UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 27, 2025
Sensei Biotherapeutics, Inc.
(Exact Name of Registrant as Specified in its Charter)
| Delaware | 001-39980 | 83-1863385 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 1405 Research Blvd, Suite 125 Rockville, MD |
20850 | |
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s telephone number, including area code: (240) 243-8000
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Securities Exchange Act of 1934:
| Title of each class |
Trading symbol |
Name of each exchange on which registered |
||
| Common Stock | SNSE | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On March 27, 2025, Sensei Biotherapeutics, Inc. (the “Company”) issued a press release titled “Sensei Biotherapeutics Reports Favorable Preliminary Dose Expansion Data for Solnerstotug in PD-(L)1 Resistant Tumors.” The press release also included information regarding a conference call to discuss the clinical trial results. A copy of the press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The Company is also updating its corporate presentation on its website, which the Company will reference during the conference call described above. A copy of the presentation is furnished as Exhibit 99.2 to this Current Report on Form 8-K.
The information in Item 7.01 and the exhibits attached hereto are being furnished and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall they be deemed incorporated by reference into any filing under the Exchange Act or the Securities Act of 1933, as amended, whether filed before or after the date hereof and regardless of any general incorporation language in such filing.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit |
Exhibit Description |
|
| 99.1 | Press release dated March 27, 2025 | |
| 99.2 | Sensei Biotherapeutics, Inc. corporate presentation, dated March 2025 | |
| 104 | The cover page from this Current Report on Form 8-K, formatted in Inline XBRL | |
2
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Sensei Biotherapeutics, Inc. | ||||
| Date: March 27, 2025 | /s/ Christopher W. Gerry |
|||
| Christopher W. Gerry | ||||
| General Counsel and Secretary | ||||
3
Exhibit 99.1
Sensei Biotherapeutics Reports Favorable Preliminary Dose Expansion Data for Solnerstotug in PD-(L)1 Resistant Tumors
– Initial clinical activity in a PD-(L)1 resistant population, with an ORR almost three times higher
than historical PD-(L)1 rechallenge response rates, with data still maturing –
– One durable complete response in a Merkel Cell Carcinoma (MCC) patient and two partial responses (PR), including one in a second MCC patient and one in a microsatellite instability-high (MSI-H) Colorectal Cancer (CRC) patient, all of whom were previously treated with and progressed on immunotherapy –
– All PD-(L)1 resistant “hot” tumor patients with tumor shrinkage remain on study, suggesting potential for deepening responses over time –
– Sensei to host investor webcast today at 5:30 p.m. ET –
BOSTON, MA – March 27, 2025 – Sensei Biotherapeutics, Inc. (Nasdaq: SNSE), a clinical-stage biotechnology company focused on the discovery and development of next-generation therapeutics for cancer patients, today announced initial results from the dose expansion portion of its Phase 1/2 trial evaluating solnerstotug (formerly SNS-101), a conditionally active monoclonal antibody targeting VISTA (V-domain Ig suppressor of T cell activation).
“Checkpoint inhibitor resistance remains a significant challenge for patients with advanced cancer, with limited treatment options beyond chemotherapy or clinical trials,” said Ron Weitzman, M.D., Chief Medical Officer of Sensei Biotherapeutics. “Historically, patients who progress on PD-(L)1 therapy are estimated to have a ≤5% likelihood of response to PD-(L)1 rechallenge, making this an extremely difficult-to-treat population, and a large unmet medical need. The initial 14% response rate seen with solnerstotug is nearly three times higher than what would typically be expected in this setting. We believe these early data suggest solnerstotug may provide a meaningful clinical benefit in select tumor types, and we look forward to further evaluating its potential in Phase 2.”
Phase 1 Dose Expansion Preliminary Results
The Phase 1 dose expansion trial is a multi-center, open-label, dose expansion study evaluating solnerstotug as monotherapy and in combination with Libtayo® (cemiplimab, Regeneron’s PD-1 inhibitor) in both a basket of “hot” tumors that typically respond to immunotherapy but have progressed on prior PD-(L)1 therapy and a single “cold” tumor histology (microsatellite stable (MSS) CRC) that is typically unresponsive to immunotherapy.
As of March 17, 2025, the study has enrolled 60 patients, including:
| • | 40 patients with “hot” tumors, including Non-Small Cell Lung Cancer (NSCLC), Head and Neck (H&N) cancer, Melanoma, Renal Cell Carcinoma (RCC), Merkel Cell Carcinoma (MCC), MSI-H Colorectal Cancer (CRC), and other tumor types. All patients received the combination of solnerstotug (3 mg/kg or 15 mg/kg) and cemiplimab. |
| • | At the time of this data cut, 21 “hot” tumor PD-(L)1 resistant patients were considered evaluable for efficacy, having received at least one post-baseline scan. An additional 11 patients have not yet reached the first baseline scan, and an additional 8 patients discontinued the study prior to any post-baseline scan. |
| • | 20 patients with PD-(L)1 non-responsive microsatellite stable (MSS) Colorectal Cancer (CRC) were included to assess potential activity in “cold” tumors. Of these patients, 10 were enrolled in a monotherapy (15 mg/kg) cohort and 10 received the combination of solnerstotug (15 mg/kg) and cemiplimab. |
| • | 17 MSS CRC patients were considered evaluable for efficacy, having received at least one post-baseline scan. Three patients discontinued the study prior to any post-baseline scan. |
Key findings include:
| • | 14% ORR (3 patients) and 62% DCR (disease control rate) (13 patients) among 21 evaluable PD-(L)1 resistant “hot” tumor patients. |
| • | Durable complete response (CR) in a MCC patient treated with 15 mg/kg solnerstotug + cemiplimab. Patient continues on treatment at 42+ weeks. Previously received a PD-(L)1 therapy for 15 months in the adjuvant setting prior to progressing on therapy. |
| • | Partial response (PR) at Week 12 in a MCC patient treated with 15 mg/kg solnerstotug + cemiplimab. Patient continues on treatment at 12+ weeks. Previously received several lines of checkpoint therapy, including the combination of PD-1 and CTLA-4, with a best response of stable disease prior to progressing on therapy. |
| • | Partial response (PR) at Week 36 in an MSI-H CRC patient treated with 15 mg/kg solnerstotug + cemiplimab. Patient had durable stable disease (SD) through the course of treatment before converting to a PR at Week 36. Patient continues on treatment at 36+ weeks. Previously received a PD-(L)1 therapy for more than 4 years, where the patient achieved a CR prior to progressing on therapy. |
| • | Six PD-(L)1 resistant patients with SD remain on treatment past 12+ weeks, with tumor reductions ranging from 0% to 17%, suggesting durable disease control in a subset of patients. |
| • | All PD-(L)1 resistant patients on study with tumor shrinkage remain on treatment, suggesting potential for prolonged benefit. |
| • | None of the MSS CRC patients experienced a CR or PR, consistent with prior checkpoint therapy in this “cold” tumor type. |
Solnerstotug continues to be well tolerated, with no dose-limiting toxicities and the majority of AEs Grade 1 or 2 in severity. Out of 60 patients, there have been four (7%) cases of Grade 1 cytokine release syndrome (CRS), all mild and manageable. Two patients in the combination cohort experienced immune-mediated events.
A Challenging Treatment Landscape for PD-(L)1 Resistant Tumors
Patients who progress following treatment with PD-1 or PD-L1 inhibitors (“secondary resistance”) face a particularly poor prognosis, as resistance to immune checkpoint blockade is a significant challenge in oncology. According to the Society for Immunotherapy of Cancer (SITC), secondary resistance is defined as disease progression following an initial period of disease control. For patients who develop secondary resistance after treatment with PD-(L)1 immune checkpoint inhibitors, the likelihood of benefiting from a rechallenge with the same therapy is estimated to be 5% or less.1 Currently, treatment options for PD-(L)1 resistant tumors are limited, with many patients receiving chemotherapy, experimental therapies in clinical trials, or palliative care in the absence of effective alternatives. Existing immune checkpoint inhibitor (ICI) combination therapies have not been approved in this setting. They are either highly toxic, such as CTLA-4+PD-1 in which up to 40% of patients discontinue due to severe immune-related adverse events (irAEs), or offer limited treatment potential, such as LAG-3+PD-1 where the ORR has been 9-12%.
| 1. | Kluger H, et al. J immunother Cancer 2023 |
“While we remain in the early stages of evaluating solnerstotug’s therapeutic potential, the observed responses—particularly in MCC and MSI-H CRC—are encouraging given the historically poor prognosis of these patients once they have progressed on checkpoint therapy,” said Dr. Shiraj Sen, M.D., Medical Oncologist and Director of Clinical Research at NEXT Oncology - Dallas, and a Principal Investigator for the solnerstotug study. “Continued clinical evaluation will be key in determining which patients are most likely to benefit from this approach.”
Next Steps: Preparing for Phase 2
Subject to raising sufficient capital, Sensei plans to initiate a Phase 2 study in Q1 2026, with the trial design and patient selection strategies to be informed by the ongoing dose expansion results. Further analyses, including biomarker-based patient selection, are underway to optimize the Phase 2 design.
Investor Webcast Information
Sensei will host an investor webcast today at 5:30 p.m. ET, featuring company leadership and Dr. Shiraj Sen, M.D., Ph.D., Medical Oncologist and Director of Clinical Research at NEXT Oncology-Dallas, an investigator in the Phase 1/2 study.
Access the live event here: https://lifescievents.com/event/sensei-bio-2/
A replay will be available after the webcast on the Investor Relations page of Sensei’s website: https://investors.senseibio.com
About Sensei Biotherapeutics
Sensei Biotherapeutics (Nasdaq: SNSE) is a clinical-stage biotechnology company developing next-generation immunotherapies for cancer. Through its TMAb™ (Tumor Microenvironment Activated biologics) platform, Sensei engineers conditionally active therapeutics designed to modulate immune responses within the tumor microenvironment. The company’s lead product candidate, solnerstotug, is a VISTA-targeting monoclonal antibody designed to restore T cell activity in checkpoint inhibitor-resistant tumors. For more information, visit www.senseibio.com and follow Sensei on X @SenseiBio and LinkedIn.
Cautionary Note Regarding Forward-Looking Statements
Any statements contained in this press release that do not describe historical facts may constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. These statements may be identified by words and phrases such as “believe”, “designed to,” “expect”, “may”, “plan”, “potential”, “will”, and similar expressions, and are based on Sensei’s current beliefs and expectations.
These forward-looking statements include expectations regarding the development and potential therapeutic benefits of Sensei’s product candidates, the timing of Sensei’s Phase 1/2 clinical trial of solnerstotug (SNS-101), including reporting of data therefrom, and its plans to initiate a Phase 2 study in the first quarter of 2026, subject to raising sufficient capital. These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements. Risks and uncertainties that may cause actual results to differ materially include uncertainties inherent in the development of therapeutic product candidates, such as the risk that any one or more of Sensei’s product candidates will not be successfully developed or commercialized; the risk of delay or cessation of any planned clinical trials of Sensei’s product candidates; the risk that prior results, such as signals of safety, activity or durability of effect, observed from preclinical studies and clinical trials, will not be replicated or will not continue in ongoing or future studies or clinical trials involving Sensei’s product candidates; the risk that Sensei’s product candidates or procedures in connection with the administration thereof will not have the safety or efficacy profile that Sensei anticipates; risks associated with Sensei’s dependence on third-party suppliers and manufacturers, including sole source suppliers, over which Sensei may not always have full control; risks regarding the accuracy of Sensei’s estimates of expenses, capital requirements and needs for additional financing; and other risks and uncertainties that are described in Sensei’s Quarterly Report on Form 10-Q filed with the U.S. Securities and Exchange Commission (SEC) on November 14, 2024 and Sensei’s other Periodic Reports filed with the SEC. Any forward-looking statements speak only as of the date of this press release and are based on information available to Sensei as of the date of this release, and Sensei assumes no obligation to, and does not intend to, update any forward-looking statements, whether as a result of new information, future events or otherwise.
Investor Contact:
Michael Biega
Senior Director, Investor Relations
Sensei Biotherapeutics
mbiega@senseibio.com
Media Contact:
Joyce Allaire
LifeSci Advisors
Jallaire@lifesciadvisors.com

Conditionally Active Antibodies for Immuno-oncology March 2025 Exhibit 99.2

Disclaimer This presentation has been prepared by Sensei Biotherapeutics, Inc. (the “Company,” "we," "us") and is made for informational purposes only. The information set forth herein does not purport to be complete or to contain all of the information you may desire. Statements contained herein are made as of the date of this presentation unless stated otherwise, and the delivery of this presentation at any time shall not under any circumstances create an implication that the information contained herein is correct as of any time after such date or that information will be updated or revised to reflect information that subsequently becomes available or changes occurring after the date hereof. This presentation contains estimates and other statistical data made by independent parties and by us relating to market shares and other data about our industry. This presentation also contains "forward-looking" statements as that term is defined in the Private Securities Litigation Reform Act of 1995 that are based on our management's beliefs and assumptions and on information currently available to management. These forward-looking statements include, without limitation, expectations regarding the development and potential therapeutic benefits of our product candidates; the expected safety, pharmacokinetic and efficacy profile of our product candidates, including solnerstotug; the expected timing of clinical data from our Phase 1/2 clinical trial of solnerstotug; and expectations regarding the initiation of a Phase 2 clinical trial of solnerstotug. When used in this presentation, the words and phrases "designed to," "may," "believes," "intends," "seeks," "anticipates," "plans," "estimates," "expects," "should," "assumes," "continues," "could," "will," "future" and the negative of these or similar terms and phrases are intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Risks and uncertainties that may cause actual results to differ materially include uncertainties inherent in the development of therapeutic product candidates, such as preclinical discovery and development; conduct of clinical trials and related regulatory requirements, including the risk of delay or cessation of any clinical trials of Sensei’s product candidates; the risk that prior results, such as signals of safety, activity or durability of effect, observed from preclinical trials and early results from the clinical trial of solnerstotug, will not be replicated or will not continue in ongoing or future studies or clinical trials involving Sensei’s product candidates, including solnerstotug; our reliance on third parties over which we may not always have full control; risks regarding the accuracy of our estimates of expenses, capital requirements and needs for additional financing; and other risk and uncertainties that are described in our Quarterly Report on Form 10-Q filed with the SEC on November 14, 2024 and our other Periodic Reports filed with the SEC. Forward-looking statements represent our management's beliefs and assumptions only as of the date of this presentation and include all matters that are not historical facts. Our actual future results may be materially different from what we expect. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. Certain information contained in this presentation relates to, or is based on, studies, publications, surveys and other data obtained from third-party sources and the Company's own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source.

Leadership Team with History of Oncology Antibody Success Josiah Craver, CPA SVP, Finance John Celebi, MBA President and CEO Christopher Gerry, J.D. SVP, General Counsel Edward van der Horst, Ph.D. Chief Scientific Officer Stephanie Krebs, M.S., MBA Chief Business Officer Ron Weitzman, M.D. Chief Medical Officer (part-time)

Sensei Investment Highlights Unique, conditionally active MOA of lead VISTA targeted candidate solnerstotug designed to maximize anti-tumor activity and minimize negative side effects characteristic of other first generation, VISTA targeted approaches Expression of VISTA in multiple solid tumor types provides the opportunity to pursue several indications, many of which are large markets with high unmet need Recent data from ongoing Phase 1/2 dose expansion trial showing favorable early efficacy results with 2/2 patients enrolled with PD-(L)1 resistant Merkel Cell Carcinoma achieved responses, including 1 durable complete response and 1 partial response, and 1 partial response in a PD-(L)1 resistant MSI-H CRC patient and clinically meaningful activity in multiple additional tumor types Demonstrated favorable safety profile and potential best-in-class PK profile Very few public company opportunities to invest in novel, VISTA-targeted approaches – most other companies pursuing VISTA are either private or large pharma companies with market caps >$100bn

Program (Target) Indication Discovery IND-enabling Phase 1 Phase 2 Upcoming Milestone Full expansion data by year-end 2025 Innovative Pipeline of IO Drugs with Broad Commercial Potential *Sensei has entered into a clinical supply agreement with Regeneron supporting the planned evaluation of solnerstotug in combination with Regeneron’s anti-PD-1 therapy Libtayo® (cemiplimab) in a Phase 1/2 clinical trial in solid tumors. Solid Tumors SNS-102 (VSIG4) Solid Tumors SNS-103 (ENTPDase1/CD39) Solid Tumors SNS-201 (VISTAxCD28) Solid Tumors Solnerstotug* (VISTA)

Differentiated Features of Solnerstotug (SNS-101) VISTA Targeting mAb

Solnerstotug is a Potential First-in-Class VISTA Targeting Mab That Binds Selectively in the Tumor Microenvironment Tumor cells Periphery Solnerstotug bound to VISTA Solnerstotug unbound Targets VISTA: An immune checkpoint protein and B7 family member that drives immunosuppression analogous to PD-1/PD-L1 Unique and extensive expression pattern, found on tumors and myeloid-lineage cells Acts in a pH-sensitive matter, engaging with its T-cell receptor PSGL-1 selectively under acidic conditions Plays a potentially key role in both primary (innate) and secondary (acquired) resistance to checkpoint blockade Unique MOA: Selectively inhibits VISTA:PSGL-1 interaction within the acidic TME Drives anti-tumor activity by reversing immunosuppression Solnerstotug is a pioneering approach designed to overcome toxicities associated with 1st-generation antibodies *Gao et al. Nat Med. 2017 Kakavand et al. Mod Pathol. 2017

The VISTA Commercial Opportunity Source: Nishizaki, D. et al. ESMO Open, Volume 9, Issue 4, 102942, Incidence and Survival: NCI SEER Data 2024, Expression: internal data and publications Unlike, PD-(L)1, VISTA is Found in Nearly All Solid Tumors with High Unmet Need Tumors with VISTA expression Tumors with no evidence of VISTA expression 32.3% (166/514) Source: Merck, BMS, Roche, AstraZeneca, Regeneron VISTA Expression Levels Are Relatively High Across Cancer Indications Sales of Top 5 PD-(L)1 Targeted Therapies PD-(L)1 Targeted Therapies Are One of the Largest Classes of Drugs Across All Therapeutic Areas VISTA Expression is Found in the Majority of Solid Tumor Indications $37 $43 $49

The Challenge of Targeting VISTA Dose-limiting toxicity Grade 3 CRS-associated encephalopathy JNJ-61610588 Human Plasma Concentration JNJ-61610588 (CI-8993) was the first anti-VISTA antibody to be studied in clinical trials in 2016 (NCT02671955)1 Transient Cytokine Release Syndrome (CRS) observed in several patients at 0.15 mg/kg Transient Grade 3 CRS-associated encephalopathy observed at 0.3 mg/kg, after which Janssen halted the study Challenging PK profile Non-linear PK, short t1/2 1 Curis, Inc., Corporate Presentation, Feb 2022 Competitors Halted Development of VISTA Antibodies as a Result of Toxicities and Poor PK Key issues likely driven by extensive off-tumor expression of VISTA
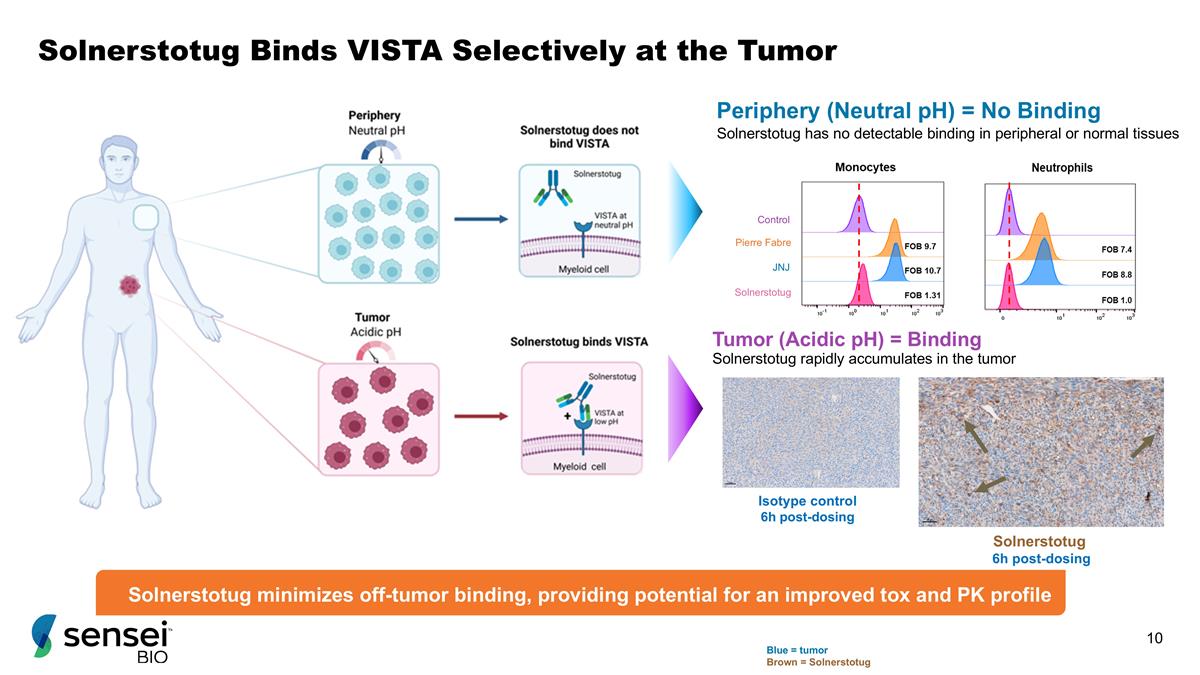
Solnerstotug Binds VISTA Selectively at the Tumor Pierre Fabre JNJ Solnerstotug Control Periphery (Neutral pH) = No Binding Solnerstotug has no detectable binding in peripheral or normal tissues Solnerstotug rapidly accumulates in the tumor Solnerstotug 6h post-dosing Isotype control 6h post-dosing Blue = tumor Brown = Solnerstotug Tumor (Acidic pH) = Binding Solnerstotug minimizes off-tumor binding, providing potential for an improved tox and PK profile
Clinical Trial Collaboration to Evaluate Solnerstotug with Libtayo® (cemiplimab) Clinical supply agreement with Regeneron’s Libtayo (cemiplimab) to evaluate solnerstotug + cemiplimab as a treatment for solid tumors Libtayo® (cemiplimab): anti-PD-1 monoclonal antibody Solnerstotug: Conditionally active monoclonal antibody targeting VISTA
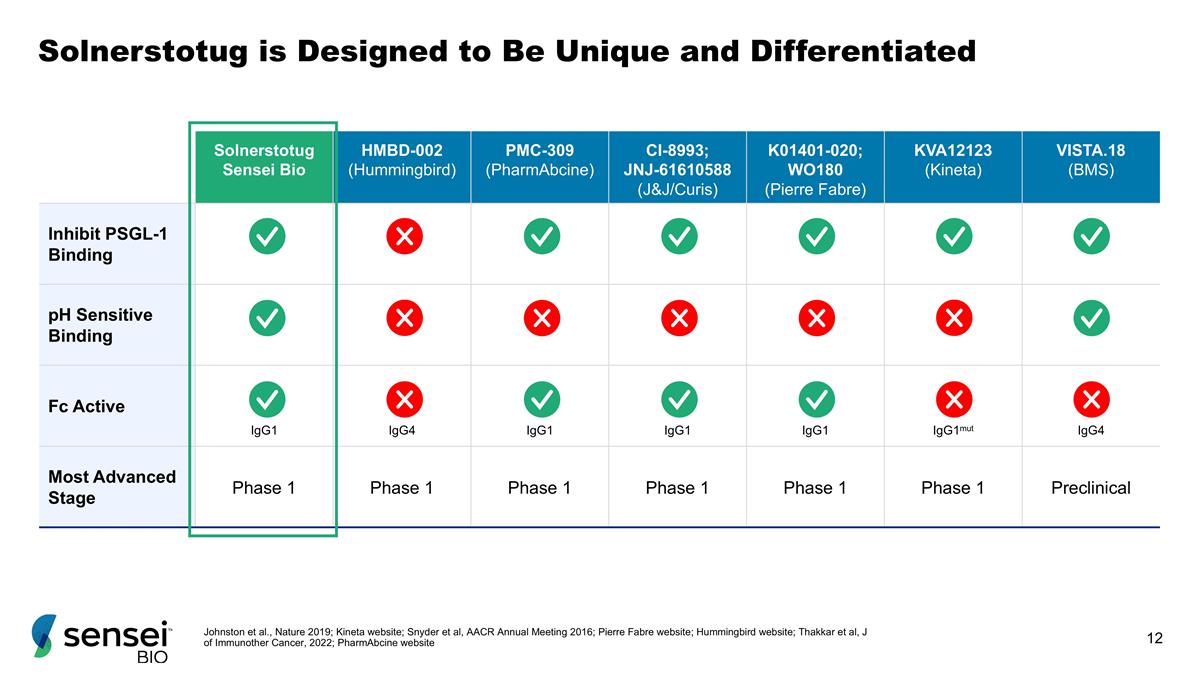
Solnerstotug is Designed to Be Unique and Differentiated Johnston et al., Nature 2019; Kineta website; Snyder et al, AACR Annual Meeting 2016; Pierre Fabre website; Hummingbird website; Thakkar et al, J of Immunother Cancer, 2022; PharmAbcine website Solnerstotug Sensei Bio HMBD-002 (Hummingbird) PMC-309 (PharmAbcine) CI-8993; JNJ-61610588 (J&J/Curis) K01401-020; WO180 (Pierre Fabre) KVA12123 (Kineta) VISTA.18 (BMS) Inhibit PSGL-1 Binding pH Sensitive Binding Fc Active IgG1 IgG4 IgG1 IgG1 IgG1 IgG1mut IgG4 Most Advanced Stage Phase 1 Phase 1 Phase 1 Phase 1 Phase 1 Phase 1 Preclinical

Solnerstotug Dose Escalation Data

Solnerstotug Phase 1 Dose Escalation Study Cohort A1 0.3 mg/kg N=1 Cohort B1 3.0 mg/kg + cemi* N=6 Cohort A2 1.0 mg/kg N=3 Cohort A3 3.0 mg/kg N=3 Cohort A4 10.0 mg/kg N=3 Cohort A5 15.0 mg/kg N=6 Cohort B2 10.0 mg/kg + cemi* N=6 Cohort B3 15.0 mg/kg + cemi* N=6 Monotherapy Dose Escalation Solnerstotug (Q3W) Combination Dose Escalation Solnerstotug + Cemiplimab* (Q3W) Phase 1 Dose Escalation BOIN Design in Patients with Advanced Solid Tumors ü ü ü ü Phase 1 Study Objectives Primary Safety, tolerability, MTD/RP2D Secondary PK, immunogenicity & anti-tumor activity RP2D = Recommended Phase 2 Dose MTD = Maximum Tolerated Dose * cemi = Libtayo (cemiplimab) 350 mg ü ü ü = cleared DLT assessment period ü ü Given prior history of VISTA antibodies, Sensei prioritized establishing: Lack of severe CRS Acceptable PK Dosing at pharmacologically relevant levels

Phase 1 Dose Escalation Data Affirms Solnerstotug’s MOA and Focused Patient Population in Dose Expansion Solnerstotug positioned to be the first VISTA-targeted mAb to test the VISTA IO hypothesis Well-tolerated Commercially acceptable and potentially best-in-class PK profile First and only agent to be dosed at pharmacologically relevant levels Forward focus on patients with ”hot” tumors more likely to respond to immunotherapy Safety Profile Summary (Dose Escalation) No dose-limiting toxicities observed Majority of AEs were Grade 1 or 2 Two patients experienced Grade 1 CRS, providing further evidence that CRS is a class effect of VISTA-targeting antibodies

Solnerstotug Dose Expansion Data

Dose Expansion Cohort Designed to Explore Efficacy in "Hot" Tumor Population * Libtayo (cemiplimab) 350 mg "Hot" tumors: Responsive to PD-1 monotherapy "Cold" tumors: Unresponsive to PD-1 monotherapy MSS CRC “Cold” N=10 MSS CRC “Cold” N=10 Phase 1 Dose Expansion ~ 60 patients Phase 1 Study Objectives Primary Safety, tolerability, MTD/RP2D Secondary PK, immunogenicity & anti-tumor activity RP2D = Recommended Phase 2 Dose MTD = Maximum Tolerated Dose MSS = Microsatellite stable CRC = Colorectal cancer NSCLC = Non small cell lung cancer H&N = Head and neck cancer Monotherapy 15 mg/kg Combination 3 mg/kg and 15 mg/kg + cemiplimab* Focused on the activity profile and optimizing dose / patient population for Phase 2 Focused on a basket of “hot” tumors (combination therapy) and one “cold” tumor type (monotherapy and combination therapy) Nearly all patients in “hot” tumor cohort: Will have received and progressed on a prior anti-PD-1 therapy; or Are PD-L1 negative Historical response rates to PD-1 rechallenge following progression on PD-1 are in the single digits1,2 Favorable activity was observed in patients with “hot” tumors No signal of activity was observed in patients with “cold” tumors (MSS CRC; see appendix) Livanou et al., Cancers 2024 Kluger H, et al. J Immunother Cancer 2023 “Hot” Basket (NSCLC, H&N, Melanoma) N=40

Patient Disposition Phase 1 Dose Expansion Expansion Cohort Basket of “Hot” Tumors Monotherapy Combination solnerstotug N = 10 (%) solnerstotug + cemi N = 50 (%) Enrolled 10 50 Dose Received 3 mg/kg 15 mg/kg 0 10 (100) 16 (32) 34 (68) Treatment Ongoing 1 (10) 23 (46) Discontinued 9 (90) 27 (54) Reason for Discontinuation Progressive Disease 6 (60) 20 (40) Adverse Event 0 (0) 0 (0) Withdrew Consent 0 (0) 1 (2) Death 1 (10)* 2 (4)** Clinical Progression 0 (0) 3 (6) Physician Decision/Lack of clinical benefit 1 (0) 1 (2) Lost to Follow-up 1 (10) 0 (0) Combination solnerstotug + cemi N = 40 (%) Enrolled 40 Dose Received 3 mg/kg 15 mg/kg 16 (40) 24 (60) Treatment Ongoing 23 (58) Discontinued 17 (42) Reason for Discontinuation Progressive Disease 10 (25) Adverse Event 0 (0) Withdrew Consent 1 (3) Death 2 (5)** Clinical Progression 3 (8) Physician Decision/Lack of clinical benefit 1 (3) Lost to Follow-up 0 (0) *Death due to Myocardial Infarction, that resulted in death; not related to SNS-101 **Death due to Hypoxia that resulted in death; secondary to the overall event of disease progression, not related to SNS-101 or Cemi; death due to PD Entire Expansion Cohort Data as of March 17, 2025

Patient Demographics (Patients with “Hot” Tumors) Phase 1 Dose Expansion solnerstotug + cemi N = 40 (%) Gender, n (%) Male 29 (73) Female 11 (27) Age, years Median (min, max) 73.5 (28, 85) Baseline ECOG, n (%) 0 11 (27) 1 29 (73) Prior lines metastatic therapy Median (min/max) 2 (0, 7) Prior PD-1/PD-L1, n (%) % Yes* 36 (90) Prior PD-1/PD-L1 regimen preceding enrollment in the study, n (%) % Yes 24 (60) solnerstotug + cemi N = 40 (%) Cancer Type, n (%) Responsive to PD-1 (e.g. “hot” tumors) Head and Neck 11 (28) NSCLC 10 (25) Melanoma 5 (13) Renal 5 (13) Merkel Cell 2 (5) CRC- MSI High 2 (5) Cholangiocarcinoma- MSI High 1 (3) Endometrial 1 (3) Esophageal 1 (3) Hepatocellular 1 (3) Pleomorphic Spindle cell 1 (3) *Two NSCLC patients were I/O naïve because 1 had very low PD-L1 expression and the other was EGFR mutant. One pleomorphic cancer patient was I/O naïve due to PD-1 not being approved in that setting. 1 patient does not have data entered about prior treatment with PD-1. Data as of March 17, 2025 90% of patients received and progressed on a prior PD-1/PD-L1 therapy

Historical Response Rates Highlight Difficulties of Re-Challenging with PD-(L)1 NSCLC Example 1 Fujita et al, 2018 Watanabe et al, 2019 Katayama et al, 2019 Fujita et al, 2020 No. of Patients 12 14 35 15 Reason for discontinuation PD PD PD PD 1st course Re-challenge 1st course Re-challenge 1st course Re-challenge 1st course Re-challenge Treatment used Anti-PD-1 Anti-PD-1 Anti-PD-(L)1 Anti-PD-(L)1 Anti-PD-(L)1 Anti-PD-L1 Anti-PD-1 Anti-PD-1 ORR, N (%) 7 (58.3) 1 (8.3) 3 (21.4) 1 (7.1) 12 (34.3) 1 (2.9) 0 (0) 0 (0) 1 Livanou et al., 2024 2 Kluger H, et al. J immunother Cancer 2023 Spagnolo et al., BMC Cancer 2021 Aligned with SITC definition of acquired resistance, which defines ≤5% chance of benefit with ICI therapy past progression 2

Preliminary Efficacy Data in PD-(L)1-Refractory “Hot” Tumor Patients is Favorable Phase 1 Dose Expansion Data as of March 17, 2025 -100% * * * * * * * -30% * Patients who received a prior PD-(L)1 (n=21) solnerstotug (3 or 15 mg/kg) + cemiplimab -100% All of PD-(L)1 resistant patients on study with tumor shrinkage remain on study with potential for further clinical benefit * * 21 “hot” tumor PD-(L)1 refractory patients were considered evaluable for efficacy having received at least one post-baseline scan. An additional 11 patients have not yet reached first baseline scan with an additional 8 patients discontinued study prior to initial scan.

“Hot” Tumor PD-(L)1 Refractory Patients Have Favorable Duration on Study and Achieved Responses or Long-Term Stable Disease Data as of March 17, 2025 Patients who received a prior PD-(L)1 (n=21) solnerstotug (3 or 15 mg/kg) + cemiplimab Ongoing treatment 07-001 (H&N) and 08-001 (NSCLC): continued beyond progression

Merkel Cell Carcinoma Patient (03-009) Complete Response 15 mg/kg solnerstotug + cemiplimab 85 yo male with Merkel Cell Carcinoma of skin (right nasal sidewall) Diagnosed June 2022 TMB 91, MSS. ATR, BLM, RB1 TP53 RASA1, CDKN1B and ARID1B K1250fs Prior treatment: 20Oct2022 to 18Jan2024: Avelumab (Bavencio) with Signatera of 553 MTM/ml, best response stable disease, stopped due to progression. 06Dec2022 to 24Jan2023: underwent RT (36 Gy) to nose and neck; sustained a prompt and robust response (PR) to Rx Cycle 1 Day 1: 24Apr2024 Treatment ongoing *Missed 2 cycles (Cycle 12 and 13) of drug due to extended vacation. Data as of March 17, 2025

Merkel Cell Carcinoma Patient (01-046) Partial Response 15 mg/kg solnerstotug + cemiplimab 58 yo female with Metastatic Merkel cell carcinoma Diagnosed 2Dec2022 TMB 15.16 mut/Mb KMT2D, DPTOR, EPHA5, CREBBP+ Hx of follicular lymphoma (received chemo followed by Zydelig maintenance until 2021, remission) Prior Treatment: March 2023 to July 2023: Pembro (adjuvant setting), best response unknown, stopped due to progression Aug 2023 to Oct 2023: Nivolumab + Ipilimumab, best response and reason for stopping unknown Nov 2023 to Apr 2024: Continued on Nivolumab alone, best response SD, stopped due to progression May 2024 to October 2024: DM919 (MICA/B Antibody), best response SD, stopped due to progression Cycle 1 Day 1: 20Nov2024 Treatment ongoing Data as of March 17, 2025
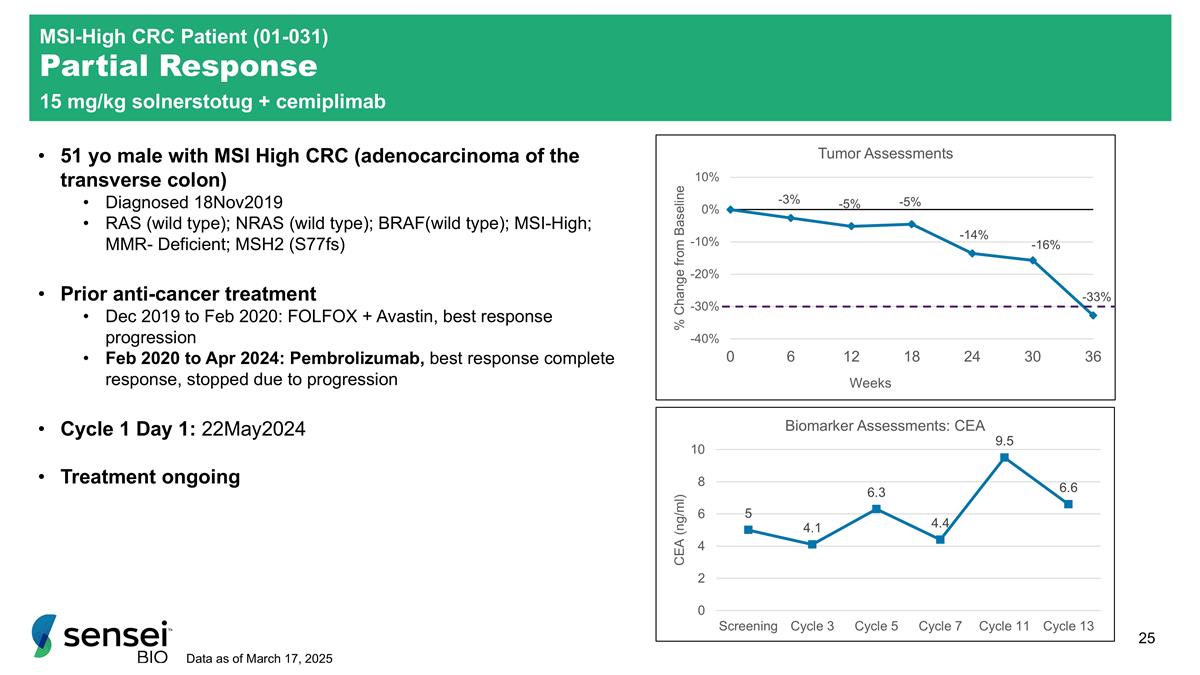
MSI-High CRC Patient (01-031) Partial Response 15 mg/kg solnerstotug + cemiplimab 51 yo male with MSI High CRC (adenocarcinoma of the transverse colon) Diagnosed 18Nov2019 RAS (wild type); NRAS (wild type); BRAF(wild type); MSI-High; MMR- Deficient; MSH2 (S77fs) Prior anti-cancer treatment Dec 2019 to Feb 2020: FOLFOX + Avastin, best response progression Feb 2020 to Apr 2024: Pembrolizumab, best response complete response, stopped due to progression Cycle 1 Day 1: 22May2024 Treatment ongoing Data as of March 17, 2025

59 yo male with metastatic Renal Clear Cell Carcinoma (malignant neoplasm of left kidney, except renal pelvis) Diagnosed 8Mar2017 Evidence of a PTEN deletion in his tumor MSI-high not detected Prior Treatment/Surgery: Apr 2018 to Feb 2019: Pembro + Lenvatinib, best response SD, stopped due to progression Mar 2019 to Jul 2020: Sutent, best response progression Aug 2019 to Sep 2019: CB-839 (Glutaminase Inhibitor) + Talazoparib, best response progression Oct 2019 to Dec 2019 : Nivo + Ipi, best response progression Jan 2020 for 3 weeks: Cabometyx, stopped due to adverse event/toxicity Feb 2020 to Aug 2023: XL092 (TKI), best response partial response, stopped due to progression Aug 2023 to Sep 2024: AB521 (HIF-alpha Inhibitor), best response partial response, stopped due to progression Cycle 1 Day 1: 21Oct2024 Treatment Ongoing Renal Cell Carcinoma Patient (03-011) Stable Disease (SD) with Tumor Regression 3 mg/kg solnerstotug + cemiplimab Data as of March 17, 2025

Hepatocellular Patient (01-038) Durable Stable Disease and Tumor Kinetics and AFP Consistent with a Pattern of Pseudo-Progression 15 mg/kg solnerstotug + cemiplimab 67 yo male with hepatocellular carcinoma Diagnosed 2017 RAD51C; ATM; TP53; CTNNB1 Prior Treatment/Surgery: 2017 and 2018: Chemoembolization x 2, Radiofrequency Ablation. Jun-Aug 2019 : Sorafenib, stopped due to Toxicity Sep 2019 to Dec 2019: Nivolumab, best response progression Dec 2019 to 2020: Lenvima, best response stable disease, stopped due to progression Aug 2020 to Jan 2022: Pembrolizumab, best response stable disease, stopped due to progression Feb 2022 to Jan 2024: Cabozantinib, best response stable disease, stopped due to progression Mar 2024 to Jun 24: OR502 (anti-LILRB mAB) + Cemiplimab, best response stable disease, stopped due to progression Cycle 1, Day 1: 14Aug2024 Treatment ongoing Data as of March 17, 2025

Solnerstotug is demonstrating favorable early signs of efficacy in patients with acquired resistance to PD-(L)1 therapy 2/2 patients enrolled with PD-(L)1 resistant Merkel Cell Carcinoma achieved responses, including 1 durable CR and 1 PR. Additionally, a PD-(L)1 resistant MSI-H CRC patient experienced a PR at Week 36 Many additional PD-(L)1 resistant patients on study are experiencing slow and durable tumor shrinkage, a hallmark of I/O agents1 All PD-(L)1 resistant patients on study with tumor shrinkage remain on study with potential for further clinical benefit Sensei believes future studies in patients that are checkpoint naïve could have even greater potential for clinical benefit Data as of March 17, 2025 Clinical Activity Highlights Phase 1 Dose Expansion 1. Pardoll, Nature Communications, April 2012, Volume 12

Solnerstotug Continues to be Well Tolerated as Monotherapy and in Combination with Cemiplimab Phase 1 Dose Expansion Monotherapy Combination Solnerstotug 15 mg/kg n=10 (%) Solnerstotug 3 or 15 mg/kg* + cemi N=50* (%) At least 1 TEAE 9 (90) 37 (74) At least 1 SAE 3 (30) 12 (24) ≥Grade 3 TEAE 2 (20) 14 (28) At least 1 TEAE leading to discontinuation 0 (0) 2 (5)** AESI 1 (10) 3 (6) Immune-mediated 0 (0) 2 (4) CRS 1 (10) 3 (6) Dose Expansion Safety Profile Summary *16 patients received 3 mg/kg and 34 patients received 15 mg/kg solnerstotug in combination with cemiplimab **1 patient had Grade 3 acute kidney injury, not related to solnerstotug or cemi; the primary reason for discontinuation is clinical progression. 1 patient had Grade 3 ischemic stroke, not related to solnerstotug or cemi; the primary reason for discontinuation is progressive disease. Majority of adverse events were Grade 1 or 2 All CRS events were Grade 1 (4 of 60 patients; all at 15 mg/kg) Data as of March 17, 2025

Preferred Term Monotherapy Solnerstotug 15 mg/kg n=10 Combination Solnerstotug 3 or 15 mg/kg + cemi n=50 Total n=60 Hypomagnesaemia 2 8 10 Constipation 2 6 8 Nausea 1 7 8 Fatigue 1 5 6 Infusion related reaction 2 4 6 Anaemia 1 5 6 Diarrhoea 1 4 5 Vomiting 0 5 5 Cytokine Release Syndrome 1 3 4 Dyspnoea 0 4 4 Lymphocyte count decreased 0 4 4 Decreased appetite 0 3 3 Dehydration 0 3 3 Dizziness 0 3 3 Myalgia 1 2 3 Pyrexia 0 3 3 Upper Respiratory Tract Infection 0 3 3 Data as of March 17, 2025 Most Frequently Occurring AEs Regardless of Causality Phase 1 Dose Expansion

Pharmacokinetic Data Support Once Every Three Week (or Greater) Dosing Detectable in blood for thousands of hours (e.g., weeks) Supports Q3W dosing in humans Dose proportional exposure (0.3 to 15.0 mg/kg) consistent with lack of TMDD No apparent effect on PK with combination Some increase with repeat dosing, but no notable accumulation Data as of December 26, 2024 Solnerstotug Monotherapy (15 mg/kg) Includes Dose Escalation + Expansion Solnerstotug (15 mg/kg) + cemiplimab Includes Dose Escalation + Expansion Solnerstotug (3 mg/kg) + cemiplimab Includes Dose Escalation + Expansion

Approved Checkpoint Inhibitor Treatment Options Studies Performed in PD-(L)1 Resistant Patients 1 VanderWalde, A. et al., Nat Med. 2023 (SWOG S1616) 2 Ascierto et al, J Clin Onc, 2023 (RELATIVITY-020) 3 Albrecht et al, Current Oncology Reports 2023 4 Kluger H, et al. J Immunother Cancer 2023 Checkpoint Target(s) Therapy ORR DCR Duration of Response (Months, Median) Tolerability Stage Benchmark: Anti-PD-1 Rechallenge Various ≤5% 4 Not reported Not reported Comparable to initial anti-PD-1 therapy Standard of care (No formal approval for anti-PD-1 resistant tumors) Anti-VISTA + Anti-PD-1 Solnerstotug (SNSE) + Cemiplimab (REGN) 14% (Ongoing, 3x higher than historical PD-1) 62% Too soon to assess; Durable responses still evolving Well-tolerated Phase 1/2 ongoing Anti-CTLA-4 + Anti-PD-1 Nivolumab + Ipilimumab (BMS) 28%1 Not reported 40.91 High toxicity3 (~40% discontinue early) Approved for 1st line therapy in several tumor types (melanoma, NSCLC, RCC)* Anti-LAG-3 + Anti-PD-1 Relatlimab + Nivolumab (BMS) ~12%2 (9–12% across cohorts) ~40%2 12.82 Well-tolerated Approved for 1st line unresectable or metastatic melanoma* *No formal approval for PD-(L)1 resistant patients

Visit C1D1 C1D8 C2D1 C2D8 C3D1 C3D8 C4D1 C5D1 50 40 30 20 10 0 -10 -20 -30 -40 -50 Visit % Change From C1D1 CD8+ TEM / TEMRA 3.0 mg/kg Solnerstotug TEM TEMRA C1D1 C1D8 C2D1 C2D8 C3D1 C3D8 C4D1 C5D1 C6D1 C7D1 C8D1 C9D1 C10D1 C11D1 C12D1 C13D1 C14D1 C15D1 C16D1 C17D1 C18D1 C19D1 -30 -20 -10 0 10 20 30 Visit % Change From C1D1 CD8+ TEM / TEMRA 3.0 mg/kg Solnerstotug + cemiplimab TEM TEMRA C1D1 C1D8 C2D1 C2D8 C3D1 C3D8 C4D1 C5D1 50 40 30 20 10 0 -10 -20 -30 -40 -50 Visit % Change From C1D1 CD8+ TEM / TEMRA 10.0 mg/kg Solnerstotug TEM TEMRA C1D1 C1D8 C2D1 C2D8 C3D1 C3D8 C4D1 C5D1 C6D1 C7D1 C8D1 C9D1 C10D1 C11D1 C12D1 C13D1 (EOT) -30 -20 -10 0 10 20 30 % Change From C1D1 CD8+ TEM / TEMRA 10.0 mg/kg Solnerstotug + cemiplimab TEM TEMRA C1D1 C1D8 C2D1 C2D8 C3D1 C3D8 C4D1 5 (EOT C5D1 C5D1 ) C6D1 C7D1 C8D1 C9D1 C10D1 C11D1 C12D1 C13D1 (EOT) -30 -20 -10 0 10 20 30 Visit % Change From C1D1 CD8+ TEM / TEMRA 15.0 mg/kg Solnerstotug TEM TEMRA C1D1 C1D8 C2D1 C2D8 C3D1 -30 -20 -10 0 10 20 30 Visit % Change From C1D1 CD8+ TEM / TEMRA 15.0 mg/kg Solnerstotug + cemiplimab TEM TEMRA C1D1 C1D8 C2D1 C2D8 C3D1 C3D8 C4D1 C5D1 C6D1 C7D1 -30 -20 -10 0 10 20 30 Visit % Change From C1D1 CD8+ TEM / TEMRA 15.0 mg/kg Solnerstotug TEM TEMRA C1D1 C1D8 C2D1 C2D8 C3D1 C3D8 C4D1 C5D1 C6D1 C7D1 C8D1 C9D1 C10D1 -30 -20 -10 0 10 20 30 Visit % Change From C1D1 CD8+ TEM / TEMRA 15.0 mg/kg Solnerstotug + cemiplimab TEM TEMRA Dose-Dependent Changes in Memory T-cell Phenotypes Occur in Mono and Combination Therapy Escalation & Expansion Cohorts A3 A4 A5 Mono Exp B1 B2 B3 ComboExp Data as of November 26, 2024

Compares baseline tumor RNA expression between a cohort of patients with observed clinical benefit (“response”) and a subset of non-responders with similar tumor types and demographics Exploratory Gene Expression Analysis Identifies Potential Biomarkers of Clinical Benefit Preliminary analysis that is subject to change with additional patient data Gene A Gene B Gene C Gene D Baseline Gene Expression Patient Detail by Gene NR – “no response”; R – “response” based on potential clinical benefit of drug/combo as defined by CR, PR, or tumor shrinkage Responder group included 1 CR, 1 PR, and 4 SD patients with tumor shrinkage ranging from -12.3% to -27% NR R 1 10 100 1000 10000 0 Gene count Gene A NR R 1 10 100 1000 10000 Gene count Gene B NR R 10 100 1000 10000 Gene count Gene C NR R 1 10 100 1000 10000 Gene count Gene D

Phase 2 Strategy Solnerstotug 3 or 15 mg/kg* + Anti-PD-1 Phase 2 Study Objectives Primary Anti-tumor activity Secondary Safety, tolerability, PK Anti-PD-1 Randomized * Phase 2 dose dependent on FDA feedback and additional data from dose expansion; possibility that 2 dose levels will be required in Phase 2 Patient Population: Current considerations: NSCLC H&N Melanoma RCC May consider a second indication (e.g., Merkel Cell carcinoma) in a single arm study to pursue accelerated path to approval Indication: TBD hot tumor type(s) N= ~80 patients

Completed and Anticipated Solnerstotug Clinical Milestones First patient dosed in combination with cemi Initial combination dose escalation PK & safety data Full Phase 1 dose expansion data First patient dosed with solnerstotug Initial monotherapy dose escalation PK & safety data Topline Phase 1 monotherapy & combination dose escalation data Sept 2023 Feb 2024 Year-end 2025 May 2023 Nov 2023 May 2024 Initiation of Phase 2 study* Q1 2026 *Subject to execution of clinical and financial objectives

Sensei Investment Highlights Unique, conditionally active MOA of lead VISTA targeted candidate solnerstotug designed to maximize anti-tumor activity and minimize negative side effects characteristic of other first generation, VISTA targeted approaches Expression of VISTA in multiple solid tumor types provides the opportunity to pursue several indications, many of which are large markets with high unmet need Recent data from ongoing Phase 1/2 dose expansion trial showing favorable early efficacy results with 2/2 patients enrolled with PD-(L)1 resistant Merkel Cell Carcinoma achieved responses, including 1 durable complete response and 1 partial response, 1 partial response in a PD-(L)1 resistant MSI-H CRC patient and clinically meaningful activity in multiple additional tumor types Demonstrated favorable safety profile and potential best-in-class PK profile Very few public company opportunities to invest in novel, VISTA-targeted approaches – most other companies pursuing VISTA are either private or large pharma companies with market caps >$100bn

Maryland 1405 Research Blvd Suite 125, Rockville MD 20850 Massachusetts 22 Boston Wharf Rd 7th floor Boston, MA 02210 senseibio.com

Evaluable “Hot” Tumor PD-(L)1 Refractory Patients on Combination Therapy Phase 1 Dose Expansion -30% solnerstotug (3 or 15 mg/kg) + cemiplimab All patients who received a prior PD-(L)1 Data as of March 17, 2025

78 yo male with squamous cell carcinoma of the lung Diagnosed 26Oct 2021 PD-1/PD-L1 positive, PD-L1 80% GNAS (R201C), TP53(Y126N), CCDKN2A (E33), TB53 (c.920-2del), ARID1A(P697fs), TMB-17.72 mut/mb Prior Treatment/Radiation Nov 2021: Paclitaxel + Carboplatin + Pembrolizumab in the metastatic setting Paclitaxel and Carboplatin were discontinued by Dec 2021 due to Adverse Event/Toxicity Pembrolizumab was continued until May 2024, best response PR then stopped due to progression Radiation therapy to the right upper lung in Feb 2023 Cycle 1 Day 1: 02Jul2024 Treatment Ongoing NSCLC Patient (01-035) Durable Stable Disease and Tumor Kinetics Consistent with a Pattern of Pseudo-Progression 15 mg/kg solnerstotug + cemiplimab Data as of March 17, 2025

Head and Neck Squamous Cell Carcinoma Patient (07-002) Durable Stable Disease 3 mg/kg solnerstotug + cemiplimab 73 yo male with P16+ squamous cell carcinoma Diagnosed 20June 2020 Positive PD1/PDL1 screening MSS stable, ARID1A , TMB 7muts/mb Prior Treatment/Surgery: Jul20 to Aug 20: Carboplatin + Paclitaxel (neoadjuvant setting), best response progression, completed treatment course Sep 2020 to Nov 2020: Cisplatin (adjuvant setting), best response PR, stopped due to AE/toxicity Apr 2023 for 1 year: 5FU + Carboplatin + Pembro + Magrolimab, best response SD, stopped due to progression Aug 2024 for 1 week: Lenvima + Pembro, best response non-evaluable (reason for stopping) / study closed Cycle 1 Day 1: 10Oct2024 Treatment Ongoing Data as of March 17, 2025

Renal Cell Carcinoma Patient (04-024) Stable Disease Accompanied by Tumor Regression 3 mg/kg solnerstotug + cemiplimab 59 yo male with renal cell carcinoma Diagnosed 26 November 2014 Prior Treatment/Surgery: 2014: Right nephrectomy Dec 2020 to Feb 2022: Pembrolizumab and Axitinib, best response PR, stopped due to progression Mar 2022 to Jul 2022: Pazopanib, stopped due to progression Sep 2022 to Nov 2022: Temsirolimus, stopped due to progression Dec 2022 to Aug 2024: Nivolumab, best response SD, stopped due to progression Cycle 1 Day 1: 18Dec2024 Treatment Ongoing Data as of March 17, 2025

Patient Demographics (MSS CRC Patients Only) Phase 1 Dose Expansion Monotherapy Combination Solnerstotug 15 mg/kg n=10 (%) Solnerstotug 15 mg/kg + cemi n=10 (%) Gender, n (%) Male 7 (70) 7 (70) Female 3 (30) 3 (30) Age, years Median (min,max) 53 (41,68) 54.5 (42,72) Baseline ECOG, n (%) 0 3 (30) 4 (40) 1 7 (70) 6 (60) Prior lines metastatic therapy, n Median (min, max) 2 (1, 3) 3 (1, 4) Prior PD-1/PDL-1, n % % Yes 1 (10) 0 (0) Data as of February 3, 2025 MSS CRC = microsatellite stable colorectal cancer Data as of March 17, 2025

Dose Expansion Patients with “Cold” Tumors Followed Trend Observed in Dose Escalation Study Phase 1 Dose Expansion Data as of March 17, 2025 -100% -30% Monotherapy Combination