--12-31Q30001806310falsehttp://fasb.org/us-gaap/2025#Liabilitieshttp://fasb.org/us-gaap/2025#AccruedLiabilitiesAndOtherLiabilitieshttp://fasb.org/us-gaap/2025#AccruedLiabilitiesAndOtherLiabilitieshttp://fasb.org/us-gaap/2025#OtherLiabilitiesNoncurrenthttp://fasb.org/us-gaap/2025#PrimeRateMemberhttp://fasb.org/us-gaap/2025#PrimeRateMember1P1Yhttp://fasb.org/srt/2025#ChiefExecutiveOfficerMember10001806310tsha:PreFundedWarrantsMember2024-01-012024-09-300001806310tsha:TwoThousandTwentyFiveTrinityTermLoanAgreementMemberus-gaap:GeneralAndAdministrativeExpenseMembertsha:TwoThousandTwentyFiveTrinityTermLoansMembertsha:TrinityLendersMember2025-01-012025-09-300001806310us-gaap:RetainedEarningsMember2025-07-012025-09-300001806310us-gaap:AccumulatedOtherComprehensiveIncomeMember2025-06-300001806310us-gaap:MoneyMarketFundsMember2025-09-300001806310tsha:UnderwrittenPublicOfferingMemberus-gaap:CommonStockMember2025-01-012025-09-300001806310us-gaap:PerformanceSharesMembertsha:ModifiedOptionsMember2025-07-012025-09-300001806310tsha:DurhamLeaseMember2025-01-012025-09-300001806310tsha:UnderwrittenPublicOfferingMember2024-07-012024-09-300001806310tsha:TwoThousandTwentyFiveTrinityTermLoanAgreementMembertsha:AugustThroughAugustMembertsha:TwoThousandTwentyFiveTrinityTermLoansMembertsha:TrinityLendersMember2025-08-072025-08-070001806310tsha:TwoThousandTwentyStockIncentivePlanMember2025-01-012025-09-300001806310tsha:EmployeeStockPurchasePlansMember2020-09-162020-09-160001806310tsha:SalesAgreementMember2022-04-012022-04-300001806310tsha:TwoThousandTwentyFiveTrinityTermLoanAgreementMembertsha:TwoThousandTwentyFiveTrinityTermLoansMembertsha:TrinityLendersMember2025-09-300001806310tsha:SecuritiesPurchaseAgreementMembertsha:AstellasMemberus-gaap:PrivatePlacementMember2022-10-310001806310tsha:AbeonaRettAgreementMembertsha:AbeonaTherapeuticsIncMember2023-01-012023-12-310001806310tsha:AstellasMember2022-10-310001806310us-gaap:AccumulatedOtherComprehensiveIncomeMember2025-07-012025-09-300001806310us-gaap:PerformanceSharesMembertsha:InitialOptionsAndOriginalOptionsMember2023-05-012023-05-310001806310tsha:AstellasMembertsha:LicenseForRettMember2025-01-012025-09-3000018063102022-10-012022-10-310001806310tsha:AstellasMember2022-10-012022-10-310001806310us-gaap:RestrictedStockUnitsRSUMembersrt:MinimumMembertsha:TwoThousandTwentyStockIncentivePlanMember2025-01-012025-09-300001806310tsha:SuccessFeeLiabilityMember2025-07-012025-09-300001806310tsha:PegasusParkLLCMembertsha:DallasLeaseMember2021-01-112021-01-110001806310tsha:UTSouthwesternAgreementMember2020-04-012020-04-3000018063102025-11-040001806310tsha:TwoThousandTwentyFiveTrinityTermLoanAgreementMembertsha:ThroughMarchThirtyOneTwentyTwentyNineMembertsha:TwoThousandTwentyFiveTrinityTermLoansMembertsha:TrinityLendersMember2025-08-070001806310tsha:SecuritiesPurchaseAgreementMembertsha:TwentyTwentyThreePreFundedWarrantsMember2023-08-140001806310tsha:UnderwrittenPublicOfferingMember2025-01-012025-09-300001806310tsha:UnderwritingAgreementMembertsha:MayTwentyTwentyFivePreFundedWarrantsMember2025-05-282025-05-280001806310us-gaap:FairValueInputsLevel1Member2025-09-3000018063102024-09-300001806310us-gaap:ResearchAndDevelopmentExpenseMember2024-07-012024-09-300001806310us-gaap:CommonStockMember2024-01-012024-09-300001806310tsha:UnderwritingAgreementMember2024-06-260001806310us-gaap:RetainedEarningsMember2024-07-012024-09-300001806310tsha:UnderwritingAgreementMemberus-gaap:CommonStockMember2024-06-260001806310us-gaap:GeneralAndAdministrativeExpenseMember2024-01-012024-09-300001806310us-gaap:AdditionalPaidInCapitalMember2025-07-012025-09-300001806310tsha:UnderwrittenPublicOfferingMemberus-gaap:AdditionalPaidInCapitalMember2024-01-012024-09-3000018063102024-06-300001806310us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-01-012024-09-300001806310tsha:InducementPlanMember2024-12-310001806310tsha:SalesAgreementMembertsha:SalesAgentMember2021-10-052021-10-050001806310tsha:EmployeeStockPurchasePlansMember2025-01-012025-09-300001806310us-gaap:EmployeeStockOptionMember2025-01-012025-09-300001806310tsha:TwoThousandTwentyStockIncentivePlanMember2025-01-010001806310tsha:TwoThousandTwentyFiveTrinityTermLoansMembertsha:TrinityLendersMembertsha:SuccessFeeAgreementMember2025-08-070001806310us-gaap:GeneralAndAdministrativeExpenseMember2025-01-012025-09-300001806310us-gaap:MeasurementInputSharePriceMember2023-04-050001806310tsha:UnderwritingAgreementMembertsha:JuneTwentyTwentyFourPreFundedWarrantsMember2024-06-262024-06-260001806310tsha:UnderwrittenPublicOfferingMember2025-07-012025-09-300001806310tsha:TwoThousandTwentyFiveTrinityTermLoanAgreementMembertsha:ThroughMarchThirtyOneTwentyTwentyNineMembertsha:TwoThousandTwentyFiveTrinityTermLoansMembertsha:TrinityLendersMember2025-08-072025-08-070001806310us-gaap:PerformanceSharesMembertsha:ModifiedOptionsMember2023-01-012023-12-310001806310us-gaap:PerformanceSharesMembertsha:TwoThousandTwentyStockIncentivePlanMember2025-01-012025-09-300001806310us-gaap:AdditionalPaidInCapitalMember2024-06-300001806310tsha:AstellasMembertsha:AcquisitionOfWorldwideRightsForTSHA120ForTreatmentOfGiantAxonalNeuropathyMemberus-gaap:PrivatePlacementMember2025-01-012025-09-300001806310tsha:TwoThousandTwentyFiveTrinityTermLoanAgreementMembertsha:TwoThousandTwentyFiveTrinityTermLoansMembertsha:AugustThroughButExcludingTheMaturityDateMembertsha:TrinityLendersMember2025-08-072025-08-070001806310tsha:AbeonaCLN1AgreementsMember2020-10-012020-10-310001806310us-gaap:PerformanceSharesMembertsha:ModifiedOptionsMember2025-01-012025-09-300001806310tsha:DurhamLeaseMember2020-12-172020-12-170001806310us-gaap:FurnitureAndFixturesMember2025-09-300001806310us-gaap:MeasurementInputRiskFreeInterestRateMember2023-04-050001806310tsha:UTSouthwesternAgreementMember2019-11-300001806310tsha:TwoThousandTwentyThreeSuccessFeeAgreementMembertsha:TwoThousandTwentyThreeTrinityTermLoansMembertsha:TrinityLendersMember2023-11-132023-11-130001806310us-gaap:AdditionalPaidInCapitalMember2024-01-012024-09-300001806310us-gaap:FairValueInputsLevel1Member2024-12-310001806310us-gaap:MeasurementInputSharePriceMember2025-09-300001806310us-gaap:CommonStockMember2024-06-300001806310tsha:SalesAgreementMembersrt:MaximumMember2025-03-282025-03-280001806310tsha:AbeonaCLN1AgreementsMember2020-01-012020-12-310001806310srt:MinimumMembertsha:DallasLeaseAmendmentMembertsha:PegasusParkLLCMember2021-12-142021-12-140001806310tsha:EmployeeStockPurchasePlansMembersrt:MaximumMember2020-09-160001806310us-gaap:AdditionalPaidInCapitalMember2024-12-310001806310us-gaap:ComputerEquipmentMember2024-12-310001806310us-gaap:WarrantMember2024-01-012024-09-300001806310tsha:SuccessFeeAgreementMember2025-09-300001806310us-gaap:CommonStockMember2025-09-300001806310tsha:SsiWarrantsMember2023-04-052023-04-050001806310tsha:TrinityTermLoansMember2024-01-012024-09-300001806310tsha:TwoThousandTwentyFiveTrinityTermLoanAgreementMembertsha:TwoThousandTwentyFiveTrinityTermLoansMembertsha:TrinityLendersMembertsha:TrinityRefinanceDateThroughAugust2026Member2025-08-072025-08-070001806310tsha:SecuritiesPurchaseAgreementMembertsha:AugustTwoThousandAndTwentyThreePrivatePlacementMember2023-08-140001806310us-gaap:EmployeeStockOptionMembersrt:MinimumMembertsha:TwoThousandTwentyStockIncentivePlanAndTwoThousandTwentyThreeInducementPlanMember2025-01-012025-09-300001806310tsha:UnderwritingAgreementMemberus-gaap:CommonStockMember2025-05-2800018063102025-06-300001806310us-gaap:PerformanceSharesMembertsha:TwoThousandTwentyStockIncentivePlanMember2023-02-282023-02-280001806310tsha:Performance-BasedRestrictedStockUnitsMember2025-08-310001806310tsha:TwentyTwentyThreePreFundedWarrantsMember2025-04-300001806310tsha:Performance-BasedRestrictedStockUnitsMembertsha:TwoThousandTwentyStockIncentivePlanMember2025-09-300001806310tsha:SecuritiesPurchaseAgreementMembertsha:AstellasMemberus-gaap:PrivatePlacementMember2022-10-210001806310us-gaap:ConstructionInProgressMember2024-12-310001806310tsha:DurhamLeaseMember2024-12-310001806310tsha:UnderwritingAgreementMembertsha:JuneTwentyTwentyFourPreFundedWarrantsMember2024-06-260001806310us-gaap:ComputerEquipmentMember2025-09-300001806310us-gaap:CommonStockMember2024-12-310001806310us-gaap:GeneralAndAdministrativeExpenseMembertsha:TwoThousandTwentyFiveTrinityTermLoansMembertsha:TrinityLendersMember2025-01-012025-09-300001806310us-gaap:ResearchAndDevelopmentExpenseMember2025-07-012025-09-300001806310us-gaap:CommonStockMember2024-07-012024-09-300001806310tsha:AbeonaCLN1AgreementsMembersrt:MaximumMember2020-08-310001806310us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-07-012024-09-300001806310us-gaap:CommonStockMember2025-01-012025-09-300001806310tsha:TwoThousandTwentyFiveTrinityTermLoanAgreementMembertsha:TwoThousandTwentyFiveTrinityTermLoansMembertsha:TrinityLendersMember2025-08-070001806310us-gaap:EmployeeStockOptionMember2025-01-012025-09-300001806310us-gaap:AdditionalPaidInCapitalMember2024-07-012024-09-300001806310tsha:AbeonaRettAgreementMember2025-01-012025-09-300001806310tsha:UnderwritingAgreementMembertsha:JuneTwentyTwentyFourPreFundedWarrantsOptionOneMember2024-06-262024-06-260001806310us-gaap:CommonStockMember2023-12-310001806310tsha:AbeonaRettAgreementMembertsha:AbeonaTherapeuticsIncMember2020-01-012020-12-310001806310tsha:SecuritiesPurchaseAgreementMembertsha:AstellasMemberus-gaap:PrivatePlacementMember2022-10-212022-10-210001806310us-gaap:PerformanceSharesMembertsha:OriginalOptionsMember2023-05-012023-05-310001806310tsha:AbeonaRettAgreementMembertsha:AstellasMember2025-01-012025-09-300001806310us-gaap:PerformanceSharesMembertsha:TwoThousandTwentyStockIncentivePlanMember2025-09-300001806310tsha:TrinityTermLoansMember2025-06-300001806310tsha:AssetsCapitalizedAsFinanceLeasesMember2024-12-310001806310us-gaap:RetainedEarningsMember2024-09-3000018063102022-02-012022-02-280001806310us-gaap:AdditionalPaidInCapitalMember2023-12-310001806310tsha:UnderwritingAgreementMembertsha:MayTwentyTwentyFivePreFundedWarrantsOptionOneMember2025-05-282025-05-2800018063102022-02-280001806310us-gaap:MeasurementInputExpectedTermMember2023-04-050001806310tsha:SecuritiesPurchaseAgreementMembertsha:TwentyTwentyThreePreFundedWarrantsMembertsha:AugustTwoThousandAndTwentyThreePrivatePlacementMember2023-08-140001806310tsha:AstellasMember2022-10-212022-10-210001806310us-gaap:RetainedEarningsMember2024-01-012024-09-300001806310us-gaap:EmployeeStockOptionMember2025-09-300001806310tsha:TwoThousandTwentyThreeAndTwoThousandTwentyFiveTrinityTermLoansMember2025-01-012025-09-300001806310us-gaap:FairValueInputsLevel3Member2024-12-310001806310us-gaap:CommonStockMember2025-06-300001806310tsha:TwoThousandTwentyThreeAndTwoThousandTwentyFiveTrinityTermLoansMember2024-12-310001806310tsha:SuccessFeeLiabilityMember2025-01-012025-09-300001806310us-gaap:RestrictedStockUnitsRSUMembertsha:TwoThousandTwentyStockIncentivePlanMember2025-01-012025-09-300001806310tsha:TrinityLendersMembertsha:TrinityTermLoansMembertsha:SuccessFeeAgreementMember2025-09-300001806310tsha:UnderwritingAgreementMembertsha:JuneTwentyTwentyFourPreFundedWarrantsMember2024-07-012024-07-310001806310tsha:SecuritiesPurchaseAgreementMemberus-gaap:PrivatePlacementMember2023-08-162023-08-160001806310us-gaap:RetainedEarningsMember2024-12-310001806310tsha:SuccessFeeLiabilityMember2024-12-310001806310us-gaap:EmployeeStockOptionMember2024-07-012024-09-300001806310tsha:AstellasMembertsha:AcquisitionOfWorldwideRightsForTSHA120ForTreatmentOfGiantAxonalNeuropathyMember2025-01-012025-09-300001806310tsha:TwoThousandTwentyStockIncentivePlanMember2025-09-300001806310us-gaap:AdditionalPaidInCapitalMember2025-06-3000018063102025-07-012025-09-300001806310tsha:TSHA120RettSyndromeMember2024-12-310001806310tsha:DurhamLeaseMember2020-12-170001806310tsha:TrinityTermLoansMember2025-09-300001806310tsha:TwoThousandTwentyThreeAndTwoThousandTwentyFiveTrinityTermLoansMember2025-09-300001806310us-gaap:ResearchAndDevelopmentExpenseMember2025-01-012025-09-3000018063102024-07-012024-09-300001806310us-gaap:MeasurementInputRiskFreeInterestRateMember2025-09-300001806310tsha:UnderwritingAgreementMember2025-05-280001806310tsha:AstellasMember2025-01-012025-09-300001806310us-gaap:EmployeeStockOptionMember2024-01-012024-12-310001806310tsha:TwoThousandTwentyFiveTrinityTermLoanAgreementMemberus-gaap:GeneralAndAdministrativeExpenseMembertsha:TwoThousandTwentyFiveTrinityTermLoansMembertsha:ThirdPartiesMember2025-01-012025-09-300001806310us-gaap:WarrantMember2025-01-012025-09-300001806310tsha:SsiWarrantsMember2025-09-300001806310tsha:MayTwentyTwentyFivePreFundedWarrantsOptionTwoMembertsha:UnderwritingAgreementMember2025-05-282025-05-280001806310us-gaap:AccumulatedOtherComprehensiveIncomeMember2025-09-300001806310us-gaap:RestrictedStockUnitsRSUMember2025-01-012025-09-300001806310us-gaap:RetainedEarningsMember2023-12-310001806310tsha:TwoThousandTwentyFiveTrinityTermLoansMembertsha:TrinityLendersMembertsha:SuccessFeeAgreementMember2025-09-300001806310tsha:SecuritiesPurchaseAgreementMemberus-gaap:PrivatePlacementMembertsha:SsiStrategyHoldingsLlcMember2023-04-012023-04-300001806310tsha:TwoThousandTwentyFiveTrinityTermLoansMembertsha:TrinityLendersMembertsha:SuccessFeeAgreementMember2025-08-072025-08-070001806310tsha:SecuritiesPurchaseAgreementMembertsha:AstellasMemberus-gaap:PrivatePlacementMember2022-10-012022-10-310001806310us-gaap:AccumulatedOtherComprehensiveIncomeMember2025-01-012025-09-300001806310us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-12-310001806310tsha:AssetsCapitalizedAsFinanceLeasesMember2025-09-300001806310us-gaap:LeaseholdImprovementsMember2024-12-310001806310us-gaap:MoneyMarketFundsMember2024-12-310001806310tsha:TwoThousandTwentyFiveTrinityTermLoanAgreementMembertsha:TwoThousandTwentyFiveTrinityTermLoansMembertsha:TrinityRefinanceDateUntilMarchThirtyOneTwentyTwentyEightMembertsha:TrinityLendersMember2025-08-072025-08-070001806310tsha:TwoThousandTwentyStockIncentivePlanMember2020-09-162020-09-160001806310us-gaap:RestrictedStockUnitsRSUMembersrt:MaximumMembertsha:TwoThousandTwentyStockIncentivePlanMember2025-01-012025-09-300001806310tsha:AbeonaCLN1AgreementsMember2025-01-012025-09-3000018063102025-01-012025-09-300001806310tsha:UnderwrittenPublicOfferingMemberus-gaap:AdditionalPaidInCapitalMember2024-07-012024-09-300001806310us-gaap:RestrictedStockUnitsRSUMember2025-09-300001806310tsha:UnderwrittenPublicOfferingMemberus-gaap:AdditionalPaidInCapitalMember2025-01-012025-09-300001806310us-gaap:RetainedEarningsMember2025-09-300001806310tsha:InducementPlanMember2024-12-120001806310tsha:AbeonaRettAgreementMember2024-01-012024-09-300001806310tsha:SalesAgreementMembersrt:MaximumMember2025-09-300001806310tsha:SalesAgreementMembertsha:SalesAgentMembersrt:MaximumMember2021-10-050001806310us-gaap:EmployeeStockOptionMembertsha:TwoThousandTwentyStockIncentivePlanAndTwoThousandTwentyThreeInducementPlanMembersrt:MaximumMember2025-01-012025-09-300001806310tsha:Performance-BasedRestrictedStockUnitsMember2025-08-012025-08-310001806310tsha:TwoThousandTwentyThreeTrinityTermLoanAgreementMembertsha:TwoThousandTwentyThreeTrinityTermLoansMembertsha:TrinityLendersMembertsha:ClosingDateThroughNovember132024Member2023-11-132023-11-130001806310us-gaap:CommonStockMember2024-09-300001806310us-gaap:RestrictedStockUnitsRSUMember2024-12-310001806310us-gaap:MeasurementInputPriceVolatilityMember2023-04-050001806310us-gaap:AdditionalPaidInCapitalMember2024-09-300001806310tsha:AbeonaRettAgreementMember2025-07-012025-09-300001806310us-gaap:AdditionalPaidInCapitalMember2025-01-012025-09-300001806310tsha:AbeonaRettAgreementMember2024-07-012024-09-300001806310tsha:EmployeeStockPurchasePlansMember2020-09-160001806310tsha:SecuritiesPurchaseAgreementMemberus-gaap:PrivatePlacementMembertsha:SsiStrategyHoldingsLlcMember2023-04-300001806310us-gaap:PerformanceSharesMembertsha:InitialOptionsAndOriginalOptionsMember2025-01-012025-09-300001806310us-gaap:PerformanceSharesMembertsha:ModifiedOptionsMember2025-09-300001806310tsha:AstellasMembertsha:LicenseForGanMember2025-01-012025-09-300001806310us-gaap:GeneralAndAdministrativeExpenseMember2025-07-012025-09-300001806310tsha:SalesAgreementMembersrt:MaximumMember2025-01-012025-09-300001806310us-gaap:RetainedEarningsMember2024-06-3000018063102024-12-310001806310tsha:LaboratoryEquipmentMember2025-09-300001806310us-gaap:ConstructionInProgressMember2025-09-300001806310us-gaap:EmployeeStockOptionMembertsha:TwoThousandTwentyStockIncentivePlanAndTwoThousandTwentyThreeInducementPlanMember2025-01-012025-09-300001806310us-gaap:EmployeeStockOptionMember2024-01-012024-09-300001806310tsha:AbeonaRettAgreementMember2024-12-310001806310tsha:UnderwrittenPublicOfferingMember2024-01-012024-09-300001806310tsha:EmployeeStockPurchasePlansMember2025-01-010001806310us-gaap:MoneyMarketFundsMemberus-gaap:FairValueInputsLevel1Member2025-09-300001806310tsha:TwoThousandTwentyFiveTrinityTermLoanAgreementMember2025-09-300001806310us-gaap:EmployeeStockOptionMember2024-12-310001806310tsha:SuccessFeeLiabilityMember2025-06-300001806310tsha:TwoThousandTwentyStockIncentivePlanMember2024-12-310001806310us-gaap:AdditionalPaidInCapitalMember2025-09-300001806310us-gaap:RestrictedStockUnitsRSUMember2025-01-012025-09-300001806310tsha:UnderwrittenPublicOfferingMemberus-gaap:AdditionalPaidInCapitalMember2025-07-012025-09-300001806310us-gaap:LeaseholdImprovementsMember2025-09-300001806310tsha:TrinityTermLoansMember2024-07-012024-09-300001806310tsha:UnderwrittenPublicOfferingMemberus-gaap:CommonStockMember2024-07-012024-09-300001806310tsha:TwoThousandTwentyThreeTrinityTermLoanAgreementMembertsha:TwoThousandTwentyThreeTrinityTermLoansMembertsha:TrinityLendersMember2023-11-132023-11-130001806310tsha:Performance-BasedRestrictedStockUnitsMembertsha:TwoThousandTwentyStockIncentivePlanMember2025-07-012025-09-300001806310tsha:TwoThousandTwentyThreeTrinityTermLoanAgreementMembertsha:TwoThousandTwentyThreeTrinityTermLoansMembertsha:TrinityLendersMember2023-11-130001806310us-gaap:ResearchAndDevelopmentExpenseMember2024-01-012024-09-300001806310us-gaap:FairValueInputsLevel3Member2025-09-300001806310us-gaap:EmployeeStockOptionMember2024-01-012024-09-300001806310tsha:SsiWarrantsMember2025-01-012025-09-300001806310tsha:TrinityTermLoansMember2025-01-012025-09-300001806310us-gaap:RestrictedStockUnitsRSUMember2024-01-012024-09-300001806310tsha:DallasLeaseAmendmentMembertsha:PegasusParkLLCMember2021-12-140001806310tsha:InducementPlanMember2025-01-012025-09-300001806310tsha:SecuritiesPurchaseAgreementMemberus-gaap:PrivatePlacementMember2023-08-142023-08-140001806310tsha:SuccessFeeLiabilityMember2025-09-300001806310tsha:MayTwentyTwentyFivePreFundedWarrantsMembertsha:UnderwritingAgreementMember2025-06-012025-06-300001806310tsha:Performance-BasedRestrictedStockUnitsMembertsha:TwoThousandTwentyStockIncentivePlanMember2025-08-310001806310us-gaap:MeasurementInputPriceVolatilityMember2025-09-300001806310tsha:TwoThousandTwentyFiveTrinityTermLoanAgreementMembertsha:TwoThousandTwentyFiveTrinityTermLoansMembertsha:TrinityLendersMember2025-08-072025-08-070001806310tsha:TwoThousandTwentyFiveTrinityTermLoanAgreementMembertsha:TwoThousandTwentyFiveTrinityTermLoansMembertsha:TrinityRefinanceDateUntilMarchThirtyOneTwentyTwentyEightMembertsha:TrinityLendersMember2025-08-070001806310us-gaap:EmployeeStockOptionMember2025-07-012025-09-300001806310us-gaap:MoneyMarketFundsMemberus-gaap:FairValueInputsLevel1Member2024-12-310001806310tsha:SalesAgreementMember2025-09-300001806310tsha:TSHA120RettSyndromeMember2025-09-300001806310tsha:DurhamLeaseMember2025-09-300001806310tsha:UnderwritingAgreementMembertsha:JuneTwentyTwentyFourPreFundedWarrantsOptionTwoMember2024-06-262024-06-260001806310tsha:LaboratoryEquipmentMember2024-12-310001806310tsha:Performance-BasedRestrictedStockUnitsMembertsha:TwoThousandTwentyStockIncentivePlanMember2025-08-012025-08-310001806310tsha:TrinityTermLoansMember2025-07-012025-09-300001806310tsha:UnderwritingAgreementMember2025-05-282025-06-060001806310us-gaap:EmployeeStockOptionMembertsha:InducementPlanMember2025-07-012025-09-300001806310tsha:TwoThousandTwentyThreeTrinityTermLoanAgreementMembertsha:November132024ThroughNovember132025Membertsha:TwoThousandTwentyThreeTrinityTermLoansMembertsha:TrinityLendersMember2023-11-132023-11-130001806310us-gaap:CommonStockMember2025-07-012025-09-300001806310us-gaap:RetainedEarningsMember2025-01-012025-09-300001806310tsha:AbeonaRettAgreementMembertsha:AbeonaTherapeuticsIncMembersrt:MaximumMember2020-10-290001806310us-gaap:GeneralAndAdministrativeExpenseMember2024-07-012024-09-300001806310us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-09-300001806310us-gaap:FurnitureAndFixturesMember2024-12-310001806310tsha:TwoThousandTwentyThreeTrinityTermLoanAgreementMembertsha:TwoThousandTwentyThreeTrinityTermLoansMembertsha:TrinityLendersMembertsha:November132025ThroughButExcludingTheMaturityDateMember2023-11-132023-11-130001806310us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-06-300001806310tsha:TSHA120RettSyndromeMember2025-07-012025-09-300001806310tsha:AbeonaRettAgreementMembertsha:AbeonaTherapeuticsIncMember2025-01-012025-09-300001806310tsha:UnderwritingAgreementMembertsha:MayTwentyTwentyFivePreFundedWarrantsMember2025-05-280001806310tsha:PegasusParkLLCMembertsha:DallasLeaseMember2021-01-1100018063102025-09-300001806310tsha:AbeonaCLN1AgreementsMember2021-01-012021-12-310001806310tsha:AbeonaCLN1AgreementsMember2020-08-012020-08-310001806310us-gaap:RetainedEarningsMember2025-06-300001806310tsha:InducementPlanMember2025-09-300001806310us-gaap:MeasurementInputExpectedTermMember2025-09-3000018063102023-12-310001806310tsha:UnderwrittenPublicOfferingMemberus-gaap:CommonStockMember2024-01-012024-09-3000018063102024-01-012024-09-30tsha:Segmentxbrli:pureutr:sqftxbrli:sharestsha:Productiso4217:USDutr:Y
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-Q
(Mark One)
|
|
☒ |
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended September 30, 2025
OR
|
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ___________ to ___________
Commission File Number: 001-39536
Taysha Gene Therapies, Inc.
(Exact Name of Registrant as Specified in its Charter)
|
|
Delaware |
84-3199512 |
|
( State or other jurisdiction of
incorporation or organization)
|
(I.R.S. Employer
Identification No.) |
|
3000 Pegasus Park Drive Ste 1430
Dallas, Texas
|
75247 |
(Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code: (214) 612-0000
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class |
|
Trading
Symbol(s)
|
|
Name of each exchange on which registered |
Common stock, par value $0.00001 per share |
|
TSHA |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
|
|
|
|
|
|
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
|
|
|
|
Non-accelerated filer |
|
☒ |
|
Smaller reporting company |
|
☒ |
|
|
|
|
|
|
|
Emerging growth company |
|
☒ |
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of November 4, 2025, the registrant had 273,919,373 shares of common stock, $0.00001 par value per share, outstanding.
PART I—FINANCIAL INFORMATION
Item 1. Financial Statements.
Taysha Gene Therapies, Inc.
Condensed Consolidated Balance Sheets
(in thousands, except share and per share data)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30,
2025 |
|
|
December 31,
2024 |
|
ASSETS |
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
297,344 |
|
|
$ |
139,036 |
|
Restricted cash |
|
|
449 |
|
|
|
449 |
|
Prepaid expenses and other current assets |
|
|
2,158 |
|
|
|
2,645 |
|
Total current assets |
|
|
299,951 |
|
|
|
142,130 |
|
Restricted cash |
|
|
2,151 |
|
|
|
2,151 |
|
Property, plant and equipment, net |
|
|
6,805 |
|
|
|
7,485 |
|
Operating lease right-of-use assets |
|
|
7,463 |
|
|
|
8,381 |
|
Other non-current assets |
|
|
184 |
|
|
|
217 |
|
Total assets |
|
$ |
316,554 |
|
|
$ |
160,364 |
|
LIABILITIES AND STOCKHOLDERS' EQUITY |
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
Accounts payable |
|
$ |
5,438 |
|
|
$ |
3,592 |
|
Accrued expenses and other current liabilities |
|
|
17,708 |
|
|
|
12,862 |
|
Deferred revenue |
|
|
5,485 |
|
|
|
9,773 |
|
Total current liabilities |
|
|
28,631 |
|
|
|
26,227 |
|
Term loan, net |
|
|
50,852 |
|
|
|
43,942 |
|
Operating lease liability, net of current portion |
|
|
16,506 |
|
|
|
17,361 |
|
Other non-current liabilities |
|
|
1,576 |
|
|
|
1,309 |
|
Total liabilities |
|
|
97,565 |
|
|
|
88,839 |
|
Commitments and contingencies - Note 13 |
|
|
|
|
|
|
Stockholders' equity |
|
|
|
|
|
|
Preferred stock, $0.00001 par value per share; 10,000,000 shares authorized and no shares issued and outstanding as of September 30, 2025 and December 31, 2024 |
|
|
— |
|
|
|
— |
|
Common stock, $0.00001 par value per share; 700,000,000 shares authorized and 273,915,373 issued and outstanding as of September 30, 2025 and 400,000,000 shares authorized and 204,943,306 issued and outstanding as of December 31, 2024 |
|
|
3 |
|
|
|
2 |
|
Additional paid-in capital |
|
|
903,578 |
|
|
|
677,859 |
|
Accumulated other comprehensive loss |
|
|
(1,143 |
) |
|
|
(4,031 |
) |
Accumulated deficit |
|
|
(683,449 |
) |
|
|
(602,305 |
) |
Total stockholders’ equity |
|
|
218,989 |
|
|
|
71,525 |
|
Total liabilities and stockholders' equity |
|
$ |
316,554 |
|
|
$ |
160,364 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
Taysha Gene Therapies, Inc.
Condensed Consolidated Statements of Operations
(in thousands, except share and per share data)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Three Months
Ended September 30, |
|
|
For the Nine Months
Ended September 30, |
|
|
|
2025 |
|
|
2024 |
|
|
2025 |
|
|
2024 |
|
Revenue |
|
$ |
— |
|
|
$ |
1,788 |
|
|
$ |
4,288 |
|
|
$ |
6,311 |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
|
25,745 |
|
|
|
14,946 |
|
|
|
61,451 |
|
|
|
50,676 |
|
General and administrative |
|
|
8,279 |
|
|
|
7,902 |
|
|
|
25,035 |
|
|
|
22,324 |
|
Impairment of long-lived assets |
|
|
— |
|
|
|
4,838 |
|
|
|
— |
|
|
|
4,838 |
|
Total operating expenses |
|
|
34,024 |
|
|
|
27,686 |
|
|
|
86,486 |
|
|
|
77,838 |
|
Loss from operations |
|
|
(34,024 |
) |
|
|
(25,898 |
) |
|
|
(82,198 |
) |
|
|
(71,527 |
) |
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
Change in fair value of warrant liability |
|
|
(292 |
) |
|
|
75 |
|
|
|
(463 |
) |
|
|
(67 |
) |
Change in fair value of term loan |
|
|
(1,534 |
) |
|
|
(1,703 |
) |
|
|
(4,525 |
) |
|
|
(4,035 |
) |
Interest income |
|
|
3,169 |
|
|
|
2,107 |
|
|
|
6,354 |
|
|
|
5,240 |
|
Interest expense |
|
|
(15 |
) |
|
|
(24 |
) |
|
|
(51 |
) |
|
|
(80 |
) |
Other expense |
|
|
(37 |
) |
|
|
(81 |
) |
|
|
(261 |
) |
|
|
(44 |
) |
Total other income, net |
|
|
1,291 |
|
|
|
374 |
|
|
|
1,054 |
|
|
|
1,014 |
|
Net loss |
|
$ |
(32,733 |
) |
|
$ |
(25,524 |
) |
|
$ |
(81,144 |
) |
|
$ |
(70,513 |
) |
Net loss per common share, basic and diluted |
|
$ |
(0.09 |
) |
|
$ |
(0.10 |
) |
|
$ |
(0.26 |
) |
|
$ |
(0.29 |
) |
Weighted average common shares outstanding, basic and diluted |
|
|
353,309,524 |
|
|
|
267,824,045 |
|
|
|
307,175,982 |
|
|
|
244,052,057 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
Taysha Gene Therapies, Inc.
Condensed Consolidated Statements of Comprehensive Loss
(in thousands, except share and per share data)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
For the Nine Months Ended September 30, |
|
|
|
2025 |
|
|
2024 |
|
|
2025 |
|
|
2024 |
|
Net loss |
|
$ |
(32,733 |
) |
|
$ |
(25,524 |
) |
|
$ |
(81,144 |
) |
|
$ |
(70,513 |
) |
Other comprehensive income (loss): |
|
|
|
|
|
|
|
|
|
|
|
|
Change in fair value of term loan attributable to instrument specific credit risk |
|
|
(444 |
) |
|
|
(4,733 |
) |
|
|
2,888 |
|
|
|
(2,328 |
) |
Comprehensive loss |
|
$ |
(33,177 |
) |
|
$ |
(30,257 |
) |
|
$ |
(78,256 |
) |
|
$ |
(72,841 |
) |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
Taysha Gene Therapies, Inc.
Condensed Consolidated Statements of Stockholders’ Equity
(in thousands, except share data)
(Unaudited)
For the Three Months Ended September 30, 2025
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
|
|
|
Accumulated |
|
|
Total |
|
|
|
Common Stock |
|
|
Paid-in |
|
|
Accumulated |
|
|
Other |
|
|
Stockholders' |
|
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Comprehensive Loss |
|
|
Equity |
|
Balance as of June 30, 2025 |
|
|
272,730,060 |
|
|
$ |
3 |
|
|
$ |
900,139 |
|
|
$ |
(650,716 |
) |
|
$ |
(699 |
) |
|
$ |
248,727 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
3,285 |
|
|
|
— |
|
|
|
— |
|
|
|
3,285 |
|
Issuance of common stock and pre-funded warrants upon closing of underwritten public offering, net of underwriting discounts and commissions, and other offering costs of $(6) |
|
|
— |
|
|
|
— |
|
|
|
(6 |
) |
|
|
— |
|
|
|
— |
|
|
|
(6 |
) |
Issuance of common stock upon vesting and settlement of restricted stock units, net |
|
|
1,049,338 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Issuance of common stock under ESPP |
|
|
64,825 |
|
|
|
— |
|
|
|
84 |
|
|
|
— |
|
|
|
— |
|
|
|
84 |
|
Issuance of common stock upon exercise of stock options |
|
|
71,150 |
|
|
|
— |
|
|
|
76 |
|
|
|
— |
|
|
|
— |
|
|
|
76 |
|
Loss on instrument-specific credit risk |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(444 |
) |
|
|
(444 |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(32,733 |
) |
|
|
— |
|
|
|
(32,733 |
) |
Balance as of September 30, 2025 |
|
|
273,915,373 |
|
|
$ |
3 |
|
|
$ |
903,578 |
|
|
$ |
(683,449 |
) |
|
$ |
(1,143 |
) |
|
$ |
218,989 |
|
For the Three Months Ended September 30, 2024
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
|
|
|
Accumulated |
|
|
Total |
|
|
|
Common Stock |
|
|
Paid-in |
|
|
Accumulated |
|
|
Other |
|
|
Stockholders' |
|
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Comprehensive Loss |
|
|
Equity |
|
Balance as of June 30, 2024 |
|
|
201,381,450 |
|
|
$ |
2 |
|
|
$ |
664,457 |
|
|
$ |
(557,996 |
) |
|
$ |
2,405 |
|
|
$ |
108,868 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
3,357 |
|
|
|
— |
|
|
|
— |
|
|
|
3,357 |
|
Issuance of common stock and pre-funded warrants upon closing of underwritten public offering, net of underwriting discounts and commissions, and other offering costs of $542 |
|
|
3,235,000 |
|
|
|
— |
|
|
|
6,737 |
|
|
|
— |
|
|
|
— |
|
|
|
6,737 |
|
Issuance of common stock under ESPP |
|
|
47,170 |
|
|
|
— |
|
|
|
63 |
|
|
|
— |
|
|
|
— |
|
|
|
63 |
|
Issuance of common stock upon exercise of stock options, net |
|
|
56,770 |
|
|
|
— |
|
|
|
38 |
|
|
|
— |
|
|
|
— |
|
|
|
38 |
|
Issuance of common stock upon vesting and settlement of restricted stock units, net |
|
|
222,916 |
|
|
|
— |
|
|
|
(9 |
) |
|
|
— |
|
|
|
— |
|
|
|
(9 |
) |
Loss on instrument-specific credit risk |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(4,733 |
) |
|
|
(4,733 |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(25,524 |
) |
|
|
— |
|
|
|
(25,524 |
) |
Balance as of September 30, 2024 |
|
|
204,943,306 |
|
|
$ |
2 |
|
|
$ |
674,643 |
|
|
$ |
(583,520 |
) |
|
$ |
(2,328 |
) |
|
$ |
88,797 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
Taysha Gene Therapies, Inc.
Condensed Consolidated Statements of Stockholders’ Equity
(in thousands, except share data)
(Unaudited)
For the Nine Months Ended September 30, 2025
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock |
|
|
Paid-in |
|
|
Accumulated |
|
|
Accumulated Other |
|
|
Total |
|
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Comprehensive Loss |
|
|
Stockholders' Equity |
|
Balance as of December 31, 2024 |
|
|
204,943,306 |
|
|
$ |
2 |
|
|
$ |
677,859 |
|
|
$ |
(602,305 |
) |
|
$ |
(4,031 |
) |
|
$ |
71,525 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
9,769 |
|
|
|
— |
|
|
|
— |
|
|
|
9,769 |
|
Issuance of common stock and pre-funded warrants upon closing of underwritten public offering, net of underwriting discounts and commissions, and other offering costs of $14,329 |
|
|
57,777,777 |
|
|
|
1 |
|
|
|
215,645 |
|
|
|
— |
|
|
|
— |
|
|
|
215,646 |
|
Issuance of common stock upon exercise of pre-funded warrants |
|
|
9,607,145 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Issuance of common stock upon vesting and settlement of restricted stock units, net |
|
|
1,299,156 |
|
|
|
— |
|
|
|
(51 |
) |
|
|
— |
|
|
|
— |
|
|
|
(51 |
) |
Issuance of stock upon exercise of stock options |
|
|
164,838 |
|
|
|
— |
|
|
|
197 |
|
|
|
— |
|
|
|
— |
|
|
|
197 |
|
Issuance of common stock under ESPP |
|
|
123,151 |
|
|
|
— |
|
|
|
159 |
|
|
|
— |
|
|
|
— |
|
|
|
159 |
|
Gain on instrument-specific credit risk |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,888 |
|
|
|
2,888 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(81,144 |
) |
|
|
— |
|
|
|
(81,144 |
) |
Balance as of September 30, 2025 |
|
|
273,915,373 |
|
|
$ |
3 |
|
|
$ |
903,578 |
|
|
$ |
(683,449 |
) |
|
$ |
(1,143 |
) |
|
$ |
218,989 |
|
For the Nine Months Ended September 30, 2024
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
|
|
|
|
|
|
Total |
|
|
|
Common Stock |
|
|
Paid-in |
|
|
Accumulated |
|
|
Accumulated Other |
|
|
Stockholders' |
|
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Comprehensive Loss |
|
|
Equity |
|
Balance as of December 31, 2023 |
|
|
186,960,193 |
|
|
$ |
2 |
|
|
$ |
587,942 |
|
|
$ |
(513,007 |
) |
|
$ |
— |
|
|
$ |
74,937 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
9,887 |
|
|
|
— |
|
|
|
— |
|
|
|
9,887 |
|
Issuance of common stock and pre-funded warrants upon closing of underwritten public offering, net of underwriting discounts and commissions, and other offering costs of $5,566 |
|
|
17,596,113 |
|
|
|
— |
|
|
|
76,694 |
|
|
|
— |
|
|
|
— |
|
|
|
76,694 |
|
Issuance of common stock upon vesting and settlement of restricted stock units, net |
|
|
234,198 |
|
|
|
|
|
|
(9 |
) |
|
|
— |
|
|
|
— |
|
|
|
(9 |
) |
Issuance of common stock under ESPP |
|
|
93,970 |
|
|
|
— |
|
|
|
89 |
|
|
|
— |
|
|
|
— |
|
|
|
89 |
|
Issuance of common stock upon exercise of stock options |
|
|
58,832 |
|
|
|
— |
|
|
|
40 |
|
|
|
— |
|
|
|
— |
|
|
|
40 |
|
Loss on instrument-specific credit risk |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(2,328 |
) |
|
|
(2,328 |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(70,513 |
) |
|
|
— |
|
|
|
(70,513 |
) |
Balance as of September 30, 2024 |
|
|
204,943,306 |
|
|
$ |
2 |
|
|
$ |
674,643 |
|
|
$ |
(583,520 |
) |
|
$ |
(2,328 |
) |
|
$ |
88,797 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
Taysha Gene Therapies, Inc.
Condensed Consolidated Statements of Cash Flows
(in thousands)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
For the Nine Months
Ended September 30, |
|
|
|
2025 |
|
|
2024 |
|
Cash flows from operating activities |
|
|
|
|
|
|
Net loss |
|
$ |
(81,144 |
) |
|
$ |
(70,513 |
) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
Depreciation expense |
|
|
846 |
|
|
|
941 |
|
Stock-based compensation |
|
|
9,769 |
|
|
|
9,887 |
|
Change in fair value of warrant liability |
|
|
463 |
|
|
|
67 |
|
Non-cash change in fair value of term loan |
|
|
627 |
|
|
|
135 |
|
Debt issuance costs expensed under the fair value option |
|
|
147 |
|
|
|
— |
|
Impairment of long-lived assets |
|
|
— |
|
|
|
4,838 |
|
Non-cash lease expense |
|
|
959 |
|
|
|
916 |
|
Other |
|
|
264 |
|
|
|
678 |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
Prepaid expenses and other assets |
|
|
401 |
|
|
|
(612 |
) |
Accounts payable |
|
|
1,973 |
|
|
|
(1,416 |
) |
Accrued expenses and other liabilities |
|
|
3,615 |
|
|
|
(1,527 |
) |
Deferred revenue |
|
|
(4,288 |
) |
|
|
(6,311 |
) |
Net cash used in operating activities |
|
|
(66,368 |
) |
|
|
(62,917 |
) |
Cash flows from investing activities |
|
|
|
|
|
|
Purchase of property, plant and equipment |
|
|
(488 |
) |
|
|
(376 |
) |
Other |
|
|
131 |
|
|
|
— |
|
Net cash used in investing activities |
|
|
(357 |
) |
|
|
(376 |
) |
Cash flows from financing activities |
|
|
|
|
|
|
Proceeds from issuance of common stock and pre-funded warrants from underwritten public offering, net of underwriting discounts and sales commissions and other offering costs |
|
|
215,716 |
|
|
|
77,140 |
|
Prepayment of 2023 Trinity term loan |
|
|
(40,612 |
) |
|
|
— |
|
Proceeds from 2025 Trinity term loan, net |
|
|
49,853 |
|
|
|
— |
|
Debt issuance costs for term loan |
|
|
— |
|
|
|
(18 |
) |
Payment of shelf registration costs |
|
|
(33 |
) |
|
|
— |
|
Proceeds from common stock issuances under ESPP |
|
|
159 |
|
|
|
89 |
|
Proceeds from stock option exercises |
|
|
197 |
|
|
|
40 |
|
Other |
|
|
(247 |
) |
|
|
(210 |
) |
Net cash provided by financing activities |
|
|
225,033 |
|
|
|
77,041 |
|
Net increase in cash, cash equivalents and restricted cash |
|
|
158,308 |
|
|
|
13,748 |
|
Cash, cash equivalents and restricted cash at the beginning of the period |
|
|
141,636 |
|
|
|
146,540 |
|
Cash, cash equivalents and restricted cash at the end of the period |
|
$ |
299,944 |
|
|
$ |
160,288 |
|
Cash and cash equivalents |
|
|
297,344 |
|
|
|
157,688 |
|
Restricted cash |
|
|
2,600 |
|
|
|
2,600 |
|
Cash, cash equivalents and restricted cash at the end of the period |
|
$ |
299,944 |
|
|
$ |
160,288 |
|
Supplemental disclosure of cash flow information: |
|
|
|
|
|
|
Cash paid for interest |
|
$ |
3,949 |
|
|
$ |
3,980 |
|
Supplemental disclosure of noncash investing and financing activities: |
|
|
|
|
|
|
Proceeds allocated to Success Fee liability |
|
217 |
|
|
|
— |
|
Property, plant and equipment in accounts payable and accrued expenses |
|
41 |
|
|
133 |
|
Offering costs not yet paid |
|
|
25 |
|
|
|
360 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
Taysha Gene Therapies, Inc.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Note 1—Organization and Description of Business Operations
Taysha Gene Therapies, Inc. (the “Company” or “Taysha”) was originally formed under the laws of the State of Texas on September 20, 2019. Taysha converted to a Delaware corporation on February 13, 2020, which had no impact to the Company’s par value or issued and authorized capital structure.
Taysha is a clinical-stage biotechnology company focused on advancing AAV-based gene therapies for severe monogenic diseases of the central nervous system.
Sales Agreement
On October 5, 2021, the Company entered into a Sales Agreement (the “Sales Agreement”) with SVB Securities LLC (f/k/a SVB Leerink LLC) and Wells Fargo Securities, LLC (collectively, the “Sales Agents”), pursuant to which the Company may issue and sell, from time to time in its sole discretion, shares of its common stock having an aggregate offering price of up to $150.0 million through the Sales Agents. In March 2022, the Company amended the Sales Agreement to, among other things, include Goldman Sachs & Co. LLC as an additional Sales Agent. The Sales Agents may sell common stock by any method permitted by law deemed to be an “at-the-market offering” as defined in Rule 415(a)(4) of the Securities Act (the “ATM”), including sales made directly on or through the Nasdaq Global Select Market or any other existing trade market for the common stock, in negotiated transactions at market prices prevailing at the time of sale or at prices related to prevailing market prices, or any other method permitted by law. The Sales Agents are entitled to receive 3.0% of the gross sales price per share of common stock sold under the Sales Agreement. In April 2022, the Company sold 2,000,000 shares of common stock under the Sales Agreement and received $11.6 million in net proceeds. No other shares of common stock have been issued and sold pursuant to the Sales Agreement as of September 30, 2025.
On December 13, 2024, the Company filed a new shelf registration statement on Form S-3 following the expiration of its prior registration statement, in relation to the registration of common stock, preferred stock, debt securities, warrants and units or any combination thereof up to a total aggregate offering price of $300.0 million, including up to $100.0 million shares of common stock that may be offered and sold pursuant to the Sales Agreement.
On May 28, 2025, the Company notified the Sales Agents that it was suspending and terminating the prospectus (the “ATM Prospectus”) related to up to $100.0 million of the Company’s common stock issuable pursuant to the Sales Agreement. The Company will not make any sales of its securities pursuant to the Sales Agreement unless and until a new prospectus, prospectus supplement or a new registration statement is filed. Other than the termination of the ATM Prospectus, the Sales Agreement remains in full force and effect.
Liquidity and Capital Resources
The Company has incurred operating losses since inception and expects to continue to incur significant operating losses for the foreseeable future and may never become profitable. Losses are expected to continue as the Company continues to invest in its research and development activities. As of September 30, 2025, the Company had an accumulated deficit of $683.4 million. The Company expects to continue to incur significant expenses and operating losses for the foreseeable future.
On May 28, 2025, the Company entered into an underwriting agreement (the “May 2025 Underwriting Agreement”) with Jefferies LLC, BofA Securities, Inc., Piper Sandler & Co. and Barclays Capital Inc, as representatives of the several underwriters set forth therein (collectively, the “Underwriters”), to issue and sell 46,868,687 shares of the Company’s common stock and, in lieu of common stock to certain investors, pre-funded warrants to purchase 25,858,586 shares of its common stock in an underwritten public offering (the “May 2025 Offering”), pursuant to an effective shelf registration statement on Form S-3 and a related prospectus and prospectus supplement. The offering price to the public was $2.75 per share of common stock and $2.749 per pre-funded warrant, which was the price to the public of each share of common stock sold in the May 2025 Offering minus the $0.001 exercise price per pre-funded warrant. The Underwriters purchased the shares and the pre-funded warrants from the Company pursuant to the May 2025 Underwriting Agreement at a price of $2.585 per share and $2.584 per pre-funded warrant, respectively. See Note 10 for additional information. The initial closing of the May 2025 Offering occurred on May 30, 2025. In addition, the Company granted the Underwriters an option to purchase, for a period of 30 days, up to an additional 10,909,090 shares of common stock, which the Underwriters exercised in full on June 6, 2025. The total net proceeds from the May 2025 Offering were approximately $215.6 million, including the proceeds from the Underwriter’s option, after deducting underwriting discounts and commissions and offering expenses.
Future capital requirements will depend on many factors, including the timing and extent of spending on research and development and the market acceptance of the Company’s products. The Company will need to obtain additional financing in order to complete clinical studies and launch and commercialize any product candidates for which it receives regulatory approval. There can be no assurance that such financing will be available or will be on terms acceptable to the Company. As of September 30, 2025, the Company had cash and cash equivalents of $297.3 million, which the Company believes will be sufficient to fund its planned operations for a period of at least twelve months from the date of issuance of these unaudited condensed consolidated financial statements. The Company has based this estimate on assumptions that may prove to be wrong, and its operating plan may change as a result of many factors currently unknown to it. As a result, the Company could deplete its capital resources sooner than it currently expects. The Company expects to finance its future cash needs through a combination of equity offerings, debt financings, collaborations, strategic alliances or licensing arrangements. If the Company is unable to obtain funding, the Company would be forced to delay, reduce or eliminate some or all of its research and development programs, preclinical and clinical testing or commercialization efforts, which could adversely affect its business prospects.
Note 2—Summary of Significant Accounting Policies
Basis of Presentation
The unaudited condensed consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States (“GAAP”) as determined by the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) for interim financial information and the instructions to Form 10-Q and Article 10 of Regulation S-X and are consistent in all material respects with those included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2024, filed with the Securities and Exchange Commission (“SEC”) on February 26, 2025 (the “2024 Annual Report”). In the opinion of management, the unaudited condensed consolidated financial statements reflect all adjustments, which include only normal recurring adjustments necessary for the fair statement of the balances and results for the periods presented. The condensed consolidated balance sheet as of December 31, 2024 is derived from audited financial statements, however, it does not include all of the information and footnotes required by GAAP for complete financial statements. These unaudited condensed consolidated financial statements should be read in conjunction with the consolidated financial statements and related notes in the Company’s 2024 Annual Report.
Principles of Consolidation
The accompanying interim condensed consolidated financial statements include the accounts of Taysha and its wholly owned subsidiaries. All intercompany transactions and balances have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. The most significant estimates and assumptions in the Company’s financial statements relate to the determination of the fair value of the common stock prior to the initial public offering (“IPO”) (as an input into stock-based compensation), estimating manufacturing accruals and accrued or prepaid research and development expenses, the measurement of impairment of long-lived assets, the valuation of the Trinity Term Loans (as defined below) that are carried at fair value and the allocation of consideration received in connection with the Astellas Transactions (as defined below). These estimates and assumptions are based on current facts, historical experience and various other factors believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the recording of expenses that are not readily apparent from other sources. Actual results may differ materially from these estimates. To the extent there are material differences between the estimates and actual results, the Company’s future results of operations will be affected.
Significant Accounting Policies
There have been no changes in the Company’s significant accounting policies as disclosed in Note 2 to the audited consolidated financial statements included in the 2024 Annual Report.
Recently Adopted Accounting Pronouncements
There have been no significant changes in recently adopted accounting pronouncements from those disclosed in the section titled “Financial Statements and Supplementary Data” included in the 2024 Annual Report.
Recently Issued Accounting Pronouncements
In November 2024, the FASB issued ASU No. 2024-03, Income Statement - Reporting Comprehensive Income - Expense Disaggregation Disclosures, to improve expense disclosure requirements under ASC 220, Income Statement - Reporting Comprehensive Income, through enhancing disclosures about significant segment expenses. The guidance requires entities to provide additional disclosure about specific expenses by requiring entities to disaggregate, in a tabular presentation, each relevant expense caption on the face of the income statement that includes any of the following natural expenses (1) purchases of inventory, (2) employees compensation, (3) depreciation, (4) intangible asset amortization, and (5) depreciation, depletion and amortization recognized as part of oil - and gas - producing activities or other types of depletion expenses. The tabular disclosure would also include certain other expenses, when applicable. The ASU also enhances interim segment reporting requirements by aligning interim disclosures with information that must be disclosed annually in accordance with ASC 220. The guidance is effective for annual periods beginning after December 15, 2026, and interim periods beginning after December 15, 2027, applied either prospectively or retrospectively with early adoption permitted. The Company is still evaluating the impact this ASU will have on its disclosures.
Note 3—Fair Value Measurements
The Company’s financial instruments that are measured at fair value on a recurring basis consist of money market funds, the Trinity Term Loans, a success fee derivative liability and certain of the Company’s warrant liabilities.
The following tables present information about the Company’s financial assets and liabilities measured at fair value on a recurring basis and indicate the level of the fair value hierarchy used to determine such fair values (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2025 |
|
|
Total |
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
Cash equivalents – money market funds |
$ |
295,708 |
|
|
$ |
295,708 |
|
|
$ |
— |
|
|
$ |
— |
|
Total assets |
$ |
295,708 |
|
|
$ |
295,708 |
|
|
$ |
— |
|
|
$ |
— |
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
Trinity Term Loans |
$ |
50,852 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
50,852 |
|
Success Fee Derivative liabilities |
|
1,519 |
|
|
|
— |
|
|
|
— |
|
|
|
1,519 |
|
SSI Warrant liability |
|
901 |
|
|
|
— |
|
|
|
— |
|
|
|
901 |
|
Total liabilities |
$ |
53,272 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
53,272 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2024 |
|
|
Total |
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
Cash equivalents – money market funds |
$ |
138,308 |
|
|
$ |
138,308 |
|
|
$ |
— |
|
|
$ |
— |
|
Total assets |
$ |
138,308 |
|
|
$ |
138,308 |
|
|
$ |
— |
|
|
$ |
— |
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
Trinity Term Loans |
$ |
43,942 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
43,942 |
|
Success Fee Derivative liability |
|
930 |
|
|
|
— |
|
|
|
— |
|
|
|
930 |
|
SSI Warrant liability |
|
438 |
|
|
|
— |
|
|
|
— |
|
|
|
438 |
|
Total liabilities |
$ |
45,310 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
45,310 |
|
The Company classifies its money market funds, which are valued based on quoted market prices in an active market with no valuation adjustment, as Level 1 assets within the fair value hierarchy.
The Company’s Trinity Term Loans and Success Fee liabilities are classified as Level 3 measurements under the fair value hierarchy as the fair values were determined based on significant inputs not observable in the market. The fair values were determined utilizing a probability-weighted income approach, including variables for the timing of a success event and other probability estimates. See Note 7 for additional information on the Trinity Term Loans and Success Fees.
The Company’s SSI Warrant liability is classified as Level 3 measurements under the fair value hierarchy as the fair values were determined based on significant inputs not observable in the market. The fair values were determined using the Black-Scholes-Merton option pricing model to determine the fair value of the SSI Warrants (as defined below). See Note 10 for additional information on the SSI Warrants.
Note 4—Balance Sheet Components
Prepaid expenses and other current assets consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
September 30,
2025 |
|
|
December 31, 2024 |
|
Prepaid research and development |
|
$ |
839 |
|
|
$ |
841 |
|
Prepaid clinical trial |
|
|
760 |
|
|
|
1,112 |
|
Deferred offering costs |
|
|
57 |
|
|
|
135 |
|
Prepaid insurance |
|
|
46 |
|
|
|
249 |
|
Other |
|
|
456 |
|
|
|
308 |
|
Total prepaid expenses and other current assets |
|
$ |
2,158 |
|
|
$ |
2,645 |
|
Property, plant and equipment, net consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
September 30,
2025 |
|
|
December 31,
2024 |
|
Leasehold improvements |
|
$ |
2,117 |
|
|
$ |
2,117 |
|
Laboratory equipment |
|
|
3,170 |
|
|
|
3,130 |
|
Computer equipment |
|
|
728 |
|
|
|
707 |
|
Furniture and fixtures |
|
|
853 |
|
|
|
864 |
|
Construction in progress |
|
|
4,322 |
|
|
|
4,251 |
|
|
|
|
11,190 |
|
|
|
11,069 |
|
Accumulated depreciation |
|
|
(4,385 |
) |
|
|
(3,584 |
) |
Property, plant and equipment, net |
|
$ |
6,805 |
|
|
$ |
7,485 |
|
Property, plant and equipment, net includes $0.4 million and $0.7 million of assets capitalized as finance leases as of September 30, 2025 and December 31, 2024, respectively. During the nine months ended September 30, 2025, the Company sold equipment with a book value of zero and recorded a gain on the disposal of $0.1 million.
Depreciation expense was $0.2 million and $0.3 million for the three months ended September 30, 2025 and 2024, respectively. Depreciation expense was $0.8 million and $0.9 million for the nine months ended September 30, 2025 and 2024, respectively.
Accrued expenses and other current liabilities consisted of the following (in thousands):
|
|
|
|
|
|
|
|
|
|
|
September 30,
2025 |
|
|
December 31,
2024 |
|
Accrued compensation |
|
$ |
5,347 |
|
|
$ |
5,242 |
|
Accrued research and development |
|
|
5,139 |
|
|
|
1,714 |
|
Accrued clinical trial |
|
|
3,382 |
|
|
|
1,907 |
|
Lease liabilities, current portion |
|
|
1,668 |
|
|
|
1,877 |
|
Warrant liability |
|
|
901 |
|
|
|
438 |
|
Accrued professional and consulting fees |
|
|
522 |
|
|
|
725 |
|
Accrued property, plant and equipment |
|
|
— |
|
|
|
207 |
|
Other |
|
|
749 |
|
|
|
752 |
|
Total accrued expenses and other current liabilities |
|
$ |
17,708 |
|
|
$ |
12,862 |
|
Note 5— Leases
The Company leases certain office, laboratory, and manufacturing space.
Dallas Lease
On January 11, 2021, the Company entered into a lease agreement (the “Dallas Lease”) with Pegasus Park, LLC, a Delaware limited liability company (the “Dallas Landlord”), pursuant to which the Company leases approximately 15,000 square feet of office space at 3000 Pegasus Park Drive, Dallas, Texas 75247 (the “Office Space”).
The Dallas Lease commenced on May 27, 2021, and has a term of approximately ten years. The Company has an option to extend the term of the Dallas Lease for one additional period of five years.
The Dallas Landlord has the right to terminate the Dallas Lease, or the Company’s right to possess the Office Space without terminating the Dallas Lease, upon specified events of default, including the Company’s failure to pay rent in a timely manner and upon the occurrence of certain events of insolvency with respect to the Company.
Dallas Lease Expansion
On December 14, 2021, the Company amended the Dallas Lease (the “Dallas Lease Amendment”) with the Dallas Landlord, pursuant to which the Company leases approximately 18,000 square feet of office space adjacent to the Office Space at 3000 Pegasus Park Drive, Dallas, Texas 75247 (the “Expansion Premises”).
The Dallas Lease Amendment commenced on July 1, 2022, and has a term of approximately ten years.
The Company is obligated to pay operating costs and utilities applicable to the Expansion Premises. Total future minimum lease payments under the Dallas Lease Amendment over the initial 10 year term are approximately $6.0 million. The Company is responsible for costs of constructing interior improvements within the Expansion Premises that exceed a $40.00 per rentable square foot construction allowance provided by the Dallas Landlord.
The Company has a right of first refusal with respect to certain additional office space on the 15th floor at 3000 Pegasus Park Drive, Dallas, Texas 75247 before the Dallas Landlord accepts any offer for such space.
Durham Lease
On December 17, 2020, the Company entered into a lease agreement (the “Durham Lease”) with Patriot Park Partners II, LLC, a Delaware limited liability company (the “Durham Landlord”), pursuant to which the Company agreed to lease approximately 187,500 square feet of a manufacturing facility located at 5 National Way, Durham, North Carolina (the “Facility”). The Durham Lease commenced on April 1, 2021 and is expected to have a term of approximately fifteen years and six months. The Company has two options to extend the term of the Durham Lease, each for a period of an additional five years.
The Company was not required to provide a security deposit in connection with its entry into the Durham Lease. The Company was responsible for constructing interior improvements within the Facility. The Company was required to place $2.6 million in an escrow account which was to be released when the improvements were substantially complete. In December 2023, the Company entered into an agreement with the landlord whereby the Company agreed to remove specified leasehold improvements which will be funded by the escrowed funds. The escrow funds are recorded as restricted cash on the condensed consolidated balance sheets as of September 30, 2025 and December 31, 2024 with $0.5 million recorded in current assets and $2.1 million in noncurrent assets. The Durham Landlord has the right to terminate the Durham Lease upon specified events of default, including the Company’s failure to pay rent in a timely manner and upon the occurrence of certain events of insolvency with respect to the Company.
Summary of all lease costs recognized under ASC 842
The following table summarizes the lease costs recognized under ASC 842 and other information pertaining to the Company’s operating leases for the three and nine months ended September 30, 2025 and 2024 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
|
For the Nine Months Ended September 30, |
|
|
2025 |
|
2024 |
|
|
2025 |
|
|
2024 |
|
Operating lease cost |
$ |
665 |
|
$ |
673 |
|
|
$ |
2,000 |
|
|
$ |
1,994 |
|
Variable lease cost |
|
407 |
|
|
212 |
|
|
|
1,148 |
|
|
|
608 |
|
Total lease cost |
$ |
1,072 |
|
$ |
885 |
|
|
$ |
3,148 |
|
|
$ |
2,602 |
|
Supplemental information related to the remaining lease term and discount rate are as follows:
|
|
|
|
|
|
|
|
|
|
September 30, 2025 |
|
December 31, 2024 |
|
Weighted average remaining lease term (in years) – Finance leases |
|
|
1.39 |
|
|
1.88 |
|
Weighted average remaining lease term (in years) – Operating leases |
|
|
9.44 |
|
|
9.98 |
|
|
|
|
|
|
|
Weighted average discount rate – Finance leases |
|
|
10.56 |
% |
|
10.54 |
% |
Weighted average discount rate – Operating leases |
|
|
7.87 |
% |
|
7.84 |
% |
As of September 30, 2025, future minimum commitments under ASC 842 under the Company’s operating and finance leases were as follows (in thousands):
|
|
|
|
|
|
|
Year Ending December 31, |
Operating |
|
Finance |
|
2025 |
|
738 |
|
|
114 |
|
2026 |
|
2,485 |
|
|
399 |
|
2027 |
|
2,577 |
|
|
— |
|
2028 |
|
2,673 |
|
|
— |
|
2029 |
|
2,779 |
|
|
— |
|
Thereafter |
|
14,284 |
|
|
— |
|
Total lease payments |
|
25,536 |
|
|
513 |
|
Less: imputed interest |
|
(7,785 |
) |
|
(33 |
) |
Total lease liabilities |
$ |
17,751 |
|
$ |
480 |
|
Lease liabilities, current |
|
1,245 |
|
|
423 |
|
Lease liabilities, non-current |
|
16,506 |
|
|
57 |
|
Total lease liabilities |
$ |
17,751 |
|
$ |
480 |
|
Note 6—Astellas Agreements
On October 21, 2022 (the “Effective Date”), the Company entered into the Option Agreement (the “Option Agreement”) with Astellas Gene Therapies, Inc. (f/k/a Audentes Therapeutics, Inc. (d/b/a Astellas Gene Therapy))(“Astellas”), pursuant to which the Company granted to Astellas an exclusive option to obtain an exclusive, worldwide, royalty and milestone-bearing right and license (A) to research, develop, make, have made, use, sell, offer for sale, have sold, import, export and otherwise exploit, or, collectively, exploit, the product known, as of the Effective Date, as TSHA-120 (the “120 GAN Product”), and any backup products with respect thereto for use in the treatment of Giant Axonal Neuropathy (“GAN”) or any other gene therapy product for use in the treatment of GAN that is controlled by Taysha or any of its affiliates or with respect to which the Company or any of its affiliates controls intellectual property rights covering the exploitation thereof (a “GAN Product”) and (B) under any intellectual property rights controlled by Taysha or any of its affiliates with respect to such exploitation (the “GAN Option”). Subject to certain extensions, the GAN Option was exercisable from the Effective Date through a specified period of time following Astellas’ receipt of (i) the formal minutes from the Type B end-of-Phase 2 meeting between Taysha and the FDA in response to the Company’s meeting request sent to the FDA on September 19, 2022 for the 120 GAN Product (the “Type B end-of-Phase 2 Meeting”), (ii) all written feedback from the FDA with respect to the Type B end-of-Phase 2 Meeting, and (iii) all briefing documents sent by Taysha to the FDA with respect to the Type B end-of-Phase 2 Meeting. In September 2023, Astellas provided written notice of its decision not to exercise the GAN Option.
Under the Option Agreement, the Company also granted to Astellas an exclusive option to obtain an exclusive, worldwide, royalty and milestone-bearing right and license (A) to exploit any Rett Product (as defined below), and (B) under any intellectual property rights controlled by Taysha or any of its affiliates with respect to such exploitation (the “Rett Option”). Subject to certain extensions, the Rett Option was exercisable from the Effective Date through a specified period of time following Astellas’ receipt of (i) certain clinical data from the female pediatric trial and (ii) certain specified data with respect to TSHA-102, such data package the “Rett Data Package” and such period, the “Rett Option Period,” related to (i) the product known, as of the Effective Date, as TSHA-102 and any backup products with respect thereto for use in the treatment of Rett syndrome, and (ii) any other gene therapy product for use in the treatment of Rett syndrome that is controlled by Taysha or any of its affiliates or with respect to which the Company or any of its affiliates controls intellectual property rights covering the exploitation thereof (a “Rett Product”). The Company delivered the Rett Data Package to Astellas in mid-2025. Under the Option Agreement, Astellas was required to decide whether to exercise the Rett Option within 90 days after Astellas’ receipt of the Rett Data Package. In October 2025, the Rett Option expired without being exercised.
Following the expiration of the Option Agreement, the Company now holds unencumbered rights to the TSHA-102 program.
During the Rett Option Period, which has now expired, the Company agreed to (A) not solicit or encourage any inquiries, offers or proposals for, or that could reasonably be expected to lead to, a Change of Control (as defined in the Option Agreement), or (B) otherwise initiate a process for a potential Change of Control, in each case, without first notifying Astellas and offering Astellas the opportunity to submit an offer or proposal to the Company for a transaction that would result in a Change of Control. If Astellas failed or declined to submit any such offer within a specified period after the receipt of such notice, the Company would have the ability to solicit third party bids for a Change of Control transaction. If Astellas delivered an offer to the Company for a transaction that would result in a Change of Control, the Company and Astellas would have attempted to negotiate in good faith the potential terms and conditions for such potential transaction that would have resulted in a Change of Control for a specified period, which period may have been shortened or extended by mutual agreement. All of Astellas’ rights with respect to a Change of Control of the Company terminated as a result of the expiration of the Rett Option Period and the Option Agreement in October 2025.
As partial consideration for the rights granted to Astellas under the Option Agreement, Astellas paid the Company an upfront payment of $20.0 million (the “Upfront Payment”). Astellas or any of its affiliates had the right, in its or their discretion and upon written notice to the Company, to offset the amount of the Upfront Payment (in whole or in part, until the full amount of the Upfront Payment has been offset) against (a) any payment(s) owed to Taysha or any of its affiliates (or to any third party on behalf of the Company) under or in connection with any license agreement entered into with respect to any GAN Product or Rett Product, including, any upfront payment, milestone payment or royalties owed to Taysha or any of its affiliates (or to any third party on behalf of the Company) under or in connection with any such license agreement or (b) any amount owed to Taysha or any of its affiliates in connection with a Change of Control transaction with Astellas or any of its affiliates. As further consideration for the rights granted to Astellas under the Option Agreement, the Company and Astellas also entered into the Astellas Securities Purchase Agreement (as defined below).
Astellas Securities Purchase Agreement
On October 21, 2022, the Company entered into a securities purchase agreement with Astellas (the “Astellas Securities Purchase Agreement”), pursuant to which the Company agreed to issue and sell to Astellas in a private placement (the “Astellas Private Placement”), an aggregate of 7,266,342 shares (the “Astellas Private Placement Shares”), of its common stock, for aggregate gross proceeds of $30.0 million. The Astellas Private Placement closed on October 24, 2022. Pursuant to the Astellas Securities Purchase Agreement, in connection with the Astellas Private Placement, Astellas has the right to designate one individual to attend all meetings of the Board in a non-voting observer capacity. The Company also granted Astellas certain registration rights with respect to the Astellas Private Placement Shares.
Accounting Treatment
In October 2022, upon closing of the Astellas Private Placement and transferring the 7,266,342 shares to Astellas, the Company recorded the issuance of shares at fair value. Fair value of the shares transferred to Astellas was calculated in accordance with ASC 820, Fair Value Measurement by analyzing the Company’s stock price for a short period of time prior to and after the transaction date as traded on the NASDAQ. The NASDAQ trading data is considered an active market and a Level 1 measurement under ASC 820. The fair value was determined to be approximately $13.95 million or $1.92 per share. The $16.1 million difference between the $30.0 million paid by Astellas and the fair market value of shares issued was allocated to the transaction price of the Option Agreement.
The Company determined that the Option Agreement falls within the scope of ASC 606, Revenue from Contracts with Customers as the development of TSHA-102 for the treatment of Rett Syndrome and TSHA-120 for the treatment of GAN are considered ordinary activities for the Company. In accordance with ASC 606, the Company evaluated the Option Agreement and identified three separate performance obligations: (1) option to obtain licensing right to GAN, (2) option to obtain licensing right to Rett and (3) performance of research and development activities in the Rett development plan. The transaction price is determined to be $36.1 million which is comprised of the $20.0 million Upfront Payment and the $16.1 million allocated from the Astellas Private Placement.
To determine the standalone selling price (“SSP”) of the Rett and GAN options, which the Company concluded to be material rights, the Company utilized the probability-weighted expected return (“PWERM”) method. The PWERM method contemplates the probability and timing of an option exercise. At contract inception, the Company estimated that the probability of exercise was 50% for each of the GAN and Rett options. The SSP of the Rett research and development activities was estimated using an expected cost-plus margin approach. The standalone selling prices of the material rights and Rett research and development activities were then used to proportionately allocate the $36.1 million transaction price to the three performance obligations. The $36.1 million transaction price was recorded as deferred revenue on the condensed consolidated balance sheet at the inception of the Astellas Transactions.
The following table summarizes the allocation of the transaction price to the three performance obligations at contract inception (amounts in thousands):
|
|
|
|
|
|
|
Transaction Price Allocation |
|
Option to obtain license for Rett |
|
$ |
5,485 |
|
Option to obtain license for GAN |
|
|
2,317 |
|
Rett research and development activities |
|
|
28,257 |
|
Total |
|
$ |
36,059 |
|
Revenue allocated to the material rights will be recognized at a point in time when each option period expires or when a decision is made by Astellas to exercise or not exercise each option. Revenue from the Rett research and development activities will be recognized as activities are performed using an input method, according to the costs incurred as related to the total costs expected to be incurred to satisfy the performance obligation. The transfer of control occurs over this time period and is a reliable measure of progress towards satisfying the performance obligation.
The Company recognized no revenue from Rett research and development activities for the three months ended September 30, 2025 and recognized $1.8 million for the three months ended September 30, 2024. The Company recognized revenue of $4.3 million and $6.3 million from Rett research and development activities for the nine months ended September 30, 2025 and 2024, respectively.
The Company had $5.5 million of deferred revenue on the condensed consolidated balance sheet as of September 30, 2025 comprised of $5.5 million for the Rett Option. The $5.5 million of deferred revenue for the Rett Option will be recognized in the fourth quarter of 2025 following the expiration of the Rett Option in October 2025. The Company had $9.8 million of deferred revenue on the condensed consolidated balance sheets as of December 31, 2024 comprised of $5.5 million for the Rett Option and $4.3 million of Rett research and development activities.
Note 7 – Term Loans
2025 Loan with Trinity Capital
On August 7, 2025 (the “Trinity Refinance Date”), the Company entered into a Loan and Security Agreement (the “2025 Trinity Term Loan Agreement”), by and among the Company, the lenders party thereto from time to time (the “2025 Trinity Lenders”) and Trinity Capital Inc., as administrative agent and collateral agent for the 2025 Trinity Lenders (“Trinity”). The 2025 Trinity Term Loan Agreement provides for (i) on the Trinity Refinance Date, $50.0 million aggregate principal amount of term loans (“Tranche A”), (ii) from the Trinity Refinance Date until March 31, 2028 an additional $25.0 million term loan facility contingent on delivering evidence satisfactory to Trinity that the Company has submitted a biologics license application (“BLA”) to the FDA for TSHA-102 in Rett Syndrome (“Tranche B”), (iii) from the Trinity Refinance Date until March 31, 2029, an additional $25.0 million term loan facility contingent on delivering evidence satisfactory to Trinity that the Company has received BLA approval from the FDA for TSHA-102 in Rett Syndrome (collectively, the “2025 Trinity Term Loans”). The Company drew $50.0 million in term loans on the Trinity Refinance Date. The existing 2023 Trinity Term Loan Agreement was terminated and the existing 2023 Trinity Term Loans were repaid concurrently with entry into the 2025 Trinity Term Loan Agreement and the draw of Tranche A.
The interest rate applicable to the 2025 Trinity Term Loans is the greater of (a) the WSJ Prime Rate plus 4.00% or (b) 11.50% per annum. The 2025 Trinity Term Loans are interest only from the Trinity Refinance Date through 48 months from the Trinity Refinance Date, which may be extended to 60 months from the Trinity Refinance Date upon the satisfaction of certain milestones set forth in the 2025 Trinity Term Loan Agreement, after which the Company is required to pay equal monthly installments of principal through August 1, 2030 (the “New Maturity Date”). As of September 30, 2025, $50.0 million was outstanding on the 2025 Trinity Term Loan, recorded as Term Loan, net on the condensed consolidated balance sheet.
Future principal debt payments on the 2025 Trinity Term Loan Agreement as of September 30, 2025 are as follows (in thousands):
|
|
|
|
|
Year Ending December 31, |
|
|
|
2025 |
|
$ |
— |
|
2026 |
|
|
— |
|
2027 |
|
|
— |
|
2028 |
|
|
— |
|
2029 |
|
|
11,969 |
|
2030 |
|
|
38,031 |
|
Total principal payments |
|
$ |
50,000 |
|
On the Trinity Refinance Date, the Company paid a commitment fee of $0.1 million to Trinity. Upon any future draw of 2025 Trinity Term Loans, the Company is required to pay an additional commitment fee of 1.00% of the aggregate principal amount of such 2025 Trinity Term Loans.
The 2025 Trinity Term Loans may be prepaid in full (i) from the Trinity Refinance Date through August 7, 2026, with payment of a 3.00% prepayment premium, (ii) from August 8, 2026 through August 7, 2027, with payment of a 2.00% prepayment premium, and (iii) from August 8, 2027 through, but excluding, the New Maturity Date, with payment of a 1.00% prepayment premium. Upon repayment in full of the 2025 Trinity Term Loans, the Company will pay to Trinity an end of term payment equal to 5.00% of the original principal amount of the 2025 Trinity Term Loans.
The obligations under the 2025 Trinity Term Loan Agreement are secured by a perfected security interest in all of the Company’s assets except for certain customarily excluded property pursuant to the terms of the 2025 Trinity Term Loan Agreement. There are no financial or minimum cash balance covenants and no warrants associated with the 2025 Trinity Term Loan Agreement. The 2025 Trinity Term Loan Agreement contains various covenants that limit the Company’s ability to engage in specified types of transactions without the consent of Trinity and the New Trinity Lenders which include, among others, incurring or assuming certain debt; merging, consolidating or acquiring all or substantially all of the capital stock or property of another entity; changing the nature of the Company’s business; changing the Company’s organizational structure or type; licensing, transferring or disposing of certain assets; granting certain types of liens on the Company’s assets; making certain investments; and paying cash dividends.
The 2025 Trinity Term Loan Agreement contains customary representations and warranties, affirmative and negative covenants and events of default, including payment default, breach of covenants, change of control, and material adverse effects. Upon the occurrence of an event of default, a default interest rate of an additional 5.00% per annum may be applied to the outstanding loan balances, and the New Trinity Lenders may declare all outstanding obligations immediately due and payable and exercise all of its rights and remedies as set forth in the 2025 Trinity Term Loan Agreement and under applicable law.
The Company assessed the terms and features of the 2025 Trinity Term Loans and determined that the 2025 Trinity Term Loan constituted a debt modification under ASC 470-50, Debt - Modifications and Extinguishments (“ASC 450”). As such, the 2025 Trinity Term Loans are accounted for as a continuation of the original debt, and no gain or loss on extinguishment was recognized. The Company continues to apply the fair value option under ASC 825, Financial Instruments (“ASC 825”) which was initially elected at the time of the 2023 Trinity Term Loan Agreement.
Under the fair value option, the 2025 Trinity Term Loans are measured at fair value on a recurring basis, with changes in fair value recognized in other income (expense) in the condensed consolidated statements of operations. The Company has not elected to present interest expense separately from changes in fair value and therefore will not present interest expense associated with the 2025 Trinity Term Loans. Any changes in fair value attributable to instrument-specific credit risk are presented in other comprehensive income or loss, if material. Under the fair value option, debt issuance costs are expensed as incurred. The Company incurred $1.1 million of debt issuance costs, of which $0.1 million were paid to Trinity and $1.0 were paid to third parties associated with the 2025 Trinity Term Loans. The debt issuance costs were recorded within general and administrative expense in the condensed consolidated statements of operations for the nine months ended September 30, 2025.
In connection with the 2025 Trinity Term Loan Agreement, the Company entered into a Success Fee Agreement with Trinity which specifies the terms regarding a fee in the amount of $0.5 million plus 5% of the principal amount of the funded 2025 Trinity Term Loans under Tranche B (the “2025 Success Fee”). The 2025 Success Fee is payable upon the achievement of certain corporate development value-inflection milestones. The 2025 Success Fee survives the termination of the 2025 Trinity Term Loans and expires on the earlier of ten years from the Trinity Refinance Date, or payment in full in cash of the 2025 Success Fee.
The Company determined that the 2025 Success Fee represents a freestanding financial instrument and should be accounted for as a derivative liability under ASC 815, Derivatives and Hedging (“ASC 815”) and recorded a liability within other non-current liabilities on the consolidated balance sheet, at fair value on the Trinity Refinance Date and will be marked-to-market at the end of each reporting period with gains and losses recognized as a component of other income (expense) in the condensed consolidated statements of operations.
The proceeds from the 2025 Trinity Term Loans were allocated to the 2025 Success Fee and 2025 Trinity Term Loans based on their respective fair values on the Trinity Refinance Date. The fair values were determined utilizing a probability-weighted income approach, including variables for the timing of a success event and other probability estimates.
The Company determined the fair value of the 2025 Trinity Term Loans and the 2025 Success Fee using a probability-weighted income approach and recorded the loan at fair value of $49.8 million and the 2025 Success Fee liability at fair value of $0.2 million in the condensed consolidated balance sheet at issuance. The Company calculated the discounted cash flows of the 2025 Trinity Term Loans using a discount rate of 13.86% and adjusted for the probability of various repayment scenarios. The Company calculated the discounted cash flows of the 2025 Success Fee liability, using a discount rate of 13.86% then adjusted for the probability of achievement of certain corporate development value-inflection milestones.
2023 Loan with Trinity Capital
On November 13, 2023 (the “Trinity Closing Date”), the Company entered into a Loan and Security Agreement (the “2023 Trinity Term Loan Agreement”), by and among the Company, the lenders party thereto from time to time (the “Trinity Lenders”) and Trinity Capital Inc., as administrative agent and collateral agent for the Trinity Lenders (“Trinity”). The 2023 Trinity Term Loan Agreement provided for, on the Trinity Closing Date, $40.0 million aggregate principal amount of term loans (collectively, the “2023 Trinity Term Loans”). The Company drew the Trinity Term Loans in full on the Trinity Closing Date.
The interest rate applicable to the 2023 Trinity Term Loans was the greater of (a) the Wall Street Journal (“WSJ”) Prime Rate plus 4.50% or (b) 12.75% per annum. The 2023 Trinity Term Loans were interest only from the Trinity Closing Date through 36 months from the Trinity Closing Date, which could have been extended to 48 months from the Trinity Closing Date upon the satisfaction of certain milestones set forth in the 2023 Trinity Term Loan Agreement, after which the Company was required to pay equal monthly installments of principal through November 13, 2028 (the “Maturity Date”).
The 2023 Trinity Term Loans could have been prepaid in full (i) from the Trinity Closing Date through November 13, 2024, with payment of a 3.00% prepayment premium, (ii) from November 13, 2024 through November 13, 2025, with payment of a 2.00% prepayment premium, and (iii) from November 13, 2025 through, but excluding, the Maturity Date, with payment of a 1.00% prepayment premium. On the Trinity Closing Date, the Company paid to Trinity a commitment fee of 1.00% of the original principal amount of the 2023 Trinity Term Loans. Upon repayment in full of the 2023 Trinity Term Loans, the Company paid to Trinity an end of term payment equal to $0.6 million.
The obligations under the 2023 Trinity Term Loan Agreement were secured by a perfected security interest in all of the Company’s assets except for certain customarily excluded property pursuant to the terms of the 2023 Trinity Term Loan Agreement.
The Company assessed the terms and features of the 2023 Trinity Term Loans and determined that the Company was eligible to elect the fair value option under ASC 825. The 2023 Trinity Term Loans contain various embedded features and the election of the fair value option allowed the Company to bypass analysis of potential embedded derivatives and further analysis of bifurcation of any recognized financial liabilities. Under the fair value option, the financial liability is initially measured at its fair value on the issue date and subsequently remeasured at estimated fair value on a recurring basis at each reporting date. Changes in the fair value of the 2023 Trinity Term Loans, which include accrued interest, if any, are recorded as a component of other expense (income) in the condensed consolidated statements of operations. The Company did not elect to present interest expense separately from changes in fair value and therefore did not present interest expense associated with the 2023 Trinity Term Loans. Any changes in fair value caused by instrument-specific credit risk were presented separately in other comprehensive income or loss if material.
In connection with the 2023 Trinity Term Loans, the Company entered into a Success Fee Agreement with Trinity which specifies the terms regarding a fee in the amount of 10% of the principal amount of the funded 2023 Trinity Term Loans (the “2023 Success Fee”). The 2023 Success Fee is payable upon the achievement of certain corporate development value-inflection milestones. The 2023 Success Fee survives the termination of the 2023 Trinity Term Loans and expires on the earlier of ten years, or payment in full in cash of the Success Fee. The 2023 Success Fee remains in effect after the termination of the 2023 Trinity Term Loans upon entry into the 2025 Trinity Term Loan Agreement and the draw of Tranche A.
The Company determined that the Success Fee represents a freestanding financial instrument and should be accounted for as a derivative liability under ASC 815 and recorded a liability within other non-current liabilities on the consolidated balance sheet, at fair value on the Trinity Closing Date and will be marked-to-market at the end of each reporting period with gains and losses recognized as a component of other income (expense) in the condensed consolidated statements of operations.
Fair Value
The 2025 Trinity Term Loans are accounted for as a continuation of the original debt in accordance with ASC 450, and the Company continues to apply the fair value option under ASC 825, which was initially elected at the time of the 2023 Trinity Term Loan Agreement. The Company remeasured the 2025 Trinity Term Loans, 2025 Success Fee and 2023 Success Fee as of September 30, 2025 using a probability weighted income approach. The Company calculated the discounted cash flows of the 2025 Trinity Term Loans using a discount rate of 13.43% and adjusted for the probability of various repayment scenarios. The Company calculated the discounted cash flows of the 2023 Success Fee and 2025 Success Fee, (the “Success Fees”), using a discount rate of 13.43% then adjusted for the probability of achievement of certain corporate development value-inflection milestones. As of September 30, 2025, the Company recorded the Tranche A Loan at a fair value of $50.9 million and the Success Fees at $1.5 million in the consolidated balance sheet.
The following table reconciles the change in fair value of the 2023 Trinity Term Loans and 2025 Trinity Term Loans, (the “Trinity Term Loans”), during the nine months ended September 30, 2025 (in thousands):
|
|
|
|
|
Trinity Term Loans |
|
|
|
Beginning fair value balance as of January 1, 2025 |
|
$ |
43,942 |
|
Repayment of 2023 Term Loan |
|
|
(40,612 |
) |
Beginning fair value balance of 2025 Trinity Term Loan on August 7, 2025 |
|
|
49,783 |
|
Change in fair value reported in statements of operations |
|
|
627 |
|
Change in fair value reported in comprehensive loss |
|
|
(2,888 |
) |
Ending fair value balance as of September 30, 2025 |
|
$ |
50,852 |
|
The following table reconciles the change in fair value of the Trinity Term Loans during the three months ended September 30, 2025 (in thousands):
|
|
|
|
|
Trinity Term Loans |
|
|
|
Beginning fair value balance as of July 1, 2025 |
|
$ |
41,051 |
|
Repayment of 2023 Term Loan |
|
|
(40,612 |
) |
Beginning fair value balance of 2025 Trinity Term Loan on August 7, 2025 |
|
|
49,783 |
|
Change in fair value reported in statements of operations |
|
|
186 |
|
Change in fair value reported in comprehensive loss |
|
|
444 |
|
Ending fair value balance as of September 30, 2025 |
|
$ |
50,852 |
|
During the three and nine months ended September 30, 2025, the Company recorded $1.3 million and $3.9 million, respectively, of interest expense within change in fair value of term loans, all of which was paid as of September 30, 2025. During the three and nine months ended September 30, 2024, the Company recorded $1.3 million and $3.9 million, respectively, of interest expense within change in fair value of term loans, all of which was paid as of September 30, 2024.
The following table reconciles the change in fair value of the Success Fees liability during the nine months ended September 30, 2025 (in thousands):
|
|
|
|
|
Success Fees |
|
|
|
Beginning fair value balance as of January 1, 2025 |
|
$ |
930 |
|
Beginning fair value balance of 2025 Success Fee on August 7, 2025 |
|
|
217 |
|
Change in fair value of Success Fees |
|
|
372 |
|
Ending fair value balance as of September 30, 2025 |
|
$ |
1,519 |
|
The following table reconciles the change in fair value of the Success Fees liability during the three months ended September 30, 2025 (in thousands):
|
|
|
|
|
Success Fees |
|
|
|
Beginning fair value balance as of July 1, 2025 |
|
$ |
1,229 |
|
Beginning fair value balance of 2025 Success Fee on August 7, 2025 |
|
|
217 |
|
Change in fair value of Success Fees |
|
|
73 |
|
Ending fair value balance as of September 30, 2025 |
|
$ |
1,519 |
|
Note 8—Research, Collaboration and License Agreements
UT Southwestern Agreement
On November 19, 2019, the Company entered into a research, collaboration and license agreement (“UT Southwestern Agreement”) with the Board of Regents of the University of Texas System on behalf of The University of Texas Southwestern Medical Center (“UT Southwestern”). Under the UT Southwestern Agreement, UT Southwestern is primarily responsible for preclinical development activities with respect to licensed products for use in certain specified indications (up to investigational new drug application-enabling studies), and the Company is responsible for all subsequent clinical development and commercialization activities with respect to the licensed products. UT Southwestern will conduct such preclinical activities for a two-year period under mutually agreed upon sponsored research agreements that were entered into beginning in April 2020. During the initial research phase, the Company has the right to expand the scope of specified indications under the UT Southwestern Agreement.
In connection with the UT Southwestern Agreement, the Company obtained an exclusive, worldwide, royalty-free license under certain patent rights of UT Southwestern and a non-exclusive, worldwide, royalty-free license under certain know-how of UT Southwestern, in each case to make, have made, use, sell, offer for sale and import licensed products for use in certain specified indications. Additionally, the Company obtained a non-exclusive, worldwide, royalty-free license under certain patents and know-how of UT Southwestern for use in all human uses, with a right of first refusal to obtain an exclusive license under certain of such patent rights and an option to negotiate an exclusive license under other of such patent rights. The Company is required to use commercially reasonable efforts to develop, obtain regulatory approval for, and commercialize at least one licensed product.
On April 2, 2020, the Company amended the UT Southwestern Agreement to include the addition of another licensed product and certain indications, and a right of first refusal to the Company over certain patient dosing patents. No additional consideration was transferred in connection with this amendment. In March 2022, the Company and UT Southwestern mutually agreed to revise the payment schedules and current performance expectations of the current sponsored research agreements under the UT Southwestern Agreement and defer payments by fifteen months. In December 2023, the Company and UT Southwestern mutually agreed to terminate specific sponsored research agreements.
The UT Southwestern Agreement expires on a country-by-country and licensed product-by-licensed product basis upon the expiration of the last valid claim of a licensed patent in such country for such licensed product. After the initial research term, the Company may terminate the agreement, on an indication-by-indication and licensed product-by-licensed product basis, at any time upon specified written notice to UT Southwestern. Either party may terminate the agreement upon an uncured material breach of the agreement or insolvency of the other party. In December 2023 and March 2025, the Company transferred rights to specific indications back to UT Southwestern.
In November 2019, as partial consideration for the license rights granted under the UT Southwestern Agreement, the Company issued 2,179,000 shares of its common stock, or 20% of its then outstanding fully-diluted common stock, to UT Southwestern. The Company does not have any future milestone or royalty obligations to UT Southwestern under the UT Southwestern Agreement other than costs related to maintenance of patents.
Abeona CLN1 Agreements
In August 2020, the Company entered into license and inventory purchase agreements with Abeona Therapeutics Inc. (“Abeona”) for worldwide exclusive rights to certain intellectual property rights and know-how relating to the research, development and manufacture of ABO-202, an AAV-based gene therapy for CLN1 disease (also known as infantile Batten disease). Under the terms of the agreements, the Company made initial cash payments to Abeona of $3.0 million for the license fee and $4.0 million for purchase of clinical materials and reimbursement for previously incurred development costs in October 2020. In exchange for the license rights, the Company recorded an aggregate of $7.0 million within research and development expenses in the consolidated statements of operations for the year ended December 31, 2020 since the acquired license or acquired inventory do not have an alternative future use.
The Company is obligated to make up to $26.0 million in regulatory-related milestones and up to $30.0 million in sales-related milestones per licensed CLN1 product. The Company will also pay an annual earned royalty in the high single digits on net sales of any licensed CLN1 products. The license agreement expires on a country-by-country and licensed product-by-licensed product basis upon the expiration of the last royalty term of a licensed product. Either party may terminate the agreement upon an uncured material breach of the agreement or insolvency of the other party. The Company may terminate the license agreement for convenience upon specified prior written notice to Abeona.
In December 2021, a regulatory milestone was triggered in connection with this agreement and therefore the Company recorded $3.0 million within research and development expenses in the consolidated statements of operations for the year ended December 31, 2021. The milestone fee was paid in January 2022 and classified as an investing cash outflow in the consolidated statements of cash flows. No additional milestone payments were triggered in connection with this agreement during the nine months ended September 30, 2025.
Abeona Rett Agreement
On October 29, 2020, the Company entered into a license agreement (the “Abeona Rett Agreement”) with Abeona pursuant to which the Company obtained an exclusive, worldwide, royalty-bearing license, with the right to grant sublicenses under certain patents, know-how and materials originally developed by the University of North Carolina at Chapel Hill, the University of Edinburgh and Abeona to research, develop, manufacture, have manufactured, use, and commercialize licensed products for gene therapy and the use of related transgenes for Rett syndrome.
Subject to certain obligations of Abeona, the Company is required to use commercially reasonable efforts to develop at least one licensed product and commercialize at least one licensed product in the United States.
In connection with the Abeona Rett Agreement, the Company paid Abeona a one-time upfront license fee of $3.0 million which was recorded in research and development expenses in the consolidated statements of operations for the year ended December 31, 2020 since the acquired license does not have an alternative future use. The Company is obligated to pay Abeona up to $26.5 million in regulatory-related milestones and up to $30.0 million in sales-related milestones per licensed Rett product and high single-digit royalties on net sales of licensed Rett products. Royalties are payable on a licensed product-by-licensed product and country-by-country basis until the latest of the expiration or revocation or complete rejection of the last licensed patent covering such licensed product in the country where the licensed product is sold, the loss of market exclusivity in such country where the product is sold, or, if no licensed product exists in such country and no market exclusivity exists in such country, ten years from first commercial sale of such licensed product in such country.
The Abeona Rett Agreement expires on a country-by-country and licensed product-by-licensed product basis upon the expiration of the last royalty term of a licensed product. Either party may terminate the agreement upon an uncured material breach of the agreement or insolvency of the other party. The Company may terminate the agreement for convenience upon specified prior written notice to Abeona.
In March 2022, the Company’s clinical trial application, (“CTA”) filing for TSHA-102 for the treatment of Rett Syndrome was approved by Health Canada and therefore triggered a regulatory milestone payment in connection with this agreement. The Company recorded $1.0 million within research and development expenses and classified the payment as an investing cash outflow in the consolidated statements of cash flows. In May 2023, the Company dosed the first patient with TSHA-102 in the Phase 1/2 REVEAL trial evaluating the safety and preliminary efficacy of TSHA-102 in adult patients with Rett syndrome and therefore triggered a milestone payment in connection with the Abeona Rett Agreement. The Company recorded $3.5 million within research and development expenses in the condensed consolidated statements of operations for the year ended December 31, 2023. This milestone fee was paid in August 2023 and classified as an investing cash outflow in the consolidated statements of cash flows for the year ended December 31, 2023. No additional milestone payments were made or triggered in connection with this agreement during the nine months ended September 30, 2025.
Note 9—Stock-Based Compensation
On July 1, 2020, the Company’s board of directors approved the 2020 Equity Incentive Plan (“Previous Plan”) which permitted the granting of incentive stock options, non-statutory stock options, stock appreciation rights, restricted stock awards (“RSAs”), restricted stock units (“RSUs”) and other stock-based awards to employees, directors, officers and consultants. As of September 16, 2020, the approval date of the New Plan (as defined below), no additional awards will be granted under the Previous Plan. The terms of the Previous Plan will continue to govern the terms of outstanding equity awards that were granted prior to approval of the New Plan.
On September 16, 2020, the Company’s stockholders approved the 2020 Stock Incentive Plan (“New Plan”), which became effective upon the execution of the underwriting agreement in connection with the IPO. The number of shares of common stock reserved for issuance under the New Plan automatically increases on January 1 of each year, for a period of ten years, from January 1, 2021, continuing through January 1, 2030, by 5% of the total number of shares of common stock outstanding on December 31 of the preceding calendar year, or a lesser number of shares as may be determined by the Company’s board of directors (the “New Plan Evergreen Provision”). Pursuant to this provision, on January 1, 2025, the Company increased the number of shares of common stock reserved for issuance under the New Plan by 10,247,165 shares.
Furthermore, on September 16, 2020, the Company’s stockholders approved the Employee Stock Purchase Plan (“ESPP”), which became effective upon the execution of the underwriting agreement in connection with the IPO. The maximum number of shares of common stock that may be issued under the ESPP will not exceed 362,000 shares of common stock, plus the number of shares of common stock that are automatically added on January 1st of each year for a period of up to ten years, commencing on the first January 1 following the IPO and ending on (and including) January 1, 2030, in an amount equal to the lesser of (i) one percent (1.0%) of the total number of shares of capital stock outstanding on December 31st of the preceding calendar year, and (ii) 724,000 shares of common stock. Pursuant to this provision, on January 1, 2025 the Company increased the number of shares of common stock reserved for issuance under the ESPP by 724,000. The Company has issued an aggregate of 358,514 shares of common stock under the ESPP as of September 30, 2025.
On December 15, 2023, the Company’s board of directors adopted the Taysha Gene Therapies, Inc. 2023 Inducement Plan (the “Inducement Plan”). The Inducement Plan was adopted without stockholder approval pursuant to Nasdaq Listing Rule 5635(c)(4). The Board reserved 4,000,000 shares of the Company’s common stock for issuance under the Inducement Plan. On December 12, 2024, the Company reserved an additional 2,000,000 shares of the Company’s common stock for issuance under the Inducement Plan.
The only persons eligible to receive grants of Inducement Awards (as defined below) under the Inducement Plan are individuals who satisfy the standards for inducement grants under Nasdaq Listing Rule 5635(c)(4). The Inducement Plan will be administered by the Board and the Company’s Compensation Committee. Inducement Awards may only be granted by: (i) the Compensation Committee, provided such committee is comprised solely of “independent directors” (as defined by Nasdaq Listing Rule 5605(a)(2)) or (ii) a majority of the Company’s “independent directors.” An “Inducement Award” means any right to receive the Company’s common stock, cash or other property granted under the Inducement Plan (including nonstatutory stock options, restricted stock awards, restricted stock unit awards, stock appreciation rights, performance stock awards, performance cash awards or other stock-based awards).
The number of shares available for grant under the Company’s incentive plans were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
New |
|
|
Inducement |
|
|
|
|
|
|
Plan |
|
|
Plan |
|
|
Total |
|
Available for grant - January 1, 2025 |
|
|
701,430 |
|
|
|
2,999,700 |
|
|
|
3,701,130 |
|
Plan adjustments and amendments |
|
|
10,247,165 |
|
|
|
— |
|
|
|
10,247,165 |
|
Grants |
|
|
(15,959,976 |
) |
|
|
(2,640,000 |
) |
|
|
(18,599,976 |
) |
Forfeitures |
|
|
1,136,093 |
|
|
|
1,283,950 |
|
|
|
2,420,043 |
|
Available for grant net of reserve - September 30, 2025 |
|
|
(3,875,288 |
) |
|
|
1,643,650 |
|
|
|
(2,231,638 |
) |
In August 2025, the Company’s board of directors approved the grant of 5,765,000 PSUs (as defined below) under the New Plan that are eligible to vest upon the achievement of various clinical and regulatory performance conditions. The grant of these awards exhausted the existing share reserve under the New Plan, and the Company used 3,875,288 shares of the shares to be issued on January 1, 2026 under the New Plan Evergreen Provision, which is permissible under section 2(c) of the New Plan, which limits the number of shares of common stock that may be issued pursuant to awards but does not limit the granting of awards. The Company expects to increase the shares of common stock reserved for issuance under the New Plan to cover the deficit on January 1, 2026 pursuant to the New Plan Evergreen Provision.
Stock Options
For the three months ended September 30, 2025, a total of 345,000 shares of common stock under the Inducement Plan were awarded with a weighted-average grant date fair value per share of $2.09. For the nine months ended September 30, 2025, a total of 9,503,984 shares of common stock under the New Plan and the Inducement Plan were awarded with a weighted-average grant date fair value per share of $1.44. The stock options vest over one to four years and have a ten-year contractual term.
The following weighted-average assumptions were used to estimate the fair value of time-based vesting stock options that were granted during the three and nine months ended September 30, 2025 and 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
|
Nine Months Ended September 30, |
|
|
|
2025 |
|
|
2024 |
|
|
2025 |
|
|
2024 |
|
Risk-free interest rate |
|
|
3.88 |
% |
|
|
3.99 |
% |
|
|
4.32 |
% |
|
|
4.04 |
% |
Expected dividend yield |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Expected term (in years) |
|
|
6.1 |
|
|
|
6.1 |
|
|
|
6.0 |
|
|
|
6.1 |
|
Expected volatility |
|
|
90 |
% |
|
|
90 |
% |
|
|
89 |
% |
|
|
89 |
% |
The following table summarizes time-based vesting stock option activity during the nine months ended September 30, 2025:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted |
|
|
|
|
|
|
|
|
|
Weighted |
|
|
Average |
|
|
Aggregate |
|
|
|
|
|
|
Average |
|
|
Remaining |
|
|
Intrinsic |
|
|
|
Stock |
|
|
Exercise |
|
|
Contractual |
|
|
Value |
|
|
|
Options |
|
|
Price |
|
|
Life (in years) |
|
|
(in thousands) |
|
Options outstanding at January 1, 2025 |
|
|
16,220,605 |
|
|
$ |
3.29 |
|
|
|
8.7 |
|
|
$ |
1,967 |
|
Options granted |
|
|
9,503,984 |
|
|
|
1.89 |
|
|
|
|
|
|
|
Exercised |
|
|
(110,228 |
) |
|
|
1.44 |
|
|
|
|
|
|
|
Options cancelled or forfeited |
|
|
(1,931,407 |
) |
|
|
2.00 |
|
|
|
|
|
|
|
Options expired |
|
|
(96,043 |
) |
|
|
2.13 |
|
|
|
|
|
|
|
Outstanding at September 30, 2025 |
|
|
23,586,911 |
|
|
$ |
2.85 |
|
|
|
8.4 |
|
|
$ |
31,133 |
|
Options exercisable at September 30, 2025 |
|
|
8,247,474 |
|
|
$ |
4.55 |
|
|
|
7.5 |
|
|
$ |
10,314 |
|
The aggregate intrinsic value in the above table is calculated as the difference between the fair value of the Company’s common stock at the respective reporting date and the exercise price of the stock options. As of September 30, 2025, the total unrecognized compensation related to unvested stock option awards granted was $19.6 million, which the Company expects to recognize over a weighted-average period of approximately 2.6 years.
Performance Stock Options
In February 2023, the Company issued options to purchase 70,235 shares of common stock to employees under the New Plan that contain performance-based vesting conditions, subject to continued employment through each anniversary and achievement of the performance conditions. The grant date fair value of these awards was not material. During the nine months ended September 30, 2025, 1,065 performance-based stock options were exercised. As of September 30, 2025, 57,281 of the shares subject to the performance-based options were vested and outstanding.
In May 2023, the Company issued options to purchase 2,166,653 shares of common stock to employees under the New Plan that contain both service and performance-based vesting conditions (the “Original Options”), with a weighted average grant date fair value per share of $0.50. These Original Options were expected to vest over a 3.6 year term if a combination of clinical, regulatory and financing performance conditions were achieved. No compensation expense was recognized in 2023 related to the Original Options as achievement of the performance conditions was not considered probable. The following weighted-average assumptions were used to estimate the fair value of the options granted in February 2023 and the Original Options that were granted in May 2023:
|
|
|
|
|
|
|
|
|
|
|
|
|
Risk-free interest rate |
|
|
4.02 |
% |
Expected dividend yield |
|
|
— |
|
Expected term (in years) |
|
|
6.0 |
|
Expected volatility |
|
|
81 |
% |
In December 2023, the Company modified all of the Original Options to amend the clinical and regulatory performance conditions and decreased the number of options granted to 1,516,655 (the “Modified Options”). The Company accounted for the changes in award terms as a modification in accordance with ASC 718, Compensation - Stock Compensation. Total compensation cost is equal to the modification date fair value.
The Modified Options have a grant date fair value per share of $1.28. The following assumptions were used to estimate the fair value of the Modified Options:
|
|
|
|
|
|
|
|
|
Risk-free interest rate |
|
|
3.90 |
% |
Expected dividend yield |
|
|
— |
|
Expected term (in years) |
|
|
5.8 |
|
Expected volatility |
|
|
88 |
% |
The Modified Options will vest over 3.0 years. The Company recognized stock-based compensation expense of $0.1 million and $0.4 million for the three and nine months ended September 30, 2025, respectively, related to the Modified Options. As of September 30, 2025, the total unrecognized compensation expense related to the Modified Options was $0.3 million, which the Company expects to recognize over a weighted average period of approximately 1.0 years using the accelerated attribution method. During the nine months ended September 30, 2025, 53,545 of the Modified Options were exercised. As of September 30, 2025, 1,463,110 of the Modified Options were outstanding, of which 452,007 were vested. No Modified Options vested during the nine months ended September 30, 2025.
Restricted Stock Units
For the nine months ended September 30, 2025, the Company issued 3,330,992 RSUs under the New Plan. The RSUs are subject to a service-based vesting condition. The service-based RSUs vest over a period of one to four year years. The Company at any time may accelerate the vesting of the RSUs. Such shares are not accounted for as outstanding until they vest.
The Company’s default tax withholding method for RSUs granted prior to 2023 is the sell-to-cover method, in which shares with a market value equivalent to the tax withholding obligation are sold on behalf of the holder of the RSUs upon vesting and settlement to cover the tax withholding liability and the cash proceeds from such sales are remitted by the Company to taxing authorities. For RSUs granted in 2023, the Company’s tax withholding policy allows the RSU holder to choose to either pay cash to the Company for the tax withholding obligation or elect the net withholding method, in which shares with a market equivalent to the tax withholding obligation are withheld and the net shares are issued to the RSU holder. For RSUs granted in 2024 and later, the Company’s tax withholding policy allows the RSU holder to choose to either pay cash to the Company for the tax withholding obligation or to elect the sell-to-cover method, in which shares with a market value equivalent to the tax withholding obligation are sold on behalf of the holder of the RSUs upon vesting and settlement to cover the tax withholding liability and the cash proceeds from such sales are remitted by the Company to taxing authorities.
The Company’s RSU activity for the nine months ended September 30, 2025 was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted |
|
|
|
|
|
|
Average |
|
|
|
|
|
|
Grant Date |
|
|
|
Number |
|
|
Fair Value |
|
|
|
of Shares |
|
|
per Share |
|
Nonvested at January 1, 2025 |
|
|
4,549,154 |
|
|
$ |
1.82 |
|
Restricted units granted |
|
|
3,330,992 |
|
|
|
1.91 |
|
Vested |
|
|
(1,246,218 |
) |
|
|
1.94 |
|
Cancelled or forfeited |
|
|
(362,750 |
) |
|
|
2.40 |
|
Nonvested at September 30, 2025 |
|
|
6,271,178 |
|
|
$ |
1.84 |
|
As of September 30, 2025, the total unrecognized compensation cost related to the unvested RSU's was $8.8 million which is expected to be amortized on a straight-line basis over a weighted-average period of approximately 2.7 years.
Performance-based Restricted Stock Units
In August 2025, the Company issued 5,765,000 performance-based restricted stock units (“PSUs”) to employees under the New Plan that are eligible to vest upon the achievement of certain clinical and regulatory performance conditions. The grant date fair value of the PSUs was $17.3 million, which is expected to be recognized at a point in time when achievement of the underlying performance conditions is considered probable, using management’s best estimates, which consider the inherent risk and uncertainty regarding the future outcomes of the performance conditions. During the three months ended September 30, 2025, no compensation expense was recorded related to the PSUs as achievement of the performance conditions was not considered probable.
As of September 30, 2025, 5,765,000 of the PSUs were unvested and outstanding.
Employee Stock Purchase Plan
In February 2022, the Company’s board of directors authorized the first offering under the ESPP. Under the ESPP, eligible employees may purchase shares of Taysha common stock through payroll deductions at a price equal to 85% of the lower of the fair market values of the stock as of the beginning or the end of six-month offering periods. An employee’s payroll deductions under the ESPP are limited to 15% of the employee’s compensation and employees may not purchase more than 1,800 shares of Taysha common stock during any offering period. During each of the three and nine months ended September 30, 2025 and 2024, stock-based compensation expense related to the ESPP was not material.
The following table summarizes the total stock-based compensation expense for the stock options, ESPP and RSUs recorded in the condensed consolidated statements of operations for the three and nine months ended September 30, 2025 and 2024 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
For the Nine Months Ended September 30, |
|
|
|
2025 |
|
|
2024 |
|
|
2025 |
|
|
2024 |
|
Research and development expense |
|
$ |
1,601 |
|
|
$ |
1,425 |
|
|
$ |
4,483 |
|
|
$ |
4,029 |
|
General and administrative expense |
|
|
1,684 |
|
|
|
1,932 |
|
|
|
5,286 |
|
|
|
5,858 |
|
Total |
|
$ |
3,285 |
|
|
$ |
3,357 |
|
|
$ |
9,769 |
|
|
$ |
9,887 |
|
Note 10—Warrants
Pre-Funded Warrants
Pre-Funded Warrants Issued in May 2025
On May 28, 2025, the Company entered into the May 2025 Underwriting Agreement with the Underwriters to issue and sell 46,868,687 shares of common stock, and, in lieu of common stock to certain investors, pre-funded warrants to purchase 25,858,586 shares of common stock (the “May 2025 Pre-Funded Warrants”) in the May 2025 Offering. The offering price to the public was $2.75 per share of common stock and $2.749 per May 2025 Pre-Funded Warrant, which was the price to the public of each share of common stock sold in the May 2025 Offering, minus the $0.001 exercise price per May 2025 Pre-Funded Warrant. The Underwriters agreed to purchase the shares and the May 2025 Pre-Funded Warrants from the Company pursuant to the May 2025 Underwriting Agreement at a price of $2.585 per share and $2.584 per May 2025 Pre-Funded Warrant, respectively. The initial closing of the May 2025 Offering occurred on May 30, 2025; no additional May 2025 Pre-Funded Warrants were sold upon the exercise of the Underwriters’ option in June 2025.
Each May 2025 Pre-Funded Warrant has an initial exercise price per share of $0.001, subject to certain adjustments. The May 2025 Pre-Funded Warrants may be exercised at any time until exercised in full, except that a holder will not be entitled to exercise any portion of any pre-funded warrant, which, upon giving effect to such exercise would cause (i) the aggregate number of shares of the Company’s common stock beneficially owned by the holder (together with its affiliates) to exceed 4.99% or 9.99%, as the case may be, of the number of shares of the Company’s common stock outstanding immediately prior to or after giving effect to the exercise, or (ii) the combined voting power of the Company’s securities beneficially owned by the holder (together with its affiliates) to exceed 4.99% or 9.99%, as the case may be, of the combined voting power of all of the Company’s securities then outstanding immediately prior to or after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the pre-funded warrants, subject to such holder’s rights under the May 2025 Pre-Funded Warrant to increase or decrease such percentage to another percentage not in excess of 19.99% upon at least 61 days’ prior notice from such holder to the Company.
The Company concluded that the May 2025 Pre-Funded Warrants meet the criteria for equity classification at issuance and were recorded as a component of stockholders’ equity within additional paid-in capital. The May 2025 Pre-funded Warrants are equity classified because they (i) are freestanding financial instruments that are legally detachable and separately exercisable from the equity instruments, (ii) are immediately exercisable, (iii) do not embody an obligation for the Company to repurchase its shares, (iv) permit the holders to receive a fixed number of shares of common stock upon exercise, (v) are indexed to the Company’s common stock and (vi) meet the equity classification criteria. In addition, such pre-funded warrants do not provide any guarantee of value or return.
Pre-Funded Warrants Issued in June 2024
On June 26, 2024, the Company entered into the June 2024 Underwriting Agreement with the Underwriters to issue and sell 14,361,113 shares of common stock, and, in lieu of common stock to certain investors, pre-funded warrants to purchase 18,972,221 shares of common stock (the “June 2024 Pre-Funded Warrants”) in the June 2024 Offering. The offering price to the public was $2.25 per share of common stock and $2.249 per June 2024 Pre-Funded Warrant, which was the price to the public of each share of common stock sold in the June 2024 Offering, minus the $0.001 exercise price per June 2024 Pre-Funded Warrant.
The Underwriters agreed to purchase the shares and the June 2024 Pre-Funded Warrants from the Company pursuant to the June 2024 Underwriting Agreement at a price of $2.115 per share and $2.114 per June 2024 Pre-Funded Warrant, respectively. The initial closing of the June 2024 Offering occurred on June 27, 2024; no additional June 2024 Pre-Funded Warrants were sold upon the exercise of the Underwriters’ option in July 2024.
Each June 2024 Pre-Funded Warrant has an initial exercise price per share of $0.001, subject to certain adjustments. The June 2024 Pre-Funded Warrants may be exercised at any time until exercised in full, except that a holder will not be entitled to exercise any portion of any pre-funded warrant, which, upon giving effect to such exercise would cause (i) the aggregate number of shares of the Company’s common stock beneficially owned by the holder (together with its affiliates) to exceed 4.99% or 9.99%, as the case may be, of the number of shares of the Company’s common stock outstanding immediately prior to or after giving effect to the exercise, or (ii) the combined voting power of the Company’s securities beneficially owned by the holder (together with its affiliates) to exceed 4.99% or 9.99%, as the case may be, of the combined voting power of all of the Company’s securities then outstanding immediately prior to or after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the pre-funded warrants, subject to such holder’s rights under the June 2024 Pre-Funded Warrant to increase or decrease such percentage to another percentage not in excess of 19.99% upon at least 61 days’ prior notice from such holder to the Company.
The Company concluded that the June 2024 Pre-Funded Warrants meet the criteria for equity classification at issuance and were recorded as a component of stockholders’ equity within additional paid-in capital. The June 2024 Pre-funded Warrants are equity classified because they (i) are freestanding financial instruments that are legally detachable and separately exercisable from the equity instruments, (ii) are immediately exercisable, (iii) do not embody an obligation for the Company to repurchase its shares, (iv) permit the holders to receive a fixed number of shares of common stock upon exercise, (v) are indexed to the Company’s common stock and (vi) meet the equity classification criteria. In addition, such pre-funded warrants do not provide any guarantee of value or return.
Pre-Funded Warrants Issued in August 2023
On August 14, 2023, the Company entered into a Securities Purchase Agreement (the “August 2023 Purchase Agreement”) with certain institutional and other accredited investors (the “Purchasers”), pursuant to which the Company agreed to sell and issue to the Purchasers in a private placement transaction (the “August 2023 Private Placement”) (i) 122,412,376 shares (the “PIPE Shares”) of the Company’s common stock, and (ii) with respect to certain Purchasers, pre-funded warrants to purchase 44,250,978 shares of the Company’s common stock (the “2023 Pre-Funded Warrants”) in lieu of shares of the Company’s common stock. The purchase price per share of common stock was $0.90 per share (the “PIPE Purchase Price”), and the purchase price for the 2023 Pre-Funded Warrants was the PIPE Purchase Price minus $0.001 per 2023 Pre-Funded Warrant.
The 2023 Pre-Funded Warrants have a per share exercise price of $0.001, subject to proportional adjustments in the event of stock splits or combinations or similar events. The 2023 Pre-Funded Warrants will not expire until exercised in full. The 2023 Pre-Funded Warrants may not be exercised if the aggregate number of shares of common stock beneficially owned by the holder thereof immediately following such exercise would exceed a specified beneficial ownership limitation; provided, however, that a holder may increase or decrease the beneficial ownership limitation by giving 61 days’ notice to the Company, but not to any percentage in excess of 19.99%.
The closing of the August 2023 Private Placement occurred on August 16, 2023 (the “PIPE Closing”). The total gross proceeds to the Company at the PIPE Closing were $150.0 million, and after deducting placement agent commissions and offering expenses payable by the Company, net proceeds were $140.3 million.
In April 2025, 9,615,000 of the 2023 Pre-Funded Warrants were exercised on a cashless basis in exchange for 9,607,145 shares of common stock.
SSI Warrants
In April 2023, the Company entered into a securities purchase agreement (the “SSI Securities Purchase Agreement”), with two affiliates of SSI Strategy Holdings LLC (“SSI”), named therein (the “SSI Investors”) pursuant to which the Company agreed to issue and sell to the SSI Investors in a private placement (the “SSI Private Placement”), 705,218 shares of its common stock (the “SSI Shares”) and warrants (the “SSI Warrants”) to purchase an aggregate of 525,000 shares of the Company’s common stock (the “Warrant Shares”). SSI provides certain consulting services to the Company. Each SSI Warrant has an exercise price of $0.7090 per Warrant Share, which was the closing price of the Company’s common stock on the Nasdaq Global Market on April 4, 2023 and expire ten years after issuance. The SSI Warrants issued in the SSI Private Placement provide that the holder of the SSI Warrants will not have the right to exercise any portion of its SSI Warrants until the achievement of certain clinical and regulatory milestones related to the Company’s clinical programs. The SSI Private Placement closed on April 5, 2023. Gross proceeds of the SSI Private Placement were $0.5 million.
The Company concluded that the SSI Warrants do not meet the criteria for equity classification under the guidance of ASC 815 due to settlement provisions that permit the holder to receive a variable number of shares in the event of a specified fundamental transaction as well as provisions that permit the holder to participate in dividends. As the SSI Warrants do not meet the criteria for equity classification, the Company recorded the warrants as liabilities at their fair value. This liability is subject to remeasurement at each balance sheet date until the warrants are exercised or expire, and any change in fair value is recognized in the Company’s condensed consolidated statements of operations.
The Company determined the fair value of the SSI Warrants at issuance was $0.3 million using the Black-Scholes-Merton option pricing model. The following assumptions were used to estimate the fair value of the warrants at issuance:
|
|
|
|
|
Risk-free interest rate |
|
|
3.46 |
% |
Expected dividend yield |
|
— |
|
Expected term (in years) |
|
|
5.2 |
|
Expected volatility |
|
|
81 |
% |
Market value of common stock (per share) |
|
$ |
0.71 |
|
The fair value adjustment as of September 30, 2025 was $0.5 million using the Black-Scholes-Merton option pricing model. As of September 30, 2025, 316,667 of the SSI Warrants have vested and are exercisable. No warrants were exercised during the period.
The Company estimated the fair value of the SSI Warrant liability using the following assumptions as of September 30, 2025:
|
|
|
|
|
Risk-free interest rate |
|
|
3.63 |
% |
Expected dividend yield |
|
|
— |
|
Expected term (in years) |
|
|
3.8 |
|
Expected volatility |
|
|
93 |
% |
Market value of common stock (per share) |
|
$ |
3.27 |
|
The following table summarizes changes in the Company’s warrant liability during the nine months ended September 30, 2025 (in thousands):
|
|
|
|
|
|
|
Warrant Liability |
|
Balance at January 1, 2025 |
|
$ |
438 |
|
Change in fair value |
|
|
463 |
|
Balance at September 30, 2025 |
|
$ |
901 |
|
Note 11—Net Loss Per Common Share
Basic net loss per common share is computed by dividing net loss attributable to common stockholders by the weighted-average number of shares of common stock outstanding for the period. Since the Company had a net loss in all periods presented, basic and diluted net loss per common share are the same.
In accordance with ASC 260, Earnings Per Share, shares issuable for little to no cash consideration should be included in the number of outstanding shares used to calculate basic loss per share as long as all conditions necessary for exercise are met. The 2023 Pre-Funded Warrants, the June 2024 Pre-Funded Warrants and the May 2025 Pre-Funded Warrants are therefore included as outstanding shares as of September 30, 2025 to calculate the weighted average number of shares outstanding to calculate basic loss per share.
The following table represents the calculation of basic and diluted net loss per common share for the three and nine months ended September 30, 2025 and 2024, respectively (in thousands, except share and per share data):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
For the Nine Months Ended September 30, |
|
|
|
2025 |
|
|
2024 |
|
|
2025 |
|
|
2024 |
|
Net loss |
|
$ |
(32,733 |
) |
|
$ |
(25,524 |
) |
|
$ |
(81,144 |
) |
|
$ |
(70,513 |
) |
Weighted-average shares of common stock outstanding used to compute net loss per common share, basic and diluted |
|
|
353,309,524 |
|
|
|
267,824,045 |
|
|
|
307,175,982 |
|
|
|
244,052,057 |
|
Net loss per common share, basic and diluted |
|
$ |
(0.09 |
) |
|
$ |
(0.10 |
) |
|
$ |
(0.26 |
) |
|
$ |
(0.29 |
) |
The following common stock equivalents outstanding as of September 30, 2025 and 2024 were excluded from the computation of diluted net loss per share attributable to common stockholders for the periods presented because including them would have been anti‑dilutive:
|
|
|
|
|
|
|
|
|
|
|
September 30,
2025 |
|
|
September 30,
2024 |
|
Unvested RSUs |
|
|
6,271,178 |
|
|
|
4,549,154 |
|
Stock options |
|
|
25,107,302 |
|
|
|
16,985,006 |
|
SSI Warrants |
|
|
316,667 |
|
|
|
316,667 |
|
Pre-Funded Warrants |
|
|
— |
|
|
|
63,223,199 |
|
Total |
|
|
31,695,147 |
|
|
|
85,074,026 |
|
Note 12—Income Taxes
Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Valuation allowances are provided if based upon the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized. The Company has evaluated the positive and negative evidence bearing upon the realizability of its deferred tax assets. There is no provision for income taxes because the Company has incurred operating losses and capitalized certain items for income tax purposes since its inception and maintains a full valuation allowance against its net deferred tax assets. The reported amount of income tax expense for the period differs from the amount that would result from applying the federal statutory tax rate to net loss before taxes primarily because of the change in valuation allowance.
As of September 30, 2025, there were no material changes to either the nature or the amounts of the uncertain tax positions previously determined for the year ended December 31, 2024.
On July 4, 2025, the One Big Beautiful Bill Act (“OBBBA”) was enacted in the United States. The OBBBA includes significant changes, such as the permanent extension of certain expiring provisions of the Tax Cuts and Jobs Act, modifications to certain aspects of the international tax framework and the restoration of favorable tax treatment for certain business expense provisions. The legislation has multiple effective dates, with certain provisions effective in 2025 and others implemented through 2027. The Company is currently assessing its impact on its consolidated financial statements.
Note 13—Commitments and Contingencies
Litigation
From time to time, the Company may be subject to various legal proceedings and claims that arise in the ordinary course of its business activities. The Company records a liability when a particular contingency is probable and estimable.
In January 2024 and April 2024, the Company was named a nominal defendant in two putative stockholder derivative actions filed by stockholders of the Company in the Court of Chancery of the State of Delaware. The lawsuits have since been consolidated and a lead plaintiff has been appointed. In October 2024, the lead plaintiff filed an amended complaint asserting claims relating to the Company’s August 2023 Private Placement against (i) certain of the Company’s current and former directors and officers for breach of fiduciary duty and unjust enrichment; and (ii) certain participants in the Company’s August 2023 Private Placement for aiding and abetting breach of fiduciary duty and unjust enrichment. Among other things, the complaints seek an unspecified award of damages in the Company’s favor, plus pre-judgment and post-judgment interest, and an award to the plaintiffs for the costs and disbursement of the action, including fees for their attorneys and experts.
The board of directors of the Company has formed a special litigation committee to investigate the claims and allegations in the amended complaint. On January 27, 2025, the court entered an order staying the litigation until June 30, 2025, while the special litigation committee conducts its investigation. On April 30, 2025, the court extended the stay until September 30, 2025. On August 29, 2025, the court again extended the stay until December 2, 2025. The Company has not recorded a liability related to these lawsuits because, at this time, the Company is unable to reasonably estimate possible losses or gains or determine whether an unfavorable outcome is either probable or remote.
In connection with an investigation captioned In the Matter of Taysha Gene Therapies, Inc. (D-04192), Taysha and certain of its officers and directors received subpoenas in late 2024 from the United States Securities and Exchange Commission (“SEC”) for materials relating to Taysha’s August 2023 PIPE and certain public offerings. Production of materials in response to the subpoenas was completed in April 2025. The SEC investigation is neither a determination that the Company or any individuals have violated any law nor a charge of any wrongdoing.
Commitments
In the normal course of business, the Company enters into contracts that contain a variety of indemnifications with its directors, officers, employees, licensors, suppliers and service providers. The Company’s maximum exposure under these arrangements is unknown at September 30, 2025. The Company does not anticipate recognizing any significant losses relating to these arrangements.
Note 14 – Retirement Plan
In July 2021, the Company adopted a 401(k) retirement savings plan that provides retirement benefits to all full-time employees. Eligible employees may contribute a percentage of their annual compensation, subject to Internal Revenue Service limitations. The Company contributed $0.1 million to the 401(k) retirement savings plan for each of the three months ended September 30, 2025 and 2024. The Company contributed $0.4 million to the 401(k) retirement savings plan for each of the nine months ended September 30, 2025 and 2024.
Note 15 – Segment Information
The Company’s Chief Executive Officer (“CEO”) is the Chief Operating Decision Maker (“CODM”). The CODM allocates resources and makes operating decisions based on financial information presented on a consolidated basis. The CODM does not evaluate profitability below the level of the consolidated company. Accordingly, the Company has determined that it has a single reportable segment and operating segment structure. The Company views its operations and manages its business as a single operating segment, the gene therapy segment, which is the business of developing AAV-based gene therapies for the treatment of severe monogenic diseases of the central nervous system.
The gene therapy segment derives revenue solely from the Astellas Agreements (see Note 6). The accounting policies of the gene therapy segment are the same as those described in the summary of significant accounting policies.
The CODM reviews significant segment expenses including direct program expenses and compensation expenses as part of the assessment of segment profit and loss. The measure of segment assets is reported on the balance sheet as total consolidated assets.
The following table presents significant segment expenses for the three and nine months ended September 30, 2025 and 2024 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
For the Nine Months Ended September 30, |
|
|
|
2025 |
|
|
2024 |
|
|
2025 |
|
|
2024 |
|
Revenue |
|
$ |
— |
|
|
$ |
1,788 |
|
|
$ |
4,288 |
|
|
$ |
6,311 |
|
Less: |
|
|
|
|
|
|
|
|
|
|
|
|
Research and development program expense |
|
|
|
|
|
|
|
|
|
|
|
|
Program expense |
|
|
14,366 |
|
|
|
4,673 |
|
|
|
26,737 |
|
|
|
20,159 |
|
Consultants and contractors expense |
|
|
2,913 |
|
|
|
3,662 |
|
|
|
9,385 |
|
|
|
11,756 |
|
Compensation expense |
|
|
11,111 |
|
|
|
9,283 |
|
|
|
33,231 |
|
|
|
26,707 |
|
Other segment expense(a) |
|
|
4,343 |
|
|
|
9,694 |
|
|
|
16,079 |
|
|
|
18,202 |
|
Consolidated net loss |
|
$ |
(32,733 |
) |
|
$ |
(25,524 |
) |
|
$ |
(81,144 |
) |
|
$ |
(70,513 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) Other segment expense included in consolidated net loss includes interest income, depreciation, insurance, travel, software and subscription services, legal, professional and consulting expense, rent and facilities expense, other general and administrative expense, impairment of long-lived assets, change in fair value of term loan, change in fair value of warrant liability and other expense. |
|
|
|
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our unaudited condensed consolidated financial statements and related notes included in this Quarterly Report on Form 10-Q and the audited financial statements and notes thereto as of and for the year ended December 31, 2024 and the related Management’s Discussion and Analysis of Financial Condition and Results of Operations, included in our Annual Report on Form 10-K for the year ended December 31, 2024, or Annual Report, filed with the Securities and Exchange Commission, or the SEC, on February 26, 2025. Unless the context requires otherwise, references in this Quarterly Report on Form 10-Q to “we,” “us,” and “our” refer to Taysha Gene Therapies, Inc. together with its consolidated subsidiaries.
Forward-Looking Statements
The information in this discussion contains forward-looking statements and information within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, which are subject to the “safe harbor” created by those sections. These forward-looking statements include, but are not limited to, statements concerning our strategy, future operations, future financial position, future revenues, projected costs, prospects and plans and objectives of management. The words “anticipates,” “believes,” “estimates,” “expects,” “intends,” “may,” “plans,” “projects,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions, or expectations disclosed in our forward-looking statements and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements that we make. These forward-looking statements involve risks and uncertainties that could cause our actual results to differ materially from those in the forward-looking statements, including, without limitation, the risks set forth in Part I, Item 1A, “Risk Factors” in our Annual Report. The forward-looking statements are applicable only as of the date on which they are made, and we do not assume any obligation to update any forward-looking statements.
Note Regarding Trademarks
All brand names or trademarks appearing in this report are the property of their respective holders. Unless the context requires otherwise, references in this report to the “Company,” “we,” “us,” and “our” refer to Taysha Gene Therapies, Inc.
Overview
We are a clinical-stage biotechnology company focused on advancing AAV-based gene therapies for the treatment of severe monogenic diseases of the central nervous system, or CNS. Our lead clinical program TSHA-102 is in development for the treatment of Rett syndrome, a rare neurodevelopmental disorder with no approved disease-modifying therapies that address the genetic root cause of the disease. With a singular focus on developing transformative medicines, we aim to address severe unmet medical needs and dramatically improve the lives of patients and their caregivers. Our management team has proven experience in gene therapy development and commercialization. We leverage this experience, our manufacturing process and a clinically and commercially proven AAV9 capsid in an effort to rapidly translate treatments from bench to bedside.
We are evaluating TSHA-102 for the treatment of females with Rett syndrome in our REVEAL clinical trials. The clinical trials are divided into two parts – Part A and Part B.
Part A includes the REVEAL Phase 1/2 Adolescent and Adult Trial, which is a first-in-human, open-label, randomized, dose-escalation and dose-expansion study evaluating the safety and preliminary efficacy of TSHA-102 as a single lumbar intrathecal administration in adolescent and adult females aged 12 years and older with Rett syndrome due to MECP2 loss-of-function mutation. The trial is taking place in Canada and the United States. A total of six participants aged 15 to 21 years were treated with TSHA-102 in this study, and enrollment for this trial is complete. Part A also includes the REVEAL Phase 1/2 Pediatric Trial, which is a first-in-human, open-label, randomized, dose-escalation and dose-expansion study evaluating the safety and preliminary efficacy of TSHA-102 as a single lumbar intrathecal administration in pediatric females aged 5 to 8 years with Rett syndrome due to MECP2 loss-of-function mutation. The trial is taking place in the United States, Canada and the United Kingdom. A total of six participants aged 6 to 8 years were treated with TSHA-102 in this study, and enrollment for this trial is also complete.
We have completed dosing of the 12 patients in Part A of both REVEAL trials, which includes eight patients in cohort two (high dose, 1x1015 total vg) and four patients in cohort one (low dose, 5.7x1014 total vg). As of the October 2025 data cutoff, TSHA-102 continues to be generally well tolerated with no treatment-related serious adverse events, or SAEs, or dose-limiting toxicities, or DLTs, in the 12 patients dosed in Part A of the REVEAL trials.
Part B is the pivotal phase of the trial, which includes the REVEAL Part B pivotal trial, a single-arm, open-label study evaluating the efficacy and safety of TSHA-102 in females with Rett syndrome, with each patient serving as their own control. Each participant will receive a single administration of the high dose (1x1015 total vg) of TSHA-102 delivered by lumbar intrathecal injection. The study will enroll 15 females aged 6 to <22 years in the developmental plateau population of Rett syndrome; these patients have an exceedingly low likelihood (0% to <6.7%) of gaining new or regaining developmental milestones that were lost after a defined number of years, based on our analysis of the NIH-funded International Rett Syndrome Foundation’s natural history study data. The primary endpoint will assess response rate, defined as the percentage of patients who gain or regain ≥ one developmental milestone from a list of 28 defined milestones across the core functional domains of communication, fine motor and gross motor, following TSHA-102. Standardized milestone assessments will be administered and captured on video at pre- and post-treatment timepoints, with determination of milestone gain/regain upon video-evidence review by independent, blinded central raters based on prespecified definitions of achievement for each milestone.
We have finalized FDA alignment on the REVEAL pivotal trial protocol and statistical analysis plan, which includes a 6-month interim analysis that may serve as the basis for Biologics License Application, or BLA, submission. Dosing of the first patient in the REVEAL pivotal trial is scheduled for Q4 2025, with enrollment of additional patients expected to continue at multiple sites this quarter.
We also obtained written alignment from the FDA on an extrapolation approach in a separate safety-focused study, which is planned in addition to the REVEAL pivotal trial. The study will evaluate the safety and preliminary efficacy of TSHA-102 in females aged 2 to <6 years who are in the pre-developmental plateau population of Rett syndrome. Efficacy will be extrapolated from the REVEAL pivotal trial. This approach is intended to enable access across a broad population of patients with Rett syndrome.
We have received orphan drug designation and rare pediatric disease designation from the United States Food and Drug Administration, or FDA, and orphan drug designation from the European Commission for TSHA-102 for the treatment of Rett syndrome. We also received Fast Track Designation from the FDA for TSHA-102 for the treatment of Rett syndrome. In February 2024, we received Innovative Licensing and Access Pathway, or ILAP, designation for TSHA-102 from the U.K. MHRA. The ILAP aims to facilitate patient access to novel treatments by accelerating time to market through opportunities for enhanced engagements with U.K. regulatory authorities and other stakeholders.
In April 2024, the FDA granted Regenerative Medicine Advanced Therapy, or RMAT, designation for TSHA-102 in Rett syndrome following the FDA’s review of available safety and efficacy data from the first three patients with Rett syndrome dosed with the low dose of TSHA-102 in Part A of the REVEAL Phase 1/2 Adolescent and Adult trial and the REVEAL Phase 1/2 Pediatric trial. In September 2025, the FDA granted Breakthrough Therapy designation to TSHA-102 following the FDA’s review of positive clinical evidence across the 12 patients treated with TSHA-102 in Part A of the REVEAL Phase 1/2 trials. The FDA grants Breakthrough Therapy designation to expedite the development and regulatory review of an investigational therapy intended to treat a serious condition. A drug is eligible for this designation if it demonstrates preliminary clinical evidence of substantial improvement over available treatments in one or more clinically significant endpoints.
Our Pipeline
We are focused on discovering, developing and commercializing gene therapies for the treatment of monogenic diseases of the CNS, in both rare and large patient populations. Our primary focus is advancing our lead TSHA-102 clinical program in Rett syndrome, while our pipeline of CNS programs offers the potential for additional development opportunities in the future. The stage of development of our Rett syndrome program, including the progress in our ongoing clinical trials, is represented in the table below:
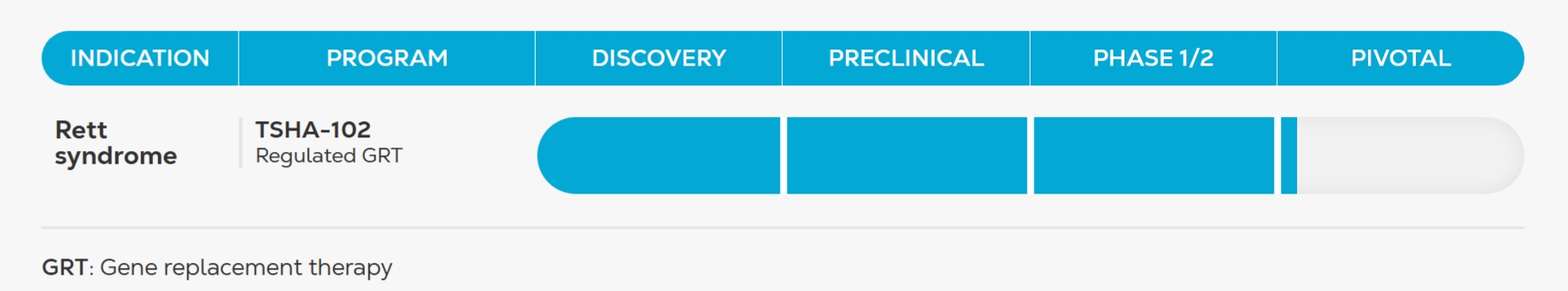
We have a limited operating history. Since our inception, our operations have focused on organizing and staffing our company, business planning, raising capital and entering into collaboration agreements for conducting preclinical and clinical development activities for our product candidates. Our lead product candidate is still in the clinical stage. We do not have any product candidates approved for sale and have not generated any revenue from product sales.
Through September 30, 2025, we have funded our operations primarily through: (i) the sale of equity, raising an aggregate of $911.0 million of gross proceeds from our initial public offering, or the IPO, sales of common stock pursuant to our Sales Agreement (as defined below), our October 2022 follow-on offering, our 2023 private placement, our June 2024 Offering (as defined below) and our May 2025 Offering (as defined below); (ii) pre-IPO private placements of our convertible preferred stock; (iii) our Term Loan Agreement (as defined below) and subsequently the 2025 Trinity Term Loan Agreement (as defined below); and (iv) the Astellas Transactions.
On November 13, 2023, or the 2023 Trinity Closing Date, we entered into a Loan and Security Agreement, or the 2023 Trinity Term Loan Agreement, by and among us, the lenders party thereto from time to time, or the Trinity Lenders, and Trinity Capital Inc., as administrative agent and collateral agent for the Trinity Lenders, or Trinity. The 2023 Trinity Term Loan Agreement provided for, on the Trinity Closing Date, $40.0 million aggregate principal amount of term loans, or, collectively, the 2023 Trinity Term Loans. We drew the 2023 Trinity Term Loans in full on the Trinity Closing Date. On August 7, 2025, we entered into the 2025 Trinity Term Loan Agreement (as defined below) with the 2025 Trinity Lenders (as defined below). We drew $50.0 million in term loans on the Trinity Refinance Date (as defined below). The existing 2023 Trinity Term Loan Agreement with the Trinity Lenders was terminated and the existing 2023 Trinity Term Loans were repaid in full concurrently with entry into the 2025 Trinity Term Loans (as defined below).
Since our inception, we have incurred significant operating losses. Our net losses were $81.1 million for the nine months ended September 30, 2025. As of September 30, 2025, we had an accumulated deficit of $683.4 million. We expect to continue to incur significant expenses and operating losses for the foreseeable future. We anticipate that our expenses will increase significantly in connection with our ongoing activities, as we:
•
continue to advance the clinical development of our product candidates and, if we determine to do so in the future, reprioritize the advancement of our preclinical and discovery programs;
•
conduct our ongoing clinical trials of TSHA-102 and any other current and future product candidates that we advance;
•
seek regulatory approval for any product candidates that successfully complete clinical trials;
•
continue to develop our gene therapy product candidate pipeline;
•
scale up our clinical and regulatory capabilities;
•
work with contract manufacturing organizations, or CMOs, for the manufacture of current Good Manufacturing Practice, or cGMP, material for clinical trials or potential commercial sales;
•
establish a commercialization infrastructure and scale up internal and external manufacturing and distribution capabilities to commercialize any product candidates for which we may obtain regulatory approval;
•
adapt our regulatory compliance efforts to incorporate requirements applicable to marketed products;
•
maintain, expand and protect our intellectual property portfolio;
•
hire additional clinical, manufacturing quality control, regulatory, manufacturing and scientific and administrative personnel;
•
add operational, financial and management information systems and personnel, including personnel to support our product development and planned future commercialization efforts; and
•
incur additional legal, accounting and other expenses in operating as a public company.
Clinical and Regulatory Update on TSHA-102
On May 28, 2025, we announced new clinical data from Part A of the REVEAL Phase 1/2 Adolescent/Adult and Pediatric trials evaluating TSHA-102 in Rett syndrome, as well as key elements of the REVEAL pivotal Part B trial design for TSHA-102 following written alignment from the FDA. The alignment reached with the FDA was supported by our analysis of the longitudinal Rett syndrome natural history study, or NHS, data from the International Rett Syndrome Foundation, or IRSF, as well as clinical data from the ongoing REVEAL Phase 1/2 trials. Subsequently, we commenced site activation activities for the REVEAL pivotal trial in accordance with previously aligned upon key design elements, following receipt of a No Objection Letter, or NOL, from Health Canada and feedback from the FDA. In October 2025, we announced that we finalized alignment with the FDA on the REVEAL pivotal trial protocol and statistical analysis plan, or SAP, that are intended to support the planned BLA submission for TSHA-102 following the resolution of remaining clinical and statistical queries. The previously aligned upon key design elements for the REVEAL pivotal trial protocol and SAP in support of the planned BLA submission remain unchanged.
Natural History Study
The NHS dataset from the IRSF contains data from approximately 1,100 females with confirmed Rett syndrome diagnosis and has up to 14 years of follow-up data. The NHS captures longitudinal data examining the gain, loss, and regain of developmental milestones across core functional domains of Rett syndrome: communication, fine motor function and gross motor function. According to independent third-party caregiver research, these functional skills and activities of daily living are highly important to caregivers of girls with Rett syndrome.
We leveraged a cohort of approximately 1,100 females with up to 14 years of follow up that met our REVEAL trial criteria to develop age- and time-based cumulative incidence curves that demonstrate the incidence of gaining a new milestone or regaining a milestone that was previously lost. The cumulative incidence models demonstrated distinct age-and time-based trends in developmental milestone acquisition that strengthened our understanding of the longitudinal disease progression in Rett syndrome, substantiated the disease-modifying potential of TSHA-102 and informed our discussions with the FDA on the proposed pivotal trial design for TSHA-102.
The NHS cumulative incidence models demonstrated that patients of six years of age or greater have reached a developmental plateau, with an exceedingly low (ranging from 0% to <6.7%) likelihood of gaining new developmental milestones and regaining developmental milestones that were lost after a defined number of years. We leveraged these findings to establish the developmental plateau population of patients who are six or more years of age.
These models of developmental milestone NHS data informed our discussion with the FDA on our proposed pivotal trial design for TSHA-102. We believe the NHS models provide a data-driven, objective approach to assessing clinically meaningful functional gains in a single-arm trial.
Regulatory Update and Pivotal Part B Trial Design
On May 28, 2025, we announced that we obtained written alignment from the FDA on key elements of our pivotal Part B REVEAL clinical trial design, following formal discussions enabled by the RMAT mechanism. Per guidance from the FDA, we submitted our IND application amendment to the FDA and CTA amendment to Health Canada in the second quarter of 2025. Taysha commenced site activation activities for the REVEAL pivotal trial, following receipt of a NOL from Health Canada and feedback from the FDA. In October 2025, we announced that we finalized alignment with the FDA on the REVEAL pivotal trial protocol and SAP that are intended to support the planned BLA submission for TSHA-102, following the resolution of remaining clinical and statistical queries. Previously aligned upon key design elements remain unchanged, including a 6-month interim analysis that may serve as the basis for the BLA submission, which was enabled by the rigorous, objective evaluation criteria used to evaluate developmental milestone achievement in Part A of the REVEAL Phase 1/2 trials and the unprecedented, based on natural history, response rate seen at six months post-treatment with TSHA-102 that deepened over time. We believe the 6-month interim analysis has the potential to expedite our BLA submission for TSHA-102 by at least two full quarters.
The REVEAL pivotal trial is a single-arm, open-label trial that will enroll 15 females aged 6 to <22 years who are in the developmental plateau population of Rett syndrome. Each patient will serve as their own control, and the primary endpoint will assess response rate, defined as the percentage of patients who gain or regain ≥ one developmental milestone from a list of 28 defined milestones across the core functional domains of communication, fine motor and gross motor, following TSHA-102. A response rate of 33% (5 out of 15 patients) is the minimum threshold for success sufficient to reject the null hypothesis. Based on our natural history data analysis, the null hypothesis is that one out of 15 patients aged ≥ 6 years may gain/regain one of the 28 natural history defined developmental milestone without treatment, corresponding to a response rate of 6.7%.
Standardized milestone assessments will be administered and captured on video at pre- and post-treatment timepoints. Video-evidenced determination of milestone gain/regain will be determined by independent, blinded central raters based on prespecified definitions of achievement for each milestone. The following 28 developmental milestones are being evaluated in the REVEAL pivotal Part B trial as part of the primary endpoint. These 28 developmental milestones from the NHS dataset were selected based on our caregiver research that demonstrated these milestones would reflect meaningful functional gains and are closely linked to activities of daily living, and based on our cumulative incidence models of the NHS data that demonstrated these milestones have a 0% to <6.7% likelihood of being achieved in the untreated population of females with Rett syndrome aged six years and older. The 28 developmental milestones are depicted in the chart below:
Key secondary endpoints in the REVEAL pivotal trial include the average number of total developmental milestones gained or regained per patient following TSHA-102, as well as clinician-assessed outcome measures, including the Revised Motor Behavior Assessment, or R-MBA, and Clinician Global Impression – Improvement, or CGI-I. Dosing of the first patient in the REVEAL pivotal trial is scheduled for Q4 2025, with enrollment of additional patients expected to continue at multiple sites this quarter.
We also obtained written alignment from the FDA on an extrapolation approach in a separate safety-focused study, which is planned in addition to the REVEAL pivotal trial. The study will evaluate the safety and preliminary efficacy of TSHA-102 in females aged 2 to <6 years who are in the pre-developmental plateau population of Rett syndrome. Efficacy will be extrapolated from the REVEAL pivotal trial. This approach is intended to enable access across a broad population of patients with Rett syndrome.
Clinical Data from Part A of Ongoing REVEAL Phase 1/2 Adolescent/Adult and Pediatric Trials from May 2025 Data Cutoff
The clinical trial efficacy data is based on the May 19, 2025, data cutoff, and included a total of 10 females with Rett syndrome (high dose, N=6; low dose, N=4) aged 6-21 years at dosing treated with the high dose (1x1015 total vg) or low dose (5.7x1014 total vg) of TSHA-102. In both clinical trials, 100% of the pediatric, adolescent and adult patients gained or regained one or more defined developmental milestone across the core functional domains of communication, fine motor function, and gross motor function post-TSHA-102 administration, with an approximately 0% likelihood of being achieved without treatment based on natural history data. The developmental milestone gains and regains were determined by multiple independent, central raters, who evaluated functional skills through video evidence at baseline and post-treatment, against predefined binary criteria.
Milestones achieved post TSHA-102 treatment for all 10 patients are depicted in the chart below:
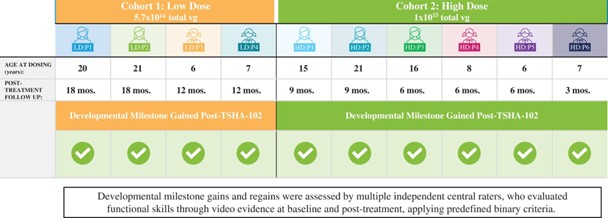
A total of 22 developmental milestones were achieved across the 10 patients. Developmental milestones were achieved early post-TSHA-102 administration, with new gains/regains demonstrated over time. The high dose cohort achieved a 100% responder rate 25% faster than the low dose cohort, supporting the accelerated functional benefit observed with the high dose.
A summary of the developmental milestones that were gained or regained in the REVEAL trials across the core functional domains of Rett syndrome post-TSHA-102 treatment is summarized in the chart below:
In October 2025, we announced results from a new supplemental data analysis that provide supportive evidence of additional functional gains in skills and improvements across core disease characteristics outside of the 28 natural history defined developmental milestones that further reinforce TSHA-102’s consistent, multi-domain impact on activities of daily living. Skills and improvements were derived from structured, validated scales administered in Part A. Findings demonstrated that in addition to the developmental milestones achieved across the treatment cohort in Part A, all patients gained multiple additional functional skills/improvements across the domains of communication, fine motor, gross motor and autonomic function post-TSHA-102. A total of 165 additional skills/improvements were achieved across the 10 patients, further supporting the potential broad and consistent therapeutic impact of TSHA-102 on functional abilities and activities of daily living that are important to caregivers and clinicians.
The supplemental data analysis was based on additional skills and improvements achieved in core disease characteristics outside of the natural history defined developmental milestones that were derived from structured efficacy scales assessed in Part A that are validated in Rett syndrome. The scales include the Adapted Mullen Scales of Early Learning (MSEL-A) evaluating defined communication skills (N=5 patients with available data), the Revised Motor Behavior Assessment (R-MBA) evaluating specific Rett syndrome disease characteristics (N=10 patients with available data), and the Observer-Reported Communication Ability (ORCA) evaluating communication skills (N=5 patients with available data).
A summary of the skills and improvements achieved across cored disease characteristics outside of the the natural history defined developmental milestones is summarized in the chart below:
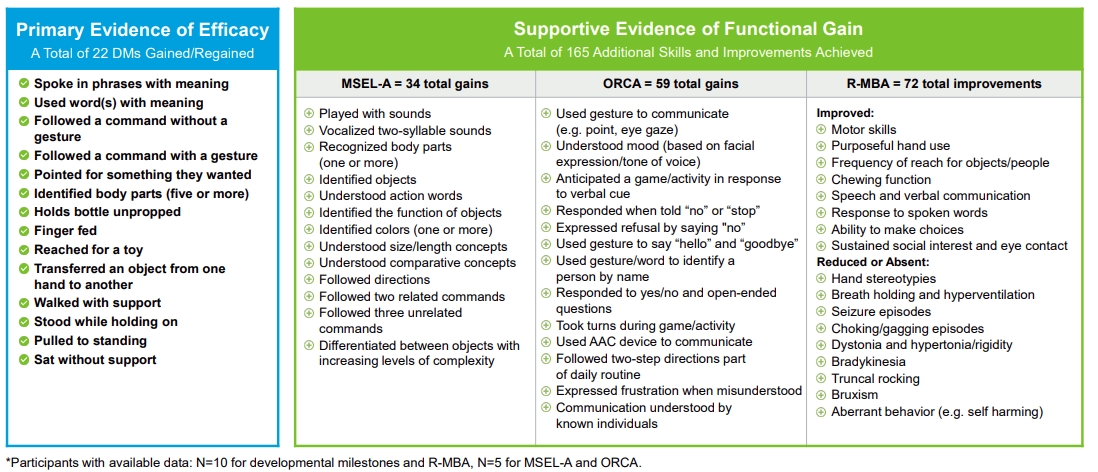
Examples of multi-domain foundational gains and improvements demonstrated post-TSHA-102 impacting activities of daily living, based on developmental milestones and additional skills/improvements achieved are summarized in the chart below:
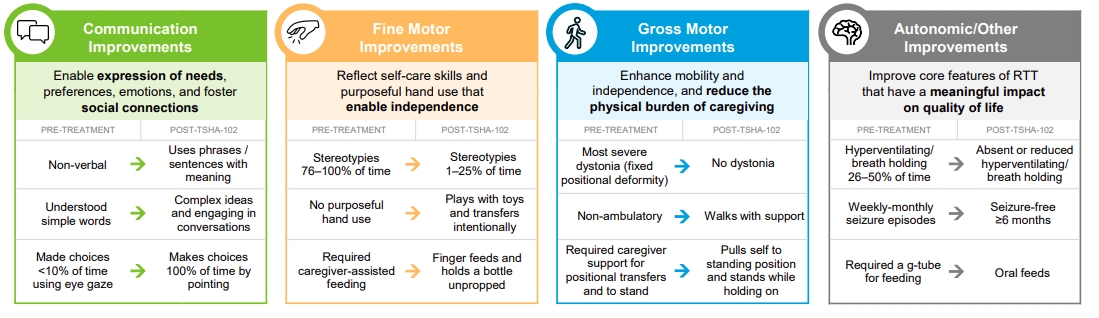
The high dose cohort consistently outperformed the low dose cohort in Part A of the REVEAL trials across multiple outcome measures. Specifically, improvements were observed across multiple clinician-assessed outcome measures, including R-MBA, and CGI-I, which corroborated the developmental milestone gains/regains demonstrated post-TSHA-102.
Patients demonstrated a statistically significant mean R-MBA score improvement post-TSHA-102 administration compared to natural history data at both six- and 12-months post-treatment. The improvement in R-MBA score at six- and 12-months post-treatment is depicted in the graphs below:
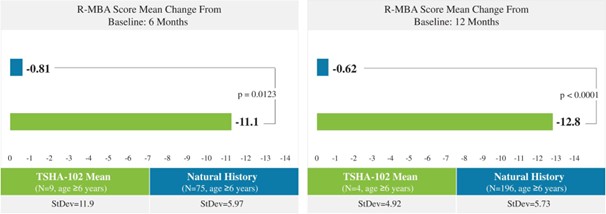
TSHA-102 also demonstrated early global improvement, with dose-dependent effects deepening over time, in CGI-I. The high dose cohort consistently outperformed the low dose cohort in CGI-I at the comparable time points of three months, six months and nine months post-treatment. The CGI-I score improvement for both cohorts are summarized in the below table:
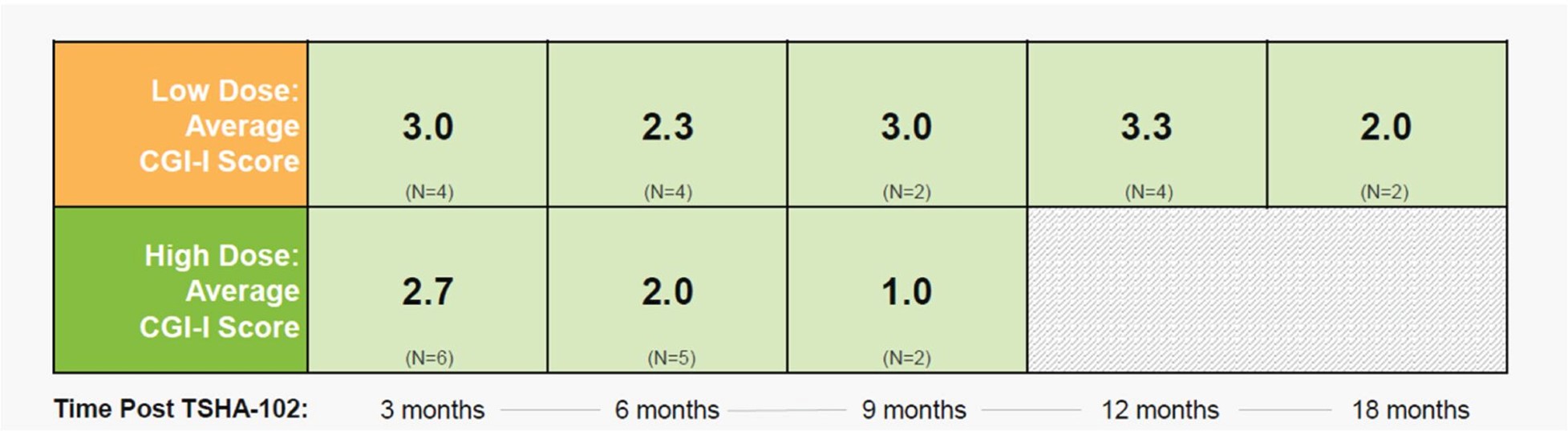
The high dose cohort continually outperformed the low dose cohort across key outcome measures six months post-treatment, with dose-dependent effects deepening over time at nine months or greater post-treatment. A summary of the dose-dependent effects is depicted in the chart below:
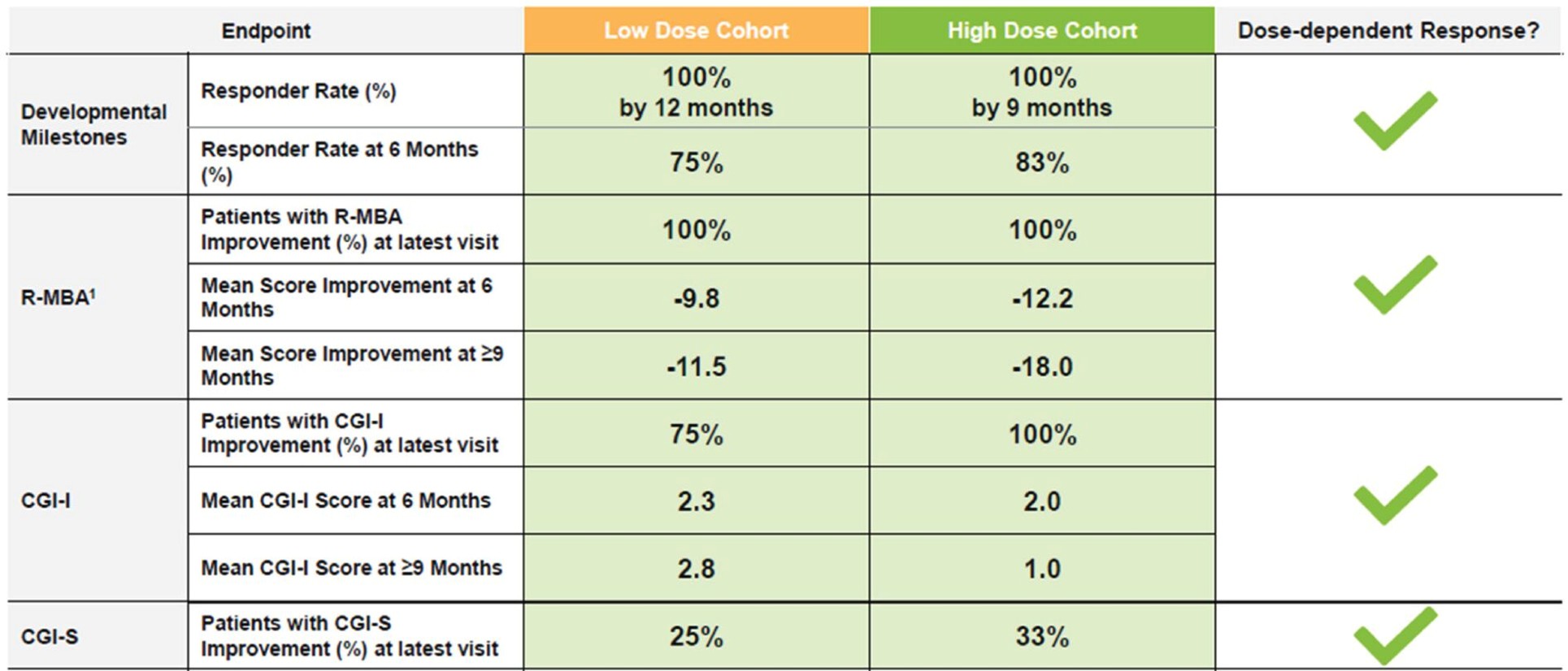
|
R-MBA mean score change post-TSHA-102 is relative to baseline; lower score=improvement from baseline |
In both the high dose and low dose cohorts of the TSHA-102 REVEAL clinical trials, TSHA-102 was generally well tolerated with no treatment-related serious adverse events, or SAEs, or dose limiting toxicities, or DLTs, as of the October 2025 data cutoff for 12 patients aged 6-21 years (high dose, N=8; low dose, N=4).
Detailed safety data from the May 20, 2025 data cutoff for 12 patients aged 6-21 years (high dose, N=8; low dose, N=4) are detailed below. The treatment-emergent adverse events, or AEs, related to TSHA-102 were mild to moderate in severity. In addition, seizures have generally been well controlled following TSHA-102 administration. The number of adverse events across 12 pediatric, adolescent and adult patients is summarized in the table below:
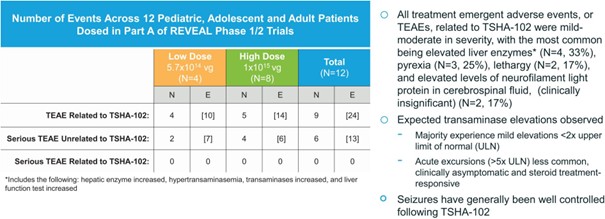
License Agreements
Research, Collaboration and License Agreement with The University of Texas Southwestern Medical Center
In November 2019, we entered into a research, collaboration and license agreement, or the UT Southwestern Agreement, with The Board of Regents of the University of Texas System on behalf of The University of Texas Southwestern Medical Center, or UT Southwestern, as amended in April 2020.
In connection with the UT Southwestern Agreement, we obtained an exclusive, worldwide, royalty-free license under certain patent rights of UT Southwestern and a non-exclusive, worldwide, royalty-free license under certain know-how of UT Southwestern, in each case to make, have made, use, sell, offer for sale and import licensed products for use in certain specified indications. Additionally, we obtained a non-exclusive, worldwide, royalty-free license under certain patents and know-how of UT Southwestern for use in all human uses, with a right of first refusal to obtain an exclusive license under certain of such patent rights and an option to negotiate an exclusive license under other of such patent rights. We are required to use commercially reasonable efforts to develop, obtain regulatory approval for, and commercialize at least one licensed product.
On April 2, 2020, we amended the UT Southwestern Agreement to include the addition of another licensed product and certain indications, and a right of first refusal to us over certain patient dosing patents. No additional consideration was transferred in connection with this amendment. In March 2022, we and UT Southwestern mutually agreed to revise the payment schedules and current performance expectations of the current sponsored research agreements under the UT Southwestern Agreement and defer payments by fifteen months. In December 2023, we and UT Southwestern mutually agreed to terminate specific sponsored research agreements. There are no outstanding payments due for these terminated programs as of September 30, 2025.
In connection with the UT Southwestern Agreement, we issued to UT Southwestern 2,179,000 shares of our common stock. We do not have any future milestone or royalty obligations to UT Southwestern under the UT Southwestern Agreement, other than costs related to the maintenance of patents.
The UT Southwestern Agreement expires on a country-by-country and licensed product-by-licensed product basis upon the expiration of the last valid claim of a licensed patent in such country for such licensed product. After the initial research term, we may terminate the agreement, on an indication-by-indication and licensed product-by-licensed product basis, at any time upon specified written notice to UT Southwestern. Either party may terminate the agreement upon an uncured material breach of the agreement or insolvency of the other party. In December 2023 and March 2025, we transferred rights to specific indications back to UT Southwestern.
License Agreement with Abeona (CLN1 Disease)
In August 2020, we entered into a license agreement, or the Abeona CLN1 Agreement, with Abeona Therapeutics Inc., or Abeona. In connection with the Abeona CLN1 Agreement, we obtained an exclusive, worldwide, royalty-bearing license, with the right to grant sublicenses under certain patents, know-how and materials originally developed by the University of North Carolina at Chapel Hill and Abeona to research, develop, manufacture, have manufactured, use, and commercialize licensed products for gene therapy for the prevention, treatment, or diagnosis of CLN1 Disease (one of the forms of Batten disease) in humans.
Subject to certain obligations of Abeona, we are obligated to use commercially reasonable efforts to develop at least one product and commercialize at least one product in the United States.
In connection with the license grant, we paid Abeona a one-time upfront license fee of $3.0 million during fiscal year 2020. We are obligated to pay Abeona up to $26.0 million in regulatory-related milestones and up to $30.0 million in sales-related milestones per licensed product and high single-digit royalties on net sales of licensed products. Royalties are payable on a licensed product-by-licensed product and country-by-country basis until the latest of the expiration or revocation or complete rejection of the last licensed patent covering such licensed product in the country where the licensed product is sold, the loss of market exclusivity in such country where the product is sold, or, if no licensed product exists in such country and no market exclusivity exists in such country, ten years from first commercial sale of such licensed product in such country. In addition, concurrent with the Abeona CLN1 Agreement, we entered into a purchase and reimbursement agreement with Abeona, pursuant to which we purchased specified inventory from Abeona and reimbursed Abeona for certain research and development costs previously incurred for total consideration of $4.0 million paid in fiscal year 2020.
In December 2021, our CTA filing for TSHA-118 for the treatment of CLN1 disease was approved by Health Canada and therefore triggered a $3.0 million regulatory milestone payment in connection with the Abeona CLN1 Agreement. No additional milestone payments were made or triggered during the nine months ended September 30, 2025.
The Abeona CLN1 Agreement expires on a country-by-country and licensed product-by-licensed product basis upon the expiration of the last royalty term of a licensed product in such country. Either party may terminate the agreement upon an uncured material breach of the agreement or insolvency of the other party. We may terminate the agreement for convenience upon specified prior written notice to Abeona.
License Agreement with Abeona (Rett Syndrome)
In October 2020, we entered into a license agreement, or the Abeona Rett Agreement, with Abeona pursuant to which we obtained an exclusive, worldwide, royalty-bearing license, with the right to grant sublicenses under certain patents, know-how and materials originally developed by the University of North Carolina at Chapel Hill, the University of Edinburgh and Abeona to research, develop, manufacture, have manufactured, use, and commercialize licensed products for gene therapy and the use of related transgenes for Rett syndrome.
Subject to certain obligations of Abeona, we are required to use commercially reasonable efforts to develop at least one licensed product and commercialize at least one licensed product in the United States.
In connection with the Abeona Rett Agreement, we paid Abeona a one-time upfront license fee of $3.0 million during fiscal year 2020. We are obligated to pay Abeona up to $26.5 million in regulatory-related milestones and up to $30.0 million in sales-related milestones per licensed product and high single-digit royalties on net sales of licensed products. Royalties are payable on a licensed product-by-licensed product and country-by-country basis until the latest of the expiration or revocation or complete rejection of the last licensed patent covering such licensed product in the country where the licensed product is sold, the loss of market exclusivity in such country where the product is sold, or, if no licensed product exists in such country and no market exclusivity exists in such country, ten years from first commercial sale of such licensed product in such country.
In March 2022, our CTA filing for TSHA-102 for the treatment of Rett Syndrome was approved by Health Canada and therefore triggered a regulatory milestone payment of $1.0 million in connection with the Rett Agreement. In May 2023, we dosed the first patient with TSHA-102 in the Phase 1/2 REVEAL trial evaluating the safety and preliminary efficacy of TSHA-102 in adult patients with Rett syndrome and therefore triggered a milestone payment of $3.5 million in connection with the Rett Agreement, which was paid in August 2023. No additional milestone payments were made during the nine months ended September 30, 2025.
The Abeona Rett Agreement expires on a country-by-country and licensed product-by-licensed product basis upon the expiration of the last royalty term of a licensed product in such country. Either party may terminate the agreement upon an uncured material breach of the agreement or insolvency of the other party. We may terminate the agreement for convenience upon specified prior written notice to Abeona.
Option Agreement with Astellas
On October 21, 2022, or the Effective Date, we entered into an Option Agreement, or the Option Agreement, with Astellas Gene Therapies, Inc. (f/k/a Audentes Therapeutics, Inc. (d/b/a Astellas Gene Therapy)), or Astellas.
TSHA-120 Giant Axonal Neuropathy
Under the Option Agreement, we granted to Astellas an exclusive option to obtain an exclusive, worldwide, royalty and milestone-bearing right and license (A) to research, develop, make, have made, use, sell, offer for sale, have sold, import, export and otherwise exploit, or, collectively, Exploit or the Exploitation, the product known, as of the Effective Date, as TSHA-120, or the 120 GAN Product, and any backup products with respect thereto for use in the treatment of GAN or any other gene therapy product for use in the treatment of GAN that is controlled by us or any of our affiliates or with respect to which we or any of our affiliates controls intellectual property rights covering the Exploitation thereof, or a GAN Product, and (B) under any intellectual property rights controlled by us or any of our affiliates with respect to such Exploitation, or the GAN Option. Subject to certain extensions, the GAN Option was exercisable from the Effective Date through a specified period of time following Astellas’ receipt of (i) the formal minutes from the Type B end-of-Phase 2 meeting between us and the FDA in response to our meeting request sent to the FDA on September 19, 2022 for the 120 GAN Product, (ii) all written feedback from the FDA with respect to the Type B end-of-Phase 2 Meeting, and (iii) all briefing documents sent by us to the FDA with respect to the Type B end-of-Phase 2 Meeting. Following the receipt of Type C meeting feedback from the FDA regarding a registrational path for TSHA-120 in September 2023, Astellas elected not to exercise the GAN Option, and we recognized revenue related to this expiration during the third quarter of 2023.
TSHA-102 Rett Syndrome
Under the Option Agreement, we also granted to Astellas an exclusive option to obtain an exclusive, worldwide, royalty and milestone-bearing right and license (A) to Exploit any Rett Product (as defined below), and (B) under any intellectual property rights controlled by us or any of our affiliates with respect to such Exploitation, or the Rett Option. Subject to certain extensions, the Rett Option was exercisable from the Effective Date through a specified period of time following Astellas’ receipt of (1) certain clinical data from the female pediatric trial and (2) certain specified data with respect to TSHA-102, such data package the “Rett Data Package” and such period the Rett Option Period, related to (i) the product known, as of the Effective Date, as TSHA-102 and any backup products with respect thereto for use in the treatment of Rett syndrome, and (ii) any other gene therapy product for use in the treatment of Rett syndrome that is controlled by us or any of our affiliates or with respect to which we or any of our affiliates controls intellectual property rights covering the Exploitation thereof, or a Rett Product. We delivered the Rett Data Package to Astellas in mid-2025. Under the Option Agreement, Astellas was required to decide whether to exercise the Rett Option within 90 days after Astellas’ receipt of the Rett Data Package. In October 2025, the Rett Option expired without being exercised. Following the expiration of the Option Agreement, we now hold unencumbered rights to the TSHA-102 program.
Components of Results of Operations
Revenue
Revenue for the nine months ended September 30, 2025 and 2024 was derived from the Astellas Transactions. We recognize revenue as research and development activities related to our Rett program are performed. Revenue related to the material rights associated with the Rett Option and must be recognized at a point in time when the option is exercised or the option period expires.
To date, we have not recognized any revenue from product sales, and we do not expect to generate any revenue from the sale of products, if approved, in the foreseeable future. If our development efforts for our product candidates are successful and result in regulatory approval, or license agreements with third parties, we may generate revenue in the future from product sales. However, there can be no assurance as to when we will generate such revenue, if at all.
Operating Expenses
Research and Development Expenses
Research and development expenses primarily consist of clinical and preclinical development of our product candidates and discovery efforts, including conducting preclinical studies, manufacturing development efforts, preparing for and conducting clinical trials and activities related to regulatory filings for our product candidates. Research and development expenses are recognized as incurred and payments made prior to the receipt of goods or services to be used in research and development are capitalized until the goods or services are received. Costs incurred in obtaining technology licenses through asset acquisitions are charged to research and development expense if the licensed technology has not reached technological feasibility and has no alternative future use.
Research and development expenses include or could include:
•
employee-related expenses, including salaries, bonuses, benefits, stock-based compensation, severance costs and other related costs for those employees involved in research and development efforts;
•
license maintenance fees and milestone fees incurred in connection with various license agreements;
•
external research and development expenses incurred under agreements with consultants, contract research organizations, or CROs, investigative sites and consultants to conduct our preclinical studies;
•
costs related to manufacturing material for our preclinical studies and clinical trials, including fees paid to CMOs;
•
laboratory supplies and research materials;
•
costs related to compliance with regulatory requirements; and
•
facilities, depreciation and other allocated expenses, which include direct and allocated expenses for rent, maintenance of facilities, insurance and equipment.
Research and development activities are central to our business model. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We have increased, and expect to continue to increase for the foreseeable future, our research and development spend with respect to the Rett clinical trials as we continue the development of TSHA-102 and manufacturing processes and conduct discovery and research activities for our preclinical programs. We cannot determine with certainty the timing of initiation, the duration or the completion costs of current or future preclinical studies and clinical trials of our product candidates due to the inherently unpredictable nature of preclinical and clinical development. Clinical and preclinical development timelines, the probability of success and development costs can differ materially from expectations. We anticipate that we will make determinations as to which product candidates to pursue and how much funding to direct to each product candidate on an ongoing basis in response to the results of ongoing and future preclinical studies and clinical trials, regulatory developments and our ongoing assessments as to each product candidate’s commercial potential. We will need to raise substantial additional capital in the future. Our clinical development costs are expected to increase significantly as we commence clinical trials. Our future expenses may vary significantly each period based on factors such as:
•
expenses incurred to conduct preclinical studies required to advance our product candidates into clinical development;
•
per patient trial costs, including based on the number of doses that patients received;
•
the number of patients who enroll in each trial;
•
the number of trials required for approval;
•
the number of sites included in the trials;
•
the countries in which the trials are conducted;
•
the length of time required to enroll eligible patients;
•
the drop-out or discontinuation rates of patients;
•
potential additional safety monitoring requested by regulatory agencies;
•
the duration of patient participation in the trials and follow-up;
•
the phase of development of the product candidate;
•
third-party contractors failing to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all;
•
the ability of our CMOs to manufacture our product candidates;
•
regulators or institutional review boards requiring that we or our investigators suspend or terminate clinical development for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks; and
•
the efficacy and safety profile of our product candidates.
General and Administrative Expenses
General and administrative expenses consist principally of salaries and related costs for personnel in executive and administrative functions, including stock-based compensation, severance costs, travel expenses and recruiting expenses. Other general and administrative expenses include professional fees for legal, consulting, accounting and audit and tax-related services and insurance costs.
We anticipate that our general and administrative expenses may increase in the future as a result of payments for accounting, audit, legal, consulting services, costs associated with maintaining compliance with Nasdaq listing rules and SEC requirements, director and officer liability insurance, investor and public relations activities and other expenses associated with operating as a public company to support planned future Rett program development.
Other Income (Expense)
Other income (expense) consists primarily of dividends earned from our money market fund and interest income on our cash and cash equivalents, interest expense on borrowings under the Trinity Term Loans (as defined below), and non-cash changes in the fair value of our outstanding warrant liability and the Trinity Term Loans.
Results of Operations
Results of Operations for the Three Months ended September 30, 2025 and 2024
The following table summarizes our results of operations for the three months ended September 30, 2025 and 2024 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended September 30, |
|
|
|
2025 |
|
|
2024 |
|
Revenue |
|
$ |
— |
|
|
$ |
1,788 |
|
Operating expenses: |
|
|
|
|
|
|
Research and development |
|
|
25,745 |
|
|
|
14,946 |
|
General and administrative |
|
|
8,279 |
|
|
|
7,902 |
|
Impairment of long-lived assets |
|
|
— |
|
|
|
4,838 |
|
Total operating expenses |
|
|
34,024 |
|
|
|
27,686 |
|
Loss from operations |
|
|
(34,024 |
) |
|
|
(25,898 |
) |
Other income (expense): |
|
|
|
|
|
|
Change in fair value of warrant liability |
|
|
(292 |
) |
|
|
75 |
|
Change in fair value of term loan |
|
|
(1,534 |
) |
|
|
(1,703 |
) |
Interest income |
|
|
3,169 |
|
|
|
2,107 |
|
Interest expense |
|
|
(15 |
) |
|
|
(24 |
) |
Other expense |
|
|
(37 |
) |
|
|
(81 |
) |
Total other income, net |
|
|
1,291 |
|
|
|
374 |
|
Net loss |
|
$ |
(32,733 |
) |
|
$ |
(25,524 |
) |
Revenue
Revenue related to the Astellas Transactions was zero for the three months ended September 30, 2025, compared to $1.8 million for the three months ended September 30, 2024. The revenue recorded for the three months ended September 30, 2024 was the result of Rett research and development activities performed during the period.
Research and Development Expenses
Research and development expenses were $25.7 million for the three months ended September 30, 2025, compared to $14.9 million for the three months ended September 30, 2024. The $10.8 million increase was primarily driven by BLA-enabling process performance qualification manufacturing initiatives, clinical trial activities from the Rett syndrome REVEAL adolescent/adult and pediatric trials and higher compensation expenses during the three months ended September 30, 2025.
General and Administrative Expenses
General and administrative expenses were $8.3 million for the three months ended September 30, 2025, compared to $7.9 million for the three months ended September 30, 2024. The increase of $0.4 million was primarily due to debt issuance costs incurred in connection with the 2025 Trinity Term Loans that are recorded in general and administrative expense under the fair value option and was partially offset by lower legal and professional fees.
Other Income (Expense)
Change in fair value of warrant liability
Change in fair value of warrant liability was a non-cash expense totaling $0.3 million for the three months ended September 30, 2025 related to the SSI Warrants (as defined below). Change in fair value of warrant liability was a non-cash gain totaling $0.1 million for the three months ended September 30, 2024 due to the increase in the fair value of the common stock underlying the SSI Warrants.
Change in fair value of term loan
We elected the fair value option for the Trinity Term Loans and changes to fair value, other than changes that were directly attributed to instrument-specific credit risk, were recorded as a component of other income (expense). The change in fair value was $1.5 million of expense for the three months ended September 30, 2025 compared to $1.7 million of expense for the three months ended September 30, 2024.
Interest Income
Interest income was $3.2 million for the three months ended September 30, 2025 compared to $2.1 million for the three months ended September 30, 2024. The increase in income was attributable to higher dividends earned from our money market fund and higher interest earned on our savings account due to the May 2025 financing proceeds.
Results of Operations for the Nine Months ended September 30, 2025 and 2024
The following table summarizes our results of operations for the nine months ended September 30, 2025 and 2024 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
For the Nine Months
Ended September 30, |
|
|
|
2025 |
|
|
2024 |
|
Revenue |
|
$ |
4,288 |
|
|
$ |
6,311 |
|
Operating expenses: |
|
|
|
|
|
|
Research and development |
|
|
61,451 |
|
|
|
50,676 |
|
General and administrative |
|
|
25,035 |
|
|
|
22,324 |
|
Impairment of long-lived assets |
|
|
— |
|
|
|
4,838 |
|
Total operating expenses |
|
|
86,486 |
|
|
|
77,838 |
|
Loss from operations |
|
|
(82,198 |
) |
|
|
(71,527 |
) |
Other income (expense): |
|
|
|
|
|
|
Change in fair value of warrant liability |
|
|
(463 |
) |
|
|
(67 |
) |
Change in fair value of term loan |
|
|
(4,525 |
) |
|
|
(4,035 |
) |
Interest income |
|
|
6,354 |
|
|
|
5,240 |
|
Interest expense |
|
|
(51 |
) |
|
|
(80 |
) |
Other expense |
|
|
(261 |
) |
|
|
(44 |
) |
Total other income, net |
|
|
1,054 |
|
|
|
1,014 |
|
Net loss |
|
$ |
(81,144 |
) |
|
$ |
(70,513 |
) |
Revenue
Revenue related to the Astellas Transactions was $4.3 million for the nine months ended September 30, 2025, compared to $6.3 million for the nine months ended September 30, 2024. The revenue recorded is the result of Rett research and development activities performed during the respective nine month periods ended September 30, 2025 and 2024.
Research and Development Expenses
Research and development expenses were $61.5 million for the nine months ended September 30, 2025, compared to $50.7 million for the nine months ended September 30, 2024. Compensation expenses increased during the nine months ended September 30, 2025 as a result of higher research and development headcount. Clinical trial expenses also increased during the nine months ended September 30, 2025 due to ongoing clinical trial efforts in the Rett REVEAL adolescent/adult and pediatric studies.
General and Administrative Expenses
General and administrative expenses were $25.0 million for the nine months ended September 30, 2025, compared to $22.3 million for the nine months ended September 30, 2024. The increase of $2.7 million was primarily due to debt issuance costs incurred in connection with the 2025 Trinity Term Loan that are recorded in general and administrative expense under the fair value option as well as higher compensation expenses and legal and professional fees.
Other Income (Expense)
Change in fair value of warrant liability
Change in fair value of warrant liability was a non-cash loss totaling $0.5 million for the nine months ended September 30, 2025 related to the SSI Warrants (as defined below). Change in fair value of warrant liability was a non-cash loss totaling $0.1 million for the nine months ended September 30, 2024.
Change in fair value of term loan
We elected the fair value option for the Trinity Term Loans and changes to fair value, other than changes that were directly attributed to instrument-specific credit risk, were recorded as a component of other income (expense). The change in fair value was $4.5 million of expense for the nine months ended September 30, 2025 compared to $4.0 million of expense for the nine months ended September 30, 2024.
Interest Income
Interest income was $6.4 million for the nine months ended September 30, 2025 compared to $5.2 million for the nine months ended September 30, 2024. The increase in income was attributable higher dividends earned from our money market fund and higher interest earned on our savings account due to the May 2025 financing proceeds.
Liquidity and Capital Resources
Overview
Since our inception, we have not generated any revenue from product sales and have incurred significant operating losses. As of September 30, 2025, we had cash and cash equivalents of $297.3 million. We have funded our operations primarily through equity financings, raising an aggregate of $911.0 million in gross proceeds from equity financings, including from pre-IPO private placements of convertible preferred stock, our IPO, and subsequent sales of common stock in public and private securities offerings, our term loans and the Astellas Transactions.
On August 7, 2025, or the Trinity Refinance Date, we entered into a Loan and Security Agreement, or the 2025 Trinity Term Loan Agreement, by and among us, the lenders party thereto from time to time, or the New Trinity Lenders, and Trinity Capital Inc., as administrative agent and collateral agent for the New Trinity Lenders, or Trinity. The 2025 Trinity Term Loan Agreement provides for (i) on the Trinity Refinance Date, $50.0 million aggregate principal amount of term loans, or Tranche A (ii) from the Trinity Refinance Date until March 31, 2028 an additional $25.0 million term loan facility contingent on delivering evidence satisfactory to Trinity that we have submitted a biologics license application, or BLA, acceptance to the FDA for TSHA-102 in Rett Syndrome, or Tranche B, (iii) from the Trinity Refinance Date until March 31, 2029, an additional $25.0 million term loan facility contingent on delivering evidence satisfactory to Trinity that we have received BLA approval from the FDA for TSHA-102 in Rett Syndrome (collectively, the “2025 Trinity Term Loans” and, together with the 2023 Trinity Term Loans, the “Trinity Term Loans”). We drew $50.0 million in term loans on the Trinity Refinance Date. The existing 2023 Trinity Term Loan Agreement was terminated and the existing 2023 Trinity Term Loans were repaid concurrently with entry into the 2025 Trinity Term Loan Agreement and the draw of Tranche A. The interest rate applicable to the 2025 Trinity Term Loans is the greater of (a) the WSJ Prime Rate plus 4.00% or (b) 11.50% per annum. The 2025 Trinity Term Loans are interest only from the Trinity Refinance Date through 48 months from the Trinity Refinance Date, which may be extended to 60 months from the Trinity Refinance Date upon the satisfaction of certain milestones set forth in the 2025 Trinity Term Loan Agreement, after which we are required to pay equal monthly installments of principal through August 1, 2030, or the New Maturity Date.
The 2025 Trinity Term Loans may be prepaid in full (i) from the Trinity Refinance Date through August 7, 2026, with payment of a 3.00% prepayment premium, (ii) from August 8, 2026 through August 7, 2027, with payment of a 2.00% prepayment premium, and (iii) from August 8, 2027 through, but excluding, the New Maturity Date, with payment of a 1.00% prepayment premium. Upon repayment in full of the 2025 Trinity Term Loans, we will pay to Trinity an end of term payment equal to 5.00% of the original principal amount of the 2025 Trinity Term Loans.
In connection with the 2025 Trinity Term Loan Agreement, we entered into a Success Fee Agreement with Trinity which specifies the terms regarding a fee in the amount of $0.5 million plus 5% of the principal amount of the funded 2025 Trinity Term Loans under Tranche B, or the 2025 Success Fee. The 2025 Success Fee is payable upon the achievement of certain corporate development value-inflection milestones. The 2025 Success Fee survives the termination of the 2025 Trinity Term Loans and expires on the earlier of ten years from the Trinity Refinance Date, or payment in full in cash of the 2025 Success Fee.
The obligations under the 2025 Trinity Term Loan Agreement are secured by a perfected security interest in all of our assets except for certain customarily excluded property pursuant to the terms of the 2025 Trinity Term Loan Agreement. There are no financial covenants and no warrants associated with the 2025 Trinity Term Loan Agreement. The 2025 Trinity Term Loan Agreement contains various covenants that limit our ability to engage in specified types of transactions without the consent of Trinity and the New Trinity Lenders which include, among others, incurring or assuming certain debt; merging, consolidating or acquiring all or substantially all of the capital stock or property of another entity; changing the nature of our business; changing our organizational structure or type; licensing, transferring or disposing of certain assets; granting certain types of liens on our assets; making certain investments; and paying cash dividends.
On October 5, 2021, we filed a shelf registration statement on Form S-3 with the SEC in relation to the registration of common stock, preferred stock, debt securities, warrants and units or any combination thereof up to a total aggregate offering price of $350.0 million. We also simultaneously entered into a Sales Agreement, or the Sales Agreement, with SVB Leerink LLC and Wells Fargo Securities, LLC, or the Sales Agents, pursuant to which we may issue and sell, from time to time at our discretion, shares of our common stock having an aggregate offering price of up to $150.0 million through the Sales Agents. In March 2022, we amended the Sales Agreement to, among other things, include Goldman Sachs & Co. LLC as an additional Sales Agent. In April 2022, we sold 2,000,000 shares of common stock pursuant to the Sales Agreement and received net proceeds of $11.6 million. No other shares of common stock have been issued and sold pursuant to the Sales Agreement as of June 30, 2025. On December 13, 2024, we filed a new shelf registration statement on Form S-3 following the expiration of our prior registration statement, in relation to the registration of common stock, preferred stock, debt securities, warrants and units or any combination thereof up to a total aggregate offering price of $300.0 million, including up to $100.0 million shares of common stock that may be offered and sold pursuant to the Sales Agreement. On May 28, 2025, we notified the Sales Agents that we were suspending and terminating the prospectus, or the ATM Prospectus, related to up to $100.0 million of our common stock issuable pursuant to the Sales Agreement. We will not make any sales of our securities pursuant to the Sales Agreement unless and until a new prospectus, prospectus supplement or a new registration statement is filed. Other than the termination of the ATM Prospectus, the Sales Agreement remains in full force and effect.
On October 21, 2022, we entered into the Option Agreement with Astellas granting Astellas an exclusive option to obtain exclusive, worldwide, royalty and milestone-bearing rights and licenses related to TSHA-120 and TSHA-102. As partial consideration for the rights granted to Astellas under the Option Agreement, Astellas paid us a one-time payment in the amount of $20.0 million, or the Upfront Payment, in November 2022.
Also on October 21, 2022, we entered into a securities Purchase Agreement with Astellas, or the Astellas Securities Purchase Agreement, and together with the Option Agreement, the Astellas Transactions, pursuant to which we agreed to issue and sell to Astellas in a private placement, or the Astellas Private Placement, an aggregate of 7,266,342 shares of our common stock, or the Astellas Private Placement Shares, for aggregate proceeds of approximately $30.0 million. The Astellas Private Placement closed on October 24, 2022. Pursuant to the Astellas Securities Purchase Agreement, in connection with the Astellas Private Placement, Astellas has the right to designate one individual to attend all meetings of the Board in a non-voting observer capacity. We also granted Astellas certain registration rights with respect to the Astellas Private Placement Shares.
On October 26, 2022, we entered into an Underwriting Agreement to issue and sell 14,000,000 shares of our common stock, par value $0.00001 per share, in an underwritten public offering pursuant to effective registration statement on Form S-3 and a related prospectus and prospectus supplement, or the Underwriting Agreement. The offering price to the public was $2.00 per share and the Underwriter purchased the shares from us pursuant to the Underwriting Agreement at a price of $1.88 per share. In addition, we granted the Underwriter an option to purchase, for a period of 30 days, up to an additional 2,100,000 shares of our common stock. The Follow-on Offering closed on October 31, 2022 and we received net proceeds of $26.0 million after deducting underwriting discounts, commissions and offering expenses. On November 10, 2022, the Underwriter exercised their option to purchase an additional 765,226 shares of our common stock and we received net proceeds of $1.4 million after deducting underwriting discounts and commissions.
In April 2023, we entered into a securities purchase agreement, or the SSI Securities Purchase Agreement, with two affiliates of SSI Strategy Holdings LLC, or SSI, named therein, or the SSI Investors, pursuant to which we agreed to issue and sell to the SSI Investors in a private placement, or the SSI Private Placement, 705,218 shares of our common stock, or the SSI Shares, and warrants, or the SSI Warrants, to purchase an aggregate of 525,000 shares of our common stock, or the Warrant Shares. SSI provides certain consulting services to us. Each SSI Warrant has an exercise price of $0.7090 per Warrant Share, which was the closing price of our common stock on the Nasdaq Global Market on April 4, 2023. The SSI Warrants issued in the SSI Private Placement provide that the holder of the SSI Warrants will not have the right to exercise any portion of its SSI Warrants until the achievement of certain clinical and regulatory milestones related to our clinical programs.
The SSI Private Placement closed on April 5, 2023. Gross proceeds of the SSI Private Placement were $0.5 million.
On August 14, 2023, we entered into a securities purchase agreement, or the August 2023 Securities Purchase Agreement, with certain institutional and other accredited investors, or the Purchasers, pursuant to which we agreed to sell and issue to the Purchasers in a private placement transaction, or the August 2023 Private Placement, that closed on August 16, 2023: (i) 122,412,376 shares of our common stock and (ii) with respect to certain Purchasers, pre-funded warrants, or the 2023 Pre-Funded Warrants, to purchase 44,250,978 shares of common stock in lieu of shares of common stock. The closing of the August 2023 Private Placement, or the PIPE Closing, occurred on August 16, 2023. The total gross proceeds to us at the PIPE Closing were $150.0 million, and after deducting placement agent commissions and offering expenses payable by us, net proceeds were $140.3 million.
On June 26, 2024, we entered into an underwriting agreement, or the June 2024 Underwriting Agreement, with Jefferies LLC and Goldman Sachs & Co. LLC, as representatives of the several underwriters set forth therein, or, collectively, the Underwriters, to issue and sell 14,361,113 shares of our common stock and pre-funded warrants to purchase 18,972,221 shares of our common stock, or the June 2024 Pre-Funded Warrants, pursuant to an effective shelf registration statement on Form S-3 and a related prospectus and prospectus supplement, or the June 2024 Offering. The offering price to the public was $2.25 per share of common stock and $2.249 per June 2024 Pre-Funded Warrant, which is the price to the public of each share of common stock sold in the June 2024 Offering, minus the $0.001 exercise price per June 2024 Pre-Funded Warrant. The Underwriters purchased the shares and the June 2024 Pre-Funded Warrants from us pursuant to the June 2024 Underwriting Agreement at a price of $2.115 per share and $2.114 per pre-funded warrant, respectively. The initial closing of the June 2024 Offering occurred on June 27, 2024 and we received net proceeds of $70.0 million, after deducting underwriting discounts and commissions and offering expenses. In addition, we granted the Underwriters an option to purchase, for a period of 30 days, up to an additional 5,000,000 shares of our common stock. On July 9, 2024, the Underwriters exercised their option to purchase an additional 3,235,000 shares of common stock and we received additional net proceeds of $6.7 million, after deducting underwriting discounts and commissions and offering expenses. The total net proceeds received from the June 2024 Offering were $76.7 million after deducting underwriting discounts, commissions and other offering expenses payable by us.
On May 28, 2025, we entered into an underwriting agreement, or the May 2025 Underwriting Agreement, with Jefferies LLC, BofA Securities, Inc., Piper Sandler & Co. and Barclays Capital Inc., as representatives of the several underwriters set forth therein, or, collectively, the Underwriters, to issue and sell 46,868,687 shares of our common stock and pre-funded warrants to purchase 25,858,586 shares of our common stock, or the May 2025 Pre-Funded Warrants, pursuant to an effective shelf registration statement on Form S-3 and a related prospectus and prospectus supplement, or the May 2025 Offering. The offering price to the public was $2.75 per share of common stock and $2.749 per May 2025 Pre-Funded Warrant, which is the price to the public of each share of common stock sold in the May 2025 Offering, minus the $0.001 exercise price per May 2025 Pre-Funded Warrant. The Underwriters purchased the shares and the May 2025 Pre-Funded Warrants from us pursuant to the May 2025 Underwriting Agreement at a price of $2.585 per share and $2.584 per pre-funded warrant, respectively. The initial closing of the May 2025 Offering occurred on May 30, 2025. In addition, we granted the Underwriters an option to purchase, for a period of 30 days, up to an additional 10,909,090 shares of our common stock, which the Underwriters exercised in full on June 6, 2025. The total net proceeds received from the May 2025 Offering were $215.6 million after deducting underwriting discounts, commissions and other offering expenses payable by us.
Funding Requirements
To date, we have not generated any revenues from the commercial sale of approved drug products, and we do not expect to generate substantial revenue for at least the next few years. If we fail to complete the development of our product candidates in a timely manner or fail to obtain their regulatory approval, our ability to generate future revenue will be compromised. We do not know when, or if, we will generate any revenue from our product candidates, and we do not expect to generate significant revenue unless and until we obtain regulatory approval of, and commercialize, our product candidates. We have increased and expect to continue to increase our research and development and general and administrative expenses, particularly with respect to the Rett clinical trials, for the foreseeable future as we continue the development of our product candidates and manufacturing processes and conduct discovery and research activities for our preclinical programs. If we obtain approval for any of our product candidates, we expect to incur significant commercialization expenses related to sales, marketing, manufacturing and distribution. We anticipate that we will need substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we could be forced to delay, reduce or eliminate our research and development programs or future commercialization efforts.
As of September 30, 2025, our material cash requirements consisted of $26.0 million in total lease payments under our noncancelable leases for equipment, laboratory space and office space. These leases are described in further detail in Note 5 to our unaudited condensed consolidated financial statements located in “Part I – Financial Information, Item 1. Financial Statements” in this Quarterly Report on Form 10-Q.
Our most significant purchase commitments consist of approximately $25.2 million in cancellable purchase obligations to our CROs and other clinical trial vendors.
We believe that our existing cash and cash equivalents will enable us to fund our operating expenses and capital requirements into 2028. We will require additional capital to fund the research and development of our product candidates, to fund our manufacturing activities, to fund precommercial activities of our programs and for working capital and general corporate purposes. The assessment of our ability to meet our future obligations is inherently judgmental, subjective and susceptible to change.
Because of the numerous risks and uncertainties associated with research, development and commercialization of biological products, we are unable to estimate the exact amount of our operating capital requirements. Our future funding requirements will depend on many factors, including, but not limited to:
•
the scope, progress, costs and results of discovery, preclinical development, laboratory testing and clinical trials for TSHA-102 and any current and future product candidates that we advance;
•
our ability to access sufficient additional capital on a timely basis and on favorable terms;
•
the extent to which we develop, in-license or acquire other product candidates and technologies in our gene therapy product candidate pipeline;
•
the costs and timing of process development and manufacturing scale-up activities associated with our product candidates and other programs as we advance them through preclinical and clinical development;
•
the number and development requirements of product candidates that we may pursue;
•
the costs, timing and outcome of regulatory review of our product candidates;
•
our headcount growth and associated costs as we expand our research and development capabilities and establish a commercial infrastructure;
•
the costs and timing of future commercialization activities, including product manufacturing, marketing, sales, and distribution, for any of our product candidates for which we receive marketing approval;
•
the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending any intellectual property-related claims;
•
the costs incurred in defending ourselves in any legal proceedings that we may be subject to;
•
the revenue, if any, received from commercial sales of our product candidates for which we receive marketing approval; and
•
the costs of operating as a public company.
Identifying potential product candidates and conducting preclinical studies and clinical trials is a time-consuming, expensive and uncertain process that takes many years to complete, and we may never generate the necessary data or results required to obtain marketing approval and achieve product sales. In addition, our product candidates, if approved, may not achieve commercial success. Our commercial revenues, if any, will be derived from sales of product candidates that we do not expect to be commercially available in the near term, if at all. Accordingly, we will need to continue to rely on additional financing to achieve our business objectives. Adequate additional financing may not be available to us on acceptable terms, or at all. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the terms of these equity securities or this debt may restrict our ability to operate. The 2025 Trinity Term Loan Agreement contains negative covenants, including, among other things, restrictions on indebtedness, liens investments, mergers, dispositions, prepayment of other indebtedness and dividends and other distributions. Any future additional debt financing and equity financing, if available, may involve agreements that include covenants limiting and restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures, entering into profit-sharing or other arrangements or declaring dividends. If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may be required to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us.
Cash Flows
The following table shows a summary of our cash flows for the nine months ended September 30, 2025 and 2024 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
For the Nine Months Ended September 30, |
|
|
|
2025 |
|
|
2024 |
|
Net cash used in operating activities |
|
$ |
(66,368 |
) |
|
$ |
(62,917 |
) |
Net cash used in investing activities |
|
|
(357 |
) |
|
|
(376 |
) |
Net cash provided by financing activities |
|
|
225,033 |
|
|
|
77,041 |
|
Net change in cash, cash equivalents and restricted cash |
|
$ |
158,308 |
|
|
$ |
13,748 |
|
Operating Activities
For the nine months ended September 30, 2025, our net cash used in operating activities of $66.4 million primarily consisted of a net loss of $81.1 million, primarily attributable to our spending on research and development expenses. The net loss of $81.1 million was partially offset by adjustments for non-cash items, primarily stock-based compensation expense of $9.8 million and other non-cash items of $3.3 million, net. Additional cash used in operating assets and liabilities of $1.7 million was primarily attributable to decreases in deferred revenue and partially offset by increases in accrued expenses and accounts payable.
For the nine months ended September 30, 2024, our net cash used in operating activities of $62.9 million primarily consisted of a net loss of $70.5 million, primarily attributable to our spending on research and development expenses. The net loss of $70.5 million was partially offset by adjustments for non-cash items, primarily stock-based compensation expense of $9.9 million, an impairment charge of $4.8 million related to the North Carolina manufacturing facility and other non-cash items of $2.8 million, net. Additional cash used in operating assets and liabilities of $9.9 million was primarily attributable to a decrease in deferred revenue of $6.3 million.
Investing Activities
During the nine months ended September 30, 2025, investing activities used $0.4 million of cash primarily attributable to the purchase of lab equipment and computer equipment.
During the nine months ended September 30, 2024, investing activities used $0.4 million of cash primarily attributable to the purchase of lab equipment.
Financing Activities
During the nine months ended September 30, 2025, financing activities provided $225.0 million of cash, which is primarily attributable to the closing of the May 2025 Offering and net proceeds from the 2025 Trinity Term Loans.
During the nine months ended September 30, 2024, financing activities provided $77.0 million of cash, which is primarily attributable to the closing of the June 2024 Offering.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
Critical Accounting Policies and Significant Judgments and Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the unaudited condensed consolidated financial statements and the reported amounts of expenses during the reporting period. A description of our significant accounting policies is included in our Annual Report. Please read the unaudited condensed consolidated financial statements in conjunction with our audited financial statements and accompanying notes in our Annual Report.
Our critical accounting policies that require significant judgments and estimates are more fully described under the heading “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Significant Judgments and Estimates” in our Annual Report and in Note 2 to our audited consolidated financial statements contained in our Annual Report.
There have been no significant changes to our critical accounting policies that require significant judgments and estimates from those disclosed in our Annual Report.
Recent Accounting Pronouncements
See Note 2 to our unaudited condensed consolidated financial statements located in “Part I – Financial Information, Item 1. Financial Statements” in this Quarterly Report on Form 10-Q for a description of recent accounting pronouncements applicable to our condensed consolidated financial statements.
Emerging Growth Company and Smaller Reporting Company Status
In April 2012, the Jumpstart Our Business Startups Act of 2012, or JOBS Act, was enacted. Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, for complying with new or revised accounting standards. Thus, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We elected the extended transition period for complying with new or revised accounting standards, which delays the adoption of these accounting standards until they would apply to private companies.
In addition, as an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies. These provisions include:
•
an exception from compliance with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended;
•
reduced disclosure about our executive compensation arrangements in our periodic reports, proxy statements and registration statements;
•
exemptions from the requirements of holding non-binding advisory votes on executive compensation or golden parachute arrangements; and
•
an exemption from compliance with the requirements of the Public Company Accounting Oversight Board regarding the communication of critical audit matters in the auditor’s report on financial statements.
We may take advantage of these provisions until we no longer qualify as an emerging growth company. We will cease to qualify as an emerging growth company on the date that is the earliest of: (i) December 31, 2025, (ii) the last day of the fiscal year in which we have more than $1.235 billion in total annual gross revenues, (iii) the date on which we are deemed to be a “large accelerated filer” under the rules of the SEC, which means the market value of our common stock that is held by non-affiliates exceeds $700 million as of the prior June 30th, or (iv) the date on which we have issued more than $1.0 billion of non-convertible debt over the prior three-year period. We may choose to take advantage of some but not all of these reduced reporting burdens. We have taken advantage of certain reduced reporting requirements in this Quarterly Report on Form 10-Q and our other filings with the SEC. Accordingly, the information contained herein may be different than you might obtain from other public companies in which you hold equity interests.
We are also a “smaller reporting company,” meaning that the market value of our shares held by non-affiliates is less than $700 million and our annual revenue was less than $100 million during the most recently completed fiscal year. We may continue to be a smaller reporting company if either (i) the market value of our shares held by non-affiliates is less than $250 million or (ii) our annual revenue was less than $100 million during the most recently completed fiscal year and the market value of our shares held by non-affiliates is less than $700 million. If we are a smaller reporting company at the time we cease to be an emerging growth company, we may continue to rely on exemptions from certain disclosure requirements that are available to smaller reporting companies. Specifically, as a smaller reporting company, we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Report on Form 10-K and, similar to emerging growth companies, smaller reporting companies have reduced disclosure obligations regarding executive compensation.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide the information required under this Item.
Item 4. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act ), as of the end of the period covered by this Quarterly Report on Form 10-Q. Based on such evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that as of June 30, 2025, our disclosure controls and procedures were effective to provide reasonable assurance that the information required to be disclosed by us in this Quarterly Report on Form 10-Q was (a) reported within the time periods specified by SEC rules and regulations, and (b) communicated to our management, including our Chief Executive Officer and Chief Financial Officer, to allow timely decisions regarding any required disclosure.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting identified in management’s evaluation pursuant to Rules 13a-15(d) or 15d-15(d) of the Exchange Act during the period covered by this Quarterly Report on Form 10-Q that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on Effectiveness of Internal Controls
In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable, not absolute, assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply judgment in evaluating the benefits of possible controls and procedures relative to their costs. Our management, including our Chief Executive Officer and Chief Financial Officer, believes that our disclosure controls and procedures and internal control over financial reporting are designed to provide reasonable assurance of achieving their objectives and are effective at the reasonable assurance level. However, our management does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent all errors and all fraud.
PART II—OTHER INFORMATION
Item 1. Legal Proceedings.
In January 2024 and April 2024, the Company was named a nominal defendant in two putative stockholder derivative actions filed by stockholders of the Company in the Court of Chancery of the State of Delaware. The lawsuits have since been consolidated and a lead plaintiff has been appointed. In October 2024, the lead plaintiff filed an amended complaint asserting claims relating to the Company’s August 2023 Private Placement against (i) certain of the Company’s current and former directors and officers for breach of fiduciary duty and unjust enrichment; and (ii) certain participants in the Company’s August 2023 Private Placement for aiding and abetting breach of fiduciary duty and unjust enrichment. Amont other things, the complaints seek an unspecified award of damages in the Company’s favor, plus pre-judgment and post-judgment interest, and an award to the plaintiffs for the costs and disbursement of the action, including fees for their attorneys and experts. The board of directors of the Company has formed a special litigation committee to investigate the claims and allegations in the amended complaint. On January 27, 2025, the court entered an order staying the litigation until June 30, 2025, while the special litigation committee conducts its investigation. On April 30, 2025, the court extended the stay until September 30, 2025. On August 29, 2025, the court again extended the stay until December 2, 2025. The Company has not recorded a liability related to these lawsuits because, at this time, the Company is unable to reasonably estimate possible losses or gains or determine whether an unfavorable outcome is either probable or remote.
In connection with an investigation captioned In the Matter of Taysha Gene Therapies, Inc. (D-04192), Taysha and certain of its officers and directors received subpoenas in late 2024 from the United States Securities and Exchange Commission, or the SEC, for materials relating to Taysha’s August 2023 PIPE and certain public offerings. Production of materials in response to the subpoenas was completed in April 2025. The SEC investigation is neither a determination that the Company or any individuals have violated any law nor a charge of any wrongdoing.
From time to time, we may be involved in additional legal or regulatory proceedings. Regardless of outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
Item 1A. Risk Factors.
Our business is subject to risks and events that, if they occur, could adversely affect our financial condition and results of operations and the trading price of our securities. In addition to the other information set forth in this Quarterly Report on Form 10-Q, you should carefully consider the factors described in Part I, Item 1A. “Risk Factors” of our Annual Report for the fiscal year ended December 31, 2024, filed with the Securities and Exchange Commission on February 26, 2025.
Risks Related to the Development of our Product Candidates
Interim “top-line” and preliminary results from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we may publish interim top-line or preliminary results from our clinical trials. Interim results from clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available. Preliminary or top-line results also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published. As a result, interim and preliminary data should be viewed with caution until the final data are available. For example, in May 2025, we announced clinical data from adult/adolescent and pediatric patients treated in the Phase 1/2 REVEAL trials of TSHA-102. However, those observations may not endure as patients continue their follow-up visits as part of the trial. Initial clinical observations also may not translate into success on primary endpoints of the REVEAL trial. Differences between preliminary or interim data and final data could significantly harm our business prospects and may cause the trading price of our common stock to fluctuate significantly.
Further, others, including regulatory authorities, may not accept or agree with our assumptions, estimates, calculations, conclusions or analyses or may interpret or weigh the importance of data differently, which could impact the approvability or commercialization of the particular product candidate or product and our business in general. In addition, the information we choose to publicly disclose regarding a particular study or clinical trial is based on what is typically extensive information, and you or others may not agree with what we determine is the material or otherwise appropriate information to include in our disclosure, and any information we determine not to disclose may ultimately be deemed significant with respect to future decisions, conclusions, views, activities or otherwise regarding a particular drug, product candidate or our business. If the top-line data that we report differ from actual results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for and commercialize any of our product candidates, our business, operating results, prospects or financial condition may be harmed.
We are substantially dependent on the success of our lead product candidate, TSHA-102. There is no guarantee that we will be able to successfully develop and commercialize TSHA-102. If we are unable to complete development of, obtain regulatory approval for and commercialize TSHA-102 in one or more indications and in a timely manner, our business, financial condition, results of operations and prospects will be significantly harmed.
We are substantially dependent on the success of our lead product candidate, TSHA-102, which is currently our sole product candidate in clinical development. We are still developing TSHA-102 and it cannot be marketed or sold in the United States or in foreign markets until regulatory approval has been obtained from the FDA or applicable foreign regulatory agencies. The process of obtaining regulatory approval is expensive and time consuming. The FDA and foreign regulatory authorities may never approve TSHA-102 for sale and marketing, and even if TSHA-102 is ultimately approved, regulatory approval may be delayed or limited in the United States or in other jurisdictions. Even if we are authorized to sell and market TSHA-102 in one or more markets, there is no assurance that we will be able to successfully market TSHA-102 or that TSHA-102 will achieve market acceptance sufficient to generate profits. If we are unable to successfully develop and commercialize TSHA-102 due to failure to obtain regulatory approval for TSHA-102, to successfully market TSHA-102, to generate profits from the sale of TSHA-102, or due to other risk factors outlined in this report, it would have material adverse effects on our business, financial condition, and results of operations.
We have not yet completed testing of any product candidate in clinical trials. Success in preclinical studies or earlier clinical trials, including our Phase 1/2 REVEAL trials of TSHA-102, may not be indicative of results in future clinical trials, or provide support for the submission of a marketing application.
Success in preclinical testing and early clinical trials does not ensure that later clinical trials will generate the same results or otherwise provide adequate data to demonstrate the efficacy and safety of a product candidate. Preclinical tests and Phase 1 and Phase 2 clinical trials are primarily designed to test safety, to study pharmacokinetics and pharmacodynamics and to understand the side effects of product candidates at various doses and schedules. Success in preclinical or animal studies and early clinical trials does not ensure that later large-scale efficacy trials will be successful nor does it predict final results. For example, we may be unable to identify suitable animal disease models for our product candidates, which could delay or frustrate our ability to proceed into clinical trials or obtain marketing approval. Our product candidates, including TSHA-102, may fail to show the desired safety and efficacy in clinical development despite positive results in preclinical studies or having successfully advanced through initial clinical trials. Any such outcome would cause us to cease development of TSHA-102, which is currently our only product candidate in clinical development. Our Phase 1/2 clinical trials of TSHA-102 involve small patient populations and duration of treatment has been relatively short. Because of the small sample sizes, the results of these trials may not be indicative of results of future clinical trials. Further, although other gene therapy clinical trials conducted by others also utilized AAV9 vectors, these trials should not be relied upon as evidence that our planned clinical trials will succeed.
Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials even after achieving promising results in preclinical testing and earlier-stage clinical trials. Data obtained from preclinical and clinical activities are subject to varying interpretations, which may delay, limit or prevent regulatory approval. In addition, we may experience regulatory delays or rejections as a result of many factors, including changes in regulatory policy during the period of our product candidate development. Any such delays could negatively impact our business, financial condition, results of operations and prospects.
As an organization, we have never conducted pivotal clinical trials, and may be unable to do so for any product candidates we may develop, including TSHA-102.
We will need to successfully complete our ongoing and planned clinical trials, including pivotal clinical trials, in order to obtain FDA and comparable foreign regulatory approval to market our product candidates. Carrying out later-stage clinical trials and the submission of a successful biologics license application, or BLA, and comparable foreign application is a complicated process. As an organization, we have initiated Phase 1/2 clinical trials, have not previously conducted any later stage or pivotal clinical trials, but have limited experience in preparing, submitting and prosecuting regulatory filings and have not previously submitted a BLA or comparable foreign application for any product candidate.
In addition, we have had limited interactions with the FDA and comparable foreign regulatory authorities and cannot be certain how many additional clinical trials of TSHA-102 will be required or how such trials should be designed. Although we have received feedback from the FDA on the design of Part B of our clinical trial of TSHA-102, there is no assurance that our trial design will be sufficient for FDA approval. Consequently, we may be unable to successfully and efficiently execute and complete necessary clinical trials in a way that leads to BLA submission or comparable foreign application and approval of TSHA-102. We may require more time and incur greater costs than our competitors and may not succeed in obtaining regulatory approvals of TSHA-102. Failure to commence or complete, or delays in, our planned clinical trials, could prevent us from or delay us in commercializing TSHA-102.
Risks Related to Legal and Regulatory Compliance Matters
We are subject to legal proceedings and claims from time to time that may seek material damages or otherwise may have a material adverse effect on our business. The costs we incur in defending ourselves or associated with settling any of these proceedings, as well as a material final judgment or decree against us, could materially adversely affect our financial condition.
We are subject to legal proceedings and claims from time to time that may seek material damages or otherwise may have a material adverse effect on our business. For example, in January 2024 and April 2024, we were named a nominal defendant in stockholder derivative lawsuits asserting claims against certain of our current and former directors, among others, in the Court of Chancery of the State of Delaware. These lawsuits, which have since been consolidated, seek, among other relief, a declaration that certain defendants were unjustly enriched, unspecified monetary damages, disgorgement of certain alleged profits received by defendants, and reasonable costs and expenses, including attorneys’ fees, and other relief. We have not recorded a liability related to these lawsuits because, at this time, we are unable to reasonably estimate possible losses or gains or determine whether an unfavorable outcome is either probable or remote. Our board of directors has formed a special litigation committee to investigate the claims and allegations in the amended complaint, which is ongoing. In connection with an investigation captioned In the Matter of Taysha Gene Therapies, Inc. (D-04192), we and certain of our officers and directors received subpoenas in late 2024 from the SEC for materials relating to our August 2023 PIPE and certain public offerings. The SEC investigation is neither a determination that the Company or any individuals have violated any law nor a charge of any wrongdoing. See “Item 3-Legal Proceedings” and “Part II, Item 8, Note 13-Commitments and Contingencies” in the 2024 Form 10-K for more information and “Part I, Item 2, Note 13-Commitments and Contingencies” in our First Quarter 2025 Form 10-Q for more information about each of these matters.
Due to the inherent uncertainties in legal proceedings, we cannot accurately predict the duration, scope or ultimate outcome of any such proceedings. This or any future litigation, regardless of the merits of any such proceeding, could harm our reputation and result in substantial costs and diversion of management’s attention and resources, which could adversely impact our business. Although we have directors’ and officers’ liability insurance, it provides for a substantial retention of liability and is subject to limitations and may not cover a significant portion, or any, of the expenses or liabilities we may incur or be subject to in connection with these lawsuits or other litigation to which we are party. The costs we incur in defending ourselves or associated with settling such proceedings, as well as a material final judgment or decree against us, that are not covered by our directors’ and officers’ liability insurance could materially adversely affect our financial condition. In addition, additional lawsuits may be filed, the conclusion of which in a manner adverse to us and for which we incur substantial costs or damages not covered by our directors’ and officers’ liability insurance could have a material adverse effect on our financial condition and business.
Healthcare legislative or regulatory reform measures may have a negative impact on our business and results of operations.
In the United States and some foreign jurisdictions, there have been, and continue to be, several legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of product candidates, restrict or regulate post-approval activities, and affect our ability to profitably sell any product candidates for which we obtain marketing approval.
Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives. For example, The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively the ACA, substantially changed the way healthcare is financed by both the government and private insurers, and significantly impacts the U.S. pharmaceutical industry.
There have been judicial, congressional, and executive branch challenges to certain aspects of the ACA, including efforts to repeal or replace certain aspects of the ACA. For example, on August 16, 2022, the Inflation Reduction Act of 2022, or the IRA, was signed into law, which among other things, extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025. The IRA also eliminates the “donut hole” under the Medicare Part D program beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost and creating a new manufacturer discount program. It is possible that the ACA will be subject to judicial or Congressional challenges in the future. It is also unclear how any additional healthcare reform measures of the second Trump administration will impact the ACA and our business.
Other legislative changes have been proposed and adopted since the ACA was enacted. For example, on July 4, 2025, the annual reconciliation bill, the "One Big Beautiful Bill Act", or the OBBBA, was signed into law which, is expected to reduce Medicaid spending and enrollment by implementing work requirements for some beneficiaries, capping state-directed payments, reducing federal funding, and limiting provider taxes used to fund the program. OBBBA also narrows access to ACA marketplace exchange enrollment and declines to extend the ACA enhanced advanced premium tax credits, set to expire at the end of 2025, which, among other provisions in the law, are anticipated to reduce the number of Americans with health insurance.
Additional changes include aggregate reductions to Medicare payments to providers of 2% per fiscal year pursuant to the Budget Control Act of 2011, which began in 2013, and due to subsequent legislative amendments to the statute, will remain in effect until 2032, unless additional congressional action is taken. These laws may result in additional reductions in Medicare and other healthcare funding, which could have an adverse effect on customers for our product candidates, if approved, and, accordingly, our financial operations. Additionally, on March 11, 2021, the American Rescue Plan Act of 2021 was signed into law, which eliminates the statutory Medicaid drug rebate cap, currently set at 100% of a drug’s average manufacturer price, for single source and innovator multiple source drugs, effective January 1, 2024.
Additionally, there has been heightened governmental scrutiny in the United States of pharmaceutical pricing practices in light of the rising cost of prescription drugs and biologics. Such scrutiny has resulted in several recent Presidential executive orders, congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for products. At the federal level, the IRA, among other things, (i) directs Health and Human Services, or HHS, to negotiate the price of certain high-expenditure, single-source biologics that have been on the market for at least 11 years covered under Medicare, or the Medicare Drug Price Negotiation Program, and subject drug manufacturers to civil monetary penalties and a potential excise tax by offering a price that is not equal to or less than the negotiated “maximum fair price” for such drugs and biologics under the law, and (ii) imposes rebates with respect to certain drugs and biologics covered under Medicare Part B or Medicare Part D to penalize price increases that outpace inflation. The IRA permits HHS to implement many of these provisions through guidance, as opposed to regulation, for the initial years. These provisions began to take effect progressively starting in fiscal year 2023. On August 15, 2024, HHS announced the agreed-upon price of the first ten drugs that were subject to price negotiations, although the Medicare Drug Price Negotiation Program is currently subject to legal challenges. On January 17, 2025, HHS selected fifteen additional products covered under Part D for price negotiation in 2025. Each year thereafter more Part B and Part D products will become subject to the Medicare Drug Price Negotiation Program. Further, on December 7, 2023, an initiative to control the price of prescription drugs through the use of march-in rights under the Bayh-Dole Act was announced. On December 8, 2023, the National Institute of Standards and Technology published for comment a Draft Interagency Guidance Framework for Considering the Exercise of March-In Rights which for the first time includes the price of a product as one factor an agency can use when deciding to exercise march-in rights. While march-in rights have not previously been exercised, it is uncertain if that will continue under the new framework. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
We expect that these and other healthcare reform measures that may be adopted in the future may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved drug. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our drugs. The current Trump administration is pursuing policies to reduce regulations and expenditures across government including at HHS, the FDA, Centers for Medicare & Medicaid Services and related agencies. These actions, presently directed by executive orders or memoranda from the Office of Management and Budget, may propose policy changes that create additional uncertainty for our business. For example, on September 30, 2025, the current administration announced the first agreement with a major pharmaceutical company that requires the drug manufacturer to offer, through a direct to consumer platform, U.S. patients and Medicaid programs prescription drug Most-Favored Nation pricing equal to or lower than those paid in other developed nations, with additional mandates for direct-to-patient discounts and repatriation of foreign revenues. Other recent actions include, for example, (1) directives to reduce agency workforce and cut programs, (2) rescinding a Biden administration executive order tasking the Center for Medicare & Medicaid Innovation to consider new payment and healthcare models to limit drug spending, (3) eliminating the Biden administration’s executive order that directed HHS to establishing an AI task force and developing a strategic plan, (4) directing HHS to lower prescription drug costs for Medicare through a variety of initiatives, including by improving upon the Medicare Drug Price Negotiation Program and establishing Most-Favored-Nation pricing for pharmaceutical products, (5) imposing tariffs of imported pharmaceutical products, (6) directing certain federal agencies to enforce existing law regarding hospital and plan price transparency and by standardizing prices across hospitals and health plans and (7) as part of the Make America Healthy Again (MAHA) Commission’s recent Strategy Report, working across government agencies to increase enforcement on direct-to-consumer pharmaceutical advertising. These actions and policies may significantly reduce U.S. drug prices, potentially impacting manufacturers’ global pricing strategies and profitability, while increasing their operational costs and compliance risks. Additionally, in its June 2024 decision in Loper Bright Enterprises v. Raimondo, the U.S. Supreme Court overturned the longstanding Chevron doctrine, under which courts were required to give deference to regulatory agencies’ reasonable interpretations of ambiguous federal statutes. The Loper Bright decision could result in additional legal challenges to current regulations and guidance issued by federal agencies applicable to our operations, including those issued by the FDA.
In addition, FDA and comparable foreign regulatory regulations and guidance may be revised or reinterpreted by the FDA or the comparable foreign regulatory in ways that may significantly affect our business and our products. Any new regulations or guidance, or revisions or reinterpretations of existing regulations or guidance, may impose additional costs or lengthen FDA review times for TSHA-102 or any future product candidates. We cannot determine how changes in regulations, statutes, policies, or interpretations when and if issued, enacted or adopted, may affect our business in the future. Such changes could, among other things, require:
• additional clinical trials to be conducted prior to obtaining approval;
• changes to manufacturing methods;
• recalls, replacements, or discontinuance of one or more of our products; and
• additional recordkeeping.
For instance, the regulatory landscape related to clinical trials in the European Union recently evolved. The EU Clinical Trials Regulation, or CTR, which was adopted in April 2014 and repeals the EU Clinical Trials Directive, became applicable on January 31, 2022. The CTR allows sponsors to make a single submission to both the competent authority and an ethics committee in each EU Member State, leading to a single decision for each EU Member State. The assessment procedure for the authorization of clinical trials has been harmonized as well, including a joint assessment by all EU Member States concerned, and a separate assessment by each EU Member State with respect to specific requirements related to its own territory, including ethics rules. Each EU Member State’s decision is communicated to the sponsor via the centralized EU portal. Once the clinical trial is approved, clinical study development may proceed. The CTR foresees a three-year transition period. The extent to which ongoing and new clinical trials will be governed by the CTR varies. For clinical trials in relation to which application for approval was made on the basis of the Clinical Trials Directive before January 31, 2023, the Clinical Trials Directive continued to apply on a transitional basis until January 31, 2025, by which date all ongoing trials under the Clinical Trials Directive became subject to the provisions of the CTR and all new trials since January 31, 2023 have been subject to the CTR. Compliance with the CTR requirements by us and our third-party service providers, such as CROs, may impact our developments plans.
In addition, on April 26, 2023, the European Commission adopted a proposal for a new Directive and Regulation to revise the existing pharmaceutical legislation. The proposed revisions remain to be agreed and adopted by the European Council. Moreover, on December 1, 2024, a new European Commission took office. The proposal could, therefore, still be subject to revisions. If adopted in the form proposed, the recent European Commission proposals to revise the existing EU laws governing authorization of medicinal products may result in a decrease in data and market exclusivity opportunities for our product candidates in the European Union and make them open to generic or biosimilar competition earlier than is currently the case with a related reduction in reimbursement status.
If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, our development plans may be impacted.
Such changes would likely require substantial time and impose significant costs, or could reduce the potential commercial value of TSHA-102 or other product candidates, and could materially harm our business and our financial results. In addition, delays in receipt of or failure to receive regulatory clearances or approvals for any other products would harm our business, financial condition, and results of operations.
Disruptions at the FDA, the SEC and other government agencies and regulatory authorities caused by funding shortages or global health concerns could hinder their ability to hire and retain key leadership and other personnel, prevent new products and services from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of our business may rely, which could negatively impact our business.
The ability of the FDA and comparable foreign regulatory authorities to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of the U.S. Securities and Exchange Commission, or SEC, and other government agencies on which our operations may rely, including those that fund research and development activities, is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies and comparable regulatory authorities may also slow the time necessary for new drugs or biologics to be reviewed and/or approved by necessary government agencies and regulatory authorities, which would adversely affect our business. For example, over the last several years, including most recently in October 2025, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical FDA, SEC and other government employees and stop critical activities.
If the current government shutdown continues or a prolonged government shutdown occurs, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business.
The ability of the FDA and other government agencies to properly administer their functions is highly dependent on the levels of government funding and the ability to fill key leadership appointments, among various factors. Delays in filling or replacing key positions could significantly impact the ability of the FDA and other agencies to fulfill their functions, and could greatly impact healthcare and the pharmaceutical industry. In addition, the current administration has implemented substantial reductions in force at various government agencies including the FDA, which could significantly reduce the FDA’s capacity to perform its functions in a manner consistent with its past practices and could delay reviews and negatively impact our business. There is increased uncertainty as to how the FDA and other regulatory agencies will regulate our products.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
(a) Recent Sales of Unregistered Equity Securities
None.
(b) Use of Proceeds
None.
(c) Issuer Purchases of Equity Securities
None.
Item 3. Defaults Upon Senior Securities.
Not applicable.
Item 4. Mine Safety Disclosures.
Not applicable.
Item 5. Other Information.
During the three months ended September 30, 2025, none of our directors or officers (as defined in Rule 16a-1(f) under the Exchange Act) adopted or terminated any “Rule 10b5-1 trading arrangement” or “non-Rule 10b5-1 trading arrangement,” as those terms are defined in Item 408 of Regulation S-K.
Item 6. Exhibits.
The exhibits listed on the Exhibit Index are either filed or furnished with this report or incorporated herein by reference.
|
|
|
|
Exhibit
Number
|
|
Description |
3.1 |
|
Amended and Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K (File No. 001-39536), filed with the Securities and Exchange Commission on September 29, 2020). |
3.2 |
|
Amended and Restated Bylaws (incorporated by reference to Exhibit 3.4 to the Company’s Current Report on Form 8-K (File No. 001-39536), filed with the Securities and Exchange Commission on September 29, 2020). |
3.3 |
|
Certificate of Amendment to the Amended and Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K (File No. 001-39536), filed with the Securities and Exchange Commission on November 15, 2023). |
3.4 |
|
Certificate of amendment to the Amended and Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K (File No. 001-39536), filed with the Securities and Exchange Commission on June 3, 2025). |
10.1*†+ |
|
Loan and Security Agreement, dated August 7, 2025, by and among the Company, the lenders from time to time party thereto as lenders, and Trinity Capital Inc. as administrative agent and collateral agent. |
31.1* |
|
Certification of Principal Executive Officer Pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
31.2* |
|
Certification of Principal Financial Officer Pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
32.1# |
|
Certification of Principal Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
32.2# |
|
Certification of Principal Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
101.INS |
|
XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document |
101.SCH |
|
Inline XBRL Taxonomy Extension Schema Document |
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document |
101.LAB |
|
Inline XBRL Taxonomy Extension Label Linkbase Document |
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
104 |
|
Cover Page Interactive Data File (formatted as inline XBRL and contained in Exhibit 101). |
* Filed herewith.
† Portions of this agreement (indicated by asterisks) have been omitted because the registrant has determined they are not material and would likely cause competitive harm to the registrant if publicly disclosed.
+ Certain schedules to this agreement have been omitted in accordance with Item 601(b)(2) of Regulation S-K. A copy of any omitted schedules will be furnished supplementally to the SEC upon request.
# These certifications are being furnished solely to accompany this quarterly report pursuant to 18 U.S.C. Section 1350, and are not being filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and are not to be incorporated by reference into any filing of the registrant, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
Taysha Gene Therapies, Inc. |
|
|
|
|
Date: November 4, 2025 |
|
By: |
/s/ Sean Nolan |
|
|
|
Sean Nolan |
|
|
|
Chief Executive Officer
(Principal Executive Officer)
|
|
|
|
|
Date: November 4, 2025 |
|
By: |
/s/ Kamran Alam |
|
|
|
Kamran Alam |
|
|
|
Chief Financial Officer
(Principal Financial and Accounting Officer)
|
EX-10.1
13
tsha-ex10_1.htm
EX-10.1
EX-10.1
Exhibit 10.1
CERTAIN INFORMATION HAS BEEN EXCLUDED FROM THIS AGREEMENT (INDICATED BY “[***]”) BECAUSE TAYSHA GENE THERAPIES, INC. HAS DETERMINED SUCH INFORMATION (I) IS NOT MATERIAL AND (II) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED.
LOAN AND SECURITY AGREEMENT
THIS LOAN AND SECURITY AGREEMENT is made as of August 7, 2025 (the “Closing Date”), by and among TAYSHA GENE THERAPIES, INC., a Delaware corporation (“Borrower”), the lenders from time to time party hereto (each, a “Lender” and collectively, the “Lenders”) and TRINITY CAPITAL INC., a Maryland corporation, as administrative agent and collateral agent for the Lenders (“Administrative Agent).
RECITALS
WHEREAS, Borrower may, from time to time, desire to borrow from Lenders, and Lenders, may, from time to time, make available to Borrower, term loans (each a “Loan” and collectively the “Loans”); and
WHEREAS, Borrower and Lenders desire that this Agreement shall serve as a master agreement which sets forth the terms and conditions governing any Loan by Lenders to Borrower.
NOW, THEREFORE, in consideration of the agreements and covenants contained herein, and for other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, the parties hereto agree as follows:
As used herein, all capitalized terms shall have the meanings set forth below. All other capitalized terms used but not defined herein shall have the meaning given to such terms in the UCC. Any accounting term used but not defined herein shall be construed in accordance with GAAP and all calculations shall be made in accordance with GAAP. The term “financial statements” shall include the accompanying notes and schedules.
“Account” is, as to any Person, any “account” of such Person as “account” is defined in the UCC with such additions to such term as may hereafter be made, and includes, without limitation, all accounts receivable and other sums owing to such Person.
“Account Control Agreement” means any deposit account control agreement or securities account control agreement in a form acceptable to Administrative Agent required to perfect Administrative Agent’s security interest in all Deposit Accounts and Securities Accounts of Borrower and each of its Subsidiaries.
“Account Debtor” means, with respect to any Account, the Person or Persons obligated to make payments with respect to such Account, including any guarantor thereof.
“Administrative Agent” means Trinity Capital Inc., in its capacity as administrative agent and collateral agent under the Loan Documents, or any successor administrative agent and collateral agent appointed in accordance with Article 5.
“Administrative Agent’s Expenses” means all reasonable and documented out of pocket costs or expenses (including reasonable and documented attorneys’ fees and expenses) incurred by Administrative Agent in connection with the preparation, negotiation, documentation, drafting, amendment, modification, administration, perfection and funding of the Loan Documents; and all of Administrative Agent’s reasonable and documented attorneys’ fees, costs and expenses incurred in enforcing or defending the Loan Documents (including fees and expenses of appeal or review) and the rights of Administrative Agent in and to the Loans and the Collateral or otherwise hereunder, including the exercise of any rights or remedies afforded hereunder or under applicable law, whether or not suit is brought, whether before or after bankruptcy or insolvency, including all fees and costs incurred by Administrative Agent in connection with its enforcement of its rights in a bankruptcy or insolvency proceeding filed by or against Borrower, any Subsidiary or their respective Property.
“Advance” means any Loan funds advanced under this Agreement.
“Affiliate” means, with respect to any Person, any other Person that owns or controls directly or indirectly ten percent (10%) or more of the stock of another entity of such Person, any other Person that controls or is controlled by or is under common control with such Person and each of such Person’s officers, directors, managers, joint venturers or partners. For purposes of this definition, the term “control” of a Person means the possession, direct or indirect, of the power to direct or cause the direction of the management and policies of such Person, whether through the ownership of voting Equity Securities, by contract or otherwise and the terms “controlled by” and “under common control with” shall have correlative meanings.
“Agreement” means this Loan and Security Agreement and all Schedules and Exhibits annexed hereto and made a part hereof, as the same may be amended, supplemented and or modified from time to time by the parties hereto.
“Amortization Date” is set forth on Schedule 1 hereto.
“Amortization Schedule” has the meaning provided in Section 2.1(a).
“Anti-Terrorism Laws” means any laws relating to terrorism or money laundering, including Executive Order No. 13224 (effective September 24, 2001), the USA PATRIOT Act, the laws comprising or implementing the Bank Secrecy Act, and the laws administered by OFAC.
“Applicable Rate” is set forth on Schedule 1 hereto.
“Assignment and Acceptance” means an assignment and acceptance entered into by an assigning Lender and an eligible assignee and, to the extent required, consented to by the Administrative Agent and Borrower in accordance with Section 5.4 hereof and substantially in form reasonably acceptable to the Administrative Agent and Borrower.
“BLA” means a Biologics License Application submitted to the FDA pursuant to 42 U.S.C. § 262 and 21 C.F.R. Part 601, or any successor application or procedure, seeking approval to market a biological product in the United States, including all amendments, supplements, and updates thereto, and all correspondence and communications with the FDA relating to such application.
“Blocked Person” means any Person: (a) listed in the annex to, or is otherwise subject to the provisions of, Executive Order No. 13224, (b) owned or controlled by, or acting for or on behalf of, any Person that is listed in the annex to, or is otherwise subject to the provisions of, Executive Order No. 13224,
(c) with which any Lender is prohibited from dealing or otherwise engaging in any transaction by any Anti- Terrorism Law, (d) that commits, threatens or conspires to commit or supports “terrorism” as defined in Executive Order No. 13224, or (e) that is named a “specially designated national” or “blocked person” on the most current list published by OFAC or other similar list.
“Business Day” means a day when the banks in Phoenix, Arizona are open for business.
“Change of Control” means the closing of any transaction or series of transactions by which Borrower shall merge with (whether or not Borrower is the surviving entity) or consolidate into any other Person or lease or sell substantially all of its and its subsidiaries’ assets substantially as an entirety to any other Person or by which any Person, entity or group (within the meaning of Rule 13d-5 under the Securities Exchange Act of 1934) acquires, directly or indirectly, forty-nine percent (49%) or more of Borrower’s outstanding capital stock that has ordinary voting power for the election of directors of Borrower (determined on a fully diluted basis), unless in each case, as a condition to the closing and consummation of such transaction that would otherwise constitute a “Change of Control,” the Obligations will be paid in full.
“Closing Date” has the meaning set forth in the preamble hereto.
“Code” means the Internal Revenue Code of 1986.
“Collateral” has the meaning provided in Article 3.
“Commitments” means, with respect to each Lender, such Lender’s obligation to make Loans to the Borrower hereunder in a principal amount equal to the amount set forth under the heading “Commitment” opposite such Lender’s name on Schedule 2.
“Commitment Fee” is set forth on Schedule 1 hereto.
“Compliance Certificate” is that certain certificate in substantially the form attached hereto as Exhibit C.
“Debt” means (a) all indebtedness for borrowed money; (b) all indebtedness for the deferred purchase price of property or services (other than (i) trade payables and accrued expenses incurred in the Ordinary Course of Business, (ii) any earn-out, purchase price adjustment or similar obligation until such obligation appears in the liabilities section of the balance sheet and (iii) any amounts being disputed in good faith by Borrower where such dispute would not cause, or be reasonably expected to cause, a Material Adverse Change); (c) all obligations evidenced by notes, bonds, debentures or other similar instruments;
(d) all indebtedness created or arising under any conditional sale or other title retention agreement with respect to property acquired (even though the rights and remedies of the seller or lender under such agreement in the event of default are limited to repossession or sale of such property); (e) equity securities subject to repurchase or redemption by the holder of such equity securities (other than in connection with a Change of Control or asset sale) prior to 91 days after the Maturity Date, (f) all obligations, contingent or otherwise, as an account party or applicant under acceptance, letter of credit or similar facilities in respect of obligations of the kind referred to in subsections (a) through (e) of this definition; and (g) all obligations of the kind referred to in subsections (a) through (f) above secured by (or which the holder of such obligation has an existing right, contingent or otherwise, to be secured by) any Lien on property (including accounts and contract rights). Notwithstanding anything herein to the contrary, operating leases shall not constitute Debt hereunder.
“Default Rate” has the meaning set forth in Section 2.2(c).
“Defaulting Lender” means any Lender that (a) has failed to (i) fund all or any portion of its Loans within two Business Days of the date such Loans were required to be funded hereunder unless such Lender notifies the Administrative Agent and the Borrower in writing that such failure is the result of such Lender’s determination that one or more conditions precedent to funding (each of which conditions precedent, together with any applicable default, shall be specifically identified in such writing) has not been satisfied, or (ii) pay to the Administrative Agent or any other Lender any other amount required to be paid by it hereunder within two Business Days of the date when due, (b) has notified the Borrower, or the Administrative Agent in writing that it does not intend to comply with its funding obligations hereunder, or has made a public statement to that effect (unless such writing or public statement relates to such Lender’s obligation to fund a Loan hereunder and states that such position is based on such Lender’s determination that a condition precedent to funding (which condition precedent, together with any applicable default, shall be specifically identified in such writing or public statement) cannot be satisfied), (c) has failed, within three Business Days after written request by the Administrative Agent or the Borrower, to confirm in writing to the Administrative Agent and the Borrower that it will comply with its prospective funding obligations hereunder (provided that such Lender shall cease to be a Defaulting Lender pursuant to this clause (c) upon receipt of such written confirmation by the Administrative Agent and the Borrower), or (d) has, or has a direct or indirect parent company that has, (i) become the subject of a proceeding under any debtor relief law, (ii) had appointed for it a receiver, custodian, conservator, trustee, administrator, assignee for the benefit of creditors or similar Person charged with reorganization or liquidation of its business or assets, including the Federal Deposit Insurance Corporation or any other state or federal regulatory authority acting in such a capacity or (iii) become the subject of a bail-in action.
Notwithstanding anything to the contrary herein, a Lender shall not be a Defaulting Lender solely by virtue of the ownership or acquisition of any Equity Security in that Lender or any direct or indirect parent company thereof by a Governmental Authority so long as such ownership interest does not result in or provide such Lender with immunity from the jurisdiction
of courts within the United States or from the enforcement of judgments or writs of attachment on its assets or permits such Lender (or such Governmental Authority) to reject, repudiate, disavow or disaffirm any contracts or agreements made with such Lender. Any determination by the Administrative Agent that a Lender is a Defaulting Lender under clauses (a) through (d) above shall be conclusive and binding absent manifest error, and such Lender shall be deemed to be a Defaulting Lender upon delivery of written notice of such determination to the Borrower and each Lender.
“Deposit Account” means any “deposit account” as defined in the UCC with such additions to the term as may hereafter be made, and includes any checking account, savings account, or certificate of deposit.
“Documentation and Funding Fees” has the meaning set forth in Section 2.1(c).
“End of Term Payment” is set forth on Schedule 1 hereto.
“Equity Securities” of any Person means (a) all common stock, preferred stock, participations, shares, partnership interests, membership interests or other equity interests in and of such Person (regardless of how designated and whether or not voting or non-voting) and (b) all warrants, options and other rights to acquire any of the foregoing, but excluding any debt securities convertible into such shares or other such equity interests unless such debt securities are converted into such shares or other such equity interests.
“Event of Default” means any of the following events and conditions at any time, unless waived in writing by Administrative Agent and the Required Lenders, and shall constitute an Event of Default:
(a)
failure on the part of Borrower to remit to Administrative Agent (i) any payment of principal or interest when due or (ii) any other amounts required to be remitted under this Agreement or any Loan Documents on or before such amount is due, and such failure continues for three (3) Business Days (which three (3) Business Day cure period shall not apply to payments due on the Maturity Date). During such cure period, the failure to make or pay any payment specified under clause (ii) is not an Event of Default (but no Advance will be made during the cure period);
(b)
failure on the part of Borrower: (A) to perform any obligation arising under Section 4.2 or to comply with any covenants of Section 4.3 or (B) duly to observe or perform in any other of its respective covenants or agreements in this Agreement or any other Loan Document, which failure continues for a period of ten (10) Business Days after the occurrence of such breach;
(c)
there is (a) a default in any agreement to which Borrower or any of its Subsidiaries is a party with a third party or parties resulting in the acceleration of the maturity of any Debt in an amount in excess of One Million Dollars ($1,000,000.00) provided, however, that the Event of Default under this clause (c) caused by the occurrence of a breach or default under such other agreement shall be cured or waived for purposes of this Agreement upon Administrative Agent receiving written notice from the party asserting such breach or default of such cure or waiver of the breach or default under such other agreement, if at the time of such cure or waiver under such other agreement (x) Administrative Agent has not declared an Event of Default under this Agreement and/or exercised any rights with respect thereto; and (y) any such cure or waiver does not result in an Event of Default under any other provision of this Agreement or any Loan Document;
(d)
if any representation or warranty of Borrower made in this Agreement or in any certificate or other writing delivered pursuant hereto or any other related document is materially incorrect or misleading as of the time when the same shall have been made;
(e)
any provision of this Agreement or any Lien or security interest of Administrative Agent in the Collateral ceases for any reason to be valid, binding and in full force and effect other than as expressly permitted hereunder;
(f)
any bankruptcy, insolvency or other similar proceeding is filed by Borrower or any of its Subsidiaries;
(g)
any involuntary bankruptcy, insolvency or other similar proceeding is filed against Borrower or any of its Subsidiaries and such proceeding or petition shall not be dismissed within forty-five (45) days after filing;
(h)
any assignment is made by Borrower or any attempt by Borrower to assign any of its duties or rights hereunder;
(i)
Borrower is consolidated with, merged with (other than if a Subsidiary merges into Borrower and Borrower is the surviving entity), or sells its properties and assets substantially as an entity to another entity without Administrative Agent’s and Required Lender’s prior written consent, provided that no consent of Administrative Agent or Required Lenders shall be required if, in connection with such merger or sale of properties and assets the Obligations will be paid in full;
(j)
(i) If any material portion of Borrower’s or any of its Subsidiaries’ assets (A) is attached, seized, subjected to a writ or distress warrant, or is levied upon or (B) comes into the possession of any trustee, receiver or person acting in a similar capacity and such attachment, seizure, writ or distress warrant or levy has not been removed, discharged or rescinded within ten (10) Business Days, (ii) if Borrower or any of its Subsidiaries is enjoined, restrained or in way prevented by court order from continuing to conduct all or any material part of its business affairs, (iii) if a judgment or other claim becomes a Lien or encumbrance upon any material portion of Borrower’s or any of its Subsidiaries’ assets or (iv) if a notice of Lien, levy or assessment if filed of record with respect to any material portion of Borrower’s or any of its Subsidiaries’ assets by the United States Government, or any department agency or instrumentality thereof, or by any state, county municipal, or governmental agency, and the same is not paid within ten (10) Business Days after Borrower or any Subsidiary receives notice thereof; provided that none of the foregoing shall constitute an Event of Default where such action or event is stayed or an adequate bond has been posted pending a good faith contest by Borrower;
(k)
If any of the Loan Documents shall cease to be, or Borrower shall assert that any of the Loan Documents is not, a legal, valid and binding obligation of Borrower enforceable in accordance with its terms;
(l)
If there occurs a Material Adverse Change to Borrower;
(m)
there is a Change of Control, unless, as a condition to the closing of such change of control the Obligations will be paid in full; or
(n)
a final, non-appealable judgment which is not covered by insurance is entered against Borrower or any Subsidiary for an amount in excess of One Million Dollars ($1,000,000.00), which is not paid or bonded within twenty (20) days of entry.
“Excluded Account” means (a) any account specifically used, and identified in writing as such to Administrative Agent, for payroll, payroll taxes, workers’ compensation or unemployment compensation premiums or benefits, pension benefits, and other employee wage and benefit payments to or for the benefit of the employees of the Borrower and its Subsidiaries, not to exceed the amounts to fund the next two (2) succeeding payroll periods, (b) any deposit account, securities account, commodities account or other account to the extent solely used to hold any cash or cash equivalents pledged as a Permitted Lien, as may be designated in writing to Administrative Agent from time to time after the Closing Date, (c) escrow account maintained with Metro Title Company, LLC, as escrow agent, holding cash collateral so secure lease at Patriot Park, (d) any Deposit Account used exclusively to maintain deposits or cash collateral for the benefit of unaffiliated third parties as expressly permitted by this Agreement, as may be designated in writing to Administrative Agent from time to time after the Closing Date, and (e) foreign deposit accounts of Inactive Subsidiaries so long as the aggregate balance for all such accounts does not exceed $50,000 at any time.
“Excluded Taxes” means any of the following Taxes imposed on or with respect to a Lender or Administrative Agent or required to be withheld or deducted from a payment to a Lender or Administrative Agent: (a) Taxes imposed on or measured by net income (however denominated), franchise Taxes and branch profits Taxes, in each case, (i) imposed as a result of such Lender or Administrative Agent being organized under the laws of, or having its principal office or, in the case of any Lender, its applicable lending office located in, the jurisdiction imposing such Tax (or any political subdivision thereof) or (ii) that are Other Connection Taxes, (b) in the case of a Lender, any U.S. federal withholding Taxes imposed on amounts payable to or for the account of such Lender with respect to an applicable interest in a Loan or Commitment pursuant to a law in effect on the date on which such Lender acquires the applicable interest in such Loan or Commitment or changes its lending office, except in each case to the extent that, pursuant to Section 2.11, additional amounts with respect to such Taxes were payable either to such Lender’s assignor immediately before such Lender became a party hereto or to such Lender immediately before it changes its lending office, (c) Taxes attributable to such Lender’s or Administrative Agent’s failure to comply with Section 2.11(g), and (d) any Taxes imposed under FATCA.
“Existing Indebtedness” means Indebtedness owing by Borrower to Lenders pursuant to the Original Loan Agreement.
“FATCA” means Sections 1471 through 1474 of the Code, as of the date of this Agreement (or any amended or successor version to the extent such version is substantively comparable and not materially more onerous to comply with), any current or future Treasury Regulations or official administrative interpretations thereof, any agreements entered into pursuant to current Section 1471(b)(1) of the Code (or any amended or successor version described above), any intergovernmental agreement, treaty or convention among Governmental Authorities (and any related fiscal or regulatory legislation, rules or official practices) implementing the foregoing.
“FDA” means the United States Food and Drug Administration, and any successor agency or authority thereto having the same or similar responsibilities.
“GAAP” means generally accepted accounting principles, consistently applied, as in effect from time to time in the United States.
“Governmental Approval” is any consent, authorization, approval, order, license, franchise, permit, certificate, accreditation, registration, filing or notice, of, issued by, from or to, or other act by or in respect of, any Governmental Authority.
“Governmental Authority” is any nation or government, any state or other political subdivision thereof, any agency, authority, instrumentality, regulatory body, court, central bank or other entity exercising executive, legislative, judicial, taxing, regulatory or administrative functions of or pertaining to government, any securities exchange and any self-regulatory organization.
“Inactive Subsidiary” means each Subsidiary identified by Borrower as “inactive” on the Perfection Certificate.
“Indemnified Taxes” means (a) all Taxes, other than Excluded Taxes, imposed on or with respect to any payment made by or on account of any obligation of any Borrower under any Loan Document and (b) to the extent not otherwise described in (a), Other Taxes.
“Intellectual Property” means any and all intellectual property, including copyrights, copyright licenses, patents, patent licenses, trademarks, trademark licenses, technology, know-how and processes, all rights therein, and all rights to sue at law or in equity for any past present or future infringement, violation, misuse, misappropriation or other impairment thereof, whether arising under United States, multinational or foreign laws or otherwise, including the right to receive injunctive relief and all proceeds and damages therefrom.
“Interest Only Period” is set forth on Schedule 1 hereto.
“Investment” means the purchase or acquisition of any capital stock, equity interest, or any obligations or other securities of, or any interest in, any Person, or the extension of any advance, loan, extension of credit or capital contribution to, or any other investment in, or deposit with, any Person.
“IP Security Agreement” means the Intellectual Property Security Agreement, dated as of the date hereof, by and among Administrative Agent and each grantor party thereto (as amended, amended and restated, supplemented or otherwise modified from time to time).
“Key Person” is each of Borrower’s (i) Chief Executive Officer, who is Sean P. Nolan as of the Closing Date and (ii) Chief Financial Officer, who is Kamran Alam as of the Closing Date.
“Knowledge” or “Knowledge of Borrower” means the actual knowledge of the chief executive officer, chief operating officer or chief financial officer of Borrower and such knowledge that would be obtained upon due inquiry and reasonable investigation by such Persons.
“Lender’s Expenses” means all reasonable and documented out of pocket costs or expenses (including reasonably and documented attorneys’ fees and expenses) incurred in connection with the preparation, negotiation, documentation, drafting, amendment, modification, administration, perfection and funding of the Loan Documents; and all of Lenders’ reasonable and documented attorneys’ fees, costs and expenses incurred in enforcing or defending the Loan Documents (including fees and expenses of appeal or review) and the rights of a Lender in and to the Loans and the Collateral or otherwise hereunder, including the exercise of any rights or remedies afforded hereunder or under applicable law, whether or not suit is brought, whether before or after bankruptcy or insolvency, including all reasonable and documented fees and costs incurred by any Lender in connection with such Lender’s enforcement of its rights in a bankruptcy or insolvency proceeding filed by or against Borrower, any Subsidiary or their respective Property.
“Lien” means a claim, mortgage, deed of trust, levy, charge, pledge, security interest or other encumbrance of any kind, whether voluntarily incurred or arising by operation of law or otherwise against any property.
“Loans” has the meaning set forth in the preamble above.
“Loan Advance Request Form” is that certain form attached hereto as Exhibit D.
“Loan Documents” means this Agreement and any schedules, exhibits, certificates, notices, and any other documents related to this Agreement, [***], every Account Control Agreement, the IP Security Agreement, and any other intercreditor agreement, or subordination agreement, any documents pertaining to a mortgage, any landlord waivers and bailee waivers, the Perfection Certificate, each Compliance Certificate, each Loan Advance Request Form and every other document evidencing, securing or relating to the Loans, in each case as amended, amended and restated, supplemented or otherwise modified from time to time.
“Loan Termination Date” is set forth on Schedule 1 hereto.
“Material Adverse Change” means (i) a materially adverse effect on the business, financial condition, operations, performance or Property of Borrower and its Subsidiaries, taken as a whole, or (ii) a material impairment of the ability of Borrower to perform its obligations under or remain in compliance with this Agreement and the other Loan Documents, or any documents executed in connection therewith.
“Maturity Date” is set forth on Schedule 1 hereto.
“Obligations” means all present and future obligations owing by Borrower to Administrative Agent and the Lenders governed or evidenced by the Loan Documents whether or not for the payment of money, whether or not evidenced by any note or other instrument, whether direct or indirect, absolute or contingent, due or to become due, joint or several, primary or secondary, liquidated or unliquidated, secured or unsecured, original or renewed or extended, whether arising before, during or after the commencement of any bankruptcy case in which Borrower is a debtor (specifically including interest accruing after the commencement of any bankruptcy, insolvency or similar proceeding with respect to Borrower, whether or not a claim for such post-commencement interest is allowed), including but not limited to any obligations arising pursuant to letters of credit or acceptance transactions or any other financial accommodations.
“OFAC” means the United States Department of the Treasury’s Office of Foreign Assets Control.
“Operating Documents” means, for any Person, such Person’s formation documents, as certified by the Secretary of State (or equivalent agency) of such Person’s jurisdiction of organization on a date that is no earlier than thirty (30) days prior to the Closing Date, and, (a) if such Person is a corporation, its bylaws in current form, (b) if such Person is a limited liability company, its limited liability company agreement (or similar agreement), and (c) if such Person is a partnership, its partnership agreement (or similar agreement), each of the foregoing with all current amendments or modifications thereto.
“Ordinary Course of Business” means, in respect of any transaction involving any Person, the ordinary course of such Person’s business as conducted by any such Person in accordance with the usual and customary customs and practices in the kind of business in which such Person is engaged, and undertaken by such Person in good faith and not for purposes of evading any covenant or restriction in any Loan Document.
“Other Connection Taxes” means, with respect to any Lender or Administrative Agent, Taxes imposed as a result of a present or former connection between such Lender or Administrative Agent and the jurisdiction imposing such Tax (other than connections arising from such Lender or Administrative Agent having executed, delivered, become a party to, performed its obligations under, received payments under, received or perfected a security interest under, engaged in any other transaction pursuant to or enforced any Loan Document, or sold or assigned an interest in any Loan or Loan Document).
“Other Taxes” means all present or future stamp, court or documentary, intangible, recording, filing or similar Taxes that arise from any payment made under, from the execution, delivery, performance, enforcement or registration of, from the receipt or perfection of a security interest under, or otherwise with respect to, any Loan Document, except any such Taxes that are Other Connection Taxes imposed with respect to an assignment.
“Participant” shall have the meaning provided in Section 8.18.
“Participant Register” shall have the meaning provided in Section 8.18.
“Payment Date” means the first (1st) day of each month, or if such day is not a Business Day, the next Business Day.
“Perfection Certificate” means the perfection certificate delivered to Administrative Agent dated as of the Closing Date.
“Permitted Debt” means and includes:
(o)
Debt of Borrower to Lenders under this Agreement;
(p)
Debt of Borrower in an aggregate principal amount not to exceed One Million Dollars ($1,000,000.00) at any time, secured by Liens permitted under clause (g) of the definition of Permitted Liens;
(q)
Debt consisting of reimbursement obligations with respect to letters of credit in an aggregate face amount not to exceed Two Hundred Fifty Thousand Dollars ($250,000.00) at any time, and any other letters of credit, provided that any such letters of credit are designated in writing to the Administrative Agent and approved by Administrative Agent from time to time after the Closing Date;
(r)
Debt incurred on corporate credit cards in the Ordinary Course of Business with JP Morgan Chase Bank, N.A. and Ramp, provided that the aggregate outstanding principal amount shall not exceed Six Hundred Fifty Thousand Dollars ($650,000.00);
(s)
Debt of Borrower existing on the date hereof and set forth on the Perfection Certificate;
(t)
extensions, refinancings, modifications, amendments and restatements of any items of Permitted Debt under subsections (a)-(c) above; provided that the principal amount thereof is not increased or the terms thereof are not modified to impose materially more burdensome terms upon Borrower;
(u)
Debt of Borrower subordinated to the Obligations pursuant to a subordination, intercreditor, or similar agreement in form and substance, and on terms, satisfactory to Administrative Agent in Administrative Agent’s sole reasonable discretion;
(v)
unsecured Debt to trade creditors incurred in the Ordinary Course of Business;
(w)
Debt incurred as a result of endorsing negotiable instruments received in the Ordinary Course of Business;
(x)
Debt incurred in connection with insurance premium financings in the Ordinary Course of Business; provided that any Lien securing such Indebtedness is limited to the unearned premium of such insurance;
(y)
other unsecured Debt not otherwise permitted hereunder in an aggregate outstanding principal amount not to exceed Five Hundred Thousand Dollars ($500,000); and
(z)
to the extent constituting Debt, investments permitted in clause (g) of the definition of “Permitted Investments”; provided that intercompany Debt shall be subject to a subordination agreement in favor of, and in form and substance reasonably acceptable to, Agent; and
(aa)
Debt in respect of performance bonds, bid bonds, appeal bonds, surety bonds and similar obligations incurred in the Ordinary Course of Business.
“Permitted Investment” means
(bb)
Deposits and Deposit Accounts (which shall be subject to Account Control Agreements as required herein) with commercial banks organized under the laws of the United States or a state thereof to the extent: (i) the Deposit Accounts of each such institution are insured by the Federal Deposit Insurance Corporation up to the legal limit; and (ii) each such institution has an aggregate capital and surplus of not less than One Hundred Million Dollars ($100,000,000.00);
(cc)
Investments in marketable obligations issued or fully guaranteed by the United States and maturing not more than one (1) year from the date of issuance;
(dd)
Investments in open market commercial paper rated at least “A1” or “P1” or higher by a national credit rating agency and maturing not more than one (1) year from the creation thereof;
(ee)
other highly liquid investments consistent with Borrower’s investment policy approved by Borrower’s board of directors as in effect, and as provided to and reviewed and approved by Administrative Agent, prior to the Closing Date (together with Amendments thereto, as provided to and reviewed and approved in writing by Administrative Agent);
(ff)
Investments consisting of the endorsement of negotiable instruments for deposit or collection or similar transactions in the ordinary course of Borrower;
(gg)
Investments outstanding on the date hereof and set forth on the Perfection Certificate;
(hh)
Investments (i) from one Borrower into another Borrower, (ii) by Borrower in Subsidiaries that are not Guarantors not to exceed Fifty Thousand Dollars ($50,000.00) in the aggregate in any fiscal year, and (iii) by Subsidiaries that are not Guarantors in other Subsidiaries that are not Guarantors;
(ii)
Investments (including debt obligations) received in connection with the bankruptcy or reorganization of customers or suppliers and in settlement of delinquent obligations of, and other disputes with, customers or suppliers arising in the Ordinary Course of Business;
(jj)
Investments consisting of notes receivable of, or prepaid royalties and other credit extensions, to customers and suppliers who are not Affiliates, in the Ordinary Course of Business; provided that this paragraph shall not apply to Investments of Borrower in any Subsidiary; and
(kk)
Investments accepted in connection with transfers permitted by this Agreement, in accordance with Section 4.3 (d);
(ll)
Investments consisting of (i) travel advances and employee relocation loans and other employee loans and advances in the Ordinary Course of Business, and (ii) loans to employees, officers or directors relating to the purchase of equity securities of Borrower or its Subsidiaries pursuant to employee stock purchase plans or agreements approved by Borrower’s board of directors; not to exceed Two Hundred Fifty Thousand Dollars ($250,000.00) in the aggregate in any fiscal year;
(mm)
Investments consisting of deposits to secure the performance of bids, trade contracts, statutory obligations, surety and appeal bonds (other than bonds related to judgments or litigation) or performance bonds, in each case in the Ordinary Course of Business;
(nn)
Other Investments aggregating not in excess of Five Hundred Thousand Dollars ($500,000.00) per year.
“Permitted License” means (A) licenses of over-the-counter software that is commercially available to the public, and (B) non-exclusive and exclusive licenses for the use of the Intellectual Property of Borrower or any of its Subsidiaries entered into in the Ordinary Course of Business, provided, that, with respect to each such license described in clause (B), (i) no Event of Default has occurred or is continuing at the time of such license; (ii) the license constitutes an arm’s length transaction, the terms of which, on their face, do not provide for a sale or assignment of any Intellectual Property and do not restrict the ability of Borrower or any of its Subsidiaries, as applicable, to pledge, grant a security interest in or lien on, or assign or otherwise Transfer any Intellectual Property; (iii) in the case of any exclusive license, (x) Borrower delivers ten (10) days’ prior written notice and a brief summary of the terms of the proposed license to Lender and delivers to Lender copies of the final executed licensing documents in connection with the exclusive license promptly upon consummation thereof, and (y) any such license could not result in a legal transfer of title of the licensed property but may be exclusive in respects other than territory and may be exclusive as to territory only as to discrete geographical areas outside of the United States; and (iv) all upfront payments, royalties, milestone payments or other proceeds arising from the licensing agreement that are payable to Borrower or any of its Subsidiaries are paid to a Deposit Account that is governed by an Account Control Agreement.
“Permitted Liens” means any of the following:
(oo)
Liens of the Administrative Agent pursuant to this Agreement;
(pp)
Liens outstanding on the date hereof and set forth on the Perfection Certificate;
(qq)
Liens for taxes and assessments not yet due and payable or, if due and payable, those being contested in good faith by appropriate proceedings and for which appropriate reserves are maintained in accordance with GAAP;
(rr)
Liens arising in the Ordinary Course of Business (such as Liens of carriers, warehousemen, mechanics, and materialmen) and other similar Liens imposed by law for sums not yet due and payable or, if due and payable, those being contested in good faith by appropriate proceedings and for which appropriate reserves are maintained in accordance with GAAP;
(ss)
easements, rights of way, restrictions, minor defects or irregularities in title or other similar Liens which alone or in the aggregate do not interfere in any material way with the ordinary conduct of the business of Borrower;
(tt)
Liens consisting of Permitted Licenses;
(uu)
Liens consisting of purchase money security interests for new equipment financing not to exceed the amount permitted in clause (b) of the definition of “Permitted Debt”;
(vv)
Liens on cash collateral securing letters of credit that constitute Permitted Debt;
(ww)
Liens to secure payment of workers’ compensation, employment insurance, old- age pensions, social security and other like obligations incurred in the Ordinary Course of Business (other than Liens imposed by ERISA);
(xx)
Liens to secure leases or subleases of real property granted in the ordinary course of Borrower’s business (or, if referring to another Person, in the ordinary course of such Person’s business), and leases, subleases, non-exclusive licenses or sublicenses of personal property (other than Intellectual Property) granted in the ordinary course of Borrower’s business (or, if referring to another Person, in the ordinary course of such Person’s business), if the leases, subleases, licenses and sublicenses do not prohibit granting Lender a security interest therein;
(yy)
Liens arising from attachments or judgments, orders, or decrees in circumstances not constituting an Event of Default;
(zz)
Liens arising from the filing of any precautionary financing statement on operating leases covering the leased property, to the extent such operating leases are permitted under this Agreement; Liens in favor or other financial institutions arising in connection with Borrower’s deposit or investment accounts held at such institutions to secure customary fees and charges (but not credit/debt relationships or margin accounts), provided that Administrative Agent has a perfected security interest in the amounts held in such deposit accounts to the extent required by this Agreement;
(bbb)
Liens on insurance proceeds granted solely as a security for financed premiums to the extent the Debt qualifies as Permitted Debt; and
(ccc)
(n) cash pledges and deposits to secure the performance of bids, trade, commercial and government contracts, tenders, leases, statutory or regulatory obligations (including ERISA obligations and bonds), surety and appeal bonds, performance bonds and other obligations of a like nature, in each case in the Ordinary Course of Business; in any case other than statutory or regulatory obligations, such as but not limited to ERISA and ERISA bonds, not representing an obligation for borrowed money.
“Person” means and includes any individual, any partnership, any corporation, any business trust, any joint stock company, any limited liability company, any unincorporated association or any other entity and any domestic or foreign national, state or local government, foregoing.
“Potential Event of Default” means any event or circumstance, which, with the giving of notice or lapse of time or both, would become an Event of Default.
“Prime Rate” means, at any time, the rate of interest noted in The Wall Street Journal, Money Rates section, as the “Prime Rate.” In the event that The Wall Street Journal quotes more than one rate, or a range of rates, as the Prime Rate, then the Prime Rate shall mean the average of the quoted rates. In the event that The Wall Street Journal ceases to publish a Prime Rate, then the Prime Rate shall be as announced by Administrative Agent.
“Pro Rata Share” means, with respect to:
(ddd)
a Lender’s obligation to make Loans and the right to receive payments of interest, fees and principal with respect thereto, the percentage obtained by dividing (i) such Lender’s Commitments, by (ii) the Total Commitments, provided that if the Total Commitments have been reduced to zero, the numerator shall be the aggregate unpaid principal amount of such Lender’s portion of the Loans and the denominator shall be the aggregate unpaid principal amount of the Loans, and
(eee)
all other matters (including, without limitation, the indemnification obligations arising under Section 5.7), the percentage obtained by dividing (i) the sum of the unpaid principal amount of such Lender’s portion of the Loans, by (ii) the sum of the aggregate unpaid principal amount of the Loans.
“Property” means any interest in any kind of property or asset, whether real, personal or mixed, whether tangible or intangible.
“Register” shall have the meaning provided in Section 8.18.
“Required Lenders” means Lenders (other than Defaulting Lenders) whose Pro Rata Shares (without giving effect to the Pro Rata Share of Defaulting Lenders) aggregate at least fifty and one-tenth percent (50.1%); provided that such Lenders must include Administrative Agent (unless Administrative Agent is a Defaulting Lender).
“Responsible Officer” means each of the chief executive officer, the chief operating officer, the chief financial officer, president, treasurer, vice president of finance and the controller of Borrower, as well as any other officer or employee identified as an authorized officer in the corporate resolution delivered by Borrower to Administrative Agent in connection with this Agreement.
“Restricted License” means any license or other agreement with respect to which Borrower is the licensee and such license or agreement is material to Borrower’s business and that prohibits or otherwise restricts Borrower from granting a security interest in Borrower’s interest in such license or agreement or any other property.
“Secured Parties” means the Lenders, Administrative Agent, each other Indemnified Person and any other holder of any Obligation.
“Securities Account” means any “securities account” as defined in the UCC with such additions to such term as may hereafter be made.
“Solvent” with respect to any person or entity as of any date of determination, means that on such date (a) the present fair salable value of the property and assets of such person or entity exceeds the debts and liabilities, including contingent liabilities, of such person or entity, (b) the present fair salable value of the property and assets of such person or entity is greater than the amount that will be required to pay the probable liability of such person or entity on its debts and other liabilities, including contingent liabilities, as such debts and other liabilities become absolute and matured, (c) such person or entity does not intend to incur, or believe (nor should it reasonably believe) that it will incur, debts and liabilities, including contingent liabilities, beyond its ability to pay such debts and liabilities as they become absolute and matured, and (d) such person or entity does not have unreasonably small capital with which to conduct the business in which it is engaged as such business is now conducted and is proposed to be conducted. The amount of contingent liabilities at any time shall be computed as the amount that, in the light of all the facts and circumstances existing at such time, represents the amount that can reasonably be expected to become an actual or matured liability.
“Subsidiary” as to any Person, means any corporation, partnership, limited liability company, joint venture, trust or estate of or in which more than fifty percent (50%) of (a) the issued and outstanding capital stock having ordinary voting power to elect a majority of the board of directors of such corporation (irrespective of whether at the time capital stock of any other class of such corporation may have voting power upon the happening of a contingency), (b) the interest in the capital or profits of such partnership, limited liability company, or joint venture or (c) the beneficial interest in such trust or estate is at the time directly or indirectly owned or controlled through one or more intermediaries, or both, by such Person. Unless otherwise qualified, all references to a “Subsidiary” or to “Subsidiaries” in this Agreement shall refer to a Subsidiary or Subsidiaries of the Borrower.
[***].
[***].
“Taxes” means all present or future taxes, levies, imposts, duties, deductions, withholdings (including backup withholding), assessments, fees or other charges in the nature of a tax imposed by any Governmental Authority, including any interest, additions to tax or penalties applicable thereto.
"Tranche A Availability Amount” is set forth on Schedule 1 hereto.
“Tranche A Loan” shall have the meaning provided in Section 2.1(b).
"Tranche B Availability Amount” is set forth on Schedule 1 hereto.
“Tranche B Loan” shall have the meaning provided in Section 2.1(b).
“Tranche B Milestone” means [***].
"Tranche C Availability Amount” is set forth on Schedule 1 hereto.
“Tranche C Loan” shall have the meaning provided in Section 2.1(b).
“Tranche C Milestone” means [***].
“Total Commitments” means the sum of the amounts of the Lenders’ Commitments.
“Transfer” means to convey, sell, lease, transfer, assign, or otherwise dispose of.
“UCC” means the Uniform Commercial Code as the same may from time to time be in effect in the State of California; provided, however, in the event, by reason of mandatory provisions of law, any and all of the attachment, perfection or priority of the security interest of Administrative Agent in and to the Collateral is governed by the Uniform Commercial Code as in effect in a jurisdiction other than California, the term “UCC” shall mean the Uniform Commercial Code as in effect in such other jurisdiction for purposes of the provisions relating to such attachment, perfection or priority and for purposes of definitions related to such provisions; provided, further, that the term “UCC” shall include Article 9 thereof as in effect on the Closing Date.
“U.S. Person” means any Person that is a “United States person” as defined in Section 7701(a)(30) of the Code.
(a)
Subject to the terms and conditions of this Agreement, each Lender severally hereby agrees to make a Loan to the Borrower in a principal amount not to exceed the amount of such Lender’s Commitments. If the aggregate outstanding principal amount of Loans at any time exceeds the Total Commitments, Borrower shall immediately repay such excess in full. The Obligations of Borrower under this Agreement shall at all times be absolute and unconditional. Borrower acknowledges and agrees that any obligation of any Lender to make any Loan hereunder is strictly contingent upon the satisfaction of the conditions set forth in Sections 2.4, 2.5, 2.6 and 2.7 (as applicable). For each Loan, Borrower shall make (i) monthly payments of interest only in arrears at the Applicable Rate during the Interest Only Period, and (ii) beginning on the Amortization Date, equal monthly payments on each subsequent Payment Date in an amount determined through a calculation fully amortizing the outstanding principal balance due under each Loan at the Applicable Rate over the period from the Amortization Date through (and including) the Maturity Date. For clarity, the payment schedule with respect to the Tranche A Loan as of the Closing Date is reflected in Exhibit A attached hereto, and Administrative Agent may update such payment schedule from time to time in accordance with the terms of the Loan Documents (as amended from time to time, the “Amortization Schedule”). In the event of any inconsistency between the Amortization Schedule and the terms of the Loan Documents (including this Section 2.1), the terms of the Loan Documents shall prevail. Borrower shall continue to comply with all of the terms and provisions hereof until all of the Obligations are paid and satisfied in full. After the Loan Termination Date, no further Loans shall be available from Lender.
(b)
The initial Advance hereunder, to be funded on the date hereof upon satisfaction of the conditions in Sections 2.4 and 2.5, shall be an amount equal to the Tranche A Availability Amount (the “Tranche A Loan”). Thereafter, upon satisfaction of the conditions set forth in Section 2.4 and Section 2.6, or 2.7, as applicable, Borrower may request additional Advances of up to (i) the Tranche B Availability Amount (the “Tranche B Loan”), or (ii) the Tranche C Availability Amount (the “Tranche C Loan”).
(c)
At the time of the Advance of the Tranche A Loan, Borrower will pay Administrative Agent and the Lenders for all reasonable and documented out of pocket costs related to the Tranche A Loan including travel, UCC search, filing, insurance, and legal costs for the Tranche A Loan (the “Tranche A Documentation and Funding Fee”).
(d)
At the time of the Advance of the Tranche B Loan, Borrower will pay Administrative Agent and the Lenders for all reasonable costs related to the Tranche B Loan including travel, UCC search, filing, insurance, and legal costs for the Tranche B Loan (the “Tranche B Documentation and Funding Fees”).
(e)
At the time of the Advance of the Tranche C Loan, Borrower will pay Administrative Agent and the Lenders for all reasonable costs related to the Tranche C Loan including travel, UCC search, filing, insurance, and legal costs for the Tranche C Loan (the “Tranche C Documentation and Funding Fees”). The Tranche A Documentation and Funding Fee, the Tranche B Documentation and Funding Fee, the Tranche C Documentation and Funding Fee and any such additional costs due related to additional Loans (if any) shall be collectively referred to hereunder as “Documentation and Funding Fees.”
2.2
Advances and Interest.
(a)
All Loans requested by Borrower must be requested by 11:00 A.M. Arizona time, five (5) Business Days prior to the date of such requested Loan. All requests or confirmations of requests for a Loan are to be in writing to Administrative Agent and may be sent by telecopy or facsimile transmission or by email provided that Administrative Agent shall have the right to require that receipt of such request not be effective unless confirmed via telephone with Lender. Borrower may not request more than one (1) Loan per calendar month. As express conditions precedent to Lender making each Loan to Borrower, Borrower shall deliver to Administrative Agent the documents, instruments and agreements required pursuant to Sections 2.4, 2.5, 2.6, and 2.7 (as applicable) of this Agreement (including, without limitation, the Loan Advance Request Form). Except as otherwise provided in this Section 2.2(a), all Loans under this Agreement shall be made by the Lenders simultaneously and proportionately to their Pro Rata Shares of the Total Commitments, as the case may be, it being understood that no Lender shall be responsible for any default by any other Lender in that other Lender’s obligations to make a Loan requested hereunder, nor shall the Commitment of any Lender be increased or decreased as a result of the default by any other Lender in that other Lender’s obligation to make a Loan requested hereunder, and each Lender shall be obligated to make the Loans required to be made by it by the terms of this Agreement regardless of the failure by any other Lender.
(b)
The following amounts shall be deducted from each Loan advanced hereunder: (i) as to the Tranche A Loan advanced hereunder, the applicable Commitment Fee and the Tranche A Documentation and Funding Fees, (ii) as to the Tranche B Loan, the Tranche B Documentation and Funding Fees and (iii) as to the Tranche C Loan, the Tranche C Documentation and Funding Fees.
(c)
Beginning on the date of each Advance, the unpaid principal balance of all advanced Loans and all other Obligations hereunder shall bear interest, subject to the terms hereof, at the Applicable Rate. All payments shall be due to Administrative Agent on the applicable Payment Date, or if such day is not a Business Day, the next succeeding Business Day. If Borrower fails to make a monthly payment due within five (5) Business Days after the date such payment is due, Administrative Agent, on behalf of the Lenders, shall have the right to require Borrower to pay to Lender a late charge equal to five percent (5%) of the past due payment. After the occurrence and during the continuance of an Event of Default hereunder, Administrative Agent, on behalf of the Lenders, shall have the right to increase the per annum effective rate of interest on all Loans outstanding hereunder to a rate equal to 500 basis points in excess of the Applicable Rate (the “Default Rate”). All contractual rates of interest chargeable on outstanding Loans, shall continue to accrue and be paid even after default, maturity, acceleration, judgment, bankruptcy, insolvency proceedings of any kind or the happening of any event or occurrence similar or dissimilar. In no contingency or event whatsoever shall the aggregate of all amounts deemed interest hereunder and charged or collected pursuant to the terms of this Agreement exceed the highest rate permissible under any law which a court of competent jurisdiction shall, in a final determination, deem applicable hereto. In the event that such court determines Lenders have charged or received interest hereunder in excess of the highest applicable rate, Administrative Agent, shall in its sole discretion and acting on behalf of the Lenders, apply and set off such excess interest received by Lenders against other Obligations hereunder due or to become due and such rate shall automatically be reduced to the maximum rate permitted by such law.
(d)
Interest shall be computed on the basis of a 360-day year, and twelve 30-day months. For any partial month interest periods, interest will be charged for the actual number of days elapsed. In computing interest, (i) all payments received after 12:00 p.m. Arizona time on any day shall be deemed received at the opening of business on the next Business Day, and (ii) the date of the making of the Loans shall be included and the date of payment shall be excluded. Changes to the Applicable Rate based on changes to the Prime Rate, shall be effective as of the day immediately following the date of such change, and to the extent, of such change.
(e)
Upon the occurrence and during the continuance of an Event of Default and/or the maturity of any portion of the Obligations, any moneys on deposit with Administrative Agent may, at the direction of the Required Lenders, be applied against the Obligations in such order and manner as Administrative Agent may elect or as may otherwise be required under this Agreement.
2.3
Administrative Agent Accounts. Administrative Agent shall maintain accounts in which it shall record (i) the amount of each Loan made hereunder, (ii) the amount of any principal or interest due and payable or to become due and payable from the Borrower to each Lender hereunder, and (iii) the amount of any sum received by Administrative Agent hereunder for the account of the Lenders and each Lender’s share thereof.
2.4
Conditions Precedent to Each Advance. It shall be an express condition precedent to each Lender’s obligation to make an Advance of each Loan that (i) the representations and warranties contained in Section 4.1 shall be true and correct in all material respects as of the date of such Advance (provided, however, that those representations and warranties expressly referring to another date shall be true and correct in all material respects as of such other date), (ii) no Event of Default or Potential Event of Default shall have occurred and be continuing, (iii) receipt by Administrative Agent of an executed Loan Advance Request Form in the form of Exhibit D attached hereto, (iv) no circumstance shall exist that could reasonably be expected to have a Material Adverse Change, (v) all governmental and third party approvals necessary in connection with the Loan and this Agreement shall have been obtained and be in full force and effect, and (vi) Administrative Agent’s satisfaction, in Administrative Agent’s sole discretion, with the results of Administrative Agent’s due diligence investigation, including, without limitation, review of the financial statements of Borrower dated no more than thirty (30) days prior to the funding of such Advance.
2.5
Conditions Precedent to the Tranche A Loan. It shall be an express condition precedent to a Lender’s obligation to make an Advance of the Tranche A Loan that Borrower shall provide or cause to be provided to Administrative Agent all of the following items:
(a)
UCC-1 financing statements designating Borrower, as debtor, and Administrative Agent, as secured party for the benefit of Lenders, for filing in the state of Borrower’s incorporation or formation, as applicable, the state of Borrower’s chief executive office, the place where Borrower transacts business or in any other state required by Administrative Agent with respect to all Collateral which may be perfected under the UCC by the filing of a UCC-1 financing statement, together with any other documents Administrative Agent deems necessary to evidence or perfect Administrative Agent’s security interest with respect to the Collateral;
(b)
a certificate as to authorizing resolutions and Operating Documents of Borrower with specimen signatures, substantially in the form of Exhibit B;
(c)
the Operating Documents of Borrower and good standing certificates from each of Borrower’s jurisdiction of organization and chief executive office location, and each jurisdiction in which Borrower is qualified to conduct business where the failure to be so qualified could reasonably be expected to result in a Material Adverse Change;
(d)
landlord waivers and bailee waivers in the form reasonably acceptable to Administrative Agent for each location where the Collateral in excess of $200,000 is located; insurance certificates and endorsements evidencing that the Borrower, its Subsidiaries, and the Collateral are insured in accordance with the requirements of Section 4.2(q) hereof;
(f)
a recent Lien search in each of the jurisdictions where the Borrower and each Subsidiary is organized and the assets of Borrower and each Subsidiary are located, and such searches reveal no Liens on any of the assets of Borrower or any Subsidiary, except for Permitted Liens;
(g)
payment in full of the applicable Commitment Fee and the Tranche A Documentation and Funding Fees;
(h)
a fully executed copy of this Agreement;
(i)
fully executed Account Control Agreements in respect of each of Borrower’s Deposit Accounts and Securities Accounts (other than Excluded Accounts) disclosed on the Perfection Certificate as of the date hereof;
(j)
fully executed copies of each other Loan Document;
(k)
a duly executed legal opinion of counsel to Borrower dated as of the Closing Date;
(l)
a copy of each applicable stockholders’ agreement, investors rights agreement, voting agreement, or other similar equity financing documents of Borrower, and any amendments thereto;
(m)
a completed Perfection Certificate for Borrower and each of its Subsidiaries;
(n)
a payoff letter with respect to the Existing Indebtedness; and
(o)
such other documents and completion of such other matters as Administrative Agent may reasonably deem necessary and appropriate.
2.6
Conditions Precedent to the Tranche B Loan. It shall be an express condition precedent to a Lender’s obligation to make the Advance of the Tranche B Loan that:
(a)
the Advance under the Tranche B Loan shall occur on or after achievement of the Tranche B Milestone and prior to the Loan Termination Date;
(b)
the amount of such Advance shall be at least Five Million Dollars ($5,000,000), provided that the amount of all Advances under the Tranche B Loan shall not exceed the Tranche B Availability Amount.
(c)
Administrative Agent shall have received payment in full of the Tranche B Documentation and Funding Fees and the applicable Commitment Fee; and
(d)
Borrower shall have achieved the Tranche B Milestone.
2.7
Conditions Precedent to the Tranche C Loan. It shall be an express condition precedent to a Lender’s obligation to make the Advance of the Tranche C Loan that:
(a)
the Advance under the Tranche C Loan shall occur on or after achievement of the Tranche C Milestone and prior to the Loan Termination Date;
(b)
the amount of such Advance shall be at least Five Million Dollars ($5,000,000), provided that the amount of all Advances under the Tranche C Loan shall not exceed the Tranche C Availability Amount.
(c)
Administrative Agent shall have received payment in full of the Tranche C Documentation and Funding Fees and the applicable Commitment Fee; and
(d)
Borrower shall have achieved the Tranche C Milestone.
2.8
Voluntary Prepayment. Borrower may prepay in whole or in part, the Loans at any time, subject to payment of the premium set forth below (“Prepayment Premium”). The calculated pre-payment amount shall include the outstanding principal due under each Loan at the time of retirement, any partially accrued interest thereon, and a Prepayment Premium based on the following schedule:
(a)
On or before the first anniversary of the Closing Date the Prepayment Premium shall be equal to three percent (3.00%) of the principal being repaid.
(b)
After the first anniversary of the Closing Date and on or before the second anniversary of the Closing Date the Prepayment Premium shall be equal to two percent (2.00%) of the principal being repaid.
(c)
After the second anniversary of the Closing Date and before the Maturity Date the Prepayment Premium shall be equal to one percent (1.00%) of the principal repaid.
2.9
Mandatory Prepayment. If a Change of Control occurs or the Loans are accelerated following the occurrence of an Event of Default, Borrower shall immediately pay to Administrative Agent, for the benefit of Lenders, an amount equal to the sum of: (i) all outstanding principal of the Loans plus accrued and unpaid interest thereon through the prepayment date, (ii) the Prepayment Premium, plus (iii) all other Obligations that are due and payable, including, without limitation, Administrative Agent Expenses and Lender’s Expenses and interest at the rate set forth in Section 2.2(c) with respect to any past due amounts.
2.10
End of Term Payment. On the Maturity Date or on the date of the earlier prepayment of the Loans by Borrower pursuant to Section 2.8 or Section 2.9 or acceleration of the balance of the Loans by Administrative Agent pursuant to Section 7.1, Borrower shall pay to Administrative Agent, for the benefit of Lenders, the End of Term Payment.
2.11
Proceeds of Collateral. Following the occurrence and during the continuance of an Event of Default, upon the written notice of Administrative Agent, all proceeds from the Collateral shall be immediately delivered to Administrative Agent, at the direction of the Required Lenders, may apply such proceeds and payments to any of the Obligations in such order as Administrative Agent may decide in its sole discretion.
(a)
Withholding. Payments received by the Administrative Agent or a Lender from Borrower hereunder will be made free and clear of and without deduction for any Taxes, except as required by any Governmental Authority, law, regulation or international agreement. If (i) at any time any Governmental Authority, law, regulation or international agreement requires Borrower to make any withholding or deduction of any Tax from any such payment or other sum payable hereunder to the Administrative Agent or a Lender, and (ii) such Tax is an Indemnified Tax, Borrower hereby covenants and agrees that the amount due from Borrower with respect to such payment or additional amounts payable pursuant to this Section 2.11(a) will be increased to the extent necessary to ensure that, after the making of such required withholding or deduction for Indemnified Taxes, Administrative Agent or such Lender receives a net sum equal to the sum which it would have received had no withholding or deduction for Indemnified Taxes been required and Borrower shall pay the full amount withheld or deducted to the relevant Governmental Authority. Borrower will, upon request, furnish the Administrative Agent with proof reasonably satisfactory to the Administrative Agent indicating that Borrower has made such withholding payment; provided, however, that Borrower need not make any withholding payment if the amount or validity of such withholding payment is contested in good faith by appropriate and timely proceedings and as to which payment in full is bonded or reserved against by Borrower.
The agreements and obligations of Borrower contained in this Section 2.11 shall survive the termination of this Agreement.
(b)
Borrower shall timely pay to the relevant Governmental Authority in accordance with applicable law, or at the option of the Administrative Agent timely reimburse it for the payment of, any Other Taxes.
(c)
The Borrower shall indemnify each Lender and Administrative Agent, within 10 days after demand therefor, for the full amount of any Indemnified Taxes (including Indemnified Taxes imposed or asserted on or attributable to amounts payable under this Section 2.11) payable or paid by such Lender or Administrative Agent, or required to be withheld or deducted from a payment to such Lender or Administrative Agent, and any reasonable expenses arising therefrom or with respect thereto, whether or not such Indemnified Taxes were correctly or legally imposed or asserted by the relevant Governmental Authority. The relevant Lender or Administrative Agent shall notify the Borrower of the imposition of any Indemnified Tax reasonably promptly after becoming aware of the imposition of such Tax. A certificate as to the amount of such payment or liability delivered to the Borrower by a Lender (with a copy to the Administrative Agent), or by the Administrative Agent on its own behalf or on behalf of a Lender, shall be conclusive absent manifest error.
(d)
Each Lender shall severally indemnify the Administrative Agent, within 10 days after demand therefor, for (i) any Indemnified Taxes attributable to such Lender (but only to the extent that Borrower has not already indemnified the Administrative Agent for such Indemnified Taxes and without limiting the obligation of Borrower to do so), (ii) any Taxes attributable to such Lender’s failure to comply with the provisions of Section 8.18 relating to the maintenance of a Participant Register and (iii) any Excluded Taxes attributable to such Lender, in each case, that are payable or paid by the Administrative Agent in connection with any Loan Document, and any reasonable expenses arising therefrom or with respect thereto, whether or not such Taxes were correctly or legally imposed or asserted by the relevant Governmental Authority. A certificate as to the amount of such payment or liability delivered to any Lender by the Administrative Agent shall be conclusive absent manifest error. Each Lender hereby authorizes the Administrative Agent to set off and apply any and all amounts at any time owing to such Lender under any Loan Document or otherwise payable by the Administrative Agent to the Lender from any other source against any amount due to the Administrative Agent under this Section 2.11(d).
(e)
As soon as practicable after any payment of Taxes by Borrower to a Governmental Authority pursuant to this Section 2.11, Borrower shall deliver to the Administrative Agent the original or a certified copy of a receipt issued by such Governmental Authority evidencing such payment, a copy of the return reporting such payment or other evidence of such payment reasonably satisfactory to the Administrative Agent.
(f)
If any Lender or the Administrative Agent determines, in its sole discretion exercised in good faith, that it has received a refund of any Indemnified Taxes as to which it has been indemnified pursuant to Section 2.11 (including by the payment of additional amounts pursuant to Section 2.11(a)), it shall pay to the indemnifying party an amount equal to such refund (but only to the extent of indemnity payments made under Section 2.11 with respect to the Indemnified Taxes giving rise to such refund), net of all out-of-pocket expenses (including Taxes) of such indemnifying party and without interest (other than any interest paid by the relevant Governmental Authority with respect to such refund). Such indemnifying party, upon the request of such indemnified party, shall promptly repay to such indemnified party the amount paid over pursuant to this Section 2.11(f) (plus any penalties, interest or other charges imposed by the relevant Governmental Authority) in the event that such indemnified party is required to repay such refund to such Governmental Authority. Notwithstanding anything to the contrary in this Section 2.11(f), in no event will the indemnified party be required to pay any amount to an indemnifying party pursuant to this Section 2.11(f) the payment of which would place the indemnified party in a less favorable net after-Tax position than the indemnified party would have been in if the Tax subject to indemnification and giving rise to such refund had not been deducted, withheld or otherwise imposed and the indemnification payments or additional amounts with respect to such Tax had never been paid.
This Section 2.11(f) shall not be construed to require any indemnified party to make available its Tax returns (or any other information relating to its Taxes that it deems confidential) to the indemnifying party or any other Person.
(g)
(i) Any Lender that is entitled to an exemption from or reduction of withholding Tax with respect to any payments made under any Loan Document shall deliver to the Borrower and the Administrative Agent, at the time or times reasonably requested by the Borrower or the Administrative Agent, such properly completed and executed documentation reasonably requested by the Borrower or the Administrative Agent as will permit such payments to be made without withholding or at a reduced rate of withholding. In addition, any Lender, if reasonably requested by the Borrower or the Administrative Agent, shall deliver such other documentation prescribed by applicable law or reasonably requested by the Borrower or the Administrative Agent as will enable the Borrower or the Administrative Agent to determine whether or not such Lender is subject to backup withholding or information reporting requirements. Notwithstanding anything to the contrary in the preceding two sentences, the completion, execution and submission of such documentation (other than such documentation set forth in Section 2.11(g)(ii)(A), (ii)(B), (ii)(D) and (ii)(E) below) shall not be required if in the Lender’s reasonable judgment such completion, execution or submission would subject such Lender to any material unreimbursed cost or expense or would materially prejudice the legal or commercial position of such Lender.
(i)
Without limiting the generality of the foregoing,
(A)
any Lender that is a U.S. Person shall deliver to the Borrower and the Administrative Agent (in such number of copies as shall be reasonably requested by the recipient) on or prior to the date on which such Lender becomes a Lender under this Agreement (and from time to time thereafter upon the reasonable request of the Borrower or the Administrative Agent) executed copies of Internal Revenue Service (“IRS”) Form W-9 (or any successor form) certifying that such Lender is exempt from U.S. federal backup withholding tax;
(B)
any Lender that is not a U.S. Person (a “Non-U.S. Lender”) shall, to the extent it is legally entitled to do so, deliver to the Borrower and the Administrative Agent (in such number of copies as shall be reasonably requested by the recipient) on or prior to the date on which such Non-U.S. Lender becomes a Lender under this Agreement (and from time to time thereafter upon the reasonable request of the Borrower or the Administrative Agent), whichever of the following is applicable:
(a)
in the case of a Non-U.S. Lender claiming the benefits of an income tax treaty to which the United States is a party, (x) with respect to payments of interest under any Loan Document, executed copies of IRS Form W-8BEN or IRS Form W-8BEN-E establishing an exemption from, or reduction of, U.S. federal withholding Tax pursuant to the “interest” article of such tax treaty and (y) with respect to any other applicable payments under any Loan Document, IRS Form W-8BEN or IRS Form W-8BEN- E establishing an exemption from, or reduction of, U.S. federal withholding Tax pursuant to the “business profits” or “other income” article of such tax treaty;
(b)
executed copies of IRS Form W-8ECI (or any successor form);
(c)
in the case of a Non-U.S. Lender claiming the benefits of the exemption for portfolio interest under Section 871(h) or Section 881(c) of the Code, (x) a certificate substantially in the form of Exhibit G-1 to the effect that such Non-U.S. Lender is not a “bank” within the meaning of Section 881(c)(3)(A) of the Code, a “10-percent shareholder” of the Borrower within the meaning of Section 881(c)(3)(B) of the Code, a “controlled foreign corporation” related to Borrower as described in Section 881(c)(3)(C) of the Code (a “U.S. Tax Compliance Certificate”) and (y) executed copies of IRS Form W- 8BEN or IRS Form W-8BEN-E, as applicable (or any successor form); or to the extent a Non-U.S.
(d)
Lender is not the beneficial owner, executed copies of IRS Form W-8IMY, accompanied by IRS Form W-8ECI, IRS Form W-8BEN, IRS Form W-8BEN-E, a certificate substantially in the form of Exhibit G-2 or Exhibit G-3, IRS Form W-9, and/or other certification documents from each beneficial owner, as applicable; provided that if the Non-U.S. Lender is a partnership and one or more direct or indirect partners of such Non-U.S. Lender are claiming the portfolio interest exemption, such Non-U.S. Lender may provide a certificate substantially in the form of Exhibit G-4 on behalf of each such direct and indirect partner;
(C)
any Non-U.S. Lender shall, to the extent it is legally entitled to do so, deliver to the Borrower or the Administrative Agent (in such number of copies as shall be reasonably requested by the recipient) on or prior to the date on which such Non-U.S. Lender becomes a Lender under this Agreement (and from time to time thereafter upon the reasonable request of the Borrower or the Administrative Agent), executed copies of any other form prescribed by applicable law as a basis for claiming exemption from or a reduction in U.S. federal withholding Tax, duly completed, together with such supplementary documentation as may be prescribed by applicable law to permit the Borrower or the Administrative Agent to determine the withholding or deduction required to be made;
(D)
each Lender and the Administrative Agent shall deliver to the Borrower and the Administrative Agent at the time or times prescribed by law and at such time or times reasonably requested by the Borrower or the Administrative Agent such documentation prescribed by applicable law (including as prescribed by Section 1471(b)(3)(C)(i) of the Code) and such additional documentation reasonably requested by the Borrower or the Administrative Agent as may be necessary for the Borrower and the Administrative Agent to (i) comply with their obligations under FATCA and (ii) determine whether such Lender (or the Administrative Agent, as applicable) has complied with such Lender’s (or the Administrative Agent’s, as applicable) obligations under FATCA or to determine the amount to deduct and withhold from such payment. Solely for purposes of this clause (D), “FATCA” shall include any amendments made to FATCA after the date of this Agreement; and
(E)
the Administrative Agent, and any successor or supplemental Administrative Agent, shall deliver to the Borrower (in such number of copies as shall be requested by the recipient) on or prior to the date on which the Administrative Agent becomes the administrative agent hereunder or under any other Loan Document (and from time to time thereafter upon the reasonable request of the Borrower) executed copies of either (i) IRS Form W-9 (or any successor form) or (ii) a U.S. branch withholding certificate on IRS Form W-8IMY (or any successor form) evidencing its agreement with the Borrower to be treated as a U.S. Person (with respect to amounts received on account of any Lender) and IRS Form W-8ECI (with respect to amounts received on its own account), with the effect that, in either case, the Borrower will be entitled to make payments hereunder to the Administrative Agent without withholding or deduction on account of U.S. federal withholding Tax.
Each Lender and the Administrative Agent agrees that if any documentation it previously delivered expires or becomes obsolete or inaccurate in any respect, it shall promptly update and deliver such form or certification to the Borrower and the Administrative Agent or promptly notify the Borrower and the Administrative Agent in writing of its legal ineligibility to do so.
(h)
For the avoidance of doubt, the term “applicable law” includes FATCA.
(i)
The agreements and obligations of Borrower contained in this Section 2.11 shall survive the termination of this Agreement, the resignation and/or replacement of the Administrative Agent, any assignment of rights by, or the replacement of, a Lender, the termination of the Commitments and the repayment, satisfaction or discharge of all other Obligations.
(j)
Borrower and the Lenders hereby acknowledge and agree that, for U.S. federal income tax purposes, the issue price (within the meaning of Section 1273(b) of the Code) of the Loan will be determined pursuant to Section 1272 through 1275 of the Code and the Treasury Regulations thereunder, including Section 1.1273-2(h)(1) of the Treasury Regulations
2.13
Apportionment of Payments. All payments of principal and interest in respect of outstanding Loans, all payments of fees and all other payments in respect of any other Obligations, shall be allocated by the Administrative Agent among such of the Lenders as are entitled thereto, in proportion to their respective Pro Rata Shares, or as otherwise provided herein.
2.14
Defaulting Lenders. Notwithstanding anything to the contrary contained in this Agreement, if any Lender becomes a Defaulting Lender, then, until such time as such Lender is no longer a Defaulting Lender, to the extent permitted by applicable law:
(a)
Such Defaulting Lender’s right to approve or disapprove any amendment, waiver or consent with respect to this Agreement shall be restricted as set forth in Section 5.10.
(b)
Administrative Agent shall not be obligated to transfer to such Defaulting Lender any payments made by Borrower to Administrative Agent for such Defaulting Lender’s benefit, and, in the absence of such transfer to such Defaulting Lender, the Administrative Agent shall transfer any such payments to each other non-Defaulting Lender ratably in accordance with their Pro Rata Shares (without giving effect to the Pro Rata Shares of such Defaulting Lender) (but only to the extent that such Defaulting Lender’s Loans were funded by the other Lenders).
(c)
The operation of this Section shall not be construed to increase or otherwise affect the Commitments of any Lender, to relieve or excuse the performance by such Defaulting Lender or any other Lender of its duties and obligations hereunder, or to relieve or excuse the performance by the Borrower of its duties and obligations hereunder to Administrative Agent or to the Lenders other than such Defaulting Lender.
2.15
Covenant to Deliver; Post-Closing Matters.
(a)
Subject to clause (b) hereof, Borrower hereby agrees to deliver to Administrative Agent each item required to be delivered to Administrative Agent under this Agreement as a condition precedent to any Loan. Borrower expressly agrees that a Loan made prior to the receipt by Administrative Agent of any such item shall not constitute a waiver by Administrative Agent of Borrower’s obligation to deliver such item, and the making of any Loan in the absence of a required item shall be in Administrative Agent’s sole discretion.
(b)
Unless otherwise provided in writing, as soon as possible following the Closing Date (but in no event later than thirty (30) days following the Closing Date (or such later date as Administrative Agent may agree)), Borrower shall provide to Administrative Agent insurance endorsements as required by Section 4.2(q), in form and substance reasonably satisfactory to Administrative Agent.
ARTICLE 3
CREATION OF SECURITY INTEREST; COLLATERAL
3.1
Grant of Security Interests. Borrower grants to Administrative Agent, for the benefit of the Lenders, a valid, continuing security interest in all presently existing and hereafter acquired or arising Collateral in order to secure prompt, full and complete payment of any and all Obligations and in order to secure prompt, full and complete performance by Borrower of each of its covenants and duties under each of the Loan Documents. The “Collateral” shall mean and include all right, title, interest, claims and demands of Borrower in the following:
(a)
All goods (and embedded computer programs and supporting information included within the definition of “goods” under the UCC) and equipment now owned or hereafter acquired, including all laboratory equipment, computer equipment, office equipment, machinery, fixtures, vehicles (including motor vehicles and trailers), and other equipment and any interest in any of the foregoing, and all attachments, accessories, accessions, replacements, substitutions, additions, and improvements to any of the foregoing, wherever located;
(b)
All inventory now owned or hereafter acquired, including all merchandise, raw materials, parts, supplies, packing and shipping materials, work in process and finished products including such inventory as is temporarily out of Borrower’s custody or possession or in transit and including any returns upon any accounts or other proceeds, including insurance proceeds, resulting from the sale or disposition of any of the foregoing and any documents of title representing any of the above, and Borrower’s books relating to any of the foregoing;
(c)
All contract rights and general intangibles (including Intellectual Property), now owned or hereafter acquired, including goodwill, license agreements, franchise agreements, blueprints, drawings, purchase orders, customer lists, route lists, infringements, claims, software, computer programs, computer disks, computer tapes, literature, reports, catalogs, design rights, income tax refunds, payment intangibles, commercial tort claims, payments of insurance and rights to payment of any kind;
(d)
All now existing and hereafter arising accounts, contract rights, royalties, license rights, license fees and all other forms of obligations owing to Borrower arising out of the sale or lease of goods, the licensing of technology or the rendering of services by Borrower (subject, in each case, to the contractual rights of third parties to require funds received by Borrower to be expended in a particular manner), whether or not earned by performance, and any and all credit insurance, guaranties, and other security therefor, as well as all merchandise returned to or reclaimed by Borrower and Borrower’s books relating to any of the foregoing;
(e)
All documents, cash, Deposit Accounts, letters of credit and letters of credit rights (whether or not the letter of credit is evidenced by a writing) and other supporting obligations, certificates of deposit, instruments, promissory notes, chattel paper (whether tangible or electronic) and investment property, including all securities, whether certificated or uncertificated, security entitlements, Securities Accounts, commodity contracts and commodity accounts, and all financial assets held in any Securities Account or otherwise, wherever located, now owned or hereafter acquired and Borrower’s books relating to the foregoing; and
(f)
To the extent not covered by clauses (a) through (e), all other personal property of the Borrower, whether tangible or intangible, and any and all rights and interests in any of the above and the foregoing and, any and all claims, rights and interests in any of the above and all substitutions for, additions and accessions to and proceeds thereof, including insurance, condemnation, requisition or similar payments and proceeds of the sale or licensing of Intellectual Property and all of Borrower’s books and records related to any items of other Collateral.
Notwithstanding the foregoing, or anything to the contrary herein, the Collateral does not include (i) any Excluded Account, (ii) the assets to be sold in connection with the Brady Equipment Sale, (iii) “intent- to-use” trademarks at all times prior to the first use thereof, whether by the actual use thereof in commerce, the recording of a statement of use with the United States Patent and Trademark Office or otherwise, but only to the extent and solely during such period that granting a security interest in the “intent-to-use” trademarks would be contrary to applicable law or may interfere with Borrower’s rights to obtain and maintain such trademarks; provided that after such period, Borrower acknowledges that such interest in such trademark application or trademark shall be subject to a security interest in favor of Administrative Agent and shall be included in the Collateral, (iv) (x) rights of Borrower held under a license that are not assignable by their terms without the consent of the licensor thereof (but only to the extent such restriction on assignment is enforceable under applicable law), and (y) any interest of Borrower as a lessee or sublessee under a real property lease or an Equipment lease if Borrower is prohibited by the terms of such lease from granting a security interest in such lease or under which such an assignment or Lien would cause a default to occur under such lease (but only to the extent that such prohibition is enforceable under all applicable laws including, without limitation, the UCC); provided, however, that upon termination of such prohibition, such interest shall immediately become Collateral without any action by Borrower or Administrative Agent; provided, in each case, that no asset or property shall be excluded from the Collateral to the extent the restriction described in the foregoing clauses (x) and (y) would be rendered ineffective pursuant to Section 9-406, 9-407, 9-408 or 9-409 of the UCC or any other applicable law or principles of equity, or to the extent that any necessary consents or waivers have been obtained to allow the security interest in such asset or property notwithstanding such restriction, (v) leased equipment or equipment financed (and related software) by indebtedness with a third party (and any accessions, attachments, replacements or improvements thereon) and specifically identifiable sale proceeds thereof (but only in the amount of the lien secured thereby) that is subject to a lien that is permitted pursuant to the definition of “Permitted Liens”, provided, that (a) the foregoing exclusion shall apply only to the extent the applicable documents relating to such financing prohibits the granting of a security interest in favor of Administrative Agent and (b) upon the release of any such lien, such equipment (and any accessions, attachments, replacements or improvements thereon), software and specifically identifiable sale proceeds shall automatically be deemed to be Collateral hereunder and shall be subject to the security interest granted herein and (vi) with respect to stock in foreign Subsidiaries and so long as Borrower identifies to Administrative Agent a present and existing adverse tax consequence as a direct result of a grant of more than sixty-five percent (65.0%), more than sixty-five percent (65.0%) of the presently existing and hereafter arising issued and outstanding shares of capital stock owned by Borrower of any foreign Subsidiary which shares entitle the holder thereof to vote for directors or any other matter.
3.2
After-Acquired Property. If Borrower shall at any time acquire a commercial tort claim with a value in excess of $500,000, as defined in the UCC, Borrower shall promptly notify Administrative Agent in writing signed by Borrower of the brief details thereof and grant to Administrative Agent in such writing a security interest therein and in the proceeds thereof, all upon the terms of this Agreement, with such writing to be in form and substance reasonably satisfactory to Administrative Agent.
3.3
Location and Possession of Collateral. The Collateral is and shall remain in the possession of Borrower or its bailee at its location as set forth in the Perfection Certificate (the “Permitted Locations”) or any other location as long as Borrower provides Administrative Agent with notice of such new location within 10 days of entry into such location and, in the event that the Collateral at any new location is valued in excess of Two Hundred Thousand Dollars ($200,000.00) in the aggregate, at Administrative Agent’s election, Borrower shall use commercially reasonable efforts to cause such bailee or landlord, as applicable, to execute and deliver a bailee waiver or landlord waiver, as applicable, in form and substance reasonably satisfactory to Administrative Agent. Borrower shall remain in full possession, enjoyment and control of the Collateral (except only as may be otherwise required by Administrative Agent for perfection of the security interests therein created hereunder) and so long as no Event of Default has occurred and is continuing, shall be entitled to manage, operate and use the same and each part thereof with the rights and franchises appertaining thereto; provided that the possession, enjoyment, control and use of the Collateral shall at all times be subject to the observance and performance of the terms of this Agreement.
3.4
Delivery of Additional Documentation Required. Borrower shall from time to time execute and deliver to Administrative Agent, at the request of Administrative Agent, all financing statements and other documents Administrative Agent may reasonably request, in form satisfactory to Administrative Agent, to perfect and continue Administrative Agent’s perfected security interests in the Collateral and in order to consummate fully all of the transactions contemplated under the Loan Documents.
3.5
Right to Inspect. Administrative Agent (through any of its officers, employees, or agents) shall have the right, upon reasonable prior notice, from time to time during Borrower’s usual business hours, to inspect the books and records of Borrower and Subsidiaries and to make copies thereof and to inspect, test, and appraise the Collateral in order to verify Borrower’s financial condition or the amount, condition of, or any other matter relating to, the Collateral up to once per year (unless an Event of Default has occurred and is continuing, in which case such inspections and appraisals are in Lender’s discretion).
3.6
Intellectual Property. Borrower shall notify Administrative Agent before the federal registration or filing by Borrower of any copyright or copyright application and shall promptly execute and deliver to Lender any grants of security interests in same, in form acceptable to Administrative Agent, to file with the United States Copyright Office. In addition, Borrower shall deliver to Administrative Agent within 30 days after the end of each calendar quarter, a report (each, a “Patent and Trademark Report”) reflecting (i) the patents, patent applications, trademarks and trademark applications that were registered or filed by Borrower during such quarter and (ii) Intellectual Property that Borrower deems “immaterial” to its business, if any, and shall promptly execute and deliver to Administrative Agent, on behalf of the Lenders, any grants of security interests in same, in form reasonably acceptable to Administrative Agent, to file with the United States Patent and Trademark Office.
3.7
Protection of Intellectual Property. With respect to Intellectual Property that is material to the Borrower’s business, Borrower shall and shall cause its Subsidiaries to:
(a)
protect, defend and maintain the validity and enforceability of its Intellectual Property and promptly advise Administrative Agent in writing of material infringements;
(b)
not allow any Intellectual Property material to Borrower’s or its Subsidiaries business to be abandoned, forfeited or dedicated to the public without Administrative Agent’s written consent;
(c)
provide written notice to the Administrative Agent within ten (10) days of entering or becoming bound by any Restricted License (other than over-the-counter software that is commercially available to the public); and
(d)
take such commercially reasonable steps as Administrative Agent requests to obtain the consent of, or waiver by, any person whose consent or waiver is necessary for (i) any Restricted License to be deemed “Collateral” and for Administrative Agent to have a security interest in it that might otherwise be restricted or prohibited by law or by the terms of any such Restricted License, whether now existing or entered into in the future, and (ii) Administrative Agent to have the ability in the event of a liquidation of any Collateral to dispose of such Collateral in accordance with the Administrative Agent’s rights and remedies under this Agreement and the other Loan Documents.
[***]
ARTICLE 4
REPRESENTATIONS, WARRANTIES AND COVENANTS
4.1
Representations and Warranties. Borrower hereby warrants, represents and covenants that:
(a)
Borrower and each Subsidiary is duly organized, validly existing and in good standing under the laws of the state set forth in the Perfection Certificate. Borrower and each Subsidiary is duly qualified to do business and is in good standing in every other jurisdiction where the nature of its business requires it to be qualified, except where failure to be so qualified would not result in a Material Adverse Change, and is not subject to any bankruptcy, insolvency or other similar proceedings. Borrower’s and each Subsidiary’s chief executive office, principal place of business and the place where Borrower maintains its records concerning the Collateral are located at the addresses set forth in the Perfection Certificate. The Collateral is presently located at the address set forth on the Perfection Certificate or as otherwise agreed by Administrative Agent pursuant to Section 3.3;
(b)
Borrower and each Subsidiary has full power, authority and legal right to execute, deliver and perform each Loan Document to which it is a party, and the execution, delivery and performance hereof and thereof have been duly authorized by all necessary action; Each Loan Document has been duly executed and delivered by Borrower and each constitutes a legal, valid and binding obligation of Borrower and each Subsidiary party thereto, enforceable in accordance with its terms, except as enforceability may be limited by bankruptcy, insolvency or other laws affecting enforcement of creditors’ rights generally and general equitable principles;
(d)
The execution, delivery and performance of the Loan Documents (i) are not in contravention of any material agreement or indenture by which Borrower or any Subsidiary is bound, or by which its properties may be affected, (ii) do not require any shareholder approval, or any approval or consent of, or filing or registration with, any governmental body or regulatory authority or agency (other than the filing of UCC financing statements and filings with the United States Patent and Trademark Office and United States Copyright Office, in connection with the registration of the security interest granted hereunder), or any approval or consent of any trustees or holders of any of its indebtedness or obligations, unless such approval or consent has been obtained and (iii) do not contravene any material law, regulation, judgment or decree applicable to it or its Operating Documents;
(e)
Borrower is not a “bank holding company” or a direct or indirect subsidiary of a “bank holding company” as defined in the Bank Holding Company Act of 1956, as amend, and Regulation Y thereunder of the Board of Governors of the Federal Reserve System. Borrower is not an “investment company” or a company controlled by an “investment company” under the Investment Company Act of 1940. Borrower is not engaged in the business of extending credit for the purpose of purchasing or carrying margin stock (as defined in Regulation U of the Board of Governors of the Federal Reserve System) and no proceeds of any Loan will be used to purchase or carry margin stock or to extend credit to others for the purpose of purchasing or carrying any margin stock;
(f)
To Borrower’s Knowledge, Borrower and each Subsidiary is in compliance with all requirements of law and orders, rules or regulations of any regulatory authority except to the extent that failure to be in compliance would not reasonably be expected to cause a Material Adverse Change, and no such requirement applicable to Borrower or any Subsidiary or any item of Collateral could reasonably be expected to cause a Material Adverse Change;
(g)
Borrower is the owner and holder of all right, title and interest in and to the Collateral (other than the right, title and interests granted under the Permitted Liens), and Borrower has not assigned or pledged and hereby covenants that it will not assign or pledge, so long as this Agreement shall remain in effect, the whole or any part of the rights in the Collateral hereby and thereby assigned, to anyone other than Administrative Agent, its designee, its successors or assigns, other than Permitted Liens;
(h)
Borrower has good and marketable title to the Collateral, and the Collateral is free and clear of all Liens, claims and encumbrances, other than Permitted Liens;
(i)
Borrower has delivered to Administrative Agent copies of the most recent annual reviewed financial statements and most recent monthly and quarterly unaudited financial statements required to be delivered pursuant to Section 4.2(f) hereof, or as may hereafter be delivered in connection with the Loans (the “Financial Statements”). Since the date of the last Financial Statement provided to Administrative Agent, no event has occurred which would have a Material Adverse Change on Borrower or any Subsidiary. The Financial Statements are true and correct and fairly present in all material respects the financial condition of Borrower and its Subsidiaries;
(j)
No default or event of default has occurred and is continuing under or with respect to any contractual obligation, loan or indenture of Borrower or any Subsidiary in which the default could reasonably be expected to result in a Material Adverse Change;
(k)
Other than customary prosecution proceedings before the USPTO and customary licensing application proceedings with federal and state regulatory authorities, no action, suit, litigation, or proceeding of or before any arbitrator or governmental or regulatory authority is pending or, to the Knowledge of Borrower threatened, by or against Borrower or against any of its property or assets which could reasonably be expected to result in a Material Adverse Change; To Borrower’s Knowledge, no facilities or properties leased or operated by Borrower contains any “hazardous materials” in amount or concentrations that could constitute a violation of any federal, state or local law, rule, regulation, order or permit (the “Environmental Laws”).
(l)
Borrower has not received notice of any suspected or actual violations of any material Environmental Laws and Borrower’s business has been operated in conformity with all Environmental Laws in all material respects;
(m)
Borrower has no Subsidiaries other than those listed on the Perfection Certificate. Neither Borrower nor any Subsidiary has done business in the past 5 years under any name other than that specified on the Perfection Certificate;
(n)
To the best of Borrower’s Knowledge, as of the date hereof and at all times throughout the term of this Agreement, including after giving effect to any transfers of interests permitted pursuant to the Loan Documents, (1) none of the funds or other assets of Borrower, any of their Affiliates constitute (or will constitute) property of, or are (or will be) beneficially owned, directly or indirectly, by any Blocked Person; (2) no Blocked Person has (or will have) any interest of any nature whatsoever in Borrower, in their Affiliates, with the result that the investment in the respective party (whether directly or indirectly), is prohibited by applicable law or the Loans are in violation of applicable law; and (3) none of the funds of Borrower, or of their Affiliates have been (or will be) derived from any unlawful activity with the result that the investment in the respective party (whether directly or indirectly), is prohibited by applicable law or the Loans are in violation of applicable law;
(o)
To Borrower’s Knowledge, the Property of Borrower and the Collateral are insured with financially sound and reputable insurance companies in such amounts, with such deductibles and covering such risks as are customarily carried by companies engaged in similar businesses and owning similar properties in localities where the Borrower operates;
(p)
To Borrower’s Knowledge, Borrower owns, or is licensed to use, all Intellectual Property necessary for the conduct of its business as currently conducted or proposed to be conducted. No material claim has been asserted and is pending by any other person or entity challenging the use, validity or effectiveness of any Intellectual Property, nor does the Borrower have Knowledge of any basis for any such claim;
(q)
Borrower and each Subsidiary has filed all federal, state and other tax returns that are required to be filed and has paid all taxes shown thereon to be due, together with applicable interest and penalties, and all other taxes, fees or other charges imposed on it or any of its property by any governmental or regulatory authority except (a) to the extent such taxes, fees and/or other charges are being contested in good faith by appropriate proceedings promptly instituted and diligently conducted, so long as such reserve or other appropriate provision, if any, as shall be required in conformity with GAAP shall have been made therefor, or (b) if such taxes, assessments, deposits and contributions do not, individually or in the aggregate, exceed $250,000 at any time. No tax Liens have been filed, and, to the Knowledge of Borrower, no claim is being asserted, with respect to any such tax, fee or other charge other than Permitted Liens. Neither Borrower nor any Subsidiary is a party to any tax sharing agreement;
(r)
This Agreement creates in favor of Administrative Agent, for the benefit of the Lenders, a legal, valid and continuing and enforceable security interest in the Collateral, the enforceability of which is subject to applicable bankruptcy, insolvency, reorganization, moratorium or other laws affecting creditor’s rights generally and subject to general principles of equity. To the Knowledge of Borrower, upon Administrative Agent filing UCC-1 financing statements with the central filing location in the state of Borrower’s formation or incorporation and/or the obtaining of “control” (as defined under the UCC) through an Account Control Agreement or otherwise, Administrative Agent, for the benefit of the Lenders, will have a perfected first priority Lien on and security interest in the Collateral subject to Permitted Liens;
(t)
Borrower and its Subsidiaries, on a consolidated basis are, and after giving effect to the incurrence of the debt evidenced by this Agreement and all obligations hereunder will be, Solvent; (i) The Perfection Certificate lists all of Borrower’s and each Subsidiary’s patents and pending applications, registered trademarks and pending applications, registered domain names, registered copyrights and pending applications and material Intellectual Property licenses owned by Borrower and each Subsidiary; (ii) all of Borrower’s and each Subsidiary’s Intellectual Property that is material to its business is valid, subsisting, unexpired and enforceable and has not been abandoned; (iii) except as described on the Perfection Certificate and except for Permitted Liens, Borrower and each Subsidiary is the exclusive owner of all right, title and interest in and to, or has the right to use, all of such Borrower’s or Subsidiary’s Intellectual Property that is material to its business; (iv) consummation and performance of this Agreement will not result in the invalidity, unenforceability or impairment of any of Borrower’s or any Subsidiary’s Intellectual Property that is material to its business, or in default or termination of any material Intellectual Property license of Borrower or any Subsidiary; (v) except as described on the Perfection Certificate or in connection with customary prosecution proceedings before the USPTO, there are no outstanding holdings, decisions, consents, settlements, decrees, orders, injunctions, rulings or judgments that would limit, cancel or question the validity or enforceability of any of Borrower’s or any Subsidiary’s Intellectual Property that is material to its business or Borrower’s or such Subsidiary’s rights therein or use thereof; (vi) to Borrower’s Knowledge, except as described on the Perfection Certificate, the operation of Borrower’s and each Subsidiary’s business and Borrower’s or such Subsidiary’s use of Intellectual Property material to its business in connection therewith, does not infringe or misappropriate the intellectual property rights of any other person or entity; (vii) except as described in the Perfection Certificate or in connection with customary prosecution proceedings before the USPTO, no action or proceeding is pending or, to Borrower’s Knowledge, threatened (1) seeking to limit, cancel or question the validity of any of Borrower’s or any Subsidiary’s Intellectual Property, (2) which, if adversely determined, could be reasonably expected to cause a Material Adverse Change on the value of any such Intellectual Property or (3) alleging that any such Intellectual Property, or Borrower’s or such Subsidiary’s use thereof in the operation of its business, infringes or misappropriates the intellectual property rights of any person or entity and (viii) to Borrower’s Knowledge, there has been no Material Adverse Change on Borrower’s or any Subsidiary’s rights in its material trade secrets as a result of any unauthorized use, disclosure or appropriation by or to any person, including Borrower’s and each Subsidiary’s current and former employees, contractors and agents; and
(u)
No statement or information contained in this Agreement or any document or certificate executed or delivered, or hereafter delivered, in connection with this Agreement or the Loans contains or will contain any untrue statement of a material fact or omits to state a material fact necessary to make the statements contained herein or therein not misleading (it being recognized by Lender that projections and forecasts provided by Borrower in good faith and based upon reasonable assumptions are not to be viewed as facts and that actual results during the period or periods covered by any such projections and forecasts may differ from the projected or forecasted results).
4.2
Affirmative Covenants of Borrower. Borrower shall, and shall cause each of its Subsidiaries to, do all of the following, so long as any of the Loan Documents remain outstanding:
(a)
maintain its corporate existence and its good standing in its jurisdiction of incorporation and maintain qualification in each jurisdiction in which the failure to so qualify could reasonably be expected to cause a Material Adverse Change;
(b)
maintain in force all licenses, approvals, agreements and Governmental Approvals, the loss of which could reasonably be expected to cause a Material Adverse Change;
(c)
comply with all statutes, laws, ordinances and government rules and regulations to which it is subject, noncompliance with which could reasonably be expected to cause a Material Adverse Change;
(d)
if required by applicable law, pay and discharge or cause to be paid and discharged, all sales, use, rental and personal property or similar taxes and fees (excluding any taxes on any Lender’s net income) which arise and are due prior to each Advance in connection with the Collateral except (a) to the extent such taxes are being contested in good faith by appropriate proceedings promptly instituted and diligently conducted, so long as such reserve or other appropriate provision, if any, as shall be required in conformity with GAAP shall have been made therefor, or (b) if such taxes, assessments, deposits and contributions do not, individually or in the aggregate, exceed $250,000 at any time;
(e)
assist Administrative Agent in obtaining and filing UCC-1 financing statements against the Collateral and Account Control Agreements to the extent that Administrative Agent deems such action necessary or desirable;
(f)
deliver the following to Administrative Agent:
(i)
as soon as available, but no later than thirty (30) days after the last day of each month:
(A)
copies of Borrower’s bank statements on all Deposit Accounts
(B)
copies of any material Governmental Approvals obtained by Borrower or any of its Subsidiaries;
(C)
written notice of the commencement of, and any material development in, the proceedings contemplated by Section 4.2(i) hereof;
(D)
a duly completed Compliance Certificate signed by a Responsible Officer of Borrower, certifying that as of the end of such month Borrower was in full compliance with all of the terms and conditions of this Agreement;
(E)
written notice of any litigation or governmental proceedings pending or threatened (in writing) against Borrower or any of its Subsidiaries, which could reasonably be expected to result in damages or costs to Borrower or any of its Subsidiaries in excess of One Million Dollars ($1,000,000.00); and
(F)
written notice of all returns, recoveries, disputes and claims regarding Inventory that involve more than One Million Dollars ($1,000,000.00) individually or in the aggregate in any calendar year;
(ii)
(within forty five (45) days after the end of each fiscal quarter:
(A)
unaudited financial statements pertaining to the results of operations for the month then ended covering the consolidated operations of Borrower and its Subsidiaries for such month and certified as true and correct by a Responsible Officer of Borrower, consisting of a consolidated balance sheet, income statement and cash flow statement, prepared in accordance with GAAP applied on a consistent basis subject to normal year end audit adjustments and the absence of footnotes; provided that to the extent the foregoing documents are included in materials otherwise filed with the Securities and Exchange Commission, such documents shall be deemed to have been delivered on the date on which Borrower posts such documents, or provides a link thereto, on Borrower’s website;
(B)
any updates to Sections 4 (Encumbrances and Liens) and 13 (Indebtedness) of the Perfection Certificate to reflect amendments, modifications and updates to these sections to the extent such amendments, modifications and updates are permitted by one or more specific provisions in the Agreement;
(C)
together with the quarterly financial reports, reports as to the following, in a form acceptable to Administrative Agent: accounts receivable, accounts payable aging, and primary key performance indicators, in each case, in form and substance satisfactory to Administrative Agent; provided that to the extent the foregoing documents are included in materials otherwise filed with the Securities and Exchange Commission, such documents shall be deemed to have been delivered on the date on which Borrower posts such documents, or provides a link thereto, on Borrower’s website;
(iii)
(within one hundred eighty (180) days following the end of each fiscal year, a copy of Borrower’s annual, audited financial statements consisting of a consolidated balance sheet, income statement and cash flow statement prepared in conformity with GAAP applied on a basis consistent with that of the preceding fiscal year and presenting fairly Borrower’s financial condition as at the end of that fiscal year and the results of its operations for the twelve (12) month period then ended and certified as true and correct by Borrower’s chief financial officer, together with an unqualified opinion (other than a qualification as to a going concern typical for venture backed companies similar to Borrower) on the financial statements from an independent certified public accounting firm acceptable to Administrative Agent in its reasonable discretion; provided that Borrower’s certified public accounting firm as of the Closing Date, and any such firm of national recognized standing, is acceptable to Administrative Agent; provided further that to the extent the foregoing documents are included in materials otherwise filed with the Securities and Exchange Commission, such documents shall be deemed to have been delivered on the date on which Borrower posts such documents, or provides a link thereto, on Borrower’s website;
(iv)
within thirty (30) days of the effective date or filing date thereof, a copy of any amendment to Borrower’s Operating Documents; provided that to the extent the foregoing documents are included in materials otherwise filed with the Securities and Exchange Commission, such documents shall be deemed to have been delivered on the date on which Borrower posts such documents, or provides a link thereto, on Borrower’s website;
(v)
as requested by Administrative Agent, have Borrower’s chief financial or chief operating officer participate in quarterly management update calls with Administrative Agent to discuss such information about the operations and financial condition of the business of the Borrower as Administrative Agent shall reasonably inquire into, at such times reasonably scheduled by Administrative Agent; and
(vi)
deliver such other financial information as Administrative Agent shall reasonably request from time-to-time.
(g)
deliver to Administrative Agent within ten (10) days after approval by the Borrower’s board of directors, and in any event no later than within sixty (60) days after the end of each fiscal year of Borrower, annual operating budgets and financial projections approved by the Borrower’s board of directors, in a form acceptable to Administrative Agent;
(h)
deliver to Administrative Agent, promptly as they are available and in any event: (i) at the time of filing of Borrower’s Form 10-K with the Securities and Exchange Commission after the end of each fiscal year of Borrower, the financial statements of Borrower filed with such Form 10-K; and (ii) at the time of filing of Borrower’s Form 10-Q with the Securities and Exchange Commission after the end of each of the first three fiscal quarters of Borrower, the consolidated financial statements of Borrower filed with such Form 10-Q; provided that to the extent the foregoing documents are included in materials otherwise filed with the Securities and Exchange Commission, such documents shall be deemed to have been delivered on the date on which Borrower posts such documents, or provides a link thereto, on Borrower’s website;
(i)
deliver to Administrative Agent (A) promptly upon becoming available, copies of all statements, reports and notices sent or made available generally by Borrower to its security holders and (B) immediately upon receipt of written notice thereof, a report of any material legal actions pending or threatened against Borrower or any of its Subsidiaries or the commencement of any action, proceeding or governmental investigation involving Borrower or any of its Subsidiaries is commenced that is reasonably expected to result in damages or costs to Borrower or any of its Subsidiaries in excess of One Million Dollars ($1,000,000.00); provided that to the extent the foregoing documents are included in materials otherwise filed with the Securities and Exchange Commission, such documents shall be deemed to have been delivered on the date on which Borrower posts such documents, or provides a link thereto, on Borrower’s website;
(j)
deliver the following to Administrative Agent: (i) as of the date of each Compliance Certificate, a list of all Intellectual Property owned or licensed to Borrower and a list of items within the definition of Collateral hereunder since the date of the last Compliance Certificate in such form as reasonably required by Administrative Agent; (ii) promptly after the same are sent by Borrower, copies of any statements, reports, or correspondence required to be delivered to any other Lender; (iii) promptly upon receipt of the same, copies of all notices, requests and other documents received by any other party pursuant any other material contract, instrument, indenture regarding or relating to any breach or default alleged by or against any party thereto or any other event that could materially impair the value of the interests or rights of Administrative Agent or any Lender or could otherwise be reasonably expected to cause a Material Adverse Change; and (iv) such other information respecting the business, condition (financial or otherwise), operations, performance, properties or prospects of Borrower as Administrative Agent may from time to time reasonably request;
(k)
make due and timely payment or deposit of all federal, state, and local taxes, assessments, or contributions required of it by law or imposed upon any Property belonging to it, and will execute and deliver to Administrative Agent, on demand, appropriate certificates attesting to the payment or deposit thereof; and Borrower will make timely payment or deposit of all tax payments and withholding taxes required of it by applicable laws, including those laws concerning F.I.C.A., F.U.T.A., state disability, and local, state, and federal income taxes, and will, upon request, furnish Administrative Agent with proof satisfactory to Administrative Agent indicating that Borrower and each Subsidiary has made such payments or deposits; provided that Borrower need not make any payment if the amount or validity of such payment is contested in good faith by appropriate proceedings which suspend the collection thereof (provided that such proceedings do not involve any substantial danger of the sale, forfeiture or loss of any material item of Collateral or Collateral which in the aggregate is material to Borrower and that Borrower has adequately bonded such amounts or reserves sufficient to discharge such amounts have been provided on the books of Borrower); provided further that Borrower shall not change its respective jurisdiction of residence for taxation purposes, without the prior written consent of Administrative Agent or if such contested taxes, assessments, deposits and contributions do not, individually or in the aggregate, exceed $250,000 at any time;
(l)
make or cause to be made all filings in respect of, and pay or cause to be paid when due, all taxes, assessments, fines, fees and other liabilities (including all taxes and other claims in respect of the Collateral) unless being contested in good faith and for which Borrower maintains adequate reserves or if such contested taxes, assessments, deposits and contributions do not, individually or in the aggregate, exceed $250,000 at any time;
(m)
perform all of Borrower’s and each Subsidiary’s obligations imposed by applicable law, rule or regulation in all material respects with respect to the Collateral;
(n)
as soon as possible, and in any event within two (2) Business Days after Borrower having obtained Knowledge of the occurrence of any Event of Default or Potential Event of Default, provide a written notice setting forth the details of such Event of Default or Potential Event of Default and the action, if any is permitted, which is proposed to be taken by Borrower with respect thereto;
(o)
as soon as possible, and in any event, no later than three (3) Business Days after receipt, provide Administrative Agent with a copy of any notice of default, notice of termination or similar notice pertaining to a lease of real property where any Collateral is located with a value in excess of $200,000;
(p)
from time to time execute and deliver such further documents and do such further acts and things as Administrative Agent may reasonably request in order to fully effect the purposes of this Agreement and to protect Administrative Agent’s security interest in the Collateral, and Borrower hereby authorizes Administrative Agent to execute and deliver on behalf of Borrower and to file such financing statements (including an indication that the financing statement covers “all assets or all personal property” of Borrower in accordance with Section 9-504 of the UCC), collateral assignments, notices, control agreements, security agreements and other documents without the signature of Borrower either in Administrative Agent’s name or in the name of Administrative Agent as agent and attorney-in-fact for Borrower;
(q)
keep Borrower’s and its Subsidiaries’ business and the Collateral insured for risks and in amounts standard for companies in Borrower’s and its Subsidiaries’ industry and location and as Administrative Agent may reasonably request, including, but not limited to, D&O insurance reasonably satisfactory to Administrative Agent. Insurance policies shall be in a form, with companies, and in amounts that are reasonably satisfactory to Administrative Agent. All property policies shall have a lender’s loss payable endorsement showing Administrative Agent as lender loss payee and waive subrogation against Administrative Agent, and all liability policies shall show, or have endorsements showing Administrative Agent, as additional insured. Administrative Agent shall be named as lender loss payee and/or additional insured with respect to any such insurance providing coverage in respect of any Collateral, and each provider of any such insurance shall agree, by endorsement upon the policy or policies issued by it or by independent instruments furnished to Administrative Agent, that it will give Administrative Agent thirty (30) days prior written notice before any such policy or policies shall be materially altered by a decrease in coverage or exclusion to coverage or canceled (other than cancellation for non-payment of premiums, for which ten (10) days’ prior written notice shall be required). At Administrative Agent’s request, Borrower shall deliver certified copies of policies and evidence of all premium payments. Proceeds payable under any policy shall, at Administrative Agent’s option, be payable to Administrative Agent, on account of the Obligations. If Borrower or any of its Subsidiaries fails to obtain insurance as required under this Section 4.2(q) or to pay any amount or furnish any required proof of payment to third persons, Administrative Agent may make (but has no obligation to do so), at Borrower’s expense, all or part of such payment or obtain such insurance policies required in this Section 4.2(q), and take any action under the policies Administrative Agent deems prudent;
(r)
during all times any amounts remain due from Borrower to Administrative Agent or Lenders under this Agreement or Borrower has any Obligations under the Loan Documents, (i) preserve, renew and maintain in full force and effect its corporate existence and take all commercially reasonable action to maintain all rights, privileges and franchises necessary or desirable in the normal course of business; (ii) perform and observe all the terms and provisions of any material contract, instrument, or indenture to be performed or observed by it, maintain each such contract, instrument, or indenture in full force and effect, and enforce such rights under any material contract instrument, or indenture, unless the failure to do so could not be reasonably expected to cause a Material Adverse Change; (iii) keep proper books and records and accounts in which in all material respects full, true and correct entries in conformity with GAAP and all requirements of any governmental or regulatory authorities shall be made of all dealings and transactions and assets in relations to its business and activities; and (iv) permit Administrative Agent to visit and inspect any of its assets and properties and examine and make abstracts from any of its books and records at any time with or without prior written notice and as often as may be reasonably desired at any time during an Event of Default or upon prior written notice at reasonable times when no Event of Default is continuing up to two (2) times per year, and to discuss its business operations, properties and financial and other conditions with its officers and employees and accountants;
(s)
make available to the Administrative Agent, without expense to the Administrative Agent, Borrower and each of Borrower’s officers, employees and agents and Borrower’s books, to the extent that the Administrative Agent may reasonably deem them necessary to prosecute or defend any third party suit or proceeding instituted by or against the Administrative Agent or any Lender with respect to any Collateral or relating to Borrower;
If, after the Closing Date, any Borrower intends to form any direct or indirect Subsidiary, or acquire any direct or indirect Subsidiary, the Borrower shall (or shall cause such Borrower to): (i) ten (10) Business Days prior to such formation or acquisition, provide written notice to Administrative Agent of the formation of such Subsidiary, and, upon Administrative Agent’s request, copies of the Operating Documents of such Subsidiary, and (ii) promptly upon Administrative Agent’s request, and in any event within thirty (30) days of such request (or such later date as Administrative Agent may agree in its sole discretion) of such formation or creation: (A) take all such action as may be reasonably required by Administrative Agent to cause such new Subsidiary to either: (x) provide to Administrative Agent a joinder to this Agreement pursuant to which such Subsidiary becomes a Borrower hereunder, or (y) guarantee the Obligations of Borrowers under the Loan Documents, (B) grant a security interest in and to the assets which constitute Collateral of such Subsidiary (substantially in accordance with this Agreement), in each case together with such Account Control Agreements and other documents, instruments and agreements reasonably requested by Administrative Agent in accordance with the terms of this Agreement, all in form and substance reasonably satisfactory to Administrative Agent (including being sufficient to grant Administrative Agent a first priority Lien, subject to Permitted Liens) and (C) to pledge all of the direct or beneficial Equity Securities in such Subsidiary to the extent constituting Collateral;
(u)
Use the proceeds of the Loan solely to refinance Existing Indebtedness, as working capital and to fund its general corporate purposes; and
(v)
use Lumonic or any other reporting and administration platform designated by Administrative Agent and provide Administrative Agent with continuous online viewing access to the account balance and activity of each Deposit Account and Securities Account subject to an Account Control Agreement through such platform.
4.3
Negative Covenants of Borrower. Borrower shall not, and shall not permit any of its Subsidiaries to, do any of the following without the prior written consent of Administrative Agent, which may be conditioned or withheld in its sole discretion:
(a)
change its name, jurisdiction of incorporation, chief executive office, or principal place of business without ten (10) days’ prior written notice to Administrative Agent;
(b)
(i) create, incur, assume, or permit to exist any Lien or security interest on any Property or Collateral now or hereafter acquired by Borrower or any Subsidiary or on any income or rights in respect of any thereof, (including sale of any Accounts) except Liens and security interests created pursuant to this Agreement or Permitted Liens or (ii) or enter into any agreement with any Person other than Administrative Agent not to grant a security interest in. or otherwise encumber, any of its property, or permit any Subsidiary to do so except for agreements governing Permitted Liens;
(c)
(i) merge into or consolidate with any other entity, or permit any other entity to merge or consolidate with Borrower or any Subsidiary; provided that any Subsidiary may merge or consolidated with Borrower, (ii) liquidate or dissolve; provided that any Subsidiary may liquidate or dissolve as long as any assets of such Subsidiary are transferred to Borrower prior to liquidation or dissolution, (iii) acquire, or permit any of its Subsidiaries to acquire, all or substantially all of the capital stock, shares or property of another Person other than Permitted Investments or (iv) engage in any business other than the business of the type conducted by Borrower on the date hereof and business reasonably related, supplemental or ancillary thereto;
(d)
Transfer any of its Property, whether now owned or hereafter acquired except: (i) dispositions of worn-out, obsolete or surplus Equipment or assets in the Ordinary Course of Business that is, in the reasonable judgment of such Borrower or Subsidiary as applicable, no longer economically practicable to maintain or useful; (ii) the sale of Inventory in the Ordinary Course of Business; (iii) in connection with Permitted Liens, Permitted Investments and Permitted Licenses; (iv) consisting of Borrower’s or its Subsidiaries’ use or transfer of money or Cash Equivalents in the ordinary course of its business for the payment of ordinary course business expenses in a manner that is not prohibited by the terms of this Agreement or the other Loan Documents; (v) other assets in an aggregate amount not to exceed $500,000 per year; and (vi) the termination of Intellectual Property licenses for Borrower’s de-prioritized or immaterial programs and the transfer of assets back to the licensor in connection therewith, so long as any such Intellectual Property licenses are identified to Administrative Agent in writing, in advance of any transfer; amend, supplement or otherwise modify (pursuant to waiver or otherwise) its Operating Documents , in any respect that would reasonably be expected to result in a Material Adverse Change;
(f)
move any Collateral from the Permitted Locations except in compliance with Section 3.3 above;
(g)
(i) pay any dividends or make any distributions, on its Equity Securities; (ii) purchase, redeem, retire, defease or otherwise acquire, for value any of its Equity Securities (other than repurchases pursuant to the terms of employee stock purchase plans, employee restricted stock agreements or similar arrangements in an aggregate amount not to exceed One Hundred Thousand Dollars ($100,000.00) in any fiscal year); (iii) return any capital to any holder of its Equity Securities as such; (iv) make, any distribution of Property, Equity Securities, obligations or securities to any holder of its Equity Securities; or (v) set apart any sum for any such purpose; provided, however, that Borrower may (A) convert any of its convertible securities into other securities pursuant to the terms of such convertible securities or otherwise in exchange thereof, (B) pay dividends solely in the form of common stock; (C) pay cash in lieu of fractional shares upon exercise or conversion of any option, warrant or other convertible security; and (D) pay dividends and distributions by any Subsidiary to Borrower or another Subsidiary that is a co-Borrower;
(h)
any Key Person to cease to be actively engaged in the management of Borrower unless written notice thereof is provided to Administrative Agent within ten (10) days;
(i)
enter into any contractual obligation with any Affiliate or engage in any other transaction with any Affiliate except (i) upon terms at least as favorable to Borrower as an arms-length transaction with Persons who are not Affiliates of Borrower, (ii) sales of equity securities to its investors in bona fide equity financings so long as a Change in Control does not occur, (iii) transactions between Borrower and its Subsidiaries permitted under this Agreement, (d) reasonable and customary compensation arrangements and benefit plans for officers and other employees of Borrower entered into or maintained in the Ordinary Course of Business and approved by Borrower’s board of directors, and (e) reasonable and customary fees paid to independent members of Borrower’s board of directors in the Ordinary Course of Business;
(j)
(i) prepay, redeem, purchase, defease or otherwise satisfy in any manner prior to the scheduled repayment thereof any subordinated Debt for borrowed money other than as permitted by the applicable subordination or intercreditor agreement applicable thereto), or (ii) amend, modify or otherwise change the terms of any subordinated Debt for borrowed money or capital lease obligations so as to accelerate the scheduled repayment thereof except pursuant to the terms of any subordination or intercreditor agreement applicable thereto or (iii) repay any notes to officers, directors or shareholders, provided that Borrower may convert any such notes into Borrower’s Equity Securities or repay or otherwise satisfy such notes by the issuance of Borrower’s Equity Securities except pursuant to the terms of any subordination or intercreditor agreement applicable thereto;
(k)
create, incur, assume or permit to exist any Debt except Permitted Debt; provided however, notwithstanding any Debt that is permitted under the definition of Permitted Debt, Borrower shall not create, incur, assume to exist any Debt involving the sale or financing of its accounts receivables or any Debt secured or supported by its accounts receivables without the prior written consent of Administrative Agent;
(l)
make, or permit any Subsidiary to make, any Investment except for Permitted Investments;
(i) become an “investment company” or a company controlled by an “investment company” under the Investment Company Act of 1940 or undertake as one of its important activities extending credit to purchase or carry margin stock (as defined in Regulation U of the Board of Governors of the Federal Reserve System), or use the proceeds of any Loan for that purpose; (ii) become subject to any other federal or state law or regulation which purports to restrict or regulate its ability to borrow money; or (iii) fail to meet the minimum funding requirements of the Employment Retirement Income Security Act of 1974, and its regulations, as amended from time to time (“ERISA”), permit, or permit any Subsidiary to permit, a Reportable Event or Prohibited Transaction, as defined in ERISA, to occur; (iv) fail to comply with the Federal Fair Labor Standards Act or violate any other law or regulation, if the violation could reasonably be expected to have a Material Adverse Change;
(n)
(x) directly or indirectly, enter into any documents, instruments, agreements or contracts with any Blocked Person or (y) directly or indirectly, (A) conduct any business or engage in any transaction or dealing with any Blocked Person, including the making or receiving of any contribution of funds, goods or services to or for the benefit of any Blocked Person, (B) deal in, or otherwise engage in any transaction relating to, any property or interests in property blocked pursuant to Executive Order No. 13224, any similar executive order or other Anti-Terrorism Law or (C) engage in or conspire to engage in any transaction that evades or avoids, or has the purpose of evading or avoiding, or attempts to violate, any of the prohibitions set forth in Executive Order No. 13224 or other Anti-Terrorism Law. Each Lender hereby notifies Borrower that pursuant to the requirements of Anti-Terrorism Laws and such Lender’s policies and practices, such Lender is required to obtain, verify and record certain information and documentation that identifies Borrower and its principals, which information includes the name and address of Borrower and its principals and such other information that will allow each Lender to identify such party in accordance with Anti-Terrorism Laws. Borrower shall immediately notify Administrative Agent if Borrower has knowledge that Borrower is listed on the OFAC Lists or (i) is convicted on, (ii) pleads nolo contendere to, (iii) is indicted on or (iv) is arraigned and held over on charges involving money laundering or predicate crimes to money laundering;
(o)
(i) maintain any Deposit Account or Securities Account except accounts with respect to which Administrative Agent is able to take such actions as Administrative Agent deems necessary to obtain a perfected security interest in such accounts through one or more Account Control Agreements or other agreements giving Administrative Agent “control” as defined under the UCC (other than with respect to any Excluded Account) or (ii) grant or allow any other Person (other than a Lender) to perfect a security interest in, or enter into any agreements with any Persons (other than Lender) accomplishing perfection via control as to, any of its Deposit Accounts or Securities Accounts, other with respect to any Excluded Account; or
(p)
Cause or permit any Inactive Subsidiary to maintain cash or assets in excess of Fifty Thousand Dollars ($50,000) at any time or to own or maintain any Intellectual Property.
(a)
Each Lender hereby irrevocably designates and appoints Trinity Capital Inc., or its successor or assignee, as Administrative Agent under this Agreement and the other Loan Documents, and each such Lender irrevocably authorizes the Administrative Agent, in such capacity, to take such action on its behalf under the provisions of this Agreement and the other Loan Documents (including without limitation any subordination and intercreditor agreements (or similar agreements)) and to exercise such rights, powers and perform such duties as are expressly delegated to the Administrative Agent by the terms of this Agreement and the other Loan Documents (including without limitation any subordination and intercreditor agreements (or similar agreements)), together with such other powers as are reasonably incidental thereto. Notwithstanding any provision to the contrary elsewhere in this Agreement, the Administrative Agent shall not have any duties or responsibilities, except those expressly set forth herein and in the other Loan Documents, or any fiduciary relationship with any Lender, and no implied covenants, functions, responsibilities, duties, obligations or liabilities shall be read into this Agreement or any other Loan Document or otherwise exist against the Administrative Agent.
(b)
Each of the Lenders hereby irrevocably appoints and authorizes the Administrative Agent to act as the agent of such Lender for purposes of acquiring, holding and enforcing any and all Liens on Collateral granted by any Borrower to secure any of the Obligations and to take all other actions, exercise all powers and perform such duties as are delegated to Administrative Agent under the Loan Documents, together with such powers and discretion as are reasonably incidental thereto. In furtherance thereof, the Administrative Agent and any co-agents, sub-agents and attorneys-in-fact appointed by the Administrative Agent pursuant to Section 5.2 for purposes of holding or enforcing any Lien on the Collateral (or any portion thereof) granted under this Agreement or any other Loan Document, or for exercising any rights and remedies thereunder (at the direction of the Administrative Agent), shall be entitled to the benefits of all provisions of this Article 5, as though such co-agents, sub-agents and attorneys-in-fact were the “collateral agent” under the Loan Documents as if set forth in full herein with respect thereto.
5.2
Delegation of Duties. Administrative Agent may execute any of its duties under this Agreement and the other Loan Documents by or through its agents or attorneys-in-fact shall be entitled to advice of counsel concerning all matters pertaining to such duties. The exculpatory and indemnification provisions of this Article 5 shall apply to attorney-in-fact and shall apply to their respective activities in connection with the syndication of the Loans as well as activities as the Administrative Agent. The Administrative Agent shall not be responsible for the negligence or misconduct of any agents or attorneys- in-fact selected by it with reasonable care.
5.3
Exculpatory Provisions. Neither Administrative Agent nor any of its Affiliates nor any of their respective officers, directors, employees, agents, advisors or attorneys-in-fact shall be (i) liable for any action taken or omitted to be taken, (including the making of (or omitting to make) any determination, calculations, selection, request or providing any approval or consent or enter into any amendments, modifications or supplements) by it or such Person under or in connection with this Agreement or any other Loan Document (except to the extent that any of the foregoing are found by a final and nonappealable judgment of a court of competent jurisdiction to have resulted from its or such Person’s own gross negligence or willful misconduct; provided, that no action taken or not taken in accordance with the directions of the Required Lenders or such other percentage of Lenders as shall be necessary hereunder, as applicable, shall be deemed to constitute gross negligence or willful misconduct) or (ii) responsible in any manner to any of the Lenders for (A) any recitals, statements, representations or warranties made by Borrower or any officer thereof contained in this Agreement or any other Loan Document or in any certificate, report, instrument, statement or other document referred to or provided for in, or received by the Administrative Agent or Lenders under or in connection with, this Agreement or any other Loan Document or the transactions contemplated herein or therein, (B) the value, validity, effectiveness, genuineness, enforceability, execution, collectability or sufficiency of this Agreement or any other Loan Document or for any failure of any Borrower a party thereto to perform its obligations hereunder or thereunder, (C) the financial condition or business affairs of Borrower or any other Person liable for the payment of any Obligations or (D) the attachment, creation and/or perfection of the Liens granted or purported to be granted in the Collateral pursuant to this Agreement or the continuation and/or amendment of any financing statements filed to perfect the Liens in the applicable Collateral (other than to the extent expressly directed by the Required Lenders). The Administrative Agent shall not be under any obligation to any Lender (i) to ascertain or to inquire as to the observance or performance of any of the agreements, terms, covenants or provisions contained in, or conditions of, this Agreement or any other Loan Document, (ii) to inspect the properties, books or records of any Borrower, (iii) to ascertain or to inquire as to the use of the proceeds of the Loans, (iv) to ascertain or to inquire as to the existence or possible existence of any Event of Default, (v) to ascertain or to inquire as to any statement, warranty or representation made in or in connection with this Agreement or any other Loan Document, (vi) to ascertain or to inquire as to the contents of any certificate, report or other document delivered hereunder or under any Loan Documents or in connection herewith or therewith, (vii) to ascertain or to inquire as to the validity, enforceability, effectiveness or genuineness of this Agreement, any other Loan Document or any other agreement, instrument or document, or the creation, perfection or priority of any Lien purported to be created by this Agreement, (viii) to ascertain or to inquire as to the value or the sufficiency of any Collateral, or (ix) to ascertain or to inquire as to the satisfaction of any condition set forth in Article 2 or elsewhere herein, other than to confirm receipt of items expressly required to be delivered to the Administrative Agent or (x) to make any disclosures with respect to the foregoing or otherwise relating to any Borrower unless expressly required herein.
Anything contained herein to the contrary notwithstanding, the Administrative Agent shall not have any liability to the Lenders arising from confirmations of the amount of outstanding Loans or the component amounts thereof. Additionally, the Administrative Agent shall not be responsible or have any liability for, or have any duty to ascertain, inquire into, monitor or enforce, compliance with the provisions hereof relating to Defaulting Lenders, Affiliates of a Lender (or otherwise determine whether a Person qualifies as a Defaulting Lender or Affiliate of a Lender). Without limiting the generality of the foregoing, the Administrative Agent shall not (x) be obligated to ascertain, monitor or inquire as to whether any Lender or participant or prospective Lender or participant qualifies as a Defaulting Lender or Affiliate of a Lender and, absent actual knowledge to the contrary (which may be by written notice), shall be permitted to treat each Lender, participant, prospective Lender or prospective participant as if it is not a Defaulting Lender or Affiliate of a Lender or (y) have any liability with respect to or arising out of any assignment or participation of Loans, or disclosure of confidential information, to any Defaulting Lender or Affiliate of a Lender.
5.4
Reliance by the Administrative Agent. Administrative Agent shall be entitled to rely, and shall be fully protected in relying, upon (and shall not be liable for so relaying upon) any communication, request, instrument, writing, resolution, notice, consent, certificate, affidavit, letter, telecopy or email message, internet or intranet website posting, statement, order or other document (or other writing) or conversation believed by it to be genuine and correct and to have been signed, sent or made (or authenticated) by the proper Person or Persons and upon advice and statements of legal counsel (including counsel to the Borrower), independent accountants and other experts and professional advisors selected by the Administrative Agent. In determining compliance with any condition hereunder to the making of a Loan, that by its terms must be fulfilled to the satisfaction of a Lender, the Administrative Agent may presume that such condition is satisfactory to such Lender unless the Administrative Agent shall have received notice to the contrary from such Lender prior to the making of such Loan. The Administrative Agent may request instructions from the Required Lenders (or such number or percentage of the Lenders as shall be necessary under the circumstances as provided for herein or in the other Loan Documents) prior to taking any action or enter into any amendments, modifications or supplements, making any determination (including as to whether any agreement, document or instrument is in form and substance satisfactory to the Administrative Agent), making any calculation (which may be confirmed by the Required Lenders), sending any notice, making a selection or request (including failing to make a selection or request), exercising any voting rights or powers (including failing to exercise any voting rights or powers) or providing any consent or approval (including failing to provide any consent or approval) in connection with this Agreement or any of the other Loan Documents and may refrain (and shall incur no liability from so refraining) from taking or omitting to take any act or making any such determination, calculation, selection, request, exercising such voting rights or powers or providing such notice, approval or consent or entering into or any amendments, modifications or supplements until it receives such instruction (or calculation, as applicable) from the Required Lenders (or such number or percentage of the Lenders as shall be necessary under the circumstances as provided for herein or in the other Loan Documents), in each case as it reasonably deems appropriate (and until such instructions and indemnity, as applicable, are received, the Administrative Agent may (but shall not be obligated to) act, or refrain from acting, as it deems advisable in good faith in the interests of the Lenders). The Administrative Agent shall in all cases be fully protected in acting, or in refraining from acting, under this Agreement and the other Loan Documents in accordance with a request of the Required Lenders (or such number or percentage of the Lenders as shall be necessary under the circumstances as provided for herein or in the other Loan Documents), and such request and any action taken or failure to act pursuant thereto shall be binding upon all the Lenders and all future holders of the Loans. Notwithstanding any other provisions set forth in this Agreement or any other Loan Documents, the Administrative Agent shall not be required to take any action that is in its opinion contrary to applicable requirement of law (including, for the avoidance of doubt, any action that may be in violation of the automatic stay under the Bankruptcy Code (or any similar laws)) or that may effect a forfeiture, modification or termination of property of a Defaulting Lender in violation of Bankruptcy Code (or any similar laws) or the terms of any of the Loan Documents or that would in its reasonable opinion subject it or any of its officers, employees or directors to personal liability. Each Lender, by delivering its signature page to this Agreement, an Assignment and Acceptance and/or funding its Loans, shall be deemed to have acknowledged receipt of, and consented to and approved, each Loan Document and each other document required to be approved by the Administrative Agent, Required Lenders or Lenders, as applicable on the Closing Date or as of the date of funding such Loan. On any applicable date of determination, upon request, the Administrative Agent shall be required to calculate whether a particular group of Lenders constitutes the Required Lenders.
The Administrative Agent shall not be required to remit payments, the proceeds of Collateral or any other funds to the Lenders or any other Secured Parties herein except in accordance with the Loan Documents.
5.5
Notice of Default. Administrative Agent shall not be deemed to have knowledge or notice of the occurrence of any Potential Event of Default or Event of Default unless the Administrative Agent has received written notice from a Lender or the Borrower referring to this Agreement, describing such Potential Event of Default or Event of Default and stating that such notice is a “notice of default”. In the event that the Administrative Agent receives such a notice, the Administrative Agent shall give notice thereof to the Lenders. The Administrative Agent shall take such action with respect to such Potential Event of Default or Event of Default as shall be reasonably directed by the Required Lenders (or, if so specified by this Agreement, all Lenders or such number or percentage of the Lenders as shall be necessary under the circumstances as provided for herein or in the other Loan Documents); provided that unless and until the Administrative Agent shall have received such directions, the Administrative Agent may (but shall not be obligated to) take such action, or refrain from taking such action, with respect to such Potential Event of Default or Event of Default as it shall deem advisable in good faith in the interests of the Lenders.
5.6
Non-Reliance on Administrative Agent and Other Lenders. Each Lender expressly acknowledges that neither the Administrative Agent nor any of its Affiliates nor any of their respective officers, directors, employees, agents, advisors or attorneys in fact have made any representations or warranties to it and that no act by the Administrative Agent hereafter taken, including any review of the affairs of a Borrower or any affiliate of a Borrower, shall be deemed to constitute any representation or warranty by the Administrative Agent to any Lender. Each Lender represents to the Administrative Agent that it has, independently and without reliance upon the Administrative Agent or any other Lender, and based on such documents and information as it has deemed appropriate, made its own appraisal of an investigation into the business, operations, property, financial and other condition and creditworthiness of the Borrower and its affiliates and made its own decision to make its Loans and other extensions of credit hereunder and enter into this Agreement. Each Lender also represents that it will, independently and without reliance upon the Administrative Agent or any other Lender, and based on such documents and information as it shall deem appropriate at the time, continue to make its own credit analysis, appraisals and decisions in taking or not taking action under this Agreement and the other Loan Documents, and to make such investigation as it deems necessary to inform itself as to the business, operations, property, financial and other condition and creditworthiness of the Borrower and its affiliates. Except for notices, reports and other documents expressly required hereunder or otherwise requested by the Borrower in writing to be furnished to the Lenders by the Administrative Agent, the Administrative Agent shall not have any duty or responsibility to provide any Lender with any credit or other information concerning the business, operations, property, condition (financial or otherwise), prospects or creditworthiness of Borrower or any affiliate of Borrower that may come into the possession of the Administrative Agent or any of its officers, directors, employees, agents, advisors, attorneys in fact or affiliates.
5.7
Indemnification. The Lenders agree to indemnify, hold harmless and defend the Administrative Agent and its Affiliates and their respective officers, directors, employees, agents, advisors and controlling persons (each, an “Agent Indemnitee”) (to the extent not timely reimbursed by the Borrower and without limiting the obligation of the Borrower to do so), ratably according to their respective Pro Rata Shares in effect on the date on which indemnification is sought under this Section 5.7 (or, if indemnification is sought after the date upon which the Commitments shall have terminated and the Loans shall have been paid in full, ratably in accordance with such Pro Rata Shares immediately prior to such date), from and against any and all liabilities, obligations, losses, damages, penalties, actions, judgments, suits, costs, expenses or disbursements of any kind whatsoever that may at any time (whether before or after the payment of the Loans) be imposed on, incurred by or asserted against such Agent Indemnitee in any way relating to or arising out of, the Commitments, this Agreement, any of the other Loan Documents or any documents contemplated by or referred to herein or therein or the transactions contemplated hereby or thereby or any action taken or omitted by such Agent Indemnitee under or in connection with any of the foregoing including without limitation, exercising any of the Administrative Agent’s powers, rights, and remedies and performing their duties hereunder and thereunder (or omitting to do the same); provided that no Lender shall be liable to any Agent Indemnitee for the payment of any portion of such liabilities, obligations, losses, damages, penalties, actions, judgments, suits, costs, expenses or disbursements that are found by a final and nonappealable decision of a court of competent jurisdiction to have resulted from such Agent Indemnitee’s bad faith, gross negligence or willful misconduct, provided, however, no action taken or not taken in accordance with the directions of the Administrative Agent, Required Lenders or such other percentage of Lenders as shall be necessary hereunder, as applicable, shall be deemed to constitute gross negligence or willful misconduct.
The agreements in this Section 5.7 shall survive the termination of this Agreement and the payment of the Loans and all other amounts payable hereunder.
5.8
Administrative Agent in Its Individual Capacity. Administrative Agent and its affiliates may make loans to, accept deposits from and generally engage in any kind of business with Borrower as though the Administrative Agent were not the Administrative Agent. With respect to its Loans made or renewed by it, the Administrative Agent shall have the same rights and powers under this Agreement and the other Loan Documents as any Lender and may exercise the same as though it were not the Administrative Agent, and the terms “Lender” and “Lenders” shall include the Administrative Agent in its individual capacity.
5.9
Successor Administrative Agent. Administrative Agent may resign as Administrative Agent (which shall include the Administrative Agent’s capacities as administrative agent and collateral agent) upon 30 days’ notice to the Lenders and the Borrower. If the Administrative Agent shall resign as Administrative Agent under this Agreement and the other Loan Documents, then the Required Lenders shall appoint from among the Lenders a successor agent for the Lenders, which successor agent shall (unless an Event of Default with respect to the Borrower shall have occurred and be continuing) be subject to written approval by the Borrower (which approval shall not be unreasonably withheld or delayed), whereupon such successor agent shall succeed to the rights, powers and duties of the Administrative Agent (other than any rights to indemnity payments or other amounts owed to the retiring Administrative Agent as of the Resignation Effective Date), and the term “Administrative Agent” shall mean such successor agent effective upon such appointment and approval, and the former Administrative Agent’s rights, powers and duties as Administrative Agent shall be terminated, without any other or further act or deed on the part of such former Administrative Agent or any of the parties to this Agreement or any holders of the Loans. Any successor Administrative Agent appointed pursuant to this Section 5.9 shall, upon its acceptance of such appointment, become the successor Administrative Agent for all purposes hereunder unless otherwise agreed. If no successor agent has accepted appointment as Administrative Agent by the date that is 30 days following a retiring Administrative Agent’s delivery of its notice of resignation, the retiring Administrative Agent may (but shall not be obligated to), on behalf of the Lenders and with the written consent of the Borrower (such consent not to be unreasonably withheld or delayed or required if an Event of Default shall have occurred and be continuing) appoint a successor Administrative Agent, which shall be a commercial bank organized or licensed under the laws of the United States of America or of any State thereof and having a combined capital and surplus of at least Five Hundred Million Dollars ($500,000,000.00). If no successor agent has accepted appointment as Administrative Agent by the date that is 30 days following a retiring Administrative Agent’s notice of resignation (“Resignation Effective Date”), the retiring Administrative Agent’s resignation shall nevertheless thereupon become effective in accordance with such notice, and (i) the Lenders shall assume and perform all of the duties of the Administrative Agent hereunder until such time, if any, as the Required Lenders appoint a successor agent as provided for above, (ii) the retiring Administrative Agent shall be discharged from its duties and obligations hereunder and under the other Loan Documents and (iii) except for any indemnity payments or other amounts then owed to the retiring Administrative Agent, all payments, communications and determinations provided to be made by, to or through the Administrative Agent shall instead be made by or to each Lender directly, until such time, if any, as the successor Administrative Agent is appointed as provided for above. After any retiring Administrative Agent’s resignation as Administrative Agent, the provisions of this Article 5 shall continue to inure to its benefit as to any actions taken or omitted to be taken by it while it was Administrative Agent. Notwithstanding anything to the contrary, in no event shall a successor agent be a Defaulting Lender.
5.10
Authorization for Intercreditor Agreement and Subordination Agreement. The Lenders irrevocably authorize the Administrative Agent to enter into and perform its obligations under any Subordination Agreement or other similar arrangement permitted under this Agreement and any amendments, restatements, supplements or other modifications thereto approved in accordance with the terms thereof (without limiting the provisions set forth in Section 5.4 hereof).
5.11
Administrative Agent May File Proofs of Claim. In case of the pendency of any receivership, insolvency, liquidation, bankruptcy, reorganization, arrangement, adjustment, composition or other judicial proceeding relative to any Borrower, the Administrative Agent (on behalf of the Lenders) (irrespective of whether the principal of any Loan shall then be due and payable as herein expressed or by declaration or otherwise and irrespective of whether the Administrative Agent shall have made any demand on the Borrower) shall be entitled and empowered, by intervention in such proceeding or otherwise:
(a)
To file a verified statement pursuant to rule 2019 of the Federal Rules of Bankruptcy Procedure that, in its sole opinion, complies with such rule’s disclosure requirements for entities representing more than one creditor;
(b)
To file and prove a claim for the whole amount of the principal and interest owing and unpaid in respect of the Loans and all other Obligations that are owing and unpaid and to file such other documents as may be necessary or advisable in order to have the claims of the Secured Parties (including any claim for the reasonable compensation, expenses, disbursements and advances of the Secured Parties and their respective agents and counsel and all other amounts due the Secured Parties hereunder) allowed in such judicial proceeding;
(c)
To collect and receive any monies or other property payable or deliverable on any such claims and to distribute the same; and
(d)
Any custodian, receiver, assignee, trustee, liquidator, sequestrator or other similar official in any such judicial proceeding is hereby authorized by each applicable Lender to make such payments to the Administrative Agent, as applicable, and, in the event that the Administrative Agent shall consent to the making of such payments directly to the Lenders, to pay to the Administrative Agent any amount due for the reasonable compensation, expenses, disbursements and advances of the Administrative Agent and their respective agents and counsel, and any other amounts due to the Administrative Agent.
Each Lender further agrees that it shall not propose, vote in favor of, or otherwise support any plan of reorganization that is in contravention of any plan of reorganization that is proposed or supported by the Administrative Agent, and shall affirmatively vote to “reject” any plan of reorganization that is not affirmatively supported by the Administrative Agent.
(a)
Administrative Agent is hereby authorized on behalf of the Lenders, without the necessity of any notice to or further consent from the Lenders, from time to time (but without any obligation) to take any action with respect to the Collateral and this Agreement or any other Loan Document that may be necessary to perfect and maintain perfected Liens upon the Collateral granted pursuant to this Agreement or any other Loan Document if required or expressly permitted under the terms of any of the other Loan Documents.
(b)
Each of the Lenders hereby irrevocably authorize and instruct the Administrative Agent to, and the Administrative Agent shall:
(i)
Release (or confirm any release) any Lien granted to or held by the Administrative Agent upon any Collateral (A) upon the date on which all Obligations have been repaid in full, (B) constituting property sold or to be sold or otherwise disposed of as part of or in connection with any disposition permitted hereunder or under any other Loan Document or to which the Required Lenders have consented, (C) that does not constitute (or ceases to constitute) Collateral, (D) otherwise pursuant to and in accordance with the provisions of any applicable Loan Document or (E) subject to Section 5.11, if approved, authorized or ratified in writing by the Required Lenders, provided, however, that if any action is required by the Administrative Agent to so release such Lien, upon the request of the Administrative Agent, the Borrower shall have delivered to the Administrative Agent a certificate certifying to the permissibility of such release hereunder (and the Administrative Agent shall be permitted to rely upon such certificate without incurring any liability therefor);
(ii)
Enter into any Subordination Agreement and/or similar agreement contemplated hereunder, including with respect to Debt that is (i) required or permitted to be subordinated in right of payment hereunder and/or (ii) secured by Liens and required or permitted to be pari passu with or junior to the Liens securing the Obligations, and with respect to which Debt, a Subordination Agreement or similar agreement is contemplated under this Agreement.
(c)
Anything contained in any of the Loan Documents to the contrary notwithstanding, the Borrower, the Administrative Agent and each Lender hereby agree that (i) no Lender (other than the Administrative Agent) shall have any right individually to realize upon any of the Collateral, (ii) no Lender shall have any right to enforce the Obligations, it being understood and agreed that all powers, rights and remedies hereunder and under any of the Loan Documents may be exercised solely by the Administrative Agent for the benefit of the Lenders in accordance with the terms hereof and thereof, and (iii) in the event of a foreclosure or similar enforcement action by the Administrative Agent on any of the Collateral pursuant to a public or private sale or other disposition (including pursuant to Section 363(k), Section 1129(b)(2)(a)(ii) or otherwise of the Bankruptcy Code), the Administrative Agent (or any Lender, except with respect to a “credit bid” pursuant to Section 363(k), Section 1129(b)(2)(a)(ii) or otherwise of the Bankruptcy Code), may be the purchaser or licensor of any or all of such Collateral at any such sale or other disposition and the Administrative Agent, as agent for and representative of Lenders (but not any Lender or the Lenders in its or their respective individual capacities) shall be entitled, upon instructions from Required Lenders, for the purpose of bidding and making settlement or payment of the purchase price for all or any portion of the Collateral sold at any such sale or disposition, to use and apply any of the Obligations as a credit on account of the purchase price for any collateral payable by the Administrative Agent at such sale or other disposition.
(d)
Administrative Agent shall not be responsible for or have a duty to ascertain or inquire into any representation or warranty regarding the existence or collectability of the Collateral, the existence, priority or perfection of the Administrative Agent’s Lien thereon, or any certificate prepared by Borrower in connection therewith, nor shall the Administrative Agent be responsible or liable to the Lenders for any failure to monitor or maintain any portion of the Collateral, Liens therein or financing statements filed in connection therewith. Upon request by the Administrative Agent at any time, the Lenders will confirm in writing the Administrative Agent’s authority to release or subordinate its interest in particular types or items of property, or to release any Borrower from its obligations under the Loan Documents or its Lien on any Collateral pursuant this Section 5.12. In each case as specified in this Article 5, the Administrative Agent will (and each Lender hereby authorizes the Administrative Agent to, at the Borrower’s expense, promptly execute and deliver to Borrower such documents, filings and recordings as Borrower may reasonably request to evidence the release of such item of Collateral from the assignment and security interest granted under this Agreement or any other Loan Document or to subordinate its interest therein, in accordance with the terms of the Loan Documents and this Article 5. Additionally, upon the reasonable request of the Borrower, the Administrative Agent will return possessory Collateral held by it that is released from the security interests of the Loan Documents pursuant to this Article 5; provided that, in the event that any possessory collateral in the possession of the Administrative Agent gets lost or misplaced upon the reasonable request of the Borrower, the Administrative Agent shall provide a loss affidavit to the Borrower in the form customarily provided by the Administrative Agent in such circumstances.
ARTICLE 6
BORROWER’S INDEMNITY
6.1
Indemnity By Borrower.
Borrower covenants and agrees, at its sole cost and expense and without limiting any other rights which Administrative Agent and Lenders have hereunder, to indemnify, protect and save Administrative Agent, each Lender, and each of their directors, officers, employees, consultants, agents, attorneys, or any other Person affiliated with or representing Administrative Agent or any Lender (each, an “Indemnified Person”) harmless against and from any and all claims, damages, losses, liabilities, obligations, demands, defenses, judgments, costs, disbursements Administrative Agent’s Expenses or Lender Expenses of any kind or of any nature whatsoever which may be imposed upon, incurred by or asserted or awarded against Administrative Agent or a Lender and related to or arising from the following, unless such claim, loss or damage shall be based upon the gross negligence or willful misconduct of Administrative Agent or such Lender:
(a)
the transactions contemplated by the Loan Documents (including reasonable attorneys’ fees and expenses);
(b)
any investigative, response, remedial, administrative or judicial matter or proceeding, whether or not such Indemnified Person shall be designated a party thereto and including any such proceeding initiated by or on behalf of Borrower, and the reasonable expenses of investigation by engineers, environmental consultants and similar technical personnel and any commission, fee or compensation claimed by any broker (other than any broker retained by a Lender) asserting any right to payment for the transactions contemplated hereby which may be imposed on, incurred by or asserted against such Indemnified Person as a result of or in connection with the transactions contemplated hereby and the use or intended use of the proceeds of the loan proceeds;
(c)
any breach by Borrower of the representations, warranties, covenants, or other obligations or agreements made by Borrower in this Agreement or in any agreement related hereto or thereto;
(d)
the violation by Borrower of any state or federal law, rule or regulation;
(e)
a material misrepresentation made by Borrower to Administrative Agent or a Lender; and
(f)
any governmental fees, charges, taxes or penalties levied or imposed in respect to any Collateral.
6.2
Defense of Claims. Borrower agrees to pay all amounts due under this Article 6 promptly on notice thereof from Administrative Agent. To the extent that Borrower may make or provide, to Administrative Agent’s satisfaction, for payment of all amounts due under this Article 6, Borrower shall be subrogated to Administrative Agent’s rights with respect to such events or conditions. So long as no Event of Default has occurred and is continuing, Borrower may defend any claims with counsel of its own choosing reasonably acceptable to Administrative Agent, provided if the claim creates a significant exposure for the Lenders in Administrative Agent’s its sole judgment, or attempts to establish legal principle adverse to any Lender or Administrative Agent, Administrative Agent, on behalf of Lenders, shall select the defense counsel. Borrower may settle any claims against Administrative Agent or a Lender, provided such settlement includes a complete release of Administrative Agent and Lenders from any claims at no cost to Administrative Agent or Lenders.
6.3
Survival. All of the indemnities and agreements contained in this Article 6 shall survive and continue in full force and effect notwithstanding termination of this Agreement, the full payment of any Loans or Borrower’s performance of all Obligations.
7.1
Lender’s Rights on Default. If an Event of Default occurs and is continuing, Administrative Agent, on behalf of Lenders, shall be entitled to:
(a)
declare the unpaid balance of the Loans and this Agreement immediately due and payable, whether then due or thereafter arising;
(b)
modify the terms and conditions upon which the Lenders may be willing to consider making Loans hereunder or immediately and automatically terminate any further obligations to make Loans under this Agreement;
(c)
require Borrower to, and Borrower hereby agrees that it will at its expense and upon request of Administrative Agent, assemble the Collateral or any part thereof, as directed by Administrative Agent and make it available to Administrative Agent at a place and time to be designated by Administrative Agent, for cash, on credit or for future delivery, and upon such other terms as the Administrative Agent deems commercially reasonable;
(d)
ship, reclaim, recover, store, finish, maintain, repair, prepare for sale, advertise for sale, and sell (in the manner provided for herein) the Collateral. Administrative Agent and its agents and any purchasers at or after foreclosure are hereby granted a non-exclusive, irrevocable, perpetual, fully paid, royalty-free license or other right, solely pursuant to the provisions of this Section 7.1, to use, without charge, Borrower’s Intellectual Property, including labels, patents, copyrights, rights of use of any name, trade secrets, trade names, trademarks, service marks, and advertising matter, or any Property of a similar nature, now or at any time hereafter owned or acquired by Borrower or in which Borrower now or at any time hereafter has any rights; provided that such license shall only be exercisable in connection with the disposition of Collateral upon Administrative Agent’s exercise of its remedies hereunder;
(e)
without notice except as specified below, sell, resell, assign and deliver or grant a license to use or otherwise dispose of the Collateral or any part thereof, in one or more parcels at public or private sale, at any place designated by Administrative Agent;
(f)
occupy any premises owned or leased by Borrower where the Collateral or any part thereof is assembled or located for a reasonable period in order to effectuate its rights and remedies hereunder or under law, without obligation to Borrower in respect of such occupation;
(g)
commence and prosecute any bankruptcy, insolvency or other similar proceeding or consent to Borrower commencing any bankruptcy, insolvency or other similar proceeding;
(h)
place a “hold” on any account maintained with Administrative Agent and/or deliver a notice of exclusive control, any entitlement order, or other directions or instructions pursuant to any Account Control Agreement or similar agreements providing control of any Collateral;
(i)
exercise any and all rights and remedies of Borrower under or in connection with the Collateral, or otherwise in respect of the Collateral, including without limitation, (A) any and all rights of Borrower to demand or otherwise require payment of any amount under, or performance of any provision of, the accounts receivables and the other Collateral, (B) withdraw, or cause or direct the withdrawal, of all funds with respect to any Deposit Accounts, (C) exercise all other rights and remedies with respect to the accounts receivables and the other Collateral, including without limitation, those set forth in Section 9-607 of the UCC and (D) exercise any and all voting, consensual and other rights with respect to any Collateral; and
(j)
exercise all rights and remedies available to Administrative Agent and Lenders under the Loan Documents or at law or equity, including all remedies provided under the UCC (including disposal of the Collateral pursuant to the terms thereof).
Borrower agrees that, to the extent notice of sale shall be required by law, at least ten (10) days’ notice to Borrower of the time and place of any public sale or the time after which any private sale is to be made shall constitute reasonable notification. At any sale of the Collateral, if permitted by applicable law, the Administrative Agent and Lenders may be the purchaser, licensee, assignee or recipient of the Collateral or any part thereof and shall be entitled, for the purpose of bidding and making settlement or payment of the purchase price for all or any portion of the Collateral sold, assigned or licensed at such sale, to use and apply any of the Obligations as a credit on account of the purchase price of the Collateral or any part thereof payable at such sale.
To the extent permitted by applicable law, Borrower waives all claims, damages and demands it may acquire against the Administrative Agent and Lenders arising out of the exercise by it of any rights hereunder. Borrower hereby waives and releases to the fullest extent permitted by law any right or equity of redemption with respect to the Collateral, whether before or after sale hereunder, and all rights, if any, of marshalling the Collateral and any other security for the Obligations or otherwise. The Administrative Agent and Lenders shall not be liable for failure to collect or realize upon any or all of the Collateral or for any delay in so doing nor shall it be under any obligation to take any action with regard thereto. The Administrative Agent and Lenders shall not be obligated to make any sale of the Collateral regardless of notice of sale having been given. The Administrative Agent and Lenders may adjourn any public or private sale from time to time by announcement at the time and place fixed therefore, and such sale may, without further notice, be made at the time and place to which it was so adjourned. The Administrative Agent and Lenders shall not be obligated to clean-up or otherwise prepare the Collateral for sale.
(k)
all payments received by Borrower in respect of the Collateral shall be received in trust for the benefit of the Administrative Agent and Lenders, shall be segregated from other funds of Borrower and shall be forthwith paid over the Administrative Agent, for the benefit of the Lenders, in the same form as so received (with any necessary endorsement);
(l)
the Administrative Agent may, without notice to Borrower except as required by law and at any time or from time to time, charge, set off and otherwise apply all or part of the Obligations against any funds deposited with it or held by it;
(m)
upon the written demand of the Administrative Agent, Borrower shall execute and deliver to the Administrative Agent a collateral assignment or assignments of any or all of Borrower’s Intellectual Property and such other documents and take such other actions as are necessary or appropriate to carry out the intent and purposes hereof;
(n)
if Borrower fails to pay any amounts or furnish any required proof of payment due to third persons or entities, as required under the terms of this Agreement, then Administrative Agent may do any or all of the following: (a) make payment of the same or any part thereof; or (b) obtain and maintain insurance policies of the type discussed in Section 4.2(q) of this Agreement, and take any action with respect to such policies as Administrative Agent deems prudent. Any amounts paid or deposited by Administrative Agent shall constitute Administrative Agent’s Expenses, shall be immediately due and payable, shall bear interest at the Default Rate and shall be secured by the Collateral. Any payments made by Administrative Agent shall not constitute an agreement by Administrative Agent to make similar payments in the future or a waiver by Administrative Agent of any Event of Default under this Agreement. Borrower shall pay all reasonable fees and expenses, including Administrative Agent’s Expenses, incurred by Administrative Agent in the enforcement or attempt to enforce any of the Obligations hereunder not performed when due;
(o)
Lenders’ rights and remedies under this Agreement, the Loan Documents, and all other agreements shall be cumulative. Lenders shall have all other rights and remedies not inconsistent herewith as provided under the UCC, by law, or in equity. No exercise by Administrative Agent or any Lender of one right or remedy shall be deemed an election, and no waiver by Administrative Agent or any Lender of any Event of Default on Borrower’s part shall be deemed a continuing waiver. No delay by Administrative Agent or any Lender shall constitute a waiver, election, or acquiescence by it. The Obligations of Borrower to any Lender may be enforced against Borrower in accordance with the terms of this Agreement and the other Loan Documents and, to the fullest extent permitted by applicable law, it shall not be necessary for any other party to be joined as an additional party in any proceeding to enforce such Obligations; the proceeds and/or avails of the Collateral, or any part thereof, and the proceeds and the avails of any remedy hereunder (as well as any other amounts of any kind held by Administrative Agent, for the benefit of Lenders, at the time of or received by Administrative Agent after the occurrence of an Event of Default hereunder) shall be paid to and applied as follows:
First, to the payment of out-of-pocket costs and expenses, including all amounts expended to preserve the value of the Collateral, of foreclosure or suit, if any, and of such sale and the exercise of any other rights or remedies, and of all proper fees, expenses, liability and advances, including reasonable legal expenses and attorneys’ fees, incurred or made hereunder by Administrative Agent, including Administrative Agent’s Expenses;
Second, to the payment to Administrative Agent, on behalf of the Lenders of the amount then owing or unpaid on the Loans for any accrued and unpaid interest, the amounts which would have otherwise come due under Sections 2.8 or 2.9, if the Loans had been voluntarily prepaid, the principal balance of the Loans, and all other Obligations with respect to the Loans (provided, however, if such proceeds shall be insufficient to pay in full the whole amount so due, owing or unpaid upon the Loans, then first, to the unpaid interest thereon ratably, second, to the amounts which would have otherwise come due under Section 2.8 or 2.9 ratably, if the Loans had been voluntarily prepaid, third, to the principal balance of the Loans ratably, and fourth, to the ratable payment of other amounts then payable to Lenders under any of the Loan Documents); and
Third, to the payment of the surplus, if any, to Borrower, its successors and assigns or to the Person lawfully entitled to receive the same;
(q)
Administrative Agent shall have proceeded to enforce any right under this Agreement or any other of the Loan Documents by foreclosure, sale, entry or otherwise, and such proceedings shall have been discontinued or abandoned for any reason or shall have been determined adversely, then and in every such case (unless otherwise ordered by a court of competent jurisdiction), Administrative Agent shall be restored to its former position and rights hereunder with respect to the Property subject to the security interest created under this Agreement.
7.2
Rights Cumulative; Waivers. All rights, remedies and powers granted to Administrative Agent and Lenders hereunder are irrevocable and cumulative, and not alternative or exclusive, and shall be in addition to all other rights, remedies and powers given hereunder, or in or by any other instrument, or available in law or equity. Administrative Agent’s and Lender’s knowledge at any time of any breach of, or non-compliance with, any representations, warranties, covenants or agreements hereunder shall not constitute or be deemed a waiver of any of such rights or remedies hereunder, and any waiver of any default shall not constitute a waiver of any other default. Notwithstanding any foreclosure or sale of any item of Collateral by Administrative Agent as permitted under this Agreement, Borrower shall remain liable for any deficiency. All amounts realized by Administrative Agent in furtherance of its rights to sell or foreclose upon the Collateral shall first be applied to all costs of the action and all costs of enforcement or interpretation of this Agreement, including any court costs, legal or expert fees and filing fees, then to any outstanding interest or penalties payable under this Agreement, then to repayment of principal of all Loans.
8.1
Costs and Expenses. Borrower will pay all Administrative Agent’s Expenses and Lender’s Expenses on demand.
Borrower hereby irrevocably constitutes and appoints Administrative Agent as Borrower’s attorney-in-fact with full power of substitution, for Borrower and any of its Subsidiary’s and in Borrower’s or any of its Subsidiary’s name to do, at Administrative Agent’s option and at Borrower’s expense upon the occurrence and during the continuance of an Event of Default, to (a) ask, demand, collect (including, but not limited to the execution, in Borrower’s or any Subsidiary’s name, of notification letters), sue for, compound and give acquittance for any and all payments assigned hereunder and to endorse, in writing or by stamp, Borrower’s name or otherwise on all checks for any monies in respect of the Collateral; (b) sign Borrower’s or any of its Subsidiaries’ name on any invoice or bill of lading for any account or drafts against Account Debtors; (c) settle and adjust disputes and claims about any accounts directly with Account Debtors, for amounts and on terms Administrative Agent determines reasonable; (d) make, settle, and adjust all claims under Borrower’s insurance policies; (e) pay, contest or settle any Lien, charge, encumbrance, security interest, and adverse claim in or to the Collateral, or any judgment based thereon, or otherwise take any action to terminate or discharge the same; and (f) transfer the Collateral into the name of Administrative Agent or a third party as the UCC or any applicable law permits. Borrower hereby appoints Administrative Agent as its lawful attorney in fact to sign Borrower’s or any of its Subsidiaries’ name on any documents necessary to perfect or continue the perfection of Administrative Agent’s security interest in the Collateral regardless of whether an Event of Default has occurred until all Obligations (other than inchoate indemnity obligations) have been satisfied in full and Lenders are under no further obligation to make or extend Loans hereunder. Administrative Agent’s foregoing appointment as Borrower’s or any of its Subsidiaries’ attorney in fact, and all of Administrative Agent’s and Lenders’ rights and powers, coupled with an interest, are irrevocable until all Obligations (other than inchoate indemnity obligations) have been fully repaid and performed and Lenders’ obligation to provide Loans terminates.
8.3
Survival. All representations, warranties and indemnities contained in this Agreement (and any and each other agreement or instrument delivered pursuant hereto) shall survive (i) the execution and delivery of this Agreement, (ii) the consummation of the transactions contemplated hereby, (iii) the payment of the Loans, (iv) the performance of all Obligations, and (v) termination of this Agreement.
8.4
Assignments. Except as herein provided, this Agreement shall be binding upon and inure to the benefit of Administrative Agent, Lenders, and Borrower and their respective representatives, successors and assigns. Any Lender may assign this Agreement in whole or in part or sell participations therein without notice to Borrower or Borrower’s consent. Notwithstanding the foregoing, Borrower may not assign, transfer or otherwise convey this Agreement, in whole or in part, without Administrative Agent’s and each Lender’s prior written consent. Notwithstanding the foregoing, so long as no Event of Default shall have occurred and is continuing, no Lender shall assign its interests in the Loan Documents to any Person who, in the reasonable estimation of such Lender, is (a) a direct competitor of Borrower or (b) a vulture fund or distressed debt fund.
8.5
No Brokers. Borrower represents to Lenders that no brokers or advisors have been or will be retained in connection with the transactions contemplated herein.
8.6
Notice. All notices, consents, requests, instructions, approvals and communications provided herein shall be validly given, made or served, effective only if in writing, except as otherwise provided herein, and sent by overnight courier, certified U.S. mail, postage prepaid, or by electronic mail, and shall be deemed received within five (5) Business Days from the date of posting if sent by mail, one Business Day after delivery thereto if sent by overnight courier service, or on the day of transmission if sent by electronic mail with a confirmation receipt obtained, or if such day is not a Business Day, then on the following Business Day. All such notices, consents, requests, instructions, approvals and communications shall be sent to a party at the address set forth for such party on the signature pages hereto, or to such other address as such party may designate in writing.
8.7
Governing Law; Consent to Jurisdiction and Service of Process. THIS AGREEMENT SHALL BE SUBJECT TO AND GOVERNED BY THE LAWS OF THE STATE OF CALIFORNIA (WITHOUT REGARD TO THE CONFLICT OF LAW PRINCIPLES THEREOF THAT WOULD RESULT IN THE APPLICATION OF ANY LAWS OTHER THAN THE LAWS OF SUCH STATE). IN THE EVENT THAT ADMINISTRATIVE AGENT OR ANY LENDER INITIATES AGAINST BORROWER ANY DISPUTE, CLAIM, OR SUIT WHETHER DIRECTLY OR INDIRECTLY ARISING OUT OF OR RELATING TO THIS AGREEMENT, OR ANY OTHER LOAN DOCUMENT OR ANY OF BORROWER’S OBLIGATIONS OR INDEBTEDNESS HEREUNDER OR THEREUNDER, EACH PARTY DOES HEREBY IRREVOCABLY SUBMIT TO THE JURISDICTION AND VENUE OF ANY COURTS (FEDERAL, STATE OR LOCAL) HAVING A LOCATION IN THE STATE OF CALIFORNIA.
IN THE EVENT THAT BORROWER INITIATES AGAINST ADMINISTRATIVE AGENT OR ANY LENDER ANY DISPUTE, CLAIM, OR SUIT WHETHER DIRECTLY OR INDIRECTLY ARISING OUT OF OR RELATING TO THIS AGREEMENT, OR ANY RELATED ASSIGNMENT OR ANY OF BORROWER’S OBLIGATIONS OR INDEBTEDNESS HEREUNDER, EACH PARTY DOES HEREBY IRREVOCABLY SUBMIT TO THE JURISDICTION AND VENUE OF ANY COURTS (FEDERAL, STATE OR LOCAL) HAVING A LOCATION IN THE STATE OF CALIFORNIA. EACH PARTY EXPRESSLY WAIVES PERSONAL SERVICE OF PROCESS AND CONSENTS TO SERVICE BY CERTIFIED MAIL, POSTAGE PREPAID, DIRECTED TO ITS LAST KNOWN ADDRESS WHICH SERVICE SHALL BE DEEMED COMPLETED WITHIN FIVE (5) DAYS AFTER THE DATE OF MAILING THEREOF. EACH PARTY HEREBY IRREVOCABLY WAIVES ANY CLAIM THAT THE STATE OF CALIFORNIA IS AN INCONVENIENT FORUM OR AN IMPROPER FORUM BASED ON LACK OF VENUE AS WELL AS ANY RIGHT IT MAY NOW OR HEREAFTER HAVE TO REMOVE ANY SUCH ACTION OR PROCEEDING, ONCE COMMENCED TO ANOTHER COURT ON THE GROUNDS OF FORUM NON CONVENIENS OR OTHERWISE. THE EXCLUSIVE CHOICE OF FORUM SET FORTH HEREIN SHALL NOT BE DEEMED TO PRECLUDE THE ENFORCEMENT BY EITHER PARTY OF ANY JUDGMENT OBTAINED IN SUCH FORUM OR THE TAKING OF ANY ACTION BY SUCH PARTY TO ENFORCE THE SAME IN ANY OTHER APPROPRIATE JURISDICTION.
8.8
Other Documents. Borrower shall execute such other documents and shall otherwise cooperate with Administrative Agent as Administrative Agent reasonably requires to effectuate the transactions contemplated hereby.
8.9
Severability. If any part of this Agreement shall be contrary to any law which a party might seek to apply or enforce or should otherwise be defective, the other provisions hereof shall not be affected thereby but shall continue in full force and effect, to which end they are hereby declared severable.
8.10
Entirety; Amendments. This Agreement and the Exhibits referred to herein constitute the entire agreement between Administrative Agent, Lenders, and Borrower as to the subject matter contemplated herein, and supersedes all prior agreements and understandings relating thereto. Each of the parties hereto acknowledges that no party hereto nor any agent of any other party whomsoever has made any promise, representation or warranty whatsoever, express or implied, not contained herein, concerning the subject matter hereof, to induce it to execute this Agreement. No other agreements will be effective to change, modify or terminate this Agreement in whole or in part unless such agreement is in writing and duly executed by the party to be charged except as expressly set forth herein.
8.11
WAIVER OF JURY TRIAL. EACH PARTY HEREBY UNCONDITIONALLY WAIVES ITS RIGHT TO A JURY TRIAL OF ANY CLAIM OR CAUSE OF ACTION BASED UPON OR ARISING OUT OF, DIRECTLY OR INDIRECTLY, THIS AGREEMENT, ANY RELATED DOCUMENTS, ANY DEALINGS BETWEEN THE PARTIES RELATING TO THE SUBJECT MATTER OF THIS AGREEMENT, AND/OR THE RELATIONSHIP THAT IS BEING ESTABLISHED BY THE PARTIES. THE SCOPE OF THIS WAIVER IS INTENDED TO BE ALL ENCOMPASSING OF ANY AND ALL DISPUTES THAT MAY BE FILED IN ANY COURT (INCLUDING, WITHOUT LIMITATION, TRANSACTION CLAIMS, TORT CLAIMS, BREACH OF DUTY CLAIMS, AND ALL OTHER COMMON LAW AND STATUTORY CLAIMS). THIS WAIVER IS IRREVOCABLE AND MAY NOT BE MODIFIED ORALLY OR IN WRITING, AND SHALL APPLY TO ANY SUBSEQUENT AMENDMENTS, RENEWALS, SUPPLEMENTS AND MODIFICATIONS TO THIS AGREEMENT. IN THE EVENT OF LITIGATION, THIS AGREEMENT MAY BE FILED AS A WRITTEN CONSENT TO TRIAL BY THE COURT.
WITHOUT INTENDING IN ANY WAY TO LIMIT THE PARTIES’ AGREEMENT TO WAIVE THEIR RESPECTIVE RIGHT TO A TRIAL BY JURY, if the above waiver of the right to a trial by jury is not enforceable, any disputes or controversies of any nature between them arising at any time shall be decided by a reference to a private judge, mutually selected by the parties (or, if they cannot agree, by the Presiding Judge of the Santa Clara County, California Superior Court) appointed in accordance with California Code of Civil Procedure Section 638 (or pursuant to comparable provisions of federal law if the dispute falls within the exclusive jurisdiction of the federal courts), sitting without a jury, in Santa Clara County, California; and the parties hereby submit to the jurisdiction of such court. The reference proceedings shall be conducted pursuant to and in accordance with the provisions of California Code of Civil Procedure §§ 638 through 645.1, inclusive.
The private judge shall have the power to decide all issues in the action or proceeding, whether of fact or of law, and shall report a statement of decision thereon pursuant to California Code of Civil Procedure § 644(a). Nothing in this paragraph shall limit the right of any party at any time to exercise self-help remedies, foreclose against collateral, or obtain provisional remedies. The private judge shall also determine all issues relating to the applicability, interpretation, and enforceability of this paragraph.
8.12
Publicity. Each Lender will have the right to (a) make a public announcement and include on its website, social media sites, and other marketing materials information related to this transaction; provided that Lender and Borrower shall mutually agree on the timing and content of any publicity permitted by this Section 8.12(a), and (b) include information about this transaction, including but not limited to Borrower’s name, the type of investment, principal amount, interest rate and maturity date, in its periodic reports with the Securities and Exchange Commission (“SEC”), to the extent required by SEC rules and regulations.
8.13
Demand Waiver. Borrower waives, to the fullest extent permitted by law, demand, notice of default or dishonor, notice of payment and nonpayment, notice of any default, nonpayment at maturity, release, compromise, settlement, extension, or renewal of accounts, documents, instruments, chattel paper, and guarantees held by the Lenders on which Borrower or any Subsidiary is liable.
8.14
Counterparts. This Agreement may be executed in any number of counterparts and by different parties on separate counterparts, each of which, when executed and delivered, is an original, and all taken together, constitute one Agreement. Delivery of an executed counterpart of a signature page of this Agreement by facsimile, portable document format (.pdf) or other electronic transmission will be as effective as delivery of a manually executed counterpart hereof.
8.15
Electronic Execution of Certain Other Documents. The words “execution,” “execute”, “signed,” “signature,” and words of like import in or related to any document to be signed in connection with this Agreement and the transactions contemplated hereby (including without limitation assignments, assumptions, amendments, waivers and consents) shall be deemed to include electronic signatures, the electronic matching of assignment terms and contract formations on electronic platforms approved by the Administrative Agent, or the keeping of records in electronic form, each of which shall be of the same legal effect, validity or enforceability as a manually executed signature or the use of a paper-based recordkeeping system, as the case may be, to the extent and as provided for in any applicable law, including the Federal Electronic Signatures in Global and National Commerce Act, the California Electronic Transactions Act, or any other similar state laws based on the Uniform Electronic Transactions Act.
8.16
Correction of Loan Documents. Administrative Agent, on behalf of Lenders, may correct patent errors and fill in any blanks in the Loan Documents consistent with the agreement of the parties so long as Administrative Agent provides Borrower with notice of such correction.
8.17
Right of Set Off. Borrower hereby grants to Administrative Agent, for the benefit of Lenders, a Lien, security interest and right of set off as security for all Obligations to Lenders hereunder, whether now existing or hereafter arising upon and against all deposits, credits, collateral and property, now or hereafter in the possession, custody, safekeeping or control of the Administrative Agent or any entity under the control of the Lenders (including a Lender affiliate) or in transit to any of them. At any time after the occurrence and during the continuance of an Event of Default, without demand or notice, the Administrative Agent, on behalf of Lenders, may set off the same or any part thereof and apply the same to any liability or obligation of Borrower even though unmatured and regardless of the adequacy of any other collateral securing the Obligations. ANY AND ALL RIGHTS TO REQUIRE LENDERS TO EXERCISE THEIR RIGHTS OR REMEDIES WITH RESPECT TO ANY OTHER COLLATERAL WHICH SECURES THE OBLIGATIONS, PRIOR TO EXERCISING THEIR RIGHT OF SETOFF WITH RESPECT TO SUCH DEPOSITS, CREDITS OR OTHER PROPERTY OF BORROWER ARE HEREBY KNOWINGLY, VOLUNTARILY AND IRREVOCABLY WAIVED BY BORROWER.
(a)
Register. The Administrative Agent, acting solely for this purpose as an agent of the Borrower, shall maintain a copy of each Assignment and Assumption delivered to it and a register for the recordation of the names and addresses of the Lenders, and the Commitments of, and principal amounts (and stated interest) of the Loans owing to, each Lender pursuant to the terms hereof from time to time (the “Register”). The entries in the Register shall be conclusive absent manifest error, and the Borrower, the Administrative Agent and the Lenders shall treat each Person whose name is recorded in the Register pursuant to the terms hereof as a Lender hereunder for all purposes of this Agreement. The Register shall be available for inspection by the Borrower and any Lender, at any reasonable time and from time to time upon reasonable prior notice.
(b)
Participant Register. Each Lender that sells a participation shall, acting solely for this purpose as a non-fiduciary agent of the Borrower, maintain a register on which it enters the name and address of each participant and the principal amounts (and stated interest) of each participant’s interest in the Loans or other obligations under the Loan Documents (the “Participant Register”); provided that no Lender shall have any obligation to disclose all or any portion of the Participant Register (including the identity of any participant or any information relating to a participant’s interest in any commitments, loans, its other obligations under any Loan Document) to any Person except to the extent that such disclosure is necessary to establish that such commitment, loan, letter of credit or other obligation is in registered form under Section 5f.103-1(c) of the United States Treasury Regulations. The entries in the Participant Register shall be conclusive absent manifest error, and such Lender shall treat each Person whose name is recorded in the Participant Register as the owner of such participation for all purposes of this Agreement notwithstanding any notice to the contrary. For the avoidance of doubt, the Administrative Agent (in its capacity as Administrative Agent) shall have no responsibility for maintaining a Participant Register. Borrower agrees that each participant shall be entitled to the benefits of the provisions in Section 2.11 (subject to the requirements and limitations therein, including the requirements under Section 2.11(g) (it being understood that the documentation required under Section 2.11(g) shall be delivered to the participating Lender)) to the same extent as if it were a Lender and had acquired its interest by assignment pursuant to Section 8.4; provided that such participant shall not be entitled to receive any greater payment under Section 2.11, with respect to any participation, than its participating Lender would have been entitled to receive, except to the extent such entitlement to receive a greater payment results from a change in law that occurs after the participant acquired the applicable participation.
8.19
Confidentiality. In handling any confidential information of Borrower, the Lenders and Administrative Agent shall exercise the same degree of care that it exercises for their own proprietary information, but disclosure of information may be made: (a) subject to the terms and conditions of this Agreement, to the Lenders’ and Administrative Agent’s Subsidiaries or Affiliates; (b) to prospective transferees (other than those identified in (a) above) or purchasers of any interest in the Loans (provided, however, the Lenders and Administrative Agent shall, except upon the occurrence and during the continuance of an Event of Default, obtain such prospective transferee’s or purchaser’s agreement to the terms of this provision or to similar confidentiality terms); (c) as required by law, regulation, subpoena, or other order; (d) to Lenders’ or Administrative Agent’s regulators or as otherwise required in connection with an examination or audit; (e) as Administrative Agent reasonably considers appropriate in exercising remedies under the Loan Documents; and (f) to third party service providers of the Lenders and/or Administrative Agent so long as such service providers have executed a confidentiality agreement with the Lenders and Administrative Agent with terms no less restrictive than those contained herein. Confidential information does not include information that either: (i) is in the public domain or in the Lenders’ and/or Administrative Agent’s possession when disclosed to the Lenders and/or Administrative Agent, or becomes part of the public domain after disclosure to the Lenders and/or Administrative Agent; or (ii) is disclosed to the Lenders and/or Administrative Agent by a third party, if the Lenders and/or Administrative Agent does not know that the third party is prohibited from disclosing the information. Administrative Agent and the Lenders may use confidential information for any purpose, including, without limitation, for the development of client databases, reporting purposes, and market analysis. The provisions of the immediately preceding sentence shall survive the termination of this Agreement. The agreements provided under this Section 8.19 supersede all prior agreements, understanding, representations, warranties, and negotiations between the parties about the subject matter of this Section 8.19.
[SIGNATURES ON FOLLOWING PAGE]
IN WITNESS WHEREOF, the parties hereto have caused this Loan and Security Agreement to be duly executed as of the day and year first above written.
|
|
LENDER:
|
|
TRINITY CAPITAL INC.,
a Maryland corporation
|
By: /s/ Sarah Stanton |
Name: Sarah Stanton |
|
Its: General Counsel and Chief Compliance Officer
|
|
Address for Notices:
Trinity Capital Inc.
1 N. 1st Street, Floor 3
Phoenix, AZ 85004
Attention: Legal Department Telephone: (480) 374-5350
Email: legal@trincapinvestment.com
|
|
EPT 16 LLC,
a Delaware limited liability company
By: Trinity Capital Adviser LLC, its Manager
By: Trinity Capital Inc., its Sole Member
|
By: /s/ Sarah Stanton |
Name: Sarah Stanton |
|
Its: General Counsel and Chief Compliance Officer
|
|
Address for Notices:
EPT 16 LLC
1 N. 1st Street, Floor 3
Phoenix, AZ 85004
Attention: Legal Department
Telephone: (480) 374-5350
Email: legal@trincapinvestment.com
|
[Signature Page to Loan and Security Agreement]
|
|
|
BORROWER:
|
|
TAYSHA GENE THERAPIES, INC.,
a Delaware corporation
|
By: /s/ Kamran Alam |
Name: Kamran Alam |
|
Its: Chief Financial Officer
|
|
Address for Notices:
3000 Pegasus Park Dr., Suite 1430
Dallas, Texas 75247
Attention: Kamran Alam
Telephone: [***]
Email Address: kalam@tayshagtx.com
|
|
|
[Signature Page to Loan and Security Agreement]
|
|
ADMINISTRATIVE AGENT:
|
|
TRINITY CAPITAL INC.,
a Maryland corporation
|
By: /s/ Sarah Stanton |
Name: Sarah Stanton |
|
Its: General Counsel and Chief Compliance Officer
|
|
Address for Notices:
Trinity Capital Inc.
1 N. 1st Street, Floor 3
Phoenix, AZ 85004
Attention: Legal Department
Telephone: (480) 374-5350
Email: legal@trincapinvestment.com
|
[Signature Page to Loan and Security Agreement]
SCHEDULE 1
LSA PROVISIONS
|
|
LSA Section |
LSA Provision |
Article 1 – “Amortization Date” |
“Amortization Date” means the first Payment Date after expiration of the Interest Only Period. |
Article 1 – “Applicable Rate” |
“Applicable Rate” means a variable annual interest rate equal to the greater of (i) the Prime Rate plus four percent (4.00%) or (ii) eleven and one-half percent (11.50%). |
Article 1 – “Commitment Fee” |
“Commitment Fee” is for each Advance (other than the Tranche A Loan) the fully earned and non-refundable commitment fee equal to one percent (1.00%) of the aggregate principal amount of such Advance. The Commitment Fee due and payable on the Closing Date with respect to the Tranche A Loan shall be equal to One Hundred Thousand Dollars ($100,000.00). |
Article 1 – “End of Term Payment” |
“End of Term Payment” is an amount equal to five percent (5.00%) of the original principal amount of the Loans in addition to all sums payable hereunder |
Article 1 – “Interest Only Period” |
“Interest Only Period” means the period from and including the Closing Date and through but excluding the forty-ninth (49th) Payment Date following the Closing Date, provided that if Borrower advances the full Tranche B Availability Amount, the Interest Only Period shall be the period beginning on the Closing Date and through but excluding the fifty-ninth (59th) Payment Date following the Closing Date. |
Article 1 – “Loan Termination Date” |
“Loan Termination Date” means, with respect to the Tranche B Loan, March 31, 2028 and with respect to the Tranche C Loan, March 31, 2029. |
Article 1 – “Maturity Date” |
“Maturity Date” means August 1, 2030. |
Article 1 – “Tranche A Availability Amount” |
“Tranche A Availability Amount” means an aggregate principal amount equal to Fifty Million Dollars ($50,000,000). |
Article 1 – “Tranche B Availability Amount” |
“Tranche B Availability Amount” means an aggregate principal amount equal to Twenty Five Million Dollars ($25,000,000). |
Article 1 – “Tranche C Availability Amount” |
“Tranche C Availability Amount” means an aggregate principal amount equal to Twenty Five Million Dollars ($25,000,000). |
SCHEDULE 2
COMMITMENTS
|
|
|
|
|
|
Lender
|
Tranche A Loan Commitment
|
Tranche B Loan Commitment
|
Tranche C Loan Commitment
|
Total Commitment
|
|
Trinity Capital Inc.
|
$48,000,000.00
|
$24,000,000.00 |
$24,000,000.00 |
$96,000,000.00 |
EPT 16 LLC |
$2,000,000.00 |
$1,000,000.00 |
$1,000,000.00 |
$4,000,000.00 |
Total |
$50,000,000.00
|
$25,000,000.00 |
$25,000,000.00
|
$100,000,000.00 |
EXHIBIT A
AMORTIZATION SCHEDULE
EXHIBIT B
SECRETARY’S CERTIFICATE
|
|
|
BORROWER: |
TAYSHA GENE THERAPIES, INC. |
Date: August 7, 2025 |
|
ADMINISTRATIVE
AGENT:
|
Trinity Capital Inc., as Administrative Agent |
|
Pursuant to the Loan and Security Agreement, dated as of August 7, 2025, by and among Borrower the Lenders party thereto, and Trinity Capital Inc., as administrative agent and collateral agent for the Lenders (“Administrative Agent”) (the “Loan Agreement”, unless otherwise defined herein, terms defined in the Loan Agreement and used herein shall have the meanings given to them in the Loan Agreement), I hereby certify as follows, as of the date set forth above:
1.
I am the Secretary or other Responsible Officer of Borrower. My title is as set forth below.
2.
Borrower’s exact legal name is set forth above. Borrower is a corporation existing under the laws of the State of Delaware.
3.
Attached hereto as Annex I and Annex II, respectively, are true, correct and complete copies of (i) Borrower’s Articles of Incorporation (including amendments), as filed with the Secretary of State of the state in which Borrower is incorporated as set forth in paragraph 2 above; and (ii) Borrower’s Bylaws. Neither such Articles of Incorporation nor such Bylaws have been amended, annulled, rescinded, revoked or supplemented, and such Articles of Incorporation and such Bylaws remain in full force and effect as of the date hereof.
4.
The resolutions attached hereto as Annex III were duly and validly adopted by Borrower’s board of directors at a duly held meeting of such directors (or pursuant to a unanimous written consent or other authorized corporate action). Such resolutions are in full force and effect as of the date hereof and have not been in any way modified, repealed, rescinded, amended or revoked, and the Lenders may rely on them until each Lender receives written notice of revocation from Borrower.
5.
Any one of the following officers or employees of Borrower, whose names, titles and signatures are below, may act on behalf of Borrower:
|
|
|
|
Name |
Title |
Signature |
Authorized to Add or Remove Signatories |
|
|
|
□ |
|
|
|
□ |
|
|
|
□ |
|
|
|
□ |
[Balance of Page Intentionally Left Blank]
IN WITNESS WHEREOF, the undersigned has executed and delivered this Secretary’s Certificate on behalf of TAYSHA GENE THERAPIES, INC. as of the date first set forth above.
The undersigned, [_____], [_____] of TAYSHA GENE THERAPIES, INC., does hereby certify that [_____] is the duly elected and presently incumbent [_____] of TAYSHA GENE THERAPIES, INC., and that the statements and signatures in the foregoing Secretary’s Certificate are true and correct on the date hereof.
[Signature Page to Secretary's Certificate]
ANNEX I
Articles of Incorporation (including amendments)
[see attached]
ANNEX II
Bylaws
[see attached]
ANNEX III
Resolutions
[see attached]
EXHIBIT C
FORM OF COMPLIANCE CERTIFICATE
|
|
TO: |
Trinity Capital Inc., as Administrative Agent |
FROM: |
TAYSHA GENE THERAPIES, INC. |
The undersigned authorized officer (“Officer”) of TAYSHA GENE THERAPIES, INC. (“Borrower”), hereby certifies that in accordance with the terms and conditions of the Loan and Security Agreement dated as of August 7, 2025, by and among Borrower, the Lenders party thereto, and Trinity Capital Inc., as administrative agent and collateral agent for the Lenders (“Administrative Agent”) (the “Loan Agreement;” capitalized terms used but not otherwise defined herein shall have the meanings given them in the Loan Agreement),
(a)
Borrower is in complete compliance for the period ending with all required covenants except as noted below;
(b)
There are no Potential Events of Default or Events of Default, except as noted below;
(c)
Except as noted below, all representations and warranties of Borrower stated in the Loan Documents are true and correct in all material respects on this date and for the period described in (a), above; provided, however, that such materiality qualifier shall not be applicable to any representations and warranties that already are qualified or modified by materiality in the text thereof; and provided, further that those representations and warranties expressly referring to a specific date shall be true, accurate and complete in all material respects as of such date.
(d)
Borrower and each Subsidiary has filed all federal, state and other tax returns that are required to be filed and has paid all taxes shown thereon to be due, together with applicable interest and penalties, and all other taxes, fees or other charges imposed on it or any of its property by any governmental or regulatory authority in accordance with the terms of the Loan Agreement. No tax Liens have been filed, and, to the Knowledge of Borrower, no claim is being asserted, with respect to any such tax, fee or other charge.
(e)
No Liens have been levied or claims made against Borrower or any of its Subsidiaries relating to unpaid employee payroll or benefits of which Borrower has not previously provided written notification to Administrative Agent.
Attached are the required documents, if any, supporting our certification(s). The Officer, on behalf of Borrower, further certifies that the attached financial statements are prepared in accordance with GAAP applied on a consistent basis from one period to the next except as explained in an accompanying letter or footnotes and except, in the case of unaudited financial statements, for the absence of footnotes and subject to year-end audit adjustments as to the interim financial statements.
Please indicate compliance status since the last Compliance Certificate by circling Yes, No, or N/A under “Complies” column.
|
|
|
|
|
|
|
|
Reporting Covenant |
Requirement |
Actual |
Complies |
1. |
Quarterly financial statements |
Quarterly within 45 days |
|
Yes |
No |
N/A |
2. |
Compliance Certificate |
Quarterly within 45 days |
|
Yes |
No |
N/A |
3. |
Annual (CPA Audited) statements |
Within 180 days after FYE |
|
Yes |
No |
N/A |
4. |
Annual Financial Projections |
Within 10 days of board of directors Approval but no later than 60 days after FYE |
|
Yes |
No |
N/A |
5. |
8-K, 10-K and 10-Q Filings |
At time of filing |
|
Yes |
No |
N/A |
6. |
IP Report |
Concurrently with Compliance Certificate |
|
Yes |
No |
N/A |
*To the extent the foregoing documents are included in materials otherwise filed with Securities and Exchange Commission, such documents shall be deemed to have been delivered on the date on which Borrower posts such documents, or provides a link thereto, on Borrower’s website.
Deposit and Securities Account
(Please list all accounts; attach separate sheet if additional space needed)
|
|
|
|
|
|
|
|
Institution Name |
Account Number |
New Account? |
Account Control Agreement in place? |
1. |
|
|
Yes |
No |
Yes |
No |
2. |
|
|
Yes |
No |
Yes |
No |
3. |
|
|
Yes |
No |
Yes |
No |
4. |
|
|
Yes |
No |
Yes |
No |
Other Matters
|
|
|
|
1. |
Have there been any changes in Key Persons since the last Compliance Certificate? |
Yes |
No |
2. |
Have there been any transfers/sales/dispositions/retirement of Collateral or IP prohibited by the Loan Agreement? |
Yes |
No |
3. |
Have there been any new or pending material claims or causes of action against Borrower? |
Yes |
No |
4. |
Has Borrower provided the Administrative Agent with all notices required to be delivered under Sections 3.2, 3.7, 3.8(c), 4.2 and 4.3 of the Loan Agreement? |
Yes |
No |
5. |
Have there been any material updates to the contents of the Perfection Certificate last delivered? If yes, please explain. |
Yes |
No |
Exceptions
Please explain any exceptions with respect to the certification above: (If no exceptions exist, state “No exceptions.” Attach separate sheet if additional space needed.)
|
|
TAYSHA GENE THERAPIES, INC.
|
By: |
Name: Kamran Alam |
Title: Chief Financial Officer |
Date: |
|
|
|
ADMINISTRATIVE AGENT USE ONLY |
Received by: |
Date: |
Verified by: |
Date: |
Compliance Status: |
Yes |
No |
EXHIBIT D LOAN ADVANCE REQUEST FORM I, Sean Nolan, certify that:
EX-31.1
14
tsha-ex31_1.htm
EX-31.1
EX-31.1
Exhibit 31.1
CERTIFICATION PURSUANT TO
RULES 13a-14(a) AND 15d-14(a) UNDER THE SECURITIES EXCHANGE ACT OF 1934,
AS ADOPTED PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
1.
I have reviewed this Quarterly Report on Form 10-Q of Taysha Gene Therapies, Inc.;
2.
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.
The registrant's other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in exchange act rules 13a-15(f) and 15d-15(f)) for the registrant and have:
(a)
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b)
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c)
Evaluated the effectiveness of the registrant's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d)
Disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter (the registrant's fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and
5.
The registrant's other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant's auditors and the audit committee of the registrant's board of directors (or persons performing the equivalent functions):
(a)
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant's ability to record, process, summarize and report financial information; and
(b)
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal control over financial reporting.
|
|
|
|
Date: November 4, 2025 |
|
By: |
/s/ Sean Nolan |
|
|
|
Sean Nolan |
|
|
|
Chief Executive Officer
(Principal Executive Officer)
|
EX-31.2
15
tsha-ex31_2.htm
EX-31.2
EX-31.2
Exhibit 31.2
CERTIFICATION PURSUANT TO
RULES 13a-14(a) AND 15d-14(a) UNDER THE SECURITIES EXCHANGE ACT OF 1934,
AS ADOPTED PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Kamran Alam, certify that:
1.
I have reviewed this Quarterly Report on Form 10-Q of Taysha Gene Therapies, Inc.;
2.
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.
The registrant's other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in exchange act rules 13a-15(f) and 15d-15(f)) for the registrant and have:
(a)
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b)
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c)
Evaluated the effectiveness of the registrant's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d)
Disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter (the registrant's fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and
5.
The registrant's other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant's auditors and the audit committee of the registrant's board of directors (or persons performing the equivalent functions):
(a)
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant's ability to record, process, summarize and report financial information; and
(b)
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal control over financial reporting.
|
|
|
|
Date: November 4, 2025 |
|
By: |
/s/ Kamran Alam |
|
|
|
Kamran Alam |
|
|
|
Chief Financial Officer
(Principal Financial Officer and Principal Accounting Officer)
|
EX-32.1
16
tsha-ex32_1.htm
EX-32.1
EX-32.1
Exhibit 32.1
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
Pursuant to the requirement set forth in Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. §1350), Sean Nolan, Chief Executive Officer of Taysha Gene Therapies, Inc. (the “Company”) hereby certifies that, to the best of his knowledge:
(1)
The Company’s Quarterly Report on Form 10-Q for the period ended September 30, 2025, to which this Certification is attached as Exhibit 32.1 (the “Periodic Report”) fully complies with the requirements of Section 13(a) or Section 15(d) of the Exchange Act; and
(2)
The information contained in the Periodic Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
|
|
|
Date: November 4, 2025 |
|
By: |
/s/ Sean Nolan |
|
|
|
Sean Nolan |
|
|
|
Chief Executive Officer
(Principal Executive Officer)
|
EX-32.2
17
tsha-ex32_2.htm
EX-32.2
EX-32.2
Exhibit 32.2
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
Pursuant to the requirement set forth in Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. §1350), Kamran Alam, Chief Financial Officer of Taysha Gene Therapies, Inc. (the “Company”) hereby certifies that, to the best of his knowledge:
(1)
The Company’s Quarterly Report on Form 10-Q for the period ended September 30, 2025, to which this Certification is attached as Exhibit 32.2 (the “Periodic Report”) fully complies with the requirements of Section 13(a) or Section 15(d) of the Exchange Act; and
(2)
The information contained in the Periodic Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
|
|
|
Date: November 4, 2025 |
|
By: |
/s/ Kamran Alam |
|
|
|
Kamran Alam |
|
|
|
Chief Financial Officer
(Principal Financial Officer and Principal Accounting Officer)
|









