UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): August 12, 2025
SPERO THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-38266 | 46-4590683 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
| 675 Massachusetts Avenue, 14th Floor | ||
| Cambridge, Massachusetts | 02139 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code (857) 242-1600
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange on which registered |
||
| Common Stock, $0.001 par value | SPRO | The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 2.02 | Results of Operations and Financial Condition. |
On August 12, 2025, Spero Therapeutics, Inc. (the “Company”) issued a press release announcing its results for the third quarter ended June 30, 2025. A copy of the press release issued in connection with the announcement is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information contained in this Item 2.02 and in the press release furnished as Exhibit 99.1 hereto shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended (the “Securities Act”), nor shall it be deemed incorporated by reference in any filing under the Securities Act or the Exchange Act, except as expressly set forth by specific reference in such filing.
| Item 7.01 | Regulation FD Disclosure. |
On August 12, 2025, the Company released an investor presentation (the “Investor Presentation”), which includes updates regarding the Company’s business and operations that management intends to use from time to time in investor communications and conferences. A copy of the Investor Presentation is attached and furnished hereto as Exhibit 99.2 and is also available on the “Investor Relations” portion of the Company’s website at https://www.sperotherapeutics.com/investor-relations#ir-corp-presentation.
The information in this Item 7.01 and Exhibit 99.2, is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that section or sections 11 and 12(a)(2) of the Securities Act, nor shall it be deemed incorporated by reference in any filing under the Securities Act or the Exchange Act, except as expressly set forth by specific reference in such filing.
| Item 9.01 | Financial Statements and Exhibits |
| (d) | Exhibits |
| Exhibit |
Description |
|
| 99.1 | Press release issued by Spero Therapeutics, Inc. on August 12, 2025 | |
| 99.2 | Corporate Investor Presentation of Spero Therapeutics, Inc. as of August 12, 2025 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document and incorporated as Exhibit 101) | |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: August 12, 2025 | SPERO THERAPEUTICS, INC. | |||||
| By: | /s/ Esther Rajavelu |
|||||
| Esther Rajavelu | ||||||
| Chief Executive Officer, Chief Financial Officer and Chief Business Officer |
||||||
Exhibit 99.1
Spero Therapeutics Announces Second Quarter 2025 Operating Results and Provides a Business Update
| • | PIVOT-PO Phase 3 trial evaluating tebipenem Hbr in complicated urinary tract infection (cUTI) patients stopped early for efficacy following review by independent data monitoring committee (IDMC) |
| • | Spero, along with its development partner, GSK, plans to submit data from the PIVOT-PO trial as part of a planned US Food and Drug Administration (FDA) filing in 2H 2025 |
| • | Existing cash and earned and non-contingent development milestone payments from GSK provide anticipated runway to fund the Company’s current operating expenses and capital expenditures into 2028 |
CAMBRIDGE, Mass., Aug 12, 2025 — Spero Therapeutics, Inc. (Nasdaq: SPRO), a clinical-stage biopharmaceutical company focused on identifying and developing novel treatments for rare diseases and multi-drug resistant (MDR) bacterial infections, today announced financial results for the second quarter ended June 30, 2025, and provided a business update.
“During the second quarter, we announced that the tebipenem Phase 3 PIVOT-PO trial met its primary endpoint, marking a significant milestone for this program. We look forward to working with GSK on next steps for this program which include completion of the Phase 3 data analysis and submission of the data package to the FDA,” said Esther Rajavelu, Chief Executive Officer and Chief Financial Officer of Spero. “There remains a critical unmet need for an oral carbapenem to treat complicated urinary tract infections, including pyelonephritis. If approved, we believe tebipenem HBr could set a new standard of care for these infections, with the potential to shorten hospital stays, improve patient outcomes, and reduce pressure on healthcare resources.”
Pipeline Update
Tebipenem HBr
Tebipenem HBr is an investigational oral carbapenem antibiotic being developed for the treatment of cUTI, including pyelonephritis, to help patients potentially reduce the duration of in-patient therapy. Spero granted GSK an exclusive license to commercialize tebipenem HBr in all territories, except certain Asian territories where Meiji holds development and commercialization rights.
| • | In May 2025, Spero and its development partner GSK announced that the Phase 3 PIVOT-PO trial met the primary endpoint of non-inferiority of tebipenem HBr compared to intravenous imipenem-cilastatin in hospitalized adult patients with cUTI, including pyelonephritis, on overall response (composite of clinical cure plus microbiological eradication) at the test-of-cure visit. |
| • | The IDMC review did not identify any new safety concerns beyond what has been reported in other studies with tebipenem, with diarrhea and headache as the two most reported adverse events. |
| • | GSK plans to work with U.S. regulatory authorities to include these data as part of a filing in 2H 2025. |
| • | Full results will be submitted for presentation at an upcoming scientific meeting and for publication in a peer-reviewed journal. For more information on the PIVOT-PO trial, please refer to ClinicalTrials.gov ID NCT06059846. |
SPR720
SPR720 is an investigational, chemically stable phosphate ester prodrug that is converted rapidly in vivo to SPR719, the active moiety, after oral administration. SPR719 targets the ATPase site of DNA gyrase B in mycobacteria, a mechanism that is distinct from that of other antibiotics in use for nontuberculous mycobacterium pulmonary disease (NTM-PD).
| • | The oral development program in NTM-PD was suspended in 4Q 2024. This followed a planned interim analysis of 16 patients dosed in the Phase 2a trial, which demonstrated the trial did not meet its primary endpoint. |
| • | The Company is determining next steps for the program. |
Corporate Update
| • | Esther Rajavelu was appointed as Spero’s President and Chief Executive Officer, effective May 2, 2025. Ms. Rajavelu was also elected to the Board of Directors at Spero’s 2025 annual meeting of stockholders. She continues to serve as the Company’s Chief Financial Officer and Treasurer. |
| • | As of June 30, 2025, Spero had cash and cash equivalents of $31.2 million. Spero estimates that its existing cash and cash equivalents, together with earned and noncontingent development milestone payments from GSK, including the final payment under the GSK License Agreement of $23.8 million received in August 2025, will be sufficient to fund its operating expenses and capital expenditures into 2028. |
| • | Pursuant to the GSK License Agreement, Spero has adjusted the aggregate potential commercial milestone payments contingent upon first sales from up to $150.0 million to up to $101.0 million after PIVOT-PO was stopped early for efficacy. The trial was stopped early following completion of a pre-specified interim analysis with 1,690 patients enrolled in the trial, reducing the overall costs to Spero; the maximum potential milestone payment of $150.0 million was contingent upon the trial continuing to full enrollment with 2,637 patients enrolled in the trial. For further detail on upcoming milestones, please review the disclosure in our Form 10-Q, filed today. |
Second Quarter 2025 Financial Results
| • | Spero reported a net loss of $1.7 million for the second quarter of 2025 compared to a net loss of $17.9 million for the second quarter of 2024, or a diluted net loss per share of common stock of $(0.03) and $(0.33), respectively. |
| • | Total revenue for the second quarter of 2025 was $14.2 million, compared with total revenue of $10.2 million for the second quarter of 2024. The revenue increase for the second quarter of 2025 was primarily due to collaboration revenue from GSK. |
| • | Research and development expenses for the second quarter of 2025 were $10.7 million, compared to $23.7 million of research and development expenses for the same period in 2024. The decrease in research and development expenses compared with the prior year period was primarily due to reduced clinical expense related to the PIVOT-PO trial. |
| • | General and administrative expenses for the second quarter of 2025 were $5.9 million, compared to $5.5 million of general and administrative expenses for the same period in 2024. This increase compared with the prior year-period was primarily due to increased personnel and professional service expense. |
For further details on Spero’s financials, refer to Spero’s Quarterly Report on Form 10-Q, filed with the U.S. Securities and Exchange Commission (SEC) today.
Conference Call and Live Webcast
Spero management will host a conference call and live audio webcast at 4:30 p.m. ET today, August 12, 2025, to discuss the second quarter financial results and provide a business update.
To access the call, please dial 1-844-825-9789 (domestic) or 1-412-317-5180 (international) and refer to conference ID 10200686, or click on this link and request a return call with passcode 0605709. The audio webcast can be accessed live on this link and also on the “Investor Relations” page of the Spero Corporate Website at https://www.sperotherapeutics.com/. The archived webcast will also be available on Spero’s website for 30 days following the call.
Government Agency Research Support
The views expressed in this press release are those of the authors and may not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.
Tebipenem HBr Research Support
Select tebipenem HBr studies have been funded in part with federal funds from the Department of Health and Human Services; Administration for Strategic Preparedness and Response; and Biomedical Advanced Research and Development Authority, under contract number HHSO100201800015C.
About Spero Therapeutics
Spero Therapeutics, headquartered in Cambridge, Massachusetts, is a clinical-stage biopharmaceutical company focused on identifying and developing novel treatments for rare diseases and MDR bacterial infections with high unmet need. For more information, visit www.sperotherapeutics.com.
Forward Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, statements regarding the timing, progress and results of Spero’s Phase 3 PIVOT-PO trial; the timing of a planned FDA filing in 2H 2025 for tebipenem HBr; the potential of tebipenem HBr to be the first oral carbapenem antibiotic for US patients with cUTI, including pyelonephritis, and to set a new standard of care; the potential receipt of milestone payments under Spero’s license and collaboration agreements; Spero’s anticipated cash runway; and the potential benefits of any of Spero’s current or future product candidates in treating patients. In some cases, forward-looking statements may be identified by terms such as “may,” “will,” “should,” “expect,” “plan,” “aim,” “anticipate,” “could,” “intent,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue,” the negative of these terms or other similar expressions. Any forward-looking statements in this press release are based on management’s current expectations and beliefs and are subject to a number of important risks, uncertainties and other factors that may cause actual results to differ materially from those indicated by such forward looking statements, including whether tebipenem HBr will advance through the clinical development process, or at all, taking into account the effects of possible regulatory delays, slower than anticipated patient enrollment, manufacturing challenges, clinical trial design and clinical outcomes; whether the results of such trials will warrant submission for approval from the FDA or equivalent foreign regulatory agencies; whether the FDA will ultimately approve tebipenem HBr and, if so, the timing of any such approval; whether the FDA will require any additional clinical data or place labeling restrictions on the use of tebipenem HBr that would delay approval and/or reduce the commercial prospects of tebipenem HBr; whether a successful commercial launch can be achieved and market acceptance of tebipenem HBr can be established; whether results obtained in preclinical studies and clinical trials will be indicative of results obtained in future clinical trials; Spero’s reliance on third parties to manufacture, develop, and commercialize its product candidates, if approved, including, in the case of tebipenem HBr, Spero’s reliance on GSK pursuant to the exclusive GSK License Agreement to develop tebipenem HBr and GSK’s right thereunder to determine, in its sole discretion, whether to continue the PIVOT-PO trial or otherwise further develop tebipenem HBr; Spero’s need for additional funding; the ability to commercialize Spero’s product candidates, if approved; Spero’s ability to retain key personnel; Spero’s leadership transitions; whether Spero’s cash resources will be sufficient to fund its continuing operations for the periods and/or trials anticipated; and other factors discussed in the “Risk Factors” set forth in filings that Spero periodically makes with the SEC. The forward-looking statements included in this press release represent Spero’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date. Except as required by law, Spero explicitly disclaims any obligation to update any forward-looking statements.
Investor Relations Contact:
Shai Biran, PhD
Spero Therapeutics
IR@Sperotherapeutics.com
Media Inquiries:
media@sperotherapeutics.com
Spero Therapeutics, Inc.
Condensed Consolidated Balance Sheet Data
(in thousands)
(Unaudited)
| June 30, 2025 |
December 31, 2024 |
|||||||
| Cash and cash equivalents |
$ | 31,194 | $ | 52,889 | ||||
| Other assets |
30,925 | 57,654 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 62,119 | $ | 110,543 | ||||
|
|
|
|
|
|||||
| Total liabilities |
29,291 | 64,420 | ||||||
| Total stockholder’s equity |
32,828 | 46,123 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 62,119 | $ | 110,543 | ||||
|
|
|
|
|
|||||
Spero Therapeutics, Inc.
Condensed Consolidated Statements of Operations
(in thousands, except share and per share data)
(unaudited)
| Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||
| 2025 | 2024 | 2025 | 2024 | |||||||||||||
| Revenues: |
||||||||||||||||
| Grant revenue |
$ | 2,387 | $ | 4,180 | $ | 3,150 | $ | 9,243 | ||||||||
| Collaboration revenue - related party |
11,802 | 5,903 | 16,901 | 9,967 | ||||||||||||
| Collaboration revenue |
— | 114 | 12 | 254 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenues |
14,189 | 10,197 | 20,063 | 19,464 | ||||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
10,672 | 23,725 | 24,278 | 41,057 | ||||||||||||
| General and administrative |
5,878 | 5,533 | 12,702 | 11,450 | ||||||||||||
| Restructuring |
83 | — | 258 | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
16,633 | 29,258 | 37,238 | 52,507 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(2,444 | ) | (19,061 | ) | (17,175 | ) | (33,043 | ) | ||||||||
| Total other income, net |
744 | 1,199 | 1,609 | 2,512 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (1,700 | ) | $ | (17,862 | ) | $ | (15,566 | ) | $ | (30,531 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per share attributable to common shareholders per share, basic and diluted |
$ | (0.03 | ) | $ | (0.33 | ) | $ | (0.28 | ) | $ | (0.57 | ) | ||||
| Weighted average shares outstanding, basic and diluted: |
56,026,767 | 53,957,766 | 55,703,275 | 53,740,901 | ||||||||||||

Corporate Presentation August 2025 Exhibit 99.2

Forward-looking Statement This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended, including without limitation, statements regarding, among other things, the potential number of patients who could be treated by tebipenem HBr and market demand for tebipenem HBr generally; the potential regulatory path forward for tebipenem HBr, the potential approval of tebipenem HBr by the U.S. Food and Drug Administration (FDA) and the timing thereof; the potential commercialization of tebipenem HBr and its future value, the potential receipt of milestone payments and royalties on future sales of tebipenem HBr under the GlaxoSmithKline Intellectual Property (No. 3) Limited (GSK) license agreement; the effectiveness of tebipenem HBr and its potential impact on healthcare resource utilizations; the potential for SPR720; the initiation, timing, progress and results of the Company’s preclinical studies and clinical trials and its research and development programs, including management’s assessment of such results; the timing of the availability of data from the Company’s clinical trials; the timing of the Company’s filings with regulatory agencies; product candidate benefits; competitive position; cash runway, business strategies; potential growth opportunities; potential market size; projected costs and the availability of additional non-dilutive funding from governmental agencies beyond any initially funded awards. In some cases, forward-looking statements can be identified by terms such as “may,” “will,” “should,” “expect,” “plan,” “aim,” “anticipate,” “could,” “intent,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions. All statements other than statements of historical facts contained in this presentation are forward-looking statements. The Company may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various factors, including whether the FDA will ultimately approve tebipenem HBr and, if so, the timing of any such approval; whether the FDA will require any additional clinical data or place labeling restrictions on the use of tebipenem HBr that would add costs for the Company, delay approval and/or reduce the commercial prospects of tebipenem HBr; the Company’s need for additional funding; the lengthy, expensive, and uncertain process of clinical drug development; the Company’s reliance on third parties to manufacture, develop, and commercialize its product candidates, if approved; the ability to develop and commercialize the Company’s product candidates, if approved; the Company’s ability to retain key personnel; whether results obtained in preclinical studies and clinical trials will be indicative of results obtained in future clinical trials and whether preliminary data from the Company’s clinical trials will be predictive of final results from such trials; the Company’s dependence on raising capital and whether the Company’s product candidates will advance through the preclinical development and clinical trial process on a timely basis, or at all, taking into account such factors as the effects of possible regulatory delays, slower than anticipated patient enrollment, manufacturing challenges, clinical trial design, clinical data requirements and clinical outcomes; whether the results of such clinical trials will warrant submission for approval from the FDA or equivalent foreign regulatory agencies; decisions made by the FDA and equivalent foreign regulatory agencies with respect to the development and commercialization of the Company’s product candidates; the commercial potential of the Company’s product candidates; the Company’s ability to obtain adequate third-party reimbursement for its product candidates; whether the Company will satisfy all of the pre-conditions to receipt of the milestone payments under its various license and collaboration agreements; the Company’s ability to implement its strategic plans; the Company’s ability to obtain, maintain and enforce intellectual property and other proprietary rights for its product candidates; the risks and uncertainties related to market conditions; whether the Company’s cash resources will be sufficient to fund its continuing operations for the periods and/or trials anticipated; and other factors discussed in the “Risk Factors” section of the Company’s periodic reports filed with the U.S. Securities and Exchange Commission (SEC), and risks described in other filings the Company may make with the SEC in the future. The forward-looking statements included in this presentation represent the Company’s views as of the date of this presentation. The Company anticipates that subsequent events and developments will cause its views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this presentation.
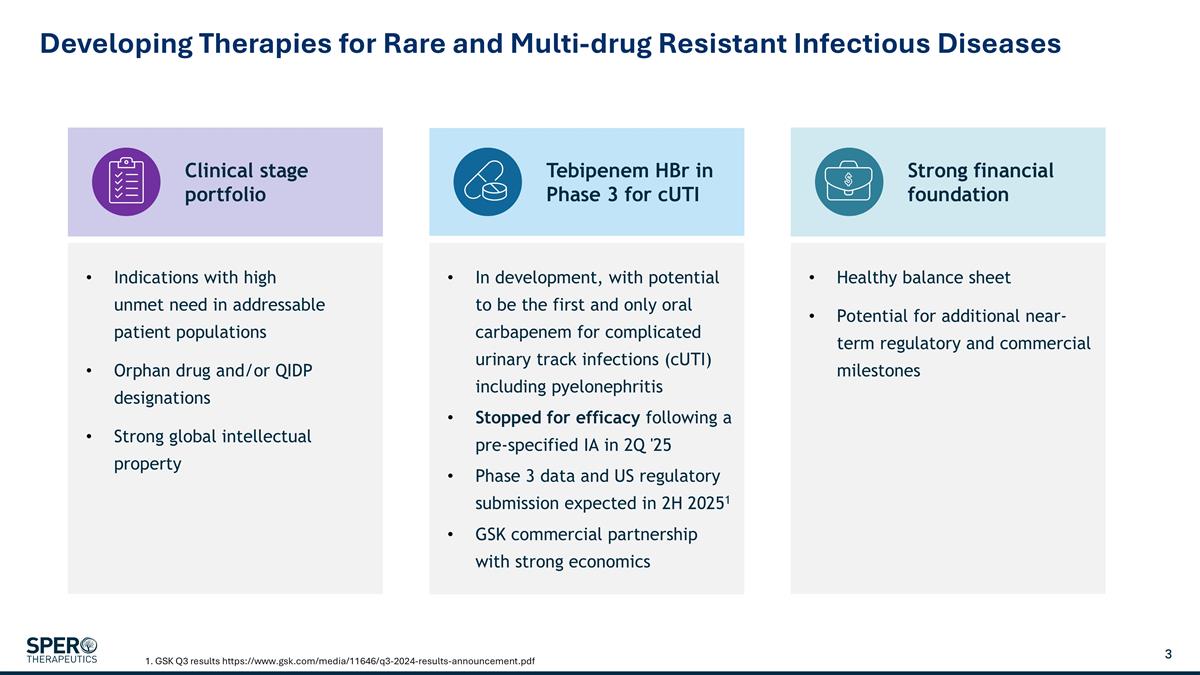
Clinical stage portfolio Strong financial foundation Tebipenem HBr in Phase 3 for cUTI Indications with high unmet need in addressable patient populations Orphan drug and/or QIDP designations Strong global intellectual property Developing Therapies for Rare and Multi-drug Resistant Infectious Diseases 1. GSK Q3 results https://www.gsk.com/media/11646/q3-2024-results-announcement.pdf Healthy balance sheet Potential for additional near-term regulatory and commercial milestones In development, with potential to be the first and only oral carbapenem for complicated urinary track infections (cUTI) including pyelonephritis Stopped for efficacy following a pre-specified IA in 2Q '25 Phase 3 data and US regulatory submission expected in 2H 20251 GSK commercial partnership with strong economics
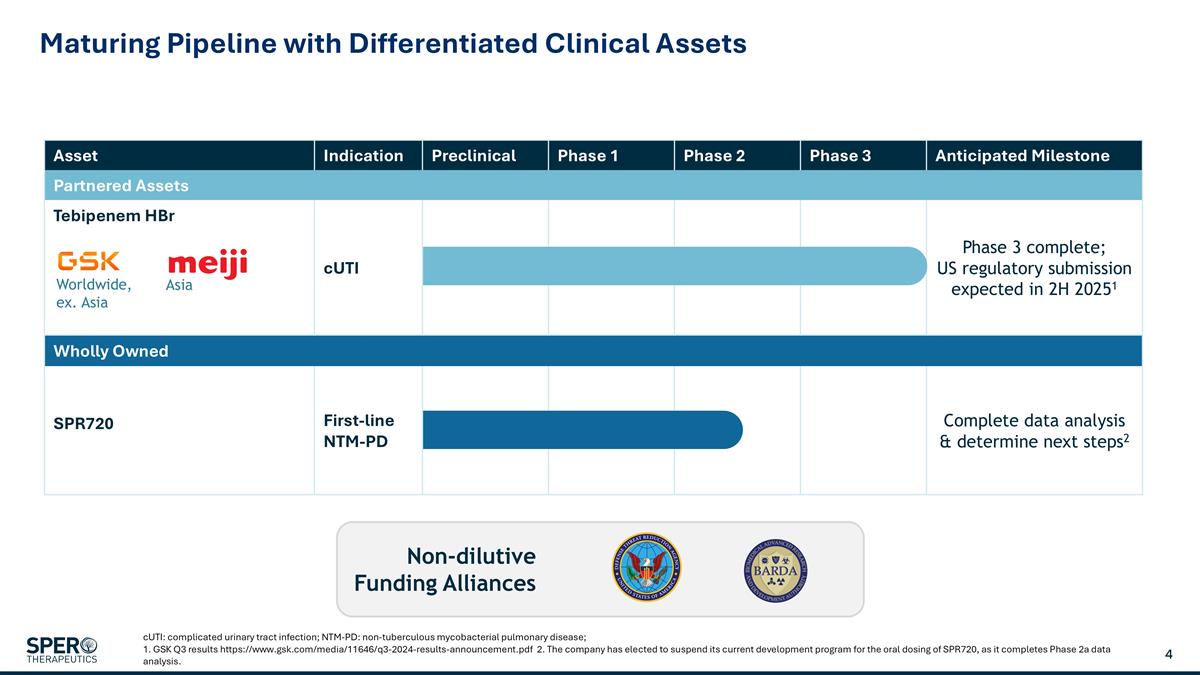
Asset Indication Preclinical Phase 1 Phase 2 Phase 3 Anticipated Milestone Partnered Assets Tebipenem HBr cUTI Phase 3 complete; US regulatory submission expected in 2H 20251 Wholly Owned SPR720 First-line NTM-PD Complete data analysis & determine next steps2 Maturing Pipeline with Differentiated Clinical Assets cUTI: complicated urinary tract infection; NTM-PD: non-tuberculous mycobacterial pulmonary disease; 1. GSK Q3 results https://www.gsk.com/media/11646/q3-2024-results-announcement.pdf 2. The company has elected to suspend its current development program for the oral dosing of SPR720, as it completes Phase 2a data analysis. Non-dilutive Funding Alliances Worldwide, ex. Asia Asia

Tebipenem HBr Oral Carbapenem for cUTI
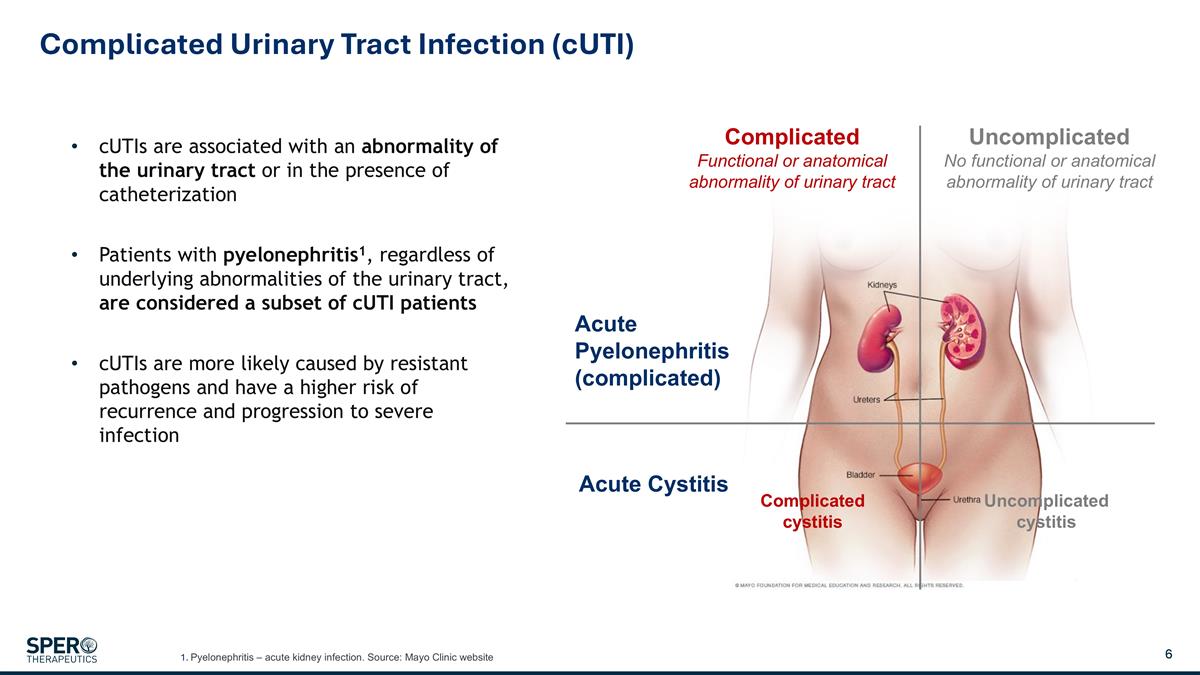
Complicated Urinary Tract Infection (cUTI) cUTIs are associated with an abnormality of the urinary tract or in the presence of catheterization Patients with pyelonephritis1, regardless of underlying abnormalities of the urinary tract, are considered a subset of cUTI patients cUTIs are more likely caused by resistant pathogens and have a higher risk of recurrence and progression to severe infection Acute Pyelonephritis (complicated) Acute Cystitis Complicated Functional or anatomical abnormality of urinary tract Uncomplicated No functional or anatomical abnormality of urinary tract Complicated cystitis Uncomplicated cystitis 1. Pyelonephritis – acute kidney infection. Source: Mayo Clinic website
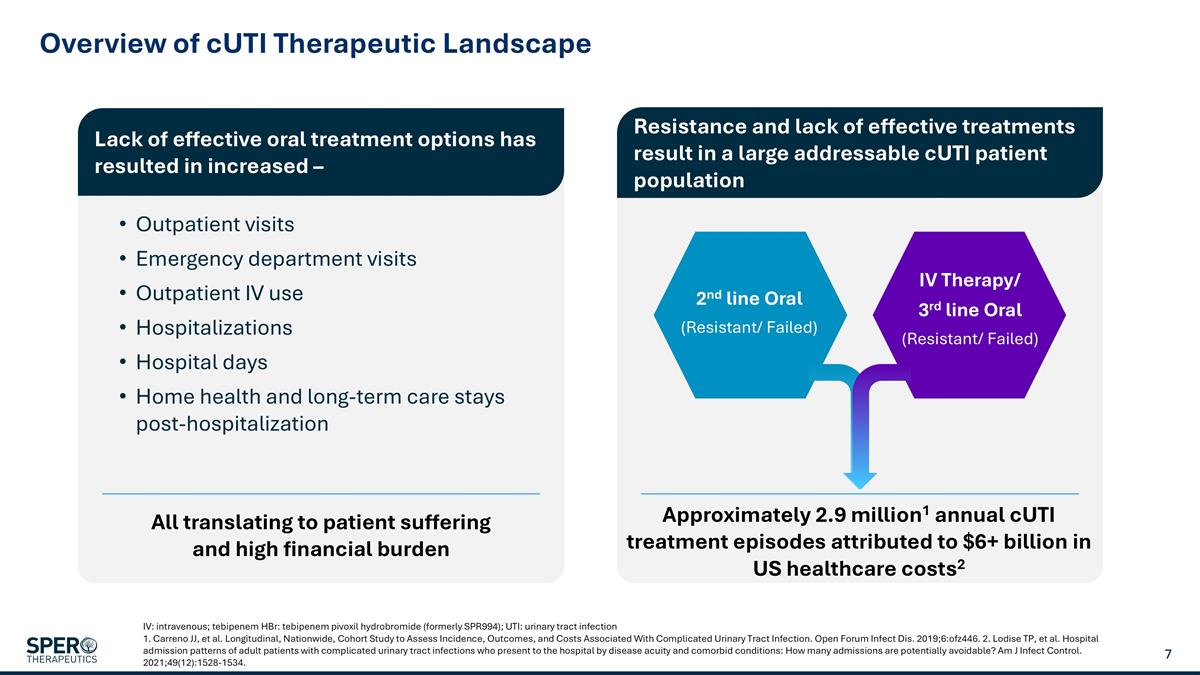
Overview of cUTI Therapeutic Landscape IV: intravenous; tebipenem HBr: tebipenem pivoxil hydrobromide (formerly SPR994); UTI: urinary tract infection 1. Carreno JJ, et al. Longitudinal, Nationwide, Cohort Study to Assess Incidence, Outcomes, and Costs Associated With Complicated Urinary Tract Infection. Open Forum Infect Dis. 2019;6:ofz446. 2. Lodise TP, et al. Hospital admission patterns of adult patients with complicated urinary tract infections who present to the hospital by disease acuity and comorbid conditions: How many admissions are potentially avoidable? Am J Infect Control. 2021;49(12):1528-1534. Lack of effective oral treatment options has resulted in increased – Outpatient visits Emergency department visits Outpatient IV use Hospitalizations Hospital days Home health and long-term care stays post-hospitalization Approximately 2.9 million1 annual cUTI treatment episodes attributed to $6+ billion in US healthcare costs2 Resistance and lack of effective treatments result in a large addressable cUTI patient population All translating to patient suffering and high financial burden 2nd line Oral (Resistant/ Failed) IV Therapy/ 3rd line Oral (Resistant/ Failed)

Tebipenem HBr: Positioned to Change the Treatment Landscape for cUTI Patients Potential first-to-market oral carbapenem Phase 3 stopped for efficacy Global commercial partnership Orally bioavailable carbapenem prodrug that rapidly converts to active moiety tebipenem Potential use as an effective oral treatment taken at home vs an IV therapy in hospitalized setting Robust IP through 2041 QIDP designation Trial met primary endpoint following pre-specified IA of data from 1,690 enrolled patients No new safety concerns identified Global trial with centers in North and South America, Europe, Africa, and Asia PIVOT-PO trial protocol approved under FDA Special Protocol Assessment (SPA) Out-licensed global commercial rights ex-Asia to GSK Japan and certain other Asian countries retained by Meiji Robust financial terms including developmental, regulatory, and commercial milestones, as well as tiered sales royalties QIDP: qualified infectious disease product.
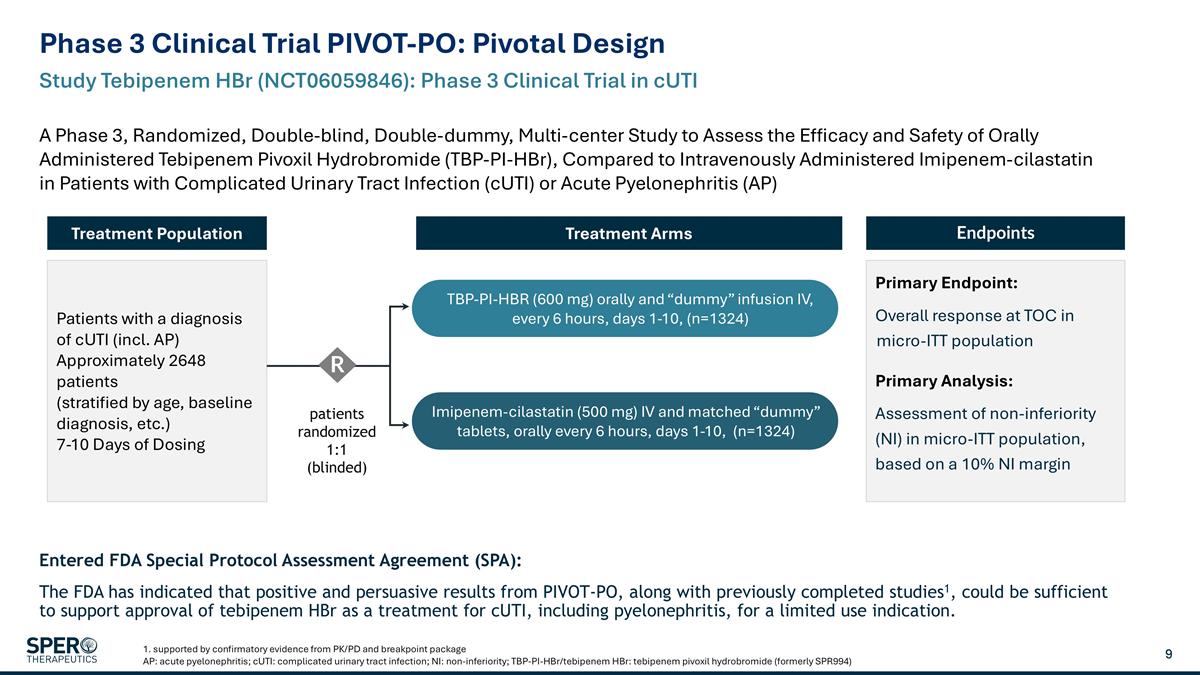
Phase 3 Clinical Trial PIVOT-PO: Pivotal Design 1. supported by confirmatory evidence from PK/PD and breakpoint package AP: acute pyelonephritis; cUTI: complicated urinary tract infection; NI: non-inferiority; TBP-PI-HBr/tebipenem HBr: tebipenem pivoxil hydrobromide (formerly SPR994) Study Tebipenem HBr (NCT06059846): Phase 3 Clinical Trial in cUTI A Phase 3, Randomized, Double-blind, Double-dummy, Multi-center Study to Assess the Efficacy and Safety of Orally Administered Tebipenem Pivoxil Hydrobromide (TBP-PI-HBr), Compared to Intravenously Administered Imipenem-cilastatin in Patients with Complicated Urinary Tract Infection (cUTI) or Acute Pyelonephritis (AP) Patients with a diagnosis of cUTI (incl. AP) Approximately 2648 patients (stratified by age, baseline diagnosis, etc.) 7-10 Days of Dosing Treatment Population Endpoints Primary Endpoint: Overall response at TOC in micro-ITT population Primary Analysis: Assessment of non-inferiority (NI) in micro-ITT population, based on a 10% NI margin Treatment Arms patients randomized 1:1 (blinded) R TBP-PI-HBR (600 mg) orally and “dummy” infusion IV, every 6 hours, days 1-10, (n=1324) Imipenem-cilastatin (500 mg) IV and matched “dummy” tablets, orally every 6 hours, days 1-10, (n=1324) Entered FDA Special Protocol Assessment Agreement (SPA): The FDA has indicated that positive and persuasive results from PIVOT-PO, along with previously completed studies1, could be sufficient to support approval of tebipenem HBr as a treatment for cUTI, including pyelonephritis, for a limited use indication.
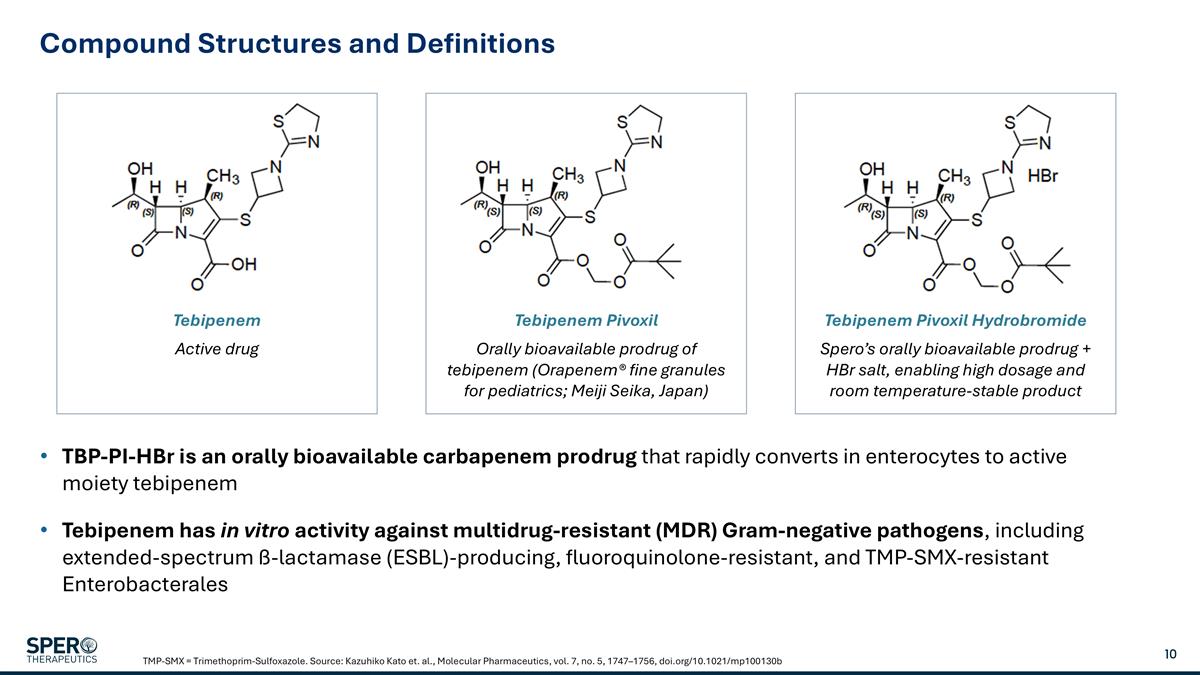
Compound Structures and Definitions TBP-PI-HBr is an orally bioavailable carbapenem prodrug that rapidly converts in enterocytes to active moiety tebipenem Tebipenem has in vitro activity against multidrug-resistant (MDR) Gram-negative pathogens, including extended-spectrum ß-lactamase (ESBL)-producing, fluoroquinolone-resistant, and TMP-SMX-resistant Enterobacterales TMP-SMX = Trimethoprim-Sulfoxazole. Source: Kazuhiko Kato et. al., Molecular Pharmaceutics, vol. 7, no. 5, 1747–1756, doi.org/10.1021/mp100130b Tebipenem Active drug Tebipenem Pivoxil Orally bioavailable prodrug of tebipenem (Orapenem® fine granules for pediatrics; Meiji Seika, Japan) Tebipenem Pivoxil Hydrobromide Spero’s orally bioavailable prodrug + HBr salt, enabling high dosage and room temperature-stable product

741 Adult subjects evaluated, across 17 efficacy and pharmacology trials 440 pediatric subjects evaluated, across 6 efficacy and pharmacology trials Generally well tolerated, comparable to common, approved oral beta lactam antibiotics and IV carbapenems Met its primary endpoint in 3 double blind controlled efficacy trials in pediatric pneumonia, otitis media, and sinusitis Approved for pediatric pneumonia, otitis media, sinusitis, over 4 million patients dosed to date Extensive post-marketing safety and efficacy surveillance completed, covering 3,331 patients No issues of safety were observed, and adequate efficacy was demonstrated 964 subjects have received at least one dose of TBP-PI-HBr 279 healthy volunteers or patients with renal impairment across Phase 1 studies 685 patients with cUTI including pyelonephritis in a previous Phase 3 study (SPR994-301; ADAPT-PO) Clinical Experience Supports Safety, Efficacy of Tebipenem1 1. Akash Jain, Luke Utley, Thomas R. Parr, Thomas Zabawa & Michael J. Pucci (2018): Tebipenem, the first oral carbapenem antibiotic, Expert Review of Anti-infective Therapy, DOI: 10.1080/14787210.2018.1496821 Tebipenem Pivoxil evaluated in 23 trials enrolling over 1,300 subjects (Meiji-Seika experience) Tebipenem Pivoxil approved in Japan since 2009 Tebipenem HBr evaluated in 10 trials enrolling over 950 subjects till date (Spero experience)

Activity of Tebipenem and Comparator Carbapenems against Gram-negative and -positive Uropathogens Pathogen N MIC90 (µg/mL) Pathogen N Tebipenem Imipenem Meropenem Ertapenem Enterobacterales (Surveillance US & EU, 2022) E. coli 1,444 0.03 ≤0.12 0.03 0.015 K. pneumoniae 404 0.12 0.5 0.06 0.25 P. mirabilis 170 0.25 4 0.12 0.015 E. cloacae (species complex) 72 0.25 0.5 0.12 2 K. oxytoca 69 0.06 0.25 0.03 0.03 K. aerogenes 40 0.12 1 0.06 0.25 S. marcescens 22 0.25 2 0.12 0.5 C. koseri 43 0.03 ≤0.12 0.03 0.008 Gram-positive uropathogens (NCRPT-0089) E. faecalis 30 0.5 NA 4 >4 S. aureus (MSSA) 20 0.03 NA 0.25 0.25 S. Saprophyticus (MS) 25 0.25 NA 0.25 1 * Spero data on file. MIC = Minimum Inhibitory Concentration; MSSA = Methicillin Susceptible Staph Aureus; MS = Methicillin Susceptible

Tebipenem is Active Against Clinically Important Resistant Enterobacterales UTI Pathogens from Europe and the USA in 2022 * Spero data on file. ESBL = Extended spectrum beta-lactamases; TMP-SMX = Trimethoprim-Sulfoxazole; R = Resistant; NS= Non susceptible; CRE = Carbapenem Resistant Enterobacterales; MIC = Minimum Inhibitory Concentration Organism/ phenotype Phenotype N MIC90 (µg/mL) Tebipenem Imipenem Meropenem Ertapenem Enterobacterales All 2,447 0.12 1 0.06 0.06 E. coli All 1,444 0.03 ≤0.12 0.03 0.015 ESBL* 205 0.03 0.25 0.03 0.12 Levofloxacin-NS* 317 0.03 0.25 0.03 0.06 TMP-SMX-R* 399 0.03 ≤0.12 0.03 0.03 K. pneumoniae All 404 0.12 0.5 0.06 0.25 ESBL* 96 0.12 0.5 0.12 1 Levofloxacin-NS* 74 0.25 0.5 0.12 1 TMP-SMX-R* 102 0.06 0.5 0.06 0.25 *Non-CRE (Imipenem-susceptible, MIC ≤1 µg/mL): 2022 European and US Surveillance Isolates (JMI Laboratories)
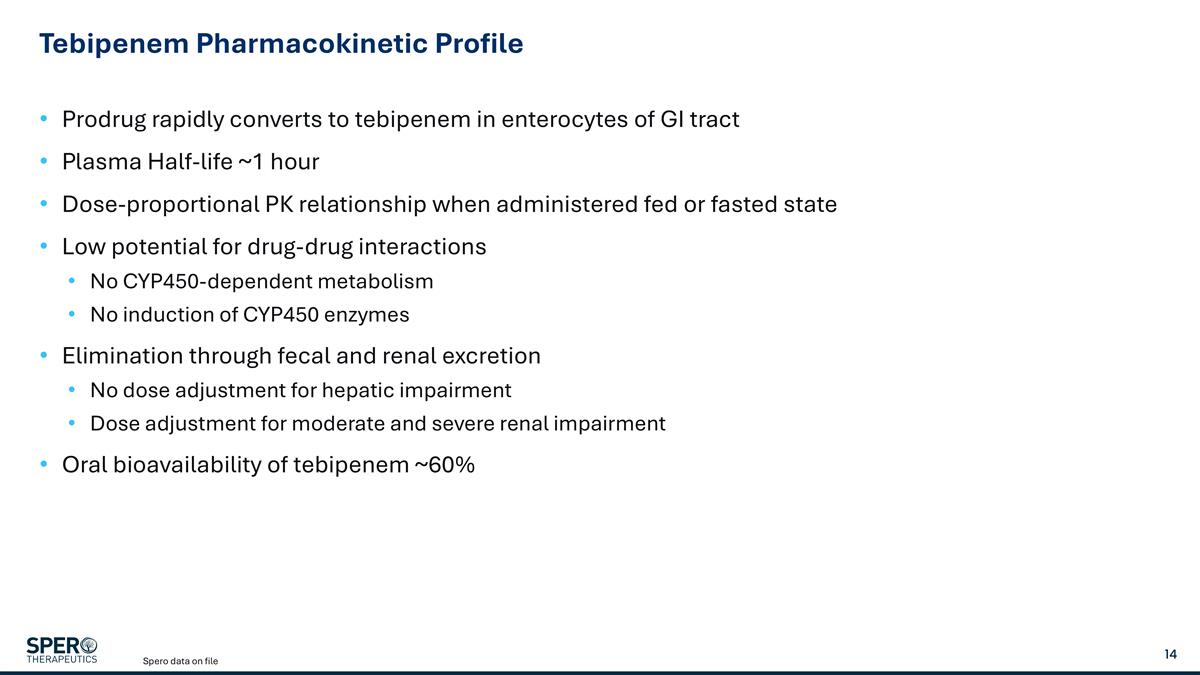
Tebipenem Pharmacokinetic Profile Prodrug rapidly converts to tebipenem in enterocytes of GI tract Plasma Half-life ~1 hour Dose-proportional PK relationship when administered fed or fasted state Low potential for drug-drug interactions No CYP450-dependent metabolism No induction of CYP450 enzymes Elimination through fecal and renal excretion No dose adjustment for hepatic impairment Dose adjustment for moderate and severe renal impairment Oral bioavailability of tebipenem ~60% Spero data on file
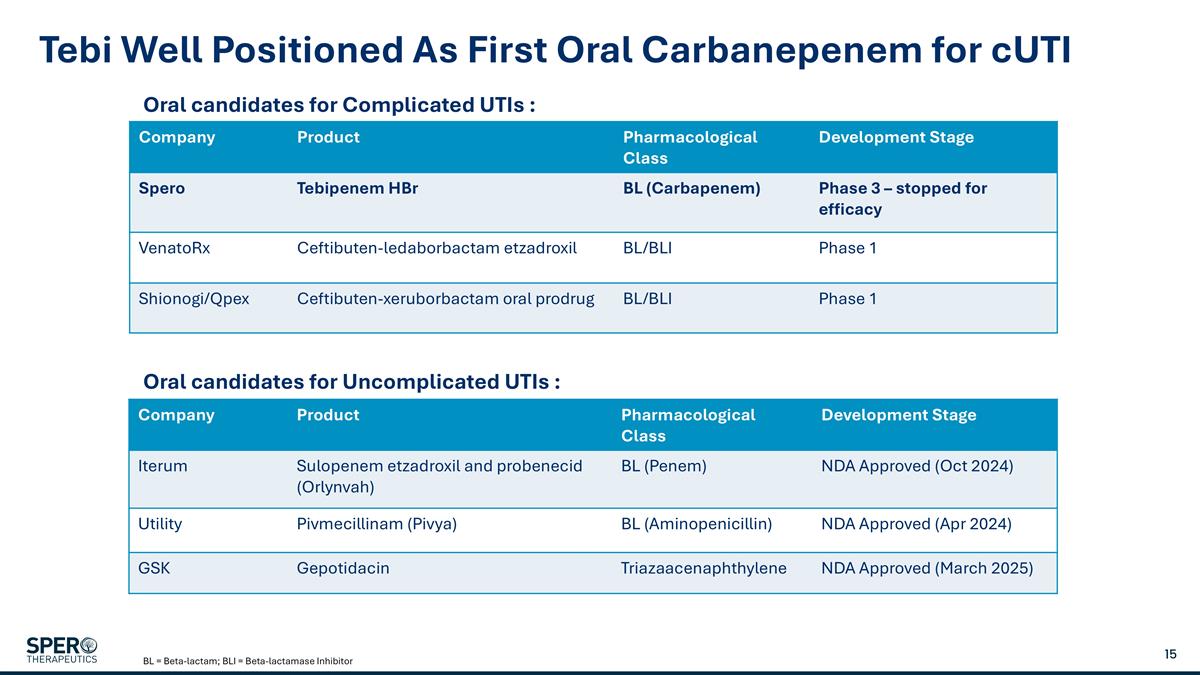
Tebi Well Positioned As First Oral Carbanepenem for cUTI Company Product Pharmacological Class Development Stage Spero Tebipenem HBr BL (Carbapenem) Phase 3 – stopped for efficacy VenatoRx Ceftibuten-ledaborbactam etzadroxil BL/BLI Phase 1 Shionogi/Qpex Ceftibuten-xeruborbactam oral prodrug BL/BLI Phase 1 Company Product Pharmacological Class Development Stage Iterum Sulopenem etzadroxil and probenecid (Orlynvah) BL (Penem) NDA Approved (Oct 2024) Utility Pivmecillinam (Pivya) BL (Aminopenicillin) NDA Approved (Apr 2024) GSK Gepotidacin Triazaacenaphthylene NDA Approved (March 2025) Oral candidates for Complicated UTIs : Oral candidates for Uncomplicated UTIs : BL = Beta-lactam; BLI = Beta-lactamase Inhibitor

Exclusive License Agreement with GSK for Tebipenem HBr and Equity Investment FPFD: first patient first dose. SPA: Special Protocol Assessment. 1. Under the terms of the GSK License Agreement, the milestone amount was revised from $150M after PIVOT-PO was stopped early for efficacy following completion of a pre-specified interim analysis of data from 1,690 patients enrolled in the trial, thereby reducing the overall cost of the study to the Company; the maximum potential milestone payment of $150M was contingent upon the trial continuing to full enrollment, with 2,637 patients enrolled in the trial. Global Collaboration (ex-Asia) Financial Terms SPRO granted GSK exclusive license to: Develop Tebipenem in territories outside of United States (not including Japan and certain other Asian countries where rights are held by Meiji Seika); and Obtain regulatory approval and commercialize tebipenem HBr in all territories, except Meiji Seika Territories Received $66 Million upfront and $9 Million in common stock investment Received $30 Million upon SPA agreement with the FDA Received $95 Million in development milestone following FPFD Spero is eligible to receive up to $351 million in additional potential regulatory, commercial, and sales milestone payments, as well as royalties: $25 Million to be paid upon GSK’s submission of tebipenem HBr’s New Drug Application (NDA) Up to $1011 million in potential commercial milestones based on first commercial sales (US/EU) Up to $225 million in sales related milestone payments Spero to receive royalties on annual net sales: 1% for annual sales up to $750.0 million each year High single-digit % on annual net sales above $750.0 million each year Low double-digit % on annual net sales above $1,000 million each year

The company has suspended its current development for SPR720 SPR720 Antibiotic for Non-Tuberculosis Mycobacterium Pulmonary Disease (NTM-PD)
SPR720: Inhibits Gyrase B in Bacterial Cells Novel mechanism of action for NTM-PD not exploited by current antibiotics No evidence of cross resistance against marketed antibiotics based on in vitro trials Low propensity for development of resistance as monotherapy and in combination with SOC antibiotics in vitro Currently formulated as an oral drug 1. Keith A. Rodvold et. Al., Antimicrobial Agents and Chemotherapy, https://doi.org/10.1128/aac.01103-24 Preclinical Studies Support activity against a broad spectrum of NTM-PD pathogens, as well as low propensity for development of resistance Proof of Concept Phase 2a oral study While the data showed antimicrobial activity associated with SPR720, an interim analysis did not show sufficient separation from placebo, highlighted potential oral dose limiting safety issues in subjects dosed at 1,000 mg orally once daily Next Steps The Company has completed data analysis of all enrolled patients (n=25) and is currently determining the next steps for the SPR720 program

Thank You