UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 26, 2025
CANDEL THERAPEUTICS, INC.
(Exact name of Registrant as Specified in Its Charter)
| Delaware | 001-40629 | 52-2214851 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 117 Kendrick St., Suite 450 Needham, MA |
02494 | |||
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s Telephone Number, Including Area Code: (617) 916-5445
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange |
||
| Common Stock, $0.01 par value per share | CADL | The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On March 26, 2025, Candel Therapeutics, Inc. (the “Company”) issued a press release announcing positive final survival data from its phase 2a clinical trial of CAN-2409 in patients with stage III/IV non-small cell lung cancer (“NSCLC”), inadequately responding to Immune Checkpoint Inhibitor (“ICI”) treatment.
A copy of the full press release is attached as Exhibit 99.1 to this Current Report on Form 8-K and incorporated by reference herein. A copy of the presentation is attached as Exhibit 99.2 to this Current Report on Form 8-K and incorporated by reference herein. The presentation will also be available on the investor relations section of the Company’s website at https://ir.candeltx.com/. Information contained on the Company’s website is not incorporated by reference into this Current Report on Form 8-K, and you should not consider any information on, or that can be accessed from, the Company’s website as part of this Current Report on Form 8-K.
The information in this Item 7.01 and Exhibits 99.1 and 99.2 of this Current Report on Form 8-K are furnished and shall not be deemed to be “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section. The information in this Item 7.01 and Exhibits 99.1 and 99.2 of this Current Report on Form 8-K shall not be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, whether made before or after the date of this Current Report on Form 8-K, regardless of any general incorporation language in any such filing.
| Item 8.01 | Other Events. |
Phase 2a Trial Results for CAN-2409 in Advanced Non-Small Cell Lung Cancer Inadequately Responding to Immune Checkpoint Inhibitor Treatment
On March 26, 2025, the Company announced final survival data from its phase 2a clinical trial of CAN-2409 in patients with stage III/IV NSCLC, inadequately responding to ICI treatment. This final analysis included extended follow-up data (one year after the latest data cut) with a median follow-up time for all the enrolled patients of 29 months). This data showed a sizeable percentage of patients with survival exceeding 24 months, evidence of a longer tail of survival, with 37% of patients with progressive disease despite treatment with ICI alive 2 years after CAN-2409 administration. Data highlights include:
| • | Survival data: |
| • | In patients with an inadequate response to ICI treatment (Cohort 1+2, n=46), median overall survival (“mOS”) was 24.5 months |
| • | In patients with progressive disease, despite ICI treatment (Cohort 2, n=41), mOS was 21.5 months, which is markedly longer than the 9.8–11.8 months of survival reported in published literature in a similar patient population receiving standard of care of docetaxel chemotherapy |
| • | 37% of patients exceeding 24 months survival were still alive at the time of the March 2, 2025 data cut |
| • | Potential precision medicine approach: |
| • | Patients with non-squamous histology predominated amongst the long-term survivors: 14/15 patients with overall survival (OS) >24 months and 9/9 patients with OS> 30 months had non-squamous NSCLC. |
| • | Patients with non-squamous histology exhibited larger changes in T cells, B cells, and dendritic cells after CAN-2409 administration, compared to patients with squamous NSCLC. |
| • | mOS of 25.4 months observed in non-squamous NSCLC patients with progressive disease, despite ICI treatment (n=33). |
| • | Although a phase 2a open-label experimental medicine clinical trial is not designed for an intention to treat (ITT) analysis, Candel conducted an exploratory ITT analysis and observed mOS of 16.7 months after CAN-2409 in non-squamous NSCLC patients with progressive disease despite ICI treatment (n=53). Recent trials have reported a mOS of 9.9–12.3 months in ICI-refractory, non-squamous NSCLC patients receiving standard of care docetaxel chemotherapy. |
| • | Systemic anti-tumor response (abscopal effect) and safety profile: |
| • | Decrease in size of uninjected tumors was observed in 69% of patients with multiple lesions (n=35), indicating that local injection may induce a systemic anti-tumor immune response (abscopal effect). |
| • | CAN-2409 maintained its generally favorable safety and tolerability profile throughout the extended follow-up period. |
Based on these positive findings, the Company will advance its development program for CAN-2409 in NSCLC, including preparation and enabling work for a future, potentially registrational, clinical trial in patients with NSCLC with non-squamous histology.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit Number |
Description |
|
| 99.1 | Press Release dated March 26, 2025 | |
| 99.2 | Presentation dated March 26, 2025 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Candel Therapeutics, Inc. | ||||||
| Date: March 26, 2025 | By: | /s/ Paul Peter Tak |
||||
| Paul Peter Tak, M.D., Ph.D., FMedSci | ||||||
| President and Chief Executive Officer | ||||||
Exhibit 99.1

Candel Therapeutics Reports Both Prolonged Median Overall Survival and Long Tail of Survival in Phase 2a Clinical Trial of CAN-2409 in Advanced Non-Small Cell Lung Cancer (NSCLC) Patients Non-Responsive to Immune Checkpoint Inhibitor (ICI) Treatment
| • | Experimental treatment with CAN-2409 was associated with a median overall survival (mOS) of 24.5 months in patients with advanced NSCLC who had an inadequate response to ICI treatment, failed several chemotherapy regimens, and presented with multiple negative prognostic factors at enrollment (90% of the patients had stage IV disease, most patients had low or undetectable PDL-1 expression, > 90% were current or former smokers, and most patients had failed multiple lines of chemotherapy) |
| • | mOS of 21.5 months was observed in patients with progressive disease at baseline despite ICI therapy (cohort 2), markedly exceeding mOS which has been reported in published literature for this population with standard of care of docetaxel chemotherapy (mOS of 9.8-11.8 months) |
| • | Long tail of survival observed in 37% (15/41) of patients with progressive disease despite ICI treatment at enrollment, who were still alive more than 2 years after CAN-2409 administration at the time of data cutoff (March 3, 2025) |
| • | Evidence of a systemic immune response with regression of both injected and uninjected lesions observed in approximately two-thirds of patients with metastatic disease and at least one uninjected tumor (abscopal effect) |
| • | Statistically significant improved overall survival in non-squamous NSCLC compared to squamous NSCLC after experimental treatment with CAN-2409, supported by immunological biomarker data: mOS of 25.4 months in per protocol population of patients with non-squamous NSCLC with progressive disease at baseline despite ICI; intention to treat analysis showed mOS of 16.7 months compared to published data for standard of care chemotherapy of 9.9 –12.3 months, recently reported in the non-squamous patient population, progressing despite ICI |
| • | CAN-2409 continued to exhibit a generally favorable safety and tolerability profile throughout the extended follow-up period, with no new safety signals identified |
NEEDHAM, Mass., March 26, 2025 (GLOBE NEWSWIRE) – Candel Therapeutics, Inc. (Candel or the Company) (Nasdaq: CADL), a clinical stage biopharmaceutical company focused on developing multimodal biological immunotherapies to help patients fight cancer, today announced final survival data from a phase 2a clinical trial of CAN-2409 in patients with stage III/IV NSCLC, inadequately responding to ICI treatment. mOS was 24.5 months in 46 evaluable patients receiving 2 courses of CAN-2409 (per protocol population; cohort 1 and 2) and 21.5 months in evaluable patients from cohort 2 (n=41) that presented with progressive disease at baseline, despite ICI treatment. mOS in patients with progressive disease despite ICI treatment, was 9.8-11.8 months in other studies, including those with standard of care of docetaxel chemotherapy, which has a very poor prognosis, did not exceed 12 months in other published studies. (1, 2) This final analysis included extended follow-up data (1 year after the previous data cut) with a median follow up time for the per protocol population of 32.4 months. Data showed a sizeable percentage of patients with survival exceeding 24 months, evidence of a long tail of survival, with 37% of patients with progressive disease despite treatment with ICI alive 2 years after CAN-2409 administration.
Biomarker research showed an enhanced immunological and clinical response after CAN-2409 administration in patients with non-squamous histology compared to squamous histology, and improved mOS was in this population (25.4 months in patients with progressive disease despite ICI treatment and non-squamous NSCLC, n=33).
“Treatment options are quite limited for patients with unresectable NSCLC who progress on anti-PD-1 therapy,” said Charu Aggarwal, MD, MPH, Leslye Heisler Professor for Lung Cancer Excellence at the University of Pennsylvania’s Perelman School of Medicine and Principal Investigator of the study. “The survival benefit seen in this study is striking, especially when compared to both the current standard of care treatment of docetaxel chemotherapy and other therapies under investigation for this patient group,” she added.
Data Highlights:
Pre-treatment and mid-treatment dropout rates were comparable to those reported in other clinical trials in similar populations of patients with advanced NSCLC.(1, 3) Three patients were enrolled, but did not receive treatment, 22 patients received only one injection of CAN-2409, 51 patients received at least 2 injections of CAN-2409, but 5 patients did not complete treatment. 46 patients received complete treatment (2 courses of CAN-2409 plus prodrug) and were included in the evaluable, per protocol population. The per protocol population was representative of the overall enrolled population in terms of baseline demographics and prognostic factors.
| • | Survival data: |
| • | In patients with an inadequate response to ICI treatment (Cohort 1+2, n=46), mOS was 24.5 months. |
| • | In patients with progressive disease, despite ICI treatment (Cohort 2, n=41), mOS was 21.5 months, which is markedly longer than the 9.8–11.8 months of survival reported in published literature in a similar patient population receiving standard of care of docetaxel chemotherapy.1,2 |
| • | 37% of patients exceeding 24 months survival were still alive at the time of the March 3, 2025 data cut. |
| • | Potential precision medicine approach: |
| • | Patients with non-squamous histology predominated amongst the long-term survivors: 14/15 patients with OS > 24 months and 9/9 patients with OS > 30 months had non-squamous NSCLC. |
| • | Patients with non-squamous histology exhibited larger changes in T cells, B cells, and dendritic cells after CAN-2409 administration compared to patients with squamous NSCLC. |
| • | mOS of 25.4 months observed in non-squamous NSCLC patients with progressive disease, despite ICI treatment (n=33). |
| • | Although a phase 2a open-label experimental medicine clinical trial is not designed for an intention to treat (ITT) analysis, we conducted an exploratory ITT analysis and observed mOS of 16.7 months after CAN-2409 in non-squamous NSCLC patients with progressive disease administration despite ICI treatment (n=53). Recent trials have reported a mOS of 9.9–12.3 months in ICI-refractory, non-squamous NSCLC patients receiving standard of care docetaxel chemotherapy.(1, 2) |
| • | Systemic anti-tumor response (abscopal effect) and safety profile: |
| • | Decrease in size of uninjected tumors was observed in 69% of patients with multiple lesions (n=35), indicating that local injection may induce a systemic anti-tumor immune response (abscopal effect). |
| • | CAN-2409 maintained its generally favorable safety and tolerability profile throughout the extended follow-up period. |
“These updated survival data confirm and strengthen our previously reported findings, demonstrating that CAN-2409 has the potential to extend survival for patients with advanced NSCLC, who have limited treatment options after failing to respond to, or progressing, despite immune checkpoint inhibitor therapy,” said Paul Peter Tak, MD, PhD, FMedSci, President and Chief Executive Officer of Candel. “CAN-2409 may represent an entirely new approach to solid tumor treatment, with its unique mechanism of action and favorable safety profile to date, enabling potentially meaningful improvements in outcomes beyond current standard of care. These compelling results mark a potentially transformative advance in our fight against this aggressive disease.”
“The extension of survival in patients with non-squamous disease is notable even when compared to data that have been reported for other investigational products, such as antibody-drug conjugates, for this patient population,” said W. Garrett Nichols, MD, CMO of Candel. “CAN-2409, in addition to continued ICI treatment, may prolong survival beyond that offered by docetaxel chemotherapy, and has the potential to be better tolerated.”
Based on these positive findings, the Company will advance its development program for CAN-2409 in NSCLC, including preparation and enabling work for a future, potentially registrational, clinical trial in patients with NSCLC with non-squamous histology. The U.S. Food and Drug Administration (FDA) previously granted Fast Track Designation for CAN-2409 plus valacyclovir in combination with ICI treatment for the treatment of stage III/IV NSCLC in patients who are resistant to first line PD-(L)1 inhibitor therapy and who do not have activating molecular driver mutations or have progressed on directed molecular therapy.
About CAN-2409
CAN-2409, Candel’s most advanced multimodal biological immunotherapy candidate, is an investigational, off-the-shelf, replication-defective adenovirus engineered to deliver the herpes simplex virus thymidine kinase (HSV-tk) gene to a patient’s specific tumor and induce an individualized, systemic immune response against the tumor. HSV-tk is an enzyme that locally converts orally administered valacyclovir into a toxic nucleotide analogue that kills nearby cancer cells. Together, this regimen is designed to induce an individualized and specific CD8+ T cell-mediated response against the injected tumor and uninjected distant metastases for broad anti-tumor activity, based on in-situ vaccination against a variety of tumor antigens.
Because of its versatility, CAN-2409 has the potential to treat a broad range of solid tumors. Encouraging monotherapy activity, as well as combination activity with standard of care radiotherapy, surgery, chemotherapy, and immune checkpoint inhibitors, have previously been shown in several preclinical and clinical settings. More than 1,000 patients have been dosed with CAN-2409 with a favorable tolerability profile to date, supporting the potential for combination with other therapeutic strategies.
Candel’s clinical development program for CAN-2409 includes completed phase 2a clinical trials in both non-small cell lung cancer (NSCLC) and pancreatic ductal adenocarcinoma (PDAC), as well as a positive pivotal randomized, placebo-controlled phase 3 clinical trial of CAN-2409 in localized, non-metastatic prostate cancer. In December 2024, Candel announced that CAN-2409 achieved its primary endpoint in a phase 3 clinical trial in men with intermediate-to-high-risk, localized prostate cancer, demonstrating statistically significant and clinically meaningful improvement in disease-free survival when added to SoC radiation therapy +/- androgen deprivation therapy. In the Company’s randomized controlled phase 2a clinical trial of CAN-2409 in borderline resectable PDAC, positive survival data showed notable improvement in estimated median overall survival of 31.4 months after experimental treatment with CAN-2409 plus standard of care versus 12.5 months in the control group in patients with PDAC, who received only standard of care. Median survival post-progression was 21.2 months in patients who received CAN-2409 compared to 6.4 months in the control arm. CAN-2409 plus prodrug has been granted Fast Track Designation by the FDA for the treatment of PDAC, stage III/IV NSCLC in patients who are resistant to first line PD-(L)1 inhibitor therapy and who do not have activating molecular driver mutations or have progressed on directed molecular therapy, and localized primary prostate cancer. The FDA has also granted Orphan Drug Designation to CAN-2409 for the treatment of PDAC. Candel’s pivotal phase 3 clinical trial in newly diagnosed, localized prostate cancer was conducted under a Special Protocol Assessment (SPA) agreed with the FDA.
About Candel Therapeutics
Candel is a BLA ready clinical stage biopharmaceutical company focused on developing off-the-shelf multimodal biological immunotherapies that elicit an individualized, systemic anti-tumor immune response to help patients fight cancer. CAN-2409 is the lead product candidate from the adenovirus platform. CAN-3110 is the lead product candidate from the HSV platform and is currently in an ongoing phase 1b clinical trial in recurrent high-grade glioma. In October 2023, the Company announced that Nature published initial results from this ongoing clinical trial: CAN-3110 was well tolerated and the investigators observed nearly two-fold increase in median overall survival compared to historical controls after a single CAN-3110 injection in this therapy-resistant condition.4 Finally, Candel’s enLIGHTEN™ Discovery Platform is a systematic, iterative HSV-based discovery platform leveraging human biology and advanced analytics to create new viral immunotherapies for solid tumors.
For more information about Candel, visit: www.candeltx.com
Forward-Looking Statements
This press release includes certain disclosures that contain “forward-looking statements,” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, express or implied statements regarding the timing and advancement of current and future development programs; including the timing and availability of additional data and key data readout milestones and presentations; expectations regarding early biological readouts as predictor of clinical response; and expectations regarding the therapeutic benefit of the Company’s programs, including the ability of CAN-2409 to treat a broad range of solid tumors and improve disease-free survival, overall survival, and post-progression survival. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
Any forward-looking statements in this press release are based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this press release, including, without limitation, those risks and uncertainties related to the timing and advancement of development programs; expectations regarding the therapeutic benefit of the Company’s programs; that final data from the Company’s pre-clinical studies and completed clinical trials may differ materially from reported interim data from ongoing studies and trials; the Company’s ability to efficiently discover and develop product candidates; the Company’s ability to obtain and maintain regulatory approval of product candidates; the Company’s ability to maintain its intellectual property; the implementation of the Company’s business model, including strategic plans for the Company’s business and product candidates; and other risks identified in the Company’s filings with the U.S. Securities and Exchange Commission (SEC) including the Company’s most recent Annual Report on Form 10-K filed with the SEC and any subsequent filings with the SEC. The Company cautions you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. The Company disclaims any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions, or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. Any forward-looking statements contained in this press release represent the Company’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date.
Investor Contact:
Theodore Jenkins
VP, Investor Relations and Business Development
Candel Therapeutics, Inc.
tjenkins@candeltx.com
Media Contact:
Ben Shannon
ICR Healthcare
CandelPR@icrhealthcare.com
| 1 | Paz-Ares LG et al. J Clin Oncol 2024;42:2860-2872 |
| 2 | Ahn MJ et al. J Clin Onc 2024;43:260-272 |
| 3 | Reckamp,KL et al. J Clin Oncol. 2022; 40 :2295-2306 |
| 4 | Ling AL, et al. Nature. 2023;623(7985):157-166. |

Exhibit 99.2 Tipping the balance in favor of the immune system to fight cancer Corporate Presentation | March 2025 NASDAQ: CADL

Forward Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts contained in this presentation, including express or implied statements regarding our strategy, future financial condition, future operations, projected costs, prospects, plans, objectives of management and expected market size, plans and timelines for the preclinical and clinical development of our product candidates, including the therapeutic potential, clinical benefits and safety profiles of such product candidates, expectations regarding timing, success and data announcements of ongoing preclinical studies and clinical trials, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “target,” “seek,” “predict,” “potential,” “continue” or the negative of these terms or other comparable terminology. Although we believe that the expectations reflected in these forward-looking statements are reasonable, these statements relate to our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans, objectives of management and expected market size, and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by these forward-looking statements. Forward-looking statements in this presentation include, but are not limited to, statements about: the initiation, timing, progress, results, and cost of our research and development programs and our current and future preclinical and clinical studies, including those for CAN-2409 and CAN-3110, and statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the period during which the results of the trials will become available, and our research and development programs; the therapeutic benefit of our programs, including the potential for our programs to extend patient survival; our ability to efficiently discover and develop product candidates; our ability to initiate, recruit and enroll patients in and conduct our clinical trials at the pace that we project; our ability to obtain and maintain regulatory approval of our product candidates; our ability to compete with companies currently marketing or engaged in the development of treatments that our product candidates are designed to target; our reliance on third parties to conduct our clinical trials and to manufacture drug substance for use in our clinical trials; the size and growth potential of the markets for our product candidates and our ability to serve those markets; the ability and willingness of our third-party strategic collaborators to continue research and development activities relating to our development candidates and product candidates; our ability to obtain and maintain adequate intellectual property rights; our estimates of our future expenses, revenue, capital requirements or our need for or ability to obtain additional financing; our ability to continue as a going concern, the potential benefits of strategic collaboration agreements, our ability to enter into additional strategic collaborations or arrangements, and our ability to attract collaborators with development, regulatory and commercialization expertise; our financial performance; and developments and projections relating to our competitors or our industry. We caution the recipient not to place considerable reliance on the forward-looking statements contained in this presentation. The forward-looking statements in this presentation speak only as of the date of this document, and we undertake no obligation to update or revise any of these statements. Our business is subject to substantial risks and uncertainties, including those referenced above. Certain information contained in this presentation relates to or is based on estimates, projections and other information concerning the Company’s industry, its business and the markets for its programs and product candidates and studies, publications, surveys and other data obtained from third-party sources and the Company's own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions; there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source. 2
Candel at a glance • Positive phase 3 randomized, triple-blinded, placebo-controlled, clinical trial of CAN-2409 in intermediate-to-high-risk, localized prostate cancer • Positive overall survival data from randomized phase 2a clinical trial of CAN-2409 in borderline resectable pancreatic ductal adenocarcinoma (PDAC) • Proof of concept in NSCLC: mOS of 21.5 months in patients with progressive disease at baseline despite ICI (vs. published historical controls of mOS in PD-1 refractory population with SoC chemo of 9.8 – 11.8 mos), and evidence of systemic immune CAN-2409: Off-the-shelf response pan-solid tumor therapy, • Fast Track Designation in NSCLC, pancreatic cancer, and prostate cancer individualized anti-cancer • Orphan Drug Designation in pancreatic cancer immune response • “Pipeline in a product” strategy advancing multiple programs in several large indications • BLA filing for CAN-2409 in prostate cancer expected in Q4 2026 • Proof of concept in patients with recurrent high-grade glioma published in Nature (“Clinical trial links oncolytic immunoactivation to survival in glioblastoma”) • Fast Track Designation, Orphan Drug Designation CAN-3110: Oncolytic HSV-1 • Opportunity for creation of “pipeline in a product” by expansion into indications beyond brain cancers • Upcoming catalyst: designed for tumor-specific − Initial survival and immunological biomarker data expected in Q4 of 2025, evaluating repeat dosing regimen of CAN-3110 replication • Experienced Executive Team and strong scientific support from high-profile Research Advisory Board • Cash and cash equivalents of $102.7 million as of Dec 31, 2024; current expected runway into Q1 2027 • IP protection: CAN-2409 (2034, method of use); CAN-3110 (2036, composition of matter); potential 12 years regulatory exclusivity Corporate Highlights • Low-cost manufacturing 3
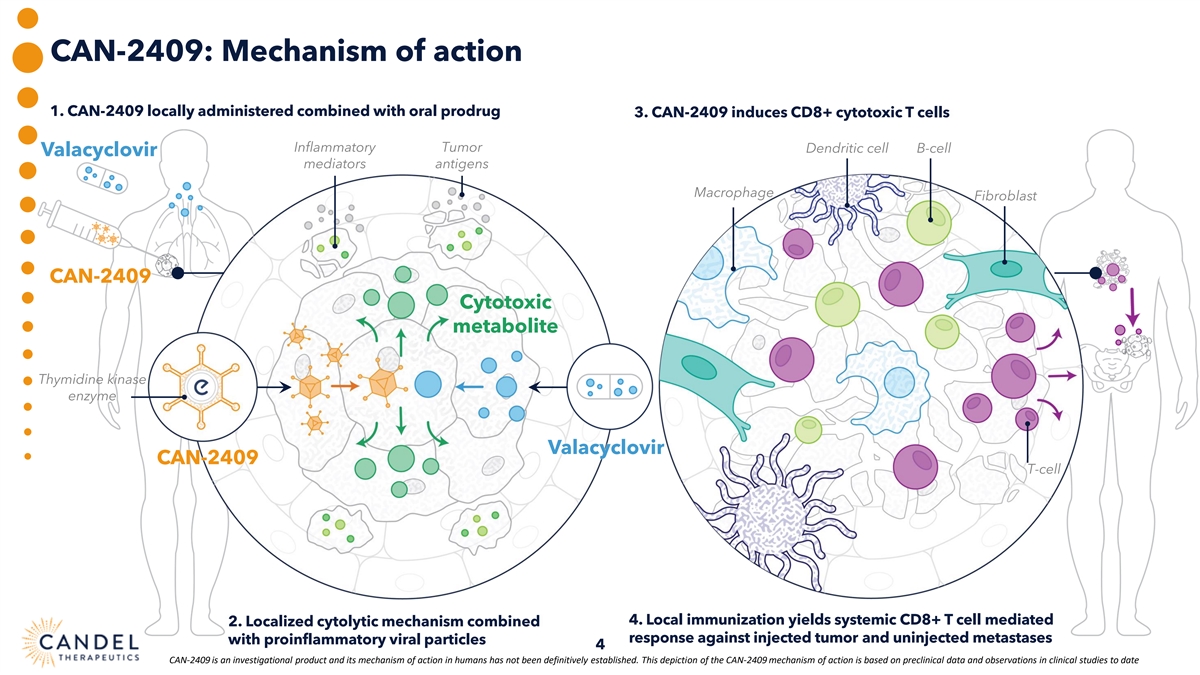
CAN-2409: Mechanism of action 1. CAN-2409 locally administered combined with oral prodrug 3. CAN-2409 induces CD8+ cytotoxic T cells Inflammatory Tumor Dendritic cell B-cell Valacyclovir mediators antigens Macrophage Fibroblast CAN-2409 Cytotoxic metabolite Thymidine kinase enzyme Valacyclovir CAN-2409 T-cell 4. Local immunization yields systemic CD8+ T cell mediated 2. Localized cytolytic mechanism combined response against injected tumor and uninjected metastases with proinflammatory viral particles 4 CAN-2409 is an investigational product and its mechanism of action in humans has not been definitively established. This depiction of the CAN-2409 mechanism of action is based on preclinical data and observations in clinical studies to date
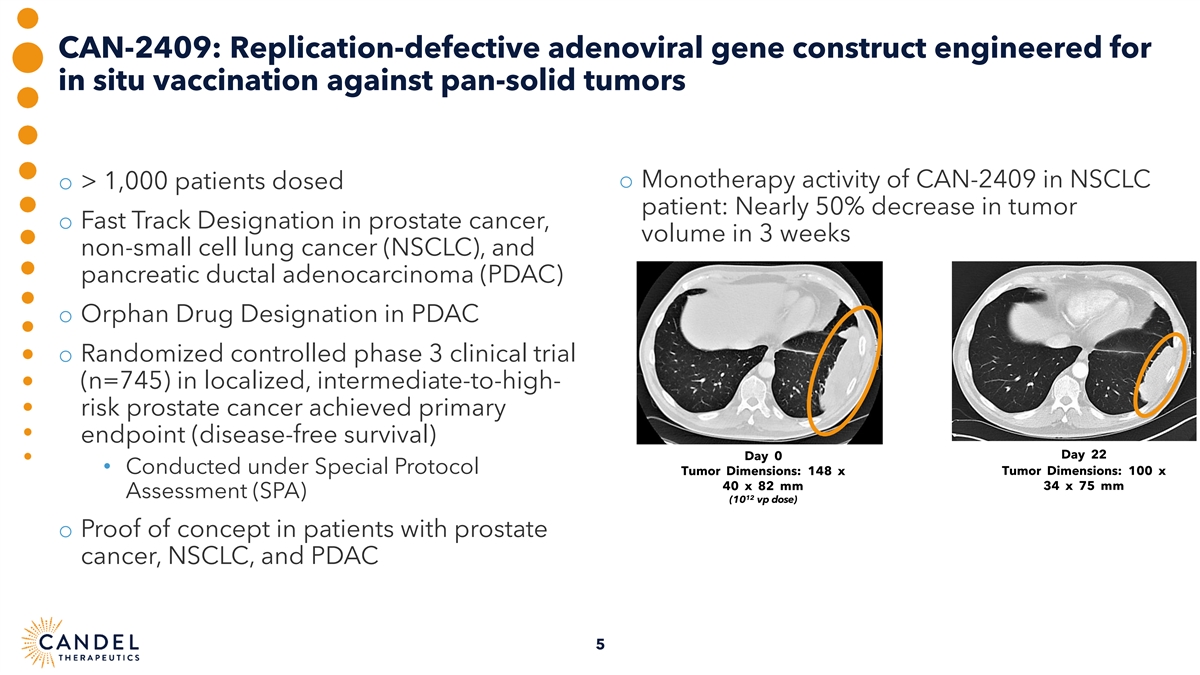
CAN-2409: Replication-defective adenoviral gene construct engineered for in situ vaccination against pan-solid tumors o Monotherapy activity of CAN-2409 in NSCLC o > 1,000 patients dosed patient: Nearly 50% decrease in tumor o Fast Track Designation in prostate cancer, volume in 3 weeks non-small cell lung cancer (NSCLC), and pancreatic ductal adenocarcinoma (PDAC) o Orphan Drug Designation in PDAC o Randomized controlled phase 3 clinical trial (n=745) in localized, intermediate-to-high- risk prostate cancer achieved primary endpoint (disease-free survival) Day 22 Day 0 • Conducted under Special Protocol Tumor Dimensions: 148 x Tumor Dimensions: 100 x 40 x 82 mm 34 x 75 mm Assessment (SPA) 12 (10 vp dose) o Proof of concept in patients with prostate cancer, NSCLC, and PDAC 5
CAN-2409 Lung Cancer Phase 2a clinical trial of CAN-2409 + continued immune checkpoint inhibitor (ICI) in stage III/IV NSCLC patients with an inadequate response to ICI Enabling work underway for anticipated phase 3 clinical trial (Protocol development, Scientific and Regulatory Advisory Boards, engagement with FDA) 6
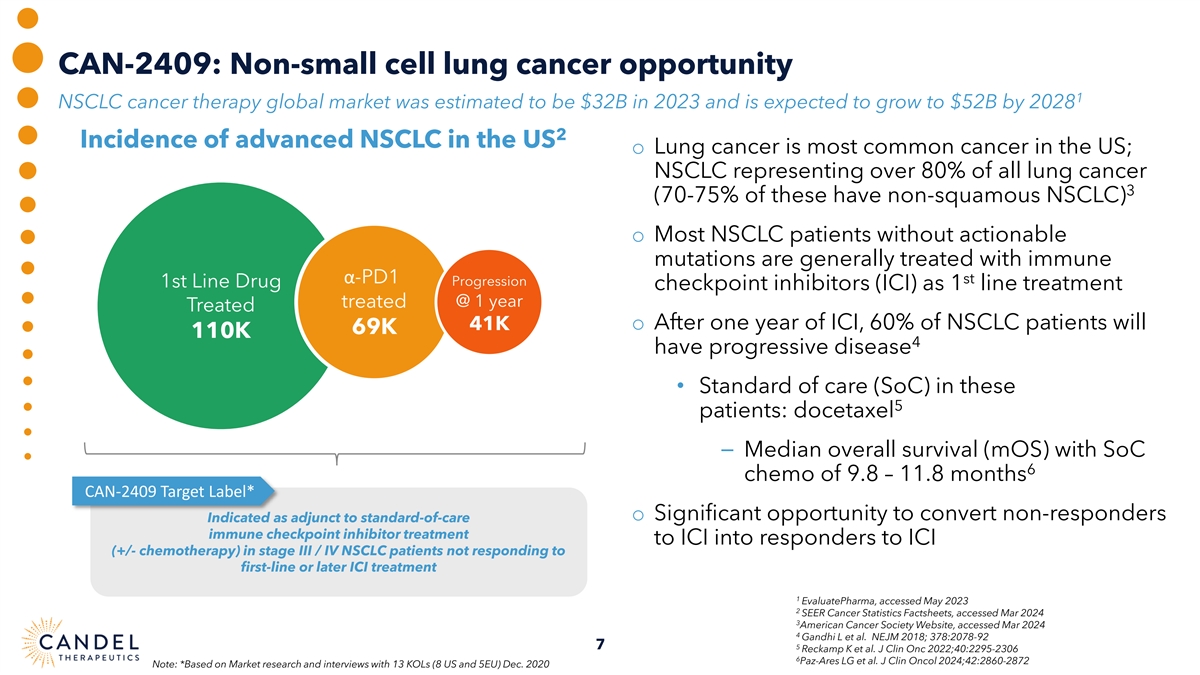
CAN-2409: Non-small cell lung cancer opportunity 1 NSCLC cancer therapy global market was estimated to be $32B in 2023 and is expected to grow to $52B by 2028 2 Incidence of advanced NSCLC in the US o Lung cancer is most common cancer in the US; NSCLC representing over 80% of all lung cancer 3 (70-75% of these have non-squamous NSCLC) o Most NSCLC patients without actionable mutations are generally treated with immune α-PD1 st Progression 1st Line Drug checkpoint inhibitors (ICI) as 1 line treatment treated @ 1 year Treated 41K o After one year of ICI, 60% of NSCLC patients will 69K 110K 4 have progressive disease • Standard of care (SoC) in these 5 patients: docetaxel ⎯ Median overall survival (mOS) with SoC 6 chemo of 9.8 – 11.8 months CAN-2409 Target Label* o Significant opportunity to convert non-responders Indicated as adjunct to standard-of-care immune checkpoint inhibitor treatment to ICI into responders to ICI (+/- chemotherapy) in stage III / IV NSCLC patients not responding to first-line or later ICI treatment 1 EvaluatePharma, accessed May 2023 2 SEER Cancer Statistics Factsheets, accessed Mar 2024 3 American Cancer Society Website, accessed Mar 2024 4 Gandhi L et al. NEJM 2018; 378:2078-92 7 5 Reckamp K et al. J Clin Onc 2022;40:2295-2306 6 Paz-Ares LG et al. J Clin Oncol 2024;42:2860-2872 Note: *Based on Market research and interviews with 13 KOLs (8 US and 5EU) Dec. 2020
Positive overall survival data in phase 2a NSCLC clinical trial o Experimental treatment of CAN-2409 + valacyclovir in NSCLC patients with an inadequate response to (1) ICI was well tolerated, with median overall survival (mOS) of 24.5 months after only two administrations o We observed mOS of 21.5 months in patients with progressive disease at baseline, markedly exceeding mOS reported in this population using standard of care chemotherapy (9.8 – 11.8 months) o Longer tail of survival with 37% of patients alive > 2 years after CAN-2409 administration o Potential for precision medicine approach in patients with the greatest unmet medical needs o mOS of 25.4 months after CAN-2409 treatment in non-squamous NSCLC patients (70-75% of patients) with progressive disease despite ICI o 90% of the patients had stage IV disease; an abscopal effect was observed in ~two-thirds of the patients presenting with at least one uninjected lesion o This observation supports the hypothesis that only one or two tumors need to be injected to teach the immune cells how to recognize the patient’s tumor and induce systemic and durable anti-tumor immunity associated with improved survival 8 (1) The comparisons in mOS for NSCLC are not head-to-head.
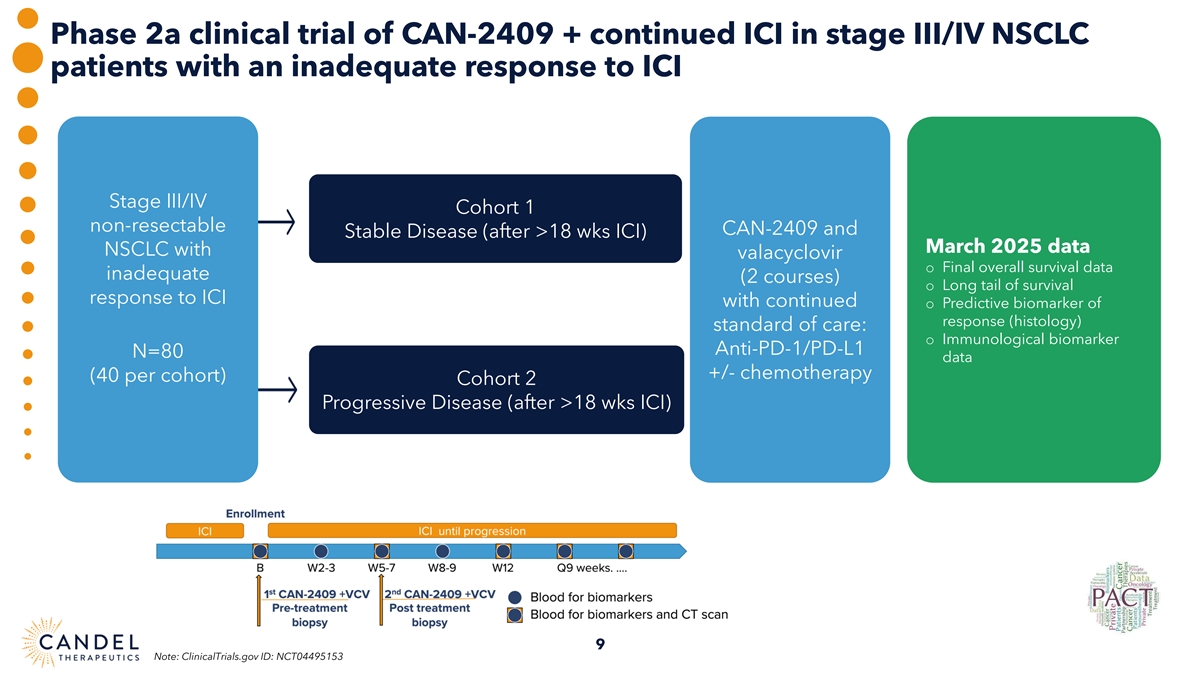
Phase 2a clinical trial of CAN-2409 + continued ICI in stage III/IV NSCLC patients with an inadequate response to ICI Stage III/IV Cohort 1 non-resectable CAN-2409 and Stable Disease (after >18 wks ICI) March 2025 data NSCLC with valacyclovir o Final overall survival data inadequate (2 courses) o Long tail of survival response to ICI with continued o Predictive biomarker of response (histology) standard of care: o Immunological biomarker Anti-PD-1/PD-L1 N=80 data +/- chemotherapy (40 per cohort) Cohort 2 Progressive Disease (after >18 wks ICI) 9 Note: ClinicalTrials.gov ID: NCT04495153

Bronchoscopic delivery of CAN-2409 is an extension of existing care for NSCLC patients Most lung and thoracic lymph node lesions are accessible through outpatient bronchoscopic injection o Therapeutic delivery tool based on extensive experience with bronchoscopic biopsy, a routine outpatient procedure (~30 min, outpatient setting) o Transbronchial needle injection (TBNI) presents similar complication rate as biopsy (extremely rare) o Latest generation of TBNI includes ultrasound-guided transbronchial injection of lymph nodes and robotic bronchoscopy (already in use in phase 2a clinical trial of CAN-2409 in NSCLC) DeMaio A, Sterman D. Eur Respir Rev 2020; 29: 200028 10
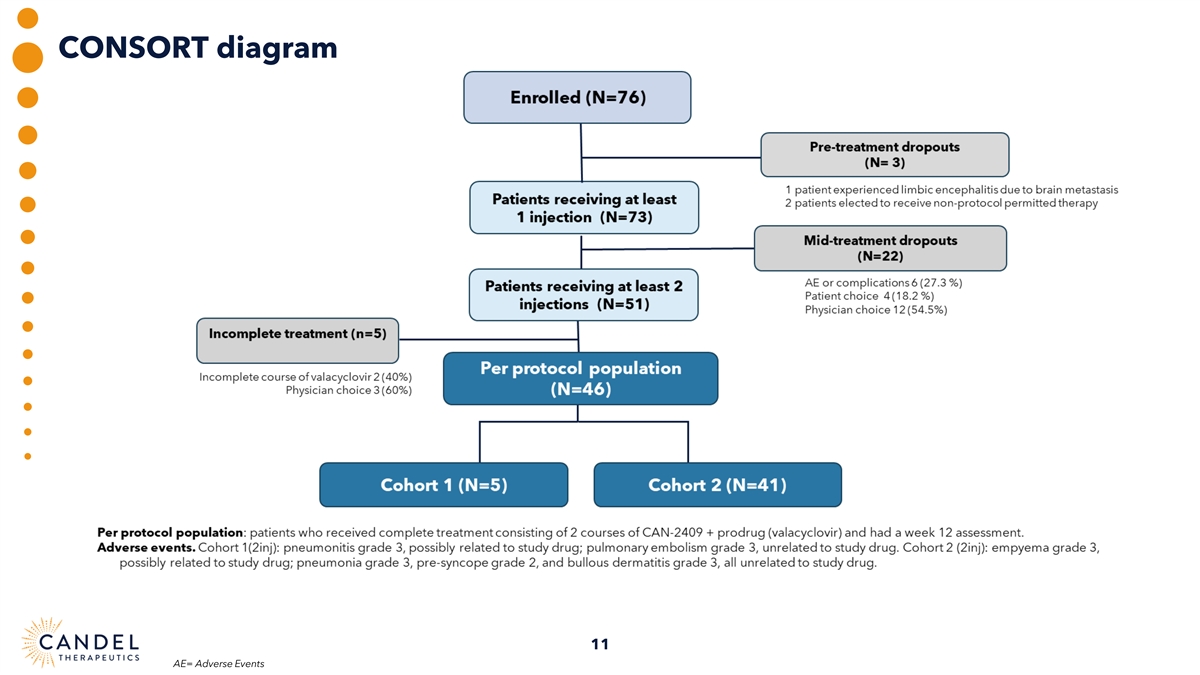
CONSORT diagram 11 AE= Adverse Events
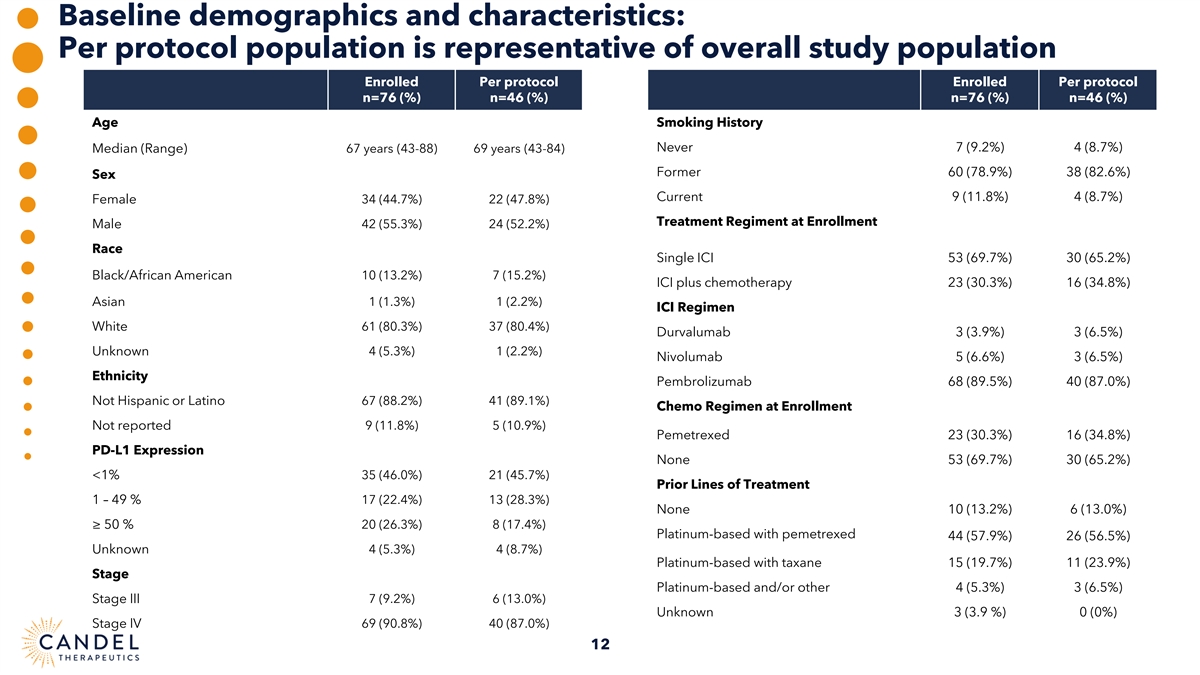
Baseline demographics and characteristics: Per protocol population is representative of overall study population Enrolled Per protocol Enrolled Per protocol n=76 (%) n=46 (%) n=76 (%) n=46 (%) Age Smoking History Never 7 (9.2%) 4 (8.7%) Median (Range) 67 years (43-88) 69 years (43-84) Former 60 (78.9%) 38 (82.6%) Sex Current 9 (11.8%) 4 (8.7%) Female 34 (44.7%) 22 (47.8%) Treatment Regiment at Enrollment Male 42 (55.3%) 24 (52.2%) Race Single ICI 53 (69.7%) 30 (65.2%) Black/African American 10 (13.2%) 7 (15.2%) ICI plus chemotherapy 23 (30.3%) 16 (34.8%) Asian 1 (1.3%) 1 (2.2%) ICI Regimen White 61 (80.3%) 37 (80.4%) Durvalumab 3 (3.9%) 3 (6.5%) 4 (5.3%) 1 (2.2%) Unknown Nivolumab 5 (6.6%) 3 (6.5%) Ethnicity Pembrolizumab 68 (89.5%) 40 (87.0%) Not Hispanic or Latino 67 (88.2%) 41 (89.1%) Chemo Regimen at Enrollment Not reported 9 (11.8%) 5 (10.9%) Pemetrexed 23 (30.3%) 16 (34.8%) PD-L1 Expression None 53 (69.7%) 30 (65.2%) 35 (46.0%) 21 (45.7%) <1% Prior Lines of Treatment 1 – 49 % 17 (22.4%) 13 (28.3%) None 10 (13.2%) 6 (13.0%) ≥ 50 % 20 (26.3%) 8 (17.4%) Platinum-based with pemetrexed 44 (57.9%) 26 (56.5%) Unknown 4 (5.3%) 4 (8.7%) Platinum-based with taxane 15 (19.7%) 11 (23.9%) Stage Platinum-based and/or other 4 (5.3%) 3 (6.5%) Stage III 7 (9.2%) 6 (13.0%) Unknown 3 (3.9 %) 0 (0%) Stage IV 69 (90.8%) 40 (87.0%) 12
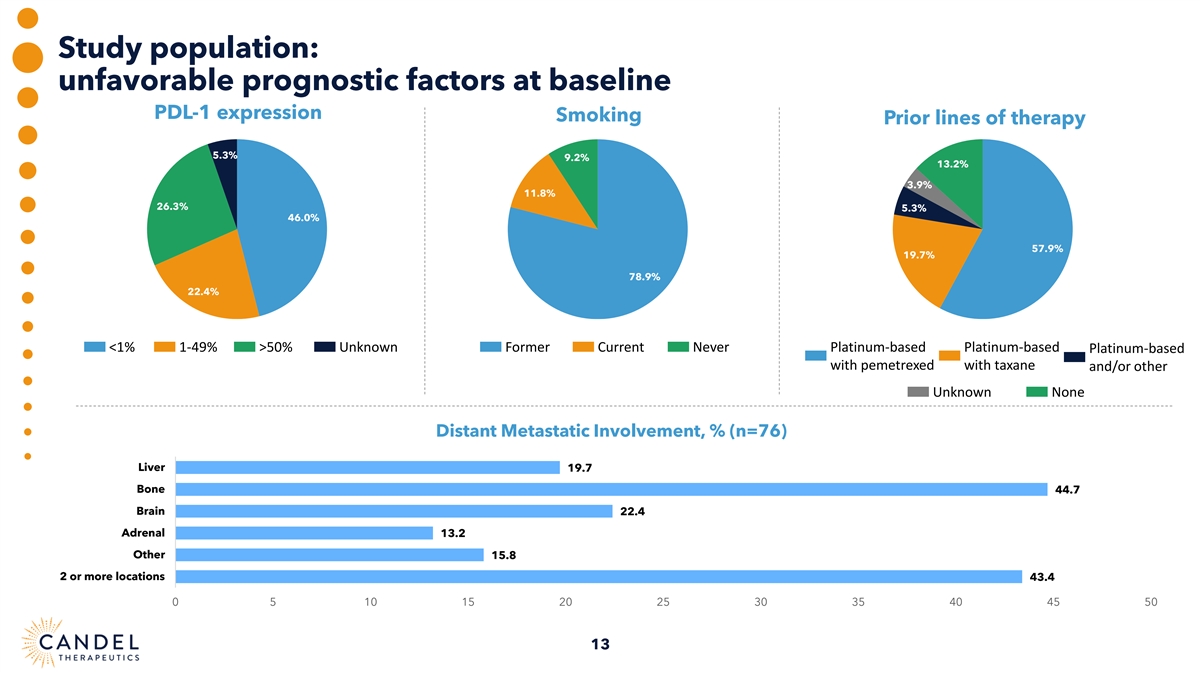
Study population: unfavorable prognostic factors at baseline PDL-1 expression Smoking Prior lines of therapy 5.3% 9.2% 13.2% 3.9% 11.8% 26.3% 5.3% 46.0% 57.9% 19.7% 78.9% 22.4% <1% 1-49% >50% Unknown Former Current Never Platinum-based Platinum-based Platinum-based with pemetrexed with taxane and/or other Unknown None Distant Metastatic Involvement, % (n=76) Liver 19.7 Bone 44.7 Brain 22.4 Adrenal 13.2 Other 15.8 2 or more locations 43.4 0 5 10 15 20 25 30 35 40 45 50 13
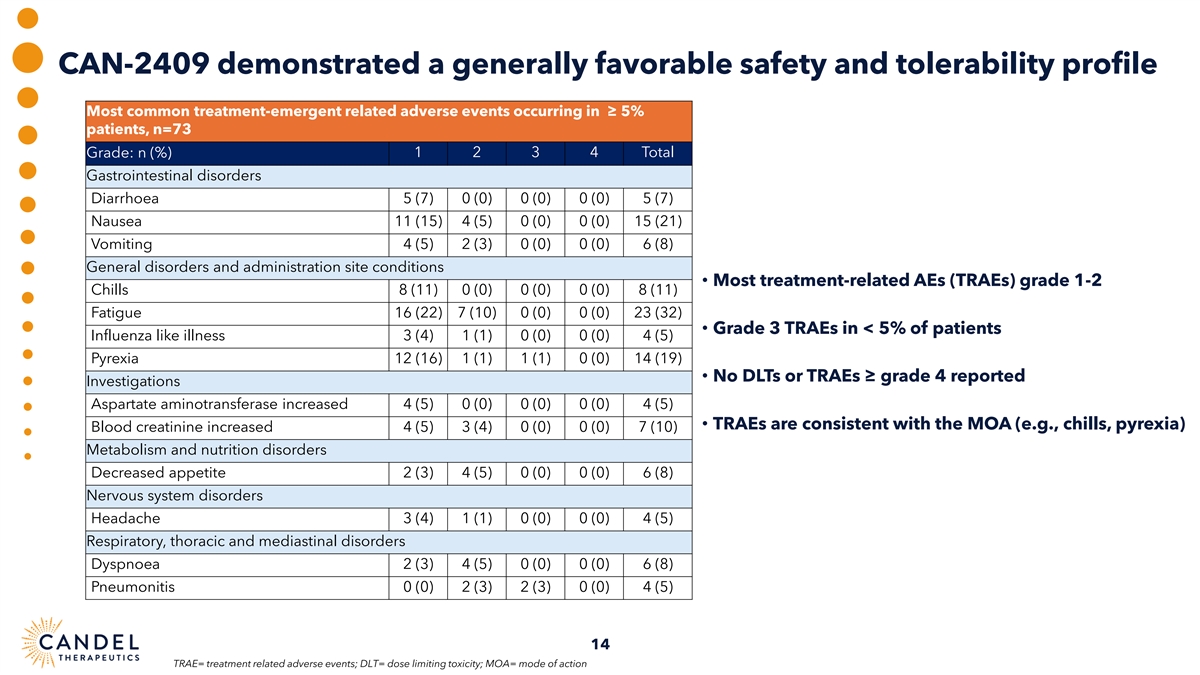
CAN-2409 demonstrated a generally favorable safety and tolerability profile Most common treatment-emergent related adverse events occurring in ≥ 5% patients, n=73 Grade: n (%) 1 2 3 4 Total Gastrointestinal disorders Diarrhoea 5 (7) 0 (0) 0 (0) 0 (0) 5 (7) Nausea 11 (15) 4 (5) 0 (0) 0 (0) 15 (21) Vomiting 4 (5) 2 (3) 0 (0) 0 (0) 6 (8) General disorders and administration site conditions • Most treatment-related AEs (TRAEs) grade 1-2 Chills 8 (11) 0 (0) 0 (0) 0 (0) 8 (11) Fatigue 16 (22) 7 (10) 0 (0) 0 (0) 23 (32) • Grade 3 TRAEs in < 5% of patients Influenza like illness 3 (4) 1 (1) 0 (0) 0 (0) 4 (5) Pyrexia 12 (16) 1 (1) 1 (1) 0 (0) 14 (19) • No DLTs or TRAEs ≥ grade 4 reported Investigations Aspartate aminotransferase increased 4 (5) 0 (0) 0 (0) 0 (0) 4 (5) • TRAEs are consistent with the MOA (e.g., chills, pyrexia) Blood creatinine increased 4 (5) 3 (4) 0 (0) 0 (0) 7 (10) Metabolism and nutrition disorders Decreased appetite 2 (3) 4 (5) 0 (0) 0 (0) 6 (8) Nervous system disorders Headache 3 (4) 1 (1) 0 (0) 0 (0) 4 (5) Respiratory, thoracic and mediastinal disorders Dyspnoea 2 (3) 4 (5) 0 (0) 0 (0) 6 (8) Pneumonitis 0 (0) 2 (3) 2 (3) 0 (0) 4 (5) 14 TRAE= treatment related adverse events; DLT= dose limiting toxicity; MOA= mode of action
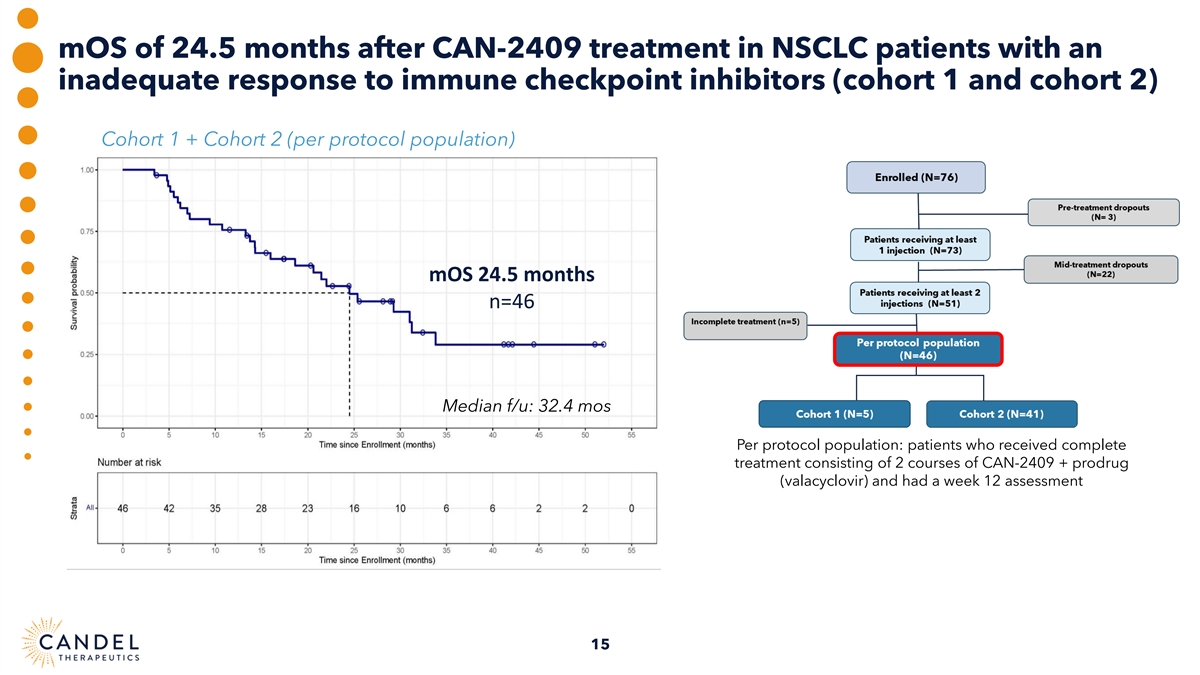
mOS of 24.5 months after CAN-2409 treatment in NSCLC patients with an inadequate response to immune checkpoint inhibitors (cohort 1 and cohort 2) Cohort 1 + Cohort 2 (per protocol population) mOS 24.5 months n=46 Median f/u: 32.4 mos Per protocol population: patients who received complete treatment consisting of 2 courses of CAN-2409 + prodrug (valacyclovir) and had a week 12 assessment 15
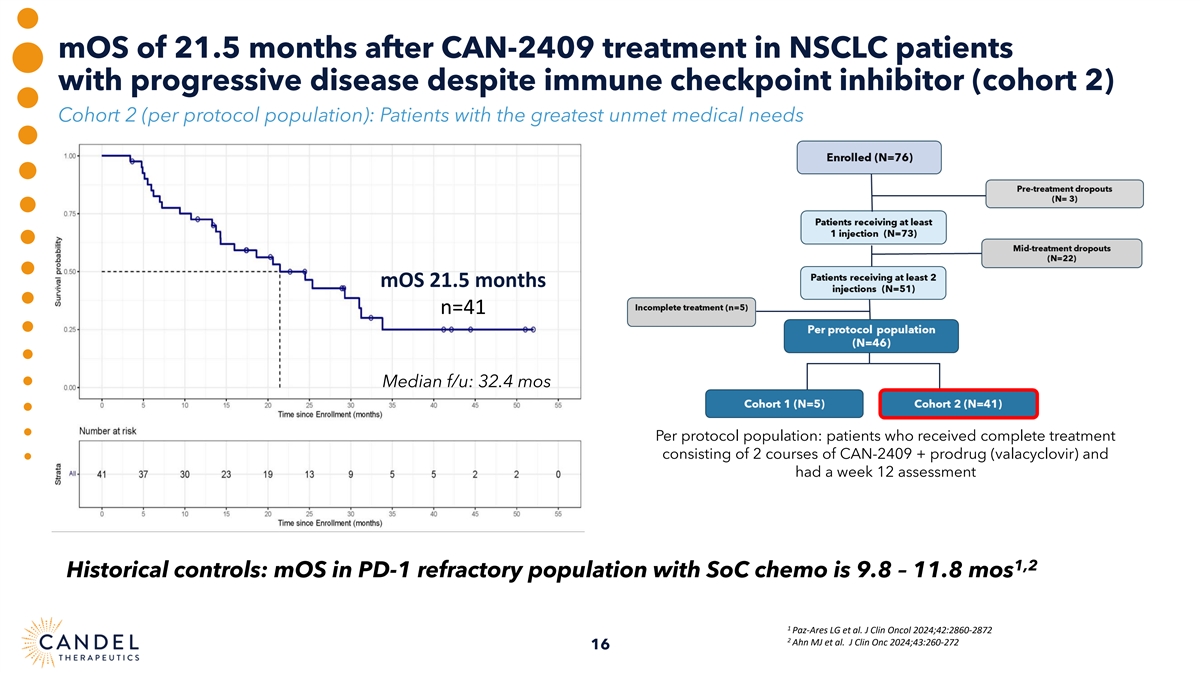
mOS of 21.5 months after CAN-2409 treatment in NSCLC patients with progressive disease despite immune checkpoint inhibitor (cohort 2) Cohort 2 (per protocol population): Patients with the greatest unmet medical needs mOS 21.5 months n=41 Median f/u: 32.4 mos Per protocol population: patients who received complete treatment consisting of 2 courses of CAN-2409 + prodrug (valacyclovir) and had a week 12 assessment 1,2 Historical controls: mOS in PD-1 refractory population with SoC chemo is 9.8 – 11.8 mos 1 Paz-Ares LG et al. J Clin Oncol 2024;42:2860-2872 2 Ahn MJ et al. J Clin Onc 2024;43:260-272 16
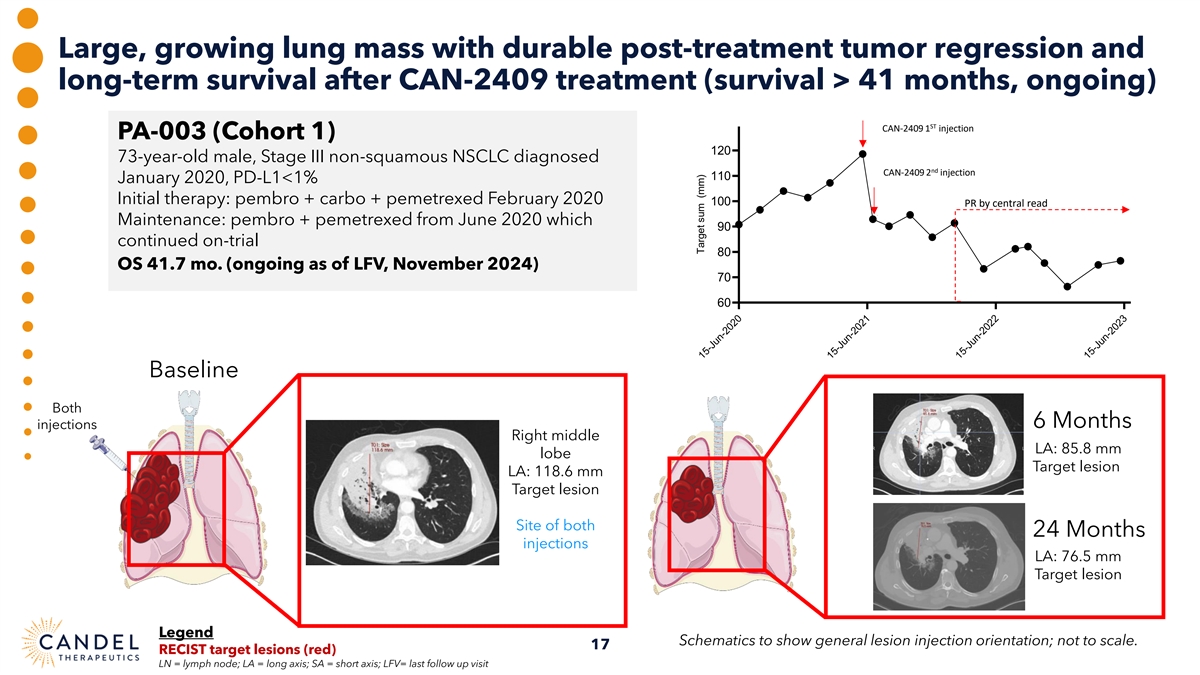
Large, growing lung mass with durable post-treatment tumor regression and long-term survival after CAN-2409 treatment (survival > 41 months, ongoing) ST CAN-2409 1 injection PA-003 (Cohort 1) 120 73-year-old male, Stage III non-squamous NSCLC diagnosed nd CAN-2409 2 injection 110 January 2020, PD-L1<1% Initial therapy: pembro + carbo + pemetrexed February 2020 100 PR by central read Maintenance: pembro + pemetrexed from June 2020 which 90 continued on-trial 80 OS 41.7 mo. (ongoing as of LFV, November 2024) 70 60 Baseline Both 6 Months injections Right middle LA: 85.8 mm lobe Target lesion LA: 118.6 mm Target lesion Site of both 24 Months injections LA: 76.5 mm Target lesion Legend Schematics to show general lesion injection orientation; not to scale. 17 RECIST target lesions (red) LN = lymph node; LA = long axis; SA = short axis; LFV= last follow up visit 15-Jun-2020 15-Jun-2021 15-Jun-2022 15-Jun-2023 Target sum (mm)
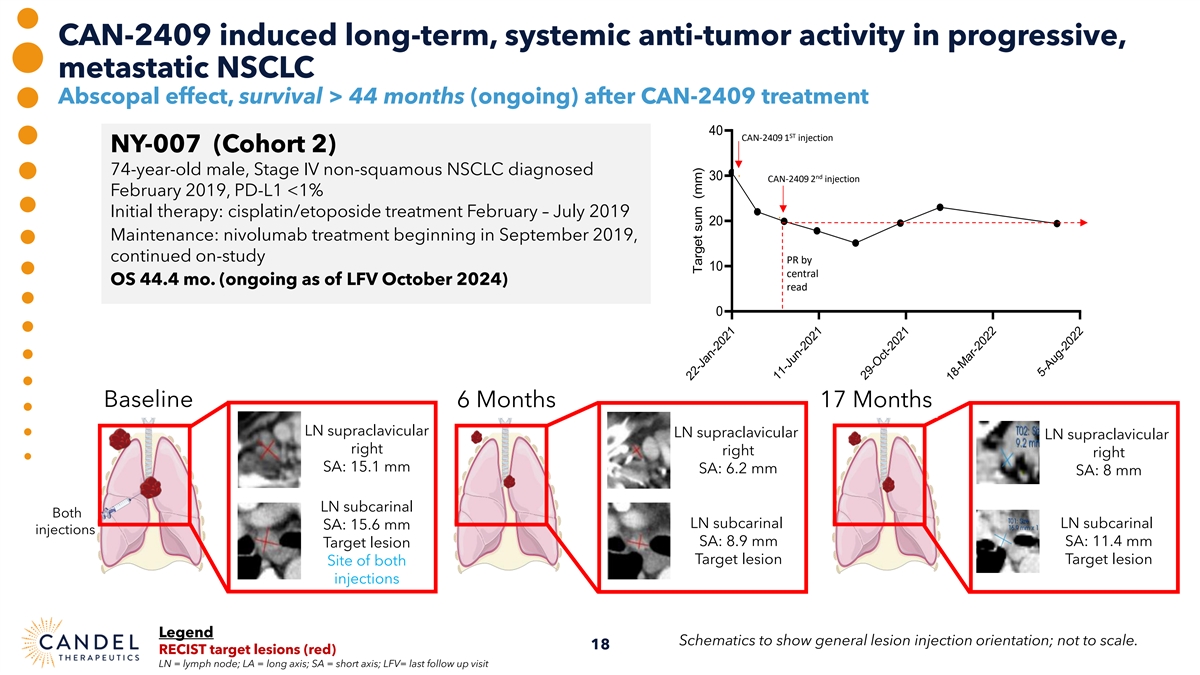
CAN-2409 induced long-term, systemic anti-tumor activity in progressive, metastatic NSCLC Abscopal effect, survival > 44 months (ongoing) after CAN-2409 treatment 40 ST CAN-2409 1 injection NY-007 (Cohort 2) 74-year-old male, Stage IV non-squamous NSCLC diagnosed nd 30 CAN-2409 2 injection February 2019, PD-L1 <1% Initial therapy: cisplatin/etoposide treatment February – July 2019 20 Maintenance: nivolumab treatment beginning in September 2019, continued on-study PR by 10 central OS 44.4 mo. (ongoing as of LFV October 2024) read 0 Baseline 6 Months 17 Months LN supraclavicular LN supraclavicular LN supraclavicular right right right SA: 15.1 mm SA: 6.2 mm SA: 8 mm LN subcarinal Both LN subcarinal LN subcarinal SA: 15.6 mm injections SA: 8.9 mm SA: 11.4 mm Target lesion Target lesion Target lesion Site of both injections Legend Schematics to show general lesion injection orientation; not to scale. 18 RECIST target lesions (red) LN = lymph node; LA = long axis; SA = short axis; LFV= last follow up visit 22-Jan-2021 11-Jun-2021 29-Oct-2021 18-Mar-2022 5-Aug-2022 Target sum (mm)
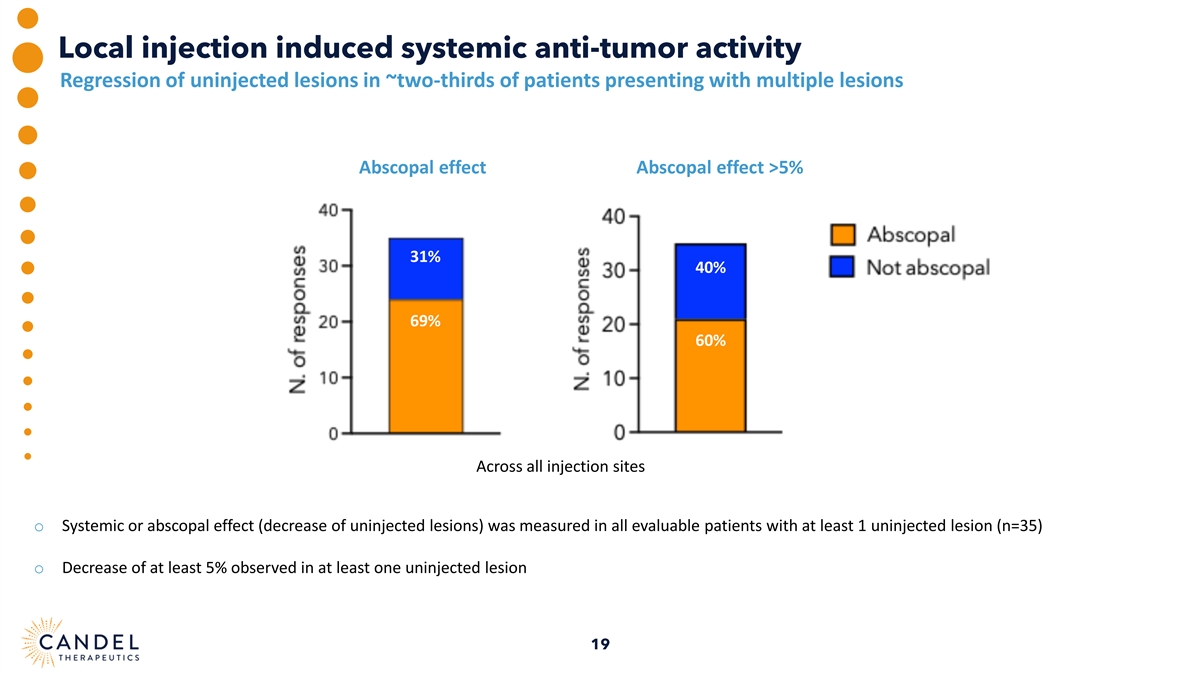
Local injection induced systemic anti-tumor activity Regression of uninjected lesions in ~two-thirds of patients presenting with multiple lesions Abscopal effect Abscopal effect >5% 31% 40% 69% 60% Across all injection sites o Systemic or abscopal effect (decrease of uninjected lesions) was measured in all evaluable patients with at least 1 uninjected lesion (n=35) o Decrease of at least 5% observed in at least one uninjected lesion 19
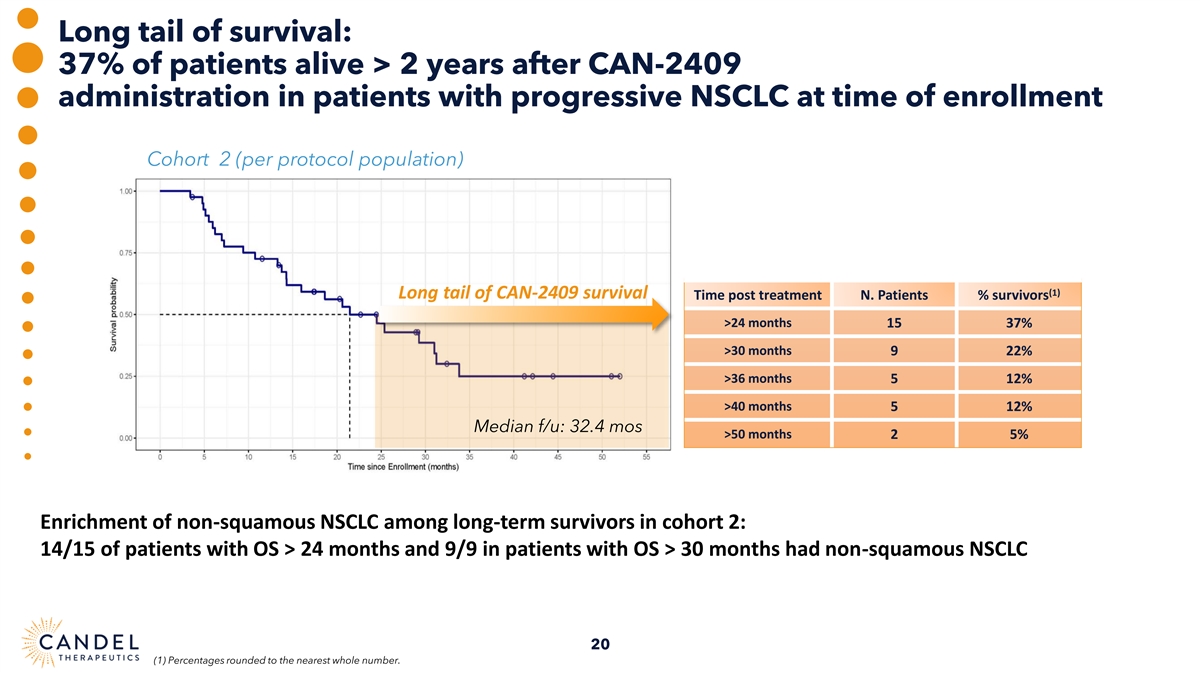
Long tail of survival: 37% of patients alive > 2 years after CAN-2409 administration in patients with progressive NSCLC at time of enrollment Cohort 2 (per protocol population) (1) Long tail of CAN-2409 survival Time post treatment N. Patients % survivors >24 months 15 37% >30 months 9 22% >36 months 5 12% >40 months 5 12% Median f/u: 32.4 mos >50 months 2 5% Enrichment of non-squamous NSCLC among long-term survivors in cohort 2: 14/15 of patients with OS > 24 months and 9/9 in patients with OS > 30 months had non-squamous NSCLC 20 (1) Percentages rounded to the nearest whole number.

Towards a precision medicine approach: Non-squamous (~70-75%) and squamous PD(L)-1 refractory NSCLC (~25- 30%) are distinct disease subsets with a differential response to treatment 1 Survival by histology in TROPION-Lung01 study Survival by histology in CAN-2409 treated patients 16.7 14.6 12.3 HR 0.444; p=0.026 9.4 7.6 7.2 Dato DXd Docetaxel Non-SQ SQ Non-SQ SQ 1 Ahn MJ et al. J Clin Onc 2024;43:260-272 Patients with progressive disease at enrollment Non-SQ= non squamous, SQ = squamous, HR = Hazard Ratio (statistical measure used (Cohort 2) who received at least one CAN-2409 in survival analysis to compare the risk of an event (such as death) occurring between injection two groups over time) Median represented (Non-SQ=51; SQ=15) 21 Median OS in months Median OS in months
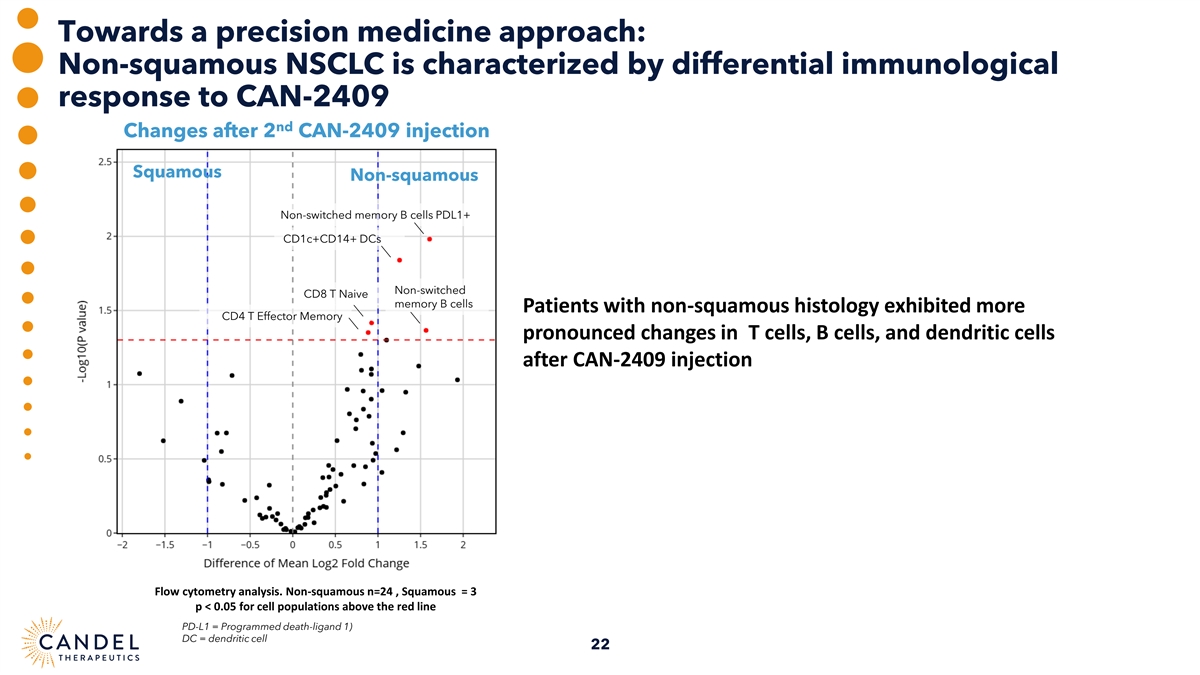
Towards a precision medicine approach: Non-squamous NSCLC is characterized by differential immunological response to CAN-2409 nd Changes after 2 CAN-2409 injection Squamous Non-squamous Non-switched memory B cells PDL1+ CD1c+CD14+ DCs Non-switched CD8 T Naive memory B cells Patients with non-squamous histology exhibited more CD4 T Effector Memory pronounced changes in T cells, B cells, and dendritic cells after CAN-2409 injection Flow cytometry analysis. Non-squamous n=24 , Squamous = 3 p < 0.05 for cell populations above the red line PD-L1 = Programmed death-ligand 1) DC = dendritic cell 22
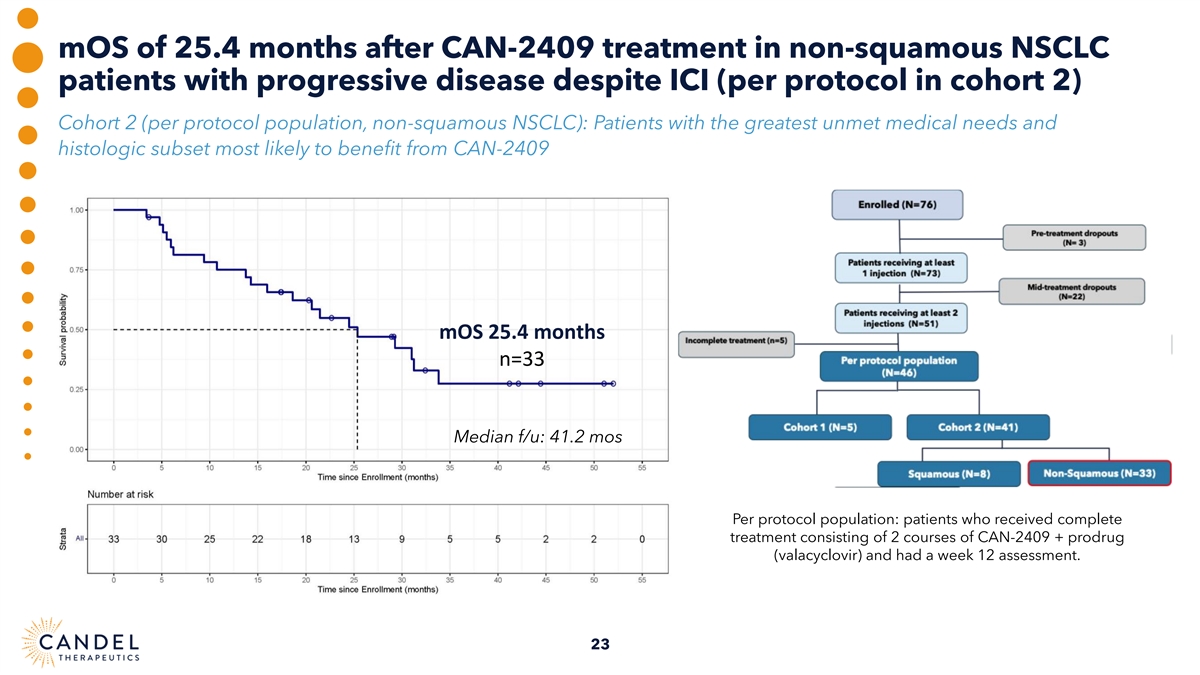
mOS of 25.4 months after CAN-2409 treatment in non-squamous NSCLC patients with progressive disease despite ICI (per protocol in cohort 2) Cohort 2 (per protocol population, non-squamous NSCLC): Patients with the greatest unmet medical needs and histologic subset most likely to benefit from CAN-2409 mOS 25.4 months n=33 Median f/u: 41.2 mos Per protocol population: patients who received complete treatment consisting of 2 courses of CAN-2409 + prodrug (valacyclovir) and had a week 12 assessment. 23
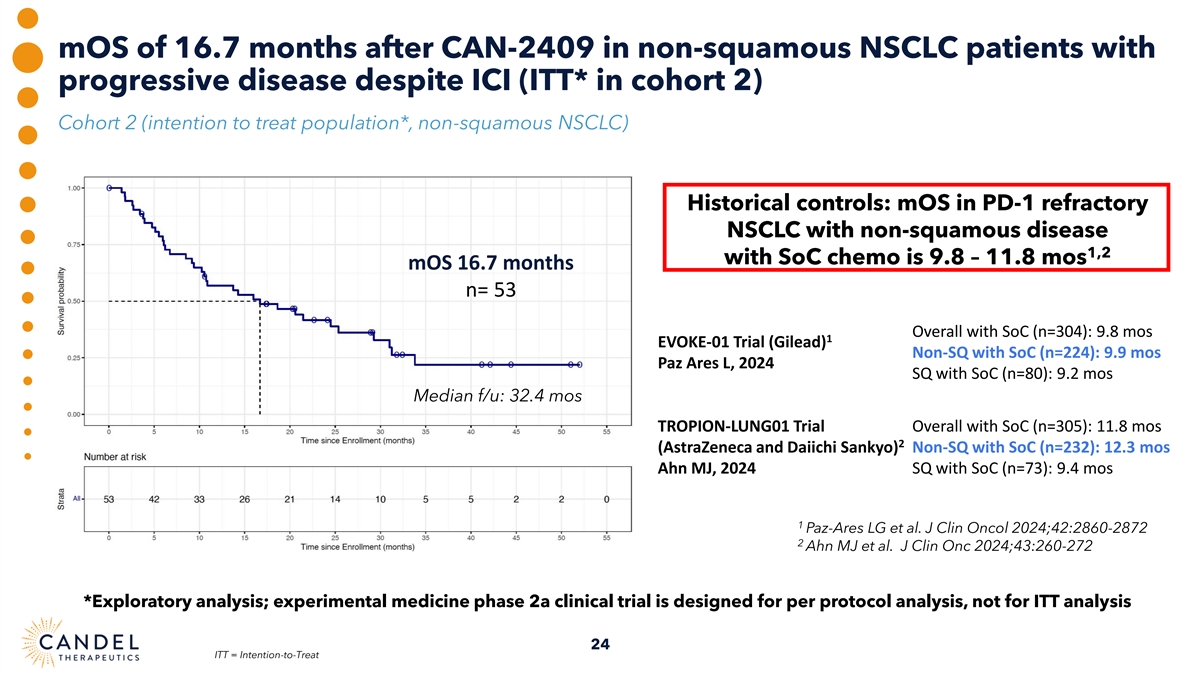
mOS of 16.7 months after CAN-2409 in non-squamous NSCLC patients with progressive disease despite ICI (ITT* in cohort 2) Cohort 2 (intention to treat population*, non-squamous NSCLC) Historical controls: mOS in PD-1 refractory NSCLC with non-squamous disease 1,2 with SoC chemo is 9.8 – 11.8 mos mOS 16.7 months n= 53 Overall with SoC (n=304): 9.8 mos 1 EVOKE-01 Trial (Gilead) Non-SQ with SoC (n=224): 9.9 mos Paz Ares L, 2024 SQ with SoC (n=80): 9.2 mos Median f/u: 32.4 mos TROPION-LUNG01 Trial Overall with SoC (n=305): 11.8 mos 2 (AstraZeneca and Daiichi Sankyo) Non-SQ with SoC (n=232): 12.3 mos Ahn MJ, 2024 SQ with SoC (n=73): 9.4 mos 1 Paz-Ares LG et al. J Clin Oncol 2024;42:2860-2872 2 Ahn MJ et al. J Clin Onc 2024;43:260-272 *Exploratory analysis; experimental medicine phase 2a clinical trial is designed for per protocol analysis, not for ITT analysis 24 ITT = Intention-to-Treat

CAN-2409 Pancreatic Cancer Off-the-shelf therapy, individualized cancer response Enabling work underway (Protocol development, Scientific and Regulatory Advisory Boards, engagement with FDA) 25
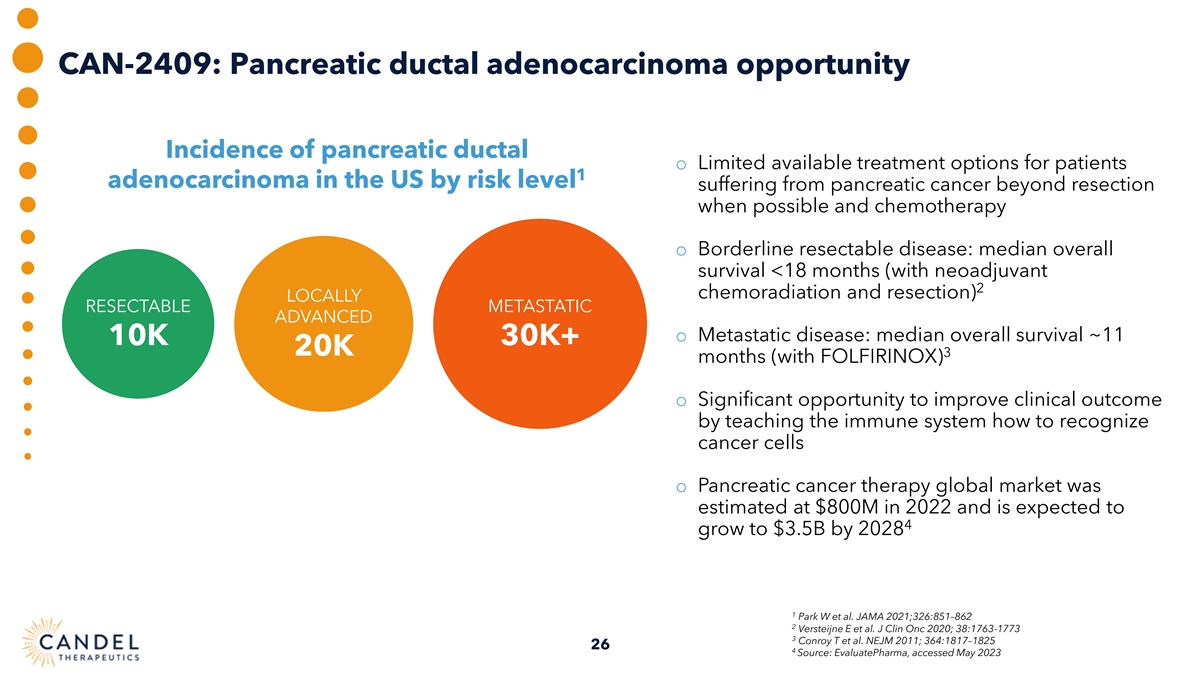
CAN-2409: Pancreatic ductal adenocarcinoma opportunity Incidence of pancreatic ductal o Limited available treatment options for patients 1 adenocarcinoma in the US by risk level suffering from pancreatic cancer beyond resection when possible and chemotherapy o Borderline resectable disease: median overall survival <18 months (with neoadjuvant 2 chemoradiation and resection) LOCALLY RESECTABLE METASTATIC ADVANCED o Metastatic disease: median overall survival ~11 10K 30K+ 20K 3 months (with FOLFIRINOX) o Significant opportunity to improve clinical outcome by teaching the immune system how to recognize cancer cells o Pancreatic cancer therapy global market was estimated at $800M in 2022 and is expected to 4 grow to $3.5B by 2028 1 Park W et al. JAMA 2021;326:851–862 2 Versteijne E et al. J Clin Onc 2020; 38:1763-1773 3 Conroy T et al. NEJM 2011; 364:1817–1825 26 4 Source: EvaluatePharma, accessed May 2023
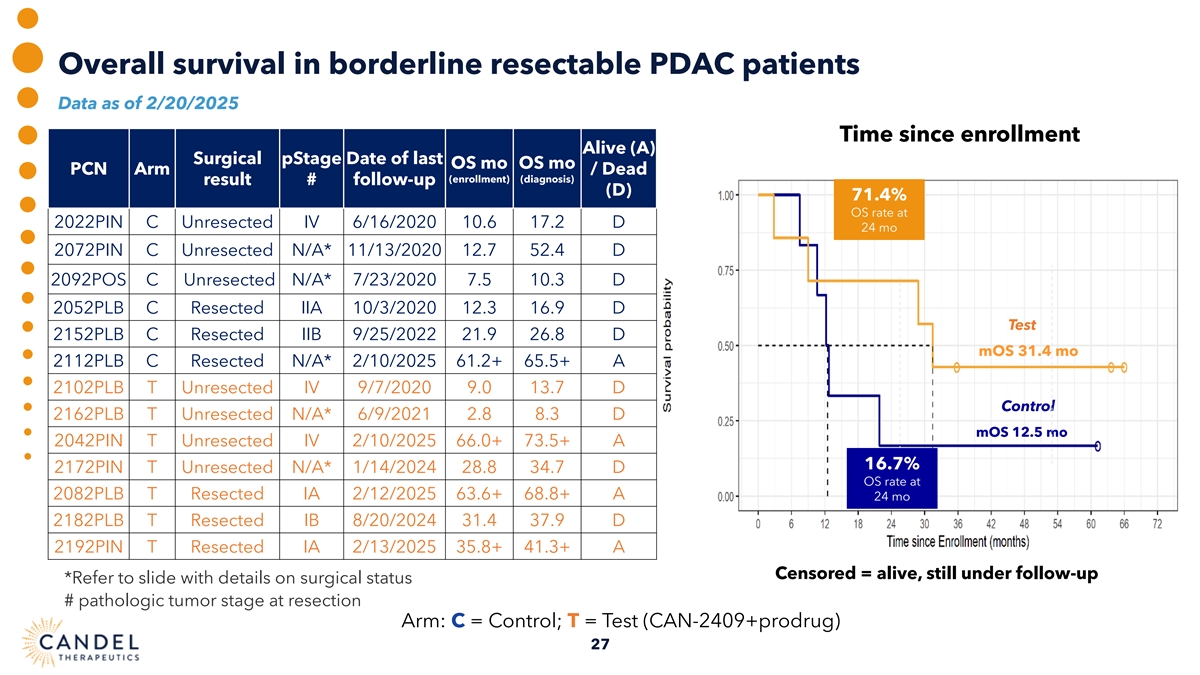
Overall survival in borderline resectable PDAC patients Data as of 2/20/2025 Time since enrollment Alive (A) Surgical pStage Date of last OS mo OS mo PCN Arm / Dead (enrollment) (diagnosis) result # follow-up (D) 71.4% OS rate at 2022PIN C Unresected IV 6/16/2020 10.6 17.2 D 24 mo 2072PIN C Unresected N/A* 11/13/2020 12.7 52.4 D 2092POS C Unresected N/A* 7/23/2020 7.5 10.3 D 2052PLB C Resected IIA 10/3/2020 12.3 16.9 D Test 2152PLB C Resected IIB 9/25/2022 21.9 26.8 D mOS 31.4 mo 2112PLB C Resected N/A* 2/10/2025 61.2+ 65.5+ A 2102PLB T Unresected IV 9/7/2020 9.0 13.7 D Control 2162PLB T Unresected N/A* 6/9/2021 2.8 8.3 D mOS 12.5 mo 2042PIN T Unresected IV 2/10/2025 66.0+ 73.5+ A 16.7% 2172PIN T Unresected N/A* 1/14/2024 28.8 34.7 D OS rate at 2082PLB T Resected IA 2/12/2025 63.6+ 68.8+ A 24 mo 2182PLB T Resected IB 8/20/2024 31.4 37.9 D 2192PIN T Resected IA 2/13/2025 35.8+ 41.3+ A Censored = alive, still under follow-up *Refer to slide with details on surgical status # pathologic tumor stage at resection Arm: C = Control; T = Test (CAN-2409+prodrug) 27
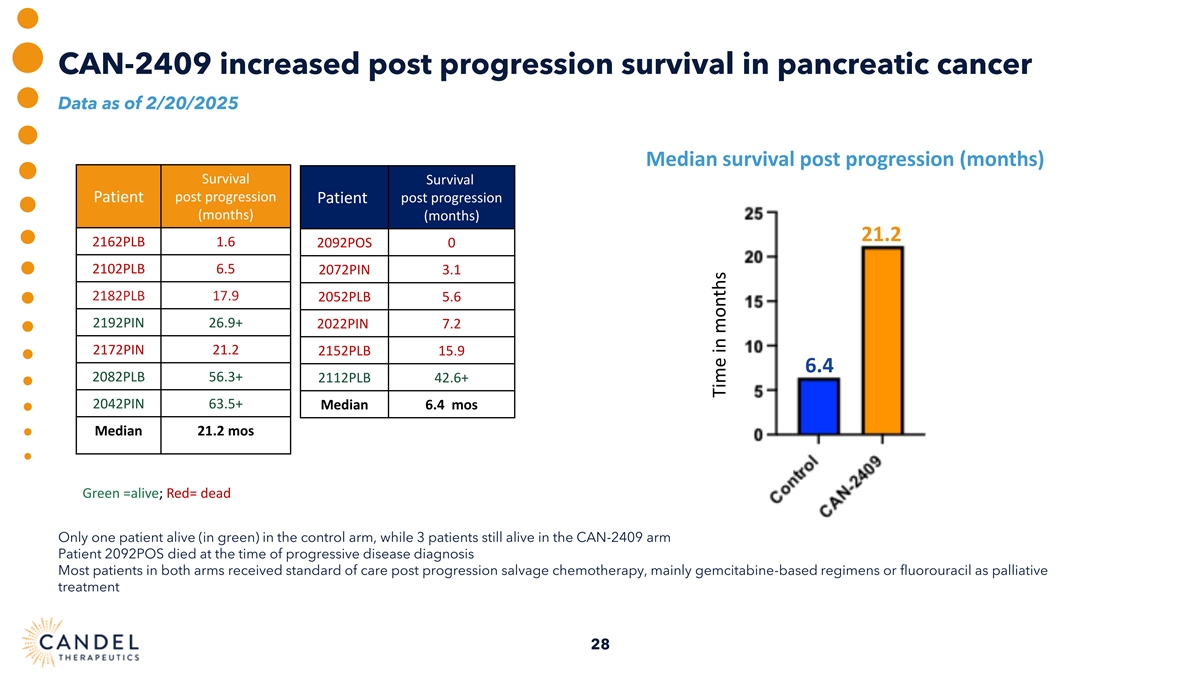
CAN-2409 increased post progression survival in pancreatic cancer Data as of 2/20/2025 Median survival post progression (months) Survival Survival post progression Patient post progression Patient (months) (months) 21.2 2162PLB 1.6 2092POS 0 2102PLB 6.5 2072PIN 3.1 2182PLB 17.9 2052PLB 5.6 2192PIN 26.9+ 2022PIN 7.2 2172PIN 21.2 2152PLB 15.9 6.4 2082PLB 56.3+ 2112PLB 42.6+ 2042PIN 63.5+ Median 6.4 mos Median 21.2 mos Green =alive; Red= dead Only one patient alive (in green) in the control arm, while 3 patients still alive in the CAN-2409 arm Patient 2092POS died at the time of progressive disease diagnosis Most patients in both arms received standard of care post progression salvage chemotherapy, mainly gemcitabine-based regimens or fluorouracil as palliative treatment 28 Time in months

CAN-2409 Prostate Cancer Preparatory activities underway for BLA submission and potential commercialization 29
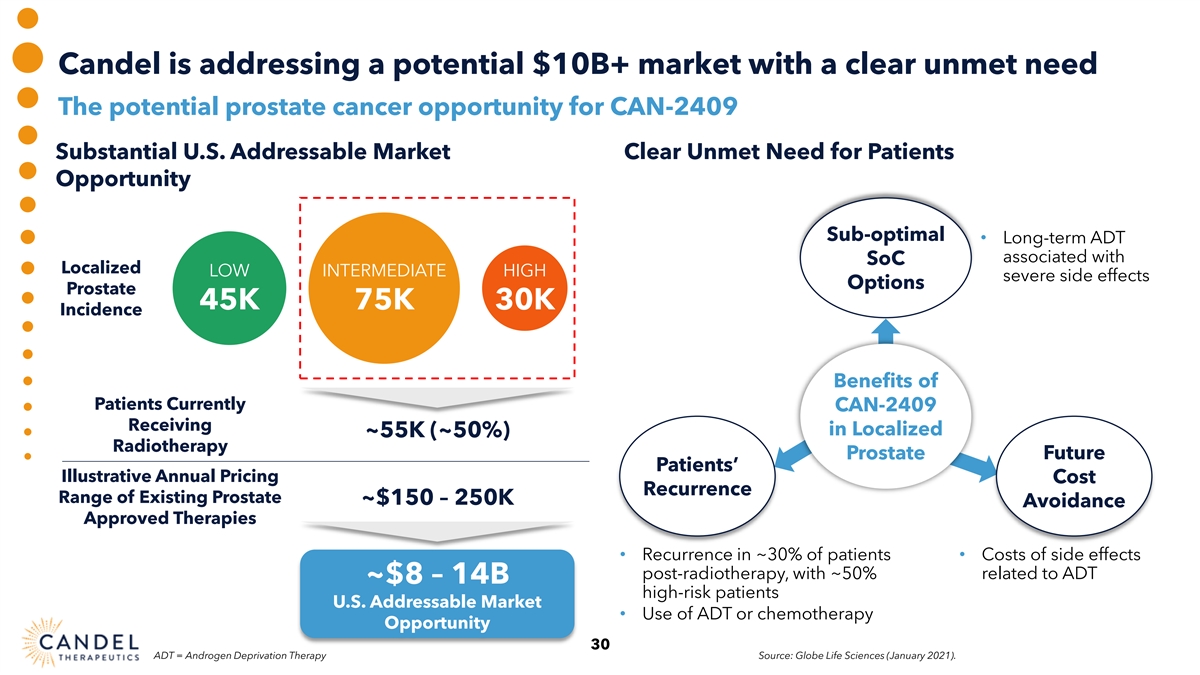
Candel is addressing a potential $10B+ market with a clear unmet need The potential prostate cancer opportunity for CAN-2409 Substantial U.S. Addressable Market Clear Unmet Need for Patients Opportunity Sub-optimal • Long-term ADT associated with SoC Localized LOW INTERMEDIATE HIGH severe side effects Options Prostate 45K 75K 30K Incidence Benefits of Patients Currently CAN-2409 Receiving in Localized ~55K (~50%) Radiotherapy Prostate Future Patients’ Illustrative Annual Pricing Cost Recurrence Range of Existing Prostate ~$150 – 250K Avoidance Approved Therapies • Recurrence in ~30% of patients • Costs of side effects post-radiotherapy, with ~50% related to ADT ~$8 – 14B high-risk patients U.S. Addressable Market • Use of ADT or chemotherapy Opportunity 30 ADT = Androgen Deprivation Therapy Source: Globe Life Sciences (January 2021).
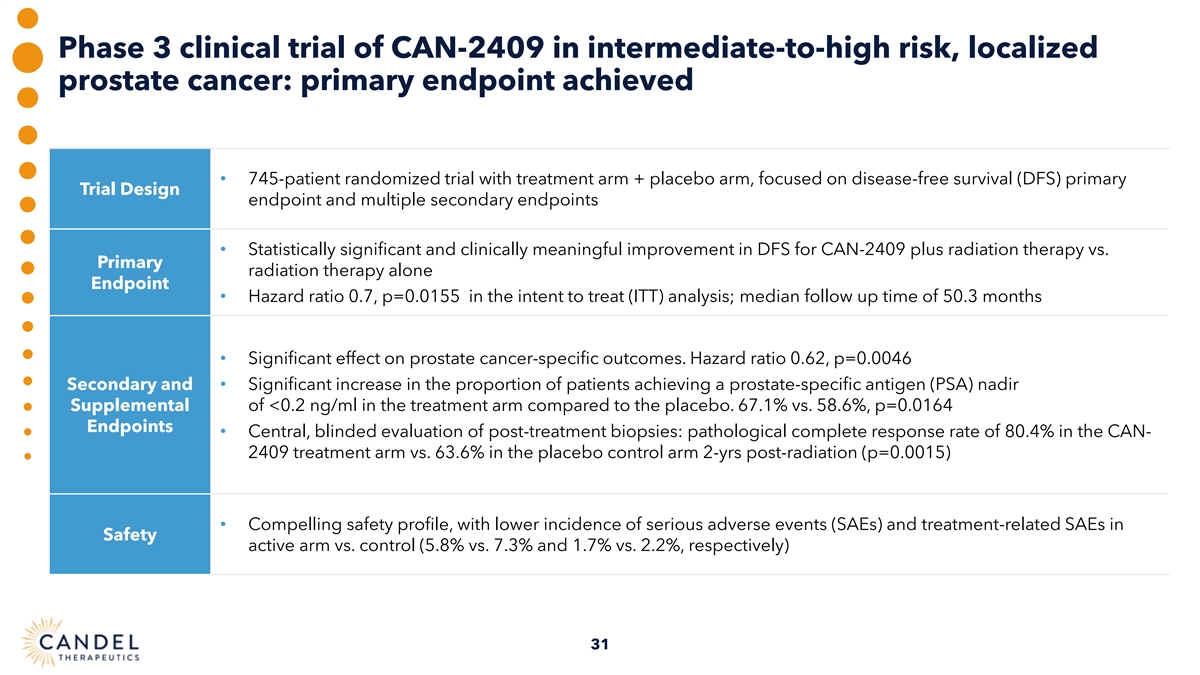
Phase 3 clinical trial of CAN-2409 in intermediate-to-high risk, localized prostate cancer: primary endpoint achieved • 745-patient randomized trial with treatment arm + placebo arm, focused on disease-free survival (DFS) primary Trial Design endpoint and multiple secondary endpoints • Statistically significant and clinically meaningful improvement in DFS for CAN-2409 plus radiation therapy vs. Primary radiation therapy alone Endpoint • Hazard ratio 0.7, p=0.0155 in the intent to treat (ITT) analysis; median follow up time of 50.3 months • Significant effect on prostate cancer-specific outcomes. Hazard ratio 0.62, p=0.0046 Secondary and • Significant increase in the proportion of patients achieving a prostate-specific antigen (PSA) nadir Supplemental of <0.2 ng/ml in the treatment arm compared to the placebo. 67.1% vs. 58.6%, p=0.0164 Endpoints • Central, blinded evaluation of post-treatment biopsies: pathological complete response rate of 80.4% in the CAN- 2409 treatment arm vs. 63.6% in the placebo control arm 2-yrs post-radiation (p=0.0015) • Compelling safety profile, with lower incidence of serious adverse events (SAEs) and treatment-related SAEs in Safety active arm vs. control (5.8% vs. 7.3% and 1.7% vs. 2.2%, respectively) 31
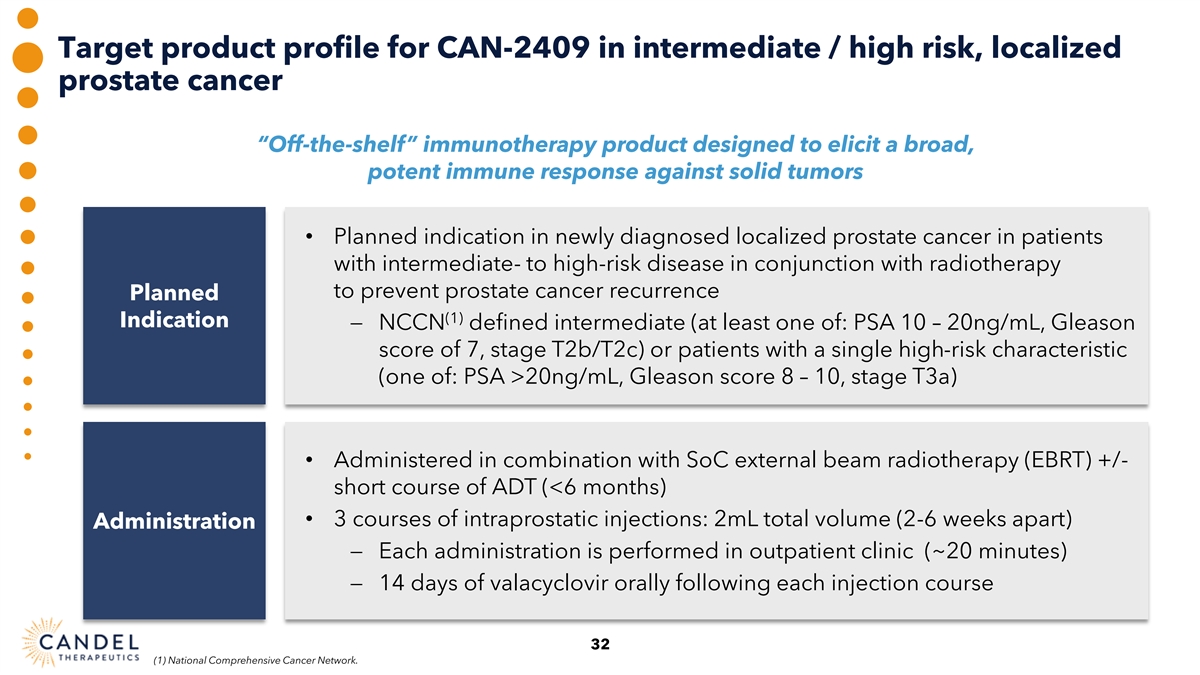
Target product profile for CAN-2409 in intermediate / high risk, localized prostate cancer “Off-the-shelf” immunotherapy product designed to elicit a broad, potent immune response against solid tumors • Planned indication in newly diagnosed localized prostate cancer in patients with intermediate- to high-risk disease in conjunction with radiotherapy to prevent prostate cancer recurrence Planned (1) Indication − NCCN defined intermediate (at least one of: PSA 10 – 20ng/mL, Gleason score of 7, stage T2b/T2c) or patients with a single high-risk characteristic (one of: PSA >20ng/mL, Gleason score 8 – 10, stage T3a) • Administered in combination with SoC external beam radiotherapy (EBRT) +/- short course of ADT (<6 months) • 3 courses of intraprostatic injections: 2mL total volume (2-6 weeks apart) Administration − Each administration is performed in outpatient clinic (~20 minutes) − 14 days of valacyclovir orally following each injection course 32 (1) National Comprehensive Cancer Network.
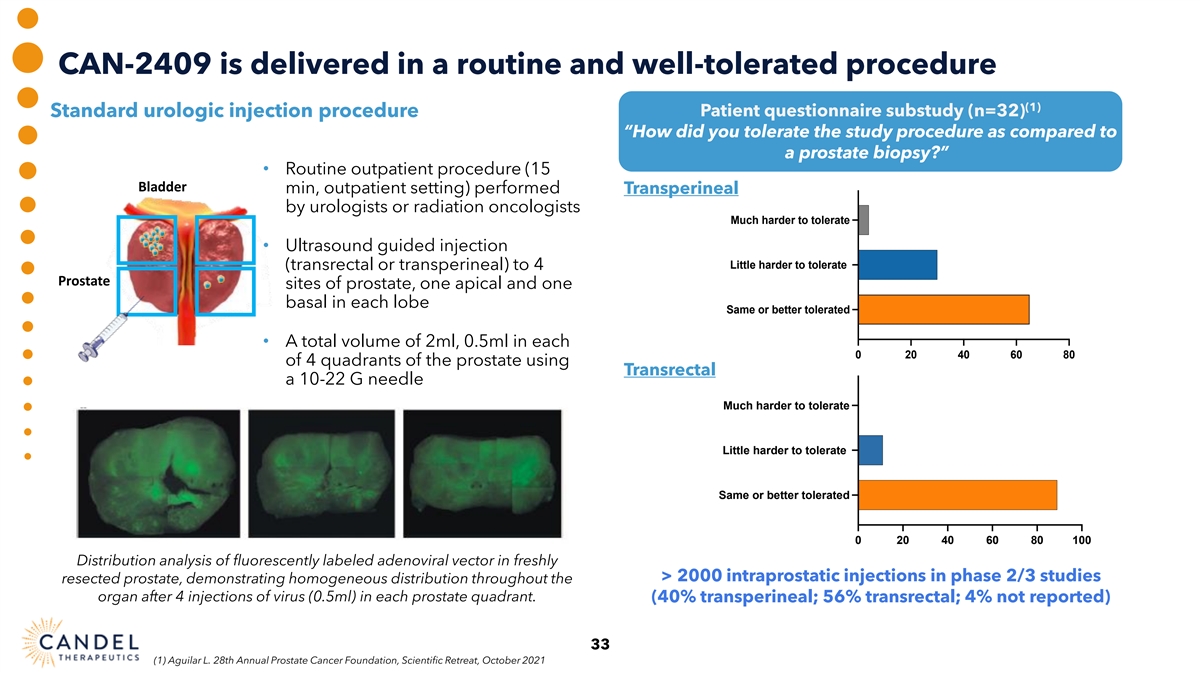
CAN-2409 is delivered in a routine and well-tolerated procedure (1) Patient questionnaire substudy (n=32) Standard urologic injection procedure “How did you tolerate the study procedure as compared to Tolerability of study procedure a prostate biopsy?” compared to prostate biopsy • Routine outpatient procedure (15 Bladder min, outpatient setting) performed Transperineal by urologists or radiation oncologists Much harder to tolerate • Ultrasound guided injection Little harder to tolerate (transrectal or transperineal) to 4 Prostate sites of prostate, one apical and one basal in each lobe Same or better tolerated • A total volume of 2ml, 0.5ml in each 0 20 40 60 80 of 4 quadrants of the prostate using Transrectal a 10-22 G needle Much harder to tolerate Little harder to tolerate Same or better tolerated 0 20 40 60 80 100 Distribution analysis of fluorescently labeled adenoviral vector in freshly > 2000 intraprostatic injections in phase 2/3 studies resected prostate, demonstrating homogeneous distribution throughout the organ after 4 injections of virus (0.5ml) in each prostate quadrant. (40% transperineal; 56% transrectal; 4% not reported) 33 (1) Aguilar L. 28th Annual Prostate Cancer Foundation, Scientific Retreat, October 2021
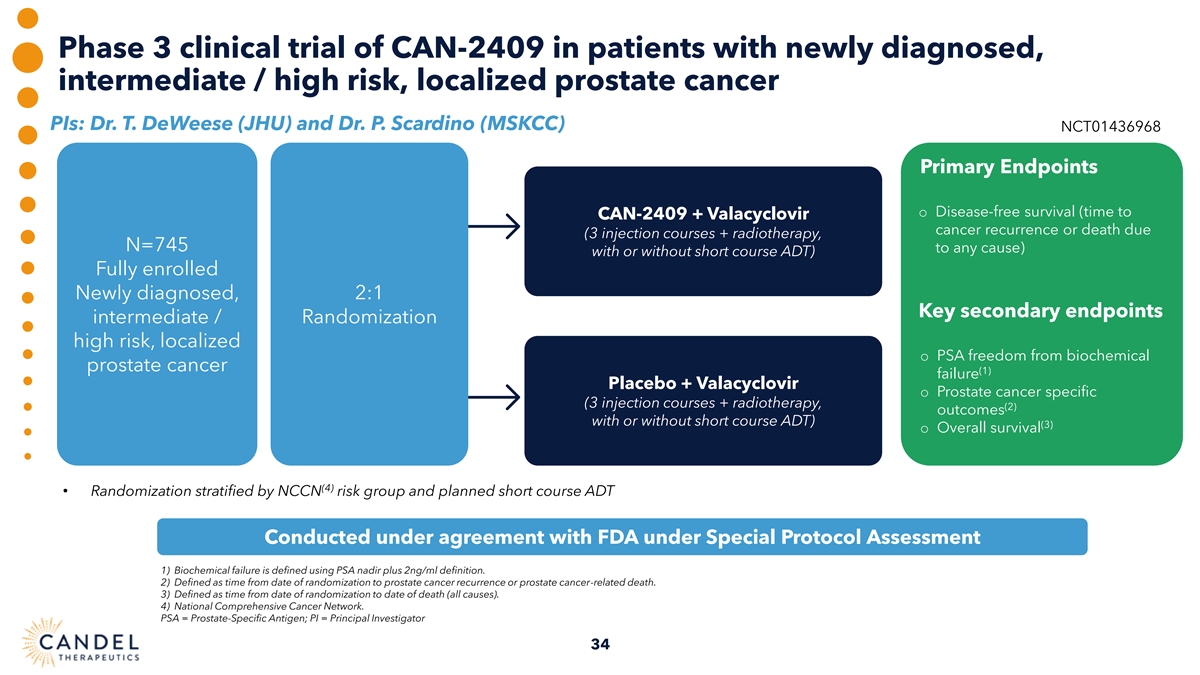
Phase 3 clinical trial of CAN-2409 in patients with newly diagnosed, intermediate / high risk, localized prostate cancer PIs: Dr. T. DeWeese (JHU) and Dr. P. Scardino (MSKCC) NCT01436968 Primary Endpoints o Disease-free survival (time to CAN-2409 + Valacyclovir cancer recurrence or death due (3 injection courses + radiotherapy, N=745 to any cause) with or without short course ADT) Fully enrolled Newly diagnosed, 2:1 Key secondary endpoints intermediate / Randomization high risk, localized o PSA freedom from biochemical prostate cancer (1) failure Placebo + Valacyclovir o Prostate cancer specific (3 injection courses + radiotherapy, (2) outcomes with or without short course ADT) (3) o Overall survival (4) • Randomization stratified by NCCN risk group and planned short course ADT Conducted under agreement with FDA under Special Protocol Assessment 1) Biochemical failure is defined using PSA nadir plus 2ng/ml definition. 2) Defined as time from date of randomization to prostate cancer recurrence or prostate cancer-related death. 3) Defined as time from date of randomization to date of death (all causes). 4) National Comprehensive Cancer Network. PSA = Prostate-Specific Antigen; PI = Principal Investigator 34
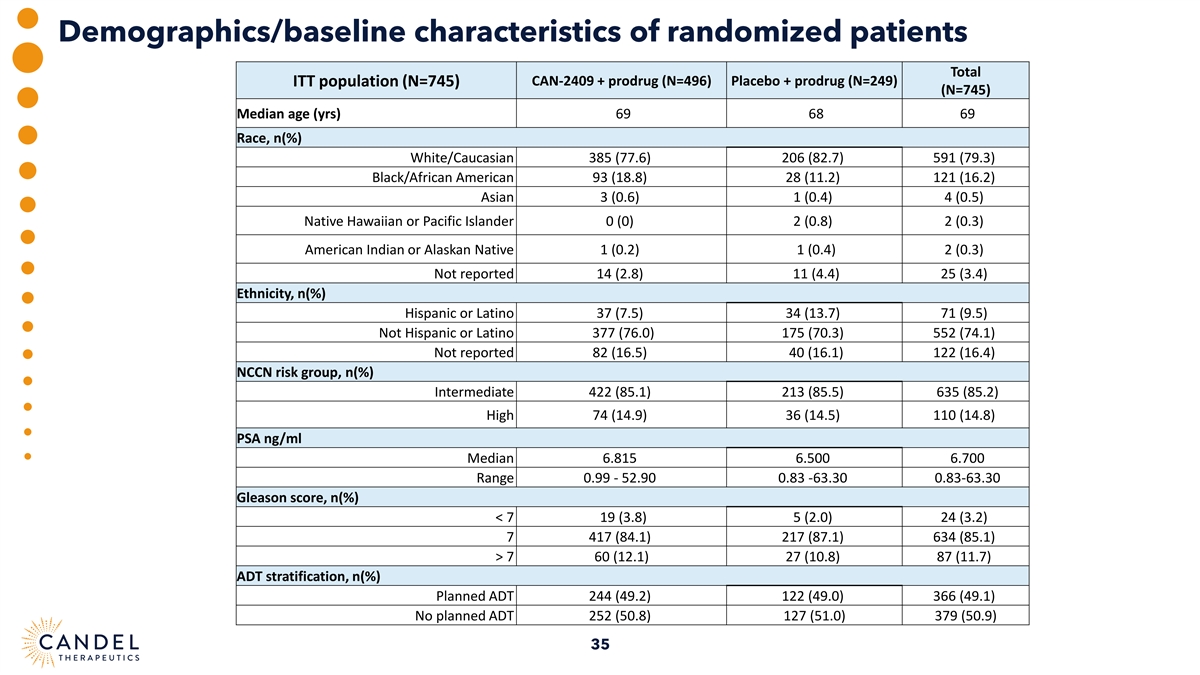
Demographics/baseline characteristics of randomized patients Total ITT population (N=745) CAN-2409 + prodrug (N=496) Placebo + prodrug (N=249) (N=745) Median age (yrs) 69 68 69 Race, n(%) White/Caucasian 385 (77.6) 206 (82.7) 591 (79.3) Black/African American 93 (18.8) 28 (11.2) 121 (16.2) Asian 3 (0.6) 1 (0.4) 4 (0.5) Native Hawaiian or Pacific Islander 0 (0) 2 (0.8) 2 (0.3) American Indian or Alaskan Native 1 (0.2) 1 (0.4) 2 (0.3) Not reported 14 (2.8) 11 (4.4) 25 (3.4) Ethnicity, n(%) Hispanic or Latino 37 (7.5) 34 (13.7) 71 (9.5) Not Hispanic or Latino 377 (76.0) 175 (70.3) 552 (74.1) Not reported 82 (16.5) 40 (16.1) 122 (16.4) NCCN risk group, n(%) Intermediate 422 (85.1) 213 (85.5) 635 (85.2) High 74 (14.9) 36 (14.5) 110 (14.8) PSA ng/ml Median 6.815 6.500 6.700 Range 0.99 - 52.90 0.83 -63.30 0.83-63.30 Gleason score, n(%) < 7 19 (3.8) 5 (2.0) 24 (3.2) 7 417 (84.1) 217 (87.1) 634 (85.1) > 7 60 (12.1) 27 (10.8) 87 (11.7) ADT stratification, n(%) Planned ADT 244 (49.2) 122 (49.0) 366 (49.1) No planned ADT 252 (50.8) 127 (51.0) 379 (50.9) 35
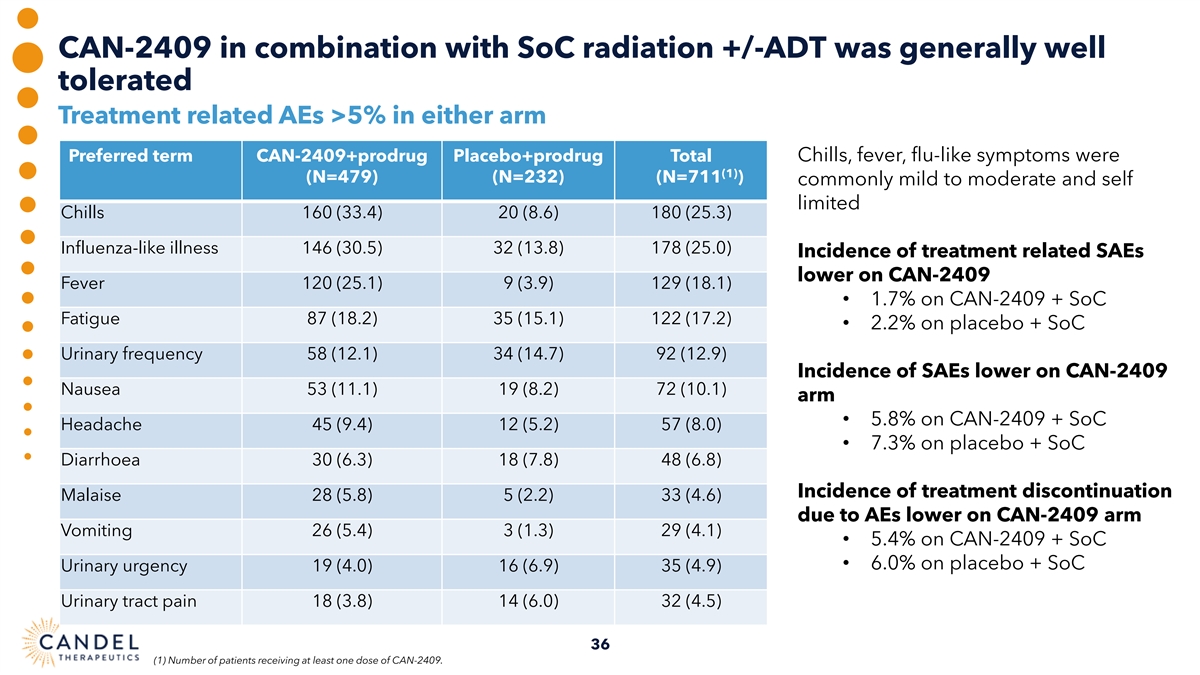
CAN-2409 in combination with SoC radiation +/-ADT was generally well tolerated Treatment related AEs >5% in either arm Preferred term CAN-2409+prodrug Placebo+prodrug Total Chills, fever, flu-like symptoms were (1) (N=479) (N=232) (N=711 ) commonly mild to moderate and self limited Chills 160 (33.4) 20 (8.6) 180 (25.3) Influenza-like illness 146 (30.5) 32 (13.8) 178 (25.0) Incidence of treatment related SAEs lower on CAN-2409 Fever 120 (25.1) 9 (3.9) 129 (18.1) • 1.7% on CAN-2409 + SoC Fatigue 87 (18.2) 35 (15.1) 122 (17.2) • 2.2% on placebo + SoC Urinary frequency 58 (12.1) 34 (14.7) 92 (12.9) Incidence of SAEs lower on CAN-2409 Nausea 53 (11.1) 19 (8.2) 72 (10.1) arm • 5.8% on CAN-2409 + SoC Headache 45 (9.4) 12 (5.2) 57 (8.0) • 7.3% on placebo + SoC Diarrhoea 30 (6.3) 18 (7.8) 48 (6.8) Incidence of treatment discontinuation Malaise 28 (5.8) 5 (2.2) 33 (4.6) due to AEs lower on CAN-2409 arm Vomiting 26 (5.4) 3 (1.3) 29 (4.1) • 5.4% on CAN-2409 + SoC • 6.0% on placebo + SoC Urinary urgency 19 (4.0) 16 (6.9) 35 (4.9) Urinary tract pain 18 (3.8) 14 (6.0) 32 (4.5) 36 (1) Number of patients receiving at least one dose of CAN-2409.
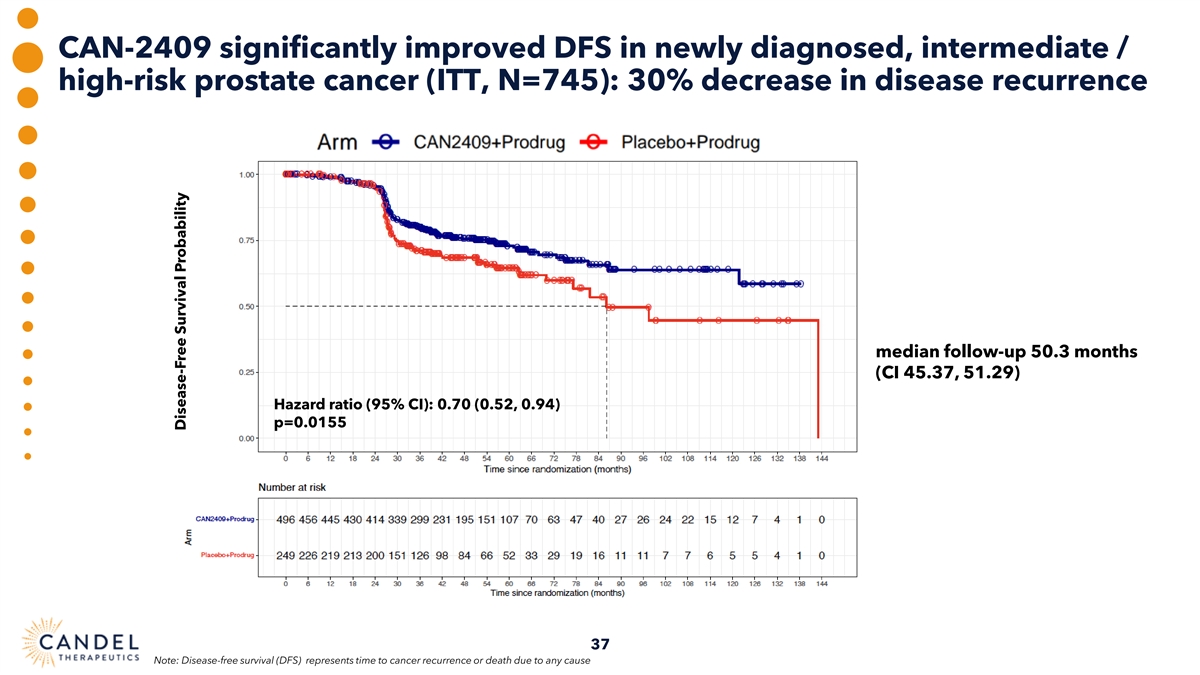
CAN-2409 significantly improved DFS in newly diagnosed, intermediate / high-risk prostate cancer (ITT, N=745): 30% decrease in disease recurrence median follow-up 50.3 months (CI 45.37, 51.29) Hazard ratio (95% CI): 0.70 (0.52, 0.94) p=0.0155 37 Note: Disease-free survival (DFS) represents time to cancer recurrence or death due to any cause Disease-Free Survival Probability
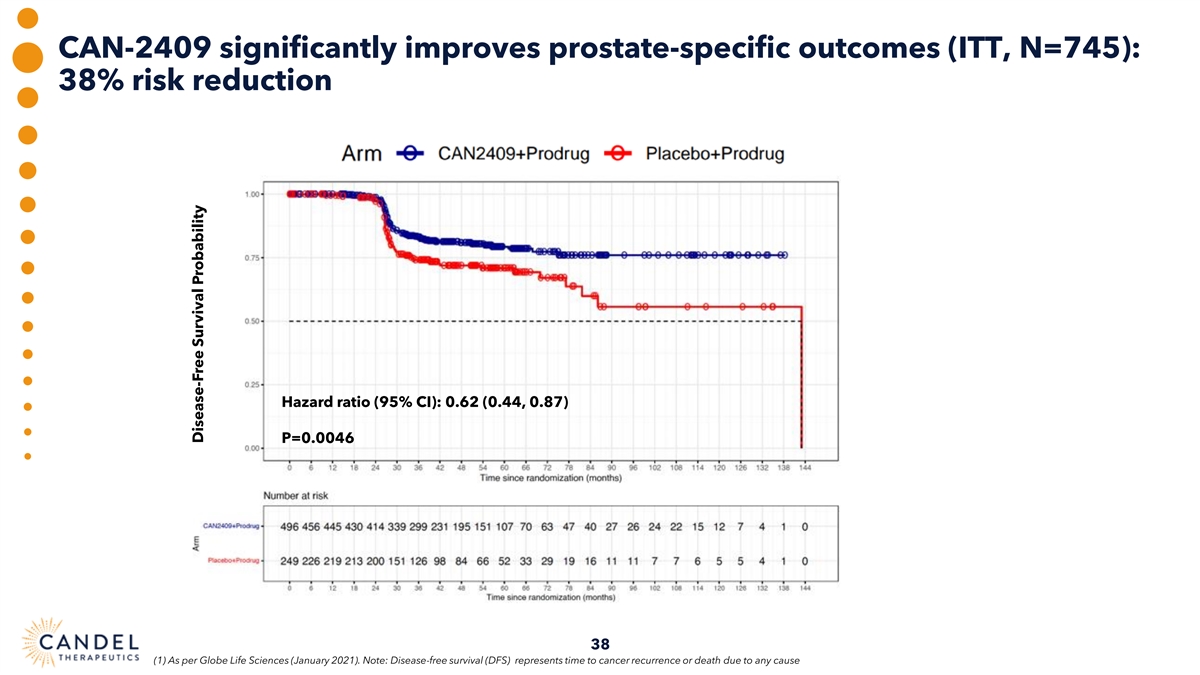
CAN-2409 significantly improves prostate-specific outcomes (ITT, N=745): 38% risk reduction Hazard ratio (95% CI): 0.62 (0.44, 0.87) P=0.0046 38 (1) As per Globe Life Sciences (January 2021). Note: Disease-free survival (DFS) represents time to cancer recurrence or death due to any cause Disease-Free Survival Probability
CAN-2409: other key secondary endpoints • Significant increase in the proportion of patients achieving a prostate-specific antigen (PSA) nadir of <0.2 ng/ml in the treatment arm compared to the placebo • 67.1% vs. 58.6%, respectively (p=0.0164) • Only 2 deaths due to prostate cancer over 10+ years (one CAN-2409, one placebo) 1 • Consistent with expected prostate-specific survival during median follow-up < 6 years • 50 patients died due to other causes, unrelated to treatment 39 39 1 Hamdy FC et al. N Engl J Med 2023;388:1547-1558
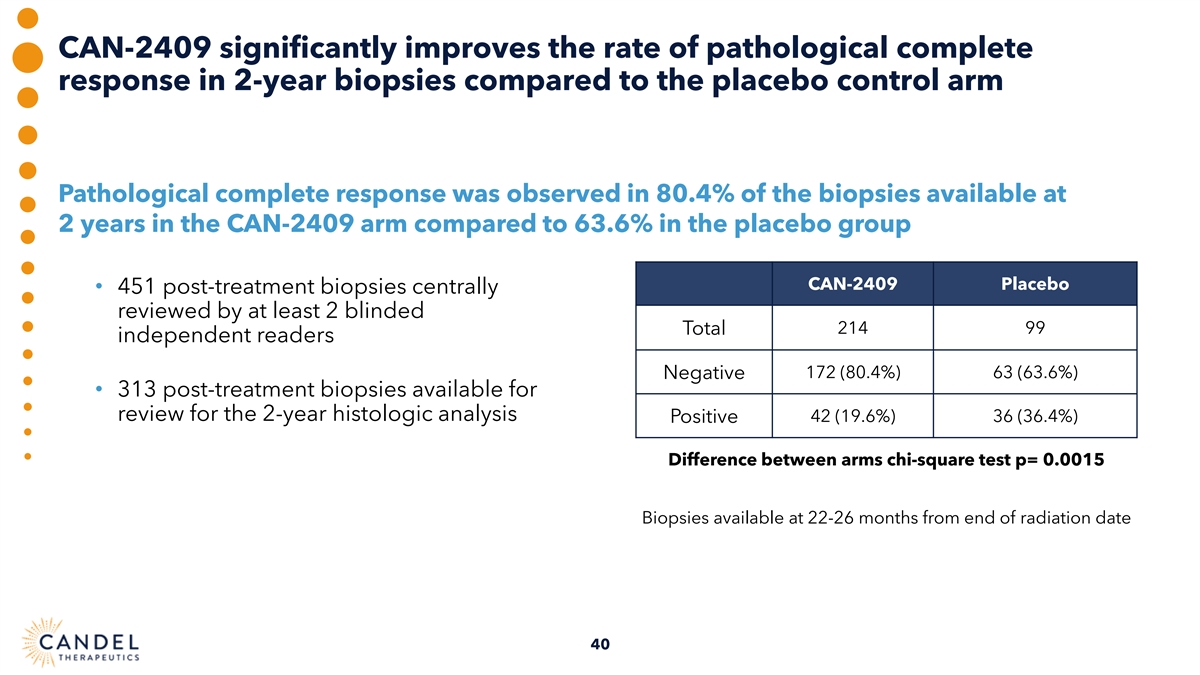
CAN-2409 significantly improves the rate of pathological complete response in 2-year biopsies compared to the placebo control arm Pathological complete response was observed in 80.4% of the biopsies available at 2 years in the CAN-2409 arm compared to 63.6% in the placebo group CAN-2409 Placebo • 451 post-treatment biopsies centrally reviewed by at least 2 blinded 214 99 Total independent readers Negative 172 (80.4%) 63 (63.6%) • 313 post-treatment biopsies available for review for the 2-year histologic analysis Positive 42 (19.6%) 36 (36.4%) Difference between arms chi-square test p= 0.0015 Biopsies available at 22-26 months from end of radiation date 40
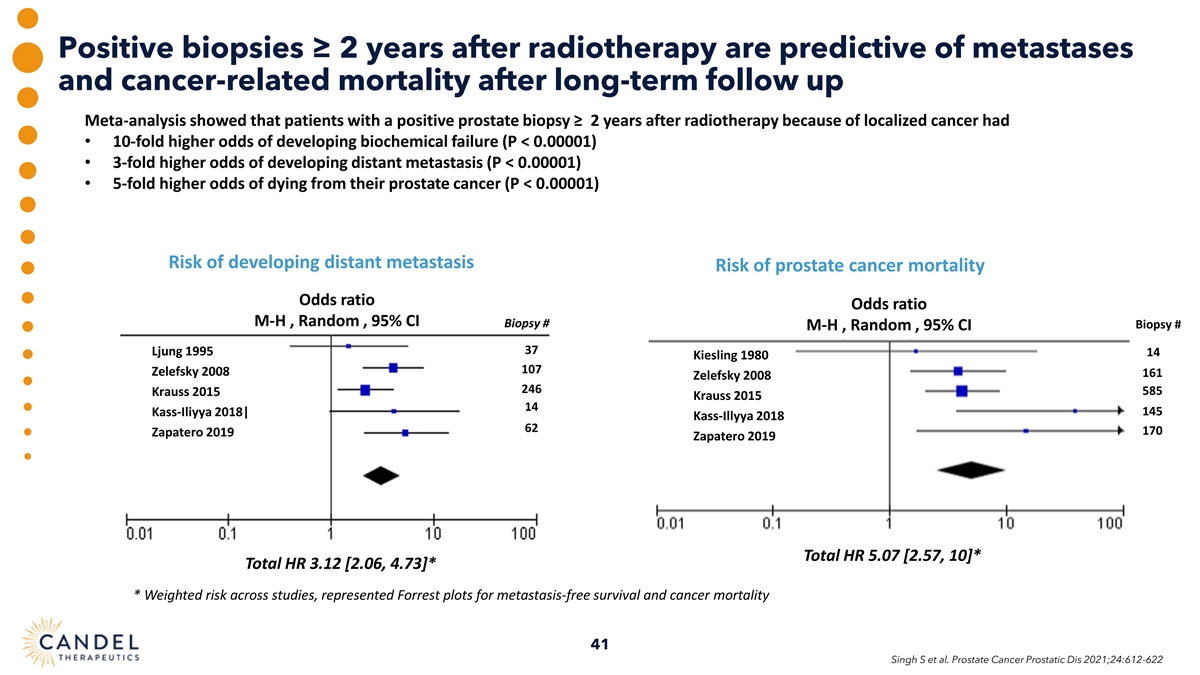
Positive biopsies ≥ 2 years after radiotherapy are predictive of metastases and cancer-related mortality after long-term follow up Meta-analysis showed that patients with a positive prostate biopsy ≥ 2 years after radiotherapy because of localized cancer had • 10-fold higher odds of developing biochemical failure (P < 0.00001) • 3-fold higher odds of developing distant metastasis (P < 0.00001) • 5-fold higher odds of dying from their prostate cancer (P < 0.00001) Risk of developing distant metastasis Risk of prostate cancer mortality Odds ratio Odds ratio M-H , Random , 95% CI Biopsy # Biopsy # M-H , Random , 95% CI 37 Ljung 1995 14 Kiesling 1980 107 Zelefsky 2008 161 Zelefsky 2008 246 585 Krauss 2015 Krauss 2015 14 145 Kass-Iliyya 2018| Kass-Illyya 2018 62 170 Zapatero 2019 Zapatero 2019 Total HR 5.07 [2.57, 10]* Total HR 3.12 [2.06, 4.73]* * Weighted risk across studies, represented Forrest plots for metastasis-free survival and cancer mortality 41 Singh S et al. Prostate Cancer Prostatic Dis 2021;24:612-622
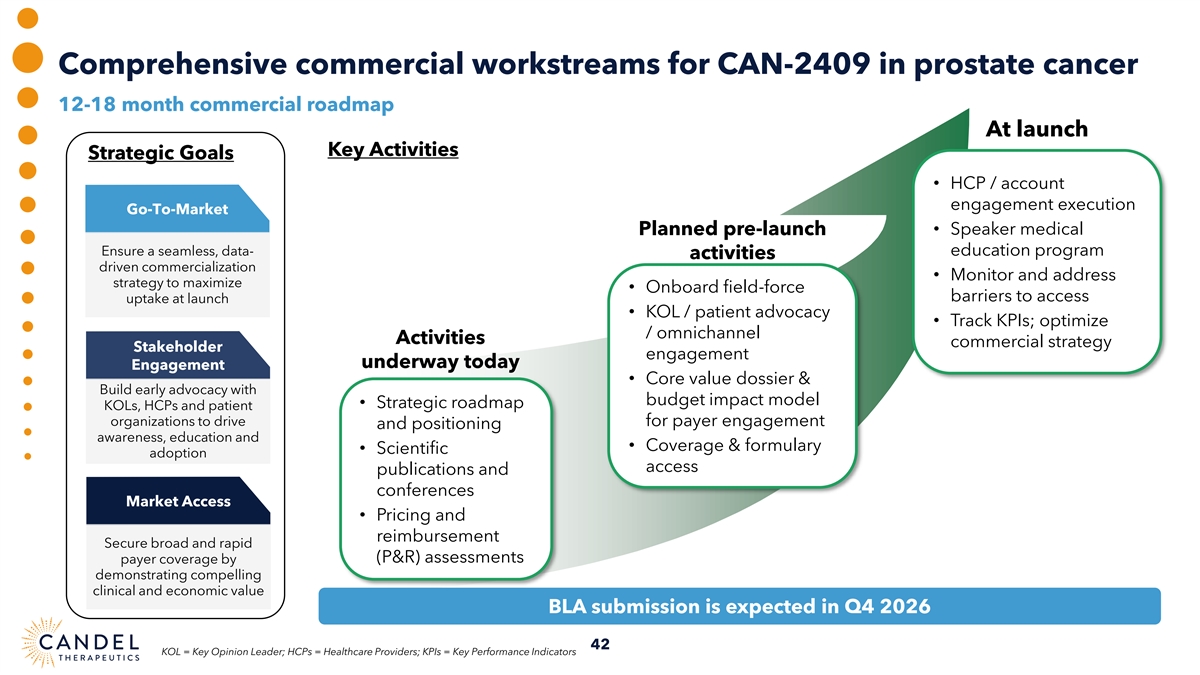
Comprehensive commercial workstreams for CAN-2409 in prostate cancer 12-18 month commercial roadmap At launch Key Activities Strategic Goals • HCP / account engagement execution Go-To-Market Planned pre-launch • Speaker medical Ensure a seamless, data- education program activities driven commercialization • Monitor and address strategy to maximize • Onboard field-force barriers to access uptake at launch • KOL / patient advocacy • Track KPIs; optimize / omnichannel Activities commercial strategy Stakeholder engagement underway today Engagement • Core value dossier & Build early advocacy with budget impact model • Strategic roadmap KOLs, HCPs and patient organizations to drive for payer engagement and positioning awareness, education and • Coverage & formulary • Scientific adoption access publications and conferences Market Access • Pricing and reimbursement Secure broad and rapid (P&R) assessments payer coverage by demonstrating compelling clinical and economic value BLA submission is expected in Q4 2026 42 KOL = Key Opinion Leader; HCPs = Healthcare Providers; KPIs = Key Performance Indicators
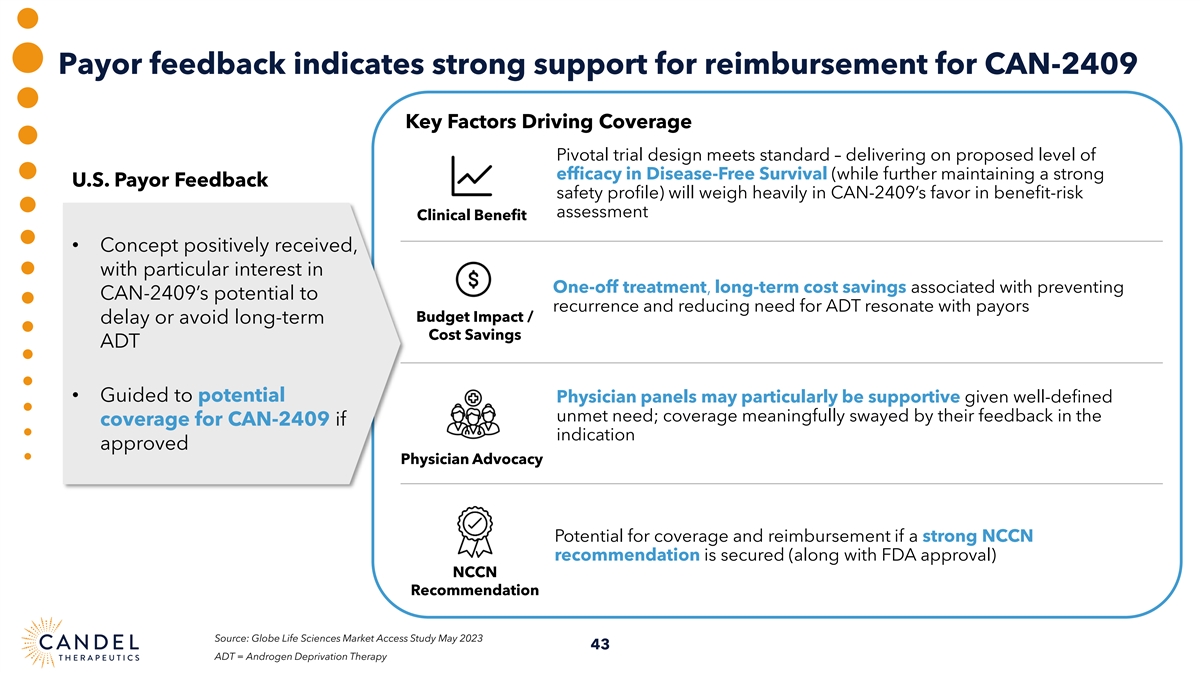
Payor feedback indicates strong support for reimbursement for CAN-2409 Key Factors Driving Coverage Pivotal trial design meets standard – delivering on proposed level of efficacy in Disease-Free Survival (while further maintaining a strong U.S. Payor Feedback safety profile) will weigh heavily in CAN-2409’s favor in benefit-risk assessment Clinical Benefit • Concept positively received, with particular interest in One-off treatment, long-term cost savings associated with preventing CAN-2409’s potential to recurrence and reducing need for ADT resonate with payors Budget Impact / delay or avoid long-term Cost Savings ADT • Guided to potential Physician panels may particularly be supportive given well-defined unmet need; coverage meaningfully swayed by their feedback in the coverage for CAN-2409 if indication approved Physician Advocacy Potential for coverage and reimbursement if a strong NCCN recommendation is secured (along with FDA approval) NCCN Recommendation Source: Globe Life Sciences Market Access Study May 2023 43 ADT = Androgen Deprivation Therapy
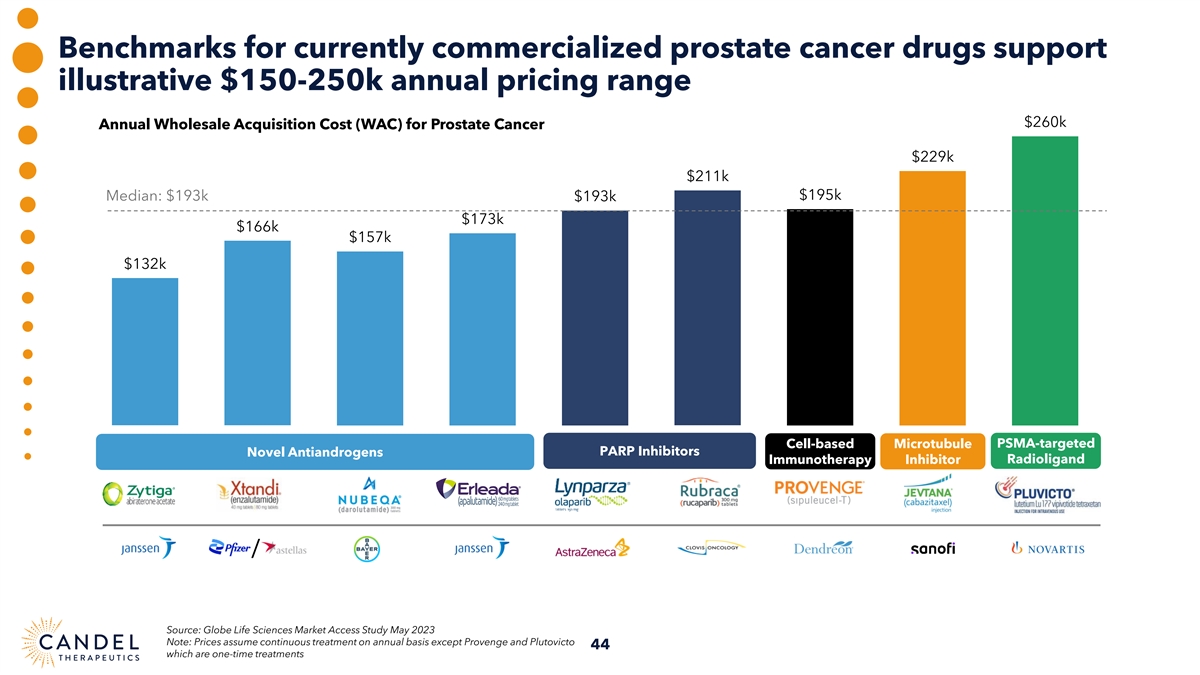
Benchmarks for currently commercialized prostate cancer drugs support illustrative $150-250k annual pricing range $260k Annual Wholesale Acquisition Cost (WAC) for Prostate Cancer $229k $211k $195k Median: $193k $193k $173k $166k $157k $132k PSMA-targeted Cell-based Microtubule Novel Antiandrogens PARP Inhibitors Immunotherapy Inhibitor Radioligand / Source: Globe Life Sciences Market Access Study May 2023 Note: Prices assume continuous treatment on annual basis except Provenge and Plutovicto 44 which are one-time treatments
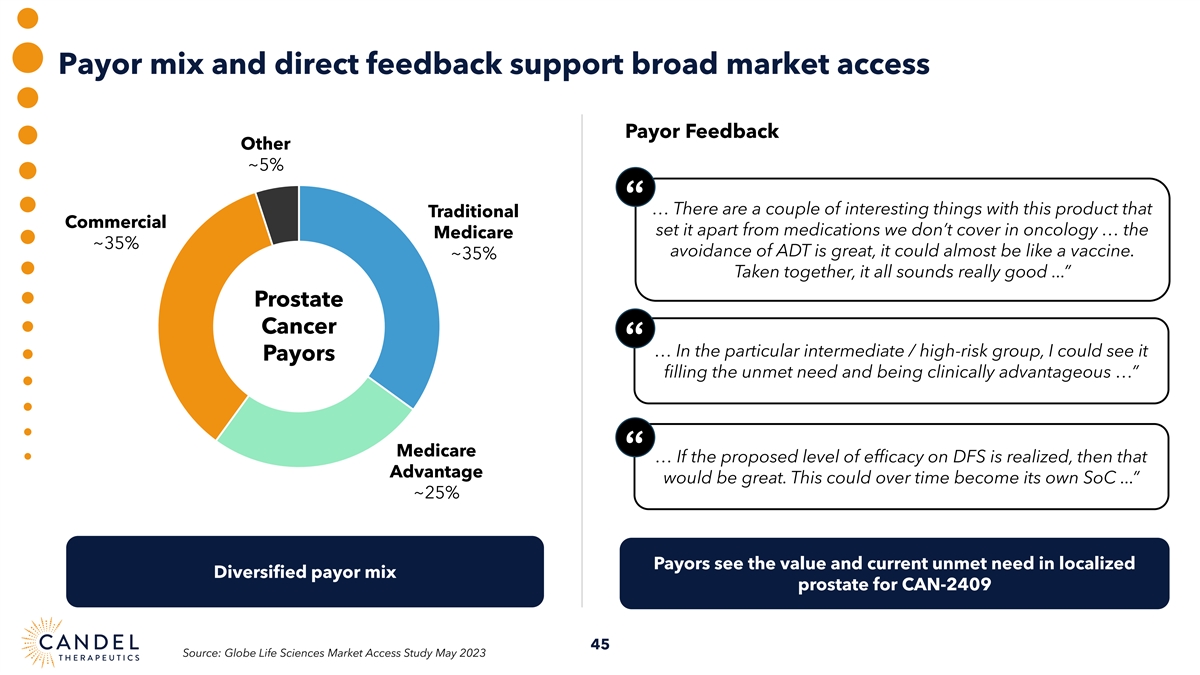
Payor mix and direct feedback support broad market access Payor Feedback Other ~5% “ … There are a couple of interesting things with this product that Traditional Commercial set it apart from medications we don’t cover in oncology … the Medicare ~35% avoidance of ADT is great, it could almost be like a vaccine. ~35% Taken together, it all sounds really good ...” Prostate Cancer “ … In the particular intermediate / high-risk group, I could see it Payors filling the unmet need and being clinically advantageous …” Medicare “ … If the proposed level of efficacy on DFS is realized, then that Advantage would be great. This could over time become its own SoC ...” ~25% Payors see the value and current unmet need in localized Diversified payor mix prostate for CAN-2409 45 Source: Globe Life Sciences Market Access Study May 2023
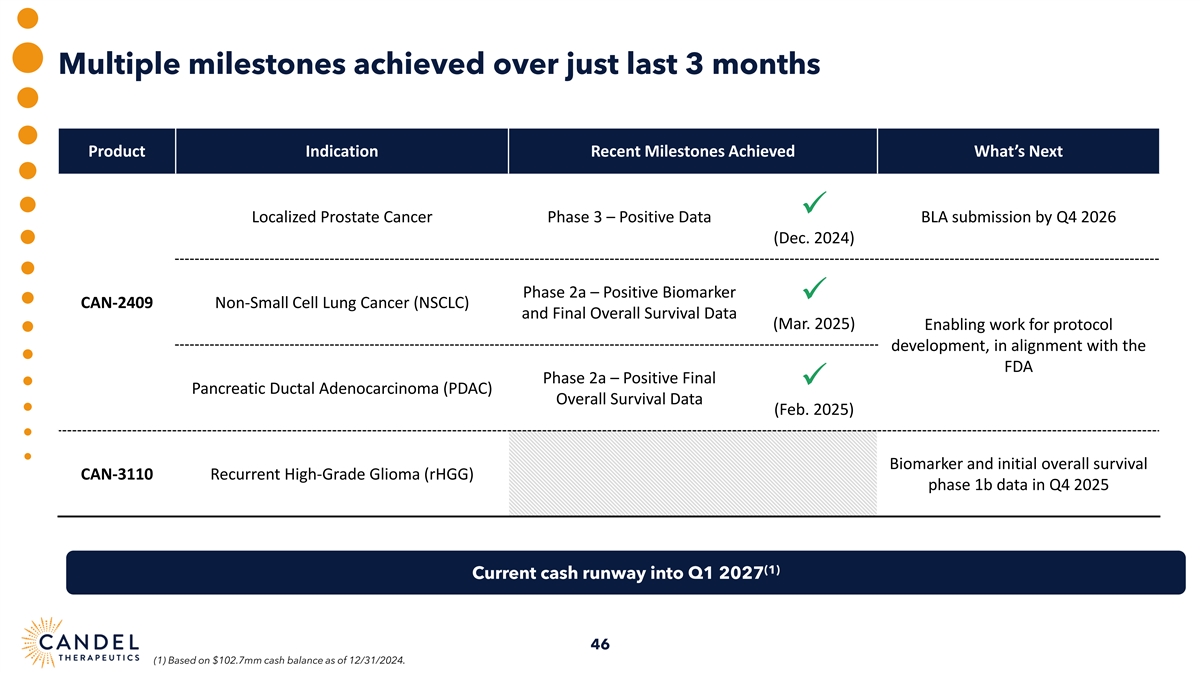
Multiple milestones achieved over just last 3 months Product Indication Recent Milestones Achieved What’s Next ✓ Localized Prostate Cancer Phase 3 – Positive Data BLA submission by Q4 2026 (Dec. 2024) Phase 2a – Positive Biomarker ✓ CAN-2409 Non-Small Cell Lung Cancer (NSCLC) and Final Overall Survival Data (Mar. 2025) Enabling work for protocol development, in alignment with the FDA Phase 2a – Positive Final ✓ Pancreatic Ductal Adenocarcinoma (PDAC) Overall Survival Data (Feb. 2025) Biomarker and initial overall survival CAN-3110 Recurrent High-Grade Glioma (rHGG) phase 1b data in Q4 2025 (1) Current cash runway into Q1 2027 46 (1) Based on $102.7mm cash balance as of 12/31/2024.
Candel at a glance • Positive phase 3 randomized, triple-blinded, placebo-controlled, clinical trial of CAN-2409 in intermediate-to-high-risk, localized prostate cancer • Positive overall survival data from randomized phase 2a clinical trial of CAN-2409 in borderline resectable pancreatic ductal adenocarcinoma (PDAC) • Proof of concept in NSCLC: mOS of 21.5 months in patients with progressive disease at baseline despite ICI (vs. published historical controls of mOS in PD-1 refractory population with SoC chemo of 9.8 – 11.8 mos), and evidence of systemic immune CAN-2409: Off-the-shelf response pan-solid tumor therapy, • Fast Track Designation in NSCLC, pancreatic cancer, and prostate cancer individualized anti-cancer • Orphan Drug Designation in pancreatic cancer immune response • “Pipeline in a product” strategy advancing multiple programs in several large indications • BLA filing for CAN-2409 in prostate cancer expected in Q4 2026 • Proof of concept in patients with recurrent high-grade glioma published in Nature (“Clinical trial links oncolytic immunoactivation to survival in glioblastoma”) • Fast Track Designation, Orphan Drug Designation CAN-3110: Oncolytic HSV-1 • Opportunity for creation of “pipeline in a product” by expansion into indications beyond brain cancers • Upcoming catalyst: designed for tumor-specific − Initial survival and immunological biomarker data expected in Q4 of 2025, evaluating repeat dosing regimen of CAN-3110 replication • Experienced Executive Team and strong scientific support from high-profile Research Advisory Board • Cash and cash equivalents of $102.7 million as of Dec 31, 2024; current expected runway into Q1 2027 • IP protection: CAN-2409 (2034, method of use); CAN-3110 (2036, composition of matter); potential 12 years regulatory exclusivity Corporate Highlights • Low-cost manufacturing 47