UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): November 5, 2025
SERES THERAPEUTICS, INC.
(Exact name of Registrant as Specified in Its Charter)
| Delaware | 001-37465 | 27-4326290 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 101 Cambridgepark Drive | ||
| Cambridge, MA | 02140 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (617) 945-9626
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange on which registered |
||
| Common stock, par value $0.001 per share | MCRB | The Nasdaq Stock Market LLC | ||
| (Nasdaq Global Select Market) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 2.02. | Results of Operations and Financial Condition. |
On November 5, 2025, Seres Therapeutics, Inc. (the “Company”) announced its financial results for the quarter ended September 30, 2025 and provided operational updates. The full text of the press release issued in connection with the announcement is furnished as Exhibit 99.1 to this Current Report on Form 8-K (the “Current Report”).
| Item 7.01. | Regulation FD Disclosure. |
On November 5, 2025, the Company posted an updated corporate presentation in the “Investors and News” portion of its website at www.serestherapeutics.com. A copy of the slide presentation is attached as Exhibit 99.2 to this Current Report and incorporated herein by reference.
The information in Items 2.02 and 7.01 of this Current Report, including Exhibits 99.1 and 99.2 attached hereto, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
| Item 9.01. | Financial Statements and Exhibits. |
(d) Exhibits
The following Exhibits 99.1 and 99.2 relate to Items 2.02 and 7.01, respectively, and shall be deemed to be furnished, and not filed:
| Exhibit No. |
Description | |
| 99.1 | Seres Therapeutics, Inc. Press Release issued November 5, 2025 | |
| 99.2 | Seres Therapeutics, Inc. Corporate Presentation as of November 2025 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| SERES THERAPEUTICS, INC. | ||||||
| Date: November 5, 2025 | By: | /s/ Thomas J. DesRosier |
||||
| Name: | Thomas J. DesRosier | |||||
| Title: | Co-Chief Executive Officer, Co-President, Executive Vice President, Chief Legal Officer and Secretary |
|||||
Exhibit 99.1

SERES THERAPEUTICS REPORTS THIRD QUARTER 2025 FINANCIAL RESULTS AND
PROVIDES BUSINESS UPDATES
Following constructive FDA feedback, Seres is finalizing its SER-155 Phase 2 study protocol for
the prevention of bloodstream infections in adults undergoing allogeneic hematopoietic stem
cell transplant for the treatment of hematological malignancies
Efforts are ongoing to obtain capital and other resources to support SER-155 Phase 2 study;
pending securing funding, interim clinical results anticipated within 12 months of study initiation
Ongoing investigator-sponsored study in immune checkpoint related enterocolitis expected to
inform broader SER-155 opportunity; initial study results anticipated in early 2026
Seres recently implemented actions to reduce operating costs; based on these actions and
current operating plans, Seres expects to fund operations through Q2 2026
CAMBRIDGE, Mass.- November 5, 2025 — Seres Therapeutics, Inc. (Nasdaq: MCRB), (Seres or the Company), a leading live biotherapeutics company, today reported third quarter 2025 financial results and provided business updates.
“Following constructive feedback from the FDA, we are working to finalize our SER-155 Phase 2 study protocol for the prevention of bloodstream infections in adults undergoing allogeneic hematopoietic stem cell transplant (allo-HSCT) for hematological malignancies,” said Thomas DesRosier and Marella Thorell, co-Chief Executive Officers of Seres. “As we prepare for the next phase of SER-155 development, we continue our efforts aimed at securing the funding needed to conduct the Phase 2 study. We expect to be ready to rapidly operationalize the study once financing is in place, and we expect to obtain interim efficacy and safety results within 12 months of the study start. If these results are consistent with our prior successful Phase 1b study, and supportive of continued development, we believe this milestone should be a significant value-creating event for the Company and shareholders. Based on our on-going engagement with the medical community and assessment of the commercial landscape, we remain highly enthusiastic about the broad potential for SER-155—in allo-HSCT and other medically vulnerable populations.”
Mr. DesRosier and Ms. Thorell continued, “In addition to SER-155 development in allo-HSCT, we are expanding our understanding of the broader SER-155 therapeutic opportunity through an ongoing investigator-sponsored study in immune checkpoint related enterocolitis, initiated by the investigator at Memorial Sloan Kettering Cancer Center, and we look forward to obtaining initial efficacy results in early 2026. Additionally, during the third quarter, we implemented cost-reduction measures, including decreasing our workforce. Considering these actions and our current operating plans, we expect to fund operations through the second quarter of 2026, providing flexibility to advance our strategic priorities.”
Recent Highlights
SER-155 and Bloodstream Infection (BSI) Prevention
| • | Efforts are ongoing to obtain capital and other resources to support the SER-155 Phase 2 study. The Company is evaluating a range of potential deal structures that could leverage Seres’ live biotherapeutics expertise and success, as demonstrated by bringing VOWST™, the first FDA-approved oral microbiome therapeutic, from early development through FDA approval. |
| • | The Company received constructive feedback from the FDA during the third quarter and is working to finalize the planned Phase 2 study protocol for SER-155 in allo-HSCT, which previously received Breakthrough Therapy designation. The study is expected to enroll approximately 248 participants and incorporate an adaptive design and an interim data analysis when approximately half of the enrolled participants have reached the primary endpoint. The Company expects to obtain the interim clinical results within 12 months following study initiation, which it believes will facilitate timely engagement with the FDA on the design of a Phase 3 study, and inform development in adjacent medically vulnerable patient populations. The Company believes that positive results from the Phase 2 study, if achieved, could create significant value and enable advancement into a single Phase 3 trial to support registration. |
| • | In the third quarter, CARB-X (Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator), a global non-profit partnership accelerating antibacterial products to address drug-resistant bacteria, awarded Seres a grant of up to $3.6 million, representing the second grant the Company has received from CARB-X. The funds will support the development of an oral liquid formulation of SER-155, potentially expanding patient access for medically vulnerable populations who cannot be dosed with oral capsules. |
| • | In October, Seres presented an oral presentation at IDWeek 2025. The presentation included new post-hoc analysis from the completed SER-155 Phase 1b study describing differences between the SER-155 and placebo groups, including the bacterial and fungal organisms causing BSIs, BSI event clinical outcomes, antibacterial prophylaxis use, and patterns of antimicrobial resistance (AMR) among the bacterial BSI organisms. These new data illustrated that BSIs occurred despite antibacterial prophylaxis, and that BSI bacteria exhibited AMR. Resistance to multiple antibacterial agents was observed only in the BSI bacteria from placebo-treated participants, two of whom had fatal outcomes related to their BSIs. These new data further support the potential of SER-155 as an innovative alternative approach to the significant unmet medical need for prevention of BSIs in HSCT patients, especially those BSIs associated with AMR that increase the risk of morbidity and mortality. |
Development of Biotherapeutics for the Treatment of Inflammatory and Immune Diseases
| • | The Company is collaborating with Memorial Sloan Kettering Cancer Center on an investigator-sponsored trial (IST) which is evaluating SER-155 in participants with immune checkpoint related enterocolitis (irEC). Enrollment is anticipated to be completed by the end of 2025, with clinical readout expected in early 2026. IrEC is among the most frequent and severe immune-related adverse events (irAEs) in recipients of immune checkpoint-inhibitor therapy and can be observed in up to 50% of patients, with rates varying based on cancer drug and treatment regimen. Immune checkpoint inhibitors can cause a wide range of irAEs with links to T cell biology and epithelial barrier inflammation, both of which are biological functions shown in our preclinical and clinical pharmacology data to be positively impacted by SER-155. Supportive data from this IST could provide further support for the expansion of indications that may be well suited for our biotherapeutic approach. |
| • | Seres continues to explore potential R&D partnerships to advance development of its investigational live biotherapeutics in inflammatory and immune diseases, including ulcerative colitis and Crohn’s disease. |
Financial Results
The Company has classified all historical operating results for the VOWST business within discontinued operations in the consolidated statements of operations for the comparative periods presented (three and nine months ended September 30, 2024). There is no activity in the current period related to discontinued operations.
| • | Seres reported net income from continuing operations of $8.2 million for the third quarter of 2025, as compared to a net loss from continuing operations of $51.0 million for the same period in 2024. The net income of $8.2 million is comprised primarily of a $22.5 million loss from operations offset by a $27.2 million gain on sale of VOWST resulting primarily from the $25 million installment payment received, as expected, from Nestle during the third quarter. The net loss of $51.0 million in 2024 is primarily comprised of a $29.2 million loss from operations plus a $23.4 million loss on the extinguishment of the Oaktree debt, which was retired at completion of the VOWST sale in September 2024. |
| • | Research and development (R&D) expenses for the third quarter of 2025 were $12.6 million, compared with $16.5 million for the third quarter of 2024. The decrease in R&D expenses was primarily driven by a decrease in personnel and related costs, a decrease in platform investments, and a reduction in clinical expenses resulting from completion of the SER-155 Phase 1b study. |
| • | General and administrative (G&A) expenses for the third quarter of 2025 were $9.5 million, compared with $12.7 million for the third quarter of 2024. The decrease in G&A expenses was primarily a result of lower personnel and related expenses, including IT-related expenses. |
| • | Manufacturing services expenses were $0.7 million for the third quarter of 2025. These costs relate to the provision of manufacturing services under the Transition Services Agreement (TSA) with Nestlé, which began in the fourth quarter of 2024. The reimbursement received from Nestlé related to these expenses is recognized in other income. |
Cash Runway
As of September 30, 2025, Seres had $47.6 million in cash and cash equivalents. Based on the Company’s current cash position, VOWST transaction-related obligations, and current operating plans, the Company expects to fund operations through the second quarter of 2026. The Company continues to evaluate further opportunities to extend its cash runway.
Conference Call Information
Seres’ management will host a conference call today, November 5, 2025, at 8:30 a.m. ET. The conference call may be accessed by calling 1-800-715-9871 (international callers dial 1-646-307-1963) and referencing the conference ID number 8471287. To join the live webcast, please visit the “Investors and News” section of the Seres website at www.serestherapeutics.com. A webcast replay will be available on the Seres website shortly after the event and will be archived for at least 21 days.
About SER-155
SER-155 is an investigational, oral, live biotherapeutic designed to decolonize gastrointestinal (GI) pathogens, improve epithelial barrier integrity, and induce immune homeostasis, to prevent bacterial bloodstream infections, including those that can harbor antimicrobial resistance (AMR), as well as other pathogen associated negative clinical outcomes, in patients undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT).
SER-155 has been evaluated in a Phase 1b placebo-controlled study in patients undergoing allo-HSCT, which demonstrated a significant reduction in both bacterial bloodstream infections (BSIs) (77% relative risk reduction) and systemic antibiotic exposure, as well as lower incidence of febrile neutropenia. SER-155 has received Breakthrough Therapy designation for the reduction of bloodstream infections in adults undergoing allo-HCST and Fast Track designation for reducing the risk of infection and graft-versus-host disease in patients undergoing allo-HCST. The early development of the program was supported by Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X), a global non-profit partnership accelerating antibacterial products to address drug-resistant bacteria.
About Seres Therapeutics
Seres Therapeutics, Inc. (Nasdaq: MCRB) is a clinical-stage company focused on improving patient outcomes in medically vulnerable populations through novel live biotherapeutics. Seres led the successful development and approval of VOWST™, the first FDA-approved orally administered microbiome therapeutic, which was sold to Nestlé Health Science in September 2024. The Company is developing SER-155, which has received Breakthrough Therapy designation for the reduction of bloodstream infections in adults undergoing allo-HSCT and Fast Track designation for reducing the risk of infection and graft-versus-host disease in adults undergoing allo-HSCT, and which has demonstrated a significant reduction in bloodstream infections and related complications (as compared to placebo) in a Phase 1b clinical study in patients undergoing allo-HSCT. SER-155 and the Company’s other pipeline programs are designed to target multiple disease-relevant pathways and are manufactured from standard clonal cell banks via cultivation, rather than from the donor-sourced production process used for VOWST. In addition to allo-HSCT, the Company intends to evaluate SER-155 and other cultivated live biotherapeutic candidates in other medically vulnerable patient populations including autologous-HSCT patients, cancer patients with neutropenia, CAR-T recipients, individuals with chronic liver disease, solid organ transplant recipients, as well as patients in the intensive care unit and long-term acute care facilities. For more information, visit www.serestherapeutics.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including statements about: the design, timing and results of our clinical studies and data readouts; current or future product candidates and their potential benefits; clinical development plans and commercial opportunities; communications with, feedback from, or submissions to the FDA; operating plans; cost reduction actions and their anticipated benefits; our cash runway; our ability to secure a strategic, R&D, or other partnership and/or generate or obtain additional capital, financing or other resources; our ability to operationalize a study upon receipt of any financing; our planned strategic focus; the anticipated timing of any of the foregoing; and other statements that are not historical fact.
These forward-looking statements are based on management’s current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: (1) our need for additional funding; (2) our ability to continue as a going concern; (3) we have incurred significant losses, are not currently profitable and may never become profitable; (4) our cost reduction actions may not achieve their intended benefits, including an extended cash runway; (5) our limited operating history; (6) the expected payments from the VOSWT sale are subject to risks and uncertainties; (7) we may not be able to realize the anticipated benefits of the VOWST sale, and may face new challenges as a smaller, less diversified company; (8) we have in the past and may in the future receive notice of the failure to satisfy a continued listing rule from The Nasdaq Stock Market LLC; (9) our novel approach to therapeutic intervention; (10) our reliance on third parties to conduct our clinical trials and manufacture our product candidates; (11) our ability to achieve market acceptance necessary for commercial success; (12) the competition we will face; (13) our ability to protect our intellectual property; (14) our ability to manage our recent CEO transition, to retain key personnel and to manage our growth; and (15) disruptions at the FDA or other government agencies. These and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (SEC) on August 6, 2025 and our Quarterly Report on Form 10-Q to be filed with the SEC on November 5, 2025, as well as our other reports filed with the SEC, could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management’s estimates as of the date of this press release. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this press release.
SERES THERAPEUTICS, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(unaudited, in thousands, except share and per share data)
| September 30, 2025 |
December 31, 2024 |
|||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 47,638 | $ | 30,793 | ||||
| Accounts receivable due from SPN - related party |
426 | 2,068 | ||||||
| Accounts receivable |
351 | — | ||||||
| Prepaid expenses and other current assets (1) |
3,112 | 5,813 | ||||||
|
|
|
|
|
|||||
| Total current assets |
51,527 | 38,674 | ||||||
| Property and equipment, net |
8,578 | 11,534 | ||||||
| Operating lease assets |
74,669 | 80,903 | ||||||
| Restricted cash |
8,668 | 8,668 | ||||||
| Other non-current assets |
31 | 31 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 143,473 | $ | 139,810 | ||||
|
|
|
|
|
|||||
| Liabilities and Stockholders’ Equity |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 1,677 | $ | 4,079 | ||||
| Accrued expenses and other current liabilities |
6,382 | 10,719 | ||||||
| Accrued liabilities due to SPN - related party |
4,450 | 17,750 | ||||||
| Operating lease liabilities |
9,920 | 8,674 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
22,429 | 41,222 | ||||||
| Operating lease liabilities, net of current portion |
75,333 | 82,966 | ||||||
| Other long-term liabilities |
2,014 | 1,838 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
99,776 | 126,026 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies (Note 9) |
||||||||
| Stockholders’ equity (deficit): |
||||||||
| Preferred stock, $0.001 par value; 10,000,000 shares authorized at September 30, 2025 and December 31, 2024; no shares issued and outstanding at September 30, 2025 and December 31, 2024 |
— | — | ||||||
| Common stock, $0.001 par value; 360,000,000 shares authorized at September 30, 2025 and December 31, 2024; 8,764,664 and 8,650,227 shares issued and outstanding at September 30, 2025 and December 31, 2024, respectively |
9 | 9 | ||||||
| Additional paid-in capital |
1,000,756 | 991,874 | ||||||
| Accumulated other comprehensive loss |
— | — | ||||||
| Accumulated deficit |
(957,068 | ) | (978,099 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
43,697 | 13,784 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 143,473 | $ | 139,810 | ||||
|
|
|
|
|
|||||
| [1] | Includes $53 as of September 30, 2025 and $2,683 as of December 31, 2024 of unbilled receivable from SPN (related party) related to certain costs of the transition services performed by the Company. See Note 3, Discontinued Operations and TSA, for further details. |
SERES THERAPEUTICS, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME
(unaudited, in thousands, except share and per share data)
| Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||
| 2025 | 2024 | 2025 | 2024 | |||||||||||||
| Revenue: |
||||||||||||||||
| Grant revenue |
351 | — | 351 | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
351 | — | 351 | — | ||||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development expenses |
12,616 | 16,460 | 37,376 | 51,759 | ||||||||||||
| General and administrative expenses |
9,476 | 12,710 | 31,617 | 40,721 | ||||||||||||
| Manufacturing services |
736 | — | 5,952 | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
22,828 | 29,170 | 74,945 | 92,480 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(22,477 | ) | (29,170 | ) | (74,594 | ) | (92,480 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Other income (expense): |
||||||||||||||||
| Gain on sale of VOWST Business |
27,222 | — | 79,588 | — | ||||||||||||
| Interest income |
618 | 652 | 1,782 | 3,530 | ||||||||||||
| Other income (expense) (2) |
2,841 | (22,517 | ) | 14,255 | (21,184 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total other income (expense), net |
30,681 | (21,865 | ) | 95,625 | (17,654 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net income (loss) from continuing operations |
$ | 8,204 | $ | (51,035 | ) | $ | 21,031 | $ | (110,134 | ) | ||||||
| Net income from discontinued operations, net of tax |
$ | — | $ | 139,811 | $ | — | $ | 125,907 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net income and comprehensive income |
$ | 8,204 | $ | 88,776 | $ | 21,031 | $ | 15,773 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net income (loss) from continuing operations per share attributable to common stockholders - basic |
$ | 0.94 | $ | (6.69 | ) | $ | 2.41 | $ | (14.68 | ) | ||||||
| Net income from discontinued operations per share attributable to common stockholders - basic |
$ | — | $ | 18.32 | $ | — | $ | 16.78 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net income per share attributable to common stockholders - basic |
$ | 0.94 | $ | 11.63 | $ | 2.41 | $ | 2.10 | ||||||||
| Net income (loss) from continuing operations per share attributable to common stockholders - diluted |
$ | 0.94 | $ | (6.69 | ) | $ | 2.40 | $ | (14.68 | ) | ||||||
| Net income from discontinued operations per share attributable to common stockholders - diluted |
$ | — | $ | 18.32 | $ | — | $ | 16.78 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net income per share attributable to common stockholders - diluted |
$ | 0.94 | $ | 11.63 | $ | 2.40 | $ | 2.10 | ||||||||
| Weighted average common shares outstanding - basic |
8,758,692 | 7,632,242 | 8,735,418 | 7,504,763 | ||||||||||||
| Weighted average common shares outstanding - diluted |
8,771,519 | 7,632,242 | 8,744,797 | 7,504,763 | ||||||||||||
| [2] | Includes $2,028 and $11,827 for the three and nine months ended September 30, 2025 related to reimbursement received from SPN (related party) for transition services provided by the Company. |
Investor and Media Contact:
IR@serestherapeutics.com
Carlo Tanzi, Ph.D.
Kendall Investor Relations
ctanzi@kendallir.com

Seres Therapeutics Investor Presentation November 2025 Exhibit 99.2

Disclaimers Forward Looking Statements This communication contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this communication that do not relate to matters of historical fact should be considered forward-looking statements, including statements about: the timing and results of our current or planned clinical trials, studies and data readouts; current or future products or product candidates and their potential benefits; our clinical development plans; communications with, feedback from, or submissions to the FDA; future product candidates; our ability to secure additional capital, financing, or other resources; the potential market and commercial opportunity for SER-155 and other product candidates, if approved; operating plans, cost reductions actions, and our future cash runway, including potential payments due from Nestlé related to the VOWST sale; our planned strategic focus; the timing of any of the foregoing; and other statements that are not historical fact. These forward-looking statements are based on management's current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: (1) our need for additional funding; (2) our ability to continue as a going concern; (3) we have incurred significant losses, are not currently profitable and may never become profitable; (4) our limited operating history; (5) the expected payments from the VOSWT sale are subject to risks and uncertainties; (6) we may not be able to realize the anticipated benefits of the VOWST sale, and may face new challenges as a smaller, less diversified company; (7) we have in the past and may in the future receive notice of the failure to satisfy a continued listing rule from The Nasdaq Stock Market LLC; (8) our novel approach to therapeutic intervention; (9) our reliance on third parties to conduct our clinical trials and manufacture our product candidates; (10) our ability to achieve market acceptance necessary for commercial success; (11) the competition we will face; (12) our ability to protect our intellectual property; and (13) our ability to manage our recent CEO transition, to retain key personnel and to manage our growth. These and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q to be filed with the Securities and Exchange Commission (SEC) on November 5, 2025, as well as our other reports filed with the SEC, could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management's estimates as of the date of this communication. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this communication. Seres Therapeutics, Inc. © 2025
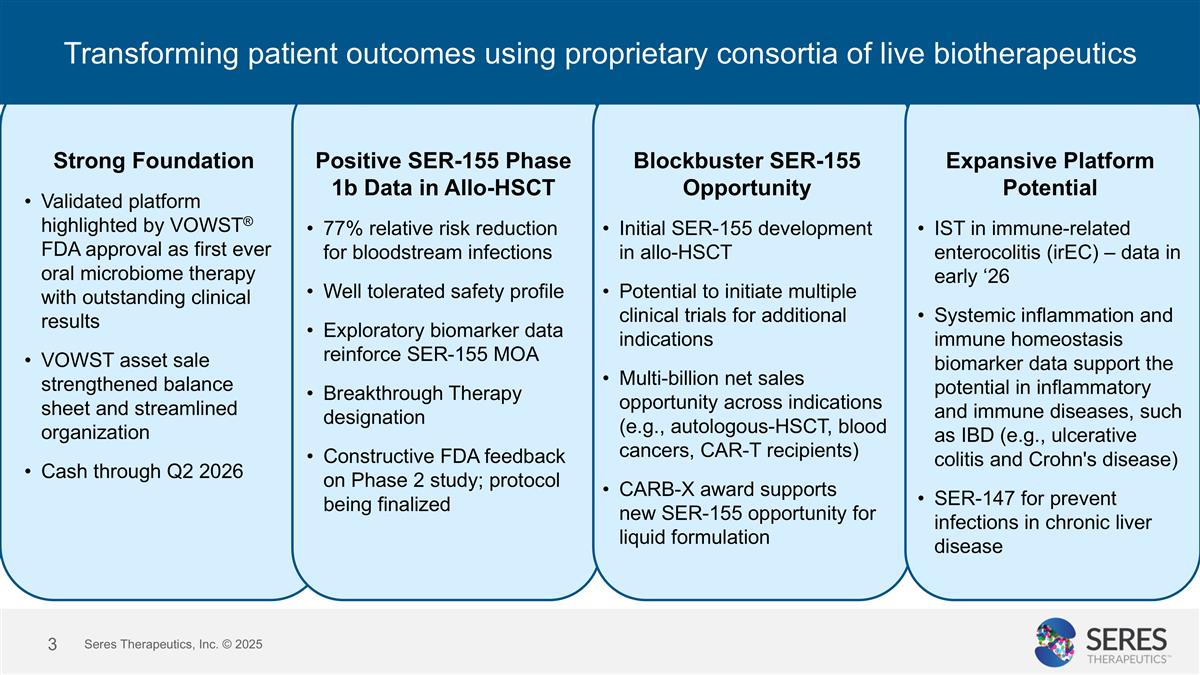
Transforming patient outcomes using proprietary consortia of live biotherapeutics Strong Foundation Validated platform highlighted by VOWST® FDA approval as first ever oral microbiome therapy with outstanding clinical results VOWST asset sale strengthened balance sheet and streamlined organization Cash through Q2 2026 Positive SER-155 Phase 1b Data in Allo-HSCT 77% relative risk reduction for bloodstream infections Well tolerated safety profile Exploratory biomarker data reinforce SER-155 MOA Breakthrough Therapy designation Constructive FDA feedback on Phase 2 study; protocol being finalized Blockbuster SER-155 Opportunity Initial SER-155 development in allo-HSCT Potential to initiate multiple clinical trials for additional indications Multi-billion net sales opportunity across indications (e.g., autologous-HSCT, blood cancers, CAR-T recipients) CARB-X award supports new SER-155 opportunity for liquid formulation Expansive Platform Potential IST in immune-related enterocolitis (irEC) – data in early ‘26 Systemic inflammation and immune homeostasis biomarker data support the potential in inflammatory and immune diseases, such as IBD (e.g., ulcerative colitis and Crohn's disease) SER-147 for prevent infections in chronic liver disease Seres Therapeutics, Inc. © 2025
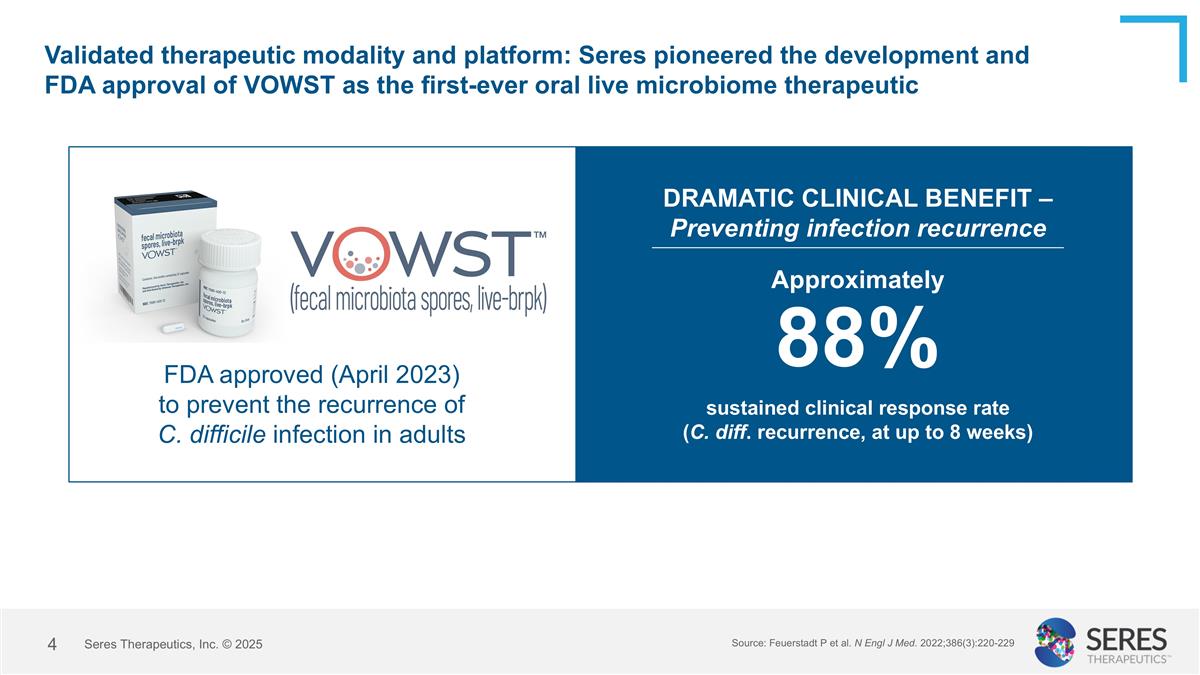
Validated therapeutic modality and platform: Seres pioneered the development and FDA approval of VOWST as the first-ever oral live microbiome therapeutic FDA approved (April 2023) to prevent the recurrence of C. difficile infection in adults Approximately 88% sustained clinical response rate (C. diff. recurrence, at up to 8 weeks) DRAMATIC CLINICAL BENEFIT – Preventing infection recurrence Source: Feuerstadt P et al. N Engl J Med. 2022;386(3):220-229 Seres Therapeutics, Inc. © 2025

VOWST asset sale to Nestle Healh Science completed in September 2024: transformational for Seres – provided resources to support SER-155 development KEY FINANCIAL TERMS VOWST asset purchase agreement provided infusion of capital to support SER-155 development Asset sale extended operational runway Retired debt and other obligations Majority of transition activities complete as of Q2 2025; manufacturing services through end of 2025 $100M upfront payment to Seres, less ~$20M in net obligations due to an affiliate of SPN* $15M equity investment by SPN at closing $60M prepaid sales-based milestone at closing $50M received in January 2025 $25M (offset by $1.4M in employment-related payment) received in July 2025 $275M in potential future sales-based milestone payments (subject to reductions for interest on prepaid milestone payment) Transaction results in a more streamlined, focused Seres organization and lower cash burn rate *SPN: Société des Produits Nestlé S.A. Seres Therapeutics, Inc. © 2025
The gut microbiome has substantial untapped therapeutic potential to both prevent and treat diseases The genetic content of the microbes living within and on your body is at least 100-fold greater than that contained in the human genome Microbes co-evolved for millions of years with and are integral to immune & metabolic function of the human body Sources: Ley et al. (2006) Cell; Qin et al. (2010) Nature Seres Therapeutics, Inc. © 2025
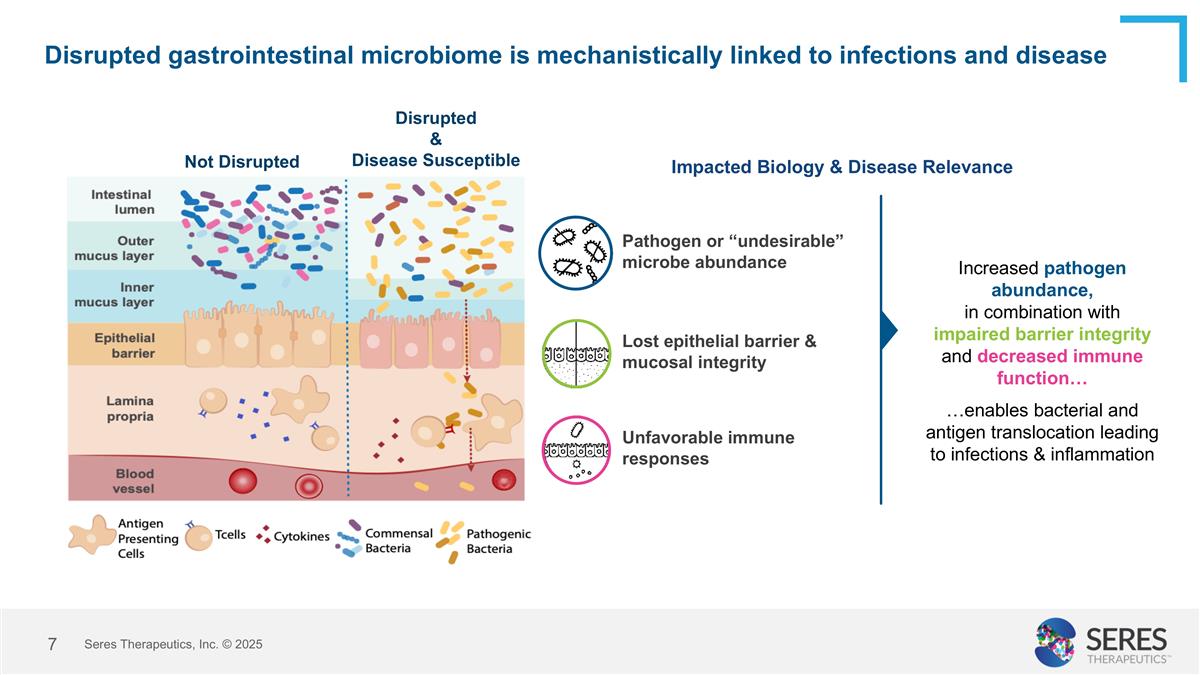
Disrupted gastrointestinal microbiome is mechanistically linked to infections and disease Impacted Biology & Disease Relevance Increased pathogen abundance, in combination with impaired barrier integrity and decreased immune function… …enables bacterial and antigen translocation leading to infections & inflammation Disrupted & Disease Susceptible Not Disrupted Lost epithelial barrier & mucosal integrity Unfavorable immune responses Pathogen or “undesirable” microbe abundance Seres Therapeutics, Inc. © 2025
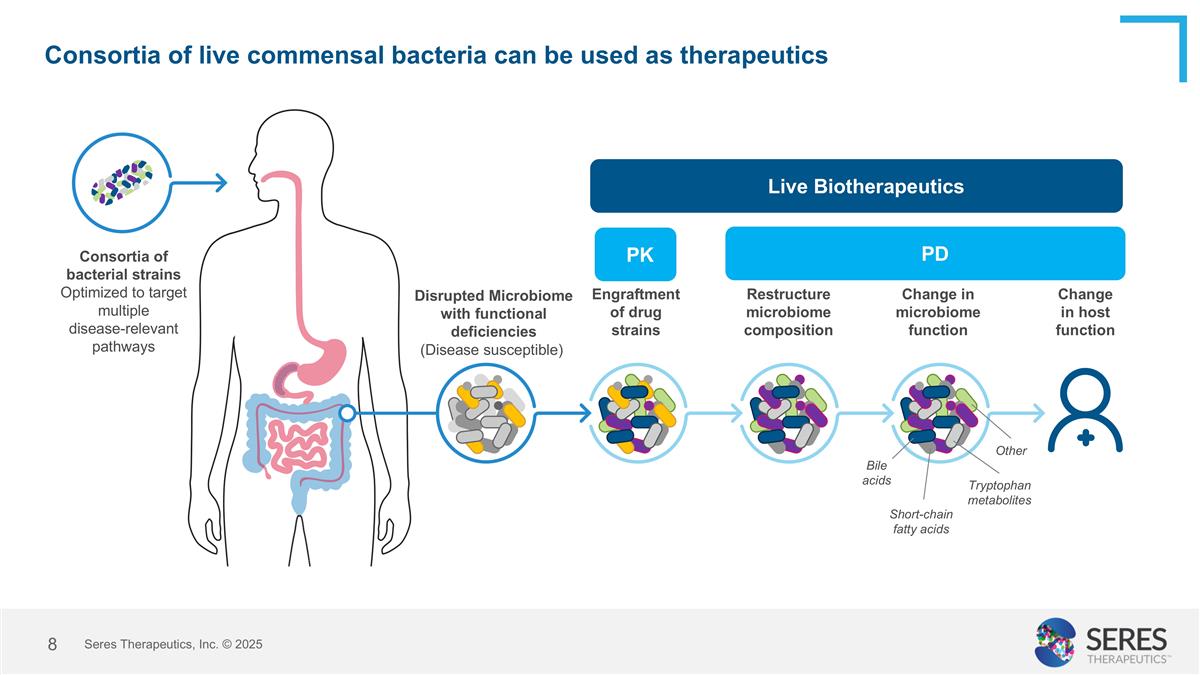
Consortia of live commensal bacteria can be used as therapeutics Change in microbiome function Bile acids Short-chain fatty acids Tryptophan metabolites Other Restructure microbiome composition Engraftment of drug strains Disrupted Microbiome with functional deficiencies (Disease susceptible) Change in host function Consortia of bacterial strains Optimized to target multiple disease-relevant pathways Live Biotherapeutics PK PD Seres Therapeutics, Inc. © 2025

Seres’ biotherapeutics and pipeline candidates are expected to have well tolerated safety profile, reducing development risk Sources: Nanayakkara et al, CA Cancer J Clin 2021; Penack et al, Blood Adv 2020; Zheng et al, Infect Dis Ther 2021 Based on GI bacteria naturally found in healthy humans, and not associated with disease VOWST product profile includes well tolerated safety without drug-related serious adverse events Well tolerated safety profile in multiple clinical trials and patient populations, including medically vulnerable allo-HSCT recipients Safety profile has potential to mitigate a primary cause of drug development failure Seres Therapeutics, Inc. © 2025
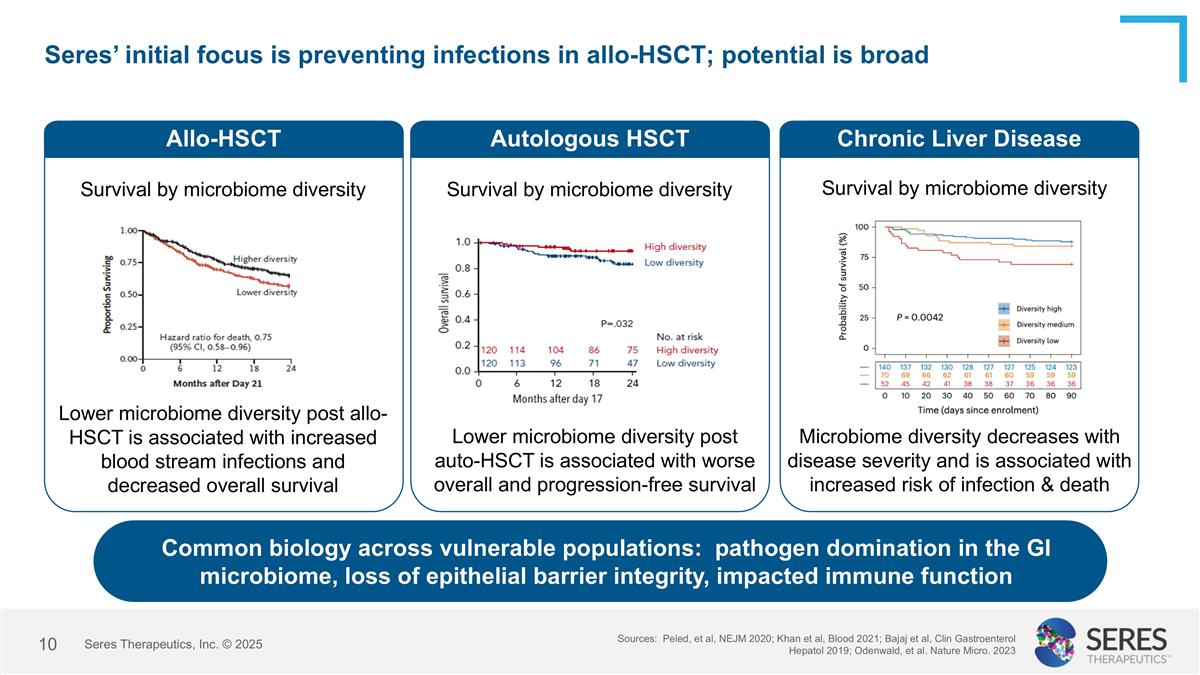
Seres’ initial focus is preventing infections in allo-HSCT; potential is broad Allo-HSCT Survival by microbiome diversity Lower microbiome diversity post allo-HSCT is associated with increased blood stream infections and decreased overall survival Autologous HSCT Survival by microbiome diversity Lower microbiome diversity post auto-HSCT is associated with worse overall and progression-free survival Chronic Liver Disease Microbiome diversity decreases with disease severity and is associated with increased risk of infection & death Sources: Peled, et al, NEJM 2020; Khan et al, Blood 2021; Bajaj et al, Clin Gastroenterol Hepatol 2019; Odenwald, et al. Nature Micro. 2023 Common biology across vulnerable populations: pathogen domination in the GI microbiome, loss of epithelial barrier integrity, impacted immune function Survival by microbiome diversity Seres Therapeutics, Inc. © 2025
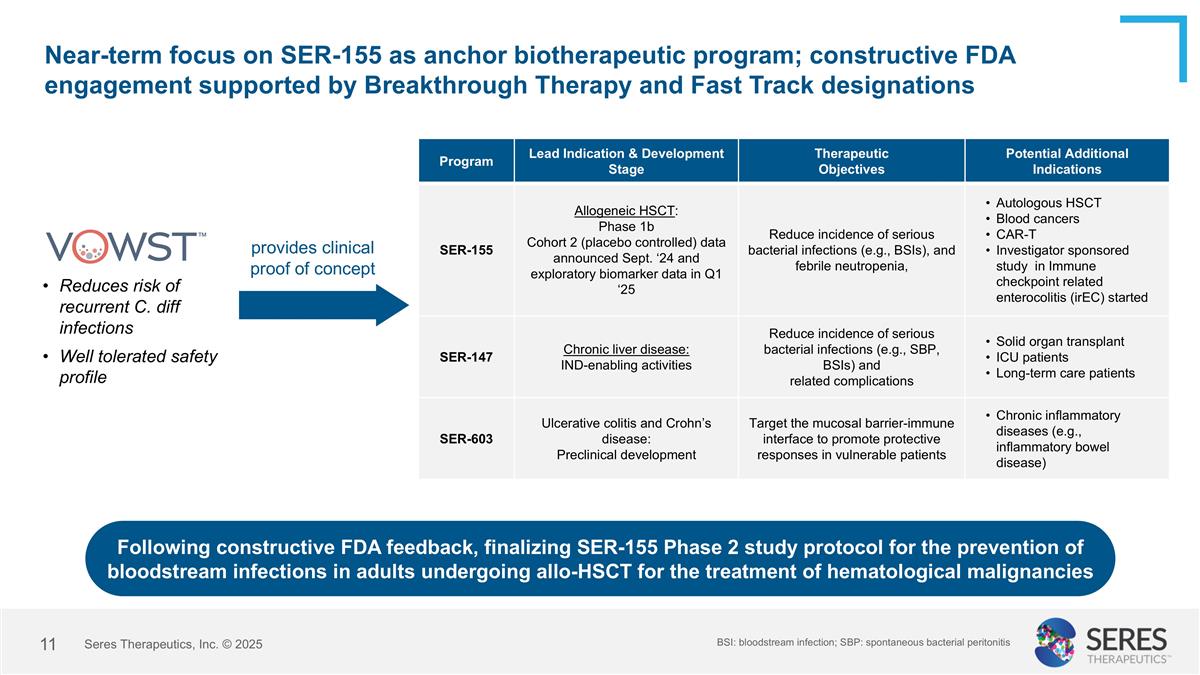
Near-term focus on SER-155 as anchor biotherapeutic program; constructive FDA engagement supported by Breakthrough Therapy and Fast Track designations Program Lead Indication & Development Stage Therapeutic Objectives Potential Additional Indications SER-155 Allogeneic HSCT: Phase 1b Cohort 2 (placebo controlled) data announced Sept. ‘24 and exploratory biomarker data in Q1 ‘25 Reduce incidence of serious bacterial infections (e.g., BSIs), and febrile neutropenia, Autologous HSCT Blood cancers CAR-T Investigator sponsored study in Immune checkpoint related enterocolitis (irEC) started SER-147 Chronic liver disease: IND-enabling activities Reduce incidence of serious bacterial infections (e.g., SBP, BSIs) and related complications Solid organ transplant ICU patients Long-term care patients SER-603 Ulcerative colitis and Crohn’s disease: Preclinical development Target the mucosal barrier-immune interface to promote protective responses in vulnerable patients Chronic inflammatory diseases (e.g., inflammatory bowel disease) provides clinical proof of concept Reduces risk of recurrent C. diff infections Well tolerated safety profile Following constructive FDA feedback, finalizing SER-155 Phase 2 study protocol for the prevention of bloodstream infections in adults undergoing allo-HSCT for the treatment of hematological malignancies BSI: bloodstream infection; SBP: spontaneous bacterial peritonitis Seres Therapeutics, Inc. © 2025
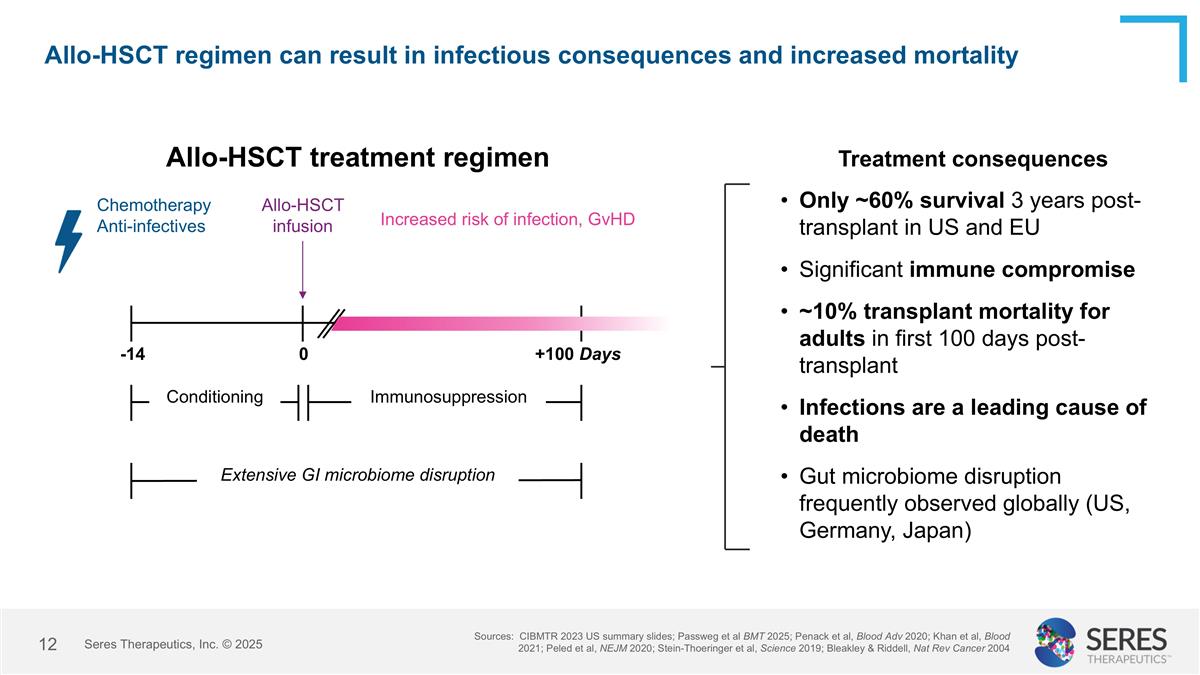
Allo-HSCT regimen can result in infectious consequences and increased mortality Sources: CIBMTR 2023 US summary slides; Passweg et al BMT 2025; Penack et al, Blood Adv 2020; Khan et al, Blood 2021; Peled et al, NEJM 2020; Stein-Thoeringer et al, Science 2019; Bleakley & Riddell, Nat Rev Cancer 2004 Seres Therapeutics, Inc. © 2025 Only ~60% survival 3 years post-transplant in US and EU Significant immune compromise ~10% transplant mortality for adults in first 100 days post-transplant Infections are a leading cause of death Gut microbiome disruption frequently observed globally (US, Germany, Japan) Allo-HSCT treatment regimen -14 0 +100 Days Conditioning Immunosuppression Chemotherapy Anti-infectives Increased risk of infection, GvHD Allo-HSCT infusion Extensive GI microbiome disruption Treatment consequences
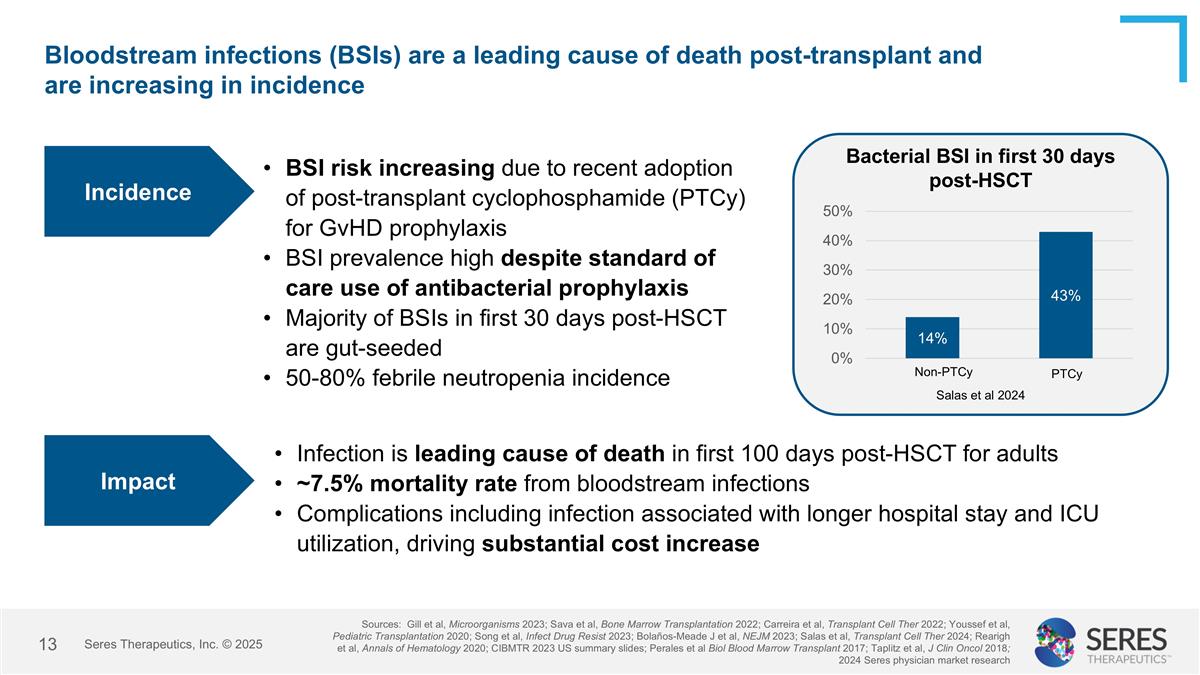
Bloodstream infections (BSIs) are a leading cause of death post-transplant and are increasing in incidence BSI risk increasing due to recent adoption of post-transplant cyclophosphamide (PTCy) for GvHD prophylaxis BSI prevalence high despite standard of care use of antibacterial prophylaxis Majority of BSIs in first 30 days post-HSCT are gut-seeded 50-80% febrile neutropenia incidence Incidence Sources: Gill et al, Microorganisms 2023; Sava et al, Bone Marrow Transplantation 2022; Carreira et al, Transplant Cell Ther 2022; Youssef et al, Pediatric Transplantation 2020; Song et al, Infect Drug Resist 2023; Bolaños-Meade J et al, NEJM 2023; Salas et al, Transplant Cell Ther 2024; Rearigh et al, Annals of Hematology 2020; CIBMTR 2023 US summary slides; Perales et al Biol Blood Marrow Transplant 2017; Taplitz et al, J Clin Oncol 2018; 2024 Seres physician market research Bacterial BSI in first 30 days post-HSCT Salas et al 2024 Impact Infection is leading cause of death in first 100 days post-HSCT for adults ~7.5% mortality rate from bloodstream infections Complications including infection associated with longer hospital stay and ICU utilization, driving substantial cost increase PTCy Non-PTCy Seres Therapeutics, Inc. © 2025
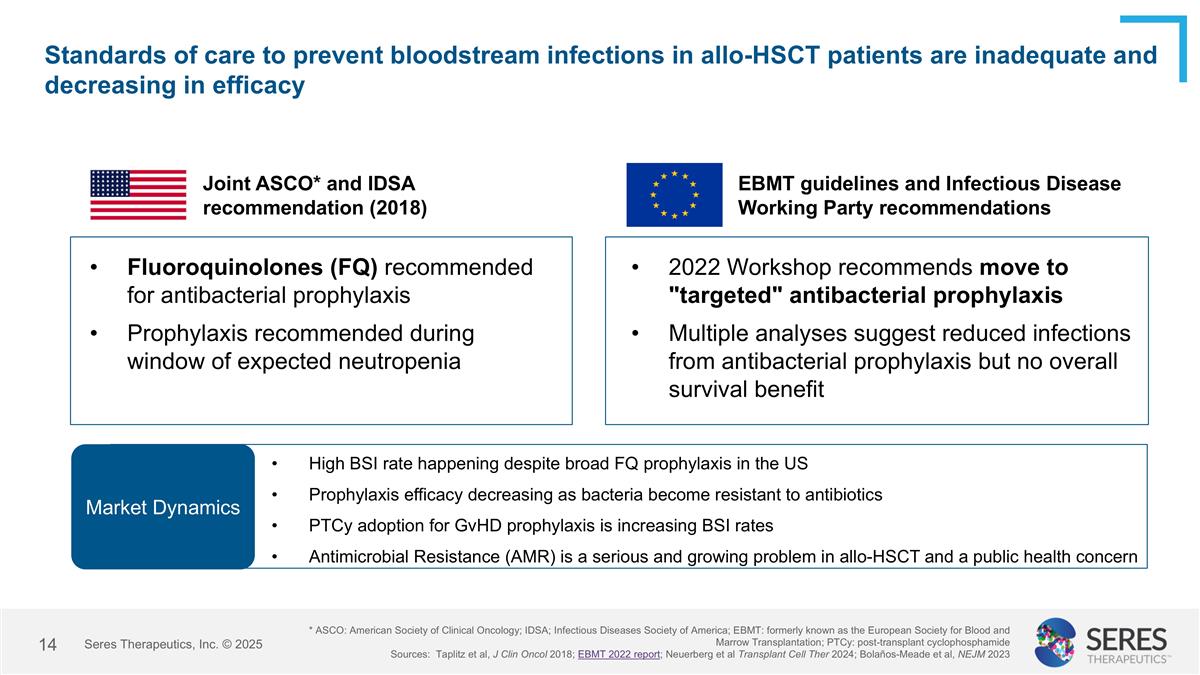
Standards of care to prevent bloodstream infections in allo-HSCT patients are inadequate and decreasing in efficacy * ASCO: American Society of Clinical Oncology; IDSA; Infectious Diseases Society of America; EBMT: formerly known as the European Society for Blood and Marrow Transplantation; PTCy: post-transplant cyclophosphamide Sources: Taplitz et al, J Clin Oncol 2018; EBMT 2022 report; Neuerberg et al Transplant Cell Ther 2024; Bolaños-Meade et al, NEJM 2023 Joint ASCO* and IDSA recommendation (2018) Fluoroquinolones (FQ) recommended for antibacterial prophylaxis Prophylaxis recommended during window of expected neutropenia EBMT guidelines and Infectious Disease Working Party recommendations 2022 Workshop recommends move to "targeted" antibacterial prophylaxis Multiple analyses suggest reduced infections from antibacterial prophylaxis but no overall survival benefit Market Dynamics High BSI rate happening despite broad FQ prophylaxis in the US Prophylaxis efficacy decreasing as bacteria become resistant to antibiotics PTCy adoption for GvHD prophylaxis is increasing BSI rates Antimicrobial Resistance (AMR) is a serious and growing problem in allo-HSCT and a public health concern Seres Therapeutics, Inc. © 2025
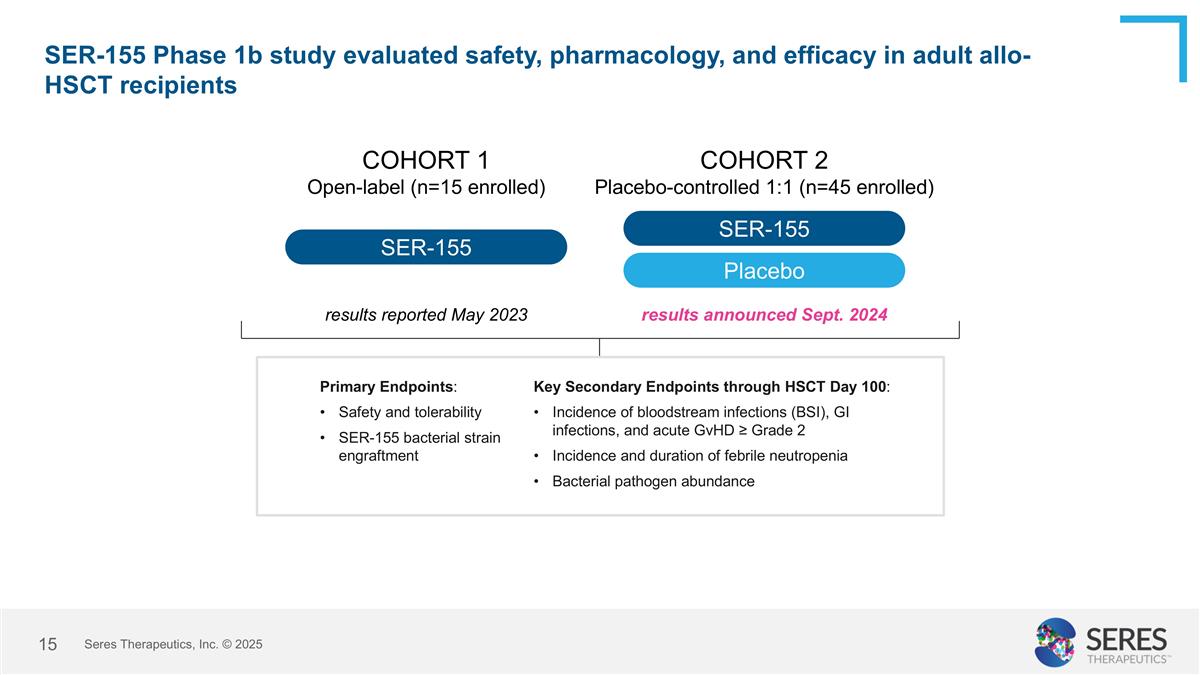
SER-155 Phase 1b study evaluated safety, pharmacology, and efficacy in adult allo-HSCT recipients SER-155 COHORT 1 Open-label (n=15 enrolled) COHORT 2 Placebo-controlled 1:1 (n=45 enrolled) SER-155 Placebo results reported May 2023 results announced Sept. 2024 Primary Endpoints: Safety and tolerability SER-155 bacterial strain engraftment Key Secondary Endpoints through HSCT Day 100: Incidence of bloodstream infections (BSI), GI infections, and acute GvHD ≥ Grade 2 Incidence and duration of febrile neutropenia Bacterial pathogen abundance Seres Therapeutics, Inc. © 2025
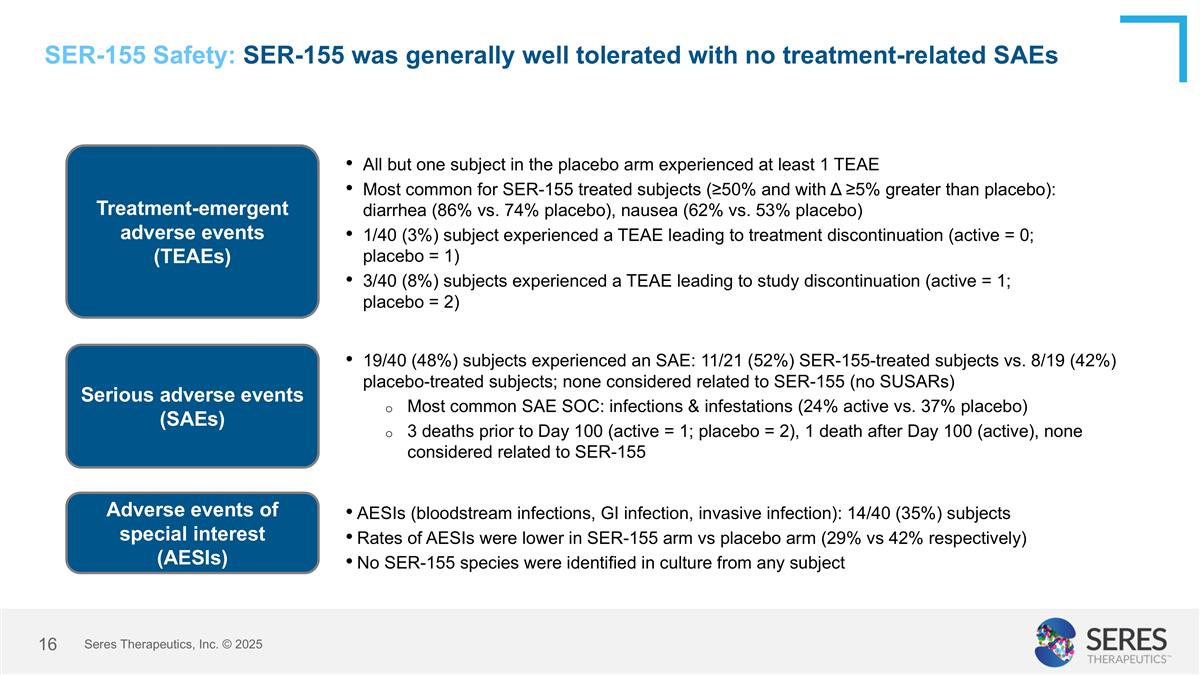
SER-155 Safety: SER-155 was generally well tolerated with no treatment-related SAEs Treatment-emergent adverse events (TEAEs) All but one subject in the placebo arm experienced at least 1 TEAE Most common for SER-155 treated subjects (≥50% and with Δ ≥5% greater than placebo): diarrhea (86% vs. 74% placebo), nausea (62% vs. 53% placebo) 1/40 (3%) subject experienced a TEAE leading to treatment discontinuation (active = 0; placebo = 1) 3/40 (8%) subjects experienced a TEAE leading to study discontinuation (active = 1; placebo = 2) Serious adverse events (SAEs) 19/40 (48%) subjects experienced an SAE: 11/21 (52%) SER-155-treated subjects vs. 8/19 (42%) placebo-treated subjects; none considered related to SER-155 (no SUSARs) Most common SAE SOC: infections & infestations (24% active vs. 37% placebo) 3 deaths prior to Day 100 (active = 1; placebo = 2), 1 death after Day 100 (active), none considered related to SER-155 Adverse events of special interest (AESIs) AESIs (bloodstream infections, GI infection, invasive infection): 14/40 (35%) subjects Rates of AESIs were lower in SER-155 arm vs placebo arm (29% vs 42% respectively) No SER-155 species were identified in culture from any subject Seres Therapeutics, Inc. © 2025
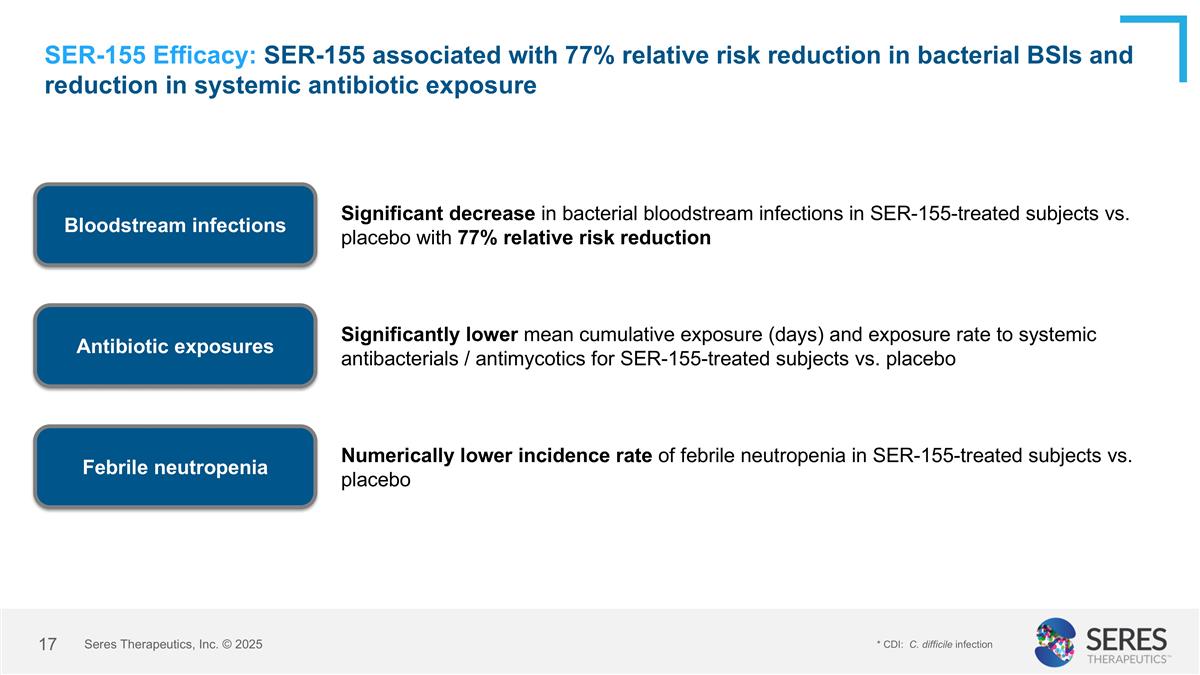
SER-155 Efficacy: SER-155 associated with 77% relative risk reduction in bacterial BSIs and reduction in systemic antibiotic exposure Bloodstream infections Significant decrease in bacterial bloodstream infections in SER-155-treated subjects vs. placebo with 77% relative risk reduction Numerically lower incidence rate of febrile neutropenia in SER-155-treated subjects vs. placebo Febrile neutropenia Antibiotic exposures Significantly lower mean cumulative exposure (days) and exposure rate to systemic antibacterials / antimycotics for SER-155-treated subjects vs. placebo * CDI: C. difficile infection Seres Therapeutics, Inc. © 2025
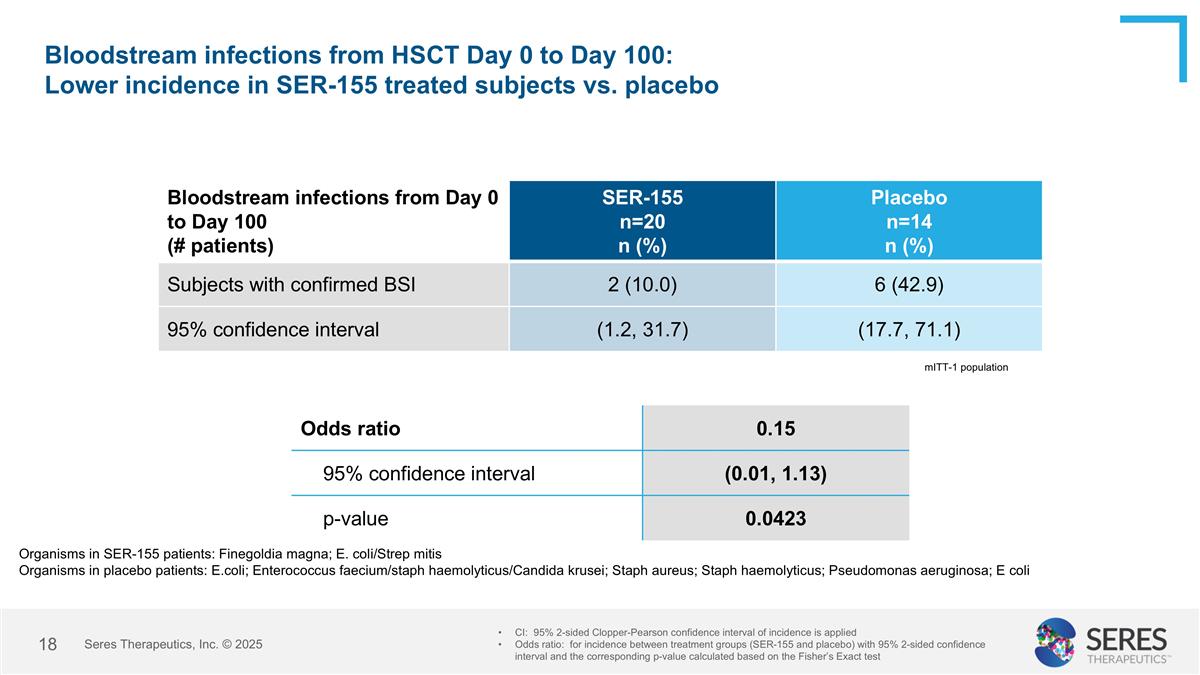
Bloodstream infections from HSCT Day 0 to Day 100: Lower incidence in SER-155 treated subjects vs. placebo Bloodstream infections from Day 0 to Day 100 (# patients) SER-155 n=20 n (%) Placebo n=14 n (%) Subjects with confirmed BSI 2 (10.0) 6 (42.9) 95% confidence interval (1.2, 31.7) (17.7, 71.1) CI: 95% 2-sided Clopper-Pearson confidence interval of incidence is applied Odds ratio: for incidence between treatment groups (SER-155 and placebo) with 95% 2-sided confidence interval and the corresponding p-value calculated based on the Fisher’s Exact test Odds ratio 0.15 95% confidence interval (0.01, 1.13) p-value 0.0423 mITT-1 population Organisms in SER-155 patients: Finegoldia magna; E. coli/Strep mitis Organisms in placebo patients: E.coli; Enterococcus faecium/staph haemolyticus/Candida krusei; Staph aureus; Staph haemolyticus; Pseudomonas aeruginosa; E coli Seres Therapeutics, Inc. © 2025
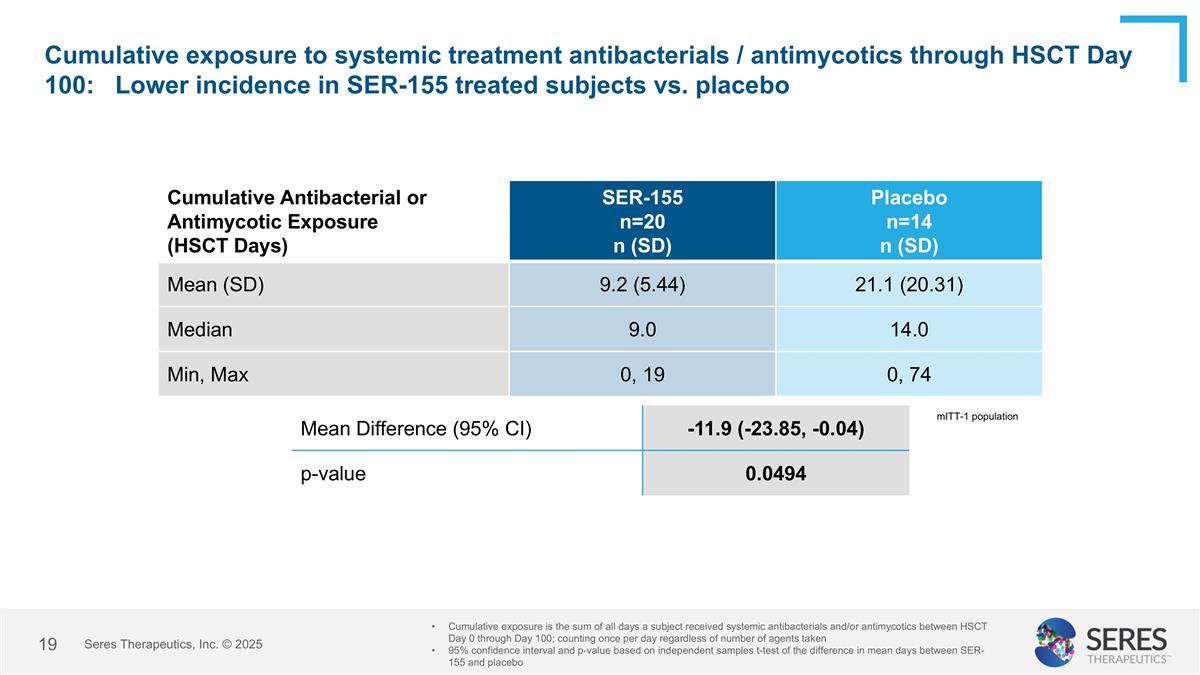
Cumulative exposure to systemic treatment antibacterials / antimycotics through HSCT Day 100: Lower incidence in SER-155 treated subjects vs. placebo Cumulative Antibacterial or Antimycotic Exposure (HSCT Days) SER-155 n=20 n (SD) Placebo n=14 n (SD) Mean (SD) 9.2 (5.44) 21.1 (20.31) Median 9.0 14.0 Min, Max 0, 19 0, 74 Cumulative exposure is the sum of all days a subject received systemic antibacterials and/or antimycotics between HSCT Day 0 through Day 100; counting once per day regardless of number of agents taken 95% confidence interval and p-value based on independent samples t-test of the difference in mean days between SER-155 and placebo Mean Difference (95% CI) -11.9 (-23.85, -0.04) p-value 0.0494 mITT-1 population Seres Therapeutics, Inc. © 2025
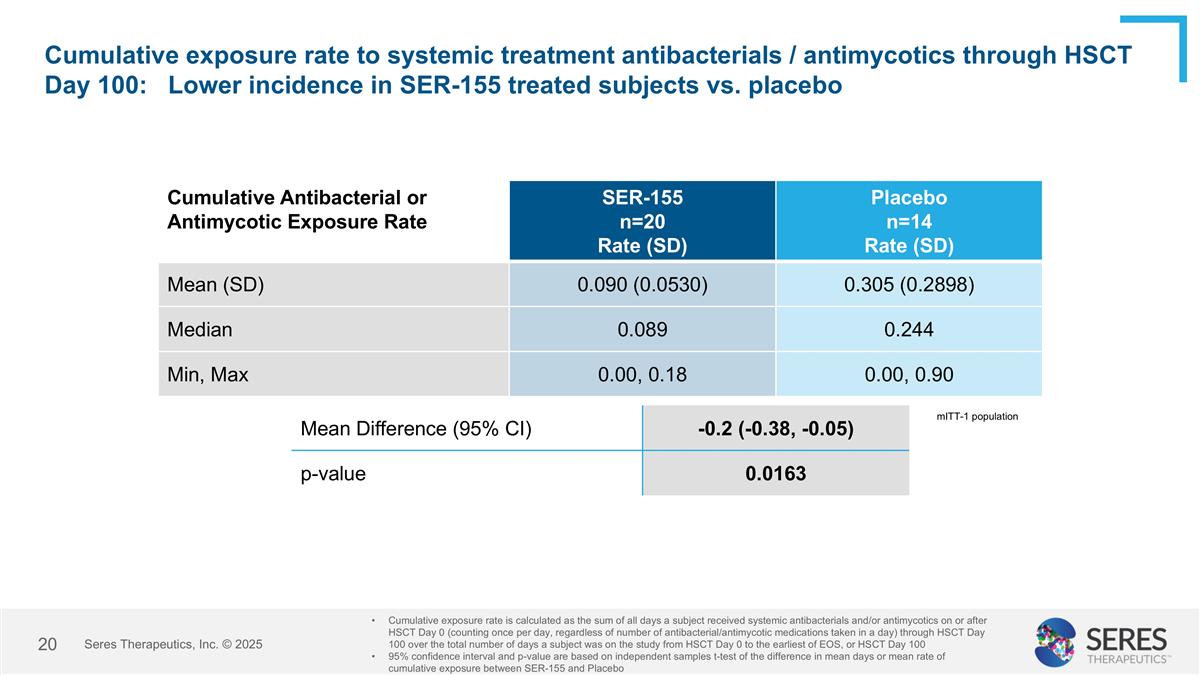
Cumulative exposure rate to systemic treatment antibacterials / antimycotics through HSCT Day 100: Lower incidence in SER-155 treated subjects vs. placebo Cumulative Antibacterial or Antimycotic Exposure Rate SER-155 n=20 Rate (SD) Placebo n=14 Rate (SD) Mean (SD) 0.090 (0.0530) 0.305 (0.2898) Median 0.089 0.244 Min, Max 0.00, 0.18 0.00, 0.90 Cumulative exposure rate is calculated as the sum of all days a subject received systemic antibacterials and/or antimycotics on or after HSCT Day 0 (counting once per day, regardless of number of antibacterial/antimycotic medications taken in a day) through HSCT Day 100 over the total number of days a subject was on the study from HSCT Day 0 to the earliest of EOS, or HSCT Day 100 95% confidence interval and p-value are based on independent samples t-test of the difference in mean days or mean rate of cumulative exposure between SER-155 and Placebo Mean Difference (95% CI) -0.2 (-0.38, -0.05) p-value 0.0163 mITT-1 population Seres Therapeutics, Inc. © 2025
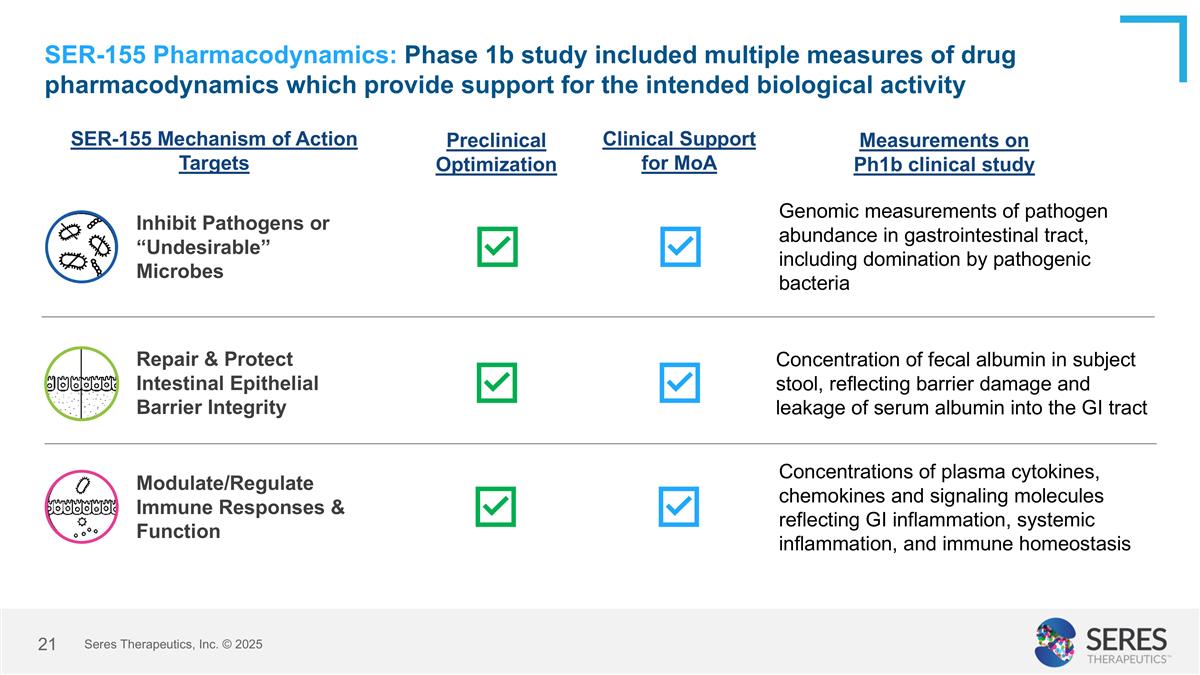
Measurements on Ph1b clinical study Preclinical Optimization SER-155 Pharmacodynamics: Phase 1b study included multiple measures of drug pharmacodynamics which provide support for the intended biological activity SER-155 Mechanism of Action Targets Concentration of fecal albumin in subject stool, reflecting barrier damage and leakage of serum albumin into the GI tract Repair & Protect Intestinal Epithelial Barrier Integrity Concentrations of plasma cytokines, chemokines and signaling molecules reflecting GI inflammation, systemic inflammation, and immune homeostasis Modulate/Regulate Immune Responses & Function Genomic measurements of pathogen abundance in gastrointestinal tract, including domination by pathogenic bacteria Inhibit Pathogens or “Undesirable” Microbes Clinical Support for MoA Seres Therapeutics, Inc. © 2025
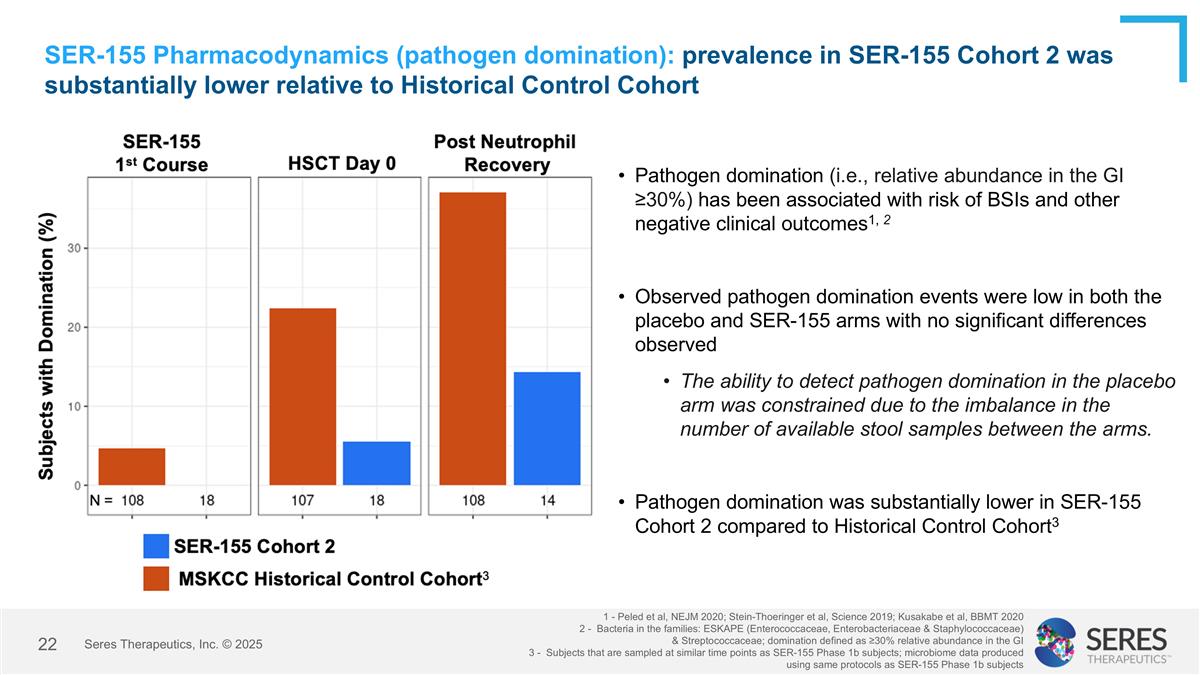
SER-155 Pharmacodynamics (pathogen domination): prevalence in SER-155 Cohort 2 was substantially lower relative to Historical Control Cohort Pathogen domination (i.e., relative abundance in the GI ≥30%) has been associated with risk of BSIs and other negative clinical outcomes1, 2 Observed pathogen domination events were low in both the placebo and SER-155 arms with no significant differences observed The ability to detect pathogen domination in the placebo arm was constrained due to the imbalance in the number of available stool samples between the arms. Pathogen domination was substantially lower in SER-155 Cohort 2 compared to Historical Control Cohort3 1 - Peled et al, NEJM 2020; Stein-Thoeringer et al, Science 2019; Kusakabe et al, BBMT 2020 2 - Bacteria in the families: ESKAPE (Enterococcaceae, Enterobacteriaceae & Staphylococcaceae) & Streptococcaceae; domination defined as ≥30% relative abundance in the GI 3 - Subjects that are sampled at similar time points as SER-155 Phase 1b subjects; microbiome data produced using same protocols as SER-155 Phase 1b subjects Seres Therapeutics, Inc. © 2025
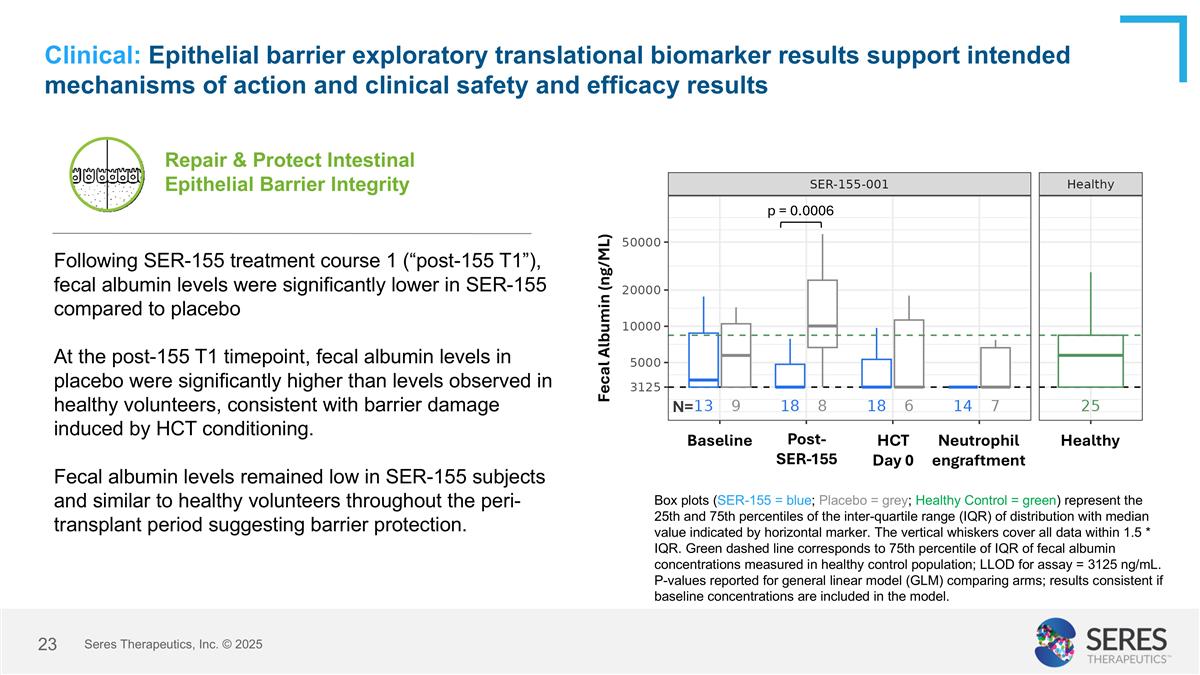
Clinical: Epithelial barrier exploratory translational biomarker results support intended mechanisms of action and clinical safety and efficacy results Box plots (SER-155 = blue; Placebo = grey; Healthy Control = green) represent the 25th and 75th percentiles of the inter-quartile range (IQR) of distribution with median value indicated by horizontal marker. The vertical whiskers cover all data within 1.5 * IQR. Green dashed line corresponds to 75th percentile of IQR of fecal albumin concentrations measured in healthy control population; LLOD for assay = 3125 ng/mL. P-values reported for general linear model (GLM) comparing arms; results consistent if baseline concentrations are included in the model. Repair & Protect Intestinal Epithelial Barrier Integrity Following SER-155 treatment course 1 (“post-155 T1”), fecal albumin levels were significantly lower in SER-155 compared to placebo At the post-155 T1 timepoint, fecal albumin levels in placebo were significantly higher than levels observed in healthy volunteers, consistent with barrier damage induced by HCT conditioning. Fecal albumin levels remained low in SER-155 subjects and similar to healthy volunteers throughout the peri-transplant period suggesting barrier protection. p = 0.0006 Baseline Post- SER-155 HCT Day 0 Healthy Neutrophil engraftment N= Fecal Albumin (ng/ML) Seres Therapeutics, Inc. © 2025
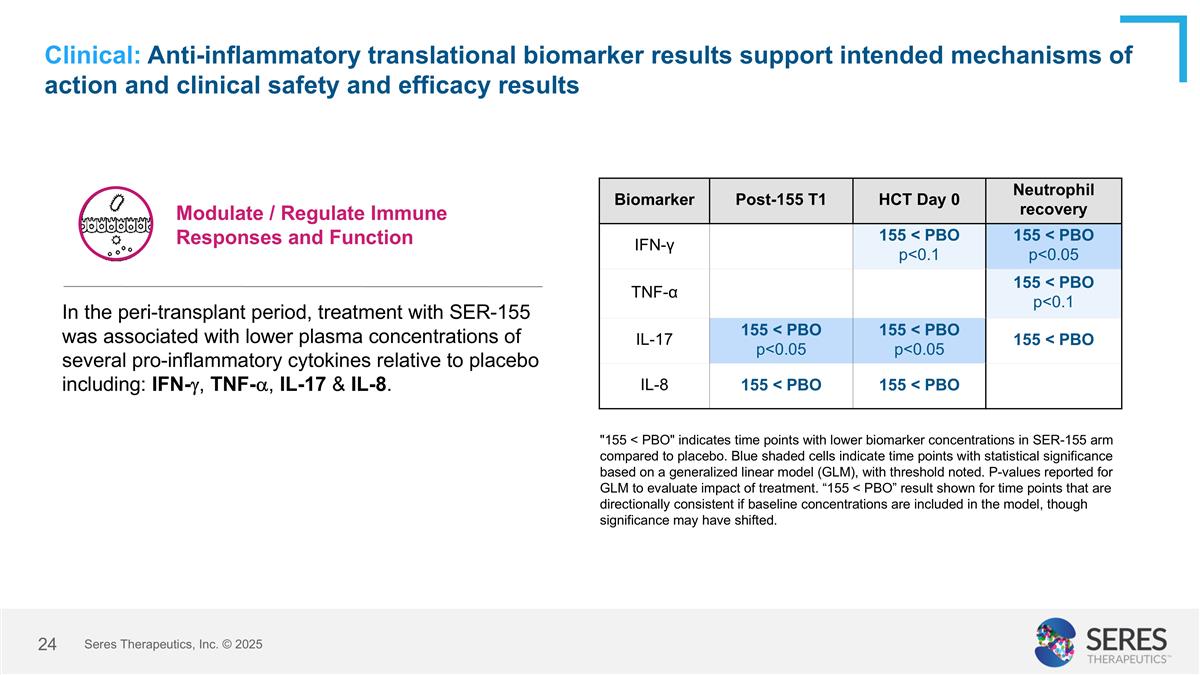
Clinical: Anti-inflammatory translational biomarker results support intended mechanisms of action and clinical safety and efficacy results "155 < PBO" indicates time points with lower biomarker concentrations in SER-155 arm compared to placebo. Blue shaded cells indicate time points with statistical significance based on a generalized linear model (GLM), with threshold noted. P-values reported for GLM to evaluate impact of treatment. “155 < PBO” result shown for time points that are directionally consistent if baseline concentrations are included in the model, though significance may have shifted. Modulate / Regulate Immune Responses and Function Biomarker Post-155 T1 HCT Day 0 Neutrophil recovery IFN-γ 155 < PBO p<0.1 155 < PBO p<0.05 TNF-α 155 < PBO p<0.1 IL-17 155 < PBO p<0.05 155 < PBO p<0.05 155 < PBO IL-8 155 < PBO 155 < PBO In the peri-transplant period, treatment with SER-155 was associated with lower plasma concentrations of several pro-inflammatory cytokines relative to placebo including: IFN-g, TNF-a, IL-17 & IL-8. Seres Therapeutics, Inc. © 2025
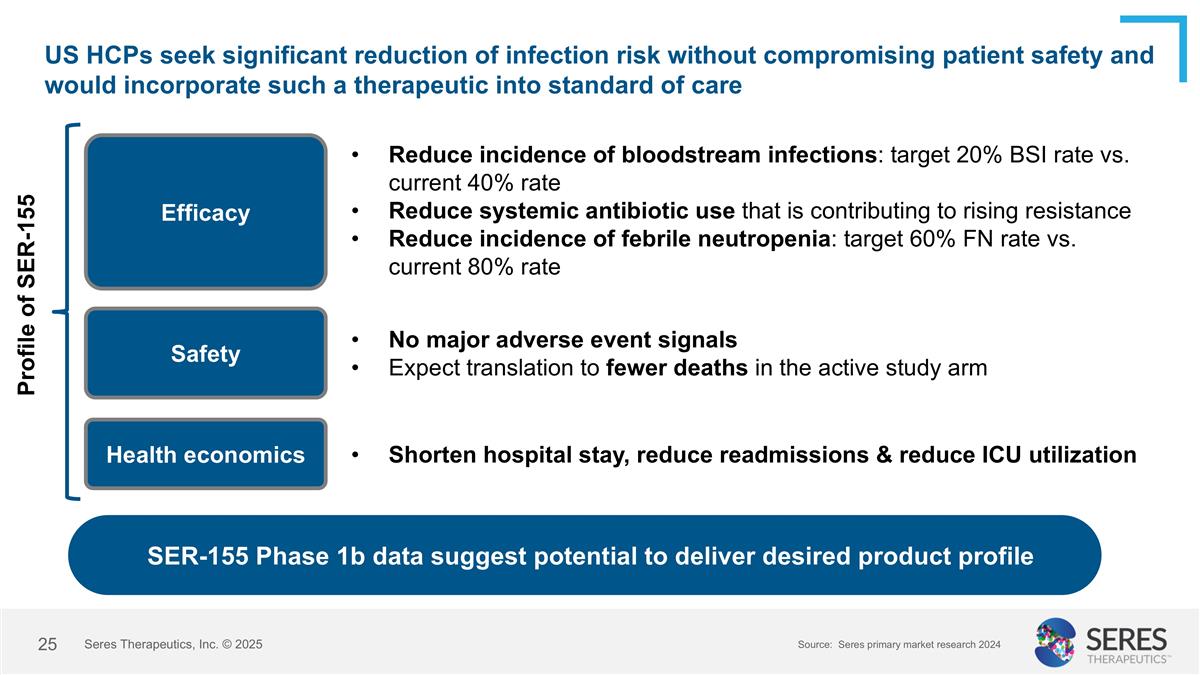
US HCPs seek significant reduction of infection risk without compromising patient safety and would incorporate such a therapeutic into standard of care Efficacy Safety Health economics Reduce incidence of bloodstream infections: target 20% BSI rate vs. current 40% rate Reduce systemic antibiotic use that is contributing to rising resistance Reduce incidence of febrile neutropenia: target 60% FN rate vs. current 80% rate No major adverse event signals Expect translation to fewer deaths in the active study arm SER-155 Phase 1b data suggest potential to deliver desired product profile Shorten hospital stay, reduce readmissions & reduce ICU utilization Source: Seres primary market research 2024 Profile of SER-155 Seres Therapeutics, Inc. © 2025
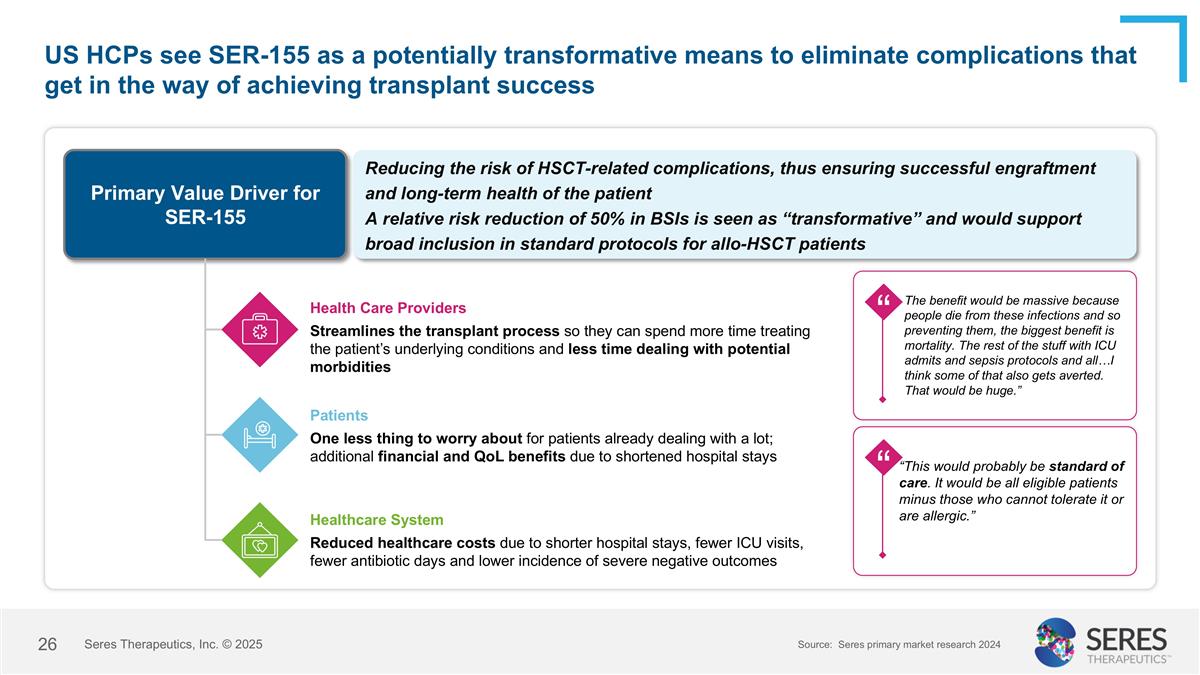
US HCPs see SER-155 as a potentially transformative means to eliminate complications that get in the way of achieving transplant success Primary Value Driver for SER-155 Reducing the risk of HSCT-related complications, thus ensuring successful engraftment and long-term health of the patient A relative risk reduction of 50% in BSIs is seen as “transformative” and would support broad inclusion in standard protocols for allo-HSCT patients Health Care Providers Streamlines the transplant process so they can spend more time treating the patient’s underlying conditions and less time dealing with potential morbidities Patients One less thing to worry about for patients already dealing with a lot; additional financial and QoL benefits due to shortened hospital stays Healthcare System Reduced healthcare costs due to shorter hospital stays, fewer ICU visits, fewer antibiotic days and lower incidence of severe negative outcomes “This would probably be standard of care. It would be all eligible patients minus those who cannot tolerate it or are allergic.” “ “ “ The benefit would be massive because people die from these infections and so preventing them, the biggest benefit is mortality. The rest of the stuff with ICU admits and sepsis protocols and all…I think some of that also gets averted. That would be huge.” Source: Seres primary market research 2024 Seres Therapeutics, Inc. © 2025
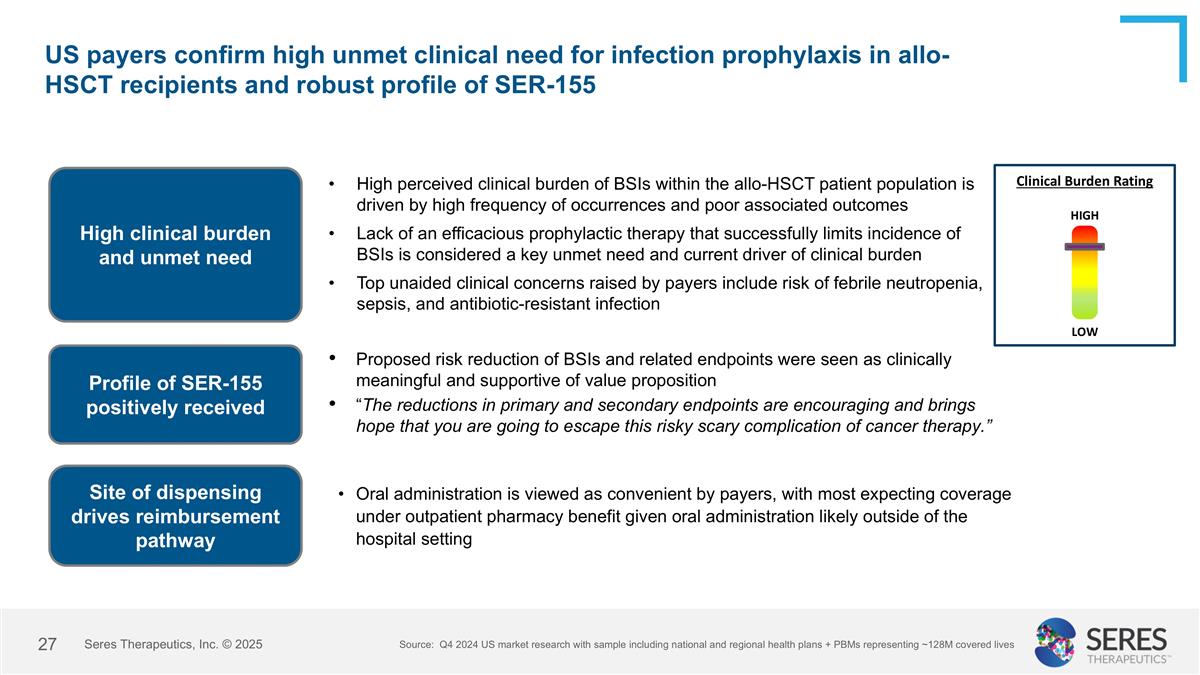
US payers confirm high unmet clinical need for infection prophylaxis in allo-HSCT recipients and robust profile of SER-155 High clinical burden and unmet need High perceived clinical burden of BSIs within the allo-HSCT patient population is driven by high frequency of occurrences and poor associated outcomes Lack of an efficacious prophylactic therapy that successfully limits incidence of BSIs is considered a key unmet need and current driver of clinical burden Top unaided clinical concerns raised by payers include risk of febrile neutropenia, sepsis, and antibiotic-resistant infection Profile of SER-155 positively received Proposed risk reduction of BSIs and related endpoints were seen as clinically meaningful and supportive of value proposition “The reductions in primary and secondary endpoints are encouraging and brings hope that you are going to escape this risky scary complication of cancer therapy.” Site of dispensing drives reimbursement pathway Oral administration is viewed as convenient by payers, with most expecting coverage under outpatient pharmacy benefit given oral administration likely outside of the hospital setting LOW HIGH Source: Q4 2024 US market research with sample including national and regional health plans + PBMs representing ~128M covered lives Clinical Burden Rating Seres Therapeutics, Inc. © 2025
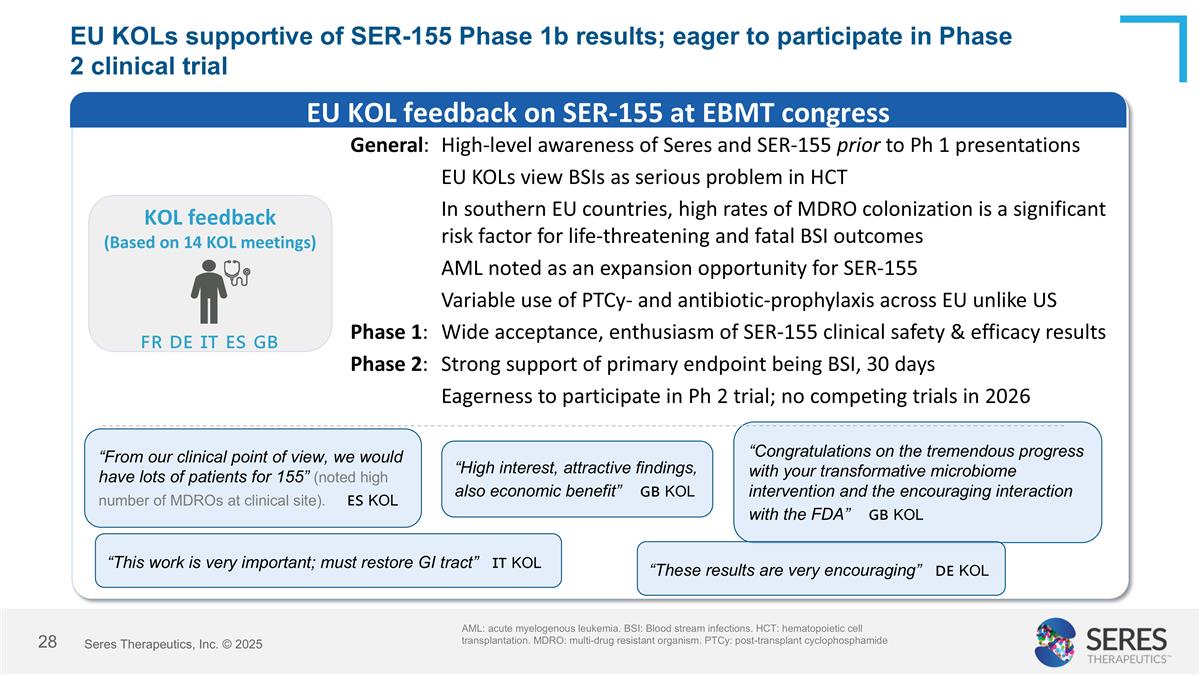
EU KOLs supportive of SER-155 Phase 1b results; eager to participate in Phase 2 clinical trial AML: acute myelogenous leukemia. BSI: Blood stream infections. HCT: hematopoietic cell transplantation. MDRO: multi-drug resistant organism. PTCy: post-transplant cyclophosphamide Ω EU KOL feedback on SER-155 at EBMT congress KOL feedback (Based on 14 KOL meetings) General:High-level awareness of Seres and SER-155 prior to Ph 1 presentations EU KOLs view BSIs as serious problem in HCT In southern EU countries, high rates of MDRO colonization is a significant risk factor for life-threatening and fatal BSI outcomes AML noted as an expansion opportunity for SER-155 Variable use of PTCy- and antibiotic-prophylaxis across EU unlike US Phase 1:Wide acceptance, enthusiasm of SER-155 clinical safety & efficacy results Phase 2:Strong support of primary endpoint being BSI, 30 days Eagerness to participate in Ph 2 trial; no competing trials in 2026 “Congratulations on the tremendous progress with your transformative microbiome intervention and the encouraging interaction with the FDA” 🇬🇧 KOL “High interest, attractive findings, also economic benefit” 🇬🇧 KOL “From our clinical point of view, we would have lots of patients for 155” (noted high number of MDROs at clinical site). 🇪🇸 KOL “This work is very important; must restore GI tract” 🇮🇹 KOL “These results are very encouraging” 🇩🇪 KOL 🇫🇷 🇩🇪 🇮🇹 🇪🇸 🇬🇧 Seres Therapeutics, Inc. © 2025
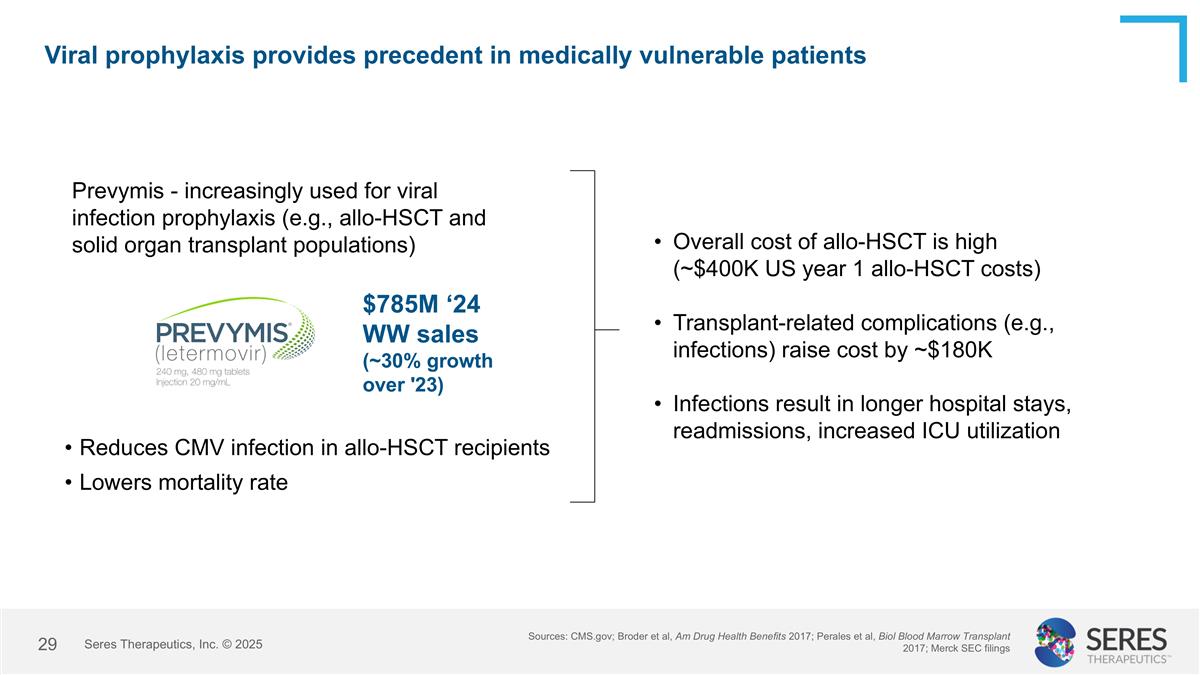
Viral prophylaxis provides precedent in medically vulnerable patients Prevymis - increasingly used for viral infection prophylaxis (e.g., allo-HSCT and solid organ transplant populations) $785M ‘24 WW sales (~30% growth over '23) Reduces CMV infection in allo-HSCT recipients Lowers mortality rate Overall cost of allo-HSCT is high (~$400K US year 1 allo-HSCT costs) Transplant-related complications (e.g., infections) raise cost by ~$180K Infections result in longer hospital stays, readmissions, increased ICU utilization Sources: CMS.gov; Broder et al, Am Drug Health Benefits 2017; Perales et al, Biol Blood Marrow Transplant 2017; Merck SEC filings Seres Therapeutics, Inc. © 2025
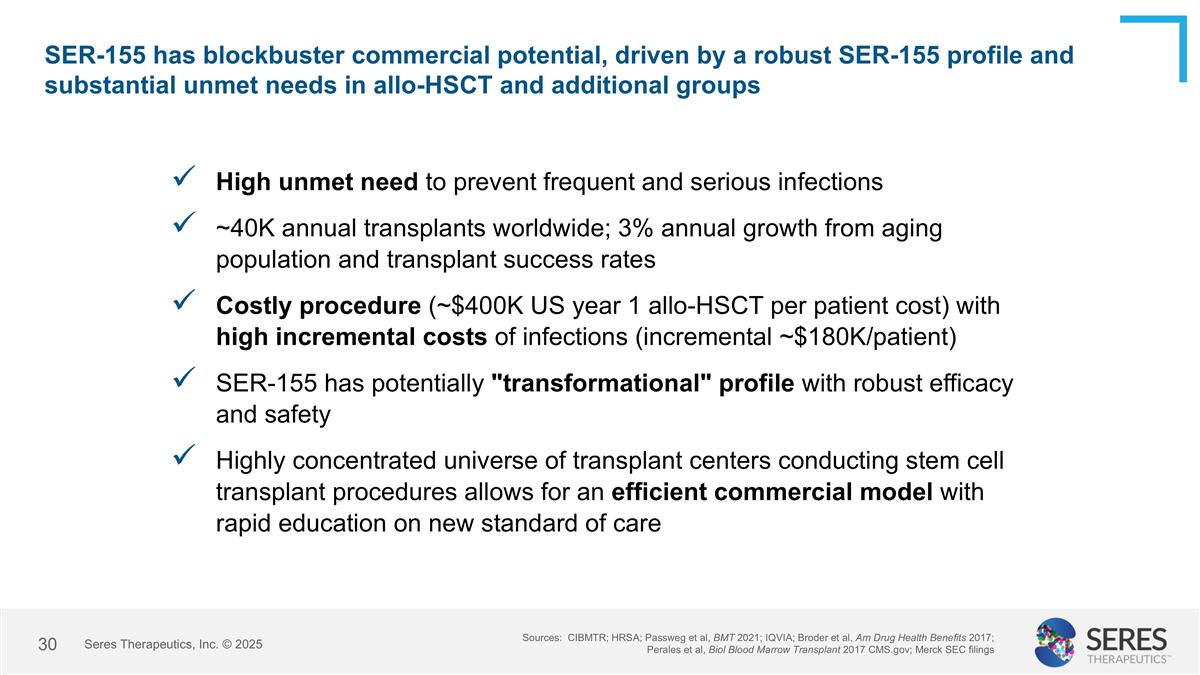
SER-155 has blockbuster commercial potential, driven by a robust SER-155 profile and substantial unmet needs in allo-HSCT and additional groups Sources: CIBMTR; HRSA; Passweg et al, BMT 2021; IQVIA; Broder et al, Am Drug Health Benefits 2017; Perales et al, Biol Blood Marrow Transplant 2017 CMS.gov; Merck SEC filings High unmet need to prevent frequent and serious infections ~40K annual transplants worldwide; 3% annual growth from aging population and transplant success rates Costly procedure (~$400K US year 1 allo-HSCT per patient cost) with high incremental costs of infections (incremental ~$180K/patient) SER-155 has potentially "transformational" profile with robust efficacy and safety Highly concentrated universe of transplant centers conducting stem cell transplant procedures allows for an efficient commercial model with rapid education on new standard of care Seres Therapeutics, Inc. © 2025
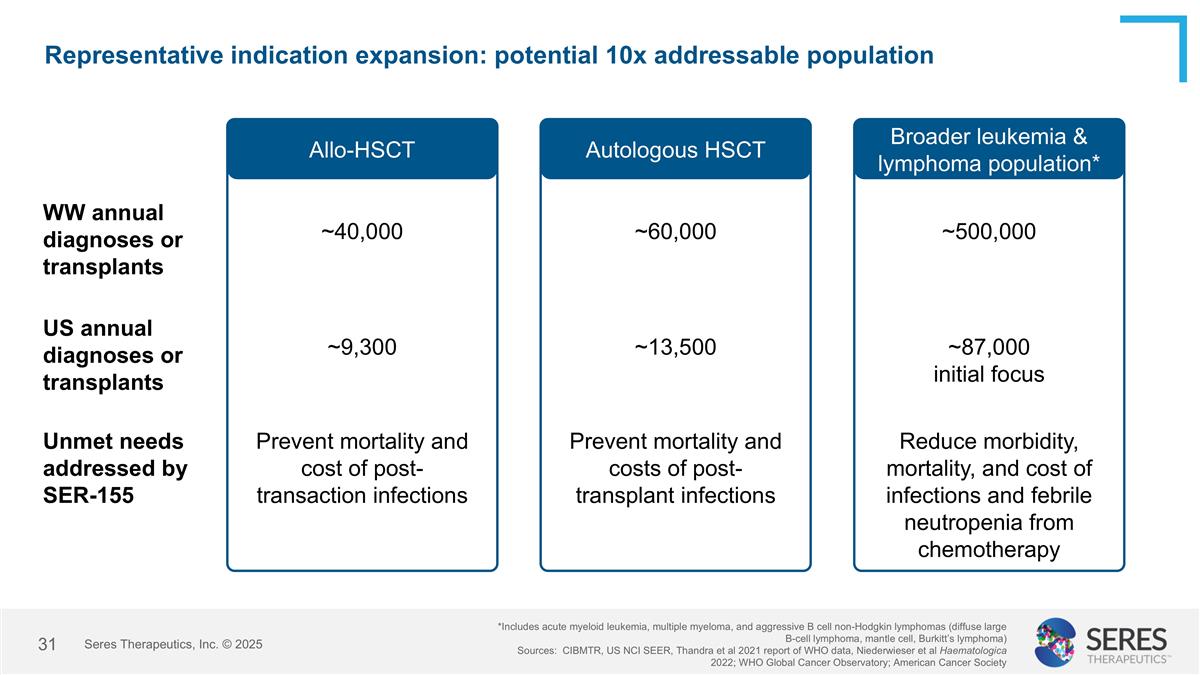
Representative indication expansion: potential 10x addressable population US annual diagnoses or transplants Unmet needs addressed by SER-155 Allo-HSCT ~9,300 Prevent mortality and cost of post-transaction infections Autologous HSCT ~13,500 Prevent mortality and costs of post-transplant infections Broader leukemia & lymphoma population* ~87,000 initial focus Reduce morbidity, mortality, and cost of infections and febrile neutropenia from chemotherapy *Includes acute myeloid leukemia, multiple myeloma, and aggressive B cell non-Hodgkin lymphomas (diffuse large B-cell lymphoma, mantle cell, Burkitt’s lymphoma) Sources: CIBMTR, US NCI SEER, Thandra et al 2021 report of WHO data, Niederwieser et al Haematologica 2022; WHO Global Cancer Observatory; American Cancer Society WW annual diagnoses or transplants ~40,000 ~60,000 ~500,000 Seres Therapeutics, Inc. © 2025
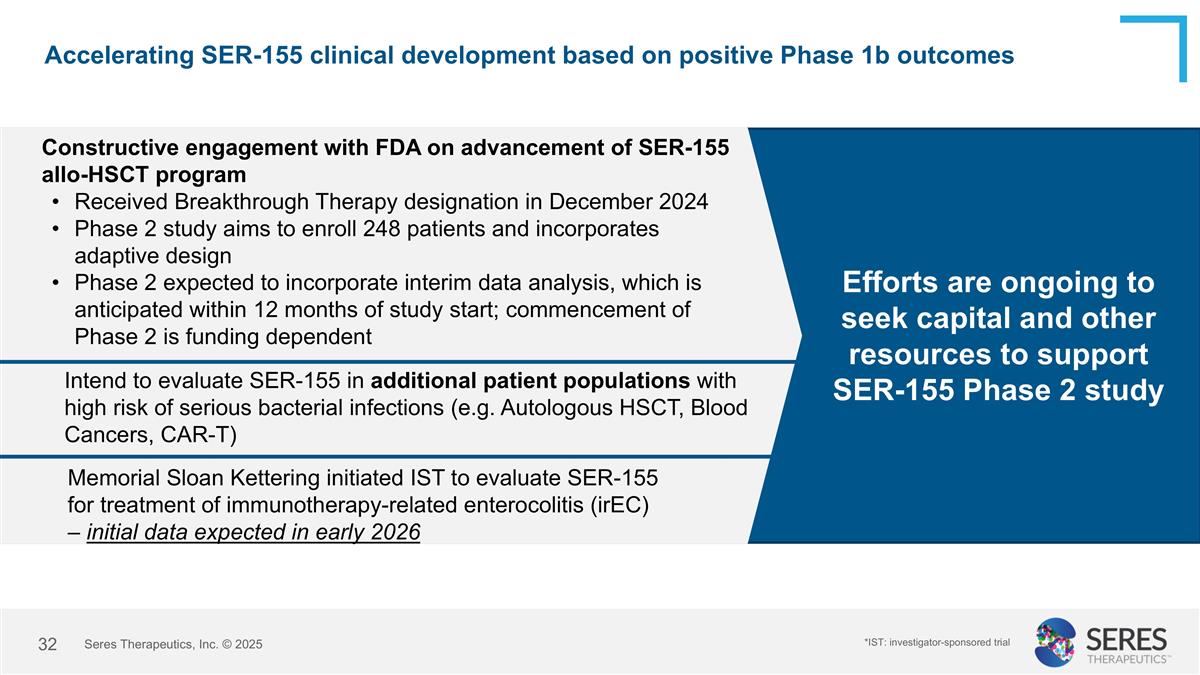
Accelerating SER-155 clinical development based on positive Phase 1b outcomes Efforts are ongoing to seek capital and other resources to support SER-155 Phase 2 study Constructive engagement with FDA on advancement of SER-155 allo-HSCT program Received Breakthrough Therapy designation in December 2024 Phase 2 study aims to enroll 248 patients and incorporates adaptive design Phase 2 expected to incorporate interim data analysis, which is anticipated within 12 months of study start; commencement of Phase 2 is funding dependent Intend to evaluate SER-155 in additional patient populations with high risk of serious bacterial infections (e.g. Autologous HSCT, Blood Cancers, CAR-T) Seres Therapeutics, Inc. © 2025 Memorial Sloan Kettering initiated IST to evaluate SER-155 for treatment of immunotherapy-related enterocolitis (irEC) – initial data expected in early 2026 *IST: investigator-sponsored trial
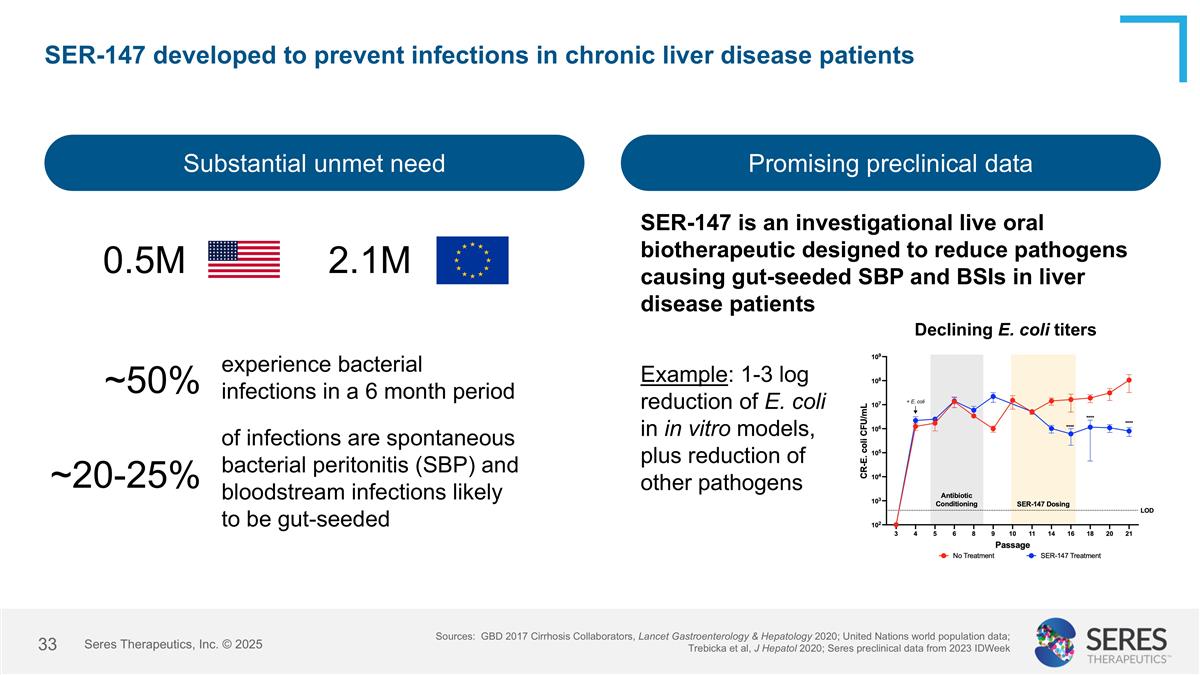
SER-147 developed to prevent infections in chronic liver disease patients Substantial unmet need Promising preclinical data Sources: GBD 2017 Cirrhosis Collaborators, Lancet Gastroenterology & Hepatology 2020; United Nations world population data; Trebicka et al, J Hepatol 2020; Seres preclinical data from 2023 IDWeek 0.5M 2.1M ~50% SER-147 is an investigational live oral biotherapeutic designed to reduce pathogens causing gut-seeded SBP and BSIs in liver disease patients Declining E. coli titers experience bacterial infections in a 6 month period ~20-25% of infections are spontaneous bacterial peritonitis (SBP) and bloodstream infections likely to be gut-seeded Example: 1-3 log reduction of E. coli in in vitro models, plus reduction of other pathogens Seres Therapeutics, Inc. © 2025

SER-603 designed to target inflammatory drivers of Inflammatory Bowel Disease (IBD) that current standards of care cannot Seres Therapeutics, Inc. © 2025 IBD is a mucosal barrier-immune disease Seres IBD Program Sources: Seres data on file Inflammatory microbiome Overabundance of pathogenic and inflammatory bacteria Loss of GI barrier integrity Barrier damage allows translocation of inflammatory bacteria and molecules Inflammatory immune responses Induce inflammatory responses in APCs and CD4+ T cells Nominated & validated novel, microbial-linked biomarkers that are predictive of response to current IBD therapies Demonstrated in preclinical models that SER-603, a novel cultivated LBP, can target all three drivers of disease without immunosuppression as a monotherapy and can improve response to advanced therapies in combination Supported by Crohn's & Colitis Foundation IBD Ventures award Engaged with potential partners on potential research collaboration SER-603 targets upstream of current IBD therapies and without immune suppression
Manufacturing platform delivers defined consortia in oral formulation using cost-effective production Novel formulations enabling consistent drug product composition, drug stability for distribution, and targeted drug delivery Quality systems to ensure product quality and stability, extending prior regulatory successes, including developing product release specifications with the FDA Highly intensive strain bioprocessing leveraging flexible, single-use manufacturing technology for cost-effective production Strain isolation and characterization pipeline to rapidly identify cGMP-suitable medium components Seres Therapeutics, Inc. © 2025
Maximizing opportunity of live biotherapeutics going forward SER-155 Bloodstream infections in Allo-HSCT: Finalizing SER-155 Phase 2 study protocol based on FDA feedback and supported by Breakthrough Therapy designation Evaluate in additional cancer treatment populations w/ high risk of serious bacterial infections (e.g. Autologous HSCT, Blood Cancers, CAR-T) Investigator-sponsored trial treating immune checkpoint-related enterocolitis underway SER-603 to treat immune-related diseases (e.g., IBD, UC, Crohn’s) Prevent life-threatening bacterial infections, including antimicrobial resistant infections in additional populations SER-603 SER-147 Infections in Chronic liver disease: IND enabling activities Indication expansion (e.g., radiation enteritis, ICU and long-term care patients, organ transplant) rCDI: Proven clinical and regulatory success; asset sale to Nestlé; Seres eligible for future milestones VOWST Seres Therapeutics, Inc. © 2025
Summary and path forward SER-155 Phase 1b placebo-controlled results promising, Phase 2 planning ongoing Finalizing SER-155 Phase 2 study protocol based on constructive FDA engagement; Commencement of study is funding dependent Administration associated with 77% relative risk reduction for BSIs, significant reduction in systemic antibiotic exposure, and lower incidence of febrile neutropenia vs placebo Exploratory biomarker data support SER-155 MOA and potential role for Seres’ platform to provide clinical benefit to patients with inflammatory & immune diseases (e.g., UC & Crohn’s disease) Capital Strategy & Financial position Efforts aimed at obtaining capital and other resources to support SER-155 Phase 2 study and additional live biotherapeutic product candidates are on-going ~$47.6M in cash/cash equivalents at September 30, 2025; cash runway projected through Q2 2026 VOWST asset sale closed in September 2024; $50M received in January 2025 and $25M received in July 2025 (offset by ~$1.4M in employment-related payments) + $275M potential future milestones Pipeline of novel live biotherapeutics in areas with large commercial potential Pipeline aims to bring transformative medicines to a wider set of patients, led by SER-155 Evaluating SER-603 to treat immune-related diseases (e.g., IBD, UC, Crohn’s), and SER-147 for individual living with chronic liver disease Memorial Sloan Kettering IST for treatment of immunotherapy-related enterocolitis (irEC) – data expected in early 2026 Seres Therapeutics, Inc. © 2025