UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): May 20, 2024
Dyne Therapeutics, Inc.
(Exact Name of Registrant as Specified in Charter)
| Delaware | 001-39509 | 36-4883909 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
||
| 1560 Trapelo Road Waltham, Massachusetts |
02451 | |||
| (Address of Principal Executive Offices) | (Zip Code) | |||
Registrant’s telephone number, including area code: (781) 786-8230
Not applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading symbol(s) |
Name of each exchange on which registered |
||
| Common stock, $0.0001 par value per share | DYN | Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01. | Regulation FD Disclosure. |
On May 20, 2024, Dyne Therapeutics, Inc. (the “Company”) issued a press release announcing positive clinical data from its ongoing Phase 1/2 ACHIEVE clinical trial of DYNE-101 in patients with myotonic dystrophy type 1 (“DM1”) and its ongoing Phase 1/2 DELIVER clinical trial of DYNE-251 in patients with Duchenne muscular dystrophy (“DMD”) who are amenable to exon 51 skipping. A copy of the press release is furnished as Exhibit 99.1 hereto and is incorporated herein by reference.
The information furnished under this Item 7.01, including Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
| Item 8.01. | Other Events. |
On May 20, 2024, the Company issued a press release announcing positive clinical data from its ongoing Phase 1/2 ACHIEVE clinical trial of DYNE-101 in patients with DM1 and its ongoing Phase 1/2 DELIVER clinical trial of DYNE-251 in patients with DMD who are amenable to exon 51 skipping.
Phase 1/2 ACHIEVE Trial of DYNE-101 in DM1
Efficacy Data
The Company reported efficacy data from 40 adult DM1 patients enrolled in the randomized, placebo-controlled multiple ascending dose (“MAD”) portion of the DYNE-101 ACHIEVE trial, including 12-month data from the 1.8 mg/kg Q4W (approximate antisense oligonucleotide (“ASO”) dose) cohort (n=16), 6-month data from the 3.4 mg/kg Q4W cohort (n=16), and 3-month data from the 5.4 mg/kg Q8W cohort (n=8).
In the ACHIEVE trial, DYNE-101 demonstrated robust muscle delivery and dose-dependent, consistent splicing correction while also showing improvement in multiple functional endpoints and patient reported outcomes.
Key findings from ACHIEVE include:
Splicing
| • | DYNE-101 continued to show dose dependent splicing correction as seen in earlier cohorts. Patients in the 5.4 mg/kg Q8W cohort had a 27% mean splicing correction from baseline across a broad, 22-gene panel at 3 months, with all participants demonstrating splicing correction. |
Function
| • | Myotonia (vHOT): DYNE-101 demonstrated an improvement in myotonia as measured by video hand opening time (“vHOT”) in all reported cohorts. The 1.8 mg/kg Q4W group had a mean 3.1 second benefit in myotonia at 3 months that increased to 4.4 seconds at 12 months. In addition, the 5.4 mg/kg Q8W cohort had a mean 4.5 second improvement in myotonia at 3 months. |
| • | Strength and Timed Assessments: DYNE-101 demonstrated an improvement in muscle strength as measured by Quantitative Myometry Testing (“QMT”), a test of muscle strength and fatigue, and early and sustained potential benefit in 10-Meter Walk/Run Test and 5 Times Sit to Stand Test. |
Patient Reported Outcomes
| • | Myotonic Dystrophy Health Index (“MDHI”): DYNE-101 demonstrated an overall improvement in the MDHI and benefit in all 17 subscales, including those that assess peripheral muscles, central nervous system, and gastrointestinal measures. These represent some of the most burdensome manifestations of DM1 and daily quality of life issues for patients and their families. |
| • | Myotonic Dystrophy Type 1 Activity and Participation Scale (“DM1-ACTIVc”): Treatment with DYNE-101 resulted in an improvement in the assessment of various activities of daily living, as measured by DM1-ACTIVc. |
Safety and Tolerability Data
| • | The Company also reported safety and tolerability data from 56 patients enrolled through the 6.8 mg/kg Q8W cohort of the MAD portion of the ACHIEVE trial. DYNE-101 demonstrated a favorable safety profile as of the DYNE-101 safety data cut-off date of May 8, 2024. The majority of treatment emergent adverse events were mild or moderate and no related serious treatment emergent adverse events have been identified. |
| • | Enrollment is complete through the 6.8 mg/kg Q8W cohort. Approximately 500 doses have been administered to date, representing over 40-patient years of follow-up and supporting dose escalation up to 10.2 mg/kg. |
Phase 1/2 DELIVER Trial of DYNE-251 in DMD
Efficacy Data
The Company reported efficacy data from 8 male patients with DMD amenable to exon 51 skipping enrolled in the 10 mg/kg (approximate phosphorodiamidate morpholino oligomer (“PMO”) dose) cohort of the randomized, placebo-controlled MAD portion of the DYNE-251 DELIVER trial. Patients were randomized to receive either DYNE-251 (n=6) or placebo (n=2) once every four weeks for 6 months.
In the 10 mg/kg dose cohort, DYNE-251 Q4W demonstrated dose-dependent exon skipping and dystrophin expression. DYNE-251 reached levels of dystrophin expression that exceeded levels reported in a clinical trial for the current weekly standard of care for DMD exon 51, eteplirsen, at 6 months with a 12-fold lower PMO dose. DYNE-251 also demonstrated encouraging trends in multiple functional endpoints.
Key findings from DELIVER include:
Dystrophin expression measured by Western blot
| • | Patients treated with 10 mg/kg of DYNE-251 Q4W had a mean absolute dystrophin level of 3.22% of normal and a 2.97% change (unadjusted for muscle content) from baseline at 6 months. Eteplirsen reached a mean absolute unadjusted dystrophin level of 0.30% of normal and a 0.06% change from baseline at 6 months. When adjusting for muscle content, the DYNE-251 treated group reached 7.64% mean absolute dystrophin, which is greater than levels reported by peptide conjugate PMOs in clinical development. |
Function
| • | Trends in improvement were observed in multiple functional endpoints in the 10 mg/kg DYNE-251 Q4W group at 6 months, including North Star Ambulatory Assessment (“NSAA”), Stride Velocity 95th Centile (“SV95C”), 10-Meter Walk/Run Time, and Time to Rise from Floor. |
The DELIVER trial does not compare DYNE-251 to eteplirsen or SRP-5051, and no head-to-head trials have been conducted comparing DYNE-251 to eteplirsen or SRP-5051. Eteplirsen data and SRP-5051 data may not be directly comparable to the DELIVER data due to differences between the trials in trial protocols, dosing regimens, methodologies for calculating muscle content adjusted dystrophin and patient populations. Accordingly, these cross-trial comparisons may not be reliable.
Safety and Tolerability Data
| • | Dyne also reported safety and tolerability data from 48 patients enrolled through the 40 mg/kg Q8W cohort of the MAD portion of the DELIVER trial. DYNE-251 demonstrated a favorable safety profile as of the DYNE-251 safety data cut-off date of April 30, 2024. The majority of treatment emergent adverse events were mild or moderate and no related serious treatment emergent adverse events have been identified. |
| • | Enrollment is complete through the 40 mg/kg cohort. Approximately 480 doses have been administered to date, representing over 35 patient-years of follow-up, supporting dose escalation up to 40 mg/kg. |
KEY ANTICIPATED MILESTONES
ACHIEVE and DELIVER Trials
| • | The Company plans to continue to engage with global regulators this year on ACHIEVE and DELIVER, and anticipates providing an update on the path to registration for both DYNE-101 and DYNE-251 by the end of 2024. Both trials are designed to be registrational, and the Company is pursuing expedited approval pathways for both programs. |
| • | In DM1, based on recent dialogue with the Center for Drug Evaluation and Research (“CDER”) division of the U.S. Food and Drug Administration (“FDA”), The Company continues to pursue an accelerated approval pathway for DYNE-101, including leveraging splicing as a potential surrogate biomarker. |
| • | In DMD, the Company has confirmed that the FDA precedent for using dystrophin as a surrogate biomarker for accelerated approval remains available. |
FORCE™ Platform and Pipeline
| • | With ACHIEVE and DELIVER data continuing to reinforce the promise of the FORCE platform, the Company expects to provide updates on programs, including facioscapulohumeral muscular dystrophy (“FSHD”) and other pipeline opportunities during 2024. |
Data Presentation
On May 20, 2024, the Company made available a presentation to be used with investors to discuss the clinical data from the ACHIEVE and DELIVER clinical trials. A copy of the presentation is filed as Exhibit 99.2 hereto and is incorporated herein by reference.
Cautionary Note Regarding Forward-Looking Statements
This Current Report on Form 8-K contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this Current Report on Form 8-K, including statements regarding the Company’s strategy, future operations, prospects and plans, objectives of management, the potential of the FORCE platform, the anticipated timelines for reporting additional data from the ACHIEVE and DELIVER clinical trials and initiating registrational cohorts, expectations regarding the timing and outcome of interactions with global regulatory authorities and the availability of accelerated approval pathways for DYNE-101 and DYNE-251, and plans to provide future updates on pipeline programs, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “objective,” “ongoing,” “plan,” “predict,” “project,” “potential,” “should,” or “would,” or the negative of these terms, or other comparable terminology are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The Company may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements.
Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various important factors, including: uncertainties inherent in the identification and development of product candidates, including the initiation and completion of preclinical studies and clinical trials; uncertainties as to the availability and timing of results from preclinical studies and clinical trials; the timing of and the Company’s ability to enroll patients in clinical trials; whether results from preclinical studies and initial data from early clinical trials will be predictive of the final results of the clinical trials or future trials; uncertainties as to the FDA’s and other regulatory authorities’ interpretation of the data from the Company’s clinical trials and acceptance of the Company’s clinical programs and the regulatory approval process; whether the Company’s cash resources will be sufficient to fund the Company’s foreseeable and unforeseeable operating expenses and capital expenditure requirements; as well as the risks and uncertainties identified in the Company’s filings with the Securities and Exchange Commission (“SEC”), including the Company’s most recent Form 10-Q and in subsequent filings the Company may make with the SEC. In addition, the forward-looking statements included in this Current Report on Form 8-K represent the Company’s views as of the date hereof. The Company anticipates that subsequent events and developments will cause its views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date hereof.
| Item 9.01. | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit No. |
Description | |
| 99.1 | Press Release, dated May 20, 2024. | |
| 99.2 | Presentation, dated May 20, 2024. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| DYNE THERAPEUTICS, INC. | ||||||
| Date: May 20, 2024 | By: | /s/ John G. Cox |
||||
| Name: | John G. Cox | |||||
| Title: | President and Chief Executive Officer | |||||
Exhibit 99.1

Dyne Therapeutics Announces New Clinical Data from ACHIEVE Trial of DYNE-101 in DM1 and DELIVER Trial of DYNE-251 in DMD Demonstrating Compelling Impact on Key Disease Biomarkers and Improvement in Multiple Functional Endpoints
- In Phase 1/2 ACHIEVE Trial, DYNE-101 Demonstrated Dose Dependent 27% Mean Splicing Correction Across All Patients in the 5.4 mg/kg Cohort at 3 Months -
- DYNE-101 Showed Improvement in Myotonia, Muscle Strength, and Timed Function Tests and in DM1-ACTIVc and MDHI Patient Reported Outcomes -
- In Phase 1/2 DELIVER Trial, DYNE-251 Showed 3.2% Mean Unadjusted (7.6% Mean Muscle Adjusted) Dystrophin Expression at 6 Months in 10 mg/kg Cohort Administered Monthly; 10 Times Higher Level of Dystrophin Than Reported for Weekly Administered Standard of Care1-
- DYNE-251 Demonstrated Trends in Functional Improvement in NSAA, Time to Rise from Floor, 10-Meter Walk/Run Time, and Stride Velocity 95th Centile at 6 Months in 10 mg/kg Cohort -
- Favorable Safety Profiles for DYNE-101 and DYNE-251 with Enrollment Complete in the 6.8 mg/kg Cohort of ACHIEVE and 40 mg/kg Cohort of DELIVER -
- Based on Recent Regulatory Interactions, Continuing to Pursue Expedited Approvals for Both Programs with Update on Registrational Pathways Expected by Year-End -
- Virtual Investor Event Today, May 20 at 8:00 a.m. ET -
WALTHAM, Mass., May 20, 2024 – Dyne Therapeutics, Inc. (Nasdaq: DYN), a clinical-stage muscle disease company focused on advancing innovative life-transforming therapeutics for people living with genetically driven diseases, today announced positive clinical data from its ongoing Phase 1/2 ACHIEVE trial of DYNE-101 in patients with myotonic dystrophy type 1 (DM1) and its ongoing Phase 1/2 DELIVER trial of DYNE-251 in patients with Duchenne muscular dystrophy (DMD) who are amenable to exon 51 skipping. New data from both trials demonstrated compelling impact on key disease biomarkers as well as improvement in multiple functional endpoints and favorable safety profiles.
“We are excited to report new clinical data from both our ACHIEVE and DELIVER trials demonstrating meaningful impact on key biomarkers and functional improvement in multiple clinical endpoints that matter to patients. We believe these data reflect the best-in-class potential for these product candidates and reinforce the opportunity to transform the treatment of DM1 and DMD as well as the potential of the FORCE platform to address other rare muscle diseases,” said John Cox, Dyne’s president and chief executive officer.
“We believe the breadth and depth of these data are truly differentiating. Our robust preclinical work is translating into clinical benefit along with favorable safety profiles for both DYNE-101 and DYNE-251. In ACHIEVE, treatment with DYNE-101 demonstrated consistent, dose-dependent splicing correction, which led to an improvement in muscle strength, function, and patient reported outcomes.
1

In DELIVER, treatment with DYNE-251 resulted in dystrophin expression that exceeded levels that have been reported for the standard of care for DMD as well as trends in functional improvement earlier than expected,” said Wildon Farwell, M.D., MPH, Dyne’s chief medical officer. “The DM1 and Duchenne communities have waited too long for new and better therapeutic options. Building on the strength of these encouraging data and recent regulatory interactions, we look forward to continuing to engage with global regulatory authorities throughout this year to pursue expedited approval pathways and address the urgent unmet medical needs in these communities. We are thankful to the participants and clinicians in these trials and the communities for their continued partnership.”
Phase 1/2 ACHIEVE Trial of DYNE-101 in DM1
Efficacy Data
Dyne reported efficacy data from 40 adult DM1 patients enrolled in the randomized, placebo-controlled multiple ascending dose (MAD) portion of the DYNE-101 ACHIEVE trial, including 12-month data from the 1.8 mg/kg Q4W (approximate ASO dose) cohort (n=16), 6-month data from the 3.4 mg/kg Q4W cohort (n=16), and 3-month data from the 5.4 mg/kg Q8W cohort (n=8).
In the ACHIEVE trial, DYNE-101 demonstrated robust muscle delivery and dose-dependent, consistent splicing correction while also showing improvement in multiple functional endpoints and patient reported outcomes.
Key findings from ACHIEVE include:
Splicing
| • | DYNE-101 continued to show dose dependent splicing correction as seen in earlier cohorts. Patients in the 5.4 mg/kg Q8W cohort had a 27% mean splicing correction from baseline across a broad, 22-gene panel at 3 months, with all participants demonstrating splicing correction. |
Function
| • | Myotonia (vHOT): DYNE-101 demonstrated an improvement in myotonia as measured by video hand opening time (vHOT) in all reported cohorts. The 1.8 mg/kg Q4W group had a mean 3.1 second benefit in myotonia at 3 months that increased to 4.4 seconds at 12 months. In addition, the 5.4 mg/kg Q8W cohort had a mean 4.5 second improvement in myotonia at 3 months. |
| • | Strength and Timed Assessments: DYNE-101 demonstrated an improvement in muscle strength as measured by Quantitative Myometry Testing (QMT), a test of muscle strength and fatigue, and early and sustained potential benefit in 10-Meter Walk/Run Test and 5 Times Sit to Stand Test. |
Patient Reported Outcomes (PROs)
| • | Myotonic Dystrophy Health Index (MDHI): DYNE-101 demonstrated an overall improvement in the MDHI and benefit in all 17 subscales, including those that assess peripheral muscles, central nervous system, and gastrointestinal measures. These represent some of the most burdensome manifestations of DM1 and daily quality of life issues for patients and their families. |
2

| • | Myotonic Dystrophy Type 1 Activity and Participation Scale (DM1-ACTIVc): Treatment with DYNE-101 resulted in an improvement in the assessment of various activities of daily living, as measured by DM1-ACTIVc. |
Safety and Tolerability Data
| • | Dyne also reported safety and tolerability data from 56 patients enrolled through the 6.8 mg/kg Q8W cohort of the MAD portion of the ACHIEVE trial. DYNE-101 demonstrated a favorable safety profile.2 The majority of treatment emergent adverse events were mild or moderate and no related serious treatment emergent adverse events have been identified. |
| • | Enrollment is complete through the 6.8 mg/kg Q8W cohort. Approximately 500 doses have been administered to date, representing over 40-patient years of follow-up and supporting dose escalation up to 10.2 mg/kg. |
Phase 1/2 DELIVER Trial of DYNE-251 in DMD
Efficacy Data
Dyne reported efficacy data from 8 male patients with DMD amenable to exon 51 skipping enrolled in the 10 mg/kg (approximate PMO dose) cohort of the randomized, placebo-controlled MAD portion of the DYNE-251 DELIVER trial. Patients were randomized to receive either DYNE-251 (n=6) or placebo (n=2) once every four weeks for 6 months.
10 mg/kg of DYNE-251 Q4W demonstrated dose-dependent exon skipping and dystrophin expression. DYNE-251 reached levels of dystrophin expression that exceeded levels reported in a clinical trial for the current weekly standard of care for DMD exon 51, eteplirsen, at 6 months1 with a 12-fold lower PMO dose. DYNE-251 also demonstrated encouraging trends in multiple functional endpoints.
Key findings from DELIVER include:
Dystrophin expression measured by Western blot
| • | Patients treated with 10 mg/kg of DYNE-251 Q4W had a mean absolute dystrophin level of 3.22% of normal and a 2.97% change (unadjusted for muscle content) from baseline at 6 months. Eteplirsen reached a mean absolute unadjusted dystrophin level of 0.30% of normal and a 0.06% change from baseline at 6 months.1 When adjusting for muscle content, the DYNE-251 treated group reached 7.64% mean absolute dystrophin, which is greater than levels reported by peptide conjugate PMOs in clinical development.3 |
Function
| • | Trends in improvement were observed in multiple functional endpoints in the 10 mg/kg DYNE-251 Q4W group at 6 months, including North Star Ambulatory Assessment (NSAA), Stride Velocity 95th Centile (SV95C), 10-Meter Walk/Run Time, and Time to Rise from Floor. |
3

Safety and Tolerability Data
| • | Dyne also reported safety and tolerability data from 48 patients enrolled through the 40 mg/kg Q8W cohort of the MAD portion of the DELIVER trial. DYNE-251 demonstrated a favorable safety profile.4 The majority of treatment emergent adverse events were mild or moderate and no related serious treatment emergent adverse events have been identified. |
| • | Enrollment is complete through the 40 mg/kg cohort. Approximately 480 doses have been administered to date, representing over 35 patient-years of follow-up, supporting dose escalation up to 40 mg/kg. |
Key Milestones
ACHIEVE and DELIVER Trials
| • | Dyne plans to continue to engage with global regulators this year on ACHIEVE and DELIVER, and anticipates providing an update on the path to registration for both DYNE-101 and DYNE-251 by the end of 2024. Both trials are designed to be registrational, and the company is pursuing expedited approval pathways for both programs. |
| • | In DM1, based on recent dialogue with the Center for Drug Evaluation and Research (CDER) division of the U.S. Food and Drug Administration (FDA), Dyne continues to pursue an accelerated approval pathway for DYNE-101, including leveraging splicing as a potential surrogate biomarker. |
| • | In DMD, Dyne has confirmed that the FDA precedent for using dystrophin as a surrogate biomarker for accelerated approval remains available. |
FORCE™ Platform and Pipeline
| • | With ACHIEVE and DELIVER data continuing to reinforce the promise of the FORCE platform, Dyne expects to provide updates on programs, including facioscapulohumeral muscular dystrophy (FSHD) and other pipeline opportunities during 2024. |
Virtual Investor Event
Dyne will host a live video webcast event to discuss these ACHIEVE and DELIVER data today, May 20, 2024, at 8:00 a.m. ET. The live webcast will be available on the Events & Presentations page of the Investors & Media section of Dyne’s website and a replay will be accessible for 90 days following the presentation. An accompanying slide presentation will also be available. To access the presentation, register for the live webcast and replay, please visit https://investors.dyne-tx.com/news-and-events/events-and-presentations.
About ACHIEVE
ACHIEVE is a Phase 1/2 global clinical trial evaluating DYNE-101, consisting of a 24-week multiple ascending dose (MAD) randomized placebo-controlled period, a 24-week open-label extension and a 96-week long-term extension. The trial, which is designed to be registrational, is enrolling adult patients with myotonic dystrophy type 1 (DM1) who are 18 to 49 years of age. The primary endpoints are safety and tolerability, with secondary endpoints of pharmacokinetics and pharmacodynamics, including change from baseline in splicing, as well as measures of muscle strength and function. For more information on the ACHIEVE trial, visit https://www.clinicaltrials.gov/ (NCT05481879).
4

About DYNE-101
DYNE-101 is an investigational therapeutic being evaluated in the Phase 1/2 global ACHIEVE clinical trial for people living with DM1. DYNE-101 consists of an antisense oligonucleotide (ASO) conjugated to a fragment antibody (Fab) that binds to the transferrin receptor 1 (TfR1) which is highly expressed on muscle. It is designed to enable targeted muscle tissue delivery with the goal of reducing toxic DMPK RNA in the nucleus, releasing splicing proteins, allowing normal mRNA processing and translation of normal proteins, and potentially stopping or reversing the disease progression. DYNE-101 has been granted orphan drug designation by the U.S. Food and Drug Administration and the European Medicines Agency for the treatment of DM1.
About Myotonic Dystrophy Type 1 (DM1)
DM1 is a rare, progressive, genetic disease that affects skeletal, cardiac and smooth muscle. It is a monogenic, autosomal dominant disease caused by an abnormal trinucleotide expansion in a region of the DMPK gene. This expansion of CTG repeats causes toxic RNA to cluster in the nucleus, forming nuclear foci and altering the splicing of multiple proteins essential for normal cellular function. This altered splicing, or spliceopathy, results in a wide range of symptoms. People living with DM1 typically experience myotonia and progressive weakness of major muscle groups, which can affect mobility, breathing, heart function, speech, digestion and vision as well as cognition. DM1 is estimated to affect more than 40,000 people in the United States and over 74,000 people in Europe, but there are currently no approved disease-modifying therapies.
About the DELIVER Trial
DELIVER is a Phase 1/2 global clinical trial evaluating DYNE-251, consisting of a 24-week multiple ascending dose (MAD) randomized placebo-controlled period, a 24-week open-label extension and a 96-week long-term extension. The trial, which is designed to be registrational, is enrolling ambulant and non-ambulant males with Duchenne muscular dystrophy (DMD) who are ages 4 to 16 and have mutations amenable to exon 51 skipping. The primary endpoints are safety, tolerability and change from baseline in dystrophin levels as measured by Western blot. Secondary endpoints include measures of muscle function, exon skipping and pharmacokinetics. For more information on the DELIVER trial, visit https://www.clinicaltrials.gov/ (NCT05524883).
About DYNE-251
DYNE-251 is an investigational therapeutic being evaluated in the Phase 1/2 global DELIVER clinical trial for people living with DMD who are amenable to exon 51 skipping. DYNE-251 consists of a phosphorodiamidate morpholino oligomer (PMO) conjugated to a fragment antibody (Fab) that binds to the transferrin receptor 1 (TfR1) which is highly expressed on muscle. It is designed to enable targeted muscle tissue delivery and promote exon skipping in the nucleus, allowing muscle cells to create a truncated, functional dystrophin protein, with the goal of stopping or reversing disease progression. DYNE-251 has been granted fast track, orphan drug and rare pediatric disease designations by the U.S. Food and Drug Administration for the treatment of DMD mutations amenable to exon 51 skipping.
In addition to DYNE-251, Dyne is building a global DMD franchise and has preclinical programs targeting other exons, including 53, 45 and 44.
5

About Duchenne Muscular Dystrophy (DMD)
DMD is a rare disease caused by mutations in the gene that encodes for dystrophin, a protein critical for the normal function of muscle cells. These mutations, the majority of which are deletions, result in the lack of dystrophin protein and progressive loss of muscle function. DMD occurs primarily in males and affects an estimated 12,000 to 15,000 individuals in the U.S. and 25,000 in Europe. Loss of strength and function typically first appears in pre-school age boys and worsens as they age. As the disease progresses, the severity of damage to skeletal and cardiac muscle often results in patients experiencing total loss of ambulation by their early teenage years and includes worsening cardiac and respiratory symptoms and loss of upper body function by the later teens. There is no cure for DMD and currently approved therapies provide limited benefit.
About Dyne Therapeutics
Dyne Therapeutics is a clinical-stage muscle disease company focused on advancing innovative life-transforming therapeutics for people living with genetically driven diseases. With its proprietary FORCE™ platform, Dyne is developing modern oligonucleotide therapeutics that are designed to overcome limitations in delivery to muscle tissue. Dyne has a broad pipeline for serious muscle diseases, including clinical programs for myotonic dystrophy type 1 (DM1) and Duchenne muscular dystrophy (DMD) and a preclinical program for facioscapulohumeral muscular dystrophy (FSHD). For more information, please visit https://www.dyne-tx.com, and follow us on X, LinkedIn and Facebook.
Forward-Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this press release, including statements regarding Dyne’s strategy, future operations, prospects and plans, objectives of management, the potential of the FORCE platform, the anticipated timelines for reporting additional data from the ACHIEVE and DELIVER clinical trials and initiating registrational cohorts, expectations regarding the timing and outcome of interactions with global regulatory authorities and the availability of accelerated approval pathways for DYNE-101 and DYNE-251, and plans to provide future updates on pipeline programs, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “objective,” “ongoing,” “plan,” “predict,” “project,” “potential,” “should,” or “would,” or the negative of these terms, or other comparable terminology are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Dyne may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements.
6

Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various important factors, including: uncertainties inherent in the identification and development of product candidates, including the initiation and completion of preclinical studies and clinical trials; uncertainties as to the availability and timing of results from preclinical studies and clinical trials; the timing of and Dyne’s ability to enroll patients in clinical trials; whether results from preclinical studies and initial data from early clinical trials will be predictive of the final results of the clinical trials or future trials; uncertainties as to the FDA’s and other regulatory authorities’ interpretation of the data from Dyne’s clinical trials and acceptance of Dyne’s clinical programs and the regulatory approval process; whether Dyne’s cash resources will be sufficient to fund its foreseeable and unforeseeable operating expenses and capital expenditure requirements; as well as the risks and uncertainties identified in Dyne’s filings with the Securities and Exchange Commission (SEC), including the Company’s most recent Form 10-Q and in subsequent filings Dyne may make with the SEC. In addition, the forward-looking statements included in this press release represent Dyne’s views as of the date of this press release. Dyne anticipates that subsequent events and developments will cause its views to change. However, while Dyne may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing Dyne’s views as of any date subsequent to the date of this press release.
| 1. | No head-to-head trials have been conducted comparing DYNE-251 to eteplirsen. Eteplirsen data may not be directly comparable due to differences in trial protocols, dosing regimens and patient populations. Accordingly, these cross-trial comparisons may not be reliable. Eteplirsen data from J Neuromuscul Dis. 2021; 8(6): 989–1001 |
| 2. | DYNE-101 safety data as of May 8, 2024 |
| 3. | No head-to-head trials have been conducted comparing DYNE-251 to SRP-5051. SRP-5051 data may not be directly comparable due to differences in trial protocols, dosing regimens, methodologies for calculating muscle content adjusted dystrophin and patient populations. Accordingly, these cross-trial comparisons may not be reliable. SRP-5051 data from Clinical Update: MOMENTUM (Study SRP-5051-201, Part B) Jan. 29, 2024. |
| 4. | DYNE-251 safety data as of April 30, 2024 |
Contacts:
Investors
Amy Reilly
areilly@dyne-tx.com
857-341-1203

Media
Stacy Nartker
snartker@dyne-tx.com
781-317-1938
7

Exhibit 99.2 Achieving the Promise of FORCE to Deliver for Patients F O R C E ACHIEVE & DELIVER CLINICAL UPDATE | MAY 20, 2024

Forward-Looking Statements & Disclaimer This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding Dyne’s strategy, future operations, prospects and plans, objectives of management, the potential of the FORCE platform, the anticipated timelines for reporting additional data from the ACHIEVE and DELIVER clinical trials and initiating registrational cohorts, expectations regarding the timing and outcome of interactions with global regulatory authorities and the availability of accelerated approval pathways for DYNE-101 and DYNE-251, and plans to provide future updates on pipeline programs, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “objective,” “ongoing,” “plan,” “predict,” “project,” “potential,” “should,” or “would,” or the negative of these terms, or other comparable terminology are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Dyne may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various important factors, including: uncertainties inherent in the identification and development of product candidates, including the initiation and completion of preclinical studies and clinical trials; uncertainties as to the availability and timing of results from preclinical studies and clinical trials; the timing of and Dyne’s ability to enroll patients in clinical trials; whether results from preclinical studies and initial data from early clinical trials will be predictive of the final results of the clinical trials or future trials; uncertainties as to the FDA’s and other regulatory authorities’ interpretation of the data from Dyne's clinical trials and acceptance of Dyne's clinical programs and the regulatory approval process; whether Dyne’s cash resources will be sufficient to fund its foreseeable and unforeseeable operating expenses and capital expenditure requirements; as well as the risks and uncertainties identified in Dyne’s filings with the Securities and Exchange Commission (SEC), including the Company’s most recent Form 10-Q and in subsequent filings Dyne may make with the SEC. In addition, the forward-looking statements included in this presentation represent Dyne’s views as of the date of this presentation. Dyne anticipates that subsequent events and developments will cause its views to change. However, while Dyne may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing Dyne’s views as of any date subsequent to the date of this presentation. This presentation also contains estimates, projections and other statistical data made by independent parties and by the Company relating to market size and growth and other data about the Company’s industry and business. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. The Company has not independently verified the accuracy and completeness of the information obtained by third parties included in this presentation. In addition, projections, assumptions and estimates of the Company’s future performance and the future performance of the markets in which the Company operates are necessarily subject to a high degree of uncertainty and risk. 2

Program Opening remarks John Cox, President & CEO DYNE-101 ACHIEVE Trial in DM1 Data DYNE-251 DELIVER Trial in DMD Data Wildon Farwell, M.D., MPH, Chief Medical Officer Q&A Closing Remarks John Cox, President & CEO 3

OUR MISSION Life-transforming therapies for patients with serious muscle diseases 4
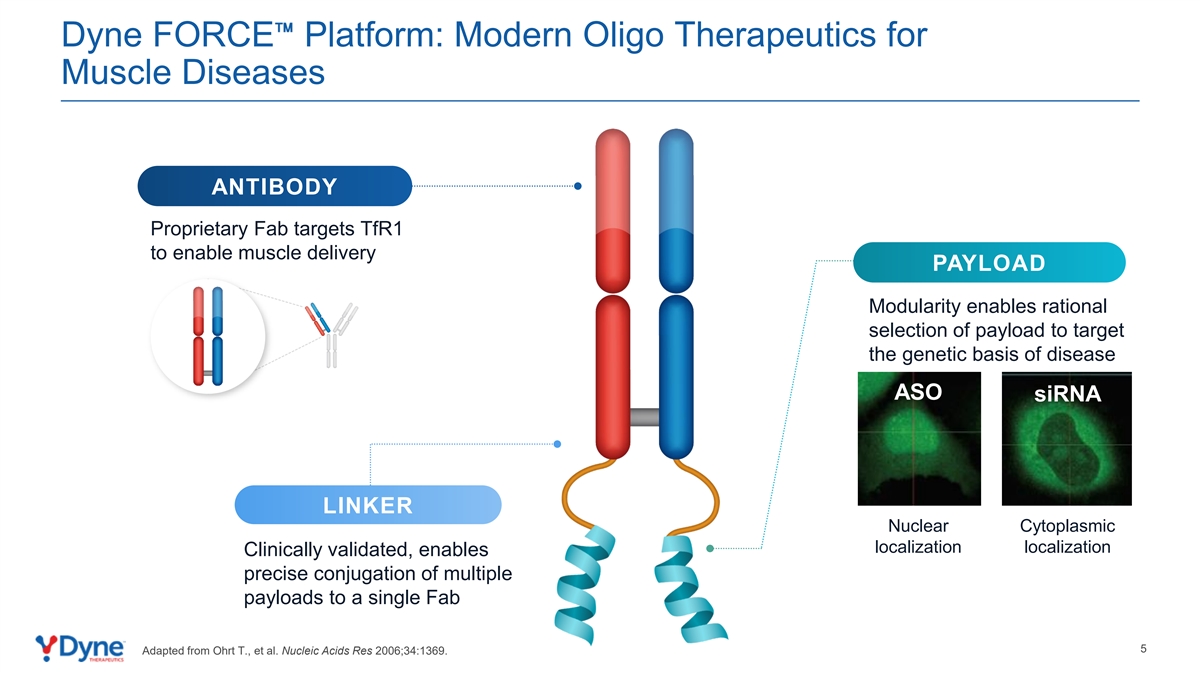
Dyne FORCE Platform: Modern Oligo Therapeutics for Muscle Diseases ANTIBODY Proprietary Fab targets TfR1 to enable muscle delivery PAYLOAD Modularity enables rational selection of payload to target the genetic basis of disease ASO siRNA LINKER Nuclear Cytoplasmic localization localization Clinically validated, enables precise conjugation of multiple payloads to a single Fab 5 Adapted from Ohrt T., et al. Nucleic Acids Res 2006;34:1369.
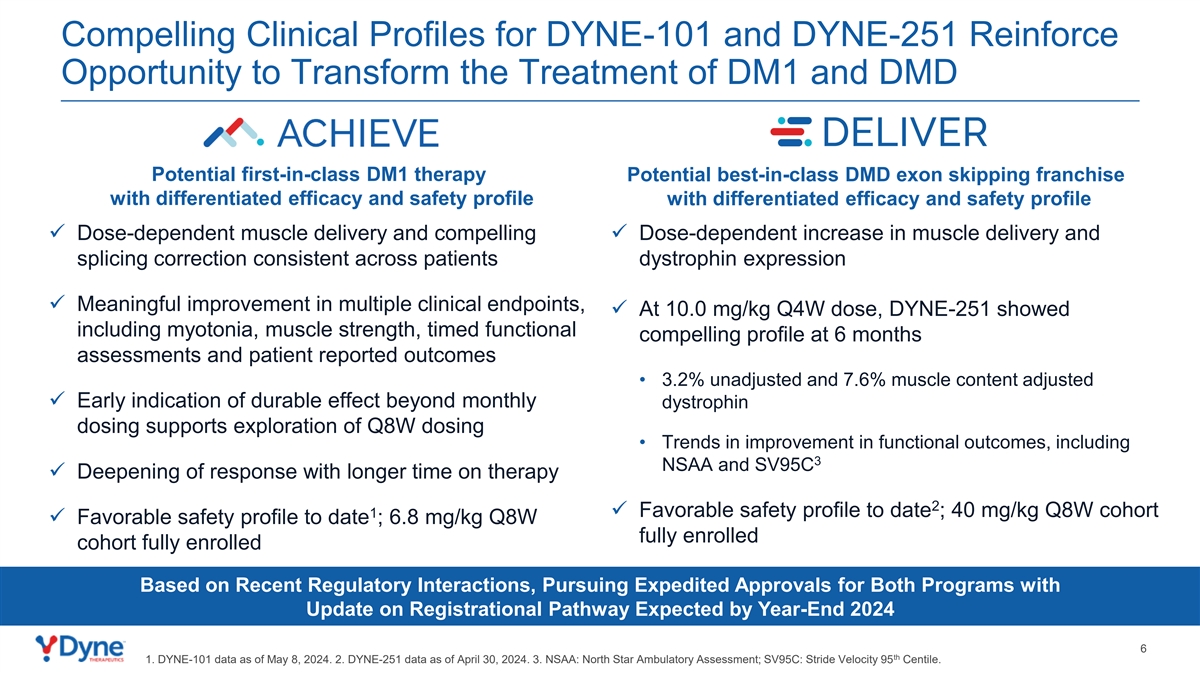
Compelling Clinical Profiles for DYNE-101 and DYNE-251 Reinforce Opportunity to Transform the Treatment of DM1 and DMD Potential first-in-class DM1 therapy Potential best-in-class DMD exon skipping franchise with differentiated efficacy and safety profile with differentiated efficacy and safety profile ✓ Dose-dependent muscle delivery and compelling ✓ Dose-dependent increase in muscle delivery and splicing correction consistent across patients dystrophin expression ✓ Meaningful improvement in multiple clinical endpoints, ✓ At 10.0 mg/kg Q4W dose, DYNE-251 showed including myotonia, muscle strength, timed functional compelling profile at 6 months assessments and patient reported outcomes • 3.2% unadjusted and 7.6% muscle content adjusted ✓ Early indication of durable effect beyond monthly dystrophin dosing supports exploration of Q8W dosing • Trends in improvement in functional outcomes, including 3 NSAA and SV95C ✓ Deepening of response with longer time on therapy 2 1✓ Favorable safety profile to date ; 40 mg/kg Q8W cohort ✓ Favorable safety profile to date ; 6.8 mg/kg Q8W fully enrolled cohort fully enrolled Based on Recent Regulatory Interactions, Pursuing Expedited Approvals for Both Programs with Update on Registrational Pathway Expected by Year-End 2024 6 th 1. DYNE-101 data as of May 8, 2024. 2. DYNE-251 data as of April 30, 2024. 3. NSAA: North Star Ambulatory Assessment; SV95C: Stride Velocity 95 Centile.

Program Opening remarks John Cox, President & CEO DYNE-101 ACHIEVE Trial in DM1 Data DYNE-251 DELIVER Trial in DMD Data Wildon Farwell, M.D., MPH, Chief Medical Officer Q&A Closing Remarks John Cox, President & CEO 7
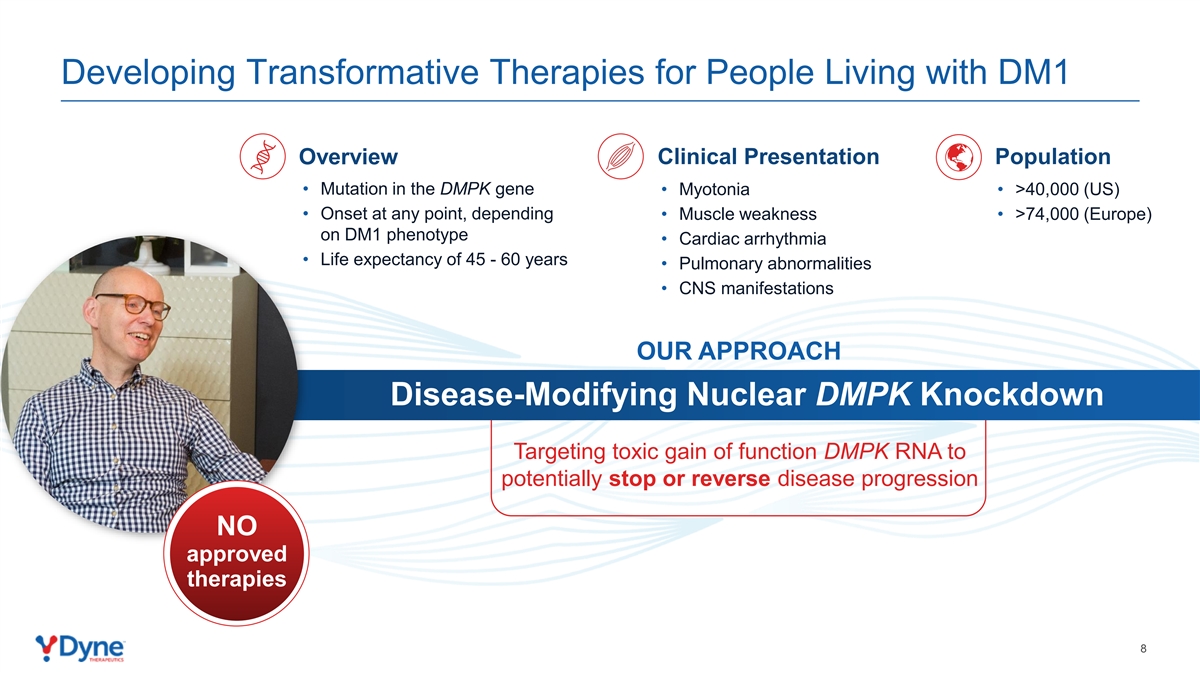
Developing Transformative Therapies for People Living with DM1 Clinical Presentation Population Overview • Mutation in the DMPK gene • Myotonia • >40,000 (US) • Onset at any point, depending • Muscle weakness • >74,000 (Europe) on DM1 phenotype • Cardiac arrhythmia • Life expectancy of 45 - 60 years • Pulmonary abnormalities • CNS manifestations OUR APPROACH Disease-Modifying Nuclear DMPK Knockdown Targeting toxic gain of function DMPK RNA to potentially stop or reverse disease progression NO approved therapies 8

DM1 Community Urgently Needs Treatment Options “In a nutshell, it's a huge, complex disease. It not only affects every muscle in your body, but also your brain, cognition, your stamina, your endurance. And also, I think myotonic dystrophy is not just a physical disability, it also involves mental health.” Sarah, living with DM1 9
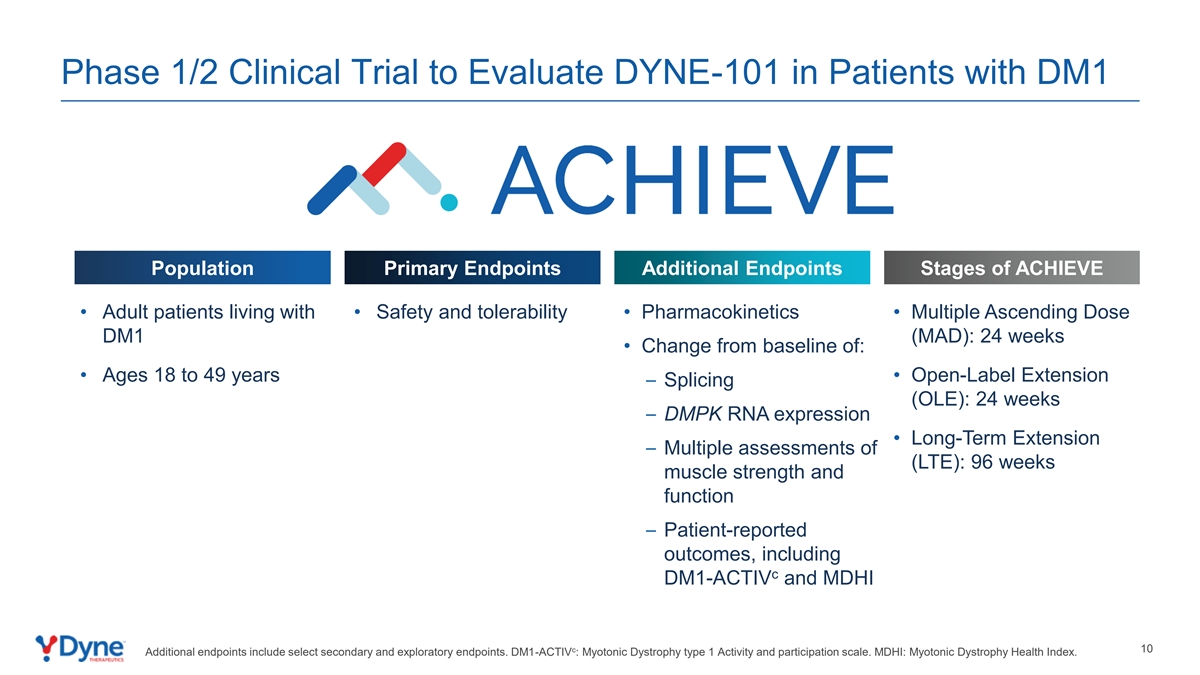
Phase 1/2 Clinical Trial to Evaluate DYNE-101 in Patients with DM1 Population Primary Endpoints Additional Endpoints Stages of ACHIEVE • Adult patients living with • Safety and tolerability • Pharmacokinetics • Multiple Ascending Dose DM1 (MAD): 24 weeks • Change from baseline of: • Ages 18 to 49 years • Open-Label Extension – Splicing (OLE): 24 weeks – DMPK RNA expression • Long-Term Extension – Multiple assessments of (LTE): 96 weeks muscle strength and function – Patient-reported outcomes, including c DM1-ACTIV and MDHI c 10 Additional endpoints include select secondary and exploratory endpoints. DM1-ACTIV : Myotonic Dystrophy type 1 Activity and participation scale. MDHI: Myotonic Dystrophy Health Index.

ACHIEVE Trial Design Global, Randomized, Placebo-Controlled Stage Evaluating Once Monthly or Less Frequent Administration of DYNE-101 in Adult Patients Living with DM1 MAD Study Details Registrational Cohort Dose: TBD (Up to 10.2 mg/kg, Q4W or Q8W) • IV administration of DYNE- 101 or placebo every 4 6.8 mg/kg (Cohort 3A) Fully enrolled weeks or every 8 weeks N=8 (3:1) Q8W with Booster, Placebo 5.4 mg/kg (Cohort 3B) Fully enrolled • Muscle biopsies: Baseline, 12 N=8 (3:1) Q8W with Booster, Placebo weeks, 24 weeks 3.4 mg/kg (Cohort 2B) N=8 (3:1) Q8W with Booster, Placebo • Patients in MAD study Fully enrolled 3.4 mg/kg (Cohort 2A) escalated to highest tolerable N=16 (3:3:2) Q4W, Recovery, Placebo dose in OLE and LTE 1.8 mg/kg (Cohort 1) Fully enrolled N=16 (3:3:2) Q4W, Recovery, Placebo Global Strategy & Adaptive Trial Design To Enable Rapid Achievement of Potentially Registrational Clinical Data Doses provided refer to ASO component of DYNE-101. Recovery cohort Q4W x 2 doses then placebo for the remainder of the 24W placebo-controlled period. Q8W with booster includes Q4W x 3 11 doses then Q8W dosing. Study protocol allows for dosing up to 10.2 mg/kg. Randomization

Dosing Schedules for Treatment Arms 1 Biopsy Placebo DYNE-101 Q4W Arm Recovery Arm Q8W Arm Baseline Week 4 Week 8 Week 12 Week 16 Week 20 Week 24 1. Needle biopsies taken from alternating TA muscles prior to dosing at baseline, Day 85, and Day 169. 12 Note: Represents dosing during 24-week multiple ascending dose (MAD) stage. Patients in MAD study escalated to highest tolerable dose in 24-week OLE and 96-week LTE.
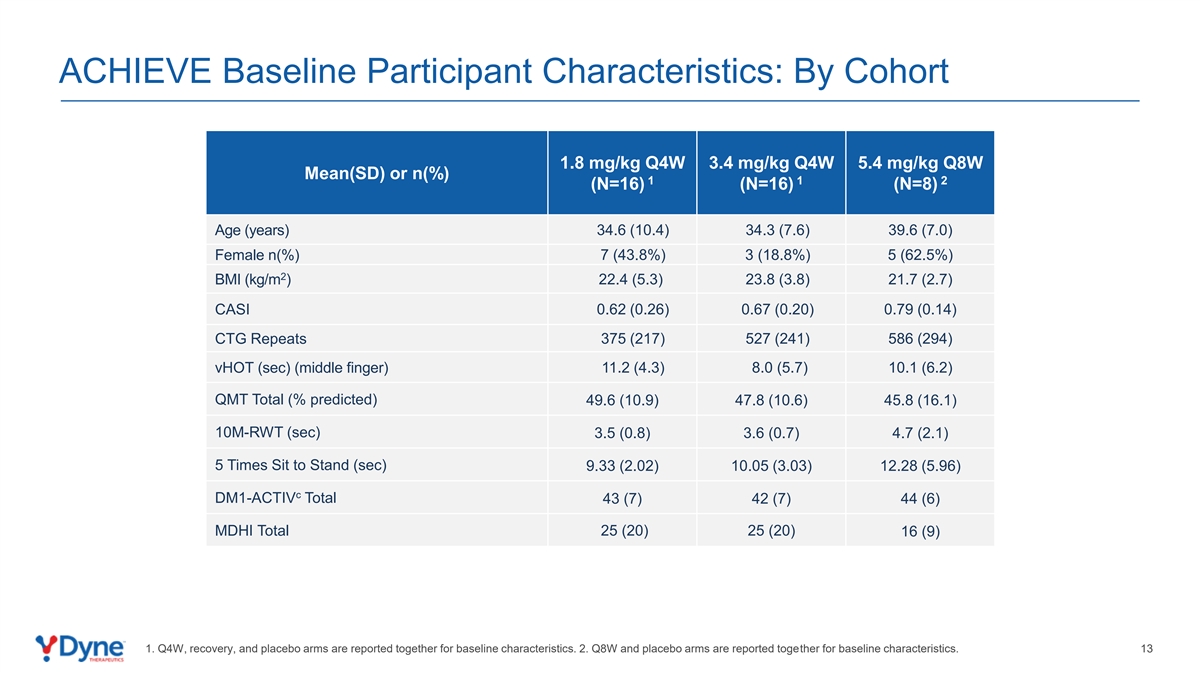
ACHIEVE Baseline Participant Characteristics: By Cohort 1.8 mg/kg Q4W 3.4 mg/kg Q4W 5.4 mg/kg Q8W Mean(SD) or n(%) 1 1 2 (N=16) (N=16) (N=8) Age (years) 34.6 (10.4) 34.3 (7.6) 39.6 (7.0) Female n(%) 7 (43.8%) 3 (18.8%) 5 (62.5%) 2 BMI (kg/m ) 22.4 (5.3) 23.8 (3.8) 21.7 (2.7) CASI 0.62 (0.26) 0.67 (0.20) 0.79 (0.14) CTG Repeats 375 (217) 527 (241) 586 (294) vHOT (sec) (middle finger) 11.2 (4.3) 8.0 (5.7) 10.1 (6.2) QMT Total (% predicted) 49.6 (10.9) 47.8 (10.6) 45.8 (16.1) 10M-RWT (sec) 3.5 (0.8) 3.6 (0.7) 4.7 (2.1) 5 Times Sit to Stand (sec) 9.33 (2.02) 10.05 (3.03) 12.28 (5.96) c DM1-ACTIV Total 43 (7) 42 (7) 44 (6) MDHI Total 25 (20) 25 (20) 16 (9) 1. Q4W, recovery, and placebo arms are reported together for baseline characteristics. 2. Q8W and placebo arms are reported together for baseline characteristics. 13

PRO Safety Muscle Delivery Splicing Function DYNE-101 Safety Profile Is Favorable to Date 1 Summary of Treatment Emergent Adverse Events (TEAEs) Most TEAEs Were Mild or Moderate in Intensity • 4 serious TEAEs unrelated to study drug 3 Participants with ≥1 TEAE – n (%)▪ Atrioventricular block first degree (1) ▪ Pneumonia (1) TEAE 1.8 mg/kg 3.4 mg/kg 3.4 mg/kg 5.4 mg/kg 6.8 mg/kg Overall 4 Category ▪ Pulmonary embolism (2) Q4W+Rec. Q4W+Rec. Q8W Q8W Q8W 5 N=16 N=16 N=8 N=8 N=8 (N=56) • Most common TEAEs (≥10% participant incidence) ▪ Nasopharyngitis (20%) Any TEAE 16 (100%) 16 (100%) 7 (88%) 8 (100%) 6 (75%) 53 (95%) ▪ Procedural pain (18%) Any related 7 (44%) 6 (38%) 1 (13%) 3 (38%) 5 (63%) 22 (39%)▪ Influenza; pyrexia (each 16%) TEAE ▪ Diarrhea; headache (each 14%) Any serious ▪ Back pain (13%) 4 (25%) 0 0 0 0 4 (7%) TEAE Additional Safety Data Any serious related 0 0 0 0 0 0 • Liver enzyme elevations have been observed in ~19% of TEAE participants ▪ No impact on liver function (bilirubin or coagulation) Any TEAE 2 2 ▪ Interpretation is complicated by underlying disease and elevated leading to 1 (6%) 0 0 0 0 1 (2%) baseline values up to ~2.5x greater than the upper limit of normal withdrawal • No participants have demonstrated persistent related anemia or Any TEAE thrombocytopenia leading to 0 0 0 0 0 0 death ~500 Doses Administered to Date Representing Over 40-patient Years of Follow-Up 1. Data as of May 8, 2024. 2. Single participant withdrawal due to infusion related reaction in absence of respiratory signs or symptoms after completing MAD and receiving multiple doses of study drug in OLE. 3.Transient worsening of atrioventricular (AV) block in a participant with ongoing medical history of first-degree AV block. 4. Attributed to risk factors for pulmonary embolism. 5. All cohorts combined; preferred terms are reported 14
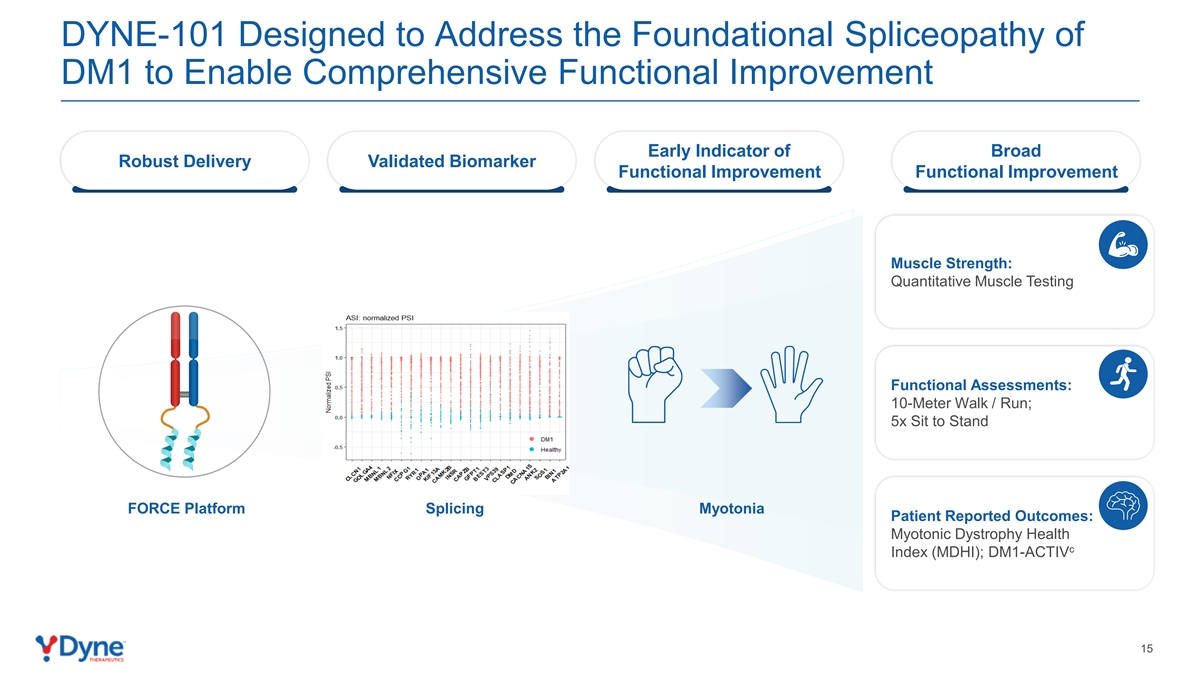
DYNE-101 Designed to Address the Foundational Spliceopathy of DM1 to Enable Comprehensive Functional Improvement Early Indicator of Broad Robust Delivery Validated Biomarker Functional Improvement Functional Improvement Muscle Strength: Quantitative Muscle Testing Functional Assessments: 10-Meter Walk / Run; 5x Sit to Stand FORCE Platform Splicing Myotonia Patient Reported Outcomes: Myotonic Dystrophy Health c Index (MDHI); DM1-ACTIV 15

PRO Safety Muscle Delivery Splicing Function DMCRN NHS Enabled Establishment of Composite Alternative Splicing Index (CASI) as Biomarker Correlating with Clinical Function in DM1 CASI: Composite Alternative PSI = Percent Spliced In ASI: Alternative Splicing Index Splicing Index Compute the mean of normalized PSI Normalize to reference PSI from Targeted sequencing reads to from a panel of 22 genes. healthy controls and patients calculate Percent Spliced In (PSI) of 0 representing median score of healthy 1 from DM1 natural history studies specific exons subjects; 1 representing 95th percentile severity of DM1 patients 1. Reference PSI described within Thornton CA et al., Lancet Neurol, 2023; 22: 218–228; DMCRN NHS: Myotonic Dystrophy Clinical Research Network natural history study. 16
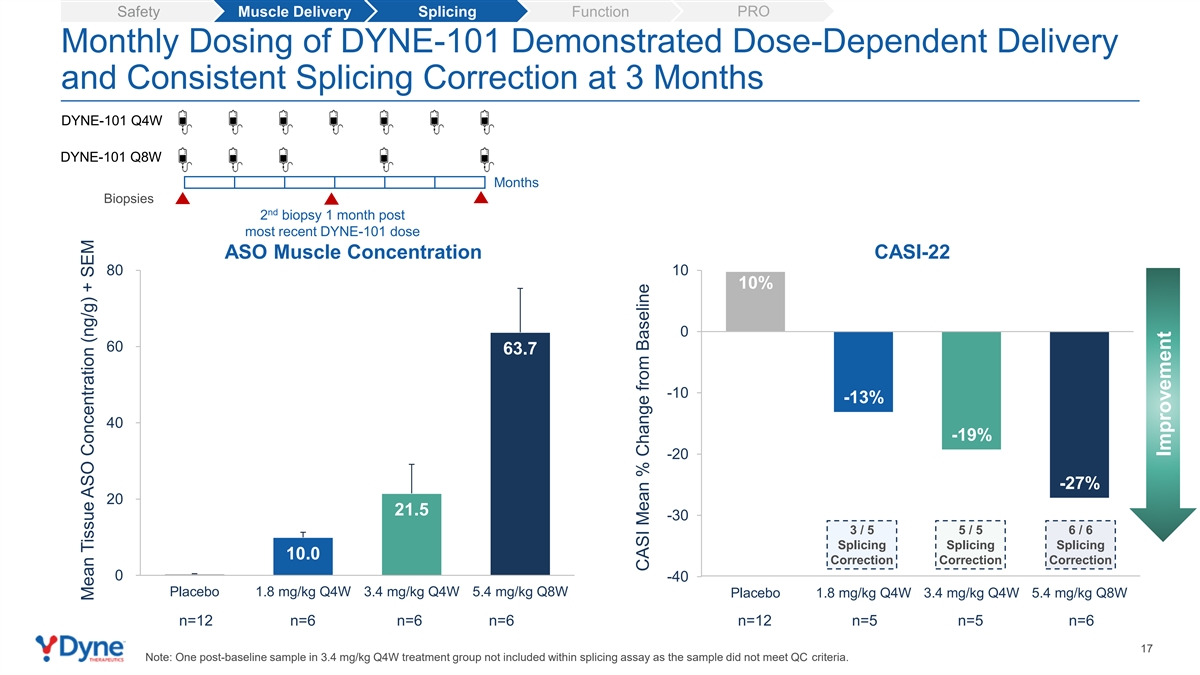
PRO Safety Muscle Delivery Splicing Function Monthly Dosing of DYNE-101 Demonstrated Dose-Dependent Delivery and Consistent Splicing Correction at 3 Months DYNE-101 Q4W DYNE-101 Q8W Months Biopsies nd 2 biopsy 1 month post most recent DYNE-101 dose ASO Muscle Concentration CASI-22 80 10 10% 0 60 63.7 -10 -13% 40 -19% -20 -27% 20 21.5 -30 3 / 5 5 / 5 6 / 6 Splicing Splicing Splicing 10.0 Correction Correction Correction 0 -40 Placebo 1.8 mg/kg Q4W 3.4 mg/kg Q4W 5.4 mg/kg Q8W Placebo 1.8 mg/kg Q4W 3.4 mg/kg Q4W 5.4 mg/kg Q8W n=12 n=6 n=6 n=6 n=12 n=5 n=5 n=6 17 Note: One post-baseline sample in 3.4 mg/kg Q4W treatment group not included within splicing assay as the sample did not meet QC criteria. Mean Tissue ASO Concentration (ng/g) + SEM CASI Mean % Change from Baseline Improvement

PRO Safety Muscle Delivery Splicing Function Recovery Data Supports Less Frequent Dosing Regimen CASI-22 Q4W Active Arm CASI-22 at 3 Months 15 DYNE-101 Months 10% Biopsies 5 nd 2 biopsy 1 month post most recent DYNE-101 dose -5 Q4W Recovery Arm -15 Placebo -19% 3% DYNE-101 -22% Months -25 Biopsies nd Placebo 3.4 mg/kg Q4W 3.4 mg/kg Recovery 2 biopsy 2 months post most recent DYNE-101 dose n=12 n=5 n=6 Robust Splicing Correction in Both Q4W and Recovery Arm with 3.4 mg/kg -22% Dose 22% 18 CASI Mean % Change from Baseline Improvement
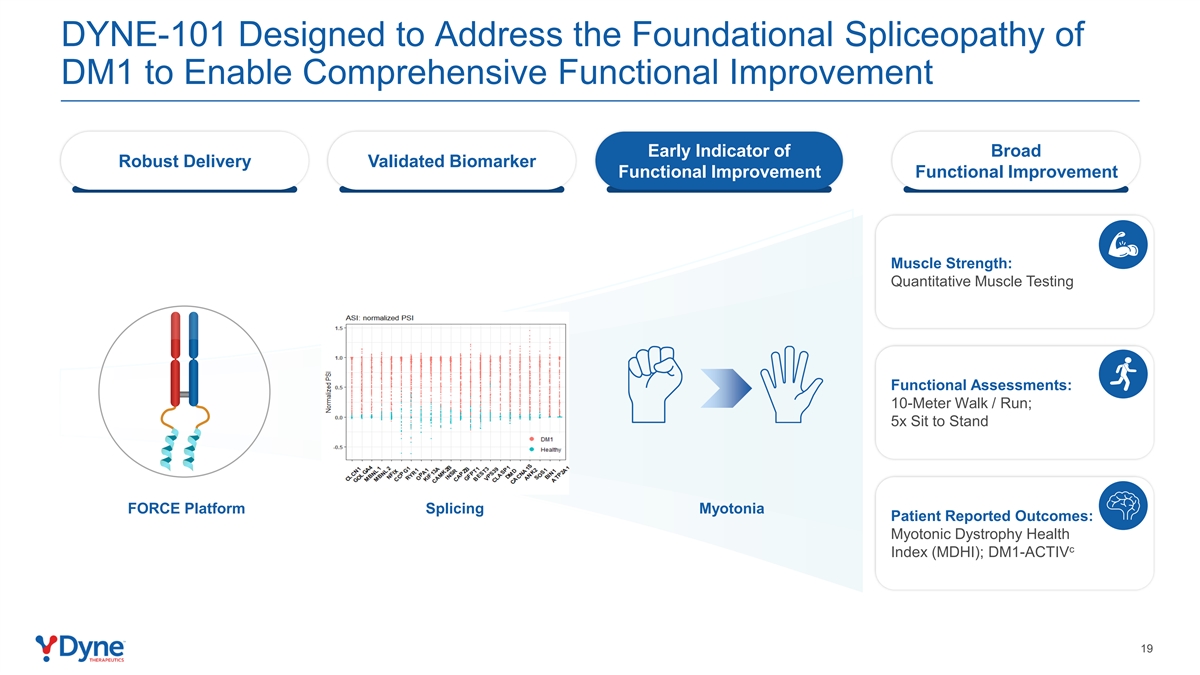
DYNE-101 Designed to Address the Foundational Spliceopathy of DM1 to Enable Comprehensive Functional Improvement Early Indicator of Broad Robust Delivery Validated Biomarker Functional Improvement Functional Improvement Muscle Strength: Quantitative Muscle Testing Functional Assessments: 10-Meter Walk / Run; 5x Sit to Stand FORCE Platform Splicing Myotonia Patient Reported Outcomes: Myotonic Dystrophy Health c Index (MDHI); DM1-ACTIV 19

PRO Safety Muscle Delivery Splicing Function Continued Improvement in Functional Myotonia at 6 and 12 Months 1.8 mg/kg Q4W myotonia benefit increased from 3.1 seconds at 3 Months to 4.4 seconds at 12 Months vHOT Middle Finger Change from Baseline 2.5 +0.7 sec., +9% change from baseline 0.0 -2.5 -3.1 sec., -5.0 -28% change from baseline -3.8 sec., -4.4 sec., -34% change from baseline -39% change from baseline -7.5 Baseline Day 85 Day 169 OLE Day 337 Visit: Cohort 3.4 mg/kg Q4W (n = 6) 5.4 mg/kg Q8W (n = 6) Placebo (n = 12) 1.8 mg/kg Q4W (n = 6) Baseline vHot: 7.46 (0.92) 11.25 (1.78) 6.58 (1.6) 11.92 (2.32) Mean (SE) 1. Middle Finger (sec) is the average of all myotonia trials for an individual participant in ACHIEVE. Note: Placebo group includes 12 participants at Day 85 and 8 participants at Day 169. Mean percent change from baseline for placebo group are based on baseline values from 12 patients. 20 1 Middle Finger (Sec) Change from Baseline +/- SEM Improvement
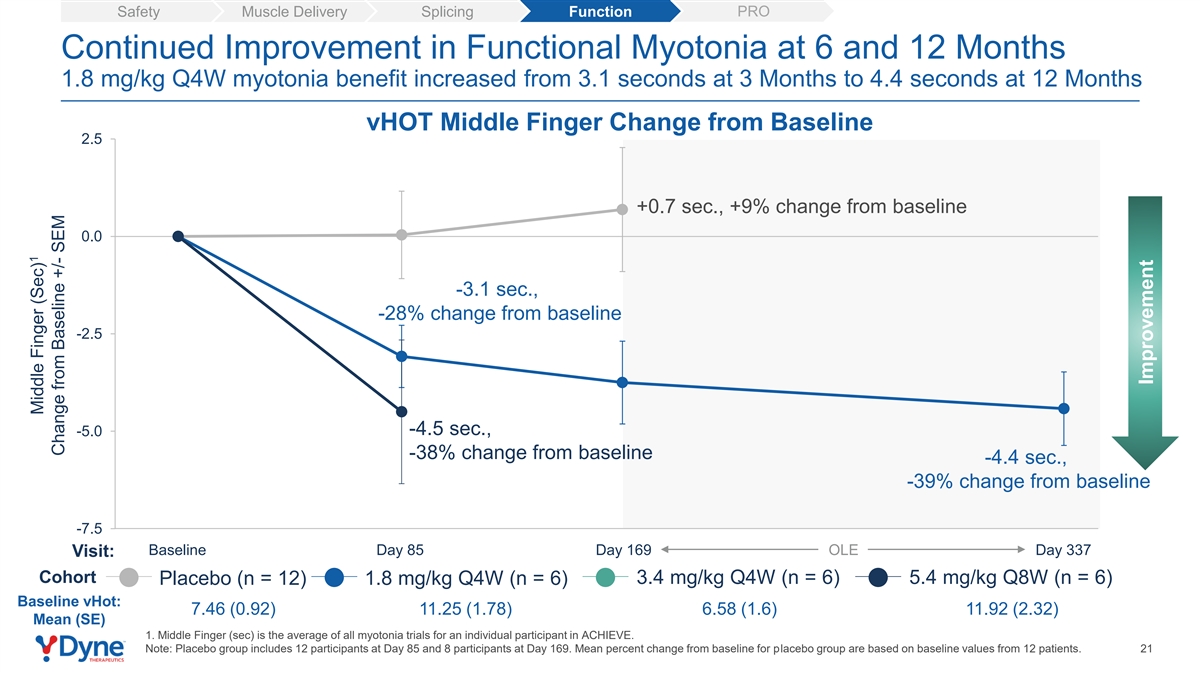
PRO Safety Muscle Delivery Splicing Function Continued Improvement in Functional Myotonia at 6 and 12 Months 1.8 mg/kg Q4W myotonia benefit increased from 3.1 seconds at 3 Months to 4.4 seconds at 12 Months vHOT Middle Finger Change from Baseline 2.5 +0.7 sec., +9% change from baseline 0.0 -3.1 sec., -28% change from baseline -2.5 -4.5 sec., -5.0 -38% change from baseline -4.4 sec., -39% change from baseline -7.5 Baseline Day 85 Day 169 OLE Day 337 Visit: Cohort 3.4 mg/kg Q4W (n = 6) 5.4 mg/kg Q8W (n = 6) Placebo (n = 12) 1.8 mg/kg Q4W (n = 6) Baseline vHot: 7.46 (0.92) 11.25 (1.78) 6.58 (1.6) 11.92 (2.32) Mean (SE) 1. Middle Finger (sec) is the average of all myotonia trials for an individual participant in ACHIEVE. Note: Placebo group includes 12 participants at Day 85 and 8 participants at Day 169. Mean percent change from baseline for placebo group are based on baseline values from 12 patients. 21 1 Middle Finger (Sec) Change from Baseline +/- SEM Improvement
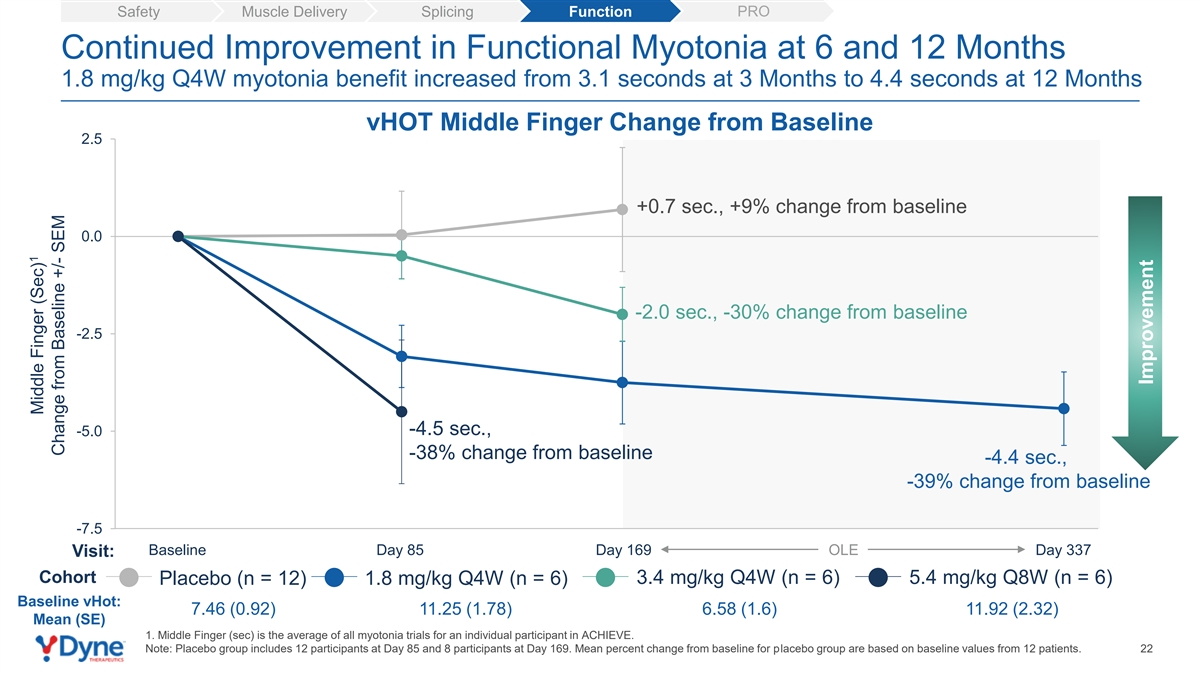
PRO Safety Muscle Delivery Splicing Function Continued Improvement in Functional Myotonia at 6 and 12 Months 1.8 mg/kg Q4W myotonia benefit increased from 3.1 seconds at 3 Months to 4.4 seconds at 12 Months vHOT Middle Finger Change from Baseline 2.5 +0.7 sec., +9% change from baseline 0.0 -2.0 sec., -30% change from baseline -2.5 -4.5 sec., -5.0 -38% change from baseline -4.4 sec., -39% change from baseline -7.5 Baseline Day 85 Day 169 OLE Day 337 Visit: Cohort 3.4 mg/kg Q4W (n = 6) 5.4 mg/kg Q8W (n = 6) Placebo (n = 12) 1.8 mg/kg Q4W (n = 6) Baseline vHot: 7.46 (0.92) 11.25 (1.78) 6.58 (1.6) 11.92 (2.32) Mean (SE) 1. Middle Finger (sec) is the average of all myotonia trials for an individual participant in ACHIEVE. Note: Placebo group includes 12 participants at Day 85 and 8 participants at Day 169. Mean percent change from baseline for placebo group are based on baseline values from 12 patients. 22 1 Middle Finger (Sec) Change from Baseline +/- SEM Improvement

DYNE-101 Designed to Address the Foundational Spliceopathy of DM1 to Enable Comprehensive Functional Improvement Early Indicator of Broad Robust Delivery Validated Biomarker Functional Improvement Functional Improvement Muscle Strength: Quantitative Muscle Testing Functional Assessments: 10-Meter Walk / Run; 5x Sit to Stand FORCE Platform Splicing Myotonia Patient Reported Outcomes: Myotonic Dystrophy Health c Index (MDHI); DM1-ACTIV 23

PRO Safety Muscle Delivery Splicing Function DYNE-101 Demonstrated Improvement in Muscle Strength Measured by Quantitative Muscle Testing (QMT) QMT Total Score 5.0 2.5 0.0 -2.5 -5.0 -7.5 OLE Baseline Day 85 Day 169 Day 337 Visit: 3.4 mg/kg Q4W (n = 6) 5.4 mg/kg Q8W (n = 6) Placebo (n = 12) 1.8 mg/kg Q4W (n = 6) Note: Placebo group includes 12 participants at Day 85 and 8 participants at Day 169. 24 QMT Total (%p) Change from Baseline +/- SEM Improvement
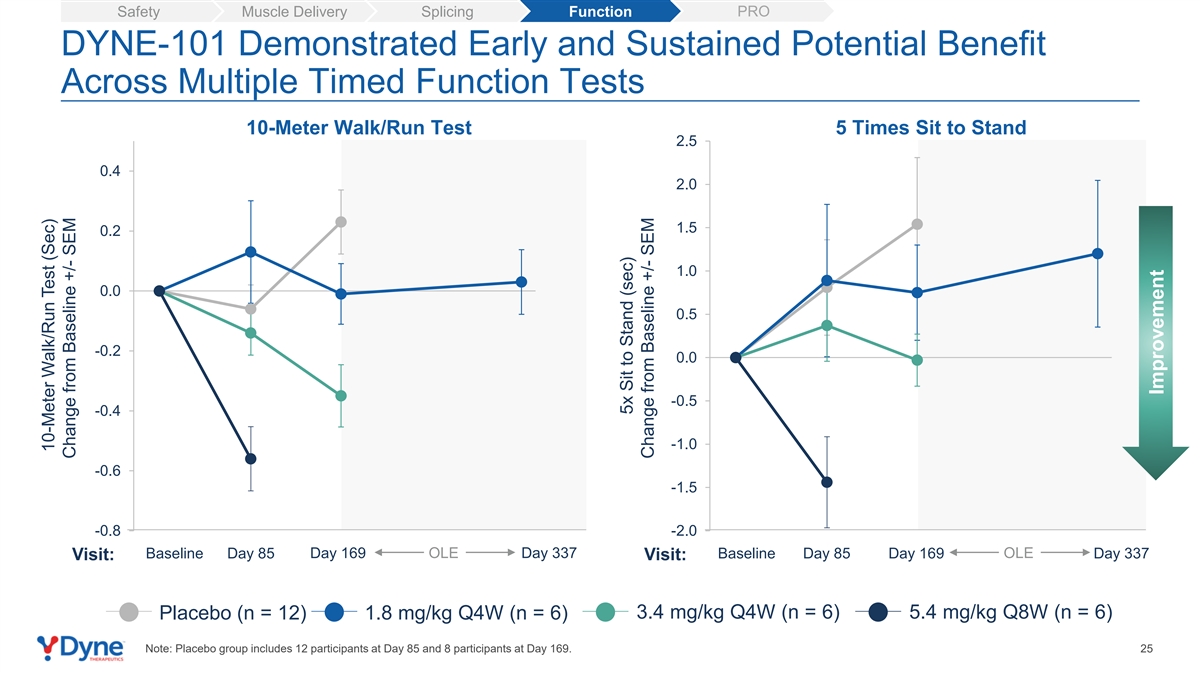
PRO Safety Muscle Delivery Splicing Function DYNE-101 Demonstrated Early and Sustained Potential Benefit Across Multiple Timed Function Tests 10-Meter Walk/Run Test 5 Times Sit to Stand 2.5 0.4 2.0 1.5 0.2 1.0 0.0 0.5 -0.2 0.0 -0.5 -0.4 -1.0 -0.6 -1.5 -0.8 -2.0 Baseline Day 85 Day 169 OLE Day 337 Baseline Day 85 Day 169 OLE Day 337 Visit: Visit: 3.4 mg/kg Q4W (n = 6) 5.4 mg/kg Q8W (n = 6) Placebo (n = 12) 1.8 mg/kg Q4W (n = 6) Note: Placebo group includes 12 participants at Day 85 and 8 participants at Day 169. 25 10-Meter Walk/Run Test (Sec) Change from Baseline +/- SEM 5x Sit to Stand (sec) Change from Baseline +/- SEM Improvement

PRO Safety Muscle Delivery Splicing Function DYNE-101 Showed Improvement from Baseline in Activities of Daily Living c Measured by DM1-ACTIV Patient Reported Outcome c DM1-ACTIV 5.0 2.5 0.0 -2.5 -5.0 -7.5 OLE Baseline Day 169 Day 337 Visit: 3.4 mg/kg Q4W (n = 6) Placebo (n = 8) 1.8 mg/kg Q4W (n = 6) Notes: Patient-reported outcomes (PRO), including DM1-ACTIVc, collected at baseline, day 169 and day 337. 26 c DM1-ACTIV Change from Baseline +/- SEM Improvement

PRO Safety Muscle Delivery Splicing Function DYNE-101 Demonstrated Clinical Benefit Based on Well-Validated PRO Showed Benefit in 17 out of 17 MDHI Subscales Upper Extremity Function Improved MDHI Total Score 15.0 20.0 10.0 0.0 -10.0 10.0 -20.0 Baseline Day 169 OLE Day 337 5.0 Gastrointestinal Issues 10.0 0.0 0.0 -10.0 -20.0 -5.0 OLE Baseline Day 169 Day 337 Fatigue -10.0 10.0 0.0 -10.0 -15.0 -20.0 -30.0 Visit: Baseline Day 169 OLE Day 337 -40.0 OLE Baseline Day 169 Day 337 Placebo (n = 8) 1.8 mg/kg Q4W (n = 6) 3.4 mg/kg Q4W (n = 6) Note: Patient-reported outcomes (PRO), including Myotonic Dystrophy Health Index (MDHI) collected at baseline, day 169 and day 337. 27 MDHI Total Score Change from Baseline +/- SEM MDHI Total and Subscale Improvement
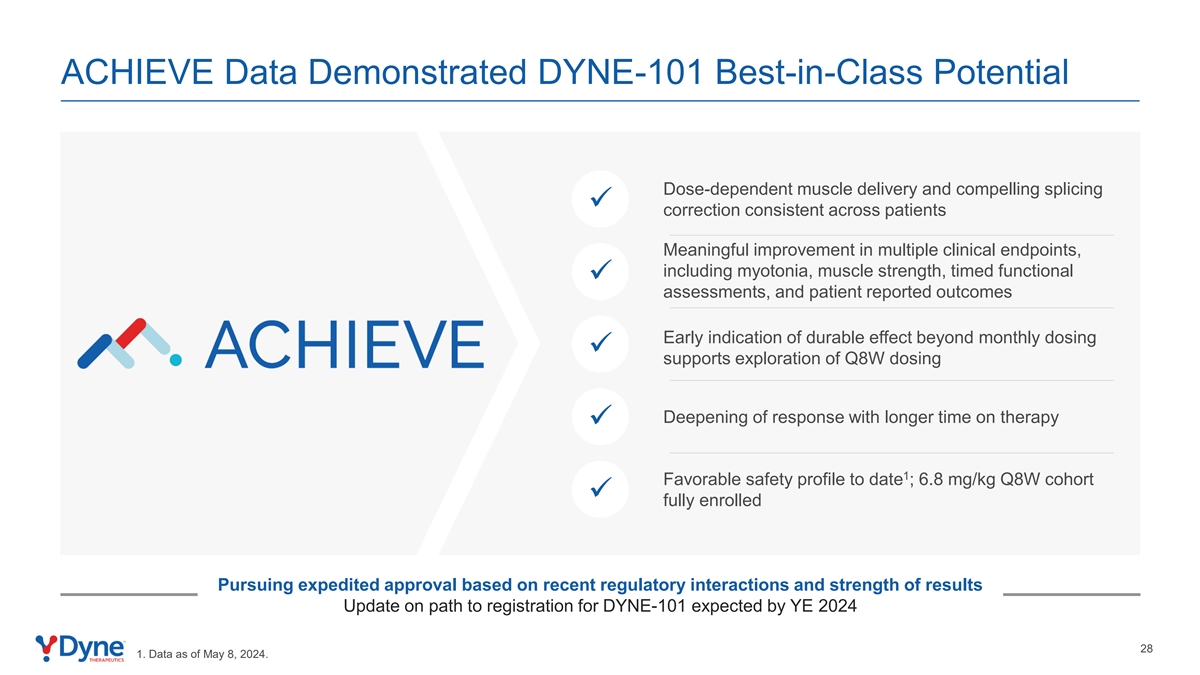
ACHIEVE Data Demonstrated DYNE-101 Best-in-Class Potential Dose-dependent muscle delivery and compelling splicing ✓ correction consistent across patients Meaningful improvement in multiple clinical endpoints, including myotonia, muscle strength, timed functional ✓ assessments, and patient reported outcomes Early indication of durable effect beyond monthly dosing ✓ supports exploration of Q8W dosing Deepening of response with longer time on therapy ✓ 1 Favorable safety profile to date ; 6.8 mg/kg Q8W cohort ✓ fully enrolled Pursuing expedited approval based on recent regulatory interactions and strength of results Update on path to registration for DYNE-101 expected by YE 2024 28 1. Data as of May 8, 2024.

Building a Global DMD Franchise of Transformative Therapies Clinical Presentation Population Overview • Mutation in the DMD gene that • Muscle weakness • ~12,000 - 15,000 (US) encodes for dystrophin • Progressive loss of function • ~ 25,000 (Europe) • Onset in first few years of life • Loss of ambulation • Life expectancy ~30 years • Respiratory/cardiac failure OUR APPROACH Potential Best-in-class Targeted Exon Skipping Increase dystrophin expression and enable less frequent dosing to potentially Current Approved stop or reverse disease progression Exon 51 Therapies Only Increased Dystrophin Production <1% 29

DMD Community Has Urgent Need for Improved Treatment Options “A potentially transformative treatment for me would be halting the progression of DMD and that would change everything for me and so many other people with it. And because right now the progression of it, yeah, it's very tough. Great people are dying every day from it. It's always a big surprise, people die so suddenly from this. So, I think halting the progression would really change everything.” Alan, living with DMD 30

Phase 1/2 Clinical Trial to Evaluate DYNE-251 in Patients with DMD Additional Endpoints Population Primary Endpoints Stages of DELIVER • Male patients with DMD • Safety and tolerability • Pharmacokinetics • Multiple Ascending Dose with mutations amenable (MAD): 24 weeks • Change from baseline in • Change from baseline of: to exon 51 skipping dystrophin protein levels • Open-Label Extension – Exon 51 skipping therapy by Western Blot (OLE): 24 weeks levels • Ages 4 to 16 years • Long-Term Extension – Muscle tissue PDPF • Ambulant and non- (LTE): 96 weeks – Multiple assessments ambulant of muscle function, including NSAA score, SV95C and certain timed functional tests Note: Additional endpoints include select secondary and exploratory endpoints 31 th PDPF: percent dystrophin-positive fibers; NSAA: North Star Ambulatory Assessment; SV95C: Stride Velocity 95 Centile.

DELIVER Trial Design Global, Randomized, Placebo-Controlled Stage Evaluating Administration of DYNE-251 in Ambulant and Non-Ambulant Male DMD Patients with Mutations Amenable to Exon 51 Skipping Therapy Registrational Cohort MAD Study Details Dose: TBD (Up to 40 mg/kg, Q4W or Q8W) Fully • IV administration of DYNE- 40 mg/kg Q8W Dose Optimization N=8 (3:1) enrolled 251 or placebo every 4 weeks Cohorts or every 8 weeks 20 mg/kg Q4W Fully enrolled N=8 (3:1) • Muscle biopsies: Baseline 10 mg/kg Q4W Fully enrolled and 24 weeks* N=8 (3:1) 5 mg/kg Q4W • Patients in MAD study Fully enrolled Starting Dose N=6 (2:1) escalated to highest tolerable Cohorts 2.8 mg/kg Q4W dose in OLE and LTE Fully enrolled N=6 (2:1) 1.4 mg/kg Q4W* Fully enrolled N=6 (2:1) 0.7 mg/kg Q4W* Fully enrolled N=6 (2:1) Global Trial Designed to be Registrational and to Enable Rapid Achievement of Predicted Pharmacologically Active Dose Levels Doses provided refer to PMO component of DYNE-251. Cohorts randomized to active arm or placebo. Study protocol allows for dosing up to 40 mg/kg. 32 * Muscle biopsies taken at baseline and 24 weeks in 2.8 mg/kg Q4W cohort to 20 mg/kg Q4W cohort; muscle biopsies taken at baseline and 48 weeks in 40 mg/kg Q8W cohort; biopsies not taken in 0.7 mg/kg and 1.4 mg/kg cohorts. Ran Rando domi miz zat atiio on n

DELIVER Baseline Participant Characteristics: By Cohort mean (SD) or n(%) 0.7 mg/kg 1.4 mg/kg 2.8 mg/kg 5 mg/kg 10 mg/kg (N=6) (N=6) (N=6) (N=6) (N=8) Age (years) 10.8 (2.2) 7.8 (3.3) 10.7 (2.9) 8.3 (2.8) 6.6 (2.2) 2 BMI (kg/m ) 19.5 (3.4) 18.6 (2.3) 22.6 (6.3) 20.9 (1.6) 18.3 (3.2) Age of Symptom Onset (years) 3.7 (1.8) 4.5 (2.1) 2.8 (1.8) 3.7 (3.1) 2.8 (1.6) 1 Corticosteroid dosing regimen (n (%)) Daily 4 (66.7%) 4 (66.7%) 5 (83.3%) 6 (100.0%) 8 (100.0%) Other 2 (33.3%) 3 (50.0%) 1 (16.7%) 0 0 Prior DMD Therapy (n (%)) Eteplirsen 4 (66.7%) 2 (33.3%) 5 (83.3%) 1 (16.7%) 1 (12.5%) Other 2 (33.3%) 1 (16.7%) 0 0 1 (12.5%) NSAA Total Score 22.2 (7.2) 22.8 (10.5) 20.3 (9.0) 21.0 (7.0) 25.3 (6.4) 10 Meter Run/Walk (sec) 6.1 (1.5) 6.3 (5.2) 6.9 (3.6) 5.1 (1.5) 4.6 (1.9) Time Rise From Floor (sec) 8.5 (4.0) 3.1 (0.3) 6.9 (4.9) 5.0 (2.6) 6.3 (5.6) th Stride Velocity 95 Percentile (m/sec) N/A N/A N/A N/A 1.9 (0.5) 33 Note: Q4W and placebo arms are reported together for baseline characteristics. N/A: not applicable as data not collected. 1. Historical corticosteroid regimen based on medical history; a participant can be counted in multiple categories.
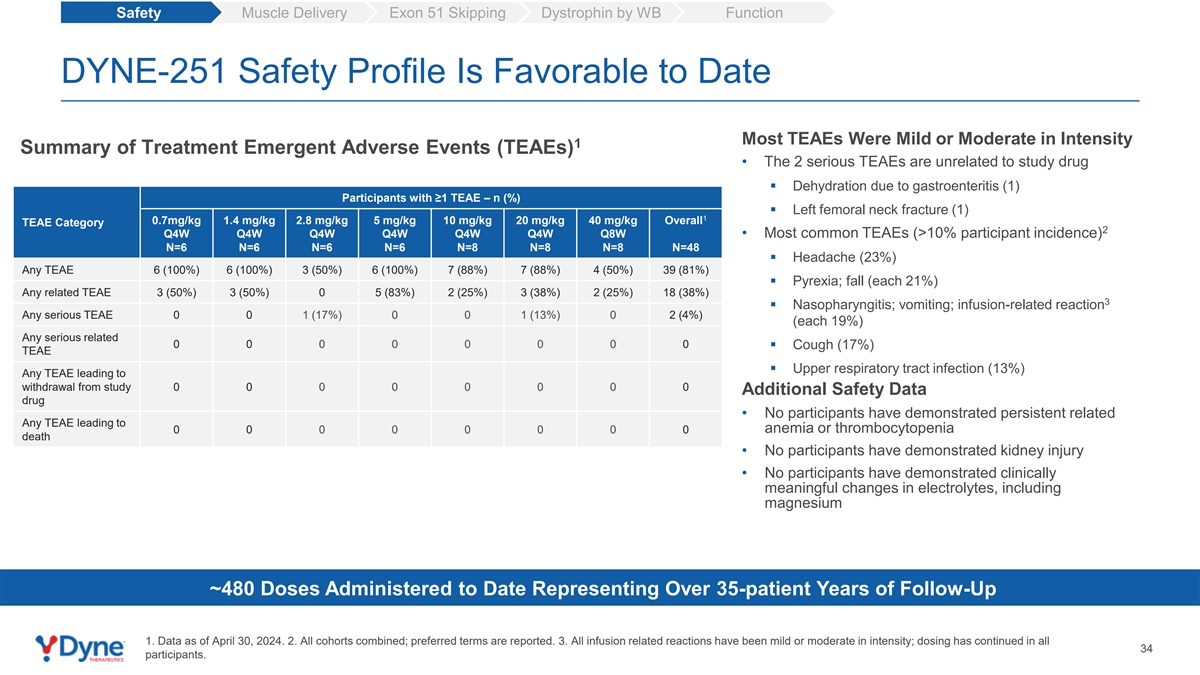
Safety Muscle Delivery Exon 51 Skipping Dystrophin by WB Function DYNE-251 Safety Profile Is Favorable to Date Most TEAEs Were Mild or Moderate in Intensity 1 Summary of Treatment Emergent Adverse Events (TEAEs) • The 2 serious TEAEs are unrelated to study drug ▪ Dehydration due to gastroenteritis (1) Participants with ≥1 TEAE – n (%) ▪ Left femoral neck fracture (1) 1 0.7mg/kg 1.4 mg/kg 2.8 mg/kg 5 mg/kg 10 mg/kg 20 mg/kg 40 mg/kg Overall TEAE Category 2 Q4W Q4W Q4W Q4W Q4W Q4W Q8W • Most common TEAEs (>10% participant incidence) N=6 N=6 N=6 N=6 N=8 N=8 N=8 N=48 ▪ Headache (23%) Any TEAE 6 (100%) 6 (100%) 3 (50%) 6 (100%) 7 (88%) 7 (88%) 4 (50%) 39 (81%) ▪ Pyrexia; fall (each 21%) Any related TEAE 3 (50%) 3 (50%) 0 5 (83%) 2 (25%) 3 (38%) 2 (25%) 18 (38%) 3 ▪ Nasopharyngitis; vomiting; infusion-related reaction Any serious TEAE 0 0 1 (17%) 0 0 1 (13%) 0 2 (4%) (each 19%) Any serious related 0 0 0 0 0 0 0 0 ▪ Cough (17%) TEAE ▪ Upper respiratory tract infection (13%) Any TEAE leading to withdrawal from study 0 0 0 0 0 0 0 0 Additional Safety Data drug • No participants have demonstrated persistent related Any TEAE leading to anemia or thrombocytopenia 0 0 0 0 0 0 0 0 death • No participants have demonstrated kidney injury • No participants have demonstrated clinically meaningful changes in electrolytes, including magnesium ~480 Doses Administered to Date Representing Over 35-patient Years of Follow-Up 1. Data as of April 30, 2024. 2. All cohorts combined; preferred terms are reported. 3. All infusion related reactions have been mild or moderate in intensity; dosing has continued in all 34 participants.
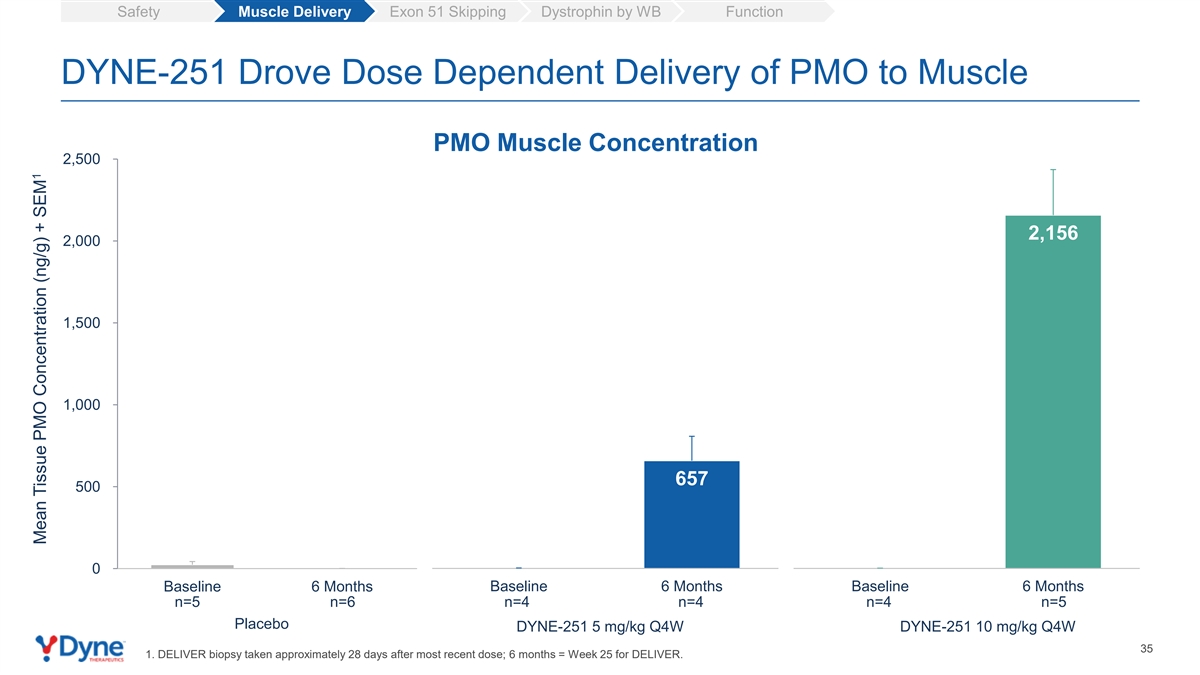
Safety Muscle Delivery Exon 51 Skipping Dystrophin by WB Function DYNE-251 Drove Dose Dependent Delivery of PMO to Muscle PMO Muscle Concentration 2,500 2,156 2,000 1,500 1,000 657 500 0 Baseline 6 Months Baseline 6 Months Baseline 6 Months n=5 n=6 n=4 n=4 n=4 n=5 Placebo DYNE-251 5 mg/kg Q4W DYNE-251 10 mg/kg Q4W 35 1. DELIVER biopsy taken approximately 28 days after most recent dose; 6 months = Week 25 for DELIVER. 1 Mean Tissue PMO Concentration (ng/g) + SEM
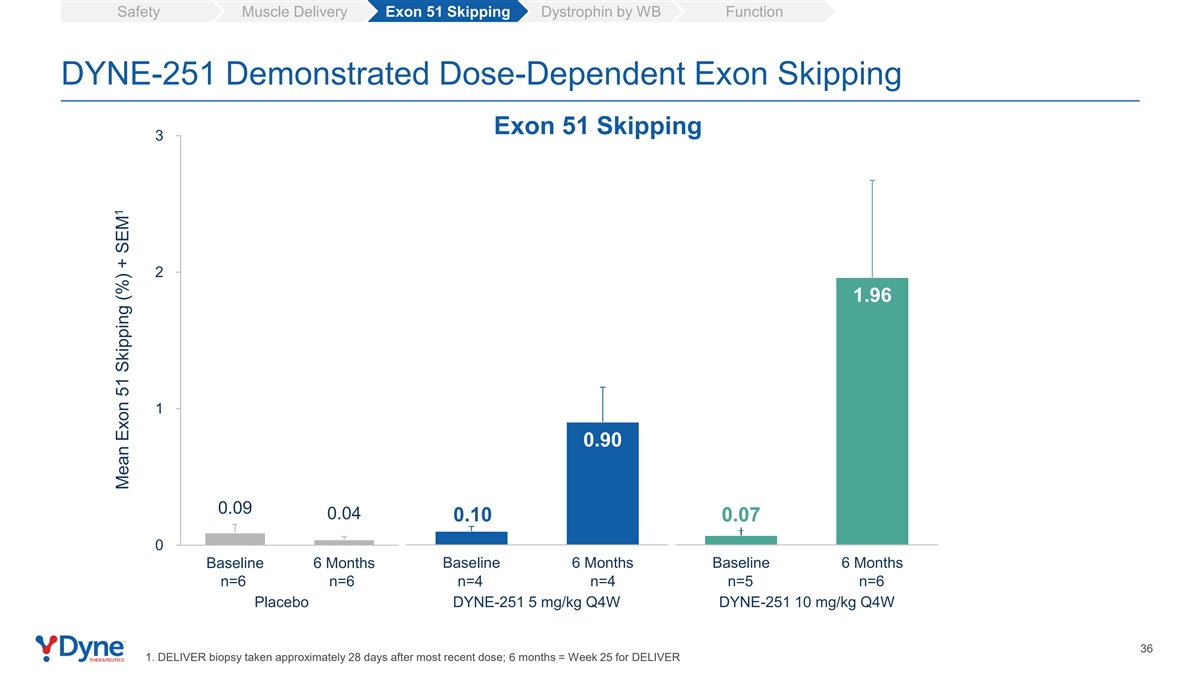
Safety Muscle Delivery Exon 51 Skipping Dystrophin by WB Function DYNE-251 Demonstrated Dose-Dependent Exon Skipping Exon 51 Skipping 3 2 1.96 1 0.90 0.09 0.04 0.10 0.07 0 Baseline 6 Months Baseline 6 Months Baseline 6 Months n=6 n=6 n=4 n=4 n=5 n=6 Placebo DYNE-251 5 mg/kg Q4W DYNE-251 10 mg/kg Q4W 36 1. DELIVER biopsy taken approximately 28 days after most recent dose; 6 months = Week 25 for DELIVER 1 Mean Exon 51 Skipping (%) + SEM
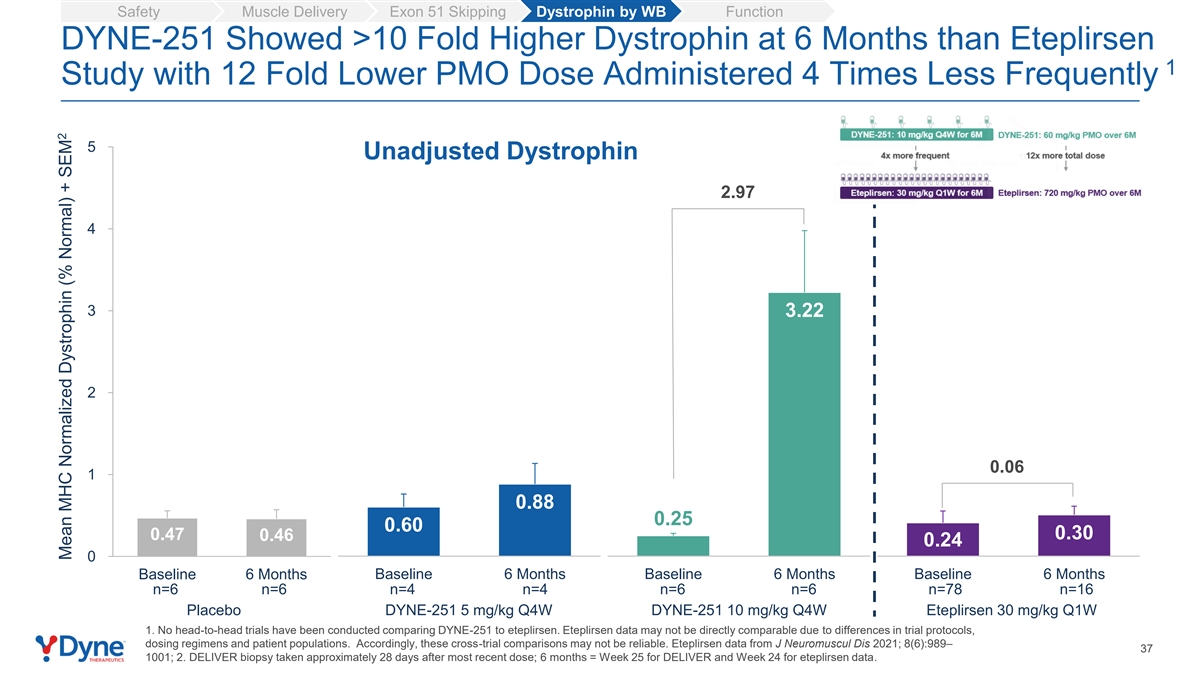
Safety Muscle Delivery Exon 51 Skipping Dystrophin by WB Function DYNE-251 Showed >10 Fold Higher Dystrophin at 6 Months than Eteplirsen 1 Study with 12 Fold Lower PMO Dose Administered 4 Times Less Frequently 5 Unadjusted Dystrophin 2.97 4 3 3.22 2 0.06 1 0.88 0.25 0.60 0.47 0.30 0.46 0.24 0 Baseline 6 Months Baseline 6 Months Baseline 6 Months Baseline 6 Months n=6 n=6 n=4 n=4 n=6 n=6 n=78 n=16 Placebo DYNE-251 5 mg/kg Q4W DYNE-251 10 mg/kg Q4W Eteplirsen 30 mg/kg Q1W 1. No head-to-head trials have been conducted comparing DYNE-251 to eteplirsen. Eteplirsen data may not be directly comparable due to differences in trial protocols, dosing regimens and patient populations. Accordingly, these cross-trial comparisons may not be reliable. Eteplirsen data from J Neuromuscul Dis 2021; 8(6):989– 37 1001; 2. DELIVER biopsy taken approximately 28 days after most recent dose; 6 months = Week 25 for DELIVER and Week 24 for eteplirsen data. 2 Mean MHC Normalized Dystrophin (% Normal) + SEM

Safety Muscle Delivery Exon 51 Skipping Dystrophin by WB Function DYNE-251 Achieved 7.6% Muscle Content Adjusted Dystrophin at 6 Months DYNE-251 dosed 10 mg/kg Q4W, SRP-5051 (PPMO) dosed 30 mg/kg Q4W 10 6.94 Muscle Content Adjusted Dystrophin Muscle-content 8 Dystrophin (MHC normalized) adjusted = 1 % Muscle Content dystrophin 4.53 7.64 6 5.17 4 2 1.63 1.63 1.61 1.19 0.70 0.65 0 Baseline 6 Months Baseline 6 Months Baseline 6 Months Baseline 6 Months n=5 n=6 n=4 n=4 n=5 n=6 n=20 n=20 1 Placebo DYNE-251 5 mg/kg Q4W DYNE-251 10 mg/kg Q4W SRP-5051 (PPMO) 30 mg/kg Q4W 1. No head-to-head trials have been conducted comparing DYNE-251 to SRP-5051. SRP-5051 data may not be directly comparable due to differences in trial protocols, dosing regimens, 38 methodologies for calculating muscle content adjusted dystrophin and patient populations. Accordingly, these cross-trial comparisons may not be reliable. SRP-5051 data from Clinical Update: MOMENTUM (Study SRP-5051-201, Part B) Jan. 29, 2024 ; 2. DELIVER biopsy taken approximately 28 days after most recent dose; 6 months = Week 25 for DELIVER and Week 28 for SRP-5051 data. Mean MCA MHC Normalized Dystrophin (% Normal) + SEM

Safety Muscle Delivery Exon 51 Skipping Dystrophin by WB Function Encouraging Trends Across Multiple Functional Endpoints Baseline values inform interpretation of data; ongoing exploration of higher dose cohorts and longer time points th NSAA Total Score Stride Velocity 95 Centile 1.5 0.4 0.0 0.2 -1.5 0.0 -3.0 -0.2 -4.5 -0.4 Baseline Day 85 Day 169 Baseline Day 85 Day 169 Timed Rise from Floor Timed 10-Meter Walk / Run Test 10.0 1.2 8.0 6.0 0.6 4.0 0.0 2.0 0.0 -2.0 -0.6 Baseline Day 85 Day 169 Baseline Day 85 Day 169 Placebo (n=3 for SV95C DYNE-251 10 mg/kg Q4W (n = 5 for all endpoints) and n=9 for other endpoints) 39 Timed Rise from Floor (Sec) NSAA Total Score Change from Baseline +/- SEM Change from Baseline +/- SEM Improvement Improvement SV95C (m/sec) Timed 10m Run / Walk Test Change from Baseline +/- SEM Change from Baseline +/- SEM Improvement Improvement

Opportunity to Build a Global DMD Franchise: Leading with DYNE-251, Payloads Identified for Exons 53, 45, 44 Exon 51 – 13% Exon 53 – 8% Approximately Exon 45 – 8% Exon 44 – 6% Exon 50 – 4% 80% of patients Exon 52 – 4% Exon 43 – 4% have genotypes amenable Exon 55 – 2% to exon skipping Exon 8 – 2% Other exon skips – ~30% May not be amenable to exon skipping – ~20% 40
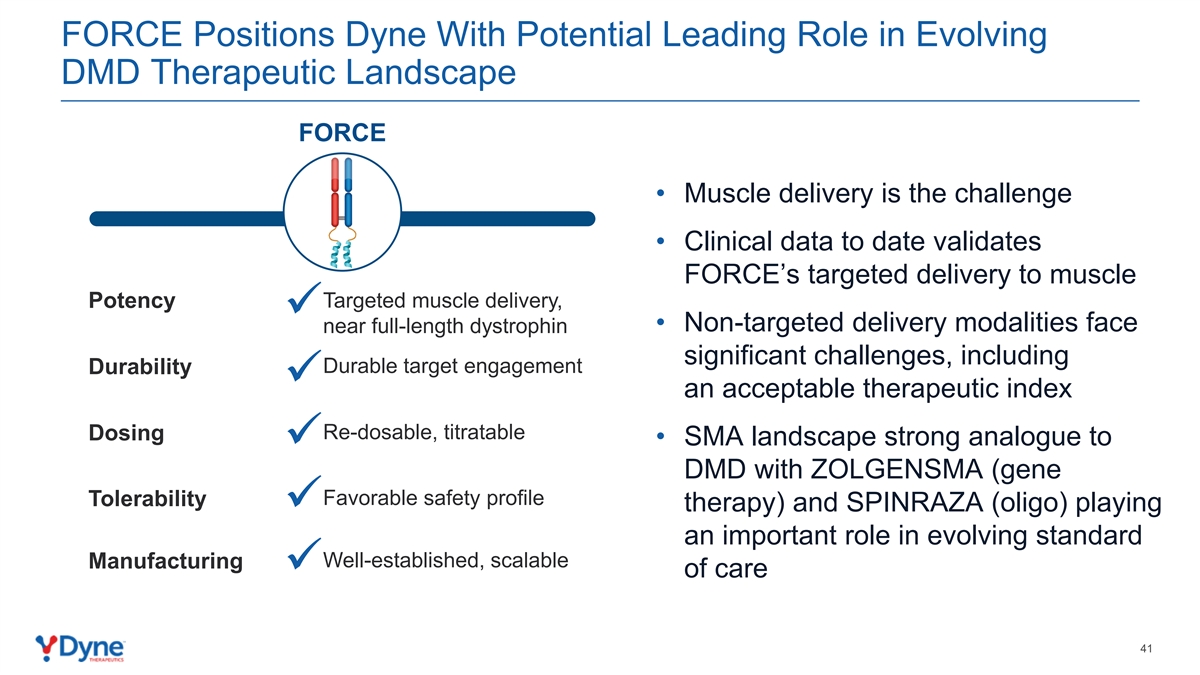
FORCE Positions Dyne With Potential Leading Role in Evolving DMD Therapeutic Landscape FORCE • Muscle delivery is the challenge • Clinical data to date validates FORCE’s targeted delivery to muscle Targeted muscle delivery, Potency ✓ • Non-targeted delivery modalities face near full-length dystrophin significant challenges, including Durable target engagement Durability ✓ an acceptable therapeutic index Re-dosable, titratable Dosing • SMA landscape strong analogue to ✓ DMD with ZOLGENSMA (gene Favorable safety profile Tolerability therapy) and SPINRAZA (oligo) playing ✓ an important role in evolving standard Well-established, scalable Manufacturing ✓ of care 41

DELIVER Data Demonstrated Potential for DMD Exon Skipping Franchise with Differentiated Efficacy and Safety Profile At 10.0 mg/kg Q4W dose, DYNE-251 showed compelling ✓ 1 profile vs. eteplirsen standard-of-care reported at 6 months : • 3.2% unadjusted and 7.6% muscle content adjusted dystrophin expression • Trends in improvement in functional outcomes, including NSAA and SV95C 2 Favorable safety profile to date ; 40 mg/kg Q8W cohort ✓ fully enrolled Supports further development of DMD global franchise ✓ Pursuing expedited approval based on recent regulatory interactions and strength of results Update on path to registration for DYNE-251 expected by YE 2024 1. No head-to-head trials have been conducted comparing DYNE-251 to eteplirsen. Eteplirsen data may not be directly comparable due to differences in trial protocols, dosing regimens and patient populations. Accordingly, these cross-trial comparisons may not be reliable. Eteplirsen data from J Neuromuscul Dis 2021; 8(6):989–1001; 42 2. Data as of April 30, 2024.

Program Opening remarks John Cox, President & CEO DYNE-101 ACHIEVE Trial in DM1 Data DYNE-251 DELIVER Trial in DMD Data Wildon Farwell, M.D., MPH, Chief Medical Officer Q&A Closing Remarks John Cox, President & CEO 43

Driving Towards Potentially Transformative DM1 and DMD Therapies Delivered on the Promise of FORCE: Enhanced Delivery of Therapeutics to Muscle Compelling Impact on Key Disease Biomarkers and Improvement in Multiple Functional Endpoints in Both DM1 and DMD Favorable Safety & Tolerability Profile Supporting Dose Escalation Fully Enrolled Through 6.8 mg/kg Fully Enrolled Through 40 mg/kg Pursuing Expedited Approvals for Both Programs with Update on Registrational Pathway by YE 2024 44

Advancing Robust Portfolio Focused on Muscle Diseases Pipeline Update by YE 2024 Including FSHD and Other Pipeline Programs DISEASE TARGET DISCOVERY PRECLINICAL PHASE 1/2 ESTIMATED PATIENTS Myotonic Dystrophy US: >40,000 DMPK DYNE-101 Type 1 (DM1) Europe: >74,000 DYNE-251 Exon 51 Exon 53 Duchenne Muscular US: ~12,000-15,000 Exon 45 Dystrophy (DMD) Europe: ~25,000 Exon 44 Other Exons Facioscapulohumeral US: ~16,000-38,000 DYNE-301 Muscular Dystrophy DUX4 Europe: ~35,000 (FSHD) Pipeline Expansion Opportunities Rare Skeletal CNS Cardiac Metabolic 45

Building the World’s Leading Muscle Disease Company Dynamo Culture Own Muscle Delivery & Win in DM1, DMD, FSHD Leverage FORCE 46

Achieving the Promise of FORCE to Deliver for Patients F O R C E ACHIEVE & DELIVER CLINICAL UPDATE | MAY 20, 2024