UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): November 19, 2025
IMAGENEBIO, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-40287 | 81-1697316 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
| 12526 High Bluff Drive, Suite 345 | ||
| San Diego, California | 92130 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (858) 345-6265
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
||
| Common Stock, $0.001 par value | IMA | The Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01. | Regulation FD Disclosure. |
On November 19, 2025, the Company updated its corporate presentation for use in meetings with investors, analysts and others. The presentation is being pubished on the Company’s website and a copy is attached as Exhibit 99.1 to this Current Report on Form 8-K and incorporated by reference herein.
The information in this Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.1, is furnished and shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or incorporated by reference in any filing under the Securities Act of 1933, as amended, unless expressly incorporated by specific reference in such filing.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit Number |
Description | |
| 99.1 | Corporate Presentation of the Company Dated November 19, 2025 | |
| 104 | Cover Page Interactive Data File (embedded with the Inline XBRL document). | |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| IMAGENEBIO, INC. | ||||||
| Date: November 19, 2025 | By: | /s/ Kristin Yarema |
||||
| Kristin Yarema, Ph.D. | ||||||
| Chief Executive Officer | ||||||

Exhibit 99.1 ImageneBio Corporate Overview Fourth Quarter 2025

Disclaimer and forward-looking statements This investor presentation and any accompanying oral commentary have been prepared by ImageneBio, Inc. (the Company) for informational purposes only and not for any other purpose. This investor presentation and any accompanying oral commentary contain forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, including, without limitation, statements regarding: the potential benefits of OX40/OX40L antagonists generally and IMG-007 specifically, including as a treatment option for AD and AA, its best-in-class potential, its differentiating features and as a pipeline in a product; expected cash runway; the current estimated global market size in autoimmune and inflammatory indications where OX40/OX40L signaling has been implicated; the estimated market opportunity, patient population and demographics of patients with AD; the ongoing Phase 2b ADAPTIVE study, including the aims thereof and the expected timing for topline data; and other statements regarding management's intentions, plans, beliefs, expectations or forecasts for the future. Words such as will, can, expect, may, plan, potential, opportunity, goal, believe, or other words that convey uncertainty of future events or outcomes are used to identify these forward-looking statements. These statements are subject to a number of risks, uncertainties and assumptions, including, but not limited to: risks associated with the nonclinical and clinical development and regulatory approval of IMG-007, including potential delays in the completion of clinical trials and potential safety and other complications thereof; the timing of the availability of data from the Company's clinical trials; the clinical utility, potential differentiation and/or benefits and market acceptance of IMG-007; the requirement for additional capital to continue to advance the IMG-007 program, which may not be available on favorable terms or at all; the Company's ability to attract, hire, and retain skilled executive officers and employees; the Company's ability to protect its intellectual property and proprietary technologies; the Company's reliance on third parties, contract manufacturers, and contract research organizations; the possibility that the Company may be adversely affected by other economic, political, business, or competitive factors; and risks associated with changes in applicable laws or regulations or government resources and policies. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties. These and other risks and uncertainties are more fully described in the Company's filings with the Securities and Exchange Commission (the SEC), including the factors described in the section titled Risk Factors in the Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 2025, filed with the SEC on November 12, 2025, and in the Company’s other documents subsequently filed with the SEC. You should not place undue reliance on these forward-looking statements, which are made only as of the date hereof. The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law. This investor presentation also contains estimates and other statistical data made by independent parties and by us related to patient demographics and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. This investor presentation contains trademarks, service marks, trade names and copyrights of the Company and other companies which are the property of their respective owners. This investor presentation shall not constitute an offer to sell or the solicitation of an offer to buy securities of the Company, nor shall there be any sale of such securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. 2

Innovating in immunology Established as a public company (NASDAQ: Anchored on IMG-007, a differentiated anti- IMA) in July 2025 through Inmagene-Ikena OX40 monoclonal antibody (mAb) in Phase 2b reverse merger and concurrent financing for atopic dermatitis, the most common type of eczema Well-funded with strong investor group and cash runway expected through end of 2027 Worldwide commercialization rights Headquartered in San Diego, CA Designed to be different: developing a potential best-in-class anti-OX40 treatment with features that matter to patients 3

In the large atopic dermatitis (AD) population, patients are being underserved 1 As of today, it is estimated that there are… The global AD biologics market is estimated at $15 billion in 2025, and People living in the US with AD 26 million 2 growing year-over-year Children living in the US with AD 7 million Persistent symptoms despite treatment: Even with biologics and JAK inhibitors, 30- 40% of patients report inadequate disease Adults living in the US have 6.8 million 3 control moderate-to-severe AD Quality of life crisis: Itch, sleep loss, and People living around the world with AD 237 million mental health impacts plague patients Children living around the world with AD Burdensome regimens: Existing products 96 million require daily or bi-weekly dosing Adults living around the world have Access and adoption gap: Only ~15% of 49 million moderate-to-severe AD 3 biologic-eligible patients receive treatment JAKi: JAK inhibitor (Janus Kinase inhibitor) https://nationaleczema.org/ patient submitted pictures 1. Market research report sourced from: National Eczema Association, 2024; Fuxench et al., 2019; CDC, January 2023; Global Data; TARGET-DERM-AD registry; Laughter et al., 2021; Tian et al., 2024; Ab Hadi et al., 2021; Suaini et al., 2020; ,International League of Dermatological Societies, 2022 Report. 2. Global Data 3. Multiple sources on market reporting and publications including Ann P Quick, J Silverberg, et Al. 707 - Contemporary systemic treatment patterns in atopic dermatitis, British Journal of Dermatology, 2024 4
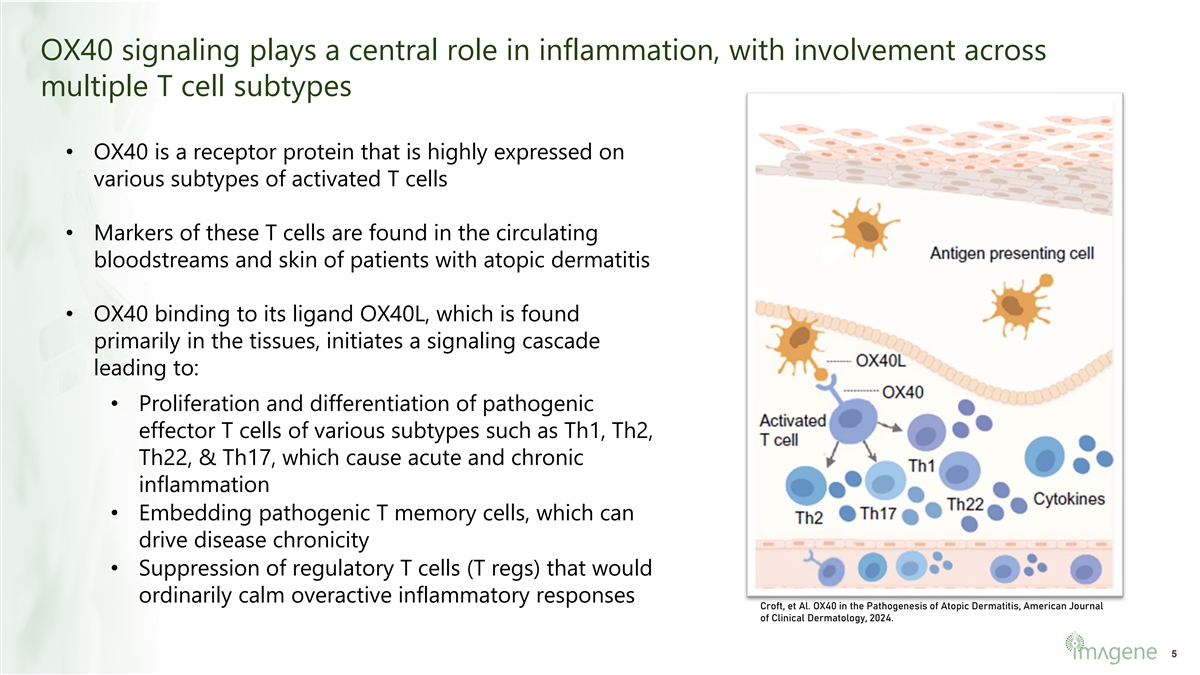
OX40 signaling plays a central role in inflammation, with involvement across multiple T cell subtypes • OX40 is a receptor protein that is highly expressed on various subtypes of activated T cells • Markers of these T cells are found in the circulating bloodstreams and skin of patients with atopic dermatitis • OX40 binding to its ligand OX40L, which is found primarily in the tissues, initiates a signaling cascade leading to: • Proliferation and differentiation of pathogenic effector T cells of various subtypes such as Th1, Th2, Th22, & Th17, which cause acute and chronic inflammation • Embedding pathogenic T memory cells, which can drive disease chronicity • Suppression of regulatory T cells (T regs) that would ordinarily calm overactive inflammatory responses Croft, et Al. OX40 in the Pathogenesis of Atopic Dermatitis, American Journal of Clinical Dermatology, 2024. 5

OX40’s role across T cell subsets underscores its potential in atopic dermatitis • Atopic dermatitis is well known as a heterogeneous disease where patients can have multiple pathways over-activated simultaneously • Inhibiting OX40/OX40L signal cascade is a promising novel therapeutic approach because it may help restore balance to multiple inflammation-related pathways at once • OX40/OX40L blockade contrasts with biologic therapies that inhibit a single pathway; for example, anti-IL-13 or anti-IL4Rα drug, like dupillimab, primarily block Th2 signaling • We believe OX40 inhibition could be a very effective and novel monotherapy approach and may also have potential in combinations with other agents given its broad, rebalancing (vs. immunosuppressing) mechanism https://nationaleczema.org/ patient submitted pictures Guttman-Yassky, Croft, M., Esfandiari, E., Chong, C. et al. OX40 in the Pathogenesis of Atopic Dermatitis—A New Therapeutic Target. Am J Clin Dermatol 25, 447–461 (2024) Chovatiya R, Silverberg JI. The Heterogeneity of Atopic Dermatitis. J Drugs Dermatol. 2022 Feb 1;21(2):172-176. doi: 10.36849/JDD.6408. PMID: 35133102; PMCID: PMC10119386. 6

Designed to be different: IMG-007, a next generation anti-OX40 mAb 1. Receptor targeting for optimal efficacy profile IMG-007 has a trifecta of 2. T cell-preserving for safety and tolerability differentiating features 3. Extended half-life for patient- and physician-friendly dosing schedules 7
Designed to be different: IMG-007, a next generation anti-OX40 mAb 1. Receptor targeting for optimal efficacy profile Targeting OX40 receptor (OX40) rather than OX40 ligand (OX40L) allows IMG-007 to inhibit OX40-OX40L signaling in both blood and tissues. 2. T cell-preserving for safety and tolerability 3. Extended half-life for patient- and physician-friendly dosing schedules 8
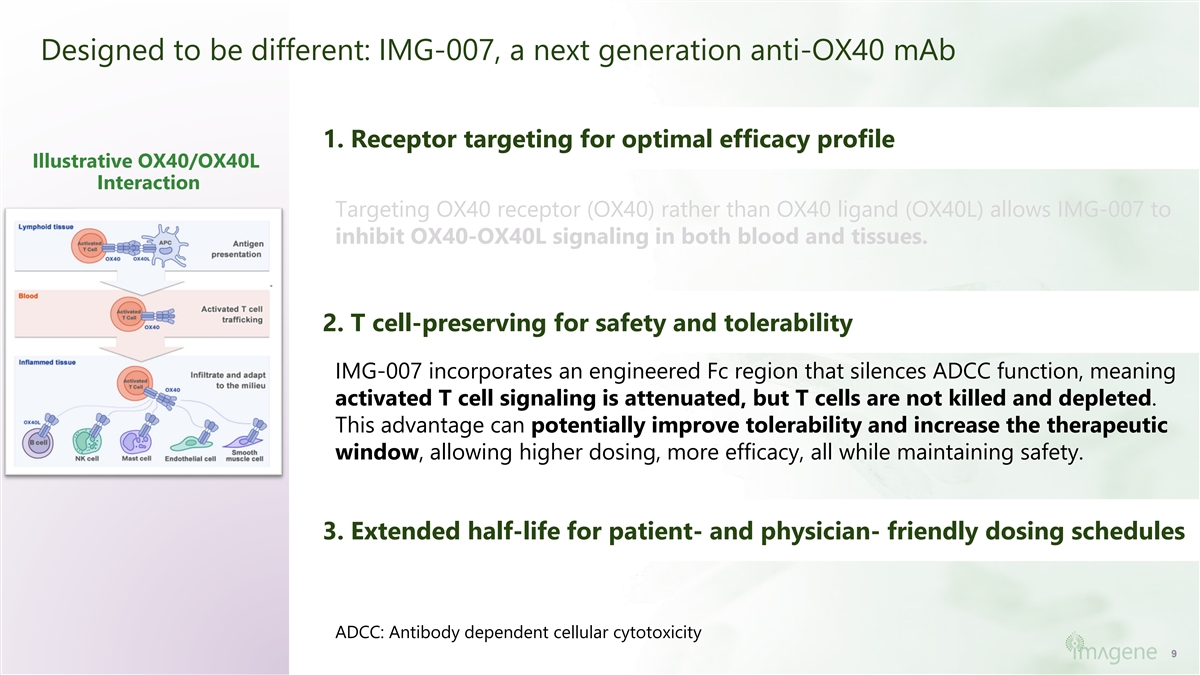
Designed to be different: IMG-007, a next generation anti-OX40 mAb 1. Receptor targeting for optimal efficacy profile Illustrative OX40/OX40L Interaction Targeting OX40 receptor (OX40) rather than OX40 ligand (OX40L) allows IMG-007 to inhibit OX40-OX40L signaling in both blood and tissues. 2. T cell-preserving for safety and tolerability IMG-007 incorporates an engineered Fc region that silences ADCC function, meaning activated T cell signaling is attenuated, but T cells are not killed and depleted. This advantage can potentially improve tolerability and increase the therapeutic window, allowing higher dosing, more efficacy, all while maintaining safety. 3. Extended half-life for patient- and physician- friendly dosing schedules ADCC: Antibody dependent cellular cytotoxicity 9

Designed to be different: IMG-007, a next generation anti-OX40 mAb 1. Receptor targeting for optimal efficacy profile Half-Life Targeting OX40 receptor (OX40) rather than OX40 ligand (OX40L) allows IMG-007 to inhibit OX40-OX40L signaling in both blood and tissues. Roca 2. T cell-preserving for safety and tolerability Amli IMG-007 incorporates an engineered Fc region that silences ADCC function, meaning activated T cell signaling is attenuated, but T cells are not killed and depleted. This advantage can potentially improve tolerability and increase the therapeutic IMG-007 window, allowing higher dosing, more efficacy, all while maintaining safety. 0 10 20 30 40 (Days) 3. Extended half-life for patient- and physician- friendly dosing schedules SC formulation, available for IMG-007 and rocatinlimab, IMG-007's engineering has resulted in a half-life of approximately 5 weeks in a are presented. Half-life data for amlitelimab SC is not available, therefore data from the IV PK study is patient’s bloodstream, opening the potential for dosing intervals of once every presented. (see slide 12 for further detail on studies) No head-to-head trials have been conducted among the results shown and cross-trial comparisons must be few months or beyond. interpreted with caution. As a result, conclusive cross- trial comparisons cannot be made 10

Designed to be different: IMG-007, a next generation anti-OX40 mAb 1. Receptor targeting for optimal efficacy profile Efficacy, safety, and convenience all drive IMG-007 has a product choice among physicians and patients in trifecta of inflammatory and autoimmune disease 2. T cell-preserving for safety and tolerability differentiating features As a non-T cell depleting, receptor targeting anti- OX40, with a roughly 5-week half-life and well tolerated profile, investigational IMG-007 is well positioned to emerge as a best-in-class OX40 treatment 3. Extended half-life for patient- and physician-friendly dosing schedules 11
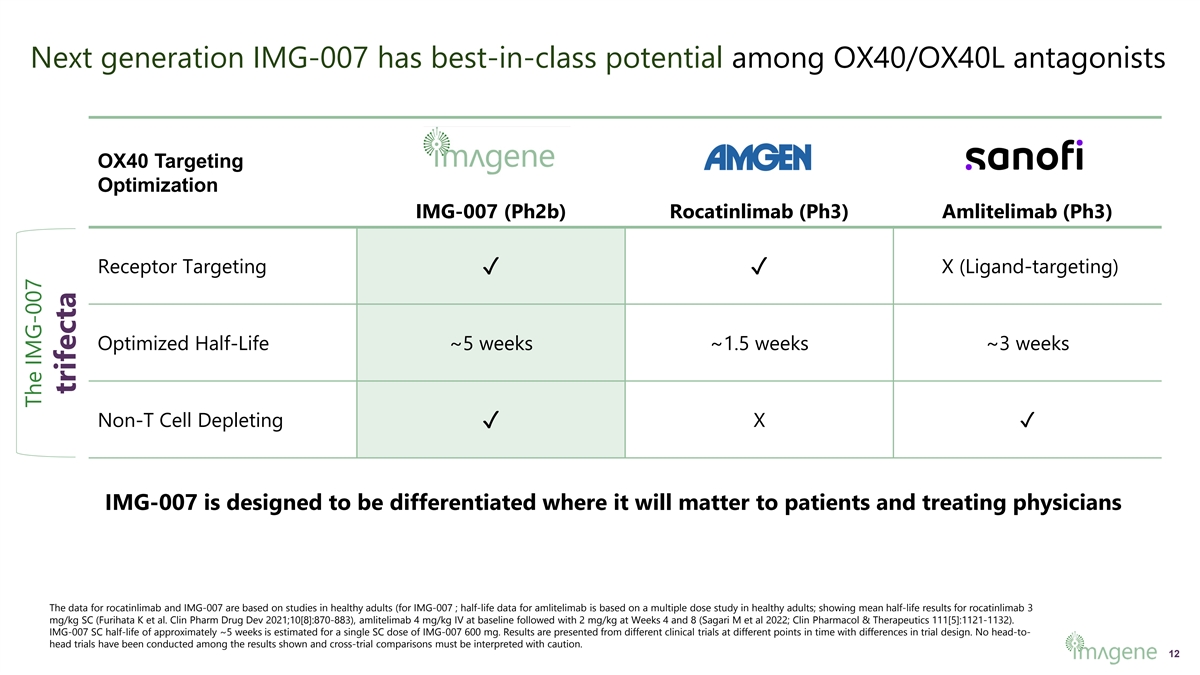
Next generation IMG-007 has best-in-class potential among OX40/OX40L antagonists OX40 Targeting Optimization IMG-007 (Ph2b) Rocatinlimab (Ph3) Amlitelimab (Ph3) Receptor Targeting✓✓ X (Ligand-targeting) Optimized Half-Life ~5 weeks ~1.5 weeks ~3 weeks Non-T Cell Depleting✓ X✓ IMG-007 is designed to be differentiated where it will matter to patients and treating physicians The data for rocatinlimab and IMG-007 are based on studies in healthy adults (for IMG-007 ; half-life data for amlitelimab is based on a multiple dose study in healthy adults; showing mean half-life results for rocatinlimab 3 mg/kg SC (Furihata K et al. Clin Pharm Drug Dev 2021;10[8]:870-883), amlitelimab 4 mg/kg IV at baseline followed with 2 mg/kg at Weeks 4 and 8 (Sagari M et al 2022; Clin Pharmacol & Therapeutics 111[5]:1121-1132). IMG-007 SC half-life of approximately ~5 weeks is estimated for a single SC dose of IMG-007 600 mg. Results are presented from different clinical trials at different points in time with differences in trial design. No head-to- head trials have been conducted among the results shown and cross-trial comparisons must be interpreted with caution. 12 The IMG-007 trifecta
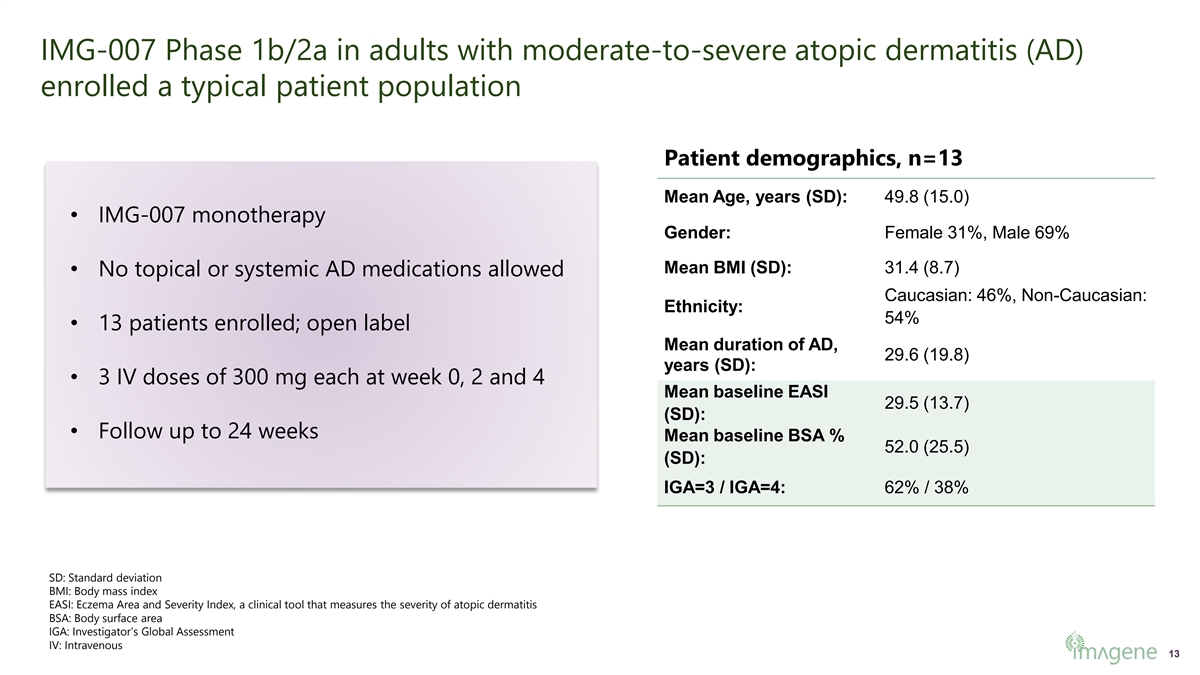
IMG-007 Phase 1b/2a in adults with moderate-to-severe atopic dermatitis (AD) enrolled a typical patient population Patient demographics, n=13 Mean Age, years (SD): 49.8 (15.0) • IMG-007 monotherapy Gender: Female 31%, Male 69% Mean BMI (SD): 31.4 (8.7) • No topical or systemic AD medications allowed Caucasian: 46%, Non-Caucasian: Ethnicity: 54% • 13 patients enrolled; open label Mean duration of AD, 29.6 (19.8) years (SD): • 3 IV doses of 300 mg each at week 0, 2 and 4 Mean baseline EASI 29.5 (13.7) (SD): • Follow up to 24 weeks Mean baseline BSA % 52.0 (25.5) (SD): IGA=3 / IGA=4: 62% / 38% SD: Standard deviation BMI: Body mass index EASI: Eczema Area and Severity Index, a clinical tool that measures the severity of atopic dermatitis BSA: Body surface area IGA: Investigator’s Global Assessment IV: Intravenous 13
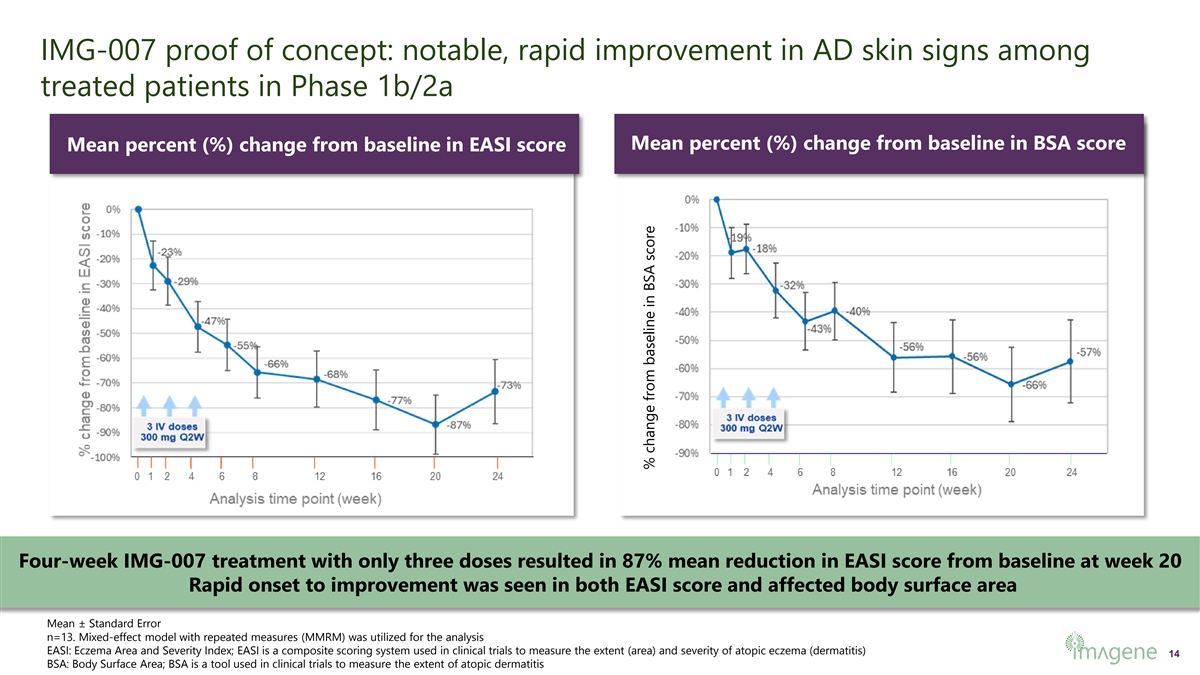
IMG-007 proof of concept: notable, rapid improvement in AD skin signs among treated patients in Phase 1b/2a Mean percent (%) change from baseline in BSA score Mean percent (%) change from baseline in EASI score Four-week IMG-007 treatment with only three doses resulted in 87% mean reduction in EASI score from baseline at week 20 Rapid onset to improvement was seen in both EASI score and affected body surface area Mean ± Standard Error n=13. Mixed-effect model with repeated measures (MMRM) was utilized for the analysis EASI: Eczema Area and Severity Index; EASI is a composite scoring system used in clinical trials to measure the extent (area) and severity of atopic eczema (dermatitis) 14 BSA: Body Surface Area; BSA is a tool used in clinical trials to measure the extent of atopic dermatitis % change from baseline in BSA score
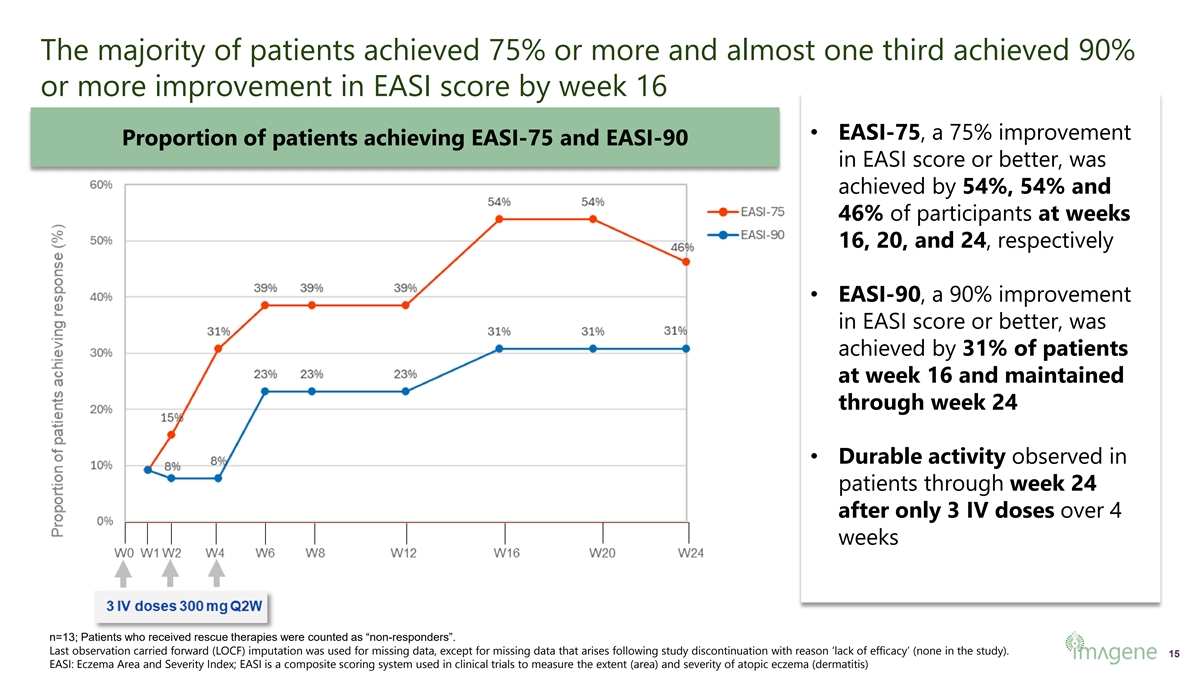
The majority of patients achieved 75% or more and almost one third achieved 90% or more improvement in EASI score by week 16 • EASI-75, a 75% improvement Proportion of patients achieving EASI-75 and EASI-90 in EASI score or better, was achieved by 54%, 54% and 46% of participants at weeks 16, 20, and 24, respectively • EASI-90, a 90% improvement in EASI score or better, was achieved by 31% of patients at week 16 and maintained through week 24 • Durable activity observed in patients through week 24 after only 3 IV doses over 4 weeks n=13; Patients who received rescue therapies were counted as “non-responders”. Last observation carried forward (LOCF) imputation was used for missing data, except for missing data that arises following study discontinuation with reason ‘lack of efficacy’ (none in the study). 15 EASI: Eczema Area and Severity Index; EASI is a composite scoring system used in clinical trials to measure the extent (area) and severity of atopic eczema (dermatitis)

Early IMG-007 data support a favorable emerging safety profile; no fever or chills observed Treatment-emergent adverse events in Phase 1b/2a Participants with at least one TEAE 9 (69%) • In the Phase 1b/2a of IMG-007 in moderate- to-severe AD there were Study treatment related TEAEs 0 • No serious adverse events Serious AE 0 • No treatment-related AEs TEAE by CTCAE grade • No infusion-related reactions Grade 1 (Mild) 3 (23%) • No reports of pyrexia or chills reported Grade 2 (Moderate) 5 (38%) • All AEs were of mild or moderate intensity, Grade 3 (Severe) 1 (8%) except for one patient who experienced an unrelated severe AE of AD flare TEAE that are infusion-related reactions 0 • The well-tolerated profile can potentially be TEAE of pyrexia (fever) or chills 0 attributed to IMG-007’s silenced ADCC TEAE leading to 4-week dosing period 0 function and resulting lack of T cell depletion discontinuation IMG-007 safety profile has been consistent across all four clinical studies conducted to date, including the AD proof-of-concept, alopecia areata proof-of-concept, and two healthy volunteer studies AE: adverse event CTCAE: Common terminology criteria for adverse events TEAE: treatment-emergent adverse event 16

Th1, Th2, and Th17 biomarkers were reduced to within healthy volunteer ranges in IMG-007 treated AD patients in the Phase 1b/2a proof-of-concept study Th2 serum proteins Th17 serum proteins Th1 serum proteins th 50 percentile th of healthy 50 percentile th subjects of healthy 50 percentile subjects of healthy subjects (Mean ± SEM) (Mean ± SEM) (Mean ± SEM) AD: Atopic dermatitis Two-way ANOVA with Dunnett’s multiple comparisons test; SEM: standard error of the mean n numbers at baseline, wk16, and 24 were 13, 6 and 6, respectively Post-systemic rescue treatment results were censored from the analysis 17

ADAPTIVE: the IMG-007 phase 2b clinical trial in atopic dermatitis (AD) The phase 2b ADAPTIVE trial is a randomized, double-blind, placebo-controlled dose-finding study of IMG-007 in adults with moderate-to-severe AD, recruiting both biologic- and/or JAK inhibitor-experienced and naive patients and using a subcutaneous formulation Aims of the ADAPTIVE study: • Continue to assess overall efficacy and safety of IMG-007 in AD • Currently recruiting in North • More fully characterize the clinical profile, including at a range of America, Europe to follow drug exposures • Expert team with specialty CRO to • Understand the role of short- and longer-term treatment drive trial execution • Characterize the role of loading doses in driving the magnitude of efficacy and time to onset of effect • Topline data expected in 2027 • Evaluate patient-friendly dosing intervals 18
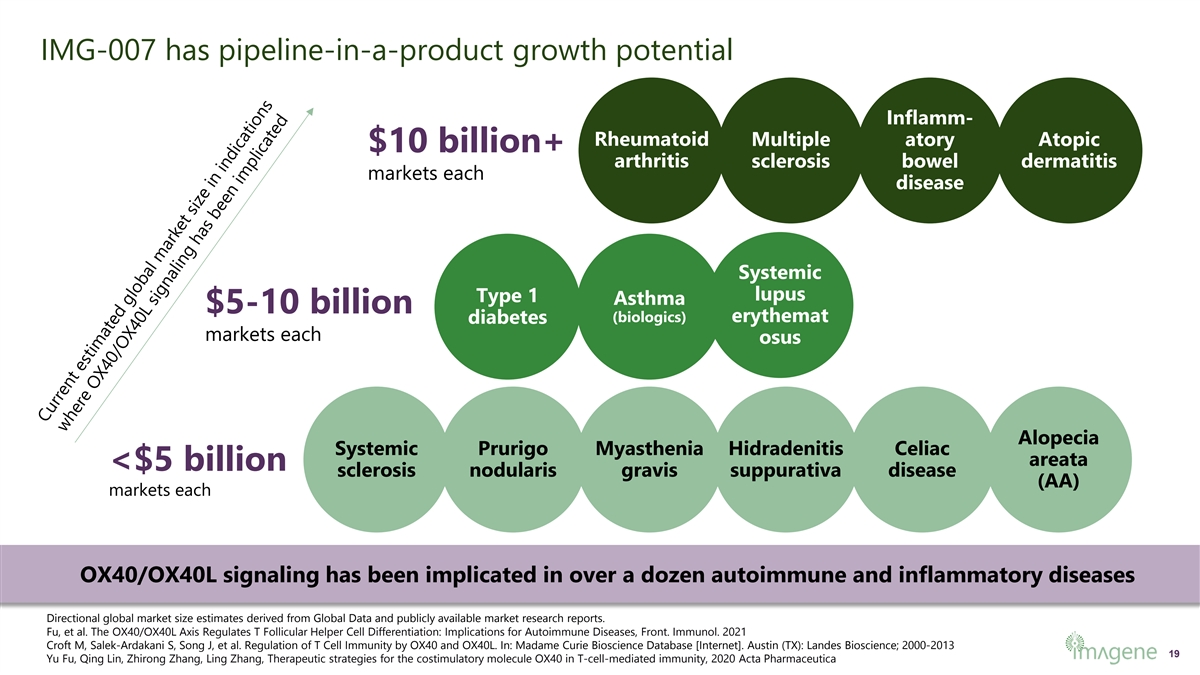
IMG-007 has pipeline-in-a-product growth potential Inflamm- Rheumatoid Multiple atory Atopic $10 billion+ arthritis sclerosis bowel dermatitis markets each disease Systemic lupus Type 1 Asthma $5-10 billion (biologics) erythemat diabetes markets each osus Alopecia Systemic Prurigo Myasthenia Hidradenitis Celiac areata <$5 billion sclerosis nodularis gravis suppurativa disease (AA) markets each OX40/OX40L signaling has been implicated in over a dozen autoimmune and inflammatory diseases Directional global market size estimates derived from Global Data and publicly available market research reports. Fu, et al. The OX40/OX40L Axis Regulates T Follicular Helper Cell Differentiation: Implications for Autoimmune Diseases, Front. Immunol. 2021 Croft M, Salek-Ardakani S, Song J, et al. Regulation of T Cell Immunity by OX40 and OX40L. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013 19 Yu Fu, Qing Lin, Zhirong Zhang, Ling Zhang, Therapeutic strategies for the costimulatory molecule OX40 in T-cell-mediated immunity, 2020 Acta Pharmaceutica
IMG-007 has shown proof-of-concept in a second indication: alopecia areata • Alopecia areata (AA): chronic autoimmune Alopecia areata is a difficult, disease characterized by hair loss chronic autoimmune disease with • OX40 association: Upregulation of OX40 / high unmet need; People OX40L in the scalp and blood of AA patients • Treatment options: JAK inhibitors are the only worldwide have a approved targeted treatment; only for severe disease, carry a boxed warning, require 2x/day dosing lifetime risk of ~2% • Unmet need: desperate need of new treatments of developing AA, which can affect that are safe, offer durable efficacy, and can all ages, ethnicities and races address the full spectrum of disease severity JAKi: JAK inhibitor (Janus Kinase inhibitor) Sample article on Immunology background in AA: Oratt, et Al. Alopecia areata, Nat Rev Dis Primers, 2017 Ding, H., Yu, Z., Yao, H. et al. Global burden of alopecia areata from 1990 to 2019 and emerging treatment trends analyzed through GBD 2019 and bibliometric data. Sci Rep 15, 25869 (2025) 20
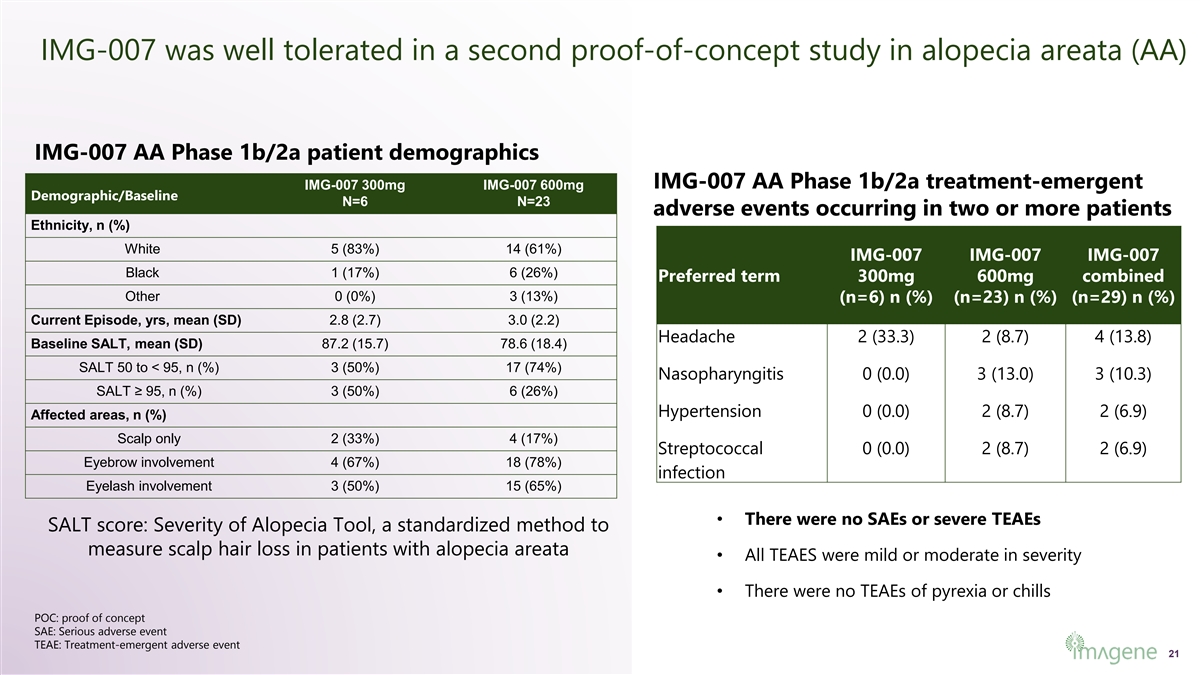
IMG-007 was well tolerated in a second proof-of-concept study in alopecia areata (AA) IMG-007 AA Phase 1b/2a patient demographics IMG-007 AA Phase 1b/2a treatment-emergent IMG-007 300mg IMG-007 600mg Demographic/Baseline N=6 N=23 adverse events occurring in two or more patients Ethnicity, n (%) White 5 (83%) 14 (61%) IMG-007 IMG-007 IMG-007 Black 1 (17%) 6 (26%) Preferred term 300mg 600mg combined Other 0 (0%) 3 (13%) (n=6) n (%) (n=23) n (%) (n=29) n (%) Current Episode, yrs, mean (SD) 2.8 (2.7) 3.0 (2.2) Headache 2 (33.3) 2 (8.7) 4 (13.8) Baseline SALT, mean (SD) 87.2 (15.7) 78.6 (18.4) SALT 50 to < 95, n (%) 3 (50%) 17 (74%) Nasopharyngitis 0 (0.0) 3 (13.0) 3 (10.3) SALT ≥ 95, n (%) 3 (50%) 6 (26%) Hypertension 0 (0.0) 2 (8.7) 2 (6.9) Affected areas, n (%) Scalp only 2 (33%) 4 (17%) Streptococcal 0 (0.0) 2 (8.7) 2 (6.9) Eyebrow involvement 4 (67%) 18 (78%) infection Eyelash involvement 3 (50%) 15 (65%) • There were no SAEs or severe TEAEs SALT score: Severity of Alopecia Tool, a standardized method to measure scalp hair loss in patients with alopecia areata • All TEAES were mild or moderate in severity • There were no TEAEs of pyrexia or chills POC: proof of concept SAE: Serious adverse event TEAE: Treatment-emergent adverse event 21

Four-week IMG-007 treatment resulted in SALT score improvement, with marked improvement in AA patients with baseline SALT score 50 to <95 Mean % change from baseline in SALT score Mean % change from baseline in SALT score by dose by baseline disease severity (600mg) IMG-007 300 mg (N=6) IMG-007 600 mg (N=23) -1% -7% -7% -20% -25% Baseline SALT >= 95 in 600 mg (n=6) -30% Baseline SALT 50 to < 95 in 600 mg (n=17) 3 doses @ week 0,2,4 Deepening SALT reduction without plateauing by Four-week (600mg) treatment led to deeper improvement in week 36 -- ~8 months after the last dose at week 4 patients with baseline SALT score 50 to <95 than in patients with baseline SALT 95 - 100 AA: Alopecia areata SALT score: Severity of Alopecia Tool, a standardized method to measure scalp hair loss in patients with alopecia areata All assessments after the start date of prohibited medication were set to missing. All the collected data available after treatment discontinuation were included in the analysis. 22

Photographs of select AA patients after treatment with three IMG-007 doses showed hair regrowth Baseline Week 24 Week 16 Week 24 Week 36 Baseline Week 24 Baseline All three patients treated with 600mg doses at weeks 0, 2, and 4 23 Patient 1 Patient 2 Patient 3

IMG-007 value creation trajectory; well resourced to execute AD Phase 2b study IMG-007 designed Build value: with a trifecta Focus on execution, raise company profile, drive IMG-007 of differentiating POC data in AD shows Phase 2b forward features promising EASI score changes and favorable Build value: 2020- tolerability 2026 Evaluate expansion strategy in autoimmune and inflammation 2024 2025 2027 Phase 2b initiated in AD Established as a public company Data in a second POC study (AA) Topline data expected from through reverse merger and highlights IMG-007’s potential as a IMG-007 AD Phase 2b concurrent financing ‘pipeline in a product’ across autoimmune and inflammatory diseases $142.6 million in cash and securities at close of Q3 2025; Runway estimated through 2027 Company executing across all verticals to deliver high quality clinical program and corporate strategy POC: proof of concept AD: Atopic dermatitis AA: Alopecia areata EASI: Eczema Area and Severity Index; EASI is a composite scoring system used in clinical trials to measure the extent (area) and severity of atopic eczema (dermatitis) 24
