UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): June 17, 2025
SPYRE THERAPEUTICS, INC.
(Exact name of Registrant as Specified in Its Charter)
| Delaware | 001-37722 | 46-4312787 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 221 Crescent Street | ||
| Building 23 | ||
| Suite 105 | ||
| Waltham, MA | 02453 | |
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s Telephone Number, Including Area Code: 617 651-5940
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange |
||
| Common Stock, $0.0001 Par Value Per Share | SYRE | The Nasdaq Stock Market LLC (Nasdaq Global Select Market) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On June 17, 2025, Spyre Therapeutics, Inc. (“Spyre” or the “Company”) issued a press release announcing positive interim Phase 1 results from its first-in-human trials of SPY002 (formerly SPY002-091) and SPY072 (formerly SPY002-072), two investigational, novel, extended half-life monoclonal antibodies targeting TL1A, and clinical development updates, including the initiation of its SKYLINE-UC platform trial and unveiling of its SKYWAY-RD basket trial evaluating anti-TL1A in rheumatoid arthritis (“RA”), psoriatic arthritis (“PsA”) and axial spondyloarthritis (“axSpA”). The Company will host a conference call and webcast today, Tuesday, June 17, 2025 at 8:00 a.m., Eastern Time, to discuss the anti-TL1A Phase 1 interim results and its Phase 2 clinical development plans.
A copy of the press release and data presentation that will be referenced during the conference call are attached hereto as Exhibit 99.1 and Exhibit 99.2, respectively. The information in this Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.1 and Exhibit 99.2 attached hereto, is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or under the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 8.01 Other Events.
On June 17, 2025, the Company announced positive interim Phase 1 results from its first-in-human trials of SPY002 (formerly SPY002-091) and SPY072 (formerly SPY002-072), two investigational, novel, extended half-life monoclonal antibodies targeting TL1A. In addition, the Company announced the initiation of its SKYLINE-UC platform trial and unveiled its SKYWAY-RD basket trial evaluating anti-TL1A targeted therapy in three rheumatologic indications.
Single doses of up to 1500 mg for SPY002 and SPY072 were well tolerated with no serious adverse events reported, exhibited a prolonged half-life supportive of quarterly or less frequent dosing, and suppressed free TL1A through 20 weeks of follow up available for the lowest dose tested.
The Company is advancing SPY002 into the SKYLINE-UC platform trial – initiated in May 2025 – for ulcerative colitis. SKYLINE-UC is expected to include SPY001 (anti-α4ß7), SPY002 (anti-TL1A), SPY003 (anti-IL-23), and combinations thereof under a single master protocol.
SPY072 will be advanced via the newly announced SKYWAY-RD basket trial for three rheumatologic conditions. The SKYWAY-RD study is a Phase 2 basket trial investigating Spyre’s anti-TL1A as a treatment for RA, PsA, and axSpA and is expected to initiate in the third quarter of 2025. In addition to a planned Phase 1 interim readout of SPY003 (anti-IL-23)
in the second half of 2025, in 2026 the Company expects to readout open-label monotherapy data for its three investigational long-acting antibodies from the SKYLINE-UC trial along with three placebo-controlled readouts for SPY072 in RA, PsA, and axSpA from the SKYWAY-RD trial. In 2027, the Company expects to read out placebo-controlled data of its monotherapies and combination therapies from the SKYLINE-UC study.
Anti-TL1A Phase 1 Interim Findings
The SPY002 and SPY072 Phase 1 trials are first-in-human, randomized, double-blind, placebo-controlled trials designed to evaluate safety, pharmacokinetic (“PK”), and pharmacodynamic (“PD”) data in healthy volunteers. To date, each trial has enrolled 40 healthy adult participants into five single-ascending dose (“SAD”) cohorts. Doses of SPY002 and SPY072 evaluated included 100 mg subcutaneous (“SC”), 300 mg SC, 300 mg intravenous (“IV”), 1000 mg IV, and 1500 mg IV. Interim findings from the trial are as follows:
| • | Safety – well-tolerated across all dose groups |
| • | Single doses of SPY002 and SPY072 up to 1500 mg were well-tolerated with a favorable safety profile consistent with existing third-party data of the anti-TL1A class. |
| • | The most common (i.e., occurring in more than two subjects) treatment-emergent adverse events (“TEAEs”) for SPY002 and SPY072 were COVID-19 and nausea, respectively. |
| • | There were no TEAEs greater than Grade 2, and no adverse events led to trial discontinuation. |
| • | PK – observed differentiated profile relative to first-generation anti-TL1As |
| • | SPY002 half-life is estimated at ~75 days across IV and SC SAD cohorts, more than 3-fold greater than first-generation anti-TL1As. |
| • | SPY072 showed comparable PK to SPY002 at clinically relevant doses through available follow up; waiting for comparable follow-up to accurately estimate half-life. |
| • | PK for SPY002 and SPY072 supports potential for chronic dosing in a single SC injection on a quarterly or twice annual basis. |
| • | PD – observed complete suppression of free TL1A at the latest time points available |
| • | Both SPY002 and SPY072 demonstrated dose-dependent increases in total TL1A as expected. |
| • | A single 100 mg dose of either SPY002 or SPY072 observed to suppress free TL1A to below the lower-limit of quantitation through 20-weeks of follow-up (longest follow-up available with PD data). |
| • | Immunogenicity – no apparent impact of anti-drug antibodies was observed on PK or PD |
SKYLINE-UC: Platform Phase 2 trial in Ulcerative Colitis
SKYLINE-UC (NCT07012395) is a Phase 2 randomized and placebo-controlled induction and maintenance platform trial of SPY001, SPY002, SPY003, and pairwise combinations thereof (six investigational agents in total) in patients with moderately to severely active ulcerative colitis with two parts:
| • | Part A: open-label assessment of the safety and preliminary efficacy of monotherapies |
| • | Investigating a single dose level of each monotherapy as induction and maintenance therapies. |
| • | Induction data are expected in 2026. |
| • | Part B: randomized and placebo-controlled assessment of the safety and efficacy of monotherapies and combination therapies |
| • | Enrollment expected after completion of enrollment of Part A. |
| • | Designed to provide dose-ranging data on monotherapies, proof-of-concept and contribution of components for combination therapies. |
| • | Induction data are expected in 2027. |
SKYWAY-RD: Basket Phase 2 trial of anti-TL1A in Three Rheumatologic Conditions
SKYWAY-RD is a planned Phase 2 randomized and placebo-controlled basket trial of SPY072 in patients with moderately to severely active RA, PsA, or axSpA with inadequate response to conventional or advanced therapies.
| • | RA sub-study: Double-blind, placebo-controlled safety and efficacy study of two dose levels of SPY072 through Week 12 with open-label follow-up through Week 36. |
| • | PsA sub-study: Double-blind, placebo-controlled safety and efficacy study of a single dose level of SPY072 through Week 16 with open-label follow-up through Week 40. |
| • | axSpA sub-study: Double-blind, placebo-controlled safety and efficacy study of a single dose level of SPY072 through Week 16 with open-label follow-up through Week 40. |
Topline proof-of-concept data are expected in 2026.
Forward-Looking Statements
This Current Report on Form 8-K contains “forward-looking” statements within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. All statements contained in this Current Report on Form 8-K, other than statements of historical fact are forward-looking statements. These forward-looking statements include statements regarding the Company’s ability to achieve the expected benefits or opportunities with respect to its pipeline of product candidates such as the potential efficacy, tolerability, convenience, commercial viability, dosing regimen and safety profile of SPY002 and SPY072 in humans; its plans to advance both SPY002 and SPY072 programs into Phase 2 clinical trials; expectations regarding the drug delivery of SPY002 and SPY072, including in the form of a single SC injection; its ongoing and future clinical development activities, including the expected design and timing of the planned SKYWAY-RD Phase 2 basket trial, including timing of data readouts, enrollment of clinical trials and timing of each part, cohort and data readout for the ongoing SKYLINE-UC Phase 2 platform trial; the expected SPY003 readout in the second half of 2025; the potential consistency of the SPY002 and SPY072 Phase 1 trial final data readouts with the interim Phase 1 results; the potential therapeutic benefits of its product candidates as monotherapies or in combinations and their extended half-life, including the expected duration of half-life in comparison to competitor products; and the timing and results of clinical trials. The words “believe,” “may,” “will,” “potentially,” “estimate,” “continue,” “anticipate,” “predict,” “target,” “intend,” “could,” “would,” “should,” “project,” “plan,” “expect,” the negatives of these terms, and similar expressions that convey uncertainty of future events or outcomes are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond the Company’s control) or other assumptions that may cause actual results or performance, final clinical trial data readouts and clinical trial designs, including the planned SKYWAY-RD Phase 2 basket trial design, to be materially different from those expressed or implied by these forward-looking statements.
These risks and uncertainties include, but are not limited, to the final SPY002 and SPY072 Phase 1 trial data readouts not being consistent with or being different than the interim SPY002 and SPY072 Phase 1 trial results reported in this Current Report on Form 8-K; regulatory feedback including potential disagreement by regulatory authorities with the Company’s interpretation of data and the Company’s planned clinical trials for its product candidates, including the Company’s planned SKYWAY-RD Phase 2 clinical trial design; and those uncertainties and factors described under the heading “Risk Factors,” “Risk Factor Summary” and “Note about Forward-Looking Statements” in Spyre’s most recent Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission, as well as discussions of potential risks, uncertainties, and other important factors included in other filings by Spyre from time to time. Should one or more of these risks or uncertainties materialize, or should any of Spyre’s assumptions prove incorrect, actual results may vary in material respects from those projected in these forward-looking statements. Nothing in this Current Report on Form 8-K should be regarded as a representation by any person that the forward-looking statements set forth therein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements in this Current Report on Form 8-K, which speak only as of the date they are made and are qualified in their entirety by reference to the cautionary statements herein. Spyre does not undertake or accept any duty to make any updates or revisions to any forward-looking statements. This Current Report on Form 8-K does not purport to summarize all of the conditions, risks and other attributes of an investment in Spyre.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit Number |
Description |
|
| 99.1 | Press Release of the Company Regarding Data, dated June 17, 2025 | |
| 99.2 | Spyre Therapeutics, Inc. Data Presentation, dated June 17, 2025 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| SPYRE THERAPEUTICS, INC. | ||||||
| Date: June 17, 2025 | By: | /s/ Cameron Turtle |
||||
| Cameron Turtle Chief Executive Officer |
||||||
Exhibit 99.1

Spyre Therapeutics Announces Positive Interim Phase 1 Results for Two Next-
Generation TL1A Antibody Programs, and Provides Clinical Development
Updates Expected to Deliver 9 Phase 2 Readouts
SPY002 and SPY072 were well tolerated, exhibited PK that supports quarterly or less frequent
dosing, and fully engaged TL1A through up to 20 weeks of follow-up; ~75 day half-life
demonstrated, more than 3-fold greater than first-generation anti-TL1A antibodies
SKYLINE-UC platform study evaluating three optimized monotherapies and three potentially
paradigm-changing combinations in ulcerative colitis, initiated in May 2025
SKYWAY-RD basket study evaluating SPY072 in rheumatoid arthritis (RA), psoriatic arthritis
(PsA), and axial spondyloarthritis (axSpA) announced, with initiation expected in Q3 2025
Management to host a webcast and conference call today at 8:00 a.m. ET
WALTHAM, Mass., June 17, 2025 (PR NEWSWIRE) – Spyre Therapeutics, Inc. (NASDAQ: SYRE), a clinical-stage biotechnology company advancing best-in-class antibody engineering, dose optimization, and rational therapeutic combinations for the treatment of Inflammatory Bowel Disease (“IBD”) and other immune-mediated diseases, today announced positive interim Phase 1 results from its first-in-human trials of SPY002 (formerly SPY002-091) and SPY072 (formerly SPY002-072), two investigational, novel, extended half-life monoclonal antibodies targeting TL1A. In addition, the company announced the initiation of its SKYLINE-UC platform trial and unveiled its SKYWAY-RD basket trial evaluating anti-TL1A targeted therapy in three rheumatologic indications.
Interim results from the Phase 1 trials for SPY002 and SPY072, with data reported as of May 30, 2025, met all Phase 1 objectives, supporting their potential to become next-generation anti-TL1A monotherapies in immune-mediated diseases or as elements of combination therapies. Single doses of up to 1500 mg for SPY002 and SPY072 were well tolerated with no serious adverse events reported, exhibited a prolonged half-life supportive of quarterly or less frequent dosing, and suppressed free TL1A through 20 weeks of follow up available for the lowest dose tested.
“These interim results demonstrate clear benefits of our anti-TL1A approach versus first-generation molecules, underscoring the promise and potential of SPY002 and SPY072 as transformational therapies in immune-mediated diseases,” said Josh Friedman, M.D., Ph.D., SVP of Clinical Development at Spyre. “We are excited to advance both programs into Phase 2, with the planned addition of SPY002 to our ongoing SKYLINE-UC trial and initiation of a novel basket trial, SKYWAY-RD, with SPY072.”
1

Building on these encouraging Phase 1 results, Spyre is advancing SPY002 into the SKYLINE-UC platform trial – initiated in May 2025 – for ulcerative colitis. SKYLINE-UC is expected to include SPY001 (anti-α4b7), SPY002 (anti-TL1A), SPY003 (anti-IL-23), and combinations thereof under an efficient single master protocol.
SPY072 will be advanced via the newly announced SKYWAY-RD basket trial for three rheumatologic conditions. The SKYWAY-RD study is a Phase 2 basket trial investigating Spyre’s improved anti-TL1A as a treatment for RA, PsA, and axSpA and is expected to initiate in Q3 2025.
“The recently initiated SKYLINE-UC platform trial and the planned SKYWAY-RD basket trial leverage innovative and efficient designs to potentially deliver impactful results for the patients, physicians, and caregivers we aim to serve,” said Sheldon Sloan, MD, Chief Medical Officer at Spyre. “The SKYLINE-UC study will test optimized versions of the best monotherapies in IBD and combinations of those monotherapies, aiming to identify therapies that deliver a step change in efficacy and convenience compared to today’s standard of care. The SKYWAY-RD study will explore safety and efficacy of our anti-TL1A therapy in three rheumatologic diseases with favorable scientific rationale and large patient populations.”
In addition to a planned Phase 1 interim readout of SPY003 (anti-IL-23) in the second half of 2025, in 2026 the company expects to readout open-label monotherapy data for its three investigational long-acting antibodies from the SKYLINE-UC trial along with three placebo-controlled readouts for SPY072 in RA, PsA, and axSpA from the SKYWAY-RD trial. In 2027, the company expects to read out placebo-controlled data of its monotherapies and combination therapies from the SKYLINE-UC study.
“Initiation of our Phase 2 SKYLINE-UC trial and planned initiation of our Phase 2 SKYWAY-RD trial marks a significant inflection point as we begin to explore the potential of our pipeline to deliver breakthroughs for patients with hard-to-treat inflammatory diseases,” said Cameron Turtle, DPhil, Chief Executive Officer at Spyre. “With cash runway into the second half of 2028, we are well-funded to deliver 9 proof-of-concept readouts over the next two years in markets totaling >$60B of annual revenue. We believe our high-probability science and efficient development program provides the potential for exceptional stockholder value creation.”
Anti-TL1A Phase 1 Interim Findings
The SPY002 and SPY072 Phase 1 trials are first-in-human, randomized, double-blind, placebo-controlled trials designed to evaluate safety, PK, and PD in healthy volunteers. To date, each trial has enrolled 40 healthy adult participants into five single-ascending dose (SAD) cohorts. Doses of SPY002 and SPY072 evaluated included 100 mg SC, 300 mg SC, 300 mg IV, 1000 mg IV, and 1500 mg IV. Interim findings from the trial are as follows:
2

| • | Safety – well-tolerated across all dose groups |
| • | Single doses of SPY002 and SPY072 up to 1500 mg were well-tolerated with a favorable safety profile consistent with existing third-party data of the anti-TL1A class. |
| • | The most common (i.e., occurring in more than two subjects) treatment-emergent adverse events (TEAEs) for SPY002 and SPY072 were COVID-19 and nausea, respectively. |
| • | There were no TEAEs greater than Grade 2, and no AEs led to trial discontinuation. |
| • | PK – differentiated profile relative to first-generation anti-TL1As |
| • | SPY002 half-life is estimated at ~75 days across IV and SC SAD cohorts, more than 3-fold greater than first-generation anti-TL1As. |
| • | SPY072 showed comparable PK to SPY002 at clinically relevant doses through available follow up; waiting for comparable follow-up to accurately estimate half-life. |
| • | PK for SPY002 and SPY072 supports potential for chronic dosing in a single SC injection on a quarterly or twice annual basis. |
| • | PD – complete suppression of free TL1A at the latest time points available |
| • | Both SPY002 and SPY072 demonstrated dose-dependent increases in total TL1A as expected. |
| • | A single 100 mg dose of either SPY002 or SPY072 suppressed free TL1A to below the lower-limit of quantitation (LLOQ) through 20-weeks of follow-up (longest follow-up available with PD data). |
| • | Immunogenicity – no apparent impact of anti-drug-antibodies was observed on PK or PD |
SKYLINE-UC: Platform Phase 2 trial in Ulcerative Colitis
SKYLINE-UC (NCT07012395) is a Phase 2 randomized and placebo-controlled induction and maintenance platform trial of SPY001, SPY002, SPY003, and pairwise combinations thereof (six investigational agents in total) in patients with moderately to severely active ulcerative colitis with two parts:
| • | Part A: open-label assessment of the safety and preliminary efficacy of monotherapies |
| • | Investigating a single dose level of each monotherapy as induction and maintenance therapies. |
3

| • | Induction data are expected in 2026. |
| • | Part B: randomized and placebo-controlled assessment of the safety and efficacy of monotherapies and combination therapies |
| • | Seamless enrollment expected after completion of enrollment of Part A. |
| • | Designed to provide dose-ranging data on monotherapies, proof-of-concept and contribution of components for combination therapies. |
| • | Induction data are expected in 2027. |
SKYWAY-RD: Basket Phase 2 trial of anti-TL1A in Three Rheumatologic Conditions
SKYWAY-RD is a planned Phase 2 randomized and placebo-controlled basket trial of SPY072 in patients with moderately to severely active RA, PsA, or axSpA with inadequate response to conventional or advanced therapies.
| • | RA sub-study: Double-blind, placebo-controlled safety and efficacy study of two dose levels of SPY072 through Week 12 with open-label follow-up through Week 36. |
| • | PsA sub-study: Double-blind, placebo-controlled safety and efficacy study of a single dose level of SPY072 through Week 16 with open-label follow-up through Week 40. |
| • | axSpA sub-study: Double-blind, placebo-controlled safety and efficacy study of a single dose level of SPY072 through Week 16 with open-label follow-up through Week 40. |
Topline proof-of-concept data are expected in 2026.
Conference Call and Webcast
Spyre will host a conference call and webcast today, June 17, 2025, at 8:00 a.m. ET to discuss the anti-TL1A Phase 1 interim results and its Phase 2 clinical development plans. A live webcast of the call will be available on the Investor Relations website at https://ir.spyre.com/events-and-presentations. The webcast will be made available for replay on the company’s website following completion of the event.
About SPY002
SPY002 is an investigational, extended half-life monoclonal antibody targeting TL1A for the potential treatment of IBD. IBD is a chronic condition characterized by inflammation in the gastrointestinal tract and encompasses two main disorders: ulcerative colitis and Crohn’s disease. Together, these conditions affect more than 2.4 million individuals in the United States. In head-to-head preclinical studies, SPY002 demonstrated potency equivalent to or better than first-generation anti-TL1As. Interim data from a Phase 1 trial demonstrated that SPY002 was well tolerated, exhibited prolonged pharmacokinetics, and rapidly and durably suppressed free TL1A. Based on Phase 1 clinical data, the company plans to evaluate SPY002 in its SKYLINE-UC Phase 2 platform study.
4

About SPY072
SPY072 is an investigational, extended half-life monoclonal antibody targeting TL1A for the potential treatment of rheumatologic diseases including rheumatoid arthritis, psoriatic arthritis, and axial spondyloarthritis. Together, these conditions affect more than 3 million individuals in the United States. In head-to-head preclinical studies, SPY072 demonstrated potency equivalent to or better than first-generation anti-TL1As. Interim data from a Phase 1 trial demonstrated that SPY072 was well tolerated, exhibited prolonged pharmacokinetics, and rapidly and durably suppressed free TL1A. Based on Phase 1 clinical data, the company plans to evaluate SPY002 in a planned SKYWAY-RD Phase 2 basket study.
About Spyre Therapeutics
Spyre Therapeutics is a clinical-stage biotechnology company that aims to create next-generation inflammatory bowel disease (IBD) and other immune-mediated disease products by combining best-in-class antibody engineering, dose optimization, and rational therapeutic combinations. Spyre’s pipeline includes investigational extended half-life antibodies targeting α4b7, TL1A, and IL-23.
For more information, please visit http://spyre.com.
Forward-Looking Statements
Certain statements in this press release, other than purely historical information, may constitute “forward-looking statements” within the meaning of the federal securities laws, including for purposes of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995, concerning Spyre and other matters.
5

These forward-looking statements include, but are not limited to, express or implied statements relating to Spyre’s management team’s expectations, hopes, beliefs, intentions or strategies regarding the future including, without limitation, Spyre’s ability to achieve the expected benefits or opportunities with respect to its pipeline of product candidates such as the potential efficacy, tolerability, convenience, commercial viability, dosing regimen and safety profile of SPY002 and SPY072 in humans; the potential for SPY002 and SPY072 to become next-generation anti-TL1A therapies in immune-mediated diseases as monotherapies or as elements for combination therapies; Spyre’s plans to advance both SPY002 and SPY072 programs into Phase 2 clinical trials; expectations regarding the drug delivery of SPY002 and SPY072, including in the form of a single SC injection; Spyre’s ongoing and future clinical development activities, including the expected design and timing of the planned SKYWAY-RD Phase 2 basket trial, including timing of data readouts, timing of each part, cohort and data readout for the ongoing SKYLINE-UC Phase 2 platform trial, enrollment of clinical trials and number of data readouts expected to be delivered in 2026 and 2027; the expected SPY003 readout in the second half of 2025; the potential consistency of the SPY002 and SPY072 Phase 1 trial final data readouts with interim Phase 1 results; the potential therapeutic benefits of Spyre’s product candidates as monotherapies or in combinations and their extended half-life, including the expected duration of half-life in comparison to competitor products and the potential efficacy and convenience compared to today’s standard of care; the sufficiency of the Company’s funding to support the development of its assets, including the expectation of being well-funded to deliver 9 proof-of-concept readouts in 2026 and 2027; the length of time that the Company believes its existing cash resources will fund its operations, including expectations of cash runway extending into the second half of 2028; estimated market sizes and potential growth opportunities; the potential for exceptional stockholder value creation; and the timing and results of clinical trials. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words “opportunity,” “potential,” “milestones,” “pipeline,” “can,” “goal,” “aim,” “strategy,” “target,” “seek,” “anticipate,” “achieve,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “predict,” “project,” “should,” “will,” “would,” and similar expressions (including the negatives of these terms or variations of them) may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. These forward-looking statements are based on current expectations and beliefs concerning future developments and their potential effects. There can be no assurance that future developments affecting Spyre will be those that have been anticipated. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond Spyre’s control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements. These risks and uncertainties include, but are not limited, uncertainties and risks arising from regulatory feedback, including potential disagreement by regulatory authorities with the Company’s interpretation of data and the Company’s planned clinical trials for its product candidates, including the Company’s planned SKYWAY-RD Phase 2 clinical trial design; the potential for final data not being consistent with or different than the interim data reported for our programs; the potential impact of Trump Administration policies and changes in law on our business; and those uncertainties and factors described under the heading “Risk Factors,” “Risk Factor Summary” and “Note about Forward-Looking Statements” in Spyre’s most recent Annual Report on Form 10-K, as supplemented and updated by subsequent Quarterly Reports on Form 10-Q and Current Reports on Form 8-K that the Company has filed or will file with the SEC, as well as discussions of potential risks, uncertainties, and other important factors included in other filings by Spyre from time to time. Should one or more of these risks or uncertainties materialize, or should any of Spyre’s assumptions prove incorrect, actual results may vary in material respects from those projected in these forward-looking statements.
6

Nothing in this press release should be regarded as a representation by any person that the forward-looking statements set forth therein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements in this press release, which speak only as of the date they are made and are qualified in their entirety by reference to the cautionary statements herein. Spyre does not undertake or accept any duty to make any updates or revisions to any forward-looking statements. This press release does not purport to summarize all of the conditions, risks and other attributes of an investment in Spyre.
For Investors:
Eric McIntyre
VP of Finance and Investor Relations
Spyre Therapeutics
Eric.mcintyre@spyre.com
For Media:
Josie Butler, 1AB
josie@1abmedia.com
7

Exhibit 99.2 Anti-TL1A Phase 1 Results and Phase 2 Development Updates June 2025

Disclosures The information contained in this presentation has been prepared by Spyre Therapeutics, Inc. and its affiliates (“Spyre” or the “Company”) and contains information pertaining to the business and operations of the Company. The information contained in this presentation: (a) is provided as at the date hereof, is subject to change without notice, and is based on publicly available information, internally developed data as well as third party information from other sources; (b) does not purport to contain all the information that may be necessary or desirable to fully and accurately evaluate an investment in the Company; (c) is not to be considered as a recommendation by the Company that any person make an investment in the Company; (d) is for information purposes only and shall not constitute an offer to buy, sell, issue or subscribe for, or the solicitation of an offer to buy, sell or issue, or subscribe for any securities of the Company in any jurisdiction in which such offer, solicitation or sale would be unlawful. Where any opinion or belief is expressed in this presentation, it is based on certain assumptions and limitations and is an expression of present opinion or belief only. This presentation should not be construed as legal, financial or tax advice to any individual, as each individual’s circumstances are different. This document is for informational purposes only and should not be considered a solicitation or recommendation to purchase, sell or hold a security. Forward-Looking Information Certain information set forth in this presentation contains “forward-looking statements” within the meaning of applicable United States securities legislation. Except for statements of historical fact, certain information contained herein constitutes forward- looking statements which include but are not limited to statements regarding: our business strategy, including our ability to develop best-in-class and first-in-class therapeutics for inflammatory bowel disease (IBD), rheumatoid arthritis (RA), psoriatic arthritis (PsA), axial spondyloarthritis (axSpA) and other immune-mediated diseases that meaningfully improve both efficacy and convenience compared to today’s standard of care; the potential consistency of the SPY001, SPY002 and SPY072 Phase 1 trial final data readouts with interim Phase 1 results; the efficacy, safety profile, dosing regime, convenience, commercial viability and tolerability of SPY002, SPY072 and our other product candidates; our plans to advance both SPY002 and SPY072 programs into Phase 2 clinical trials; Spyre’s ongoing and future clinical development activities, including the expected design and timing of the planned platform SKYWAY-RD Phase 2 basket trial, including timing of data readouts, timing of data readout for the ongoing SPY003 Phase 1 trial, timing of each part, cohort and data readouts for the ongoing SKYLINE-UC Phase 2 platform trial, enrollment of clinical trials and number of data readouts expected to be delivered in 2026 and 2027; our ability to provide anticipated readouts ahead of any disclosed bispecific approaches against our targets; the planned induction and maintenance dosing regimen for SPY001 and our other product candidates and combinations thereof, including the potential for a Q3M-Q6M dosing profile; the potential therapeutic benefits of our product candidates as monotherapies or in combinations and their extended half-life, including the expected duration of half-life in comparison to competitor products; potential cost savings from the trial designs of our Phase 2 platform trial and Phase 2 basket trial; potential alignment with regulatory authorities and anticipated regulatory submissions; expected timing for regulatory feedback; estimated market sizes, potential growth opportunities, potential value creation and sample transactions; the length of time that the Company believes its existing cash resources will fund its operations, including expectations of cash runway extending into the second half of 2028; expectations regarding our potential therapeutic combinations, including dosing regime, and the potential benefits thereof; and management’s assessment of future plans and operations which are based on current internal expectations, estimates, projections, assumptions and beliefs, which may prove to be incorrect. Forward-looking statements can often be identified by the use of words such as “may”, “will”, “could”, “would”, “anticipate”, ‘believe”, expect”, “intend”, “potential”, “estimate”, “scheduled”, “plans”, “planned”, “forecasts”, “goals” and similar expressions or the negatives thereof. Forward-looking statements are neither historical facts nor assurances of future performance. Forward-looking statements are based on a number of factors and assumptions made by management and considered reasonable at the time such information is provided, and forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause the actual results, performance or achievements to be materially different from those expressed or implied by the forward-looking statements, including uncertainties and risks arising from regulatory feedback, including potential disagreement by regulatory authorities with the Company’s interpretation of data and the Company’s planned clinical trials for its product candidates, including the Company’s planned SKYWAY-RD Phase 2 clinical trial design; the potential for final data not being consistent with or different than the interim data reported for our programs; the potential impact of Trump Administration policies and changes in law on our business; and those uncertainties and factors described under the heading “Risk Factors,” “Risk Factor Summary” and “Note about Forward-Looking Statements” in the Company’s most recent Annual Report on Form 10-K, as supplemented and updated by subsequent Quarterly Reports on Form 10-Q and Current Reports on Form 8-K that the Company has filed or will file with the SEC, as well as discussions of potential risks, uncertainties, and other filings by the Company from time to time, as well as risk factors associated with companies that operate in the biopharma industry, including those associated with the uncertainties of drug development. All of the forward-looking statements made in this presentation are qualified by these cautionary statements and other cautionary statements or other factors contained herein. Although management believes that the expectations conveyed by forward-looking statements herein are reasonable based on information available on the date such forward-looking statements are made, there can be no assurance that forward looking statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. The Company undertakes no obligation to update forward-looking statements if circumstances or management’s estimates or opinions should change except as required by applicable securities laws. The forward-looking statements contained herein are presented for the purposes of assisting readers in understanding the Company’s plan, objectives and goals and may not be appropriate for other purposes. The reader is cautioned not to place undue reliance on forward-looking statements. Industry Information This presentation also contains or references certain industry data that is based upon information from independent industry publications, market research, and surveys and other publicly available sources. Although the Company believes these sources to be generally reliable, such information is subject to interpretation and cannot be verified with complete certainty due to limits on the availability and reliability of data, the voluntary nature of the data gathering process and other inherent limitations and uncertainties. The Company has not independently verified any of the data from third party sources referred to in this presentation and accordingly, the Company makes no representation or warranty as to the origin, validity, accuracy, completeness, currency or reliability of the information in this presentation. © 2025 Spyre Therapeutics, Inc. 2 All rights reserved.

Agenda Cameron Turtle, DPhil Introduction and Executive Summary Chief Executive Officer Joshua Friedman, MD, PhD SPY002 & SPY072 Phase 1 Interim Results SVP, Clinical Development Deanna Nguyen, MD SKYLINE-UC Phase 2 Study SVP, Clinical Development Joshua Friedman, MD PhD SKYWAY-RD Phase 2 Study SVP, Clinical Development Cameron Turtle, CEO Joshua Friedman, SVP Sheldon Sloan, CMO Closing and Analyst Q&A Deanna Nguyen, SVP Scott Burrows, CFO © 2025 Spyre Therapeutics, Inc. 3 All rights reserved.

Introduction Today’s 1 2 3 Objectives Report Launch Unveil Anti-TL1A data SKYLINE-UC SKYWAY-RD Phase 1 results for two A Phase 2 platform study A Phase 2 basket study potential best-in-class in ulcerative colitis evaluating anti-TL1A in molecules evaluating three three rheumatologic monotherapies & three conditions combinations Cameron Turtle Chief Executive Officer © 2025 Spyre Therapeutics, Inc. 4 All rights reserved.

Engineering for new heights in the treatment of IBD & beyond Validated targets Half-life extension Fixed-dose combinations Engineered for prolonged activity to enable Rationale combinations to address distinct MOAs rationally chosen based on attractive infrequent administration disease drivers risk-benefit profiles α4β7 TL1A IL-23 YTE Coformulations Potential best-in-class monotherapies & Potential first-in-class anti-TL1A in combinations in IBD rheumatologic conditions © 2025 Spyre Therapeutics, Inc. Concept image of drug delivery device shown for illustrative purposes only. 5 All rights reserved.

Recall: We advanced two anti-TL1A antibodies into Ph1 with best-in-class preclinical properties SPY002 (formerly SPY002-091) SPY072 (formerly SPY002-072) In vitro potency (TF-1 apoptosis) In vitro potency (TF-1 apoptosis) 100 100 80 80 60 60 40 40 20 20 SPY002 SPY072 Tulisokibart 0 Tulisokibart 0 0.01 0.1 1 10 100 0.01 0.1 1 10 100 mAb Concentration (nM) mAb Concentration (nM) Distinct epitopes | Superior-to-comparable in vitro potency | NHP half-life >2-3x vs. first-generation anti-TL1As © 2025 Spyre Therapeutics, Inc. Data on file. 6 All rights reserved. Inhibition %

SPY002 and SPY072 met Phase 1 objectives, and Spyre plans to advance both into Phase 2 SPY002 SPY072 Simulated mean PK profiles Median PD profiles Simulated mean PK profiles Median PD profiles SPY002 SPY072 Placebo Placebo st st 1 gen 1 gen SPY002 SPY072 anti-TL1As anti-TL1As Well tolerated PK supports quarterly or Rapid & sustained No apparent impact of favorable safety profile less frequent dosing target engagement ADAs on PK/PD © 2025 Spyre Therapeutics, Inc. Interim analysis as of May 30, 2025 data cutoff. PK=pharmacokinetics; PD=pharmacodynamics; ADA=anti-drug antibodies. 7 All rights reserved.

Transitioning to a mid-stage development company… PHASE 1 PHASE 2 PHASE 3 P PH HA AS SE E 2 2 S SP PY Y0 00 01 1 S SP PY Y0 00 02 2 S SP PY Y0 00 01 1 S Su uc cc ce es ss sffu ull iin nv ve es sttiig ga attiio on na all a ag ge en ntts s 2H S SP PY Y0 00 03 3 P Ph h2 2 p plla attffo or rm m ttr riia all o off s siix x iin nv ve es sttiig ga attiio on na all a ag ge en ntts s iin n U UC C S SP PY Y0 07 72 2 S SP PY Y0 00 01 1 S Su uc cc ce es ss sffu ull iin nd diic ca attiio on ns s P Ph h2 2 b ba as sk ke ett ttr riia all o off S SP PY Y0 07 72 2 iin n R RA A,, P Ps sA A,, a ax xS Sp pA A Rationale for advancing two anti-TL1As: Strategic: Partnering optionality Commercial: Pricing flexibility, separate IRA timing and revenue © 2025 Spyre Therapeutics, Inc. UC=ulcerative colitis; RD=rheumatological diseases; IRA=Inflation Reduction Act. 8 All rights reserved. Check marks refer to interim Ph1 results.

…with two innovative and capital-efficient Phase 2 trials Ph2 platform trial evaluating SPY001, SPY002, SPY003 Ph2 basket trial evaluating SPY072 in rheumatoid and pairwise combinations in ulcerative colitis arthritis, psoriatic arthritis, and axial spondyloarthritis SPY072 Dose A SPY001 RA UC SPY072 Dose B SPY002 Placebo SPY003 SPY072 Dose A SPY120 PsA Placebo SPY130 SPY230 SPY072 Dose A axSpA Placebo Placebo 40% 35% 6 1 1 3 1 1 AGENTS INDICATION COST SAVINGS AGENT INDICATIONS COST SAVINGS 1 © 2025 Spyre Therapeutics, Inc. Estimated savings relative to separate Ph2 trials for each program (SKYLINE) or indication (SKYWAY) 9 All rights reserved. RD=rheumatologic diseases. Combo Mono

Anticipated 9 proof-of-concept readouts in 2026-27 for the treatment of IBD & beyond Trial Indication Program Target Phase 1 Phase 2 Phase 3 Anticipated Milestones α4β7 UC SPY001 2026: Ph2 open-label POC TL1A SPY002 2027: Ph2 pbo-controlled data IL-23 SPY003 SPY120 α4β7 + TL1A 2027: Ph2 POC SPY130 α4β7 + IL-23 SPY230 TL1A + IL-23 RA 2026: Ph2 POC PsA SPY072 TL1A axSpA © 2025 Spyre Therapeutics, Inc. Milestones expected as of the date of this presentation. 10 All rights reserved. UC=ulcerative colitis; RD=rheumatological disease; RA=rheumatoid arthritis; PsA=psoriatic arthritis; axSpA=axial spondyloarthritis; POC=proof of concept.

Potential additive paths to significant value creation in 2026-27 mid- 2026 2027 2025 Trial Phase 1s 2H 2025 Programs SPY001 SPY002 SPY072 SPY003 SPY001 SPY002 SPY003 SPY072 SPY120 SPY130 SPY230 Interim safety, PK, PD Monotherapy OL POC Anti-TL1A POC Combination POC Readout HVs UC RA, PsA, axSpA UC Analog Validated MOA, First-in-class MOA Paradigm-changing improved convenience in $30B+ market combinations type 1 Precedent 2 $3B+ acquisition $10B+ acquisition $10B+ post-POC 1 2 Milestones expected as of the date of this presentation. Precedent valuations provided for illustrative purposes only and may not be indicative of potential Spyre valuation; Vertex © 2025 Spyre Therapeutics, Inc. valuation following proof-of-concept for first Phase 2 combination study of VX-770 and VX-809. 11 All rights reserved. OL=open label; Check marks refer to interim Ph1 results.

SPY002 and SPY072 Phase 1 interim results Today’s 1 2 3 Objectives Report Launch Unveil Anti-TL1A data SKYLINE-UC SKYWAY-RD Phase 1 results for two A Phase 2 basket study A Phase 2 platform study potential best-in-class evaluating anti-TL1A in in ulcerative colitis molecules three rheumatologic evaluating three conditions monotherapies & three combinations Josh Friedman SVP, Clinical Development © 2025 Spyre Therapeutics, Inc. 12 All rights reserved.

Both molecules studied in similar Ph1 SAD studies, with interim data up to 20-weeks of follow up Trial design elements SPY002 and SPY072 SAD PK and safety follow up Design 20 weeks 100 mg SC • Double-blind, pbo-controlled, first-in-human trial 16 weeks • Escalating SAD cohorts 16 weeks 300 mg SC 12 weeks Population • Healthy adult volunteers 12 weeks 300 mg IV • N=8/cohort (3:1 randomization) 8 weeks 8 weeks Endpoints 1000 mg IV 4 weeks • Primary: Safety • Secondary: Pharmacokinetics, anti-drug SPY002 4 weeks antibodies (ADAs) 1500 mg IV SPY072 <4 weeks • Exploratory: Pharmacodynamic markers © 2025 Spyre Therapeutics, Inc. Interim analysis as of May 30, 2025 data cutoff. 13 All rights reserved.

SPY002 and SPY072 were well tolerated with a favorable safety profile (pooled, blinded data) SPY002 safety profile SPY072 safety profile SAD SAD N (%) 100 mg 300 mg 300 mg 1000 mg 1500 mg Pooled 100 mg 300 mg 300 mg 1000 mg 1500 mg Pooled N= 8 8 8 8 8 40 8 8 8 8 8 40 At least one 3 (38%) 2 (25%) 3 (38%) 3 (38%) 0 11 (28%) 2 (25%) 5 (63%) 5 (63%) 2 (25%) 1 (13%) 14 (35%) TEAE At least one 0 0 0 0 0 0 0 0 0 0 0 0 TESAE At least one 1 2 3 Treatment- 2 (25%) 0 0 0 0 2 (5%) 0 2 (25%) 0 1 (13%) 0 3 (8%) related AE At least one 0 0 0 0 0 0 0 0 0 0 0 0 Grade 3/4 TEAE © 2025 Spyre Therapeutics, Inc. 1 2 3 Interim analysis as of May 30, 2025 data cutoff. Headache and migraine. Chest tightness; Gastroenteritis, rash, and abdominal pain in one subject. Chest tightness. 14 All rights reserved.

SPY002 and SPY072 demonstrated prolonged and dose proportional PK SPY002 mean PK profiles SPY072 mean PK profiles ~75-day t Pending t 1/2 1/2 Waiting for comparable follow-up to accurately estimate half-life © 2025 Spyre Therapeutics, Inc. Interim analysis as of May 30, 2025 data cutoff. 15 All rights reserved.

SPY072 showed comparable PK to SPY002 at clinically relevant doses through available follow up SPY002 & SPY072 mean PK profiles (IV) SPY002 & SPY072 mean PK profiles (SC) 1000 mg IV (N=6) 300 mg SC (N=6) Remove 1500 SPY072 Pending t Pending t SPY072 300 mg IV (N=6) 1/2 100 mg SC (N=6) 1/2 1000 mg IV (N=6) 300 mg SC (N=6) ~75-day t SPY002 SPY002 ~75-day t 1/2 300 mg IV (N=6) 1/2 100 mg SC (N=6) © 2025 Spyre Therapeutics, Inc. Interim analysis as of May 30, 2025 data cutoff. 16 All rights reserved.

SPY002 and SPY072 suppressed free TL1A through 20- weeks of follow-up at the lowest dose tested SPY002 median free sTL1A PD profiles SPY072 median free sTL1A PD profiles © 2025 Spyre Therapeutics, Inc. Interim analysis as of May 30, 2025 data cutoff. Median values below LLOQ of 8pg/mL plotted as one-half of LLOQ per convention. 17 All rights reserved.
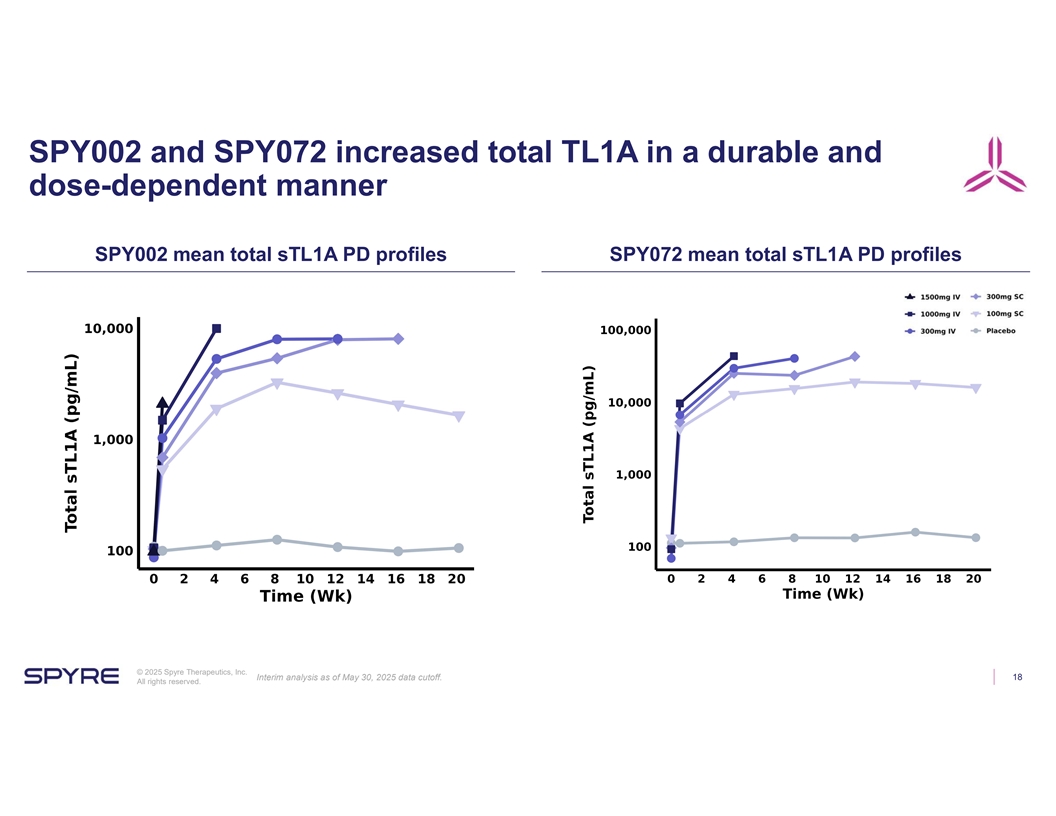
SPY002 and SPY072 increased total TL1A in a durable and dose-dependent manner SPY002 mean total sTL1A PD profiles SPY072 mean total sTL1A PD profiles © 2025 Spyre Therapeutics, Inc. Interim analysis as of May 30, 2025 data cutoff. 18 All rights reserved.

Interim PK supports quarterly or twice annual dosing Afimkibart Ph2 UC results SPY002 simulated concentration-time profile SPY002 Q3M 50 mg Q4W Induction Maintenance Clinical remission by mMS SPY002 Q6M 150 mg Q4W % 2-4x SC maintenance injections per year 450 mg Q4W 50 39% 40 38% 36% Afimkibart simulated 450mg C trough 31% 30% 29% 30 Afimkibart simulated 150mg C trough 20 Afimkibart simulated 50mg C trough 10 0 SPY002 Q3M SC Week 14 Week 56 N=46 N=29 N=28 N=42 N=26 N=28 SPY002 Q6M SC Afimkibart QM SC © 2025 Spyre Therapeutics, Inc. Simulations based on interim analysis as of May 30, 2025 data cutoff. 19 All rights reserved. mMS = Modified Mayo Score. Danese, Silvio, et al. Official journal of the American College of Gastroenterology | ACG 119.10S (2024).

SPY002 and SPY072 Phase 1 interim results summary xx Favorable safety profile across all dose levels Well-tolerated Extended half-lives support potential for quarterly or twice annual xx Prolonged PK exposure dosing Rapid & sustained target xx PD was durable through 20-weeks at the lowest dose tested engagement No apparent xx No apparent impact of ADAs on PK or PD through available follow up immunogenicity impact SPY002 expected to advance to the SKYLINE-UC study and SPY072 expected to advance to SKYWAY-RD study © 2025 Spyre Therapeutics, Inc. Interim analysis as of May 30, 2025 data cutoff. 20 All rights reserved.

SKYLINE-UC Phase 2 study Today’s 1 2 3 Objectives Report Launch Unveil Anti-TL1A data SKYLINE-UC SKYWAY-RD Phase 1 results for two A Phase 2 platform study A Phase 2 basket study potential best-in-class in ulcerative colitis evaluating anti-TL1A in molecules evaluating three three rheumatologic monotherapies & three conditions combinations Deanna Nguyen SVP, Clinical Development © 2025 Spyre Therapeutics, Inc. 21 All rights reserved.

SKYLINE-UC: Phase 2 platform study evaluating three monotherapies and three combinations in UC Part A: Open-label monotherapy evaluation (N=~100) KEY ENDPOINTS OBJECTIVES UC mMS 5-9 INDUCTION MAINTENANCE P Monotherapy POC Change in RHI at W12 SPY001 (α4β7) Sequential S activation SPY002 (TL1A) Clinical remission at W12 after Ph1 SPY003 (IL-23) S Endoscopic improvement at W12 W12 W48 S Change in mMS at W12 P Part B: PBO-controlled factorial combination evaluation (N=~500) INDUCTION MAINTENANCE UC mMS 5-9 SPY001 (α4β7) Monotherapy dose optimization P Clinical remission at W12 Seamless SPY002 (TL1A) enrollment after Part A S Combination POC Endoscopic improvement at W12 SPY003 (IL-23) S Contribution of components Clinical response at W12 SPY120 (α4β7+TL1A) S Histological improvement at W12 SPY130 (α4β7+IL-23) S SPY230 (TL1A+IL-23) HEMI at W12 Placebo S Clinical remission at W48 W12 W48 P © 2025 Spyre Therapeutics, Inc. mMS=modified mayo score; RHI=Robarts Histopathology Index; HEMI=Histo-Endoscopic Mucosal Improvement; P=primary endpoint; S=secondary endpoint 22 All rights reserved. Initiation of each cohort subject to regulatory clearance.

Capital-efficient trial that is designed to be attractive to patients and investigators CAPTIAL EFFICIENT PATIENT CENTRIC STREAMLINED UNIFIED DOSING PART A PART B INDUCTION MAINTENANCE 6 actives 40% vs. Open-label 1 placebo Cost savings and reduced sample size compared to individual Ph2 studies for each program © 2025 Spyre Therapeutics, Inc. 23 All rights reserved. Placebo Combos Monos

Spyre’s portfolio uniquely enables product profiles with potential superior efficacy and convenience ILLUSTRATIVE 80 60 Potential to break the efficacy ceiling with 40 SPY120 SPY130 SPY230 combinations JNJ’4804 TNF+IL-23 combo Potential for best-in-class 1 (VEGA ) efficacy with higher exposures SPY001 SPY002 SPY003 20 Standard of care IBD biologics Potential for Q3M & Q6M dosing profiles 0 1 2 3 4 5 6 Maintenance dosing interval (months) 1 VEGA trial was not pbo-controlled. Positioning of Spyre programs is illustrative and based on Phase 1 results for SPY001 and SPY002 only and illustrates what we believe we can © 2025 Spyre Therapeutics, Inc. potentially achieve. Placebo-adjusted clinical remission data are derived from different clinical trials conducted at different times, with differences in trial design and patient populations. As a 24 All rights reserved. result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. Pbo-adjusted clinical remission (%)

Co-administration of mAbs can reduce immunogenicity whereas bispecific formats can increase immunogenicity ADA rates for TNF or TL1A bsAbs vs. coformulations Case studies Asset Target Immunogenicity Population Structure IL-23 dimer JNJ-4804 coformulation TNF trimer guselkumab Gol golimumab + Separate Gus golimumab guselkumab TNFα + IL-23 PHASE 2 UC 6% human IgG1 mAbs (JNJ-4804) IgG-fynomer … Combination therapy appeared to JNJ-3539 TNFα / IL-17 DISCONTINUED HVs 93% (2x2) decrease the incidence of antibodies to No impact on drug exposure golimumab … Time Duobody JNJ-8104 TNFα / IL-17 DISCONTINUED HVs 100% (1x1) TL1A trimer AMG-966 bispecific TNF trimer AMG-966 Dual variable domain ABT-122 TNFα / IL-17 DISCONTINUED HVs 99% (2x2) AMG-966 Four unique heavy ADAs AMG-966 TNFα / TL1A DISCONTINUED HVs and light chain pairs 98% (1x1) IgG-scFv DISCONTINUED MEDI-7352 TNFα / NGF OA 72% (2x2) … We demonstrate here that AMG966 forms large immune complexes ... which drive an antibody response and resulted Loss of drug exposure RO7837195 CrossmAb TL1A / p40 PHASE 2 100% HVs (PF-07261271) (1x1) in loss of exposures … Time © 2025 Spyre Therapeutics, Inc. Clin Pharmacol Ther. 2017;101(S1):S81; J. of Clin Pharmacol. 2019; 59(7): 968-978; J. of Clin Pharmacol. 2018; 58(6): 803-813; Front Immunol. 2021 Dec 14:12:782788; Shao et al. Clin 25 All rights reserved. Pharmacol Ther. 2024 Jun;115(6):1418-1427. doi: 10.1002/cpt.3235. OA=osteoarthritis; UC=ulcerative colitis; HV=healthy volunteers. bsAbs Combo Exposure Exposure

SKYLINE-UC: First patients in screening and on-track for expected 6 POC readouts through 2027 2H 2025 2026 2027 6 POC readouts 3 POC readouts Phase 1 q SPY003 Ph1 ü q Ph2 Initiation Part A monotherapy results Part B monotherapy results q SPY001 (α4β7) open-label POCq SPY001 (α4β7) pbo-controlled data Phase 2 platform study q SPY002 (TL1A) open-label POCq SPY002 (TL1A) pbo-controlled data q SPY003 (IL-23) open-label POCq SPY003 (IL-23) pbo-controlled data Part B combination results q SPY120 (α4β7+TL1A) POC q SPY130 (α4β7+IL-23) POC q SPY230 (TL1A+IL-23) POC q Ph2 Initiation Ph2 results q SPY072 (TL1A) RA POC Phase 2 basket study q SPY072 (TL1A) PsA POC q SPY072 (TL1A) axSpA POC © 2025 Spyre Therapeutics, Inc. Milestones expected as of the date of this presentation. 26 All rights reserved.

SKYWAY-RD Phase 2 study Today’s 1 2 3 Objectives Report Launch Unveil Anti-TL1A data SKYLINE-UC SKYWAY-RD Phase 1 results for two A Phase 2 platform study A Phase 2 basket study potential best-in-class in ulcerative colitis evaluating anti-TL1A in molecules evaluating three three rheumatologic monotherapies & three conditions combinations Josh Friedman SVP, Clinical Development © 2025 Spyre Therapeutics, Inc. 27 All rights reserved.

SKYWAY-RD: Phase 2 basket study evaluating SPY072 (anti-TL1A) in RA, PsA, and axSpA Sub-study A: SPY072 in moderate-to-severely active rheumatoid arthritis (RA) DOUBLE-BLIND OPEN-LABEL FOLLOW UP KEY ENDPOINTS KEY INCLUSION SPY072 Dose A P Swollen joint count ≥ 4 Change in DAS28-CRP at W12 RA 1:1:1 S Tender joint count ≥ 4 Proportion ACR20 at W12 N=120 SPY072 Dose B RF+, ACPA+, or bony erosions E ACR50/70 at W12 Placebo IR to cs/b/tsDMARDs W12 W36 Sub-study B: SPY072 in moderate-to-severely active psoriatic arthritis (PsA) SPY072 Dose A Tender joint count ≥ 3/68 P Proportion ACR20 at W16 PsA 2:1 S Swollen joint count ≥ 3/66 Change in DAPSA at W16 Placebo N=90 E Active PsO lesion or history ACR50/70 at W16 W16 W40 IR to NSAIDs or cs/b/tsDMARDs Sub-study C: SPY072 in moderate-to-severely active axial spondyloarthritis (axSpA) Radiographic / non-radiographic P Change in ASDAS at W16 SPY072 Dose A axSpA 2:1 S BASDAI ≥ 4 & back pain ≥ 4 Proportion ASAS40 at W16 Placebo N=75 IR to NSAIDs or b/tsDMARDs W16 W40 © 2025 Spyre Therapeutics, Inc. RF=rheumatoid factor; ACPA= Anti-citrullinated protein antibodies; cs/b/tsDMARD=conventional synthetic, biologic, or targeted synthetic disease modifying antirheumatic drugs; 28 All rights reserved. BASDAI=Bath Ankylosing Spondylitis Disease Activity Index; P=primary endpoint; S=secondary endpoint; E=exploratory endpoint. Pending regulatory feedback.
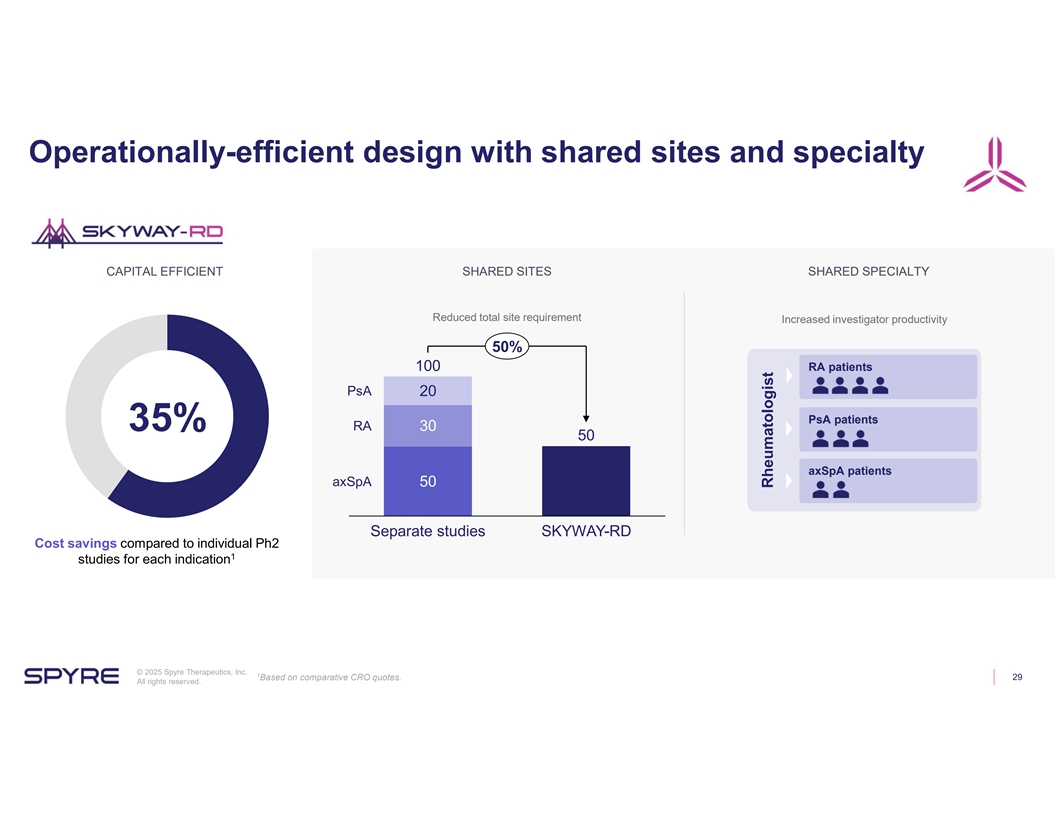
Operationally-efficient design with shared sites and specialty CAPITAL EFFICIENT SHARED SITES SHARED SPECIALTY Reduced total site requirement Increased investigator productivity 50% 100 RA patients PsA 20 PsA patients RA 30 35% 50 axSpA patients axSpA 50 Separate studies SKYWAY-RD Cost savings compared to individual Ph2 1 studies for each indication © 2025 Spyre Therapeutics, Inc. 1 Based on comparative CRO quotes. 29 All rights reserved. Rheumatologist

TL1A is upregulated in RA, PsA, and axSpA relative to healthy controls Axial spondyloarthritis Rheumatoid arthritis Psoriatic arthritis 800 ✱✱✱✱ ✱✱ ✱✱✱ ✱✱ 600 400 200 0 Healthy axSpA Control Source Whole blood Skin biopsy Whole blood Sequencing Microarray Bulk RNA seq Microarray Sample size N=192 RA, 30 HC N=9 per cohort N=52 axSpa, 20 HC Spyre analysis of RA-MAP, Li, Sifang, et al. BMC Musculoskeletal Disorders 25.1 (2024), and Johnsson, Hanna, et al. Arthritis Research & Therapy. © 2025 Spyre Therapeutics, Inc. ** P≤0.01, *** P ≤0.001, **** P ≤0.0001. Unpaired tailed t-test used for RA and axSpA analysis, One-way ANOVA used in PsA. 30 All rights reserved. RA=rheumatoid arthritis; PsA=psoriatic arthritis; axSpA=axial spondyloarthritis; HC=healthy controls. TNFSF15 Counts TNFSF15 Counts

Spyre anti-TL1A antibody met or exceeded the efficacy of etanercept (anti-TNF) in rat models of arthritis Superior efficacy vs. anti-TNF in semi-preventative model Comparable efficacy vs. anti-TNF in therapeutic model Collagen Treatment period Collagen Treatment period * * * * * © 2025 Spyre Therapeutics, Inc. * P< 0.0001 vs. isotype control; 2-way ANOVA using Dunnett's correction for multiple comparisons. 31 All rights reserved. Disease improvement Arthritis score Arthritis score

SPY072 is a potential first-in-class therapy for rheumatologic conditions with anticipated Q3M-Q6M dosing ILLUSTRATIVE 60 50 40 axSpA Potential for novel MOA with comparable-to-better PsA efficacy and improved SC dosing frequency (Q3M-Q6M) 30 RA 20 Today’s SOC 10 0 1 2 3 4 5 6 Dosing interval (months) © 2025 Spyre Therapeutics, Inc. Positioning of Spyre programs is illustrative and based on Phase 1 interim results. Placebo-adjusted clinical data are derived from different clinical trials conducted at different times, with 32 All rights reserved. differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. Pbo-adjusted ACR20 / ASAS20 (%)

SKYWAY-RD: 3 POC readouts expected through 2026 2H 2025 2026 2027 6 POC readouts 3 POC readouts Phase 1 q SPY003 Ph1 ü q Ph2 Initiation Part A monotherapy results Part B monotherapy results q SPY001 (α4β7) open-label POCq SPY001 (α4β7) pbo-controlled data Phase 2 platform study q SPY002 (TL1A) open-label POCq SPY002 (TL1A) pbo-controlled data q SPY003 (IL-23) open-label POCq SPY003 (IL-23) pbo-controlled data Part B combination results q SPY120 (α4β7+TL1A) POC q SPY130 (α4β7+IL-23) POC q SPY230 (TL1A+IL-23) POC q Ph2 Initiation Ph2 results q SPY072 (TL1A) RA POC Phase 2 basket study q SPY072 (TL1A) PsA POC q SPY072 (TL1A) axSpA POC © 2025 Spyre Therapeutics, Inc. Milestones expected as of the date of this presentation. 33 All rights reserved.

Closing and Q&A Today’s Spokespeople Cameron Turtle Josh Friedman Deanna Nguyen Sheldon Sloan Scott Burrows Chief Executive Officer Chief Financial Officer SVP, Clinical SVP, Clinical Chief Medical Officer Development Development © 2025 Spyre Therapeutics, Inc. 34 All rights reserved.

Anticipated 9 proof-of-concept readouts in 2026-27 for the treatment of IBD & beyond 1 $565M cash 2H 2025 2026 2027 Runway into 2H 2028 6 POC readouts 3 POC readouts Phase 1 q SPY003 Ph1 ü q Ph2 Initiation Part A monotherapy results Part B monotherapy results q SPY001 (α4β7) open-label POCq SPY001 (α4β7) pbo-controlled data Phase 2 platform study q SPY002 (TL1A) open-label POCq SPY002 (TL1A) pbo-controlled data q SPY003 (IL-23) open-label POCq SPY003 (IL-23) pbo-controlled data Part B combination results q SPY120 (α4β7+TL1A) POC q SPY130 (α4β7+IL-23) POC q SPY230 (TL1A+IL-23) POC q Ph2 Initiation Ph2 results q SPY072 (TL1A) RA POC Phase 2 basket study q SPY072 (TL1A) PsA POC q SPY072 (TL1A) axSpA POC © 2025 Spyre Therapeutics, Inc. 1 Milestones expected as of the date of this presentation. Cash includes cash, cash equivalents, & marketable securities as of 3/31/25. 35 All rights reserved.

Thank you Engineering for new heights in the treatment of IBD and beyond