
Updated Safety, Efficacy and Biomarker Analysis from the Phase I Monotherapy Study of Givastomig, a Novel Claudin 18.2/4-1BB Bispecific Antibody, in Claudin 18.2 Positive Advanced Gastroesophageal Carcinoma (GEC) Samuel J. Klempner, MD Massachusetts General Hospital, Boston, MA, United States

Disclosure Information Samuel J. Klempner I have the following relevant financial relationships to disclose: Employee of: Massachusetts General Hospital Consultant for: Bristol-Myers Squibb, Merck, Astellas, Daiichi-Sankyo, Natera, Novartis, AstraZeneca, Mersana, Sanofi-Aventis, Amgen, Boehringer-Ingelheim, Taiho Oncology, Eisai, BeiGene, Elevation Oncology, EsoBiotec, and Gilead Speaker’s Bureau for: none Grant/Research support: none Stockholder in: Turning Point Therapeutics and Nuvalent Honoraria from: none - and - My additional financial relationship disclosures are: currently participating as an investigator on the study being presented. 2
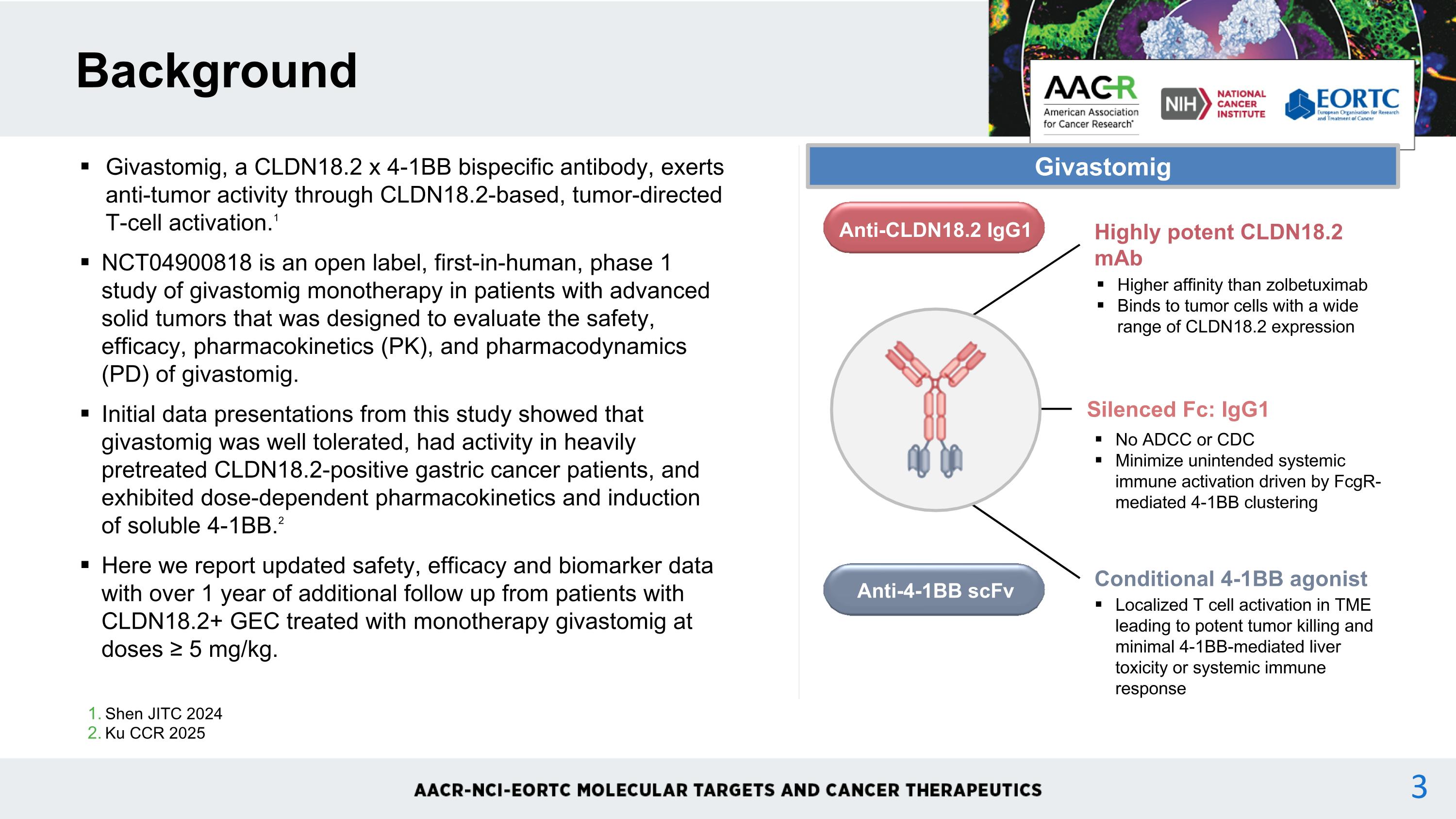
Shen JITC 2024 Ku CCR 2025 Background Givastomig, a CLDN18.2 x 4-1BB bispecific antibody, exerts anti-tumor activity through CLDN18.2-based, tumor-directed T-cell activation.1 NCT04900818 is an open label, first-in-human, phase 1 study of givastomig monotherapy in patients with advanced solid tumors that was designed to evaluate the safety, efficacy, pharmacokinetics (PK), and pharmacodynamics (PD) of givastomig. Initial data presentations from this study showed that givastomig was well tolerated, had activity in heavily pretreated CLDN18.2-positive gastric cancer patients, and exhibited dose-dependent pharmacokinetics and induction of soluble 4-1BB.2 Here we report updated safety, efficacy and biomarker data with over 1 year of additional follow up from patients with CLDN18.2+ GEC treated with monotherapy givastomig at doses ≥ 5 mg/kg. Highly potent CLDN18.2 mAb Higher affinity than zolbetuximab Binds to tumor cells with a wide range of CLDN18.2 expression Silenced Fc: IgG1 No ADCC or CDC Minimize unintended systemic immune activation driven by FcgR-mediated 4-1BB clustering Conditional 4-1BB agonist Localized T cell activation in TME leading to potent tumor killing and minimal 4-1BB-mediated liver toxicity or systemic immune response Anti-CLDN18.2 IgG1 Anti-4-1BB scFv Givastomig 3

Multi-center, dose-escalation and expansion phase Ib study Monotherapy: US and China. Combination: US only Escalation via BOIN design with at least four subjects per dose Phase 1 Study Design BOIN = Bayesian Optimal Interval Design CLDN18.2-positive = membrane intensity ≥1+ on ≥1% of tumor cells Dose Escalation n=4 per cohort Adv or metastatic solid tumors Parallel Dose Expansion n=6 per cohort, CLDN18.2 + GEC, PDAC & cholangioca 12mg/kg Q2W 0.1 mg/kg Q2W 0.3 mg/kg Q2W 1 mg/kg Q2W 3 mg/kg Q2W 5 mg/kg Q2W 8 mg/kg Q2W 12 mg/kg Q2W 15 mg/kg Q2W Dose Expansion n~20, CLDN18.2 + GEC Based on safety, PK/PD and efficacy Accelerated Titration (n=1 per cohort) 5 mg/kg Q2W 8 mg/kg Q2W 12 mg/kg Q2W 15 mg/kg Q2W CLDN18.2+ GEC 18 mg/kg Q3W CLDN18.2+ GEC Part 1 Givastomig Monotherapy 1L HER2(-) CLDN18.2+ unresectable or metastatic GC/GEJ/EAC Part 2 Givastomig + Nivolumab + mFOLFOX Dose Escalation (n=6) Giva 8 mg/kg + nivo + mFOLFOX6 Q2W Lead-In (n=5) Giva 5 mg/kg + nivo + mFOLFOX6 Q2W Dose Escalation (n=17) Dose Escalation (n=6) Giva 12 mg/kg + nivo + mFOLFOX6 Q2W Completed Dose Expansion (n=40) 8 mg/kg (n=20) – enrolled Giva 8 mg/kg + nivo + mFOLFOX6 Q2W 12 mg/kg (n=20) – enrolled Giva 12 mg/kg + nivo + mFOLFOX6 Q2W Ongoing 18 mg/kg Q3W CLDN18.2+ GEC; n=6 4
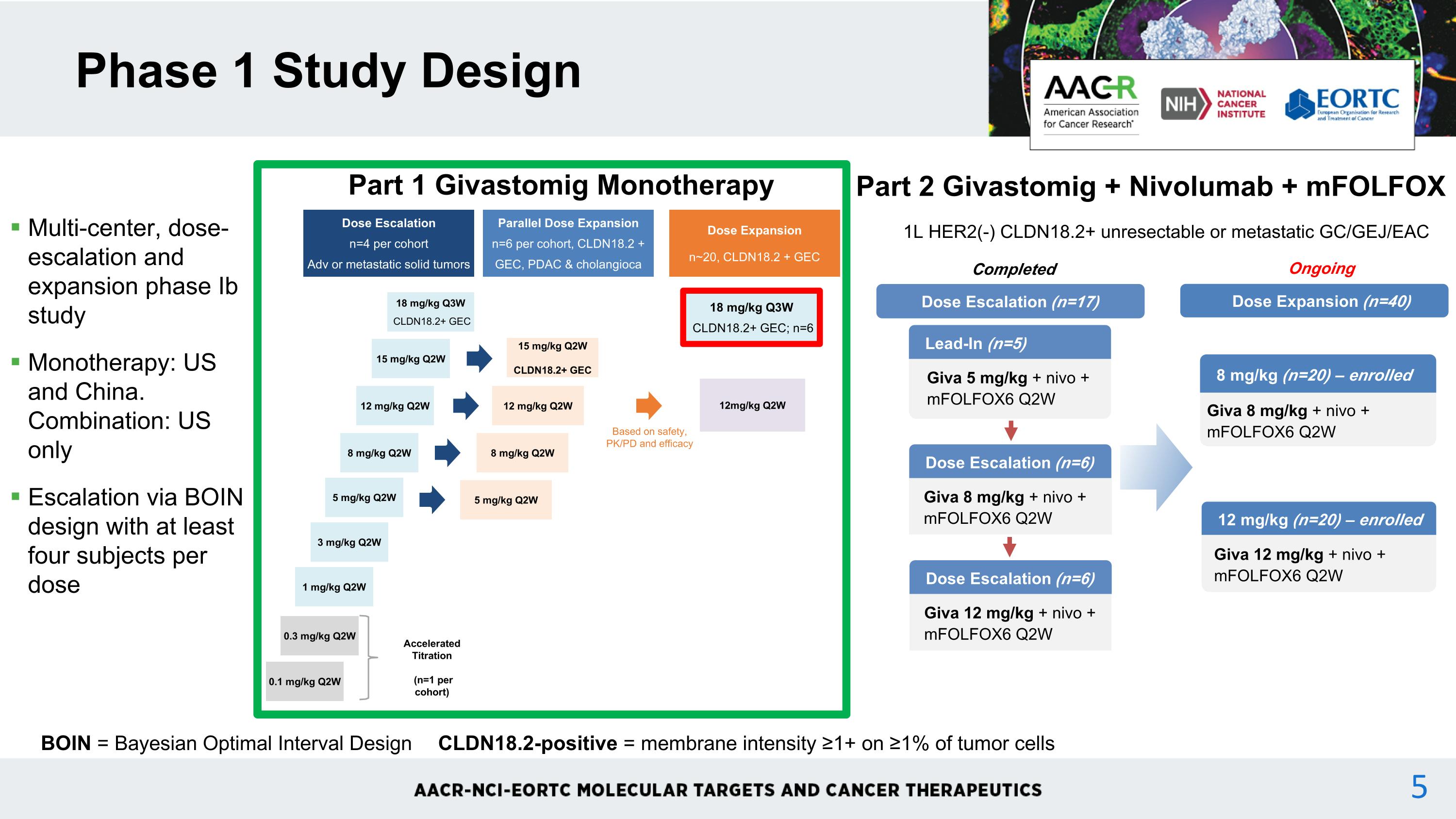
Multi-center, dose-escalation and expansion phase Ib study Monotherapy: US and China. Combination: US only Escalation via BOIN design with at least four subjects per dose Phase 1 Study Design BOIN = Bayesian Optimal Interval Design CLDN18.2-positive = membrane intensity ≥1+ on ≥1% of tumor cells Dose Escalation n=4 per cohort Adv or metastatic solid tumors Parallel Dose Expansion n=6 per cohort, CLDN18.2 + GEC, PDAC & cholangioca 12mg/kg Q2W 0.1 mg/kg Q2W 0.3 mg/kg Q2W 1 mg/kg Q2W 3 mg/kg Q2W 5 mg/kg Q2W 8 mg/kg Q2W 12 mg/kg Q2W 15 mg/kg Q2W Dose Expansion n~20, CLDN18.2 + GEC Based on safety, PK/PD and efficacy Accelerated Titration (n=1 per cohort) 5 mg/kg Q2W 8 mg/kg Q2W 12 mg/kg Q2W 15 mg/kg Q2W CLDN18.2+ GEC 18 mg/kg Q3W CLDN18.2+ GEC Part 1 Givastomig Monotherapy 1L HER2(-) CLDN18.2+ unresectable or metastatic GC/GEJ/EAC Part 2 Givastomig + Nivolumab + mFOLFOX Dose Escalation (n=6) Giva 8 mg/kg + nivo + mFOLFOX6 Q2W Lead-In (n=5) Giva 5 mg/kg + nivo + mFOLFOX6 Q2W Dose Escalation (n=17) Dose Escalation (n=6) Giva 12 mg/kg + nivo + mFOLFOX6 Q2W Completed Dose Expansion (n=40) 8 mg/kg (n=20) – enrolled Giva 8 mg/kg + nivo + mFOLFOX6 Q2W 12 mg/kg (n=20) – enrolled Giva 12 mg/kg + nivo + mFOLFOX6 Q2W Ongoing 18 mg/kg Q3W CLDN18.2+ GEC; n=6 5
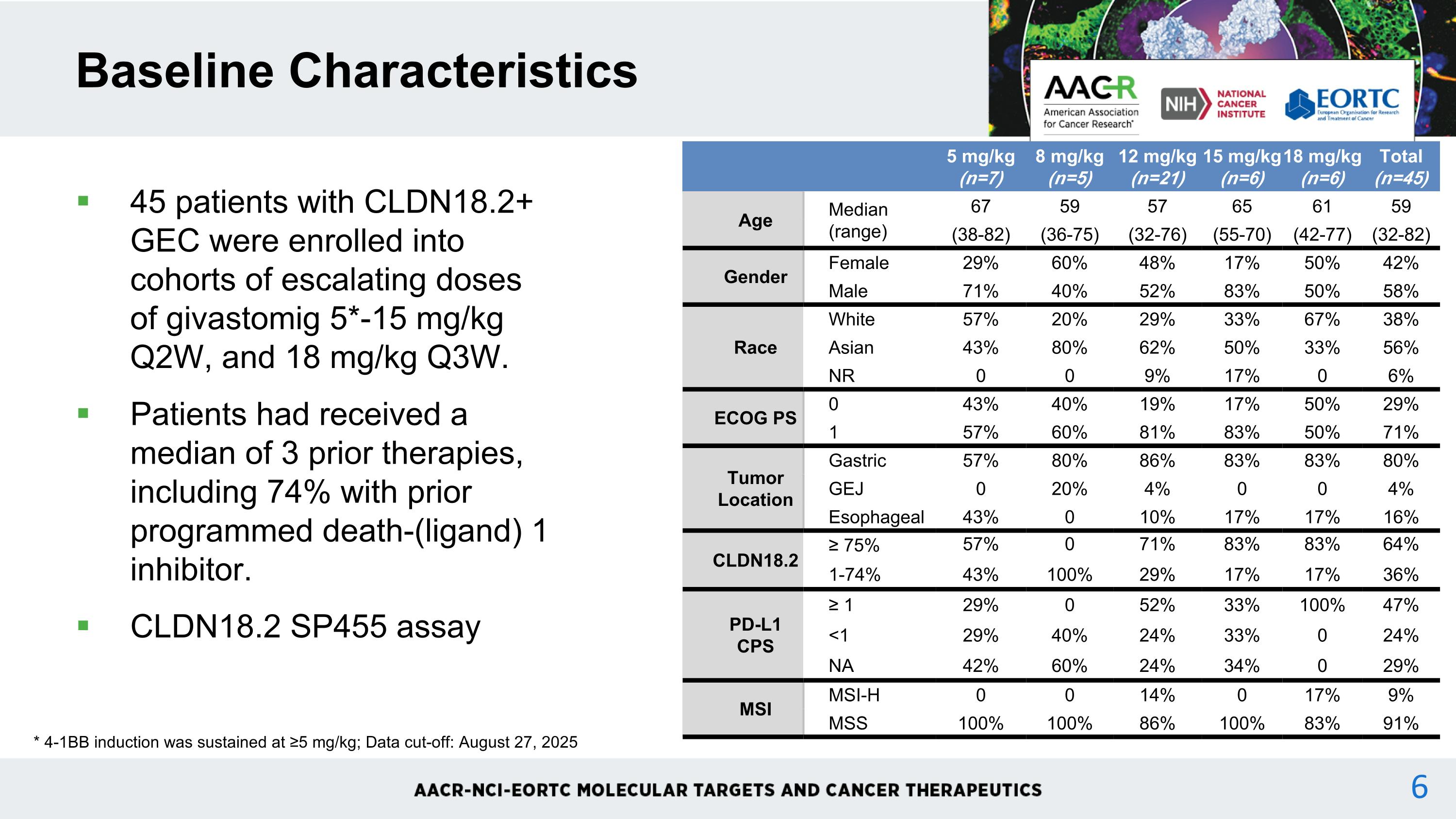
45 patients with CLDN18.2+ GEC were enrolled into cohorts of escalating doses of givastomig 5*-15 mg/kg Q2W, and 18 mg/kg Q3W. Patients had received a median of 3 prior therapies, including 74% with prior programmed death-(ligand) 1 inhibitor. CLDN18.2 SP455 assay Baseline Characteristics * 4-1BB induction was sustained at ≥5 mg/kg; Data cut-off: August 27, 2025 5 mg/kg (n=7) 8 mg/kg (n=5) 12 mg/kg (n=21) 15 mg/kg (n=6) 18 mg/kg (n=6) Total (n=45) Age Median (range) 67 59 57 65 61 59 (38-82) (36-75) (32-76) (55-70) (42-77) (32-82) Gender Female 29% 60% 48% 17% 50% 42% Male 71% 40% 52% 83% 50% 58% Race White 57% 20% 29% 33% 67% 38% Asian 43% 80% 62% 50% 33% 56% NR 0 0 9% 17% 0 6% ECOG PS 0 43% 40% 19% 17% 50% 29% 1 57% 60% 81% 83% 50% 71% Tumor Location Gastric 57% 80% 86% 83% 83% 80% GEJ 0 20% 4% 0 0 4% Esophageal 43% 0 10% 17% 17% 16% CLDN18.2 ≥ 75% 57% 0 71% 83% 83% 64% 1-74% 43% 100% 29% 17% 17% 36% PD-L1 CPS ≥ 1 29% 0 52% 33% 100% 47% <1 29% 40% 24% 33% 0 24% NA 42% 60% 24% 34% 0 29% MSI MSI-H 0 0 14% 0 17% 9% MSS 100% 100% 86% 100% 83% 91%

No dose-limiting toxicities were observed up to 15mg/kg Q2W and 18 mg/kg Q3W. Maximum-tolerated-dose was not reached. Grade ≥3 TRAE occurred in 33% of patients. One patient experienced a grade 4 platelet count decreased*. Grade 3 TRAE occurring in more than one patient were anemia (9%), lymphocyte count decreased (9%), WBC decreased (7%), AST increased (4%), neutropenia (4%), and upper GI hemorrhage* (4%). TRAE leading to permanent withdrawal of givastomig occurred in 9% of patients. These events were grade 3 ALT increased (n=1, 5 mg/kg) grade 3 infusion related reaction (n=1, 5 mg/kg), grade 2 pulmonary embolism (n=1, 12 mg/kg) and grade 3 nausea (n=1, 18 mg/kg). There were no grade 5 treatment-related adverse events. Safety Data cut-off: August 27, 2025 * patient with grade 4 platelet count decreased also experienced grade 3 upper GU hemorrhage
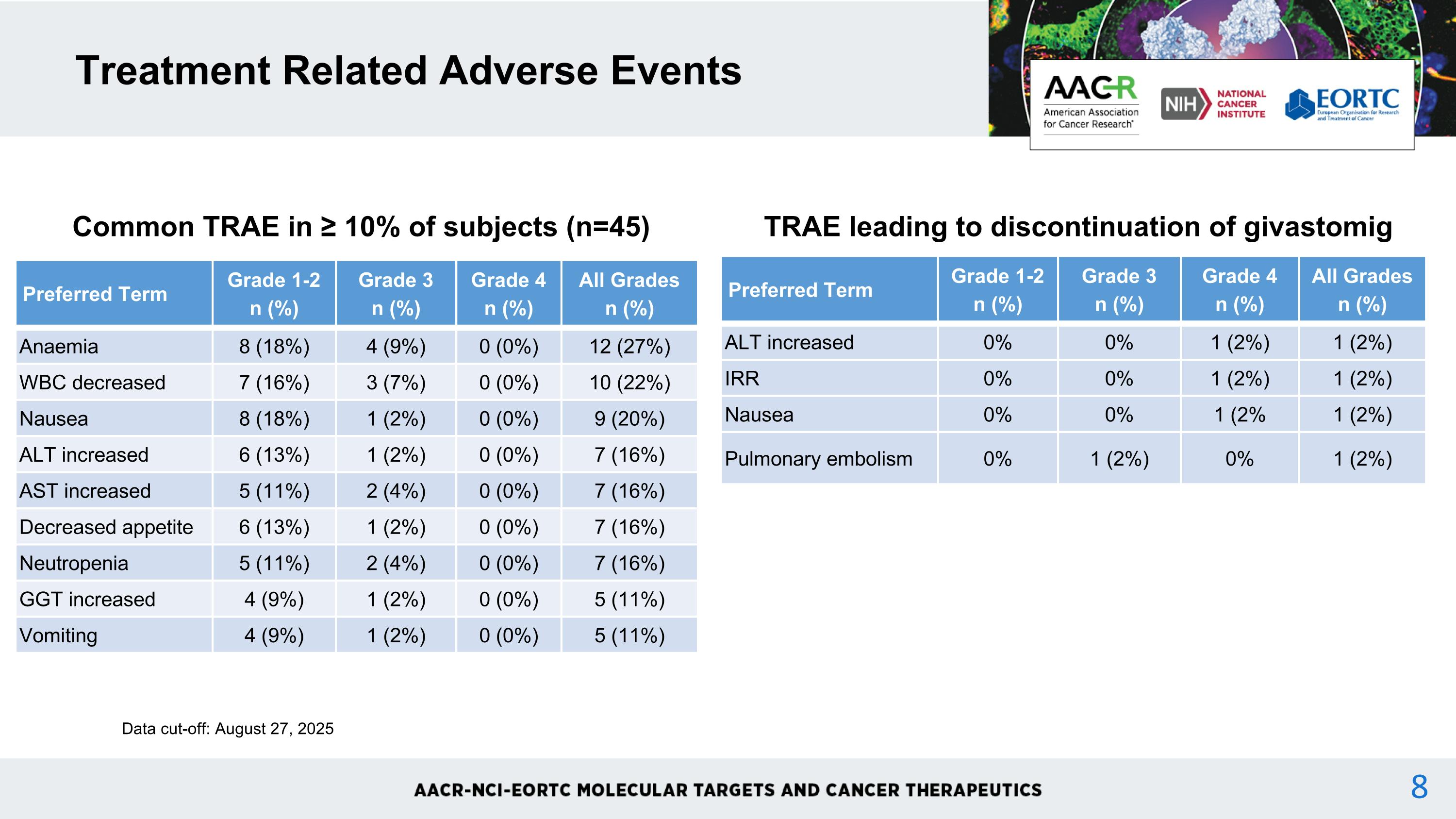
Treatment Related Adverse Events Common TRAE in ≥ 10% of subjects (n=45) Preferred Term Grade 1-2n (%) Grade 3n (%) Grade 4n (%) All Gradesn (%) Anaemia 8 (18%) 4 (9%) 0 (0%) 12 (27%) WBC decreased 7 (16%) 3 (7%) 0 (0%) 10 (22%) Nausea 8 (18%) 1 (2%) 0 (0%) 9 (20%) ALT increased 6 (13%) 1 (2%) 0 (0%) 7 (16%) AST increased 5 (11%) 2 (4%) 0 (0%) 7 (16%) Decreased appetite 6 (13%) 1 (2%) 0 (0%) 7 (16%) Neutropenia 5 (11%) 2 (4%) 0 (0%) 7 (16%) GGT increased 4 (9%) 1 (2%) 0 (0%) 5 (11%) Vomiting 4 (9%) 1 (2%) 0 (0%) 5 (11%) Data cut-off: August 27, 2025 TRAE leading to discontinuation of givastomig Preferred Term Grade 1-2n (%) Grade 3n (%) Grade 4n (%) All Gradesn (%) ALT increased 0% 0% 1 (2%) 1 (2%) IRR 0% 0% 1 (2%) 1 (2%) Nausea 0% 0% 1 (2% 1 (2%) Pulmonary embolism 0% 1 (2%) 0% 1 (2%)
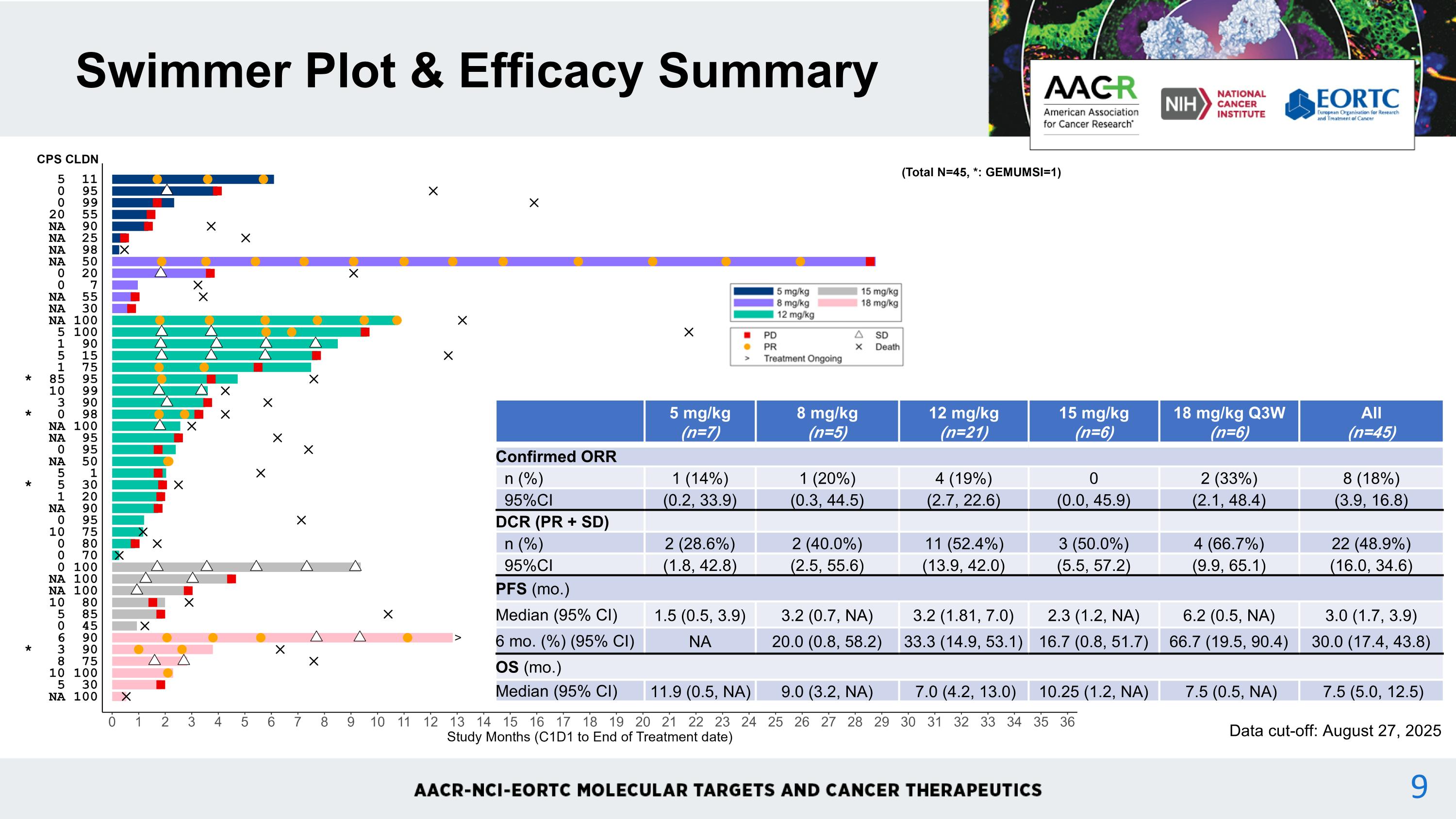
Swimmer Plot & Efficacy Summary 5 mg/kg (n=7) 8 mg/kg (n=5) 12 mg/kg (n=21) 15 mg/kg (n=6) 18 mg/kg Q3W (n=6) All (n=45) Confirmed ORR n (%) 1 (14%) 1 (20%) 4 (19%) 0 2 (33%) 8 (18%) 95%CI (0.2, 33.9) (0.3, 44.5) (2.7, 22.6) (0.0, 45.9) (2.1, 48.4) (3.9, 16.8) DCR (PR + SD) n (%) 2 (28.6%) 2 (40.0%) 11 (52.4%) 3 (50.0%) 4 (66.7%) 22 (48.9%) 95%CI (1.8, 42.8) (2.5, 55.6) (13.9, 42.0) (5.5, 57.2) (9.9, 65.1) (16.0, 34.6) PFS (mo.) Median (95% CI) 1.5 (0.5, 3.9) 3.2 (0.7, NA) 3.2 (1.81, 7.0) 2.3 (1.2, NA) 6.2 (0.5, NA) 3.0 (1.7, 3.9) 6 mo. (%) (95% CI) NA 20.0 (0.8, 58.2) 33.3 (14.9, 53.1) 16.7 (0.8, 51.7) 66.7 (19.5, 90.4) 30.0 (17.4, 43.8) OS (mo.) Median (95% CI) 11.9 (0.5, NA) 9.0 (3.2, NA) 7.0 (4.2, 13.0) 10.25 (1.2, NA) 7.5 (0.5, NA) 7.5 (5.0, 12.5) Data cut-off: August 27, 2025 9
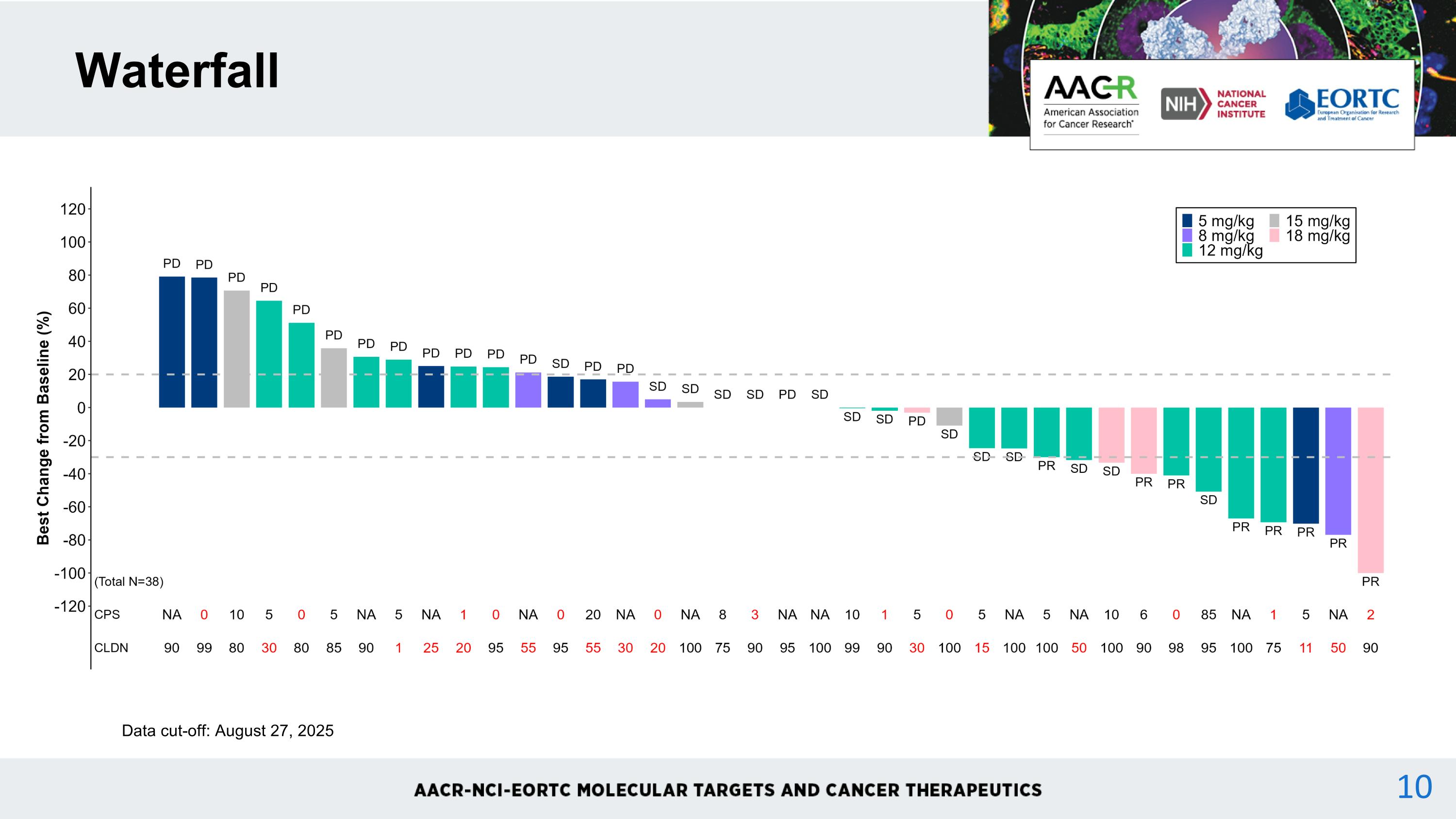
NA = not available red font = CPS <5 and CLDN <75% Waterfall Data cut-off: August 27, 2025
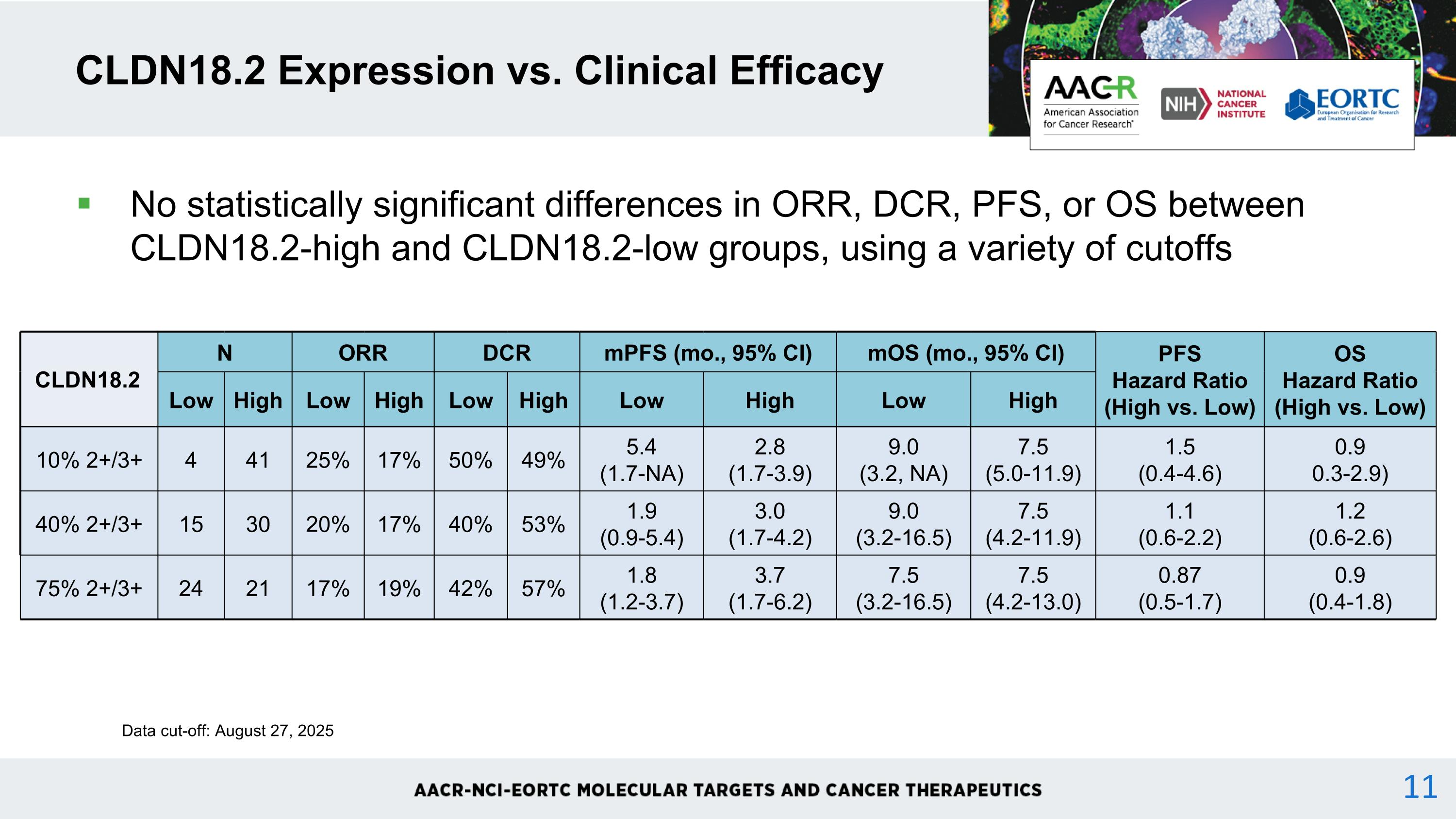
CLDN18.2 Expression vs. Clinical Efficacy CLDN18.2 N ORR DCR mPFS (mo., 95% CI) mOS (mo., 95% CI) PFS Hazard Ratio (High vs. Low) OS Hazard Ratio (High vs. Low) Low High Low High Low High Low High Low High 10% 2+/3+ 4 41 25% 17% 50% 49% 5.4 (1.7-NA) 2.8 (1.7-3.9) 9.0 (3.2, NA) 7.5 (5.0-11.9) 1.5 (0.4-4.6) 0.9 0.3-2.9) 40% 2+/3+ 15 30 20% 17% 40% 53% 1.9 (0.9-5.4) 3.0 (1.7-4.2) 9.0 (3.2-16.5) 7.5 (4.2-11.9) 1.1 (0.6-2.2) 1.2 (0.6-2.6) 75% 2+/3+ 24 21 17% 19% 42% 57% 1.8 (1.2-3.7) 3.7 (1.7-6.2) 7.5 (3.2-16.5) 7.5 (4.2-13.0) 0.87 (0.5-1.7) 0.9 (0.4-1.8) Data cut-off: August 27, 2025 No statistically significant differences in ORR, DCR, PFS, or OS between CLDN18.2-high and CLDN18.2-low groups, using a variety of cutoffs 11
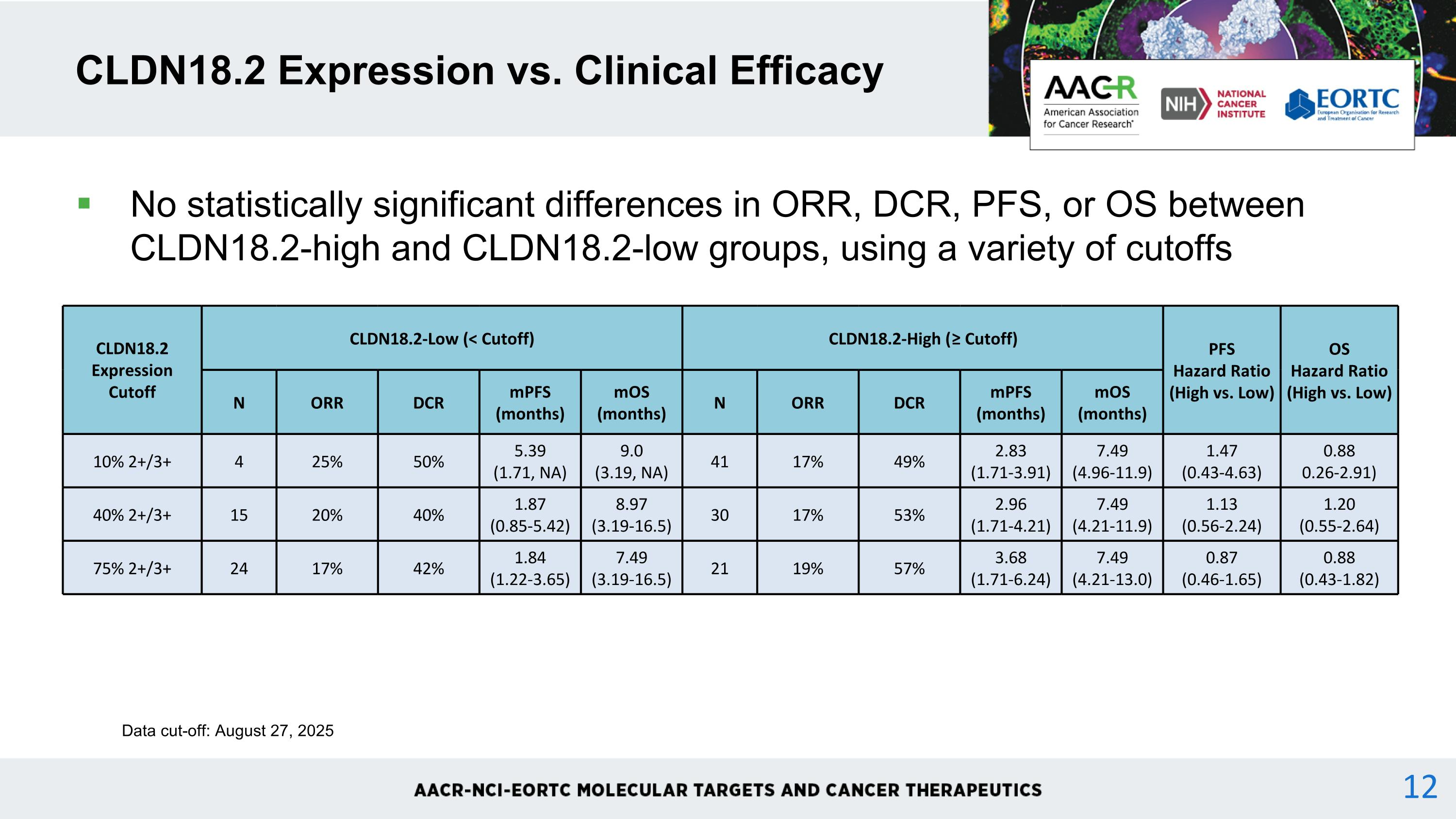
No statistically significant differences in ORR, DCR, PFS, or OS between CLDN18.2-high and CLDN18.2-low groups, using a variety of cutoffs CLDN18.2 Expression vs. Clinical Efficacy CLDN18.2 Expression Cutoff CLDN18.2-Low (< Cutoff) CLDN18.2-High (≥ Cutoff) PFS Hazard Ratio (High vs. Low) OS Hazard Ratio (High vs. Low) N ORR DCR mPFS (months) mOS (months) N ORR DCR mPFS (months) mOS (months) 10% 2+/3+ 4 25% 50% 5.39 (1.71, NA) 9.0 (3.19, NA) 41 17% 49% 2.83 (1.71-3.91) 7.49 (4.96-11.9) 1.47 (0.43-4.63) 0.88 0.26-2.91) 40% 2+/3+ 15 20% 40% 1.87 (0.85-5.42) 8.97 (3.19-16.5) 30 17% 53% 2.96 (1.71-4.21) 7.49 (4.21-11.9) 1.13 (0.56-2.24) 1.20 (0.55-2.64) 75% 2+/3+ 24 17% 42% 1.84 (1.22-3.65) 7.49 (3.19-16.5) 21 19% 57% 3.68 (1.71-6.24) 7.49 (4.21-13.0) 0.87 (0.46-1.65) 0.88 (0.43-1.82) Data cut-off: August 27, 2025 12
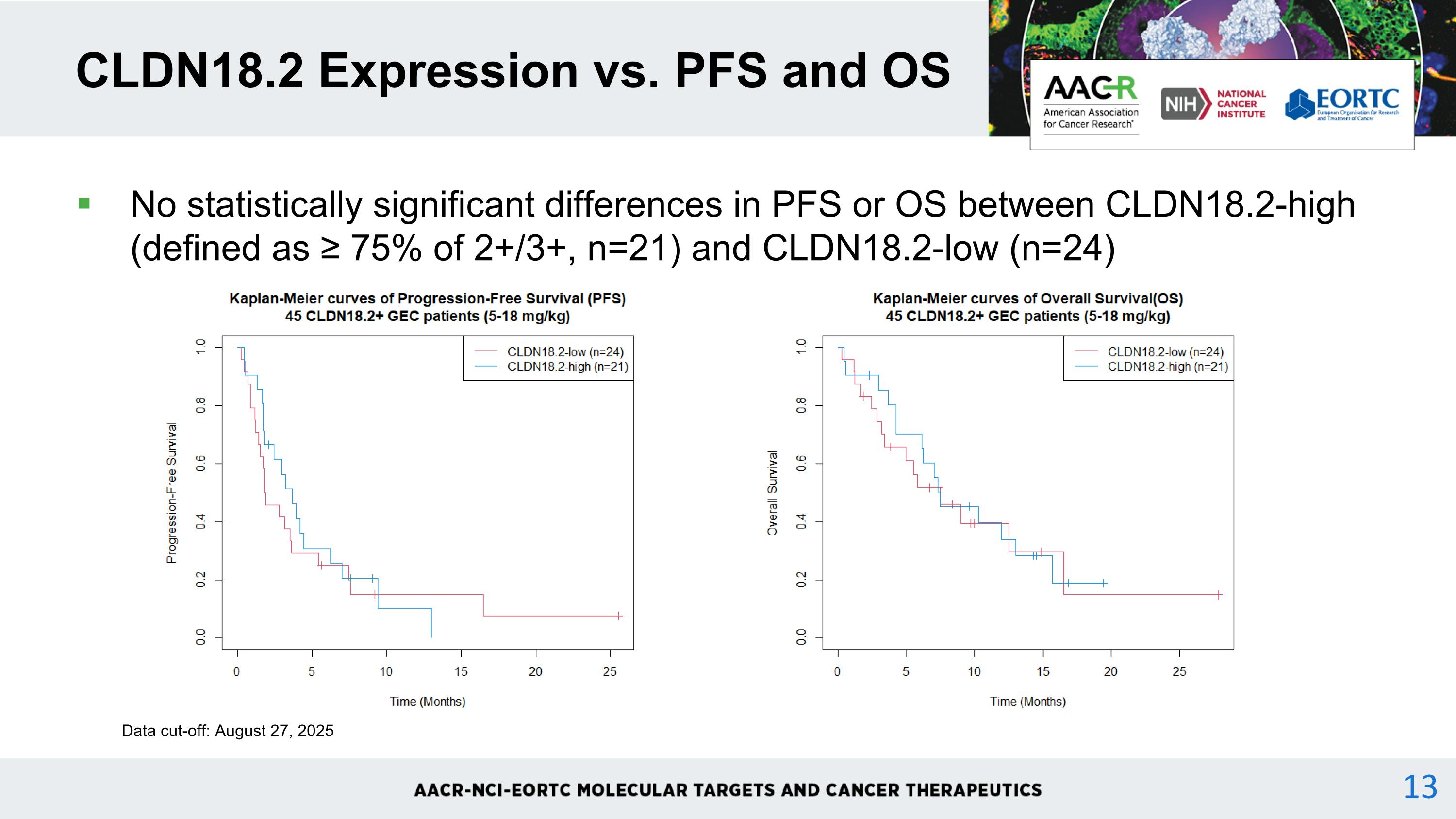
No statistically significant differences in PFS or OS between CLDN18.2-high (defined as ≥ 75% of 2+/3+, n=21) and CLDN18.2-low (n=24) CLDN18.2 Expression vs. PFS and OS Data cut-off: August 27, 2025 13
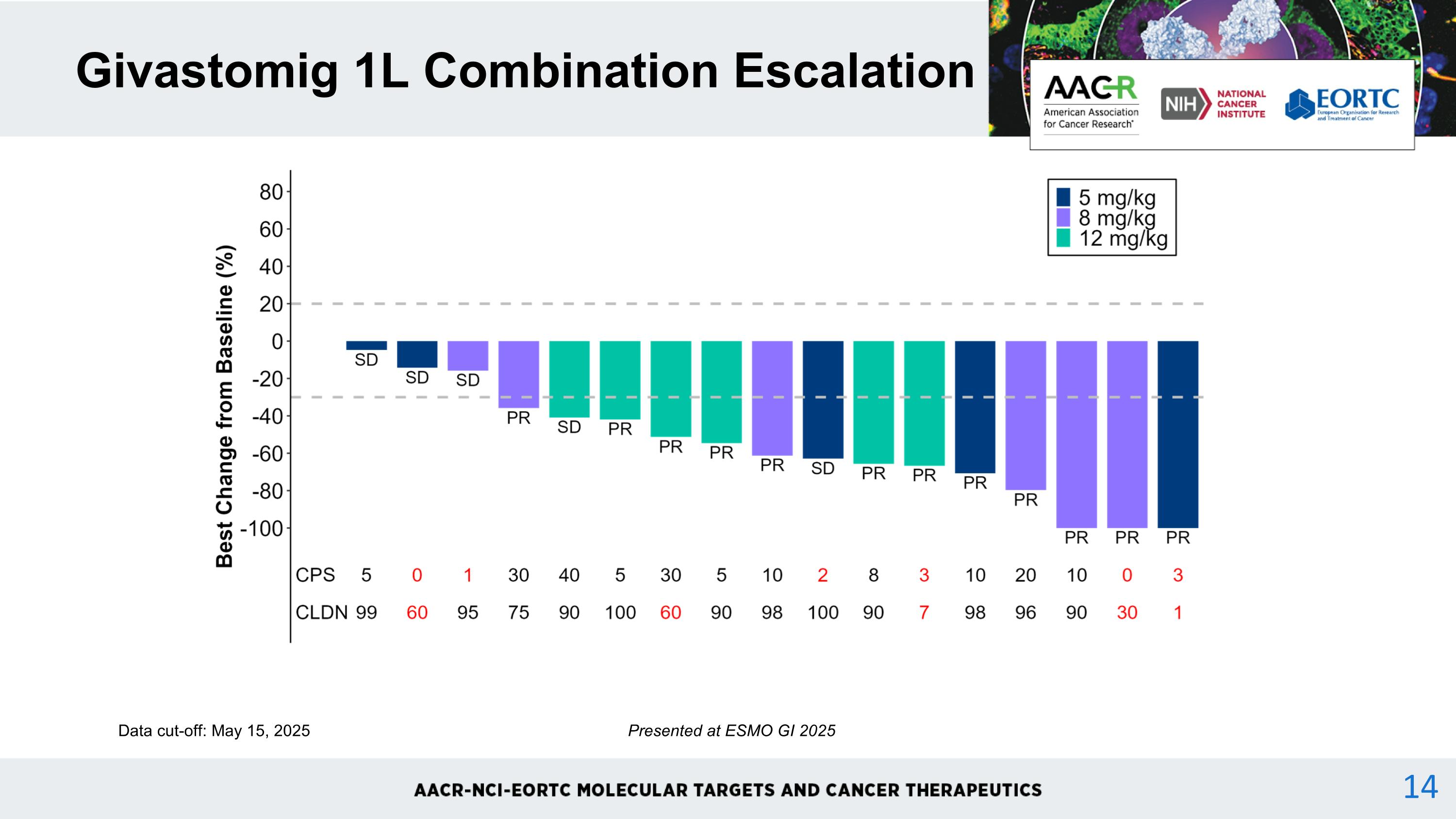
Givastomig 1L Combination Escalation Data cut-off: May 15, 2025 Presented at ESMO GI 2025 14

With over a year of additional follow up, givastomig continues to be well tolerated up to 15 mg/kg Q2W and 18 mg/kg Q3W and continues to show encouraging monotherapy activity in heavily pre-treated GEC patients with a wide range of CLDN18.2 expression (confirmed ORR 18%). There was no statistically significant difference in ORR, DCR, PFS, or OS between CLDN18.2-high and CLDN18.2-low groups, using a variety of cutoffs. Givastomig may have utility in patients with lower CLDN18.2 expression compared with other CLDN18.2 agents. The sustained tolerability and efficacy support the development of givastomig as an add on to 1L therapy in combination with nivolumab and mFOLFOX6 in advanced or metastatic gastric, gastroesophageal and esophageal adenocarcinomas (NCT04900818), as well as other CLDN+ GI malignancies. Conclusions 15














