UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported): October 29, 2025
SAVARA INC.
(Exact name of Registrant as specified in its charter)
| Delaware | 001-32157 | 84-1318182 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
1717 Langhorne Newtown Road, Suite 300
Langhorne, PA 19047
(Address of principal executive offices, including zip code)
(512) 614-1848
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange |
||
| Common Stock, par value $0.001 per share | SVRA | The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 1.01 | Entry into a Material Definitive Agreement. |
On October 29, 2025, Savara Inc. (the “Company”) announced its entry into a purchase and sale agreement (the “Purchase Agreement”) with funds managed by RTW Investments, LP (the “Purchaser”). Under the terms of the Purchase Agreement, the Purchaser has agreed to pay the Company $75 million (the “Purchase Price”) upon approval of the Company’s investigational inhaled biologic, molgramostim inhalation solution (“MOLBREEVI”), for the treatment of autoimmune pulmonary alveolar proteinosis (“autoimmune PAP”) by the FDA on or before March 31, 2027 and subject to satisfaction of other customary closing conditions, in exchange for a true sale of assigned interests, including the right to receive royalty payments equal to a percentage of Net Sales (as defined in the Purchase Agreement) of MOLBREEVI in the U.S. The royalty rate is tiered, with the payments ranging from 7.0% to 1.0% of Net Sales in each calendar year, with the 7.0% tier increasing to 9.5% for a calendar year if the prior year’s Net Sales do not achieve a specified level. The royalty payments commence in the first calendar quarter in which there is a commercial sale of MOLBREEVI in the U.S. and end upon the receipt by the Purchaser of $187.5 million (the “Maximum Payment”). Based on the Company’s current projections, the Company expects the effective royalty rate over the life of the Purchase Agreement will be in the low-single digits. The Purchase Agreement includes a buy-back option that may allow the Company to pay a specified amount up to the Maximum Payment to terminate the Purchase Agreement and all obligations in the event of certain changes of control within two years of receipt of the Purchase Price. Unless otherwise agreed with the Purchaser, the Company is required to use a portion of the Purchase Price to repay all outstanding indebtedness.
The Purchase Agreement contains customary affirmative and negative covenants, including covenants that limit or restrict the Company’s ability to, among other things, incur indebtedness (which restrictions are eliminated after the achievement by the Company of a specified amount of Net Sales), and other provisions customary for transactions of this nature, in each case subject to certain exceptions set forth in the Purchase Agreement.
The foregoing description of the Purchase Agreement does not purport to be complete and is qualified in its entirety by reference to the full text of the Purchase Agreement, a copy of which will be included as an exhibit to the Company’s Annual Report on Form 10-K for the year ending December 31, 2025 to be filed with the Securities and Exchange Commission (the “SEC”).
| Item 7.01 | Regulation FD Disclosure. |
On October 29, 2025, the Company issued a press release announcing the Purchase Agreement described above. A copy of the press release is furnished as Exhibit 99.1 and incorporated herein by reference.
The information contained under Item 7.01 of this Current Report on Form 8-K (this “Current Report”) (including Exhibit 99.1), shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act except as may be expressly set forth by specific reference in such filing.
| Item 8.01 | Other Events. |
Corporate Presentation
An abbreviated version of the Company’s corporate presentation is filed as Exhibit 99.2 to this Current Report on Form 8-K and incorporated by reference herein. The Company undertakes no duty or obligation to update or revise the information contained in this presentation, although it may do so from time to time. Any such updates may be made through the Investor Relations page of the Company’s website, the filing of other reports or documents with the SEC, press releases, or other public disclosure.
Litigation
On September 8, 2025, a stockholder filed putative securities class action claims against the Company and certain of our executive officers in the United States District Court for the Eastern District of Pennsylvania, purportedly on behalf of classes of the Company’s investors who purchased or otherwise acquired the Company’s common stock and securities between March 7, 2024 and May 23, 2025.
The complaint alleges violations of various sections of the Exchange Act and Rule 10b-5 promulgated thereunder in connection with various public statements made by the Company regarding its regulatory filings for MOLBREEVI as a therapy to treat patients with autoimmune PAP. The action seeks unspecified damages, costs and expenses, including attorneys’ fees. The Company intends to vigorously defend against the allegations. Given the nature of the case, including that the proceeding is in its early stages, the Company is unable to predict the ultimate outcome of the case or estimate the range of potential loss, if any. Regardless of the outcome, litigation can have an adverse impact on the Company because of defense and settlement costs, diversion of management attention and resources, and other factors.
Forward-Looking Statements
The Company cautions you that statements in this Current Report and the accompanying press release that are not a description of historical fact are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements may be identified by the use of words referencing future events or circumstances such as “expect,” “intend,” “plan,” “anticipate,” “believe,” and “will,” among others. Such statements include, but are not limited to, statements related to the Company’s expectations with respect to the description of the Purchase Agreement and the transactions contemplated thereby and statements regarding the pending litigation. The Company may not actually achieve any of the matters referred to in such forward-looking statements, and you should not place undue reliance on these forward-looking statements. These forward-looking statements are based upon the Company’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, the Company’s ability to satisfy the conditions to closing in the Purchase Agreement; the risks associated with the Company’s ability to successfully develop, obtain regulatory approval for, and commercialize MOLBREEVI for autoimmune PAP; the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations; the availability of sufficient resources for the Company’s operations and to conduct or continue planned clinical development programs; and the timing and ability of the Company to raise additional capital as needed to fund continued operations. All forward-looking statements are expressly qualified in their entirety by these cautionary statements. For a detailed description of the Company’s risks and uncertainties, you are encouraged to review the Company’s documents filed with the SEC including its recent filings on Form 8-K, Form 10-K and Form 10-Q. You are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date on which they were made. The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as may be required by law.
| Item 9.01 | Financial Statements and Exhibits |
(d) Exhibits.
| Exhibit |
Description |
|
| 99.1 | Press Release of the Company issued on October 29, 2025. | |
| 99.2 | Corporate Presentation. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document). | |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Dated: October 29, 2025 | SAVARA INC. a Delaware corporation |
|||||
| By: | /s/ Dave Lowrance |
|||||
| Dave Lowrance | ||||||
| Chief Financial & Administrative Officer | ||||||
Exhibit 99.1

SAVARA ANNOUNCES $75M ROYALTY FUNDING AGREEMENT WITH RTW TO
SUPPORT THE POTENTIAL LAUNCH OF MOLBREEVI* IN AUTOIMMUNE
PULMONARY ALVEOLAR PROTEINOSIS (AUTOIMMUNE PAP)
— RTW Committed $75M in Launch Funding, Subject to U.S. Food and Drug Administration (FDA) Approval of MOLBREEVI —
— Royalties Based on Annual Net Sales of MOLBREEVI in the U.S. —
— Funds Will Strengthen the Company’s Balance Sheet for Potential U.S. Commercialization of MOLBREEVI in Autoimmune PAP —
LANGHORNE, PA – October 29, 2025 – Savara Inc. (Nasdaq: SVRA) (the Company), a clinical stage biopharmaceutical company focused on rare respiratory diseases, announced a $75 million royalty funding agreement with funds managed by RTW Investments, LP (RTW), subject to FDA approval of MOLBREEVI.
“This non-dilutive strategic financing will support the U.S. launch of MOLBREEVI, assuming FDA approval, and allow us to further invest in commercial priorities,” said Matt Pauls, J.D., M.B.A., Chair and Chief Executive Officer, Savara. “We remain on track to resubmit the MOLBREEVI BLA this December and, given there are no approved medicines in the U.S. and Europe for autoimmune PAP, we believe this drug could fundamentally change the way this rare and chronic disease is treated. We are grateful to work with RTW to bring this important therapy to market.”
“The pivotal IMPALA-2 clinical trial demonstrated the potential of MOLBREEVI to treat autoimmune PAP and today’s investment reflects our confidence in Savara and the strong commercial potential of the therapy,” said Roderick Wong, M.D., Managing Partner and Chief Investment Officer, RTW Investments. “We are proud to partner with the Savara management team and look forward to supporting their efforts to bring this meaningful treatment to patients.”
The royalty financing will become available upon FDA’s approval of MOLBREEVI, and satisfaction of certain customary conditions. Under the terms of the agreement, RTW will receive a tiered single digit percentage of annual net sales of MOLBREEVI in the U.S. for the treatment of autoimmune PAP, subject to a cap.

About Autoimmune Pulmonary Alveolar Proteinosis (aPAP)
Autoimmune PAP is a rare lung disease characterized by the abnormal build-up of surfactant in the alveoli of the lungs. Surfactant consists of proteins and lipids and is an important physiological substance that lines the alveoli to prevent them from collapsing. In a healthy lung, excess surfactant is cleared and digested by immune cells called alveolar macrophages. Alveolar macrophages need to be stimulated by granulocyte-macrophage colony-stimulating factor (GM-CSF) to function properly in clearing surfactant, but in aPAP, GM-CSF is neutralized by antibodies against GM-CSF, rendering macrophages unable to adequately clear surfactant. As a result, an excess of surfactant accumulates in the alveoli, causing impaired gas transfer, resulting in clinical symptoms of shortness of breath, often with cough and frequent fatigue. Patients may also experience episodes of fever, chest pain, or coughing up blood, especially if secondary infection develops. In the long-term, the disease can lead to serious complications, including lung fibrosis and the need for a lung transplant.
About Savara
Savara is a clinical stage biopharmaceutical company focused on rare respiratory diseases. Our lead program, MOLBREEVI*, is a recombinant human granulocyte-macrophage colony stimulating factor (GM-CSF) in Phase 3 development for autoimmune pulmonary alveolar proteinosis (autoimmune PAP). MOLBREEVI is delivered via a proprietary investigational eFlow® Nebulizer System (PARI Pharma GmbH) specifically developed for inhalation of MOLBREEVI. Our management team has significant experience in rare respiratory diseases and pulmonary medicine, identifying unmet needs, and effectively advancing product candidates to approval and commercialization. More information can be found at www.savarapharma.com and LinkedIn.
| * | MOLBREEVI is the FDA and EMA conditionally accepted trade name for molgramostim inhalation solution. It is not approved in any indication. MOLBREEVI is a trademark of Savara Inc. |
Forward-Looking Statements
The Company cautions you that statements in this Current Report and the accompanying press release that are not a description of historical fact are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements may be identified by the use of words referencing future events or circumstances such as “expect,” “intend,” “plan,” “anticipate,” “believe,” and “will,” among others. Such statements include, but are not limited to, statements related to expected regulatory filing timelines, the impact of the royalty financing and planned use of the funds, the belief that MOLBREEVI could fundamentally change the way autoimmune PAP is treated, and the Company’s plans to bring MOLBREEVI to market. The Company may not actually achieve any of the matters referred to in such forward-looking statements, and you should not place undue reliance on these forward-looking statements.

These forward-looking statements are based upon the Company’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, the Company’s ability to satisfy the conditions to closing in the royalty funding agreement; the risks associated with the Company’s ability to successfully develop, obtain regulatory approval for, and commercialize MOLBREEVI for autoimmune PAP; the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations; the availability of sufficient resources for the Company’s operations and to conduct or continue planned clinical development programs; and the timing and ability of the Company to raise additional capital as needed to fund continued operations. All forward-looking statements are expressly qualified in their entirety by these cautionary statements. For a detailed description of the Company’s risks and uncertainties, you are encouraged to review the Company’s documents filed with the SEC including its recent filings on Form 8-K, Form 10-K and Form 10-Q. You are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date on which they were made. The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as may be required by law.
Media and Investor Relations Contact
Savara Inc.
Temre Johnson, Executive Director, Corporate Affairs
ir@savarapharma.com

Corporate Overview October 2025 © Savara Inc. All Rights Reserved. Exhibit 99.2

Safe Harbor Statement © Savara Inc. All Rights Reserved. Savara Inc. (“Savara” or the “Company”) cautions you that statements in this presentation that are not a description of historical fact are forward-looking statements which may be identified by the use of words such as “expect,” “intend,” “plan,” “anticipate,” “believe,” and “will,” among others. Such statements include, but are not limited to, statements regarding the planned use of proceeds from the proposed equity financing; statements related to the planned royalty financing; the potential health benefits and risks and projected development timeline of MOLBREEVI; the timing of regulatory submissions; the potential for and impact of regulatory approval; the potential addressable patient population, market size, commercial opportunity, and competitive landscape for MOLBREEVI; Savara’s commercial launch planning activities and the potential impact of those activities; GM-CSF autoantibody testing and its potential impact; and the sufficiency of our resources to fund the advancement of our development program and potential sources of additional capital. Savara may not actually achieve any of its plans or product development goals in a timely manner, if at all, or otherwise carry out its current intentions or meet the expectations or projections disclosed in its forward-looking statements, and you should not place undue reliance on these forward-looking statements. These forward-looking statements are based upon Savara's current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, the risks associated with our ability to successfully develop, obtain regulatory approval for and commercialize MOLBREEVI for autoimmune PAP; risks and uncertainties associated with the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations; the ability to successfully conduct clinical trials for our product candidate; the availability of sufficient resources and the timing and ability of Savara to raise additional capital as needed to fund continued operations. The risks and uncertainties facing Savara are described more fully in Savara's filings with the Securities and Exchange Commission, including our filings on Form 8-K, our Annual Report on Form 10-K for the fiscal year ended December 31, 2024, and our Quarterly Report on Form 10-Q for the quarter ended June 30, 2025. You are cautioned not to place undue reliance on our forward-looking statements, which speak only as of the date on which they were made. Savara undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as may be required by law. Third-party information included herein has been obtained from sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, and should not be construed as a representation by, the Company. Additionally, this presentation includes internal research and estimates performed by the Company, which have not been independently verified. MOLBREEVI (molgramostim inhalation solution) is an investigational product that has not been approved for sale or determined to be safe or effective by the U.S. Food & Drug Administration or any regulatory authority. MOLBREEVI and aPAP ClearPath are trademarks of Savara. All other trademarks included herein are the property of the owners thereof and are used for reference purposes only.
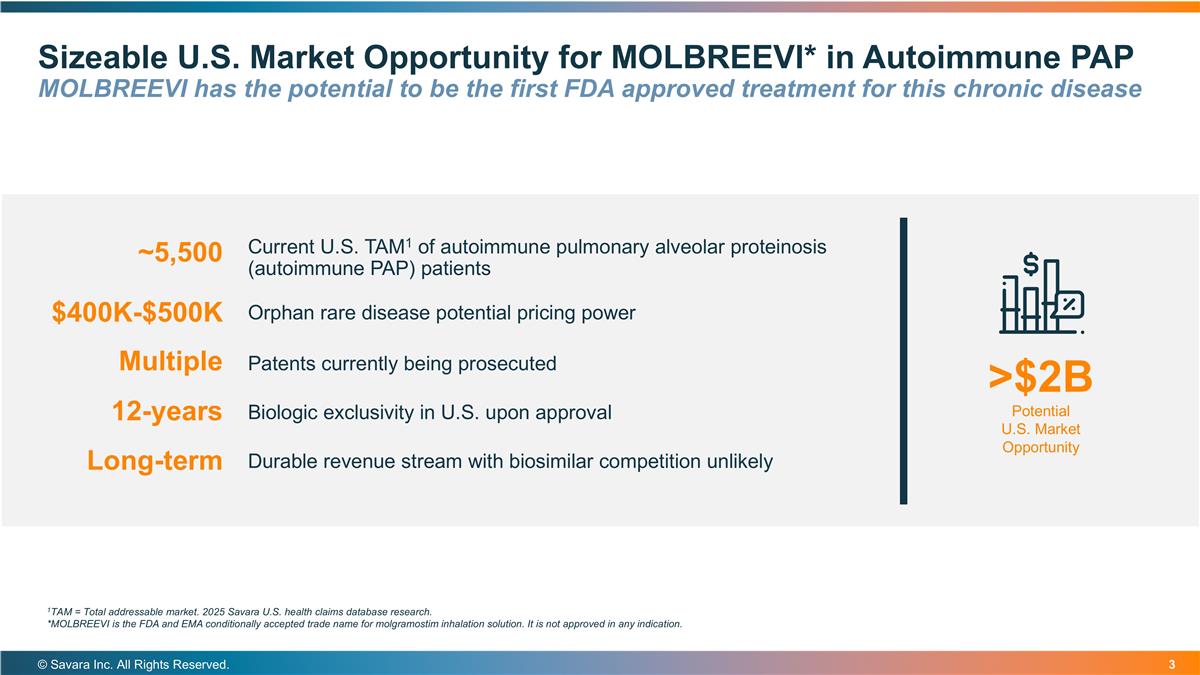
Sizeable U.S. Market Opportunity for MOLBREEVI* in Autoimmune PAP MOLBREEVI has the potential to be the first FDA approved treatment for this chronic disease © Savara Inc. All Rights Reserved. Current U.S. TAM1 of autoimmune pulmonary alveolar proteinosis (autoimmune PAP) patients >$2B Potential U.S. Market Opportunity $400K-$500K ~5,500 Orphan rare disease potential pricing power Patents currently being prosecuted Multiple Biologic exclusivity in U.S. upon approval Durable revenue stream with biosimilar competition unlikely 12-years Long-term 1TAM = Total addressable market. 2025 Savara U.S. health claims database research. *MOLBREEVI is the FDA and EMA conditionally accepted trade name for molgramostim inhalation solution. It is not approved in any indication.

Overview of Royalty Financing with RTW © Savara Inc. All Rights Reserved. $75M non-dilutive strategic financing from RTW for MOLBREEVI Funded upon FDA approval, subject to satisfaction of customary closing conditions Tiered royalty payments, based on U.S. net sales thresholds, range from 7% initially and scale down to 1% Based on U.S. sales only First tier of royalty rate could increase to 9.5% if net sales do not achieve specified levels DEBT Cap: Royalty payments will stop on RTW’s receipt of $187.5M Advance corporate objectives, including further investment in the global commercialization of MOLBREEVI If necessary, paying down our current debt USE OF PROCEEDS RTW has also submitted an indication to make a $25M equity investment in Savara.
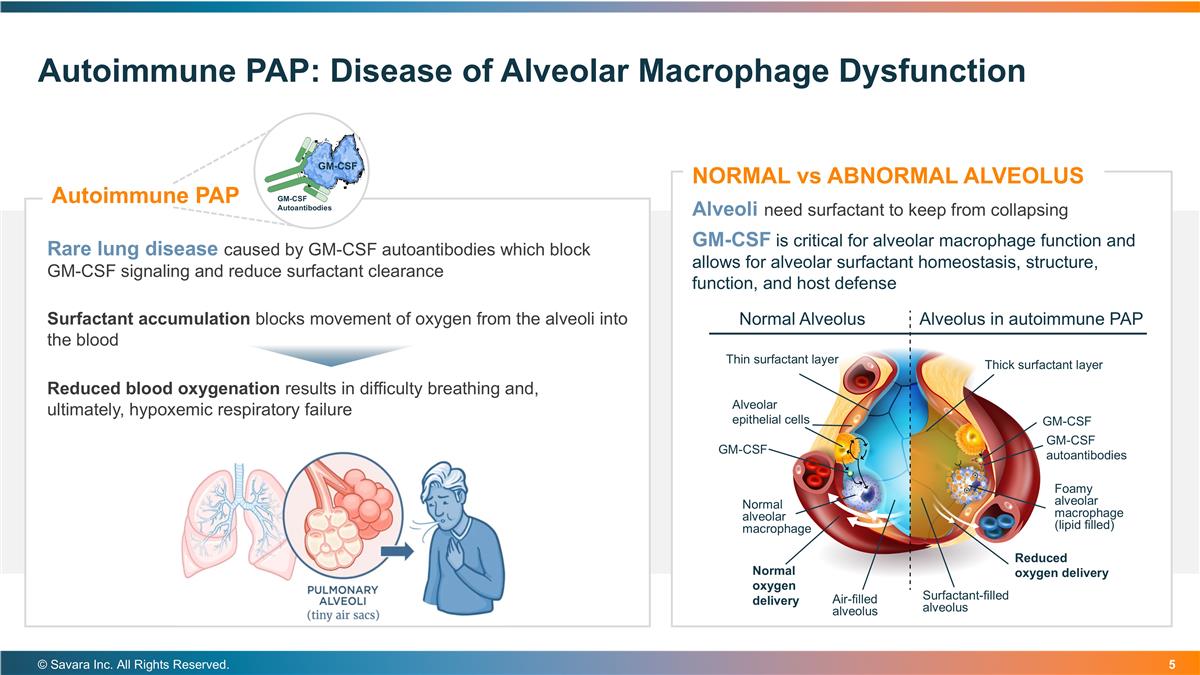
Autoimmune PAP: Disease of Alveolar Macrophage Dysfunction © Savara Inc. All Rights Reserved. Alveoli need surfactant to keep from collapsing GM-CSF is critical for alveolar macrophage function and allows for alveolar surfactant homeostasis, structure, function, and host defense NORMAL vs ABNORMAL ALVEOLUS Surfactant accumulation blocks movement of oxygen from the alveoli into the blood Reduced blood oxygenation results in difficulty breathing and, ultimately, hypoxemic respiratory failure Rare lung disease caused by GM-CSF autoantibodies which block GM-CSF signaling and reduce surfactant clearance Autoimmune PAP GM-CSF Autoantibodies GM-CSF GM-CSF autoantibodies Alveolus in autoimmune PAP Normal Alveolus Thin surfactant layer Thick surfactant layer Alveolar epithelial cells GM-CSF GM-CSF Normal oxygen delivery Reduced oxygen delivery Foamy alveolar macrophage (lipid filled) Normal alveolar macrophage Air-filled alveolus Surfactant-filled alveolus
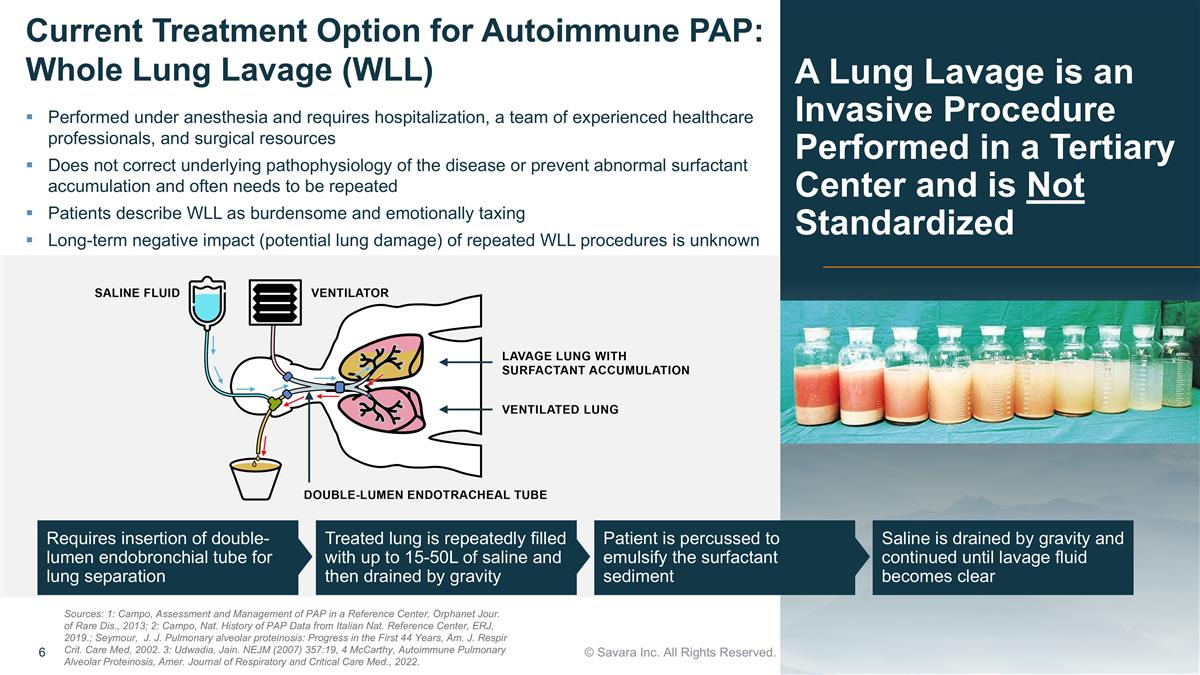
Requires insertion of double-lumen endobronchial tube for lung separation Treated lung is repeatedly filled with up to 15-50L of saline and then drained by gravity Patient is percussed to emulsify the surfactant sediment Saline is drained by gravity and continued until lavage fluid becomes clear A Lung Lavage is an Invasive Procedure Performed in a Tertiary Center and is Not Standardized © Savara Inc. All Rights Reserved. Current Treatment Option for Autoimmune PAP: Whole Lung Lavage (WLL) Performed under anesthesia and requires hospitalization, a team of experienced healthcare professionals, and surgical resources Does not correct underlying pathophysiology of the disease or prevent abnormal surfactant accumulation and often needs to be repeated Patients describe WLL as burdensome and emotionally taxing Long-term negative impact (potential lung damage) of repeated WLL procedures is unknown Sources: 1: Campo, Assessment and Management of PAP in a Reference Center, Orphanet Jour. of Rare Dis., 2013; 2: Campo, Nat. History of PAP Data from Italian Nat. Reference Center, ERJ, 2019.; Seymour, J. J. Pulmonary alveolar proteinosis: Progress in the First 44 Years, Am. J. Respir Crit. Care Med, 2002. 3: Udwadia, Jain. NEJM (2007) 357:19, 4 McCarthy, Autoimmune Pulmonary Alveolar Proteinosis, Amer. Journal of Respiratory and Critical Care Med., 2022.
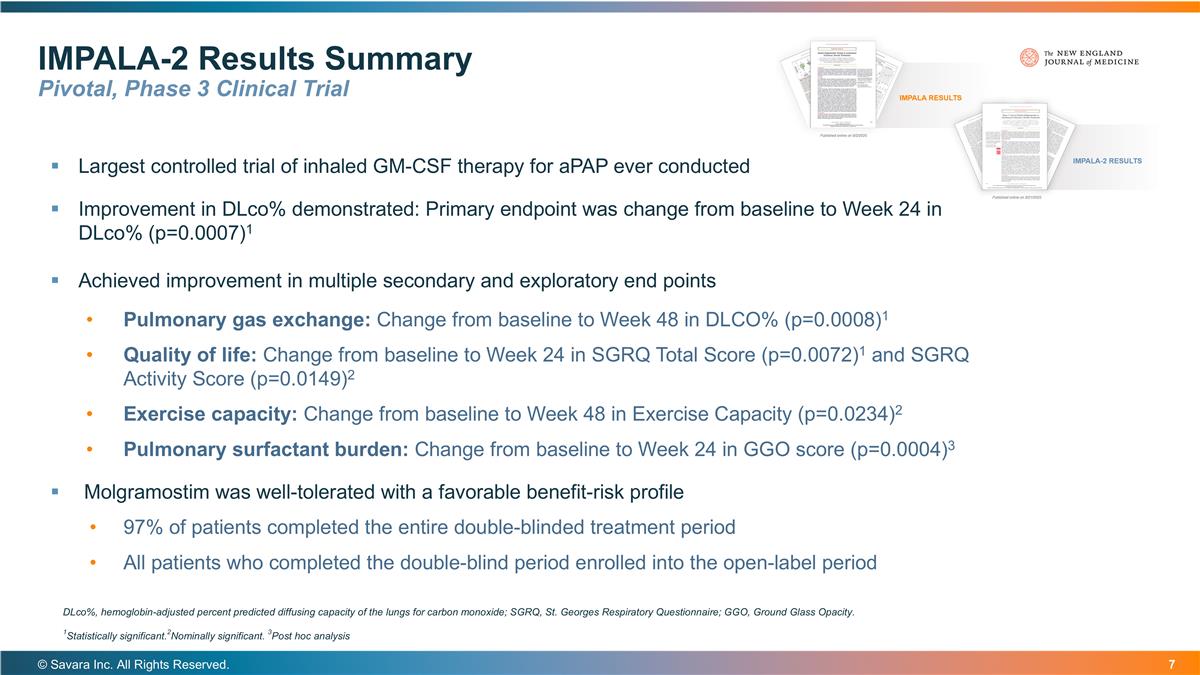
Largest controlled trial of inhaled GM-CSF therapy for aPAP ever conducted Improvement in DLco% demonstrated: Primary endpoint was change from baseline to Week 24 in DLco% (p=0.0007)1 Achieved improvement in multiple secondary and exploratory end points Pulmonary gas exchange: Change from baseline to Week 48 in DLCO% (p=0.0008)1 Quality of life: Change from baseline to Week 24 in SGRQ Total Score (p=0.0072)1 and SGRQ Activity Score (p=0.0149)2 Exercise capacity: Change from baseline to Week 48 in Exercise Capacity (p=0.0234)2 Pulmonary surfactant burden: Change from baseline to Week 24 in GGO score (p=0.0004)3 Molgramostim was well-tolerated with a favorable benefit-risk profile 97% of patients completed the entire double-blinded treatment period All patients who completed the double-blind period enrolled into the open-label period IMPALA-2 Results Summary Pivotal, Phase 3 Clinical Trial © Savara Inc. All Rights Reserved. DLco%, hemoglobin-adjusted percent predicted diffusing capacity of the lungs for carbon monoxide; SGRQ, St. Georges Respiratory Questionnaire; GGO, Ground Glass Opacity. 1Statistically significant.2Nominally significant. 3Post hoc analysis
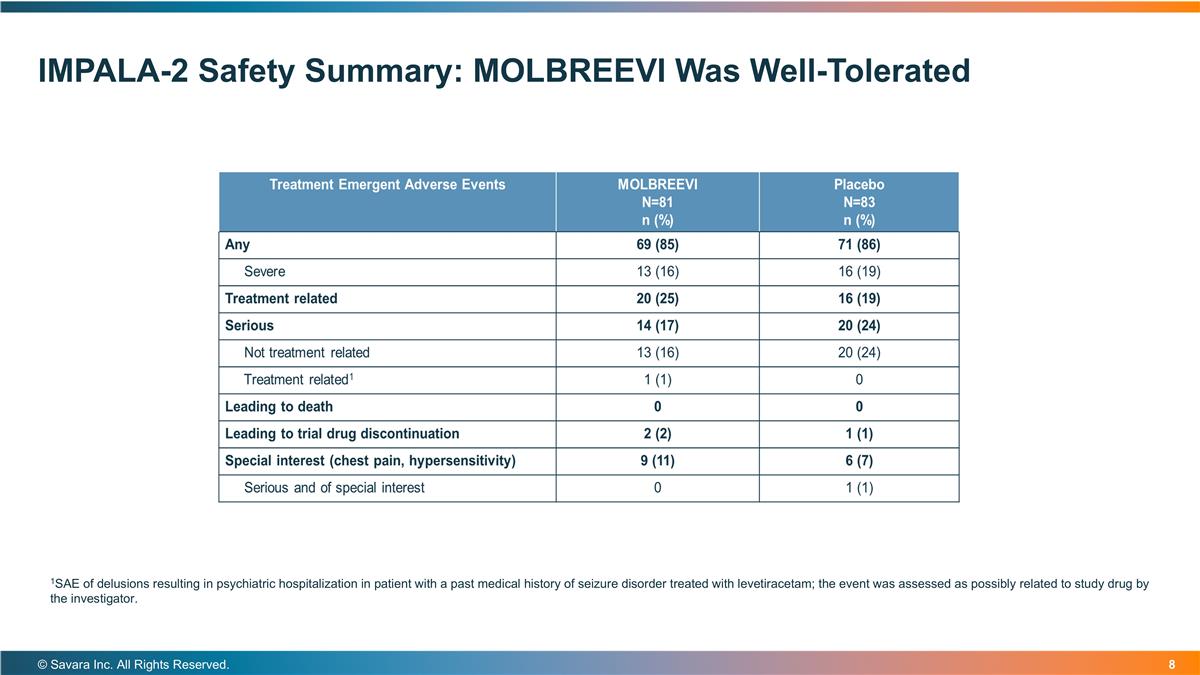
IMPALA-2 Safety Summary: MOLBREEVI Was Well-Tolerated © Savara Inc. All Rights Reserved. 1SAE of delusions resulting in psychiatric hospitalization in patient with a past medical history of seizure disorder treated with levetiracetam; the event was assessed as possibly related to study drug by the investigator.
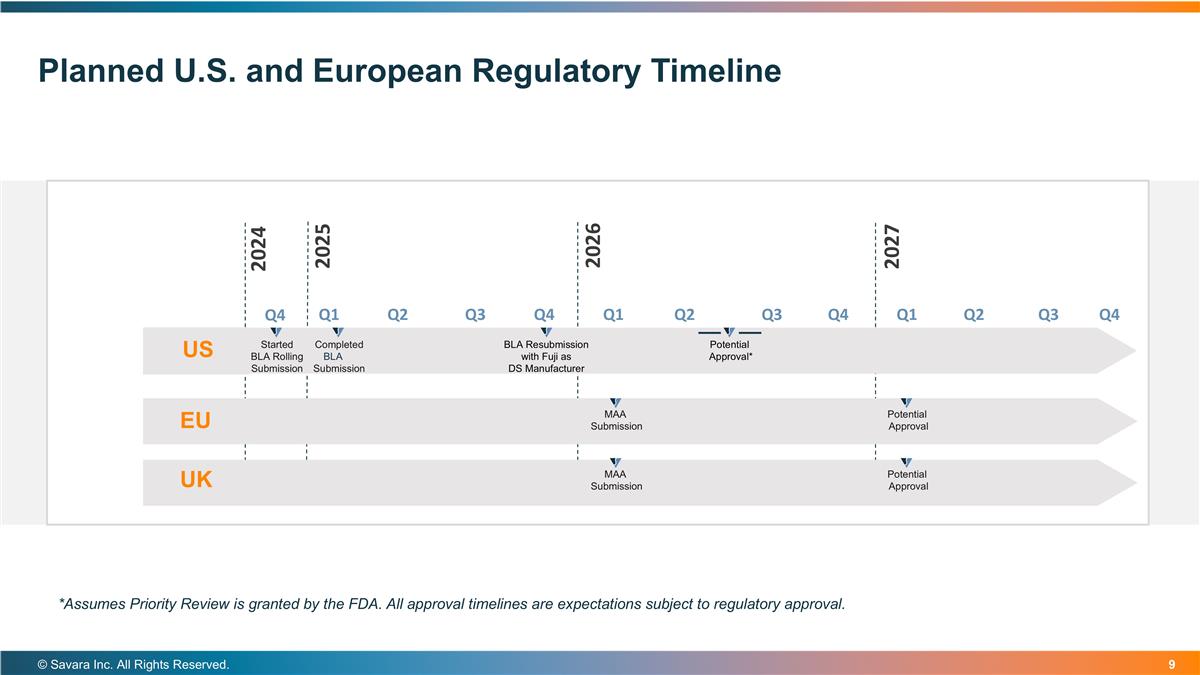
Planned U.S. and European Regulatory Timeline US Started BLA Rolling Submission EU Completed BLALA Submission BLA Resubmission with Fuji as DS Manufacturer Potential Approval* MAA Submission Potential Approval © Savara Inc. All Rights Reserved. *Assumes Priority Review is granted by the FDA. All approval timelines are expectations subject to regulatory approval. UK MAA Submission Potential Approval
Autoimmune PAP Disease State Awareness Campaign Multi-channel effort across healthcare professionals (HCP) and patients © Savara Inc. All Rights Reserved. HCP Disease State Awareness Campaign Patient DSA Campaign
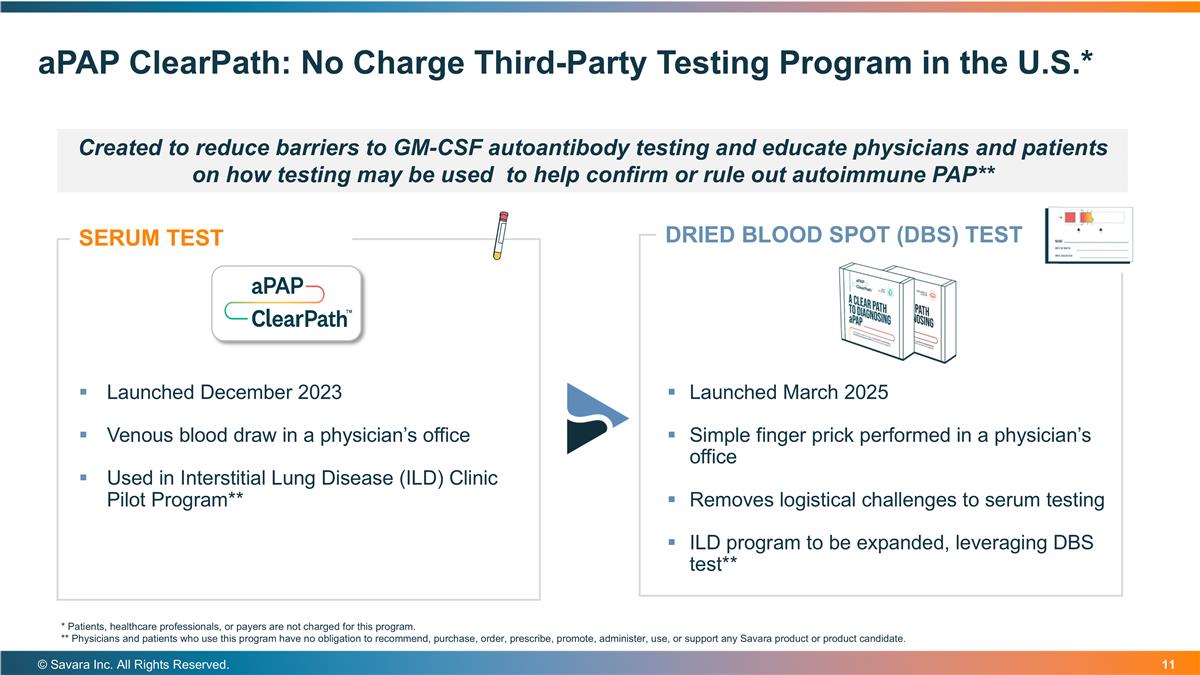
aPAP ClearPath: No Charge Third-Party Testing Program in the U.S.* © Savara Inc. All Rights Reserved. Launched March 2025 Simple finger prick performed in a physician’s office Removes logistical challenges to serum testing ILD program to be expanded, leveraging DBS test** SERUM TEST Launched December 2023 Venous blood draw in a physician’s office Used in Interstitial Lung Disease (ILD) Clinic Pilot Program** DRIED BLOOD SPOT (DBS) TEST * Patients, healthcare professionals, or payers are not charged for this program. ** Physicians and patients who use this program have no obligation to recommend, purchase, order, prescribe, promote, administer, use, or support any Savara product or product candidate. Created to reduce barriers to GM-CSF autoantibody testing and educate physicians and patients on how testing may be used to help confirm or rule out autoimmune PAP**
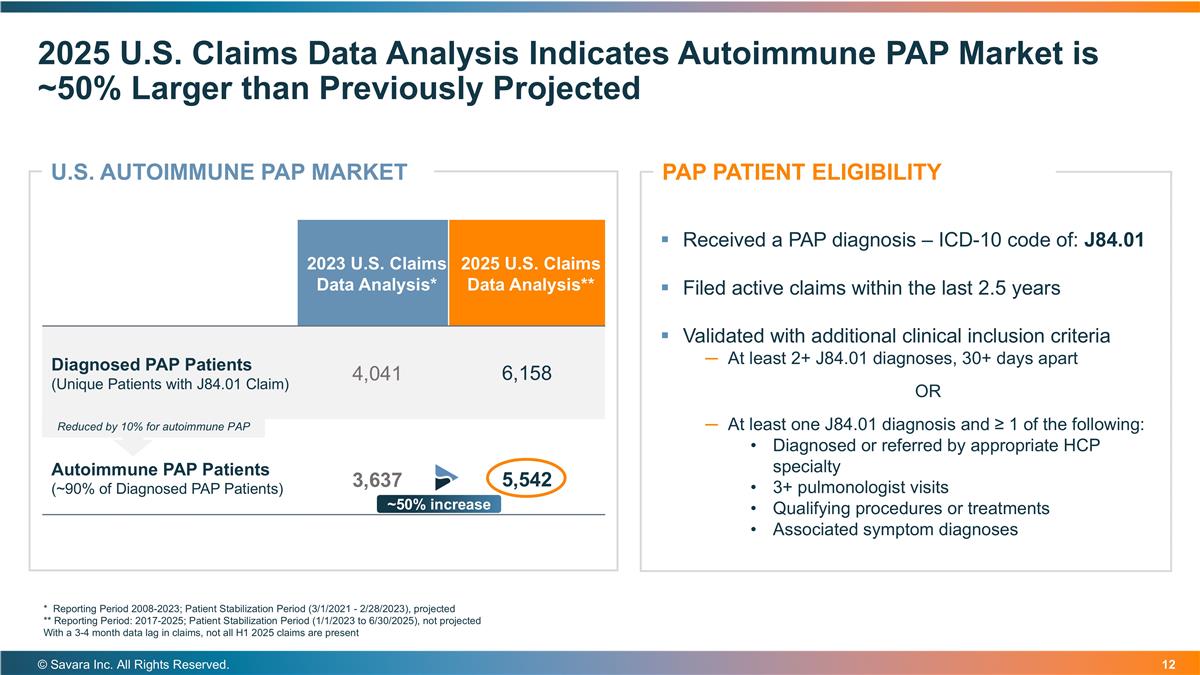
2025 U.S. Claims Data Analysis Indicates Autoimmune PAP Market is ~50% Larger than Previously Projected © Savara Inc. All Rights Reserved. * Reporting Period 2008-2023; Patient Stabilization Period (3/1/2021 - 2/28/2023), projected ** Reporting Period: 2017-2025; Patient Stabilization Period (1/1/2023 to 6/30/2025), not projected With a 3-4 month data lag in claims, not all H1 2025 claims are present Received a PAP diagnosis – ICD-10 code of: J84.01 Filed active claims within the last 2.5 years Validated with additional clinical inclusion criteria At least 2+ J84.01 diagnoses, 30+ days apart OR At least one J84.01 diagnosis and ≥ 1 of the following: Diagnosed or referred by appropriate HCP specialty 3+ pulmonologist visits Qualifying procedures or treatments Associated symptom diagnoses PAP PATIENT ELIGIBILITY 2023 U.S. Claims Data Analysis* 2025 U.S. Claims Data Analysis** Diagnosed PAP Patients (Unique Patients with J84.01 Claim) 4,041 6,158 Autoimmune PAP Patients (~90% of Diagnosed PAP Patients) 3,637 5,542 U.S. AUTOIMMUNE PAP MARKET ~50% increase Reduced by 10% for autoimmune PAP
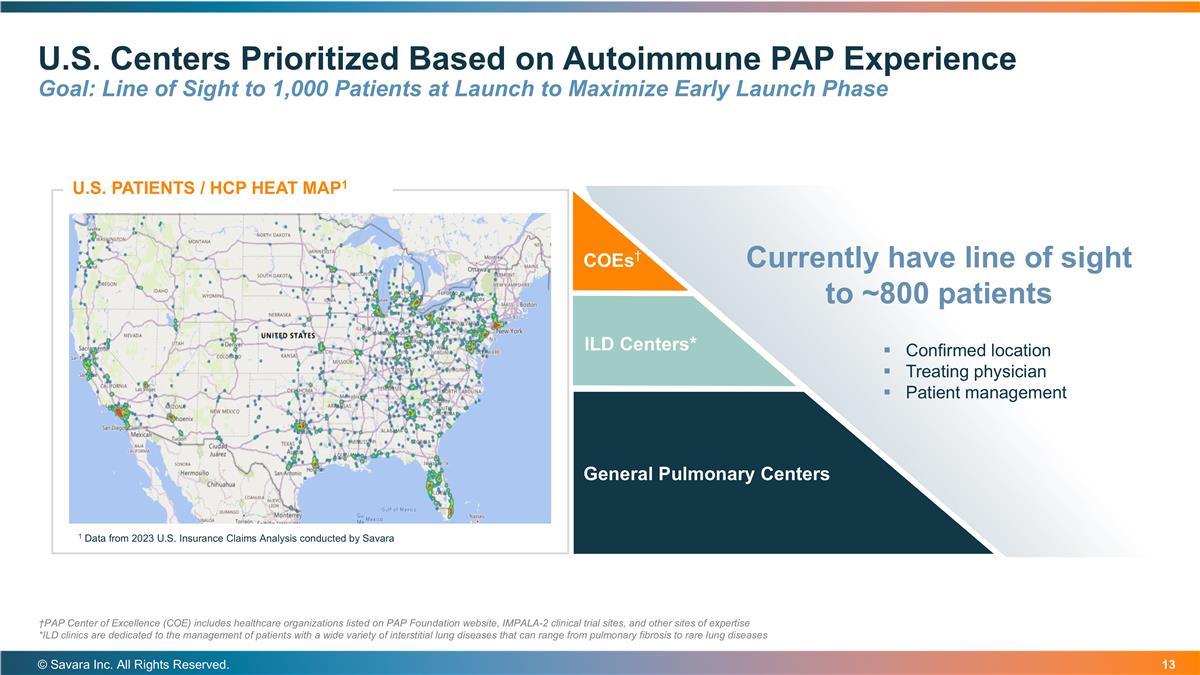
U.S. Centers Prioritized Based on Autoimmune PAP Experience Goal: Line of Sight to 1,000 Patients at Launch to Maximize Early Launch Phase © Savara Inc. All Rights Reserved. COEs† ILD Centers* General Pulmonary Centers U.S. PATIENTS / HCP HEAT MAP1 1 Data from 2023 U.S. Insurance Claims Analysis conducted by Savara Currently have line of sight to ~800 patients Confirmed location Treating physician Patient management †PAP Center of Excellence (COE) includes healthcare organizations listed on PAP Foundation website, IMPALA-2 clinical trial sites, and other sites of expertise *ILD clinics are dedicated to the management of patients with a wide variety of interstitial lung diseases that can range from pulmonary fibrosis to rare lung diseases
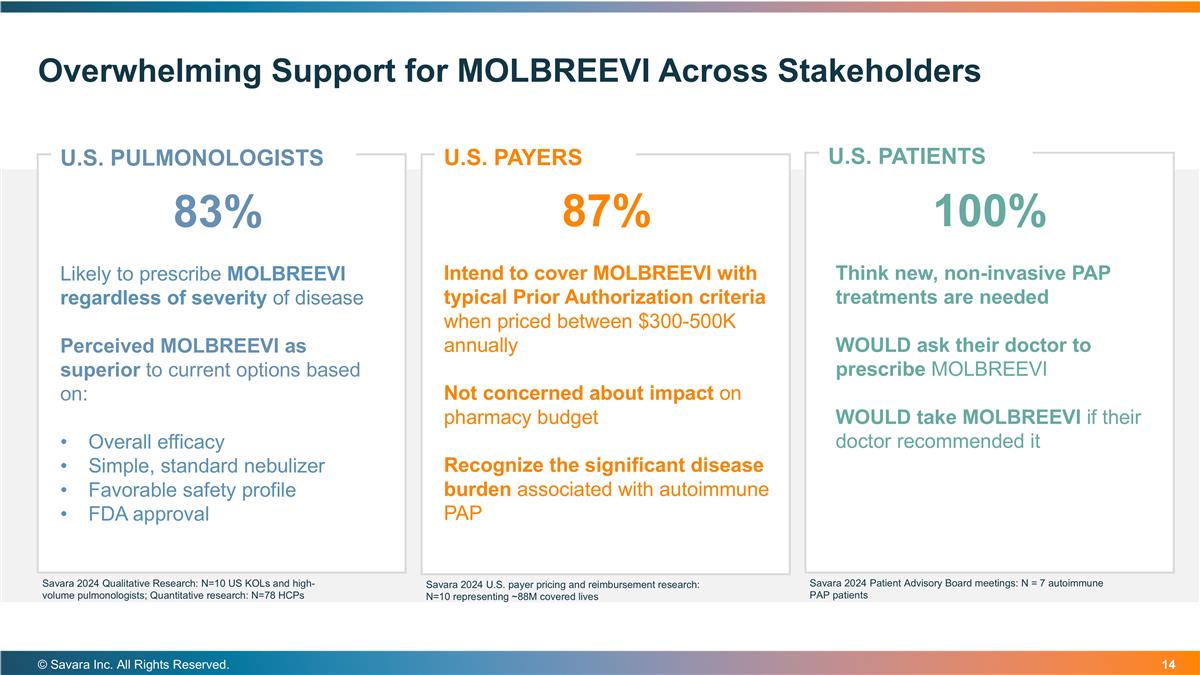
Overwhelming Support for MOLBREEVI Across Stakeholders © Savara Inc. All Rights Reserved. U.S. PATIENTS U.S. PULMONOLOGISTS 83% Likely to prescribe MOLBREEVI regardless of severity of disease Perceived MOLBREEVI as superior to current options based on: Overall efficacy Simple, standard nebulizer Favorable safety profile FDA approval U.S. PAYERS 87% Intend to cover MOLBREEVI with typical Prior Authorization criteria when priced between $300-500K annually Not concerned about impact on pharmacy budget Recognize the significant disease burden associated with autoimmune PAP 100% Think new, non-invasive PAP treatments are needed WOULD ask their doctor to prescribe MOLBREEVI WOULD take MOLBREEVI if their doctor recommended it Savara 2024 Qualitative Research: N=10 US KOLs and high-volume pulmonologists; Quantitative research: N=78 HCPs Savara 2024 U.S. payer pricing and reimbursement research: N=10 representing ~88M covered lives Savara 2024 Patient Advisory Board meetings: N = 7 autoimmune PAP patients

THANK YOU © Savara Inc. All Rights Reserved.