UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): September 29, 2025
Larimar Therapeutics, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 001-36510 | 20-3857670 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
| Three Bala Plaza East, Suite 506 | ||
| Bala Cynwyd, Pennsylvania | 19004 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (844) 511-9056
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange on which registered |
||
| Common Stock, par value $0.001 per share | LRMR | Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 8.01 | Other Events. |
Press Release
On September 29, 2025, Larimar Therapeutics, Inc. (the “Company”) issued a press release announcing positive 25 mg and 50 mg data from the Company’s ongoing long-term open label study evaluating daily subcutaneous injections of nomlabofusp self-administered or administered by a caregiver in participants with Friedreich’s ataxia. A copy of the press release is attached as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
Investor Presentation
On September 29, 2025, the Company will host a conference call to discuss a regulatory update and use a slide presentation in conjunction with the call. A copy of the presentation is filed herewith as Exhibit 99.2, and incorporated herein by reference.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
Below is a list of exhibits included with this Current Report on Form 8-K.
| Exhibit |
Document |
|
| 99.1 | Press Release issued by Larimar Therapeutics, Inc. on September 29, 2025* | |
| 99.2 | Larimar Therapeutics, Inc. Conference Call Presentation, dated September 29, 2025* | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |
| * | Filed herewith |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Larimar Therapeutics, Inc. | ||
| By: | /s/ Carole S. Ben-Maimon, M.D. |
|
| Name: | Carole S. Ben-Maimon, M.D. | |
| Title: | President and Chief Executive Officer | |
Date: September 29, 2025
Exhibit 99.1

Larimar Therapeutics Announces Positive Data from Ongoing Long-term Open Label Study and Updates to Nomlabofusp Program for Friedreich’s Ataxia
| • | In 4 completed studies and the ongoing OL study, 65 participants received at least 1 dose of nomlabofusp, including 39 in the OL study, with 14 on treatment for at least 6 months and 8 for over 1 year in the OL study |
| • | Increases in skin FXN levels with short- and long-term daily nomlabofusp; 10/10 participants with data at 6 months achieved skin FXN levels over 50% of median levels in healthy volunteers (which is similar to levels in asymptomatic carriers) |
| • | Consistent directional improvement across 4 key clinical outcomes (mFARS, FARS-ADL, 9-HPT, MFIS) observed after 1 year of nomlabofusp treatment could suggest potential for clinical benefit relative to a worsening in a FACOMS natural history study reference population |
| • | Anaphylaxis has been reported in 7 participants in the OL study, with most events occurring on the initial day of administration and all occurring within the first 6 weeks of dosing; excluding these events, long term dosing of nomlabofusp was generally well tolerated |
| • | Following the 2 most recent cases of anaphylaxis, Larimar consulted its experts and decided to modify its starting dose regimen; Larimar provided the FDA a full update on the clinical development program including the safety, FXN, and clinical data in this press release and FDA agreed with our approach |
| • | BLA submission seeking accelerated approval targeted in Q2 2026 |
| • | Company management to host webcast and conference call today at 8:00 a.m. ET |
Bala Cynwyd, PA, September 29, 2025 – Larimar Therapeutics, Inc. (Larimar) (Nasdaq: LRMR), a clinical-stage biotechnology company focused on developing treatments for complex rare diseases, today announced positive 25 mg and 50 mg data from the ongoing long-term open label (OL) study evaluating daily subcutaneous injections of nomlabofusp self-administered or administered by a caregiver in participants with Friedreich’s ataxia (FA), a rare, progressive, and systemic disease with neurologic deterioration. The Company also provided a nomlabofusp development program update.
“We are excited to announce the consistent directional improvements across 4 key clinical outcomes observed in the OL study relative to a Friedrich’s Ataxia Clinical Outcomes Measure Study (FACOMS) reference population and the observed increase in skin frataxin (FXN) levels. These new data, as well as the improvement in abnormal lipid profiles observed in prior completed studies, provide support that nomlabofusp increases FXN in patients with FA and that the strategy of FXN replacement has the potential to result in a clinical benefit. Importantly, achieving tissue FXN levels equivalent to more than 50% of those found in healthy volunteers means participants are at levels found in asymptomatic carriers who do not develop the disease,” said Carole Ben-Maimon, MD, President, and Chief Executive Officer of Larimar. “Long-term treatment with daily nomlabofusp, including 8 participants for over 1 year, was generally well-tolerated. Through the Support for Clinical Trials Advancing Rare Disease Therapeutics (START) pilot program, Larimar has regularly updated the FDA regarding all aspects of the clinical development program, including the data presented in this press release. We continue to hear strong interest in the nomlabofusp clinical program directly from patients with FA and their parents. The long-term clinical data presented today reinforce our conviction in the potential of nomlabofusp to address the root cause of FA and be the first potential disease modifying therapy.”
Dr. Rusty Clayton, Chief Medical Officer of Larimar added, “The changes observed in skin FXN levels, lipid profiles, and clinical outcomes after nomlabofusp administration across diverse participants with FA – including individuals with advanced disease – are all directionally consistent and suggest a potential treatment effect. Allergic reactions, including anaphylaxis, are a known risk associated with nomlabofusp, similar to many other approved therapies, particularly proteins. To date, all anaphylaxis events have occurred within the first 6 weeks of nomlabofusp administration. In this rare neurodegenerative disease with limited therapeutic options, patients with FA continue to express interest in having access to new potentially disease modifying agents.”
Jennifer Farmer, Chief Executive Officer of the Friedreich’s Ataxia Research Alliance (FARA) added, “FA is a relentlessly progressive disease that is life-altering and can be life-shortening. Treatment approaches, like nomlabofusp, that target the root cause of FA by FXN supplementation are of great interest to the FA community. We are encouraged by the increases in FXN protein and improved clinical outcomes relative to the FACOMS reference population observed in the individuals who have maintained nomlabofusp therapy. FA patients and their families are informed and engaged. They understand that therapies come with side effects and risks that must be evaluated in the context of potential benefit. We appreciate Larimar’s commitment to patient safety and their regular communications and updates on study outcomes.”
The OL study is evaluating the safety and tolerability, pharmacokinetics (PK), and FXN levels in skin and buccal cells, along with exploratory pharmacodynamic (PD) markers (lipid profiles) and clinical outcomes following long-term subcutaneous administration of nomlabofusp. Participants were initially administered 25 mg of nomlabofusp daily. The dose was increased to 50 mg in the fourth quarter of 2024, with all newly enrolled patients receiving the 50 mg dose since November of 2024. Participants who completed treatment in a Phase 1 or Phase 2 study evaluating nomlabofusp were the first group of eligible patients to screen for the OL study. The OL study protocol has now been amended to include adolescent and adult patients who have not participated in a prior nomlabofusp study.
As of August 27, 2025, 39 participants in the OL study had received at least one dose of nomlabofusp and 25 participants (19 adults, 6 adolescents) were receiving daily dosing of nomlabofusp for up to 527 days (mean 154 days). This includes the time from the initial dose of 25 or 50 mg to the last dose of nomlabofusp prior to the data cut off. Among the study participants, approximately 50% were non-ambulatory at baseline.
Long-term Safety with Daily Nomlabofusp in OL Study
| • | In participants receiving long term continuous treatment, including 14 participants on nomlabofusp for at least 6 months, 8 of whom continue to be on nomlabofusp for over 1 year, daily administration of nomlabofusp was generally well-tolerated. |
| • | Most common adverse events continue to be local injection site reactions that were mild to moderate and did not lead to any participant withdrawal from the study. |
| • | 65 patients have received at least one dose of nomlabofusp in 4 completed studies and the ongoing OL study. Seven OL study participants experienced anaphylaxis and were withdrawn from the study.1 Most of the events occurred on the initial day of administration and all occurred within the first 6 weeks of dosing; all participants returned to their usual state of health after receiving standard treatment. |
| • | Larimar consulted its experts and decided to modify its starting dose regimen. Larimar provided the FDA with a full update on the clinical development program including the safety, FXN, and clinical data in this press release and FDA agreed with our proposal. |
| 1 | Other discontinuations include 3 cases of generalized urticaria, 1 seizure (the same event as reported in December 2024), 1 vasovagal event, and 2 non-treatment related discontinuations |
Observed Increases in FXN with Long-term Daily Nomlabofusp in All Participants
|
Absolute Median Skin FXN Levels pg/µg (IQR), n*
|
||||||
|
Baseline
|
1 month
|
3 months
|
6 months
|
|||
| 2.70 (2.14, 4.13), n = 18 |
6.87 (5.34, 10.37), n = 18 |
7.50 (6.99, 13.73), n = 14 |
13.44 (10.10, 26.71), n = 10 |
|||
FXN = frataxin; IQR = interquartile range
Note: Median skin FXN levels in Larimar’s noninterventional healthy volunteer study= 16.34 pg/µg
| * | Data include all participants with quantifiable FXN levels at each measurement point who had received 25 mg, 50 mg or had the dose increased from 25 mg to 50 mg |
All Participants who Received Nomlabofusp for 6 months Achieved Skin FXN Levels Similar to Levels Found in Asymptomatic Carriers without Disease
|
Percentage of Participants* with Skin FXN Levels > 8.2 pg/µg** (50% of the median FXN concentration found in Larimar’s healthy volunteer study)
|
||||||
|
Baseline
|
1 month
|
3 months
|
6 months
|
|||
| 0% (0/18) | 33% (6/18) | 43% (6/14) | 100% (10/10) | |||
| * | Data include all participants with quantifiable FXN levels at each measurement point who had received 25 mg, 50 mg or had the dose increased from 25 mg to 50 mg |
| ** | 8.2 pg/µg represents 50% of the median FXN concentration |
Consistent Directional Improvement Across 4 Key Clinical Outcomes
| • | Trends towards improvement were observed in modified Friedreich Ataxia Rating Scale (mFARS), FARS-Activities of Daily Living (ADL), 9 Hole Peg Test (9-HPT), and Modified Fatigue Impact Scale (MFIS) at 1 year relative to baseline |
| • | These clinical findings support that FXN increases after treatment with nomlabofusp may lead to a potential clinical benefit across a broad spectrum of patients with FA, including those with advanced disease |
OL Study Clinical Data Relative to FA Natural History Study Data Supports Potential for Clinical Benefit with Nomlabofusp
| • | Friedreich’s Ataxia Clinical Outcome Measures Study (FACOMS), a longitudinal natural history study (N = 955), includes patients with confirmed FA diagnosis |
| • | Based on the range of baseline characteristics of participants in the OL study, Larimar identified patients from the FACOMS dataset with similar characteristics using data recorded over the last 4 years for each patient |
| • | mFARS has been used as a primary outcome measure in other clinical trials. OL study participants treated for 1 year with nomlabofusp daily demonstrated a median improvement in mFARS score of 2.25 relative to a worsening of 1.00 observed in patients in the FACOMS reference population. Directional improvements in the other three clinical outcomes (FARS-ADL, 9-HPT, MFIS) were also observed in OL study participants, while worsening in these outcomes was observed in the FACOMS reference population. |
|
Median (IQR) Clinical Outcome Measure Change from Baseline at 1 year
|
||||||||||||||
|
mFARS [0- 93]
|
FARS-ADL [0- 36]
|
9-HPT: Dominant Hand
|
MFIS [0- 84]
|
|||||||||||
|
Nomlabofusp n = 8 |
FACOMS n = 185 |
Nomlabofusp n = 8 |
FACOMS n = 237 |
Nomlabofusp n = 7 |
FACOMS n = 219 |
Nomlabofusp n = 8 |
FACOMSa n = 136 |
|||||||
| -2.25 (-3.8, -0.3) |
1.00 (-1.5, 4.0) |
-0.50 (-2.0, 1.0) |
0.50 (-1.0, 2.5) |
-7.40 (-38.8, -2.5) |
3.4 (-4.5, 18.0) |
-6.5 (-17.5, 4.0) |
1.5 (-9.5, 11.0) |
|||||||
IQR = interquartile range
aMFIS presented here is at 2 years because it was not assessed at 1 year
Long-term Pharmacokinetic Profile Consistent with Prior Studies
| • | Rapid absorption after subcutaneous administration |
| • | Exposure appeared to reach steady state in plasma by Day 30 at both the 25 mg and 50 mg doses with no further accumulation |
| • | Pharmacokinetic profile consistent with Phase 1 and Phase 2 studies |
Nomlabofusp Program Updates
| • | Informative Adolescent PK Run-In Data: Adolescents 12 to 17 years of age received a weight-based equivalent of 50 mg for 7 days. Exposure and PK in adolescents spanning 12 to 17 years of age (n = 14, 5 on placebo) were similar to adults on 50 mg of nomlabofusp. Six adolescents are currently enrolled in the OL study |
| • | Planned Enrollment in OL Study: The OL study protocol was amended to include adolescent and adult patients who have not participated in a prior nomlabofusp study. Recently, Larimar modified the starting dose regimen and is implementing this change. The new dosing regimen will include a 5 mg test dose followed by a 25 mg dose one hour later under observation. Nomlabofusp 25 mg will then be administered once daily through Day 30 and then the dose will be increased to 50 mg once daily. Larimar plans to enroll patients 2-11 years of age directly into the OL study in the future |
| • | Global Phase 3 Study: Sites are identified and being qualified in the U.S., Europe, U.K., Canada, and Australia |
| • | Developments in Chemistry Manufacturing and Control (CMC): Received agreement from FDA on analytical testing requirements including potency testing of nomlabofusp. Process performance qualification (PPQ) on the commercial scale drug substance is planned in Q4 2025, in preparation of data for BLA submission. Drug substance manufactured during PPQ activities are expected to be used as the initial commercial launch supply |
Key Upcoming Milestones
| • | Implement the new dosing regimen in the OL study in Q4 2025 |
| • | PPQ on commercial scale drug substance planned in Q4 2025 |
| • | BLA submission seeking accelerated approval targeted in Q2 2026 |
Conference Call and Webcast
Larimar will host a conference call and webcast today, September 29, 2025, at 8:00 a.m. EDT. To access the webcast, please visit this link to the event. To participate by phone, please dial 1-877-407-9716 (domestic) or 1-201-493-6779 (international) and refer to conference ID 13756144 or click on this link and request a return call. Following the live event, an archived webcast will be available on the “Events & Presentations” page of the Larimar website.
About Larimar Therapeutics
Larimar Therapeutics, Inc. (Nasdaq: LRMR), is a clinical-stage biotechnology company focused on developing treatments for complex rare diseases. Larimar’s lead compound, nomlabofusp, is being developed as a potential treatment for Friedreich’s ataxia. Larimar also plans to use its intracellular delivery platform to design other fusion proteins to target additional rare diseases characterized by deficiencies in intracellular bioactive compounds. For more information, please visit: https://larimartx.com.
Forward-Looking Statements
This press release contains forward-looking statements that are based on Larimar’s management’s beliefs and assumptions and on information currently available to management. All statements contained in this release other than statements of historical fact are forward-looking statements, including but not limited to statements regarding Larimar’s ability to develop and commercialize nomlabofusp and other planned product candidates, Larimar’s planned research and development efforts, including the timing of its nomlabofusp clinical trials, interactions and filings with the FDA, expectations regarding potential for accelerated approval or accelerated access and time to market and overall development plan and other matters regarding Larimar’s business strategies, ability to raise capital, use of capital, results of operations and financial position, and plans and objectives for future operations.
In some cases, you can identify forward-looking statements by the words “may,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These statements involve risks, uncertainties and other factors that may cause actual results, performance, or achievements to be materially different from the information expressed or implied by these forward-looking statements. These risks, uncertainties and other factors include, among others, the success, cost and timing of Larimar’s product development activities, nonclinical studies and clinical trials, including nomlabofusp clinical and regulatory milestones and continued interactions with the FDA, and Larimar’s ability to timely implement the revised dosing regimen in its clinical program for nomlabofusp; that preliminary clinical trial results may differ from final clinical trial results, that earlier non-clinical and clinical data and testing of nomlabofusp may not be predictive of the results or success of later clinical trials, and assessments; that the FDA may not ultimately agree with Larimar’s nomlabofusp development strategy; the potential impact of public health crises on Larimar’s future clinical trials, manufacturing, regulatory, nonclinical study timelines and operations, and general economic conditions; Larimar’s ability and the ability of third-party manufacturers Larimar engages, to optimize and scale nomlabofusp’s manufacturing process; Larimar’s ability to obtain regulatory approvals for nomlabofusp and future product candidates; Larimar’s ability to develop sales and marketing capabilities, whether alone or with potential future collaborators, and to successfully commercialize any approved product candidates; Larimar’s ability to raise the necessary capital to conduct its product development activities; and other risks described in the filings made by Larimar with the Securities and Exchange Commission (SEC), including but not limited to Larimar’s periodic reports, including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the SEC and available at www.sec.gov. These forward-looking statements are based on a combination of facts and factors currently known by Larimar and its projections of the future, about which it cannot be certain. As a result, the forward-looking statements may not prove to be accurate. The forward-looking statements in this press release represent Larimar’s management’s views only as of the date hereof. Larimar undertakes no obligation to update any forward-looking statements for any reason, except as required by law.
Investor Contact:
Joyce Allaire
LifeSci Advisors
jallaire@lifesciadvisors.com
(212) 915-2569
Company Contact:
Michael Celano
Chief Financial Officer
mcelano@larimartx.com
(484) 414-2715

pro September 2025 Larimar Therapeutics Program Update Exhibit 99.2

Forward-Looking Statements This presentation contains forward-looking statements that are based on the beliefs and assumptions of Larimar Therapeutics, Inc. ( “Company”) and on information currently available to management. All statements contained in this presentation other than statements of historical fact are forward-looking statements, including but not limited to Larimar’s ability to develop and commercialize nomlabofusp (CTI-1601) and any other planned product candidates, Larimar’s planned research and development efforts, including the timing of its nomlabofusp clinical trials and non-clinical investigations and overall development plan expectations with respect to the FDA START pilot program, interactions with FDA, expectations regarding potential for accelerated approval or accelerated access and time to market and other matters regarding Larimar’s business strategies, ability to raise capital, use of capital, results of operations and financial position, and plans and objectives for future operations. In some cases, you can identify forward-looking statements by the words “may,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These statements involve risks, uncertainties and other factors that may cause actual results, performance, or achievements to be materially different from the information expressed or implied by these forward-looking statements. These risks, uncertainties and other factors include, among others, the success, cost and timing of Larimar’s product development activities, nonclinical studies and clinical trials, including nomlabofusp clinical milestones and continued interactions with the FDA, and Larimar’s ability to timely implement the revised dosing regimen in its clinical program for nomlabofusp; that preliminary clinical trial results may differ from final clinical trial results, that earlier non-clinical and clinical data and testing of nomlabofusp may not be predictive of the results or success of later non-clinical or clinical trials, and assessments; delays in patient recruitment, including as a result of changes in clinical protocols and adverse events; that the FDA may not ultimately agree with Larimar’s nomlabofusp development strategy; the potential impact of public health crises on Larimar’s future clinical trials, manufacturing, regulatory, nonclinical study timelines and operations, and general economic conditions; Larimar’s ability and the ability of third-party manufacturers Larimar engages, to optimize and scale nomlabofusp’s manufacturing process; Larimar’s ability to obtain regulatory approvals for nomlabofusp and future product candidates; Larimar’s ability to develop sales and marketing capabilities, whether alone or with potential future collaborators, and to successfully commercialize any approved product candidates; Larimar’s ability to raise the necessary capital to conduct its product development activities; and other risks described in the filings made by Larimar with the Securities and Exchange Commission (SEC), including but not limited to Larimar’s periodic reports, including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the SEC and available at www.sec.gov. These forward-looking statements are based on a combination of facts and factors currently known by Larimar and its projections of the future, about which it cannot be certain. As a result, the forward-looking statements may not prove to be accurate. The forward-looking statements in this presentation represent Larimar’s management’s views only as of the date hereof. Larimar undertakes no obligation to update any forward-looking statements for any reason, except as required by law.

Initial 50 mg OL Study Data Supports Therapeutic Potential of Nomlabofusp Increased skin FXN levels and consistent directional improvement in clinical outcomes Long-term safety observations with daily nomlabofusp 100% of participants with data at 6 months achieved skin FXN levels over 50% of median levels in healthy volunteers (which is similar to levels in asymptomatic carriers) mFARS (primary outcome measure in other clinical studies) median score improvement of 2.25 in OL study participants after 1 year relative to a worsening of a median 1.00 observed in FACOMS natural history reference population Consistent directional improvements observed after 1 year across 4 key clinical outcomes (mFARS, FARS-ADL, 9-HPT, MFIS) suggest a potential for clinical benefit relative to a worsening in the FACOMS reference population Of 39 participants in OL study (65 total participants who received at least 1 dose in all nomlabofusp studies), 7 experienced anaphylaxis within the first 6 weeks of dosing; all returned to usual state of health after standard treatment Following the 2 most recent cases of anaphylaxis, Larimar consulted its experts and decided to modify its starting dose regimen. Larimar provided FDA a full data update on the clinical development program and FDA agreed with our approach Nomlabofusp was generally well tolerated with long-term daily dosing including 14 on treatment for at least 6 months and 8 for over 1 year; most common AEs were mild/moderate local ISRs and did not lead to any withdrawals FXN: frataxin; FACOMS: Friedreich’s Ataxia Clinical Outcome Measures Study; AE: Adverse events; ISRs: Injection site reactions; BLA: Biologics License Application *Pro forma cash and investments reflect our $138.5 M of cash and investments as of June 30, 2025, combined with the $65.1 M in net proceeds from our recently completed July 2025 public offering. Continue targeting BLA submission Q2 2026 The BLA will pursue accelerated approval based on increases in skin FXN levels as further demonstrated in this data Based on these compelling data, we continue to target the BLA filing for Q2 2026 and believe that nomlabofusp could be the first disease modifying therapy for patients with FA $203.6 million in pro forma* cash and investments as of June 30, 2025, with projected cash runway into Q4 2026

Friedreich’s Ataxia (FA): A rare and progressive disease * E.C. Deutsch et al. Molecular Genetics and Metabolism 101 (2010) 238–245. Genetic defect on both alleles lowers frataxin levels Affects ~20,000 patients globally Progressive disease Unmet Medical Need Larimar is developing nomlabofusp, the first potential disease modifying therapy designed to systemically address the underlying FXN deficiency in FA Most patients with FA only produce ~20-40% of normal frataxin (FXN) levels depending on the tissue, sampling technique, and assay considered* Initial symptoms include unsteady posture and frequent falling, and patients are eventually confined to a wheelchair Life expectancy of 30-50 years with an early death usually caused by heart disease ~5,000 patients in the U.S., with most remaining patients in Europe ~70% of patients present before age 14 The only treatment currently approved for FA does not address frataxin deficiency
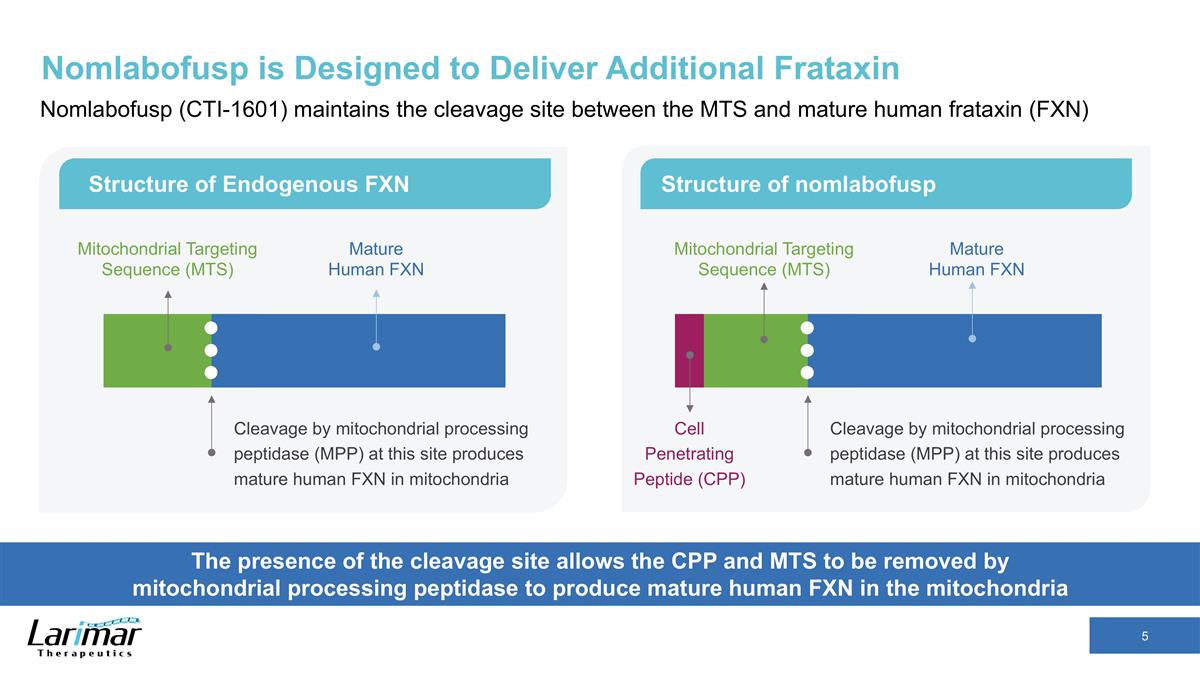
Nomlabofusp is Designed to Deliver Additional Frataxin Nomlabofusp (CTI-1601) maintains the cleavage site between the MTS and mature human frataxin (FXN) The presence of the cleavage site allows the CPP and MTS to be removed by mitochondrial processing peptidase to produce mature human FXN in the mitochondria Structure of Endogenous FXN Structure of nomlabofusp Cleavage by mitochondrial processing peptidase (MPP) at this site produces mature human FXN in mitochondria Mitochondrial Targeting Sequence (MTS) Mature Human FXN Cleavage by mitochondrial processing peptidase (MPP) at this site produces mature human FXN in mitochondria Mature Human FXN Cell Penetrating Peptide (CPP) Mitochondrial Targeting Sequence (MTS)
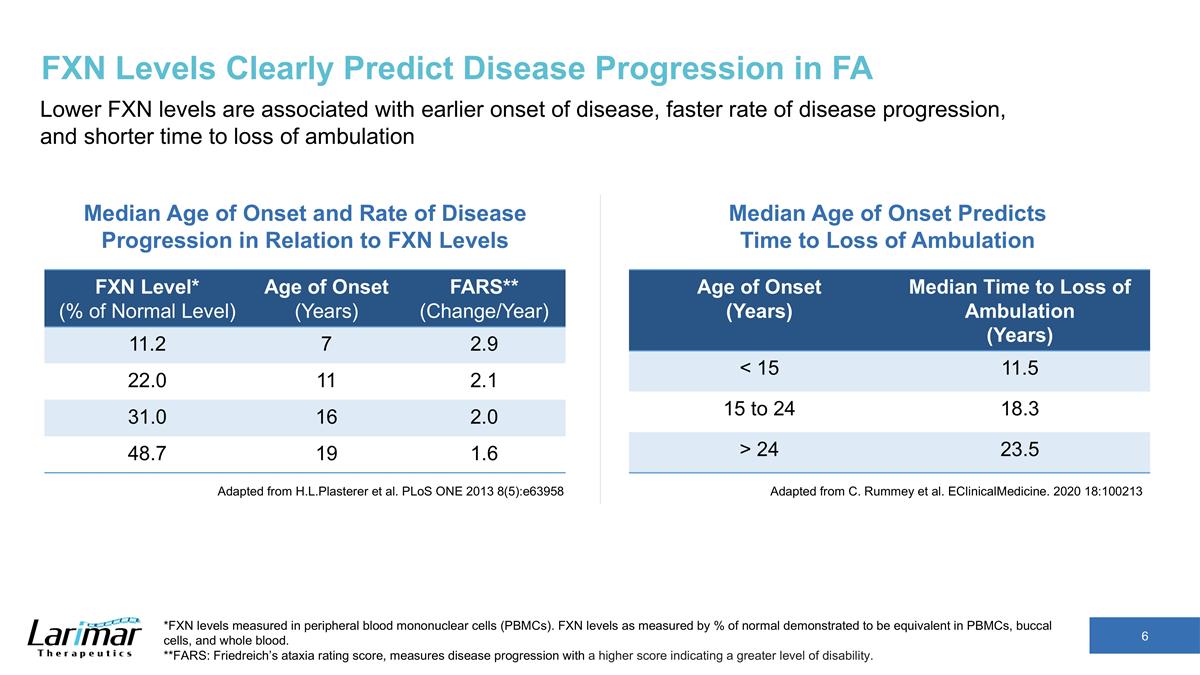
FXN Levels Clearly Predict Disease Progression in FA Lower FXN levels are associated with earlier onset of disease, faster rate of disease progression, and shorter time to loss of ambulation Adapted from H.L.Plasterer et al. PLoS ONE 2013 8(5):e63958 Age of Onset (Years) Median Time to Loss of Ambulation (Years) < 15 11.5 15 to 24 18.3 > 24 23.5 Median Age of Onset and Rate of Disease Progression in Relation to FXN Levels *FXN levels measured in peripheral blood mononuclear cells (PBMCs). FXN levels as measured by % of normal demonstrated to be equivalent in PBMCs, buccal cells, and whole blood. **FARS: Friedreich’s ataxia rating score, measures disease progression with a higher score indicating a greater level of disability. FXN Level* (% of Normal Level) Age of Onset (Years) FARS** (Change/Year) 11.2 7 2.9 22.0 11 2.1 31.0 16 2.0 48.7 19 1.6 Adapted from C. Rummey et al. EClinicalMedicine. 2020 18:100213 Median Age of Onset Predicts Time to Loss of Ambulation

Expanding Open Label Study*: Now Includes Adolescents and Participants not in Prior Nomlabofusp Studies Initially, participation in a prior Phase 1 or Phase 2 trial required Expanded study criteria to include: Adolescents (12-17 yrs) from the PK run-in study Participants not in prior studies Plan to enroll children (2 to 11 yrs) directly in study Key Study Objectives Safety and tolerability Long-term PK Skin FXN concentrations Clinical efficacy measures compared to FACOMS** database using propensity matching at the time of BLA *Due to inclusion of participants who have not participated in prior nomlabofusp clinical studies, this study is now referred to as Open Label Study (previously called the Open Label Extension study) **FACOMS: Friedreich’s Ataxia Clinical Outcome Measures Study Daily subcutaneous injections self-administered or by a caregiver 25 mg nomlabofusp 50 mg nomlabofusp*** Participants switched from 25 mg to 50 mg dose from Nov 2024 to Q1 2025 Patient Population ***Following the 2 most recent cases of anaphylaxis, Larimar consulted its experts and decided to modify its starting dose regimen. Larimar provided the FDA a full data update on the clinical development program and FDA agreed with our approach Antihistamines 5 days prior to first dose and for 90 days after first dose 5 mg test dose followed by a 25 mg dose 1 hour later and then 25 mg daily for the first 30 days; after 30 days the dose will be increased to 50 mg once daily

Median Change from Baseline in Skin FXN Levels* \ * Median Skin FXN Levels* Increases in Skin FXN Levels are Sustained Over Time 100% of Participants at Day 180 had Skin FXN Levels >50% of Healthy Volunteers Dotted Line indicates 50% of healthy volunteers FXN level *FXN levels measured via detection of peptide derived from mature FXN; FXN concentrations are normalized to total cellular protein content in each sample. Data represent median and 25th and 75th percentiles. Data include all participants with quantifiable FXN levels at baseline and at least 1 post-baseline FXN level.

100% of OL Study Participants on Nomlabofusp Achieved FXN Levels by Day 180 that are Over 50% of Healthy Volunteers Percentage of Participants* with Skin FXN Levels > 8.2 pg/µg** (50% of the median FXN concentration found in healthy volunteers) Baseline Day 30 Day 90 Day 180 0% 0/18 33% 6/18 43% 6/14 100% 10/10 *Data include all participants with quantifiable levels at each measurement point who had received 25 mg, 50 mg or had the dose increased from 25 mg to 50 mg **8.2 pg/µg represents 50% of the median FXN concentration

Skin FXN Levels Achieve Higher % of Healthy Volunteers’ FXN Levels* Following Daily Nomlabofusp % of Average HV Baseline < 12.5% 12.5 ≤ 25% 25% ≤ 37.5% > 37.5% *% of average healthy volunteers (HV) FXN level is calculated by dividing each participant's FXN level by the average FXN level (16.34 pg/µg) from the noninterventional healthy volunteer study (N=60) Data include all participants with quantifiable FXN levels at baseline and Day 90/Day 180 Baseline as a percentage of average FXN level in HV FXN levels increased from baseline and reached > 50% of average FXN level in HV FXN levels increased from baseline and reached 25% to < 50% of average FXN level in HV Day 90 Baseline Day 180
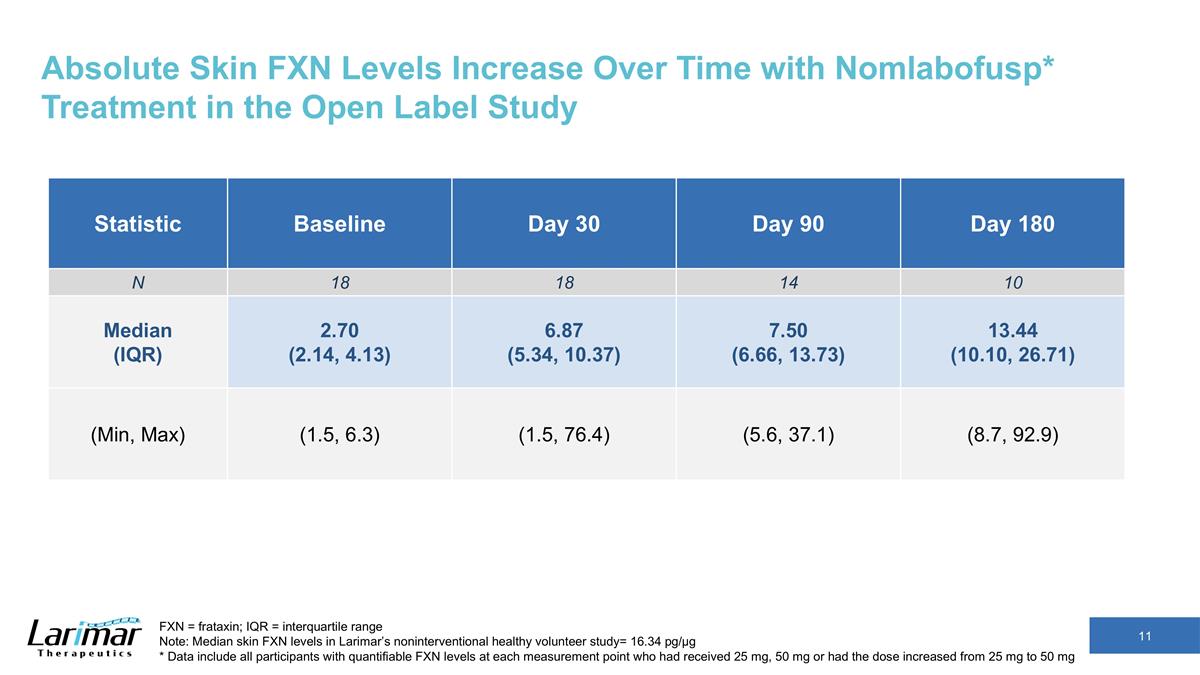
Absolute Skin FXN Levels Increase Over Time with Nomlabofusp* Treatment in the Open Label Study Statistic Baseline Day 30 Day 90 Day 180 N 18 18 14 10 Median (IQR) 2.70 (2.14, 4.13) 6.87 (5.34, 10.37) 7.50 (6.66, 13.73) 13.44 (10.10, 26.71) (Min, Max) (1.5, 6.3) (1.5, 76.4) (5.6, 37.1) (8.7, 92.9) FXN = frataxin; IQR = interquartile range Note: Median skin FXN levels in Larimar’s noninterventional healthy volunteer study= 16.34 pg/µg * Data include all participants with quantifiable FXN levels at each measurement point who had received 25 mg, 50 mg or had the dose increased from 25 mg to 50 mg
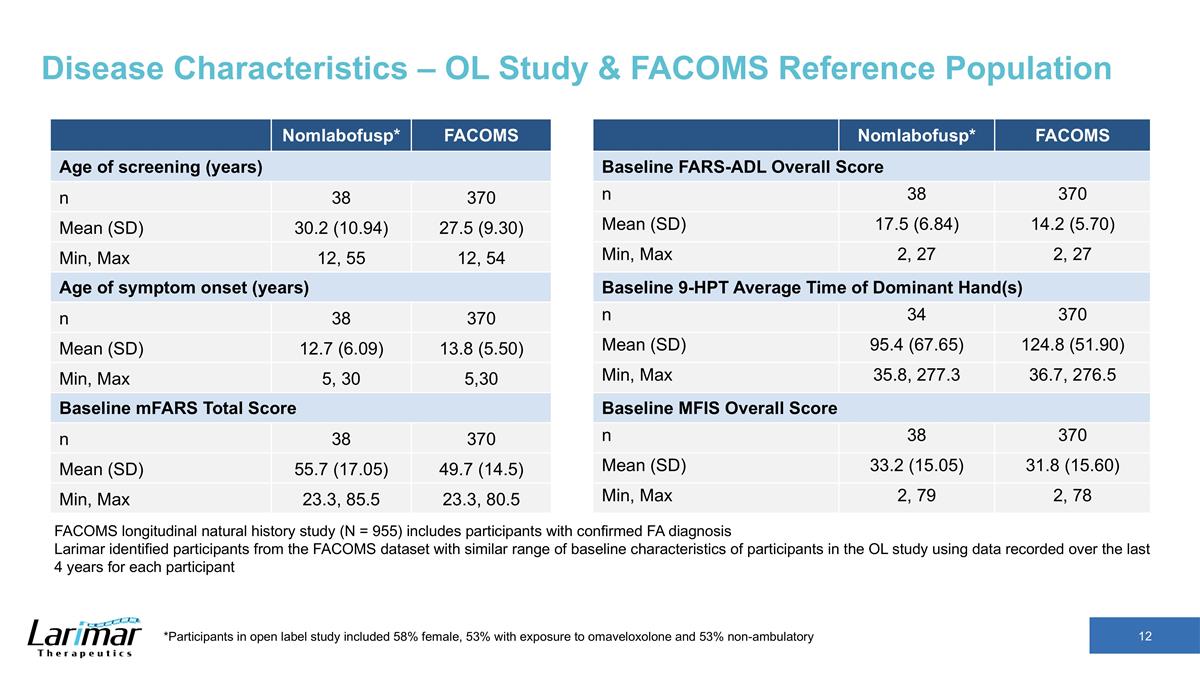
Disease Characteristics – OL Study & FACOMS Reference Population Nomlabofusp* FACOMS Age of screening (years) n 38 370 Mean (SD) 30.2 (10.94) 27.5 (9.30) Min, Max 12, 55 12, 54 Age of symptom onset (years) n 38 370 Mean (SD) 12.7 (6.09) 13.8 (5.50) Min, Max 5, 30 5,30 Baseline mFARS Total Score n 38 370 Mean (SD) 55.7 (17.05) 49.7 (14.5) Min, Max 23.3, 85.5 23.3, 80.5 Nomlabofusp* FACOMS Baseline FARS-ADL Overall Score n 38 370 Mean (SD) 17.5 (6.84) 14.2 (5.70) Min, Max 2, 27 2, 27 Baseline 9-HPT Average Time of Dominant Hand(s) n 34 370 Mean (SD) 95.4 (67.65) 124.8 (51.90) Min, Max 35.8, 277.3 36.7, 276.5 Baseline MFIS Overall Score n 38 370 Mean (SD) 33.2 (15.05) 31.8 (15.60) Min, Max 2, 79 2, 78 FACOMS longitudinal natural history study (N = 955) includes participants with confirmed FA diagnosis Larimar identified participants from the FACOMS dataset with similar range of baseline characteristics of participants in the OL study using data recorded over the last 4 years for each participant *Participants in open label study included 58% female, 53% with exposure to omaveloxolone and 53% non-ambulatory

Improvements Across Clinical Outcomes with Nomlabofusp Relative to Worsening in FACOMS Study Supports Potential Clinical Benefits mFARS [0- 93] FARS-ADL [0- 36] 9-HPT Dominant Hand MFIS [0- 84] Statistic Nomlabofusp FACOMS1 Nomlabofusp FACOMS1 Nomlabofusp FACOMS1 Nomlabofusp FACOMS1 Baseline Median (IQR) 54.75 (41.2, 71.0) 50.00 (37.0, 61.0) 17.75 (13.0, 24.5) 14.50 (10.0, 18.5) 71.95 (49.6, 114.8) 113.50 (86.5, 148.5) 34.00 (20.0, 34.0) 32.00 (21.0, 42.0) n 38 370 38 370 34 370 38 370 Change from Baseline at 1 year Median (IQR) -2.25 (-3.75, -0.25) 1.00 (-1.5, 4.0) -0.50 (-2.0, 1.0) 0.50 (-1.0, 2.5) -7.40 (-38.8, -2.5) 3.40 (-4.5, 18.0) -6.50 (-17.5, 4.0) 1.502 (-9.5, 11.0) n 8 185 8 237 7 219 8 136 IQR = interquartile range 1 Based on the range of baseline characteristics of participants in the OL study, Larimar identified patients from the FACOMS dataset with similar characteristics using data recorded over the last 4 years for each patient 2 Modified Fatigue scale presented here is at Month 24 because it was not assessed at Month 12

Nomlabofusp Safety Observations with Long-term Treatment Following the 2 most recent cases of anaphylaxis, Larimar is modifying the starting dose regimen to include: Antihistamines 5 days prior to first dose and for 90 days after first dose 5 mg test dose followed by a 25 mg dose 1 hour later and then 25 mg daily for the first 30 days; after 30 days the dose will be increased to 50 mg daily Continue dispensing epinephrine auto-injector, such as EpiPen, to be administered in the event of anaphylaxis Larimar provided the FDA a full data update including the safety, FXN, and clinical data. FDA agreed with the new dosing regimen and Larimar is implementing the change 7 of 39 participants in OL study (65 total participants received at least 1 dose of nomlabofusp across all studies) experienced anaphylaxis Most events occurred on the initial day of administration, and all occurred within the first 6 weeks of dosing All returned to usual state of health after standard treatment Nomlabofusp with long-term daily dosing was generally well tolerated, including 14 on treatment for at least 6 months and 8 for over 1 year Most common AEs were mild/moderate local ISRs and did not lead to any withdrawals AE: Adverse events; ISR: Injection site reaction
Nomlabofusp PK Profile Consistent Across Studies Rapid absorption after subcutaneous administration Steady state reached by Day 30 at both the 25 mg and 50 mg doses with no further accumulation Pharmacokinetic profile consistent with Phase 1 and Phase 2 studies Long-term PK Profile Consistent with Phase 1 and Phase 2 Studies Adolescents 12 to 17 years of age received a weight-based equivalent of 50 mg for 7 days Exposure and PK in 9 adolescents 12 to 17 years of age on nomlabofusp was similar to adults on 50 mg of nomlabofusp Adolescent PK Profile Consistent with Adult

Global Phase 3 Double-blind Placebo-controlled Study Qualifying sites in U.S., Europe, U.K., Canada, and Australia 18 months of treatment Ambulatory participants 2 - 40 years of age (~2/3 under 21 years of age) n = 100 – 150 Key Study Objectives Safety and tolerability Upright stability (U.S.) and mFARS (Europe) as primary outcome measures Daily subcutaneous injections self-administered or by a caregiver Placebo 50 mg nomlabofusp Patient Population

Potential Path to Bring Nomlabofusp to Patients Worldwide Received feedback from FDA and EMA on study protocol Sites in the U.S., Europe, U.K., Canada, and Australia currently being qualified Global Phase 3 Study* BLA submission seeking accelerated approval targeted in Q2 2026 U.S. launch targeted for early 2027 Next Steps Plan to continue enrolling participants on new starting dose regimen with long-term 50 mg dose, including adolescents and those new to a nomlabofusp study Continue introducing lyophilized dosage form Planning to enroll children (2 - 11 yrs of age) directly into the study Open Label Study *Study will initiate with participants 12-40 yrs of age and will change to 2-40 yrs when dose is confirmed in children 2-11 yrs of age Orphan Drug (US & EU) Rare Pediatric Disease (US) Fast Track (US) PRIME (EU) ILAP (UK-MHRA) START Pilot (US) Received FDA Agreement on strategy for potency testing of nomlabofusp; commercial scale PPQ in progress

Nomlabofusp Advancing Towards BLA Submission for FA First potential disease modifying therapy Designed to systemically address FXN deficiency in FA FDA clarity on key BLA elements BLA based on skin FXN levels as potential surrogate endpoint Positive long-term data Increased skin FXN levels similar to asymptomatic carriers and consistent directional improvement across 4 key clinical outcome measures BLA submission seeking accelerated approval targeted Q2 2026 U.S. launch targeted for early 2027