UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16
under the Securities Exchange Act of 1934
Date of Report: October 16, 2025
Commission File Number: 001-36891
Cellectis S.A.
(Exact Name of registrant as specified in its charter)
8, rue de la Croix Jarry
75013 Paris, France
+33 1 81 69 16 00
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Cellectis S.A.
The information included in this report on Form 6-K under the caption “—BALLI-01 Study” below shall be deemed to be incorporated by reference in the registration statements of Cellectis S.A. (collectively, the “Registration Statements”) on Form F-3 (No. 333-288491 and 333-284302) and Form S-8 (Nos. 333-204205, 333-214884, 333-222482, 333-227717, 333-258514, 333-267760, 333-273777, 333-284301 and 333-290218), to the extent not superseded by documents or reports subsequently filed.
The information under the caption “Investors R&D Day” as well as the information included as “Exhibits” to this Form 6-K shall not be deemed incorporated by reference in any filing of Cellectis S.A. under the Securities Act of 1933 or under the Exchange Act of 1934, except as shall be expressly set forth by specific reference in such a filing.
Investors R&D Day
Cellectis S.A. (the “Company”) hosted an Investors R&D Day on October 16, 2025. In connection with the R&D Day, the Company issued a press release, attached as Exhibit 99.1 hereto. Attached as Exhibit 99.2 hereto is a copy of the presentation deck that accompanied the Investors R&D Day.
BALLI-01 Study
Highlights:
| • | Efficacy: ORR of 68% with lasme-cel Process 2 (n=22), 83% at RP2D (n=12) and 100% in the target Phase 2 population (n=9) |
| • | Safety: in Phase 1 (n=40), lasme-cel was generally well-tolerated (including 1 case of grade 2 IEC-HS which resolved) |
| • | Durability: in patients who achieved MRD-negative CR/CRi, median OS was 14.8 months |
| • | In the target Phase 2 population, CR/CRi rate of 56% with ~80% of patients achieving MRD-negative status |
| • | In the target Phase 2 population, 100% patients became transplant eligible with 78% proceeding to transplant |
| • | Among 11 subjectspatients previously treated with all 3 targeted therapies (inotuzumab, blinatumomab, and CD19 CAR-T), 8 responded and 7 achieved MRD-negative status |
| • | BALLI-01 pivotal Phase 2 in r/r B-ALL initiated |
On October 16, 2025, the Company released promising clinical data from the BALLI-01 Phase 1 study of lasme-cel (UCART22) for transplant ineligible patients with relapsed/refractory B-cell acute lymphoblastic leukemia (r/r B-ALL) in the third line or beyond (3L+).
The BALLI-01 Phase 1 clinical study was designed to evaluate the safety and clinical activity of lasme-cel (UCART22) in patients with r/r B-ALL. The BALLI-01 trial enrolled 40 patients aged 15–70 years expressing >70% CD22 on leukemic blasts. Patients were heavily pretreated with a median of 4 prior lines of therapy: 80% of patients had received prior blinatumomab, approximately half had received prior inotuzumab and prior CD19 autologous CAR-T therapy.
Lasme-cel (UCART22) was given at escalating dose levels following lymphodepletion with either fludarabine and cyclophosphamide (FC) or FC with alemtuzumab (FCA). The addition of alemtuzumab was implemented to sustain host T-cell and Natural Killer (NK)-cell depletion and to support lasme-cel expansion and persistence.
Phase 1 Safety Data: The Phase 1 safety data confirm that lasme-cel was generally well-tolerated, with expectations for CAR-T therapies, with manageable adverse events, including cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS).
| • | Dose-limiting toxicities (DLTs) were uncommon, with only 1 case reported at Dose Level 3 (DL3) |
| • | Adverse event of special interest (AESI) of CRS occurred in 2.5% of patients and ICANS in 5% of patients |
| • | 8 lasme-cel related serious adverse events (SAEs) were reported |
Phase 1 Activity Data: In the BALLI-01 Phase 1 study, 40 transplant ineligible 3L+ patients were dosed with lasme-cel (UCART22):18 patients were dosed with product manufactured by an external CDMO (Process 1, or P1) and 22 patients were dosed with Cellectis-manufactured product (Process 2, or P2). In this dataset, P2 was associated with higher response rate than P1:
| • | Complete Remission (CR) / Complete Remission with complete hematologic Recovery (CRi) rate: 18% for P1 vs 36% for P2 |
At Dose Level 3, Process 2 (DL3), the recommended Phase 2 dose (RP2D), 12 patients were dosed (n=12):
| • | The CR/CRi rate was 42%, with 80% of these responders achieving MRD-negative status |
For the subset of 9 patients who met the criteria of the pivotal Phase 2 population (Process 2, DL3, age ≤ 50):
| • | The CR/CRi rate was 56% with 80% of responders MRD-negative |
| • | The ORR was 100% with MRD-negative in 78% |
In patients treated with P2, 13 patients had relapsed after prior CD22 targeted therapy (Inotuzumab). Of these 13 patients, 4 (31%) achieved CR/CRi with MRD-negative status and all 4 achieved hematopoietic stem cell transplantation (HSCT). In the overall P2 cohort, the ORR was 68% with MRD-negativity in 83% of responders.
At the RP2D (DL3) subset (n=12), 7 of these 12 patients had prior inotuzumab exposure with 43% achieving MRD-negative CR/CRi, and all of these patients achieved HSCT.
In the P2 cohort (n=22), 11 of 22 patients (50%) received 3 prior targeted therapies-CD19 CAR-T, blinatumumab and inotuzumab. Among these heavily pretreated patients, 36% achieved CR/CRi with MRD-negative status.
The survival curve for this study suggests a clear benefit: patients who proceeded to HSCT after lasme-cel (UCART22) therapy showed a trend to longer overall survival than those who did not undergo transplant.
The Phase 1 data showed that lasme-cel (UCART22) maintained its efficacy regardless of the number or type of previous treatments, including CAR-T (60% of patients), transplant (50% of patients), and blinatumomab (80% of patients).
Following successful End-of-Phase 1 meetings with the U.S Food and Drug Administration (FDA) and the European Medicines Agency (EMA), Cellectis provided a registration path for lasme-cel as a bridge to transplant in r/r ALL. The first patient in pivotal Phase 2 is expected to be enrolled in Q4 2025. Cellectis anticipates submitting a Biologics License Application (BLA) in 2028.
EXHIBITS
| Exhibit |
Title |
|
| 99.1 | Press release dated October 16, 2025 | |
| 99.2 | Investors R&D Day Presentation dated October 16, 2025 | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| CELLECTIS S.A. | ||||||
| October 16, 2025 | By: | /s/ André Choulika |
||||
| André Choulika | ||||||
| Chief Executive Officer | ||||||
Exhibit 99.1

PRESS RELEASE
Cellectis’ R&D Day Highlights Lasme-cel’s
Potential to Address Significant Unmet Need for Patients with r/r B-ALL
o Efficacy: ORR of 68% with lasme-cel Process 2 (n=22), 83% at RP2D (n=12) and 100% in the target Phase 2 population (n=9)
o Safety: in Phase 1 (n=40), lasme-cel was generally well-tolerated (including 1 case of grade 2 IEC-HS which resolved)
o Durability: in patients who achieved MRD-negative CR/CRi, median OS was 14.8 months
o In the target Phase 2 population, CR/CRi rate of 56% with ~80% of patients achieving MRD-negative status
o In the target Phase 2 population, 100% patients became transplant eligible with 78% proceeding to transplant
o Among 11 patients previously treated with all 3 targeted therapies (inotuzumab, blinatumomab, and CD19 CAR-T), 8 responded and 7 achieved MRD-negative status
o BALLI-01 pivotal Phase 2 in r/r B-ALL initiated
o Potential peak gross sales of up to ~$700 million across the U.S., EU4, UK
New York, NY – October 16, 2025—Cellectis (the “Company”) (Euronext Growth: ALCLS—NASDAQ: CLLS), a clinical-stage biotechnology company using its pioneering gene editing platform to develop life-saving cell and gene therapies, today hosted an R&D Day providing promising clinical data from the Phase 1 BALLI-01 study of lasme-cel (UCART22) for transplant ineligible patients with relapsed/refractory B-cell acute lymphoblastic leukemia (r/r B-ALL) in the third line or beyond (3L+). The Company unveiled the design of the pivotal Phase 2, its planned registration path for lasme-cel’s initial indication, as well as the commercial opportunity. The event also included panel discussions with global hematology experts on the potential of lasme-cel to address the significant unmet need for heavily pretreated patients.
“We are encouraged by the Phase 1 results of the BALLI-01 study in a population that had largely exhausted treatment options and faced a poor prognosis. The depth of response we observed, enabling a high percentage of patients in the study to proceed to transplant, with the potential for durable remission, is clinically meaningful. We are focused on rapidly advancing our pivotal Phase 2 program and bringing lasme1-cel to patients with this significant unmet need” said Adrian Kilcoyne, MD, MPH, MBA, Chief Medical Officer of Cellectis.
“Today, Cellectis’ has reached a tipping point as we move lasme-cel into a pivotal Phase 2 trial. The need in refractory or relapsed B-ALL, especially for adult patients for whom every day counts, is significant. Our strategy is to achieve minimal residual disease-negative (MRD-negative) complete remission to create a window for hematopoietic stem cell transplantation and meaningfully improve overall survival. Cellectis is pioneering allogeneic CAR-T therapies that aim to be safer, faster, and more scalable and accessible therapy. Lasme-cel is designed to be off-the-shelf, ready when the patient is ready, so it may enable rapid disease control instead of waiting, potentially bringing more patients to the point where transplant becomes possible” said André Choulika, Ph.D., Chief Executive Officer of Cellectis. “That is why an allogeneic CD22 approach is not just another CD19 program, it is potential solution for a broader patient population facing r/r B-ALL.”
BALLI-01 Phase 1 Study Evaluating lasme-cel
The Phase 1 of BALLI-01 clinical study was designed to evaluate the safety and clinical activity of lasme-cel in patients with r/r B-ALL.
The BALLI-01 trial enrolled 40 patients aged 15–70 years expressing >70% CD22 on leukemic blasts. Subjects were heavily pretreated with a median of 4 prior lines of therapy: 80% of subjects had received prior blinatumomab, approximately half had received prior inotuzumab and prior CD19 autologous CAR-T therapy.
Lasme-cel was given at escalating dose levels following lymphodepletion with either fludarabine and cyclophosphamide (FC) or FC with alemtuzumab (FCA). The addition of alemtuzumab was implemented to sustain host T-cell and Natural Killer (NK)-cell depletion and to support lasme-cel expansion and persistence.
Phase 1 Safety Data:
The Phase 1 safety data confirm that lasme-cel was generally well-tolerated, with expectations for CAR-T therapies, with manageable adverse events, including cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS).
| • | Dose-limiting toxicities (DLTs) were uncommon, with only 1 case reported at Dose Level 3 (DL3) |
| • | Adverse event of special interest (AESI) of CRS occurred in 2.5% of patients and ICANS in 5% of patients |
| • | 8 lasme-cel related serious adverse events (SAEs) were reported |
Phase 1 Activity Data
In the Phase 1 of BALLI-01 study, 40 transplant ineligible 3L+ patients were dosed with lasme-cel :18 patients were dosed with product manufactured by an external CDMO (Process 1, orP1) and 22 patients were dosed with Cellectis-manufactured product (Process 2, or P2). In this dataset, P2 was associated with higher response rate than P1:
Complete Remission (CR) / Complete Remission with complete hematologic Recovery (CRi) rate: 18% for P1 vs 36% for P2.
Lasme-cel is not a commercialized, FDA-approved product.
At Dose Level 3 P2 (DL3), the recommended Phase 2 dose (RP2D), 12 patients were dosed (n=12):
| o | The CR/CRi rate was 42%, with 80% of these responders achieving MRD-negative status |
For the subset of 9 patients who met the criteria of the pivotal Phase 2 population (Process 2, DL3, age ≤ 50):
| o | The CR/CRi rate was 56% with 80% of responders MRD-negative |
| o | The ORR was 100% with MRD-negative in 78% |
In patients treated with P2, 13 patients had relapsed after prior CD22 targeted therapy (Inotuzumab). Of these 13 patients, 4 (31%) achieved CR/CRi with MRD-negative status and all 4 achieved hematopoietic stem cell transplantation (HSCT). In the overall P2 cohort, the ORR was 69% with MRD-negativity in 83% of responders.
At the RP2D (DL3) subset, 7 of these 12 patients had prior inotuzumab exposure with 43% achieving MRD-negative CR/CRi, and all of these achieved HSCT.
In the P2 cohort, 11 of 22 patients received 3 prior targeted therapies-CD19 CAR-T, blinatumumab and inotuzumab. Among these heavily pretreated patients, 36% achieved CR/CRi with MRD-negative status.
The survival curve for this study suggests a clear benefit: patients who proceeded to HSCT after lasme-cel therapy showed a trend to longer overall survival than those who did not undergo transplant.
The Phase 1 data showed that lasme-cel maintained its efficacy regardless of the number or type of previous treatments, including CAR-T (60% of subjects), transplant (50% of patients), and blinatumomab (80% of subjects).
Taken together, these findings position lasme-cel as a potentially game-changing therapy for patients with r/r B-ALL.
Following successful End-of-Phase 1 meetings with the U.S Food and Drug Administration (FDA) and the European Medicines Agency (EMA), Cellectis provided a registration path for lasme-cel as a bridge to transplant in r/r ALL. The first patient in pivotal Phase 2 is expected to be enrolled in Q4 2025. Cellectis anticipates submitting a Biologics License Application (BLA) in 2028.
B-ALL Panel
Cellectis’ R&D Day also included a panel discussion on the potential of lasme-cel to address the significant unmet need for r/r B-ALL patients. Panelists included:
Pr. Nitin Jain, Professor of Medicine Department of Leukemia at MD Anderson Cancer Center
Pr. Nicolas Boissel, Head of the Adolescents and Young Adults Unit of the Hematology Department of Hôpital Saint-Louis AP-HP
Dr. Aravind Ramakrishnan, Leading Oncologist and Program medical Director for the Sarah Cannon Transplant & Cellular Therapy Program
Commercial Opportunity for Lasme-cel
As part of the R&D Day presentation, the Company discussed the potential commercial opportunity for lasme-cel in r/r B-ALL.
If approved for commercialization, Cellectis estimates that lasme-cel has the potential to reach up to approximately 1,900 addressable 3L+ B-ALL patients annually in 2035 across the U.S., EU4 (Germany, France, Spain, Italy) and UK. Of the projected incident 1L treated B-ALL population of approximately 9,200 patients, about 45% are expected to progress to 2L (mostly adolescents and adults given better outcomes for children) and two-thirds of patients treated in the 2L setting are expected to go on to require further therapeutic intervention. Finally, approximately 70% of patients treated in the 3L setting are considered for clinical allogeneic CAR-T eligibility based on patient fitness and comorbidities.
Lasme-cel is positioned to potentially capture a majority of such addressable market given that it provides an alternative target to CD19, one-time dosing, off-the-shelf availability and deep, MRD-responses in the 3L+ setting. Interviewed physician key opinion leaders indicated an expectation to use lasme-cel in a majority of 3L+ patients if available, and oncology analogs suggest an illustrative lasme-cel preference share of about 65% among eligible patients.
Finally, lasme-cel has high pricing potential across the U.S., EU4 and UK. For the EU4 and UK, Cellectis triangulated an illustrative lasme-cel 2025 anchor price of approximately $365,000 from the averaged CAR-T price range, with a projected list price in 2035 assumed to remain constant with 2025. For the U.S., Cellectis triangulated an illustrative lasme-cel 2025 anchor price of approximately $515,000 from the averaged CAR-T price range, with a projected 2035 list price, assumed to grow at a ~5% CAGR (Compound Annual Growth Rate) based on the 2021-2025 price growth.2
With the above assumptions, lasme-cel could achieve up to approximately $700 million in potential peak gross sales across the U.S., EU4 and UK in 2035, corresponding to an estimation of about 1,100 patients treated annually. Furthermore, gross peak sales could increase to up to approximately $1.3 billion with potential label expansion to second line and first line MRD+ consolidation. These estimates highlight that lasme-cel has the potential to drive meaningful growth of the CAR-T market in B-ALL, leading to a robust peak sales potential with highly attractive margins stemming from the allogeneic approach.
NatHaLi-01 study evaluating eti-cel (UCART20x22)
Cellectis unveiled preliminary data on eti-cel, its allogeneic CAR-T product candidate for relapsed or refractory non-Hodgkin lymphora (r/r NHL). These preliminary results demonstrate an encouraging overall response rate (ORR) of 86% and a complete response (CR) rate of 57% at the current dose level (n=7), with 4 out of 7 patients achieving a complete response.
| 2 | Pricing assumptions for illustrative purposes only – Cellectis has not started price negotiation nor made any pricing decisions for lasme-cel. |
The preliminary high rate of complete responses underscores the potential of this innovative approach to transform outcomes for r/r NHL patients. Further updates on the program are expected at the end of the year 2025.
Strategic Partnership with AstraZeneca
In its presentation during the R&D Day, AstraZeneca highlighted the significance of its strategic investment and research collaboration with Cellectis to accelerate its cell therapy and genomic medicine ambitions. The collaboration leverages Cellectis’ gene editing expertise and manufacturing capabilities to develop up to 10 novel cell and gene therapy products for areas of high unmet medical need, including oncology, immunology and rare genetic disorders.
Webcast details
A replay of today’s event and copy of the presentation will be published on Cellectis’ website.
About Cellectis
Cellectis is a clinical-stage biotechnology company using its pioneering gene-editing platform to develop life-saving cell and gene therapies. The company utilizes an allogeneic approach for CAR T immunotherapies in oncology, pioneering the concept of off-the-shelf and ready-to-use gene-edited CAR T-cells to treat cancer patients, and a platform to develop gene therapies in other therapeutic indications. With its in-house manufacturing capabilities, Cellectis is one of the few end-to-end gene editing companies that controls the cell and gene therapy value chain from start to finish.
Cellectis’ headquarters are in Paris, France, with locations in New York and Raleigh, NC. Cellectis is listed on the Nasdaq Global Market (ticker: CLLS) and on Euronext Growth (ticker: ALCLS). To find out more, visit www.cellectis.com and follow Cellectis on LinkedIn and X.
Cautionary Statement
This press release contains “forward-looking” statements within the meaning of applicable securities laws, including the Private Securities Litigation Reform Act of 1995. Forward-looking statements may be identified by words such as “designed to,” “anticipate,” “can,” “could,” “estimate,” “expectation,” “expected,” “illustrative,” “plan,” “potential,” “potentially, “positioned,” “projected,” “should,” “suggest,” and “will,” “would,” or the negative of these and similar expressions. These forward-looking statements, which are based on our management’s current expectations and assumptions and on information currently available to management, include statements regarding the market market opportunities with respect to lasme-cel (and the assumptions on which such determinations are based, including with respect to addressable populations and potential pricing), the potential of the Phase 2 BALLI-01 trial to be a registrational phase, the advancement, timing and progress of clinical trials (including with respect to patient enrollment and follow-up), the timing of our presentation of data and submission of regulatory filings (including without limitation, the date of BLA filing), the sufficiency of cash to fund operations, the potential benefit of our product candidates and technologies, and the financial position of Cellectis. These forward-looking statements are made in light of information currently available to us and are subject to significant risks and uncertainties, including with respect to the numerous risks associated with biopharmaceutical product candidate development. Among these are significant risks that the BALLI-01 Phase 1 data may not be validated by data from later stage of clinical trials and that our product candidate may not receive regulatory approval for commercialization.
Particular caution should be exercised when interpreting results from Phase 1 studies and results relating to a small number of patients – such results should not be viewed as predictive of future results. Furthermore, many other important factors, including those described in our Annual Report on Form 20-F as amended and in our annual financial report (including the management report) for the year ended December 31, 2024 and subsequent filings Cellectis makes with the Securities Exchange Commission from time to time, which are available on the SEC’s website at www.sec.gov, as well as other known and unknown risks and uncertainties may adversely affect such forward-looking statements and cause our actual results, performance or achievements to be materially different from those expressed or implied by the forward-looking statements. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons why actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future.
For further information on Cellectis, please contact:
Media contacts:
Pascalyne Wilson, Director, Communications, + 33 (0)7 76 99 14 33,
media@cellectis.com
Patricia Sosa Navarro, Chief of Staff to the CEO, +33 (0)7 76 77 46 93
Investor Relations contact:
Arthur Stril, Chief Financial Officer & Chief Business Officer, investors@cellectis.com

Exhibit 99.2 R&D Day BALLI-01 Phase 1 & Phase 2 Strategy DRAFT

Forward-Looking Statements Forward Looking Statement This presentation contains “forward-looking” statements within These forward-looking statements are made in light of the meaning of applicable securities laws, including the Private information currently available to us and are subject to Securities Litigation Reform Act of 1995. Forward-looking numerous risks and uncertainties, including with respect to the statements may be identified by words such as “designed to,” significant risks associated with biopharmaceutical product “anticipate,” “can,” “could,” “expected,” “on track,” “plan,” candidate development. Among these are significant risks that “potential,” “positioned,” “scheduled,” should, and “will,” the BALLI-01 Phase 1 data may not be validated by data from would, or the negative of these and similar expressions. later stage of clinical trials and that our product candidate may not receive regulatory approval. Particular caution should be These forward-looking statements, which are based on our exercised when interpreting the results from phase 1 studies management’s current expectations and assumptions and on and results relating to a small number of patients, such results information currently available to management, include should not be viewed as predictive or future results. statements regarding the market opportunities with respect to lasme-cel (and the assumptions on which such determinations Furthermore, many other important factors, including those are based, including with respect to addressable populations described in our Annual Report on Form 20-F and the financial and potential pricing), the potential of the phase 2 study to be a report (including the management report) for the year ended registrational phase, the advancement, timing and progress of December 31, 2024 and subsequent filings Cellectis makes with clinical trials (including with respect to patient enrollment and the Securities Exchange Commission from time to time, as well follow-up), the timing of our presentation of data and submission as other known and unknown risks and uncertainties may of regulatory filings (including, without limitation, the date of BLA adversely affect such forward-looking statements and cause our filing), the sufficiency of cash to fund operations, the potential actual results, performance or achievements to be materially benefit of our product candidates and technologies, and the different from those expressed or implied by the forward-looking financial position of Cellectis. statements. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons why actual results could differ materially from those anticipated in the forward-looking statements, even if P2 new information becomes available in the future.
Lasmecabtagene Timgedleucel or Lasme-cel (UCART22) Lasme-cel could be a game changer in refractory or relapsed B-ALL Lasme-cel is not FDA approved P3

Today’s Reveal 1. The BALLI-01 Study for Patients with r/r B-ALL • Phase 1 Update → Lessons Learned • Phase 2 Design → Path to BLA and Market Panel Discussion with Oncology Experts 2. Lasme-cel Market Opportunity • Patient Need & Addressable Market • Where We Create Value 4. Building Tomorrow’s Cell & Gene Therapies with AstraZeneca • Our Strategic Collaboration Q&A Session P4
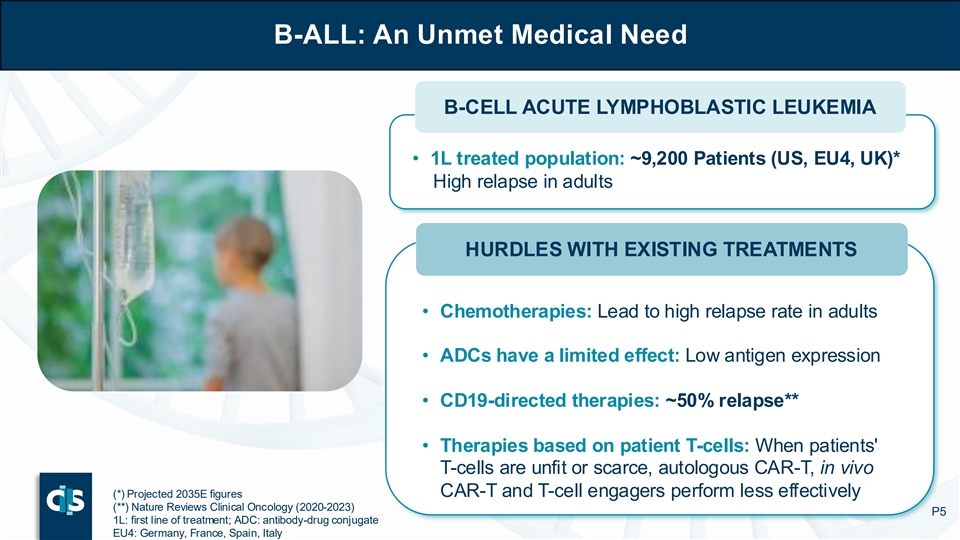
B-ALL: An Unmet Medical Need ALL: an Unmet Medical Need B-CELL ACUTE LYMPHOBLASTIC LEUKEMIA • 1L treated population: ~9,200 Patients (US, EU4, UK)* High relapse in adults HURDLES WITH EXISTING TREATMENTS • Chemotherapies: Lead to high relapse rate in adults • ADCs have a limited effect: Low antigen expression • CD19-directed therapies: ~50% relapse** • Therapies based on patient T-cells: When patients' T-cells are unfit or scarce, autologous CAR-T, in vivo CAR-T and T-cell engagers perform less effectively (*) Projected 2035E figures (**) Nature Reviews Clinical Oncology (2020-2023) P5 1L: first line of treatment; ADC: antibody-drug conjugate EU4: Germany, France, Spain, Italy

Why an Allogeneic CD22 CAR-T cell Product for r/r B-ALL? Why an Allogeneic CD22 CAR-T cell product for r/r B-ALL? Allogeneic CAR-T Starts with Healthy-donor T Cells Healthier and less exhausted than autologous cells from heavily pretreated patients Off-the-Shelf is designed for “Speed” – in B-ALL Every Day Counts Standardized, Repeatable Quality All patients would get the same product CD22 Complements/Preempts CD19 (CD19-naïve and post-CD19) Engaging CD22 could potentially rescue CD19 failures P6
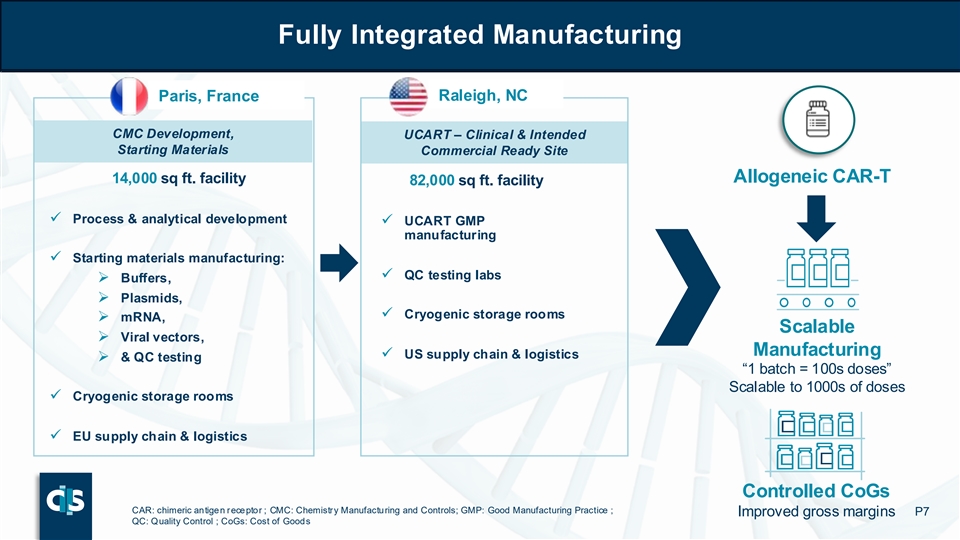
Fully Integrated Manufacturing Fully Integrated Manufacturing Paris, France Raleigh, NC CMC Development, UCART – Clinical & Intended Starting Materials Commercial Ready Site Allogeneic CAR-T 14,000 sq ft. facility 82,000 sq ft. facility ✓ Process & analytical development ✓ UCART GMP manufacturing ✓ Starting materials manufacturing: ✓ QC testing labs ➢ Buffers, ➢ Plasmids, ✓ Cryogenic storage rooms ➢ mRNA, Scalable ➢ Viral vectors, Manufacturing ✓ US supply chain & logistics ➢ & QC testing “1 batch = 100s doses” Scalable to 1000s of doses ✓ Cryogenic storage rooms ✓ EU supply chain & logistics Controlled CoGs CAR: chimeric antigen receptor ; CMC: Chemistry Manufacturing and Controls; GMP: Good Manufacturing Practice ; Improved gross margins P7 QC: Quality Control ; CoGs: Cost of Goods
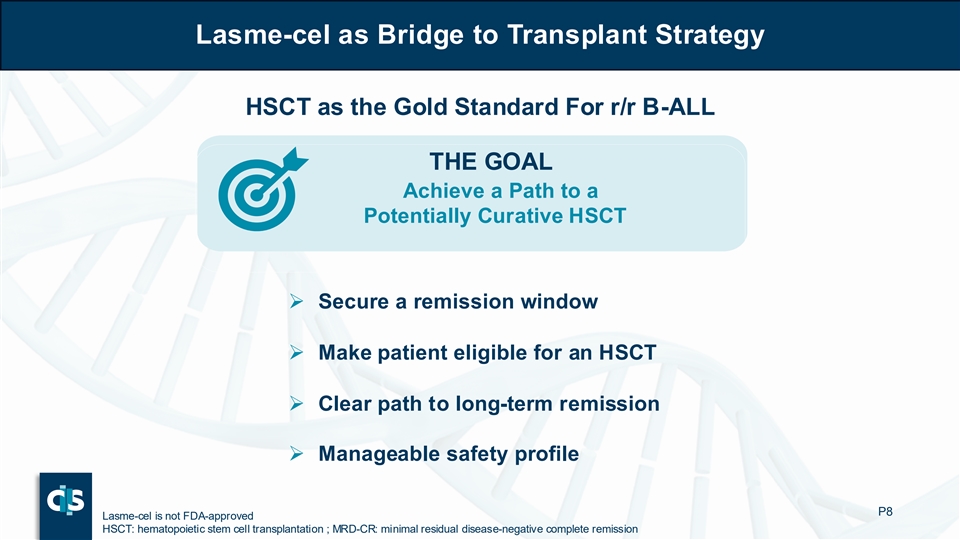
Lasme-cel as Bridge to Transplant Strategy Lasme-cel as Bridge to Transplant Strategy HSCT as the Gold Standard For r/r B-ALL THE GOAL Achieve a Path to a Potentially Curative HSCT ➢ Secure a remission window ➢ Make patient eligible for an HSCT ➢ Clear path to long-term remission ➢ Manageable safety profile P8 Lasme-cel is not FDA-approved HSCT: hematopoietic stem cell transplantation ; MRD-CR: minimal residual disease-negative complete remission

R&D Day BALLI-01 Phase 1 Data Review & Design of Pivotal Phase 2 DRAFT
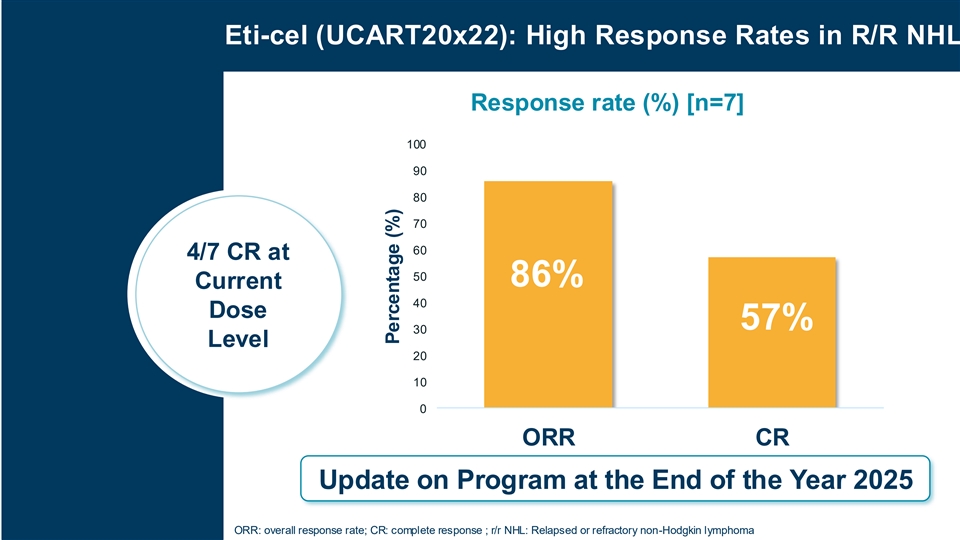
Eti-cel (UCART20x22): High Response Rates in R/R NHL Response rate (%) [n=7] 100 90 80 70 60 4/7 CR at 50 86% Current 40 Dose 57% 30 Level 20 10 0 ORR CR Update on Program at the End of the Year 2025 ORR: overall response rate; CR: complete response ; r/r NHL: Relapsed or refractory non-Hodgkin lymphoma Percentage (%)
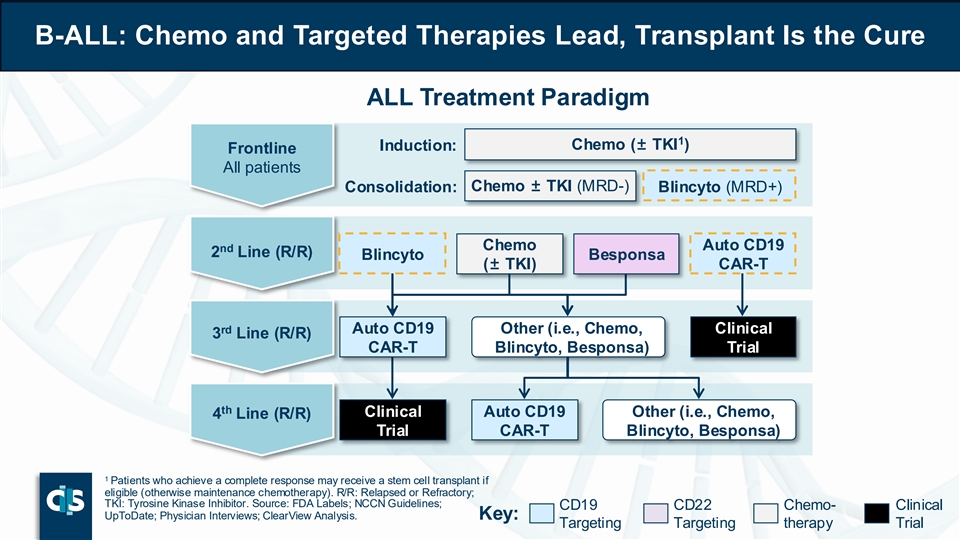
B-ALL: Chemo and Targeted Therapies Lead, Transplant Is the Cure ALL Treatment Paradigm 1 Induction: Chemo (± TKI ) Frontline All patients Consolidation: Chemo ± TKI (MRD-) Blincyto (MRD+) Chemo Auto CD19 nd 2 Line (R/R) Blincyto Besponsa (± TKI) CAR-T rd Auto CD19 Other (i.e., Chemo, Clinical 3 Line (R/R) CAR-T Blincyto, Besponsa) Trial th Clinical Auto CD19 Other (i.e., Chemo, 4 Line (R/R) Trial CAR-T Blincyto, Besponsa) 1 Patients who achieve a complete response may receive a stem cell transplant if eligible (otherwise maintenance chemotherapy). R/R: Relapsed or Refractory; TKI: Tyrosine Kinase Inhibitor. Source: FDA Labels; NCCN Guidelines; CD19 CD22 Chemo- Clinical UpToDate; Physician Interviews; ClearView Analysis. Key: Targeting Targeting therapy Trial
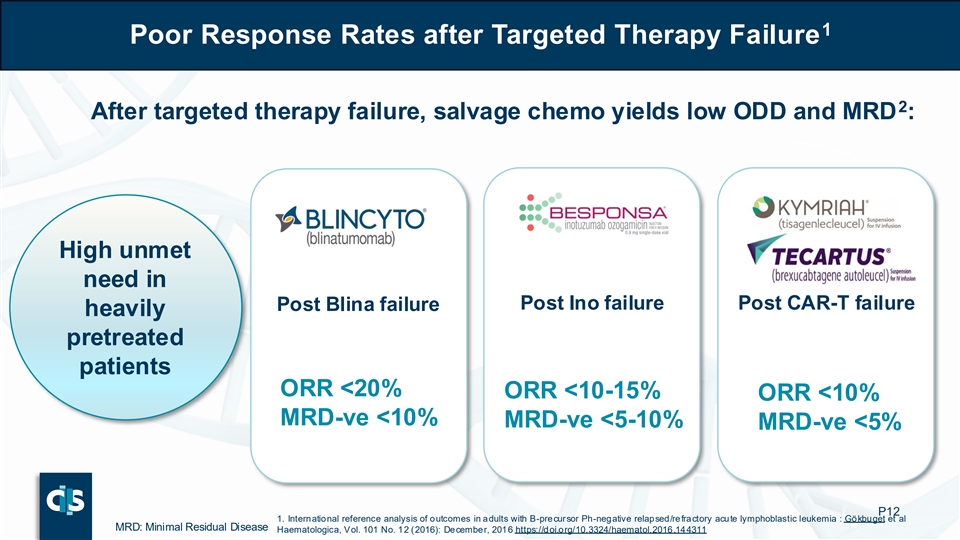
1 Poor Response Rates after Targeted Therapy Failure 2 After targeted therapy failure, salvage chemo yields low ODD and MRD : High unmet need in Post Ino failure Post CAR-T failure Post Blina failure heavily pretreated patients ORR <20% ORR <10-15% ORR <10% MRD-ve <10% MRD-ve <5-10% MRD-ve <5% P12 1. International reference analysis of outcomes in adults with B-precursor Ph-negative relapsed/refractory acute lymphoblastic leukemia : Gökbuget et al MRD: Minimal Residual Disease Haematologica, Vol. 101 No. 12 (2016): December, 2016 https://doi.org/10.3324/haematol.2016.144311
Key Questions Being Addressed in BALLI-01 Trial Is Alemtuzumab needed to Is Cellectis’ manufactured optimize response? product superior? Which endpoint best What is the optimal dose? reflects benefit? P13

BALLI-01 Study Design Is our internal manufacturing superior? Process 1 vs Process 2 5 DL1=1×10 cells/kg 6 DL2= 1×10 cells/kg 6 DL2i= 2.5×10 cells/kg 6 DL 3 DL 3 DL3= 5×10 cells/kg DL 2i DL 2i Study What is the design optimal dose? DL 2 DL 2 DL 2 DL 1 FC vs FCA Is alemtuzumab necessary? P14 DL1: Dose Level 1; DL2: Dose Level 2; DL2i: Intermediate Dose Level 2; DL3: Dose Level 3; FC: Fludarabine and cyclophosphamide; FCA: FC and alemtuzumab Process 1 or P1 refers to the Cellectis product manufactured by CDMO. Process 2 or P2 refers to the Cellectis product manufactured in-house at Cellectis. Both products were tested in the BALLI-01 study
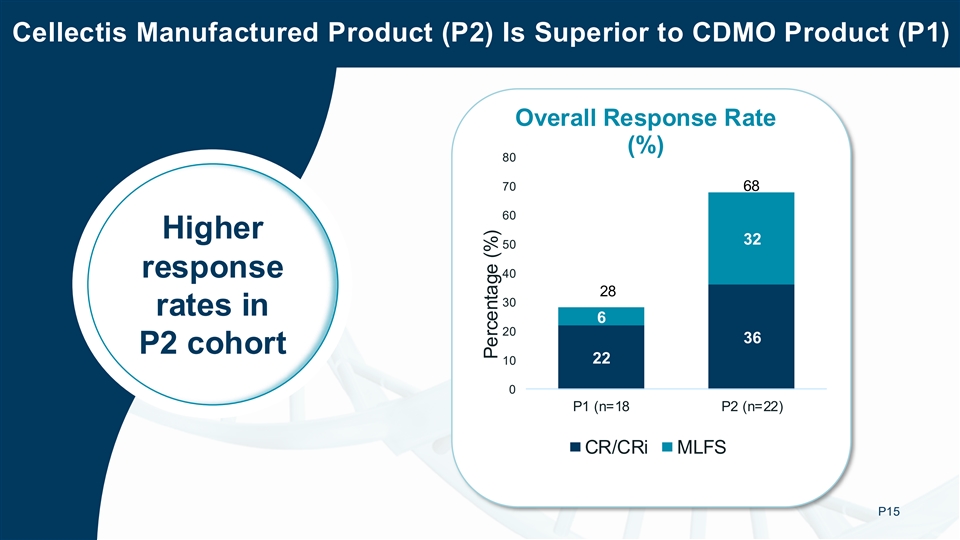
Cellectis Manufactured Product (P2) Is Superior to CDMO Product (P1) Overall Response Rate (%) 80 70 68 60 Higher 32 50 response 40 28 30 rates in 6 20 36 P2 cohort 22 10 0 P1 (n=18 P2 (n=22) CR/CRi MLFS P15 Percentage (%)

R&D Day BALLI-01 Phase 1 Data DRAFT
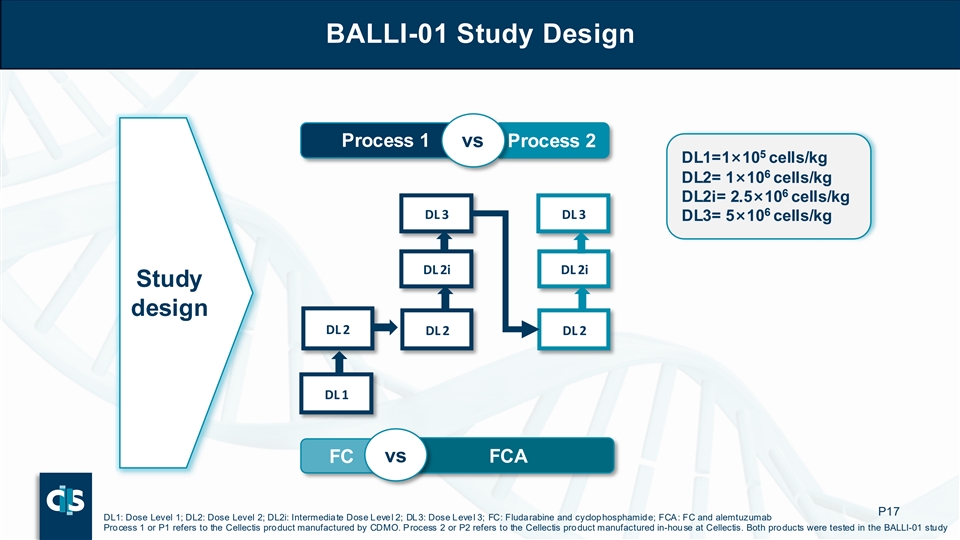
BALLI-01 Study Design Process 1 vs Process 2 5 DL1=1×10 cells/kg 6 DL2= 1×10 cells/kg 6 DL2i= 2.5×10 cells/kg 6 DL 3 DL 3 DL3= 5×10 cells/kg DL 2i DL 2i Study design DL 2 DL 2 DL 2 DL 1 FC vs FCA P17 DL1: Dose Level 1; DL2: Dose Level 2; DL2i: Intermediate Dose Level 2; DL3: Dose Level 3; FC: Fludarabine and cyclophosphamide; FCA: FC and alemtuzumab Process 1 or P1 refers to the Cellectis product manufactured by CDMO. Process 2 or P2 refers to the Cellectis product manufactured in-house at Cellectis. Both products were tested in the BALLI-01 study
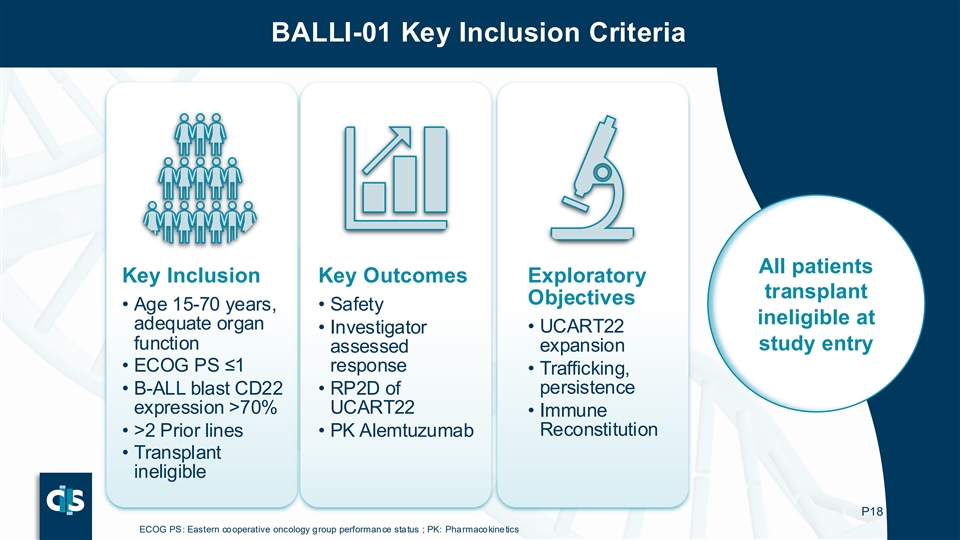
BALLI-01 Key Inclusion Criteria All patients Key Inclusion Key Outcomes Exploratory transplant Objectives • Age 15-70 years, • Safety ineligible at adequate organ • Investigator • UCART22 function study entry expansion assessed • ECOG PS ≤1 response • Trafficking, persistence • B-ALL blast CD22 • RP2D of expression >70% UCART22 • Immune Reconstitution • >2 Prior lines • PK Alemtuzumab • Transplant ineligible P18 ECOG PS: Eastern cooperative oncology group performance status ; PK: Pharmacokinetics
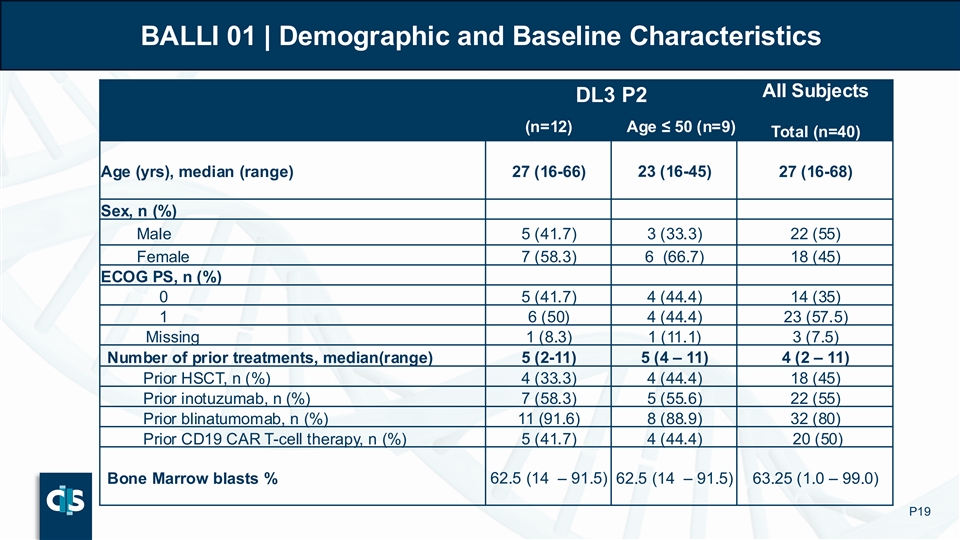
BALLI 01 | Demographic and Baseline Characteristics All Subjects DL3 P2 (n=12) Age ≤ 50 (n=9) Total (n=40) Age (yrs), median (range) 27 (16-66) 23 (16-45) 27 (16-68) Sex, n (%) Male 5 (41.7) 3 (33.3) 22 (55) Female 7 (58.3) 6 (66.7) 18 (45) ECOG PS, n (%) 0 5 (41.7) 4 (44.4) 14 (35) 1 6 (50) 4 (44.4) 23 (57.5) Missing 1 (8.3) 1 (11.1) 3 (7.5) Number of prior treatments, median(range) 5 (2-11) 5 (4 – 11) 4 (2 – 11) Prior HSCT, n (%) 4 (33.3) 4 (44.4) 18 (45) Prior inotuzumab, n (%) 7 (58.3) 5 (55.6) 22 (55) Prior blinatumomab, n (%) 11 (91.6) 8 (88.9) 32 (80) 5 (41.7) 4 (44.4) 20 (50) Prior CD19 CAR T-cell therapy, n (%) Bone Marrow blasts % 62.5 (14 – 91.5) 62.5 (14 – 91.5) 63.25 (1.0 – 99.0) P19
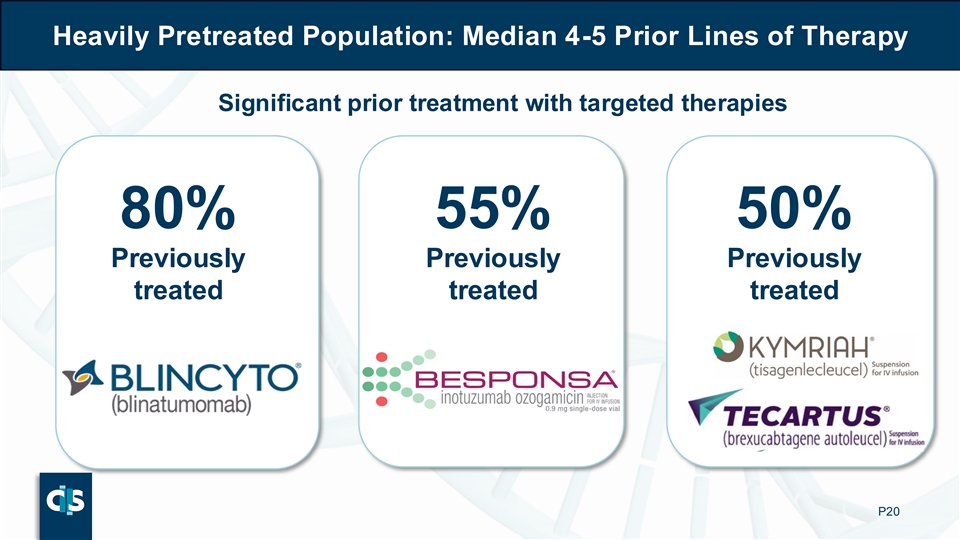
Heavily Pretreated Population: Median 4-5 Prior Lines of Therapy Significant prior treatment with targeted therapies 80% 55% 50% Previously Previously Previously treated treated treated P20

High Response Rates in P2 Cohort Recommended Phase 2 Dose: DL3 Target Phase 2 population: ▪ DL3 ≤ 50 years P21
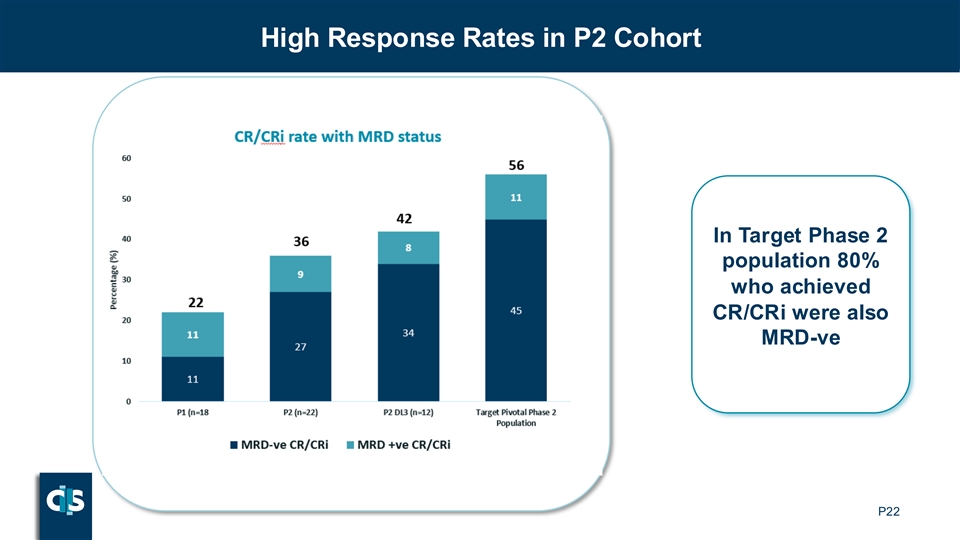
High Response Rates in P2 Cohort In Target Phase 2 population 80% who achieved CR/CRi were also MRD-ve P22
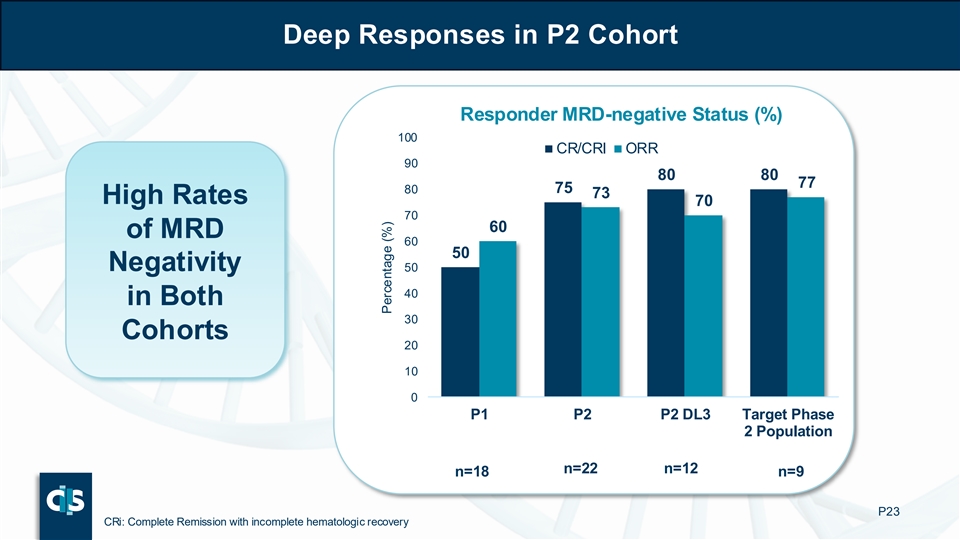
Deep Responses in P2 Cohort Responder MRD-negative Status (%) 100 CR/CRI ORR 90 80 80 77 80 75 73 High Rates 70 70 60 of MRD 60 50 Negativity 50 40 in Both 30 Cohorts 20 10 0 P1 P2 P2 DL3 Target Phase 2 Population n=22 n=12 n=18 n=9 P23 CRi: Complete Remission with incomplete hematologic recovery Percentage (%)
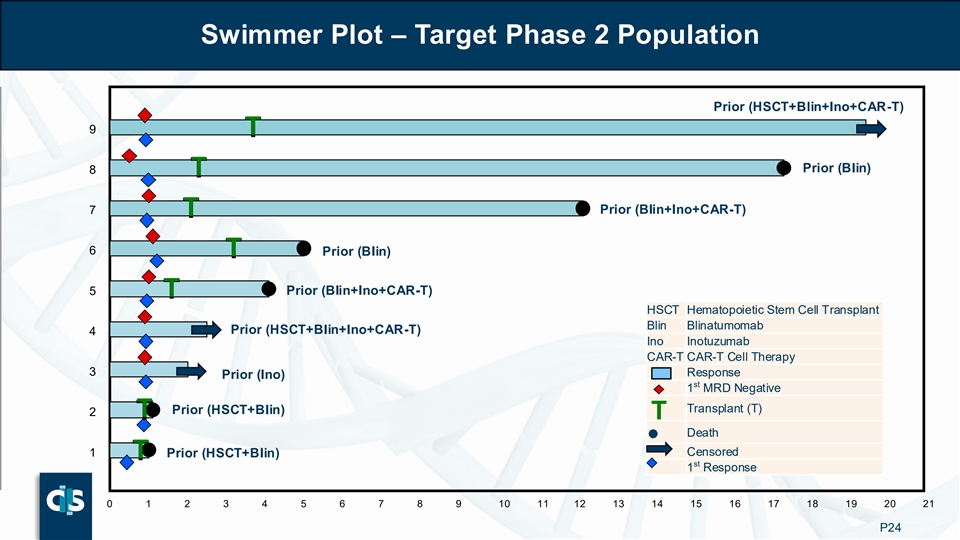
Swimmer Plot – Target Phase 2 Population Prior (HSCT+Blin+Ino+CAR-T) 9 T T Prior (Blin) 8 T T 7 Prior (Blin+Ino+CAR-T) T T 6 T Prior (Blin) T 5 Prior (Blin+Ino+CAR-T) T T HSCT Hematopoietic Stem Cell Transplant Blin Blinatumomab Prior (HSCT+Blin+Ino+CAR-T) 4 Ino Inotuzumab CAR-T CAR-T Cell Therapy 3 Response Prior (Ino) st 1 MRD Negative Transplant (T) Prior (HSCT+Blin) 2 T T T Death ● Censored 1 Prior (HSCT+Blin) T T st 1 Response 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 P24
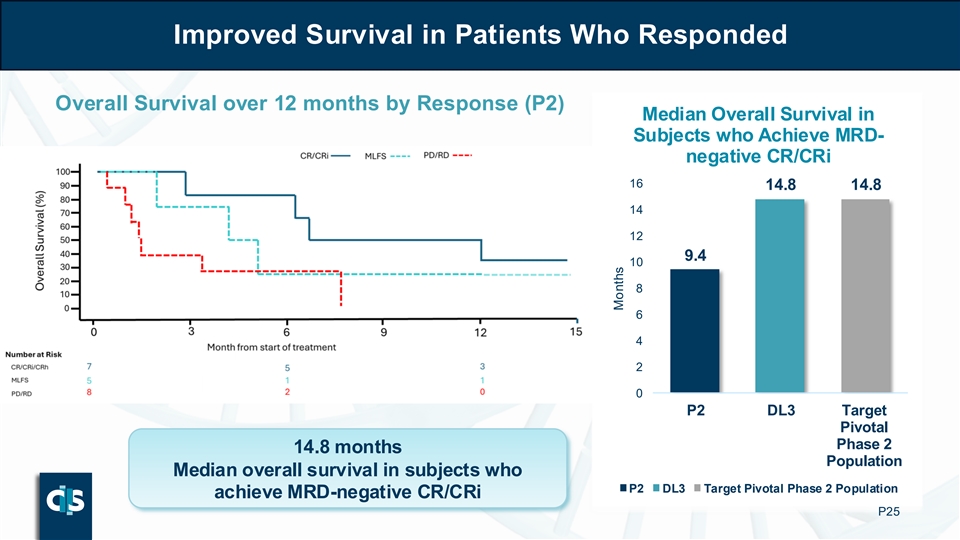
Improved Survival in Patients Who Responded Overall Survival over 12 months by Response (P2) Median Overall Survival in Subjects who Achieve MRD- negative CR/CRi 16 14.8 14.8 14 12 9.4 10 8 6 4 2 0 P2 DL3 Target Pivotal Phase 2 14.8 months Population Median overall survival in subjects who P2 DL3 Target Pivotal Phase 2 Population achieve MRD-negative CR/CRi P25 Months
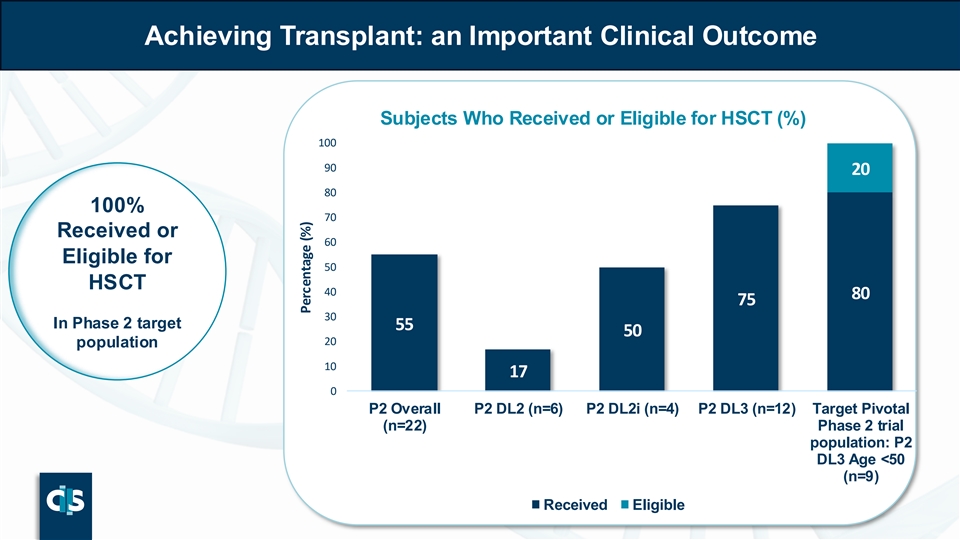
Achieving Transplant: an Important Clinical Outcome Subjects Who Received or Eligible for HSCT (%) 100 90 20 80 100% 70 Received or 60 Eligible for 50 HSCT 40 80 75 30 In Phase 2 target 55 50 20 population 10 17 0 P2 Overall P2 DL2 (n=6) P2 DL2i (n=4) P2 DL3 (n=12) Target Pivotal (n=22) Phase 2 trial population: P2 DL3 Age <50 (n=9) Received Eligible Percentage (%)
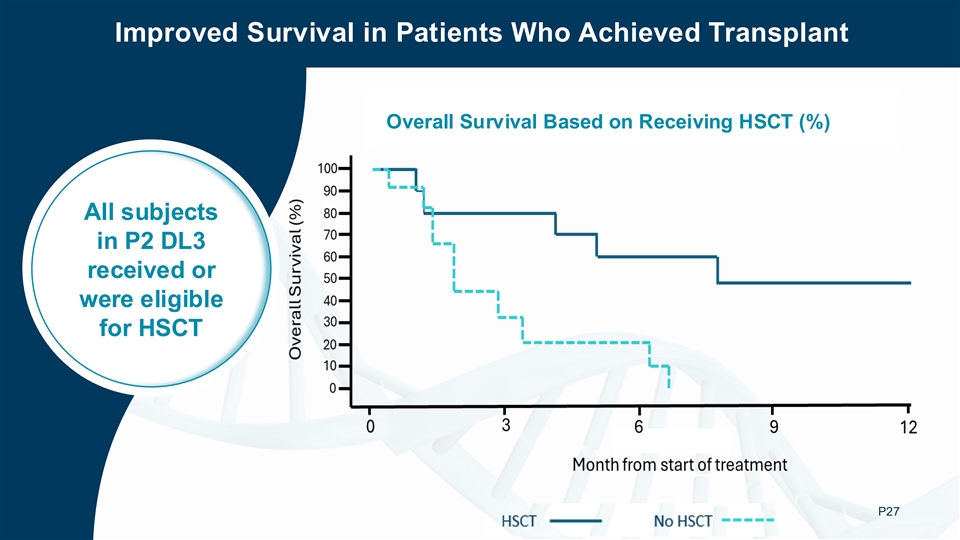
Improved Survival in Patients Who Achieved Transplant Overall Survival Based on Receiving HSCT (%) All subjects All subjects in P2 DL3 in P2 DL3 received or received or were eligible were eligible for HSCT for HSCT P27
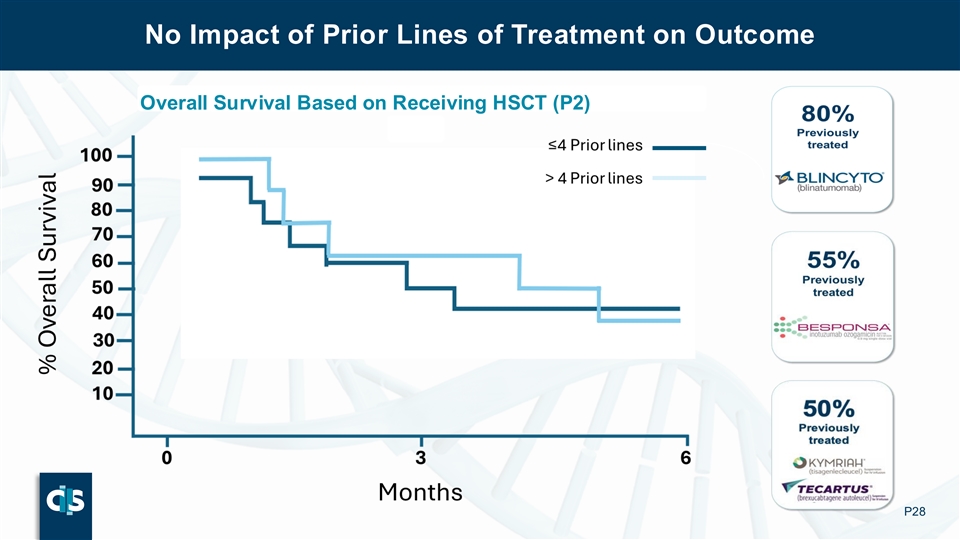
No Impact of Prior Lines of Treatment on Outcome No Impact of Prior Lines of Treatment on Outcome Overall Survival Based on Receiving HSCT (P2) P28

High ORR in Patients Who Had Prior Inotuzumab ORR in Patients with Prior Exposure to Inotuzumab 100 90 Deep 80 15 responses 70 8 60 with prior 50 100 CD22 40 71 30 61 targeted 20 11 therapy 10 11 0 P1 (n (n == 4) 9) P2 (n (n =1 =1 1)3) P2 DL3 (n=5) Target Pivotal Phase 2 Population (n=4) MRD -ve ORR MRD +ve ORR P29 Percentage (%)
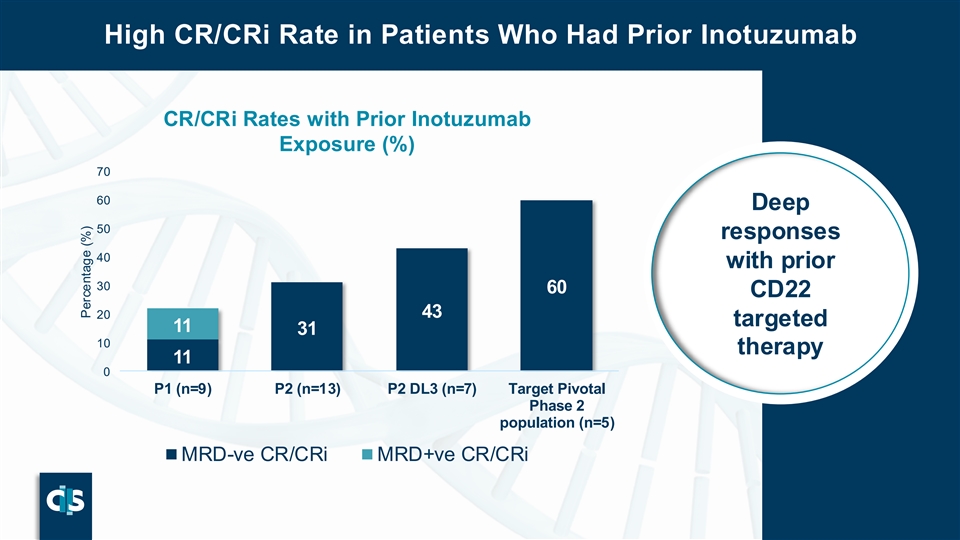
High CR/CRi Rate in Patients Who Had Prior Inotuzumab CR/CRi Rates with Prior Inotuzumab Exposure (%) 70 60 Deep 50 responses 40 with prior 30 60 CD22 20 43 targeted 11 31 10 therapy 11 0 P1 (n=9) P2 (n=13) P2 DL3 (n=7) Target Pivotal Phase 2 population (n=5) MRD-ve CR/CRi MRD+ve CR/CRi P30 Percentage (%)

High ORR in Patients Exposed to 3 Prior Targeted Therapies: Inotuzumab, Blinatumomab and CD19 CAR-T ORR in Patients with Prior Exposure to 3 Targeted Therapies: Blin, Ino and CD19 CAR-T Deep 100 responses if 90 20 80 received all 3 70 9 60 available 50 100 40 targeted 80 64 30 therapies 20 25 10 0 P1 (n=4) P2 (n=11) P2 DL3 (n=5) Target Pivotal Phase 2 Population (n=4) MRD -ve ORR MRD +ve ORR P31 Percentage (%)
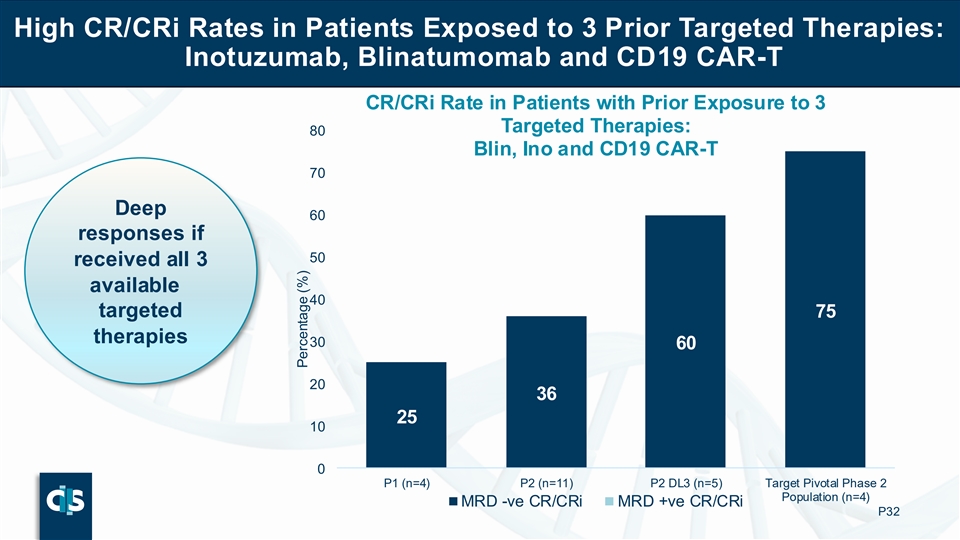
High CR/CRi Rates in Patients Exposed to 3 Prior Targeted Therapies: Inotuzumab, Blinatumomab and CD19 CAR-T CR/CRi Rate in Patients with Prior Exposure to 3 Targeted Therapies: 80 Blin, Ino and CD19 CAR-T 70 Deep 60 responses if 50 received all 3 available 40 targeted 75 therapies 30 60 20 36 25 10 0 P1 (n=4) P2 (n=11) P2 DL3 (n=5) Target Pivotal Phase 2 Population (n=4) MRD -ve CR/CRi MRD +ve CR/CRi P32 Percentage (%)
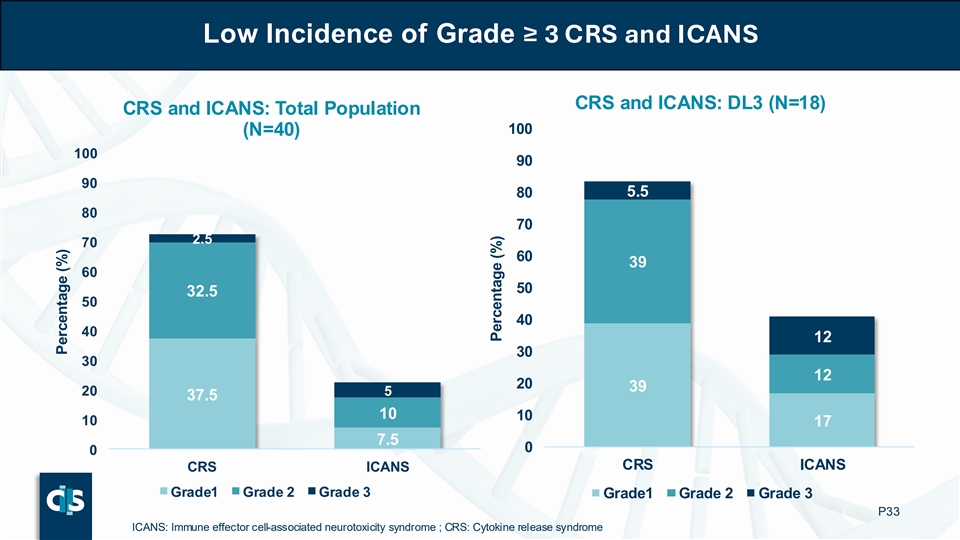
Low Incidence of Grade ≥ 3 CRS and ICANS CRS and ICANS: DL3 (N=18) CRS and ICANS: Total Population 100 (N=40) 100 90 90 80 5.5 80 70 2.5 70 60 39 60 50 32.5 50 40 40 12 30 30 12 20 39 20 5 37.5 10 10 10 17 7.5 0 0 CRS ICANS CRS ICANS Grade1 Grade 2 Grade 3 Grade1 Grade 2 Grade 3 P33 ICANS: Immune effector cell-associated neurotoxicity syndrome ; CRS: Cytokine release syndrome Percentage (%) Percentage (%)

No Current Signal for IEC-HS Significant interest in the risk of IEC-HS based on prior CD22 targeting autologous CAR-T No ▪ One case observed evidence of in BALLI-01 IEC-HS CD22 target ▪ Grade 2 HLH Day 5 incidence related ▪ Resolved with Anakinra/Dex effect P34 IEC-HS: Immune Effector Cell–Associated Hemophagocytic Syndrome ; HLH: Hemophagocytic Lymphohistiocytosis
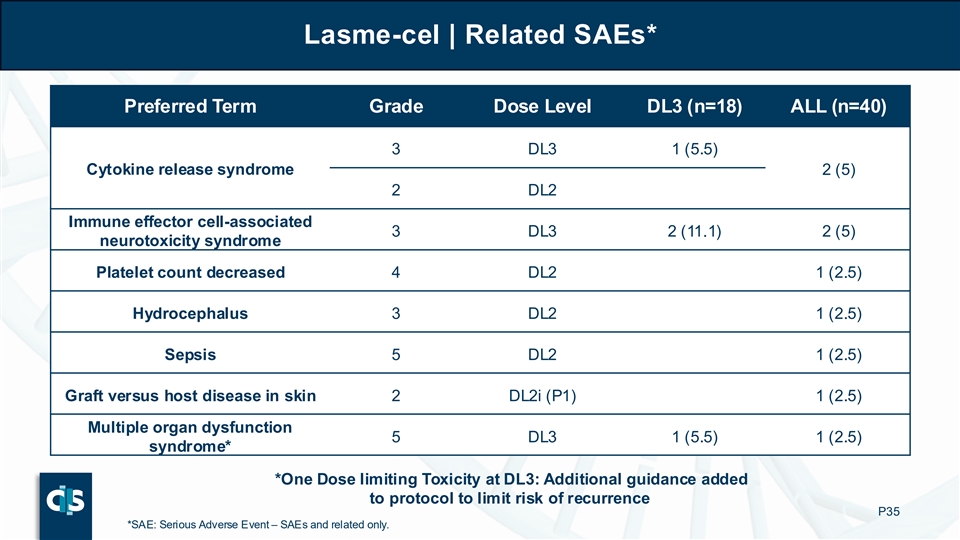
Lasme-cel | Related SAEs* Preferred Term Grade Dose Level DL3 (n=18) ALL (n=40) 3 DL3 1 (5.5) Cytokine release syndrome 2 (5) 2 DL2 Immune effector cell-associated 3 DL3 2 (11.1) 2 (5) neurotoxicity syndrome Platelet count decreased 4 DL2 1 (2.5) Hydrocephalus 3 DL2 1 (2.5) Sepsis 5 DL2 1 (2.5) Graft versus host disease in skin 2 DL2i (P1) 1 (2.5) Multiple organ dysfunction 5 DL3 1 (5.5) 1 (2.5) syndrome* *One Dose limiting Toxicity at DL3: Additional guidance added to protocol to limit risk of recurrence P35 *SAE: Serious Adverse Event – SAEs and related only.
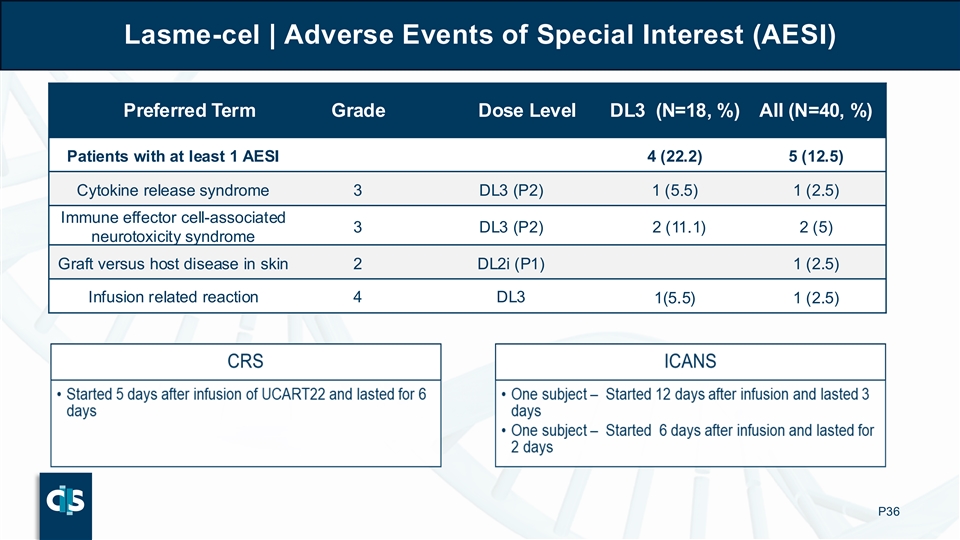
Lasme-cel | Adverse Events of Special Interest (AESI) Preferred Term Grade Dose Level DL3 (N=18, %) All (N=40, %) Patients with at least 1 AESI 4 (22.2) 5 (12.5) Cytokine release syndrome 3 DL3 (P2) 1 (5.5) 1 (2.5) Immune effector cell-associated 3 DL3 (P2) 2 (11.1) 2 (5) neurotoxicity syndrome Graft versus host disease in skin 2 DL2i (P1) 1 (2.5) Infusion related reaction 4 DL3 1(5.5) 1 (2.5) P36
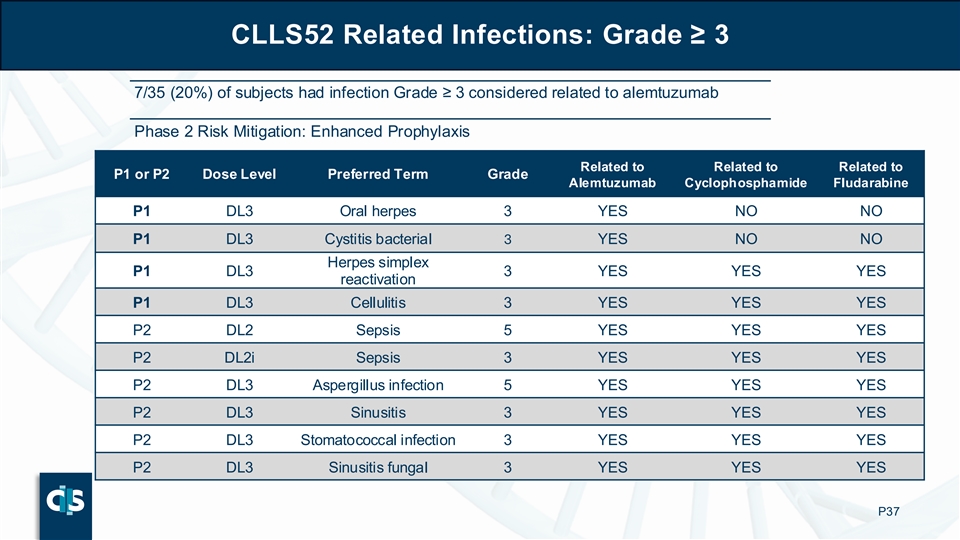
CLLS52 Related Infections: Grade ≥ 3 7/35 (20%) of subjects had infection Grade ≥ 3 considered related to alemtuzumab Phase 2 Risk Mitigation: Enhanced Prophylaxis Related to Related to Related to P1 or P2 Dose Level Preferred Term Grade Alemtuzumab Cyclophosphamide Fludarabine P1 DL3 Oral herpes 3 YES NO NO P1 DL3 Cystitis bacterial 3 YES NO NO Herpes simplex P1 DL3 3 YES YES YES reactivation P1 DL3 Cellulitis 3 YES YES YES P2 DL2 Sepsis 5 YES YES YES P2 DL2i Sepsis 3 YES YES YES P2 DL3 Aspergillus infection 5 YES YES YES P2 DL3 Sinusitis 3 YES YES YES P2 DL3 Stomatococcal infection 3 YES YES YES P2 DL3 Sinusitis fungal 3 YES YES YES P37
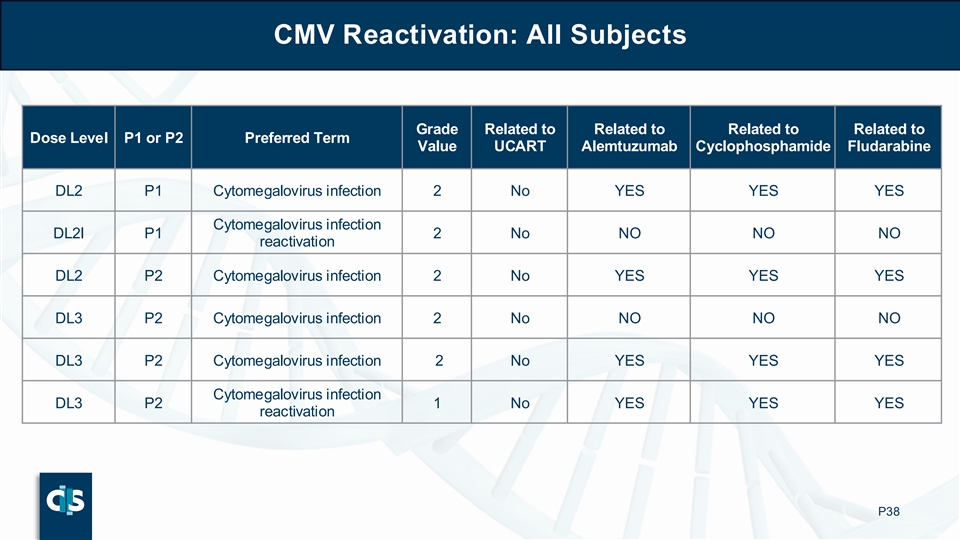
CMV Reactivation: All Subjects Grade Related to Related to Related to Related to Dose Level P1 or P2 Preferred Term Value UCART Alemtuzumab Cyclophosphamide Fludarabine DL2 P1 Cytomegalovirus infection 2 No YES YES YES Cytomegalovirus infection DL2I P1 2 No NO NO NO reactivation DL2 P2 Cytomegalovirus infection 2 No YES YES YES DL3 P2 Cytomegalovirus infection 2 No NO NO NO DL3 P2 Cytomegalovirus infection 2 No YES YES YES Cytomegalovirus infection DL3 P2 1 No YES YES YES reactivation P38
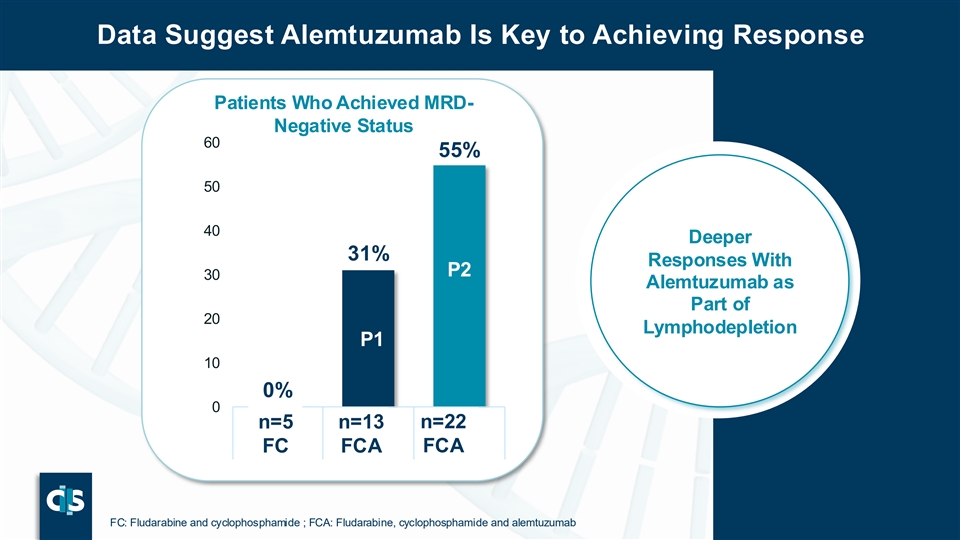
Data Suggest Alemtuzumab Is Key to Achieving Response Patients Who Achieved MRD- Negative Status 60 55% 50 40 Deeper 31% Responses With P2 30 Alemtuzumab as P Part of 20 2 Lymphodepletion P1 P 10 1 0% 0 n=22 n n= =5 5 n= n= 113 3 n=22 FCA FC FCA FC FCA FCA P39 FC: Fludarabine and cyclophosphamide ; FCA: Fludarabine, cyclophosphamide and alemtuzumab

R&D Day BALLI-01 Transition to Pivotal Phase 2 DRAFT

Clear Registration Path for Lasme-Cel in r/r-ALL P41

Phase 2 Pivotal Phase 2 Study Design: Pivotal Phase 2 Interim analysis 2: Primary analysis Interim analysis 1: Overall 50% pivotal Cohort for BLA Dose Optimization Survival Primary Endpoint: CR / CRi, evaluated within 3 months (from Day 28 to Day 84) Age 12-50 years P42
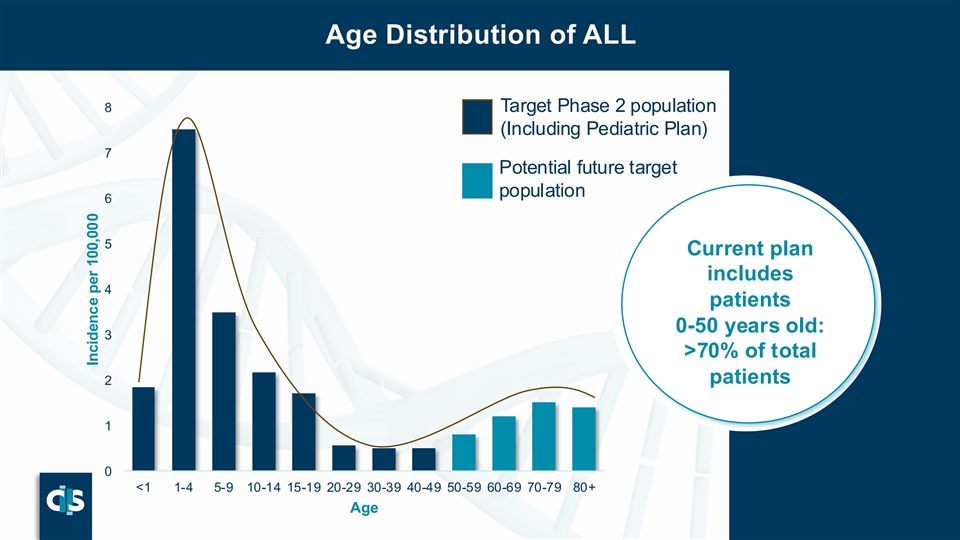
Age Distribution of ALL 8 Target Phase 2 population (Including Pediatric Plan) 7 Potential future target population 6 5 Current plan includes 4 patients 0-50 years old: 3 >70% of total patients 2 1 0 <1 1-4 5-9 10-14 15-19 20-29 30-39 40-49 50-59 60-69 70-79 80+ Age P43 Incidence per 100,000
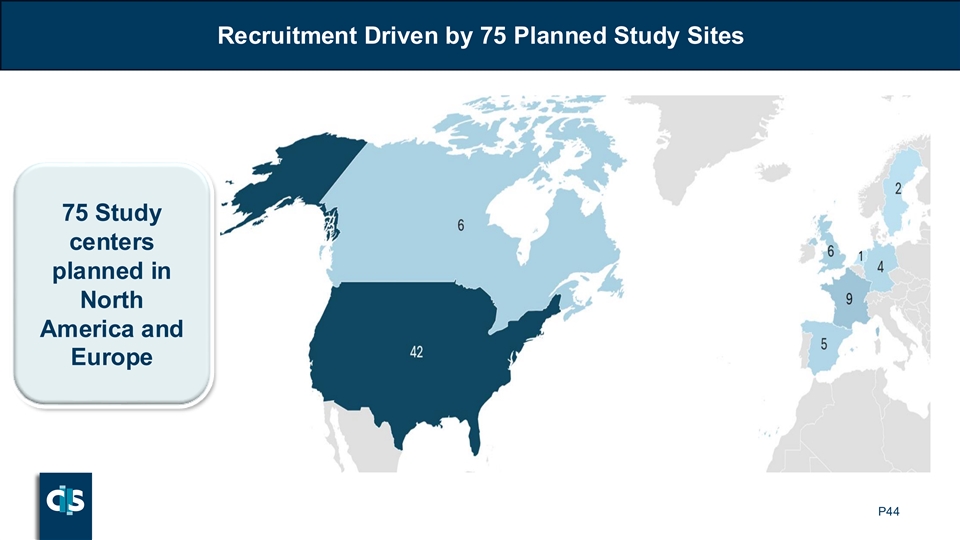
Recruitment Driven by 75 Planned Study Sites 75 Study centers planned in North America and Europe P44
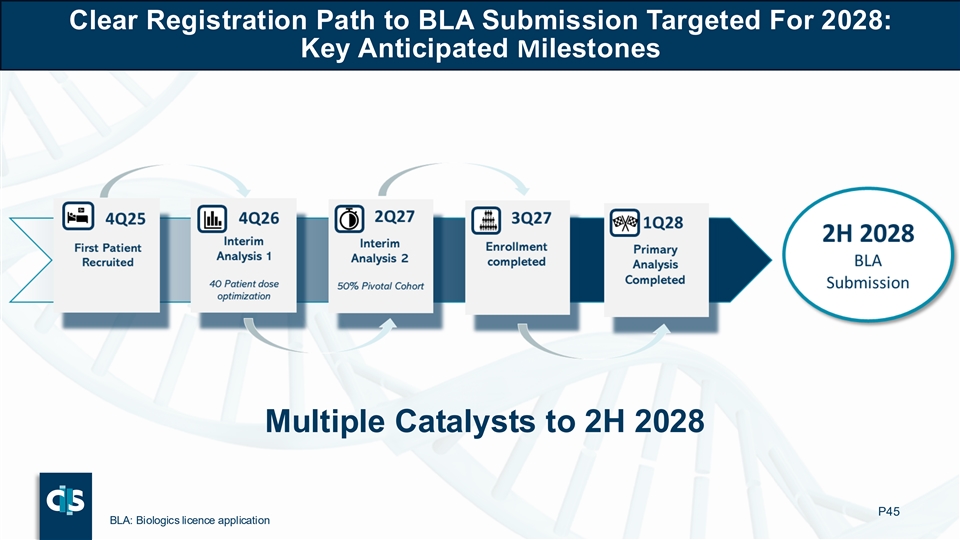
Clear Registration Path to BLA Submission Targeted For 2028: Key Anticipated Milestones Multiple Catalysts to 2H 2028 P45 BLA: Biologics licence application

R&D Day Lasme-cel Commercial Opportunity DRAFT
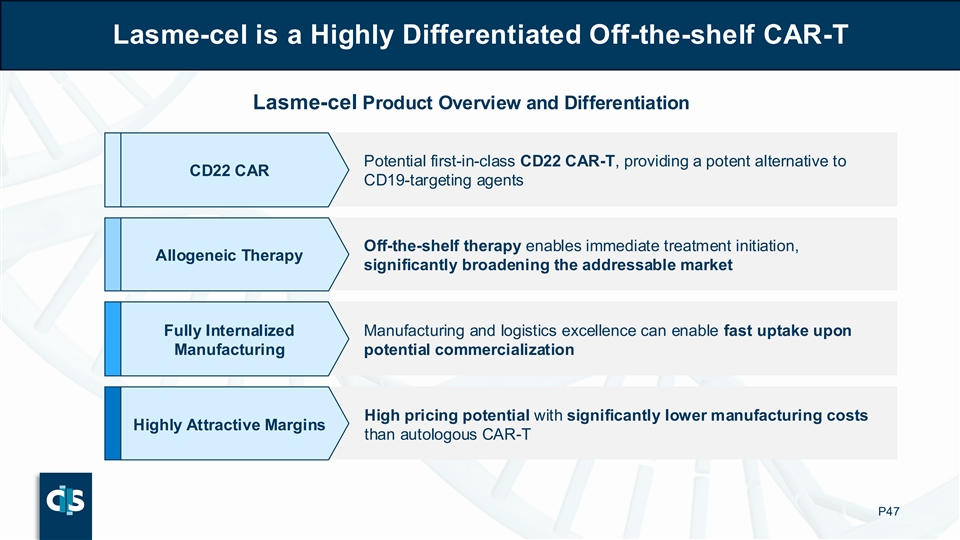
Lasme-cel is a Highly Differentiated Off-the-shelf CAR-T Lasme-cel Product Overview and Differentiation Potential first-in-class CD22 CAR-T, providing a potent alternative to CD22 CAR CD19-targeting agents Off-the-shelf therapy enables immediate treatment initiation, Allogeneic Therapy significantly broadening the addressable market Fully Internalized Manufacturing and logistics excellence can enable fast uptake upon Manufacturing potential commercialization High pricing potential with significantly lower manufacturing costs Highly Attractive Margins than autologous CAR-T P47
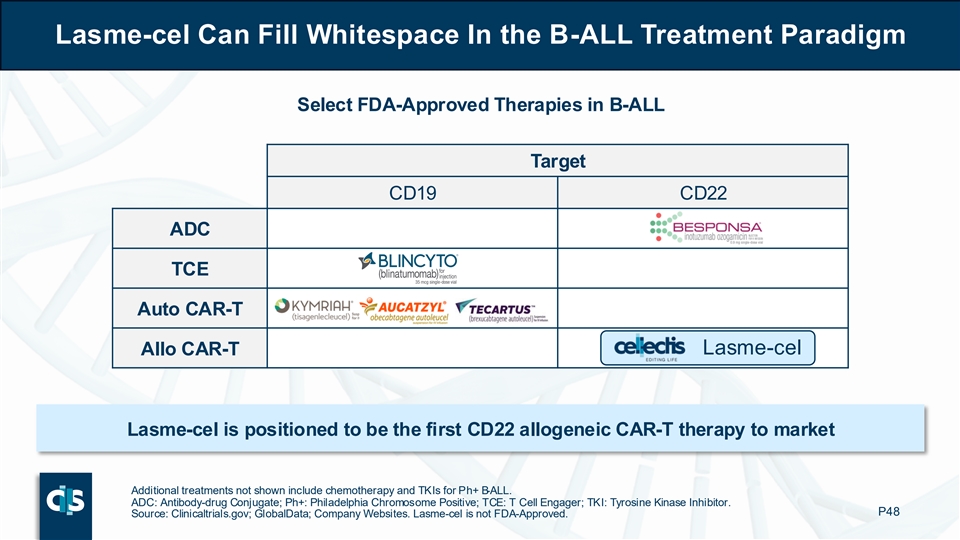
Lasme-cel Can Fill Whitespace In the B-ALL Treatment Paradigm Select FDA-Approved Therapies in B-ALL Target CD19 CD22 ADC TCE Auto CAR-T Lasme-cel Allo CAR-T Lasme-cel is positioned to be the first CD22 allogeneic CAR-T therapy to market Additional treatments not shown include chemotherapy and TKIs for Ph+ B-ALL. ADC: Antibody-drug Conjugate; Ph+: Philadelphia Chromosome Positive; TCE: T Cell Engager; TKI: Tyrosine Kinase Inhibitor. P48 Source: Clinicaltrials.gov; GlobalData; Company Websites. Lasme-cel is not FDA-Approved.

Chemo and CD19-directed Therapies are Mainstays of B-ALL Treatment ALL Treatment Paradigm 1 Induction: Chemo (± TKI ) Initial treatment with chemo ± TKI, with Blincyto Frontline (CD19 TCE) consolidation increasingly used in ± Chemo ± TKI Consolidation: Blincyto 1L following 2024 label expansion 1 Patients who achieve a complete response may receive a stem cell transplant if eligible (otherwise maintenance chemotherapy). HCP: Health Care Provider; IO: Immuno-oncology; R/R: Relapsed or Refractory; TCE: T Cell Engager; TKI: CD19 CD22 Chemo- Clinical Key: Tyrosine Kinase Inhibitor. Source: FDA Labels; NCCN Guidelines; UpToDate; P49 Targeting Targeting therapy Trial Physician Interviews; ClearView Analysis.
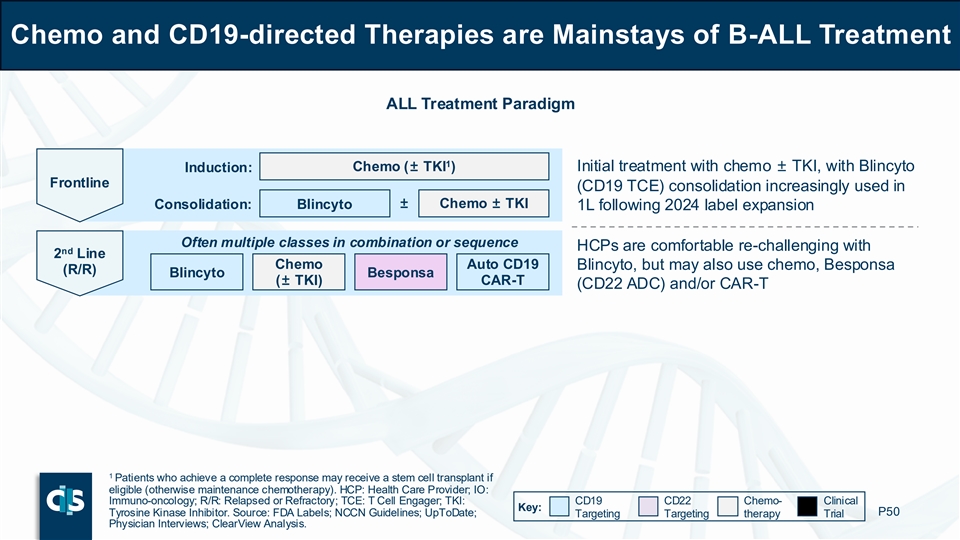
Chemo and CD19-directed Therapies are Mainstays of B-ALL Treatment ALL Treatment Paradigm 1 Induction: Chemo (± TKI ) Initial treatment with chemo ± TKI, with Blincyto Frontline (CD19 TCE) consolidation increasingly used in ± Chemo ± TKI Consolidation: Blincyto 1L following 2024 label expansion Often multiple classes in combination or sequence HCPs are comfortable re-challenging with nd 2 Line Chemo Auto CD19 Blincyto, but may also use chemo, Besponsa (R/R) Blincyto Besponsa (± TKI) CAR-T (CD22 ADC) and/or CAR-T 1 Patients who achieve a complete response may receive a stem cell transplant if eligible (otherwise maintenance chemotherapy). HCP: Health Care Provider; IO: Immuno-oncology; R/R: Relapsed or Refractory; TCE: T Cell Engager; TKI: CD19 CD22 Chemo- Clinical Key: Tyrosine Kinase Inhibitor. Source: FDA Labels; NCCN Guidelines; UpToDate; P50 Targeting Targeting therapy Trial Physician Interviews; ClearView Analysis.
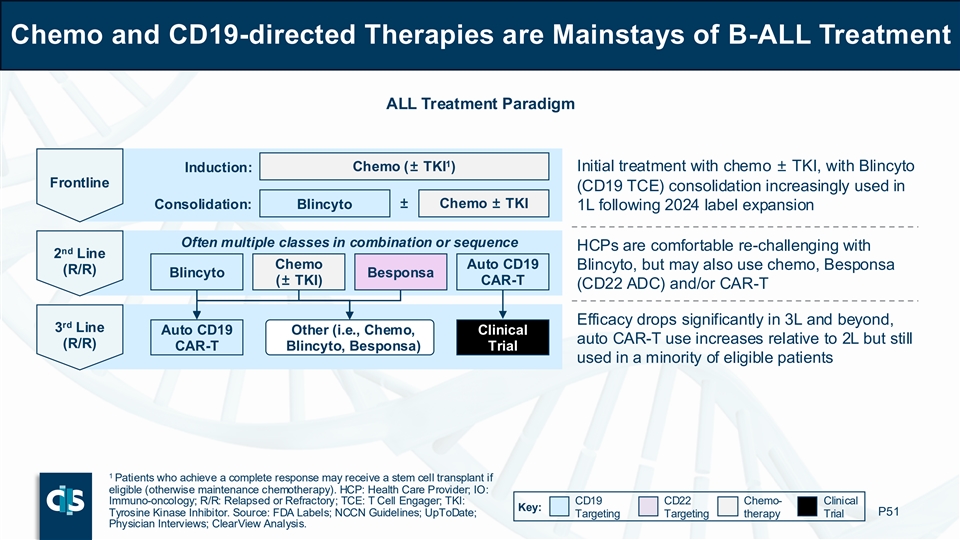
Chemo and CD19-directed Therapies are Mainstays of B-ALL Treatment ALL Treatment Paradigm 1 Induction: Chemo (± TKI ) Initial treatment with chemo ± TKI, with Blincyto Frontline (CD19 TCE) consolidation increasingly used in ± Chemo ± TKI Consolidation: Blincyto 1L following 2024 label expansion Often multiple classes in combination or sequence HCPs are comfortable re-challenging with nd 2 Line Chemo Auto CD19 Blincyto, but may also use chemo, Besponsa (R/R) Blincyto Besponsa (± TKI) CAR-T (CD22 ADC) and/or CAR-T Efficacy drops significantly in 3L and beyond, rd 3 Line Auto CD19 Other (i.e., Chemo, Clinical auto CAR-T use increases relative to 2L but still (R/R) CAR-T Blincyto, Besponsa) Trial used in a minority of eligible patients 1 Patients who achieve a complete response may receive a stem cell transplant if eligible (otherwise maintenance chemotherapy). HCP: Health Care Provider; IO: Immuno-oncology; R/R: Relapsed or Refractory; TCE: T Cell Engager; TKI: CD19 CD22 Chemo- Clinical Key: Tyrosine Kinase Inhibitor. Source: FDA Labels; NCCN Guidelines; UpToDate; P51 Targeting Targeting therapy Trial Physician Interviews; ClearView Analysis.
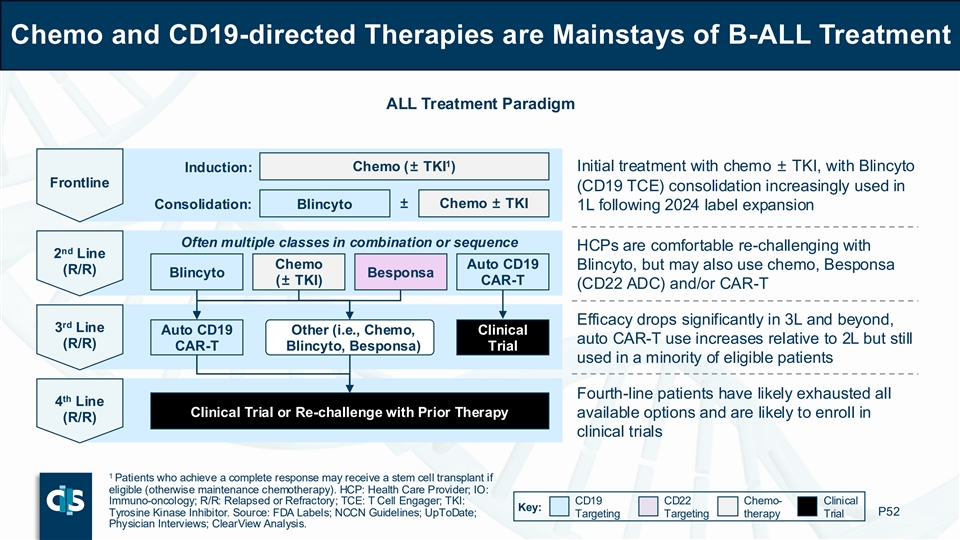
Chemo and CD19-directed Therapies are Mainstays of B-ALL Treatment ALL Treatment Paradigm 1 Induction: Chemo (± TKI ) Initial treatment with chemo ± TKI, with Blincyto Frontline (CD19 TCE) consolidation increasingly used in ± Chemo ± TKI Consolidation: Blincyto 1L following 2024 label expansion Often multiple classes in combination or sequence HCPs are comfortable re-challenging with nd 2 Line Chemo Auto CD19 Blincyto, but may also use chemo, Besponsa (R/R) Blincyto Besponsa (± TKI) CAR-T (CD22 ADC) and/or CAR-T Efficacy drops significantly in 3L and beyond, rd 3 Line Auto CD19 Other (i.e., Chemo, Clinical auto CAR-T use increases relative to 2L but still (R/R) CAR-T Blincyto, Besponsa) Trial used in a minority of eligible patients Fourth-line patients have likely exhausted all th 4 Line Clinical Trial or Re-challenge with Prior Therapy available options and are likely to enroll in (R/R) clinical trials 1 Patients who achieve a complete response may receive a stem cell transplant if eligible (otherwise maintenance chemotherapy). HCP: Health Care Provider; IO: Immuno-oncology; R/R: Relapsed or Refractory; TCE: T Cell Engager; TKI: CD19 CD22 Chemo- Clinical Key: Tyrosine Kinase Inhibitor. Source: FDA Labels; NCCN Guidelines; UpToDate; P52 Targeting Targeting therapy Trial Physician Interviews; ClearView Analysis.
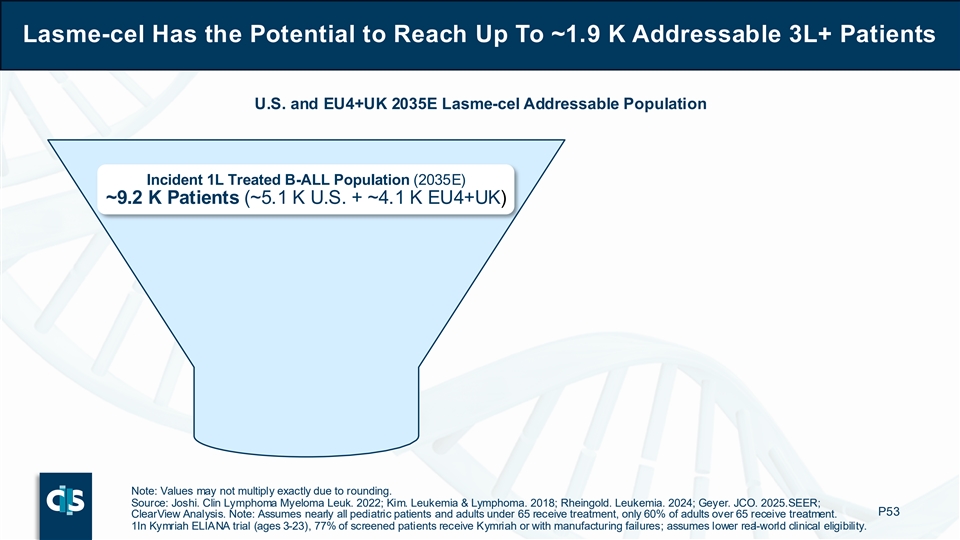
Lasme-cel Has the Potential to Reach Up To ~1.9 K Addressable 3L+ Patients U.S. and EU4+UK 2035E Lasme-cel Addressable Population Incident 1L Treated B-ALL Population (2035E) ~9.2 K Patients (~5.1 K U.S. + ~4.1 K EU4+UK) Note: Values may not multiply exactly due to rounding. Source: Joshi. Clin Lymphoma Myeloma Leuk. 2022; Kim. Leukemia & Lymphoma. 2018; Rheingold. Leukemia. 2024; Geyer. JCO. 2025. SEER; P53 ClearView Analysis. Note: Assumes nearly all pediatric patients and adults under 65 receive treatment, only 60% of adults over 65 receive treatment. 1In Kymriah ELIANA trial (ages 3-23), 77% of screened patients receive Kymriah or with manufacturing failures; assumes lower real-world clinical eligibility.
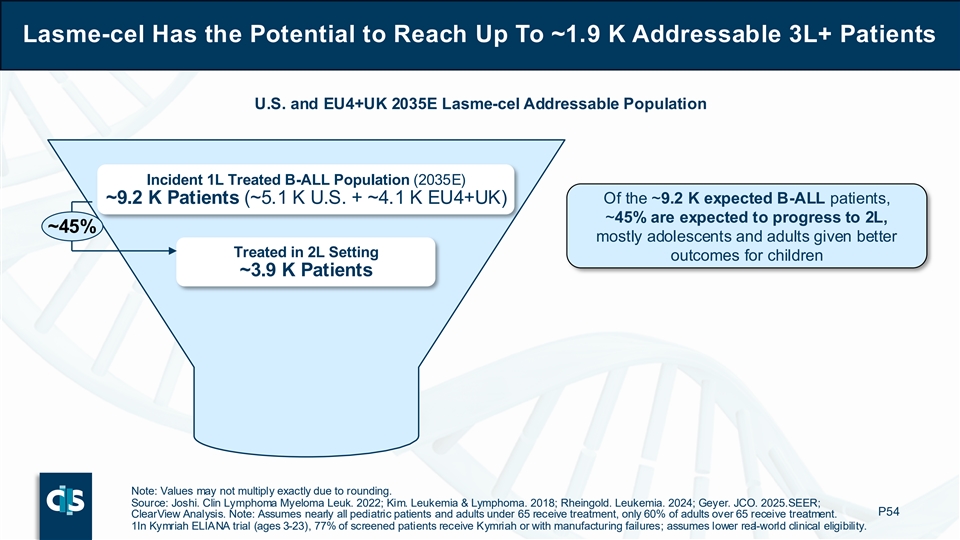
Lasme-cel Has the Potential to Reach Up To ~1.9 K Addressable 3L+ Patients U.S. and EU4+UK 2035E Lasme-cel Addressable Population Incident 1L Treated B-ALL Population (2035E) ~9.2 K Patients (~5.1 K U.S. + ~4.1 K EU4+UK) Of the ~9.2 K expected B-ALL patients, ~45% are expected to progress to 2L, ~45% mostly adolescents and adults given better Treated in 2L Setting outcomes for children ~3.9 K Patients Note: Values may not multiply exactly due to rounding. Source: Joshi. Clin Lymphoma Myeloma Leuk. 2022; Kim. Leukemia & Lymphoma. 2018; Rheingold. Leukemia. 2024; Geyer. JCO. 2025. SEER; P54 ClearView Analysis. Note: Assumes nearly all pediatric patients and adults under 65 receive treatment, only 60% of adults over 65 receive treatment. 1In Kymriah ELIANA trial (ages 3-23), 77% of screened patients receive Kymriah or with manufacturing failures; assumes lower real-world clinical eligibility.
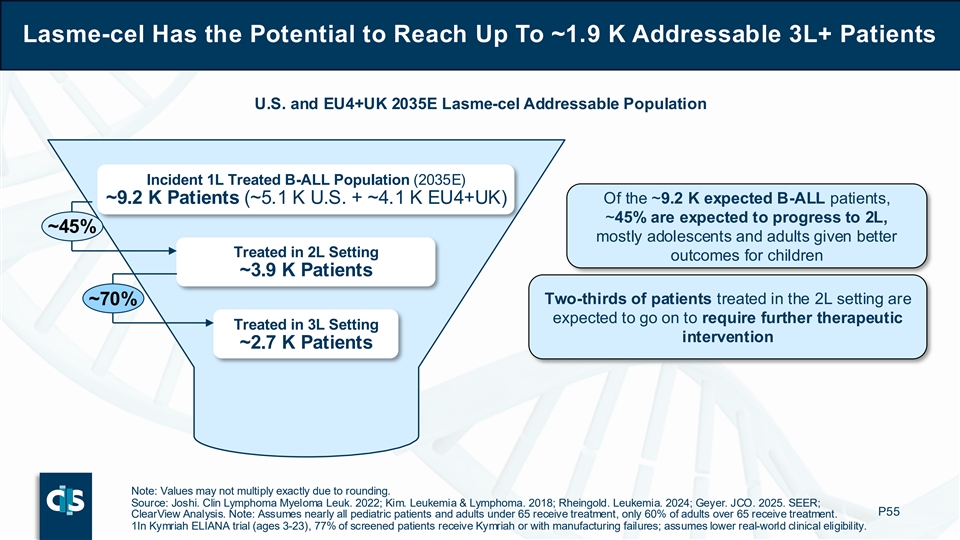
Lasme-cel Has the Potential to Reach Up To ~1.9 K Addressable 3L+ Patients U.S. and EU4+UK 2035E Lasme-cel Addressable Population Incident 1L Treated B-ALL Population (2035E) ~9.2 K Patients (~5.1 K U.S. + ~4.1 K EU4+UK) Of the ~9.2 K expected B-ALL patients, ~45% are expected to progress to 2L, ~45% mostly adolescents and adults given better Treated in 2L Setting outcomes for children ~3.9 K Patients Two-thirds of patients treated in the 2L setting are ~70% expected to go on to require further therapeutic Treated in 3L Setting intervention ~2.7 K Patients Note: Values may not multiply exactly due to rounding. Source: Joshi. Clin Lymphoma Myeloma Leuk. 2022; Kim. Leukemia & Lymphoma. 2018; Rheingold. Leukemia. 2024; Geyer. JCO. 2025. SEER; P55 ClearView Analysis. Note: Assumes nearly all pediatric patients and adults under 65 receive treatment, only 60% of adults over 65 receive treatment. 1In Kymriah ELIANA trial (ages 3-23), 77% of screened patients receive Kymriah or with manufacturing failures; assumes lower real-world clinical eligibility.
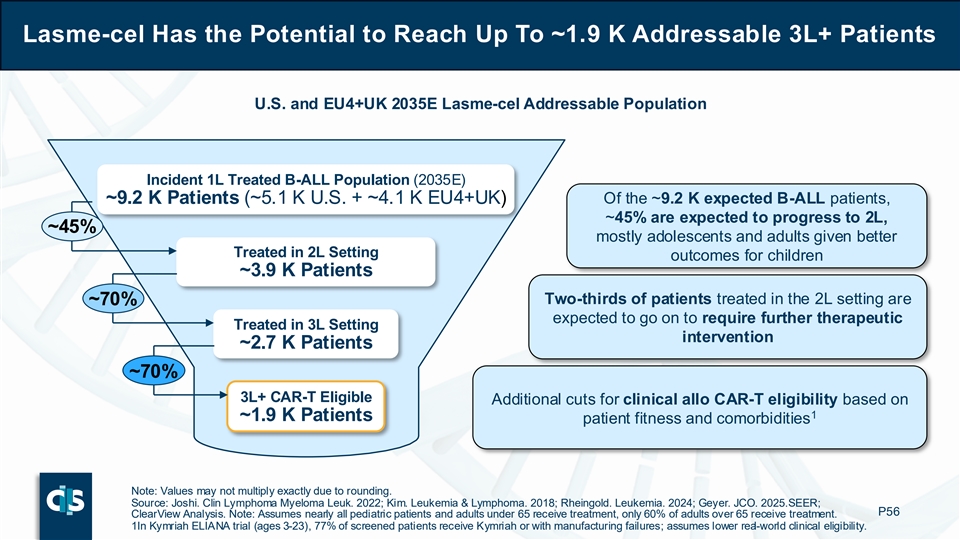
Lasme-cel Has the Potential to Reach Up To ~1.9 K Addressable 3L+ Patients U.S. and EU4+UK 2035E Lasme-cel Addressable Population Incident 1L Treated B-ALL Population (2035E) ~9.2 K Patients (~5.1 K U.S. + ~4.1 K EU4+UK) Of the ~9.2 K expected B-ALL patients, ~45% are expected to progress to 2L, ~45% mostly adolescents and adults given better Treated in 2L Setting outcomes for children ~3.9 K Patients Two-thirds of patients treated in the 2L setting are ~70% expected to go on to require further therapeutic Treated in 3L Setting intervention ~2.7 K Patients ~70% 3L+ CAR-T Eligible Additional cuts for clinical allo CAR-T eligibility based on 1 ~1.9 K Patients patient fitness and comorbidities Note: Values may not multiply exactly due to rounding. Source: Joshi. Clin Lymphoma Myeloma Leuk. 2022; Kim. Leukemia & Lymphoma. 2018; Rheingold. Leukemia. 2024; Geyer. JCO. 2025. SEER; P56 ClearView Analysis. Note: Assumes nearly all pediatric patients and adults under 65 receive treatment, only 60% of adults over 65 receive treatment. 1In Kymriah ELIANA trial (ages 3-23), 77% of screened patients receive Kymriah or with manufacturing failures; assumes lower real-world clinical eligibility.

Lasme-cel Has the Potential To Capture a Majority of the 3L+ Market Value Proposition of 3L+ Treatment Options “MRD negativity is very Lasme-cel encouraging compared to what we have in third Ç Alternative target to CD19Ç Off-the shelf availability line.” – ALL Key Opinion Leader Ç One-time dosingÇ Deep, MRD- responses in 3L+ Interviewed physicians indicated an expectation to use lasme-cel in a Illustrative Lasme-cel Pref. majority of eligible 3L+ patients if available; oncology analogs suggest ~65% Share Among Eligible Patients preferred drug class could achieve 45 – 85% market share “You could infuse this rapidly, in a small number Blincyto Besponsa Auto CD19 Chemo- of days, which would be CAR-T therapy favorable compared to CD19 CAR-T.” È Complex È Limited efficacy in – ALL Key Opinion Leader È Often used in È Repeat dosing manufacturing 3L+ 1L / 2L È Poor durability “After blina up front, then È Re-challenges È Highly toxic È Re-challenges È Often already maybe a CD19 CAR-T… CD19 CD19 then you really need used in 2L something else after another relapse.” – ALL Key Opinion Leader P57 MRD: Minimal Residual Disease. Source: Kantarjian. N Engl J Med. 2016; FDA Labels; Physician Interviews; ClearView Analysis.
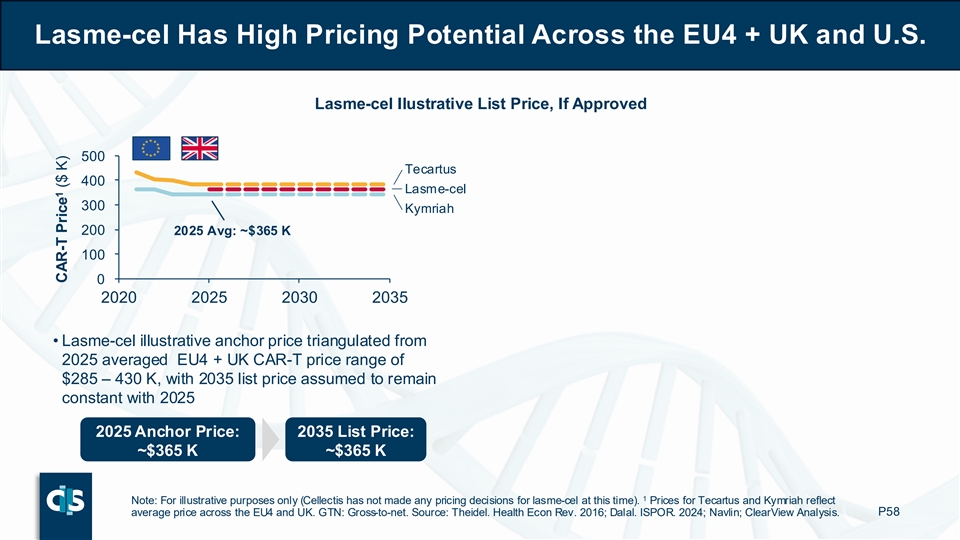
Lasme-cel Has High Pricing Potential Across the EU4 + UK and U.S. Lasme-cel Ilustrative List Price, If Approved 500 Tecartus 400 Lasme-cel 300 Kymriah 200 2025 Avg: ~$365 K 100 0 2020 2025 2030 2035 • Lasme-cel illustrative anchor price triangulated from 2025 averaged EU4 + UK CAR-T price range of $285 – 430 K, with 2035 list price assumed to remain constant with 2025 2025 Anchor Price: 2035 List Price: ~$365 K ~$365 K 1 Note: For illustrative purposes only (Cellectis has not made any pricing decisions for lasme-cel at this time). Prices for Tecartus and Kymriah reflect average price across the EU4 and UK. GTN: Gross-to-net. Source: Theidel. Health Econ Rev. 2016; Dalal. ISPOR. 2024; Navlin; ClearView Analysis. P58 1 CAR-T Price ($ K)
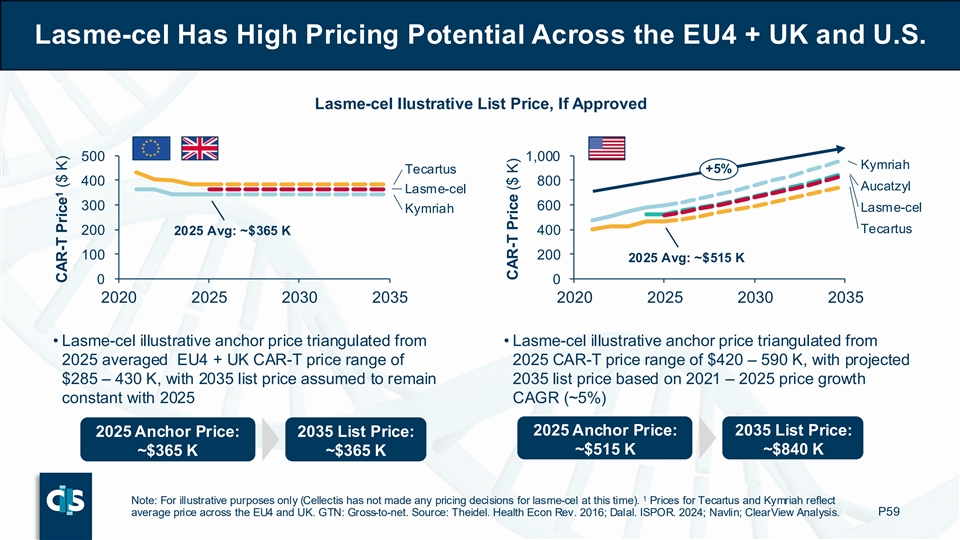
Lasme-cel Has High Pricing Potential Across the EU4 + UK and U.S. Lasme-cel Ilustrative List Price, If Approved 500 1,000 Kymriah Tecartus +5% 400 800 Aucatzyl Lasme-cel 300 600 Lasme-cel Kymriah Tecartus 200 400 2025 Avg: ~$365 K 100 200 2025 Avg: ~$515 K 0 0 2020 2025 2030 2035 2020 2025 2030 2035 • Lasme-cel illustrative anchor price triangulated from • Lasme-cel illustrative anchor price triangulated from 2025 averaged EU4 + UK CAR-T price range of 2025 CAR-T price range of $420 – 590 K, with projected $285 – 430 K, with 2035 list price assumed to remain 2035 list price based on 2021 – 2025 price growth constant with 2025 CAGR (~5%) 2025 Anchor Price: 2035 List Price: 2025 Anchor Price: 2035 List Price: ~$515 K ~$840 K ~$365 K ~$365 K 1 Note: For illustrative purposes only (Cellectis has not made any pricing decisions for lasme-cel at this time). Prices for Tecartus and Kymriah reflect average price across the EU4 and UK. GTN: Gross-to-net. Source: Theidel. Health Econ Rev. 2016; Dalal. ISPOR. 2024; Navlin; ClearView Analysis. P59 1 CAR-T Price ($ K) CAR-T Price ($ K)
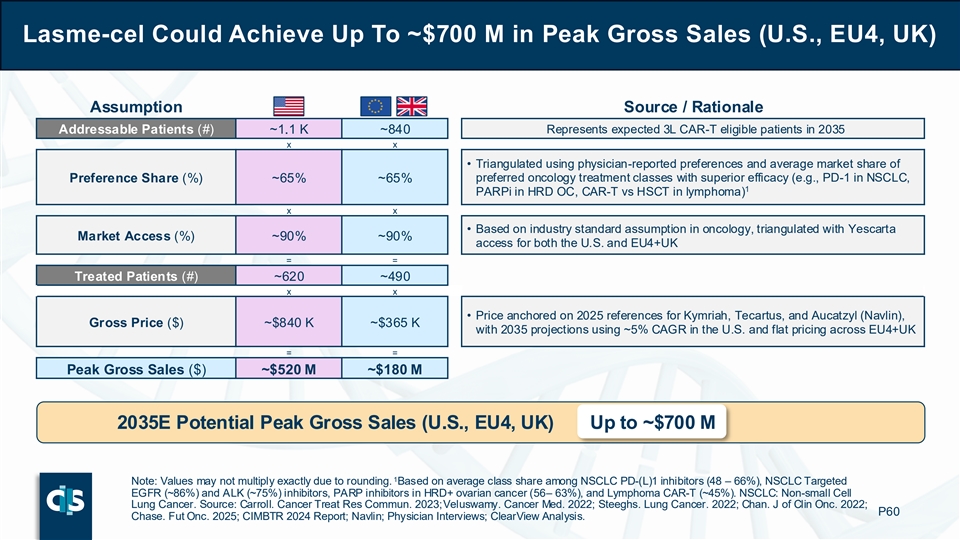
Lasme-cel Could Achieve Up To ~$700 M in Peak Gross Sales (U.S., EU4, UK) Assumption Source / Rationale Addressable Patients (#) ~1.1 K ~840 Represents expected 3L CAR-T eligible patients in 2035 x x • Triangulated using physician-reported preferences and average market share of Preference Share (%) ~65% ~65% preferred oncology treatment classes with superior efficacy (e.g., PD-1 in NSCLC, 1 PARPi in HRD OC, CAR-T vs HSCT in lymphoma) x x • Based on industry standard assumption in oncology, triangulated with Yescarta Market Access (%) ~90% ~90% access for both the U.S. and EU4+UK = = Treated Patients (#) ~620 ~490 x x • Price anchored on 2025 references for Kymriah, Tecartus, and Aucatzyl (Navlin), Gross Price ($) ~$840 K ~$365 K with 2035 projections using ~5% CAGR in the U.S. and flat pricing across EU4+UK = = Peak Gross Sales ($) ~$520 M ~$180 M 2035E Potential Peak Gross Sales (U.S., EU4, UK) Up to ~$700 M 1 Note: Values may not multiply exactly due to rounding. Based on average class share among NSCLC PD-(L)1 inhibitors (48 – 66%), NSCLC Targeted EGFR (~86%) and ALK (~75%) inhibitors, PARP inhibitors in HRD+ ovarian cancer (56 – 63%), and Lymphoma CAR-T (~45%). NSCLC: Non-small Cell Lung Cancer. Source: Carroll. Cancer Treat Res Commun. 2023; Veluswamy. Cancer Med. 2022; Steeghs. Lung Cancer. 2022; Chan. J of Clin Onc. 2022; P60 Chase. Fut Onc. 2025; CIMBTR 2024 Report; Navlin; Physician Interviews; ClearView Analysis.
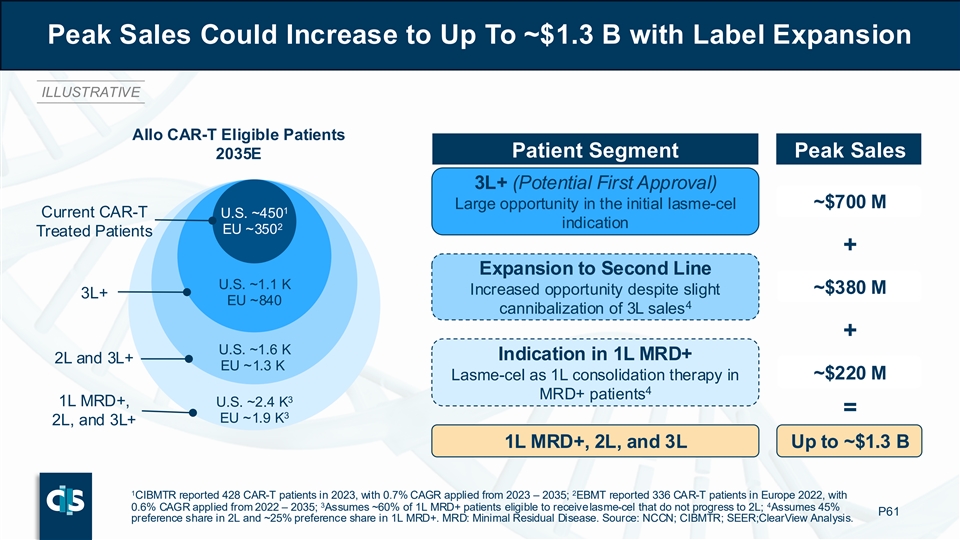
Peak Sales Could Increase to Up To ~$1.3 B with Label Expansion ILLUSTRATIVE Allo CAR-T Eligible Patients Patient Segment Peak Sales 2035E 3L+ (Potential First Approval) ~$700 M Large opportunity in the initial lasme-cel 1 Current CAR-T U.S. ~450 indication 2 EU ~350 Treated Patients + Expansion to Second Line U.S. ~1.1 K Increased opportunity despite slight ~$380 M 3L+ EU ~840 4 cannibalization of 3L sales + U.S. ~1.6 K Indication in 1L MRD+ 2L and 3L+ EU ~1.3 K Lasme-cel as 1L consolidation therapy in ~$220 M 4 MRD+ patients 3 1L MRD+, U.S. ~2.4 K = 3 EU ~1.9 K 2L, and 3L+ 1L MRD+, 2L, and 3L Up to ~$1.3 B 1 2 CIBMTR reported 428 CAR-T patients in 2023, with 0.7% CAGR applied from 2023 – 2035; EBMT reported 336 CAR-T patients in Europe 2022, with 3 4 0.6% CAGR applied from 2022 – 2035; Assumes ~60% of 1L MRD+ patients eligible to receive lasme-cel that do not progress to 2L; Assumes 45% P61 preference share in 2L and ~25% preference share in 1L MRD+. MRD: Minimal Residual Disease. Source: NCCN; CIBMTR; SEER;C learView Analysis.

Lasme-cel Can Drive Meaningful Growth of the CAR-T Market In B-ALL First-in-class Immunotherapy Beyond CD19 Lasme-cel Deep Responses with High CR Rates Opportunity and Impressive MRD Negativity Robust Peak Sales Potential (Up To ~$700 M) With Highly Attractive Margins CR: Complete Remission; MRD: Minimal Residual Disease. Source: ClearView Analysis. P62
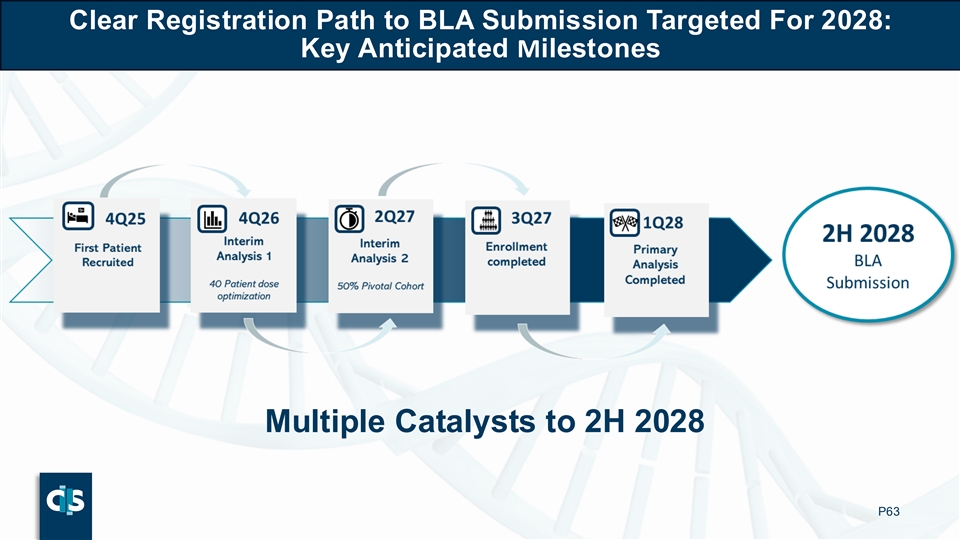
Clear Registration Path to BLA Submission Targeted For 2028: Key Anticipated Milestones Multiple Catalysts to 2H 2028 P63
Cellectis Strategic Roadmap Lasme-cel Potential BLA Filing in r/r B-ALL (2028) Eti-cel Potential EoP1 in r/r NHL (2026) Preclinical PoC for In Vivo Gene Therapy (2026) P64 EoP1: End-of-Phase 1 ; PoC: Proof-of-concept

Thank You Reach us at: investors@cellectis.com Cellectis Raleigh Cellectis New York Cellectis Paris 2500 Sumner Boulevard 8, rue de la Croix Jarry 75013 430 East 29th Street Raleigh, NC, 27616 – USA Paris – France New York, NY, 10016 – USA Cellectis®, TALEN® and our corporate logos are registered trademarks owned by Cellectis.