UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported)
December 6, 2025
ORIC Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 001-39269 | 47-1787157 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
240 E. Grand Ave, 2nd Floor
South San Francisco, CA 94080
(Address of principal executive offices, including zip code)
(650) 388-5600
(Registrant’s telephone number, including area code)
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
||
| Common stock, par value $0.0001 per share | ORIC | The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒ ORIC Pharmaceuticals, Inc. (the “Company”) presented an enozertinib (ORIC-114) program update (the “Program Update”) on December 6, 2025. The Program Update covered recently announced data from the Company’s Phase 1b trial of enozertinib. A presentation containing the data presented in the Program Update is attached as Exhibit 99.1 hereto and is incorporated herein by reference.
| Item 8.01 | Other Events. |
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits.
| Exhibit Number |
Description |
|
| 99.1 | Presentation | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| ORIC PHARMACEUTICALS, INC. | ||||||
| Date: December 8, 2025 | By: | /s/ Christian V. Kuhlen |
||||
| Christian V. Kuhlen, M.D., J.D. | ||||||
| General Counsel | ||||||

Exhibit 99.1 Enozertinib (ORIC-114) Program Update December 6, 2025

Forward-Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements other than statements of historical facts contained in this presentation, including statements regarding ORIC Pharmaceuticals, Inc.’s (“ORIC”, “we”, “us” or “our”) future financial condition, results of operations, business strategy and plans, and objectives of management for future operations, as well as statements regarding industry trends, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potentially,” “predict,” “should,” “will” or the negative of these terms or other similar expressions. Forward-looking statements contained in this presentation also include, but are not limited to, statements regarding: the potential best-in-class profile of enozertinib (ORIC-114), including antitumor activity that exceeds competitor benchmarks; our development plans and timelines; the potential advantages of enozertinib; plans for the clinical trials and development of enozertinib and ORIC-944; enozertinib clinical outcomes, which may materially change as patient enrollment continues or more patient data becomes available; the expected timing of reporting data from our clinical trials; our anticipated milestones and clinical updates; and the period over which we estimate our existing cash and investments will be sufficient to fund our current operating plan. We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including, among other things: the timing of the initiation, progress and results of our preclinical studies and clinical trials; risks associated with the process of developing and commercializing drugs that are safe and effective for use in humans and operating as an early clinical stage company; negative impacts of health emergencies, economic instability or international conflicts on our operations, including clinical trials; the potential for current or future clinical trials of product candidates to differ from preclinical, initial, interim, preliminary or expected results; our ability to advance product candidates into, and successfully complete, clinical trials; the timing or likelihood of regulatory filings and approvals; changes in our plans to develop and commercialize our product candidates; our estimates of the number of patients who suffer from the diseases we are targeting and the number of patients that may enroll in our clinical trials; the commercializing of our product candidates, if approved; our ability to successfully manufacture and supply our product candidates for clinical trials and for commercial use, if approved; potential benefits and costs of strategic arrangements, licensing and/or collaborations; the risk of the occurrence of any event, change or other circumstance that could give rise to the termination of our license or collaboration agreements; our estimates regarding expenses, future revenue, capital requirements and needs for financing and our ability to obtain capital; the sufficiency of our existing cash and investments to fund our future operating expenses and capital expenditure requirements; our ability to retain the continued service of our key personnel and to identify, hire and retain additional qualified professionals; the implementation of our business model and strategic plans for our business and product candidates; the scope of protection we are able to establish and maintain for intellectual property rights, product candidates and our pipeline; our ability to contract with third-party contract research organizations, suppliers and manufacturers and their ability to perform adequately; the pricing, coverage and reimbursement of our product candidates, if approved; developments relating to our competitors and our industry, including competing product candidates and therapies; regulatory developments in the United States and foreign countries; general economic and market conditions; and the other risks, uncertainties and assumptions discussed in the public filings we have made and will make with the Securities and Exchange Commission (“SEC”). These risks are not exhaustive. New risk factors emerge from time to time and it is not possible for our management to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data and estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Except as required by law, we undertake no obligation to update any statements in this presentation for any reason after the date of this presentation. We have filed Current Reports on Form 8-K, Quarterly Reports on Form 10-Q, Annual Reports on Form 10-K, and other documents with the SEC. You should read these documents for more complete information about us. You may obtain these documents for free by visiting EDGAR on the SEC website at www.sec.gov. This presentation discusses our product candidates that are under preclinical or clinical study, and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of our product candidates for the therapeutic use for which they are being studied. 2

Enozertinib Program Update Agenda • Executive Summary • Preclinical Differentiation • ESMO Asia Clinical Highlights – 2L EGFR exon 20 – 1L EGFR exon 20 – Pretreated EGFR PACC • 1L EGFR PACC (Preliminary Data) • Executive Summary and Next Steps ORIC Participants • Jacob Chacko, Chief Executive Officer • Lori Friedman, Chief Scientific Officer • Pratik Multani, Chief Medical Officer • Dominic Piscitelli, Chief Financial Officer • Matt Panuwat, Chief Business Officer • Keith Lui, SVP Commercial and Medical Affairs 3

Executive Summary
Enozertinib Establishes Potential Best-in-Class Profile in EGFR-Mutated NSCLC Ø Systemic activity in 2L EGFR exon 20 and pretreated EGFR PACC exceeds competitor benchmarks Ø Highly competitive preliminary 1L systemic activity, with 67% ORR in EGFR exon 20 and 80% ORR in EGFR PACC Ø Convincing 1L CNS activity, with 100% intracranial ORR in EGFR exon 20 and 100% intracranial ORR in EGFR PACC, including in patients with active brain metastases Ø Competitive safety profile, with no significant off-target toxicity and manageable on-target toxicity, resulting in low rate of discontinuations Ø 80 mg once-daily selected as recommended dose for potential Phase 3 development Ø Enrollment and follow-up continues in 1L EGFR exon 20 and 1L EGFR PACC with next update (1) expected mid-2026, ahead of potential initiation of Phase 3 trial(s) Source: John et al. ESMO Asia (2025), Hong et al. ESMO Asia (2025), and ORIC data on file. 5 (1) Enrollment in HER2 exon 20 has been completed with no further development planned in this patient population.

Enozertinib Is Pursuing a Significant Commercial Opportunity Across Patient Populations that Lack an Approved CNS Active Agent Estimated Enozertinib Commercial Opportunity (US Only) 10,000 • ~9,000 patients (~5% of NSCLC) diagnosed annually 8,000 • EGFR exon 20 mutant NSCLC: no approved CNS-active 5,000 patients therapies 6,000 • EGFR PACC mutant NSCLC: no approved therapies 4,000 Potential commercial opportunity of 2,000 4,000 patients ~$3.0 to $3.5 billion in the US annually 0 EGFR Exon 20 EGFR PACC Enozertinib has the potential to address ~9,000 patients with NSCLC annually, representing a commercial opportunity of ~$3.0 to $3.5 billion in the US alone Source: American Cancer Society Cancer Facts & Figures 2025, Heymach et al. WCLC (2018), and Robichaux et al. Nature (2021). Note: Estimated addressable market assumes 11.4-month treatment duration for EGFR exon 20 based on Girard et al. N Engl J Med (2023), 16.0-month treatment duration based on Le et al. WCLC (2025), and current price of tyrosine kinase 6 inhibitors for NSCLC. Estimated Annual Incidence in the US

Best-in-Class Profile Yet to Emerge in Treatment Landscape for NSCLC Patients Harboring EGFR Exon 20 and EGFR PACC Mutations What is a Best-In-Class Target Product Profile for an EGFR Exon 20 and EGFR PACC Inhibitor? q Manageable on-target toxicities (e.g., GI and skin) Safety / q No significant off-target toxicities (e.g., cardiac, hematologic and liver) Tolerability q Low rate of treatment discontinuations q Strong systemic antitumor activity Systemic and CNSq Treatment of active brain metastases Activity q Prevention of CNS progression Durability of q Strong systemic and CNS activity with a manageable safety profile Response translates to differentiated long-term clinical benefit There remains an unmet need for a highly selective EGFR inhibitor that is also brain-penetrant to effectively treat and prevent intracranial disease 7

High Burden of CNS Disease in NSCLC Patients with EGFR Mutations Leads to Disease Progression and Limited Survival with Current Therapies Initial Diagnosis Disease Progression on Treatment CNS Disease Highly ~50% of patients eventually develop Prevalent in EGFR ~30% of patients have known brain metastases, often as the first (1) CNS disease at diagnosis Mutated NSCLC… (2) site of progression (3) (4) Mobocertinib Amivantamab + Chemotherapy Median Progression Free Survival Risk of Progression or Death vs. Control Arm …And Patients With Patients Without 8.1 Patients Without 67% Risk CNS Metastases Derive Baseline CNS Mets months History of Brain Mets Reduction Limited Benefit From Non-CNS-Active Drugs 3.7 37% Risk Patients With Patients With months Reduction Baseline CNS Mets History of Brain Mets An effective, brain-penetrant therapy can potentially drive long-term outcomes and extend survival through durable CNS control (1) Patil et al. Clin Lung Cancer (2021). (2) Wilcox et al. Ann Oncol. (3) Janne et al. ASCO (2019) and Ramalingam et al. ASCO (2021). (4) Girard et al. ESMO Presentation (2023). 8

Enozertinib Phase 1b Data Establishes Potential Best-in-Class Profile in EGFR Exon 20 and PACC Mutated NSCLC Enozertinib Updated Phase 1b Data Highlights 2L EGFR Exon 20 Median 3L EGFR PACC 45% 36% ORR ORR Enozertinib Enozertinib Previously Treated 22% 40% Benchmark ORR Benchmark ORR 1L EGFR Exon 20 (Preliminary Data) 1L EGFR PACC (Preliminary Data) 80% ORR 67% ORR Enozertinib Enozertinib Treatment- Naïve (1L) Intracranial ORR Enozertinib 100% Intracranial ORR Enozertinib 100% Enozertinib data in previously treated patients with EGFR exon 20 and PACC mutations exceed competitor benchmarks; preliminary 1L systemic and intracranial activity establishes potential best-in-class profile Source: John et al. ESMO Asia (2025), Hong et al. ESMO Asia (2025), and ORIC data on file. Benchmark ORR: Piotrowska et al. J Clin Oncol (2025) and Udagawa et al. WCLC (2025). Note: All data in previously treated patients represent confirmed ORR. All data in 1L patients represent best ORR, due to preliminary nature of data. 1L EGFR exon 20 intracranial ORR data in all patients with measurable CNS disease by BICR-RANO 9 and 1L EGFR PACC intracranial ORR data in all patients with measurable CNS disease by investigator assessment using RECIST.

Preclinical Differentiation

Enozertinib Is a Promising Candidate for NSCLC Patients with EGFR Exon 20 and PACC Mutations, Including Those with Brain Metastases Enozertinib Target Candidate Profile Differentiated Profile Validated in Phase 1b in Patients with • Strong potency against EGFR exon 20 and EGFR Exon 20 and PACC Mutations atypical mutations; superior to competitors • Tumor regressions in multiple in vivo models Robust Mutant Potency • Exquisite selectivity with limited potential for off-target activity ü Potency → responses in breadth of • Kinome selectivity superior to competitors Exquisite Selectivity EGFR mutants ü Selectivity → minimized off-target tox • High unbound (free) brain exposures in vivo ü Brain-penetrance → robust • Substantial tumor regression in intracranial intracranial responses efficacy studies Highly Brain Penetrant Enozertinib is a potential best-in-class inhibitor of EGFR exon 20 and PACC mutations, with superior potency and selectivity, and excellent brain-penetrance driving intracranial responses 11

Enozertinib Best-in-Class Potency Across Breadth of EGFR Exon 20 and Atypical Mutations In Vitro Potency Comparison EC50 (nM) >100 Zipalertinib Firmonertinib Silevertinib Enozertinib 100 L858R Classical del19 763_FQEA 769_ASV Exon 20 770_NPG Insertion 770_SVD 80 773_NPH E709A E709K L718Q L718V 60 G719A G719C G719S G724S Atypical L747P PACC L747S 40 S768I E709A/ G719S G719A/ L861Q del19 / L792H del19 / G796S L858R / L718V 20 L858R / L718Q M277E A289V Atypical A289T Other L861Q Enozertinib displays superior potency across EGFR exon 20 and atypical mutations in vitro, including PACC singleton and complex mutations Source: Junttila et al. Cancer Research 2025. 12

Enozertinib Was Designed to Selectively Target EGFR with High Potency Against Exon 20 and Atypical Mutations Kinome Selectivity Comparison Zipalertinib Firmonertinib Silevertinib Enozertinib Off-target Wildtype Kinases Inhibited ≥80% at 1µM Enozertinib Zipalertinib Firmonertinib Silevertinib 0 7 4 25 Enozertinib has demonstrated an exquisitely clean kinome panel, mitigating the potential for off-target toxicities Source: Junttila et al. Cancer Research 2025. Note: Kinase binding profiles across 468 kinases at 1 μM assessed using KINOMEscan. Red circles indicate kinases impacted within 10% of control. Table reports the number of off-target (non-EGFR/HER2) wildtype kinases inhibited 80% or more. 13

Superior Brain Penetration of Enozertinib Differentiates from Comparator Agents Enozertinib Exhibits High Ratio of Free (Unbound) Enozertinib CNS Efficacy vs. Mobocertinib in Intracranial Brain/Plasma Exposure in Mice NSCLC EGFR Mutant In Vivo Model 9 10 10 Vehicle 8 10 1 7 10 0.1 Mobocertinib 6 (30 mg/kg QD) 10 0.01 5 10 Enozertinib (2.5 mg/kg QD) BQL 4 10 0.001 0 5 10 15 Osimertinib Mobocertinib Zipalertinib Enozertinib Days After Treatment Enozertinib preclinical profile demonstrates superior CNS properties and strong tumor regressions in an intracranial NSCLC model, with potential to treat patients with brain metastases and delay CNS progression Source: Junttila et al. Cancer Research 2025. Note: BQL – below quantifiable limits. 14 Bioluminescence (photons/sec)

ESMO Asia Clinical Highlights EGFR Exon 20 Mutant NSCLC

Enozertinib Phase 1b Trial Enrolled Patients with Previously Treated and Treatment- Naïve NSCLC with EGFR Exon 20 Mutations Enozertinib Phase 1b Trial Design in EGFR Exon 20 Mutations Key Eligibility Criteria: Enozertinib 2L post-chemotherapy 1:1 Randomization 80 mg QD • Locally advanced or Primary endpoint: • Patients with advanced metastatic NSCLC • Selection of recommended NSCLC and EGFR Enozertinib phase 2 dose (RP2D) with EGFR exon 20 exon 20 mutation 120 mg QD mutation Secondary endpoints: • Investigator assessed • Untreated, stable, objective response rate asymptomatic brain (ORR) metastases allowed 1L treatment-naïve • BICR-RANO CNS response Enozertinib Enozertinib • Patients with advanced (1L) • Treatment-naïve or NSCLC and EGFR 120 mg QD 80 mg QD • Safety received 1L exon 20 mutation platinum-based chemotherapy Enrolled 1L and 2L NSCLC patients with EGFR exon 20 mutations, including those with active untreated brain metastases Note: Tumor restaging, including with brain MRI, performed at 4 weeks and every 8 weeks thereafter. BICR – blinded independent central review; QD – once-daily. 16

Phase 1b Trial Patient Demographics and Baseline Characteristics 2L post-chemotherapy, advanced NSCLC with EGFR exon 20 mutations 80 mg 120 mg (n=24) (n=21) Age, years, median (range) 63 (44-75) 70 (28-86) Female, n (%) 17 (71) 16 (76) Non-smoker, n (%) 22 (92) 21 (100) Race: Asian / White / Other, % 42 / 50 / 8 57 / 43 / 0 ECOG performance: 0 / 1, % 29 / 71 19 / 81 Brain metastases at baseline*, n (%) 10 (42) 7 (33) Prior chemotherapy 24 (100) 21 (100) † Prior EGFR targeted therapies 0 2 (10) 38% of 2L patients had brain metastases at study entry, including those with active CNS disease Source: John et al. ESMO Asia (2025). Note: Data as of August 29, 2025. * Patients with brain metastases at study entry, including active brain metastases. † One patient each received prior erlotinib or afatinib. 17

Enozertinib Has Been Generally Well Tolerated Despite Enrolling Heavily-Pretreated Patients and a Less Stringent Enrollment Criteria for Baseline CNS Disease 2L post-chemotherapy, advanced NSCLC with EGFR exon 20 mutations Treatment-Related Adverse Events (TRAEs) in ≥20% of Patients 80 mg 120 mg 80 mg 120 mg Event, n (%) Event, n (%) (n=24) (n=21) (n=24) (n=21) Preferred term, n (%) Grade 1-2 Grade 3 Grade 1-2 Grade 3 TRAEs Grade ≥3 10 (42) 7 (33) Diarrhea 19 (79) 2 (8) 12 (57) 5 (24) Dose reduction due to 8 (33) 12 (57) TRAE Paronychia 20 (83) 0 14 (67) 0 Discontinued due to 3 (13) 0 Stomatitis 10 (42) 0 12 (57) 1 (5) TRAE Dermatitis acneiform 9 (38) 1 (4) 4 (19) 0 • Well tolerated safety profile with TRAEs Rash 9 (38) 1 (4) 12 (57) 1 (5) predominantly Grades 1-2 Nausea 8 (33) 0 9 (43) 0 • One Grade 4 TRAE (pneumonitis at 120 mg); no Grade 5 TRAEs Decreased appetite 6 (25) 0 6 (29) 0 • No significant off-target toxicities (e.g., cardiac, Mucosal inflammation 6 (25) 0 3 (14) 0 hematologic and liver) Alopecia 6 (25) 0 5 (24) 0 • Low rate of discontinuations due to TRAEs Dysgeusia 8 (33) 0 2 (10) 0 Enozertinib was generally well tolerated with mainly Grade 1 or 2 adverse events and no significant off-target toxicities; 80 mg cohort experienced lower rate of dose reductions compared to 120 mg cohort Source: John et al. ESMO Asia (2025). Note: Data as of August 29, 2025. 18

Enozertinib Achieved Robust Circulating Tumor DNA (ctDNA) Responses 2L post-chemotherapy, advanced NSCLC with EGFR exon 20 mutations Patients with Available ctDNA (n=25) 40 80 mg 120 mg 20 0 • ctDNA clearance rate of 69% (11/16) at 80 -20 mg and 67% (6/9) at 120 mg -40 -60 -80 -100 Enozertinib achieved robust ctDNA responses across doses in 2L NSCLC patients with EGFR exon 20 mutations Source: John et al. ESMO Asia (2025). Note: Data as of August 29, 2025. ctDNA assessed with Guardant360 Liquid assay. Clearance is defined as change from detected to undetected EGFR exon 20 in plasma after one treatment cycle and is shown as -100% on waterfall plot. 19 C2D1 – cycle 2 day 1, day 29. Change From Baseline to C2D1 in EGFR Ex20ins ctDNA, %

Enozertinib Achieved Strong Antitumor Activity, Including in Patients with CNS Disease at Baseline 2L post-chemotherapy, advanced NSCLC with EGFR exon 20 mutations 80 mg (1) Evaluable Population 80 mg (n=20) (n=20) 20 † Best ORR, % [95% CI] 45 [23, 69] Confirmed ORR, % [95% CI] 45 [23, 69] * * * * * * * * 0 Partial response, n (%) 9 (45) Stable disease, n (%) 11 (55) -20 Progressive disease, n (%) 0 Disease control rate (CR + PR + SD), % [95% CI] 100 [83, 100] -40 ‡ With CNS disease at baseline, % (n) 40 (8) † 38 [9, 76] Best ORR, % [95% CI] -60 38 [9, 76] Confirmed ORR, % [95% CI] Partial response, n (%) 3 (38) -80 Stable disease, n (%) 5 (63) Patients with brain metastases at study entry, Progressive disease, n (%) 0 * including active brain metastases -100 Disease control rate (CR + PR + SD), % [95% CI] 100 [63, 100] Enozertinib demonstrates strong systemic and CNS antitumor activity in 2L NSCLC patients with EGFR exon 20 mutations Source: John et al. ESMO Asia (2025). Note: Data as of August 29, 2025. Percentages in the table may not total 100% due to rounding. CR – complete response; PR – partial response; SD – stable disease. (1) Reported in the evaluable population which includes participants who have received ≥1 dose, have ≥1 measurable lesion at baseline, and have had the opportunity for ≥3 postbaseline scans. † Best objective response rate includes both confirmed and unconfirmed responses. 20 ‡ CNS disease at baseline includes patients with brain metastases at study entry, including active brain metastases. Change From Baseline in Target Lesions, %

£ * £ £ £ £ £ £ £ £ £ * * * * * * * * Tumor Responses on Enozertinib Were Generally Achieved Early, and Most Patients Remain on Treatment 2L post-chemotherapy, advanced NSCLC with EGFR exon 20 mutations CNS Disease at Baseline Confirmed PR Start £ Treatment Ongoing 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 Time on Treatment, wks • Responses generally occur by 4 weeks, but tumor regression continues over time, with late responses seen after 4+ months on treatment • Median follow-up of 30.3 weeks; 67% (6/9) of responders remain on treatment Progression-free survival and duration of response data are immature; 67% of responders are still on treatment Source: John et al. ESMO Asia (2025). Note: Data as of August 29, 2025. 21 80 mg QD (n=20)
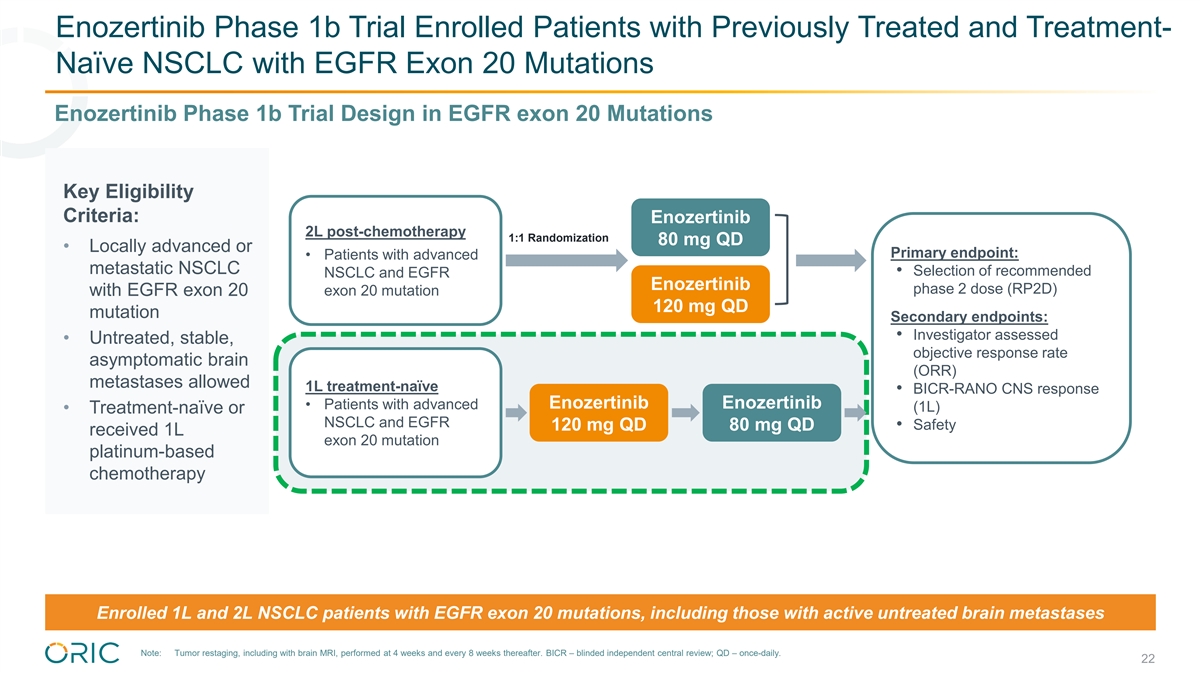
Enozertinib Phase 1b Trial Enrolled Patients with Previously Treated and Treatment- Naïve NSCLC with EGFR Exon 20 Mutations Enozertinib Phase 1b Trial Design in EGFR exon 20 Mutations Key Eligibility Criteria: Enozertinib 2L post-chemotherapy 1:1 Randomization 80 mg QD • Locally advanced or Primary endpoint: • Patients with advanced metastatic NSCLC • Selection of recommended NSCLC and EGFR Enozertinib phase 2 dose (RP2D) with EGFR exon 20 exon 20 mutation 120 mg QD mutation Secondary endpoints: • Investigator assessed • Untreated, stable, objective response rate asymptomatic brain (ORR) metastases allowed 1L treatment-naïve • BICR-RANO CNS response Enozertinib Enozertinib • Patients with advanced (1L) • Treatment-naïve or NSCLC and EGFR • Safety 120 mg QD 80 mg QD received 1L exon 20 mutation platinum-based chemotherapy Enrolled 1L and 2L NSCLC patients with EGFR exon 20 mutations, including those with active untreated brain metastases Note: Tumor restaging, including with brain MRI, performed at 4 weeks and every 8 weeks thereafter. BICR – blinded independent central review; QD – once-daily. 22

Phase 1b Trial Patient Demographics and Baseline Characteristics 1L treatment-naïve, advanced NSCLC with EGFR exon 20 mutations 80 mg 120 mg (n=18) (n=15) Age (years), median (range) 72 (43-95) 66 (48-82) Female, n (%) 13 (72) 11 (73) Non-smoker, n (%) 18 (100) 15 (100) Race: Asian / White / Other, % 17 / 78 / 6 20 / 73 / 7 ECOG performance: 0 / 1, % 39 / 61 40 / 60 Brain metastases at baseline*, n (%) 5 (28) 8 (53) Prior chemotherapy 0 0 Prior EGFR targeted therapies 0 0 39% of 1L patients had brain metastases at study entry, including those with active CNS disease Source: John et al. ESMO Asia (2025). Note: Data as of August 29, 2025. Percentages in the table may not total 100% due to rounding. * Patients with brain metastases at study entry, including active brain metastases. 23

Enozertinib Has Been Generally Well Tolerated Despite Less Stringent Enrollment Criteria for Baseline CNS Disease 1L treatment-naïve, advanced NSCLC with EGFR exon 20 mutations Treatment-Related Adverse Events (TRAEs) in ≥20% of Patients 80 mg 120 mg 80 mg 120 mg Event, n (%) Event, n (%) (n=18) (n=15) (n=18) (n=15) Preferred term, n (%) Grade 1-2 Grade 3 Grade 1-2 Grade 3 TRAEs Grade ≥3 4 (22) 9 (60) Diarrhea 15 (83) 2 (11) 9 (60) 1 (7) Dose reduction due to TRAE 3 (17) 12 (80) Paronychia 8 (44) 0 11 (73) 1 (7) Discontinued due to TRAE 2 (11) 0 Stomatitis 7 (39) 1 (6) 4 (27) 0 Dermatitis acneiform 5 (28) 0 4 (27) 6 (40) • Well tolerated safety profile with TRAEs predominantly Grades 1-2 Rash 4 (22) 1 (6) 2 (13) 1 (7) • No significant off-target toxicities (e.g., Nausea 6 (33) 0 3 (20) 0 myelosuppression, QTc prolongation, Pruritis 4 (22) 0 3 (20) 0 hepatotoxicity) Mucosal inflammation 4 (22) 0 5 (33) 1 (7) • Low rate of discontinuations due to TRAEs Dry skin 1 (6) 0 6 (40) 0 • Higher rate of dose reductions at 120 mg (80%) vs 80 mg (17%) Alopecia 1 (6) 0 8 (53) 0 – 58% of reductions at 120 mg dose by ~8 weeks Rash maculo-papular 5 (28) 0 2 (13) 0 (2 cycles) High rate of dose reductions in 120 mg cohort led to subsequent cohort of patients being dosed at 80 mg QD Source: John et al. ESMO Asia (2025). Note: Data as of August 29, 2025. 24

Enozertinib Achieved Robust ctDNA Responses 1L treatment-naïve, advanced NSCLC with EGFR exon 20 mutations Patients with Available ctDNA (n=14) 40 80 mg 120 mg 20 0 • ctDNA clearance rate of 71% (10/14) -20 -40 -60 -80 -100 Enozertinib achieved robust ctDNA responses in 1L NSCLC patients with EGFR exon 20 mutations Source: John et al. ESMO Asia (2025). Note: Data as of August 29, 2025. ctDNA assessed with Guardant360 Liquid assay. Clearance is defined as change from detected to undetected EGFR exon 20 in plasma after one treatment cycle and is shown as -100% on waterfall plot. 25 C2D1 – cycle 2 day 1, day 29. Change From Baseline to C2D1 in EGFR Ex20ins ctDNA, %

Enozertinib Achieved Strong Antitumor Activity, Including in Patients with CNS Disease at Baseline 1L treatment-naïve, advanced NSCLC with EGFR exon 20 mutations Best % Change in Lesions Systemic Objective Response Rate in Patients Receiving 120 mg Dose (n=15) 120 mg 20 (1) Evaluable Population (n=15) * † Best ORR, % [95% CI] 67 [38, 88] 0 * * * * * * * Confirmed ORR, % [95% CI] 60 [32, 84] -20 Partial response, n (%) 9 (60) Stable disease, n (%) 5 (33) -40 Progressive disease, n (%) 1 (7) Disease control rate (CR + PR -60 93 [68, 100] + SD), % [95% CI] -80 Patients with brain metastases at study entry, * including active brain metastases -100 • Initial cohort of efficacy evaluable patients were treated at 120 mg; given 80% dose reduction rate, most patients effectively received 80 mg • Subsequent cohort of patients were treated at 80 mg; follow-up is still in progress Enozertinib demonstrates strong ORR and disease control in 1L NSCLC patients with EGFR exon 20 mutations Source: John et al. ESMO Asia (2025). Note: Data as of August 29, 2025. CR – complete response; PR – partial response; SD – stable disease. (1) Reported in the evaluable population which includes participants who have received ≥1 dose, have ≥1 measurable lesion at baseline, and 26 have had the opportunity for ≥3 postbaseline scans. † Best objective response rate includes both confirmed and unconfirmed responses. Change From Baseline in Target Lesions, %

£ £ £ £ * £ £ £ £ £ £ £ ** **** ** Tumor Responses on Enozertinib Were Generally Achieved Early and the Vast Majority of Patients Remain on Treatment 1L treatment-naïve, advanced NSCLC with EGFR exon 20 mutations • Initial cohort of efficacy evaluable patients were treated at 120 mg; given 80% dose reduction rate, most patients effectively received 80 mg • Subsequent cohort of patients were treated at 80 mg; follow-up is still in progress CNS Disease at Baseline Confirmed PR Start Unconfirmed PR Start £ Treatment Ongoing 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 Time on Treatment, wks • Responses generally occur by 4 weeks, but tumor regression continues over time, with late responses seen after 4+ months on treatment • Median follow-up of 32.6 weeks; 80% (8 of 10) of responders are still on treatment 80% of responders are still on therapy, most at a reduced 80 mg dose; all subsequent enrollment was at 80 mg, with follow-up in progress Source: John et al. ESMO Asia (2025). Note: Data as of August 29, 2025. 27 120 mg QD (n=15)

Enozertinib Achieved Strong CNS Antitumor Activity as Measured by BICR-RANO 1L treatment-naïve, advanced NSCLC with EGFR exon 20 mutations Best % Change in CNS Lesions 120 mg in Patients Receiving 120 mg Dose (n=7) † CNS Response (1) (n=7) 20 ‡ Best ORR, % [95% CI] 71 [29, 96] Confirmed ORR, % [95% CI] 71 [29, 96] # # # # 0 Complete response, n (%) 2 (29) CNS non-target lesions with PD Partial response, n (%) 3 (43) -20 Stable disease, n (%) 0 -40 Progressive disease, n (%) 2 (29) ** Disease control rate (CR + PR 71 [29, 96] -60 ** + SD), % [95% CI] ** • 3 patients with measurable CNS disease: -80 – 100% confirmed intracranial ORR Patients with measurable CNS disease ** -100 # Patients with active brain metastases • 4 patients with non-measurable CNS disease: CNS non-target – 2 confirmed complete responses lesions with CR Strong CNS ORR (100% in measurable CNS disease by BICR-RANO), including in patients with active brain metastases, showcases enozertinib’s CNS activity and positions it favorably for future clinical development in 1L NSCLC patients with EGFR exon 20 Source: John et al. ESMO Asia (2025). (1) One patient was deemed not evaluable by blinded independent central review (BICR). † Per RANO by BICR. ‡ Best objective response rate includes both confirmed and unconfirmed responses. Note: Data as of August 29, 2025. Percentages in the table may not total 100% due to rounding. CR – complete response; PR – partial response; SD – stable disease. Measurable disease includes patients with target lesions ≥1 cm in diameter. 28 Change From Baseline in Target Lesions, %
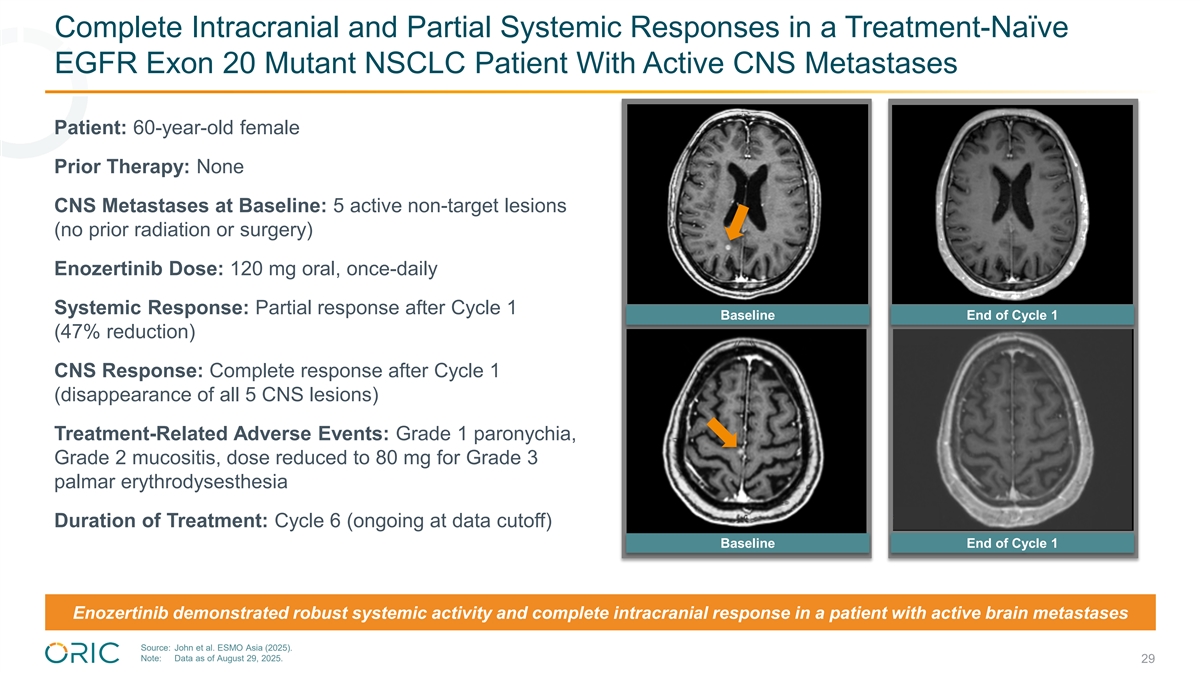
Complete Intracranial and Partial Systemic Responses in a Treatment-Naïve EGFR Exon 20 Mutant NSCLC Patient With Active CNS Metastases Patient: 60-year-old female Prior Therapy: None CNS Metastases at Baseline: 5 active non-target lesions (no prior radiation or surgery) Enozertinib Dose: 120 mg oral, once-daily Systemic Response: Partial response after Cycle 1 Baseline End of Cycle 1 (47% reduction) CNS Response: Complete response after Cycle 1 (disappearance of all 5 CNS lesions) Treatment-Related Adverse Events: Grade 1 paronychia, Grade 2 mucositis, dose reduced to 80 mg for Grade 3 palmar erythrodysesthesia Duration of Treatment: Cycle 6 (ongoing at data cutoff) Baseline End of Cycle 1 Enozertinib demonstrated robust systemic activity and complete intracranial response in a patient with active brain metastases Source: John et al. ESMO Asia (2025). Note: Data as of August 29, 2025. 29

ESMO Asia Clinical Highlights Previously Treated EGFR PACC NSCLC

Enozertinib Phase 1b Trial Enrolled Patients with Previously Treated NSCLC with EGFR Atypical Mutations Enozertinib Phase 1b Trial Design and Patient Demographics in EGFR Atypical Mutations 80 mg 120 mg Patient Characteristic (n=25) (n=22) Key Eligibility Criteria: Age (years), median (range) 65 (37-84) 66 (37-76) • Locally adv. or met. NSCLC with EGFR Female, n (%) 16 (64) 16 (73) Primary endpoint: atypical mutation Non-smoker, n (%) 25 (100) 22 (100) • Selection of • PACC, Classical-like, recommended phase 2 Race: Asian / White / Other, % 64 / 32 / 4 64 / 32 / 5 del19 non-PACC Enozertinib dose (RP2D) • Single and compound 1:1 80 mg QD ECOG performance: 0 / 1, % 28 / 72 36 / 64 mutations Secondary endpoints: Brain metastases at baseline*, n (%) 14 (56) 12 (55) Enozertinib • Untreated, stable, • Investigator assessed 120 mg QD asymptomatic brain Median prior therapies, n 2 2 objective response rate metastases allowed (ORR) Prior chemotherapy, n (%) 12 (48) 11 (50) • Prior therapies allowed, • Safety including chemotherapy Prior immunotherapy, n (%) 5 (20) 4 (18) and prior EGFR TKI Prior EGFR targeted therapy, n (%) 21 (84) 17 (77) (e.g., afatinib, osimertinib) PACC mutation, n (%) 12 (48) 13 (59) 55% of previously treated patients had brain metastases at study entry, including those with active CNS disease Source: Hong et al. ESMO Asia (2025). Note: Data as of August 29, 2025. * CNS disease at baseline includes patients with brain metastases at study entry, including active brain metastases. 31

Enozertinib Has Been Generally Well Tolerated Despite More Heavily-Pretreated Patients and Less Stringent Enrollment Criteria for Prior Therapy and Baseline CNS Disease Previously treated, advanced NSCLC with EGFR atypical mutations Treatment-Related Adverse Events (TRAEs) in ≥20% of Patients 80 mg 120 mg Event, n (%) (n=25) (n=22) 80 mg 120 mg Event, n (%) (n=25) (n=22) TRAEs Grade ≥3 7 (28) 8 (36) Preferred term, n (%) Grade 1-2 Grade 3 Grade 1-2 Grade 3 Dose reduction due to 5 (20) 15 (68) TRAE Diarrhea 12 (48) 2 (8) 14 (64) 3 (14) Discontinued due to Paronychia 12 (48) 0 13 (59) 1 (5) 0 0 TRAE Stomatitis 10 (40) 0 10 (45) 1 (5) • Well tolerated safety profile with TRAEs predominantly Dermatitis acneiform 8 (32) 0 8 (36) 0 Grades 1-2 • No Grade 4 or 5 TRAEs Rash 8 (32) 0 4 (18) 0 • No significant off-target toxicities (e.g., Nausea 3 (12) 0 7 (32) 0 myelosuppression, QTc prolongation, hepatotoxicity) Pruritis 5 (20) 1 (4) 5 (23) 0 • No discontinuations due to TRAEs Mucosal inflammation 4 (16) 0 4 (18) 2 (9) • Higher rate of dose reductions at 120 mg (68%) vs. 80 mg (20%) – 80% of dose reductions at 120 mg dose down to 80 mg occurred before ~8 weeks on treatment (2 cycles) 80 mg dose generally well tolerated; given high rate of dose reductions at 120 mg dose, most patients received an effective dose of 80 mg QD Source: Hong et al. ESMO Asia (2025). Note: Data as of August 29, 2025. 32

Enozertinib Achieved Strong Antitumor Activity, Including in Patients with CNS Disease at Baseline Previously treated (median 3L), advanced NSCLC with EGFR PACC mutations Best % Change in Lesions Effective Dose 80 mg in Patients Receiving 80 mg Effective Dose (n=22) (1) Efficacy Evaluable Population (n=22) 40 ‡ Best ORR, % [95% CI] 36 [17, 59] * 36 [17, 59] Confirmed ORR, % [95% CI] 20 Partial response, n (%) 8 (36) * 12 (55) Stable disease, n (%) 0 * * * * * * * * * * * Progressive disease, n (%) 2 (9) Disease control rate (CR + PR + SD), -20 91 [71, 99] % [95% CI] § With CNS disease at baseline, % (n) 59 (13) -40 ‡ 31 [9, 61] Best ORR, % [95% CI] Confirmed ORR, % [95% CI] 31 [9, 61] -60 4 (31) Partial response, n (%) Stable disease, n (%) 7 (54) -80 Progressive disease, n (%) 2 (15) Patients with brain metastases at study entry, including * active brain metastases Disease control rate (CR + PR + SD), -100 85 [55, 98] % [95% CI] Enozertinib demonstrates strong systemic and CNS antitumor activity in a median 3L EGFR PACC mutant NSCLC patient population, 59% of which had brain metastases at study entry Source: Hong et al. ESMO Asia (2025). Note: Data as of August 29, 2025. CR – complete response; PR – partial response; SD – stable disease. (1) Reported in the efficacy evaluable population which includes participants who have received ≥1 dose, have ≥1 measurable lesion at baseline, and have had the opportunity for ≥3 postbaseline scans. † 22 patients were efficacy evaluable and received an effective dose of 80 mg: in the 80 mg cohort n=12 patients were efficacy evaluable; of the efficacy evaluable patients in the 120 mg cohort, 77% (n=10) required an early dose reduction to 80 mg. ‡ Best objective response rate includes both confirmed and unconfirmed responses. § CNS disease at baseline includes patients with 33 brain metastases at study entry, including active brain metastases. Change From Baseline in Target Lesions, %

£ £ * £ £ £ £ £ £ £ £ ££ * ** ** *** *** ** Tumor Responses on Enozertinib Were Generally Achieved Early, and Most Patients Remain on Treatment Previously treated (median 3L), advanced NSCLC with EGFR PACC mutations Effective Dose 80 mg (n=22) CNS Disease at Baseline Confirmed PR Start £ Treatment Ongoing 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 Time on Treatment, weeks • Median follow-up of 32.6 weeks; 75% (6/8) of responders remain on treatment Enozertinib demonstrates early and durable responses in a median 3L EGFR PACC mutant NSCLC patient population Source: Hong et al. ESMO Asia (2025). Note: Data as of August 29, 2025. * CNS disease at baseline includes patients with brain metastases at study entry, including active brain metastases. 34 Effective Dose 80 mg QD (n=22)
1L EGFR PACC NSCLC Preliminary Data
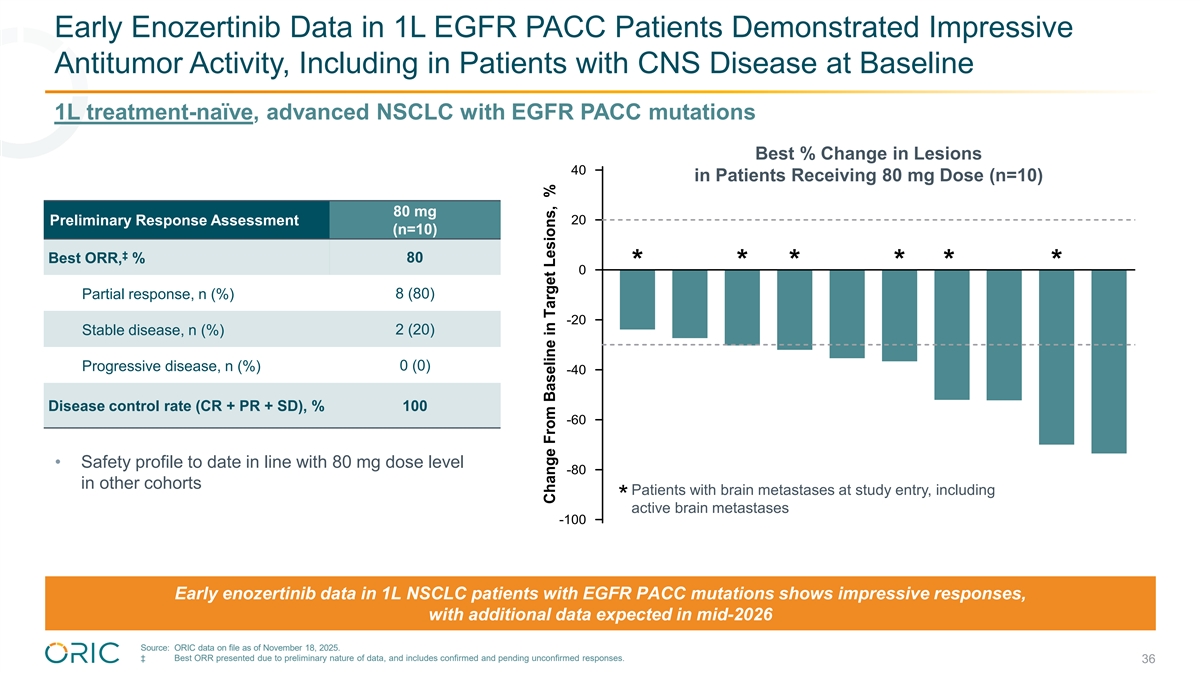
Early Enozertinib Data in 1L EGFR PACC Patients Demonstrated Impressive Antitumor Activity, Including in Patients with CNS Disease at Baseline 1L treatment-naïve, advanced NSCLC with EGFR PACC mutations Best % Change in Lesions 40 in Patients Receiving 80 mg Dose (n=10) 80 mg Preliminary Response Assessment 20 (n=10) ‡ 80 Best ORR, % * * * * * * 0 Partial response, n (%) 8 (80) -20 Stable disease, n (%) 2 (20) Progressive disease, n (%) 0 (0) -40 Disease control rate (CR + PR + SD), % 100 -60 • Safety profile to date in line with 80 mg dose level -80 in other cohorts Patients with brain metastases at study entry, including * active brain metastases -100 Early enozertinib data in 1L NSCLC patients with EGFR PACC mutations shows impressive responses, with additional data expected in mid-2026 Source: ORIC data on file as of November 18, 2025. ‡ Best ORR presented due to preliminary nature of data, and includes confirmed and pending unconfirmed responses. 36 Change From Baseline in Target Lesions, %

Early Enozertinib Data in 1L EGFR PACC Patients Demonstrated Strong CNS Antitumor Activity 1L treatment-naïve, advanced NSCLC with EGFR PACC mutations Best % Change in CNS Lesions 80 mg 40 Preliminary CNS Response (1) in Patients Receiving 80 mg Dose (n=5) (n=5) ‡ 80 Best ORR, % 20 1 (20) Complete response, n (%) # # # # # 0 3 (60) Partial response, n (%) CNS non- target disease Stable disease, n (%) * 1 (20) with non- -20 CR/non-PD* Progressive disease, n (%) 0 (0) -40 ** Disease control rate (CR + PR + SD), % 100 ** -60 • 4 patients with measurable CNS disease: – 100% intracranial ORR, including 1 complete response -80 • 1 patient with non-measurable CNS disease: – Best response of non-CR / non-PD Patients with measurable CNS disease -100 ** # Patients with active brain metastases ** ** Early enozertinib data in 1L NSCLC patients with PACC mutations shows impressive CNS antitumor activity (100% ORR in measurable CNS disease), including in patients with active brain metastases, with additional data expected in mid-2026 Source: ORIC data on file as of November 18, 2025. (1) Excludes one patient with no post-baseline CNS assessment. ‡ Best ORR (investigator assessed using RECIST) presented due to preliminary nature of data and includes confirmed and pending unconfirmed responses. * CNS non-target disease with best response of non-CR / non-PD. 37 Change From Baseline in Target Lesions, %

Partial Intracranial and Systemic Responses in a Treatment-Naïve EGFR PACC Mutant NSCLC Patient with Active CNS Disease Patient: 67-year-old male PACC Mutation: G719A Prior Therapy: None CNS Metastases at Baseline: 1 target lesion Baseline BOR Timepoint (no prior radiation or surgery) Enozertinib Dose: 80 mg oral, once-daily Systemic Response: Partial response after Cycle 1 (43% reduction) CNS Response: Partial response after Cycle 1 (66% reduction); complete response after Cycle 4 Baseline BOR Timepoint Treatment-Related Adverse Events: Grade 1 acneiform rash Duration of Treatment: Cycle 5 (ongoing at data cutoff) Enozertinib demonstrated robust systemic activity and complete intracranial response in a patient with active brain metastasis Source: ORIC data on file as of November 18, 2025. 38

Executive Summary and Next Steps

Enozertinib Phase 1b Data Establishes Potential Best-in-Class Profile in EGFR Exon 20 and PACC Mutated NSCLC Enozertinib Updated Phase 1b Data Highlights 2L EGFR Exon 20 Median 3L EGFR PACC 45% 36% ORR ORR Enozertinib Enozertinib Previously Treated 22% 40% Benchmark ORR Benchmark ORR 1L EGFR Exon 20 (Preliminary Data) 1L EGFR PACC (Preliminary Data) 80% ORR 67% ORR Enozertinib Enozertinib Treatment- Naïve (1L) Intracranial ORR Enozertinib 100% Intracranial ORR Enozertinib 100% Enozertinib data in previously treated patients with EGFR exon 20 and PACC mutations exceed competitor benchmarks; preliminary 1L systemic and intracranial activity establishes potential best-in-class profile Source: John et al. ESMO Asia (2025), Hong et al. ESMO Asia (2025), and ORIC data on file. Benchmark ORR: Piotrowska et al. J Clin Oncol (2025) and Udagawa et al. WCLC (2025). Note: All data in previously treated patients represent confirmed ORR. All data in 1L patients represent best ORR, due to preliminary nature of data. 1L EGFR exon 20 intracranial ORR data in all patients with measurable CNS disease by BICR-RANO 40 and 1L EGFR PACC intracranial ORR data in all patients with measurable CNS disease by investigator assessment using RECIST.

Enozertinib Establishes Potential Best-in-Class Profile in EGFR-Mutated NSCLC Ø Systemic activity in 2L EGFR exon 20 and pretreated EGFR PACC exceeds competitor benchmarks Ø Highly competitive preliminary 1L systemic activity, with 67% ORR in EGFR exon 20 and 80% ORR in EGFR PACC Ø Convincing 1L CNS activity, with 100% intracranial ORR in EGFR exon 20 and 100% intracranial ORR in EGFR PACC, including in patients with active brain metastases Ø Competitive safety profile, with no significant off-target toxicity and manageable on-target toxicity, resulting in low rate of discontinuations Ø 80 mg once-daily selected as recommended dose for potential Phase 3 development Ø Enrollment and follow-up continues in 1L EGFR exon 20 and 1L EGFR PACC with next update (1) expected mid-2026, ahead of potential initiation of Phase 3 trial(s) Source: John et al. ESMO Asia (2025), Hong et al. ESMO Asia (2025), and ORIC data on file. 41 (1) Enrollment in HER2 exon 20 has been completed with no further development planned in this patient population.

ORIC Pharmaceuticals: Dedicated to Overcoming Resistance In Cancer • Potential best-in-class PRC2 inhibitor for prostate cancer Validated Targets in High Unmet Need Populations • Potential best-in-class TKI for NSCLC with EGFR exon 20 and EGFR PACC mutations • ORIC-944 and enozertinib are both rapidly advancing towards potential Late-Stage Clinical Pipeline Phase 3 initiations in 2026 • Heritage of discovering, developing, and commercializing oncology therapies Experienced Management Team at Ignyta, Medivation, Aragon, Pharmacyclics, Deciphera, and Genentech (1) • Cash and investments of $413 million expected to fund company into 2H 2028 Strong Financial Position • Funding through primary endpoint readouts from first Phase 3 trials for ORIC-944 and enozertinib • ORIC-944 for mCRPC: ü 1H25: Combination dose exploration with AR inhibitor(s) ü 2H25: Updated combination data with AR inhibitors(s) ─ 1Q26: Combination dose optimization with AR inhibitor(s) Anticipated Data Milestones • Enozertinib for NSCLC: ü Dec 2025: 1L EGFR exon 20, 2L EGFR exon 20, 2L+ EGFR atypical, and 2L+ HER2 exon 20 ─ Mid-2026: 1L EGFR PACC and 1L EGFR exon 20 combination with SC amivantamab Two potential best-in-class programs with initiation of registrational trials expected in 2026; Cash runway into 2H 2028, beyond Phase 3 data readouts from both programs (1) Represents cash and investments of approximately $413 million as of September 30, 2025. 42

Thank You