| ☒ | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Delaware |
||
|
(State or other jurisdiction of
incorporation or organization)
|
(IRS Employer
Identification No.)
|
Title of each class |
Trading
Symbol(s)
|
Name of each exchange
on which registered
|
||
Common Stock, par value $0.0001 |
BNTC |
The Nasdaq Stock Market LLC |
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
|
Non-accelerated filer |
☒ | Smaller reporting company | ☒ | |||
| Emerging growth company | ☐ | |||||
BENITEC BIOPHARMA INC.
INDEX TO FORM 10-Q
| SPECIAL NOTE REGARDING FORWARD LOOKING STATEMENTS | 3 | |||||
| PART I-FINANCIAL INFORMATION | 5 | |||||
| ITEM 1. | 5 | |||||
| Consolidated Balance Sheets as of September 30, 2025 (Unaudited) and June 30, 2025 |
5 | |||||
| 6 | ||||||
| 7 | ||||||
| 9 | ||||||
| 10 | ||||||
| ITEM 2. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
22 | ||||
| ITEM 3. | 39 | |||||
| ITEM 4. | 40 | |||||
| PART II-OTHER INFORMATION | ||||||
| ITEM 1. | 41 | |||||
| ITEM 1A. | 41 | |||||
| ITEM 2. | 41 | |||||
| ITEM 3. | 41 | |||||
| ITEM 4. | 41 | |||||
| ITEM 5. | 41 | |||||
| ITEM 6. | 42 | |||||
| SIGNATURES | 43 | |||||
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Report contains forward-looking statements that are subject to a number of risks and uncertainties, many of which are beyond our control. Our forward-looking statements relate to future events or our future performance and include, but are not limited to, statements concerning our business strategy, future commercial revenues, market growth, capital requirements, new product introductions, expansion plans and the adequacy of our funding. All statements, other than statements of historical fact included in this Report, are forward-looking statements. When used in this Report, the words “could,” “believe,” “anticipate,” “intend,” “estimate,” “expect,” “may,” “continue,” “predict,” “potential,” “project,” or the negative of these terms, and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain such identifying words. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements.
Some of the risks and uncertainties that may cause our actual results, performance or achievements to differ materially from those expressed or implied by forward-looking statements include the following:
| • | the success of our plans to develop and potentially commercialize our product candidates; |
| • | the timing of the completion of preclinical studies and clinical trials; |
| • | the timing and sufficiency of patient enrollment and dosing in any future clinical trials; |
| • | the timing of the availability of data from our clinical trials; |
| • | the timing and outcome of regulatory filings and approvals; |
| • | the development of novel AAV vectors; |
| • | our potential future out-licenses and collaborations; |
| • | the plans of licensees of our technology; |
| • | the clinical utility and potential attributes and benefits of ddRNAi and our product candidates, including the potential duration of treatment effects and the potential for a “one shot” cure; |
| • | our intellectual property position and the duration of our patent portfolio; |
| • | expenses, ongoing losses, future revenue, capital needs and needs for additional financing, and our ability to access additional financing given market conditions and other factors; |
| • | the length of time over which we expect our cash and cash equivalents to be sufficient to execute on our business plan; |
| • | unanticipated delays; |
| • | further research and development and the results of clinical trials possibly being unsuccessful or insufficient to meet applicable regulatory standards or warrant continued development; |
| • | the ability to enroll sufficient numbers of subjects in clinical trials; |
| • | determinations made by the U.S. Food and Drug Administration and other governmental authorities; |
| • | regulatory developments in the United States of America; |
| • | our ability to protect and enforce our patents and other intellectual property rights; |
| • | our dependence on our relationships with our collaboration partners and other third parties; |
| • | the efficacy or safety of our products and the products of our collaboration partners; |
| • | the acceptance of our products and the products of our collaboration partners in the marketplace and market competition; |
| • | sales, marketing, manufacturing and distribution requirements; |
| • | greater than expected expenses, expenses relating to litigation or strategic activities; |
| • | the impact of, and our ability to remediate, the identified material weakness in our internal controls over financial reporting; |
3
| • | our ability to satisfy our capital needs through increasing revenue and obtaining additional financing; and |
| • | the impact of local, regional and national and international economic conditions and events; |
as well as other risks detailed under the caption “Risk Factors” in this Report and in other reports filed with the SEC. Although we believe that we have a reasonable basis for each forward-looking statement contained in this Report, we caution you that these statements are based on a combination of facts and important factors currently known by us and our expectations of the future, about which we cannot be certain. Such statements are based on assumptions and the actual outcome will be affected by known and unknown risks, trends, uncertainties and factors that are beyond our control or ability to predict. We have based the forward-looking statements included in this Report on information available to us on the date of this Report or on the date thereof. Except as required by law we undertake no obligation to revise or update any forward-looking statements, whether as a result of new information, future events or otherwise. You are advised to consult any additional disclosures that we may make directly to you or through reports that we, in the future, may file with the SEC, including annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K.
All forward-looking statements included herein or in documents incorporated herein by reference are expressly qualified in their entirety by the cautionary statements contained or referred to elsewhere in this Report.
4
| September 30, 2025 |
June 30, 2025 |
|||||||
| (Unaudited) | ||||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 94,479 | $ | 97,744 | ||||
| Restricted cash |
113 | 113 | ||||||
| Trade and other receivables |
7 | 33 | ||||||
| Prepaid and other assets |
480 | 628 | ||||||
| |
|
|
|
|||||
| Total current assets |
95,079 | 98,518 | ||||||
| Property and equipment, net |
118 | 131 | ||||||
| Deposits |
55 | 55 | ||||||
| Prepaid and other assets |
20 | 28 | ||||||
| Right-of-use |
755 | 860 | ||||||
| |
|
|
|
|||||
| Total assets |
$ | 96,027 | $ | 99,592 | ||||
| |
|
|
|
|||||
| Liabilities and stockholders’ equity |
||||||||
| Current liabilities: |
||||||||
| Trade and other payables |
$ | 1,148 | $ | 1,022 | ||||
| Accrued employee benefits |
463 | 426 | ||||||
| Lease liabilities, current portion |
414 | 354 | ||||||
| |
|
|
|
|||||
| Total current liabilities |
2,025 | 1,802 | ||||||
| Lease liabilities, less current portion |
403 | 495 | ||||||
| |
|
|
|
|||||
| Total liabilities |
2,428 | 2,297 | ||||||
| |
|
|
|
|||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $0.0001 par value—5,000,000 shares authorized; no shares issued and outstanding at September 30, 2025 and June 30, 2025, respectively |
— | — | ||||||
| Common stock, $0.0001 par value—160,000,000 shares authorized; 26,250,469 shares issued and outstanding at September 30, 2025 and June 30, 2025, respectively |
2 | 2 | ||||||
| Additional paid-in capital |
331,488 | 326,308 | ||||||
| Accumulated deficit |
(237,141 | ) | (228,176 | ) | ||||
| Accumulated other comprehensive loss |
(750 | ) | (839 | ) | ||||
| |
|
|
|
|||||
| Total stockholders’ equity |
93,599 | 97,295 | ||||||
| |
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 96,027 | $ | 99,592 | ||||
| |
|
|
|
|||||
| Three Months Ended | ||||||||
| September 30, | ||||||||
| 2025 | 2024 | |||||||
| Revenue: |
||||||||
| |
$ | — | $ | — | ||||
| |
|
|
|
|||||
| Total revenues |
— | — | ||||||
| Operating expenses |
||||||||
| Research and development |
3,370 | 3,585 | ||||||
| General and administrative |
6,433 | 2,206 | ||||||
| |
|
|
|
|||||
| Total operating expenses |
9,803 | 5,791 | ||||||
| |
|
|
|
|||||
| Loss from operations |
(9,803 | ) | (5,791 | ) | ||||
| Other income (loss): |
||||||||
| Foreign currency transaction gain (loss) |
(89 | ) | 93 | |||||
| Interest income, net |
1,011 | 604 | ||||||
| Other income (expense), net |
(84 | ) | 35 | |||||
| |
|
|
|
|||||
| Total other income, net |
838 | 732 | ||||||
| |
|
|
|
|||||
| Net loss |
$ | (8,965 | ) | $ | (5,059 | ) | ||
| |
|
|
|
|||||
| Other comprehensive income: |
||||||||
| Unrealized foreign currency translation gain (loss) |
89 | (101 | ) | |||||
| |
|
|
|
|||||
| Total other comprehensive income (loss) |
89 | (101 | ) | |||||
| |
|
|
|
|||||
| Total comprehensive loss |
$ | (8,876 | ) | $ | (5,160 | ) | ||
| |
|
|
|
|||||
| Net loss |
$ | (8,965 | ) | $ | (5,059 | ) | ||
| |
|
|
|
|||||
| Net loss attributable to common shareholders |
$ | (8,965 | ) | $ | (5,059 | ) | ||
| |
|
|
|
|||||
| Net loss per share: |
||||||||
| Basic and diluted |
$ | (0.22 | ) | $ | (0.18 | ) | ||
| |
|
|
|
|||||
| Weighted average number of shares outstanding: basic and diluted |
41,521,280 | 27,883,624 | ||||||
| |
|
|
|
|||||
| Common Stock | Additional Paid-in
|
Accumulated | Accumulated Other Comprehensive |
Total Stockholders’ |
||||||||||||||||||||
| Shares | Amount | Capital | Deficit | Loss | Equity | |||||||||||||||||||
| Balance at June 30, 2024 |
10,086,119 | $ | 1 | $ | 238,398 | $ | (190,259 | ) | $ | (892 | ) | $ | 47,248 | |||||||||||
| Exercise of pre-funded warrants |
1,768,454 | — | — | — | — | — | ||||||||||||||||||
| Exercise of Series 2 warrants |
857,845 | — | 1,655 | — | — | 1,655 | ||||||||||||||||||
| Exercise of common warrants |
5,181,347 | — | 20,002 | — | — | 20,002 | ||||||||||||||||||
| Share-based compensation |
— | — | 435 | — | — | 435 | ||||||||||||||||||
| Foreign currency translation loss |
— | — | — | — | (101 | ) | (101 | ) | ||||||||||||||||
| Net loss |
— | — | — | (5,059 | ) | — | (5,059 | ) | ||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at September 30, 2024 |
17,893,765 | $ | 1 | $ | 260,490 | $ | (195,318 | ) | $ | (993 | ) | $ | 64,180 | |||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Common Stock | Additional Paid-in
|
Accumulated | Accumulated Other Comprehensive |
Total Stockholders’ |
||||||||||||||||||||
| Shares | Amount | Capital | Deficit | Loss | Equity | |||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at June 30, 2025 |
26,250,469 | $ | 2 | $ | 326,308 | $ | (228,176 | ) | $ | (839 | ) | $ | 97,295 | |||||||||||
| Share-based compensation |
— | — | 5,180 | — | — | 5,180 | ||||||||||||||||||
| Foreign currency translation gain |
— | — | — | — | 89 | 89 | ||||||||||||||||||
| Net loss |
— | — | — | (8,965 | ) | — | (8,965 | ) | ||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at September 30, 2025 |
26,250,469 | $ | 2 | $ | 331,488 | $ | (237,141 | ) | $ | (750 | ) | $ | 93,599 | |||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Three Months Ended September 30, |
||||||||
| 2025 | 2024 | |||||||
| Cash flows from operating activities: |
||||||||
| Net loss |
$ | (8,965 | ) | $ | (5,059 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Depreciation and amortization |
13 | 25 | ||||||
| Amortization of right-of-use |
105 | 66 | ||||||
| Share-based compensation expense |
5,180 | 435 | ||||||
| Changes in operating assets and liabilities: |
||||||||
| Trade and other receivables |
30 | 226 | ||||||
| Prepaid and other assets |
156 | 100 | ||||||
| Trade and other payables |
125 | (322 | ) | |||||
| Accrued employee benefits |
37 | 16 | ||||||
| Lease liabilities |
(32 | ) | (73 | ) | ||||
| |
|
|
|
|||||
| Net cash used in operating activities |
(3,351 | ) | (4,586 | ) | ||||
| |
|
|
|
|||||
| Cash flows from investing activities: |
||||||||
| |
|
|
|
|||||
| Net cash used in investing activities |
— | — | ||||||
| Cash flows from financing activities: |
||||||||
| Proceeds from exercise of pre-funded warrants, Series 2 warrants and common warrants |
— | 21,657 | ||||||
| |
|
|
|
|||||
| Net cash provided by financing activities |
— | 21,657 | ||||||
| |
|
|
|
|||||
| Effects of exchange rate changes on cash, cash equivalents, and restricted cash |
86 | (95 | ) | |||||
| |
|
|
|
|||||
| Net increase (decrease) in cash, cash equivalents, and restricted cash |
(3,265 | ) | 16,976 | |||||
| Cash, cash equivalents, and restricted cash, beginning of period |
97,857 | 50,929 | ||||||
| |
|
|
|
|||||
| Cash, cash equivalents, and restricted cash, end of period |
$ | 94,592 | $ | 67,905 | ||||
| |
|
|
|
|||||
| Principal place of business/country of incorporation | ||
| Benitec Biopharma Proprietary Limited (“BBL”) | Australia | |
| Benitec Australia Proprietary Limited | Australia | |
| Benitec Limited | United Kingdom | |
| Benitec, Inc. | USA | |
| Benitec LLC | USA | |
| RNAi Therapeutics, Inc. | USA | |
| Tacere Therapeutics, Inc. | USA | |
| Benitec IP Holdings, Inc. | USA |
| Level 1: | Observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities. | |
| Level 2: | Inputs, other than quoted prices that are observable, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active. | |
| Level 3: | Unobservable inputs in which little or no market data exists, therefore developed using estimates and assumptions developed by us, which reflect those that a market participant would use. | |
| Software |
3 -4 |
|
| Lab equipment |
3-7 years |
|
| Computer hardware |
3-5 years |
|
| Leasehold improvements | shorter of the lease term or estimated useful lives |
| (US$’000) | September 30, 2025 |
June 30, 2025 |
||||||
Cash at bank |
$ | 94,479 | $ | 97,744 | ||||
Restricted cash |
113 | 113 | ||||||
Total |
$ | 94,592 | $ | 97,857 | ||||
| (US$’000) | September 30, 2025 |
June 30, 2025 |
||||||
Prepaid expenses |
$ | 499 | $ | 655 | ||||
Market value of listed shares |
1 | 1 | ||||||
Total other assets |
500 | 656 | ||||||
Less: non-current portion |
(20 | ) | (28 | ) | ||||
Current portion |
$ | 480 | $ | 628 | ||||
| (US$’000) | September 30, 2025 |
June 30, 2025 |
||||||
Software |
$ | 6 | $ | 6 | ||||
Lab equipment |
1,533 | 1,533 | ||||||
Computer hardware |
32 | 32 | ||||||
Furniture and fixtures |
6 | 6 | ||||||
Leasehold improvements |
24 | 24 | ||||||
Total property and equipment, gross |
1,601 | 1,601 | ||||||
Accumulated depreciation and amortization |
(1,483 | ) | (1,470 | ) | ||||
Total property and equipment, net |
$ | 118 | $ | 131 | ||||
| (US$’000) | September 30, 2025 |
June 30, 202 5
|
||||||
Trade payable |
$ | 271 | $ | 201 | ||||
Accrued consultant fees |
20 | 36 | ||||||
Accrued professional fees |
125 | 62 | ||||||
Filing fees |
76 | — | ||||||
Accrued clinical development project costs |
473 | 656 | ||||||
Accrued patent fees |
36 | — | ||||||
Other payables |
147 | 67 | ||||||
Total |
$ | 1,148 | $ | 1,022 | ||||
| (US$’000) | Operating lease right- of- use assets |
|||
Balance at July 1, 2025 |
$ | 860 | ||
Amortization of right of use asset |
(105 | ) | ||
Operating lease right-of-use |
$ | 755 | ||
| (US$’000) | Operating lease liabilities |
|||
Balance at July 1, 2025 |
$ | 849 | ||
Principal payments on operating lease liabilities |
(32 | ) | ||
Operating lease liabilities at September 30, 2025 |
817 | |||
Less: non-current portion |
(403 | ) | ||
Current portion at September 30, 2025 |
$ | 414 | ||
| (US$’000) | September 30, 2025 |
|||
2025 |
$ | 100 | ||
2026 |
435 | |||
2027 |
331 | |||
Total operating lease payments |
866 | |||
Less imputed interest |
(49 | ) | ||
Present value of operating lease liabilities |
$ | 817 | ||
| Common Stock from Warrants |
Weighted- average Exercise Price (per share) |
|||||||
Outstanding at July 1, 2024 |
34,271,146 | $ | 1.8453 | |||||
Pre-funded warrants issued March 25, 2025 |
300,000 | $ | 0.0001 | |||||
Pre-funded warrants exercised |
(2,374,583 | ) | $ | 0.0001 | ||||
Series 2 warrants exercised |
(1,553,927 | ) | $ | 1.9299 | ||||
Common warrants exercised |
(10,192,840 | ) | $ | 3.86 | ||||
Purchase warrants expired |
(6,300 | ) | $ | 178.50 | ||||
Outstanding and exercisable at June 30, 2025 |
20,443,496 | $ | 0.9672 | |||||
Outstanding and exercisable at September 30, 2025 |
20,443,496 | $ | 0.9672 | |||||
| Stock Options |
Weighted- average Exercise Price |
Weighted- average Remaining Contractual Term |
Aggregate Intrinsic Value |
|||||||||||||
Outstanding at June 30, 2025 |
4,902,140 | $ | 10.81 | 9.24 years | $ | 7,728,384 | ||||||||||
Granted |
— | — | — | |||||||||||||
Expired |
— | — | — | |||||||||||||
Forfeited |
— | — | — | |||||||||||||
Outstanding at September 30, 2025 |
4,902,140 | $ | 10.81 | 8.99 years | $ | 17,368,566 | ||||||||||
Exercisable at September 30, 2025 |
1,066,502 | $ | 10.97 | 8.73 years | $ | 4,855,540 | ||||||||||
Three Months Ended |
||||||||
September 30, |
||||||||
2025 |
2024 |
|||||||
Expected volatility |
120.9 | % | 120.3 | % | ||||
Expected term |
6 years | 6 years | ||||||
Risk-free interest rate |
4.09 | % | 3.99 | % | ||||
Expected dividend yield |
— | % | — | % | ||||
| Three Months Ended | ||||||||
| September 30, | ||||||||
| (US$’000) | 2025 | 2024 | ||||||
Research and development |
$ | 868 | $ | 113 | ||||
General and administrative |
4,312 | 322 | ||||||
Total share-based compensation expense |
$ | 5,180 | $ | 435 | ||||
Three Months Ended |
||||||||
(US$’000) |
September 30, |
|||||||
Operating Expenses |
2025 |
2024 |
||||||
Research and development |
$ | 3,370 | $ | 3,585 | ||||
General and administrative |
6,433 | 2,206 | ||||||
Other segment items |
(838 | ) | (732 | ) | ||||
Net loss |
$ | (8,965 | ) | $ | (5,059 | ) | ||
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of financial condition and operating results together with our consolidated financial statements and the related notes and other financial information included elsewhere in this document.
Company Overview
We endeavor to become the leader in discovery, development, and commercialization of therapeutic agents capable of addressing significant unmet medical need via the application of the silence and replace approach to the treatment of genetic disorders.
Benitec Biopharma Inc. (“Benitec” or the “Company” or in the third person, “we” or “our”) is a clinical-stage biotechnology company focused on the advancement of novel genetic medicines with headquarters in Hayward, California. The proprietary platform, called DNA-directed RNA interference, or ddRNAi, combines RNA interference, or RNAi, with gene therapy to create medicines that facilitate sustained silencing of disease-causing genes following a single administration. The unique therapeutic constructs also enable the simultaneous delivery of wildtype replacement genes, facilitating the proprietary “silence and replace” approach to the treatment of genetically defined diseases. The Company is developing a silence and replace-based therapeutic (BB-301) for the treatment of Oculopharyngeal Muscular Dystrophy (OPMD), a chronic, life-threatening genetic disorder.
BB-301 is a silence and replace-based genetic medicine currently under development by Benitec. BB-301 is an AAV-based gene therapy designed to permanently silence the expression of the disease-causing gene (to slow, or halt, the biological mechanisms underlying disease progression in OPMD) and to simultaneously replace the mutant gene with a wildtype gene (to drive restoration of function in diseased cells). This fundamental therapeutic approach to disease management is called “silence and replace.” The silence and replace mechanism offers the potential to restore the normative physiology of diseased cells and tissues and to improve treatment outcomes for patients suffering from the chronic, and potentially fatal, effects of OPMD. BB-301 has been granted Orphan Drug Designation in the United States and the European Union.
The targeted gene silencing effects of RNAi, in conjunction with the durable transgene expression achievable via the use of modified viral vectors, imbues the silence and replace approach with the potential to produce permanent silencing of disease-causing genes along with simultaneous replacement of the wild type gene function following a single administration of the proprietary genetic medicine. We believe that this novel mechanistic profile of the current and future investigational agents developed by Benitec could facilitate the achievement of robust and durable clinical activity while greatly reducing the frequency of drug administration traditionally expected for medicines employed for the management of chronic diseases. Additionally, the achievement of permanent gene silencing and gene replacement may significantly reduce the risk of patient non-compliance during the course of medical management of potentially fatal clinical disorders.
We will require additional financing to progress our product candidates through to key inflection points.
Our proprietary technology platforms are designated as DNA-directed RNA interference, or “ddRNAi”, and “silence and replace.” ddRNAi is designed to produce permanent silencing of disease-causing genes, by combining RNA interference, or RNAi, with viral delivery agents typically associated with the field of gene therapy (i.e., viral vectors). Modified AAV vectors are employed to deliver genetic constructs which encode short hairpin RNAs that are, then, serially expressed and processed to produce siRNA molecules within the transduced cell for the duration of the life of the target cell. These newly introduced siRNA molecules drive permanent silencing of the expression of the disease-causing gene. The silence and replace approach further bolsters the biological benefits of permanent silencing of disease-causing genes by incorporating multifunctional genetic constructs within the modified AAV vectors to create an AAV-based gene therapy agent that is designed to silence the expression of disease-causing genes (to slow, or halt, the underlying mechanism of disease progression) and to simultaneously replace the mutant genes with normal, “wildtype” genes (to drive restoration of function in diseased cells). This fundamentally distinct therapeutic approach to disease management offers the potential to restore the underlying physiology of the treated tissues and, in the process, improve treatment outcomes for patients suffering from the chronic and, potentially, fatal effects of diseases like Oculopharyngeal Muscular Dystrophy (OPMD).
Traditional gene therapy is defined by the introduction of an engineered transgene to correct the pathophysiological derangements derived from mutated or malfunctioning genes. Mutated genes can facilitate the intracellular production of disease-causing proteins or hamper the production of critical, life-sustaining, proteins. The introduction of a new transgene can facilitate the restoration of production of normal proteins within the diseased cell, thus restoring natural biological function. Critically, the implementation of this traditional method of gene therapy cannot eliminate the expression, or the potential deleterious effects of, the underlying mutant gene (as mutant proteins may be continually expressed and aggregate or drive the aggregation of other native proteins within the diseased cell). In this regard, the dual capabilities of the proprietary silence and replace approach to silence a disease-causing gene via ddRNAi and simultaneously replace the wild type activity of a mutant gene via the delivery of an engineered transgene could facilitate the development of differentially efficacious treatments for a range of genetic disorders.
22
Overview of RNAi and the siRNA Approach
The mutation of a single gene can cause a chronic disease via the resulting intracellular production of a disease-causing protein (i.e., an abnormal form of the protein of interest), and many chronic and/or fatal disorders are known to result from the inappropriate expression of a single gene or multiple genes. In some cases, genetic disorders of this type can be treated exclusively by “silencing” the intracellular production of the disease-causing protein through well-validated biological approaches like RNA interference (“RNAi”). RNAi employs small nucleic acid molecules to activate an intracellular enzyme complex, and this biological pathway temporarily reduces the production of the disease-causing protein. In the absence of the disease-causing protein, normal cellular function is restored and the chronic disease that initially resulted from the presence of the mutant protein is partially or completely resolved. RNAi is potentially applicable to over 20,000 human genes and a large number of disease-causing microorganism-specific genes.
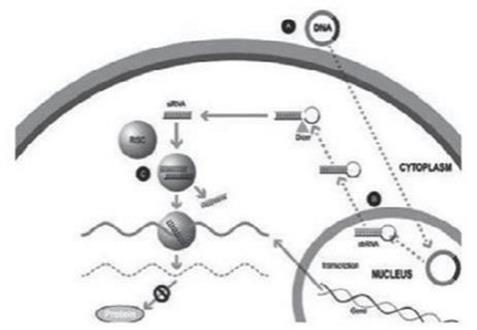
Figure 1

A small double stranded RNA, or dsRNA, molecule (A, Figure 1), comprising one strand known as the sense strand and another strand known as the antisense strand, which are complementary to each other, is synthesized in the laboratory. These small dsRNAs are called small interfering RNAs, or siRNAs. The sequence of the sense strand corresponds to a short region of the target gene mRNA. The siRNA is delivered to the target cell (B, Figure 1), where a group of enzymes, referred to as the RNA-Induced Silencing Complex, or RISC, process the siRNA (C, Figure 1), where one of the strands (usually the sense strand) is released (D, Figure 1). RISC uses the antisense strand to find the mRNA that has a complementary sequence (E, Figure 1) leading to the cleavage of the target mRNA (F, Figure 1). As a consequence, the output of the mRNA (protein production) does not occur (G, Figure 1). Several companies, including Alnylam Pharmaceuticals Inc. (“Alnylam”), utilize this approach in their RNAi product candidates.
Importantly, many genetic disorders are not amenable to the traditional gene silencing approach outlined in Figure 1, as the diseased cells may produce a mixture of the wild type protein of interest and the disease-causing mutant variant of the protein, and the underlying genetic mutation may be too small to allow for selective targeting of the disease-causing variant of the protein through the use of siRNA-based approaches exclusively. In these cases, it is extraordinarily difficult to selectively silence the disease-causing protein without simultaneously silencing the wild type intracellular protein of interest whose presence is vital to the conduct of normal cellular functions.
Our proprietary silence and replace technology utilizes the unique specificity and robust gene silencing capabilities of RNAi while overcoming many of the key limitations of siRNA-based approaches to disease management.
23
In the standard RNAi approach, double-stranded siRNA is produced synthetically and, subsequently, introduced into the target cell via chemical modification of the RNA or alternative methods of delivery. While efficacy has been demonstrated in several clinical indications through the use of this approach, siRNA-based approaches maintain a number of limitations, including:
| • | Clinical management requires repeat administration of the siRNA-based therapeutic agent for multiple cycles to maintain efficacy; |
| • | Long-term patient compliance challenges due to dosing frequencies and treatment durations; |
| • | Therapeutic concentrations of siRNA are not stably maintained because the levels of synthetic siRNA in the target cells decrease over time; |
| • | Novel chemical modifications or novel delivery materials are typically required to introduce the siRNA into the target cells, making it complicated to develop a broad range of therapeutics agents; |
| • | Potential adverse immune responses, resulting in serious adverse effects; |
| • | Requirement for specialized delivery formulations for genetic disorders caused by mutations of multiple genes; and |
| • | siRNA acts only to silence genes and cannot be used to replace defective genes with normally functioning genes. |
Our Approach to the Treatment of Genetic Diseases—ddRNAi and Silence and Replace
Our proprietary silence and replace approach to the treatment of genetic diseases combines RNAi with wild type gene replacement to drive permanent silencing of disease-causing genes and concomitant restoration of functional wild type genes following a single administration of the therapeutic agent. Benitec employs ddRNAi in combination with classical gene therapy (i.e., transgene delivery via viral vectors) to overcome several of the fundamental limitations of RNAi.
The silence and replace approach to the treatment of genetic disorders employs adeno-associated viral vectors (“AAVs”) to deliver genetic constructs which may, after a single administration to the target tissues:
| • | Chronically express RNAi molecules inside of the target, diseased, cells (to serially silence the intracellular production of mutant, disease-causing, protein and the wild type protein of interest); |
| • | Simultaneously drive the expression of a wild type variant of the protein of interest (to restore native intracellular biological processes); and |
| • | AAV vectors can accommodate the multi-functional DNA expression cassettes containing the engineered wild type transgenes and the novel genes encoding short hairpinRNA/microRNA molecules (shRNA/miRNA) that are required to support the development of therapeutic agents capable of the achievement of the goals of the silence and replace approach to therapy. |
Our silence and replace technology utilizes proprietary DNA expression cassettes to foster continuous production of gene silencing shRNAs and wild type proteins (via expression of the wild type transgene). A range of viral gene therapy vectors can be used to deliver the DNA construct into the nucleus of the target cell and, upon delivery, shRNA molecules are expressed and subsequently processed by intracellular enzymes into siRNA molecules that silence the expression of the mutant, disease-causing protein (Figure 2).
In the silence and replace approach (Figure 2):
| • | A DNA construct is delivered to the nucleus of the target cell by a gene therapy vector (A) such as an AAV vector; |
| • | Once inside of the nucleus, the DNA construct drives the continuous production of shRNA molecules (B) which are processed by an enzyme called Dicer into siRNAs (C); |
| • | The processed siRNA is incorporated into RISC and silences the target gene using the same mechanism shown in Figure 1; and |
| • | When the DNA expression cassette is additionally comprised of a wild type transgene, upon entry of the DNA construct into the nucleus of the target cell via the use of the AAV vector, the DNA construct also drives the continuous production of wild type protein (to restore native intracellular biological processes). |
24
Figure 2
Our strategy is to discover, develop and commercialize treatments that leverage the capabilities of ddRNAi and the silence and replace approach to disease management.
For selected product candidates, at the appropriate stage, we may collaborate with large biopharmaceutical companies to further co-develop and, if approved, commercialize our ddRNAi-based and silence and replace-based products to achieve broad clinical and commercial distribution. For specific clinical indications that we deem to be outside of our immediate areas of focus, we will continue to out-license, where appropriate, applications of our ddRNAi and silence and replace technology to facilitate the development of differentiated therapeutics, which could provide further validation of our proprietary technology and approach to disease management.
Our cash and cash equivalents will be deployed for the advancement of our product candidate BB-301 for the treatment of OPMD-derived dysphagia, including the natural history lead-in study and the Phase 1b/2a BB-301 treatment study, for the continued advancement of development activities for other existing and new product candidates, for general corporate purposes and for strategic growth opportunities.
Oculopharyngeal Muscular Dystrophy—OPMD
OPMD is an insidious, autosomal-dominant, late-onset degenerative muscle disorder that typically presents in patients at 40-to-50 years of age. The disease is characterized by progressive swallowing difficulties (dysphagia) and eyelid drooping (ptosis). OPMD is caused by a specific mutation in the poly(A)-binding protein nuclear 1, or PABPN1, gene. OPMD is a rare disease; however, patients have been diagnosed with OPMD in at least 33 countries. Patient populations suffering from OPMD are well-identified, and significant geographical clustering has been noted for patients with this disorder, which could simplify clinical development and global commercialization efforts.
BB-301 is an AAV-based gene therapy designed to silence the expression of disease-causing genes (to slow, or halt, the underlying mechanism of disease progression) and to simultaneously replace the mutant genes with normal, “wildtype” genes (to drive restoration of function in diseased cells). This fundamental therapeutic approach to disease management is called “silence and replace” and this biological mechanism offers the potential to restore the underlying physiology of the treated tissues and, in the process, improve treatment outcomes for patients suffering from the chronic and, potentially, fatal effects of Oculopharyngeal Muscular Dystrophy (OPMD). BB-301 has been granted Orphan Drug Designation in the United States and the European Union.
25
Our Pipeline
The following table sets forth our current product candidate and the development status:
Table 1. Pipeline: Oculopharyngeal Muscular Dystrophy

We are developing BB-301 for the treatment of Oculopharyngeal Muscular Dystrophy (OPMD)-related dysphagia. The Investigational New Drug (IND) application for BB-301 was approved to proceed by the U.S. Food and Drug Administration in June 2023. The first study subject was safely treated in the BB-301 Phase 1b/2a clinical trial (NCT06185673) in November 2023. The second study subject was safely treated in February 2024. The third study subject was safely treated in October 2024. The fourth study subject was safely treated in December 2024. The fifth study subject was safely treated in February 2025, and the sixth study subject was safely treated in April 2025. BB-301 is the lead investigational gene therapy agent under development by Benitec, and the key attributes of BB-301 are outlined in Figure 3.
26
Figure 3
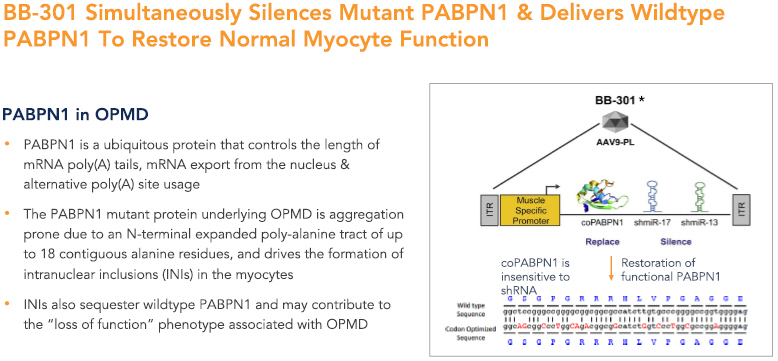
BB-301 is a first-in-class genetic medicine employing the “silence and replace” approach for the treatment of OPMD. OPMD is an insidious, autosomal-dominant, late-onset, degenerative muscle disorder that typically presents in patients at 40-to-50 years of age. The disease is characterized by progressive swallowing difficulties (dysphagia) and eyelid drooping (ptosis). OPMD is caused by a specific mutation in the poly(A)-binding protein nuclear 1 gene (PABPN1).
OPMD is a rare disease, however, patients have been diagnosed with OPMD in at least 33 countries. Patient populations suffering from OPMD are well-identified, and significant geographical clustering has been noted for patients with this disorder. Each of these attributes could facilitate efficient clinical development and global commercialization of BB-301.
PABPN1 is a ubiquitous factor that promotes the interaction between the poly(A) polymerase and CPSF (cleavage and polyadenylation specificity factor) and, thus, controls the length of mRNA poly(A) tails, mRNA export from the nucleus, and alternative poly(A) site usage. The characteristic genetic mutation underlying OPMD results in trinucleotide repeat expansion(s) within exon 1 of PABPN1 and results in an expanded poly-alanine tract at the N-terminal end of PABPN1. The mutation generates a protein with an N-terminal expanded poly-alanine tract of up to 18 contiguous alanine residues, and the mutant protein is prone to the formation of intranuclear aggregates designated as intranuclear inclusions (INIs). The INIs that sequester wildtype PABPN1 may contribute to the “loss of function” phenotype associated with OPMD.
No therapeutic agents are approved for the treatment of OPMD. Additionally, there are no surgical interventions available to OPMD patients that modify the natural history of the disease, which is principally comprised of chronic deterioration of swallowing function. BB-301 has received Orphan Drug Designation in the United States and the European Union and, upon achievement of regulatory approval for BB-301 in these respective jurisdictions, the Orphan Drug Designations would provide commercial exclusivity independent of intellectual property protection. While OPMD is a rare medical disorder, we believe the commercial opportunity for a safe and efficacious therapeutic agent in this clinical indication exceeds $1 billion over the course of the commercial life of the product.
BB-301 is our Lead, Silence and Replace-Based, OPMD Therapeutic Agent
BB-301 is composed of a modified AAV serotype 9 (AAV9) capsid that expresses a bifunctional construct under the control of a single muscle specific Spc5-12 promoter to achieve co-expression of both the codon-optimized PABPN1 mRNA and two shmiR molecules directed against wild type and mutant PABPN1. BB-301 is designed to correct the genetic defect underlying OPMD following a single localized administration.
27
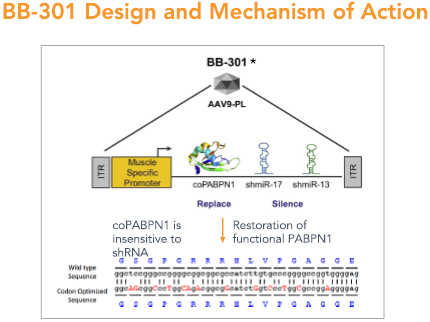
BB-301—Design and Mechanism of Action
BB-301 is designed to target two distinct regions of the PABPN1 mRNA to accomplish gene silencing via the concomitant expression of two distinct shmiRs from a single DNA construct (Figure 4). BB-301 is also engineered to drive the simultaneous expression of a codon-optimized, siRNA-resistant, version of the wild type PABPN1 gene (Figure 4).
28
Figure 4
29
Summary of the Key Regulatory Interactions:
| • | In June 2023 the U.S. Food and Drug Administration (FDA) cleared the Investigational New Drug (IND) application for BB-301 which allowed dosing of BB-301 to begin for OPMD subjects that are eligible for enrollment into the Phase 1b/2a treatment study (NCT06185673) described below. |
Operational Updates
The key milestones related to the development of BB-301 for the treatment of OPMD, along with other corporate updates, are outlined below:
BB-301 Clinical Development Program Overview:
| • | The BB-301 clinical development program is being conducted in the United States, and the primary elements of the program are summarized below: |
The program comprises approximately 76 weeks of follow-up which will consist of:
| • | The OPMD Natural History (NH) Study: 6-month pre-treatment observation periods for the evaluation of baseline disposition and natural history of OPMD-derived dysphagia (swallowing impairment) in each study participant. |
| • | Dosing with BB-301: 1-day of BB-301 dosing to initiate participation in the Phase 1b/2a single-arm, open-label, sequential, dose-escalation cohort study (NCT06185673). BB-301 is delivered directly to the pharyngeal muscles of each study subject. |
| • | Phase 1b/2a Treatment Evaluation: 52-weeks of post-dosing follow-up for conclusive evaluation of the primary and secondary endpoints of the BB-301 Phase 1b/2a treatment study (NCT06185673), with interim safety and efficacy results expected to be available at the end of each 180-day period following the administration of BB-301. |
30
| • | The OPMD NH Study will characterize the level of dysphagia borne by each OPMD subject at baseline and assess subsequent progression of dysphagia via the use of the following quantitative radiographic measures (i.e., videofluoroscopic swallowing studies or “VFSS”). The VFSS outlined below collectively provide objective assessments of global swallowing function and the function of the pharyngeal constrictor muscles (i.e., the muscles whose functional deterioration drives disease progression in OPMD): |
| • | Total Pharyngeal Residue %(C2-4)2 |
| • | Pharyngeal Area at Maximum Constriction (PhAMPC) |
| • | Dynamic Imaging Grade of Swallowing Toxicity Scale (DIGEST) |
| • | Vallecular Residue %(C2-4)2, Pyriform Sinus Residue %(C2-4)2, and Other Pharyngeal Residue %(C2-4)2 |
| • | Normalized Residue Ratio Scale (NRRSv, NRRSp) |
| • | Pharyngeal Construction Ratio (PCR) |
| • | The NH study will also employ clinical measures of global swallowing capacity and oral-pharyngeal dysphagia, along with two distinct patient-reported outcome instruments targeting the assessment of oral-pharyngeal dysphagia. |
| • | Upon the achievement of 6-months of follow-up in the NH Study, participants will, potentially, be eligible for enrollment into the BB-301 Phase 1b/2a treatment study (NCT06185673). |
| • | BB-301 Phase 1b/2a Treatment Study (NCT06185673): |
| • | This first-in-human (FIH) study will evaluate the safety and clinical activity of intramuscular doses of BB-301 administered to subjects with OPMD. |
| • | The primary endpoint of the FIH study will be safety. |
| • | Secondary endpoints are designed to determine the impact of BB-301 on swallowing efficiency, swallowing safety, and pharyngeal constrictor muscle function in subjects diagnosed with OPMD with dysphagia via the use of serial clinical and videofluoroscopic assessments. Critically, each of the clinical and videofluoroscopic assessments employed in the FIH study will be equivalent to those employed for the NH study to facilitate comparative clinical and statistical analyses for each study subject. |
| • | The primary and secondary endpoints will be evaluated during each 90-day period following BB-301 intramuscular injection (Day 1). |
| • | The NH of dysphagia observed for each OPMD NH Study participant, as characterized by the VFSS and clinical swallowing assessments carried out during the NH Study, will serve as the baseline for comparative assessments of safety and efficacy of BB-301 upon rollover from the NH Study onto the BB-301 Phase 1b/2a Treatment Study (NCT06185673). |
All six subjects have been safely treated with BB-301. No treatment-related Severe Adverse Events have been observed for the Subjects treated with BB-301.
Recent Interim Clinical Study Results and FDA Fast Track Designation
On November 3, 2025, we announced positive interim clinical results for the BB-301 Phase 1b/2a Clinical Trial. Following administration of BB-301, Cohort 1 patients demonstrated significant and sustained improvements across multiple clinical measures including dysphagic symptom burden, post-swallow residue accumulation, time required to consume fixed volumes of liquid, as well as improved pharyngeal closure during swallowing. All six patients enrolled into Cohort 1 met the formal statistical criteria for response to BB-301, representing a 100% response rate. Following review of these encouraging interim data, the U.S. Food and Drug Administration granted Fast Track designation to BB-301 for the treatment of OPMD with dysphagia. BB-301 was also previously granted Orphan Drug Designation from both the FDA and European Medical Association.
The pre-treatment data for Cohort 1 patients reflect the first six months of Natural History Study follow-up and the final pre-treatment visit (i.e., the Phase 1 Screening Visit)
The interim post-treatment data for Cohort 1 patients reflect the following:
| • | 12-months of post-BB-301-treatment follow-up for Patient 1 and Patient 2 |
| • | 9-months of post-BB-301-treatment follow-up for Patient 3 |
| • | 6-months of post-BB-301-treatment follow-up for Patient 4 and Patient 5; and |
| • | 3-months of post-BB-301-treatment follow-up for Patient 6 |
As the total dysphagic symptom burden experienced by OPMD patients has several known underlying contributors, the development of a multi-component composite endpoint to evaluate the potential treatment effects of BB-301 allows for incorporation of multiple discrete assessments that, in total, assess disease progression and treatment benefit of BB-301.
The BB-301 Responder Analysis (the multi-component composite endpoint) is comprised of a combination of patient-reported outcome results, objective assessment results, and swallowing capacity assessment results:
| • | Patient-Reported Outcome assessment results include: Sydney Swallow Questionnaire or “SSQ” results |
| • | Objective Assessment Results include: Videofluoroscopic swallowing study results (Pharyngeal Area at Maximum Constriction or “PhAMPC”, Post-Swallow Pharyngeal Residue as measured by Total Pharyngeal Residue or “TPR” and Normalized Residue Ratio Scale or “NRRS”, Frequency of sequential swallows or “SEQ”) |
| • | Functional Swallowing Capacity Assessment Results include: Clinically administered drinking assessment results (as measured by the cold-water timed drinking test or “CWDT”) |
Following the administration of BB-301, Cohort 1 patients experienced clinically significant reductions, and met the formal statistical criteria for response, in the following assessments:

31
Manufacturing
The manufacture of the biological products required for gene therapy is complex and difficult. We do not currently own or operate manufacturing facilities for the production of preclinical, clinical or commercial quantities of any of our product candidates. We are exploring long-term manufacturing alliances with a number of potential partners to investigate manufacturing processes in order to produce materials at reasonable scale and cost of goods to support future commercialization efforts. We do not have a long-term agreement with any third-party manufacturer, but we plan to establish such a relationship with an appropriate manufacturer to serve our long-term needs.
Manufacturing is subject to extensive regulations that impose various procedural and documentation requirements, which govern record keeping, manufacturing processes and controls, personnel, quality control and quality assurance, among others. Our contract manufacturing organizations manufacture our product candidates under cGMP conditions. cGMP is a regulatory standard for the production of pharmaceuticals that will be used in humans.
Sales and Marketing
We have not yet established sales, marketing or product distribution operations because our product candidates are in preclinical or clinical development. If we receive marketing and commercialization approval for any of our product candidates, we intend to market the product through strategic alliances and distribution agreements with third parties. In certain cases, we may market an approved product directly worldwide or in selected geographical segments. The ultimate implementation of our strategy for realizing the financial value of our product candidates is dependent on the results of clinical trials for our product candidates, the availability of funds and the ability to negotiate acceptable commercial terms with third parties.
Competition
The biopharmaceutical industry is characterized by intense and dynamic competition to develop new technologies and proprietary therapies.
Any product candidates that we successfully develop and commercialize will have to compete with existing therapies and new therapies that may become available in the future.
32
While we believe that our proprietary technology and scientific expertise in gene silencing using ddRNAi provide us with competitive advantages, we face potential competition from many different sources, including larger and better-funded pharmaceutical, specialty pharmaceutical and biotechnology companies, as well as from academic institutions and governmental agencies and public and private research institutions that may develop potentially competitive products or technologies. We are aware of several companies focused on developing gene therapy or gene silencing product candidates.
We are not aware of any companies developing a gene therapy or gene silencing approach for OPMD. Our product candidates, if approved, would also compete with treatments that have already been approved and accepted by the medical community, patients and third-party payers.
Many of our competitors and potential competitors, alone or with their strategic partners, have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of product candidates, obtaining FDA and other regulatory approvals of treatments and the commercialization of those treatments. Mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated among a smaller number of our competitors. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical study sites and subject registration for clinical studies, as well as in acquiring technologies complementary to, or necessary for, our programs. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
We anticipate that we will face intense and increasing competition as new products enter the market and advanced technologies become available. We expect any treatments that we develop and commercialize to compete on the basis of, among other things, efficacy, safety, convenience of administration and delivery, price, the level of competition and the availability of reimbursement from government and other third party-payers.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. In addition, we expect that our therapeutic products, if approved, will be priced at a significant premium over competitive products and our ability to compete may be affected in many cases by insurers or other third-party payers seeking to encourage the use of competitive products including biosimilar or generic products.
This increasingly competitive landscape may compromise the development of our product candidates.
Royalties, milestone payments and other license fees
We are required to pay royalties, milestone payments and other license fees in connection with our licensing of intellectual property from third parties, including as discussed below.
Foreign Currency Translation and Other Comprehensive Income (Loss)
The Company’s functional currency and reporting currency is the United States dollar. BBL’s functional currency is the Australian dollar (AUD). Assets and liabilities are translated at the exchange rate in effect at the balance sheet date. Revenues and expenses are translated at the average rate of exchange prevailing during the reporting period. Equity transactions are translated at each historical transaction date spot rate. Translation adjustments arising from the use of different exchange rates from period to period are included as a component of stockholders’ equity as “Accumulated other comprehensive loss.” Gains and losses resulting from foreign currency translation are included in the consolidated statements of operations and comprehensive loss as other comprehensive income (loss).
33
ATM Agreement
On October 11, 2024, the Company entered into a Sales Agreement (the “Sales Agreement”) with Leerink Partners LLC (the “Agent”). Pursuant to the terms of the Sales Agreement, the Company may offer and sell shares of the Company’s common stock having an aggregate offering amount of up to $75 million from time to time through the Agent. The Agent will use its commercially reasonable efforts, as the agent and subject to the terms of the Sales Agreement, to sell the shares offered. Sales of the shares, if any, may be made in sales deemed to be an “at-the-market offering” as defined in Rule 415 under the Securities Act of 1933, as amended. The Company may also agree to sell shares to the Agent as principal for its own account on terms agreed to by the Company and the Agent. The Agent will be entitled to a commission from the Company of 3.0% of the gross proceeds from the sale of shares sold under the Sales Agreement. In addition, the Company has agreed to reimburse certain expenses incurred by the Agent in connection with the offering. Shares sold pursuant to the Sales Agreement, if any, will be sold pursuant to the Company’s shelf registration statement on Form S-3 (File No. 333-277310), that was filed with the Securities and Exchange Commission, including the related prospectus, dated March 5, 2024, as supplemented by a prospectus supplement.
34
Results of Operations
Revenues
The Company has not generated any revenues from the sales of products. Revenues from licensing fees are included in the revenue from customers line item on our consolidated statements of operations and comprehensive loss. Our licensing fees have been generated through the licensing of our ddRNAi technology to biopharmaceutical companies. The Company did not recognize any revenue during the three months ended September 30, 2025 and September 30, 2024.
Research and Development Expenses
Research and development expenses relate primarily to the cost of conducting clinical and preclinical trials. Preclinical and clinical development costs are a significant component of research and development expenses. We record accrued liabilities for estimated costs of research and development activities conducted by third-party service providers, which include the conduct of preclinical studies and clinical trials, and contract manufacturing activities. We record the estimated costs of research and development activities based upon the estimated amount of services provided but not yet invoiced and includes these costs in trade and other payables on the consolidated balance sheets and within research and development expenses on the consolidated statements of operations and comprehensive loss.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries, related benefits, travel, and equity-based compensation expense. General and administrative expenses also include facility expenses, professional fees for legal, consulting, accounting and audit services and other related costs.
We anticipate that our general and administrative expenses may increase as we focus on the continued development of the clinical OPMD program. We also anticipate an increase in expenses relating to accounting, legal and regulatory-related services associated with maintaining compliance with exchange listing and SEC requirements, director and officer insurance premiums and other similar costs.
35
Operating Expenses
The following tables sets forth a summary of our expenses for each of the periods:
| Three Months Ended September 30, |
||||||||
| 2025 | 2024 | |||||||
| (US$’000) | ||||||||
| Operating Expenses: |
||||||||
| Research and development |
$ | 3,370 | $ | 3,585 | ||||
| General and administrative |
6,433 | 2,206 | ||||||
|
|
|
|
|
|||||
| Total operating expenses |
$ | 9,803 | $ | 5,791 | ||||
|
|
|
|
|
|||||
During the three months ended September 30, 2025, we incurred $3.4 million in research and development expenses, as compared to $3.6 million for the comparable period ended September 30, 2024. Research and development expenses relate primarily to ongoing clinical development of BB-301 for the treatment of OPMD. The decrease for the three months ended September 30, 2025 reflects the timing of contract manufacturing activity and the timing of payments for the OPMD Natural History and Dosing study.
General and administrative expense totaled $6.4 million for the three months ended September 30, 2025, compared to $2.2 million for the comparable period ended September 30, 2024. The increase for the three months ended September 30, 2025, relates primarily to an increase in share based compensation of $4.0 million and salaries and wages of $267 thousand.
Other Income (Expense)
The following tables sets forth a summary of our other income (loss) for each of the periods:
| Three Months Ended | ||||||||
| September 30, | ||||||||
| 2025 | 2024 | |||||||
| (US$’000) | ||||||||
| Other Income (Loss): |
||||||||
| Foreign currency transaction gain (loss) |
$ | (89 | ) | $ | 93 | |||
| Interest income, net |
1,011 | 604 | ||||||
| Other income (expense), net |
(84 | ) | 35 | |||||
|
|
|
|
|
|||||
| Total other income (loss), net |
$ | 838 | $ | 732 | ||||
|
|
|
|
|
|||||
Other income (loss), net during the three months ended September 30, 2025, which consists of foreign currency transaction gain (loss), interest income, and other expense, net, totaled $838 thousand. Other income (loss), net during the three months ended September 30, 2024, which consists of foreign currency transaction gain (loss), interest income, and other income, net, totaled $732 thousand. Foreign currency transaction gains and losses reflect changes in foreign exchange rates. Net interest income for the three month period ended September 30, 2025, in comparison to the three month period ended September 30, 2024, reflects the increase in the Company’s cash and cash equivalent balances. Other expense recognized during the three months ended September 30, 2025 relates to current period accrued franchise tax expenses, in comparison to the three month period ended September 30, 2024, which relates to recognition of a refund of overpaid franchise tax expenses offset by the period accruals.
36
Liquidity and Capital Resources
The Company has incurred cumulative losses and negative cash flows from operations since our predecessor’s inception in 1995. The Company had accumulated losses of $237 million as of September 30, 2025. We expect that our research and development expenses will increase due to the continued development of the OPMD program. It is also likely that there will be an increase in the general and administrative expenses due to the obligations of being a domestic public company in the United States.
We had no borrowings as of September 30, 2025 and do not currently have a credit facility. As of September 30, 2025, we had outstanding warrants to purchase 20,443,496 shares of Common Stock consisting of the following:
| September 30, 2025 |
June 30, 2025 |
|||||||
| September 2022 Pre-Funded Warrants to purchase Common Stock |
588,236 | 588,236 | ||||||
| Series 2 Warrants to purchase Common Stock |
101,537 | 101,537 | ||||||
| August 2023 Pre-Funded Warrants to purchase Common Stock |
12,178,739 | 12,178,739 | ||||||
| Common Warrants to purchase Common Stock |
5,071,148 | 5,071,148 | ||||||
| April 2024 Pre-Funded Warrants to purchase Common Stock |
2,202,836 | 2,202,836 | ||||||
| March 2025 Pre-Funded Warrants to purchase Common Stock |
300,000 | 300,000 | ||||||
|
|
|
|
|
|||||
| Total |
20,443,496 | 20,443,496 | ||||||
|
|
|
|
|
|||||
As of September 30, 2025, we had cash and cash equivalents of approximately $94.5 million. Cash in excess of immediate requirements is invested in accordance with our investment policy, primarily with a view to liquidity and capital preservation. Currently, our cash and cash equivalents are held in bank accounts. On October 11, 2024, we entered into the Sales Agreement as discussed above, which provides for the sale of up to $75 million of our common stock from time-to-time in “at-the-market offerings”.
The following table sets forth a summary of the net cash flow activity for each of the periods set forth below:
| Three Months Ended | ||||||||
| September 30, | ||||||||
| 2025 | 2024 | |||||||
| (US$’000) | ||||||||
| Net cash provided by (used in): |
||||||||
| Operating activities |
$ | (3,352 | ) | $ | (4,586 | ) | ||
| Investing activities |
— | — | ||||||
| Financing activities |
— | 21,655 | ||||||
| Effects of exchange rate changes on cash and cash equivalents |
87 | (93 | ) | |||||
|
|
|
|
|
|||||
| Net increase (decrease) in cash, cash equivalents, and restricted cash |
$ | (3,265 | ) | $ | 16,976 | |||
|
|
|
|
|
|||||
Operating activities
Net cash used in operating activities for the three months ended September 30, 2025 and 2024 was $3.4 million and $4.6 million, respectively. Net cash used in operating activities was primarily the result of our net loss, partially offset by non-cash expenses, and changes in working capital, including increases trade and other receivables, prepaid expenses, and liabilities.
Investing activities
Net cash used in investing activities for the three month periods ended September 30, 2025 and 2024 was zero, respectively.
Financing activities
Net cash provided by financing activities was $0 and $21.7 million for the three months ended September 30, 2025 and 2024, respectively. Cash from financing activities for the three month period ended September 30, 2025 was zero. Cash from financing activities in the three months ended September 30, 2024 was related to the issuance of common stock for the exercise of pre-funded warrants, Series 2 warrants, and common warrants, with gross proceeds of $21.7 million.
The future of the Company as an operating business will depend on its ability to manage operating costs and budgeted amounts and obtain adequate financing. While we continue to progress discussions and advance opportunities to engage with pharmaceutical companies and continue to seek licensing partners for ddRNAi in disease areas that are not our focus, there can be no assurance as to whether we will enter into such arrangements or what the terms of any such arrangement could be.
37
We do not have any products approved for sale and have not generated any revenue from product sales. We do not know when, or if, we will generate any revenue from product sales. We do not expect to generate significant revenue from product sales unless and until we obtain regulatory approval of and commercialize one of our current or future product candidates.
Unless and until we establish significant revenues from licensing programs, strategic alliances or collaboration arrangements with pharmaceutical companies, or from product sales, we anticipate that we will continue to generate losses for the foreseeable future, and we expect the losses to increase as we continue the development of product candidates and begin to prepare to commercialize any product that receives regulatory approval. We are subject to the risks inherent in the development of new gene therapy products, and we may encounter unforeseen expenses, difficulties, complications, delays, and other unknown factors that may adversely affect our business. We estimate that our cash and cash equivalents will be sufficient to fund the Company’s operations for at least the next twelve months from the date of this report.
We have based our projections of operating capital requirements on assumptions that may prove to be incorrect and we may use all of our available capital resources sooner than we expect. Because of the numerous risks and uncertainties associated with research, development, and commercialization of pharmaceutical products, we are unable to estimate the exact amount of our operating capital requirements. Our future funding requirements will depend on many factors, including, but not limited to:
| • | the timing and costs of our clinical trials for our ddRNAi and silence and replace product candidates; |
| • | the timing and costs of our preclinical studies for our ddRNAi and silence and replace product candidates; |
| • | the number and characteristics of product candidates that we pursue; |
| • | the outcome, timing, and costs of seeking regulatory approvals; |
| • | revenue received from commercial sales of any of our product candidates that may receive regulatory approval; |
| • | the terms and timing of any future collaborations, licensing, consulting, or other arrangements that we may establish; |
| • | the amount and timing of any payments we may be required to make, or that we may receive, in connection with the licensing, filing, prosecution, defense and enforcement of any patents or other intellectual property rights; |
| • | the costs of preparing, filing and prosecuting patent applications, maintaining and protecting our intellectual property rights and defending against intellectual property related claims; and |
| • | the extent to which we need to in-license or acquire other products and technologies. |
Contractual Obligations and Commercial Commitments
On October 1, 2016, the Company entered into an operating lease for office space in Hayward, California that originally expired in April 2018. The Company has entered into lease amendments that extended the lease through December 2027. The Company also entered into a new lease in Los Angeles, California, which has an initial expiration date in July 2026. See Note 8 of the Notes to Consolidated Financial Statements included in this Quarterly Report on Form 10-Q.
The Company enters into contracts in the normal course of business with third-party contract research organizations, contract development and manufacturing organizations and other service providers and vendors. These contracts generally provide for termination on notice and, therefore, are cancellable contracts and not considered contractual obligations and commitments.
Critical Accounting Policies and Significant Accounting Estimates
The preparation of consolidated financial statements and related disclosures in conformity with accounting principles generally accepted in the United States of America requires management to make judgments, assumptions and estimates that affect the amounts reported. Note 2 of the Notes to Consolidated Financial Statements included in this Quarterly Report on Form 10-Q describes the significant accounting policies used in the preparation of the consolidated financial statements. Certain of these significant accounting policies are considered to be critical accounting policies.
A critical accounting policy is defined as one that is both material to the presentation of the Company’s consolidated financial statements and requires management to make difficult, subjective, or complex judgments that could have a material effect on the Company’s financial condition or results of operations. Specifically, these policies have the following attributes: (1) the Company is required to make assumptions about matters that are highly uncertain at the time of the estimate; and (2) different estimates the Company could reasonably have used, or changes in the estimate that are reasonably likely to occur, would have a material effect on the Company’s financial condition or results of operations.
Estimates and assumptions about future events and their effects cannot be determined with certainty. The Company bases its estimates on historical experience and on various other assumptions believed to be applicable and reasonable under the circumstances. These estimates may change as new events occur, as additional information is obtained and as the Company’s operating environment changes.
38
These changes have historically been minor and have been included in the consolidated financial statements as soon as they became known. In addition, management is periodically faced with uncertainties, the outcomes of which are not within its control and will not be known for prolonged periods of time. These uncertainties are discussed in the section above entitled “Risk Factors.” Based on a critical assessment of its accounting policies and the underlying judgments and uncertainties affecting the application of those policies, management believes that the Company’s consolidated financial statements are fairly stated in accordance with accounting principles generally accepted in the United States of America and provide a meaningful presentation of the Company’s financial condition and results of operations.
Management believes that the following are critical accounting policies:
Research and Development Expense
Preclinical and clinical trial costs are a significant component of our research and development expenses. We accrue for preclinical and clinical development costs based on factors such as estimates of the work completed and in accordance with agreements established with our third-party service providers. We make significant judgments and estimates in determining the accrued liabilities balance at the end of each reporting period. As actual costs become known, we adjust our accrued liabilities accordingly on a prospective basis and will do so in the period in which the facts that give rise to the revision become reasonably certain.
Share-based Compensation Expense
We record share-based compensation in accordance with ASC 718, Stock Compensation. ASC 718 requires the fair value of all share-based employee compensation awarded to employees and non-employees to be recorded as an expense over the shorter of the service period or the vesting period. We determine employee and non-employee share-based compensation based on grant-date fair value using the Black-Scholes Option Pricing Model and allocate the resulting compensation expense over the corresponding requisite service period using the graded vesting attribution method. We account for forfeitures of share-based awards as they occur.
Recent Accounting Pronouncements
For a discussion of recent accounting pronouncements that we have adopted and have not yet adopted, see Note 2 to our consolidated financial statements.
Item 3. Quantitative and Qualitative Disclosures About Market Risk
As a smaller reporting company, we are not required to provide the information pursuant to this Item.
39
Item 4. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
We have established disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended). As of the end of the period covered by this Report we carried out an evaluation under the supervision and with the participation of our management, including our principal executive officer and principal financial and accounting officer, of the effectiveness of our disclosure controls and procedures pursuant to Rule 13a-15 of the Securities and Exchange Act of 1934, as amended. Based on this evaluation, and as a result of the material weakness in our internal control over financial reporting further described in Management’s Report on Internal Control Over Financial Reporting in Item 9A of our Form 10-K for the fiscal year ended June 30, 2025 (relating to inadequate design and implementation of controls over our share-based compensation calculation review process; specifically, we did not design and/or implement process level controls to ensure all inputs used in share-based compensation expense calculations are complete and accurate, including review of the vesting allocation method applied by the equity system), our principal executive officer and principal financial officer, concluded that as of September 30, 2025, our disclosure controls and procedures were not effective to provide reasonable assurance that information we are required to disclose in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer as appropriate to allow timely decisions regarding required disclosure. In order to remediate this matter, we have updated the equity system’s default vesting allocation method configuration and have established enhancements to our quarterly share-based compensation review process to identify and verify all relevant inputs of the share-based compensation expense calculation, including review over the completeness and accuracy of the vesting allocation method applied by the equity system. We will consider the material weakness to be fully remediated once the applicable controls operate for a sufficient period of time and our management has concluded, through testing, that these controls are operating effectively. These processes will be tested in the coming months.
We do not expect that our disclosure controls and procedures or our internal controls will prevent all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected.
Changes in Internal Control over Financial Reporting
Other than the efforts towards remediating the material weakness as previously described above, there were no changes in our internal controls over financial reporting during the quarter ended September 30, 2025 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
40
Item 6. Exhibits.
| * | Filed herewith. |
| ** | Furnished, not filed. |
42
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on our behalf by the undersigned thereunto duly authorized.
| Benitec Biopharma Inc. | ||||
| Dated: November 14, 2025 | /s/ Jerel Banks |
|||
| Dr. Jerel Banks | ||||
| Executive Chairman and Chief Executive Officer | ||||
| (principal executive officer) | ||||
| /s/ Megan Boston |
||||
| Megan Boston | ||||
| Chief Financial Officer and Secretary (principal financial and accounting officer) | ||||
43
Exhibit 10.4
REGISTRATION RIGHTS AGREEMENT
This Registration Rights Agreement (this “Agreement”) is made and entered into as of November 7, 2025, by and among Benitec Biopharma Inc., a Delaware corporation (the “Company”), Averill Master Fund, Ltd. (“Averill”) and Averill Madison Master Fund, Ltd. (“Averill Madison,” and together with Averill, each a “Purchaser” and collectively, the “Purchasers”).
This Agreement is made pursuant to that certain Securities Purchase Agreement, dated November 5, 2025, between the Company, Averill and Averill Madison (the “Registered Direct Purchase Agreement”).
The Company and each Purchaser hereby agrees as follows:
1. Definitions.
Capitalized terms used and not otherwise defined herein that are defined in the Registered Direct Purchase Agreement shall have the meanings given to such terms in the Registered Direct Purchase Agreement. As used in this Agreement, the following terms shall have the following meanings:
“Advice” shall have the meaning set forth in Section 6(b).
“Commission” means the U.S. Securities and Exchange Commission.
“Effectiveness Date” means, with respect to the Initial Registration Statement required to be filed hereunder, the 90th calendar day following the closing date of the transactions contemplated by the Registered Direct Purchase Agreement (the “Closing Date”) (or, in the event of a “full review” by the Commission, the 120th calendar day following the Closing Date) and with respect to any additional Registration Statements which may be required pursuant to Section 2(c) or Section 3(c), the 60th calendar day following the date on which an additional Registration Statement is required to be filed hereunder (or, in the event of a “full review” by the Commission, the 90th calendar day following the date such additional Registration Statement is required to be filed hereunder); provided, however, that in the event the Company is notified by the Commission that one or more of the above Registration Statements will not be reviewed or is no longer subject to further review and comments, the Company will submit a request for acceleration of the effectiveness no later than the fifth Trading Day following the date on which the Company is so notified and the Effectiveness Date shall be no later than the second Trading Day following the submission of the request for acceleration (or an earlier date in the Company’s discretion if such earlier date is acceptable to the Commission) if such date precedes the dates otherwise required above (unless the Company is informed by the Commission that it will not take the Registration Statement effective on such date), provided, further, if such Effectiveness Date falls on a day that is not a Trading Day, then the Effectiveness Date shall be the next succeeding Trading Day, provided, further, that if the Commission is closed for operations due to a government shutdown or lapse in appropriations after the date on which the Company filed the Initial Registration Statement with the Commission, the Effectiveness Date shall be extended by the same number of calendar days that the Commission remains closed and/or shut down for operations after the date on which the Company filed the Initial Registration Statement with the Commission.
“Effectiveness Period” shall have the meaning set forth in Section 2(a).
“Event” shall have the meaning set forth in Section 2(d).
“Event Date” shall have the meaning set forth in Section 2(d).
“Filing Date” means, with respect to the Initial Registration Statement required hereunder, no later than the 60th calendar day following the Closing Date and, with respect to any additional Registration Statements which may be required pursuant to Section 2(c) or Section 3(c), the earliest practical date on which the Company is permitted by SEC Guidance to file such additional Registration Statement related to the Registrable Securities.
“Indemnified Party” shall have the meaning set forth in Section 5(c).
“Indemnifying Party” shall have the meaning set forth in Section 5(c).
“Initial Registration Statement” means the initial Registration Statement filed pursuant to this Agreement.
“Losses” shall have the meaning set forth in Section 5(a).
“Plan of Distribution” shall have the meaning set forth in Section 2(a).
“Prospectus” means the prospectus included in a Registration Statement (including, without limitation, a prospectus that includes any information previously omitted from a prospectus filed as part of an effective registration statement in reliance upon Rule 430A promulgated by the Commission pursuant to the Securities Act), as amended or supplemented by any prospectus supplement, with respect to the terms of the offering of any portion of the Registrable Securities covered by a Registration Statement, and all other amendments and supplements to the Prospectus, including post-effective amendments, and all material incorporated by reference or deemed to be incorporated by reference in such Prospectus.
“Registered Direct Shares” means the shares of the Company’s common stock, par value $0.0001 per share, purchased by the Purchasers pursuant to the Registered Direct Purchase Agreement.
“Registrable Securities” means, as of any date of determination, all Registered Direct Shares, and any securities issued or then issuable upon any stock split, dividend or other distribution, recapitalization or similar event with respect to the foregoing; provided, however, that any such Registrable Securities shall cease to be Registrable Securities (and the Company shall not be required to maintain the effectiveness of any, or file another, Registration Statement hereunder with respect thereto) for so long as (i) a Registration Statement with respect to the sale of such Registrable Securities was declared effective by the Commission under the Securities Act and such Registrable Securities have been disposed of by the Purchaser in accordance with such effective Registration Statement, (ii) such Registrable Securities have been previously sold in accordance with Rule 144, or (iii) such securities become eligible for resale without volume or manner-of-sale restrictions and without current public information pursuant to Rule 144 as set forth in a written opinion letter to such effect, addressed, delivered and acceptable to the Transfer Agent and the affected Purchasers (assuming that such securities and any securities issuable upon exercise, conversion or exchange of which, or as a dividend upon which, such securities were issued or are issuable, were at no time held by any Affiliate of the Company), as reasonably determined by the Company, upon the advice of counsel to the Company.
“Registration Statement” means any registration statement required to be filed hereunder pursuant to Section 2(a) and any additional registration statements contemplated by Section 2(c) or Section 3(c), including (in each case) the Prospectus, amendments and supplements to any such registration statement or Prospectus, including pre- and post-effective amendments, all exhibits thereto, and all material incorporated by reference or deemed to be incorporated by reference in any such registration statement.
“Rule 415” means Rule 415 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended or interpreted from time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same purpose and effect as such Rule.
“Rule 424” means Rule 424 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended or interpreted from time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same purpose and effect as such Rule.
“Selling Stockholder Questionnaire” shall have the meaning set forth in Section 3(a).
“SEC Guidance” means (i) any publicly available written or oral guidance of the Commission staff, or any comments, requirements or requests of the Commission staff and (ii) the Securities Act.
2. Shelf Registration.
(a) On or prior to each Filing Date, the Company shall prepare and file with the Commission a Registration Statement covering the resale of all of the Registrable Securities for an offering to be made on a continuous basis pursuant to Rule 415. Each Registration Statement filed hereunder shall be on Form S-3 (or, at the election of the Company and if available, such other form available to register for resale the Registrable Securities as a secondary offering), and shall contain substantially the “Plan of Distribution” attached hereto as Annex A and substantially the “Selling Stockholder” section attached hereto as Annex B; provided, however, that no Purchasers shall be required to be named as an “underwriter” without such Purchaser’s express prior written consent. Subject to the terms of this Agreement, the Company shall use its best efforts to cause a Registration Statement filed under this Agreement (including, without limitation, under Section 3(c)) to be declared effective under the Securities Act as promptly as possible after the filing thereof, but in any event no later than the applicable Effectiveness Date, and shall use its best efforts to keep such Registration Statement continuously effective under the Securities Act until the date that all Registrable Securities covered by such Registration Statement (i) have been sold, thereunder or pursuant to Rule 144, or (ii) may be sold without volume or manner-of-sale restrictions pursuant to Rule 144 and without the requirement for the Company to be in compliance with the current public information requirement under Rule 144, as determined by the counsel to the Company pursuant to a written opinion letter to such effect, addressed and acceptable to the Transfer Agent and the affected Purchaser (the “Effectiveness Period”). The Company shall telephonically request effectiveness of a Registration Statement as of 5:00 p.m. (New York City time) on a Trading Day. The Company shall immediately notify the Purchasers via e-mail of the effectiveness of a Registration Statement on the same Trading Day that the Company telephonically confirms effectiveness with the Commission, which shall be the date requested for effectiveness of such Registration Statement. The Company shall, by 9:30 a.m. (New York City time) on the second Trading Day after the effective date of such Registration Statement, file a final Prospectus with the Commission as required by Rule 424. Failure to so notify the Purchaser within one (1) Trading Day of such notification of effectiveness or failure to file a final Prospectus as foresaid shall be deemed an Event under Section 2(d).
(b) Notwithstanding the registration obligations set forth in Section 2(a), if the Commission informs the Company that all of the Registrable Securities cannot, as a result of the application of Rule 415, be registered for resale as a secondary offering on a single registration statement, the Company agrees to promptly inform each of the Purchasers thereof and use its commercially reasonable efforts to file amendments to the Initial Registration Statement as required by the Commission, covering the maximum number of Registrable Securities permitted to be registered by the Commission, on Form S-3 (or, at the election of the Company and if available, such other form available to register for resale the Registrable Securities as a secondary offering); with respect to filing on Form S-1 or other appropriate form; provided, however, that prior to filing such amendment, the Company shall be obligated to use reasonable efforts to advocate with the Commission for the registration of all of the Registrable Securities in accordance with the SEC Guidance, including without limitation, Compliance and Disclosure Interpretation 612.09.
(c) Notwithstanding any other provision of this Agreement, if the Company elects to use Form S-3, and the Commission or any SEC Guidance sets forth a limitation on the number of Registrable Securities permitted to be registered on a particular Registration Statement as a secondary offering (and notwithstanding that the Company used reasonable efforts to advocate with the Commission for the registration of all or a greater portion of Registrable Securities), unless otherwise directed in writing by the Purchasers, the number of Registrable Securities to be registered on such Registration Statement will be reduced as follows:
a. First, the Company shall reduce or eliminate any securities to be included other than Registrable Securities;
b. Second, the Company shall reduce Registrable Securities represented by Registrable Securities.
In the event of a cutback hereunder, the Company shall give the Purchasers at least three (3) Trading Days prior written notice. In the event the Company amends the Initial Registration Statement in accordance with the foregoing, the Company will use its best efforts to file with the Commission, as promptly as allowed by Commission or SEC Guidance provided to the Company or to registrants of securities in general, one or more registration statements on Form S-3 or such other form available to register for resale those Registrable Securities that were not registered for resale on the Initial Registration Statement, as amended.
(d) Notwithstanding anything to the contrary contained herein, in no event shall the Company be permitted to name any Purchaser or affiliate of a Purchaser as any Underwriter without the prior written consent of such Purchaser, except as required by law.
3. Registration Procedures.
In connection with the Company’s registration obligations hereunder, the Company shall:
(a) Not less than five (5) Trading Days prior to the filing of each Registration Statement and not less than one (1) Trading Day prior to the filing of any related Prospectus or any amendment or supplement thereto (including any document that would be incorporated or deemed to be incorporated therein by reference, but not including any quarterly report on Form 10-Q, annual report on Form 10-K, proxy statement on Schedule 14A, or other filings with the Commission that do not include information about the Purchaser), the Company shall (i) furnish to each Purchaser copies of all such documents proposed to be filed, which documents (other than those incorporated or deemed to be incorporated by reference) will be subject to the review of such Purchasers, and (ii) cause its officers and directors, counsel and independent registered public accountants to respond to such inquiries as shall be necessary, in the reasonable opinion of respective counsel to the Purchasers, to conduct a reasonable investigation within the meaning of the Securities Act.
The Company shall not file a Registration Statement or any such Prospectus or any amendments or supplements thereto to which the Purchaser shall reasonably object in good faith, provided that, the Company is notified of such objection in writing no later than five (5) Trading Days after the Purchases have been so furnished copies of a Registration Statement or one (1) Trading Day after the Purchasers have been so furnished copies of any related Prospectus or amendments or supplements thereto. Each Purchaser agrees to furnish to the Company a completed questionnaire in the form attached to this Agreement as Annex B (a “Selling Stockholder Questionnaire”) on a date that is not less than two (2) Trading Days prior to the Filing Date or by the end of the fourth (4th) Trading Day following the date on which such Purchaser receives draft materials in accordance with this Section.
(b) (i) Prepare and file with the Commission such amendments, including post-effective amendments, to a Registration Statement and the Prospectus used in connection therewith as may be necessary to keep a Registration Statement continuously effective as to the applicable Registrable Securities for the Effectiveness Period and prepare and file with the Commission such additional Registration Statements in order to register for resale under the Securities Act all of the Registrable Securities, (ii) cause the related Prospectus to be amended or supplemented by any required Prospectus supplement (subject to the terms of this Agreement), and, as so supplemented or amended, to be filed pursuant to Rule 424, (iii) respond as promptly as reasonably possible to any comments received from the Commission with respect to a Registration Statement or any amendment thereto and provide as promptly as reasonably possible to the Purchasers true and complete copies of all correspondence from and to the Commission relating to a Registration Statement (provided that, the Company shall excise any information contained therein which would constitute material non-public information regarding the Company or any of its Subsidiaries), and (iv) comply in all material respects with the applicable provisions of the Securities Act and the Exchange Act with respect to the disposition of all Registrable Securities covered by a Registration Statement during the applicable period in accordance (subject to the terms of this Agreement) with the intended methods of disposition by the Holders thereof set forth in such Registration Statement as so amended or in such Prospectus as so supplemented.
(c) If during the Effectiveness Period, the number of Registrable Securities at any time exceeds 100% of the number of shares of Common Stock constituting Registerable Securities then registered in a Registration Statement, then the Company shall file as soon as reasonably practicable, but in any case prior to the applicable Filing Date, an additional Registration Statement covering the resale by the Purchasers of not less than the number of such Registrable Securities.
(d) Notify the Purchasers of Registrable Securities to be sold (which notice shall, pursuant to clauses (iii) through (vi) hereof, be accompanied by an instruction to suspend the use of the Prospectus until the requisite changes have been made) as promptly as reasonably possible (and, in the case of (i)(A) below, not less than one (1) Trading Day prior to such filing) and (if requested by any such Person) confirm such notice in writing no later than one (1) Trading Day following the day (i)(A) when a Prospectus or any Prospectus supplement or post-effective amendment to a Registration Statement is proposed to be filed, (B) when the Commission notifies the Company whether there will be a “review” of such Registration Statement and whenever the Commission comments in writing on such Registration Statement, and (C) with respect to a Registration Statement or any post-effective amendment, when the same has become effective, (ii) of any request by the Commission or any other federal or state governmental authority for amendments or supplements to a Registration Statement or Prospectus or for additional information, (iii) of the issuance by the Commission or any other federal or state governmental authority of any stop order suspending the effectiveness of a Registration Statement covering any or all of the Registrable Securities or the initiation of any Proceedings for that purpose, (iv) of the receipt by the Company of any notification with respect to the suspension of the qualification or exemption from qualification of any of the Registrable Securities for sale in any jurisdiction, or the initiation or threatening of any Proceeding for such purpose, (v) of the occurrence of any event or passage of time that makes the financial statements included in a Registration Statement ineligible for inclusion therein or any statement made in a Registration Statement or Prospectus or any document incorporated or deemed to be incorporated therein by reference untrue in any material respect or that requires any revisions to a Registration Statement, Prospectus or other documents so that, in the case of a Registration Statement or the Prospectus, as the case may be, it will not contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which they were made, not misleading, and (vi) of the occurrence or existence of any pending corporate development with respect to the Company that the Company believes may be material and that, in the determination of the Company, makes it not in the best interest of the Company to allow continued availability of a Registration Statement or Prospectus; provided, however, that in no event shall any such notice contain any information which would constitute material, non-public information regarding the Company or any of its Subsidiaries, and the Company agrees that the Purchasers shall not, solely as a result of the receipt of such notice, have any duty of confidentiality to the Company or any of its Subsidiaries and shall not, solely as a result of the receipt of such notice, have any duty to the Company or any of its Subsidiaries not to trade on the basis of such information.
(e) Use its best efforts to avoid the issuance of, or, if issued, obtain the withdrawal of (i) any order stopping or suspending the effectiveness of a Registration Statement, or (ii) any suspension of the qualification (or exemption from qualification) of any of the Registrable Securities for sale in any jurisdiction, at the earliest practicable moment.
(f) Furnish to each Purchaser, without charge, at least one conformed copy of each such Registration Statement and each amendment thereto, including financial statements and schedules, all documents incorporated or deemed to be incorporated therein by reference to the extent requested by such Person, and all exhibits to the extent requested by such Person (including those previously furnished or incorporated by reference) promptly after the filing of such documents with the Commission, provided that any such item which is available on the EDGAR system (or successor thereto) need not be furnished in physical form.
(g) Subject to the terms of this Agreement, the Company hereby consents to the use of such Prospectus and each amendment or supplement thereto by each of the Purchasers in connection with the offering and sale of the Registrable Securities covered by such Prospectus and any amendment or supplement thereto, except after the giving of any notice pursuant to Section 3(d).
(h) Prior to any resale of Registrable Securities by a Purchaser, use its commercially reasonable efforts to register or qualify or cooperate with the selling Purchaser in connection with the registration or qualification (or exemption from the Registration or qualification) of such Registrable Securities for the resale by the Purchaser under the securities or Blue Sky laws of such jurisdictions within the United States as any Purchaser reasonably requests in writing, to keep each registration or qualification (or exemption therefrom) effective during the Effectiveness Period and to do any and all other acts or things reasonably necessary to enable the disposition in such jurisdictions of the Registrable Securities covered by each Registration Statement, provided that the Company shall not be required to qualify generally to do business in any jurisdiction where it is not then so qualified, subject the Company to any material tax in any such jurisdiction where it is not then so subject or file a general consent to service of process in any such jurisdiction.
(i) If requested by a Purchaser, cooperate with such Purchaser to facilitate the timely preparation and delivery of certificates representing Registrable Securities to be delivered to a transferee pursuant to a Registration Statement, which certificates shall be free of all restrictive legends, and to enable such Registrable Securities to be in such denominations and registered in such names as any such Purchaser may request.
(j) Upon the occurrence of any event contemplated by Section 3(d), as promptly as reasonably possible under the circumstances taking into account the Company’s good faith assessment of any adverse consequences to the Company and its stockholders of the premature disclosure of such event, prepare a supplement or amendment, including a post-effective amendment, to a Registration Statement or a supplement to the related Prospectus or any document incorporated or deemed to be incorporated therein by reference, and file any other required document so that, as thereafter delivered, neither a Registration Statement nor such Prospectus will contain an untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which they were made, not misleading. If the Company notifies the Purchasers in accordance with clauses (iii) through (vi) of Section 3(d) above to suspend the use of any Prospectus until the requisite changes to such Prospectus have been made, then the Purchasers shall suspend use of such Prospectus. The Company will use its best efforts to ensure that the use of the Prospectus may be resumed as promptly as is practicable. The Company shall be entitled to exercise its right under this Section 3(j) to suspend the availability of a Registration Statement and Prospectus, subject to the payment of liquidated damages otherwise required pursuant to Section 2(d), for a period not to exceed sixty (60) consecutive calendar days or ninety (90) calendar days (which need not be consecutive calendar days) in any 12-month period.
(k) Otherwise use commercially reasonable efforts to comply with all applicable rules and regulations of the Commission under the Securities Act and the Exchange Act, including, without limitation, Rule 172 under the Securities Act, file any final Prospectus, including any supplement or amendment thereof, with the Commission pursuant to Rule 424 under the Securities Act, promptly inform the Purchasers in writing if, at any time during the Effectiveness Period, the Company does not satisfy the conditions specified in Rule 172 and, as a result thereof, the Purchasers are required to deliver a Prospectus in connection with any disposition of Registrable Securities and take such other actions as may be reasonably necessary to facilitate the registration of the Registrable Securities hereunder.
(l) The Company may require the Purchaser to furnish to the Company a certified statement as to the number of shares of Common Stock beneficially owned by the Purchaser and, if required by the Commission, the natural persons thereof that have voting and dispositive control over the shares.
4. Registration Expenses. All fees and expenses incident to the performance of or compliance with this Agreement by the Company shall be borne by the Company whether or not any Registrable Securities are sold pursuant to a Registration Statement. The fees and expenses referred to in the foregoing sentence shall include, without limitation, (i) all registration and filing fees (including, without limitation, fees and expenses of the Company’s counsel and independent registered public accountants) (A) with respect to filings made with the Commission, (B) with respect to filings required to be made with any Trading Market on which the Common Stock is then listed for trading, and (C) in compliance with applicable state securities or Blue Sky laws reasonably agreed to by the Company in writing (including, without limitation, fees and disbursements of counsel for the Company in connection with Blue Sky qualifications or exemptions of the Registrable Securities), (ii) printing expenses (including, without limitation, expenses of printing certificates for Registrable Securities), (iii) messenger, telephone and delivery expenses, (iv) fees and disbursements of counsel for the Company, (v) Securities Act liability insurance, if the Company so desires such insurance, and (vi) fees and expenses of all other Persons retained by the Company in connection with the consummation of the transactions contemplated by this Agreement.
In addition, the Company shall be responsible for all of its internal expenses incurred in connection with the consummation of the transactions contemplated by this Agreement (including, without limitation, all salaries and expenses of its officers and employees performing legal or accounting duties), the expense of any annual audit and the fees and expenses incurred in connection with the listing of the Registrable Securities on any securities exchange as required hereunder. In no event shall the Company be responsible for any broker, selling, underwriting or similar commissions of any Purchaser, any stock transfer expenses, or, except to the extent provided for in the Transaction Documents, any legal fees or other costs of the Purchasers.
5. Indemnification.
(a) Indemnification by the Company. To the extent permitted by applicable law, the Company shall, notwithstanding any termination of this Agreement, indemnify and hold harmless each Purchaser, the officers, directors, members, partners, agents, brokers (including brokers who offer and sell Registrable Securities as principal as a result of a pledge or any failure to perform under a margin call of Common Stock), investment advisors and employees (and any other Persons with a functionally equivalent role of a Person holding such titles, notwithstanding a lack of such title or any other title) of each of them, each Person who controls any such Purchaser (within the meaning of Section 15 of the Securities Act or Section 20 of the Exchange Act) and the officers, directors, members, stockholders, partners, agents and employees (and any other Persons with a functionally equivalent role of a Person holding such titles, notwithstanding a lack of such title or any other title) of each such controlling Person, to the fullest extent permitted by applicable law, from and against any and all losses, claims, damages, liabilities, costs (including, without limitation, reasonable attorneys’ fees) and expenses (collectively, “Losses”), as incurred, arising out of or relating to (1) any untrue or alleged untrue statement of a material fact contained in a Registration Statement, any Prospectus or any form of prospectus or in any amendment or supplement thereto or in any preliminary prospectus, or arising out of or relating to any omission or alleged omission of a material fact required to be stated therein or necessary to make the statements therein (in the case of any Prospectus or supplement thereto, in light of the circumstances under which they were made) not misleading or (2) any violation or alleged violation by the Company of the Securities Act, the Exchange Act or any state securities law, or any rule or regulation thereunder, in connection with the performance of its obligations under this Agreement, except to the extent, but only to the extent, that (i) such untrue statements or omissions are based solely upon information regarding such Purchaser furnished in writing to the Company by such Purchaser expressly for use therein, or to the extent that such information relates to such Purchaser or such Purchaser’s proposed method of distribution of Registrable Securities and was reviewed and expressly approved in writing by such Purchaser expressly for use in a Registration Statement, such Prospectus or in any amendment or supplement thereto (it being understood that the Purchaser has approved Annex A hereto for this purpose) or (ii) in the case of an occurrence of an event of the type specified in Section 3(d)(iii)-(vi), the use by such Purchaser of an outdated, defective or otherwise unavailable Prospectus after the Company has notified such Purchaser in writing that the Prospectus is outdated, defective or otherwise unavailable for use by such Purchaser and prior to the receipt by such Purchaser of the Advice contemplated in Section 6(c), or (iii) in the case of a sale directly by a Purchaser of Registrable Securities, such untrue statement or alleged untrue statement or omission or alleged omission was corrected in a final or amended prospectus, and such Purchaser failed to deliver a copy of the final or amended prospectus at or prior to the confirmation of the sale of the Registrable Securities to the Person asserting any such loss, claim, damage or liability in any case in which such delivery is required by the Securities Act.
The indemnity agreement contained in this section shall not apply to amounts paid in settlement of any loss, claim, damage, liability or action if such settlement is effected without the prior written consent of the Company (which consent shall not be unreasonably withheld or delayed). The Company shall notify the Purchasers promptly of the institution, threat or assertion of any Proceeding arising from or in connection with the transactions contemplated by this Agreement of which the Company is aware. Such indemnity shall remain in full force and effect regardless of any investigation made by or on behalf of such indemnified person and shall survive the transfer of any Registrable Securities by any of the Purchasers in accordance with Section 6(f).
(b) Indemnification by Purchasers. Each Purchaser shall, severally and not jointly, indemnify and hold harmless the Company, its directors, officers, agents and employees, each Person who controls the Company (within the meaning of Section 15 of the Securities Act and Section 20 of the Exchange Act), and the directors, officers, agents or employees of such controlling Persons, to the fullest extent permitted by applicable law, from and against all Losses, as incurred, to the extent arising out of or based solely upon: any untrue or alleged untrue statement of a material fact contained in any Registration Statement, any Prospectus, or in any amendment or supplement thereto or in any preliminary prospectus, or arising out of or relating to any omission or alleged omission of a material fact required to be stated therein or necessary to make the statements therein (in the case of any Prospectus or supplement thereto, in light of the circumstances under which they were made) not misleading (i) to the extent, but only to the extent, that such untrue statement or omission is contained in any information so furnished in writing by such Purchaser to the Company expressly for inclusion in such Registration Statement or such Prospectus or (ii) to the extent, but only to the extent, that such information relates to such Purchaser’s information provided in the Selling Stockholder Questionnaire or the proposed method of distribution of Registrable Securities and was reviewed and expressly approved in writing by such Purchaser expressly for use in a Registration Statement (it being understood that the Purchaser has approved Annex A hereto for this purpose), such Prospectus or in any amendment or supplement thereto.
In no event shall the liability of a selling Purchaser be greater in amount than the dollar amount of the proceeds (net of all expenses paid by such Purchaser in connection with any claim relating to this Section 5 and the amount of any damages such Purchaser has otherwise been required to pay by reason of such untrue statement or omission) received by such Purchaser upon the sale of the Registrable Securities included in the Registration Statement giving rise to such indemnification obligation.
(c) Conduct of Indemnification Proceedings. If any Proceeding shall be brought or asserted against any Person entitled to indemnity hereunder (an “Indemnified Party”), such Indemnified Party shall promptly notify the Person from whom indemnity is sought (the “Indemnifying Party”) in writing, and the Indemnifying Party shall have the right to assume the defense thereof, including the employment of counsel reasonably satisfactory to the Indemnified Party and the payment of all fees and expenses incurred in connection with defense thereof, provided that the failure of any Indemnified Party to give such notice shall not relieve the Indemnifying Party of its obligations or liabilities pursuant to this Agreement, except (and only) to the extent that it shall be finally determined by a court of competent jurisdiction (which determination is not subject to appeal or further review) that such failure shall have materially and adversely prejudiced the Indemnifying Party.
An Indemnified Party shall have the right to employ separate counsel in any such Proceeding and to participate in the defense thereof, but the fees and expenses of such counsel shall be at the expense of such Indemnified Party or Parties unless: (1) the Indemnifying Party has agreed in writing to pay such fees and expenses, (2) the Indemnifying Party shall have failed promptly to assume the defense of such Proceeding and to employ counsel reasonably satisfactory to such Indemnified Party in any such Proceeding, or (3) the named parties to any such Proceeding (including any impleaded parties) include both such Indemnified Party and the Indemnifying Party, and counsel to the Indemnified Party shall reasonably believe that a material conflict of interest is likely to exist if the same counsel were to represent such Indemnified Party and the Indemnifying Party (in which case, if such Indemnified Party notifies the Indemnifying Party in writing that it elects to employ separate counsel at the expense of the Indemnifying Party, the Indemnifying Party shall not have the right to assume the defense thereof and the reasonable fees and expenses of no more than one separate counsel shall be at the expense of the Indemnifying Party). The Indemnifying Party shall not be liable for any settlement of any such Proceeding effected without its written consent, which consent shall not be unreasonably withheld or delayed. No Indemnifying Party shall, without the prior written consent of the Indemnified Party, effect any settlement of any pending Proceeding in respect of which any Indemnified Party is a party, unless such settlement includes an unconditional release of such Indemnified Party from all liability on claims that are the subject matter of such Proceeding.
Subject to the terms of this Agreement, all reasonable fees and expenses of the Indemnified Party (including reasonable fees and expenses to the extent incurred in connection with investigating or preparing to defend such Proceeding in a manner not inconsistent with this Section) shall be paid to the Indemnified Party, as incurred, within ten Trading Days of written notice thereof to the Indemnifying Party, provided that the Indemnified Party shall promptly reimburse the Indemnifying Party for that portion of such fees and expenses applicable to such actions for which such Indemnified Party is finally determined by a court of competent jurisdiction (which determination is not subject to appeal or further review) not to be entitled to indemnification hereunder.
(d) Contribution. If the indemnification under Section 5(a) or 5(b) is unavailable to an Indemnified Party or insufficient to hold an Indemnified Party harmless for any Losses, then each Indemnifying Party shall contribute to the amount paid or payable by such Indemnified Party, in such proportion as is appropriate to reflect the relative fault of the Indemnifying Party and Indemnified Party in connection with the actions, statements or omissions that resulted in such Losses as well as any other relevant equitable considerations. The relative fault of such Indemnifying Party and Indemnified Party shall be determined by reference to, among other things, whether any action in question, including any untrue or alleged untrue statement of a material fact or omission or alleged omission of a material fact, has been taken or made by, or relates to information supplied by, such Indemnifying Party or Indemnified Party, and the parties’ relative intent, knowledge, access to information and opportunity to correct or prevent such action, statement or omission. The amount paid or payable by a party as a result of any Losses shall be deemed to include, subject to the limitations set forth in this Agreement, any reasonable attorneys’ or other fees or expenses incurred by such party in connection with any Proceeding to the extent such party would have been indemnified for such fees or expenses if the indemnification provided for in this Section was available to such party in accordance with its terms.
The parties hereto agree that it would not be just and equitable if contribution pursuant to this Section 5(d) were determined by pro rata allocation or by any other method of allocation that does not take into account the equitable considerations referred to in the immediately preceding paragraph. In no event shall the contribution obligation of a Purchaser be greater in amount than the dollar amount of the proceeds (net of all expenses paid by such Purchaser in connection with any claim relating to this Section 5 and the amount of any damages such Purchaser has otherwise been required to pay by reason of such untrue or alleged untrue statement or omission or alleged omission) received by it upon the sale of the Registrable Securities giving rise to such contribution obligation.
The indemnity and contribution agreements contained in this Section are in addition to any liability that the Indemnifying Parties may have to the Indemnified Parties.
6. Miscellaneous.
(a) Remedies. In the event of a breach by the Company or by a Purchaser of any of their respective obligations under this Agreement, each Purchaser or the Company, as the case may be, in addition to being entitled to exercise all rights granted by law and under this Agreement, including recovery of damages, shall be entitled to specific performance of its rights under this Agreement. Each of the Company and each Purchaser agrees that monetary damages would not provide adequate compensation for any losses incurred by reason of a breach by it of any of the provisions of this Agreement and hereby further agrees that, in the event of any action for specific performance in respect of such breach, it shall not assert or shall waive the defense that a remedy at law would be adequate.
(b) Discontinued Disposition. By its acquisition of Registrable Securities, each Purchaser agrees that, upon receipt of a notice from the Company of the occurrence of any event of the kind described in Section 3(d)(iii) through (vi), each Purchaser will forthwith discontinue disposition of such Registrable Securities under a Registration Statement until it is advised in writing (the “Advice”) by the Company that the use of the applicable Prospectus (as it may have been supplemented or amended) may be resumed. The Company will use its best efforts to ensure that the use of the Prospectus may be resumed as promptly as is practicable.
(c) Amendments and Waivers. The provisions of this Agreement, including the provisions of this sentence, may not be amended, modified or supplemented, and waivers or consents to departures from the provisions hereof may not be given, unless the same shall be in writing and signed by the Company and each Purchaser. No consideration shall be offered or paid to any Person to amend or consent to a waiver or modification of any provision of this Agreement unless the same consideration also is offered to all of the parties to this Agreement.
(d) Notices. Any and all notices or other communications or deliveries required or permitted to be provided hereunder shall be delivered as set forth in the Registered Direct Purchase Agreement.
(e) Successors and Assigns. This Agreement shall inure to the benefit of and be binding upon the successors and permitted assigns of each of the parties and shall inure to the benefit of each Purchaser. The Company may not assign (except by merger) its rights or obligations hereunder without the prior written consent of the Purchasers. Each Purchaser may assign their respective rights hereunder in the manner and to the Persons as permitted under Section 5.7 of the Registered Direct Purchase Agreement.
(f) No Inconsistent Agreements. Neither the Company nor any of its Subsidiaries has entered, as of the date hereof, nor shall the Company or any of its Subsidiaries, on or after the date of this Agreement, enter into any agreement with respect to its securities, that would have the effect of impairing the rights granted to the Purchasers in this Agreement or otherwise conflicts with the provisions hereof. Except as set forth on Schedule 6(f), neither the Company nor any of its Subsidiaries has previously entered into any agreement granting any registration rights with respect to any of its securities to any Person that have not been satisfied in full.
(g) Execution and Counterparts. This Agreement may be executed in two or more counterparts, all of which when taken together shall be considered one and the same agreement and shall become effective when counterparts have been signed by each party and delivered to the other party, it being understood that both parties need not sign the same counterpart. In the event that any signature is delivered by e-mail delivery of a “.pdf” format data file or any electronic signature complying with the U.S. federal ESIGN Act of 2000 (e.g., www.docusign.com), such signature shall create a valid and binding obligation of the party executing (or on whose behalf such signature is executed) with the same force and effect as if such “.pdf” signature page were an original thereof.
(h) Governing Law. All questions concerning the construction, validity, enforcement and interpretation of this Agreement shall be determined in accordance with the provisions of the Registered Direct Purchase Agreement.
(i) Cumulative Remedies. The remedies provided herein are cumulative and not exclusive of any other remedies provided by law.
(j) Severability. If any term, provision, covenant or restriction of this Agreement is held by a court of competent jurisdiction to be invalid, illegal, void or unenforceable, the remainder of the terms, provisions, covenants and restrictions set forth herein shall remain in full force and effect and shall in no way be affected, impaired or invalidated, and the parties hereto shall use their commercially reasonable efforts to find and employ an alternative means to achieve the same or substantially the same result as that contemplated by such term, provision, covenant or restriction. It is hereby stipulated and declared to be the intention of the parties that they would have executed the remaining terms, provisions, covenants and restrictions without including any of such that may be hereafter declared invalid, illegal, void or unenforceable.
(k) Headings. The headings in this Agreement are for convenience only, do not constitute a part of the Agreement and shall not be deemed to limit or affect any of the provisions hereof.
********************
(Signature Pages Follow)
IN WITNESS WHEREOF, the parties have executed this Registration Rights Agreement as of the date first written above.
| BENITEC BIOPHARMA INC. |
||
| By: |
/s/ Jerel Banks |
|
| Name: |
Dr. Jerel Banks |
|
| Title: |
Chief Executive Officer |
|
[SIGNATURE PAGE OF PURCHASERS FOLLOWS]
[Company signature page to RRA]
[SIGNATURE PAGE OF PURCHASERS TO BNTC RRA]
Name of Purchaser: Averill Master Fund, Ltd.
Signature of Authorized Signatory of Purchaser: /s/ Andrew Nathanson
Name of Authorized Signatory: Andrew Nathanson
Title of Authorized Signatory: Authorized Signatory
[SIGNATURE PAGES CONTINUE]
[signature page to RRA]
[SIGNATURE PAGE OF PURCHASERS TO BNTC RRA]
Name of Purchaser: Averill Madison Master Fund, Ltd.
Name of Authorized Signatory: Andrew Nathanson
Title of Authorized Signatory: Authorized Signatory
[SIGNATURE PAGES CONTINUE]
[signature page to RRA]
Annex A
Plan of Distribution
Signature of Authorized Signatory of Purchaser: /s/ Andrew Nathanson Each Selling Stockholder (the “Selling Stockholders”) of the securities and any of their pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their securities covered hereby on the principal Trading Market or any other stock exchange, market or trading facility on which the securities are traded or in private transactions. These sales may be at fixed or negotiated prices. A Selling Stockholder may use any one or more of the following methods when selling securities:
| • | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| • | block trades in which the broker-dealer will attempt to sell the securities as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| • | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| • | an exchange distribution in accordance with the rules of the applicable exchange; |
| • | privately negotiated transactions; |
| • | settlement of short sales; |
| • | in transactions through broker-dealers that agree with the Selling Stockholders to sell a specified number of such securities at a stipulated price per security; |
| • | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| • | a combination of any such methods of sale; or |
| • | any other method permitted pursuant to applicable law. |
The Selling Stockholders may also sell securities under Rule 144 or any other exemption from registration under the Securities Act of 1933, as amended (the “Securities Act”), if available, rather than under this prospectus.
Broker-dealers engaged by the Selling Stockholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the Selling Stockholders (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser) in amounts to be negotiated, but, except as set forth in a supplement to this Prospectus, in the case of an agency transaction not in excess of a customary brokerage commission in compliance with FINRA Rule 2121; and in the case of a principal transaction a markup or markdown in compliance with FINRA Rule 2121.
In connection with the sale of the securities or interests therein, the Selling Stockholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they assume. The Selling Stockholders may also sell securities short and deliver these securities to close out their short positions, or loan or pledge the securities to broker-dealers that in turn may sell these securities. The Selling Stockholders may also enter into option or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which require the delivery to such broker-dealer or other financial institution of securities offered by this prospectus, which securities such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The Selling Stockholders and any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Each Selling Stockholder has informed the Company that it does not have any written or oral agreement or understanding, directly or indirectly, with any person to distribute the securities.
The Company is required to pay certain fees and expenses incurred by the Company incident to the registration of the securities. The Company has agreed to indemnify the Selling Stockholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
We agreed to keep this prospectus effective until the earlier of (i) the date on which the securities may be resold by the Selling Stockholders without registration and without regard to any volume or manner-of-sale limitations by reason of Rule 144, without the requirement for the Company to be in compliance with the current public information under Rule 144 under the Securities Act or any other rule of similar effect or (ii) all of the securities have been sold pursuant to this prospectus or Rule 144 under the Securities Act or any other rule of similar effect. The resale securities will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in certain states, the resale securities covered hereby may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
Under applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously engage in market making activities with respect to the common stock for the applicable restricted period, as defined in Regulation M, prior to the commencement of the distribution.
In addition, the Selling Stockholders will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of the common stock by the Selling Stockholders or any other person. We will make copies of this prospectus available to the Selling Stockholders and have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including by compliance with Rule 172 under the Securities Act).
Annex B
SELLING STOCKHOLDERS
The common stock being offered by the selling stockholders are those previously issued to the selling stockholders, and those issuable to the selling stockholders, upon exercise of the pre-funded warrants. For additional information regarding the issuances of those shares of common stock and warrants, see “Private Placement of Shares of Common Stock and Warrants” above. We are registering the shares of common stock in order to permit the selling stockholders to offer the shares for resale from time to time.
The table below lists the selling stockholders and other information regarding the beneficial ownership of the shares of common stock by each of the selling stockholders. The second column lists the number of shares of common stock beneficially owned by each selling shareholder, based on its ownership of the shares of common stock and pre-funded warrants, as of ________, 2025, assuming exercise of the pre-funded warrants held by the selling stockholders on that date, without regard to any limitations on exercises.
The third column lists the shares of common stock being offered by this prospectus by the selling stockholders.
In accordance with the terms of a registration rights agreement with the selling stockholders, this prospectus generally covers the resale of the sum of (i) the number of shares of common stock issued to the selling stockholders in the “Private Placement of Shares of Common Stock and Warrants” described above and (ii) the maximum number of shares of common stock issuable upon exercise of the related pre-funded warrants, determined as if the outstanding pre-funded warrants were exercised in full as of the trading day immediately preceding the date this registration statement was initially filed with the SEC, each as of the trading day immediately preceding the applicable date of determination and all subject to adjustment as provided in the registration right agreement, without regard to any limitations on the exercise of the warrants. The fourth column assumes the sale of all of the shares offered by the selling stockholders pursuant to this prospectus.
Under the terms of the pre-funded warrants, a selling stockholder may not exercise the warrants to the extent such exercise would cause such selling stockholder, together with its affiliates and attribution parties, to beneficially own a number of shares of common stock which would exceed 4.99%, 9.99% or 19.99%, as applicable, of our then outstanding common stock following such exercise, excluding for purposes of such determination shares of common stock issuable upon exercise of such pre-funded warrants which have not been exercised. The number of shares in the second and fourth columns do not reflect this limitation. The selling stockholders may sell all, some or none of their shares in this offering. See “Plan of Distribution.”
| Name of Selling Stockholder |
Number of shares of Common Stock Owned Prior to Offering |
Maximum Number of shares of Common Stock to be Sold Pursuant to this Prospectus |
Number of shares of Common Stock Owned After Offering |
Annex C
BENITEC BIOPHARMA INC.
Selling Stockholder Notice and Questionnaire
The undersigned beneficial owner of common stock (the “Registrable Securities”) of Benitec Biopharma Inc., a Delaware corporation (the “Company”), understands that the Company has filed or intends to file with the Securities and Exchange Commission (the “Commission”) a registration statement (the “Registration Statement”) for the registration and resale under Rule 415 of the Securities Act of 1933, as amended (the “Securities Act”), of the Registrable Securities, in accordance with the terms of the Registration Rights Agreement (the “Registration Rights Agreement”) to which this document is annexed. A copy of the Registration Rights Agreement is available from the Company upon request at the address set forth below. All capitalized terms not otherwise defined herein shall have the meanings ascribed thereto in the Registration Rights Agreement.
Certain legal consequences arise from being named as a selling stockholder in the Registration Statement and the related prospectus. Accordingly, holders and beneficial owners of Registrable Securities are advised to consult their own securities law counsel regarding the consequences of being named or not being named as a selling stockholder in the Registration Statement and the related prospectus.
NOTICE
The undersigned beneficial owner (the “Selling Stockholder”) of Registrable Securities hereby elects to include the Registrable Securities owned by it in the Registration Statement.
The undersigned hereby provides the following information to the Company and represents and warrants that such information is accurate:
QUESTIONNAIRE
| 1. | Name. |
| (a) | Full Legal Name of Selling Stockholder |
|
|
| (b) | Full Legal Name of Registered Holder (if not the same as (a) above) through which Registrable Securities are held: |
|
|
| (c) | Full Legal Name of Natural Control Person (which means a natural person who directly or indirectly alone or with others has power to vote or dispose of the securities covered by this Questionnaire): |
|
|
| 2. | Address for Notices to Selling Stockholder. |
|
|
|
|
|
|
| Telephone: |
|
|
| E-Mail: |
|
|
| Contact Person: |
| 3. | Broker-Dealer Status. |
| (a) | Are you a broker-dealer? |
Yes ☐ No ☐
| (b) | If “yes” to Section 3(a), did you receive your Registrable Securities as compensation for investment banking services to the Company? |
Yes ☐ No ☐
| Note: | If “no” to Section 3(b), the Commission’s staff has indicated that you should be identified as an underwriter in the Registration Statement. |
| (c) | Are you an affiliate of a broker-dealer? |
Yes ☐ No ☐
| (d) | If you are an affiliate of a broker-dealer, do you certify that you purchased the Registrable Securities in the ordinary course of business, and at the time of the purchase of the Registrable Securities to be resold, you had no agreements or understandings, directly or indirectly, with any person to distribute the Registrable Securities? |
Yes ☐ No ☐
| Note: | If “no” to Section 3(d), the Commission’s staff has indicated that you should be identified as an underwriter in the Registration Statement. |
| 4. | Beneficial Ownership of Securities of the Company Owned by the Selling Stockholder. |
Except as set forth below in this Item 4, the undersigned is not the beneficial or registered owner of any securities of the Company other than the securities issuable pursuant to the Purchase Agreement.
| (a) | Type and Amount of other securities beneficially owned by the Selling Stockholder: |
|
|
|
|
| 5. | Organizational Structure. |
Please indicate or (if applicable) describe how the selling securityholder is organized.
Is the selling securityholder a natural person?
(If so, please mark the box and skip to the next question)
Yes ☐ No ☐
Is the selling securityholder a reporting company under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)? Is the selling securityholder a majority-owned subsidiary of a reporting company under the Exchange Act?
(If so, please mark the box and skip to the next question)
Yes ☐ No ☐
(If so, please mark the box and skip to the next question)
Yes ☐ No ☐
Is the selling securityholder a registered investment company under the Investment Company Act of 1940?
(If so, please mark the box and skip to the next question)
Yes ☐ No ☐
If the answer to all of the foregoing questions is “no,” please describe: (i) the exact legal description of the selling securityholder (e.g., corporation, partnership, limited liability company, etc.); (ii) whether the legal entity so described is managed by another entity and the exact legal description of such entity (repeat this step until the last entity described is managed by a person or persons, each of whom is described in any one of (a) through (d) above); (iii) the names of each person or persons having voting and investment control over the Company’s securities that the entity owns (e.g., director(s), general partner(s), managing member(s), etc.).
| (a) |
Legal Description of Entity: |
|
|
|
||
| (b) |
Name of Entit(ies)/(y) Managing Such Entity (if any): |
|
|
|
||
|
|
||
| (c) |
Name of Entit(ies)/(y) Managing such Entit(ies)/(y) (if any): |
|
|
|
||
|
|
||
| (d) |
Name(s) of Natural Person(s) Having Voting or Investment Control Over the Shares Held by such Entit(ies)/(y): |
|
|
|
||
| 6. | Relationships with the Company. |
Except as set forth below, neither the undersigned nor any of its affiliates, officers, directors or principal equity holders (owners of 5% of more of the equity securities of the undersigned) has held any position or office or has had any other material relationship with the Company (or its predecessors or affiliates) during the past three years.
State any exceptions here:
|
|
|
|
| 7. | Reliance on Responses. |
The undersigned acknowledges and agrees that the Company and its legal counsel shall be entitled to rely on its responses in this Questionnaire in all matters pertaining to the Registration Statement and the sale of any Registrable Securities pursuant to the Registration Statement.
The undersigned hereby acknowledges and is advised of the SEC’s Compliance and Disclosure Interpretation 239.10 regarding short selling:
An Issuer filed a Form S-3 registration statement for a secondary offering of common stock which is not yet effective. One of the selling shareholders wanted to do a short sale of common stock “against the box” and cover the short sale with registered shares after the effective date. The issuer was advised that the short sale could not be made before the registration statement become effective, because the shares underlying the short sale are deemed to be sold at the time such sale is made. There would, therefore, be a violation of Section 5 if the shares were effectively sold prior to the effective date.
The undersigned agrees to promptly notify the Company of any material inaccuracies or changes in the information provided herein that may occur subsequent to the date hereof at any time while the Registration Statement remains effective; provided, that the undersigned shall not be required to notify the Company of any changes to the number of securities held or owned by the undersigned or its affiliates.
By signing below, the undersigned consents to the disclosure of the information contained herein in its answers to Items 1 through 5 and the inclusion of such information in the Registration Statement and the related prospectus and any amendments or supplements thereto. The undersigned understands that such information will be relied upon by the Company in connection with the preparation or amendment of the Registration Statement and the related prospectus and any amendments or supplements thereto.
IN WITNESS WHEREOF the undersigned, by authority duly given, has caused this Notice and Questionnaire to be executed and delivered either in person or by its duly authorized agent.
|
Date: |
Beneficial Owner: |
|||||
|
|
||||||
| By: |
||||||
|
|
||||||
| Name: |
||||||
| Title: |
||||||
PLEASE EMAIL A .PDF COPY OF THE COMPLETED AND EXECUTED NOTICE AND QUESTIONNAIRE TO: [***]
EXHIBIT 31.1
Statement Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 by
Principal Executive Officer
Regarding Facts and Circumstances Relating to Exchange Act Filings
I, Jerel Banks, certify that:
| 1. | I have reviewed this quarterly report on Form 10-Q of Benitec Biopharma Inc.; |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
| 4. | The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
| a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| c) | Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| d) | Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
| 5. | The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
| a) | all significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
| b) | any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
Date: November 14, 2025
| /s/ Jerel Banks |
| Jerel Banks |
| Executive Chairman and Chief Executive Officer |
EXHIBIT 31.2
Statement Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 by
Principal Financial Officer
Regarding Facts and Circumstances Relating to Exchange Act Filings
I, Megan Boston, certify that:
| 1. | I have reviewed this quarterly report on Form 10-Q of Benitec Biopharma Inc.; |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
| 4. | The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
| a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| c) | Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| d) | Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
| 5. | The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions): |
| a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
| b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
Date: November 14, 2025
| /s/ Megan Boston |
| Megan Boston |
| Chief Financial Officer and Secretary (principal financial and accounting officer) |
EXHIBIT 32.1
Statement Pursuant to Section 906 the Sarbanes-Oxley Act of 2002
By
Principal Executive Officer
Regarding Facts and Circumstances Relating to Exchange Act Filings
Dated: November 14, 2025
I, Jerel Banks, Chief Executive Officer of Benitec Biopharma Inc., hereby certify, to my knowledge, that:
| 1. | the accompanying Quarterly Report on Form 10-Q of Benitec Biopharma Inc. for the three month period ended September 30, 2025 (the “Report”) fully complies with the requirements of Section 13(a) or 15(d), as applicable, of the Securities and Exchange Act of 1934, as amended; and |
| 2. | the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of Benitec Biopharma Inc. |
IN WITNESS WHEREOF, the undersigned has executed this Statement as of the date first written above.
| /s/ Jerel Banks |
| Jerel Banks |
| Executive Chairman and Chief Executive Officer |
EXHIBIT 32.2
Statement Pursuant to Section 906 the Sarbanes-Oxley Act of 2002
By
Principal Financial Officer
Regarding Facts and Circumstances Relating to Exchange Act Filings
Dated: November 14, 2025
I, Megan Boston, Chief Financial Officer (principal accounting officer) of Benitec Biopharma Inc., hereby certify, to my knowledge, that:
| 1. | the accompanying Quarterly Report on Form 10-Q of Benitec Biopharma Inc. for the three month period ended September 30, 2025 (the “Report”) fully complies with the requirements of Section 13(a) or 15(d), as applicable, of the Securities and Exchange Act of 1934, as amended; and |
| 2. | the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of Benitec Biopharma Inc. |
IN WITNESS WHEREOF, the undersigned has executed this Statement as of the date first written above.
| /s/ Megan Boston |
| Megan Boston |
| Chief Financial Officer and Secretary (principal financial and accounting officer) |