UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): September 4, 2024
Syros Pharmaceuticals, Inc.
(Exact Name of Registrant as Specified in its Charter)
| Delaware | 001-37813 | 45-3772460 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 35 CambridgePark Drive Cambridge, Massachusetts |
02140 | |
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s telephone number, including area code: (617) 744-1340
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered or to be registered pursuant to Section 12(b) of the Act.
| Title of each class |
Trading |
Name of each exchange on which registered |
||
| Common Stock, $0.001 par value | SYRS | Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On September 4, 2024, Syros Pharmaceuticals, Inc. presented a poster at the Society of Hematologic Oncology 12th Annual Meeting. A copy of the poster is attached to this Current Report on Form 8-K as Exhibit 99.1 and is incorporated herein by reference. The information responsive to Item 7.01 of this Form 8-K and Exhibit 99.1 hereto shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934 (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933 or the Exchange Act, except as expressly set forth by specific reference in such a filing.
| Item 9.01 | Financial Statements and Exhibits. |
| Exhibit No. |
Description |
|
| 99.1 | Poster, dated September 4, 2024. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document). | |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| SYROS PHARMACEUTICALS, INC. | ||||||
| Date: September 5, 2024 | By: | /s/ Gerald Quirk |
||||
| Gerald Quirk Chief Legal & Compliance Officer; Chief Business Officer |
||||||
Exhibit 99.1
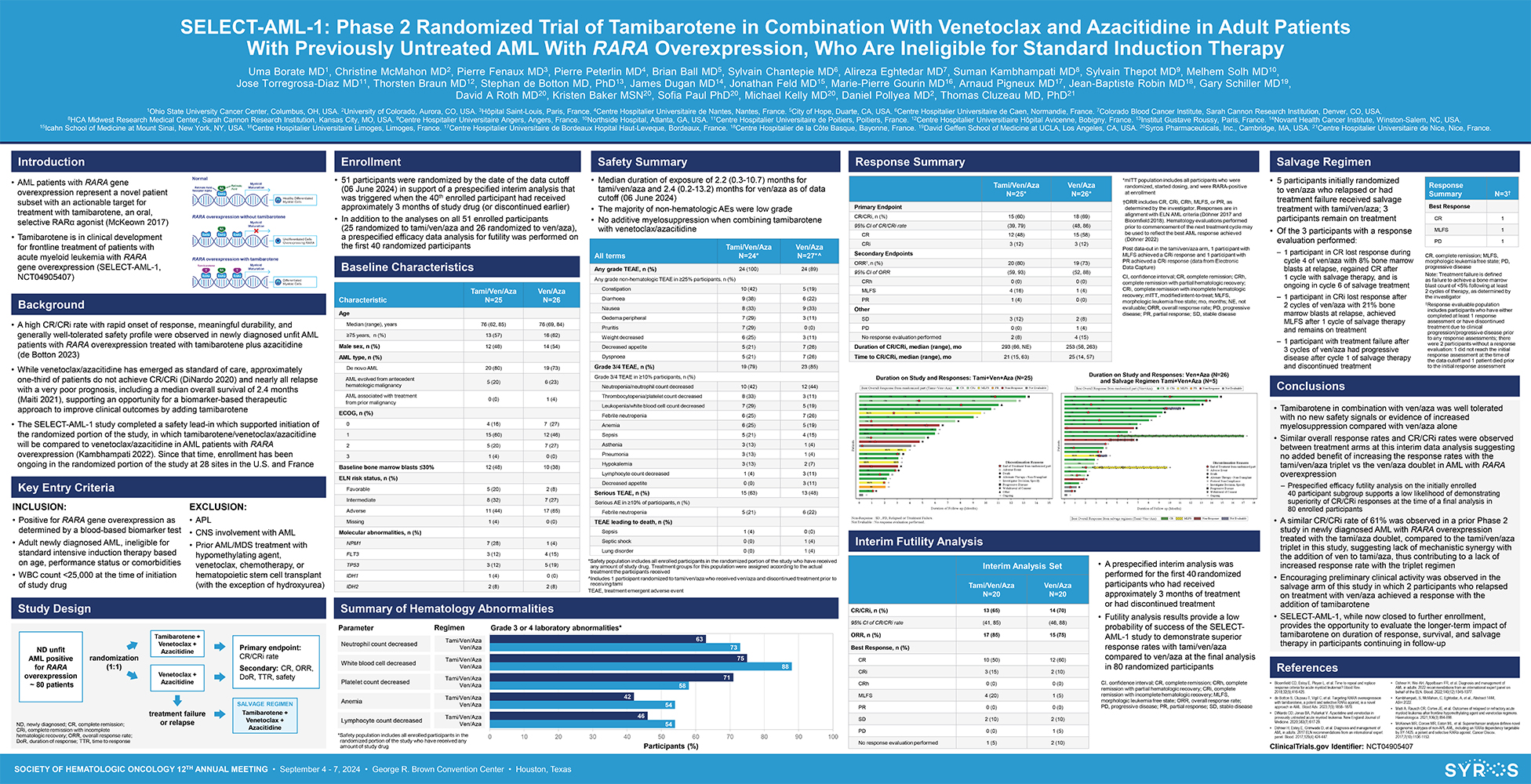
SOCIETY OF HEMATOLOGIC ONCOLOGY 12TH ANNUAL MEETING • September 4—7, 2024 • George R. Brown Convention Center • Houston, Texas SELECT-AML-1: Phase 2 Randomized Trial of Tamibarotene in Combination With Venetoclax and Azacitidine in Adult Patients With Previously Untreated AML With RARA Overexpression, Who Are Ineligible for Standard Induction Therapy Uma Borate MD1, Christine McMahon MD2, Pierre Fenaux MD3, Pierre Peterlin MD4, Brian Ball MD5, Sylvain Chantepie MD6, Alireza Eghtedar MD7, Suman Kambhampati MD8, Sylvain Thepot MD9, Melhem Solh MD10, Jose Torregrosa-Diaz MD11, Thorsten Braun MD12, Stephan de Botton MD, PhD13, James Dugan MD14, Jonathan Feld MD15, Marie-Pierre Gourin MD16, Arnaud Pigneux MD17, Jean-Baptiste Robin MD18, Gary Schiller MD19, David A Roth MD20, Kristen Baker MSN20, Sofia Paul PhD20, Michael Kelly MD20, Daniel Pollyea MD2, Thomas Cluzeau MD, PhD21 1Ohio State University Cancer Center, Columbus, OH, USA. 2University of Colorado, Aurora, CO, USA. 3Hôpital Saint-Louis, Paris, France. 4Centre Hospitalier Universitaire de Nantes, Nantes, France. 5City of Hope, Duarte, CA, USA. 6Centre Hospitalier Universitaire de Caen, Normandie, France. 7Colorado Blood Cancer Institute, Sarah Cannon Research Institution, Denver, CO, USA. 8HCA Midwest Research Medical Center, Sarah Cannon Research Institution, Kansas City, MO, USA. 9Centre Hospitalier Universitaire Angers, Angers, France. 10Northside Hospital, Atlanta, GA, USA. 11Centre Hospitalier Universitaire de Poitiers, Poitiers, France. 12Centre Hospitalier Universitiaire Hôpital Avicenne, Bobigny, France. 13Institut Gustave Roussy, Paris, France. 14Novant Health Cancer Institute, Winston-Salem, NC, USA. 15Icahn School of Medicine at Mount Sinai, New York, NY, USA. 16Centre Hospitalier Universitaire Limoges, Limoges, France. 17Centre Hospitalier Universitaire de Bordeaux Hopital Haut-Leveque, Bordeaux, France. 18Centre Hospitalier de la Côte Basque, Bayonne, France. 19David Geffen School of Medicine at UCLA, Los Angeles, CA, USA. 20Syros Pharmaceuticals, Inc., Cambridge, MA, USA. 21Centre Hospitalier Universitaire de Nice, Nice, France. • Tamibarotene in combination with ven/aza was well tolerated with no new safety signals or evidence of increased myelosuppression compared with ven/aza alone • Similar overall response rates and CR/CRi rates were observed between treatment arms at this interim data analysis suggesting no added benefit of increasing the response rates with the tami/ven/aza triplet vs the ven/aza doublet in AML with RARA overexpression – Prespecified efficacy futility analysis on the initially enrolled 40 participant subgroup supports a low likelihood of demonstrating superiority of CR/CRi responses at the time of a final analysis in 80 enrolled participants • A similar CR/CRi rate of 61% was observed in a prior Phase 2 study in newly diagnosed AML with RARA overexpression treated with the tami/aza doublet, compared to the tami/ven/aza triplet in this study, suggesting lack of mechanistic synergy with the addition of ven to tami/aza, thus contributing to a lack of increased response rate with the triplet regimen • Encouraging preliminary clinical activity was observed in the salvage arm of this study in which 2 participants who relapsed on treatment with ven/aza achieved a response with the addition of tamibarotene • SELECT-AML-1, while now closed to further enrollment, provides the opportunity to evaluate the longer-term impact of tamibarotene on duration of response, survival, and salvage therapy in participants continuing in follow-up Salvage Regimen • A high CR/CRi rate with rapid onset of response, meaningful durability, and generally well-tolerated safety profile were observed in newly diagnosed unfit AML patients with RARA overexpression treated with tamibarotene plus azacitidine (de Botton 2023) • While venetoclax/azacitidine has emerged as standard of care, approximately one-third of patients do not achieve CR/CRi (DiNardo 2020) and nearly all relapse with a very poor prognosis, including a median overall survival of 2.4 months (Maiti 2021), supporting an opportunity for a biomarker-based therapeutic approach to improve clinical outcomes by adding tamibarotene • The SELECT-AML-1 study completed a safety lead-in which supported initiation of the randomized portion of the study, in which tamibarotene/venetoclax/azacitidine will be compared to venetoclax/azacitidine in AML patients with RARA overexpression (Kambhampati 2022). Since that time, enrollment has been ongoing in the randomized portion of the study at 28 sites in the U.S. and France Introduction Background Key Entry Criteria INCLUSION: • Positive for RARA gene overexpression as determined by a blood-based biomarker test • Adult newly diagnosed AML, ineligible for standard intensive induction therapy based on age, performance status or comorbidities • WBC count <25,000 at the time of initiation of study drug EXCLUSION: • APL • CNS involvement with AML • Prior AML/MDS treatment with hypomethylating agent, venetoclax, chemotherapy, or hematopoietic stem cell transplant (with the exception of hydroxyurea) • AML patients with RARA gene overexpression represent a novel patient subset with an actionable target for treatment with tamibarotene, an oral, selective RARα agonist (McKeown 2017) • Tamibarotene is in clinical development for frontline treatment of patients with acute myeloid leukemia with RARA gene overexpression (SELECT-AML-1, NCT04905407) Conclusions • Bloomfield CD, Estey E, Pleyer L, et al. Time to repeal and replace response criteria for acute myeloid leukemia? Blood Rev. 2018;32(5);416-425. • de Botton S, Cluzeau T, Vigil C, et al. Targeting RARA overexpression with tamibarotene, a potent and selective RARα agonist, is a novel approach in AML. Blood Adv. 2023;7(9):1858–1870. • DiNardo CD, Jonas BA, Pullarkat V. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. New England Journal of Medicine. 2020;383(7):617-29. • Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447. Ven/Aza N=26 Tami/Ven/Aza Characteristic N=25 Age Median (range), years 76 (62, 85) 76 (69, 84) ≥75 years, n (%) 13 (57) 16 (62) Male sex, n (%) 12 (48) 14 (54) AML type, n (%) De novo AML 20 (80) 19 (73) AML evolved from antecedent 5 (20) 6 (23) hematologic malignancy AML associated with treatment 0 (0) 1 (4) from prior malignancy ECOG, n (%) 0 4 (16) 7 (27) 1 15 (60) 12 (46) 2 5 (20) 7 (27) 3 1 (4) 0 (0) Baseline bone marrow blasts ≤30% 12 (48) 10 (38) ELN risk status, n (%) Favorable 5 (20) 2 (8) Intermediate 8 (32) 7 (27) Adverse 11 (44) 17 (65) Missing 1 (4) 0 (0) Molecular abnormalities, n (%) NPM1 7 (28) 1 (4) FLT3 3 (12) 4 (15) TP53 3 (12) 5 (19) IDH1 1 (4) 0 (0) IDH2 2 (8) 2 (8) *Safety population includes all enrolled participants in the randomized portion of the study who have received any amount of study drug. Treatment groups for this population were assigned according to the actual treatment the participants received ^Includes 1 participant randomized to tami/ven/aza who received ven/aza and discontinued treatment prior to receiving tami TEAE, treatment emergent adverse event Safety Summary Baseline Characteristics • Median duration of exposure of 2.2 (0.3-10.7) months for tami/ven/aza and 2.4 (0.2-13.2) months for ven/aza as of data cutoff (06 June 2024) • The majority of non-hematologic AEs were low grade • No additive myelosuppression when combining tamibarotene with venetoclax/azacitidine Summary of Hematology Abnormalities Parameter 54 54 58 88 73 46 42 71 75 63 0 10 20 30 40 50 60 70 80 90 100 Participants (%) *Safety population includes all enrolled participants in the randomized portion of the study who have received any amount of study drug Anemia Lymphocyte count decreased Neutrophil count decreased White blood cell decreased Platelet count decreased Tami/Ven/Aza Ven/Aza Tami/Ven/Aza Ven/Aza Tami/Ven/Aza Ven/Aza Tami/Ven/Aza Ven/Aza Tami/Ven/Aza Ven/Aza Regimen Grade 3 or 4 laboratory abnormalities* References Enrollment • 51 participants were randomized by the date of the data cutoff (06 June 2024) in support of a prespecified interim analysis that was triggered when the 40th enrolled participant had received approximately 3 months of study drug (or discontinued earlier) • In addition to the analyses on all 51 enrolled participants (25 randomized to tami/ven/aza and 26 randomized to ven/aza), a prespecified efficacy data analysis for futility was performed on the first 40 randomized participants Study Design Response Summary Ven/Aza N=26* Tami/Ven/Aza N=25* Primary Endpoint CR/CRi, n (%) 15 (60) 18 (69) 95% CI of CR/CRi rate (39, 79) (48, 86) CR 12 (48) 15 (58) CRi 3 (12) 3 (12) Secondary Endpoints ORR†, n (%) 20 (80) 19 (73) 95% CI of ORR (59, 93) (52, 88) CRh 0 (0) 0 (0) MLFS 4 (16) 1 (4) PR 1 (4) 0 (0) Other SD 3 (12) 2 (8) PD 0 (0) 1 (4) No response evaluation performed 2 (8) 4 (15) Duration of CR/CRi, median (range), mo 293 (66, NE) 253 (56, 263) Time to CR/CRi, median (range), mo 21 (15, 63) 25 (14, 57) *mITT population includes all participants who were randomized, started dosing, and were RARA-positive at enrollment †ORR includes CR, CRi, CRh, MLFS, or PR, as determined by the investigator. Responses are in alignment with ELN AML criteria (Döhner 2017 and Bloomfield 2018). Hematology evaluations performed prior to commencement of the next treatment cycle may be used to reflect the best AML response achieved (Döhner 2022) Post data-cut in the tami/ven/aza arm, 1 participant with MLFS achieved a CRi response and 1 participant with PR achieved a CRi response (data from Electronic Data Capture) CI, confidence interval; CR, complete remission; CRh, complete remission with partial hematologic recovery; CRi, complete remission with incomplete hematologic recovery; mITT, modified intent-to-treat; MLFS, morphologic leukemia free state; mo, months; NE, not evaluable; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease • 5 participants initially randomized to ven/aza who relapsed or had treatment failure received salvage treatment with tami/ven/aza; 3 participants remain on treatment • Of the 3 participants with a response evaluation performed: – 1 participant in CR lost response during cycle 4 of ven/aza with 8% bone marrow blasts at relapse, regained CR after 1 cycle with salvage therapy, and is ongoing in cycle 6 of salvage treatment – 1 participant in CRi lost response after 2 cycles of ven/aza with 21% bone marrow blasts at relapse, achieved MLFS after 1 cycle of salvage therapy and remains on treatment – 1 participant with treatment failure after 3 cycles of ven/aza had progressive disease after cycle 1 of salvage therapy and discontinued treatment N=3† Response Summary Best Response CR 1 MLFS 1 PD 1 CR, complete remission; MLFS, morphologic leukemia free state; PD, progressive disease Note: Treatment failure is defined as failure to achieve a bone marrow blast count of <5% following at least 2 cycles of therapy, as determined by the investigator †Response evaluable population includes participants who have either completed at least 1 response assessment or have discontinued treatment due to clinical progression/progressive disease prior to any response assessments; there were 2 participants without a response evaluation: 1 did not reach the initial response assessment at the time of the data cutoff and 1 patient died prior to the initial response assessment ND, newly diagnosed; CR, complete remission; CRi, complete remission with incomplete hematologic recovery; ORR, overall response rate; DoR, duration of response; TTR, time to response Primary endpoint: CR/CRi rate Secondary: CR, ORR, DoR, TTR, safety randomization (1:1) Venetoclax + Azacitidine Tamibarotene + Venetoclax + ND unfit Azacitidine AML positive for RARA overexpression ~ 80 patients SALVAGE REGIMEN Tamibarotene + Venetoclax + Azacitidine treatment failure or relapse Ven/Aza N=27*^ Tami/Ven/Aza All terms N=24* Any grade TEAE, n (%) 24 (100) 24 (89) Any grade non-hematologic TEAE in ≥25% participants, n (%) Constipation 10 (42) 5 (19) Diarrhoea 9 (38) 6 (22) Nausea 8 (33) 9 (33) Oedema peripheral 7 (29) 3 (11) Pruritis 7 (29) 0 (0) Weight decreased 6 (25) 3 (11) Decreased appetite 5 (21) 7 (26) Dyspnoea 5 (21) 7 (26) Grade 3/4 TEAE, n (%) 19 (79) 23 (85) Grade 3/4 TEAE in ≥10% participants, n (%) Neutropenia/neutrophil count decreased 10 (42) 12 (44) Thrombocytopenia/platelet count decreased 8 (33) 3 (11) Leukopenia/white blood cell count decreased 7 (29) 5 (19) Febrile neutropenia 6 (25) 7 (26) Anemia 6 (25) 5 (19) Sepsis 5 (21) 4 (15) Asthenia 3 (13) 1 (4) Pneumonia 3 (13) 1 (4) Hypokalemia 3 (13) 2 (7) Lymphocyte count decreased 1 (4) 3 (11) Decreased appetite 0 (0) 3 (11) Serious TEAE, n (%) 15 (63) 13 (48) Serious AE in ≥10% of participants, n (%) Febrile neutropenia 5 (21) 6 (22) TEAE leading to death, n (%) Sepsis 1 (4) 0 (0) Septic shock 0 (0) 1 (4) Lung disorder 0 (0) 1 (4) Interim Analysis Set Ven/Aza N=20 Tami/Ven/Aza N=20 CR/CRi, n (%) 13 (65) 14 (70) 95% CI of CR/CRi rate (41, 85) (46, 88) ORR, n (%) 17 (85) 15 (75) Best Response, n (%) CR 10 (50) 12 (60) CRi 3 (15) 2 (10) CRh 0 (0) 0 (0) MLFS 4 (20) 1 (5) PR 0 (0) 0 (0) SD 2 (10) 2 (10) PD 0 (0) 1 (5) No response evaluation performed 1 (5) 2 (10) CI, confidence interval; CR, complete remission; CRh, complete remission with partial hematologic recovery; CRi, complete remission with incomplete hematologic recovery; MLFS, morphologic leukemia free state; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease • A prespecified interim analysis was performed for the first 40 randomized participants who had received approximately 3 months of treatment or had discontinued treatment • Futility analysis results provide a low probability of success of the SELECTAML- 1 study to demonstrate superior response rates with tami/ven/aza compared to ven/aza at the final analysis in 80 randomized participants Interim Futility Analysis Duration on Study and Responses: Tami+Ven+Aza (N=25) Duration on Study and Responses: Ven+Aza (N=26) and Salvage Regimen Tami+Ven+Aza (N=5) ClinicalTrials.gov Identifier: NCT04905407 • Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345-1377. • Kambhampati, S, McMahon, C, Eghtedar, A, et al., Abstract 1444, ASH 2022. • Maiti A, Rausch CR, Cortes JE, et al. Outcomes of relapsed or refractory acute myeloid leukemia after frontline hypomethylating agent and venetoclax regimens. Haematologica. 2021;106(3):894-898. • McKeown MR, Corces MR, Eaton ML, et al. Superenhancer analysis defines novel epigenomic subtypes of non-APL AML, including an RARα dependency targetable by SY-1425, a potent and selective RARα agonist. Cancer Discov. 2017;7(10):1136-1153.