UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
April 17, 2024
Poseida Therapeutics, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 001-39376 | 47-2846548 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
| 9390 Towne Centre Drive, Suite 200 | ||
| San Diego, California | 92121 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (858) 779-3100
N/A
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange on which registered |
||
| Common Stock, par value $0.0001 per share | PSTX | Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On April 17, 2024, Poseida Therapeutics, Inc. (the “Company”) issued a press release announcing that members of its management and external advisors are providing an update on the Company’s genetic medicine research and development programs and making available a corporate presentation. A copy of the press release and the corporate presentation are attached as Exhibit 99.1 and Exhibit 99.2, respectively, to this report. The corporate presentation will also be available under the “Investors & Media” section of the Company’s website.
The information in this Item 7.01 of this report (including Exhibits 99.1 and 99.2) is furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information shall not be deemed incorporated by reference into any other filing with the Securities and Exchange Commission made by the Company, whether made before or after today’s date, regardless of any general incorporation language in such filing, except as shall be expressly set forth by specific references in such filing.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits.
| Exhibit No. |
Description | |
| 99.1 | Press Release, dated April 17, 2024 | |
| 99.2 | Corporate Presentation, dated April 17, 2024 | |
| 104 | Cover Page Interactive Data File | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Poseida Therapeutics, Inc. | ||||||
| Date: April 17, 2024 | By: | /s/ Harry J. Leonhardt, Esq. |
||||
| Name: | Harry J. Leonhardt, Esq. | |||||
| Title: | General Counsel, Chief Compliance Officer & Corporate Secretary | |||||
Exhibit 99.1
Poseida Therapeutics Hosts Gene Therapy R&D Day Highlighting New Scientific Advancements and Pipeline Focus
Fully non-viral approach to genetic medicine employs differentiated gene delivery, gene editing and gene insertion technology
Progressing two fully non-viral programs in rare disease with significant unmet patient need
Virtual R&D Day featuring academic experts and Poseida’s leadership and scientific teams to be held today at 10:00am ET / 7:00am PT
SAN DIEGO, April 17, 2024 — Poseida Therapeutics, Inc. (Nasdaq: PSTX), a clinical-stage cell therapy and genetic medicines company advancing a new class of treatments for patients with cancer and rare diseases, today announced that the Company plans to highlight progress across its proprietary non-viral genetic engineering and delivery platform and rare disease pipeline during a virtual R&D Day to be held today at 10:00am ET / 7:00am PT.
“Poseida is forging ahead with a renewed focus on our genetic medicine portfolio. Our system of proprietary, non-viral tools used individually or together has the capacity to treat rare genetic diseases as well as address much more prevalent diseases,” said Kristin Yarema, Ph.D., President & Chief Executive Officer of Poseida Therapeutics. “In the short-term, we are laser-focused on progressing our two lead non-viral candidates within areas of significant opportunity: P-KLKB1-101 for Hereditary Angioedema, and P-FVIII-101, which utilizes our fully non-viral stable gene insertion technique to treat Hemophilia A, a condition affecting approximately 30,000 adults and children in the U.S. alone.”
“Poseida has developed a broad suite of fully non-viral, differentiated genetic engineering technologies, including stable, potentially site-specific insertion of whole genes, high-fidelity gene editing, and strength in delivery systems including lipid nanoparticles,” said Blair Madison, Ph.D., Chief Scientific Officer of Gene Therapy at Poseida Therapeutics. “We believe this uniquely positions us in the industry to deliver on the hope and promise of genetic medicines and bring much-needed therapies to patients in need.”
The event will highlight the Company’s proprietary genetic engineering and delivery platform, including its non-viral gene insertion and gene editing programs. External expert speakers will include:
| • | Marc Riedl, M.D., M.S., Professor of Medicine and Clinical Director of the U.S. HAEA Angioedema Center at University of California, San Diego; and |
| • | Steven W. Pipe, M.D., Professor of Pediatrics and Pathology, University of Michigan |
Key Gene Therapy R&D Day Topics and Highlights
Gene Therapy Programs
The Company will present advancements in fully non-viral liver-directed gene therapies highlighting the potential for functional cures across commercially viable indications.
| • | P-KLKB1-101 is the Company’s lead liver-directed investigational gene therapy program for the treatment of hereditary angioedema (HAE), a rare, inherited disorder which results in the swelling of the limbs, intestinal tract, and airways which can be both debilitating and life-threatening. The Company will share data highlighting durable disease correction and high fidelity in pre-clinical studies using the Company’s enhanced editing technology, Cas-CLOVERTM. |
| • | P-FVIII-101, the Company’s second non-viral gene therapy program, is a liver-directed investigational in vivo gene therapy for the treatment of Hemophilia A, a hereditary disorder caused by a deficiency in Factor VIII (FVIII) production resulting in excessive bleeding occurring either spontaneously or due to trauma. The Company will share data demonstrating durability and restoration of FVIII deficiency to near-normal levels in adult mouse models. |
Technology Innovation in Gene Therapy
The Company will highlight significant advancements in its emerging platform technologies.
| • | Site-specific Super piggyBac® is a single enzyme fusion system for site-specific integration, executing clean DNA gene insertion without double strand breaks, unintended mutations, or the need for DNA repair. The Company will announce that the current molecular evolution of its platform yields a 30-fold improvement of DNA expression and efficient targeted cargo integration at single- and multi-copy sites. These data support the potential for the Company’s non-viral insertion technology as an efficient and safe approach to achieve sustained DNA integration and expression to remediate disease. |
| • | Cas-CLOVER is a proprietary high-fidelity nuclease for enabling clean site-specific gene editing that is engineered for high specificity. The Company will present data confirming and validating the benefits and advantages of Cas-CLOVER in multiple applications and disease areas. |
| • | Novel Lipids and DNA Delivery: The Company is leveraging proprietary lipids notable for their low immunogenicity, dose titration potential, and ability to be manufactured at scale and favorable cost. The Company has achieved multiple breakthroughs in its delivery technology, including novel lipids for in vivo delivery of its technology as well as innovations enabling improved in vivo non-viral DNA delivery. |
Video Webcast and Replay
This virtual event and access to the live webcast is available to through the following registration link: https://wsw.com/webcast/cc/pstx6/1466622.
Registration for this virtual event and access to a replay of the live webcast will be available on the Investors & Media section of www.poseida.com. A replay of the webcast will be available for approximately 90 days following the presentation.
About Poseida Therapeutics, Inc.
Poseida Therapeutics is a clinical-stage biopharmaceutical company advancing differentiated cell therapies and genetic medicines with the capacity to cure certain cancers and rare diseases. The Company’s pipeline includes investigational allogeneic CAR-T cell therapies for both solid tumors and hematologic cancers as well as investigational in vivo genetic medicines that address patient populations with high unmet medical need. The Company’s approach is based on its proprietary genetic editing platforms, including its non-viral piggyBac® DNA Delivery System, Cas-CLOVER™ Site-Specific Gene Editing System, Booster Molecule and nanoparticle gene delivery technologies, as well as in-house GMP cell therapy manufacturing. The Company has formed a global strategic collaboration with Roche to unlock the promise of cell therapies for patients with hematologic malignancies. Learn more at www.poseida.com and connect with Poseida on X and LinkedIn.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include statements regarding, among other things, expected plans with respect to clinical trials, including timing of clinical data updates; anticipated timelines and milestones with respect to the Company’s development programs and manufacturing activities and capabilities; the potential capabilities and benefits of the Company’s technology platforms and product candidates, including the efficacy and safety profile of such product candidates; the quotes from Drs. Yarema and Madison; and the Company’s plans and strategy with respect to developing its technologies and product candidates. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. These forward-looking statements are based upon the Company’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, the Company’s reliance on third parties for various aspects of its business; risks and uncertainties associated with development and regulatory approval of novel product candidates in the biopharmaceutical industry; the Company’s ability to retain key scientific or management personnel; and the other risks described in the Company’s filings with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made. The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
# # #
Poseida Investor and Media Relations:
Alex Chapman
Senior Vice President, IR & Corporate Communications
IR@poseida.com
Sarah Thailing
Senior Director, IR & Corporate Communications
PR@poseida.com
Exhibit 99.2 Gene Therapy R&D Day Advancing next -generation non -viral genetic medicines with the capacity to cure th APRIL 17 , 2024

Disclaimer This presentation and any accompanying oral commentary contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts and include, without limitation, statements related to future events; our future financial performance or condition; business strategy; expected timing and plans with respect to development milestones, clinical trials, and regulatory and manufacturing activities; estimated market opportunities for product candidates; potential capabilities and benefits of our technology platforms and product candidates, including the efficacy and safety profile of such product candidates; the quotes from Peter Marks; our plans and strategy with respect to developing our technologies and product candidates; our ability to exploit and consummate additional business development opportunities; and future results of anticipated development efforts. Words such as expect(s), feel(s), believe(s), will, may, anticipate(s) , “potentially” or negative of these terms or similar expressions are intended to identify forward-looking statements. These forward-looking statements are based on management's current expectations of future events only as of the date of this presentation and are subject to a number of important risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to: risks associated with conducting clinical trials; whether any of our product candidates will be shown to be safe and effective; our ability to finance continued operations; our reliance on third parties for various aspects of our business; competition in our target markets; our ability to protect our intellectual property; our ability to retain key scientific or management personnel; risks and uncertainties associated with development and regulatory approval of novel product candidates in the biopharmaceutical industry; and other risks and uncertainties described in our filings with the Securities and Exchange Commission, including under the heading “Risk Factors”. Except as required by law, we assume no obligation to update these forward-looking statements, or to update the reasons why actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. 2 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.

Welcome & Introduction P r e s e n t e r : K r i s t i n Ya r e m a , P h D

Agenda Introduction Kristin Yarema, PhD P-KLKB1-101 Program / Platform Marc Riedl, MD, MS / Blair Madison, PhD / Bonnie Jacques, PhD Non-viral system Jack Rychak, PhD P-FVIII-101 Program / Platform Steven Pipe, MD / Blair Madison, PhD Site specific SPB Blair Madison, PhD Conclusion Kristin Yarema, PhD Q&A Executive and Scientific Leadership 4 © © 2024 2024 Po Pos se eid ida a T Th he era rap pe eu utics tics, , In Inc. c. All All Rig Righ hts ts Res Rese erv rve ed d. .
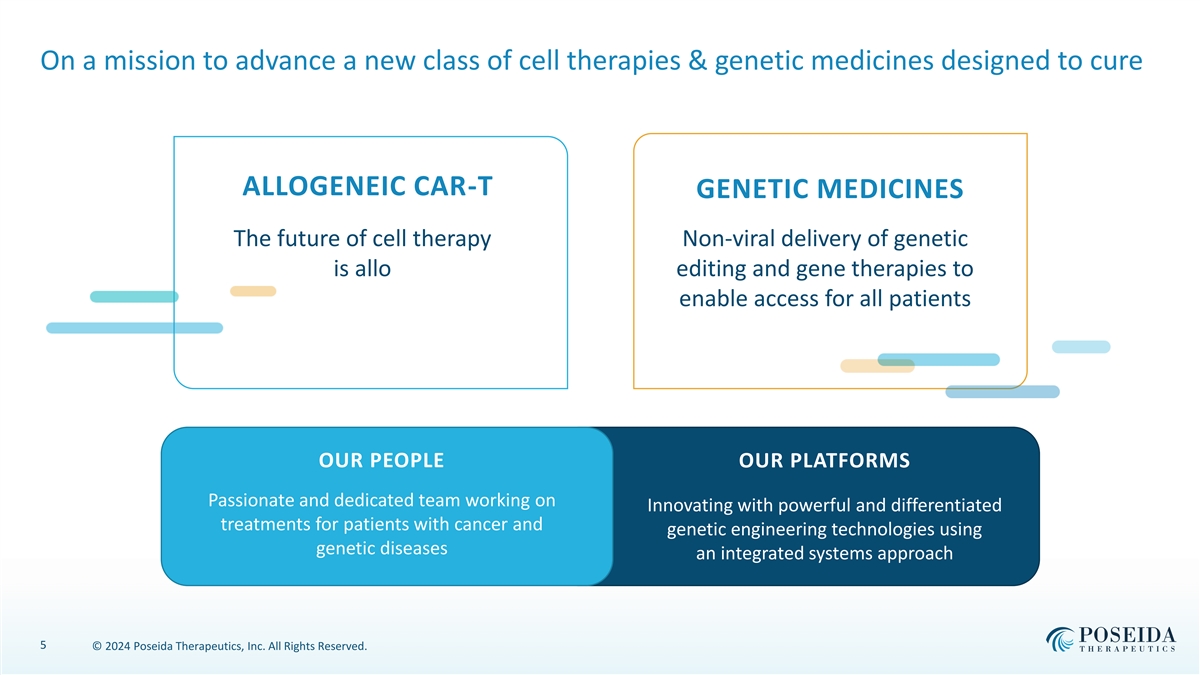
On a mission to advance a new class of cell therapies & genetic medicines designed to cure ALLOGENEIC CAR-T GENETIC MEDICINES The future of cell therapy Non-viral delivery of genetic is allo editing and gene therapies to enable access for all patients OUR PEOPLE OUR PLATFORMS Passionate and dedicated team working on Innovating with powerful and differentiated treatments for patients with cancer and genetic engineering technologies using genetic diseases an integrated systems approach 5 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
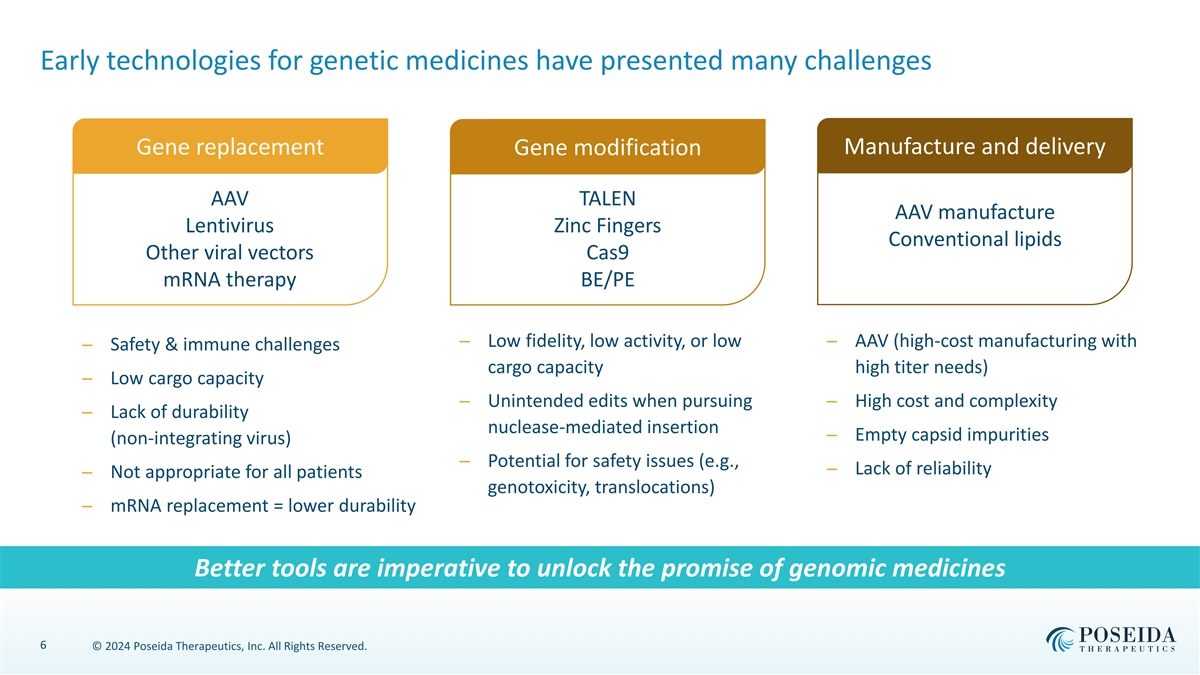
Early technologies for genetic medicines have presented many challenges Manufacture and delivery Gene replacement Gene modification AAV TALEN AAV manufacture Lentivirus Zinc Fingers Conventional lipids Other viral vectors Cas9 mRNA therapy BE/PE ─ Low fidelity, low activity, or low ─ AAV (high-cost manufacturing with ─ Safety & immune challenges cargo capacity high titer needs) ─ Low cargo capacity ─ Unintended edits when pursuing ─ High cost and complexity ─ Lack of durability nuclease-mediated insertion ─ Empty capsid impurities (non-integrating virus) ─ Potential for safety issues (e.g., ─ Lack of reliability ─ Not appropriate for all patients genotoxicity, translocations) ─ mRNA replacement = lower durability Better tools are imperative to unlock the promise of genomic medicines 6 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.

“We are enthusiastic to see the development of non- viral vectors for gene therapy and look forward to working with sponsors on these programs as they work to achieve the necessary efficiency needed for effective gene transfer.” – Peter Marks, Director of the Center for Biologics Evaluation and Research (CBER) at the Food and Drug Administration 7
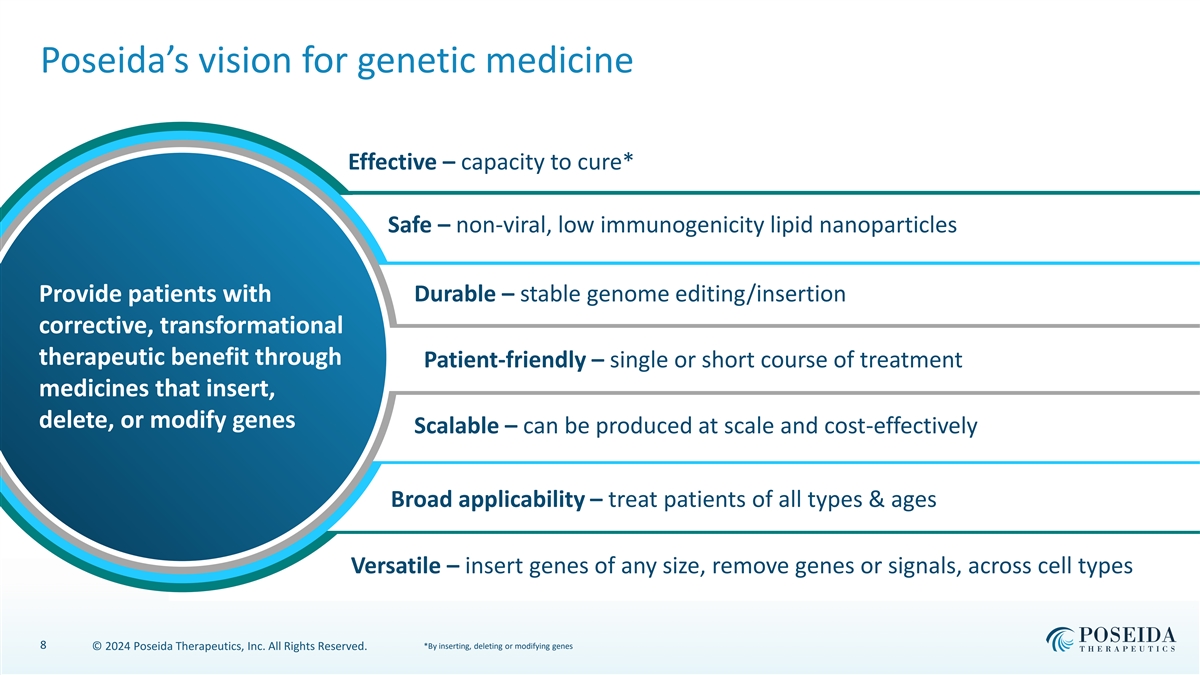
Poseida’s vision for genetic medicine Effective – capacity to cure* Safe – non-viral, low immunogenicity lipid nanoparticles Durable – stable genome editing/insertion Provide patients with corrective, transformational therapeutic benefit through Patient-friendly – single or short course of treatment medicines that insert, delete, or modify genes Scalable – can be produced at scale and cost-effectively Broad applicability – treat patients of all types & ages Versatile – insert genes of any size, remove genes or signals, across cell types 8 *By inserting, deleting or modifying genes © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
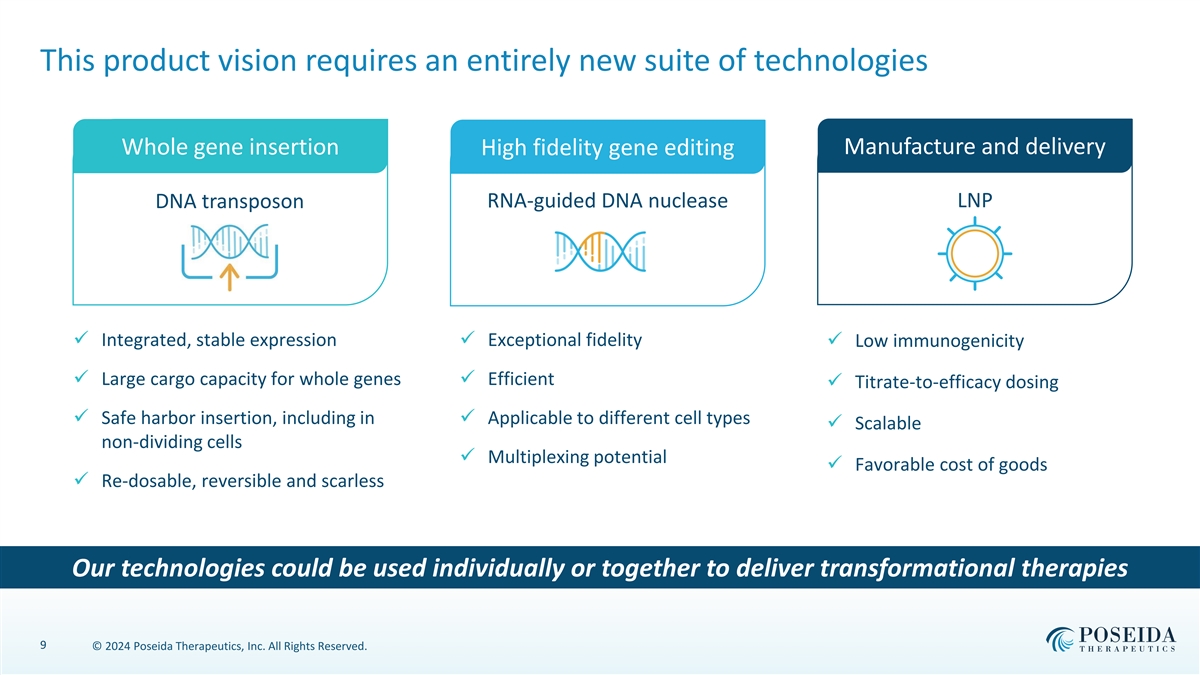
This product vision requires an entirely new suite of technologies Manufacture and delivery Whole gene insertion High fidelity gene editing RNA-guided DNA nuclease LNP DNA transposon ✓ Integrated, stable expression✓ Exceptional fidelity ✓ Low immunogenicity ✓ Large cargo capacity for whole genes✓ Efficient ✓ Titrate-to-efficacy dosing ✓ Safe harbor insertion, including in ✓ Applicable to different cell types ✓ Scalable non-dividing cells ✓ Multiplexing potential ✓ Favorable cost of goods ✓ Re-dosable, reversible and scarless Our technologies could be used individually or together to deliver transformational therapies 9 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
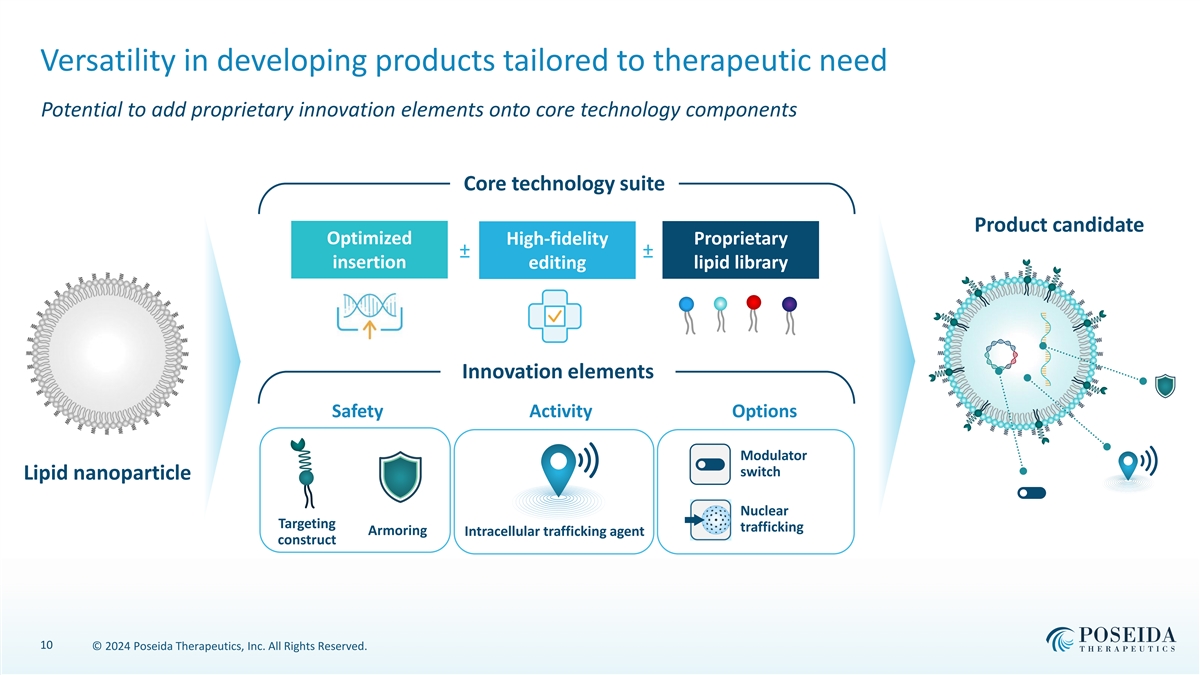
Versatility in developing products tailored to therapeutic need Potential to add proprietary innovation elements onto core technology components Core technology suite Product candidate Optimized High-fidelity Proprietary ± a ± a insertion editing lipid library Innovation elements Safety Activity Options Modulator switch Lipid nanoparticle Nuclear Targeting trafficking Armoring Intracellular trafficking agent construct 10 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
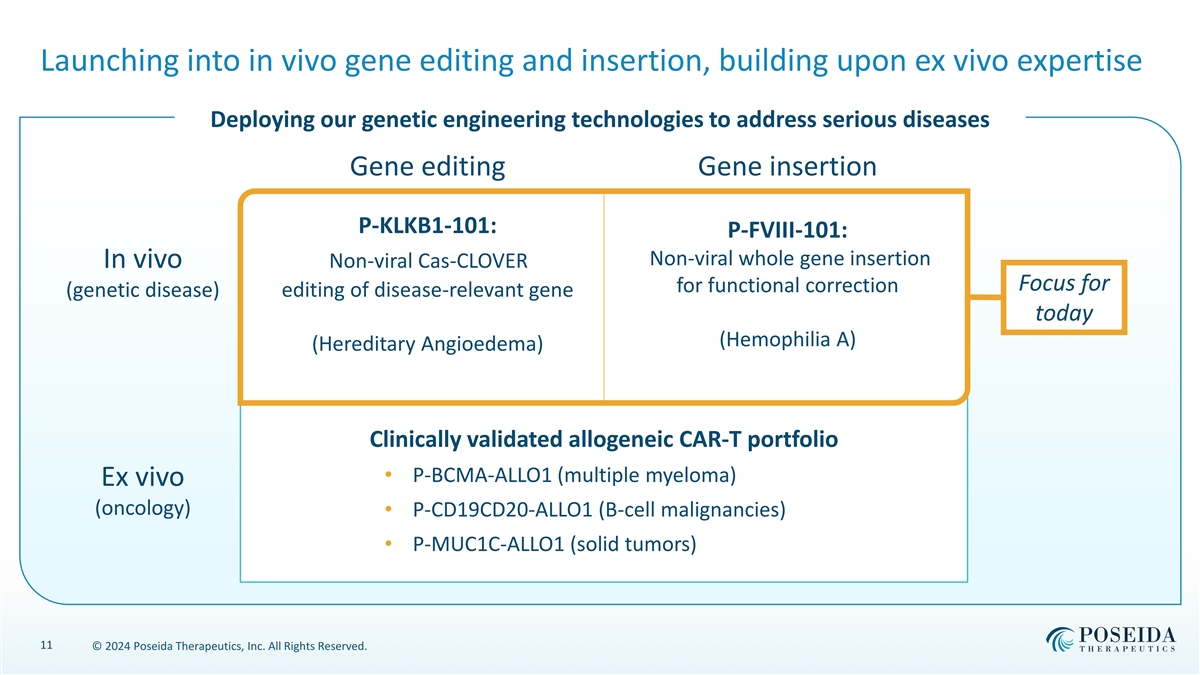
Launching into in vivo gene editing and insertion, building upon ex vivo expertise Deploying our genetic engineering technologies to address serious diseases Gene editing Gene insertion P-KLKB1-101: P-FVIII-101: Non-viral whole gene insertion In vivo Non-viral Cas-CLOVER Focus for for functional correction (genetic disease) editing of disease-relevant gene today (Hemophilia A) (Hereditary Angioedema) Clinically validated allogeneic CAR-T portfolio • P-BCMA-ALLO1 (multiple myeloma) Ex vivo (oncology) • P-CD19CD20-ALLO1 (B-cell malignancies) • P-MUC1C-ALLO1 (solid tumors) 11 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
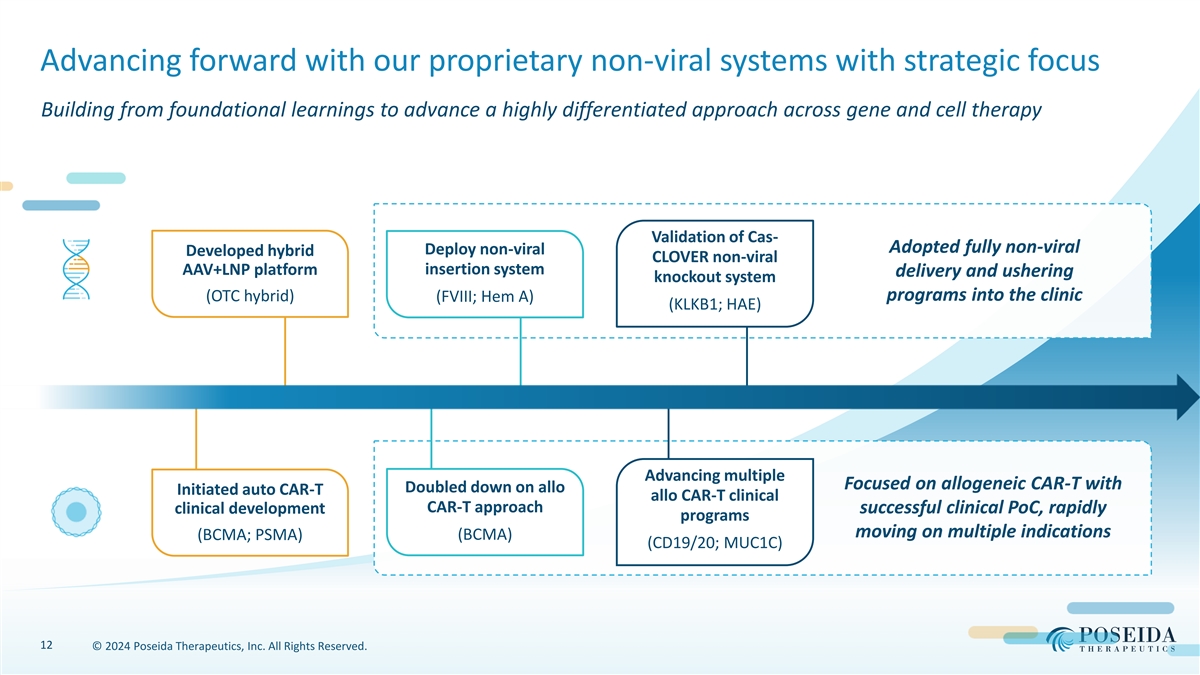
Advancing forward with our proprietary non-viral systems with strategic focus Building from foundational learnings to advance a highly differentiated approach across gene and cell therapy Validation of Cas- Adopted fully non-viral Deploy non-viral Developed hybrid CLOVER non-viral AAV+LNP platform insertion system delivery and ushering knockout system (OTC hybrid) programs into the clinic (FVIII; Hem A) (KLKB1; HAE) Advancing multiple Focused on allogeneic CAR-T with Doubled down on allo Initiated auto CAR-T allo CAR-T clinical CAR-T approach clinical development successful clinical PoC, rapidly programs moving on multiple indications (BCMA; PSMA) (BCMA) (CD19/20; MUC1C) 12 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
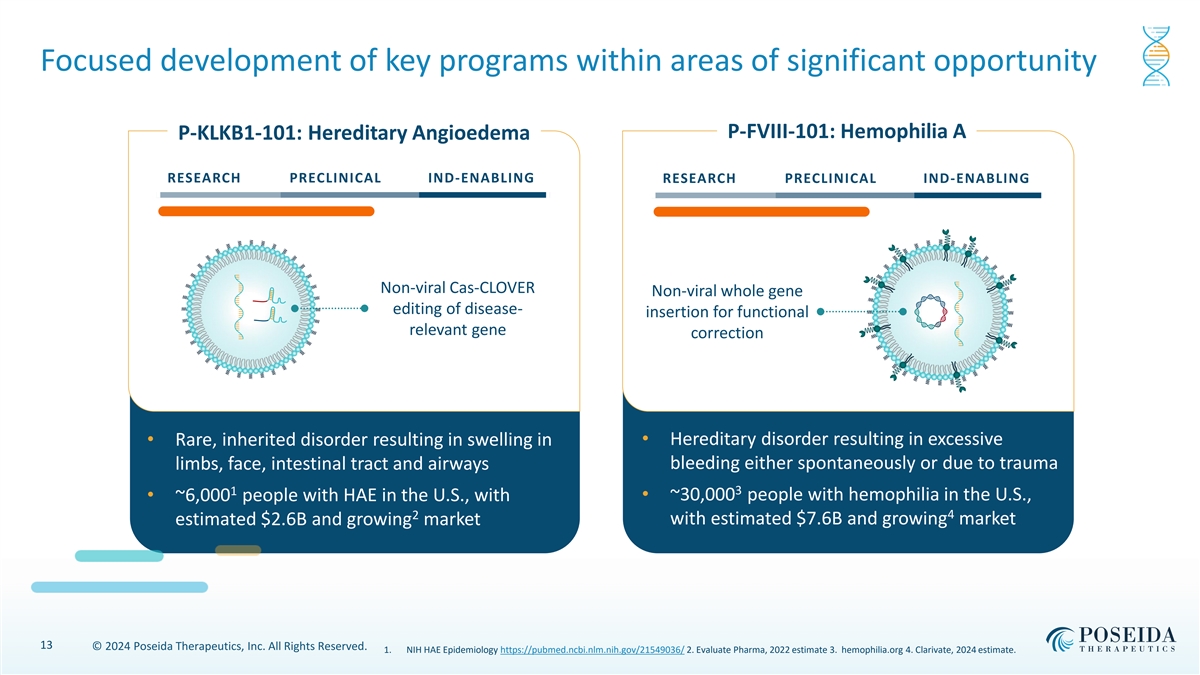
Focused development of key programs within areas of significant opportunity P-FVIII-101: Hemophilia A P-KLKB1-101: Hereditary Angioedema RESEARCH PRECLINICAL IND-ENABLING RESEARCH PRECLINICAL IND-ENABLING Non-viral Cas-CLOVER Non-viral whole gene editing of disease- insertion for functional relevant gene correction • Hereditary disorder resulting in excessive • Rare, inherited disorder resulting in swelling in bleeding either spontaneously or due to trauma limbs, face, intestinal tract and airways 1 3 • ~6,000 people with HAE in the U.S., with • ~30,000 people with hemophilia in the U.S., 4 2 estimated $2.6B and growing market with estimated $7.6B and growing market 13 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. 1. NIH HAE Epidemiology https://pubmed.ncbi.nlm.nih.gov/21549036/ 2. Evaluate Pharma, 2022 estimate 3. hemophilia.org 4. Clarivate, 2024 estimate.

Guest speakers Marc Riedl, MD, MS Professor of Medicine at University of California, San Diego Steven W. Pipe, MD Professor of Pediatrics and Pathology, University of Michigan 14 © © 2024 2024 Po Pos se eid ida a T Th he era rap pe eu utics tics, , In Inc. c. All All Rig Righ hts ts Res Rese erv rve ed d. .

Hereditary Angioedema (HAE): Where Are We Now? Marc Riedl MD, MS Professor of Medicine at University of California, San Diego
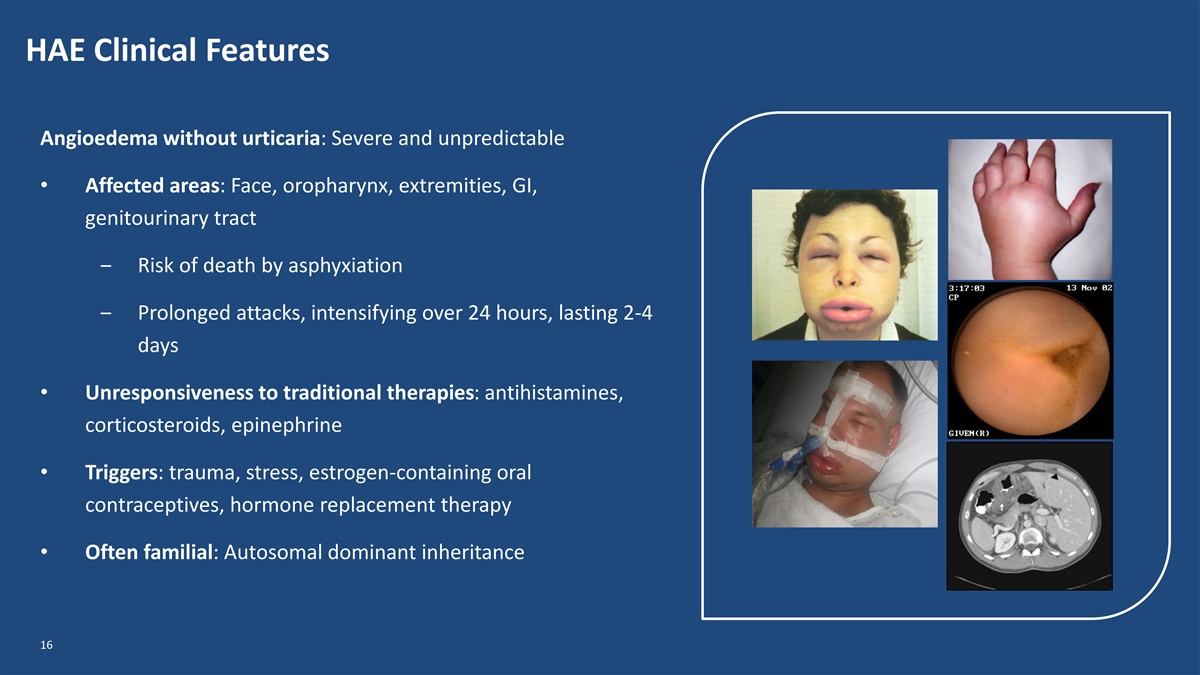
HAE Clinical Features Angioedema without urticaria: Severe and unpredictable • Affected areas: Face, oropharynx, extremities, GI, genitourinary tract ‒ Risk of death by asphyxiation ‒ Prolonged attacks, intensifying over 24 hours, lasting 2-4 days • Unresponsiveness to traditional therapies: antihistamines, corticosteroids, epinephrine • Triggers: trauma, stress, estrogen-containing oral contraceptives, hormone replacement therapy • Often familial: Autosomal dominant inheritance 16
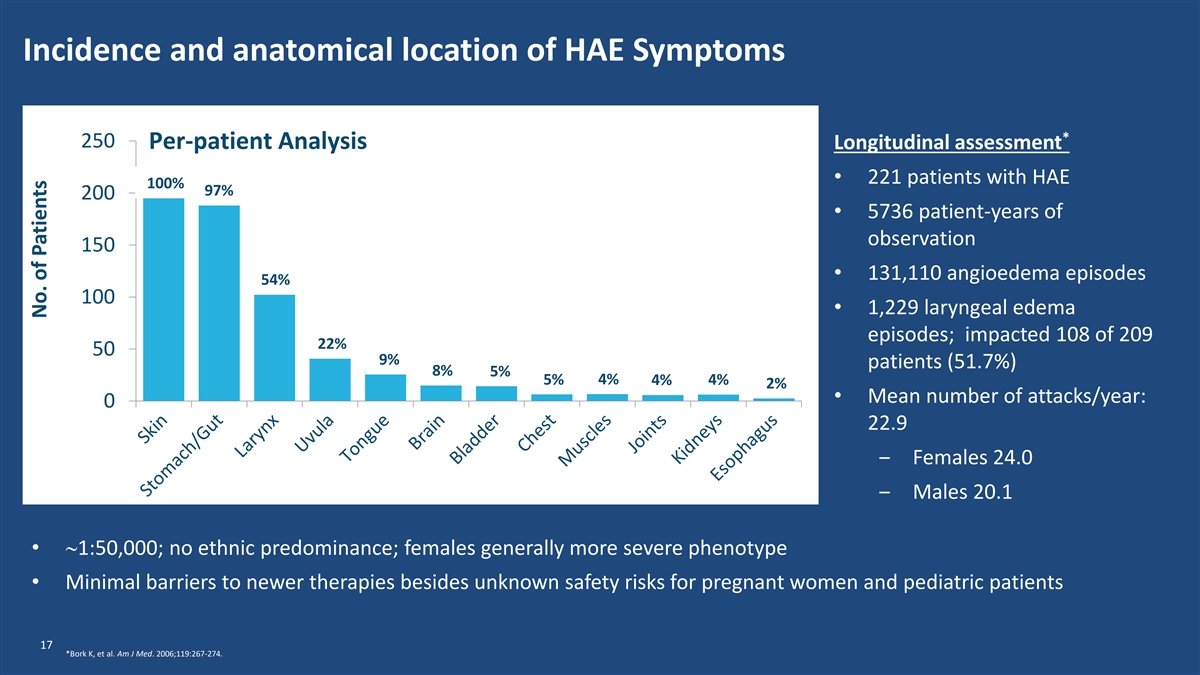
Incidence and anatomical location of HAE Symptoms * 250 Per-patient Analysis Longitudinal assessment • 221 patients with HAE 100% 97% 200 • 5736 patient-years of observation 150 • 131,110 angioedema episodes 54% 100 • 1,229 laryngeal edema episodes; impacted 108 of 209 22% 50 9% patients (51.7%) 8% 5% 5% 4% 4% 4% 2% • Mean number of attacks/year: 0 22.9 ‒ Females 24.0 ‒ Males 20.1 •~1:50,000; no ethnic predominance; females generally more severe phenotype • Minimal barriers to newer therapies besides unknown safety risks for pregnant women and pediatric patients 17 *Bork K, et al. Am J Med. 2006;119:267-274. No. of Patients
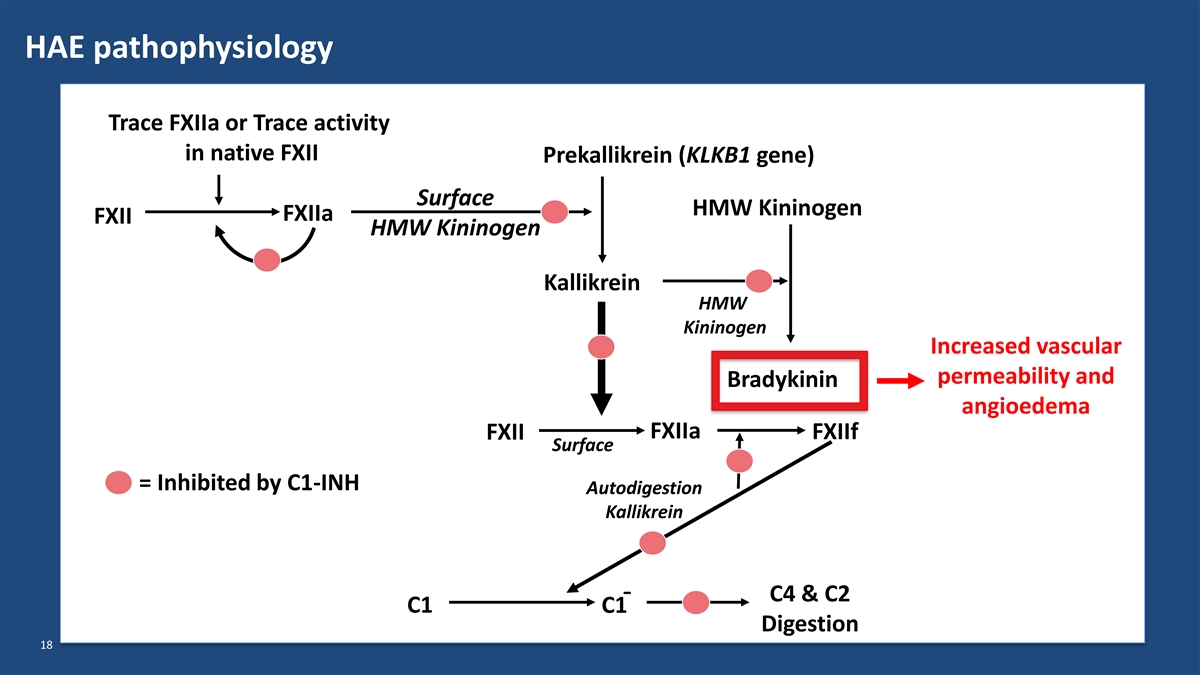
HAE pathophysiology Trace FXIIa or Trace activity in native FXII Prekallikrein (KLKB1 gene) Surface HMW Kininogen FXIIa FXII HMW Kininogen Kallikrein HMW Kininogen Increased vascular permeability and Bradykinin angioedema FXIIa FXII FXIIf Surface = Inhibited by C1-INH Autodigestion Kallikrein C4 & C2 ¯ C1 C1 Digestion 18
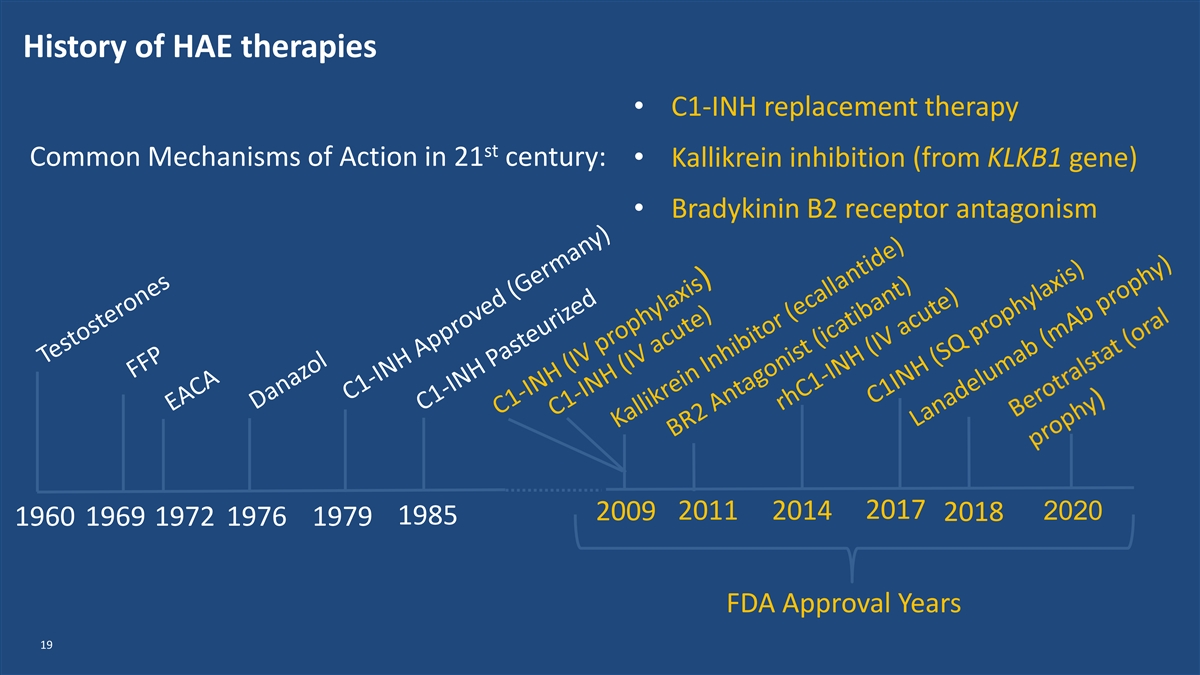
History of HAE therapies • C1-INH replacement therapy st Common Mechanisms of Action in 21 century: • Kallikrein inhibition (from KLKB1 gene) • Bradykinin B2 receptor antagonism 2017 2009 2011 2014 2020 2018 1985 1960 1969 1972 1976 1979 FDA Approval Years 19
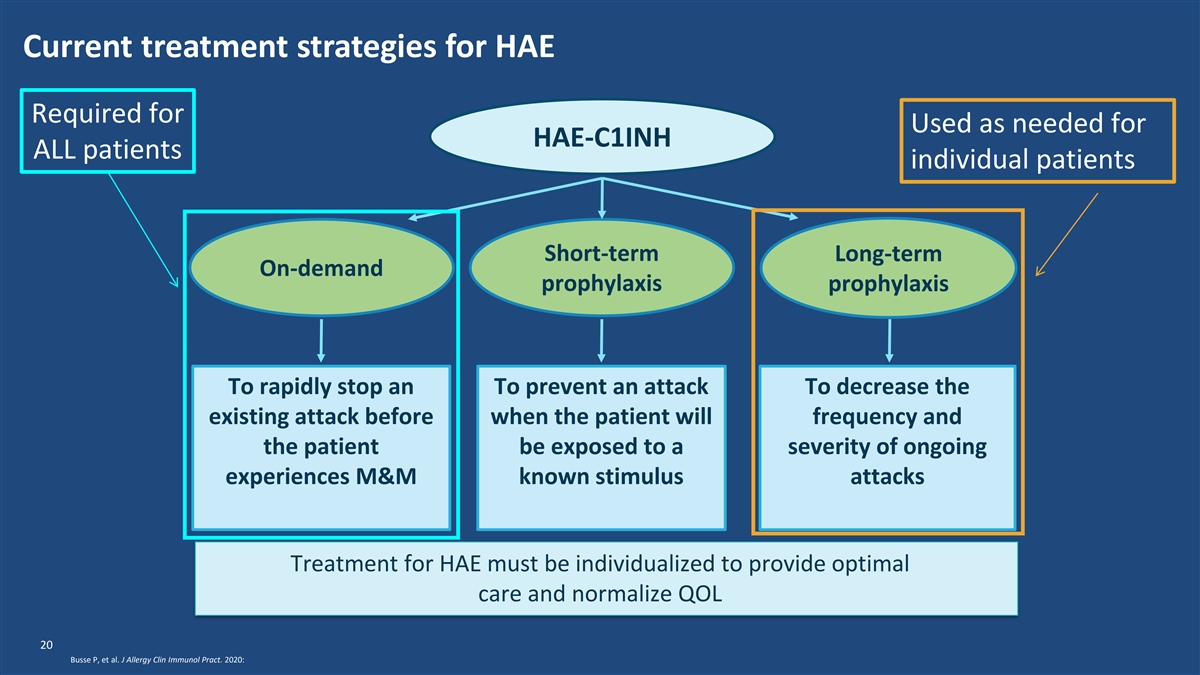
Current treatment strategies for HAE Required for Used as needed for HAE-C1INH ALL patients individual patients Short-term Long-term On-demand prophylaxis prophylaxis To rapidly stop an To prevent an attack To decrease the existing attack before when the patient will frequency and the patient be exposed to a severity of ongoing experiences M&M known stimulus attacks Treatment for HAE must be individualized to provide optimal care and normalize QOL 20 Busse P, et al. J Allergy Clin Immunol Pract. 2020:
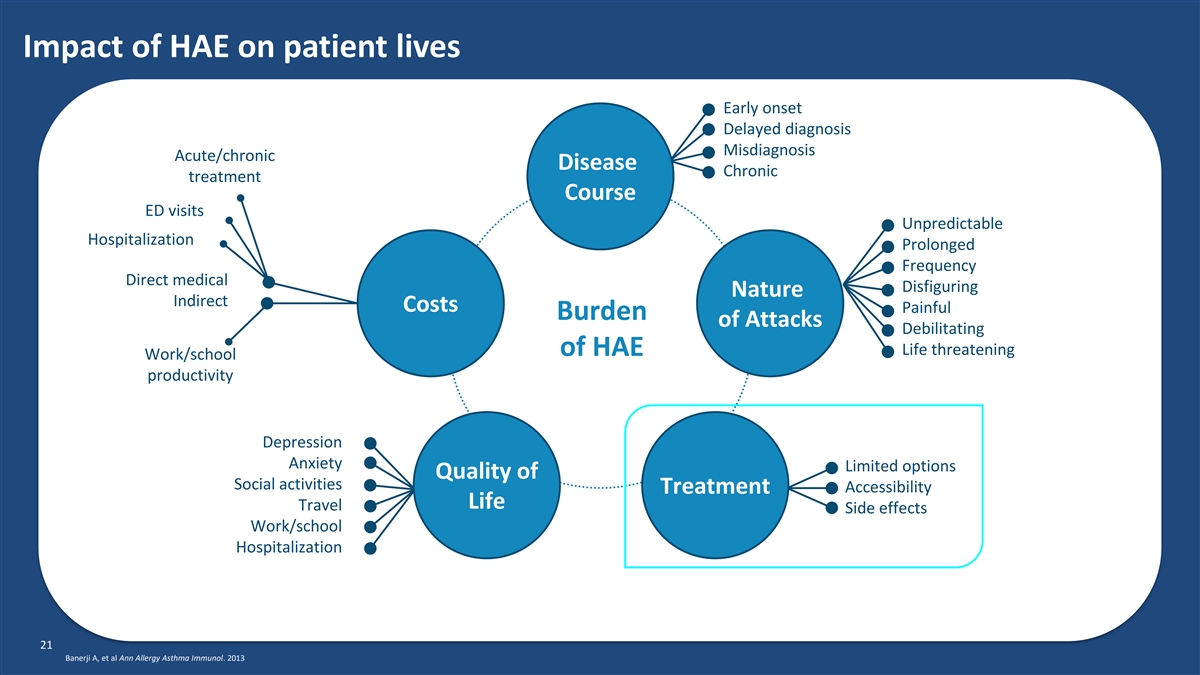
Impact of HAE on patient lives Early onset Delayed diagnosis Misdiagnosis Acute/chronic Disease Chronic treatment Course ED visits Unpredictable Hospitalization Prolonged Frequency Direct medical Disfiguring Nature Indirect Costs Painful Burden of Attacks Debilitating Life threatening of HAE Work/school productivity Depression Anxiety Limited options Quality of Social activities Accessibility Treatment Life Travel Side effects Work/school Hospitalization 21 Banerji A, et al Ann Allergy Asthma Immunol. 2013
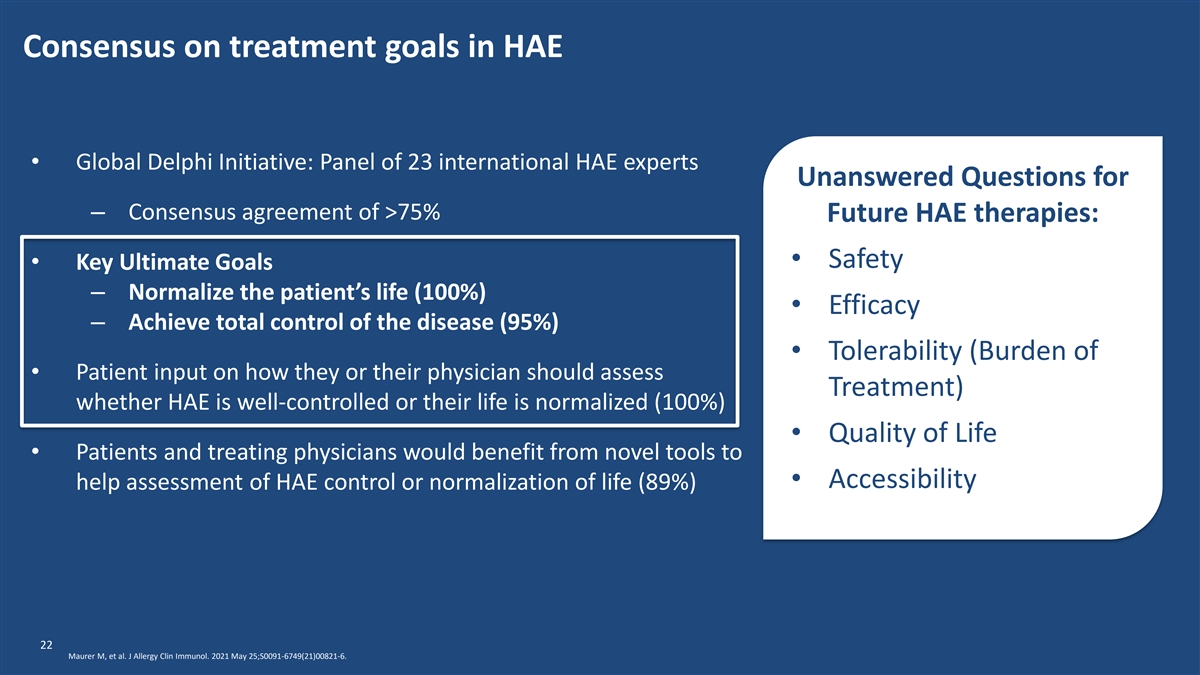
Consensus on treatment goals in HAE • Global Delphi Initiative: Panel of 23 international HAE experts Unanswered Questions for – Consensus agreement of >75% Future HAE therapies: • Safety • Key Ultimate Goals – Normalize the patient’s life (100%) • Efficacy – Achieve total control of the disease (95%) • Tolerability (Burden of • Patient input on how they or their physician should assess Treatment) whether HAE is well-controlled or their life is normalized (100%) • Quality of Life • Patients and treating physicians would benefit from novel tools to • Accessibility help assessment of HAE control or normalization of life (89%) 22 Maurer M, et al. J Allergy Clin Immunol. 2021 May 25;S0091-6749(21)00821-6.

The road forward for unmet needs • Current state of patient management: – Prevention of death and excessive pain – Reduced hospitalizations and disability • Unmet Needs: – Reduced treatment burden and frequency – Life without interference from HAE • Potential Next-Generation Therapies – KLKB1-targeting gene editing (e.g. Poseida) – KLKB1-targeting anti-sense oligonucleotides (e.g. Ionis) – C1-INH AAV-based gene therapy (e.g. BioMarin) – Targeted oral therapies (kallikrein inhibition, B2 receptor antagonism) 23

Thank you

P-KLKB1-101 for the treatment of Hereditary Angioedema (HAE) Ap p lication of Cas - CLOV ER P r e s e n t e r : B l a i r M a d i s o n , P h D
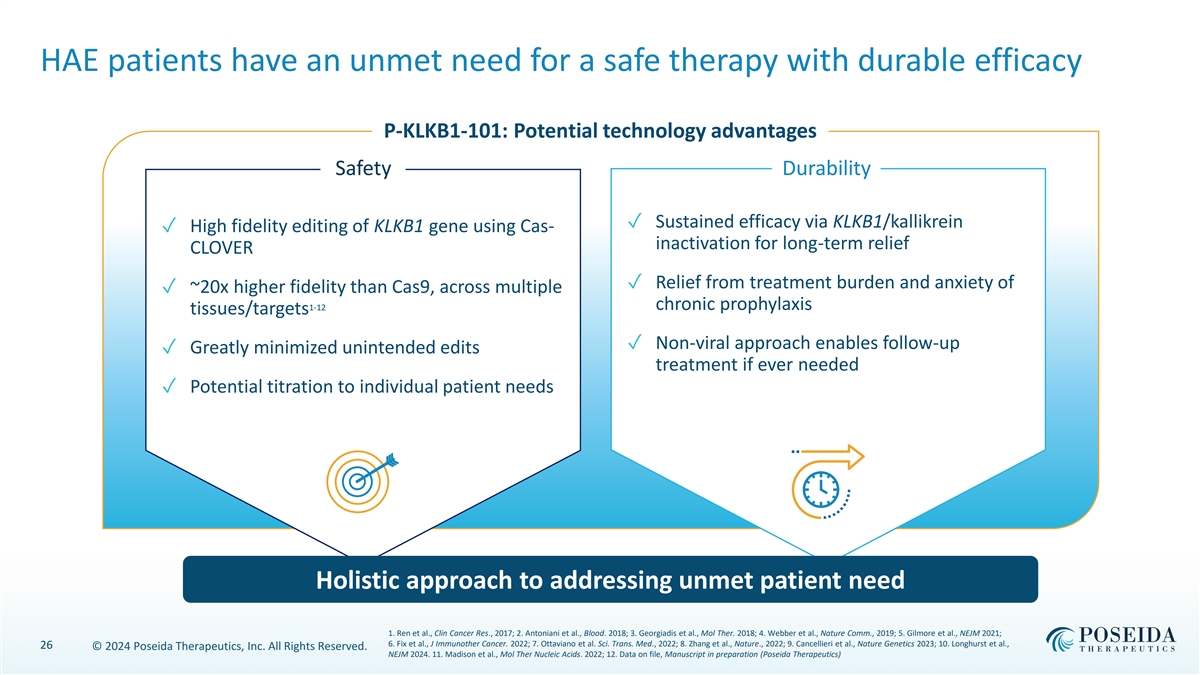
HAE patients have an unmet need for a safe therapy with durable efficacy P-KLKB1-101: Potential technology advantages Safety Durability ✓ Sustained efficacy via KLKB1/kallikrein ✓ High fidelity editing of KLKB1 gene using Cas- inactivation for long-term relief CLOVER ✓ Relief from treatment burden and anxiety of ✓ ~20x higher fidelity than Cas9, across multiple 1-12 chronic prophylaxis tissues/targets ✓ Non-viral approach enables follow-up ✓ Greatly minimized unintended edits treatment if ever needed ✓ Potential titration to individual patient needs Holistic approach to addressing unmet patient need 1. Ren et al., Clin Cancer Res., 2017; 2. Antoniani et al., Blood. 2018; 3. Georgiadis et al., Mol Ther. 2018; 4. Webber et al., Nature Comm., 2019; 5. Gilmore et al., NEJM 2021; 6. Fix et al., J Immunother Cancer. 2022; 7. Ottaviano et al. Sci. Trans. Med., 2022; 8. Zhang et al., Nature., 2022; 9. Cancellieri et al., Nature Genetics 2023; 10. Longhurst et al., 26 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. NEJM 2024. 11. Madison et al., Mol Ther Nucleic Acids. 2022; 12. Data on file, Manuscript in preparation (Poseida Therapeutics)
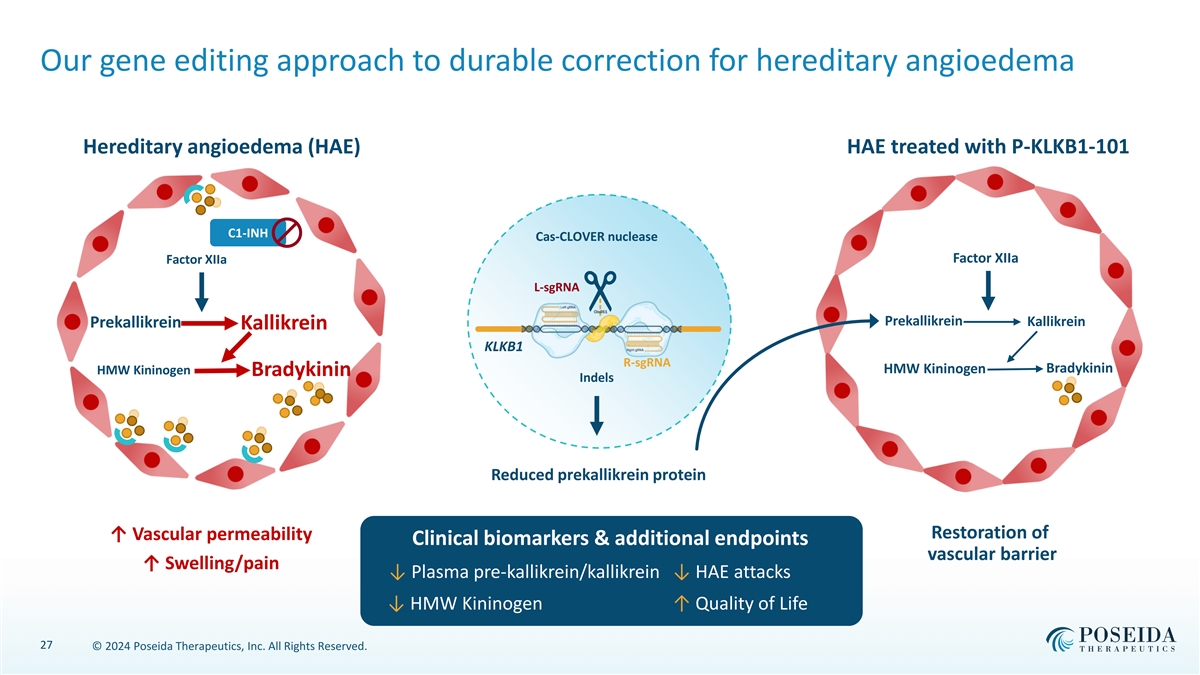
Our gene editing approach to durable correction for hereditary angioedema Hereditary angioedema (HAE) HAE treated with P-KLKB1-101 C1-INH Cas-CLOVER nuclease Factor XIIa Factor XIIa L-sgRNA Prekallikrein Kallikrein Prekallikrein Kallikrein KLKB1 R-sgRNA Bradykinin HMW Kininogen HMW Kininogen Bradykinin Indels Reduced prekallikrein protein Restoration of ↑ Vascular permeability Clinical biomarkers & additional endpoints vascular barrier ↑ Swelling/pain ↓ Plasma pre-kallikrein/kallikrein ↓ HAE attacks ↓ HMW Kininogen ↑ Quality of Life 27 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
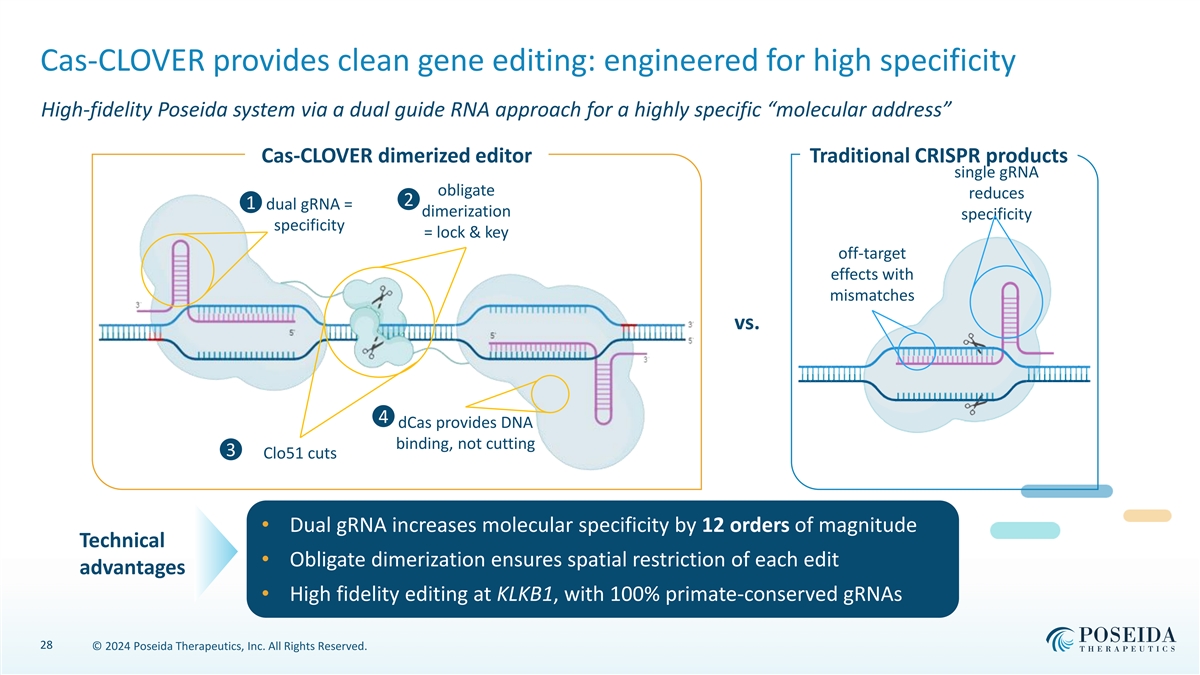
Cas-CLOVER provides clean gene editing: engineered for high specificity High-fidelity Poseida system via a dual guide RNA approach for a highly specific “molecular address” Cas-CLOVER dimerized editor Traditional CRISPR products single gRNA obligate reduces 2 1 dual gRNA = dimerization specificity specificity = lock & key off-target effects with mismatches vs. 4 dCas provides DNA binding, not cutting 3 Clo51 cuts • Dual gRNA increases molecular specificity by 12 orders of magnitude Technical • Obligate dimerization ensures spatial restriction of each edit advantages • High fidelity editing at KLKB1, with 100% primate-conserved gRNAs 28 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
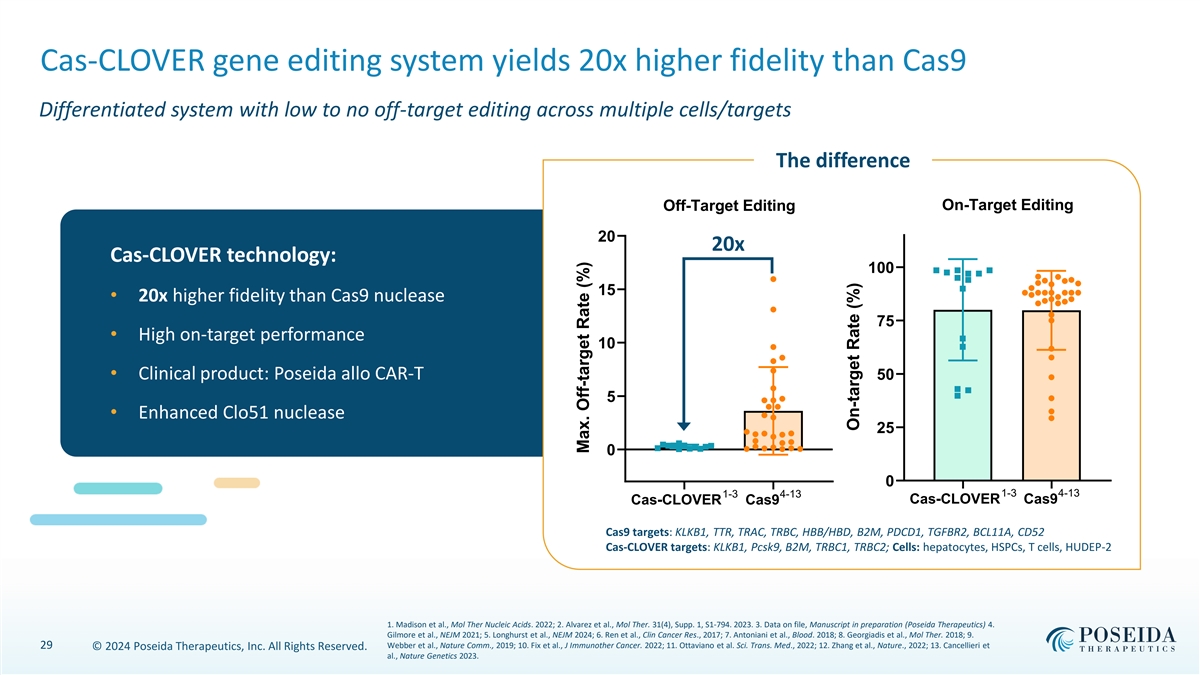
Cas-CLOVER gene editing system yields 20x higher fidelity than Cas9 Differentiated system with low to no off-target editing across multiple cells/targets The difference On-Target Editing Off-Target Editing 20 20x Cas-CLOVER technology: 100 15 • 20x higher fidelity than Cas9 nuclease 75 • High on-target performance 10 • Clinical product: Poseida allo CAR-T 50 5 • Enhanced Clo51 nuclease 25 0 0 1-3 4-13 1-3 4-13 Cas-CLOVER Cas9 Cas-CLOVER Cas9 Cas9 targets: KLKB1, TTR, TRAC, TRBC, HBB/HBD, B2M, PDCD1, TGFBR2, BCL11A, CD52 Cas-CLOVER targets: KLKB1, Pcsk9, B2M, TRBC1, TRBC2; Cells: hepatocytes, HSPCs, T cells, HUDEP-2 1. Madison et al., Mol Ther Nucleic Acids. 2022; 2. Alvarez et al., Mol Ther. 31(4), Supp. 1, S1-794. 2023. 3. Data on file, Manuscript in preparation (Poseida Therapeutics) 4. Gilmore et al., NEJM 2021; 5. Longhurst et al., NEJM 2024; 6. Ren et al., Clin Cancer Res., 2017; 7. Antoniani et al., Blood. 2018; 8. Georgiadis et al., Mol Ther. 2018; 9. 29 Webber et al., Nature Comm., 2019; 10. Fix et al., J Immunother Cancer. 2022; 11. Ottaviano et al. Sci. Trans. Med., 2022; 12. Zhang et al., Nature., 2022; 13. Cancellieri et © 2024 Poseida Therapeutics, Inc. All Rights Reserved. al., Nature Genetics 2023. Max. Off-target Rate (%) On-target Rate (%)
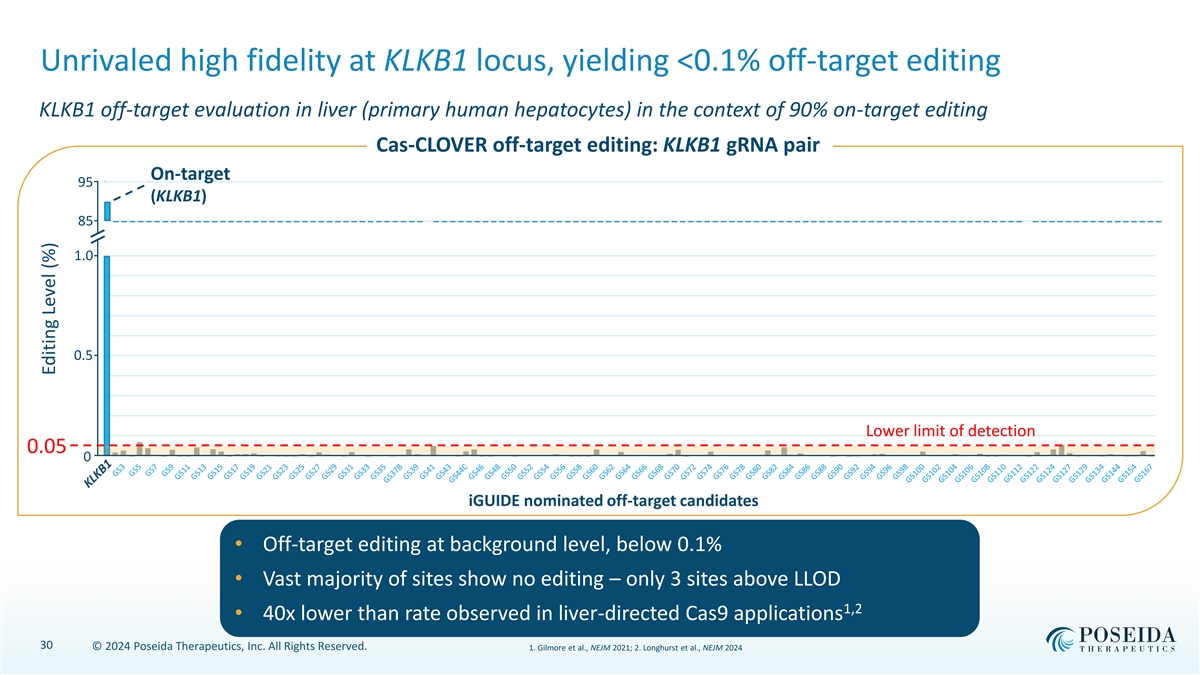
Unrivaled high fidelity at KLKB1 locus, yielding <0.1% off-target editing KLKB1 off-target evaluation in liver (primary human hepatocytes) in the context of 90% on-target editing Cas-CLOVER off-target editing: KLKB1 gRNA pair On-target 95 95 (KLKB1) 85 85 1.0 1.0 0.9 0.8 0.7 0.6 0.5 0.5 0.4 0.3 0.2 Lower limit of detection 0.1 0.05 0.0 0 iGUIDE nominated off-target candidates • Off-target editing at background level, below 0.1% • Vast majority of sites show no editing – only 3 sites above LLOD 1,2 • 40x lower than rate observed in liver-directed Cas9 applications 30 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. 1. Gilmore et al., NEJM 2021; 2. Longhurst et al., NEJM 2024 Editing Level (%)
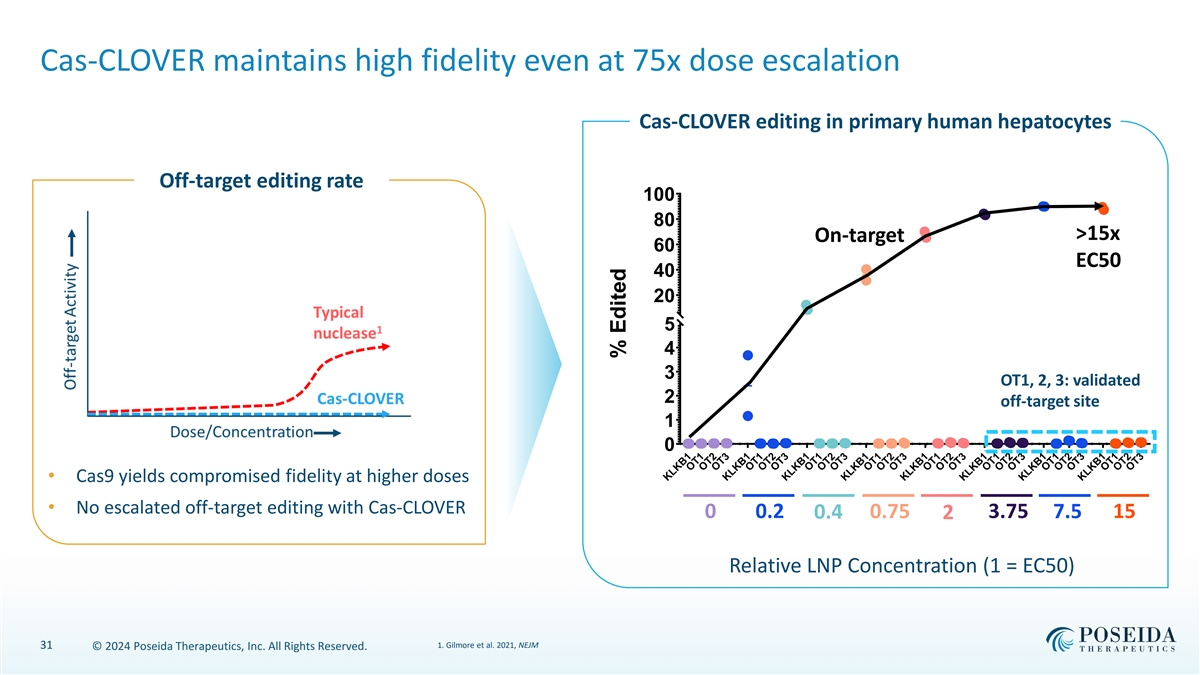
Cas-CLOVER maintains high fidelity even at 75x dose escalation Cas-CLOVER editing in primary human hepatocytes Off-target editing rate 100 80 >15x On-target 60 EC50 40 20 5 4 3 OT1, 2, 3: validated 2 off-target site 1 0 • Cas9 yields compromised fidelity at higher doses • No escalated off-target editing with Cas-CLOVER 0 0.2 0.4 0.75 3.75 7.5 15 2 Relative LNP Concentration (1 = EC50) 1. Gilmore et al. 2021, NEJM 31 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. KLKB1 OT1 OT2 OT3 KLKB1 OT1 OT2 OT3 KLKB1 OT1 OT2 OT3 KLKB1 OT1 OT2 OT3 KLKB1 OT1 OT2 OT3 KLKB1 OT1 OT2 OT3 KLKB1 OT1 OT2 OT3 KLKB1 OT1 OT2 OT3 % Edited

P-KLKB1-101 for the treatment of Hereditary Angioedema (HAE) In vivo ap p lication of Cas - CLOV ER: Ph armacology stu dies P r e s e n t e r : B o n n i e J a c q u e s , P h D
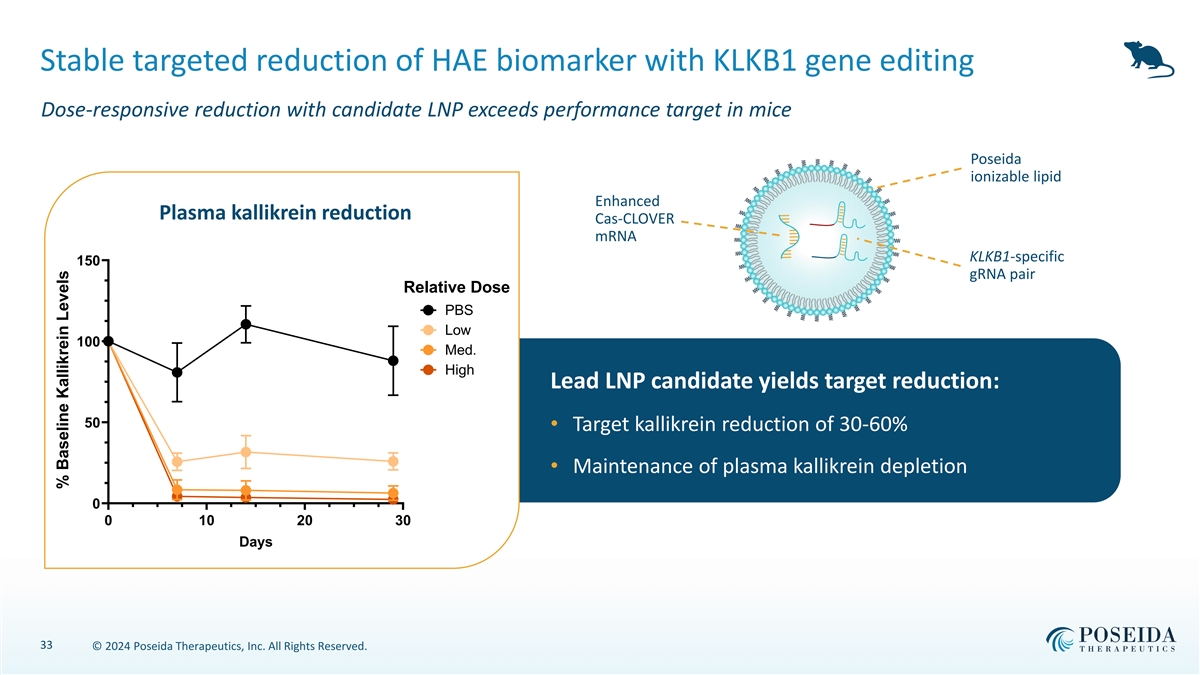
Stable targeted reduction of HAE biomarker with KLKB1 gene editing Dose-responsive reduction with candidate LNP exceeds performance target in mice Poseida ionizable lipid Enhanced Plasma kallikrein reduction Cas-CLOVER mRNA KLKB1-specific 150 gRNA pair Relative Dose PBS Low 100 Med. High Lead LNP candidate yields target reduction: 50 • Target kallikrein reduction of 30-60% • Maintenance of plasma kallikrein depletion 0 0 10 20 30 Days 33 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. % Baseline Kallikrein Levels
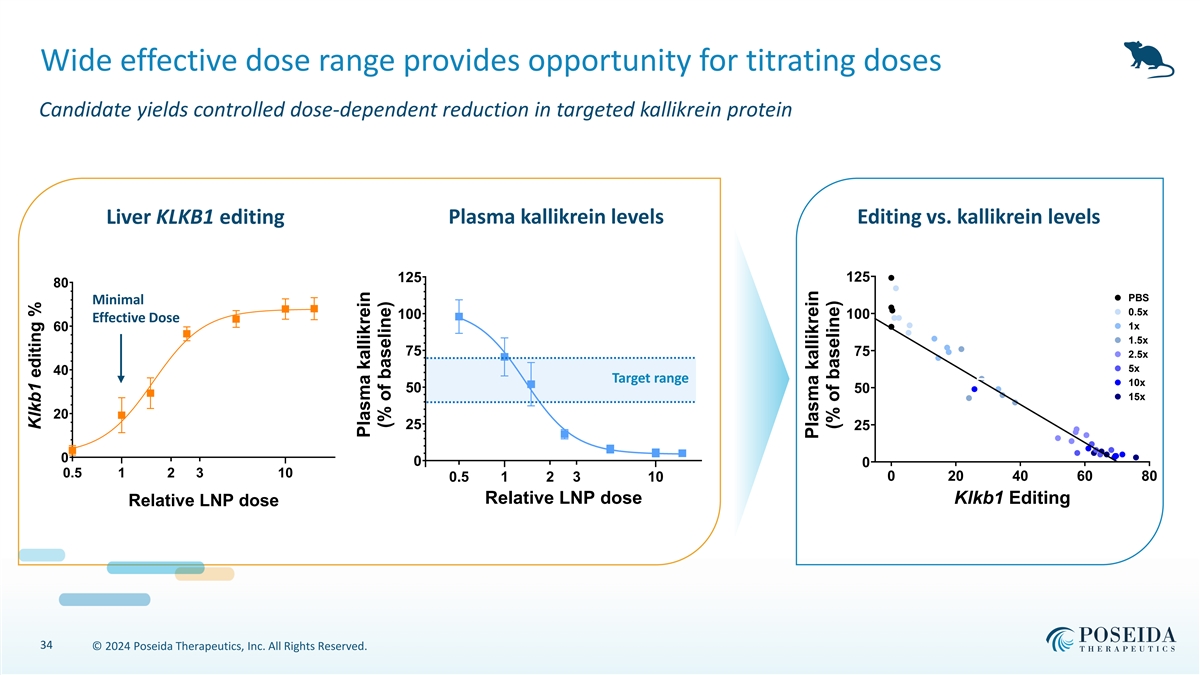
Wide effective dose range provides opportunity for titrating doses Candidate yields controlled dose-dependent reduction in targeted kallikrein protein Liver KLKB1 editing Plasma kallikrein levels Editing vs. kallikrein levels 125 125 80 PBS Minimal 0.5x 100 100 Effective Dose 1x 60 1.5x 75 75 2.5x 5x 40 L L Target range 10x 50 50 15x 20 25 25 0 0 0 0.5 1 2 3 10 0 20 40 60 80 0.3 0.5 1 2 3 10 Relative LNP dose Klkb1 Editing Relative LNP dose 34 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. Klkb1 editing % Plasma kallikrein (% of baseline) Plasma kallikrein (% of baseline)
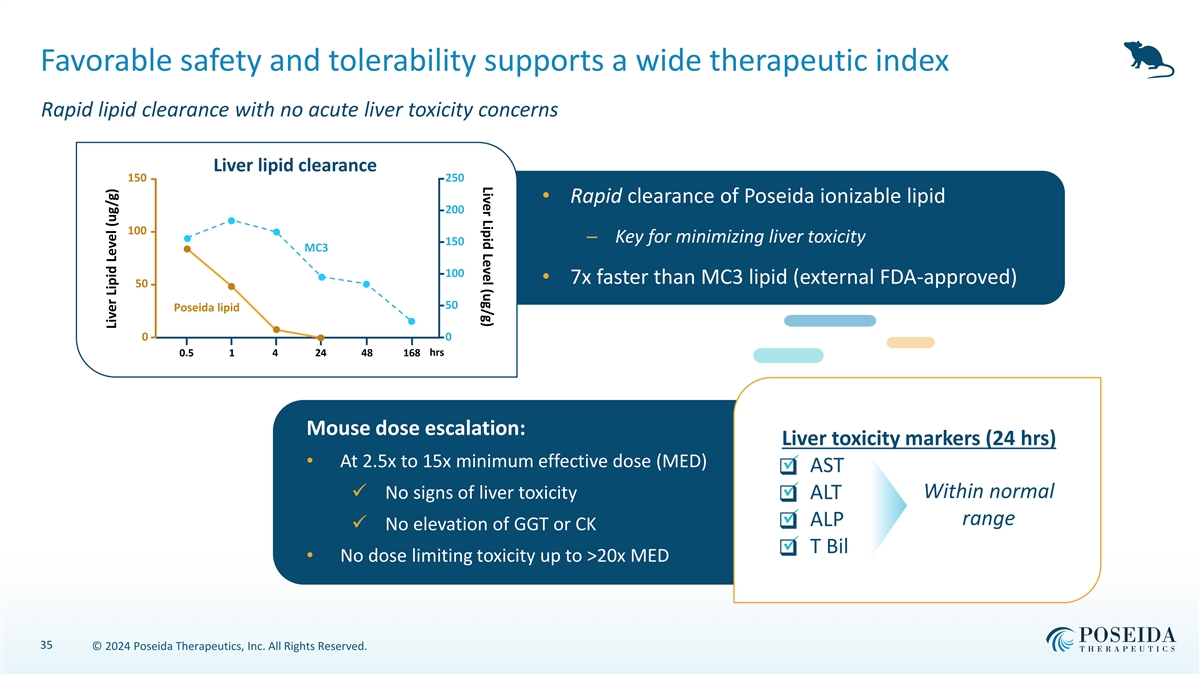
Liver Lipid Level (ug/g) Favorable safety and tolerability supports a wide therapeutic index Rapid lipid clearance with no acute liver toxicity concerns Liver lipid clearance 150 250 • Rapid clearance of Poseida ionizable lipid 200 100 ─ Key for minimizing liver toxicity 150 MC3 100 • 7x faster than MC3 lipid (external FDA-approved) 50 50 Poseida lipid 0 0 0.5 1 4 24 48 168 hrs Mouse dose escalation: Liver toxicity markers (24 hrs) • At 2.5x to 15x minimum effective dose (MED) ✓ AST ❑ ✓ No signs of liver toxicity✓ ALT Within normal ❑ range ✓ ALP ❑ ✓ No elevation of GGT or CK ✓ T Bil ❑ • No dose limiting toxicity up to >20x MED 35 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. Liver Lipid Level (ug/g)
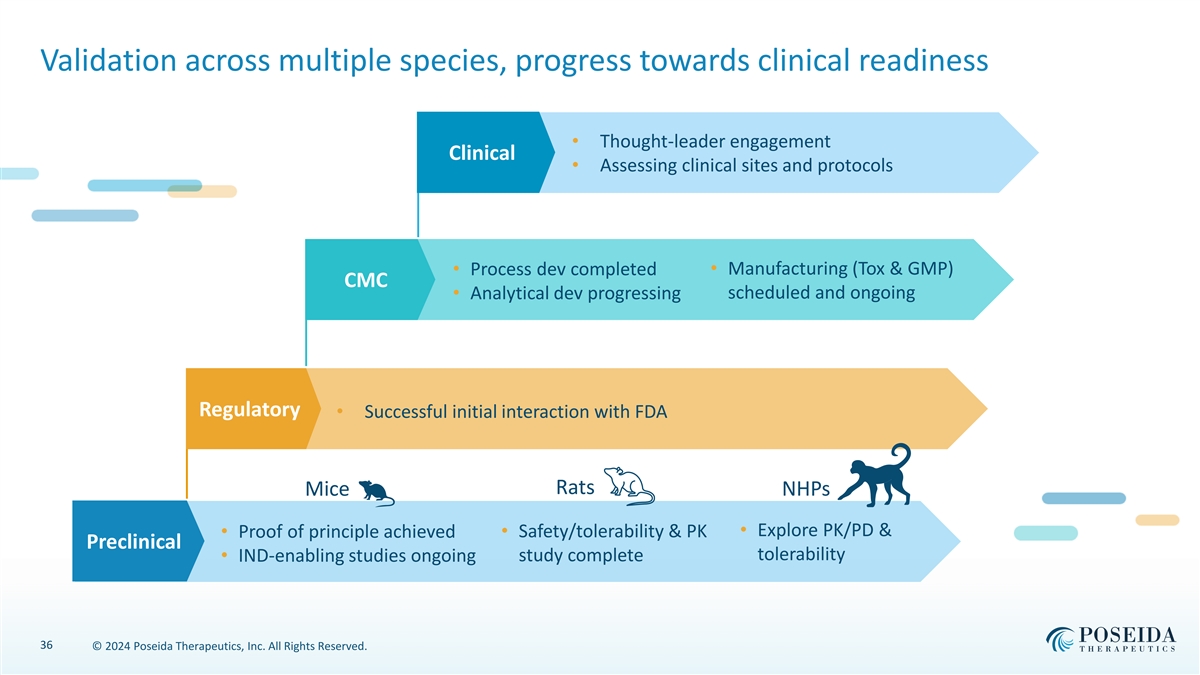
Validation across multiple species, progress towards clinical readiness • Thought-leader engagement Clinical • Assessing clinical sites and protocols • Process dev completed• Manufacturing (Tox & GMP) CMC scheduled and ongoing • Analytical dev progressing Regulatory • Successful initial interaction with FDA Rats Mice NHPs • Explore PK/PD & • Proof of principle achieved• Safety/tolerability & PK Preclinical tolerability study complete • IND-enabling studies ongoing 36 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.

Poseida’s non-viral gene insertion system P r e s e n t e r : J a c k Ry c h a k , P h D
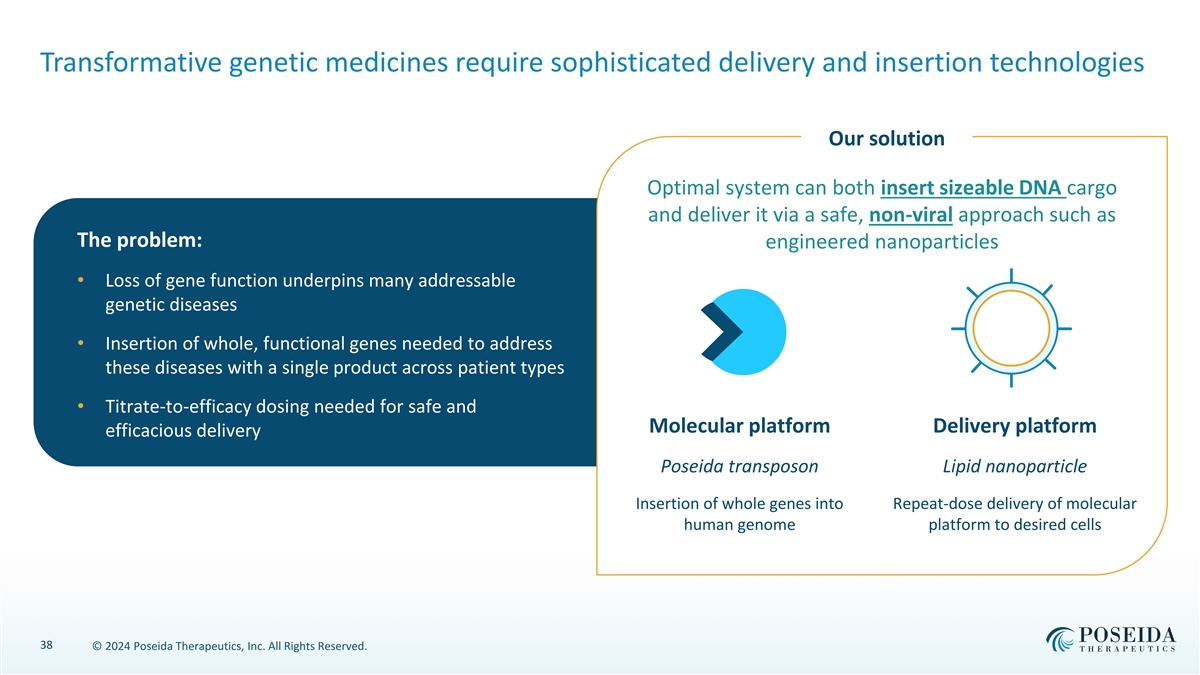
Transformative genetic medicines require sophisticated delivery and insertion technologies Our solution Optimal system can both insert sizeable DNA cargo and deliver it via a safe, non-viral approach such as The problem: engineered nanoparticles • Loss of gene function underpins many addressable genetic diseases • Insertion of whole, functional genes needed to address these diseases with a single product across patient types • Titrate-to-efficacy dosing needed for safe and Molecular platform Delivery platform efficacious delivery Poseida transposon Lipid nanoparticle Insertion of whole genes into Repeat-dose delivery of molecular human genome platform to desired cells 38 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
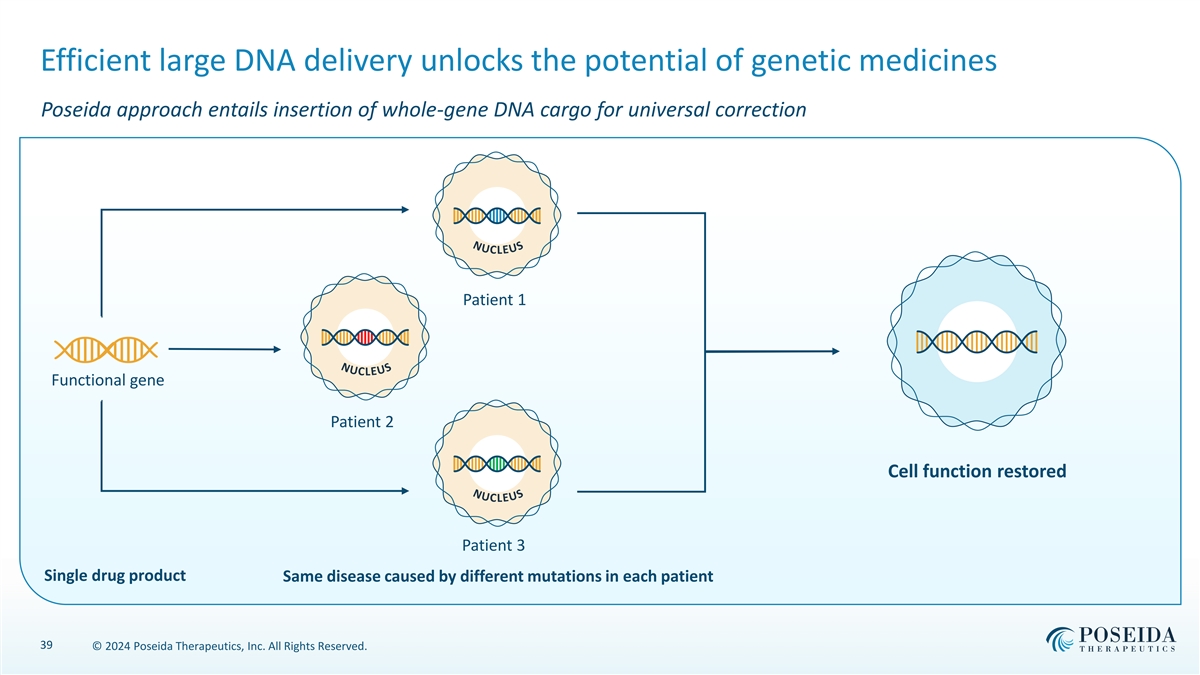
N N N U U U C C C Efficient large DNA delivery unlocks the potential of genetic medicines Poseida approach entails insertion of whole-gene DNA cargo for universal correction Patient 1 Functional gene Patient 2 Cell function restored Patient 3 Single drug product Same disease caused by different mutations in each patient 39 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. L L L E E E U U U S S S
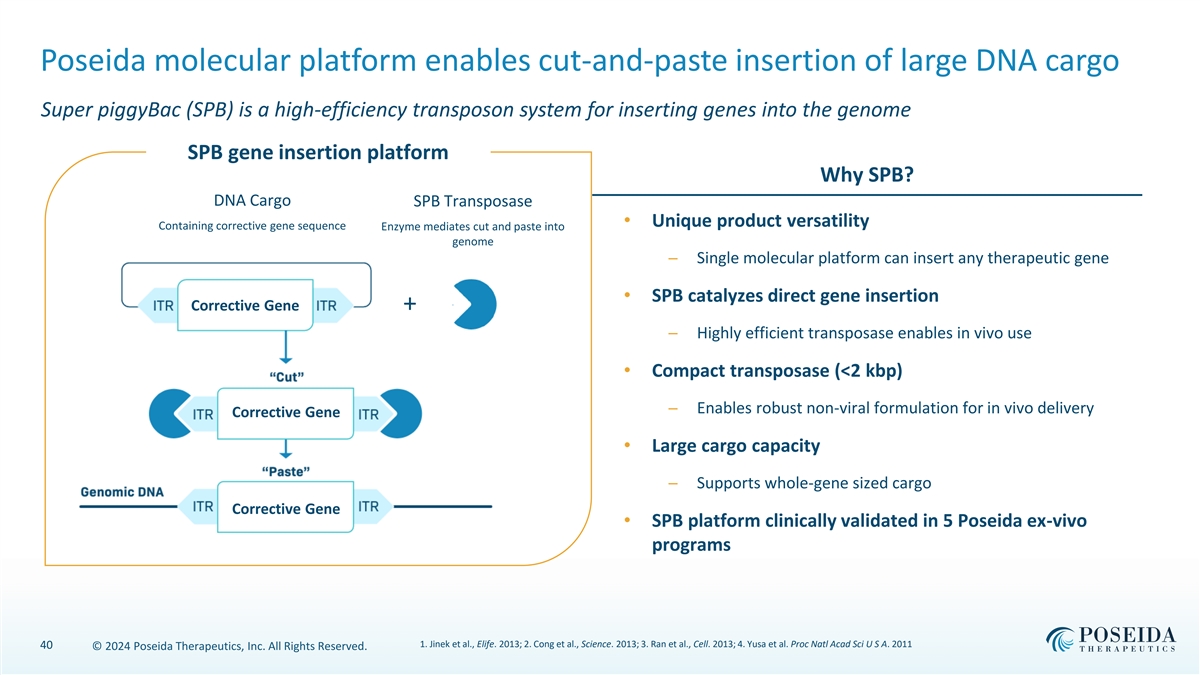
Poseida molecular platform enables cut-and-paste insertion of large DNA cargo Super piggyBac (SPB) is a high-efficiency transposon system for inserting genes into the genome SPB gene insertion platform Why SPB? DNA Cargo SPB Transposase • Unique product versatility Containing corrective gene sequence Enzyme mediates cut and paste into genome ─ Single molecular platform can insert any therapeutic gene • SPB catalyzes direct gene insertion Corrective Gene + ─ Highly efficient transposase enables in vivo use • Compact transposase (<2 kbp) ─ Enables robust non-viral formulation for in vivo delivery Corrective Gene • Large cargo capacity ─ Supports whole-gene sized cargo Corrective Gene • SPB platform clinically validated in 5 Poseida ex-vivo programs 1. Jinek et al., Elife. 2013; 2. Cong et al., Science. 2013; 3. Ran et al., Cell. 2013; 4. Yusa et al. Proc Natl Acad Sci U S A. 2011 40 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
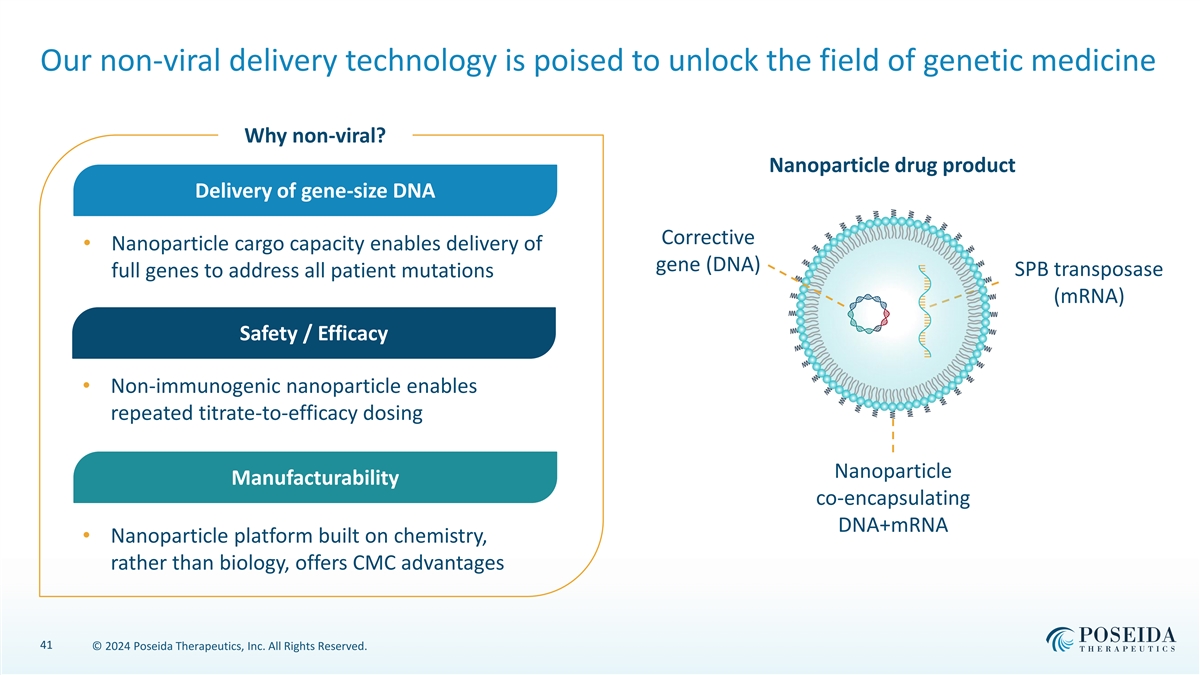
Our non-viral delivery technology is poised to unlock the field of genetic medicine Why non-viral? Nanoparticle drug product Delivery of gene-size DNA Corrective • Nanoparticle cargo capacity enables delivery of gene (DNA) SPB transposase full genes to address all patient mutations (mRNA) Safety / Efficacy • Non-immunogenic nanoparticle enables repeated titrate-to-efficacy dosing Nanoparticle Manufacturability co-encapsulating DNA+mRNA • Nanoparticle platform built on chemistry, rather than biology, offers CMC advantages 41 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
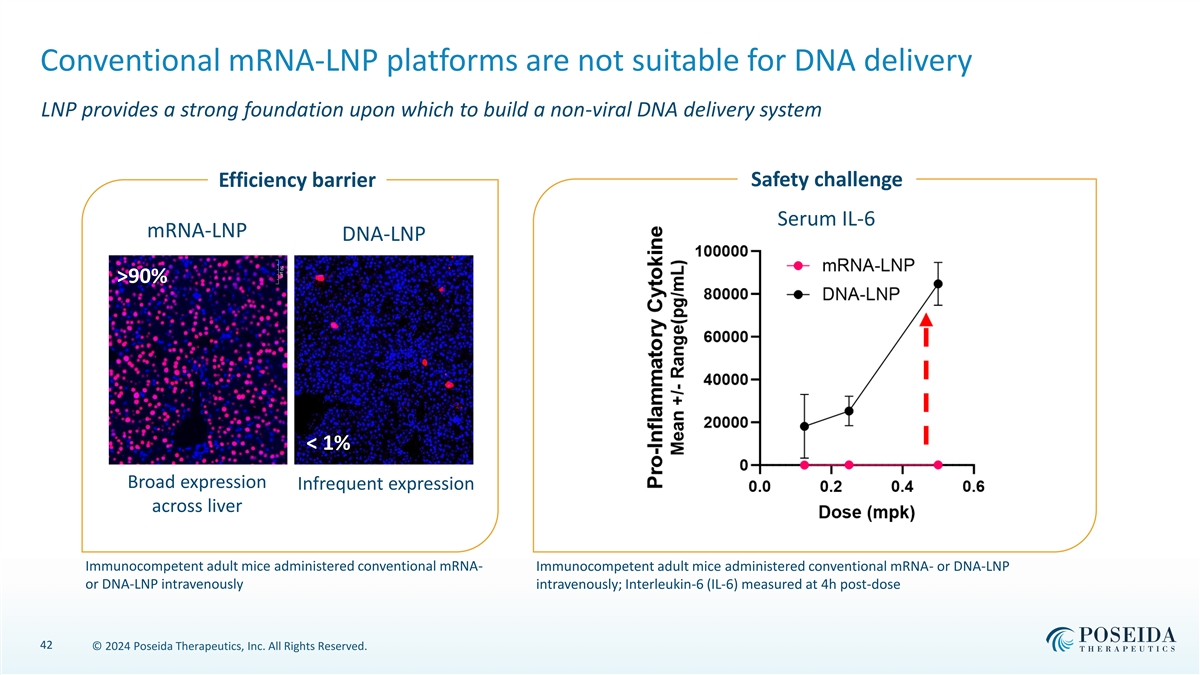
Conventional mRNA-LNP platforms are not suitable for DNA delivery LNP provides a strong foundation upon which to build a non-viral DNA delivery system Safety challenge Efficiency barrier Serum IL-6 mRNA-LNP DNA-LNP >90% < 1% Broad expression Infrequent expression across liver Immunocompetent adult mice administered conventional mRNA- Immunocompetent adult mice administered conventional mRNA- or DNA-LNP or DNA-LNP intravenously intravenously; Interleukin-6 (IL-6) measured at 4h post-dose 42 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
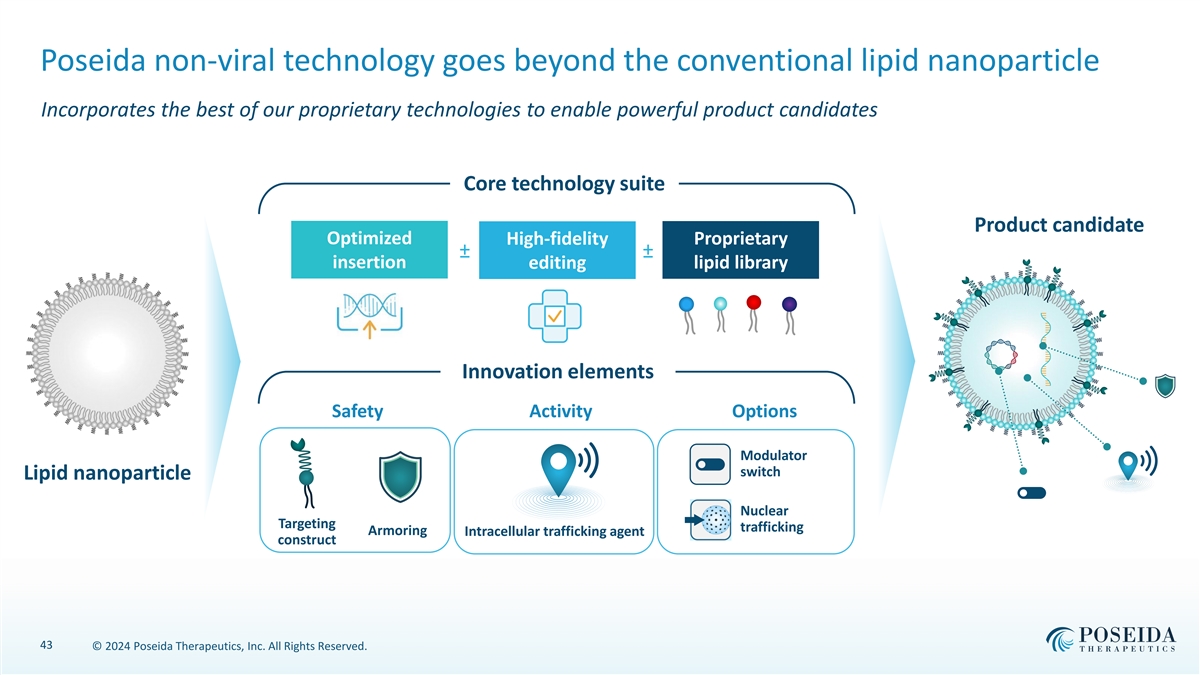
Poseida non-viral technology goes beyond the conventional lipid nanoparticle Incorporates the best of our proprietary technologies to enable powerful product candidates Core technology suite Product candidate Optimized High-fidelity Proprietary ± a ± a insertion editing lipid library Innovation elements Safety Activity Options Modulator switch Lipid nanoparticle Nuclear Targeting trafficking Armoring Intracellular trafficking agent construct 43 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
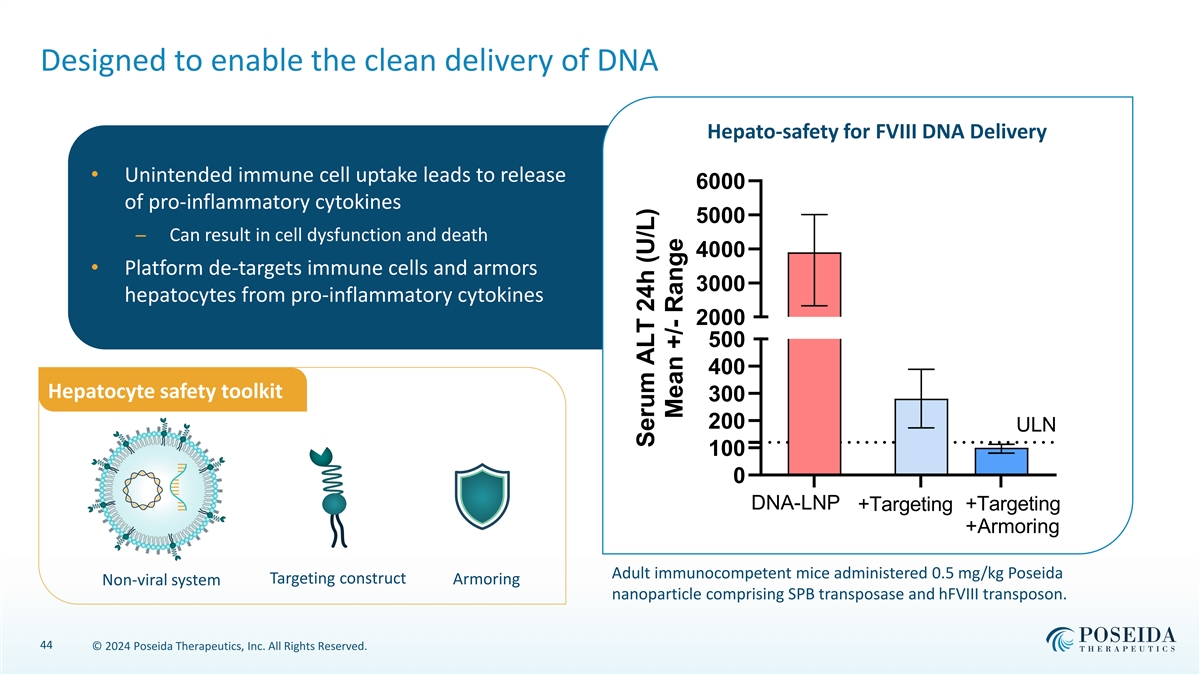
Designed to enable the clean delivery of DNA Hepato-safety for FVIII DNA Delivery • Unintended immune cell uptake leads to release 6000 of pro-inflammatory cytokines 5000 ─ Can result in cell dysfunction and death 4000 • Platform de-targets immune cells and armors 3000 hepatocytes from pro-inflammatory cytokines 2000 500 400 Hepatocyte safety toolkit 300 200 ULN 100 0 DNA-LNP +Targeting +Targeting +Armoring Adult immunocompetent mice administered 0.5 mg/kg Poseida Targeting construct Non-viral system Armoring nanoparticle comprising SPB transposase and hFVIII transposon. 44 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. Serum ALT 24h (U/L) Mean +/- Range
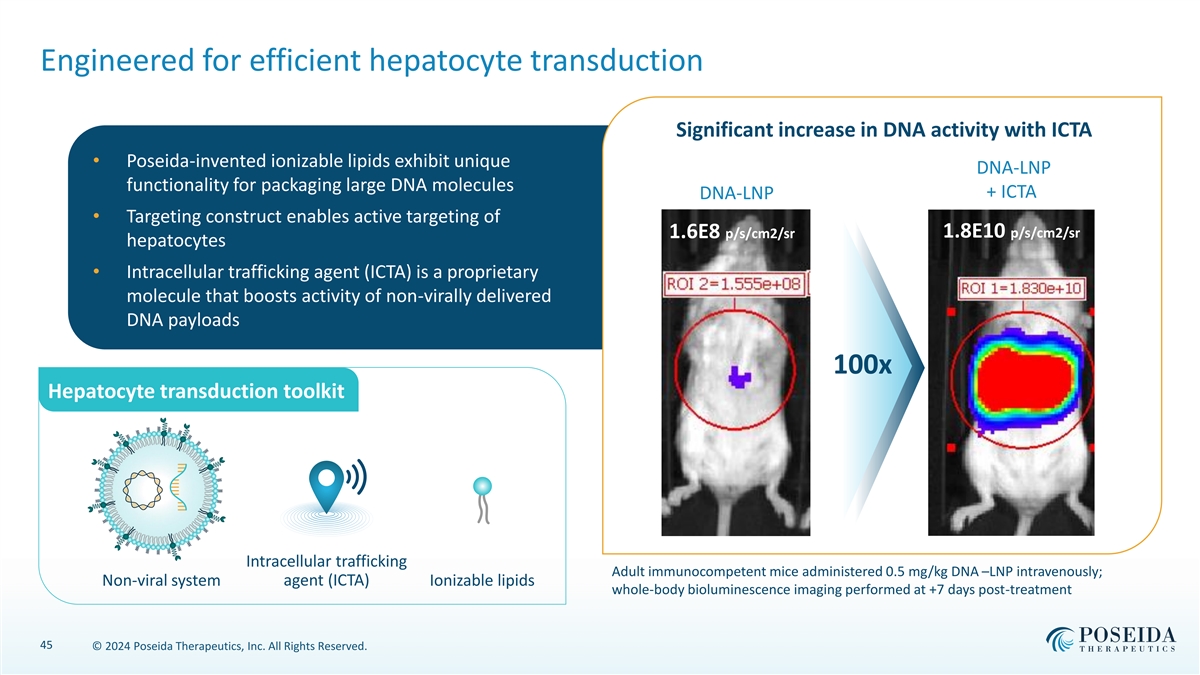
Engineered for efficient hepatocyte transduction Significant increase in DNA activity with ICTA • Poseida-invented ionizable lipids exhibit unique DNA-LNP functionality for packaging large DNA molecules + ICTA DNA-LNP • Targeting construct enables active targeting of 1.8E10 p/s/cm2/sr 1.6E8 p/s/cm2/sr hepatocytes • Intracellular trafficking agent (ICTA) is a proprietary molecule that boosts activity of non-virally delivered DNA payloads 100x Hepatocyte transduction toolkit Intracellular trafficking Adult immunocompetent mice administered 0.5 mg/kg DNA –LNP intravenously; Non-viral system agent (ICTA) Ionizable lipids whole-body bioluminescence imaging performed at +7 days post-treatment 45 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
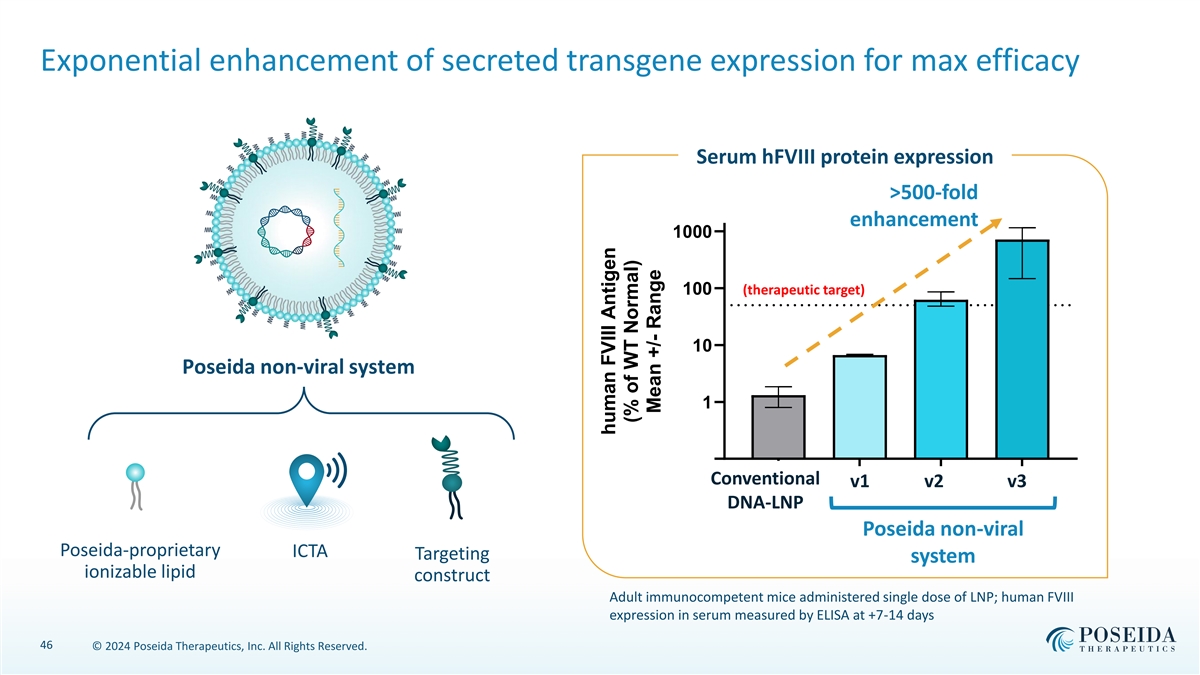
Exponential enhancement of secreted transgene expression for max efficacy Serum hFVIII protein expression >500-fold enhancement 1000 100 (therapeutic target) 10 Poseida non-viral system 1 0.1 Conventional LNP-A LN vP 1- B v2 LNP -1 v3 LNP-2 DNA-LNP Poseida non-viral Poseida-proprietary ICTA Targeting system ionizable lipid construct Adult immunocompetent mice administered single dose of LNP; human FVIII expression in serum measured by ELISA at +7-14 days 46 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. human FVIII Antigen (% of WT Normal) Mean +/- Range
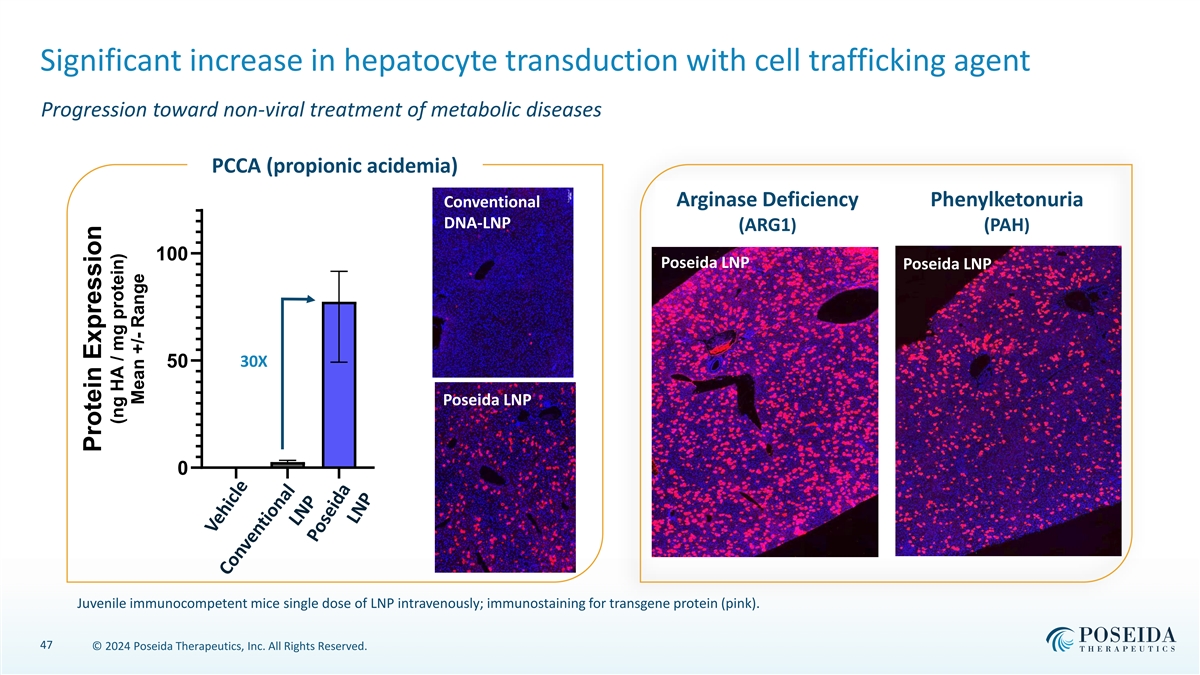
Significant increase in hepatocyte transduction with cell trafficking agent Progression toward non-viral treatment of metabolic diseases C12 – SPB PCCA (propionic acidemia) Arginase Deficiency Phenylketonuria Conventional DNA-LNP (ARG1) (PAH) 100 Poseida LNP Poseida LNP 50 30X Poseida LNP 0 Juvenile immunocompetent mice single dose of LNP intravenously; immunostaining for transgene protein (pink). 47 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. Protein Expression (ng HA / mg protein) Mean +/- Range
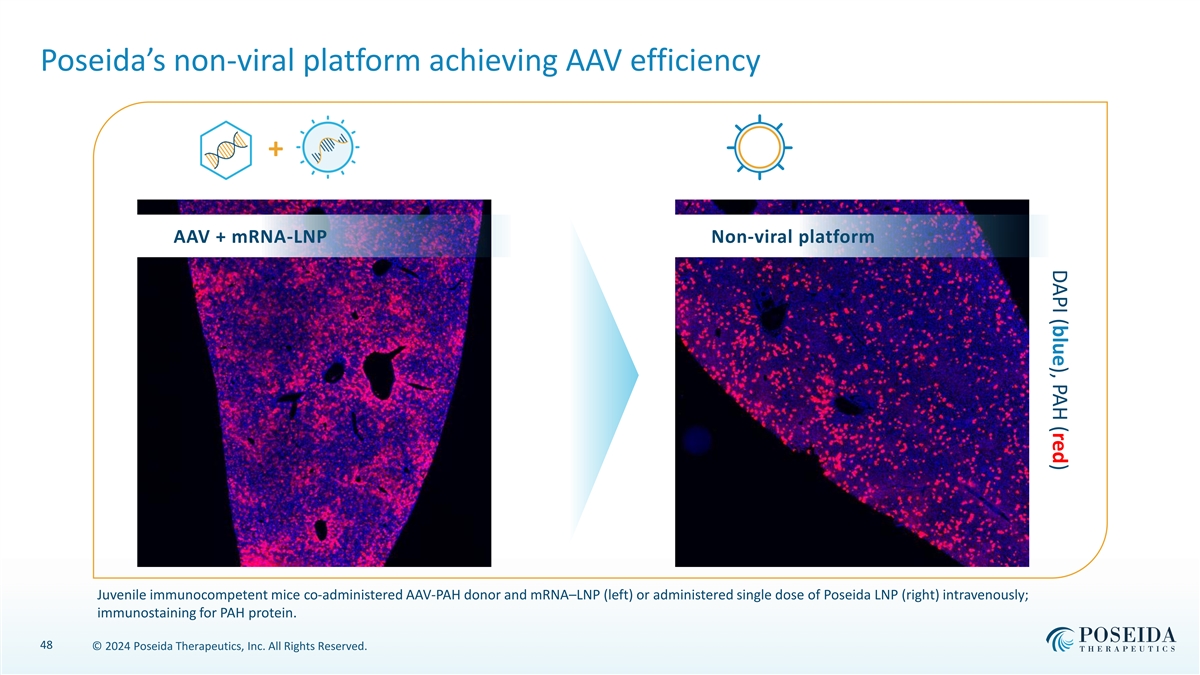
DAPI (blue), PAH (red) Poseida’s non-viral platform achieving AAV efficiency + AAV + mRNA-LNP Non-viral platform Juvenile immunocompetent mice co-administered AAV-PAH donor and mRNA–LNP (left) or administered single dose of Poseida LNP (right) intravenously; immunostaining for PAH protein. 48 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
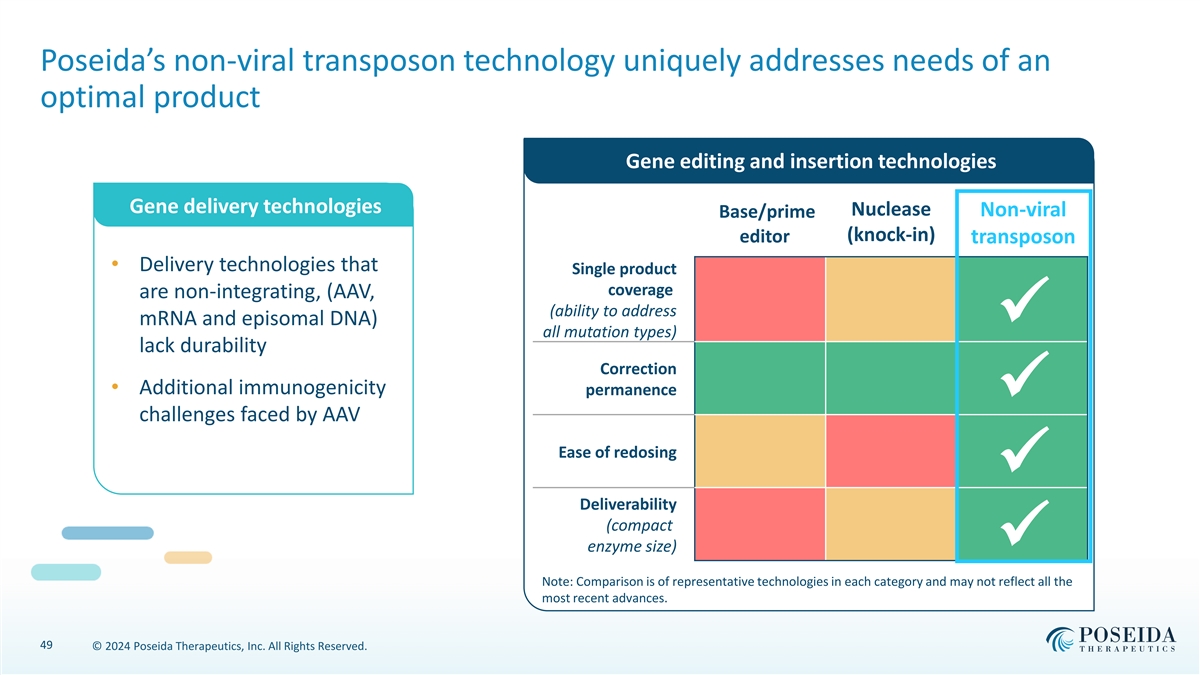
Poseida’s non-viral transposon technology uniquely addresses needs of an optimal product Gene editing and insertion technologies Gene delivery technologies Nuclease Non-viral Base/prime (knock-in) editor transposon • Delivery technologies that Single product coverage are non-integrating, (AAV, (ability to address mRNA and episomal DNA) ✓ all mutation types) lack durability Correction • Additional immunogenicity permanence ✓ challenges faced by AAV Ease of redosing ✓ Deliverability (compact enzyme size)✓ Note: Comparison is of representative technologies in each category and may not reflect all the most recent advances. 49 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
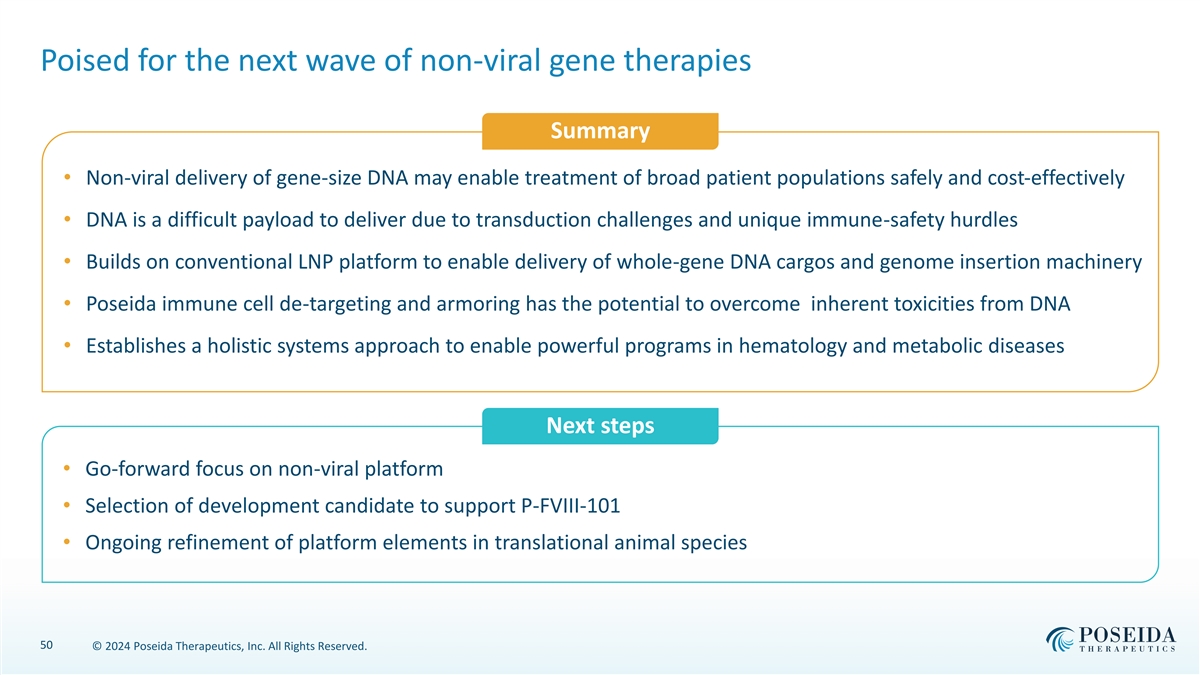
Poised for the next wave of non-viral gene therapies Summary • Non-viral delivery of gene-size DNA may enable treatment of broad patient populations safely and cost-effectively • DNA is a difficult payload to deliver due to transduction challenges and unique immune-safety hurdles • Builds on conventional LNP platform to enable delivery of whole-gene DNA cargos and genome insertion machinery • Poseida immune cell de-targeting and armoring has the potential to overcome inherent toxicities from DNA • Establishes a holistic systems approach to enable powerful programs in hematology and metabolic diseases Next steps • Go-forward focus on non-viral platform • Selection of development candidate to support P-FVIII-101 • Ongoing refinement of platform elements in translational animal species 50 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.

Treatment landscape for Hemophilia A: Available Therapies and Unmet Needs Steven W. Pipe, MD Professor of Pediatrics and Pathology, University of Michigan © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
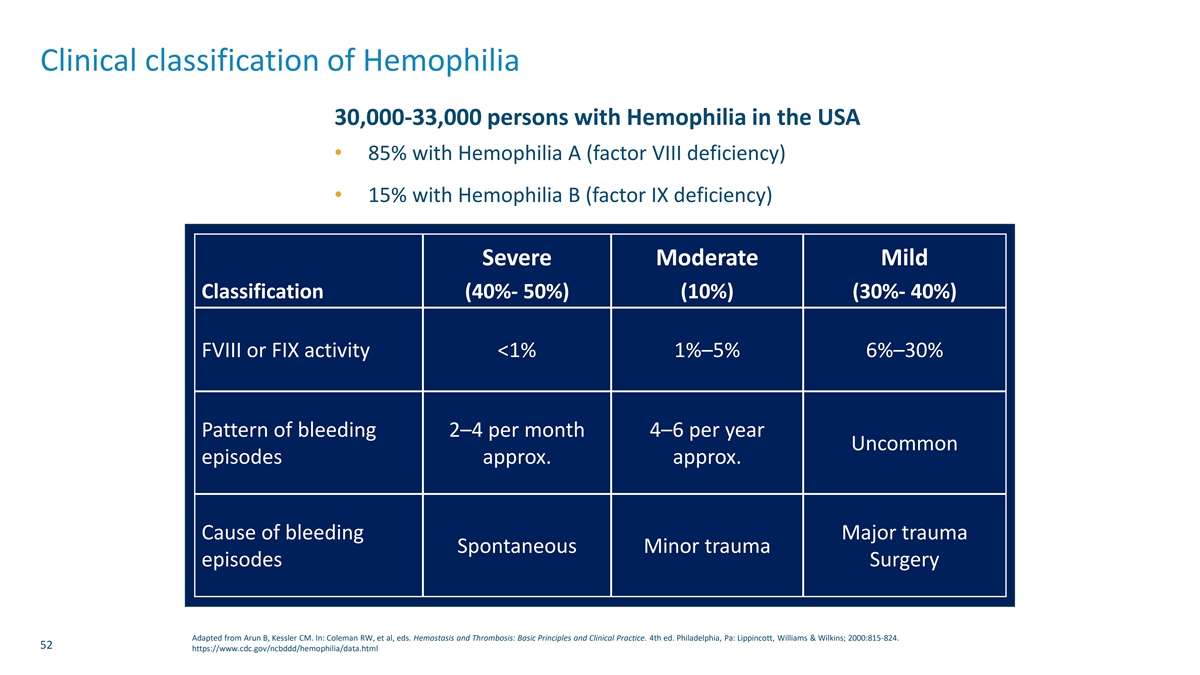
Clinical classification of Hemophilia 30,000-33,000 persons with Hemophilia in the USA • 85% with Hemophilia A (factor VIII deficiency) • 15% with Hemophilia B (factor IX deficiency) Severe Moderate Mild Classification (40%- 50%) (10%) (30%- 40%) FVIII or FIX activity <1% 1%–5% 6%–30% Pattern of bleeding 2–4 per month 4–6 per year Uncommon episodes approx. approx. Cause of bleeding Major trauma Spontaneous Minor trauma episodes Surgery Adapted from Arun B, Kessler CM. In: Coleman RW, et al, eds. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. 4th ed. Philadelphia, Pa: Lippincott, Williams & Wilkins; 2000:815-824. 52 https://www.cdc.gov/ncbddd/hemophilia/data.html
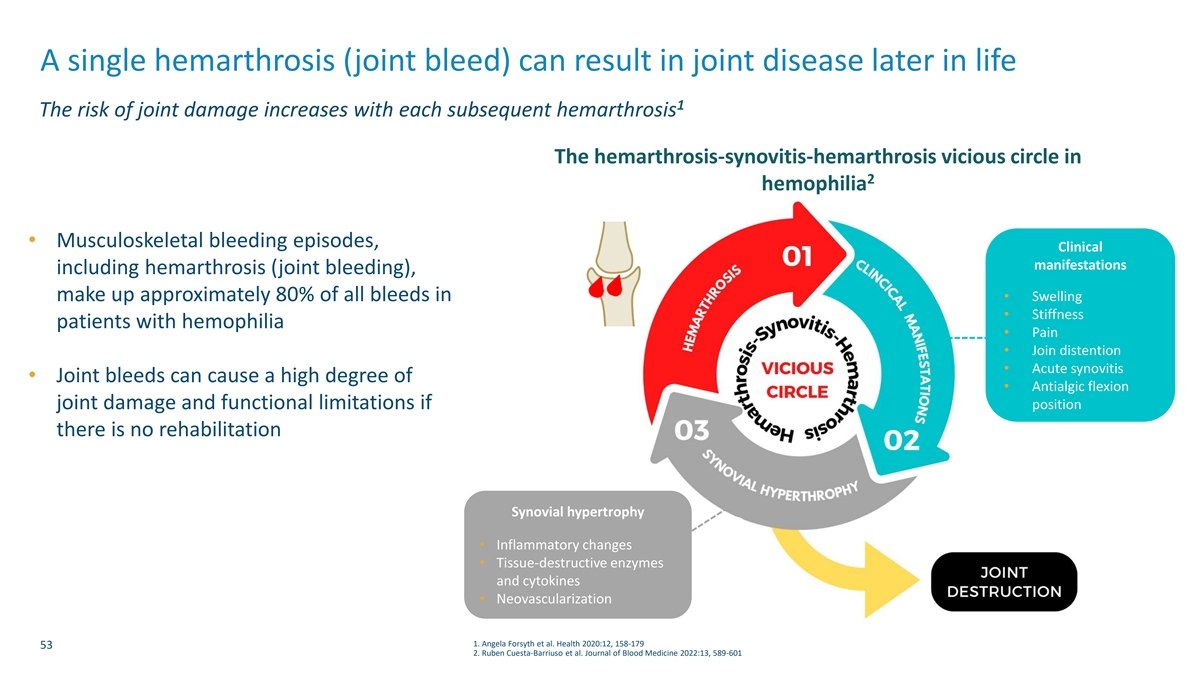
A single hemarthrosis (joint bleed) can result in joint disease later in life 1 The risk of joint damage increases with each subsequent hemarthrosis The hemarthrosis-synovitis-hemarthrosis vicious circle in 2 hemophilia • Musculoskeletal bleeding episodes, Clinical manifestations including hemarthrosis (joint bleeding), make up approximately 80% of all bleeds in • Swelling • Stiffness patients with hemophilia • Pain • Join distention • Acute synovitis • Joint bleeds can cause a high degree of • Antialgic flexion position joint damage and functional limitations if there is no rehabilitation Synovial hypertrophy • Inflammatory changes • Tissue-destructive enzymes and cytokines • Neovascularization 1. Angela Forsyth et al. Health 2020:12, 158-179 53 2. Ruben Cuesta-Barriuso et al. Journal of Blood Medicine 2022:13, 589-601
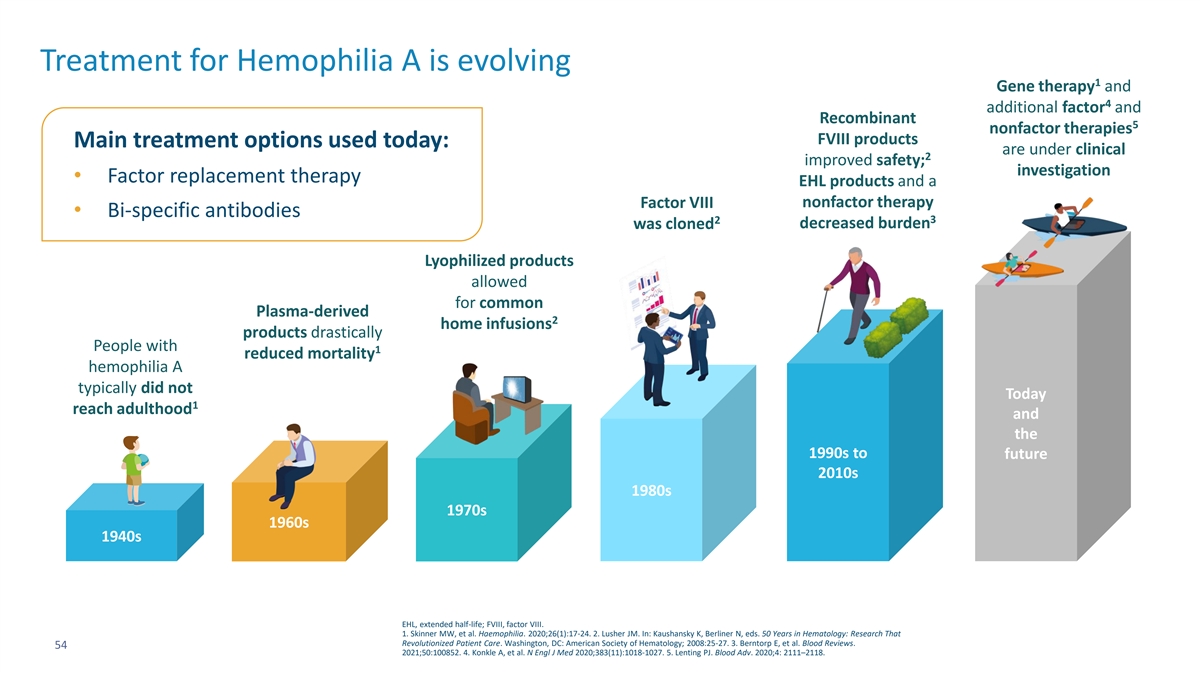
Treatment for Hemophilia A is evolving 1 Gene therapy and 4 additional factor and Recombinant 5 nonfactor therapies FVIII products Main treatment options used today: are under clinical 2 improved safety; investigation • Factor replacement therapy EHL products and a nonfactor therapy Factor VIII • Bi-specific antibodies 2 3 was cloned decreased burden Lyophilized products allowed for common Plasma-derived 2 home infusions products drastically People with 1 reduced mortality hemophilia A typically did not Today 1 reach adulthood and the 1990s to future 2010s 1980s 1970s 1960s 1940s EHL, extended half-life; FVIII, factor VIII. 1. Skinner MW, et al. Haemophilia. 2020;26(1):17-24. 2. Lusher JM. In: Kaushansky K, Berliner N, eds. 50 Years in Hematology: Research That Revolutionized Patient Care. Washington, DC: American Society of Hematology; 2008:25-27. 3. Berntorp E, et al. Blood Reviews. 54 2021;50:100852. 4. Konkle A, et al. N Engl J Med 2020;383(11):1018-1027. 5. Lenting PJ. Blood Adv. 2020;4: 2111–2118.
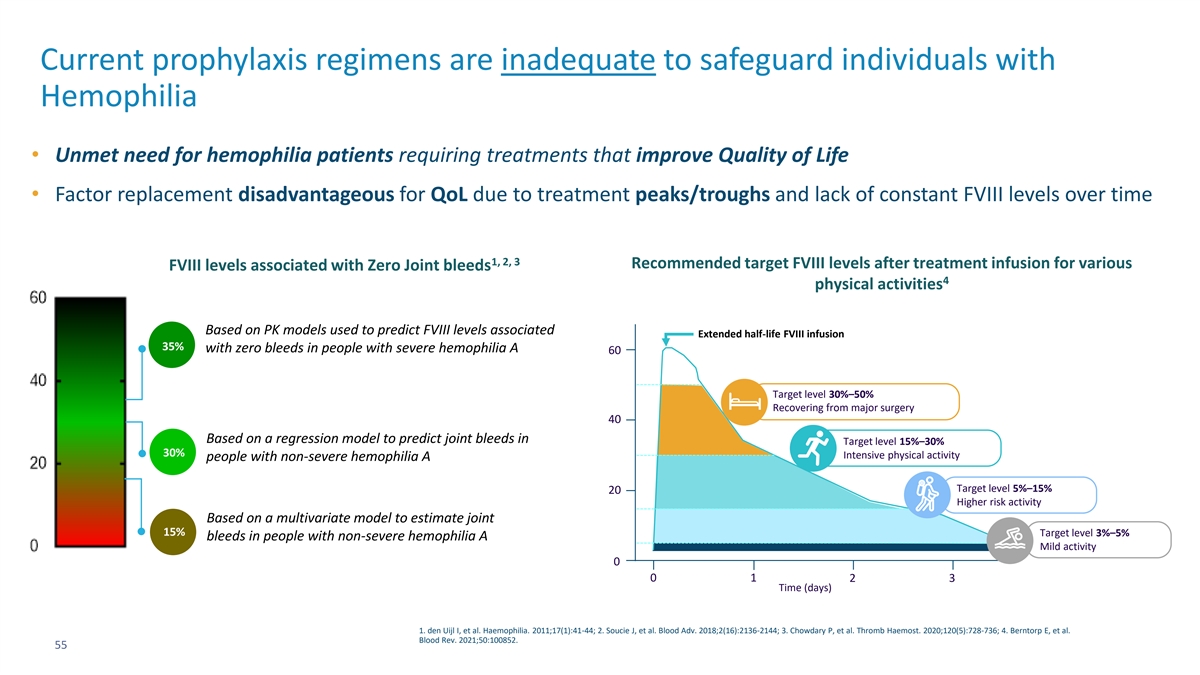
Current prophylaxis regimens are inadequate to safeguard individuals with Hemophilia • Unmet need for hemophilia patients requiring treatments that improve Quality of Life • Factor replacement disadvantageous for QoL due to treatment peaks/troughs and lack of constant FVIII levels over time 1, 2, 3 Recommended target FVIII levels after treatment infusion for various FVIII levels associated with Zero Joint bleeds 4 physical activities Based on PK models used to predict FVIII levels associated Extended half-life FVIII infusion 35% with zero bleeds in people with severe hemophilia A 60 Target level 30%–50% Recovering from major surgery 40 Based on a regression model to predict joint bleeds in Target level 15%–30% 30% Intensive physical activity people with non-severe hemophilia A Target level 5%–15% 20 Higher risk activity Based on a multivariate model to estimate joint 15% Target level 3%–5% bleeds in people with non-severe hemophilia A Mild activity 0 0 1 2 3 Time (days) 1. den Uijl I, et al. Haemophilia. 2011;17(1):41-44; 2. Soucie J, et al. Blood Adv. 2018;2(16):2136-2144; 3. Chowdary P, et al. Thromb Haemost. 2020;120(5):728-736; 4. Berntorp E, et al. Blood Rev. 2021;50:100852. 55
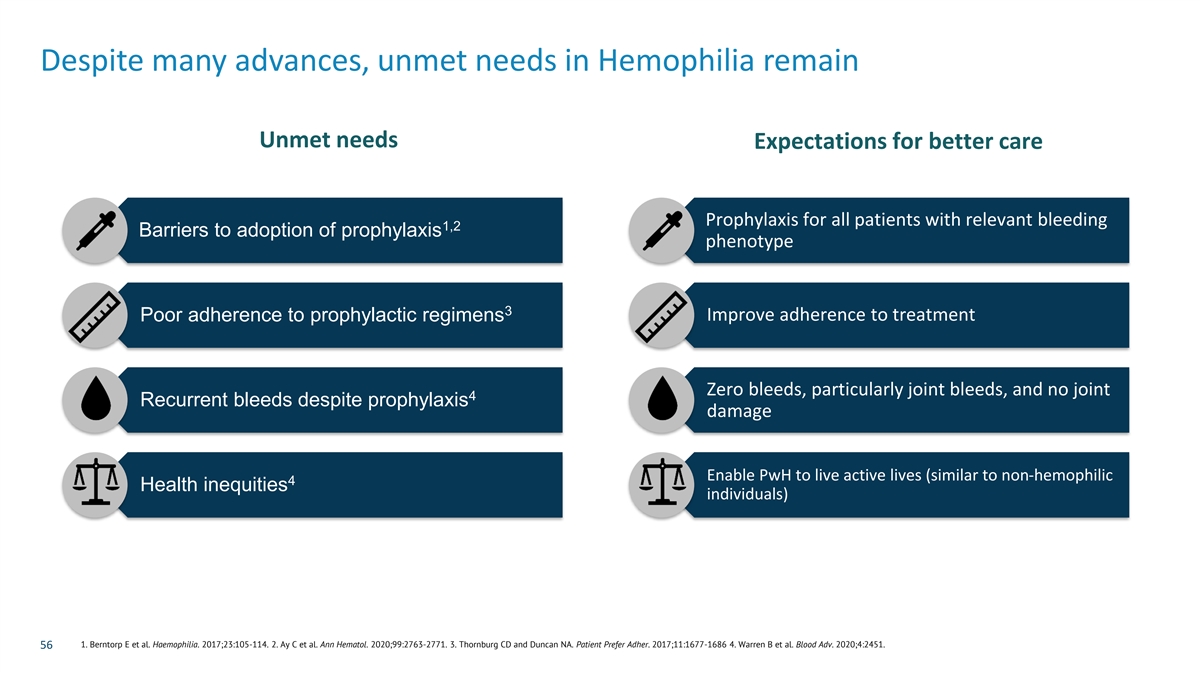
Despite many advances, unmet needs in Hemophilia remain Unmet needs Expectations for better care Prophylaxis for all patients with relevant bleeding 1,2 Barriers to adoption of prophylaxis phenotype 3 Poor adherence to prophylactic regimens Improve adherence to treatment Zero bleeds, particularly joint bleeds, and no joint 4 Recurrent bleeds despite prophylaxis damage Enable PwH to live active lives (similar to non-hemophilic 4 Health inequities individuals) 1. Berntorp E et al. Haemophilia. 2017;23:105-114. 2. Ay C et al. Ann Hematol. 2020;99:2763-2771. 3. Thornburg CD and Duncan NA. Patient Prefer Adher. 2017;11:1677-1686 4. Warren B et al. Blood Adv. 2020;4:2451. 56
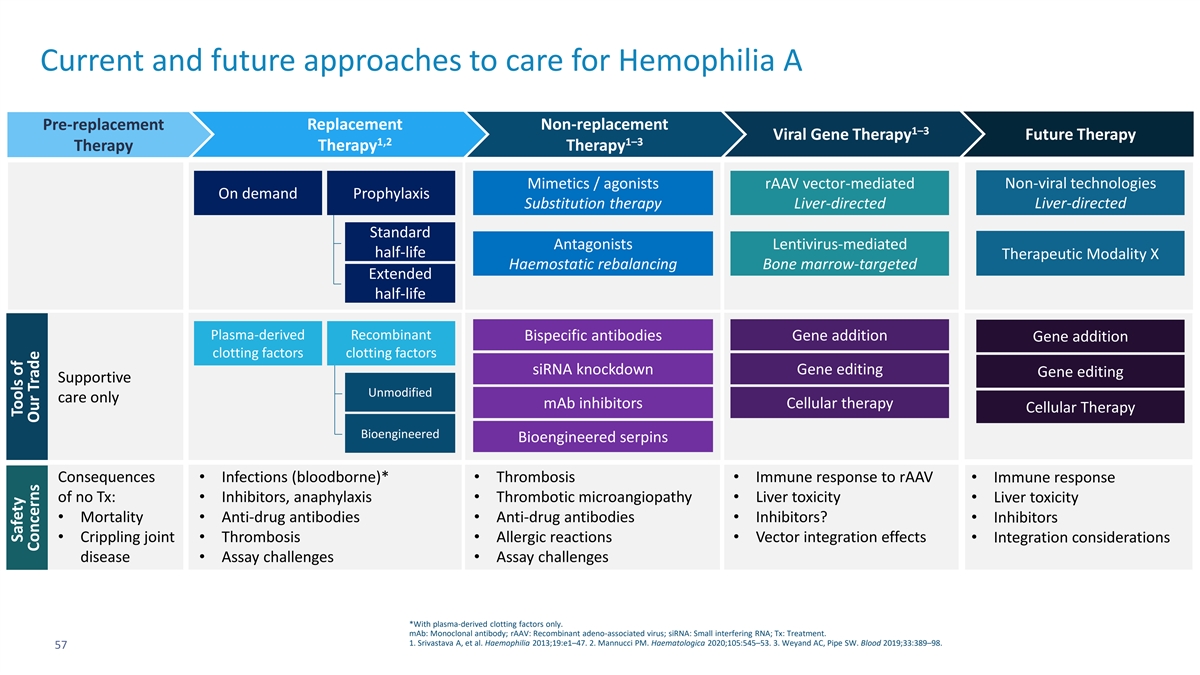
Current and future approaches to care for Hemophilia A Pre-replacement Replacement Non-replacement 1–3 Viral Gene Therapy Future Therapy 1,2 1–3 Therapy Therapy Therapy Mimetics / agonists rAAV vector-mediated Non-viral technologies On demand Prophylaxis Substitution therapy Liver-directed Liver-directed Standard Antagonists Lentivirus-mediated half-life Therapeutic Modality X Haemostatic rebalancing Bone marrow-targeted Extended half-life Plasma-derived Recombinant Bispecific antibodies Gene addition Gene addition clotting factors clotting factors siRNA knockdown Gene editing Gene editing Supportive Unmodified care only mAb inhibitors Cellular therapy Cellular Therapy Bioengineered Bioengineered serpins Consequences • Infections (bloodborne)*• Thrombosis• Immune response to rAAV• Immune response of no Tx:• Inhibitors, anaphylaxis• Thrombotic microangiopathy• Liver toxicity• Liver toxicity • Mortality• Anti-drug antibodies• Anti-drug antibodies• Inhibitors?• Inhibitors • Crippling joint • Thrombosis• Allergic reactions• Vector integration effects• Integration considerations disease• Assay challenges• Assay challenges *With plasma-derived clotting factors only. mAb: Monoclonal antibody; rAAV: Recombinant adeno-associated virus; siRNA: Small interfering RNA; Tx: Treatment. 1. Srivastava A, et al. Haemophilia 2013;19:e1–47. 2. Mannucci PM. Haematologica 2020;105:545–53. 3. Weyand AC, Pipe SW. Blood 2019;33:389–98. 57 Safety Tools of Concerns Our Trade
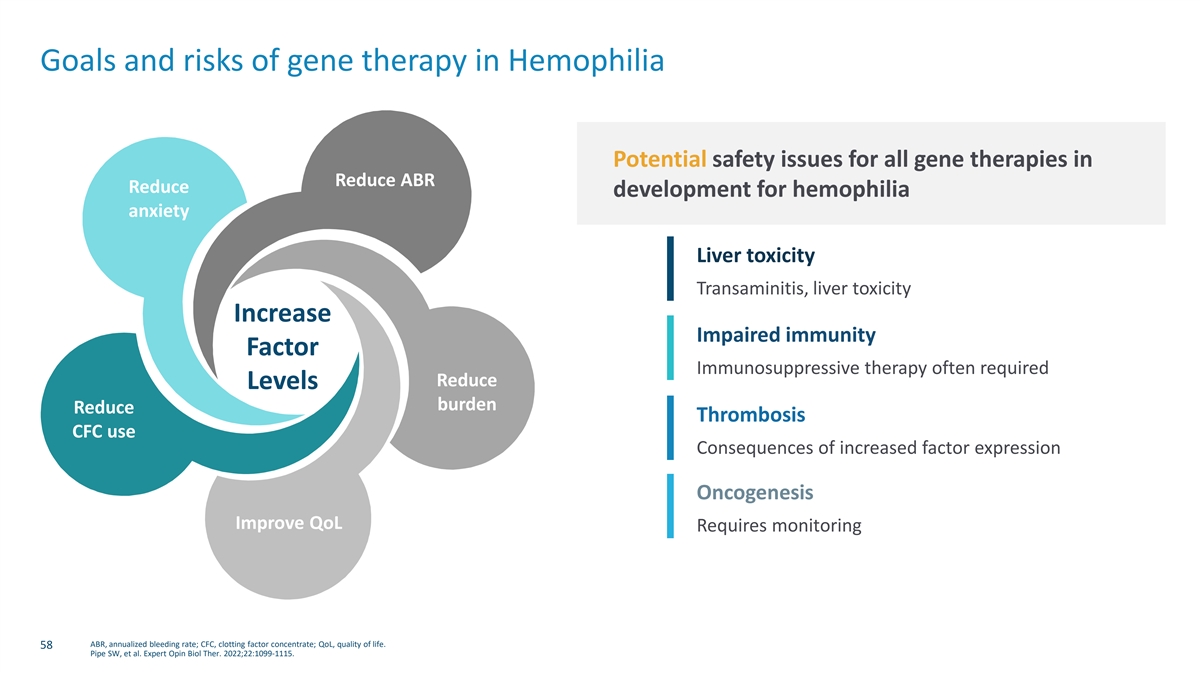
Goals and risks of gene therapy in Hemophilia Potential safety issues for all gene therapies in Reduce ABR Reduce development for hemophilia anxiety Liver toxicity Transaminitis, liver toxicity Increase Impaired immunity Factor Immunosuppressive therapy often required Reduce Levels burden Reduce Thrombosis CFC use Consequences of increased factor expression Oncogenesis Improve QoL Requires monitoring ABR, annualized bleeding rate; CFC, clotting factor concentrate; QoL, quality of life. 58 Pipe SW, et al. Expert Opin Biol Ther. 2022;22:1099-1115.
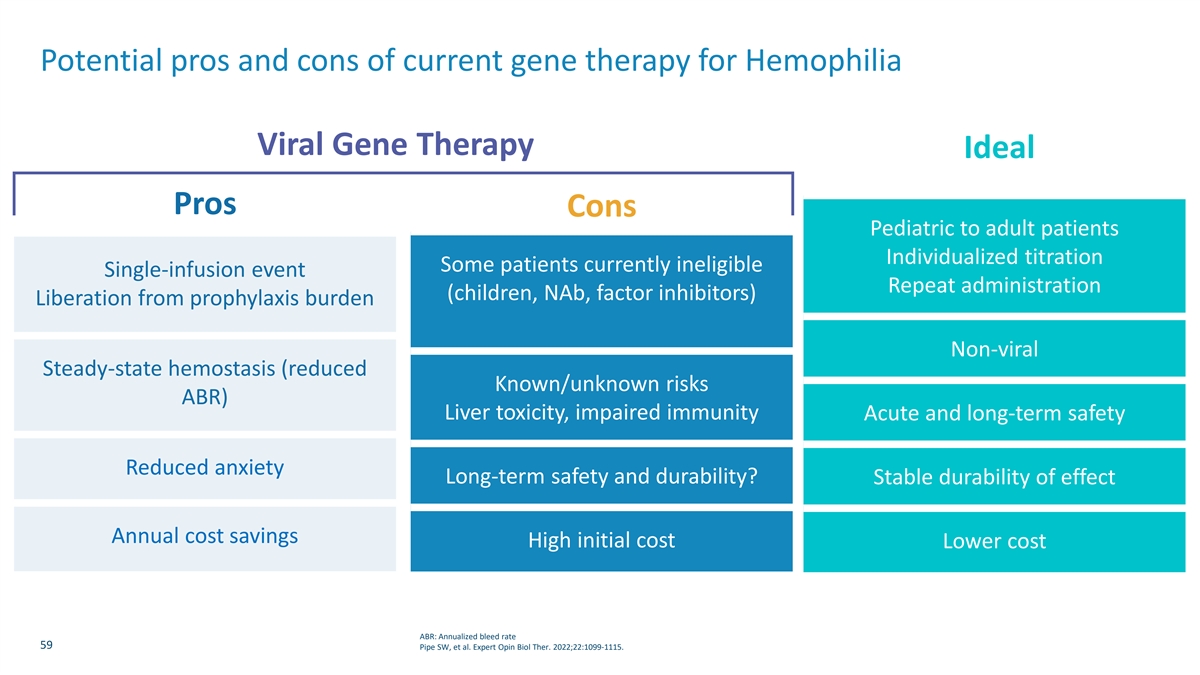
Potential pros and cons of current gene therapy for Hemophilia Viral Gene Therapy Ideal Pros Cons Pediatric to adult patients Individualized titration Some patients currently ineligible Single-infusion event Repeat administration (children, NAb, factor inhibitors) Liberation from prophylaxis burden Non-viral Steady-state hemostasis (reduced Known/unknown risks ABR) Liver toxicity, impaired immunity Acute and long-term safety Reduced anxiety Long-term safety and durability? Stable durability of effect Annual cost savings High initial cost Lower cost ABR: Annualized bleed rate 59 Pipe SW, et al. Expert Opin Biol Ther. 2022;22:1099-1115.
Thank you

P-FVIII-101 for the treatment of Hemophilia A In vivo ap p lication o f n o n -viral syste m P r e s e n t e r : B l a i r M a d i s o n , P h D
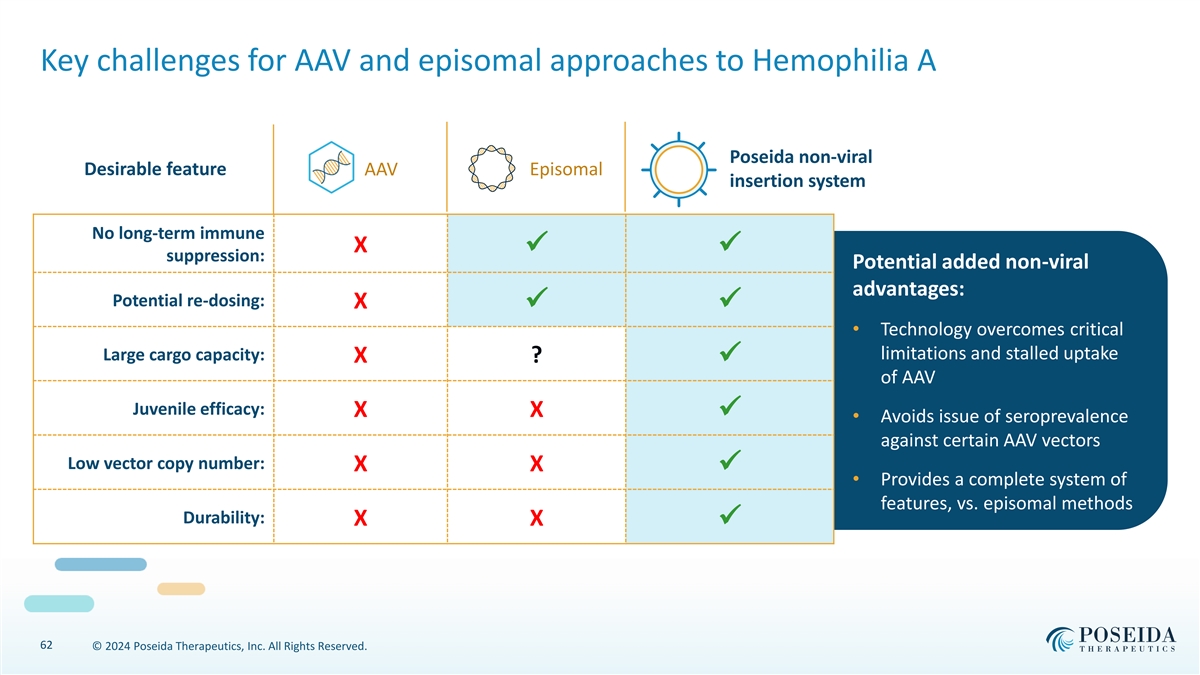
Key challenges for AAV and episomal approaches to Hemophilia A Poseida non-viral Desirable feature Episomal AAV insertion system No long-term immune X ✓✓ suppression: Potential added non-viral advantages: Potential re-dosing: X ✓✓ • Technology overcomes critical limitations and stalled uptake Large cargo capacity: X ? ✓ of AAV Juvenile efficacy: X X ✓ • Avoids issue of seroprevalence against certain AAV vectors Low vector copy number: X X ✓ • Provides a complete system of features, vs. episomal methods Durability: X X ✓ 62 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
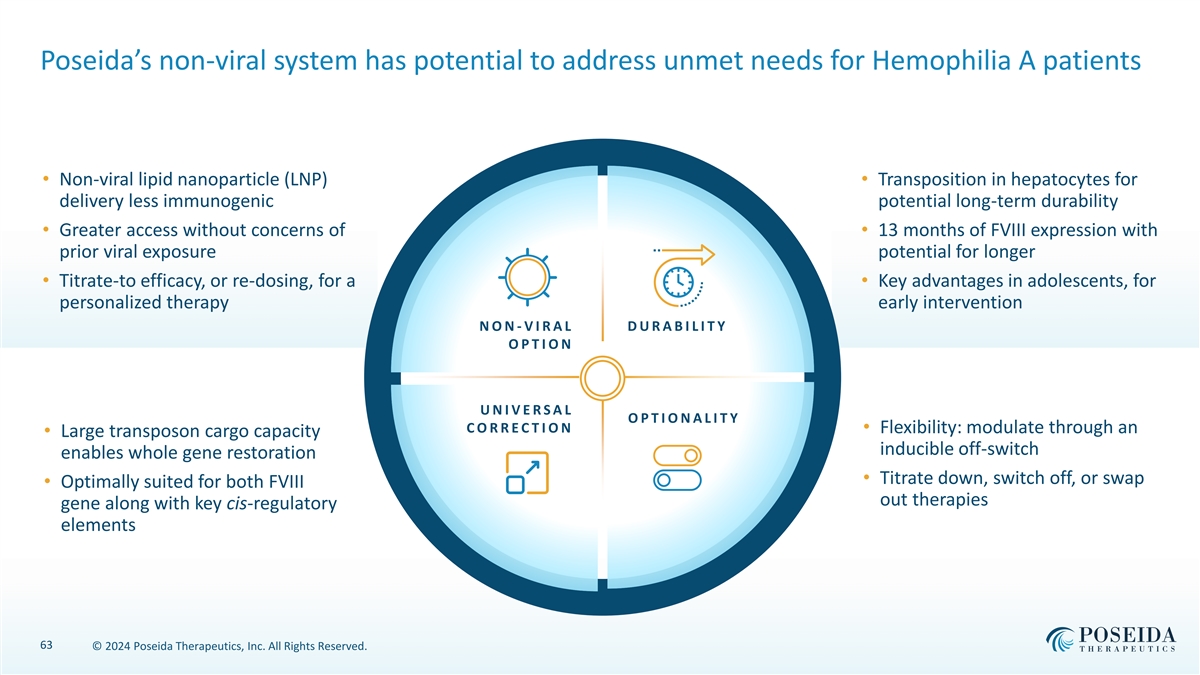
Poseida’s non-viral system has potential to address unmet needs for Hemophilia A patients • Non-viral lipid nanoparticle (LNP) • Transposition in hepatocytes for delivery less immunogenic potential long-term durability • Greater access without concerns of • 13 months of FVIII expression with prior viral exposure potential for longer • Titrate-to efficacy, or re-dosing, for a • Key advantages in adolescents, for personalized therapy early intervention N O N - V I R A L D U R A B I L I T Y O P T I O N U N I V E R S A L O P T I O N A L I T Y C O R R E C T I O N • Flexibility: modulate through an • Large transposon cargo capacity inducible off-switch enables whole gene restoration • Titrate down, switch off, or swap • Optimally suited for both FVIII out therapies gene along with key cis-regulatory elements 63 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
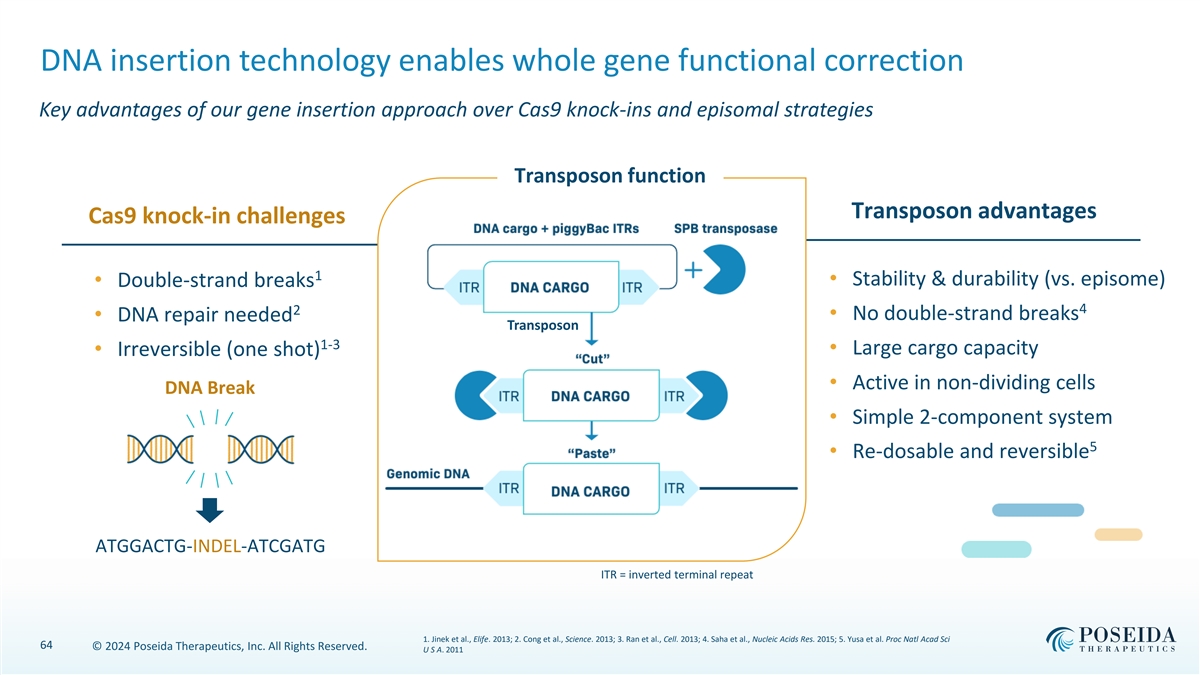
DNA insertion technology enables whole gene functional correction Key advantages of our gene insertion approach over Cas9 knock-ins and episomal strategies Transposon function Transposon advantages Cas9 knock-in challenges 1 • Stability & durability (vs. episome) • Double-strand breaks 4 2 • No double-strand breaks • DNA repair needed Transposon 1-3 • Large cargo capacity • Irreversible (one shot) • Active in non-dividing cells DNA Break • Simple 2-component system 5 • Re-dosable and reversible ATGGACTG-INDEL-ATCGATG ITR = inverted terminal repeat 1. Jinek et al., Elife. 2013; 2. Cong et al., Science. 2013; 3. Ran et al., Cell. 2013; 4. Saha et al., Nucleic Acids Res. 2015; 5. Yusa et al. Proc Natl Acad Sci 64 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. U S A. 2011
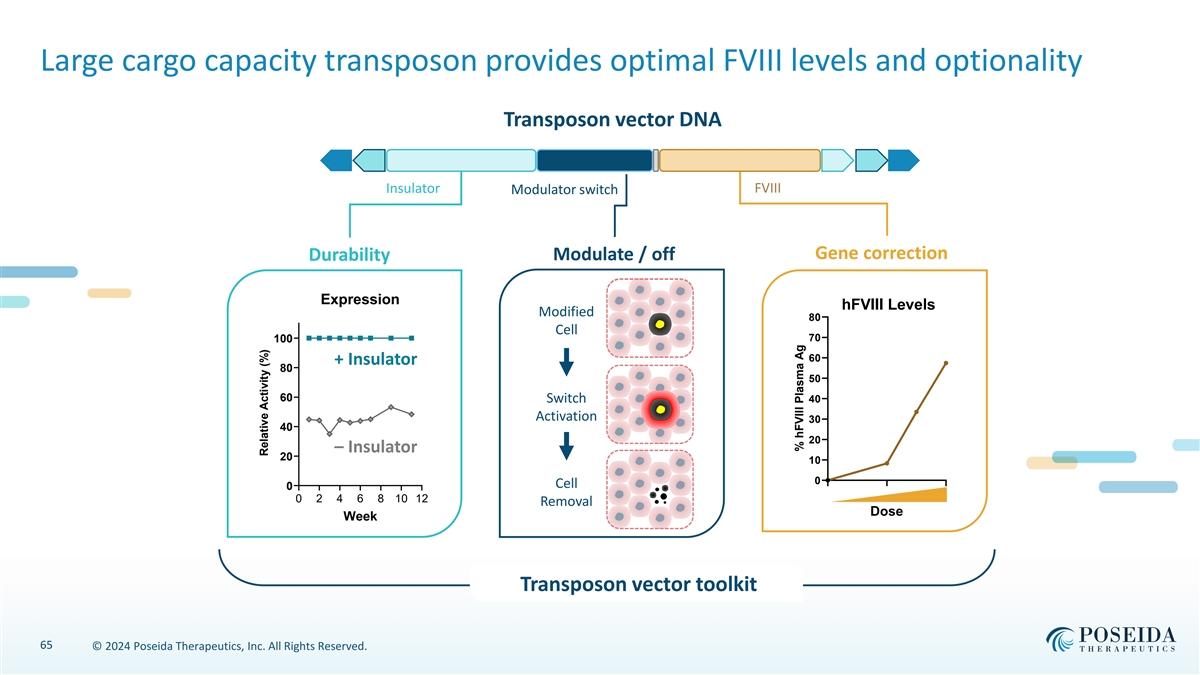
Large cargo capacity transposon provides optimal FVIII levels and optionality Transposon vector DNA Insulator FVIII Modulator switch Gene correction Modulate / off Durability Expression hFVIII Levels Modified 80 Cell 70 100 60 + Insulator 80 50 60 Switch 40 Activation 30 40 20 – Insulator 20 10 0 Cell 0 0.0 0.5 1.0 0 2 4 6 8 10 12 Removal Dose Week Transposon vector toolkit 65 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. Relative Activity (%) % hFVIII Plasma Ag
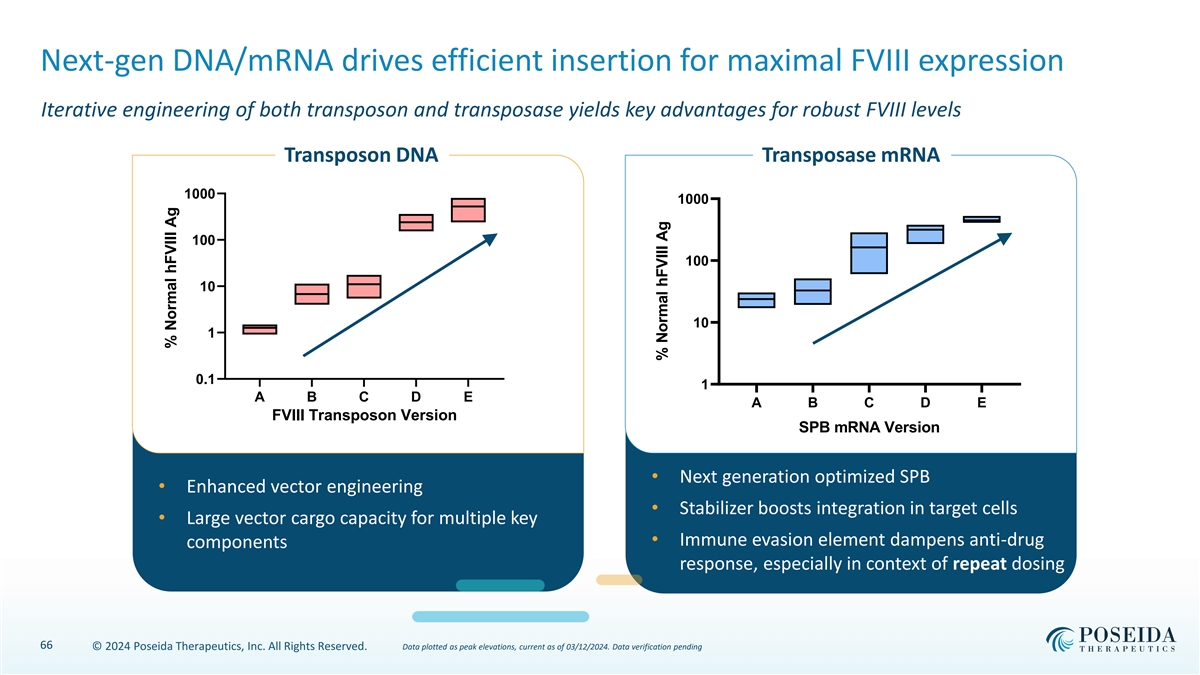
Next-gen DNA/mRNA drives efficient insertion for maximal FVIII expression Iterative engineering of both transposon and transposase yields key advantages for robust FVIII levels Transposon DNA Transposase mRNA 1000 1000 100 100 10 10 1 0.1 1 A B C D E A B C D E FVIII Transposon Version SPB mRNA Version • Next generation optimized SPB • Enhanced vector engineering • Stabilizer boosts integration in target cells • Large vector cargo capacity for multiple key • Immune evasion element dampens anti-drug components response, especially in context of repeat dosing 66 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. Data plotted as peak elevations, current as of 03/12/2024. Data verification pending % Normal hFVIII Ag % Normal hFVIII Ag
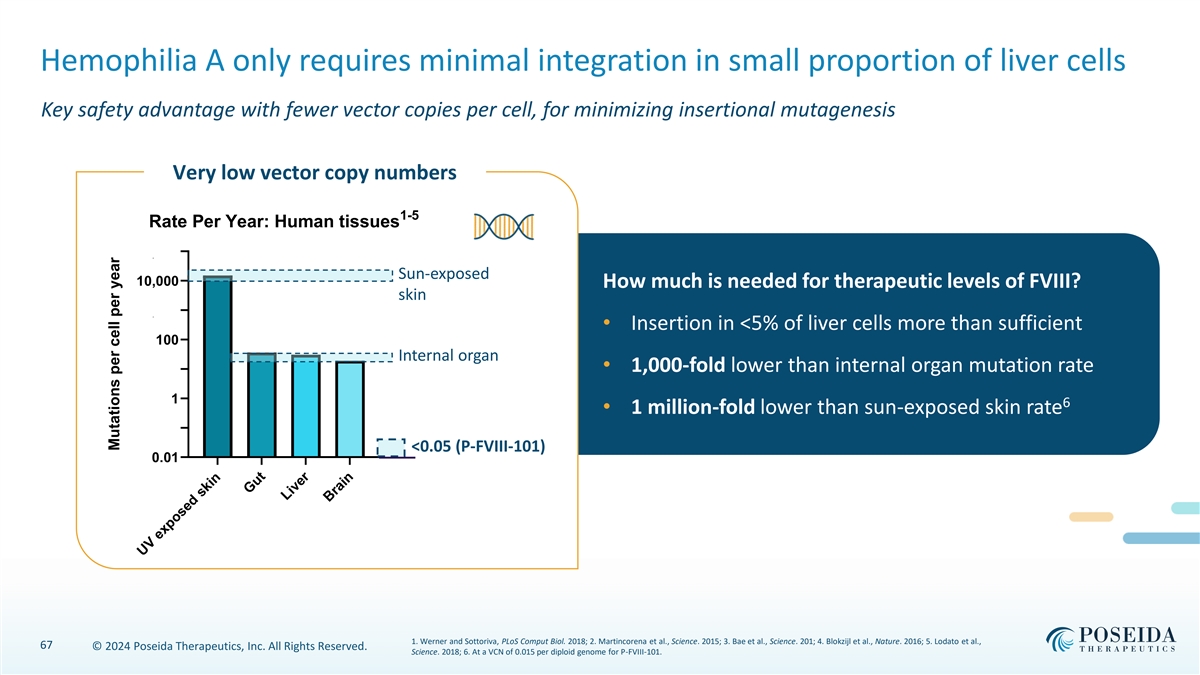
Hemophilia A only requires minimal integration in small proportion of liver cells Key safety advantage with fewer vector copies per cell, for minimizing insertional mutagenesis Very low vector copy numbers 1-5 Rate Per Year: Human tissues 100,000 Sun-exposed 10,000 How much is needed for therapeutic levels of FVIII? skin 1,000 • Insertion in <5% of liver cells more than sufficient 100 Internal organ • 1,000-fold lower than internal organ mutation rate 10 1 6 • 1 million-fold lower than sun-exposed skin rate 0.1 <0.05 (P-FVIII-101) 0.01 1. Werner and Sottoriva, PLoS Comput Biol. 2018; 2. Martincorena et al., Science. 2015; 3. Bae et al., Science. 201; 4. Blokzijl et al., Nature. 2016; 5. Lodato et al., 67 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. Science. 2018; 6. At a VCN of 0.015 per diploid genome for P-FVIII-101. UV exposed skin Gut Liver Brain Mutations per cell per year
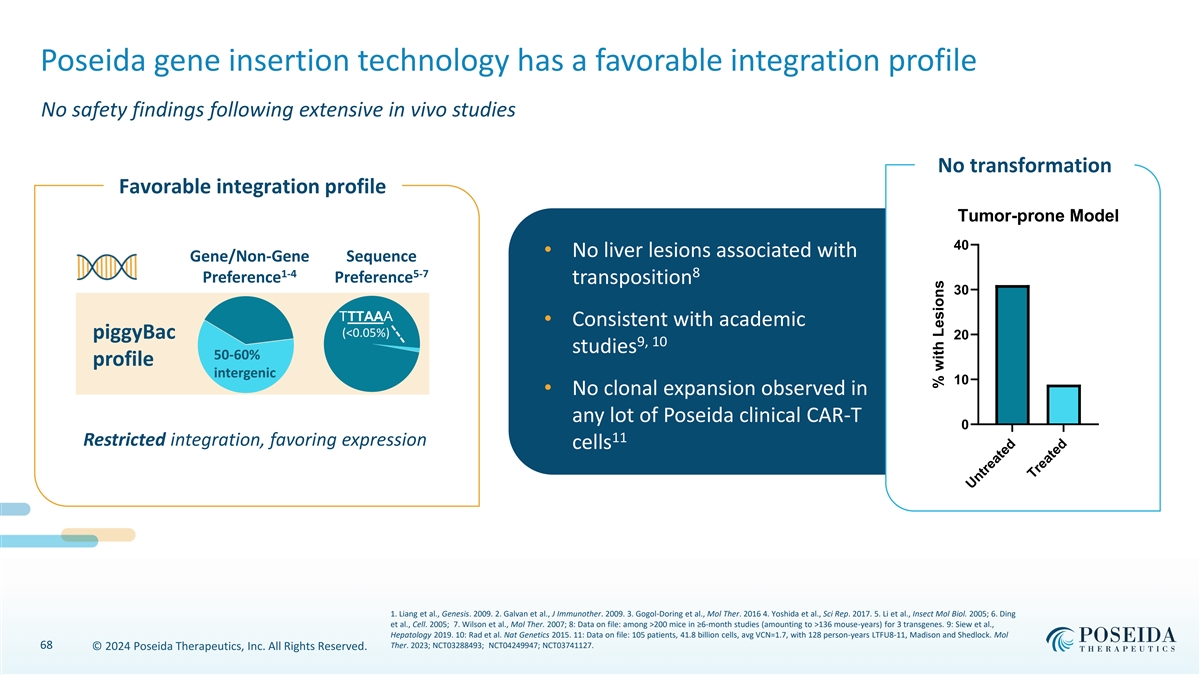
Poseida gene insertion technology has a favorable integration profile No safety findings following extensive in vivo studies No transformation Favorable integration profile Tumor-prone Model 40 • No liver lesions associated with Gene/Non-Gene Sequence 1-4 5-7 8 Preference Preference transposition 30 TTTAAA • Consistent with academic (<0.05%) piggyBac 20 9, 10 studies 50-60% profile intergenic 10 • No clonal expansion observed in any lot of Poseida clinical CAR-T 0 11 Restricted integration, favoring expression cells 1. Liang et al., Genesis. 2009. 2. Galvan et al., J Immunother. 2009. 3. Gogol-Doring et al., Mol Ther. 2016 4. Yoshida et al., Sci Rep. 2017. 5. Li et al., Insect Mol Biol. 2005; 6. Ding et al., Cell. 2005; 7. Wilson et al., Mol Ther. 2007; 8: Data on file: among >200 mice in ≥6-month studies (amounting to >136 mouse-years) for 3 transgenes. 9: Siew et al., Hepatology 2019. 10: Rad et al. Nat Genetics 2015. 11: Data on file: 105 patients, 41.8 billion cells, avg VCN=1.7, with 128 person-years LTFU8-11, Madison and Shedlock. Mol 68 Ther. 2023; NCT03288493; NCT04249947; NCT03741127. © 2024 Poseida Therapeutics, Inc. All Rights Reserved. Untreated Treated % with Lesions
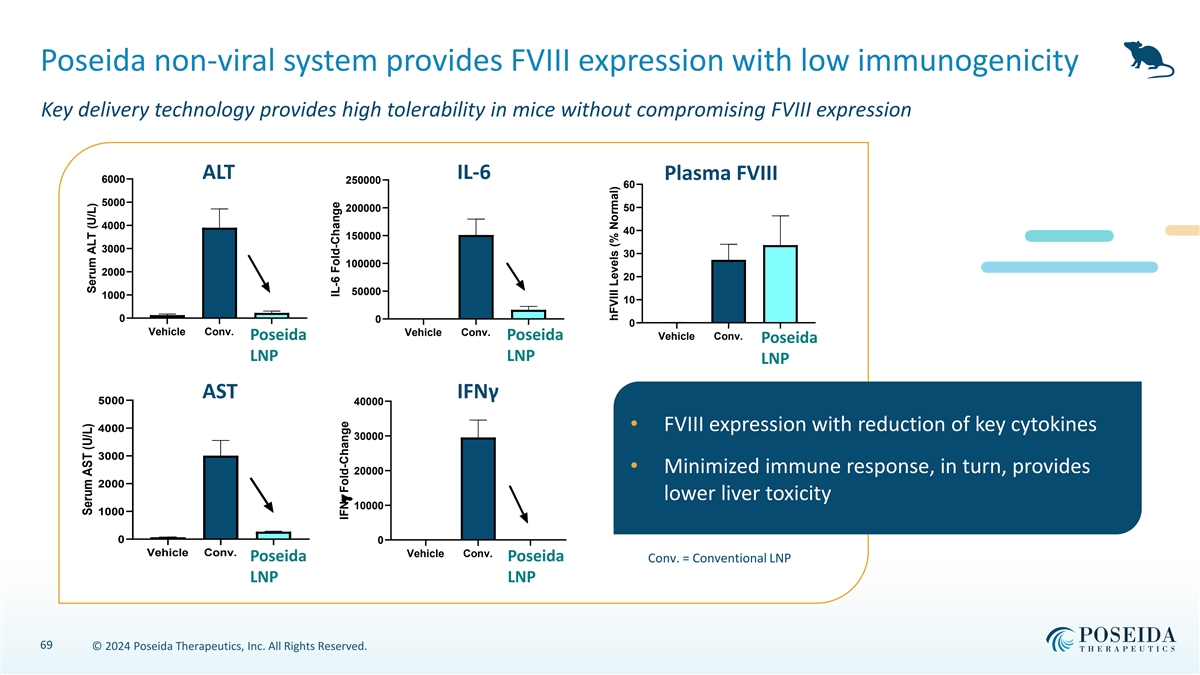
Poseida non-viral system provides FVIII expression with low immunogenicity Key delivery technology provides high tolerability in mice without compromising FVIII expression ALT IL-6 Plasma FVIII 6000 250000 60 5000 50 200000 4000 40 150000 3000 30 100000 2000 20 50000 1000 10 0 0 0 Vehicle Conv. Vehicle Conv. Poseida Poseida Vehicle Conv. Poseida LNP LNP LNP AST IFNγ 5000 40000 • FVIII expression with reduction of key cytokines 4000 30000 3000 • Minimized immune response, in turn, provides 20000 2000 lower liver toxicity 10000 1000 0 0 Vehicle Conv. Vehicle Conv. Poseida Poseida Conv. = Conventional LNP LNP LNP 69 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. Serum AST (U/L) Serum ALT (U/L) IL-6 Fold-Change IFNg Fold-Change hFVIII Levels (% Normal)
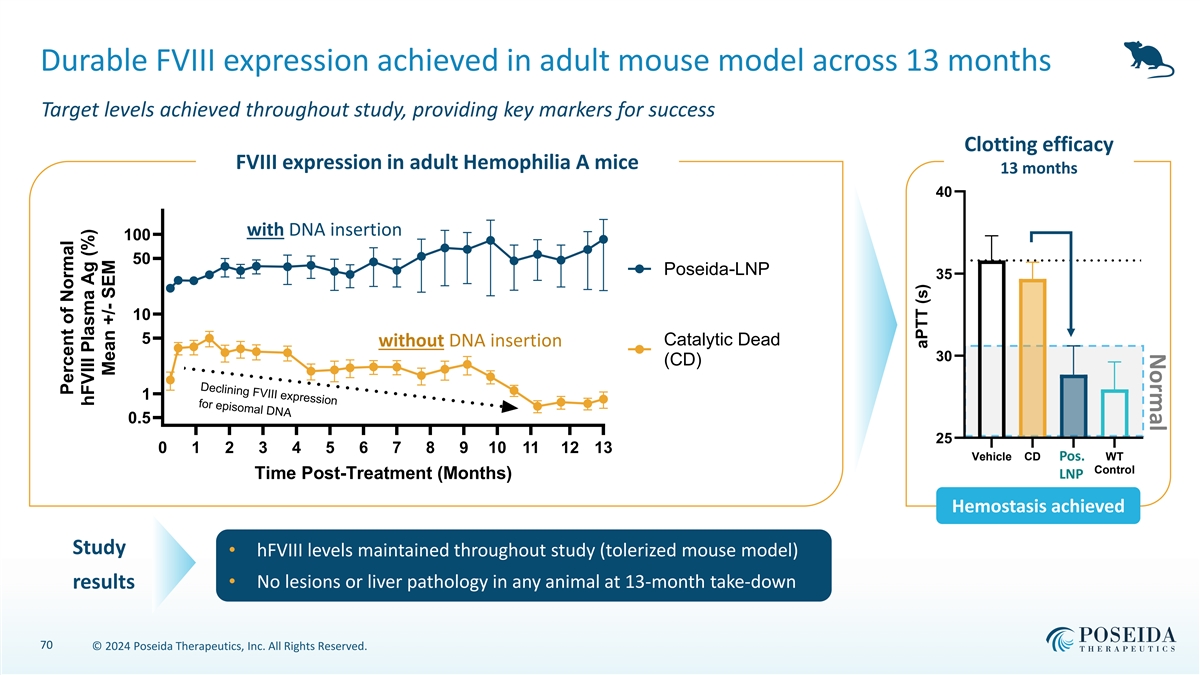
Normal Declining FVIII expression for episomal DNA Durable FVIII expression achieved in adult mouse model across 13 months Target levels achieved throughout study, providing key markers for success Clotting efficacy FVIII expression in adult Hemophilia A mice 13 months 40 with DNA insertion 100 50 Poseida-LNP 35 10 5 without DNA insertion Catalytic Dead 30 (CD) 1 0.5 25 0 1 2 3 4 5 6 7 8 9 10 11 12 13 Vehicle CD Pos. WT Control Time Post-Treatment (Months) LNP Hemostasis achieved Study • hFVIII levels maintained throughout study (tolerized mouse model) • No lesions or liver pathology in any animal at 13-month take-down results 70 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. Percent of Normal hFVIII Plasma Ag (%) Mean +/- SEM aPTT (s)
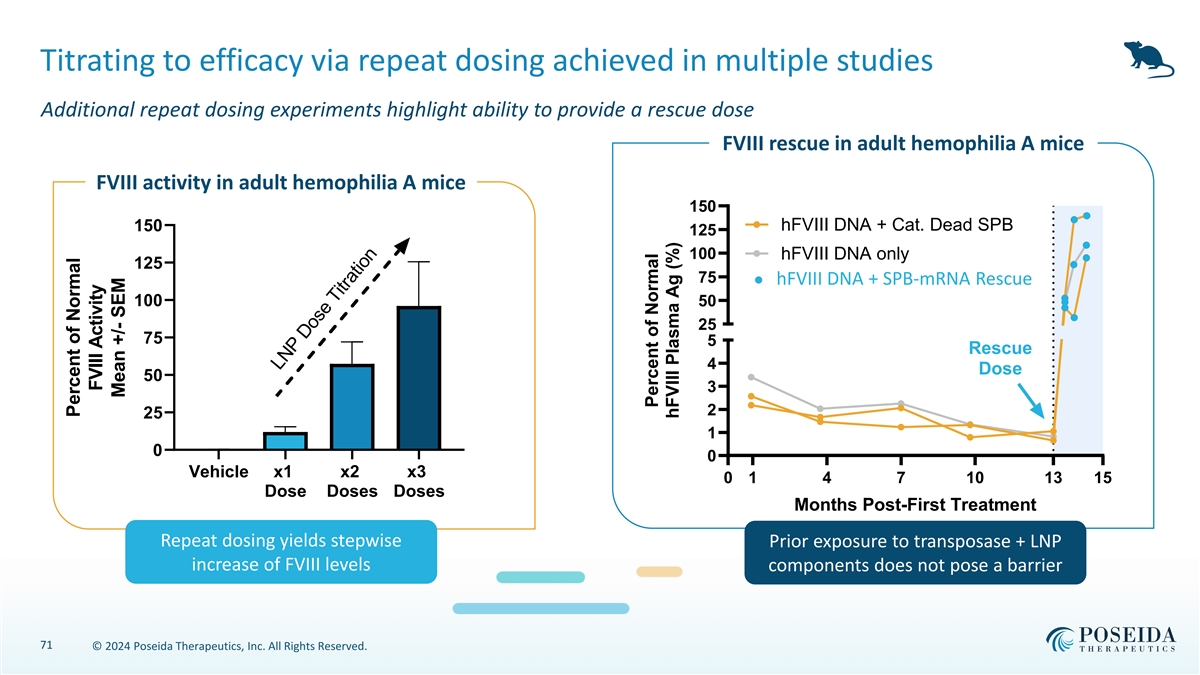
Titrating to efficacy via repeat dosing achieved in multiple studies Additional repeat dosing experiments highlight ability to provide a rescue dose FVIII rescue in adult hemophilia A mice FVIII activity in adult hemophilia A mice 150 150 hFVIII DNA + Cat. Dead SPB 125 100 hFVIII DNA only 125 75 hFVIII DNA + SPB-mRNA Rescue 100 50 25 75 5 Rescue 4 Dose 50 3 2 25 1 0 0 Vehicle x1 x2 x3 0 1 4 7 10 13 15 Dose Doses Doses Months Post-First Treatment Repeat dosing yields stepwise Prior exposure to transposase + LNP increase of FVIII levels components does not pose a barrier 71 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. LNP Dose Titration Percent of Normal FVIII Activity Mean +/- SEM Percent of Normal hFVIII Plasma Ag (%)
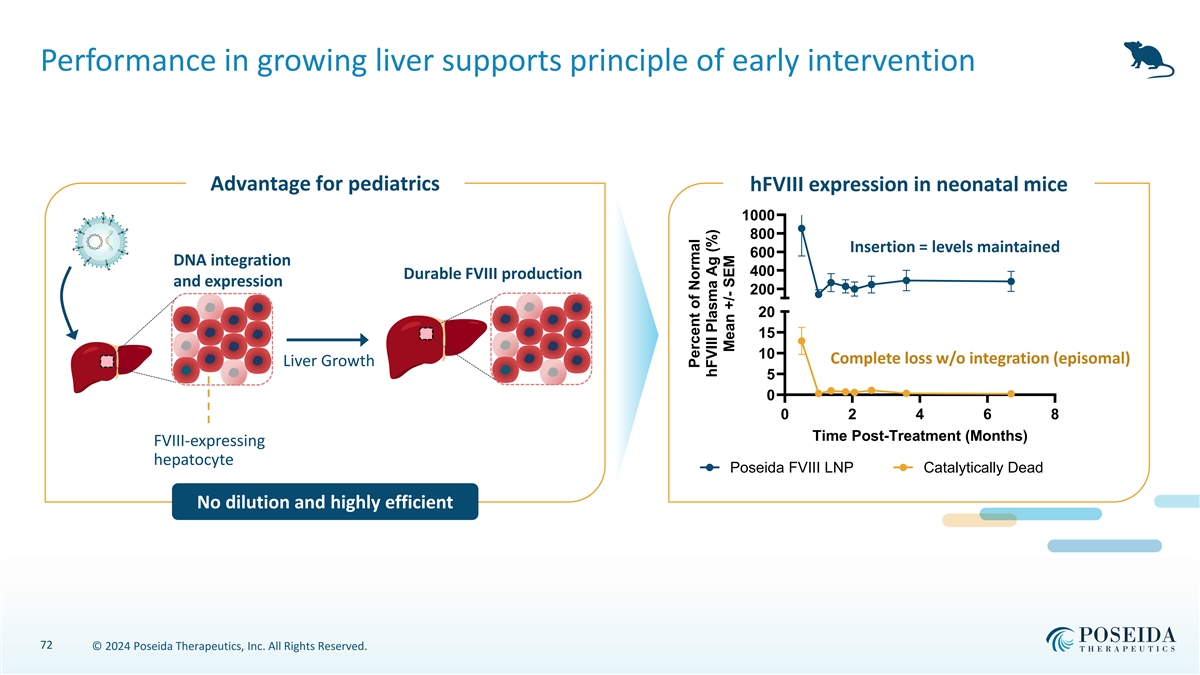
Performance in growing liver supports principle of early intervention Advantage for pediatrics hFVIII expression in neonatal mice 1000 800 Insertion = levels maintained 600 DNA integration 400 Durable FVIII production and expression 200 20 15 10 Complete loss w/o integration (episomal) Liver Growth 5 0 0 2 4 6 8 Time Post-Treatment (Months) FVIII-expressing hepatocyte Poseida FVIII LNP Catalytically Dead No dilution and highly efficient 72 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. Percent of Normal hFVIII Plasma Ag (%) Mean +/- SEM
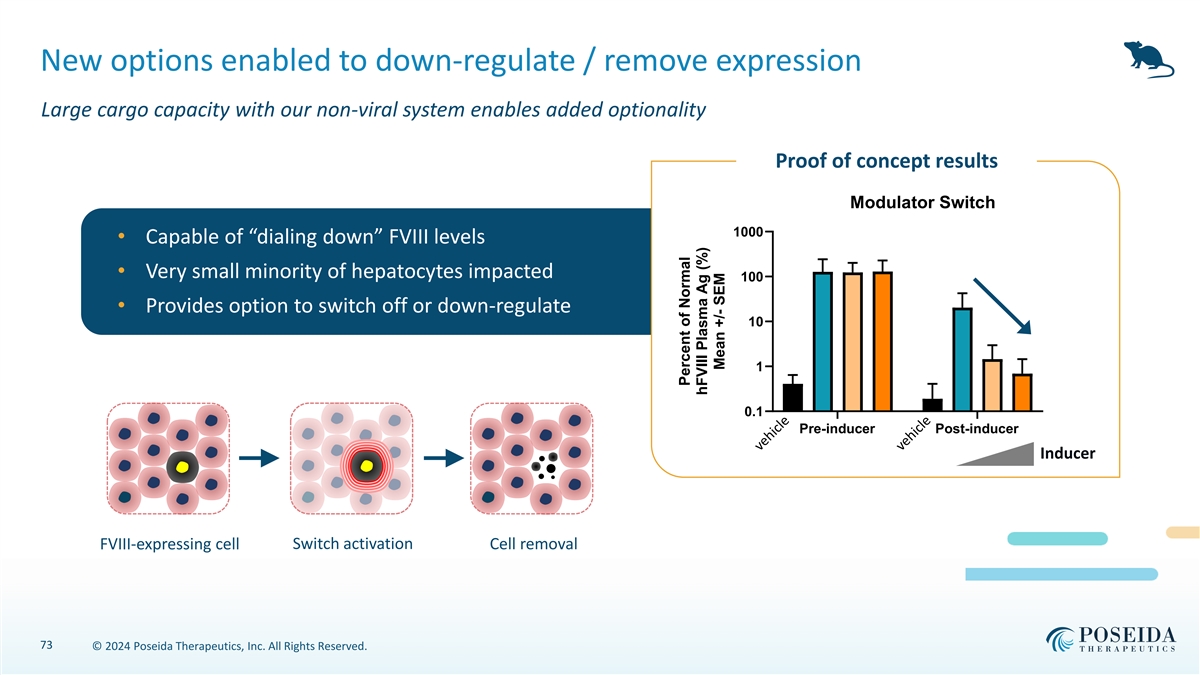
New options enabled to down-regulate / remove expression Large cargo capacity with our non-viral system enables added optionality Proof of concept results Off/Modulator Switch 1000 • Capable of “dialing down” FVIII levels • Very small minority of hepatocytes impacted 100 • Provides option to switch off or down-regulate 10 1 0.1 Pre-inducer Post-inducer Inducer Switch activation FVIII-expressing cell Cell removal 73 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. Percent of Normal hFVIII Plasma Ag (%) Mean +/- SEM
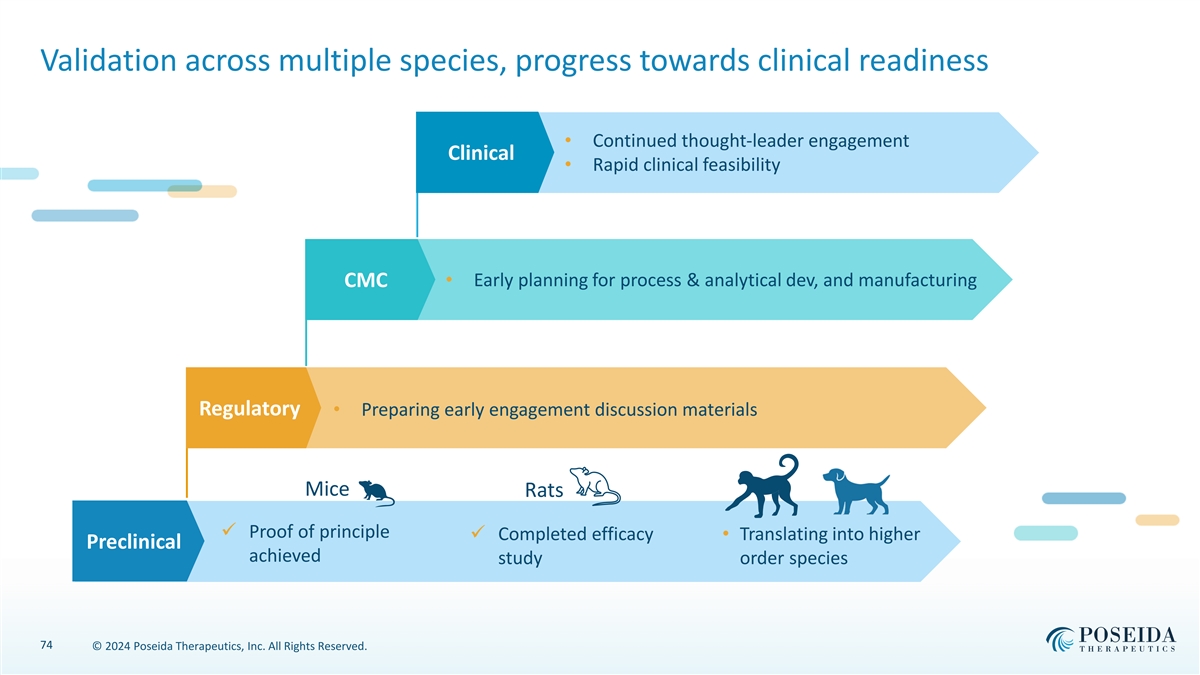
Validation across multiple species, progress towards clinical readiness • Continued thought-leader engagement Clinical • Rapid clinical feasibility • Early planning for process & analytical dev, and manufacturing CMC Regulatory • Preparing early engagement discussion materials Mice Rats ✓ Proof of principle ✓ Completed efficacy • Translating into higher Preclinical achieved study order species 74 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.

Site-Specific Super piggyBac (ssSPB) Advancements Up d ate on site -specific ge n e in sertion ap p roach P r e s e n t e r : B l a i r M a d i s o n , P h D 75
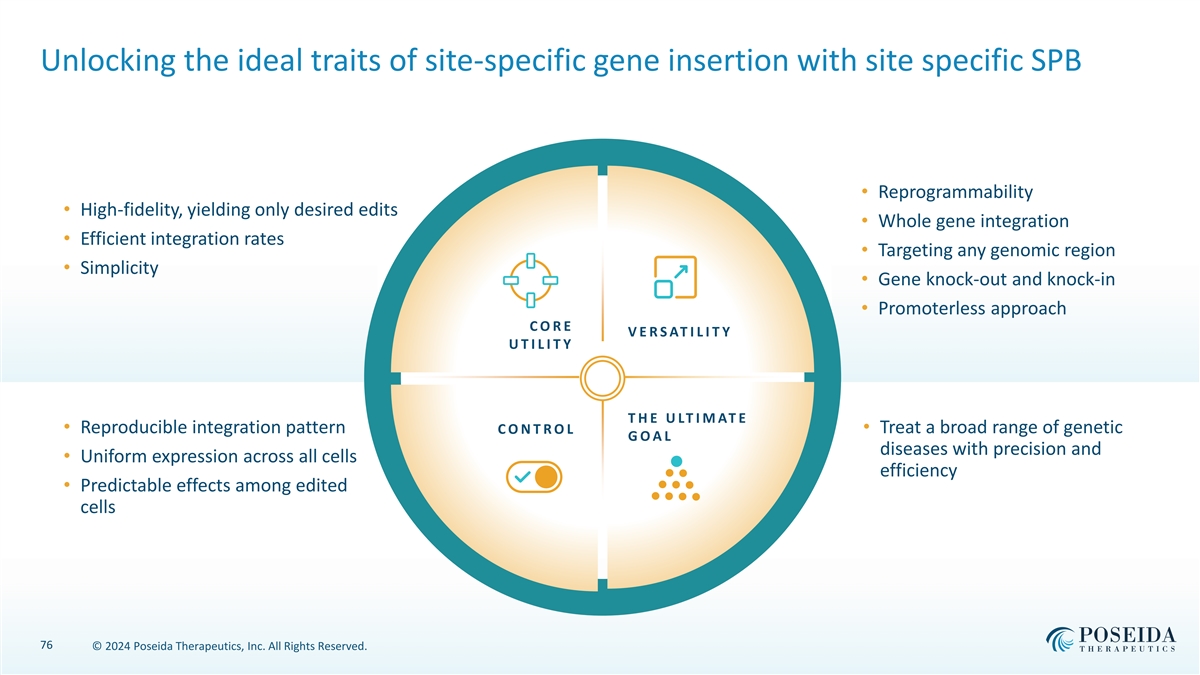
Unlocking the ideal traits of site-specific gene insertion with site specific SPB • Reprogrammability • High-fidelity, yielding only desired edits • Whole gene integration • Efficient integration rates • Targeting any genomic region • Simplicity • Gene knock-out and knock-in • Promoterless approach C O R E V E R S AT I L I T Y U T I L I T Y T H E U LT I M AT E • Reproducible integration pattern C O N T R O L • Treat a broad range of genetic G O A L diseases with precision and • Uniform expression across all cells efficiency • Predictable effects among edited cells 76 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
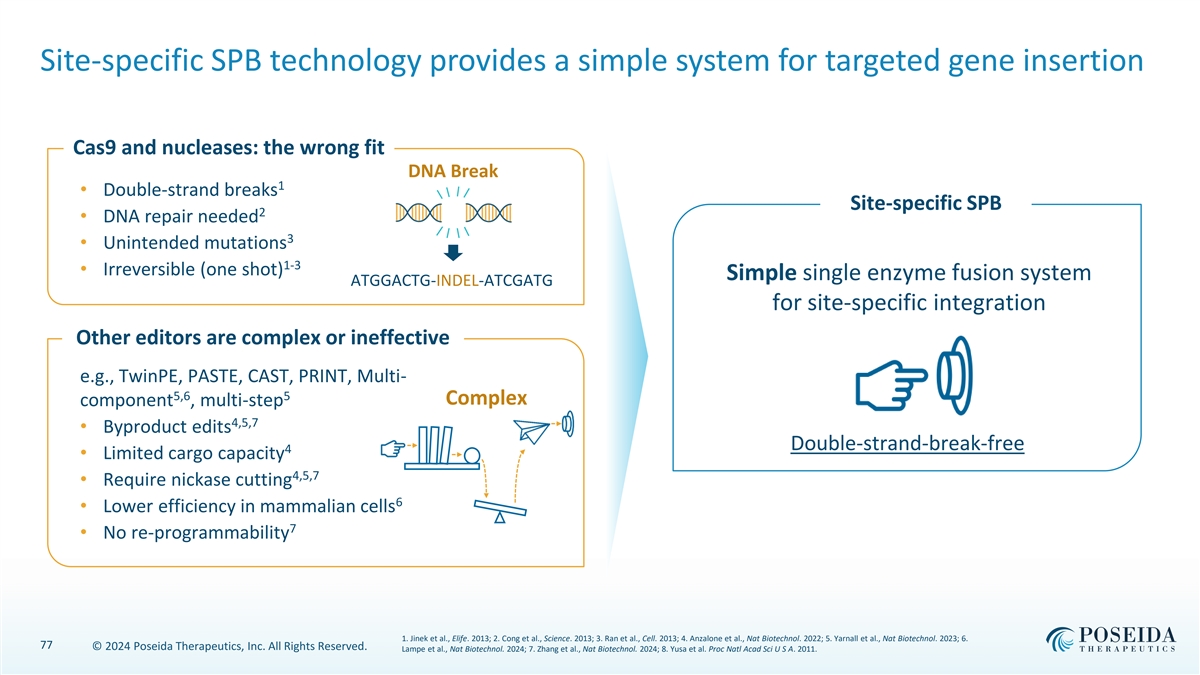
Site-specific SPB technology provides a simple system for targeted gene insertion Cas9 and nucleases: the wrong fit DNA Break 1 • Double-strand breaks Site-specific SPB 2 • DNA repair needed 3 • Unintended mutations 1-3 • Irreversible (one shot) Simple single enzyme fusion system ATGGACTG-INDEL-ATCGATG for site-specific integration Other editors are complex or ineffective e.g., TwinPE, PASTE, CAST, PRINT, Multi- 5,6 5 Complex component , multi-step 4,5,7 • Byproduct edits Double-strand-break-free 4 • Limited cargo capacity 4,5,7 • Require nickase cutting 6 • Lower efficiency in mammalian cells 7 • No re-programmability 1. Jinek et al., Elife. 2013; 2. Cong et al., Science. 2013; 3. Ran et al., Cell. 2013; 4. Anzalone et al., Nat Biotechnol. 2022; 5. Yarnall et al., Nat Biotechnol. 2023; 6. 77 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. Lampe et al., Nat Biotechnol. 2024; 7. Zhang et al., Nat Biotechnol. 2024; 8. Yusa et al. Proc Natl Acad Sci U S A. 2011.
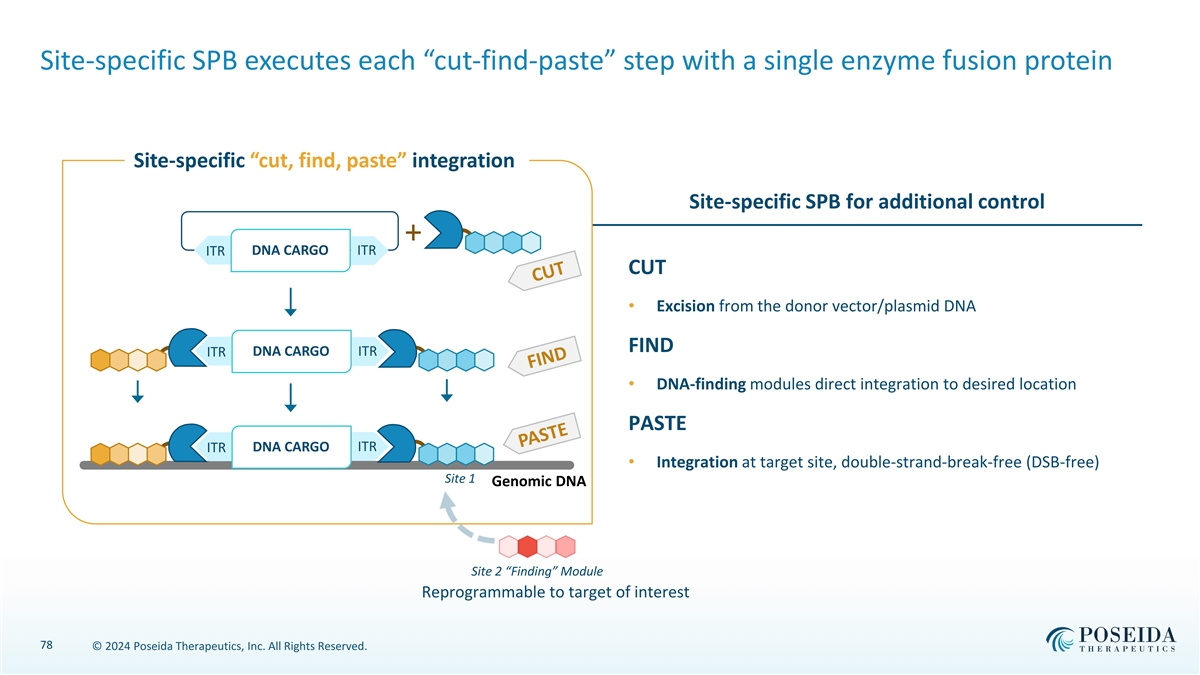
Site-specific SPB executes each “cut-find-paste” step with a single enzyme fusion protein Site-specific “cut, find, paste” integration Site-specific SPB for additional control + DNA CARGO ITR ITR CUT • Excision from the donor vector/plasmid DNA FIND ITR DNA CARGO ITR • DNA-finding modules direct integration to desired location PASTE DNA CARGO ITR ITR • Integration at target site, double-strand-break-free (DSB-free) Site 1 Genomic DNA Site 2 “Finding” Module Reprogrammable to target of interest 78 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
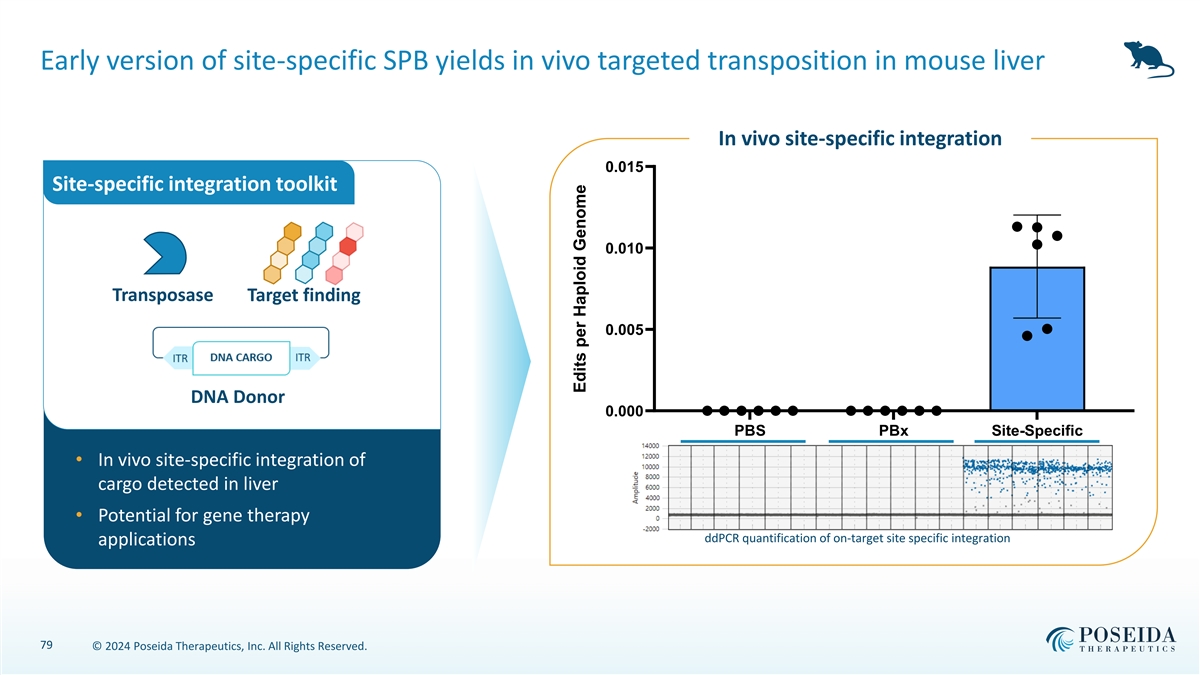
Early version of site-specific SPB yields in vivo targeted transposition in mouse liver In vivo site-specific integration 0.015 Site-specific integration toolkit 0.010 Transposase Target finding 0.005 DNA Donor 0.000 PBS PBx Site-Specific • In vivo site-specific integration of cargo detected in liver • Potential for gene therapy ddPCR quantification of on-target site specific integration applications 79 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. Edits per Haploid Genome
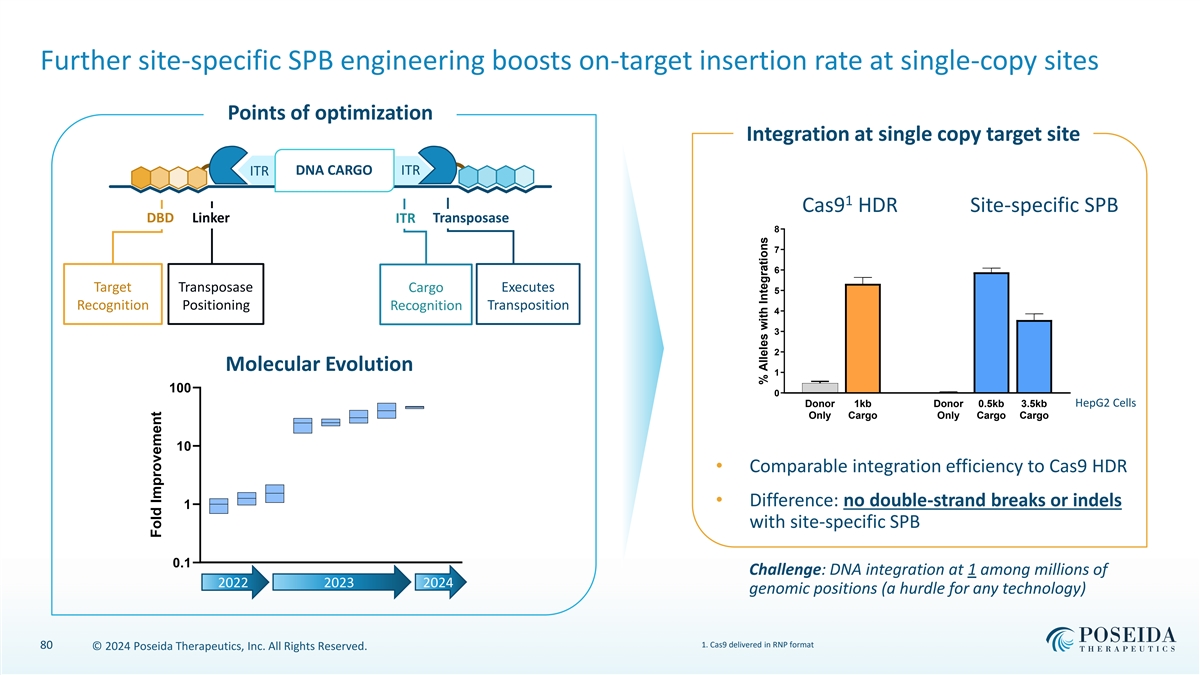
Further site-specific SPB engineering boosts on-target insertion rate at single-copy sites Points of optimization Integration at single copy target site DNA CARGO ITR ITR 1 Cas9 HDR Site-specific SPB DBD Linker ITR Transposase 8 7 6 Target Transposase Cargo Executes 5 Recognition Positioning Recognition Transposition 4 3 2 Molecular Evolution 1 100 0 Donor 1kb Donor 0.5kb 3.5kb HepG2 Cells Only Cargo Only Cargo Cargo 10 • Comparable integration efficiency to Cas9 HDR • Difference: no double-strand breaks or indels 1 with site-specific SPB 0.1 Challenge: DNA integration at 1 among millions of 2022 2023 2024 genomic positions (a hurdle for any technology) 1. Cas9 delivered in RNP format 80 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. Fold Improvement % Alleles with Integrations
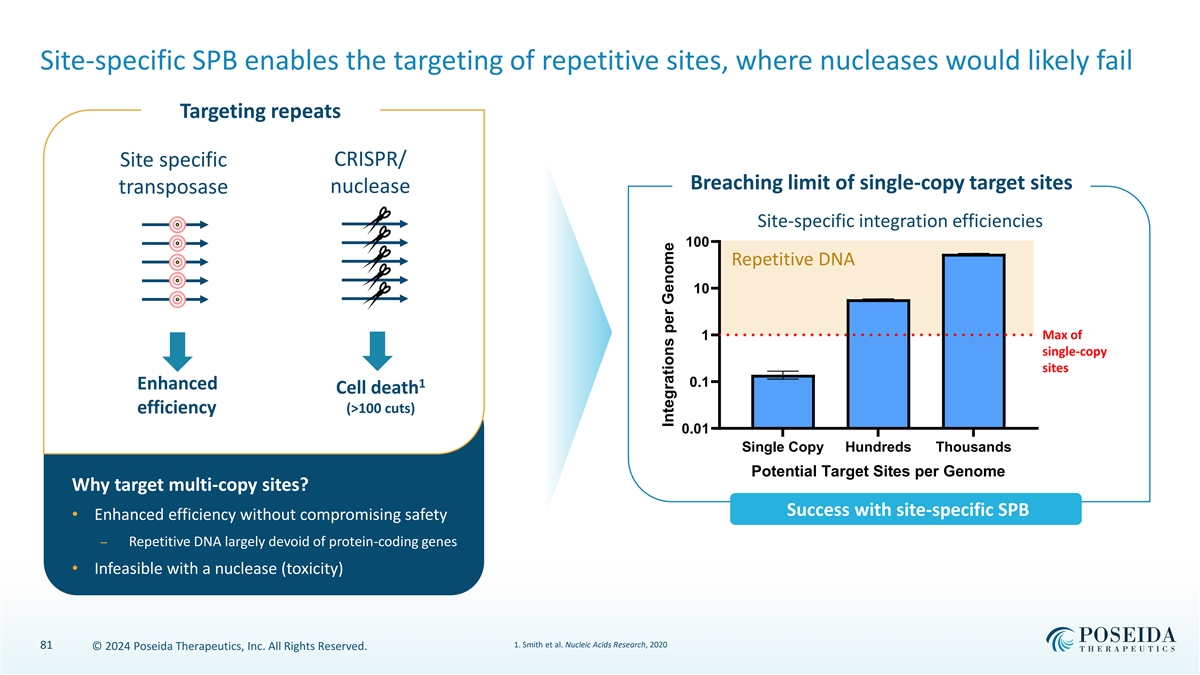
Site-specific SPB enables the targeting of repetitive sites, where nucleases would likely fail Targeting repeats Site specific CRISPR/ Breaching limit of single-copy target sites transposase nuclease Site-specific integration efficiencies 100 Repetitive DNA 10 1 Max of single-copy sites 1 0.1 Enhanced Cell death efficiency (>100 cuts) 0.01 Single Copy Hundreds Thousands Potential Target Sites per Genome Why target multi-copy sites? Success with site-specific SPB • Enhanced efficiency without compromising safety ⎯ Repetitive DNA largely devoid of protein-coding genes • Infeasible with a nuclease (toxicity) 1. Smith et al. Nucleic Acids Research, 2020 81 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. Integrations per Genome
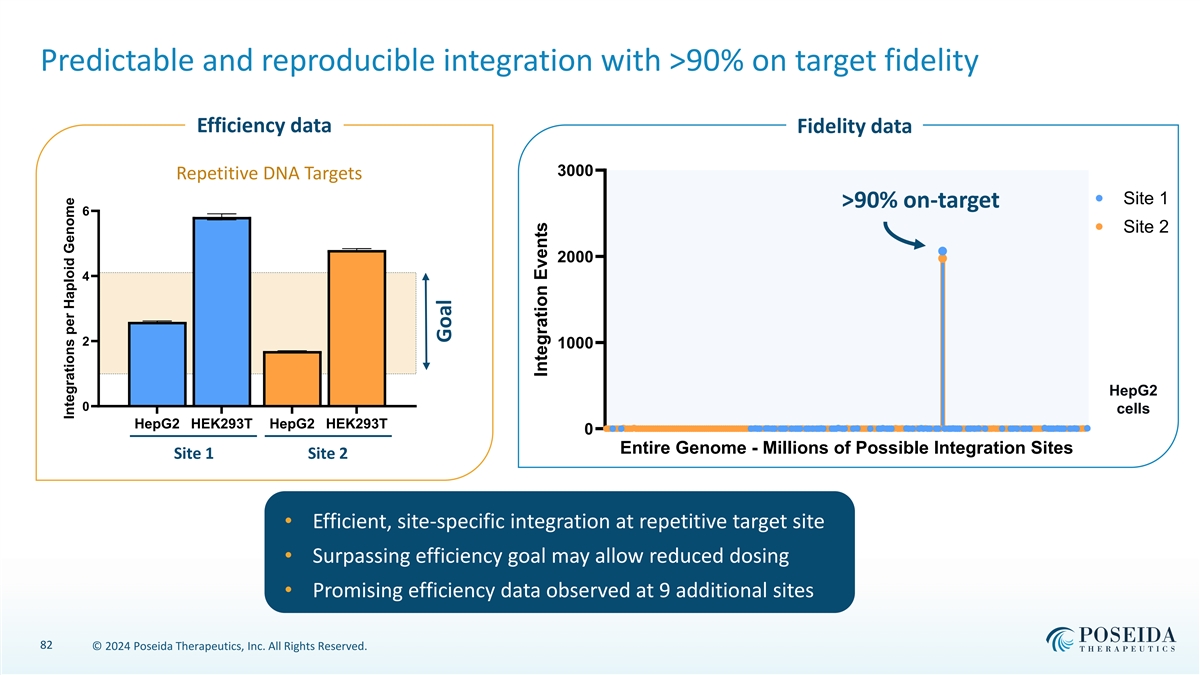
Predictable and reproducible integration with >90% on target fidelity Efficiency data Fidelity data 3000 Repetitive DNA Targets Site 1 >90% on-target 6 Site 2 2000 4 2 1000 HepG2 0 cells HepG2 HEK293T HepG2 HEK293T 0 Entire Genome - Millions of Possible Integration Sites Site 1 Site 2 • Efficient, site-specific integration at repetitive target site • Surpassing efficiency goal may allow reduced dosing • Promising efficiency data observed at 9 additional sites 82 © 2024 Poseida Therapeutics, Inc. All Rights Reserved. Integrations per Haploid Genome Goal Integration Events
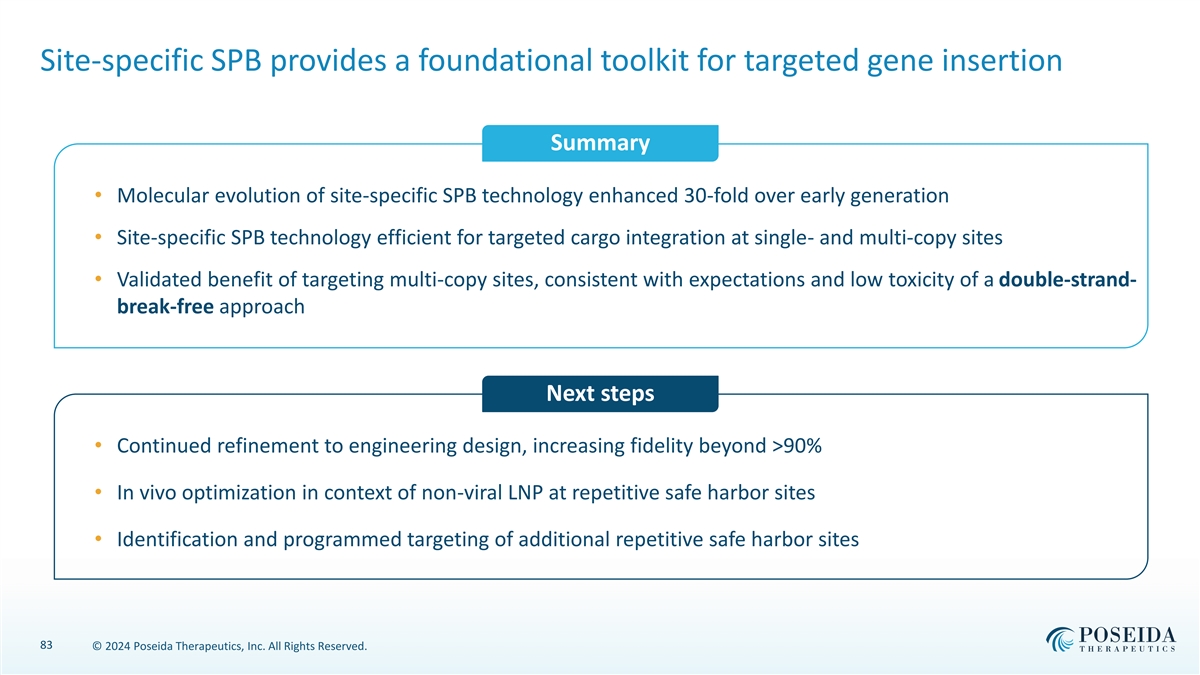
Site-specific SPB provides a foundational toolkit for targeted gene insertion Summary • Molecular evolution of site-specific SPB technology enhanced 30-fold over early generation • Site-specific SPB technology efficient for targeted cargo integration at single- and multi-copy sites • Validated benefit of targeting multi-copy sites, consistent with expectations and low toxicity of a double-strand- break-free approach Next steps • Continued refinement to engineering design, increasing fidelity beyond >90% • In vivo optimization in context of non-viral LNP at repetitive safe harbor sites • Identification and programmed targeting of additional repetitive safe harbor sites 83 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.

Conclusion P r e s e n t e r : K r i s t i n Ya r e m a , P h D 84

“If we don’t lean into accelerated approval, we’re going to leave a lot of patients behind” “I think the possibility of genome editing… could be an incredible game changer, not just for rare diseases but more common disease.” – Peter Marks, Director of the Center for Biologics Evaluation and Research (CBER) at the Food and Drug Administration 85
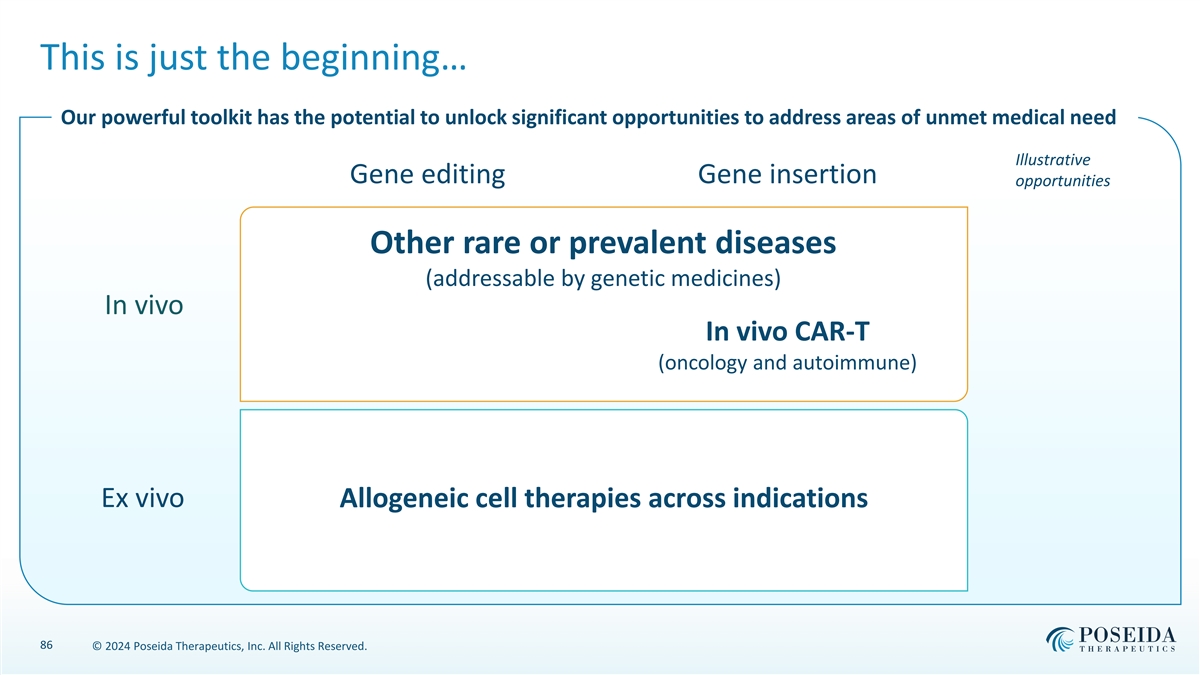
This is just the beginning… Our powerful toolkit has the potential to unlock significant opportunities to address areas of unmet medical need Illustrative Gene editing Gene insertion opportunities Other rare or prevalent diseases (addressable by genetic medicines) In vivo In vivo CAR-T (oncology and autoimmune) Ex vivo Allogeneic cell therapies across indications 86 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.
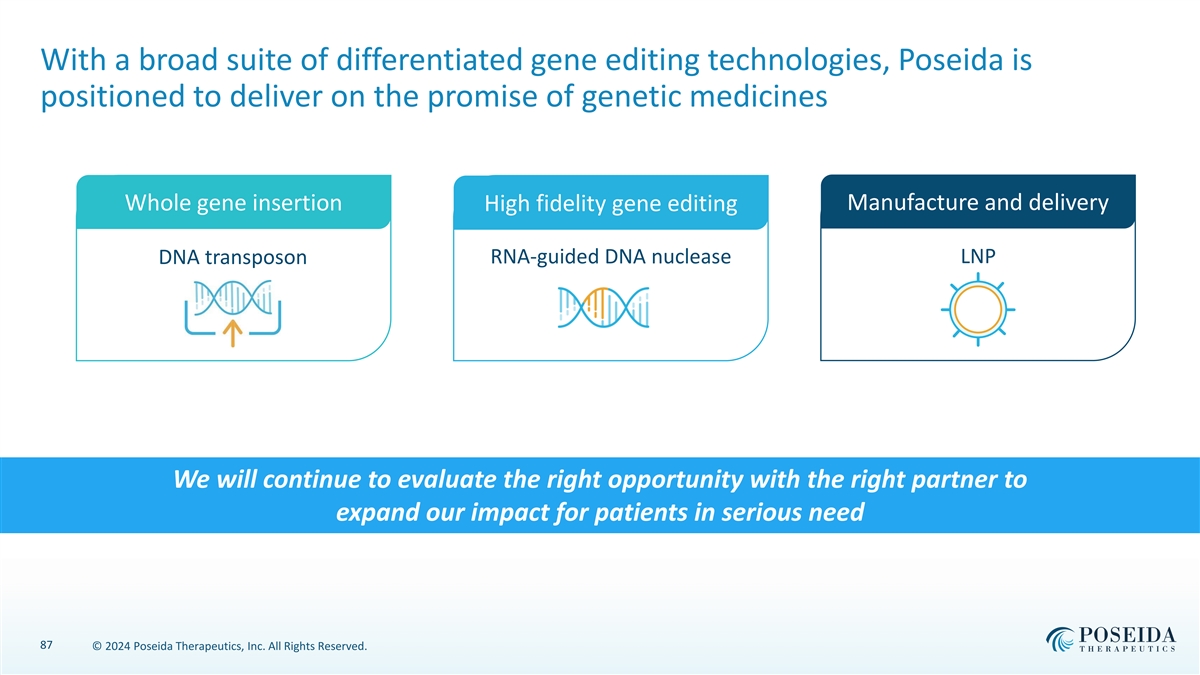
With a broad suite of differentiated gene editing technologies, Poseida is positioned to deliver on the promise of genetic medicines Manufacture and delivery Whole gene insertion High fidelity gene editing DNA transposon RNA-guided DNA nuclease LNP We will continue to evaluate the right opportunity with the right partner to expand our impact for patients in serious need 87 © 2024 Poseida Therapeutics, Inc. All Rights Reserved.