UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 27, 2025
Regulus Therapeutics Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 001-35670 | 26-4738379 | ||
| (State of | (Commission | (IRS Employer | ||
| incorporation) | File No.) | Identification No.) |
| 4224 Campus Point Court, Suite 210 | ||
| San Diego, CA | 92121 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (858) 202-6300
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) Securities registered pursuant to Section 12(b) of the Act: |
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
||
| Common Stock, par value $0.001 per share | RGLS | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
Furnished as Exhibit 99.1 to this report is a presentation of Regulus Therapeutics Inc. (the “Company”), relating to topline results from the fourth cohort of patients in the Company’s Phase 1b multiple-ascending dose (“MAD”) study of farabursen (RGLS8429) for the treatment of autosomal dominant polycystic kidney disease (“ADPKD”).
The information in this Item 7.01, including Exhibit 99.1, is being furnished and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section, nor shall it be deemed incorporated by reference in any filing made by the Company under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
| Item 8.01 | Other Events. |
On March 27, 2025, the Company announced positive topline results from the fourth cohort of patients in its Phase 1b MAD study of farabursen (RGLS8429) for the treatment of ADPKD, as summarized below:
In the fourth cohort, 26 subjects received a fixed dose of 300 mg of farabursen every other week for three months. Consistent with the previously announced interim analysis of efficacy data from the first 14 subjects of this fixed-dose cohort, the full cohort of 26 patients demonstrated similar mechanistic response based on urinary PC1 and PC2 levels as well as a mean halting of htTKV growth over the four-month study period.
Cohort 4 data highlights:
| • | Increases in urinary PC1 and PC2 levels were similar to cohort 3 and reached a similar level of statistical significance compared to placebo (PC1 p=0.026; PC2 p=0.014). |
| • | Mean htTKV growth rate over 4 months was 0.05% (SE -0.86% to +0.92%) while placebo subjects in the trial experienced a mean growth rate of 2.58% (SE +1.09% to +4.10%). |
| • | Changes in htTKV were highly correlated with changes in renal cyst volume, suggesting farabursen directly impacts disease progression by limiting abnormal cyst growth. |
| • | In an exploratory analysis, the combination of patients treated with 3 mg/kg or 300 mg fixed dose farabursen (n=35) were compared to a combined historical control group of placebo-treated patients from prior pivotal ADPKD trials (n=550). Farabursen-treated patients experienced mean reduction in htTKV growth rate (-0.14%) compared to a mean increase of (+1.87%) in placebo-treated patients (p=0.0056). |
| • | Farabursen at 300 mg demonstrated a favorable safety and tolerability profile in this study, consistent with earlier cohorts. |
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits.
| Exhibit No. |
Description |
|
| 99.1 | PowerPoint slides summarizing Cohort 4 data from the Company’s Phase 1b Multiple Ascending Dose study. | |
| 104 | Cover Page Interactive Data File (the cover page XBRL tags are embedded within the inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: March 27, 2025 | REGULUS THERAPEUTICS INC. | |||||
| By: | /s/ Joseph Hagan |
|||||
| Joseph Hagan | ||||||
| Chief Executive Officer | ||||||

Phase 1b Trial Cohort 4 Final Efficacy Results. March 27, 2025 Exhibit 99.1

Statements contained in this presentation regarding matters that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995, including statements associated with Regulus’ farabursen (RGLS8429) program, the potential that farabursen may be eligible for an accelerated approval pathway, the potential to achieve therapeutic efficacy and address the underlying genetic causes of ADPKD, predictions based on and future clinical results and outcomes suggested by the Cohort 4 data, our Phase 3 trial design including whether the FDA will ultimately determine its components and the overall design to be acceptable and sufficient to serve as a single pivotal study, the advancing of farabursen into a pivotal study in the third quarter of 2025, farabursen potentially being a safe and effective treatment option for ADPKD, next steps for the farabursen program and their timing, the timing and future occurrence of other preclinical and clinical activities, and other statements relating to future events or conditions. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as "plans," "will," "potential," “suggest,” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon Regulus' current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, the risk that the approach we are taking to discover and develop drugs is novel and may never lead to marketable products; that preliminary or topline results are based on a preliminary analysis of key efficacy and safety data, and such data may change following a more comprehensive review of the data related to the clinical trial and may not be indicative of future results; an accelerated approval pathway designation may not be received, or even if it is received, lead to a faster development, regulatory review or approval process, and does not increase the likelihood that farabursen will receive marketing approval; the risk that clinical studies may not be successful; risks related to regulatory review and approval; risks related to our reliance on third-party collaborators and other third parties; risks associated with the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics; the risk that additional data may be negative; and risks related to our ability to successfully secure and deploy capital. These and other risks are described in additional detail in Regulus' filings with the Securities and Exchange Commission, including under the "Risk Factors" heading of Regulus’ annual report on Form 10-K for the quarter and year ended December 31, 2024. All forward-looking statements contained in this presentation speak only as of the date on which they were made. Regulus undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. Forward Looking Statements and Disclaimers.
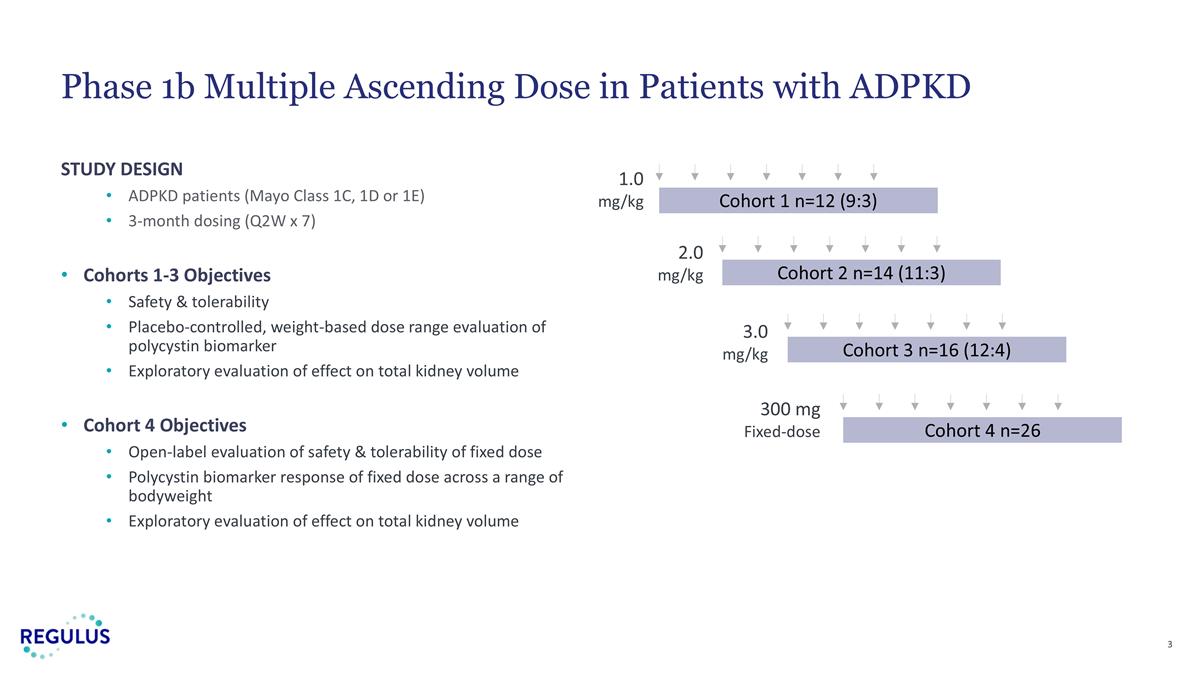
STUDY DESIGN ADPKD patients (Mayo Class 1C, 1D or 1E) 3-month dosing (Q2W x 7) Cohorts 1-3 Objectives Safety & tolerability Placebo-controlled, weight-based dose range evaluation of polycystin biomarker Exploratory evaluation of effect on total kidney volume Cohort 4 Objectives Open-label evaluation of safety & tolerability of fixed dose Polycystin biomarker response of fixed dose across a range of bodyweight Exploratory evaluation of effect on total kidney volume Phase 1b Multiple Ascending Dose in Patients with ADPKD 1.0 mg/kg Cohort 1 n=12 (9:3) 2.0 mg/kg 3.0 mg/kg Cohort 2 n=14 (11:3) Cohort 3 n=16 (12:4) 300 mg Fixed-dose Cohort 4 n=26
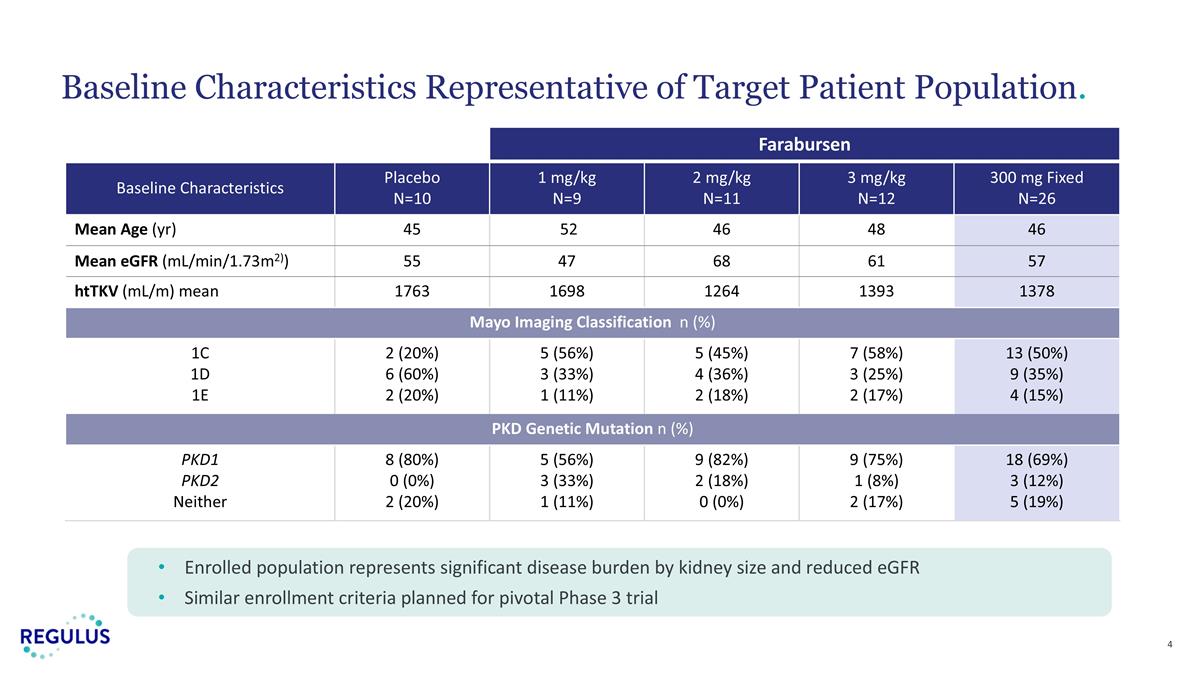
Baseline Characteristics Representative of Target Patient Population. Enrolled population represents significant disease burden by kidney size and reduced eGFR Similar enrollment criteria planned for pivotal Phase 3 trial Farabursen Baseline Characteristics Placebo N=10 1 mg/kg N=9 2 mg/kg N=11 3 mg/kg N=12 300 mg Fixed N=26 Mean Age (yr) 45 52 46 48 46 Mean eGFR (mL/min/1.73m2)) 55 47 68 61 57 htTKV (mL/m) mean 1763 1698 1264 1393 1378 Mayo Imaging Classification n (%) 1C 1D 1E 2 (20%) 6 (60%) 2 (20%) 5 (56%) 3 (33%) 1 (11%) 5 (45%) 4 (36%) 2 (18%) 7 (58%) 3 (25%) 2 (17%) 13 (50%) 9 (35%) 4 (15%) PKD Genetic Mutation n (%) PKD1 PKD2 Neither 8 (80%) 0 (0%) 2 (20%) 5 (56%) 3 (33%) 1 (11%) 9 (82%) 2 (18%) 0 (0%) 9 (75%) 1 (8%) 2 (17%) 18 (69%) 3 (12%) 5 (19%)
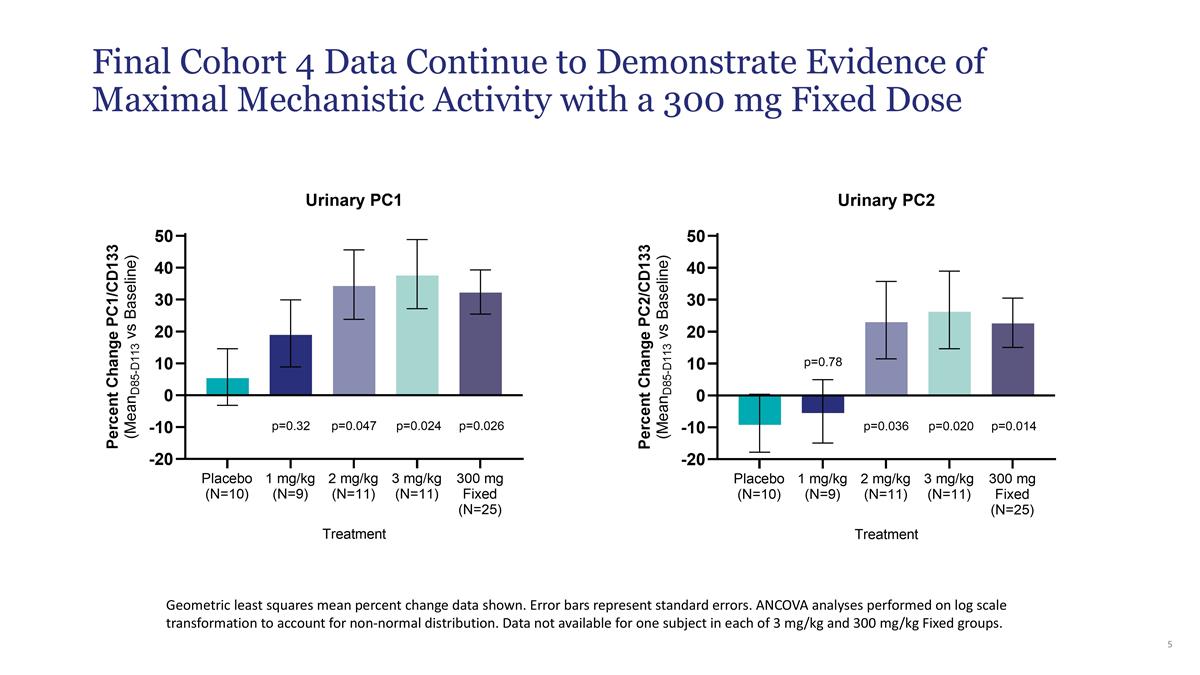
Geometric least squares mean percent change data shown. Error bars represent standard errors. ANCOVA analyses performed on log scale transformation to account for non-normal distribution. Data not available for one subject in each of 3 mg/kg and 300 mg/kg Fixed groups. Final Cohort 4 Data Continue to Demonstrate Evidence of Maximal Mechanistic Activity with a 300 mg Fixed Dose
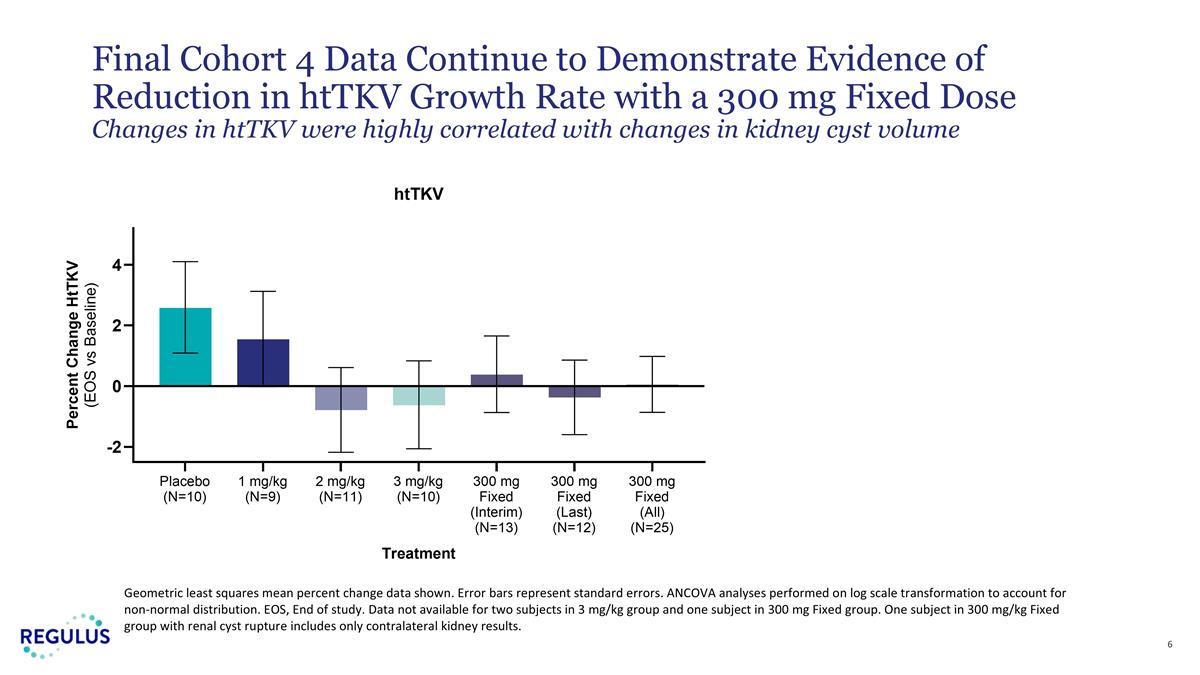
Geometric least squares mean percent change data shown. Error bars represent standard errors. ANCOVA analyses performed on log scale transformation to account for non-normal distribution. EOS, End of study. Data not available for two subjects in 3 mg/kg group and one subject in 300 mg Fixed group. One subject in 300 mg/kg Fixed group with renal cyst rupture includes only contralateral kidney results. Final Cohort 4 Data Continue to Demonstrate Evidence of Reduction in htTKV Growth Rate with a 300 mg Fixed Dose Changes in htTKV were highly correlated with changes in kidney cyst volume
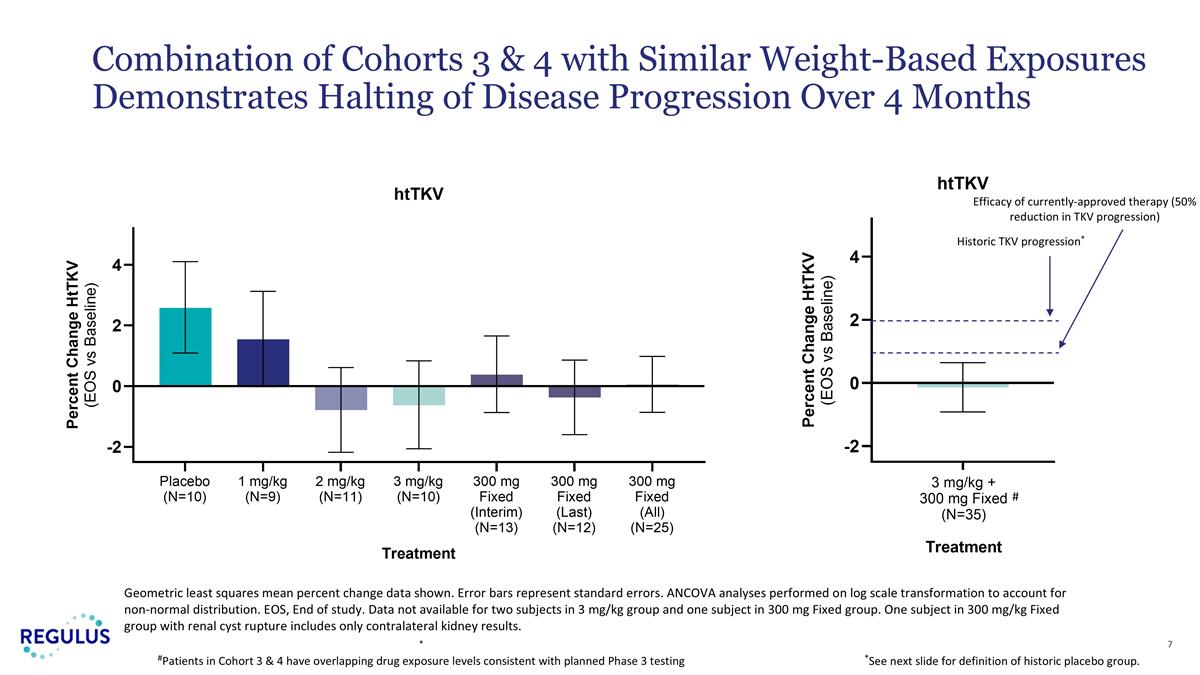
Geometric least squares mean percent change data shown. Error bars represent standard errors. ANCOVA analyses performed on log scale transformation to account for non-normal distribution. EOS, End of study. Data not available for two subjects in 3 mg/kg group and one subject in 300 mg Fixed group. One subject in 300 mg/kg Fixed group with renal cyst rupture includes only contralateral kidney results. Combination of Cohorts 3 & 4 with Similar Weight-Based Exposures Demonstrates Halting of Disease Progression Over 4 Months Efficacy of currently-approved therapy (50% reduction in TKV progression) Historic TKV progression* *See next slide for definition of historic placebo group. # * #Patients in Cohort 3 & 4 have overlapping drug exposure levels consistent with planned Phase 3 testing
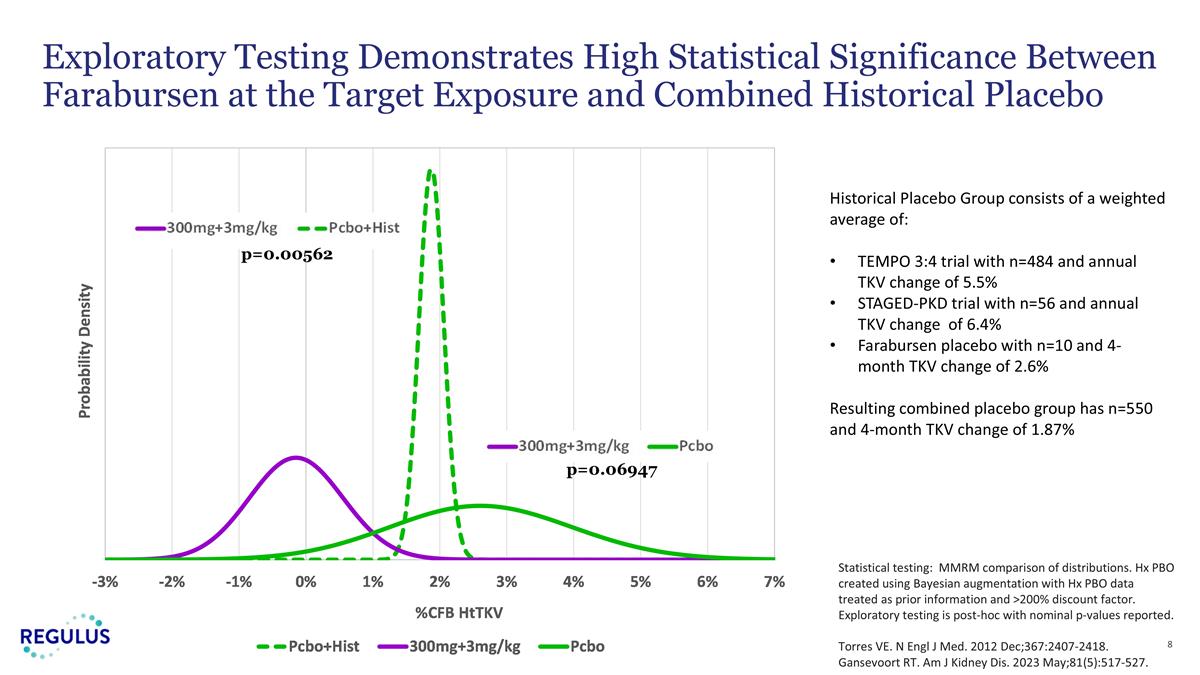
p=0.00562 p=0.06947 Exploratory Testing Demonstrates High Statistical Significance Between Farabursen at the Target Exposure and Combined Historical Placebo Statistical testing: MMRM comparison of distributions. Hx PBO created using Bayesian augmentation with Hx PBO data treated as prior information and >200% discount factor. Exploratory testing is post-hoc with nominal p-values reported. Torres VE. N Engl J Med. 2012 Dec;367:2407-2418. Gansevoort RT. Am J Kidney Dis. 2023 May;81(5):517-527. Historical Placebo Group consists of a weighted average of: TEMPO 3:4 trial with n=484 and annual TKV change of 5.5% STAGED-PKD trial with n=56 and annual TKV change of 6.4% Farabursen placebo with n=10 and 4-month TKV change of 2.6% Resulting combined placebo group has n=550 and 4-month TKV change of 1.87%
Increases in polycystin biomarkers consistent with top end of dose range 300 mg provided evidence of maximal mechanistic activity with no indication that higher doses will achieve greater levels of urinary polycystin Farabursen continues to show evidence of a notable effect on TKV growth rate 300 mg demonstrated halting of htTKV growth rate over 4 months (based on 3 months of dosing) Combining all data from patients with overlapping drug exposure of 3 mg/kg or 300 mg fixed dose demonstrated a statistically significant reduction in htTKV growth rate compared to historical placebo-treated ADPKD patients Changes in htTKV were highly correlated with changes in renal cyst volume, suggesting farabursen directly impacts disease progression by limiting abnormal cyst growth The Phase 3 12-month htTKV endpoint is meaningfully derisked based on the observed results in Phase 1b Cohort 4 Final Efficacy Analysis Confirms Selection of 300 mg Fixed Dose for Phase 3. Along with the previously reported favorable safety & tolerability profile, these final efficacy data confirm appropriateness of 300 mg for the Phase 3 pivotal trial
