UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): October 29, 2025
Tectonic Therapeutic, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 001-38537 | 81-0710585 | ||
| (state or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
| 490 Arsenal Way, Suite 210 Watertown, Massachusetts |
02472 | |||
| (Address of principal executive offices) | (Zip Code) | |||
Registrant’s telephone number, including area code: (339) 666-3320
Not applicable
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange on which registered |
||
| Common Stock, $0.0001 par value per share | TECX | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 8.01 | Other Events. |
On October 29, 2025, Tectonic Therapeutic, Inc. (the “Company”) posted a presentation titled “TX45 Phase 1b (Part B) PH-HFrEF Data Release Single Dose Hemodynamic Trial” on its website, a copy of which is attached as Exhibit 99.1 to this Current Report on Form 8-K.
On October 29, 2025, the Company issued a press release titled “Tectonic Therapeutic Announces Positive Topline Data from Phase 1b Part B Clinical Trial for TX45 in Patients with Group 2 Pulmonary Hypertension in HFrEF.” A copy of the press release is attached as Exhibit 99.2 to this Current Report on Form 8-K and, other than the quotes contained therein, is incorporated herein by reference.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit |
Description |
|
| 99.1 | Presentation dated October 29, 2025. | |
| 99.2 | Press Release dated October 29, 2025. | |
| 104 | Cover Page Interactive Data File (the cover page XBRL tags are embedded within the Inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| TECTONIC THERAPEUTIC, INC. | ||
| By: | /s/ Daniel Lochner |
|
| Daniel Lochner Chief Financial Officer |
||
Dated: October 29, 2025

Exhibit 99.1 TX45 Phase 1b (Part B) PH-HFrEF Data Release Single Dose Hemodynamic Trial O c t o b e r 2025

2 DISCLAIMER Statements contained in this presentation regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Words such as anticipates, believes, expects, intends, “plans,” “potential,” projects,” “would” and future or similar expressions are intended to identify forward-looking statements. Each of these forward-looking statements involves substantial risks and uncertainties that could cause actual results to differ significantly from those expressed or implied by such forward- looking statements. Forward-looking statements contained in this presentation include, but are not limited to, statements regarding: the design, objectives, initiation, timing, progress and results of current and future preclinical studies and clinical trials of our product candidates, including the ongoing Phase 2 clinical trial for TX45, in Group 2 Pulmonary Hypertension; the expected timing of program updates and data disclosures; the timing of filing INDs and other regulatory documents; the competitive landscape for and market potential of our product candidates; and the potential properties and benefits of TX45. These forward-looking statements reflect our current beliefs and expectations. Many factors may cause differences between current expectations and actual results, including the early stage of our development efforts; success in preclinical testing and earlier clinical trials does not ensure that later clinical trials will generate the same results or otherwise provide adequate data to demonstrate the efficacy and safety of a product candidates; clinical site activation rates or clinical trial enrollment rates that are lower than expected; changes in expected or existing competition; changes in the regulatory environment; the uncertainties and timing of the regulatory approval process; the impact of macroeconomic conditions, including the conflict in Ukraine and the conflict in the Middle East, heightened inflation and uncertain credit and financial markets, on our business, clinical trials and financial position; and unexpected litigation or other disputes. These and other risks are described more fully in our filings with the Securities and Exchange Commission (“SEC”), including the risks detailed in our Quarterly Report on Form 10-Q filed with the SEC on August 7, 2025, and other documents we subsequently filed with or furnished to the SEC. All forward-looking statements contained in this presentation speak only as of the date on which they were made. Except as required by law, we assume no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such data after the date of this presentation. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.
3 Positive Results in TX45 Single Dose Study in PH-HFrEF Patients Trial Goal • Assess safety and tolerability of TX45 in patients with PH-HFrEF • Observe trends in hemodynamic effects similar to Phase 1b (Part A) PH-HFpEF study (Topline data reported January 2025) Results • TX45 was observed to be well tolerated • Improvements in left ventricular function and pulmonary hemodynamics in PH-HFrEF were consistent with effects demonstrated in PH-HFpEF Outlook • Phase 1b Part B data support further clinical investigation of TX45 in PH-HFrEF, pending data in APEX Phase 2, expanding potential market opportunity • Ongoing APEX Phase 2 trial is evaluating chronic treatment in ~180 PH-HFpEF patients ‒ Study enriched for CpcPH (PVR≥3), primary endpoint is in this patient population PH-HFrEF = pulmonary hypertension, heart failure with reduced ejection fraction PH-HFpEF = pulmonary hypertension, heart failure with preserved ejection fraction = pulmonary vascular resistance PVR CpcPH = combined post- and pre-capillary pulmonary hypertension
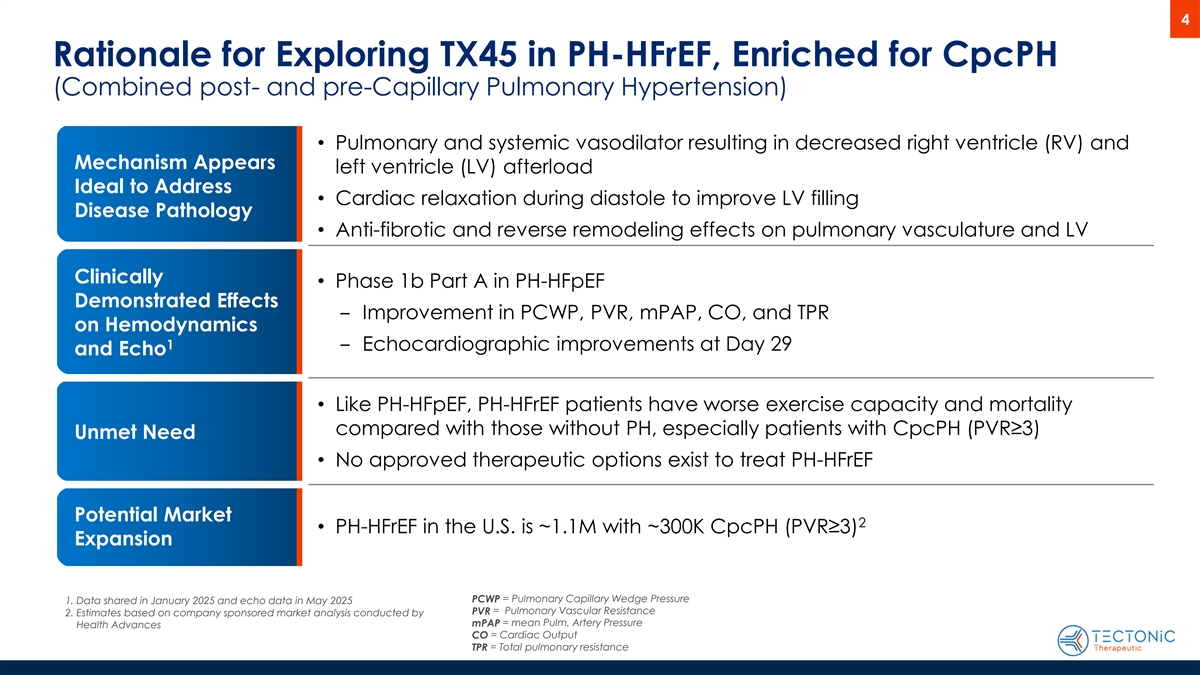
4 Rationale for Exploring TX45 in PH-HFrEF, Enriched for CpcPH (Combined post- and pre-Capillary Pulmonary Hypertension) • Pulmonary and systemic vasodilator resulting in decreased right ventricle (RV) and Mechanism Appears left ventricle (LV) afterload Ideal to Address • Cardiac relaxation during diastole to improve LV filling Disease Pathology • Anti-fibrotic and reverse remodeling effects on pulmonary vasculature and LV Clinically • Phase 1b Part A in PH-HFpEF Demonstrated Effects ‒ Improvement in PCWP, PVR, mPAP, CO, and TPR on Hemodynamics 1 ‒ Echocardiographic improvements at Day 29 and Echo • Like PH-HFpEF, PH-HFrEF patients have worse exercise capacity and mortality compared with those without PH, especially patients with CpcPH (PVR≥3) Unmet Need • No approved therapeutic options exist to treat PH-HFrEF Potential Market 2 • PH-HFrEF in the U.S. is ~1.1M with ~300K CpcPH (PVR≥3) Expansion PCWP = Pulmonary Capillary Wedge Pressure 1. Data shared in January 2025 and echo data in May 2025 PVR = Pulmonary Vascular Resistance 2. Estimates based on company sponsored market analysis conducted by mPAP = mean Pulm. Artery Pressure Health Advances CO = Cardiac Output TPR = Total pulmonary resistance

5 Different LV Pathology but Similar Pulmonary Vascular and RV Consequences Thickened, Weakened Normal less distensible heart muscle heart heart muscle HFpEF HFrEF Characteristics (Heart Failure with Preserved Ejection Fraction) (Heart Failure with Reduced Ejection Fraction) ≤40% >40%* Ejection Fraction % Myocardial infarction, Hypertension, aging, Common Causes cardiomyopathy diabetes, obesity Less distensible, pumping impairment filling Dilated, normal-sized during systole, impairment Left Ventricle State weak or concentric sensitive to SVR in diastole hypertrophy Driver of PH, Elevated left heart filling pressures can lead to PH and RV failure RV failure Hemodynamic Increases in PCWP, mPAP, PVR; Decrease in CO Changes * Current definition of HFmrEF is 41% to 49% EF and HFpEF is EF≥50%

6 Phase 1b (Part B) Trial Design A Single Dose, Open-Label, Acute Hemodynamic Trial in PH-HFrEF (n = 14) TX45 IV n = 1 @ 0.3 mg/kg n = 1 @ 1 mg/kg n = 12 @ 3 mg/kg Days 2, 15, 29: ECHO Days 8, 43: Safety Right Heart Catheter (RHC) is inserted Screening Follow-ups and hemodynamic measurements are Admit to Unit Day -28 to -2 captured at the timepoints listed above Day -1 Day 1 - Hemodynamic data was prespecified to be pooled across all doses. After IV administration, all dose levels result in exposures which are in the predicted efficacious range during the 8-hour assessment period (i.e., above trough exposure of 2 ug/ml) - Pure intention to treat (ITT) analysis performed: all data points included in the analysis

7 Key Hemodynamic Measures Goal: Treatment for PH due to heart failure needs to both increase LV function and improve pulmonary vascular and right ventricular component of the disease Hemodynamic Definition Significance PCWP• Measure of left atrial pressure• Key marker of left ventricular (LV) function (Pulmonary Capillary Wedge Pressure) • Measure of resistance to blood flow in • Health of the pulmonary vessels PVR (Pulmonary Vascular pulmonary vessels Resistance) • PVR = (mPAP – PCWP)/CO SVR • Measure of resistance to blood flow • Critical indicator of cardiovascular heath (Systemic Vascular through the entire systemic circulation Resistance) • SVR = (mAP – CVP)/CO TPR• Measure of right ventricular afterload• Key marker of resistance, how hard must the (Total Pulmonary • TPR = mPAP/CO right ventricle (RV) work Resistance) CO• Amount of blood heart pumps • How well is the heart working (both RV and LV) (Cardiac Output) (volume/time) • CO = heart rate x stroke volume SV• Amount of blood ejected from ventricle • Effectiveness of the heart at pumping blood (Stroke Volume) per beat (both RV and LV) Note: mPAP = mean Pulmonary Artery Pressure = average pressure required to pump blood through the lungs, mAP = mean Arterial Pressure, CO = Cardiac Output, CVP = central venous pressure
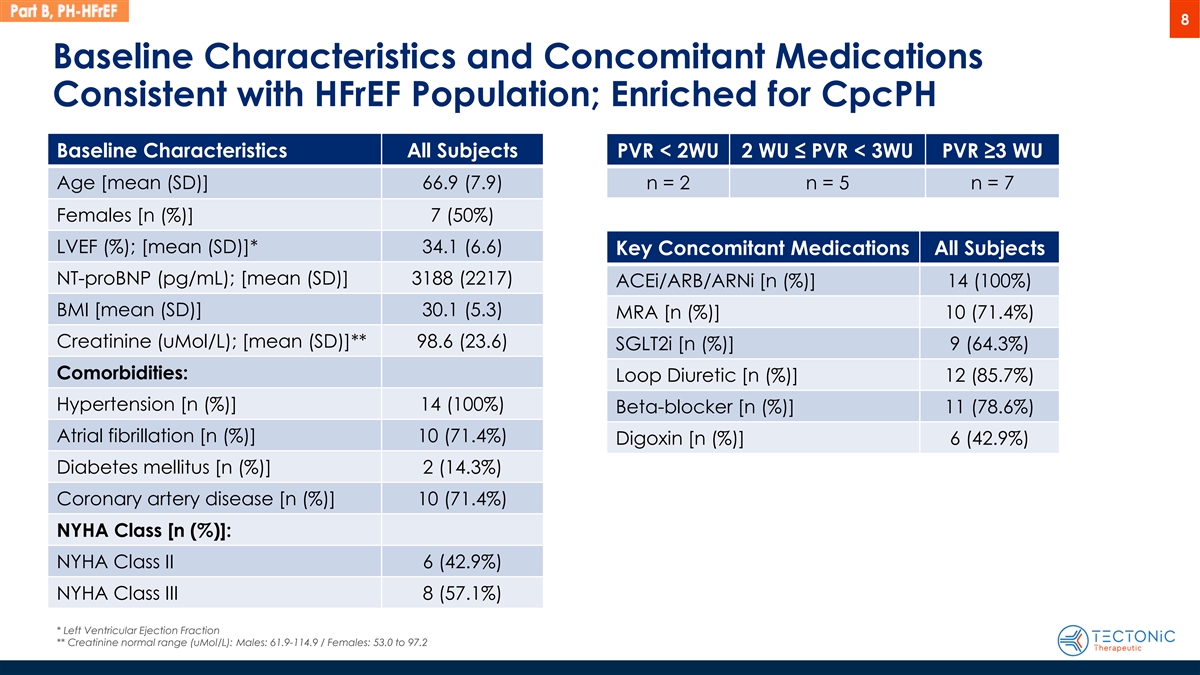
8 Baseline Characteristics and Concomitant Medications Consistent with HFrEF Population; Enriched for CpcPH Baseline Characteristics All Subjects PVR < 2WU 2 WU ≤ PVR < 3WU PVR ≥3 WU Age [mean (SD)] 66.9 (7.9) n = 2 n = 5 n = 7 Females [n (%)] 7 (50%) LVEF (%); [mean (SD)]* 34.1 (6.6) Key Concomitant Medications All Subjects NT-proBNP (pg/mL); [mean (SD)] 3188 (2217) ACEi/ARB/ARNi [n (%)] 14 (100%) BMI [mean (SD)] 30.1 (5.3) MRA [n (%)] 10 (71.4%) Creatinine (uMol/L); [mean (SD)]** 98.6 (23.6) SGLT2i [n (%)] 9 (64.3%) Comorbidities: Loop Diuretic [n (%)] 12 (85.7%) Hypertension [n (%)] 14 (100%) Beta-blocker [n (%)] 11 (78.6%) Atrial fibrillation [n (%)] 10 (71.4%) Digoxin [n (%)] 6 (42.9%) Diabetes mellitus [n (%)] 2 (14.3%) Coronary artery disease [n (%)] 10 (71.4%) NYHA Class [n (%)]: NYHA Class II 6 (42.9%) NYHA Class III 8 (57.1%) * Left Ventricular Ejection Fraction ** Creatinine normal range (uMol/L): Males: 61.9-114.9 / Females: 53.0 to 97.2

9 Baseline Hemodynamics are Consistent with PH-HFrEF Generally Accepted All Patients Baseline Value Parameter Normal Ranges Mean (SD) (At Rest) Systolic Blood Pressure (mm Hg) 136 (14.1) 90-120 Diastolic Blood Pressure (mm Hg) 78 (11.3) 60-80 Pulmonary Capillary Wedge Pressure (mm Hg) 21.1 (5.2) 4-12 Pulmonary Vascular Resistance (Wood Units) 3.26 (1.46) <2 Cardiac Output (L/min) 4.23 (1.53) 4-8 Stroke Volume (mL) 62.9 (23.9) 60-100 Total Pulmonary Resistance (Wood Units) 8.94 (3.31) <3 Mean Pulmonary Artery Pressure (mm Hg) 34.1 (6.8) 12-16 Systemic Vascular Resistance (Wood Units) 22.4 (6.5) 10-15 Right Atrial Pressure (mm Hg) 10.3 (3.6) 2-8

10 TX45 was Well-Tolerated in PH-HFrEF After a Single Dose • No serious or severe adverse events, Treatment-emergent adverse events (TEAE) discontinuations, infusion related reactions or drug related adverse 0.3 mg/kg 1 mg/kg 3 mg/kg Total Preferred Term events (n = 1) (n = 1) (n = 12) (n = 14) • All TEAEs were mild/moderate and Procedural back pain* 1 - 5 6 (42.9%) self-limited * TEAE of procedural back pain due to Right Heart Catheterization • There were no clinically significant changes in vital signs, ECG or safety laboratory values ‒ Transient asymptomatic drop of sBP (5-10 mm Hg) on day 1 • No signs or symptoms of congestion • No TEAE of fatigue
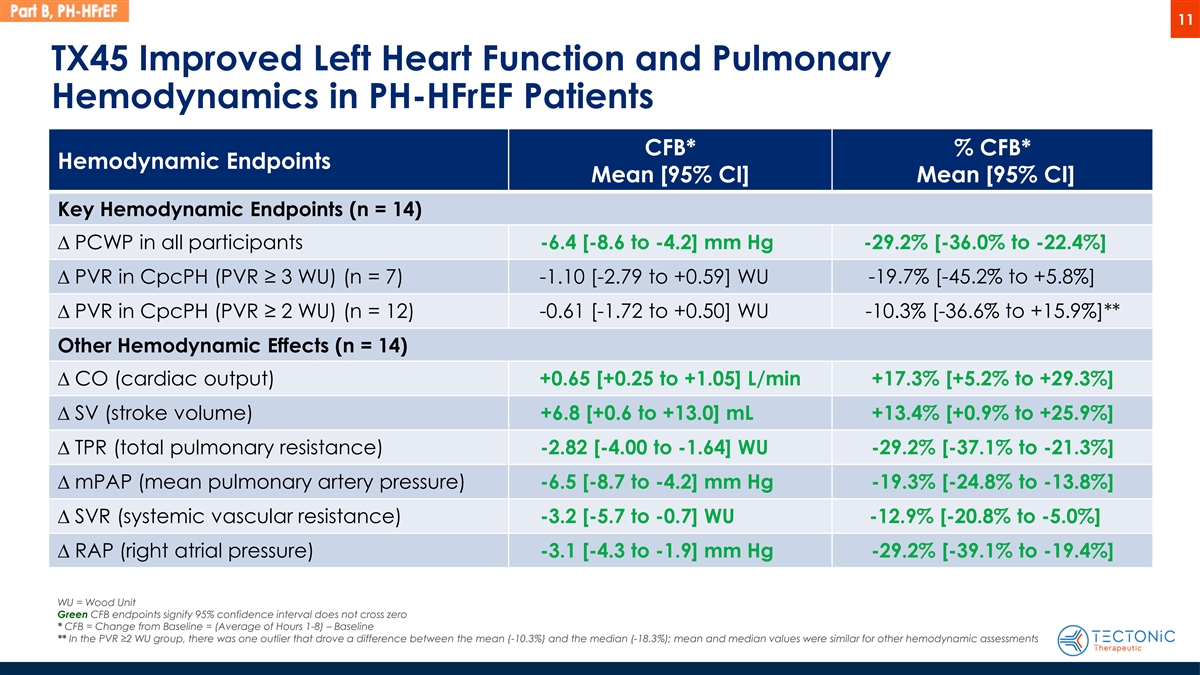
11 TX45 Improved Left Heart Function and Pulmonary Hemodynamics in PH-HFrEF Patients CFB* % CFB* Hemodynamic Endpoints Mean [95% CI] Mean [95% CI] Key Hemodynamic Endpoints (n = 14) D PCWP in all participants -6.4 [-8.6 to -4.2] mm Hg -29.2% [-36.0% to -22.4%] D PVR in CpcPH (PVR ≥ 3 WU) (n = 7) -1.10 [-2.79 to +0.59] WU -19.7% [-45.2% to +5.8%] -0.61 [-1.72 to +0.50] WU -10.3% [-36.6% to +15.9%]** D PVR in CpcPH (PVR ≥ 2 WU) (n = 12) Other Hemodynamic Effects (n = 14) D CO (cardiac output) +0.65 [+0.25 to +1.05] L/min +17.3% [+5.2% to +29.3%] D SV (stroke volume) +6.8 [+0.6 to +13.0] mL +13.4% [+0.9% to +25.9%] D TPR (total pulmonary resistance) -2.82 [-4.00 to -1.64] WU -29.2% [-37.1% to -21.3%] D mPAP (mean pulmonary artery pressure) -6.5 [-8.7 to -4.2] mm Hg -19.3% [-24.8% to -13.8%] D SVR (systemic vascular resistance) -3.2 [-5.7 to -0.7] WU -12.9% [-20.8% to -5.0%] D RAP (right atrial pressure) -3.1 [-4.3 to -1.9] mm Hg -29.2% [-39.1% to -19.4%] WU = Wood Unit Green CFB endpoints signify 95% confidence interval does not cross zero * CFB = Change from Baseline = (Average of Hours 1-8) – Baseline ** In the PVR ≥2 WU group, there was one outlier that drove a difference between the mean (-10.3%) and the median (-18.3%); mean and median values were similar for other hemodynamic assessments
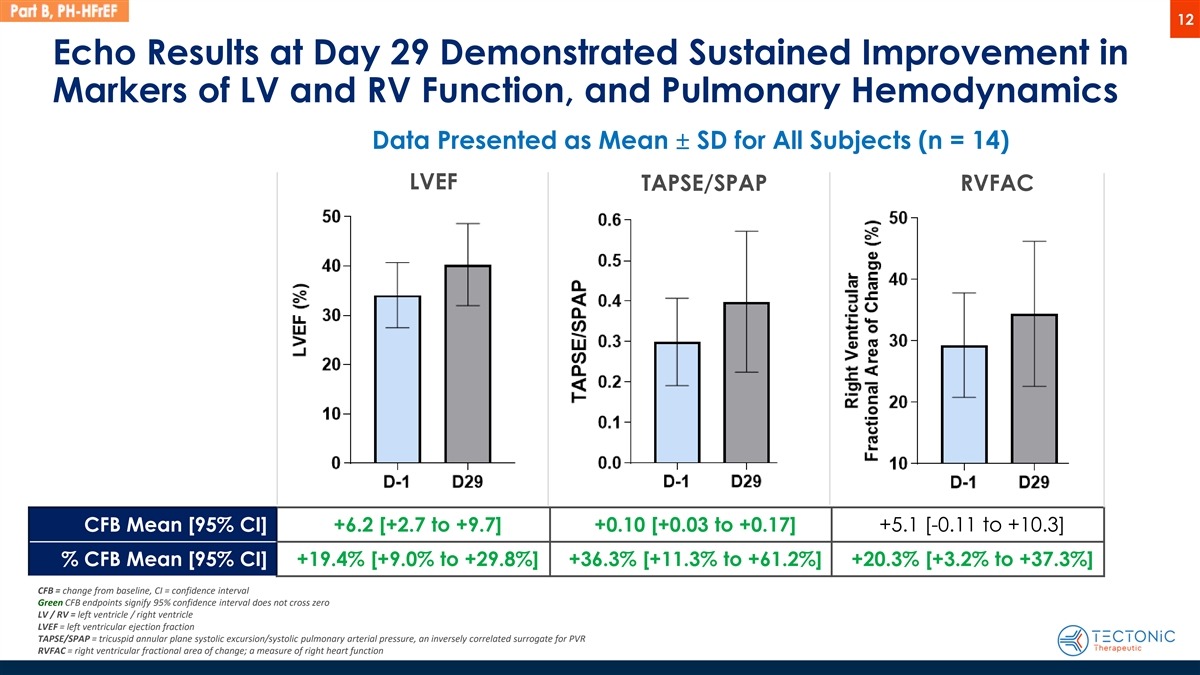
12 Echo Results at Day 29 Demonstrated Sustained Improvement in Markers of LV and RV Function, and Pulmonary Hemodynamics Data Presented as Mean ± SD for All Subjects (n = 14) LVEF TAPSE/SPAP RVFAC CFB Mean [95% CI] +6.2 [+2.7 to +9.7] +0.10 [+0.03 to +0.17] +5.1 [-0.11 to +10.3] % CFB Mean [95% CI] +19.4% [+9.0% to +29.8%] +36.3% [+11.3% to +61.2%] +20.3% [+3.2% to +37.3%] CFB = change from baseline, CI = confidence interval Green CFB endpoints signify 95% confidence interval does not cross zero LV / RV = left ventricle / right ventricle LVEF = left ventricular ejection fraction TAPSE/SPAP = tricuspid annular plane systolic excursion/systolic pulmonary arterial pressure, an inversely correlated surrogate for PVR RVFAC = right ventricular fractional area of change; a measure of right heart function
13 Summary: TX45 Improves Cardiac and Pulmonary Hemodynamics in PH-HFrEF and PH-HFpEF Patients • TX45 was well-tolerated in both patient populations • TX45 demonstrated hemodynamic improvements in both left heart function and in the pulmonary vasculature in PH-HFrEF, consistent with effects in PH-HFpEF ‒ Acute improvement in left ventricular hemodynamics and pulmonary vascular / RV hemodynamics (PCWP, CO, PVR, TPR, mPAP) ‒ Echocardiographic improvements at Day 29 (LVEF, TAPSE/SPAP, RVFAC) ‒ The magnitude of the reduction in PCWP and PVR, demonstrated in our HFpEF and HFrEF 1-3 cohorts, have been associated with meaningful changes in 6MWD in previous studies ‒ Right ventricular afterload, as assessed by TPR, was reduced. Reduced RV afterload has been associated with improved mortality and outcomes in cohort studies of patients with 4 Group 2 PH • Results support future expansion of TX45’s addressable Group 2 PH patient population to PH-HFrEF, pending results of ongoing APEX Phase 2 clinical trial 1. Lewis GD et al. Circ. Heart Failure 2023 2. Lewis GD et al. Circulation 2007 3. Zhang H et al. JACC Cardiovasc. Interv. 2019 4. Tampakakis,, Circ Heart Failure, 2018
Exhibit 99.2
Tectonic Therapeutic Announces Positive Topline Data from Phase 1b Part B Clinical Trial for TX45 in Patients with Group 2 Pulmonary Hypertension in HFrEF
| • | TX45 improved both left heart function and pulmonary hemodynamics in patients with Group 2 Pulmonary Hypertension in Heart Failure with reduced Ejection Fraction (“PH-HFrEF”) |
| • | TX45 was well tolerated in patients with PH-HFrEF with no serious or severe adverse events, no clinically significant changes in blood pressure and no immune related reactions |
| • | Results support potential expansion of TX45’s addressable Group 2 PH patient population to PH-HFrEF |
| • | Company to host webcast today, October 29th at 4:30 p.m. ET |
WATERTOWN, Mass., October 29, 2025 (GLOBE NEWSWIRE) — Tectonic Therapeutic, Inc. (NASDAQ: TECX) (“Tectonic”), today announced positive topline results from the Phase 1b Part B acute hemodynamic clinical trial of TX45, a long-acting, Fc-relaxin fusion protein, in patients with Group 2 PH-HFrEF. The topline data showed that a single intravenous dose of TX45 was well tolerated in this patient population and resulted in meaningful improvements in both left heart function and pulmonary hemodynamics.
The Phase 1b Part B trial was designed to evaluate TX45 in the expanded patient population of Group 2 PH-HFrEF, building on the positive results from the Phase 1b Part A trial of TX45 in patients with Group 2 Pulmonary Hypertension in Heart Failure with preserved Ejection Fraction (“PH-HFpEF”). Tectonic is currently conducting the APEX Phase 2 clinical trial to evaluate TX45 over a 24-week treatment period in patients with PH-HFpEF, with topline results expected in 2026 (ClinicalTrials.gov NCT06616974). The APEX clinical trial is enriched for patients with combined pre- and post-capillary pulmonary hypertension (“CpcPH”) with pulmonary vascular resistance >3, and the primary endpoint is in this patient population.
“We met the goal of this exploratory study in patients with PH-HFrEF, which was to observe improvements in hemodynamic effects, including PCWP, PVR, CO, TPR and mPAP, that were directionally similar to the positive results of our Phase 1b Part A study in PH-HFpEF,” said Alise Reicin, M.D., President and Chief Executive Officer of Tectonic. “These results in PH-HFrEF open up the potential to expand into this additional patient population with significant unmet need and no approved therapies, pending results from the ongoing APEX Phase 2 clinical trial.”
“Similar to what was seen with TX45 in patients with PH-HFpEF, this study in patients with PH-HFrEF demonstrated clinically important changes across multiple hemodynamic measures, showing the broad potential of TX45 across different patient populations with pulmonary hypertension associated with heart failure,” said John Teerlink, M.D., Professor of Medicine, University of California, San Francisco. “It is encouraging to see that TX45 reduced PCWP along with the decrease in afterload on the right ventricle, which appears to address the underlying pathologies of CpcPH that lead to impairment of exercise capacity, poor outcomes and increased mortality in both PH-HFrEF and PH-HFpEF.”
Highlights from Phase 1b Part B Topline Results and Clinical Trial Overview
The topline results from the Phase 1b Part B open label clinical trial are based on 14 enrolled patients with PH-HFrEF. Within the 14 patients, 7 patients had CpcPH as measured by pulmonary vascular resistance >3 Wood units (“PVR” >3 “WU”), 5 patients had CpcPH as measured by PVR >2 WU and <3 WU, and 2 patients had isolated post-capillary pulmonary hypertension (“IpcPH”) as measured by PVR <2 WU.
Hemodynamic Results: TX45 administration resulted in meaningful improvement in both left heart function and pulmonary hemodynamics, endpoints that most strongly match the severe pathophysiology of the subpopulation of patients with CpcPH.
Hemodynamic measures evaluating left heart function included pulmonary capillary wedge pressure (“PCWP”), and cardiac output (“CO”). Hemodynamic measures evaluating the pulmonary vasculature included PVR, total pulmonary resistance (“TPR”) and mean pulmonary artery pressure (“mPAP”). PCWP and PVR are endpoints known to correlate with exercise capacity, morbidity and mortality in patients with Group 2 pulmonary hypertension. TPR is an important measure of right ventricular afterload, and persistent elevations of afterload often lead to right heart failure and worse outcomes.
| Hemodynamic measure |
Number of subjects |
% Change from baseline* mean |
[95% Confidence Interval, “CI”] |
|||||||
| Pulmonary Capillary Wedge Pressure (PCWP) |
14 | -29.2% | [-36.0% to -22.4%] | |||||||
| Pulmonary Vascular Resistance (PVR): CpcPH with PVR >3 Wood units |
7 | -19.7% | [-45.2% to +5.8%] | |||||||
| CpcPH with PVR >2 Wood units |
12 | -10.3% | ** | [-36.6% to +15.9%] | ||||||
| Cardiac Output (CO) |
14 | +17.3% | [+5.2% to +29.3%] | |||||||
| Total Pulmonary Resistance (TPR) |
14 | -29.2% | [-37.1% to -21.3%] | |||||||
| Mean Pulmonary Artery Pressure (mPAP) |
14 | -19.3% | [-24.8% to -13.8%] | |||||||
| * | Change from baseline = (Average of Hours, 1-8) - Baseline |
| ** | In the PVR>2 group, there was one outlier that drove a difference between the mean (-10.3%) and the median (-18.3%). Mean and median values were similar for other hemodynamic assessments. |
Echocardiography Results: The echocardiography data support persistence of TX45 single dose improvement in left and right ventricular function and on pulmonary hemodynamics at 29 days post dose.
| • | For LVEF (left ventricular ejection fraction), TX45 demonstrated a mean percent change from baseline improvement of +19.4% [95% CI, +9.0% to +29.8%] at day 29. |
| • | For RVFAC (right ventricular fractional area of change), a measure correlated with right ventricular contractile function, TX45 demonstrated a mean percent change from baseline improvement of +20.3% [95% CI, +3.2% to +37.3%] at day 29. |
| • | For TAPSE/SPAP (tricuspid annular plane systolic excursion/systolic pulmonary artery pressure), a ratio that is inversely correlated with PVR, TX45 demonstrated a mean percent change from baseline improvement of +36.3% [95% CI, +11.3% to +61.2%] at day 29. |
Safety Results: TX45 was well tolerated with no serious or severe adverse events, discontinuations, infusion reactions or drug-related adverse events.
| • | There were no clinically significant changes in vital signs, physical exam or safety laboratory values. |
| • | Transient asymptomatic decreases in blood pressure were observed over the first 24 hours after TX45 dosing. |
| • | There were no symptoms or signs of congestion, and no adverse experience of fatigue was reported. |
“We are highly encouraged that these Part B data in PH-HFrEF demonstrated hemodynamic improvements in both left heart function and in the pulmonary vasculature. It is also gratifying to observe that a single dose of TX45 showed persistent effects at day 29 based on the echocardiographic data, as well as being well tolerated in PH-HFrEF patients,” said Marcella K. Ruddy, M.D., Chief Medical Officer of Tectonic. “Our team is focused on continuing to work toward completing the APEX Phase 2 trial, enriched for patients with CpcPH with PVR >3 where the benefit of a relaxin therapy may be the greatest.”
The Phase 1b open label clinical trial evaluated the safety and hemodynamic effect of single doses of TX45 in patients with Group 2 pulmonary hypertension. The design of the clinical trial was as follows: after obtaining informed consent, a right heart catheter, which is the gold standard for the measurement of cardiopulmonary hemodynamics, was inserted and baseline measurements were obtained, an intravenous dose of TX45 were administered, and hemodynamic effects were evaluated over 8 hours post dose. Participants were then followed for 45 days post dose for safety and exploratory biomarker endpoints.
Conference Call
Tectonic will host a webcast today, October 29, 2025, at 4:30 p.m. ET. The live webcast of the event will be available here and under Events and Presentations in the Investors section of the Company’s website at www.tectonictx.com. A replay of the webcast will also be available on the Company’s website after the call’s conclusion.
About TX45, a Long-Acting Fc-Relaxin Fusion Protein
TX45 is an Fc-relaxin fusion protein with optimized pharmacokinetics and biophysical properties that activates the RXFP1 receptor, the G-protein coupled receptor target of the hormone relaxin. Relaxin is an endogenous protein, expressed at low levels in both men and women that is a pulmonary and systemic vasodilator with lusitropic, anti-fibrotic and anti-inflammatory activity. In normal human physiology, relaxin is upregulated during pregnancy where it exerts vasodilative effects, reduces systemic and pulmonary vascular resistance and increases cardiac output to accommodate the increased demand for oxygen and nutrients from the developing fetus. Relaxin also exerts anti-fibrotic effects on pelvic ligaments to facilitate delivery of the baby.
About Tectonic
Tectonic is a biotechnology company focused on the discovery and development of therapeutic proteins and antibodies that modulate the activity of G-protein coupled receptors (“GPCRs”). Leveraging its proprietary technology platform called GEODe™ (GPCRs Engineered for Optimal Discovery), Tectonic is focused on developing biologic medicines that overcome the existing challenges of GPCR-targeted drug discovery and harness the human body to modify the course of disease. Tectonic focuses on areas of significant unmet medical need, often where therapeutic options are poor or nonexistent, as these are areas where new medicines have the potential to improve patient quality of life. Tectonic is headquartered in Watertown, Massachusetts. For more information, please visit www.tectonictx.com and follow on LinkedIn.
Forward-Looking Statements
This press release contains “forward-looking statements” within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. All statements in this press release other than statements of historical facts are “forward-looking statements.” These statements may be identified by words such as “aims,” “anticipates,” “believes,” “could,” “estimates,” “expects,” “forecasts,” “goal,” “intends,” “may,” “plans,” “possible,” “potential,” “seeks,” “will” and variations of these words or similar expressions that are intended to identify forward-looking statements, although not all forward-looking statements contain these words. Forward-looking statements in this press release include, but are not limited to, statements regarding: the design, objectives, initiation, timing, progress and results of current and future preclinical studies and clinical trials of Tectonic’s product candidates, including the ongoing Phase 1b and Phase 2 clinical trials for its lead program, TX45, in Group 2 PH-HFpEF and Group 2 PH-HFrEF; and the potential properties and benefits of TX45. These forward-looking statements are based on Tectonic’s expectations and assumptions as of the date of this press release. Each of these forward-looking statements involves risks and uncertainties that could cause Tectonic’s clinical development programs, future results or performance to differ materially from those expressed or implied by the forward-looking statements. Many factors may cause differences between current expectations and actual results, including: the potential that success in preclinical testing and earlier clinical trials does not ensure that later clinical trials will generate the same results or otherwise provide adequate data to demonstrate the efficacy and safety of a product candidate; the impacts of macroeconomic conditions, including the conflict in Ukraine and the conflict in the Middle East, heightened inflation and uncertain credit and financial markets, on Tectonic’s business, clinical trials and financial position; unexpected safety or efficacy data observed during preclinical studies or clinical trials; clinical trial site activation or enrollment rates that are lower than expected; Tectonic’s ability to realize the benefits of its collaborations and license agreements; changes in expected or existing competition; changes in the regulatory environment; the uncertainties and timing of the regulatory approval process; and unexpected litigation or other disputes. Other factors that may cause Tectonic’s actual results to differ from those expressed or implied in the forward-looking statements in this press release are identified under the heading “Risk Factors” in Tectonic’s quarterly report on Form 10-Q filed with the SEC on August 7, 2025, and in other filings that Tectonic makes and will make with the SEC in the future. Tectonic expressly disclaims any obligation to update any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law. For more information, please visit www.tectonictx.com and follow @TectonicTx on X (formerly Twitter) and LinkedIn.
Contacts:
Investors:
Dan Ferry
LifeSci Advisors
daniel@lifesciadvisors.com
(617) 430-7576
Media:
Kathryn Morris
The Yates Network
kathryn@theyatesnetwork.com
(914) 204-6412