UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): October 29, 2025
Kyverna Therapeutics, Inc.
(Exact name of Registrant as Specified in Its Charter)
| Delaware | 001-41947 | 83-1365441 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 5980 Horton St., Suite 550 | ||
| Emeryville, California | 94608 | |
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s Telephone Number, Including Area Code: (510) 925-2492
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange |
||
| Common Stock, par value $0.00001 per share | KYTX | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On October 29, 2025, Kyverna Therapeutics, Inc. (the “Company”) issued a press release announcing positive interim data from the Phase 2 portion from its KYSA-6 Phase 2/3 trial of KYV-101 in generalized myasthenia gravis. The Company will host a conference call at 8:00 a.m. Eastern Time on October 29, 2025 to review the results (the “Conference Call”). A copy of the slides to be used in connection with the Conference Call is furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
The information contained in Item 7.01 of this Current Report on Form 8-K (including Exhibit 99.1 attached hereto) shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly provided by specific reference in such a filing.
| Item 9.01 | Financial Statements and Exhibits. |
| Exhibit |
Description |
|
| 99.1 | Kyverna Therapeutics, Inc. Presentation - Interim Data for KYSA-6 Phase 2 Clinical Trial of KYV-101 in Generalized Myasthenia Gravis, Conference Call, October 29, 2025. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document). | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| KYVERNA THERAPEUTICS, INC. | ||||||
| Date: October 29, 2025 | By: | /s/ Marc Grasso |
||||
| Marc Grasso Chief Financial Officer |
||||||

Interim Data for KYSA-6 Phase 2 Clinical Trial of KYV-101 in Generalized Myasthenia Gravis Conference Call October 29, 2025 Exhibit 99.1

This presentation contains forward-looking statements that are based on management’s beliefs and assumptions and information currently available to management of Kyverna Therapeutics, Inc. (“Kyverna”, “we”, “our,” or the “Company”). All statements other than statements of historical facts contained in this presentation are forward-looking statements. Forward looking statements include, but are not limited to, statements concerning: the Company’s future results of operations and financial position, business strategy, drug candidates, planned preclinical studies and clinical trials, results of preclinical studies, named-patient access data, ongoing clinical trials, research and development costs, plans for manufacturing, regulatory approvals, timing and likelihood of success, as well as plans and objectives of management for future operations. These forward-looking statements are subject to risks and uncertainties, including the factors described under the Risk Factors section of the Company’s most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q that the Company has filed or may subsequently file with the U.S. Securities and Exchange Commission. Actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. When evaluating Kyverna’s business and prospects, careful consideration should be given to these risks and uncertainties. These statements speak only as of the date of this presentation, and Kyverna undertakes no obligation to update or revise these statements. This presentation also contains estimates made by independent parties relating to industry market size and other data. These estimates involve a number of assumptions and limitations and you are cautioned not to give undue weight on such estimates. We have not independently verified the accuracy or completeness of such information, and we do not take any responsibility for the accuracy or completeness of such information. This presentation contains references to trademarks and marks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this presentation may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that the applicable licensor will not assert, to the fullest extent under applicable law, its rights to these trademarks and trade names. The Company does not intend its use or display of other companies’ trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of the Company by any other companies. This presentation includes results from named-patient basis access. Similar to expanded access or compassionate use in the United States, “IH” or “Individueller Heilversuch,” also known as “named-patient basis access,” is a regulatory scheme in Germany that allows for the supply of a treatment that has not received marketing authorization for an individual patient in response to a request by the treating physician on behalf of the named patient. This option can be pursued for the expected benefit of a patient who has exhausted all available treatment options, under the discretion of the treating physician, with the patient’s consent. The use of KYV-101 in the IH settings is not a substitute for, or intended to replace, our clinical trials. The goal is not to assess the effectiveness of a potential therapy, but rather to provide an individual patient with a possible efficacious approach when all other treatment options have failed, as determined by the patient’s physician. While we do not expect to be able to use the results from these activities as the basis for approval in our applications for marketing approval to the U.S. Food and Drug Administration (FDA) or other foreign regulatory agencies, we believe such activities may provide additional clinical insights beyond highly focused clinical trials in specific geographies. Disclaimer and Forward-Looking Statements ©2025 Kyverna Therapeutics, Inc.

Introduction Warner Biddle – Chief Executive Officer

Today’s Agenda Warner Biddle Chief Executive Officer Sri Muppidi M.D. Stanford Medicine Naji Gehchan M.D., MSc, MBA Chief Medical & Development Officer Marc Grasso M.D. Chief Financial Officer Introduction Interim Phase 2 gMG Data Results Neuroimmunology Franchise and Future Growth Opportunities Q&A Speakers Q&A
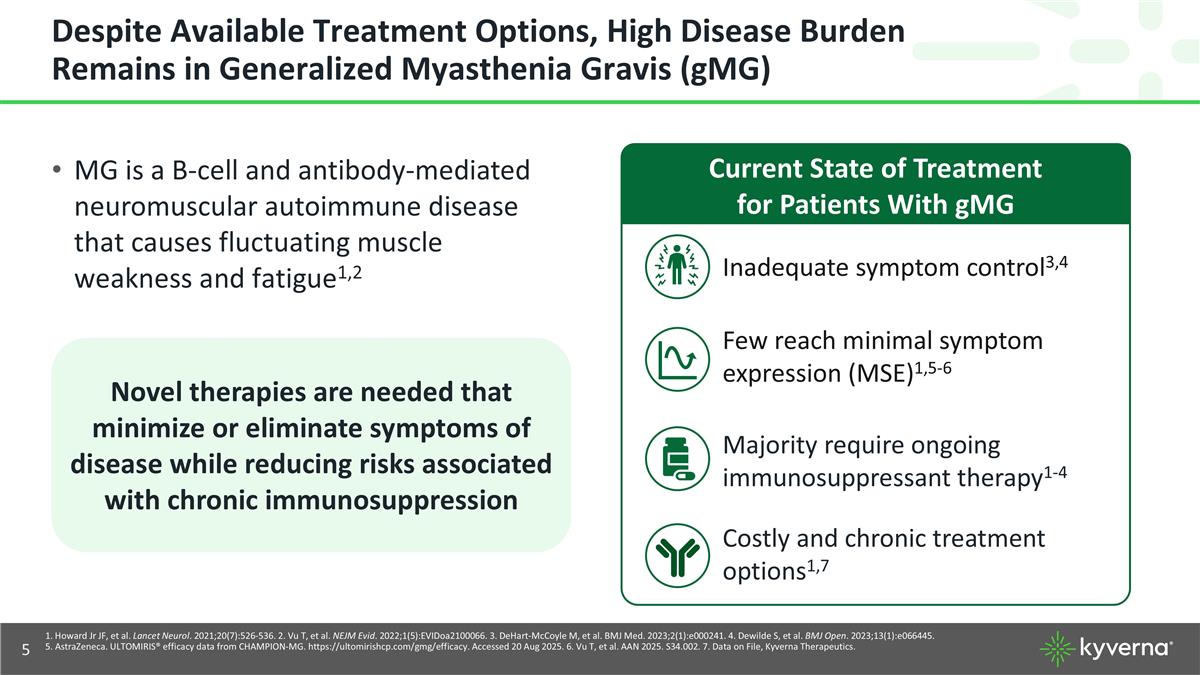
Despite Available Treatment Options, High Disease Burden Remains in Generalized Myasthenia Gravis (gMG) Novel therapies are needed that minimize or eliminate symptoms of disease while reducing risks associated with chronic immunosuppression MG is a B-cell and antibody-mediated neuromuscular autoimmune disease that causes fluctuating muscle weakness and fatigue1,2 Current State of Treatment for Patients With gMG Inadequate symptom control3,4 Few reach minimal symptom expression (MSE)1,5-6 Majority require ongoing immunosuppressant therapy1-4 Costly and chronic treatment options1,7 1. Howard Jr JF, et al. Lancet Neurol. 2021;20(7):526-536. 2. Vu T, et al. NEJM Evid. 2022;1(5):EVIDoa2100066. 3. DeHart-McCoyle M, et al. BMJ Med. 2023;2(1):e000241. 4. Dewilde S, et al. BMJ Open. 2023;13(1):e066445. 5. AstraZeneca. ULTOMIRIS® efficacy data from CHAMPION-MG. https://ultomirishcp.com/gmg/efficacy. Accessed 20 Aug 2025. 6. Vu T, et al. AAN 2025. S34.002. 7. Data on File, Kyverna Therapeutics.
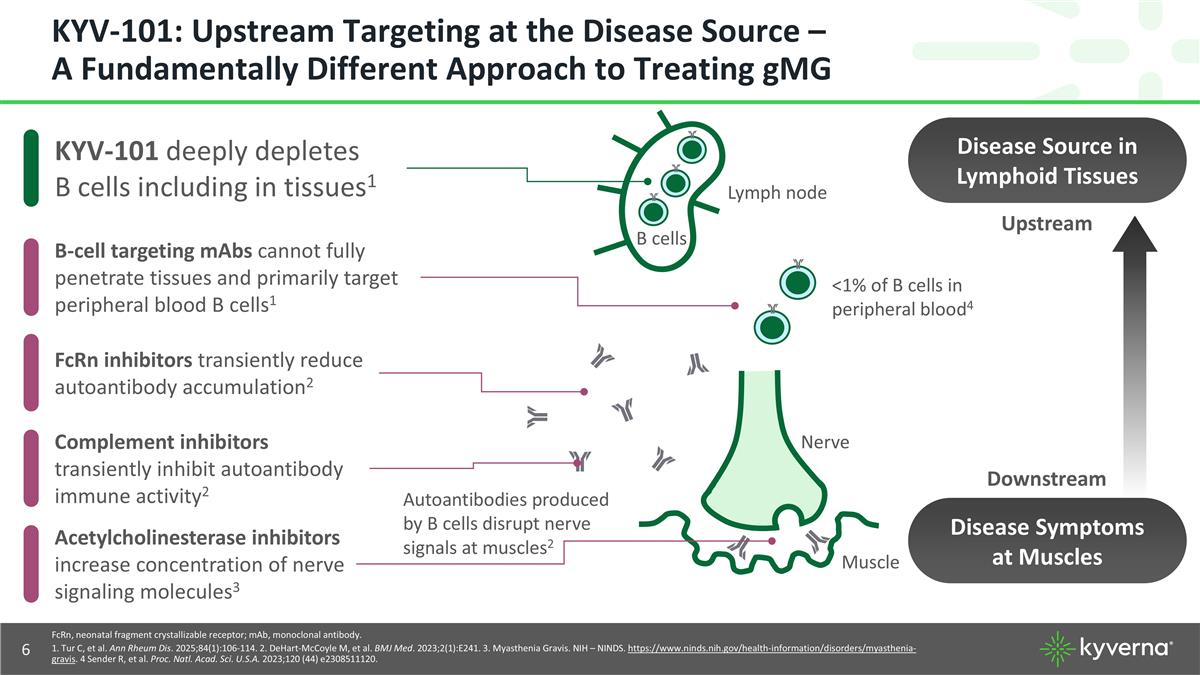
KYV-101: Upstream Targeting at the Disease Source – A Fundamentally Different Approach to Treating gMG FcRn, neonatal fragment crystallizable receptor; mAb, monoclonal antibody. 1. Tur C, et al. Ann Rheum Dis. 2025;84(1):106-114. 2. DeHart-McCoyle M, et al. BMJ Med. 2023;2(1):E241. 3. Myasthenia Gravis. NIH – NINDS. https://www.ninds.nih.gov/health-information/disorders/myasthenia-gravis. 4 Sender R, et al. Proc. Natl. Acad. Sci. U.S.A. 2023;120 (44) e2308511120. Complement inhibitors transiently inhibit autoantibody immune activity2 KYV-101 deeply depletes B cells including in tissues1 B-cell targeting mAbs cannot fully penetrate tissues and primarily target peripheral blood B cells1 FcRn inhibitors transiently reduce autoantibody accumulation2 Upstream Downstream Disease Source in Lymphoid Tissues B cells Lymph node Autoantibodies produced by B cells disrupt nerve signals at muscles2 <1% of B cells in peripheral blood4 Disease Symptoms at Muscles Nerve Muscle Acetylcholinesterase inhibitors increase concentration of nerve signaling molecules3
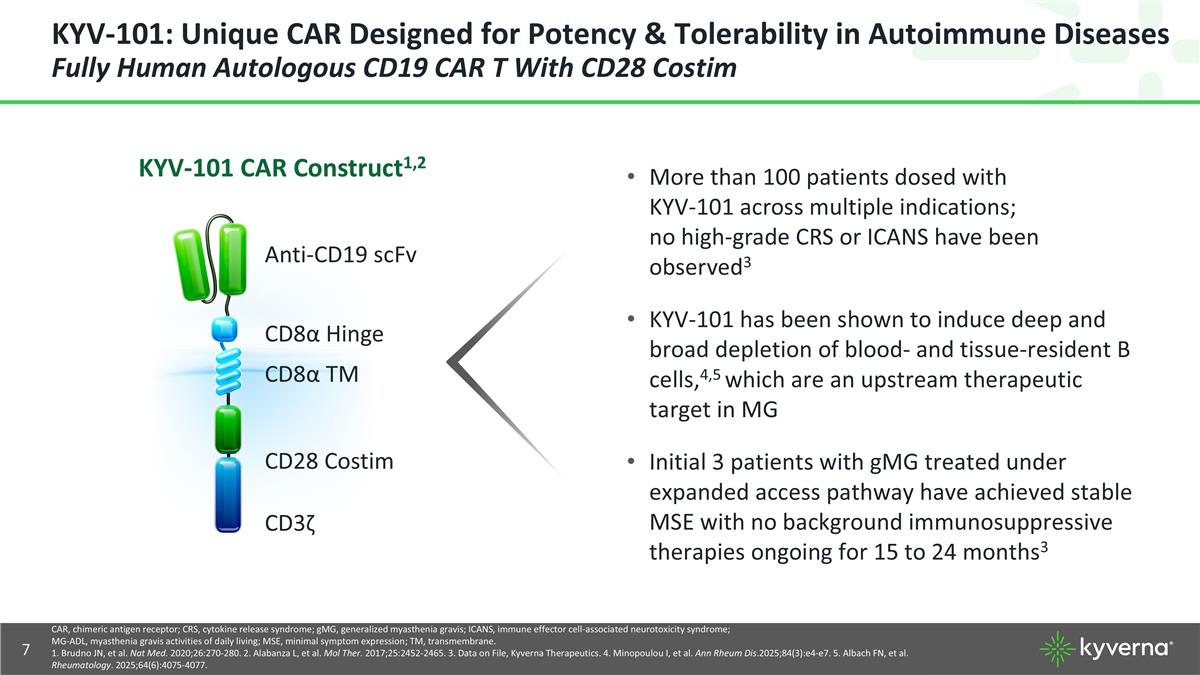
KYV-101: Unique CAR Designed for Potency & Tolerability in Autoimmune Diseases Fully Human Autologous CD19 CAR T With CD28 Costim © 2025 Kyverna Therapeutics, Inc. | Confidential – Internal Use Only Anti-CD19 scFv CD8α TM CD28 Costim CD3ζ CD8α Hinge KYV-101 CAR Construct1,2 More than 100 patients dosed with KYV-101 across multiple indications; no high-grade CRS or ICANS have been observed3 KYV-101 has been shown to induce deep and broad depletion of blood- and tissue-resident B cells,4,5 which are an upstream therapeutic target in MG Initial 3 patients with gMG treated under expanded access pathway have achieved stable MSE with no background immunosuppressive therapies ongoing for 15 to 24 months3 CAR, chimeric antigen receptor; CRS, cytokine release syndrome; gMG, generalized myasthenia gravis; ICANS, immune effector cell-associated neurotoxicity syndrome; MG-ADL, myasthenia gravis activities of daily living; MSE, minimal symptom expression; TM, transmembrane. 1. Brudno JN, et al. Nat Med. 2020;26:270-280. 2. Alabanza L, et al. Mol Ther. 2017;25:2452-2465. 3. Data on File, Kyverna Therapeutics. 4. Minopoulou I, et al. Ann Rheum Dis.2025;84(3):e4-e7. 5. Albach FN, et al. Rheumatology. 2025;64(6):4075-4077.
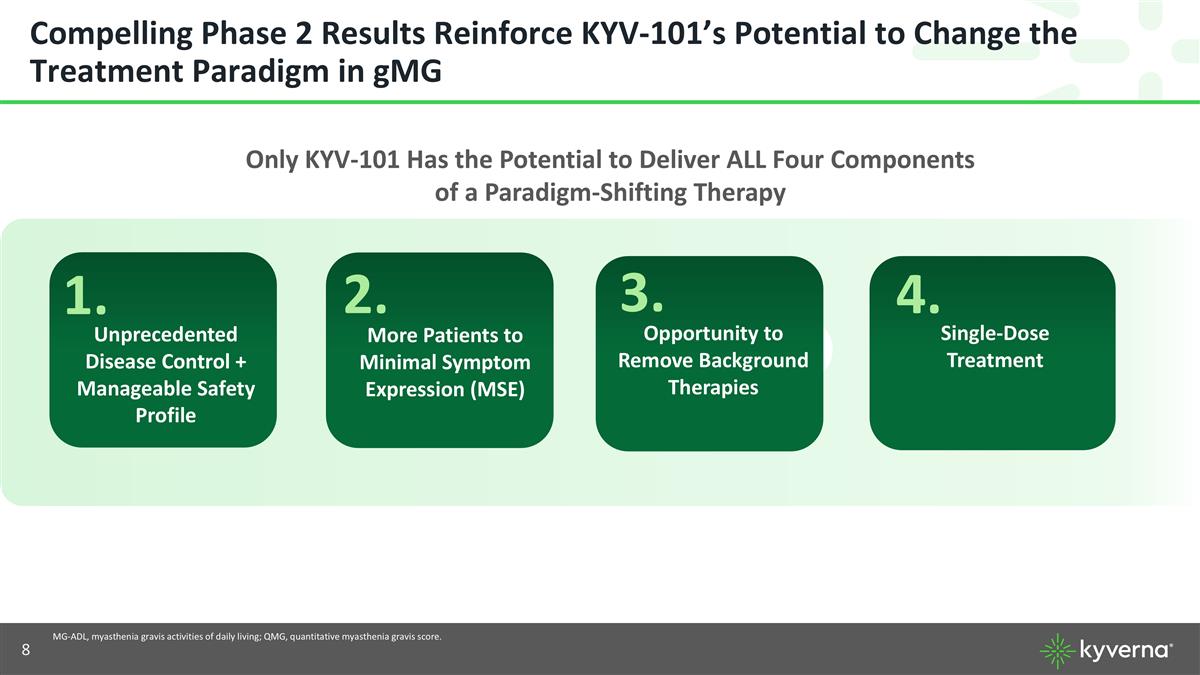
Compelling Phase 2 Results Reinforce KYV-101’s Potential to Change the Treatment Paradigm in gMG MG-ADL, myasthenia gravis activities of daily living; QMG, quantitative myasthenia gravis score. More Patients to Minimal Symptom Expression (MSE) Single-Dose Treatment Unprecedented Disease Control + Manageable Safety Profile Opportunity to Remove Background Therapies Only KYV-101 Has the Potential to Deliver ALL Four Components of a Paradigm-Shifting Therapy 1. 2. 3. 4.

Changing the Treatment Paradigm in gMG Naji Gehchan, MD, MSc, MBA – Chief Medical and Development Officer

KYSA-6: Phase 2/3 Study of KYV-101 in gMG AchR, acetylcholine receptor; Cy/Flu, cyclophosphamide and fludarabine; gMG, generalized myasthenia gravis; LRP4, low-density lipoprotein receptor-related protein 4; MG-ADL, myasthenia gravis Activities of Daily Living; MGC, Myasthenia Gravis Composite; MGFA, Myasthenia Gravis Foundation of America; MuSK, muscle-specific kinase; QMG, Quantitative Myasthenia Gravis. N = 6 Age 18 to 75 years Diagnosis of gMG, Class IIB-IV per MGFA criteria Autoantibodies to AChR, MuSK, or LRP4 MG-ADL ≥6 Failed ≥2 immunosuppressive/ immunomodulatory therapies OR failed ≥1 immunosuppressive therapy and required chronic plasmapheresis or IVIg to control symptoms Primary endpoints MG-ADL at 24 weeks Adverse events Key secondary endpoints QMG and MGC scores PK/PD Phase 2 design: Open-label, single-arm, multicenter study KYV-101 Cy/Flu lymphodepletion + Single infusion of 1×108 CAR T cells 18-month follow up Interim analysis of ongoing phase 2 study with data cutoff of October 3, 2025

Patients Treated With KYV-101 Had Moderate to Severe gMG All patients had robust CAR T-cell expansion and B-cell depletion AChE-I, acetylcholinesterase inhibitor; AChR, acetylcholine receptor; FcRn, neonatal fragment crystallizable receptor; IVIG, Intravenous Immunoglobulin; MG-ADL, myasthenia gravis activities of daily living; MGC, Myasthenia Gravis Composite; MGFA, MG Foundation of America; MuSK, muscle-specific kinase; NSIST, nonsteroidal immunosuppressive therapy; PLEX, plasma exchange; QMG, quantitative myasthenia gravis. Characteristic N=6 Age, mean years (range) 45.5 (21-62) Sex, female | male, n (%) 5 (83%) | 1 (17%) Duration of MG, mean years (range) 5.3 (1.7-13.3) Total outcome score, mean (range) MG-ADL 11.2 (7-16) QMG 17.3 (9-28) MGC 21.8 (15-30) MGFA Class IIIb | Class IV at screening, n (%) 4 (67%) | 2 (33%) AChR | MuSK-positive at screening/historically, n (%) 5 (83%) | 1 (17%) Prior therapies, n (%) AChE inhibitors 6 (100%) NSISTs 6 (100%) FcRn and/or complement inhibitors 5 (83%) Steroids 6 (100%) IVIG and/or PLEX 4 (67%) Rituximab 3 (50%)

KYV-101 Demonstrated Rapid, Robust, and Sustained Reductions in MG-ADL and QMG Data cutoff: October 3, 2025. BL, baseline; MG-ADL, myasthenia gravis activities of daily living; QMG, quantitative myasthenia gravis. MG-ADL score QMG score Baseline Change from BL Week 2 Week 24 Mean MG-ADL 11.2 -7.8 -8.0 Baseline Change from BL Week 2 Week 24 Mean QMG 17.3 -7.8 -7.7
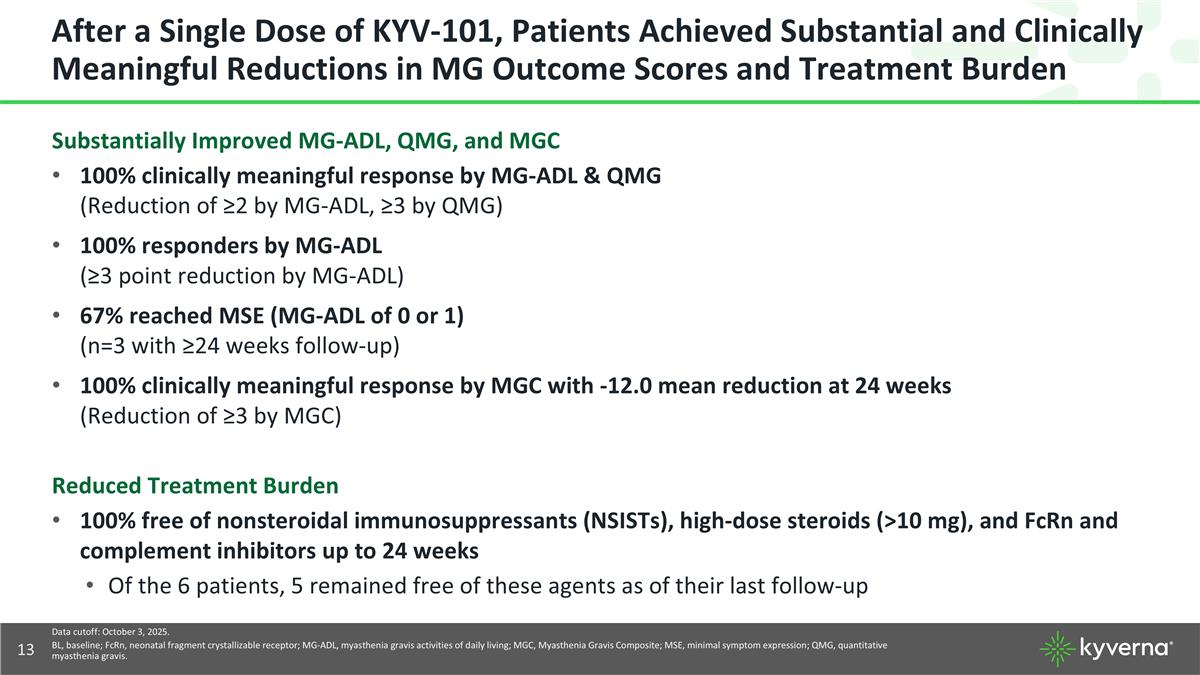
After a Single Dose of KYV-101, Patients Achieved Substantial and Clinically Meaningful Reductions in MG Outcome Scores and Treatment Burden Substantially Improved MG-ADL, QMG, and MGC 100% clinically meaningful response by MG-ADL & QMG (Reduction of ≥2 by MG-ADL, ≥3 by QMG) 100% responders by MG-ADL (≥3 point reduction by MG-ADL) 67% reached MSE (MG-ADL of 0 or 1) (n=3 with ≥24 weeks follow-up) 100% clinically meaningful response by MGC with -12.0 mean reduction at 24 weeks (Reduction of ≥3 by MGC) Reduced Treatment Burden 100% free of nonsteroidal immunosuppressants (NSISTs), high-dose steroids (>10 mg), and FcRn and complement inhibitors up to 24 weeks Of the 6 patients, 5 remained free of these agents as of their last follow-up Data cutoff: October 3, 2025. BL, baseline; FcRn, neonatal fragment crystallizable receptor; MG-ADL, myasthenia gravis activities of daily living; MGC, Myasthenia Gravis Composite; MSE, minimal symptom expression; QMG, quantitative myasthenia gravis.

KYV-101 was Well-Tolerated with No High-Grade CRS and No ICANS Observed Safety Patients (n=6) CRS (any Grade), n (%) 6 (100) Grade 1 4 (67) Grade 2 2 (33) ICANS (any Grade), n (%) 0 (0) Grade 3/4 TEAEs, n (%) 3 (50) Neutropenia 3 (50) Lymphopenia 1 (17) Lymphocyte count decreased 1 (17) Any treatment-related serious AE, n (%) 1 (17) No ICANS observed CRS was low-grade and manageable in all patients 4 of 6 patients only experienced fever (Grade 1 CRS) 3 patients with expected AEs associated with lymphodepletion and CAR T-cell therapy 2 patients had transient Grade 3/4 neutropenia that resolved within 10 days of infusion 1 Grade 4 neutropenia SAE was manageable with G-CSF, not associated with infections, and had improved to Grade 1 by data cutoff Data cutoff: October 3, 2025 CRS and ICANS graded using ASTCT criteria; other AEs graded using CTCAE criteria. AE, adverse event; ASTCT, American Society for Transplantation and Cellular Therapy; CTCAE, Common Terminology Criteria for Adverse Events; CRS, cytokine release syndrome; G-CSF, granulocyte colony-stimulating factor; ICANS, immune effector cell-associated neurotoxicity syndrome; TEAE, treatment-emergent adverse event.
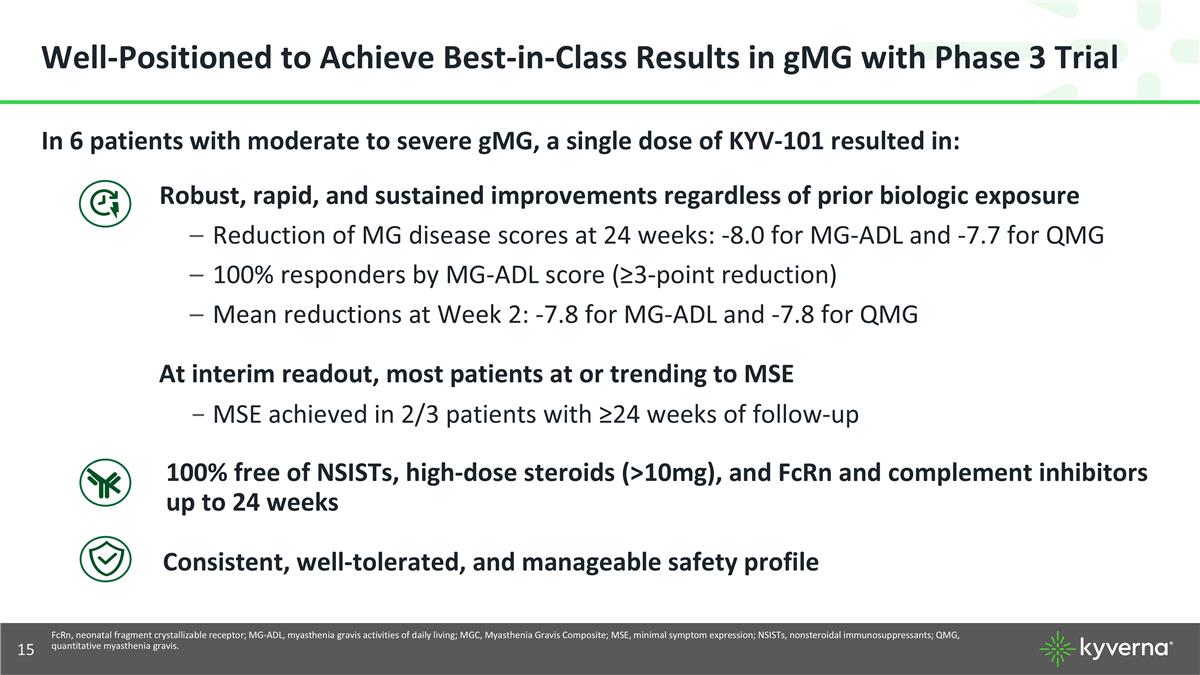
Well-Positioned to Achieve Best-in-Class Results in gMG with Phase 3 Trial In 6 patients with moderate to severe gMG, a single dose of KYV-101 resulted in: Robust, rapid, and sustained improvements regardless of prior biologic exposure Reduction of MG disease scores at 24 weeks: -8.0 for MG-ADL and -7.7 for QMG 100% responders by MG-ADL score (≥3-point reduction) Mean reductions at Week 2: -7.8 for MG-ADL and -7.8 for QMG At interim readout, most patients at or trending to MSE MSE achieved in 2/3 patients with ≥24 weeks of follow-up 100% free of NSISTs, high-dose steroids (>10mg), and FcRn and complement inhibitors up to 24 weeks Consistent, well-tolerated, and manageable safety profile FcRn, neonatal fragment crystallizable receptor; MG-ADL, myasthenia gravis activities of daily living; MGC, Myasthenia Gravis Composite; MSE, minimal symptom expression; NSISTs, nonsteroidal immunosuppressants; QMG, quantitative myasthenia gravis.

Robust Phase 2 Results Strengthen Confidence in Phase 3 Powering Assumptions, Efficient Trial-Size, and Co-primary Endpoint Measurement MG-ADL, myasthenia gravis activities of daily living; QMG, quantitative myasthenia gravis score. Interim Phase 2 Results Increase Phase 3 Probability of Success Reductions in MG-ADL and QMG exceeded the magnitude of effect assumed for Phase 3 co-primary endpoints Deep, sustained treatment effect was observed at week 24 - the timepoint of assessment for Phase 3 co-primary endpoints

Innovative and FDA-Aligned Registrational Phase 3 Trial Design ~60-patient, global, open-label, randomized controlled Phase 2/3 trial with crossover design KYV-101 Cy/Flu lymphodepletion + Single infusion of 1×108 CAR T cells Standard of Care Traditional agents or complement pathway inhibitors N = ~60 Randomized 1:1 Baseline Measurement Screening, washout, apheresis, and resume standard of care 24 weeks 18 Months Co-Primary Endpoints (MG-ADL & QMG) KYV-101 Crossover Follow Up Follow Up Standard of care may consist of traditional agents (e.g., prednisone, azathioprine, mycophenolate, methotrexate, chronic IVIG/PLEX) or complement pathway inhibitors (e.g., eculizumab, ravulizumab). Anti-CD20 or -CD19 monoclonal antibodies or FcRn inhibitors not allowed as defined in inclusion criteria. Cy/Flu, cyclophosphamide and fludarabine; FcRn, neonatal fragment crystallizable receptor; MG-ADL, myasthenia gravis activities of daily living; PLEX, plasma exchange; QMG, quantitative myasthenia gravis score.
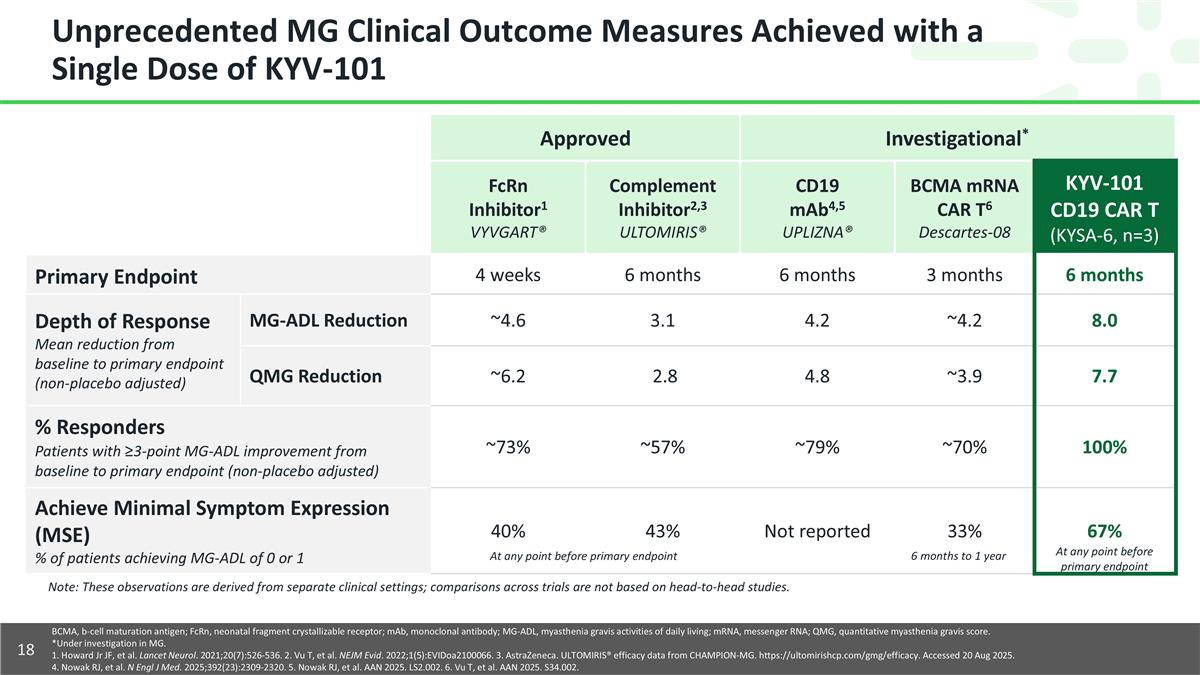
Unprecedented MG Clinical Outcome Measures Achieved with a Single Dose of KYV-101 Approved Investigational* FcRn Inhibitor1 VYVGART® Complement Inhibitor2,3 ULTOMIRIS® CD19 mAb4,5 UPLIZNA® BCMA mRNA CAR T6 Descartes-08 KYV-101 CD19 CAR T (KYSA-6, n=3) Primary Endpoint 4 weeks 6 months 6 months 3 months 6 months Depth of Response Mean reduction from baseline to primary endpoint (non-placebo adjusted) MG-ADL Reduction ~4.6 3.1 4.2 ~4.2 8.0 QMG Reduction ~6.2 2.8 4.8 ~3.9 7.7 % Responders Patients with ≥3-point MG-ADL improvement from baseline to primary endpoint (non-placebo adjusted) ~73% ~57% ~79% ~70% 100% Achieve Minimal Symptom Expression (MSE) % of patients achieving MG-ADL of 0 or 1 40% 43% Not reported 33% 67% BCMA, b-cell maturation antigen; FcRn, neonatal fragment crystallizable receptor; mAb, monoclonal antibody; MG-ADL, myasthenia gravis activities of daily living; mRNA, messenger RNA; QMG, quantitative myasthenia gravis score. *Under investigation in MG. 1. Howard Jr JF, et al. Lancet Neurol. 2021;20(7):526-536. 2. Vu T, et al. NEJM Evid. 2022;1(5):EVIDoa2100066. 3. AstraZeneca. ULTOMIRIS® efficacy data from CHAMPION-MG. https://ultomirishcp.com/gmg/efficacy. Accessed 20 Aug 2025. 4. Nowak RJ, et al. N Engl J Med. 2025;392(23):2309-2320. 5. Nowak RJ, et al. AAN 2025. LS2.002. 6. Vu T, et al. AAN 2025. S34.002. At any point before primary endpoint 6 months to 1 year At any point before primary endpoint Note: These observations are derived from separate clinical settings; comparisons across trials are not based on head-to-head studies.

Accelerating Potential First-in-Class CAR T Franchise with MG and SPS Warner Biddle – Chief Executive Officer

Potential to Change the Treatment Paradigm in a Large and Growing Market with KYV-101 ~80k U.S. Diagnosed gMG Patients1,2 Addressable Market1,3 KYV-101 ~12k Patients 15% of total diagnosed Patients with inadequate response to > 1 biologic* ~40k Patients 50% of total diagnosed Patients with inadequate response to immunosuppressants Initial Priority Total KYV-101 Addressable Market ©2025 Kyverna Therapeutics, Inc. gMG, generalized myasthenia gravis. *Biologics defined as immunomodulatory therapies including FcRN blockers, complement inhibitors, rituximab or chronic IVIg use. 1. Rodriguez E, et al. Muscle. Nerve. 2024;69(2):166-171. 2. Hendricks TM, et al. Am J Opthamol. 2019; 205:99-105. 3. Clarivate DRG Report (2024).

Robust Market Opportunity Supported by Efficient and Scalable Commercial Strategy in Neuroimmunology Neuroimmunology Franchise Synergies SPS MG Physicians CMC Approach Treatment Centers Commercial and Medical Infrastructure Rapid market entry and uptake in MG to be supported by first-mover advantage in stiff person syndrome (SPS) and neuroimmunology franchise synergies
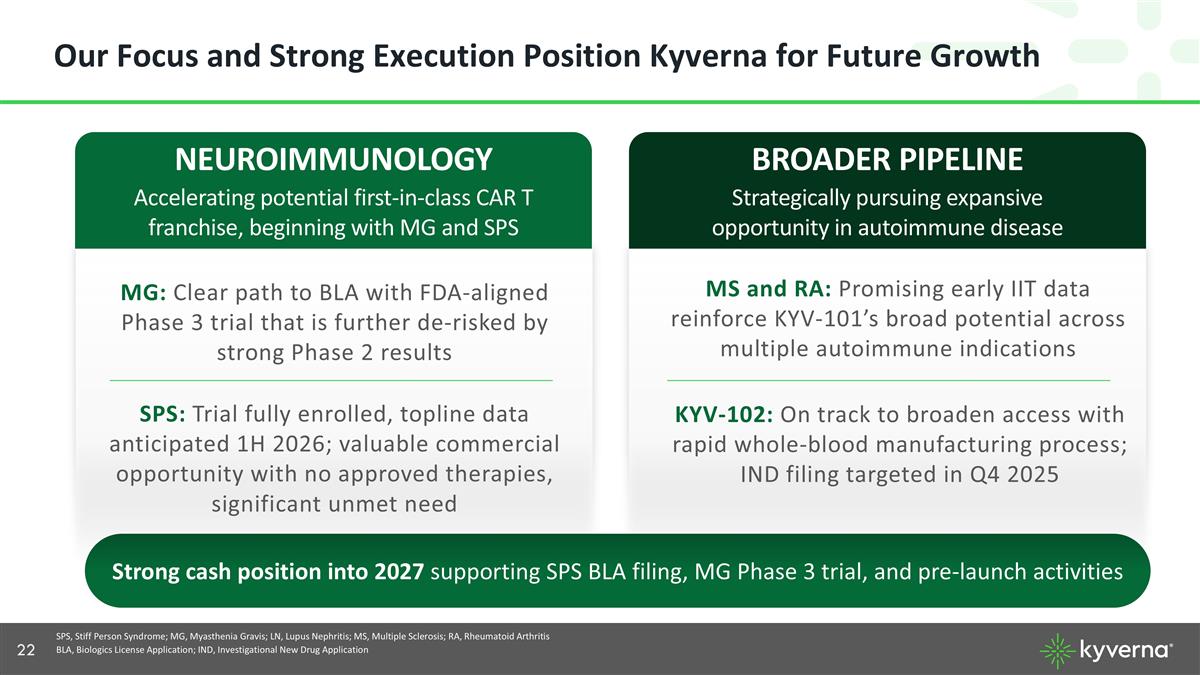
Our Focus and Strong Execution Position Kyverna for Future Growth NEUROIMMUNOLOGY Accelerating potential first-in-class CAR T franchise, beginning with MG and SPS MG: Clear path to BLA with FDA-aligned Phase 3 trial that is further de-risked by strong Phase 2 results BROADER PIPELINE Strategically pursuing expansive opportunity in autoimmune disease KYV-102: On track to broaden access with rapid whole-blood manufacturing process; IND filing targeted in Q4 2025 SPS, Stiff Person Syndrome; MG, Myasthenia Gravis; LN, Lupus Nephritis; MS, Multiple Sclerosis; RA, Rheumatoid Arthritis BLA, Biologics License Application; IND, Investigational New Drug Application Strong cash position into 2027 supporting SPS BLA filing, MG Phase 3 trial, and pre-launch activities SPS: Trial fully enrolled, topline data anticipated 1H 2026; valuable commercial opportunity with no approved therapies, significant unmet need MS and RA: Promising early IIT data reinforce KYV-101’s broad potential across multiple autoimmune indications
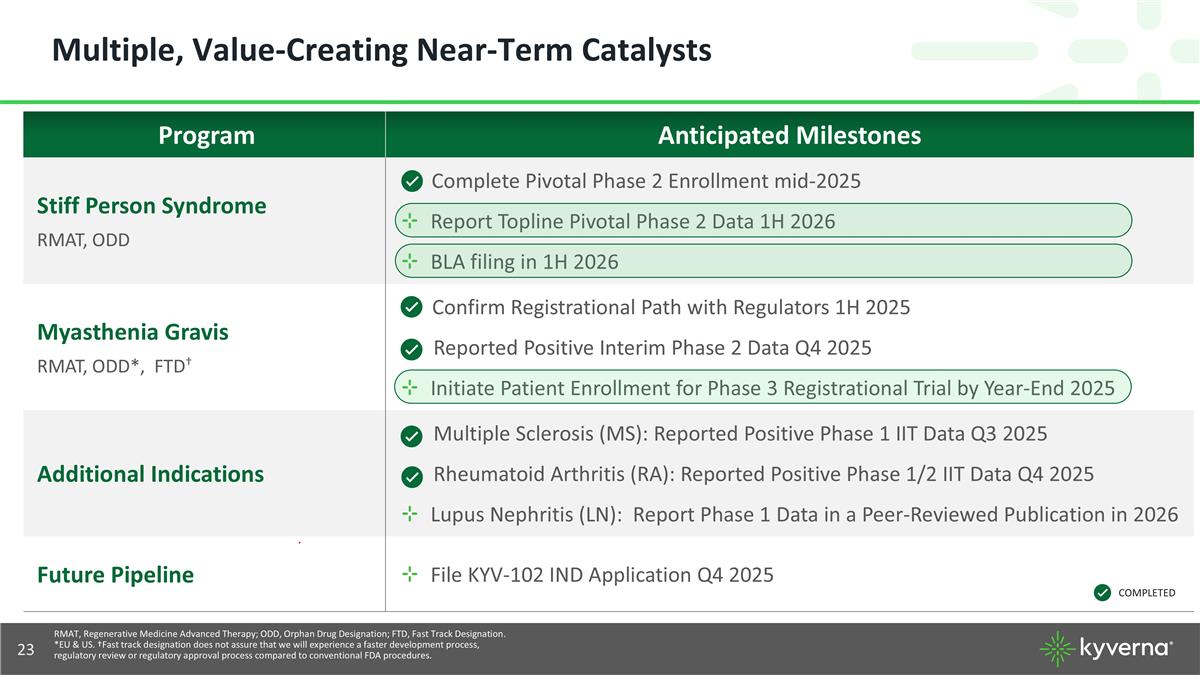
Multiple, Value-Creating Near-Term Catalysts Program Anticipated Milestones Stiff Person Syndrome RMAT, ODD Complete Pivotal Phase 2 Enrollment mid-2025 Report Topline Pivotal Phase 2 Data 1H 2026 BLA filing in 1H 2026 Myasthenia Gravis RMAT, ODD*, FTD† Confirm Registrational Path with Regulators 1H 2025 Reported Positive Interim Phase 2 Data Q4 2025 Initiate Patient Enrollment for Phase 3 Registrational Trial by Year-End 2025 Additional Indications Multiple Sclerosis (MS): Reported Positive Phase 1 IIT Data Q3 2025 Rheumatoid Arthritis (RA): Reported Positive Phase 1/2 IIT Data Q4 2025 Lupus Nephritis (LN): Report Phase 1 Data in a Peer-Reviewed Publication in 2026 Future Pipeline File KYV-102 IND Application Q4 2025 COMPLETED RMAT, Regenerative Medicine Advanced Therapy; ODD, Orphan Drug Designation; FTD, Fast Track Designation. *EU & US. †Fast track designation does not assure that we will experience a faster development process, regulatory review or regulatory approval process compared to conventional FDA procedures.

Q&A
Key Takeaways from Today 1 2 3 Kyverna is uniquely positioned to fundamentally change the treatment paradigm in gMG Data reinforces KYV-101’s potential to deliver durable, drug-free, disease-free remission with a single dose Kyverna is well positioned to deliver on a compelling commercial opportunity in gMG, a large and growing market ©2025 Kyverna Therapeutics, Inc. Today’s unprecedented clinical trial results increase confidence in Kyverna’s registrational Phase 3 superiority trial and path to BLA