UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): July 25, 2025
IMAGENEBIO, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-40287 | 81-1697316 | ||
|
(State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
|
12526 High Bluff Drive Suite 345 San Diego, California |
92130 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: 617-901-7098
Ikena Oncology, Inc.
645 Summer Street
Suite 101
Boston, Massachusetts
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
Trading |
Name of each exchange on which registered |
||
| Common Stock, $0.001 par value | IMA | The Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Explanatory Note
On July 25, 2025, the Delaware corporation formerly known as “Ikena Oncology, Inc.” completed its previously announced merger with Inmagene Biopharmaceuticals, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands (“Inmagene”), in accordance with the terms of the Agreement and Plan of Merger, dated as of December 23, 2024 (the “Merger Agreement”), by and among Ikena Oncology, Inc. (“Ikena”), Insight Merger Sub I, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands and a direct, wholly owned subsidiary of Ikena (“Merger Sub I”), Insight Merger Sub II, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands and a direct, wholly owned subsidiary of Ikena (“Merger Sub II”), and Inmagene, providing for the merger of Merger Sub I with and into Inmagene, with Inmagene surviving as a wholly owned subsidiary of Ikena (such transaction, the “First Merger”), and the subsequent merger of the surviving entity of the First Merger with and into Insight Merger Sub II, with Merger Sub II surviving as a wholly owned subsidiary of Ikena (such transaction, the “Second Merger” and, collectively with the First Merger, as appropriate, the “Merger”). Also on July 25, 2025, Ikena changed its name from “Ikena Oncology, Inc.” to “ImageneBio, Inc.” (together with its subsidiaries, the “Company,” “we,” “our,” or “us”). See Item 2.01 for additional information regarding completion of the Merger.
| Item 1.01 | Entry into a Material Definitive Agreement. |
Registration Rights Agreement
To the extent required by Item 1.01 of Form 8-K, the information set forth under Item 3.02 of this Current Report on Form 8-K regarding the Registration Rights Agreement (as defined below) is hereby incorporated herein by reference.
Ikena Contingent Value Rights Agreement
On July 25, 2025, immediately prior to the First Effective Time (as defined below), Ikena and Computershare Trust Company, N.A., the designated rights agent, entered into a Contingent Value Rights Agreement (the “Ikena CVR Agreement”), pursuant to which Ikena shareholders of record as of the close of business on July 24, 2025 received one contingent value right (each, an “Ikena CVR”) for each outstanding share of Ikena Common Stock (as defined below) held by such stockholder on such date.
Pursuant to the Ikena CVR Agreement, each Ikena CVR holder will be entitled to certain rights to receive (i) 100% of the net proceeds, if any, received by Ikena as a result of contingent payments (“Ikena CVR Payments”) made to Ikena, such as milestone, royalty or earnout payments, received under any disposition agreements related to Ikena’s pre-Merger assets, including but not limited to IK-595 (the “Ikena CVR Assets”), including pursuant to any out-license agreements, entered into prior to the closing of the Merger and (ii) 90% of the net proceeds, if any, received by Ikena as a result of Ikena CVR Payments received under any disposition agreements related to the Ikena CVR Assets entered into after the closing date of the Merger and prior to the first anniversary of the closing of the Merger (the “Disposition Period”). Such proceeds are subject to certain permitted deductions, including for applicable tax payments, certain expenses incurred by Ikena or its affiliates, and losses incurred or reasonably expected to be incurred by Ikena or its affiliates due to a third-party proceeding in connection with a disposition and certain wind-down costs.
During and following the Disposition Period, the Company has no obligation to attempt to sell or dispose of the Ikena CVR Assets.
The Ikena CVR Payments, if any, will become payable to Computershare Trust Company, N.A. for subsequent distribution to the Ikena CVR holders. In the event that no such proceeds are received during the CVR Term (as defined in the Ikena CVR Agreement), holders of the Ikena CVRs will not receive any payment pursuant to the Ikena CVR Agreement. There can be no assurance that any Ikena CVR holders will receive any Ikena CVR Payments.
The right to the contingent payments contemplated by the Ikena CVR Agreement is a contractual right only and is not transferable, except in the limited circumstances specified in the Ikena CVR Agreement. The Ikena CVRs are not evidenced by a certificate or any other instrument and are not registered with U.S. Securities and Exchange Commission (the “SEC”). The Ikena CVRs do not have any voting or dividend rights and do not represent any equity or ownership interest in the Company or any of its affiliates. No interest will accrue on any amounts payable in respect of the Ikena CVRs.
Inmagene Contingent Value Rights Agreement
On July 25, 2025, immediately prior to the First Effective Time, Ikena, Inmagene and Computershare Trust Company, N.A., the designated rights agent, entered into a Contingent Value Rights Agreement (the “Inmagene CVR Agreement”), pursuant to which Inmagene shareholders of record as of immediately prior to the First Effective Time received one contingent value right (each, an “Inmagene CVR”) for each outstanding Inmagene share held by such shareholder on such date.
Pursuant to the Inmagene CVR Agreement, each Inmagene CVR holder will be entitled to certain rights to receive (i) 100% of the net proceeds, if any, received by Ikena as a result of contingent payments (“Inmagene CVR Payments”) made to Ikena under any disposition agreement related to the programs and projects controlled by Inmagene any time prior to the closing date of the Merger (other than its anti-OX40 monoclonal antibody asset, IMG-007) (the “Inmagene CVR Assets”), which agreement is entered into prior to the closing of the Merger and (ii) 90% of the net proceeds, if any, received by Ikena as a result of Inmagene CVR Payments received under any disposition agreement related to the Inmagene CVR Assets entered into during the Disposition Period. Such proceeds are subject to certain permitted deductions, including for applicable tax payments, certain expenses incurred by Ikena or its affiliates, and losses incurred or reasonably expected to be incurred by Ikena or its affiliates due to a third-party proceeding in connection with a disposition.
During and following the Disposition Period, the Company has no obligation to attempt to sell or dispose of the Inmagene CVR Assets.
The Inmagene CVR Payments, if any, will become payable to Computershare Trust Company, N.A. for subsequent distribution to the Inmagene CVR holders. In the event that no such proceeds are received during the CVR Term, holders of Inmagene CVRs will not receive any payment pursuant to the Inmagene CVR Agreement. There can be no assurance that any Inmagene CVR holders will receive any Inmagene CVR Payments.
The right to the contingent payments contemplated by the Inmagene CVR Agreement is a contractual right only and is not transferable, except in the limited circumstances specified in the Inmagene CVR Agreement. The Inmagene CVRs are not evidenced by a certificate or any other instrument and are not registered with SEC. The Inmagene CVRs do not have any voting or dividend rights and do not represent any equity or ownership interest in the Company or any of its affiliates. No interest will accrue on any amounts payable in respect of the Inmagene CVRs.
Transition Services Agreement
In connection with the consummation of the Legacy Asset Transaction (as defined below), on July 25, 2025, Inmagene entered into a Transition Services Agreement (the “Transition Services Agreement”) with SellCo (as defined below) for the provision by Miragene of certain transitional services related to the ongoing operations of Inmagene’s business with respect to the IMG-007 program, which may include services related to chemistry, manufacturing and controls, regulatory affairs, clinical trial support and operations, translational science research and support, bioanalysis and pharmacovigilance (collectively, the “Miragene Services”).
The initial term of the Transition Services Agreement is six months, which shall be automatically extended for an additional six months unless during the first three months of the Initial Term, the Company provides written notice to terminate the Transition Services Agreement (as may be extended, the “Initial Term”). In addition, the Company may extend the term for the receipt of the Miragene Services for up to an additional 12 months upon 60 days’ prior written notice prior to the end of the Initial Term.
Upon the closing of the Merger, the Company paid SellCo $1.25 million as pre-payment for the Miragene Services to be provided during the Initial Term. Up to $1.25 million may be payable if the Initial Term is automatically extended for the Miragene Services to be provided during such period. If the Transition Services Agreement is extended beyond the Initial Term, the Miragene Services shall be provided at an annual FTE rate of $200,000.
In addition, pursuant to the Transition Services Agreement, SellCo shall be permitted to use the Company’s principal executive offices under specified circumstances at an agreed monthly rate.
The Transition Services Agreement may be terminated after the Initial Term by either party upon 60 days’ prior written notice or by SellCo after the completion by the Company of the sale or other disposition of any portion of the Company’s business, assets or properties constituting all or a majority of the IMG-007 Business (as defined in the Transition Services Agreement).
The legacy shareholders of Inmagene own and control SellCo in the same proportions as their previous ownership interests in Inmagene, including each of Inmagene’s greater than 5% shareholders prior to the Merger, an entity affiliated with Guoliang Yu, and one of Inmagene’s former directors, Jonathan Jian Wang, Ph.D., MBA, who is SellCo’s current Chief Executive Officer and Inmagene’s former Chief Executive Officer and Chairman, as well as a current member of the Company’s board of directors (the “Board”).
The foregoing summary of the Transition Services Agreement does not purport to be complete and is qualified in its entirety by reference to the full text of the Transition Services Agreement, which is attached hereto as Exhibit 10.4 and incorporated herein by reference.
Hutchmed Collaboration, Option and License Agreement
In January 2021, Inmagene entered into a collaboration, option and license agreement (the “Hutchmed Agreement”), pursuant to which HUTCHMED Limited (formerly known as Hutchinson Medipharma Limited) (“Hutchmed”) granted Inmagene the exclusive option to the worldwide license with the right to sublicense to develop, manufacture and commercialize several licensed compounds including humanized antagonistic OX40 receptor mAb (IMG-007) (the “Licensed Compounds”) for the treatment or prevention of all diseases and conditions except oncology. The exclusive option was granted on a Licensed Compound-by-Licensed Compound basis, exercisable at Inmagene’s sole discretion upon payment of an option exercise fee in cash or the issuance of Inmagene ordinary shares.
On February 2, 2024, Inmagene exercised the option under the Hutchmed Agreement by entering into a share subscription agreement to issue 140,636,592 Inmagene ordinary shares to Hutchmed and obtained an exclusive, worldwide, royalty-bearing license with the right to sublicense through multiple tiers, under certain patents and know-how controlled by Hutchmed and Hutchmed’s right, title and interest in the joint intellectual property to develop, manufacture and commercialize any product that contains, incorporates, or otherwise includes the humanized OX40 antagonistic monoclonal antibody (anti-OX40 mAb) (“Licensed Product”).
Under the Hutchmed Agreement, we are required to pay an aggregate of up to $92.5 million for each Licensed Product upon the achievement of various development, regulatory and commercialization milestones with respect to such Licensed Product, $20.0 million of which would be due prior to the first approval of a Licensed Product in the United States, and an aggregate of up to $135.0 million for each Licensed Compound upon the achievement of various worldwide aggregate cumulative annual net sales milestones for the Licensed Products that contain such Licensed Compound. We are also obligated to pay tiered royalty rates in the high single-digit to low tens percentages to Hutchmed on a Licensed Compound-by-Licensed Compound basis for net sales of such Licensed Compounds worldwide, subject to reduction in certain circumstances. Royalties will be payable on a Licensed Product-by-Licensed Product and country-by-country basis for a period commencing upon the first commercial sale of the Licensed Product in such country and continuing until the later of (a) the expiration of all valid patent claims or regulatory exclusivity covering the Licensed Product in such country and (b) 10 years after such first commercial sale.
The Hutchmed Agreement will remain in effect until the expiration of all royalty payment obligations on a country-by-country and Licensed Product-by-Licensed Product basis, and may be earlier terminated by either party for the other party’s uncured material breach or insolvency. In addition, Hutchmed may terminate the Hutchmed Agreement if we challenge any of the licensed patents, or terminate the Hutchmed Agreement with respect to a particular Licensed Compound if we do not conduct any material development or commercialization activities for a specified period of time after we exercise the applicable exclusive option, and we have the right to terminate the Hutchmed Agreement for convenience upon advance notice to Hutchmed.
The foregoing summary of the Hutchmed Agreement does not purport to be complete and is qualified in its entirety by reference to the full text of the Hutchmed Agreement, which is attached hereto as Exhibit 10.5 and incorporated herein by reference.
Cell Line License Agreement with WuXi Biologics
In February 2021, Inmagene and WuXi Biologics (Hong Kong) Limited (“WuXi Biologics”) entered into a Cell Line License Agreement (the “Cell Line License Agreement”). Under the Cell Line License Agreement, Inmagene received a non-exclusive, worldwide, conditionally sublicensable license to certain of WuXi Biologics’ know-how, cell line, biological materials and media and feeds (the “WuXi Biologics Licensed Technology”) to make, have made, use, sell and import certain therapeutic products produced through the use of the cell line licensed by WuXi Biologics under the Cell Line License Agreement (the “WuXi Biologics Licensed Products”). Specifically, the WuXi Biologics Licensed Technology is used to manufacture a component of our IMG-007 program. In consideration of the license, Inmagene agreed to pay WuXi Biologics a non-refundable license fee of $150,000. Additionally, if we manufacture all of our commercial supplies of WuXi Biologics Licensed Products with a manufacturer other than WuXi Biologics or its affiliates, we are required to make royalty payments to WuXi Biologics in an amount equal a fraction of a single digit percentage of global net sales of WuXi Biologics Licensed Products manufactured by a third-party manufacturer. The Cell Line License Agreement will continue indefinitely unless terminated (i) by us upon a certain time period’s prior written notice and our payment of all undisputed amounts due to WuXi Biologics through the effective date of termination, (ii) by either party for a material breach by the other party that remains uncured for certain a period of time after written notice, or (iii) by WuXi Biologics if we fail to make a payment and such failure continues for a certain period of time after receiving notice of such failure.
The foregoing summary of the Cell Line License Agreement does not purport to be complete and is qualified in its entirety by reference to the full text of the Cell Line License Agreement, which is attached hereto as Exhibit 10.6 and incorporated herein by reference.
| Item 2.01 | Completion of Acquisition or Disposition of Assets. |
The Merger
On July 25, 2025, Ikena, Merger Sub I, Merger Sub II and Inmagene consummated the transactions contemplated by the Merger Agreement. Effective at 8:45 a.m. eastern time on July 25, 2025, Ikena effected a 1-for-12 reverse stock split of its common stock (the “Reverse Stock Split”). Effective at 10:30 a.m. eastern time on July 25, 2025, pursuant to the Plan of Merger for the First Merger, Merger Sub I was merged with and into Inmagene and Inmagene became a wholly owned subsidiary of the Company. Effective at 10:31 a.m. eastern time on July 25, 2025, pursuant to the Plan of Merger for the Second Merger, Merger Sub II was merged with and into Inmagene and Merger Sub II became a wholly owned subsidiary of the Company. Effective at 11:00 a.m. eastern time on July 25, 2025, the Company changed its name to “ImageneBio, Inc.” (the “Name Change”). Following the completion of the Merger, the business conducted by the Company became primarily the business conducted by Inmagene, immediately prior to the Merger, which is a clinical-stage biopharmaceutical company focused on the development of innovative and differentiated therapies for immunological and inflammatory (“I&I”) diseases. Unless noted otherwise, all references to share and per share amounts in this Current Report on Form 8-K reflect the Reverse Stock Split.
Under the terms of the Merger Agreement, at the effective time of the First Merger (the “First Effective Time”), (a) each ordinary share and preferred share of Inmagene (each such share, an “Inmagene Share”) held as treasury shares immediately prior to the First Effective Time were canceled and ceased to exist, and no consideration was delivered in exchange therefor, (b) each then-outstanding Inmagene Share was converted into the right to receive 0.0030510 shares of Ikena Common Stock, par value $0.001 per share (“Ikena Common Stock”) (such ratio, the “Exchange Ratio”) and (c) each then-outstanding option to purchase Inmagene Shares was converted into an option to purchase Ikena Common Stock, subject to adjustment as set forth in the Merger Agreement. At the First Effective Time, Ikena issued an aggregate of 4,601,368 shares of Ikena Common Stock to Inmagene shareholders based on the Exchange Ratio, resulting in approximately 11,181,676 shares of our common stock outstanding immediately following the Merger and the PIPE Financing (as defined below), which is based on an estimated number of shares outstanding immediately following the Reverse Stock Split and may be adjusted following confirmation by the Company’s transfer agent of the actual number of shares outstanding immediately following the Reverse Stock Split.
Following the closing of the First Merger, the pre-Merger Inmagene equityholders owned approximately 55.0% of the Company and the pre-Merger Ikena equityholders owned approximately 45.0% of the Company, in each case, on a fully diluted basis using the treasury stock method. Following the closing of the PIPE Financing (as defined below), the pre-Merger Inmagene equityholders owned approximately 43.5% of the Company, the pre-Merger Ikena equityholders owned approximately 35.0% of the Company, in each case of Inmagene and Ikena, on a fully diluted basis calculated using the treasury stock method, and the investors who were issued shares of Ikena Common Stock in the PIPE Financing owned approximately 21.5% of the Company.
The issuance of the shares of the Company’s common stock to the former shareholders of Inmagene was registered with the SEC on the Company’s Registration Statement on Form S-4 (File No. 333-285881), as amended and declared effective by the SEC on June 11, 2025 (the “Registration Statement”).
The shares of the Company’s common stock, which traded on The Nasdaq Global Market through the close of business on Friday, July 25, 2025 under the ticker symbol “IKNA,” commenced trading on The Nasdaq Capital Market on a post-Reverse Stock Split adjusted basis under the ticker symbol “IMA” on July 28, 2025. The Company’s common stock is represented by a new CUSIP number, 45175G 207.
The foregoing description of the Merger and the Merger Agreement contained herein does not purport to be complete and is qualified in its entirety by reference to the full text of the Merger Agreement, which is attached hereto as Exhibit 2.1 and incorporated herein by reference.
The Non-OX40 Divestiture
On July 25, 2025, immediately prior to consummation of the Merger, Inmagene consummated the divestiture of the non-IMG-007 business related assets, business and operations (the “Non-OX40 Business”) controlled by Inmagene immediately prior to the Merger (the “Non-OX40 Divestiture”). Specifically, Inmagene sold and transferred (including via sublicense) all of the Non-OX40 Business to Miragene Inc, a newly formed private company and wholly owned subsidiary of Inmagene (“SellCo”).
As part of the Non-OX40 Divestiture, Miragene Co, a newly formed private company (“BuyCo”) held by the holders of Inmagene’s outstanding shares prior to the Merger, purchased from Inmagene all of the outstanding share capital of SellCo (holding the Non-OX40 Business) in exchange for a promissory note in the amount of $8,900,000 issued by BuyCo to Inmagene. Any payments made under the promissory note from BuyCo to Inmagene will be distributed to Inmagene CVR holders as Inmagene CVR Payments.
As a result of the Non-OX40 Divestiture, IMG-007, a non-depleting anti-OX40 monoclonal antibody, for the treatment of atopic dermatitis and other potential indications, is the only product candidate of the Company in clinical development and the only product candidate the Company plans to initially develop.
FORM 10 INFORMATION
Cautionary Note Regarding Forward-Looking Statements
This Current Report on Form 8-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”) and 21E of the Exchange Act of 1934, as amended (the “Exchange Act”), including statements regarding the anticipated benefits of the Merger and the financial condition, results of operations, and prospects of the Company. These statements may discuss goals, intentions and expectations as to future plans, trends, events, results of operations or financial condition, or otherwise, based on current expectations and beliefs of the management of the Company, as well as assumptions made by, and information currently available to, the management of the Company. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as “may,” “will,” “should,” “would,” “expect,” “anticipate,” “plan,” “likely,” “believe,” “estimate,” “project,” “intend,” “seek,”
“predict,” “pro forma,” “possible,” “potential,” and other similar expressions or the negative or plural of these words, or other similar expressions that are predictions or indicate future events or prospects, although not all forward-looking statements contain these words. Statements that are not historical facts are forward-looking statements. Forward-looking statements in this communication include, but are not limited to, the section titled “Business, Facilities and Legal Proceedings” and the disclosures incorporated by reference into the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in this Current Report on Form 8-K. Forward-looking statements are based on current beliefs and assumptions that are subject to risks and uncertainties and are not guarantees of future performance. Forward-looking statements include, but are not limited to, any statements regarding the strategies, prospects, plans, expectations or objectives of management the Company for future operations, the progress, scope or timing of the development of IMG-007, the commercial or market opportunity and potential benefits of IMG-007, the expectations surrounding the potential safety, efficacy, and regulatory and clinical progress of IMG-007 and anticipated milestones and timing therefor, the Company’s ongoing and planned clinical trials, including the expected timing for data readouts, the ability of the Company to protect its intellectual property rights, the anticipated operations, financial position, ability to raise capital to fund operations, revenues, costs or expenses of the Company, cash runway, the expected benefits from the Merger, the potential of the Ikena CVR holders or the Inmagene CVR holders to receive any payments under the Ikena CVR Agreement or Inmagene CVR Agreement, respectively, the estimated number of shares outstanding immediately following the Reverse Stock Split, statements regarding future economic conditions or performance, statements of belief and any statement of assumptions underlying any of the foregoing.
The foregoing review of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the disclosures incorporated by reference into the “Risk Factors” section of this Current Report on Form 8-K and other documents to be filed by the Company from time to time with the SEC discussions of potential risks, uncertainties, and other important factors in the Company’s subsequent filings with the SEC, and risk factors associated with companies, such as the Company, that operate in the biopharma industry. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond the Company’s control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements. Nothing in this communication should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that the contemplated results of any such forward-looking statements will be achieved. Forward-looking statements in this communication speak only as of the day they are made and are qualified in their entirety by reference to the cautionary statements herein. Except as required by applicable law, the Company undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events or otherwise.
Business and Facilities
The information set forth in the section of the definitive proxy statement/prospectus (the “Proxy Statement/Prospectus”) included in the Registration Statement entitled “Inmagene’s Business” beginning on page 291 is incorporated herein by reference.
On July 1, 2025, Inmagene announced the successful dosing of the first patient in its global multicenter Phase 2b dose-finding study (ADAPTIVE Trial, NCT07037901) of IMG-007 in patients with moderate-to-severe atopic dermatitis (“AD”).
Recent Developments
On July 25, 2025, Inmagene completed the Merger and the Non-OX40 Divestiture. The information set forth in Item 2.01 of this Current Report on Form 8-K under the headings “The Merger” and “The Non-OX40 Divestiture” are incorporated herein by reference.
Risk Factors
The information set forth in the section of the Proxy Statement/Prospectus entitled “Risk Factors” beginning on page 30 is incorporated herein by reference.
Financial Information
The information set forth in Item 9.01 of this Current Report on Form 8-K concerning the financial information of the Company is incorporated herein by reference. The unaudited pro forma condensed combined financial information of the Company as of and for the three months ended March 31, 2025 and the year ended December 31, 2024 is set forth in Exhibit 99.2 hereto and is incorporated herein by reference.
Management’s Discussion and Analysis of Financial Condition and Results of Operations
Management’s Discussion and Analysis of Financial Condition and Results of Operations of Ikena for the years ended December 31, 2023 and 2024 is set forth in the section of the Proxy Statement/Prospectus entitled “Ikena Management’s Discussion and Analysis of Financial Condition and Results of Operations” beginning on page 328 and is incorporated herein by reference. Management’s Discussion and Analysis of Financial Condition and Results of Operations of Inmagene for the years ended December 31, 2023 and 2024 and the three months ended March 31, 2024 and 2025 is set forth in the section of the Proxy Statement/Prospectus entitled “Inmagene Management’s Discussion and Analysis of Financial Condition and Results of Operations” beginning on page 342 and is incorporated herein by reference.
Management’s Discussion and Analysis of Financial Condition and Results of Operations for Ikena for the quarter ended June 30, 2025 is set forth in the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” beginning on page 13 of Ikena’s Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2025, filed with the SEC on July 24, 2025, and is incorporated herein by reference.
Security Ownership of Certain Beneficial Owners and Management
The following table and the related notes present certain information with respect to the beneficial ownership of our common stock as of immediately following the consummation of the Merger and the PIPE Financing by: (i) each person or group of affiliated persons known by us to be the beneficial owner of more than 5% of our common stock; (ii) each of our directors; (iii) each of our named executive officers; and (iv) all of our executive officers and directors as a group.
Unless otherwise indicated in the footnotes to this table, we believe that each of the persons named in this table have sole voting and investment power with respect to the shares indicated as beneficially owned.
The following table lists applicable percentage ownership based on an estimated 11,181,676 shares of our common stock outstanding immediately following the Merger and gives effect to the Reverse Stock Split. Shares of our common stock that may be acquired by an individual or group within 60 days of July 25, 2025, pursuant to the exercise of options or warrants, are deemed to be outstanding for the purpose of computing the percentage ownership of such individual or group, but are not deemed to be outstanding for the purpose of computing the percentage ownership of our common stock of any other person shown in the table.
Unless otherwise indicated, the address for the following stockholders is: c/o ImageneBio, Inc., 12526 High Bluff Drive, Suite 345, San Diego, CA 92130.
Name of Beneficial Owner |
Number of Shares Beneficially Owned (#) |
Percentage of Shares Beneficially Owned (%) |
||||||
Greater than 5% Holders: |
|
|||||||
Engene Inc.(1) |
971,173 | 8.69 | % | |||||
Entities affiliated with OrbiMed Advisors LLC(2) |
909,648 | 8.13 | % | |||||
Deep Track Capital, LP(3) |
878,516 | 7.86 | % | |||||
Entities affiliated with Biotechnology Value Fund, L.P.(4) |
802,102 | 7.17 | % | |||||
Directors and Named Executive Officers: |
||||||||
Kristin Yarema, Ph.D. |
— | — | ||||||
Jotin Marango, M.D., Ph.D.(5) |
11,146 | * | ||||||
Yufang Lu, M.D., Ph.D.(6) |
38,779 | * | ||||||
Erin Butler(7) |
4,878 | * | ||||||
Jonathan Jian Wang, Ph.D., MBA(8) |
1,115,005 | 9.97 | % | |||||
Stephen Hui Wang, MBA(9) |
250,721 | 2.24 | % | |||||
Weiguo Su, Ph.D. |
— | — | ||||||
Otello Stampacchia, Ph.D.(10) |
379 | * | ||||||
David P. Bonita, M.D. |
— | — | ||||||
All directors and executive officers as a group (9 persons)(11) |
1,420,908 | 12.65 | % | |||||
| * | Represents beneficial ownership of less than 1%. |
| (1) | The registered address of Engene Inc. is Trident Chambers, P.O. Box 146, Road Town, Tortola, British Virgin Islands. Engene Inc. is controlled by Jonathan Jian Wang. |
| (2) | Represents (i) 83,611 shares of common stock issued to OrbiMed Private Investments VI, LP (“OPI VI”) upon the closing of the PIPE Financing, (ii) 825,659 shares of common stock beneficially owned prior to the Merger and (iii) 379 shares of common stock held by David P. Bonita, M.D. Except for the shares reported in clause (iii), the information herein for shares held prior to the Merger is based on the Schedule 13D/A filed with the SEC on June 18, 2025 and the Form 4/A filed with the SEC on September 21, 2023 by OrbiMed Advisors LLC (“OrbiMed Advisors”), OrbiMed Capital GP VI LLC (“OrbiMed GP”), OrbiMed Genesis GP LLC (“OrbiMed Genesis GP”), and OrbiMed Capital LLC (“OrbiMed Capital”) (collectively, the “Reporting Persons”), except that (x) the number of non-voting shares of common stock as disclosed on the Schedule 13D/A has been revised herein because it exceeds the number of non-voting shares outstanding by one share and (y) the number of shares have been adjusted to effect to the Reverse Stock Split. OrbiMed Advisors has shared voting and dispositive power over 211,858 voting shares of common stock, comprised of: (a) 204,274 voting shares of common stock held by OrbiMed Private Investments VI, LP (“OPI VI”), of which 29,432 shares were acquired in connection with Ikena’s acquisition of Pionyr, and over which OrbiMed GP has shared voting and dispositive power, and (b) 7,584 voting shares of common stock held by OrbiMed Genesis Master Fund, L.P. (“OrbiMed Genesis”), over which OrbiMed Genesis GP has shared voting and dispositive power. Worldwide Healthcare Trust PLC (“WWH”) holds 83,087 voting shares of common stock, which may be deemed to be beneficially owned by OrbiMed Capital. Additionally, OPI VI holds 465,178 shares of non-voting common stock, OrbiMed Genesis holds 13,107 shares of non-voting common stock, and WWH holds 52,429 shares of non-voting common stock. Furthermore, pursuant to an agreement between Dr. Bonita and OPI VI, OPI VI, has sole voting and dispositive power over any shares of common stock held by Dr. Bonita. OrbiMed GP is the general partner of OPI VI, pursuant to the terms of the limited partnership agreement of OPI VI, and OrbiMed Advisors is the managing member of OrbiMed GP, pursuant to the terms of the limited liability company agreement of OrbiMed GP. As a result, OrbiMed Advisors and OrbiMed GP share power to direct the vote and disposition of the shares held by OPI VI and may be deemed directly or indirectly, including by reason of their mutual affiliation, to be the beneficial owners of the shares held by OPI VI. OrbiMed Advisors exercises this investment and voting power through a management committee comprised of Carl L. Gordon, Sven H. Borho, and W. Carter Neild, each of whom disclaims beneficial ownership of the shares held by OPI VI. OrbiMed Genesis GP is the general partner of OrbiMed Genesis, pursuant to the terms of the limited partnership agreement of OrbiMed Genesis, and |
| OrbiMed Advisors is the managing member of OrbiMed Genesis GP, pursuant to the terms of the limited liability company agreement of OrbiMed Genesis GP. As a result, OrbiMed Advisors and OrbiMed Genesis GP share power to direct the vote and disposition of the shares held by OrbiMed Genesis and may be deemed, directly or indirectly, including by reason of their mutual affiliation, to be the beneficial owners of the shares held by OrbiMed Genesis. OrbiMed Advisors exercises this investment and voting power through a management committee comprised of Carl L. Gordon, Sven H. Borho, and W. Carter Neild, each of whom disclaims beneficial ownership of the shares held by OrbiMed Genesis. OrbiMed Capital is the investment advisor of WWH. As a result, OrbiMed Capital has the power to direct the vote and disposition of the shares held by WWH and may be deemed directly or indirectly, including by reason of mutual affiliation, to be the beneficial owner of the shares held by WWH. OrbiMed Capital exercises this investment and voting power through a management committee comprised of Carl L. Gordon, Sven H. Borho, and W. Carter Neild, each of whom disclaims beneficial ownership of the shares held by WWH. The principal business address of each of these entities and individuals is c/o OrbiMed Advisors LLC, 601 Lexington Avenue 54th Floor, New York, NY 10022. |
| (3) | Represents (i) 668,890 shares of common stock issued to Deep Track Biotechnology Master Fund, Ltd. (“Deep Track Master Fund”) upon the closing of the PIPE Financing and (ii) 209,626 shares of common stock beneficially owned prior to the Merger, after giving effect to the Reverse Stock Split. The information herein for shares held prior to the Merger is based on the Schedule 13G filed with the SEC on February 14, 2025 by Deep Track Capital, LP (“Deep Track”), Deep Track Master Fund and David Kroin, except that the number of shares have been adjusted to effect to the Reverse Stock Split. Deep Track, Deep Track Master Fund and David Kroin each have shared voting and shared dispositive power with respect to the 898,786 shares. David Kroin is the managing member of Deep Track Master Fund and may be considered a control person of Deep Track. The address of Deep Track is 200 Greenwich Ave, 3rd Floor, Greenwich, CT 06830. The address of Deep Track Master Fund is c/o Walkers Corporate Limited, 190 Elgin Ave, George Town, KY1-9001, Cayman Islands. The address of David Kroin is c/o Deep Track Capital, LP, 200 Greenwich Ave, 3rd Floor, Greenwich, CT 06830. |
| (4) | Represents (i) an aggregate of 468,223 shares of common stock issued to entities affiliated with Biotechnology Value Fund, L.P. (“BVF”) upon the closing of the PIPE Financing, of which 243,528 were issued to BVF, 186,504 were issued Biotechnology Value Fund II, L.P. (“BVF2”), 27,181 were issued to Biotechnology Value Trading Fund OS LP (“Trading Fund OS”) and 11,010 were issued to MSI BVF SPV, LLC (“MSI”), and (ii) 333,879 shares of common stock beneficially owned prior to the Merger, after giving effect to the Reverse Stock Split. The information herein for shares held prior to the Merger is based on the Schedule 13G/A filed with the SEC on February 14, 2025 by BVF, except that the number of shares have been adjusted to effect to the Reverse Stock Split, which consists of (i) 172,237 shares beneficially owned by BVF, (ii) 139,092 shares beneficially owned by BVF2 and (iii) 17,397 shares beneficially owned by Trading Fund OS. BVF I GP LLC (“BVF GP”), as the general partner of BVF, may be deemed to beneficially own the 172,237 shares beneficially owned by BVF. BVF II GP LLC (“BVF2 GP”), as the general partner of BVF2, may be deemed to beneficially own the 139,092 shares beneficially owned by BVF2. BVF Partners OS Ltd. (“Partners OS”), as the general partner of Trading Fund OS, may be deemed to beneficially own the 17,397 shares beneficially owned by Trading Fund OS. BVF GP Holdings LLC (“BVF GPH”), as the sole member of each of BVF GP and BVF2 GP, may be deemed to beneficially own the 311,329 shares beneficially owned in the aggregate by BVF and BVF2. BVF Partners L.P. (“Partners”), as the investment manager of BVF, BVF2, Trading Fund 0S and MSI, beneficially own the 333,879 shares beneficially owned in the aggregate by BVF, BVF2 and Trading Fund OS and held in a certain Partners managed account (the “Partners Managed Account”), including 5,153 shares held in the Partners Managed Account, and any shares held by MSI upon the closing of the Ikena concurrent financing. BVF Inc., as the general partner of Partners, may be deemed to beneficially own the 333,879 shares beneficially owned by Partners. Mark N. Lampert, as a director and officer of BVF Inc., may be deemed to beneficially own the 333,879 shares beneficially owned by BVF Inc. BVF GP disclaims beneficial ownership of the shares beneficially owned by BVF. BVF2 GP disclaims beneficial ownership of the shares beneficially owned by BVF2. Partners OS disclaims beneficial ownership of the shares beneficially owned by Trading Fund OS. BVF GPH disclaims beneficial ownership of the shares beneficially owned by BVF and BVF2. Each of Partners, BVF Inc. and Mr. Lampert disclaims beneficial ownership of the shares beneficially owned by BVF, BVF2 and Trading Fund OS and held in the Partners Managed Account. The address of the principal business office of BVF, BVF GP, BVF2, BVF2 GP, BVF GPH, MSI and Partners is 44 Montgomery St., 40th Floor, San Francisco, California 94104 and the address of the principal business office of Trading Fund OS and Partners OS is PO Box 309 Ugland House, Grand Cayman, KY1-1104, Cayman Islands. |
| (5) | Consists of 11,146 shares of common stock. |
| (6) | Consists of 38,779 shares of common stock that are issuable upon the exercise of stock options within 60 days of July 25, 2025. |
| (7) | Consists of (i) 3,051 shares of common stock and (ii) 1,827 shares of common stock that are issuable upon the exercise of stock options within 60 days of July 25, 2025. |
| (8) | Consists of (i) the shares listed in footnote (1) above and (ii) 143,832 shares of common stock held by Dr. Wang. |
| (9) | Represents (i) 188,042 shares of common stock held by HLC Healthcare HK Limited, a corporation incorporated in Hong Kong, (ii) 50,144 shares of common stock held by Galaxy Alpha L.P., a limited partnership incorporated in Cayman Islands, and (iii) 12,535 shares of common stock held by Magic Hat L.P., a limited partnership incorporated in Cayman Islands. The registered address of HLC Healthcare HK Limited is Suite 603, 6/F, Laws Commercial Plaza, 788 Cheung Sha Wan Road, Kowloon, Hong Kong. The registered address of Galaxy Alpha L.P. is Maricorp Services Ltd., P.O. Box 2075, #31 the Strand, 46 Canal Point Drive, Grand Cayman KY1-1105, Cayman Islands. The registered address of Magic Hat L.P. is Maricorp Services Ltd., P.O. Box 2075, #31 the Strand, 46 Canal Point Drive, Grand Cayman KY1-1105, Cayman Islands. HLC Healthcare HK Limited is controlled by HLC Partners III L.P., whose general partner is HLC GP III Company Limited (“HLC GP”). HLC GP is wholly owned by Mr. Stephen Hui Wang. HLC GP also acts as the general partner of Galaxy Alpha L.P. and Magic Hat L.P. The voting and investment power of shares held by HLC Healthcare HK Limited, Galaxy Alpha L.P. and Magic Hat L.P. is exercised by Mr. Stephen Hui Wang. |
| (10) | Consists of 379 shares of common stock. |
| (11) | Consists of the shares listed in footnotes (5)-(10) above. |
Information about Directors and Executive Officers; Director Compensation and Director Independence; Executive Compensation
The information set forth in Item 5.02 of this Current Report on Form 8-K is incorporated herein by reference. The information set forth in the section of the Proxy Statement/Prospectus entitled “Management Following the Merger” beginning on page 355 is incorporated herein by reference.
A description of the compensation of the named executive officers and directors of Inmagene and the compensation of the named executive officers and directors of Ikena before the consummation of the Merger is set forth in the Proxy Statement/Prospectus in the sections titled “Inmagene Executive and Director Compensation” beginning on page 234 of the Proxy Statement/Prospectus and “Ikena Executive and Director Compensation” beginning on page 227 of the Proxy Statement/Prospectus, respectively, and that information is incorporated herein by reference.
Certain Relationships and Related Party Transactions
The information set forth in the section of the Proxy Statement/Prospectus entitled “Certain Relationships and Related Person Transactions of the Combined Company” beginning on page 362 is incorporated herein by reference.
The information set forth in Item 1.01 of this Current Report on Form 8-K under the heading “Transition Services Agreement” is incorporated herein by reference.
The information set forth in Item 2.01 of this Current Report on Form 8-K under the heading “The Non-OX40 Divestiture” is incorporated herein by reference.
The information set forth in Item 3.02 of this Current Report on Form 8-K is incorporated herein by reference.
Legal Proceedings
The information set forth in the sections of the Proxy Statement/Prospectus entitled “Ikena’s Business—Legal Proceedings” on page 290 and “Inmagene’s Business—Legal Proceedings” on page 318 are incorporated herein by reference.
Since the announcement of the Merger Agreement on December 23, 2024, two lawsuits (captioned captions Smith v. Ikena Oncology, Inc., et al., No. 653576/2025 (N.Y. Sup. Ct.) and Kent v. Ikena Oncology, Inc., et al., No. 653588/2025 (N.Y. Sup. Ct.)) were filed in the Supreme Court of the State of New York against Ikena and its directors. The complaints, filed by purported stockholders of Ikena, assert negligence claims under New York common law and allege that the prospectus filed in connection with the Merger omitted certain purportedly material information which rendered the prospectus incomplete and misleading. The complaints seek equitable and money damages. The Company believes that the allegations in the complaints described above are without merit, and that the disclosures set forth in the prospectus comply fully with all applicable law.
Market Price of and Dividends on the Registrant’s Common Equity and Related Stockholder Matters
Shares of Ikena’s common stock were historically listed on The Nasdaq Global Market under the symbol “IKNA.” Shares of the Company’s common stock commenced trading on The Nasdaq Capital Market on a post-Reverse Stock Split adjusted basis on July 28, 2025 under the symbol “IMA.”
As of the closing and following the completion of the Merger and after giving effect to the Reverse Stock Split, the Company had an estimated 11,181,676 shares of common stock issued and outstanding, which is based on an estimated number of shares outstanding immediately following the Reverse Stock Split and may be adjusted following confirmation by the Company’s transfer agent of the actual number of shares outstanding immediately following the Reverse Stock Split, held of record by an estimated number of approximately 93 holders. The number of holders of record does not include a substantially greater number of “street name” holders or beneficial holders whose shares of Company common stock are held of record by banks, brokers and other financial institutions.
The information set forth in the section of the Proxy Statement/Prospectus entitled “Market Price and Dividend Information—Dividends” on page 153 is incorporated herein by reference.
Recent Sales of Unregistered Securities
The information set forth in Item 3.02 of this Current Report on Form 8-K is incorporated herein by reference.
Description of Registrant’s Securities
The information set forth in the section of the Proxy Statement/Prospectus entitled “Description of Ikena Capital Stock” beginning on page 381 and in the section entitled “Comparison of Rights of Holders of Ikena Capital Stock and Inmagene Capital Stock” beginning on page 383 is incorporated herein by reference.
Indemnification of Directors and Officers
The information set forth in Item 5.02 of this Current Report on Form 8-K under the heading “Indemnification Agreements” is incorporated herein by reference.
A description of the Company’s indemnification obligations in respect of its directors and officers is included in the Proxy Statement/Prospectus in the section entitled “The Merger Agreement—Limitations of Liability and Indemnification” beginning on page 187 and is incorporated herein by reference.
Financial Statements and Supplementary Data
The information set forth in Item 9.01 of this Current Report on Form 8-K is incorporated herein by reference.
WHERE YOU CAN FIND MORE INFORMATION
The Company is subject to the informational requirements of the Exchange Act and in accordance therewith, files annual, quarterly and current reports, proxy statements and other information with the SEC electronically, and the SEC maintains a website that contains the Company’s filings as well as reports, proxy and information statements, and other information issuers file electronically with the SEC at www.sec.gov.
The Company also makes available free of charge on or through its website at inmagenebio.com/investors, its Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after the Company electronically files such material with or otherwise furnishes it to the SEC. The website addresses for the SEC and the Company are inactive textual references and except as specifically incorporated by reference into this Current Report on Form 8-K, information on those websites is not part of this Current Report on Form 8-K.
If you would like to request documents from the Company, please send a request in writing or by telephone to the following address:
ImageneBio, Inc.
12526 High Bluff Drive
Suite 345
San Diego, CA 92130
617-901-7098
Attn: Investor Relations
| Item 3.02 | Unregistered Sales of Equity Securities |
Concurrently with the execution of the Merger Agreement, Ikena entered into a subscription agreement (the “Subscription Agreement”) with certain accredited investors (the “PIPE Investors”), pursuant to which, following the closing of the Merger, the PIPE Investors subscribed for and purchased an aggregate of 2,508,337 shares of Ikena Common Stock, after giving effect to the Reverse Stock Split, at a price of approximately $29.90 per share for aggregate gross proceeds of approximately $75.0 million (the “PIPE Financing”). The shares of Ikena Common Stock issued pursuant to the Subscription Agreement (the “PIPE Shares”) have not been registered under the Securities Act in reliance upon the exemption provided in Section 4(a)(2) of the Securities Act and Rule 506 of Regulation D promulgated by the SEC under the Securities Act.
Pursuant to the Subscription Agreement:
| • | an entity affiliated with Blue Owl, which was a greater than 5% holder of Ikena Common Stock prior to the Merger purchased 217,389 shares of Ikena Common Stock at an aggregate purchase price of $6.5 million; |
| • | entities affiliated with Biotechnology Value Fund, which was a greater than 5% holder of Ikena Common Stock prior to the Merger and, immediately following the Merger and PIPE Financing, is a greater than 5% stockholder of the Company, purchased 468,223 shares of Ikena Common Stock at an aggregate purchase price of $14.0 million; |
| • | an entity affiliated with Deep Track, which was a greater than 5% holder of Ikena Common Stock prior to the Merger and, immediately following the Merger and PIPE Financing, is a greater than 5% stockholder of the Company, purchased 668,890 shares of Ikena Common Stock at an aggregate purchase price of $20.0 million; |
| • | an entity affiliated with Omega, which was a greater than 5% holder of Ikena Common Stock, and Ikena’s former and the Company’s current director Otello Stampacchia purchased 267,556 shares of Ikena Common Stock at an aggregate purchase price of $8.0 million; and |
| • | an entity affiliated with OrbiMed, which was a greater than 5% holder of Ikena Common Stock prior to the Merger and, immediately following the Merger and PIPE Financing, is a greater than 5% stockholder of the Company, and Ikena directors Iain D. Dukes and David P. Bonita purchased 83,611 shares of Ikena Common Stock at an aggregate purchase price of $2.5 million. |
In connection with the consummation of the Merger, the Company entered into a registration rights agreement (the “Registration Rights Agreement”) with the PIPE Investors, pursuant to which the Company agreed that, within 45 calendar days after the closing of the Merger, we will file with the SEC (at our sole cost and expense) a registration statement registering the resale of the PIPE Shares. The foregoing descriptions of the Subscription Agreement and Registration Rights Agreement do not purport to be complete and are qualified in their entirety by the terms and conditions thereof, the forms of which are attached hereto as Exhibit 10.7 and Exhibit 10.8, respectively, and are incorporated herein by reference.
| Item 3.03 | Material Modification to Rights of Security Holders |
To the extent required by Item 3.03 of Form 8-K, the information contained in Item 2.01 of this Current Report on Form 8-K is incorporated by reference herein.
As previously disclosed, at the annual meeting of Ikena’s stockholders held on July 15, 2025 (the “Annual Meeting”), Ikena’s stockholders approved, among other things, an amendment to Ikena’s Fifth Amended and Restated Certificate of Incorporation to effect the Reverse Stock Split. On July 25, 2025, Ikena amended its Fifth Amended and Restated Certificate of Incorporation to effect the Reverse Stock Split, effective as of 8:45 a.m. eastern time on July 25, 2025.
As a result of the Reverse Stock Split, each 12 shares of Ikena Common Stock held by a stockholder immediately prior to the Reverse Stock Split were combined and reclassified, automatically and without any action on the part of Ikena or its stockholders, into one new share of Ikena Common Stock. As such, immediately following the Reverse Stock Split, there were an estimated 4,021,502 shares of Ikena’s Common Stock outstanding, which may be adjusted following confirmation by the Company’s transfer agent of the actual number of shares outstanding immediately following the Reverse Stock Split.
No fractional shares were issued in connection with the Reverse Stock Split. Any fractional shares resulting from the Reverse Stock Split were rounded down to the nearest whole number, and each Ikena stockholder who would otherwise be entitled to a fraction of a share of Ikena Common Stock upon the Reverse Stock Split is entitled to receive a cash payment in lieu thereof at a price equal to the fraction to which the stockholder would otherwise be entitled multiplied by the closing price of the Company’s common stock on July 25, 2025.
On July 25, 2025, the Company also amended its amended and restated certificate of incorporation to effect the Name Change, effective as of 11:00 a.m. eastern time on July 25, 2025.
The foregoing descriptions of the certificates of amendment to Ikena’s Fifth Amended and Restated Certificate of Incorporation are not complete and are subject in their entirety by reference to the certificates of amendment, copies of which are attached hereto as Exhibit 3.1 and Exhibit 3.2, respectively, and are incorporated herein by reference.
| Item 5.01 | Changes in Control of Registrant |
The information set forth in Item 2.01 of this Current Report on Form 8-K regarding the Merger and the information set forth in Item 5.02 of this Current Report on Form 8-K regarding the Company’s board of directors and executive officers following the Merger are incorporated by reference into this Item 5.01.
| Item 5.02 | Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers |
(a)
Resignation of Directors
In accordance with the Merger Agreement, and effective upon the closing of the Merger, Iain Dukes, D.Phil., Maria Koehler, M.D., Ph.D., Jean-François Formela, M.D., Richard Wooster, Ph.D., Owen Hughes and Mark Manfredi, Ph.D. resigned from the Board and committees of the Board on which they respectively served, which resignations were not the result of any disagreements with the Company relating to the Company’s operations, policies or practices.
(b)
Departure of Officers
On July 25, 2025, effective upon the closing of the Merger, Mark Manfredi, Ph.D. resigned from all positions held at Ikena and its subsidiaries. In accordance with the employment agreement, effective as of Ikena’s first underwritten public offering, between Ikena and Dr. Manfredi (the “Manfredi Agreement”), effective upon Dr. Manfredi’s separation of employment with Ikena and his execution of a separation agreement with Ikena, Dr. Manfredi is entitled to the following severance benefits, (i) a lump-sum payment equal to 1.5 times the sum of (a) Dr. Manfredi’s then-current base salary or the base salary in effect immediately prior to the change in control, if higher, plus (b) Dr. Manfredi’s annual target bonus for the then-current year; (ii) acceleration of all time-based stock options and other stock-based awards subject to time-based vesting held by Dr. Manfredi, as of the later of the date of his termination or a “change in control” (the “Manfredi Accelerated Vesting Date”); and (iii) up to 18 months of the employer portion of COBRA premium payments. Subject to the closing of the Merger, Dr. Manfredi will receive a one-time transaction bonus of $1,050,000, payable promptly following the closing, subject to any reduction required under the best net after-tax cutback provision in Dr. Manfredi’s employment agreement.
In addition, Ikena entered into a retention award agreement with Dr. Manfredi in July 2024 (the “Manfredi Retention Award”) which generally provides for a one-time discretionary bonus of $300,000 to be paid within two business days of the closing of the Merger.
On July 25, 2025, effective upon the closing of the Merger, Jonathan Jian Wang, Ph.D.’s employment as Inmagene’s President and Chief Executive Officer was terminated. Pursuant to that certain Separation Agreement, dated as of July 23, 2025, by and between Inmagene and Dr. Wang (the “Wang Separation Agreement”) and in accordance with that certain Severance Rights Agreement, dated as of March 6, 2025, by and between Inmagene and Dr. Wang (the “Wang Severance Rights Agreement”), Dr. Wang is entitled to the following severance benefits: (i) severance payments equal to ten months of continued base salary, (ii) employer paid medical benefits for up to ten months following termination, and (iii) full acceleration of his equity awards.
The above descriptions of the Manfredi Agreement, Manfredi Retention Award, Wang Separation Agreement and the Wang Severance Agreement do not purport to be complete and are subject to and qualified in their entirety by reference to the copies of the Manfredi Agreement, Wang Separation Agreement and the Wang Severance Agreement included as Exhibits 10.14, 10.25, 10.18, and 10.17, respectively, to this Current Report on Form 8-K, which are incorporated herein by reference.
(c)
Appointment of Officers
On July 25, 2025, the board of directors of the Company appointed the following persons to serve as officers of the Company, in each case, effective immediately after the closing of the Merger, until their respective successor is duly elected and qualified or until their earlier resignation or removal:
Name |
Title |
|
Kristin Yarema, Ph.D. |
Chief Executive Officer (Principal Executive Officer) | |
Jotin Marango, M.D., Ph.D. |
Chief Financial Officer (Principal Financial Officer) and Corporate Secretary | |
Yufang Lu, M.D., Ph.D. |
Chief Medical Officer | |
Erin Butler |
Senior Vice President, Finance and Administration (Principal Accounting Officer) | |
Each of the persons above was designated by the board of directors as an “officer” of the Company as such term is defined in Rule 16a-1(f) promulgated under the “Exchange Act”, and, with the exception of Ms. Butler, an “executive officer” of the Company as such term is defined in Rule 3b-7 promulgated under the Exchange Act.
There are no family relationships among any of the Company’s newly appointed principal officers and any director or executive officer of the Company.
Each of the newly appointed executive officers’ biographical information is set forth below.
Kristin Yarema, Ph.D., age 54, Dr. Yarema was appointed as our Chief Executive Officer and a member of our Board effective immediately after the closing of the Merger. Dr. Yarema brings over two decades of leadership experience in human therapeutics to the Company. Dr. Yarema most recently served as President and Chief Executive Officer of Poseida Therapeutics, Inc. (formerly Nasdaq: PSTX) from January 2024 through June 2025, continuing in her leadership role following the company’s acquisition of the oncology, autoimmune, and rare disease company by Roche in January 2025. She also served as a member of Poseida’s Board of Directors from January 2024 until the acquisition in January 2025. She previously held the position of President, Cell Therapy at Poseida from April 2023 to December 2023. Prior to Poseida, Dr. Yarema served as Chief Commercial Officer at Atara Biotherapeutics, Inc., a publicly held oncology and autoimmune T-cell immunotherapy company, from February 2020 to November 2022. She also held numerous senior positions at Amgen, including Vice President and Therapeutic Area Head roles in Inflammation (autoimmune), Bone, Nephrology, Hematology, Cardiovascular, Metabolism, and Neuroscience along with various other U.S. and global commercial leadership positions of increasing responsibility from 2013 to 2020, including U.S. commercial responsibilities for dermatology and rheumatology. Earlier in her career, Dr. Yarema held various clinical development and commercial leadership roles in at Novartis from 2007 to 2013, including Global Program Head for multiple therapeutics, and Global Head, Global Strategic Marketing. She began her industry career at management consultancy McKinsey & Company, where she provided strategic advice to many healthcare companies from 2000 to 2007, ultimately as Associate Principal. Dr. Yarema currently serves on the boards of directors of the Celiac Disease Foundation, a global patient advocacy group, and the Alliance for Regenerative Medicine, a cell and gene therapy industry association. She holds a B.S. in Chemical Engineering and B.A. in English from Stanford University, and a Ph.D. in Chemical Engineering from the University of California, Berkeley. We believe Dr. Yarema is qualified to serve as a member of our Board because of her extensive scientific background and leadership positions at multiple biopharmaceutical companies and her strong academic background.
In July 2025, the Company entered into an offer letter, dated July 23, 2025, with Dr. Yarema (the “Yarema Offer Letter”), which provides for the following compensation: (i) an initial annual base salary of $630,000, (ii) a one-time signing bonus of $180,000 payable within 30 days of the Effective Date, and (iii) eligibility to receive an annual discretionary performance bonus, with a target bonus percentage of 50% of her base salary, pro-rated in case of a partial calendar year.
In addition, pursuant to the Yarema Offer Letter, subject to approval by the Board of the Company, Dr. Yarema is entitled to a stock option to purchase shares of the Company’s common stock equal to 3.75% of the Company’s fully diluted shares of common stock as of the date of grant at an exercise price equal to the closing price of the common stock on the grant date as reported on The Nasdaq Stock Market. One-fourth of the shares subject to the option vests on the first anniversary of the Effective Date, and 1/48th of the shares subject to the option vest monthly thereafter, subject to Dr. Yarema’s continuous service with the Company as of such vesting date. In addition, subject to approval by the Board of the Company, Dr. Yarema is entitled to a grant of restricted stock units (the “RSUs”) for shares of common stock equal to 1.25% of the Company’s fully diluted shares of common stock as of the date of grant. One-fourth of the shares subject to the RSUs shall vest on the first anniversary of the Effective Date, and 1/16th of the shares subject to the RSUs shall vest every three months thereafter, subject to Dr. Yarema’s continuous service with the Company as of such vesting date. The option and RSUs will be granted with the terms of the 2025 Plan (as defined in Item 5.02(d) below) and the applicable form of stock option agreement and restricted stock unit agreement.
Under the terms of the Yarema Offer Letter, if Dr. Yarema’s employment is terminated by the Company without cause or by Dr. Yarema for good reason not in connection with a change of control, and subject to the execution of a release of claims in favor of the Company, she is entitled to receive (i) payments aggregating to twelve months of Dr. Yarema’s base salary for the year in which the termination occurs, which shall be paid ratably in accordance with the Company’s payroll procedures over twelve months and (ii) reimbursement for COBRA premiums for up to twelve months following the date of termination. Such severance benefits may not be aggregated with any other severance benefits made available by the Company pursuant to any written agreement, plan or policy.
Additionally, under the terms of the Yarema Offer Letter, if Dr. Yarema’s employment is terminated without cause or by Dr. Yarema for good reason in connection with a change of control and subject to the execution of a release of claims in favor of the Company, she is entitled to receive (i) payments aggregating to eighteen months of Dr. Yarema’s base salary for the year in which the termination occurs, which shall be paid ratably in accordance with the Company’s payroll procedures over eighteen months, provided that if such change of control also qualifies as a Section 409A change in control, then such amount will be paid in a lump sum on the first regularly-scheduled payroll date following the sixtieth (60th) day after such termination; (ii) reimbursement for COBRA premiums for up to eighteen months following the date of termination; (iii) a lump sum payment equal to her target cash bonus for the bonus year in which such termination occurs; and (iv) accelerated vesting of outstanding time-based equity awards. Such severance benefits may not be aggregated with any other severance benefits made available by the Company pursuant to any written agreement, plan or policy.
There is no arrangement or understanding between Dr. Yamara and any other person pursuant to which Dr. Yamara was selected as an officer or director, and there are no actual or proposed transactions between the Company and Dr. Yamara or any related person that would require disclosure under Item 404(a) of Regulation S-K.
Jotin Marango, M.D., Ph.D., age 46, Dr. Marango has served as the Chief Financial Officer and Head of Corporate Development of the Company since April 2022 and as Chief Operating Officer of the Company since July 2024. Prior to this, he served as senior vice president, chief business officer at Aptose Biosciences Inc. (Nasdaq: APTO) from June 2019 to April 2022 and also as their chief financial officer from May 2021 to April 2022. Before that, from September 2017 to April 2019, Dr. Marango worked as an equity research analyst at Roth Capital Partners covering small and mid-cap biotechnology companies focused on hematology, oncology and rare diseases. Dr. Marango also served as chief operating officer at the Samuel Waxman Cancer Research Foundation from 2012 to 2015, where he oversaw venture philanthropy initiatives in therapeutic development. Through his education and career, Dr. Marango has solidified a passion for working in oncology and facilitating growth for businesses looking to make a difference in cancer research. Dr. Marango holds a B.A. in Chemistry with Honors from Harvard University and earned his M.D. and Ph.D. from the Mount Sinai School of Medicine of New York University.
In April 2022, the Company entered into an employment agreement with Dr. Marango (the “Marango Agreement”). In the event that Dr. Marango’s service with the Company is terminated “without cause” or for “good reason” (in each case, as defined in the Marango Agreement), on or within 45 days immediately preceding or 12 months after the closing of a “change in control” (as defined in the Marango Agreement), Dr. Marango will be entitled to the following severance benefits, subject to Dr. Marango executing a separation agreement and it becoming effective: (i) a lump-sum payment equal to the sum of (a) Dr. Marango’s then-current base salary or the base salary in effect immediately prior to the “change in control,” if higher, plus (b) Dr. Marango’s annual target bonus for the then-current year or the target bonus in effect immediately prior to the “change in control,” if higher; and (ii) up to 12 months of the employer portion of COBRA premium payments.
In the event that Dr. Marango’s service with the Company is terminated without “cause” or for “good reason” (in each case, as defined in the Marango Agreement), other than in connection with a “change in control” (as defined in the Marango Agreement), Dr. Marango will be entitled to the following severance benefits, subject to Dr. Marango executing a separation agreement and release and it becoming effective: (i) a lump-sum payment equal to nine months of Dr. Marango’s then-current base salary and (ii) up to 9 months of the employer portion of COBRA premium payments.
In addition, Ikena entered into a retention award agreement with Dr. Marango in July 2024, and approved an amendment of such agreement on January 10, 2025 (the “Marango 2024 Retention Award”) which generally provides for payment of his retention bonus in three installments, with $82,500 paid on July 15, 2025, $82,500 paid at the time of the signing of the Merger Agreement, and $85,000 payable within two business days of the closing of the Merger. Additionally, Ikena also approved a retention bonus agreement with Dr. Marango in February 2025 (the “Marango 2025 Retention Award” and together with the Marango 2024 Retention Award, the “Marango Retention Awards”) which generally provides for the payment of a one-time discretionary bonus of $70,000, to be paid within three business days following the closing of the Merger.
On July 10, 2025, the Company and Dr. Marango entered into an amendment to the Marango Agreement (the “Marango Agreement Amendment”) which provides for certain additional benefits to Dr. Marango upon termination in the event that his employment is terminated by the Company without “cause” or Dr. Marango resigns for any reason during the “change in control period” (in each case, as defined in the Marango Agreement). Under such circumstances, all time-based stock options and awards held by Dr. Marango will accelerate as of the later of the date of his termination or a “change in control” (the “Marango Accelerated Vesting Date”).
The termination or forfeiture of any unvested stock options or awards will also be delayed until the Marango Accelerated Vesting Date if such awards do not otherwise vest pursuant to a separation agreement and release.
Yufang Lu, M.D., Ph.D., age 61, Dr. Lu was appointed as our Chief Medical Officer effective immediately after the closing of the Merger. Dr. Lu served as Chief Medical Officer of Inmagene from 2023 to the closing of the Merger. Dr. Lu has more than 20 years of experience in drug development and medical affairs. She has successfully led clinical development for both small molecules and biologics across diverse therapeutic areas, including gastroenterology, dermatology, allergy, and rheumatology. Prior to Inmagene, Dr. Lu was Vice President of Clinical Development at Celldex Therapeutics between 2020 and 2023, where she drove the advancement of innovative immunology assets, including barzovolimab. When at Regeneron Pharmaceuticals, Inc. as an Executive Director in Medical Affairs Immunology between 2016 and 2020, she led cross-functional medical teams, in collaboration with Sanofi, in the global launches of Dupixent® in atopic dermatitis and asthma. Earlier in her career, she held roles in clinical development and medical affairs at Celgene, Eisai US, and GlaxoSmithKline. Dr. Lu received her Bachelor of Medicine degree from Nanjing Medical University with clinical training in Dermatology and a PhD in Toxicology with a specialization in Cellular & Molecular Toxicology from Texas A&M University.
On December 11, 2022, Inmagene and Dr. Lu entered into an offer letter, pursuant to which she was entitled to an initial base salary of $432,000 and an annual target performance bonus of 30% of her annual base salary based on achievement of certain performance objectives. In addition, Dr. Lu was granted an option to purchase 12,500,000 Inmagene ordinary shares under the 2019 Plan (as defined below) which vest over a period of four years with 25% of the shares underlying the option vesting on the one-year anniversary of the vesting commencement date and 1/48th of the shares underlying the option vesting on a monthly basis thereafter, subject to continued service through each vesting date.
On October 8, 2024, Inmagene and Dr. Lu entered into a severance rights agreement. Under the terms of the agreement, if Dr. Lu is terminated without cause or resigns for good reason (in each case as defined in the agreement), then she will be entitled to severance payments equal to six months of continued base salary, employer paid medical benefits for up to six months following termination, and additionally, if such termination or resignation occurs upon or within 12 months of a change in control, full acceleration of her equity awards, provided that in either case, she executes an effective release of claims.
Erin Butler, age 46, Ms. Butler was appointed as our Senior Vice President of Finance & Administration effective immediately after the closing of the Merger. Ms. Butler served as Inmagene’s Vice President of Finance and Administration from April 2024 to the closing of the Merger. Prior to her appointment, Ms. Butler served as Inmagene’s Vice President, Corporate Controller from October 2023 to April 2024. Prior to joining Inmagene, from March 2022 to May 2023, Ms. Butler was Vice President, Finance and Administration at Armata Pharmaceuticals, Inc. and from September 2017 to February 2022 she served as its Senior Director, Corporate Controller. Prior to joining Armata Pharmaceuticals, Ms. Butler held accounting and finance leadership positions of increasing responsibility with AmpliPhi Biosciences, Inc., Inovio, Inc., and Apricus Biosciences, Inc. Ms. Butler began her career as an auditor for Deloitte and Touche, LLP, a public accounting firm. Ms. Butler has extensive experience in all aspects of financial and administrative operations and has led or participated in equity multiple financing, M&A transactions, IPOs, system implementations, clinical trial accounting oversight, as well as private and public financial reporting. She holds a B.S. in Business Administration, Accounting from San Diego State University.
On October 10, 2023, Inmagene and Ms. Butler entered into an offer letter, pursuant to which she was entitled to an initial annual base salary of $300,000 and an annual target performance bonus of 30% of her annual base salary based on achievement of certain performance objectives. In addition, Ms. Butler was granted an option to purchase 500,000 Inmagene ordinary shares under the 2019 Plan which vest over a period of four years with 25% of the shares underlying the option vesting on the one-year anniversary of the vesting commencement date and 1/48th of the shares underlying the option vesting on a monthly basis thereafter, subject to continued service through each vesting date.
On October 21, 2024, Inmagene and Ms. Butler entered into a severance rights agreement. Under the terms of the agreement, if Ms. Butler is terminated without cause or resigns for good reason (in each case as defined in the agreement), then she will be entitled to severance payments equal to four months of continued base salary, employer paid medical benefits for up to six months following termination, and additionally, if such termination or resignation occurs upon or within 12 months of a change in control, full acceleration of her equity awards, provided that in either case, she executes an effective release of claims.
The above descriptions of the offer letter agreements for Dr. Yarema, Dr. Marango, Dr. Lu and Ms. Butler and the severance rights agreements for Dr. Lu and Ms. Butler do not purport to be complete and are subject to and qualified in their entirety by reference to the copies of the employment related agreements for Dr. Yarema, Dr. Marango, Dr. Lu and Ms. Butler included as Exhibits 10.9, 10.10, 10.11 and 10.12, respectively, the Marango Agreement Amendment included as Exhibit 10.24, and the Marango Retention Awards included as Exhibits 10.26 and 10.27 and the severance rights agreements for Dr. Lu and Ms. Butler included as Exhibits 10.15 and 10.16, respectively, to this Current Report on Form 8-K, which are incorporated herein by reference.
(d)
Appointment of Directors
Effective immediately after the closing of the Merger, the size of the Board was decreased from eight to six members, and the Board was reconstituted as follows: (i) Otello Stampacchia, Ph.D. and David P. Bonita, M.D. (designated by Ikena), (ii) Jonathan Jian Wang, Ph.D., MBA, Stephen Hui Wang, MBA and Weiguo Su, Ph.D. (designated by Inmagene), and (iii) Kristin Yarema, Ph.D. (designated by each of Ikena and Inmagene). The classification of the Board was confirmed as follows: Otello Stampacchia, Ph.D., an existing Class I director, will continue to serve as a Class I director, and Jonathan Jian Wang, Ph.D., MBA was appointed as a Class I director (whose terms expire at the Company’s 2028 annual meeting), David P. Bonita, M.D., an existing Class II director, will continue to serve as a Class II director, and Stephen Hui Wang, MBA, was appointed as a Class II director (whose terms expire at the Company’s 2026 annual meeting), and Weiguo Su, Ph.D. and Kristin Yarema, Ph.D. were appointed as Class III directors (whose terms expire at the Company’s 2027 annual meeting). Jonathan Jian Wang, Ph.D., MBA was also appointed as Chair of the Board and David P. Bonita, M.D. was appointed as its lead independent director.
Under the Nasdaq Listing Rules, a majority of the members of the board of directors must qualify as “independent,” as affirmatively determined by the board of directors. Under the Nasdaq Listing Rules, a director will only qualify as an “independent director” if, in the opinion of that company’s board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. The Board has determined that each of its directors other than Jonathan Jian Wang, Ph.D., MBA and Kristin Yarema, Ph.D. qualify as “independent directors” as defined by the Nasdaq Listing Rules.
In addition, Stephen Hui Wang, MBA, David P. Bonita, M.D. and Otello Stampacchia, Ph.D. were appointed to the audit committee of the board of directors, Mr. Wang was appointed the chair of the audit committee, and each of Dr. Bonita and Mr. Wang was designated an “audit committee financial expert.” David P. Bonita, M.D. and Weiguo Su, Ph.D. were appointed to the compensation committee of the Board, and David P. Bonita, M.D. was appointed the chair of the compensation committee. Otello Stampacchia, Ph.D. and David P. Bonita, M.D. were appointed to the nominating and corporate governance committee of the Board, and Otello Stampacchia, Ph.D. was appointed the chair of the nominating and corporate governance committee.
Other than pursuant to the Merger Agreement, there were no arrangements or understandings between the Company’s newly appointed directors and any person pursuant to which they were elected. There are no family relationships between the Company’s newly appointed directors and any director or executive officer of the Company.
The Company intends to adopt a new Non-Employee Director Compensation Policy at the next meeting of our board of directors following the closing of the Merger, but the terms of such policy have not yet been determined.
Each of the newly appointed directors’ biographical information is set forth below.
Kristin Yarema, Ph.D. age, 54, Dr. Yarema’s biographical information is disclosed in Item 5.02(c) above.
Jonathan Jian Wang, Ph.D., MBA, age 55, Dr. Wang was appointed as a member of our Board effective immediately after the closing of the Merger. Dr. Wang is the founder of Inmagene and served as its Chairman of the Board of Directors and Chief Executive Officer from July 2019 to the closing of the Merger. Dr. Wang has more than 30 years of healthcare and life sciences experience, spanning entrepreneurship, investment, and research.
Previously, he was a healthcare investor for 22 years. For 12 years, Dr. Wang was a Partner at OrbiMed and a co-founder at OrbiMed Asia, where he worked with his partners to establish and manage US$1.1 billion of private equity and venture capital funds. Under the supervision of Eric Kandel, a Nobel laureate, Dr. Wang obtained his Ph.D. in Neurobiology from Columbia University where he was rewarded the Howard Hughes Medical Institute Research Fellowship. Dr. Wang has also obtained an MBA from Stanford University and a B.S. in Biology from Wuhan University in China. Dr. Wang is a co-founder and former Chairman of the BayHelix Group and has authored two philosophy of science books. We believe that Dr. Wang is qualified to serve as a member of our Board because of his extensive experience in the healthcare and life sciences sectors, including his experience as Inmagene’s former Chief Executive Officer, his proven track record in entrepreneurship and investment, and his strong academic background.
Stephen Hui Wang, MBA, age 51, Mr. Wang was appointed as a member of our Board effective immediately after the closing of the Merger. Mr. Wang brings over 20 years of experience in global capital markets, with a strong track record in leading and participating in transactions related to mergers and acquisitions, public company restructuring, and growth and early-stage investments. Mr. Wang is the founder and Chief Executive Officer of HighLight Capital, a position he has held since 2014. Previously, Mr. Wang was a Senior Partner and Investment Committee member at CDH Investments from 2009 to 2014. Mr. Wang is currently a director of Viva Biotech Holdings (HKEX: 1873). He received a Bachelor’s degree in Biology from the University of Science and Technology of China, a Master’s degree in Chemistry and Doctoral qualifications from New York University, and an MBA from the London Business School. We believe that Mr. Wang is qualified to serve as a member of our Board because of his extensive experience in global capital markets, proven leadership in managing and executing complex transactions, and strong academic background.
Weiguo Su, Ph.D., age 67, Dr. Su was appointed as a member of our Board effective immediately after the closing of the Merger. Dr. Su has served as the Chief Executive Officer of HUTCHMED (China) Limited (“HUTCHMED (China)”), a commercial stage biopharmaceutical company listed on The Stock Exchange of Hong Kong Limited, AIM market of the London Stock Exchange and in the form of American depositary shares on the Nasdaq Global Select Market, since 2022, its Chief Scientific Officer since 2012, and as one of its executive directors since 2017. Dr. Su has headed all drug discovery and research since he joined HUTCHMED (China), including master-minding the scientific strategy of the company, being a key leader of its Oncology/Immunology operations, and being responsible for the discovery of each and every small molecule drug candidate in its pipeline. Prior to joining HUTCHMED (China) in 2005, Dr. Su worked with the U.S. research and development department of Pfizer, Inc. In 2017, Dr. Su was granted the prestigious award by the China Pharmaceutical Innovation and Research Development Association (PhIRDA) as one of the Most Influential Drug R&D Leaders in China. Dr. Su received a Bachelor of Science degree in Chemistry from Fudan University in Shanghai and completed a Ph.D. and Post-Doctoral Fellowship in Chemistry at Harvard University under the guidance of Nobel Laureate Professor E. J. Corey. We believe that Dr. Su is qualified to serve as a member of our Board because of his extensive experience in the healthcare and life sciences sector, proven executive leadership, and strong academic background.
David P. Bonita, M.D., age 48, Dr. Bonita has served as a member of our Board since March 2016. Dr. Bonita is a member of OrbiMed Advisors LLC, an investment firm. Dr. Bonita currently serves on the boards of directors of Acutus Medical Inc. (Nasdaq: AFIB), Prelude Therapeutics, Inc. (Nasdaq: PRLD), Repare and Third Harmonic Bio, Inc. (Nasdaq: THRD), as well as several private companies. Dr. Bonita also previously served on the board of directors of IMARA, Inc. (Nasdaq: IMRA) and Tricida, Inc. (Nasdaq: TCDA). Prior to OrbiMed, Dr. Bonita worked as a corporate finance analyst in the healthcare investment banking groups of Morgan Stanley and UBS. He received his B.A. in biology from Harvard University and his joint M.D./M.B.A. from Columbia University. We believe that Dr. Bonita is qualified to serve on our Board based on his roles on several public and private boards of directors as well as his extensive experience in investing in healthcare companies.
Otello Stampacchia, Ph.D., age 55, Dr. Stampacchia has served as a member of our Board since December 2020. Dr. Stampacchia is founder, managing director and member of the investment committee at Omega Funds LLC. Dr. Stampacchia currently serves on the boards of directors of several private companies. Dr. Stampacchia previously served on the board of directors of Kronos Bio, Inc. (Nasdaq: KRON), Median Technologies, Inc., Nuvation Bio, Inc. (NYSE: NUVB) and Replimune Group, Inc. (Nasdaq: REPL). Prior to founding Omega in January 2004, Dr. Stampacchia was a Partner at AlpInvest Partners (now part of The Carlyle Group). Before AlpInvest Partners, he was the portfolio manager of the Lombard Odier Immunology Fund, an investment vehicle in Geneva, Switzerland, investing in public and private healthcare companies worldwide. Previously, Dr. Stampacchia was a member of the HealthCare corporate finance and M&A team at Goldman Sachs.
Before Goldman Sachs, he helped co-found the healthcare investment activities at Index Securities (now Index Ventures). Dr. Stampacchia received a Masters of Science in Plant Genetics from the University of Pavia, a Masters of Science in Molecular Biology, a Doctorate of Philosophy in Molecular Biology from the University of Geneva and a Doctorate of Philosophy in Biotechnology from European Union Strasbourg. We believe Dr. Stampacchia is qualified to serve on our Board because of his venture capital experience in the life sciences industry and his service on the boards of directors of other public and private life sciences companies.
Information regarding transactions between the Company and the newly appointed directors and executive officers is included in the section of the Proxy Statement/Prospectus entitled “Certain Relationships and Related Person Transactions of the Combined Company” beginning on page 362 and is incorporated herein by reference.
Compensatory Plans
2019 Stock Incentive Plan
The Company assumed, effective as of the closing of the Merger, the Inmagene 2019 Stock Incentive Plan (the “2019 Plan”), as well as the outstanding awards granted thereunder, the award agreements evidencing the grants of such awards and the remaining shares available under the 2019 Plan, including any awards granted to the Company’s named executive officers, in each case subject to applicable adjustments in the manner set forth in the Merger Agreement to such awards. A copy of the 2019 Plan, as well as the forms of stock option award notice and stock option award agreement and forms of restricted share unit grant notice and unit award agreement are filed as Exhibit 10.19, to this Current Report on Form 8-K and are incorporated herein by reference.
2025 Equity Incentive Plan
At the Annual Meeting, Ikena’s stockholders considered and approved the Company’s 2025 Equity Incentive Plan (the “2025 Plan”), which became effective on the date immediately following the consummation of the Merger and following the Reverse Stock Split. As of the Second Effective Time, there were an estimated 1,118,168 shares of the Company’s common stock available for grant under the 2025 Plan, which is based on an estimated number of shares outstanding immediately following the Reverse Stock Split and may be adjusted following confirmation by the Company’s transfer agent of the actual number of shares outstanding immediately following the Reverse Stock Split. In addition, the number of shares reserved and available for issuance under the 2025 Plan will automatically increase on January 1 of each year for a period of 10 years, commencing on January 1, 2026 and ending on January 1, 2035, in an amount equal to 5% of the total number of shares of the Company’s capital stock outstanding on the last day of the calendar month before the date of each automatic increase, or a lesser number of shares determined by our Board.
A more complete summary of the terms of the 2025 Plan is set forth in the Proxy Statement/Prospectus under the section titled “Proposal No. 5 (The 2025 Plan Proposal)” beginning on page 255 of the Proxy Statement/Prospectus and is incorporated by reference herein. That summary and the foregoing description of the 2025 Plan do not purport to be complete and are qualified in their entirety by reference to the text of the 2025 Plan, forms of option grant notices and option agreements and forms of restricted stock unit grant notice and unit award agreement, copies of which are attached to this Current Report on Form 8-K as Exhibits 10.20, 10.21 and 10.22, respectively, and are incorporated herein by reference.
2025 Employee Stock Purchase Plan
At the Annual Meeting, Ikena’s stockholders considered and approved the Company’s 2025 Employee Stock Purchase Plan (the “2025 ESPP”), which became effective on the date immediately following the consummation of the Merger and following the Reverse Stock Split. As of the Second Effective Time, there were an estimated 111,817 shares of the Company’s common stock reserved for issuance under the 2025 ESPP, which is based on an estimated number of shares outstanding immediately following the Reverse Stock Split and may be adjusted following confirmation by the Company’s transfer agent of the actual number of shares outstanding immediately following the Reverse Stock Split. Additionally, the number of shares of common stock reserved for issuance under the 2025 ESPP will automatically increase on January 1 of each year for a period of 10 years, beginning on January 1, 2026 and continuing through and including January 1, 2035, by an amount equal to the lesser of (i) 1% of the total number of shares of the Company’s capital stock outstanding on the last day of the calendar month before the date of the automatic increase, and (ii) 227,944 shares; provided that before the date of any such increase, the Board may determine that such increase will be less than the amount set forth in clauses (i) and (ii).
A more complete summary of the terms of the 2025 ESPP is set forth in the Proxy Statement/Prospectus under the section titled “Proposal No. 6 (The ESPP Proposal)” beginning on page 262 of the Proxy Statement/Prospectus and is incorporated by reference herein. That summary and the foregoing description of the 2025 ESPP do not purport to be complete and are qualified in their entirety by reference to the text of the 2025 ESPP, a copy of which is attached to this Current Report on Form 8-K as Exhibit 10.23 hereto and are incorporated herein by reference.
Indemnification Agreements
In connection with the Merger, the Company entered into indemnification agreements with each of its new directors and executive officers. Each indemnification agreement provides for indemnification and advancements by the Company of certain expenses and costs relating to claims, suits or proceedings arising from each individual’s service to the Company as an officer or director, as applicable, to the maximum extent permitted by applicable law.
The foregoing description of the indemnification agreements is qualified in its entirety by the full text of the form of indemnification agreement, which is attached hereto as Exhibit 10.1 and incorporated herein by reference.
| Item 5.03 | Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year. |
To the extent required by Item 5.03 of Form 8-K, the information contained in Item 2.01 and Item 3.03 of this Current Report on Form 8-K is incorporated by reference herein.
Commencing on July 28, 2025, the Company expects its common stock, which is currently listed on The Nasdaq Global Market and will be listed on The Nasdaq Capital Market, to trade under the ticker symbol “IMA.” The change in trading symbol is related solely to the Name Change.
| Item 5.06 | Change in Shell Company Status. |
As a result of the Merger, the Company ceased to be a shell company (as defined in Rule 12b-2 of the Exchange Act) as of the closing of the Merger. The material terms and provisions of the Merger Agreement are included in the Proxy Statement/Prospectus in the sections titled “The Merger Agreement” beginning on page 195 of the Proxy Statement/Prospectus and are incorporated by reference herein. Further reference is made to the information contained in Item 2.01 of this Current Report on Form 8-K.
| Item 7.01 | Regulation FD Disclosure. |
On July 25, 2025, the Company posted a corporate presentation regarding the business of the Company to its website at inmagenebio.com/investors. A copy of the corporate presentation is attached as Exhibit 99.1 hereto and incorporated herein by reference.
The information set forth under this Item 7.01, including Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or incorporated by reference in any filing under the Securities Act unless expressly incorporated by specific reference in such filing.
| Item 9.01 | Financial Statements and Exhibits |
| (a) | Financial Statements of Business Acquired |
The audited consolidated financial statements of Ikena as of and for the years ended December 31, 2023 and 2024 and the related notes thereto are included in Ikena’s Annual Report on Form 10-K, filed with the SEC on March 6, 2025, and are incorporated herein by reference.
The audited financial statements of Inmagene as of and for the years ended December 31, 2023 and 2024 and related notes thereto are included in the Proxy Statement/Prospectus, and are incorporated by reference herein.
The unaudited interim condensed consolidated financial statements of Ikena as of and for the six months ended June 31, 2024 and 2025 are included in Ikena’s Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2025, filed with the SEC on July 24, 2025.
The unaudited interim condensed consolidated financing statements of Inmagene as of and for the three months ended March 31, 2024 and 2025 and related notes thereto are included in the Proxy Statement/Prospectus, and are incorporated by reference herein.
| (b) | Pro Forma Financial Information |
The unaudited pro forma condensed combined financial information of the Company as of and for the three months ended March 31, 2025 and the year ended December 31, 2024 is set forth in Exhibit 99.2 hereto and is incorporated herein by reference.
| * | Filed herewith |
| ** | Previously filed. |
| # | Indicates a management contract or any compensatory plan, contract or arrangement. |
| † | Certain portions of this exhibit (indicated by asterisks) have been omitted because they are not material and are the type of information that the Company treats as private or confidential. |
| + | The annexes, schedules, and certain exhibits to this agreement have been omitted pursuant to Item 601(b)(2) of Regulation S-K. The registrant hereby agrees to furnish supplementally a copy of any omitted annex, schedule or exhibit to the Commission upon request. |
| ‡ | Schedules and exhibits have been omitted pursuant to Item 601(a)(5) of Regulation S-K. The registrant undertakes to furnish supplemental copies of any of the omitted schedules upon request by the SEC. |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| IMAGENEBIO, INC. | ||||||
| Date: July 29, 2025 | By: | /s/ Kristin Yarema, Ph.D. |
||||
| Kristin Yarema, Ph.D. | ||||||
| Chief Executive Officer | ||||||
Exhibit 3.1
CERTIFICATE OF AMENDMENT
TO
FIFTH AMENDED AND RESTATED
CERTIFICATE OF INCORPORATION
OF
IKENA ONCOLOGY, INC.
IKENA ONCOLOGY, INC., a corporation organized and existing under the laws of the State of Delaware (the “Corporation”), hereby certifies as follows:
| 1. | The name of the Corporation is Ikena Oncology, Inc. The date of the filing of its original Certificate of Incorporation with the Secretary of State of the State of Delaware was March 2, 2016 (the “Original Certificate”). The name under which the Corporation filed the Original Certificate was Kyn Therapeutics Inc. The Fifth Amended and Restated Certificate of Incorporation was filed with the Secretary of State of the State of Delaware on March 30, 2021 (the Fifth Amended and Restated Certificate of Incorporation, as so amended, the “Certificate of Incorporation”). |
| 2. | This Certificate of Amendment hereby amends Article IV of the Certificate of Incorporation by adding the following paragraphs after the first paragraph of Article IV, as follows: |
“Effective as of 8:45 a.m. (Eastern Time) on July 25, 2025 (such time, the “Effective Time”), a one-for-twelve reverse stock split of the shares of Common Stock, pursuant to which every twelve shares of the Common Stock issued and held of record by each stockholder of the Corporation (including treasury shares) immediately prior to the Effective Time shall be reclassified and combined into one validly issued, fully paid and non-assessable share of Common Stock from and after the Effective Time, without any action on the part of the Corporation or the respective stockholders thereof (such reclassification and combination of shares, the “Reverse Stock Split”). The par value of the Common Stock following the Reverse Stock Split shall remain at $0.001 per share. No fractional shares of Common Stock shall be issued as a result of the Reverse Stock Split. In lieu of any fractional shares, if upon aggregating all of the shares of Common Stock held by a record holder immediately following the Reverse Stock Split such holder would otherwise be entitled to a fractional share of Common Stock as a result of the Reverse Stock Split, the Corporation shall pay in cash (without interest) to each such holder an amount equal to the product of such resulting fractional interest in one share of Common Stock multiplied by the closing trading price on The Nasdaq Global Market LLC of a share of Common Stock on the last trading day immediately prior to the date on which the Effective Time occurs (with such price proportionately adjusted to give effect to the Reverse Stock Split).
Each stock certificate or book entry share that, immediately prior to the Effective Time, represented shares of Common Stock that were issued and outstanding immediately prior to the Effective Time shall, from and after the Effective Time, automatically and without the necessity of presenting the same for exchange, represent that number of whole shares of Common Stock after the Effective Time into which the shares formerly represented by such certificate or book entry share have been combined (as well as the right to receive cash in lieu of fractional shares of Common Stock after the Effective Time); provided, however, that each stockholder of record holding a certificate that represented shares of Common Stock that were issued and outstanding immediately prior to the Effective Time shall receive, upon surrender of such certificate, a new certificate evidencing and representing the number of whole shares of Common Stock after the Effective Time into which the shares of Common Stock formerly represented by such certificate shall have been combined.”
| 3. | The stockholders of the Corporation have duly approved the foregoing amendment in accordance with the provisions of Section 211 and 242 of the General Corporation Law of the State of Delaware. |
IN WITNESS WHEREOF, the Corporation has caused this Certificate of Amendment to be duly adopted and executed in its corporate name and on its behalf by its duly authorized officer as of this 25th day of July, 2025.
| IKENA ONCOLOGY, INC. | ||
| By: | /s/ Mark Manfredi |
|
| Name: Mark Manfredi, Ph.D. | ||
| Title: President and Chief Executive Officer | ||
Exhibit 3.2
CERTIFICATE OF AMENDMENT
TO
FIFTH AMENDED AND RESTATED CERTIFICATE OF INCORPORATION
OF
IKENA ONCOLOGY, INC.
Ikena Oncology, Inc. (the “Corporation”), a corporation organized and existing under and by virtue of the General Corporation Law of the State of Delaware (the “DGCL”), does hereby certify that:
FIRST: The name of this corporation is Ikena Oncology, Inc., and the date on which the Certificate of Incorporation of this corporation was originally filed with the Secretary of State of the State of Delaware was March 2, 2016, under the original name Kyn Therapeutics Inc. A Fifth Amended and Restated Certificate of Incorporation was filed with the Secretary of State of the State of Delaware on March 30, 2021 (as amended and restated, the “Restated Certificate of Incorporation”). Pursuant to Sections 141 and 242 of the DGCL this Certificate of Amendment (this “Amendment”) amends certain provisions of the Restated Certificate of Incorporation.
SECOND: The Board of Directors of the Corporation duly adopted resolutions approving the Amendment and declaring the Amendment to be advisable.
THIRD: Article I of the Restated Certificate of Incorporation is hereby amended and restated in its entirety to read as follows:
“The name of the Corporation is ImageneBio, Inc.”
FOURTH: All other references to “Ikena Oncology, Inc.” in the Restated Certificate of Incorporation shall be replaced with “ImageneBio, Inc.”
FIFTH: This Amendment shall become effective at 11:00 a.m. Eastern Time on July 25, 2025.
[SIGNATURE PAGE FOLLOWS]
IN WITNESS WHEREOF, the Corporation has caused this Certificate of Amendment to be duly adopted and executed in its corporate name and on its behalf by its duly authorized officer as of July 25, 2025.
| IKENA CONCOLOGY, INC. | ||
| By: | /s/ Jotin Marango |
|
| Name: | Jotin Marango, M.D., Ph.D. | |
| Title: | Corporate Secretary | |
Exhibit 10.2
INSIGHT CONTINGENT VALUE RIGHTS AGREEMENT
THIS CONTINGENT VALUE RIGHTS AGREEMENT (this “Agreement”), dated as of July 25, 2025, is entered into by and among Ikena Oncology, Inc., a Delaware corporation (“Insight”), and Computershare Inc., a Delaware corporation (“Computershare”), and its affiliate, Computershare Trust Company, N.A., a federally chartered trust company (collectively, as “Rights Agent”).
RECITALS
A. Insight, Insight Merger Sub I, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands and direct wholly owned subsidiary of Insight, Insight Merger Sub II, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands and direct wholly owned subsidiary of Insight, and Inmagene Biopharmaceuticals, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands (the “Company”) and the other parties thereto, have entered into an Agreement and Plan of Merger, dated as of December 23, 2024 (the “Merger Agreement”).
B. Pursuant to the Merger Agreement, and in accordance with the terms and conditions thereof, Insight has agreed to provide to the Holders (as defined herein) contingent value rights as hereinafter described.
C. The parties have done all things reasonably necessary to make the contingent value rights, when issued pursuant to the Merger Agreement and hereunder, the valid obligations of Insight and to make this Agreement a valid and binding agreement of Insight, in accordance with its terms.
NOW, THEREFORE, in consideration of the premises and the consummation of the transactions referred to above, it is mutually covenanted and agreed, for the proportionate benefit of all Holders, as follows:
ARTICLE 1
DEFINITIONS
Section 1.1 Definitions.
Capitalized terms used but not otherwise defined herein have the meanings ascribed thereto in the Merger Agreement. The following terms have the meanings ascribed to them as follows:
“Acting Holders” means, at the time of determination, the Holders of at least 40% of the outstanding CVRs, as reflected on the CVR Register.
“Assignee” has the meaning set forth in Section 7.5.
“Calendar Quarter” means the successive periods of three (3) consecutive calendar months ending on March 31, June 30, September 30 or December 31, for so long as this Agreement is in effect; provided, however that (a) the first Calendar Quarter shall commence on the date of this Agreement and shall end on the first September 30 thereafter, and (b) the last Calendar Quarter shall commence on the first day after the full Calendar Quarter immediately preceding the effective date of the termination or expiration of this Agreement and shall end on the effective date of the termination or expiration of this Agreement.
“Change of Control” means (a) the acquisition in one transaction or a series of related transactions, by any Person or group (within the meaning of Section 13(d)(3) of the Securities Exchange Act of 1934) of the securities of Insight possessing more than 50% of the total combined voting power of all outstanding securities of Insight (provided, however, that a Change of Control will not result upon such acquisition of ownership if such acquisition occurs as a result of: (i) a public offering of Insight’s securities or any
financing transaction or series of financing transactions, in each case for bona fide financing purposes or (ii) a merger or consolidation involving Insight where the holders of the outstanding voting securities of Insight immediately prior to such merger or consolidation (taken in the aggregate) possess beneficial ownership of 50% or more of the total combined voting power of all outstanding voting securities of Insight, the surviving entity, the acquiring entity or a parent or holding company of the acquiring entity, immediately after such merger or consolidation); or (b) the sale, transfer, exclusive license or other disposition (in one transaction or a series of related transactions) of all or substantially all of the assets of Insight (on a consolidated basis), except for a transaction in which the holders of the outstanding voting securities of Insight immediately prior to such transaction(s) (taken in the aggregate) receive as a distribution with respect to securities of Insight more than 50% of the total combined voting power of all outstanding voting securities of the acquiring entity or a parent or holding company of the acquiring entity immediately after such transaction(s).
“Common Stock” means the common stock, $0.001 par value, of Insight.
“CVR” means a contingent contractual right of Holders to receive CVR Payments pursuant to the Merger Agreement and this Agreement.
“CVR Payment” means a cash payment equal to (i) ninety percent (90%) of the Net Proceeds received by Insight in a given CVR Payment Period to the extent such payment relates to a Disposition Agreement entered into after the Closing Date, if any, with the remaining ten percent (10%) being retained by Insight, (ii) one hundred percent (100%) of the Net Proceeds received by Insight to the extent such payment relates to a Disposition Agreement entered into prior to the Closing Date (other than agreements covered by clause (iii)), or (iii) one hundred percent (100%) of the Net Proceeds received by Insight to the extent such payment relates to a Disposition Agreement entered into prior to the Closing Date with respect to the Pionyr Assets that exceed the aggregate amounts payable by Insight to the holders of contingent value rights pursuant to that certain Contingent Value Rights Agreement, by and between Insight and Computershare, dated as of August 4, 2023; provided, that with respect to clauses (i), (ii) and (iii), Insight, in its reasonable discretion as resolved by the Insight Board, may withhold from any CVR Payment to provide for the satisfaction of indemnity obligations or other potential post-disposition Liabilities under any Disposition Agreement in excess of any escrow fund established therein, in each case to the extent not already deducted as Permitted Deductions; provided, further, that any such withheld Net Proceeds shall be distributed (net of any Permitted Deductions satisfied therefrom) to the Holders no later than the CVR Payment Period in which occurs the extinction of any such indemnity obligations or other post-disposition Liabilities.
“CVR Payment Amount” means with respect to each CVR Payment and each Holder, an amount equal to such CVR Payment divided by the total number of CVRs and then multiplied by the total number of CVRs held by such Holder as reflected on the CVR Register.
“CVR Payment Period” means a period equal to two (2) consecutive Calendar Quarters (or portion thereof) beginning on the First Effective Time of the Merger and ending on March 31 or September 30 of any given calendar year during the CVR Term (provided, that if the last CVR Payment Period would end subsequent to the expiration of the CVR Term, such CVR Payment Period will end on the Termination Date).
“CVR Payment Statement” means, for a given CVR Payment Period during the CVR Term, a written statement of Insight, signed on behalf of Insight, setting forth in reasonable detail the calculation of the applicable CVR Payment for such CVR Payment Period.
“CVR Register” has the meaning set forth in Section 2.3(b).
“CVR Term” means the period beginning on the Closing and ending upon the tenth (10th) anniversary of this Agreement; provided that, solely with respect to any Disposition Agreement set forth on Schedule 1.1, the CVR Term shall extend until the latest date that Insight may earn a contingent, earnout, milestone or similar payment pursuant to such Disposition Agreement if (i) the first patient has been dosed in a registrational trial of an asset covered by such Disposition Agreement before the second (2nd) anniversary of this Agreement or (ii) FDA approval of an NDA for an asset covered by such Disposition Agreement has been received by Insight before the fifth (5th) anniversary of this Agreement.
“Disposition” means the sale, exclusive license, transfer or other disposition of any Insight CVR Asset (including any such sale or disposition of equity securities in any Subsidiary established by Insight to hold any right, title or interest in or to any Insight CVR Asset), in each case during the Disposition Period.
“Disposition Agreement” means a definitive written agreement providing for a transaction or series of transactions between Insight or its Affiliates and any Person who is not an Affiliate of Insight regarding a Disposition.
“Disposition Period” means the period beginning on the execution date of the Merger Agreement and ending on the first anniversary of the Closing Date.
“Gross Proceeds” means, without duplication, the sum of all cash consideration and all cash proceeds from the sale of non-cash consideration of any kind that is paid to Insight or any of its Affiliates, or is received by Insight or any of its Affiliates, during the CVR Term with respect to a Disposition, solely as such consideration relates to an Insight CVR Asset.
“Holder” means, at the relevant time, a Person in whose name CVRs are registered in the CVR Register.
“Insight CVR Assets” means (a) Insight’s targeted oncology programs, IK-930 and IK-595, (b) Insight’s kynurenine degrading enzyme program, IK-412, (c) Insight’s antibody assets PY314, PY159, and PY265 (the “Pionyr Assets”), (d) Insight’s aryl hydrocarbon receptor antagonist program, IK-175 and (e) any other assets used or owned by Insight prior to the Closing.
“Liability” means any liability, indebtedness, obligation, expense, claim, deficiency, guaranty or endorsement of any kind, whether accrued, absolute, contingent, matured, unmatured or otherwise.
“Loss” has the meaning set forth in Section 3.2(g).
“Net Proceeds” means, for any CVR Payment Period, Gross Proceeds minus Permitted Deductions, all as calculated, to the extent in accordance with GAAP, in a manner consistent with Insight’s accounting practices and the most recently filed annual audited financial statements with the SEC, except as otherwise set forth herein. For clarity, to the extent Permitted Deductions exceed Gross Proceeds for any CVR Payment Period, any excess Permitted Deductions shall be applied against Gross Proceeds in subsequent CVR Payment Periods.
“Notice” has the meaning set forth in Section 7.1.
“Officer’s Certificate” means a certificate signed by the chief executive officer or the chief financial officer of Insight, in their respective official capacities.
“Party” means Insight or the Rights Agent.
“Permitted Deductions” means the sum of:
(a) any applicable Tax (including any applicable value added or sales taxes) imposed on Gross Proceeds and payable by Insight or any of its Affiliates (regardless of whether the due date for such Taxes arises during or after the Disposition Period) and, without duplication, any income or other similar Taxes payable by Insight or any of its Affiliates that would not have been incurred by Insight or any of its Affiliates but for the Gross Proceeds; provided that, for purposes of calculating income Taxes incurred by Insight or its Affiliates in respect of the Gross Proceeds, any such income Taxes shall be computed based on the gain recognized by Insight or its Affiliates from the Disposition after reduction for any net operating loss carryforwards or other Tax attributes of Insight or its Affiliates as of the Closing Date that are available to offset such gain after taking into account any limits of the usability of such attributes, including under Section 382 of the Code as determined by Insight’s tax advisers (and for the sake of clarity such income taxes shall be calculated without taking into account any net operating losses or other tax attributes generated by Insight or its Affiliates after the Closing Date); (b) any reasonable and documented expenses incurred by Insight or any of its Affiliates to preserve or ready the Insight CVR Assets for sale or in respect of its performance of this Agreement following the Closing Date or in respect of its performance of any Contract in connection with any Insight CVR Asset, including any damages, liabilities arising under any Contract or Disposition Agreement, including any incurred in litigation or other dispute resolution therefor, and any costs related to the prosecution, maintenance or enforcement by Insight or any of its Subsidiaries of intellectual property rights (but excluding any costs related to a breach of this Agreement, including costs incurred in litigation or dispute resolution in respect of the same);
(c) any reasonable and documented expenses incurred or accrued by Insight or any of its Affiliates in connection with the negotiation, entry into and closing of any Disposition of any Insight CVR Asset or the sale of any marketable securities received in any Disposition, including any brokerage fee, finder’s fee, opinion fee, success fee, transaction fee, service fee or other fee, commission or expense owed to any broker, finder, investment bank, auditor, accountant, counsel, advisor or other third party in relation thereto;
(d) any Losses incurred or reasonably expected to be incurred by Insight or any of its Affiliates arising out of any third-party claims, demands, actions, or other proceedings relating to or in connection with any Disposition, including indemnification obligations of Insight or any of its Affiliates set forth in any Disposition Agreement;
(e) any proceeds in consideration for a Disposition pursuant to a Disposition Agreement included in the final determination of the Net Cash of Insight in accordance with the Merger Agreement (for the purpose of avoiding overpayment of any duplicative amounts);
(f) any Liabilities borne by Insight or any of its Affiliates pursuant to Contracts related to Insight CVR Assets, including costs arising from the termination thereof; and
(g) any amounts payable to the Rights Agent in connection with the distribution of any CVR Payment Amount.
“Permitted Transfer” means a transfer of CVRs (a) upon death of a Holder by will or intestacy; (b) pursuant to a court order; (c) by operation of law (including by consolidation or merger) or without consideration in connection with the dissolution, liquidation or termination of any corporation, limited liability company, partnership or other entity; (d) in the case of CVRs held in book-entry or other similar nominee form, from a nominee to a beneficial owner and, if applicable, through an intermediary, to the extent allowable by DTC; or (e) as provided in Section 2.6.
“Rights Agent” means the Rights Agent named in the first paragraph of this Agreement, until a successor Rights Agent will have become the Rights Agent pursuant to the applicable provisions of this Agreement, and thereafter “Rights Agent” will mean such successor Rights Agent.
ARTICLE 2
CONTINGENT VALUE RIGHTS
Section 2.1 Holders of CVRs; Appointment of Rights Agent.
(a) The CVRs represent the rights of Holders to receive contingent CVR Payments pursuant to this Agreement. The initial Holders will be the holders of Common Stock as of immediately prior to the First Effective Time (excluding, for the avoidance of doubt, those shares of Common Stock issued in respect of the Concurrent Investment). One CVR will be issued with respect to each share of Common Stock that is outstanding as of immediately prior to the First Effective Time.
(b) Insight hereby appoints the Rights Agent to act as Rights Agent for Insight in accordance with the express terms and conditions set forth in this Agreement (and no implied terms and conditions), and the Rights Agent hereby accepts such appointment.
Section 2.2 Non-transferable.
The CVRs may not be sold, assigned, transferred, pledged, encumbered or in any other manner transferred or disposed of, in whole or in part, other than through a Permitted Transfer. Any attempted sale, assignment, transfer, pledge, encumbrance or disposal that is not a Permitted Transfer shall be void ab initio and of no effect. The CVRs will not be listed on any quotation system or traded on any securities exchange.
Section 2.3 No Certificate; Registration; Registration of Transfer; Change of Address.
(a) The CVRs will be issued in book-entry form only and will not be evidenced by a certificate or other instrument. Holders’ rights and obligations in respect of CVRs derive solely from this Agreement.
(b) Subject to the receipt by the Rights Agent of the information and instructions described in Section 4.1, the Rights Agent shall create and maintain a register (the “CVR Register”) for the purpose of registering CVRs and Permitted Transfers. The CVR Register will be created, and CVRs will be distributed, pursuant to written instructions to the Rights Agent from Insight. The CVR Register will initially show one position for Cede & Co. representing shares of Common Stock held by DTC on behalf of the street holders of the shares of Common Stock held by such Holders as of immediately prior to the First Effective Time. The Rights Agent will have no responsibility whatsoever directly or indirectly to the street name holders with respect to transfers of CVRs. With respect to any payments or issuances to be made under Section 2.4 below, the Rights Agent will accomplish the payment to any former street name holders of shares Common Stock by sending one lump-sum payment or issuance to DTC. The Rights Agent will have no responsibilities whatsoever with regard to the distribution of payments or shares of Common Stock by DTC to such street name holders.
(c) Subject to the restrictions on transferability set forth in Section 2.2, every request made to transfer a CVR must be in writing and accompanied by a written instrument of transfer in form reasonably satisfactory to the Rights Agent pursuant to its guidelines or procedures, including a guaranty of signature by an “eligible guarantor institution” that is a member or participant in the Securities Transfer Agents Medallion Program, duly executed and properly completed by the Holder thereof, the Holder’s attorney duly authorized in writing, the Holder’s personal representative or the Holder’s survivor, and setting forth in reasonable detail the circumstances relating to the transfer. Upon receipt of such written notice, the Rights Agent shall, subject to its reasonable determination that the transfer instrument is in proper form and the transfer otherwise complies with the other terms and conditions of this Agreement (including the provisions of Section 2.2), register the transfer of the CVRs in the CVR Register. Insight and Rights Agent shall not be responsible for, and may require evidence of payment of a sum sufficient to cover (or evidence that such Taxes and charges are not applicable), any stamp, documentary, registration, or other Tax or governmental charge that is imposed in connection with any such registration of transfer. The Rights Agent shall have no duty or obligation to take any action under any section of this Agreement that requires the payment by a Holder of a CVR of applicable taxes or charges unless and until the Rights Agent is satisfied that all such taxes or charges have been paid. All duly transferred CVRs registered in the CVR Register will be the valid obligations of Insight and will entitle the transferee to the same benefits and rights under this Agreement as those held immediately prior to the transfer by the transferor. No transfer of a CVR will be valid until registered in the CVR Register and any transfer not duly registered in the CVR Register shall be void.
(d) A Holder may make a written request to the Rights Agent to change such Holder’s address of record in the CVR Register. The written request must be duly executed by the Holder. Upon receipt of such written notice, the Rights Agent shall, subject to its reasonable determination that the transfer instrument is in proper form, promptly record the change of address in the CVR Register. The Acting Holders may, without duplication, make a written request to the Rights Agent for a list containing the names, addresses and number of CVRs of the Holders that are registered in the CVR Register. Upon receipt of such written request from the Acting Holders, the Rights Agent shall promptly deliver a copy of such list to the Acting Holders.
(e) Insight will provide written instructions to the Rights Agent for the distribution of CVRs to holders of Common Stock as of end of Business Day one day prior to the First Effective Time (the “Record Time”). Subject to the terms and conditions of this Agreement and Insight’s prompt confirmation of the First Effective Time, the Rights Agent shall effect the distribution of the CVRs, less any applicable tax withholding, to each holder of Common Stock as of the Record Time by the mailing of a statement of holding reflecting such CVRs.
Section 2.4 Payment Procedures.
(a) No later than forty-five (45) days following the end of each CVR Payment Period during the CVR Term, Insight shall deliver to the Rights Agent a CVR Payment Statement for such CVR Payment Period. Concurrent with the delivery of each CVR Payment Statement, on the terms and conditions of this Agreement, Insight shall pay the Rights Agent in U.S. dollars an amount equal to one-hundred percent (100%) of the Net Proceeds (if any) (subject to the proviso in the definition of the term “CVR Payment”) for the applicable CVR Payment Period; provided, however, that in the event that the aggregate CVR Payment on any CVR Payment Statement shall be less than $1,000,000, no CVR Payment Amount shall be due and instead such CVR Payment shall be added to subsequent CVR Payments until: (i) the aggregate CVR Payment shall be at least $1,000,000 or (ii) final CVR Payment Period. Such amount of Net Proceeds will be transferred by wire transfer of immediately available funds to an account designated in writing by the Rights Agent not less than ten (10) Business Days prior to the date of the applicable payment. Upon receipt of the wire transfer referred to in the foregoing sentence, the Rights Agent shall promptly (and in any event, within ten (10) Business Days) pay, by check mailed, first-class postage prepaid, to the address each Holder set forth in the CVR Register at such time or by other method of deliver as specified by the applicable Holder in writing to the Rights Agent, an amount equal to such Holder’s CVR Payment Amount. The Rights Agent shall, upon any Holder’s request in writing and as soon as practicable after receipt of a CVR Payment Statement under this Section 2.4(a), send such Holder at its registered address a copy of such statement. For the avoidance of doubt Insight shall have no further liability in respect of the relevant CVR Payment upon delivery of such CVR Payment in accordance with this Section 2.4(a) and the satisfaction of each of Insight’s obligations set forth in this Section 2.4(a).
(b) The Rights Agent shall solicit from each Holder an IRS Form W-9 or applicable IRS Form W-8 at such time or times as is necessary to permit any payment under this Agreement to be made without U.S. federal backup withholding. That notwithstanding, Insight shall be entitled to deduct and withhold and hereby authorizes the Rights Agent to deduct and withhold, any tax or similar governmental charge or levy, that is required to be deducted or withheld under applicable law from any amounts payable pursuant to this Agreement (“Withholding Taxes”). To the extent the amounts are so withheld by Insight or the Rights Agent, as the case may be, such withheld amounts shall be treated for all purposes of this Agreement as having been paid to the person in respect of whom such deduction and withholding was made. In the event Insight becomes aware that a payment under this Agreement is subject to Withholding Taxes (other than U.S. federal backup withholding), Insight shall use commercially reasonable efforts to provide written notice to the Rights Agent and the Rights Agent shall use commercially reasonable efforts to provide written notice of such Withholding Taxes to the applicable Holders and a reasonable opportunity for the Holder to provide any necessary Tax forms, including an IRS Form W-9 or appropriate IRS Form W-8, as applicable, in order to reduce such withholding amounts; provided that the time period for payment of a CVR Payment by the Rights Agent set forth in Section 2.4(a) will be extended by a period equal to any delay caused by the Holder providing such forms. For the avoidance of doubt, in the event that notice has been provided to an applicable Holder pursuant to this Section 2.4(b), no further notice shall be required to be given for any future payments of such Withholding Tax. Insight will use commercially reasonable efforts to provide withholding and reporting instructions in writing (email being sufficient) to the Rights Agent from time to time as relevant, and upon reasonable request of the Rights Agent. The Rights Agent shall have no responsibilities with respect to tax withholding, reporting or payment except specifically instructed by Insight.
(c) Any portion of a CVR Payment that remains undistributed to the Holders six (6) months after the end of an applicable CVR Payment Period (including by means of uncashed checks or invalid addresses on the CVR Register) will be delivered by the Rights Agent to Insight or a person nominated in writing by Insight (with written notice thereof from Insight to the Rights Agent), and any Holder will thereafter look only to Insight for payment of such CVR Payment (which shall be without interest).
(d) If any CVR Payment (or portion thereof) remains unclaimed by a Holder two (2) years after the end of an applicable CVR Payment Period (or immediately prior to such earlier date on which such CVR Payment would otherwise escheat to or become the property of any Governmental Authority), such CVR Payment (or portion thereof) will, to the extent permitted by applicable Law, become the property of Insight and will be transferred to Insight or a person nominated in writing by Insight (with written notice thereof from Insight to the Rights Agent), free and clear of all claims or interest of any Person previously entitled thereto, and no consideration or compensation shall be payable therefor. Neither Insight nor the Rights Agent will be liable to any Person in respect of a CVR Payment delivered to a public official pursuant to any applicable abandoned property, escheat or similar legal requirement under applicable Law. In addition to and not in limitation of any other indemnity obligation herein, Insight agrees to indemnify and hold harmless the Rights Agent with respect to any liability, penalty, cost or expense the Rights Agent may incur or be subject to in connection with transferring such property to Insight, a public office or a person nominated in writing by Insight.
Section 2.5 No Voting, Dividends or Interest; No Equity or Ownership Interest.
(a) The CVRs will not have any voting or dividend rights, and interest will not accrue on any amounts payable in respect of CVRs to any Holder.
(b) The CVRs will not represent any equity or ownership interest in Insight or in any constituent company to the Merger. The sole right of the Holders to receive property hereunder is the right to receive CVR Payments, if any, in accordance with the terms hereof. It is hereby acknowledged and agreed that a CVR shall not constitute a security of Insight.
(c) Nothing contained in this Agreement shall be construed as conferring upon any Holder, by virtue of the CVRs, any rights or obligations of any kind or nature whatsoever as a stockholder or member of Insight or any of its subsidiaries either at law or in equity. The rights of any Holder and the obligations of Insight and its Affiliates and their respective officers, directors and controlling Persons are contract rights limited to those expressly set forth in this Agreement.
(d) It is hereby acknowledged and agreed that the CVRs and the possibility of any payment hereunder with respect thereto are highly speculative and subject to numerous factors outside of Insight’s control, and there is no assurance that Holders will receive any payments under this Agreement or in connection with the CVRs. Each Holder acknowledges that it is highly possible that no Disposition will occur prior to the expiration of the Disposition Period and that there will not be any Gross Proceeds that may be the subject of a CVR Payment Amount. It is further acknowledged and agreed that neither Insight nor its Affiliates owe, by virtue of their obligations under this Agreement, a fiduciary duty or any implied duties to the Holders and the parties hereto intend solely the express provisions of this Agreement to govern their contractual relationship with respect to the CVRs. It is acknowledged and agreed that this Section 2.5(d) is an essential and material term of this Agreement.
Section 2.6 Ability to Abandon CVR.
A Holder may at any time, at such Holder’s option, abandon all of such Holder’s remaining rights represented by CVRs by transferring such CVR to Insight or a Person nominated in writing by Insight (with written notice thereof from Insight to the Rights Agent) without consideration in compensation therefor, and such rights will be cancelled, with the Rights Agent being promptly notified in writing by Insight of such transfer and cancellation. Nothing in this Agreement is intended to prohibit Insight or its Affiliates from offering to acquire or acquiring CVRs, in private transactions or otherwise, for consideration in its sole discretion.
ARTICLE 3
THE RIGHTS AGENT
Section 3.1 Certain Duties and Responsibilities.
(a) The Rights Agent will not have any liability for any actions taken or not taken in connection with this Agreement, except to the extent such liability arises as a result of the willful misconduct, bad faith, fraud or gross negligence of the Rights Agent (in each case as determined by a final non-appealable judgment of court of competent jurisdiction). Notwithstanding anything in this Agreement to the contrary, any liability of the Rights Agent under this Agreement will be limited to the amount of annual fees paid by Insight to the Rights Agent (but not including reimbursable expenses and other charges) during the twelve (12) months immediately preceding the event for which recovery from the Rights Agent is being sought. Anything to the contrary notwithstanding, in no event will the Rights Agent be liable for special, punitive, indirect, incidental or consequential loss or damages of any kind whatsoever (including, without limitation, lost profits), even if the Rights Agent has been advised of the likelihood of such loss or damages, and regardless of the form of action.
(b) The Rights Agent shall not have any duty or responsibility in the case of the receipt of any written demand from any Holder with respect to any action or default by any person or entity, including, without limiting the generality of the foregoing, any duty or responsibility to initiate or attempt to initiate any proceedings at law or otherwise or to make any demand upon Insight or the Company. The Rights Agent may (but shall not be required to) enforce all rights of action under this Agreement and any related claim, action, suit, audit, investigation or proceeding instituted by the Rights Agent may be brought in its name as the Rights Agent and any recovery in connection therewith will be for the proportionate benefit of all the Holders, as their respective rights or interests may appear on the CVR Register.
Section 3.2 Certain Rights of Rights Agent.
(a) The Rights Agent undertakes to perform such duties and only such duties as are specifically set forth in this Agreement, and no implied covenants or obligations will be read into this Agreement against the Rights Agent.
(b) The Rights Agent may rely and will be protected by Insight in acting or refraining from acting upon any resolution, certificate, statement, instrument, opinion, report, notice, request, direction, consent, order or other paper or document reasonably believed by it to be genuine and to have been signed or presented by or on behalf of Insight or, with respect to Section 2.3(d), the Acting Holders.
(c) Whenever the Rights Agent deems it desirable that a matter be proved or established prior to taking, suffering or omitting any action hereunder, the Rights Agent may rely upon an Officer’s Certificate, which certificate shall be full authorization and protection to the Rights Agent, and the Rights Agent shall, in the absence of bad faith, fraud, gross negligence or willful misconduct (each as determined by a final non-appealable judgment of a court of competent jurisdiction) on its part, not incur any liability and shall be held harmless by Insight for or in respect of any action taken, suffered or omitted to be taken by it under the provisions of this Agreement in reliance upon such Officer’s Certificate.
(d) The Rights Agent may engage and consult with counsel of its selection, and the advice or opinion of such counsel will, in the absence of bad faith, fraud, gross negligence or willful misconduct (in each case, as determined by a final, non-appealable judgment of a court of competent jurisdiction) on the part of the Rights Agent, be full and complete authorization and protection in respect of any action taken, suffered or not taken by the Rights Agent in reliance thereon.
(e) Any permissive rights of the Rights Agent hereunder will not be construed as a duty.
(f) The Rights Agent will not be required to give any note or surety in respect of the execution of its powers or otherwise under this Agreement.
(g) Insight agrees to indemnify the Rights Agent for, and to hold the Rights Agent harmless from and against, any loss, liability, damage, judgment, fine, penalty, claim, demand, settlement, cost or expense (including the reasonable and documented fees and expenses of legal counsel) (each, a “Loss”) which may be paid, incurred or suffered by or to which it may become subject, arising from or out of, directly or indirectly, any claims or liability resulting from any action taken, suffered or omitted by the Rights Agent in connection the execution, acceptance, administration, exercise and performance of its duties under this Agreement, including the costs and expenses of defending against any claim of liability arising therefrom, directly or indirectly, or enforcing its rights hereunder, except to the extent such Loss has been determined by a final non-appealable decision of a court of competent jurisdiction to have resulted from the Rights Agent’s willful misconduct, bad faith, fraud or gross negligence; provided that this Section 3.2(g) shall not apply with respect to income, receipt, franchise or similar Taxes levied against the Rights Agent by a Governmental Authority.
(h) Insight agrees (i) to pay the fees of the Rights Agent in connection with the Rights Agent’s performance of its obligations hereunder as set forth in a fee schedule agreed upon in writing by the Rights Agent and Insight on or prior to the date of this Agreement (the “Fee Schedule”), and (ii) to reimburse the Rights Agent for all reasonable and documented out-of-pocket expenses and other disbursements incurred in the preparation, delivery, negotiation, amendment, administration and execution of this Agreement and the exercise and performance of its duties hereunder, including all stamp and transfer Taxes (and excluding for the avoidance of doubt, any income, receipt, franchise or similar Taxes levied against the Rights Agent by a Governmental Authority) and governmental charges, incurred by the Rights Agent in the performance of its obligations under this Agreement.
(i) No provision of this Agreement shall require the Rights Agent to expend or risk its own funds or otherwise incur any financial liability in the performance of any of its duties hereunder or in the exercise of any of its rights or powers if it believes that repayment of such funds or adequate indemnification against such risk or liability is not reasonably assured to it.
(j) The Rights Agent shall have no responsibility to Insight, any holders of CVRs, any holders of shares of Common Stock or any other Person for interest or earnings on any moneys held by the Rights Agent pursuant to this Agreement.
(k) The Rights Agent shall not be subject to, nor be required to comply with, or determine if any Person has complied with, the Merger Agreement or any other agreement between or among any Insight, the Company or Holders, even though reference thereto may be made in this Agreement, or to comply with any notice, instruction, direction, request or other communication, paper or document other than as expressly set forth in this Agreement.
(l) Subject to applicable Law, (i) the Rights Agent and any shareholder, affiliate, director, officer or employee of the Rights Agent may buy, sell or deal in any securities of Insight or the Company or become peculiarly interested in any transaction in which such parties may be interested, or contract with or lend money to such parties or otherwise act as fully and freely as though it were not the Rights Agent under this Agreement, and (ii) nothing herein will preclude the Rights Agent from acting in any other capacity for Insight or for any other Person.
(m) In the event the Rights Agent reasonably believes any ambiguity or uncertainty exists hereunder or in any notice, instruction, direction, request or other communication, paper or document received by the Rights Agent hereunder, the Rights Agent shall, as soon as practicable, provide notice to Insight, and the Rights Agent, may, in its sole discretion, refrain from taking any action, and shall be fully protected and shall not be liable in any way to Insight or any Holder or any other Person for refraining from taking such action, unless the Rights Agent receives written instructions from Insight or such Holder or other Person which eliminate such ambiguity or uncertainty to the reasonable satisfaction of the Rights Agent.
(n) The Rights Agent may execute and exercise any of the rights or powers hereby vested in it or perform any duty hereunder either itself or by or through its attorney or agents and the Rights Agent shall not be answerable or accountable for any act, default, neglect or misconduct of any such attorney or agents or for any loss to Insight or the Company resulting from any such act, default, neglect or misconduct, absent willful misconduct, bad faith, fraud or gross negligence (each as determined by a final non- appealable judgment of a court of competent jurisdiction) in the selection and continued employment thereof.
(o) The Rights Agent shall not be liable for or by reason of any of the statements of fact or recitals contained in this Agreement (except its countersignature thereof) or be required to verify the same, and all such statements and recitals are and shall be deemed to have been made by Insight only.
(p) The Rights Agent shall act hereunder solely as agent for Insight and shall not assume any obligations or relationship of agency or trust with any of the owners or holders of the CVRs. The Rights Agent shall not have any duty or responsibility in the case of the receipt of any written demand from any Holders with respect to any action or default by Insight, including, without limiting the generality of the foregoing, any duty or responsibility to initiate or attempt to initiate any proceedings at law or otherwise or to make any demand upon Insight.
(q) The Rights Agent may rely on and be fully authorized and protected in acting or failing to act upon (a) any guaranty of signature by an “eligible guarantor institution” that is a member or participant in the Securities Transfer Agents Medallion Program or other comparable “signature guarantee program” or insurance program in addition to, or in substitution for, the foregoing; or (b) any law, act, regulation or any interpretation of the same even though such law, act, or regulation may thereafter have been altered, changed, amended or repealed.
(r) The Rights Agent shall not be liable or responsible for any failure of Insight to comply with any of its obligations relating to any registration statement filed with the Securities and Exchange Commission or this Agreement, including without limitation obligations under applicable regulation or law.
(s) The obligations of Insight and the rights of the Rights Agent under this Section 3.2, Section 3.1. and Section 2.4 shall survive the expiration of the CVRs and the termination of this Agreement and the resignation, replacement or removal of the Rights Agent.
(t) The Rights Agent will not be deemed to have knowledge of any event of which it was supposed to receive notice hereunder but has not received written notice of such event, and the Rights Agent will not incur any liability for failing to take action in connection therewith, in each case, unless and until it has received such notice in writing.
(u) The Rights Agent will have no liability and shall be held harmless by Insight in respect of the validity of this Agreement and the execution and delivery hereof (except the due execution and delivery hereof by the Rights Agent and the enforceability of this Agreement against the Rights Agent assuming the due execution and delivery hereof by Insight), nor shall it be responsible for any breach by Insight of any covenant or condition contained in this Agreement.
Section 3.3 Resignation and Removal; Appointment of Successor.
(a) The Rights Agent may resign at any time by written notice to Insight. Any such resignation notice shall specify the date on which such resignation will take effect (which shall be at least thirty (30) days following the date that such resignation notice is delivered), and such resignation will be effective on the earlier of (x) the date so specified and (y) the appointment of a successor Rights Agent.
(b) Insight will have the right to remove the Rights Agent at any time by written notice to the Rights Agent, specifying the date on which such removal will take effect. Such notice will be given at least thirty (30) days prior to the date so specified (or, if earlier, the appointment of the successor Rights Agent).
(c) If the Rights Agent resigns, is removed or becomes incapable of acting, Insight will promptly appoint a qualified successor Rights Agent. Notwithstanding the foregoing, if Insight fails to make such appointment within a period of thirty (30) days after giving notice of such removal or after it has been notified in writing of such resignation or incapacity by the resigning or incapacitated Rights Agent, then the any Holder may apply to any court of competent jurisdiction for the appointment of a new Rights Agent. The successor Rights Agent so appointed will, upon its acceptance of such appointment in accordance with this Section 3.3(c) and Section 3.4, become the Rights Agent for all purposes hereunder.
(d) Insight will give notice to the Holders of each resignation or removal of the Rights Agent and each appointment of a successor Rights Agent in accordance with Section 7.2. Each notice will include the name and address of the successor Rights Agent. If Insight fails to send such notice within ten (10) Business Days after acceptance of appointment by a successor Rights Agent, the successor Rights Agent will cause the notice to be mailed at the expense of Insight.
(e) Notwithstanding anything to the contrary in this Section 3.3, unless consented to in writing by the Acting Holders, Insight will not appoint as a successor Rights Agent any Person that is not a stock transfer agent of national reputation or the corporate trust department of a commercial bank.
(f) The Rights Agent will reasonably cooperate with Insight and any successor Rights Agent in connection with the transition of the duties and responsibilities of the Rights Agent to the successor Rights Agent, including the transfer of all relevant data, including the CVR Register, to the successor Rights Agent, but such predecessor Rights Agent shall not be required to make any additional expenditure or assume any additional liability in connection with the foregoing.
Section 3.4 Acceptance of Appointment by Successor.
Every successor Rights Agent appointed hereunder will, at or prior to such appointment, execute, acknowledge and deliver to Insight and to the resigning or removed Rights Agent an instrument accepting such appointment and a counterpart of this Agreement, and such successor Rights Agent, without any further act, deed or conveyance, will become vested with all the rights, powers, trusts and duties of the Rights Agent; provided that upon the request of Insight or the successor Rights Agent, such resigning or removed Rights Agent will execute and deliver an instrument transferring to such successor Rights Agent all the rights, powers and trusts of such resigning or removed Rights Agent, except such rights which survive its resignation or removal under the terms hereunder.
ARTICLE 4
COVENANTS
Section 4.1 List of Holders.
Insight will furnish or cause to be furnished to the Rights Agent, in such form as Insight receives from Insight’s transfer agent (or other agent performing similar services for Insight), the names and addresses of the Holders within fifteen (15) Business Days following the Closing Date.
Section 4.2 Obligations of Public Company.
(a) Notwithstanding anything herein to the contrary, (a) Insight and its Affiliates shall have the power and right to control all aspects of their businesses and operations (and all of their assets and products, including, subject to Section 4.2(b), with respect to (i) managing, directing and controlling the exploitation of the Insight CVR Assets in all respects and (ii) conducting any sale process (including engagement of advisors) with respect to any Insight CVR Assets), and subject to Insight’s compliance with the terms of this Agreement, Insight and its Affiliates may exercise or refrain from exercising such power and right as it may deem appropriate and in the best overall interests of Insight and its Affiliates and its and their stockholders, rather than the interest of the Holders, (b) none of Insight or any of its Affiliates (or any directors, officer, employee, or other representative of the foregoing) owes any fiduciary duty or similar duty or any other implied duties (including the implied covenant of good faith and fair dealing) to any Holder in respect of the CVRs or the Insight CVR Assets, (c) except as specifically provided in Section 4.2(b), Insight shall have no obligation to operate, use, sell, transfer, convey, license, develop, commercialize or otherwise exploit in any particular manner any of its or its Affiliates’ business or operations (or any of their assets or products) or to negotiate or enter into any agreement, including any Disposition Agreement, including in order to obtain, maximize or expedite the receipt of any Gross Proceeds or minimize Permitted Deductions, and (d) following the Disposition Period, Insight shall be permitted to take any action in respect of the Insight CVR Assets in order to satisfy any wind-down and termination Liabilities of the Insight CVR Assets.
(b) Insight will allocate reasonable resources to reasonably maintain the Insight CVR Assets during the Disposition Period; provided, that Insight will not in any event be required to exceed the Expense Reserve in maintaining the Insight CVR Assets.
(c) None of Insight or any of its Affiliates shall have any obligation or liability whatsoever to any Person relating to or in connection with any action, or failure to act, with respect to the sale of the Insight CVR Assets, except for the obligation to make CVR Payment Amounts to the Holders of CVRs pursuant to this Agreement.
(d) Except for the obligations to the Rights Agent set forth in this Agreement, neither Insight nor its Subsidiaries will have any responsibility or liability whatsoever to any Person other than the Holders.
(e) Solely with respect to the sale of an Insight CVR Asset and for up to twelve (12) months after receipt thereof, Insight will use commercially reasonable efforts to sell any securities listed on a U.S. national exchange (subject to any restrictions associated with such securities received as consideration for such sale); provided, that Insight will have no liability for its failure to monetize any such non-cash consideration received.
Section 4.3 Prohibited Actions.
Unless approved by the Insight Board, prior to the end of the Disposition Period, Insight shall not grant any lien, security interest, pledge or similar interest in any Insight CVR Assets or any Net Proceeds.
Section 4.4 Books and Records.
Until the end of the CVR Term, Insight shall, and shall cause its Affiliates to, keep true, complete and accurate records in sufficient detail to support the applicable CVR Payments, if any, payable hereunder in accordance with the terms specified in this Agreement.
Section 4.5 Audits.
Until the Termination Date and for a period of one (1) year thereafter, Insight shall keep complete and accurate records in sufficient detail to support the accuracy of the payments due hereunder. The Acting Holders shall have the right to cause an independent accounting firm reasonably acceptable to Insight to audit such records for the sole purpose of confirming payments for a period covering not more than the date commencing with the first CVR Payment Period in which Insight or its Affiliates receives Gross Proceeds and ending on the last day of the CVR Term. Insight may require such accounting firm to execute a reasonable confidentiality agreement with Insight prior to commencing the audit. The accounting firm shall disclose to Rights Agent or the Acting Holders, as applicable, only whether the reports are correct or not and the specific details concerning any discrepancies. No other information shall be shared, and in no event shall Insight be required to provide any Tax returns or any other Tax information it deems confidential to the Acting Holders or any other party.
Such audits may be conducted during normal business hours upon reasonable prior written notice to Insight, but no more than frequently than once per year. No accounting period of Insight shall be subject to audit more than one time by the Acting Holders, as applicable. Adjustments (including remittances of underpayments or overpayments disclosed by such audit) shall be made by Insight to reflect the results of such audit, which adjustments shall be paid promptly following receipt of an invoice therefor. Whenever such an adjustment is made, Insight shall promptly prepare a certificate setting forth such adjustment, and a brief, reasonably detailed statement of the facts, computation and methodology accounting for such adjustment to the extent not already reflected in the audit report and promptly file with the Rights Agent a copy of such report and promptly deliver to the Rights Agent a revised CVR Payment Statement for the relevant CVR Payment Period. The Rights Agent shall be fully protected in relying on any such report and on any adjustment or statement therein contained and shall have no duty or liability with respect to, and shall not be deemed to have knowledge of any such adjustment or any such event unless and until it shall have received such report. The Acting Holders, as applicable, shall bear the full cost and expense of such audit unless such audit discloses an underpayment by Insight of twenty percent (20%) or more of the CVR Payment due under this Agreement, in which case Insight shall bear the full cost and expense of such audit. The Rights Agent shall be entitled to rely on any audit report delivered by the independent accounting firm pursuant to this Section 4.5.
ARTICLE 5
AMENDMENTS
Section 5.1 Amendments Without Consent of Holders or Rights Agent.
(a) Insight, at any time and from time to time, may (without the consent of any Person, including the Holders, other than the Rights Agent, with such consent not to be unreasonably withheld, conditioned or delayed) enter into one or more amendments to this Agreement for any of the following purposes:
(i) to evidence the appointment of another Person as a successor Rights Agent and the assumption by any successor Rights Agent of the covenants and obligations of the Rights Agent herein in accordance with the provisions hereof;
(ii) subject to Section 6.1, to evidence the succession of another person to Insight and the assumption of any such successor of the covenants of Insight outlined herein in a transaction contemplated by Section 6.1;
(iii) to add to the covenants of Insight such further covenants, restrictions, conditions or provisions as Insight will consider to be for the protection and benefit of the Holders; provided that in each case, such provisions do not adversely affect the interests of the Holders;
(iv) to cure any ambiguity, to correct or supplement any provision in this Agreement that may be defective or inconsistent with any other provision in this Agreement, or to make any other provisions with respect to matters or questions arising under this Agreement; provided that, in each case, such provisions do not adversely affect the interests of the Holders;
(v) as may be necessary or appropriate to ensure that the CVRs are not subject to registration under the Securities Act or the Exchange Act and the rules and regulations promulgated thereunder, or any applicable state securities or “blue sky” laws;
(vi) as may be necessary or appropriate to ensure that Insight is not required to produce a prospectus or an admission document in order to comply with applicable Law;
(vii) to cancel the CVRs (i) in the event that any Holder has abandoned its rights in accordance with Section 2.6, or (ii) following a transfer of such CVRs to Insight or its Affiliates in accordance with Section 2.2 or Section 2.3;
(viii) as may be necessary or appropriate to ensure that Insight complies with applicable Law; or
(ix) to effect any other amendment to this Agreement for the purpose of adding, eliminating or changing any provisions of this Agreement, provided that, in each case, such additions, eliminations or changes do not adversely affect the interests of the Holders.
(b) Promptly after the execution by Insight of any amendment pursuant to this Section 5.1, Insight will (or will cause the Rights Agent to) notify the Holders in general terms of the substance of such amendment in accordance with Section 7.2.
Section 5.2 Amendments with Consent of Holders.
(a) In addition to any amendments to this Agreement that may be made by Insight without the consent of any Holder pursuant to Section 5.1, with the consent of the Acting Holders (whether evidenced in a writing or taken at a meeting of the Holders), Insight and the Rights Agent may enter into one or more amendments to this Agreement for the purpose of adding, eliminating or amending any provisions of this Agreement, even if such addition, elimination or amendment is adverse to the interests of the Holders.
(b) Promptly after the execution by Insight and the Rights Agent of any amendment pursuant to the provisions of this Section 5.2, Insight will (or will cause the Rights Agent to) notify the Holders in general terms of the substance of such amendment in accordance with Section 7.2.
Section 5.3 Effect of Amendments.
Upon the execution of any amendment under this Article 5, this Agreement will be modified in accordance therewith, such amendment will form a part of this Agreement for all purposes and every Holder will be bound thereby. Upon the delivery of a certificate from an appropriate officer of Insight which states that the proposed supplement or amendment is in compliance with the terms of this Article 5, the Rights Agent shall execute such supplement or amendment. Notwithstanding anything in this Agreement to the contrary, the Rights Agent shall not be required to execute any supplement or amendment to this Agreement that it has determined would adversely affect its own rights, duties, obligations or immunities under this Agreement. No supplement or amendment to this Agreement shall be effective unless duly executed by the Rights Agent.
ARTICLE 6
CONSOLIDATION, MERGER, SALE OR CONVEYANCE
Section 6.1 Change of Control.
Prior to the Termination Date, Insight shall not consummate a Change of Control without the consent of the Acting Holders (such consent not to be unreasonably withheld, conditioned or delayed), unless:
(a) the Person that is the relevant transferee, assignee, acquiror, delegate or other successor (including by operation of law) in such Change of Control (the “Surviving Person”) shall assume or succeed to the obligations on all CVRs (when and as due hereunder); and
(b) Insight has delivered to the Rights Agent an Officer’s Certificate stating that such Change of Control complies with this Article 6 and that all conditions precedent herein provided for relating to such Change of Control have been complied with.
Section 6.2 Successor Substituted.
Upon the consummation of any Change of Control in accordance with Section 6.1, the Surviving Person shall succeed to, and be substituted for, and may exercise every right and power of, and shall assume all of the obligations of Insight under this Agreement with the same effect as if the Surviving Person had been named as Insight herein.
ARTICLE 7
MISCELLANEOUS
Section 7.1 Notices to Rights Agent and to Insight.
All notices, requests and other communications (each, a “Notice”) to any party hereunder shall be in writing and shall be deemed to have been duly delivered and received hereunder when sent (a) fees prepaid, via a reputable international overnight courier service in the case of delivery in person, by FedEx or other internationally recognized overnight courier service or (b) on the date sent in the place of delivery if sent by email (with a written or electronic confirmation of delivery) prior to 6:00 p.m. (New York City time), otherwise on the next succeeding Business Day, in each case to the intended recipient as set forth below:
if to the Rights Agent, to:
Computershare Trust Company, N.A.
Computershare Inc.
150 Royall Street
2nd Floor
Canton, MA 02021
if to Insight, to:
ImageneBio, Inc.
12526 High Bluff Drive, Suite 345
San Diego, CA 92130
Attention: Legal
Email: legal@inmagenebio.com
with a copy, which shall not constitute notice, to:
Cooley LLP
10265 Science Center Drive
San Diego, CA 92121-1117
Attention: Patrick Loofbourrow
Email: loof@cooley.com
or to such other address as such party may hereafter specify for the purpose by notice to the other parties hereto.
Section 7.2 Notice to Holders.
All Notices required to be given to the Holders will be given (unless otherwise herein expressly provided) in writing and mailed, first-class postage prepaid, to each Holder at such Holder’s address as set forth in the CVR Register, not later than the latest date, and not earlier than the earliest date, prescribed for the sending of such Notice, if any, and will be deemed given on the date of mailing. In any case where notice to the Holders is given by mail, neither the failure to mail such Notice, nor any defect in any Notice so mailed, to any particular Holder will affect the sufficiency of such Notice with respect to other Holders.
Section 7.3 Entire Agreement.
As between Insight and the Rights Agent, this Agreement constitutes the entire agreement between the parties with respect to the subject matter of this Agreement, notwithstanding the reference to any other agreement herein, and supersedes all prior agreements and understandings, both written and oral, among or between any of the parties with respect to the subject matter of this Agreement.
Section 7.4 Merger or Consolidation or Change of Name of Rights Agent.
Any Person into which the Rights Agent or any successor Rights Agent may be merged or with which it may be consolidated, or Person resulting from any merger or consolidation to which the Rights Agent or any successor Rights Agent shall be a party, or any Person succeeding to the stock transfer or other shareholder services business of the Rights Agent or any successor Rights Agent, shall be the successor to the Rights Agent under this Agreement without the execution or filing of any paper or any further act on the part of any of the parties hereto, provided that such Person would be eligible for appointment as a successor Rights Agent under the provisions of Section 3.3. The purchase of all or substantially all of the Rights Agent’s assets employed in the performance of transfer agent activities shall be deemed a merger or consolidation for purposes of this Section 7.4.
Section 7.5 Successors and Assigns.
This Agreement will be binding upon, and will be enforceable by and inure solely to the benefit of, the Holders, Insight and the Rights Agent and their respective successors and assigns. Except for assignments pursuant to Section 7.4, the Rights Agent may not assign this Agreement without Insight’s prior written consent. Subject to Section 5.1(a)(ii) and Article 6 hereof, Insight may assign, in its sole discretion and without the consent of any other party, any or all of its rights, interests and obligations hereunder to one or more of its Affiliates or to any Person with whom Insight is merged or consolidated, or any entity resulting from any merger or consolidation to which Insight shall be a party (each, an “Assignee”); provided, that in connection with any assignment to an Assignee, Insight shall agree to remain liable for the performance by Insight of its obligations hereunder (to the extent Insight exists following such assignment). Insight or an Assignee may not otherwise assign this Agreement without the prior consent of the Acting Holders (such consent not to be unreasonably withheld, conditioned or delayed). Any attempted assignment of this Agreement in violation of this Section 7.5 will be void ab initio and of no effect.
Section 7.6 Benefits of Agreement; Action by Acting Holders.
Nothing in this Agreement, express or implied, will give to any Person (other than Insight, the Rights Agent, the Holders and their respective permitted successors and assigns hereunder) any benefit or any legal or equitable right, remedy or claim under this Agreement or under any covenant or provision herein contained, all such covenants and provisions being for the sole benefit of Insight, the Rights Agent, the Holders and their permitted successors and assigns. The Holders will have no rights hereunder except as are expressly set forth herein. Except for the rights of the Rights Agent set forth herein, the Acting Holders will have the sole right, on behalf of all Holders, by virtue of or under any provision of this Agreement, to institute any action or proceeding at law or in equity with respect to this Agreement, and no individual Holder or other group of Holders will be entitled to exercise such rights.
Section 7.7 Governing Law.
This Agreement and the CVRs will be governed by, and construed in accordance with, the laws of the State of Delaware, without giving effect to any provision of law or rule (whether of the State of Delaware or any other jurisdictions) that would cause the application of the laws of any jurisdictions other than the State of Delaware.
Section 7.8 Jurisdiction.
In any action or proceeding between any of the parties hereto arising out of or relating to this Agreement or any of the transactions contemplated hereby, each of the parties hereto: (a) irrevocably and unconditionally consents and submits to the exclusive jurisdiction and venue of the Court of Chancery of the State of Delaware or, to the extent such court does not have subject matter jurisdiction, the Superior Court of the State of Delaware or the United States District Court for the District of Delaware, and appellate courts thereof; (b) agrees that all claims in respect of such action or proceeding shall be heard and determined exclusively in accordance with clause (a) of this Section 7.8; (c) waives any objection to laying venue in any such action or proceeding in such courts; (d) waives any objection that such courts are an inconvenient forum or do not have jurisdiction over any Party; and (e) agrees that service of process upon such Party in any such action or proceeding shall be effective if notice is given in accordance with Section 7.1 or Section 7.2 of this Agreement; provided that nothing contained herein shall be deemed to limit in any way any right to serve process in any manner permitted by Law.
Section 7.9 WAIVER OF JURY TRIAL.
EACH OF THE PARTIES HERETO HEREBY IRREVOCABLY WAIVES ANY AND ALL RIGHT TO TRIAL BY JURY IN ANY LEGAL PROCEEDING ARISING OUT OF OR RELATED TO THIS AGREEMENT OR THE TRANSACTIONS CONTEMPLATED HEREBY. EACH PARTY CERTIFIES AND ACKNOWLEDGES THAT (I) NO REPRESENTATIVE, AGENT OR ATTORNEY OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF LITIGATION, SEEK TO ENFORCE THE FOREGOING WAIVER, (II) EACH PARTY UNDERSTANDS AND HAS CONSIDERED THE IMPLICATION OF THIS WAIVER, (III) EACH PARTY MAKES THIS WAIVER VOLUNTARILY, AND (IV) EACH PARTY HAS BEEN INDUCED TO ENTER INTO THIS AGREEMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS AND CERTIFICATIONS IN THIS SECTION 7.9.
Section 7.10 Severability Clause.
In the event that any provision of this Agreement, or the application of any such provision to any Person or set of circumstances, is for any reason determined to be invalid, unlawful, void or unenforceable to any extent, the remainder of this Agreement, and the application of such provision to Persons or circumstances other than those as to which it is determined to be invalid, unlawful, void or unenforceable, will not be impaired or otherwise affected and will continue to be valid and enforceable to the fullest extent permitted by applicable Law. Upon such a determination, the parties hereto will negotiate in good faith to modify this Agreement so as to effect the original intent of the parties as closely as possible in a mutually acceptable manner in order that the transactions contemplated hereby be consummated as originally contemplated to the fullest extent possible; provided, however, that if an excluded provision shall affect the rights, immunities, liabilities, duties or obligations of the Rights Agent, the Rights Agent shall be entitled to resign immediately upon written Notice to Insight.
Section 7.11 Counterparts; Effectiveness.
This Agreement may be signed in any number of counterparts, each of which will be deemed an original, with the same effect as if the signatures thereto and hereto were upon the same instrument. This Agreement or any counterpart may be executed and delivered by facsimile copies or delivered by electronic communications by portable document format (.pdf), each of which shall be deemed an original. This Agreement will become effective when each party hereto will have received a counterpart hereof signed by the other party hereto. Until and unless each party has received a counterpart hereof signed by the other party hereto, this Agreement will have no effect and no party will have any right or obligation hereunder (whether by virtue of any oral or written agreement or any other communication).
Section 7.12 Termination.
This Agreement will automatically terminate and be of no further force or effect and, except the rights, protections and immunities of the Rights Agent under Article 3, the parties hereto will have no further liability hereunder, and the CVRs will expire without any consideration or compensation therefor, upon the earliest to occur of (a) the expiration of the CVR Term, (b) the expiration of all payment obligations under the Disposition Agreements, or (c) the delivery of a written notice of termination duly executed by Insight and the Acting Holders (such date, the “Termination Date”). The termination of this Agreement will not affect or limit the right of Holders to receive the CVR Payments under Section 2.4 to the extent earned prior to the termination of this Agreement, and the provisions applicable thereto will survive the expiration or termination of this Agreement until such CVR Payments, if any, have been made, if applicable.
Section 7.13 Funds.
All funds received by Computershare under this Agreement that are to be distributed or applied by Computershare in the performance of services hereunder (the “Funds”) shall be held by Computershare, as agent for Insight, and deposited in one or more bank accounts to be maintained by Computershare in its name as agent for Insight. Until paid pursuant to the terms of this Agreement, Computershare may hold or invest the Funds through such accounts in: (a) funds backed by obligations of, or guaranteed by, the United States of America; (b) debt or commercial paper obligations rated A-1 or P-1 or better by S&P Global Inc. (“S&P”) or Moody’s Investors Service, Inc. (“Moody’s”), respectively; (c) Government and Treasury backed AAA-rated Fixed NAV money market funds that comply with Rule 2a-7 of the Investment Company Act of 1940, as amended; or (d) short term certificates of deposit, bank repurchase agreements, and bank accounts with commercial banks with Tier 1 capital exceeding $1 billion, or with an investment grade rating by S&P (LT Local Issuer Credit Rating), Moody’s (Long Term Rating) and Fitch Ratings, Inc. (LT Issuer Default Rating) (each as reported by Bloomberg Finance L.P.). Computershare shall have no responsibility or liability for any diminution of the Funds that may result from any deposit or investment made by Computershare in accordance with this paragraph, including any losses resulting from a default by any bank, financial institution or other third party. Computershare may from time to time receive interest, dividends or other earnings in connection with such deposits or investments. Computershare shall not be obligated to pay such interest, dividends or earnings to Insight, any holder or any other party.
Section 7.14 Further Assurance by Insight.
Insight agrees that it will perform, execute, acknowledge and deliver or cause to be performed, executed, acknowledged and delivered all such further and other acts, instruments and assurances as may reasonably be required or requested by the Rights Agent for the carrying out or performing by the Rights Agent of the provisions of this Agreement.
Section 7.15 Confidentiality. The Rights Agent and Insight agree that all books, records, information and data pertaining to the business of the other party, including inter alia, personal, non-public CVR holder information, which are exchanged or received pursuant to the negotiation or the carrying out of this Agreement including the Fee Schedule shall remain confidential, and shall not be voluntarily disclosed to any other person other than representatives of the Rights Agent or Insight, except as may be required by Law, including, without limitation, pursuant to subpoenas from state or federal government authorities (e.g., in divorce and criminal actions).
Section 7.16 Force Majeure. Notwithstanding anything to the contrary contained herein, the Rights Agent will not be liable for any delays or failures in performance resulting from acts beyond its reasonable control including, without limitation, acts of God, epidemic, pandemic, terrorist acts, shortage of supply, breakdowns or malfunctions, interruptions or malfunction of computer facilities, or loss of data due to power failures or mechanical difficulties with information storage or retrieval systems, labor difficulties, war, or civil unrest.
Section 7.17 Construction.
(a) For purposes of this Agreement, whenever the context requires: singular terms will include the plural, and vice versa; the masculine gender will include the feminine and neuter genders; the feminine gender will include the masculine and neuter genders; and the neuter gender will include the masculine and feminine genders.
(b) As used in this Agreement, the words “include” and “including,” and variations thereof, will not be deemed to be terms of limitation, but rather will be deemed to be followed by the words “without limitation.”
(c) The headings contained in this Agreement are for convenience of reference only, will not be deemed to be a part of this Agreement and will not be referred to in connection with the construction or interpretation of this Agreement.
(d) Unless stated otherwise, “Article” and “Section” followed by a number or letter mean and refer to the specified Article or Section of this Agreement. The term “Agreement” and any reference in this Agreement to this Agreement or any other agreement or document includes, and is a reference to, this Agreement or such other agreement or document as it may have been, or may from time to time be, amended, restated, replaced, supplemented or novated and includes all schedules to it.
(e) A period of time is to be computed as beginning on the day following the event that began the period and ending at 4:30 p.m. on the last day of the period, if the last day of the period is a Business Day, or at 4:30 p.m. on the next Business Day if the last day of the period is not a Business Day.
(f) Any reference in this Agreement to a date or time shall be deemed to be such date or time in New York City, United States, unless otherwise specified. The parties hereto and Insight have participated jointly in the negotiation and drafting of this Agreement. In the event an ambiguity or question of intent or interpretation arises, this Agreement shall be construed as if drafted jointly by the parties and Insight and no presumption or burden of proof shall arise favoring or disfavoring any Person by virtue of the authorship of any provision of this Agreement.
(g) All references herein to “$” are to United States Dollars.
[Remainder of page intentionally left blank]
IN WITNESS WHEREOF, each of the parties has caused this Agreement to be executed as of the day and year first above written.
| IKENA ONCOLOGY, INC. | ||
| By: | /s/ Jotin Marango |
|
| Name: | Jotin Marango | |
| Title: | Chief Financial Officer, Chief Operating Officer and Head of Corporate Development |
|
| COMPUTERSHARE TRUST COMPANY, N.A. and COMPUTERSHARE INC., |
||
| On behalf of both entities | ||
| By: | /s/ Collin Ekeogu |
|
| Name: | Collin Ekeogu | |
| Title: | Senior Manager, Corporate Actions | |
Schedule 1.1
| 1. | Patent Assignment, by and between Insight and Aryl Therapeutics, Inc. (“Aryl”), dated as of November 8, 2024. |
| 2. | Bill of Sale and Assignment and Assumption Agreement, by and between Insight and Aryl, dated as of November 8, 2024. |
| 3. | Exclusive License Agreement, by and between Insight and Foundery Immune Studio, LLC, dated as of December 3, 2024. |
Exhibit 10.3
COMPANY CONTINGENT VALUE RIGHTS AGREEMENT
THIS CONTINGENT VALUE RIGHTS AGREEMENT (this “Agreement”), dated as of July 25, 2025, is entered into by and among Ikena Oncology, Inc., a Delaware corporation (“Insight”), Inmagene Biopharmaceuticals, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands (the “Company”), and Computershare Inc., a Delaware corporation (“Computershare”), and its affiliate, Computershare Trust Company, N.A., a federally chartered trust company (collectively, as “Rights Agent”).
RECITALS
A. Insight, Insight Merger Sub I, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands and direct wholly owned subsidiary of Insight, Insight Merger Sub II, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands and direct wholly owned subsidiary of Insight, and the Company have entered into an Agreement and Plan of Merger, dated as of December 23, 2024 (the “Merger Agreement”).
B. Pursuant to the Merger Agreement, and in accordance with the terms and conditions thereof, Insight has agreed to provide to the Holders (as defined herein) contingent value rights as hereinafter described.
C. The parties have done all things reasonably necessary to make the contingent value rights, when issued pursuant to the Merger Agreement and hereunder, the valid obligations of Insight and to make this Agreement a valid and binding agreement of Insight, in accordance with its terms.
NOW, THEREFORE, in consideration of the premises and the consummation of the transactions referred to above, it is mutually covenanted and agreed, for the proportionate benefit of all Holders, as follows:
ARTICLE 1
DEFINITIONS
Section 1.1 Definitions.
Capitalized terms used but not otherwise defined herein have the meanings ascribed thereto in the Merger Agreement. The following terms have the meanings ascribed to them as follows:
“Acting Holders” means, at the time of determination, the Holders of at least 25% of the outstanding CVRs, as reflected on the CVR Register.
“Assignee” has the meaning set forth in Section 7.5.
“Calendar Quarter” means the successive periods of three (3) consecutive calendar months ending on March 31, June 30, September 30 or December 31, for so long as this Agreement is in effect; provided, however, that (a) the first Calendar Quarter shall commence on the date of this Agreement and shall end on the first September 30 thereafter, and (b) the last Calendar Quarter shall commence on the first day after the full Calendar Quarter immediately preceding the effective date of the termination or expiration of this Agreement and shall end on the effective date of the termination or expiration of this Agreement.
“Change of Control” means (a) the acquisition in one transaction or a series of related transactions, by any Person or group (within the meaning of Section 13(d)(3) of the Securities Exchange Act of 1934) of the securities of Insight possessing more than 50% of the total combined voting power of all outstanding securities of Insight (provided, however, that a Change of Control will not result upon such acquisition of ownership if such acquisition occurs as a result of: (i) a public offering of Insight’s securities or any
financing transaction or series of financing transactions, in each case for bona fide financing purposes or (ii) a merger or consolidation involving Insight where the holders of the outstanding voting securities of Insight immediately prior to such merger or consolidation (taken in the aggregate) possess beneficial ownership of 50% or more of the total combined voting power of all outstanding voting securities of Insight, the surviving entity, the acquiring entity or a parent or holding company of the acquiring entity, immediately after such merger or consolidation); or (b) the sale, transfer, exclusive license or other disposition (in one transaction or a series of related transactions) of all or substantially all of the assets of Insight (on a consolidated basis), except for a transaction in which the holders of the outstanding voting securities of Insight immediately prior to such transaction(s) (taken in the aggregate) receive as a distribution with respect to securities of Insight more than 50% of the total combined voting power of all outstanding voting securities of the acquiring entity or a parent or holding company of the acquiring entity immediately after such transaction(s).
“Company CVR Assets” means the programs and projects controlled by the Company or any of its Affiliates any time prior to the date of this Agreement (other than IMG-007), as may be further developed by or on behalf of Insight after the date of this Agreement.
“Company Shares” means the Company Ordinary Shares, the Company Series Seed Preferred Shares, the Company Series A Preferred Shares, the Company Series B Preferred Shares, the Company Series C-1 Preferred Shares and the Company Series C-2 Preferred Shares, in each case as defined in the Merger Agreement.
“CVR” means a contingent contractual right of Holders to receive CVR Payments pursuant to the Merger Agreement and this Agreement.
“CVR Payment” means a payment equal to (i) ninety percent (90%) of the Net Proceeds received by Insight in a given CVR Payment Period to the extent such payment relates to a Disposition Agreement entered into after the Closing Date, if any, with the remaining ten percent (10%) being retained by Insight, or (ii) one hundred percent (100%) of the Net Proceeds received by Insight to the extent such payment relates to a Disposition Agreement entered into prior to the Closing Date; provided, that with respect to clauses (i) and (ii), Insight, in its reasonable discretion as resolved by the Insight Board, may withhold from any CVR Payment to provide for the satisfaction of indemnity obligations or other potential post-disposition Liabilities under any Disposition Agreement in excess of any escrow fund established therein, in each case to the extent not already deducted as Permitted Deductions; provided, further, that any such withheld Net Proceeds shall be distributed (net of any Permitted Deductions satisfied therefrom) to the Holders no later than the CVR Payment Period in which occurs the extinction of any such indemnity obligations or other post-disposition Liabilities.
“CVR Payment Amount” means with respect to each CVR Payment and each Holder, an amount equal to such CVR Payment divided by the total number of CVRs and then multiplied by the total number of CVRs held by such Holder as reflected on the CVR Register.
“CVR Payment Period” means a period equal to two (2) consecutive Calendar Quarters (or portion thereof) beginning on the First Effective Time of the Merger and ending on March 31 or September 30 of any given calendar year during the CVR Term (provided, that if the last CVR Payment Period would end subsequent to the expiration of the CVR Term, such CVR Payment Period will end on the Termination Date).
“CVR Payment Statement” means, for a given CVR Payment Period during the CVR Term, a written statement of Insight, signed on behalf of Insight, setting forth in reasonable detail the calculation of the applicable CVR Payment for such CVR Payment Period.
“CVR Register” has the meaning set forth in Section 2.3(b).
“CVR Term” means the period beginning on the Closing and ending upon the tenth (10th) anniversary of this Agreement; provided that, solely with respect to any Disposition Agreement set forth on Schedule 1.1, the CVR Term shall extend until the latest date that Insight may earn a contingent, earnout, milestone or similar payment pursuant to such Disposition Agreement if (i) the first patient has been dosed in a registrational trial of an asset covered by such Disposition Agreement before the second (2nd) anniversary of this Agreement or (ii) FDA approval of an NDA for an asset covered by such Disposition Agreement has been received by Insight before the fifth (5th) anniversary of this Agreement.
“Disposition” means the sale, exclusive license, transfer or other disposition of all or any portion of a Company CVR Asset (including any such sale or disposition of equity securities in any Subsidiary established to hold any right, title or interest in or to any Company CVR Asset), in each case during the Disposition Period.
“Disposition Agreement” means a definitive written agreement providing for a transaction or series of transactions between the Company or Insight or their respective Affiliates, as applicable, and any Person who is not an Affiliate of the Company or Insight, as applicable, regarding a Disposition.
“Disposition Period” means the period beginning on the execution date of the Merger Agreement and ending on the first anniversary of the Closing Date.
“Gross Proceeds” means, without duplication, the sum of all cash consideration and all equity consideration of any kind that is paid to, or received by, Insight or any of its Affiliates (including, after the Closing Date, the Company), during the CVR Term with respect to all Dispositions, solely as such consideration relates to each and every Company CVR Asset. The value of any equity securities constituting Gross Proceeds shall be determined as follows: (i) if a value was ascribed to any such securities in connection with such Disposition, such value so ascribed or (ii) if no value was ascribed, then the value of securities that have no established public market, shall be the fair market value, as determined by the Board of Directors of Insight, thereof as of the date of payment to, or receipt by, Insight or its relevant Affiliate.
“Holder” means, at the relevant time, a Person in whose name CVRs are registered in the CVR Register.
“Liability” means any liability, indebtedness, obligation, expense, claim, deficiency, guaranty or endorsement of any kind, whether accrued, absolute, contingent, matured, unmatured or otherwise.
“Loss” has the meaning set forth in Section 3.2(g).
“Net Proceeds” means, for any CVR Payment Period, Gross Proceeds minus Permitted Deductions, all as calculated, to the extent in accordance with GAAP, in a manner consistent with Insight’s accounting practices and the most recently filed annual audited financial statements with the SEC, except as otherwise set forth herein. For clarity, to the extent Permitted Deductions exceed Gross Proceeds for any CVR Payment Period, any excess Permitted Deductions shall be applied against Gross Proceeds in subsequent CVR Payment Periods.
“Notice” has the meaning set forth in Section 7.1.
“Officer’s Certificate” means a certificate signed by the chief executive officer or the chief financial officer of Insight, in their respective official capacities.
“Party” means Insight, the Company or the Rights Agent.
“Permitted Deductions” means the sum of:
(a) any applicable Tax (including any applicable value added or sales taxes) imposed on Gross Proceeds and payable by Insight or any of its Affiliates (regardless of whether the due date for such Taxes arises during or after the Disposition Period) and, without duplication, any income or other similar Taxes payable by Insight or any of its Affiliates that would not have been incurred by Insight or any of its Affiliates but for the Gross Proceeds; provided that, for purposes of calculating income Taxes incurred by Insight or its Affiliates in respect of the Gross Proceeds, any such income Taxes shall be computed based on the gain recognized by Insight or its Affiliates from the Disposition after reduction for any net operating loss carryforwards or other Tax attributes of Insight or its Affiliates as of the Closing Date that are available to offset such gain after taking into account any limits of the usability of such attributes, including under Section 382 of the Code as determined by Insight’s tax advisers (and for the sake of clarity such income taxes shall be calculated without taking into account any net operating losses or other tax attributes generated by Insight or its Affiliates after the Closing Date);
(b) any reasonable and documented expenses incurred by Insight or any of its Affiliates to develop or otherwise ready the Company CVR Assets for sale or in respect of its performance of this Agreement following the Closing Date or in respect of its performance of any Contract in connection with any Company CVR Asset, including any damages or liabilities arising under any Contract or Disposition Agreement, including any incurred in litigation or other dispute resolution therefor, and any costs related to the prosecution, maintenance or enforcement by Insight or any of its Subsidiaries of intellectual property rights (but excluding any costs related to a breach of this Agreement, including costs incurred in litigation or dispute resolution in respect of the same);
(c) any reasonable and documented expenses incurred or accrued by Insight or any of its Affiliates in connection with the negotiation, entry into and closing of any Disposition of any Company CVR Asset, including any brokerage fee, finder’s fee, opinion fee, success fee, transaction fee, service fee or other fee, commission or expense owed to any broker, finder, investment bank, auditor, accountant, counsel, advisor or other third party in relation thereto;
(d) any Losses incurred or reasonably expected to be incurred by Insight or any of its Affiliates arising out of any third-party claims, demands, actions, or other proceedings relating to or in connection with any Disposition, including indemnification obligations of Insight or any of its Affiliates set forth in any Disposition Agreement;
(e) any Liabilities borne by Insight or any of its Affiliates pursuant to Contracts related to Company CVR Assets, including costs arising from the termination thereof; and
(f) any amounts payable to the Rights Agent in connection with the distribution of any CVR Payment Amount.
“Permitted Transfer” means a transfer of CVRs (a) upon death of a Holder by will or intestacy; (b) pursuant to a court order; (c) by operation of law (including by consolidation or merger) or without consideration in connection with the dissolution, liquidation or termination of any corporation, limited liability company, partnership or other entity; (d) in the case of CVRs held in book-entry or other similar nominee form, from a nominee to a beneficial owner and, if applicable, through an intermediary, to the extent allowable by DTC; or (e) as provided in Section 2.6.
“Rights Agent” means the Rights Agent named in the first paragraph of this Agreement, until a successor Rights Agent will have become the Rights Agent pursuant to the applicable provisions of this Agreement, and thereafter “Rights Agent” will mean such successor Rights Agent.
ARTICLE 2
CONTINGENT VALUE RIGHTS
Section 2.1 Holders of CVRs; Appointment of Rights Agent.
(a) The CVRs represent the rights of Holders to receive contingent CVR Payments pursuant to this Agreement. The initial Holders will be the holders of Company Shares as of immediately prior to the First Effective Time. One CVR will be issued with respect to each Company Share that is outstanding as of immediately prior to the First Effective Time.
(b) Insight hereby appoints the Rights Agent to act as Rights Agent in accordance with the express terms and conditions set forth in this Agreement (and no implied terms and conditions), and the Rights Agent hereby accepts such appointment.
Section 2.2 Non-transferable.
The CVRs may not be sold, assigned, transferred, pledged, encumbered or in any other manner transferred or disposed of, in whole or in part, other than through a Permitted Transfer. Any attempted sale, assignment, transfer, pledge, encumbrance or disposal that is not a Permitted Transfer shall be void ab initio and of no effect. The CVRs will not be listed on any quotation system or traded on any securities exchange.
Section 2.3 No Certificate; Registration; Registration of Transfer; Change of Address.
(a) The CVRs will be issued in book-entry form only and will not be evidenced by a certificate or other instrument. Holders’ rights and obligations in respect of CVRs derive solely from this Agreement.
(b) Subject to the receipt by the Rights Agent of the information and instructions described in Section 4.1, the Rights Agent shall create and maintain a register (the “CVR Register”) for the purpose of registering CVRs and Permitted Transfers. The CVR Register will be created, and CVRs will be distributed, pursuant to written instructions to the Rights Agent from Insight. The CVR Register will initially show one position for Cede & Co. representing all of the Company Shares held by DTC on behalf of the street holders of Company Shares held by such Holders as of immediately prior to the First Effective Time. The Rights Agent will have no responsibility whatsoever directly or indirectly to the street name holders with respect to transfers of CVRs. With respect to any payments or issuances to be made under Section 2.4 below, the Rights Agent will accomplish the payment to any former street name holders of Company Shares by sending one lump-sum payment or issuance to DTC. The Rights Agent will have no responsibilities whatsoever with regard to the distribution of payments by DTC to such street name holders.
(c) Subject to the restrictions on transferability set forth in Section 2.2, every request made to transfer a CVR must be in writing and accompanied by a written instrument of transfer in form reasonably satisfactory to the Rights Agent pursuant to its guidelines or procedures, including a guaranty of signature by an “eligible guarantor institution” that is a member or participant in the Securities Transfer Agents Medallion Program, duly executed and properly completed by the Holder thereof, the Holder’s attorney duly authorized in writing, the Holder’s personal representative or the Holder’s survivor, and setting forth in reasonable detail the circumstances relating to the transfer. Upon receipt of such written notice, the Rights Agent shall, subject to its reasonable determination that the transfer instrument is in proper form and the transfer otherwise complies with the other terms and conditions of this Agreement (including the provisions of Section 2.2), register the transfer of the CVRs in the CVR Register. None of Insight, the Company or Rights Agent shall be responsible for, and each such party may require evidence of payment of a sum sufficient to cover (or evidence that such Taxes and charges are not applicable), any stamp, documentary, registration, or other Tax or governmental charge that is imposed in connection with any such registration of transfer. The Rights Agent shall have no duty or obligation to take any action under any section of this Agreement that requires the payment by a Holder of a CVR of applicable taxes or charges unless and until the Rights Agent is satisfied that all such taxes or charges have been paid. All duly transferred CVRs registered in the CVR Register will be the valid obligations of Insight and the Company and will entitle the transferee to the same benefits and rights under this Agreement as those held immediately prior to the transfer by the transferor. No transfer of a CVR will be valid until registered in the CVR Register and any transfer not duly registered in the CVR Register shall be void.
(d) A Holder may make a written request to the Rights Agent to change such Holder’s address of record in the CVR Register. The written request must be duly executed by the Holder. Upon receipt of such written notice, the Rights Agent shall, subject to its reasonable determination that the transfer instrument is in proper form, promptly record the change of address in the CVR Register. The Acting Holders may, without duplication, make a written request to the Rights Agent for a list containing the names, addresses and number of CVRs of the Holders that are registered in the CVR Register. Upon receipt of such written request from the Acting Holders, the Rights Agent shall promptly deliver a copy of such list to the Acting Holders.
(e) Insight will, or will cause the Company to, provide written instructions to the Rights Agent for the distribution of CVRs to holders of Company Shares as of immediately prior to the First Effective Time (the “Record Time”). Subject to the terms and conditions of this Agreement and Insight’s prompt confirmation of the First Effective Time, the Rights Agent shall effect the distribution of the CVRs, less any applicable tax withholding, to each holder of Company Shares as of the Record Time by the mailing of a statement of holding reflecting such CVRs.
Section 2.4 Payment Procedures.
(a) No later than forty-five (45) days following the end of each CVR Payment Period during the CVR Term, Insight shall deliver to the Rights Agent a CVR Payment Statement for such CVR Payment Period. Concurrent with the delivery of each CVR Payment Statement, on the terms and conditions of this Agreement, Insight shall pay the Rights Agent in U.S. dollars an amount equal to one-hundred percent (100%) of the Net Proceeds (if any) (subject to the proviso in the definition of the term “CVR Payment”) for the applicable CVR Payment Period; provided, however, that in the event that the aggregate CVR Payment on any CVR Payment Statement shall be less than $1,000,000, no CVR Payment Amount shall be due and instead such CVR Payment shall be added to subsequent CVR Payments until: (i) the aggregate CVR Payment shall be at least $1,000,000 or (ii) final CVR Payment Period. Such amount of Net Proceeds will be transferred by wire transfer of immediately available funds to an account designated in writing by the Rights Agent not less than ten (10) Business Days prior to the date of the applicable payment. Upon receipt of the wire transfer referred to in the foregoing sentence, the Rights Agent shall promptly (and in any event, within ten (10) Business Days) pay, by check mailed, first-class postage prepaid, to the address each Holder set forth in the CVR Register at such time or by other method of deliver as specified by the applicable Holder in writing to the Rights Agent, an amount equal to such Holder’s CVR Payment Amount. The Rights Agent shall, upon any Holder’s request in writing and as soon as practicable after receipt of a CVR Payment Statement under this Section 2.4(a), send such Holder at its registered address a copy of such statement. For the avoidance of doubt neither Insight nor the Company shall have any further liability in respect of the relevant CVR Payment upon delivery of such CVR Payment in accordance with this Section 2.4(a) and the satisfaction of each of Insight’s obligations set forth in this Section 2.4(a).
(b) The Rights Agent shall solicit from each Holder an IRS Form W-9 or applicable IRS Form W-8 at such time or times as is necessary to permit any payment under this Agreement to be made without U.S. federal backup withholding. That notwithstanding, Insight shall be entitled to deduct and withhold and hereby authorizes the Rights Agent to deduct and withhold, any tax or similar governmental charge or levy, that is required to be deducted or withheld under applicable law from any amounts payable pursuant to this Agreement (“Withholding Taxes”). To the extent the amounts are so withheld by Insight or the Rights Agent, as the case may be, such withheld amounts shall be treated for all purposes of this Agreement as having been paid to the person in respect of whom such deduction and withholding was made. In the event Insight becomes aware that a payment under this Agreement is subject to Withholding Taxes (other than U.S. federal backup withholding), Insight shall use commercially reasonable efforts to provide written notice to the Rights Agent and the Rights Agent shall use commercially reasonable efforts to provide written notice of such Withholding Taxes to the applicable Holders and a reasonable opportunity for the Holder to provide any necessary Tax forms, including an IRS Form W-9 or appropriate IRS Form W-8, as applicable, in order to reduce such withholding amounts; provided that the time period for payment of a CVR Payment by the Rights Agent set forth in Section 2.4(a) will be extended by a period equal to any delay caused by the Holder providing such forms. For the avoidance of doubt, in the event that notice has been provided to an applicable Holder pursuant to this Section 2.4(b), no further notice shall be required to be given for any future payments of such Withholding Tax. Insight will use commercially reasonable efforts to provide withholding and reporting instructions in writing (email being sufficient) to the Rights Agent from time to time as relevant, and upon reasonable request of the Rights Agent. The Rights Agent shall have no responsibilities with respect to tax withholding, reporting or payment except specifically instructed by Insight.
(c) Any portion of a CVR Payment that remains undistributed to the Holders six (6) months after the end of an applicable CVR Payment Period (including by means of uncashed checks or invalid addresses on the CVR Register) will be delivered by the Rights Agent to Insight or a person nominated in writing by Insight (with written notice thereof from Insight to the Rights Agent), and any Holder will thereafter look only to Insight for payment of such CVR Payment (which shall be without interest).
(d) If any CVR Payment (or portion thereof) remains unclaimed by a Holder two (2) years after the end of an applicable CVR Payment Period (or immediately prior to such earlier date on which such CVR Payment would otherwise escheat to or become the property of any Governmental Authority), such CVR Payment (or portion thereof) will, to the extent permitted by applicable Law, become the property of Insight and will be transferred to Insight or a person nominated in writing by Insight (with written notice thereof from Insight to the Rights Agent), free and clear of all claims or interest of any Person previously entitled thereto, and no consideration or compensation shall be payable therefor. Neither Insight nor the Rights Agent will be liable to any Person in respect of a CVR Payment delivered to a public official pursuant to any applicable abandoned property, escheat or similar legal requirement under applicable Law. In addition to and not in limitation of any other indemnity obligation herein, Insight agrees to indemnify and hold harmless the Rights Agent with respect to any liability, penalty, cost or expense the Rights Agent may incur or be subject to in connection with transferring such property to Insight, a public office or a person nominated in writing by Insight.
Section 2.5 No Voting, Dividends or Interest; No Equity or Ownership Interest.
(a) The CVRs will not have any voting or dividend rights, and interest will not accrue on any amounts payable in respect of CVRs to any Holder.
(b) The CVRs will not represent any equity or ownership interest in Insight, the Company or in any constituent company to the Merger. The sole right of the Holders to receive property hereunder is the right to receive CVR Payments, if any, in accordance with the terms hereof. It is hereby acknowledged and agreed that a CVR shall not constitute a security of Insight.
(c) Nothing contained in this Agreement shall be construed as conferring upon any Holder, by virtue of the CVRs, any rights or obligations of any kind or nature whatsoever as a stockholder or member of Insight or any of its subsidiaries either at law or in equity. The rights of any Holder and the obligations of Insight, the Company and their respective Affiliates and their respective officers, directors and controlling Persons are contract rights limited to those expressly set forth in this Agreement.
(d) It is hereby acknowledged and agreed that the CVRs and the possibility of any payment hereunder with respect thereto are highly speculative and subject to numerous factors outside of Insight’s control, and there is no assurance that Holders will receive any payments under this Agreement or in connection with the CVRs. Each Holder acknowledges that it is highly possible that no Disposition will occur prior to the expiration of the Disposition Period and that there will not be any Gross Proceeds that may be the subject of a CVR Payment Amount. It is further acknowledged and agreed that neither Insight nor the Company nor their respective Affiliates owe, by virtue of their obligations under this Agreement, a fiduciary duty or any implied duties to the Holders and the parties hereto intend solely the express provisions of this Agreement to govern their contractual relationship with respect to the CVRs. It is acknowledged and agreed that this Section 2.5(d) is an essential and material term of this Agreement.
Section 2.6 Ability to Abandon CVR.
A Holder may at any time, at such Holder’s option, abandon all of such Holder’s remaining rights represented by CVRs by transferring such CVR to Insight or a Person nominated in writing by Insight (with written notice thereof from Insight to the Rights Agent) without consideration in compensation therefor, and such rights will be cancelled, with the Rights Agent being promptly notified in writing by Insight of such transfer and cancellation. Nothing in this Agreement is intended to prohibit Insight or its Affiliates from offering to acquire or acquiring CVRs, in private transactions or otherwise, for consideration in its sole discretion.
ARTICLE 3
THE RIGHTS AGENT
Section 3.1 Certain Duties and Responsibilities.
(a) The Rights Agent will not have any liability for any actions taken or not taken in connection with this Agreement, except to the extent such liability arises as a result of the willful misconduct, bad faith, fraud or gross negligence of the Rights Agent (in each case as determined by a final non-appealable judgment of court of competent jurisdiction). Notwithstanding anything in this Agreement to the contrary, any liability of the Rights Agent under this Agreement will be limited to the amount of annual fees paid by Insight to the Rights Agent (but not including reimbursable expenses and other charges) during the twelve (12) months immediately preceding the event for which recovery from the Rights Agent is being sought. Anything to the contrary notwithstanding, in no event will the Rights Agent be liable for special, punitive, indirect, incidental or consequential loss or damages of any kind whatsoever (including, without limitation, lost profits), even if the Rights Agent has been advised of the likelihood of such loss or damages, and regardless of the form of action.
(b) The Rights Agent shall not have any duty or responsibility in the case of the receipt of any written demand from any Holder with respect to any action or default by any person or entity, including, without limiting the generality of the foregoing, any duty or responsibility to initiate or attempt to initiate any proceedings at law or otherwise or to make any demand upon Insight or the Company. The Rights Agent may (but shall not be required to) enforce all rights of action under this Agreement and any related claim, action, suit, audit, investigation or proceeding instituted by the Rights Agent may be brought in its name as the Rights Agent and any recovery in connection therewith will be for the proportionate benefit of all the Holders, as their respective rights or interests may appear on the CVR Register.
Section 3.2 Certain Rights of Rights Agent.
(a) The Rights Agent undertakes to perform such duties and only such duties as are specifically set forth in this Agreement, and no implied covenants or obligations will be read into this Agreement against the Rights Agent.
(b) The Rights Agent may rely and will be protected by Insight in acting or refraining from acting upon any resolution, certificate, statement, instrument, opinion, report, notice, request, direction, consent, order or other paper or document reasonably believed by it to be genuine and to have been signed or presented by or on behalf of Insight or, with respect to Section 2.3(d), the Acting Holders.
(c) Whenever the Rights Agent deems it desirable that a matter be proved or established prior to taking, suffering or omitting any action hereunder, the Rights Agent may rely upon an Officer’s Certificate, which certificate shall be full authorization and protection to the Rights Agent, and the Rights Agent shall, in the absence of bad faith, fraud, gross negligence or willful misconduct (each as determined by a final non-appealable judgment of a court of competent jurisdiction) on its part, not incur any liability and shall be held harmless by Insight for or in respect of any action taken, suffered or omitted to be taken by it under the provisions of this Agreement in reliance upon such Officer’s Certificate.
(d) The Rights Agent may engage and consult with counsel of its selection, and the advice or opinion of such counsel will, in the absence of bad faith, fraud, gross negligence or willful misconduct (in each case, as determined by a final, non-appealable judgment of a court of competent jurisdiction) on the part of the Rights Agent, be full and complete authorization and protection in respect of any action taken, suffered or not taken by the Rights Agent in reliance thereon.
(e) Any permissive rights of the Rights Agent hereunder will not be construed as a duty.
(f) The Rights Agent will not be required to give any note or surety in respect of the execution of its powers or otherwise under this Agreement.
(g) Insight agrees to indemnify the Rights Agent for, and to hold the Rights Agent harmless from and against, any loss, liability, damage, judgment, fine, penalty, claim, demand, settlement, cost or expense (including the reasonable and documented fees and expenses of legal counsel) (each, a “Loss”) which may be paid, incurred or suffered by or to which it may become subject, arising from or out of, directly or indirectly, any claims or liability resulting from any action taken, suffered or omitted by the Rights Agent in connection the execution, acceptance, administration, exercise and performance of its duties under this Agreement, including the costs and expenses of defending against any claim of liability arising therefrom, directly or indirectly, or enforcing its rights hereunder, except to the extent such Loss has been determined by a final non-appealable decision of a court of competent jurisdiction to have resulted from the Rights Agent’s willful misconduct, bad faith, fraud or gross negligence; provided that this Section 3.2(g) shall not apply with respect to income, receipt, franchise or similar Taxes levied against the Rights Agent by a Governmental Authority.
(h) Insight agrees (i) to pay the fees of the Rights Agent in connection with the Rights Agent’s performance of its obligations hereunder as set forth in a fee schedule agreed upon in writing by the Rights Agent and Insight on or prior to the date of this Agreement (the “Fee Schedule”), and (ii) to reimburse the Rights Agent for all reasonable and documented out-of-pocket expenses and other disbursements incurred in the preparation, delivery, negotiation, amendment, administration and execution of this Agreement and the exercise and performance of its duties hereunder, including all stamp and transfer Taxes (and excluding for the avoidance of doubt, any income, receipt, franchise or similar Taxes levied against the Rights Agent by a Governmental Authority) and governmental charges, incurred by the Rights Agent in the performance of its obligations under this Agreement.
(i) No provision of this Agreement shall require the Rights Agent to expend or risk its own funds or otherwise incur any financial liability in the performance of any of its duties hereunder or in the exercise of any of its rights or powers if it believes that repayment of such funds or adequate indemnification against such risk or liability is not reasonably assured to it.
(j) The Rights Agent shall have no responsibility to Insight, the Company, any holders of CVRs, any holders of Company Shares or any other Person for interest or earnings on any moneys held by the Rights Agent pursuant to this Agreement.
(k) The Rights Agent shall not be subject to, nor be required to comply with, or determine if any Person has complied with, the Merger Agreement or any other agreement between or among any Insight, the Company or Holders, even though reference thereto may be made in this Agreement, or to comply with any notice, instruction, direction, request or other communication, paper or document other than as expressly set forth in this Agreement.
(l) Subject to applicable Law, (i) the Rights Agent and any shareholder, affiliate, director, officer or employee of the Rights Agent may buy, sell or deal in any securities of Insight or the Company or become peculiarly interested in any transaction in which such parties may be interested, or contract with or lend money to such parties or otherwise act as fully and freely as though it were not the Rights Agent under this Agreement, and (ii) nothing herein will preclude the Rights Agent from acting in any other capacity for Insight or for any other Person.
(m) In the event the Rights Agent reasonably believes any ambiguity or uncertainty exists hereunder or in any notice, instruction, direction, request or other communication, paper or document received by the Rights Agent hereunder, the Rights Agent shall, as soon as practicable, provide notice to Insight, and the Rights Agent, may, in its sole discretion, refrain from taking any action, and shall be fully protected and shall not be liable in any way to Insight, the Company or any Holder or any other Person for refraining from taking such action, unless the Rights Agent receives written instructions from Insight or such Holder or other Person which eliminate such ambiguity or uncertainty to the reasonable satisfaction of the Rights Agent.
(n) The Rights Agent may execute and exercise any of the rights or powers hereby vested in it or perform any duty hereunder either itself or by or through its attorney or agents and the Rights Agent shall not be answerable or accountable for any act, default, neglect or misconduct of any such attorney or agents or for any loss to Insight or the Company resulting from any such act, default, neglect or misconduct, absent willful misconduct, bad faith, fraud or gross negligence (each as determined by a final non- appealable judgment of a court of competent jurisdiction) in the selection and continued employment thereof.
(o) The Rights Agent shall not be liable for or by reason of any of the statements of fact or recitals contained in this Agreement (except its countersignature thereof) or be required to verify the same, and all such statements and recitals are and shall be deemed to have been made by Insight only.
(p) The Rights Agent shall act hereunder solely as agent for Insight and shall not assume any obligations or relationship of agency or trust with any of the owners or holders of the CVRs. The Rights Agent shall not have any duty or responsibility in the case of the receipt of any written demand from any Holders with respect to any action or default by Insight or the Company, including, without limiting the generality of the foregoing, any duty or responsibility to initiate or attempt to initiate any proceedings at law or otherwise or to make any demand upon Insight or the Company.
(q) The Rights Agent may rely on and be fully authorized and protected in acting or failing to act upon (a) any guaranty of signature by an “eligible guarantor institution” that is a member or participant in the Securities Transfer Agents Medallion Program or other comparable “signature guarantee program” or insurance program in addition to, or in substitution for, the foregoing; or (b) any law, act, regulation or any interpretation of the same even though such law, act, or regulation may thereafter have been altered, changed, amended or repealed.
(r) The Rights Agent shall not be liable or responsible for any failure of Insight or the Company to comply with any of its obligations relating to any registration statement filed with the Securities and Exchange Commission or this Agreement, including without limitation obligations under applicable regulation or law.
(s) The obligations of Insight and the Company and the rights of the Rights Agent under this Section 3.2, Section 3.1. and Section 2.4 shall survive the expiration of the CVRs and the termination of this Agreement and the resignation, replacement or removal of the Rights Agent.
(t) The Rights Agent will not be deemed to have knowledge of any event of which it was supposed to receive notice hereunder but has not received written notice of such event, and the Rights Agent will not incur any liability for failing to take action in connection therewith, in each case, unless and until it has received such notice in writing.
(u) The Rights Agent will have no liability and shall be held harmless by Insight in respect of the validity of this Agreement and the execution and delivery hereof (except the due execution and delivery hereof by the Rights Agent and the enforceability of this Agreement against the Rights Agent assuming the due execution and delivery hereof by Insight and the Company), nor shall it be responsible for any breach by Insight or the Company of any covenant or condition contained in this Agreement.
Section 3.3 Resignation and Removal; Appointment of Successor.
(a) The Rights Agent may resign at any time by written notice to Insight. Any such resignation notice shall specify the date on which such resignation will take effect (which shall be at least thirty (30) days following the date that such resignation notice is delivered), and such resignation will be effective on the earlier of (x) the date so specified and (y) the appointment of a successor Rights Agent.
(b) Insight will have the right to remove the Rights Agent at any time by written notice to the Rights Agent, specifying the date on which such removal will take effect. Such notice will be given at least thirty (30) days prior to the date so specified (or, if earlier, the appointment of the successor Rights Agent).
(c) If the Rights Agent resigns, is removed or becomes incapable of acting, Insight will promptly appoint a qualified successor Rights Agent. Notwithstanding the foregoing, if Insight fails to make such appointment within a period of thirty (30) days after giving notice of such removal or after it has been notified in writing of such resignation or incapacity by the resigning or incapacitated Rights Agent, then the any Holder may apply to any court of competent jurisdiction for the appointment of a new Rights Agent. The successor Rights Agent so appointed will, upon its acceptance of such appointment in accordance with this Section 3.3(c) and Section 3.4, become the Rights Agent for all purposes hereunder.
(d) Insight will give notice to the Holders of each resignation or removal of the Rights Agent and each appointment of a successor Rights Agent in accordance with Section 7.2. Each notice will include the name and address of the successor Rights Agent. If Insight fails to send such notice within ten (10) Business Days after acceptance of appointment by a successor Rights Agent, the successor Rights Agent will cause the notice to be mailed at the expense of Insight.
(e) Notwithstanding anything to the contrary in this Section 3.3, unless consented to in writing by the Acting Holders, Insight will not appoint as a successor Rights Agent any Person that is not a stock transfer agent of national reputation or the corporate trust department of a commercial bank.
(f) The Rights Agent will reasonably cooperate with Insight and any successor Rights Agent in connection with the transition of the duties and responsibilities of the Rights Agent to the successor Rights Agent, including the transfer of all relevant data, including the CVR Register, to the successor Rights Agent, but such predecessor Rights Agent shall not be required to make any additional expenditure or assume any additional liability in connection with the foregoing.
Section 3.4 Acceptance of Appointment by Successor.
Every successor Rights Agent appointed hereunder will, at or prior to such appointment, execute, acknowledge and deliver to Insight and to the resigning or removed Rights Agent an instrument accepting such appointment and a counterpart of this Agreement, and such successor Rights Agent, without any further act, deed or conveyance, will become vested with all the rights, powers, trusts and duties of the Rights Agent; provided that upon the request of Insight or the successor Rights Agent, such resigning or removed Rights Agent will execute and deliver an instrument transferring to such successor Rights Agent all the rights, powers and trusts of such resigning or removed Rights Agent, except such rights which survive its resignation or removal under the terms hereunder.
ARTICLE 4
COVENANTS
Section 4.1 List of Holders.
Insight will furnish or cause to be furnished to the Rights Agent, in such form as Insight receives from Insight’s transfer agent (or other agent performing similar services for Insight), the names and addresses of the Holders within fifteen (15) Business Days following the Closing Date.
Section 4.2 Obligations of Public Company.
(a) Notwithstanding anything herein to the contrary, (a) Insight and its Affiliates (including, after the Closing Date, the Company) shall have the power and right to control all aspects of their businesses and operations (and all of their assets and products, including, subject to Section 4.2(b), with respect to (i) managing, directing and controlling the exploitation of the Company CVR Assets in all respects and (ii) conducting any sale process (including engagement of advisors) with respect to any Company CVR Assets), and subject to Insight’s and the Company’s compliance with the terms of this Agreement, Insight and its Affiliates may exercise or refrain from exercising such power and right as it may deem appropriate and in the best overall interests of Insight and its Affiliates and its and their stockholders, rather than the interest of the Holders, (b) none of Insight or any of its Affiliates (including, after the Closing Date, the Company) (or any directors, officer, employee, or other representative of the foregoing) owes any fiduciary duty or similar duty or any other implied duties (including the implied covenant of good faith and fair dealing) to any Holder in respect of the CVRs or the Company CVR Assets, (c) except as specifically provided in Section 4.2(b), Insight shall have no obligation to operate, use, sell, transfer, convey, license, develop, commercialize or otherwise exploit in any particular manner any of its or its Affiliates’ business or operations (or any of their assets or products) or to negotiate or enter into any agreement, including any Disposition Agreement, including in order to obtain, maximize or expedite the receipt of any Gross Proceeds or minimize Permitted Deductions, and (d) following the Disposition Period, Insight shall be permitted to take any action in respect of the Company CVR Assets in order to satisfy any wind-down and termination Liabilities of the Company CVR Assets.
(b) Insight will allocate reasonable resources to reasonably maintain the Company CVR Assets during the Disposition Period.
(c) None of Insight, the Company or any of their respective Affiliates shall have any obligation or liability whatsoever to any Person relating to or in connection with any action, or failure to act, with respect to the sale of the Company CVR Assets, except for the obligation to make CVR Payment Amounts to the Holders of CVRs pursuant to this Agreement.
(d) Except for the obligations to the Rights Agent set forth in this Agreement, neither Insight nor its Subsidiaries (including, after the Closing Date, the Company) will have any responsibility or liability whatsoever to any Person other than the Holders.
Section 4.3 Prohibited Actions.
Unless approved by the Insight Board, prior to the end of the Disposition Period, neither of Insight nor the Company shall grant any lien, security interest, pledge or similar interest in any Company CVR Assets or any Net Proceeds.
Section 4.4 Books and Records.
Until the end of the CVR Term, Insight shall, and shall cause its Affiliates to, keep true, complete and accurate records in sufficient detail to support the applicable CVR Payments, if any, payable hereunder in accordance with the terms specified in this Agreement.
Section 4.5 Audits.
Until the Termination Date and for a period of one (1) year thereafter, Insight shall keep complete and accurate records in sufficient detail to support the accuracy of the payments due hereunder. The Acting Holders shall have the right to cause an independent accounting firm reasonably acceptable to Insight to audit such records for the sole purpose of confirming payments for a period covering not more than the date commencing with the first CVR Payment Period in which Insight or its Affiliates receives Gross Proceeds and ending on the last day of the CVR Term. Insight may require such accounting firm to execute a reasonable confidentiality agreement with Insight prior to commencing the audit. The accounting firm shall disclose to Rights Agent or the Acting Holders, as applicable, only whether the reports are correct or not and the specific details concerning any discrepancies. No other information shall be shared, and in no event shall Insight be required to provide any Tax returns or any other Tax information it deems confidential to the Acting Holders or any other party. Such audits may be conducted during normal business hours upon reasonable prior written notice to Insight, but no more than frequently than once per year. No accounting period of Insight shall be subject to audit more than one time by the Acting Holders, as applicable. Adjustments (including remittances of underpayments or overpayments disclosed by such audit) shall be made by Insight to reflect the results of such audit, which adjustments shall be paid promptly following receipt of an invoice therefor.
Whenever such an adjustment is made, Insight shall promptly prepare a certificate setting forth such adjustment, and a brief, reasonably detailed statement of the facts, computation and methodology accounting for such adjustment to the extent not already reflected in the audit report and promptly file with the Rights Agent a copy of such report and promptly deliver to the Rights Agent a revised CVR Payment Statement for the relevant CVR Payment Period. The Rights Agent shall be fully protected in relying on any such report and on any adjustment or statement therein contained and shall have no duty or liability with respect to, and shall not be deemed to have knowledge of any such adjustment or any such event unless and until it shall have received such report. The Acting Holders, as applicable, shall bear the full cost and expense of such audit unless such audit discloses an underpayment by Insight of twenty percent (20%) or more of the CVR Payment due under this Agreement, in which case Insight shall bear the full cost and expense of such audit. The Rights Agent shall be entitled to rely on any audit report delivered by the independent accounting firm pursuant to this Section 4.5.
ARTICLE 5
AMENDMENTS
Section 5.1 Amendments Without Consent of Holders or Rights Agent.
(a) Insight and the Company, at any time and from time to time, may (without the consent of any Person, including the Holders, other than each other and the Rights Agent, with such consent not to be unreasonably withheld, conditioned or delayed) enter into one or more amendments to this Agreement for any of the following purposes:
(i) to evidence the appointment of another Person as a successor Rights Agent and the assumption by any successor Rights Agent of the covenants and obligations of the Rights Agent herein in accordance with the provisions hereof;
(ii) subject to Section 6.1, to evidence the succession of another person to Insight and the assumption of any such successor of the covenants of Insight outlined herein in a transaction contemplated by Section 6.1;
(iii) to add to the covenants of Insight such further covenants, restrictions, conditions or provisions as Insight will consider to be for the protection and benefit of the Holders; provided that in each case, such provisions do not adversely affect the interests of the Holders;
(iv) to cure any ambiguity, to correct or supplement any provision in this Agreement that may be defective or inconsistent with any other provision in this Agreement, or to make any other provisions with respect to matters or questions arising under this Agreement; provided that, in each case, such provisions do not adversely affect the interests of the Holders;
(v) as may be necessary or appropriate to ensure that the CVRs are not subject to registration under the Securities Act or the Exchange Act and the rules and regulations promulgated thereunder, or any applicable state securities or “blue sky” laws;
(vi) as may be necessary or appropriate to ensure that Insight is not required to produce a prospectus or an admission document in order to comply with applicable Law;
(vii) to cancel the CVRs (i) in the event that any Holder has abandoned its rights in accordance with Section 2.6, or (ii) following a transfer of such CVRs to Insight or its Affiliates in accordance with Section 2.2 or Section 2.3;
(viii) as may be necessary or appropriate to ensure that Insight complies with applicable Law; or
(ix) to effect any other amendment to this Agreement for the purpose of adding, eliminating or changing any provisions of this Agreement, provided that, in each case, such additions, eliminations or changes do not adversely affect the interests of the Holders.
(b) Promptly after the execution by Insight of any amendment pursuant to this Section 5.1, Insight will (or will cause the Rights Agent to) notify the Holders in general terms of the substance of such amendment in accordance with Section 7.2.
Section 5.2 Amendments with Consent of Holders.
(a) In addition to any amendments to this Agreement that may be made by Insight, the Company and the Rights Agent without the consent of any Holder pursuant to Section 5.1, with the consent of the Acting Holders (whether evidenced in a writing or taken at a meeting of the Holders), the Company, Insight and the Rights Agent may enter into one or more amendments to this Agreement for the purpose of adding, eliminating or amending any provisions of this Agreement, even if such addition, elimination or amendment is adverse to the interests of the Holders.
(b) Promptly after the execution by Insight, the Company and the Rights Agent of any amendment pursuant to the provisions of this Section 5.2, Insight will (or will cause the Rights Agent to) notify the Holders in general terms of the substance of such amendment in accordance with Section 7.2.
Section 5.3 Effect of Amendments.
Upon the execution of any amendment under this Article 5, this Agreement will be modified in accordance therewith, such amendment will form a part of this Agreement for all purposes and every Holder will be bound thereby. Upon the delivery of an Officer’s Certificate which states that the proposed supplement or amendment is in compliance with the terms of this Article 5, the Rights Agent shall execute such supplement or amendment. Notwithstanding anything in this Agreement to the contrary, the Rights Agent shall not be required to execute any supplement or amendment to this Agreement that it has determined would adversely affect its own rights, duties, obligations or immunities under this Agreement. No supplement or amendment to this Agreement shall be effective unless duly executed by the Rights Agent.
ARTICLE 6
CONSOLIDATION, MERGER, SALE OR CONVEYANCE
Section 6.1 Change of Control.
Prior to the Termination Date, Insight shall not consummate a Change of Control without the consent of the Acting Holders (such consent not to be unreasonably withheld, conditioned or delayed), unless:
(a) the Person that is the relevant transferee, assignee, acquiror, delegate or other successor (including by operation of law) in such Change of Control (the “Surviving Person”) shall assume or succeed to the obligations on all CVRs (when and as due hereunder); and
(b) Insight has delivered to the Rights Agent an Officer’s Certificate stating that such Change of Control complies with this Article 6 and that all conditions precedent herein provided for relating to such Change of Control have been complied with.
Section 6.2 Successor Substituted.
Upon the consummation of any Change of Control in accordance with Section 6.1, the Surviving Person shall succeed to, and be substituted for, and may exercise every right and power of, and shall assume all of the obligations of Insight under this Agreement with the same effect as if the Surviving Person had been named as Insight herein.
Section 7.5 Successors and Assigns.
This Agreement will be binding upon, and will be enforceable by and inure solely to the benefit of, the Holders, Insight, the Company and the Rights Agent and their respective successors and assigns. Except for assignments pursuant to Section 7.4, the Rights Agent may not assign this Agreement without Insight’s prior written consent. Subject to Section 5.1(a)(ii) and Article 6 hereof, Insight may assign, in its sole discretion and without the consent of any other party, any or all of its rights, interests and obligations hereunder to one or more of its Affiliates or to any Person with whom Insight is merged or consolidated, or any entity resulting from any merger or consolidation to which Insight shall be a party (each, an “Assignee”); provided, that in connection with any assignment to an Assignee, Insight shall agree to remain liable for the performance by Insight of its obligations hereunder (to the extent Insight exists following such assignment). Insight or an Assignee may not otherwise assign this Agreement without the prior consent of the Acting Holders (such consent not to be unreasonably withheld, conditioned or delayed). Any attempted assignment of this Agreement in violation of this Section 7.5 will be void ab initio and of no effect.
Section 7.6 Benefits of Agreement; Action by Acting Holders.
Nothing in this Agreement, express or implied, will give to any Person (other than Insight, the Company, the Rights Agent, the Holders and their respective permitted successors and assigns hereunder) any benefit or any legal or equitable right, remedy or claim under this Agreement or under any covenant or provision herein contained, all such covenants and provisions being for the sole benefit of Insight, the Company, the Rights Agent, the Holders and their permitted successors and assigns. The Holders will have no rights hereunder except as are expressly set forth herein. Except for the rights of the Rights Agent set forth herein, the Acting Holders will have the sole right, on behalf of all Holders, by virtue of or under any provision of this Agreement, to institute any action or proceeding at law or in equity with respect to this Agreement, and no individual Holder or other group of Holders will be entitled to exercise such rights.
Section 7.7 Governing Law.
This Agreement and the CVRs will be governed by, and construed in accordance with, the laws of the State of Delaware, without giving effect to any provision of law or rule (whether of the State of Delaware or any other jurisdictions) that would cause the application of the laws of any jurisdictions other than the State of Delaware.
Section 7.8 Jurisdiction.
In any action or proceeding between any of the parties hereto arising out of or relating to this Agreement or any of the transactions contemplated hereby, each of the parties hereto: (a) irrevocably and unconditionally consents and submits to the exclusive jurisdiction and venue of the Court of Chancery of the State of Delaware or, to the extent such court does not have subject matter jurisdiction, the Superior Court of the State of Delaware or the United States District Court for the District of Delaware, and appellate courts thereof; (b) agrees that all claims in respect of such action or proceeding shall be heard and determined exclusively in accordance with clause (a) of this Section 7.8; (c) waives any objection to laying venue in any such action or proceeding in such courts; (d) waives any objection that such courts are an inconvenient forum or do not have jurisdiction over any Party; and (e) agrees that service of process upon such Party in any such action or proceeding shall be effective if notice is given in accordance with Section 7.1 or Section 7.2 of this Agreement; provided that nothing contained herein shall be deemed to limit in any way any right to serve process in any manner permitted by Law.
Section 7.5 Successors and Assigns.
This Agreement will be binding upon, and will be enforceable by and inure solely to the benefit of, the Holders, Insight, the Company and the Rights Agent and their respective successors and assigns. Except for assignments pursuant to Section 7.4, the Rights Agent may not assign this Agreement without Insight’s prior written consent. Subject to Section 5.1(a)(ii) and Article 6 hereof, Insight may assign, in its sole discretion and without the consent of any other party, any or all of its rights, interests and obligations hereunder to one or more of its Affiliates or to any Person with whom Insight is merged or consolidated, or any entity resulting from any merger or consolidation to which Insight shall be a party (each, an “Assignee”); provided, that in connection with any assignment to an Assignee, Insight shall agree to remain liable for the performance by Insight of its obligations hereunder (to the extent Insight exists following such assignment). Insight or an Assignee may not otherwise assign this Agreement without the prior consent of the Acting Holders (such consent not to be unreasonably withheld, conditioned or delayed). Any attempted assignment of this Agreement in violation of this Section 7.5 will be void ab initio and of no effect.
Section 7.6 Benefits of Agreement; Action by Acting Holders.
Nothing in this Agreement, express or implied, will give to any Person (other than Insight, the Company, the Rights Agent, the Holders and their respective permitted successors and assigns hereunder) any benefit or any legal or equitable right, remedy or claim under this Agreement or under any covenant or provision herein contained, all such covenants and provisions being for the sole benefit of Insight, the Company, the Rights Agent, the Holders and their permitted successors and assigns. The Holders will have no rights hereunder except as are expressly set forth herein. Except for the rights of the Rights Agent set forth herein, the Acting Holders will have the sole right, on behalf of all Holders, by virtue of or under any provision of this Agreement, to institute any action or proceeding at law or in equity with respect to this Agreement, and no individual Holder or other group of Holders will be entitled to exercise such rights.
Section 7.7 Governing Law.
This Agreement and the CVRs will be governed by, and construed in accordance with, the laws of the State of Delaware, without giving effect to any provision of law or rule (whether of the State of Delaware or any other jurisdictions) that would cause the application of the laws of any jurisdictions other than the State of Delaware.
Section 7.8 Jurisdiction.
In any action or proceeding between any of the parties hereto arising out of or relating to this Agreement or any of the transactions contemplated hereby, each of the parties hereto: (a) irrevocably and unconditionally consents and submits to the exclusive jurisdiction and venue of the Court of Chancery of the State of Delaware or, to the extent such court does not have subject matter jurisdiction, the Superior Court of the State of Delaware or the United States District Court for the District of Delaware, and appellate courts thereof; (b) agrees that all claims in respect of such action or proceeding shall be heard and determined exclusively in accordance with clause (a) of this Section 7.8; (c) waives any objection to laying venue in any such action or proceeding in such courts; (d) waives any objection that such courts are an inconvenient forum or do not have jurisdiction over any Party; and (e) agrees that service of process upon such Party in any such action or proceeding shall be effective if notice is given in accordance with Section 7.1 or Section 7.2 of this Agreement; provided that nothing contained herein shall be deemed to limit in any way any right to serve process in any manner permitted by Law.
Section 7.9 WAIVER OF JURY TRIAL.
EACH OF THE PARTIES HERETO HEREBY IRREVOCABLY WAIVES ANY AND ALL RIGHT TO TRIAL BY JURY IN ANY LEGAL PROCEEDING ARISING OUT OF OR RELATED TO THIS AGREEMENT OR THE TRANSACTIONS CONTEMPLATED HEREBY. EACH PARTY CERTIFIES AND ACKNOWLEDGES THAT (I) NO REPRESENTATIVE, AGENT OR ATTORNEY OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF LITIGATION, SEEK TO ENFORCE THE FOREGOING WAIVER, (II) EACH PARTY UNDERSTANDS AND HAS CONSIDERED THE IMPLICATION OF THIS WAIVER, (III) EACH PARTY MAKES THIS WAIVER VOLUNTARILY, AND (IV) EACH PARTY HAS BEEN INDUCED TO ENTER INTO THIS AGREEMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS AND CERTIFICATIONS IN THIS SECTION 7.9.
Section 7.10 Severability Clause.
In the event that any provision of this Agreement, or the application of any such provision to any Person or set of circumstances, is for any reason determined to be invalid, unlawful, void or unenforceable to any extent, the remainder of this Agreement, and the application of such provision to Persons or circumstances other than those as to which it is determined to be invalid, unlawful, void or unenforceable, will not be impaired or otherwise affected and will continue to be valid and enforceable to the fullest extent permitted by applicable Law. Upon such a determination, the parties hereto will negotiate in good faith to modify this Agreement so as to effect the original intent of the parties as closely as possible in a mutually acceptable manner in order that the transactions contemplated hereby be consummated as originally contemplated to the fullest extent possible; provided, however, that if an excluded provision shall affect the rights, immunities, liabilities, duties or obligations of the Rights Agent, the Rights Agent shall be entitled to resign immediately upon written Notice to Insight.
Section 7.11 Counterparts; Effectiveness.
This Agreement may be signed in any number of counterparts, each of which will be deemed an original, with the same effect as if the signatures thereto and hereto were upon the same instrument. This Agreement or any counterpart may be executed and delivered by facsimile copies or delivered by electronic communications by portable document format (.pdf), each of which shall be deemed an original. This Agreement will become effective when each party hereto will have received a counterpart hereof signed by the other party hereto. Until and unless each party has received a counterpart hereof signed by the other party hereto, this Agreement will have no effect and no party will have any right or obligation hereunder (whether by virtue of any oral or written agreement or any other communication).
Section 7.12 Termination.
This Agreement will automatically terminate and be of no further force or effect and, except the rights, protections and immunities of the Rights Agent under Article 3, the parties hereto will have no further liability hereunder, and the CVRs will expire without any consideration or compensation therefor, upon the earliest to occur of (a) the expiration of the CVR Term, (b) the expiration of all payment obligations under the Disposition Agreements, or (c) the delivery of a written notice of termination duly executed by Insight and the Acting Holders (such date, the “Termination Date”). The termination of this Agreement will not affect or limit the right of Holders to receive the CVR Payments under Section 2.4 to the extent earned prior to the termination of this Agreement, and the provisions applicable thereto will survive the expiration or termination of this Agreement until such CVR Payments, if any, have been made, if applicable.
Section 7.13 Funds.
All funds received by Computershare under this Agreement that are to be distributed or applied by Computershare in the performance of services hereunder (the “Funds”) shall be held by Computershare, as agent for Insight, and deposited in one or more bank accounts to be maintained by Computershare in its name as agent for Insight. Until paid pursuant to the terms of this Agreement, Computershare may hold or invest the Funds through such accounts in: (a) funds backed by obligations of, or guaranteed by, the United States of America; (b) debt or commercial paper obligations rated A-1 or P-1 or better by S&P Global Inc.
(“S&P”) or Moody’s Investors Service, Inc. (“Moody’s”), respectively; (c) Government and Treasury backed AAA-rated Fixed NAV money market funds that comply with Rule 2a-7 of the Investment Company Act of 1940, as amended; or (d) short term certificates of deposit, bank repurchase agreements, and bank accounts with commercial banks with Tier 1 capital exceeding $1 billion, or with an investment grade rating by S&P (LT Local Issuer Credit Rating), Moody’s (Long Term Rating) and Fitch Ratings, Inc. (LT Issuer Default Rating) (each as reported by Bloomberg Finance L.P.). Computershare shall have no responsibility or liability for any diminution of the Funds that may result from any deposit or investment made by Computershare in accordance with this paragraph, including any losses resulting from a default by any bank, financial institution or other third party. Computershare may from time to time receive interest, dividends or other earnings in connection with such deposits or investments. Computershare shall not be obligated to pay such interest, dividends or earnings to Insight, the Company, any holder or any other party.
Section 7.14 Further Assurance by Insight and the Company.
Each of Insight and the Company agrees that it will perform, execute, acknowledge and deliver or cause to be performed, executed, acknowledged and delivered all such further and other acts, instruments and assurances as may reasonably be required or requested by the Rights Agent for the carrying out or performing by the Rights Agent of the provisions of this Agreement.
Section 7.15 Confidentiality. The Rights Agent, the Company and Insight agree that all books, records, information and data pertaining to the business of the other party, including inter alia, personal, non-public CVR holder information, which are exchanged or received pursuant to the negotiation or the carrying out of this Agreement including the Fee Schedule shall remain confidential, and shall not be voluntarily disclosed to any other person, except as may be required by Law, including, without limitation, pursuant to subpoenas from state or federal government authorities (e.g., in divorce and criminal actions).
Section 7.16 Force Majeure. Notwithstanding anything to the contrary contained herein, the Rights Agent will not be liable for any delays or failures in performance resulting from acts beyond its reasonable control including, without limitation, acts of God, epidemic, pandemic, terrorist acts, shortage of supply, breakdowns or malfunctions, interruptions or malfunction of computer facilities, or loss of data due to power failures or mechanical difficulties with information storage or retrieval systems, labor difficulties, war, or civil unrest.
Section 7.17 Construction.
(a) For purposes of this Agreement, whenever the context requires: singular terms will include the plural, and vice versa; the masculine gender will include the feminine and neuter genders; the feminine gender will include the masculine and neuter genders; and the neuter gender will include the masculine and feminine genders.
(b) As used in this Agreement, the words “include” and “including,” and variations thereof, will not be deemed to be terms of limitation, but rather will be deemed to be followed by the words “without limitation.”
(c) The headings contained in this Agreement are for convenience of reference only, will not be deemed to be a part of this Agreement and will not be referred to in connection with the construction or interpretation of this Agreement.
(d) Unless stated otherwise, “Article” and “Section” followed by a number or letter mean and refer to the specified Article or Section of this Agreement. The term “Agreement” and any reference in this Agreement to this Agreement or any other agreement or document includes, and is a reference to, this Agreement or such other agreement or document as it may have been, or may from time to time be, amended, restated, replaced, supplemented or novated and includes all schedules to it.
(e) A period of time is to be computed as beginning on the day following the event that began the period and ending at 4:30 p.m. on the last day of the period, if the last day of the period is a Business Day, or at 4:30 p.m. on the next Business Day if the last day of the period is not a Business Day.
(f) Any reference in this Agreement to a date or time shall be deemed to be such date or time in New York City, United States, unless otherwise specified. The parties hereto have participated jointly in the negotiation and drafting of this Agreement. In the event an ambiguity or question of intent or interpretation arises, this Agreement shall be construed as if drafted jointly by the parties and no presumption or burden of proof shall arise favoring or disfavoring any Person by virtue of the authorship of any provision of this Agreement.
(g) All references herein to “$” are to United States Dollars.
[Remainder of page intentionally left blank]
IN WITNESS WHEREOF, each of the parties has caused this Agreement to be executed as of the day and year first above written.
| IKENA ONCOLOGY, INC. | ||
| By: | /s/ Jotin Marango |
|
| Name: | Jotin Marango | |
| Title: | Chief Financial Officer, Chief Operating Officer and Head of Corporate Development |
|
| INMAGENE BIOPHARMACEUTICALS | ||
| By: | /s/ Jonathan Wang |
|
| Name: | Jonathan Wang, Ph.D. | |
| Title: | Chief Executive Officer | |
| COMPUTERSHARE TRUST COMPANY, N.A. and COMPUTERSHARE INC., |
||
| On behalf of both entities | ||
| By: | /s/ Collin Ekeogu |
|
| Name: | Collin Ekeogu | |
| Title: | Senior Manager, Corporate Actions | |
Schedule 1.1
| 1. | Promissory Note by and between Miragene Co and Inmagene Biopharmaceuticals, dated on or about the date hereof. |
| 2. | Share Purchase Agreement by and between Miragene Co and Inmagene Biopharmaceuticals, dated on or about the date hereof. |
Exhibit 10.4
TRANSITION SERVICES AGREEMENT
dated as of
July 25, 2025
by and between
Miragene Inc
and
Inmagene Biopharmaceuticals
TABLE OF CONTENTS
| PAGE | ||||||
| ARTICLE 1 DEFINITIONS |
1 | |||||
| 1.1 |
Definitions | 1 | ||||
| 1.2 |
Other Definitional and Interpretative Provisions | 2 | ||||
| ARTICLE 2 SERVICES |
2 | |||||
| 2.1 |
Provision of Services | 2 | ||||
| 2.2 |
Discontinuation of Services | 4 | ||||
| 2.3 |
Compliance with Law; Consents | 4 | ||||
| ARTICLE 3 SERVICE COSTS; OTHER CHARGES |
5 | |||||
| 3.1 |
Service Fees Generally | 5 | ||||
| 3.2 |
Third Party Costs | 5 | ||||
| 3.3 |
Compensation of Personnel | 5 | ||||
| 3.4 |
Taxes | 6 | ||||
| 3.5 |
Invoicing and Settlement of Service Costs | 6 | ||||
| ARTICLE 4 THE SERVICES |
7 | |||||
| 4.1 |
Standards of Service | 7 | ||||
| 4.2 |
Changes to the Services | 8 | ||||
| 4.3 |
Suspension of Services | 9 | ||||
| 4.4 |
Management of Services | 9 | ||||
| 4.5 |
Limitations | 9 | ||||
| ARTICLE 5 INTELLECTUAL PROPERTY |
10 | |||||
| 5.1 |
Work for Hire | 10 | ||||
| 5.2 |
Inventions and Assignment | 10 | ||||
| 5.3 |
Performing Party Property | 10 | ||||
| 5.4 |
No Other License Grant | 11 | ||||
| 5.5 |
Third Party Intellectual Property | 11 | ||||
| 5.6 |
Use of Third Party Facilities | 11 | ||||
| ARTICLE 6 DISCLAIMER, LIABILITY AND INDEMNIFICATION |
11 | |||||
| 6.1 |
Indemnification of Miragene by IMA | 11 | ||||
| 6.2 |
Indemnification of IMA by Miragene | 12 | ||||
| 6.3 |
Exclusion Of Warranties | 12 | ||||
| 6.4 |
Limitation of Liability | 12 | ||||
| ARTICLE 7 TERM AND TERMINATION |
13 | |||||
| 7.1 |
Term | 13 | ||||
| 7.2 |
Termination | 13 | ||||
| 7.3 |
Effect of Expiration or Termination | 13 | ||||
| ARTICLE 8 ADDITIONAL AGREEMENTS |
14 | |||||
i
TABLE OF CONTENTS
Continued
| PAGE | ||||||
| 8.1 |
Confidential Information | 14 | ||||
| 8.2 |
Access to Information | 15 | ||||
| 8.3 |
Data Transfer | 15 | ||||
| 8.4 |
Employment and Labor Matters | 16 | ||||
| ARTICLE 9 MISCELLANEOUS |
16 | |||||
| 9.1 |
No Agency; Independent Contractor Status | 16 | ||||
| 9.2 |
Force Majeure | 16 | ||||
| 9.3 |
Entire Agreement | 17 | ||||
| 9.4 |
Information | 17 | ||||
| 9.5 |
Notices | 17 | ||||
| 9.6 |
Governing law | 18 | ||||
| 9.7 |
Jurisdiction | 18 | ||||
| 9.8 |
Service | 18 | ||||
| 9.9 |
Waiver Of Jury Trial | 18 | ||||
| 9.10 |
Severability | 19 | ||||
| 9.11 |
Amendments and Waivers | 19 | ||||
| 9.12 |
Successors and Assigns | 19 | ||||
| 9.13 |
Specific Performance | 19 | ||||
| 9.14 |
Counterparts | 20 | ||||
| 9.15 |
No Benefit to Third Parties | 20 | ||||
| 9.16 |
Further Assurances | 20 | ||||
| Schedule A | Miragene Services | |
| Schedule B | IMA Services | |
| Schedule C | Form of Statement of Work |
ii
TRANSITION SERVICES AGREEMENT
TRANSITION SERVICES AGREEMENT (this “Agreement”) dated as of July 25, 2025 between MIRAGENE INC, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands (“Miragene”) and INMAGENE BIOPHARMACEUTICALS, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands (“IMA”, together with Miragene, the “Parties” and each a “Party”).
RECITALS
A. Miragene and IMA entered into a Share Purchase Agreement of even date herewith (the “Purchase Agreement”), pursuant to which Miragene Co, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands, agreed to purchase from IMA all of the outstanding shares of Miragene, a wholly owned subsidiary of IMA, in accordance with the terms of and as further set forth in the Purchase Agreement.
B. The closing of the transactions under the Purchase Agreement will occur immediately prior to the consummation of the merger transactions as contemplated by, and in accordance with the terms of, that certain Agreement and Plan of Merger entered into on December 23, 2024, by and among IMA, Ikena Oncology, Inc., a Delaware corporation (“Ikena”), Insight Merger Sub I, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands and direct wholly owned subsidiary of Ikena, and Insight Merger Sub II, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands and direct wholly owned subsidiary of Ikena (the “Merger”).
C. In connection with the transactions contemplated by the Purchase Agreement, Miragene and IMA desire to enter into this Agreement to set forth the terms and conditions pursuant to which Miragene (or one of its Affiliates) shall provide (or cause to be provided) to IMA (or one of its Affiliates) the Miragene Services (as defined herein) for a transition period and IMA may provide to Miragene (or one of its Affiliates) certain IMA services (as defined herein) for a transition period.
NOW, THEREFORE, in consideration of the foregoing and the representations, warranties, covenants and agreements contained herein, the Parties hereto agree as follows:
ARTICLE 1
DEFINITIONS
1.1 Definitions. For purposes of this Agreement, any capitalized term used herein shall have the meaning assigned to such term herein.
(a) “Affiliate” means, with respect to any Person, any Person that owns or controls directly or indirectly such Person, any Person that controls or is controlled by or is under common control with such Person.
1
(b) “Law” means any federal, state, national, supra-national, foreign, local or municipal or other law, statute, constitution, principle of common law, resolution, ordinance, code, edict, decree, decision, act, rule, regulation, ruling or requirement issued, enacted, adopted, promulgated, implemented or otherwise put into effect by or under the authority of any governmental authority.
(c) “Person” means any individual, corporation, partnership, limited liability company or other entity.
(d) “Tax” means any U.S. federal, state or local, non-U.S. or other tax, including any income tax, franchise tax, capital gains tax, gross receipts tax, value-added tax, surtax, estimated tax, unemployment tax, national health insurance tax, excise tax, ad valorem tax, transfer tax, stamp tax, sales tax, use tax, property tax, business tax, withholding tax, payroll tax, customs duty, alternative or add-on minimum or other tax of any kind whatsoever, and including any fine, penalty, addition to tax or interest imposed by a governmental authority with respect thereto, whether disputed or not.
1.2 Other Definitional and Interpretative Provisions. When a reference is made in this Agreement to Articles, Sections or Schedules such reference shall be to an Article, Section or Schedule to this Agreement unless otherwise indicated. For purposes of this Agreement, the words “include,” “includes” and “including,” when used herein, shall be deemed in each case to be followed by the words “without limitation.” The table of contents and headings contained in this Agreement are for reference purposes only and shall not affect in any way the meaning or interpretation of this Agreement.
ARTICLE 2
SERVICES
2.1 Provision of Services.
(a) On the terms and subject to the conditions of this Agreement, from and after the date hereof, and for the applicable periods specified under Schedule A attached hereto under the heading “Service Period” or under an applicable Statement of Work (as defined below), if any (each such period, a “Miragene Service Period”), Miragene shall use commercially reasonable efforts to provide (or cause to be provided) to IMA the services set forth on Schedule A or in any Statement of Work entered into in accordance with Section 2.1(c) (as such Schedule or Statement of Work may be amended from time to time), which services are referred to individually as a “Miragene Service” and collectively the “Miragene Services.” Except for the Miragene Services expressly contemplated to be provided in accordance with the provisions of this Agreement or as agreed to between the Parties pursuant to a Statement of Work, neither Miragene, nor any of its Affiliates, shall have any obligation under this Agreement or otherwise to provide any services to IMA or its Affiliates. For the avoidance of doubt, none of the Miragene Services shall require Miragene to provide any legal, financial, accounting or tax advice in connection with any such Miragene Service or otherwise. No person providing Miragene Services to IMA under this Agreement or any Statement of Work shall have the authority to negotiate or bind IMA to any contracts.
2
(b) On the terms and subject to the conditions of this Agreement, from and after the date hereof, and for the applicable periods specified under Schedule B attached hereto under the heading “Service Period” or under an applicable Statement of Work (as defined below), if any (each such period, a “IMA Service Period”), IMA shall use commercially reasonable efforts to provide (or cause to be provided) to Miragene (or one of its Affiliates) the services set forth on Schedule B or in any Statement of Work entered into in accordance with Section 2.1(c) (as such Schedule or Statement of Work may be amended from time to time), which services are referred to individually as a “IMA Service” and collectively the “IMA Services,” together with the Miragene Services, the “Services.” Except for the IMA Services expressly contemplated to be provided in accordance with the terms of this Agreement or arranged to between the Parties pursuant to a Statement of Work, neither IMA, nor any of its Affiliates, shall have any obligation under this Agreement or otherwise to provide any services to Miragene or its Affiliates. No person providing IMA Services to Miragene under this Agreement or any Statement of Work shall have the authority to negotiate or bind Miragene to any contracts.
(c) Miragene and IMA may further agree to additional Miragene Services or IMA Services (collectively, the “Services”) pursuant to this Agreement by entering into one or more statements of work in the form attached hereto as Schedule C (each, a “Statement of Work”) from time to time during the Term (and no Statement of Work will become effective until it has been executed by both of the Service Coordinators (as defined below)), each of which will include the following:
(i) a reference to this Agreement, which reference will be deemed to incorporate all applicable provisions of this Agreement;
(ii) the date as of which the applicable Statement of Work will be effective and, if applicable, the term or period of time during which Miragene or IMA, as applicable, will provide or cause to be provided the applicable Services and resources to the other Party (or its Affiliate) pursuant to that Statement of Work;
(iii) a description of the Services to be provided by one Party to the other Party (or its Affiliate) pursuant to that Statement of Work, including the location at which the applicable Services are to be provided, and all deliverables to be provided as part of the applicable services pursuant to that Statement of Work, as well as the associated fees; and
(iv) any additional provisions applicable to the Services provided under that Statement of Work that are not otherwise set forth in this Agreement or that are exceptions to the provisions set forth in this Agreement.
(d) Miragene and IMA shall each nominate a representative to act as the primary contact person with respect to the provision of the Services (the “Service Coordinators”). Each Party hereto may change its Service Coordinator from time to time at its discretion by providing prior written notice to the other Party (including contact information for such Service Coordinator). Unless otherwise agreed to in writing by the Parties hereto, all communications relating to the day-to-day provision of the Services shall be directed to the Service Coordinators.
3
2.2 Discontinuation of Services. Either Party may discontinue the provision by the other Party of any Service by providing no less than [***] advance written notice to the other Party (“Termination Notice”) at any time, and, in such case, the applicable Service shall terminate on the termination date specified in the Termination Notice (or such other date mutually agreed in writing by IMA and Miragene) and Schedule A, Schedule B or the applicable Statement of Work shall be deemed amended to delete such Service as of the termination date, and this Agreement shall be of no further force and effect for such Service, except as to liabilities accrued prior to the date of termination of such Service; provided that, Service Fees for any Service that is terminated prior to the last day of any calendar quarter will be prorated. Any Service may be terminated without the termination of any other Service. Within a reasonable period of time following receipt of any Termination Notice (but in no event more than [***] following receipt of any Termination Notice), to the extent the termination of the applicable Service will result in the non-terminating Party incurring any incremental or out-of-pocket costs, such as any Service Costs, including for commitments made to, or in respect of, third party service providers (including contractors or subcontractors), due to the requirement to provide the Services through their respective Miragene Service Period or IMA Service Period, or prepaid expenses related to the applicable Miragene Service or IMA Service that cannot or will not be reimbursed or costs related to terminating such commitments (for clarity, such costs and expenses shall include, and IMA shall reimburse Miragene for, the required severance payments under applicable Law related to terminating the employees performing the applicable Miragene Service in an amount not to exceed [***] of [***]; provided, that, any such required severance payments under applicable Law shall first be netted against the portion of the Prepaid Service Fees or Prepaid Further Service Fees, as applicable, that are no longer required to pay the salary of such terminated employees for the remainder of the Initial Term or Initial Term Extension, as applicable) (collectively, the “Early Termination Costs”), the non-terminating Party will provide the terminating Party with written notice thereof (“Response Notice”), which notice shall set forth an estimate (to the extent then known by Miragene or IMA, as applicable) of the amount of the Early Termination Costs. A Party may withdraw its Termination Notice by delivering a withdrawal notice within five business days following the receipt of such Response Notice. If the terminating Party does not withdraw the Termination Notice within such period, such Termination Notice will be final and irrevocable, and such Party shall reimburse the non-terminating Party for all Early Termination Costs reasonably incurred by non-terminating Party as a result of such early termination; provided that, the non-terminating Party shall use commercially reasonable efforts to mitigate such costs. Such reimbursement shall be made in accordance with Section 3.5.
2.3 Compliance with Law; Consents. Each Party shall comply with all applicable Laws in connection with its performance or receipt of any Services. At the Recipient Party’s sole cost, each Performing Party shall obtain all licenses and consents, and pay all fees, required by applicable Law and necessary for such Performing Party’s performance and Recipient Party’s receipt of the applicable Services.
4
ARTICLE 3
SERVICE COSTS; OTHER CHARGES
3.1 Service Fees Generally. The service fees to be paid by IMA to Miragene (or one of its Affiliates) for each Miragene Service to be provided under this Agreement shall be as set forth opposite such Miragene Service on Schedule A or in the applicable Statement of Work (the “Miragene Service Fees”); except that the Service Fees in respect of the Services set forth on Schedule A, to be provided during the Initial Term shall be [***] (the “Prepaid Service Fees”), and, in case of the Initial Term Extension, to be provided during the second [***] month period of the extended Initial Term shall be an amount equal to [***] prorated for the number of FTEs still providing Miragene Services as of the end of the Initial Term (with each FTE hour of Miragene Services to be weighted equally for purposes of this calculation) (the “Prepaid Further Service Fees”). The service fees to be paid by Miragene (or one of its Affiliates) to IMA for each IMA Service to be provided under this Agreement shall be as set forth on Schedule B or in the applicable Statement of Work (the “IMA Service Fees,” and together with the Miragene Service Fees, the “Service Fees”). Other than in respect of the Prepaid Service Fees or Prepaid Further Service Fees, Service Fees for any Service that is terminated prior to the last day of any calendar quarter will be prorated. For purposes of this Agreement, “FTE” means a full-time scientific, technical or administrative person, or in the case of less than a full-time person, a full-time equivalent person’s year or portion thereof reasonably and directly allocated to the provision of the Miragene Services, where “full-time” is based upon a total of 1,800 working hours per calendar year; provided that any such full-time person constituting an FTE will be dedicated solely to the provision (and shall not be providing any other services or be engaged in any other work for Miragene) of Miragene Services during the Miragene Service Period.
3.2 Third Party Costs. In addition to the Miragene Service Fees or IMA Service Fees, as applicable, the Party receiving services (the “Recipient Party”) shall reimburse the Party performing services (the “Performing Party”) for all reasonable and documented out-of-pocket costs and expenses of third parties actually incurred by the Performing Party in the provision of the Services at the cost (without markup) to the Performing Party; provided, that the Performing Party shall seek and obtain prior written consent from the Receiving Party for any costs and expenses in excess of [***] in the aggregate per calendar quarter. Any such third party costs and expenses described in this Section 3.2 are the “Third Party Costs” (together with the Service Fees, the “Service Costs”).
3.3 Compensation of Personnel. Each Party is solely responsible for compensating its personnel providing its respective Services (the “Performing Party Personnel”), for maintaining worker’s compensation, personal liability, property, and other insurance coverage relating to its Performing Party Personnel, and for any and all other liabilities associated with or related to its Performing Party Personnel.
5
3.4 Taxes.
(a) The Recipient Party receiving Services (or its applicable Affiliate) shall bear any and all taxes and other similar charges (and any related interest and penalties) imposed by applicable taxing authorities with respect to any fees or cost reimbursement, payable by it pursuant to this Agreement, including all sales, use, value-added and similar Taxes, but excluding Taxes based on net income. Such Taxes shall be incremental to other payments or charges identified in this Agreement. Each Party shall, and shall cause its Affiliates to, use commercially reasonable efforts to avail itself of any available exemptions from such Taxes and to cooperate with the other Party in providing any information or documentation that may be necessary to obtain such exemptions.
(b) All sums payable under this Agreement shall be paid free and clear of all deductions or withholdings in respect of any Taxes unless the deduction or withholding is required by applicable law, in which event the amount of the payment due from the Party required to make such payment shall be increased (such increase, an “Additional Amount”) to an amount which after any withholding or deduction leaves an amount equal to the payment which would have been due if no such deduction or withholding had been required. The Parties agree to use commercially reasonable efforts to reduce or eliminate Taxes for which an Additional Amount would otherwise be payable under this Section 3.4(b).
3.5 Invoicing and Settlement of Service Costs.
(a) Upon the consummation of the Merger, Miragene shall deliver an invoice to IMA and IMA shall pay, or cause to be paid, an amount equal to the Prepaid Service Fees by wire transfer of immediately available funds payable to such account or accounts designated by Miragene. Upon the Initial Term Extension, Miragene shall deliver an invoice to IMA and IMA shall pay, or cause to be paid, an amount equal to the Prepaid Further Service Fees by wire transfer of immediately available funds payable to such account or accounts designated by Miragene. IMA is entitled to invoice Miragene, and Miragene is obligated to repay IMA, for the amount of any unused Prepaid Services Fees pursuant to this Section 3.5 at the end of the Initial Term if some or all Services are terminated prior to the full duration thereof.
(b) Unless the Parties hereto agree in writing to a different arrangement and, with respect to Miragene Services, except for the Prepaid Service Fees or Prepaid Further Service Fees, each Party (or one of its Affiliates) shall invoice or notify in writing the other Party on a quarterly basis for all Service Costs incurred hereunder in respect of the prior calendar quarter to the extent such Service Costs are reasonably determinable during such calendar quarter, and to the extent any such amounts are not reasonably determinable, each Party shall continue to invoice such amounts (including after the termination of this Agreement) as promptly as practicable after such amounts are determined until all amounts are invoiced and paid (the date of delivery of such invoice or notification, the “Invoice Date”); provided that, each Party will use commercially reasonable efforts to provide the other Party with notice of the existence of amounts that are not reasonably determinable with respect to any calendar quarter and when such amounts are expected to be invoiced. In the event an invoice includes payments to subcontractors or other third-party service providers, the Performing Party will provide the Recipient Party with copies of such subcontractors’ or other third-party service providers’ applicable invoices upon the Recipient Party’s reasonable request. All such invoices and payments shall be made in U.S. dollars.
(c) Each Party agrees to pay, on or before the date that is 45 days after the Invoice Date, by wire transfer of immediately available funds payable to such account or accounts designated by the invoicing Party, all amounts specified in the invoice or notification delivered pursuant to the preceding clause “(a)” payable by such Party.
6
(d) If there is a dispute (an “Invoice Dispute”) between Miragene and IMA regarding any amount set forth in any invoice, the disputing Party shall notify the other Party in writing (each such notice, an “Invoice Dispute Notice”) of each amount that is the subject of the Invoice Dispute and the basis therefor, and such amount shall be excluded from the amount due until resolution of the Invoice Dispute in accordance with the procedures set forth below in this Section 3.5(c); provided that, any undisputed portion of such amount shall be included in the calculation of the amount due pursuant to Section 3.5(b) and paid in accordance with Section 3.5(b). Miragene and IMA shall cooperate and use their commercially reasonable efforts to resolve such Invoice Dispute. If Miragene and IMA are unable to resolve the Invoice Dispute within 10 business days after the applicable Party’s receipt of the Invoice Dispute Notice, then either Miragene or IMA may refer the Invoice Dispute for resolution to an independent accounting firm of recognized national standing as Miragene and IMA may mutually agree, which agreement shall not be unreasonably withheld, conditioned or delayed (the “Invoice Accounting Referee”), which shall determine the disputed amounts. The determinations of the Invoice Accounting Referee shall be final and binding on Miragene and IMA. The fees and expenses of the Invoice Accounting Referee shall be borne by Miragene and IMA in the same proportion that the aggregate amount of the disputed items submitted to the Invoice Accounting Referee that are unsuccessfully disputed by each such Party (as finally determined by the Invoice Accounting Referee) bears to the total amount of such disputed items so submitted. If the amount owed by the applicable Party based on the determinations of the Invoice Accounting Referee exceeds the amount paid by such Party, then such Party shall promptly pay to the other Party to the Invoice Dispute the full amount of such excess with interest, from the date that such amount was required to be paid pursuant to Section 3.5(b) to (but excluding) the date of payment by the applicable Party of such excess. The interest rate shall be prime rate as published in the Wall Street Journal plus [***] percent per annum calculated based on a 360 day year.
ARTICLE 4
THE SERVICES
4.1 Standards of Service.
(a) Miragene shall perform the Miragene Services and IMA shall perform the IMA Services (i) in accordance with the provisions of this Agreement, (ii) in a professional, competent and workmanlike manner in accordance with generally accepted industry standards and practices, and (iii) at a level of quality substantially similar to that at which the Services were provided immediately prior to the date hereof and consistent with the manner in which the Performing Party provides its respective Services for its own business. Either Party shall notify the other Party promptly if such Party becomes aware of any circumstances that adversely impact or are likely to adversely impact such Party’s performance of its respective Services or the other Party’s receipt of such Services; provided that, nothing in this Section 4.1(a) shall limit or relieve the Performing Party from its obligation to provide any and all of the Services required hereunder.
7
(b) The Parties will use good faith efforts to cooperate with each other in all matters relating to the provision and receipt of Services. Such cooperation shall include exchanging information (subject to Section 8.2 of this Agreement), providing electronic access to systems used in connection with Services, performing true-ups and adjustments and using commercially reasonable efforts to obtain all applicable consents, licenses, sublicenses or approvals (collectively, “Consents”) necessary to permit each Party to perform its obligations hereunder. Any Performing Party shall promptly inform the Recipient Party of any Consents required in connection with the provisions of Services hereunder. The Recipient Party shall be responsible for the costs of obtaining such Consents required in connection with the provision of Services hereunder. If the Parties are not able to obtain a Consent, then such Performing Party shall use its commercially reasonable efforts (subject to the terms and conditions (including the limitations on the Performing Party’s obligations) contained in this Agreement) to provide or cause to be provided the affected Service(s) (or as much of such Service(s) as is reasonably practicable to provide) or adopt alternative approaches to enable the Recipient Party to receive the benefit of this Agreement until the earlier of the receipt of the missing Consent or the term of the Service(s) in question has terminated.
(c) If, after the date hereof, IMA shall purchase, lease or otherwise acquire any businesses, assets or properties or rights in respect thereof, Miragene, or any of its Affiliates, shall have no obligation to, or cause any Affiliate to, provide any Miragene Services hereunder to or in respect of such acquired businesses, assets or properties.
(d) If, after the date hereof, any service providers (including any employee of a Party) with respect to any of the Services hereunder are terminated, for any reason, then (i) the Performing Party which maintains the relationship with such service provider shall provide the Recipient Party with notice of such termination and (ii) upon the delivery of notice of the termination of such service provider, the Service which was being provided by such service provider, shall be deemed to have been terminated without any further action of any Party; provided, however, that a replacement for such service may be agreed to between the Parties pursuant to a Statement of Work.
(e) Miragene shall not be responsible for any inability to provide a Miragene Service or any delay in doing so to the extent that such inability or delay is the result of the failure of IMA to timely provide the information, access or other cooperation necessary for Miragene to provide such Miragene Service. IMA shall not be responsible for any inability to provide an IMA Service or any delay in doing so to the extent that such inability or delay is the result of the failure of Miragene to timely provide the information, access or other cooperation necessary for IMA to provide such IMA Service.
4.2 Changes to the Services. It is understood and agreed that a Party may from time to time modify or change the manner, nature, quality and/or standard of care of any Service as set forth on Schedule A, Schedule B or a Statement of Work (such Party, the “Modifying Party”) provided to the other Party (the “Receiving Party”) to the extent that the Modifying Party is making a similar change in the performance of such services for itself or its Affiliates; provided that, any such modification or change may not be undertaken (i) with the intent or purpose of disadvantaging the Receiving Party or (ii) if such modification or change would reasonably be expected to have a materially and disproportionately adverse effect on the Receiving Party and its subsidiaries, taken as a whole.
8
4.3 Suspension of Services. If the performance of any Service subjects Miragene or IMA, in its capacity as the provider of such Service (as applicable), to a reasonable risk of violating applicable laws or would reasonably be expected to, individually or in the aggregate, materially and adversely affect the business of such Party or its ultimate parent, then such Party (the “At-Risk Party”) (i) in the case of a violation of applicable laws, may immediately upon providing written notice of such fact to the other Party (it being understood that the At-Risk Party shall provide the other Party with as much advance notice as is reasonably practicable under the circumstances and permitted by applicable laws) suspend performance of such Service without liability and (ii) in the case of a material and adverse effect to the business of the At-Risk Party or its ultimate parent, may, upon providing written notice of such fact to the other Party sufficiently in advance to permit the other Party (acting reasonably) to arrange for replacement services, suspend performance of such Service without liability; provided that, following delivery of such notice, the Parties hereto will use commercially reasonable efforts to promptly amend this Agreement to the extent necessary to eliminate such violation of applicable laws or such effect while as nearly as possible accomplishing the purpose of the intended Service in a mutually satisfactory manner. If the Parties are unable to agree upon such an amendment to this Agreement within 15 days of such notification, then either Party may terminate its obligation with respect to such Service upon written notice to the other Party pursuant to Section 2.2.
4.4 Management of Services. Except as may otherwise be expressly provided in this Agreement, the management of and control over the provision of the Miragene Services by Miragene shall reside solely with Miragene and notwithstanding anything to the contrary, Miragene shall be permitted to choose the methodology, systems and applications it utilizes in the provision of such Miragene Services; provided that, Miragene shall remain responsible for the performance of the Miragene Services in accordance with this Agreement. The provision, use of and access to the Miragene Services shall be subject to (i) any technical and operational changes that may be required to manage any reasonable restrictions imposed by Miragene in respect of data access; (ii) any third party services, resources or dependencies; (iii) any applicable laws; and (iv) the terms of this Agreement.
4.5 Limitations.
(a) It is understood that the Services to be provided to IMA under this Agreement shall only be provided for the purposes of operating the IMG-007 programs and projects (the “IMG-007 Business”) substantially as conducted immediately prior to the date hereof and facilitating an orderly transition of the operation of the IMG-007 Business. Neither IMA, nor any of its Affiliates, may resell, license the use of or otherwise permit the use by others of any Services, except with the prior written consent of Miragene.
(b) In providing the Services, Miragene shall not be obligated to: (i) hire any additional employees; (ii) maintain the employment of any specific employee; (iii) purchase, lease or license any additional equipment, hardware, intellectual property or software; or (iv) pay any costs related to the transfer or conversion of IMA’s data to IMA or any alternate supplier of Services, all of which shall be paid by IMA.
9
ARTICLE 5
INTELLECTUAL PROPERTY
5.1 Work for Hire. Except as provided in Section 5.3, all deliverables resulting from the Services provided under this Agreement are “Works Made for Hire” as defined in the U.S. Copyright Act and as such, all copyrightable works will be deemed, upon creation, to be assigned to the Recipient Party and are hereby perpetually and irrevocably assigned to Recipient Party.
5.2 Inventions and Assignment. Except as provided in Section 5.3, any materials, data, processes, documents, deliverables, information (including Confidential Information (as defined in Section 8.1(a))), discoveries, inventions, know-how and the like developed or generated by or on behalf of the Performing Party during the course of performing the Services, whether or not patentable, and all related patent, copyright and other intellectual property rights in any of the foregoing (collectively the “Inventions”) shall be the sole and exclusive property of the Recipient Party. The Performing Party hereby perpetually and irrevocably assigns, and to the extent it cannot presently assign, agrees to assign and shall use reasonable efforts to obtain the right to assign, to the Recipient Party all of the Performing Party’s worldwide right, title and interest in and to such Inventions. The Performing Party shall assist the Recipient Party in securing for the Recipient Party any patents, copyrights or other proprietary rights in such Inventions, and shall take such actions and execute such documents as the Recipient Party may reasonably request in connection with providing such assistance or otherwise to vest in the Recipient Party all right, title and interest in and to such Inventions, including any and all applications, assignments or other instruments. The Performing Party shall be compensated for all of its reasonable out-of-pocket costs and expenses associated with such requested assistance. To the extent Inventions cannot be assigned to IMA in accordance with this Article 5, the Performing Party hereby grants to the Recipient Party an exclusive perpetual, irrevocable, transferable, royalty-free, fully paid-up, worldwide license, with the right to grant sublicenses (through multiple tiers), under such Inventions for any and all purposes.
5.3 Performing Party Property. Any (a) processes or process improvements developed or generated by or on behalf of the Performing Party that are specifically related to the Performing Party’s pre-existing technology, are general in nature and are not unique or specific to the targets or work performed for the Recipient Party, or (b) pre-existing patents, know-how or other technology or information controlled by the Performing Party prior to the date hereof or developed outside the scope of this Agreement that are incorporated into or embodied in any Inventions or deliverables or results provided by the Performing Party under this Agreement (clauses (a) and (b) collectively, the “Performing Party Technology”) will be owned by the Performing Party. For clarity, Performing Party Technology shall not include any technology, patents, know-how or information which has been assigned or exclusively licensed to the Recipient Party or to any third party. The Performing Party hereby grants to the Recipient Party a perpetual, irrevocable, non-exclusive, worldwide, royalty-free, fully paid-up license (with a right to grant sublicenses through multiple tiers) under the Performing Party’s intellectual property rights in Performing Party Technology solely to the extent necessary for the Recipient Party to utilize the Inventions and the other deliverables or results of Services for any purpose. The foregoing license may be sublicensed by the Recipient Party (through multiple tiers) in connection with the transfer, use or other exploitation by the Recipient Party of the deliverables, results or Inventions to which the license relates.
10
5.4 No Other License Grant. Except as expressly set forth in this Agreement, nothing in this Agreement, nor the delivery of any information or materials to the Performing Party by the Recipient Party (or any third party acting on its behalf) in connection with the Performing Party’s performance of Services under this Agreement shall be deemed to grant to either Party any right or license under any patents, patent applications, know-how, technology, inventions or other intellectual property of the other Party. Notwithstanding anything in this Agreement to the contrary, the Recipient Party shall own all right, title and interest in and to all inventions, know-how, information and materials, and all related intellectual property rights, that arise from the Recipient Party’s use of Inventions and the other deliverables and results of Services.
5.5 Third Party Intellectual Property. The Performing Party will not knowingly utilize in the performance of Services under this Agreement or incorporate into any deliverable or materials or other results of the Services provided to the Recipient Party any technology or materials covered by proprietary rights of a third party unless the Performing Party is freely permitted to utilize or incorporate such technology or materials and the Recipient Party is freely permitted to use such work, deliverable or materials or any other results of the Services without further compensation by any Party to any third party.
5.6 Use of Third Party Facilities. The Performing Party will not make any use of any funds, space, personnel, facilities, equipment or other resources of a third party in performing Services, or take any other action, that would result in a third party owning or having a right in the results of Services or Inventions, unless agreed upon in writing in advance by the Recipient Party.
ARTICLE 6
DISCLAIMER, LIABILITY AND INDEMNIFICATION
6.1 Indemnification of Miragene by IMA. IMA agrees to indemnify and hold harmless Miragene, its Affiliates, and their respective directors, officers, agents, employees or other Representatives (each, a “Miragene Indemnified Person”) from and against any and all damage, loss, liability and expense (including reasonable expenses of investigation and reasonable attorneys’ fees and expenses in connection with any Action (as defined below) involving a third party claim) (“Damages”), and to reimburse each Miragene Indemnified Person for all reasonable and documented expenses (including reasonable attorneys’ fees) as they are incurred in investigating, preparing, pursuing or defending any action, suit, investigation or proceeding, in each case by or before any arbitrator or governmental body (collectively, “Actions”), whether or not in connection with pending or threatened litigation and whether or not any Miragene Indemnified Person is a Party, in each case to the extent arising out of the Services rendered or to be rendered by or on behalf of any Miragene Indemnified Person pursuant to this Agreement; provided that, IMA shall not be responsible for any Damages or expenses of any Miragene Indemnified Person to the extent such Damages or expenses have resulted from a Miragene Indemnified Person’s gross negligence or willful misconduct in connection with any such Services, actions or inactions.
11
6.2 Indemnification of IMA by Miragene. Miragene agrees to indemnify and hold harmless IMA, its Affiliates, and its and their respective directors, officers, agents, consultants, Representatives and/or employees (each, a “IMA Indemnified Person” and, together with the Miragene Indemnified Persons, the “Indemnified Persons”)) from and against any Damages, and to reimburse each IMA Indemnified Person for all reasonable expenses (including reasonable attorneys’ fees) as they are incurred in investigating, preparing, pursuing or defending any Action, whether or not in connection with pending or threatened litigation and whether or not any IMA Indemnified Person is a Party, in each case to the extent arising out of Miragene’s gross negligence or willful misconduct in connection with the provision of any such Services; provided that, Miragene shall not be responsible for any Damages or expenses of any IMA Indemnified Person to the extent such Damages or expenses have resulted from an IMA Indemnified Person’s gross negligence or willful misconduct in connection with any such Services, actions or inactions.
6.3 Exclusion Of Warranties. EXCEPT AS EXPRESSLY SET FORTH IN THIS AGREEMENT, THE SERVICES AND RIGHTS GRANTED HEREUNDER ARE PROVIDED AND GRANTED “AS-IS” WITH NO WARRANTIES, AND EACH OF THE PARTIES EXPRESSLY EXCLUDES AND DISCLAIMS ANY WARRANTIES UNDER OR ARISING AS A RESULT OF THIS AGREEMENT, WHETHER EXPRESS, IMPLIED OR STATUTORY, INCLUDING THE IMPLIED WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, TITLE, NON-INFRINGEMENT OR ANY OTHER WARRANTY WHATSOEVER.
6.4 Limitation of Liability.
(a) Each Party hereto, agrees that (i) no Party shall have any liability, whether direct or indirect, in contract or tort or otherwise, to any Indemnified Person or any other person for or in connection with the Services rendered or to be rendered by or on behalf of any such Party pursuant to this Agreement, the transactions contemplated hereby or any actions or inactions by or on behalf of such Party in connection with any such Services except to the extent any Damages have resulted from a such Party’s gross negligence or willful misconduct in connection with any such Services, actions or inactions and (ii) the aggregate liability of either Party with respect to this Agreement shall not exceed the aggregate amount of such Service Costs paid hereunder to such Party; provided, however, that the foregoing shall not limit any liability arising from (A) the gross negligence or willful misconduct of such Party or any of any of its Affiliates in connection with any such Services, actions or inactions, (B) a breach of confidentiality obligations or (C) the indemnification obligations set forth in Section 6.1 or Section 6.2, as applicable.
(b) Notwithstanding the provisions of Section 6.4(a) no Party shall be liable for any (i) consequential, indirect, incidental, special or other speculative form of damages or any damages based on lost profits, diminution in value, a multiple of EBITDA (earnings before interest, taxes, depreciation and amortization) or of any other financial metric (in each case, whether trailing, forward or otherwise), (ii) punitive or exemplary damages (except to the extent actually paid by the Indemnified Person to a third party pursuant to a claim from a third party) or (iii) Damages that would not exist if not for, or to the extent aggravated by, any act or wrongful omission of the Indemnified Person.
12
(c) In addition to the foregoing, each Party agrees that it shall, in all circumstances, use commercially reasonable efforts to mitigate and otherwise minimize its Damages and those of any of its Affiliates, whether direct or indirect, due to, resulting from or arising in connection with any failure by it to comply fully with its obligations under this Agreement.
ARTICLE 7
TERM AND TERMINATION
7.1 Term. This Agreement is effective on the date hereof and will continue in effect for [***] months (the “Initial Term,” and the period for which this Agreement is in effect, the “Term”); provided that, (i) if IMA does not provide its written notice to terminate this Agreement pursuant to Section 7.2(a) during the first [***] months of the Initial Term, the Initial Term shall automatically extend for an additional [***] months (the “Initial Term Extension”) and (ii) each Party may, upon delivery of written notice no less than [***] days prior to the end of the Initial Term, extend the Term for up to [***] additional months with respect to any or all of the Miragene Services set forth on Schedule A, whereby such Party shall identify in such notice the Miragene Services or IMA Services to be continued and the new respective Service Period(s). Notwithstanding the foregoing, to the extent that any Statement of Work entered into during the Term (including any extension thereof) provides for a performance period that extends beyond the Term, then the Term shall be automatically extended, and this Agreement shall remain effective, for the duration of the performance period under such Statement of Work, provided, however such extension shall only apply to the Services being performed under such Statement of Work (and no new Services may be added during the term of such extension unless mutually agreed to by the Parties).
7.2 Termination. This Agreement may be terminated:
(a) after the Initial Term, by either Party by providing no less than [***] days’ advance written notice to the other Party; or
(b) by Miragene, after the completion by IMA of the sale or other disposition of any portion of IMA’s business, assets or properties constituting all or a majority of the IMG-007 Business.
7.3 Effect of Expiration or Termination.
(a) Other than as required by applicable Law, upon expiration of the Term or termination of this Agreement pursuant to Section 7.2, Miragene or IMA, as applicable, shall have no further obligation to provide any future Services and IMA shall have no obligation to pay any Service Costs relating to such Services; provided that, notwithstanding such expiration or termination, the Receiving Party shall remain liable for (i) all Service Costs accrued or owed and payable in respect of Services provided for the benefit of the Receiving Party prior to the effective date of the expiration or termination and (ii) all Service Costs incurred between the time of such expiration or termination and the time the provision of the relevant Service(s) would have been stopped absent such expiration or termination to the extent the Performing Party cannot avoid the incurrence of such costs using commercially reasonable efforts.
13
(b) Termination of this Agreement as provided for herein shall not prejudice or affect any rights or remedies which shall have accrued or shall thereafter accrue to either Party.
ARTICLE 8
ADDITIONAL AGREEMENTS
8.1 Confidential Information.
(a) The Parties hereto hereby covenant and agree to maintain confidential all Confidential Information relating to the other Party or any of such other Party’s Affiliates. Without limiting the generality of the foregoing, each Party shall cause its employees, agents and other Representatives to exercise the same level of care with respect to Confidential Information relating to the other Party or any of its Affiliates as it would with respect to proprietary information, materials and processes relating to itself or any of its Affiliates. “Confidential Information” shall mean all information, materials and processes relating to a Party or any Affiliate of such Party obtained by the other Party or any Affiliate thereof at any time (whether prior to or after the date hereof) in any format whatsoever (whether orally, visually, in writing, electronically or in any other form) relating to, arising out of or in connection with the Services rendered or to be rendered hereunder and shall include, but not be limited to, economic and business information or data, business plans, computer software and information relating to employees, vendors, customers, products, financial performance and projections, processes, strategies and systems but shall not include (i) information which becomes generally available to the public other than by release in violation of the provisions of this Section 8.1(a), (ii) information which becomes available on a non-confidential basis to a Party from a source other than the other Party to this Agreement, provided that, the Party in question reasonably believes that such source is not or was not bound to hold such information confidential, (iii) information acquired or developed independently by a Party without violating this Section 8.1(a) or any other confidentiality agreement with the other Party and (iv) information that is known by the Receiving Party at the time of receiving such information, as evidenced by pre-existing records. Except with the prior written consent of the other Party, each Party will use the other Party’s Confidential Information only in connection with the performance of its obligations hereunder and each Party shall use commercially reasonable efforts to restrict access to the other Party’s Confidential Information to those employees of such Party and its Affiliates and its and their Representatives requiring access for the purpose of providing Services hereunder who are contractually or legally bound by obligations of non-disclosure and non-use at least as stringent as those contained herein. Notwithstanding any provision of this Section 8.1(a) to the contrary, a Party may disclose such portion of the Confidential Information relating to the other Party to the extent, but only to the extent, the disclosing Party reasonably believes that such disclosure is required by judicial or administrative process or by other laws or pursuant to any listing agreement with any national securities exchange to which a Party is subject; provided that, if permissible under laws and practicable, the disclosing Party first notifies the other Party hereto of such requirement and allows such Party a reasonable opportunity to seek a protective order or other appropriate remedy to prevent such disclosure. The Parties acknowledge that money damages would not be a sufficient remedy for any breach of the provisions of this Section 8.1(a) and that the non-breaching Party shall be entitled to equitable relief in a court of law in the event of, or to prevent, a breach or threatened breach of this Section 8.1(a).
14
(b) Upon the termination of this Agreement, except to the extent otherwise required by Laws and/or its internal policies and procedures, each Party hereto shall, at its election, promptly return to the other Party or destroy all Confidential Information of the other received under or pursuant to the performance of this Agreement (including, to the extent practicable, all copies (in any and all media) and summaries thereof) that is within such Party’s or its Affiliates’ or its or their Representatives’ possession, power, custody or control; provided that, neither Party hereto nor such Party’s Affiliates or its or their Representatives shall be required to delete Confidential Information from back-up, archival electronic storage; provided, further, that any Confidential Information retained by such Party or its Affiliates shall continue to be subject to Section 8.1(a). Promptly upon the request of a Party, the other Party shall confirm in writing to such first Party that it has complied with this Section 8.1(b).
8.2 Access to Information. Subject to applicable laws and the confidentiality provisions of Section 8.1, with respect to any Service during the term of such Service (unless otherwise provided on Schedule A, Schedule B or any Statement of Work, as applicable), each Party shall, and shall cause its Affiliates to, upon reasonable advance notice, afford the other Party and its Representatives reasonable access, during normal business hours, to the employees, properties, systems, books and records and other documents that are reasonably requested in connection with the provision and receipt of such Service hereunder; provided that, any such access shall not unreasonably interfere with the conduct of the business of the Party providing such access; and provided, further, that in the event any Party reasonably determines that affording any such access to the other Party would be commercially detrimental in any material respect or violate any applicable laws or agreement to which such Party or any Affiliate thereof is a Party, or waive any attorney-client privilege, work-product doctrine or similar privilege applicable to such Party or any Affiliate thereof, the Parties shall use commercially reasonable efforts to permit the compliance with such request in a manner that avoids any such harm or consequence.
8.3 Data Transfer. Each Party shall (a) comply with all Data Protection Laws with respect to the collection, use, transfer, storage, destruction, aggregation or other use of Personal Data (as defined in the applicable Data Protection Laws, collectively, “Personal Data”) in connection with its activities under or in connection with this Agreement, (b) implement appropriate and reasonable security processes and controls in connection with its activities under or in connection with this Agreement so as to protect the security and privacy of Personal Data in accordance with Data Protection Laws, and (c) take such steps as necessary to comply with Data Protection Laws, including entering into relevant supplemental documentation as may be required under applicable Data Protection Laws, to permit such Party to disclose, transfer, use or process Personal Data to the other Party and to permit the other Party to use, process and disclose such Personal Data in accordance with this Agreement. As used herein, “Data Protection Laws” means any applicable law, rule, regulation, ordinance, directive, interpretation, judgment, or decision of any governmental authority in relation to data protection, privacy, restrictions on, or requirements in respect of, the processing of Personal Data of any kind, including the Health Insurance Portability and Accountability Act of 1996 (as amended by the Health Information Technology for Economic and Clinical Health Act) (HIPAA), the General Data Protection Regulation (Regulation (EU) 2016/679) (GDPR), and any equivalent applicable Laws in any other jurisdiction, as any of the foregoing may be amended from time to time.
15
8.4 Employment and Labor Matters. All employment and labor matters relating to employees of Miragene (including, without limitation, employees involved in the provision of Services to IMA) shall be within the exclusive control of Miragene, and IMA shall not take any action affecting such matters. Nothing in this Agreement is intended to transfer the employment of employees engaged in the provision of any Service from Miragene to IMA. All employees and Representatives of Miragene and any of its Affiliates will be deemed for all compensation, employee benefits, Tax and social security contribution purposes to be employees or Representatives of Miragene or its Affiliates (or their subcontractors) and not employees or Representatives of IMA or any of its Affiliates. In providing the Services, such employees and Representatives of Miragene and its Affiliates (or their subcontractors) will be under the direction, control and supervision of Miragene or its Affiliates (or their subcontractors) and not of IMA or its Affiliates.
ARTICLE 9
MISCELLANEOUS
9.1 No Agency; Independent Contractor Status. Nothing in this Agreement shall constitute or be deemed to constitute a partnership or joint venture between the Parties hereto or constitute or be deemed to constitute any Party the agent or employee of the other Party for any purpose whatsoever and neither Party shall have authority or power to bind the other or to contract in the name of, or create a liability against, the other in any way or for any purpose. The Parties hereto acknowledge and agree that the other Party is an independent contractor in the performance of each and every part of this Agreement and nothing herein shall be construed to be inconsistent with this status. Each Party shall have the authority to select the means, methods and manner by which any Service is performed.
9.2 Force Majeure.
(a) For purposes of this Section 9.2, “force majeure” means an event beyond the reasonable control of either Party, which by its nature could not have been foreseen by such Party, or, if it could have been foreseen, was unavoidable, and includes without limitation, acts of God, storms, floods, riots, fires, sabotage, civil commotion or civil unrest, interference by civil or military authorities, threat, declaration, continuation, escalation or acts of war (declared or undeclared) or acts of terrorism, cyber-attacks, embargo, strike, walkout, lockout or other labor trouble or shortage, and acts, omissions or delays in acting by any governmental body or the other Party.
16
(b) Without limiting the generality of Section 6.4(a), a Performing Party shall not be liable to the Recipient Party or any other person for interruption of service, any delays or failure to fulfill any obligations under this Agreement, so long as and to the extent the interruption, delay or fulfillment of such obligation is prevented, frustrated, hindered, or delayed as a consequence of circumstances of force majeure; provided that, the Performing Party shall have used commercially reasonable efforts to minimize to the extent practicable the effect of force majeure on its obligations hereunder; provided, further, that nothing in this Section 9.2 shall be construed to require the settlement of any strike, walkout, lockout or other labor dispute on terms which, in the reasonable judgment of the Performing Party, are contrary to its interests. It is understood that the settlement of a strike, walkout, lockout or other labor dispute will be entirely within the discretion of the Performing Party. The Performing Party shall use commercially reasonable efforts to promptly notify the Recipient Party upon learning of the occurrence of such event. In the event the Performing Party is unable to provide any Service due to a force majeure, (a) the Parties shall agree in good faith on a reasonable reduction to the fees owed pursuant to this Agreement for the time period in which the applicable Service is not provided and (b) if the Recipient Party so requests, the applicable term of the Service so interrupted or suspended shall be extended for a period of time equal to the time lost by reason of such interruption or suspension.
9.3 Entire Agreement. This Agreement (including Schedule A and Schedule B, each constituting a part of this Agreement), any Statement of Work and any other writing signed by the Parties that specifically references this Agreement constitute the entire agreement between the Parties hereto with respect to the subject matter hereof and thereof and supersede all prior agreements and understandings, both oral and written, between the Parties hereto with respect to the subject matter hereof and thereof.
9.4 Information. Subject to applicable laws and privileges, each Party hereto covenants and agrees to provide the other Party with all information regarding itself and transactions under this Agreement that the other Party reasonably believes are required to comply with all applicable laws, including, but not limited to, securities laws and regulations.
9.5 Notices. Any notice shall be in writing, shall refer specifically to this Agreement and shall be deemed given only if delivered by hand or sent by electronic mail (with transmission confirmed) or by internationally recognized overnight delivery service that maintains records of delivery, addressed to the Parties at their respective addresses specified below or to such other address as the Party to whom notice is to be given may have provided to the other Party at least ten calendar days’ prior to such address taking effect in accordance with this Section 9.5. Such notice shall be deemed to have been given as of the date delivered by hand or internationally recognized overnight delivery service (with receipt confirmed by telephone or email). Any notice delivered by electronic mail shall be confirmed by a hard copy delivered as soon as practicable thereafter:
if to IMA, to:
Inmagene Biopharmaceuticals
12526 High Bluff Drive, Suite 345
San Diego, CA 92130, USA
Attention: Legal
Email: legal@inmagenebio.com
17
if to Miragene, to:
Miragene Inc
12526 High Bluff Drive, Suite 345
San Diego, CA 92130, USA
Attention: Jonathan Jian Wang
Email: [***]
and in each case, with a copy (which shall not constitute notice) to:
Cooley LLP
3175 Hanover Street
Palo Alto, CA 94304
Attention: Lila Hope, Ph.D.
Email: lhope@cooley.com
or such other address or email as such Party may hereafter specify for the purpose by notice to the other Parties hereto. All such notices, requests and other communications shall be deemed received on the date of receipt thereof if received prior to 5:00 p.m. in the place of receipt and such day is a business day in the place of receipt. Otherwise, any such notice, request or communication shall be deemed not to have been received until the next succeeding business day in the place of receipt.
9.6 Governing law. This Agreement shall be governed by and construed in accordance with the laws of the State of Delaware, without giving effect to any conflicts or choice of law rule or principle (whether of the State of Delaware or any other jurisdiction) that might otherwise refer construction or interpretation of this Agreement to the substantive law of another jurisdiction.
9.7 Jurisdiction. Subject to Section 9.13 the Parties hereby irrevocably and unconditionally consent to the exclusive jurisdiction of the Court of Chancery of the State of Delaware (or, only if the Court of Chancery of the State of Delaware declines to accept or does not have jurisdiction over a particular matter, any federal or other state court sitting in New Castle County within the State of Delaware) for any action, suit or proceeding (other than appeals therefrom) arising out of or relating to this Agreement, and agree not to commence any action, suit or proceeding (other than appeals therefrom) related thereto except in such courts.
9.8 Service. Each Party further agrees that service of any process, summons, notice or document by registered mail to its address set forth in Section 9.5 shall be effective service of process for any action, suit or proceeding brought against it under this Agreement in any such court.
9.9 Waiver Of Jury Trial. Each Party hereby waives, to the fullest extent permitted by applicable law, any right it may have to a trial by jury in respect of any action, suit or proceeding arising out of this Agreement. Each Party (a) certifies that no Representative of any other Party has represented, expressly or otherwise, that such Party would not, in the event of any action, suit or proceeding, seek to enforce the foregoing waiver and (b) acknowledges that it and the other Party have been induced to enter into this Agreement by, among other things, the mutual waiver and certifications in this Section 9.9.
18
9.10 Severability. If any provision of this Agreement is held to be illegal, invalid or unenforceable under any present or future law, and if the rights or obligations of either Party under this Agreement will not be materially and adversely affected thereby, (a) such provision shall be fully severable, (b) this Agreement shall be construed and enforced as if such illegal, invalid or unenforceable provision had never comprised a part hereof, (c) the remaining provisions of this Agreement shall remain in full force and effect and shall not be affected by the illegal, invalid or unenforceable provision or by its severance herefrom and (d) in lieu of such illegal, invalid or unenforceable provision, there shall be added automatically as a part of this Agreement a legal, valid and enforceable provision as similar in terms to such illegal, invalid or unenforceable provision as may be possible and reasonably acceptable to the Parties.
9.11 Amendments and Waivers.
(a) This Agreement (including Schedule A and Schedule B hereto) may not be modified, amended, altered or supplemented except upon the execution and delivery of a written agreement executed by both Parties.
(b) Any term or condition of this Agreement may be waived at any time by the Party that is entitled to the benefit thereof, but no such waiver shall be effective unless set forth in a written instrument duly executed by or on behalf of the Party waiving such term or condition. The waiver by either Party of any right hereunder or of the failure to perform or of a breach by the other Party shall not be deemed a waiver of any other right hereunder or of any other breach or failure by said other Party whether of a similar nature or otherwise. The rights and remedies provided herein are cumulative and do not exclude any other right or remedy provided by applicable law or otherwise available, and the exercise by a Party of any one right or remedy will not preclude the exercise of any other right or remedy, except as expressly set forth herein.
9.12 Successors and Assigns. Neither this Agreement nor either Party’s rights or obligations hereunder may be assigned or delegated by such Party without the prior written consent of the other Party, and any attempted assignment or delegation of this Agreement or any of such rights or obligations by either Party without the prior written consent of the other Party shall be void and of no effect; provided, however, that either Party may assign or delegate any or all of its rights or obligations hereunder to an Affiliate without the prior written consent of the other Party. Subject to the preceding sentence, this Agreement will be binding upon, inure to the benefit of, and be enforceable by, the Parties and their respective successors and permitted assigns.
9.13 Specific Performance. The Parties agree that irreparable damage would occur in the event that any of the provisions of this Agreement were not performed in accordance with their specific terms or were otherwise breached, and that money damages or other legal remedies would not be an adequate remedy for any such damages. It is accordingly agreed that the Parties shall be entitled to an injunction or injunctions to prevent breaches or threatened breaches, or to enforce compliance with, the covenants and obligations of this Agreement and to enforce specifically the terms and provisions of this Agreement in any court of the United States or any state having jurisdiction, this being in addition to any other remedy to which they are entitled at law or in equity. Each Party hereby waives (a) any requirement that the other Party post a bond or other security as a condition for obtaining any such relief, and (b) any defenses in any action for specific performance, including the defense that a remedy at law would be adequate.
19
9.14 Counterparts. This Agreement may be executed in any number of counterparts, and each such counterpart hereof shall be deemed to be an original instrument, but all such counterparts together shall constitute but one agreement. Delivery of an executed counterpart of a signature page of this Agreement by portable document format (PDF) or other electronic transmission shall be effective as delivery of a manually executed original counterpart of this Agreement.
9.15 No Benefit to Third Parties. The covenants and agreements set forth in this Agreement are for the sole benefit of the Parties hereto and their successors and permitted assigns, and, they shall not be construed as conferring any rights or remedies of any nature whatsoever under or by reason of this Agreement on any other persons.
9.16 Further Assurances. Each Party covenants and agrees that, without any additional consideration, it shall execute and deliver any further legal instruments and perform any acts that are or may become necessary to effectuate this Agreement.
[Remainder of page intentionally left blank]
20
IN WITNESS WHEREOF, the Parties hereto have caused this Agreement to be duly executed by their respective authorized officers as of the date first above written.
| MIRAGENE INC | ||
| By: | /s/ Jonathan Jian Wang |
|
| Name: | Jonathan Jian Wang | |
| Title: | Director | |
| INMAGENE BIOPHARMACEUTICALS | ||
| By: | /s/ Jonathan Jian Wang |
|
| Name: | Jonathan Jian Wang | |
| Title: | Chief Executive Officer | |
[SIGNATURE PAGE TO TRANSITION SERVICES AGREEMENT]
SCHEDULE A
MIRAGENE SERVICES
[***]
SCHEDULE B
IMA SERVICES
[***]
SCHEDULE C
FORM - STATEMENT OF WORK
Statement of Work No. ___
This Statement of Work No. ____ (this “Statement of Work”), effective as of ________________, is entered into by and between MIRAGENE INC (“Miragene”) and INMAGENE BIOPHARMACEUTICALS (“IMA”). This Statement of Work is a part of and subject to the terms and conditions set forth in the Transition Services Agreement, dated as of [•], 2025 between Miragene and IMA, as may be amended from time to time.
| 1. | Scope of Work. |
| 2. | Service Period. |
| 3. | Management Representatives. |
| 4. | Fees and Charges. |
| 5. | Other Provisions. |
Exhibit 10.8
REGISTRATION RIGHTS AGREEMENT
This REGISTRATION RIGHTS AGREEMENT (the “Agreement”) is made as of July 25, 2025 by and among ImageneBio, Inc. (formerly Ikena Oncology, Inc.), (the “Company”), and the several purchasers signatory hereto (each, a “Purchaser” and collectively, the “Purchasers”).
RECITALS
WHEREAS, the Company and the Purchasers are parties to a Subscription Agreement, dated as of December 23, 2024 (the “Purchase Agreement”), pursuant to which the Purchasers have committed to purchase shares of common stock of the Company; and
WHEREAS, in connection with the consummation of the transactions contemplated by the Purchase Agreement, and pursuant to the terms of the Purchase Agreement, the parties desire to enter into this Agreement in order to grant certain rights to the Purchasers as set forth below.
NOW, THEREFORE, in consideration of the covenants and promises set forth herein, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties hereby agree as follows:
AGREEMENT
Section 1. Certain Definitions. Unless the context otherwise requires, the following terms, for all purposes of this Agreement, shall have the meanings specified in this Section 1.
“Affiliate” has the meaning set forth in Rule 12b-2 of the rules and regulations promulgated under the Exchange Act; provided, however, that for purposes of this Agreement, the Purchasers and their Affiliates, on the one hand, and the Company and its Affiliates, on the other, shall not be deemed to be “Affiliates” of one another.
“Allowed Delay” has the meaning set forth in Section 2.1(b)(ii).
“Board” means the board of directors of the Company.
“Business Day” has the meaning ascribed to such term in the Purchase Agreement.
“Closing Date” has the meaning ascribed to such term in the Purchase Agreement.
“Common Stock” means shares of the common stock, par value $0.001 per share, of the Company.
“Effective Date” means the date that a Registration Statement filed pursuant to Section 2.1(a) is first declared effective by the SEC.
“Effectiveness Deadline” means, with respect to the Resale Registration Statement or New Registration Statement, the forty-fifth (45th) calendar day following the Filing Date for such Resale Registration Statement or New Registration Statement (or, if earlier than such filing date, the Filing Deadline) (or, in the event the SEC reviews and has written comments to the Resale Registration Statement or the New Registration Statement, the ninetieth (90th) calendar day following such Filing Date (or, if earlier than such filing date, the Filing Deadline)); provided, however, that if the Company is notified by the SEC (either orally or in writing, whichever is earlier) that the Resale Registration Statement or the New Registration Statement will not be reviewed or is no longer subject to further review and comments, the Effectiveness Deadline as to such Resale Registration Statement shall be the fifth (5th) Business Day following the date on which the Company is so notified if such date precedes the dates otherwise required above; provided, further, that if the Effectiveness Deadline falls on a Saturday, Sunday or other day that the SEC is closed for business, the Effectiveness Deadline shall be extended to the next Business Day on which the SEC is open for business; provided, further, that if the SEC is closed for operations due to a government shutdown, the Effectiveness Deadline shall be extended by the same amount of days that the SEC remains closed for operations and provided, further, that notwithstanding anything herein to the contrary, if the audited financial statements of any acquired company or other entity or pro forma financial statements that are required by the Securities Act to be included in the Resale Registration Statement or New Registration Statement are unavailable as of the Effectiveness Deadline provided for above, the Effectiveness Deadline shall be delayed until such time as such financial statements are prepared or obtained by the Company, it being understood that such date shall in no event extend beyond the one hundred twentieth (120th) calendar day following the Closing Date.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations of the SEC promulgated thereunder.
“Filing Date” means the date the applicable Resale Registration Statement or New Registration Statement is first filed with the SEC.
1
“Filing Deadline” means, (i) with respect to the Resale Registration Statement, forty-five (45) days following the Closing Date and (ii) with respect to a New Registration Statement, the later of (A) forty-five (45) days following the Closing Date and (B) forty-five (45) days after the Company determines that filing a New Registration Statement is necessary pursuant to Section 2.1(a).
“FINRA” means the Financial Industry Regulatory Authority.
“Form S-1” means a registration statement on Form S-1 under the Securities Act as in effect on the date hereof or any successor or similar registration form under the Securities Act subsequently adopted by the SEC.
“Form S-3” means a registration statement on Form S-3 under the Securities Act as in effect on the date hereof or any successor or similar registration form under the Securities Act subsequently adopted by the SEC that permits inclusion or incorporation of substantial information by reference to other documents filed by the Company with the SEC.
“Free Writing Prospectus” means an issuer free writing prospectus, as defined in Rule 433 under the Securities Act, relating to an offer of Registrable Securities.
“Holder” means any Purchaser owning or having the right to acquire Registrable Securities.
“Liquidated Damages” has the meaning set forth in Section 2.1(c).
“National Exchange” means each of the following, together with any successor thereto: the NYSE American, The New York Stock Exchange, the Nasdaq Global Market, the Nasdaq Global Select Market and the Nasdaq Capital Market.
“New Registration Statement” has the meaning set forth in Section 2.1(a).
“Participating Holder” means with respect to any registration, any Holder of Registrable Securities covered by the applicable Registration Statement.
“Person” has the meaning ascribed to such term in the Purchase Agreement.
“Prospectus” means the prospectus included in any Registration Statement, all amendments and supplements to such prospectus, including pre- and post-effective amendments to such Registration Statement, and all other material incorporated by reference in such prospectus.
“Register,” “registered” and “registration” refer to a registration effected by preparing and filing a registration statement in compliance with the Securities Act, and the declaration or ordering of effectiveness of such registration statement or document.
“Registrable Securities” means the Shares and any Common Stock issued as (or issuable upon the conversion or exercise of any warrant, right or other security which is issued as) a dividend or other distribution with respect to, or in exchange for or in replacement of, such Shares, provided, that the Holder has completed and delivered to the Company a selling stockholder questionnaire and any other information regarding the Holder and the distribution of the Registrable Securities as the Company may, from time to time, reasonably request for inclusion in a Registration Statement pursuant to applicable law. Notwithstanding the foregoing, Shares or any such Common Stock, as applicable, shall cease to be Registrable Securities for all purposes hereunder upon the earliest to occur of the following: (A) the sale by any Person of such Shares or any such Common Stock, as applicable, pursuant to a registration statement under the Securities Act or under Rule 144 (in which case, only such Shares or any such Common Stock, as applicable, sold shall cease to be Registrable Securities), (B) such Shares or any such Common Stock, as applicable, becoming eligible for sale by the Holder pursuant to Rule 144 without restriction and without the requirement for the Company to be in compliance with the current public information requirement under Rule 144(c)(1) (or Rule 144(i)(2), if applicable) or (C) such Shares or any shares of Common Stock cease to be outstanding.
“Registration Expenses” has the meaning set forth in Section 2.2 .
“Registration Statement” means any registration statement of the Company that covers Registrable Securities pursuant to the provisions of this Agreement filed with, or to be filed with, the SEC under the rules and regulations promulgated under the Securities Act, including the related Prospectus, amendments and supplements to such registration statement, including pre- and post-effective amendments, and all exhibits and all material incorporated by reference in such registration statement.
“Remainder Registration Statement” has the meaning set forth in Section 2.1.
“Resale Registration Statement” has the meaning set forth in Section 2.1(a).
“Rule 144” means Rule 144 as promulgated by the SEC under the Securities Act, as such rule may be amended from time to time, or any similar successor rule that may be promulgated by the SEC.
2
“Rule 145” means Rule 145 promulgated by the SEC pursuant to the Securities Act, as such rule may be amended from time to time, or any similar rule or regulation hereafter adopted by the SEC having substantially the same effect as such rule.
“SEC” means the Securities and Exchange Commission or any other federal agency at the time administering the Securities Act.
“SEC Guidance” means any publicly available written guidance, comments, requirements or requests of the SEC staff under the Securities Act.
“Securities Act” means the Securities Act of 1933, as amended, or any similar successor federal statute and the rules and regulations thereunder, all as the same shall be in effect from time to time.
“Shares” means the shares of Common Stock issued pursuant to the Purchase Agreement.
“Transaction Documents” means this Agreement and the Purchase Agreement, all exhibits and schedules thereto and hereto and any other documents or agreement executed in connection with the transactions contemplated hereunder or thereunder.
Section 2. Registration Rights.
(a) Registration Statements. On or prior to the date of the Filing Deadline, the Company shall prepare and file with the SEC a Registration Statement on Form S-1 (or, if Form S-3 is then available to the Company, on Form S-3) for the resale of the Registrable Securities pursuant to an offering to be made on a continuous basis pursuant to Rule 415 under the Securities Act (the “Resale Registration Statement”). Such Resale Registration Statement shall, subject to the limitations of Form S-1 (or Form S-3, if available), include the aggregate amount of Registrable Securities to be registered therein and shall contain (except if otherwise required pursuant to written comments received from the SEC upon a review of such Resale Registration Statement) the “Plan of Distribution” in substantially the form attached hereto as Annex A. To the extent the staff of the SEC does not permit all of the Registrable Securities to be registered on the Resale Registration Statement filed pursuant to this Section 2.1(a) or for any other reason any Registrable Securities are not then included in a Registration Statement filed under this Agreement, the Company shall (i) inform each of the Holders thereof and use its commercially reasonable efforts to file amendments to the Resale Registration Statement as required by the SEC and/or (ii) withdraw the Resale Registration Statement and file a new registration statement (a “New Registration Statement”), in either case covering the maximum number of Registrable Securities permitted to be registered by the SEC, on Form S-1 (or Form S-3, if available) or such other form available to register for resale the Registrable Securities as a secondary offering; provided, however, that prior to filing such amendment or New Registration Statement, the Company shall be obligated to use its commercially reasonable efforts to advocate with the SEC for the registration of all of the Registrable Securities in accordance with the SEC Guidance, including without limitation, the Compliance and Disclosure Interpretation Securities Act Rules No. 612.09. Notwithstanding any other provision of this Agreement and subject to the payment of any liquidated damages that may be required to be paid pursuant to Section 2.1(c), if any SEC Guidance sets forth a limitation of the number of Registrable Securities permitted to be registered on a particular Registration Statement as a secondary offering (and notwithstanding that the Company used commercially reasonable efforts to advocate with the SEC for the registration of all or a greater number of Registrable Securities), unless otherwise directed in writing by a Holder as to its Registrable Securities, the number of Registrable Securities to be registered on such Registration Statement will first be reduced by Registrable Securities not acquired pursuant to the Purchase Agreement, and second by Registrable Securities represented by Shares (applied, in the case that some Shares may be registered, to the Holders on a pro rata basis based on the total number of unregistered Shares held by such Holders, subject to a determination by the SEC that certain Holders must be excluded or must be reduced first based on the number of Shares held by such Holders). In the event the Company amends the Resale Registration Statement or files a New Registration Statement, as the case may be, under clauses (i) or (ii) above, the Company will use its commercially reasonable efforts to file with the SEC, as promptly as allowed by SEC or SEC Guidance provided to the Company or to registrants of securities in general, one or more Registration Statements on Form S-1 (or Form S-3, if available) or such other form available to register for resale those Registrable Securities that were not registered for resale on the Resale Registration Statement, as amended, or the New Registration Statement (the “Remainder Registration Statement”) and use commercially reasonable efforts to have such Remainder Registration Statement(s) to become effective as promptly as practicable after the filing thereof, but in any event no later than ninety (90) calendar days after the filing of such Remainder Registration Statement (the “Additional Effectiveness Deadline”); provided, however, that if the Company is notified by the SEC (either orally or in writing, whichever is earlier) that the Remainder Registration Statement will not be reviewed or is no longer subject to further review and comments, the Additional Effectiveness Deadline as to such Remainder Registration Statement shall be the fifth (5th) Business Day following the date on which the Company is so notified if such date precedes the dates otherwise required above; provided further, that if the Additional Effectiveness Deadline falls on a Saturday, Sunday or other day that the SEC is closed for business, the Additional Effectiveness Deadline shall be extended to the next Business Day on which the SEC is open for business. In no event shall any Participating Holder be identified as a statutory underwriter in the Registration Statement unless in response to a comment or request from the staff of the SEC or another regulatory agency; provided, however, that if the SEC requests that a Participating Holder be identified as a statutory underwriter in the Registration Statement, such Holder will have an opportunity to withdraw from the Registration Statement.
3
(b) Effectiveness.
(i) The Company shall use commercially reasonable efforts to have the Resale Registration Statement or New Registration Statement declared effective as soon as practicable but in no event later than the Effectiveness Deadline (including filing with the SEC a request for acceleration of effectiveness in accordance with Rule 461 promulgated under the Securities Act), and shall use its commercially reasonable efforts to keep the Resale Registration Statement or New Registration Statement continuously effective under the Securities Act until the earlier of (A) such time as all of the Registrable Securities covered by such Registration Statement have been publicly sold by the Holders or (B) the date that all Registrable Securities covered by such Registration Statement may be sold by non-affiliates without volume or manner-of-sale restrictions pursuant to Rule 144, without the requirement for the Company to be in compliance with the current public information requirement under Rule 144(c)(1) (or Rule 144(i)(2), if applicable) as determined by counsel to the Company pursuant to a written opinion letter to such effect, addressed and reasonably acceptable to the Company’s transfer agent (the “Effectiveness Period”); provided that the Company will not be obligated to update the Registration Statement and no sales may be made under the applicable Registration Statement during any Allowed Delay (as defined below) of which the Holders have received notice. The Company shall notify the Purchasers by e-mail as promptly as reasonably practicable after any Registration Statement is declared effective and shall simultaneously provide the Purchasers with copies of any related Prospectus to be used in connection with the sale or other disposition of the securities covered thereby.
(ii) On no more than two (2) occasions and for not more than a total of thirty (30) consecutive days or a total of not more than ninety (90) days, in each case, in any twelve (12) month period, the Company may suspend the use of any Prospectus included in any Registration Statement contemplated by this Section 2 in the event that the Company determines, in good faith and upon the advice of legal counsel, that such suspension is necessary to (A) delay the disclosure of material non-public information concerning the Company, which the Company has a bona fide business purpose for keeping confidential and the non-disclosure of which in the Registration Statement would be expected, in the reasonable determination of the Board, upon the advice of legal counsel, to cause the Registration Statement to comply with applicable disclosure requirements or (B) amend or supplement the Registration Statement or the related Prospectus so that such Registration Statement or Prospectus shall not include an untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary to make the statements therein, in the case of the Prospectus in light of the circumstances under which they were made, not misleading (an “Allowed Delay”); provided, that the Company shall promptly (1) notify each Purchaser in writing of the commencement of and the reasons for an Allowed Delay, but shall not (without the prior written consent of a Purchaser) disclose to such Purchaser any material non-public information giving rise to an Allowed Delay, (2) advise the Purchasers in writing to cease all sales under the Registration Statement until the end of the Allowed Delay and (3) use commercially reasonable efforts to terminate an Allowed Delay as promptly as reasonably practicable.
(c) If: (i) the Resale Registration Statement is not filed with the SEC on or prior to the Filing Deadline, (ii) the Resale Registration Statement or the New Registration Statement, as applicable, is not declared effective by the SEC (or otherwise does not become effective) for any reason on or prior to the Effectiveness Deadline, (iii) after its Effective Date and other than for an Allowed Delay, (A) such Registration Statement ceases for any reason (including without limitation by reason of a stop order, or the Company’s failure to update the Registration Statement), to remain continuously effective as to all Registrable Securities included in such Registration Statement or (B) the Company suspends the use of the Prospectus contained in the Registration Statement, (iv) an Allowed Delay applicable to a required Registration Statement exceeds the length of the Allowed Delay, (v) the Company fails to satisfy the current public information requirement pursuant to Rule 144(c)(1) (or Rule 144(i)(2), if applicable) as a result of which the Holders who are not affiliates are unable to sell Registrable Securities without restriction under Rule 144 (or any successor thereto) and fails to cure any such failure to satisfy the requirements of Rule 144(c)(1) (or Rule 144(i)(2), if applicable) within 10 Business Days following the date upon which the Holder notifies the Company in writing that such Holder is unable to sell Registrable Securities as a result thereof, or (vi) following the date that is one (1) year following the Closing Date, the Common Stock is not listed on a National Exchange, or trading of the Common Stock is suspended or halted for more than three (3) consecutive Business Days (any such failure or breach in clauses (i) through (vi) above being referred to as an “Event,” and, for purposes of clauses (i), (ii), (iii), (v) or (vi), the date on which such Event occurs, or for purposes of clause (iv) the date on which such Allowed Delay is exceeded, being referred to as an “Event Date”), then in addition to any other rights the Holders may have hereunder or under applicable law, on each such Event Date and on each monthly anniversary of each such Event Date (if the applicable Event shall not have been cured by such date) until the earlier of (1) the applicable Event is cured or (2) the Registrable Securities are eligible for resale pursuant to Rule 144 without manner of sale or volume restrictions and without the requirement for the Company to be in compliance with the current public information requirement under Rule 144(c)(1) (or Rule 144(i)(2), if applicable), the Company shall pay to each Holder an amount in cash, as partial liquidated damages and not as a penalty (“Liquidated Damages”), equal to one percent (1.0%) of the aggregate purchase price paid by such Holder pursuant to the Purchase Agreement for any unregistered Registrable Securities then held by such Holder on the Event Date. The parties agree that (1) notwithstanding anything to the contrary herein or in the Purchase Agreement, no Liquidated Damages shall be payable with respect to any period after the expiration of the Effectiveness Period (it being understood that this sentence shall not relieve the Company of any Liquidated Damages accruing prior to the Effectiveness Deadline) and in no event shall, the aggregate amount of Liquidated Damages payable to a Holder exceed, in the aggregate, five percent (5.0%) of the aggregate purchase price paid by such Holder pursuant to the Purchase Agreement and (2) in no event shall the Company be liable in any thirty (30) day period for Liquidated Damages under this Agreement in excess of one percent (1.0%) of the aggregate purchase price paid by the Holders pursuant to the Purchase Agreement.
4
If the Company fails to pay any Liquidated Damages pursuant to this Section 2.1(c) in full within ten (10) Business Days after the date payable, the Company will pay interest thereon at a rate of one percent (1.0%) per month (or such lesser maximum amount that is permitted to be paid by applicable law) to the Holder, accruing daily from the date such Liquidated Damages are due until such amounts, plus all such interest thereon, are paid in full. The Liquidated Damages pursuant to the terms hereof shall apply on a daily pro-rata basis for any portion of a month prior to the cure of an Event, except in the case of the first Event Date. The Company shall not be liable for Liquidated Damages under this Agreement as to any Registrable Securities which are not permitted by the SEC to be included in a Registration Statement due solely to SEC Guidance from the time that it is determined that such Registrable Securities are not permitted to be registered until such time as the provisions of this Agreement as to the Remainder Registration Statements required to be filed hereunder are triggered, in which case the provisions of this Section 2.1(c) shall once again apply, if applicable. In such case, the Liquidated Damages shall be calculated to only apply to the percentage of Registrable Securities which are permitted in accordance with SEC Guidance to be included in such Registration Statement. The Effectiveness Deadline for a Registration Statement shall be extended without default or Liquidated Damages hereunder in the event that the Company’s failure to obtain the effectiveness of such Registration Statement on a timely basis results from the failure of a Purchaser to timely provide the Company with information requested by the Company and necessary to complete the Registration Statement in accordance with the requirements of the Securities Act (in which the Effectiveness Deadline would be extended with respect to Registrable Securities held by such Purchaser).
(d) In the event that Form S-3 is not available for the registration of the resale of Registrable Securities hereunder, the Company shall (i) register the resale of the Registrable Securities on Form S-1 or another appropriate form reasonably acceptable to the Holder and (ii) undertake to register the Registrable Securities on Form S-3 promptly after such form is available, provided that the Company shall maintain the effectiveness of the Registration Statement then in effect until such time as a Registration Statement on Form S-3 covering the Registrable Securities has been declared effective by the SEC.
2.2. Expenses. All expenses incident to the Company’s performance of or compliance with this Agreement shall be paid by the Company, other than any transfer tax or underwriting discounts or commissions deducted from the proceeds in respect of any sale of the Registrable Securities, including (a) all registration and filing fees, and any other fees and expenses associated with filings required to be made with the SEC, FINRA or any other regulatory authority, (b) all fees and expenses in connection with compliance with any securities or “Blue Sky” laws (including fees and disbursements of counsel for the underwriters in connection with “Blue Sky” qualifications of the Registrable Securities), (c) all printing, duplicating, word processing, messenger, telephone, facsimile and delivery expenses (including expenses of printing certificates for the Registrable Securities in a form eligible for deposit with The Depository Trust Company and of printing Prospectuses and Free Writing Prospectuses), (d) all fees and disbursements of counsel for the Company and of all independent certified public accountants of the Company (including the expenses of any special audit and cold comfort letters required by or incident to such performance), (e) Securities Act liability insurance or similar insurance if the Company so desires or the underwriters so require in accordance with then-customary underwriting practice, (f) all fees and expenses incurred in connection with the listing of Registrable Securities on any securities exchange or quotation of the Registrable Securities on any inter-dealer quotation system, (g) any reasonable fees and disbursements of underwriters customarily paid by issuers of securities, (h) all fees and expenses of any special experts or other Persons retained by the Company in connection with any registration, and (i) all of the Company’s internal expenses (including all salaries and expenses of its officers and employees performing legal or accounting duties). All such expenses are referred to herein as “Registration Expenses.”
2.3. Company Obligations. The Company will use commercially reasonable efforts to effect the registration of the Registrable Securities in accordance with the terms hereof, and pursuant thereto the Company will:
(a) prepare the required Registration Statement including all exhibits and financial statements required under the Securities Act to be filed therewith, and before filing a Registration Statement, Prospectus or any Free Writing Prospectus, or any amendments or supplements thereto, (i) provide copies to permit each Participating Holder to review copies of all documents prepared to be filed and a reasonable opportunity to provide comments thereon (it being acknowledged and agreed that if a Participating Holder does not object to or comment on the aforementioned documents, then the Participating Holder shall be deemed to have consented to and approved the use of such documents), provided that, with respect to the filing of the initial Registration Statement, the Company shall provide such Registration Statement for review by the Participating Holders at least five (5) Business Days prior to filing;
(b) if the Registration Statement is subject to review by the SEC, use its commercially reasonable efforts to promptly respond to all comments, diligently pursue resolution of any comments to the satisfaction of the SEC and file all amendments and supplements to such Registration Statement as may be required to respond to comments from the SEC and otherwise to enable such Registration Statement to be declared effective; (c) file with the SEC a Registration Statement relating to the Registrable Securities including all exhibits and financial statements required by the SEC to be filed therewith, and use commercially reasonable efforts to cause such Registration Statement to become effective under the Securities Act;
5
(d) prepare and file with the SEC such pre- and post-effective amendments to such Registration Statement, supplements to the Prospectus and such amendments or supplements to any Free Writing Prospectus as may be necessary to keep such registration effective for the period of time required by this Agreement, and comply with provisions of the applicable securities laws with respect to the sale or other disposition of all securities covered by such Registration Statement during such period in accordance with the intended method or methods of disposition by the sellers thereof set forth in such Registration Statement;
(e) notify the Participating Holders by facsimile or email as promptly as practicable after notice thereof is received by the Company (i) when the applicable Registration Statement or any amendment thereto has been filed or becomes effective, and when the applicable Prospectus or Free Writing Prospectus or any amendment or supplement thereto has been filed (provided, that the Company will not have any obligation to provide any document pursuant to this clause that is available on the SEC’s EDGAR website), (ii) of any written comments by the SEC or any request by the SEC for amendments or supplements to such Registration Statement, Prospectus or Free Writing Prospectus or for additional information, (iii) of the issuance by the SEC of any stop order suspending the effectiveness of such Registration Statement or any order by the SEC preventing or suspending the use of any preliminary or final Prospectus or any Free Writing Prospectus or the initiation or threatening of any proceedings for such purposes, (iv) of the receipt by the Company of any notification with respect to the suspension of the qualification of the Registrable Securities for offering or sale in any jurisdiction and (v) of the receipt by the Company of any notification with respect to the initiation or threatening of any proceeding for the suspension of the qualification of the Registrable Securities for offering or sale in any jurisdiction;
(f) promptly notify the Participating Holders, at any time prior to the end of the Effectiveness Period, when the Company becomes aware of the happening of any event as a result of which the Registration Statement, the Prospectus included in such Registration Statement (as then in effect) or any Free Writing Prospectus contains any untrue statement of a material fact or omits to state a material fact necessary to make the statements therein (in the case of such Prospectus, any preliminary Prospectus or any Free Writing Prospectus, in light of the circumstances under which they were made) not misleading, when any Free Writing Prospectus includes information that may conflict with the information contained in the Registration Statement, or, if for any other reason it shall be necessary during such time period to amend or supplement such Registration Statement, Prospectus or Free Writing Prospectus in order to comply with the Securities Act and, in either case as promptly as reasonably practicable thereafter, prepare and file with the SEC and furnish without charge to the Participating Holders an amendment or supplement to such Registration Statement, Prospectus or Free Writing Prospectus which shall correct such misstatement or omission or effect such compliance;
(g) promptly incorporate in a Prospectus supplement, Free Writing Prospectus or post-effective amendment to the applicable Registration Statement such information as the Participating Holders agree should be included therein relating to the plan of distribution with respect to such Registrable Securities, and make all required filings of such Prospectus supplement, Free Writing Prospectus or post-effective amendment as soon as reasonably practicable after being notified of the matters to be incorporated in such Prospectus supplement, Free Writing Prospectus or post-effective amendment;
(h) furnish to each Participating Holder, if requested by the Participating Holder, without charge, one (1) conformed copy of the applicable Registration Statement and any amendment or post-effective amendment thereto, including financial statements and schedules, all documents incorporated therein by reference and all exhibits (including those incorporated by reference) (other than any portion thereof which contains information for which the Company has sought confidential treatment), except if such documents are available on the SEC’s EDGAR website;
(i) deliver to each Participating Holder, if requested by the Participating Holder, without charge, as many copies of the applicable Prospectus (including each preliminary Prospectus), any Free Writing Prospectus and any amendment or supplement thereto as such Participating Holder may reasonably request (it being understood that the Company consents to the use of such Prospectus, any Free Writing Prospectus and any amendment or supplement thereto by such Participating Holder in connection with the offering and sale of the Registrable Securities thereby) and such other documents as such Participating Holder may reasonably request in order to facilitate the disposition of the Registrable Securities by such Participating Holder (other than any portion thereof which contains information for which the Company has sought confidential treatment);
(j) on or prior to the date on which the Registration Statement is declared effective, use its commercially reasonable efforts to register or qualify, and cooperate with the Participating Holders and their respective counsel, in connection with the registration or qualification of such Registrable Securities for offer and sale under the securities or “Blue Sky” laws of each state and other jurisdiction of the United States as any Participating Holder or their respective counsel reasonably request in writing and do any and all other acts or things reasonably necessary or advisable to keep such registration or qualification in effect for such period as required by this Agreement, provided that the Company shall not be required to qualify generally to do business in any jurisdiction where it is not then so qualified or to take any action which would subject it to taxation or general service of process in any such jurisdiction where it is not then so subject; (k) use its commercially reasonable efforts to cause the Registrable Securities covered by the Registration Statement to be registered with or approved by such other governmental agencies or authorities as may be necessary to enable the Participating Holders to consummate the disposition of such Registrable Securities;
6
(l) enter into such customary agreements (including underwriting agreements) and take all such other actions as the Participating Holders reasonably request in order to expedite or facilitate the registration and disposition of such Registrable Securities;
(m) for any Participating Holder that is required under applicable securities laws to be described as an “underwriter” in a Registration Statement, obtain for delivery to such Participating Holder (i) an opinion or opinions from counsel for the Company dated the effective date of the Registration Statement or, in the event of an underwritten offering, the date of the closing under the underwriting agreement, and (ii) in the event of any underwritten offering, a “comfort” letter signed by the independent public accountants who have certified the Company’s financial statements included in, or incorporated by reference into, the Registration Statement, on such date or dates as may be required under the underwriting agreement, in each case in customary form, scope and substance, which opinions and auditor comfort letters shall be reasonably satisfactory to such Participating Holders or underwriters, as the case may be, and their respective counsel;
(n) cooperate with each Participating Holder participating in the disposition of such Registrable Securities and their respective counsel in connection with any filings required to be made with FINRA or any other securities regulatory authority;
(o) use its commercially reasonable efforts to comply with all applicable securities laws and, if any Holders are required under applicable securities laws to be described as an “underwriter” in a Registration Statement, make available to its security holders, as soon as reasonably practicable, an earnings statement satisfying the provisions of Section 11(a) of the Securities Act and the rules and regulations promulgated thereunder;
(p) provide and cause to be maintained a transfer agent and registrar for all Registrable Securities covered by the applicable Registration Statement from and after a date not later than the effective date of such Registration Statement;
(q) use commercially reasonable efforts to cause all Registrable Securities covered by the Registration Statement to be listed on each securities exchange on which any of the Common Stock is then listed or quoted and on each inter-dealer quotation system on which any of the Common Stock is then quoted;
(r) if any Participating Holders are required under applicable securities laws to be described as an “underwriter” in a Registration Statement, in connection with such Participating Holders’ due diligence requirements, if any, make available, during normal business hours, for inspection and review by the Participating Holders, advisors to and representatives of the Participating Holders (collectively, “Inspectors”) (who may or may not be affiliated with the Participating Holders and who are reasonably acceptable to the Company), all financial and other records, all SEC Reports (as defined in the Purchase Agreement) and other filings with the SEC, and all other corporate documents and properties of the Company as may be reasonably deemed necessary for the purpose of establishing a due diligence defense to underwriter liability under the Securities Act, and cause the Company’s officers, directors and employees, within a reasonable time period, to supply all such information reasonably requested by the Inspectors in connection with such Registration Statement (including, without limitation, in response to all questions and other inquiries reasonably made or submitted by any of them), prior to and from time to time after the filing and effectiveness of the Registration Statement for the sole purpose of enabling the Inspectors to conduct initial and ongoing due diligence with respect to the Company and the accuracy of such Registration Statement; and
(s) with a view to making available to the Purchasers the benefits of Rule 144 (or its successor rule) and any other rule or regulation of the SEC that may at any time permit the Purchasers to sell shares of Common Stock to the public without registration, the Company covenants and agrees to: (i) make and keep public information available, as those terms are understood and defined in Rule 144, until the earlier of (A) the date as all of the Registrable Securities may be sold without restriction by the Holders thereof pursuant to Rule 144 and without the requirement for the Company to be in compliance with the current public information requirement under Rule 144(c)(1) (or Rule 144(i)(2), if applicable), or any other rule of similar effect or (B) such date as all of the Registrable Securities shall have been resold; (ii) file with the SEC in a timely manner all reports and other documents required of the Company under the Exchange Act; and (iii) furnish to each Purchaser upon request, as long as such Purchaser owns any Registrable Securities, (A) a written statement by the Company that, if true, it has complied with the reporting requirements of the Exchange Act, (B) a copy of the Company’s most recent Annual Report on Form 10-K or Quarterly Report on Form 10-Q, and (C) such other information as may be reasonably requested in order to avail such Purchaser of any rule or regulation of the SEC that permits the selling of any such Registrable Securities without registration.
All such information made available or provided pursuant to this Section 2.3 shall be treated as confidential information and shall not be disclosed by the Participating Holders or Inspectors to any other Person other than, in the case of a Participating Holder, such Participating Holder’s respective officers, directors, employees, accountants, consultants, legal counsel, investment bankers, advisors and authorized agents (collectively, the “Holder Representatives”); provided, that, each such Holder Representative shall be informed that such confidential information is strictly confidential and shall be subject to confidentiality restrictions in favor of the disclosing Participating Holder with respect to the confidential information disclosed by the Participating Holder to such Holder Representative.
7
Notwithstanding anything to the contrary herein, the foregoing restrictions shall not prevent the disclosure by a Participating Holder of any information (x) that is required to be disclosed by order of a court of competent jurisdiction, administrative body or other Governmental Entity (as defined in the Purchase Agreement), or by subpoena, summons or legal process, or by law, rule or regulation or (y) that is publicly available (other than by a breach of such Participating Holder’s or Inspector’s confidentiality obligations to the Company), provided that, to the extent permitted by applicable law, in the event a Participating Holder or Holder Representative is required to make a disclosure pursuant to clause (x) hereof, it shall provide to the Board prompt notice of such disclosure (other than any such disclosure required by any administrative body or other Governmental Entity in the exercise of its regulatory or other oversight authority with respect to such Participating Holder or Holder Representative). The confidentiality obligations herein shall, with respect to any particular Participating Holder, expire on the second (2nd) anniversary of the date on which such Purchaser ceases to hold any Shares.
2.4. Obligations of the Holders.
(a) Each Holder shall furnish in writing to the Company such information regarding itself, the Registrable Securities held by it and the intended method of disposition of the Registrable Securities held by it, as shall be reasonably required to effect the registration of such Registrable Securities and shall execute such documents in connection with such registration as the Company may reasonably request. At least five (5) Business Days prior to the first anticipated filing date of any Registration Statement, the Company shall notify each Holder of the information the Company requires from such Holder if such Holder elects to have any of its Registrable Securities included in the Registration Statement. A Holder shall provide such information to the Company at least three (3) Business Days prior to the first anticipated filing date of such Registration Statement if such Holder elects to have any of its Registrable Securities included in the Registration Statement. Each Holder acknowledges and agrees that the information in the selling shareholder questionnaire or request for further information as described in this Section 2.4(a) will be used by the Company in the preparation of the Registration Statement and hereby consents to the inclusion of such information in the Registration Statement, subject to its right to review the Registration Statement as provided herein. The Company shall not be obligated to file more than one post-effective amendment or supplement in any sixty (60) day period following the date such Registration Statement is declared effective for the purposes of naming Holders as selling security holders who are not named in such Registration Statement at the time of effectiveness.
(b) Each Holder, by its acceptance of the Registrable Securities agrees to cooperate with the Company as reasonably requested by the Company in connection with the preparation and filing of a Registration Statement hereunder, unless such Holder has notified the Company in writing of its election to exclude all of its Registrable Securities from such Registration Statement. The Company may require each selling Holder to furnish the Company a certified statement as to (i) the number of shares of Common Stock beneficially owned by such Holder or any Affiliate thereof, (ii) any FINRA affiliations, (iii) any natural persons who have the power to vote or dispose of the Common Stock and (iv) any other information as may be requested by the SEC, FINRA or any state securities commission.
(c) Each Holder agrees that, upon receipt of any notice from the Company of either (i) the commencement of an Allowed Delay pursuant to Section 2.1(b) or (ii) the happening of an event pursuant to Section 2.3(e) and Section 2.3(f) hereof, such Holder will immediately discontinue disposition of Registrable Securities pursuant to the Registration Statement covering such Registrable Securities, until the Holder is advised by the Company that such dispositions may again be made.
2.5. Indemnification.
(a) Indemnification by the Company. The Company will indemnify and hold harmless each Holder and its officers, directors, members, employees, advisors and agents, successors and assigns, and each other Person, if any, who controls such Holder within the meaning of the Securities Act, against any losses, claims, damages, liabilities and reasonable and documented out-of-pocket expenses (including reasonable and documented out-of-pocket attorney fees) (or actions in respect thereof), joint or several, to which any of them may become subject under the Securities Act or otherwise, insofar as such losses, claims, damages, liabilities or reasonable and documented out-of-pocket expenses (or actions in respect thereof) arise out of or are based upon: (i) any untrue statement or alleged untrue statement of any material fact contained in any Registration Statement, any preliminary Prospectus or final Prospectus, or any amendment or supplement thereof or any omission or alleged omission to state a material fact required to be stated therein or necessary to make the statements therein (in the case of any Prospectus or form of prospectus or supplement thereto, in light of the circumstances under which they were made) not misleading; (ii) any “Blue Sky” application or other document executed by the Company specifically for that purpose or based upon written information furnished by the Company filed in any state or other jurisdiction in order to qualify any or all of the Registrable Securities under the securities laws thereof (any such application, document or information herein called a “Blue Sky Application”); (iii) the omission or alleged omission to state in a Blue Sky Application a material fact required to be stated therein or necessary to make the statements therein not misleading, in light of the circumstances in which they were made; (iv) any violation by the Company or its agents of any rule or regulation promulgated under the Securities Act applicable to the Company or its agents and relating to action or inaction required of the Company in connection with such registration; or (v) any failure to register or qualify the Registrable Securities included in any such Registration Statement in any state where the Company or its agents has affirmatively undertaken or agreed in writing that the Company will undertake such registration or qualification on a Holder’s behalf and will reimburse such indemnified person for any legal or other expenses reasonably incurred by them in connection with investigating or defending any such loss, claim, damage, liability or action; provided, however, that the Company will not be liable in any such case if and only to the extent that any such loss, claim, damage, liability or expense arises out of or is based upon (x) an untrue statement or alleged untrue statement or omission or alleged omission so made in conformity with information furnished by such Holder or any such controlling person in writing specifically for use in such Registration Statement or Prospectus, (y) the use by a Holder of an outdated or defective Prospectus after the Company has notified such Holder in writing that such Prospectus is outdated or defective or (z) a Holder’s (or any other indemnified Person’s) failure to send or give a copy of the Prospectus or supplement (as then amended or supplemented), if required, pursuant to Rule 172 under the Securities Act (or any successor rule) to the Persons asserting any untrue statement or alleged untrue statement or omission or alleged omission at or prior to the written confirmation of the sale of Registrable Securities to such Person if such statement or omission was corrected in such Prospectus or supplement.
8
(b) Indemnification by the Holders. Each Holder agrees, severally and not jointly, to indemnify and hold harmless, to the fullest extent permitted by law, the Company, its directors, officers, employees, stockholders, agents, successors and assigns and each Person who controls the Company (within the meaning of the Securities Act) against any losses, claims, damages, liabilities and reasonable and documented out-of-pocket expenses (including reasonable and documented out-of-pocket attorney fees) (or actions in respect thereof) arising out of or based upon any untrue statement or alleged untrue statement of a material fact or any omission or alleged omission of a material fact required to be stated in the Registration Statement or Prospectus or preliminary Prospectus or amendment or supplement thereto or necessary to make the statements therein (in the case of any Prospectus or form of prospectus or supplement thereto, in light of the circumstances under which they were made) not misleading, to the extent, but only to the extent that such untrue statement or alleged untrue statement or omission or alleged omission is contained in any information furnished in writing by or on behalf of such Holder to the Company specifically for inclusion in such Registration Statement or Prospectus or amendment or supplement thereto. In no event shall the liability of a Holder be greater in amount than the dollar amount of the net proceeds received by such Holder upon the sale of the Registrable Securities included in the Registration Statement giving rise to such indemnification obligation.
(c) Conduct of Indemnification Proceedings. Any Person entitled to indemnification hereunder shall (i) give prompt notice to the indemnifying party of any claim with respect to which it seeks indemnification and (ii) permit such indemnifying party to assume the defense of such claim with counsel reasonably satisfactory to the indemnified party; provided that, subject to the preceding sentence, any Person entitled to indemnification hereunder shall have the right to employ separate counsel and to participate in the defense of such claim, but the fees and expenses of such counsel shall be at the expense of such Person unless (a) the indemnifying party has agreed to pay such fees or expenses, or (b) in the reasonable judgment of any such Person, based upon written advice of its counsel, a conflict of interest exists between such Person and the indemnifying party with respect to such claims (in which case, if the Person notifies the indemnifying party in writing that such Person elects to employ separate counsel at the expense of the indemnifying party, the indemnifying party shall not have the right to assume the defense of such claim on behalf of such Person); and provided, further, that the failure of any indemnified party to give notice as provided herein shall not relieve the indemnifying party of its obligations hereunder, except to the extent that such failure to give notice shall materially adversely affect the indemnifying party in the defense of any such claim or litigation. It is understood that the indemnifying party shall not, in connection with any proceeding in the same jurisdiction, be liable for fees or expenses of more than one separate firm of attorneys at any time for all such indemnified parties. No indemnifying party will, except with the consent of the indemnified party, consent to entry of any judgment or enter into any settlement that does not include as an unconditional term thereof the giving by the claimant or plaintiff to such indemnified party of a release from all liability in respect of such claim or litigation or that would require an admission of guilt.
(d) Contribution. If for any reason the indemnification provided for in the preceding paragraphs (a) and (b) is unavailable to an indemnified party or insufficient to hold it harmless, other than as expressly specified therein, then the indemnifying party shall contribute to the amount paid or payable by the indemnified party as a result of such loss, claim, damage, liability or expense in such proportion as is appropriate to reflect the relative fault of the indemnified party and the indemnifying party, as well as any other relevant equitable considerations. No Person guilty of fraudulent misrepresentation within the meaning of Section 11(f) of the Securities Act shall be entitled to contribution from any Person not guilty of such fraudulent misrepresentation. In no event shall the contribution obligation of a Holder be greater in amount than the dollar amount of the net proceeds received by it upon the sale of the Registrable Securities giving rise to such contribution obligation.
2.6. Termination of Registration Rights. The registration rights provided to the Holders under Section 2 shall terminate in their entirety upon the earlier to occur of: (a) the date that is three (3) years from the Effective Date; or (b) at such time as there are no Registrable Securities. Notwithstanding the foregoing, Section 2, Section 2.5 and Section 3 shall survive the termination of such registration rights.
9
3.1. Governing Law; Jurisdiction. This Agreement shall be governed by, and construed in accordance with, the internal laws of the State of Delaware without regard to the choice of law principles thereof. Each of the parties hereto irrevocably submits to the exclusive jurisdiction of the state and federal courts located in the State of Delaware for the purpose of any suit, action, proceeding or judgment relating to or arising out of this Agreement and the transactions contemplated hereby. Service of process in connection with any such suit, action or proceeding may be served on each party hereto anywhere in the world by the same methods as are specified for the giving of notices under this Agreement. Each of the parties hereto irrevocably consents to the jurisdiction of any such court in any such suit, action or proceeding and to the laying of venue in such court. Each party hereto irrevocably waives any objection to the laying of venue of any such suit, action or proceeding brought in such courts and irrevocably waives any claim that any such suit, action or proceeding brought in any such court has been brought in an inconvenient forum.
3.2. Successors and Assigns. Except as otherwise expressly provided herein, the provisions hereof shall inure to the benefit of, and be binding upon, the successor and assigns of the parties hereto (other than the rights of any Holder under Section 2 hereof, which shall not be assignable and shall not inure to the benefit of any successor or assign of a Holder). The Company may not assign its rights or obligations hereunder except with the prior written consent of each Holder. Each Holder may assign their respective rights hereunder (other than the rights of any Holder under Section 2 hereof, which shall not be assignable and shall not inure to the benefit of any successor or assign of a Holder) in the manner and to the Persons permitted under the Purchase Agreement.
3.3. Entire Agreement; Amendment. This Agreement and the other Transaction Documents constitute the full and entire understanding and agreement between the parties with regard to the subjects hereof and thereof. Any previous agreements among the parties relative to the specific subject matter hereof are superseded by this Agreement. Neither this Agreement nor any provision hereof may be amended, changed, waived, discharged or terminated other than by a written instrument signed by the party against whom enforcement of any such amendment, change, waiver, discharge or termination is sought.
3.4. Notices. All notices and other communications provided for or permitted hereunder shall be made as set forth in Section 10.02 of the Purchase Agreement.
3.5. Severability. If any term, provision, covenant or restriction of this Agreement is held by a court of competent jurisdiction to be invalid, illegal, void or unenforceable, the remainder of the terms, provisions, covenants and restrictions set forth herein shall remain in full force and effect and shall in no way be affected, impaired or invalidated, and the parties hereto shall use their commercially reasonable efforts to find and employ an alternative means to achieve the same or substantially the same result as that contemplated by such term, provision, covenant or restriction. It is hereby stipulated and declared to be the intention of the parties that they would have executed the remaining terms, provisions, covenants and restrictions without including any of such that may be hereafter declared invalid, illegal, void or unenforceable.
3.6. Headings. The headings herein are for convenience only, do not constitute a part of this Agreement and shall not be deemed to limit or affect any of the provisions hereof.
3.7. Counterparts. This Agreement may be executed in counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. Counterparts may be delivered via electronic mail (including pdf or any electronic signature complying with the U.S. federal ESIGN Act of 2000, Uniform Electronic Transactions Act or other applicable law) or other transmission method and any counterpart so delivered shall be deemed to have been duly and validly delivered and be valid and effective for all purposes.
3.8. Delays or Omissions. It is agreed that no delay or omission to exercise any right, power or remedy accruing to any party upon any breach or default of any other party under this Agreement shall impair any such right, power or remedy, nor shall it be construed to be a waiver of any such breach or default, or any acquiescence therein, or of any similar breach or default thereafter occurring; nor shall any waiver of any single breach or default be deemed a waiver of any other breach or default theretofore or thereafter occurring. It is further agreed that any waiver, permit, consent or approval of any kind or character of any breach or default under this Agreement, or any waiver of any provisions or conditions of this Agreement must be in writing and shall be effective only to the extent specifically set forth in writing, and that all remedies, either under this Agreement, by law or otherwise, shall be cumulative and not alternative.
3.9. Consents. Any permission, consent, or approval of any kind or character under this Agreement shall be in writing and shall be effective only to the extent specifically set forth in such writing.
3.10. SPECIFIC PERFORMANCE. THE PARTIES HERETO AGREE THAT IRREPARABLE DAMAGE WOULD OCCUR IN THE EVENT THAT ANY OF THE PROVISIONS OF THIS AGREEMENT WERE NOT PERFORMED IN ACCORDANCE WITH ITS SPECIFIC INTENT OR WERE OTHERWISE BREACHED. IT IS ACCORDINGLY AGREED THAT THE PARTIES SHALL BE ENTITLED TO AN INJUNCTION OR INJUNCTIONS, WITHOUT BOND, TO PREVENT OR CURE BREACHES OF THE PROVISIONS OF THIS AGREEMENT AND TO ENFORCE SPECIFICALLY THE TERMS AND PROVISIONS HEREOF, THIS BEING IN ADDITION TO ANY OTHER REMEDY TO WHICH THEY MAY BE ENTITLED BY LAW OR EQUITY, AND ANY PARTY SUED FOR BREACH OF THIS AGREEMENT EXPRESSLY WAIVES ANY DEFENSE THAT A REMEDY IN DAMAGES WOULD BE ADEQUATE.
10
3.11. Construction of Agreement. No provision of this Agreement shall be construed against either party as the drafter thereof.
3.12. Section References. Unless otherwise stated, any reference contained herein to a Section or subsection refers to the provisions of this Agreement.
3.13. Variations of Pronouns. All pronouns and all variations thereof shall be deemed to refer to the masculine, feminine, or neuter, singular or plural, as the context in which they are used may require.
[Remainder of Page Intentionally Left Blank; Signature Pages Follow]
11
IN WITNESS WHEREOF, the parties have caused this Registration Rights Agreement to be duly executed and delivered by their proper and duly authorized officers as of the day and year first written above.
| ImageneBio, Inc. | ||
| By: | /s/ Jotin Marango |
|
| Name: Jotin Marango | ||
| Title: Chief Financial Officer | ||
IN WITNESS WHEREOF, the parties have caused this Registration Rights Agreement to be duly executed and delivered by their proper and duly authorized officers as of the day and year first written above.
| PURCHASERS: | ||
| BLUE OWL HEALTHCARE OPPORTUNITIES IV PUBLIC INVESTMENTS LP | ||
| By: Blue Owl Healthcare Opportunities GP IV LLC, its general partner | ||
| By: | /s/ Kevin Raidy |
|
| Name: Kevin Raidy | ||
| Title: Authorized Signatory | ||
[Signature Page to Registration Rights Agreement]
IN WITNESS WHEREOF, the parties have caused this Registration Rights Agreement to be duly executed and delivered by their proper and duly authorized officers as of the day and year first written above.
| PURCHASERS: | ||
| Biotechnology Value Fund, L.P. | ||
| By: | /s/ Mark Lampert |
|
| Name: Mark Lampert | ||
| Title: Chief Executive Officer of BVF I GP LLC, General Partner of Biotechnology Value Fund, L.P. | ||
| Biotechnology Value Fund II, L.P. | ||
| By: | /s/ Mark Lampert |
|
| Name: Mark Lampert | ||
| Title: Chief Executive Officer of BVF II GP LLC, General Partner of Biotechnology Value Fund II, L.P. | ||
| Biotechnology Value Trading Fund OS LP | ||
| By: | /s/ Mark Lampert |
|
| Name: Mark Lampert | ||
| Title: President of BVF Inc., General Partner of BVF Partners L.P., Sole Member of BVF Partners OS Ltd., General Partner of Biotechnology Value Trading Fund OS LP | ||
| MSI BVF SPV, LLC | ||
| By: | /s/ Mark Lampert |
|
| Name: Mark Lampert | ||
| Title: President of BVF Inc., General Partner of BVF Partners L.P., Attorney in Fact of MSI BVF SPV, LLC | ||
[Signature Page to Registration Rights Agreement]
IN WITNESS WHEREOF, the parties have caused this Registration Rights Agreement to be duly executed and delivered by their proper and duly authorized officers as of the day and year first written above.
| PURCHASERS: | ||
| DEEP TRACK BIOTECHNOLOGY MASTER FUND, LTD. | ||
| By: | /s/ Nir Messafi |
|
| Name: Nir Messafi | ||
| Title: Authorized Person | ||
[Signature Page to Registration Rights Agreement]
IN WITNESS WHEREOF, the parties have caused this Registration Rights Agreement to be duly executed and delivered by their proper and duly authorized officers as of the day and year first written above.
| PURCHASERS: | ||
| Foresite Capital Fund VI LP | ||
| By: Foresite Capital Management VI LLC Its: General Partner |
||
| By: | /s/ Dennis D. Ryan |
|
| Name: Dennis D. Ryan |
||
| Title: Chief Financial Officer |
||
| Address: | ||
| 900 Larkspur Landing Circle, Suite 150 | ||
| Larkspur, CA 94939 | ||
| EMAIL: Docs-Investments@foresitecapital.com | ||
[Signature Page to Registration Rights Agreement]
IN WITNESS WHEREOF, the parties have caused this Registration Rights Agreement to be duly executed and delivered by their proper and duly authorized officers as of the day and year first written above.
| PURCHASERS: | ||
| Omega Fund VI, L.P. | ||
| By: Omega Fund VI GP, L.P., its General Partner By: Omega Fund VI GP Manager, Ltd., its General Partner |
||
| By: | /s/ Otello Stampacchia |
|
| Name: Otello Stampacchia | ||
| Title: Director | ||
[Signature Page to Registration Rights Agreement]
IN WITNESS WHEREOF, the parties have caused this Registration Rights Agreement to be duly executed and delivered by their proper and duly authorized officers as of the day and year first written above.
| PURCHASERS: | ||
| RTW Master Fund, Ltd. | ||
| By: | /s/ Darshan Patel |
|
| Name: Darshan Patel | ||
| Title: Director | ||
| RTW Innovation Master Fund, Ltd. | ||
| By: | /s/ Darshan Patel |
|
| Name: Darshan Patel | ||
| Title: Director | ||
| RTW Biotech Opportunities Operating Ltd. | ||
| By: RTW Investments, LP, its Investment Manager | ||
| By: | /s/ Roderick Wong |
|
| Name: Roderick Wong, M.D. | ||
| Title: Managing Partner | ||
[Signature Page to Registration Rights Agreement]
IN WITNESS WHEREOF, the parties have caused this Registration Rights Agreement to be duly executed and delivered by their proper and duly authorized officers as of the day and year first written above.
| PURCHASERS: | ||
| ORBIMED PRIVATE INVESTMENTS VI, LP | ||
| By: OrbiMed Capital GP VI LLC, its General Partner |
||
| By: OrbiMed Advisors LLC, its Managing Member |
||
| By: | /s/ David Bonita |
|
| Name: David Bonita | ||
| Title: Member | ||
[Signature Page to Registration Rights Agreement]
Annex A
PLAN OF DISTRIBUTION
Each Selling Stockholder (the “Selling Stockholders”) of the securities and any of their pledgees, assignees, donees, transferees or other successors-in-interest may, from time to time, sell, transfer or otherwise dispose of any or all of their securities covered hereby on the principal Trading Market or any other stock exchange, market or trading facility on which the securities are traded or in private transactions. These sales may be at fixed or negotiated prices. A Selling Stockholder may use any one or more of the following methods when selling securities:
| • | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| • | block trades in which the broker-dealer will attempt to sell the securities as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| • | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| • | an exchange distribution in accordance with the rules of the applicable exchange; |
| • | privately negotiated transactions; |
| • | settlement of short sales; |
| • | in transactions through broker-dealers that agree with the Selling Stockholders to sell a specified number of such securities at a stipulated price per security; |
| • | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| • | a combination of any such methods of sale; or |
| • | any other method permitted pursuant to applicable law. |
The Selling Stockholders may also sell securities under Rule 144 or any other exemption from registration under the Securities Act of 1933, as amended (the “Securities Act”), if available, rather than under this prospectus. Broker-dealers engaged by the Selling Stockholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the Selling Stockholders (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser) in amounts to be negotiated, but, except as set forth in a supplement to this Prospectus, in the case of an agency transaction not in excess of a customary brokerage commission in compliance with FINRA Rule 2121; and in the case of a principal transaction a markup or markdown in compliance with FINRA Rule 2121.
In connection with the sale of the securities or interests therein, the Selling Stockholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they assume. The Selling Stockholders may also sell securities short and deliver these securities to close out their short positions, or loan or pledge the securities to broker-dealers that in turn may sell these securities. The Selling Stockholders may also enter into option or other transactions with broker-dealers or other financial institutions or create one or more derivative securities that require the delivery to such broker-dealer or other financial institution of securities offered by this prospectus, which securities such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction). The Selling Stockholders also may transfer the securities in other circumstances, in which case the transferees, pledgees, donees or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
The Selling Stockholders and any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Each Selling Stockholder has informed us that it does not have any written or oral agreement or understanding, directly or indirectly, with any person to distribute the securities.
We are required to pay certain fees and expenses incurred by us incident to the registration of the securities. We have agreed to indemnify the Selling Stockholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
We agreed to keep this prospectus effective until the earlier of the date that the securities (i) have been sold, pursuant to this prospectus or pursuant to Rule 144, or (ii) the date on which the securities may be resold by the Selling Stockholders without registration and without regard to any volume or manner-of-sale limitations by reason of Rule 144, and without the requirement for us to be in compliance with the current public information under Rule 144 under the Securities Act or any other rule of similar effect. The resale securities will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in certain states, the resale securities covered hereby may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
Under applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously engage in market making activities with respect to the common stock for the applicable restricted period, as defined in Regulation M, prior to the commencement of the distribution. In addition, the Selling Stockholders will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of the common stock by the Selling Stockholders or any other person. We will make copies of this prospectus available to the Selling Stockholders and have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including by compliance with Rule 172 under the Securities Act).
2
Exhibit 10.9
July 23, 2025
Kristin Yarema, Ph.D.
[***]
| Re: | Offer of Employment |
Dear Dr. Yarema:
We are pleased to offer you at-will employment in the position of Chief Executive Officer (“CEO”) of Ikena Oncology, Inc. (to be renamed ImageneBio, Inc. upon consummation of the Merger, the “Company”) on the terms and conditions set forth in this letter agreement (the “Agreement”). The “Merger” shall refer to the previously-announced merger of a wholly-owned subsidiary of the Company with and into Inmagene Biopharmaceuticals.
1. Employment by the Company. Your employment with the Company shall begin upon, and subject to, the effectiveness of the Merger, and is anticipated to begin no later than July 25, 2025 (such actual date your employment begins (the “Start Date”)). This is an exempt position, and during your employment with the Company, you will devote your best efforts and substantially all of your business time and attention to the business of the Company, except for approved vacation periods and reasonable periods of illness or other incapacities permitted by the Company’s general employment policies. You shall perform such duties as are required by the Company’s Board of Directors (“Board”), to whom you will report. You will be nominated to serve as a member of the Board during the term of your employment and, if elected, will serve on the Board for no additional compensation. You represent to the Company that you are not subject to or a party to any employment agreement, non-competition covenant, or other agreement that would be breached by, or prohibit you from, executing this Agreement and performing fully your duties and responsibilities hereunder. Your primary work location shall be the Company’s office located in San Diego, California, although you are permitted to work remotely from your personal residence location in California as reasonably necessary to perform your assigned duties. The Company reserves the right to reasonably require you to perform your duties at places other than your primary office location from time to time, and to require reasonable business travel. Subject to the “Good Reason” provision set forth in Section 7, the Company may modify your job title and duties as it deems necessary and appropriate in light of the Company’s needs and interests from time to time.
2. Compensation.
2.1 Base Salary. For services to be rendered hereunder, you shall receive a base salary at the rate of $630,000 per year (the “Base Salary”), subject to standard payroll deductions and withholdings and payable in accordance with the Company’s regular payroll schedule. Following an annual review by the Board, you will be eligible for increases to your Base Salary at the Board’s sole discretion.
2.2 Annual Bonus. You shall be eligible to earn an annual bonus with a target amount of up to 50% of your then current annual Base Salary, prorated for the number of days employed in a calendar year (the “Annual Bonus”). Whether you receive the Annual Bonus for any given year, and the amount of the Annual Bonus, shall be determined by the Board and/or its Compensation Committee in its discretion based upon the achievement of preestablished corporate and/or individual objectives and milestones that are determined in the sole discretion of the Board. You must continue to be employed through the date the Annual Bonus is paid to earn and be paid such bonus.
1.
2.3 Signing Bonus. Upon the commencement of your employment, you will receive a one-time signing bonus in the amount of $180,000 (the “Signing Bonus”). The Signing Bonus will be paid to you as an advance in a single lump sum in accordance with the Company’s standard payroll processes within 30 days after your Start Date, and is provided to you prior to your earning of such Signing Bonus. You will not earn the Signing Bonus unless you remain actively and continuously employed with the Company through the second anniversary of your Start Date. If your employment terminates under any circumstances other than due to your termination without Cause by the Company, you agree to repay to the Company, within 10 days of your employment termination date: (i) 100% of the gross amount of the Signing Bonus if such termination occurs before the first anniversary of your Start Date, and (ii) 50% of the gross amount of the Signing Bonus if such termination occurs before the second anniversary of your Start Date.
2.4 Equity. Subject to approval by the Board, as soon as practicable following the Start Date, you shall be granted an option (the “Option”) to purchase a number of shares of common stock, par value $0.001 per share of the Company (“Common Stock”) equal to approximately 3.75% of the fully diluted shares of Common Stock calculated as of the grant date. The Option shall have an exercise price equal to the fair market value on the grant date. The Option shall be governed in all respects by the terms of the Company’s 2025 Equity Incentive Plan (the “Equity Plan”) and option agreement between you and the Company. The Option shall vest over a period of four years, with 1/4 of the shares of Common Stock subject to the Option vesting on the first anniversary of the Start Date, and the balance vesting in a series of thirty-six (36) successive equal monthly installments thereafter, subject to your Continuous Service (as defined in the Plan) through each vesting date. In the event your Continuous Service terminates for any reason other than by the Company for Cause, you may exercise the vested shares subject to the Option until the earliest of: (i) the day immediately preceding the tenth anniversary of the grant date of the Option, (ii) twelve (12) months after the last day of your Continuous Service, or (iii) their termination in accordance with Sections 6(b) or 6(c) of the Equity Plan. Subject to approval by the Board, as soon as practicable following the Start Date, you shall be granted restricted stock units (the “RSUs”) covering a number of shares of Common Stock equal to approximately 1.25% of the fully diluted shares of Common Stock calculated as of the grant date. The RSUs shall be governed in all respects by the terms of the Equity Plan and RSU award grant notice and award agreement between you and the Company. The RSUs shall vest over four years, with 1/4 of the RSUs vesting on the first anniversary of the Start Date, and the balance vesting in a series of twelve (12) successive equal quarterly installments thereafter, subject to your Continuous Service through each vesting date. You shall be eligible for consideration for annual grants of additional equity awards pursuant to the process applicable to other members of the executive leadership team, with the terms of any such grants to be determined in the sole discretion of the Board.
3. Reasonable Business Expenses. You shall be eligible for reimbursement of all reasonable, necessary and documented out-of-pocket business, entertainment, and travel expenses incurred by you in connection with the performance of your duties hereunder in accordance with the Company’s expense reimbursement policies and procedures.
2.
4. Company Policies; Standard Company Benefits. The employment relationship between the parties shall be governed by the general employment policies and practices of the Company, except that when the terms of this Agreement differ from or are in conflict with the Company’s general employment policies or practices, this Agreement shall control. You shall be entitled to participate in all employee benefit programs for which you are eligible under the terms and conditions of the benefit plans that may be in effect from time to time and provided by the Company to its employees. The Company reserves the right to cancel or change the benefit plans or programs it offers to its employees at any time.
5. At-Will Employment. Your employment relationship is at-will. Either you or the Company may terminate the employment relationship at any time, with or without cause or advance notice. Upon termination of your employment for any reason, you shall resign from all positions and terminate any relationships as an employee, advisor, officer or director with the Company and any of its affiliates, each effective on the date of termination.
6. Outside Activities During Employment. Except with the prior written consent of the Board, you will not during the term of your employment with the Company undertake or engage in any other employment, occupation or business enterprise, other than ones in which you are a passive investor. You may engage in civic, not-for-profit activities, including with the Alliance for Regenerative Medicine (ARM), and serve as a director for a single for-profit entity, as approved by the Board in its sole discretion, with such approval not being unreasonably withheld, so long as such activities do not materially interfere with the performance of your duties hereunder or conflict in any way with the business of the Company. You agree not to acquire, assume or participate in, directly or indirectly, any position, investment or interest known to be adverse or antagonistic to the Company, its business or prospects, financial or otherwise.
7. Termination; Severance.
7.1 Term and Termination. The term of this Agreement shall be the period commencing on the Start Date and ending on the date that your employment is terminated by either party pursuant to the provisions of this Agreement. You are employed at-will, meaning that, subject to the terms and conditions set forth herein, either the Company or you may terminate your employment at any time, with or without Cause.
7.2 Compensation upon Termination. Upon the termination of your employment for any reason, the Company shall pay you all of your accrued and unpaid wages earned through your last day of employment (the “Separation Date”).
7.3 Involuntary Termination Unrelated to a Change in Control. If you are subject to an Involuntary Termination (that does not occur within the Change in Control Period (as defined below)), and provided that you remain in compliance with the terms of this Agreement (including the conditions described in Section 7.6 below), the Company shall provide you with the following benefits (the “Severance Benefits”):
3.
(a) Cash Severance. The Company shall pay you, as severance, the equivalent of twelve (12) months (the “Severance Period”) of your Base Salary in effect as of the Separation Date, subject to standard payroll deductions and withholdings (the “Severance”). The Severance will be paid as a continuation on the Company’s regular payroll, beginning no later than the first regularly-scheduled payroll date following the sixtieth (60th) day after your Separation from Service, provided the Separation Agreement (as discussed in Section 7.6) has become effective.
(b) Payment of Continued Group Health Plan Benefits. If you are eligible for and timely elect continued group health plan coverage under the Consolidated Omnibus Budget Reconciliation Act of 1985 or any state law of similar effect (“COBRA”) following your Involuntary Termination, the Company will pay your COBRA group health insurance premiums for you and your eligible dependents directly to the insurer until the earliest of (A) the end of the period immediately following your Involuntary Termination that is equal to the Severance Period (the “COBRA Payment Period”), (B) the expiration of your eligibility for continuation coverage under COBRA, or (C) the date when you become eligible for substantially equivalent health insurance coverage in connection with new employment or self-employment. For purposes of this Section, references to COBRA premiums shall not include any amounts payable by you under a Section 125 health care reimbursement plan under the Code. Notwithstanding the foregoing, if at any time the Company determines, in its sole discretion, that it cannot pay the COBRA premiums without potentially incurring financial costs or penalties under applicable law (including, without limitation, Section 2716 of the Public Health Service Act), then regardless of whether you elect continued health coverage under COBRA, and in lieu of providing the COBRA premiums, the Company will instead pay you on the last day of each remaining month of the COBRA Payment Period, a fully taxable cash payment equal to the COBRA premiums for that month, subject to applicable tax withholdings (such amount, the “Special Severance Payment”), which payments shall continue until the earlier of expiration of the COBRA Payment Period or the date when you become eligible for substantially equivalent health insurance coverage in connection with new employment or self-employment. On the first payroll date following the effectiveness of the Separation Agreement, the Company will make the first payment to the insurer under this clause (and, in the case of the Special Severance Payment, such payment will be to you, in a lump sum) equal to the aggregate amount of payments that the Company would have paid through such date had such payments instead commenced on the Separation Date, with the balance of the payments paid thereafter on the schedule described above. If you become eligible for coverage under another employer’s group health plan, you must immediately notify the Company of such event, and all payments and obligations under this subsection shall cease.
7.4 Involuntary Termination in Connection with a Change in Control. If you are subject to an Involuntary Termination during the Change in Control Period, and provided that you remain in compliance with the terms of this Agreement (including the conditions described in Section 7.6 below), the Company shall provide you with the following benefits (the “Change in Control Severance Benefits”):
(a) Cash Severance. The Company shall pay you, as severance, the equivalent of eighteen (18) months (the “CIC Severance Period”) of your Base Salary in effect as of the Separation Date, subject to standard payroll deductions and withholdings (the “CIC Severance”). If the Change in Control first occurring during the Change in Control Period does not also qualify as a Section 409A Change in Control, then the CIC Severance will be paid as a continuation on the Company’s regular payroll, beginning no later than the first regularly-scheduled payroll date following the sixtieth (60th) day after your Separation from Service, provided the Separation Agreement (as discussed in Section 7.6) has become effective. If the Change in Control first occurring during the Change in Control Period also qualifies as a Section 409A Change in Control, then the CIC Severance will be paid in a lump sum on the first regularly-scheduled payroll date following the sixtieth (60th) day after your Separation from Service, provided the Separation Agreement (as discussed in Section 7.6) has become effective.
4.
(b) Payment of Continued Group Health Plan Benefits. If you are eligible for and timely elect continued group health plan coverage under COBRA following your Involuntary Termination, the Company will pay your COBRA group health insurance premiums for you and your eligible dependents directly to the insurer until the earliest of (A) the end of the period immediately following your Involuntary Termination that is equal to the CIC Severance Period (the “CIC COBRA Payment Period”), (B) the expiration of your eligibility for continuation coverage under COBRA, or (C) the date when you become eligible for substantially equivalent health insurance coverage in connection with new employment or self-employment. For purposes of this Section, references to COBRA premiums shall not include any amounts payable by you under a Section 125 health care reimbursement plan under the Code. Notwithstanding the foregoing, if at any time the Company determines, in its sole discretion, that it cannot pay the COBRA premiums without potentially incurring financial costs or penalties under applicable law (including, without limitation, Section 2716 of the Public Health Service Act), then regardless of whether you elect continued health coverage under COBRA, and in lieu of providing the COBRA premiums, the Company will instead pay you on the last day of each remaining month of the CIC COBRA Payment Period, a fully taxable cash payment equal to the COBRA premiums for that month, subject to applicable tax withholdings (such amount, the “CIC Special Severance Payment”), which payments shall continue until the earlier of expiration of the CIC COBRA Payment Period or the date when you become eligible for substantially equivalent health insurance coverage in connection with new employment or self-employment. On the first payroll date following the effectiveness of the Separation Agreement, the Company will make the first payment to the insurer under this clause (and, in the case of the CIC Special Severance Payment, such payment will be to you, in a lump sum) equal to the aggregate amount of payments that the Company would have paid through such date had such payments instead commenced on the Separation Date, with the balance of the payments paid thereafter on the schedule described above. If you become eligible for coverage under another employer’s group health plan, you must immediately notify the Company of such event, and all payments and obligations under this subsection shall cease.
(c) Bonus. The Company will pay you, as an additional severance benefit, a one-time, lump-sum payment equal to your full on-target Annual Bonus for the bonus year in which your Involuntary Termination occurs (the “CIC Bonus Severance”). The CIC Bonus Severance will be paid to you at the same time as the first installment payment of the CIC Severance, subject to all applicable deductions and withholdings.
(d) Accelerated Vesting. The vesting and exercisability of all outstanding time-based stock options and other time-based equity awards covering the Common Stock that are held by you as of immediately prior to the Separation Date shall fully accelerate.
In no event shall you be entitled to benefits under both Section 7.3 and this Section 7.4.
5.
7.5 Termination for Cause; Resignation Without Good Reason; Death or Disability. If you resign without Good Reason, or the Company terminates your employment for Cause, upon dissolution or cessation of the Company, or upon your death or disability, then all payments of compensation by the Company to you hereunder will terminate immediately (except as to amounts already earned), and you will not be entitled to any Severance Benefits or Change in Control Severance Benefits.
7.6 Conditions to Receipt of Severance Benefits and Change in Control Severance Benefits. The receipt of the Severance Benefits and Change in Control Severance Benefits will be subject to you signing and not revoking a separation agreement and general release of claims in a form reasonably satisfactory to the Company (that includes mutual non-disparagement provisions) (the “Separation Agreement”) by no later than the sixtieth (60th) day after the Separation Date (“Release Deadline”). No Severance Benefits or Change in Control Severance Benefits will be paid or provided until the Separation Agreement becomes effective. You must also resign from all positions and terminate any relationships as an employee, advisor, officer or director with the Company and all of its affiliates, each effective on the Separation Date.
8. Definitions.
8.1 Cause. For purposes of this Agreement, “Cause” shall mean the occurrence of any of the following events: (i) your performance of any act of personal dishonesty or other unlawful act committed by you that results in harm to the Company or any parent or subsidiary of the Company; (ii) your commission of (A) a felony or (B) any misdemeanor involving moral turpitude, deceit, dishonesty or fraud, or in each case the equivalent in any relevant jurisdiction in which you perform service for the Company; (iii) your failure to substantially perform your assigned duties and responsibilities with a level of competence and diligence that would customarily be expected from an executive having your position and responsibilities, which failure continues, in the reasonable judgment of the Board, after written notice given to you by the Board and you have been provided at least fourteen days to cure; (iv) your gross negligence, willful misconduct or insubordination with respect to the Company; or (v) your material violation of any provision of any written agreement(s) between you and the Company or any parent or subsidiary of the Company relating to noncompetition, nonsolicitation, nondisclosure and/or assignment of inventions.
8.2 Change in Control. For purposes of this Agreement, a “Change in Control” shall have the meaning as set forth in the Equity Plan, but shall not include the Merger.
8.3 Change in Control Period. For purposes of this Agreement, the “Change in Control Period” means the period commencing on the effective date of a Change in Control and ending twelve (12) months following the effective date of the Change in Control.
8.4 Code. For purposes of this Agreement, “Code” means the U.S. Internal Revenue Code of 1986 (as it has been and may be amended from time to time) and any regulations and guidance that has been promulgated or may be promulgated from time to time thereunder and any state law of similar effect.
6.
8.5 Good Reason. For purposes of this Agreement, “Good Reason” shall specifically mean the occurrence of any of the following, without your express written consent: (a) a material reduction in your Base Salary (unless made pursuant to a salary reduction program applicable generally to the Company’s executive officers); (b) a material diminution in your title, duties, responsibilities and/or authorities, including but not limited to, no longer reporting directly to the Board, an assignment of duties and responsibilities that are not the customary duties and responsibilities of a chief executive officer; (c) the relocation of the principal place of your employment to a location that is more than twenty-five (25) miles away from its current location; or (d) the uncured breach of any material provision of this Agreement by the Company. In order to resign for Good Reason, you must (i) provide written notice to the Company’s Board within 30 days after the first occurrence of the event giving rise to Good Reason setting forth the basis for your resignation, (ii) allow the Company at least 30 days from receipt of such written notice to cure such event, and (iii) if such event is not reasonably cured within such period, your resignation from all positions you then hold with the Company is effective not later than 30 days after the expiration of the cure period.
8.6 Involuntary Termination. For purposes of this Agreement, “Involuntary Termination” means a termination of your employment with the Company pursuant to either (i) a termination initiated by the Company without Cause, or (ii) your resignation for Good Reason, and provided in either case such termination constitutes a Separation from Service. An Involuntary Termination does not include any other termination of your employment, including a termination due to your death or disability.
8.7 Section 409A Change in Control. For purposes of this Agreement, a “Section 409A Change in Control” shall have the meaning as set forth in the Equity Plan.
8.8 Separation from Service. For purposes of this Agreement, “Separation from Service” means a “separation from service”, as defined under Treasury Regulation Section 1.409A-1(h).
9. Proprietary Information Obligations. As a condition of employment, you shall execute and abide by the Company’s standard form of Confidential Information and Invention Assignment Agreement (the “Confidentiality Agreement”), attached as Exhibit A. In your work for the Company, you will be expected not to use or disclose any confidential information, including trade secrets, of any former employer or other person to whom you have an obligation of confidentiality. Rather, you will be expected to use only that information which is generally known and used by persons with training and experience comparable to your own, which is common knowledge in the industry or otherwise legally in the public domain, or which is otherwise provided or developed by the Company. You agree that you will not bring onto Company premises any unpublished documents or property belonging to any former employer or other person to whom you have an obligation of confidentiality. You hereby represent that you have disclosed to the Company any contract you have signed that may restrict your activities on behalf of the Company.
7.
10. Section 409A. It is intended that all of the severance benefits and other payments payable under this Agreement satisfy, to the greatest extent possible, the exemptions from the application of Code Section 409A provided under Treasury Regulations Sections 1.409A-1(b)(4), 1.409A-1(b)(5) and 1.409A-1(b)(9), and this Agreement will be construed to the greatest extent possible as consistent with those provisions, and to the extent not so exempt, this Agreement (and any definitions hereunder) will be construed in a manner that complies with Section 409A. For all purposes of Code Section 409A (including, without limitation, for purposes of Treasury Regulations Sections 1.409A-2(b)(2)(i) and (iii)), your right to receive any installment payments under this Agreement (whether severance payments, reimbursements or otherwise) shall be treated as a right to receive a series of separate payments and, accordingly, each installment payment hereunder shall at all times be considered a separate and distinct payment. Notwithstanding any provision to the contrary in this Agreement, if you are deemed by the Company at the time of your Separation from Service to be a “specified employee” for purposes of Code Section 409A(a)(2)(B)(i), and if any of the payments upon Separation from Service set forth herein and/or under any other agreement with the Company are deemed to be “deferred compensation,” then to the extent delayed commencement of any portion of such payments is required in order to avoid a prohibited distribution under Code Section 409A(a)(2)(B)(i) and the related adverse taxation under Section 409A, such payments shall not be provided to you prior to the earliest of (i) the first date following expiration of the six-month period following the date of your Separation from Service with the Company, (ii) the date of your death or (iii) such earlier date as permitted under Section 409A without the imposition of adverse taxation. Upon the first business day following the expiration of such applicable Code Section 409A(a)(2)(B)(i) period, all payments deferred pursuant to this Paragraph shall be paid in a lump sum to you, and any remaining payments due shall be paid as otherwise provided herein or in the applicable agreement. No interest shall be due on any amounts so deferred. If the severance benefits are not covered by one or more exemptions from the application of Section 409A and the Release Deadline occurs in the calendar year following the calendar year of your Separation from Service, the Separation Agreement will not be deemed effective any earlier than the Release Deadline for purposes of determining the timing of provision of any severance benefits.
8.
11. Arbitration of All Disputes. To aid the rapid and economical resolution of disputes that may arise in connection with your employment with the Company, and in exchange for the mutual promises contained in this offer letter, you and the Company agree that any and all disputes, claims, or causes of action, in law or equity, including but not limited to statutory claims, arising from or relating to the enforcement, breach, performance, or interpretation of this letter agreement, your employment with the Company, or the termination of your employment, shall be resolved, to the fullest extent permitted by law, by final, binding and confidential arbitration conducted by JAMS, Inc. (“JAMS”) or its successor, under JAMS’ then applicable rules and procedures appropriate to the relief being sought (available upon request and also currently available at the following web address: (i) https://www.jamsadr.com/rules-employment-arbitration/ and (ii) https://www.jamsadr.com/rules-comprehensive-arbitration/) at a location closest to where you last worked for the Company or another mutually agreeable location. Notwithstanding the foregoing, if JAMS is unavailable due to location or otherwise, or if the parties mutually agree, then the arbitration shall be conducted by the American Arbitration Association (“AAA”) or its successor, under AAA’s then applicable rules and procedures appropriate to the relief being sought (available upon request and also currently available at the following web address: https://www.adr.org/sites/default/files/EmploymentRules-Web.pdf), at a location closest to where you last worked for the Company or another mutually agreeable location. Any demand for arbitration must be made within the statute of limitations applicable to the claim asserted as if such claim were asserted in court. Failure to demand arbitration (or, where applicable, file a counterclaim, crossclaim, or third-party claim) within such time limitation shall serve as a waiver and release with respect to all such claims. You acknowledge that by agreeing to this arbitration procedure, both you and the Company waive the right to resolve any such dispute through a trial by jury or judge. The Federal Arbitration Act, 9 U.S.C. § 1 et seq., will, to the fullest extent permitted by law, govern the interpretation and enforcement of this arbitration agreement and any arbitration proceedings. This provision shall not be mandatory for any claim or cause of action to the extent applicable law prohibits subjecting such claim or cause of action to mandatory arbitration and such applicable law is not preempted by the Federal Arbitration Act or otherwise invalid (collectively, the “Excluded Claims”), such as non-individual claims that cannot be waived under applicable law, claims or causes of action alleging sexual harassment or a nonconsensual sexual act or sexual contact, or unemployment or workers’ compensation claims brought before the applicable state governmental agency. In the event you or the Company intend to bring multiple claims, including one of the Excluded Claims listed above, the Excluded Claims may be filed with a court, while any other claims will remain subject to mandatory arbitration. You acknowledge and agree that proceedings of any non-individual claim(s) under the California Private Attorneys General Act (“PAGA”) that may be brought in court shall be stayed for the duration and pending a final resolution of the arbitration of any individual or individual PAGA claim. Nothing herein prevents you from filing and pursuing proceedings before a federal or state governmental agency, although if you choose to pursue a claim following the exhaustion of any applicable administrative remedies, that claim would be subject to this provision. In addition, with the exception of Excluded Claims arising out of 9 U.S.C. § 401 et seq., all claims, disputes, or causes of action under this section, whether by you or the Company, must be brought in an individual capacity, and shall not be brought as a plaintiff (or claimant) or class member in any purported class, representative, or collective proceeding, nor joined or consolidated with the claims of any other person or entity. You acknowledge that by agreeing to this arbitration procedure, both you and the Company waive all rights to have any dispute be brought, heard, administered, resolved, or arbitrated on a class, representative, or collective action basis. The arbitrator may not consolidate the claims of more than one person or entity, and may not preside over any form of representative or class proceeding. If a court finds, by means of a final decision, not subject to any further appeal or recourse, that the preceding sentences regarding class, representative, or collective claims or proceedings violate applicable law or are otherwise found unenforceable as to a particular claim or request for relief, the parties agree that any such claim(s) or request(s) for relief be severed from the arbitration and may proceed in a court of law rather than by arbitration. All other claims or requests for relief shall be arbitrated. You will have the right to be represented by legal counsel at any arbitration proceeding. Questions of whether a claim is subject to arbitration and procedural questions which grow out of the dispute and bear on the final disposition are matters for the arbitrator to decide, provided however, that if required by applicable law, a court and not the arbitrator may determine the enforceability of this paragraph with respect to Excluded Claims. The arbitrator shall: (a) have the authority to compel adequate discovery for the resolution of the dispute and to award such relief as would otherwise be permitted by law; and (b) issue a written statement signed by the arbitrator regarding the disposition of each claim and the relief, if any, awarded as to each claim, the reasons for the award, and the arbitrator’s essential findings and conclusions on which the award is based. The arbitrator shall be authorized to award all relief that you or the Company would be entitled to seek in a court of law. The Company shall pay all arbitration administrative fees in excess of the administrative fees that you would be required to pay if the dispute were decided in a court of law. Each party is responsible for its own attorneys’ fees, except as may be expressly set forth in your Employee Confidential Information and Inventions Assignment Agreement or as otherwise provided under applicable law. Nothing in this letter agreement is intended to prevent either you or the Company from obtaining injunctive relief in court to prevent irreparable harm pending the conclusion of any such arbitration. Any awards or orders in such arbitrations may be entered and enforced as judgments in the federal and state courts of any competent jurisdiction.
12. General Provisions. This Agreement, together with the Confidentiality Agreement, constitutes the entire agreement between you and the Company with regard to this subject matter and is the complete, final, and exclusive embodiment of the parties’ agreement with regard to this subject matter. This Agreement is entered into without reliance on any promise or representation, written or oral, other than those expressly contained herein, and it supersedes any other such promises, warranties or representations. Modifications or amendments to this Agreement, other than those changes
9.
expressly reserved to the Company’s discretion in this letter, must be made in a written agreement signed by you and the Company at the direction of the Board. Whenever possible, each provision of this Agreement will be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Agreement is held to be invalid, illegal or unenforceable in any respect under any applicable law or rule in any jurisdiction, such invalidity, illegality or unenforceability will not affect any other provision or any other jurisdiction, but this Agreement will be reformed, construed and enforced in such jurisdiction to the extent possible in keeping with the intent of the parties. Any waiver of any breach of any provisions of this Agreement must be in writing to be effective, and it shall not thereby be deemed to have waived any preceding or succeeding breach of the same or any other provision of this Agreement. This Agreement is intended to bind and inure to the benefit of and be enforceable by you and the Company, and their respective successors, assigns, heirs, executors and administrators. The Company may freely assign this Agreement, without your prior written consent. You may not assign any of your duties hereunder and you may not assign any of your rights hereunder without the written consent of the Company. This Agreement shall become effective as of the Start Date and shall terminate upon your termination of employment with the Company. The obligations as forth under Sections 7, 8, 9, 10, 11, and 12 will survive the termination of this Agreement and your employment. All questions concerning the construction, validity and interpretation of this Agreement will be governed by the laws of the State of California.
This offer is subject to satisfactory proof of your identity and right to work in the United States and other applicable pre-employment screenings.
We look forward to having you join us. If you have any questions about this Agreement, please do not hesitate to call me.
| Best regards, |
| INMAGENE BIOPHARMACEUTICALS |
| /s/ Jonathan Jian Wang |
| Jonathan Jian Wang, Ph.D., MBA |
| Chief Executive Officer |
| IKENA ONCOLOGY, INC. |
| /s/ Mark Manfredi |
| Mark Manfredi, Ph.D. |
| President and Chief Executive Officer |
10.
| Accepted and agreed: |
| /s/ Kristin Yarema |
| Kristin Yarema |
| Date: July 23, 2025 |
11.
Exhibit A
EMPLOYEE CONFIDENTIAL INFORMATION AND INVENTIONS ASSIGNMENT AGREEMENT
12.
Exhibit 10.10
EMPLOYMENT AGREEMENT
This Employment Agreement (this “Agreement”), effective on April 25, 2022 (the “Effective Date”), is made and entered into by and between Ikena Oncology, Inc., a Delaware corporation (the “Company”), and Jotin Marango (the “Executive”)
WHEREAS, the Company desires to continue to employ the Executive and the Executive desires to continue to be employed by the Company on the new terms and conditions contained herein.
NOW, THEREFORE, in consideration of the mutual covenants and agreements herein contained and other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, the parties agree as follows:
1. Employment.
(a) Term. The Company shall employ the Executive and the Executive shall be employed by the Company pursuant to this Agreement commencing as of the Effective Date and continuing until such employment is terminated in accordance with the provisions hereof (the “Term”). The Executive’s employment with the Company shall continue to be “at will,” meaning that the Executive’s employment may be terminated by the Company or the Executive at any time and for any reason subject to the terms of this Agreement.
(b) Position and Duties. The Executive shall serve as the Chief Financial Officer, Head of Corporate Development of the Company and shall have such powers and duties as may from time to time be prescribed by the Chief Executive Officer (the “CEO”) or other duly authorized executive. The Executive shall devote the Executive’s full working time and best efforts to the business and affairs of the Company. Notwithstanding the foregoing, the Executive may serve on other boards of directors, with the approval of the Board of Directors of the Company (the “Board”), or engage in religious, charitable or other community activities as long as such services and activities do not interfere with the Executive’s performance of the Executive’s duties to the Company.
2. Compensation and Related Matters.
(a) Base Salary. The Executive’s initial base salary shall be paid at the rate of $425,000 per year. The Executive’s base salary shall be subject to periodic review by the Board or the Compensation Committee of the Board (the “Compensation Committee”). The base salary in effect at any given time is referred to herein as “Base Salary.” The Base Salary shall be payable in a manner that is consistent with the Company’s usual payroll practices for its executive officers.
(b) Incentive Compensation. The Executive shall be eligible to receive cash incentive compensation as determined by the Board or the Compensation Committee from time to time. The Executive’s initial target annual incentive compensation shall be 40 percent of the Executive’s Base Salary. The target annual incentive compensation in effect at any given time is referred to herein as the “Target Bonus.” The actual amount of the Executive’s annual incentive compensation, if any, shall be determined in the sole discretion of the Board or the Compensation Committee, subject to the terms of any applicable incentive compensation plan that may be in effect from time to time.
1
Except as otherwise provided herein, as may be provided by the Board or the Compensation Committee or as may otherwise be set forth in the applicable incentive compensation plan, the Executive must be employed by the Company on the date such incentive compensation is paid in order to earn or receive any annual incentive compensation. For the avoidance of doubt, the Target Bonus for incentive compensation to be paid with respect to 2022 performance shall be based on 40 percent of the Executive’s full annual salary without prorating for commencing employment after the start of 2022; provided that the actual incentive compensation shall be subject to the other terms of this Section 2(b).
(c) Expenses. The Executive shall be entitled to receive prompt reimbursement for all reasonable expenses incurred by the Executive during the Term in performing services hereunder, in accordance with the policies and procedures then in effect and established by the Company for its executive officers.
(d) Other Benefits. The Executive shall be eligible to participate in or receive benefits under the Company’s employee benefit plans in effect from time to time, subject to the terms of such plans.
(e) Paid Time Off. The Executive shall be entitled to take paid time off in accordance with the Company’s applicable paid time off policy for executives, as may be in effect from time to time.
(f) Equity. The equity awards held by the Executive shall continue to be governed by the terms and conditions of the Company’s applicable equity incentive plan(s) and the applicable award agreement(s) governing the terms of such equity awards (collectively, the “Equity Documents”); provided, however, and notwithstanding anything to the contrary in the Equity Documents, Section 6(a)(ii) of this Agreement shall apply in the event of a termination by the Company without Cause or by the Executive for Good Reason in either event within the Change in Control Period (as such terms are defined below).
3. Termination. The Executive’s employment hereunder may be terminated without any breach of this Agreement under the following circumstances:
(a) Death. The Executive’s employment hereunder shall terminate upon death.
(b) Disability. The Company may terminate the Executive’s employment if the Executive is disabled and unable to perform or expected to be unable to perform the essential functions of the Executive’s then existing position or positions under this Agreement with or without reasonable accommodation for a period of 180 days (which need not be consecutive) in any 12-month period. If any question shall arise as to whether during any period the Executive is disabled so as to be unable to perform or expected to be unable to perform the essential functions of the Executive’s then existing position or positions with or without reasonable accommodation for the applicable period, the Executive may, and at the request of the Company shall, submit to the Company a certification in reasonable detail by a physician selected by the Company to whom the Executive or the Executive’s guardian has no reasonable objection as to whether the Executive is so disabled and unable to perform and, if so, for how long such disability and inability to perform is expected to continue, and such certification shall for the purposes of this Agreement be conclusive of the issue.
2
The Executive shall cooperate with any reasonable request of the physician in connection with such certification. If such question shall arise and the Executive shall fail to submit such certification, the Company’s determination of such issue shall be binding on the Executive. Nothing in this Section 3(b) shall be construed to waive the Executive’s rights, if any, under existing law including, without limitation, the Family and Medical Leave Act of 1993, 29 U.S.C. §2601 et seq. and the Americans with Disabilities Act, 42 U.S.C. §12101 et seq.
(c) Termination by the Company for Cause. The Company may terminate the Executive’s employment hereunder for Cause. For purposes of this Agreement, “Cause” shall mean any of the following:
(i) conduct by the Executive constituting a material act of misconduct in connection with the performance of the Executive’s duties, including, without limitation, (A) willful failure or refusal to perform material responsibilities that have been requested by the CEO; (B) dishonesty to the CEO with respect to any material matter; or (C) misappropriation of funds or property of the Company or any of its subsidiaries or affiliates other than the occasional, customary and de minimis use of Company property for personal purposes;
(ii) the commission by the Executive of acts satisfying the elements of (A) any felony or (B) a misdemeanor involving moral turpitude, deceit, dishonesty or fraud;
(iii) any misconduct by the Executive, regardless of whether or not in the course of the Executive’s employment, that may reasonably be expected to result in material injury or reputational harm to the Company or any of its subsidiaries or affiliates if the Executive were to continue to be employed in the same position;
(iv) continued unsatisfactory performance or non-performance by the Executive of the Executive’s duties hereunder (other than by reason of the Executive’s physical or mental illness, incapacity or disability) which has continued for more than 30 days following written notice of such unsatisfactory performance or non-performance from the CEO;
(v) a breach by the Executive of any of the provisions contained in Section 8 of this Agreement or the Restrictive Covenants Agreements (as defined below);
(vi) a material violation by the Executive of any of the Company’s written employment policies; or
(vii) the Executive’s failure to cooperate with a bona fide internal investigation or an investigation by regulatory or law enforcement authorities, after being instructed by the Company to cooperate, or the willful destruction or failure to preserve documents or other materials known to be relevant to such investigation or the inducement of others to fail to cooperate or to produce documents or other materials in connection with such investigation.
3
(d) Termination by the Company without Cause. The Company may terminate the Executive’s employment hereunder at any time without Cause. Any termination by the Company of the Executive’s employment under this Agreement which does not constitute a termination for Cause under Section 3(c) and does not result from the death or disability of the Executive under Section 3(a) or (b) shall be deemed a termination without Cause.
(e) Termination by the Executive. The Executive may terminate employment hereunder at any time for any reason, including but not limited to, Good Reason. For purposes of this Agreement, “Good Reason” shall mean that the Executive has completed all steps of the Good Reason Process (hereinafter defined) following the occurrence of any of the following events without the Executive’s consent (each, a “Good Reason Condition”):
(i) a material diminution in the Executive’s responsibilities, authority or duties;
(ii) a material diminution in the Executive’s Base Salary except for across-the-board salary reductions based on the Company’s financial performance similarly affecting all or substantially all senior management employees of the Company;
(iii) a material change in the geographic location of the principal office of the Company to which the Executive is assigned, such that there is an increase of at least thirty (30) miles of driving distance to such location from the Executive’s principal residence as of such change; or
(iv) a material breach of this Agreement by the Company.
The “Good Reason Process” consists of the following steps:
(i) the Executive reasonably determines in good faith that a Good Reason Condition has occurred;
(ii) the Executive notifies the Company in writing of the first occurrence of the Good Reason Condition within 60 days of the first occurrence of such condition;
(iii) the Executive cooperates in good faith with the Company’s efforts, for a period of not less than 30 days following such notice (the “Cure Period”), to remedy the Good Reason Condition;
(iv) notwithstanding such efforts, the Good Reason Condition continues to exist at the end of the Cure Period; and
(v) the Executive terminates employment within 60 days after the end of the Cure Period.
If the Company cures the Good Reason Condition during the Cure Period, Good Reason shall be deemed not to have occurred.
4
4. Matters related to Termination.
(a) Notice of Termination. Except for termination as specified in Section 3(a), any termination of the Executive’s employment by the Company or any such termination by the Executive shall be communicated by written Notice of Termination to the other party hereto. For purposes of this Agreement, a “Notice of Termination” shall mean a notice which shall indicate the specific termination provision in this Agreement relied upon.
(b) Date of Termination. “Date of Termination” shall mean: (i) if the Executive’s employment is terminated by death, the date of death; (ii) if the Executive’s employment is terminated on account of disability under Section 3(b) or by the Company for Cause under Section 3(c), the date on which Notice of Termination is given; (iii) if the Executive’s employment is terminated by the Company without Cause under Section 3(d), the date on which a Notice of Termination is given or the date otherwise specified by the Company in the Notice of Termination; (iv) if the Executive’s employment is terminated by the Executive under Section 3(e) other than for Good Reason, 30 days after the date on which a Notice of Termination is given, and (v) if the Executive’s employment is terminated by the Executive under Section 3(e) for Good Reason, the date on which a Notice of Termination is given after the end of the Cure Period. Notwithstanding the foregoing, in the event that the Executive gives a Notice of Termination to the Company, the Company may unilaterally accelerate the Date of Termination and such acceleration shall not result in a termination by the Company for purposes of this Agreement.
(c) Accrued Obligations. If the Executive’s employment with the Company is terminated for any reason, the Company shall pay or provide to the Executive (or to the Executive’s authorized representative or estate) (i) any Base Salary earned through the Date of Termination and, if applicable, any accrued but unused vacation through the Date of Termination; (ii) unpaid expense reimbursements (subject to, and in accordance with, Section 2(c) of this Agreement); and (iii) any vested benefits the Executive may have under any employee benefit plan of the Company through the Date of Termination, which vested benefits shall be paid and/or provided in accordance with the terms of such employee benefit plans (collectively, the “Accrued Obligations”).
(d) Resignation of All Other Positions. To the extent applicable, the Executive shall be deemed to have resigned from all officer and board member positions that the Executive holds with the Company or any of its respective subsidiaries and affiliates upon the termination of the Executive’s employment for any reason. The Executive shall execute any documents in reasonable form as may be requested to confirm or effectuate any such resignations.
5. Severance Pay and Benefits Upon Termination by the Company without Cause or by the Executive for Good Reason Outside the Change in Control Period. If the Executive’s employment is terminated by the Company without Cause as provided in Section 3(d), or the Executive terminates employment for Good Reason as provided in Section 3(e), in each case outside of the Change in Control Period (as defined below), then, in addition to the Accrued Obligations, and subject to (i) the Executive signing a separation agreement and release in a form and manner satisfactory to the Company, which shall include, without limitation, a general release of claims against the Company and all related persons and entities that shall not release the Executive’s rights under this Agreement, a reaffirmation of all of the Executive’s Continuing Obligations (as defined below), and, in the Company’s sole discretion, a one-year post-employment noncompetition agreement, and shall provide that if the Executive breaches any of the Continuing Obligations, all payments of the Severance Amount shall immediately cease (the “Separation Agreement”), and (ii) the Separation Agreement becoming irrevocable, all within 60 days after the Date of Termination (or such shorter period as set forth in the Separation Agreement), which shall include a seven (7) business day revocation period:
5
(a) the Company shall pay the Executive an amount equal to nine months of the Executive’s Base Salary (the “Severance Amount”); provided that in the event the Executive is entitled to any payments pursuant to the Restrictive Covenants Agreement, the Severance Amount received in any calendar year will be reduced by the amount the Executive is paid in the same such calendar year pursuant to the Restrictive Covenants Agreement (the “Restrictive Covenants Agreement Setoff”); and
(b) subject to the Executive’s copayment of premium amounts at the applicable active employees’ rate and the Executive’s proper election to receive benefits under the Consolidated Omnibus Budget Reconciliation Act of 1985, as amended (“COBRA”), the Company shall pay to the group health plan provider(s) or the COBRA provider a monthly payment equal to the monthly employer contribution that the Company would have made to provide health insurance to the Executive if the Executive had remained employed by the Company until the earliest of (A) the nine month anniversary of the Date of Termination; (B) the date that the Executive becomes eligible for group medical plan benefits under any other employer’s group medical plan; or (C) the cessation of the Executive’s health continuation rights under COBRA; provided, however, that if the Company determines that it cannot pay such amounts to the group health plan provider(s) or the COBRA provider (if applicable) without potentially violating applicable law (including, without limitation, Section 2716 of the Public Health Service Act), then the Company shall convert such payments to payroll payments directly to the Executive for the time period specified above. Such payments to the Executive shall be subject to tax-related deductions and withholdings and paid on the Company’s regular payroll dates.
The amounts payable under Section 5, to the extent taxable, shall be paid out in substantially equal installments in accordance with the Company’s payroll practice over nine months commencing within 60 days after the Date of Termination; provided, however, that if the 60-day period begins in one calendar year and ends in a second calendar year, such payments, to the extent they qualify as “non-qualified deferred compensation” within the meaning of Section 409A of the Internal Revenue Code of 1986, as amended (the “Code”), shall begin to be paid in the second calendar year by the last day of such 60-day period; provided, further, that the initial payment shall include a catch-up payment to cover amounts retroactive to the day immediately following the Date of Termination. Each payment pursuant to this Agreement is intended to constitute a separate payment for purposes of Treasury Regulation Section 1.409A-2(b)(2).
6
6. Severance Pay and Benefits Upon Termination by the Company without Cause or by the Executive for Good Reason within the Change in Control Period. The provisions of this Section 6 shall apply in lieu of, and expressly supersede, the provisions of Section 5 if (i) the Executive’s employment is terminated either (a) by the Company without Cause as provided in Section 3(d), or (b) by the Executive for Good Reason as provided in Section 3(e), and (ii) the Date of Termination is on or within forty-five (45) days immediately preceding or twelve (12) months immediately following the occurrence of the first event constituting a Change in Control (such period, the “Change in Control Period”). These provisions shall terminate and be of no further force or effect after the Change in Control Period.
(a) If the Executive’s employment is terminated by the Company without Cause as provided in Section 3(d) or the Executive terminates employment for Good Reason as provided in Section 3(e) and in each case the Date of Termination occurs during the Change in Control Period, then, in addition to the Accrued Obligations, and subject to the signing of a general release of claims against the Company and all related persons and entities that shall not release the Executive’s rights under this Agreement (the “Release”) by the Executive and the Release becoming fully effective, all within the time frame set forth in the Release but in no event more than 60 days after the Date of Termination:
(i) the Company shall pay the Executive a lump sum in cash in an amount equal to the sum of (A) the Executive’s then-current Base Salary (or the Executive’s Base Salary in effect immediately prior to the Change in Control, if higher) plus (B) the Executive’s Target Bonus for the then-current year (or the Executive’s Target Bonus in effect immediately prior to the Change in Control, if higher) (the “Change in Control Payment”); provided that the Change in Control Payment shall be reduced by the amount of the Restrictive Covenants Agreement Setoff, if applicable; and
(ii) the equity awards held by the Executive shall continue to be governed by the terms and conditions of the Company’s applicable equity incentive plan(s) and the applicable award agreement(s) governing the terms of such equity awards (collectively, the “Equity Documents”); provided, however, and notwithstanding anything to the contrary in the Equity Documents, Section 6(a)(ii) of this Agreement shall apply in the event of a termination by the Company without Cause or by the Executive for Good Reason in either event within the Change in Control Period (as such terms are defined below); and
(iii) subject to the Executive’s copayment of premium amounts at the applicable active employees’ rate and the Executive’s proper election to receive benefits under COBRA, the Company shall pay to the group health plan provider(s) or the COBRA provider a monthly payment equal to the monthly employer contribution that the Company would have made to provide health insurance to the Executive if the Executive had remained employed by the Company until the earliest of (A) the twelve month anniversary of the Date of Termination; (B) the date that the Executive becomes eligible for group medical plan benefits under any other employer’s group medical plan; or (C) the cessation of the Executive’s health continuation rights under COBRA; provided, however, that if the Company determines that it cannot pay such amounts to the group health plan provider(s) or the COBRA provider (if applicable) without potentially violating applicable law (including, without limitation, Section 2716 of the Public Health Service Act), then the Company shall convert such payments to payroll payments directly to the Executive for the time period specified above. Such payments to the Executive shall be subject to tax-related deductions and withholdings and paid on the Company’s regular payroll dates.
7
The amounts payable under this Section 6(a), to the extent taxable, shall be paid or commence to be paid within 60 days after the Date of Termination; provided, however, that if the 60-day period begins in one calendar year and ends in a second calendar year, such payments to the extent they qualify as “non-qualified deferred compensation” within the meaning of Section 409A of the Code, shall be paid or commence to be paid in the second calendar year by the last day of such 60-day period.
(b) Additional Limitation.
(i) Anything in this Agreement to the contrary notwithstanding, in the event that the amount of any compensation, payment or distribution by the Company to or for the benefit of the Executive, whether paid or payable or distributed or distributable pursuant to the terms of this Agreement or otherwise, calculated in a manner consistent with Section 280G of the Code, and the applicable regulations thereunder (the “Aggregate Payments”), would be subject to the excise tax imposed by Section 4999 of the Code, then the Aggregate Payments shall be reduced (but not below zero) so that the sum of all of the Aggregate Payments shall be $1.00 less than the amount at which the Executive becomes subject to the excise tax imposed by Section 4999 of the Code; provided that such reduction shall only occur if it would result in the Executive receiving a higher After Tax Amount (as defined below) than the Executive would receive if the Aggregate Payments were not subject to such reduction. In such event, the Aggregate Payments shall be reduced in the following order, in each case, in reverse chronological order beginning with the Aggregate Payments that are to be paid the furthest in time from consummation of the transaction that is subject to Section 280G of the Code: (1) cash payments not subject to Section 409A of the Code; (2) cash payments subject to Section 409A of the Code; (3) equity-based payments and acceleration; and (4) non-cash forms of benefits; provided that in the case of all the foregoing Aggregate Payments all amounts or payments that are not subject to calculation under Treas. Reg. §1.280G-1, Q&A-24(b) or (c) shall be reduced before any amounts that are subject to calculation under Treas. Reg. §1.280G-1, Q&A-24(b) or (c).
(ii) For purposes of this Section 6(b), the “After Tax Amount” means the amount of the Aggregate Payments less all federal, state, and local income, excise and employment taxes imposed on the Executive as a result of the Executive’s receipt of the Aggregate Payments. For purposes of determining the After Tax Amount, the Executive shall be deemed to pay federal income taxes at the highest marginal rate of federal income taxation applicable to individuals for the calendar year in which the determination is to be made, and state and local income taxes at the highest marginal rates of individual taxation in each applicable state and locality, net of the maximum reduction in federal income taxes which could be obtained from deduction of such state and local taxes.
(iii) The determination as to whether a reduction in the Aggregate Payments shall be made pursuant to Section 6(b)(i) shall be made by a nationally recognized accounting firm selected by the Company (the “Accounting Firm”), which shall provide detailed supporting calculations both to the Company and the Executive within 15 business days of the Date of Termination, if applicable, or at such earlier time as is reasonably requested by the Company or the Executive. Any determination by the Accounting Firm shall be binding upon the Company and the Executive.
8
(c) Definitions. For purposes of this Section 6, “Change in Control” shall mean a “Sale Event” as defined in the Company’s 2021 Stock Option and Incentive Plan.
7. Section 409A.
(a) Anything in this Agreement to the contrary notwithstanding, if at the time of the Executive’s separation from service within the meaning of Section 409A of the Code, the Company determines that the Executive is a “specified employee” within the meaning of Section 409A(a)(2)(B)(i) of the Code, then to the extent any payment or benefit that the Executive becomes entitled to under this Agreement or otherwise on account of the Executive’s separation from service would be considered deferred compensation otherwise subject to the 20 percent additional tax imposed pursuant to Section 409A(a) of the Code as a result of the application of Section 409A(a)(2)(B)(i) of the Code, such payment shall not be payable and such benefit shall not be provided until the date that is the earlier of (A) six months and one day after the Executive’s separation from service, or (B) the Executive’s death. If any such delayed cash payment is otherwise payable on an installment basis, the first payment shall include a catch-up payment covering amounts that would otherwise have been paid during the six-month period but for the application of this provision, and the balance of the installments shall be payable in accordance with their original schedule.
(b) All in-kind benefits provided and expenses eligible for reimbursement under this Agreement shall be provided by the Company or incurred by the Executive during the time periods set forth in this Agreement. All reimbursements shall be paid as soon as administratively practicable, but in no event shall any reimbursement be paid after the last day of the taxable year following the taxable year in which the expense was incurred. The amount of in-kind benefits provided or reimbursable expenses incurred in one taxable year shall not affect the in-kind benefits to be provided or the expenses eligible for reimbursement in any other taxable year (except for any lifetime or other aggregate limitation applicable to medical expenses). Such right to reimbursement or in-kind benefits is not subject to liquidation or exchange for another benefit.
(c) To the extent that any payment or benefit described in this Agreement constitutes “non-qualified deferred compensation” under Section 409A of the Code, and to the extent that such payment or benefit is payable upon the Executive’s termination of employment, then such payments or benefits shall be payable only upon the Executive’s “separation from service.” The determination of whether and when a separation from service has occurred shall be made in accordance with the presumptions set forth in Treasury Regulation Section 1.409A-1(h).
(d) The parties intend that this Agreement will be administered in accordance with Section 409A of the Code. To the extent that any provision of this Agreement is ambiguous as to its compliance with Section 409A of the Code, the provision shall be read in such a manner so that all payments hereunder comply with Section 409A of the Code. Each payment pursuant to this Agreement or the Restrictive Covenants Agreement is intended to constitute a separate payment for purposes of Treasury Regulation Section 1.409A-2(b)(2). The parties agree that this Agreement may be amended, as reasonably requested by either party, and as may be necessary to fully comply with Section 409A of the Code and all related rules and regulations in order to preserve the payments and benefits provided hereunder without additional cost to either party.
9
(e) The Company makes no representation or warranty and shall have no liability to the Executive or any other person if any provisions of this Agreement are determined to constitute deferred compensation subject to Section 409A of the Code but do not satisfy an exemption from, or the conditions of, such Section.
8. Continuing Obligations.
(a) Restrictive Covenants Agreements. The terms of the Invention and Non-Disclosure Agreement dated January 28, 2019 and the Non-Competition and Non-Solicitation Agreement dated January 28, 2019 (together, the “Restrictive Covenants Agreements”), between the Company and the Executive, attached hereto as Exhibit A and Exhibit B, respectively, continue to be in full force and effect. For purposes of this Agreement, the obligations in this Section 8 and those that arise in the Restrictive Covenants Agreements and any other agreement relating to confidentiality, assignment of inventions, or other restrictive covenants shall collectively be referred to as the “Continuing Obligations.”
(b) Third-Party Agreements and Rights. The Executive hereby confirms that the Executive is not bound by the terms of any agreement with any previous employer or other party which restricts in any way the Executive’s use or disclosure of information, other than confidentiality restrictions (if any), or the Executive’s engagement in any business. The Executive represents to the Company that the Executive’s execution of this Agreement, the Executive’s employment with the Company and the performance of the Executive’s proposed duties for the Company will not violate any obligations the Executive may have to any such previous employer or other party. In the Executive’s work for the Company, the Executive will not disclose or make use of any information in violation of any agreements with or rights of any such previous employer or other party, and the Executive will not bring to the premises of the Company any copies or other tangible embodiments of non-public information belonging to or obtained from any such previous employment or other party.
(c) Litigation and Regulatory Cooperation. During and after the Executive’s employment, the Executive shall cooperate fully with the Company in (i) the defense or prosecution of any claims or actions now in existence or which may be brought in the future against or on behalf of the Company which relate to events or occurrences that transpired while the Executive was employed by the Company, and (ii) the investigation, whether internal or external, of any matters about which the Company believes the Executive may have knowledge or information. The Executive’s full cooperation in connection with such claims, actions or investigations shall include, but not be limited to, being available to meet with counsel to answer questions or to prepare for discovery or trial and to act as a witness on behalf of the Company at mutually convenient times. During and after the Executive’s employment, the Executive also shall cooperate fully with the Company in connection with any investigation or review of any federal, state or local regulatory authority as any such investigation or review relates to events or occurrences that transpired while the Executive was employed by the Company. The Company shall reimburse the Executive for any out-of-pocket expenses, including but not limited to, attorneys’ fees, incurred in connection with the Executive’s performance of obligations pursuant to this Section 8(c).
10
(d) Relief. The Executive agrees that it would be difficult to measure any damages caused to the Company which might result from any breach by the Executive of the Continuing Obligations, and that in any event money damages would be an inadequate remedy for any such breach. Accordingly, the Executive agrees that if the Executive breaches, or proposes to breach, any portion of the Continuing Obligations, the Company shall be entitled, in addition to all other remedies that it may have, to seek an injunction or other appropriate equitable relief to restrain any such breach without showing or proving any actual damage to the Company.
9. Indemnification. Executive will be provided with indemnification against third party claims related to his work for the Company as required by applicable law, including advancement of attorneys’ fees and costs related to any such indemnification as provided by applicable law. The Company shall provide Executive with directors and officers liability insurance coverage at least as favorable as that which the Company may maintain from time to time for the directors of the Company or the other executive officers.
10. Consent to Jurisdiction. The parties hereby submit to the exclusive jurisdiction of the state and federal courts of the Commonwealth of Massachusetts with respect to any dispute arising under this Agreement.
11. Waiver of Jury Trial. Each of the Executive and the Company irrevocably and unconditionally WAIVES ALL RIGHT TO TRIAL BY JURY IN ANY PROCEEDING (WHETHER BASED ON CONTRACT, TORT OR OTHERWISE) ARISING OUT OF OR RELATING TO THIS AGREEMENT OR THE EXECUTIVE’S EMPLOYMENT BY THE COMPANY OR ANY AFFILIATE OF THE COMPANY, INCLUDING WITHOUT LIMITATION THE EXECUTIVE’S OR THE COMPANY’S PERFORMANCE UNDER, OR THE ENFORCEMENT OF, THIS AGREEMENT.
12. Integration. This Agreement constitutes the entire agreement between the parties with respect to the subject matter hereof and supersedes all prior agreements between the parties concerning such subject matter, including the Prior Agreement, provided that the Restrictive Covenants Agreements and the Equity Documents remain in full force and effect.
13. Withholding; Tax Effect. All payments made by the Company to the Executive under this Agreement shall be net of any tax or other amounts required to be withheld by the Company under applicable law. Nothing in this Agreement shall be construed to require the Company to make any payments to compensate the Executive for any adverse tax effect associated with any payments or benefits or for any deduction or withholding from any payment or benefit.
14. Assignment; Successors and Assigns. Neither the Executive nor the Company may make any assignment of this Agreement or any interest in it, by operation of law or otherwise, without the prior written consent of the other; provided, however, that the Company may assign its rights and obligations under this Agreement (including the Restrictive Covenants Agreements) without the Executive’s consent to any affiliate or to any person or entity with whom the Company shall hereafter effect a reorganization or consolidation, into which the Company merges or to whom it transfers all or substantially all of its properties or assets; provided, further that if the Executive remains employed or becomes employed by the Company, the purchaser or any of their affiliates in connection with any such transaction, then the Executive shall not be entitled to any payments, benefits or vesting pursuant to Section 5 or pursuant to Section 6 of this Agreement solely as a result of such transaction.
11
This Agreement shall inure to the benefit of and be binding upon the Executive and the Company, and each of the Executive’s and the Company’s respective successors, executors, administrators, heirs and permitted assigns. In the event of the Executive’s death after the Executive’s termination of employment but prior to the completion by the Company of all payments due to the Executive under this Agreement, the Company shall continue such payments to the Executive’s beneficiary designated in writing to the Company prior to the Executive’s death (or to the Executive’s estate, if the Executive fails to make such designation).
15. Enforceability. If any portion or provision of this Agreement (including, without limitation, any portion or provision of any section of this Agreement) shall to any extent be declared illegal or unenforceable by a court of competent jurisdiction, then the remainder of this Agreement, or the application of such portion or provision in circumstances other than those as to which it is so declared illegal or unenforceable, shall not be affected thereby, and each portion and provision of this Agreement shall be valid and enforceable to the fullest extent permitted by law.
16. Survival. For the avoidance of doubt, this Agreement shall survive the termination of the Executive’s employment to the extent necessary to effectuate the terms contained herein.
17. Waiver. No waiver of any provision hereof shall be effective unless made in writing and signed by the waiving party. The failure of any party to require the performance of any term or obligation of this Agreement, or the waiver by any party of any breach of this Agreement, shall not prevent any subsequent enforcement of such term or obligation or be deemed a waiver of any subsequent breach.
18. Notices. Any notices, requests, demands and other communications provided for by this Agreement shall be sufficient if in writing and delivered in person or sent by a nationally recognized overnight courier service or by registered or certified mail, postage prepaid, return receipt requested, to the Executive at the last address the Executive has filed in writing with the Company or, in the case of the Company, at its main offices, attention of the Board. Notices may also be sent by email to the last email address of the Executive or the Chairman of the Board, as the case may be; provided that such email notice is promptly thereafter confirmed by one of the foregoing methods. For purposes of email notice, the applicable email address of the Executive shall be the most recent email address that the Executive has provided to the Company, whereas the Chairman’s email address shall be the Chairman’s regular business email address as of the date of notice. Notices delivered in person or by email shall be effective on the date of notice. Notices delivered by overnight courier service shall be effective on the next business day after mailing. Notices delivered by registered or certified mail shall be effective three business days after mailing.
19. Amendment. This Agreement may be amended or modified only by a written instrument signed by the Executive and by a duly authorized representative of the Company.
12
20. Effect on Other Plans and Agreements. An election by the Executive to resign for Good Reason under the provisions of this Agreement shall not be deemed a voluntary termination of employment by the Executive for the purpose of interpreting the provisions of any of the Company’s benefit plans, programs or policies. Nothing in this Agreement shall be construed to limit the rights of the Executive under the Company’s benefit plans, programs or policies except as otherwise provided in Section 8 hereof, and except that the Executive shall have no rights to any severance benefits under any Company severance pay plan, offer letter or otherwise. Except for the Restrictive Covenants Agreement, in the event that the Executive is party to an agreement with the Company providing for payments or benefits under such plan or agreement and under this Agreement, the terms of this Agreement shall govern and the Executive may receive payment under this Agreement only and not both. Further, Section 5 and Section 6 of this Agreement are mutually exclusive and in no event shall the Executive be entitled to payments or benefits pursuant to both Section 5 and Section 6 of this Agreement.
21. Governing Law. This is a Massachusetts contract and shall be construed under and be governed in all respects by the laws of the Commonwealth of Massachusetts, without giving effect to the conflict of laws principles thereof. With respect to any disputes concerning federal law, such disputes shall be determined in accordance with the law as it would be interpreted and applied by the United States Court of Appeals for the First Circuit.
22. Counterparts. This Agreement may be executed in separate counterparts. When both counterparts are signed, they shall be treated together as one and the same document. PDF copies of signed counterparts shall be equally effective as originals.
13
IN WITNESS WHEREOF, the parties have executed this Agreement effective on the Effective Date.
| IKENA ONCOLOGY, INC. | ||
| By: | /s/ Mark Manfredi |
|
| Its: | President and CEO | |
| EXECUTIVE | ||
| /s/ Jotin Marango |
||
| Jotin Marango | ||
| 8 April 2022 |
||
| Date | ||
14
EXHIBIT A
IKENA ONCOLOGY INC.
EMPLOYEE CONFIDENTIALITY, ASSIGNMENT,
NONSOLICITATION AND NONCOMPETITION
AGREEMENT
15
EXHIBIT B
16
Exhibit 10.14
EMPLOYMENT AGREEMENT
This Employment Agreement (this “Agreement”) is made between Ikena Oncology, Inc., a Delaware corporation (the “Company”), and Mark Manfredi, Ph.D. (the “Executive”) and is effective as of the closing of the Company’s first underwritten public offering of its equity securities pursuant to an effective registration statement under the Securities Act of 1933, as amended (the “Effective Date”). Except with respect to the Restrictive Covenants Agreements and the Equity Documents (each as defined below), this Agreement supersedes in all respects all prior agreements between the Executive and the Company regarding the subject matter herein, including without limitation (i) the Employment Agreement between the Executive and the Company dated December 8, 2017 (the “Prior Agreement”), and (ii) any offer letter, employment agreement or severance agreement.
WHEREAS, the Company desires to continue to employ the Executive and the Executive desires to continue to be employed by the Company on the new terms and conditions contained herein.
NOW, THEREFORE, in consideration of the mutual covenants and agreements herein contained and other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, the parties agree as follows:
1. Employment.
(a) Term. The Company shall employ the Executive and the Executive shall be employed by the Company pursuant to this Agreement commencing as of the Effective Date and continuing until such employment is terminated in accordance with the provisions hereof (the “Term”). The Executive’s employment with the Company shall continue to be “at will,” meaning that the Executive’s employment may be terminated by the Company or the Executive at any time and for any reason subject to the terms of this Agreement.
(b) Position and Duties. The Executive shall serve as the Chief Executive Officer of the Company and shall have such powers and duties as may from time to time be prescribed by the Board of Directors (the “Board”). In addition, the Company shall cause the Executive to be nominated for election to the Board and to be recommended to the stockholders for election to the Board as long as the Executive remains the Chief Executive Officer of the Company (the “CEO”), provided that the Executive shall be deemed to have resigned from the Board and from any related positions upon ceasing to serve as CEO for any reason. The Executive shall devote the Executive’s full working time and best efforts to the business and affairs of the Company. Notwithstanding the foregoing, the Executive may serve on other boards of directors, with the approval of the Board, or engage in religious, charitable or other community activities as long as such services and activities do not interfere with the Executive’s performance of the Executive’s duties to the Company.
2. Compensation and Related Matters.
(a) Base Salary. The Executive’s initial base salary shall be paid at the rate of $530,000 per year. The Executive’s base salary shall be subject to periodic review by the Board or the Compensation Committee of the Board (the “Compensation Committee”). The base salary in effect at any given time is referred to herein as “Base Salary.” The Base Salary shall be payable in a manner that is consistent with the Company’s usual payroll practices for its executive officers.
1
(b) Incentive Compensation. The Executive shall be eligible to receive cash incentive compensation as determined by the Board or the Compensation Committee from time to time. The Executive’s initial target annual incentive compensation shall be fifty percent of the Executive’s Base Salary. The target annual incentive compensation in effect at any given time is referred to herein as the “Target Bonus.” The actual amount of the Executive’s annual incentive compensation, if any, shall be determined in the sole discretion of the Board or the Compensation Committee, subject to the terms of any applicable incentive compensation plan that may be in effect from time to time. Except as otherwise provided herein, as may be provided by the Board or the Compensation Committee or as may otherwise be set forth in the applicable incentive compensation plan, the Executive must be employed by the Company on the date such incentive compensation is paid in order to earn or receive any annual incentive compensation.
(c) Expenses. The Executive shall be entitled to receive prompt reimbursement for all reasonable expenses incurred by the Executive during the Term in performing services hereunder, in accordance with the policies and procedures then in effect and established by the Company for its executive officers.
(d) Other Benefits. The Executive shall be eligible to participate in or receive benefits under the Company’s employee benefit plans in effect from time to time, subject to the terms of such plans.
(e) Paid Time Off. The Executive shall be entitled to take paid time off in accordance with the Company’s applicable paid time off policy for executives, as may be in effect from time to time.
(f) Equity. The equity awards held by the Executive shall continue to be governed by the terms and conditions of the Company’s applicable equity incentive plan(s) and the applicable award agreement(s) governing the terms of such equity awards (collectively, the “Equity Documents”); provided, however, and notwithstanding anything to the contrary in the Equity Documents, Section 6(a)(ii) of this Agreement shall apply in the event of a termination by the Company without Cause or by the Executive for Good Reason in either event within the Change in Control Period (as such terms are defined below).
3. Termination. The Executive’s employment hereunder may be terminated without any breach of this Agreement under the following circumstances:
(a) Death. The Executive’s employment hereunder shall terminate upon death.
(b) Disability. The Company may terminate the Executive’s employment if the Executive is disabled and unable to perform or expected to be unable to perform the essential functions of the Executive’s then existing position or positions under this Agreement with or without reasonable accommodation for a period of 180 days (which need not be consecutive) in any 12-month period.
2
If any question shall arise as to whether during any period the Executive is disabled so as to be unable to perform or expected to be unable to perform the essential functions of the Executive’s then existing position or positions with or without reasonable accommodation for the applicable period, the Executive may, and at the request of the Company shall, submit to the Company a certification in reasonable detail by a physician selected by the Company to whom the Executive or the Executive’s guardian has no reasonable objection as to whether the Executive is so disabled and unable to perform and, if so, for how long such disability and inability to perform is expected to continue, and such certification shall for the purposes of this Agreement be conclusive of the issue. The Executive shall cooperate with any reasonable request of the physician in connection with such certification. If such question shall arise and the Executive shall fail to submit such certification, the Company’s determination of such issue shall be binding on the Executive. Nothing in this Section 3(b) shall be construed to waive the Executive’s rights, if any, under existing law including, without limitation, the Family and Medical Leave Act of 1993, 29 U.S.C. §2601 et seq. and the Americans with Disabilities Act, 42 U.S.C. §12101 et seq.
(c) Termination by the Company for Cause. The Company may terminate the Executive’s employment hereunder for Cause. For purposes of this Agreement, “Cause” shall mean any of the following:
(i) conduct by the Executive constituting a material act of misconduct in connection with the performance of the Executive’s duties, including, without limitation, (A) willful failure or refusal to perform material responsibilities that have been requested by the Board; (B) dishonesty to the Board with respect to any material matter; or (C) misappropriation of funds or property of the Company or any of its subsidiaries or affiliates other than the occasional, customary and de minimis use of Company property for personal purposes;
(ii) the commission by the Executive of acts satisfying the elements of (A) any felony or (B) a misdemeanor involving moral turpitude, deceit, dishonesty or fraud;
(iii) any misconduct by the Executive, regardless of whether or not in the course of the Executive’s employment, that may reasonably be expected to result in material injury or reputational harm to the Company or any of its subsidiaries or affiliates if the Executive were to continue to be employed in the same position;
(iv) continued unsatisfactory performance or non-performance by the Executive of the Executive’s duties hereunder (other than by reason of the Executive’s physical or mental illness, incapacity or disability) which has continued for more than 30 days following written notice of such unsatisfactory performance or non-performance from the Board;
(v) a breach by the Executive of any of the provisions contained in Section 8 of this Agreement or the Restrictive Covenants Agreements (as defined below);
(vi) a material violation by the Executive of any of the Company’s written employment policies; or
(vii) the Executive’s failure to cooperate with a bona fide internal investigation or an investigation by regulatory or law enforcement authorities, after being instructed by the Company to cooperate, or the willful destruction or failure to preserve documents or other materials known to be relevant to such investigation or the inducement of others to fail to cooperate or to produce documents or other materials in connection with such investigation.
3
(d) Termination by the Company without Cause. The Company may terminate the Executive’s employment hereunder at any time without Cause. Any termination by the Company of the Executive’s employment under this Agreement which does not constitute a termination for Cause under Section 3(c) and does not result from the death or disability of the Executive under Section 3(a) or (b) shall be deemed a termination without Cause.
(e) Termination by the Executive. The Executive may terminate employment hereunder at any time for any reason, including but not limited to, Good Reason. For purposes of this Agreement, “Good Reason” shall mean that the Executive has completed all steps of the Good Reason Process (hereinafter defined) following the occurrence of any of the following events without the Executive’s consent (each, a “Good Reason Condition”):
(i) a material diminution in the Executive’s responsibilities, authority or duties;
(ii) a material diminution in the Executive’s Base Salary except for across-the-board salary reductions based on the Company’s financial performance similarly affecting all or substantially all senior management employees of the Company;
(iii) a material change in the geographic location of the principal office of the Company to which the Executive is assigned, such that there is an increase of at least thirty (30) miles of driving distance to such location from the Executive’s principal residence as of such change; or
(iv) a material breach of this Agreement by the Company.
The “Good Reason Process” consists of the following steps:
(i) the Executive reasonably determines in good faith that a Good Reason Condition has occurred;
(ii) the Executive notifies the Company in writing of the first occurrence of the Good Reason Condition within 60 days of the first occurrence of such condition;
(iii) the Executive cooperates in good faith with the Company’s efforts, for a period of not less than 30 days following such notice (the “Cure Period”), to remedy the Good Reason Condition;
(iv) notwithstanding such efforts, the Good Reason Condition continues to exist at the end of the Cure Period; and
(v) the Executive terminates employment within 60 days after the end of the Cure Period.
4
If the Company cures the Good Reason Condition during the Cure Period, Good Reason shall be deemed not to have occurred.
4. Matters related to Termination.
(a) Notice of Termination. Except for termination as specified in Section 3(a), any termination of the Executive’s employment by the Company or any such termination by the Executive shall be communicated by written Notice of Termination to the other party hereto. For purposes of this Agreement, a “Notice of Termination” shall mean a notice which shall indicate the specific termination provision in this Agreement relied upon.
(b) Date of Termination. “Date of Termination” shall mean: (i) if the Executive’s employment is terminated by death, the date of death; (ii) if the Executive’s employment is terminated on account of disability under Section 3(b) or by the Company for Cause under Section 3(c), the date on which Notice of Termination is given; (iii) if the Executive’s employment is terminated by the Company without Cause under Section 3(d), the date on which a Notice of Termination is given or the date otherwise specified by the Company in the Notice of Termination; (iv) if the Executive’s employment is terminated by the Executive under Section 3(e) other than for Good Reason, 30 days after the date on which a Notice of Termination is given, and (v) if the Executive’s employment is terminated by the Executive under Section 3(e) for Good Reason, the date on which a Notice of Termination is given after the end of the Cure Period. Notwithstanding the foregoing, in the event that the Executive gives a Notice of Termination to the Company, the Company may unilaterally accelerate the Date of Termination and such acceleration shall not result in a termination by the Company for purposes of this Agreement.
(c) Accrued Obligations. If the Executive’s employment with the Company is terminated for any reason, the Company shall pay or provide to the Executive (or to the Executive’s authorized representative or estate) (i) any Base Salary earned through the Date of Termination and, if applicable, any accrued but unused vacation through the Date of Termination; (ii) unpaid expense reimbursements (subject to, and in accordance with, Section 2(c) of this Agreement); and (iii) any vested benefits the Executive may have under any employee benefit plan of the Company through the Date of Termination, which vested benefits shall be paid and/or provided in accordance with the terms of such employee benefit plans (collectively, the “Accrued Obligations”).
(d) Resignation of All Other Positions. To the extent applicable, the Executive shall be deemed to have resigned from all officer and board member positions that the Executive holds with the Company or any of its respective subsidiaries and affiliates upon the termination of the Executive’s employment for any reason. The Executive shall execute any documents in reasonable form as may be requested to confirm or effectuate any such resignations.
5. Severance Pay and Benefits Upon Termination by the Company without Cause or by the Executive for Good Reason Outside the Change in Control Period.
5
If the Executive’s employment is terminated by the Company without Cause as provided in Section 3(d), or the Executive terminates employment for Good Reason as provided in Section 3(e), in each case outside of the Change in Control Period (as defined below), then, in addition to the Accrued Obligations, and subject to (i) the Executive signing a separation agreement and release in a form and manner satisfactory to the Company, which shall include, without limitation, a general release of claims against the Company and all related persons and entities that shall not release the Executive’s rights under this Agreement, a reaffirmation of all of the Executive’s Continuing Obligations (as defined below), and, in the Company’s sole discretion, a one-year post-employment noncompetition agreement, and shall provide that if the Executive breaches any of the Continuing Obligations, all payments of the Severance Amount shall immediately cease (the “Separation Agreement”), and (ii) the Separation Agreement becoming irrevocable, all within 60 days after the Date of Termination (or such shorter period as set forth in the Separation Agreement), which shall include a seven (7) business day revocation period:
(a) the Company shall pay the Executive an amount equal to twelve months of the Executive’s Base Salary (the “Severance Amount”); and
(b) subject to the Executive’s copayment of premium amounts at the applicable active employees’ rate and the Executive’s proper election to receive benefits under the Consolidated Omnibus Budget Reconciliation Act of 1985, as amended (“COBRA”), the Company shall pay to the group health plan provider(s) or the COBRA provider a monthly payment equal to the monthly employer contribution that the Company would have made to provide health insurance to the Executive if the Executive had remained employed by the Company until the earliest of (A) the twelve month anniversary of the Date of Termination; (B) the date that the Executive becomes eligible for group medical plan benefits under any other employer’s group medical plan; or (C) the cessation of the Executive’s health continuation rights under COBRA; provided, however, that if the Company determines that it cannot pay such amounts to the group health plan provider(s) or the COBRA provider (if applicable) without potentially violating applicable law (including, without limitation, Section 2716 of the Public Health Service Act), then the Company shall convert such payments to payroll payments directly to the Executive for the time period specified above. Such payments to the Executive shall be subject to tax-related deductions and withholdings and paid on the Company’s regular payroll dates.
The amounts payable under Section 5, to the extent taxable, shall be paid out in substantially equal installments in accordance with the Company’s payroll practice over twelve months commencing within 60 days after the Date of Termination; provided, however, that if the 60-day period begins in one calendar year and ends in a second calendar year, such payments, to the extent they qualify as “non-qualified deferred compensation” within the meaning of Section 409A of the Internal Revenue Code of 1986, as amended (the “Code”), shall begin to be paid in the second calendar year by the last day of such 60-day period; provided, further, that the initial payment shall include a catch-up payment to cover amounts retroactive to the day immediately following the Date of Termination. Each payment pursuant to this Agreement is intended to constitute a separate payment for purposes of Treasury Regulation Section 1.409A-2(b)(2).
6
6. Severance Pay and Benefits Upon Termination by the Company without Cause or by the Executive for Good Reason within the Change in Control Period. The provisions of this Section 6 shall apply in lieu of, and expressly supersede, the provisions of Section 5 if (i) the Executive’s employment is terminated either (a) by the Company without Cause as provided in Section 3(d), or (b) by the Executive for Good Reason as provided in Section 3(e), and (ii) the Date of Termination is on or within 12 months after the occurrence of the first event constituting a Change in Control (such period, the “Change in Control Period”). These provisions shall terminate and be of no further force or effect after the Change in Control Period.
(a) If the Executive’s employment is terminated by the Company without Cause as provided in Section 3(d) or the Executive terminates employment for Good Reason as provided in Section 3(e) and in each case the Date of Termination occurs during the Change in Control Period, then, in addition to the Accrued Obligations, and subject to the signing of a general release of claims against the Company and all related persons and entities that shall not release the Executive’s rights under this Agreement (the “Release”) by the Executive and the Release becoming fully effective, all within the time frame set forth in the Release but in no event more than 60 days after the Date of Termination:
(i) the Company shall pay the Executive a lump sum in cash in an amount equal to 1.5 times the sum of (A) the Executive’s then-current Base Salary (or the Executive’s Base Salary in effect immediately prior to the Change in Control, if higher) plus (B) the Executive’s Target Bonus for the then-current year (or the Executive’s Target Bonus in effect immediately prior to the Change in Control, if higher); and
(ii) notwithstanding anything to the contrary in any applicable option agreement or other stock-based award agreement, all time-based stock options and other stock-based awards subject to time-based vesting held by the Executive (the “Time-Based Equity Awards”) shall immediately accelerate and become fully exercisable or nonforfeitable as of the later of (i) the Date of Termination or (ii) the effective date of the Separation Agreement and Release (the “Accelerated Vesting Date”); provided that any termination or forfeiture of the unvested portion of such Time-Based Equity Awards that would otherwise occur on the Date of Termination in the absence of this Agreement will be delayed until the effective date of the Separation Agreement and Release and will only occur if the vesting pursuant to this subsection does not occur due to the absence of the Separation Agreement and Release becoming fully effective within the time period set forth therein. Notwithstanding the foregoing, no additional vesting of the Time-Based Equity Awards shall occur during the period between the Executive’s Date of Termination and the Accelerated Vesting Date; and
(iii) subject to the Executive’s copayment of premium amounts at the applicable active employees’ rate and the Executive’s proper election to receive benefits under COBRA, the Company shall pay to the group health plan provider(s) or the COBRA provider a monthly payment equal to the monthly employer contribution that the Company would have made to provide health insurance to the Executive if the Executive had remained employed by the Company until the earliest of (A) the eighteen month anniversary of the Date of Termination; (B) the date that the Executive becomes eligible for group medical plan benefits under any other employer’s group medical plan; or (C) the cessation of the Executive’s health continuation rights under COBRA; provided, however, that if the Company determines that it cannot pay such amounts to the group health plan provider(s) or the COBRA provider (if applicable) without potentially violating applicable law (including, without limitation, Section 2716 of the Public Health Service Act), then the Company shall convert such payments to payroll payments directly to the Executive for the time period specified above.
7
Such payments to the Executive shall be subject to tax-related deductions and withholdings and paid on the Company’s regular payroll dates.
The amounts payable under this Section 6(a), to the extent taxable, shall be paid or commence to be paid within 60 days after the Date of Termination; provided, however, that if the 60-day period begins in one calendar year and ends in a second calendar year, such payments to the extent they qualify as “non-qualified deferred compensation” within the meaning of Section 409A of the Code, shall be paid or commence to be paid in the second calendar year by the last day of such 60-day period.
(b) Additional Limitation.
(i) Anything in this Agreement to the contrary notwithstanding, in the event that the amount of any compensation, payment or distribution by the Company to or for the benefit of the Executive, whether paid or payable or distributed or distributable pursuant to the terms of this Agreement or otherwise, calculated in a manner consistent with Section 280G of the Code, and the applicable regulations thereunder (the “Aggregate Payments”), would be subject to the excise tax imposed by Section 4999 of the Code, then the Aggregate Payments shall be reduced (but not below zero) so that the sum of all of the Aggregate Payments shall be $1.00 less than the amount at which the Executive becomes subject to the excise tax imposed by Section 4999 of the Code; provided that such reduction shall only occur if it would result in the Executive receiving a higher After Tax Amount (as defined below) than the Executive would receive if the Aggregate Payments were not subject to such reduction. In such event, the Aggregate Payments shall be reduced in the following order, in each case, in reverse chronological order beginning with the Aggregate Payments that are to be paid the furthest in time from consummation of the transaction that is subject to Section 280G of the Code: (1) cash payments not subject to Section 409A of the Code; (2) cash payments subject to Section 409A of the Code; (3) equity-based payments and acceleration; and (4) non-cash forms of benefits; provided that in the case of all the foregoing Aggregate Payments all amounts or payments that are not subject to calculation under Treas. Reg. §1.280G-1, Q&A-24(b) or (c) shall be reduced before any amounts that are subject to calculation under Treas. Reg. §1.280G-1, Q&A-24(b) or (c).
(ii) For purposes of this Section 6(b), the “After Tax Amount” means the amount of the Aggregate Payments less all federal, state, and local income, excise and employment taxes imposed on the Executive as a result of the Executive’s receipt of the Aggregate Payments. For purposes of determining the After Tax Amount, the Executive shall be deemed to pay federal income taxes at the highest marginal rate of federal income taxation applicable to individuals for the calendar year in which the determination is to be made, and state and local income taxes at the highest marginal rates of individual taxation in each applicable state and locality, net of the maximum reduction in federal income taxes which could be obtained from deduction of such state and local taxes.
8
(iii) The determination as to whether a reduction in the Aggregate Payments shall be made pursuant to Section 6(b)(i) shall be made by a nationally recognized accounting firm selected by the Company (the “Accounting Firm”), which shall provide detailed supporting calculations both to the Company and the Executive within 15 business days of the Date of Termination, if applicable, or at such earlier time as is reasonably requested by the Company or the Executive. Any determination by the Accounting Firm shall be binding upon the Company and the Executive.
(c) Definitions. For purposes of this Section 6, “Change in Control” shall mean a “Sale Event” as defined in the Company’s 2021 Stock Option and Incentive Plan.
7. Section 409A.
(a) Anything in this Agreement to the contrary notwithstanding, if at the time of the Executive’s separation from service within the meaning of Section 409A of the Code, the Company determines that the Executive is a “specified employee” within the meaning of Section 409A(a)(2)(B)(i) of the Code, then to the extent any payment or benefit that the Executive becomes entitled to under this Agreement or otherwise on account of the Executive’s separation from service would be considered deferred compensation otherwise subject to the 20 percent additional tax imposed pursuant to Section 409A(a) of the Code as a result of the application of Section 409A(a)(2)(B)(i) of the Code, such payment shall not be payable and such benefit shall not be provided until the date that is the earlier of (A) six months and one day after the Executive’s separation from service, or (B) the Executive’s death. If any such delayed cash payment is otherwise payable on an installment basis, the first payment shall include a catch-up payment covering amounts that would otherwise have been paid during the six-month period but for the application of this provision, and the balance of the installments shall be payable in accordance with their original schedule.
(b) All in-kind benefits provided and expenses eligible for reimbursement under this Agreement shall be provided by the Company or incurred by the Executive during the time periods set forth in this Agreement. All reimbursements shall be paid as soon as administratively practicable, but in no event shall any reimbursement be paid after the last day of the taxable year following the taxable year in which the expense was incurred. The amount of in-kind benefits provided or reimbursable expenses incurred in one taxable year shall not affect the in-kind benefits to be provided or the expenses eligible for reimbursement in any other taxable year (except for any lifetime or other aggregate limitation applicable to medical expenses). Such right to reimbursement or in-kind benefits is not subject to liquidation or exchange for another benefit.
(c) To the extent that any payment or benefit described in this Agreement constitutes “non-qualified deferred compensation” under Section 409A of the Code, and to the extent that such payment or benefit is payable upon the Executive’s termination of employment, then such payments or benefits shall be payable only upon the Executive’s “separation from service.” The determination of whether and when a separation from service has occurred shall be made in accordance with the presumptions set forth in Treasury Regulation Section 1.409A-1(h).
9
(d) The parties intend that this Agreement will be administered in accordance with Section 409A of the Code. To the extent that any provision of this Agreement is ambiguous as to its compliance with Section 409A of the Code, the provision shall be read in such a manner so that all payments hereunder comply with Section 409A of the Code. Each payment pursuant to this Agreement is intended to constitute a separate payment for purposes of Treasury Regulation Section 1.409A-2(b)(2). The parties agree that this Agreement may be amended, as reasonably requested by either party, and as may be necessary to fully comply with Section 409A of the Code and all related rules and regulations in order to preserve the payments and benefits provided hereunder without additional cost to either party.
(e) The Company makes no representation or warranty and shall have no liability to the Executive or any other person if any provisions of this Agreement are determined to constitute deferred compensation subject to Section 409A of the Code but do not satisfy an exemption from, or the conditions of, such Section.
8. Continuing Obligations.
(a) Restrictive Covenants Agreements. The terms of the Invention and Non-Disclosure Agreement dated April 15, 2016 and the Non-Competition and Non-Solicitation Agreement dated April 15, 2016 (together, the “Restrictive Covenants Agreements”), between the Company and the Executive, attached hereto as Exhibit A and Exhibit B, respectively, continue to be in full force and effect. For purposes of this Agreement, the obligations in this Section 8 and those that arise in the Restrictive Covenants Agreements and any other agreement relating to confidentiality, assignment of inventions, or other restrictive covenants shall collectively be referred to as the “Continuing Obligations.”
(b) Third-Party Agreements and Rights. The Executive hereby confirms that the Executive is not bound by the terms of any agreement with any previous employer or other party which restricts in any way the Executive’s use or disclosure of information, other than confidentiality restrictions (if any), or the Executive’s engagement in any business. The Executive represents to the Company that the Executive’s execution of this Agreement, the Executive’s employment with the Company and the performance of the Executive’s proposed duties for the Company will not violate any obligations the Executive may have to any such previous employer or other party. In the Executive’s work for the Company, the Executive will not disclose or make use of any information in violation of any agreements with or rights of any such previous employer or other party, and the Executive will not bring to the premises of the Company any copies or other tangible embodiments of non-public information belonging to or obtained from any such previous employment or other party.
(c) Litigation and Regulatory Cooperation. During and after the Executive’s employment, the Executive shall cooperate fully with the Company in (i) the defense or prosecution of any claims or actions now in existence or which may be brought in the future against or on behalf of the Company which relate to events or occurrences that transpired while the Executive was employed by the Company, and (ii) the investigation, whether internal or external, of any matters about which the Company believes the Executive may have knowledge or information. The Executive’s full cooperation in connection with such claims, actions or investigations shall include, but not be limited to, being available to meet with counsel to answer questions or to prepare for discovery or trial and to act as a witness on behalf of the Company at mutually convenient times.
10
During and after the Executive’s employment, the Executive also shall cooperate fully with the Company in connection with any investigation or review of any federal, state or local regulatory authority as any such investigation or review relates to events or occurrences that transpired while the Executive was employed by the Company. The Company shall reimburse the Executive for any reasonable out-of-pocket expenses incurred in connection with the Executive’s performance of obligations pursuant to this Section 8(c).
(d) Relief. The Executive agrees that it would be difficult to measure any damages caused to the Company which might result from any breach by the Executive of the Continuing Obligations, and that in any event money damages would be an inadequate remedy for any such breach. Accordingly, the Executive agrees that if the Executive breaches, or proposes to breach, any portion of the Continuing Obligations, the Company shall be entitled, in addition to all other remedies that it may have, to an injunction or other appropriate equitable relief to restrain any such breach without showing or proving any actual damage to the Company.
9. Consent to Jurisdiction. The parties hereby submit to the exclusive jurisdiction of the state and federal courts of the Commonwealth of Massachusetts with respect to any dispute arising under this Agreement.
10. Waiver of Jury Trial. Each of the Executive and the Company irrevocably and unconditionally WAIVES ALL RIGHT TO TRIAL BY JURY IN ANY PROCEEDING (WHETHER BASED ON CONTRACT, TORT OR OTHERWISE) ARISING OUT OF OR RELATING TO THIS AGREEMENT OR THE EXECUTIVE’S EMPLOYMENT BY THE COMPANY OR ANY AFFILIATE OF THE COMPANY, INCLUDING WITHOUT LIMITATION THE EXECUTIVE’S OR THE COMPANY’S PERFORMANCE UNDER, OR THE ENFORCEMENT OF, THIS AGREEMENT.
11. Integration. This Agreement constitutes the entire agreement between the parties with respect to the subject matter hereof and supersedes all prior agreements between the parties concerning such subject matter, including the Prior Agreement, provided that the Restrictive Covenants Agreements and the Equity Documents remain in full force and effect.
12. Withholding; Tax Effect. All payments made by the Company to the Executive under this Agreement shall be net of any tax or other amounts required to be withheld by the Company under applicable law. Nothing in this Agreement shall be construed to require the Company to make any payments to compensate the Executive for any adverse tax effect associated with any payments or benefits or for any deduction or withholding from any payment or benefit.
13. Assignment; Successors and Assigns. Neither the Executive nor the Company may make any assignment of this Agreement or any interest in it, by operation of law or otherwise, without the prior written consent of the other; provided, however, that the Company may assign its rights and obligations under this Agreement (including the Restrictive Covenants Agreements) without the Executive’s consent to any affiliate or to any person or entity with whom the Company shall hereafter effect a reorganization or consolidation, into which the Company merges or to whom it transfers all or substantially all of its properties or assets; provided, further that if the Executive remains employed or becomes employed by the Company, the purchaser or any of their affiliates in connection with any such transaction, then the Executive shall not be entitled to any payments, benefits or vesting pursuant to Section 5 or pursuant to Section 6 of this Agreement solely as a result of such transaction.
11
This Agreement shall inure to the benefit of and be binding upon the Executive and the Company, and each of the Executive’s and the Company’s respective successors, executors, administrators, heirs and permitted assigns. In the event of the Executive’s death after the Executive’s termination of employment but prior to the completion by the Company of all payments due to the Executive under this Agreement, the Company shall continue such payments to the Executive’s beneficiary designated in writing to the Company prior to the Executive’s death (or to the Executive’s estate, if the Executive fails to make such designation).
14. Enforceability. If any portion or provision of this Agreement (including, without limitation, any portion or provision of any section of this Agreement) shall to any extent be declared illegal or unenforceable by a court of competent jurisdiction, then the remainder of this Agreement, or the application of such portion or provision in circumstances other than those as to which it is so declared illegal or unenforceable, shall not be affected thereby, and each portion and provision of this Agreement shall be valid and enforceable to the fullest extent permitted by law.
15. Survival. For the avoidance of doubt, this Agreement shall survive the termination of the Executive’s employment to the extent necessary to effectuate the terms contained herein.
16. Waiver. No waiver of any provision hereof shall be effective unless made in writing and signed by the waiving party. The failure of any party to require the performance of any term or obligation of this Agreement, or the waiver by any party of any breach of this Agreement, shall not prevent any subsequent enforcement of such term or obligation or be deemed a waiver of any subsequent breach.
17. Notices. Any notices, requests, demands and other communications provided for by this Agreement shall be sufficient if in writing and delivered in person or sent by a nationally recognized overnight courier service or by registered or certified mail, postage prepaid, return receipt requested, to the Executive at the last address the Executive has filed in writing with the Company or, in the case of the Company, at its main offices, attention of the Board. Notices may also be sent by email to the last email address of the Executive or the Chairman of the Board, as the case may be; provided that such email notice is promptly thereafter confirmed by one of the foregoing methods. For purposes of email notice, the applicable email address of the Executive shall be the most recent email address that the Executive has provided to the Company, whereas the Chairman’s email address shall be the Chairman’s regular business email address as of the date of notice. Notices delivered in person or by email shall be effective on the date of notice. Notices delivered by overnight courier service shall be effective on the next business day after mailing. Notices delivered by registered or certified mail shall be effective three business days after mailing.
18. Amendment. This Agreement may be amended or modified only by a written instrument signed by the Executive and by a duly authorized representative of the Company.
12
19. Effect on Other Plans and Agreements. An election by the Executive to resign for Good Reason under the provisions of this Agreement shall not be deemed a voluntary termination of employment by the Executive for the purpose of interpreting the provisions of any of the Company’s benefit plans, programs or policies. Nothing in this Agreement shall be construed to limit the rights of the Executive under the Company’s benefit plans, programs or policies except as otherwise provided in Section 8 hereof, and except that the Executive shall have no rights to any severance benefits under any Company severance pay plan, offer letter or otherwise. In the event that the Executive is party to an agreement with the Company providing for payments or benefits under such plan or agreement and under this Agreement, the terms of this Agreement shall govern and the Executive may receive payment under this Agreement only and not both. Further, Section 5 and Section 6 of this Agreement are mutually exclusive and in no event shall the Executive be entitled to payments or benefits pursuant to both Section 5 and Section 6 of this Agreement.
20. Governing Law. This is a Massachusetts contract and shall be construed under and be governed in all respects by the laws of the Commonwealth of Massachusetts, without giving effect to the conflict of laws principles thereof. With respect to any disputes concerning federal law, such disputes shall be determined in accordance with the law as it would be interpreted and applied by the United States Court of Appeals for the First Circuit.
21. Counterparts. This Agreement may be executed in separate counterparts. When both counterparts are signed, they shall be treated together as one and the same document. PDF copies of signed counterparts shall be equally effective as originals.
13
IN WITNESS WHEREOF, the parties have caused this Agreement to be signed as of the day and year first above written.
| IKENA ONCOLOGY, INC. | ||
| By: | /s/ Douglas R. Carlson |
|
| Name: Douglas R. Carlson | ||
| Title: Chief Operating Officer | ||
| EXECUTIVE |
| /s/ Mark Manfredi, Ph.D. |
| Mark Manfredi, Ph.D. |
14
Exhibit A
Invention and Non-Disclosure Agreement
ATTACHMENT A
LIST OF PRIOR INVENTIONS AND ORIGINAL WORKS OF AUTHORSHIP EXCLUDED UNDER
SECTION 3(A) OR CONFLICTING AGREEMENTS DISCLOSED UNDER SECTION 4
Exhibit B
Non-Competition and Non-Solicitation Agreement
EXHIBIT B
KVN THERAPEUTICS INC.
NON-COMPETITION AND NON-SOLICITATION AGREEMENT
Exhibit 10.18
July 23, 2025
Jonathan Jian Wang, Ph.D., MBA
Via email to [***]
| Re: | Separation Agreement |
Dear Dr. Wang:
This letter sets forth the substance of the separation agreement (the “Agreement”) that Inmagene Biopharmaceuticals (the “Company”) is offering to you to aid in your employment transition.
1. SEPARATION. As you know, the Company has entered into that certain Agreement and Plan of Merger by and between the Company, Ikena Oncology, Inc. (“Ikena”), and the other parties thereto (the “Merger Agreement”), pursuant to which the Company and Ikena intend to undertake a series of merger transactions (collectively, the “Merger”). Your employment termination date will be simultaneous with the Second Effective Time, as defined in the Meger Agreement, which is currently anticipated to be July 25, 2025 (such actual date, the “Separation Date”). In the event the Merger does not close, this Agreement shall be of no force and effect and shall terminate as of the termination of the Merger Agreement.
2. FINAL COMPENSATION. The Company will pay you all compensation earned through the Separation Date, subject to standard payroll deductions and withholdings. To the extent provided by the federal COBRA law or, if applicable, state insurance laws (collectively, “COBRA”), and by the Company’s current group health insurance policies, you will be eligible to continue your group health insurance benefits after the Separation Date at your own expense. Later, you may be able to convert to an individual policy through the provider of the Company’s health insurance, if you wish. You are entitled to these payments and benefits regardless of whether or not you sign this Agreement. Vesting of any equity awards you may have received from the Company will cease as of the Separation Date. Your equity awards shall continue to be governed by the terms of the applicable grant notices, award agreements, and governing plan documents.
3. SEVERANCE BENEFITS. Although the Company has no obligation to do so, if you: (i) sign and return this Agreement to the Company on or within twenty-one (21) calendar days after the date hereof (but not prior to the Separation Date); (ii) allow the releases contained herein to become effective; and (iii) comply with all of your legal and contractual obligations to the Company, then the Company will provide you with the following severance benefits (the “Severance Benefits”): (1) an amount equivalent to ten (10) months of your current base salary, in the gross amount of $405,450.00, subject to standard payroll deductions and withholdings and (2) ten (10) months of COBRA continuation, in each case, pursuant to the terms set forth in the Severance Rights Agreement between you and the Company, dated March 6, 2025 (the “Severance Agreement”).
Page 2
4. NO OTHER COMPENSATION OR BENEFITS. You acknowledge that, except as expressly provided in this Agreement, you have not earned and will not receive from the Company any additional compensation (including base salary, bonus, incentive compensation, equity, equity acceleration, or vesting), severance, or benefits before or after the Separation Date, with the exception of any vested right you may have under the express terms of a written ERISA-qualified benefit plan (e.g., 401(k) account). You agree that the Severance Benefits provided in this Agreement are in full satisfaction of any obligation of the Company to provide you with benefits under the Severance Agreement and that you will not receive any other separation benefits, including severance, equity acceleration, or health care continuation, other than as set forth in this Agreement.
5. EXPENSE REIMBURSEMENTS. You agree that, within thirty (30) calendar days after the Separation Date, you will submit your final documented expense reimbursement statement reflecting all business expenses you incurred through the Separation Date, if any, for which you seek reimbursement. The Company will reimburse you for these expenses pursuant to its regular business practice.
6. CONFIDENTIALITY. The provisions of this Agreement will be held in strictest confidence by you and will not be publicized or disclosed by you in any manner whatsoever; provided, however, that: (a) you may disclose this Agreement in confidence to your immediate family and to your attorneys, accountants, tax preparers and financial advisors; (b) you may disclose this Agreement pursuant to a government investigation, if necessary to enforce its terms, or as otherwise required by law; and (c) you may disclose this Agreement to the extent permitted by the “Protected Rights” section below.
7. NO ADMISSIONS. You understand and agree that the promises and payments in consideration of this Agreement shall not be construed to be an admission of any liability or obligation by the Company to you or to any other person, and that the Company makes no such admission.
8. RELEASE OF CLAIMS
(a) General Release. In exchange for the consideration provided to you under this Agreement to which you would not otherwise be entitled, you hereby generally and completely release the Company, Ikena Oncology, Inc., and each of its and their past, present, and future affiliated, related, parent and subsidiary entities, and its and their directors, officers, employees, shareholders, partners, agents, attorneys, predecessors, successors, insurers, affiliates, and assigns (collectively, the “Released Parties”) from any and all claims, liabilities and obligations, both known and unknown, that arise out of or are in any way related to events, acts, conduct, or omissions occurring prior to or on the date you sign this Agreement (collectively, the “Released Claims”).
Page 3
(b) Scope of Release. The Released Claims include, but are not limited to: (i) all claims arising out of or in any way related to your employment with the Company, or the termination of that employment; (ii) all claims related to your compensation or benefits from the Company, including salary, bonuses, commissions, vacation, expense reimbursements, severance pay, fringe benefits, stock, stock options, or any other ownership, equity, or profits interests in the Company; (iii) all claims for breach of contract, wrongful termination, and breach of the implied covenant of good faith and fair dealing; (iv) all tort claims, including claims for fraud, defamation, emotional distress, and discharge in violation of public policy; and (v) all federal, state, and local statutory claims, including claims for discrimination, harassment, retaliation, attorneys’ fees, or other claims arising under the federal Civil Rights Act of 1964 (as amended), the federal Americans with Disabilities Act of 1990, the federal Age Discrimination in Employment Act of 1967 (as amended) (the “ADEA”), the California Labor Code (as amended), the California Family Rights Act (as amended), and the California Fair Employment and Housing Act (as amended). You acknowledge that you have been advised, as required by California Government Code Section 12964.5(b)(4), that you have the right to consult an attorney regarding this Agreement and that you were given a reasonable time period of not less than five (5) business days in which to do so. You further acknowledge and agree that, in the event you sign this Agreement prior to the end of the reasonable time period provided by the Company, your decision to accept such shortening of time is knowing and voluntary and is not induced by the Company through fraud, misrepresentation, or a threat to withdraw or alter the offer prior to the expiration of the reasonable time period, or by providing different terms to employees who sign such an agreement prior to the expiration of the time period. You hereby represent that you have been paid all compensation owed and for all hours worked, have received all the leave and leave benefits and protections for which you are eligible pursuant to the Family and Medical Leave Act, the California Family Rights Act, or otherwise, and have not suffered any on-the-job injury for which you have not already filed a workers’ compensation claim.
(c) Excluded Claims. Notwithstanding the foregoing, the following are not included in the Released Claims (the “Excluded Claims”): (i) any rights or claims for indemnification you may have pursuant to any written indemnification agreement with the Company to which you are a party, applicable directors and officers liability insurance policy, or under applicable law; (ii) any rights which are not waivable as a matter of law; (iii) any claims for breach of this Agreement; and (iv) any rights or claims to receive consideration pursuant to the terms of the Merger Agreement.
(d) Protected Rights. You understand that nothing in this Agreement limits your ability to file a charge or complaint with the Equal Employment Opportunity Commission, the Department of Labor, the National Labor Relations Board, the Occupational Safety and Health Administration, the California Civil Rights Department, the Department of Justice, the Securities and Exchange Commission or any other federal, state or local governmental agency or commission (“Government Agencies”). You further understand this Agreement does not limit your ability to communicate with any Government Agencies or otherwise participate in any investigation or proceeding that may be conducted by any Government Agency, including providing documents or other information, without notice to the Company. While this Agreement does not limit your right to receive a government-issued award for information provided to any Government Agencies in connection with a government whistleblower program or protected whistleblower activity, you understand and agree that, to the maximum extent permitted by law, you are otherwise waiving any and all rights you may have to individual relief based on any claims that you have released and any rights you have waived by signing this Agreement. Nothing in this Agreement (i) prevents you from discussing or disclosing information about unlawful acts in the workplace, such as harassment or discrimination or any other conduct that you have reason to believe is unlawful; or (ii) waives any rights you may have under Section 7 of the National Labor Relations Act, if applicable (subject to the release of claims set forth herein).
Page 4
(e) ADEA Waiver. You acknowledge that you are knowingly and voluntarily waiving and releasing any rights you may have under the ADEA, and that the consideration given for the waiver and release in this section is in addition to anything of value to which you are already entitled. You further acknowledge that you have been advised, as required by the ADEA, that: (i) your waiver and release do not apply to any rights or claims that may arise after the date that you sign this Agreement; (ii) you should consult with an attorney prior to signing this Agreement (although you may choose voluntarily not to do so); (iii) you have twenty-one (21) calendar days to consider this Agreement (although you may choose voluntarily to sign it earlier, and changes to this Agreement, whether material or immaterial, do not restart the running of the twenty-one (21) calendar day period); (iv) you have seven (7) calendar days following the date you sign this Agreement to revoke it (by providing written notice of your revocation to me); and (v) this Agreement will not be effective until the date upon which the revocation period has expired, which will be the eighth calendar day after the date that this Agreement is signed by you provided that you do not revoke it (the “Effective Date”).
9. SECTION 1542 WAIVER. In giving the release herein, which includes claims which may be unknown to you at present, you acknowledge that you have read and understand Section 1542 of the California Civil Code, which reads as follows: “A general release does not extend to claims that the creditor or releasing party does not know or suspect to exist in his or her favor at the time of executing the release and that, if known by him or her, would have materially affected his or her settlement with the debtor or released party.” You hereby expressly waive and relinquish all rights and benefits under that section and any law of any other jurisdiction of similar effect with respect to your release of claims herein, including but not limited to your release of unknown claims.
10. CONTINUING OBLIGATIONS. You acknowledge and reaffirm your continuing obligations under any applicable confidentiality, intellectual property assignment, and restrictive covenant agreement, which is incorporated herein by reference, and agree to abide by those continuing obligations. You agree to comply with the return of property requirements set forth in the Severance Agreement as and when requested by the Company.
11. NON-DISPARAGEMENT. Except to the extent permitted by the “Protected Rights” section above, you agree not to disparage the Released Parties in any manner likely to be harmful to its or their business, business reputation, or personal reputation; provided that you may respond accurately and fully to any request for information if required by legal process, or in connection with a government investigation. In addition, nothing in this provision or this Agreement prohibits or restrains you from making disclosures protected under whistleblower provisions of federal or state law or from exercising your rights to engage in legally protected speech.
12. MISCELLANEOUS. This Agreement constitutes the complete, final and exclusive embodiment of the entire agreement between you and the Company with regard to its subject matter. It is entered into without reliance on any promise or representation, written or oral, other than those expressly contained herein, and it supersedes any other such promises, warranties or
Page 5
representations. This Agreement may not be modified or amended except in a writing signed by both you and a duly authorized officer of the Company. This Agreement will bind the heirs, personal representatives, successors and assigns of both you and the Company, and inure to the benefit of both you and the Company, their heirs, successors and assigns. The Company may freely assign this Agreement, without your prior written consent. You may not assign any of your duties hereunder and you may not assign any of your rights hereunder without the written consent of the Company. If any provision of this Agreement is determined to be invalid or unenforceable, in whole or in part, this determination will not affect any other provision of this Agreement and the provision in question will be modified so as to be rendered enforceable. This Agreement will be deemed to have been entered into and will be construed and enforced in accordance with the laws of the State of California without regard to conflict of laws principles. Any ambiguity in this Agreement shall not be construed against either party as the drafter. Any waiver of a breach of this Agreement shall be in writing and shall not be deemed to be a waiver of any successive breach. This Agreement may be executed in counterparts which shall be deemed to be part of one original, and facsimile and electronic image signatures (including .pdf or any electronic signature complying with the U.S. federal ESIGN Act of 2000, Uniform Electronic Transactions Act, or other applicable law) shall be equivalent to original signatures.
If this Agreement is acceptable to you, please sign below and return the original to me. You have twenty-one (21) calendar days from the date hereof to decide whether you would like to accept this Agreement, and the Company’s offer contained herein will automatically expire if you do not sign and return it within this timeframe. Although you may be provided this Agreement prior to the Separation Date for your review, you may not sign it until on or after the Separation Date.
We wish you the best in your future endeavors.
Sincerely,
| INMAGENE BIOPHARMACEUTICALS | ||
| By: | /s/ Guoliang Yu | |
| Guoliang Yu | ||
| Director | ||
I HAVE READ, UNDERSTAND AND AGREE FULLY TO THE FOREGOING AGREEMENT:
| /s/ Jonathan Jian Wang |
| Jonathan Jian Wang, Ph.D., MBA |
Date: July 23, 2025
Exhibit 10.20
IMAGENEBIO, INC.
2025 EQUITY INCENTIVE PLAN
ADOPTED BY THE BOARD OF DIRECTORS: DECEMBER 12, 2024
APPROVED BY THE STOCKHOLDERS: JULY 15, 2025
1. GENERAL.
(a) Successor to and Continuation of Prior Plan. The Plan is the successor to and continuation of the Prior Plan. As of the Effective Date, (i) no additional awards may be granted under the Prior Plan; (ii) any Returning Shares will become available for issuance pursuant to Awards granted under this Plan; and (iii) all outstanding awards granted under the Prior Plan will remain subject to the terms of the Prior Plan (except to the extent such outstanding awards result in Returning Shares that become available for issuance pursuant to Awards granted under this Plan). All Awards granted under this Plan will be subject to the terms of this Plan.
(b) Plan Purpose. The Company, by means of the Plan, seeks to secure and retain the services of Employees, Directors and Consultants, to provide incentives for such persons to exert maximum efforts for the success of the Company and any Affiliate and to provide a means by which such persons may be given an opportunity to benefit from increases in value of the Common Stock through the granting of Awards.
(c) Available Awards. The Plan provides for the grant of the following Awards: (i) Incentive Stock Options; (ii) Nonstatutory Stock Options; (iii) SARs; (iv) Restricted Stock Awards; (v) RSU Awards; (vi) Performance Awards; and (vii) Other Awards.
(d) Adoption Date; Effective Date. The Plan will come into existence on the Adoption Date, but no Award may be granted prior to the Effective Date.
2. SHARES SUBJECT TO THE PLAN.
(a) Share Reserve. Subject to adjustment in accordance with Section 2(c) and any adjustments as necessary to implement any Capitalization Adjustments, the aggregate number of shares of Common Stock that may be issued pursuant to Awards will not exceed 1,118,168 shares, which is the sum of: (i) 1,118,168 new shares, plus (ii) up to 0 Returning Shares, as such shares become available from time to time. In addition, subject to any adjustments as necessary to implement any Capitalization Adjustments, such aggregate number of shares of Common Stock will automatically increase on January 1 of each year for a period of ten years commencing on January 1, 2026 and ending on (and including) January 1, 2035, in an amount equal to five percent (5%) of the total number of shares of Capital Stock outstanding on December 31 of the preceding year; provided, however, that the Board may act prior to January 1st of a given year to provide that the increase for such year will be a lesser number of shares of Common Stock.
(b) Aggregate Incentive Stock Option Limit. Notwithstanding anything to the contrary in Section 2(a) and subject to any adjustments as necessary to implement any Capitalization Adjustments, the aggregate maximum number of shares of Common Stock that may be issued pursuant to the exercise of Incentive Stock Options is 3,419,172 shares.
1
(c) Share Reserve Operation.
(i) Limit Applies to Common Stock Issued Pursuant to Awards. For clarity, the Share Reserve is a limit on the number of shares of Common Stock that may be issued pursuant to Awards and does not limit the granting of Awards, except that the Company will keep available at all times the number of shares of Common Stock reasonably required to satisfy its obligations to issue shares pursuant to such Awards. Shares may be issued in connection with a merger or acquisition as permitted by, as applicable, Nasdaq Listing Rule 5635(c), NYSE Listed Company Manual Section 303A.08, NYSE American Company Guide Section 711 or other applicable rule, and such issuance will not reduce the number of shares available for issuance under the Plan.
(ii) Actions that Do Not Constitute Issuance of Common Stock and Do Not Reduce Share Reserve. The following actions do not result in an issuance of shares under the Plan and accordingly do not reduce the number of shares subject to the Share Reserve and available for issuance under the Plan: (1) the expiration or termination of any portion of an Award without the shares covered by such portion of the Award having been issued; (2) the settlement of any portion of an Award in cash (i.e., the Participant receives cash rather than Common Stock); (3) the withholding of shares that would otherwise be issued by the Company to satisfy the exercise, strike or purchase price of an Award; or (4) the withholding of shares that would otherwise be issued by the Company to satisfy a tax withholding obligation in connection with an Award.
(iii) Reversion of Previously Issued Shares of Common Stock to Share Reserve. The following shares of Common Stock previously issued pursuant to an Award and accordingly initially deducted from the Share Reserve will be added back to the Share Reserve and again become available for issuance under the Plan: (1) any shares that are forfeited back to or repurchased by the Company because of a failure to meet a contingency or condition required for the vesting of such shares; (2) any shares that are reacquired by the Company to satisfy the exercise, strike or purchase price of an Award; and (3) any shares that are reacquired by the Company to satisfy a tax withholding obligation in connection with an Award.
3. ELIGIBILITY AND LIMITATIONS.
(a) Eligible Award Recipients. Subject to the terms of the Plan, Employees, Directors and Consultants are eligible to receive Awards.
(b) Specific Award Limitations.
(i) Limitations on Incentive Stock Option Recipients. Incentive Stock Options may be granted only to Employees of the Company or a “parent corporation” or “subsidiary corporation” thereof (as such terms are defined in Sections 424(e) and (f) of the Code).
(ii) Incentive Stock Option $100,000 Limitation. To the extent that the aggregate Fair Market Value (determined at the time of grant) of Common Stock with respect to which Incentive Stock Options are exercisable for the first time by any Optionholder during any calendar year (under all plans of the Company and any Affiliates) exceeds $100,000 (or such other limit established in the Code) or otherwise does not comply with the rules governing Incentive Stock Options, the Options or portions thereof that exceed such limit (according to the order in which they were granted) or otherwise do not comply with such rules will be treated as Nonstatutory Stock Options, notwithstanding any contrary provision of the applicable Option Agreement(s).
2
(iii) Limitations on Incentive Stock Options Granted to Ten Percent Stockholders. A Ten Percent Stockholder may not be granted an Incentive Stock Option unless (1) the exercise price of such Option is at least 110% of the Fair Market Value on the date of grant of such Option and (2) the Option is not exercisable after the expiration of five years from the date of grant of such Option.
(iv) Limitations on Nonstatutory Stock Options and SARs. Nonstatutory Stock Options and SARs may not be granted to Employees, Directors and Consultants unless the stock underlying such Awards is treated as “service recipient stock” under Section 409A or unless such Awards otherwise comply with the requirements of Section 409A.
(c) Aggregate Incentive Stock Option Limit. The aggregate maximum number of shares of Common Stock that may be issued pursuant to the exercise of Incentive Stock Options is the number of shares specified in Section 2(b).
(d) Non-Employee Director Compensation Limit. The aggregate value of all compensation granted or paid, as applicable, to any individual for service as a Non-Employee Director with respect to any calendar year, including Awards granted and cash fees paid by the Company to such Non-Employee Director, will not exceed (1) $750,000 in total value or (2) in the event such Non-Employee Director is first appointed or elected to the Board during such calendar year, $1,000,000 in total value, in each case, calculating the value of any equity awards based on the grant date fair value of such equity awards for financial reporting purposes.
4. OPTIONS AND STOCK APPRECIATION RIGHTS.
Each Option and SAR will have such terms and conditions as determined by the Board. Each Option will be designated in writing as an Incentive Stock Option or Nonstatutory Stock Option at the time of grant; provided, however, that if an Option is not so designated or if an Option designated as an Incentive Stock Option fails to qualify as an Incentive Stock Option, then such Option will be a Nonstatutory Stock Option, and the shares purchased upon exercise of each type of Option will be separately accounted for. Each SAR will be denominated in shares of Common Stock equivalents. The terms and conditions of separate Options and SARs need not be identical; provided, however, that each Option Agreement and SAR Agreement will conform (through incorporation of provisions hereof by reference in the Award Agreement or otherwise) to the substance of each of the following provisions:
(a) Term. Subject to Section 3(b) regarding Ten Percent Stockholders, no Option or SAR will be exercisable after the expiration of ten years from the date of grant of such Award or such shorter period specified in the Award Agreement.
(b) Exercise or Strike Price. Subject to Section 3(b) regarding Ten Percent Stockholders, the exercise or strike price of each Option or SAR will not be less than 100% of the Fair Market Value on the date of grant of such Award. Notwithstanding the foregoing, an Option or SAR may be granted with an exercise or strike price lower than 100% of the Fair Market Value on the date of grant of such Award if such Award is granted pursuant to an assumption of or substitution for another option or stock appreciation right pursuant to a Corporate Transaction and in a manner consistent with the provisions of Sections 409A and, if applicable, 424(a) of the Code.
3
(c) Exercise Procedure and Payment of Exercise Price for Options. In order to exercise an Option, the Participant must provide notice of exercise to the Plan Administrator in accordance with the procedures specified in the Option Agreement or otherwise provided by the Company. The Board has the authority to grant Options that do not permit all of the following methods of payment (or otherwise restrict the ability to use certain methods) and to grant Options that require the consent of the Company to utilize a particular method of payment. The exercise price of an Option may be paid, to the extent permitted by Applicable Law and as determined by the Board, by one or more of the following methods of payment to the extent set forth in the Option Agreement:
(i) by cash or check, bank draft or money order payable to the Company;
(ii) pursuant to a “cashless exercise” program developed under Regulation T as promulgated by the U.S. Federal Reserve Board that, prior to the issuance of the Common Stock subject to the Option, results in either the receipt of cash (or check) by the Company or the receipt of irrevocable instructions to pay the exercise price to the Company from the sales proceeds;
(iii) by delivery to the Company (either by actual delivery or attestation) of shares of Common Stock that are already owned by the Participant free and clear of any liens, claims, encumbrances or security interests, with a Fair Market Value on the date of exercise that does not exceed the exercise price, provided that (1) at the time of exercise the Common Stock is publicly traded, (2) any remaining balance of the exercise price not satisfied by such delivery is paid by the Participant in cash or other permitted form of payment, (3) such delivery would not violate any Applicable Law or agreement restricting the redemption of the Common Stock, (4) any certificated shares are endorsed or accompanied by an executed assignment separate from certificate, and (5) such shares have been held by the Participant for any minimum period necessary to avoid adverse accounting treatment as a result of such delivery;
(iv) if the Option is a Nonstatutory Stock Option, by a “net exercise” arrangement pursuant to which the Company will reduce the number of shares of Common Stock issuable upon exercise by the largest whole number of shares with a Fair Market Value on the date of exercise that does not exceed the exercise price, provided that (1) such shares used to pay the exercise price will not be exercisable thereafter and (2) any remaining balance of the exercise price not satisfied by such net exercise is paid by the Participant in cash or other permitted form of payment; or
(v) in any other form of consideration that may be acceptable to the Board and permissible under Applicable Law.
4
(d) Exercise Procedure and Payment of Appreciation Distribution for SARs. In order to exercise any SAR, the Participant must provide notice of exercise to the Plan Administrator in accordance with the SAR Agreement. The appreciation distribution payable to a Participant upon the exercise of a SAR will not be greater than an amount equal to the excess of (i) the aggregate Fair Market Value on the date of exercise of a number of shares of Common Stock equal to the number of Common Stock equivalents that are vested and being exercised under such SAR, over (ii) the strike price of such SAR. Such appreciation distribution may be paid to the Participant in the form of Common Stock or cash (or any combination of Common Stock and cash) or in any other form of payment, as determined by the Board and specified in the SAR Agreement.
(e) Transferability. Options and SARs may not be transferred to third party financial institutions for value. The Board may impose such additional limitations on the transferability of an Option or SAR as it determines. In the absence of any such determination by the Board, the following restrictions on the transferability of Options and SARs will apply, provided that except as explicitly provided herein, neither an Option nor a SAR may be transferred for consideration and provided, further, that if an Option is an Incentive Stock Option, such Option may be deemed to be a Nonstatutory Stock Option as a result of such transfer:
(i) Restrictions on Transfer. An Option or SAR will not be transferable, except by will or by the laws of descent and distribution, and will be exercisable during the lifetime of the Participant only by the Participant; provided, however, that the Board may permit transfer of an Option or SAR in a manner that is not prohibited by applicable tax and securities laws upon the Participant’s request, including to a trust if the Participant is considered to be the sole beneficial owner of such trust (as determined under Section 671 of the Code and applicable state law) while such Option or SAR is held in such trust, provided that the Participant and the trustee enter into a transfer and other agreements required by the Company.
(ii) Domestic Relations Orders. Notwithstanding the foregoing, subject to the execution of transfer documentation in a format acceptable to the Company and subject to the approval of the Board or a duly authorized Officer, an Option or SAR may be transferred pursuant to a domestic relations order.
(f) Vesting. The Board may impose such restrictions on or conditions to the vesting and/or exercisability of an Option or SAR as determined by the Board. Except as otherwise provided in the applicable Award Agreement or other written agreement between a Participant and the Company or an Affiliate, vesting of Options and SARs will cease upon termination of the Participant’s Continuous Service.
(g) Termination of Continuous Service for Cause. Except as explicitly otherwise provided in the Award Agreement or other written agreement between a Participant and the Company or an Affiliate, if a Participant’s Continuous Service is terminated for Cause, the Participant’s Options and SARs will terminate and be forfeited immediately upon such termination of Continuous Service, and the Participant will be prohibited from exercising any portion (including any vested portion) of such Awards on and after the date of such termination of Continuous Service and the Participant will have no further right, title or interest in such forfeited Award, the shares of Common Stock subject to the forfeited Award, or any consideration in respect of the forfeited Award.
5
(h) Post-Termination Exercise Period Following Termination of Continuous Service for Reasons Other than Cause. Subject to Section 4(i), if a Participant’s Continuous Service terminates for any reason other than for Cause, the Participant may exercise his or her Option or SAR to the extent vested, but only within the following period of time or, if applicable, such other period of time provided in the Award Agreement or other written agreement between a Participant and the Company or an Affiliate; provided, however, that in no event may such Award be exercised after the expiration of its maximum term (as set forth in Section 4(a)):
(i) three months following the date of such termination if such termination is a termination without Cause (other than any termination due to the Participant’s Disability or death);
(ii) 12 months following the date of such termination if such termination is due to the Participant’s Disability;
(iii) 12 months following the date of such termination if such termination is due to the Participant’s death; or
(iv) 12 months following the date of the Participant’s death if such death occurs following the date of such termination but during the period such Award is otherwise exercisable (as provided in (i) or (ii) above).
Following the date of such termination, to the extent the Participant does not exercise such Award within the applicable Post-Termination Exercise Period (or, if earlier, prior to the expiration of the maximum term of such Award), such unexercised portion of the Award will terminate, and the Participant will have no further right, title or interest in the terminated Award, the shares of Common Stock subject to the terminated Award, or any consideration in respect of the terminated Award.
(i) Restrictions on Exercise; Extension of Exercisability. A Participant may not exercise an Option or SAR at any time that the issuance of shares of Common Stock upon such exercise would violate Applicable Law. Except as otherwise provided in the Award Agreement or other written agreement between a Participant and the Company or an Affiliate, if a Participant’s Continuous Service terminates for any reason other than for Cause and, at any time during the last thirty days of the applicable Post-Termination Exercise Period: (i) the exercise of the Participant’s Option or SAR would be prohibited solely because the issuance of shares of Common Stock upon such exercise would violate Applicable Law, or (ii) the immediate sale of any shares of Common Stock issued upon such exercise would violate the Company’s Trading Policy, then the applicable Post-Termination Exercise Period will be extended to the last day of the calendar month that commences following the date the Award would otherwise expire, with an additional extension of the exercise period to the last day of the next calendar month to apply if any of the foregoing restrictions apply at any time during such extended exercise period, generally without limitation as to the maximum permitted number of extensions; provided, however, that in no event may such Award be exercised after the expiration of its maximum term (as set forth in Section 4(a)).
6
(j) Non-Exempt Employees. No Option or SAR, whether or not vested, granted to an Employee who is a non-exempt employee for purposes of the U.S. Fair Labor Standards Act of 1938, as amended, will be first exercisable for any shares of Common Stock until at least six months following the date of grant of such Award. Notwithstanding the foregoing, in accordance with the provisions of the U.S. Worker Economic Opportunity Act, any vested portion of such Award may be exercised earlier than six months following the date of grant of such Award in the event of (i) such Participant’s death or Disability, (ii) a Corporate Transaction in which such Award is not assumed, continued or substituted, (iii) a Change in Control, or (iv) such Participant’s retirement (as such term may be defined in the Award Agreement or another applicable agreement or, in the absence of any such definition, in accordance with the Company’s then current employment policies and guidelines). This Section 4(j) is intended to operate so that any income derived by a non-exempt employee in connection with the exercise or vesting of an Option or SAR will be exempt from his or her regular rate of pay.
(k) Whole Shares. Options and SARs may be exercised only with respect to whole shares of Common Stock or their equivalents.
5. AWARDS OTHER THAN OPTIONS AND STOCK APPRECIATION RIGHTS.
(a) Restricted Stock Awards and RSU Awards. Each Restricted Stock Award and RSU Award will have such terms and conditions as determined by the Board; provided, however, that each Restricted Stock Award Agreement and RSU Award Agreement will conform (through incorporation of the provisions hereof by reference in the Award Agreement or otherwise) to the substance of each of the following provisions:
(i) Form of Award.
(1) Restricted Stock Awards: To the extent consistent with the Company’s Bylaws, at the Board’s election, shares of Common Stock subject to a Restricted Stock Award may be (A) held in book entry form subject to the Company’s instructions until such shares become vested or any other restrictions lapse, or (B) evidenced by a certificate, which certificate will be held in such form and manner as determined by the Board. Unless otherwise determined by the Board, a Participant will have voting and other rights as a stockholder of the Company with respect to any shares subject to a Restricted Stock Award.
(2) RSU Awards: An RSU Award represents a Participant’s right to be issued on a future date the number of shares of Common Stock that is equal to the number of restricted stock units subject to the RSU Award. As a holder of an RSU Award, a Participant is an unsecured creditor of the Company with respect to the Company’s unfunded obligation, if any, to issue shares of Common Stock in settlement of such Award and nothing contained in the Plan or any RSU Award Agreement, and no action taken pursuant to its provisions, will create or be construed to create a trust of any kind or a fiduciary relationship between a Participant and the Company or an Affiliate or any other person. A Participant will not have voting or any other rights as a stockholder of the Company with respect to any RSU Award (unless and until shares are actually issued in settlement of a vested RSU Award).
(ii) Consideration.
(1) Restricted Stock Awards: A Restricted Stock Award may be granted in consideration for (A) cash or check, bank draft or money order payable to the Company, (B) services to the Company or an Affiliate, or (C) any other form of consideration as the Board may determine and permissible under Applicable Law.
7
(2) RSU Awards: Unless otherwise determined by the Board at the time of grant, an RSU Award will be granted in consideration for the Participant’s services to the Company or an Affiliate, such that the Participant will not be required to make any payment to the Company (other than such services) with respect to the grant or vesting of the RSU Award, or the issuance of any shares of Common Stock pursuant to the RSU Award. If, at the time of grant, the Board determines that any consideration must be paid by the Participant (in a form other than the Participant’s services to the Company or an Affiliate) upon the issuance of any shares of Common Stock in settlement of the RSU Award, such consideration may be paid in any form of consideration as the Board may determine and permissible under Applicable Law.
(iii) Vesting. The Board may impose such restrictions on or conditions to the vesting of a Restricted Stock Award or RSU Award as determined by the Board. Except as otherwise provided in the Award Agreement or other written agreement between a Participant and the Company or an Affiliate, vesting of Restricted Stock Awards and RSU Awards will cease upon termination of the Participant’s Continuous Service.
(iv) Termination of Continuous Service. Except as otherwise provided in the Award Agreement or other written agreement between a Participant and the Company or an Affiliate, if a Participant’s Continuous Service terminates for any reason, (1) the Company may receive through a forfeiture condition or a repurchase right any or all of the shares of Common Stock held by the Participant under his or her Restricted Stock Award that have not vested as of the date of such termination as set forth in the Restricted Stock Award Agreement and the Participant will have no further right, title or interest in the Restricted Stock Award, the shares of Common Stock subject to the Restricted Stock Award, or any consideration in respect of the Restricted Stock Award and (2) any portion of his or her RSU Award that has not vested will be forfeited upon such termination and the Participant will have no further right, title or interest in the RSU Award, the shares of Common Stock issuable pursuant to the RSU Award, or any consideration in respect of the RSU Award.
(v) Dividends and Dividend Equivalents. Dividends or dividend equivalents may be paid or credited, as applicable, with respect to any shares of Common Stock subject to a Restricted Stock Award or RSU Award, as determined by the Board and specified in the Award Agreement.
(vi) Settlement of RSU Awards. An RSU Award may be settled by the issuance of shares of Common Stock or cash (or any combination thereof) or in any other form of payment, as determined by the Board and specified in the RSU Award Agreement. At the time of grant, the Board may determine to impose such restrictions or conditions that delay such delivery to a date following the vesting of the RSU Award.
(b) Performance Awards. With respect to any Performance Award, the length of any Performance Period, the Performance Goals to be achieved during the Performance Period, the other terms and conditions of such Award, and the measure of whether and to what degree such Performance Goals have been attained will be determined by the Board.
8
(c) Other Awards. Other forms of Awards valued in whole or in part by reference to, or otherwise based on, Common Stock, including the appreciation in value thereof may be granted either alone or in addition to Awards provided for under Section 4 and the preceding provisions of this Section 5. Subject to the provisions of the Plan, the Board will have sole and complete discretion to determine the persons to whom and the time or times at which such Other Awards will be granted, the number of shares of Common Stock (or the cash equivalent thereof) to be granted pursuant to such Other Awards and all other terms and conditions of such Other Awards.
6. ADJUSTMENTS UPON CHANGES IN COMMON STOCK; OTHER CORPORATE EVENTS.
(a) Capitalization Adjustments. In the event of a Capitalization Adjustment, the Board shall appropriately and proportionately adjust: (i) the class(es) and maximum number of shares of Common Stock subject to the Plan and the maximum number of shares by which the Share Reserve may annually increase pursuant to Section 2(a); (ii) the class(es) and maximum number of shares that may be issued pursuant to the exercise of Incentive Stock Options pursuant to Section 2(b); and (iii) the class(es) and number of securities and exercise price, strike price or purchase price of Common Stock subject to outstanding Awards. The Board shall make such adjustments, and its determination shall be final, binding and conclusive. Notwithstanding the foregoing, no fractional shares or rights for fractional shares of Common Stock shall be created in order to implement any Capitalization Adjustment. The Board shall determine an appropriate equivalent benefit, if any, for any fractional shares or rights to fractional shares that might be created by the adjustments referred to in the preceding provisions of this Section.
(b) Dissolution or Liquidation. Except as otherwise provided in the Award Agreement, in the event of a dissolution or liquidation of the Company, all outstanding Awards (other than Awards consisting of vested and outstanding shares of Common Stock not subject to a forfeiture condition or the Company’s right of repurchase) will terminate immediately prior to the completion of such dissolution or liquidation, and the shares of Common Stock subject to the Company’s repurchase rights or subject to a forfeiture condition may be repurchased or reacquired by the Company notwithstanding the fact that the holder of such Award is providing Continuous Service, provided, however, that the Board may determine to cause some or all Awards to become fully vested, exercisable and/or no longer subject to repurchase or forfeiture (to the extent such Awards have not previously expired or terminated) before the dissolution or liquidation is completed but contingent on its completion.
(c) Corporate Transaction. The following provisions will apply to Awards in the event of a Corporate Transaction, except as set forth in Section 11, unless otherwise provided in the instrument evidencing the Award or any other written agreement between the Company or any Affiliate and the Participant or unless otherwise expressly provided by the Board at the time of grant of an Award.
(i) Awards May Be Assumed. In the event of a Corporate Transaction, any surviving corporation or acquiring corporation (or the surviving or acquiring corporation’s parent company) may assume or continue any or all Awards outstanding under the Plan or may substitute similar awards for Awards outstanding under the Plan (including but not limited to, awards to acquire the same consideration paid to the stockholders of the Company pursuant to the Corporate Transaction), and any reacquisition or repurchase rights held by the Company in respect of Common Stock issued pursuant to Awards may be assigned by the Company to the successor of the Company (or the successor’s parent company, if any), in connection with such Corporate Transaction. A surviving corporation or acquiring corporation (or its parent) may choose to assume or continue only a portion of an Award or substitute a similar award for only a portion of an Award, or may choose to assume, continue or substitute the Awards held by some, but not all Participants. The terms of any assumption, continuation or substitution will be set by the Board.
9
(ii) Awards Held by Current Participants. In the event of a Corporate Transaction in which the surviving corporation or acquiring corporation (or its parent company) does not assume or continue such outstanding Awards or substitute similar awards for such outstanding Awards, then with respect to Awards that have not been assumed, continued or substituted and that are held by Participants whose Continuous Service has not terminated prior to the effective time of the Corporate Transaction (referred to as the “Current Participants”), the vesting of such Awards (and, with respect to Options and Stock Appreciation Rights, the time when such Awards may be exercised) will be accelerated in full to a date prior to the effective time of such Corporate Transaction (contingent upon the effectiveness of the Corporate Transaction) as the Board determines (or, if the Board does not determine such a date, to the date that is five days prior to the effective time of the Corporate Transaction), and such Awards will terminate if not exercised (if applicable) at or prior to the effective time of the Corporate Transaction, and any reacquisition or repurchase rights held by the Company with respect to such Awards will lapse (contingent upon the effectiveness of the Corporate Transaction). With respect to the vesting of Performance Awards that will accelerate upon the occurrence of a Corporate Transaction pursuant to this subsection (ii) and that have multiple vesting levels depending on the level of performance, unless otherwise provided in the Award Agreement, the vesting of such Performance Awards will accelerate at 100% of the target level upon the occurrence of the Corporate Transaction in which the Awards are not assumed, continued or substituted in accordance with Section 6(c)(i). With respect to the vesting of Awards that will accelerate upon the occurrence of a Corporate Transaction pursuant to this subsection (ii) and are settled in the form of a cash payment, such cash payment will be made no later than 30 days following the occurrence of the Corporate Transaction or such later date as required to comply with Section 409A of the Code.
(iii) Awards Held by Persons other than Current Participants. In the event of a Corporate Transaction in which the surviving corporation or acquiring corporation (or its parent company) does not assume or continue such outstanding Awards or substitute similar awards for such outstanding Awards, then with respect to Awards that have not been assumed, continued or substituted and that are held by persons other than Current Participants, such Awards will terminate if not exercised (if applicable) prior to the occurrence of the Corporate Transaction; provided, however, that any reacquisition or repurchase rights held by the Company with respect to such Awards will not terminate and may continue to be exercised notwithstanding the Corporate Transaction.
(iv) Payment for Awards in Lieu of Exercise. Notwithstanding the foregoing, in the event an Award will terminate if not exercised prior to the effective time of a Corporate Transaction, the Board may provide, in its sole discretion, that the holder of such Award may not exercise such Award but will receive a payment, in such form as may be determined by the Board, equal in value, at the effective time, to the excess, if any, of (1) the value of the property the Participant would have received upon the exercise of the Award (including, at the discretion of the Board, any unvested portion of such Award), over (2) any exercise price payable by such holder in connection with such exercise.
10
(d) Appointment of Stockholder Representative. As a condition to the receipt of an Award under this Plan, a Participant will be deemed to have agreed that the Award will be subject to the terms of any agreement governing a Corporate Transaction involving the Company, including, without limitation, a provision for the appointment of a stockholder representative that is authorized to act on the Participant’s behalf with respect to any escrow, indemnities and any contingent consideration.
(e) No Restriction on Right to Undertake Transactions. The grant of any Award under the Plan and the issuance of shares pursuant to any Award does not affect or restrict in any way the right or power of the Company, the Board or the stockholders of the Company to make or authorize any adjustment, recapitalization, reorganization or other change in the Company’s capital structure or its business, any Change in Control, any Corporate Transaction, any merger or consolidation of the Company, any issue of stock or of options, rights or options to purchase stock or of bonds, debentures, preferred or prior preference stocks whose rights are superior to or affect the Common Stock or the rights thereof or which are convertible into or exchangeable for Common Stock, or the dissolution or liquidation of the Company, or any sale or transfer of all or any part of its assets or business, or any other corporate act or proceeding, whether of a similar character or otherwise.
7. ADMINISTRATION.
(a) Administration by Board. The Board will administer the Plan unless and until the Board delegates administration of the Plan to a Committee or Committees, as provided in subsection (c) below.
(b) Powers of Board. The Board will have the power, subject to, and within the limitations of, the express provisions of the Plan:
(i) To determine from time to time (1) which of the persons eligible under the Plan will be granted Awards; (2) when and how each Award will be granted; (3) what type or combination of types of Award will be granted; (4) the provisions of each Award granted (which need not be identical), including the time or times when a person will be permitted to receive an issuance of Common Stock or other payment pursuant to an Award; (5) the number of shares of Common Stock or cash equivalent with respect to which an Award will be granted to each such person; (6) the Fair Market Value applicable to an Award; and (7) the terms of any Performance Award that is not valued in whole or in part by reference to, or otherwise based on, the Common Stock, including the amount of cash payment or other property that may be earned and the timing of payment.
(ii) To construe and interpret the Plan and Awards granted under it, and to establish, amend and revoke rules and regulations for its administration. The Board, in the exercise of this power, may correct any defect, omission or inconsistency in the Plan or in any Award Agreement, in a manner and to the extent it deems necessary or expedient to make the Plan or Award fully effective.
(iii) To settle all controversies regarding the Plan and Awards granted under it.
11
(iv) To accelerate the time at which an Award may first be exercised or the time during which an Award or any part thereof will vest, notwithstanding the provisions in the Award Agreement stating the time at which it may first be exercised or the time during which it will vest.
(v) To prohibit the exercise of any Option, SAR or other exercisable Award during a period of up to 30 days prior to the consummation of any pending stock dividend, stock split, combination or exchange of shares, merger, consolidation or other distribution (other than normal cash dividends) of Company assets to stockholders, or any other change affecting the shares of Common Stock or the share price of the Common Stock, including any Corporate Transaction, for reasons of administrative convenience.
(vi) To suspend or terminate the Plan at any time. Suspension or termination of the Plan will not Materially Impair rights and obligations under any Award granted while the Plan is in effect except with the written consent of the affected Participant.
(vii) To amend the Plan in any respect the Board deems necessary or advisable; provided, however, that stockholder approval will be required for any amendment to the extent required by Applicable Law. Except as provided above, rights under any Award granted before amendment of the Plan will not be Materially Impaired by any amendment of the Plan unless (1) the Company requests the consent of the affected Participant, and (2) such Participant consents in writing.
(viii) To submit any amendment to the Plan for stockholder approval.
(ix) To approve forms of Award Agreements for use under the Plan and to amend the terms of any one or more Awards, including, but not limited to, amendments to provide terms more favorable to the Participant than previously provided in the Award Agreement, subject to any specified limits in the Plan that are not subject to Board discretion; provided however, that, a Participant’s rights under any Award will not be Materially Impaired by any such amendment unless (1) the Company requests the consent of the affected Participant, and (2) such Participant consents in writing.
(x) Generally, to exercise such powers and to perform such acts as the Board deems necessary or expedient to promote the best interests of the Company and that are not in conflict with the provisions of the Plan or Awards.
(xi) To adopt such procedures and sub-plans as are necessary or appropriate to permit and facilitate participation in the Plan by, or take advantage of specific tax treatment for Awards granted to, Employees, Directors or Consultants who are non-U.S. nationals or employed outside the United States (provided that Board approval will not be necessary for immaterial modifications to the Plan or any Award Agreement to ensure or facilitate compliance with the laws of the relevant non-U.S. jurisdiction).
(xii) To effect, at any time and from time to time, subject to the consent of any Participant whose Award is Materially Impaired by such action, (1) the reduction of the exercise price (or strike price) of any outstanding Option or SAR; (2) the cancellation of any outstanding Option or SAR and the grant in substitution therefor of (A) a new Option, SAR, Restricted Stock Award, RSU Award or Other Award, under the Plan or another equity plan of the Company, covering the same or a different number of shares of Common Stock, (B) cash and/or (C) other valuable consideration (as determined by the Board); or (3) any other action that is treated as a repricing under generally accepted accounting principles.
12
(c) Delegation to Committee.
(i) General. The Board may delegate some or all of the administration of the Plan to a Committee or Committees. If administration of the Plan is delegated to a Committee, the Committee will have, in connection with the administration of the Plan, the powers theretofore possessed by the Board that have been delegated to the Committee, including the power to delegate to another Committee or a subcommittee of the Committee any of the administrative powers the Committee is authorized to exercise (and references in this Plan to the Board will thereafter be to the Committee or subcommittee), subject, however, to such resolutions, not inconsistent with the provisions of the Plan, as may be adopted from time to time by the Board. Each Committee may retain the authority to concurrently administer the Plan with the Committee or subcommittee to which it has delegated its authority hereunder and may, at any time, revest in such Committee some or all of the powers previously delegated. The Board may retain the authority to concurrently administer the Plan with any Committee and may, at any time, revest in the Board some or all of the powers previously delegated.
(ii) Rule 16b-3 Compliance. To the extent an Award is intended to qualify for the exemption from Section 16(b) of the Exchange Act that is available under Rule 16b-3 of the Exchange Act, the Award will be granted by the Board or a Committee that consists solely of two or more Non-Employee Directors, as determined under Rule 16b-3(b)(3) of the Exchange Act and thereafter any action establishing or modifying the terms of the Award will be approved by the Board or a Committee meeting such requirements to the extent necessary for such exemption to remain available.
(d) Effect of Board’s Decision. All determinations, interpretations and constructions made by the Board or any Committee in good faith will not be subject to review by any person and will be final, binding and conclusive on all persons.
(e) Delegation to Other Person or Body. The Board or any Committee may delegate to one or more persons or bodies the authority to do one or more of the following to the extent permitted by Applicable Law: (i) designate recipients, other than Officers, of Awards, provided that no person or body may be delegated authority to grant an Award to themself; (ii) determine the number of shares subject to such Awards; and (iii) determine the terms of such Awards; provided, however, that the Board or Committee action regarding such delegation will fix the terms of such delegation in accordance with Applicable Law, including without limitation Sections 152 and 157 of the Delaware General Corporation Law. Unless provided otherwise in the Board or Committee action regarding such delegation, each Award granted pursuant to this section will be granted on the applicable form of Award Agreement most recently approved for use by the Board or the Committee, with any modifications necessary to incorporate or reflect the terms of such Award. Notwithstanding anything to the contrary herein, neither the Board nor any Committee may delegate to any person or body (who is not a Director or that is not comprised solely of Directors, respectively) the authority to determine the Fair Market Value.
13
8. TAX WITHHOLDING
(a) Withholding Authorization. As a condition to acceptance of any Award under the Plan, a Participant authorizes withholding from payroll and any other amounts payable to such Participant, and otherwise agrees to make adequate arrangements to satisfy the Tax-Related Items withholding obligations, if any, of the Company and/or an Affiliate that arise in connection with the grant, vesting, exercise or settlement of such Award, as applicable. Accordingly, a Participant may not be able to exercise an Award even though the Award is vested, and the Company shall have no obligation to issue shares of Common Stock subject to an Award, unless and until such obligations are satisfied.
(b) Satisfaction of Withholding Obligation. To the extent permitted by the terms of an Award Agreement, the Company may, in its sole discretion, satisfy any Tax-Related Items withholding obligation relating to an Award by any of the following means or by a combination of such means: (i) causing the Participant to tender a cash payment; (ii) withholding shares of Common Stock from the shares of Common Stock issued or otherwise issuable to the Participant in connection with the Award; (iii) withholding cash from an Award settled in cash; (iv) withholding payment from any amounts otherwise payable to the Participant; (v) by allowing a Participant to effectuate a “cashless exercise” pursuant to a program developed under Regulation T as promulgated by the U.S. Federal Reserve Board; or (vi) by such other method as may be set forth in the Award Agreement.
(c) No Obligation to Notify or Minimize Taxes; No Liability to Claims. Except as required by Applicable Law, the Company has no duty or obligation to any Participant to advise such holder as to the time or manner of exercising such Award. Furthermore, the Company has no duty or obligation to warn or otherwise advise such holder of a pending termination or expiration of an Award or a possible period in which the Award may not be exercised. The Company has no duty or obligation to minimize the tax consequences of an Award to the holder of such Award and will not be liable to any holder of an Award for any adverse tax consequences to such holder in connection with an Award. As a condition to accepting an Award under the Plan, each Participant (i) agrees to not make any claim against the Company, or any of its Officers, Directors, Employees or Affiliates related to tax liabilities arising from such Award or other Company compensation and (ii) acknowledges that such Participant was advised to consult with his or her own personal tax, financial and other legal advisors regarding the tax consequences of the Award and has either done so or knowingly and voluntarily declined to do so. Additionally, each Participant acknowledges any Option or SAR granted under the Plan is exempt from Section 409A only if the exercise or strike price is at least equal to the “fair market value” of the Common Stock on the date of grant as determined by the U.S. Internal Revenue Service and there is no other impermissible deferral of compensation associated with the Award. Additionally, as a condition to accepting an Option or SAR granted under the Plan, each Participant agrees to not make any claim against the Company, or any of its Officers, Directors, Employees or Affiliates in the event that the U.S. Internal Revenue Service asserts that such exercise price or strike price is less than the “fair market value” of the Common Stock on the date of grant as subsequently determined by the U.S. Internal Revenue Service.
14
(d) Withholding Indemnification. The Company and/or its Affiliate may withhold or account for Tax-Related Items by considering statutory or other withholding rates, including minimum or maximum rates applicable in a Participant’s jurisdiction. In the event of overwithholding, the Participant may receive a refund of any over-withheld amount in cash (with no entitlement to the equivalent in Common Stock) or, if not refunded, the Participant may seek a refund from the local tax authorities. In the event of under-withholding, the Participant may be required to pay any additional Tax-Related Items directly to the applicable tax authority or to the Company and/or its Affiliate. As a condition to accepting an Award under the Plan, in the event that the amount of the Company’s and/or its Affiliate’s withholding obligation in connection with such Award was greater than the amount actually withheld by the Company and/or its Affiliates, each Participant agrees to indemnify and hold the Company and/or its Affiliates harmless from any failure by the Company and/or its Affiliates to withhold the proper amount. Further, if the obligation for Tax-Related Items is satisfied by withholding in shares of Common Stock, for tax purposes, the Participant will be deemed to have been issued the full number of shares subject to the Award, notwithstanding that a number of the shares is held back solely for the purpose of paying the Tax-Related Items.
9. MISCELLANEOUS.
(a) Source of Shares. The stock issuable under the Plan will be shares of authorized but unissued or reacquired Common Stock, including shares repurchased by the Company on the open market or otherwise.
(b) Use of Proceeds from Sales of Common Stock. Proceeds from the sale of shares of Common Stock pursuant to Awards will constitute general funds of the Company.
(c) Corporate Action Constituting Grant of Awards. Corporate action constituting a grant by the Company of an Award to any Participant will be deemed completed as of the date of such corporate action, unless otherwise determined by the Board, regardless of when the instrument, certificate, or letter evidencing the Award is communicated to, or actually received or accepted by, the Participant. In the event that the corporate records (e.g., Board consents, resolutions or minutes) documenting the corporate action approving the grant contain terms (e.g., exercise price, vesting schedule or number of shares) that are inconsistent with those in the Award Agreement or related grant documents as a result of a clerical error in the Award Agreement or related grant documents, the corporate records will control and the Participant will have no legally binding right to the incorrect term in the Award Agreement or related grant documents.
(d) Stockholder Rights. No Participant will be deemed to be the holder of, or to have any of the rights of a holder with respect to, any shares of Common Stock subject to such Award unless and until (i) such Participant has satisfied all requirements for exercise of the Award pursuant to its terms, if applicable, and (ii) the issuance of the Common Stock subject to such Award is reflected in the records of the Company.
15
(e) No Employment or Other Service Rights. Nothing in the Plan, any Award Agreement or any other instrument executed thereunder or in connection with any Award granted pursuant thereto will confer upon any Participant any right to continue to serve the Company or an Affiliate in the capacity in effect at the time the Award was granted or affect the right of the Company or an Affiliate to terminate at will (unless otherwise required under Applicable Law) and without regard to any future vesting opportunity that a Participant may have with respect to any Award (i) the employment of an Employee with or without notice and with or without Cause, (ii) the service of a Consultant pursuant to the terms of such Consultant’s agreement with the Company or an Affiliate, or (iii) the service of a Director pursuant to the Bylaws of the Company or an Affiliate, and any applicable provisions of the corporate law of the U.S. state or non-U.S. jurisdiction in which the Company or the Affiliate is incorporated, as the case may be. Further, nothing in the Plan, any Award Agreement or any other instrument executed thereunder or in connection with any Award will constitute any promise or commitment by the Company or an Affiliate regarding the fact or nature of future positions, future work assignments, future compensation or any other term or condition of employment or service or confer any right or benefit under the Award or the Plan unless such right or benefit has specifically accrued under the terms of the Award Agreement and/or Plan.
(f) Change in Time Commitment. In the event a Participant’s regular level of time commitment in the performance of his or her services for the Company and any Affiliates is reduced (for example, and without limitation, if the Participant is an Employee of the Company and the Employee has a change in status from a full-time Employee to a part-time Employee or takes an extended leave of absence) after the date of grant of any Award to the Participant, the Board may determine, to the extent permitted by Applicable Law, to (i) make a corresponding reduction in the number of shares or cash amount subject to any portion of such Award that is scheduled to vest or become payable after the date of such change in time commitment, and (ii) in lieu of or in combination with such a reduction, extend the vesting or payment schedule applicable to such Award. In the event of any such reduction, the Participant will have no right with respect to any portion of the Award that is so reduced or extended.
(g) Execution of Additional Documents. As a condition to accepting an Award under the Plan, the Participant agrees to execute any additional documents or instruments necessary or desirable, as determined in the Plan Administrator’s sole discretion, to carry out the purposes or intent of the Award, or facilitate compliance with securities and/or other regulatory requirements, in each case at the Plan Administrator’s request.
(h) Electronic Delivery and Participation. Any reference herein or in an Award Agreement to a “written” agreement or document will include any agreement or document delivered electronically, filed publicly at www.sec.gov (or any successor website thereto) or posted on the Company’s intranet (or other shared electronic medium controlled by the Company to which the Participant has access). By accepting any Award the Participant consents to receive documents by electronic delivery and to participate in the Plan through any on-line electronic system established and maintained by the Plan Administrator or another third party selected by the Plan Administrator. The form of delivery of any Common Stock (e.g., a stock certificate or electronic entry evidencing such shares) shall be determined by the Company.
(i) Clawback/Recovery. All Awards granted under the Plan will be subject to recoupment in accordance with any clawback policy that the Company is required to adopt pursuant to the listing standards of any national securities exchange or association on which the Company’s securities are listed or as is otherwise required by the Dodd-Frank Wall Street Reform and Consumer Protection Act or other Applicable Law and any clawback policy that the Company otherwise adopts, to the extent applicable and permissible under Applicable Law. In addition, the Board may impose such other clawback, recovery or recoupment provisions in an Award Agreement as the Board determines necessary or appropriate, including but not limited to a reacquisition right in respect of previously acquired shares of Common Stock or other cash or property upon the occurrence of Cause. No recovery of compensation under such a clawback policy will be an event giving rise to a Participant’s right to voluntarily terminate employment upon a “resignation for good reason,” or for a “constructive termination” or any similar term under any plan of or agreement with the Company.
16
(j) Securities Law Compliance. A Participant will not be issued any shares in respect of an Award unless either (i) the shares are registered under the Securities Act or (ii) the Company has determined that such issuance would be exempt from the registration requirements of the Securities Act. Each Award also must comply with other Applicable Law governing the Award, and a Participant will not receive such shares if the Company determines that such receipt would not be in material compliance with Applicable Law.
(k) Transfer or Assignment of Awards; Issued Shares. Except as expressly provided in the Plan or the form of Award Agreement, Awards granted under the Plan may not be transferred or assigned by the Participant. After the vested shares subject to an Award have been issued, or in the case of a Restricted Stock Award and similar awards, after the issued shares have vested, the holder of such shares is free to assign, hypothecate, donate, encumber or otherwise dispose of any interest in such shares provided that any such actions are in compliance with the provisions herein, the terms of the Trading Policy and Applicable Law.
(l) Effect on Other Employee Benefit Plans. The value of any Award granted under the Plan, as determined upon grant, vesting or settlement, shall not be included as compensation, earnings, salaries, or other similar terms used when calculating any Participant’s benefits under any employee benefit plan sponsored by the Company or any Affiliate, except as such plan otherwise expressly provides. The Company expressly reserves its rights to amend, modify, or terminate any of the Company’s or any Affiliate’s employee benefit plans.
(m) Deferrals. To the extent permitted by Applicable Law, the Board, in its sole discretion, may determine that the delivery of Common Stock or the payment of cash, upon the exercise, vesting or settlement of all or a portion of any Award may be deferred and may also establish programs and procedures for deferral elections to be made by Participants. Deferrals will be made in accordance with the requirements of Section 409A.
(n) Section 409A. Unless otherwise expressly provided for in an Award Agreement, the Plan and Award Agreements will be interpreted to the greatest extent possible in a manner that makes the Plan and the Awards granted hereunder exempt from Section 409A, and, to the extent not so exempt, in compliance with the requirements of Section 409A. If the Board determines that any Award granted hereunder is not exempt from and is therefore subject to Section 409A, the Award Agreement evidencing such Award will incorporate the terms and conditions necessary to avoid the consequences specified in Section 409A(a)(1) of the Code, and to the extent an Award Agreement is silent on terms necessary for compliance, such terms are hereby incorporated by reference into the Award Agreement. Notwithstanding anything to the contrary in this Plan (and unless the Award Agreement specifically provides otherwise), if the shares of Common Stock are publicly traded, and if a Participant holding an Award that constitutes “deferred compensation” under Section 409A is a “specified employee” for purposes of Section 409A, no distribution or payment of any amount that is due because of a “separation from service” (as defined in Section 409A without regard to alternative definitions thereunder) will be issued or paid before the date that is six months and one day following the date of such Participant’s “separation from service” or, if earlier, the date of the Participant’s death, unless such distribution or payment can be made in a manner that complies with Section 409A, and any amounts so deferred will be paid in a lump sum on the day after such six month period elapses, with the balance paid thereafter on the original schedule.
17
(o) Choice of Law. This Plan and any controversy arising out of or relating to this Plan shall be governed by, and construed in accordance with, the internal laws of the State of Delaware, without regard to conflict of law principles that would result in any application of any law other than the law of the State of Delaware.
10. COVENANTS OF THE COMPANY.
The Company will seek to obtain from each regulatory commission or agency, as may be deemed to be necessary, having jurisdiction over the Plan such authority as may be required to grant Awards and to issue and sell shares of Common Stock upon exercise or vesting of the Awards; provided, however, that this undertaking will not require the Company to register under the Securities Act the Plan, any Award or any Common Stock issued or issuable pursuant to any such Award. If, after reasonable efforts and at a reasonable cost, the Company is unable to obtain from any such regulatory commission or agency the authority that counsel for the Company deems necessary or advisable for the lawful issuance and sale of Common Stock under the Plan, the Company will be relieved from any liability for failure to issue and sell Common Stock upon exercise or vesting of such Awards unless and until such authority is obtained. A Participant is not eligible for the grant of an Award or the subsequent issuance of Common Stock pursuant to the Award if such grant or issuance would be in violation of any Applicable Law.
11. ADDITIONAL RULES FOR AWARDS SUBJECT TO SECTION 409A.
(a) Application. Unless the provisions of this Section of the Plan are expressly superseded by the provisions in the form of Award Agreement, the provisions of this Section shall apply and shall supersede anything to the contrary set forth in the Award Agreement for a Non-Exempt Award.
(b) Non-Exempt Awards Subject to Non-Exempt Severance Arrangements. To the extent a Non-Exempt Award is subject to Section 409A due to application of a Non-Exempt Severance Arrangement, the following provisions of this subsection (b) apply.
(i) If the Non-Exempt Award vests in the ordinary course during the Participant’s Continuous Service in accordance with the vesting schedule set forth in the Award Agreement, and does not accelerate vesting under the terms of a Non-Exempt Severance Arrangement, in no event will the shares be issued in respect of such Non-Exempt Award any later than the later of: (i) December 31st of the calendar year that includes the applicable vesting date; or (ii) the 60th day that follows the applicable vesting date.
18
(ii) If vesting of the Non-Exempt Award accelerates under the terms of a Non-Exempt Severance Arrangement in connection with the Participant’s Separation from Service, and such vesting acceleration provisions were in effect as of the date of grant of the Non-Exempt Award and, therefore, are part of the terms of such Non-Exempt Award as of the date of grant, then the shares will be earlier issued in settlement of such Non-Exempt Award upon the Participant’s Separation from Service in accordance with the terms of the Non-Exempt Severance Arrangement, but in no event later than the 60th day that follows the date of the Participant’s Separation from Service. However, if at the time the shares would otherwise be issued the Participant is subject to the distribution limitations contained in Section 409A applicable to “specified employees,” as defined in Section 409A(a)(2)(B)(i) of the Code, such shares shall not be issued before the date that is six months following the date of such Participant’s Separation from Service, or, if earlier, the date of the Participant’s death that occurs within such six-month period.
(iii) If vesting of a Non-Exempt Award accelerates under the terms of a Non-Exempt Severance Arrangement in connection with a Participant’s Separation from Service, and such vesting acceleration provisions were not in effect as of the date of grant of the Non-Exempt Award and, therefore, are not a part of the terms of such Non-Exempt Award on the date of grant, then such acceleration of vesting of the Non-Exempt Award shall not accelerate the issuance date of the shares, but the shares shall instead be issued on the same schedule as set forth in the Grant Notice as if they had vested in the ordinary course during the Participant’s Continuous Service, notwithstanding the vesting acceleration of the Non-Exempt Award. Such issuance schedule is intended to satisfy the requirements of payment on a specified date or pursuant to a fixed schedule, as provided under U.S. Treasury Regulations Section 1.409A-3(a)(4).
(c) Treatment of Non-Exempt Awards Upon a Corporate Transaction for Employees and Consultants. The provisions of this subsection (c) shall apply and shall supersede anything to the contrary set forth in the Plan with respect to the permitted treatment of any Non-Exempt Award in connection with a Corporate Transaction if the Participant was either an Employee or Consultant upon the applicable date of grant of the Non-Exempt Award.
(i) Vested Non-Exempt Awards. The following provisions shall apply to any Vested Non-Exempt Award in connection with a Corporate Transaction:
(1) If the Corporate Transaction is also a Section 409A Change in Control then the Acquiring Entity may not assume, continue or substitute the Vested Non-Exempt Award. Upon the Section 409A Change in Control the settlement of the Vested Non-Exempt Award will automatically be accelerated and the shares will be immediately issued in respect of the Vested Non-Exempt Award. Alternatively, the Company may instead provide that the Participant will receive a cash settlement equal to the Fair Market Value of the shares that would otherwise be issued to the Participant upon the Section 409A Change in Control.
(2) If the Corporate Transaction is not also a Section 409A Change in Control, then the Acquiring Entity must either assume, continue or substitute each Vested Non-Exempt Award. The shares to be issued in respect of the Vested Non-Exempt Award shall be issued to the Participant by the Acquiring Entity on the same schedule that the shares would have been issued to the Participant if the Corporate Transaction had not occurred. In the Acquiring Entity’s discretion, in lieu of an issuance of shares, the Acquiring Entity may instead substitute a cash payment on each applicable issuance date, equal to the Fair Market Value of the shares that would otherwise be issued to the Participant on such issuance dates, with the determination of the Fair Market Value of the shares made on the date of the Corporate Transaction.
19
(ii) Unvested Non-Exempt Awards. The following provisions shall apply to any Unvested Non-Exempt Award unless otherwise determined by the Board pursuant to subsection (e) of this Section.
(1) In the event of a Corporate Transaction, the Acquiring Entity shall assume, continue or substitute any Unvested Non-Exempt Award. Unless otherwise determined by the Board, any Unvested Non-Exempt Award will remain subject to the same vesting and forfeiture restrictions that were applicable to the Award prior to the Corporate Transaction. The shares to be issued in respect of any Unvested Non-Exempt Award shall be issued to the Participant by the Acquiring Entity on the same schedule that the shares would have been issued to the Participant if the Corporate Transaction had not occurred. In the Acquiring Entity’s discretion, in lieu of an issuance of shares, the Acquiring Entity may instead substitute a cash payment on each applicable issuance date, equal to the Fair Market Value of the shares that would otherwise be issued to the Participant on such issuance dates, with the determination of Fair Market Value of the shares made on the date of the Corporate Transaction.
(2) If the Acquiring Entity will not assume, substitute or continue any Unvested Non-Exempt Award in connection with a Corporate Transaction, then such Award shall automatically terminate and be forfeited upon the Corporate Transaction with no consideration payable to any Participant in respect of such forfeited Unvested Non-Exempt Award. Notwithstanding the foregoing, to the extent permitted and in compliance with the requirements of Section 409A, the Board may in its discretion determine to elect to accelerate the vesting and settlement of the Unvested Non-Exempt Award upon the Corporate Transaction, or instead substitute a cash payment equal to the Fair Market Value of such shares that would otherwise be issued to the Participant, as further provided in subsection (e)(ii) below. In the absence of such discretionary election by the Board, any Unvested Non-Exempt Award shall be forfeited without payment of any consideration to the affected Participants if the Acquiring Entity will not assume, substitute or continue the Unvested Non-Exempt Awards in connection with the Corporate Transaction.
(3) The foregoing treatment shall apply with respect to all Unvested Non-Exempt Awards upon any Corporate Transaction, and regardless of whether or not such Corporate Transaction is also a Section 409A Change in Control.
(d) Treatment of Non-Exempt Awards Upon a Corporate Transaction for Non-Employee Directors. The following provisions of this subsection (d) shall apply and shall supersede anything to the contrary that may be set forth in the Plan with respect to the permitted treatment of a Non-Exempt Director Award in connection with a Corporate Transaction.
(i) If the Corporate Transaction is also a Section 409A Change in Control then the Acquiring Entity may not assume, continue or substitute the Non-Exempt Director Award. Upon the Section 409A Change in Control the vesting and settlement of any Non-Exempt Director Award will automatically be accelerated and the shares will be immediately issued to the Participant in respect of the Non-Exempt Director Award. Alternatively, the Company may provide that the Participant will instead receive a cash settlement equal to the Fair Market Value of the shares that would otherwise be issued to the Participant upon the Section 409A Change in Control pursuant to the preceding provision.
20
(ii) If the Corporate Transaction is not also a Section 409A Change in Control, then the Acquiring Entity must either assume, continue or substitute the Non-Exempt Director Award. Unless otherwise determined by the Board, the Non-Exempt Director Award will remain subject to the same vesting and forfeiture restrictions that were applicable to the Award prior to the Corporate Transaction. The shares to be issued in respect of the Non-Exempt Director Award shall be issued to the Participant by the Acquiring Entity on the same schedule that the shares would have been issued to the Participant if the Corporate Transaction had not occurred. In the Acquiring Entity’s discretion, in lieu of an issuance of shares, the Acquiring Entity may instead substitute a cash payment on each applicable issuance date, equal to the Fair Market Value of the shares that would otherwise be issued to the Participant on such issuance dates, with the determination of Fair Market Value made on the date of the Corporate Transaction.
(e) If the RSU Award is a Non-Exempt Award, then the provisions in this Section 11(e) shall apply and supersede anything to the contrary that may be set forth in the Plan or the Award Agreement with respect to the permitted treatment of such Non-Exempt Award:
(i) Any exercise by the Board of discretion to accelerate the vesting of a Non-Exempt Award shall not result in any acceleration of the scheduled issuance dates for the shares in respect of the Non-Exempt Award unless earlier issuance of the shares upon the applicable vesting dates would be in compliance with the requirements of Section 409A.
(ii) The Company explicitly reserves the right to earlier settle any Non-Exempt Award to the extent permitted and in compliance with the requirements of Section 409A, including pursuant to any of the exemptions available in U.S. Treasury Regulations Section 1.409A-3(j)(4)(ix).
(iii) To the extent the terms of any Non-Exempt Award provide that it will be settled upon a Change in Control or Corporate Transaction, to the extent it is required for compliance with the requirements of Section 409A, the Change in Control or Corporate Transaction event triggering settlement must also constitute a Section 409A Change in Control. To the extent the terms of a Non-Exempt Award provide that it will be settled upon a termination of employment or termination of Continuous Service, to the extent it is required for compliance with the requirements of Section 409A, the termination event triggering settlement must also constitute a Separation From Service. However, if at the time the shares would otherwise be issued to a Participant in connection with a “separation from service” such Participant is subject to the distribution limitations contained in Section 409A applicable to “specified employees,” as defined in Section 409A(a)(2)(B)(i) of the Code, such shares shall not be issued before the date that is six months following the date of the Participant’s Separation From Service, or, if earlier, the date of the Participant’s death that occurs within such six month period.
(iv) The provisions in this subsection (e) for delivery of the shares in respect of the settlement of an RSU Award that is a Non-Exempt Award are intended to comply with the requirements of Section 409A so that the delivery of the shares to the Participant in respect of such Non-Exempt Award will not trigger the additional tax imposed under Section 409A, and any ambiguities herein will be so interpreted.
21
12. SEVERABILITY.
If all or any part of the Plan or any Award Agreement is declared by any court or governmental authority to be unlawful or invalid, such unlawfulness or invalidity shall not invalidate any portion of the Plan or such Award Agreement not declared to be unlawful or invalid. Any Section of the Plan or any Award Agreement (or part of such a Section) so declared to be unlawful or invalid shall, if possible, be construed in a manner which will give effect to the terms of such Section or part of a Section to the fullest extent possible while remaining lawful and valid.
13. TERMINATION OF THE PLAN.
The Board may suspend or terminate the Plan at any time. No Incentive Stock Options may be granted after the tenth anniversary of the earlier of: (i) the Adoption Date; or (ii) the date the Plan is approved by the Company’s stockholders. No Awards may be granted under the Plan while the Plan is suspended or after it is terminated.
22
14. DEFINITIONS.
As used in the Plan, the following definitions apply to the capitalized terms indicated below:
(a) “Acquiring Entity” means the surviving or acquiring corporation (or its parent company) in connection with a Corporate Transaction.
(b) “Adoption Date” means the date the Plan is first approved by the Board or Compensation Committee, as applicable.
(c) “Affiliate” means, at the time of determination, any “parent” or “subsidiary” of the Company as such terms are defined in Rule 405 promulgated under the Securities Act. The Board may determine the time or times at which “parent” or “subsidiary” status is determined within the foregoing definition.
(d) “Applicable Law” means the Code and any applicable U.S and non-U.S. securities, exchange, control, tax, federal, state, material local or municipal or other law, statute, constitution, principle of common law, resolution, ordinance, code, edict, decree, rule, listing rule, regulation, judicial decision, ruling or requirement issued, enacted, adopted, promulgated, implemented or otherwise put into effect by or under the authority of any Governmental Body (including under the authority of any applicable self-regulating organization such as the Nasdaq Stock Market, New York Stock Exchange, or the Financial Industry Regulatory Authority).
(e) “Award” means any right to receive Common Stock, cash or other property granted under the Plan (including an Incentive Stock Option, a Nonstatutory Stock Option, a Restricted Stock Award, an RSU Award, a SAR, a Performance Award or any Other Award).
(f) “Award Agreement” means a written or electronic agreement between the Company and a Participant evidencing the terms and conditions of an Award. The Award Agreement generally consists of the Grant Notice and the agreement containing the written summary of the general terms and conditions applicable to the Award and which is provided, including through electronic means, to a Participant along with the Grant Notice.
(g) “Board” means the Board of Directors of the Company (or its designee). Any decision or determination made by the Board shall be a decision or determination that is made in the sole discretion of the Board (or its designee), and such decision or determination shall be final and binding on all Participants.
(h) “Capital Stock” means each and every class of common stock of the Company, regardless of the number of votes per share.
(i) “Capitalization Adjustment” means any change that is made in, or other events that occur with respect to, the Common Stock subject to the Plan or subject to any Award after the Adoption Date without the receipt of consideration by the Company through merger, consolidation, reorganization, recapitalization, reincorporation, stock dividend, dividend in property other than cash, large nonrecurring cash dividend, stock split, reverse stock split, liquidating dividend, combination of shares, exchange of shares, change in corporate structure or any similar equity restructuring transaction, as that term is used in Statement of Financial Accounting Standards Board Accounting Standards Codification Topic 718 (or any successor thereto). Notwithstanding the foregoing, the conversion of any convertible securities of the Company will not be treated as a Capitalization Adjustment.
23
(j) “Cause” has the meaning ascribed to such term in any written agreement between a Participant and the Company or any Affiliate of the Company defining such term and, in the absence of such agreement, such term means, with respect to a Participant, the occurrence of any of the following events: (i) the Participant’s dishonest statements or acts with respect to the Company or any Affiliate of the Company, or any current or prospective customers, suppliers, vendors or other third parties with which such entity does business; (ii) the Participant’s commission of (A) a felony or (B) any misdemeanor involving moral turpitude, deceit, dishonesty or fraud, or in each case the equivalent in any relevant jurisdiction; (iii) the Participant’s failure to perform the Participant’s assigned duties and responsibilities to the reasonable satisfaction of the Company or any Affiliate of the Company which failure continues, in the reasonable judgment of the Company, after written notice given to the Participant by the Company or any Affiliate of the Company; (iv) the Participant’s gross negligence, willful misconduct or insubordination with respect to the Company or any Affiliate of the Company; or (v) the Participant’s material violation of any provision of any agreement(s) between the Participant and the Company or any Affiliate of the Company relating to noncompetition, nonsolicitation, nondisclosure and/or assignment of inventions. The determination that a termination of the Participant’s Continuous Service is either for Cause or without Cause will be made by the Board with respect to Participants who are executive officers of the Company and by the Company’s Chief Executive Officer with respect to Participants who are not executive officers of the Company. Any determination by the Company that the Continuous Service of a Participant was terminated with or without Cause for the purposes of outstanding Awards held by such Participant will have no effect upon any determination of the rights or obligations of the Company or any Affiliate of the Company or such Participant for any other purpose.
(k) “Change in Control” or “Change of Control” means the occurrence, in a single transaction or in a series of related transactions, of any one or more of the following events:
(i) any Exchange Act Person becomes the Owner, directly or indirectly, of securities of the Company representing more than 50% of the combined voting power of the Company’s then outstanding securities other than by virtue of a merger, consolidation or similar transaction. Notwithstanding the foregoing, a Change in Control shall not be deemed to occur (A) on account of the acquisition of securities of the Company directly from the Company, (B) on account of the acquisition of securities of the Company by an investor, any affiliate thereof or any other Exchange Act Person that acquires the Company’s securities in a transaction or series of related transactions the primary purpose of which is to obtain financing for the Company through the issuance of equity securities, or (C) solely because the level of Ownership held by any Exchange Act Person (the “Subject Person”) exceeds the designated percentage threshold of the outstanding voting securities as a result of a repurchase or other acquisition of voting securities by the Company reducing the number of shares outstanding, provided that if a Change in Control would occur (but for the operation of this sentence) as a result of the acquisition of voting securities by the Company, and after such share acquisition, the Subject Person becomes the Owner of any additional voting securities that, assuming the repurchase or other acquisition had not occurred, increases the percentage of the then outstanding voting securities Owned by the Subject Person over the designated percentage threshold, then a Change in Control shall be deemed to occur;
24
(ii) there is consummated a merger, consolidation or similar transaction involving (directly or indirectly) the Company and, immediately after the consummation of such merger, consolidation or similar transaction, the stockholders of the Company immediately prior thereto do not Own, directly or indirectly, either (A) outstanding voting securities representing more than 50% of the combined outstanding voting power of the Acquiring Entity in such merger, consolidation or similar transaction or (B) more than 50% of the combined outstanding voting power of the parent of the Acquiring Entity in such merger, consolidation or similar transaction, in each case in substantially the same proportions as their Ownership of the outstanding voting securities of the Company immediately prior to such transaction;
(iii) there is consummated a sale, lease, exclusive license or other disposition of all or substantially all of the consolidated assets of the Company and its Subsidiaries, other than a sale, lease, license or other disposition of all or substantially all of the consolidated assets of the Company and its Subsidiaries to an Entity, more than 50% of the combined voting power of the voting securities of which are Owned by stockholders of the Company in substantially the same proportions as their Ownership of the outstanding voting securities of the Company immediately prior to such sale, lease, license or other disposition; or
(iv) individuals who, on the Adoption Date, are members of the Board (the “Incumbent Board”) cease for any reason to constitute at least a majority of the members of the Board; provided, however, that if the appointment or election (or nomination for election) of any new Board member was approved or recommended by a majority vote of the members of the Incumbent Board then still in office, such new member shall, for purposes of this Plan, be considered as a member of the Incumbent Board.
Notwithstanding the foregoing or any other provision of this Plan, (A) the term Change in Control shall not include a sale of assets, merger or other transaction effected exclusively for the purpose of changing the domicile of the Company, (B) the definition of Change in Control (or any analogous term) in an individual written agreement between the Company or any Affiliate and the Participant shall supersede the foregoing definition with respect to Awards subject to such agreement; provided, however, that if no definition of Change in Control or any analogous term is set forth in such an individual written agreement, the foregoing definition shall apply, and (C) with respect to any nonqualified deferred compensation that becomes payable on account of the Change in Control, the transaction or event described in clause (i), (ii), (iii), or (iv) also constitutes a Section 409A Change in Control if required in order for the payment not to violate Section 409A of the Code.
(l) “Code” means the U.S. Internal Revenue Code of 1986, as amended, including any applicable regulations and guidance thereunder.
(m) “Committee” means the Compensation Committee and any other committee of one or more Directors to whom authority has been delegated by the Board or Compensation Committee in accordance with the Plan.
25
(n) “Common Stock” means the common stock of the Company.
(o) “Company” means Ikena Oncology, Inc., a Delaware corporation, and as of the Effective Date, shall mean ImageneBio, Inc., a Delaware corporation, and any successor corporation thereto.
(p) “Compensation Committee” means the Compensation Committee of the Board.
(q) “Consultant” means any person, including an advisor, who is (i) engaged by the Company or an Affiliate to render consulting or advisory services and is compensated for such services, or (ii) serving as a member of the board of directors of an Affiliate and is compensated for such services. However, service solely as a Director, or payment of a fee for such service, will not cause a Director to be considered a “Consultant” for purposes of the Plan. Notwithstanding the foregoing, a person is treated as a Consultant under this Plan only if a Form S-8 Registration Statement under the Securities Act is available to register either the offer or the sale of the Company’s securities to such person.
(r) “Continuous Service” means that the Participant’s service with the Company or an Affiliate, whether as an Employee, Director or Consultant, is not interrupted or terminated. A change in the capacity in which the Participant renders service to the Company or an Affiliate as an Employee, Director or Consultant or a change in the Entity for which the Participant renders such service, provided that there is no interruption or termination of the Participant’s service with the Company or an Affiliate, will not terminate a Participant’s Continuous Service; provided, however, that if the Entity for which a Participant is rendering services ceases to qualify as an Affiliate, as determined by the Board, such Participant’s Continuous Service will be considered to have terminated on the date such Entity ceases to qualify as an Affiliate. For example, a change in status from an Employee of the Company to a Consultant of an Affiliate or to a Director will not constitute an interruption of Continuous Service. To the extent permitted by Applicable Law, the Board or the chief executive officer of the Company, in that party’s sole discretion, may determine whether Continuous Service will be considered interrupted in the case of (i) any leave of absence approved by the Company or an Affiliate, including sick leave, military leave or any other personal leave, or (ii) transfers between the Company, an Affiliate, or their successors. Notwithstanding the foregoing, a leave of absence will be treated as Continuous Service for purposes of vesting in an Award only to such extent as may be provided in the Company’s leave of absence policy, in the written terms of any leave of absence agreement or policy applicable to the Participant, or as otherwise required by Applicable Law. In addition, to the extent required for exemption from or compliance with Section 409A, the determination of whether there has been a termination of Continuous Service will be made, and such term will be construed, in a manner that is consistent with the definition of “separation from service” as defined under U.S. Treasury Regulation Section 1.409A-1(h) (without regard to any alternative definition thereunder).
(s) “Corporate Transaction” means the consummation, in a single transaction or in a series of related transactions, of any one or more of the following events:
(i) a sale or other disposition of all or substantially all, as determined by the Board, of the consolidated assets of the Company and its Subsidiaries;
26
(ii) a sale or other disposition of at least 50% of the outstanding securities of the Company;
(iii) a merger, consolidation or similar transaction following which the Company is not the surviving corporation; or
(iv) a merger, consolidation or similar transaction following which the Company is the surviving corporation but the shares of Common Stock outstanding immediately preceding the merger, consolidation or similar transaction are converted or exchanged by virtue of the merger, consolidation or similar transaction into other property, whether in the form of securities, cash or otherwise.
Notwithstanding the foregoing or any other provision of this Plan, (A) the term Corporate Transaction shall not include a sale of assets, merger or other transaction effected exclusively for the purpose of changing the domicile of the Company, (B) the definition of Corporate Transaction (or any analogous term) in an individual written agreement between the Company or any Affiliate and the Participant shall supersede the foregoing definition with respect to Awards subject to such agreement; provided, however, that if no definition of Corporate Transaction or any analogous term is set forth in such an individual written agreement, the foregoing definition shall apply, and (C) with respect to any nonqualified deferred compensation that becomes payable on account of the Corporate Transaction, the transaction or event described in clause (i), (ii), (iii), or (iv) also constitutes a Section 409A Change in Control if required in order for the payment not to violate Section 409A of the Code.
(t) “determine” or “determined” means as determined by the Board or the Committee (or its designee) in its sole discretion.
(u) “Director” means a member of the Board.
(v) “Disability” means, with respect to a Participant, such Participant is unable to engage in any substantial gainful activity by reason of any medically determinable physical or mental impairment which can be expected to result in death or which has lasted or can be expected to last for a continuous period of not less than 12 months, as provided in Section 22(e)(3) of the Code, and will be determined by the Board on the basis of such medical evidence as the Board deems warranted under the circumstances.
(w) “Effective Date” means the date of the closing of the transactions contemplated by that Agreement and Plan of Merger, dated December 23, 2024, between the Ikena Oncology, Inc., Insight Merger Sub I, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands and direct wholly owned subsidiary of Ikena Oncology, Inc., Insight Merger Sub II, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands and direct wholly owned subsidiary of Ikena Oncology, Inc., and Inmagene Biopharmaceuticals, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands.
(x) “Employee” means any person employed by the Company or an Affiliate. However, service solely as a Director, or payment of a fee for such services, will not cause a Director to be considered an “Employee” for purposes of the Plan.
27
(y) “Employer” means the Company or the Affiliate that employs the Participant.
(z) “Entity” means a corporation, partnership, limited liability company or other entity.
(aa) “Exchange Act” means the U.S. Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
(bb) “Exchange Act Person” means any natural person, Entity or “group” (within the meaning of Section 13(d) or 14(d) of the Exchange Act), except that “Exchange Act Person” will not include (i) the Company or any Subsidiary of the Company, (ii) any employee benefit plan of the Company or any Subsidiary of the Company or any trustee or other fiduciary holding securities under an employee benefit plan of the Company or any Subsidiary of the Company, (iii) an underwriter temporarily holding securities pursuant to a registered public offering of such securities, (iv) an Entity Owned, directly or indirectly, by the stockholders of the Company in substantially the same proportions as their Ownership of stock of the Company, or (v) any natural person, Entity or “group” (within the meaning of Section 13(d) or 14(d) of the Exchange Act) that, as of the Effective Date, is the Owner, directly or indirectly, of securities of the Company representing more than 50% of the combined voting power of the Company’s then outstanding securities.
(cc) “Fair Market Value” means, as of any date, unless otherwise determined by the Board, the value of the Common Stock (as determined on a per share or aggregate basis, as applicable) determined as follows:
(i) If the Common Stock is listed on any established stock exchange or traded on any established market, the Fair Market Value will be the closing sales price for such stock as quoted on such exchange or market (or the exchange or market with the greatest volume of trading in the Common Stock) on the date of determination, as reported in a source the Board deems reliable.
(ii) If there is no closing sales price for the Common Stock on the date of determination, then the Fair Market Value will be the closing selling price on the last preceding date for which such quotation exists.
(iii) In the absence of such markets for the Common Stock, or if otherwise determined by the Board, the Fair Market Value will be determined by the Board in good faith and in a manner that complies with Sections 409A and 422 of the Code.
(dd) “Governmental Body” means any: (i) nation, state, commonwealth, canton, province, territory, county, municipality, district or other jurisdiction of any nature; (ii) U.S. or non-U.S. federal, state, local, municipal, or other government; (iii) governmental or regulatory body, or quasi-governmental body of any nature (including any governmental division, department, administrative agency or bureau, commission, authority, instrumentality, official, ministry, fund, foundation, center, organization, unit, body or Entity and any court or other tribunal, and for the avoidance of doubt, any tax authority) or other body exercising similar powers or authority; or (iv) self-regulatory organization (including the Nasdaq Stock Market, New York Stock Exchange, and the Financial Industry Regulatory Authority).
28
(ee) “Grant Notice” means the notice provided to a Participant that he or she has been granted an Award under the Plan and which includes the name of the Participant, the type of Award, the date of grant of the Award, number of shares of Common Stock subject to the Award or potential cash payment right, (if any), the vesting schedule for the Award (if any) and other key terms applicable to the Award.
(ff) “Incentive Stock Option” means an option granted pursuant to Section 4 of the Plan that is intended to be, and qualifies as, an “incentive stock option” within the meaning of Section 422 of the Code.
(gg) “Materially Impair” means any amendment to the terms of the Award that materially adversely affects the Participant’s rights under the Award. A Participant’s rights under an Award will not be deemed to have been Materially Impaired by any such amendment if the Board, in its sole discretion, determines that the amendment, taken as a whole, does not materially impair the Participant’s rights. For example, the following types of amendments to the terms of an Award do not Materially Impair the Participant’s rights under the Award: (i) imposition of reasonable restrictions on the minimum number of shares subject to an Option or SAR that may be exercised; (ii) to maintain the qualified status of the Award as an Incentive Stock Option under Section 422 of the Code; (iii) to change the terms of an Incentive Stock Option in a manner that disqualifies, impairs or otherwise affects the qualified status of the Award as an Incentive Stock Option under Section 422 of the Code; (iv) to clarify the manner of exemption from, or to bring the Award into compliance with or qualify it for an exemption from, Section 409A; or (v) to comply with other Applicable Laws.
(hh) “Non-Employee Director” means a Director who either (i) is not a current employee or officer of the Company or an Affiliate, does not receive compensation, either directly or indirectly, from the Company or an Affiliate for services rendered as a consultant or in any capacity other than as a Director (except for an amount as to which disclosure would not be required under Item 404(a) of Regulation S-K promulgated pursuant to the Securities Act (“Regulation S-K”)), does not possess an interest in any other transaction for which disclosure would be required under Item 404(a) of Regulation S-K, and is not engaged in a business relationship for which disclosure would be required pursuant to Item 404(b) of Regulation S-K; or (ii) is otherwise considered a “non-employee director” for purposes of Rule 16b-3.
(ii) “Non-Exempt Award” means any Award that is subject to, and not exempt from, Section 409A, including as the result of (i) a deferral of the issuance of the shares subject to the Award which is elected by the Participant or imposed by the Company, or (ii) the terms of any Non-Exempt Severance Arrangement.
(jj) “Non-Exempt Director Award” means a Non-Exempt Award granted to a Participant who was a Director but not an Employee on the applicable grant date.
(kk) “Non-Exempt Severance Arrangement” means a severance arrangement or other agreement between the Participant and the Company that provides for acceleration of vesting of an Award and issuance of the shares in respect of such Award upon the Participant’s termination of employment or separation from service (as such term is defined in Section 409A(a)(2)(A)(i) of the Code (and without regard to any alternative definition thereunder)) (“Separation from Service”) and such severance benefit does not satisfy the requirements for an exemption from application of Section 409A provided under U.S. Treasury Regulations Section 1.409A-1(b)(4), 1.409A-1(b)(9) or otherwise.
29
(ll) “Nonstatutory Stock Option” means any option granted pursuant to Section 4 of the Plan that does not qualify as an Incentive Stock Option.
(mm) “Officer” means a person who is an officer of the Company within the meaning of Section 16 of the Exchange Act.
(nn) “Option” means an Incentive Stock Option or a Nonstatutory Stock Option to purchase shares of Common Stock granted pursuant to the Plan.
(oo) “Option Agreement” means a written or electronic agreement between the Company and the Optionholder evidencing the terms and conditions of the Option grant. The Option Agreement includes the Grant Notice for the Option and the agreement containing the written summary of the general terms and conditions applicable to the Option and which is provided including through electronic means, to a Participant along with the Grant Notice. Each Option Agreement will be subject to the terms and conditions of the Plan.
(pp) “Optionholder” means a person to whom an Option is granted pursuant to the Plan or, if applicable, such other person who holds an outstanding Option.
(qq) “Other Award” means an award valued in whole or in part by reference to, or otherwise based on, Common Stock, including the appreciation in value thereof (e.g., options or stock rights with an exercise price or strike price less than 100% of the Fair Market Value at the time of grant) that is not an Incentive Stock Option, Nonstatutory Stock Option, SAR, Restricted Stock Award, RSU Award or Performance Award.
(rr) “Other Award Agreement” means a written or electronic agreement between the Company and a holder of an Other Award evidencing the terms and conditions of an Other Award grant. Each Other Award Agreement will be subject to the terms and conditions of the Plan.
(ss) “Own,” “Owned,” “Owner,” or “Ownership” means that a person or Entity will be deemed to “Own,” to have “Owned,” to be the “Owner” of, or to have acquired “Ownership” of securities if such person or Entity, directly or indirectly, through any contract, arrangement, understanding, relationship or otherwise, has or shares voting power, which includes the power to vote or to direct the voting, with respect to such securities.
(tt) “Participant” means an Employee, Director or Consultant to whom an Award is granted pursuant to the Plan or, if applicable, such other person who holds an outstanding Award.
(uu) “Performance Award” means an Award that may vest or may be exercised or a cash award that may vest or become earned and paid contingent upon the attainment during a Performance Period of certain Performance Goals and which is granted under the terms and conditions of Section 5(b) pursuant to such terms as are approved by the Board. In addition, to the extent permitted by Applicable Law and set forth in the applicable Award Agreement, the Board may determine that cash or other property may be used in payment of Performance Awards. Performance Awards that are settled in cash or other property are not required to be valued in whole or in part by reference to, or otherwise based on, the Common Stock.
30
(vv) “Performance Criteria” means one or more criteria that the Board will select for purposes of establishing the Performance Goals for a Performance Period. The Performance Criteria that will be used to establish such Performance Goals may be based on any one of, or combination of, the following as determined by the Board: earnings (including earnings per share and net earnings); earnings before interest, taxes and depreciation; earnings before interest, taxes, depreciation and amortization; total stockholder return; relative stockholder return; return on equity or average stockholder’s equity; return on assets, investment, or capital employed; stock price; margin (including gross margin); income (before or after taxes); operating income; operating income after taxes; pre-tax profit; operating cash flow; sales, annual recurring revenue or revenue targets; increases in revenue or product revenue; expenses and cost reduction goals; improvement in or attainment of working capital levels; economic value added (or an equivalent metric); market share; cash flow; cash flow per share; share price performance; debt reduction; customer satisfaction; stockholders’ equity; capital expenditures; debt levels; operating profit or net operating profit; workforce diversity; growth of net income or operating income; billings; financing; regulatory milestones; stockholder liquidity; corporate governance and compliance; intellectual property; personnel matters; progress of internal research; progress of partnered programs; partner satisfaction; budget management; partner or collaborator achievements; internal controls, including those related to the U.S. Sarbanes-Oxley Act of 2002; investor relations, analysts and communication; implementation or completion of projects or processes; employee retention; number of users, including unique users; strategic partnerships or transactions (including in-licensing and out-licensing of intellectual property); establishing relationships with respect to the marketing, distribution and sale of the Company’s products; supply chain achievements; co-development, co-marketing, profit sharing, joint venture or other similar arrangements; individual performance goals; corporate development and planning goals; and other measures of performance selected by the Board or Committee whether or not listed herein.
(ww) “Performance Goals” means, for a Performance Period, one or more goals established by the Board for the Performance Period based upon the Performance Criteria. Performance Goals may be based on a Company-wide basis, with respect to one or more business units, divisions, Affiliates, or business segments, and in either absolute terms or relative to the performance of one or more comparable companies or the performance of one or more relevant indices. Unless specified otherwise by the Board (i) in the Award Agreement at the time the Award is granted or (ii) in such other document setting forth the Performance Goals at the time the Performance Goals are established, the Board will appropriately make adjustments in the method of calculating the attainment of Performance Goals for a Performance Period as follows: (1) to exclude restructuring and/or other nonrecurring charges; (2) to exclude exchange rate effects; (3) to exclude the effects of changes to generally accepted accounting principles; (4) to exclude the effects of any statutory adjustments to corporate tax rates; (5) to exclude the effects of items that are “unusual” in nature or occur “infrequently” as determined under generally accepted accounting principles; (6) to exclude the dilutive effects of acquisitions or joint ventures; (7) to assume that any business divested by the Company achieved performance objectives at targeted levels during the balance of a Performance Period following such divestiture; (8) to exclude the effect of any change in the outstanding shares of Common Stock by reason of any stock dividend or split, stock repurchase, reorganization, recapitalization, merger, consolidation, spin-off, combination or exchange of shares or other similar corporate change, or any distributions to common stockholders other than regular cash dividends; (9) to exclude the effects of stock based compensation and the award of bonuses under the Company’s bonus plans; (10) to exclude costs incurred in connection with potential acquisitions or divestitures that are required to be expensed under generally accepted accounting principles; and (11) to exclude the goodwill and intangible asset impairment charges that are required to be recorded under generally accepted accounting principles. In addition, the Board may establish or provide for other adjustment items in the Award Agreement at the time the Award is granted or in such other document setting forth the Performance Goals at the time the Performance Goals are established. In addition, the Board retains the discretion to reduce or eliminate the compensation or economic benefit due upon attainment of Performance Goals and to define the manner of calculating the Performance Criteria it selects to use for such Performance Period. Partial achievement of the specified criteria may result in the payment or vesting corresponding to the degree of achievement as specified in the Award Agreement or the written terms of a Performance Award.
31
(xx) “Performance Period” means the period of time selected by the Board over which the attainment of one or more Performance Goals will be measured for the purpose of determining a Participant’s right to vesting or exercise of an Award. Performance Periods may be of varying and overlapping duration, at the sole discretion of the Board.
(yy) “Plan” means this Ikena Oncology, Inc. 2025 Equity Incentive Plan, as amended from time to time.
(zz) “Plan Administrator” means the person, persons, and/or third-party administrator designated by the Company to administer the day-to-day operations of the Plan and the Company’s other equity incentive programs.
(aaa) “Post-Termination Exercise Period” means the period following termination of a Participant’s Continuous Service within which an Option or SAR is exercisable, as specified in Section 4(h).
(bbb) “Prior Plan” means the Ikena Oncology, Inc. 2021 Stock Option and Incentive Plan, as amended from time to time.
(ccc) “Prospectus” means the document containing the Plan information specified in Section 10(a) of the Securities Act.
(ddd) “Restricted Stock Award” or “RSA” means an Award of shares of Common Stock which is granted pursuant to the terms and conditions of Section 5(a).
(eee) “Restricted Stock Award Agreement” means a written or electronic agreement between the Company and a holder of a Restricted Stock Award evidencing the terms and conditions of a Restricted Stock Award grant. The Restricted Stock Award Agreement includes the Grant Notice for the Restricted Stock Award and the agreement containing the written summary of the general terms and conditions applicable to the Restricted Stock Award and which is provided including by electronic means, to a Participant along with the Grant Notice. Each Restricted Stock Award Agreement will be subject to the terms and conditions of the Plan.
32
(fff) “Returning Shares” means shares subject to outstanding stock awards granted under the Prior Plan and that following the Effective Date: (A) are not issued because such stock award or any portion thereof expires or otherwise terminates without all of the shares covered by such stock award having been issued; (B) are not issued because such stock award or any portion thereof is settled in cash; (C) are forfeited back to or repurchased by the Company because of the failure to meet a contingency or condition required for the vesting of such shares; (D) are withheld or reacquired to satisfy the exercise, strike or purchase price; or (E) are withheld or reacquired to satisfy a tax withholding obligation.
(ggg) “RSU Award” or “RSU” means an Award of restricted stock units representing the right to receive an issuance of shares of Common Stock which is granted pursuant to the terms and conditions of Section 5(a).
(hhh) “RSU Award Agreement” means a written or electronic agreement between the Company and a holder of an RSU Award evidencing the terms and conditions of an RSU Award grant. The RSU Award Agreement includes the Grant Notice for the RSU Award and the agreement containing the written summary of the general terms and conditions applicable to the RSU Award and which is provided including by electronic means, to a Participant along with the Grant Notice. Each RSU Award Agreement will be subject to the terms and conditions of the Plan.
(iii) “Rule 16b-3” means Rule 16b-3 promulgated under the Exchange Act or any successor to Rule 16b-3, as in effect from time to time.
(jjj) “Rule 405” means Rule 405 promulgated under the Securities Act.
(kkk) “SAR Agreement” means a written or electronic agreement between the Company and a holder of a SAR evidencing the terms and conditions of a SAR grant. The SAR Agreement includes the Grant Notice for the SAR and the agreement containing the written summary of the general terms and conditions applicable to the SAR and which is provided, including by electronic means, to a Participant along with the Grant Notice. Each SAR Agreement will be subject to the terms and conditions of the Plan.
(lll) “Section 409A” means Section 409A of the Code and the regulations and other guidance thereunder.
(mmm) “Section 409A Change in Control” means a change in the ownership or effective control of the Company, or in the ownership of a substantial portion of the Company’s assets, as provided in Section 409A(a)(2)(A)(v) of the Code and U.S. Treasury Regulations Section 1.409A-3(i)(5) (without regard to any alternative definition thereunder).
(nnn) “Securities Act” means the U.S. Securities Act of 1933, as amended.
(ooo) “Share Reserve” means the number of shares available for issuance under the Plan as set forth in Section 2(a).
(ppp) “Stock Appreciation Right” or “SAR” means a right to receive the appreciation on Common Stock that is granted pursuant to the terms and conditions of Section 4.
33
(qqq) “Subsidiary” means, with respect to the Company, (i) any corporation of which more than 50% of the outstanding capital stock having ordinary voting power to elect a majority of the board of directors of such corporation (irrespective of whether, at the time, stock of any other class or classes of such corporation will have or might have voting power by reason of the happening of any contingency) is at the time, directly or indirectly, Owned by the Company, and (ii) any partnership, limited liability company or other entity in which the Company has a direct or indirect interest (whether in the form of voting or participation in profits or capital contribution) of more than 50%.
(rrr) “Tax-Related Items” means any income tax, social insurance, payroll tax, fringe benefit tax, payment on account or other tax-related items arising out of or in relation to a Participant’s participation in the Plan and legally applicable or deemed applicable to the Participant.
(sss) “Ten Percent Stockholder” means a person who Owns (or is deemed to Own pursuant to Section 424(d) of the Code) stock possessing more than 10% of the total combined voting power of all classes of stock of the Company or any Affiliate.
(ttt) “Trading Policy” means the Company’s policy permitting certain individuals to sell Company shares only during certain “window” periods and/or otherwise restricts the ability of certain individuals to transfer or encumber Company shares, as in effect from time to time.
(uuu) “Unvested Non-Exempt Award” means the portion of any Non-Exempt Award that had not vested in accordance with its terms upon or prior to the date of any Corporate Transaction.
(vvv) “Vested Non-Exempt Award” means the portion of any Non-Exempt Award that had vested in accordance with its terms upon or prior to the date of a Corporate Transaction.
34
Exhibit 10.21
IMAGENEBIO, INC.
STOCK OPTION GRANT NOTICE
(2025 EQUITY INCENTIVE PLAN)
ImageneBio, Inc. (the “Company”), pursuant to the Company’s 2025 Equity Incentive Plan (the “Plan”), has granted to you (“Optionholder”) an option to purchase the number of shares of the Common Stock set forth below (the “Option”). Your Option is subject to all of the terms and conditions as set forth herein and in the Plan, and the Stock Option Agreement and the Notice of Exercise, all of which are attached hereto and incorporated herein in their entirety. Capitalized terms not explicitly defined herein but defined in the Plan or the Stock Option Agreement shall have the meanings set forth in the Plan or the Stock Option Agreement, as applicable.
| Optionholder: |
|
|||
| Date of Grant: |
|
|||
| Vesting Commencement Date: |
|
|||
| Number of Shares of Common Stock Subject to Option: |
|
|||
| Exercise Price (Per Share): |
|
|||
| Total Exercise Price: |
|
|||
| Expiration Date: |
|
| Type of Grant: | [Incentive Stock Option] OR [Nonstatutory Stock Option] | |
| Exercise and | ||
| Vesting Schedule: | Subject to the Optionholder’s Continuous Service through each applicable vesting date, the Option will vest as follows: | |
| [__________________________________________________________] | ||
Optionholder Acknowledgements: By your signature below or by electronic acceptance or authentication in a form authorized by the Company, you understand and agree that:
| • | The Option is governed by this Stock Option Grant Notice (this “Grant Notice”), and the provisions of the Plan and the Stock Option Agreement and the Notice of Exercise, all of which are made a part of this document. Unless otherwise provided in the Plan, this Grant Notice and the Stock Option Agreement (together, the “Option Agreement”) may not be modified, amended or revised except in a writing signed by you and a duly authorized officer of the Company. |
| • | If the Option is an Incentive Stock Option, it (plus other outstanding Incentive Stock Options granted to you) cannot be first exercisable for more than $100,000 in value (measured by exercise price) in any calendar year. Any excess over $100,000 is a Nonstatutory Stock Option. |
| • | You consent to receive this Grant Notice, the Stock Option Agreement, the Plan, the Prospectus and any other Plan-related documents by electronic delivery and to participate in the Plan through an online or electronic system established and maintained by the Company or another third party designated by the Company. |
| • | You have read and are familiar with the provisions of the Plan, the Stock Option Agreement, the Notice of Exercise and the Prospectus. In the event of any conflict between the provisions in this Grant Notice, the Option Agreement, the Notice of Exercise, or the Prospectus and the terms of the Plan, the terms of the Plan shall control. |
| • | The Option Agreement sets forth the entire understanding between you and the Company regarding the acquisition of Common Stock and supersedes all prior oral and written agreements, promises and/or representations on that subject with the exception of other equity awards previously granted to you and any written employment agreement, offer letter, severance agreement, written severance plan or policy, or other written agreement between the Company and you in each case that specifies the terms that should govern this Option. |
| • | Counterparts may be delivered via facsimile, electronic mail (including pdf or any electronic signature complying with the U.S. federal ESIGN Act of 2000, Uniform Electronic Transactions Act or other applicable law) or other transmission method and any counterpart so delivered will be deemed to have been duly and validly delivered and be valid and effective for all purposes. |
| IMAGENEBIO, INC. | OPTIONHOLDER: | |||||||
| By: |
|
|
||||||
| Signature | Signature | |||||||
| Title: |
|
Date: |
|
|||||
| Date: |
|
|||||||
IMAGENEBIO, INC.
STOCK OPTION AGREEMENT
(2025 EQUITY INCENTIVE PLAN)
ATTACHMENTS: Stock Option Agreement, Equity Incentive Plan, Notice of Exercise, Prospectus As reflected by your Stock Option Grant Notice (“Grant Notice”), ImageneBio, Inc. (the “Company”) has granted you an option under the Company’s 2025 Equity Incentive Plan (the “Plan”) to purchase a number of shares of Common Stock at the exercise price indicated in your Grant Notice (the “Option”). Capitalized terms not explicitly defined in this Agreement but defined in the Grant Notice or the Plan shall have the meanings set forth in the Grant Notice or Plan, as applicable. The terms of your Option as specified in the Grant Notice and this Stock Option Agreement constitute your Option Agreement.
The general terms and conditions applicable to your Option are as follows:
1. GOVERNING PLAN DOCUMENT. Your Option is subject to all the provisions of the Plan, including but not limited to the provisions in:
(a) Section 6 regarding the impact of a Capitalization Adjustment, dissolution, liquidation, or Corporate Transaction on your Option;
(b) Section 8 regarding certain tax consequences of your Option; and
(c) Section 9(e) regarding the Company’s retained rights to terminate your Continuous Service notwithstanding the grant of the Option.
Your Option is further subject to all interpretations, amendments, rules and regulations, which may from time to time be promulgated and adopted pursuant to the Plan. In the event of any conflict between the Option Agreement and the provisions of the Plan, the provisions of the Plan shall control.
2. VESTING. Your Option will vest as provided in your Grant Notice, subject to the provisions contained herein and the terms of the Plan. Vesting will cease upon the termination of your Continuous Service.
3. EXERCISE.
(a) You may generally exercise the vested portion of your Option for whole shares of Common Stock at any time during its term by delivery of payment of the exercise price and applicable withholding taxes and other required documentation to the Plan Administrator in accordance with the exercise procedures established by the Plan Administrator, which may include an electronic submission. Please review Sections 4(i), 4(j) and 7(b)(v) of the Plan, which may restrict or prohibit your ability to exercise your Option during certain periods.
(b) To the extent permitted by Applicable Law, you may pay your Option exercise price as follows:
(i) cash, check, bank draft, or money order;
(ii) subject to Company and/or Committee consent at the time of exercise, pursuant to a “cashless exercise” program as further described in Section 4(c)(ii) of the Plan if at the time of exercise, the Common Stock is publicly traded;
(iii) subject to Company and/or Committee consent at the time of exercise, by delivery of previously owned shares of Common Stock as further described in Section 4(c)(iii) of the Plan; or
(iv) subject to Company and/or Committee consent at the time of exercise, if the Option is a Nonstatutory Stock Option, by a “net exercise” arrangement as further described in Section 4(c)(iv) of the Plan.
(c) By accepting your Option, you agree that you will not sell, dispose of, transfer, make any short sale of, grant any option for the purchase of, or enter into any hedging or similar transaction with the same economic effect as a sale with respect to any shares of Common Stock, or other securities of the Company held by you, for a period of one hundred and eighty (180) days following the effective date of a registration statement of the Company filed under the Securities Act or such longer period as the underwriters or the Company will request to facilitate compliance with FINRA Rule 2241 or any successor or similar rules or regulation (the “Lock-Up Period”); provided, however, that nothing contained in this section will prevent the exercise of a repurchase option, if any, in favor of the Company during the Lock-Up Period. You further agree to execute and deliver such other agreements as may be reasonably requested by the Company or the underwriters that are consistent with the foregoing or that are necessary to give further effect thereto. In order to enforce the foregoing covenant, the Company may impose stop-transfer instructions with respect to your shares of Common Stock until the end of such period. You also agree that any transferee of any shares of Common Stock (or other securities) of the Company held by you will be bound by this Section 3(c). The underwriters of the Company’s stock are intended third party beneficiaries of this Section 3(c) and will have the right, power and authority to enforce the provisions hereof as though they were a party hereto.
4. TERM. You may not exercise your Option before the commencement of its term or after its term expires. The term of your Option commences on the Date of Grant and expires upon the earliest of the following:
(a) immediately upon the termination of your Continuous Service for Cause;
(b) three (3) months after the termination of your Continuous Service for any reason other than Cause, Disability or death;
(c) twelve (12) months after the termination of your Continuous Service due to your Disability;
(d) twelve (12) months after your death if you die during your Continuous Service;
(e) immediately upon a Corporate Transaction if the Board has determined that the Option will terminate in connection with a Corporate Transaction;
(f) the Expiration Date indicated in your Grant Notice; or
(g) the day before the tenth (10th) anniversary of the Date of Grant.
Notwithstanding the foregoing, if you die during the period provided in Section4(b) or 4(c) above, the term of your Option shall not expire until the earlier of (i) twelve (12) months after your death, (ii) upon any termination of the Option in connection with a Corporate Transaction, (iii) the Expiration Date indicated in your Grant Notice, or (iv) the day before the tenth anniversary of the Date of Grant. Additionally, the Post-Termination Exercise Period of your Option may be extended as provided in Section 4(i) of the Plan.
To obtain the federal income tax advantages associated with an Incentive Stock Option, the Code requires that at all times beginning on the date of grant of your Option and ending on the day three months before the date of your Option’s exercise, you must be an employee of the Company or an Affiliate, except in the event of your death or Disability. If the Company provides for the extended exercisability of your Option under certain circumstances for your benefit, your Option will not necessarily be treated as an Incentive Stock Option if you exercise your Option more than three months after the date your employment terminates.
5. WITHHOLDING OBLIGATIONS. As further provided in Section 8 of the Plan: (a) you may not exercise your Option unless the applicable tax withholding obligations are satisfied; and (b) at the time you exercise your Option, in whole or in part, or at any time thereafter as requested by the Company, you hereby authorize withholding from payroll and any other amounts payable to you, and otherwise agree to make adequate provision for (including by means of a “cashless exercise” pursuant to a program developed under Regulation T as promulgated by the Federal Reserve Board to the extent permitted by the Company), any sums required to satisfy the federal, state, local and foreign tax withholding obligations, if any, which arise in connection with the exercise of your Option in accordance with the withholding procedures established by the Company. Accordingly, you may not be able to exercise your Option even though the Option is vested, and the Company shall have no obligation to issue shares of Common Stock subject to your Option, unless and until such obligations are satisfied. In the event that the amount of the Company’s withholding obligation in connection with your Option was greater than the amount actually withheld by the Company, you agree to indemnify and hold the Company harmless from any failure by the Company to withhold the proper amount.
6. INCENTIVE STOCK OPTION DISPOSITION REQUIREMENT. If your Option is an Incentive Stock Option, you must notify the Company in writing within fifteen (15) days after the date of any disposition of any of the shares of the Common Stock issued upon exercise of your Option that occurs within two years after the date of your Option grant or within one year after such shares of Common Stock are transferred upon exercise of your Option.
7. TRANSFERABILITY. Except as otherwise provided in Section 4(e) of the Plan, your Option is not transferable, except by will or by the applicable laws of descent and distribution and is exercisable during your life only by you.
8. CORPORATE TRANSACTION. Your Option is subject to the terms of any agreement governing a Corporate Transaction involving the Company, including, without limitation, a provision for the appointment of a stockholder representative that is authorized to act on your behalf with respect to any escrow, indemnities, and any contingent consideration.
9. NO LIABILITY FOR TAXES. As a condition to accepting the Option, you hereby (a) agree to not make any claim against the Company, or any of its Officers, Directors, Employees or Affiliates related to tax liabilities arising from the Option or other Company compensation and (b) acknowledge that you were advised to consult with your own personal tax, financial and other legal advisors regarding the tax consequences of the Option and have either done so or knowingly and voluntarily declined to do so. Additionally, you acknowledge that the Option is exempt from Section 409A only if the exercise price is at least equal to the “fair market value” of the Common Stock on the date of grant as determined by the Internal Revenue Service and there is no other impermissible deferral of compensation associated with the Option. Additionally, as a condition to accepting the Option, you agree not make any claim against the Company, or any of its Officers, Directors, Employees or Affiliates in the event that the Internal Revenue Service asserts that such exercise is less than the “fair market value” of the Common Stock on the date of grant as subsequently determined by the Internal Revenue Service.
10. SEVERABILITY. If any part of this Option Agreement or the Plan is declared by any court or governmental authority to be unlawful or invalid, such unlawfulness or invalidity will not invalidate any portion of this Option Agreement or the Plan not declared to be unlawful or invalid. Any Section of this Option Agreement (or part of such a Section) so declared to be unlawful or invalid will, if possible, be construed in a manner which will give effect to the terms of such Section or part of a Section to the fullest extent possible while remaining lawful and valid
11. OTHER DOCUMENTS. You hereby acknowledge receipt of or the right to receive a document providing the information required by Rule 428(b)(1) promulgated under the Securities Act, which includes the Prospectus. In addition, you acknowledge receipt of the Company’s Trading Policy.
12. QUESTIONS. If you have questions regarding these or any other terms and conditions applicable to your Option, including a summary of the applicable federal income tax consequences, please see the Prospectus.
* * * *
IMAGENEBIO, INC.
NOTICE OF EXERCISE
(2025 EQUITY INCENTIVE PLAN)
IMAGENEBIO, INC.
12526 High Bluff Drive, Suite 345
San Diego, CA 92130
Date of Exercise: _______________
This constitutes notice to ImageneBio, Inc. (the “Company”) that I elect to purchase the below number of shares of Common Stock of the Company (the “Shares”) by exercising my Option for the price set forth below. Capitalized terms not explicitly defined in this Notice of Exercise but defined in the Stock Option Grant Notice, Stock Option Agreement or 2025 Equity Incentive Plan (the “Plan”) shall have the meanings set forth in the Stock Option Grant Notice, Stock Option Agreement, or Plan, as applicable. Use of certain payment methods is subject to Company and/or Committee consent and certain additional requirements set forth in the Stock Option Agreement and the Plan.
| Type of option (check one): |
Incentive ☐ | Nonstatutory | ☐ | |||||
| Date of Grant: |
||||||||
|
|
|
|||||||
| Number of Shares as to which Option is exercised: |
||||||||
|
|
|
|||||||
| Certificates to be issued in name of: |
||||||||
|
|
|
|||||||
| Total exercise price: |
$ | ______________ | ||||||
| Cash, check, bank draft or money order delivered herewith: |
$ | ______________ | ||||||
| Value of ________ Shares delivered herewith: |
$ | ______________ | ||||||
| Regulation T Program (cashless exercise): |
$ | _____________ | ||||||
| Value of _______ Shares pursuant to net exercise: |
$ | _____________ |
By this exercise, I agree (i) to provide such additional documents as you may require pursuant to the terms of the Plan; (ii) to satisfy the tax withholding obligations, if any, relating to the exercise of this Option as set forth in the Stock Option Agreement; and, (iii) if this exercise relates to an incentive stock option, to notify you in writing within fifteen (15) days after the date of any disposition of any of the Shares issued upon exercise of this Option that occurs within two (2) years after the Date of Grant or within one year after such Shares are issued upon exercise of this Option.
I further agree that, if required by the Company (or a representative of the underwriters) in connection with the first underwritten registration of the offering of any securities of the Company under the Securities Act, I will not sell, dispose of, transfer, make any short sale of, grant any option for the purchase of, or enter into any hedging or similar transaction with the same economic effect as a sale with respect to any shares of Common Stock or other securities of the Company, for a period of one hundred and eighty (180) days following the effective date of a registration statement of the Company filed under the Securities Act (or such longer period as the underwriters or the Company shall request to facilitate compliance with FINRA Rule 2241 or any successor or similar rule or regulation) (the “Lock-Up Period”); provided, however, that nothing contained in this paragraph will prevent the exercise of a repurchase option, if any, in favor of the Company during the Lock-Up Period. I further agree to execute and deliver such other agreements as may be reasonably requested by the Company or the underwriters that are consistent with the foregoing or that are necessary to give further effect thereto. To enforce the foregoing covenant, the Company may impose stop-transfer instructions with respect to securities subject to the foregoing restrictions until the end of such period.
| Very truly yours, |
|
|
Exhibit 10.22
IMAGENEBIO, INC.
RSU AWARD GRANT NOTICE
(2025 EQUITY INCENTIVE PLAN)
ImageneBio, Inc. (the “Company”) has awarded to you (the “Participant”) the number of restricted stock units specified and on the terms set forth below in consideration of your services (the “RSU Award”). Your RSU Award is subject to all of the terms and conditions as set forth herein and in the Company’s 2025 Equity Incentive Plan (the “Plan”) and the Award Agreement (the “Agreement”), which are attached hereto and incorporated herein in their entirety. Capitalized terms not explicitly defined herein but defined in the Plan or the Agreement shall have the meanings set forth in the Plan or the Agreement.
| Participant: |
|
|||
| Date of Grant: |
|
|||
| Vesting Commencement Date: |
|
|||
| Number of Restricted Stock Units: |
|
| Vesting Schedule: | Subject to the Participant’s Continuous Service through each applicable vesting date, the RSU Award will vest as follows: [_____] | |
| Notwithstanding the foregoing, vesting shall terminate upon the Participant’s termination of Continuous Service. |
||
| Issuance Schedule: | Subject to any Capitalization Adjustment, one share of Common Stock will be issued at the time set forth in Section 5 of the Agreement for each restricted stock unit which vests. | |
Participant Acknowledgements: By your signature below or by electronic acceptance or authentication in a form authorized by the Company, you understand and agree that:
| • | The RSU Award is governed by this RSU Award Grant Notice (the “Grant Notice”), and the provisions of the Plan and the Agreement, all of which are made a part of this document. Unless otherwise provided in the Plan, this Grant Notice and the Agreement (together, the “RSU Award Agreement”) may not be modified, amended or revised except in a writing signed by you and a duly authorized officer of the Company. |
| • | You have read and are familiar with the provisions of the Plan, the RSU Award Agreement and the Prospectus. In the event of any conflict between the provisions in the RSU Award Agreement, or the Prospectus and the terms of the Plan, the terms of the Plan shall control. |
| • | The RSU Award Agreement sets forth the entire understanding between you and the Company regarding the acquisition of Common Stock and supersedes all prior oral and written agreements, promises and/or representations on that subject with the exception of: (i) other equity awards previously granted to you; and (ii) any written employment agreement, offer letter, severance agreement, written severance plan or policy, or other written agreement between the Company and you in each case that specifies the terms that should govern this RSU Award. |
| IMAGENEBIO, INC. | PARTICIPANT: | |||||||
| By: |
|
|
||||||
| Signature | Signature | |||||||
| Title: |
|
Date: |
|
|||||
| Date: |
|
|||||||
IMAGENEBIO, INC.
AWARD AGREEMENT
(2025 EQUITY INCENTIVE PLAN)
ATTACHMENTS: Award Agreement, 2025 Equity Incentive Plan, Prospectus As reflected by your RSU Award Grant Notice (“Grant Notice”), ImageneBio, Inc. (the “Company”) has granted you a RSU Award under the Company’s 2025 Equity Incentive Plan (the “Plan”) for the number of restricted stock units as indicated in your Grant Notice (the “RSU Award”). The terms of your RSU Award as specified in this Award Agreement for your RSU Award (this “Agreement”) and the Grant Notice constitute your “RSU Award Agreement.” Defined terms not explicitly defined in this Agreement but defined in the Grant Notice or the Plan shall have the same definitions as in the Grant Notice or Plan, as applicable.
The general terms applicable to your RSU Award are as follows:
1. GOVERNING PLAN DOCUMENT. Your RSU Award is subject to all the provisions of the Plan, including but not limited to the provisions in:
(a) Section 6 of the Plan regarding the impact of a Capitalization Adjustment, dissolution, liquidation, or Corporate Transaction on your RSU Award;
(b) Section 8 of the Plan regarding the tax consequences of your RSU Award; and
(c) Section 9(e) of the Plan regarding the Company’s retained rights to terminate your Continuous Service notwithstanding the grant of the RSU Award.
Your RSU Award is further subject to all interpretations, amendments, rules and regulations, which may from time to time be promulgated and adopted pursuant to the Plan. In the event of any conflict between the RSU Award Agreement and the provisions of the Plan, the provisions of the Plan shall control.
2. GRANT OF THE RSU AWARD. This RSU Award represents your right to be issued on a future date the number of shares of the Company’s Common Stock that is equal to the number of restricted stock units indicated in the Grant Notice as modified to reflect any Capitalization Adjustment and subject to your satisfaction of the vesting conditions set forth therein (the “Restricted Stock Units”). Any additional Restricted Stock Units that become subject to the RSU Award pursuant to Capitalization Adjustments as set forth in the Plan and the provisions of Section 3 below, if any, shall be subject, in a manner determined by the Board, to the same forfeiture restrictions, restrictions on transferability, and time and manner of delivery as applicable to the other Restricted Stock Units covered by your RSU Award.
3. DIVIDENDS. You shall receive no benefit or adjustment to this RSU Award with respect to any cash dividend, stock dividend, or other distribution that does not result from a Capitalization Adjustment; provided, however, that this sentence will not apply with respect to any shares of Common Stock that are delivered to you in connection with your RSU Award after such shares have been delivered to you.
4. WITHHOLDING OBLIGATIONS. As further provided in Section 8 of the Plan, you hereby authorize withholding from payroll and any other amounts payable to you, and otherwise agree to make adequate provision for, any sums required to satisfy the federal, state, local and foreign tax withholding obligations, if any, which arise in connection with your RSU Award (the “Withholding Obligation”) in accordance with the withholding procedures established by the Company. Unless the Withholding Obligation is satisfied, the Company shall have no obligation to deliver to you any Common Stock in respect of the RSU Award. In the event the Withholding Obligation of the Company arises prior to the delivery to you of Common Stock or it is determined after the delivery of Common Stock to you that the amount of the Withholding Obligation was greater than the amount withheld by the Company, you agree to indemnify and hold the Company harmless from any failure by the Company to withhold the proper amount.
5. DATE OF ISSUANCE.
(a) The issuance of shares in respect of the Restricted Stock Units is intended to comply with Treasury Regulations Section 1.409A-1(b)(4) and will be construed and administered in such a manner. Subject to the satisfaction of the Withholding Obligation, if any, in the event one or more Restricted Stock Units vests, the Company shall issue to you one (1) share of Common Stock for each Restricted Stock Unit that vests on the applicable vesting date(s) (subject to any adjustment under Section 3 above, and subject to any different provisions in the Grant Notice) . Each issuance date determined by this paragraph is referred to as an “Original Issuance Date.”
(b) If the Original Issuance Date falls on a date that is not a business day, delivery shall instead occur on the next following business day. In addition, if:
(i) the Original Issuance Date does not occur (1) during an “open window period” applicable to you, as determined by the Company in accordance with the Company’s then-effective policy on trading in Company securities; or (2) on a date when you are otherwise permitted to sell shares of Common Stock on an established stock exchange or stock market (including but not limited to under a previously established written trading plan that meets the requirements of Rule 10b5-1 under the Exchange Act and was entered into in compliance with the Company’s policies (a “10b5-1 Arrangement”)), and
(ii) either (1) a Withholding Obligation does not apply; or (2) the Company decides, prior to the Original Issuance Date, (A) not to satisfy the Withholding Obligation by withholding shares of Common Stock from the shares otherwise due, on the Original Issuance Date, to you under this Award; and (B) not to permit you to enter into a “same day sale” commitment with a broker-dealer (including but not limited to a commitment under a 10b5-1 Arrangement); and (C) not to permit you to pay your Withholding Obligation in cash, then the shares that would otherwise be issued to you on the Original Issuance Date will not be delivered on such Original Issuance Date and will instead be delivered on the first business day when you are not prohibited from selling shares of the Company’s Common Stock in the open public market, but in no event later than December 31st of the calendar year in which the Original Issuance Date occurs (that is, the last day of your taxable year in which the Original Issuance Date occurs), or, if and only if permitted in a manner that complies with Treasury Regulations Section 1.409A-1(b)(4), no later than the date that is the 15th day of the 3rd calendar month of the applicable year following the year in which the shares of Common Stock under this Award are no longer subject to a “substantial risk of forfeiture” within the meaning of Treasury Regulations Section 1.409A-1(d).
(c) To the extent the RSU Award is a Non-Exempt Award, the provisions of Section 11 of the Plan shall apply.
6. TRANSFERABILITY. Except as otherwise provided in the Plan, your RSU Award is not transferable, except by will or by the applicable laws of descent and distribution.
7. CORPORATE TRANSACTION. Your RSU Award is subject to the terms of any agreement governing a Corporate Transaction involving the Company, including, without limitation, a provision for the appointment of a stockholder representative that is authorized to act on your behalf with respect to any escrow, indemnities, and any contingent consideration.
8. NO LIABILITY FOR TAXES. As a condition to accepting the RSU Award, you hereby (a) agree to not make any claim against the Company, or any of its Officers, Directors, Employees, or Affiliates related to tax liabilities arising from the RSU Award or other Company compensation; and (b) acknowledge that you were advised to consult with your own personal tax, financial, and other legal advisors regarding the tax consequences of the RSU Award and have either done so or have knowingly and voluntarily declined to do so.
9. SEVERABILITY. If any part of this Agreement or the Plan is declared by any court or governmental authority to be unlawful or invalid, such unlawfulness or invalidity will not invalidate any portion of this Agreement or the Plan not declared to be unlawful or invalid. Any Section of this Agreement (or part of such a Section) so declared to be unlawful or invalid will, if possible, be construed in a manner which will give effect to the terms of such Section or part of a Section to the fullest extent possible while remaining lawful and valid.
10. OTHER DOCUMENTS. You hereby acknowledge receipt of or the right to receive a document providing the information required by Rule 428(b)(1) promulgated under the Securities Act, which includes the Prospectus. In addition, you acknowledge receipt of the Company’s Trading Policy.
11. QUESTIONS. If you have questions regarding these or any other terms and conditions applicable to your RSU Award, including a summary of the applicable federal income tax consequences, please see the Prospectus.
* * * *
Exhibit 10.23
ImageneBio, Inc.
2025 Employee Stock Purchase Plan
Adopted by the Board of Directors: December 12, 2024
Approved by the Stockholders: July 15, 2025
Effective Date: July 26, 2025
1. GENERAL; PURPOSE.
(a) The Plan provides a means by which Eligible Employees of the Company and certain Designated Companies may be given an opportunity to purchase shares of Common Stock. The Plan permits the Company to grant a series of Purchase Rights to Eligible Employees under an Employee Stock Purchase Plan. In addition, the Plan permits the Company to grant a series of Purchase Rights to Eligible Employees that do not meet the requirements of an Employee Stock Purchase Plan.
(b) The Plan includes two components: a 423 Component and a Non-423 Component. The Company intends (but makes no undertaking or representation to maintain) the 423 Component to qualify as an Employee Stock Purchase Plan. The provisions of the 423 Component, accordingly, will be construed in a manner that is consistent with the requirements of Section 423 of the Code. In addition, this Plan authorizes grants of Purchase Rights under the Non-423 Component that do not meet the requirements of an Employee Stock Purchase Plan. Except as otherwise provided in the Plan or determined by the Board, the Non-423 Component will operate and be administered in the same manner as the 423 Component. In addition, the Company may make separate Offerings which vary in terms (provided that such terms are not inconsistent with the provisions of the Plan or the requirements of an Employee Stock Purchase Plan to the extent the Offering is made under the 423 Component), and the Company will designate which Designated Company is participating in each separate Offering.
(c) The Company, by means of the Plan, seeks to retain the services of Eligible Employees, to secure and retain the services of new Employees and to provide incentives for such persons to exert maximum efforts for the success of the Company and its Related Corporations.
2. ADMINISTRATION.
(a) The Board will administer the Plan unless and until the Board delegates administration of the Plan to a Committee or Committees, as provided in Section 2(c).
(b) The Board will have the power, subject to, and within the limitations of, the express provisions of the Plan:
(i) To determine how and when Purchase Rights will be granted and the provisions of each Offering (which need not be identical).
(ii) To designate from time to time (A) which Related Corporations will be eligible to participate in the Plan as Designated 423 Companies, (B) which Related Corporations or Affiliates will be eligible to participate in the Plan as Designated Non-423 Companies, (C) which Affiliates or Related Corporations may be excluded from participation in the Plan, and (D) which Designated Companies will participate in each separate Offering (to the extent that the Company makes separate Offerings).
(iii) To construe and interpret the Plan and Purchase Rights, and to establish, amend and revoke rules and regulations for its administration. The Board, in the exercise of this power, may correct any defect, omission or inconsistency in the Plan, in a manner and to the extent it deems necessary or expedient to make the Plan fully effective.
(iv) To settle all controversies regarding the Plan and Purchase Rights granted under the Plan.
(v) To suspend or terminate the Plan at any time as provided in Section 12.
(vi) To amend the Plan at any time as provided in Section 12.
(vii) Generally, to exercise such powers and to perform such acts as it deems necessary or expedient to promote the best interests of the Company, its Related Corporations and Affiliates, and to carry out the intent that the Plan be treated as an Employee Stock Purchase Plan with respect to the 423 Component.
(viii) To adopt such rules, procedures and sub-plans as are necessary or appropriate to permit or facilitate participation in the Plan by Employees who are non-U.S. nationals or employed or located outside the United States. Without limiting the generality of, and consistent with, the foregoing, the Board specifically is authorized to adopt rules, procedures, and sub-plans regarding, without limitation, eligibility to participate in the Plan, the definition of eligible “earnings,” handling and making of Contributions, establishment of bank or trust accounts to hold Contributions, payment of interest, conversion of local currency, obligations to pay payroll tax, determination of beneficiary designation requirements, withholding procedures and handling of share issuances, any of which may vary according to applicable requirements, and which, if applicable to a Designated Non-423 Company, do not have to comply with the requirements of Section 423 of the Code.
(c) The Board may delegate some or all of the administration of the Plan to a Committee or Committees. If administration is delegated to a Committee, the Committee will have, in connection with the administration of the Plan, the powers theretofore possessed by the Board that have been delegated to the Committee, including the power to delegate to a subcommittee any of the administrative powers the Committee is authorized to exercise (and references in this Plan and any applicable Offering Document to the Board will thereafter be to the Committee or subcommittee), subject, however, to such resolutions, not inconsistent with the provisions of the Plan, as may be adopted from time to time by the Board. Further, to the extent not prohibited by Applicable Law, the Board or Committee may, from time to time, delegate some or all of its authority under the Plan to one or more officers of the Company or other persons or groups of persons as it deems necessary, appropriate or advisable under conditions or limitations that it may set at or after the time of the delegation. The Board may retain the authority to concurrently administer the Plan with the Committee (or its delegate) and may, at any time, revest in the Board some or all of the powers previously delegated. Whether or not the Board has delegated administration of the Plan to a Committee (or a delegate of the Committee), the Board will have the final power to determine all questions of policy and expediency that may arise in the administration of the Plan.
(d) All determinations, interpretations and constructions made by the Board will not be subject to review by any person and will be final, binding and conclusive on all persons.
2
3. SHARES OF COMMON STOCK SUBJECT TO THE PLAN.
(a) Subject to the provisions of Section 11(a) relating to Capitalization Adjustments, the maximum number of shares of Common Stock that may be issued under the Plan will not exceed 111,817 shares of Common Stock, plus the number of shares of Common Stock that are automatically added on January 1st of each year for a period of up to ten years, commencing on January 1, 2026 and ending on (and including) January 1, 2035, in an amount equal to the lesser of (x) one percent (1%) of the total number of shares of Capital Stock outstanding on December 31st of the preceding calendar year, and (y) 227,944 shares of Common Stock. Notwithstanding the foregoing, the Board may act prior to the first day of any calendar year to provide that there will be no January 1st increase in the share reserve for such calendar year or that the increase in the share reserve for such calendar year will be a lesser number of shares of Common Stock than would otherwise occur pursuant to the preceding sentence. For the avoidance of doubt, up to the maximum number of shares of Common Stock reserved under this Section 3(a) may be used to satisfy purchases of Common Stock under the 423 Component and any remaining portion of such maximum number of shares may be used to satisfy purchases of Common Stock under the Non-423 Component.
(b) If any Purchase Right granted under the Plan terminates without having been exercised in full, the shares of Common Stock not purchased under such Purchase Right will again become available for issuance under the Plan.
(c) The stock purchasable under the Plan will be shares of authorized but unissued or reacquired Common Stock, including shares repurchased by the Company on the open market.
4. GRANT OF PURCHASE RIGHTS; OFFERING.
(a) The Board may from time to time grant or provide for the grant of Purchase Rights to Eligible Employees under an Offering (consisting of one or more Purchase Periods) on an Offering Date or Offering Dates selected by the Board. Each Offering will be in such form and will contain such terms and conditions as the Board will deem appropriate, and with respect to the 423 Component, will comply with the requirement of Section 423(b)(5) of the Code that all Employees granted Purchase Rights will have the same rights and privileges. The terms and conditions of an Offering shall be incorporated by reference into the Plan and treated as part of the Plan. The provisions of separate Offerings need not be identical, but each Offering will include (through incorporation of the provisions of this Plan by reference in the document comprising the Offering or otherwise) the period during which the Offering will be effective, which period will not exceed 27 months beginning with the Offering Date, and the substance of the provisions contained in Sections 5 through 8, inclusive.
(b) If a Participant has more than one Purchase Right outstanding under the Plan, unless such Participant otherwise indicates in forms delivered to the Company or a third party designated by the Company (each, a “Company Designee”): (i) each form will apply to all of such Participant’s Purchase Rights under the Plan, and (ii) a Purchase Right with a lower exercise price (or an earlier-granted Purchase Right, if different Purchase Rights have identical exercise prices) will be exercised to the fullest possible extent before a Purchase Right with a higher exercise price (or a later-granted Purchase Right if different Purchase Rights have identical exercise prices) will be exercised.
(c) The Board will have the discretion to structure an Offering so that if the Fair Market Value of a share of Common Stock on the first Trading Day of a new Purchase Period within that Offering is less than or equal to the Fair Market Value of a share of Common Stock on the Offering Date for that Offering, then (i) that Offering will terminate immediately as of that first Trading Day, and (ii) the Participants in such terminated Offering will be automatically enrolled in a new Offering beginning on the first Trading Day of such new Purchase Period.
3
5. ELIGIBILITY.
(a) Purchase Rights may be granted only to Employees of the Company or, as the Board may designate in accordance with Section 2(b), to Employees of a Related Corporation or an Affiliate. Except as provided in Section 5(b) or as required by Applicable Law, an Employee will not be eligible to be granted Purchase Rights unless, on the Offering Date, the Employee has been in the employ of the Company, the Related Corporation or the Affiliate, as the case may be, for such continuous period preceding such Offering Date as the Board may (unless prohibited by Applicable Law) require, but in no event will the required period of continuous employment be equal to or greater than two years. In addition, the Board may provide (unless prohibited by Applicable Law) that no Employee will be eligible to be granted Purchase Rights under the Plan unless, on the Offering Date, such Employee’s customary employment with the Company, the Related Corporation or the Affiliate is more than 20 hours per week and more than five months per calendar year or such other criteria as the Board may determine consistent with Section 423 of the Code with respect to the 423 Component. The Board may also exclude (unless prohibited by Applicable Law) from participation in the Plan or any Offering Employees who are “highly compensated employees” (within the meaning of Section 423(b)(4)(D) of the Code) of the Company, a Related Corporation or an Affiliate, or a subset of such highly compensated employees.
(b) The Board may provide that each person who, during the course of an Offering, first becomes an Eligible Employee will, on a date or dates specified in the Offering which coincides with the day on which such person becomes an Eligible Employee or which occurs thereafter, receive a Purchase Right under that Offering, which Purchase Right will thereafter be deemed to be a part of that Offering. Such Purchase Right will have the same characteristics as any Purchase Rights originally granted under that Offering, as described herein, except that:
(i) the date on which such Purchase Right is granted will be the “Offering Date” of such Purchase Right for all purposes, including determination of the exercise price of such Purchase Right;
(ii) the period of the Offering with respect to such Purchase Right will begin on its Offering Date and end coincident with the end of such Offering; and
(iii) the Board may provide that if such person first becomes an Eligible Employee within a specified period of time before the end of the Offering, the individual will not receive any Purchase Right under that Offering.
(c) No Employee will be eligible for the grant of any Purchase Rights under the 423 Component if, immediately after any such Purchase Rights are granted, such Employee owns stock possessing five percent or more of the total combined voting power or value of all classes of stock of the Company or of any Related Corporation. For purposes of this Section 5(c), the rules of Section 424(d) of the Code will apply in determining the stock ownership of any Employee, and stock which such Employee may purchase under all outstanding Purchase Rights and options will be treated as stock owned by such Employee.
(d) As specified by Section 423(b)(8) of the Code, an Eligible Employee may be granted Purchase Rights under the 423 Component only if such Purchase Rights, together with any other rights granted under all Employee Stock Purchase Plans of the Company and any Related Corporations, do not permit such Eligible Employee’s rights to purchase stock of the Company or any Related Corporation to accrue at a rate which, when aggregated, exceeds U.S. $25,000 of Fair Market Value of such stock (determined at the time such rights are granted, and which, with respect to the Plan, will be determined as of their respective Offering Dates) for each calendar year in which such rights are outstanding at any time.
(e) Officers of the Company and any Designated Company, if they are otherwise Eligible Employees, will be eligible to participate in Offerings under the Plan. Notwithstanding the foregoing, the Board may (unless prohibited by Applicable Law) provide in an Offering that Employees who are highly compensated Employees within the meaning of Section 423(b)(4)(D) of the Code will not be eligible to participate.
4
(f) Notwithstanding anything in this Section 5 to the contrary, in the case of an Offering under the Non-423 Component, an Eligible Employee (or group of Eligible Employees) may be excluded from participation in the Plan or an Offering if the Board has determined, in its sole discretion, that participation of such Eligible Employee(s) is not advisable or practical for any reason.
6. PURCHASE RIGHTS; PURCHASE PRICE.
(a) On each Offering Date, each Eligible Employee, pursuant to an Offering made under the Plan, will be granted a Purchase Right to purchase up to that number of shares of Common Stock purchasable either with a percentage of earnings (as such concept is defined in the Offering Document) or with a maximum dollar amount, but in either case as so specified by the Board in the Offering Document, during the period that begins on the Offering Date (or such later date as the Board determines for a particular Offering) and ends on the date stated in the Offering, which date will be no later than the end of the Offering.
(b) The Board will establish one or more Purchase Dates during an Offering on which Purchase Rights granted for that Offering will be exercised and shares of Common Stock will be purchased in accordance with such Offering.
(c) In connection with each Offering made under the Plan, the Board may specify (i) a maximum number of shares of Common Stock that may be purchased by any Participant on any Purchase Date during such Offering, (ii) a maximum aggregate number of shares of Common Stock that may be purchased by all Participants pursuant to such Offering and/or (iii) a maximum aggregate number of shares of Common Stock that may be purchased by all Participants on any Purchase Date under the Offering. If the aggregate purchase of shares of Common Stock issuable upon exercise of Purchase Rights granted under the Offering would exceed any such maximum aggregate number, then, in the absence of any Board action otherwise, a pro rata (based on each Participant’s accumulated Contributions) allocation of the shares of Common Stock (rounded down to the nearest whole share) available will be made in as nearly a uniform manner as will be practicable and equitable.
(d) The purchase price of shares of Common Stock acquired pursuant to Purchase Rights will be specified by the Board prior to commencement of an Offering and will not be less than the lesser of:
(i) an amount equal to 85% of the Fair Market Value of the shares of Common Stock on the Offering Date; or
(ii) an amount equal to 85% of the Fair Market Value of the shares of Common Stock on the applicable Purchase Date.
7. PARTICIPATION; WITHDRAWAL; TERMINATION.
(a) An Eligible Employee may elect to participate in an Offering and authorize payroll deductions as the means of making Contributions by completing and delivering to the Company or a Company Designee, within the time specified in the Offering, an enrollment form provided by the Company or a Company Designee. The enrollment form will specify the amount of Contributions not to exceed the maximum amount specified by the Board. Each Participant’s Contributions will be credited to a bookkeeping account for such Participant under the Plan and will be deposited with the general funds of the Company except where Applicable Law requires that Contributions be held separately or deposited with a third party. If permitted in the Offering, a Participant may begin such Contributions with the first practicable payroll occurring on or after the Offering Date (or, in the case of a payroll date that occurs after the end of the prior Offering but before the Offering Date of the next new Offering, Contributions from such payroll will be included in the new Offering). If permitted in the Offering, a Participant may thereafter reduce (including to zero) or increase such Participant’s Contributions. If payroll deductions are impermissible or problematic under Applicable Law or if specifically provided in the Offering and to the extent permitted by Section 423 of the Code with respect to the 423 Component, in addition to or instead of making Contributions by payroll deductions, a Participant may make Contributions through payment by cash, check or wire transfer prior to a Purchase Date.
5
(b) During an Offering, a Participant may cease making Contributions and withdraw from the Offering by delivering to the Company or a Company Designee a withdrawal form provided by the Company or a Company Designee. The Company may impose a deadline before a Purchase Date for withdrawing. Upon such withdrawal, such Participant’s Purchase Right in that Offering will immediately terminate and the Company will distribute as soon as practicable to such Participant all of such Participant’s accumulated but unused Contributions and such Participant’s Purchase Right in that Offering shall thereupon terminate. A Participant’s withdrawal from that Offering will have no effect upon such Participant’s eligibility to participate in any other Offerings under the Plan, but such Participant will be required to deliver a new enrollment form to participate in subsequent Offerings.
(c) Purchase Rights granted pursuant to any Offering under the Plan will terminate immediately if the Participant either (i) is no longer an Employee for any reason or for no reason or (ii) is otherwise no longer eligible to participate. The Company will distribute as soon as practicable to such individual all of such individual’s accumulated but unused Contributions.
(d) Unless otherwise determined by the Board, a Participant whose employment transfers or whose employment terminates with an immediate rehire (with no break in service) by or between the Company and a Designated Company or between Designated Companies will not be treated as having terminated employment for purposes of participating in the Plan or an Offering; however, if a Participant transfers from an Offering under the 423 Component to an Offering under the Non-423 Component, the exercise of the Participant’s Purchase Right will be qualified under the 423 Component only to the extent such exercise complies with Section 423 of the Code. If a Participant transfers from an Offering under the Non-423 Component to an Offering under the 423 Component, the exercise of the Purchase Right will remain non-qualified under the Non-423 Component for the remainder of the Offering. The Board may establish different and additional rules governing transfers between separate Offerings within the 423 Component and between Offerings under the 423 Component and Offerings under the Non-423 Component.
(e) During a Participant’s lifetime, Purchase Rights will be exercisable only by such Participant. Purchase Rights are not transferable by a Participant, except by will, by the laws of descent and distribution, or, if permitted by the Company and valid under Applicable Law, by a beneficiary designation as described in Section 10.
(f) Unless otherwise specified in the Offering or required by Applicable Law, the Company will have no obligation to pay interest on Contributions.
8. EXERCISE OF PURCHASE RIGHTS.
(a) On each Purchase Date, each Participant’s accumulated Contributions will be applied to the purchase of shares of Common Stock, up to the maximum number of shares of Common Stock permitted by the Plan and the applicable Offering, at the purchase price specified in the Offering. No fractional shares will be issued unless specifically provided for in the Offering.
6
(b) Unless otherwise provided in the Offering, if any amount of accumulated Contributions remains in a Participant’s account after the purchase of shares of Common Stock and such remaining amount is less than the amount required to purchase one share of Common Stock on the final Purchase Date of an Offering, then such remaining amount will be held in such Participant’s account for the purchase of shares of Common Stock under the next Offering under the Plan, unless such Participant withdraws from or is not eligible to participate in such next Offering, in which case such amount will be distributed to such Participant after the final Purchase Date without interest (unless the payment of interest is otherwise required by Applicable Law). If the amount of Contributions remaining in a Participant’s account after the purchase of shares of Common Stock is at least equal to the amount required to purchase one (1) whole share of Common Stock on the final Purchase Date of an Offering, then such remaining amount will be distributed in full to such Participant after the final Purchase Date of such Offering without interest (unless otherwise required by Applicable Law).
(c) No Purchase Rights may be exercised to any extent unless the shares of Common Stock to be issued upon such exercise under the Plan are covered by an effective registration statement pursuant to the Securities Act and the Plan is in material compliance with all applicable U.S. and non-U.S. federal, state and other securities, exchange control and other laws applicable to the Plan. If on a Purchase Date the shares of Common Stock are not so registered or the Plan is not in such compliance, no Purchase Rights will be exercised on such Purchase Date, and, subject to Section 423 of the Code with respect to the 423 Component, the Purchase Date will be delayed until the shares of Common Stock are subject to such an effective registration statement and the Plan is in material compliance, except that the Purchase Date will in no event be more than 27 months from the Offering Date. If, on the Purchase Date, as delayed to the maximum extent permissible, the shares of Common Stock are not registered and the Plan is not in material compliance with all Applicable Laws, as determined by the Company in its sole discretion, no Purchase Rights will be exercised and all accumulated but unused Contributions will be distributed to the Participants without interest (unless the payment of interest is otherwise required by Applicable Law).
9. COVENANTS OF THE COMPANY.
The Company will seek to obtain from each U.S. and non-U.S. federal, state or other regulatory commission, agency or other Governmental Body having jurisdiction over the Plan such authority as may be required to grant Purchase Rights and issue and sell shares of Common Stock thereunder unless the Company determines, in its sole discretion, that doing so is not practical or would cause the Company to incur costs that are unreasonable. If, after commercially reasonable efforts, the Company is unable to obtain the authority that counsel for the Company deems necessary for the grant of Purchase Rights or the lawful issuance and sale of Common Stock under the Plan, and at a commercially reasonable cost, the Company will be relieved from any liability for failure to grant Purchase Rights and/or to issue and sell Common Stock upon exercise of such Purchase Rights.
10. DESIGNATION OF BENEFICIARY.
(a) The Company may, but is not obligated to, permit a Participant to submit a form designating a beneficiary who will receive any shares of Common Stock and/or Contributions from the Participant’s account under the Plan if the Participant dies before such shares and/or Contributions are delivered to the Participant. The Company may, but is not obligated to, permit the Participant to change such designation of beneficiary. Any such designation and/or change must be on a form approved by the Company.
(b) If a Participant dies, and in the absence of a valid beneficiary designation, the Company will deliver any shares of Common Stock and/or Contributions to the executor or administrator of the estate of the Participant. If no executor or administrator has been appointed (to the knowledge of the Company), the Company, in its sole discretion, may deliver such shares of Common Stock and/or Contributions, without interest (unless the payment of interest is otherwise required by Applicable Law), to the Participant’s spouse, dependents or relatives, or if no spouse, dependent or relative is known to the Company, then to such other person as the Company may designate.
7
11. ADJUSTMENTS UPON CHANGES IN COMMON STOCK; CORPORATE TRANSACTIONS.
(a) In the event of a Capitalization Adjustment, the Board will appropriately and proportionately adjust: (i) the class(es) and maximum number of securities subject to the Plan pursuant to Section 3(a), (ii) the class(es) and maximum number of securities by which the share reserve is to increase automatically each year pursuant to Section 3(a), (iii) the class(es) and number of securities subject to, and the purchase price applicable to outstanding Offerings and Purchase Rights, and (iv) the class(es) and number of securities that are the subject of the purchase limits under each ongoing Offering. The Board will make these adjustments, and its determination will be final, binding and conclusive.
(b) In the event of a Corporate Transaction, then: (i) any surviving corporation or acquiring corporation (or the surviving or acquiring corporation’s parent company) may assume or continue outstanding Purchase Rights or may substitute similar rights (including a right to acquire the same consideration paid to the stockholders in the Corporate Transaction) for outstanding Purchase Rights, or (ii) if any surviving or acquiring corporation (or its parent company) does not assume or continue such Purchase Rights or does not substitute similar rights for such Purchase Rights, then the Participants’ accumulated Contributions will be used to purchase shares of Common Stock (rounded down to the nearest whole share) within ten business days (or such other period specified by the Board) prior to the Corporate Transaction under the outstanding Purchase Rights, and the Purchase Rights will terminate immediately after such purchase.
12. AMENDMENT, TERMINATION OR SUSPENSION OF THE PLAN.
(a) The Board may amend the Plan at any time in any respect the Board deems necessary or advisable. However, except as provided in Section 11(a) relating to Capitalization Adjustments, stockholder approval will be required for any amendment of the Plan for which stockholder approval is required by Applicable Law.
(b) The Board may suspend or terminate the Plan at any time. No Purchase Rights may be granted under the Plan while the Plan is suspended or after it is terminated.
(c) Any benefits, privileges, entitlements and obligations under any outstanding Purchase Rights granted before an amendment, suspension or termination of the Plan will not be materially impaired by any such amendment, suspension or termination except (i) with the consent of the person to whom such Purchase Rights were granted, (ii) as necessary to facilitate compliance with any laws, listing requirements, or governmental regulations (including, without limitation, the provisions of Section 423 of the Code and the regulations and other interpretive guidance issued thereunder relating to Employee Stock Purchase Plans) including without limitation any such regulations or other guidance that may be issued or amended after the date the Plan is adopted by the Board, or (iii) as necessary to obtain or maintain favorable tax, listing, or regulatory treatment. To be clear, the Board may amend outstanding Purchase Rights without a Participant’s consent if such amendment is necessary to ensure that the Purchase Right and/or the Plan complies with the requirements of Section 423 of the Code with respect to the 423 Component or with respect to other Applicable Laws. Notwithstanding anything in the Plan or any Offering Document to the contrary, the Board will be entitled to: (i) establish the exchange ratio applicable to amounts withheld in a currency other than U.S. dollars; (ii) permit Contributions in excess of the amount designated by a Participant in order to adjust for mistakes in the Company’s processing of properly completed Contribution elections; (iii) establish reasonable waiting and adjustment periods and/or accounting and crediting procedures to ensure that amounts applied toward the purchase of Common Stock for each Participant
8
properly correspond with amounts withheld from the Participant’s Contributions; (iv) amend any outstanding Purchase Rights or clarify any ambiguities regarding the terms of any Offering to enable the Purchase Rights to qualify under and/or comply with Section 423 of the Code with respect to the 423 Component; and (v) establish other limitations or procedures as the Board determines in its sole discretion advisable that are consistent with the Plan. The actions of the Board pursuant to this paragraph will not be considered to alter or impair any Purchase Rights granted under an Offering as they are part of the initial terms of each Offering and the Purchase Rights granted under each Offering.
13. TAX QUALIFICATION; TAX WITHHOLDING.
(a) Although the Company may endeavor to (i) qualify a Purchase Right for special tax treatment under the laws of the United States or jurisdictions outside of the United States or (ii) avoid adverse tax treatment, the Company makes no representation to that effect and expressly disavows any covenant to maintain special or to avoid unfavorable tax treatment, notwithstanding anything to the contrary in this Plan. The Company will be unconstrained in its corporate activities without regard to the potential negative tax impact on Participants.
(b) Each Participant will make arrangements, satisfactory to the Company and any applicable Related Corporation or Affiliate, to enable the Company, the Related Corporation or the Affiliate to fulfill any withholding obligation for Tax-Related Items. Without limitation to the foregoing, in the Company’s sole discretion and subject to Applicable Law, such withholding obligation may be satisfied in whole or in part by (i) withholding from the Participant’s salary or any other cash payment due to the Participant from the Company, a Related Corporation or an Affiliate; (ii) withholding from the proceeds of the sale of shares of Common Stock acquired under the Plan, either through a voluntary sale or a mandatory sale arranged by the Company; or (iii) any other method deemed acceptable by the Board. The Company shall not be required to issue any shares of Common Stock under the Plan until such obligations are satisfied.
(c) The 423 Component is exempt from the application of Section 409A of the Code, and any ambiguities herein shall be interpreted to so be exempt from Section 409A of the Code. The Non-423 Component is intended to be exempt from the application of Section 409A of the Code under the short-term deferral exception and any ambiguities shall be construed and interpreted in accordance with such intent. In furtherance of the foregoing and notwithstanding any provision in the Plan to the contrary, if the Committee determines that an option granted under the Plan may be subject to Section 409A of the Code or that any provision in the Plan would cause an option under the Plan to be subject to Section 409A, the Committee may amend the terms of the Plan and/or of an outstanding option granted under the Plan, or take such other action the Committee determines is necessary or appropriate, in each case, without the Participant’s consent, to exempt any outstanding option or future option that may be granted under the Plan from or to allow any such options to comply with Section 409A of the Code, but only to the extent any such amendments or action by the Committee would not violate Section 409A of the Code. Notwithstanding the foregoing, the Company shall have no liability to a Participant or any other party if the option under the Plan that is intended to be exempt from or compliant with Section 409A of the Code is not so exempt or compliant or for any action taken by the Committee with respect thereto.
14. EFFECTIVE DATE OF PLAN.
The Plan will become effective immediately prior to and contingent upon the Effective Date. No Purchase Rights will be exercised unless and until the Plan has been approved by the stockholders of the Company, which approval must be within 12 months before or after the date the Plan is adopted (or if required under Section 12(a) above, materially amended) by the Board.
9
15. MISCELLANEOUS PROVISIONS.
(a) Proceeds from the sale of shares of Common Stock pursuant to Purchase Rights will constitute general funds of the Company.
(b) A Participant will not be deemed to be the holder of, or to have any of the rights of a holder with respect to, shares of Common Stock subject to Purchase Rights unless and until the Participant’s shares of Common Stock acquired upon exercise of Purchase Rights are recorded in the books of the Company (or its transfer agent).
(c) The Plan and Offering do not constitute an employment contract. Nothing in the Plan or in the Offering will in any way alter the at will nature of a Participant’s employment or amend a Participant’s employment or service contract, as applicable, or be deemed to create in any way whatsoever any obligation on the part of any Participant to continue in the employ or service of the Company, a Related Corporation or an Affiliate, or on the part of the Company, a Related Corporation or an Affiliate to continue the employment or service of a Participant.
(d) The provisions of the Plan will be governed by the laws of the State of Delaware without resort to that state’s conflict of laws rules.
(e) If any particular provision of the Plan is found to be invalid or otherwise unenforceable, such provision will not affect the other provisions of the Plan, but the Plan will be construed in all respects as if such invalid provision were omitted.
(f) If any provision of the Plan does not comply with Applicable Law, such provision shall be construed in such a manner as to comply with Applicable Law.
16. DEFINITIONS.
As used in the Plan, the following definitions will apply to the capitalized terms indicated below:
(a) “423 Component” means the part of the Plan, which excludes the Non-423 Component, pursuant to which Purchase Rights that satisfy the requirements for an Employee Stock Purchase Plan may be granted to Eligible Employees.
(b) “Affiliate” means any entity, other than a Related Corporation, whether now or subsequently established, which is at the time of determination, a “parent” or “subsidiary” of the Company as such terms are defined in Rule 405 promulgated under the Securities Act. The Board may determine the time or times at which “parent” or “subsidiary” status is determined within the foregoing definition.
(c) “Applicable Law” means the Code and any applicable U.S. and non-U.S. securities, exchange control, tax, federal, state, material local or municipal or other law, statute, constitution, principle of common law, resolution, ordinance, code, edict, decree, rule, listing rule, regulation, judicial decision, ruling or requirement issued, enacted, adopted, promulgated, implemented or otherwise put into effect by or under the authority of any Governmental Body (or under the authority of the New York Stock Exchange, NASDAQ Stock Market or the Financial Industry Regulatory Authority).
(d) “Board” means the Board of Directors of the Company.
(e) “Capital Stock” means each and every class of common stock of the Company, regardless of the number of votes per share.
10
(f) “Capitalization Adjustment” means any change that is made in, or other events that occur with respect to, the Common Stock subject to the Plan or subject to any Purchase Right after the date the Plan is adopted by the Board without the receipt of consideration by the Company through merger, consolidation, reorganization, recapitalization, reincorporation, stock dividend, dividend in property other than cash, large nonrecurring cash dividend, stock split, liquidating dividend, combination of shares, exchange of shares, change in corporate structure or other similar equity restructuring transaction, as that term is used in Financial Accounting Standards Board Accounting Standards Codification Topic 718 (or any successor thereto). Notwithstanding the foregoing, the conversion of any convertible securities of the Company will not be treated as a Capitalization Adjustment.
(g) “Code” means the U.S. Internal Revenue Code of 1986, as amended, including any applicable regulations and guidance thereunder.
(h) “Committee” means a committee of one or more members of the Board to whom authority has been delegated by the Board in accordance with Section 2(c).
(i) “Common Stock” means the common stock of the Company.
(j) “Company” means Ikena Oncology Inc., a Delaware corporation, and as of the Effective Date, shall mean ImageneBio, Inc., a Delaware corporation, and any successor corporation thereto
(k) “Contributions” means the payroll deductions, contributions made by Participants in case payroll deductions are impermissible or problematic under Applicable Law and other additional payments specifically provided for in the Offering that a Participant contributes to fund the exercise of a Purchase Right. A Participant may make additional payments into the Participant’s account if specifically provided for in the Offering, and then only if the Participant has not already had the maximum permitted amount withheld during the Offering through payroll deductions or other contributions and, with respect to the 423 Component, to the extent permitted by Section 423.
(l) “Corporate Transaction” means the consummation, in a single transaction or in a series of related transactions, of any one or more of the following events:
(i) a sale or other disposition of all or substantially all, as determined by the Board in its sole discretion, of the consolidated assets of the Company and its subsidiaries;
(ii) a sale or other disposition of more than 50% of the outstanding securities of the Company;
(iii) a merger, consolidation or similar transaction following which the Company is not the surviving corporation; or
(iv) a merger, consolidation or similar transaction following which the Company is the surviving corporation but the shares of Common Stock outstanding immediately preceding the merger, consolidation or similar transaction are converted or exchanged by virtue of the merger, consolidation or similar transaction into other property, whether in the form of securities, cash or otherwise.
(m) “Designated 423 Company” means any Related Corporation selected by the Board as participating in the 423 Component.
11
(n) “Designated Company” means any Designated Non-423 Company or Designated 423 Company, provided, however, that at any given time, a Related Corporation participating in the 423 Component shall not be a Related Corporation participating in the Non-423 Component.
(o) “Designated Non-423 Company” means any Related Corporation or Affiliate selected by the Board as participating in the Non-423 Component.
(p) “Director” means a member of the Board.
(q) “Effective Date” means the date of the closing of the transactions contemplated by that Agreement and Plan of Merger, dated December 23, 2024, between Ikena Oncology, Inc, Insight Merger Sub I, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands and direct wholly owned subsidiary of Ikena Oncology, Inc., Insight Merger Sub II, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands and direct wholly owned subsidiary of Ikena Oncology, Inc, and Inmagene Biopharmaceuticals, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands.
(r) “Eligible Employee” means an Employee who meets the requirements set forth in the document(s) governing the Offering for eligibility to participate in the Offering, provided that such Employee also meets the requirements for eligibility to participate set forth in the Plan.
(s) “Employee” means any person, including an Officer or Director, who is “employed” for purposes of Section 423(b)(4) of the Code by the Company or a Related Corporation or solely with respect to the Non-423 Component, an Affiliate. However, service solely as a Director, or payment of a fee for such services, will not cause a Director to be considered an “Employee” for purposes of the Plan.
(t) “Employee Stock Purchase Plan” means a plan that grants Purchase Rights intended to be options issued under an “employee stock purchase plan,” as that term is defined in Section 423(b) of the Code.
(u) “Exchange Act” means the U.S. Securities Exchange Act of 1934, as amended and the rules and regulations promulgated thereunder.
(v) “Fair Market Value” means, as of any date, the value of the Common Stock determined as follows:
(i) If the Common Stock is listed on any established stock exchange or traded on any established market, the Fair Market Value of a share of Common Stock will be, unless otherwise determined by the Board, the closing sales price for such stock as quoted on such exchange or market (or the exchange or market with the greatest volume of trading in the Common Stock) on the date of determination, as reported in such source as the Board deems reliable. Unless otherwise provided by the Board, if there is no closing sales price for the Common Stock on the date of determination, then the Fair Market Value will be the closing sales price on the last preceding date for which such quotation exists.
(ii) In the absence of such markets for the Common Stock, the Fair Market Value will be determined by the Board in good faith in compliance with Applicable Laws and regulations and, to the extent applicable as determined in the sole discretion of the Board, in a manner that complies with Sections 409A of the Code.
(iii) Notwithstanding the foregoing, for any Offering that commences on the IPO Date, the Fair Market Value of the shares of Common Stock on the Offering Date will be the price per share at which shares are first sold to the public in the Company’s initial public offering as specified in the final prospectus for that initial public offering.
12
(w) “Governmental Body” means any: (i) nation, state, commonwealth, canton, province, territory, county, municipality, district or other jurisdiction of any nature; (ii) U.S. or non-U.S. federal, state, local, municipal or other government; (iii) governmental or regulatory body, or quasi-governmental body of any nature (including any governmental division, department, administrative agency or bureau, commission, authority, instrumentality, official, ministry, fund, foundation, center, organization, unit, body or entity and any court or other tribunal, and for the avoidance of doubt, any tax authority) or other body exercising similar powers or authority; or (iv) self-regulatory organization (including the New York Stock Exchange, the NASDAQ Stock Market and the Financial Industry Regulatory Authority).
(x) “Non-423 Component” means the part of the Plan, which excludes the 423 Component, pursuant to which Purchase Rights that are not intended to satisfy the requirements for an Employee Stock Purchase Plan may be granted to Eligible Employees.
(y) “Offering” means the grant to Eligible Employees of Purchase Rights, with the exercise of those Purchase Rights automatically occurring at the end of one or more Purchase Periods. The terms and conditions of an Offering will generally be set forth in the “Offering Document” approved by the Board for that Offering.
(z) “Offering Date” means a date selected by the Board for an Offering to commence.
(aa) “Officer” means a person who is an officer of the Company or a Related Corporation within the meaning of Section 16 of the Exchange Act.
(bb) “Participant” means an Eligible Employee who holds an outstanding Purchase Right.
(cc) “Plan” means this Ikena Oncology, Inc. 2024 Employee Stock Purchase Plan, as amended from time to time, including both the 423 Component and the Non-423 Component.
(dd) “Purchase Date” means one or more dates during an Offering selected by the Board on which Purchase Rights will be exercised and on which purchases of shares of Common Stock will be carried out in accordance with such Offering.
(ee) “Purchase Period” means a period of time specified within an Offering, generally beginning on the Offering Date or on the first Trading Day following a Purchase Date, and ending on a Purchase Date. An Offering may consist of one or more Purchase Periods.
(ff) “Purchase Right” means an option to purchase shares of Common Stock granted pursuant to the Plan.
(gg) “Related Corporation” means any “parent corporation” or “subsidiary corporation” of the Company whether now or subsequently established, as those terms are defined in Sections 424(e) and (f), respectively, of the Code.
(hh) “Securities Act” means the U.S. Securities Act of 1933, as amended.
13
(ii) “Tax-Related Items” means any income tax, social insurance, payroll tax, fringe benefit tax, payment on account or other tax-related items arising out of or in relation to a Participant’s participation in the Plan, including, but not limited to, the exercise of a Purchase Right and the receipt of shares of Common Stock or the sale or other disposition of shares of Common Stock acquired under the Plan.
(jj) “Trading Day” means any day on which the exchange(s) or market(s) on which shares of Common Stock are listed, including but not limited to the New York Stock Exchange, Nasdaq Global Select Market, the Nasdaq Global Market, the Nasdaq Capital Market or any successors thereto, is open for trading.
14

Exhibit 99.1 Corporate Overview July 2025

Disclaimer This presentation contains “forward-looking statements” under the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. ImageneBio, Inc.’s (“Imagene’s”) actual results may differ from their expectations, estimates and projections and consequently, you should not rely on these forward-looking statements as predictions of future events. Words such as “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “continue,” “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. Forward-looking statements contained in this presentation include, but are not limited to, statements about: the potential of IMG-007 in AD, AA and other indications; the market and revenue opportunity for IMG-007 in AD and AA; the potential benefits of IMG-007 as compared to approved therapies or investigational drugs of competitors, including its potential differentiated profile and potential as a maintenance therapy; the design of the planned Phase 2b trial of IMG-007 in AD; and the clinical development plan for IMG-007 in AD, including the planned clinical trials and timing thereof;. These forward-looking statements involve significant risks and uncertainties that could cause the actual results to differ materially from the expected results. Most of these factors are outside Imagene’s control and are difficult to predict. Factors that may cause such differences include, but are not limited to the risk that risks associated with: the uncertainties associated with Imagene’s product candidates, as well as the risks associated with the clinical development and regulatory approval of product candidates, including potential delays in the commencement, enrollment and completion of clinical trials and potential safety and other complications; risks related to the inability of Imagene to obtain sufficient additional capital on favorable terms or at all to continue to advance these product candidates and its preclinical programs; uncertainties in obtaining successful clinical results for product candidates and unexpected costs that may result therefrom; the significant net losses Imagene has incurred since inception; the timing of the availability of data from Imagene’s clinical trials; the outcome of preclinical testing and clinical trials of Imagene’s product candidates, including the ability of those trials to satisfy relevant governmental or regulatory requirements; preclinical and clinical results may not be indicative of results that may be observed in the future; Imagene’s ability to successfully commercialize IMG-007 and any future product candidates, if approved, the rate and degree of market acceptance of IMG-007 and any future product candidates and the favorability of pricing regulations, reimbursement practices from third-party payors or healthcare reform initiatives in the United States and abroad; developments and projections relating to Imagene’s competitors, its industry or the market opportunities for IMG-007 or any future product candidates; regulatory, political, environmental and public health developments in the United States and foreign countries; the ability of Imagene to maintain and protect its intellectual property rights; Imagene’s ability to attract, hire, and retain skilled executive officers and employees; Imagene’s reliance on third parties, contract manufacturers, and contract research organizations; the possibility that Imagene may be adversely affected by other economic, business, or competitive factors; and risks associated with changes in applicable laws or regulations. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties. These and other risks and uncertainties are more fully described in the section titled “Risk Factors” in the Registration Statement on Form S-4, as amended (File No. 333-285881) (the “Registration Statement”), initially filed by Imagene with the SEC on March 18, 2025, and declared effective on June 11, 2025, and in other filings that Imagene subsequently makes and will make with the SEC. You should not place undue reliance on these forward-looking statements, which are made only as of the date hereof or as of the dates indicated in the forward-looking statements. Imagene expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in its expectations with regard thereto or any change in events, conditions or circumstances on which any such statements are based. This presentation does not purport to summarize all of the conditions, risks and other attributes of an investment in Imagene. Imagene obtained the industry, market and competitive position data used throughout this presentation from its own internal estimates and research, as well as from industry and general publications, and research, surveys and studies conducted by third parties. Internal estimates are derived from publicly available information released by industry analysts and third party sources, Imagene’s internal research and its industry experience, and are based on assumptions made by Imagene based on such data and its knowledge of the industry and market, which it believes to be reasonable. In addition, while Imagene believes the industry, market and competitive position data included in this presentation is reliable and based on reasonable assumptions, Imagene has not independently verified any third party information, and all such data involve risks and uncertainties and are subject to change based on various factors. This presentation may contain trademarks, service marks, trade names and copyrights of other companies, which are the property of their respective owners. Solely for convenience, some of the trademarks, service marks, trade names and copyrights referred to in this presentation may be listed without the TM, SM © or ® symbols, but Imagene will assert, to the fullest extent under applicable law, the rights of the applicable owners, if any, to these trademarks, service marks, trade names and copyrights. 2
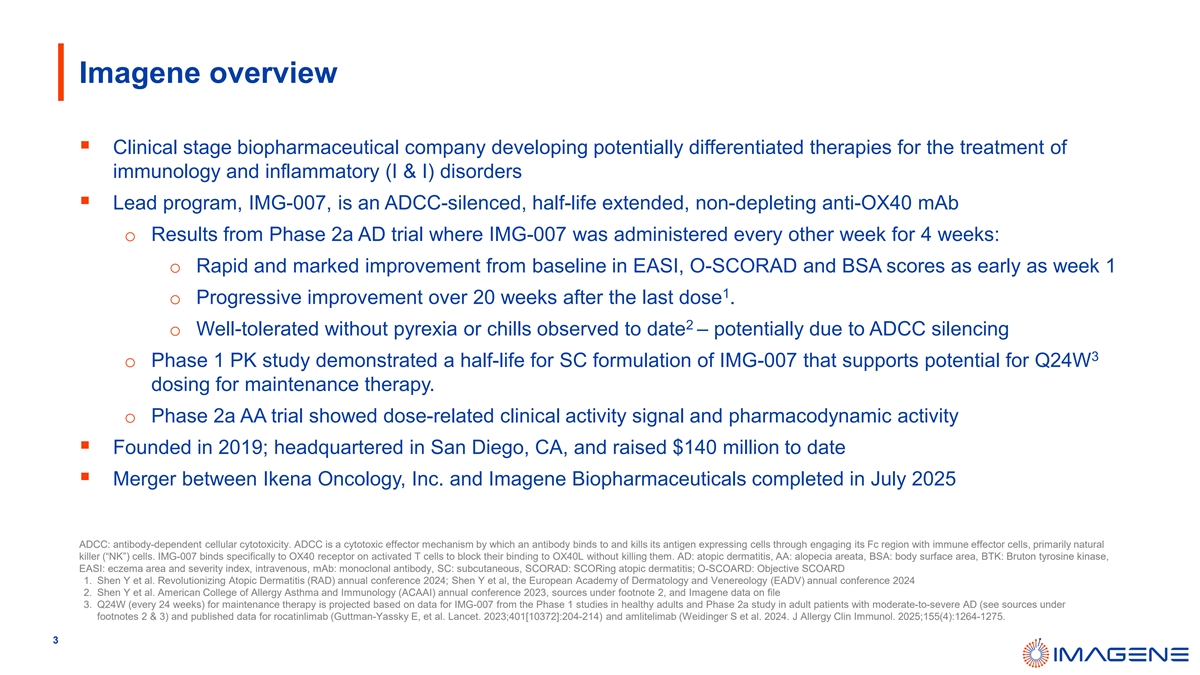
Imagene overview § Clinical stage biopharmaceutical company developing potentially differentiated therapies for the treatment of immunology and inflammatory (I & I) disorders § Lead program, IMG-007, is an ADCC-silenced, half-life extended, non-depleting anti-OX40 mAb o Results from Phase 2a AD trial where IMG-007 was administered every other week for 4 weeks: o Rapid and marked improvement from baseline in EASI, O-SCORAD and BSA scores as early as week 1 1 o Progressive improvement over 20 weeks after the last dose . 2 o Well-tolerated without pyrexia or chills observed to date – potentially due to ADCC silencing 3 o Phase 1 PK study demonstrated a half-life for SC formulation of IMG-007 that supports potential for Q24W dosing for maintenance therapy. o Phase 2a AA trial showed dose-related clinical activity signal and pharmacodynamic activity § Founded in 2019; headquartered in San Diego, CA, and raised $140 million to date § Merger between Ikena Oncology, Inc. and Imagene Biopharmaceuticals completed in July 2025 ADCC: antibody-dependent cellular cytotoxicity. ADCC is a cytotoxic effector mechanism by which an antibody binds to and kills its antigen expressing cells through engaging its Fc region with immune effector cells, primarily natural killer (“NK”) cells. IMG-007 binds specifically to OX40 receptor on activated T cells to block their binding to OX40L without killing them. AD: atopic dermatitis, AA: alopecia areata, BSA: body surface area, BTK: Bruton tyrosine kinase, EASI: eczema area and severity index, intravenous, mAb: monoclonal antibody, SC: subcutaneous, SCORAD: SCORing atopic dermatitis; O-SCOARD: Objective SCOARD 1. Shen Y et al. Revolutionizing Atopic Dermatitis (RAD) annual conference 2024; Shen Y et al, the European Academy of Dermatology and Venereology (EADV) annual conference 2024 2. Shen Y et al. American College of Allergy Asthma and Immunology (ACAAI) annual conference 2023, sources under footnote 2, and Imagene data on file 3. Q24W (every 24 weeks) for maintenance therapy is projected based on data for IMG-007 from the Phase 1 studies in healthy adults and Phase 2a study in adult patients with moderate-to-severe AD (see sources under footnotes 2 & 3) and published data for rocatinlimab (Guttman-Yassky E, et al. Lancet. 2023;401[10372]:204-214) and amlitelimab (Weidinger S et al. 2024. J Allergy Clin Immunol. 2025;155(4):1264-1275. 3

Imagene leadership team Kristin Yarema, PhD Yufang Lu, MD, PhD Jotin Marango, MD, PhD Chief Executive Officer Chief Financial Officer Chief Medical Officer 20+ years of leadership in executive 20+ years of experience in drug 20+ years of experience in management, clinical development, development and medical affairs finance, corporate development, and commercial strategy/operations and biomedical research Erin Butler Anna Vardanyan, MD, PhD VP, Finance & Administration SVP, Business and Corporate Development 20+ years of experience in finance/accounting; 10+ years in 17+ years of experience in business public biotech companies development and R&D 4

1 Market potential for IMG-007 estimated at ~ $5bn Indication(s) Eligible Population Current Status Atopic dermatitis ~3.0M Phase 2b (AD) Alopecia Areata ~0.6M Expansion Opportunity (AA) Asthma ~1.9M Expansion Opportunity IMG-007 2 Other Indications TBD Expansion Opportunity Total Opportunity ~5.5M Patients ~ $5bn Peak Sales Source: GlobalData, Sanofi R&D Day and Epidemiology Appendix (12/7/23) 1. U.S., EU5 (France, Germany, UK, Spain, Italy) and Japan 5 2. Including celiac disease, systemic sclerosis etc.

IMG-007 in Atopic Dermatitis (AD) 6

Addressing unmet need in treatment of atopic dermatitis AD presents a diverse clinical course and AD is a chronic relapsing disease that requires long- presentation due to heterogenous molecular endotypes term management Currently approved biologics target Th2 pathway (IL13, Currently approved biologics require frequent IL4), which may explain their suboptimal efficacy and 1 injections (Q2W or Q4W) 1 safety profiles Agents that require less frequent dosing, Agents modulating broader T cell pathways without especially for maintenance therapy, are desirable increased safety risks are desirable OX40/OX40L antagonists may potentially address Molecular engineering (e.g., half-life this gap by uniquely targeting diverse T cell subtypes extension) may address this gap 1. Dupixent Prescribing information, Regeneron Sanofi; ADBRY Prescribing information, LEO Pharma Inc.; EBGLYSS Prescribing information, Eli Lily and Company. 7

IMG-007: OX40 mAb with silenced ADCC and long half-life IMG-007 antibody design § IgG1 mAb targeting OX40 Fab Fab 1 § ADCC silenced to minimize safety risk 2 § Extended half-life supports the potential of 3 Q24W for maintenance therapy N297A Mutations Fc ADCC is a cytotoxic effector mechanism by which an antibody binds to and kills its antigen expressing cells through engaging its Fc region with immune effector cells, primarily natural killer (“NK”) cells. 1.Based on nonclinical and clinical evaluations, IMG-007 did not exhibit T cell depleting effect, Imagene data on file 2.IMG-007 SC half-life is approximately 34.7 days for a single SC dose of IMG-007 600 mg based on interim data from a Phase 1 study in healthy adults. 3.Q24W for maintenance therapy is projected based on data for IMG-007 from the Phase 1 studies in healthy adults (Shen Y et al. EADV annual conference 2023 and Imagene data on file) and Phase 2a study in adult patients with moderate-to-severe AD (Shen Y et al. RAD annual conference 2024 and Shen Y et al. EADV annual conference 2024) and published data for rocatinlimab (Guttman- 8 Yassky E, et al. Lancet. 2023;401[10372]:204-214) and amlitelimab (Weidinger S, et al. J Allergy Clin Immunol. 2025;155(4):1264-1275..)

Advantages of binding OX40 receptor versus OX40 ligand 1 Illustrative OX40/OX40L interaction Anti-OX40 Anti-OX40L Block OX40/L signaling in: ü Blood ü ü Tissues § An anti-OX40 mAb can engage its target in both blood and tissues vs. an anti-OX40L mAb predominantly in tissues § Targeting OX40L is limited by less efficient antibody penetration into tissues OX40L: OX40 ligand 1. Illustrative OX40/OX40L Interaction: OX40 is expressed primarily on activated T cells, including effector T cells, memory T cells and regulatory T (Tregs) cells, while sparing naïve CD4+ and CD8+ T cells, or most resting memory T cells. OX40L is expressed primarily on antigen presenting cells (APCs), including dendritic cells, macrophages, activated B cells. OX40L expression is also found on various tissue resident cells, including mast cells, endothelial cells, smooth muscle cells, and activated natural killer (NK) cells. During initial antigen recognition, professional APCs provide the OX40L signal to activated OX40-expressing T cells. The activated OX40-expressing T cells can migrate through circulation to peripheral tissues where they interact with various OX40L-expressing resident cells during the effector phase, such as B cells, NK cells, mast cells, endothelial cells, and smooth muscle cells, which results in a complex inflammatory milieu through OX40-OX40L signaling. 9

IMG-007’s potential advantages compared to anti-Th2 mAbs Target Anti-OX40 mAb Anti-Th2 (IL-4, IL- IMG-007 has the potential: pathway IMG-007 13, IL-31) mAbs § to address more diverse clinical phenotypes Th1 ü by targeting a broader range of T cell subtypes than Th2 targeting biologics Th2 üü § to provide durable pharmacodynamic effect 1 Th17 ü that supports favorable Q24W treatment regimen for maintenance therapy (vs. Q2W Th22 ü or Q4W), and could be disease modifying Memory Tü Regulatory T ü Th: T-helper, MOA: mechanism of action, Q2W: every two weeks, Q4W: every four weeks 1. Q24W for maintenance therapy is projected based on data for IMG-007 from the Phase 1 studies in healthy adults (Shen Y et al. EADV annual conference 2023 and Imagene data on file) and Phase 2a study in adult patients with moderate-to-severe AD (Shen Y et al. RAD annual conference 2024 and Shen Y et al. EADV annual conference 2024) and published data for rocatinlimab (Guttman-Yassky E, et al. Lancet. 2023;401[10372]:204-214) and amlitelimab (Weidinger S, et al. J Allergy Clin Immunol. 2025;155(4):1264-1275.) 10

IMG-007 Ph2a trial in adult patients with moderate-to-severe AD 1 1 Trial design Baseline characteristics Mean Age, years (SD): 49.8 (15.0) Sex: Female 30.8%, Male 69.2% Mean BMI (SD): 31.4 (8.7) Race: Caucasian: 46.2%, Non-Caucasian: 53.8% Mean duration of AD, years 29.6 (19.8) (SD): § Monotherapy study, topical or systemic AD Mean EASI (SD): 29.5 (13.7) medications were prohibited § 13 patients enrolled; open label Mean BSA % (SD): 52.0 (25.5) 2 § 3 IV doses of 300 mg at Week 0, 2 and 4 IGA=3 / IGA=4: 61.5% / 38.5% § Follow up to 24 weeks 1. Shen Y et al. Revolutionizing Atopic Dermatitis (RAD) annual conference 2024; Shen Y et al, the European Academy of Dermatology and Venereology (EADV) annual conference 2024. 2. The same open-label design and IV dose regimen were used in the rocatinlimab AD proof-of-concept study (H. Nakagawa et al. Journal of Dermatological Science 99 (2020) 82–89 BMI: body mass index Shen Y et al. Revolutionizing Atopic Dermatitis (RAD) annual conference 2024; Shen Y et al, the European Academy of Dermatology and Venereology (EADV) annual conference 2024, 11 EASI: eczema area and severity index, IGA: Investigator's Global Assessment SD: Standard deviation

IMG-007 was generally well-tolerated in Ph2a atopic dermatitis trial 1 Overall summary of treatment-emergent adverse events § There were no serious adverse events, Participants with at least one TEAE 9 (69.2%) no treatment-related AEs, no infusion- related reactions, no reports of pyrexia Study treatment related TEAEs 0 or chills Serious AE 0 § All AEs were of mild or moderate TEAE by CTCAE grade intensity, except for one patient who experienced a severe AE of AD flare Grade 1 (Mild) 3 (23.1%) § AEs by preferred terms that were Grade 2 (Moderate) 5 (38.5%) reported by ≥ 2 participants included: dermatitis atopic (4 of 13), hypertension Grade 3 (Severe) 1 (7.7%) (2 of 13) and urticaria (2 of 13) TEAE that are infusion-related reactions 0 § The well-tolerated profile is potentially due to silenced ADCC function TEAE of pyrexia or chills 0 TEAE leading to 4-week dosing period discontinuation 0 1. AE: adverse event; CTCAE: Common Terminology Criteria for Adverse Events; TEAE: treatment-emergent adverse event Imagene data on file. Shen Y et al. Revolutionizing Atopic Dermatitis (RAD) annual conference 2024; Shen Y et al, the European Academy of Dermatology and Venereology (EADV) annual conference 2024 1 12
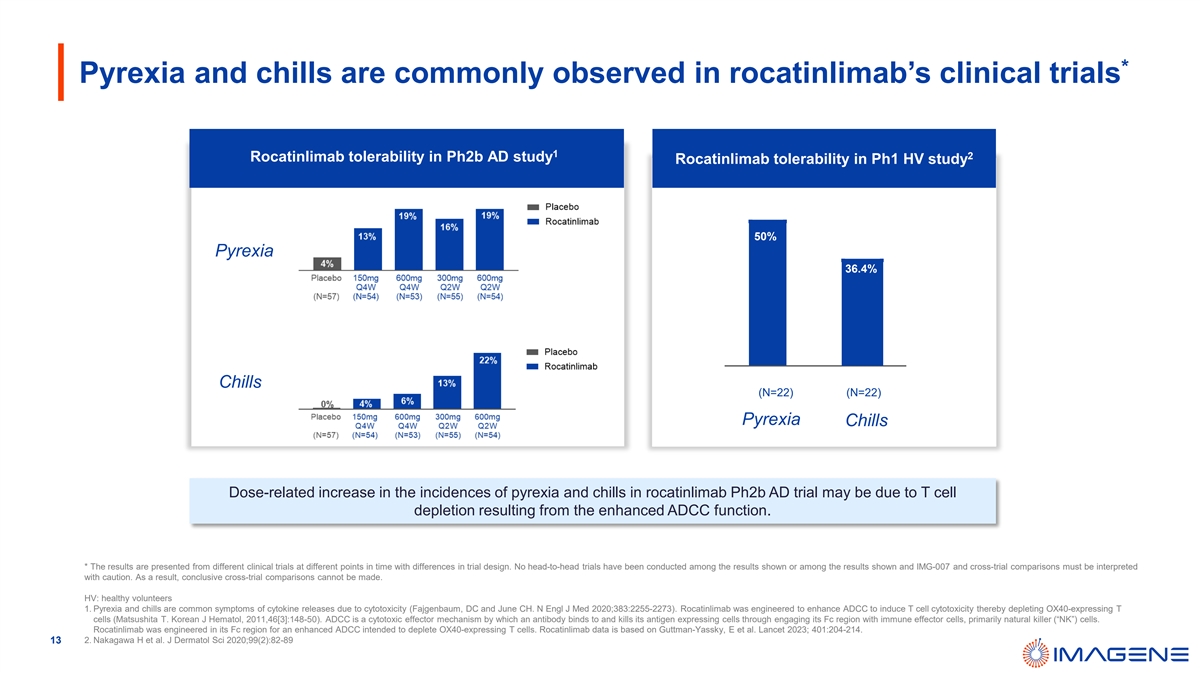
* Pyrexia and chills are commonly observed in rocatinlimab’s clinical trials 1 2 Rocatinlimab tolerability in Ph2b AD study Rocatinlimab tolerability in Ph1 HV study 50% Pyrexia 36.4% Chills (N=22) (N=22) Pyrexia Chills Dose-related increase in the incidences of pyrexia and chills in rocatinlimab Ph2b AD trial may be due to T cell depletion resulting from the enhanced ADCC function. * The results are presented from different clinical trials at different points in time with differences in trial design. No head-to-head trials have been conducted among the results shown or among the results shown and IMG-007 and cross-trial comparisons must be interpreted with caution. As a result, conclusive cross-trial comparisons cannot be made. HV: healthy volunteers 1. Pyrexia and chills are common symptoms of cytokine releases due to cytotoxicity (Fajgenbaum, DC and June CH. N Engl J Med 2020;383:2255-2273). Rocatinlimab was engineered to enhance ADCC to induce T cell cytotoxicity thereby depleting OX40-expressing T cells (Matsushita T. Korean J Hematol, 2011,46[3]:148-50). ADCC is a cytotoxic effector mechanism by which an antibody binds to and kills its antigen expressing cells through engaging its Fc region with immune effector cells, primarily natural killer (“NK”) cells. Rocatinlimab was engineered in its Fc region for an enhanced ADCC intended to deplete OX40-expressing T cells. Rocatinlimab data is based on Guttman-Yassky, E et al. Lancet 2023; 401:204-214. 13 2. Nakagawa H et al. J Dermatol Sci 2020;99(2):82-89

IMG-007 Ph2a AD study: PK sustained well above EC90 for 24 weeks 1 Drug concentration in the blood § With 3x 300 mg IV doses over 4 weeks, the mean serum drug concentration was maintained well above the EC90, concentration needed for OX40 target engagement in the blood, for 24 weeks § The robust PK profile supports a potential for differentiated SC dose regimens in future late phase studies 3 IV doses @Q2W 1 EC90 for inhibiting OX40/OX40Lsignaling 1. EC90: The 90% maximal effective concentration for the inhibition of OX40/OX40L signaling is ~1.2 ug/mL based on in vitro assays. Imagene data on file N numbers for Day 1, Day 8, wk2 pre-dose, wk2 post-dose, wk4 pre-dose, wk4 post-dose, wk 6, 8, 12, 16, 20 and 24 were 12, 12, 13, 13, 12, 10, 9, 9, 8, 6, 6, and 6, respectively. PK: pharmacokinetic 14 EC90: 90% maximal effective concentration

IMG-007 AD Ph2a: rapid onset of clinical activity; sustained for 6 months Percent (%) change from Percent (%) change from Percent (%) change from baseline in EASI score baseline in O-SCORAD score baseline in BSA score 0 1 2 4 6 8 12 16 20 24 0 1 2 4 6 8 12 16 20 24 0 1 2 4 6 8 12 16 20 24 Analysis time point (week) Analysis time point (week) Analysis time point (week) The above charts show Mean ± Standard Error N=13. Mixed-effect model with repeated measures (MMRM) was utilized for the analysis EASI: Eczema Area and Severity Index; EASI is a composite scoring system used in clinical trials to measure the extent (area) and severity of atopic eczema (dermatitis) SCORAD: SCORing Atopic Dermatitis; O-SCOARD: Objective SCOARD. SCORAD and O-SCORAD are composite scoring systems used in clinical trials to measure the extent and severity of atopic dermatitis BSA: Body Surface Area; BSA is a tool used in clinical trials to measure the extent of atopic dermatitis Source: Imagene data on file. Shen Y et al. Revolutionizing Atopic Dermatitis (RAD) annual conference 2024; Shen Y et al, the European Academy of Dermatology and Venereology (EADV) annual conference 2024 15 % change from baseline in EASI score % change from baseline in O-SCORAD score % change from baseline in BSA score

1 IMG-007’s Ph2a data and rocatinlimab’s historical POC data Percent (%) change from baseline in EASI score Percent (%) change from baseline in EASI score (Rocatinlimab’s historical POC data) (IMG-007 Ph2a data) 1.Rocatinlimab’s historical AD proof-of-concept (POC) trial: Nakagawa H et al. J Dermatol Science 2020;99(2020):82–89. Comparison is made because the overall study design, the formulation (IV) and the dose regimen (3 doses Q2W over 4 weeks) evaluated in rocatinlimab’s historical POC study and IMG-007 Phase 2a POC study are sufficiently similar. The results are presented from different clinical trials at different points in time with differences in trial design. No head-to-head trials have been conducted among the results shown and cross-trial comparisons must be interpreted with caution. As a result, conclusive cross-trial comparisons cannot be made. The above chart for rocatinlimab shows Mean ± Standard Deviation. 2.In IMG-007’s AD Ph2a study, 300 mg flat dosing was used, which is equivalent to ~4 mg/kg based on an average patient body weight of 75 kg. Imagene data on file. Shen Y et al. Revolutionizing Atopic Dermatitis (RAD) 16 annual conference 2024. The above chart for IMG-007 shows Mean ± Standard Error. % change from baseline in EASI score

IMG-007 AD Ph2a: durable activity based on EASI responder endpoints Proportion of patients achieving EASI-75 and EASI-90 W0 W1 W2 W4 W6 W8 W12 W16 W20 W24 3 IV doses 300 mg Q2W N=13; Patients who received rescue therapies were counted as “non-responders”. Last observation carried forward (LOCF) imputation was used for missing data, except for missing data that arises following study discontinuation with reason ‘lack of efficacy’ (none in the study). EASI-75: proportion of patients achieving ≥ 75% reduction from baseline in EASI EASI-90: proportion of patients achieving ≥ 90% reduction from baseline in EASI 17 Proportion of patients achieving response (%)

* Clinical activity of rocatinlimab and amlitelimab in Ph2b AD studies Key clinical activity data of amlitelimab Key clinical activity data of rocatinlimab 2 1 in Ph2b AD study in Ph2b AD study Rocatinlimab Amlitelimab Phase 2b Phase 2b (N=52) (N=77) Treatment period 18 weeks Treatment period 24 weeks SC 500 mg Dose regimens SC 300 mg Q2W Dose regimens loading+250 mg Q4W % EASI reduction from 61.1% % EASI reduction from baseline to week 16 61.5% baseline to week 16 % Patients achieving EASI- 54% % Patients achieving EASI- 75 @ week 16 40.3% 75 @ week 16 In IMG-007 Ph2a study (N=13), four-week treatment resulted in a mean 77% EASI reduction from baseline to Week 16 and 3 54% patients achieved EASI-75 response @ week 16 . * The results are presented from different clinical trials at different points in time with differences in trial design. No head-to-head trials have been conducted among the results shown or among the results shown and IMG-007 and cross-trial comparisons must be interpreted with caution. As a result, conclusive cross-trial comparisons cannot be made. 1. Rocatinlimab data is based on Guttman-Yassky, E et al. Lancet 2023; 401:204-214. 2. Amlitelimab data is based on Weidinger S et al. J Allergy Clin Immunol. 2025;155(4):1264-1275. 3. Imagene data on file. Shen Y et al. Revolutionizing Atopic Dermatitis (RAD) annual conference 2024; Shen Y et al, the European Academy of Dermatology and Venereology (EADV) annual conference 2024 18 EASI-75: Proportion of patients achieving ≥ 75% reduction from baseline in the EASI score

IMG-007 AD Ph2a: Th1/2/17 biomarkers depressed after 4-week treatment Th1 Serum Proteins (Mean ± SEM) Th2 Serum Proteins (Mean ± SEM) Th17 Serum Proteins (Mean ± SEM) * p<0.05 ** p<0.01; Two-way ANOVA with Dunnett’s multiple comparisons test; SEM: standard error of the mean N numbers at baseline, wk16, and 24 were 13, 6 and 6, respectively. Post-systemic rescue treatment results were censored from the analysis. Olink Target48 panel was used for detection of serum proteins including those related to signaling of Th1 (IL27, CXCL9, IL1B, IL18, CXCL10, IFNG, CCL3, CXCL8, CCL4, CXCL11), Th2 (CCL2, CCL8, IL4, CCL11, IL13, CCL13, CCL19,CCL7. CCL17 by ELISA) and Th17 (CXCL12, IL17F, MMP12, IL17C, IL17A, CSF2). Classification of Th1/2/17 cytokines/chemokines was primarily based on Pavel A B, Guttman-Yassky E, Allergy 2021;76(1): 314-325 th Median and 90 percentile levels of each protein in healthy subjects’ serum were derived from validation data of Olink Target48 Cytokine panel and kit information of Human CCL17/TARC Quantikine ELISA. Th: T helper 19

IMG-007 AD Ph2a: inhibition of Th1/2/17 serum markers 0 . 2 Th1 Th2 Th17 0 . 1 WK 1 4 16 24 1 4 16 24 WK 1 4 16 24 1 4 16 24 WK 1 4 16 24 1 4 16 24 0 . 0 0.0 0.0 - 0 . 1 -0.1 -0.2 - 0 . 2 -0.2 -0.4 - 0 . 3 -0.3 - 0 . 4 -0.6 -0.4 CCL17 CCL7 CXCL8 IL1B IL17F MMP12 FC: fold change, Log10 (FC vs Baseline): Log10 transform of fold change vs baseline Ns at baseline, wk 1, 4, 16, and 24 were 13, 12, 11, 6 and 6, respectively Post-systemic rescue treatment results were censored from the analysis ELISA assay for CCL17 and OLINK T48 panels were used for quantification of serum protein levels Classification of Th1/2/17 cytokines/chemokines was primarily based on Pavel A B, Guttman-Yassky E, Allergy 2021;76(1): 314-325 20 Log10 (FC vs Baseline) Mean + SEM Log10 (FC vs Baseline) Mean + SEM Log10 (FC vs Baseline) Mean + SEM

IMG-007 subcutaneous formulation has been developed § Imagene has developed a subcutaneous formulation, which will be used in Ph2b and later studies § A SC/IV PK bridging study in healthy adults has been completed § The SC formulation is at a concentration of 150 mg/mL § GMP manufacturing process has been validated § Stability data support anticipated shelf life of 2 years at 2-8°C § Could be readily developed into a commercialization format, such as pre-filled syringe or autoinjector SC: subcutaneous IV: intravenous 21 GMP: Good Manufacturing Practices
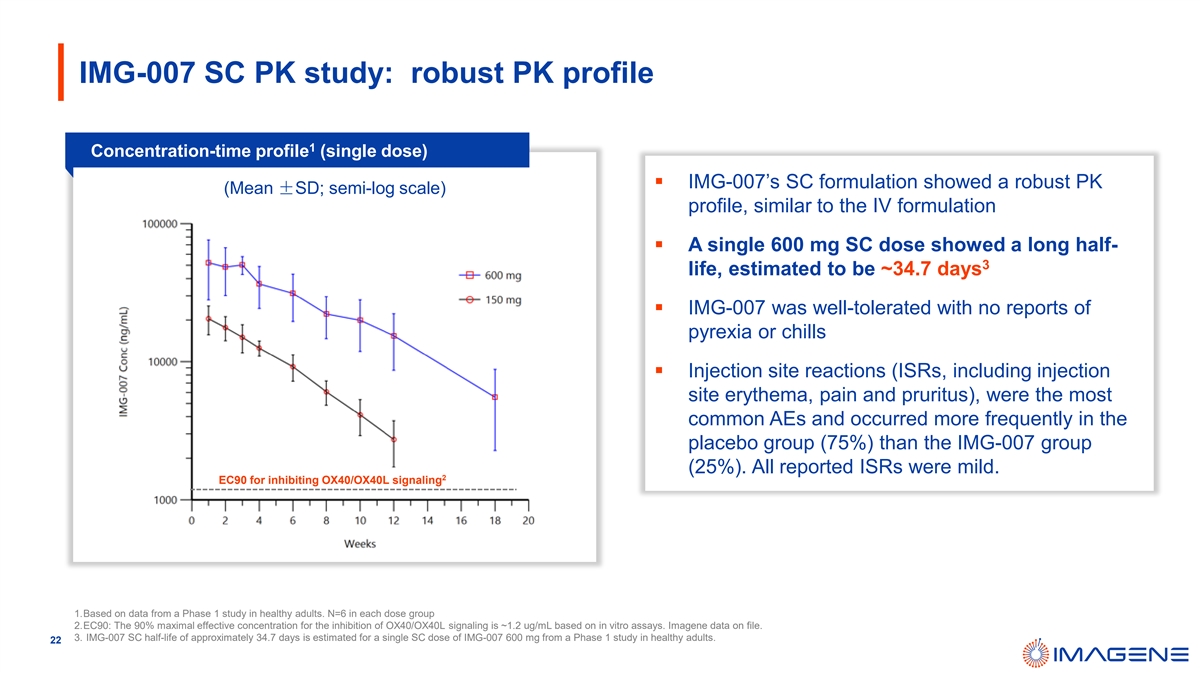
IMG-007 SC PK study: robust PK profile 1 Concentration-time profile (single dose) § IMG-007’s SC formulation showed a robust PK (Mean ±SD; semi-log scale) profile, similar to the IV formulation § A single 600 mg SC dose showed a long half- 3 life, estimated to be ~34.7 days § IMG-007 was well-tolerated with no reports of pyrexia or chills § Injection site reactions (ISRs, including injection site erythema, pain and pruritus), were the most common AEs and occurred more frequently in the placebo group (75%) than the IMG-007 group (25%). All reported ISRs were mild. 2 EC90 for inhibiting OX40/OX40L signaling 1.Based on data from a Phase 1 study in healthy adults. N=6 in each dose group 2.EC90: The 90% maximal effective concentration for the inhibition of OX40/OX40L signaling is ~1.2 ug/mL based on in vitro assays. Imagene data on file. 3. IMG-007 SC half-life of approximately 34.7 days is estimated for a single SC dose of IMG-007 600 mg from a Phase 1 study in healthy adults. 22

* Half-life data for rocatinlimab and amlitelimab Half-life data for amlitelimab Half-life data for rocatinlimab (an anti-OX40L mAb) (an anti-OX40 mAb with enhanced ADCC function) § A single IV dose of 4 mg/kg amlitelimab on Day 1 § A single SC dose of 3 mg/kg rocatinlimab followed with 2 mg/kg on Days 4 and 8 demonstrated demonstrated a mean half-life ranging from 7.4 to 12.0 a mean half-life of 20.3 days in healthy adults. days in healthy adults § Dose frequency in Ph3 maintenance studies: Q4W or § Dose frequency in Ph3 maintenance studies: Q4W or Q12W Q8W Half-life data for IMG-007 (an anti-OX40 mAb with silenced ADCC function): § A single SC dose of 600 mg IMG-007 demonstrated a mean half-life of 34.7 days in healthy adults § IMG-007 has a potential to be dosed at Q24W for long-term maintenance therapy * The results are presented from different clinical trials at different points in time with differences in trial design. No head-to-head trials have been conducted among the results shown or among the results shown and IMG-007 and cross- trial comparisons must be interpreted with caution. As a result, conclusive cross-trial comparisons cannot be made. Since SC formulation is intended for late-phase development, half-life data for the SC formulation, available for rocatinlimab, is presented. Half-life data for amlitelimab SC is not available, therefore data from the IV PK study is presented. Rocatilimab data is based on Furihata K et al. Clin Pharm Drug Dev 2021;10[8]:870-883); amlitelimab data is based on Sagari M et al 2022; Clin Pharmacol & Therapeutics 111[5]:1121-1132. IMG-007 SC half-life is approximately 34.7 days for a single SC dose of IMG-007 600 mg from a Phase 1 study in healthy adults. Q24W for maintenance therapy is projected based on data for IMG-007 from the Phase 1 studies in healthy adults (Shen Y et al. EADV annual conference 2023 and Imagene data on file) and Phase 2a study in adult patients with 23 moderate-to-severe AD (Shen Y et al. RAD annual conference 2024 and Shen Y et al. EADV annual conference 2024) and published data for rocatinlimab.

IMG-007 AD Ph2b study design Screening Period 1: Placebo-controlled Period 2: Active Treatment Follow-up Period Period Treatment IMG-007 high dose IMG-007 high dose IMG-007 medium dose IMG-007 medium dose IMG-007 low dose IMG-007 low dose IMG-007 high dose Placebo W-5 W0 W20 W52 W68 Screening Baseline § Study population: Adult patients with moderate to severe atopic dermatitis who are candidates for systemic therapy (i.e., with or without prior systemic agents such as biologics, Jak inhibitors) § A monotherapy study: Topical and systemic AD medications will be prohibited Primary analysis W: week 24 Randomization (1:1:1:1) Enter a separate long-term extension study or safety follow-up

IMG-007 clinical development plan for atopic dermatitis 2026 20232023 2024 2024 2025 2025+ 2022 2027 & + Phase 1 (IV) in Ph2a (IV) in AD Healthy Adults Interim EOS Phase 1 (SC) in 1 Ph2b (SC) Dose Ranging in AD Healthy Adults Primary PK/Safety Ph3 (SC) in AD 1 First patient dosed in the Phase 2b AD study July 1, 2025. EOS: End of study 25

IMG-007 in Alopecia Areata (AA) 26
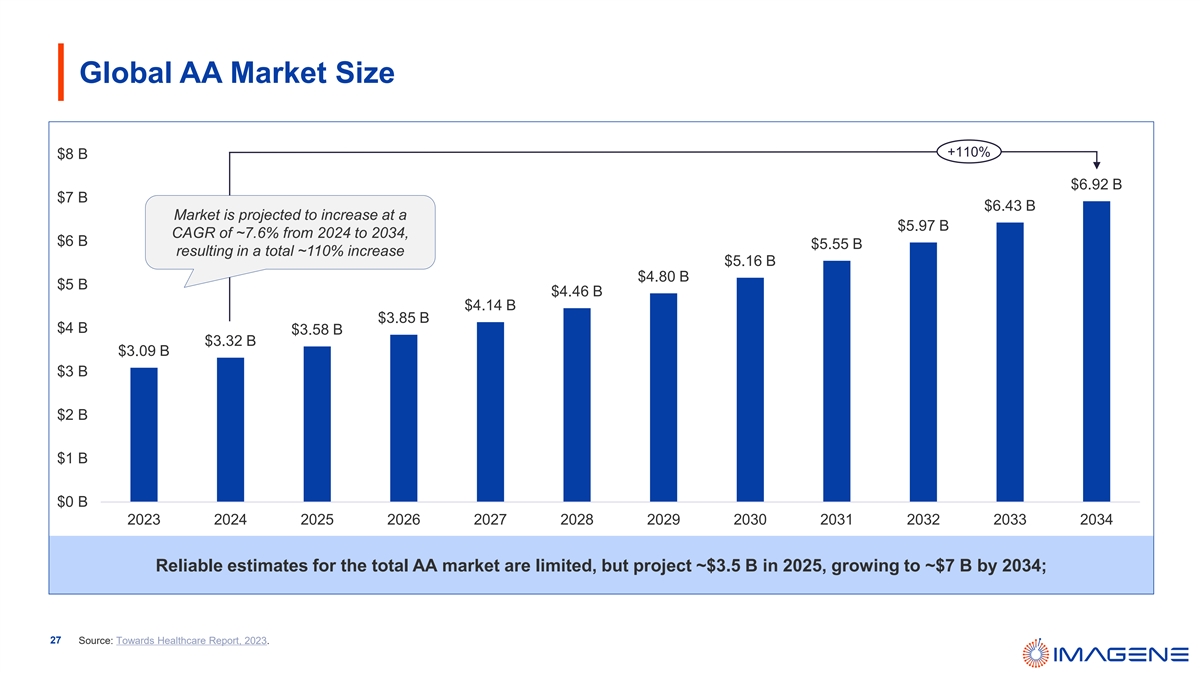
Global AA Market Size +110% $8 B $6.92 B $7 B $6.43 B Market is projected to increase at a $5.97 B CAGR of ~7.6% from 2024 to 2034, $6 B $5.55 B resulting in a total ~110% increase $5.16 B $4.80 B $5 B $4.46 B $4.14 B $3.85 B $4 B $3.58 B $3.32 B $3.09 B $3 B $2 B $1 B $0 B 2023 2024 2025 2026 2027 2028 2029 2030 2031 2032 2033 2034 Reliable estimates for the total AA market are limited, but project ~$3.5 B in 2025, growing to ~$7 B by 2034; Source: Towards Healthcare Report, 2023. 27 Source: Towards Healthcare Report, 2023.

AA is a large market with high unmet need for safe & effective drugs § The lifetime incidence risk of AA in the general 1 population was estimated to be ~2% Boxed warnings for FDA-approved JAK 4 inhibitors for AA § AA has a profound negative impact on the patient’s quality of life and wellbeing§ Serious infections § More than 80% AA patients had the first onset by § Mortality 2 age 40, and ~40% patients by age 20 § Maligancies § Approved JAK inhibitors carry boxed warnings § Major adverse cardiovascular events § No targeted biologics are available for the (MACE) treatment of AA § Thrombosis § KOLs expect systemic treatment adoption to 3 increase once safer alternatives become available 1. Mirzoyev SA et al. J Invest Dermatol. 2014;134(4):1141-1142 2. Mostaghimi A et al. JAMA Dermatol. 2023;159(4):411-418. 3. US KOL Qualitative Primary Research conducted by LifeSci, including 8 KOL interviews, 2025. 28 4. OLUMIANT Prescribing information. Eli Lilly and Co, LITFULO Prescribing information. Pfizer, LEQSELVI Prescribing information. Sun Pharmaceutical Industries.

IMG-007: Global Phase 2a AA study design IMG-007-202: a proof-of-concept study to evaluate the safe, PK and efficacy of IMG-007 in AA patients with ≥ 50% scalp hair loss Treatment Follow-up Screening (4 weeks) (up to Week 36) Cohort 1 300 mg, Q2W, 3 IV over 4 weeks (N~6) (safety run-in) W24 W36 D1 W2 W4 (EOS) Dosing When 6 participants • End of study (EOS) visit: Week 24 completed the Week 2 visit • Patients in Cohort 2 were offered an option to participate in an extended follow- 600 mg, Q2W, 3 IV over 4 weeks (N~24) Cohort 2 up period up to Week 36 • Scalp biopsies were to be taken from all participants at Baseline and Week 16 Population Adult alopecia areata patients with at least 50% scalp hair loss (SALT score ≥ 50) 1 Design Open label Key Endpoints • Safety, tolerability • % changes from baseline in SALT score over time Planned size Total ~30 patients 1. In historical AA trials, the placebo effects were generally very low (<10%), making an open-label design adequate for an AA POC study 29 SALT: Severity of Alopecia Tool

IMG-007 Ph2a AA study: key baseline AA characteristics § 29 patients were enrolled from 11 centers in the U.S. and Canada (N=6 in Cohort 1 and N=23 in Cohort 2) § Mean duration of current AA episode of 3.0 years § Mean SALT score of 80.4 § 69% of all patients had SALT scores 50 to <95, and 31% had scores of ≥ 95 § In Cohort 2, 17 patients had SALT scores 50 to <95, and 6 patients had scores of ≥ 95. § 16 patients in Cohort 2 also participated in an optional extended follow-up period up to Week 36 30

IMG-007 was overall well-tolerated in Ph2a AA study IMG-007 300 mg IMG-007 600 mg All IMG-007 (N=6) (N=23) (N=29) Main study up to 24 weeks § IMG-007 was generally well tolerated Participants with at least one TEAE 3 (50.0) 19 (82.6) 22 (75.9) § There were no SAEs Study treatment related TEAE 2 (33.3) 2 (8.7) 4 (13.8) § All TEAEs were mild or moderate in SAE 0 (0.0) 0 (0.0) 0 (0.0) severity TEAE by CTCAE grade § TEAEs which occurred in at least 2 Grade 1 (Mild) 1 (16.7) 12 (52.2) 13 (44.8) patients in any treatment group were Grade 2 (Moderate) 2 (33.3) 7 (30.4) 9 (31.0) headache (4 [13.8%]), Grade 3 (Severe) 0 (0.0) 0 (0.0) 0 (0.0) nasopharyngitis (3 [10.3%]), TEAE leading to treatment discontinuation 0 (0.0) 0 (0.0) 0 (0.0) hypertension (2 [6.9%]), and streptococcal infection (2 [6.9%]). Study extension to 36 weeks § There were no reports of pyrexia or Participants with at least one TEAE 3 (50.0) 19 (82.6) 22 (75.9) chills. Study treatment related TEAE 2 (33.3) 2 (8.7) 4 (13.8) SAE 0 (0.0) 0 (0.0) 0 (0.0) TEAE by CTCAE grade Grade 1 (Mild) 1 (16.7) 12 (52.2) 13 (44.8) Grade 2 (Moderate) 2 (33.3) 7 (30.4) 9 (31.0) Grade 3 (Severe) 0 (0.0) 0 (0.0) 0 (0.0) 31

4-week treatment resulted in a dose-related improvement in SALT score Patients in the 600 mg cohort showed greater improvement in SALT score than in the 300 mg cohort (Baseline SALT 50 - 100) Mean % change from baseline in SALT score % Patients achieving ≥ 30% improvement in SALT score (SALT30) Wk 16 Wk 24 Wk 36 -0.1% 25.0% -1.1% (6.8) (7.6) -8.6% (3.5) -14.3% 8.7% 8.7% (3.9) -21.7% (4.1) 0.0% 0.0% Wk 16 300 mg (N=6) 600 mg (N=23) 600 mg (N=16) Wk 36 Wk 24 600 mg (N=23) 300 mg (N=6) 600 mg (N=16) Least square (LS) mean percentage change from baseline in SALT is estimated from the mixed model repeated measure (MMRM). Data is presented as mean (SE). All assessments after the start date of prohibited medication were set to missing. All the collected data available after treatment discontinuation were included in the analysis. Non-responder imputation was performed for all scheduled visits following patient discontinuation from the study with the reason “lack of efficacy”. LOCF approach was used for all missing visits, except for missing data that arises following study discontinuation with reason “lack of efficacy”. 32 The number of participants in the 600 mg group was 23 at weeks 16 and 24, and 16 at week 36. LS Mean SALT percent change from baseline Proportion of patients achieving SALT30

Marked improvement seen in patients with baseline SALT score 50 to < 95 Four-week (600 mg) treatment led to deeper improvement in patients with baseline SALT score 50 - < 95 than in patients with baseline SALT 95 - 100 % Patients achieving ≥ 30% improvement in Mean % change from baseline in SALT score SALT score (SALT30) Wk 16 Wk 24 Wk 36 36.4% -0.1% -0.7% (6.8) (7.1) -6.6% (8.5) -12.3% (4.2) -20.1% (4.4) 11.8% 11.8% -30.1% 0.0% (5.4) 0.0% 0.0% Wk 36 Wk 16 Wk 24 Baseline 50-< 95 (N=17) Baseline 95-100 (N=6) Baseline 50-< 95 Baseline 95-100 Figures showing data for Cohort 2 patients who received 600 mg over 4 weeks. Least square (LS) mean percentage change from baseline in SALT is estimated from the mixed model repeated measure (MMRM). ). Data is presented as mean (SE). All assessments after the start date of prohibited medication were set to missing. All the collected data available after treatment discontinuation were included in the analysis. Non-responder imputation was performed for all scheduled visits following patient discontinuation from the study with the reason “lack of efficacy”. LOCF approach was used for all missing visits, except for missing data that arises following study discontinuation with reason “lack of efficacy”. 33 For the figure on the right showing SALT30 response, the number of participants in the 600 mg group with baseline SALT of 95-100 was 6 at weeks 16 and 24, and 5 at weeks 36. The number of participants in the 600 mg group with baseline SALT of 50 to < 95 was 17 at weeks 16 and 24, and 11 at week 36. LS Mean SALT Percent change from baseline Proportion of patients achieving SALT30

Durable suppression of inflammatory markers in the AA lesional scalp 2 Fold change in lesional vs. non-lesional scalp skin (Mean ± SEM) (three IV doses of 600 mg at Week 0, 2 and 4) Th2 CD8+ T Th1 NS ** ** 2.5 2.5 2.5 2.0 2.0 2.0 1.5 1.5 1.5 Activations of diverse (Th1 & Th2, and Levels in CD8+) T cell subtypes corresponding 1.0 1.0 1.0 in scalp tissue is Non-lesional 1 pathogenic in AA scalp skin 0.5 0.5 0.5 0.0 0.0 0.0 Baseline WK16 Baseline WK16 Baseline WK16 1. Kim M, et al. Allergy, 2024, 79(12): 3401-3414; Guttman-Yassky E, et al. JACI, 2022, 149(4): 1318-1328; Fuentes‐ Duculan J, et al. Experimental Dermatology, 2016, 25(4): 282-286. 2. Data from 4 participants who used prohibited medications have been censored (after the start of the prohibited use). * p<0.05, ** p<0.01, unpaired T-test; SEM: standard error of the mean For lesional scalp expression results (600mg), Ns at Baseline and wk16 were 23 and 17, respectively. Non-lesional scalp gene expression levels were measured in the corresponding non-lesional tissues, n=14. Bulk RNAseq was used to measure gene expression levels in Th1 (CXCL9, CXCL10, CXCL11, CXCR3, IFNG, IL12RB1, CCL3, CCL4), Th2 (IL13, CCL13, CCL26, CCL17, IL4, CCL19, CCL8, CCL2, OSM, IL13RA2) and CD8+ T cells (GZMB, GZMA, CD8A, PRF1, KLRC1, CCL5, CXCR6) Th: T helper 34

Photos showing improvement in IMG-007 Ph2a AA trial After 3 IV 600 mg doses at Week 0, 2 and 4 Week 16 Baseline Week 24 Week 36 35

Exhibit 99.2
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
Defined terms included below shall have the same meaning as terms defined and included in the Ikena’s proxy statement/prospectus filed with the U.S. Securities and Exchange Commission (the “SEC”) on June 11, 2025.
As previously announced, on December 23, 2024, Ikena Oncology, Inc., a Delaware corporation (“Ikena”), Insight Merger Sub I, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands and direct wholly owned subsidiary of Ikena (“Merger Sub I”), Insight Merger Sub II, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands and direct wholly owned subsidiary of Ikena (“Merger Sub II”), and Inmagene Biopharmaceuticals, an exempted company with limited liability incorporated and existing under the laws of the Cayman Islands (the “Inmagene”), entered into an Agreement and Plan of Merger (the “Merger Agreement”), pursuant to which, among other matters, and subject to the satisfaction or waiver of the conditions set forth in the Merger Agreement, Merger Sub I merged with and into Inmagene, pursuant to which Merger Sub I ceased to exist and was struck off the Register of Companies by the Registrar of Companies in the Cayman Islands (the “Registrar of Companies”), with Inmagene surviving (the “Surviving Entity”) the merger as a direct, wholly owned subsidiary of Ikena (the “First Merger”), and immediately after the First Merger, the Surviving Entity merged with and into Merger Sub II, pursuant to which Inmagene ceased to exist and was struck off the Register of Companies by the Registrar of Companies, with Merger Sub II surviving the merger as a direct, wholly owned subsidiary of Ikena (the “Second Merger” and, collectively with the First Merger, as appropriate, the “Merger”). In connection with the Merger, Merger Sub II changed its corporate name to “Imagene Biopharmaceuticals” and Ikena changed its name to “ImageneBio, Inc.” Ikena following the Merger is referred to herein as the “combined company.” The combined company is led by individuals mutually agreed upon by the Inmagene and Ikena (and not from either the Inmagene or Ikena in the case of the chief executive officer), and remains focused on developing IMG-007, a non-depleting anti-OX40 monoclonal antibody, for the treatment of atopic dermatitis and other potential indications.
Upon the terms and subject to the conditions set forth in the Merger Agreement, (i) any Inmagene ordinary shares and Inmagene preferred shares held as treasury shares immediately prior to effective time of the First Merger (the “First Effective Time”) were canceled and ceased to exist, and no consideration was delivered in exchange therefor and (ii) each Inmagene share outstanding immediately prior to the First Effective Time (excluding shares to be canceled pursuant to (i) and excluding Dissenting Shares) was canceled and converted solely into the right to receive such number of shares of Ikena common stock calculated by reference to the Exchange Ratio.
The Exchange Ratio was 0.003051 shares of Ikena common stock for each Inmagene share immediately prior to the First Effective Time. At the Ikena annual meeting held on July 15, 2025, Ikena stockholders approved a reverse stock split of the issued and outstanding shares of Ikena common stock at a ratio of 1:12. The reverse stock split occurred immediately prior to the Merger. Under the Exchange Ratio formula in the Merger Agreement, immediately following the closing of the First Merger and prior to the closing of the Ikena concurrent financing, on a pro forma basis and based upon the number of shares of Ikena common stock issued in the Merger, the pre-Merger Inmagene securityholders owned approximately 55.0% of the combined company’s common stock and the pre-Merger Ikena securityholders owned approximately 44.0% of the combined company’s common stock, in each case, on a fully diluted basis calculated using the treasury stock method. Under the Exchange Ratio formula in the Merger Agreement, following the closing of the Merger and the Ikena concurrent financing of $75 million, on a pro forma basis and based upon the number of shares of Ikena common stock issued in the Merger, the pre-Merger Inmagene securityholders owned approximately 43.5% of the combined company’s common stock, the pre-Merger Ikena securityholders owned approximately 35.0% of the combined company’s common stock, in each case of Inmagene and Ikena, on a fully diluted basis calculated using the treasury stock method, and the investors in the Ikena concurrent financing owned approximately 21.5% of the combined company’s common stock.
Immediately prior to the First Effective Time, Ikena and the designated rights agent entered into a CVR Agreement, pursuant to which Ikena stockholders of record as of the close of business on the last business day prior to the day on which the First Effective Time occurred received one contingent value right for each outstanding share of Ikena common stock held by such stockholder on such date.
Pursuant to the Ikena CVR Agreement, each Ikena CVR holder is entitled to certain rights to receive (i) 100% of the net proceeds, if any, received by Ikena as a result of contingent payments made to Ikena, such as milestone, royalty or earnout payments, received under any disposition agreements related to Ikena CVR Assets, entered into prior to the closing of the Merger including pursuant to any out-license agreements entered into prior to the execution of the Merger Agreement and (ii) 90% of the net proceeds, if any, received by Ikena as a result of Ikena CVR Payments received under any disposition agreements related to the Ikena CVR Assets, including but not limited to, IK-595, entered into after the closing date of the Merger and prior to the first anniversary of the closing of the Merger (the “Disposition Period”). Such proceeds are subject to certain permitted deductions, including for applicable tax payments, certain expenses incurred by Ikena or its affiliates, and losses incurred or reasonably expected to be incurred by Ikena or its affiliates due to a third-party proceeding in connection with a disposition and certain wind-down costs.
Additionally, each Ikena CVR holder is entitled to 90% of the net proceeds, if any, received by Ikena as a result of Ikena CVR Payments received under any disposition agreement related to the Ikena CVR Assets entered into during the Disposition Period. During and following the Disposition Period, the combined company has no obligation to attempt to sell or dispose of the Ikena CVR Assets.
Immediately prior to the First Effective Time, Inmagene and the designated rights agent entered into a CVR Agreement, pursuant to which Inmagene shareholders of record as of the close of business on the last business day prior to the day on which the First Effective Time occurred received one contingent value right for each outstanding Inmagene share held by such shareholder on such date.
Pursuant to the Inmagene CVR Agreement, each Inmagene CVR holder is entitled to certain rights to receive (i) 100% of the net proceeds, if any, received by Ikena as a result of contingent payments made to Ikena, such as milestone, royalty or earnout payments, received under any disposition agreements related to the programs and projects controlled by Inmagene any time prior to the closing date of the Merger (other than its anti-OX40 monoclonal antibody asset, IMG-007), as may be further developed by or on behalf of Ikena after the closing of the Merger, entered into prior to the closing of the Merger and (ii) 90% of the net proceeds, if any, received by Ikena as a result of Inmagene CVR Payments received under any disposition agreements related to the Inmagene CVR Assets entered into during the Disposition Period. Such proceeds are subject to certain permitted deductions, including for applicable tax payments, certain expenses incurred by Ikena or its affiliates, and losses incurred or reasonably expected to be incurred by Ikena or its affiliates due to a third-party proceeding in connection with a disposition.
Additionally, each Inmagene CVR holder is entitled to 90% of the net proceeds, if any, received by Ikena as a result of Inmagene CVR Payments received under any disposition agreement related to the Inmagene CVR Assets entered into during the Disposition Period. During and following the Disposition Period, the combined company has no obligation to attempt to sell or dispose of the Inmagene CVR Assets.
Any Ikena CVR Payments could materially change the combined company’s research and development activities as the legacy in-process research and development assets excluded from the Merger will not be pursued by the combined company. However, the Ikena CVR Agreement is not reflected in the following unaudited pro forma condensed combined financial statements as the value of the CVRs distributed to the respective shareholders is not expected to be significant at the time of the closing of the Merger and the potential future changes in value are unable to be determined at this time. Furthermore, an Ikena strategic option for continued development of legacy programs, if any, is not reflected in the following unaudited pro forma condensed combined financial statements as the consummation of such transactions, if any, is uncertain and not a condition of the Merger Agreement.
On July 25, 2025, immediately prior to the Merger, Inmagene consummated the divestiture of the non-IMG-007 business related assets, business and operations (the “Non-OX40 Business”) controlled by Inmagene prior to the Merger (the “Non-OX40 Divestiture”). Specifically, Inmagene sold and transferred (including via sublicense) all of the Non-OX40 Business to Miragene Inc, a newly formed private company and wholly owned subsidiary of Inmagene (“SellCo”). As part of the Non-OX40 Divestiture, Miragene Co, a newly formed private company (“BuyCo”) held by the holders of Inmagene’s outstanding shares prior to the Merger, purchased from Inmagene all of the outstanding share capital of SellCo (holding the Non-OX40 Business) in exchange for a promissory note in the amount of $8.9 million issued by BuyCo to Inmagene. Any payments made under the promissory note from BuyCo to Inmagene will be distributed to Inmagene CVR holders as Inmagene CVR Payments. The promissory note accrues interest at an annual rate of 4.61%, with interest payments due monthly in arrears, unless Miragene elects to capitalize the interest through payment-in-kind (PIK) treatment. The promissory note matures on the earlier of (i) the year 2035 or (ii) the date on which Inmagene declares the promissory note due and payable or after the occurrence of an event of default. Additionally, in the event that Miragene receives certain specified milestone or license payments, after the second anniversary of the promissory note, 50% of such proceeds must be used to prepay the outstanding balance of the promissory note.
The following unaudited pro forma condensed combined financial information gives effect to the Merger, which is accounted for as a reverse recapitalization under U.S. GAAP, the Ikena concurrent financing and the Non-OX40 Divestiture. For further details related to the accounting for the Merger and the Non-OX40 Divestiture, please see Notes 1 and 3 below. All pro forma share amounts have been adjusted to reflect Exchange Ratio of 0.003051 shares of Ikena common stock for each Inmagene share, based on a reverse stock split ratio of 1:12.
The unaudited pro forma condensed combined balance sheet combines the historical balance sheets of Ikena and Inmagene as of March 31, 2025 and depicts the accounting of the transactions prepared pursuant to Article 11 of Regulation S-X (the “pro forma balance sheet transaction accounting adjustments”). The unaudited pro forma condensed combined statement of operations for the three months ended March 31, 2025, and for the year ended December 31, 2024, for Ikena and Inmagene depict the pro forma transaction accounting adjustments assuming that those adjustments were made as of January 1, 2024 (the “pro forma statements of operations transaction accounting adjustments”).
This unaudited pro forma condensed combined financial information and related notes have been derived from and should be read in conjunction with:
| • | the historical unaudited condensed consolidated financial statements of Inmagene as of and for the three months ended March 31, 2025, and the related notes included in the proxy statement/prospectus filed with the SEC on June 11, 2025; |
| • | the historical unaudited condensed consolidated financial statements of Ikena as of and for the three months ended March 31, 2025, and the related notes included in the proxy statement/prospectus filed with the SEC on June 11, 2025; |
| • | the historical audited consolidated financial statements of Inmagene as of December 31, 2024 and for the year ended December 31, 2024, and the related notes included in the proxy statement/prospectus filed with the SEC on June 11, 2025; |
| • | the historical audited consolidated financial statements of Ikena as of and for the year ended December 31, 2024, and the related notes included in the proxy statement/prospectus filed with the SEC on June 11, 2025; |
| • | the sections titled “Ikena Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Inmagene Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and other financial information relating to Ikena and Inmagene included in the proxy statement/prospectus filed with the SEC on June 11, 2025. |
The unaudited pro forma condensed combined financial information is based on the assumptions and pro forma adjustments that are described in the accompanying notes. Adjustments have been made solely for the purpose of providing unaudited pro forma condensed combined financial information. Differences between preliminary estimates and the final accounting, completed after the closing, may occur and these differences could have a material impact on the accompanying unaudited pro forma condensed combined financial information.
The unaudited pro forma condensed combined financial information does not give effect to the potential impact of current financial conditions, regulatory matters, operating efficiencies or other savings or expenses that may be associated with the integration of the two companies. The unaudited pro forma condensed combined financial information is not necessarily indicative of the financial position or results of operations in the future periods or the result that actually would have been realized had Ikena and Inmagene been a combined organization during the specified periods. The actual results reported in periods following the Merger may differ significantly from those reflected in the unaudited condensed combined pro forma financial information presented herein for a number of reasons, including, but not limited to, differences in the assumptions used to prepare this unaudited pro forma condensed combined financial information.
UNAUDITED PRO FORMA CONDENSED COMBINED BALANCE SHEET
AS OF MARCH 31, 2025
(In thousands, except share and per share amounts)
| Historical | Historical Adjusted | |||||||||||||||||
| 5(A) Ikena Oncology, Inc. |
5(B) Inmagene Biopharmaceuticals |
Transaction Accounting Adjustments |
Notes | Pro Forma Combined |
||||||||||||||
| Assets |
||||||||||||||||||
| Current assets: |
||||||||||||||||||
| Cash and cash equivalents |
$ | 36,763 | $ | 2,001 | $ | (4,860 | ) | 5(a) | $ | 171,826 | ||||||||
| (6,498 | ) | 5(b) | ||||||||||||||||
| 77,288 | 5(f) | |||||||||||||||||
| 74,500 | 5(j) | |||||||||||||||||
| (4,118 | ) | 5(k) | ||||||||||||||||
| (3,250 | ) | 5(m) | ||||||||||||||||
| Marketable securities |
77,288 | — | (77,288 | ) | 5(f) | — | ||||||||||||
| Prepaid expenses and other current assets |
2,948 | 2,042 | (1,962 | ) | 5(c) | 3,028 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
| Total current assets |
116,999 | 4,043 | 53,812 | 174,854 | ||||||||||||||
| Non-current assets: |
||||||||||||||||||
| Property and equipment, net |
506 | — | (506 | ) | 5(d) | — | ||||||||||||
| Deferred offering costs |
— | 4,214 | (4,214 | ) | 5(k) | — | ||||||||||||
| Right-of-use asset |
3,102 | 77 | (1,965 | ) | 5(g) | 1,214 | ||||||||||||
| Promissory note receivable |
— | — | 8,900 | 5(n) | 8,900 | |||||||||||||
| Other non-current assets |
10,231 | 8 | (1,335 | ) | 5(c) | 1,286 | ||||||||||||
| (7,618 | ) | 5(h) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
| Total assets |
$ | 130,838 | $ | 8,342 | $ | 47,074 | $ | 186,254 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
| Liabilities, Redeemable Convertible Preferred Shares and Shareholders’ Deficit |
||||||||||||||||||
| Current liabilities: |
||||||||||||||||||
| Accounts payable |
$ | 805 | $ | 3,996 | $ | (184 | ) | 5(k) | $ | 4,617 | ||||||||
| Accrued expenses and other current liabilities |
4,096 | 4,555 | (3,418 | ) | 5(k) | 3,693 | ||||||||||||
| (1,540 | ) | 5(b) | ||||||||||||||||
| Term loan |
— | 7,618 | (7,618 | ) | 5(h) | — | ||||||||||||
| Lease liabilities, current |
3,892 | 77 | — | 3,969 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
| Total current liabilities |
8,793 | 16,246 | (12,760 | ) | 12,279 | |||||||||||||
| Long term liabilities: |
||||||||||||||||||
| Lease liabilities, non-current |
2,718 | — | — | 2,718 | ||||||||||||||
| Other long-term liabilities |
1,077 | — | 8,900 | 5(n) | 9,977 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
| Total liabilities |
12,588 | 16,246 | (3,860 | ) | 24,974 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
| Redeemable convertible preferred shares: |
||||||||||||||||||
| Inmagene Series Seed redeemable convertible preferred shares – $.00005 par value |
— | 955 | (955 | ) | 5(i) | — | ||||||||||||
| Inmagene Series B redeemable convertible preferred shares – $.00005 par value |
— | 29,518 | (29,518 | ) | 5(i) | — | ||||||||||||
| Inmagene Series C-1 redeemable convertible preferred shares – $.00005 par value |
— | 83,224 | (83,224 | ) | 5(i) | — | ||||||||||||
| Inmagene Series C-2 redeemable convertible preferred shares – $.00005 par value |
— | 48,393 | (48,393 | ) | 5(i) | — | ||||||||||||
| Shareholders’ equity (deficit): |
||||||||||||||||||
| Ikena preferred shares, $0.001 par value |
— | — | — | — | ||||||||||||||
| Ikena common stock, $0.001 par value |
48 | — | (44 | ) | 5(i) | 11 | ||||||||||||
| 4 | 5(i) | |||||||||||||||||
| 3 | 5(j) | |||||||||||||||||
| Inmagene Series A convertible preferred shares – $.00005 par value |
— | 18,967 | (18,967 | ) | 5(i) | — | ||||||||||||
| Inmagene ordinary shares – $.00005 par value |
— | 17 | (17 | ) | 5(i) | — | ||||||||||||
| Additional paid-in capital |
458,341 | — | 12 | 5(e) | 365,416 | |||||||||||||
| (174,667 | ) | 5(i) | ||||||||||||||||
| 44 | 5(i) | |||||||||||||||||
| 74,497 | 5(j) | |||||||||||||||||
| (4,730 | ) | 5(k) | ||||||||||||||||
| 11,919 | 5(l) | |||||||||||||||||
| Accumulated other comprehensive loss |
99 | — | (99 | ) | 5(f) | — | ||||||||||||
| Accumulated deficit |
(340,238 | ) | (188,978 | ) | (4,860 | ) | 5(a) | (204,147 | ) | |||||||||
| (4,958 | ) | 5(b) | ||||||||||||||||
| (3,297 | ) | 5(c) | ||||||||||||||||
| (506 | ) | 5(d) | ||||||||||||||||
| (12 | ) | 5(e) | ||||||||||||||||
| 99 | 5(f) | |||||||||||||||||
| (1,965 | ) | 5(g) | ||||||||||||||||
| (11,919 | ) | 5(l) | ||||||||||||||||
| 355,737 | 5(i) | |||||||||||||||||
| (3,250 | ) | 5(m) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
| Total shareholders’ equity (deficit) |
118,250 | (169,994 | ) | 213,024 | 161,280 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
| Total liabilities, redeemable convertible preferred shares and shareholders’ deficit |
$ | 130,838 | $ | 8,342 | $ | 47,074 | $ | 186,254 | ||||||||||
|
|
|
|
|
|
|
|
|
See accompanying notes to the unaudited pro forma condensed combined financial information
UNAUDITED PRO FORMA CONDENSED COMBINED STATEMENT OF OPERATIONS
FOR THE THREE MONTHS ENDED MARCH 31, 2025
(In thousands, except share and per share amounts)
| Historical | Historical Adjusted | |||||||||||||||||||
| 6(A) Ikena Oncology, Inc. |
6(B) Inmagene Biopharmaceuticals |
Transaction Accounting Adjustments |
Notes | Pro Forma Combined |
Notes | |||||||||||||||
| Revenue |
$ | — | $ | — | $ | — | $ | — | ||||||||||||
| Operating expenses: |
||||||||||||||||||||
| Research and development |
3,715 | 3,555 | (9 | ) | 6(c) | 7,261 | ||||||||||||||
| General and administrative |
5,644 | 2,739 | (78 | ) | 6(c) | 8,305 | ||||||||||||||
| Restructuring and other charges |
1,435 | — | — | 1,435 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total operating expenses |
10,794 | 6,294 | (87 | ) | 17,001 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Loss from operations |
(10,794 | ) | (6,294 | ) | 87 | (17,001 | ) | |||||||||||||
| Other income (expense): |
||||||||||||||||||||
| Investment income |
1,418 | — | 1,418 | |||||||||||||||||
| Interest expense |
— | (119 | ) | 118 | 6(i) | (1 | ) | |||||||||||||
| Other (expense) income |
785 | 35 | (118 | ) | 6(i) | 702 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total other income (expense), net |
2,203 | (84 | ) | — | 2,119 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Loss before income taxes |
(8,591 | ) | (6,378 | ) | 87 | (14,882 | ) | |||||||||||||
| Income tax expense |
(28 | ) | — | — | (28 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net loss |
$ | (8,619 | ) | $ | (6,378 | ) | $ | 87 | $ | (14,910 | ) | |||||||||
| Less: Accretion of redeemable convertible preferred shares |
— | 3,051 | (3,051 | ) | 6(h) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net loss attributable to common stockholders or ordinary shareholders |
$ | (8,619 | ) | $ | (9,429 | ) | $ | 3,138 | $ | (14,910 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Loss per share– basic and diluted: |
||||||||||||||||||||
| Common stock or Ordinary shares |
$ | (0.18 | ) | $ | (0.01 | ) | $ | (1.33 | ) | 6(j) | ||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Series A convertible preferred shares |
$ | — | $ | (0.01 | ) | $ | — | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Weighted average shares used to compute basic and diluted loss per share: |
||||||||||||||||||||
| Common stock or Ordinary shares |
48,258,111 | 462,131,592 | 11,171,910 | 6(j) | ||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Series A convertible preferred shares |
— | 326,079,495 | — | |||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
See accompanying notes to the unaudited pro forma condensed combined financial information
UNAUDITED PRO FORMA CONDENSED COMBINED STATEMENT OF OPERATIONS
FOR THE YEAR ENDED DECEMBER 31, 2024
(In thousands, except share and per share amounts)
| Historical | Historical Adjusted | |||||||||||||||||||
| 6(C) Ikena Oncology, Inc. |
6(D) Inmagene Biopharmaceuticals |
Transaction Accounting Adjustments |
Notes | Pro Forma Combined |
Notes | |||||||||||||||
| Revenue |
$ | — | $ | — | $ | — | $ | — | ||||||||||||
| Operating expenses: |
||||||||||||||||||||
| Research and development |
30,875 | 21,082 | 983 | 6(a) | 64,384 | |||||||||||||||
| 2,531 | 6(b) | |||||||||||||||||||
| (171 | ) | 6(c) | ||||||||||||||||||
| 5 | 6(e) | |||||||||||||||||||
| 9,079 | 6(f) | |||||||||||||||||||
| General and administrative |
23,679 | 8,292 | 3,877 | 6(a) | 41,107 | |||||||||||||||
| 766 | 6(b) | |||||||||||||||||||
| (319 | ) | 6(c) | ||||||||||||||||||
| 1,965 | 6(d) | |||||||||||||||||||
| 7 | 6(e) | |||||||||||||||||||
| 2,840 | 6(f) | |||||||||||||||||||
| Restructuring and other charges |
4,419 | — | — | 4,419 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total operating expenses |
58,973 | 29,374 | 21,563 | 109,910 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Loss from operations |
(58,973 | ) | (29,374 | ) | (21,563 | ) | (109,910 | ) | ||||||||||||
| Other income (expense): |
||||||||||||||||||||
| Investment income |
7,373 | — | 99 | 6(g) | 7,472 | |||||||||||||||
| Interest income |
— | 336 | 336 | |||||||||||||||||
| Other (expense) income |
2,518 | 71 | — | 2,589 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total other income (expense), net |
9,891 | 407 | 99 | 10,397 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Loss before income taxes |
(49,082 | ) | (28,967 | ) | (21,464 | ) | (99,513 | ) | ||||||||||||
| Income tax benefit (expense) |
(152 | ) | (13 | ) | — | (165 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net loss |
$ | (49,234 | ) | $ | (28,980 | ) | $ | (21,464 | ) | $ | (99,678 | ) | ||||||||
| Less: Accretion of redeemable convertible preferred shares |
— | 11,816 | (11,816 | ) | 6(h) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net loss attributable to common stockholders or ordinary shareholders |
$ | (49,234 | ) | $ | (40,796 | ) | $ | (9,648 | ) | $ | (99,678 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Loss per share– basic and diluted: |
||||||||||||||||||||
| Common stock or Ordinary shares |
$ | (1.02 | ) | $ | (0.06 | ) | $ | (8.92 | ) | 6(j) | ||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Series A convertible preferred shares |
$ | — | $ | (0.06 | ) | $ | — | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Weighted average shares used to compute basic and diluted loss per share: |
||||||||||||||||||||
| Common stock or Ordinary shares |
48,258,111 | 391,403,349 | 11,171,910 | 6(j) | ||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Series A convertible preferred shares |
— | 326,079,495 | — | |||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
See accompanying notes to the unaudited pro forma condensed combined financial information
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
| 1. | Description of the Merger and Non-OX40 Divestiture |
On December 23, 2024, Inmagene entered into the Merger Agreement with Ikena and the Merger Subs, pursuant to which, and subject to the satisfaction or waiver of the conditions set forth in the Merger Agreement, Merger Sub I merged with and into Inmagene, with Inmagene surviving as a wholly owned subsidiary of Ikena and the surviving corporation of the First Merger, and, immediately following the First Merger and as part of the same overall transaction, Inmagene merged with and into Merger Sub II, with Merger Sub II being the surviving entity of the Second Merger. The Merger was closed on July 25, 2025, following the effectiveness of the registration statement and receipt of approval by the shareholders of Inmagene and stockholders of Ikena. In connection with the Merger, Merger Sub II changed its corporate name to “Imagene Biopharmaceuticals” and Ikena changed its name to “ImageneBio, Inc.” Ikena following the Merger is referred to herein as the “combined company.”
Subject to the terms and conditions of the Merger Agreement, at the First Effective Time:
| (a) | any Inmagene shares held as treasury shares immediately prior to the First Effective Time were canceled and shall cease to exist, and no consideration was delivered in exchange therefor; |
| (b) | each Inmagene share outstanding immediately prior to the First Effective Time (excluding Inmagene shares held as treasury shares and Dissenting Shares) was automatically converted solely into the right to receive a number of validly issued, fully paid and nonassessable shares of Ikena common stock equal to the Exchange Ratio; and |
| (c) | each then-outstanding option to purchase Inmagene shares was converted into an option to purchase Ikena common stock, with the number of shares and exercise price being appropriately adjusted to reflect the Exchange Ratio between Ikena common stock and Inmagene shares, determined in accordance with the Merger Agreement. |
For purposes of calculating the Exchange Ratio, (1) shares of Ikena common stock underlying Ikena stock options outstanding as of immediately prior to the First Effective Time with an exercise price that is less than $2.3647 (subject to certain adjustments) were deemed to be outstanding, and (2) all Inmagene shares underlying outstanding Inmagene options were deemed to be outstanding.
At closing, subject to the terms and conditions of the Merger Agreement, each unexpired and unexercised Ikena option was accelerated in full. Each such Ikena option granted under the Ikena 2021 Stock Option and Incentive Plan received shares of Ikena common stock equal to the (a) product of (x) the aggregate number of shares of Ikena common stock underlying such Ikena option multiplied by (y) (i) amount by which the Ikena In-the-Money Price exceeds the exercise price on such options divided by (b) the Ikena In- the-Money Price. Each such Ikena option granted under the 2016 Stock Incentive Plan remained outstanding pursuant to its terms, unless the holder of such 2016 Ikena Option entered into an agreement with Ikena to have their 2016 Ikena Option cancelled and extinguished as of the First Effective Time in exchange for the right to receive a number of shares of Ikena common stock equal to the Option Value.
Under the Exchange Ratio formula in the Merger Agreement, following the closing of the Merger and the Ikena concurrent financing, on a pro forma basis and based upon the number of shares of Ikena common stock issued in the Merger, the pre-Merger Inmagene securityholders owned approximately 43.5% of the combined company’s common stock, the pre-Merger Ikena securityholders owned approximately 35.0% of the combined company’s common stock, in each case of Inmagene and Ikena, on a fully diluted basis, and the investors in the Ikena concurrent financing owned approximately 21.5% of the combined company’s common stock.
The following table summarizes the pro forma number of fully-diluted common stock of the combined company outstanding following the consummation of the Transactions:
| Equity Capitalization Summary (fully diluted basis) |
Pro Forma | |||||||
| Upon Consummation of the Merger and Ikena concurrent financing |
Number of Shares Owned |
% Ownership |
||||||
| Inmagene shareholders(1) |
5,086,085 | 43.51 | % | |||||
| Ikena stockholders |
4,093,621 | 35.02 | % | |||||
| Investors participating in the Ikena concurrent financing |
2,508,342 | 21.47 | % | |||||
|
|
|
|
|
|||||
| Total common stock of the combined company |
11,688,048 | 100.00 | % | |||||
| (1) | The number of shares owned by Inmagene shareholders upon consummation of the Merger includes 516,137 share options, based on 169,175,852 Inmagene ordinary shares options outstanding following the consummation of the Transactions and the Exchange Ratio of 0.0030510. |
At the First Effective Time, the combined company board consisted of seven members, three of whom were designated by Inmagene, two of whom were designated by Ikena, one of whom was mutually agreed to by Inmagene and Ikena, and one of whom was designated by the investors in the Ikena concurrent financing, led by Deep Track Master Fund.
On December 23, 2024, Ikena entered into the subscription agreements with certain accredited investors. Pursuant to the subscription agreements, and subject to the terms and conditions therein, immediately following the second effective time, Ikena consummated a private placement through the sale and issuance of shares of Ikena common stock at a purchase price per share equal to the Aggregate Valuation divided by Post-Closing Ikena Shares, for an aggregate purchase price of $75 million. The Ikena concurrent financing is exempt from registration pursuant to Section 4(a)(2) of the Securities Act, and Rule 506 of Regulation D promulgated thereunder, as a transaction by an issuer not involving a public offering.
At the closing of the Ikena concurrent financing, in connection with the subscription agreements, Ikena entered into a registration rights agreement with the investors participating in the Ikena concurrent financing providing for the registration under the Securities Act of resales of the shares of Ikena common stock sold in the Ikena concurrent financing. The consummation of the Ikena concurrent financing was conditioned upon the satisfaction or waiver of the conditions set forth in the Merger Agreement and in the subscription agreements.
Concurrent with the execution of the Merger Agreement, Ikena and Inmagene entered into the Loan Agreement, pursuant to which Ikena loaned up to $22.5 million in Term Loan Advances of at least $7.5 million with the first Term Loan Advance occurring within three days of the execution of the Loan Agreement (together with the Merger, each of the other transactions contemplated by the Merger Agreement and the Ikena concurrent financing, the “Transactions”). The Term Loan Advances bears interest, on the outstanding daily balance thereof, at a rate of 6.0% per annum, and may be prepaid at any time without premium or penalty. The Term Loan Advances is secured by all assets held or owned by Inmagene Biopharmaceuticals in respect of anti-OX40 monoclonal antibody asset, IMG-007. Upon consummation of the Merger, all Obligations (as defined in the Loan Agreement) were automatically forgiven and the Loan Agreement terminated.
On July 25, 2025, immediately prior to the Merger, Inmagene consummated the Non-OX40 Divestiture. Specifically, Inmagene sold and transferred (including via sublicense) all of the Non-OX40 Business to SellCo. As part of the Non-OX40 Divestiture BuyCo, held by the holders of Inmagene’s outstanding shares prior to the Merger, purchased from Inmagene all of the outstanding share capital of SellCo (holding the Non-OX40 Business) in exchange for a promissory note in the amount of $8.9 million issued by BuyCo to Inmagene. Any payments made under the promissory note from BuyCo to Inmagene will be distributed to Inmagene CVR holders as Inmagene CVR Payments. The promissory note accrues interest at an annual rate of 4.61%, with interest payments due monthly in arrears, unless Miragene elects to capitalize the interest through PIK treatment. The promissory note matures on the earlier of (i) the year 2035 or (ii) the date on which Inmagene declares the promissory note due and payable or after the occurrence of an event of default. Additionally, in the event that Miragene receives certain specified milestone or license payments, after the second anniversary of the promissory note, 50% of such proceeds must be used to prepay the outstanding balance of the promissory note.
In connection with the Non-OX40 Divestiture Inmagene entered into a Transition Services Agreement (the “Transition Services Agreement”) with SellCo (as defined above) for certain transitional services related to the ongoing operations of Inmagene’s business with respect to the IMG-007 program, including services related to chemistry, manufacturing and controls, regulatory affairs, clinical trial support and operations, translational science research and support, bioanalysis, pharmacovigilance, program management, accounting and finance, program management communication, administration and human resources and intellectual property support.
| 2. | Basis of Presentation |
The unaudited pro forma condensed combined financial information has been prepared in accordance with Article 11 of Regulation S-X. The adjustments presented in the unaudited pro forma condensed combined financial information have been identified and presented to provide relevant information necessary for an understanding of the combined company upon consummation of the Transactions and Non-OX40 Divestiture. The unaudited pro forma condensed combined statement of operations data for the three months ended March 31, 2025, and for the year ended December 31, 2024, gives effect to the Transactions as if they had been consummated on January 1, 2024. The unaudited pro forma condensed combined balance sheet as of March 31, 2025, gives effect to the Transactions and Non-OX40 Divestiture and combines the historical balance sheets of Ikena and Inmagene as of such date.
The unaudited pro forma condensed combined financial information is based on management’s current best estimate of the assumptions and adjustments that are described in the accompanying notes. Accordingly, the pro forma adjustments are preliminary, subject to further revision as additional information becomes available and is analyzed, and have been made solely for the purpose of providing unaudited pro forma condensed combined financial information. Differences between these preliminary accounting conclusions and estimates and the final accounting conclusions and amounts may occur as a result of, among other reasons: (i) changes in initial assumptions in the determination of the accounting acquirer and related accounting, (ii) Ikena’s net cash determined at closing, (iii) the final reverse stock split ratio, (iv) changes in initial assumptions related to the accounting for the Non-OX40 Divestiture, including the estimated fair values of the promissory note and Inmagene CVR payment liability and (v) other changes in Ikena’s assets and liabilities, which are expected to be completed after the closing, and these differences could have a material impact on the accompanying unaudited pro forma condensed combined financial information and the combined company’s future results of operations and financial position.
| 3. | Accounting for the Merger and the Non-OX40 Divestiture |
The Merger
The unaudited pro forma condensed combined financial information gives effect to the Merger, which will be accounted for under U.S. GAAP as a reverse recapitalization of Ikena by Inmagene, as the transaction is, in essence, the issuance of equity for Ikena’s net assets, which primarily consist of nominal non-operating assets and liabilities and therefore Ikena is viewed as a public shell company as of the date of the Merger. Under this method of accounting, Inmagene will be considered the accounting acquirer for financial reporting purposes. This determination is based on the fact that, immediately following the Merger:
| • | Inmagene shareholders own a majority of the voting rights in the combined company through existing ownership following the first merger; |
| • | Inmagene’s largest shareholder retains the largest interest in the combined company; |
| • | Inmagene designated three of the seven members of the combined company board; |
| • | Certain members of Inmagene’s executive management team become the management of the combined company; and |
| • | The combined company will be renamed “ImageneBio, Inc.” |
As a result of Inmagene being the accounting acquirer, Inmagene’s assets and liabilities were recorded at their pre-combination carrying amounts. Ikena’s assets and liabilities were measured and recognized at their fair values as of the effective time of the Merger, which are expected to approximate the carrying value of the acquired non-operating assets and liabilities, with no goodwill or other intangible assets recorded. Any difference between the consideration transferred and the fair value of the net assets of Ikena following the determination of the actual consideration transferred for Ikena were reflected as an adjustment to additional paid-in capital. For periods prior to the closing, the historical financial statements of Inmagene shall become the historical financial statements of the combined company.
The Non-OX40 Divestiture
The unaudited pro forma financial information gives effect to the Non-OX40 Divestiture. The Non-OX40 Divestiture represents a transaction between entities with a high degree of common ownership, and therefore, will be accounted for like a common control transaction. Accordingly, the assets and liabilities transferred were derecognized at their historical carrying values, and the estimated fair value of promissory note receivable were recognized, with any difference recorded in equity. No gain or loss was recognized in the unaudited pro forma condensed combined statement of operations in connection with this transaction. In addition, the initial estimated fair value of the corresponding Inmagene CVR liability was recorded as a dividend to the Inmagene shareholders within equity, with the related payable recorded within other liabilities.
| 4. | Shares of Ikena common stock, Preferred Shares, and Options Issued to Inmagene Shareholders upon the Closing of the Merger |
At closing, all outstanding Inmagene shares were exchanged for shares of Ikena common stock based on the Exchange Ratio of 0.0030510 shares of Ikena common stock for each Inmagene share, determined in accordance with the terms of the Merger Agreement, which reflects, among other things, Ikena net cash of $100 million and a reverse stock split ratio of 1:12. The number of shares of Ikena common stock that Ikena issued to Inmagene’s shareholders was determined as follows:
| Shares of Inmagene ordinary shares outstanding |
462,684,023 | |||
| Shares of Inmagene preferred shares outstanding |
1,035,177,195 | |||
|
|
|
|||
| Total Inmagene shares outstanding (1) |
1,497,861,218 | |||
|
|
|
|||
| The Exchange Ratio |
0.0030510 | |||
| Shares of Ikena common stock issued to Inmagene shareholders |
4,569,948 |
| (1) | The number of shares is based on a total of 1,497,861,218 shares of Inmagene outstanding at the Merger close. |
| 5. | Adjustments to Unaudited Pro Forma Condensed Combined Balance Sheet as of March 31, 2025 |
The pro forma notes and adjustments, based on preliminary estimates that could change materially as additional information is obtained, are as follows:
Pro forma notes:
| 5(A) | Derived from the unaudited condensed consolidated balance sheet of Ikena as of March 31, 2025. |
| 5(B) | Derived from the unaudited condensed consolidated balance sheet of Inmagene as of March 31, 2025, adjusted for the Non-OX40 Divestiture as follows: |
| Historical Inmagene Biopharmaceuticals as of March 31, 2025 |
Adjustment for Non-OX40 Divestiture (1) |
Adjusted Inmagene Biopharmaceuticals as of March 31, 2025 |
||||||||||
| Assets |
||||||||||||
| Current assets: |
||||||||||||
| Cash and cash equivalents |
$ | 4,918 | $ | (2,917 | ) | $ | 2,001 | |||||
| Prepaid expenses and other current assets |
2,144 | (102 | ) | 2,042 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total current assets |
7,062 | (3,019 | ) | 4,043 | ||||||||
| Non-current assets: |
||||||||||||
| Deferred offering costs |
4,214 | — | 4,214 | |||||||||
| Right-of-use asset |
471 | (394 | ) | 77 | ||||||||
| Other non-current assets |
1,016 | (1,008 | ) | 8 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total assets |
$ | 12,763 | (4,421 | ) | $ | 8,342 | ||||||
|
|
|
|
|
|
|
|||||||
| Liabilities, Redeemable Convertible Preferred Shares and Shareholders’ Deficit |
||||||||||||
| Current liabilities: |
||||||||||||
| Accounts payable |
$ | 6,912 | $ | (2,916 | ) | $ | 3,996 | |||||
| Accrued expenses and other current liabilities |
5,279 | (724 | ) | 4,555 | ||||||||
| Term loan |
7,618 | — | 7,618 | |||||||||
| Lease liabilities, current |
282 | (205 | ) | 77 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total current liabilities |
20,091 | (3,845 | ) | 16,246 | ||||||||
| Long term liabilities: |
||||||||||||
| Lease liabilities, non-current |
182 | (182 | ) | — | ||||||||
| Other long-term liabilities |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities |
20,273 | (4,027 | ) | 16,246 | ||||||||
|
|
|
|
|
|
|
|||||||
| Redeemable convertible preferred shares: |
||||||||||||
| Inmagene Series Seed redeemable convertible preferred shares - $.00005 par value |
955 | — | 955 | |||||||||
| Inmagene Series B redeemable convertible preferred shares - $.00005 par value |
29,518 | — | 29,518 | |||||||||
| Inmagene Series C-1 redeemable convertible preferred shares - $.00005 par value |
83,224 | — | 83,224 | |||||||||
| Inmagene Series C-2 redeemable convertible preferred shares - $.00005 par value |
48,393 | — | 48,393 | |||||||||
| Shareholders’ equity (deficit): |
||||||||||||
| Inmagene Series A convertible preferred shares - $.00005 par value |
18,967 | — | 18,967 | |||||||||
| Inmagene ordinary shares - $.00005 par value |
17 | — | 17 | |||||||||
| Accumulated other comprehensive loss |
(1,800 | ) | 1,800 | — | ||||||||
| Accumulated deficit |
(186,784 | ) | (2,194 | ) | (188,978 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total shareholders’ equity (deficit) |
(169,600 | ) | (394 | ) | (169,994 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities, redeemable convertible |
$ | 12,763 | (4,421 | ) | $ | 8,342 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | The balances of the entities subject to the Non-OX40 Divestiture were derived from the historical condensed consolidated balance sheet of Inmagene as of March 31, 2025, in accordance with the contractual terms of the divestiture and the legal structure of the companies involved. |
Pro forma Balance Sheet Transaction Accounting Adjustments:
| 5(a) | To reflect preliminary estimated incremental compensation expense of $4.9 million related to severance payments and retention bonuses resulting from pre-existing employment agreements or from approval from the Ikena board that is expected to be incurred upon the closing and is a one-time expense directly attributed to the Merger. There were no severance payments owed and unpaid by Ikena at March 31, 2025. The pro forma adjustment is reflected as a decrease in cash of $4.9 million for the severance and retention-bonus payments made subsequent to March 31, 2025, and an increase to accumulated deficit of $4.9 million. |
| 5(b) | To reflect preliminary estimated transaction costs of $5.0 million not yet reflected in the historical financial statements, expected to be incurred on a one-time basis by Ikena in connection with the Merger, such as advisory, legal and auditor fees, and a Directors and Officers (“D&O”) tail policy as a decrease in cash of $6.5 million upon settlement, a decrease in accrued expenses of $1.5 million and a corresponding increase in accumulated deficit. |
| 5(c) | To reflect the one-time derecognition of $3.3 million of Ikena’s prepaid expenses and other assets primarily related to prepaid research and development expenses of discontinued research and development activities and prepaid insurance related to Ikena’s current D&O insurance policy that was fully utilized at the closing. The adjustment consists of $2.0 million included in prepaid expenses and other current assets and $1.3 million included in other non-current assets. |
| 5(d) | To reflect the one-time derecognition of property and equipment of $0.5 million that was acquired for Ikena’s operations prior to the Merger and is expected to have no value to the combined company. |
| 5(e) | To reflect Ikena’s one-time stock-based compensation expense of $12 thousand in general and administrative expense related to the excess fair value of Ikena options over the fair value of the original award for Ikena in-the-money options that were converted to 72,112 shares of Ikena common stock immediately prior to the Merger pursuant to the terms of the Merger Agreement, resulting in an increase in Ikena common stock at par value and a corresponding increase in additional paid-in capital of $12 thousand in the unaudited pro forma condensed combined balance sheet. |
| 5(f) | To reflect the liquidation of $77.3 million of Ikena’s marketable securities into cash prior to the Merger. The $0.1 million in unrealized gains on marketable securities previously recorded in accumulated other comprehensive income, has been reclassified as realized gains in accumulated deficit due to the release of investments as well as an adjustment to investment income in the unaudited pro forma condensed combined statement of operations, refer to 6(g). |
| 5(g) | To reflect the anticipated impairment of Ikena’s right-of -use asset related to an office lease for $2.0 million to be incurred upon the closing and is a one-time expense directly attributed to the Merger as Inmagene expects to abandon this lease. |
| 5(h) | To reflect on a one- time basis, pursuant to the Loan Agreement, the forgiveness of the $7.5 million Term Loan Advance from Ikena to Inmagene and associated accrued interest of $0.1 million, which is automatically forgiven upon the closing of the Merger, as a decrease in other non-current assets and a corresponding decrease in term loan. See Note 1. |
| 5(i) | To reflect the one-time recapitalization of Inmagene, pursuant to the Merger Agreement, through the contribution of 462,684,023 Inmagene ordinary shares and 1,035,177,195 Inmagene convertible preferred shares, in exchange for the issuance of 4,569,948 shares of Ikena common stock, reflecting the Exchange Ratio of 0.0030510, reserving 516,137 shares of Ikena common stock for Inmagene options outstanding following the consummation of the Transactions, and to reflect the derecognition of the accumulated deficit of Ikena, with a net reduction of $174.7 million reflected in additional paid-in capital. |
The derecognition of accumulated deficit of Ikena of $355.7 million is determined as follows (in thousands):
| Accumulated Deficit of Ikena as of March 31, 2025 |
$ | (340,238 | ) | |
| Compensation expense related to Ikena severance and bonus retention payments, see Note 5(a) |
(4,860 | ) | ||
| Preliminary estimated transaction costs of Ikena, see Note 5(b) |
(4,958 | ) | ||
| Derecognition of Ikena prepaid expenses and prepaid insurance, see Note 5(c) |
(3,297 | ) | ||
| Derecognition of Ikena property plant and equipment, see Note 5(d) |
(506 | ) | ||
| One-time stock-based compensation expense related to in-the-money Ikena options, see Note 5(e) |
(12 | ) | ||
| Recognition of previously unrealized gains upon liquidation of Ikena’s marketable securities, see Note 5(f) |
99 | |||
| Ikena office impairment, see Note 5(g) |
(1,965 | ) | ||
|
|
|
|||
| Total adjustment to derecognize the accumulated deficit of Ikena |
$ | (355,737 | ) | |
|
|
|
| 5(j) | To reflect the issuance of 2,508,342 shares of Ikena common stock pursuant to the Ikena concurrent financing entered into concurrently with the execution of the Merger Agreement, for aggregate proceeds of $75.0 million. The net proceeds of $74.5 million are net of one-time estimated transaction costs deemed to be direct and incremental costs of the Ikena concurrent financing in the amount of approximately $0.5 million. The issuance of shares in connection with the Ikena concurrent financing is recorded as the issuance of Ikena common stock at par value, with the remaining amount recorded to additional paid-in capital. |
| 5(k) | To reflect one-time preliminary estimated transaction costs of $4.7 million that are expected to be incurred by Inmagene in connection with the Merger, such as advisory, legal and auditor fees, as a decrease in cash and a corresponding reduction to additional paid-in capital. $0.6 million of these costs have been paid and are included in deferred offering costs in Inmagene’s historical balance sheet as of March 31, 2025. $3.6 million of these costs have been incurred but not paid and are included in deferred offering costs, accounts payable and accrued expenses and other current liabilities. Upon closing, such costs will be derecognized from deferred offering costs, and $4.1 million will be paid in cash. As the Merger will be accounted for as a reverse recapitalization equivalent to the issuance of equity for the net assets, primarily cash, of Ikena, these direct and incremental costs are treated as a reduction of the net proceeds received within additional paid-in capital. |
| 5(l) | To reflect Inmagene’s one-time share-based compensation expense of $11.9 million related to pre-existing grant agreements which limit the exercisability of awards until certain corporate transactions occur, which will be triggered by the Merger. Inmagene’s 2019 Plan allows for, at the determination of the Chief Executive Officer, accelerated vesting of unvested awards immediately prior to the effective date of the Merger. These pro forma financial statements do not include any adjustment for the potential impact of accelerated vesting of such awards. The unrecognized share-based compensation expense for unvested awards was $3.4 million as of March 31, 2025. |
| 5(m) | To reflect the payments to Miragene of $1.3 million in service fees, and related expense, under Inmagene’s Transition Services Agreement for the first year of services, and an approximate $2.0 million payment for pre-clinical activities associated with Non-OX40 assets which were contracted subsequent to March 31, 2025, assuming the Non-OX40 Divestiture occurred as of January 1, 2024. |
| 5(n) | To reflect the estimated fair value of the promissory note receivable from Buy Co in the amount of $8.9 million and the corresponding estimated fair value of the CVR liability of $8.9 million. The fair values of both the promissory note receivable and the CVR liability represent management’s best estimates as of the Merger closing date. |
6. Adjustments to Unaudited Pro Forma Condensed Combined Statement of Operations
The pro forma notes and adjustments, based on preliminary estimates that could change materially as additional information is obtained, are as follows:
Pro forma notes:
| 6(A) | Derived from the unaudited condensed consolidated statement of operations of Ikena for the three months ended March 31, 2025. |
| 6(B) | Derived from the unaudited condensed consolidated statement of operations of Inmagene for the three months ended March 31, 2025 adjusted for Non-OX40 Divestiture as follows: |
| Historical | Adjusted Historical | |||||||||||
| Inmagene Biopharmaceuticals for the three months ended March 31, 2025 |
Adjustment for Non-OX40 Divestiture (2) |
Inmagene Biopharmaceuticals for the three months ended March 31, 2025 |
||||||||||
| Revenue |
$ | 800 | $ | (800 | ) | $ | — | |||||
| Operating expenses: |
||||||||||||
| Research and development |
4,040 | (485 | ) | 3,555 | ||||||||
| General and administrative |
2,755 | (16 | ) | 2,739 | ||||||||
| Restructuring and other charges |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
6,795 | (501 | ) | 6,294 | ||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(5,995 | ) | (299 | ) | (6,294 | ) | ||||||
| Other income (expense): |
||||||||||||
| Investment income |
— | — | — | |||||||||
| Interest expense |
(118 | ) | (1 | ) | (119 | ) | ||||||
| Other (expense) income |
36 | (1 | ) | 35 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total other income (expense), net |
(82 | ) | (2 | ) | (84 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Loss before income taxes |
(6,077 | ) | (301 | ) | (6,378 | ) | ||||||
| Income tax expense |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
(6,077 | ) | (301 | ) | (6,378 | ) | ||||||
| Less: Accretion of redeemable convertible preferred shares |
3,051 | — | 3,051 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss attributable to common stockholders |
$ | (9,128 | ) | $ | (301 | ) | $ | (9,429 | ) | |||
|
|
|
|
|
|
|
|||||||
| Loss per share – basic and diluted: |
||||||||||||
| Common stock or Ordinary shares |
$ | (0.01 | ) | $ | (0.01 | ) | ||||||
|
|
|
|
|
|||||||||
| Series A convertible preferred shares |
$ | (0.01 | ) | $ | (0.01 | ) | ||||||
|
|
|
|
|
|||||||||
| Weighted average shares used to compute basic and diluted loss per share: |
||||||||||||
| Common stock or Ordinary shares |
462,131,592 | 462,131,592 | ||||||||||
|
|
|
|
|
|||||||||
| Series A convertible preferred shares |
326,079,495 | 326,079,495 | ||||||||||
|
|
|
|
|
|||||||||
| (2) | The amounts related to the entities subject to the Non-OX40 Divestiture were derived from the historical condensed consolidated statement of operations of Inmagene for the three months ended March 31, 2025, in accordance with the contractual terms of the divestiture and the legal structure of the companies involved. Where possible, income and expenses were specifically identified and directly attributed to the products and operations transferred as part of the Non-OX40 Divestiture. Indirect expenses that could not be specifically identified were not allocated. |
| 6(C) | Derived from the audited consolidated statement of operations of Ikena for the year ended December 31, 2024. |
| 6(D) | Derived from the audited consolidated statement of operations of Inmagene for the year ended December 31, 2024 adjusted for Non-OX40 Divestiture as follows: |
| Historical | Adjusted Historical | |||||||||||
| Inmagene Biopharmaceuticals for the year ended December 31, 2024 |
Adjustment for Non-OX40 Divestiture (3) |
Inmagene Biopharmaceuticals for the year ended December 31, 2024 |
||||||||||
| Revenue |
$ | 3,500 | $ | (3,500 | ) | $ | — | |||||
| Operating expenses: |
||||||||||||
| Research and development |
32,109 | (11,027 | ) | 21,082 | ||||||||
| General and administrative |
8,391 | (99 | ) | 8,292 | ||||||||
| Restructuring and other charges |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
40,500 | (11,126 | ) | 29,374 | ||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(37,000 | ) | 7,626 | (29,374 | ) | |||||||
| Other income (expense): |
||||||||||||
| Interest income |
374 | (38 | ) | 336 | ||||||||
| Other (expense) income |
71 | — | 71 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total other income, net |
445 | (38 | ) | 407 | ||||||||
|
|
|
|
|
|
|
|||||||
| Loss before income taxes |
(36,555 | ) | 7,588 | (28,967 | ) | |||||||
| Income tax expense |
(13 | ) | — | (13 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
(36,568 | ) | 7,588 | (28,980 | ) | |||||||
| Less: Accretion of redeemable convertible preferred shares |
11,816 | — | 11,816 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss attributable to common stockholders or ordinary shareholders |
$ | (48,384 | ) | $ | 7,588 | $ | (40,796 | ) | ||||
|
|
|
|
|
|
|
|||||||
| Loss per share ordinary share– basic and diluted: |
||||||||||||
| Common stock or Ordinary shares |
$ | (0.07 | ) | $ | (0.06 | ) | ||||||
|
|
|
|
|
|||||||||
| Series A convertible preferred shares |
$ | (0.07 | ) | $ | (0.06 | ) | ||||||
|
|
|
|
|
|||||||||
| Weighted average shares used to compute basic and diluted loss per share: |
||||||||||||
| Common stock or Ordinary shares |
391,403,349 | 391,403,349 | ||||||||||
|
|
|
|
|
|||||||||
| Series A convertible preferred shares |
326,079,495 | 326,079,495 | ||||||||||
|
|
|
|
|
|||||||||
| (3) | The amounts related to the entities subject to the Non-OX40 Divestiture were derived from the historical condensed consolidated statement of operations of Inmagene for the year ended December 31, 2024, in accordance with the contractual terms of the divesture and the legal structure of the companies involved. Where possible, income and expenses were specifically identified and directly attributed to the products and operations transferred as part of the Non-OX40 Divestiture. Indirect expenses that could not be specifically identified were not allocated. |
Inmagene and Ikena recorded minimal provision or benefit for income taxes during the three months ended March 31, 2025, and during the year ended December 31, 2024, because each company expects to incur a pre-tax loss in 2024 and 2025, and each company maintains a full valuation allowance on its deferred tax assets. Accordingly, no pro forma adjustments have an impact on associated income tax.
Pro forma Statements of Operations Transaction Accounting Adjustments:
| 6(a) | To reflect one-time preliminary estimated incremental compensation expense of $4.9 million related to severance payments and retention bonus that is expected to be incurred upon the closing, assuming that the adjustment described in Note 5(a) was made on January 1, 2024. |
| 6(b) | To reflect the one-time derecognition of $3.3 million of Ikena’s prepaid research and development expenses related to discontinued research and development activities, and prepaid insurance related to Ikena’s current D&O insurance policy that will be fully utilized at the closing, assuming that the adjustment described in Note 5(c) was made on January 1, 2024. |
| 6(c) | To reflect the one-time derecognition of Ikena’s depreciation expense, related to assets that will be fully depreciated prior to the closing and expected to have no value, assuming that the adjustment described in Note 5(d) was made on January 1, 2024. |
| 6(d) | To reflect the one-time anticipated impairment of Ikena’s right-of-use asset for $2.0 million related to an office lease assuming that the adjustment described in Note 5(g) was made on January 1, 2024. |
| 6(e) | To reflect Ikena’s one-time stock-based compensation expense of $12 thousand, reflected in research and development expense and general and administrative expense related to the excess fair value of stock options over the fair value of the original award for Ikena’s in-the-money options, assuming that the adjustment described in Note 5(e) was made on January 1, 2024. |
| 6(f) | To reflect Inmagene’s one-time share-based compensation expense of $11.9 million related to pre-existing grant agreements which limit the exercisability of awards until certain corporate transactions occur, which will be triggered by the Merger, assuming that the adjustment described in Note 5(l) was made on January 1, 2024. Inmagene’s 2019 Plan allows for, at the determination of the Chief Executive Officer, accelerated vesting of unvested awards immediately prior to the effective date of the Merger. These pro forma financial statements do not include any adjustment for the potential impact of accelerated vesting of such awards. The unrecognized share-based compensation expense for unvested awards was $3.4 million as of March 31, 2025. |
| 6(g) | To reflect $0.1 million in one-time unrealized gains on marketable securities previously recorded in accumulated other comprehensive income, due to the release of investments expected to occur upon consummation of the Merger, assuming that the adjustment described in Note 5(f) was made on January 1, 2024. |
| 6(h) | To reflect the one -time derecognition of the accretion of Inmagene’s redeemable convertible preferred shares, assuming that the adjustment described in Note 5(i) was made on January 1, 2024. |
| 6(i) | To reflect the one-time derecognition, pursuant to the Loan Agreement, the forgiveness of the interest income and interest expense related to the term loan between Ikena and Inmagene which is automatically forgiven upon the closing of the Merger, assuming the adjustment described in Note 5(h) was made on January 1, 2024. |
| 6(j) | The pro forma combined basic and diluted net loss per share has been adjusted to reflect the pro forma net loss for the year ended December 31, 2024. In addition, the number of shares used in calculating the pro forma combined basic and diluted net loss per share has been adjusted to reflect the estimated total number of shares of common stock or ordinary shares of the combined company for the periods presented, which assumes a reverse stock split ratio of 1:12. For periods in which Ikena, Inmagene, or the combined company reported a net loss, diluted loss per share is the same as basic loss per share, since dilutive common stock or ordinary shares are not assumed to have been issued as their effect would be anti-dilutive. |
The pro forma weighted average shares have been calculated as follows for the periods ended:
| March 31, 2025 | December 31, 2024 | |||||||
| Basic and Diluted |
Basic and Diluted |
|||||||
| Net loss attributable to ordinary shareholders |
$ | (14,910 | ) | $ | (99,678 | ) | ||
| Historical weighted average number of Ikena common stock outstanding, after giving effect to the approved one-for-twelve reverse stock split of Ikena common stock |
4,021,508 | 4,021,508 | ||||||
| Shares of Ikena common stock issued in connection with the conversion of Ikena’s in-the-money options immediately prior to the First Effective Time, assuming consummation of the Merger as of January 1, 2024, see Note 5(e). |
72,112 | 72,112 | ||||||
| Shares of Ikena common stock issued to Inmagene shareholders upon the First Effective Time, assuming consummation of the Merger as of January 1, 2024 see Note 5(i)(l). |
4,569,948 | 4,569,948 | ||||||
| Shares of Ikena common stock issued in connection with the subscription agreements, assuming consummation of the Merger and Ikena concurrent financing as of January 1, 2024, see Note 5(j). |
2,508,342 | 2,508,342 | ||||||
|
|
|
|
|
|||||
| Pro forma combined weighted average of common stock outstanding |
11,171,910 | 11,171,910 | ||||||
|
|
|
|
|
|||||
| Net loss per share attributable to stockholders |
$ | (1.33 | ) | $ | (8.92 | ) | ||
|
|
|
|
|
|||||
| (1) | Represents the estimated shares of Ikena common stock expected to be issued to Inmagene shareholders at the First Effective Time which is based on the Exchange Ratio of 0.0030510 and a reverse stock split ratio of 1:12, excluding the outstanding Inmagene share options at the First Effective Time that will be converted into the right to receive 516,137 shares of Ikena common stock, based on 169,175,852 Inmagene ordinary share options outstanding, and the Exchange Ratio of 0.0030510, as including the outstanding ordinary share options would be anti-dilutive. |
Please see below selected financial data presenting selected share and per share data reflecting the effect of the Exchange Ratio on all periods previously reported. The selected financial data for Ikena is derived from the consolidated financial statements included in the Ikena Annual Report on Form 10-K filed with the SEC on March 6, 2025 and Quarterly Reports on Form 10-Q filed with the SEC on May 8, 2025 and July 24, 2025, as adjusted to reflect the one-for-twelve reverse stock split, for all periods presented. The selected financial data for Inmagene is derived from the consolidated financial statements included in the Ikena proxy statement/prospectus filed with the SEC on June 11, 2025, as adjusted to reflect the Exchange Ratio of 0.003051, which is reflective of a one-for-twelve reverse stock split, for all periods presented.
Ikena
AS REPORTED
(in thousands, except per share amounts)
| Year Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Net loss per share: |
||||||||
| Net loss per share, basic and diluted |
$ | (1.02 | ) | $ | (1.63 | ) | ||
|
|
|
|
|
|||||
| Weighted-average common shares outstanding, basic and diluted |
48,258,111 | 41,735,081 | ||||||
|
|
|
|
|
|||||
| Three Months Ended March 31, | ||||||||
| 2025 | 2024 | |||||||
| Net loss per share: |
||||||||
| Net loss per share, basic and diluted |
$ | (0.18 | ) | $ | (0.33 | ) | ||
|
|
|
|
|
|||||
| Weighted-average common shares outstanding, basic and diluted |
48,258,111 | 48,258,111 | ||||||
|
|
|
|
|
|||||
| Three Months Ended June 30, | ||||||||
| 2025 | 2024 | |||||||
| Net loss per share: |
||||||||
| Net loss per share, basic and diluted |
$ | (0.06 | ) | $ | (0.28 | ) | ||
|
|
|
|
|
|||||
| Weighted-average common shares outstanding, basic and diluted |
48,258,111 | 48,258,111 | ||||||
|
|
|
|
|
|||||
| Six Months Ended June 30, | ||||||||
| 2025 | 2024 | |||||||
| Net loss per share: |
||||||||
| Net loss per share, basic and diluted |
$ | (0.24 | ) | $ | (0.62 | ) | ||
|
|
|
|
|
|||||
| Weighted-average common shares outstanding, basic and diluted |
48,258,111 | 48,258,111 | ||||||
|
|
|
|
|
|||||
AS ADJUSTED FOR ONE-FOR-TWELVE REVERSE STOCK SPLIT
(in thousands, except per share amounts)
| Year Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Net loss per share: |
||||||||
| Net loss per share, basic and diluted |
$ | (12.24 | ) | $ | (19.60 | ) | ||
|
|
|
|
|
|||||
| Weighted-average common shares outstanding, basic and diluted |
4,021,509 | 3,477,923 | ||||||
|
|
|
|
|
|||||
| Three Months Ended March 31, | ||||||||
| 2025 | 2024 | |||||||
| Net loss per share: |
||||||||
| Net loss per share, basic and diluted |
$ | (2.14 | ) | $ | (4.01 | ) | ||
|
|
|
|
|
|||||
| Weighted-average common shares outstanding, basic and diluted |
4,021,509 | 4,021,509 | ||||||
|
|
|
|
|
|||||
| Three Months Ended June 30, | ||||||||
| 2025 | 2024 | |||||||
| Net loss per share: |
||||||||
| Net loss per share, basic and diluted |
$ | (0.69 | ) | $ | (3.41 | ) | ||
|
|
|
|
|
|||||
| Weighted-average common shares outstanding, basic and diluted |
4,021,509 | 4,021,509 | ||||||
|
|
|
|
|
|||||
| Six Months Ended June 30, | ||||||||
| 2025 | 2024 | |||||||
| Net loss per share: |
||||||||
| Net loss per share, basic and diluted |
$ | (2.83 | ) | $ | (7.43 | ) | ||
|
|
|
|
|
|||||
| Weighted-average common shares outstanding, basic and diluted |
4,021,509 | 4,021,509 | ||||||
|
|
|
|
|
|||||
Inmagene
AS REPORTED
(in thousands, except per share amounts)
| Year Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Loss per ordinary share– basic and diluted: |
||||||||
| Ordinary shares |
$ | (0.07 | ) | $ | (0.05 | ) | ||
|
|
|
|
|
|||||
| Series A convertible preferred shares |
$ | (0.07 | ) | $ | (0.05 | ) | ||
|
|
|
|
|
|||||
| Weighted average shares used to compute basic and diluted loss per share: |
||||||||
| Ordinary shares |
391,403,349 | 320,910,402 | ||||||
|
|
|
|
|
|||||
| Series A convertible preferred shares |
326,079,495 | 326,079,495 | ||||||
|
|
|
|
|
|||||
| Three Months Ended March 31, | ||||||||
| 2025 | 2024 | |||||||
| Loss per ordinary share– basic and diluted: |
||||||||
| Ordinary shares |
$ | (0.01 | ) | $ | (0.04 | ) | ||
|
|
|
|
|
|||||
| Series A convertible preferred shares |
$ | (0.01 | ) | $ | (0.04 | ) | ||
|
|
|
|
|
|||||
| Weighted average shares used to compute basic and diluted loss per share: |
||||||||
| Ordinary shares |
462,131,592 | 321,469,306 | ||||||
|
|
|
|
|
|||||
| Series A convertible preferred shares |
326,079,495 | 326,079,495 | ||||||
|
|
|
|
|
|||||
AS ADJUSTED FOR THE EXCHANGE RATIO OF 0.003051
(in thousands, except per share amounts)
| Year Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Loss per ordinary share– basic and diluted: |
||||||||
| Ordinary shares |
$ | (22.10 | ) | $ | (16.23 | ) | ||
|
|
|
|
|
|||||
| Series A convertible preferred shares |
$ | (22.10 | ) | $ | (16.23 | ) | ||
|
|
|
|
|
|||||
| Weighted average shares used to compute basic and diluted loss per share: |
||||||||
| Ordinary shares |
1,194,171 | 979,097 | ||||||
|
|
|
|
|
|||||
| Series A convertible preferred shares |
994,868 | 994,868 | ||||||
|
|
|
|
|
|||||
| Three Months Ended March 31, | ||||||||
| 2025 | 2024 | |||||||
| Loss per ordinary share– basic and diluted: |
||||||||
| Ordinary shares |
$ | (3.80 | ) | $ | (12.74 | ) | ||
|
|
|
|
|
|||||
| Series A convertible preferred shares |
$ | (3.80 | ) | $ | (12.74 | ) | ||
|
|
|
|
|
|||||
| Weighted average shares used to compute basic and diluted loss per share: |
||||||||
| Ordinary shares |
1,409,963 | 980,802 | ||||||
|
|
|
|
|
|||||
| Series A convertible preferred shares |
994,868 | 994,868 | ||||||
|
|
|
|
|
|||||