UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported)
November 13, 2025
ORIC Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 001-39269 | 47-1787157 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
240 E. Grand Ave, 2nd Floor
South San Francisco, CA 94080
(Address of principal executive offices, including zip code)
(650) 388-5600
(Registrant’s telephone number, including area code)
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange on which registered |
||
| Common stock, par value $0.0001 per share | ORIC | The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒ ORIC Pharmaceuticals, Inc. (the “Company”) intends to include updated ORIC-944 data slides (the “Updated Corporate Presentation Slides”) in its corporate deck for use in future investor presentations. A copy of the Updated Corporate Presentation Slides is furnished as Exhibit 99.1 hereto and is incorporated herein by reference.
| Item 7.01 | Regulation FD Disclosure. |
All of the information furnished in this Item 7.01 and Item 9.01 (including Exhibit 99.1) shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits.
| Exhibit Number |
Description |
|
| 99.1 | Updated Corporate Presentation Slides | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| ORIC PHARMACEUTICALS, INC. | ||||||
| Date: November 13, 2025 | By: | /s/ Christian V. Kuhlen |
||||
| Christian V. Kuhlen, M.D., J.D. | ||||||
| General Counsel | ||||||

ORIC-944 Dose Exploration Data Update November 13, 2025 Exhibit 99.1

Forward-Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements other than statements of historical facts contained in this presentation, including statements regarding ORIC Pharmaceuticals, Inc.’s (“ORIC”, “we”, “us” or “our”) future financial condition, results of operations, business strategy and plans, and objectives of management for future operations, as well as statements regarding industry trends, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potentially,” “predict,” “should,” “will” or the negative of these terms or other similar expressions. Forward-looking statements contained in this presentation also include, but are not limited to, statements regarding: the continued clinical development of ORIC-944; the potential best-in-class properties of ORIC-944; our development plans and timelines for ORIC-944; the potential advantages of ORIC-944; plans underlying ORIC-944’s clinical trials and development; clinical outcomes from combination studies with ORIC-944, which may materially change as patient enrollment continues or more patient data becomes available; and the expected timing of reporting data from our clinical trials. We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including, among other things: the timing of the initiation, progress and results of our preclinical studies and clinical trials; risks associated with the process of developing and commercializing drugs that are safe and effective for use in humans and operating as an early clinical stage company; negative impacts of health emergencies, economic instability or international conflicts on our operations, including clinical trials; the potential for current or future clinical trials of product candidates to differ from preclinical, initial, interim, preliminary or expected results; our ability to advance product candidates into, and successfully complete, clinical trials; the timing or likelihood of regulatory filings and approvals; changes in our plans to develop and commercialize our product candidates; our estimates of the number of patients who suffer from the diseases we are targeting and the number of patients that may enroll in our clinical trials; the commercializing of our product candidates, if approved; our ability to successfully manufacture and supply our product candidates for clinical trials and for commercial use, if approved; potential benefits and costs of strategic arrangements, licensing and/or collaborations; the risk of the occurrence of any event, change or other circumstance that could give rise to the termination of our license or collaboration agreements; our estimates regarding expenses, future revenue, capital requirements and needs for financing and our ability to obtain capital; the sufficiency of our existing cash and investments to fund our future operating expenses and capital expenditure requirements; our ability to retain the continued service of our key personnel and to identify, hire and retain additional qualified professionals; the implementation of our business model and strategic plans for our business and product candidates; the scope of protection we are able to establish and maintain for intellectual property rights, product candidates and our pipeline; our ability to contract with third-party contract research organizations, suppliers and manufacturers and their ability to perform adequately; the pricing, coverage and reimbursement of our product candidates, if approved; developments relating to our competitors and our industry, including competing product candidates and therapies; regulatory developments in the United States and foreign countries; general economic and market conditions; and the other risks, uncertainties and assumptions discussed in the public filings we have made and will make with the Securities and Exchange Commission (“SEC”). These risks are not exhaustive. New risk factors emerge from time to time and it is not possible for our management to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data and estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Except as required by law, we undertake no obligation to update any statements in this presentation for any reason after the date of this presentation. We have filed Current Reports on Form 8-K, Quarterly Reports on Form 10-Q, Annual Reports on Form 10-K, and other documents with the SEC. You should read these documents for more complete information about us. You may obtain these documents for free by visiting EDGAR on the SEC website at www.sec.gov. This presentation discusses our product candidates that are under preclinical or clinical study, and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of our product candidates for the therapeutic use for which they are being studied.

ORIC-944 Advanced into Combination Development with AR Inhibitors Key Eligibility Patients with mCRPC Previously treated with an ARPI (e.g., abiraterone, enzalutamide, apalutamide, or darolutamide) May have received up to 1 line of chemotherapy Phase 1b, Multicenter, Open-Label Trial (in Collaboration with Johnson & Johnson and Bayer) Candidate RP2Ds ORIC-944 Combination Dose Optimization ORIC-944 Combination Dose Exploration Note:ClinicalTrials.gov identifier: NCT05413421. RP2D – recommended Phase 2 dose; rPFS – radiographic progression free survival; ORR – objective response rate; DOR – duration of response: PSA – prostate-specific antigen. Abiraterone refers to abiraterone acetate. Primary endpoints: Safety and Recommended Phase 2 Dose Key secondary endpoints: rPFS, ORR, and DOR Exploratory endpoints: PSA, ctDNA, H3K27 trimethylation, PRC2 target gene expression, and genomics Dose exploration update (November 2025) includes 20 patients, 17 of which were previously disclosed in May 2025 Prior enza, apa, daro Prior abiraterone Prior enza, apa, daro ORIC-944 (QD) + darolutamide (600 mg BID) ORIC-944 (QD) + apalutamide (240 mg QD) ORIC-944 (QD) + darolutamide (600 mg BID) Prior abiraterone ORIC-944 (QD) + apalutamide (240 mg QD) November 2025 Update Ongoing

ORIC-944 Phase 1b Combination Dose Exploration in Previously Treated Patients with mCRPC Patients previously treated with a median of three prior therapies, including abiraterone and a variety of other approved and investigational treatment regimens Demographics and Baseline Characteristics Characteristic ORIC-944 + Apalutamide or Darolutamide (N=20) Age, median (range), years 71 (48-85) Race, White / Black or African American / Asian, % 70 / 25 / 5 ECOG Performance Status, 0 / 1, n (%) 12 (60) / 8 (40) Gleason Score, <8 / ≥8, n (%) 4 (20) / 15 (75) Prior lines of therapy, median (range) 3 (2-5) Prior systemic therapy, n (%) Abiraterone Docetaxel Immune therapy PARP inhibitor Radium 223 Other 20 (100) 7 (35) 7 (35) 2 (10) 2 (10) 6 (30) Metastases at baseline, n (%) Bone Lymph nodes Visceral 17 (85) 8 (40) 3 (15) Note:Data as of September 22, 2025. Includes 17 patients previously disclosed in May 2025. Gleason score was missing for one (5%) patient. Immune therapy includes nivolumab, pembrolizumab, atezolizumab, and sipuleucel-T. Other therapies include abemaciclib, cabozantinib, CYP11A, and KLK2 CAR-T. Excludes three patients previously treated with lutetium 177. Number of prior lines of therapy excludes androgen deprivation therapy or first-generation androgen receptor deprivation therapy. Abiraterone refers to abiraterone acetate.
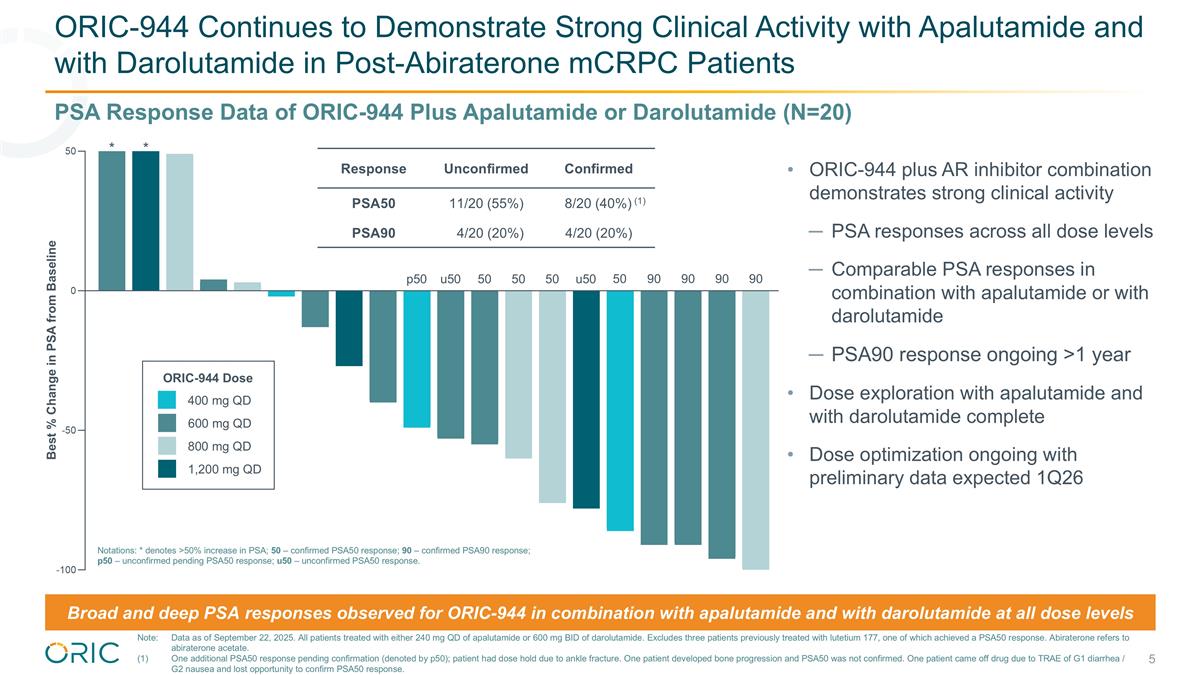
ORIC-944 Continues to Demonstrate Strong Clinical Activity with Apalutamide and with Darolutamide in Post-Abiraterone mCRPC Patients PSA Response Data of ORIC-944 Plus Apalutamide or Darolutamide (N=20) Response Unconfirmed Confirmed PSA50 11/20 (55%) 8/20 (40%) (1) PSA90 4/20 (20%) 4/20 (20%) 400 mg QD 600 mg QD ORIC-944 Dose 800 mg QD 1,200 mg QD ORIC-944 plus AR inhibitor combination demonstrates strong clinical activity PSA responses across all dose levels Comparable PSA responses in combination with apalutamide or with darolutamide PSA90 response ongoing >1 year Dose exploration with apalutamide and with darolutamide complete Dose optimization ongoing with preliminary data expected 1Q26 Broad and deep PSA responses observed for ORIC-944 in combination with apalutamide and with darolutamide at all dose levels 90 90 90 90 50 50 50 50 p50 Notations: * denotes >50% increase in PSA; 50 – confirmed PSA50 response; 90 – confirmed PSA90 response; p50 – unconfirmed pending PSA50 response; u50 – unconfirmed PSA50 response. Note:Data as of September 22, 2025. All patients treated with either 240 mg QD of apalutamide or 600 mg BID of darolutamide. Excludes three patients previously treated with lutetium 177, one of which achieved a PSA50 response. Abiraterone refers to abiraterone acetate. (1)One additional PSA50 response pending confirmation (denoted by p50); patient had dose hold due to ankle fracture. One patient developed bone progression and PSA50 was not confirmed. One patient came off drug due to TRAE of G1 diarrhea / G2 nausea and lost opportunity to confirm PSA50 response. u50 u50 Best % Change in PSA from Baseline * *

ctDNA Analysis Reveals Impressive Molecular Response for ORIC-944 Plus Apalutamide or Darolutamide in Post-Abiraterone mCRPC Patients ctDNA Response Data of ORIC-944 Plus Apalutamide or Darolutamide (n=17) (1) Note:Data as of September 22, 2025. Waterfall plot displays patients previously treated with abiraterone. Abiraterone refers to abiraterone acetate. Germline, predicted clonal hematopoiesis, and synonymous somatic variants were excluded from ctDNA analysis. (1)From the 20-patient data-cut, ctDNA data are included for 17 patients, with 2 patients lacking samples for ctDNA assessment and 1 patient without evidence of ctDNA at baseline. Patients denoted by asterisk had a ctDNA fraction <0.5% at baseline, which cleared by C2D1. Rapid and deep ctDNA reductions observed for ORIC-944 in combination with darolutamide and with apalutamide, across breadth of AR mutations and other tumor alterations Impressive ctDNA responses across all dose levels and at comparable rates in combination with apalutamide or with darolutamide Deep ctDNA reductions for the vast majority of patients, with 76% of patients demonstrating >50% ctDNA reduction ctDNA responses in breadth of genotypes including tumors with: AR mutations, AR amplified, AR wildtype Tumor suppressor and oncogene mutations, e.g., p53, SPOP, PI3K, BRCA ORIC-944 Dose 400 mg QD 600 mg QD 800 mg QD 1,200 mg QD Best % Change in ctDNA Fraction from Baseline
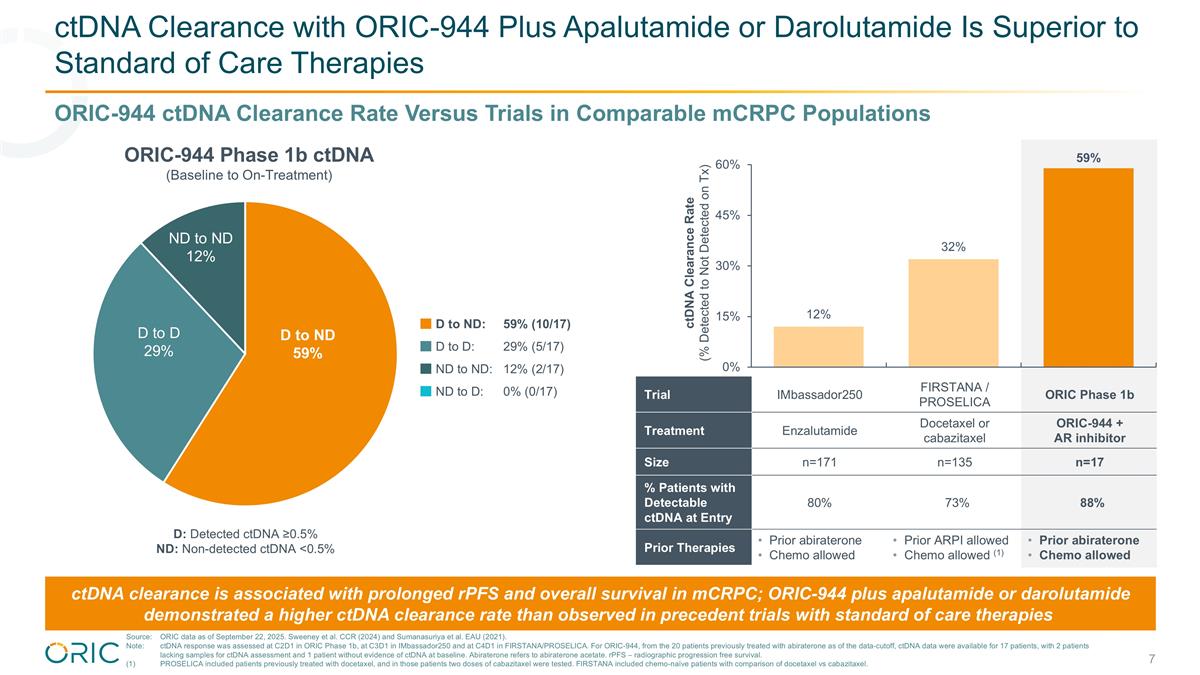
ctDNA Clearance with ORIC-944 Plus Apalutamide or Darolutamide Is Superior to Standard of Care Therapies Trial IMbassador250 FIRSTANA / PROSELICA ORIC Phase 1b Treatment Enzalutamide Docetaxel or cabazitaxel ORIC-944 + AR inhibitor Size n=171 n=135 n=17 % Patients with Detectable ctDNA at Entry 80% 73% 88% Prior Therapies Prior abiraterone Chemo allowed Prior ARPI allowed Chemo allowed (1) Prior abiraterone Chemo allowed D to ND 59% ND to ND 12% D to D 29% D: Detected ctDNA ≥0.5% ND: Non-detected ctDNA <0.5% ORIC-944 ctDNA Clearance Rate Versus Trials in Comparable mCRPC Populations ORIC-944 Phase 1b ctDNA (Baseline to On-Treatment) ctDNA Clearance Rate (% Detected to Not Detected on Tx) ctDNA clearance is associated with prolonged rPFS and overall survival in mCRPC; ORIC-944 plus apalutamide or darolutamide demonstrated a higher ctDNA clearance rate than observed in precedent trials with standard of care therapies D to ND:59% (10/17) D to D:29% (5/17) ND to ND:12% (2/17) ND to D:0% (0/17) Source:ORIC data as of September 22, 2025. Sweeney et al. CCR (2024) and Sumanasuriya et al. EAU (2021). Note:ctDNA response was assessed at C2D1 in ORIC Phase 1b, at C3D1 in IMbassador250 and at C4D1 in FIRSTANA/PROSELICA. For ORIC-944, from the 20 patients previously treated with abiraterone as of the data-cutoff, ctDNA data were available for 17 patients, with 2 patients lacking samples for ctDNA assessment and 1 patient without evidence of ctDNA at baseline. Abiraterone refers to abiraterone acetate. rPFS – radiographic progression free survival. (1)PROSELICA included patients previously treated with docetaxel, and in those patients two doses of cabazitaxel were tested. FIRSTANA included chemo-naïve patients with comparison of docetaxel vs cabazitaxel.
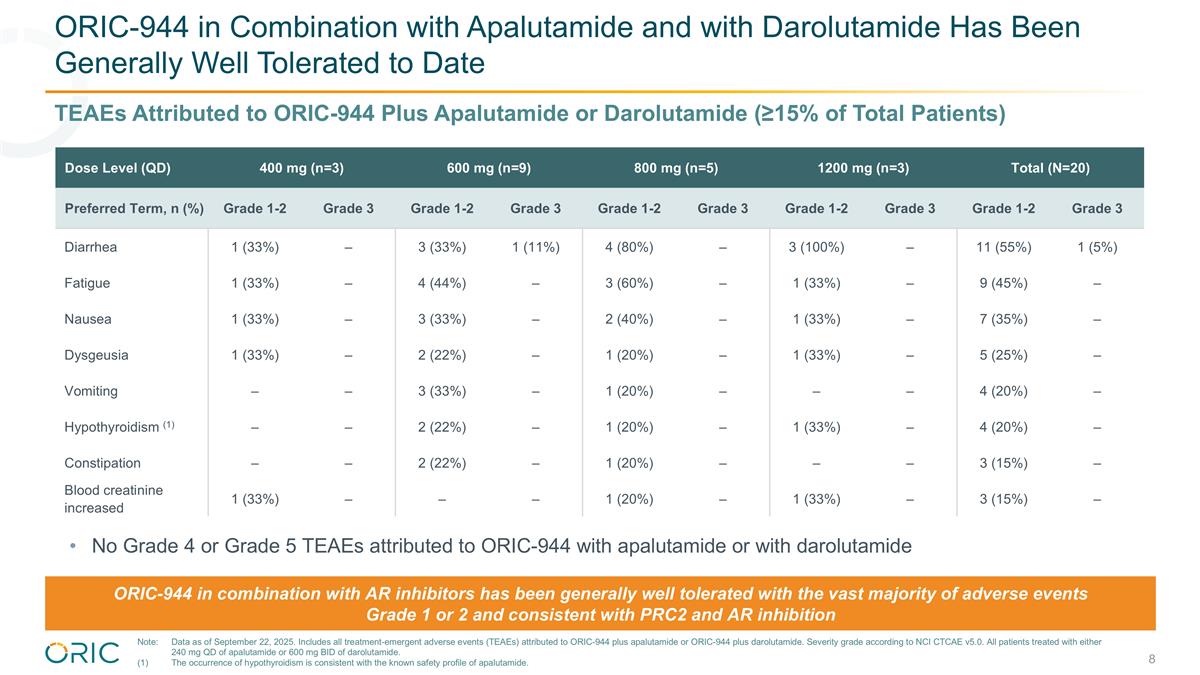
ORIC-944 in Combination with Apalutamide and with Darolutamide Has Been Generally Well Tolerated to Date Dose Level (QD) 400 mg (n=3) 600 mg (n=9) 800 mg (n=5) 1200 mg (n=3) Total (N=20) Preferred Term, n (%) Grade 1-2 Grade 3 Grade 1-2 Grade 3 Grade 1-2 Grade 3 Grade 1-2 Grade 3 Grade 1-2 Grade 3 Diarrhea 1 (33%) – 3 (33%) 1 (11%) 4 (80%) – 3 (100%) – 11 (55%) 1 (5%) Fatigue 1 (33%) – 4 (44%) – 3 (60%) – 1 (33%) – 9 (45%) – Nausea 1 (33%) – 3 (33%) – 2 (40%) – 1 (33%) – 7 (35%) – Dysgeusia 1 (33%) – 2 (22%) – 1 (20%) – 1 (33%) – 5 (25%) – Vomiting – – 3 (33%) – 1 (20%) – – – 4 (20%) – Hypothyroidism (1) – – 2 (22%) – 1 (20%) – 1 (33%) – 4 (20%) – Constipation – – 2 (22%) – 1 (20%) – – – 3 (15%) – Blood creatinine increased 1 (33%) – – – 1 (20%) – 1 (33%) – 3 (15%) – TEAEs Attributed to ORIC-944 Plus Apalutamide or Darolutamide (≥15% of Total Patients) ORIC-944 in combination with AR inhibitors has been generally well tolerated with the vast majority of adverse events Grade 1 or 2 and consistent with PRC2 and AR inhibition No Grade 4 or Grade 5 TEAEs attributed to ORIC-944 with apalutamide or with darolutamide Note:Data as of September 22, 2025. Includes all treatment-emergent adverse events (TEAEs) attributed to ORIC-944 plus apalutamide or ORIC-944 plus darolutamide. Severity grade according to NCI CTCAE v5.0. All patients treated with either 240 mg QD of apalutamide or 600 mg BID of darolutamide. (1)The occurrence of hypothyroidism is consistent with the known safety profile of apalutamide.

Combination of ORIC-944 + AR Inhibitors Compares Favorably to Competitor PRC2 Inhibitor + AR Inhibitor and to AR Inhibitor Monotherapy ORIC-944 Phase 1b Update (November 2025) PSA50 PSA90 SAFETY (All Gr / Gr ≥3) ORIC-944 + Apalutamide or Darolutamide Mevrometostat + Enzalutamide Enzalutamide Diarrhea (60% / 5%) Fatigue (45% / 0%) Nausea (35% / 0%) Dysgeusia (25% / 0%) Vomiting (20% / 0%) Hypothyroidism (20% / 0%) Constipation (15% / 0%) Blood creatinine increased (15% / 0%) AE cutoff of ≥15% Diarrhea (78% / 17%) Dysgeusia (59% / 0%) Decreased appetite (59% / 0%) Fatigue (56% / 5%) Anemia (49% / 5%) Nausea (42% / 0%) Alopecia (39% / 0%) Thrombocytopenia (29% / 2%); 2% Gr 4 Neutropenia (22% / 7%); 2% Gr 4 Vomiting (22% / 0%) Arthralgia in (22% / 0%) Rash (20% / 2%) AE cutoff of ≥20% Fatigue (43% / 3%) Nausea (25% / 0%) Anemia (23% / 3%) Diarrhea (18% / 0%) Decreased appetite (18% / 0%) Dysgeusia (8% / 0%) ORIC-944 + AR inhibitor safety profile compatible with long-term dosing, with the vast majority of AEs Gr 1 or 2, and no Gr 4/5 events Source:ORIC data on file. Enzalutamide and mevrometostat + enzalutamide data from Schweizer et al. ASCO GU (2025) and Matsubara et al. ASCO (2025). AEs <30% estimated from Matsubara et al. ASCO (2025). Note:Cross-trial comparison in previously treated mCRPC patients shown. ORIC-944 + apalutamide or darolutamide data as of September 22, 2025. (1)One additional PSA50 response pending confirmation; patient had dose hold due to ankle fracture. One patient developed bone progression and PSA50 was not confirmed. One patient came off drug due to TRAE of G1 diarrhea/G2 nausea and lost opportunity to confirm PSA50 response.