UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): September 8, 2025
Rapport Therapeutics, Inc.
(Exact name of Registrant as Specified in Its Charter)
| Delaware | 001-42121 | 88-0724208 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 99 High Street Suite 2100 |
||
| Boston, Massachusetts | 02210 | |
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s Telephone Number, Including Area Code: (857) 321-8020
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
||
| Common Stock, $0.001 par value per share | RAPP | The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01. | Regulation FD Disclosure. |
On September 8, 2025, Rapport Therapeutics, Inc. (the “Company” or “Rapport”) issued a press release titled “Rapport Announces Positive Topline Results from Phase 2a Clinical Trial of RAP-219 in Patients with Focal Onset Seizures.” A copy of the press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
Also, on September 8, 2025, the Company will host a webcast to discuss topline data from its Phase 2a clinical trial of RAP-219 in patients with drug-resistant focal onset seizures. A copy of the presentation from the webcast will be available on the “Investors” page of the Company’s website at www.rapportrx.com and is furnished as Exhibit 99.2 to this Current Report on Form 8-K.
The information under this Item 7.01, including Exhibit 99.1 and Exhibit 99.2 hereto, is being furnished herewith and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall such information be deemed incorporated by reference into any filing under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, except as expressly set forth by specific reference in such filing.
| Item 8.01. | Other Events. |
On September 8, 2025, the Company announced topline data from its Phase 2a clinical trial of RAP-219 (RAP-219-FOS-201) in patients with drug-resistant focal onset seizures. The topline results, as well as additional corporate updates, are summarized below.
Key Efficacy Results
Efficacy findings from the Phase 2a trial achieved statistically significant results for primary long episode (“LE”) endpoints and key secondary endpoints of clinical seizures. In the 8-week treatment period, 85.2% of patients achieved ≥30% reduction in LEs from baseline (p<0.0001), 72.0% achieved ≥50% reduction in clinical seizures from baseline (p<0.0001), and 24% of patients achieved seizure freedom for the 8-week treatment period (p<0.0001). Topline efficacy data are shown in the following table.
| Outcome Measures for 8-Week Treatment Period | RAP-219 | |||
| Long Episodes (LEs)— primary efficacy endpoint mITT: N=27 |
Patients with ≥30% reduction in LEs from baseline | 85.2% (p<0.0001) |
||
| Median reduction in LE frequency from baseline | 71.0% (p=0.0001) |
|||
| Clinical Seizures (CS)— key secondary endpoint mITT-CS: N=25 |
Patients with ≥50% reduction in clinical seizures from baseline | 72.0% (p<0.0001) |
||
| Patients who achieved seizure freedom | 24.0% (p<0.0001) |
|||
| Median reduction in clinical seizure frequency from baseline | 77.8% (p=0.01) |
|||
| mITT: patients with ≥3 weeks of treatment, ≥70% adherence, and no RNS system detection or stimulation setting changes. mITT-CS: mITT with clinical seizures in prospective baseline. Statistical methods: For responder analysis for LEs, clinical seizure reduction ≥50%, and seizure freedom, two-tailed p-values were calculated using a one-sample exact binomial of proportions against a null hypothesis of 10%, 20%, and 1.5% respectively. For median reduction from baseline in LEs and clinical seizures, two-tailed p-values were calculated from the Wilcoxon signed rank test against a null hypothesis of 0% and 20%, respectively.
|
||||
Key Safety and Tolerability Results
Thirty patients entered the 8-week treatment period of the Phase 2a trial and were dosed with RAP-219. There were four discontinuations during the treatment period, three of which were attributed to treatment-emergent adverse events (“TEAEs”). The safety population comprised all 30 patients receiving at least one dose of RAP-219. In the Phase 2a trial, RAP-219 was generally well-tolerated, with the majority of TEAEs being mild and a low discontinuation rate:
| • | No serious adverse events were reported during the treatment period |
| • | All TEAEs reported were mild (78.5%) or moderate (21.5%) in severity (Grades 1 or 2) |
| • | 3 (10%) patients discontinued treatment due to TEAEs |
| • | The most common TEAEs reported (≥ 10% incidence) were dizziness (n= 8, 26.7%), headache (n = 5, 16.7%), fatigue (n = 4, 13.3%), fall (n = 3, 10.0%), nausea (n = 3, 10.0%), and somnolence (n = 3, 10.0%). |
Trial Demographics and Baseline Characteristics
The demographics and baseline characteristics of patients enrolled in the Phase 2a study are consistent with that of patients expected in future registrational trials. The trial enrolled 12 women and 18 men, and the mean age of patients enrolled was 40.1 years. The mean age of enrolled patients at the time of their first seizure was 16.6 years. Patients were taking a median of 3 concomitant antiseizure medications, with the highest proportion of patients taking lamotrigine (50%), levetiracetam (40%), and cenobamate (37%) medications.
Additional Development Plans
Rapport plans to hold an end-of-Phase 2 meeting with the U.S. Food and Drug Administration in the fourth quarter of 2025. The 8-week follow-up period of the Phase 2a trial is currently ongoing with additional efficacy analyses and 8-week follow up results expected in 2026. By the end of 2025, Rapport plans to initiate an open-label long term safety trial to allow patients enrolled in the Phase 2a trial to continue on RAP-219. Preliminary results of the trial are expected in the second half of 2026. The Company plans to advance RAP-219 into two Phase 3 pivotal trials in the third quarter of 2026.
Additionally, Rapport continues development of a long-acting injectable (“LAI”) formulation of RAP-219 and expects to report initial pharmacokinetic results in 2027.
Outside of epilepsy, Rapport is evaluating RAP-219 in a Phase 2 trial in bipolar mania. The trial is currently enrolling patients and is on track, with topline results expected in the first half of 2027. An update on the plan and timeline for initiation of a Phase 2 trial in diabetic peripheral neuropathic pain is expected later in 2025.
Forward-Looking Statements
This Current Report on Form 8-K contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, each as amended. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will” and “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, but are not limited to, express or implied statements regarding: the clinical development of RAP-219 for the treatment of focal onset seizures, bipolar mania and diabetic peripheral neuropathic pain, including the initiation, timing, progress and results of the Company’s Phase 3 clinical trials in focal onset seizures, and a Phase 2 clinical trial of RAP-219 in bipolar mania, as well as other planned clinical trials; expectations for the activity, tolerability, and commercial potential of RAP-219; the future release of data from the ongoing 8-week follow-up period of the Phase 2a trial for RAP-219; expectations for a LAI formulation of RAP-219 and the potential of a LAI to improve patient adherence; the Company’s expectations for upcoming regulatory interactions; the potential of Rapport’s RAP technology platform; and expectations for Rapport’s uses of capital, expenses and financial results.
Forward looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect Rapport’s business, operating results, financial condition and stock value. Factors that could cause actual results to differ materially from those currently anticipated include: risks relating to the Company’s research and development activities; Rapport’s ability to execute on its strategy including obtaining the requisite regulatory approvals on the expected timeline, if at all; uncertainties relating to preclinical and clinical development activities; the Company’s dependence on third parties to conduct clinical trials, manufacture its product candidates and develop and commercialize its product candidates, if approved; Rapport’s ability to attract, integrate and retain key personnel; risks related to the Company’s financial condition and need for substantial additional funds in order to complete development activities and commercialize a product candidate, if approved; risks related to regulatory developments and approval processes of the U.S. Food and Drug Administration and comparable foreign regulatory authorities; risks related to establishing and maintaining Rapport’s intellectual property protections; and risks related to the competitive landscape for Rapport’s product candidates; as well as other risks described in “Risk Factors,” in the Company’s Annual Report on Form 10-K and most recent Quarterly Report on Form 10-Q, as well as discussions of potential risks, uncertainties, and other important factors in Rapport’s subsequent filings with the Securities and Exchange Commission. Any forward-looking statements represent Rapport’s views only as of today and should not be relied upon as representing its views as of any subsequent date. Rapport expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in its expectations or any changes in events, conditions or circumstances on which any such statement is based, except as required by law, and claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995.
| Item 9.01. | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit No. |
Description |
|
| 99.1 | Press Release issued by Rapport Therapeutics, Inc. on September 8, 2025, furnished herewith. | |
| 99.2 | Corporate presentation of Rapport Therapeutics, Inc., furnished herewith. | |
| 104 | Cover Page Interactive Data File (embedded within Inline XBRL document) | |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Rapport Therapeutics, Inc. | ||||||
| Date: September 8, 2025 | By: | /s/ Troy Ignelzi |
||||
| Troy Ignelzi Chief Financial Officer |
||||||
Exhibit 99.1

Rapport Announces Positive Topline Results from Phase 2a Clinical Trial of RAP-219 in Patients with Focal Onset Seizures
• Trial met primary long episode endpoints with high statistical significance, and RAP-219 was generally well tolerated
• Patients achieved 77.8% reduction in clinical seizures (p=0.01), with 24% achieving seizure freedom for the 8-week treatment period (p<0.0001)
• Data support advancement of RAP-219 into Phase 3 registrational trials
• Company to host a conference call today at 8:00 a.m. ET
BOSTON and SAN DIEGO, September 8, 2025 — Rapport Therapeutics, Inc. (Nasdaq: RAPP) (“Rapport” or the “Company”), a clinical-stage biotechnology company dedicated to the discovery and development of small molecule precision medicines for patients with neurological or psychiatric disorders, today announced that the Phase 2a clinical trial of RAP-219 (RAP-219-FOS-201) in patients with drug-resistant focal onset seizures met its primary endpoint, demonstrating a statistically significant reduction in long episodes (LEs) – an objective electrographic biomarker for clinical seizure reduction – compared with baseline over the 8-week treatment period. In the trial, RAP-219 also demonstrated a statistically significant and clinically meaningful reduction in clinical seizures compared with baseline. RAP-219 was generally well tolerated. The Company plans to advance RAP-219 into two Phase 3 pivotal trials in the third quarter of 2026. RAP-219 is a potential first-in-class, investigational TARPg8-specific AMPAR negative allosteric modulator.
“Despite the available therapies, up to 40% of patients with focal epilepsy continue to experience seizures. There is still tremendous need for additional effective anti-seizure medications with novel mechanisms of action. Physicians and patients need new options that deliver meaningful benefits and the potential to offer the promise of seizure freedom,” said Jacqueline French, M.D., principal investigator of the study and professor in the Department of Neurology at NYU Langone Health’s Comprehensive Epilepsy Center. “This trial represents the first time a novel antiseizure medication was evaluated in focal seizure patients using the RNS system with an objective biomarker of seizure activity. The magnitude of the reduction in clinical seizure frequency seen in this trial, and the corroboration of the clinical activity from the objective biomarker, give me confidence that a medication like RAP-219 has the potential to be a highly effective ASM for drug-resistant focal seizure patients.”
RAP-219 Phase 2a Focal Onset Seizures Trial Design
The Phase 2a clinical trial of RAP-219 is a proof-of-concept, multi-center, open-label study designed to evaluate the efficacy, safety, and tolerability of RAP-219 in adult patients with drug-resistant focal onset seizures. The trial enrolled 30 patients with focal onset seizures who had an implanted RNS® System. Patients received 0.75 mg RAP-219 oral tablet daily for 5 days followed by 1.25 mg RAP-219 oral tablet daily for the remainder of the 8-week treatment period. The primary efficacy endpoint was the change in frequency of RNS-recorded long episodes (LEs) in patients with focal onset seizures evaluated both as the proportion of responders achieving ≥30% reduction in LEs from baseline, which has been demonstrated to be associated with ≥50% reduction in clinical seizures, and median percent change from baseline in LE frequency. Secondary endpoints include clinical seizure frequency reductions (assessed as median percent change from baseline, responder proportion who achieve a ≥50% reduction in seizure frequency, and seizure freedom), safety, and tolerability.
Topline Results
Topline efficacy and tolerability data shared today are for the treatment period (weeks 1-8). The Phase 2a trial 8-week follow-up period is currently ongoing.
Key Efficacy Results:
Efficacy findings from the Phase 2a trial achieved statistically significant results for primary LE endpoints and key secondary endpoints of clinical seizures. In the 8-week treatment period, 85.2% of patients achieved ≥30% reduction in LEs from baseline (p<0.0001), 72.0% achieved ≥50% reduction in clinical seizures from baseline (p<0.0001), and 24% of patients achieved seizure freedom (p<0.0001). Topline efficacy data are shown in the following table.
| Outcome Measures for 8-Week Treatment Period | RAP-219 | |||
| Long Episodes (LEs)— primary efficacy endpoint mITT: N=27 |
Patients with ≥30% reduction in LEs from baseline | 85.2% (p<0.0001) |
||
| Median reduction in LE frequency from baseline | 71.0% (p=0.0001) |
|||
| Clinical Seizures (CS)— key secondary endpoint mITT-CS: N=25 |
Patients with ≥50% reduction in clinical seizures from baseline | 72.0% (p<0.0001) |
||
| Patients who achieved seizure freedom | 24.0% (p<0.0001) |
|||
| Median reduction in clinical seizure frequency from baseline | 77.8% (p=0.01) |
|||
|
mITT: patients with ≥3 weeks of treatment, ≥70% adherence, and no RNS system detection or stimulation setting changes. mITT-CS: mITT with clinical seizures in prospective baseline. Statistical methods: For responder analysis for LEs, clinical seizure reduction ≥50%, and seizure freedom, two-tailed p-values were calculated using a one-sample exact binomial of proportions against a null hypothesis of 10%, 20%, and 1.5% respectively. For median reduction from baseline in LEs and clinical seizures, two-tailed p-values were calculated from the Wilcoxon signed rank test against a null hypothesis of 0% and 20%, respectively.
|
||||
Key Safety and Tolerability Results:
RAP-219 was generally well-tolerated in the trial, with the majority of treatment-emergent adverse events (TEAEs) being mild and a low discontinuation rate:
| • | No serious adverse events (SAEs) were reported during the treatment period |
| • | All TEAEs reported were mild (78.5%) or moderate (21.5%) in severity (Grades 1 or 2) |
| • | 3 (10%) patients discontinued treatment due to TEAEs |
| • | The most common TEAEs reported (≥ 10% incidence) were dizziness (n= 8, 26.7%), headache (n = 5, 16.7%), fatigue (n = 4, 13.3%), fall (n = 3, 10.0%), nausea (n = 3, 10.0%), and somnolence (n = 3, 10.0%). |
“The efficacy data and tolerability profile seen in the Phase 2a trial demonstrate RAP-219’s potential to be an important treatment for patients with drug-resistant focal onset seizures. What’s particularly encouraging is the consistency of the significant improvements seen in the long episode biomarker responses and the clinical seizure reductions,” said Abe Ceesay, chief executive officer of Rapport. “With these data and RAP-219’s emerging best-in-class profile, if approved, we believe RAP-219 could address a significant unmet need among patients, with the potential to support broad adoption among epileptologists and neurologists treating patients living with drug-resistant focal seizures.”
Trial Patient Demographics and Baseline Characteristics
The demographics and baseline characteristics of patients enrolled in the Phase 2a study are consistent with that of patients expected in future registrational trials. The trial enrolled 12 women and 18 men, and the mean age of patients enrolled was 40.1 years. The mean age of enrolled patients at the time of their first seizure was 16.6 years. Patients were taking a median of 3 concomitant antiseizure medications, with the highest proportion of patients taking lamotrigine (50%), levetiracetam (40%), and cenobamate (37%) medications.
“We are excited by the strength of these data in both the electrographic biomarker and clinical seizure reductions. These results give us the confidence to progress RAP-219 into its next stage of clinical development,” said Dr. Jeffrey Sevigny, M.D., chief medical officer of Rapport. “Importantly, the baseline characteristics of patients enrolled, together with the broad cortical expression of TARPg8 and the robust results in the trial, give us confidence in the translatability of the data into the Phase 3 drug-resistant focal onset seizure patient population. Given the persistent unmet need in focal epilepsy, we plan to move into two Phase 3 trials using traditional clinical seizure endpoints, with initiation expected in the third quarter of 2026.”
Rapport plans to hold an end-of-Phase 2 meeting with the U.S. Food and Drug Administration (FDA) in the fourth quarter of 2025 and plans to initiate pivotal trials in the third quarter of 2026. The Company also expects to present additional efficacy analyses and 8-week follow-up results in 2026.
Additional RAP-219 Development Plans
By the end of 2025, Rapport plans to initiate an open-label long term safety trial to allow patients enrolled in the RAP-219-FOS-201 trial to continue on RAP-219. Preliminary results of the trial are expected in the second half of 2026.
Additionally, Rapport continues development of a long-acting injectable (LAI) formulation of RAP-219. Up to half of patients are nonadherent to prescribed ASMs, which can present a significant issue in optimizing treatment benefit and lead to potential breakthrough seizures. The Company believes a LAI formulation has the potential to improve patient adherence and expand the potential clinical utility across all of RAP-219’s indications.
Outside of epilepsy, Rapport is evaluating RAP-219 in a Phase 2 trial in bipolar mania. The trial is currently enrolling patients and is on track, with topline results expected in the first half of 2027. An update on the plan and timeline for initiation of a Phase 2 trial in diabetic peripheral neuropathic pain is expected later in 2025.
Conference Call Information
Rapport Therapeutics will host a conference call and live webcast at 8:00 a.m. ET / 5:00 a.m. PT on September 8, 2025, to discuss the data and provide a business update. Individuals interested in listening to the live conference call may do so by dialing (800) 715-9871 for U.S callers and (646) 307-1963 for other locations and reference conference ID 4762775, or from the webcast link in the “Investors” section of the Company’s website at www.rapportrx.com. A webcast replay will be available in the investor relations section on the Company’s website for 90 days following the completion of the call.
About RAP-219
RAP-219 is a potential first-in-class, clinical-stage TARPg8-specific AMPA receptor (AMPAR) negative allosteric modulator (NAM). Whereas AMPARs are distributed widely in the central nervous system, the receptor associated protein (RAP) TARPg8 is expressed only in discrete brain regions, including the hippocampus and neocortex, where focal seizures often originate. By contrast, TARPg8 has minimal expression in the hindbrain, where drug effects are often associated with intolerable adverse events. With this precision approach, the Company believes RAP-219 has the potential to provide a differentiated profile as compared to traditional neuroscience medications. Due to the role of AMPA biology in various neurological disorders and the selective targeting of TARPg8, the Company believes RAP-219 has pipeline-in-a-product potential and is evaluating the compound as a transformational treatment for patients with focal onset seizures, bipolar disorder, and peripheral neuropathic pain.
About Rapport Therapeutics
Rapport Therapeutics is a clinical-stage biotechnology company dedicated to discovering and developing small molecule precision medicines for patients with neurological or psychiatric disorders. The Company’s founders have made pioneering discoveries related to the function of receptor associated proteins (RAPs) in the brain. Their findings form the basis of Rapport’s RAP technology platform, which enables a differentiated approach to generate precision small molecule product candidates with the potential to overcome many limitations of conventional neurology drug discovery. Rapport’s precision neuroscience pipeline includes the Company’s lead investigational drug, RAP-219, designed to achieve neuroanatomical specificity through its selective targeting of a RAP expressed in only discrete regions of the brain. The Company is currently pursuing RAP-219 as a potential treatment for drug-resistant focal onset seizures, bipolar mania and diabetic peripheral neuropathic pain. Additional preclinical and late-stage discovery stage programs are also underway, including targeting chronic pain and hearing disorders.
Availability of Other Information About Rapport Therapeutics
Rapport Therapeutics uses and intends to continue to use its Investor Relations website and LinkedIn (Rapport Therapeutics) as a means of disclosing material nonpublic information and for complying with its disclosure obligations under Regulation FD. Accordingly, investors should monitor the Company’s Investor Relations website and LinkedIn, in addition to following the Company’s press releases, SEC filings, public conference calls, presentations, and webcasts. The contents of the Company’s website or social media shall not be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended.
Forward-Looking Statements
Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, each as amended. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, but are not limited to, express or implied statements regarding: the clinical development of RAP-219 for the treatment of focal onset seizures, bipolar mania, and diabetic peripheral neuropathic pain, including the initiation, timing, progress and results of the Company’s Phase 3 clinical trials in focal onset seizures, and a Phase 2 clinical trial of RAP-219 in bipolar mania, as well as other planned clinical trials; expectations for the activity, tolerability, and commercial potential of RAP-219; the future release of data from the ongoing 8-week follow-up period of the Phase 2a trial for RAP-219; expectations for a LAI formulation of RAP-219 and the potential of a LAI to improve patient adherence; the Company’s expectations for upcoming regulatory interactions; the potential of Rapport’s RAP technology platform; and expectations for Rapport’s uses of capital, expenses and financial results.
Forward looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect Rapport’s business, operating results, financial condition and stock value. Factors that could cause actual results to differ materially from those currently anticipated include: risks relating to the Company’s research and development activities; Rapport’s ability to execute on its strategy including obtaining the requisite regulatory approvals on the expected timeline, if at all; uncertainties relating to preclinical and clinical development activities; the Company’s dependence on third parties to conduct clinical trials, manufacture its product candidates and develop and commercialize its product candidates, if approved; Rapport’s ability to attract, integrate and retain key personnel; risks related to the Company’s financial condition and need for substantial additional funds in order to complete development activities and commercialize a product candidate, if approved; risks related to regulatory developments and approval processes of the U.S.
Food and Drug Administration and comparable foreign regulatory authorities; risks related to establishing and maintaining Rapport’s intellectual property protections; and risks related to the competitive landscape for Rapport’s product candidates; as well as other risks described in “Risk Factors,” in the Company’s Annual Report on Form 10-K and most recent Quarterly Report on Form 10-Q, as well as discussions of potential risks, uncertainties, and other important factors in Rapport’s subsequent filings with the Securities and Exchange Commission. Any forward-looking statements represent Rapport’s views only as of today and should not be relied upon as representing its views as of any subsequent date. Rapport expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in its expectations or any changes in events, conditions or circumstances on which any such statement is based, except as required by law, and claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995.
Contact
Julie DiCarlo
Head of Communications & IR
Rapport Therapeutics
jdicarlo@rapportrx.com

Exhibit 99.2 RAP-219 Phase 2a Trial in Drug-resistant Focal Onset Seizures Topline Results Conference Call Presentation September 8, 2025

Disclaimer This presentation contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, each as amended. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, but are not limited to, express or implied statements regarding: the clinical development of RAP-219 for the treatment of focal onset seizures, bipolar mania and diabetic peripheral neuropathic pain, including the initiation, timing, progress and results of the Company's Phase 3 clinical trials in focal onset seizures, and a Phase 2a clinical trial of RAP-219 in bipolar mania, as well as other planned clinical trials; expectations for the activity, tolerability, and commercial potential of RAP-219; the future release of data from the ongoing 8-week follow-up period of the Phase 2a trial for RAP-219; expectations for a long-acting injectable (LAI) formulation of RAP-219 and the potential of a LAI to improve patient adherence; the Company's expectations for upcoming regulatory interactions; the potential of Rapport's RAP technology platform; the advancement of the Company's discovery programs; and expectations for Rapport's uses of capital, expenses and financial results, including its cash runway through the end of 2026. Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect Rapport’s business, operating results, financial condition and stock value. Factors that could cause actual results to differ materially from those currently anticipated include: risks relating to the Company’s research and development activities; Rapport’s ability to execute on its strategy including obtaining the requisite regulatory approvals on the expected timeline, if at all; uncertainties relating to preclinical and clinical development activities; the Company’s dependence on third parties to conduct clinical trials, manufacture its product candidates and develop and commercialize its product candidates, if approved; Rapport’s ability to attract, integrate and retain key personnel; risks related to the Company’s financial condition and need for substantial additional funds in order to complete development activities and commercialize a product candidate, if approved; risks related to regulatory developments and approval processes of the U.S. Food and Drug Administration and comparable foreign regulatory authorities; risks related to establishing and maintaining Rapport’s intellectual property protections; and risks related to the competitive landscape for Rapport’s product candidates; as well as other risks described in “Risk Factors,” in the Company’s Annual Report on Form 10-K and most recent Quarterly Report on Form 10-Q, as well as discussions of potential risks, uncertainties, and other important factors in Rapport’s subsequent filings with the Securities and Exchange Commission. Any forward-looking statements represent Rapport’s views only as of today and should not be relied upon as representing its views as of any subsequent date. Rapport expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in its expectations or any changes in events, conditions or circumstances on which any such statement is based, except as required by law, and claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. 2

Agenda Overview 01 Abe Ceesay, Chief Executive Officer RAP-219 in Drug-resistant Focal Onset Seizures Topline Phase 2a Data 02 Jeff Sevigny, M.D., Chief Medical Officer Closing Remarks 03 Abe Ceesay Questions and Answers 04 Abe Ceesay | Jeff Sevigny | Troy Ignelzi, Chief Financial Officer 3

Agenda Overview 01 Abe Ceesay, Chief Executive Officer RAP-219 in Drug-resistant Focal Onset Seizures Topline Phase 2a Data 02 Jeff Sevigny, M.D., Chief Medical Officer Closing Remarks 03 Abe Ceesay Questions and Answers 04 Abe Ceesay | Jeff Sevigny | Troy Ignelzi, Chief Financial Officer 4

Foundational science and supportive research established our early confidence in RAP-219 Corneal Kindling Responders and Rotarod Failures in Mice TARPγ8 Clinical PET High expression in the neocortex and mesial temporal lobe, where nearly all seizures originate Relatively low expression in hindbrain Clear confirmation that TARPγ8 is broadly Robust, dose-dependent seizure protection in expressed in brain regions associated with gold-standard preclinical model the initiation and propagation of FOS 5
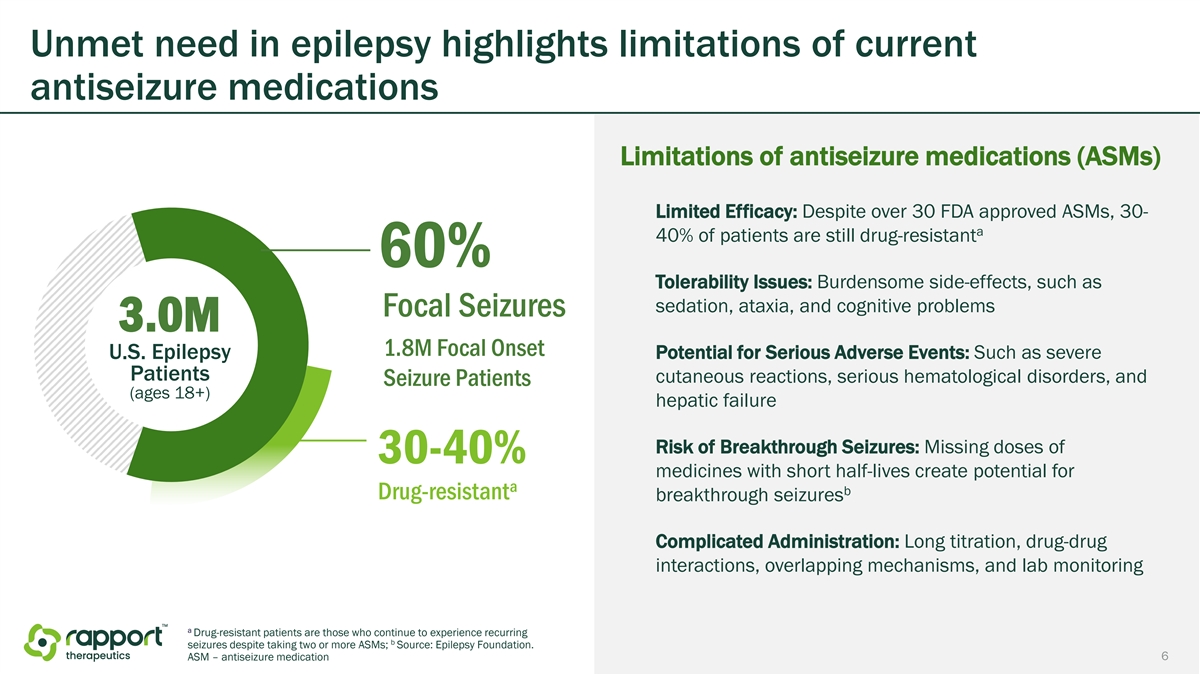
Unmet need in epilepsy highlights limitations of current antiseizure medications Limitations of antiseizure medications (ASMs) Limited Efficacy: Despite over 30 FDA approved ASMs, 30- a 40% of patients are still drug-resistant 60% Tolerability Issues: Burdensome side-effects, such as sedation, ataxia, and cognitive problems Focal Seizures 3.0M 1.8M Focal Onset U.S. Epilepsy Potential for Serious Adverse Events: Such as severe Patients cutaneous reactions, serious hematological disorders, and Seizure Patients (ages 18+) hepatic failure Risk of Breakthrough Seizures: Missing doses of 30-40% medicines with short half-lives create potential for a b Drug-resistant breakthrough seizures Complicated Administration: Long titration, drug-drug interactions, overlapping mechanisms, and lab monitoring a Drug-resistant patients are those who continue to experience recurring b seizures despite taking two or more ASMs; Source: Epilepsy Foundation. 6 ASM – antiseizure medication

Emerging best-in-class profile for RAP-219 in focal onset seizures, with multi-billion dollar commercial opportunity, if approved Long-acting Efficacy Data Tolerability Data Ease of Use Injectable Phase 2a results RAP-219 was generally Once daily dosing, low risk Advancing first ever LAI demonstrated statistically of drug-drug interactions, well-tolerated in Phase 2a for epilepsy patients, significant reductions in rational polypharmacy providing IP extension long episodes and clinical potential, and long half- leading to potential seizures life commercial upside Results support advancement into Phase 3 registrational trials and LAI development LAI – long acting injectable 7

Agenda Overview 01 Abe Ceesay, Chief Executive Officer RAP-219 in Drug-resistant Focal Onset Seizures Topline Phase 2a Data 02 Jeff Sevigny, M.D., Chief Medical Officer Closing Remarks 03 Abe Ceesay Questions and Answers 04 Abe Ceesay | Jeff Sevigny | Troy Ignelzi, Chief Financial Officer 8

Phase 2a trial in drug-resistant focal onset seizure patients 8-week open-label 8-week 12-week pre-treatment period treatment period follow-up period 4-week 8-week Retrospective Prospective 0.75 mg x 5 days then 1.25 mg QD RAP-219 Washout Baseline Baseline Clinical seizure End of Start of End of a baseline treatment treatment trial b Long Episode (LE) baseline Key Entry Criteria Key Endpoints 1. Drug-resistant focal onset seizures • LE reduction (power determinations based on this outcome measure) ® 2. RNS probe implanted in seizure onset zone within mesial temporal • Proportion of patients with ≥30% reduction compared with LE baseline lobe (MTL) ≥15 months before screening • Median percent change from LE baseline 3. Stable RNS System settings and other therapies • Clinical seizure reduction 4. ≥16 LEs during 8-week retrospective review period • Proportion of patients with ≥50% reduction compared with pre- 5. ≥1 clinical seizure reported during 8-week retrospective review period treatment baseline; proportion of patients who achieved seizure freedom 6. >50% concordance between LEs and electrographic seizures • Median percent change from baseline a Patients with no clinical seizures during the prospective baseline period were allowed to enter the treatment period. b LE baseline was defined as the 12 weeks preceding the initiation of treatment (the combination of the 8-week retrospective, and 4-week prospective baseline periods) 9 LE – long episode; MTL – mesial temporal lobe; RNS System – responsive neurostimulator; QD – once daily
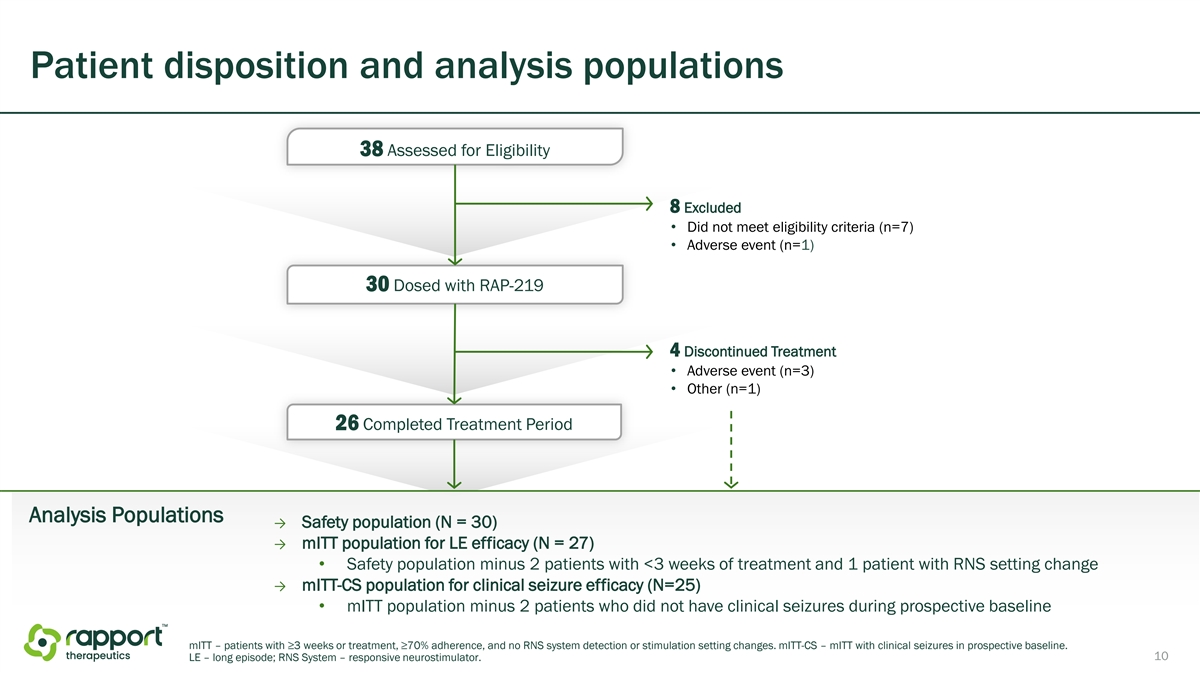
Patient disposition and analysis populations 38 Assessed for Eligibility 8 Excluded • Did not meet eligibility criteria (n=7) • Adverse event (n=1) 30 Dosed with RAP-219 4 Discontinued Treatment • Adverse event (n=3) • Other (n=1) 26 Completed Treatment Period Analysis Populations → Safety population (N = 30) → mITT population for LE efficacy (N = 27) • Safety population minus 2 patients with <3 weeks of treatment and 1 patient with RNS setting change → mITT-CS population for clinical seizure efficacy (N=25) • mITT population minus 2 patients who did not have clinical seizures during prospective baseline mITT – patients with ≥3 weeks or treatment, ≥70% adherence, and no RNS system detection or stimulation setting changes. mITT-CS – mITT with clinical seizures in prospective baseline. 10 LE – long episode; RNS System – responsive neurostimulator.
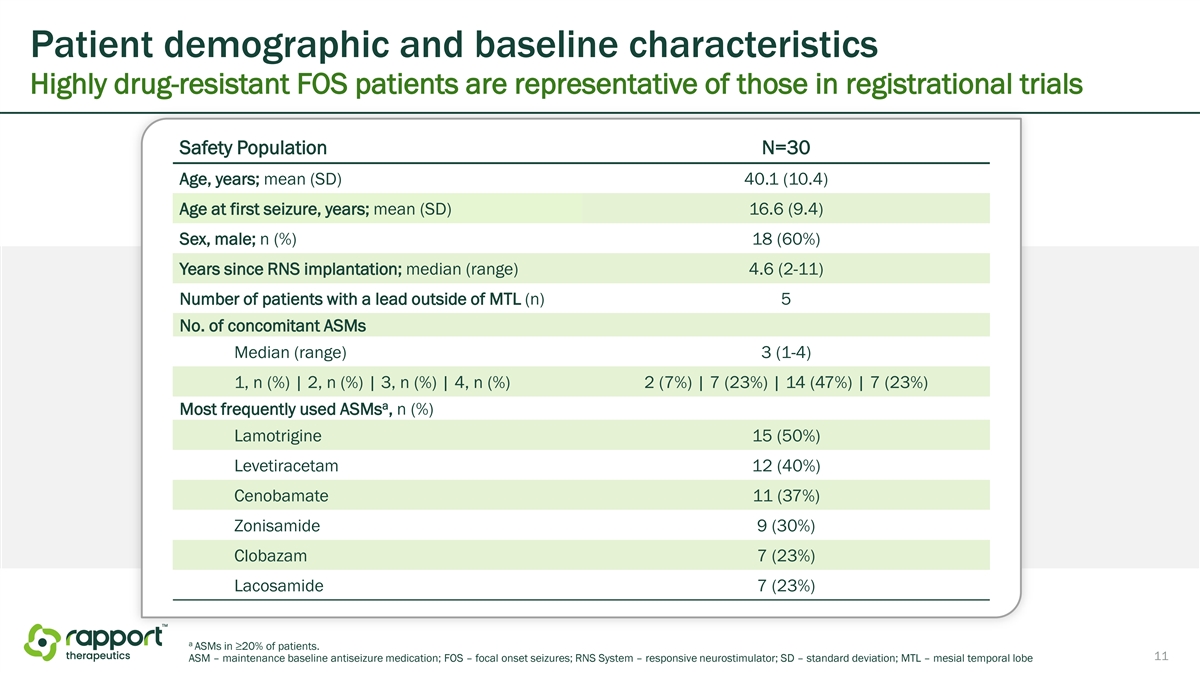
Patient demographic and baseline characteristics Highly drug-resistant FOS patients are representative of those in registrational trials Safety Population N=30 Age, years; mean (SD) 40.1 (10.4) Age at first seizure, years; mean (SD) 16.6 (9.4) Sex, male; n (%) 18 (60%) Years since RNS implantation; median (range) 4.6 (2-11) Number of patients with a lead outside of MTL (n) 5 No. of concomitant ASMs Median (range) 3 (1-4) 1, n (%) | 2, n (%) | 3, n (%) | 4, n (%) 2 (7%) | 7 (23%) | 14 (47%) | 7 (23%) a Most frequently used ASMs , n (%) Lamotrigine 15 (50%) Levetiracetam 12 (40%) Cenobamate 11 (37%) Zonisamide 9 (30%) Clobazam 7 (23%) Lacosamide 7 (23%) a ASMs in ≥20% of patients. 11 ASM – maintenance baseline antiseizure medication; FOS – focal onset seizures; RNS System – responsive neurostimulator; SD – standard deviation; MTL – mesial temporal lobe

Efficacy analyses → Efficacy analyses for 8-week treatment period: • mITT population (N=27) for long episodes • mITT-CS population (N=25) for clinical seizures → 8-week follow-up period currently ongoing; full data will be presented at future medical meetings 12
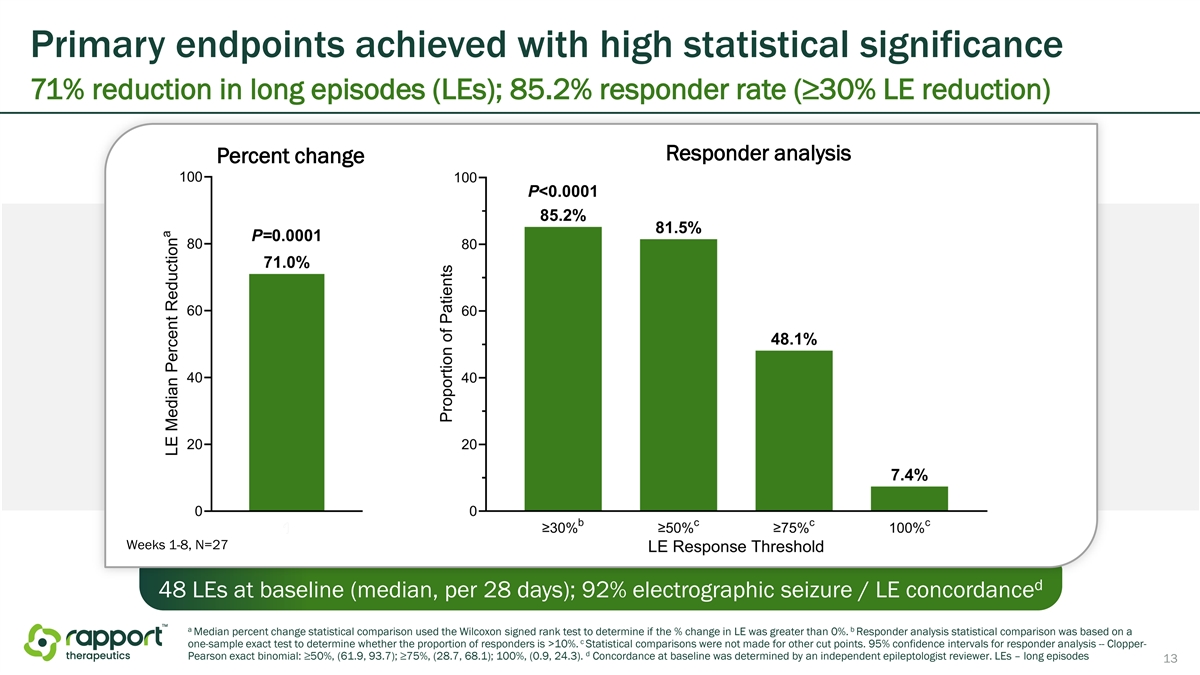
Primary endpoints achieved with high statistical significance 71% reduction in long episodes (LEs); 85.2% responder rate (≥30% LE reduction) Responder analysis Percent change Weeks 1-8, N=27 d 48 LEs at baseline (median, per 28 days); 92% electrographic seizure / LE concordance a b Median percent change statistical comparison used the Wilcoxon signed rank test to determine if the % change in LE was greater than 0%. Responder analysis statistical comparison was based on a c one-sample exact test to determine whether the proportion of responders is >10%. Statistical comparisons were not made for other cut points. 95% confidence intervals for responder analysis -- Clopper- d Pearson exact binomial: ≥50%, (61.9, 93.7); ≥75%, (28.7, 68.1); 100%, (0.9, 24.3). Concordance at baseline was determined by an independent epileptologist reviewer. LEs – long episodes 13

Clinical seizure secondary endpoints achieved with statistical significance 77.8% clinical seizure reduction; 72% achieved clinically meaningful response (≥50% reduction) Percent change Responder analysis Seizure Freedom (weeks 1-8) Weeks 1-8, N=25 10 clinical seizures at baseline (median, per 28 days) a Statistical comparison for median percent change used the Wilcoxon signed-rank test to determine if the median % reduction from baseline in CS was greater than 20%. Responder analysis statistical comparison is based on a one sample exact test to determine whether the proportion of responders is, (b) >20%; (c) >7%; (d) >1.5% for the 50% responder, 75% responder, and seizure freedom groups, respectively. CS – clinical seizures. 14

RAP-219 was generally well tolerated TEAEs: 79% were mild, 10% discontinuation rate Safety Population (N=30) TEAE SUMMARY Any TEAE, n (%) 25 (83.3%) TEAE leading to study drug 3 (10.0%) → 25 patients reported a total of 65 TEAEs discontinuation Grade 1 TEAE - Mild 15 (50.0%) • 78.5% were mild, 21.5% were moderate Grade 2 TEAE - Moderate 10 (33.3%) • No serious adverse events or clinically significant Grade ≥3 TEAE - Severe 0 laboratory, vital signs, or ECG abnormalities TEAEs reported in ≥10% of patients, n (%) Dizziness 8 (26.7%) → 3 (10%) patients discontinued RAP-219 due to TEAEs Headache 5 (16.7%) • Grade 1 worsening of preexisting memory impairment Fatigue 4 (13.3%) • Grade 1 panic attack Fall 3 (10.0%) Nausea 3 (10.0%) • Grade 2 worsening of preexisting anxiety Somnolence 3 (10.0%) TEAE – treatment emergent adverse event during treatment period; ECG – electrocardiogram. 15
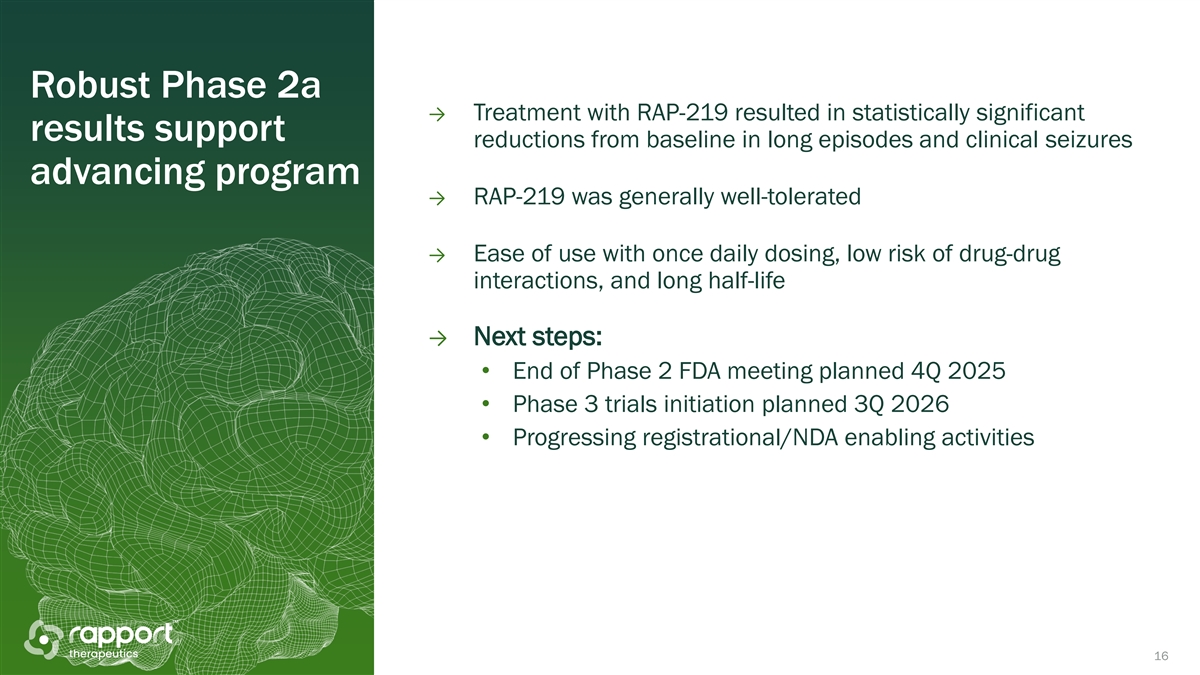
Robust Phase 2a → Treatment with RAP-219 resulted in statistically significant results support reductions from baseline in long episodes and clinical seizures advancing program → RAP-219 was generally well-tolerated → Ease of use with once daily dosing, low risk of drug-drug interactions, and long half-life → Next steps: • End of Phase 2 FDA meeting planned 4Q 2025 • Phase 3 trials initiation planned 3Q 2026 • Progressing registrational/NDA enabling activities 16

Agenda Overview 01 Abe Ceesay, Chief Executive Officer RAP-219 in Drug-resistant Focal Onset Seizures Topline Phase 2a Data 02 Jeff Sevigny, M.D., Chief Medical Officer Closing Remarks 03 Abe Ceesay Questions and Answers 04 Abe Ceesay | Jeff Sevigny | Troy Ignelzi, Chief Financial Officer 17
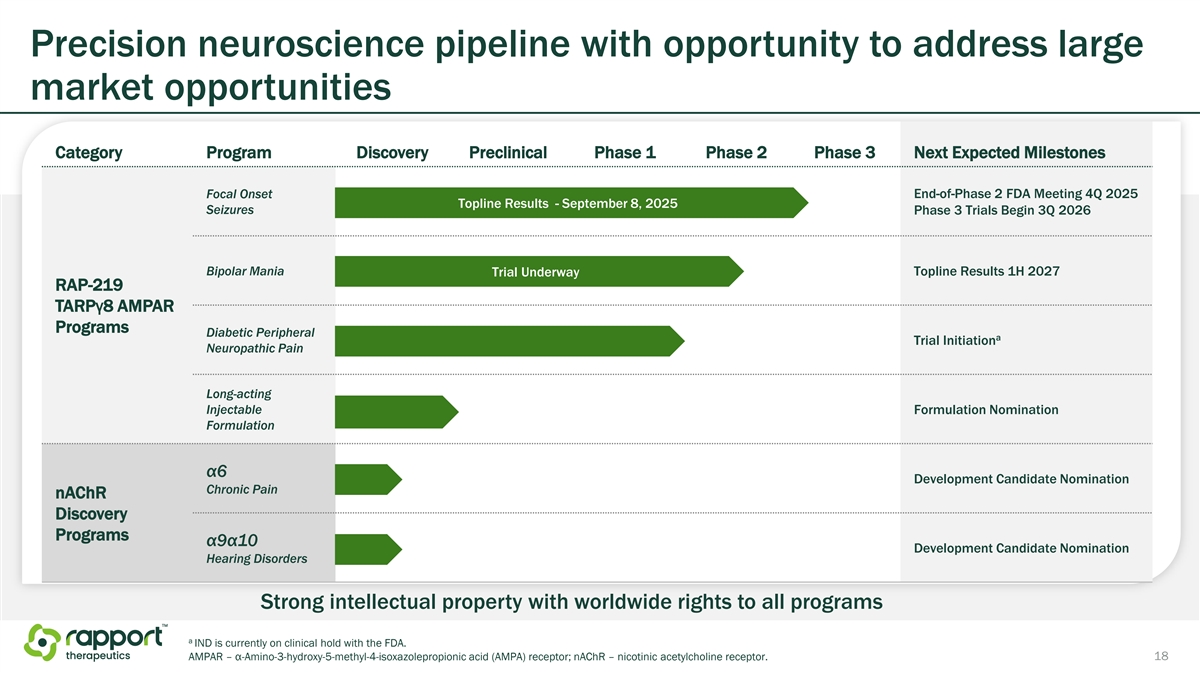
Precision neuroscience pipeline with opportunity to address large market opportunities Category Program Discovery Preclinical Phase 1 Phase 2 Phase 3 Next Expected Milestones Focal Onset End-of-Phase 2 FDA Meeting 4Q 2025 Topline Results - September 8, 2025 Seizures Phase 3 Trials Begin 3Q 2026 Bipolar Mania Trial Underway Topline Results 1H 2027 RAP-219 TARPγ8 AMPAR Programs Diabetic Peripheral a Trial Initiation Neuropathic Pain Long-acting Injectable Formulation Nomination Formulation α6 Development Candidate Nomination Chronic Pain nAChR Discovery Programs α9α10 Development Candidate Nomination Hearing Disorders Strong intellectual property with worldwide rights to all programs a IND is currently on clinical hold with the FDA. AMPAR – α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor; nAChR – nicotinic acetylcholine receptor. 18

Multiple anticipated catalysts over next 24 months 2H 2025 1H 2027 3Q 2026 RAP-219 FOS RAP-219 bipolar RAP-219 FOS open label mania Phase 2 two Phase 3 extension trial topline results trials initiation initiation 2H 2026 3Q 2025 2026 2027 RAP-219 FOS Phase RAP-219 bipolar RAP-219 FOS open RAP-219 long- 2a additional efficacy mania Phase 2 acting injectable label extension analyses and 8-wk trial initiation initial PK results interim results follow-up results 2025 2026 2027 a Cash balance of $260.4mm (as of 6/30/25) supports Rapport through end of 2026 a Cash, cash equivalents and short-term investments, excluding restricted cash. 19 FOS – focal onset seizures; PK – Pharmacokinetics.
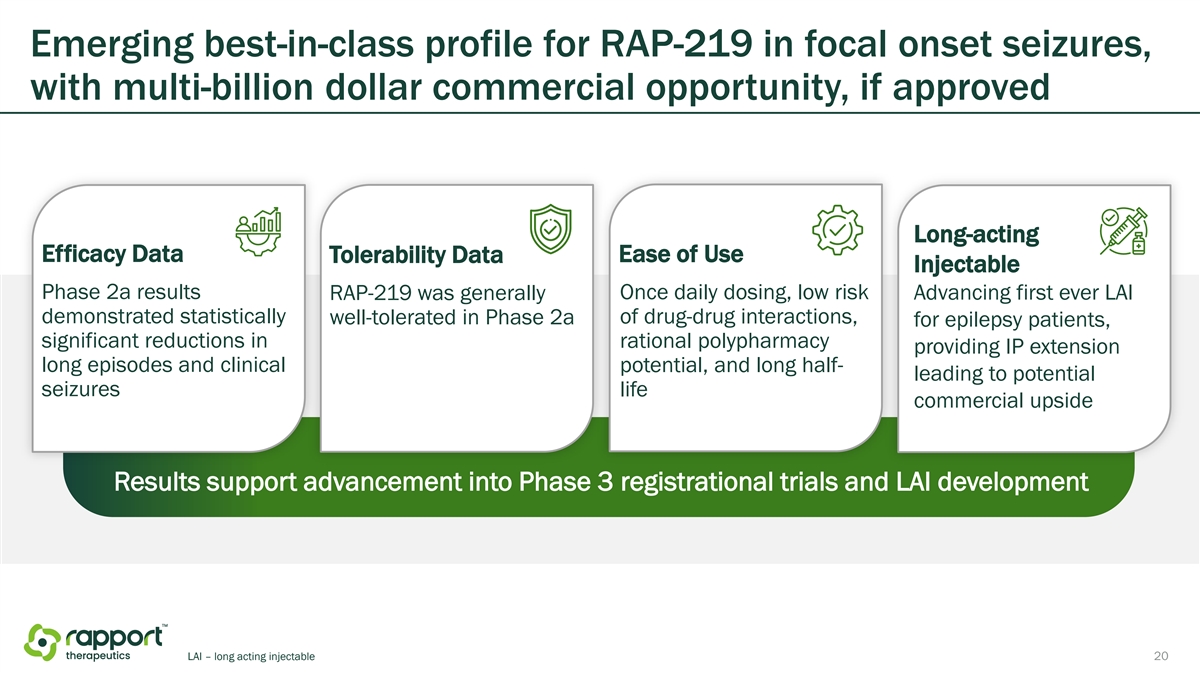
Emerging best-in-class profile for RAP-219 in focal onset seizures, with multi-billion dollar commercial opportunity, if approved Long-acting Efficacy Data Tolerability Data Ease of Use Injectable Phase 2a results RAP-219 was generally Once daily dosing, low risk Advancing first ever LAI demonstrated statistically of drug-drug interactions, well-tolerated in Phase 2a for epilepsy patients, significant reductions in rational polypharmacy providing IP extension long episodes and clinical potential, and long half- leading to potential seizures life commercial upside Results support advancement into Phase 3 registrational trials and LAI development LAI – long acting injectable 20

Agenda Overview 01 Abe Ceesay, Chief Executive Officer RAP-219 in Drug-resistant Focal Onset Seizures Topline Phase 2a Data 02 Jeff Sevigny, M.D., Chief Medical Officer Closing Remarks 03 Abe Ceesay Questions and Answers 04 Abe Ceesay | Jeff Sevigny | Troy Ignelzi, Chief Financial Officer 21

Thank you