UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 24, 2025
Surrozen, Inc.
(Exact name of Registrant as Specified in Its Charter)
| Delaware | 001-39635 | 30-1374889 | ||
|
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
||
| 171 Oyster Point Blvd | ||||
| Suite 400 | ||||
| South San Francisco, California | 94080 | |||
| (Address of Principal Executive Offices) | (Zip Code) | |||
Registrant’s Telephone Number, Including Area Code: +1 (650) 489-9000
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
||
| Common Stock, $0.0001 par value per share | SRZN | The Nasdaq Capital Market | ||
| Redeemable warrants, each whole warrant exercisable for one-fifteenth of a share of Common Stock | SRZNW | The Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure |
On March 24, 2025, Surrozen, Inc. issued a press release and also released a corporate presentation related to the foregoing press release. A copy of the press release and the corporate presentation are furnished herewith as Exhibits 99.1 and 99.2, respectively, and are incorporated herein by reference.
The information disclosed under this Item 7.01 and in the related exhibits hereto is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and shall not be deemed incorporated by reference into any filing made under the Securities Act of 1933, as amended, except as expressly set forth by specific reference in such filing. The furnishing of information pursuant to this Item 7.01 will not be deemed an admission that any information in this report is material or required to be disclosed by Regulation FD.
| Item 9.01 | Financial Statements and Exhibits. |
| (d) | Exhibits |
Exhibit |
Description |
|
| 99.1 | Press Release, dated March 24, 2025. | |
| 99.2 | Corporate Presentation, dated March 2025. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document). | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| SURROZEN, INC. | ||||
| Date: March 24, 2025 | By: | /s/ Charles Williams |
||
| Name: | Charles Williams | |||
| Title: | Chief Financial Officer and Chief Operating Officer | |||
Exhibit 99.1
Surrozen Announces an Oversubscribed $175 Million Private Placement of Securities to Focus on Selective Wnt Mimetic Therapeutics to Treat Serious Eye Diseases
Company prioritizes ophthalmology pipeline programs with potential to provide new or improved treatment options in multiple severe and disabling eye diseases
Announces an oversubscribed financing of $175 million in gross proceeds, which funds multiple ophthalmology programs through phase 1 safety, tolerability and efficacy studies
Company discontinues clinical development of SZN-043 in severe alcohol associated hepatitis
SOUTH SAN FRANCISCO, Calif., March 24, 2025 (GLOBE NEWSWIRE) — Surrozen, Inc. (“Surrozen” or the “Company”) (Nasdaq: SRZN), a company pioneering targeted therapeutics that selectively activate the Wnt Pathway for tissue repair and regeneration, today announced that the company will focus its Wnt biology expertise and Wnt signal modulation antibody technologies on its ophthalmology programs including development of new treatment options for retinopathies. The Company also announced a private placement consisting of two tranches in aggregate up to $175 million in gross proceeds to fund multiple ophthalmology programs through initial Phase 1 safety, tolerability and efficacy studies.
Modulation of Wnt signaling has the potential to be relevant in a broad range of highly prevalent eye diseases including “wet” and “dry” Age Related Macular Degeneration (AMD), diabetic retinopathy, Fuchs’ endothelial corneal dystrophy (FECD), and non-infectious uveitis as well as certain rare eye diseases like retinitis pigmentosa, Stargardt’s and Familial Exudative Vitreoretinopathy (FEVR). The two lead candidates for the treatment of retinopathies include SZN-8141 (Fzd4/VEGF) and SZN-8143 (Fzd4/VEGF/IL-6).
The Company will discontinue development of SZN-043 in severe alcohol associated hepatitis. While treatment with SZN-043 was safe and well-tolerated and demonstrated positive changes in liver function assays, there was not a sufficient early signal of clinical benefit to warrant further investment given the challenges associated with an acutely sick target population and a lengthy clinical development path.
“Given the significant progress and potential of our ophthalmology programs, we have decided to focus on our robust ophthalmology pipeline. Importantly, our retinal ophthalmology programs represent novel combinations of clinically validated targets for treating a broad spectrum of serious eye diseases. We look forward to advancing our proprietary ophthalmology candidates to the clinic including our collaboration with Boehringer Ingelheim to advance SZN-413 into development,” said Craig Parker, President and Chief Executive Officer of Surrozen. “Given the decision to discontinue SZN-043, we want to thank the subjects, our collaborators and the medical professionals who participated in clinical trials and helped advance our understanding of Wnt mimetics in acute liver disease.”
Wholly-Owned Ophthalmology Portfolio
SZN-8141 combines Frizzled 4 (Fzd4) agonism and Vascular Endothelial Growth Factor (VEGF) antagonism which has the potential to provide benefits over treatment with single agents for Diabetic Macular Edema (DME) and neovascular Age Related Macular Degeneration (wet AMD).
SZN-8143 combines Fzd4 agonism, VEGF antagonism, and interleukin-6 (IL-6) antagonism which may have benefits over single agents for treatment of DME/wet AMD/uveitic macular edema (UME).
The current standard of care for diabetic retinopathy (including DME), retinal vein occlusion and wet AMD is intravitreal administration of anti-VEGF monotherapies. In addition, Fzd4 monotherapy has demonstrated proof of concept in clinical trials. Surrozen believes SZN-8141 and SZN-8143 have the potential to treat multiple retinopathy indications and be differentiated from existing therapies. Data generated in preclinical models of retinopathy demonstrated that both SZN-8141 and SZN-8143 stimulated Wnt signaling and induced normal retinal vessel regrowth while suppressing pathological vessel growth.
Financing Overview
The Company has entered into a securities purchase agreement with certain institutional and accredited investors to purchase shares of common stock, or pre-funded warrants in lieu thereof, and accompanying warrants to purchase shares of common stock. The private placement was priced “at-the-market” under the rules and regulations of The Nasdaq Stock Market LLC.
At the initial closing, expected on or about March 26, 2025 the Company will issue to the investors 6,034,494 units, each consisting of one share of common stock, or pre-funded warrant in lieu thereof, and one half of a common stock warrant, at a purchase price of $11.60 for gross proceeds of approximately $70 million, before deducting placement agent fees and other expenses payable by the Company. Each pre-funded warrant has an exercise price of $0.0001 per share, is exercisable immediately and will not expire until exercised in full.
At the second closing, investors have committed to purchase an additional 9,051,742 units, each consisting of one share of common stock, or pre-funded warrant in lieu thereof, and one half of a common stock warrant, at a purchase price of $11.60 for gross proceeds of approximately $105 million, before deducting placement agent fees and other expenses payable by the Company. Each pre-funded warrant has an exercise price of $0.0001 per share, is exercisable immediately and will not expire until exercised in full. The second closing is subject to the receipt of clearance from the FDA of the Company’s Investigational New Drug Application for SZN-8141, expected in 2026. In connection with the private placement, the exercise prices of the Company’s outstanding Series A common stock warrants and Series B common stock warrants are being reduced and the Company’s outstanding Series C common stock warrants and Series D common stock warrants are being cancelled.
The financing was led by Venrock Healthcare Capital Partners and includes participation from new and existing investors, including The Column Group, Access Biotechnology, RA Capital Management, Vivo Capital, Spruce Street Capital, 5AM Ventures, Kalehua Capital, Samsara BioCapital, Acuta Capital Partners, StemPoint Capital, Braidwell LP and other leading life sciences investors. In connection with the financing, Tim Kutzkey, Ph.D. will be joining the Surrozen Board of Directors.
Guggenheim Securities, LLC is acting as sole placement agent for the private placement.
The securities being sold in the private placement have not been registered under the Securities Act of 1933, as amended (the “Securities Act”), or the securities laws of any state, and may not be offered or sold in the United States, except pursuant to an effective registration statement or an applicable exemption from the registration requirements of the Securities Act. Surrozen has agreed to file a registration statement with the Securities and Exchange Commission registering the resale of the shares of common stock and shares of common stock issuable upon the exercise of the warrants issued in the private placement within 30 days of the closing.
This press release does not constitute an offer to sell or the solicitation of an offer to buy, nor will there be any sales of these securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to the registration or qualification under the securities laws of such jurisdiction.
About SZN-413 for Retinal Diseases
SZN-413 is a bi-specific antibody targeting Fzd4-mediated Wnt signaling designed using Surrozen’s SWAP™ technology. It is currently being developed for the treatment of retinal vascular-associated diseases. Data generated by Surrozen with SZN-413 in preclinical models of retinopathy demonstrated that SZN-413 could potently stimulate Wnt signaling in the eye, induce normal retinal vessel regrowth, suppress pathological vessel growth and reduce vascular leakage. This novel approach could thus potentially allow for regeneration of healthy eye tissue, not only halting retinopathy, but possibly allowing for a full reversal of the patient’s disease.
In the fourth quarter of 2022, Surrozen entered into a strategic partnership with Boehringer Ingelheim for the research and development of SZN-413 for the treatment of retinal diseases. Under the terms of the agreement, Boehringer Ingelheim received an exclusive, worldwide license to develop SZN-413 and other Fzd4-specific Wnt-modulating molecules for all purposes, including as a treatment for retinal diseases, in exchange for an upfront payment to Surrozen of $12.5 million. Surrozen will also be eligible to receive up to $587.0 million in success-based development, regulatory, and commercial milestone payments, in addition to mid-single digit to low-double digit royalties on sales. After an initial period of joint research, Boehringer Ingelheim will assume all development and commercial responsibilities.
About Surrozen
Surrozen is a biotechnology company discovering and developing drug candidates to selectively modulate the Wnt pathway. Surrozen is developing tissue-specific antibodies designed to engage the body’s existing biological repair mechanisms with a current focus in ophthalmology.
Forward Looking Statements
This press release contains certain forward-looking statements within the meaning of the federal securities laws. Forward-looking statements generally are accompanied by words such as “will,” “plan,” “intend,” “potential,” “expect,” “could,” or the negative of these words and similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements regarding Surrozen’s discovery, research and development activities, in particular its development plans for its product candidates (including anticipated clinical development plans and timelines, the availability of data, the potential for such product candidates to be used to treat human disease, as well as the potential benefits of such product candidates); the Company’s partnership with Boehringer Ingelheim, including the
potential for future success-based development, regulatory, and commercial milestone payments, in addition to mid-single digit to low-double digit royalties on sales; the potential of TGF-ß to be a novel, first-in-class therapeutic to treat the pathology of Idiopathic Pulmonary Fibrosis; the anticipated initial closing of the Company’s private placement; and the anticipated second closing of the Company’s private placement, which is subject to the receipt of clearance from the FDA of the Company’s Investigational New Drug Application for SZN-8141, expected in 2026. These statements are based on various assumptions, whether or not identified in this press release, and on the current expectations of the management of Surrozen and are not predictions of actual performance. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on as a guarantee, an assurance, a prediction, or a definitive statement of fact or probability. Actual events and circumstances are difficult or impossible to predict and will differ from assumptions. Many actual events and circumstances are beyond the control of Surrozen. These forward-looking statements are subject to a number of risks and uncertainties, including the initiation, cost, timing, progress and results of research and development activities, preclinical and clinical trials with respect to its product candidates and potential future drug candidates; the Company’s ability to fund its preclinical and clinical trials and development efforts, whether with existing funds or through additional fundraising; Surrozen’s ability to identify, develop and commercialize drug candidates; Surrozen’s ability to successfully complete preclinical and clinical studies for Sits product candidates; the effects that arise from volatility in global economic, political, regulatory and market conditions; and all other factors discussed in Surrozen’s Annual Report on Form 10-K for the year ended December 31, 2023 and Surrozen’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2024 filed with the Securities and Exchange Commission (“SEC”) under the heading “Risk Factors,” and other documents Surrozen has filed, or will file, with the SEC. If any of these risks materialize or our assumptions prove incorrect, actual results could differ materially from the results implied by these forward-looking statements. There may be additional risks that Surrozen presently does not know, or that Surrozen currently believes are immaterial, that could also cause actual results to differ from those contained in the forward-looking statements. In addition, forward-looking statements reflect Surrozen’s expectations, plans, or forecasts of future events and views as of the date of this press release. Surrozen anticipates that subsequent events and developments will cause its assessments to change. However, while Surrozen may elect to update these forward-looking statements at some point in the future, Surrozen specifically disclaims any obligation to do so, except as required by law. These forward-looking statements should not be relied upon as representing Surrozen’s assessments of any date after the date of this press release. Accordingly, undue reliance should not be placed upon the forward-looking statements.
Investor/Media Contact:
Email: Investorinfo@surrozen.com

Exhibit 99.2 Targeted Regeneration March 2025

Legal Disclaimers This presentation contains certain forward-looking statements within the meaning of the federal securities laws. Forward-looking statements generally are accompanied by words such as “will,” “plan,” “intend,” “potential,” “expect,” “could,” or the negative of these words and similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements regarding Surrozen’s discovery, research and development activities, in particular its development plans for its product candidates (including anticipated clinical development plans and timelines, the availability of data, the potential for such product candidates to be used to treat human disease, as well as the potential benefits of such product candidates) and the Company’s partnership with Boehringer Ingelheim, including the potential for future success-based development, regulatory, and commercial milestone payments, in addition to mid-single digit to low-double digit royalties on sales. These statements are based on various assumptions, whether or not identified in this presentation, and on the current expectations of the management of Surrozen and are not predictions of actual performance. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on as a guarantee, an assurance, a prediction, or a definitive statement of fact or probability. Actual events and circumstances are difficult or impossible to predict and will differ from assumptions. Many actual events and circumstances are beyond the control of Surrozen. These forward-looking statements are subject to a number of risks and uncertainties, including the initiation, cost, timing, progress and results of research and development activities, preclinical and clinical trials with respect to its product candidates, and potential future drug candidates; Surrozen’s ability to fund its preclinical and clinical trials and development efforts, whether with existing funds or through additional fundraising; Surrozen’s ability to identify, develop and commercialize drug candidates; Surrozen’s ability to successfully complete preclinical and clinical studies for its product candidates; the effects that arise from volatility in global economic, political, regulatory and market conditions; and all other factors discussed in Surrozen’s Annual Report on Form 10-K for the year ended December 31, 2023 and Surrozen’s Quarterly Report on Form 10- Q for the quarter ended September 30, 2024 to be filed with the Securities and Exchange Commission ( SEC ) under the heading “Risk Factors,” and other documents Surrozen has filed, or will file, with the Securities and Exchange Commission. If any of these risks materialize or our assumptions prove incorrect, actual results could differ materially from the results implied by these forward-looking statements. There may be additional risks that Surrozen presently does not know, or that Surrozen currently believes are immaterial, that could also cause actual results to differ from those contained in the forward-looking statements. In addition, forward-looking statements reflect Surrozen’s expectations, plans, or forecasts of future events and views as of the date of this presentation. Surrozen anticipates that subsequent events and developments will cause its assessments to change. However, while Surrozen may elect to update these forward-looking statements at some point in the future, Surrozen specifically disclaims any obligation to do so, except as required by law. These forward-looking statements should not be relied upon as representing Surrozen’s assessments of any date after the date of this presentation. Accordingly, undue reliance should not be placed upon the forward-looking statements. 2 © 2025 Surrozen, Inc.

Legal Disclaimers This presentation is not an offer to sell, a solicitation of an offer to buy or a recommendation to purchase any security, nor is it a solicitation of a proxy, consent or authorization with respect to any securities transaction, nor shall there be any sale, issuance or transfer of securities in any jurisdiction in contravention of applicable law. 3 © 2025 Surrozen, Inc.

Leader in Antibody → Innovator in modulating the Wnt pathway for tissue regeneration • Novel and clinically validated treatment strategy for large markets with Engineering to high unmet need Modulate Wnt • Significant strategic interest in Wnt signaling approach: Merck, Roche, BI Signaling in → Therapeutic relevance of Wnt signal activation supported by clinical POC in DME Ophthalmology → Pipeline of multiple ophthalmology development candidates targeting highly prevalent retinal and corneal diseases → Proprietary antibody platforms with robust patent estate including FZD4/LRP antibodies • Patent applications cited as prior art to Eyebio/Merck's Restoret → Advancing potential best-in-class FZD4/LRP targeted SWAP to the clinic with partner Boehringer Ingelheim for retinal diseases 4 © 2025 Surrozen, Inc.
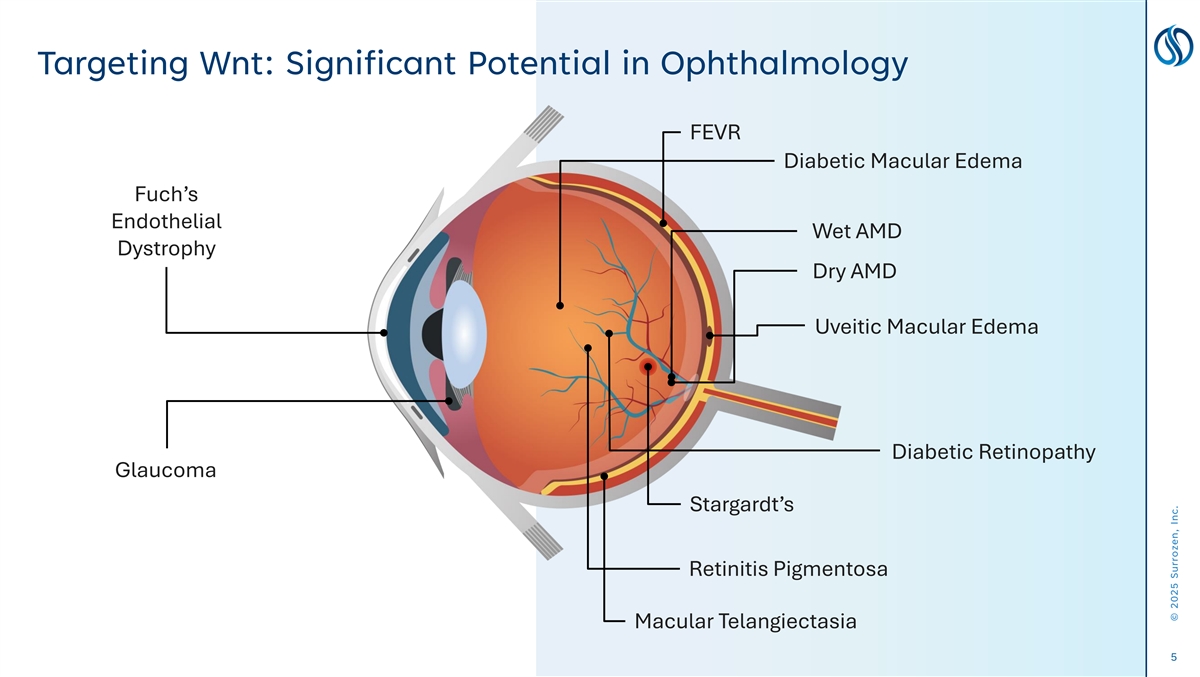
Targeting Wnt: Significant Potential in Ophthalmology FEVR Diabetic Macular Edema Fuch’s Endothelial Wet AMD Dystrophy Dry AMD Uveitic Macular Edema Diabetic Retinopathy Glaucoma Stargardt’s Retinitis Pigmentosa Macular Telangiectasia 5 © 2025 Surrozen, Inc.

Portfolio Targeting High Value Ocular Indications PROGRAM INDICATION RESEARCH PRE-CLINICAL PHASE 1 PHASE 2 SZN-413 Retinopathies FZD4 SZN-8141 wet AMD, DME FZD4, VEGF SZN-8143 wet AMD, DME, UME FZD4, VEGF, IL-6 Fuchs’ Endothelial Corneal Dystrophy (FECD) SZN-113 FZD127 Geographic Atrophy (GA) 6 © 2025 Surrozen, Inc.
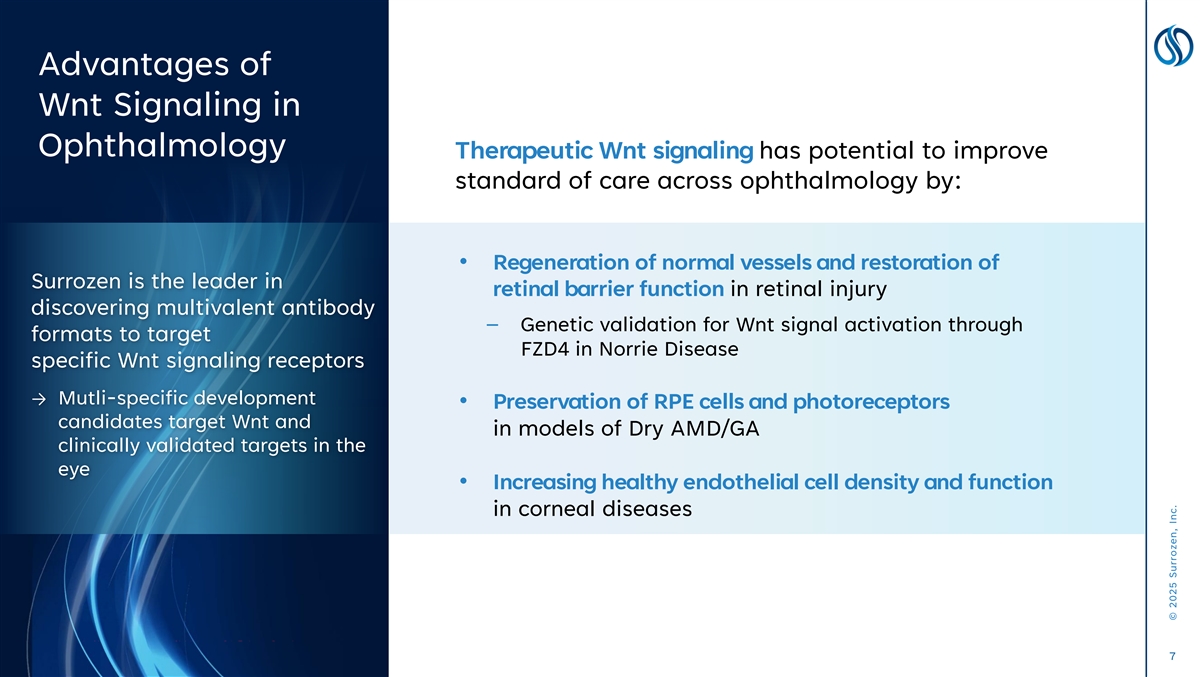
Advantages of Wnt Signaling in Ophthalmology Therapeutic Wnt signaling has potential to improve standard of care across ophthalmology by: • Regeneration of normal vessels and restoration of Surrozen is the leader in retinal barrier function in retinal injury discovering multivalent antibody – Genetic validation for Wnt signal activation through formats to target FZD4 in Norrie Disease specific Wnt signaling receptors → Mutli-specific development • Preservation of RPE cells and photoreceptors candidates target Wnt and in models of Dry AMD/GA clinically validated targets in the eye • Increasing healthy endothelial cell density and function in corneal diseases 7 © 2025 Surrozen, Inc.

Surrozen has considerable discovery capabilities in Ophthalmology ophthalmology Franchise Executive • Antibody discovery and pre-clinical models established for retinal and corneal diseases Summary Surrozen ophthalmology discovery franchise has already generated one development candidate • SZN-413 licensed to Boehringer Ingelheim in October 2022 Wnt signaling is implicated in • Potential best-in-class FZD4/LRP bi-specific antibody for multiple diseases and tissues in retinal diseases like neovascular AMD and DME the eye • Narrowly defined license enables Surrozen to pursue additional FZD4 targeted antibodies on its own Surrozen has multiple novel ophthalmology development candidates • SZN-8141 and 8143 for wet AMD, DME and UME | FZD4/VEGF combination (one molecule) and FZD4/VEGF/IL-6 combination (one molecule) • SZN-113 for Geographic Atrophy (FZD127) • SZN-113 for Fuchs' Endothelial Cell Dystrophy (FZD127) IND targeted for 2026 8 © 2025 Surrozen, Inc.

Strategic Interest in Targeting Merck acquisition of EyeBio in 2024 for $1.2BN: FZD4/LRP5 targeting for DME and nvAMD Wnt Pathway for Therapeutics Roche acquisition of AntlerA in 2024: Wnt mimetic library 9 © 2025 Surrozen, Inc.

FZD4/Multi-Target Strategy Multiple Potential Candidates FZD4 FZD4-αVEGF FZD4-αIL6 FZD4-αVEGF-αIL6 Combination of FZD4 agonism Generate new FZD4 agonists: IL6 is one of the major Combining all three in one and VEGF/VEGFR antagonism • New formats contributors to retinal vascular molecule could become the may have benefits over single • New binders to FZD4 and/or LRP inflammation. Combination of best-in-class treatment for agent alone for DME/ wet the two may have benefits in multiple type of retinopathies AMD treatment DME/wet AMD, UME 10 © 2025 Surrozen, Inc.

SZN-413 Program First Fzd4/Lrp5 Targeted Wnt Mimetic

Novel mechanism for treatment of retinopathies that can SZN-413 Program directly reduce leakage and potentially reduce VEGF Summary production Antibody Targeted FZD4/Norrin signaling in the eye is known to mediate proper to FZD4 function of retinal vascular endothelial cells Multiple preclinical models of retinal injury demonstrated that SZN-413 rapidly reduces vascular leakage and avascular areas SZN-413 was licensed to Boehringer-Ingelheim (BI) under an October 2022 collaboration and licensing agreement • Surrozen received $12.5M upfront; potential milestones of up to $586.5M; mid-single to low double-digit royalties • $10M milestone payment received in 2024 for Start of Development (SOD) • $22.5M received to date • Potential for additional near-term milestones through IND 12 © 2025 Surrozen, Inc.

SZN-413-p Retinal Vascular Program SZN-413: SZN-413 addresses retinal non-perfusion and Potential for Full vascular leakage simultaneously Fzd4 Lrp5 Fzd4 Reversal of Patient’s FZD4/Norrin signaling plays critical role in maintenance of retinal vasculature integrity Retinopathy Vessel growth Vascular barrier function Vehicle Aflibercept SZN-413 SZN-413 (FZD4 SWAP Wnt Mimetic): • Stimulated Wnt signaling increased tight junction protein expression in endothelial cells • Restored norrin function in Ndp KO mice • Reduced avascular area & pathologic NV tuft formation in OIR model • Reduced vascular leakage in VEGF-induced retinal model 13 Nguyen et al., 2022 © 2025 Surrozen, Inc.

Surrozen FZD4/LRP/VEGF/IL-6 Programs Targeting Multiple Pathways for Retinal Vascular Diseases

SZN-8141 & SZN-8143 Address Unmet Needs in Wet AMD FZD4 antibodies targeting in combination Based on recent ASRS Practice Trends survey of ophthalmologists, the with anti-VEGF and/or IL6 have the largest unmet needs for Wet AMD & DME are: potential to: Greater US 78.6% Improve anatomical outcomes: Durability INTL 74.3% – FZD4 activation complements anti-VEGF to provide stronger suppression of vascular leakage Improved US 50.4% Vision– FZD4 activation regenerates RPE and reduces RPE INTL 55.2% atrophy Longer VEGF US 47.1% – FZD4 fixes damaged vessels and restores oxygen Suppression INTL 58.2% and nutritional supply to the tissue Stable US 29.5% Extend VEGF suppression: Anatomy INTL 45.5% – Two complementary mechanisms restore vascular None of the US 1.1% function which improves oxygenation of tissue and Above suppresses VEGF production INTL 0.0% US 2.5% Enhance durability and improve vision: Other INTL 0.4% – All of these benefits can lead to greater durability and vision improvement Hahn P, ed. ASRS 2023 Preferences and Trends Membership Survey, Chicago IL. American Society of Retina Specialists, 2023. 15 © 2025 Surrozen, Inc.
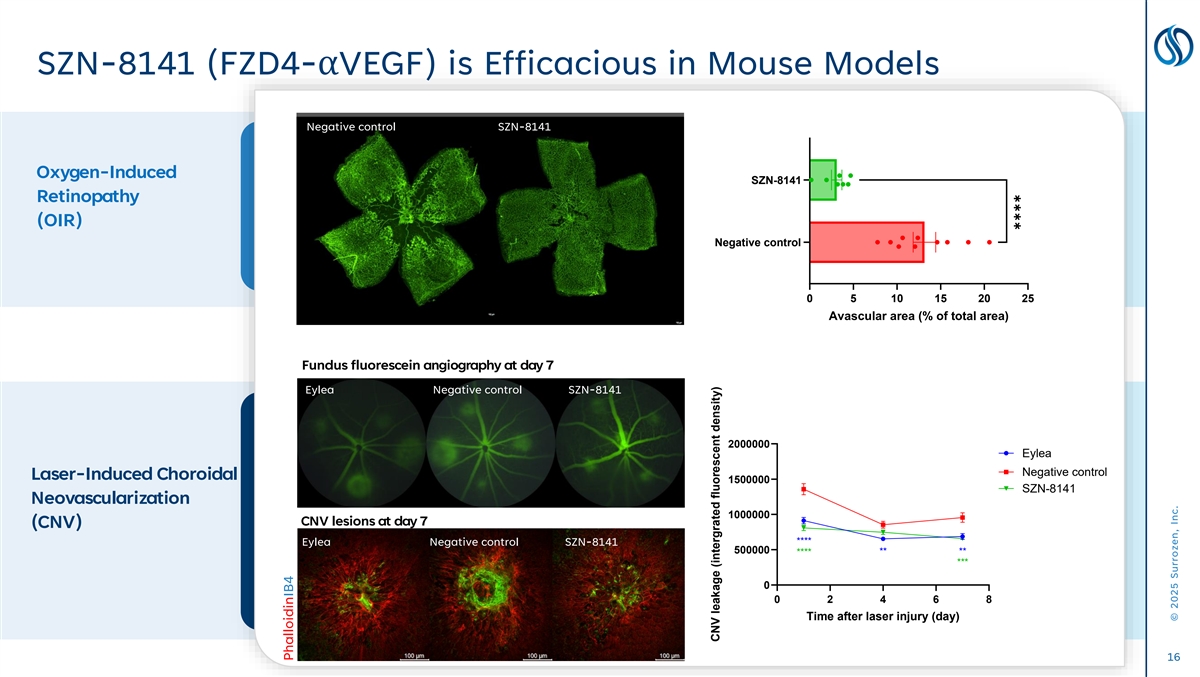
✱✱✱✱ SZN-8141 (FZD4-αVEGF) is Efficacious in Mouse Models Negative control FZD4-αVEGF Negative control SZN-8141 Oxygen-Induced SZN-8141 Retinopathy (OIR) Negative control 0 5 10 15 20 25 Avascular area (% of total area) Fundus fluorescein angiography at day 7 Eylea Negative control SZN-8141 2000000 Eylea Negative control Laser-Induced Choroidal 1500000 SZN-8141 Neovascularization 1000000 CNV lesions at day 7 (CNV) Eylea Negative control SZN-8141 **** 500000 **** ** ** *** 0 0 2 4 6 8 Time after laser injury (day) 16 PhalloidinIB4 CNV leakage (intergrated fluorescent density) © 2025 Surrozen, Inc.
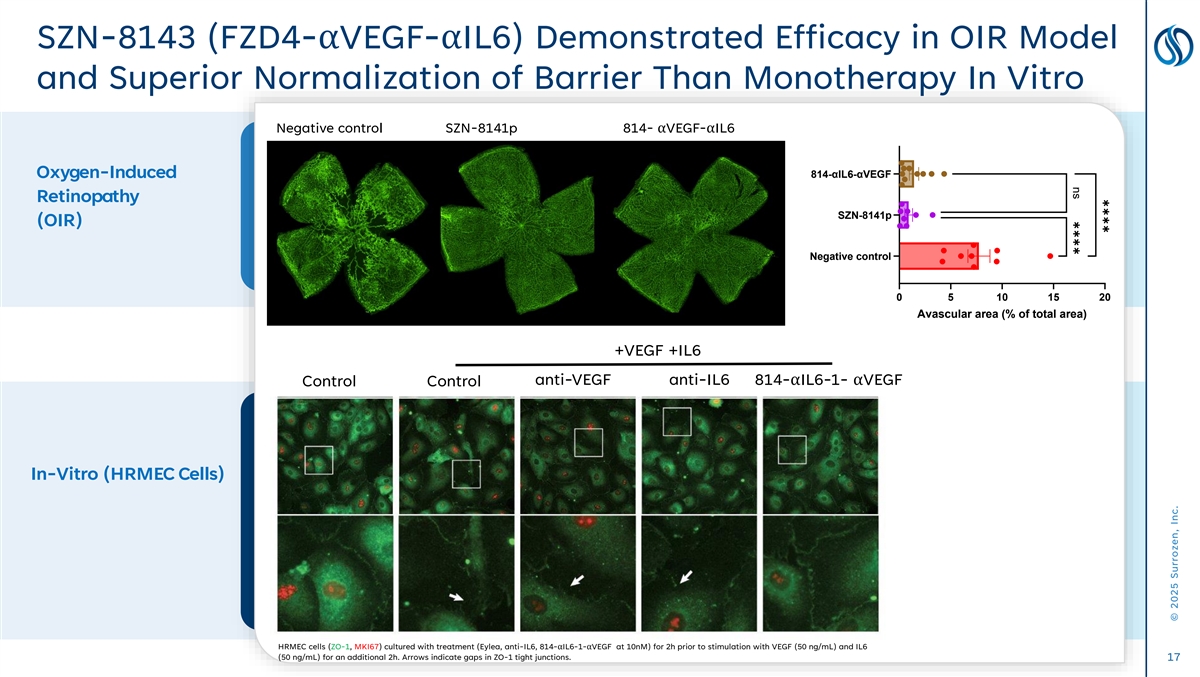
✱✱✱✱ ns ✱✱✱✱ SZN-8143 (FZD4-αVEGF-αIL6) Demonstrated Efficacy in OIR Model and Superior Normalization of Barrier Than Monotherapy In Vitro Negative control FZD4-αVEGF Negative control SZN-8141p 814-αVEGF-αIL6 Oxygen-Induced 814-αIL6-αVEGF Retinopathy SZN-8141p (OIR) Negative control 0 5 10 15 20 Avascular area (% of total area) +VEGF +IL6 anti-VEGF anti-IL6 814-αIL6-1-αVEGF Control Control In-Vitro (HRMEC Cells) HRMEC cells (ZO-1, MKI67) cultured with treatment (Eylea, anti-IL6, 814-αIL6-1-αVEGF at 10nM) for 2h prior to stimulation with VEGF (50 ng/mL) and IL6 (50 ng/mL) for an additional 2h. Arrows indicate gaps in ZO-1 tight junctions. 17 © 2025 Surrozen, Inc.

Cornea Endothelium Program

Cornea Program: FZD127 antibody is a novel approach to treating dysfunctions of corneal endothelium, like Fuchs’ Dystrophy Wnt Signal • Corneal transplantation surgery is only direct treatment option, but is Activation/Corneal infrequently used to due to: – Limit on donor corneas (~20K in US/year) Regeneration – Invasive procedure and notable re-transplantation rate – Variable outcomes on vision improvement Fuchs’ endothelial cell dystrophy • Medical therapies limited to topical symptomatic treatment • Few direct treatments being explored, but have limitations on efficacy (FECD) is a disease and invasiveness characterized by corneal swelling and ultimately vision SZN-113 (FZD127) has demonstrated preclinical evidence of: loss caused by progressive loss • Proliferation of healthy endothelial cells and reorganization of matrix in of corneal endothelial cells human explants • Recovery of cornea thickness and clarity in mouse and rabbit cryoinjury models Market research confirms large commercial opportunity as treatment for moderate-severe Fuchs’ Dystrophy ahead of surgery • Additional indication potential in endothelial damage from cataract surgery, supplement to corneal transplant surgeries 19 © 2025 Surrozen, Inc.
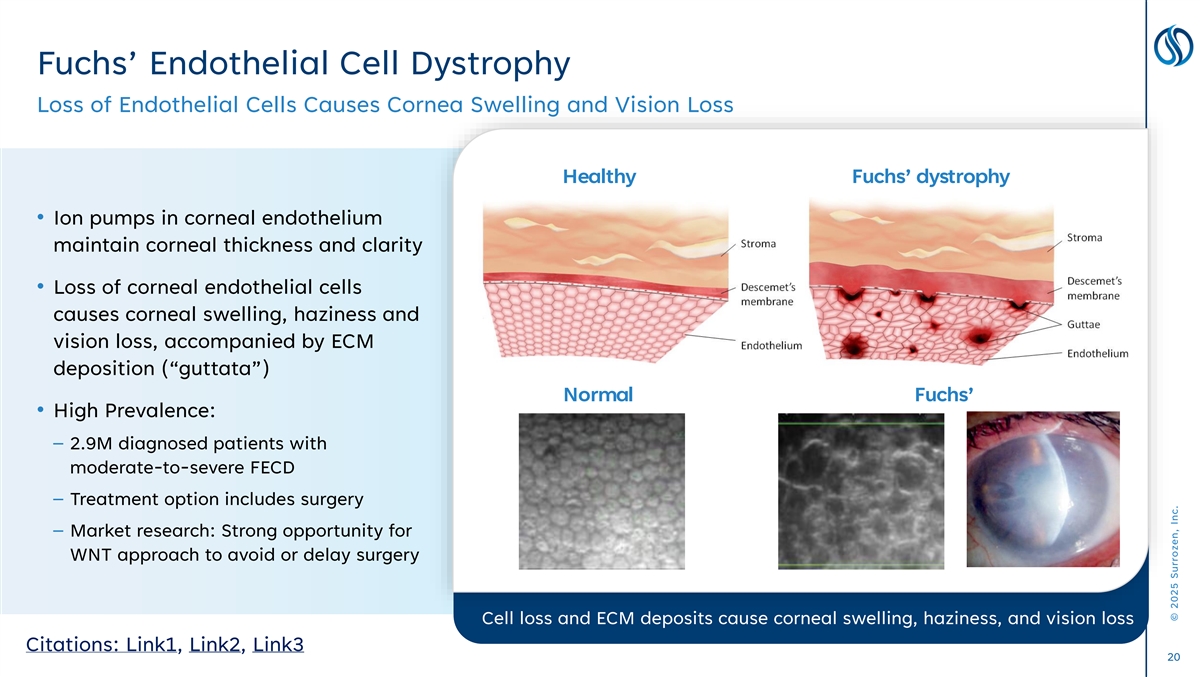
Fuchs’ Endothelial Cell Dystrophy Loss of Endothelial Cells Causes Cornea Swelling and Vision Loss Healthy Fuchs’ dystrophy • Ion pumps in corneal endothelium maintain corneal thickness and clarity • Loss of corneal endothelial cells causes corneal swelling, haziness and vision loss, accompanied by ECM deposition (“guttata”) Normal Fuchs’ • High Prevalence: – 2.9M diagnosed patients with moderate-to-severe FECD – Treatment option includes surgery – Market research: Strong opportunity for WNT approach to avoid or delay surgery Cell loss and ECM deposits cause corneal swelling, haziness, and vision loss Citations: Link1, Link2, Link3 20 © 2025 Surrozen, Inc.

SZN-113 is Efficacious in Mouse and Rabbit Cryoinjury Models SZN-113 Reduced Corneal Thickness and Improved Clarity Score Central Corneal Thickness SPOTS Clarity Score SPOTS Clarity Score Day 3 5 Anti-GFP anti-GFP 4 SZN-113p 0.5ug SZN-113p 5ug 3 SZN-113p 25ug 2 * * 1 SZN-113p 0 Pre 0 1 2 3 4 Days • Trans-corneal injury induces endothelial cell loss and corneal edema (thickening) • SZN-113 rapidly and significantly reduced central corneal thickness Improved corneal clarity 3 days after injury and treatment • SZN-113 rapidly induced improvement in corneal clarity (scoring by cornea specialist (SPOTs grading scale) blinded to treatment group) 21 Clarity Score © 2025 Surrozen, Inc.

SZN-113 Stimulates Proliferation in Human Cornea Cultures Wnt activation Proliferation ns AXIN2 - 24h Human endothelial ✱ 8 2.0 “skirts” can be removed and cultured 6 1.5 4 1.0 2 0.5 0 0.0 SZN platforms and competitors' comparison • SZN-113 activates Wnt pathway • SZN-113 as good or better than engineered FGF 22 a-GFP SZN-113 TTHX1114 a-GFP SZN-113 AXIN2 fold change relative to ACTB normalized to control + Relative Ki67 cells (normalized to a-GFP) © 2025 Surrozen, Inc.
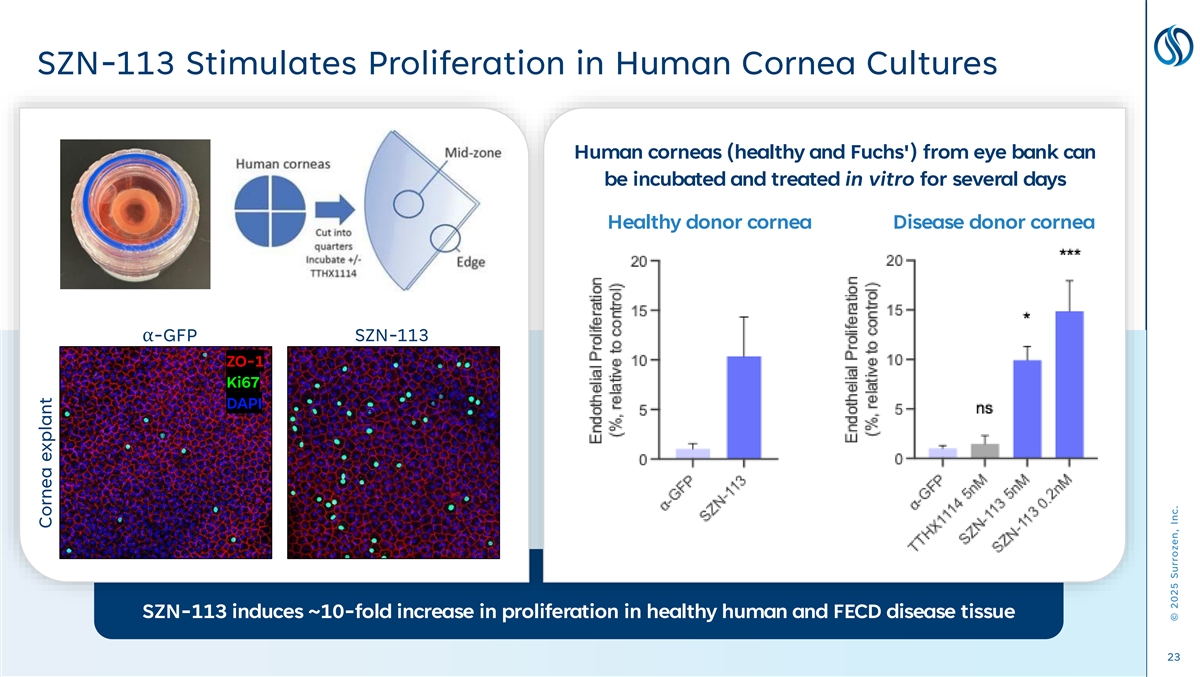
SZN-113 Stimulates Proliferation in Human Cornea Cultures Human corneas (healthy and Fuchs') from eye bank can be incubated and treated in vitro for several days Healthy donor cornea Disease donor cornea α-GFP SZN-113 ZO-1 Ki67 DAPI SZN-113 induces ~10-fold increase in proliferation in healthy human and FECD disease tissue 23 Cornea explant © 2025 Surrozen, Inc.

Dry AMD and Geographic Atrophy

SZN-113 (FZD127) has Novel Potential to Treat Dry AMD/ GA through Preservation of Critical Retinal Layers 01 02 03 FZD127 antibody targeting SZN-113 (FZD127) has Potential as monotherapy of retinal neurons is a novel demonstrated preclinical or combination with other approach to treating Dry evidence of: existing/novel GA AMD/GA treatment approaches • Neuroprotection in acute injury (MNU-induced) and progressive • Complement inhibition treatment degeneration (rd10 mutant) has shown marginal clinical utility models of photoreceptor – Lack of vision improvement, damage infrequent but significant SAE’s • Stimulation of RPE proliferation • KOLs cite “holy grail” in dry AMD is and differentiation in vitro protection/preservation of RPE and photoreceptors 25 © 2025 Surrozen, Inc.

Leveraging Activation of Wnt Signaling in the Eye Can Provide Protection and/or Regeneration Upon activation of Wnt signaling in the retina: • Muller glia could be stimulated to secrete growth factors that provide IVT injection neuroprotection Wnt mimetic • Photoreceptors could potentially be more resistant to insults • RPEs could potentially be regenerated Retina 26 Muller glia Photoreceptors RPE © 2025 Surrozen, Inc.
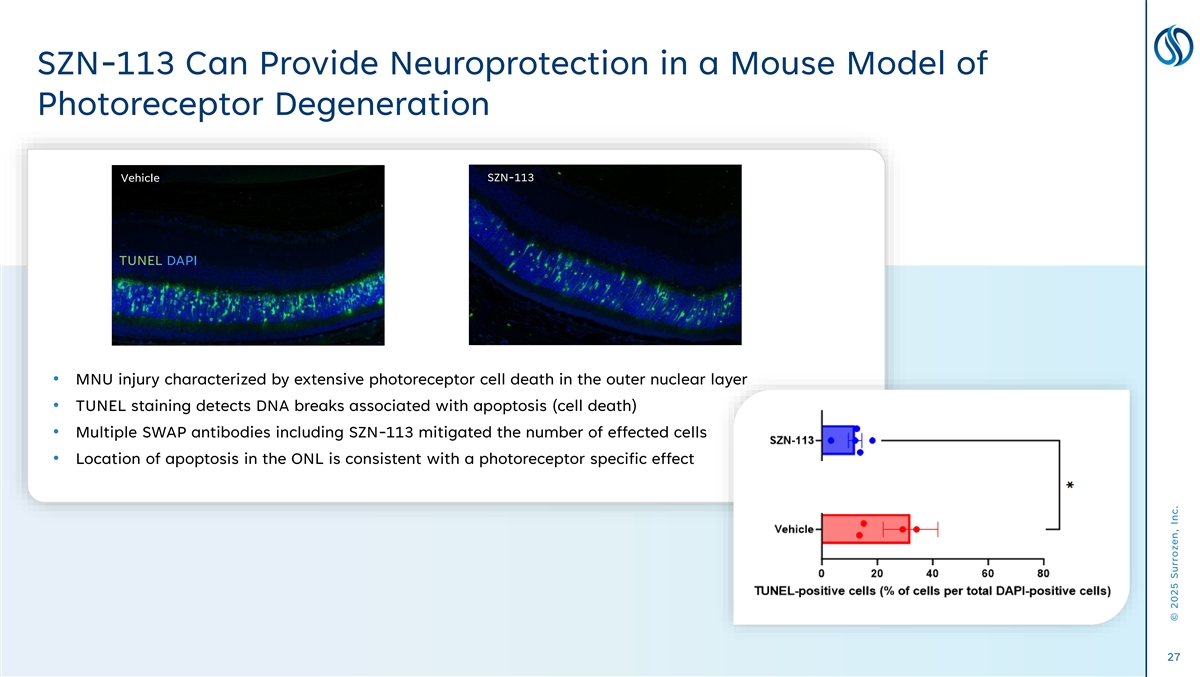
SZN-113 Can Provide Neuroprotection in a Mouse Model of Photoreceptor Degeneration Vehicle SZN-1326 SZN-113 TUNEL DAPI • MNU injury characterized by extensive photoreceptor cell death in the outer nuclear layer • TUNEL staining detects DNA breaks associated with apoptosis (cell death) • Multiple SWAP antibodies including SZN-113 mitigated the number of effected cells • Location of apoptosis in the ONL is consistent with a photoreceptor specific effect 27 © 2025 Surrozen, Inc.

SWAPs Stimulate/Accelerate RPE Proliferation and Differentiation hiPSC-RPE Melanin Deposit 3D human primary RPE expansion (Day 14) hiPSC-RPE Melanin Deposit 2.5 Negative control SWAP1+RSPO SWAP1+RSPO Negative control Negative Control SWAP1+RSPO 2.0 1.5 • FZD-specific SWAPs can stimulate proliferation of RPE cells in vitro • RPE monolayer differentiation can be facilitated by FZD-specific SWAPs 1.0 0 10 20 30 Treatment days 28 Melanin deposit (OD 405 nm, normalized) Day 58 Day 26 © 2025 Surrozen, Inc.
Glossary AMD Age-related macular degeneration Lrp Lipoprotein receptor-related protein CNV Choroidal neovascularization MNU N-methyl-N-nitrosourea (rodent models) DME Diabetic macular edema Ndp. Norrie disease gene ECM Extracellular matrix NV. Neovascularization FECD Fuchs' endothelial corneal dystrophy POC. Proof-of-concept FEVR Familial exudative vitreoretinopathy OIR Oxygen induced retinopathy Fzd Frizzled ONL Outer nuclear layer of the retina GA Geographic atophy RPE Retinal pigment epithelium GFP. Green fluorescence protein RSPO R-spondins HiPSC Human induced pluripotennt stell SOC. Standard of care HRMEC Human retinal microvascular endothelial cells SOD Start of developmennt IND. Investigational new drug SWAP. Surrozen Wnt signal activating proteins TUNEL Test to determine if eye cells indicate apoptotic cell death IL-6 Interleukin 6 UME Uveitic macular edema IVT. Intravitreal injection VEGF/R Vascular endothelial growth factor/receptors KO. Knock-out model Wnt Wingless-related integration site 29 © 2025 Surrozen, Inc.