UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): May 20, 2025

VIRIDIAN THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-36483 | 47-1187261 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 221 Crescent Street, Suite 103A Waltham, MA |
02453 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (617) 272-4600
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange on which registered |
||
| Common Stock, $0.01 par value | VRDN | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On May 20, 2025, Viridian Therapeutics, Inc. (the “Company”) issued a press release announcing long-term durability data from the THRIVE phase 3 clinical trial of veligrotug, an intravenously delivered anti-insulin-like growth factor-1 receptor (IGF-1R) antibody, in patients with active thyroid eye disease, which the Company refers to as the THRIVE trial. The Company also began using a new THRIVE data presentation, which includes the long-term durability data.
A copy of the press release and the data presentation are furnished as Exhibit 99.1 and Exhibit 99.2, respectively, to this Current Report on Form 8-K and are incorporated herein by reference. The exhibits furnished under Item 7.01 of this Current Report on Form 8-K shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall they be deemed incorporated by reference in any filing under the Exchange Act or the Securities Act, regardless of any general incorporation language in such filing.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits.
| 99.1 |
Press Release, dated May 20, 2025 | |
| 99.2 |
Data Presentation, dated May 20, 2025 | |
| 104 |
Cover Page Interactive Data File (embedded within the Inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Viridian Therapeutics, Inc. | ||||||
| Date: May 20, 2025 | By: | /s/ Stephen Mahoney |
||||
| Stephen Mahoney | ||||||
| President and Chief Executive Officer | ||||||
Exhibit 99.1

Viridian Therapeutics Announces Positive Long-Term Durability Data from the Veligrotug
Phase 3 THRIVE Clinical Trial in Patients with Active Thyroid Eye Disease (TED)
- 70% of patients treated with veligrotug in THRIVE who were proptosis responders at week 15
maintained their response at week 52 -
- Veligrotug recently received Breakthrough Therapy Designation (BTD), supporting eligibility for Priority
Review; the BTD request was based on veligrotug’s (i) consistent and robust improvement and resolution
of diplopia in chronic TED, and (ii) rapid onset of proptosis response -
- Biologics License Application (BLA) submission for veligrotug is on track for second half 2025 -
- Actively preparing organization for planned U.S. commercial launch in 2026 -
Waltham, Mass., May 20, 2025 — Viridian Therapeutics, Inc. (Nasdaq: VRDN), a biopharmaceutical company focused on discovering, developing, and commercializing potential best-in-class medicines for serious and rare diseases, today announced positive long-term durability data from the THRIVE phase 3 clinical trial of veligrotug (“veli”), an intravenously delivered anti-insulin-like growth factor-1 receptor (IGF-1R) antibody, in patients with active thyroid eye disease (TED). TED is an autoimmune condition characterized by inflammation, growth, and damage to tissues around and behind the eye.
The THRIVE phase 3 clinical trial in active TED evaluated 5 infusions of veli or placebo every three weeks with primary topline analysis at week 15 and then followed patients through week 52.
Positive Veligrotug Durability at 52 Weeks
| • | 70% of veligrotug patients (21/30) in THRIVE, who were proptosis responders at week 15 and continued follow-up to the end of the study at week 52, maintained their proptosis response. Maintenance of response is defined as responders at week 15 who still had at least a 2-millimeter (mm) reduction in proptosis compared to baseline at week 52, without worsening in the fellow eye (≥2 mm increase), as measured by exophthalmometry. |
| • | There were no changes to the safety profile in the follow-up period. The vast majority of adverse events reported at the week 15 primary analysis had resolved by week 52. |
“We view the strength of today’s durability and safety resolution data as reinforcing veli’s strong and consistently robust clinical profile,” said Steve Mahoney, Viridian’s President and CEO. “We believe that the totality of veligrotug’s clinical data continues to demonstrate its potential to be the treatment-of-choice for patients living with TED. We believe these data, together with a streamlined dosing regimen of five infusions, position veli to become a market leading TED therapeutic, if approved. We continue to make great progress towards submitting the BLA in the second half of this year and preparing for a potential launch in 2026.”

Robust Veligrotug Topline Clinical Profile for Active and Chronic TED
As announced in late 2024, veligrotug met all of its primary and secondary endpoints and was generally well-tolerated in its pivotal phase 3 clinical trials, THRIVE and THRIVE-2, for active and chronic TED, respectively. Veligrotug demonstrated a rapid onset of treatment effect and statistically significant and clinically meaningful reduction and resolution of diplopia in both clinical trials. THRIVE-2 was the first data set from a global phase 3 clinical trial in chronic TED patients to demonstrate statistically significant diplopia response and resolution. Together, THRIVE and THRIVE-2 comprise the largest pivotal program to date in TED.
About Veligrotug
Veligrotug is an intravenously (IV) delivered, anti-insulin-like growth factor-1 receptor (IGF-1R) antibody in phase 3 development for thyroid eye disease, with the potential to be the IV treatment-of-choice for active and chronic TED patients. IGF-1R is a clinically and commercially validated target for thyroid eye disease (TED) with U.S. revenues of approximately $2 billion in 2024. Veligrotug has the potential to improve patient experience with a differentiated dosing regimen that features a shorter infusion time and fewer infusions compared to the currently approved and marketed IGF-1R inhibitor.
In its pivotal phase 3 clinical trials, THRIVE and THRIVE-2, veligrotug met all of its primary and secondary endpoints. Veligrotug demonstrated a rapid onset of treatment effect and statistically significant and clinically meaningful reduction and resolution of diplopia in both clinical trials. THRIVE-2 was the first demonstration in a global phase 3 clinical trial of a statistically significant diplopia response and resolution in chronic TED patients. Veligrotug was generally well tolerated.
Viridian believes that the robust veligrotug clinical profile has the potential to establish a strong position in the TED commercial market, if approved, and may help facilitate the introduction of VRDN-003, its potential best-in-class subcutaneous IGF-1R antibody for TED.
About Viridian Therapeutics
Viridian is a biopharmaceutical company focused on discovering, developing and commercializing potential best-in-class medicines for patients with serious and rare diseases. Viridian’s expertise in antibody discovery and protein engineering enables the development of differentiated therapeutic candidates for previously validated drug targets in commercially established disease areas.
Viridian is advancing multiple candidates in the clinic for the treatment of patients with thyroid eye disease (TED). The company is conducting a pivotal program for veligrotug (VRDN-001), including two global phase 3 clinical trials (THRIVE and THRIVE-2), to evaluate its efficacy and safety in patients with active and chronic TED. Both THRIVE and THRIVE-2 reported positive topline data, meeting all the primary and secondary endpoints of each study. Viridian is also advancing VRDN-003 as a potential best-in-class subcutaneous therapy for the treatment of TED, including two ongoing global phase 3 pivotal clinical trials, REVEAL-1 and REVEAL-2, to evaluate the efficacy and safety of VRDN-003 in patients with active and chronic TED.
In addition to its TED portfolio, Viridian is advancing a novel portfolio of neonatal Fc receptor (FcRn) inhibitors, including VRDN-006 and VRDN-008, which has the potential to be developed in multiple autoimmune diseases.
Viridian is based in Waltham, Massachusetts. For more information, please visit www.viridiantherapeutics.com. Follow Viridian on LinkedIn and X.

Forward Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of words such as, but not limited to, “anticipate,” “believe,” “become,” “continue,” “could,” “design,” “estimate,” “expect,” “intend,” “may,” “might,” “on track,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” or “would” or other similar terms or expressions that concern our expectations, plans and intentions. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations, and assumptions. Forward-looking statements include, without limitation, statements regarding: clinical development and anticipated commercialization of Viridian’s product candidates, including veligrotug (formerly VRDN-001) and VRDN-003; the potential utility, efficacy, potency, safety, clinical benefits, clinical response, convenience, and number of indications of veligrotug and VRDN-003, including Viridian’s view of the strength of the THRIVE durability and safety resolution data and veligrotug’s robust clinical profile; veligrotug’s potential to be the IV treatment-of-choice for active and chronic TED; the impact of Breakthrough Therapy Designation, including eligibility for Priority Review, or any other FDA designations; regulatory interactions and anticipated timing of regulatory submissions, including the anticipated BLA submission for veligrotug in the second half of 2025; potential market sizes and market opportunities for Viridian’s product candidates, including Viridian’s belief that veligrotug is positioned to become a market leading TED therapeutic, if approved; Viridian’s product candidates potentially being best-in-class; whether veligrotug will serve an unmet need; veligrotug’s potential to improve patient experience over existing therapies; and Viridian’s expectations regarding the potential commercialization of veligrotug and VRDN-003, if approved, including the potential U.S. launch of veligrotug in 2026.
New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Such forward-looking statements are subject to a number of material risks and uncertainties including but not limited to: potential utility, efficacy, potency, safety, clinical benefits, clinical response, and convenience of Viridian’s product candidates; that results or data from completed or ongoing clinical trials may not be representative of the results of ongoing or future clinical trials; that preliminary data may not be representative of final data; expectations and changes regarding the timing for regulatory filings; regulatory interactions; uncertainty and potential delays related to clinical drug development; the timing of and our ability to obtain and maintain regulatory approvals for our therapeutic candidates; competition from other therapies or products; estimates of market size; our future operating results and financial performance; Viridian’s intellectual property position; that our product candidates may not be commercially successful, if approved; and other risks described from time to time in the “Risk Factors” section of our filings with the Securities and Exchange Commission (SEC), including those described in our most recent Annual Report on Form 10-K or Quarterly Report on Form 10-Q, as applicable, and supplemented from time to time by our Current Reports on Form 8-K. Any forward-looking statement speaks only as of the date on which it was made. Neither the company, nor its affiliates, advisors, or representatives, undertake any obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. These forward-looking statements should not be relied upon as representing the company’s views as of any date subsequent to the date hereof.

Contact:
IR@viridiantherapeutics.com
Media@viridiantherapeutics.com
Source: Viridian Therapeutics, Inc.

THRIVE in Active TED 52-Week Follow-Up Update (May 20, 2025) Exhibit 99.2

This presentation contains forward-looking statements. These statements may be identified by the use of words such as, but not limited to, “anticipate,” “believe,” “become,” “continue,” “could,” “design,” “estimate,” “expect,” “intend,” “may,” “might,” “on track,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” or “would” or other similar terms or expressions that concern our expectations, plans and intentions. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations, and assumptions. Forward-looking statements include, without limitation, statements regarding: preclinical development, clinical development, and anticipated commercialization of Viridian’s product candidates, including veligrotug (formerly VRDN-001); the potential utility, efficacy, potency, safety, clinical benefits, clinical response, convenience and number of indications of veligrotug; Viridian’s expectations regarding the potential commercialization of veligrotug, if approved; veligrotug’s potential to transform the treatment for TED and to be the IV treatment-of-choice for active and chronic TED; potential market sizes and market opportunities, including for Viridian’s product candidates; and Viridian’s product candidates potentially being best-in-class. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Such forward-looking statements are subject to a number of material risks and uncertainties including but not limited to: potential utility, efficacy, potency, safety, clinical benefits, clinical response, and convenience of Viridian’s product candidates; that results or data from completed or ongoing clinical trials may not be representative of the results of ongoing or future clinical trials; that preliminary data may not be representative of final data; the timing, progress, and plans for our ongoing or future research, preclinical and clinical development programs; changes to trial protocols for ongoing or new clinical trials; expectations and changes regarding the timing for regulatory filings; regulatory interactions; expectations and changes regarding the timing for enrollment and data; uncertainty and potential delays related to clinical drug development; the duration and impact of regulatory delays in our clinical programs; the timing of and our ability to obtain and maintain regulatory approvals for our therapeutic candidates; manufacturing risks; competition from other therapies or products; estimates of market size; other matters that could affect the sufficiency of existing cash, cash equivalents, and short-term investments to fund operations; our future operating results and financial performance; Viridian’s intellectual property position; the timing of preclinical and clinical trial activities and reporting results from the same; and those risks described from time to time under the caption “Risk Factors” in our filings with the Securities and Exchange Commission (SEC), including those described in our most recent Annual Report on Form 10-K or Quarterly Report on Form 10-Q, as applicable, and supplemented from time to time by our Current Reports on Form 8-K. The forward-looking statements in this presentation represent our views as of the date of this presentation. Neither we, nor our affiliates, advisors, or representatives, undertake any obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Trademarks used herein are the property of their respective owners. Cautionary note regarding forward-looking statements

Achieved all primary and secondary endpoints with high level of statistical significance (p < 0.0001) Source: Viridian THRIVE week 15 topline data on file (interim topline database lock) & week 52 data on file (final database lock). AE = adverse event, IGF-1R = insulin-like growth factor-1 receptor, SAE = serious adverse event, TED = thyroid eye disease. THRIVE: Veligrotug showed robust and consistent clinical activity in active TED patients Rapid onset of treatment effect in as few as 3 weeks Generally well-tolerated, with no treatment-related SAEs and low (5.5%) placebo-adjusted rate of hearing impairment AEs at week 15; consistent safety profile through week 52 Demonstrated strong durability of proptosis response: 70% of topline proptosis responders maintained response at week 52 (Active TED)
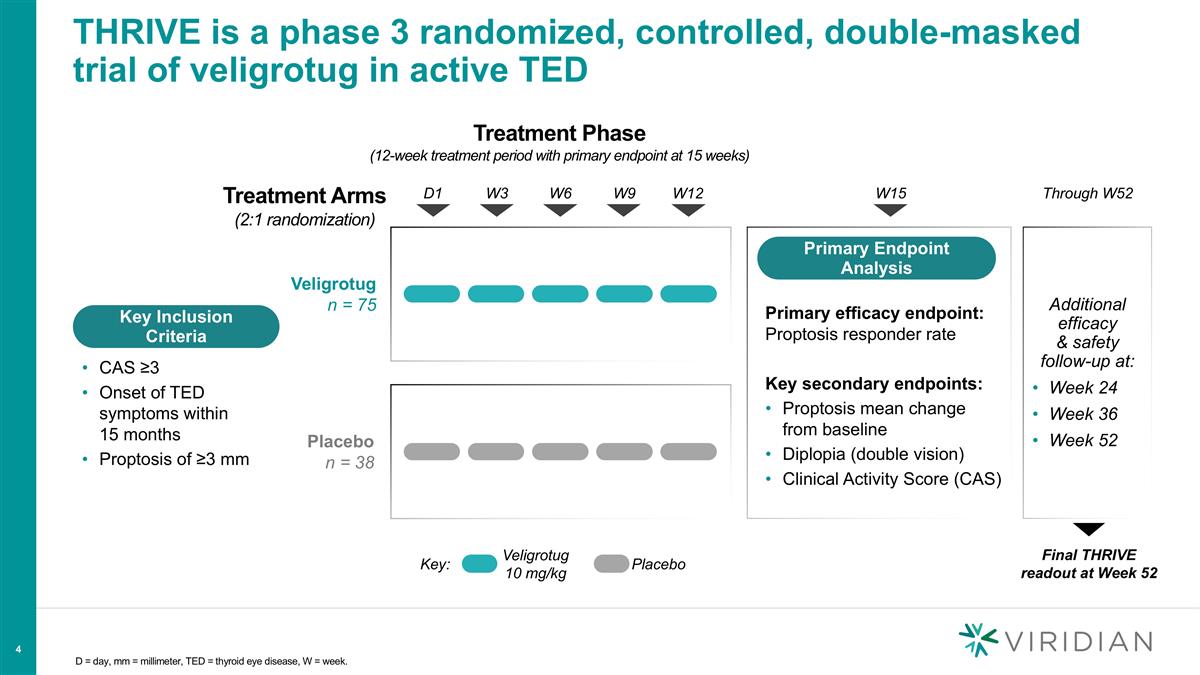
D = day, mm = millimeter, TED = thyroid eye disease, W = week. THRIVE is a phase 3 randomized, controlled, double-masked trial of veligrotug in active TED Treatment Phase (12-week treatment period with primary endpoint at 15 weeks) Veligrotug n = 75 D1 W3 W6 W9 W12 Placebo n = 38 Key: Veligrotug 10 mg/kg Placebo W15 Treatment Arms (2:1 randomization) Through W52 Additional efficacy & safety follow-up at: Week 24 Week 36 Week 52 Primary efficacy endpoint: Proptosis responder rate Key secondary endpoints: Proptosis mean change from baseline Diplopia (double vision) Clinical Activity Score (CAS) Primary Endpoint Analysis Final THRIVE readout at Week 52 Key Inclusion Criteria CAS ≥3 Onset of TED symptoms within 15 months Proptosis of ≥3 mm

7.8 Veligrotug (n = 75) Placebo (n = 38) Participant Demographics Age in years, mean (SD) 48.9 (12.4) 49.1 (12.5) Female sex, n (%) 56 (75%) 31 (82%) White race, n (%) 51 (68%) 19 (50%) Disease Characteristics Months since TED onset, mean (SD) 7.9 (3.7) 7.2 (3.8) Baseline proptosis by exophthalmometry (mm), mean (SD) 23.2 (3.1) 23.2 (3.3) Baseline CAS, mean (SD) 4.5 (1.0) 4.8 (1.1) Participants with diplopia, n (%) 50 (67%) 26 (68%) Diplopia (Gorman Score), mean (SD)1 2.0 (0.8) 2.0 (0.7) Source: Viridian THRIVE week 15 topline data on file (interim topline database lock). Note: all proptosis & CAS reported values and endpoints in the data analysis are based on study eye (defined as eye with greater proptosis at baseline). 1 Of patients with diplopia at baseline. CAS = clinical activity score, mm = millimeter, SD = standard deviation, TED = thyroid eye disease. THRIVE baseline characteristics were well-balanced between active and placebo arms

Veligrotug (n=75) Placebo (n=38) p-value Proptosis Primary Endpoint: Proptosis responder rate (exophthalmometry)1 70% 5% p < 0.0001 Proptosis mean change from baseline (exophthalmometry) -2.89 mm -0.48 mm p < 0.0001 Diplopia Diplopia complete resolution2 54% 12% p < 0.0001 Diplopia responder rate3 63% 20% p < 0.0001 CAS Clinical activity score (CAS) 0 or 1 64% 18% p < 0.0001 CAS mean change from baseline -3.4 -1.7 p < 0.0001 Overall Response Overall responder rate (ORR)4 67% 5% p < 0.0001 Source: Viridian THRIVE week 15 topline data on file (interim topline database lock). 1 Percentage of participants with ≥2 mm reduction in proptosis from baseline in the study eye, without deterioration in the fellow eye (≥2 mm increase), 2 Percentage of participants with baseline diplopia (Gorman Score >0) and a score of 0 at Week 15, 3 Percentage of participants achieving a reduction of at least 1 on the Gorman subjective diplopia scale at week 15, among patients with diplopia at baseline, 4 Percentage of participants with ≥2 mm reduction in proptosis AND ≥2-point reduction in CAS from baseline in the study eye, without corresponding deterioration [≥2 mm/point increase] in proptosis or CAS in the fellow eye. CAS = clinical activity score. THRIVE achieved high level of statistical significance across all primary and secondary endpoints at 15 weeks
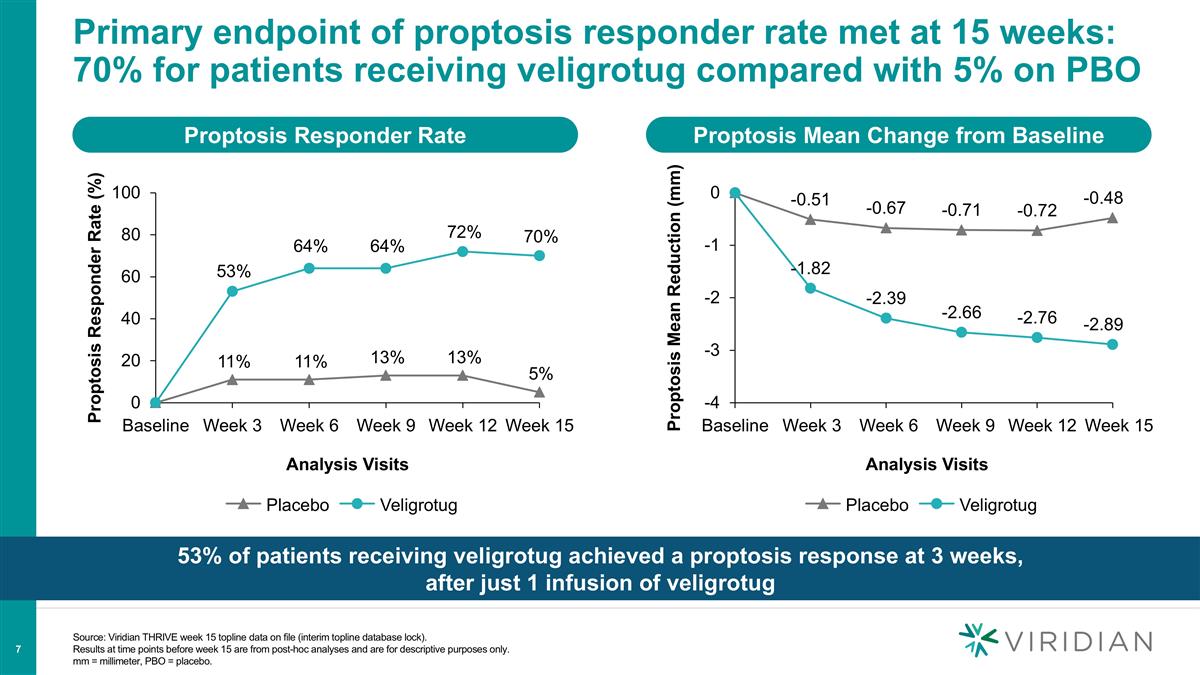
Source: Viridian THRIVE week 15 topline data on file (interim topline database lock). Results at time points before week 15 are from post-hoc analyses and are for descriptive purposes only. mm = millimeter, PBO = placebo. Primary endpoint of proptosis responder rate met at 15 weeks: 70% for patients receiving veligrotug compared with 5% on PBO 53% of patients receiving veligrotug achieved a proptosis response at 3 weeks, after just 1 infusion of veligrotug Proptosis Responder Rate Proptosis Mean Change from Baseline Proptosis Responder Rate (%) Proptosis Mean Reduction (mm) 53% 64% 64% 72% 70% Analysis Visits Veligrotug Analysis Visits Veligrotug

Source: Viridian THRIVE week 15 topline data on file (interim topline database lock). Results at time points before week 15 are from post-hoc analyses and are for descriptive purposes only. CAS = clinical activity score. Majority of patients receiving veligrotug had complete resolution of diplopia and minimal disease activity (CAS) at week 15 Diplopia Complete Resolution CAS Score 0 or 1 Analysis Visits Diplopia Resolution Rate (%) Veligrotug 26% 32% 54% Diplopia Responder Rate at Week 15 63% 20% Diplopia Responder Rate at Week 15 7% 25% 46% 24% 20% 64% 18% 64% Analysis Visits CAS 0 or 1 Rate (%) Veligrotug

Source: Viridian THRIVE week 15 topline data on file (interim topline database lock). CT = computed tomography, IGF-1R = insulin-like growth factor-1 receptor, mm = millimeter, MRI = magnetic resonance imaging. THRIVE demonstrated consistency between Hertel and MRI / CT and validates both as reliable tools for measurements of proptosis Veligrotug (n=75) Placebo (n=38) Proptosis responder rate at week 15 70% 5% Proptosis mean change from baseline at week 15 -2.89 mm -0.48 mm Veligrotug (n=75) Placebo (n=38) Proptosis responder rate at week 15 69% 9% Proptosis mean change from baseline at week 15 -2.91 mm -0.58 mm Hertel Exophthalmometry MRI / CT

Source: Viridian THRIVE week 15 topline data on file (interim topline database lock). 1 6 unrelated SAEs in 4 participants: cellulitis, appendicitis, dyspnoea, hyperthyroidism, aortic dissection (planned surgery for known Type B aortic dissection), depression (diagnosed prior to 1st dose); Includes multiple terms aggregated using standard sets of MedDRA terms. AE = adverse event, MedDRA= medical dictionary for regulatory activities, SAE = serious adverse event, TEAE = treatment-emergent adverse event. Veligrotug was generally well-tolerated at week 15, with no treatment-related SAEs, and 96% of veligrotug-treated patients completed all doses Veligrotug N=75 n (%) Placebo N=38 n (%) Participants with any treatment-emergent adverse event (TEAE) 66 (88%) 24 (63%) Participants with any serious AE (SAE) 4 (5%)1 0 Participants with any treatment-related TEAE 53 (71%) 9 (24%) Participants with any treatment-related SAE 0 0 Vast majority of TEAEs in both arms were mild Low treatment discontinuation rate 4% in veligrotug arm No treatment-related SAEs

Source: Viridian THRIVE week 15 topline data on file (interim topline database lock). 1 Includes multiple terms aggregated using standard sets of MedDRA terms, 2 Reported as percentage of menstruating women. AE = adverse event, MedDRA= medical dictionary for regulatory activities. Veligrotug was generally well-tolerated at week 15, with a 5.5% placebo-adjusted rate of hearing impairment AEs AEs occurring at ≥10% frequency in either arm Veligrotug N=75 n (%) Placebo N=38 n (%) Muscle spasms 32 (43%) 2 (5%) Headache 16 (21%) 5 (13%) Infusion related reaction (IRR) 13 (17%) 1 (3%) Hearing impairment1 12 (16%) 4 (11%) Hyperglycemia1 11 (15%) 2 (5%) Fatigue1 10 (13%) 6 (16%) Nausea 10 (13%) 3 (8%) Ear discomfort 9 (12%) 1 (3%) Diarrhea 8 (11%) 1 (3%) Alopecia 6 (8%) 4 (11%) Menstrual disorders1,2 8 / 34 (24%) 1 / 12 (8%)

Source: Viridian THRIVE week 52 data on file (final database lock). 1 Responders at week 15 who still had at least a 2-millimeter (mm) reduction in proptosis compared to baseline at week 52, without worsening in the fellow eye (≥2 mm increase), as measured by exophthalmometry. Methodology is the same as the teprotumumab durability reported in its U.S. Prescribing Information. No changes to veli’s safety profile during the follow-up period Vast majority of adverse events reported at topline resolved by Week 52 70% of proptosis responders in THRIVE maintained response at Week 52 in long-term follow up Proptosis Durability 70% (21/30 participants) of Week 15 proptosis responders maintained a proptosis response at Week 521 Safety Resolution

Appendix

Source: TEPEZZA U.S. Prescribing Information. Cross-trial comparisons should be avoided due to the lack of head-to-head clinical trial data. 1 Responders at week 24 who still had at least a 2-millimeter (mm) reduction in proptosis compared to baseline at week 52 Tepezza Prescribing Information shows 53% of proptosis responders maintained their response Tepezza Durability (Label) 53% (16/30 participants) of Week 24 proptosis responders maintained a proptosis response at Week 721