstanding anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company . Partial exercises of this Warrant resulting in purchases of a portion of the total number of Warrant Shares available hereunder shall have the effect of lowering the outstanding number of Warrant Shares purchasable hereunder in an amount equal to the applicable number of Warrant Shares purchased. The Holder and the Company shall maintain records showing the number of Warrant Shares purchased and the date of such purchases. The Company shall deliver any objection to any Notice of Exercise within one (1) Trading Day of receipt of such notice. The Holder and any assignee, by acceptance of this Warrant, acknowledge and agree that, by reason of the provisions of this paragraph, following the purchase of a portion of the Warrant Shares hereunder, the number of Warrant Shares available for purchase hereunder at any given time may be less than the amount stated on the face hereof.
(b) Exercise Price. The aggregate exercise price of this Warrant, except for a nominal exercise price of $0.0001 per Warrant Share, was pre-funded to the Company on or prior to the Initial Exercise Date and, consequently, no additional consideration (other than the nominal exercise price of $0.0001 per Warrant Share) shall be required to be paid by the Holder to any Person to effect any exercise of this Warrant. The Holder shall not be entitled to the return or refund of all, or any portion, of such pre-paid aggregate exercise price under any circumstance or for any reason whatsoever. The remaining unpaid exercise price per share of Common Stock under this Warrant shall be $0.0001, subject to adjustment hereunder (the “Exercise Price”).
(c) Cashless Exercise. This Warrant may also be exercised, in whole or in part, at such time by means of a “cashless exercise” in which the Holder shall be entitled to receive a number of Warrant Shares equal to the quotient obtained by dividing [(A-B) (X)] by (A), where:
(A) = as applicable: (i) the VWAP on the Trading Day immediately preceding the date of the applicable Notice of Exercise if such Notice of Exercise is (1) both executed and delivered pursuant to Section 2(a) hereof on a day that is not a Trading Day or (2) both executed and delivered pursuant to Section 2(a) hereof on a Trading Day prior to the opening of “regular trading hours” (as defined in Rule 600(b) of Regulation NMS promulgated under the federal securities laws) on such Trading Day, (ii) at the option of the Holder, either (y) the VWAP on the Trading Day immediately preceding the date of the applicable Notice of Exercise or (z) the Bid Price of the Common Stock on the principal Trading Market as reported by Bloomberg L.P. (“Bloomberg”) as of the time of the Holder’s execution of the applicable Notice of Exercise if such Notice of Exercise is executed during “regular trading hours” on a Trading Day and is delivered within two (2) hours thereafter (including until two (2) hours after the close of “regular trading hours” on a Trading Day) pursuant to Section 2(a) hereof or (iii) the VWAP on the date of the applicable Notice of Exercise if the date of such Notice of Exercise is a Trading Day and such Notice of Exercise is both executed and delivered pursuant to Section 2(a) hereof after the close of “regular trading hours” on such Trading Day;
(B) = the Exercise Price of this Warrant, as adjusted hereunder; and (X) = the number of Warrant Shares that would be issuable upon exercise of this Warrant in accordance with the terms of this Warrant if such exercise were by means of a cash exercise rather than a cashless exercise.
If Warrant Shares are issued in such a cashless exercise, the parties acknowledge and agree that in accordance with Section 3(a)(9) of the Securities Act, the Warrant Shares shall take on the characteristics of the Warrants being exercised, and the holding period of this Warrant may be tacked on to the holding period of the Warrant Shares. The Company agrees not to take any position contrary to this Section 2(c).
“Bid Price” means, for any date, the price determined by the first of the following clauses that applies: (a) if the Common Stock is then listed or quoted on a Trading Market, the bid price of the Common Stock for the time in question (or the nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg (based on a Trading Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if OTCQB or OTCQX is not a Trading Market, the volume weighted average price of the Common Stock for such date (or the nearest preceding date) on OTCQB or OTCQX as applicable, (c) if the Common Stock is not then listed or quoted for trading on OTCQB or OTCQX and if prices for the Common Stock is then reported on The Pink Open Market (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price per share of Common Stock so reported, or (d) in all other cases, the fair market value of a share of Common Stock as determined by an independent appraiser selected in good faith by the Holders of a majority in interest of the Securities then outstanding and reasonably acceptable to the Company, the fees and expenses of which shall be paid by the Company.
“VWAP” means, for any date, the price determined by the first of the following clauses that applies: (a) if the Common Stock is then listed or quoted on a Trading Market, the daily volume weighted average price of the Common Stock for such date (or the nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg (based on a Trading Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if OTCQB or OTCQX is not a Trading Market, the volume weighted average price of the Common Stock for such date (or the nearest preceding date) on OTCQB or OTCQX as applicable, (c) if the Common Stock is not then listed or quoted for trading on OTCQB or OTCQX and if prices for the Common Stock is then reported on The Pink Open Market (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price per share of Common Stock so reported, or (d) in all other cases, the fair market value of a share of Common Stock as determined by an independent appraiser selected in good faith by the Holders of a majority in interest of the Securities then outstanding and reasonably acceptable to the Company, the fees and expenses of which shall be paid by the Company.
(d) Mechanics of Exercise.
(i) Delivery of Warrant Shares Upon Exercise. The Company shall cause the Warrant Shares purchased hereunder to be transmitted by the Transfer Agent to the Holder by crediting the account of the Holder’s or its designee’s balance account with The Depository Trust Company through its Deposit or Withdrawal at Custodian system (“DWAC”) if the Company is then a participant in such system and either (A) there is an effective registration statement permitting the issuance of the Warrant Shares to or resale of the Warrant Shares by the Holder or (B) the Warrant Shares are eligible for resale by the Holder pursuant to Rule 144 (assuming cashless exercise of the Warrants), and otherwise by physical delivery of a certificate, registered in the Company’s share register in the name of the Holder or its designee, for the number of Warrant Shares to which the Holder is entitled pursuant to such exercise to the address specified by the Holder in the Notice of Exercise by the date that is the later of (i) the earlier of (a) two (2) Trading Days after the delivery to the Company of the Notice of Exercise and (b) the number of Trading Days comprising the Standard Settlement Period after the delivery to the Company of the Notice of Exercise, and (ii) one (1) Trading Day after delivery of the aggregate Exercise Price to the Company (providing the foregoing clause (ii) shall not apply in the event of a cashless exercise) (such date, the “Warrant Share Delivery Date”). Upon delivery of the Notice of Exercise, the Holder shall be deemed for all corporate purposes to have become the holder of record of the Warrant Shares with respect to which this Warrant has been exercised, irrespective of the date of delivery of the Warrant Shares, provided that payment of the aggregate Exercise Price (other than in the case of a cashless exercise) is received within the earlier of (i) two (2) Trading Days and (ii) the number of Trading Days comprising the Standard Settlement Period following delivery of the Notice of Exercise. If the Company fails for any reason to deliver to the Holder the Warrant Shares subject to a Notice of Exercise by the Warrant Share Delivery Date, the Company shall pay to the Holder, in cash, as liquidated damages and not as a penalty, for each $1,000 of Warrant Shares subject to such exercise (based on the VWAP of the Common Stock on the date of the applicable Notice of Exercise), $10 per Trading Day (increasing to $20 per Trading Day on the fifth Trading Day after such liquidated damages begin to accrue) for each Trading Day after such Warrant Share Delivery Date until such Warrant Shares are delivered or Holder rescinds such exercise. The Company agrees to maintain a transfer agent that is a participant in the FAST program so long as this Warrant remains outstanding and exercisable. As used herein, “Standard Settlement Period” means the standard settlement period, expressed in a number of Trading Days, on the Company’s primary Trading Market with respect to the Common Stock as in effect on the date of delivery of the Notice of Exercise.
(ii) Delivery of New Warrants Upon Exercise. If this Warrant shall have been exercised in part, the Company shall, at the request of a Holder and upon surrender of this Warrant certificate, at the time of delivery of the Warrant Shares, deliver to the Holder a new Warrant evidencing the rights of the Holder to purchase the unpurchased Warrant Shares called for by this Warrant, which new Warrant shall in all other respects be identical with this Warrant.
(iii) Rescission Rights. If the Company fails to cause the Transfer Agent to transmit to the Holder the Warrant Shares pursuant to Section 2(d)(i) by the Warrant Share Delivery Date, then the Holder will have the right to rescind such exercise.
(iv) Compensation for Buy-In on Failure to Timely Deliver Warrant Shares Upon Exercise. In addition to any other rights available to the Holder, if the Company fails to cause the Transfer Agent to transmit to the Holder the Warrant Shares in accordance with the provisions of Section 2(d)(i) above pursuant to an exercise on or before the Warrant Share Delivery Date, and if after such date the Holder is required by its broker to purchase (in an open market transaction or otherwise) or the Holder’s brokerage firm otherwise purchases, shares of Common Stock to deliver in satisfaction of a sale by the Holder of the Warrant Shares which the Holder anticipated receiving upon such exercise (a “Buy-In”), then the Company shall pay in cash to the Holder the amount, if any, by which (x) the Holder’s total purchase price (including brokerage commissions, if any) for the shares of Common Stock so purchased exceeds (y) the product of (1) the lesser of the (a) the number of shares of Common Stock so purchased and (b) the aggregate number of Warrant Shares that the Company was required to deliver to the Holder in connection with the exercise at issue multiplied by (2) the actual sale price at which the sell order giving rise to such purchase obligation was executed (including any brokerage commissions). For example, if a Holder purchases shares of Common Stock having a total purchase price of $11,000 to cover a Buy-In with respect to an attempted exercise of Common Stock with an aggregate sale price giving rise to such purchase obligation of $10,000, the Company shall be required to pay the Holder $1,000. The Holder shall provide the Company written notice after the occurrence of a Buy-In, indicating the amounts payable to the Holder in respect of the Buy-In together with applicable confirmations and other evidence reasonably requested by the Company. Nothing herein shall limit a Holder’s right to pursue any other remedies available to it hereunder, at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief with respect to the Company’s failure to timely deliver the Common Stock upon exercise of the Warrant as required pursuant to the terms hereof.
(v) No Fractional Shares or Scrip. No fractional shares or scrip representing fractional shares shall be issued upon the exercise of this Warrant. As to any fraction of a share which the Holder would otherwise be entitled to purchase upon such exercise, the Company shall, at its election, either pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the Exercise Price or round up to the next whole share.
(vi) Charges, Taxes and Expenses. Issuance of Warrant Shares shall be made without charge to the Holder for any issue or transfer tax or other incidental expense in respect of the issuance of such Warrant Shares, all of which taxes and expenses shall be paid by the Company, and such Warrant Shares shall be issued in the name of the Holder or in such name or names as may be directed by the Holder; provided, however, that, in the event that Warrant Shares are to be issued in a name other than the name of the Holder, this Warrant when surrendered for exercise shall be accompanied by the Assignment Form attached hereto duly executed by the Holder and the Company may require, as a condition thereto, the payment of a sum sufficient to reimburse it for any transfer tax incidental thereto. The Company shall pay all Transfer Agent fees required for same-day processing of any Notice of Exercise and all fees to the Depository Trust Company (or another established clearing corporation performing similar functions) required for same-day electronic delivery of the Warrant Shares.
(vii) Closing of Books. The Company will not close its stockholder books or records in any manner which prevents the timely exercise of this Warrant, pursuant to the terms hereof.
(e) Holder’s Exercise Limitations. Notwithstanding anything to the contrary herein, a Holder shall not have the right to exercise any portion of this Warrant, pursuant to Section 2 or otherwise, to the extent that after giving effect to such issuance after exercise as set forth on the applicable Notice of Exercise, the Holder (together with its Attribution Parties), would beneficially own in excess of the Beneficial Ownership Limitation. For purposes of the foregoing sentence, the number of shares of Common Stock beneficially owned by the Holder and its Attribution Parties shall include the number of shares of Common Stock issuable upon exercise of this Warrant with respect to which such determination is being made, but shall exclude the number of shares of Common Stock which would be issuable upon (i) exercise of the remaining, nonexercised portion of this Warrant beneficially owned by the Holder or any of its Attribution Parties and (ii) exercise or conversion of the unexercised or nonconverted portion of any other securities of the Company subject to a limitation on conversion or exercise analogous to the limitation contained herein beneficially owned by the Holder or any of its Attribution Parties. Except as set forth in the preceding sentence, for purposes of this Section 2(e), beneficial ownership shall be calculated in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated thereunder, it being acknowledged by the Holder that the Company is not representing to the Holder that such calculation is in compliance with Section 13(d) of the Exchange Act and the Holder is solely responsible for any schedules required to be filed in accordance therewith. To the extent that the limitation contained in this Section 2(e) applies, the determination of whether this Warrant is exercisable (in relation to other securities owned by the Holder together with its Attribution Parties) and of which portion of this Warrant is exercisable shall be in the sole discretion of the Holder, and the submission of a Notice of Exercise shall be deemed to be the Holder’s determination of whether this Warrant is exercisable (in relation to other securities owned by the Holder together and its Attribution Parties) and of which portion of this Warrant is exercisable, in each case subject to the Beneficial Ownership Limitation, and the Company shall have no obligation to verify or confirm the accuracy of such determination. In addition, a determination as to any group status as contemplated above shall be determined in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated thereunder. For purposes of this Section 2(e), in determining the number of outstanding shares of Common Stock, a Holder may rely on the number of outstanding shares of Common Stock as reflected in (A) the Company’s most recent periodic or annual report filed with the SEC, as the case may be, (B) a more recent public announcement by the Company or (C) a more recent written notice by the Company or the Transfer Agent setting forth the number of shares of Common Stock outstanding. Upon the written or oral request of a Holder, the Company shall within one Trading Day confirm orally and in writing to the Holder the number of shares of Common Stock then outstanding. In any case, the number of outstanding shares of Common Stock shall be determined after giving effect to the conversion or exercise of securities of the Company, including this Warrant, by the Holder or its Affiliates or Attribution Parties since the date as of which such number of outstanding shares of Common Stock was reported. The Holder, upon notice to the Company, may increase or decrease the Beneficial Ownership Limitation provisions of this Section 2(e), provided that the Beneficial Ownership Limitation in no event exceeds 19.99% of the number of shares of Common Stock outstanding immediately after giving effect to the issuance of shares of Common Stock upon exercise of this Warrant held by the Holder and the provisions of this Section 2(e) shall continue to apply. Any increase in the Beneficial Ownership Limitation will not be effective until the 61st day after such notice is delivered to the Company. The provisions of this paragraph shall be construed and implemented in a manner otherwise than in strict conformity with the terms of this Section 2(e) to correct this paragraph (or any portion hereof) which may be defective or inconsistent with the intended Beneficial Ownership Limitation herein contained or to make changes or supplements necessary or desirable to properly give effect to such limitation. The limitations contained in this paragraph shall apply to a successor holder of this Warrant.
Section 3. Certain Adjustments.
(a) Stock Dividends and Splits. If the Company, at any time while this Warrant is outstanding: (i) pays a stock dividend or otherwise makes a distribution or distributions on shares of its Common Stock or any other equity or equity equivalent securities payable in shares of Common Stock (which, for avoidance of doubt, shall not include any shares of Common Stock issued by the Company upon exercise of this Warrant), (ii) subdivides outstanding shares of Common Stock into a larger number of shares, (iii) combines (including by way of reverse stock split) outstanding shares of Common Stock into a smaller number of shares, or (iv) issues by reclassification of shares of the Common Stock any shares of capital stock of the Company, then in each case the Exercise Price shall be multiplied by a fraction of which the numerator shall be the number of shares of Common Stock (excluding treasury shares, if any) outstanding immediately before such event and of which the denominator shall be the number of shares of Common Stock outstanding immediately after such event, and the number of shares issuable upon exercise of this Warrant shall be proportionately adjusted such that the aggregate Exercise Price of this Warrant shall remain unchanged. Any adjustment made pursuant to this Section 3(a) shall become effective immediately after the record date for the determination of stockholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or re-classification.
(b) Subsequent Rights Offerings. In addition to any adjustments pursuant to Section 3(a) above, if at any time the Company grants, issues or sells any rights to purchase stock, warrants, securities or other property pro rata to the record holders of any class of Common Stock (the “Purchase Rights”), then the Holder will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the Holder could have acquired if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the date as of which the record holders of shares of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights (provided, however, that, to the extent that the Holder’s right to participate in any such Purchase Right would result in the Holder exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate in such Purchase Right to such extent (or beneficial ownership of such shares of Common Stock as a result of such Purchase Right to such extent) and such Purchase Right to such extent shall be held in abeyance for the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding the Beneficial Ownership Limitation).
(c) Pro Rata Distributions. During such time as this Warrant is outstanding and except as covered by Section 3(d) below, if the Company shall declare or make any dividend or other distribution of its assets (or rights to acquire its assets) to holders of shares of Common Stock, by way of return of capital or otherwise (including, without limitation, any distribution of cash, stock or other securities, property or options by way of a dividend, spin off, reclassification, corporate rearrangement, scheme of arrangement or other similar transaction) (a “Distribution”), at any time after the issuance of this Warrant, then, in each such case, the Holder shall be entitled to participate in such Distribution to the same extent that the Holder would have participated therein if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the date of which a record is taken for such Distribution, or, if no such record is taken, the date as of which the record holders of shares of Common Stock are to be determined for the participation in such Distribution (provided, however, that, to the extent that the Holder’s right to participate in any such Distribution would result in the Holder exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate in such Distribution to such extent (or in the beneficial ownership of any shares of Common Stock as a result of such Distribution to such extent) and the portion of such Distribution shall be held in abeyance for the benefit of the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding the Beneficial Ownership Limitation).
(d) Fundamental Transaction. If, at any time while this Warrant is outstanding, (i) the Company, directly or indirectly, in one or more related transactions effects any merger or consolidation of the Company with or into another Person, (ii) the Company (or any Subsidiary), directly or indirectly, effects any sale, lease, license, assignment, transfer, conveyance or other disposition of all or substantially all of its assets in one or a series of related transactions, (iii) any, direct or indirect, purchase offer, tender offer or exchange offer (whether by the Company or another Person) is completed pursuant to which holders of Common Stock are permitted to sell, tender or exchange their shares for other securities, cash or property and has been accepted by the holders of 50% or more of the outstanding shares of Common Stock, (iv) the Company, directly or indirectly, in one or more related transactions effects any reclassification, reorganization or recapitalization of the Common Stock or any compulsory share exchange pursuant to which the Common Stock is effectively converted into or exchanged for other securities, cash or property, or (v) the Company, directly or indirectly, in one or more related transactions consummates a stock or share purchase agreement or other business combination (including, without limitation, a reorganization, recapitalization, spin-off, merger or scheme of arrangement) with another Person or group of Persons whereby such other Person or group acquires securities representing more than 50% of the aggregate voting power, including the power to vote on the election of directors of the Company, of the issued and outstanding equity securities of the Company (not including any shares of Common Stock held by the other Person or other Persons making or party to, or associated or affiliated with the other Persons making or party to, such stock or share purchase agreement or other business combination) (each a “Fundamental Transaction”), then, upon any subsequent exercise of this Warrant, the Holder shall have the right to receive, for each Warrant Share that would have been issuable upon such exercise immediately prior to the occurrence of such Fundamental Transaction, at the option of the Holder (without regard to any limitation in Section 2(e) on the exercise of this Warrant), the number of shares of Common Stock of the successor or acquiring corporation or of the Company, if it is the surviving corporation, and any additional consideration (the “Alternate Consideration”) receivable as a result of such Fundamental Transaction by a holder of the number of shares of Common Stock for which this Warrant is exercisable immediately prior to such Fundamental Transaction (without regard to any limitation in Section 2(e) on the exercise of this Warrant). For purposes of any such exercise, the determination of the Exercise Price shall be appropriately adjusted to apply to such Alternate Consideration based on the amount of Alternate Consideration issuable in respect of one share of Common Stock in such Fundamental Transaction, and the Company shall apportion the Exercise Price among the Alternate Consideration in a reasonable manner reflecting the relative value of any different components of the Alternate Consideration. If holders of Common Stock are given any choice as to the securities, cash or property to be received in a Fundamental Transaction, then the Holder shall be given the same choice as to the Alternate Consideration it receives upon any exercise of this Warrant following such Fundamental Transaction. The Company shall cause any successor entity in a Fundamental Transaction in which the Company is not the survivor (the “Successor Entity”) to assume in writing all of the obligations of the Company under this Warrant prior to such Fundamental Transaction and shall, at the option of the Holder, deliver to the Holder in exchange for this Warrant a security of the Successor Entity evidenced by a written instrument substantially similar in form and substance to this Warrant which is exercisable for a corresponding number of shares of capital stock of such Successor Entity (or its parent entity) equivalent to the shares of Common Stock acquirable and receivable upon exercise of this Warrant (without regard to any limitations on the exercise of this Warrant) prior to such Fundamental Transaction, and with an exercise price which applies the exercise price hereunder to such shares of capital stock (but taking into account the relative value of the shares of Common Stock pursuant to such Fundamental Transaction and the value of such shares of capital stock, such number of shares of capital stock and such exercise price being for the purpose of protecting the economic value of this Warrant immediately prior to the consummation of such Fundamental Transaction), and which is reasonably satisfactory in form and substance to the Holder. Upon the occurrence of any such Fundamental Transaction, the Successor Entity shall succeed to, and be substituted for (so that from and after the date of such Fundamental Transaction, the provisions of this Warrant referring to the “Company” shall refer instead to the Successor Entity), and may exercise every right and power of the Company and shall assume all of the obligations of the Company under this Warrant with the same effect as if such Successor Entity had been named as the Company herein.
(e) Calculations. All calculations under this Section 3 shall be made to the nearest cent or the nearest 1/100th of a share, as the case may be. For purposes of this Section 3, the number of shares of Common Stock deemed to be issued and outstanding as of a given date shall be the sum of the number of shares of Common Stock (excluding treasury shares, if any) issued and outstanding.
(f) Notice to Holder.
(i) Adjustment to Exercise Price. Whenever the Exercise Price is adjusted pursuant to any provision of this Section 3, the Company shall promptly deliver to the Holder by facsimile or email a notice setting forth the Exercise Price after such adjustment and any resulting adjustment to the number of Warrant Shares and setting forth a brief statement of the facts requiring such adjustment.
(ii) Notice to Allow Exercise by Holder. If (A) the Company shall declare a dividend (or any other distribution in whatever form) on the Common Stock, (B) the Company shall declare a special nonrecurring cash dividend on or a redemption of the Common Stock, (C) the Company shall authorize the granting to all holders of the Common Stock rights or warrants to subscribe for or purchase any shares of capital stock of any class or of any rights, (D) the approval of any stockholders of the Company shall be required in connection with any reclassification of the Common Stock, any consolidation or merger to which the Company (or any of its Subsidiaries) is a party, any sale or transfer of all or substantially all of its assets, or any compulsory share exchange whereby the Common Stock is converted into other securities, cash or property, or (E) the Company shall authorize the voluntary or involuntary dissolution, liquidation or winding up of the affairs of the Company, then, in each case, the Company shall cause to be delivered by facsimile or email to the Holder at its last facsimile number or email address as it shall appear upon the Warrant Register of the Company, at least 10 calendar days prior to the applicable record or effective date hereinafter specified, a notice stating (x) the date on which a record is to be taken for the purpose of such dividend, distribution, redemption, rights or warrants, or if a record is not to be taken, the date as of which the holders of the Common Stock of record to be entitled to such dividend, distributions, redemption, rights or warrants are to be determined or (y) the date on which such reclassification, consolidation, merger, sale, transfer or share exchange is expected to become effective or close, and the date as of which it is expected that holders of the Common Stock of record shall be entitled to exchange their shares of Common Stock for securities, cash or other property deliverable upon such reclassification, consolidation, merger, sale, transfer or share exchange; provided that the failure to deliver such notice or any defect therein or in the delivery thereof shall not affect the validity of the corporate action required to be specified in such notice. To the extent that any notice provided in this Warrant constitutes, or contains, material, non-public information regarding the Company or any of the Subsidiaries, the Company shall simultaneously file such notice with the SEC pursuant to a Current Report on Form 8-K.The Holder shall remain entitled to exercise this Warrant during the period commencing on the date of such notice to the effective date of the event triggering such notice except as may otherwise be expressly set forth herein.
Section 4. Transfer of Warrant.
(a) Transferability. Subject to compliance with any applicable securities laws and the conditions set forth herein, this Warrant and all rights hereunder (including, without limitation, any registration rights) are transferable, in whole or in part, upon surrender of this Warrant at the principal office of the Company or its designated agent, together with a written assignment of this Warrant substantially in the form attached hereto duly executed by the Holder or its agent or attorney and funds sufficient to pay any transfer taxes payable upon the making of such transfer. Upon such surrender and, if required, such payment, the Company shall execute and deliver a new Warrant or Warrants in the name of the assignee or assignees, as applicable, and in the denomination or denominations specified in such instrument of assignment, and shall issue to the assignor a new Warrant evidencing the portion of this Warrant not so assigned, and this Warrant shall promptly be cancelled. Notwithstanding anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company unless the Holder has assigned this Warrant in full, in which case, the Holder shall surrender this Warrant to the Company within three (3) Trading Days of the date on which the Holder delivers an assignment form to the Company assigning this Warrant in full. The Warrant, if properly assigned in accordance herewith, may be exercised by a new holder for the purchase of Warrant Shares without having a new Warrant issued.
(b) New Warrants. This Warrant may be divided or combined with other Warrants upon presentation hereof at the aforesaid office of the Company, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed by the Holder or its agent or attorney. Subject to compliance with Section 4(a), as to any transfer which may be involved in such division or combination, the Company shall execute and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants to be divided or combined in accordance with such notice. All Warrants issued on transfers or exchanges shall be dated the original Issue Date and shall be identical with this Warrant except as to the number of Warrant Shares issuable pursuant thereto.
(c) Warrant Register. The Company shall register this Warrant, upon records to be maintained by the Company for that purpose (the “Warrant Register”), in the name of the record Holder hereof from time to time. The Company may deem and treat the registered Holder of this Warrant as the absolute owner hereof for the purpose of any exercise hereof or any distribution to the Holder, and for all other purposes, absent actual notice to the contrary.
(d) Representation by the Holder. The Holder, by the acceptance hereof, represents and warrants that it is acquiring this Warrant and, upon any exercise hereof, will acquire the Warrant Shares issuable upon such exercise, for its own account and not with a view to or for distributing or reselling such Warrant Shares or any part thereof in violation of the Securities Act or any applicable state securities law, except pursuant to sales registered or exempted under the Securities Act.
Section 5. Miscellaneous.
(a) No Rights as Stockholder Until Exercise; No Settlement in Cash. This Warrant does not entitle the Holder to any voting rights, dividends or other rights as a stockholder of the Company prior to the exercise hereof as set forth in Section 2(d)(i), except as expressly set forth in Section 3. Without limiting any rights of a Holder to receive Warrant Shares on a “cashless exercise” pursuant to Section 2(c) or to receive cash payments pursuant to Section 2(d)(i) and Section 2(d)(iv) herein, in no event shall the Company be required to net cash settle an exercise of this Warrant.
(b) Loss, Theft, Destruction or Mutilation of Warrant. The Company covenants that upon receipt by the Company of evidence reasonably satisfactory to it of the loss, theft, destruction or mutilation of this Warrant or any stock certificate relating to the Warrant Shares, and in case of loss, theft or destruction, of indemnity or security reasonably satisfactory to it (which, in the case of the Warrant, shall not include the posting of any bond), and upon surrender and cancellation of such Warrant or stock certificate, if mutilated, the Company will make and deliver a new Warrant or stock certificate of like tenor and dated as of such cancellation, in lieu of such Warrant or stock certificate.
(c) Saturdays, Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of any right required or granted herein shall not be a Trading Day, then such action may be taken, or such right may be exercised on the next succeeding Trading Day.
(d) Authorized Shares. The Company covenants that it will, during the period the Warrant is outstanding, reserve from its authorized and unissued Common Stock a sufficient number of shares to provide for the issuance of the Warrant Shares upon the exercise of any purchase rights under this Warrant. The Company further covenants that its issuance of this Warrant shall constitute full authority to its officers who are charged with the duty of issuing the necessary Warrant Shares upon the exercise of the purchase rights under this Warrant. The Company will take all such reasonable action as may be necessary to assure that such Warrant Shares may be issued as provided herein without violation of any applicable law or regulation, or of any requirements of the Trading Market upon which the Common Stock may be listed. The Company covenants that all Warrant Shares which may be issued upon the exercise of the purchase rights represented by this Warrant will, upon exercise of the purchase rights represented by this Warrant and payment for such Warrant Shares in accordance herewith, be duly authorized, validly issued, fully paid and nonassessable and free from all taxes, liens and charges created by the Company in respect of the issue thereof (other than taxes in respect of any transfer occurring contemporaneously with such issue). Except and to the extent as waived or consented to by the Holder, the Company shall not by any action, including, without limitation, amending its certificate of incorporation or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities or any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms of this Warrant, but will at all times in good faith assist in the carrying out of all such terms and in the taking of all such actions as may be necessary or appropriate to protect the rights of Holder as set forth in this Warrant against impairment. Without limiting the generality of the foregoing, the Company will (i) not increase the par value of any Warrant Shares above the amount payable therefor upon such exercise immediately prior to such increase in par value, (ii) take all such action as may be necessary or appropriate in order that the Company may validly and legally issue fully paid and nonassessable Warrant Shares upon the exercise of this Warrant and (iii) use commercially reasonable efforts to obtain all such authorizations, exemptions or consents from any public regulatory body having jurisdiction thereof, as may be, necessary to enable the Company to perform its obligations under this Warrant. Before taking any action that would result in an adjustment in the number of Warrant Shares for which this Warrant is exercisable or in the Exercise Price, the Company shall obtain all such authorizations or exemptions thereof, or consents thereto, as may be necessary from any public regulatory body or bodies having jurisdiction thereof.
(e) Jurisdiction. All questions concerning the construction, validity, enforcement and interpretation of this Warrant shall be determined in accordance with the provisions of the Purchase Agreement.
(f) Restrictions. The Holder acknowledges that the Warrant Shares acquired upon the exercise of this Warrant, if not registered, and the Holder does not utilize cashless exercise, will have restrictions upon resale imposed by state and federal securities laws.
(g) Nonwaiver and Expenses. No course of dealing or any delay or failure to exercise any right hereunder on the part of Holder shall operate as a waiver of such right or otherwise prejudice the Holder’s rights, powers or remedies. Without limiting any other provision of this Warrant or the Purchase Agreement, if the Company willfully and knowingly fails to comply with any provision of this Warrant, which results in any material damages to the Holder, the Company shall pay to the Holder such amounts as shall be sufficient to cover any costs and expenses including, but not limited to, reasonable attorneys’ fees, including those of appellate proceedings, incurred by the Holder in collecting any amounts due pursuant hereto or in otherwise enforcing any of its rights, powers or remedies hereunder.
(h) Notices. All notices required or permitted hereunder shall be in writing and shall be deemed effectively given: (a) upon personal delivery to the party to be notified, (b) when sent by email or facsimile if sent during normal business hours of the recipient, and if sent at a time other than during normal business hours of the recipient, then on the next Business Day (provided, with respect to notices sent by email so long as such sent email is kept on file by the sending party and the sending party does not receive an automatically generated message from the recipient’s email server that such email could not be delivered to such recipient), (c) five days after having been sent by registered or certified mail, return receipt requested, postage prepaid, or (d) one Business Day after deposit with a nationally recognized overnight courier, specifying next day delivery, with written verification of receipt. The addresses for such communications are:
(i) If to the Company, to:
Immunic, Inc.
1200 Avenue of the Americas, Suite 200
New York, NY 10036
Attention: Glenn Whaley
Email: glen.whaley@imux.com
With a copy (which will not constitute notice) to:
Dentons US LLP
Ilan Katz
1221 Avenue of the Americas
New York, NY 10020
Email: Ilan.Katz@Dentons.com
(i) If to the Holder: To the address, email address or facsimile number set forth in the Warrant Register, or as otherwise provided by the Holder to the Company in accordance with this Section 5(h).
(j) Limitation of Liability. No provision hereof, in the absence of any affirmative action by the Holder to exercise this Warrant to purchase Warrant Shares, and no enumeration herein of the rights or privileges of the Holder, shall give rise to any liability of the Holder for the purchase price of any Common Stock or as a stockholder of the Company, whether such liability is asserted by the Company or by creditors of the Company.
(k) Remedies. The Holder, in addition to being entitled to exercise all rights granted by law, including recovery of damages, will be entitled to specific performance of its rights under this Warrant. The Company agrees that monetary damages would not be adequate compensation for any loss incurred by reason of a breach by it of the provisions of this Warrant and hereby agrees to waive and not to assert the defense in any action for specific performance that a remedy at law would be adequate.
(l) Successors and Assigns. Subject to applicable securities laws, this Warrant and the rights and obligations evidenced hereby shall inure to the benefit of and be binding upon the successors and permitted assigns of the Company and the successors and permitted assigns of Holder. The provisions of this Warrant are intended to be for the benefit of any Holder from time to time of this Warrant and shall be enforceable by the Holder or holder of Warrant Shares.
(m) Amendment. This Warrant may be modified or amended, or the provisions hereof waived with the written consent of the Company and the Holder.
(n) Severability. Wherever possible, each provision of this Warrant shall be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Warrant shall be prohibited by or invalid under applicable law, such provision shall be ineffective to the extent of such prohibition or invalidity, without invalidating the remainder of such provisions or the remaining provisions of this Warrant.
(o) Headings. The headings used in this Warrant are for the convenience of reference only and shall not, for any purpose, be deemed a part of this Warrant.
(Signature Page Follows)
IN WITNESS WHEREOF, the Company has caused this Warrant to be executed by its officer thereunto duly authorized as of the date first above indicated.
| IMMUNIC, INC. | ||
| By: | ||
| Name: Daniel A. Vitt | ||
| Title: Chief Executive Officer | ||
NOTICE OF EXERCISE
TO: IMMUNIC, INC.
(1) The undersigned hereby elects to purchase ________ Warrant Shares of the Company pursuant to the terms of the attached Warrant (only if exercised in full), and tenders herewith payment of the exercise price in full, together with all applicable transfer taxes, if any.
(2) Payment shall take the form of (check applicable box):
| ________ | in lawful money of the United States; or |
| ________ | the cancellation of such number of Warrant Shares as is necessary, in accordance with the formula set forth in subsection 2(c), to exercise this Warrant with respect to the maximum number of Warrant Shares purchasable pursuant to the cashless exercise procedure set forth in subsection 2(c). |
(3) Please issue said Warrant Shares in the name of the undersigned or in such other name as is specified below:
__________________________________________________
(4) By its delivery of this Notice of Exercise, the undersigned represents and warrants to the Company that in giving effect to the exercise evidenced hereby the Holder will not beneficially own in excess of the number of shares of Common Stock (as determined in accordance with Section 13(d) of the Securities Exchange Act of 1934, as amended) permitted to be owned under Section 2(e) of the Warrant to which this notice relates.
The Warrant Shares shall be delivered to the following DWAC Account Number:
_______________________________
| Name of Investing Entity:_______________________________________ |
| Signature of Authorized Signatory of Investing Entity: ___________________________________________________________ |
| Name of Authorized Signatory:___________________________________ |
| Title of Authorized Signatory:____________________________________ |
| Date:_______________________________________________________ |
ASSIGNMENT FORM
(To assign the foregoing Warrant, execute this form and supply required information. Do not use this form to purchase shares.)
FOR VALUE RECEIVED, the foregoing Warrant and all rights evidenced thereby are hereby assigned to
| Name: | |
| (Please Print) | |
| Address: | |
| Phone Number: | (Please Print) |
| Email Address: | |
| Dated: _________________ | |
| Holder’s Signature: | |
| Holder’s Address: |
17
SECURITIES PURCHASE AGREEMENT
This SECURITIES PURCHASE AGREEMENT (this “Agreement”) is made and entered into as of January 4, 2024 (the “Effective Date”) by and among Immunic, Inc., a Delaware corporation (the “Company”), and the Investors identified on Annex I attached hereto (each an “Investor” and collectively, the “Investors”).
RECITALS
A. The Company and each Investor is executing and delivering this Agreement in reliance upon the exemption from securities registration afforded by Section 4(a)(2) of the 1933 Act (as defined below) and/or Regulation D (as defined below); and
B. The Investors, severally and not jointly, wish to purchase from the Company, and the Company wishes to sell and issue to the Investors, upon the terms and subject to the conditions stated in this Agreement, (a) shares (the “Shares”) of common stock, par value $0.0001 per share (the “Common Stock”) and/or (b) pre-funded warrants in the form attached hereto as Exhibit A to purchase shares of Common Stock (each, a “Pre-Funded Warrant” and collectively, the “Pre-Funded Warrants”).
In consideration of the mutual promises made herein and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties hereto agree as follows:
1. Definitions. For the purposes of this Agreement, the following terms shall have the meanings set forth below:
“1933 Act” means the Securities Act of 1933, as amended, or any successor statute, and the rules and regulations promulgated thereunder.
“1934 Act” means the Securities Exchange Act of 1934, as amended, or any successor statute, and the rules and regulations promulgated thereunder.
“Affiliate” means, with respect to any Person, any other Person which directly or indirectly through one or more intermediaries controls, is controlled by, or is under common control with, such Person.
“Agreement” has the meaning set forth in the Preamble.
“Anti-Money Laundering Laws” has the meaning set forth in Section 4.9.
“Applicable Closing” means the First Closing, the Second Closing and/or the Third Closing, as applicable.
“Applicable Closing Date” means the First Closing Date, the Second Closing Date and/or the Third Closing Date, as applicable.
“Attribution Parties” means, with respect to any Person, such Person’s Affiliates and any other Person whose beneficial ownership of Common Stock would be aggregated with such Person’s for purposes of Section 13(d) or Section 16 of the 1934 Act and the applicable regulations of the SEC, including any “group” of which such Person is a member.
“Beneficial Ownership Limitation” means the percentage set forth opposite such Investor’s name on Annex I under the heading “Beneficial Ownership Limitation”.
“Business Day” means a day, other than a Saturday or Sunday, on which banks in New York City are open for the general transaction of business.
“Bylaws” means the Company’s Amended and Restated Bylaws, as may be amended and/or restated from time to time.
“Certificate of Incorporation” means the Company’s Amended and Restated Certificate of Incorporation, as may be amended and/or restated from time to time.
“Cleansing Release” has the meaning set forth in Section 10.7.
“Common Stock” has the meaning set forth in the Recitals.
“Company” has the meaning set forth in the Preamble.
“Company’s Knowledge” means the actual knowledge of the executive officers (as defined in Rule 405 under the 1933 Act) of the Company.
“control” (including the terms “controlling”, “controlled by” or “under common control with”) means the possession, direct or indirect, of the power to direct or cause the direction of the management and policies of a Person, whether through the ownership of voting securities, by contract or otherwise.
“Defaulting Investor” has the meaning set forth in Section 2.2.
“DTC” has the meaning set forth in Section 8.2(c).
“EDGAR system” means the Electronic Data Gathering, Analysis, and Retrieval system.
“Effective Date” has the meaning set forth in the Preamble.
“Environmental Laws” has the meaning set forth in Section 4.15.
“Evaluation Date” has the meaning set forth in Section 4.22.
“Event” has the meaning set forth in Section 8.9(g).
“Event Date” has the meaning set forth in Section 8.9(g).
“Fair Market Value” means the last trade price of the Common Stock on the Business Day prior to the Third Closing Date on the Trading Market, as reported by Bloomberg Financial Markets, or, if such Trading Market begins to operate on an extended hours basis and does not designate the last trade price, then the last trade price of such security prior to 4:00 P.M., New York City time, as reported by Bloomberg Financial Markets, or if the foregoing do not apply, the last trade price of such security in the over-the-counter market on the electronic bulletin board for such security as reported by Bloomberg Financial Markets, or, if no last trade price is reported for such security by Bloomberg Financial Markets, the average of the bid and ask prices, of any market makers for such security as reported in the “Pink Market” by the OTC Markets Group.
“FDA” has the meaning set forth in Section 4.24.
“First Closing” has the meaning set forth in Section 3.1(a).
“First Closing Date” has the meaning set forth in Section 3.1(a).
“First Closing Overage Number” has the meaning set forth in Section 2.1.
“First Closing Subscription Amount” means, as to an Investor, the aggregate amount to be paid for the Shares and/or Pre-Funded Warrants purchased hereunder at the First Closing as specified opposite such Investor’s name on Annex I attached hereto, under the column entitled “First Closing Aggregate Purchase Price,” in U.S. Dollars and in immediately available funds.
“GAAP” has the meaning set forth in Section 4.17.
“Health Care Laws” means the Federal Food, Drug, and Cosmetic Act (21 U.S.C. §§ 301 et seq.), the regulations promulgated by other similar local, state or federal law and regulations.
“Indemnified Party” has the meaning set forth in Section 9.4.
“Indemnifying Party” has the meaning set forth in Section 9.4.
“Intellectual Property” means all patents, patent applications, trademarks, trademark applications, service marks, trade names, copyrights, trade secrets, licenses, domain names, information and proprietary rights and processes.
“Intellectual Property Rights” has the meaning set forth in Section 4.14.
“Investor” has the meaning set forth in the Preamble.
“Investor Indemnified Party” has the meaning set forth in Section 9.2.
“Investor Questionnaire” has the meaning set forth in Section 3.1(a).
“Investors” has the meaning set forth in the Preamble.
“Irrevocable Transfer Agent Instructions” has the meaning set forth in Section 8.3.
“Legend Removal Date” has the meaning set forth in Section 8.2(a).
“Majority Investors” has the meaning set forth in Section 7.1(a)(i).
“Material Adverse Effect” means a material adverse effect on (i) the assets, liabilities, results of operations, condition (financial or otherwise), prospects, business or properties of the Company and the Subsidiaries taken as a whole, (ii) the legality, validity or enforceability of any of the Transaction Documents or (iii) the ability of the Company to perform its obligations under the Transaction Documents; provided, however, that in no event shall any of the following occurring after the date hereof, alone or in combination, be deemed to constitute, or be taken into account in determining whether, a Material Adverse Effect has occurred: any effect caused by the announcement or pendency of the transactions contemplated by the Transaction Documents, or the identity of any Investor or any of its Affiliates as the purchaser in connection with the transactions contemplated by this Agreement or any change in the market price of the Common Stock.
“Material Contract” means any contract, instrument or other agreement to which the Company is a party or by which it is bound which is material to the business of the Company, including those that have been filed as an exhibit to the SEC Filings pursuant to Item 601(b)(10) of Regulation S-K.
“Nasdaq” means The Nasdaq Global Select Market or any other trading market of The Nasdaq Stock Market LLC.
“Overage Number” has the meaning set forth in Section 2.1.
“Person” means an individual, corporation, partnership, limited liability company, trust, business trust, association, joint stock company, joint venture, sole proprietorship, unincorporated organization, governmental authority or any other form of entity not specifically listed herein.
“Phase 2b Release Date” means the date that the Company files with the SEC a Current Report on Form 8-K or issues a press release to publicly disclose the Company’s Phase 2b clinical trial topline data for its IMU-838 progressive multiple sclerosis clinical trial.
“Placement Agent” means Leerink Partners LLC.
“Pre-Funded Warrant Share Instructions” has the meaning set forth in Section 8.3.
“Pre-Funded Warrant Shares” means the shares of Common Stock issuable upon exercise of the Pre-Funded Warrants.
“Pre-Funded Warrants” has the meaning set forth in the Recitals.
“Proposal” has the meaning set forth in Section 8.10.
“Qualified Investor” has the meaning set forth in Section 2.2.
“Registrable Securities” means (i) the Shares, (ii) the Pre-Funded Warrant Shares and (iii) any other securities issued upon any stock split, dividend or other distribution, recapitalization or similar event with respect to the foregoing; provided, however, that any such Registrable Securities shall cease to be Registrable Securities (and the Company shall not be required to maintain the effectiveness of any, or file another, registration statement hereunder with respect thereto) upon the first to occur of (A) a registration statement with respect to the sale of such Registrable Securities being declared effective by the SEC under the 1933 Act and such Registrable Securities having been disposed of or transferred by the holder thereof in accordance with such effective Registration Statement, (B) such Registrable Securities having been previously sold or transferred in accordance with Rule 144 (or another exemption from the registration requirements of the 1933 Act), and (C) such securities becoming eligible for resale without volume or manner-of-sale restrictions and without current public information requirements pursuant to Rule 144.
“Registration Statement” has the meaning set forth in Section 8.9(a).
“Regulation D” means Regulation D as promulgated by the SEC under the 1933 Act.
“Required Approvals” has the meaning set forth in Section 4.5.
“Rule 144” means Rule 144 promulgated under the 1933 Act (or any successor rule).
“Sanctions” has the meaning set forth in Section 4.27.
“SEC” means the United States Securities and Exchange Commission.
“SEC Filings” has the meaning set forth in Section 4.7.
“Second Closing” means the closing of the purchase and sale of Shares and/or Pre-Funded Warrants on the Second Closing Date pursuant to Section 3.2 of this Agreement.
“Second Closing Date” means the Trading Day on which all conditions precedent to (i) the Investors’ obligations to pay the Second Closing Subscription Amount and (ii) the Company’s obligations to deliver Shares and/or Pre-Funded Warrants in connection with the Second Closing, in each case, have been satisfied or waived; provided, that the Second Closing Date shall be a date that is not more than five (5) Trading Days following the date on which all such conditions have been satisfied or waived.
“Second Closing Notice” has the meaning set forth in Section 3.2(a).
“Second Closing Overage Number” has the meaning set forth in Section 2.2.
“Second Closing Subscription Amount” means, as to an Investor, the aggregate amount to be paid for the Shares and/or Pre-Funded Warrants purchased hereunder at the Second Closing as specified opposite such Investor’s name on Annex I attached hereto, under the column entitled “Second Closing Aggregate Purchase Price,” in U.S. Dollars and in immediately available funds.
“Securities” means the Shares, the Pre-Funded Warrants and the Pre-Funded Warrant Shares.
“Shares” has the meaning set forth in the Recitals.
“Short Sales” means all “short sales” as defined in Rule 200 of Regulation SHO under the 1934 Act (but shall not be deemed to include the location and/or reservation of borrowable shares of Common Stock).
“Special Meeting” has the meaning set forth in Section 8.10.
“Staff” means the staff of the SEC.
“Stockholder Approval” has the meaning set forth in Section 4.5.
“Subsidiary” has the meaning set forth in Section 4.1.
“Third Closing” means the closing of the purchase and sale of Shares and/or Pre-Funded Warrants on the Third Closing Date pursuant to Section 3.3 of this Agreement.
“Third Closing Date” means the Trading Day on which all conditions precedent to (i) the Investors’ obligations to pay the Third Closing Subscription Amount and (ii) the Company’s obligations to deliver Shares and/or Pre-Funded Warrants in connection with the Third Closing, in each case, have been satisfied or waived; provided, that the Third Closing Date shall be a date that is not more than five (5) Trading Days following the date on which all such conditions have been satisfied or waived.
“Third Closing Notice” has the meaning set forth in Section 3.3(a).
“Third Closing Overage Number” has the meaning set forth in Section 2.3.
“Third Closing Subscription Amount” means, as to an Investor, (a) the aggregate amount to be paid for the Shares and/or Pre-Funded Warrants purchased hereunder at the Third Closing as specified opposite such Investor’s name on Annex I attached hereto, under the column entitled “Third Closing Aggregate Purchase Price,” in U.S. Dollars and in immediately available funds or (b) the forfeiture of the right to receive a portion of the Shares to be issued at the Third Closing pursuant to the terms of Section 3.3(d).
“Trading Day” means a day on which the shares of Common Stock are traded on Nasdaq or, if not traded on Nasdaq, a day on which the principal Trading Market on which the shares of Common Stock are traded is open for trading.
“Trading Market” means any of the following markets or exchanges on which the shares of Common Stock are listed or quoted for trading on the date in question: the NYSE American, the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market, or the New York Stock Exchange (or any successors to any of the foregoing).
“Transaction Documents” means this Agreement, including the exhibits attached hereto, the Pre-Funded Warrants, the Irrevocable Transfer Agent Instructions and any other documents or agreements executed and delivered by the Company and the Investors in connection with the transactions contemplated hereunder.
“Transfer Agent” has the meaning set forth in Section 8.2.
2. Purchase of Securities.
2.1. First Closing Purchase and Sale of Shares and Pre-Funded Warrants. On the First Closing Date, upon the terms and subject to the conditions set forth herein, the Company will issue and sell, and each Investor will purchase, severally and not jointly, the number of Shares set forth opposite the name of such Investor under the heading “First Closing Number of Shares” on Annex I attached hereto, at a price per Share equal to $1.43. In the event the number of Shares set forth in Annex I under the heading “First Closing Number of Shares” for any Investor would result in such Investor, together with its Attribution Parties, beneficially owning in excess of the Beneficial Ownership Limitation of the outstanding Common Stock immediately after the First Closing, then (a) the number of Shares otherwise issuable to such Investor at the First Closing will be reduced by the number (such number, the “First Closing Overage Number”) of Shares that would result in such Investor beneficially owning, together with its Attribution Parties, no more than the Beneficial Ownership Limitation of the outstanding Common Stock immediately after the First Closing, (b) the Company will issue to such Investor at the First Closing a Pre-Funded Warrant that is exercisable for a number of Pre-Funded Warrant Shares equal to the Overage Number, and (c) the First Closing Subscription Amount payable by such Investor at the First Closing shall be reduced by $0.0001 for each Pre-Funded Warrant Share subject to the Pre-Funded Warrant being purchased by such Investor.
2.2. Second Closing Purchase and Sale of Shares and Pre-Funded Warrants. On the Second Closing Date, upon the terms and subject to the conditions set forth herein, the Company will issue and sell, and each Investor will purchase, severally and not jointly, the number of Shares set forth opposite the name of such Investor under the heading “Second Closing Number of Shares” on Annex I attached hereto, at a price per Share equal to $1.716. In the event the number of Shares set forth in Annex I under the heading “Second Closing Number of Shares” for any Investor would result in such Investor, together with its Attribution Parties, beneficially owning in excess of the Beneficial Ownership Limitation of the outstanding Common Stock immediately after the Second Closing, then (a) the number of Shares otherwise issuable to such Investor at the Second Closing will be reduced by the number (such number, the “Second Closing Overage Number”) of Shares that would result in such Investor beneficially owning, together with its Attribution Parties, no more than the Beneficial Ownership Limitation of the outstanding Common Stock immediately after the Second Closing, (b) the Company will issue to such Investor at the Second Closing a Pre-Funded Warrant that is exercisable for a number of Pre-Funded Warrant Shares equal to the Second Closing Overage Number, and (c) the Second Closing Subscription Amount payable by such Investor at the Second Closing shall be reduced by $0.0001 for each Pre-Funded Warrant Share subject to the Pre-Funded Warrant being purchased by such Investor. Each Investor who purchases the number of Shares (and/or Pre-Funded Warrants) set forth opposite the name of such Investor under the heading “Second Closing Number of Shares” on Annex I attached hereto at the Second Closing shall be referred to as “Qualified Investor” and each Investor who fails to purchase the number of Shares (and/or Pre-Funded Warrants) set forth opposite the name of such Investor under the heading “Second Closing Number of Shares” on Annex I attached hereto at the Second Closing shall be referred to as a “Defaulting Investor.”
2.3. Third Closing Purchase and Sale of Shares and Pre-Funded Warrants. On the Third Closing Date, upon the terms and subject to the conditions set forth herein, the Company will issue and sell, and each Qualified Investor will purchase, severally and not jointly, the number of Shares set forth opposite the name of such Qualified Investor under the heading “Third Closing Number of Shares” on Annex I attached hereto, at a price per Share equal to $1.716. In the event the number of Shares set forth in Annex I under the heading “Third Closing Number of Shares” for any Investor would result in such Qualified Investor, together with its Attribution Parties, beneficially owning in excess of the Beneficial Ownership Limitation of the outstanding Common Stock immediately after the Third Closing, then (a) the number of Shares otherwise issuable to such Qualified Investor at the Third Closing will be reduced by the number (such number, the “Third Closing Overage Number”) of Shares that would result in such Investor beneficially owning, together with its Attribution Parties, no more than the Beneficial Ownership Limitation of the outstanding Common Stock immediately after the Third Closing, (b) the Company will issue to such Qualified Investor at the Third Closing a Pre-Funded Warrant that is exercisable for a number of Pre-Funded Warrant Shares equal to the Third Closing Overage Number, and (c) the Third Closing Subscription Amount payable by such Qualified Investor at the Third Closing shall be reduced by $0.0001 for each Pre-Funded Warrant Share subject to the Pre-Funded Warrant being purchased by such Investor.
3. Closings.
3.1. First Closing.
(a) The initial closing of the purchase and sale of the Shares and Pre-Funded Warrants pursuant to this Agreement (the “First Closing”) shall be held remotely via the exchange of documents and signatures no later than 10:00 AM (Eastern Time) on January 8, 2024 (the “First Closing Date”). At or prior to the First Closing, each Investor shall execute any related agreements or other documents required to be executed hereunder, dated on or before the First Closing Date, including but not limited to the Investor Questionnaire in the form attached hereto as Appendix I (the “Investor Questionnaire”).
(b) On the First Closing Date, each Investor shall deliver or cause to be delivered to the Company the First Closing Subscription Amount via wire transfer of immediately available funds pursuant to the wire instructions delivered to such Investor by the Company on or prior to the First Closing Date.
(c) At the First Closing, the Company shall deliver or cause to be delivered to each Investor the number of Shares to be issued to such Investor pursuant to Section 2.1, registered in the name of the Investor (or its nominee in accordance with its delivery instructions) in book entry form and the number of Pre-Funded Warrants to be issued to such Investor pursuant to Section 2.1, registered in the name of the Investor.
3.2. Second Closing.
(a) If at any time within six (6) months following the Phase 2b Release Date (or at such earlier time as determined by Investors required to purchase a majority of the Shares (or Pre-Funded Warrants) at the Second Closing, including each of Biotechnology Value Fund, L.P. (“BVF”) and Avidity Private Master Fund I LP (“Avidity”)), the ten (10)-day volume weighted average price of the Common Stock (as quoted on Nasdaq and as calculated by Bloomberg Financial Markets) is at least $8.00 per share (or such lesser amount as may be approved by Investors required to purchase a majority of the Shares (or Pre-Funded Warrants) at the Second Closing, including each of BVF and Avidity) (which amount shall be appropriately adjusted for any reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction) with aggregate trading volume (measured in terms of aggregate sale prices) during the same ten (10)-day period of at least $100 million (or such lesser amount as may be approved by Investors required to purchase a majority of the Shares (or Pre-Funded Warrants) at the Second Closing, including each of BVF and Avidity), then the Company shall promptly (and in any event within two Trading Days) distribute to each Investor a notice identifying the date of the Second Closing (the “Second Closing Notice”).
(b) At the Second Closing (which, for the avoidance of doubt, shall occur on the Second Closing Date), the Company shall deliver or cause to be delivered to each Investor the number of Shares to be issued to such Investor pursuant to Section 2.2, registered in the name of the Investor (or its nominee in accordance with its delivery instructions) in book entry form and/or the number of Pre-Funded Warrants to be issued to such Investor pursuant to Section 2.2, registered in the name of the Investor.
(c) On the Second Closing Date, each Investor shall deliver or cause to be delivered to the Company the Second Closing Subscription Amount via wire transfer of immediately available funds pursuant to the wire instructions delivered to such Investor by the Company on or prior to the Second Closing Date.
(d) Without limiting any remedy available to the Company (including any equitable remedies), within five (5) Business Days after the Second Closing Date, the Company will notify each Qualified Investor in writing if any Defaulting Investor failed to purchase the number of Shares set forth opposite the name of such Defaulting Investor under the heading “Second Closing Number of Shares” on Annex I attached hereto at the Second Closing and the aggregate number of Shares not purchased (the “Available Shares”). Each Qualified Investor may notify the Company in writing, within five (5) Business Days of receipt of the notice specified above, of its binding election to purchase their pro rate portion (based on their pro rata share of the number of Shares purchased at the Second Closing) or a greater number of the Available Shares. If the Qualified Investors elect in the aggregate to purchase a greater number of Shares than the total number of Available Shares, then (i) each electing Qualified Investor electing to purchase their pro rata portion of the Available Shares shall purchase such pro rata amount of the Available Shares (and/or Pre-Funded Warrants) and (ii) any remaining Available Shares will be allocated among the electing Qualified Investors who elected to purchase more than their pro rata portion of the Available Shares, based on each such Qualified Investor’s pro rata share of the number of Shares purchased at the Second Closing by such electing Qualified Investor. The purchase of the elected portion of the Available Shares (and/or Pre-Funded Warrants) shall occur on a date designated by the Company that is not more than fifteen (15) Business Days following the Second Closing Date. Following the Second Closing, the number of Shares set forth opposite the name of such Qualified Investor under the heading “Third Closing Number of Shares” on Annex I attached hereto shall be revised to add the number of Available Shares and Pre-Funded Warrants purchased by such Qualified Investor pursuant to the terms of this Section 3.2(d).
3.3. Third Closing.
(a) If at any time within three (3) years following the Second Closing Date (or at such earlier time as determined by Qualified Investors required to purchase a majority of the Shares (or Pre-Funded Warrants) at the Third Closing, including each of BVF (for so long as BVF is a Qualified Investor) and Avidity (for so long as Avidity is a Qualified Investor)), the ten (10)-day volume weighted average price of the Common Stock (as quoted on Nasdaq and as calculated by Bloomberg Financial Markets) is at least $8.00 per share (or such lesser amount as may be approved by Qualified Investors required to purchase a majority of the Shares (or Pre-Funded Warrants) at the Third Closing, including each of BVF (for so long as BVF is a Qualified Investor) and Avidity (for so long as Avidity is a Qualified Investor)) (which amount shall be appropriately adjusted for any reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction) with aggregate trading volume (measured in terms of aggregate sale prices) during the same ten (10)-day period of at least $100 million (or such lesser amount as may be approved by Qualified Investors required to purchase a majority of the Shares (or Pre-Funded Warrants) at the Second Closing, including each of BVF (for so long as BVF is a Qualified Investor) and Avidity (for so long as Avidity is a Qualified Investor)), then the Company shall promptly (and in any event within two Trading Days) distribute to each Qualified Investor a notice identifying the date of the Third Closing (the “Third Closing Notice”).
(b) Subject to the terms of Section 3.3(d), at the Third Closing (which, for the avoidance of doubt, shall occur on the Third Closing Date), the Company shall deliver or cause to be delivered to each Qualified Investor the number of Shares to be issued to such Qualified Investor pursuant to Section 2.3, registered in the name of the Investor (or its nominee in accordance with its delivery instructions) in book entry form and/or the number of Pre-Funded Warrants to be issued to such Qualified Investor pursuant to Section 2.3, registered in the name of the Investor.
(c) Subject to the terms of Section 3.3(d), on the Third Closing Date, each Qualified Investor shall deliver or cause to be delivered to the Company the Third Closing Subscription Amount via wire transfer of immediately available funds pursuant to the wire instructions delivered to such Qualified Investor by the Company on or prior to the Third Closing Date.
(d) Each Qualified Investor shall have the right, exercisable in its sole discretion, to elect to satisfy its obligations to pay the Third Closing Subscription Amount by reducing the number of Shares or Pre-Funded Warrants to be issued at the Third Closing instead of paying the Third Closing Subscription Amount in cash. If a Qualified Investor exercises this right, such Qualified Investor shall not pay any cash at the Third Closing and such Qualified Investor shall instead receive a number of Shares and/or Pre-Funded Warrants equal to the difference between (x) the number of Shares and/or Pre-Funded Warrants that such Qualified Investor would have otherwise received at the Third Closing absent this election and (y) a number of Shares (rounded up to the nearest whole share) equal to the quotient of (A) the aggregate amount to be paid for the Shares and/or Pre-Funded Warrants purchased hereunder at the Third Closing by such Qualified Investor as specified opposite such Qualified Investor’s name on Annex I attached hereto, under the column entitled “Third Closing Aggregate Purchase Price” and (B) the Fair Market Value of a share of Common Stock.
4. Representations and Warranties of the Company. The Company hereby represents and warrants to the Investors that, except as otherwise described in the SEC Filings, which qualify these representations and warranties in their entirety to the extent of the disclosure of the SEC Filings, as of the date hereof and the First Closing Date (except for the representations and warranties that speak as of a specific date, which shall be made as of such date):
4.1. Organization, Good Standing and Qualification. The Company is an entity duly incorporated, validly existing and in good standing under the laws of the State of Delaware, with the requisite corporate power and authority to own or lease and use its properties and assets, to execute and deliver the Transaction Documents, to carry out the provisions of the Transaction Documents, to issue and sell the Securities and to carry on its business as presently conducted and as proposed to be conducted as described in the SEC Filings. Immunic AG (the “Subsidiary”) is the only material subsidiary of the Company and is wholly owned by the Company. The Subsidiary is an entity duly incorporated or otherwise organized, validly existing and in good standing (to the extent such concept exists in the relevant jurisdiction) under the laws of the jurisdiction of its incorporation or organization, as applicable, and has all requisite power and authority to carry on its business and to own and use its properties. Neither the Company nor its Subsidiary is in violation or default of any of the provisions of its respective articles of association, charter, certificate of incorporation, bylaws, limited partnership agreement or other organizational or constitutive documents. Each of the Company and its Subsidiary is duly qualified to do business as a foreign entity and is in good standing (to the extent such concept exists in the relevant jurisdiction) in each jurisdiction in which the conduct of its business or its ownership or leasing of property makes such qualification necessary, except to the extent any failure to so qualify has not had and would not reasonably be expected to have a Material Adverse Effect.
4.2. Authorization. The Company has the requisite corporate power and authority and has taken all requisite corporate action necessary for, and no further action on the part of the Company, its officers, directors and stockholders, other than in connection with the Required Approvals, is necessary for, (i) the authorization, execution and delivery of the Transaction Documents, (ii) the authorization of the performance of all obligations of the Company hereunder or thereunder, and (iii) the authorization, issuance (or reservation for issuance) and delivery of the Securities. The Company’s execution and delivery of each of the Transaction Documents and the consummation by it of the transactions contemplated hereby and thereby have been duly and validly authorized by all necessary board and stockholder action. Each of the Transaction Documents has been duly executed and delivered by the Company and, assuming due authorization, execution and delivery by the Investors, constitutes valid and binding obligations of the Company enforceable in accordance with their terms, except (a) as limited by applicable bankruptcy, insolvency, reorganization, moratorium or other laws of general application affecting enforcement of creditors’ rights, (b) general principles of equity that restrict the availability of equitable remedies and (c) to the extent that the enforceability of indemnification provisions may be limited by applicable laws.
4.3. Capitalization. The Company is authorized under the Certificate of Incorporation to issue 130,000,000 shares of Common Stock and 20,000,000 shares of preferred stock. The Company’s disclosure of its issued and outstanding capital stock in its most recent SEC Filing containing such disclosure was accurate in all material respects as of the date indicated in such SEC Filing. Since the date indicated in such SEC Filing and through the date of this Agreement, there has not been any change to the Company’s capital stock, other than as a result of the exercise of stock options or incentive awards in the ordinary course of business pursuant to the Company’s stock-based compensation plans described in the SEC Filings, and the exercise of warrants to purchase Common Stock described in the SEC Filings. All of the issued and outstanding shares of the Company’s capital stock have been duly authorized and validly issued and are fully paid and nonassessable, and none of such shares were issued in violation of any pre-emptive rights and such shares were issued in compliance in all material respects with applicable state and federal securities laws and any rights of third parties. No Person is entitled to pre-emptive or similar statutory or contractual rights with respect to the issuance by the Company of any securities of the Company. Except as described in the SEC Filings and as provided in this Agreement, as of the date hereof, there are no outstanding warrants, options, convertible securities or other rights, agreements or arrangements of any character under which the Company is or may be obligated to issue any equity securities of any kind. Except as described in the SEC Filings or for agreements filed as exhibits to the SEC Filings, as of the date hereof, there are no material voting agreements, buy-sell agreements, option or right of first purchase agreements or other agreements of any kind among the Company and any of the security holders of the Company relating to the securities of the Company held by them. Except as described in the SEC Filings and as provided in this Agreement, as of the date hereof, no Person has the right to require the Company to register any securities of the Company under the 1933 Act, whether on a demand basis or in connection with the registration of securities of the Company for its own account or for the account of any other Person. The issuance and sale of the Securities hereunder will not obligate the Company to issue shares of Common Stock or other securities to any other Person (other than the Investors) and will not result in the adjustment of the exercise, conversion, exchange or reset price of any outstanding security.
4.4. Valid Issuance. The Shares have been duly and validly authorized and, when issued and paid for pursuant to this Agreement, will be validly issued, fully paid and nonassessable, and shall be free and clear of all encumbrances and restrictions (other than those waived or created by the Investors), except for restrictions on transfer set forth in the Transaction Documents or imposed by applicable securities laws. The Pre-Funded Warrant Shares have been duly and validly authorized and reserved for issuance and, upon exercise of the Pre-Funded Warrants in accordance with their terms, including the payment of any exercise price therefor, will be validly issued, fully paid and nonassessable and will be free and clear of all encumbrances and restrictions (other than those created by the Investors), except for restrictions on transfer set forth in the Transaction Documents or imposed by applicable securities laws. Assuming the accuracy of the representations and warranties of the Investors in this Agreement, the Securities will be issued in compliance with all applicable federal and state securities laws.
4.5. Filings, Consents and Approvals. Neither the Company nor any of its Subsidiaries is required to obtain any consent, waiver, authorization or order of, give any notice to, or make any filing or registration with, any court or other federal, state, local or other governmental authority or other Person in connection with the execution, delivery and performance by the Company of the Transaction Documents (including the issuance of the Securities), other than (a) the filing with the SEC of one or more Registration Statements in accordance with the requirements hereof, (b) filings required by applicable state securities laws, (c) the filing of a Notice of Sale of Securities on Form D with the SEC under Regulation D of the 1933 Act, (d) the filing of any requisite notices and/or applications to the applicable Trading Market for the listing of the Shares and Pre-Funded Warrant Shares for trading or quotation, as the case may be, thereon in the time and manner required thereby, (e) approval of the Proposal by the stockholders of the Company (including (i) the filing with the SEC of a preliminary proxy statement and a definitive proxy statement for a vote of stockholders of the Company at a special meeting to approve the Proposal and the mailing of such definitive proxy statement to the stockholders of the Company, (ii) the filing with the SEC of a Current Report on Form 8-K to disclose the results of votes taken at such special meeting and (iii) the filing of an amendment to the Company’s Certificate of Incorporation with the Secretary of State of the State of Delaware) (the “Stockholder Approval”) and (f) those that have been made or obtained prior to the date of this Agreement (collectively, the “Required Approvals”).
4.6. No Material Adverse Change. Since September 30, 2023, except as specifically set forth in a subsequent SEC Filing, there has not been:
(i) any change in the consolidated assets, liabilities, financial condition or operating results of the Company from that reflected in the financial statements included in the Company’s Quarterly Report on Form 10-Q for the nine months ended September 30, 2023, except for changes in the ordinary course of business which have not had and would not reasonably be expected, individually or in the aggregate, to have a Material Adverse Effect;
(ii) any declaration or payment by the Company of any dividend, or any authorization or payment by the Company of any distribution, on any of the capital stock of the Company, or any redemption or repurchase by the Company of any securities of the Company;
(iii) any material damage, destruction or loss, whether or not covered by insurance, to any assets or properties of the Company;
(iv) any waiver, not in the ordinary course of business, by the Company of a material right or of a material debt owed to it;
(v) any satisfaction or discharge of a material lien, claim or encumbrance or payment of any obligation by the Company, except in the ordinary course of business;
(vi) any change or amendment to the Certificate of Incorporation or Bylaws, or termination of or material amendment to any contract of the Company that the Company is required to file with the SEC pursuant to Item 601(b)(10) of Regulation S-K;
(vii) any material labor difficulties or, to the Company’s Knowledge, labor union organizing activities with respect to employees of the Company;
(viii) any material transaction entered into by the Company other than in the ordinary course of business;
(ix) the loss of the services of any executive officer (as defined in Rule 405 under the 1933 Act) of the Company; or
(x) any other event or condition that, to the Company’s Knowledge, has had or would reasonably be expected to have a Material Adverse Effect.
4.7. SEC Filings. The Company has filed all reports, schedules, forms, statements and other documents required to be filed by the Company under the 1933 Act and the 1934 Act, including pursuant to Section 13(a) or 15(d) thereof, for the two years preceding the date hereof (or such shorter period as the Company was required by law or regulation to file such material) (the “SEC Filings”). At the time of filing thereof, the SEC Filings complied as to form in all material respects with the requirements of the 1933 Act or 1934 Act and Nasdaq rules, as applicable, and none of the SEC Filings, when filed, contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading. As of the date of this Agreement, the Company’s Common Stock is listed on Nasdaq, and except as disclosed in SEC Filings, the Company has not received any notification that the SEC or Nasdaq is contemplating suspending or terminating such listing (or the applicable registration under the 1934 Act related thereto). The Company meets the registration and transaction requirements for use of Form S-3 for the registration of the Shares and Pre-Funded Warrant Shares for resale by the Investors.
4.8. No Conflict, Breach, Violation or Default. The execution, delivery and performance of the Transaction Documents by the Company and the issuance and sale of the Securities in accordance with the provisions thereof will not (i) conflict with or result in a breach or violation of (a) any of the terms and provisions of, or constitute a default under, the Certificate of Incorporation or Bylaws, both as in effect on the date hereof (true and complete copies of which have been made available to the Investors through the EDGAR system), or (b) assuming the accuracy of the representations and warranties in Section 5, any applicable statute, rule, regulation or order of any governmental agency or body or any court, domestic or foreign, having jurisdiction over the Company and its Subsidiary, or any of their assets or properties, or (ii) conflict with, or constitute a default (or an event that with notice or lapse of time or both would become a default) under, result in the creation of any lien, encumbrance or other adverse claim upon any of the properties or assets of the Company and its Subsidiary or give to others any rights of termination, amendment, acceleration or cancellation (with or without notice, lapse of time or both) of, any Material Contract except, in the case of clauses (i)(b) and (ii) only, for such conflicts, breaches, violations and defaults as have not and would not reasonably be expected to have a Material Adverse Effect. This Section does not relate to matters with respect to tax status, which are the subject of Section 4.10, employee relations and labor matters, which are the subject of Section 4.13, intellectual property, which are the subject of Section 4.14, and environmental laws, which are the subject of Section 4.15.
4.9. Compliance. Neither the Company nor the Subsidiary is (i) in default under or in violation of (and no event has occurred that has not been waived that, with notice or lapse of time or both, would result in a default by the Company under), nor has the Company or the Subsidiary received notice of a claim that the Company or the Subsidiary is in default under or that the Company or the Subsidiary is in violation of, any indenture, loan or credit agreement or any other agreement or instrument to which the Company or the Subsidiary is a party or by which the Company or the Subsidiary or any of their properties are bound (whether or not such default or violation has been waived), (ii) in violation of any judgment, decree or order of any court, arbitrator or governmental body or (iii) in violation of any statute, rule, ordinance or regulation of any governmental authority, including without limitation all foreign, federal, state and local laws relating to environmental protection, occupational health and safety, product quality and safety and employment and labor matters, and excluding taxes, which are the subject of Section 4.10, except in each case as would not have or reasonably be expected to result in a Material Adverse Effect.
4.10. Tax Matters. The Company and its Subsidiary have filed all tax returns required to have been filed by them and have paid all taxes shown thereon or otherwise owed by them, other than (i) those taxes being contested in good faith and for which adequate reserves have been provided or (ii) where the failure to so file or pay would not reasonably be expected to result in a Material Adverse Effect. The Company has made adequate charges, accruals and reserves in the applicable financial statements referred to in Section 4.17 below in respect of all federal, state and foreign income and franchise taxes for all periods as to which the tax liability of the Company and its Subsidiary has not been finally determined, except to the extent of any inadequacy that would not reasonably be expected to result in a Material Adverse Effect. There are no tax liens or claims pending or, to the Company’s Knowledge, threatened against the Company and its Subsidiary or any of their respective assets or property, which could reasonably be expected to result in a Material Adverse Effect.
4.11. Title to Properties. The Company and its Subsidiary have good and marketable title to all real properties and all other tangible properties and assets owned by them, in each case free from liens, encumbrances and defects, except such as would not reasonably be expected, individually or in the aggregate, to have a Material Adverse Effect; and the Company and its Subsidiary hold any leased real or personal property under valid, subsisting and enforceable leases with which the Company and its Subsidiary are in compliance and with no exceptions, except such as would not reasonably be expected, individually or in the aggregate, to have a Material Adverse Effect and except that the enforcement thereof may be subject to (i) bankruptcy, insolvency, reorganization, receivership, moratorium, fraudulent conveyance or other similar laws relating to creditor’s rights generally and (ii) general principles of equity and the discretion of the court before which any proceeding therefor may be brought.
4.12. Certificates, Authorities and Permits. The Company and its Subsidiary possess certificates, authorities or permits issued by appropriate governmental agencies or bodies necessary to conduct the business now operated by them, except where the failure to so possess would not reasonably be expected, individually or in the aggregate, to result in a Material Adverse Effect. During the past three years, the Company and its Subsidiary have not received any written notice of proceedings relating to the revocation or modification of any such certificate, authority or permit that, if determined adversely to the Company and its Subsidiary, would reasonably be expected, individually or in the aggregate, to have a Material Adverse Effect.
4.13. Labor Matters.
(a) Neither the Company nor any Subsidiary are parties to or bound by any collective bargaining agreements or other agreements with labor organizations.
(b) No labor dispute before the National Labor Relations Board with the employees of the Company and its Subsidiary, or with the employees of any principal supplier, manufacturer, customer or contractor of the Company, exists or, to the Company’s Knowledge, is threatened or imminent that would reasonably be expected, individually or in the aggregate, to have a Material Adverse Effect.
(c) There are no claims pending against the Company before the Equal Employment Opportunity Commission or any other administrative body or in any court asserting any violation of Title VII of the Civil Rights Act of 1964, the Age Discrimination Act of 1967, 42 U.S.C. §§ 1981 or 1983 or any other federal, state or local law, statute or ordinance barring discrimination of employment that would reasonably be expected, individually or in the aggregate, to have a Material Adverse Effect.
4.14. Intellectual Property. Except as disclosed in the SEC Filings, and as would not reasonably be expected, individually or in the aggregate, to have a Material Adverse Effect, the Company and its Subsidiary own, possess, license or have other rights to use, the patents and patent applications, copyrights, trademarks, service marks, trade names, service names and trade secrets as necessary or material for use in connection with their businesses as described in the SEC Filings (collectively, the “Intellectual Property Rights”), and to the Company’s Knowledge, there are no material liens, security interests or encumbrances that have been filed against any of these Intellectual Property Rights. No actions, suits, proceedings or claims are pending, or to the Company’s Knowledge, asserted or threatened against the Company and its Subsidiary alleging infringement of a patent or other intellectual property right of others. To the Company’s Knowledge, there is no existing infringement by another Person of any of the Intellectual Property Rights that would materially affect the use thereof by the Company. To the Company’s Knowledge, the development, manufacture, sale, and any currently proposed use of any of the products, proposed products or processes of the Company referred to in the SEC Filings, in the current or proposed conduct of the business of the Company, do not, and will not upon commercialization, infringe any right or valid patent claim of any third party. To the Company’s Knowledge, there are no ownership rights of third parties to any Intellectual Property Rights in any field of use that is exclusively licensed to the Company, other than any licensor to the Company of such Intellectual Property Rights. No action, suit, claim or other proceeding, except for routine patent and trademark prosecution proceedings in patent offices throughout the world, is pending or, to the Company’s Knowledge, threatened challenging the validity, enforceability, scope, registration, ownership or use of any of the Intellectual Property Rights. No action, suit, claim or other proceeding is pending or, to the Company’s Knowledge, threatened, challenging the Company’s rights in or to any Intellectual Property Rights. The Company and its Subsidiary have security procedures designed to protect the secrecy, confidentiality and value of their Intellectual Property Rights. To the Company’s Knowledge, no employee is in or has been in violation in any material respect of any term of any employment contract, invention assignment agreement, noncompetition agreement, or nondisclosure agreement with a former employer, executed prior to such employee’s employment where the basis of such violation relates to such employee’s employment and such violation occurred while employed and while the contract was valid and in effect. All material licenses or other material agreements under which the Company is granted rights to Intellectual Property are, to the Company’s Knowledge, in full force and effect and, to the Company’s Knowledge, there is no material default by any other party thereto. To the Company’s Knowledge, the licensors under material licenses and other material agreements had all requisite power and authority to grant the rights to the Intellectual Property Rights purported to be granted thereby. The consummation of the transactions contemplated hereby and by the other Transaction Documents will not result in the alteration, loss, impairment of or restriction on the Company’s or any Subsidiary’s ownership or right to use any Intellectual Property Rights that is material to the conduct of the Company’s business as now conducted (or contemplated to be conducted as described in the SEC Filings (including the commercialization of products described in the SEC Filings)).
4.15. Environmental Matters. Except as disclosed in the SEC Filings and as would not reasonably be expected, individually or in the aggregate, to have a Material Adverse Effect, neither the Company nor any Subsidiary is in violation of any statute, rule, regulation, decision or order of any governmental agency or body or any court, domestic or foreign, relating to the use, disposal or release of hazardous or toxic substances or relating to the protection or restoration of the environment or human exposure to hazardous or toxic substances (collectively, “Environmental Laws”), has released any hazardous substances regulated by Environmental Law on to any real property that it owns or operates, or has received any written notice or claim that it is liable for any off-site disposal or contamination pursuant to any Environmental Laws; and to the Company’s Knowledge, there is no pending or threatened investigation that would reasonably be expected to lead to such a claim.
4.16. Legal Proceedings. Except as disclosed in the SEC Filings, there are no legal, governmental or regulatory investigations, actions, suits or proceedings pending to which the Company and its Subsidiary is a party or to which any property of the Company is subject that, individually or in the aggregate, if determined adversely to the Company and its Subsidiary, would reasonably be expected to have a Material Adverse Effect; and to the Company’s Knowledge, no such proceedings are threatened or contemplated by governmental authorities or others.
4.17. Financial Statements. The financial statements included in each SEC Filing comply in all material respects with applicable accounting requirements and the rules and regulations of the SEC with respect thereto as in effect at the time of filing (or to the extent corrected by a subsequent restatement) and present fairly, in all material respects, the financial position of the Company as of the dates shown and its results of operations and cash flows for the periods shown, subject in the case of unaudited financial statements to normal year-end audit adjustments, and such financial statements have been prepared in conformity with United States generally accepted accounting principles applied on a consistent basis during the periods involved (“GAAP”) (except as may be disclosed therein or in the notes thereto, and except that the unaudited financial statements may not contain all footnotes required by GAAP, and, in the case of quarterly financial statements, as permitted by Form 10-Q under the 1934 Act). There are no financial statements (historical or pro forma) that are required to be included in the SEC Filings that are not so included as required.
4.18. Insurance Coverage. The Company and its Subsidiary maintain insurance covering their respective properties, operations, personnel and businesses as the Company reasonably deems adequate; the Company reasonably believes such insurance insures against such losses and risks in accordance with customary industry practice to protect the Company and its Subsidiary and their respective businesses and which is commercially reasonable for the current conduct of their respective businesses; all such insurance is fully in force on the date hereof; the Company and its Subsidiary are in compliance with the terms of such policies in all material respects; neither the Company nor any Subsidiary has received notice from any insurer or agent of such insurer that capital improvements or other expenditures are required or necessary to be made in order to continue such insurance; there are no material claims by the Company and its Subsidiary under any such policy or instrument as to which any insurance company is denying liability or defending under a reservation of rights clause; and neither the Company nor any Subsidiary has any reason to believe that it will not be able to renew its existing insurance coverage as and when such coverage expires or to obtain similar coverage from similar insurers as may be necessary to continue its business at a cost that would not reasonably be expected to have a Material Adverse Effect.
4.19. Brokers and Finders. Neither the Company nor any Subsidiary is a party to any contract, agreement or understanding with any Person, other than the Placement Agent, that would give rise to a valid claim against any of them for a brokerage commission, finder’s fee or like payment in connection with the offering and sale of the Securities. Other than the Placement Agent, no Person will have, as a result of the transactions contemplated by the Transaction Documents, any valid right, interest or claim against or upon the Company or, to the Company’s Knowledge, an Investor for any commission, fee or other compensation pursuant to any agreement, arrangement or understanding entered into by or on behalf of the Company.
4.20. No Directed Selling Efforts or General Solicitation. Neither the Company nor any Subsidiary nor any Person acting on their behalf has conducted any general solicitation or general advertising (as those terms are used in Regulation D) in connection with the offer or sale of any of the Securities.
4.21. No Integrated Offering. Neither the Company nor its Subsidiary nor any Person acting on their behalf has, directly or indirectly, made any offers or sales of any Company security or solicited any offers to buy any Company security, under circumstances that would adversely affect reliance by the Company on Section 4(a)(2) for the exemption from registration for the transactions contemplated hereby or would require registration of the Securities under the 1933 Act. Neither the Company nor its Subsidiary solicited an Investor through the use of general solicitation and the Company or the Placement Agent has a substantive relationship with each Investor that was established prior to the commencement of the exempt offering contemplated herein.
4.22. Internal Controls. The Company has established and maintains disclosure controls and procedures (as defined in Rules 13a-15(c) and 15d-15(e) of the 1934 Act) for the Company and designed such disclosure controls and procedures to ensure that material information relating to the Company and required to be disclosed by the Company in the reports that it files or submits under the 1934 Act is made known to the certifying officers by others within the Company. The Company maintains a system of internal accounting controls sufficient to provide reasonable assurance that (i) receipts and expenditures are being made in accordance with management’s general or specific authorizations, and (ii) transactions are recorded as necessary to permit preparation of financial statements in conformity with GAAP. The Company’s certifying officers have evaluated the effectiveness of the Company’s controls and procedures as of the end of the period covered by the most recently filed periodic report under the 1934 Act (such date, the “Evaluation Date”). The Company presented in its most recently filed periodic report under the 1934 Act the conclusions of the certifying officers about the effectiveness of the disclosure controls and procedures based on their evaluations as of the Evaluation Date. Since the end of the Company’s most recent audited fiscal year, there have been no significant deficiencies or material weakness detected in the Company’s internal controls over financial reporting (whether or not remediated) and no change in the Company’s internal control over financial reporting that has materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting. The Company is not aware of any change in its internal control over financial reporting that has occurred during its most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting. The Company, its Subsidiary and the Company’s directors and officers, in their capacities as such, are each, in compliance in all material respects with the applicable provisions of the Sarbanes-Oxley Act and the rules and regulations promulgated thereunder.
4.23. Investment Company. The Company is not required to be registered as, and immediately following each Applicable Closing will not be required to register as, an “investment company” within the meaning of the Investment Company Act of 1940, as amended.
4.24. Tests and Preclinical and Clinical Trials. During the past three years, the studies, tests and preclinical and clinical trials conducted by or, to the Company’s Knowledge, on behalf of the Company were and, if still pending, are being, conducted in all material respects in accordance with all applicable Health Care Laws. The descriptions of the studies, tests and preclinical and clinical trials conducted by or, to the Company’s Knowledge, on behalf of the Company, contained in the SEC Filings are accurate in all material respects; the Company is not aware of any other studies, tests or preclinical and clinical trials, the results of which call into question the results described in the SEC Filings; and the Company has not received any written notices or correspondence from the U.S. Food and Drug Administration (the “FDA”), any foreign, state or local governmental body exercising comparable authority or any Institutional Review Board requiring the termination, suspension or clinical hold of any studies, tests or preclinical or clinical trials conducted by or on behalf of the Company. During the past three years, neither the Company nor, to the Company’s Knowledge, any of its officers or employees has committed any act, made any statement or failed to make any statement that would reasonably be expected to provide a basis for the FDA to invoke its policy with respect to “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” set forth in 56 Fed. Reg. 46191 (Sept. 10, 1991) and any amendments thereto. During the past three years, neither the Company nor, to the Company’s Knowledge, any officer or employee of the Company has been convicted of any crime or engaged in any conduct that has resulted in or would reasonably be expected to result in (i) debarment under 21 U.S.C. Section 335a or any similar state law or (ii) exclusion under 42 U.S.C. Section 1320a-7 or any similar state law or regulation.
4.25. Manipulation of Price. The Company has not, and, to the Company’s Knowledge, no Person acting on its behalf has taken, directly or indirectly, any action designed to cause or to result in the stabilization or manipulation of the price of any security of the Company to facilitate the sale or resale of any of the Securities.
4.26. Bad Actor Disqualification. None of the Company, any predecessor or affiliated issuer of the Company nor, to the Company’s Knowledge, any director or executive officer of the Company or any promoter connected with the Company in any capacity, is subject to any of the “bad actor” disqualifications within the meaning of Rule 506(d) under the 1933 Act, except for a disqualification event covered by Rule 506(d)(2) or (d)(3). The Company has complied, to the extent applicable, with any disclosure obligations under Rule 506(e) under the 1933 Act.
4.27. Foreign Corrupt Practices; Questionable Payments; Office of Foreign Assets Control. Neither the Company nor any Subsidiary has, and to the Company’s Knowledge, no agent or other person acting on behalf of the Company and its Subsidiary has (i) directly or indirectly, used any funds for unlawful contributions, gifts, entertainment or other unlawful expenses related to foreign or domestic political activity, (ii) made any unlawful payment to foreign or domestic government officials or employees or to any foreign or domestic political parties or campaigns from corporate funds, (iii) failed to disclose fully any contribution made by the Company and its Subsidiary (or made by any person acting on behalf of the Company and its Subsidiary of which the Company is aware) which is in violation of law, or (iv) violated in any material respect any provision of the Foreign Corrupt Practices Act of 1977, as amended. Neither the Company nor the Subsidiary, nor any or their directors, officers or employees, nor, to the Company’s Knowledge, any agent, Affiliate or representative of the Company or the Subsidiary, is an individual or entity that is, or is owned or controlled by an individual or entity that is: (i) the subject of any sanctions administered or enforced by the U.S. Department of Treasury’s Office of Foreign Assets Control, the United Nations Security Council, the European Union, His Majesty’s Treasury, or other relevant sanctions authority (collectively, “Sanctions”), nor (ii) located, organized or resident in a country or territory that is the subject of Sanctions (including, without limitation, Burma/Myanmar, Cuba, Iran, Libya, North Korea and Syria).
4.28. Anti-Money Laundering. The operations of the Company and the Subsidiary are and have been conducted at all times in material compliance with all applicable financial recordkeeping and reporting requirements, including those of the Bank Secrecy Act, as amended by Title III of the Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism Act of 2001 (USA PATRIOT Act), and the applicable anti-money laundering statutes of jurisdictions where the Company and its Subsidiary conduct business, the rules and regulations thereunder and any related or similar rules, regulations or guidelines, issued, administered or enforced by any governmental agency (collectively, the “Anti-Money Laundering Laws”), and no action, suit or proceeding by or before any court or governmental agency, authority or body or any arbitrator involving the Company and its Subsidiary with respect to the Anti-Money Laundering Laws is pending or, to the Company’s Knowledge, threatened.
4.29. Takeover Protections; Rights Agreements. The Company and the Board of Directors of the Company have taken all necessary action, if any, in order to render inapplicable any control share acquisition, business combination, poison pill (including any distribution under a rights agreement) or other similar anti-takeover provision under the Company’s charter documents or the laws of its state of incorporation that is or could reasonably be expected to become applicable to any of the Investors as a result of the Investors and the Company fulfilling their obligations or exercising their rights under the Transaction Documents, including, without limitation, the Company’s issuance of the Securities and the Investors’ ownership of the Securities.
4.30. Transactions With Affiliates and Employees. Other than as contemplated by the Transaction Documents, no relationship, direct or indirect, exists between or among the Company, on the one hand, and the directors, officers, stockholders, customers or suppliers of the Company, on the other hand, that is required to be described in the SEC Filings that is not so described.
5. Representations and Warranties of the Investors. Each of the Investors hereby, severally and not jointly, represents and warrants to the Company that:
5.1. Organization and Existence. If such Investor is a corporation, partnership, limited liability company, trust or other entity, such Investor is a validly existing corporation, limited partnership or limited liability company and has all requisite corporate, partnership or limited liability company power and authority to enter into and consummate the transactions contemplated by the Transaction Documents and to carry out its obligations hereunder and thereunder, and to invest in the Securities pursuant to this Agreement.
5.2. Authorization. The execution, delivery and performance by such Investor of the Transaction Documents to which such Investor is a party have been duly authorized and each has been duly executed and when delivered will constitute the valid and legally binding obligation of such Investor, enforceable against such Investor in accordance with their respective terms, except: (i) as limited by general equitable principles and applicable bankruptcy, insolvency, reorganization, moratorium and other laws of general application affecting enforcement of creditors’ rights generally, (ii) as limited by laws relating to the availability of specific performance, injunctive relief or other equitable remedies and (iii) insofar as indemnification and contribution provisions may be limited by applicable law.
5.3. Purchase Entirely for Own Account. The Securities to be received by such Investor hereunder will be acquired for such Investor’s own account, not as nominee or agent, and not with a view to the resale or distribution of any part thereof in violation of the 1933 Act, and such Investor has no present intention of selling, granting any participation in, or otherwise distributing the same in violation of the 1933 Act without prejudice, however, to such Investor’s right at all times to sell or otherwise dispose of all or any part of such Securities in compliance with applicable federal and state securities laws. Nothing contained herein shall be deemed a representation or warranty by any Investor to hold the Securities for any period of time. Such Investor is not a broker-dealer registered with the SEC under the 1934 Act or an entity engaged in a business that would require it to be so registered.
5.4. Investment Experience. Such Investor acknowledges that it can bear the economic risk and complete loss of its investment in the Securities and has such knowledge and experience in financial or business matters that it is capable of evaluating the merits and risks of the investment contemplated hereby.
5.5. Disclosure of Information. Such Investor has had an opportunity to receive, review and understand all information related to the Company requested by it and to ask questions of and receive answers from the Company regarding the Company, its business and the terms and conditions of the offering of the Securities, and has conducted and completed its own independent due diligence. Such Investor acknowledges receipt of copies of, or access through the EDGAR system to, the SEC Filings. Based on the information such Investor has deemed appropriate, it has independently made its own analysis and decision to enter into the Transaction Documents. Such Investor is relying exclusively on its own investment analysis and due diligence (including professional advice from its own independent advisors it deems appropriate), and without reliance on the Placement Agent, its Affiliates and representatives, with respect to the execution, delivery and performance of the Transaction Documents, the Securities and the business, condition (financial and otherwise), management, operations, properties and prospects of the Company, including but not limited to all business, legal, regulatory, accounting, credit and tax matters. Such Investor understands and agrees that the Placement Agent, its Affiliates and representatives have made no representations or warranties in connection with the transactions contemplated by the Transaction Documents or as to the completeness or accuracy of any information or materials such Investor may have received in connection therewith. Such Investor has not relied on any information, recommendation or advice furnished by or on behalf of the Placement Agent, its Affiliates and representatives in connection with the Transaction Documents or the transactions contemplated thereby. Neither such inquiries nor any other due diligence investigation conducted by such Investor shall modify, limit or otherwise affect such Investor’s right to rely on the Company’s representations and warranties contained in this Agreement.
5.6. Restricted Securities. Such Investor understands that the Securities are characterized as “restricted securities” under the U.S. federal securities laws inasmuch as they are being acquired from the Company in a transaction not involving a public offering and that under such laws and applicable regulations such securities may be resold without registration under the 1933 Act only in certain limited circumstances.
5.7. Legends.
(a) It is understood that, except as provided below, certificates or book entry positions evidencing the Securities may bear the following or any similar legend:
“THE SECURITIES REPRESENTED HEREBY HAVE NOT BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE IN RELIANCE UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED, AND, ACCORDINGLY, MAY NOT BE TRANSFERRED UNLESS (I) SUCH SECURITIES HAVE BEEN REGISTERED FOR SALE PURSUANT TO THE SECURITIES ACT OF 1933, AS AMENDED, (II) SUCH SECURITIES MAY BE SOLD PURSUANT TO RULE 144, OR (III) THE COMPANY HAS RECEIVED AN OPINION OF COUNSEL REASONABLY SATISFACTORY TO IT THAT SUCH TRANSFER MAY LAWFULLY BE MADE WITHOUT REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED. THESE SECURITIES ARE SUBJECT TO TRANSFER AND OTHER RESTRICTIONS SET FORTH IN A SECURITIES PURCHASE AGREEMENT, DATED JANUARY 4, 2024, COPIES OF WHICH ARE ON FILE WITH THE COMPANY. NOTWITHSTANDING THE FOREGOING, THE SECURITIES MAY BE PLEDGED IN CONNECTION WITH A BONA FIDE MARGIN ACCOUNT OR OTHER LOAN OR FINANCING ARRANGEMENT SECURED BY THE SECURITIES.”
(b) If required by the authorities of any state in connection with the issuance and sale of the Securities, certificates or book entry positions evidencing the Securities may bear any legend required by such state authority.
(c) Certificates evidencing the Shares and/or the Pre-Funded Warrant Shares shall not contain any legend (including the legend set forth in Section 5.7(a)) if such legend is not required under applicable requirements of applicable securities laws.
5.8. Accredited Investor. At the time such Investor was offered the Securities it was and, as of the date hereof such Investor is, an “accredited investor” within the meaning of Rule 501 under the 1933 Act and has executed and delivered to the Company its Investor Questionnaire, which such Investor represents and warrants is true, correct and complete. Such Investor is a sophisticated institutional investor with sufficient knowledge, sophistication and experience in business, including transactions involving private investments in public equity, to properly evaluate the risks and merits of its purchase of the Securities. Such Investor has determined based on its own independent review and such professional advice as it deems appropriate that its purchase of the Securities and participation in the transactions contemplated by the Transaction Documents (i) are fully consistent with its financial needs, objectives and condition, (ii) comply and are fully consistent with all investment policies, guidelines and other restrictions applicable to such Investor, (iii) if applicable, have been duly authorized and approved by all necessary action, (iv) if applicable, do not and will not violate or constitute a default under such Investor’s charter, bylaws or other constituent document, or under any law, rule, regulation, agreement or other obligation by which such Investor is bound and (v) are a fit, proper and suitable investment for such Investor, notwithstanding the substantial risks inherent in investing in or holding the Securities.
5.9. No General Solicitation. Such Investor did not learn of the investment in the Securities as a result of any general or public solicitation or general advertising, or publicly disseminated advertisements or sales literature, including (a) any advertisement, article, notice or other communication published in any newspaper, magazine, website, or similar media, or broadcast over television or radio, or (b) any seminar or meeting to which such Investor was invited by any of the foregoing means of communications.
5.10. Brokers and Finders. No Person will have, as a result of the transactions contemplated by the Transaction Documents, any valid right, interest or claim against or upon the Company or an Investor for any commission, fee or other compensation pursuant to any agreement, arrangement or understanding entered into by or on behalf of such Investor.
5.11. Short Sales and Confidentiality Prior to the Date Hereof. Other than consummating the transactions contemplated hereunder, such Investor has not, nor has any Person acting on behalf of or pursuant to any understanding with such Investor, directly or indirectly executed any purchases or sales, including Short Sales, of the securities of the Company during the period commencing as of the time that such Investor was first contacted by the Company or any other Person regarding the transactions contemplated hereby and ending immediately prior to the date hereof. Notwithstanding the foregoing, in the case of an Investor that is a multi-managed investment vehicle whereby separate portfolio managers manage separate portions of such Investor’s assets and the portfolio managers have no direct knowledge of the investment decisions made by the portfolio managers managing other portions of such Investor’s assets, the representation set forth above shall only apply with respect to the portion of assets managed by the portfolio manager that made the investment decision to purchase the Securities covered by this Agreement. Such Investor, its Affiliates and, to the knowledge of such Investor, authorized representatives and advisors of such Investor who are aware of the transactions contemplated hereby, maintained the confidentiality of all disclosures made to it in connection with this transaction (including the existence and terms of such transaction). Notwithstanding the foregoing, for the avoidance of doubt, nothing contained herein shall constitute a representation or warranty, or preclude any actions, with respect to the identification of the availability of, or securing of, available shares to borrow in order to effect Short Sales or similar transactions in the future.
5.12. No Government Recommendation or Approval. Such Investor understands that no United States federal or state agency, or similar agency of any other country, has reviewed, approved, passed upon, or made any recommendation or endorsement of the Company or the purchase of the Securities.
5.13. No Conflicts. The execution, delivery and performance by such Investor of the Transaction Documents and the consummation by such Investor of the transactions contemplated hereby and thereby will not (i) result in a violation of the organizational documents of such Investor or (ii) conflict with, or constitute a default (or an event which with notice or lapse of time or both would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, any agreement, indenture or instrument to which such Investor is a party, or (iii) result in a violation of any law, rule, regulation, order, judgment or decree (including federal and state securities laws) applicable to such Investor, except in the case of clauses (ii) and (iii) above, for such conflicts, defaults, rights or violations which would not, individually or in the aggregate, reasonably be expected to have a material adverse effect on the ability of such Investor to perform its obligations hereunder.
5.14. Residency. Such Investor’s office in which its investment decision with respect to the Securities was made is located at the address immediately below such Investor’s name on the Schedule of Investors.
5.15. Placement Agent. Such Investor hereby acknowledges and agrees that (i) the Placement Agent is acting solely as a placement agent in connection with the execution, delivery and performance of the Transaction Documents and is not acting as an underwriter or in any other capacity and is not and shall not be construed as fiduciaries for such Investor, the Company or any other Person in connection with the execution, delivery and performance of the Transaction Documents, (ii) the Placement Agent has not made and will not make any representation or warranty, whether express or implied, of any kind or character, and has not provided any advice or recommendation in connection with the execution, delivery and performance of the Transaction Documents, (iii) the Placement Agent will not have any responsibility with respect to (a) any representations, warranties or agreements made by any Person under or in connection with the execution, delivery and performance of the Transaction Documents, or the execution, legality, validity or enforceability (with respect to any Person) thereof, or (b) the business, affairs, financial condition, operations, properties or prospects of, or any other matter concerning the Company, and (iv) the Placement Agent will not have any liability or obligation (including without limitation, for or with respect to any losses, claims, damages, obligations, penalties, judgments, awards, liabilities, costs, expenses or disbursements incurred by such Investor, the Company or any other person or entity), whether in contract, tort or otherwise, to such Investor, or to any Person claiming through it, in respect of the execution, delivery and performance of the Transaction Documents, except, in each case in this clause (iv), for such party’s own gross negligence, willful misconduct or bad faith.
6. Conditions to the Closings.
6.1. First Closing.
(a) Conditions to the Investors’ Obligations. The obligation of each Investor to purchase Securities at the First Closing is subject to the fulfillment to such Investor’s satisfaction, on or prior to the First Closing Date, of the following conditions, any of which may be waived by such Investor (as to itself only):
(i) The representations and warranties made by the Company in Section 4 hereof, as qualified by the SEC Filings, shall be true and correct in all material respects (except for those representations and warranties which are qualified as to materiality or by Material Adverse Effect, in which case such representations and warranties shall be true and correct in all respects) as of the date hereof and on the First Closing Date, except to the extent any such representation or warranty expressly speaks as of an earlier date, in which case such representation or warranty shall be true and correct as of such earlier date. The Company shall have performed in all material respects all obligations and covenants herein required to be performed by it on or prior to the First Closing Date.
(ii) Other than the Stockholder Approval, the Company shall have obtained any and all consents, permits, approvals, registrations and waivers necessary for consummation of the purchase and sale of the Securities and the consummation of the other transactions contemplated by the Transaction Documents, all of which shall be in full force and effect.
(iii) The Company shall have filed with Nasdaq a Notification Form: Listing of Additional Shares for the Securities.
(iv) No judgment, writ, order, injunction, award or decree of or by any court, or judge, justice or magistrate, including any bankruptcy court or judge, or any order of or by any governmental authority, shall have been issued, and no action or proceeding shall have been instituted by any governmental authority, enjoining or preventing the consummation of the transactions contemplated hereby or in the other Transaction Documents.
(v) The Investors shall have received an opinion from Dentons US LLP, the Company’s counsel, dated as of the First Closing Date, in a customary form reasonably acceptable to the Investors.
(vi) The Investors shall have received the duly executed Irrevocable Transfer Agent Instructions instructing the Transfer Agent to deliver a book-entry statement evidencing the number of Shares to be issued to the Investors pursuant to Section 2.1, registered in the name of such Investor.
(vii) The Investors shall have received the duly executed Pre-Funded Warrant Share Instructions instructing the Transfer Agent to deliver a book-entry statement evidencing the number of Pre-Funded Warrants to be issued to the Investors pursuant to Section 2.1, registered in the name of such Investor.
(viii) No stop order or suspension of trading shall have been imposed by Nasdaq, the SEC or any other governmental or regulatory body with respect to public trading in the Common Stock.
(ix) The Company shall have delivered a certificate of the Secretary of the Company, dated as of the First Closing Date, (i) certifying the resolutions adopted by the Board of Directors of the Company or a duly authorized committee thereof approving the transactions contemplated by this Agreement and the other Transaction Documents and the issuance of the Securities, and (ii) certifying the current versions of the Certificate of Incorporation and Bylaws of the Company.
(x) Since the date hereof, no event or circumstance or series of events or circumstances shall have occurred that has had or would reasonably be expected to have a Material Adverse Effect.
(b) Conditions to Obligations of the Company. The Company’s obligation to sell and issue the Securities at the First Closing to each of the Investors is subject to the fulfillment to the satisfaction of the Company on or prior to the First Closing Date of the following conditions, any of which may be waived by the Company:
(i) The representations and warranties made by each Investor in Section 5 hereof shall be true and correct in all material respects (except for those representations and warranties which are qualified as to materiality, in which case such representations and warranties shall be true and correct in all respects) as of the date hereof and on the First Closing Date. The Investors shall have performed in all material respects all obligations and covenants herein required to be performed by them on or prior to the First Closing Date.
(ii) Each Investor shall have executed and delivered the Investor Questionnaire.
(iii) Any Investor purchasing Shares and Pre-Funded Warrants at the First Closing shall have paid in full its First Closing Subscription Amount to the Company.
6.2. Second Closing.
(a) Conditions to the Investors’ Obligations. The obligation of each Investor to purchase Securities at the Second Closing is subject to the fulfillment to such Investor’s satisfaction, on or prior to the Second Closing Date, of the following conditions, any of which may be waived by such Investor (as to itself only):
(i) The representations and warranties made by the Company in Section 4 hereof, as qualified by the SEC Filings, shall be true and correct in all material respects (except for those representations and warranties which are qualified as to materiality or by Material Adverse Effect, in which case such representations and warranties shall be true and correct in all respects) as of the date hereof and on the Second Closing Date, except to the extent any such representation or warranty expressly speaks as of an earlier date, in which case such representation or warranty shall be true and correct as of such earlier date. The Company shall have performed in all material respects all obligations and covenants herein required to be performed by it on or prior to the Second Closing Date.
(ii) The Company shall have obtained any and all consents, permits, approvals, registrations and waivers necessary for consummation of the purchase and sale of the Securities and the consummation of the other transactions contemplated by the Transaction Documents, all of which shall be in full force and effect.
(iii) The Company shall have filed with Nasdaq a Notification Form: Listing of Additional Shares for the Securities.
(iv) All obligations, covenants and agreements of the Company required to be performed at or prior to the Second Closing Date shall have been performed.
(v) No judgment, writ, order, injunction, award or decree of or by any court, or judge, justice or magistrate, including any bankruptcy court or judge, or any order of or by any governmental authority, shall have been issued, and no action or proceeding shall have been instituted by any governmental authority, enjoining or preventing the consummation of the transactions contemplated hereby or in the other Transaction Documents.
(vi) The Investors shall have received an opinion from Dentons US LLP, the Company’s counsel, dated as of the Second Closing Date, in a customary form reasonably acceptable to the Investors.
(vii) The Investors shall have received the duly executed Irrevocable Transfer Agent Instructions instructing the Transfer Agent to deliver a book-entry statement evidencing the number of Shares to be issued to the Investors pursuant to Section 2.2, registered in the name of such Investor.
(viii) The Investors shall have received the duly executed Pre-Funded Warrant Share Instructions instructing the Transfer Agent to deliver a book-entry statement evidencing the number of Pre-Funded Warrants to be issued to the Investors pursuant to Section 2.2, registered in the name of such Investor.
(ix) No stop order or suspension of trading shall have been imposed by Nasdaq, the SEC or any other governmental or regulatory body with respect to public trading in the Common Stock.
(x) The Company shall have delivered a certificate of the Secretary of the Company, dated as of the Second Closing Date, (i) certifying the resolutions adopted by the Board of Directors of the Company or a duly authorized committee thereof approving the transactions contemplated by this Agreement and the other Transaction Documents and the issuance of the Securities, and (ii) certifying the current versions of the Certificate of Incorporation and Bylaws of the Company.
(xi) Since the date hereof, no event or circumstance or series of events or circumstances shall have occurred that has had or would reasonably be expected to have a Material Adverse Effect.
(b) Conditions to Obligations of the Company. The Company’s obligation to sell and issue the Securities at the Second Closing to each of the Investors is subject to the fulfillment to the satisfaction of the Company on or prior to the Second Closing Date of the following conditions, any of which may be waived by the Company:
(i) The representations and warranties made by each Investor in Section 5 hereof shall be true and correct in all material respects (except for those representations and warranties which are qualified as to materiality, in which case such representations and warranties shall be true and correct in all respects) as of the date hereof and on the Second Closing Date. The Investors shall have performed in all material respects all obligations and covenants herein required to be performed by them on or prior to the Second Closing Date.
(ii) Any Investor purchasing Shares and Pre-Funded Warrants at the Second Closing shall have paid in full its Second Closing Subscription Amount to the Company.
6.3. Third Closing.
(a) Conditions to the Investors’ Obligations. The obligation of each Investor to purchase Securities at the Third Closing is subject to the fulfillment to such Investor’s satisfaction, on or prior to the Third Closing Date, of the following conditions, any of which may be waived by such Investor (as to itself only):
(i) The representations and warranties made by the Company in Section 4 hereof, as qualified by the SEC Filings, shall be true and correct in all material respects (except for those representations and warranties which are qualified as to materiality or by Material Adverse Effect, in which case such representations and warranties shall be true and correct in all respects) as of the date hereof and on the Third Closing Date, except to the extent any such representation or warranty expressly speaks as of an earlier date, in which case such representation or warranty shall be true and correct as of such earlier date. The Company shall have performed in all material respects all obligations and covenants herein required to be performed by it on or prior to the Third Closing Date.
(ii) The Company shall have obtained any and all consents, permits, approvals, registrations and waivers necessary for consummation of the purchase and sale of the Securities and the consummation of the other transactions contemplated by the Transaction Documents, all of which shall be in full force and effect.
(iii) All obligations, covenants and agreements of the Company required to be performed at or prior to the Third Closing Date shall have been performed.
(iv) The Company shall have filed with Nasdaq a Notification Form: Listing of Additional Shares for the Securities.
(v) No judgment, writ, order, injunction, award or decree of or by any court, or judge, justice or magistrate, including any bankruptcy court or judge, or any order of or by any governmental authority, shall have been issued, and no action or proceeding shall have been instituted by any governmental authority, enjoining or preventing the consummation of the transactions contemplated hereby or in the other Transaction Documents.
(vi) The Investors shall have received an opinion from Dentons US LLP, the Company’s counsel, dated as of the Third Closing Date, in a customary form reasonably acceptable to the Investors.
(vii) The Investors shall have received the duly executed Irrevocable Transfer Agent Instructions instructing the Transfer Agent to deliver a book-entry statement evidencing the number of Shares to be issued to the Investors pursuant to Section 2.3, registered in the name of such Investor.
(viii) The Investors shall have received the duly executed Pre-Funded Warrant Share Instructions instructing the Transfer Agent to deliver a book-entry statement evidencing the number of Pre-Funded Warrants to be issued to the Investors pursuant to Section 2.3, registered in the name of such Investor.
(ix) No stop order or suspension of trading shall have been imposed by Nasdaq, the SEC or any other governmental or regulatory body with respect to public trading in the Common Stock.
(x) The Company shall have delivered a certificate of the Secretary of the Company, dated as of the Third Closing Date, (i) certifying the resolutions adopted by the Board of Directors of the Company or a duly authorized committee thereof approving the transactions contemplated by this Agreement and the other Transaction Documents and the issuance of the Securities, and (ii) certifying the current versions of the Certificate of Incorporation and Bylaws of the Company.
(xi) Since the date hereof, no event or circumstance or series of events or circumstances shall have occurred that has had or would reasonably be expected to have a Material Adverse Effect.
(b) Conditions to Obligations of the Company. The Company’s obligation to sell and issue the Securities at the Third Closing to each of the Investors is subject to the fulfillment to the satisfaction of the Company on or prior to the Third Closing Date of the following conditions, any of which may be waived by the Company:
(i) The representations and warranties made by each Investor in Section 5 hereof shall be true and correct in all material respects (except for those representations and warranties which are qualified as to materiality, in which case such representations and warranties shall be true and correct in all respects) as of the date hereof and on the Third Closing Date. The Investors shall have performed in all material respects all obligations and covenants herein required to be performed by them on or prior to the Third Closing Date.
(ii) Any Investor purchasing Shares and Pre-Funded Warrants at the Third Closing shall have paid in full its Third Closing Subscription Amount to the Company.
7. Termination of Obligations; Effects.
7.1. Termination of Obligations to Effect the Applicable Closing(s).
(a) The obligations of the Company, on the one hand, and the Investors, on the other hand, to effect the Applicable Closing shall terminate as follows:
(i) Upon the mutual written consent of the Company and Investors that agreed to purchase a majority of the Shares and Pre-Funded Warrant Shares issuable upon exercise of the Pre-Funded Warrants in the aggregate (the “Majority Investors”) to be issued and sold pursuant to this Agreement;
(ii) By the Company if any of the conditions set forth in Section 6.1(b), Section 6.2(b) or Section 6.3(b) (as applicable) shall have become incapable of fulfillment, and shall not have been waived by the Company; or
(iii) By an Investor (with respect to itself only) if any of the conditions set forth in Section 6.1(a), Section 6.2(a) or Section 6.3(a) (as applicable) shall have become incapable of fulfillment, and shall not have been waived by the Investor.
(b) Except in the case of clause (i) above, the party seeking to terminate its obligation to effect the Applicable Closing(s) shall not then be in breach of any of its representations, warranties, covenants or agreements contained in this Agreement or the other Transaction Documents if such breach has resulted in the circumstances giving rise to such party’s seeking to terminate its obligation to effect the Applicable Closing(s).
(c) In the event of termination by the Company or any Investor of its obligations to effect the Applicable Closing pursuant to this Section 7, written notice thereof shall be given promptly to the other Investors by the Company and the other Investors shall have the right to terminate their obligations to effect the Applicable Closing(s) upon written notice to the Company and the other Investors. Nothing in this Section 7 shall be deemed to release any party from any liability for any breach by such party of the terms and provisions of this Agreement or the other Transaction Documents or to impair the right of any party to compel specific performance by any other party of its obligations under this Agreement or the other Transaction Documents.
(d) For the avoidance of doubt, a termination of this Agreement in connection with an Applicable Closing shall terminate all obligations hereunder with respect to any successive Applicable Closing(s) (i.e., if a termination of this Agreement occurs with respect to the First Closing, there shall be no further obligations under this Agreement with respect to the Second Closing or Third Closing or otherwise).
8. Covenants and Agreements of the Company.
8.1. Nasdaq Listing. From the date hereof until such time as the Shares and Pre-Funded Warrant Shares have been sold pursuant to Rule 144 or are eligible for resale under Rule 144(b)(1) or any successor provision, the Company will take such action as is necessary to continue the listing and trading of its Common Stock on Nasdaq and, in accordance, therewith, will take all actions necessary to comply in all respects with the Company’s reporting, filing and other obligations under the bylaws or rules of such market or exchange, as applicable. The Company further agrees, if the Company applies to have the Common Stock traded on any other trading market, it will then include in such application all of the Shares and Pre-Funded Warrant Shares, and will take such other action as is necessary to cause all of the Shares and Pre-Funded Warrant Shares to be listed or quoted on such other trading market as promptly as possible. The Company agrees to maintain the eligibility of the Common Stock for electronic transfer through the Depository Trust Company or another established clearing corporation, including, without limitation, by timely payment of fees to the Depository Trust Company or such other established clearing corporation in connection with such electronic transfer.
8.2. Removal of Legends.
(a) In connection with any sale or disposition of the Shares and Pre-Funded Warrant Shares by an Investor pursuant to Rule 144 or pursuant to any other exemption under the 1933 Act such that the purchaser acquires freely tradable shares and upon compliance by the Investor with the requirements of this Agreement, if requested by the Investor, the Company shall use its reasonable best efforts to request that the transfer agent for the Common Stock (the “Transfer Agent”) remove any restrictive legends related to the book entry account holding such Shares and Pre-Funded Warrant Shares and make a new, unlegended entry for such book entry shares sold or disposed of without restrictive legends within two (2) Trading Days of receipt of such request from the Investor (such date, the “Legend Removal Date”); provided that the Company has received customary representations and other documentation reasonably acceptable to the Company in connection therewith not later than 5:00 p.m. Eastern Time on the date of such request. Subject to receipt by the Company of customary representations and other documentation reasonably acceptable to the Company in connection therewith, upon the earliest of such time as the Shares and Pre-Funded Warrant Shares (i) have been sold or transferred pursuant to an effective registration statement, (ii) have been sold pursuant to Rule 144 or (iii) are eligible for resale under Rule 144(b)(1) or any successor provision without the current public information requirement under Rule 144(c), the Company shall (A) deliver to the Transfer Agent irrevocable instructions that the Transfer Agent shall make a new, unlegended entry for such book entry shares, and (B) cause its counsel (at the Company’s expense) to deliver to the Transfer Agent one or more opinions to the effect that the removal of such legends in such circumstances may be effected under the 1933 Act. The Company shall be responsible for the fees of its Transfer Agent and all the Depository Trust Company fees associated with such issuance.
(b) If the Company shall fail for any reason or for no reason to issue Shares or Pre-Funded Warrant Shares to an Investor that are free from all restrictive legends by the Legend Removal Date when all requirements of Section 8.2(a) have been met, then, in addition to all other remedies available to such Investor, if, on or after the Trading Day immediately following such two (2) Trading Day period, such Investor purchases (in an open market transaction or otherwise) shares of Common Stock to deliver in satisfaction of a sale of Shares or Pre-Funded Warrant Shares that such Investor anticipated receiving from the Company without any restrictive legend, then the Company shall, within two (2) Trading Days after such Investor’s request, pay in cash to such Investor the amount by which (x) such Investor’s total purchase price (including any brokerage commissions) for the shares of Common Stock so purchased exceeds (y) the product of (1) the aggregate number of Shares or Pre-Funded Warrant Shares that such Investor was entitled to receive without any restrictive legend, multiplied by (2) the actual sale price at which the sell order giving rise to such purchase obligation was executed (including any brokerage commissions). The Investor shall provide the Company prompt written notice of any such request and all evidence reasonably requested by the Company.
(c) Subject to receipt from the Investor by the Company and the Transfer Agent of customary representations and other documentation reasonably acceptable to the Company and the Transfer Agent in connection therewith, upon the earliest of such time as the Shares and/or Pre-Funded Warrant Shares (i) have been registered for resale under the 1933 Act pursuant to an effective registration statement, (ii) have been sold or transferred pursuant to an effective registration statement, (iii) have been sold pursuant to Rule 144, or (iv) are eligible for resale under Rule 144(b)(1) or any successor provision, the Company shall, in accordance with the provisions of this Section 8.2(c) and within two (2) Trading Days of any request therefor from an Investor accompanied by such customary and reasonably acceptable documentation referred to above, (A) deliver to the Transfer Agent irrevocable instructions that the Transfer Agent shall make a new, unlegended entry for such book entry Shares and/or Pre-Funded Warrant Shares, and (B) use reasonable efforts to cause its counsel to deliver to the Transfer Agent one or more opinions to the effect that the removal of such legends in such circumstances may be effected under the 1933 Act if required by the Transfer Agent to effect the removal of the legend in accordance with the provisions of this Agreement. Shares and/or Pre-Funded Warrant Shares subject to legend removal hereunder may be transmitted by the Transfer Agent to the Investor by crediting the account of the Investor’s prime broker with the Depository Trust Company’s (“DTC”) system as directed by such Investor. The Company shall be responsible for the fees of its Transfer Agent and all DTC fees associated with such issuance. Each Investor agrees that if, after the effective date of the registration statement covering the resale of the Shares and Pre-Funded Warrant Shares, such registration statement ceases to be effective and the Company has provided notice to such Investor to that effect, such Investor shall sell Shares and Pre-Funded Warrant Shares only in compliance with an exemption from the registration requirements of the 1933 Act.
8.3. Irrevocable Transfer Agent Instructions. On the Applicable Closing Date, the Company shall issue irrevocable instructions to its Transfer Agent, and any subsequent transfer agent to issue to the Investors (or in such nominee’s name(s) as designated by an Investor) book-entry notations representing the Shares to be issued on the Applicable Closing Date (the “Irrevocable Transfer Agent Instructions”) and, upon exercise of the Pre-Funded Warrants pursuant to their terms, the Company shall issue irrevocable instructions to its transfer agent, and any subsequent transfer agent to issue to the Investors (or in such nominee’s name(s) as designated by an Investor), book-entry notations representing the Pre-Funded Warrant Shares (the “Pre-Funded Warrant Share Instructions”). The Company represents and warrants that no instruction other than the Irrevocable Transfer Agent Instructions and the Pre-Funded Warrant Share Instructions referred to in this Section 8.3 (or instructions that are consistent therewith) will be given by the Company to its transfer agent in connection with this Agreement and that the Shares and Pre-Funded Warrant Shares shall otherwise be freely transferable on the books and records of the Company as and to the extent provided in this Agreement and the other Transaction Documents and applicable law. The Company acknowledges that a breach by it of its obligations under this Section 8.3 will cause irreparable harm to an Investor. Accordingly, the Company acknowledges that the remedy at law for a breach of its obligations under this Section 8.3 will be inadequate and agrees, in the event of a breach by the Company of the provisions of this Section 8.3, that an Investor shall be entitled, in addition to all other available remedies, to an order and/or injunction restraining any breach and requiring immediate issuance and transfer, without the necessity of showing economic loss and without any bond or other security being required.
8.4. Transfer Restrictions. Each Investor agrees that it will sell, transfer or otherwise dispose of the Securities only in compliance with all applicable state and federal securities laws. In connection with any transfer of the Securities other than (a) pursuant to an effective registration statement, (b) to the Company, or (c) pursuant to Rule 144 (provided that the Investor provides the Company with reasonable assurances (in the form of seller and, if applicable, broker representation letters) that the securities may be sold pursuant to such rule), the Company may require the transferor thereof to provide to the Company an opinion of counsel selected by the transferor and reasonably acceptable to the Company, the form and substance of which opinion shall be reasonably satisfactory to the Company, to the effect that such transfer does not require registration of such transferred Securities under the Securities Act. As a condition of a transfer other than pursuant to (a) through (c) above, any such transferee shall agree in writing to be bound by the terms of this Agreement and shall have the rights of an Investor under this Agreement with respect to such transferred Securities.
8.5. Reservation of Common Stock. As of the date hereof, the Company has reserved and the Company shall continue to reserve and keep available at all times, free of preemptive rights, a sufficient number of shares of Common Stock for the purpose of enabling the Company to issue Shares pursuant to this Agreement and Pre-Funded Warrant Shares pursuant to any exercise of the Pre-Funded Warrants.
8.6. Subsequent Equity Sales. The Company shall not, and shall use its commercially reasonable best efforts to ensure that no controlled Affiliate of the Company shall, sell, offer for sale or solicit offers to buy or otherwise negotiate in respect of any security (as defined in Section 2 of the 1933 Act) that will be integrated with the offer or sale of the Securities in a manner that would require the registration under the 1933 Act of the sale of the Securities to the Investors. The Company shall not take any action or steps that would adversely affect reliance by the Company in any material respect on Section 4(a)(2) for the exemption from registration for the transactions contemplated hereby or require registration of the Securities under the 1933 Act.
8.7. Short Sales and Confidentiality After the Date Hereof. Each Investor covenants that neither it nor any Affiliates acting on its behalf or pursuant to any understanding with it will execute any Short Sales during the period from the date hereof until the earlier of (i) the date after the transactions contemplated by this Agreement are first publicly announced or (ii) the date on which this Agreement is terminated in full. Each Investor covenants that until such time as all confidential information disclosed to it in connection with this transaction is publicly disclosed by the Company, such Investor will maintain the confidentiality of all such information, unless otherwise legally required to disclose.
8.8. Adjustments in Share Numbers and Prices. In the event of any stock split, subdivision, dividend or distribution payable in shares of Common Stock (or other securities or rights convertible into, or entitling the holder thereof to receive directly or indirectly shares of Common Stock), combination or other similar recapitalization or event occurring after the date hereof and prior to the Applicable Closing Date, each reference in any Transaction Document to a number of shares or a price per share shall be deemed to be amended to appropriately account for such event.
8.9. Registration Rights; Rule 144.
(a) The Company shall use its reasonable best efforts to register the resale by the Investors of the Registrable Securities on a registration statement on Form S-3, or if Form S-3 is not available, Form S-1 (each, a “Registration Statement”), and shall file the initial Registration Statement covering the Shares issued at the First Closing, the Pre-Funded Warrant Shares issuable upon exercise of the Pre-Funded Warrants issued at the First Closing and any other Registrable Securities associated therewith with the SEC no later than twenty (20) calendar days from the filing of the preliminary proxy statement required to be filed pursuant to Section 8.10, and shall use its reasonable best efforts to have such Registration Statement declared effective as soon as practicable, but in no event later than seventy five (75) calendar days from the First Closing Date; provided, that such deadline shall be extended to one hundred twenty (120) calendar days after the First Closing Date if the Registration Statement is reviewed by, and comments thereto are provided from, the Staff, provided, further that the Company shall have such Registration Statement declared effective within three (3) Business Days after the Staff has notified the Company that it will not review, or has completed its review, of such Registration Statement.
(b) The Company shall file a Registration Statement covering the Shares issued at the Second Closing, the Pre-Funded Warrant Shares issuable upon exercise of the Pre-Funded Warrants issued at the Second Closing and any other Registrable Securities associated therewith with the SEC no later than thirty (30) calendar days from the Second Closing Date, and shall use its reasonable best efforts to have such Registration Statement declared effective as soon as practicable, but in no event later than seventy five (75) calendar days from the Second Closing Date; provided, that such deadline shall be extended to one hundred twenty (120) calendar days after the Second Closing Date if the Registration Statement is reviewed by, and comments thereto are provided from, the Staff, provided, further that the Company shall have such Registration Statement declared effective within three (3) Business Days after the Staff has notified the Company that it will not review, or has completed its review, of such Registration Statement.
(c) The Company shall file a Registration Statement covering the Shares issued at the Third Closing and Pre-Funded Warrant Shares issuable upon exercise of the Pre-Funded Warrants issued at the Third Closing with the SEC no later than thirty (30) calendar days from the Third Closing Date, and shall use its reasonable best efforts to have such Registration Statement declared effective as soon as practicable, but in no event later than seventy five (75) calendar days from the Third Closing Date; provided, that such deadline shall be extended to one hundred twenty (120) calendar days after the Third Closing Date if the Registration Statement is reviewed by, and comments thereto are provided from, the Staff, provided, further that the Company shall have such Registration Statement declared effective within three (3) Business Days after the Staff has notified the Company that it will not review, or has completed its review, of such Registration Statement.
(d) In no event shall the Investor be identified as a statutory underwriter in a Registration Statement unless requested by the SEC; provided, that if the SEC requests that the Investor be identified as a statutory underwriter in the Registration Statement, the Investor will have the option, in its sole and absolute discretion, to either (i) have the opportunity to withdraw from such Registration Statement, in which case the Company’s obligation to register the Registrable Securities will be deemed satisfied or (ii) be included as such in the Registration Statement. No Registration Statement shall include any shares of Common Stock or other securities for the account of any other holder without the prior written consent of the Investors beneficially owning (as determined pursuant to Rule 13d-3 under the 1934 Act) a majority of the Registrable Securities.
(e) Notwithstanding anything to the contrary contained herein, the Company may, upon written notice to any holder of Registrable Securities included in a Registration Statement, suspend the use of any Registration Statement, including any prospectus that forms a part of a Registration Statement, if the Company (X) determines that it would be required to make disclosure of material information in the Registration Statement that the Company has a bona fide business purpose for preserving as confidential, (Y) the Company determines it must amend or supplement the Registration Statement or the related prospectus so that such Registration Statement or prospectus shall not include an untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary to make the statements therein, in the case of the prospectus in light of the circumstances under which they were made, not misleading or (Z) the Company has experienced or is experiencing some other material non-public event, including a pending transaction involving the Company, the disclosure of which at such time, in the good faith judgment of the Company, would adversely affect the Company; provided, however, in no event shall holders of Registrable Securities be suspended from selling Registrable Securities pursuant to any Registration Statement for a period that exceeds thirty (30) consecutive Trading Days or ninety (90) total Trading Days in any three hundred sixty (360)-day period. Upon disclosure of such information or the termination of the condition described above, the Company shall provide prompt notice to holders whose Registrable Securities are included in any Registration Statement, and shall promptly terminate any suspension of sales it has put into effect and shall take such other reasonable actions to permit registered sales of Registrable Securities as contemplated hereby.
(f) With a view to making available to the Investors the benefits of Rule 144 (or its successor rule) and any other rule or regulation of the SEC that may at any time permit the Investors to sell shares of Common Stock to the public without registration, the Company covenants and agrees to: (i) make and keep public information available, as those terms are understood and defined in Rule 144, until the earlier of (A) six months after such date on which all of the Registrable Securities may be sold without restriction by the holders thereof pursuant to Rule 144 or any other rule of similar effect or (B) such date on which there are no longer Registrable Securities; (ii) file with the SEC in a timely manner all reports and other documents required of the Company under the 1934 Act; and (iii) furnish electronically to each Investor upon request, as long as such Investor owns any Registrable Securities, (A) a written statement by the Company that it has complied with the reporting requirements of the 1934 Act, (B) a copy of or electronic access to the Company’s most recent Annual Report on Form 10-K or Quarterly Report on Form 10-Q, and (C) such other information as may be reasonably requested in order to avail such Investor of any rule or regulation of the SEC that permits the selling of any such Registrable Securities without registration.
(g) If (i) any Registration Statement is not filed within the time frame set forth in this Section 8.9, (ii) any Registration Statement is not declared effective within the time frame set forth in this Section 8.9, (iii) the Company fails to file with the SEC a request for acceleration of any Registration Statement within three (3) Business Days after the Staff has notified the Company that it will not review, or has completed its review, of such Registration Statement, (iv) prior to the effective date of any Registration Statement, the Company fails to file a pre-effective amendment and otherwise respond in writing to comments made by the SEC in respect of such Registration Statement within ten (10) Trading Days after the receipt of comments by or notice from the SEC that such amendment is required in order for such Registration Statement to be declared effective, or (v) after the effective date of any Registration Statement, holders of Registrable Securities are suspended from selling Registrable Securities pursuant to such Registration Statement for a period that exceeds thirty (30) consecutive Trading Days or ninety (90) total Trading Days in any three hundred sixty (360)-day period (any such failure or breach being referred to as an “Event”, and for purposes of clauses (i) and (ii), the date on which such Event occurs, for purpose of clause (iii), the date on which such three (3) Business Day period is exceeded, for purpose of clause (iv), the date which such ten (10) Trading Day period is exceeded, and for purpose of clause (v), the date on which such thirty (30) consecutive Trading Day or (90) total Trading Day period is exceeded, as applicable, being referred to as “Event Date”), then, in addition to any other rights the Investors may have hereunder or under applicable law, as liquidated damages on each such Event Date and on each monthly anniversary of each such Event Date (if the applicable Event shall not have been cured by such date) until the applicable Event is cured, the Company shall pay to each Investor negatively impacted by the Event, as liquidated damages, an amount in cash equal to the product of 1.0% multiplied by the aggregate subscription amount paid by such Investors for Registrable Securities impacted by such Event pursuant to this Agreement. The parties agree that the maximum aggregate liquidated damages payable to an Investor under this Agreement shall be 6.0% of the aggregate subscription amount paid by such Investor pursuant to this Agreement. The liquidated damages pursuant to the terms hereof shall apply on a daily pro rata basis for any portion of a month prior to the cure of an Event. Notwithstanding the foregoing, the Company shall not owe any liquidated damages to any Investor pursuant to this Section 8.9(g) if, at the time of the Event, such Investor may sell the Registrable Securities held by such Investor pursuant to Rule 144 without volume restrictions or current public information requirements.
(h) All fees and expenses incident to the performance of or compliance with this Section 8.9 by the Company shall be borne by the Company whether or not any Registrable Securities are sold pursuant to any Registration Statement; provided, however, that in no event shall the Company be responsible for any underwriting, broker or similar fees or commissions of any Investor.
8.10. Stockholder Approval. The Company shall, as soon as practicable following the First Closing, but not more than twenty-one (21) calendar days thereafter, file a preliminary proxy statement for a vote of its stockholders at a special meeting (the “Special Meeting”) to approve an amendment to the Company’s Certificate of Incorporation to increase the number of authorized shares of Common Stock from 130,000,000 to 500,000,000 (the “Proposal”). The Company shall set a record date for the initial Special Meeting that is not more than twenty (20) days following the First Closing. The Company shall, as soon as practicable following notification from the Staff that it has completed its review of the preliminary proxy statement or that it will not review the preliminary proxy statement (but not more than 5 Business Days thereafter), file and mail a definitive proxy statement for the vote of its stockholders to approve the Proposal. The Company covenants and agrees that its Board of Directors shall unanimously recommend that the Proposal be approved by the Company’s stockholders at the Special Meeting and all meetings in which such Proposal is considered. If the Company’s stockholders do not approve the Proposal at the first Special Meeting in which they are voted on by stockholders, the Company covenants and agrees that it will submit the Proposal for approval of the Company’s stockholders at least semi-annually until such approval is obtained.
9. Survival and Indemnification.
9.1. Survival. Subject to applicable statutes of limitations, the representations, warranties, covenants, and agreements contained in this Agreement shall survive the Applicable Closing Date and the delivery of the Securities.
9.2. Indemnification by the Company. The Company agrees to indemnify and hold harmless each of the Investors, the officers, directors, partners, agents, investment advisors, members, and employees (and any other Persons with a functionally equivalent role of a Person holding such titles, notwithstanding a lack of such title or any other title) of each Investor, each Person who controls any such Investor (within the meaning of Section 15 of the 1933 Act or Section 20 of the 1934 Act) and the officers, directors, partners, stockholders, members and employees (and any other Persons with a functionally equivalent role of a Person holding such titles, notwithstanding a lack of such title or any other title) of each such controlling Person (each, an “Investor Indemnified Party”), from and against any and all losses, claims, damages, liabilities, costs (including, without limitation, reasonable attorneys’ fees) or expenses, joint or several, to which such Investor Indemnified Party may become subject, insofar as such losses, claims, damages, liabilities, costs or expenses (or actions in respect thereof as contemplated below) arise out of or are based in whole or in part on the inaccuracy in the representations and warranties of the Company contained in this Agreement or the failure of the Company to perform its obligations hereunder, or arise out of or are based upon any untrue statement or alleged untrue statement or omission or alleged omission of any material fact contained in any Registration Statement, any preliminary prospectus or final prospectus thereto, or any amendment or supplement thereof, and will reimburse each Investor Indemnified Party for reasonable legal and other expenses reasonably incurred as such expenses are reasonably incurred by such Investor Indemnified Party in connection with investigating, defending, settling, compromising or paying such loss, claim, damage, liability, expense or action; provided, however, that the Company will not be liable in any such case to the extent that any such loss, claim, damage, liability, cost or expense arises out of or is based upon (i) the failure of such Investor Indemnified Party to materially comply with the covenants and agreements contained herein, (ii) the material inaccuracy of any representations made by such Investor Indemnified Party herein or in an Investor Questionnaire, (iii) an untrue statement or alleged untrue statement or omission or alleged omission so made in conformity with information furnished by an Investor Indemnified Party in writing specifically for use in the Registration Statement or a prospectus, (iv) the use by an Investor Indemnified Party of an outdated or defective prospectus after the Company has notified such Investor in writing that such prospectus is outdated or defective, or (v) an Investor Indemnified Party’s failure to send or give a copy of the prospectus or supplement (as then amended or supplemented), if required (and not exempted) to the Persons asserting an untrue statement or omission or alleged untrue statement or omission at or prior to the written confirmation of the sale of Registrable Securities.
9.3. Indemnification by the Investors. Each Investor agrees, severally but not jointly, to indemnify and hold harmless, to the fullest extent permitted by law, the Company, its directors, officers, employees, stockholders and each Person who controls the Company (within the meaning of the 1933 Act) against any losses, claims, damages, liabilities and expense (including reasonable attorneys’ fees) resulting from any untrue statement of a material fact or any omission of a material fact required to be stated in the Registration Statement or prospectus or preliminary prospectus or amendment or supplement thereto or necessary to make the statements therein not misleading, to the extent, but only to the extent, that such untrue statement or omission is contained in any information regarding such Investor and furnished in writing by such Investor to the Company specifically for inclusion in the Registration Statement or prospectus or amendment or supplement thereto. In no event shall the liability of an Investor be greater than the dollar amount of the proceeds received by such Investor upon the sale of the Registrable Securities included in such Registration Statement giving rise to such indemnification obligation.
9.4. Indemnification Procedure. Promptly after any indemnified party hereunder (the “Indemnified Party”) has received notice of any indemnifiable claim hereunder, or the commencement of any action, suit or proceeding by a third Person, which the Indemnified Party believes in good faith is an indemnifiable claim under this Agreement, the Indemnified Party shall give the indemnitor hereunder (the “Indemnifying Party”) written notice of such claim or the commencement of such action, suit or proceeding, but failure to so notify the Indemnifying Party will not relieve the Indemnifying Party from any liability it may have to such Indemnified Party hereunder except to the extent that the Indemnifying Party is materially prejudiced by such failure. Such notice shall state the nature and the basis of such claim to the extent then known. The Indemnifying Party shall have the right to defend and settle, at its own expense and by its own counsel, any such matter as long as the Indemnifying Party pursues the same diligently and in good faith. If the Indemnifying Party undertakes to defend or settle, it shall promptly notify the Indemnified Party of its intention to do so, and the Indemnified Party shall cooperate with the Indemnifying Party and its counsel in all commercially reasonable respects in the defense thereof and the settlement thereof. Such cooperation shall include, but shall not be limited to, furnishing the Indemnifying Party with any books, records and other information reasonably requested by the Indemnifying Party and in the Indemnified Party’s possession or control. Such cooperation of the Indemnified Party shall be at the cost of the Indemnifying Party. After the Indemnifying Party has notified the Indemnified Party of its intention to undertake to defend or settle any such asserted liability, and for so long as the Indemnifying Party diligently pursues such defense, the Indemnifying Party shall not be liable for any additional legal expenses incurred by the Indemnified Party in connection with any defense or settlement of such asserted liability; provided, however, that the Indemnified Party shall be entitled (a) at its expense, to assist the Indemnifying Party in the defense of such asserted liability and the negotiations of the settlement thereof and (b) if (i) the Indemnifying Party has failed to assume the defense or (ii) if the defendants in any such action include both the Indemnified Party and the Indemnifying Party and counsel to the Indemnified Party shall have reasonably concluded that there may be reasonable defenses available to the Indemnified Party that are different from or in addition to those available to the Indemnifying Party or if the interests of the Indemnified Party reasonably may be deemed to conflict with the interests of the Indemnifying Party, then the Indemnified Party shall have the right to select a separate counsel and to assume such legal defense and otherwise to participate in the defense of such action, with the expenses and fees of such separate counsel and other expenses related to such participation to be reimbursed by the Indemnifying Party as incurred. Notwithstanding any other provision of this Agreement, the Indemnifying Party shall not settle any indemnified claim without the consent of the Indemnified Party which consent shall not be unreasonably withheld or delayed, unless the settlement thereof imposes no liability or obligation on, and includes a complete release from liability of, and does not include any admission of wrongdoing or malfeasance by, the Indemnified Party.
9.5. Contribution.
(a) If the indemnification under Section 9.2 or Section 9.3 is unavailable to an Indemnified Party or insufficient to hold an Indemnified Party harmless for any losses, claims, damages, liabilities and expenses, then each Indemnifying Party shall contribute to the amount paid or payable by such Indemnified Party, in such proportion as is appropriate to reflect the relative fault of the Indemnifying Party and Indemnified Party in connection with the actions, statements or omissions that resulted in such loss, claim, damage, liability, expense or action as well as any other relevant equitable considerations. The relative fault of such Indemnifying Party and Indemnified Party shall be determined by reference to, among other things, whether any action in question, including any untrue or alleged untrue statement of a material fact or omission or alleged omission of a material fact, has been taken or made by, or relates to information supplied by, such Indemnifying Party or Indemnified Party, and the parties’ relative intent, knowledge, access to information and opportunity to correct or prevent such action, statement or omission; provided, however, that no Person guilty of fraudulent misrepresentation (within the meaning of Section 11(f) of the 1933 Act) will be entitled to contribution from any person who was not guilty of such fraudulent misrepresentation. The amount paid or payable by a party as a result of any losses, claims, damages, liabilities and expenses shall be deemed to include, subject to the limitations set forth in this Agreement, any reasonable attorneys’ or other fees or expenses incurred by such party in connection with any proceeding to the extent such party would have been indemnified for such fees or expenses if the indemnification provided for in Section 9.2 or Section was available to such party in accordance with its terms.
(b) The parties hereto agree that it would not be just and equitable if contribution pursuant to this Section 9.5(a) were determined by pro rata allocation or by any other method of allocation that does not take into account the equitable considerations referred to in the immediately preceding paragraph. In no event shall the contribution obligation of an Investor be greater in amount than dollar amount of the proceeds received by such Investor upon the sale of the Registrable Securities included in such Registration Statement giving rise to such contribution obligation.
10. Miscellaneous.
10.1. Successors and Assigns. This Agreement may not be assigned by a party hereto without the prior written consent of the Company or the Investors, as applicable; provided, however, that an Investor may assign its rights and delegate its duties hereunder in whole or in part to an Affiliate without the prior written consent of the Company or the other Investors, provided such assignee agrees in writing to be bound by the provisions hereof that apply to Investors. The provisions of this Agreement shall inure to the benefit of and be binding upon the respective permitted successors and assigns of the parties. Without limiting the generality of the foregoing, in the event that the Company is a party to a merger, consolidation, share exchange or similar business combination transaction in which the Common Stock is converted into the equity securities of another Person, from and after the effective time of such transaction, such Person shall, by virtue of such transaction, be deemed to have assumed the obligations of the Company hereunder, the term “Company” shall be deemed to refer to such Person and the term “Securities” shall be deemed to refer to the securities received by the Investors in connection with such transaction. Nothing in this Agreement, express or implied, is intended to confer upon any party other than the parties hereto or their respective permitted successors and assigns any rights, remedies, obligations, or liabilities under or by reason of this Agreement, except as expressly provided in this Agreement.
10.2. Counterparts; E-mail. This Agreement may be executed in one or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. Counterparts may be delivered via electronic mail (including pdf or any electronic signature complying with the U.S. federal ESIGN Act of 2000, e.g., www.docusign.com) or other transmission method and any counterpart so delivered shall be deemed to have been duly and validly delivered and be valid and effective for all purposes.
10.3. Titles and Subtitles. The titles and subtitles used in this Agreement are used for convenience only and are not to be considered in construing or interpreting this Agreement.
10.4. Notices. All notices and other communications given or made pursuant to this Agreement shall be in writing and shall be deemed effectively given upon the earlier of actual receipt, or (a) personal delivery to the party to be notified, (b) when sent, if sent by electronic mail during normal business hours of the recipient, and if not sent during normal business hours, then on the recipient’s next Business Day, (c) two Business Days after having been sent by registered or certified mail, return receipt requested, postage prepaid, or (d) one Business Day after deposit with a nationally recognized overnight courier, freight prepaid, specifying next Business Day delivery, with written verification of receipt. All communications shall be sent to the respective parties at their electronic mail address or address as set forth below, or to such electronic mail address or address as subsequently modified by written notice given in accordance with this Section 10.4.
If to the Company:
Immunic, Inc.
1200 Avenue of the Americas, Suite 200,
New York, NY 10036
Attention: Glenn Whaley
Email: glenn.whaley@imux.com
With a copy (which will not constitute notice) to:
Dentons US LLP
Ilan Katz
1221 Avenue of the Americas
New York, NY 10020
Email: Ilan.Katz@Dentons.com
If to the Investors, to the contact information set forth on Annex I.
10.5. Expenses. The parties hereto shall pay their own costs and expenses in connection herewith regardless of whether the transactions contemplated hereby are consummated; it being understood that each of the Company and each Investor has relied on the advice of its own respective counsel. The Company shall pay any Transfer Agent fees, stamp taxes and other taxes and duties levied in connection with the sale and issuance of the Securities to the Investors and shall pay all expenses in connection with the registration of the Registrable Securities as provided in Section 8.9(h). Notwithstanding the forgoing, at the First Closing, the Company shall pay the reasonable fees and expenses of BVF, in an amount not to exceed, in the aggregate, $100,000.
10.6. Amendments and Waivers. Other than as provided herein, any term of this Agreement may be amended and the observance of any term of this Agreement may be waived (either generally or in a particular instance and either retroactively or prospectively), only with the written consent of the Company and (a) prior to the First Closing, the Majority Investors, including each of BVF and Avidity and (b) following the First Closing, Investors required to purchase a majority of the Shares (or Pre-Funded Warrants) at the Second Closing, including each of BVF and Avidity. Notwithstanding the foregoing, this Agreement may not be amended and the observance of any term of this Agreement may not be waived with respect to any Investor without the written consent of such Investor (x) unless such amendment or waiver applies to all Investors in the same fashion and (y) if such amendment or waiver would require such Investor to purchase additional Shares (or Pre-Funded Warrants) beyond the amounts set forth on Annex I on the date of this Agreement. Any amendment or waiver effected in accordance with this paragraph shall be binding upon (i) prior to First Closing, each Investor and (ii) following the First Closing, each holder of any Securities purchased under this Agreement or Pre-Funded Warrant Shares at the time outstanding, and in each case, each future holder of all such Securities and the Company.
10.7. Publicity.
(a) Except as set forth below, no public release or announcement concerning the transactions contemplated hereby shall be issued by the Company or the Investors without the prior written consent of the Company (in the case of a release or announcement by the Investors) or the Investors (in the case of a release or announcement by the Company) (which consents shall not be unreasonably withheld or delayed), except as such release or announcement may be required by law or the applicable rules or regulations of any securities exchange or securities market, in which case the Company or the Investors, as the case may be, shall allow the Investors or the Company, as applicable, to the extent reasonably practicable in the circumstances, reasonable time to comment on such release or announcement in advance of such issuance. No later than the Trading Day immediately following the date of this Agreement, the Company shall issue a press release or make a public filing with the SEC (the “Cleansing Release”) disclosing all material terms of the transactions contemplated by this Agreement and any other material, nonpublic information that the Company may have provided to any Investor in connection with the transactions contemplated by this Agreement. Notwithstanding anything to the contrary, the Company shall not publicly disclose the name of an Investor or any of its Affiliates or advisers, or include the name of the Investor or any of its Affiliates or advisers in the Cleansing Release, any filing with the SEC or any regulatory agency or trading market filing, press release or any other public announcement without the prior written consent of such Investor except (A) as required by the federal securities laws, rules or regulations and (B) to the extent such disclosure is required by other laws, rules or regulations, at the request of the staff of the SEC or regulatory agency or under the regulations of Nasdaq, in which case of clause (A) or (B), the Company shall provide such Investor with prior written notice (including by e-mail) of such permitted disclosure, and shall reasonably consult with such Investor regarding such disclosure.
(b) Except with respect to the material terms and conditions concerning the transactions contemplated hereby, which shall be disclosed pursuant to Section 10.7(a), the Company covenants and agrees that neither it, nor any other Person acting on its behalf will, after the date of this Agreement, provide any Investor or its agents or counsel with any information that constitutes, or the Company reasonably believes constitutes, material non-public information, unless prior thereto such Investor shall have consented to the receipt of such information and agreed with the Company to keep such information confidential. The Company understands and confirms that each Investor shall be relying on the foregoing covenant in effecting transactions in securities of the Company.
(c) To the extent that the Company delivers any material, non-public information to an Investor without such Investor’s consent, the Company hereby covenants and agrees that such Investor shall not have any duty of confidentiality to the Company, the Subsidiaries, or any of their respective officers, directors, agents, employees or Affiliates, or a duty to the Company, the Subsidiary or any of their respective officers, directors, agents, employees or Affiliates not to trade on the basis of, such material, non-public information, provided that the Investor shall remain subject to applicable law. To the extent that any notice provided pursuant to any Transaction Document constitutes, or contains, material, non-public information regarding the Company or any Subsidiaries, the Company shall simultaneously file such notice with the Commission pursuant to a Current Report on Form 8-K. The Company understands and confirms that each Investor shall be relying on the foregoing covenant in effecting transactions in securities of the Company.
10.8. Severability. Any provision of this Agreement that is prohibited or unenforceable in any jurisdiction shall, as to such jurisdiction, be ineffective to the extent of such prohibition or unenforceability without invalidating the remaining provisions hereof but shall be interpreted as if it were written so as to be enforceable to the maximum extent permitted by applicable law, and any such prohibition or unenforceability in any jurisdiction shall not invalidate or render unenforceable such provision in any other jurisdiction. To the extent permitted by applicable law, the parties hereby waive any provision of law which renders any provision hereof prohibited or unenforceable in any respect.
10.9. Entire Agreement. This Agreement, including the signature pages, exhibits, the other Transaction Documents and any oral or written agreement between the Company (or on its behalf) and any Investor regarding confidentiality matters that was entered into in connection with the transactions contemplated hereby constitute the entire agreement among the parties hereto with respect to the subject matter hereof and thereof and supersede all prior agreements and understandings, both oral and written, between the parties with respect to the subject matter hereof and thereof.
10.10. Further Assurances. The parties shall execute and deliver all such further instruments and documents and take all such other actions as may reasonably be required to carry out the transactions contemplated hereby and to evidence the fulfillment of the agreements herein contained.
10.11. Governing Law; Consent to Jurisdiction; Waiver of Jury Trial.
(a) This Agreement shall be governed by, and construed in accordance with, the internal laws of the State of New York without regard to the choice of law principles thereof (other than Sections 5-1401 and 5-1402 of the General Obligations Law).
(b) Each of the parties hereto irrevocably submits to the exclusive jurisdiction of the courts of the State of New York located in New York County and the United States District Court for the Southern District of New York for the purpose of any suit, action, proceeding or judgment relating to or arising out of this Agreement and the transactions contemplated hereby. Service of process in connection with any such suit, action or proceeding may be served on each party hereto anywhere in the world by the same methods as are specified for the giving of notices under this Agreement. Each of the parties hereto irrevocably consents to the jurisdiction of any such court in any such suit, action or proceeding and to the laying of venue in such court. Each party hereto irrevocably waives any objection to the laying of venue of any such suit, action or proceeding brought in such courts and irrevocably waives any claim that any such suit, action or proceeding brought in any such court has been brought in an inconvenient forum.
(c) EACH OF THE PARTIES HERETO WAIVES ANY RIGHT TO REQUEST A TRIAL BY JURY IN ANY LITIGATION WITH RESPECT TO THIS AGREEMENT AND REPRESENTS THAT COUNSEL HAS BEEN CONSULTED SPECIFICALLY AS TO THIS WAIVER.
10.12. Independent Nature of Investors’ Obligations and Rights. The obligations of each Investor under any Transaction Document are several and not joint with the obligations of any other Investor, and no Investor shall be responsible in any way for the performance of the obligations of any other Investor under any Transaction Document. The decision of each Investor to purchase Securities pursuant to the Transaction Documents has been made by such Investor independently of any other Investor. Nothing contained herein or in any Transaction Document, and no action taken by any Investor pursuant thereto, shall be deemed to constitute the Investors as a partnership, an association, a joint venture or any other kind of entity, or create a presumption that the Investors are in any way acting in concert or as a group with respect to such obligations or the transactions contemplated by the Transaction Documents. Each Investor acknowledges that no other Investor has acted as agent for such Investor in connection with making its investment hereunder and that no Investor will be acting as agent of such Investor in connection with monitoring its investment in the Securities or enforcing its rights under the Transaction Documents. Each Investor shall be entitled to independently protect and enforce its rights, including, without limitation, the rights arising out of this Agreement or out of the other Transaction Documents, and it shall not be necessary for any other Investor to be joined as an additional party in any proceeding for such purpose.
10.13. Benefit of Agreement. The Placement Agent is an intended third-party beneficiary of the representations and warranties of the Company and of each Investor set forth in Section 4 and Section 5, respectively, of this Agreement.
10.14. Exculpation of the Placement Agent. Each party hereto agrees for the express benefit of the Placements Agent and its Affiliates and representatives that:
(a) none of the Placement Agent, its Affiliates or any of its representatives: (i) has any duties or obligations other than those specifically set forth in the engagement letter, dated as of December 21, 2023 (the “Engagement Letter”), between the Company and the Placement Agent; (ii) shall be liable for any improper payment made in accordance with the information provided by the Company; (iii) makes any representation or warranty, or has any responsibilities as to the validity, accuracy, value or genuineness of any information, certificates or documentation delivered by or on behalf of the Company pursuant to this Agreement or the Transaction Documents or in connection with any of the transactions contemplated hereby and thereby; or (iv) shall be liable (x) for any action taken, suffered or omitted by any of them in good faith and reasonably believed to be authorized or within the discretion or rights or powers conferred upon it by this Agreement or any Transaction Document or (y) for anything which any of them may do or refrain from doing in connection with this Agreement or any Transaction Document, except, in each case in this clause (iv), for such party’s own gross negligence, willful misconduct or bad faith.
(b) The Placement Agent and its Affiliates and representatives shall be entitled to (i) rely on, and shall be protected in acting upon, any certificate, instrument, notice, letter or any other document or security delivered to any of them by or on behalf of the Company, and (ii) be indemnified by the Company for acting as the Placement Agents hereunder pursuant to the indemnification provisions set forth in the Engagement Letter.
(remainder of page intentionally left blank)
IN WITNESS WHEREOF, the parties have executed this Agreement or caused their duly authorized officers to execute this Agreement as of the date first above written.
| COMPANY: | |||
| IMMUNIC, INC. | |||
| By: | |||
| Name: | Daniel A. Vitt | ||
| Title: | Chief Executive Officer | ||
Signature Page
IN WITNESS WHEREOF, the parties have executed this Agreement or caused their duly authorized officers to execute this Agreement as of the date first above written.
| INVESTOR: | |||
| FOR ENTITY: | |||
| Name of Entity Above | |||
| By: | |||
| (Signature) | |||
| Name: | |||
| Title: | |||
| INVESTOR: | |||
| FOR INDIVIDUAL | |||
| (Signature) | |||
| Name: | |||
Signature Page
ANNEX I
SCHEDULE OF INVESTORS
| Investor Name, Address and Email | Beneficial Ownership Limitation | First Closing Number of Shares | Second Closing Number of Shares | Third Closing Number of Shares | First Closing Aggregate Purchase Price | Second Closing Aggregate Purchase Price | Third Closing Aggregate Purchase Price |
Annex I
EXHIBIT A
FORM OF PRE-FUNDED WARRANT
(see attached)
Exhibit A – Page 1
APPENDIX I
Form of Investor Questionnaire
INVESTOR QUESTIONNAIRE
To: Immunic, Inc.,
This Investor Questionnaire (“Questionnaire”) must be completed by each potential investor in connection with the offer and sale of the shares of common stock, par value $0.0001 per share (“Common Stock”), and/or the pre-funded warrants to purchase Common Stock (collectively, the “Securities”), of Immunic, Inc., a Delaware corporation (the “Corporation”). The Securities are being offered and sold by the Corporation without registration under the Securities Act of 1933, as amended (the “Securities Act”), and the securities laws of certain states, in reliance on the exemptions contained in Section 4(a)(2) of the Securities Act and on Regulation D promulgated thereunder and in reliance on similar exemptions under applicable state laws. The Corporation must determine that a potential investor meets certain suitability requirements before offering or selling the Securities to such investor. The purpose of this Questionnaire is to assure the Corporation that each investor will meet the applicable suitability requirements. The information supplied by you will be used in determining whether you meet such criteria, and reliance upon the private offering exemptions from registration is based in part on the information herein supplied.
This Questionnaire does not constitute an offer to sell or a solicitation of an offer to buy any security. By signing this Questionnaire, you will be authorizing the Corporation to provide a completed copy of this Questionnaire to such parties as the Corporation deems appropriate in order to ensure that the offer and sale of the Securities will not result in a violation of the Securities Act or the securities laws of any state and that you otherwise satisfy the suitability standards applicable to purchasers of the Securities. All potential investors must answer all applicable questions and complete, date and sign this Questionnaire. Please print or type your responses and attach additional sheets of paper if necessary to complete your answers to any item.
PART A. BACKGROUND INFORMATION
Name of Beneficial Owner of the Securities:
Business Address:__________________________________________________________
(Number and Street)
| City: __________________ | State: ______________ | Zip Code: ______________ |
Telephone Number: _______________________
If a corporation, partnership, limited liability company, trust or other entity:
Type of entity: _________________________
State of formation: _____________________
Approximate Date of formation: __________________
Were you formed for the purpose of investing in the securities being offered?
| Yes | ☐ | No | ☐ |
If an individual:
Residence Address: _____________________________________________________
(Number and Street)
| City: __________________ | State: ______________ | Zip Code: ______________ |
Telephone Number: _______________________
| Age: __________ | Citizenship: _________ |
Where registered to vote: _______
Set forth in the space provided below the state(s), if any, in the United States in which you maintained your residence during the past two years and the dates during which you resided in each state:
__________________________________________________
__________________________________________________
| Are you a director or executive officer of the Corporation? | Yes ☐ | No ☐ |
Social Security or Taxpayer Identification No.: ________________ In order for the Corporation to offer and sell the Securities in conformance with state and federal securities laws, the following information must be obtained regarding your investor status.
PART B. ACCREDITED INVESTOR QUESTIONNAIRE
Please initial each category applicable to you as a purchaser of Securities of the Corporation.
| ☐ | (1) | A bank as defined in Section 3(a)(2) of the Securities Act, or any savings and loan association or other institution as defined in Section 3(a)(5)(A) of the Securities Act whether acting in its individual or fiduciary capacity; |
| ☐ | (2) | A broker or dealer registered pursuant to Section 15 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”); |
| ☐ | (3) | An insurance company as defined in Section 2(a)(13) of the Securities Act; |
| ☐ | (4) | An investment company registered under the Investment Company Act of 1940 or a business development company as defined in Section 2(a)(48) of that act; |
| ☐ | (5) | A Small Business Investment Company licensed by the U.S. Small Business Administration under Section 301(c) or (d) of the Small Business Investment Act of 1958; |
| ☐ | (6) | A plan established and maintained by a state, its political subdivisions, or any agency or instrumentality of a state or its political subdivisions, for the benefit of its employees, if such plan has total assets in excess of $5,000,000; |
| ☐ | (7) | An employee benefit plan within the meaning of the Employee Retirement Income Security Act of 1974, if the investment decision is made by a plan fiduciary, as defined in Section 3(21) of such act, which is either a bank, savings and loan association, insurance company or registered investment adviser, or if the employee benefit plan has total assets in excess of $5,000,000 or, if a self-directed plan, with investment decisions made solely by persons that are accredited investors; |
| ☐ | (8) | A private business development company as defined in Section 202(a)(22) of the Investment Advisers Act of 1940; |
| ☐ | (9) | An organization described in Section 501(c)(3) of the Internal Revenue Code, a corporation, Massachusetts or similar business trust, or partnership, not formed for the specific purpose of acquiring the Securities, with total assets in excess of $5,000,000; |
| ☐ | (10) | A trust, with total assets in excess of $5,000,000, not formed for the specific purpose of acquiring the Securities, whose purchase is directed by a sophisticated person who has such knowledge and experience in financial and business matters that such person is capable of evaluating the merits and risks of investing in the Corporation. |
PART C. BAD ACTOR QUESTIONNAIRE
| 1. | During the past ten years, have you been convicted of any felony or misdemeanor that is related to any securities matter? |
| Yes | ☐ (If yes, please continue to Question 1.a) |
| No | ☐ (If no, please continue to Question 2) |
| a) | If your answer to Question 1 was “yes”, was the conviction related to: (i) the purchase or sale of any security; (ii) the making of any false filing with the Securities and Exchange Commission (the “SEC”); or (iii) the conduct of an underwriter, broker, dealer, municipal securities dealer, investment adviser or paid solicitor of purchasers of securities? |
| Yes ☐ | No ☐ |
| 2. | Are you subject to any court injunction or restraining order entered during the past five years that is related to any securities matter? |
| Yes | ☐ (If yes, please continue to Question 2.a) |
| No | ☐ (If no, please continue to Question 3) |
| a) | If your answer to Question 2 was “yes”, does the court injunction or restraining order currently restrain or enjoin you from engaging or continuing to engage in any conduct or practice related to: (i) the purchase or sale of any security; (ii) the making of any false filing with the SEC; or (iii) the conduct of an underwriter, broker, dealer, municipal securities dealer, investment adviser or paid solicitor of purchasers of securities? |
| Yes ☐ | No ☐ |
| 3. | Are you subject to any final order1 of any governmental commission, authority, agency or officer2(2) related to any securities, insurance or banking matter? |
| Yes | ☐ (If yes, please continue to Question 3.a) |
__________________________
| 1 | A “final order” is defined under Rule 501(g) as a written directive or declaratory statement issued by a federal or state agency described in Rule 506(d)(1)(iii) under applicable statutory authority that provides for notice and an opportunity for a hearing, and that constitutes a final disposition or action by such federal or state agency. |
| 2 | You may limit your response to final orders of: (i) state securities commissions (or state agencies/officers that perform a similar function); (ii) state authorities that supervise or examine banks, savings associations or credit unions; (iii) state insurance commissions (or state agencies/officers that perform a similar function); (iv) federal banking agencies; (v) the U.S. Commodity Futures Trading Commission; or (vi) the U.S. National Credit Union Administration. |
| No | ☐ (If no, please continue to Question 4) |
| a) | If your answer to Question 3 was “yes”: |
| i) | Does the order currently bar you from: (i) associating with an entity regulated by such commission, authority, agency or officer; (ii) engaging in the business of securities, insurance or banking; or (iii) engaging in savings association or credit union activities? |
| Yes ☐ | No ☐ |
| ii) | Was the order (i) entered within the past ten years and (ii) based on a violation of any law or regulation that prohibits fraudulent, manipulative or deceptive conduct? |
| Yes ☐ | No ☐ |
| 4. | Are you subject to any SEC disciplinary order?3 |
| Yes | ☐ (If yes, please continue to Question 4.a) |
| No | ☐ (If no, please continue to Question 5) |
| a) | If your answer to Question 4 was “yes”, does the order currently: (i) suspend or revoke your registration as a broker, dealer, municipal securities dealer or investment adviser; (ii) place limitations on your activities, functions or operations; or (iii) bar you from being associated with any particular entity or class of entities or from participating in the offering of any penny stock? |
| 5. | Are you subject to any SEC cease and desist order entered within the past five years? |
| Yes | ☐ (If yes, please continue to Question 5.a) |
| No | ☐ (If no, please continue to Question 6) |
| a) | If your answer to Question 5 was “yes”, does the order currently require you to cease and desist from committing or causing a violation or future violation of (i) any knowledge-based anti-fraud provision of the U.S. federal securities laws[4] or (ii) Section 5 of the Securities Act? |
__________________________
| 3 | You may limit your response to disciplinary orders issued pursuant to Sections 15(b) or 15B(c) of the Exchange Act or Section 203(e) or (f) of the Investment Advisers Act of 1940 (the “Advisers Act”). |
| 4 | Including (but not limited to) Section 17(a)(1) of the Securities Act, Section 10(b) of the Exchange Act and Rule 10b-5 thereunder, Section 15(c)(1) of the Exchange Act, and Section 206(1) of the Advisers Act or any other rule or regulation thereunder. |
| Yes ☐ | No ☐ |
| 6. | Have you been suspended or expelled from membership in, or suspended or barred from association with a member of, a registered national securities exchange or a registered national or affiliated securities association? |
| Yes | ☐ (If yes, please describe the basis of any such suspension or expulsion and any related details in the space provided under Question 10 below)5 |
| No | ☐ (If no, please continue to Question 7) |
| 7. | Have you registered a securities offering with the SEC, made an offering under Regulation A or been named as an underwriter in any registration statement or Regulation A offering statement filed with the SEC? |
| Yes | ☐ (If no, please continue to Question 7.a) |
| No | ☐ (If no, please continue to Question 8) |
a) If your answer to Question 7 was “yes”:
| i) | During the past five years, was any such registration statement or Regulation A offering statement the subject of a refusal order, stop order or order suspending the Regulation A exemption? |
| Yes ☐ | No ☐ |
| ii) | Is any such registration statement or Regulation A offering statement currently the subject of an investigation or proceeding to determine whether a stop order or suspension order should be issued? |
| Yes ☐ | No ☐ |
| 8. | Are you subject to a U.S. Postal Service false representation order entered within the past five years? |
| Yes ☐ | No ☐ |
| 9. | Are you currently subject to a temporary restraining order or preliminary injunction with respect to conduct alleged by the U.S. Postal Service to constitute a scheme or device for obtaining money or property through the mail by means of false representations? |
| Yes ☐ | No ☐ |
__________________________
| 5 | In providing additional information, please explain whether or not the suspension or expulsion resulted from “any act or omission to act constituting conduct inconsistent with just and equitable principles of trade.” |
| 10. | In the space provided below, describe any facts or circumstances that caused you to answer “yes” to any Question (indicating the corresponding Question number). Attach additional pages if necessary. |
| ___________________________________________________________________ |
| ___________________________________________________________________ |
| ___________________________________________________________________ |
| ___________________________________________________________________ |
| ___________________________________________________________________ |
| ___________________________________________________________________ |
| ___________________________________________________________________ |
| ___________________________________________________________________ |
(SIGNATURE PAGE FOLLOWS)
| A. | FOR EXECUTION BY AN INDIVIDUAL: |
| By: ____________________________________________ | |
| Print Name: ______________________________________ | |
| Date: ___________________________________________ | |
| B. | FOR EXECUTION BY AN ENTITY: |
| Entity Name:______________________________________ | |
| By: ____________________________________________ | |
| Print Name: ______________________________________ | |
| Title:____________________________________________ | |
| Date:____________________________________________ |
| C. | ADDITIONAL SIGNATURES (if required by partnership, corporation or trust document): |
| Entity Name:______________________________________ | |
| By: ____________________________________________ | |
| Print Name: ______________________________________ | |
| Title:____________________________________________ | |
| Date:____________________________________________ |
| Entity Name:______________________________________ | |
| By: ____________________________________________ | |
| Print Name: ______________________________________ | |
| Title:____________________________________________ | |
| Date:____________________________________________ |

Immunic, Inc. Announces Private Placement of up to $240 Million
NEW YORK, January 5, 2024 – Immunic, Inc. (Nasdaq: IMUX) (“Immunic” or the “Company”), a biotechnology company developing a clinical pipeline of orally administered, small molecule therapies for chronic inflammatory and autoimmune diseases, today announced it has entered into a securities purchase agreement with select accredited investors to purchase shares of common stock (or pre-funded warrants in lieu thereof) in a three-tranche offering.
The first tranche is an upfront payment of $80 million at $1.43 per share, which is expected to close on January 8, 2024, subject to customary closing conditions. The second tranche is a mandatory purchase of an additional $80 million of shares of common stock (or pre-funded warrants) at $1.716 per share, representing 120% of the first tranche purchase price and is conditioned on the announcement of phase 2b topline data for the Company’s vidofludimus calcium (IMU-838) progressive multiple sclerosis clinical trial, volume weighted average share price levels, and minimum trading volumes. A third tranche, to occur no later than three years after the second tranche, provides for the issuance of $80 million of shares of common stock (or pre-funded warrants in lieu thereof) at the same price per share as the second tranche, but permits investors to fund their purchase obligations on a “cashless” or net settlement basis, which would reduce the proceeds to be raised in the financing. The third tranche is conditioned on the same volume weighted average share price levels and minimum trading volumes as the second tranche. Assuming that the second tranche is exercised, and depending on the extent to which the investors elect to fund the third tranche through a net settlement basis, total gross proceeds from the offering to the Company would be between $160 and $240 million.
The financing is being led by BVF Partners L.P., and includes participation from new and existing investors, including Avidity Partners, Janus Henderson Investors, Soleus Capital, RTW Investments and Adage Capital Partners LP.
The Company is obligated to register for resale by the investors all of the shares of common stock issued in the offering and the shares of common stock issuable on exercise of the pre-funded warrants.
Leerink Partners is acting as the lead placement agent and Ladenburg Thalmann is acting as a placement agent in connection with the financing. Piper Sandler, B. Riley Securities and Brookline Capital Markets, a division of Arcadia Securities, LLC, are acting as capital markets advisors to the Company.
The Company intends to use the net proceeds from the private placement to fund the ongoing clinical development of its three lead product candidates, vidofludimus calcium (IMU-838), IMU-856 and IMU-381, and for other general corporate purposes. The proceeds from the first tranche of this private placement, combined with current cash, cash equivalents and marketable securities, is expected to fund operating and capital expenditures into the third quarter of 2025.
The securities to be sold in this offering, including the shares of common stock issuable on exercise of the pre-funded warrants, have not been registered under the Securities Act of 1933, as amended (the “Securities Act”), or the securities laws of any state or other jurisdiction and may not be offered or sold in the United States absent registration or an applicable exemption from the registration requirements of the Securities Act and applicable state or other jurisdictions’ securities laws. Immunic has agreed to file a registration statement with the U.S. Securities and Exchange Commission registering the resale of the securities issued in the private placement. Any offering of the securities under the resale registration statement will only be made by means of a prospectus.
This press release does not constitute an offer to sell or the solicitation of an offer to buy, nor will there be any sales of these securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of such jurisdiction.

About Immunic, Inc.
Immunic, Inc. (Nasdaq: IMUX) is a biotechnology company developing a clinical pipeline of orally administered, small molecule therapies for chronic inflammatory and autoimmune diseases. The company’s lead development program, vidofludimus calcium (IMU-838), is currently in phase 3 and phase 2 clinical trials for the treatment of relapsing and progressive multiple sclerosis, respectively, and has shown therapeutic activity in phase 2 clinical trials in patients suffering from relapsing-remitting multiple sclerosis, progressive multiple sclerosis and moderate-to-severe ulcerative colitis. Vidofludimus calcium combines neuroprotective effects, through its mechanism as a first-in-class nuclear receptor related 1 (Nurr1) activator, with additional anti-inflammatory and anti-viral effects, by selectively inhibiting the enzyme dihydroorotate dehydrogenase (DHODH). IMU-856, which targets the protein Sirtuin 6 (SIRT6), is intended to restore intestinal barrier function and regenerate bowel epithelium, which could potentially be applicable in numerous gastrointestinal diseases, such as celiac disease, for which it is currently in preparations for a phase 2 clinical trial. IMU-381, which currently is in preclinical testing, is a next generation molecule being developed to specifically address the needs of gastrointestinal diseases. For further information, please visit: www.imux.com.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains “forward-looking statements” that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, included in this press release regarding expected future events, including the closing of each tranche of the Company’s private placement financing, the timely funding to the Company by each investor in the private placement, strategy, future operations, future financial position, future revenue, projected expenses, sufficiency of cash, expected timing and results of clinical trials, prospects, plans and objectives of management are forward-looking statements. Examples of such statements include, but are not limited to, statements relating to expected timing and funding to the Company from the private placement, Immunic’s development programs and the targeted diseases; the potential for Immunic’s development programs to safely and effectively target diseases; interpretation of preclinical and clinical data for Immunic’s development programs and potential effects; the timing of current and future clinical trials and anticipated clinical milestones; the nature, strategy and focus of the company and further updates with respect thereto; the development and commercial potential of any product candidates of the company; and the company’s expected cash runway. Immunic may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in the forward-looking statements and you should not place undue reliance on these forward-looking statements. Such statements are based on management’s current expectations and involve substantial risks and uncertainties. Actual results and performance could differ materially from those projected in the forward-looking statements as a result of many factors, including, without limitation, the COVID-19 pandemic, impacts of the Ukraine – Russia conflict and the conflict in the Middle East on clinical trials, risks and uncertainties associated with the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations, the availability of sufficient financial and other resources to meet business objectives and operational requirements, the fact that the results of earlier preclinical studies and clinical trials may not be predictive of future clinical trial results, the protection and market exclusivity provided by Immunic’s intellectual property, risks related to the drug development and the regulatory approval process and the impact of competitive products and technological changes. A further list and descriptions of these risks, uncertainties and other factors can be found in the section captioned “Risk Factors” in the company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed with the SEC on February 23, 2023, and in the company’s subsequent filings with the SEC. Copies of these filings are available online at www.sec.gov or ir.imux.com/sec-filings. Any forward-looking statement made in this release speaks only as of the date of this release. Immunic disclaims any intent or obligation to update these forward-looking statements to reflect events or circumstances that exist after the date on which they were made. Immunic expressly disclaims all liability in respect to actions taken or not taken based on any or all the contents of this press release.
Contact Information
Immunic, Inc.
Jessica Breu
Head of Investor Relations and Communications
+49 89 2080 477 09
jessica.breu@imux.com
Rx Communications Group
Paula Schwartz
+1 917 633 7790
immunic@rxir.com
US Media Contact
KOGS Communication
Edna Kaplan
+1 617 974 8659
kaplan@kogspr.com

Immunic Highlights 2023 Accomplishments and Upcoming Milestones
– Evidence for Neuroprotective Activity of Vidofludimus Calcium from Phase 2 CALLIPER Interim Analysis, Consistent Across the Entire Progressive Multiple Sclerosis Population and All Subtypes –
– Phase 3 ENSURE Program in Relapsing Multiple Sclerosis Ongoing –
– Improvements in Gut Health Demonstrated in Phase 1b Clinical Trial of IMU-856 in Celiac Disease –
– Expanded Vidofludimus Calcium Patent Portfolio with Additional New Patents Granted; Exclusivity Protection Expected Into 2041 in the United States, Unless Extended Further –
– Significantly Strengthened Balance Sheet; Cash Runway Based on First Tranch of January 2024 Financing Extended Into the Third Quarter of 2025 –
NEW YORK, January 5, 2024 – Immunic, Inc. (Nasdaq: IMUX), a biotechnology company developing a clinical pipeline of orally administered, small molecule therapies for chronic inflammatory and autoimmune diseases, today highlighted its 2023 accomplishments and upcoming milestones.
“The past year has been an extra-ordinarily productive and successful one for Immunic, with key clinical data releases highlighting the uniqueness and enormous value potential of each of our two latest-stage clinical assets” stated Daniel Vitt, Ph.D., Chief Executive Officer and President of Immunic. “With vidofludimus calcium (IMU-838) and IMU-856, we have two development programs beyond clinical proof-of-concept which is an outstanding achievement of our entire team. Regarding our lead asset, nuclear receptor related 1 (Nurr1) activator, vidofludimus calcium, the interim biomarker analysis from the phase 2 CALLIPER trial demonstrated clear separation from placebo in serum neurofilament light chain (NfL) levels in patients with progressive multiple sclerosis (PMS). This effect was observed across all subpopulations, including non-active secondary progressive multiple sclerosis, which we believe represents the segment of greatest unmet need in multiple sclerosis (MS). We look forward to continuing development of this potentially groundbreaking asset. Enrollment in CALLIPER is complete, with top-line data expected in April 2025. Meanwhile, enrollment in our phase 3 ENSURE program remains active, with an interim futility analysis expected in late 2024 and the read-out of the first of the ENSURE trials anticipated in the second quarter of 2026.”
Dr. Vitt continued, “Regarding IMU-856, our orally available and systemically acting small molecule modulator targeting Sirtuin 6 (SIRT6), results from our phase 1b trial demonstrated meaningful improvements over placebo in four key dimensions of celiac disease pathophysiology: histology, disease symptoms, biomarkers and nutrient absorption. We believe this data provides initial clinical proof-of-concept for a p000000000000000000000000000000000000000000000000.otentially new therapeutic approach to a multitude of gastrointestinal disorders through the regeneration of bowel architecture rather than the traditional immunomodulatory approaches in many gastrointestinal indications. We are currently preparing for phase 2 testing in ongoing active celiac disease (OACD), with further clinical applications in other gastrointestinal disorders also being considered.”

“On a scientific level, we announced preclinical evidence showing that vidofludimus calcium acts as a potent Nurr1 activator, which may be associated with both its hypothesized neuroprotective effects and the reduced disability-worsening events observed in our phase 2 EMPhASIS trial in relapsing-remitting MS patients. We have also continued to strengthen our patent portfolio for vidofludimus and its salt and free acid forms, with additional new layers of patents granted, now providing protection into 2041 in the United States and into 2038 internationally. We expect to continue to expand the patent protection around vidofludimus calcium in the future,” Dr. Vitt continued.
“Financially, we announced a three-tranche private placement of up to $240 million earlier today. We expect the first $80 million tranche to extend our cash runway into the third quarter of 2025, which well covers the top-line data readout of our CALLIPER trial in Aprill 2025. The second tranche, assuming it is excersied, would even finance us into the first quarter of 2027. Despite challenging capital markets, we are proud to see enormous support from new and existing investors, including lead investor BVF Partners, as well as Avidity Partners, Janus Henderson Investors, Soleus Capital, RTW Investments and Adage Capital Partners LP. Finally, I want to share my pride in our broader team, who were honored to win multiple, prestigious international awards in 2023 recognizing Immunic’s excellence in a number of clinical, corporate and communications categories. I am very excited to see what our team can achieve in 2024 and beyond,” concluded Dr. Vitt.
A more thorough review of recent events and upcoming milestones follows:
Corporate Highlights
| · | Announced a three-tranche private placement of up to $240 million with participation from select new and existing investors today. |
| · | Strengthened Board of Directors in April 2023 with the addition of Richard Rudick, M.D., a thought-leader in MS with decades of experience in the clinic, academia and industry. |
Vidofludimus Calcium 2023 Highlights and Upcoming Milestones
| · | Reported positive interim data from the phase 2 CALLIPER trial of vidofludimus calcium in PMS in October. Serum NfL improvements were consistently observed for vidofludimus calcium across PMS and all disease subtypes, as well as in patients who showed or did not show disease and/or magnetic resonance imaging (MRI) activity. Immunic believes that this data illustrates biomarker evidence that vidofludimus calcium’s activity extends beyond the previously observed anti-inflammatory effects, further reinforcing its neuroprotective potential. Enrollment of the trial was completed in August. In total, 467 patients with primary PMS, or active or non-active secondary PMS, were randomized to either 45 mg of vidofludimus calcium or placebo. |
| · | Published preclinical data in the peer-reviewed, high impact, Journal of Medicinal Chemistry in May, confirming that vidofludimus calcium acts as a potent Nurr1 activator, in addition to its known mode of action as a dihydroorotate dehydrogenase (DHODH) inhibitor. Data showed that activation of Nurr1 could be responsible for the drug’s postulated neuroprotective effects and may contribute to the previously reported reduction of confirmed disability worsening events seen in the phase 2 EMPhASIS trial in relapsing-remitting MS patients. |
| · | Reported positive data from the maintenance phase of the phase 2b CALDOSE-1 trial of vidofludimus calcium in patients with moderate-to-severe ulcerative colitis in April. |
| · | Significantly strengthened the multiple layers of patent protection around vidofludimus calcium, and other salt and free acid forms, with the receipt of two Notices of Allowance from the USPTO, covering the dosing regimens in MS and the dose strength for the treatment of RMS. The current patent portfolio provides protection into 2041 in the United States and into 2038 internationally, unless extended further. |

| · | Top-line data from the phase 2 CALLIPER trial of vidofludimus calcium in PMS is expected in April 2025. |
| · | An interim futility analysis of the ENSURE program is expected in late 2024. The read-out of the first of the ENSURE trials is currently anticipated in the second quarter of 2026; and the second ENSURE trial in the second half of 2026. |
IMU-856 2023 Highlights and Upcoming Milestones
| · | Announced, for the first time, IMU-856’s molecular mode of action as a highly selective and potent small molecule modulator of SIRT6, a protein which serves as a transcriptional regulator of intestinal barrier function and regeneration of bowel epithelium, in May. |
| · | Announced positive results from the part C portion of the phase 1 clinical trial of IMU-856 in patients with celiac disease in May. The data demonstrated positive effects for IMU-856 over placebo in four key dimensions of celiac disease pathophysiology: protection of the gut architecture, improvement of patients’ symptoms, biomarker response and enhancement of nutrient absorption. IMU-856 was also observed to be safe and well-tolerated in this trial. Immunic believes this data provides initial clinical proof-of-concept for an entirely new therapeutic approach to gastrointestinal disorders by promoting regeneration of bowel architecture. |
| · | The company is preparing for clinical phase 2 testing of IMU-856 in OACD patients. |
Award Recognition
| · | MS R&D webcast held in November 2022: |
| o | Gold German Stevie Awards for ‘Best Medical Event’ and ‘Best B2B Event’ |
| o | American Business Gold Awards in the ‘Conferences & Meetings – Medical Congress’ and ‘Conferences & Meetings – Scientific Congress’ categories and Bronze Award in the ‘Corporate & Community – B2B Event’ category |
| o | International Business Gold Award in the ‘Conferences & Meetings - Medical Congress’ category |
| · | Newly designed, accessibility-friendly website: |
| o | Gold German Stevie Award for ‘Best Overall Design’ and Bronze Stevie Award for ‘Special Achievement in Diversity & Inclusion’ |
| o | American Business Silver Award in the ‘Achievement in Diversity & Inclusion’ category |
| · | BioTech Breakthrough Award winner for the ‘Developmental Immunology Solution of the Year’ for the IMU-856 development program, including the positive phase 1b clinical trial results in celiac disease |
| · | Hella Kohlhof, Ph.D., Chief Scientific Officer of Immunic, was awarded a Silver Stevie Award for Women in Business in the ‘Women in Healthcare’ category |
Immunic’s management, business development and investor relations teams will be hosting one-on-one meetings in connection with the 42nd Annual J.P. Morgan Health Care Conference taking place January 8-11, 2024 in San Francisco. To schedule a meeting, please contact: Jessica Breu at jessica.breu@imux.com.

About Immunic, Inc.
Immunic, Inc. (Nasdaq: IMUX) is a biotechnology company developing a clinical pipeline of orally administered, small molecule therapies for chronic inflammatory and autoimmune diseases. The company’s lead development program, vidofludimus calcium (IMU-838), is currently in phase 3 and phase 2 clinical trials for the treatment of relapsing and progressive multiple sclerosis, respectively, and has shown therapeutic activity in phase 2 clinical trials in patients suffering from relapsing-remitting multiple sclerosis, progressive multiple sclerosis and moderate-to-severe ulcerative colitis. Vidofludimus calcium combines neuroprotective effects, through its mechanism as a first-in-class nuclear receptor related 1 (Nurr1) activator, with additional anti-inflammatory and anti-viral effects, by selectively inhibiting the enzyme dihydroorotate dehydrogenase (DHODH). IMU-856, which targets the protein Sirtuin 6 (SIRT6), is intended to restore intestinal barrier function and regenerate bowel epithelium, which could potentially be applicable in numerous gastrointestinal diseases, such as celiac disease, for which it is currently in preparations for a phase 2 clinical trial. IMU-381, which currently is in preclinical testing, is a next generation molecule being developed to specifically address the needs of gastrointestinal diseases. For further information, please visit: www.imux.com.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains “forward-looking statements” that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, included in this press release regarding expected future events, including the private placement and expected proceeds, strategy, future operations, future financial position, future revenue, projected expenses, sufficiency of cash and cash runway, expected timing, development and results of clinical trials, prospects, plans and objectives of management are forward-looking statements. Examples of such statements include, but are not limited to, statements relating to Immunic’s ability to raise additional capital, if needed; Immunic’s development programs and the targeted diseases; the potential for Immunic’s development programs to safely and effectively target diseases; preclinical and clinical data for Immunic’s development programs; the timing of current and future clinical trials and anticipated clinical milestones; the nature, strategy and focus of the company and further updates with respect thereto; the development and commercial potential of any product candidates of the company; and the company’s expected cash runway. Immunic may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in the forward-looking statements and you should not place undue reliance on these forward-looking statements. Such statements are based on management’s current expectations and involve substantial risks and uncertainties. Actual results and performance could differ materially from those projected in the forward-looking statements as a result of many factors, including, without limitation, the COVID-19 pandemic, increasing inflation, impacts of the Ukraine – Russia conflict and the conflict in the Middle East on planned and ongoing clinical trials, risks and uncertainties associated with the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations, the availability of sufficient financial and other resources to meet business objectives and operational requirements, the fact that the results of earlier preclinical studies and clinical trials may not be predictive of future clinical trial results, the protection and market exclusivity provided by Immunic’s intellectual property, risks related to the drug development and the regulatory approval process and the impact of competitive products and technological changes. A further list and descriptions of these risks, uncertainties and other factors can be found in the section captioned “Risk Factors,” in the company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed with the SEC on February 23, 2023, and in the company’s subsequent filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov or ir.imux.com/sec-filings. Any forward-looking statement made in this release speaks only as of the date of this release. Immunic disclaims any intent or obligation to update these forward-looking statements to reflect events or circumstances that exist after the date on which they were made. Immunic expressly disclaims all liability in respect to actions taken or not taken based on any or all the contents of this press release.

Contact Information
Immunic, Inc.
Jessica Breu
Head of Investor Relations and Communications
+49 89 2080 477 09
jessica.breu@imux.com
US IR Contact
Rx Communications Group
Paula Schwartz
+1 917 633 7790
immunic@rxir.com
US Media Contact
KOGS Communication
Edna Kaplan
+1 617 974 8659
kaplan@kogspr.com