
2025 Research & Development Webcast CORPORATE OVERVIEW November 13, 2025 Nasdaq: ALDX © Aldeyra Therapeutics, Inc. 2025 Exhibit 99.1

Disclaimers and Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and Section 21E of the Securities Exchange Act of 1934, as amended, including statements regarding Aldeyra’s possible or assumed future results of operations, expenses and financing needs, business strategies and plans, statements regarding Aldeyra's future expectations, plans and prospects, including, without limitation, statements regarding: Aldeyra’ cash runway; the outcome and expected timing and results of ongoing or planned clinical trials; FDA agreement with the clinical development and regulatory plan for reproxalap; the outcome and expected timing and results of the clinical development and regulatory plan; the outcome and timing of the FDA’s review and/or approval of the NDA resubmission for reproxalap and the adequacy of the data included in the NDA resubmission or the supplemental responses to the FDA; the potential for and timing of regulatory approval and commencement of commercialization of reproxalap; Aldeyra's expectations regarding the exercise of the AbbVie option; the potential profile and benefit of reproxalap in dry eye disease and allergic conjunctivitis and its other product candidates in the indications for which they are developed; the outcome and timing of any clinical trials with ADX-2191; the outcome and timing of the FDA’s acceptance, review, or approval of a potential NDA resubmission for ADX-2191 and the adequacy of the data expected to be included in such potential resubmitted NDA; the goals, opportunity and potential for reproxalap and its other product candidates; anticipated clinical or regulatory milestones for ADX-2191, ADX-248, and ADX-246, including expectations regarding the results of scheduled FDA meetings and discussions, clinical trial initiations and completions, and the timing and nature of NDA or other submissions to the FDA; Aldeyra's business, research, development and regulatory plans or expectations; political, economic, legal, social and health risks that may affect Aldeyra’s business or the global economy; the structure, timing and success of Aldeyra’s planned or pending clinical trials; and expected milestones, market sizing, pricing and reimbursement, competitive position, regulatory matters, industry environment and potential growth opportunities, among other things. The results of earlier preclinical or clinical trials may not be predictive of future results. Forward-looking statements include all statements that are not historical facts and, in some cases, can be identified by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” "could," “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “on track,” “scheduled,” “target,” “design,” “estimate,” “predict,” “contemplates,” “likely,” “potential,” “continue,” “ongoing,” “aim,” “plan,” or the negative of these terms, and similar expressions intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause Aldeyra’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. These statements reflect Aldeyra’s current views with respect to future events and are based on assumptions and subject to risks and uncertainties, including the development of, and clinical and regulatory plans or expectations for Aldeyra’s investigational new drugs (including reproxalap, ADX-2191, ADX-248, and ADX-246), and systems-based approaches, later developments with the FDA that may be inconsistent with Aldeyra’s expectations and beliefs, including the risk that the results from earlier clinical trials, portions of clinical trials, or pooled clinical data may not accurately predict results of subsequent trials or the remainder of a clinical trial for the same or different indications, inconsistent expectations regarding FDA acceptance and review of the company’s filings and submitted data sets, and Aldeyra’s continuing or post-hoc review and quality control analysis of clinical data. Important factors that could cause actual results to differ materially from those reflected in Aldeyra's forward-looking statements are described in Aldeyra’s most recent Annual Report on Form 10-K and Quarterly Report on Form 10-Q, as well as Aldeyra’s subsequent filings with the Securities and Exchange Commission (SEC). All of Aldeyra's development plans and timelines may be subject to adjustment depending on funding, recruitment rate, regulatory review, which regulatory review timeline may be flexible and subject to change based on the regulator's workload and other potential review issues, preclinical and clinical results, regulatory developments in the United States and other countries, and other factors any of which could result in changes to Aldeyra’s development plans and programs or delay the initiation, enrolment, completion, or reporting of clinical trials. In addition to the risks described above and in Aldeyra's other filings with the SEC, other unknown or unpredictable factors also could affect Aldeyra's results. No forward-looking statements can be guaranteed, and actual results may differ materially from such statements. The information in this presentation is provided only as of November 13, 2025, and Aldeyra undertakes no obligation to update any forward-looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law.

PRECLINICAL PHASE 1 PHASE 2 PHASE 3 NDA REVIEW† RASP Platform for Immune-Mediated Diseases Reproxalap Topical ocular administration Dry Eye Disease Dry Eye Disease Allergic Conjunctivitis Allergic Conjunctivitis ADX-248 Oral administration Atopic Dermatitis Sjögren-Larsson Syndrome** Obesity/Hypertryglyceridemia Moderate Alcohol-Associated Hepatitis CNS/Neuroinflammatory Disease ADX-246 Intravitreal injection Dry Age-Related Macular Degeneration/ Geographic Atrophy Sjögren-Larsson Syndrome** Vitreous Methotrexate Platform for Rare Retinal Inflammatory Diseases ADX-2191 Intravitreal injection Primary Vitreoretinal Lymphoma (U.S. FDA Orphan Drug Designation) Proliferative Vitreoretinopathy (U.S. FDA Orphan Drug and Fast Track Designation) Retinitis Pigmentosa (U.S. FDA Orphan Drug Designation) Proliferative Vitreoretinopathy (U.S. FDA Orphan Drug and Fast Track Designation) Aldeyra Is a Well-Capitalized Biotechnology Companywith a Broad Immunology Pipeline †Regulatory review timelines are flexible and subject to change based on the regulator's workload and other potential review issues. ‡Company guidance as of November 6, 2025; includes continued early and late-stage development of our product candidates in immune-mediated diseases. Guidance has not been updated or confirmed since November 6, 2025 and does not include any potential licensing or product revenue associated with reproxalap. CNS=central nervous system, NDA=New Drug Application. Option Agreement As of 9/30/2025, cash, cash equivalents, and marketable securities were $75.3M, which Aldeyra believes will be sufficient to fund the Company into the second half of 2027.‡

Modulating RASP – A First-in-Class,Systems-Based Therapeutic Approach

RASP are formed by oxidation of alcohols and other metabolic processes. RASP bind thiol (Michael addition) and amine (Schiff base) residues on proteins, leading to conformational and functional changes in certain proteins that initiate pro-inflammatory signaling cascades. RASP are also precursors of lipids and may contribute to obesity and dyslipidemia. Receptor / Kinase Modification Scavenger Receptor A Binding Protein Signaling via Binding to Thiols and Amines Inflammasome, NF-kB Activation, Cytokine Release Autoantibody Formation RASP=reactive aldehyde species. RASP Represent a Novel, Potentially Broadly Applicable Pharmaceutical Target that Modulates Many Proteins at Once Lipid Synthesis RASP

RASP Modulation Represents a Novel Pharmacology RASP=reactive aldehyde species. Traditional pharmacology targets specific proteins and is generally limited to two actions: on or off. . Activating or inhibiting specific proteins on a sustained basis, which rarely occurs in nature, may lead to toxicity and could limit activity. RASP modulation may allow for control of protein systems, without turning any single protein on or off. Systems-based pharmacology could potentially lead to broader-based activity with less toxicity associated with activation or inhibition of specific proteins. vs.

Results from a Phase 2 Proof-of-Concept Trial of ADX-629, a Signal-Finding RASP Modulator, in Alcohol-Associated Hepatitis

Development of ADX-629, a Signal-Finding, First-Generation RASP Modulator, Concluded with Positive Results in Alcohol-Associated Hepatitis ADX-629 is a first-generation RASP modulator that was tested in proof-of-concept Phase 2 clinical trials across a number of immune-mediated diseases, including: Atopic Dermatitis Psoriasis Chronic Cough Alcohol-Associated Hepatitis Across all trials in aggregate, ADX-629 was deemed to be safe and well tolerated, and demonstrated acute and chronic activity in reducing signs and symptoms of inflammation. ADX-629 is an investigational drug candidate. RASP=reactive aldehyde species.
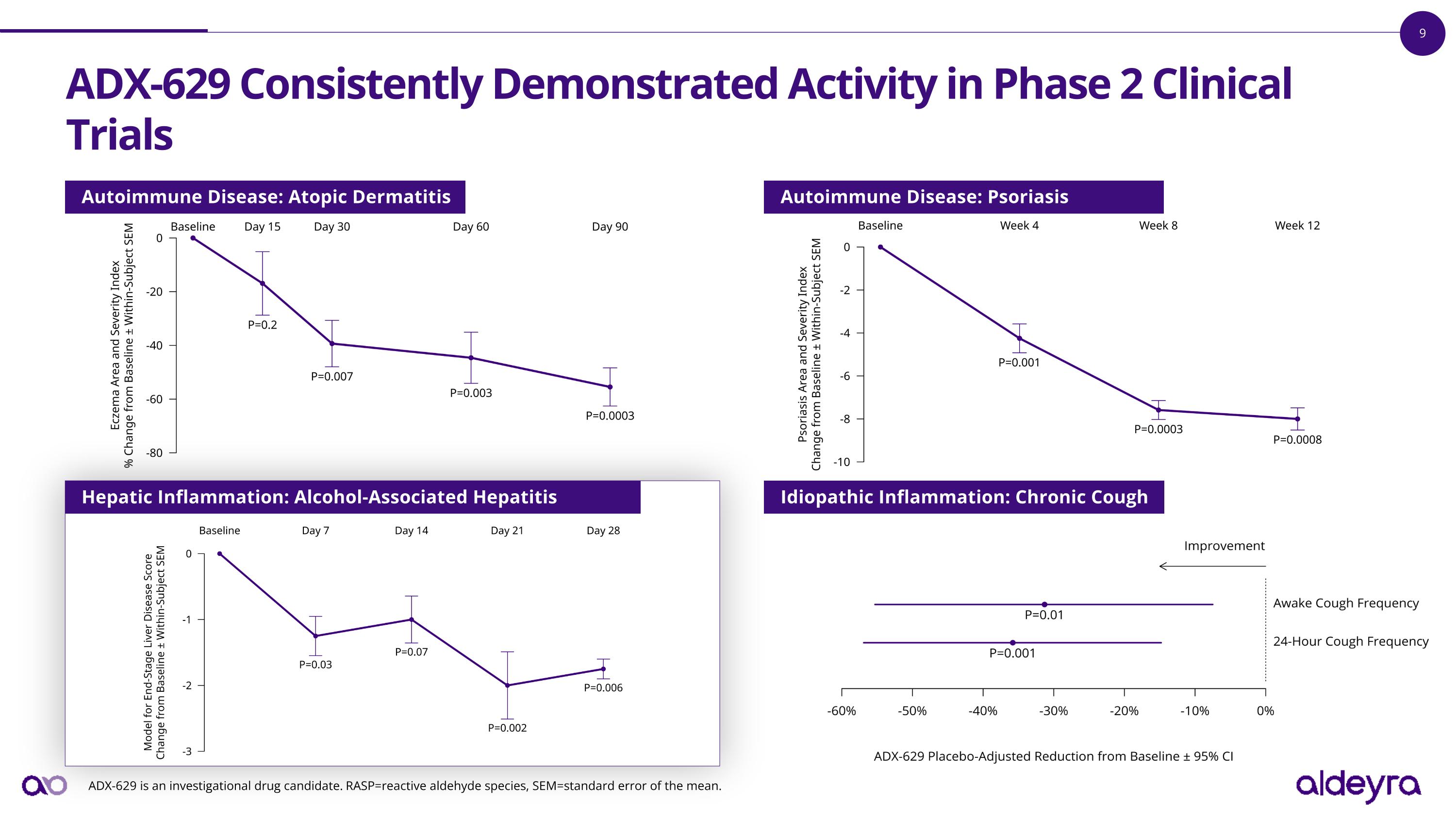
ADX-629 Consistently Demonstrated Activity in Phase 2 Clinical Trials ADX-629 is an investigational drug candidate. RASP=reactive aldehyde species, SEM=standard error of the mean. Hepatic Inflammation: Alcohol-Associated Hepatitis Autoimmune Disease: Atopic Dermatitis Autoimmune Disease: Psoriasis Idiopathic Inflammation: Chronic Cough

Statistically Significant Changes Observed in Lipid Profiles in Multiple Clinical Trials with ADX-629 ADX-629 is an investigational drug candidate. AUC=area under the curve, FFA=free fatty acids, HDL=high-density lipoprotein, LDL=low-density lipoprotein, mM=millimolar, SE=linear model standard error, RASP=reactive aldehyde species, SEM=standard error of the mean. Phase 2 Chronic Cough Clinical Trial Phase 1 Clinical Trial Phase 2 Psoriasis Clinical Trial Phase 2 Alcohol-Associated Hepatitis Clinical Trial P=0.005 P=0.036 P=0.0004 HDL(mg/dL AUC ± SE) LDL/HDL ratio (AUC ± SE) FFA(mM AUC ± SE)

ADX-629 Reduced Levels of the Inflammation Marker C-Reactive Protein in Phase 2 Clinical Trial in Alcohol-Associated Hepatitis ADX-629 is an investigational drug candidate. SEM=standard error of the mean. C-Reactive Protein has been associated with adverse cardiovascular outcomes, poor prognosis, and increased risk of death.

Activity of Next-Generation RASP Modulators in Preclinical Disease Models

By Binding the RASP Retinaldehyde, ADX-246 Potentially Represents a New Intravitreally Administered Therapy for the Treatment of Dry Age-Related Macular Degeneration (Dry AMD) Minutes Reduction in Toxic Retinaldehyde Metabolite A2E (retinal picomoles + SEM) in Abcr Knockout Mouse (Model of Dry AMD) ADX-246 Binding to RASP Retinaldehyde (absorbance units) ADX-246 Vehicle P=0.04 A2E is related to impairment in low-light vision,† one of the first symptoms of dry AMD. †J Biol Chem, 297(3):101074, 2021. ADX-246 is an investigational drug candidate. A2E=bis-retinoid N-retinyl-N-retinylidene ethanolamine, , RASP=reactive aldehyde species, SEM=standard error of the mean.

By Binding HNE, a Pro-Inflammatory RASP, ADX-248 Potentially Represents a New Orally Administered Therapy for the Treatment of Immune-Mediated Disease ADX-248 Binding to Pro-Inflammatory RASP HNE (absorbance units) Cytokine Reduction vs. Vehicle Control in LPS-Challenged Mice Epidermal Erosion Score (0-5) + SEM in Oxazolone Mouse Model of Atopic Dermatitis P=0.009 Vehicle ADX-248 Minutes *P<0.05, **P<0.01 IL-1b IL-3 IL-5 IL-6 IL-15 IL-17 ADX-248 is an investigational drug candidate. HNE=4-hydroxynonenal, LPS=lipopolysaccharide, RASP=reactive aldehyde species, SEM=standard error of the mean.

RASP Are Associated with Human Neuroinflammatory Diseases that Affect the Central Nervous System Parkinson’s Disease Multiple Sclerosis Amyotrophic Lateral Sclerosis (ALS) Environmental toxins that promote Parkinson’s disease also increase RASP. The RASP DOPAL cross-links α-synuclein and leads to dopaminergic neuron death.1 1Plotegher, N. et al. Sci Rep 7, 40699 (2017). 2Smith, R. G. et al. Ann Neurol 44, 696–699 (1998). 3Tully, M. et al. Front. Neurol. 9, (2018). 4Leung, G. et al. Neuroscience 173, 150–155 (2011). DOPAL=3,4-dihydroxyphenylacetaldehyde, HNE=4-hydroxynonenal, , RASP=reactive aldehyde species. The RASP acrolein is elevated in multiple sclerosis patients.3 Acrolein inhibition reduced disease severity in a preclinical model of multiple sclerosis.4 The RASP HNE is increased in the central nervous system of ALS patients.2 Genetic variants associated with RASP clearance influence ALS disease progression risk.

EAE Disease Model Inducible mouse model of MS Characterized by increasing paralysis Widely accepted model of neuron demyelination Study Design Legacy RASP Modulator ADX-629 Significantly Reduced Disease Severity in a Mouse Model of Multiple Sclerosis ADX-629 is an investigational drug candidate. EAE=experimental autoimmune encephalomyelitis, MS=multiple sclerosis, MOG35-55=myelin oligodendrocyte glycoprotein peptide, CFA=Complete Freund's Adjuvant, BID=twice daily, SEM=standard error of mean, PO=oral administration. EAE Disease Severity Score (0-5) (Mean ± SEM) Vehicle ADX-629 Reduced Disease Severity vs. Vehicle P=0.04

ADX-248 Binds RASP Associated with CNS/Neuroinflammatory Diseases ADX-248 binds DOPAL and HNE, neurotoxic RASP associated with neuroinflammatory diseases. ADX-248 is an investigational drug candidate. DOPAL=3, 4-dihydroxyphenylacetaldehyde, HNE=4-hydroxynonenal, , RASP=reactive aldehyde species, CNS=central nervous system. DOPAL HNE HNE DOPAL ADX-248 in vitro RASP Binding

ADX-248 Significantly Improved Rotarod Performance and Grip Strength in the Mouse MPTP Parkinson’s Disease Model Study Design MPTP Parkinson’s Model Dopaminergic neuron loss Parkinson’s-like disease symptoms Widely accepted preclinical model Rotarod Performance vs. Vehicle (time to fall, seconds ± SE) ADX-248 (PO mg/kg) 3 10 20 P=0.004 Favors drug Grip Strength vs. Vehicle (gram force ± SE) ADX-248 (PO mg/kg) 3 10 20 P=0.02 ADX-248 is an investigational drug candidate. MPTP=1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, TH+=tyrosine hydroxylase positive, QD=once daily, BID=twice daily, SE=mixed model for repeated measures standard error, PO=oral administration.

ADX-248 Significantly Increased Brain Dopamine and Dopaminergic Neuron Cell Area in the Mouse MPTP Parkinson's Disease Model Brain Dopamine vs. Vehicle (ng dopamine / mg protein ± SE) ADX-248 (PO mg/kg) 3 10 20 P=0.05 P=0.001 P=0.03 ADX-248 (PO mg/kg) 3 10 20 Substantia Nigra Dopaminergic Neuron Cell Area vs. Vehicle (TH+ mm2 ± SE) ADX-248 is an investigational drug candidate. MPTP=1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, TH+=tyrosine hydroxylase positive, ng=nanogram, SE= mixed model for repeated measures standard error, PO=oral administration. Favors drug

ADX-248 Significantly Improved Wire Hang Time in the Rat 6-OHDA Parkinson’s Disease Model ADX-248 (PO mg/kg) 3 10 20 Grip Strength vs. Vehicle (gram force ± SE) ADX-248 is an investigational drug candidate. 6-OHDA= 6-hydroxydopamine, BID=twice daily, SE= mixed model for repeated measures standard error, PO=oral administration. 6-OHDA Parkinson’s Disease Model Unilateral brain damage Dopaminergic neuron loss in the injured hemisphere Widely accepted Parkinson’s disease model Study Design ADX-248 (PO mg/kg) 3 10 20 P=0.02 Wire Hang Time vs. Vehicle (time to fall, seconds ± SE) Favors drug

ADX-248 Significantly Improved Grip Strength and Rotarod Performance in the Mouse SOD1-G93A Amyotrophic Lateral Sclerosis Disease Model ADX-248 (PO mg/kg) Rotarod Performance vs. Vehicle (time to fall, seconds ± SE) 3 10 20 P=0.005 P=0.02 ADX-248 is an investigational drug candidate. SOD1=superoxide dismutase 1, ALS=Amyotrophic lateral sclerosis, BID=twice daily, SE= mixed model for repeated measures standard error, PO=oral administration. ADX-248 (PO mg/kg) Grip Strength vs. Vehicle (gram force ± SE) 3 10 20 P=0.0002 P=0.0002 P=0.03 Favors drug SOD1-G93A ALS Disease Model Models familial ALS Translationally relevant disease pathology Widely accepted model Study Design

Key Messages ADX-248 Pharmacology Sequestered DOPAL and HNE, RASP associated with neuronal cell death Improved neuromotor function in Parkinson’s and ALS disease models Increased brain dopamine levels and TH+ cell area in the mouse MPTP Parkinson’s disease model ADX-248 Exposure & Safety Mouse in vivo and cell line studies suggest achievable therapeutic brain levels in humans Favorable preclinical safety profile, with acceptable safety margins at potentially therapeutic human doses Phase 1 healthy subject dose escalation ongoing 1 2 Favorable preclinical data in Parkinson’s, ALS, and MS disease models creates opportunities for multiple neuroinflammatory disease development paths. Potential Phase 2 trials will be supported by the ongoing human Phase 1 clinical trial and preclinical safety studies. ADX-248, a Next-Generation RASP Modulator, Demonstrated Activity in Multiple Preclinical Neuroinflammatory Disease Models ADX-248 is an investigational drug candidate. DOPAL=3,4-dihydroxyphenylacetaldehyde, HNE=4-hydroxynonenal, , RASP=reactive aldehyde species, ALS=amyotrophic lateral sclerosis, MS=multiple sclerosis, TH+=tyrosine hydroxylase positive, MPTP=1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.

ADX-248 Preclinical Benefit Observed at Doses Predicted to be Safe and Achievable Based on Ongoing Phase 1 Clinical Trial Preclinical Dose (PO mg/kg, BID) Human Equivalent Dose (PO mg, QD) 3 15 10 50 20 100 10x safety margin* expected for 100 mg human dose ADX-248 Phase 1 dose escalation in healthy subjects ongoing at doses in excess of 100 mg QD *based on preclinical safety studies in rat and dog Neurologic improvements observed at 10 and 20 mg/kg in preclinical models Ongoing pivotal safety testing expected to support Phase 2 clinical trials in 2026 6-month rat toxicity; 9-month dog toxicity; embryo-fetal toxicity in rat and rabbit Phase 1 SAD/MAD clinical trial ADX-248 is an investigational drug candidate. QD= once daily, BID=twice daily, SAD=single ascending dose, MAD=multiple ascending dose, PO=oral administration.

Reproxalap: A Novel RASP Modulator for the Treatment of Dry Eye Disease

Reproxalap Represents a Novel Potential Therapeutic Approach in Dry Eye Disease with Rapid Activity Observed in Clinical Trials Potential advantages for patients and healthcare providers could effect a paradigm shift relative to standard of care. Dry eye disease afflicts 39 million or more adults in the United States.† Rapid and sustained symptom improvement Broad symptomatic activity Acute reduction ofocular redness †Company estimates and Am J Ophthalmol. 2014;157(4):799-806. Topical ocular reproxalap is an investigational drug candidate that has not been approved by the FDA; mild and transient instillation site irritation is the most commonly reported adverse event in clinical trials. FDA=U.S. Food & Drug Administration.

The Phase 3 Dry Eye Chamber Trial Achieved the Primary Endpoint of Ocular Discomfort †Regulatory review and discussion timelines are flexible and subject to change based on the regulator's workload, governmental shutdown, and other potential review issues. P value derived from primary endpoint mixed model for repeated measures analysis. Topical ocular reproxalap is an investigational drug candidate that has not been approved by the FDA; mild and transient instillation site irritation is the most commonly reported adverse event in clinical trials. FDA=U.S. Food & Drug Administration, PDUFA=Prescription Drug User Fee Act. PDUFA Target Action Date December 16, 2025† Mean Ocular Discomfort Score (0-100) Change from Baseline Second Dose Primary Endpoint Assessment Period Minutes in Dry Eye Chamber First Dose Before Chamber Entry Reproxalap Vehicle Prespecified Analysis P=0.002 Post-Hoc Treatment Chamber Analysis P=0.004

Reproxalap Drug Substance and Drug Product Vendors Have Recently Been Inspected by theU.S. Food and Drug Administration In 2025, the U.S. Food and Drug Administration (FDA) performed routine cGMP inspections of manufacturing sites associated with the production of reproxalap. The reproxalap drug product vendor was inspected in Q1 2025. The reproxalap drug substance vendor was inspected in Q3 2025. cGMP=current Good Manufacturing Practices. Both inspections were classified as Voluntary Action Indicated (VAI), and the FDA has notified the manufacturers that the inspections are closed and that no further action was necessary.

Aldeyra has Entered into an Exclusive Option Agreement withAbbVie Inc. for License to Develop and Commercialize Reproxalap Option for AbbVie to obtain: Co-exclusive license to develop, manufacture, and commercialize reproxalap in the U.S. Exclusive license to develop, manufacture, and commercialize outside the U.S. Option terminates on the 10th business day after Aldeyra receives approval from the U.S. FDA of the NDA for reproxalap in dry eye disease Financial terms of license if option exercised: Upfront payment of $100 million less option fees $100 million milestone payment upon U.S. FDA approval in dry eye disease $200 million in additional regulatory and commercial milestones Profit and loss share (60% for AbbVie / 40% for Aldeyra) from commercialization in U.S. Tiered royalties on net sales outside of U.S. Key Terms of Reproxalap Option Agreement Topical ocular reproxalap is an investigational drug candidate that has not been approved by the FDA; mild and transient instillation site irritation is the most commonly reported adverse event in clinical trials. FDA=U.S. Food & Drug Administration, NDA=New Drug Application.

Milestones

Dry Eye Disease (Reproxalap)New Drug Application PDUFA date December 16, 2025† Atopic Dermatitis (ADX-248)Phase 2 clinical trial initiation expected in H1 2026‡ Obesity/Hypertryglyceridemia (ADX-248)Investigational New Drug application expected to be submitted in 2026 Dry Age-Related Macular Degeneration/Geographic Atrophy (ADX-246)Investigational New Drug application expected to be submitted in 2026 Primary Vitreoretinal Lymphoma (ADX-2191)Phase 3 clinical trial initiation expected in H2 2025‡ Retinitis Pigmentosa (ADX-2191) Phase 2/3 clinical trial initiation expected in H1 2026‡ Clinical and Regulatory Milestones †Regulatory review and discussion timelines are flexible and subject to change based on the regulator’s workload, governmental shutdown, and other potential review issues. ‡The timing of clinical trials depends, in part, on the availability of clinical research facilities and staffing, the ability to recruit patients, and the number of patients in the trial. PDUFA=Prescription Drug User Fee Act.





























