UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 31, 2025
Vaxcyte, Inc.
(Exact name of Registrant as Specified in Its Charter)
| Delaware | 01-39323 | 46-4233385 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 825 Industrial Road | ||
| Suite 300 | ||
| San Carlos, California | 94070 | |
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s Telephone Number, Including Area Code: 650 837-0111
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
||
| Common Stock, $0.001 par value per share | PCVX | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On March 31, 2025, Vaxcyte, Inc. (the “Company”) issued a press release announcing positive topline results from its Phase 2 dose-finding study evaluating the safety, tolerability and immunogenicity of VAX-24, the Company’s 24-valent pneumococcal conjugate vaccine candidate designed to prevent invasive pneumococcal disease, in healthy infants. The Company also announced that VAX-XL, its third-generation PCV candidate, is in development. The press release is attached as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
Exhibit 99.1 shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any registration statement or other document filed under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
| Item 8.01 | Other Events. |
On March 31, 2025, the Company also made available the slide presentation attached as Exhibit 99.2 to this Current Report on Form 8-K, which is incorporated herein by reference.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit Number |
Description | |
| 99.1 | Press Release, dated March 31, 2025. | |
| 99.2 | Slide Presentation, dated March 31, 2025. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| VAXCYTE, INC. | ||||||
| Date: March 31, 2025 | By: | /s/ Andrew Guggenhime |
||||
| Andrew Guggenhime | ||||||
| President and Chief Financial Officer | ||||||
Exhibit 99.1

Vaxcyte Announces Positive Topline Results from VAX-24 Infant Phase 2 Dose-Finding Study
— At All Doses Evaluated, VAX-24 Was Well-Tolerated and Demonstrated a Safety and Tolerability Profile Similar to Prevnar 20® (PCV20) —
— At All Doses Evaluated, VAX-24 Elicited Substantial Immune Responses Following Primary Three-Dose Immunization Series; Topline Results Also Include Interim Booster Dose IgG Data Showing Robust Memory Responses Across All Doses —
— Dose-Dependent Immune Responses Consistently Demonstrated and Little to No Evidence of Carrier Suppression Was Observed, Supporting Platform’s Potential to Deliver Broadest-Spectrum Infant Pneumococcal Conjugate Vaccine (PCV) Candidates —
— Company Selects VAX-24 Mid Dose (2.2mcg) as Basis for Optimized Dose Formulation for Advancement to Potential Infant Phase 3 Program, Pending Topline VAX-31 Infant Phase 2 Study Readout —
— Company Announces VAX-XL, Third-Generation PCV Candidate Designed to Further Expand Spectrum of Coverage —
— Company to Host Webcast/Conference Call Today at 8:00 a.m. ET / 5:00 a.m. PT —
SAN CARLOS, Calif., March 31, 2025 – Vaxcyte, Inc. (Nasdaq: PCVX), a clinical-stage vaccine innovation company engineering high-fidelity vaccines to protect humankind from the consequences of bacterial diseases, today shared positive topline results from its Phase 2 dose-finding study evaluating the safety, tolerability and immunogenicity of VAX-24, the Company’s 24-valent pneumococcal conjugate vaccine (PCV) candidate designed to prevent invasive pneumococcal disease (IPD), compared to Prevnar 20® (PCV20) in healthy infants. Based on these findings, the Company has selected the VAX-24 Mid dose as the basis for advancement of an optimized dose formulation to a potential Phase 3 program and, pending the VAX-31 infant Phase 2 study topline data results anticipated in mid-2026, plans to initiate an infant Phase 3 study with either VAX-24 or VAX-31.
In this study, VAX-24 was well-tolerated and demonstrated a safety profile similar to PCV20 across all doses studied. Frequently reported local and systemic reactions were generally mild-to-moderate, resolving within several days of vaccination, with no meaningful differences observed across the cohorts. No serious adverse events were considered to be related to study vaccines.
All VAX-24 doses evaluated (Low: 1.1 mcg, Mid: 2.2mcg and Mixed: 2.2mcg/4.4mcg) elicited substantial immunoglobulin G (IgG) and opsonophagocytic assay (OPA) immune responses at 1-month post-dose 3 (primary immunization series).
| • | Post-dose 3, the VAX-24 Mid dose met target precedent Phase 2 non-inferiority (NI) criteria on relative seroconversion rates (lower limit of the 95% confidence interval for the difference between the proportion of participants achieving the pre-defined seroconversion rate IgG concentration ≥0.35 mcg/ml is > -15% for each serotype1), particularly for the highest circulating serotypes2 contained in VAX-24 and for 20 of 24 serotypes overall. The Mid dose also met the target Phase 2 IgG Geometric Mean Ratio (GMR) point estimate of >0.63 on all currently circulating serotypes contained in VAX-24 and for 22 of 24 serotypes overall. |

| • | Post-dose 3, VAX-24 generated robust OPA responses, which are correlated with effectiveness against IPD, across all serotypes and doses. |
| • | The four serotypes unique to VAX-24 elicited robust immune responses and met all target criteria across all endpoints at all doses evaluated post-dose 3. |
| • | Dose-dependent immune responses were consistently demonstrated at 1.1mcg, 2.2mcg and 4.4mcg doses and little to no carrier suppression was observed. |
Full post-dose 4 booster data is expected by the end of 2025. An interim assessment of the IgG results was performed with currently available study samples and demonstrate:
| • | The Mid dose met the Company’s historical target Phase 2 IgG GMR point estimate of >0.6 for the highest circulating serotypes contained in VAX-24 and for 19 of 24 serotypes overall. |
| • | VAX-24 elicited robust memory responses across all doses for all serotypes. |
“Based on the strength of these data, we have selected the Mid dose as the basis of an optimized dose formulation to advance VAX-24 and, pending the VAX-31 Phase 2 dose-finding study topline data readout, plan to initiate a Phase 3 infant program with either VAX-24 or VAX-31,” said Grant Pickering, Chief Executive Officer and Co-Founder of Vaxcyte. “These results affirm the potential of our carrier-sparing platform to add coverage and maintain robust immune responses, reinforcing our confidence as we advance our PCVs into adult and infant Phase 3 programs. Building on this momentum, we are announcing VAX-XL, our third-generation PCV candidate designed to provide the broadest coverage PCV currently in development. I am incredibly proud of the entire Vaxcyte team for these achievements.”
“Despite current vaccination efforts, Streptococcus pneumoniae is the leading cause of vaccine-preventable deaths globally in children under five. Today’s results reinforce our commitment to advancing the broadest-spectrum PCVs to address the substantial invasive pneumococcal disease burden in the infant population, helping to reduce transmission and strengthen community immunity against the consequences of this devastating bacteria,” said Jim Wassil, Executive Vice President and Chief Operating Officer of Vaxcyte. “We continue to make significant progress across our PCVs, and for the infant indication, the complete VAX-24 data set is expected by the end of the year and the VAX-31 Phase 2 dose-finding study topline data is expected in mid-2026, with the balance of booster data up to 9 months later. For the adult indication, the VAX-31 Phase 3 non-interiority study initiation is expected in mid-2025 with topline data in 2026. As always, we want to thank everyone involved in this study, especially the study participants and their families, trial investigators and sites.”
About the VAX-24 Infant Phase 2 Study
The VAX-24 infant Phase 2 clinical study is a randomized, observer-blind, dose-finding two-stage clinical study evaluating the safety, tolerability and immunogenicity of VAX-24 in healthy infants that enrolled 802 participants. The study remains ongoing to continue evaluating the immunogenicity of VAX-24 1-month post-dose 4 and safety through six months post-dose 4.
| • | Stage 1 of the study evaluated the safety and tolerability of a single injection of VAX-24 at three dose levels compared to Vaxneuvance® (PCV15), which was the broadest-spectrum PCV at the time of study initiation, in 48 infants. The 36 participants from the three VAX-24 cohorts in Stage 1 proceeded to Stage 2 of the study. |

| • | Stage 2 of the study is evaluating the safety, tolerability and immunogenicity of VAX-24 at the same three dose levels and compared to PCV20, currently the broadest-spectrum PCV available, in 789 infants. |
| • | The study design includes a primary immunization series consisting of three doses given at two months, four months and six months of age, followed by a subsequent booster dose at 12-15 months of age. Other routine pediatric vaccines could be administered according to the current recommended schedule. |
| • | The key immunogenicity study endpoints include an assessment of immune responses for each of the VAX-24 dose levels in comparison with PCV20 for the 20 common and 4 unique serotypes in VAX-24. At 1-month post-dose 3, immune responses were assessed based on serotype-specific IgG seroconversion rates (IgG threshold value of ≥0.35mcg/mL). IgG GMRs were assessed at 1-month post-dose 3 and post-dose 4, along with other key immunogenicity endpoints. |
| • | Additional information about the study can be found at www.clinicaltrials.gov under the identifier NCT05844423. |
Key Anticipated PCV Franchise Milestones
Vaxcyte is advancing the clinical development of its PCV programs with several anticipated key milestones, including:
PCV Franchise Adult Indication
VAX-31
| • | Following an FDA End-of-Phase 2 meeting, initiate a Phase 3 pivotal, non-inferiority study by mid-2025 and announce topline safety, tolerability and immunogenicity data in 2026. |
| • | Initiate the remaining Phase 3 studies in 2025 and 2026 and announce data from these studies in 2026 and 2027. |
PCV Franchise Infant Indication
The Company plans to initiate an infant Phase 3 program with either VAX-24 or VAX-31, pending the VAX-31 topline Phase 2 dose-finding study readout.
VAX-24
| • | Announce the balance of the VAX-24 Phase 2 dose-finding study data, including final safety data, full post-dose 3 OPA data, and full post-dose 4 IgG and OPA data, by end of 2025. |
VAX-31
| • | Announce topline safety, tolerability and immunogenicity data for Phase 2 dose-finding study primary three-dose immunization series in mid-2026, with complete booster data up to nine months later. |

Conference Call and Webcast
Vaxcyte will hold a webcast and conference call today, March 31 at 8:00 a.m. ET to discuss the results from the VAX-24 infant Phase 2 study. To participate in the conference call, please dial 800-445-7795 (domestic) or 785-424-1699 (international) and refer to conference ID PCVX0331. A live webcast of the conference call will also be available on the investor relations page of the Vaxcyte corporate website at www.vaxcyte.com. After the live webcast, the event will remain archived on the Vaxcyte website for 30 days.
About Pneumococcal Disease
Pneumococcal disease (PD) is an infection caused by Streptococcus pneumoniae bacteria. It can result in invasive pneumococcal disease (IPD), including meningitis and bacteremia, and non-invasive PD, including pneumonia, otitis media and sinusitis. In the United States, pneumococcal pneumonia is estimated to result in approximately 150,000 hospitalizations each year. Streptococcus pneumoniae is among the World Health Organization’s top antibiotic-resistant pathogens to be urgently addressed, and the U.S. CDC lists drug-resistant Streptococcus pneumoniae as a “serious threat.” Streptococcus pneumoniae is the leading cause of vaccine-preventable deaths in children under five globally. Pneumococci also cause over 50% of all cases of bacterial meningitis in the United States. Antibiotics are used to treat PD, but some strains of the bacteria have developed resistance to treatments. The morbidity and mortality due to PD are significant, particularly for young children and older adults, underscoring the need for a broader-spectrum vaccine.
About VAX-24
VAX-24, a 24-valent PCV candidate currently being evaluated in a Phase 2 infant clinical program, is designed to prevent IPD, which is especially serious in infants, young children, older adults and those with immune deficiencies or certain chronic health conditions. IPD is associated with high case-fatality rates, antibiotic resistance and meningitis. VAX-24 has the potential to cover more serotypes than any infant pneumococcal vaccine on-market today and provide protection against both currently circulating and historically prevalent serotypes.
About Vaxcyte
Vaxcyte is a vaccine innovation company engineering high-fidelity vaccines to protect humankind from the consequences of bacterial diseases. The Company is developing broad-spectrum conjugate and novel protein vaccines to prevent or treat bacterial infectious diseases. VAX-31, a 31-valent PCV candidate advancing to a Phase 3 adult clinical program and currently being evaluated in a Phase 2 infant clinical program, is being developed for the prevention of IPD in adults and infants and is the broadest-spectrum PCV candidate in the clinic today. VAX-24, the Company’s 24-valent PCV candidate, is designed to cover more serotypes than any infant PCV on-market and is currently being evaluated in a Phase 2 infant study. Both VAX-31 and VAX-24 are designed to improve upon the standard-of-care PCVs by covering the serotypes in circulation that are responsible for a significant portion of IPD and are associated with high case-fatality rates, antibiotic resistance and meningitis, while maintaining coverage of previously circulating strains that are currently contained through continued vaccination practice. Vaxcyte is re-engineering the way highly complex vaccines are made through modern synthetic techniques, including advanced chemistry and the XpressCF™ cell-free protein synthesis platform, exclusively licensed from Sutro Biopharma, Inc. Unlike conventional cell-based approaches, the Company’s system for producing difficult-to-make proteins and antigens is intended to accelerate its ability to efficiently create and deliver high-fidelity vaccines with enhanced immunological benefits. Vaxcyte’s pipeline also includes VAX-A1, a prophylactic vaccine candidate designed to prevent Group A Strep infections; VAX-PG, a therapeutic vaccine candidate designed to slow or stop the progression of periodontal disease; and VAX-GI, a vaccine candidate designed to prevent Shigella. Vaxcyte is driven to eradicate or treat invasive bacterial infections, which have serious and costly health consequences when left unchecked. For more information, visit www.vaxcyte.com.

Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. These statements include, but are not limited to, statements related to the potential benefits of VAX-24 and VAX-31, including breadth of coverage, and the ability to improve upon the standard-of-care; the timing of the remaining VAX-24 infant Phase 2 study data readout and VAX-31 infant Phase 2 study readouts; the timing of the initiation and data read outs for the VAX-31 adult studies; the potential of the Company’s carrier-sparing platform to add coverage and maintain robust immune responses and deliver the broadest-spectrum infant PCV candidates; expectations related to the future infant Phase 3 studies; the demand for Vaxcyte’s vaccine candidates; and other statements that are not historical fact. The words “anticipate,” “believe,” “could,” “expect,” “intend,” “may,” “on track,” “potential,” “should,” “would” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) convey uncertainty of future events or outcomes and are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements are based on Vaxcyte’s current expectations and actual results and timing of events could differ materially from those anticipated in such forward-looking statements as a result of risks and uncertainties, including, without limitation, risks related to Vaxcyte’s product development programs, including development timelines, success and timing of chemistry, manufacturing and controls and related manufacturing activities, potential delays or inability to obtain and maintain required regulatory approvals for its vaccine candidates, and the risks and uncertainties inherent with preclinical and clinical development processes; the success, cost and timing of all development activities and clinical trials; and sufficiency of cash and other funding to support Vaxcyte’s development programs and other operating expenses. These and other risks are described more fully in Vaxcyte’s filings with the Securities and Exchange Commission (SEC), including its Yearly Report on Form 10-K filed with the SEC on February 25, 2025 or in other documents Vaxcyte subsequently files with or furnishes to the SEC. All forward-looking statements contained in this press release speak only as of the date on which they were made and are based on management’s assumptions and estimates as of such date, and readers should not rely upon the information in this press release as current or accurate after its publication date. Vaxcyte undertakes no duty or obligation to update any forward-looking statements contained in this release as a result of new information, future events or changes in its expectations. Readers should not rely upon the information in this press release as current or accurate after its publication date.
Contacts:
Patrick Ryan, Executive Director, Corporate Affairs Jennifer Zibuda, Senior Director, Investor Relations
Vaxcyte, Inc.
415-606-5135
media@vaxcyte.com

Vaxcyte, Inc.
860-729-8902
investors@vaxcyte.com
1Lower limit of the 95% confidence interval for the difference between the proportion of participants achieving the pre-defined seroconversion rate (IgG concentration ≥0.35 mcg/mL) is > -15% for each ST (https://pmc.ncbi.nlm.nih.gov/articles/PMC7360095/). Larger Phase 3 registration studies have required that lower limit of the 95% confidence interval for the difference between the proportion of participants achieving the pre-defined seroconversion rate (IgG concentration ≥0.35 mcg/mL) is > -10% for each ST.
2Percentage of IPD caused in individuals <5 yrs of age in the U.S. in 2023 based on ABC surveillance data ( https://data.cdc.gov/Public-Health-Surveillance/1998-2023-Serotype-Data-for-Invasive-Pneumococcal-/qvzb-qs6p/about_data).
3Target point estimate of 0.6 is based on the Company’s statistical analysis of precedent Phase 2 and Phase 3 studies.

Exhibit 99.2 VAX-24 Infant Phase 2 Dose- Finding Study Topline Results March 31, 2025

Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements include, but are not limited to, statements related to the ability of Vaxcyte’s vaccine candidates and platform to achieve the broadest coverage of any infant pneumococcal conjugate vaccine on the market; the ability for VAX-24 to provide the broadest serotype and disease coverage in infants; the ability of VAX-31 to further expand coverage; precedent criteria for licensure; the timing of the remaining VAX-24 infant Phase 2 study data readout and VAX-31 infant Phase 2 study readouts; the timing of the initiation and data read outs for the VAX-31 adult studies; the ability to deliver a potentially best-in-class pneumococcal conjugate vaccine franchise demand for Vaxcyte’s vaccine candidates; the growth and expansion of the pneumococcal vaccine market; the market opportunity for Vaxcyte’s vaccines; Vaxcyte’s expectations regarding the spectrum coverage and regulatory pathway of its vaccine candidates; and other statements that are not historical fact. The words “anticipate,” “believe,” “continue,” “could,” “designed,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements are based on Vaxcyte’s current expectations and actual results and timing of events could differ materially from those anticipated in such forward-looking statements as a result of risks and uncertainties, including, without limitation, risks related to Vaxcyte’s product development programs, including development timelines, success and timing of chemistry, manufacturing and controls and related manufacturing activities; potential delays or inability to obtain and maintain required regulatory approvals for its vaccine candidates; the risks and uncertainties inherent with preclinical and clinical development processes; the success, cost and timing of all development activities and clinical trials; and the sufficiency of cash and other funding to support Vaxcyte’s development programs and other operating expenses, any of which could materially and adversely affect Vaxcyte’s business and operations. These and other risks are described more fully in Vaxcyte’s filings with the Securities and Exchange Commission (SEC), including its Annual Report on Form 10-K filed with the SEC on February 25, 2025 or in other documents Vaxcyte subsequently files with or furnishes to the SEC. Vaxcyte undertakes no duty or obligation to update any forward-looking statements contained in this release as a result of new information, future events or changes in its expectations. March 31, 2025 2

VAXCYTE MISSION STATEMENT We are on a global mission to engineer high- fidelity vaccines that protect humankind from the consequences of bacterial diseases. March 31, 2025 3

• INTRODUCTION AND VAX-24 INFANT STUDY RESULTS OVERVIEW Agenda • VAX-24 INFANT PHASE 2 DOSE-FINDING STUDY TOPLINE RESULTS – Disposition and Demographics – Safety and Tolerability Data – Topline Immunogenicity Data § Post-Dose 3 IgG & Interim OPA § Post-Dose 4 Interim IgG • PLANNING FOR INFANT PHASE 3 PROGRAM • PCV FRANCHISE AND PIPELINE UPDATE March 31, 2025 4

Introduction and VAX-24 Infant Study Results Overview March 31, 2025 5
VAX-24 Phase 2 Infant Study Results and Platform Demonstrate Potential to Achieve Broadest Coverage of Any Infant PCV On-Market Topline study results positive and met objectives Safety and tolerability profile similar to standard-of-care VAX-24 elicited substantial IgG, OPA and memory responses and performed particularly well against currently circulating serotypes contained in the vaccine Substantial, dose-dependent immune responses and little to no evidence of carrier suppression observed Strong conviction in potential to deliver broadest-spectrum PCVs as we advance into Phase 3 in infants and adults and introduce our third-generation PCV -- VAX-XL March 31, 2025 6

Global Health Impact of Pneumococcal Disease (PD) Remains Significant The U.S. CDC lists drug- Globally, Streptococcus Over 150,000 U.S. resistant Streptococcus pneumoniae is the leading hospitalizations annually pneumoniae as a cause of vaccine- due to pneumococcal “serious threat.” preventable deaths in pneumonia. children under five, causing approximately 300,000 deaths each year. March 31, 2025 7

VAX-24 Designed to Provide Broadest Serotype and Disease Coverage in Infants with Opportunity to Further Expand Coverage with VAX-31 16.00% 14.48% 13.79% 14.00% 13.10% 12.00% 9.66% 10.00% 8.00% 6.21% 6.21% 6.00% 4.83% 4.83% 4.14% 3.45% 4.00% 3.45% 3.45% 2.00% 1.38% 1.38% 1.38% 1.38% 1.38% 0.0% 0.69% 0.69% 0.69% 0.69% 0.69% 0.0% 0.0% 0.0% 0.0% 0.0% 0.0% 0.0% 0.0% 0.0% 0.0% 0.69% 0.0% Total Coverage 0.00% 1 2 3 3 19F 15B/C 22F 15A 33F 23B 11A 35B 10A 23A 38 9N 19A 20 21 33A 18C 16F 35F 4 7F 31 1 2 5 6A/C 6B 7C 8 9V 12F 14 17F 23F (US %) 47.6% Vaxneuvance 69.0% Prevnar20 71.7% VAX-24 91.7% VAX-31 1 15C coverage due to cross protection against 15B. 2 The serogroup 20 antigen contained in VAX-24 and VAX-31, formerly known as a 20B variant, has been officially reclassified as 20C. Due to the significant structural homology between 20C and 20B, immune responses elicited by 20C have been demonstrated to be highly cross-reactive with 20B. The Company therefore expects to be able to demonstrate coverage for both serotypes, 20B and 20C, in the anticipated VAX-31 adult Phase 3 studies. Reference: Yu J, et al.; New pneumococcal serotype 20C is a WciG O-acetyltransferase deficient variant of canonical serotype 20B. Microbiol Spectr 0:e02443-24. 3 % of IPD caused in individuals <5 yrs of age in the U.S. in 2023 based on ABC surveillance data References: https://data.cdc.gov/Public-Health-Surveillance/1998-2023-Serotype-Data-for-Invasive-Pneumococcal-/qvzb-qs6p/about_data. March 31, 2025 8

VAX-24 Infant Phase 2 Dose-Finding Study Topline Results March 31, 2025 9

Study Design March 31, 2025 10

VAX-24 Infant Phase 2 Dose-Finding Clinical Study (N=802) Randomized, Observer-Blind, Active-Controlled, Dose-Finding, Clinical Study to Evaluate Safety, Tolerability and Immunogenicity of VAX-24 vs. Standard-of-Care (PCV20) in 802 Healthy Infants All 36 VAX-24 subjects from Stage 1 proceeded to Stage 2 Stage 1: Dose Escalation (n=48*) Stage 2: Main Study (n=789*) VAX-24 2.2mcg/ VAX-24 2.2mcg/4.4mcg 4.4mcg PCV15 VAX-24 2.2mcg VAX-24 2.2mcg PCV15 VAX-24 1.1mcg VAX-24 1.1 mcg PCV20 PCV15 Primary Series Boost Final Safety Safety Review Safety Review Safety Review Dose 1 Dose 1 Dose 1 Dose 1 Dose 2 Dose 3 Immunogenicity Endpoint Dose 4 Immunogenicity Endpoint Assessment at 7 Days At 7 Days at 7 Days Age 2 months Age 2 months 4 months 6 months 7 months 12-15 months 13-16 months 18-21 months *The 36 subjects from the three VAX-24 cohorts in Stage 1 proceeded to Stage 2 of the study. The 12 subjects who received PCV15 in Stage 1 were given PCV20 for Doses 2-4 and followed separately and are not included in the safety or immunogenicity evaluable populations. March 31, 2025 11 Screen Randomize (3:1) DSMB DSMB DSMB Screen Randomize (1:1:1:1)
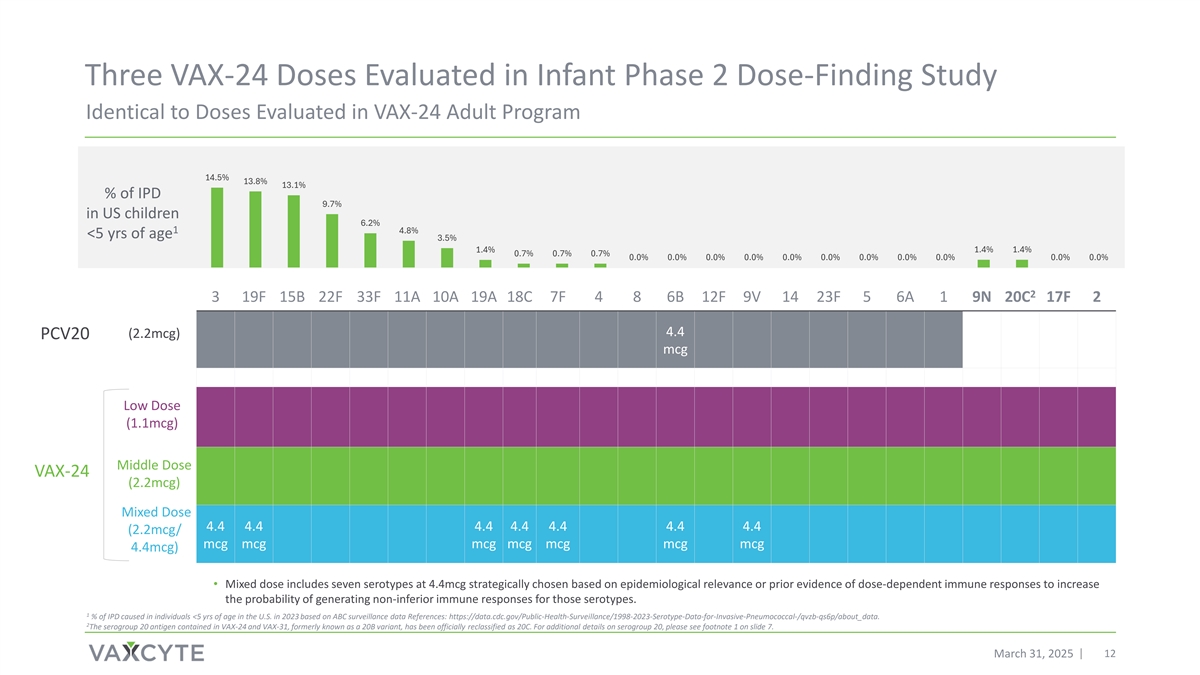
Three VAX-24 Doses Evaluated in Infant Phase 2 Dose-Finding Study Identical to Doses Evaluated in VAX-24 Adult Program 14.5% 13.8% 13.1% % of IPD 9.7% in US children 6.2% 1 4.8% <5 yrs of age 3.5% 1.4% 1.4% 1.4% 0.7% 0.7% 0.7% 0.0% 0.0% 0.0% 0.0% 0.0% 0.0% 0.0% 0.0% 0.0% 0.0% 0.0% 2 3 19F 15B 22F 33F 11A 10A 19A 18C 7F 4 8 6B 12F 9V 14 23F 5 6A 1 9N 20C 17F 2 4.4 (2.2mcg) PCV20 mcg Low Dose (1.1mcg) Middle Dose VAX-24 (2.2mcg) Mixed Dose 4.4 4.4 4.4 4.4 4.4 4.4 4.4 (2.2mcg/ mcg mcg mcg mcg mcg mcg mcg 4.4mcg) • Mixed dose includes seven serotypes at 4.4mcg strategically chosen based on epidemiological relevance or prior evidence of dose-dependent immune responses to increase the probability of generating non-inferior immune responses for those serotypes. 1 % of IPD caused in individuals <5 yrs of age in the U.S. in 2023 based on ABC surveillance data References: https://data.cdc.gov/Public-Health-Surveillance/1998-2023-Serotype-Data-for-Invasive-Pneumococcal-/qvzb-qs6p/about_data. 2 The serogroup 20 antigen contained in VAX-24 and VAX-31, formerly known as a 20B variant, has been officially reclassified as 20C. For additional details on serogroup 20, please see footnote 1 on slide 7. March 31, 2025 12

Disposition and Demographics March 31, 2025 13

Study Disposition VAX-24 Infant Phase 2 Dose-Finding Study 1 Randomized N = 802 Vaccinated Vaccinated Vaccinated Vaccinated VAX-24 Low Dose VAX-24 Mid Dose VAX-24 Mixed Dose PCV20 N = 205 N = 201 N = 195 N = 188 Included in Safety Population Included in Safety Population Included in Safety Population Included in Safety Population N = 205 N = 201 N = 195 N = 188 Included in IgG PD3 Included in IgG PD3 Included in IgG PD3 Included in IgG PD3 Immunogenicity Population Immunogenicity Population Immunogenicity Population Immunogenicity Population 2 2 2 2 N = 151 N = 152 N = 142 N = 129 Included in Interim IgG PD4 Included in Interim IgG PD4 Included in Interim IgG PD4 Included in Interim IgG PD4 Population Population Population Population N = 110 N = 100 N = 101 N = 76 1 Of the 802 randomized subjects, 12 received PCV15 for dose 1 and are not included in the PCV20 vaccinated population and 1 withdrew prior to vaccination. 2 The PD3 immunogenicity population across all cohorts excludes subjects who discontinued from the study or for whom blood samples were unavailable or ineligible. March 31, 2025 14

Population Demographics Generally Balanced Across Cohorts VAX-24 Low Dose VAX-24 Mid Dose VAX-24 Mixed Dose PCV20 205 201 195 188 Number of Subjects 1 Median Age, days (Q1, Q3) 64 ( 61, 68 ) 64 ( 61, 68 ) 64 ( 62, 68) 64 ( 62, 68) Sex, n (%) 113 ( 55.1) 103 ( 51.2 ) 94 ( 48.2 ) 87 ( 46.3 ) Female 92 ( 44.9 ) 98 ( 48.8 ) 101 ( 51.8 ) 101 ( 53.7) Male Race, n (%) 139 ( 67.8 ) 141 ( 70.1 ) 139 ( 71.3 ) 127 ( 67.6 ) White Black 38 ( 18.5 ) 35 ( 17.4 ) 29 ( 14.9 ) 31 ( 16.5 ) 3 ( 1.5 ) 0 ( 0.0 ) 5 ( 2.6 ) 2 ( 1.1 ) Asian 0 ( 0.0 ) 0 ( 0.0 ) 0 ( 0.0 ) 0 ( 0.0 ) Native Hawaiian American Indian or Native Alaskan 1 ( 0.5 ) 1 ( 0.5 ) 2 ( 1.0 ) 1 ( 0.5 ) Other/ Multiracial 24 ( 11.7 ) 24 ( 11.9 ) 20 ( 10.3 ) 27 ( 14.4 ) 5.19 5.18 5.29 5.22 Median Weight, kg (Q1, Q3) ( 4.77, 5.63 ) ( 4.73, 5.81 ) ( 4.80, 5.72 ) ( 4.73, 5.67 ) 57.66 57.79 57.80 58.09 Median Length, cm (Q1, Q3) (55.88 , 59.18) (55.88, 59.69) (55.88, 59.69) (55.88, 59.69) Median Gestational Age, weeks (Q1, 39 39 39 39 ( 38, 39 ) ( 38, 39 ) ( 38, 39 ) ( 38, 39 ) Q3) 3.29 3.27 3.27 3.22 Median Birth Weight, kg (Q1, Q3) ( 2.93, 3.60 ) ( 3.00, 3.60 ) ( 2.97, 3.63 ) ( 2.96, 3.54 ) 1 Q1 = First Quartile or 25th Percentile. Q3 = Third Quartile or 75th Percentile. *Four subjects of American Indian race redacted in order to maintain blinding. March 31, 2025 15

Safety and Tolerability Data March 31, 2025 16

VAX-24 Well Tolerated Across All Dose Cohorts Local Solicited AEs Through 7 Days After Each of Three Primary Doses 100% Mild 90% Moderate 80% Severe 70% 60% 50% 40% 30% 20% 10% 0% Low Mid Mixed PCV20 Low Mid Mixed PCV20 Low Mid Mixed PCV20 Edema* Tenderness at Injection Site Erythema * One occurrence of severe edema redacted to maintain blinding. March 31, 2025 17
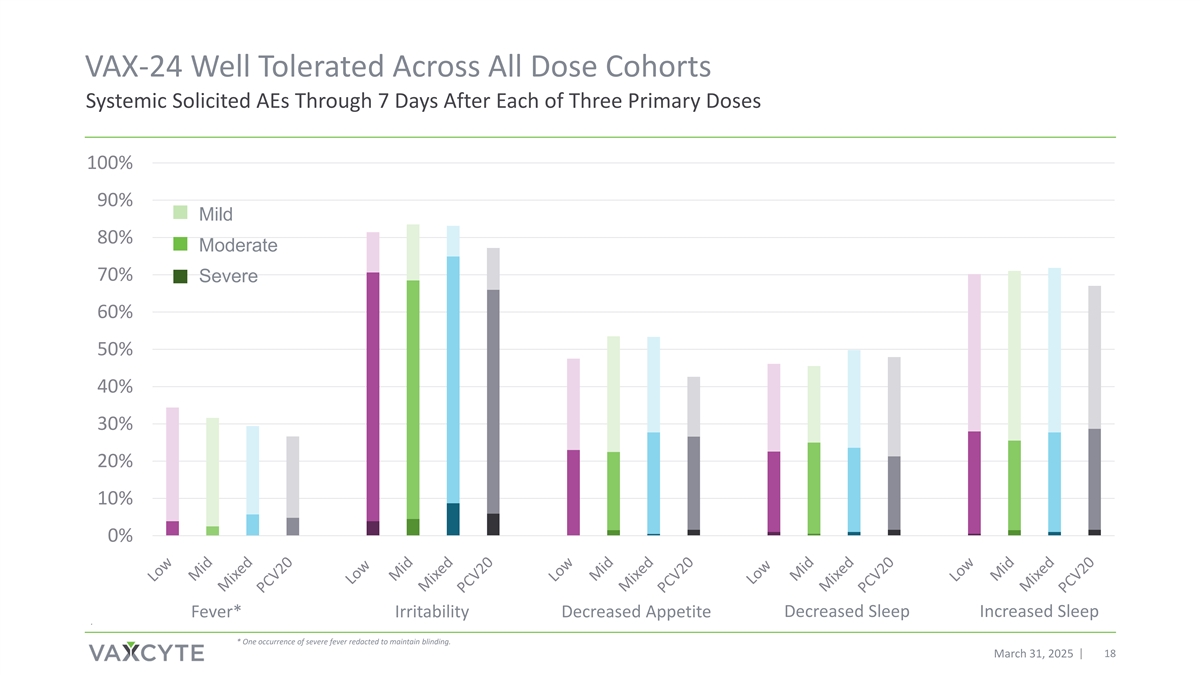
VAX-24 Well Tolerated Across All Dose Cohorts Systemic Solicited AEs Through 7 Days After Each of Three Primary Doses 100% 90% Mild 80% Moderate 70% Severe 60% 50% 40% 30% 20% 10% 0% Decreased Sleep Increased Sleep Fever* Irritability Decreased Appetite . * One occurrence of severe fever redacted to maintain blinding. March 31, 2025 18
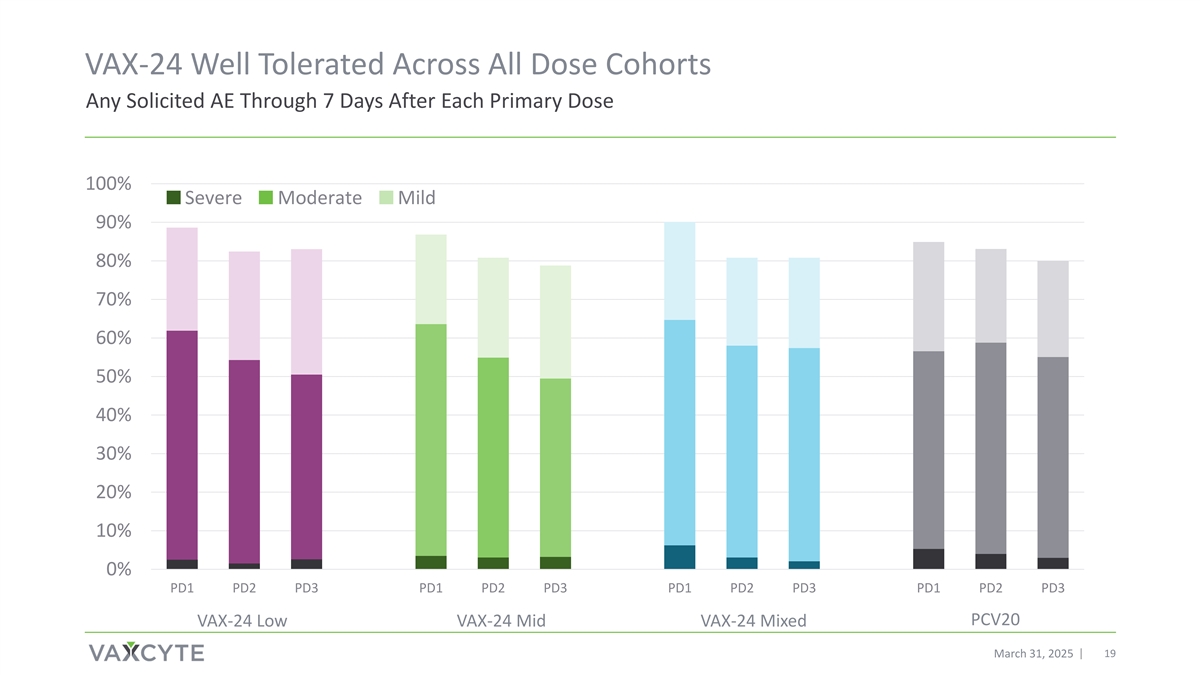
VAX-24 Well Tolerated Across All Dose Cohorts Any Solicited AE Through 7 Days After Each Primary Dose 100% Severe Moderate Mild 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% PD1 PD2 PD3 PD1 PD2 PD3 PD1 PD2 PD3 PD1 PD2 PD3 PCV20 VAX-24 Low VAX-24 Mid VAX-24 Mixed March 31, 2025 19
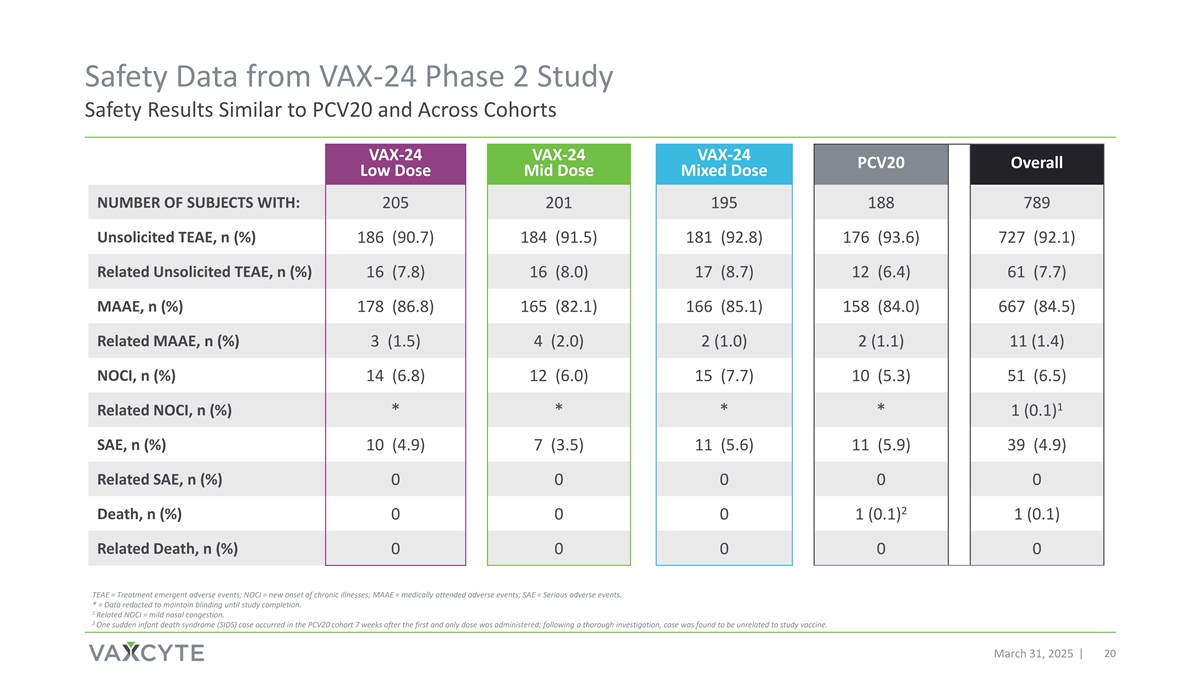
Safety Data from VAX-24 Phase 2 Study Safety Results Similar to PCV20 and Across Cohorts VAX-24 VAX-24 VAX-24 PCV20 Overall Low Dose Mid Dose Mixed Dose NUMBER OF SUBJECTS WITH: 205 201 195 188 789 Unsolicited TEAE, n (%) 186 (90.7) 184 (91.5) 181 (92.8) 176 (93.6) 727 (92.1) Related Unsolicited TEAE, n (%) 16 (7.8) 16 (8.0) 17 (8.7) 12 (6.4) 61 (7.7) MAAE, n (%) 178 (86.8) 165 (82.1) 166 (85.1) 158 (84.0) 667 (84.5) Related MAAE, n (%) 3 (1.5) 4 (2.0) 2 (1.0) 2 (1.1) 11 (1.4) NOCI, n (%) 14 (6.8) 12 (6.0) 15 (7.7) 10 (5.3) 51 (6.5) 1 Related NOCI, n (%) * * * * 1 (0.1) SAE, n (%) 10 (4.9) 7 (3.5) 11 (5.6) 11 (5.9) 39 (4.9) Related SAE, n (%) 0 0 0 0 0 2 Death, n (%) 0 0 0 1 (0.1) 1 (0.1) Related Death, n (%) 0 0 0 0 0 TEAE = Treatment emergent adverse events; NOCI = new onset of chronic illnesses; MAAE = medically attended adverse events; SAE = Serious adverse events. * = Data redacted to maintain blinding until study completion. 1 Related NOCI = mild nasal congestion. 2 One sudden infant death syndrome (SIDS) case occurred in the PCV20 cohort 7 weeks after the first and only dose was administered; following a thorough investigation, case was found to be unrelated to study vaccine. March 31, 2025 20

Topline Immunogenicity Data March 31, 2025 21
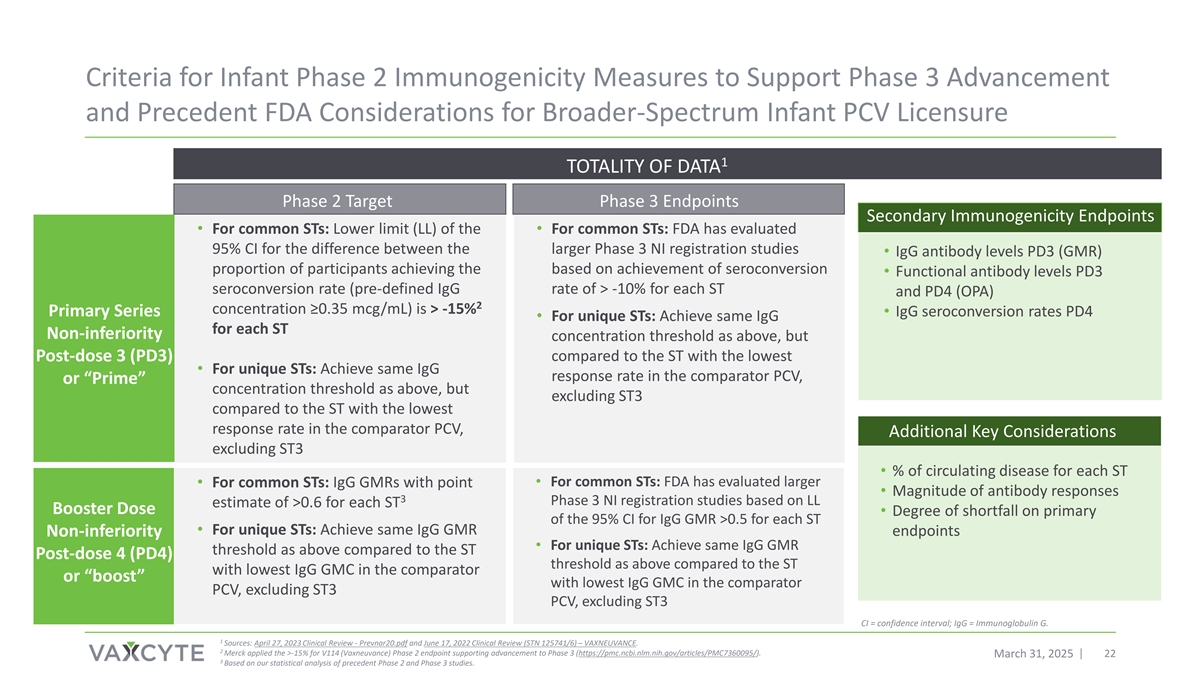
Criteria for Infant Phase 2 Immunogenicity Measures to Support Phase 3 Advancement and Precedent FDA Considerations for Broader-Spectrum Infant PCV Licensure 1 TOTALITY OF DATA Phase 2 Target Phase 3 Endpoints Secondary Immunogenicity Endpoints • For common STs: Lower limit (LL) of the • For common STs: FDA has evaluated 95% CI for the difference between the larger Phase 3 NI registration studies • IgG antibody levels PD3 (GMR) proportion of participants achieving the based on achievement of seroconversion • Functional antibody levels PD3 seroconversion rate (pre-defined IgG rate of > -10% for each ST and PD4 (OPA) 2 concentration ≥0.35 mcg/mL) is > -15% Primary Series • IgG seroconversion rates PD4 • For unique STs: Achieve same IgG for each ST Non-inferiority concentration threshold as above, but Post-dose 3 (PD3) compared to the ST with the lowest • For unique STs: Achieve same IgG response rate in the comparator PCV, or “Prime” concentration threshold as above, but excluding ST3 compared to the ST with the lowest response rate in the comparator PCV, Additional Key Considerations excluding ST3 • % of circulating disease for each ST • For common STs: FDA has evaluated larger • For common STs: IgG GMRs with point • Magnitude of antibody responses 3 Phase 3 NI registration studies based on LL estimate of >0.6 for each ST Booster Dose • Degree of shortfall on primary of the 95% CI for IgG GMR >0.5 for each ST • For unique STs: Achieve same IgG GMR Non-inferiority endpoints • For unique STs: Achieve same IgG GMR threshold as above compared to the ST Post-dose 4 (PD4) threshold as above compared to the ST with lowest IgG GMC in the comparator or “boost” with lowest IgG GMC in the comparator PCV, excluding ST3 PCV, excluding ST3 CI = confidence interval; IgG = Immunoglobulin G. 1 Sources: April 27, 2023 Clinical Review - Prevnar20.pdf and June 17, 2022 Clinical Review (STN 125741/6) – VAXNEUVANCE. 2 Merck applied the >-15% for V114 (Vaxneuvance) Phase 2 endpoint supporting advancement to Phase 3 (https://pmc.ncbi.nlm.nih.gov/articles/PMC7360095/). March 31, 2025 22 3 Based on our statistical analysis of precedent Phase 2 and Phase 3 studies.
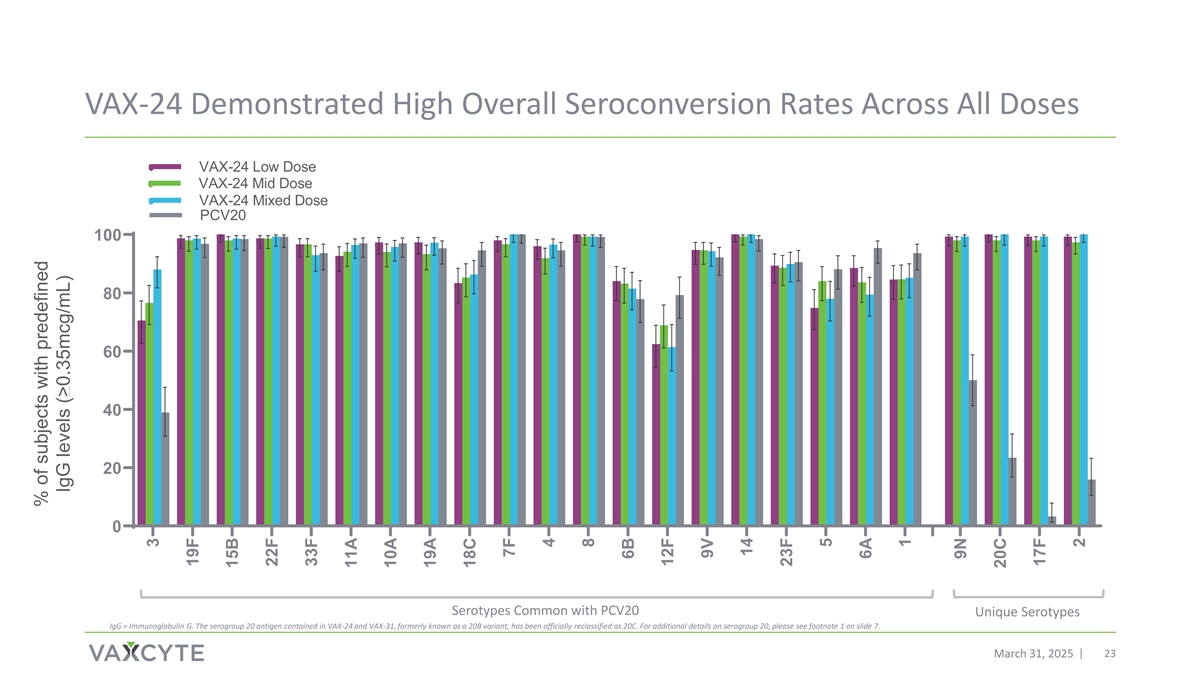
VAX-24 Demonstrated High Overall Seroconversion Rates Across All Doses VAX-24 Low Dose VAX-24 Mid Dose VAX-24 Mixed Dose PCV20 100 80 60 40 20 0 Serotypes Common with PCV20 Unique Serotypes IgG = Immunoglobulin G. The serogroup 20 antigen contained in VAX-24 and VAX-31, formerly known as a 20B variant, has been officially reclassified as 20C. For additional details on serogroup 20, please see footnote 1 on slide 7. March 31, 2025 23 % of subjects with predefined IgG levels (>0.35mcg/mL) 3 19F 15B 22F 33F 11A 10A 19A 18C 7F 4 8 6B 12F 9V 14 23F 5 6A 1 9N 20C 17F 2
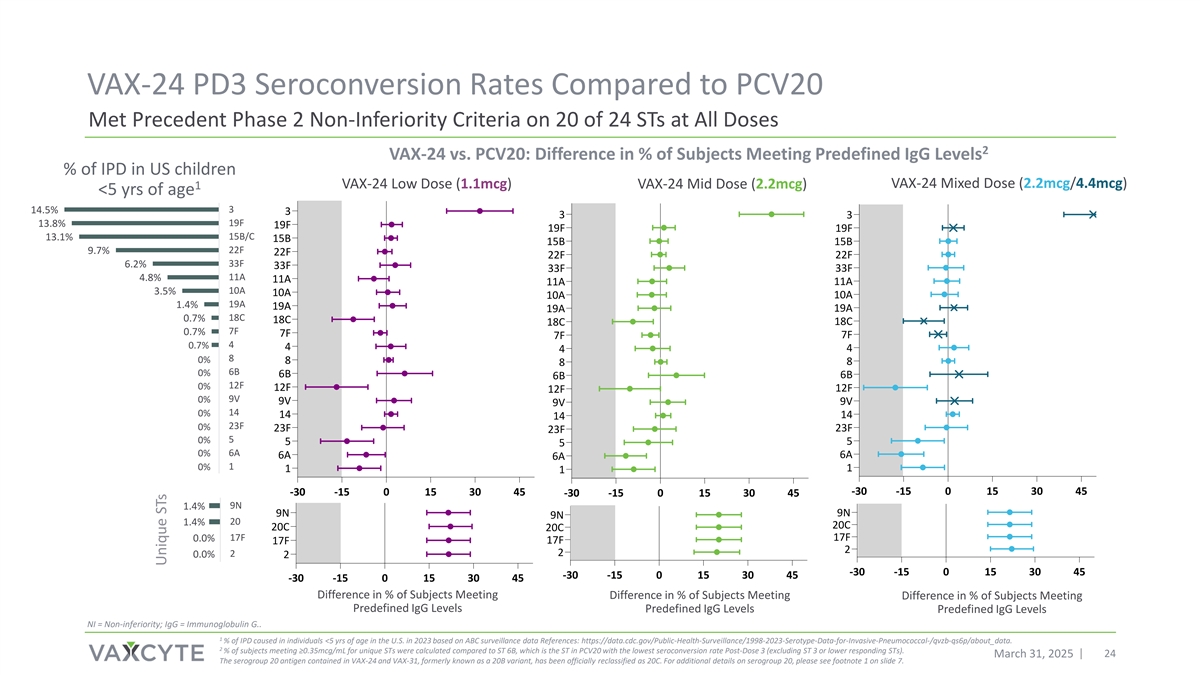
VAX-24 PD3 Seroconversion Rates Compared to PCV20 Met Precedent Phase 2 Non-Inferiority Criteria on 20 of 24 STs at All Doses 2 VAX-24 vs. PCV20: Difference in % of Subjects Meeting Predefined IgG Levels % of IPD in US children VAX-24 Mixed Dose (2.2mcg/4.4mcg) VAX-24 Low Dose (1.1mcg) VAX-24 Mid Dose (2.2mcg) 1 <5 yrs of age 3 14.5% 3 3 3 19F 13.8% 19F 19F 19F 15B/C 13.1% 15B 15B 15B 22F 9.7% 22F 22F 22F 33F 6.2% 33F 33F 33F 11A 4.8% 11A 11A 11A 10A 3.5% 10A 10A 10A 19A 1.4% 19A 19A 19A 18C 0.7% 18C 18C 18C 7F 0.7% 7F 7F 7F 4 0.7% 4 4 4 8 0% 8 8 8 6B 0% 6B 6B 6B 12F 0% 12F 12F 12F 9V 0% 9V 9V 9V 14 0% 14 14 14 23F 0% 23F 23F 23F 5 0% 5 5 5 0% 6A 6A 6A 6A 0% 1 1 1 1 15% 10% 5% 0% -30 -15 0 15 30 45 -30 -15 0 15 30 45 -30 -15 0 15 30 45 9N 1.4% 9N 9N 9N 20 1.4% 20C 20C 20C 17F 17F 0.0% 17F 17F 2 2 0.0% 2 2 -30 -15 0 15 30 45 -30 -15 0 15 30 45 15% 10% 5% 0% -30 -15 0 15 30 45 Difference in % of Subjects Meeting Difference in % of Subjects Meeting Difference in % of Subjects Meeting Predefined IgG Levels Predefined IgG Levels Predefined IgG Levels NI = Non-inferiority; IgG = Immunoglobulin G.. 1 % of IPD caused in individuals <5 yrs of age in the U.S. in 2023 based on ABC surveillance data References: https://data.cdc.gov/Public-Health-Surveillance/1998-2023-Serotype-Data-for-Invasive-Pneumococcal-/qvzb-qs6p/about_data. 2 % of subjects meeting ≥0.35mcg/mL for unique STs were calculated compared to ST 6B, which is the ST in PCV20 with the lowest seroconversion rate Post-Dose 3 (excluding ST 3 or lower responding STs). March 31, 2025 24 The serogroup 20 antigen contained in VAX-24 and VAX-31, formerly known as a 20B variant, has been officially reclassified as 20C. For additional details on serogroup 20, please see footnote 1 on slide 7. Unique STs
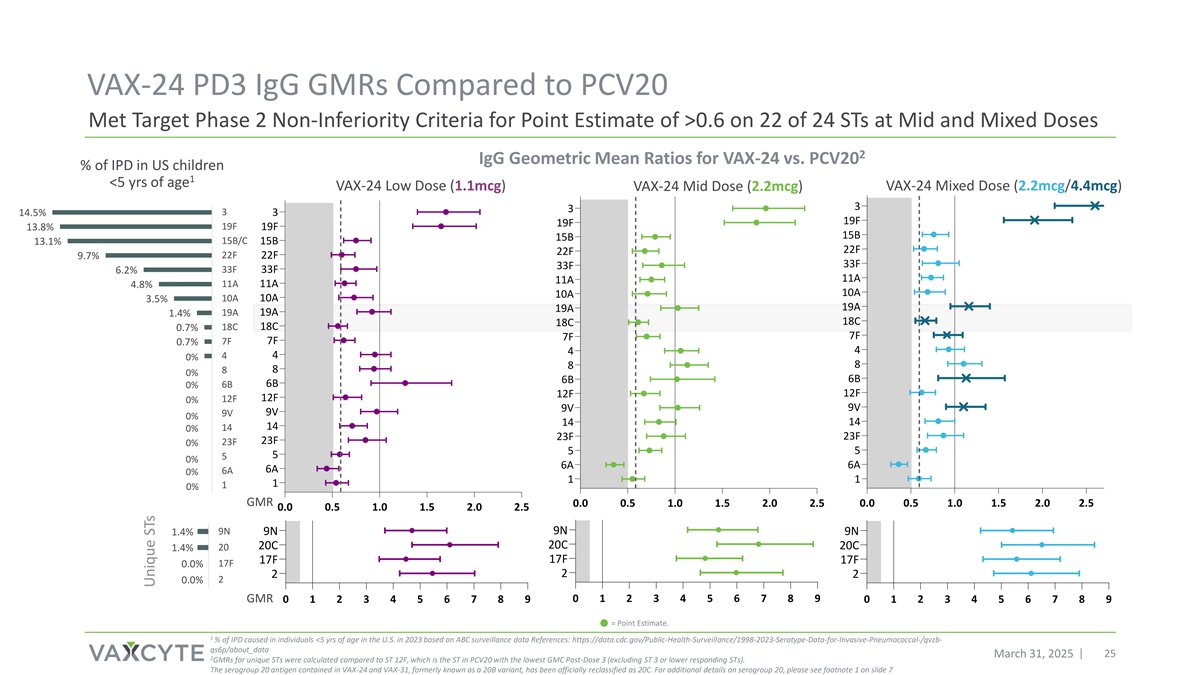
VAX-24 PD3 IgG GMRs Compared to PCV20 Met Target Phase 2 Non-Inferiority Criteria for Point Estimate of >0.6 on 22 of 24 STs at Mid and Mixed Doses 2 IgG Geometric Mean Ratios for VAX-24 vs. PCV20 % of IPD in US children 1 <5 yrs of age VAX-24 Low Dose (1.1mcg) VAX-24 Mid Dose (2.2mcg) VAX-24 Mixed Dose (2.2mcg/4.4mcg) 3 3 3 3 14.5% 19F 19F 13.8% 19F 19F 15B 15B 15B/C 13.1% 15B 22F 22F 22F 22F 9.7% 33F 33F 33F 33F 6.2% 11A 11A 4.8% 11A 11A 10A 10A 10A 10A 3.5% 19A 19A 19A 19A 1.4% 18C 18C 18C 18C 0.7% 7F 7F 7F 7F 0.7% 4 4 4 4 0% 8 8 8 8 0% 6B 6B 6B 6B 0% 12F 12F 12F 12F 0% 9V 9V 9V 9V 0% 14 14 14 14 0% 23F 23F 23F 23F 0% 5 5 5 5 0% 6A 6A 6A 6A 0% 1 1 1 1 0% GMR 0.0 0.5 1.0 1.5 2.0 2.5 0.0 0.5 1.0 1.5 2.0 2.5 15% 10% 5% 0% 0.0 0.5 1.0 1.5 2.0 2.5 9N 9N 1.4% 9N 9N 20C 20C 20C 1.4% 20 17F 17F 17F 17F 0.0% 2 2 2 2 0.0% GMR 0 1 2 3 4 5 6 7 8 9 0 1 2 3 4 5 6 7 8 9 0 1 2 3 4 5 6 7 8 9 15% 10% 5% 0% = Point Estimate. 1 % of IPD caused in individuals <5 yrs of age in the U.S. in 2023 based on ABC surveillance data References: https://data.cdc.gov/Public-Health-Surveillance/1998-2023-Serotype-Data-for-Invasive-Pneumococcal-/qvzb- qs6p/about_data March 31, 2025 25 2 GMRs for unique STs were calculated compared to ST 12F, which is the ST in PCV20 with the lowest GMC Post-Dose 3 (excluding ST 3 or lower responding STs). The serogroup 20 antigen contained in VAX-24 and VAX-31, formerly known as a 20B variant, has been officially reclassified as 20C. For additional details on serogroup 20, please see footnote 1 on slide 7 Unique STs
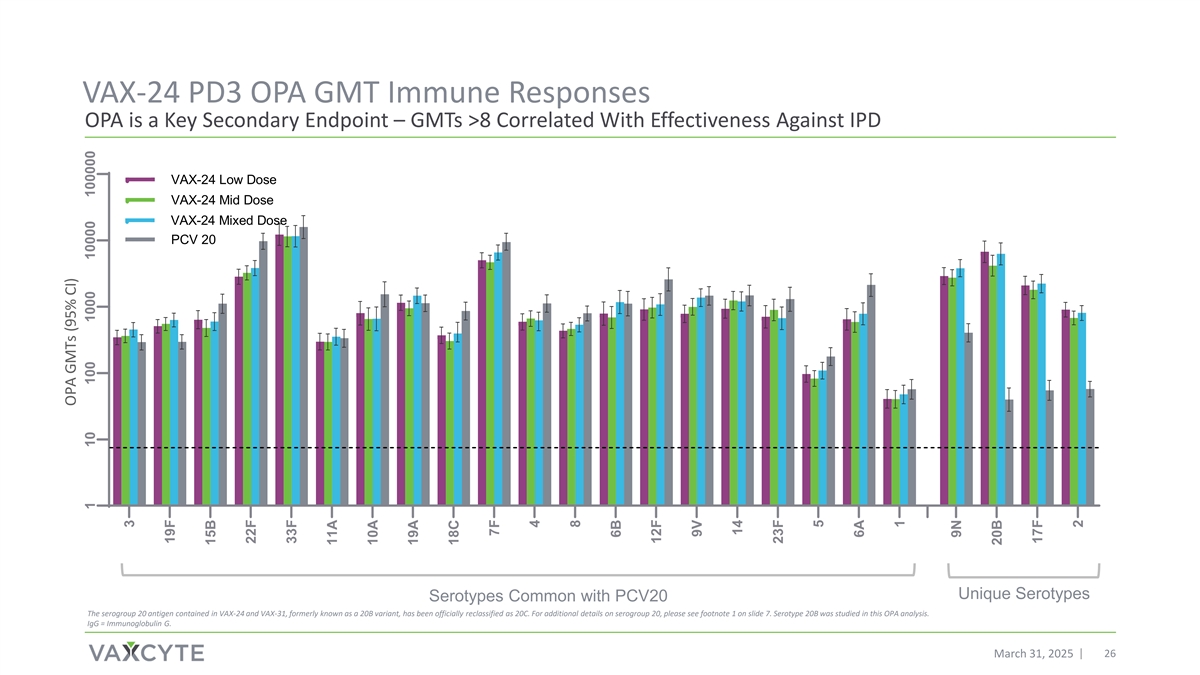
VAX-24 PD3 OPA GMT Immune Responses OPA is a Key Secondary Endpoint – GMTs >8 Correlated With Effectiveness Against IPD VAX-24 Low Dose VAX-24 Mid Dose VAX-24 Mixed Dose PCV 20 Unique Serotypes Serotypes Common with PCV20 The serogroup 20 antigen contained in VAX-24 and VAX-31, formerly known as a 20B variant, has been officially reclassified as 20C. For additional details on serogroup 20, please see footnote 1 on slide 7. Serotype 20B was studied in this OPA analysis. IgG = Immunoglobulin G. March 31, 2025 26 OPA GMTs (95% CI) 1 10 100 1000 10000 100000 3 19F 15B 22F 33F 11A 10A 19A 18C 7F 4 8 6B 12F 9V 14 23F 5 6A 1 9N 20B 17F 2

PD4 Interim IgG Immunogenicity Data March 31, 2025 27

VAX-24 PD4 Seroconversion Rates High IgG Seroconversion Rates Across All Doses VAX-24 Low Dose VAX-24 Mid Dose VAX-24 Mixed Dose PCV20 100 80 60 40 20 0 Serotypes Contained in PCV20 Non-PCV20 Serotypes The serogroup 20 antigen contained in VAX-24 and VAX-31, formerly known as a 20B variant, has been officially reclassified as 20C. For additional details on serogroup 20, please see footnote 1 on slide 7. IgG = Immunoglobulin G. March 31, 2025 28 % of subjects with predefined IgG Levels (>0.35mcg/mL) 3 19F 15B 22F 33F 11A 10A 19A 18C 7F 4 8 6B 12F 9V 14 23F 5 6A 1 9N 20C 17F 2
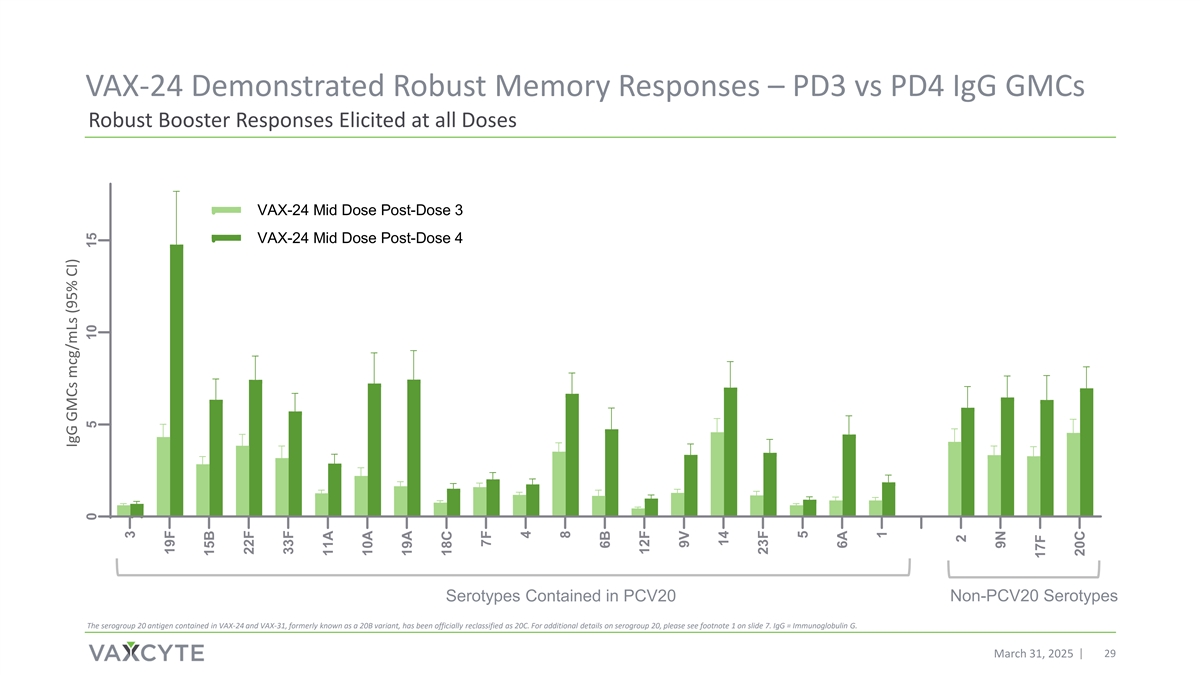
VAX-24 Demonstrated Robust Memory Responses – PD3 vs PD4 IgG GMCs Robust Booster Responses Elicited at all Doses VAX-24 Mid Dose Post-Dose 3 VAX-24 Mid Dose Post-Dose 4 Serotypes Contained in PCV20 Non-PCV20 Serotypes The serogroup 20 antigen contained in VAX-24 and VAX-31, formerly known as a 20B variant, has been officially reclassified as 20C. For additional details on serogroup 20, please see footnote 1 on slide 7. IgG = Immunoglobulin G. March 31, 2025 29 IgG GMCs mcg/mLs (95% CI) 0 5 10 15 3 19F 15B 22F 33F 11A 10A 19A 18C 7F 4 8 6B 12F 9V 14 23F 5 6A 1 2 9N 17F 20C
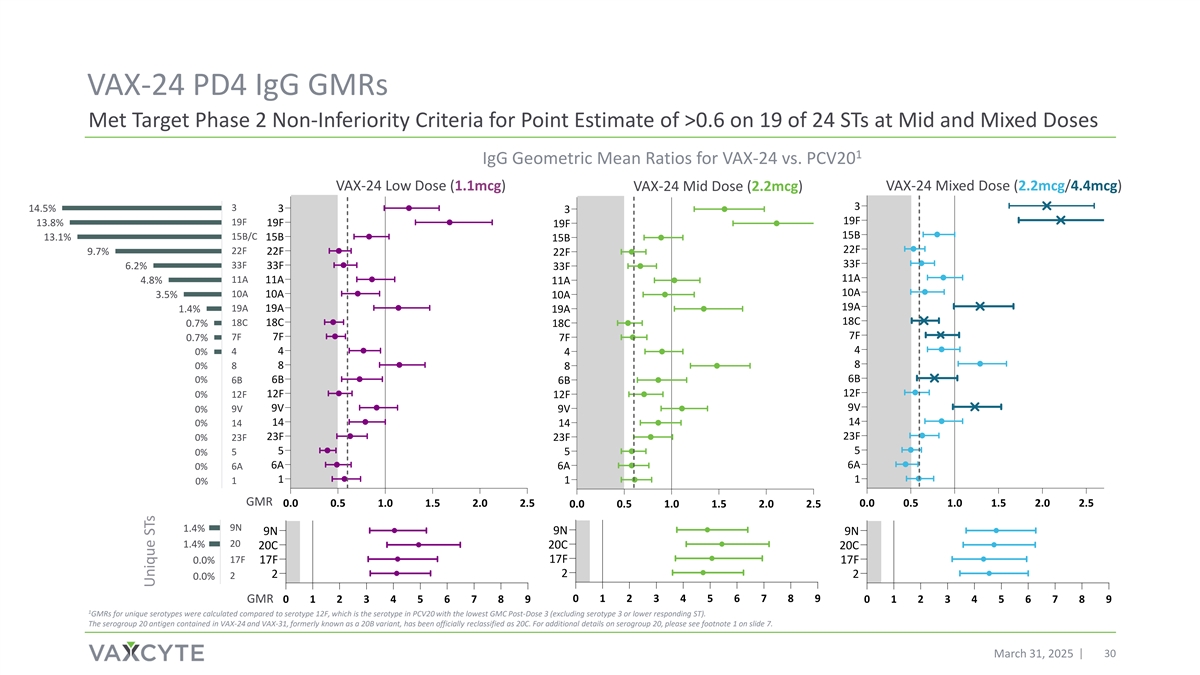
VAX-24 PD4 IgG GMRs Met Target Phase 2 Non-Inferiority Criteria for Point Estimate of >0.6 on 19 of 24 STs at Mid and Mixed Doses 1 IgG Geometric Mean Ratios for VAX-24 vs. PCV20 VAX-24 Low Dose (1.1mcg) VAX-24 Mid Dose (2.2mcg) VAX-24 Mixed Dose (2.2mcg/4.4mcg) 3 14.5% 3 3 3 19F 19F 13.8% 19F 19F 15B 15B/C 13.1% 15B 15B 22F 22F 9.7% 22F 22F 33F 33F 33F 6.2% 33F 11A 4.8% 11A 11A 11A 10A 10A 3.5% 10A 10A 19A 19A 19A 1.4% 19A 18C 18C 18C 0.7% 18C 7F 7F 7F 0.7% 7F 4 4 0% 4 4 8 0% 8 8 8 6B 0% 6B 6B 6B 12F 12F 0% 12F 12F 9V 9V 0% 9V 9V 14 0% 14 14 14 23F 0% 23F 23F 23F 5 5 0% 5 5 6A 6A 0% 6A 6A 1 1 1 0% 1 15% 10% 5% 0% GMR 0.0 0.5 1.0 1.5 2.0 2.5 0.0 0.5 1.0 1.5 2.0 2.5 0.0 0.5 1.0 1.5 2.0 2.5 9N 1.4% 9N 9N 9N 20 1.4% 20C 20C 20C 17F 17F 0.0% 17F 17F 2 2 2 2 0.0% 15% 10% 5% 0% 0 1 2 3 4 5 6 7 8 9 GMR 0 1 2 3 4 5 6 7 8 9 0 1 2 3 4 5 6 7 8 9 1 GMRs for unique serotypes were calculated compared to serotype 12F, which is the serotype in PCV20 with the lowest GMC Post-Dose 3 (excluding serotype 3 or lower responding ST). The serogroup 20 antigen contained in VAX-24 and VAX-31, formerly known as a 20B variant, has been officially reclassified as 20C. For additional details on serogroup 20, please see footnote 1 on slide 7. March 31, 2025 30 Unique STs

Planning for Infant Phase 3 Program March 31, 2025 31

VAX-24 Mid Dose (2.2mcg) Selected as Basis for Advancement Pending VAX-31 Phase 2 Topline Data Readout, Prepare for Phase 3 Study With VAX-24 or VAX-31 PD3 IgG GMR PD3 IgG Seroconversion PD4 IgG GMR % of IPD in US children <5 yrs of age 3 3 3 14.5% 3 19F 19F 19F 13.8% 19F 15B 15B 15B/C 13.1% 15B 22F 22F 22F 9.7% 22F 33F 33F 33F 6.2% 33F 11A 11A 11A 4.8% 11A 10A 10A 10A 3.5% 10A 19A 19A 19A 1.4% 19A 18C 18C 18C 0.7% 18C 7F 8 7F 0.7% 7F 4 4 4 0% 4 8 7F 8 0% 8 6B 6B 6B 6B 0% 12F 12F 12F 0% 12F 9V 9V 0% 9V 9V 14 14 0% 14 14 23F 23F 23F 0% 23F 5 5 5 0% 5 6A 0% 6A 6A 6A 1 0% 1 1 1 15% 10% 5% 0% -30 -15 0 15 30 45 0.0 0.5 1.0 1.5 2.0 2.5 0.0 0.5 1.0 1.5 2.0 2.5 9N 9N 9N 1.4% 9N 20C 20 20C 1.4% 20C 17F 17F 0.0% 17F 17F 2 2 2 0.0% 2 -30 -15 0 15 30 45 15% 10% 5% 0% 0 1 2 3 4 5 6 7 8 9 0 1 2 3 4 5 6 7 8 9 Difference in % of Subjects Meeting IgG Geometric Mean Ratios IgG Geometric Mean Ratios Predefined IgG Levels The serogroup 20 antigen contained in VAX-24 and VAX-31, formerly known as a 20B variant, has been officially reclassified as 20C. For additional details on serogroup 20, please see footnote 1 on slide 7. March 31, 2025 32 Unique STs
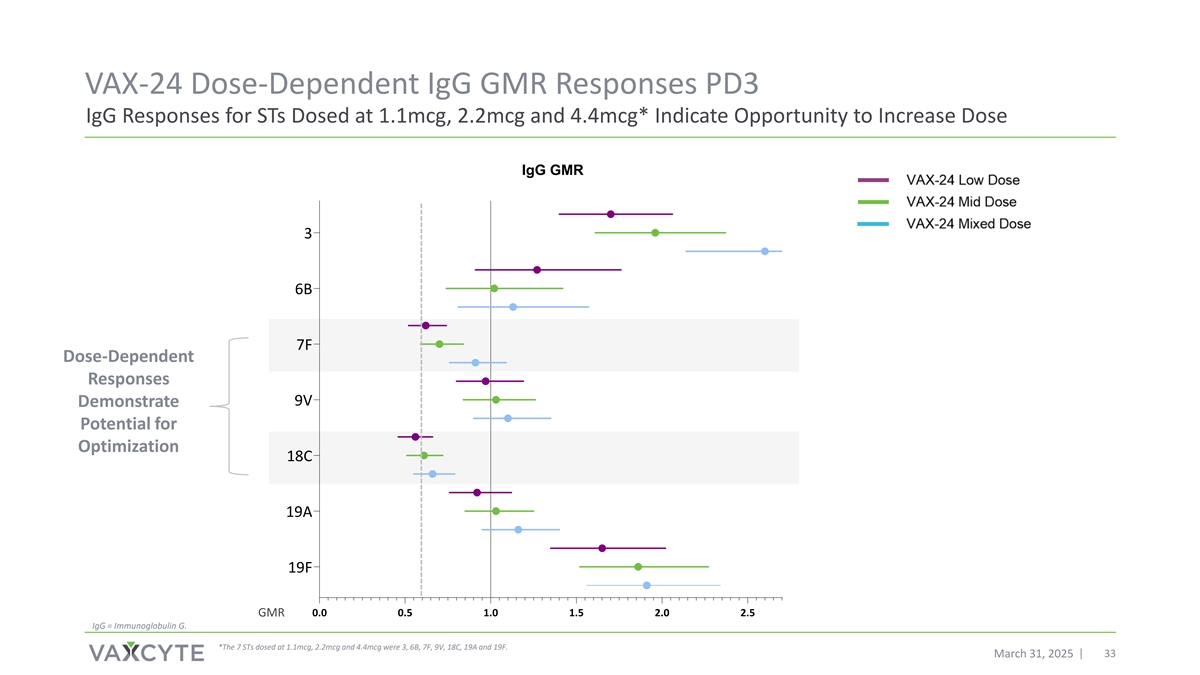
VAX-24 Dose-Dependent IgG GMR Responses PD3 IgG Responses for STs Dosed at 1.1mcg, 2.2mcg and 4.4mcg* Indicate Opportunity to Increase Dose IgG GMR 3 6B 7F Dose-Dependent Responses 9V Demonstrate Potential for Optimization 18C 19A 19F GMR 0.0 0.5 1.0 1.5 2.0 2.5 IgG = Immunoglobulin G. *The 7 STs dosed at 1.1mcg, 2.2mcg and 4.4mcg were 3, 6B, 7F, 9V, 18C, 19A and 19F. March 31, 2025 33

IgG GMCs at Mid and Mixed Doses for 13 STs Dosed at 2.2mcg Absence of Carrier Suppression Observed Indicate Opportunity to Increase Dose IgG Geometric Mean Concentrations 1-month Post-Dose 3 VAX-24 Mid Dose VAX-24 Mixed Dose The serogroup 20 antigen contained in VAX-24 and VAX-31, formerly known as a 20B variant, has been officially reclassified as 20C. For additional details on serogroup 20, please see footnote 1 on slide 7. 34 March 31, 2025 IgG GMCs mcg/mLs (95% CI) 0.1 1 10 1 4 5 6A 8 10A 11A 12F 14 15B 22F 23F 33F 2 9N 17F 20C
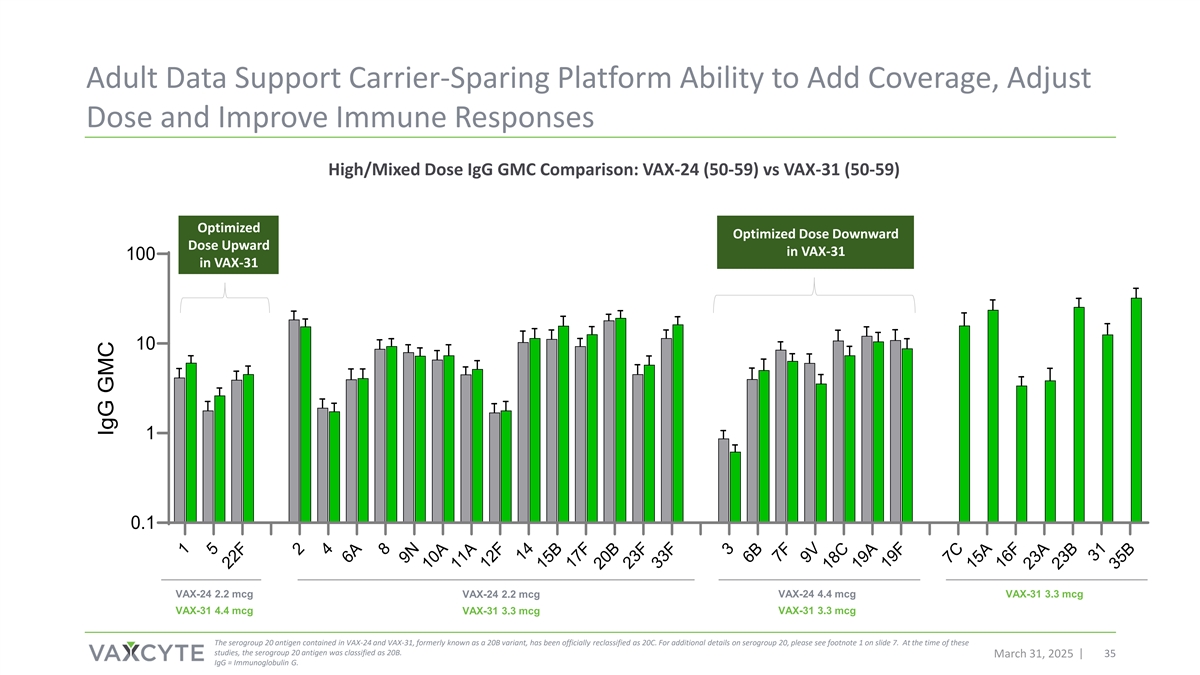
Adult Data Support Carrier-Sparing Platform Ability to Add Coverage, Adjust Dose and Improve Immune Responses High/Mixed Dose IgG GMC Comparison: VAX-24 (50-59) vs VAX-31 (50-59) Optimized Optimized Dose Downward Dose Upward in VAX-31 100 in VAX-31 10 1 0.1 VAX-24 2.2 mcg VAX-24 2.2 mcg VAX-24 4.4 mcg VAX-31 3.3 mcg VAX-31 4.4 mcg VAX-31 3.3 mcg VAX-31 3.3 mcg The serogroup 20 antigen contained in VAX-24 and VAX-31, formerly known as a 20B variant, has been officially reclassified as 20C. For additional details on serogroup 20, please see footnote 1 on slide 7. At the time of these studies, the serogroup 20 antigen was classified as 20B. March 31, 2025 35 IgG = Immunoglobulin G. 1 5 22F 2 4 6A 8 9N 10A 11A 12F 14 15B 17F 20B 23F 33F 3 6B 7F 9V 18C 19A 19F 7C 15A 16F 23A 23B 31 35B IgG GMC
VAX-24 Phase 2 Infant Study Results and Platform Demonstrate Potential to Achieve Broadest Coverage of Any Infant PCV On-Market Topline study results positive and met objectives Safety and tolerability profile similar to standard-of-care VAX-24 elicited substantial IgG, OPA and memory responses and performed particularly well against currently circulating serotypes contained in the vaccine Substantial, dose-dependent immune responses and little to no evidence of carrier suppression observed Strong conviction in potential to deliver broadest-spectrum PCVs as we advance into Phase 3 in infants and adults and introduce our third-generation PCV -- VAX-XL March 31, 2025 36

PCV Franchise and Pipeline Update March 31, 2025 37

1 Clinical Development Next Steps and Anticipated Milestones Potential Best-in-Class PCV Franchise for Adult and Infant Segments Population Investigational PCV Key Anticipated Milestones • Following FDA End-of-Phase 2 meeting, initiate Phase 3 pivotal, VAX-31 non-inferiority study by mid-2025 and announce topline safety, 31-valent PCV candidate tolerability and immunogenicity data in 2026. • Initiate remaining Phase 3 studies in 2025 and 2026 and announce data from these studies in 2026 and 2027. Adults • Announce balance of VAX-24 Phase 2 dose-finding study data, VAX-24 including final safety data, full PD3 OPA data, and full PD4 IgG and 24-valent PCV candidate OPA data by end of 2025. • Announce topline safety, tolerability and immunogenicity data for VAX-31 Phase 2 dose-finding study primary three-dose immunization 31-valent PCV candidate Infants series in mid-2026, with complete booster data up to nine months later. (1) Guidance as of March 31, 2025 March 31, 2025 38
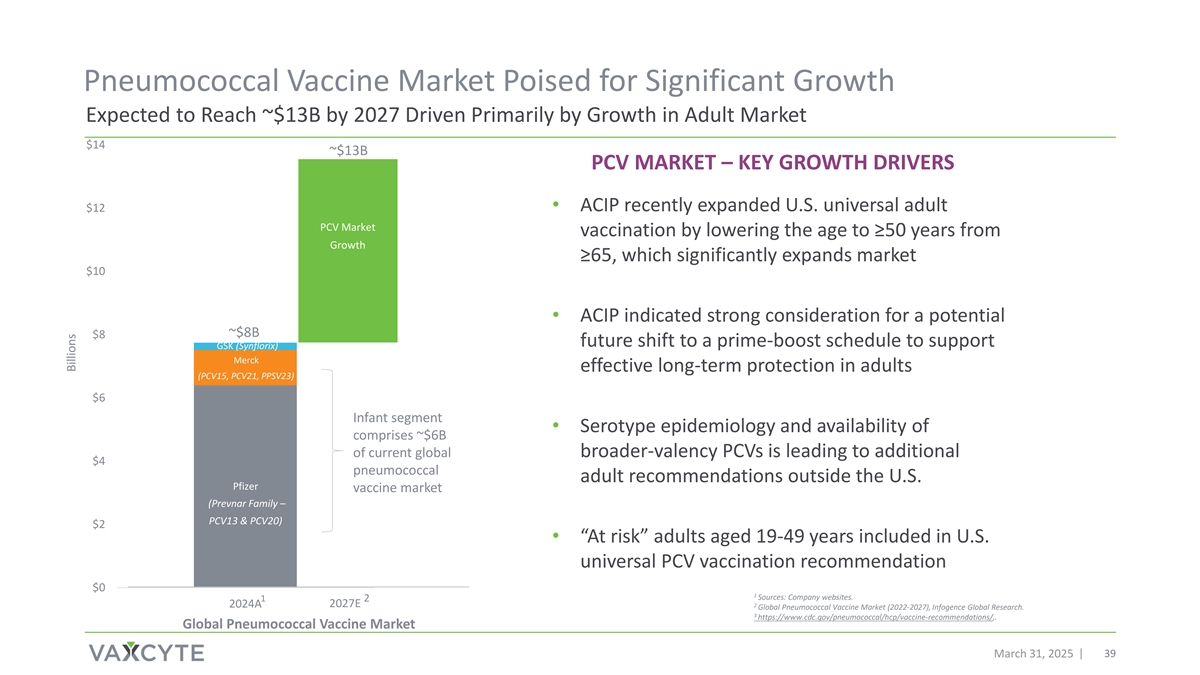
Pneumococcal Vaccine Market Poised for Significant Growth Expected to Reach ~$13B by 2027 Driven Primarily by Growth in Adult Market $14 ~$13B PCV MARKET – KEY GROWTH DRIVERS $12 • ACIP recently expanded U.S. universal adult Other PCV Market vaccination by lowering the age to ≥50 years from Market Growth ≥65, which significantly expands market Growth $10 • ACIP indicated strong consideration for a potential ~$8B $8 future shift to a prime-boost schedule to support GSK (Synflorix) Merck effective long-term protection in adults (PCV15, PCV21, PPSV23) $6 Infant segment • Serotype epidemiology and availability of comprises ~$6B of current global broader-valency PCVs is leading to additional $4 pneumococcal adult recommendations outside the U.S. Pfizer vaccine market (Prevnar Family – PCV13 & PCV20) $2 • “At risk” adults aged 19-49 years included in U.S. universal PCV vaccination recommendation $0 1 Sources: Company websites. 1 2 2024A 2027E 2 Global Pneumococcal Vaccine Market (2022-2027), Infogence Global Research. 3 https://www.cdc.gov/pneumococcal/hcp/vaccine-recommendations/,. Global Pneumococcal Vaccine Market March 31, 2025 39 39 Billions

Pipeline of High-Fidelity Vaccines Broad-Spectrum Conjugate and Novel Protein Vaccines to Prevent or Treat Bacterial Infectious Diseases Preclinical Phase 1 Phase 2 Phase 3 Adults 50+ 31-Valent VAX-31 Infants PCV Candidate 24-Valent VAX-24 Infants PCV Candidate VAX-XL Adults & Infants VAX-XL PCV Candidate Novel Group A Adults & Infants VAX-A1 Strep Vaccine Novel Therapeutic VAX- Adults PG Periodontitis Vaccine Novel Shigella Vaccine VAX-GI Adults & Infants March 31, 2025 40

Q&A with Management Grant Pickering Jim Wassil Andrew Guggenhime Chief Executive Officer, Director Executive Vice President and Chief President and Chief Financial Officer and Founder Operating Officer March 31, 2025 41


Appendix Slides

Study Safety, Tolerability and Immunogenicity Key Outcome Measures DAY 7 AFTER EACH 1 MONTH POST-DOSE 1-4; 1 MONTH POST- 1 MONTH POST- 6 MONTHS PD4 * * DOSE ONGOING DURING DOSE 3 (PD3) DOSE 4 (PD4) PRIMARY SERIES • Solicited local reactions • Unsolicited adverse events (AE) • SAE, NOCI, MAAE, TEAE • Unsolicited AE • Serious adverse events (SAE), SAFETY AND • Solicited systemic new onset of chronic illnesses • SAE, NOCI and MAAE TOLERABILITY events (NOCI), medically attended adverse events (MAAE) and OUTCOME treatment emergent AE (TEAE) MEASURES • % of subjects achieving • % of subjects achieving IgG Immunoglobulin G (IgG) antibody concentration ≥0.35 antibody concentration ≥0.35 mcg/mL mcg/mL (seroconversion rate) • IgG GMC and IgG GMC ratio • IgG Geometric Mean (GMR) Concentration (GMC) • OPA GMT IMMUNOGENICITY • Opsonophagocytic activity • IgG and OPA Geometric Mean (OPA) Geometric Mean Titer Fold Rise (GMFR) from pre-Dose 4 OUTCOME (GMT) to 1-month PD4* MEASURES • % of subjects achieving a 4-fold rise in IgG and OPA from pre-Dose 4 to 1-month PD4* • % of subjects achieving IgG concentration ≥1.0 mcg/mL* * Effect on immunogenicity of concomitant vaccination are being evaluated on a subset of subjects PD3 and PD4 based on serum availability. *Data for these outcome measures will be available with final data set by end of 2025. March 31, 2025 44