UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
OF THE SECURITIES EXCHANGE ACT OF 1934
For the Month of October 2025
(Commission File No. 001-41636)
Oculis Holding AG
(Translation of registrant’s name into English)
Bahnhofstrasse 20
CH-6300
Zug, Switzerland
(Address of registrant’s principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
INFORMATION CONTAINED IN THIS REPORT ON FORM 6-K
Press Release and Corporate Presentation
On October 6, 2025, Oculis Holding AG (“Oculis” or the “Registrant”) issued a press release announcing the advancement of Privosegtor (OCS-05) into a registrational program for neuro-ophthalmology indications following a positive meeting with the U.S. Food and Drug Administration (the “FDA”).
Privosegtor is a new peptoid small molecule candidate with the potential to become the first neuroprotective therapy for acute optic neuritis (“AON”) and non-arteritic anterior ischemic optic neuropathy (NAION), with potential in other neuro-ophthalmic and neurological diseases. Following FDA feedback, Oculis is starting the PIONEER program this year, which will include three pivotal trials to support registration plans for Privosegtor in AON and NAION.
| • | PIONEER-1 trial in AON (Q4 2025); |
| • | PIONEER-2 trial in AON (1H 2026); and |
| • | PIONEER-3 trial in NAION (mid-2026). |
The first two trials, PIONEER-1 and PIONEER-2, will evaluate Privosegtor following the acute onset of optic neuritis in a broad population. The primary endpoint will be measured as low-contrast visual acuity at 3 months. Dosing and patient enrollment criteria will mirror those of the positive Phase 2 ACUITY trial, which demonstrated improvement in visual function and anatomical preservation of the retina in patients with AON. PIONEER-1 is expected to initiate in Q4 2025, with PIONEER-2 planned to follow in the first half of 2026.
The FDA provided guidance that Privosegtor can be evaluated in other neuro-ophthalmology indications, such as NAION, under the current Investigational New Drug application. The third trial in the PIONEER program, PIONEER-3, will evaluate Privosegtor after an acute onset of NAION. This study shares the core design and operational elements with PIONEER-1 and PIONEER-2, and is expected to begin in mid-2026. Running these three studies concurrently is expected to generate operational synergies, cost efficiencies, and speed up development timelines.
Oculis also announced that as of September 30, 2025, its preliminary unaudited cash, cash equivalents and short-term investments was approximately $182 million. Oculis’ cash runway is expected to be into 2H 2027 to accelerate the Privosegtor development program, without utilization of its available loan capacity. This financial information reflects Oculis’ preliminary estimate, based on currently available information. Financial closing procedures for the quarter are not yet completed and final results may therefore vary from this estimate. This preliminary estimate has not been audited by Oculis’ independent registered public accounting firm.
On October 6, 2025, Oculis also gave a presentation on the advancement of Privosegtor (OCS-05) into a registrational program for neuro-ophthalmology indications.
The press release and presentation are attached hereto as Exhibit 99.1 and Exhibit 99.2, respectively.
Cautionary Statement Regarding Forward Looking Statements
This Report contains forward-looking statements and information. For example, statements regarding the development plans for Privosegtor; the design and timing of clinical trials of Privosegtor; potential effects of Privosegtor, including patient impact and market opportunity; the potential of Privosegtor to be a neuroprotective therapy or treatment for AON, NAION and other neuro-ophthalmic diseases; the potential for the Privosegtor clinical development plan to result in operational synergies, cost efficiencies and accelerated development timelines; Oculis’ estimated cash, cash equivalents and short-term investments and its estimated cash runway; and Oculis’ research and development programs, regulatory and business strategy, future development plans, and management, are forward-looking.
All forward-looking statements are based on estimates and assumptions that, while considered reasonable by Oculis and its management, are inherently uncertain and are inherently subject to risks, variability, and contingencies, many of which are beyond Oculis’ control. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on by an investor as, a guarantee, assurance, prediction or definitive statement of a fact or probability. Actual events and circumstances are difficult or impossible to predict and will differ from assumptions. All forward-looking statements are subject to risks, uncertainties and other factors that may cause actual results to differ materially from those that we expected and/or those expressed or implied by such forward-looking statements. Forward-looking statements are subject to numerous conditions, many of which are beyond the control of Oculis, including those set forth in the Risk Factors section of Oculis’ annual report on Form 20-F and any other documents filed with the U.S. Securities and Exchange Commission (SEC). Copies of these documents are available on the SEC’s website, www.sec.gov. Oculis undertakes no obligation to update these statements for revisions or changes after the date of this Report, except as required by law.
INCORPORATION BY REFERENCE
The information contained in this Form 6-K, excluding Exhibit 99.1 and Exhibit 99.2, is hereby incorporated by reference into the Registrant’s Registration Statements on Form S-8 (File No. 333-271938 and 333-287806) and Form F-3 (File Nos. 333-271063, 333-278409 and 333-281798).
EXHIBIT INDEX
| Exhibit |
Description |
|
| 99.1 | Press Release dated October 6, 2025 | |
| 99.2 | Presentation dated October 6, 2025 | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| OCULIS HOLDING AG | ||||||
| Date: October 6, 2025 | By: | /s/ Sylvia Cheung |
||||
| Sylvia Cheung Chief Financial Officer |
||||||
Exhibit 99.1

Oculis Accelerates Privosegtor into Registrational Trials in Acute Optic Neuritis,
Pioneering the Path for a Potential First-in-class Neuroprotective Therapy
Successful meeting with FDA enables advancement into pivotal development with
Privosegtor in Acute Optic Neuritis (AON) and Non-arteritic Anterior Ischemic Optic
Neuropathy (NAION)
Oculis will launch the PIONEER Program, which includes multiple global trials
intended to support registrations in AON and NAION
Company to host conference call and webcast at 8:30 AM ET today
ZUG, Switzerland, October 6, 2025 – Oculis Holding AG (Nasdaq: OCS / XICE: OCS) (“Oculis”), a global biopharmaceutical company focused on innovations addressing neuro-ophthalmic diseases with significant unmet medical needs, today announces the advancement of Privosegtor into a registrational program for neuro-ophthalmology indications following a positive meeting with the U.S. Food and Drug Administration (FDA).
Privosegtor is a new peptoid small molecule candidate with the potential to become the first neuroprotective therapy for acute optic neuritis (AON) and non-arteritic anterior ischemic optic neuropathy (NAION), with potential in other neuro-ophthalmic and neurological diseases. Following FDA feedback, the company is starting the PIONEER program this year, which will include three pivotal trials to support registration plans for Privosegtor in AON and NAION.
| • | PIONEER-1 trial in AON (Q4 2025) |
| • | PIONEER-2 trial in AON (1H 2026) |
| • | PIONEER-3 trial in NAION (mid-2026) |
The first two trials, PIONEER-1 and PIONEER-2, will evaluate Privosegtor following the acute onset of optic neuritis in a broad population consisting of patients with multiple sclerosis (MS) and those without MS. The primary endpoint will be measured as low-contrast visual acuity (LCVA) at 3 months. Dosing and patient enrollment criteria will mirror those of the positive Phase 2 ACUITY trial, which demonstrated improvement in visual function and anatomical preservation of the retina in patients with AON. PIONEER-1 is expected to initiate in Q4 2025, with PIONEER-2 planned to follow in the first half of 2026.
The FDA provided guidance that Privosegtor can be evaluated in other neuro-ophthalmology indications, such as NAION, under the current IND. The third trial in the PIONEER program, PIONEER-3, will evaluate Privosegtor after the acute onset of NAION. This study shares the core design and operational elements with PIONEER-1 and PIONEER-2, and is expected to begin in mid-2026. Running these three studies concurrently is expected to generate operational synergies, cost efficiencies, and speed up development timelines.
AON and NAION are two rare neuro-ophthalmic diseases with high unmet medical needs for a therapy that can provide neuroprotection and preserve vision. AON is often the first clinical symptom of multiple sclerosis. While corticosteroids are used to treat inflammation in AON, there are no approved neuroprotective therapies that can restore vision for patients suffering from acute optic neuritis. Likewise, no medical or surgical treatment has been shown to improve the prognosis in cases of acute NAION.
1

Riad Sherif, M.D., Chief Executive Officer of Oculis, commented: “The positive FDA meeting marks a significant milestone for our rapidly advancing neuroprotection candidate. The PIONEER program positions Oculis as a leader in ophthalmic neuroprotection drug development. The novel Privosegtor candidate provides the Company with multiple opportunities to target devastating diseases with significant unmet medical needs in late-stage trials. With this milestone and the upcoming top-line results from Diamond Phase 3 DME program, Oculis is now in a strong position with multiple pivotal studies, targeting multi-billion-dollar markets.”
Mark Kupersmith, M.D., Chief Medical Advisor, Neuro-Ophthalmology, added: “I am very pleased that the Company is accelerating its development plan with Privosegtor in optic neuropathies, including acute optic neuritis and NAION, given the critical unmet medical needs remaining. Neuroprotection represents a promising therapeutic approach across multiple neuro-ophthalmic conditions and although the cause of optic nerve injury can be different, patients experience permanent loss of optic nerve axons and retinal ganglion cells causing permanent visual deficits. Privosegtor’s potential neuroprotective benefits and the studies we are conducting, as part of the PIONEER Program, could have a profound impact on patients’ lives.”
Leonard A. Levin, M.D., Ph.D., Professor, Departments of Ophthalmology & Visual Sciences and Neurology & Neurosurgery, McGill University, said: “The positive ACUITY topline results represent a remarkable step forward in neuroprotection trials. I have been involved in this area for more than three decades, and this candidate is the first to show significant improvement in clinical, biological, and imaging measures of the disease in a single trial. I look forward to contributing to the testing of Privosegtor for a broad spectrum of neuro-ophthalmic and neurological diseases.”
As of September 30, 2025, Oculis’ preliminary unaudited cash, cash equivalents and short-term investments was approximately $182 million. The cash runway is expected to be into 2H 2027 to accelerate the Privosegtor development program, without utilization of the available loan capacity. The Company plans to report full Q3 2025 results on November 10, 2025.
Analyst and investor call
The Oculis management team will host an analyst and investor call today at 8:30 AM U.S. Eastern Time.
Interested parties may participate in the call via the following link:
https://lifescievents.com/event/al307vskt98/
A replay of the webcast and accompanying slides will be available for 90 days following the event through the “Events and Presentations” page of the “Investors and Media” section of the company’s website.
- END -
About Privosegtor
Privosegtor is a novel peptoid small molecule candidate with the potential to become the first neuroprotective therapy for acute optic neuritis (AON) and other neuro-ophthalmic diseases. The positive results in the ACUITY Phase 2 trial showed Privosegtor’s potential neuroprotective effects through anatomical preservation of the retina and visual function improvements after an acute episode of optic neuritis. Consistent results were observed in animal models of neuroinflammation and neurodegeneration, where Privosegtor showed preservation of retinal ganglion cell damage and was associated with improvements in mobility (clinical function disability). Privosegtor has received Orphan Drug designation from both the FDA and the EMA for AON and is now entering registrational trials for this indication as well as a registrational trial in nonarteritic anterior ischemic optic neuropathy (NAION) as part of Oculis’ PIONEER (Privosegtor Investigation in Optic Neuropathies Efficacy Evaluation Research) program. In addition to its potential effect on neuroprotection of the optic nerve, Privosegtor could also have wide applicability in treating other neuro-ophthalmic and neurology indications.
2

Privosegtor is an investigational drug and has not received regulatory approval for commercial use in any country.
About Acute Optic Neuritis
Acute Optic Neuritis (AON) is a rare condition characterized by an acute inflammation of the optic nerve that can lead to permanent visual impairment. It affects up to 8 in 100,000 people worldwide with a U.S. incidence estimated to be >30,000 and often represents the first sign of multiple sclerosis1. It mainly occurs in adults between the age of 20 and 40 years and is more frequent in women (2:1)2. The acute inflammatory process of optic neuritis leads to the loss of myelin covering the optic nerve and the axons. At the onset, patients often suffer from ocular pain that increases with eye movement and vision loss. Once the inflammation recedes, remyelination often occurs but it is incomplete. Without the myelin sheath protecting the axon, neurons located in demyelinated segments become fragile and prone to death. Unfortunately, damaged axons cannot regrow, leading to permanent visual impairment. Though most patients do not have permanent severe vision loss, visual impairment for images and things of low contrast is a common impairment. This can interfere with reading, pattern recognition and seeing on gray or cloudy days. To date there is no specific neuroprotective therapy approved for AON and unmet needs remain for therapies that can prevent vision loss after an acute episode of optic neuritis.
About Non-arteritic Anterior Ischemic Optic Neuropathy
Non-arteritic anterior ischemic optic neuropathy (NAION) is an acute optic nerve disorder and the most common cause of acute optic nerve injury in individuals over 50 years old3. It affects up to 10.2 per 100,000 people worldwide4 with a U.S. incidence estimated to be >30,0003,5,6. NAION and Acute Optic Neuritis (AON) injure the optic nerve leading to progressive axonal loss and visual decline, after the acute event. In NAION, the optic nerve head region swells and there is painless sudden vision loss. The swelling eventually resolves, but the optic nerve axons and neuronal cell bodies (in the retina) are permanently lost, leading to significant visual impairment or even irreversible blindness7. There are no approved therapies for NAION and there remains an unmet medical need for therapies that preserve vision and provide neuroprotection for patients suffering from NAION.
About Oculis
Oculis is a global biopharmaceutical company (Nasdaq: OCS; XICE: OCS) focused on innovations addressing neuro-ophthalmic conditions with significant unmet medical needs. Oculis’ highly differentiated late-stage clinical pipeline includes three core product candidates: Privosegtor, a neuroprotective candidate in the PIONEER program which consists of studies intended to support registration plans for treatment in optic neuropathies like acute optic neuritis (AON) and non-arteritic anterior ischemic optic neuropathy (NAION), with potentially broad clinical applications in various other neuro-ophthalmic and neurological diseases; OCS-01, an eye drop in pivotal registration studies, aiming to become the first non-invasive topical treatment for diabetic macular edema; and Licaminlimab, a novel, topical anti-TNF-α in Phase 2, is being developed with a genotype-based approach to drive personalized medicine in dry eye disease. Headquartered in Switzerland with operations in the U.S. and Iceland, Oculis is led by an experienced management team with a successful track record and supported by leading international healthcare investors.
For more information, please visit: www.oculis.com Corey Davis, Ph.D.
-ENDS-
3

Oculis Contacts
Ms. Sylvia Cheung, CFO
sylvia.cheung@oculis.com
Investor Relations
LifeSci Advisors
cdavis@lifesciadvisors.com
Media Relations
ICR Healthcare
Amber Fennell / David Daley / Sean Leous
oculis@icrhealthcare.com
Cautionary Statement Regarding Forward Looking Statements
This press release contains forward-looking statements and information. For example, statements regarding the development plans for Privosegtor; the design and timing of clinical trials of Privosegtor; potential effects of Privosegtor, including patient impact and market opportunity; the potential of Privosegtor to be a neuroprotective therapy or treatment for AON, NAION and other neuro-ophthalmic diseases; the potential for the Privosegtor clinical development plan to result in operational synergies, cost efficiencies and accelerated development timelines; the Company’s estimated cash, cash equivalents and short-term investments and cash runway; and Oculis’ research and development programs, regulatory and business strategy, future development plans, and management, are forward-looking. All forward-looking statements are based on estimates and assumptions that, while considered reasonable by Oculis and its management, are inherently uncertain and are inherently subject to risks, variability, and contingencies, many of which are beyond Oculis’ control. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on by an investor as, a guarantee, assurance, prediction or definitive statement of a fact or probability. Actual events and circumstances are difficult or impossible to predict and will differ from assumptions. All forward-looking statements are subject to risks, uncertainties and other factors that may cause actual results to differ materially from those that we expected and/or those expressed or implied by such forward-looking statements. Forward-looking statements are subject to numerous conditions, many of which are beyond the control of Oculis, including those set forth in the Risk Factors section of Oculis’ annual report on Form 20-F and any other documents filed with the U.S. Securities and Exchange Commission (SEC). Copies of these documents are available on the SEC’s website, www.sec.gov. Oculis undertakes no obligation to update these statements for revisions or changes after the date of this release, except as required by law.
The financial information in this release reflects the Company’s preliminary estimate, based on currently available information. Financial closing procedures for the quarter are not yet completed and final results may therefore vary from this estimate. This preliminary estimate has not been audited by the Company’s independent registered public accounting firm.
References :
| 1. | Martínez-Lapiscina EH, et al. (2014): Is the incidence of optic neuritis rising? Evidence from an epidemiological study in Barcelona (Spain) 2008-2012. J Neurol. 2014 Apr; 261(4): 759-767. |
| 2. | Pérez-Cambrodí RJ, Gómez-Hurtado Cubillana A, Merino-Suárez ML, Piñero-Llorens DP, Laria-Ochaita C. Optic neuritis in pediatric population: a review in current tendencies of diagnosis and management. J Optom. 2014 Jul-Sep;7(3):125-30. |
| 3. |
https://www.aao.org/eyenet/article/naion-diagnosis-and-management |
4

| 4. | Kuppersmith, MJ et al. (2024): Ophthalmic and Systemic Factors of Acute Nonarteritic Anterior Ischemic Optic Neuropathy in the Quark207 Treatment Trial. 2024 July;131(7):790-802. |
| 5. | Hattenhauer M G et al. (1997): Incidence of nonarteritic anterior ischemic optic neuropathy. American Journal of Ophthalmology. 1997 Jan;123(1):103-7. |
| 6. | Lee M S et al. (2011): Incidence of nonarteritic anterior ischemic optic neuropathy: increased risk among diabetic patients. Ophthalmology 2011 Mar 24;118(5):959-963 |
| 7. | North American Neuro-Ophthalmology Society website: https://www.nanosweb.org |
5

Rethinking Ophthalmology Privosegtor FDA update October 6th, 2025 Exhibit 99.2

These slides and the accompanying oral presentation contain forward-looking statements and information. The use of words such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar expressions are intended to identify forward-looking statements. For example, all statements we make regarding the initiation, timing, progress and results of our preclinical studies, our clinical studies, our research and development programs, our regulatory strategy, our future development plans, our ability to advance product candidates into, and successfully complete clinical studies, and the timing or likelihood of regulatory filings and approvals, our cash runway, and statements regarding the potential therapeutic benefits of our product candidates are forward looking. All forward-looking statements are based on estimates and assumptions by our management that, although we believe to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected. Factors that may cause actual results to differ materially from current expectations include, but are not limited to: the possibility that Oculis may be adversely affected by economic, business, and/or competitive factors; Oculis' estimates of expenses and profitability; Oculis' ability to develop, manufacture and commercialize the product candidates in its pipeline; actions of regulatory authorities, which may affect the initiation, timing and progress of clinical studies or future regulatory approvals or marketing authorizations; the ability of Oculis or its partners to enroll and retain patients in clinical studies; the ability of Oculis or its partners to gain approval from regulators for planned clinical studies, study plans or sites; Oculis' ability to obtain and maintain regulatory approval or authorizations of its products, including the timing or likelihood of expansion into additional markets or geographies; the success of Oculis' current and future collaborations, joint ventures, partnerships or licensing arrangements; financial position, strategy and anticipated milestones; and other risks and uncertainties set forth in the sections entitled “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” in documents that Oculis may from time to time file or furnish with the SEC. Any forward-looking statement speaks only as of the date on which it was made. We undertake no obligation to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. Cautionary note on forward-looking statements Copyright of this presentation is owned by Oculis. No part of this presentation may be reproduced in any manner without the permission of Oculis. Safe Harbor Statements

Corporate Update

Three registrational programs in Neuro-Ophthalmic and Retina indications provide multi-billion-dollar market opportunities Oculis (Nasdaq / XICE: OCS) Global biopharma Nasdaq listed company Innovative neuro-ophthalmology and ophthalmology candidates with significant market potential of ~$25 billion for the overall portfolio, key late-stage assets include: Privosegtor, a first-in-class neuroprotective candidate advancing to registrational programs in 2 indications: Acute Optic Neuritis (AON) and Non-arteritic Anterior Ischemic Optic Neuropathy (NAION), with potential for broad applicability in neuro-ophthalmic and neurological conditions OCS-01 eye drops, potentially the first non-invasive treatment for DME, in registrational trials with readouts anticipated in Q2 2026 Strong balance sheet, no debt, and current cash runway into 2H 2027 without utilization of the loan facility

Allows advancement of Privosegtor into the registrational phase Alignment on the acute optic neuritis (AON) registrational trial design Alignment on the regulatory pathway for non-arteritic anterior ischemic optic neuropathy (NAION), to be evaluated under the same IND AON: PIONEER-1 registrational trial to start Q4 2025 PIONEER-2 registrational trial to start in 1H 2026 Designed to mimic the successful Phase 2 ACUITY trial with: Same dose 3mg/kg/day and patient population (MS and Non-MS patients) as ACUITY Primary endpoint: Change from baseline in LCVA at Month 3 (approval) Secondary endpoints: Proportion (%) of 15 letter gainers at Month 3 and change from baseline in LCVA at Month 6 NAION: PIONEER-3 registrational trial to start in mid-2026 Establish a master global optic neuropathies program: PIONEER: Privosegtor Investigation in Optic Neuropathies Efficacy Evaluation Research Targeting multi-billion-dollar market opportunities Summary of Positive Privosegtor FDA meeting Successful meeting with U.S. FDA Accelerates Privosegtor registrational programs With solid path to approval into multi billion markets 1 2 3

Pre-clinical Phase 1 Phase 2 Phase 3 Next catalysts Start of PIONEER-1 registrational trial in Q4 2025 Start of PIONEER-2 registrational trial in 1H 2026 Start of PIONEER-3 registrational trial in mid-2026 Oculis to cross-reference Privosegtor AON IND for new IND submission in MS Relapses in 2026 AON: acute optic neuritis; NAION: non-arteritic ischemic optic neuropathy; MS: multiple sclerosis. Privosegtor is a peptoid small molecule with novel MoA targeting the activation of the trophic factor pathways. Privosegtor Development Plan Accelerated AON PRIVOSEGTOR MS RELAPSES NAION
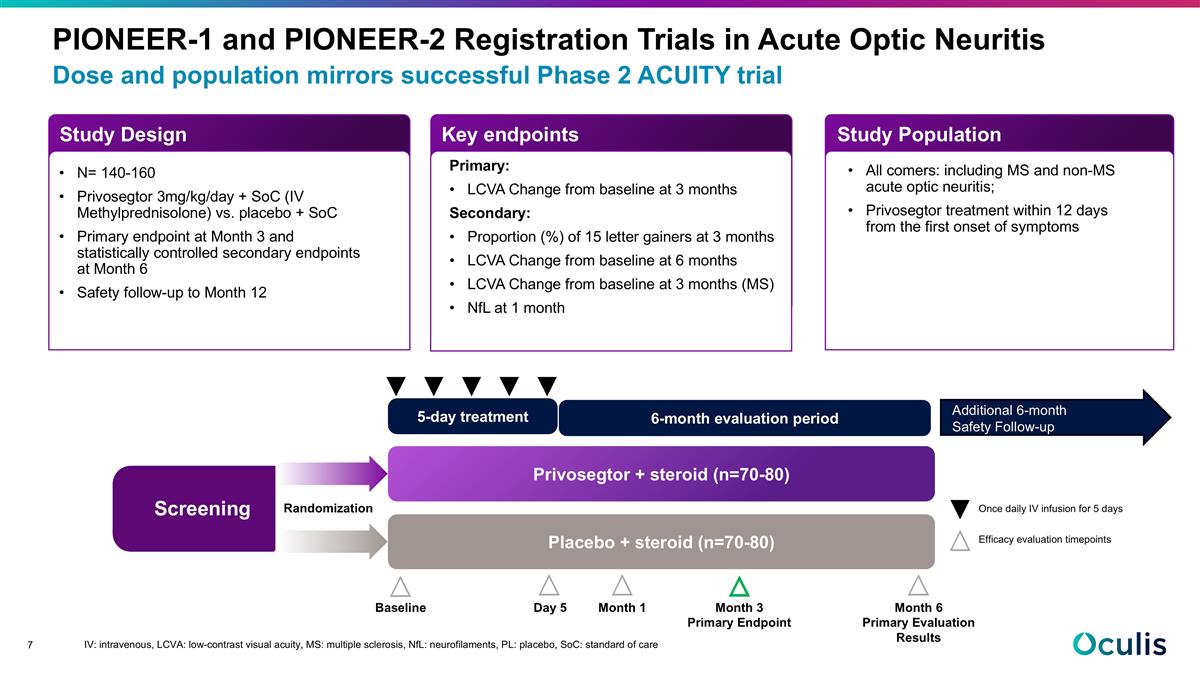
Dose and population mirrors successful Phase 2 ACUITY trial PIONEER-1 and PIONEER-2 Registration Trials in Acute Optic Neuritis Screening 5-day treatment 6-month evaluation period Privosegtor + steroid (n=70-80) Placebo + steroid (n=70-80) Month 6 Primary Evaluation Results Day 5 Month 1 Month 3 Primary Endpoint Baseline Randomization Once daily IV infusion for 5 days Efficacy evaluation timepoints N= 140-160 Privosegtor 3mg/kg/day + SoC (IV Methylprednisolone) vs. placebo + SoC Primary endpoint at Month 3 and statistically controlled secondary endpoints at Month 6 Safety follow-up to Month 12 Primary: LCVA Change from baseline at 3 months Secondary: Proportion (%) of 15 letter gainers at 3 months LCVA Change from baseline at 6 months LCVA Change from baseline at 3 months (MS) NfL at 1 month All comers: including MS and non-MS acute optic neuritis; Privosegtor treatment within 12 days from the first onset of symptoms Study Design Study Population Key endpoints Additional 6-month Safety Follow-up IV: intravenous, LCVA: low-contrast visual acuity, MS: multiple sclerosis, NfL: neurofilaments, PL: placebo, SoC: standard of care
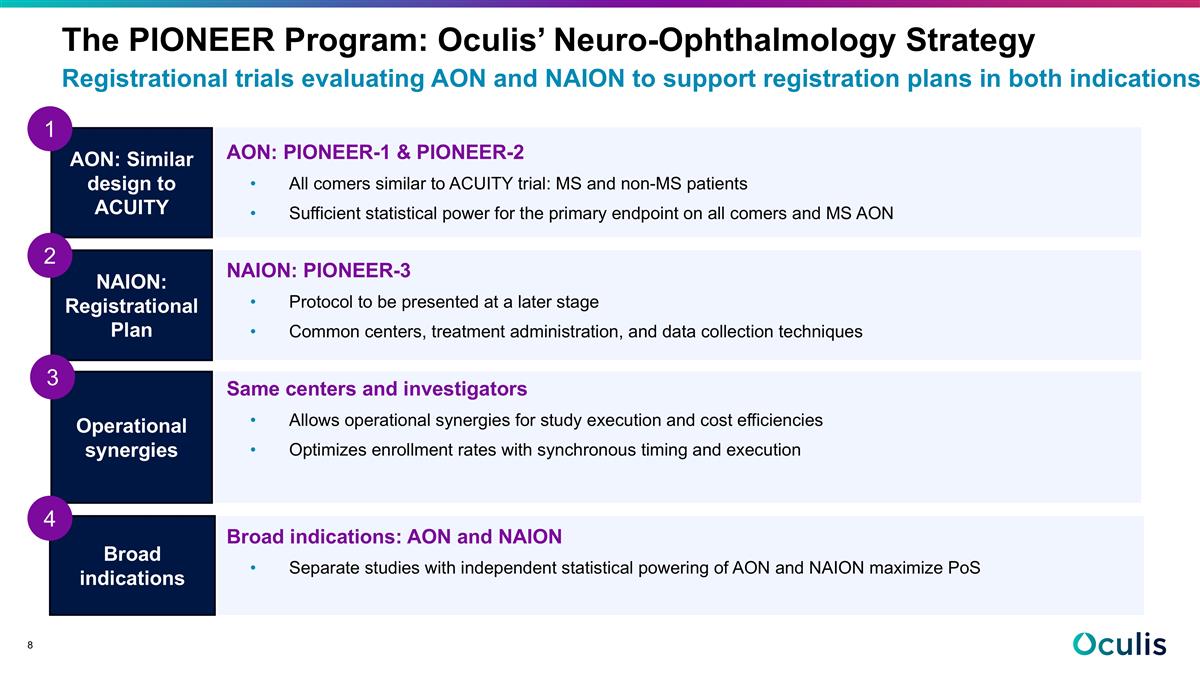
AON: Similar design to ACUITY NAION: Registrational Plan Broad indications 1 2 Registrational trials evaluating AON and NAION to support registration plans in both indications The PIONEER Program: Oculis’ Neuro-Ophthalmology Strategy AON: PIONEER-1 & PIONEER-2 All comers similar to ACUITY trial: MS and non-MS patients Sufficient statistical power for the primary endpoint on all comers and MS AON NAION: PIONEER-3 Protocol to be presented at a later stage Common centers, treatment administration, and data collection techniques Same centers and investigators Allows operational synergies for study execution and cost efficiencies Optimizes enrollment rates with synchronous timing and execution Broad indications: AON and NAION Separate studies with independent statistical powering of AON and NAION maximize PoS Operational synergies 3 4

Orphan indication with >30K patients a year (US)1,2 Acute inflammation of the optic nerve impacting Optic nerve & retinal ganglion cells Acute Optic Neuritis An acute inflammation of the optic nerve that can lead to permanent visual impairment in young adults with an average age of 32 years Inflammation in the optic nerve Acute optic neuritis is an inflammation of the optic nerve, resulting in vision loss and pain In optic neuritis, the myelin sheath is damaged or destroyed, leading to axonal and retinal ganglion cell injury and death, and subsequent vision loss High-dose IV steroids can accelerate the process, but they do not change the final outcomes, and no neuroprotective treatment is available Direct link with chronic conditions like multiple sclerosis (MS) and other autoimmune diseases Martínez-Lapiscina EH, et al. (2014): Is the incidence of optic neuritis rising? Evidence from an epidemiological study in Barcelona (Spain) 2008-2012. J Neurol. 2014 Apr; 261(4): 759-767. Weidong Gu et al. (2023) Incidence of Optic Neuritis and the Associated Risk of Multiple Sclerosis for Service Members of U.S. Armed Forces, Military Medicine, vol. 188, March/April 2023 Anatomy of a retinal ganglion cell Nucleus Dendrite Retina Optic nerve Brain Soma Myelin Axon terminal Axon

Privosegtor could be the first neuroprotective therapy to improve vision outcomes Acute Optic Neuritis – U.S. Potential Market High unmet need in rare disease with large market potential Weidong Gu et al. (2023) Incidence of Optic Neuritis and the Associated Risk of Multiple Sclerosis for Service Members of U.S. Armed Forces, Military Medicine, vol. 188, March/April 2023 https://www.medicalmex.com/oxervate-cenegermin-bkbj/ Oxervate pricing in U.S. $96k-$120k https://iovs.arvojournals.org/article.aspx?articleid=2783085 Tepezza pricing in U.S. $386k Active members of the American Academy of Ophthalmology, self-reported subspecialty, EyeNet Media Kit 2025 Acute optic neuritis incidence recently reported in U.S. to be 8.1/100K1 Rare disease without approved therapies often under diagnosed Rare disease price analogs2,3: $100-$400k per treatment Highly concentrated prescriber base: ~450 neuro-ophthalmologists in U.S.4 >30K Estimated U.S. Market Potential Estimated U.S. Incidence >$3B
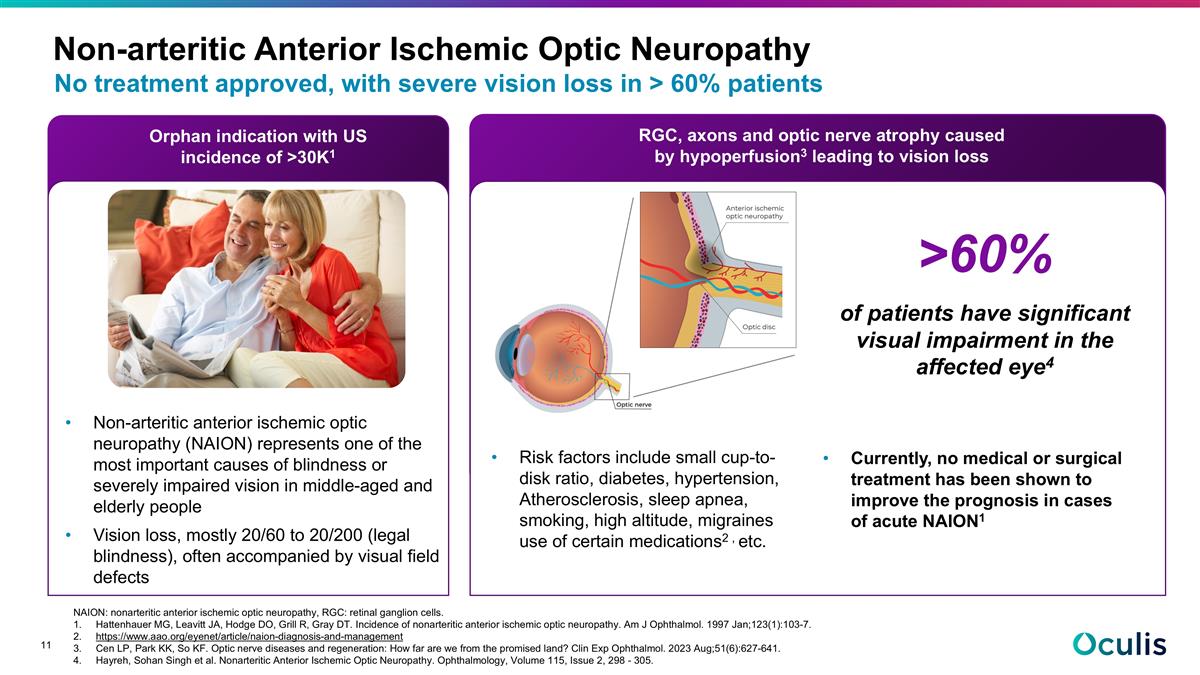
No treatment approved, with severe vision loss in > 60% patients Non-arteritic Anterior Ischemic Optic Neuropathy Orphan indication with US incidence of >30K1 RGC, axons and optic nerve atrophy caused by hypoperfusion3 leading to vision loss Non-arteritic anterior ischemic optic neuropathy (NAION) represents one of the most important causes of blindness or severely impaired vision in middle-aged and elderly people Vision loss, mostly 20/60 to 20/200 (legal blindness), often accompanied by visual field defects Currently, no medical or surgical treatment has been shown to improve the prognosis in cases of acute NAION1 Risk factors include small cup-to-disk ratio, diabetes, hypertension, Atherosclerosis, sleep apnea, smoking, high altitude, migraines use of certain medications2 , etc. NAION: nonarteritic anterior ischemic optic neuropathy, RGC: retinal ganglion cells. Hattenhauer MG, Leavitt JA, Hodge DO, Grill R, Gray DT. Incidence of nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1997 Jan;123(1):103-7. https://www.aao.org/eyenet/article/naion-diagnosis-and-management Cen LP, Park KK, So KF. Optic nerve diseases and regeneration: How far are we from the promised land? Clin Exp Ophthalmol. 2023 Aug;51(6):627-641. Hayreh, Sohan Singh et al. Nonarteritic Anterior Ischemic Optic Neuropathy. Ophthalmology, Volume 115, Issue 2, 298 - 305. >60% of patients have significant visual impairment in the affected eye4

Privosegtor could be the first neuroprotective therapy to improve vision outcomes NAION – U.S. Potential Market Significant opportunity for novel therapy to improve the current poor prognosis in NAION Incidence of nonarteritic anterior ischemic optic neuropathy – PubMed and Incidence of nonarteritic anterior ischemic optic neuropathy: increased risk among diabetic patients – PMC and discussions with experts https://www.medicalmex.com/oxervate-cenegermin-bkbj/ Oxervate pricing in U.S. $96k-$120k https://iovs.arvojournals.org/article.aspx?articleid=2783085 Tepezza pricing in U.S. $386k Active members of the American Academy of Ophthalmology, self-reported subspecialty, EyeNet Media Kit 2025 U.S. incidence up to 10.2 per 100K1 Rare disease without approved therapies often under diagnosed Rare disease price analogs2,3: $100-$400k per treatment Highly concentrated prescriber base: ~450 neuro-ophthalmologists in U.S.4 >30K Estimated U.S. Market Potential Estimated U.S. Incidence >$4B
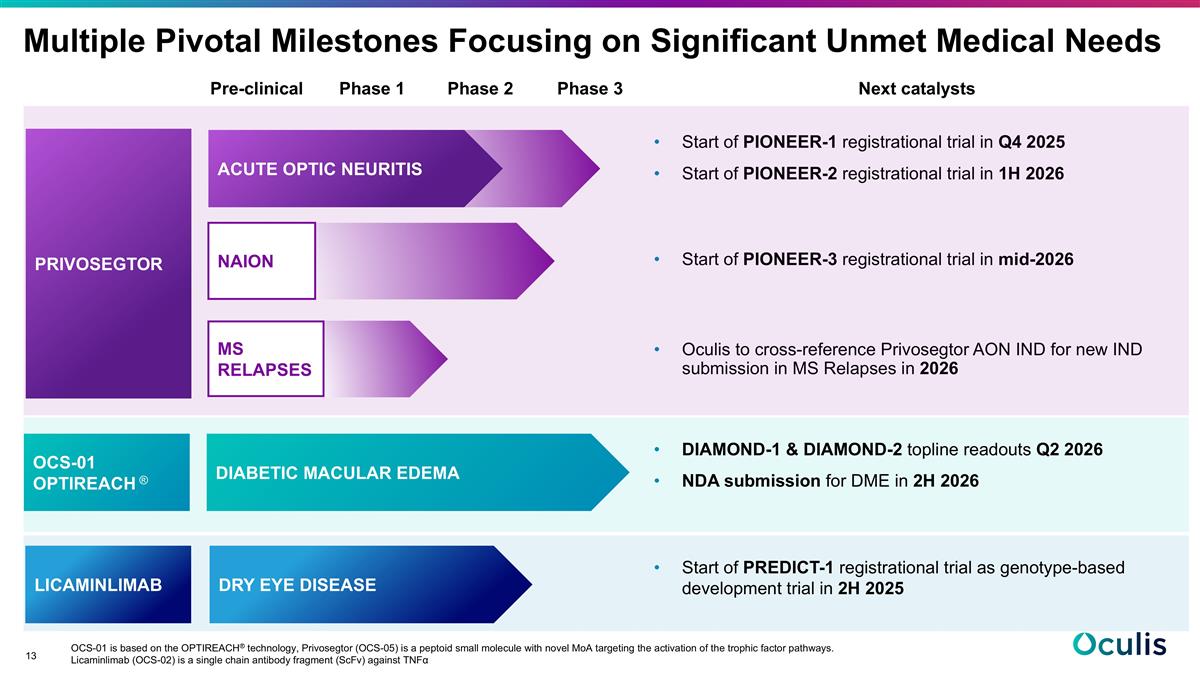
Pre-clinical Phase 1 Phase 2 Phase 3 Next catalysts Start of PIONEER-1 registrational trial in Q4 2025 Start of PIONEER-2 registrational trial in 1H 2026 Start of PIONEER-3 registrational trial in mid-2026 Oculis to cross-reference Privosegtor AON IND for new IND submission in MS Relapses in 2026 DIAMOND-1 & DIAMOND-2 topline readouts Q2 2026 NDA submission for DME in 2H 2026 Start of PREDICT-1 registrational trial as genotype-based development trial in 2H 2025 OCS-01 is based on the OPTIREACH® technology, Privosegtor (OCS-05) is a peptoid small molecule with novel MoA targeting the activation of the trophic factor pathways. Licaminlimab (OCS-02) is a single chain antibody fragment (ScFv) against TNFα Multiple Pivotal Milestones Focusing on Significant Unmet Medical Needs LICAMINLIMAB DIABETIC MACULAR EDEMA DRY EYE DISEASE OCS-01 OPTIREACH ® PRIVOSEGTOR ACUTE OPTIC NEURITIS MS RELAPSES NAION

Oculis Pipeline Development Strategic Evolution ~$10B1,2 Advance OCS-01 would be the first-ever topical treatment in DME, if approved Licaminlimab as personalized medicine in DED Privosegtor neuroprotection in Acute Optic Neuritis +$25B1-3 Expand Privosegtor in Neuro-ophthalmology and beyond NAION MS relapses +$50B1-5 Explore Potential for Privosegtor in multiple additional neuro-ophthalmology and neurology conditions DME diabetic macular edema, DED: dry eye disease, NAION: Nonarteritic Anterior Ischemic Optic Neuropathy MS: Multiple Sclerosis. 1. DR and DME Disease and Landscape report Nov. 2020 – 2024 market value estimate for G7, 2. DED Disease and Landscape report 2020 - 2024 market value estimate for G7, 3. MS Disease and Landscape report October 2024 – 2024 market value estimate for G7, 4. Optic nerve disorders, Transparency Market Research, 5. Global Market Insights, March 2024 https://www.gminsights.com/industry-analysis/neuroprotection-market OPHTHA OPHTHALMOLOGY NEURO-OPHTHALMOLOGY & BEYOND

Thank you