
November 2025 Corporate Presentation neffy – the transformative needle-free solution for severe allergic reactions Exhibit 99.2 NASDAQ: SPRY

Forward-looking statements Statements in this presentation that are not purely historical in nature are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements in this presentation include, without limitation, statements regarding: the potential market, demand and expansion opportunities for neffy; the anticipated sales of neffy; the anticipated gross-to-net percentage range; the belief that real-world outcomes data support the clinical interchangeability of neffy and epinephrine injection and that dissemination of this data will have a positive impact on neffy prescriptions; the expected intellectual property protection for neffy; guidance regarding ARS Pharma’s future performance and results of operations, including any cash or cash equivalent resource projections; the design and potential benefits of neffy, including the likelihood allergy patients and caregivers will choose to carry and dose neffy compared to needle-bearing options; the anticipated benefits of ARS Pharma’s ex-U.S. partnerships and co-promotion agreement; the expectation that the loan facility will enable ARS Pharma to execute on its strategic expansion plans and fuel continued growth; the timeline for regulatory decisions and commercialization of neffy outside of the United States; evaluations, judgments, and expectations regarding ARS Pharma’s marketing and commercialization strategies; the likelihood of neffy attaining favorable coverage and the expected timing of coverage decisions; the timing and expected percentage of commercial coverage with unrestricted access; the anticipated timing for topline data from the urticaria trial and the potential for ARS Pharma’s intranasal epinephrine technology to expand into the urticaria indication, the estimated patient population for this indication, and the belief that a majority of patients would be prescribed ARS-2, if approved; ARS Pharma’s expected competitive position; the expected composition and reach of ARS Pharma’s commercial force; the potential for the neffy Experience Program, and any statements of assumptions underlying any of the foregoing. These forward-looking statements are subject to the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “anticipate,” “demonstrate,” “expect,” “indicate,” “plan,” “potential,” “target,” “will” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon ARS Pharma’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation: the ability to obtain and maintain regulatory approval for neffy; results from clinical trials and non-clinical studies may not be indicative of results that may be observed in the future; the risk that ARS Pharma may not realize its expected return on investment from its DTC campaign; potential safety and other complications from neffy; the labeling for neffy in any future indication or patient population; the scope, progress and expansion of developing and commercializing neffy; ARS Pharma’s reliance on its licensing and co-promotion partners; the potential for payors and governments to delay, limit or deny coverage or reimbursements for neffy; the size and growth of the market therefor and the rate and degree of market acceptance thereof vis-à-vis intramuscular injectable products; net product sales may not be indicative of profitability or profitability at expected levels; reliance on survey results with small sample sizes; ARS Pharma’s ability to protect its intellectual property position; and the impact of government laws and regulations. Additional risks and uncertainties that could cause actual outcomes and results to differ materially from those contemplated by the forward-looking statements are included under the caption “Risk Factors” in ARS Pharma’s Annual Report on Form 10-Q for the quarter ended September 30, 2025, filed with the SEC on November 10, 2025. This and other documents ARS Pharma files with the SEC can also be accessed on ARS Pharma’s website at ars-pharma.com by clicking on the link “Financial Filings” under the “Investors & Media” tab. The forward-looking statements included in this presentation are made only as of the date hereof. ARS Pharma assumes no obligation and does not intend to update these forward-looking statements, except as required by law. ARS Pharmaceuticals, Inc. Investor Presentation – November 2025
Transforming the Emergency Treatment of Type I Allergic Reactions neffy®: first and only FDA approved “no needle, no injection” solution for the emergency treatment of Type I allergic reactions for those 4 years and older who weigh 33 lbs or more (2 mg and 1 mg now available) Strong execution ($31.3M net US sales) in Q3 2025, with seamless prescribing experience starting in 2026 to unlock significant growth Prescribing breadth: 18,000 HCPs have prescribed neffy (+86% q/q) Seamless prescribing: Launched $0 co-pay virtual prescriber option in November 2025 to reduce patient and HCP burden DTC: since May launch, consumer awareness +180% GTN: 50%+ overall retention including future PBM additions and $0 co-pay Potential multi-billion US market opportunity ($3.5B Rx’ed, plus $7B expansion segment) driven by HCP and patient preference and adoption1 NCE-like IP exclusivity potential with issued composition of matter and method of treatment patents until at least 2039 $288.2 million in cash, cash equivalents and short-term investments2 ARS Pharmaceuticals, Inc. Investor Presentation – November 2025 References: 1. Company estimates 2. As of 9/30/2025
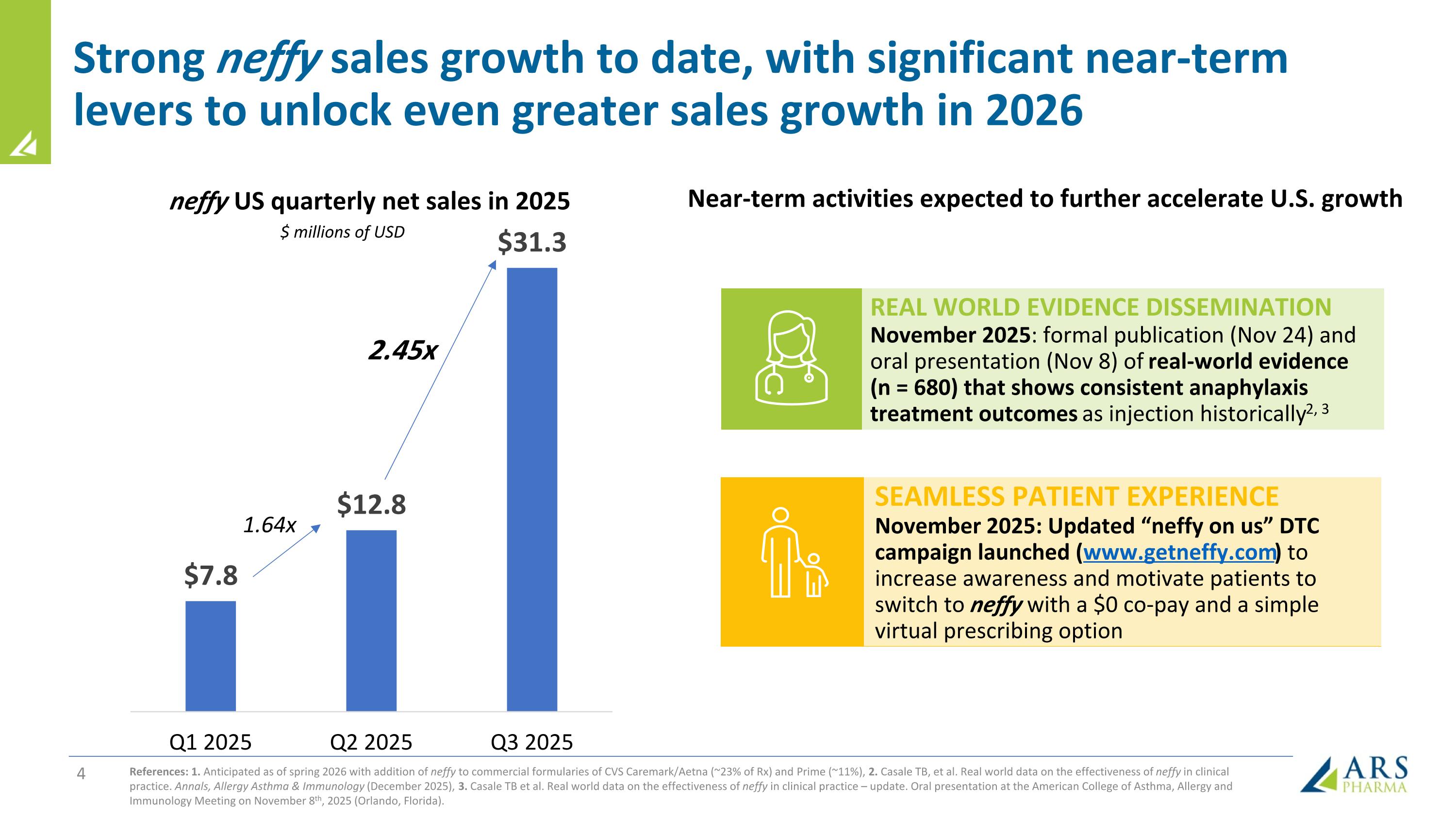
Strong neffy sales growth to date, with significant near-term levers to unlock even greater sales growth in 2026 neffy US quarterly net sales in 2025 $ millions of USD 1.64x 2.45x Near-term activities expected to further accelerate U.S. growth REAL WORLD EVIDENCE DISSEMINATION November 2025: formal publication (Nov 24) and oral presentation (Nov 8) of real-world evidence (n = 680) that shows consistent anaphylaxis treatment outcomes as injection historically2, 3 SEAMLESS PATIENT EXPERIENCE November 2025: Updated “neffy on us” DTC campaign launched (www.getneffy.com) to increase awareness and motivate patients to switch to neffy with a $0 co-pay and a simple virtual prescribing option References: 1. Anticipated as of spring 2026 with addition of neffy to commercial formularies of CVS Caremark/Aetna (~23% of Rx) and Prime (~11%), 2. Casale TB, et al. Real world data on the effectiveness of neffy in clinical practice. Annals, Allergy Asthma & Immunology (December 2025), 3. Casale TB et al. Real world data on the effectiveness of neffy in clinical practice – update. Oral presentation at the American College of Asthma, Allergy and Immunology Meeting on November 8th, 2025 (Orlando, Florida).
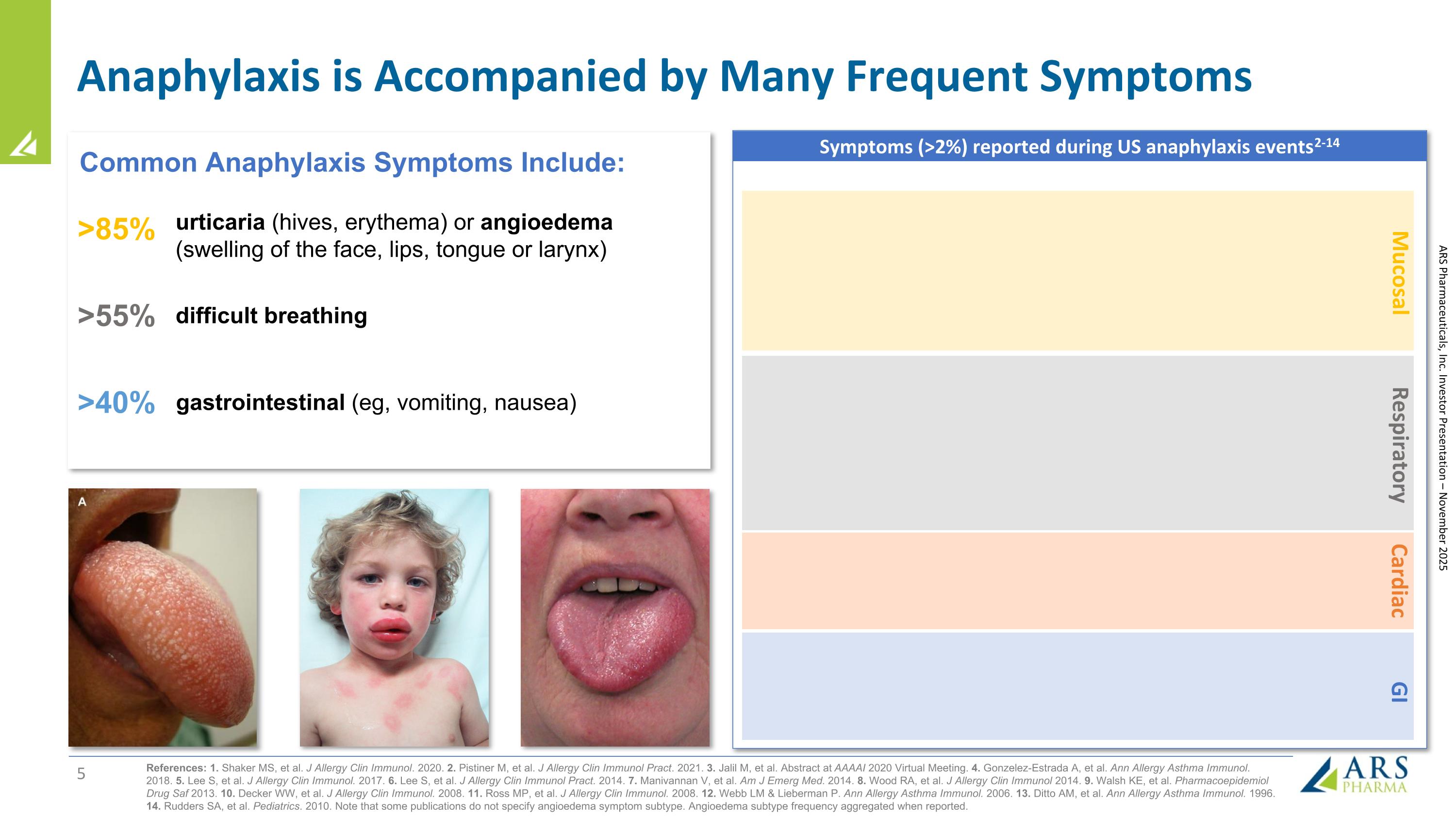
Anaphylaxis is Accompanied by Many Frequent Symptoms References: 1. Shaker MS, et al. J Allergy Clin Immunol. 2020. 2. Pistiner M, et al. J Allergy Clin Immunol Pract. 2021. 3. Jalil M, et al. Abstract at AAAAI 2020 Virtual Meeting. 4. Gonzelez-Estrada A, et al. Ann Allergy Asthma Immunol. 2018. 5. Lee S, et al. J Allergy Clin Immunol. 2017. 6. Lee S, et al. J Allergy Clin Immunol Pract. 2014. 7. Manivannan V, et al. Am J Emerg Med. 2014. 8. Wood RA, et al. J Allergy Clin Immunol 2014. 9. Walsh KE, et al. Pharmacoepidemiol Drug Saf 2013. 10. Decker WW, et al. J Allergy Clin Immunol. 2008. 11. Ross MP, et al. J Allergy Clin Immunol. 2008. 12. Webb LM & Lieberman P. Ann Allergy Asthma Immunol. 2006. 13. Ditto AM, et al. Ann Allergy Asthma Immunol. 1996. 14. Rudders SA, et al. Pediatrics. 2010. Note that some publications do not specify angioedema symptom subtype. Angioedema subtype frequency aggregated when reported. Symptoms (>2%) reported during US anaphylaxis events 2-14 Mucosal Respiratory Cardiac GI urticaria (hives, erythema) or angioedema (swelling of the face, lips, tongue or larynx) >85% >55% gastrointestinal (eg, vomiting, nausea) Common Anaphylaxis Symptoms Include: difficult breathing >40% ARS Pharmaceuticals, Inc. Investor Presentation – November 2025
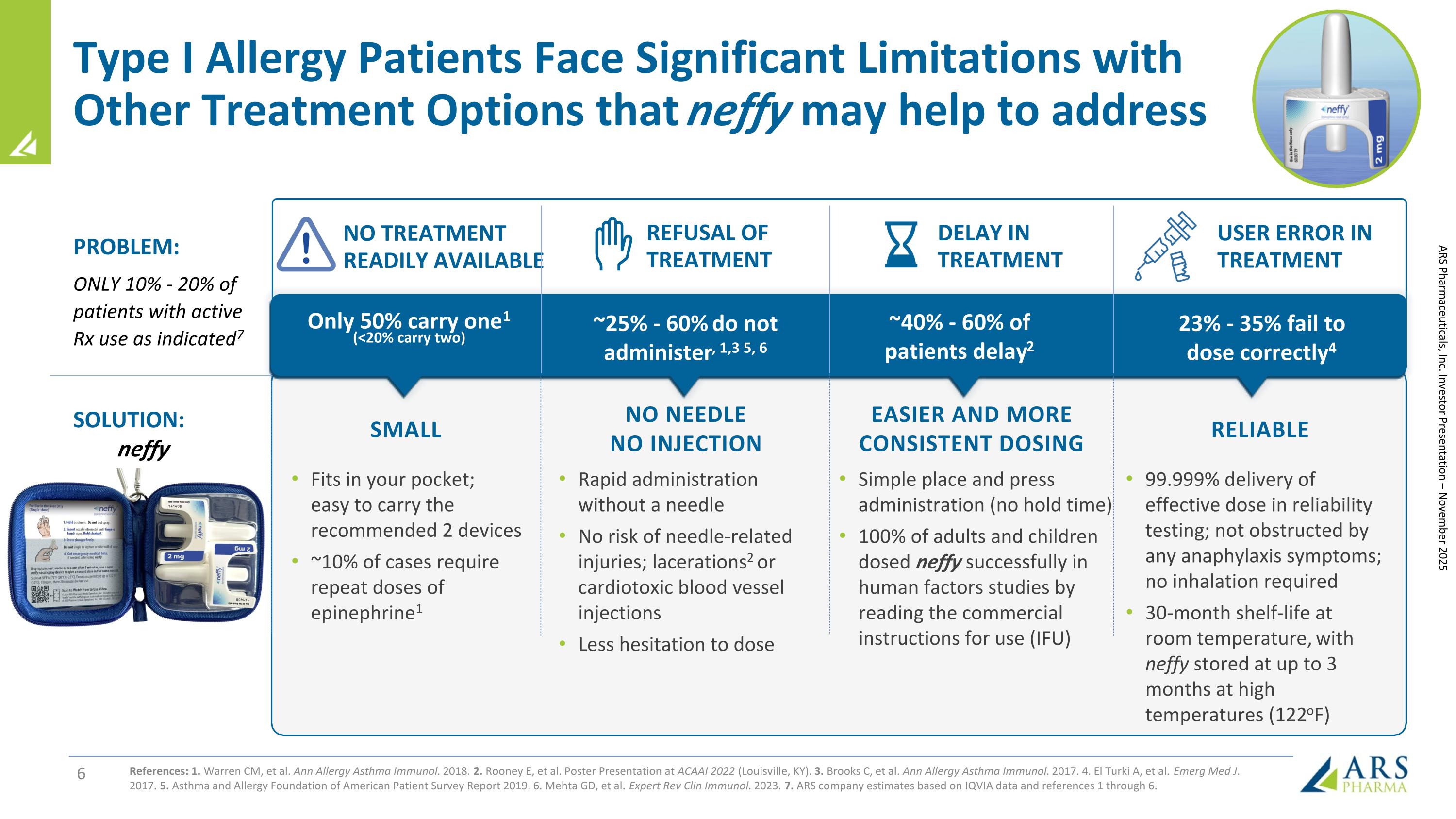
Type I Allergy Patients Face Significant Limitations with Other Treatment Options that neffy may help to address References: 1. Warren CM, et al. Ann Allergy Asthma Immunol. 2018. 2. Rooney E, et al. Poster Presentation at ACAAI 2022 (Louisville, KY). 3. Brooks C, et al. Ann Allergy Asthma Immunol. 2017. 4. El Turki A, et al. Emerg Med J. 2017. 5. Asthma and Allergy Foundation of American Patient Survey Report 2019. 6. Mehta GD, et al. Expert Rev Clin Immunol. 2023. 7. ARS company estimates based on IQVIA data and references 1 through 6. Rapid administration without a needle No risk of needle-related injuries; lacerations2 or cardiotoxic blood vessel injections Less hesitation to dose NO NEEDLE NO INJECTION Fits in your pocket; easy to carry the recommended 2 devices ~10% of cases require repeat doses of epinephrine1 EASIER AND MORE CONSISTENT DOSING Simple place and press administration (no hold time) 100% of adults and children dosed neffy successfully in human factors studies by reading the commercial instructions for use (IFU) RELIABLE 99.999% delivery of effective dose in reliability testing; not obstructed by any anaphylaxis symptoms; no inhalation required 30-month shelf-life at room temperature, with neffy stored at up to 3 months at high temperatures (122oF) Only 50% carry one1 (<20% carry two) ~25% - 60% do not administer, 1,3 5, 6 NO TREATMENT READILY AVAILABLE REFUSAL OF TREATMENT ~40% - 60% of patients delay2 DELAY IN TREATMENT 23% - 35% fail to dose correctly4 USER ERROR IN TREATMENT SOLUTION: neffy PROBLEM: ONLY 10% - 20% of patients with active Rx use as indicated7 SMALL ARS Pharmaceuticals, Inc. Investor Presentation – November 2025
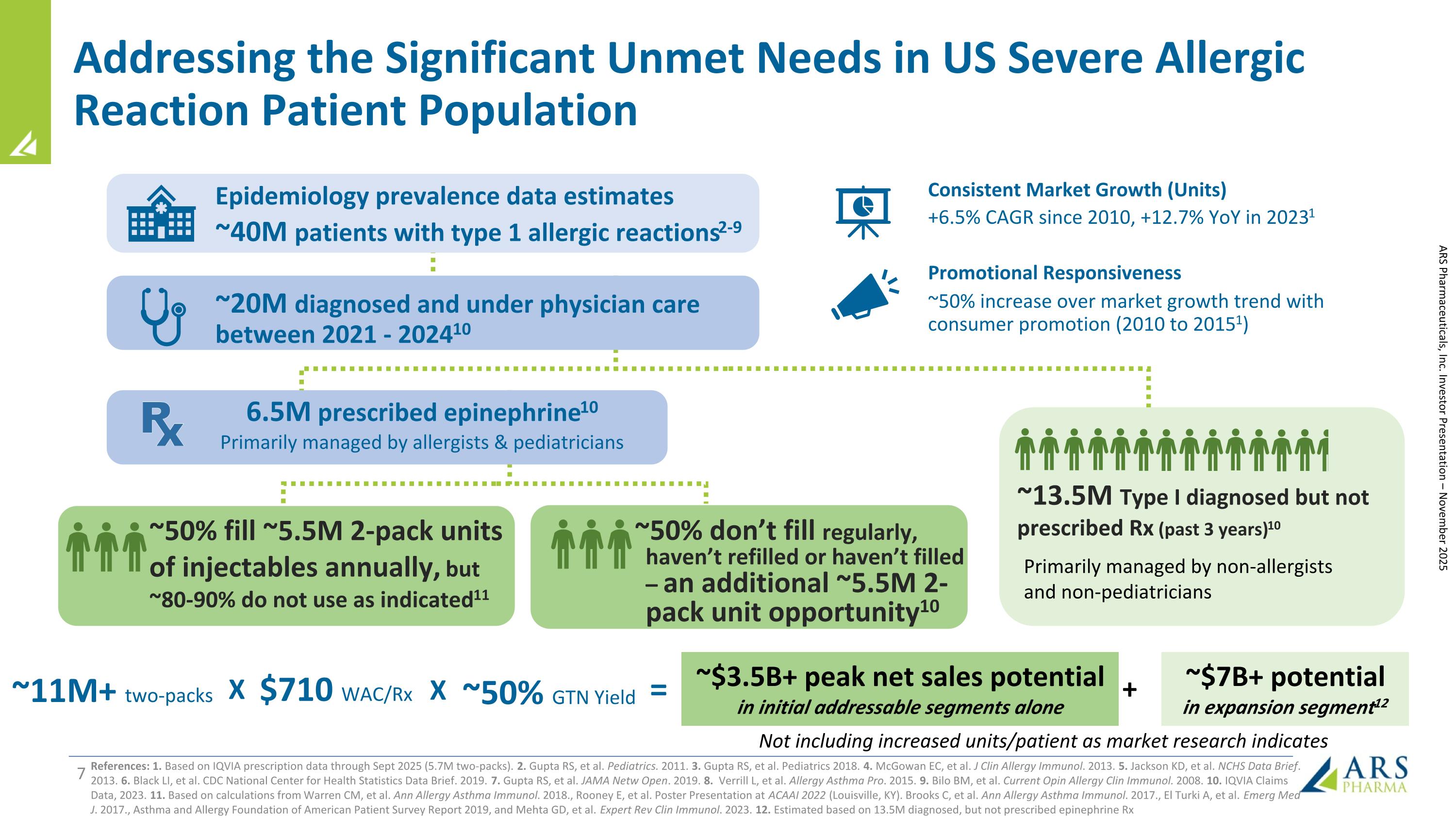
Addressing the Significant Unmet Needs in US Severe Allergic Reaction Patient Population References: 1. Based on IQVIA prescription data through Sept 2025 (5.7M two-packs). 2. Gupta RS, et al. Pediatrics. 2011. 3. Gupta RS, et al. Pediatrics 2018. 4. McGowan EC, et al. J Clin Allergy Immunol. 2013. 5. Jackson KD, et al. NCHS Data Brief. 2013. 6. Black LI, et al. CDC National Center for Health Statistics Data Brief. 2019. 7. Gupta RS, et al. JAMA Netw Open. 2019. 8. Verrill L, et al. Allergy Asthma Pro. 2015. 9. Bilo BM, et al. Current Opin Allergy Clin Immunol. 2008. 10. IQVIA Claims Data, 2023. 11. Based on calculations from Warren CM, et al. Ann Allergy Asthma Immunol. 2018., Rooney E, et al. Poster Presentation at ACAAI 2022 (Louisville, KY). Brooks C, et al. Ann Allergy Asthma Immunol. 2017., El Turki A, et al. Emerg Med J. 2017., Asthma and Allergy Foundation of American Patient Survey Report 2019, and Mehta GD, et al. Expert Rev Clin Immunol. 2023. 12. Estimated based on 13.5M diagnosed, but not prescribed epinephrine Rx Promotional Responsiveness ~20M diagnosed and under physician care between 2021 - 202410 Epidemiology prevalence data estimates ~40M patients with type 1 allergic reactions2-9 ~50% increase over market growth trend with consumer promotion (2010 to 20151) Consistent Market Growth (Units) +6.5% CAGR since 2010, +12.7% YoY in 20231 ~50% fill ~5.5M 2-pack units of injectables annually, but ~80-90% do not use as indicated11 ~13.5M Type I diagnosed but not prescribed Rx (past 3 years)10 ~50% don’t fill regularly, haven’t refilled or haven’t filled – an additional ~5.5M 2-pack unit opportunity10 6.5M prescribed epinephrine10 Primarily managed by allergists & pediatricians Primarily managed by non-allergists and non-pediatricians $710 WAC/Rx ~50% GTN Yield ~11M+ two-packs ~$3.5B+ peak net sales potential in initial addressable segments alone = X X ~$7B+ potential in expansion segment12 + ARS Pharmaceuticals, Inc. Investor Presentation – November 2025 Not including increased units/patient as market research indicates
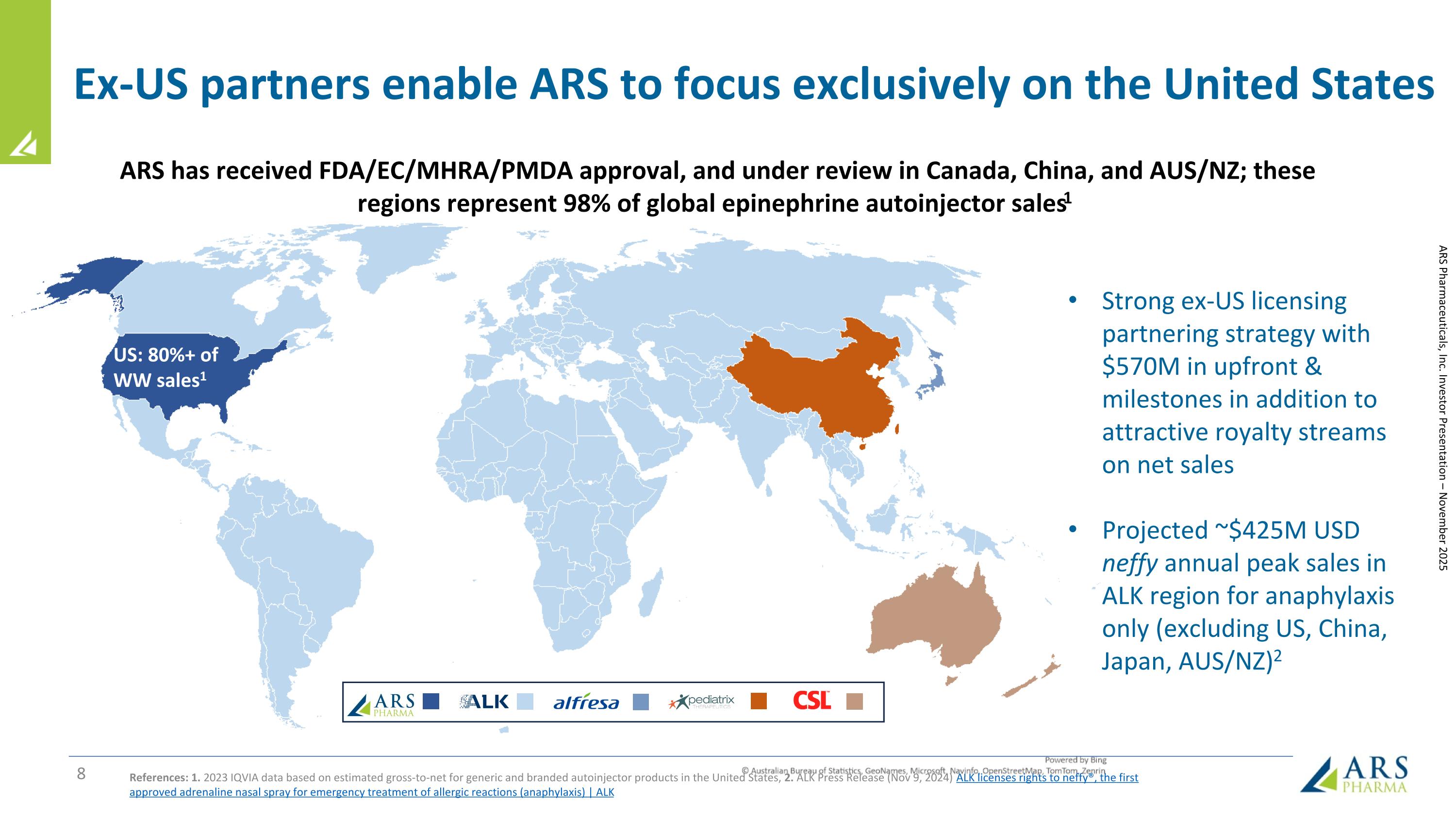
Ex-US partners enable ARS to focus exclusively on the United States References: 1. 2023 IQVIA data based on estimated gross-to-net for generic and branded autoinjector products in the United States, 2. ALK Press Release (Nov 9, 2024) ALK licenses rights to neffy®, the first approved adrenaline nasal spray for emergency treatment of allergic reactions (anaphylaxis) | ALK ARS Pharmaceuticals, Inc. Investor Presentation – November 2025 US: 80%+ of WW sales1 ARS has received FDA/EC/MHRA/PMDA approval, and under review in Canada, China, and AUS/NZ; these regions represent 98% of global epinephrine autoinjector sales1 Strong ex-US licensing partnering strategy with $570M in upfront & milestones in addition to attractive royalty streams on net sales Projected ~$425M USD neffy annual peak sales in ALK region for anaphylaxis only (excluding US, China, Japan, AUS/NZ)2

Commercialization Progress ARS Pharmaceuticals, Inc. Investor Presentation – November 2025
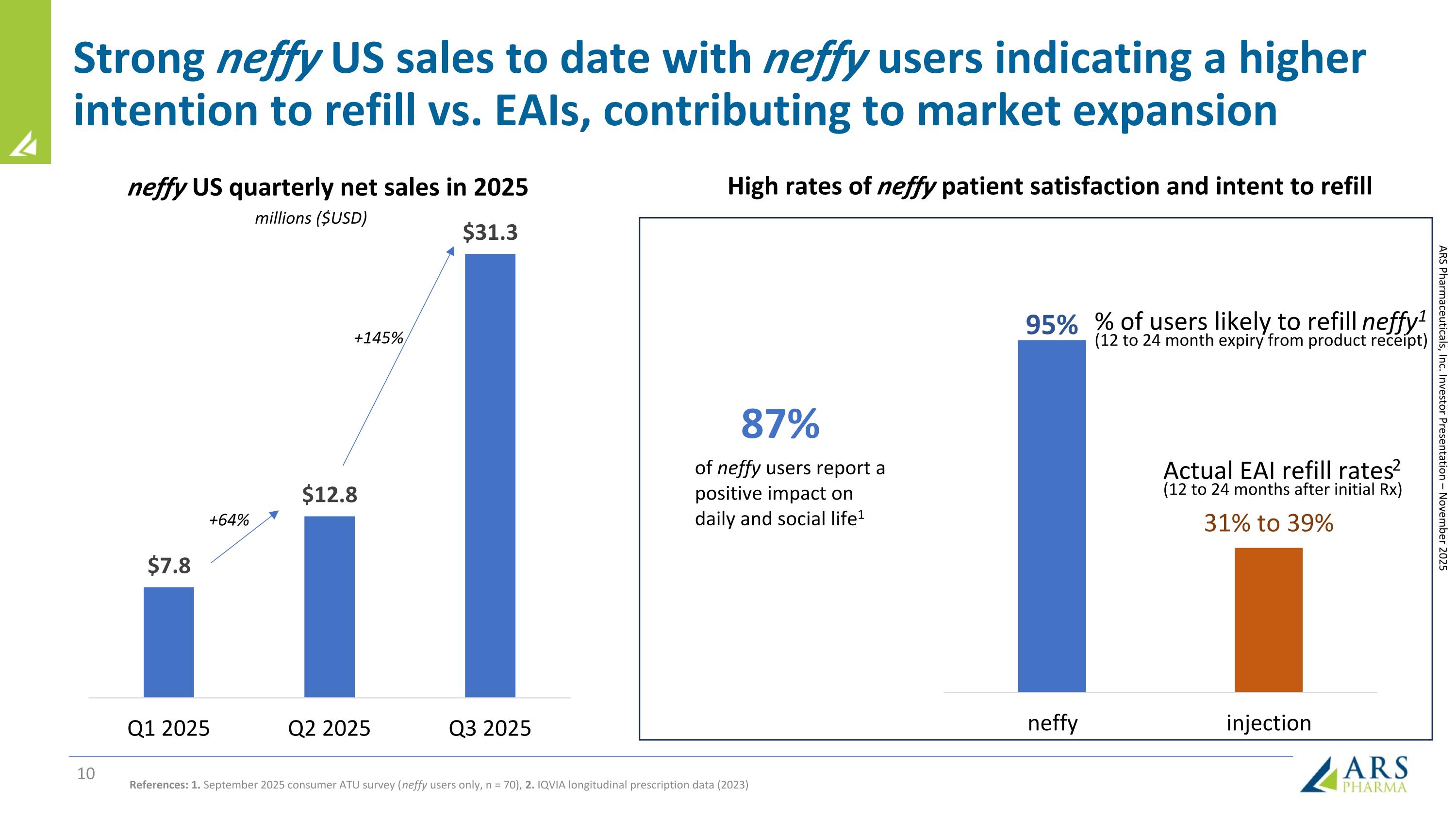
Strong neffy US sales to date with neffy users indicating a higher intention to refill vs. EAIs, contributing to market expansion % of users likely to refill neffy1 (12 to 24 month expiry from product receipt) Actual EAI refill rates2 (12 to 24 months after initial Rx) 87% of neffy users report a positive impact on daily and social life1 neffy US quarterly net sales in 2025 References: 1. September 2025 consumer ATU survey (neffy users only, n = 70), 2. IQVIA longitudinal prescription data (2023) millions ($USD) High rates of neffy patient satisfaction and intent to refill +64% +145% ARS Pharmaceuticals, Inc. Investor Presentation – November 2025
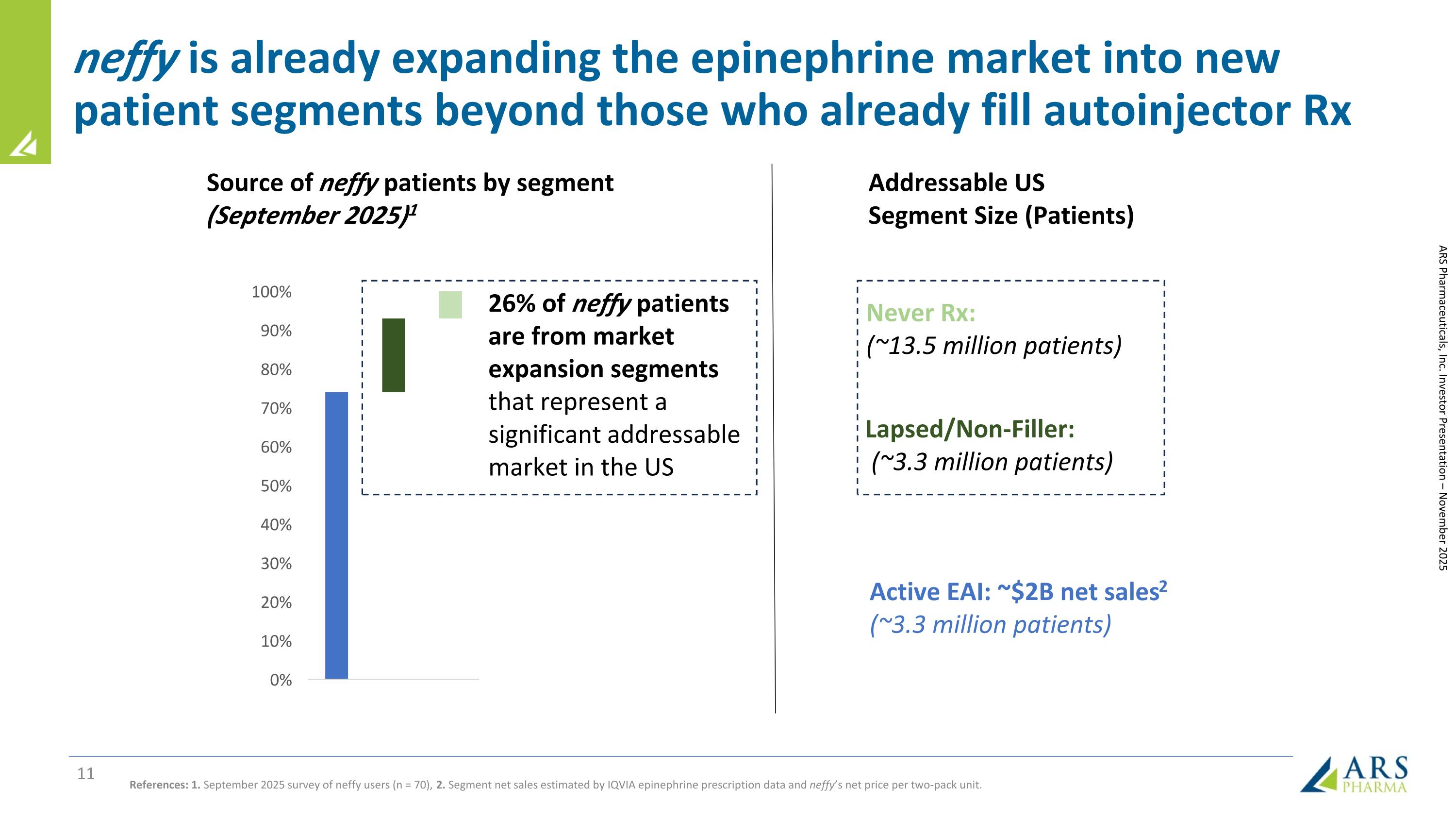
neffy is already expanding the epinephrine market into new patient segments beyond those who already fill autoinjector Rx Source of neffy patients by segment (September 2025)1 Active EAI: ~$2B net sales2 (~3.3 million patients) Addressable US Segment Size (Patients) References: 1. September 2025 survey of neffy users (n = 70), 2. Segment net sales estimated by IQVIA epinephrine prescription data and neffy’s net price per two-pack unit. ARS Pharmaceuticals, Inc. Investor Presentation – November 2025 26% of neffy patients are from market expansion segments that represent a significant addressable market in the US Lapsed/Non-Filler: (~3.3 million patients) Never Rx: (~13.5 million patients)
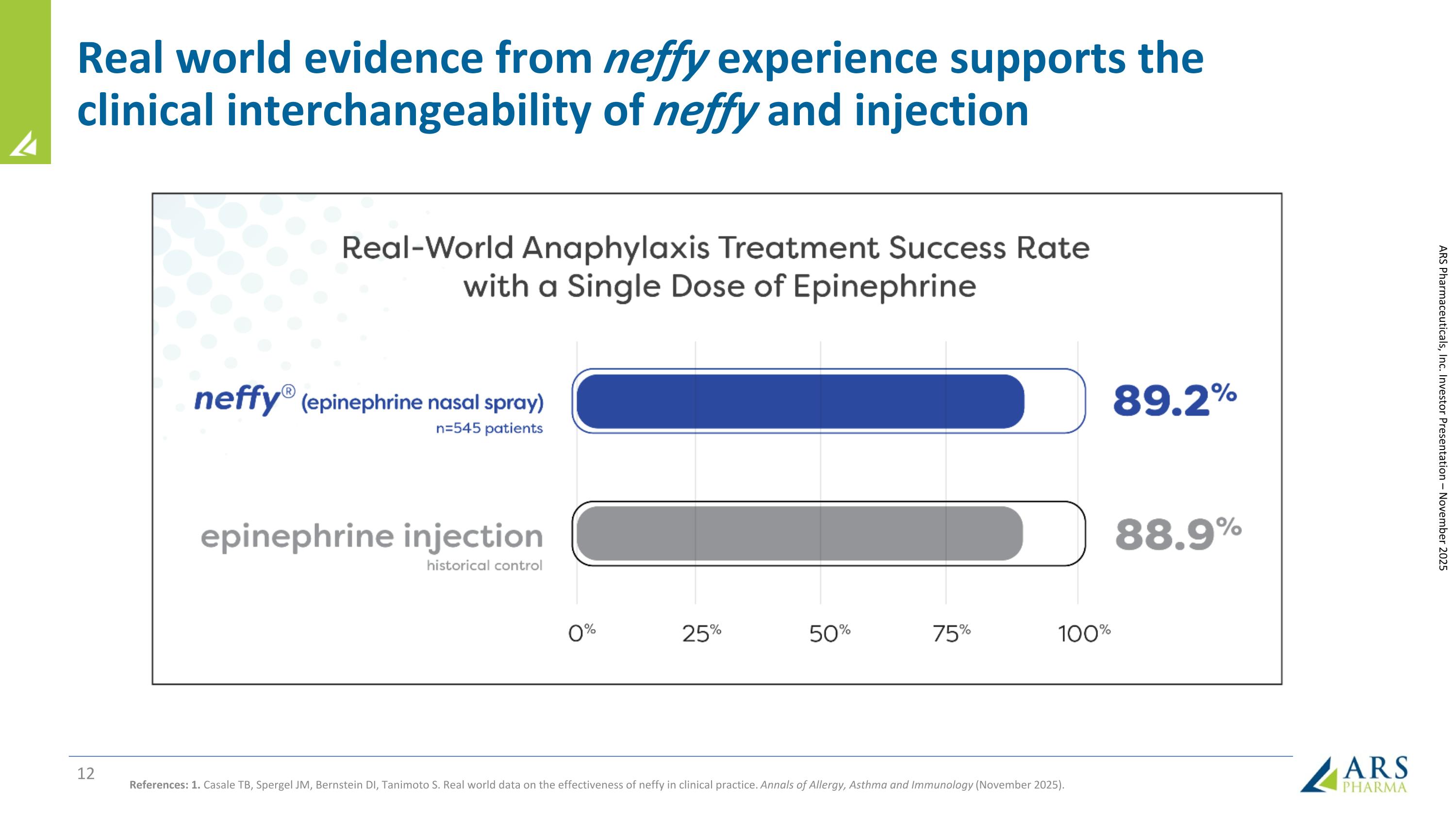
Real world evidence from neffy experience supports the clinical interchangeability of neffy and injection References: 1. Casale TB, Spergel JM, Bernstein DI, Tanimoto S. Real world data on the effectiveness of neffy in clinical practice. Annals of Allergy, Asthma and Immunology (November 2025). ARS Pharmaceuticals, Inc. Investor Presentation – November 2025
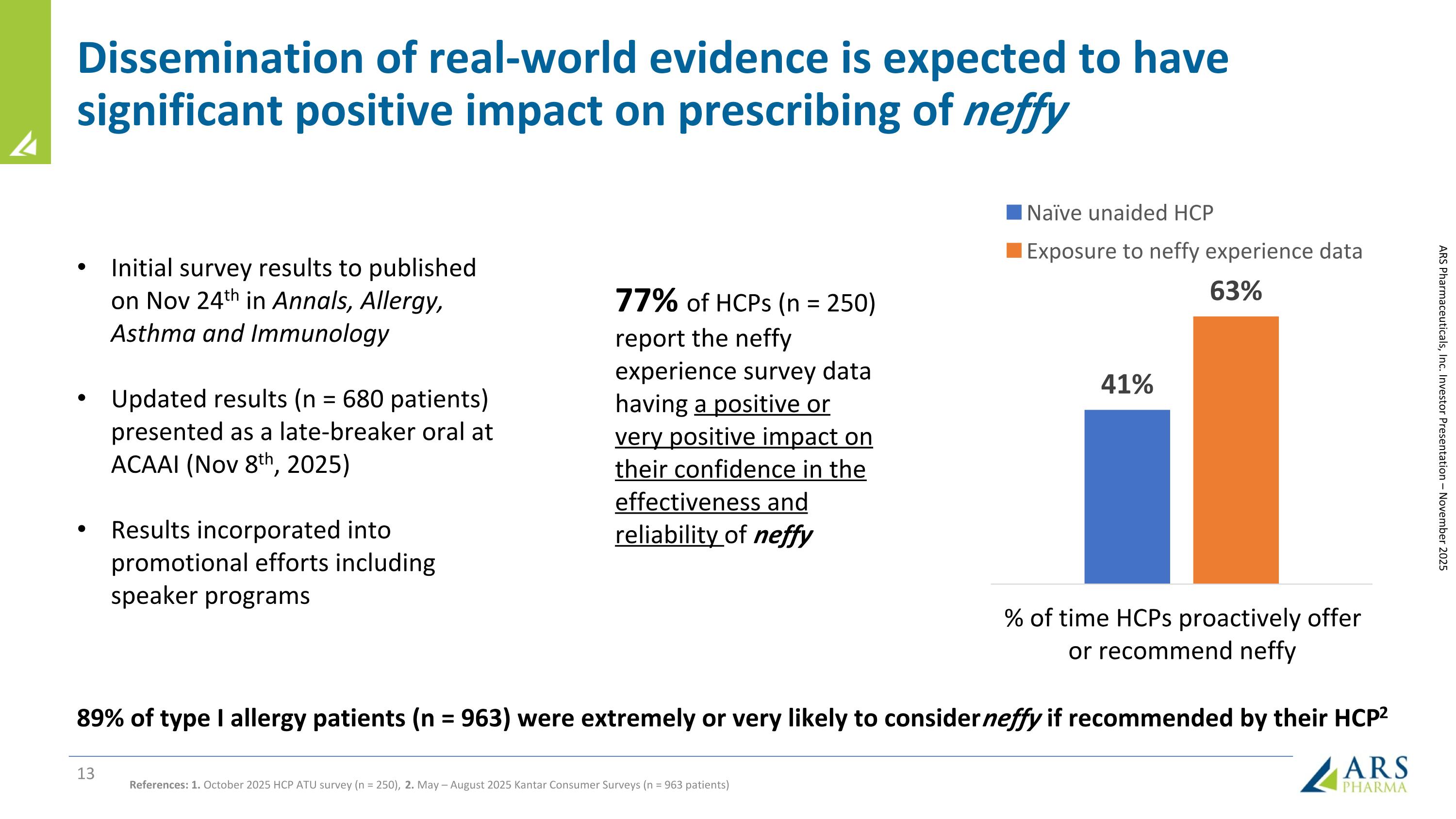
Dissemination of real-world evidence is expected to have significant positive impact on prescribing of neffy ARS Pharmaceuticals, Inc. Investor Presentation – November 2025 References: 1. October 2025 HCP ATU survey (n = 250), 2. May – August 2025 Kantar Consumer Surveys (n = 963 patients) 89% of type I allergy patients (n = 963) were extremely or very likely to consider neffy if recommended by their HCP2 Initial survey results to published on Nov 24th in Annals, Allergy, Asthma and Immunology Updated results (n = 680 patients) presented as a late-breaker oral at ACAAI (Nov 8th, 2025) Results incorporated into promotional efforts including speaker programs 77% of HCPs (n = 250) report the neffy experience survey data having a positive or very positive impact on their confidence in the effectiveness and reliability of neffy
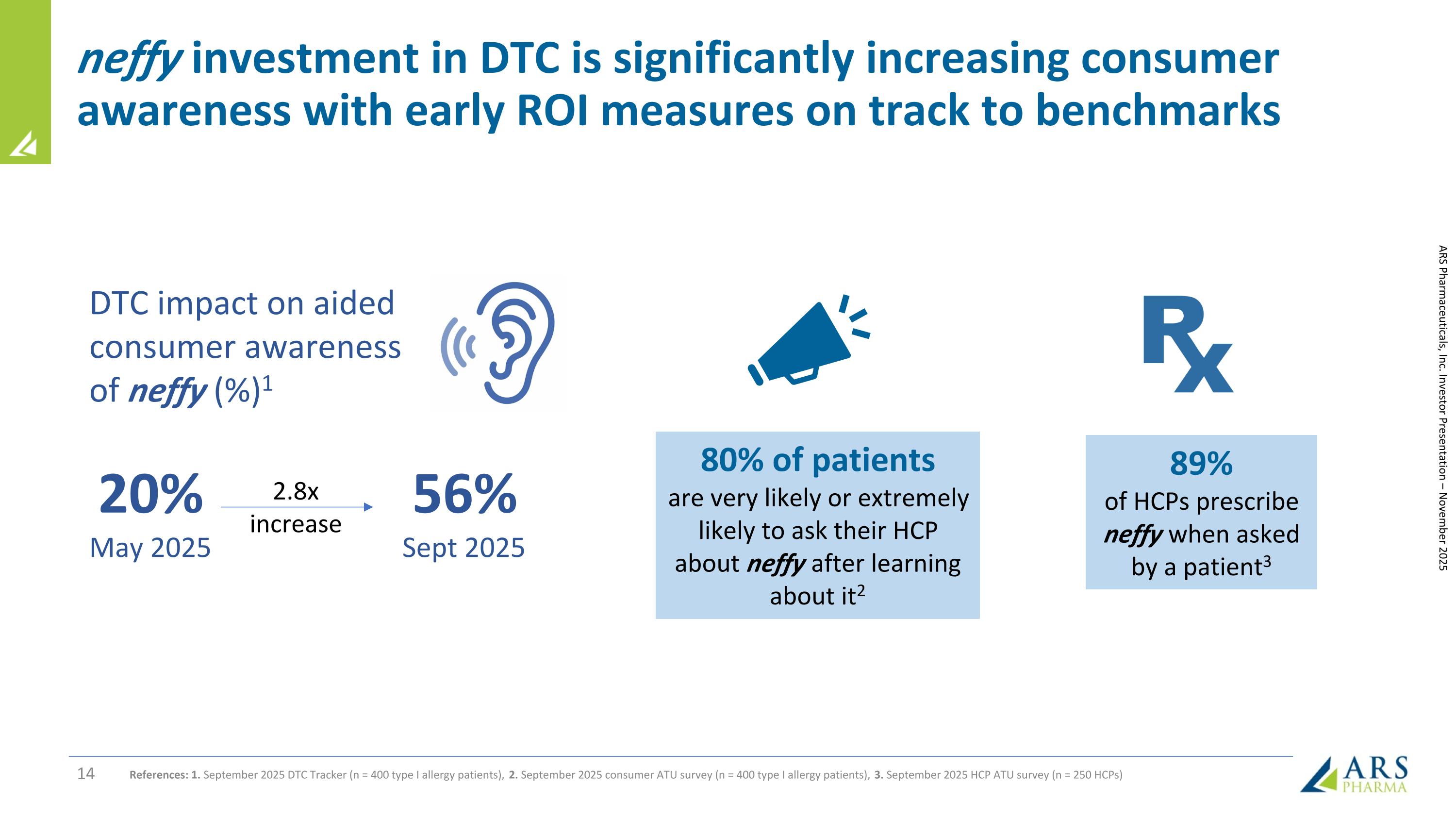
ARS Pharmaceuticals, Inc. Investor Presentation – November 2025 80% of patients are very likely or extremely likely to ask their HCP about neffy after learning about it2 89% of HCPs prescribe neffy when asked by a patient3 20% May 2025 2.8x increase 56% Sept 2025 DTC impact on aided consumer awareness of neffy (%)1 References: 1. September 2025 DTC Tracker (n = 400 type I allergy patients), 2. September 2025 consumer ATU survey (n = 400 type I allergy patients), 3. September 2025 HCP ATU survey (n = 250 HCPs) neffy investment in DTC is significantly increasing consumer awareness with early ROI measures on track to benchmarks

ARS Pharmaceuticals, Inc. Investor Presentation – November 2025 72% of patients are interested in a virtual prescriber option1 $0 co-pay for commercially eligible patients $0 visit fee <5 to 10 min appointment at the patient's convenience References: 1. August 2025 consumer ATU survey (n = 400 type I allergy patients) “Get neffy on Us” virtual prescriber with a $0 co-pay launched in November 2025 to reduce patient burden
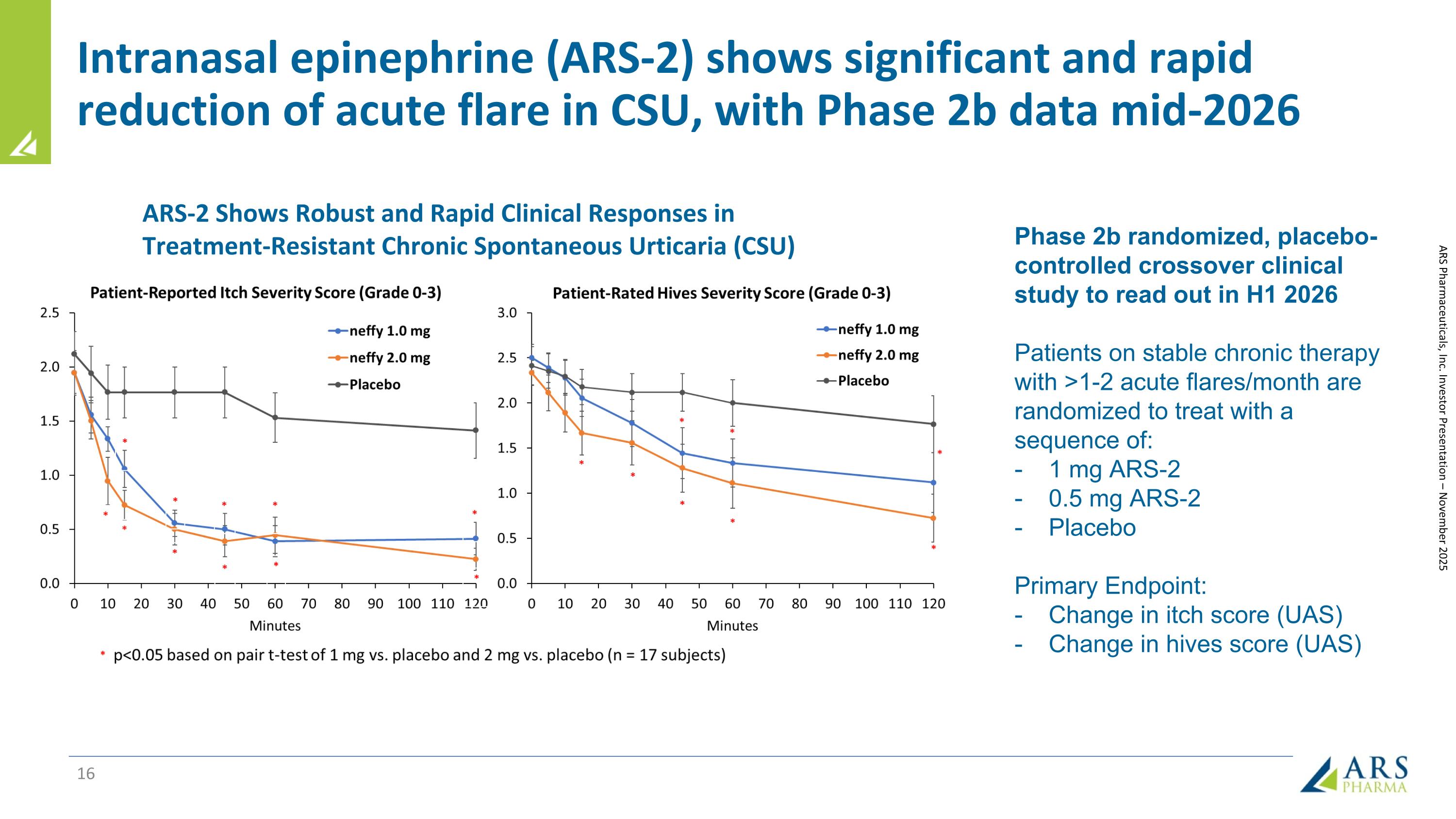
Intranasal epinephrine (ARS-2) shows significant and rapid reduction of acute flare in CSU, with Phase 2b data mid-2026 Phase 2b randomized, placebo-controlled crossover clinical study to read out in H1 2026 Patients on stable chronic therapy with >1-2 acute flares/month are randomized to treat with a sequence of: 1 mg ARS-2 0.5 mg ARS-2 Placebo Primary Endpoint: Change in itch score (UAS) Change in hives score (UAS) ARS Pharmaceuticals, Inc. Investor Presentation – November 2025 ARS-2 Shows Robust and Rapid Clinical Responses in Treatment-Resistant Chronic Spontaneous Urticaria (CSU)
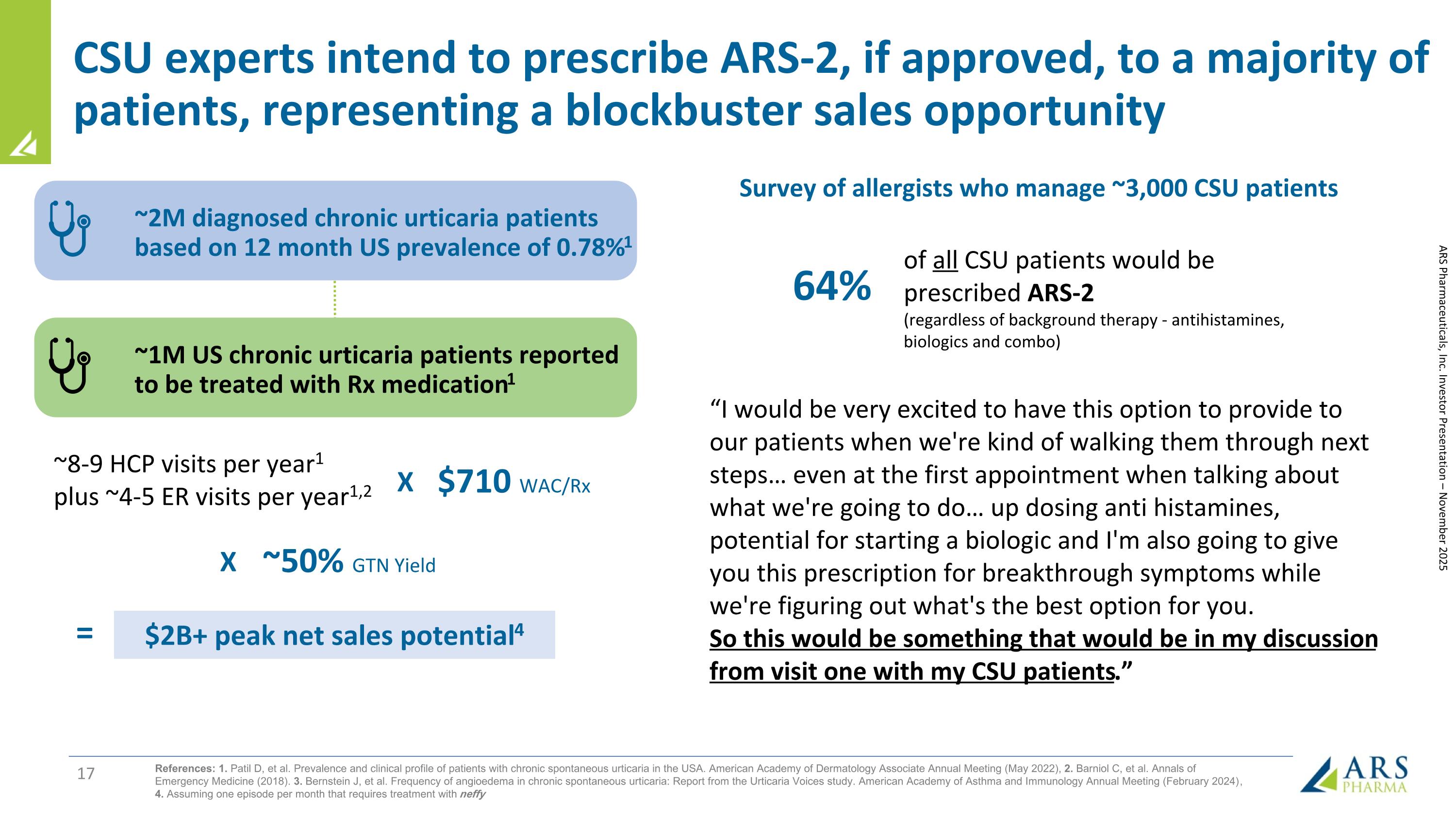
CSU experts intend to prescribe ARS-2, if approved, to a majority of patients, representing a blockbuster sales opportunity of all CSU patients would be prescribed ARS-2 (regardless of background therapy - antihistamines, biologics and combo) ~2M diagnosed chronic urticaria patients based on 12 month US prevalence of 0.78%1 ~1M US chronic urticaria patients reported to be treated with Rx medication1 ~8-9 HCP visits per year1 plus ~4-5 ER visits per year1,2 $710 WAC/Rx ~50% GTN Yield $2B+ peak net sales potential4 X X = ARS Pharmaceuticals, Inc. Investor Presentation – November 2025 References: 1. Patil D, et al. Prevalence and clinical profile of patients with chronic spontaneous urticaria in the USA. American Academy of Dermatology Associate Annual Meeting (May 2022), 2. Barniol C, et al. Annals of Emergency Medicine (2018). 3. Bernstein J, et al. Frequency of angioedema in chronic spontaneous urticaria: Report from the Urticaria Voices study. American Academy of Asthma and Immunology Annual Meeting (February 2024) , 4. Assuming one episode per month that requires treatment with neffy “I would be very excited to have this option to provide to our patients when we're kind of walking them through next steps… even at the first appointment when talking about what we're going to do… up dosing anti histamines, potential for starting a biologic and I'm also going to give you this prescription for breakthrough symptoms while we're figuring out what's the best option for you. So this would be something that would be in my discussion from visit one with my CSU patients.” 64% Survey of allergists who manage ~3,000 CSU patients
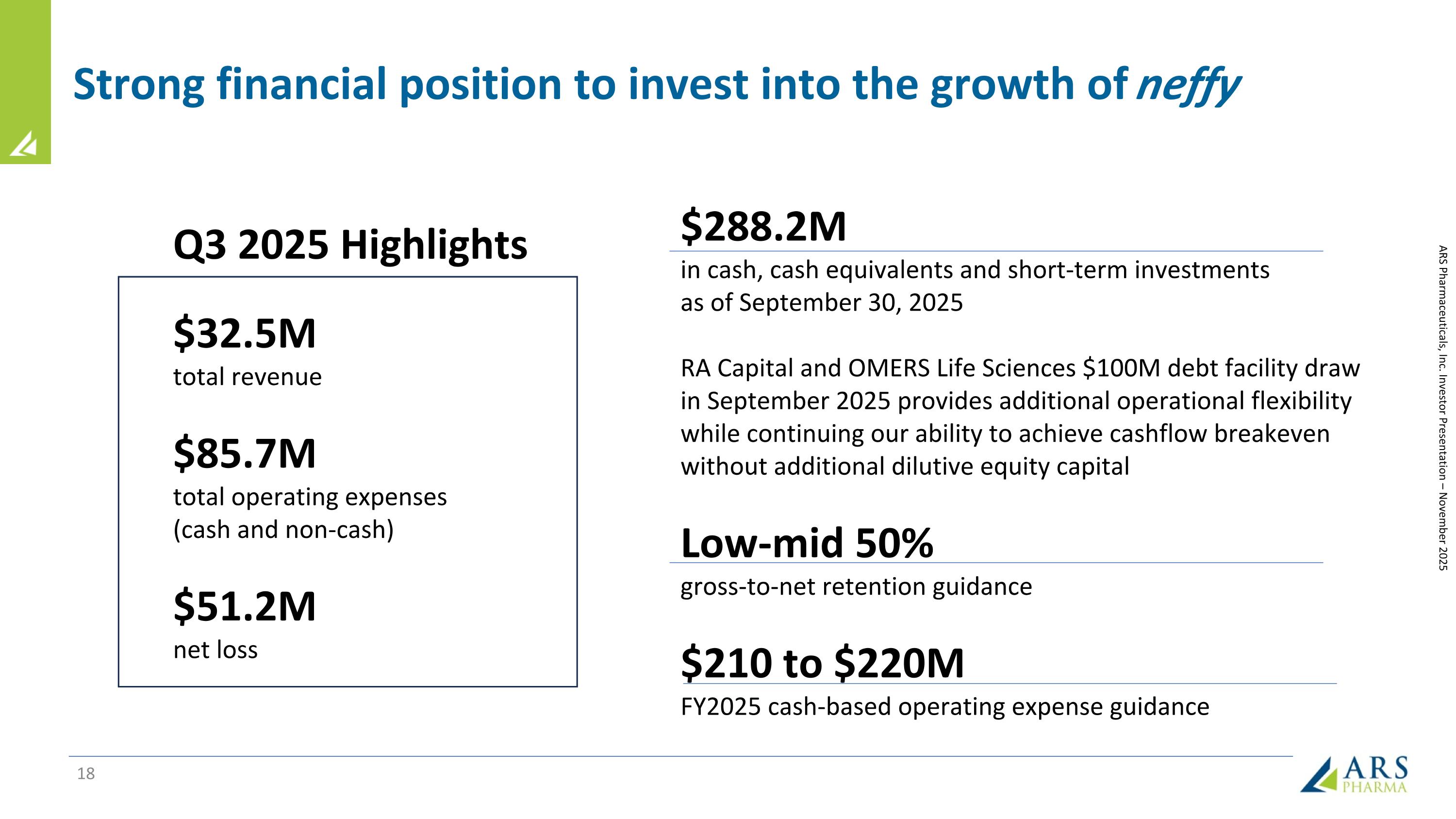
Strong financial position to invest into the growth of neffy $288.2M in cash, cash equivalents and short-term investments as of September 30, 2025 RA Capital and OMERS Life Sciences $100M debt facility draw in September 2025 provides additional operational flexibility while continuing our ability to achieve cashflow breakeven without additional dilutive equity capital Low-mid 50% gross-to-net retention guidance $210 to $220M FY2025 cash-based operating expense guidance Q3 2025 Highlights $32.5M total revenue $85.7M total operating expenses (cash and non-cash) $51.2M net loss ARS Pharmaceuticals, Inc. Investor Presentation – November 2025

A Clear Path for Continuing to Accelerate neffy Growth in 2026, and Maintain Potential Blockbuster Sales Trajectory Multi-blockbuster peak sales potential driven by initial $3.5B segment, ~$7B expansion segment and ~$2B+ CSU indication ARS Pharmaceuticals, Inc. Investor Presentation – November 2025 680+ documented cases of real-world anaphylaxis treated using neffy with a ~90% response rate to a single dose that is the same as injection 56% consumer aided awareness of neffy as of Sept 2025, with a similar or greater DTC spend investment as 2025 on an annualized basis in 2026 to further drive awareness $0 & <10 min co-pay and wait-time for getting neffy at getneffy.com, for commercially eligible patients, eliminating travel, wait time and HCP visit costs for patients for a seamless customer experience $288M Strong cash balance provides funding to cash-flow break, with GTN on track for steady-state >50% retention guidance including PBM additions and $0 co-pay ✓ ✓ ✓ ✓




















