UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
December 4, 2025
Date of Report (Date of earliest event reported)
Adverum Biotechnologies, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 001-36579 | 20-5258327 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
100 Cardinal Way
Redwood City, CA 94063
(Address of principal executive offices, including zip code)
(650) 656-9323
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligations of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange on which registered |
||
| Common Stock | ADVM | Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On December 4, 2025, Adverum Biotechnologies, Inc. (the “Company”) announced two-year long-term follow-up data from the Company’s LUNA Phase 2 clinical trial of Ixoberogene soroparvovec (“Ixo-vec”) in patients with wet age-related macular degeneration (“AMD”). In connection with the data release, the Company compiled a presentation (the “Presentation”) that includes the data referenced above. A copy of the Presentation is furnished as Exhibit 99.1. For important information about forward-looking statements, see the slide titled “Forward-Looking Statements” in Exhibit 99.1 attached hereto.
The information in this Item 7.01, including Exhibit 99.1 attached hereto, is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section. The information contained herein and in the accompanying exhibit is not incorporated by reference in any filing of the Company under the Securities Act of 1933, as amended, or the Exchange Act, whether made before or after the date hereof, regardless of any general incorporation language in such filing, except as shall be expressly set forth by specific reference in such filing.
| Item 8.01 | Other Events. |
As noted in Item 7.01, on December 4, 2025, the Company announced two-year long-term follow-up data from the Company’s LUNA Phase 2 clinical trial of Ixo-vec in patients with wet AMD. Ixo-vec is the Company’s clinical-stage gene therapy product candidate for the treatment of wet AMD.
Ixo-vec at both the Phase 3 dose (6E10 vg/eye) and the higher dose (2E11 vg/eye) demonstrated a robust, consistent, and sustained reduction over two years in anti-VEGF injection burden for previously treated, high-need wet AMD patients. Mean annualized injection rates through two years dropped from approximately 10 in the year prior to enrollment to 1.1 in the 6E10 group and 0.9 in the 2E11 group, representing a consistent ~90% reduction in both dose groups.
Sustained anatomic control and best corrected visual acuity were observed across both groups through two years. Ixo-vec continued to be well tolerated, with no new inflammation (i.e., ≥ 1+ anterior chamber or vitreous cells) observed after week 30 in participants receiving the 6E10 dose and local prophylaxis. These results reinforce the potential of Ixo-vec to meaningfully and sustainably reduce treatment burden while providing robust and durable disease control over two years in a high-need patient population.
The Company anticipates completing full enrollment of its pivotal Phase 3 ARTEMIS trial on December 5, 2025, enrolling 110% of the original target.
Forward-Looking Statements
Statements contained in this Current Report on Form 8-K regarding events or results that may occur in the future are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include but are not limited to statements regarding: the long-term potential best-in-class product profile of Ixo-vec; potential best-in-class injection-free rates and reduction in injection burden of Ixo-vec; the trial design of the Company’s clinical trials and anticipated timing of enrollment and completion; the potential of Ixo-vec to meaningfully and sustainably reduce treatment burden while providing robust and durable disease control; the potential life-long therapeutic benefit and predictable safety profile of Ixo-vec; the potential of Ixo-vec to shift the treatment paradigm for patients with wet AMD; the ability to establish gene therapy as a standard of care for wet AMD patients; the likelihood of clinical, regulatory and commercial success of Ixo-vec; and other statements that are not historical fact. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, including risks inherent to, without limitation: the Company’s novel technology, which makes it difficult to predict the timing of commencement and completion of clinical trials; regulatory uncertainties; enrollment uncertainties; the results of clinical trials not always being predictive of future clinical trials and results; the potential for future complications or side effects in connection with use of Ixo-vec; manufacturing and distribution risks of Ixo-vec that may result in additional costs or delays; our reliance on third parties to conduct our ongoing and planned clinical trials, which could delay our clinical development and be unsuccessful; and risks associated with market conditions. Additional risks and uncertainties facing the Company are set forth under the caption “Risk Factors” and elsewhere in the Company’s SEC filings and reports, including the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2025 filed with the SEC on November 12, 2025 and subsequent filings with the SEC. All forward-looking statements contained in this Current Report on Form 8-K speak only as of the date on which they were made.
1
The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits.
| Exhibit No. |
Description | |
| 99.1 | Slide presentation. | |
| 104 | The cover page of this report has been formatted in Inline XBRL. | |
2
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| ADVERUM BIOTECHNOLOGIES, INC. | ||||||
| Date: December 4, 2025 | By: | /s/ Laurent Fischer |
||||
| Laurent Fischer, M.D. | ||||||
| President and Chief Executive Officer | ||||||

Exhibit 99.1 FLORetina • December 2025 Ixo-vec Gene Therapy for nAMD: Clinical Progress from the LUNA Phase 2 Trial Charles C. Wykoff, M.D., Ph.D. Director of Research, Retina Consultants of Texas Professor of Clinical Ophthalmology, Blanton Eye Institute, Houston Methodist Hospital Disclosures: C= Consultant | R= Research Support | SO= Stock Options 4DMT (C,R), AbbVie (C,R), Adverum (C,R), AGTC (C), Alcon (C), Alexion (R), Alimera (C,R), Alkeus (C), AMC Sciences (C), Annexon (C,R), Apellis (C,R), Ascidian (R), Aviceda (R), Avirmax (R), Bausch + Lomb (C), Bayer (C,R), Beacon (R), Biocryst (C), Bionic Vision (C), Boehringer Ingelheim (C,R), Chengdu Kanghong (C), Chengdu Origen (R), Clearside (R), Curacle (C,R), Emmecell (C), EyeBiotech (C,R), EyePoint (C,R), Genentech (C,R), Gyroscope (R), InGel (C,SO), IONIS (R), IVERIC Bio (C,R), Janssen (C,R), Kalaris (R), Kiora (C), Kodiak (C,R), Kowa (C), Kyowa Kirin (R), Merck (C), Merit (C), Nanoscope (C, R), Neurotech (C, R), NGM (R), Novartis (C, R), Oak Bay Bio (C), Ocugen (R), Ocular Therapeutix (C, R), Ocuphire (C), OcuTerra (C, R), OliX (R), Ollin (C), ONL (C, SO), Opthea (C,R), Osanni (C,SO), Oxular (R), Palatin (C), Panther (SO), Perceive Bio (R), PolyPhotonix (SO), Ray (C), RecensMedical (SO), Regeneron (C,R), RegenXBio (C,R), RetinAI (C), Roche (C,R), Sandoz (C), Sanofi (C), Santen (C), Skyline (R), Stealth (C,R), THEA (C), TissueGen (SO), VH401 (C,R), Visgenx (C,SO), Vitranu (SO), Zeiss (C)

Forward-Looking Statements Statements contained in this document and any accompanying presentation regarding matters, events, statistics, or clinical or financial results that may occur in the future are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements regarding the potential benefits of Ixo-vec as a treatment of wet AMD, including the potential best-in-class product profile, clinical activity, favorable safety profile and long-term benefit including quality of life benefits; plans and milestones related to Adverum’s product candidates, clinical studies and trials, and regulatory filings; the therapeutic potential of Adverum’s product candidates; the potential of Ixo-vec to transform the TM treatment paradigm for patients with wet AMD; the potential of ixo-vec to be a One-And-Done IVT injection; the potential of ixo-vec to address the unmet need of patients with wet AMD; the design of Adverum’s clinical trials and anticipated timing of completion; the potential of Ixo-vec to meaningfully and sustainably reduce treatment burden while providing robust and durable disease control; and other statements containing the words “anticipates,” “may,” “potential,” “will” and similar expressions, all of which are based on certain assumptions made by Adverum on current conditions, expected future developments and other factors Adverum believes are appropriate under the circumstances. Adverum may not consummate any of these plans or these product, clinical development, process development, manufacturing, or regulatory goals in a timely manner, or at all, or otherwise carry out the intentions or meet the expectations or projections disclosed in its forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, the risk that Adverum’s resources will not be sufficient for Adverum to conduct or continue planned development programs and planned clinical trials, the risk that preliminary or interim data from Adverum’s clinical trials may change as more patient data become available, the risk of a delay in the enrollment of patients in Adverum’s clinical studies or in the manufacturing of products to be used in such clinical studies, risks and uncertainties inherent in the product development and the regulatory approval process, the results of clinical trials not always being predictive of future clinical trials and results, the potential for future complications or side effects in connection with use of Ixo-vec, manufacturing and distribution risks of Ixo-vec that may result in additional costs or delays, Adverum's reliance on third parties to conduct ongoing and planned clinical trials, which could delay clinical development and be unsuccessful, the risk that Adverum will not be able to successfully develop, manufacture, or commercialize any of its product candidates and the risk that Adverum will be delayed in receiving or fail to receive required regulatory approvals. Additional risks and uncertainties facing Adverum are set forth under the caption “Risk Factors” and elsewhere in Adverum’s Securities and Exchange Commission (SEC) filings and reports, including Adverum’s most recent Quarterly Report on Form 10-Q for the quarter ended September 30, 2025, filed with the SEC on November 12, 2025, and subsequent filings with the SEC. All forward-looking statements contained in this document speak only as of the date on which they were made. Adverum undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law. This document contains, and any accompanying presentation may contain, estimates, projections and other information concerning Adverum’s industry, business and the markets for certain drugs, including data regarding the estimated size of those markets, their projected growth rates and the incidence of certain medical conditions. Information that is based on estimates, forecasts, projections or similar methodologies is inherently subject to uncertainties and actual amounts may differ materially from amounts reflected in this information. Unless otherwise expressly stated, Adverum obtained this industry, business, market and other data from reports, research surveys, studies and similar data prepared by third parties, industry, medical and general publications, government data and similar sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, and should not be construed as representations made by, Adverum. The safety and efficacy of Ixo-vec for the treatment of wet AMD have not been established. Ixo-vec has not been approved for any use by any regulatory authority, including the US FDA.
Ixo-vec: A Potentially Transformative Therapy for Wet AMD Ixo-vec Gene therapy designed to preserve vision with a single intravitreal injection Single intravitreal Gold standard vector Transgene encoding Biofactory approach for injection in routine office for IVT gene therapy aflibercept, a clinically durable therapeutic setting validated anti-VEGF protein levels 3 AMD, age-related macular degeneration; IVT, intravitreal.
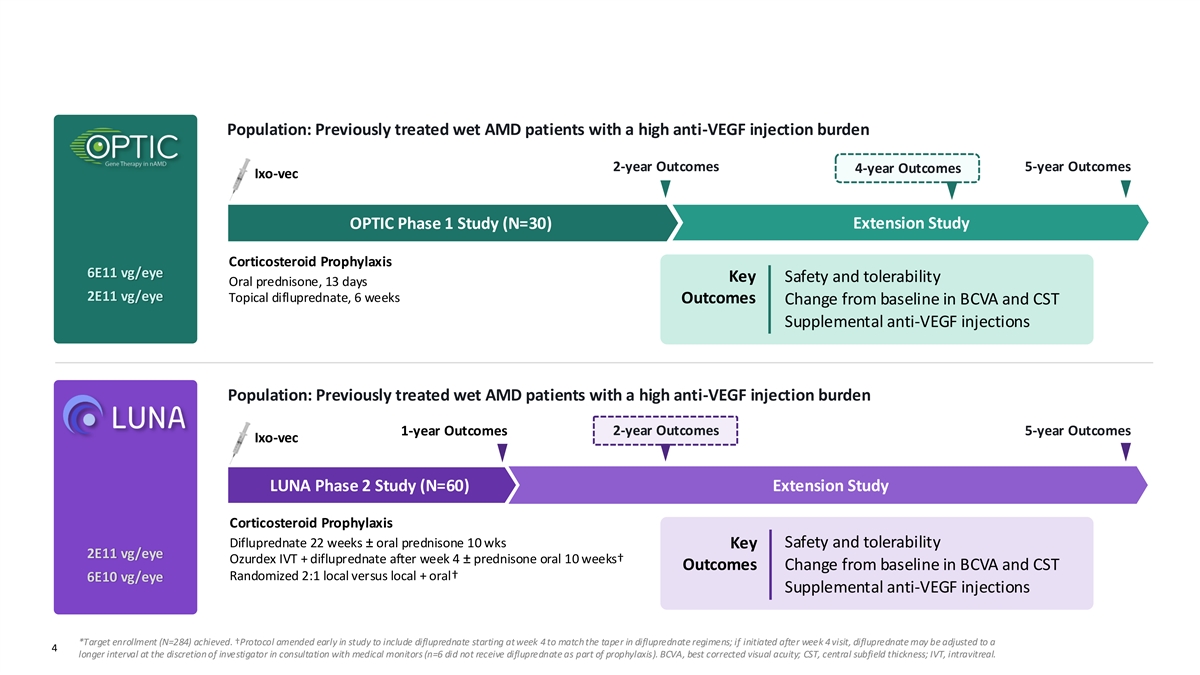
Observations from OPTIC and LUNA Informed Phase 3 Trials * ARTEMIS enrollment completed Population: Previously treated wet AMD patients with a high anti-VEGF injection burden 2-year Outcomes 5-year Outcomes 4-year Outcomes Ixo-vec OPTIC Phase 1 Study (N=30) Extension Study Corticosteroid Prophylaxis 6E11 vg/eye Key Safety and tolerability Oral prednisone, 13 days 2E11 vg/eye Topical difluprednate, 6 weeks Outcomes Change from baseline in BCVA and CST Supplemental anti-VEGF injections Population: Previously treated wet AMD patients with a high anti-VEGF injection burden 2-year Outcomes 5-year Outcomes 1-year Outcomes Ixo-vec LUNA Phase 2 Study (N=60) Extension Study Corticosteroid Prophylaxis Difluprednate 22 weeks ± oral prednisone 10 wks Safety and tolerability Key 2E11 vg/eye Ozurdex IVT + difluprednate after week 4 ± prednisone oral 10 weeks† Outcomes Change from baseline in BCVA and CST Randomized 2:1 local versus local + oral† 6E10 vg/eye Supplemental anti-VEGF injections *Target enrollment (N=284) achieved. †Protocol amended early in study to include difluprednate starting at week 4 to match the taper in difluprednate regimens; if initiated after week 4 visit, difluprednate may be adjusted to a Confidential and Proprietary 4 longer interval at the discretion of investigator in consultation with medical monitors (n=6 did not receive difluprednate as part of prophylaxis). BCVA, best corrected visual acuity; CST, central subfield thickness; IVT, intravitreal.

Phase 1 OPTIC Trial 2E11 vg/eye: Marked and sustained reduction in treatment burden (4-year results) BCVA and CST Maintained Through 4 Years Sustained Reduction in Injection Burden OPTIC OPTIC EXT Cumulative Annualized Reduction 12 9.9 500 Mean (90% CI) CST 10 84% 81% 84% 86% 400 8 6 300 4 200 1.9 1.6 1.6 1.4 2 100 0 0 12 24 36 48 60 72 84 96108120132144156168180192204 4 00 0 0.5 1 2 3 4 Prior Year 1 Year 2 Years 3 Years 4 Years Year 90 Mean (90% CI) BCVA Well Tolerated Through 4 Years 80 70 • No hypotony, vasculitis, retinitis, choroiditis, or vascular 60 * occlusions 50 • Inflammation was dose dependent, did not impact vision and, 40 when present, was responsive to local corticosteroids 30 000 12 24 36 48 60 72 84 96108120132144156168180192204 4 0 0.5 1 2 3 4 • 100% of inflammation resolved by year 1 Year 5 *Cataract surgery. BCVA, best corrected visual acuity; CST, central subfield thickness. DATA CUT-OFF: 21AUG2024 Mean (90% CI) BCVA, Mean (90% CI) CST, μm ETDRS letters Mean annualized injections
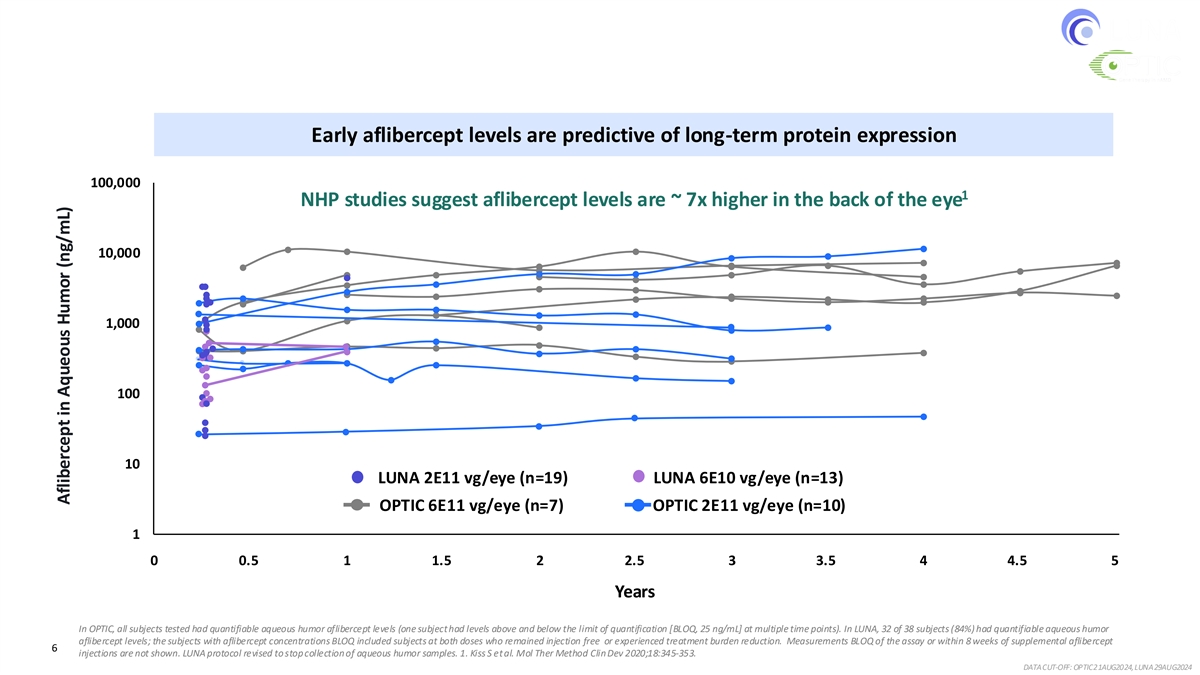
Ixo-vec Demonstrated Durable Therapeutic Levels After One Injection Overlapping expression patterns across all 3 doses & 6E10 vg/eye consistent with higher doses Early aflibercept levels are predictive of long-term protein expression 100,000 1 NHP studies suggest aflibercept levels are ~ 7x higher in the back of the eye 10,000 1,000 100 10 LUNA 2E11 vg/eye (n=19) LUNA 6E10 vg/eye (n=13) OPTIC 6E11 vg/eye (n=7) OPTIC 2E11 vg/eye (n=10) 1 0 12 24 36 48 60 72 84 96 108 120 132 144 156 168 180 192 204 216 228 240 252 0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 Years In OPTIC, all subjects tested had quantifiable aqueous humor aflibercept levels (one subject had levels above and below the limit of quantification [BLOQ, 25 ng/mL] at multiple time points). In LUNA, 32 of 38 subjects (84%) had quantifiable aqueous humor aflibercept levels; the subjects with aflibercept concentrations BLOQ included subjects at both doses who remained injection free or experienced treatment burden reduction. Measurements BLOQ of the assay or within 8 weeks of supplemental aflibercept 6 injections are not shown. LUNA protocol revised to stop collection of aqueous humor samples. 1. Kiss S et al. Mol Ther Method Clin Dev 2020;18:345-353. DATA CUT-OFF: OPTIC 21AUG2024, LUNA 29AUG2024 Aflibercept in Aqueous Humor (ng/mL)
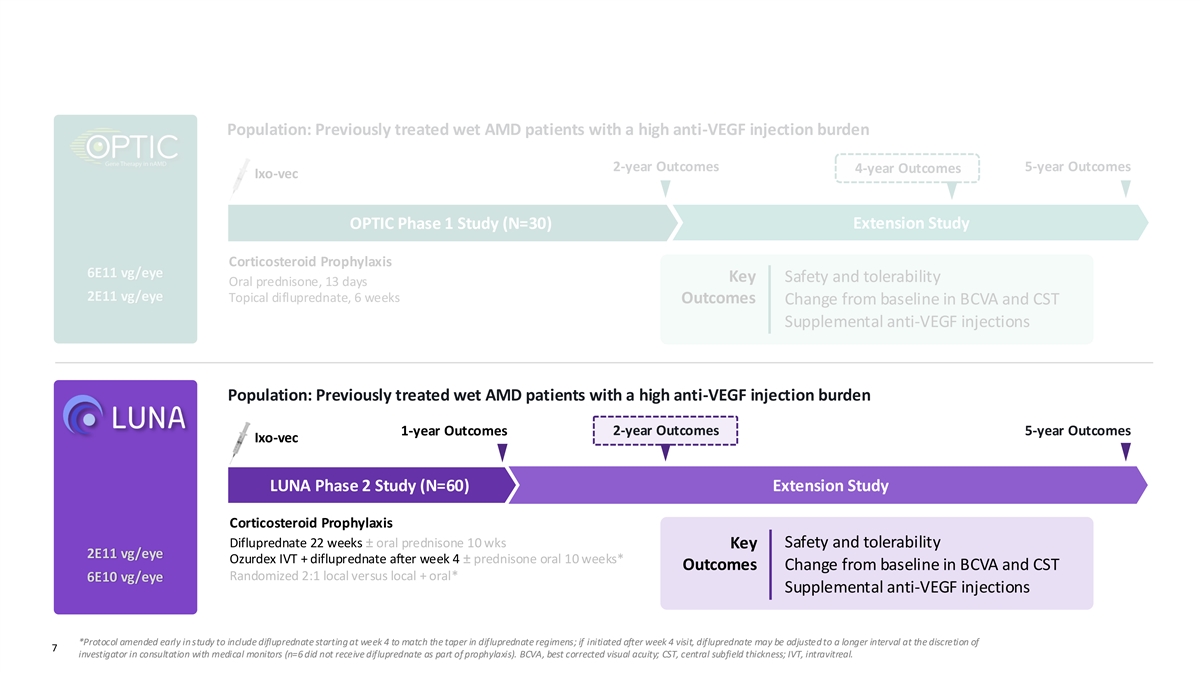
Observations from OPTIC and LUNA Informed Phase 3 Trial Design Population: Previously treated wet AMD patients with a high anti-VEGF injection burden 2-year Outcomes 5-year Outcomes 4-year Outcomes Ixo-vec OPTIC Phase 1 Study (N=30) Extension Study Corticosteroid Prophylaxis 6E11 vg/eye Key Safety and tolerability Oral prednisone, 13 days 2E11 vg/eye Topical difluprednate, 6 weeks Outcomes Change from baseline in BCVA and CST Supplemental anti-VEGF injections Population: Previously treated wet AMD patients with a high anti-VEGF injection burden 2-year Outcomes 5-year Outcomes 1-year Outcomes Ixo-vec LUNA Phase 2 Study (N=60) Extension Study Corticosteroid Prophylaxis Difluprednate 22 weeks ± oral prednisone 10 wks Safety and tolerability Key 2E11 vg/eye Ozurdex IVT + difluprednate after week 4 ± prednisone oral 10 weeks* Outcomes Change from baseline in BCVA and CST Randomized 2:1 local versus local + oral* 6E10 vg/eye Supplemental anti-VEGF injections *Protocol amended early in study to include difluprednate starting at week 4 to match the taper in difluprednate regimens; if initiated after week 4 visit, difluprednate may be adjusted to a longer interval at the discretion of Confidential and Proprietary 7 investigator in consultation with medical monitors (n=6 did not receive difluprednate as part of prophylaxis). BCVA, best corrected visual acuity; CST, central subfield thickness; IVT, intravitreal.

Demographics and Baseline Characteristics LUNA evaluated Ixo-vec in nAMD patients with a high injection burden 6E10 vg/eye 2E11 vg/eye Total N=30 N=30 N=60 Characteristics Mean (SD) age, years 75.4 (8.2) 77.7 (7.4) 76.6 (7.8) Female, n (%) 16 (53) 18 (60) 34 (57) Race, n (%) White 27 (90) 28 (93) 55 (92) Asian 2 (7) 2 (7) 4 (7) Mean (SD) years since nAMD diagnosis in study eye 3.0 (2.9) 3.0 (3.1) 3.0 (2.9) Mean (SD) annualized anti-VEGF injections in prior year 10.2 (1.7) 10.0 (3.3) 10.1 (2.6) Mean (SD) BCVA, ETDRS letters 72.9 (8.8) 71.8 (6.4) 72.3 (7.7) Mean (SD) CST, µm 360.6 (112.0) 340.5 (119.3) 350.6 (115.2) Phakic lens status, n (%) 11 (37) 11 (37) 22 (37) A total of 49 [82%] participants completed the year 2 study visit (n=26 [87%] and n=23 [77%] in the 6E10 and 2E11 groups, respectively). BCVA, best corrected visual acuity: CST, central subfield thickness; 8 ETDRS, Early Treatment Diabetic Retinopathy Study; nAMD, neovascular age-related macular degeneration; SD, standard deviation; VEGF, vascular endothelial growth factor. DATA CUT-OFF: 29AUG2025
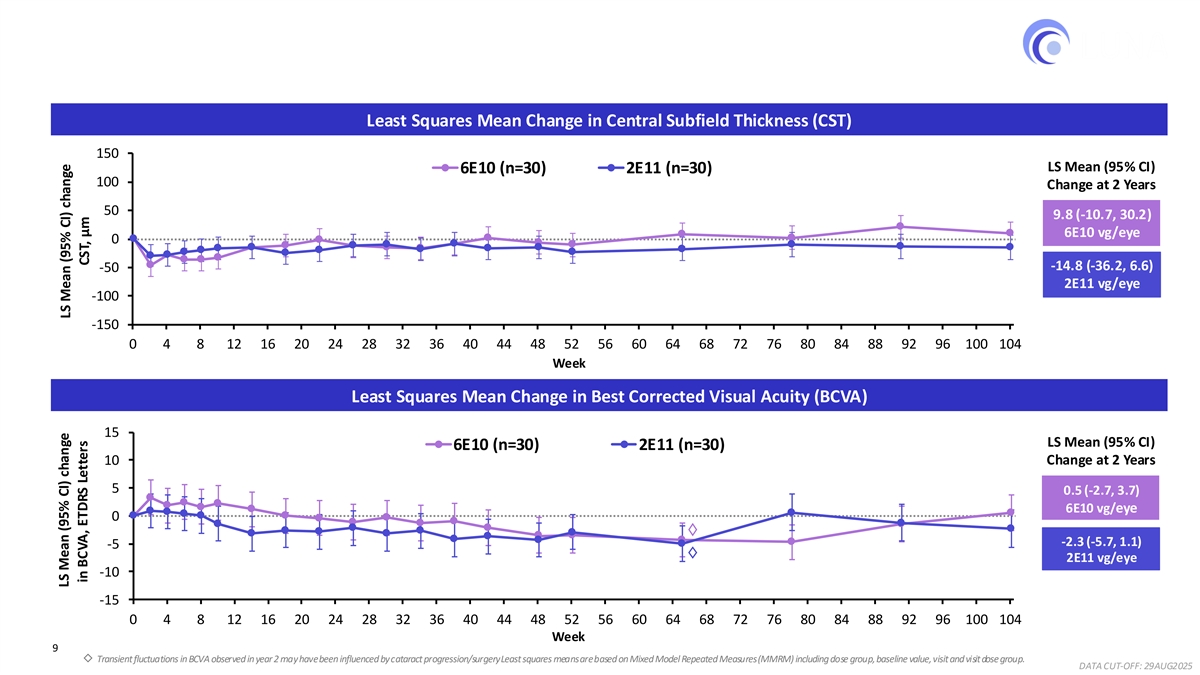
Sustained Anatomic Control and Visual Acuity Through 2 Years Robust and durable clinical activity observed in both dose groups Least Squares Mean Change in Central Subfield Thickness (CST) 150 LS Mean (95% CI) 6E10 (n=30) 2E11 (n=30) 100 Change at 2 Years 50 9.8 (-10.7, 30.2) 6E10 vg/eye 0 -14.8 (-36.2, 6.6) -50 2E11 vg/eye -100 -150 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76 80 84 88 92 96 100 104 Week Least Squares Mean Change in Best Corrected Visual Acuity (BCVA) 15 LS Mean (95% CI) 6E10 (n=30) 2E11 (n=30) 10 Change at 2 Years 5 0.5 (-2.7, 3.7) 6E10 vg/eye 0 -2.3 (-5.7, 1.1) -5 2E11 vg/eye -10 -15 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76 80 84 88 92 96 100 104 Week 9 Transient fluctuations in BCVA observed in year 2 may have been influenced by cataract progression/surgery . Least squares means are based on Mixed Model Repeated Measures (MMRM) including dose group, baseline value, visit and visit dose group. DATA CUT-OFF: 29AUG2025 LS Mean (95% CI) change LS Mean (95% CI) change in BCVA, ETDRS Letters CST, μm
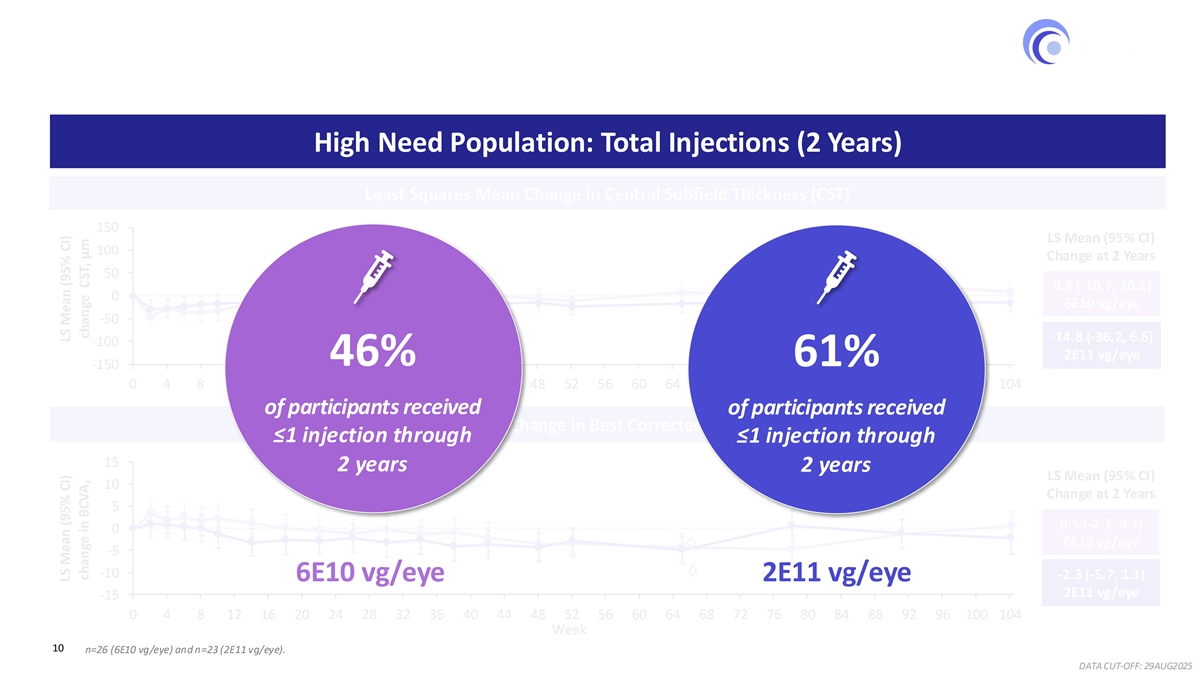
Robust and Consistent Reduction in Injection Burden 46%–61% of participants received ≤1 injection cumulatively through 2 years High Need Population: Total Injections (2 Years) Least Squares Mean Change in Central Subfield Thickness (CST) 150 LS Mean (95% CI) 100 Change at 2 Years 50 9.8 (-10.7, 30.2) 0 6E10 vg/eye -50 -14.8 (-36.2, 6.6) -100 2E11 vg/eye 46% 61% -150 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76 80 84 88 92 96 100 104 of participants received of participants received Least Squares Mean Change in Best Corrected Visual Acuity (BCVA) ≤1 injection through ≤1 injection through 15 2 years 2 years LS Mean (95% CI) 10 Change at 2 Years 5 0.5 (-2.7, 3.7) 0 6E10 vg/eye -5 -10 -2.3 (-5.7, 1.1) 6E10 vg/eye 2E11 vg/eye 2E11 vg/eye -15 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76 80 84 88 92 96 100 104 Week 10 n=26 (6E10 vg/eye) and n=23 (2E11 vg/eye). DATA CUT-OFF: 29AUG2025 LS Mean (95% CI) LS Mean (95% CI) change in BCVA, change CST, μm
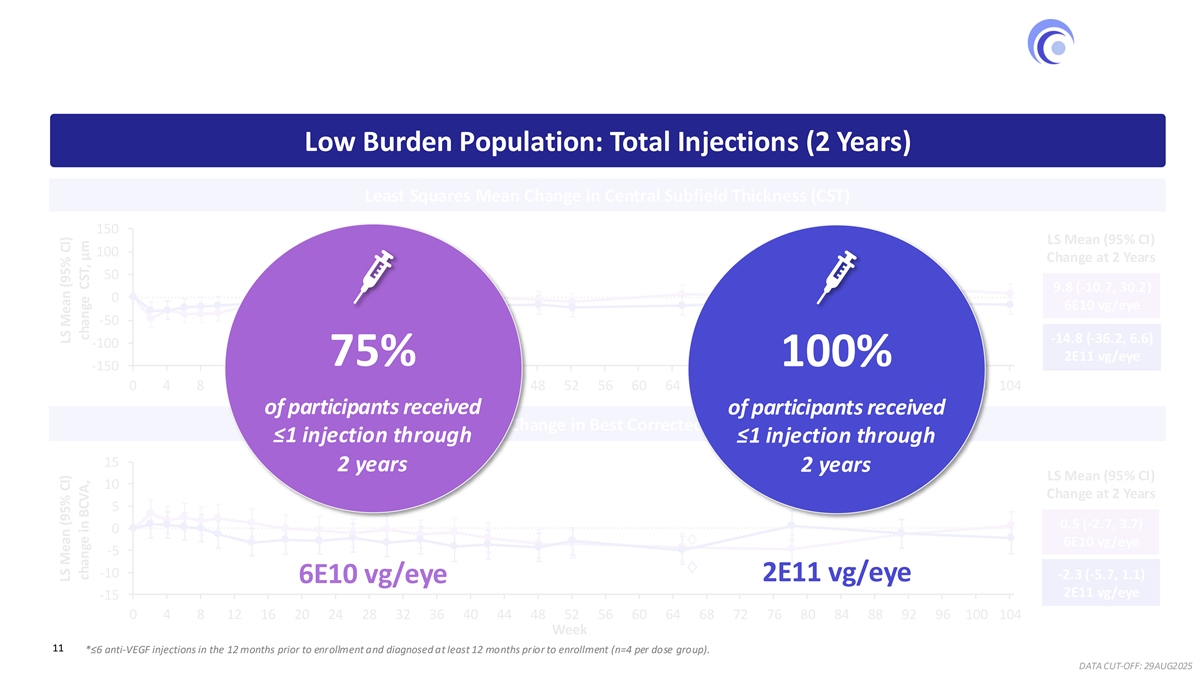
Robust and Consistent Reduction in Injection Burden * 75%−100% of participants with lower treatment burden received ≤1 injection cumulatively through 2 years Low Burden Population: Total Injections (2 Years) Least Squares Mean Change in Central Subfield Thickness (CST) 150 LS Mean (95% CI) 100 Change at 2 Years 50 9.8 (-10.7, 30.2) 0 6E10 vg/eye -50 -14.8 (-36.2, 6.6) -100 2E11 vg/eye 75% 100% -150 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76 80 84 88 92 96 100 104 of participants received of participants received Least Squares Mean Change in Best Corrected Visual Acuity (BCVA) ≤1 injection through ≤1 injection through 15 2 years 2 years LS Mean (95% CI) 10 Change at 2 Years 5 0.5 (-2.7, 3.7) 0 6E10 vg/eye -5 -10 -2.3 (-5.7, 1.1) 2E11 vg/eye 6E10 vg/eye 2E11 vg/eye -15 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76 80 84 88 92 96 100 104 Week 11 *≤6 anti-VEGF injections in the 12 months prior to enrollment and diagnosed at least 12 months prior to enrollment (n=4 per dose group). DATA CUT-OFF: 29AUG2025 LS Mean (95% CI) LS Mean (95% CI) change in BCVA, change CST, μm
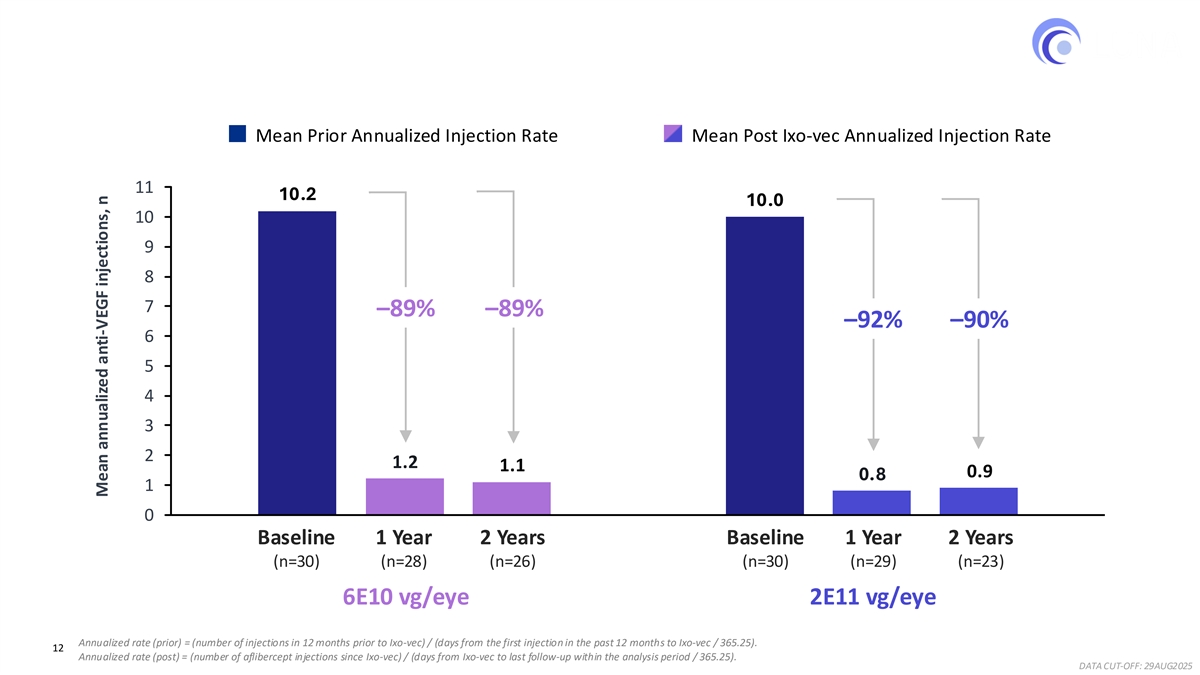
Robust and Consistent Reduction in Injection Burden Through Year 2 ~90% reduction in mean annualized anti-VEGF injections in a high need population Mean Prior Annualized Injection Rate Mean Post Ixo-vec Annualized Injection Rate 11 10.2 10.0 10 9 8 7 –89%–89% –92%–90% 6 5 4 3 2 1.2 1.1 0.9 0.8 1 0 1 2 Baseline 1 Year 2 Years Baseline 1 Year 2 Years (n=30) (n=28) (n=26) (n=30) (n=29) (n=23) 6E10 vg/eye 2E11 vg/eye Annualized rate (prior) = (number of injections in 12 months prior to Ixo-vec) / (days from the first injection in the past 12 months to Ixo-vec / 365.25). 12 Annualized rate (post) = (number of aflibercept injections since Ixo-vec) / (days from Ixo-vec to last follow-up within the analysis period / 365.25). DATA CUT-OFF: 29AUG2025 Mean annualized anti-VEGF injections, n
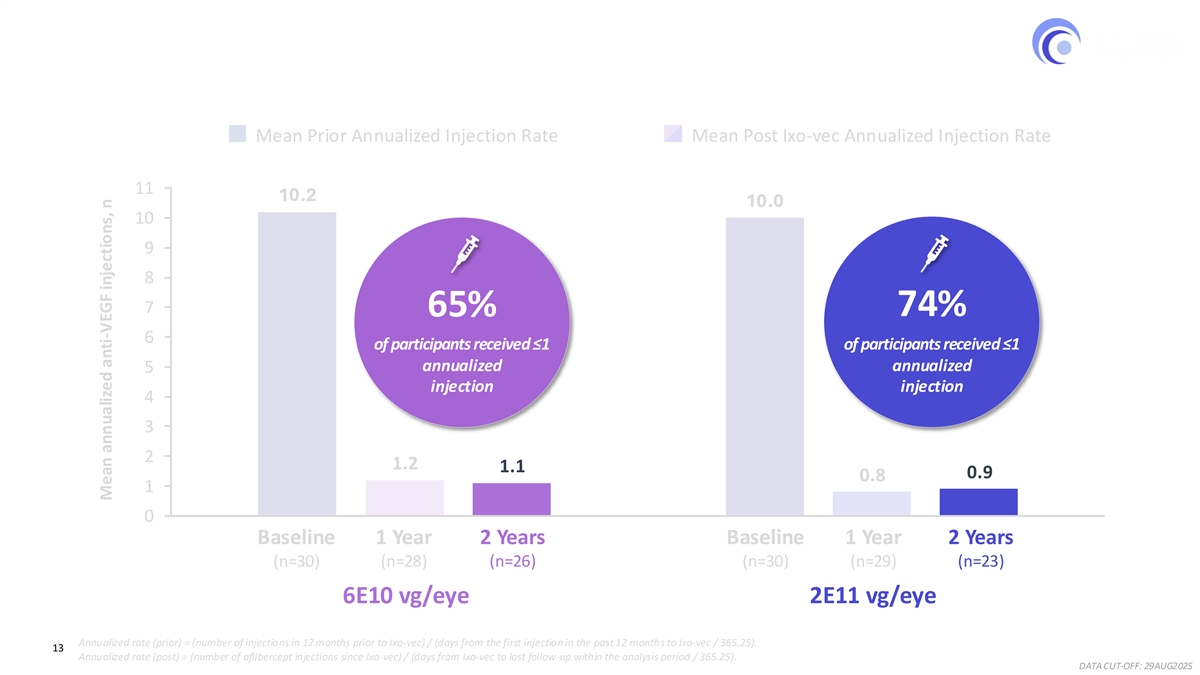
Robust Reduction in Injection Burden in a High Need Population 65%–74% of participants received ≤1 annualized injection through 2 years Mean Prior Annualized Injection Rate Mean Post Ixo-vec Annualized Injection Rate 11 10.2 10.0 10 9 8 7 65% 74% 6 of participants received ≤1 of participants received ≤1 annualized annualized 5 injection injection 4 3 2 1.2 1.1 0.9 0.8 1 0 1 2 Baseline 1 Year 2 Years Baseline 1 Year 2 Years (n=30) (n=28) (n=26) (n=30) (n=29) (n=23) 6E10 vg/eye 2E11 vg/eye Annualized rate (prior) = (number of injections in 12 months prior to Ixo-vec) / (days from the first injection in the past 12 months to Ixo-vec / 365.25). 13 Annualized rate (post) = (number of aflibercept injections since Ixo-vec) / (days from Ixo-vec to last follow-up within the analysis period / 365.25). DATA CUT-OFF: 29AUG2025 Mean annualized anti-VEGF injections, n
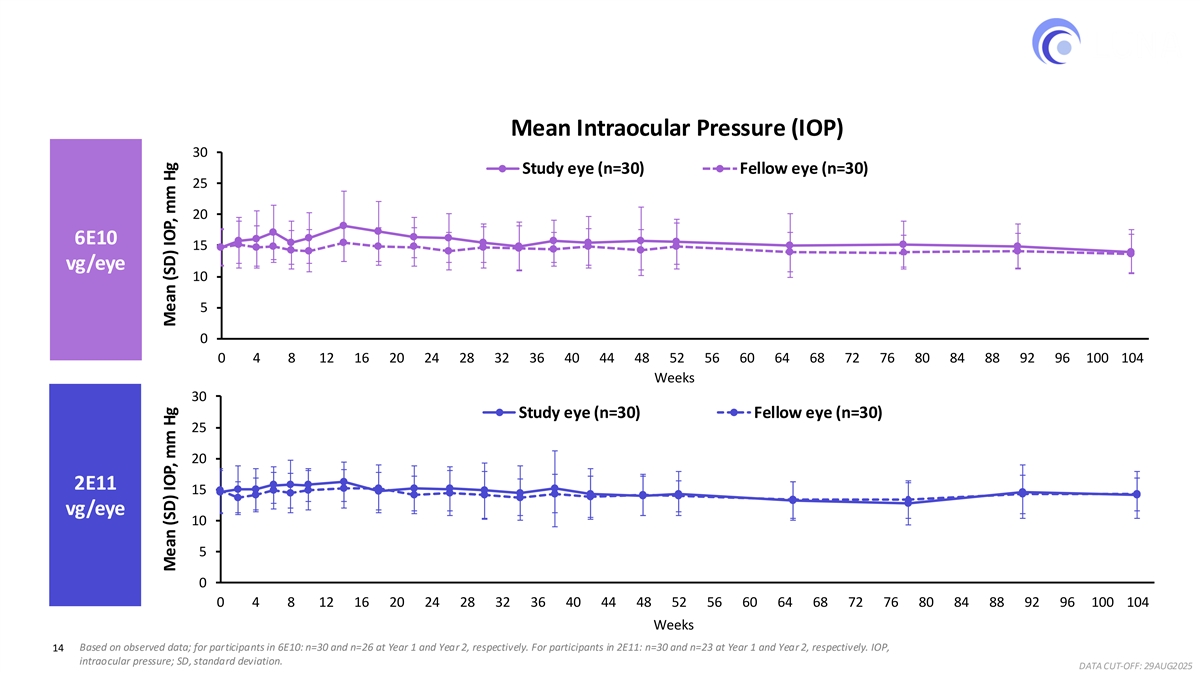
Intraocular Pressure Remained Stable in Year 2 in Both Ixo-vec Dose Groups Mean Intraocular Pressure (IOP) 30 Study eye (n=30) Fellow eye (n=30) 25 20 6E10 15 vg/eye 10 5 0 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76 80 84 88 92 96 100 104 Weeks 30 Study eye (n=30) Fellow eye (n=30) 25 20 2E11 15 vg/eye 10 5 0 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76 80 84 88 92 96 100 104 Weeks 14 Based on observed data; for participants in 6E10: n=30 and n=26 at Year 1 and Year 2, respectively. For participants in 2E11: n=30 and n=23 at Year 1 and Year 2, respectively. IOP, intraocular pressure; SD, standard deviation. DATA CUT-OFF: 29AUG2025 Mean (SD) IOP, mm Hg Mean (SD) IOP, mm Hg
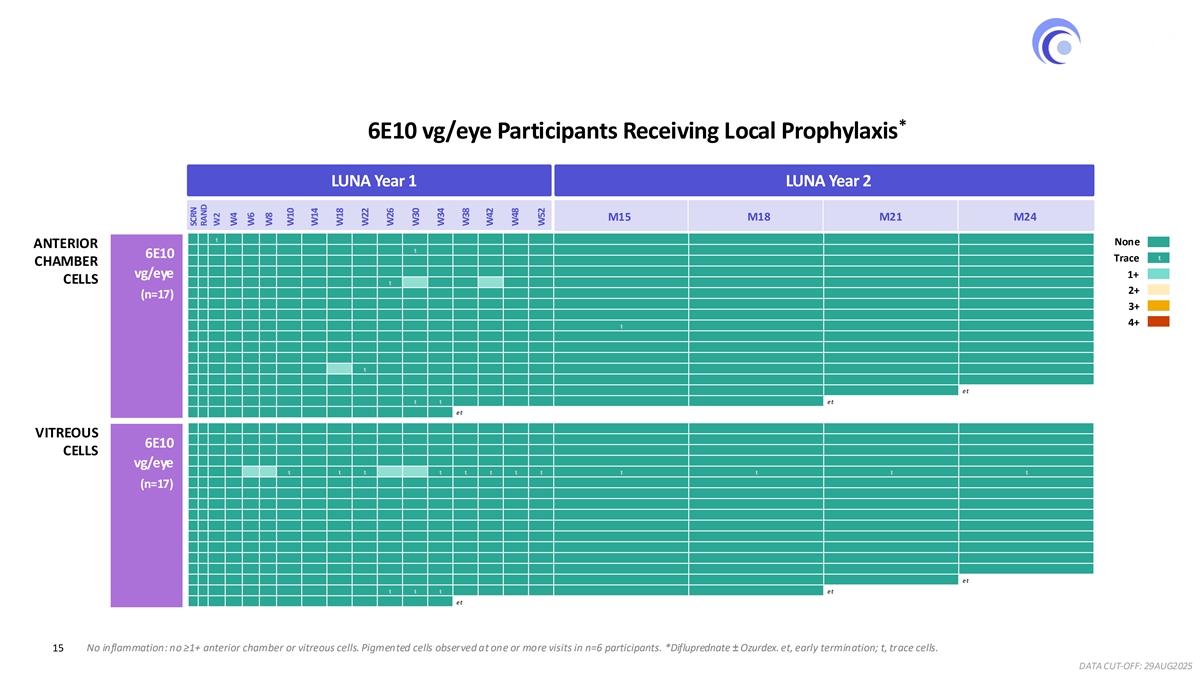
LUNA 2-year Safety Results Ixo-vec Phase 3 dose well tolerated, no new inflammation observed in year 2 * 6E10 vg/eye Participants Receiving Local Prophylaxis LUNA Year 1 LUNA Year 2 M15 M18 M21 M24 0 0 t 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 None ANTERIOR 0 0 0 0 0 0 0 0 0 0 0 t 0 0 0 0 0 0 0 0 0 6E10 t Trace 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 CHAMBER 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 vg/eye 1+ CELLS 0 0 0 0 0 0 0 0 0 0 t 1+ 0 0 1+ 0 0 0 0 0 0 2+ 0 0 0 0 0 0 0 0 0 0 0 (n=17) 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 3+ 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 4+ 0 0 0 0 0 0 0 0 0 0 0 0 0 t 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1+ t 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 et 0 0 0 0 0 0 0 0 0 0 0 t t 0 0 0 0 0 0 et 0 0 0 0 0 0 0 0 0 0 0 0 0 et 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 VITREOUS 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 6E10 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 CELLS 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 vg/eye 0 0 0 0 1+ 1+ t 0 t t 1+ 1+ t t t t t t t t t 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 (n=17) 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 et 0 0 0 0 0 0 0 0 0 0 t t t 0 0 0 0 0 0 et 0 0 0 0 0 0 0 0 0 0 0 0 0 et 15 No inflammation: no ≥1+ anterior chamber or vitreous cells. Pigmented cells observed at one or more visits in n=6 participants. *Difluprednate ± Ozurdex. et, early termination; t, trace cells. DATA CUT-OFF: 29AUG2025 SCRN RAND W2 W4 W6 W8 W10 W14 W18 W22 W26 W30 W34 W38 W42 W48 W52
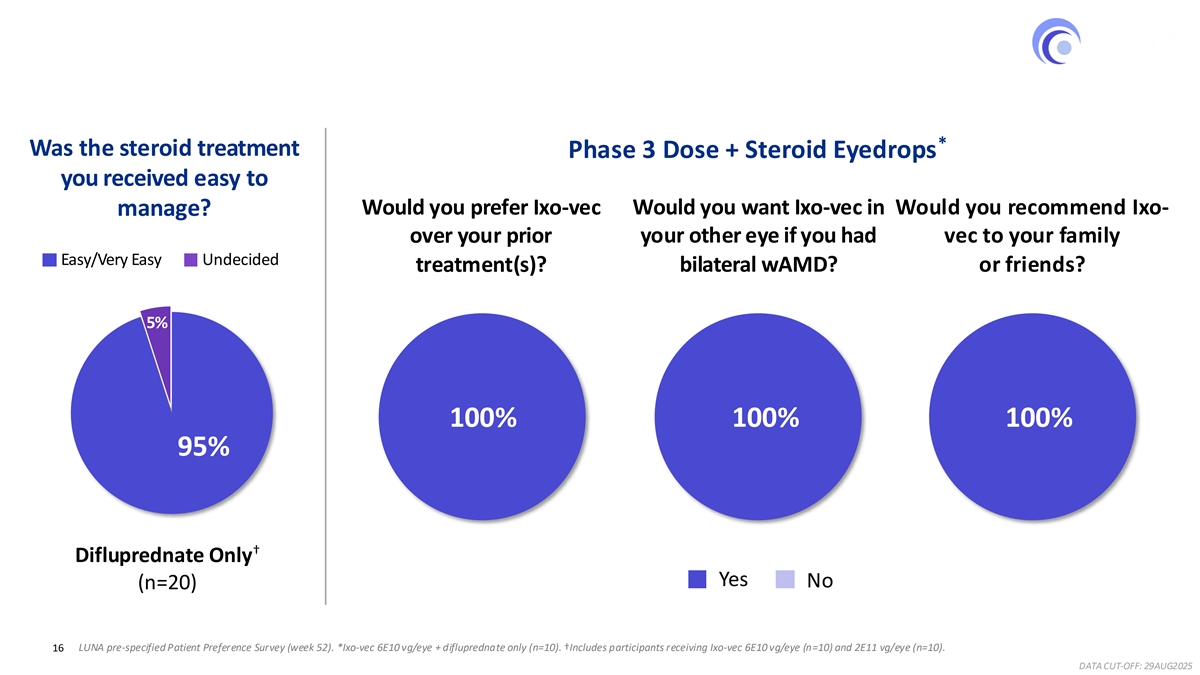
100% of Participants Receiving Phase 3 Dose + Steroid Eyedrops Alone * Preferred Ixo-vec to Prior Treatments * Was the steroid treatment Phase 3 Dose + Steroid Eyedrops you received easy to Would you prefer Ixo-vec Would you want Ixo-vec in Would you recommend Ixo- manage? over your prior your other eye if you had vec to your family Easy/Very Easy Undecided treatment(s)? bilateral wAMD? or friends? 5% 100% 100% 100% 95% † Difluprednate Only Yes No (n=20) 16 LUNA pre-specified Patient Preference Survey (week 52). *Ixo-vec 6E10 vg/eye + difluprednate only (n=10). †Includes participants receiving Ixo-vec 6E10 vg/eye (n=10) and 2E11 vg/eye (n=10). DATA CUT-OFF: 29AUG2025
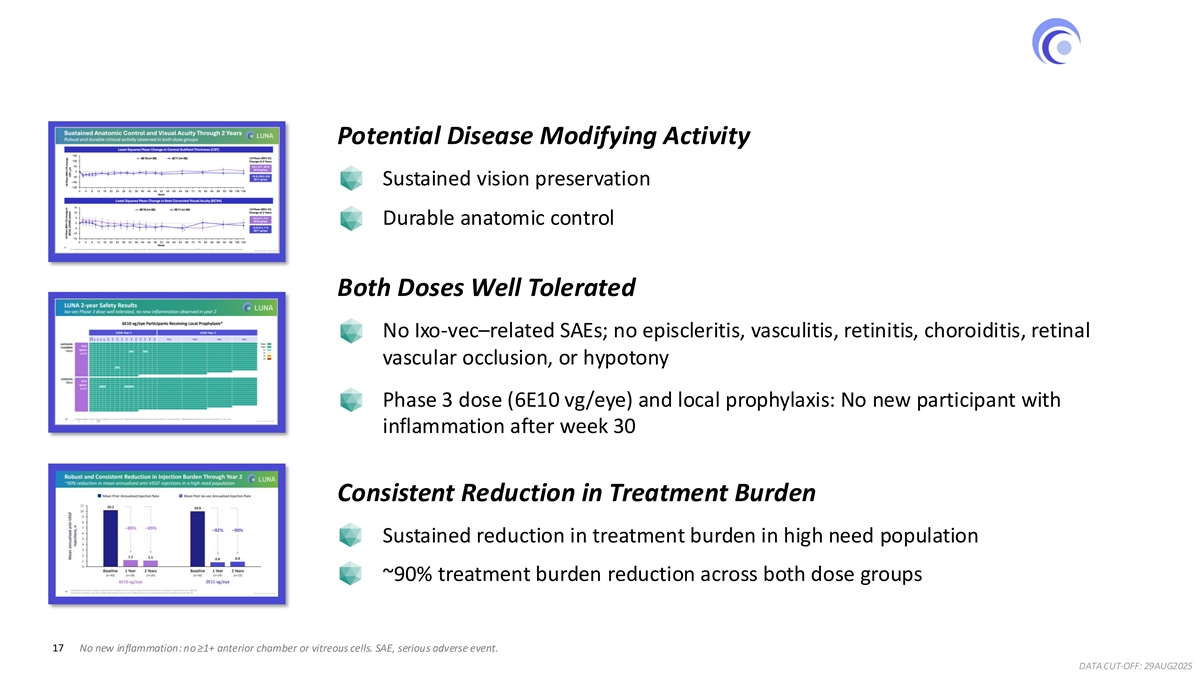
Key Findings from the LUNA Extension Study Intravitreal Ixo-vec in a high need nAMD population: 2-year Results Potential Disease Modifying Activity Sustained vision preservation Durable anatomic control Both Doses Well Tolerated No Ixo-vec–related SAEs; no episcleritis, vasculitis, retinitis, choroiditis, retinal vascular occlusion, or hypotony Phase 3 dose (6E10 vg/eye) and local prophylaxis: No new participant with inflammation after week 30 Consistent Reduction in Treatment Burden Sustained reduction in treatment burden in high need population ~90% treatment burden reduction across both dose groups 17 No new inflammation: no ≥1+ anterior chamber or vitreous cells. SAE, serious adverse event. DATA CUT-OFF: 29AUG2025
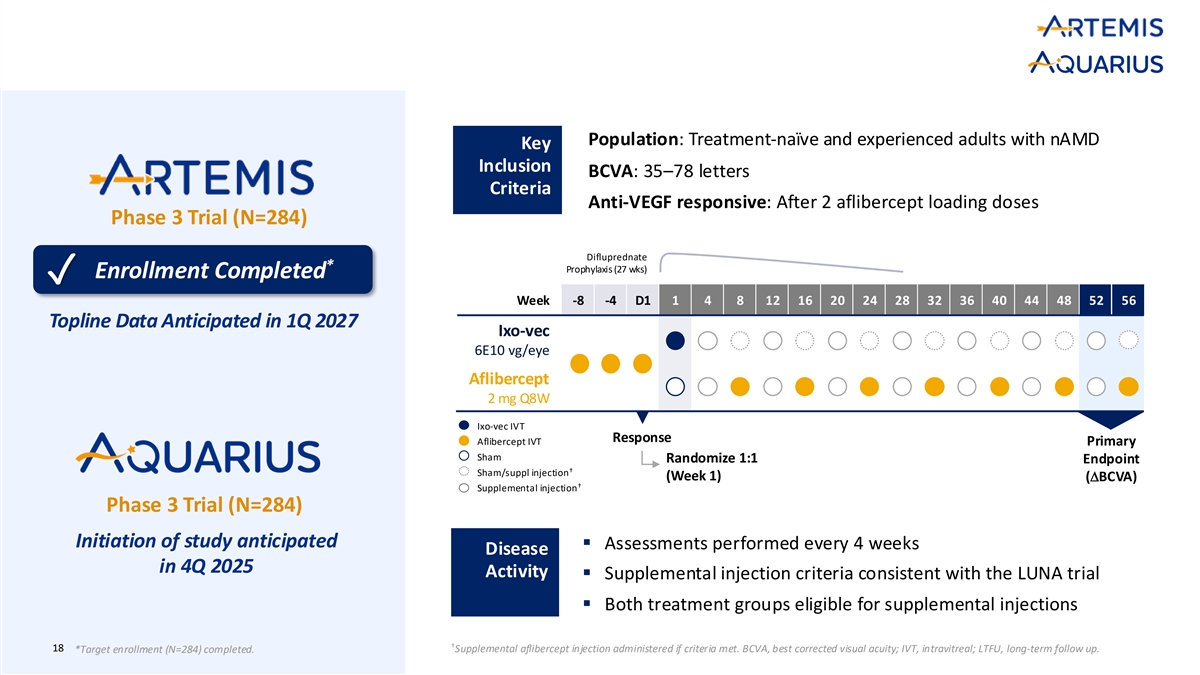
Ixo-vec Phase 3 Neovascular AMD Study Designs Population: Treatment-naïve and experienced adults with nAMD Key Inclusion BCVA: 35–78 letters Criteria Anti-VEGF responsive: After 2 aflibercept loading doses Phase 3 Trial (N=284) Difluprednate * Prophylaxis (27 wks) Enrollment Completed ✓ Week -8 -4 D1 1 4 8 12 16 20 24 28 32 36 40 44 48 52 56 Topline Data Anticipated in 1Q 2027 Ixo-vec 6E10 vg/eye Aflibercept 2 mg Q8W Ixo-vec IVT Response Aflibercept IVT Primary Sham Randomize 1:1 Endpoint † Sham/suppl injection (Week 1) (DBCVA) † Supplemental injection Phase 3 Trial (N=284) Initiation of study anticipated ▪ Assessments performed every 4 weeks Disease in 4Q 2025 Activity ▪ Supplemental injection criteria consistent with the LUNA trial ▪ Both treatment groups eligible for supplemental injections † 18 *Target enrollment (N=284) completed. Supplemental aflibercept injection administered if criteria met. BCVA, best corrected visual acuity; IVT, intravitreal; LTFU, long-term follow up.

FLORetina • December 2025 Ixo-vec Gene Therapy for nAMD: Clinical Progress from the LUNA Phase 2 Trial Charles C. Wykoff, M.D., Ph.D. Director of Research, Retina Consultants of Texas Professor of Clinical Ophthalmology, Blanton Eye Institute, Houston Methodist Hospital Disclosures: C= Consultant | R= Research Support | SO= Stock Options 4DMT (C,R), AbbVie (C,R), Adverum (C,R), AGTC (C), Alcon (C), Alexion (R), Alimera (C,R), Alkeus (C), AMC Sciences (C), Annexon (C,R), Apellis (C,R), Ascidian (R), Aviceda (R), Avirmax (R), Bausch + Lomb (C), Bayer (C,R), Beacon (R), Biocryst (C), Bionic Vision (C), Boehringer Ingelheim (C,R), Chengdu Kanghong (C), Chengdu Origen (R), Clearside (R), Curacle (C,R), Emmecell (C), EyeBiotech (C,R), EyePoint (C,R), Genentech (C,R), Gyroscope (R), InGel (C,SO), IONIS (R), IVERIC Bio (C,R), Janssen (C,R), Kalaris (R), Kiora (C), Kodiak (C,R), Kowa (C), Kyowa Kirin (R), Merck (C), Merit (C), Nanoscope (C, R), Neurotech (C, R), NGM (R), Novartis (C, R), Oak Bay Bio (C), Ocugen (R), Ocular Therapeutix (C, R), Ocuphire (C), OcuTerra (C, R), OliX (R), Ollin (C), ONL (C, SO), Opthea (C,R), Osanni (C,SO), Oxular (R), Palatin (C), Panther (SO), Perceive Bio (R), PolyPhotonix (SO), Ray (C), RecensMedical (SO), Regeneron (C,R), RegenXBio (C,R), RetinAI (C), Roche (C,R), Sandoz (C), Sanofi (C), Santen (C), Skyline (R), Stealth (C,R), THEA (C), TissueGen (SO), VH401 (C,R), Visgenx (C,SO), Vitranu (SO), Zeiss (C)