UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): December 02, 2025 |
Fractyl Health, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-41942 |
27-3553477 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
|
3 Van de Graaff Drive
Suite 200
|
|
Burlington, Massachusetts |
|
01803 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (781) 902-8800 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, $0.00001 par value per share |
|
GUTS |
|
The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On December 2, 2025, Fractyl Health, Inc. (the “Company”) issued a press release furnished as Exhibit 99.1 to this Current Report on Form 8-K and incorporated herein by reference.
The information contained in Item 7.01 of this Current Report (including Exhibit 99.1 attached hereto) shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly provided by specific reference in such a filing.
Item 8.01 Other Events.
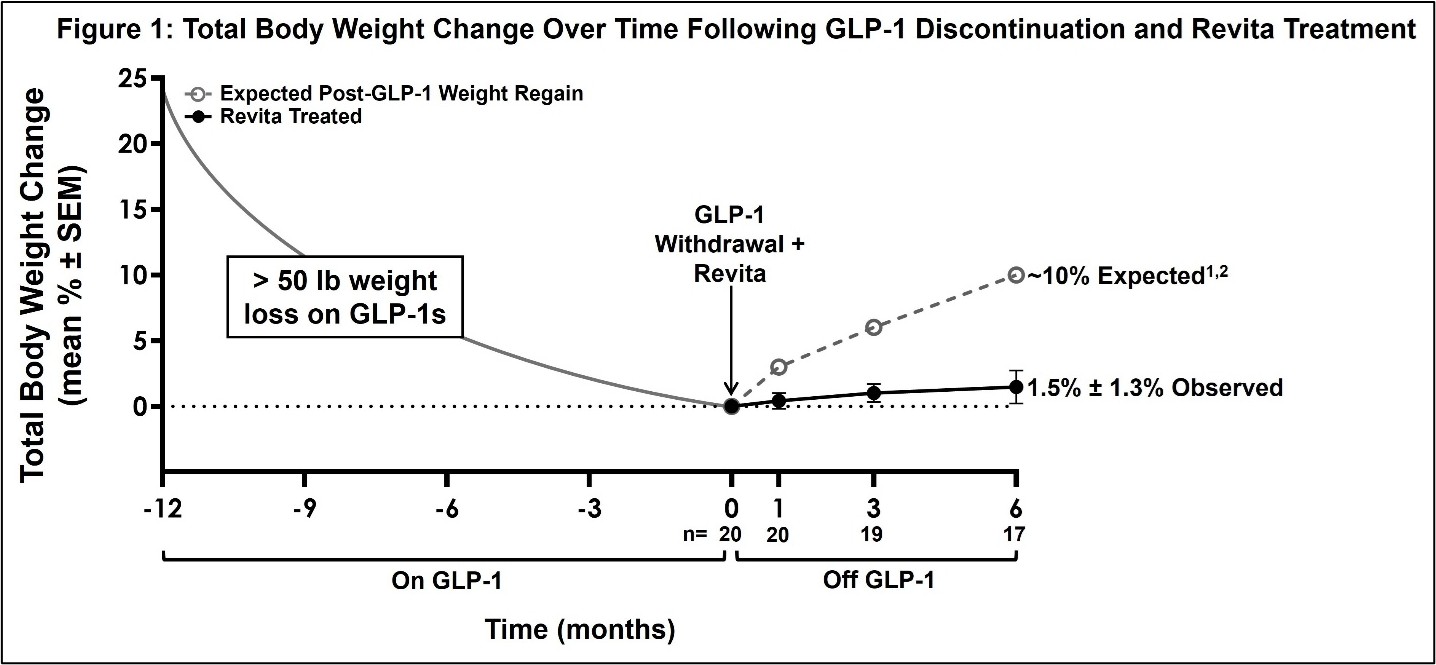
On December 2, 2025, the Company issued a press release announcing positive 6-month data from the open-label REVEAL-1 Cohort. To date, 22 participants have been treated in the REVEAL-1 Cohort and are included in safety analyses, with 6-month follow-up efficacy data reported for 17. Three participants withdrew or were lost to follow up and two participants experienced protocol deviations. Participants maintained stable weight after a single Revita procedure, with a mean total body weight change of 1.5% ± 1.3% (SEM; n=17) at 6 months. Published third-party studies report ~10% weight regain by this time point after GLP-1 withdrawal alone. Minimal change in HbA1c levels was observed after the Revita procedure (0.04% ± 0.08%; SEM; n=17), compared to the ~0.4% increase in HbA1c seen post-GLP-1 discontinuation in the STEP-1 trial extension of GLP-1 withdrawal. These results indicate the potential for Revita to help stabilize cardiometabolic parameters beyond weight loss alone. Mean body weight and HbA1c curves showed a stable and durable trajectory over time, consistent with prior Revita clinical study and real-world experience. These results are encouraging for potential longer-term durability of weight maintenance and metabolic control. No procedure-related serious adverse events were observed; 8 of 22 participants (36%) experienced mild treatment-emergent adverse events which were transient, and self-limited; all consistent with prior Revita experience and similar to routine upper endoscopy findings.
The Company also announced that it is advancing toward multiple anticipated clinical readouts from the ongoing REMAIN weight maintenance program:
•
January 2026: 6-month randomized data from the REMAIN-1 Midpoint Cohort
•
Q2 2026: 1-year REVEAL-1 Cohort data
•
Q3 2026: 1-year REMAIN-1 Midpoint Cohort data
•
H2 2026: Topline 6-month randomized data from REMAIN-1 Pivotal Cohort
•
H2 2026: Potential Revita PMA filing in post-GLP-1 weight maintenance
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact are forward-looking statements. These statements may be identified by words such as “aims,” “anticipates,” “believes,” “could,” “estimates,” “expects,” “forecasts,” “goal,” “intends,” “may,” “plans,” “possible,” “potential,” “seeks,” “will” and variations of these words or similar expressions that are intended to identify forward-looking statements, although not all forward-looking statements contain these words. Forward-looking statements in this press release include, without limitation, statements regarding the promise and potential impact of our product candidates, including Revita’s potential for preserving weight loss after GLP-1 drug discontinuation; the design, initiation, timing and results of clinical enrollment and any clinical studies or readouts, including readouts from the REVEAL-1 Cohort, REMAIN-1 Midpoint Cohort and REMAIN-1 Pivotal Cohort; the potential treatment population or benefits for any of our product candidates or products; our strategic and product development objectives and goals, including anticipated regulatory filings; and the timing of any of the foregoing. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause the Company’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: the Company’s limited operating history; the incurrence of significant net losses and the fact that the Company expects to continue to incur significant net losses for the foreseeable future; the Company’s need for substantial additional financing; the Company’s ability to continue as a going concern; the restrictive and financial covenants in the Company’s credit agreement; the lengthy and unpredictable regulatory approval process for the Company’s product candidates; uncertainty regarding its clinical studies; the fact that the Company’s product candidates may cause serious adverse events or undesirable side effects or have other properties that may cause it to suspend or discontinue clinical studies, delay or prevent regulatory development, prevent their regulatory approval, limit the commercial profile, or result in significant negative consequences; the Company’s reliance on third parties to conduct certain aspects of the Company’s preclinical studies and clinical studies; the Company’s reliance on third parties for the manufacture of sub-assembly components for Revita; the regulatory approval process of the FDA and comparable foreign regulatory authorities is lengthy, time-consuming and inherently unpredictable, and even if we complete the necessary clinical studies, we cannot predict when, or if, we will obtain regulatory approval or certification for any of our product candidates, and any such regulatory approval or certification may be for a more narrow indication than we seek; and the potential launch or commercialization of any of the Company’s product candidates or products and our strategic and product development objectives and goals, and the other factors discussed under the caption “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2024 and Quarterly Report on Form 10-Q for the quarter ended September 30, 2025, filed with the Securities and Exchange Commission on November 12, 2025 and in our other filings with the SEC. These forward-looking statements are based on management’s current estimates and expectations. While the Company may elect to update such forward-looking statements at some point in the future, the Company disclaims any obligation to do so, even if subsequent events cause its views to change. Item 9.01 Financial Statements and Exhibits. The following exhibit relates to Item 7.01 and shall be deemed to be furnished, and not filed: Exhibit No. Description 99.1 Press Release dated December 2, 2025 104 Cover Page Interactive Data File (embedded within the Inline XBRL document)
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
Fractyl Health, Inc. |
|
|
|
|
Date: |
December 2, 2025 |
By: |
/s/ Harith Rajagopalan |
|
|
|
Harith Rajagopalan, M.D., Ph.D.
Co-Founder, Chief Executive Officer and Director
(Principal Executive Officer) |