UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the month of March 2024.
Commission File Number: 001-37384
GALAPAGOS NV
(Translation of registrant’s name into English)
Generaal De Wittelaan L11 A3
2800 Mechelen, Belgium
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Note: Regulation S-T Rule 101(b)(7) only permits the submission in paper of a Form 6-K if submitted to furnish a report or other document that the registrant foreign private issuer must furnish and make public under the laws of the jurisdiction in which the registrant is incorporated, domiciled or legally organized (the registrant’s “home country”), or under the rules of the home country exchange on which the registrant’s securities are traded, as long as the report or other document is not a press release, is not required to be and has not been distributed to the registrant’s security holders, and, if discussing a material event, has already been the subject of a Form 6-K submission or other Commission filing on EDGAR.
EXPLANATORY NOTE
Enclosed hereto are copies of the following items in connection with Galapagos NV’s (the “Company”) annual report for the financial year 2023 and its Annual and Extraordinary Shareholders’ Meetings that will be held sequentially on Tuesday, April 30, 2024 at 2:00 p.m. (CET) and 3:00 p.m. (CET), respectively, at the registered office of Galapagos NV.
The information contained in this Form 6-K, including, Exhibits 99.2, 99.3, 99.4, 99.5, 99.6, 99.7, 99.8, 99.9, 99.10, 99.11, 99.12, 99.13, 99.14 and 99.15 is hereby incorporated by reference into the Company’s Registration Statements on Form S-8 (File Nos. 333-204567, 333-208697, 333-211834, 333-215783, 333-218160, 333-225263, 333-231765, 333-249416, 333-260500, 333-268756 and 333-275886). The information contained in Exhibit 99.1 to this Form 6-K is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| GALAPAGOS NV |
||
| (Registrant) | ||
| Date: March 29, 2024 | /s/ Annelies Denecker |
|
| Annelies Denecker Company Secretary |
||
Exhibit 99.1

Galapagos publishes 2023 annual report and announces Annual and Extraordinary Shareholders’ Meetings
| • | Publication of annual report for financial year 2023 |
| • | Annual Shareholders’ Meeting resolutions include approval of revised Remuneration Policy and (re)appointment of Board members |
| • | Extraordinary Shareholders’ Meeting resolutions include approval of renewal of authorized capital and issuance of Gilead Subsequent Warrant B |
Mechelen, Belgium; 28 March 2024, 21.01 CET; regulated information – Galapagos NV (Euronext & NASDAQ: GLPG) today publishes its annual report for the financial year 2023 and announces its Annual and Extraordinary Shareholders’ Meetings (AGM and EGM) to be held sequentially on Tuesday, 30 April 2024 at 2:00 pm (CET) and 3:00 pm (CET), respectively, at the registered office of the Company.
The annual report for the financial year 2023, including a review of figures and performance, is available online at https://www.glpg.com/financial-reports and can also be downloaded as PDF. Our annual 2023 Form 20-F filing with the SEC is available at www.sec.gov/edgar.
Galapagos has the honor to invite its shareholders, holders of subscription rights, Board members, and statutory auditor to its Annual (ordinary) and Extraordinary Shareholders’ Meetings that will be held sequentially on Tuesday 30 April 2024 at 2:00 pm (CET) and 3:00 pm (CET), respectively, at the Company’s registered office.
The items on the agenda of the Annual and Extraordinary Shareholders’ Meetings include, amongst other items: (i) the approval of a revised Remuneration Policy, (ii) the reappointment of Dr. Elisabeth Svanberg as Non-Executive Independent Director, (iii) the appointments of Dr. Susanne Schaffert and Mr. Simon Sturge as Non-Executive Independent Directors, and Mr. Andrew Dickinson as Non-Executive Director, (iv) the approval of the issuance of a warrant for the benefit of Gilead Therapeutics A1 Unlimited Company (“Subsequent Warrant B”), and (v) the approval of the renewal of the Company’s authorized capital by up to 20% of the share capital.
In order to be admitted to the Shareholders’ Meetings to be held on 30 April 2024, the holders of securities issued by the Company must comply with article 7:134 of the Belgian Code of Companies and Associations and article 23 of the Company’s articles of association, and fulfil the formalities described in the convening notice. The convening notice and other documents pertaining to the Annual and Extraordinary Shareholders’ Meetings can be consulted on our website at www.glpg.com/shareholders-meetings.
Biographies of proposed Board members
The biographies of Dr. Elisabeth Svanberg, Dr. Susanne Schaffert, Mr. Simon Sturge and Mr. Andrew Dickinson can be found on our website.
About Galapagos
We are a biotechnology company with operations in Europe and the US dedicated to developing transformational medicines for more years of life and quality of life. Focusing on high unmet medical needs, we synergize compelling science, technology, and collaborative approaches to create a deep pipeline of best-in-class small molecules, CAR-T therapies and biologics in oncology and immunology. With capabilities from lab to patient, including a decentralized, point-of-care CAR-T manufacturing network, we are committed to challenging the status quo and delivering results for our patients, employees and shareholders. For additional information, please visit www.glpg.com or follow us on LinkedIn or X (formerly Twitter).
1

| Contact |
||
| Media inquiries: |
Investor inquiries: | |
| Marieke Vermeersch |
Sofie Van Gijsel | |
| +32 479 490 603 |
+1 781 296 1143 | |
| media@glpg.com |
ir@glpg.com | |
| Sandra Cauwenberghs | ||
| +32 495 58 46 63 | ||
| ir@glpg.com | ||
Forward-looking statements
This release may contain forward-looking statements. Such forward-looking statements are not guarantees of future results. These statements speak only as of the date of publication of this release. We expressly disclaim any obligation to update any forward-looking statements in this release, unless specifically required by law or regulation.
Disclaimer
The contents of our website, including the annual report for the financial year 2023 and the reports prepared by the Board of Directors and the statutory auditor at the occasion of the Extraordinary Shareholders’ Meeting, and any other website that may be accessed from our website, shall not be deemed incorporated by reference in any filing under the Securities Act of 1933.
2
Exhibit 99.2
Pioneering science patient to transform outcomes Annual Report 2023


TABLE OF CONTENTS Table of Contents Our Business Disclaimer and other information 4 Our Company 7 Key achievements in 2023 15 Outlook 2024 27 Going concern statement 27 Risk management and internal control 28 Portfolio Portfolio 31 Oncology 32 Immunology 50 Risk factors Detailed description of the risk factors in Form 20-F 61 Risks related to product development and regulatory approval 61 Risks related to commercialization 65 Risks related to our financial position and need for additional capital 66 Risks related to our reliance on third parties 67 Risks related to our intellectual property 71 Risks related to our competitive position 73 Risks related to our organization, structure and operation 74 Market risks relating to the Galapagos shares 79 General statement about Galapagos’ risks 80 Sustainability report Our Sustainability Commitment – Forward, Sustainably 82 Our Ambition 83 Our Sustainability Governance 84 Our Double Materiality Assessment 84 Our Pillars 86 Reporting 99 Appendix 104 Corporate governance Galapagos’ corporate governance policies 114 Board of Directors of Galapagos NV 117 Committees 130 Executive Committee of Galapagos NV 134 Galapagos NV’s share capital and shares 140 Shareholders 144 Our Remuneration Policy 148 Remuneration Report 149 Conflict of interests and related parties 168 Code of Conduct 171 Statement by the Board of Directors 172 Financial statements Consolidated financial statements 174 Notes to the consolidated financial statements 179 Overview statutory results of Galapagos NV 254 Report of the statutory auditor Report of the statutory auditor 258 Other information Glossary 266 Financial calendar 277 Colophon 278 Contact 279 2 Galapagos NV Annual Report 2023

Our Business Overview of our company, our strategy, and 2023 key achievements Pioneering science to transform patient outcomes

OUR BUSINESS Disclaimer and other information This report contains the information required under Belgian law. Galapagos NV is a limited liability company organized under the laws of Belgium, with its registered office at Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium and registered with the Crossroads Enterprise Database (RPR Antwerp – division Mechelen) under number 0466.460.429. Throughout this report, the term “Galapagos NV” refers solely to the non-consolidated Belgian company, and references to “we,” “our,” “the group” or “Galapagos” include Galapagos NV together with its subsidiaries. This report is published in Dutch and English. Galapagos will use reasonable efforts to ensure the translation and conformity between the Dutch and English versions. In case of inconsistency between the Dutch and English versions, the Dutch version shall prevail. This document is the printed or PDF version of the Annual Report 2023 and is a free translation of the official Dutch language version in the European single electronic format (ESEF) of the Annual Report 2023. The official Dutch language ESEF version of the report prevails and is available on our website (www.glpg.com). This report, as well as the statutory financial statements of Galapagos NV, are available free of charge and upon request to be addressed to: Galapagos NV Investor Relations Generaal De Wittelaan L11 A3 2800 Mechelen, Belgium Tel: +32 15 34 29 00 Email: ir@glpg.com A digital version of this report, as well as the statutory financial statements of Galapagos NV, are available on our website (www.glpg.com). We will use our reasonable efforts to ensure the accuracy of the digital version, but do not assume responsibility if inaccuracies or inconsistencies with the printed or PDF document arise as a result of any electronic transmission. Other information on our website, or on other websites, does not form a part of this report. As a U.S. listed company, we are also subject to the reporting requirements of the U.S. Securities and Exchange Commission, or SEC. An annual report will be filed with the SEC on Form 20-F. Our annual report on Form 20-F is available in the SEC’s EDGAR database (https://www.sec.gov/edgar.shtml), and a link thereto is posted on our website. With the exception of filgotinib’s approval as Jyseleca® for the treatment of moderate to severe rheumatoid arthritis and ulcerative colitis by the European Commission, Great Britain’s Medicines and Healthcare products Regulatory Agency, and the Japanese

Ministry of Health, Labour and Welfare, our drug candidates mentioned in this report are investigational; their efficacy and safety have not been fully evaluated by any regulatory authority. Forward-looking statements This annual report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, that are based on our management’s beliefs and assumptions and on information currently available to our management. All statements other than present and historical facts and conditions contained in this annual report, including statements regarding our future results of operations and financial positions, business strategy, plans and our objectives for future operations, are forward-looking statements. When used in this annual report, the words “anticipate,” “believe,” “can,” “could,” “estimate,” “expect,” “intend,” “is designed to,” “may,” “might,” “plan,” “potential,” “predict,” “objective,” “should,” or the negative of these and similar expressions identify forward-looking statements. Forward-looking statements contained in this report include, but are not limited to, statements related to: the guidance from management regarding our financial results and expected operational use of cash, statements regarding our strategic and capital allocation priorities, statements regarding our regulatory outlook, business strategy and statements regarding preliminary, interim and topline data from our preclinical and clinical studies and any other data or analyses related to programs, and our plans and strategy with respect to such studies, statements about our ability to advance product candidates into, and successfully complete, clinical trials, statements regarding the timing and likelihood of business development projects and external innovation, statements regarding the amount and timing of potential future milestones, opt-in, royalty or other payments, statements regarding our R&D plans, strategy, and outlook, including progress on our oncology or immunology portfolio and our CAR-T portfolio, including any potential changes in such strategy, statements regarding our pipeline and complementary technology platforms facilitating future growth, statements regarding our commercialization efforts for our product candidates and any of our future approved products, if any, statements regarding the potential attributes and benefits of our product candidates, including indications, dosing and treatment modalities, and their potential competitive position with respect to other treatment alternatives, statements regarding the global R&D collaboration with Gilead, and the amendment of our arrangement with Gilead for commercialization and development of filgotinib, statements relating to the development of our commercial organization, commercial sales, and rollout of our products or product candidates (if approved) globally, statements relating to the development of our distributed manufacturing capabilities on a global basis, statements regarding our supply chain, including our reliance on third parties, and statements regarding our sustainability plans. We caution the reader that forward-looking statements are based on our management’s current expectations and beliefs and are not guarantees of any future performance. Forward-looking statements may involve known and unknown risks, uncertainties and other factors which might cause our actual results, financial condition and liquidity, performance or achievements, or the industry in which we operate, to be materially different from any historic or future

results, financial conditions, performance or achievements expressed or implied by such statements. Such risks include, but are not limited to, the risk that our beliefs, guidance, and expectations regarding our 2024 revenues, cash burn, operational expenses, or other financial metrics may be incorrect (including because one or more of our assumptions underlying our revenue or expense expectations may not be realized), the risk that ongoing and future clinical trials may not be completed in the currently envisaged timelines or at all, the inherent risks and uncertainties associated with competitive developments, clinical trials, recruitment of patients, estimated patient populations, product development activities, and regulatory approval requirements (including, but not limited to, the risk that data and timing from our ongoing and planned clinical research programs may not support registration or further development of our product candidates due to safety, or efficacy concerns, or any other reasons), risks related to the potential benefits and risks related to our current collaborations, including our plans and ability to enter into collaborations for additional programs or product candidates, risks related to the acquisitions of CellPoint and AboundBio, including the risk that we may not achieve the anticipated benefits of the acquisitions of CellPoint and AboundBio, the inherent risks and uncertainties associated with target discovery and validation, and drug discovery and development activities, the risk that the preliminary and topline data from our preclinical and clinical studies may not be reflective of the final data, risks related to our reliance on collaborations with third parties (including, but not limited to, Gilead), the risk that we will not be able to continue to execute on our currently contemplated business plan and/or will revise our business plan, including the risk that our plans with respect to CAR-T may not be achieved on the currently anticipated timeline or at all, the risk that our projections and expectations regarding the commercial potential of our product candidates or expectations regarding the revenues and costs associated with the commercialization rights may be inaccurate, the risks related to our strategic transformation exercise, including the risk that we may not achieve the anticipated benefits of such exercise on the currently envisaged timeline or at all, the risk that we will encounter challenges retaining or attracting talent, and risks related to disruption in our operations, supply chain, or ongoing studies due to conflicts or macroeconomic issues. A further list and description of these risks, uncertainties and other risks can be found in our filings and reports with the Securities and Exchange Commission (“SEC”), including in our most recent annual report on Form 20-F filed with the SEC, and our subsequent filings and reports filed with the SEC. We also refer to the “Risk Factors” section of this report. Given these risks and uncertainties, the reader is advised not to place any undue reliance on any such forward-looking statements. In addition, even if our results, performance, financial condition and liquidity, or the industry in which we operate, are consistent with such forward-looking statements, they may not be predictive of results, performance or achievements in future periods. These forward-looking statements speak only as of the date of publication of this report. We expressly disclaim any obligation to update any such statements in this report to reflect any change in our expectations with regard thereto, or any change in events, conditions or circumstances on which any such statements is based, or that may affect

the likelihood that actual results will differ from those set forth in any such statements, unless specifically required by law or regulation. Our Company Our Vision and Mission Our Vision Transforming patient outcomes through life-changing science and innovation for more years of life and quality of life. Our Mission We accelerate transformational innovation through the relentless pursuit of groundbreaking science, our entrepreneurial spirit and a collaborative mindset. Our Forward, Faster Strategy Our goal is to bring transformational medicines to patients across the globe for more years of life and quality of life. Our focus is on conditions with high unmet medical need. To achieve this, we are working to synergize compelling science, technology, and approaches to develop a deep pipeline of potentially best-in-class small molecules, CAR-T therapies and biologics in oncology and immunology. We continue to take steps to transform into an innovative pure-play biotech company by sharpening our focus on our key priority areas. Following the transfer of our entire Jyseleca® (filgotinib) business, we are moving forward with greater focus and flexibility to invest in our key technology platforms and strategic therapeutic areas. We are committed to challenging the status quo and delivering results for patients, employees, and shareholders.
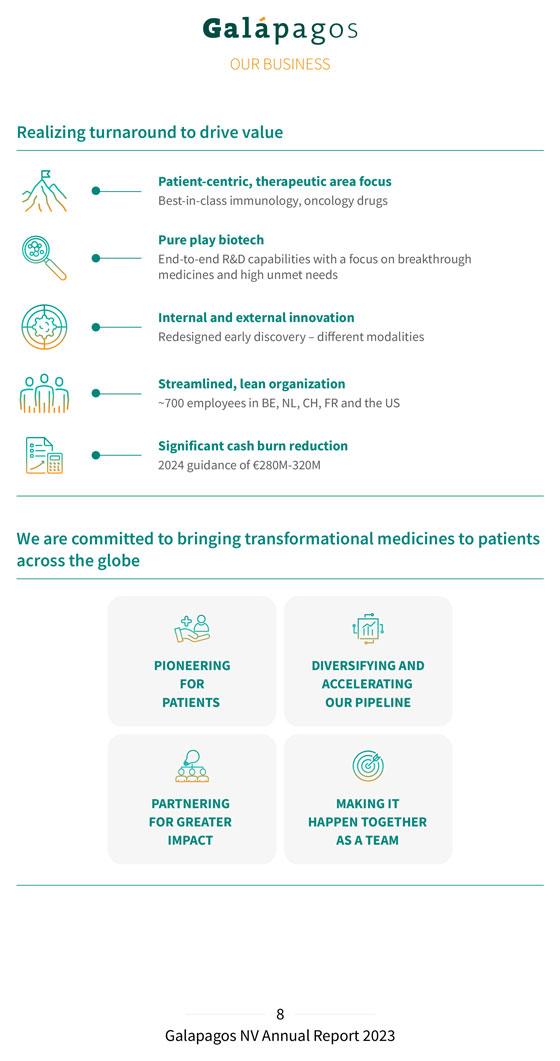
Realizing turnaround to drive value Patient-centric, therapeutic area focus Best-in-class immunology, oncology drugs Pure play biotech End-to-end R&D capabilities with a focus on breakthrough medicines and high unmet needs Internal and external innovation Redesigned early discovery – different modalities Streamlined, lean organization ~700 employees in BE, NL, CH, FR and the US Significant cash burn reduction 2024 guidance of €280M-320M We are committed to bringing transformational medicines to patients across the globe PIONEERING DIVERSIFYING AND FOR ACCELERATING PATIENTS OUR PIPELINE PARTNERING MAKING IT FOR GREATER HAPPEN TOGETHER IMPACT AS A TEAM

We have a clear path to value creation: Pioneering for patients through our targeted R&D approach. At Galapagos, we are focusing on discovering and developing best-in-class medicines in oncology and immunology. We are advancing our current clinical programs. We are developing our unique decentralized CAR-T manufactured programs in hemato-oncology. We are continuing to pursue strategic investments and partnerships to support and expand our pipeline. We are focused on validated targets and next-generation cell therapies and biologics in oncology and immunology. Diversifying our pipeline and making clear portfolio decisions to achieve our vision. We believe we have a greater chance of success by working with multiple drug modalities and combinations across our core therapeutic areas. By 2028, we aim to have: A first medicine available to patients. A robust late-stage pipeline with several programs in pivotal trials. A solid early-stage pipeline of small molecules, next-generation cell therapies and biologics in our core therapeutic areas. Accelerating and building our pipeline through strategic partnerships and M&A. To achieve our ambitious goals, we evaluate and access external innovation. We are scouting for the best science, products, and people to complement our internal assets and capabilities, with the aim of building a balanced portfolio of best-in-class medicines across modalities and development stages in our core therapeutic areas. We are open to finding the best possible deal structure or collaboration model that benefits Galapagos and our stakeholders, with a key focus on expanding and accelerating our pipeline and bringing differentiated medicines to patients. Fostering a strong culture of innovation. Our success is made possible by our incredible teams. Our employees’ relentless drive for innovation, teamwork, and a quality mindset focused on efficiency is what drives our progress. We are committed to creating a purpose-driven, inclusive workplace where our people feel safe and empowered, have opportunities to learn and grow, are recognized for their contributions, and perform at their best as individuals and as a team.

Life-changing science and innovation We combine deep disease expertise and multiple drug modalities to accelerate time-to-patients through our internal efforts and focused business development. Cell Therapy Small Molecules Biologics We have groundbreaking We have a long We are building research capabilities and a history and deep research capabilities decentralized manufacturing R&D experience in to discover novel platform for CAR-T small molecules biological medicines CAR-T cell therapy In 2022, we entered the field of CAR-T and antibody-therapy research and development through the acquisitions of CellPoint (in the Netherlands) and Abound Bio (in the U.S). The transactions provide us with end-to-end capabilities in CAR-T therapy development and offer the potential for a paradigm shift in the space through the implementation of a breakthrough, decentralized manufacturing model and cutting-edge fully human antibody-based capabilities to design next-generation CAR-Ts. CAR-T cell therapy near the point-of-care Galapagos is committed to manufacturing personalized cell therapies at or near the point-of-care (PoC). Our ambition is to reduce the manufacturing turnaround time significantly from months or weeks to days, ensuring that patients can receive their therapy in a timely manner.
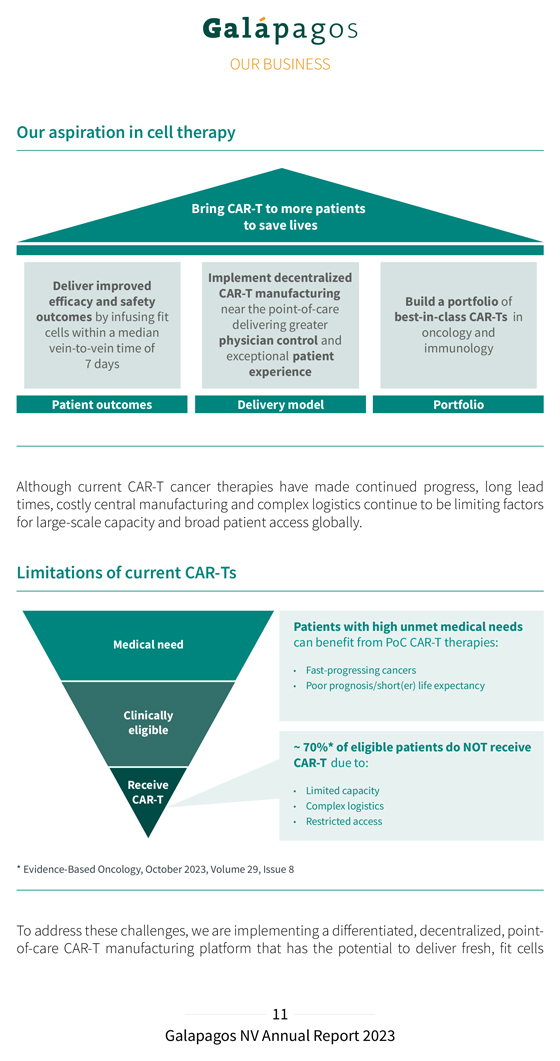
Our aspiration in cell therapy Bring CAR-T to more patients to save lives Implement decentralized Deliver improved CAR-T manufacturing efficacy and safety Build a portfolio of near the point-of-care outcomes by infusing fit best-in-class CAR-Ts in delivering greater cells within a median oncology and physician control and vein-to-vein time of immunology exceptional patient 7 days experience Patient outcomes Delivery model Portfolio Although current CAR-T cancer therapies have made continued progress, long lead times, costly central manufacturing and complex logistics continue to be limiting factors for large-scale capacity and broad patient access globally. Limitations of current CAR-Ts Patients with high unmet medical needs Medical need can benefit from PoC CAR-T therapies: Fast-progressing cancers Poor prognosis/short(er) life expectancy Clinically eligible ~ 70%* of eligible patients do NOT receive CAR-T due to: Receive Limited capacity CAR-T Complex logistics Restricted access * Evidence-Based Oncology, October 2023, Volume 29, Issue 8 To address these challenges, we are implementing a differentiated, decentralized, point-of-care CAR-T manufacturing platform that has the potential to deliver fresh, fit cells
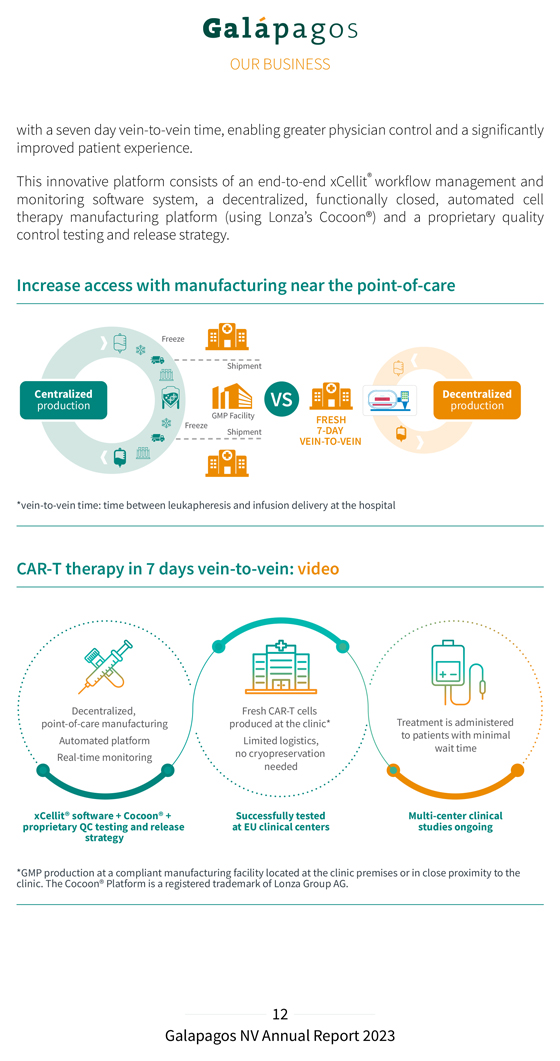
with a seven day vein-to-vein time, enabling greater physician control and a significantly improved patient experience. This innovative platform consists of an end-to-end xCellit® workflow management and monitoring software system, a decentralized, functionally closed, automated cell therapy manufacturing platform (using Lonza’s Cocoon®) and a proprietary quality control testing and release strategy. Increase access with manufacturing near the point-of-care *vein-to-vein time: time between leukapheresis and infusion delivery at the hospital CAR-T therapy in 7 days vein-to-vein: video Decentralized, Fresh CAR-T cells point-of-care manufacturing produced at the clinic* Treatment is administered Automated platform Limited logistics, to patients with minimal wait time Real-time monitoring no cryopreservation needed xCellit® software + Cocoon® + Successfully tested Multi-center clinical proprietary QC testing and release at EU clinical centers studies ongoing strategy *GMP clinic. production The Cocoon® at Platform a compliant is a manufacturing registered trademark facility of located Lonza at Group the clinic AG. premises or in close proximity to the
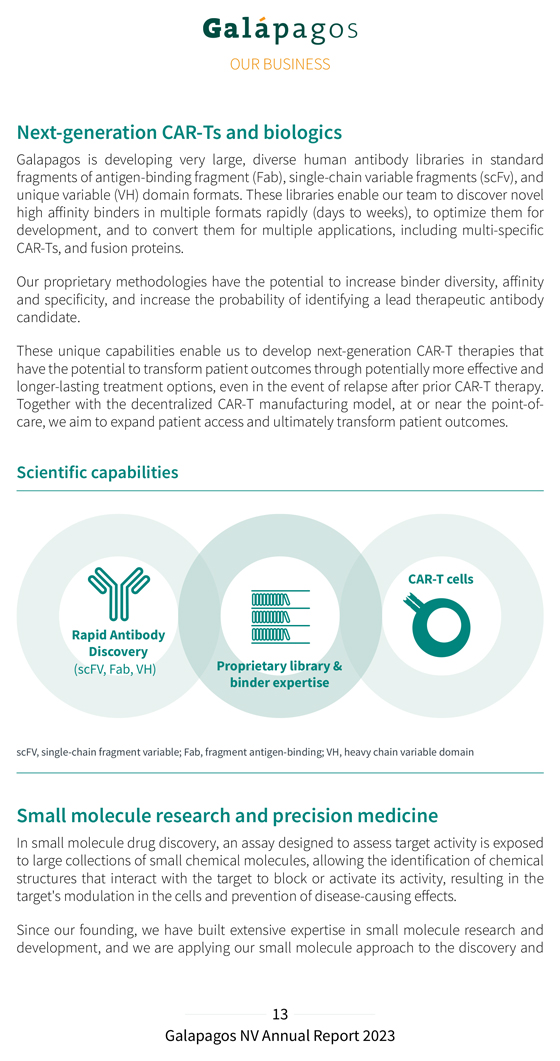
Next-generation CAR-Ts and biologics Galapagos is developing very large, diverse human antibody libraries in standard fragments of antigen-binding fragment (Fab), single-chain variable fragments (scFv), and unique variable (VH) domain formats. These libraries enable our team to discover novel high affinity binders in multiple formats rapidly (days to weeks), to optimize them for development, and to convert them for multiple applications, including multi-specific CAR-Ts, and fusion proteins. Our proprietary methodologies have the potential to increase binder diversity, affinity and specificity, and increase the probability of identifying a lead therapeutic antibody candidate. These unique capabilities enable us to develop next-generation CAR-T therapies that have the potential to transform patient outcomes through potentially more effective and longer-lasting treatment options, even in the event of relapse after prior CAR-T therapy. Together with the decentralized CAR-T manufacturing model, at or near the point-of-care, we aim to expand patient access and ultimately transform patient outcomes. Scientific capabilities CAR-T cells Rapid Antibody Discovery (scFV, Fab, VH) Proprietary library & binder expertise scFV, single-chain fragment variable; Fab, fragment antigen-binding; VH, heavy chain variable domain Small molecule research and precision medicine In small molecule drug discovery, an assay designed to assess target activity is exposed to large collections of small chemical molecules, allowing the identification of chemical structures that interact with the target to block or activate its activity, resulting in the target’s modulation in the cells and prevention of disease-causing effects. Since our founding, we have built extensive expertise in small molecule research and development, and we are applying our small molecule approach to the discovery and
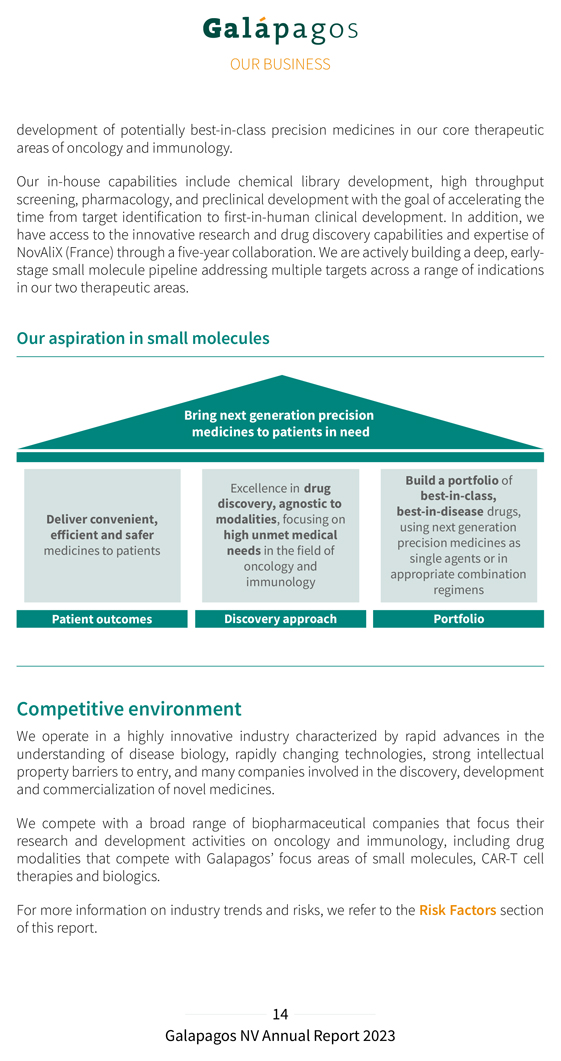
development of potentially best-in-class precision medicines in our core therapeutic areas of oncology and immunology. Our in-house capabilities include chemical library development, high throughput screening, pharmacology, and preclinical development with the goal of accelerating the time from target identification to first-in-human clinical development. In addition, we have access to the innovative research and drug discovery capabilities and expertise of NovAliX (France) through a five-year collaboration. We are actively building a deep, early-stage small molecule pipeline addressing multiple targets across a range of indications in our two therapeutic areas. Our aspiration in small molecules Bring next generation precision medicines to patients in need Excellence in drug Build a portfolio of best-in-class, discovery, agnostic to best-in-disease drugs, Deliver convenient, modalities, focusing on using next generation efficient and safer high unmet medical precision medicines as medicines to patients needs in the field of single agents or in oncology and immunology appropriate combination regimens Patient outcomes Discovery approach Portfolio Competitive environment We operate in a highly innovative industry characterized by rapid advances in the understanding of disease biology, rapidly changing technologies, strong intellectual property barriers to entry, and many companies involved in the discovery, development and commercialization of novel medicines. We compete with a broad range of biopharmaceutical companies that focus their research and development activities on oncology and immunology, including drug modalities that compete with Galapagos’ focus areas of small molecules, CAR-T cell therapies and biologics. For more information on industry trends and risks, we refer to the Risk Factors section of this report.

Key achievements in 2023 Corporate and Operational Performance 2023 Oncology portfolio GLPG5201 (CD19 CAR-T) in relapsed/refractory chronic lymphocytic leukemia (rrCLL) and Richter transformation (RT) (cut-off date: 6 September 2023) Patient recruitment of the Phase 1 dose-finding part of EUPLAGIA-1 was completed: 15 patients were enrolled (6 at dose level 1 (DL1); and 9 at dose level 2 (DL2)), all of whom were diagnosed with rrCLL and 9 with additional RT. Presented encouraging preliminary Phase 1 data at the ASH Annual Meeting, which demonstrated clinically meaningful results in severely compromised patient populations and highlighted the potential of Galapagos’ point-of-care CAR-T manufacturing platform to deliver a fresh product with a median vein-to-vein time of only seven days. GLPG5101 (CD19 CAR-T) in relapsed/refractory non-Hodgkin lymphoma (rrNHL) (cut-off date: 1 September 2023) To further build a robust data package, patient recruitment of the Phase 1 dose-finding part of ATALANTA-1 is ongoing: 14 rrNHL patients with diffuse large B cell lymphoma, mantle cell lymphoman, and indolent lymphoma were enrolled (7 at DL1 and 7 at DL2). In parallel, enrollment of the Phase 2 expansion study is ongoing, and the first 9 patients were dosed. Presented encouraging preliminary Phase 1 and Phase 2 data at the ASH Annual Meeting, which demonstrated clinically meaningful results in severely compromised patient populations and highlighted the potential of Galapagos’ point-of-care CAR-T manufacturing platform to deliver a fresh product with a median vein-to-vein time of only seven days. GLPG5301 (BCMA CAR-T) in relapsed/refractory multiple myeloma (rrMM) First patients were dosed in the PAPILIO-1 Phase 1/2 study to evaluate the safety, efficacy and feasibility of point-of-care manufactured GLPG5301 in patients with rrMM after ≥2 prior lines therapy. Continued to evolve our oncology research activities in biologics, cell therapies and small molecules To deliver best-in-class medicines for patients with high unmet medical need.

Immunology portfolio Jyseleca® (filgotinib) (JAK1): successfully transferred to Alfasigma S.p.A. Achieved reimbursement for both RA and UC across Western Europe. Sobi, the distribution and commercialization partner for filgotinib in Eastern and Central Europe, Portugal, Greece, and the Baltic countries, launched Jyseleca® in Poland and Slovenia in both rhematoid arthritis (RA) and ulcerative colitis (UC), and in Croatia and Greece for RA. The European Commission endorsed the recommendation of the Pharmaceutical Risk Assessment Committee (PRAC) to add safety measures for the JAK inhibitors class of medicines. Based on topline results from the Phase 3 DIVERSITY study in Crohn’s disease, a Marketing Authorization Application (MAA) was not submitted in Europe in this indication and the MAA for filgotinib in UC in Switzerland did not proceed. First patients dosed in the pivotal Phase 3 OLINGUITO study in axial spondyloarthritis (AxSpA). Pipeline programs First patients were dosed in the Phase 2 GALARISSO study of novel, oral, selective tyrosine kinase 2 (TYK2) inhibitor, GLPG3667, in patients with dermatomyositis (DM) and the Phase 2 GALACELA study in systemic lupus erythematosus (SLE). We initiated multiple small molecules programs to expand our research pipeline in immunology research pipeline. Corporate update Thad Huston was appointed as Chief Financial Officer (CFO) and Chief Operating Officer (COO), succeeding Bart Filius, as of 1 July 2023. The Board of Directors appointed Dr. Susanne Schaffert and Mr. Simon Sturge as Non-Executive Independent Directors by way of cooptation, replacing respectively Dr. Rajesh Parekh and Dr. Mary Kerr, who stepped down. The Board of Directors created 1,538,400 subscriptions rights under new subscription right plans, after acceptance by the beneficiaries. We successfully completed the integrated drug discovery collaboration transaction with NovAliX. We signed a letter of intent with Alfasigma to transfer the entire Jyseleca® business to Alfasigma, including the European and UK Marketing Authorizations, as well as the commercial, medical and development activities for Jyseleca® and approximately 400 Galapagos positions in 14 European countries. Galapagos and Gilead amended the Filgotinib Agreement to terminate the existing 50/50 global development cost sharing arrangement with Galapagos bearing the costs going forward, and to terminate Galapagos’ obligation to pay tiered royalties to Gilead on net sales of Jyseleca® in Europe, in addition to other amendments.

We signed an agreement with Boston-based Landmark Bio and started the technology transfer for the decentralized production of Galapagos’ CAR-T cell therapy candidates. Post-period events For strategic reasons, we decided not to continue development of our CD19 CAR-T candidate in refractory systemic lupus erythematosus (rSLE). We participated in the Series C financing round of Frontier Medicines, a pioneer in precision oncology with a unique technology platform and a pipeline of potential best-in-class assets that fit with Galapagos’ precision oncology R&D approach. The investment aligns with our innovation acceleration strategy to bring transformational medicines to patients around the world. We presented a poster at the annual EBMT-EHA congress highlighting new preliminary translational data from EUPLAGIA-1, which demonstrate that our point-of-care manufacturing platform has the potential to enable a single infusion of fresh early-phenotype CD19 CAR-T cells with robust expansion and persistence in patients with rrCLL and in patients with RT. We signed a share and asset purchase agreement with Alfasigma to transfer the entire Jyseleca® business to Alfasigma. As part of the transaction, the amended Filgotinib Agreement between Galapagos and Gilead has been assigned by Galapagos to Alfasigma. The transaction was successfully completed on 31 January 2024, which freed up resources to reinvested in R&D growth areas. Michele Manto’s mandate as Chief Commercial Officer and member of the Executive Committee of Galapagos ended in December 2023; he joined Alfasigma to lead the Jyseleca® business. We further streamlined our remaining operations, reducing approximately 100 positions across the Galapagos organization to align with the Galapagos’ renewed focus on innovation. We signed a strategic collaboration and license agreement with BridGene Biosciences to strengthen Galapagos’ growing early-stage oncology precision medicine pipeline. We entered into a strategic collaboration agreement with Thermo Fisher Scientific for CAR-T manufacturing and kitting services for Galapagos’ point-of-care CAR-T product candidate in the San Francisco area. The Board of Directors appointed Mr. Andrew Dickinson as Non-Executive Non-Independent Director by way of cooptation. Mr. Andrew Dickinson is Gilead’s Chief Financial Officer and replaces Mr. Daniel O’Day, Gilead’s Chief Executive Officer, who was a member of the Galapagos Board of Directors from 22 October 2019 to 26 March 2024.
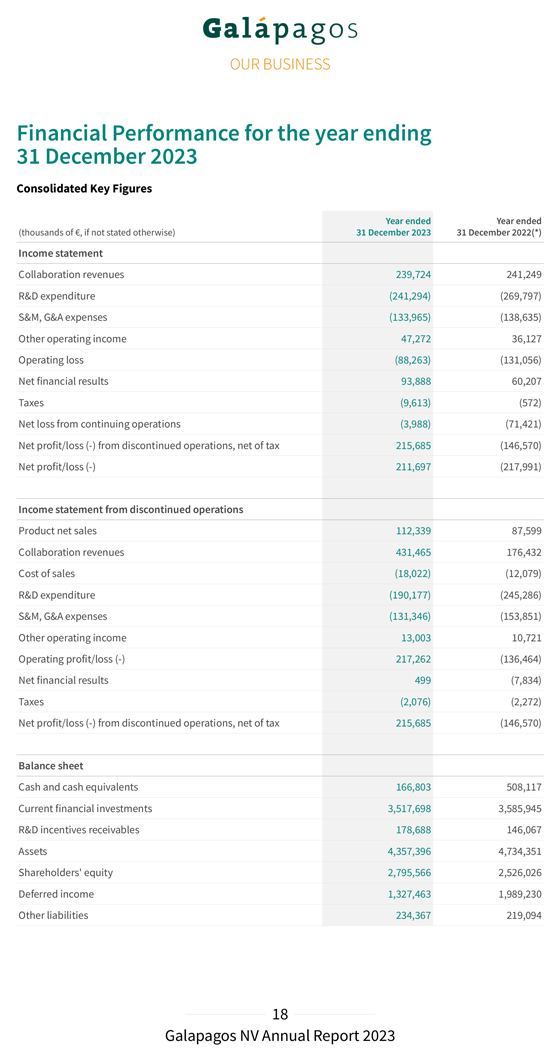
31 Financial December Performance 2023 for the year ending Consolidated Key Figures Year ended Year ended (thousands of €, if not stated otherwise) 31 December 2023 31 December 2022(*) Income statement Collaboration revenues 239,724 241,249 R&D expenditure (241,294) (269,797) S&M, G&A expenses (133,965) (138,635) Other operating income 47,272 36,127 Operating loss (88,263) (131,056) Net financial results 93,888 60,207 Taxes (9,613) (572) Net loss from continuing operations (3,988) (71,421) Net profit/loss (-) from discontinued operations, net of tax 215,685 (146,570) Net profit/loss (-) 211,697 (217,991) Income statement from discontinued operations Product net sales 112,339 87,599 Collaboration revenues 431,465 176,432 Cost of sales (18,022) (12,079) R&D expenditure (190,177) (245,286) S&M, G&A expenses (131,346) (153,851) Other operating income 13,003 10,721 Operating profit/loss (-) 217,262 (136,464) Net financial results 499 (7,834) Taxes (2,076) (2,272) Net profit/loss (-) from discontinued operations, net of tax 215,685 (146,570) Balance sheet Cash and cash equivalents 166,803 508,117 Current financial investments 3,517,698 3,585,945 R&D incentives receivables 178,688 146,067 Assets 4,357,396 4,734,351 Shareholders’ equity 2,795,566 2,526,026 Deferred income 1,327,463 1,989,230 Other liabilities 234,367 219,094
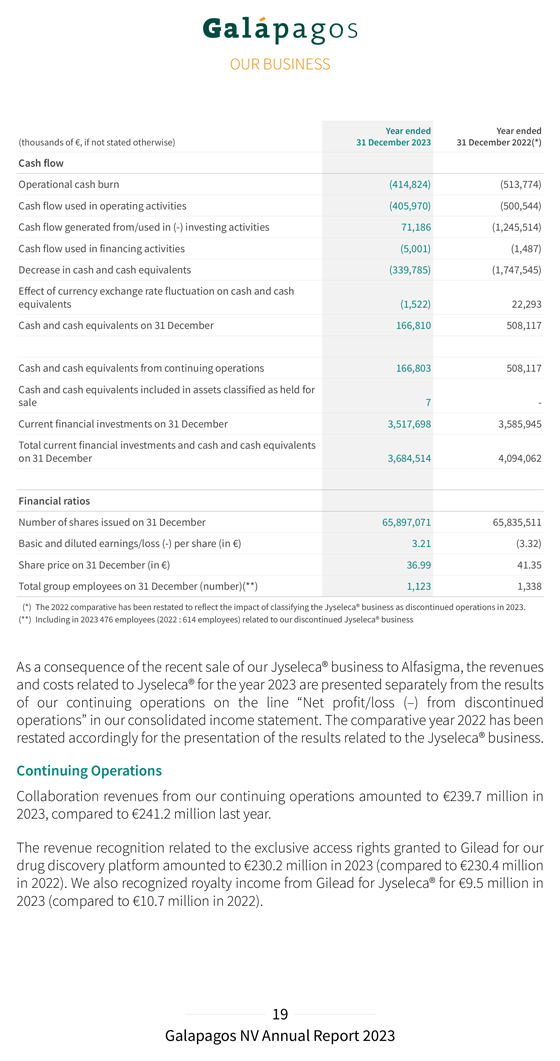
Year ended Year ended (thousands of €, if not stated otherwise) 31 December 2023 31 December 2022(*) Cash flow Operational cash burn (414,824) (513,774) Cash flow used in operating activities (405,970) (500,544) Cash flow generated from/used in (-) investing activities 71,186 (1,245,514) Cash flow used in financing activities (5,001) (1,487) Decrease in cash and cash equivalents (339,785) (1,747,545) Effect of currency exchange rate fluctuation on cash and cash equivalents (1,522) 22,293 Cash and cash equivalents on 31 December 166,810 508,117 Cash and cash equivalents from continuing operations 166,803 508,117 Cash and cash equivalents included in assets classified as held for sale 7—Current financial investments on 31 December 3,517,698 3,585,945 Total current financial investments and cash and cash equivalents on 31 December 3,684,514 4,094,062 Financial ratios Number of shares issued on 31 December 65,897,071 65,835,511 Basic and diluted earnings/loss (-) per share (in €) 3.21 (3.32) Share price on 31 December (in €) 36.99 41.35 Total group employees on 31 December (number)(**) 1,123 1,338 (*) The 2022 comparative has been restated to reflect the impact of classifying the Jyseleca® business as discontinued operations in 2023. (**) Including in 2023 476 employees (2022 : 614 employees) related to our discontinued Jyseleca® business As a consequence of the recent sale of our Jyseleca® business to Alfasigma, the revenues and costs related to Jyseleca® for the year 2023 are presented separately from the results of our continuing operations on the line “Net profit/loss (–) from discontinued operations” in our consolidated income statement. The comparative year 2022 has been restated accordingly for the presentation of the results related to the Jyseleca® business. Continuing Operations Collaboration revenues from our continuing operations amounted to €239.7 million in 2023, compared to €241.2 million last year. The revenue recognition related to the exclusive access rights granted to Gilead for our drug discovery platform amounted to €230.2 million in 2023 (compared to €230.4 million in 2022). We also recognized royalty income from Gilead for Jyseleca® for €9.5 million in 2023 (compared to €10.7 million in 2022).

Our deferred income balance at 31 December 2023 includes €1.3 billion allocated to our drug discovery platform that is recognized linearly over the remaining period of our 10-year collaboration. Our R&D expenditure in 2023 amounted to €241.3 million, compared to €269.8 million in 2022. Depreciation and impairment costs in 2023 amounted to €22.3 million (compared to €51.5 million in 2022). This decrease was primarily due to an impairment of €26.7 million of previously capitalized upfront fees related to our collaboration with Molecure and impairments of €8.9 million of intangible assets related to other discontinued projects, both recorded in 2022. Personnel costs decreased from €115.5 million in 2022 to €95.8 million in 2023 primarily related to lower accelerated non-cash cost recognition for subscription right plans related to good leavers. This was partly offset by an increase in costs from €61.2 million in 2022 to €83.0 million in 2023 following the evolution of our CAR-T programs. Our S&M expenses amounted to €5.7 million in 2023, compared to €3.5 million in 2022. Our G&A expenses amounted to €128.3 million in 2023, compared to €135.2 million in 2022. The cost decrease was explained by a decrease in personnel costs to €66.1 million in 2023 compared €76.5 million to 2022, due to lower accelerated non-cash cost recognition for subscription right plans related to good leavers. Depreciation and impairment expenses increased from €8.5 million in 2022 to €16.0 million in 2023 due to an impairment of €7.6 million on a construction project in Mechelen, Belgium. Other operating income (€47.3 million in 2023 compared to €36.1 million in 2022) increased due to higher grant income (grant from the National Institute for Health and Disability Insurance in 2023 of €6.1 million), higher other operating income (rent income) and higher R&D incentives income. We reported an operating loss amounting to €88.3 million in 2023, compared to an operating loss of €131.1 million in 2022. Net financial income in 2023 amounted to €93.9 million, compared to net financial income of €60.2 million in 2022. Net financial income in 2023 was primarily attributable to €38.3 million of net fair value gains of our current financial investments, partly offset by €20.4 million of unrealized currency exchange losses on our cash and cash equivalents and current financial investments at amortized cost in U.S. dollars. Net interest income amounted to €77.5 million in 2023 as compared to €11.2 million of net interest income in 2022. We had €9.6 million of tax expenses in 2023 (as compared to €0.6 million in 2022). This increase was primarily due to the re-assessment of net deferred tax liabilities and corporate income tax payables as a result of a one-off intercompany transaction. We reported a net loss from continuing operations in 2023 of €4.0 million, compared to a net loss from continuing operations of €71.4 million in 2022.

Discontinued operations Net profit of discontinued operations attributable to the Jyseleca® business amounted to €215.7 million in 2023, compared to €146.6 million net loss of discontinued operations in 2022. Jyseleca® product net sales in Europe amounted to €112.3 million in 2023, compared to €87.6 million in 2022. Cost of sales related to Jyseleca® net sales in Europe amounted to €18.0 million in 2023, compared to €12.1 million for the year 2022. Collaboration revenues in discontinued operations related to revenue recognition of the collaboration agreement with Gilead for the filgotinib development amounted to €429.4 million in 2023 compared to €174.4 million in 2022. This increase was explained by a substantial decrease in our assessment of the remaining costs to complete the filgotinib development following the recent sale of our Jyseleca® business to Alfasigma, including the transfer of the remaining development performance obligation after closing of the transaction. As a consequence, we saw a substantial increase of the percentage of completion of our performance obligation, and a positive catch-up released to revenues. Total operating profit from discontinued operations amounted to €217.3 million in 2023, compared to an operating loss of €136.5 million in 2022. The decrease in R&D expenditures for the development of filgotinib was mainly due to the discontinuation in early 2023 of the DIVERSITY clinical trials in CD. Personnel expenses decreased by €15.0 million, from €74.6 million in 2022 to €59.6 million in 2023, subcontracting costs decreased as well by €39.0 million, from €153.7 million in 2022 to €114.7 million in 2023. The decrease in S&M expenses from €144.1 million in 2022 to €113.4 million in 2023 is reflected in a decrease in personnel costs by €10.8 million, from €70.2 million in 2022 to €59.3 million in 2023 due to lower bonus costs and costs of our subscription right plans, while external outsourcing costs decreased by €17.0 million, from €52.8 million in 2022 to €35.8 million in 2023 primarily explained by lower costs for marketing campaigns and promotional expenses. G&A expenses attributable to the Jyseleca® business increased from €9.8 million in 2022 to €18.0 million in 2023 primarily due to an increase in costs of our subscription right plans; we experienced unusually low costs in 2022 due to a reversal of costs related to voluntary leavers and saw an increase in salaries in 2023. The G&A expenses for the year 2023 also include one-off legal fees related to the transaction with Alfasigma for €3.5 million. Other operating income attributable to the Jyseleca® business increased, mainly due to higher R&D incentives income.

The movement in other financial income/expenses is primarily explained by a lower discounting effect of long-term deferred revenue for the development of filgotinib, because we expect to recognize the remaining revenues in 2024. The financing component related to our filgotinib performance obligation was re-assessed on 31 December 2023, considering the reduced duration and the expected end of the performance obligation for the development of filgotinib. We reported a net profit in 2023 of €211.7 million, compared to a net loss of €218.0 million in 2022. Cash, cash equivalents and current financial investments Current financial investments and cash and cash equivalents totaled €3,684.5 million on 31 December 2023 (including €20.0 million of accrued interest income) as compared to €4,094.1 million on 31 December 2022 (excluding €9.9 million of net accrued interest income). Total net decrease in cash and cash equivalents and current financial investments amounted to €409.6 million in 2023, compared to a net decrease of €609.1 million in 2022. This net decrease was composed of (i) €414.8 million of operational cash burn, (ii) €20.4 million of negative exchange rate differences, (iii) €7.0 million cash-out related to the acquisition of CellPoint B.V., (iv) €14.0 million acquisition of financial assets held at fair value through profit or loss, offset by (v) €24.3 million positive changes in fair value of current financial investments, (vi) €1.8 million of cash proceeds from capital and share premium increase from exercise of subscription rights in 2023, and (vii) €12.9 million of accrued interest income on term deposits and €7.6 million accrued interest income on treasury bills. Operational cash burn (or operational cash flow if this liquidity measure is positive) is a financial measure that is not calculated in accordance with IFRS. Operational cash burn/cash flow is defined as the decrease or increase in our cash and cash equivalents (excluding the effect of exchange rate differences on cash and cash equivalents), minus: 1. the net proceeds, if any, from share capital and share premium increases included in the net cash flow generated from/used in (–) financing activities 2. the net proceeds or cash used, if any, in acquisitions or disposals of businesses and financial assets held at fair value through profit or loss; the movement in restricted cash and movement in current financial investments, if any, the loans and advances given to third parties, if any, included in the net cash flow generated from/used in (–) investing activities 3. the cash used for other liabilities related to the acquisition of businesses, if any, the accrued interest on cash and cash equivalents, if any, included in the net cash flow generated from/used in (–) operating activities. This alternative liquidity measure is, in our view, an important metric for a biotech company in the development stage.
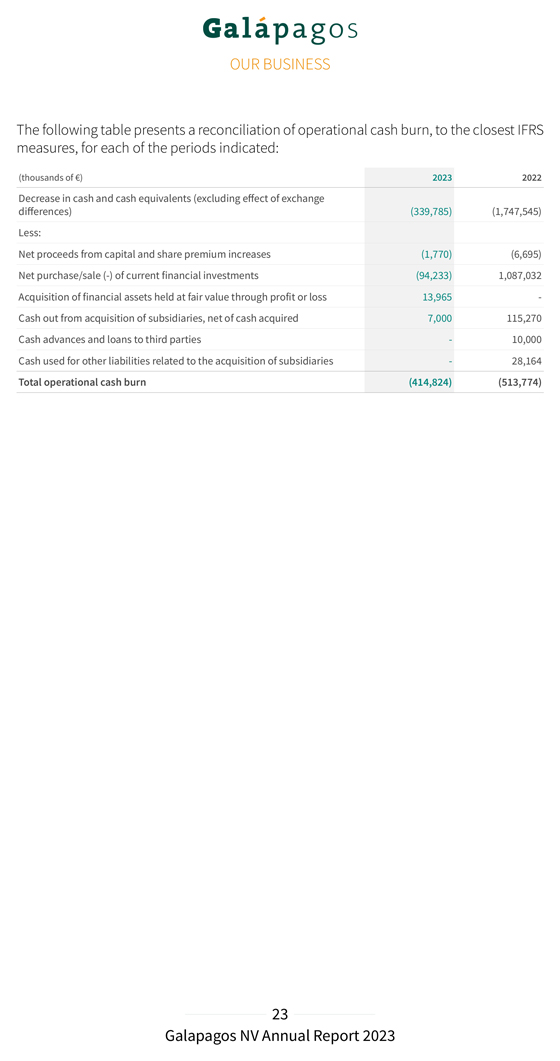
The following table presents a reconciliation of operational cash burn, to the closest IFRS measures, for each of the periods indicated: (thousands of €) 2023 2022 Decrease in cash and cash equivalents (excluding effect of exchange differences) (339,785) (1,747,545) Less: Net proceeds from capital and share premium increases (1,770) (6,695) Net purchase/sale (-) of current financial investments (94,233) 1,087,032 Acquisition of financial assets held at fair value through profit or loss 13,965—Cash out from acquisition of subsidiaries, net of cash acquired 7,000 115,270 Cash advances and loans to third parties—10,000 Cash used for other liabilities related to the acquisition of subsidiaries—28,164 Total operational cash burn (414,824) (513,774)
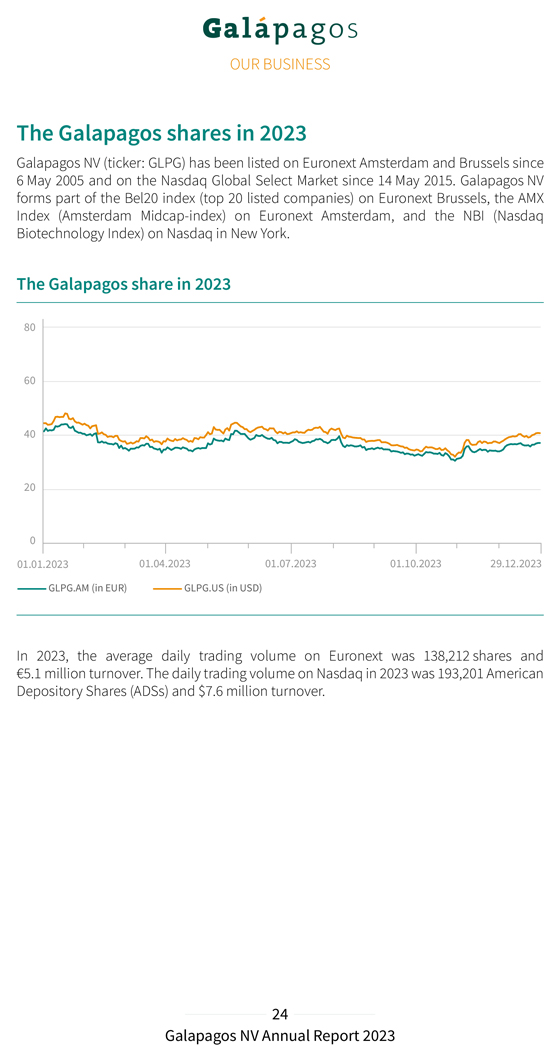
The Galapagos shares in 2023 Galapagos NV (ticker: GLPG) has been listed on Euronext Amsterdam and Brussels since 6 May 2005 and on the Nasdaq Global Select Market since 14 May 2015. Galapagos NV forms part of the Bel20 index (top 20 listed companies) on Euronext Brussels, the AMX Index (Amsterdam Midcap-index) on Euronext Amsterdam, and the NBI (Nasdaq Biotechnology Index) on Nasdaq in New York. The Galapagos share in 2023 In 2023, the average daily trading volume on Euronext was 138,212 shares and €5.1 million turnover. The daily trading volume on Nasdaq in 2023 was 193,201 American Depository Shares (ADSs) and $7.6 million turnover.
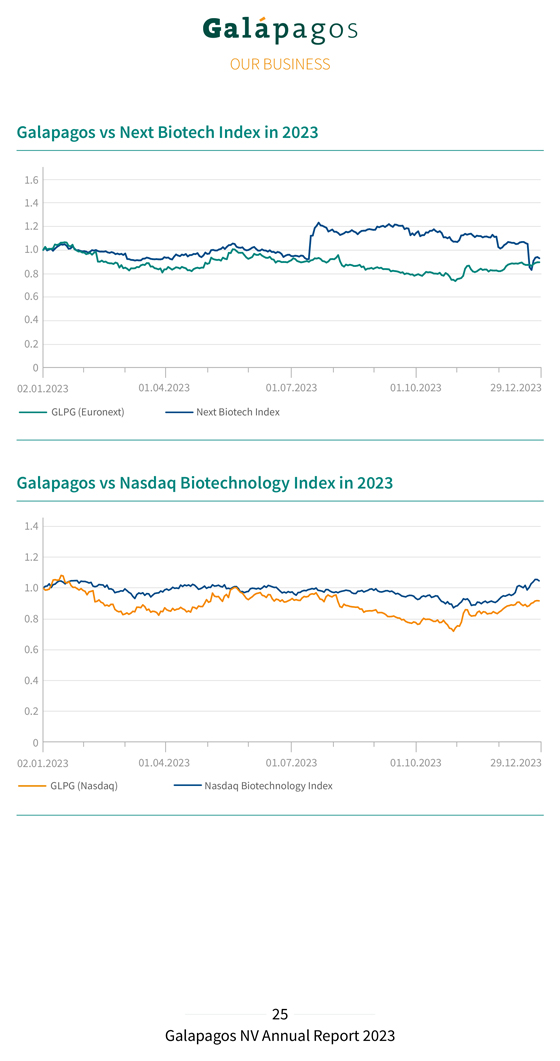
Galapagos vs Next Biotech Index in 2023 Galapagos vs Nasdaq Biotechnology Index in 2023
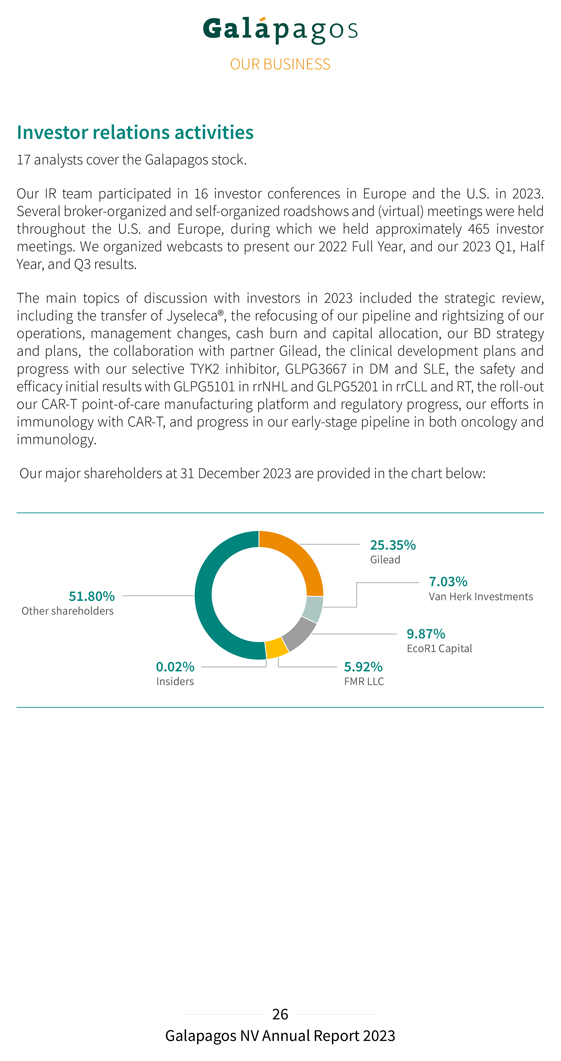
Investor relations activities 17 analysts cover the Galapagos stock. Our IR team participated in 16 investor conferences in Europe and the U.S. in 2023. Several broker-organized and self-organized roadshows and (virtual) meetings were held throughout the U.S. and Europe, during which we held approximately 465 investor meetings. We organized webcasts to present our 2022 Full Year, and our 2023 Q1, Half Year, and Q3 results. The main topics of discussion with investors in 2023 included the strategic review, including the transfer of Jyseleca®, the refocusing of our pipeline and rightsizing of our operations, management changes, cash burn and capital allocation, our BD strategy and plans, the collaboration with partner Gilead, the clinical development plans and progress with our selective TYK2 inhibitor, GLPG3667 in DM and SLE, the safety and efficacy initial results with GLPG5101 in rrNHL and GLPG5201 in rrCLL and RT, the roll-out our CAR-T point-of-care manufacturing platform and regulatory progress, our efforts in immunology with CAR-T, and progress in our early-stage pipeline in both oncology and immunology. Our major shareholders at 31 December 2023 are provided in the chart below:

Outlook 2024 Financial outlook For the full year 2024, we anticipate a further reduction in our cash burn to between €280 million and €320 million (compared to €414.8 million for the full year 2023), not including future potential business development opportunities. R&D Outlook We aim to progress three CAR-T Phase 1/2 studies in hemato-oncology: GLPG5101 in rrNHL; GLPG5201 in rrCLL, with or without RT; and GLPG5301 in rrMM. We expect to file IND applications in the U.S. to begin clinical development of our CAR-T programs in hemato-oncology. We plan to scale up our CAR-T network and operations further in the U.S. and Europe, and potentially in other key regions. Business development We will continue to evaluate multiple product candidates and business development opportunities to leverage our internal capabilities further, and accelerate and expand our pipeline of potential best-in-class investigational medicines in our therapeutic focus areas of immunology and oncology. Going concern statement To date, we have incurred significant operating losses, which are reflected in the consolidated balance sheet showing €228.3 million accumulated losses as at 31 December 2023. We realized a consolidated net profit of €211.7 million for the year ended 31 December 2023. Our existing current financial investments and cash and cash equivalents of €3,684.5 million at 31 December 2023 will enable us to fund our operating expenses and capital expenditure requirements at least for the next 12 months. The Board of Directors is also of the opinion that additional financing could be obtained, if required. Taking this into account, as well as the potential developments of our drug discovery and development activities, the Board of Directors is of the opinion that it can submit the financial statements on a going concern basis. Whilst our current financial investments and cash and cash equivalents are sufficient at least for the next 12 months, the Board of Directors points out that if the R&D activities go well, we may seek additional funding to support the continuing development of our products or to be able to execute other business opportunities.

Risk management and internal control Risk management is embedded in our strategy and is considered important for achieving our operational targets. To safeguard the proper implementation and execution of the group’s strategy, our Executive Committee has established internal risk management and control systems within Galapagos. The Board of Directors has delegated an active role to the Audit Committee members to monitor the design, implementation and effectiveness of these internal risk management and control systems. The purpose of these systems is to manage in an effective and efficient manner the significant risks to which Galapagos is exposed. The internal risk management and control system is designed to ensure: the careful monitoring of the effectiveness of our strategy Galapagos’ continuity and sustainability, through consistent accounting, reliable financial reporting and compliance with laws and regulations our focus on the most efficient and effective way to conduct our business We have defined our risk tolerance on a number of internal and external factors including: financial strength in the long run, represented by revenue growth and a solid balance sheet liquidity in the short run; cash business performance measures; operational and net profitability scientific risks and opportunities dependence on our alliance partners compliance with relevant rules and regulations reputation The identification and analysis of risks is an ongoing process that is naturally a critical component of internal control. Based on these factors and Galapagos’ risk tolerance, the key controls within Galapagos will be registered and the effectiveness will be monitored. If the assessment shows the necessity to modify the controls we will do so. This could be the situation if the external environment changes, or the laws, regulations, or the strategy of Galapagos change. The financial risks of Galapagos are managed centrally. The finance department of Galapagos coordinates the access to national and international financial markets and considers and continuously manages the financial risks concerning the activities of the group. These relate to the following financial markets risks: credit risk, liquidity risk,

currency and interest rate risk. Our interest rate risk is limited because we have nearly no financial debt. In the event of decreasing interest rates we would face a reinvestment risk on our strong cash position. The group does not buy or trade financial instruments for speculative purposes. For further reference on financial risk management, see note 34 of the notes to the consolidated financial statements. We also refer to the Risk factors section of the annual report for additional details on general risk factors. The company’s internal controls over financial reporting are a subset of internal controls and include those policies and procedures that: pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with IFRS as adopted by the EU, and that our receipts and expenditures are being made only by authorized persons provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements Our internal control over financial reporting includes controls over relevant IT systems that impact financial reporting including accuracy and completeness of our account balances. Since the company has securities registered with the U.S. Securities and Exchange Commission (SEC) and is a large accelerated filer within the meaning of Rule 12b-2 of the U.S Securities Exchange Act of 1934, the company needs to assess the effectiveness of internal control over financial reporting and provide a report on the results of this assessment. In 2023 management has reviewed its internal controls over financial reporting based on criteria established in the Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and engaged an external advisor to help assess the effectiveness of those controls. As described in Section 404 of the U.S. Sarbanes-Oxley Act of 2002 and the rules implementing such act, we will include the management and the statutory auditor’s assessment of the effectiveness of internal control over financial reporting in our annual report on Form 20-F, which is expected to be filed with the SEC on or around the publication date of the present annual report.

Our programs in oncology and immunology Pioneering science to transform patient outcomes
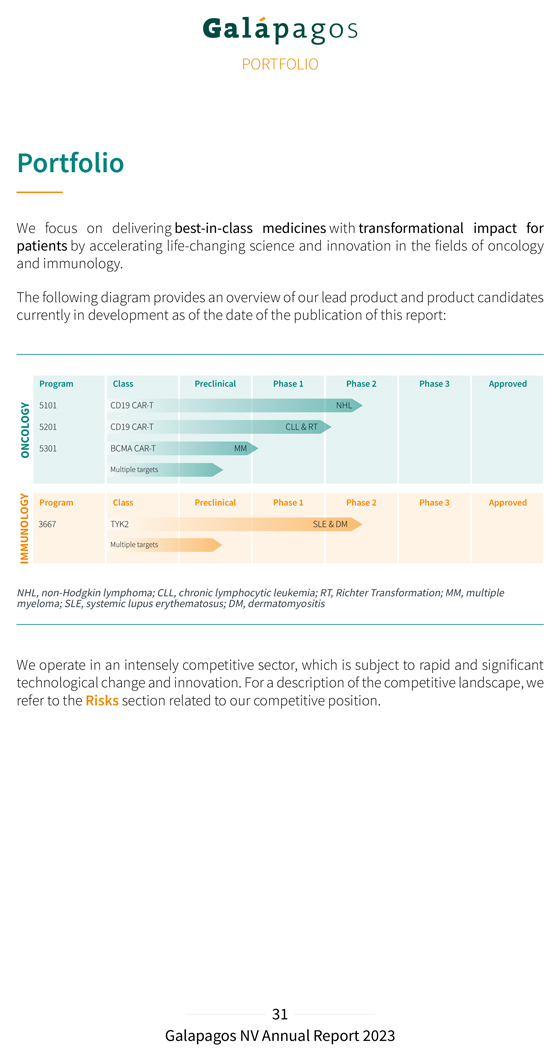
PORTFOLIO Portfolio We focus on delivering best-in-class medicines with transformational impact for patients by accelerating life-changing science and innovation in the fields of oncology and immunology. The following diagram provides an overview of our lead product and product candidates currently in development as of the date of the publication of this report: NHL, myeloma; non- Hodgkin SLE, systemic lymphoma; lupus erythematosus; CLL, chronic lymphocytic DM, dermatomyositis leukemia; RT, Richter Transformation; MM, multiple We operate in an intensely competitive sector, which is subject to rapid and significant technological change and innovation. For a description of the competitive landscape, we refer to the Risks section related to our competitive position.

Oncology Cancer leaves no one untouched, affecting many of us in one way or another. The urgency for effective, broadly accessible treatment options and novel therapies is paramount, as the outlook for patients is often grim, with survival measured in months rather than years. Advances in cancer research stands as our sole beacon of hope in addressing this disease and transforming patient outcomes. We passionately strive to turn cancers into manageable chronic conditions or even curable diseases. Our oncology researchers are determined to rise to the challenge to overcome the devastating impact of cancer by accelerating new ways to target cancer from different angles, whether through small molecules, antibody-based biological therapies, or novel chimeric antigen receptor (CAR-T) cell therapies, coupled with ingenious manufacturing technologies, and other revolutionary approaches. We believe in synergizing the most compelling science and technology from both within and outside our organization to introduce a new multi-faceted treatment paradigm for cancers with significant unmet medical needs. Our current clinical development is focused on hematological cancers for patients in need of additional and improved treatment options: non-Hodgkin’s lymphoma, chronic lymphocytic leukemia with or without Richter transformation, and multiple myeloma.

CAR care-T Pipeline manufactured at or near the point-of- GLPG5101: CD19 CAR-T in relapsed/refractory non-Hodgkin’s lymphoma Non-Hodgkin’s lymphoma (NHL) is a cancer originating from lymphocytes, a type of white blood cell which is part of the body’s immune system. NHL can occur at any age although it is more common in adults over 50 years old. Initial symptoms usually are enlarged lymph nodes, fever, and weight loss. There are many different types of NHL. These types can be divided into aggressive (fast-growing) and indolent (slow-growing) types, and they can be formed from either B lymphocytes (B cells) or in lesser extent from T lymphocytes (T cells) or Natural Killer cells (NK cells). B cell lymphoma makes up about 85% of NHL cases diagnosed in the US. Prognosis and treatment of NHL depend on the stage and type of disease. GLPG5101 is a second generation anti-CD19/4-1BB CAR-T product candidate, administered as a single fixed intravenous dose. The safety, efficacy and feasibility of point-of-care manufactured GLPG5101 are currently being evaluated in the ATALANTA-1 Phase 1/2, open-label, multicenter study in patients with relapsed/refractory non-Hodgkin lymphoma (rrNHL). The primary objective of the Phase 1 part of the study was to evaluate safety and to determine the recommended dose for the Phase 2 part of the study. Secondary objectives include assessment of efficacy and feasibility of near the point-of-care manufacturing of GLPG5101. The dose levels that were evaluated in Phase 1 are 50x106 (DL1), 110x106 (DL2) and 250x106 (DL3) CAR+ viable T cells. The primary objective of the Phase 2 part of the study is to evaluate the Objective Response Rate (ORR) while the secondary objectives include Complete Response Rate (CRR), duration of response, progression free survival, overall survival, safety, pharmacokinetic profile, and the feasibility of point-of-care manufacturing. Each enrolled patient will be followed for 24 months.

ATALANTA-1 Phase 1/2 study design of GLPG5101 in rrNHL ‘5101 basket trial in DLBCL, MCL, MZL, FL, BL & PCNSL Ph1—dose escalation (nâ‰^15) Ph2—dose expansion DL1 ‘5101 (50 x10^6 CAR T cells) (nâ‰^30 per indication) DL2 ‘5101 (110 x10^6 CAR T cells) ‘5101 RP2D dose DL3 ‘5101 (250 x10^6 CAR T cells) Key eligibility criteria r/r DLBCL, MCL, MZL, FL, BL & PCNSL population ≥ 2 prior lines of therapy, or primary refractory DLBCL or BL ≥ 1 prior line of therapy for PCNSL Not achieving CR to 2L therapy for BL and PCNSL Patient Incl. transplant ineligible No prior CD19-targeted therapy allowed BL, cell Burkitt lymphoma; lymphoma; MZL, marginal DL, dose zone level; lymphoma; DLBCL, diffuse PCNSL, large primary B-cell central lymphoma; nervous FL, follicular system lymphoma; lymphoma; rrNHL, MCL, mantle relapsed/ refractory conditioning non is- Hodgkin lymphodepleting lymphoma; chemotherapy RP2D, recommended . phase 2 dose. EudraCT 2021-003272-13. Patient
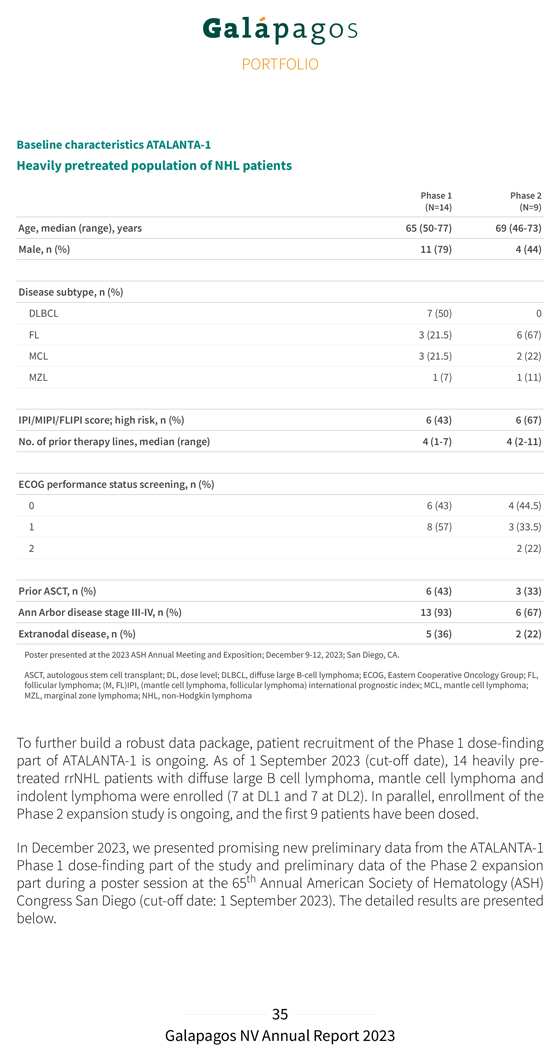
Baseline characteristics ATALANTA-1 Heavily pretreated population of NHL patients Phase 1 Phase 2 (N=14) (N=9) Age, median (range), years 65 (50-77) 69 (46-73) Male, n (%) 11 (79) 4 (44) Disease subtype, n (%) DLBCL 7 (50) 0 FL 3 (21.5) 6 (67) MCL 3 (21.5) 2 (22) MZL 1 (7) 1 (11) IPI/MIPI/FLIPI score; high risk, n (%) 6 (43) 6 (67) No. of prior therapy lines, median (range) 4 (1-7) 4 (2-11) ECOG performance status screening, n (%) 0 6 (43) 4 (44.5) 1 8 (57) 3 (33.5) 2 2 (22) Prior ASCT, n (%) 6 (43) 3 (33) Ann Arbor disease stage III-IV, n (%) 13 (93) 6 (67) Extranodal disease, n (%) 5 (36) 2 (22) Poster presented at the 2023 ASH Annual Meeting and Exposition; December 9-12, 2023; San Diego, CA. ASCT, autologous stem cell transplant; DL, dose level; DLBCL, diffuse large B-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; FL, follicular lymphoma; (M, FL)IPI, (mantle cell lymphoma, follicular lymphoma) international prognostic index; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; NHL, non-Hodgkin lymphoma To further build a robust data package, patient recruitment of the Phase 1 dose-finding part of ATALANTA-1 is ongoing. As of 1 September 2023 (cut-off date), 14 heavily pre-treated rrNHL patients with diffuse large B cell lymphoma, mantle cell lymphoma and indolent lymphoma were enrolled (7 at DL1 and 7 at DL2). In parallel, enrollment of the Phase 2 expansion study is ongoing, and the first 9 patients have been dosed. In December 2023, we presented promising new preliminary data from the ATALANTA-1 Phase 1 dose-finding part of the study and preliminary data of the Phase 2 expansion part during a poster session at the 65th Annual American Society of Hematology (ASH) Congress San Diego (cut-off date: 1 September 2023). The detailed results are presented below.
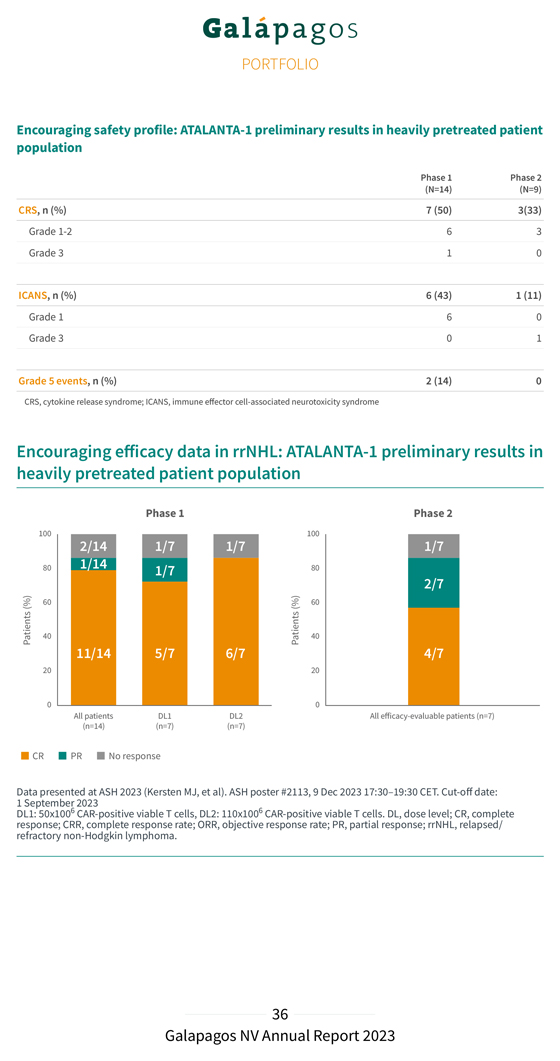
Encouraging safety profile: ATALANTA-1 preliminary results in heavily pretreated patient population Phase 1 Phase 2 (N=14) (N=9) CRS, n (%) 7 (50) 3(33) Grade 1-2 6 3 Grade 3 1 0 ICANS, n (%) 6 (43) 1 (11) Grade 1 6 0 Grade 3 0 1 Grade 5 events, n (%) 2 (14) 0 CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome Encouraging efficacy data in rrNHL: ATALANTA-1 preliminary results in heavily pretreated patient population Data 1 September presented 2023 at ASH 2023 (Kersten MJ, et al). ASH poster #2113, 9 Dec 2023 17:30–19:30 CET. Cut-off date: DL1: 50x1006 CAR-positive viable T cells, DL2: 110x1006 CAR-positive viable T cells. DL, dose level; CR, complete response; refractory CRR, non- Hodgkin complete lymphoma response .rate; ORR, objective response rate; PR, partial response; rrNHL, relapsed/

In the Phase 1 part of the study (cut-off date: 1 September 2023): GLPG5101 showed an encouraging safety profile. Most treatment emergent adverse events (TEAEs) were Grade 1 or 2 and the majority of the few Grade 3 events were hematological. No cytokine release syndrome (CRS) Grade > 3 and no immune effector cell-associated neurotoxicity syndrome (ICANS) Grade 2 were observed. 12 of 14 evaluable patients responded to treatment (ORR of 86%), with 11 of 14 patients achieving a Complete Response (CRR of 79%). 6 of 7 patients treated with the higher dose level (DL2) responded to treatment (ORR of 86%) and achieved a Complete Response (CRR of 86%). At the time of the analysis, 8 of 12 responding patients (67%) had an ongoing response, with a duration up to 15 months (median follow-up of 8.6 months); 2 of the 4 patients who progressed after an initial response had a CD19 positive relapse and 1 had confirmed CD19-negative disease. In the Phase 2 part of the study (cut-off date: 1 September 2023): GLPG5101 showed an encouraging safety profile with most TEAEs of Grade 1 or 2; the majority of Grade 3 events were hematological. No CRS Grade > 2 and ICANS was seen in one patient (Grade 3). 6 of 7 evaluable patients responded to treatment (ORR of 86%) and a Complete Response was observed in 4 of 7 patients (57%). At the time of the analysis, all 6 responding patients (100%) had an ongoing response with a median follow-up of 3.2 months. At ASH, we also showcased encouraging preliminary translational data with regard to the status of the CAR-T cells in the GLPG5101 final product (FP). A thorough characterization of the collected patient material in the ATALANTA-1 trial (19 patients) revealed an increased percentage of ‘early phenotype’ T cells (i.e. TN/SCM and T , CD4+ and CD8+) in the final product compared to the starting material (apheresed blood) CM . This was in line with the observed decrease in more differentiated, ‘late phenotype’ T cells (i.e. T , CD4+ and CD8+). EM/EFF This early phenotype reflects the differentiation status of the cells, which is associated with enhanced functionality and persistence of CAR-T cells after infusion in the patient.
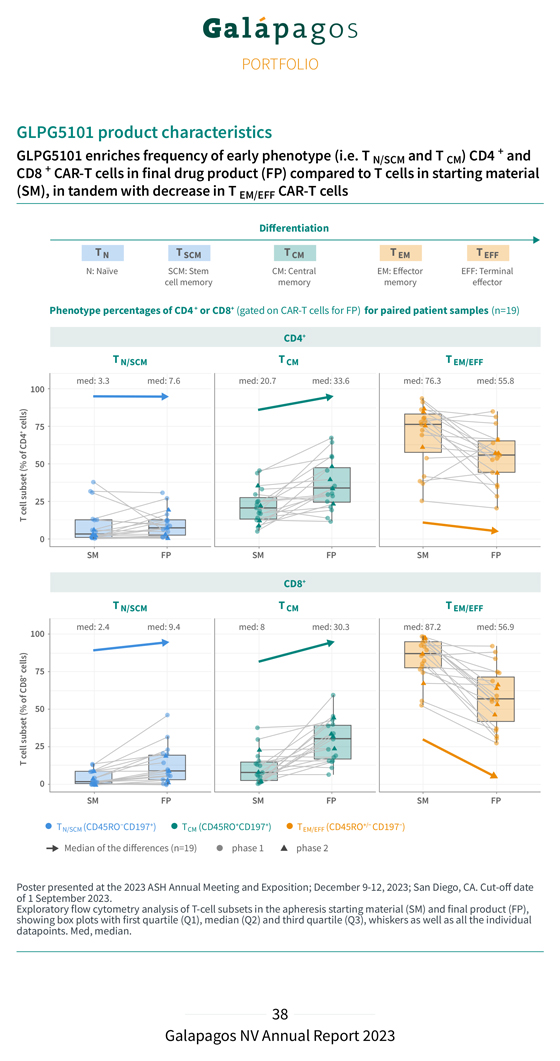
GLPG5101 product characteristics GLPG5101 enriches frequency of early phenotype (i.e. T and T ) CD4 + and N/SCM CM CD8 + CAR-T cells in final drug product (FP) compared to T cells in starting material (SM), in tandem with decrease in T EM/EFF CAR-T cells Differentiation T N T SCM T CM T EM T EFF N: Naïve SCM: Stem CM: Central EM: Effector EFF: Terminal cell memory memory memory effector Phenotype percentages of CD4+ or CD8+ (gated on CAR-T cells for FP) for paired patient samples (n=19) CD4+ CD8+ Poster of 1 September presented 2023 at the . 2023 ASH Annual Meeting and Exposition; December 9-12, 2023; San Diego, CA. Cut-off date showing Exploratory box flow plots cytometry with first analysis quartile of (Q1), T-cell median subsets (Q2) in and the third apheresis quartile starting (Q3), material whiskers (SM) as well and as final all the product individual (FP), datapoints. Med, median.
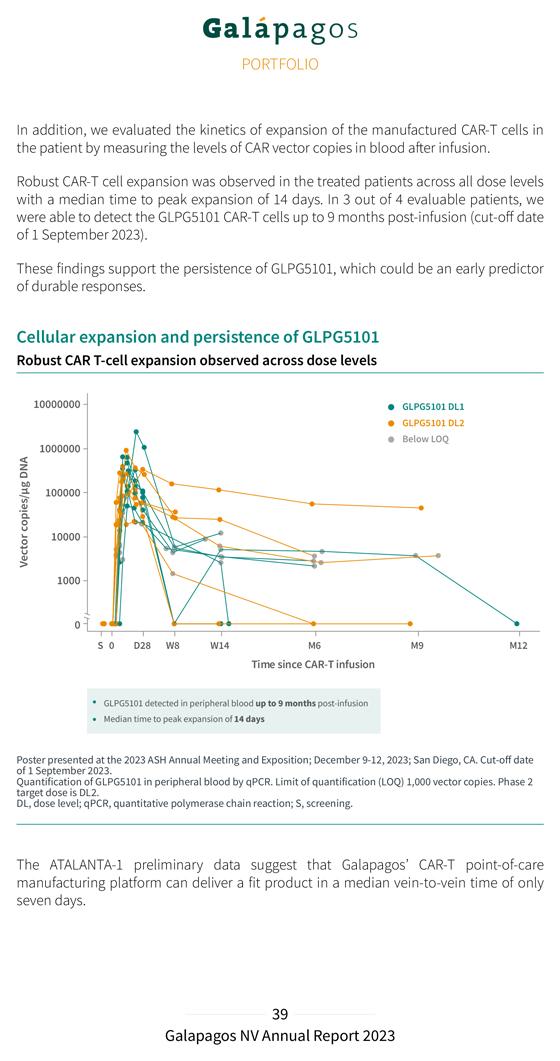
In addition, we evaluated the kinetics of expansion of the manufactured CAR-T cells in the patient by measuring the levels of CAR vector copies in blood after infusion. Robust CAR-T cell expansion was observed in the treated patients across all dose levels with a median time to peak expansion of 14 days. In 3 out of 4 evaluable patients, we were able to detect the GLPG5101 CAR-T cells up to 9 months post-infusion (cut-off date of 1 September 2023). These findings support the persistence of GLPG5101, which could be an early predictor of durable responses. Cellular expansion and persistence of GLPG5101 Robust CAR T-cell expansion observed across dose levels GLPG5101 detected in peripheral blood up to 9 months post-infusion Median time to peak expansion of 14 days Poster of 1 September presented 2023 at the . 2023 ASH Annual Meeting and Exposition; December 9-12, 2023; San Diego, CA. Cut-off date Quantification target dose is DL2 of GLPG5101 . in peripheral blood by qPCR. Limit of quantification (LOQ) 1,000 vector copies. Phase 2 DL, dose level; qPCR, quantitative polymerase chain reaction; S, screening. The ATALANTA-1 preliminary data suggest that Galapagos’ CAR-T point-of-care manufacturing platform can deliver a fit product in a median vein-to-vein time of only seven days.

GLPG5201: CD19 CAR-T in relapsed and refractory chronic lymphocytic leukemia Chronic lymphocytic leukemia (CLL) is one of the chronic lymphoproliferative disorders (lymphoid neoplasms). It is characterized by a progressive accumulation of functionally incompetent lymphocytes, which are usually monoclonal in origin. CLL affects B-cells in the blood and bone marrow.1 Richter Transformation (RT) is an uncommon clinicopathological condition observed in patients with CLL. It is characterized by the sudden transformation of the CLL into a significantly more aggressive form of large cell lymphoma and occurs in approximately 2-10% of all CLL patients. CLL usually follows an indolent course and is an incurable disease. Patients who develop relapsed and refractory disease and become resistant to new agents have a dismal prognosis and a high unmet medical need for new therapeutic options such as CAR-T cells. With estimated incidence of 4.7 new cases per 100,000 individuals, CLL is the most prevalent lymphoid malignancy and is the most common adult leukemia in the US and in Europe.2 The annual incidence of patients with RT has been estimated at 1,900 new patients in the US and 2,000 in the EU5.3 GLPG5201 is a second generation anti-CD19/4-1BB CAR-T product candidate, administered as a single fixed intravenous dose. The safety, efficacy and feasibility of point-of-care manufactured GLPG5201 are currently being evaluated in the EUPLAGIA-1 Phase 1/2, open-label, multicenter study in patients with rrCLL and rrSLL (small lymphocytic lymphoma), with or without RT. Patients with CD19 rrCLL or rrSLL with >2 lines of therapy are eligible to participate, and patients with RT are eligible, regardless of prior therapy. The primary objective of the Phase 1 part of the study is to evaluate safety and determine the recommended dose for the Phase 2 part of the study. The dose levels that are evaluated in the Phase 1 part of the study are 35x106 (DL1), 100x106 (DL2), and 300x106 (DL3) CAR+ viable T cells. The primary objective of the Phase 2 part of the study is to assess the ORR, and the secondary objectives include the analysis of the CRR, duration of response, progression free survival, overall survival, safety pharmacokinetic profile, and feasibility of point-of-care manufacturing. 1 Wierda WG. Chronic lymphocytic leukemia/ Small lymphocytic lymphoma fact sheet. In: Foundation LR, editor. 2018: 2 https://www.lymphoma.org/wp-content/uploads/2018/04/LRF_FACTSHEET_CLL_SLL.pdf Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: A Cancer Journal for Clinicians. 2021;71(1):7-33. 3 https://www.ncbi.nlm.nih.gov/books/NBK493173 IMARC report, 2023; 2-15% of incidence per Lightning Health literature review; Sigmund AM et al. 2022; Thompson PhA et al. 2022.IMARC report, 2023; 2-15% of incidence per Lightning Health literature review; Sigmund AM et al. 2022; Thompson PhA et al. 2022.
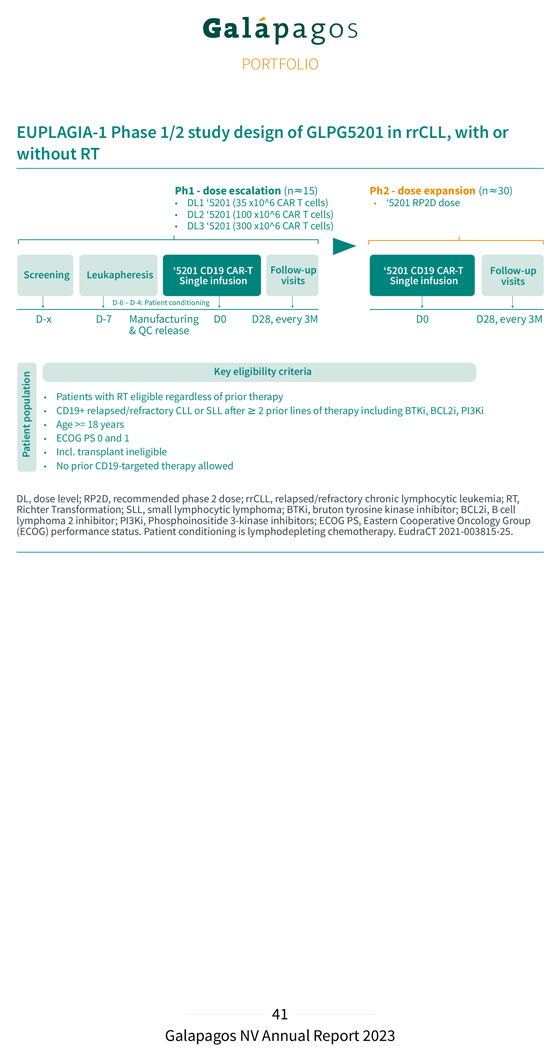
EUPLAGIA-1 Phase 1/2 study design of GLPG5201 in rrCLL, with or without RT Key eligibility criteria Patients with RT eligible regardless of prior therapy population CD19+ relapsed/refractory CLL or SLL after ≥ 2 prior lines of therapy including BTKi, BCL2i, PI3Ki Age >= 18 years ECOG PS 0 and 1 Patient Incl. transplant ineligible No prior CD19-targeted therapy allowed DL, Richter dose Transformation; level; RP2D, recommended SLL, small lymphocytic phase 2 dose; lymphoma; rrCLL, relapsed/refractory BTKi, bruton tyrosine chronic kinase lymphocytic inhibitor; leukemia; BCL2i, B cell RT, lymphoma (ECOG) performance 2 inhibitor; status PI3Ki, . Patient Phosphoinositide conditioning 3- is kinase lymphodepleting inhibitors; ECOG chemotherapy PS, Eastern . EudraCT Cooperative 2021 Oncology -003815-25 Group .
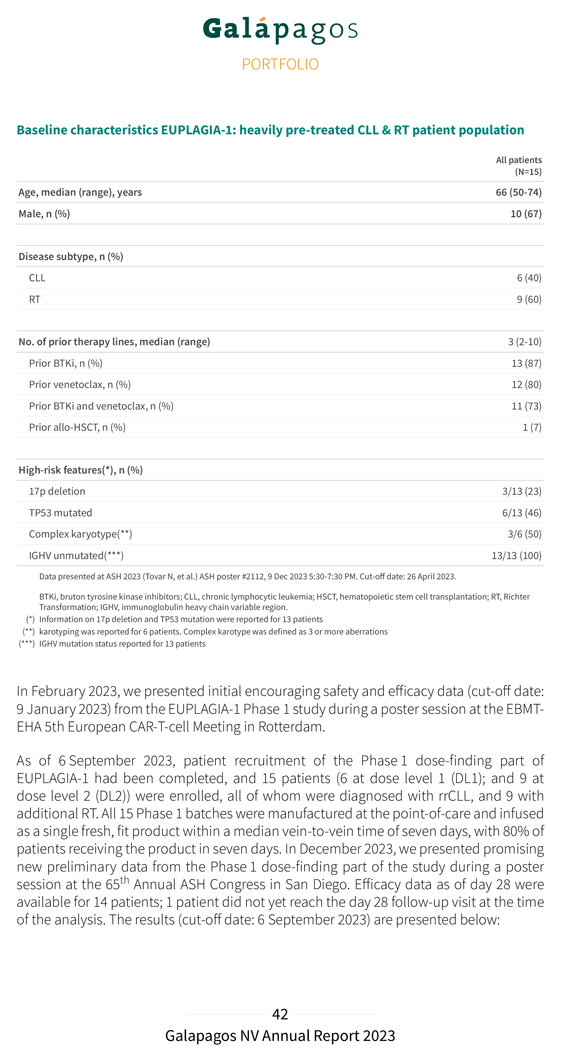
Baseline characteristics EUPLAGIA-1: heavily pre-treated CLL & RT patient population All patients (N=15) Age, median (range), years 66 (50-74) Male, n (%) 10 (67) Disease subtype, n (%) CLL 6 (40) RT 9 (60) No. of prior therapy lines, median (range) 3 (2-10) Prior BTKi, n (%) 13 (87) Prior venetoclax, n (%) 12 (80) Prior BTKi and venetoclax, n (%) 11 (73) Prior allo-HSCT, n (%) 1 (7) High-risk features(*), n (%) 17p deletion 3/13 (23) TP53 mutated 6/13 (46) Complex karyotype(**) 3/6 (50) IGHV unmutated(***) 13/13 (100) Data presented at ASH 2023 (Tovar N, et al.) ASH poster #2112, 9 Dec 2023 5:30-7:30 PM. Cut-off date: 26 April 2023. BTKi, bruton tyrosine kinase inhibitors; CLL, chronic lymphocytic leukemia; HSCT, hematopoietic stem cell transplantation; RT, Richter Transformation; IGHV, immunoglobulin heavy chain variable region. (*) Information on 17p deletion and TP53 mutation were reported for 13 patients (**) karotyping was reported for 6 patients. Complex karotype was defined as 3 or more aberrations (***) IGHV mutation status reported for 13 patients In February 2023, we presented initial encouraging safety and efficacy data (cut-off date: 9 January 2023) from the EUPLAGIA-1 Phase 1 study during a poster session at the EBMT-EHA 5th European CAR-T-cell Meeting in Rotterdam. As of 6 September 2023, patient recruitment of the Phase 1 dose-finding part of EUPLAGIA-1 had been completed, and 15 patients (6 at dose level 1 (DL1); and 9 at dose level 2 (DL2)) were enrolled, all of whom were diagnosed with rrCLL, and 9 with additional RT. All 15 Phase 1 batches were manufactured at the point-of-care and infused as a single fresh, fit product within a median vein-to-vein time of seven days, with 80% of patients receiving the product in seven days. In December 2023, we presented promising new preliminary data from the Phase 1 dose-finding part of the study during a poster session at the 65th Annual ASH Congress in San Diego. Efficacy data as of day 28 were available for 14 patients; 1 patient did not yet reach the day 28 follow-up visit at the time of the analysis. The results (cut-off date: 6 September 2023) are presented below:
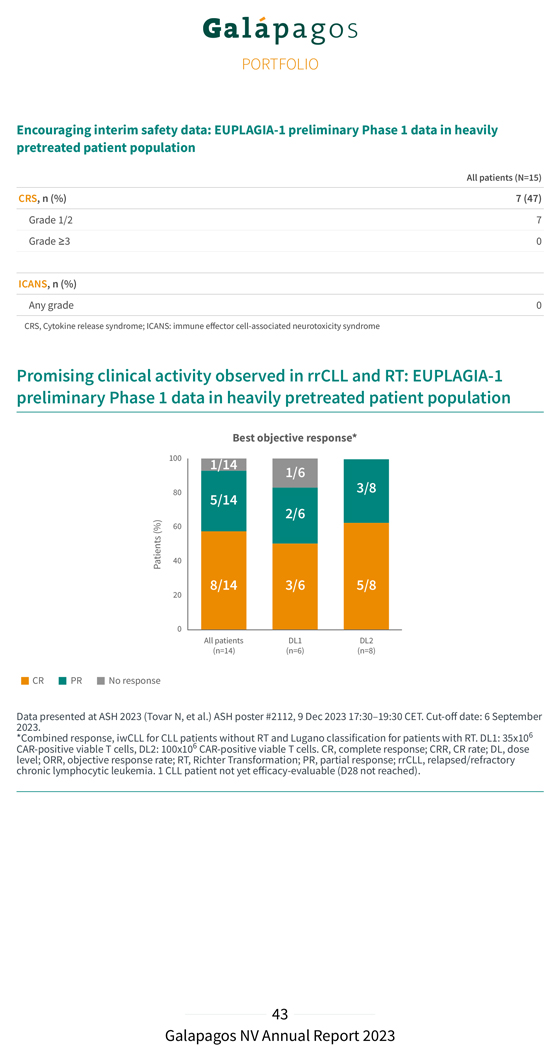
Encouraging interim safety data: EUPLAGIA-1 preliminary Phase 1 data in heavily pretreated patient population All patients (N=15) CRS, n (%) 7 (47) Grade 1/2 7 Grade ≥3 0 ICANS, n (%) Any grade 0 CRS, Cytokine release syndrome; ICANS: immune effector cell-associated neurotoxicity syndrome Promising clinical activity observed in rrCLL and RT: EUPLAGIA-1 preliminary Phase 1 data in heavily pretreated patient population Best objective response* Data 2023.presented at ASH 2023 (Tovar N, et al.) ASH poster #2112, 9 Dec 2023 17:30–19:30 CET. Cut-off date: 6 September *Combined response, iwCLL for CLL patients without RT and Lugano classification for patients with RT. DL1: 35x106 CAR-positive viable T cells, DL2: 100x106 CAR-positive viable T cells. CR, complete response; CRR, CR rate; DL, dose chronic level; ORR, lymphocytic objective leukemia response .rate; 1 CLL RT, patient Richter not Transformation; yet efficacy-evaluable PR, partial (D28 response; not reached) rrCLL, . relapsed/refractory
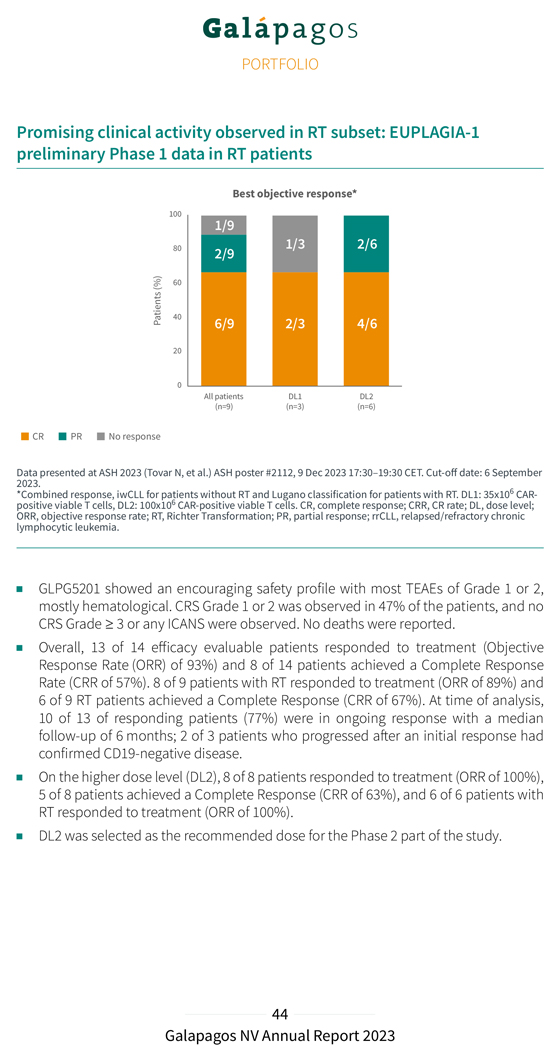
Promising clinical activity observed in RT subset: EUPLAGIA-1 preliminary Phase 1 data in RT patients Best objective response* CR PR No response Data 2023 .presented at ASH 2023 (Tovar N, et al.) ASH poster #2112, 9 Dec 2023 17:30–19:30 CET. Cut-off date: 6 September *Combined response, iwCLL for patients without RT and Lugano classification for patients with RT. DL1: 35x106 CAR-positive viable T cells, DL2: 100x106 CAR-positive viable T cells. CR, complete response; CRR, CR rate; DL, dose level; ORR, lymphocytic objective leukemia response . rate; RT, Richter Transformation; PR, partial response; rrCLL, relapsed/refractory chronic GLPG5201 showed an encouraging safety profile with most TEAEs of Grade 1 or 2, mostly hematological. CRS Grade 1 or 2 was observed in 47% of the patients, and no CRS Grade ≥ 3 or any ICANS were observed. No deaths were reported. Overall, 13 of 14 efficacy evaluable patients responded to treatment (Objective Response Rate (ORR) of 93%) and 8 of 14 patients achieved a Complete Response Rate (CRR of 57%). 8 of 9 patients with RT responded to treatment (ORR of 89%) and 6 of 9 RT patients achieved a Complete Response (CRR of 67%). At time of analysis, 10 of 13 of responding patients (77%) were in ongoing response with a median follow-up of 6 months; 2 of 3 patients who progressed after an initial response had confirmed CD19-negative disease. On the higher dose level (DL2), 8 of 8 patients responded to treatment (ORR of 100%), 5 of 8 patients achieved a Complete Response (CRR of 63%), and 6 of 6 patients with RT responded to treatment (ORR of 100%). DL2 was selected as the recommended dose for the Phase 2 part of the study.

At the annual EBMT-EHA congress in February 2024, we showcased encouraging preliminary translational data regarding the status of the CAR-T cells in the GLPG5201 final product (FP). A thorough characterization of the collected patient material in the EUPLAGIA-1 trial (10 patients) revealed an increased percentage of ‘early phenotype’ T cells (i.e. TN/SCM and T , CD4+ and CD8+) in the final product compared to the starting material (apheresed blood) CM . This was in line with the observed decrease in more differentiated, ‘late phenotype’ T cells (i.e. T , CD4+ and CD8+). EM/EFF This early phenotype reflects the differentiation status of the cells, which is associated with enhanced functionality and persistence of CAR-T cells after infusion in the patient.
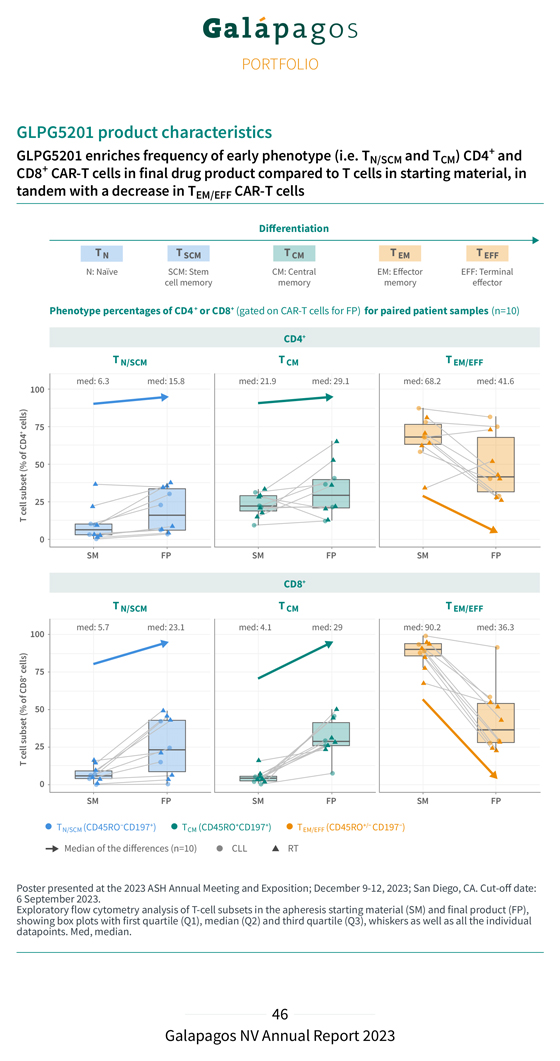
GLPG5201 product characteristics GLPG5201 enriches frequency of early phenotype (i.e. T and T ) CD4+ and N/SCM CM CD8+ CAR-T cells in final drug product compared to T cells in starting material, in tandem with a decrease in TEM/EFF CAR-T cells Differentiation T N T SCM T CM T EM T EFF N: Naïve SCM: Stem CM: Central EM: Effector EFF: Terminal cell memory memory memory effector Phenotype percentages of CD4+ or CD8+ (gated on CAR-T cells for FP) for paired patient samples (n=10) CD4+ CD8+ TN/SCM (CD45RO-CD197+) TCM (CD45RO+CD197+) TEM/EFF (CD45RO+/- CD197-) Median of the differences (n=10) CLL RT Poster 6 September presented 2023 at . the 2023 ASH Annual Meeting and Exposition; December 9-12, 2023; San Diego, CA. Cut-off date: showing Exploratory box flow plots cytometry with first analysis quartile of (Q1), T-cell median subsets (Q2) in and the third apheresis quartile starting (Q3), material whiskers (SM) as well and as final all the product individual (FP), datapoints. Med, median.
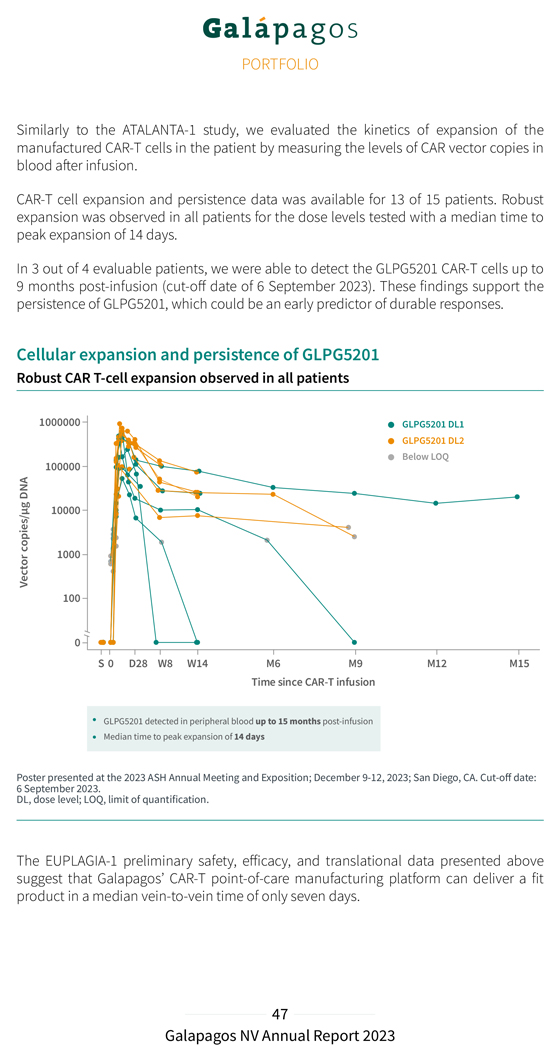
Similarly to the ATALANTA-1 study, we evaluated the kinetics of expansion of the manufactured CAR-T cells in the patient by measuring the levels of CAR vector copies in blood after infusion. CAR-T cell expansion and persistence data was available for 13 of 15 patients. Robust expansion was observed in all patients for the dose levels tested with a median time to peak expansion of 14 days. In 3 out of 4 evaluable patients, we were able to detect the GLPG5201 CAR-T cells up to 9 months post-infusion (cut-off date of 6 September 2023). These findings support the persistence of GLPG5201, which could be an early predictor of durable responses. Cellular expansion and persistence of GLPG5201 Robust CAR T-cell expansion observed in all patients Time since CAR-T infusion GLPG5201 detected in peripheral blood up to 15 months post-infusion Median time to peak expansion of 14 days Poster 6 September presented 2023 at . the 2023 ASH Annual Meeting and Exposition; December 9-12, 2023; San Diego, CA. Cut-off date: DL, dose level; LOQ, limit of quantification. The EUPLAGIA-1 preliminary safety, efficacy, and translational data presented above suggest that Galapagos’ CAR-T point-of-care manufacturing platform can deliver a fit product in a median vein-to-vein time of only seven days.

GLPG5301: BCMA CAR-T in relapsed and refractory multiple myeloma Multiple myeloma (MM) is typically characterized by the neoplastic proliferation of plasma cells producing a monoclonal immunoglobulin. The plasma cells proliferate in the bone marrow and may result in extensive skeletal destruction with osteopenia, and osteolytic lesions with or without pathologic fractures. Diagnosis is made when one (or more) of the following clinical presentations are present: bone pain with lytic lesions discovered on routine skeletal films or other imaging modalities, an increased total serum protein concentration with the presence of a monoclonal protein in the urine or serum, and anemia, hypercalcemia or renal failure. The patient may be either symptomatic or their disease may be discovered incidentally. Despite improvements in treatment, in general, patients with MM ultimately relapse or become refractory to available regiments. Triple-refractory (refractory to CD38 monoclonal antibodies [mAbs], proteasome inhibitor [PI] and immunomodulatory imide drug [IMiD] or penta-refractory (refractory to CD38 mAbs, 2 Pls and 2 IMiDs) patients have a poor prognosis and are in urgent need of novel treatment options. GLPG5301 is an autologous, second-generation/4-1BB B-cell maturation antigen (BCMA)-directed CAR-T product candidate, administered as an intravenous infusion of a fresh product in a single fixed dose, at the point-of-care. In December 2023, we announced that the first patient with rrMM was dosed in the Phase 1/2 PAPILIO-1 study. PAPILIO-1 is a Phase 1/2, open-label, multicenter study to evaluate the safety, efficacy and feasibility of point-of-care manufactured GLPG5301, a BCMA CAR-T product candidate, in patients with relapsed/refractory multiple myeloma (rrMM) after ≥2 prior lines therapy. The primary objective of the Phase 1 part of the PAPILIO-1 study is to evaluate safety and determine the recommended dose for the Phase 2 part of the study. The primary objective of the Phase 2 part of the study is to evaluate the efficacy of GLPG5301, as measured by the ORR. Secondary objectives for both Phase 1 and Phase 2 include further assessment of the safety of GLPG5301, additional efficacy endpoints, including assessment of Minimal Residual Disease (MRD), as well as the feasibility of point-of-care manufacture of GLPG5301 in rrMM patients. Each enrolled patient will be followed for 24 months. During Phase 1, up to 3 dose levels will be evaluated and at least 12 patients will be enrolled to establish the recommended Phase 2 dose. Approximately 30 additional patients will be enrolled in the Phase 2 part of the study to evaluate the safety and efficacy of GLPG5301.

PAPILIO-1 Phase 1/2 study design of GLPG5301 in rrMM Study population r/r Multiple Myeloma or Plasma cell leukemia ≥ 2 prior lines of therapy (at least IMiD, PI and anti-CD38) No prior BCMA-targeted therapy allowed BCMA, rMM, relapsed/refractory B-cell maturation antigen; multiple DL, myeloma; dose level; RP2D, IMiD, recommended immunomodulatory phase 2 dose imide . drug; PI, proteasome inhibitor; r/
Immunology By exploring new frontiers in science and technology, we strive to accelerate innovation of transformational medicines that deliver more years of life and quality of life for patients and families living with immune-mediated conditions. We recognize the complexity of developing therapies for immunological diseases, and that is why we work hand in hand with patients, patient organizations, scientists, healthcare professionals, research institutions, academia, and other partners to drive innovation. Our collaborative approach allows us to drive innovation and accelerate progress toward life-changing treatments. Our determination to bring hope to patients inspires us to develop targeted treatments that make a difference to their lives. Small molecules pipeline Jyseleca® franchise On 31 January 2024, we announced the successful completion of the transaction to transfer our Jyseleca® (filgotinib) business to Alfasigma S.p.A. (Alfasigma). The transaction includes the transfer of the entire Jyseleca® business to Alfasigma, including the European and UK Marketing Authorizations, and the commercial, medical affairs and development activities for Jyseleca®. In connection with the completion of the transaction, approximately 400 Galapagos positions in 14 European countries transferred to Alfasigma to support business continuity and ongoing patient access. Jyseleca® (filgotinib) in rheumatoid arthritis (RA) RA is a chronic autoimmune disease that affects more than three million patients in the United States and Europe. RA is characterized by inflammation and degeneration of the joints. Patients suffer from pain, stiffness, and restricted mobility due to a persistent inflammation of multiple joints, ultimately resulting in irreversible damage of the joint cartilage and bone. The current market for RA treatments in the five major European markets (EU5) is approximately €3.3 billion. Despite progress in the treatment of RA, there remains a considerable unmet need as sustained remission remains rare.4 4 Chen Y, et al. Clin Rheumatol. 2019 Mar;38(3):727-738. doi: 10.1007/s10067-018-4340-7. Epub 2018 Oct 19.

Regulatory progress of Jyseleca® in RA In 2020, Jyseleca® (filgotinib 200mg and 100mg) obtained regulatory approval in Europe, Great-Britain, and Japan for the treatment of adult patients with moderate to severe active RA. The European Summary of Product Characteristics for filgotinib, which includes contraindications and special warnings and precautions, is available at www.ema.europa.eu. The Great Britain Summary of Product Characteristics for filgotinib can be found at www.medicines.org.uk/emc and the Northern Ireland Summary of Product Characteristics for filgotinib can be found at www.emcmedicines.com/en-GB/northernireland, respectively. The interview form from the Japanese Ministry of Health, Labour and Welfare is available at www.info.pmda.go.jp. Also in 2020, Gilead Sciences, Inc (Gilead) received a Complete Response Letter (CRL) from the U.S. Food and Drug Administration (FDA) for the New Drug Application (NDA) for filgotinib. Consequently, Gilead decided not to advance with resubmission for approval of filgotinib as a treatment for RA in the U.S. In 2022, the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) concluded its Article 20 safety review of all JAK inhibitors approved in the EU for the treatment of inflammatory diseases and recommended the harmonization of all labels. PRAC concluded that JAK inhibitors should maintain their indication for the treatment of patients with RA who have responded inadequately to or who cannot tolerate disease modifying anti-rheumatic drugs (DMARDs) therapy, and for patients with UC who have responded inadequately to or who cannot tolerate conventional therapy or biologics. PRAC also recommended all JAK inhibitor product labels be updated to include a precautionary approach for use of JAK inhibitors in patients with identified risk factors only if no suitable treatment alternative is available. (Section 4.4 of the product label – Warning and Precautions). On 11 November 2022, the Committee for Medicinal Products for Human Use (CHMP), the scientific committee of the EMA, adopted PRAC’s recommendation and on 10 March 2023, this decision was approved by the European Commission. Commercialization of Jyseleca® in RA In 2021, we took full ownership of the manufacturing and commercialization of Jyseleca® in Europe and became the Marketing Authorization Holder (MAH) in 27 countries in Europe. Gilead is responsible for the commercialization and distribution of Jyseleca® outside of Europe, including in Japan where Jyseleca® is approved in RA and is co-marketed with Eisai. In Central and Eastern Europe, Portugal, Greece and the Baltic countries, Swedish Orphan Biovitrum AB (Sobi) is responsible for the distribution and commercialization of Jyseleca®.

Jyseleca® reimbursement in RA in Europe Jyseleca® in RA is currently reimbursed in Western Europe. Sobi secured reimbursement for Jyseleca® in 2023 in Poland, Slovenia, Slovakia, Estonia, Croatia, and Greece for RA. See further details regarding the revised Gilead collaboration agreement for filgotinib in our Notes to the consolidated financial statements. Safety and efficacy in the filgotinib RA development program Filgotinib showed favorable results in terms of onset of action, efficacy, safety, and tolerability from the FINCH Phase 3 and DARWIN Phase 2 clinical programs. As part of the filgotinib development program, we initiated FINCH 4 in RA. The FINCH 4 study is a multi-center, open-label, long-term extension study to assess the safety and efficacy of filgotinib in patients with RA, which enrolled subjects who completed either the FINCH 1, FINCH 2, or FINCH 3 studies. We and Gilead published integrated safety data from 7 RA studies in Annals of the Rheumatic Diseases (Winthrop et al. 2021). Data were integrated from 3 Phase 3 studies (FINCH 1 – 3), 2 Phase 2 studies (DARWIN 1, 2), and 2 long-term extension studies (DARWIN 3, FINCH 4) including up to 5.6 years of filgotinib exposure, and over a median of 1.6 years. In this pooled analysis, filgotinib was well-tolerated, and no new safety concerns were identified. Adverse events of MACE and deep venous thrombosis (DVT)/ pulmonary embolism (PE) were rare and occurred in similar numbers among all treatment groups, and with a similar incidence rate across all dose groups. The data underscore the acceptable safety and tolerability profile of filgotinib as monotherapy and in conjunction with methotrexate (MTX)/csDMARDs5 in RA. In 2023, we presented new analyses from randomized controlled trials (RCTs) and real-world evidence (RWE) studies at the European League Against Rheumatism (EULAR) congress. These included long-term efficacy and integrated safety data, post hoc analysis identifying distinct trajectories of treatment responses in patients with RA receiving filgotinib, long-term clinical profile of filgotinib in patients with RA by cardiovascular (CV) risk factors, and the added value of filgotinib on pain relief in patients with RA achieving remission in the Phase 3 FINCH 1, 2 and 3 studies. Furthermore, we published interim results from 500 patients on baseline characteristics as well as effectiveness and safety outcomes from the FILOSOPHY real-world evidence study. 5 Conventional synthetic DMARDs

Jyseleca® (filgotinib) in ulcerative colitis (UC) UC is an inflammatory bowel disease (IBD) resulting in ulcerations and inflammation of the inner layer of the colon and rectum. Regulatory progress and commercialization of Jyseleca® in UC Filgotinib obtained regulatory approval for the treatment of adults with moderate to severe UC in the European Union in 2021, and in Great Britain and Japan in January and March 2022, respectively. Filgotinib is marketed as Jyseleca® in Europe and Japan for the treatment of adult patients with moderate to severe active UC who have had an inadequate response with, lost response to, or were intolerant to either conventional therapy or a biologic agent. Jyseleca (filgotinib) 100mg and 200mg are registered in the above-mentioned territories. The European Summary of Product Characteristics for filgotinib, which includes contraindications and special warnings and precautions, is available at www.ema.europa.eu. The Great Britain Summary of Product Characteristics for filgotinib can be found at www.medicines.org.uk/emc and the Northern Ireland Summary of Product Characteristics for filgotinib can be found at www.emcmedicines.com/en-GB/northernireland, respectively. The interview from the Japanese Ministry of Health, Labour and Welfare is available at www.info.pmda.go.jp. Gilead is responsible for the distribution and commercialization of Jyseleca® outside of Europe, including in Japan where Jyseleca® is approved in UC and is co-marketed with Eisai. In Central and Eastern Europe, Portugal, Greece and the Baltic countries, Swedish Orphan Biovitrum AB (Sobi) is responsible for the distribution and commercialization of Jyseleca®. Jyseleca® reimbursements in UC Jyseleca ® in UC is currently reimbursed in Western Europe. Sobi secured reimbursement for Jyseleca® in 2023 in Poland, Portugal, Czech Republic, Slovakia, Estonia, and Slovenia in UC. Safety and efficacy in the filgotinib UC development program The SELECTION Phase 3 study is a multi-center, randomized, double-blind, placebo-controlled study to assess the safety and efficacy of the preferential JAK1 inhibitor filgotinib in adult patients with moderately to severely active UC. The SELECTION study comprises two induction trials and a maintenance trial. The Induction Study A enrolled biologic-naïve patients, and the Induction Study B enrolled biologic-experienced patients. The primary objectives of SELECTION were to evaluate the efficacy of filgotinib compared with placebo in establishing clinical remission as determined by the Mayo

endoscopic subscore of 0 or 1, rectal bleeding sub-score of 0, and 1-point decrease in stool frequency from baseline to achieve a sub-score of 0 or 1 at Week 10 in the induction studies and Week 58 in the maintenance study. Eligible patients who were enrolled in the SELECTION study were enrolled in the ongoing SELECTION long-term extension trial to evaluate the long-term safety of filgotinib in patients with UC. A majority of patients included in the SELECTION study (n=1348) had a Mayo Clinic Score (MCS) of 9 or higher at baseline, and 43% of biologic experienced patients (n=297/689) had insufficient response to a TNF antagonist and vedoluzimab as well. (Feagan et al., Lancet 2021; 397: 2372–84) In 2023, we presented additional new analyses from the SELECTION program with filgotinib at the annual ECCO congress. These include new analysis from the long-term extension (LTE) study evaluating the safety and efficacy of filgotinib in UC for nearly four years, an analysis of the prolonged benefit of filgotinib in UC, an analysis exploring factors associated with the partial Mayo Clinic Score (pMCS) over time, and an analysis of the effect of filgotinib on anaemia in UC patients. Additionally, we presented pooled data from five Phase 2/3 trials, and two long-term extension trials of filgotinib designed to further understand the safety profile of filgotinib in UC and RA. Data from the SELECTION LTE study showed that filgotinib 200mg maintained symptomatic remission and health-related quality of life (HRQoL) for up to approximately four years. Amongst subjects who completed the study, the reduction in mean pMCS in SELECTION was maintained up to LTE Week 144. In non-responders, mean pMCS decreased from LTE baseline to Week 192. The results also showed that a high proportion of completers (>80% of patients) and non-responders (>70% of patients) achieved remission according to the Inflammatory Bowel Disease Questionnaire. The safety profile of filgotinib 200mg in the SELECTIONLTE study was generally consistent with the safety profile observed in previous SELECTION studies, with no new safety signals observed. Filgotinib in Crohn’s disease (CD) CD is an IBD of unknown cause, which results in chronic inflammation of the gastrointestinal (GI) tract with a relapsing and remitting course. On 8 February 2023, we announced that both induction cohorts of the Phase 3 DIVERSITY study trial of filgotinib in CD failed to meet the co-primary endpoints of clinical remission and endoscopic response for filgotinib, 100mg and 200mg once-daily. Based on these topline data, we decided not to submit a Marketing Authorization Application in Europe for filgotinib in CD.
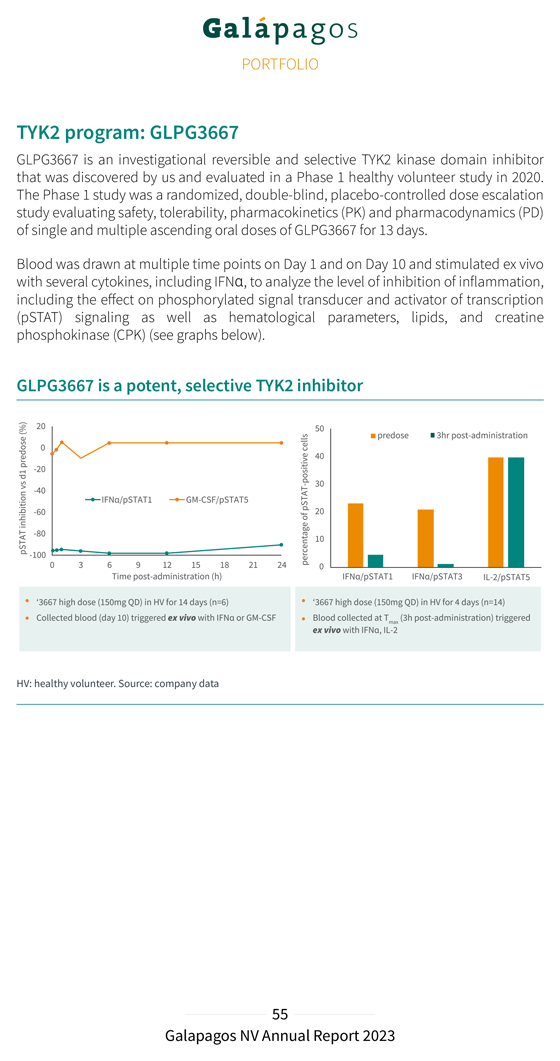
TYK2 program: GLPG3667 GLPG3667 is an investigational reversible and selective TYK2 kinase domain inhibitor that was discovered by us and evaluated in a Phase 1 healthy volunteer study in 2020. The Phase 1 study was a randomized, double-blind, placebo-controlled dose escalation study evaluating safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of single and multiple ascending oral doses of GLPG3667 for 13 days. Blood was drawn at multiple time points on Day 1 and on Day 10 and stimulated ex vivo with several cytokines, including IFNα, to analyze the level of inhibition of inflammation, including the effect on phosphorylated signal transducer and activator of transcription (pSTAT) signaling as well as hematological parameters, lipids, and creatine phosphokinase (CPK) (see graphs below). GLPG3667 is a potent, selective TYK2 inhibitor HV: healthy volunteer. Source: company data
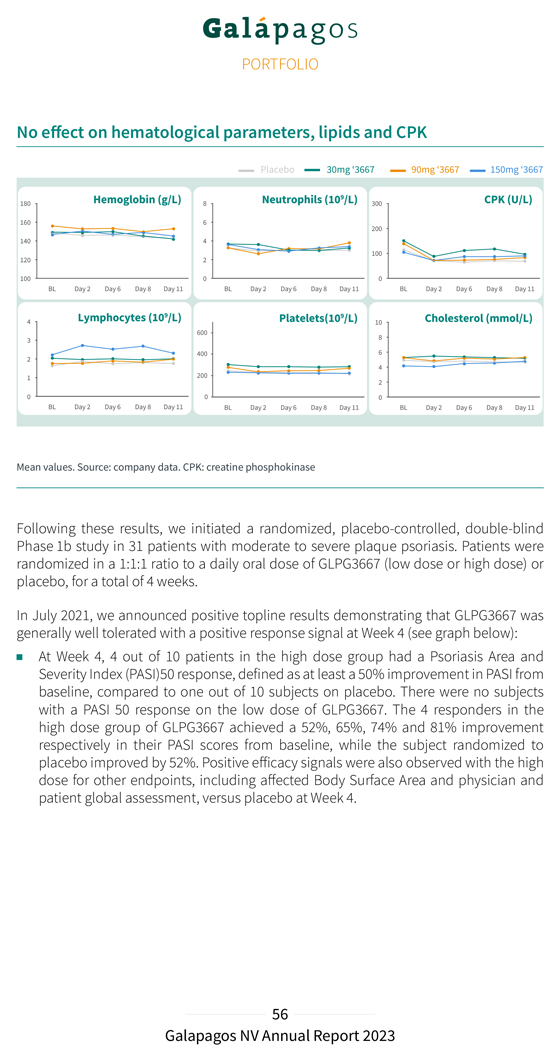
No effect on hematological parameters, lipids and CPK Mean values. Source: company data. CPK: creatine phosphokinase Following these results, we initiated a randomized, placebo-controlled, double-blind Phase 1b study in 31 patients with moderate to severe plaque psoriasis. Patients were randomized in a 1:1:1 ratio to a daily oral dose of GLPG3667 (low dose or high dose) or placebo, for a total of 4 weeks. In July 2021, we announced positive topline results demonstrating that GLPG3667 was generally well tolerated with a positive response signal at Week 4 (see graph below): At Week 4, 4 out of 10 patients in the high dose group had a Psoriasis Area and Severity Index (PASI)50 response, defined as at least a 50% improvement in PASI from baseline, compared to one out of 10 subjects on placebo. There were no subjects with a PASI 50 response on the low dose of GLPG3667. The 4 responders in the high dose group of GLPG3667 achieved a 52%, 65%, 74% and 81% improvement respectively in their PASI scores from baseline, while the subject randomized to placebo improved by 52%. Positive efficacy signals were also observed with the high dose for other endpoints, including affected Body Surface Area and physician and patient global assessment, versus placebo at Week 4.
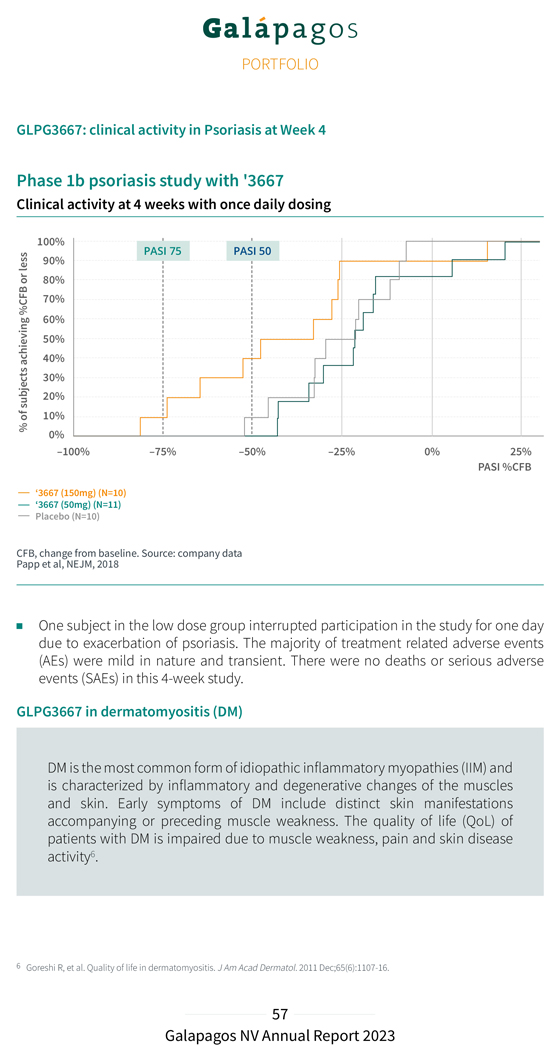
GLPG3667: clinical activity in Psoriasis at Week 4 Phase 1b psoriasis study with ‘3667 Clinical activity at 4 weeks with once daily dosing ‘3667 (150mg) (N=10) ‘3667 (50mg) (N=11) Placebo (N=10) CFB, Papp change et al, NEJM, from 2018 baseline. Source: company data One subject in the low dose group interrupted participation in the study for one day due to exacerbation of psoriasis. The majority of treatment related adverse events (AEs) were mild in nature and transient. There were no deaths or serious adverse events (SAEs) in this 4-week study. GLPG3667 in dermatomyositis (DM) DM is the most common form of idiopathic inflammatory myopathies (IIM) and is characterized by inflammatory and degenerative changes of the muscles and skin. Early symptoms of DM include distinct skin manifestations accompanying or preceding muscle weakness. The quality of life (QoL) of patients with DM is impaired due to muscle weakness, pain and skin disease activity6. 6 Goreshi R, et al. Quality of life in dermatomyositis. J Am Acad Dermatol. 2011 Dec;65(6):1107-16.
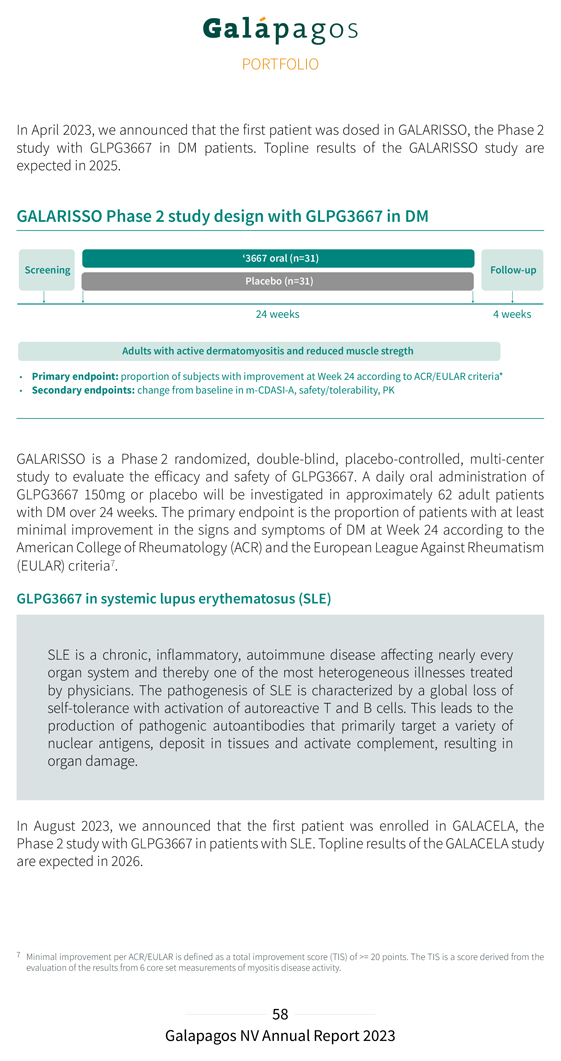
In April 2023, we announced that the first patient was dosed in GALARISSO, the Phase 2 study with GLPG3667 in DM patients. Topline results of the GALARISSO study are expected in 2025. GALARISSO Phase 2 study design with GLPG3667 in DM Adults with active dermatomyositis and reduced muscle stregth Primary endpoint: proportion of subjects with improvement at Week 24 according to ACR/EULAR criteria* Secondary endpoints: change from baseline in m-CDASI-A, safety/tolerability, PK GALARISSO is a Phase 2 randomized, double-blind, placebo-controlled, multi-center study to evaluate the efficacy and safety of GLPG3667. A daily oral administration of GLPG3667 150mg or placebo will be investigated in approximately 62 adult patients with DM over 24 weeks. The primary endpoint is the proportion of patients with at least minimal improvement in the signs and symptoms of DM at Week 24 according to the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) criteria7. GLPG3667 in systemic lupus erythematosus (SLE) SLE is a chronic, inflammatory, autoimmune disease affecting nearly every organ system and thereby one of the most heterogeneous illnesses treated by physicians. The pathogenesis of SLE is characterized by a global loss of self-tolerance with activation of autoreactive T and B cells. This leads to the production of pathogenic autoantibodies that primarily target a variety of nuclear antigens, deposit in tissues and activate complement, resulting in organ damage. In August 2023, we announced that the first patient was enrolled in GALACELA, the Phase 2 study with GLPG3667 in patients with SLE. Topline results of the GALACELA study are expected in 2026. 7 Minimal improvement per ACR/EULAR is defined as a total improvement score (TIS) of >= 20 points. The TIS is a score derived from the evaluation of the results from 6 core set measurements of myositis disease activity.

GALACELA Phase 2 study design with GLPG3667 in SLE Adults with active systemic lupus erythematosus â‰^ (N 140) Primary endpoint: proportion of subjects with improvement at Week 32 according to SLE Responder Index (SRI)-4 Secondary endpoints: proportion of subjects achieving BICLA, CLASI-A, LLDAS scores, joint count readouts, safety/tolerability, PK GALACELA is a Phase 2 randomized, double-blind, placebo-controlled, multi-center study to evaluate the efficacy, safety, tolerability, pharmacokinetics, and pharmacodynamics of GLPG3667 in adults with active SLE. A once-daily oral administration of GLPG3667 or placebo will be investigated in approximately 140 adult patients with SLE for 32 weeks. The primary endpoint is the proportion of patients who achieve the SLE responder index (SRI)-4 response at Week 32. The secondary efficacy endpoints are the proportion of patients who achieve the British Isles Lupus Assessment Group (BILAG)-based Composite Lupus Assessment (BICLA) response at Week 32, proportion of patients with >=50% reduction in Cutaneous Lupus Erythematosus Disease Area and Severity Index Activity (CLASI-A) score at Week 16, proportion of patients who achieve Lupus Low Disease Activity State (LLDAS) at Week 32 and change from baseline in the 28-joint count for tender, swollen, and tender and swollen (active) joints at Week 32.

Risk factors Description of the risks for investors Pioneering science to transform patient outcomes

RISK FACTORS in Detailed Form 20 description -F of the risk factors As a U.S. listed company, we are also subject to the reporting requirements of the U.S. Securities and Exchange Commission, or SEC. An annual report will be filed with the SEC on Form 20-F. Our annual report on Form 20-F is available in the SEC’s EDGAR database (https://www.sec.gov/edgar.shtml), and a link thereto is posted on our website. For a comprehensive, detailed description of the Risk factors, we refer to Form 20-F. regulatory Risks related approval to product development and Operating product candidates procedures, monitoring and prioritizing We operate adequate standard operating procedures to secure the integrity and protection of our research and development activities and results, and the optimum allocation of our R&D budgets. The progress of the most important research and development programs is continuously monitored by our Executive Committee, they are discussed with the Board of Directors at least once per quarter, and the members of our Board of Directors with expertise in clinical and scientific matters occasionally attend meetings with our scientific staff to discuss and assess such programs. Nevertheless, we must and have in the past and during the financial year 2023 decided to prioritize the development of certain product candidates; these decisions may prove to have been wrong and may adversely affect our business. product Strongly candidates dependent and on the the success discovery of clinical portfolio We are heavily dependent on the success of our product candidates, such as GLPG5101, GLPG5201, GLPG5301 and GLPG3667. As of year-end 2022, we implemented a new innovation R&D model focusing on the therapeutic areas of oncology and immunology. Following the strategic review announced in August 2023, we transferred the commercial, medical affairs and development activities regarding filgotinib to Alfasigma in January 2024. In addition, we are heavily investing in an early-stage product candidate pipeline, including small molecules and oncology preclinical candidates, and these drug candidates must undergo rigorous preclinical and clinical testing, the results of which are uncertain and could substantially delay or prevent the drug candidates from reaching the market.

New and complex innovative cell therapies Through the acquisitions of CellPoint and AboundBio, we gained access to an innovative, scalable, decentralized and automated point-of-care CAR-T cell therapy supply model as well as a fully human antibody-based therapeutics platform. We are heavily investing in building our therapeutic area of oncology, whereby cell therapies are novel, complex, and difficult to manufacture and require rigorous preclinical and clinical testing, the results of which are uncertain. We cannot give any assurance that any product candidate will successfully complete clinical trials or receive regulatory approval, which is necessary before it can be commercialized. Unpredictable candidates commercial viability of the product Our business and future success is substantially dependent on our ability to develop successfully, obtain regulatory approval for, and then successfully commercialize our product candidates. We are not permitted to market or promote any of our product candidates before we receive regulatory approval from the FDA, the EMA, the MHRA, the MHLW or any other comparable regulatory authority, and we may never receive such regulatory approval for any of our product candidates. We cannot give any assurances that our clinical trials for our product candidates, including our CD19 CAR-T product candidates, will be completed in a timely manner, or at all. If any of our product candidates are not approved and commercialized in certain jurisdictions, we will not be able to generate any product revenues for that product candidate. Lengthy, time-consuming regulatory processes The regulatory approval processes of the FDA, the EMA, the MHRA, the MHLW and other comparable regulatory authorities are lengthy, time-consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidates, our business, including its financial condition, will be substantially harmed. uncertain Expensive outcome clinical development process with Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Results of earlier studies and trials as well as data from any interim analysis of ongoing clinical trials may not be predictive of future trial results, and failure can occur at any time during the clinical trial process. If we experience delays in the completion of, or termination of, any clinical trial of our product candidates, the commercial prospects of our product candidates will be harmed, and our ability to generate product revenues from any of these product candidates will be delayed. If any of our product candidates are found to be unsafe or have a lack of efficacy, we will not be able to obtain or maintain regulatory approval for it and our business would be materially harmed.

Patient enrollment influence The rates at which we complete our scientific studies and clinical trials depend on many factors, including, but not limited to, patient enrollment. Patient enrollment is a significant factor in the timing of clinical trials and is affected by many factors including competing clinical trials, clinicians’ and patients’ perceptions as to the potential advantages of the drug being studied in relation to other available therapies and the relatively limited number of patients. Any of these occurrences may harm our clinical trials and by extension, our business, financial condition and prospects. effects Product or candidates serious adverse may cause events undesirable side Our product candidates may cause undesirable or unacceptable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following marketing approval, if any. Undesirable side effects caused by our product candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA, the EMA, the MHRA, the MHLW or other comparable regulatory authorities. The drug-related side effects could affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims. Any of these occurrences may harm our business, financial condition and prospects significantly and may adversely impact the viability of our other product candidates or preclinical programs. Patients receiving T cell-based immunotherapies may experience serious adverse events, including neurotoxicity and cytokine release syndrome. Serious adverse events or undesirable side effects associated with our CAR-T product candidates may result in delays, clinical holds, or terminations of our preclinical or clinical trials, impact our ability to obtain regulatory or marketing approval, and impact the commercial potential of such product candidates, which would significantly harm our business, financial condition and prospects. Public perception may be influenced by claims that cell therapy, including cell editing technologies, is unsafe, or unethical, and research activities and adverse events in the field, even if not ultimately attributable to us or our CAR-T product candidates, could result in increased governmental regulation, unfavorable public perception, challenges in recruiting patients to participate in our clinical studies, potential regulatory delays in the testing or approval of our CAR-T product candidates, labeling restrictions for any future approved CAR-T products, and a decrease in demand for any such product. For example, in November 2023, the FDA announced that it would be conducting an investigation into reports of T-cell malignancies following BCMA-directed or CD19-directed autologous CAR-T cell immunotherapies following reports of T-cell lymphoma in patients receiving these therapies. The FDA also stated that patients and clinical trial participants receiving treatment with the currently approved BCMA-directed and CD19-directed genetically modified autologous CAR-T cell immunotherapy products should be monitored life-long for new malignancies. In January 2024, the FDA

determined that new safety information related to T-cell malignancies should be included in the labeling with boxed warning language on these malignancies for all BCMA- and CD-19-directed genetically modified autologous T-cell immunotherapies. Additionally, EMA’s PRAC started a signal procedure to review data on secondary malignancies related to T-cells (cancers that begin in a type of white blood cells called T-cells), including T-cell lymphoma and leukemia, for the six approved CAR-T cell medicines. More restrictive government regulations or negative public opinion would have a negative effect on our business or financial condition and may delay or impair the development and commercialization of our CAR-T product candidates or demand for any approved products. If we are not able to obtain orphan product exclusivity, or maintain such status for future product candidates for which we seek this status, or if our competitors are able to obtain orphan product exclusivity before we do, we may not be able to obtain approval for our competing products for a significant period of time. Even if we are able to obtain orphan designation, we may not be the first to obtain marketing approval for such indication due to the uncertainties associated with developing pharmaceutical products. Orphan drug designation neither shortens the development time or regulatory review time of a drug nor gives the drug any advantage in the regulatory review or approval process. Extensive ongoing regulatory requirements If the FDA, EMA, or any other comparable regulatory authority approves any of our product candidates, the manufacturing processes, distribution, adverse event reporting, storage, advertising, and recordkeeping for the product will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, registration requirements and continued compliance with current good manufacturing practices, or cGMPs, and good clinical practices, or GCPs, for any clinical trials that we conduct post-approval. For example, the FDA stated in its January 2024 final guidance document titled “Considerations for the Development of Chimeric Antigen Receptor (CAR) T Cell Products” that subjects in clinical trials treated with CAR-T cells containing an integrated transgene should be monitored for 15 years after treatment. Failure to comply with the aforementioned practices may harm our clinical trials or regulatory process and by extension, our business, financial condition and prospects. Before we can begin to commercially manufacture our product candidates for human therapeutics, the FDA must review for the applicable manufacturing process and facilities as part of its review of our marketing application. This will likely require the manufacturing facilities to pass a pre-approval inspection by the FDA. A manufacturing authorization must also be obtained from the appropriate EU regulatory authorities or other comparable regulatory authorities. We must establish and maintain a pharmacovigilance system, including a qualified person responsible for oversight, submit safety reports to the regulators and comply with the good pharmacovigilance practice guidelines adopted by the relevant regulatory authorities. Failure to comply with these guidelines may harm our clinical trials or regulatory process and by extension, our business.

Risks related to commercialization The marketing and sale of filgotinib or future approved products may be unsuccessful or less successful than anticipated. We are dependent on the agreed and ongoing transfer to Alfasigma of the European MA for filgotinib, which is approved for the treatment of RA and UC in Europe and Japan. Degree of market acceptance The commercial success of any future products, if approved, will depend upon the degree of market acceptance by physicians, healthcare payers, patients, and the medical community. Market acceptance will depend on a number of factors, many of which are beyond our control, but not limited to (i) the wording of the product label, (ii) changes in the standard of care for the targeted indications for any product and product candidate, (iii) acceptance by physicians, patients and healthcare payers of the product as safe, effective and cost-effective and (iv) sales, marketing and distribution support. We have limited experience in the sale or marketing of pharmaceutical products. To the extent any of our product candidates for which we maintain commercial rights is approved for marketing, if we are unable to establish marketing and sales capabilities or enter into agreements with third parties to market and sell our products, we may not be able to market and sell any product effectively, or generate product revenues, which in turn would have a material adverse effect on our business, financial condition, and results of operation. reimbursement Potential adverse decisions effect of coverage and Coverage and reimbursement decisions by third-party payers may have an adverse effect on pricing and market acceptance of newly approved drugs. Legislative and regulatory activity, including enacted and future legislation, may exert downward pressure on potential pricing and reimbursement for any of our product candidates, if approved, that could materially affect the opportunity to commercialize.

Public perception and increased regulatory scrutiny Public perception may be influenced by claims that cell therapy, including cell editing technologies, is unsafe, or unethical, and research activities and adverse events in the field, even if not ultimately attributable to us or our CAR-T product candidates, could result in increased governmental regulation, unfavorable public perception, challenges in recruiting patients to participate in our clinical studies, potential regulatory delays in the testing or approval of our CAR-T product candidates, labeling restrictions for any future approved CAR -T products, and a decrease in demand for any such product. Risks need for related additional to our capital financial position and Biotechnology market We are a global biotechnology company with limited sales experience, limited historical profit from product sales and limited historical data on product revenues. Except for the commercial launch of filgotinib, our operations have been limited to developing our technology and undertaking preclinical studies and clinical trials of our product candidates. Significant operating losses Since our inception, and with the exception of the years 2019 and 2023, we have incurred significant operating losses. Our losses resulted principally from costs incurred in research and development, preclinical testing, clinical development of our product candidates as well as costs incurred for research programs, (pre-)commercial activities, primarily related to the commercial launch of Jyseleca®, and from general and administrative costs associated with our operations. We expect to continue incurring significant research, development and other expenses related to our ongoing operations, and to continue incurring operating losses for the foreseeable future. Because of the numerous risks and uncertainties associated with pharmaceutical product development, we are unable to predict the timing or amount of expenses and when we will be able to achieve or maintain profitability, if ever. Additional funding may be required We may require substantial additional future capital which may not be available to us on acceptable terms, or at all, in order to complete clinical development and, if we are successful, to commercialize any of our current product candidates, if approved. Our ability to raise additional funds will depend on financial, economic and market conditions and other factors, over which we may have no or limited control. In addition, raising additional capital may cause dilution to our existing shareholders, restrict our

operations or require us to relinquish rights to our product candidates or technologies. The incurrence of additional indebtedness could result in increased fixed payment obligations and could also result in certain additional restrictive covenants that could adversely impact our ability to conduct our business. For further reference on financial risks in particular, see note 33 of the notes to the consolidated financial statements. Risks parties related to our reliance on third Strongly with Gilead dependent and certain on collaboration other third parties agreements We are heavily dependent upon our collaboration arrangements with Gilead and certain other third parties for the development and commercialization of our products and there can be no assurance that these arrangements will deliver the benefits we expect. In July 2019, we entered into a 10-year global research and development collaboration with Gilead. In connection with our entry into the option, license and collaboration agreement, we received an upfront payment of $3.95 billion and a €960 million ($1.1 billion) equity investment from Gilead. Under the option, license and collaboration agreement, we fund and lead all discovery and development autonomously until the end of the relevant Phase 2 clinical study. After the completion of the Phase 2 clinical study (or, in certain circumstances, the first Phase 3 study), Gilead will have the option to acquire an exclusive commercial license to that program in all countries outside of Europe. If the option is exercised, we and Gilead will co-develop the compound and share costs equally. In addition, we are dependent on Gilead for the commercialization of filgotinib and the further development of filgotinib outside of Europe. Gilead may not devote sufficient resources or give sufficient priority to the programs in respect of which it acquires a commercial license pursuant to the option, license and collaboration agreement. Furthermore, Gilead may not be successful in the commercialization of filgotinib outside of Europe and further development and commercialization of filgotinib or other programs for which it acquires a commercial license, even when they do devote resources and prioritize their efforts for such programs. To the extent that Gilead is commercializing filgotinib in one or more jurisdictions via a third party, such as Eisai for certain Asian markets, we are dependent on their successful accomplishment of commercialization efforts. In addition, the terms of the collaboration with Gilead and any collaboration or other arrangement that we may establish may not ultimately prove to be favorable to us or may not be perceived as favorable, which may negatively impact the trading price of the ADSs or our ordinary shares. In addition, pursuant to the collaboration with Gilead, we are entitled to certain option payments and tiered royalties, and milestone payments

on certain products. There can be no assurance that such payments will be sufficient to cover the cost of development of the relevant product candidates. We are subject to a number of additional risks associated with our dependence on our collaborations with third parties, the occurrence of which could cause our collaboration arrangements to fail. In particular, the collaboration we entered into in July 2019 is managed by a set of joint committees comprised of equal numbers of representatives from each of us and Gilead. Conflicts may arise between us and Gilead, such as conflicts concerning the interpretation of clinical data, the achievement of milestones, the interpretation of financial provisions or the ownership of intellectual property developed during the collaboration, and there can be no assurance that the joint committees will be able to resolve any such conflicts. If any such conflicts arise, Gilead could act in a manner adverse to our best interests. Any such disagreement could result in one or more of the following, each of which could delay or prevent the development or commercialization of product candidates subject to the collaboration arrangements, and in turn prevent us from generating sufficient revenues to achieve or maintain profitability: reductions or delays in the payment of milestone payments, royalties or other payments we believe are due; actions taken by Gilead inside or outside our collaboration which could negatively impact our rights or benefits under our collaboration including termination of the collaboration for convenience; or unwillingness on the part of Gilead to keep us informed regarding the progress of its development and commercialization activities or regulatory approval or to permit public disclosure of the results of those activities. In addition to our collaboration with Gilead, we may also enter into future collaborations which will give rise to similar risks, although our ability to enter into such collaborations may be limited given the scale of our collaboration with Gilead. If our global research and development collaboration with Gilead or other collaborations on research and development candidates do not result in the successful development and commercialization of products or if Gilead or another one of our collaboration partners terminates its agreement with us, we may not receive any future research funding or milestone or royalty payments under the collaboration. If we do not receive the funding we expect under these agreements, our development of our product candidates could be delayed and we may need additional resources to develop product candidates. We may not be successful in establishing future development and commercialization collaborations, particularly given the scale of our collaborations with Gilead, and this could adversely affect, and potentially prohibit, our ability to develop our product candidates.

commercialization Potential limitation collaborations on future development and Developing pharmaceutical products, conducting clinical trials, obtaining regulatory approval, establishing manufacturing capabilities and marketing approved products are expensive. Accordingly, we have sought and may in the future seek to enter into collaborations with companies that have more resources and experience. In the future, however, our ability to do so may be limited given the scale of the 10-year global research and development collaboration that we entered into with Gilead in July 2019. If Gilead declines to exercise its option and we are otherwise unable to obtain a collaboration partner for our product candidates, we may be unable to advance the development of our product candidates through late-stage clinical development and seek approval in any market. In situations where we enter into a development and commercial collaboration arrangement for a product candidate, we may also seek to establish additional collaborations for development and commercialization in territories outside of those addressed by the first collaboration arrangement for such product candidate. If any of our product candidates receives marketing approval, we may enter into sales and marketing arrangements with third parties with respect to otherwise unlicensed or unaddressed territories. Furthermore, there are a limited number of potential collaboration partners, and we expect to face competition in seeking appropriate collaboration partners. If we are unable to enter into any development and commercial collaborations and/or sales and marketing arrangements on acceptable terms, or at all, we may be unable to successfully develop and seek regulatory approval for our product candidates and/or effectively market and sell approved products, if any. Through the acquisitions of CellPoint and AboundBio, we gained access to an innovative, scalable, decentralized and automated point-of-care cell therapy manufacturing model as well as a fully human antibody-based therapeutics platform and research capabilities for novel, differentiated CAR-T constructs. To address important limitations of current CAR-T treatments, CellPoint has developed, in a strategic collaboration with Lonza, a Swiss manufacturing company for the pharmaceutical, biotechnology and nutrition sectors, a novel decentralized delivery model designed to manufacture non-frozen CART therapies at the point-of-care. The platform consists of CellPoint’s end-to-end xCellit® workflow management and monitoring software and Lonza’s Cocoon®, a functionally closed, automated manufacturing platform for cell therapies. Clinical studies with this decentralized supply model have been approved by regulatory authorities in Belgium, Spain, and the Netherlands. If, for any reason, the collaboration is terminated or is otherwise materially changed and we are no longer entitled to use such technology platform, we may be unable to secure alternatives to such technology and, our research, development or other efforts may be interrupted or delayed, and our financial condition and results of operation may be materially adversely affected. Reliant on third party supply of materials We rely on third party suppliers for which a reliable supply of materials is required in order to avoid delays in the drug discovery and development process and commercial

supplies of any approved product. Most goods and services are provided by several different suppliers, which mitigates the risk of loss of key suppliers. Expanding the suppliers’ network can be time consuming as all source suppliers are subject to rigorous ethical and quality control standards. Our suppliers are required to adhere to contractual terms that include anti-bribery and anti-corruption provisions. Our general terms and conditions of purchase also contain a specific clause on anti-bribery and anti-corruption. They can be found on our website. No expected assurance results that or arrangements benefits will deliver We have relied on and plan to continue to rely on contract research organizations, or CROs, to monitor and manage data for our preclinical and clinical programs. We and our CROs also rely on clinical sites and investigators for the performance of our clinical trials in accordance with the applicable protocols and applicable legal, regulatory and scientific standards, including Good Clinical Practices (GCPs). Regulatory authorities enforce these GCPs through periodic inspections of trial sponsors, investigators and clinical sites. If CROs do not successfully carry out their contractual duties or obligations or meet quality standards, regulatory requirements or expectations, such as the applicable GCPs, our clinical trials may be extended, delayed or terminated, the clinical data generated in our clinical trials may be deemed unreliable and regulatory authorities may require us to perform additional clinical trials before approving our marketing applications and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. We do retain responsibility for all our studies and are required to and have put in place measures to manage, oversee, and control our studies, including the CRO selection process, audits, strong focus on deliverables, timelines, roles & responsibilities, and oversight of conduct of the studies. In addition to GCPs, our clinical trials must be conducted with products produced under current Good Manufacturing Practice (cGMP) regulations. Reliant on third party clinical data and results We rely on clinical data and results obtained by third parties that could ultimately prove to be inaccurate or unreliable. If the third-party data and the results that we rely on prove to be inaccurate, unreliable or not applicable to our product candidates, we could make inaccurate assumptions and conclusions about our product candidates and our research and development efforts could be materially adversely affected.

Risks related to our intellectual property Our ability to compete may decline if we do not adequately protect our proprietary rights. We endeavor to protect our proprietary technologies and know-how by entering into confidentiality and proprietary information agreements with our employees and partners, and by setting up special procedures (e.g. with respect to the handling of the laboratory books). The proprietary nature of, and protection for, our product candidates, their methods of use, and our platform technologies are an important part of our strategy to develop and commercialize novel medicines. We have obtained patents relating to certain of our product candidates and are pursuing additional patent protection for them and for our other product candidates and technologies. We also rely on trade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection. Additionally, we have registered and unregistered trademarks, including amongst others our company name. As of March 1, 2024, Intellectual property rights held by Galapagos NV relating to our product candidates include the following: GLPG5101 product candidate: GLPG5101 is currently being developed in our point-of-care model for the treatment of relapsed/refractory NHL. For this model, we have obtained an exclusive worldwide license from Lonza AG to use the Cocoon® for the commercial manufacture of cell therapy for the treatment of hematological malignancies at the point-of-care. GLPG5201 product candidate: GLPG5201 is currently being developed in our point-of-care model for the treatment of relapsed/refractory CLL and RT. For this model, we have obtained an exclusive worldwide license from Lonza AG to use the Cocoon® for the commercial manufacture of cell therapy for the treatment of hematological malignancies at the point-of-care. We also have a license and supply agreement on the materials to produce and use our GLPG5201 product candidate. GLPG5301 product candidate: GLPG5301 is currently being developed in our point-of-care model for the treatment of relapsed/refractory MM. For this model, we have obtained an exclusive worldwide license from Lonza AG to use the Cocoon® for the commercial manufacture of cell therapy for the treatment of hematological malignancies at the point-of-care. We also have an exclusive license and supply agreement on the materials to produce and use our GLPG5301 product candidate. GLPG3667 product candidate: We have a granted U.S. patent application, and one pending U.S. patent application. We have one patent granted via the European Patent Office (EPO) and one pending patent application at the EPO; as well as further granted patents inter alia in Japan and Australia. In addition, we have counterpart foreign patent

applications that are pending in Canada, China and other foreign countries claiming GLPG3667 compositions of matter and methods of treatment using GLPG3667. Patents, if any, that issue based on this pending patent application are estimated to expire in 2038, not including any potential extensions for the marketed product that may be available via supplementary protection certificates or patent term extensions. We also have one U.S. pending patent application as well as other foreign jurisdictions claiming dosage regimen, and any patent, if granted is estimated to expire in 2042. Finally, we have four pending applications under the Patent Cooperation Treaty (PCT) disclosing solid forms, metabolites, and/or methods for treating inflammatory disorders using GLPG3667; any patents, if granted, based on these patent applications are estimated to expire in 2043. disclosed Third parties proprietary may claim rights for wrongfully used or Our commercial success depends on obtaining and maintaining proprietary rights to our product and product candidates, as well as successfully defending these rights against third party challenges. We will only be able to protect our product candidates, and their uses from unauthorized use by third parties to the extent that valid and enforceable patents, or effectively protected trade secrets, cover them. If we fail to maintain to protect or to enforce our intellectual property rights successfully, our competitive position could suffer, which could harm our results of operations. Time can harm consuming our business and costly infringement procedures Pharmaceutical patents and patent applications involve highly complex legal and factual questions, which, if determined adversely to us, could negatively impact our patent position. Our success will depend in part on our ability to operate without infringing the intellectual property and proprietary rights of third parties. We cannot guarantee that our business, product, product candidates and methods do not or will not infringe the patents or other intellectual property rights of third parties. There is significant litigation activity in the pharmaceutical industry regarding patent and other intellectual property rights. Such litigation could result in substantial costs and be a distraction to management and other employees. law Possible or jurisprudence negative impact of developments in patent The patent positions of biotechnology and pharmaceutical companies can be highly uncertain and involve complex legal and factual questions. The interpretation and breadth of claims allowed in some patents covering pharmaceutical compositions may be uncertain and difficult to determine, and are often affected materially by the facts and circumstances that pertain to the patented compositions and the related patent claims. The standards of the United States Patent and Trademark Office, the European Patent Office, and other foreign counterparts are sometimes uncertain and could change in the future. If we fail to obtain and maintain patent protection and trade secret protection

of our product and product candidates, we could lose our competitive advantage and the competition we face would increase, reducing any potential revenues and adversely affecting our ability to attain or maintain profitability. Targeted protection and (cost) efficient intellectual property We will not seek to protect our intellectual property rights in all jurisdictions throughout the world and we may not be able to adequately enforce our intellectual property rights even in the jurisdictions where we seek protection. Filing, prosecuting and defending patents on our product candidates in all countries and jurisdictions throughout the world would be prohibitively expensive, and our intellectual property rights in some countries could be less extensive than those in the United States and Europe. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries, or from selling or importing products made using our inventions. Patent Legal uncertainty Court around new European Unitary The Unitary Patent Court (UPC) was opened in June 2023, being competent in matters of patent litigation. New case law will emerge and require risk evaluating and mitigating regarding certain intellectual property rights. Risks related to our competitive position Intensive competitive sector We face significant competition for our drug discovery and development efforts, and if we do not compete effectively, our commercial opportunities will be reduced or eliminated. The biotechnology and pharmaceutical industries are intensely competitive and subject to rapid and significant technological change and innovation. Our competitors may now or in the future develop drug products that render our products obsolete or non-competitive by developing more effective drugs or by developing their products more efficiently. In addition, our ability to develop competitive products would be limited if our competitors succeeded in obtaining regulatory approvals for drug candidates more rapidly than we were able to or in obtaining patent protection or other intellectual property rights that limited our drug development efforts. In the field of dermatomyositis (DM), physical therapy, exercise and medication including corticosteroids, immunosuppressants or recently immunoglobulin treatment are commonly used to treat DM. Treatment of this disease has relied for many years on off-

label medication. Additionally, in 2021 the FDA approved immunoglobulin treatment Octagam®, based on the Phase 3 ProDerm trial of Octapharma. In the field of SLE, corticosteroids, antimalarials and immunosuppressants are commonly used to control lupus disease activity. Only two products are approved to treat SLE, both as add-on to standard therapy: Belimumab (Benlysta®) (anti-BAFF) from GSK and recently anifrolumab (Saphnelo®) (anti-IFN) from Astra Zeneca. There are currently over 10 products in Phase 3 for SLE, of which the minority are oral –deucravacitinib (SotyktuTM) (TYK2) from BMS, upadacitinib (JAK) from Abbvie and cenerimod (S1P1) from Idorsia/Viatris. In the field of hematologic malignancies, such as Non-Hodgkin’s Lymphoma (NHL), Chronic Lymphocytic Leukemia (CLL) and Multiple Myeloma (MM), there are many approved therapies or therapies in development (including but not limited to chemotherapy, BTKi, antibodies, bispecific antibodies, antibody drug conjugates, CARTs, cytokines, NK and T-cell engagers, etc.) and many different types of cell therapy in development (allogeneic/autologous, T/NK/CAR-NK, TIL, TCR-T, dendritic, etc.). As a consequence, we are operating in a highly competitive, and rapidly evolving environment. New technologies and therapies such as in vivo modification of immune cells may further disrupt this market in the mid-to-long-term. Six CAR T treatments have been approved for hematological cancers in the US and Europe: Novartis’ Kymriah® (CD19 CAR T), Gilead/Kite’s Yescarta® (CD19 CAR T), Tecartus® (CD19 CAR T), J&J’s Carvykti® (BCMA CAR T) BMS’ Breyanzi® (CD19 CAR T) and Abecma® (BCMA CAR T). Additionally, these third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, the development of our product candidates. If we, our product candidates or our technology platforms do not compete effectively, it is likely to have a material adverse effect on our business, financial condition and results of operation. and Risks operation related to our organization, structure retaining Continuous qualified required personnel successful attracting and Our future success depends on our ability to retain the members of our Executive Committee, and to attract, retain and motivate qualified personnel to develop our business if we expand into the fields that will require additional skills and expertise, including oncology. If we are not successful in attracting and retaining highly qualified personnel, we may not be able to achieve our objectives and successfully implement our business strategy, which could have a material adverse effect on our business and prospects. Attractive development and training programs, adequate remuneration and incentive schemes, and a safe and healthy work environment mitigate this risk as they,

among others, induce valuable qualified personnel to continue their employment or services with our business. We expect that if we continue to build our development and medical organizations, including in the field of oncology, we will require significant additional investment in personnel, management and resources. Our ability to achieve our research and development objectives depends on our ability to respond effectively to these demands, expand our internal organization, systems, controls and facilities to accommodate additional anticipated growth, and upon our management developing and implementing strategies for our business to realize these objectives. If we are unable to manage our growth effectively, our business could be harmed and our ability to execute our business strategy could suffer. Potential manufacture product and production or product candidates issues We have limited experience in the field of oncology, and continue to build our therapeutic area of oncology. We expect to invest significant financial and management resources to continue to build these capabilities and to establish such therapeutic area within our business. In June 2022, we acquired CellPoint and AboundBio with the aim to enter the space of oncology. Through such acquisitions, we believe we reinforced our portfolio by gaining access to an innovative, scalable, decentralized and automated point-of-care cell therapy manufacturing model as well as fully human antibody-based therapeutics platform. Cell therapies are novel, complex, and difficult to manufacture, and we may not be successful in our efforts to develop and commercialize such therapies, in which case our financial condition and results of operation may be materially adversely affected. The manufacturing processes that we use to produce product and our product candidates for human therapeutics are complex, novel and have not been validated for commercial use. Several factors could cause production interruptions, including (without limitation) equipment malfunctions and facility contamination. Problems with the manufacturing process, even minor deviations from the normal process, could result in product defects or manufacturing failures that can result in lot failure or product liability claims. We must have a robust quality management system and team in place to ensure (continued) compliance with current good laboratory practices, current good manufacturing practices and current good clinical practices. If we are unable to comply with these practices, this may harm our clinical trials or regulatory process and by extension, our business. Information technology systems Our, our third party partners’ or vendors’, information technology systems and networks could face serious disruptions or suffer security breaches that could adversely affect our business. We rely on both internal information technology (IT) systems and networks, and those of third parties and their vendors, to process and store confidential and sensitive data, including confidential research, business plans, financial information,

intellectual property, patient data, customer data and personal data that may be subject to legal protection. The extensive information security and cybersecurity threats, which affect companies globally, pose a risk to the security and availability of these IT systems and networks, and the confidentiality, integrity, and availability of confidential and sensitive data. We continuously assess these threats and make investments to increase internal protection, detection, and response capabilities, as well as to increase our third party providers’ capabilities and controls to address this risk. However, because of the frequently changing attack techniques, along with the increased volume and sophistication of the attacks, there is the potential risk for us to be adversely impacted. Although we have invested time and resources in the protection of its information technology and other internal infrastructure systems, we and our vendors, like other companies in the industry, have experienced attacks from time to time, and we and our vendors may experience other such attacks in the future. The impact of security breaches and significant disruption in the availability of our information technology and networks could result in reputational, competitive, operational or other business harm, financial costs, litigation (including class action claims), regulatory action (for example, investigations, fines, penalties, audits and inspections), as well as interruptions in our collaborations with our partners, and delays in our research, development work, regulatory approval efforts and other work. data Potential protection non-compliances laws and requirements with evolving privacy and We have to comply with applicable data privacy laws, including the European General Data Protection Regulation (GDPR), which, among others, imposes strict obligations and restrictions on the collection and use of personal data. In the ordinary course of our business, we collect and store sensitive data. Many third-party vendors that support our business processes also have access to and process personal data. Although we have taken preventative measures and set up procedures regarding data processing, data breaches, loss of data and unauthorized access could still occur. These could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, including the GDPR, and significant regulatory penalties, disrupt our operations and damage our reputation. Any of the foregoing could materially harm our business, prospects, financial condition, and results of operation. New social risks media and usage challenges connected to increasing Despite our efforts to monitor social media and comply with applicable rules, there is a risk that the use of social media by us or our employees to communicate about our drug candidates or business may cause us to be found in violation of applicable requirements. In addition, our employees may knowingly or inadvertently make use of social media in ways that may not comply with our social media policy or other legal or contractual

requirements, which may give rise to liability, lead to the loss of trade secrets or other intellectual property, or result in public exposure of sensitive information. Furthermore, negative posts or comments in social media could seriously damage our reputation, brand image, and goodwill. Strategic difficulties, acquisitions or may not can realize result the in intended integrating advantages We may undertake strategic acquisitions in the future and any difficulties from integrating such acquisitions could adversely affect our share price, operating results and results of operations. We may acquire companies, businesses and products that complement or augment our existing business. As our programs may require the use of property rights held by third parties, the growth of our business will likely depend in part on our ability to acquire, license-in or use these proprietary rights. We may be unable to acquire or in-license any third-party proprietary rights that we identify necessary for our drug candidates, for whatsoever reason. We may not be able to integrate any acquired business successfully or operate any acquired business profitably. Integrating any newly acquired business could be expensive and time-consuming. Integration efforts often take a significant amount of time, place a significant strain on managerial, operational and financial resources, result in loss of key personnel and could prove to be more difficult or expensive than we predict. As part of our efforts to acquire companies, business or product candidates or to enter into other significant transactions, we conduct business, legal and financial due diligence with the goal of identifying and evaluating material risks involved in the transaction. Despite our efforts, we ultimately may be unsuccessful in ascertaining or evaluating all such risks and, as a result, might not realize the intended advantages of the transaction. Governance Impact of Sustainability (ESG) regulations or Environmental and potential Social impact or exposure Our business and operations are subject to numerous human rights, corruption, environmental, sustainability, health & safety laws and regulations. On the basis of our activities and the requirement to use hazardous materials, we could incur significant costs and reputational loss associated with civil and criminal fines and penalties. Although we maintain workers’ compensation insurance, this may not provide adequate coverage against potential claims and liabilities. Additionally, we may incur substantial costs in order to comply with the existing and future Sustainability and ESG regulations or permitting requirements. At the date of this report, we are subject to the EU’s Corporate Sustainability Reporting Directive (CSRD). We are required (starting next financial year) to report on a broad range of sustainability KPI’s and to formulate long-term ESG targets , policy and strategic plans under a double materiality principle. These current and future laws, regulations and permitting

requirements may impair our business, and failure to comply with them can result in substantial fines, penalties or other sanctions. Impact liabilities of tax legislative changes and exposure to tax If we are unable to use tax loss carryforwards to reduce future taxable income or benefit from favorable tax legislation, our business, results of operations and financial condition may be adversely affected. We may incur unexpected tax charges, including penalties, due to the failure of tax planning or due to the challenge by tax authorities on the basis of transfer pricing. Any changes to Belgian and international taxation legislation or the interpretation of such legislation by tax authorities may adversely affect our activities, financial situation and results. Such potential changes and their impact are monitored carefully by our management and advisors. Being active in research and development in Belgium, France and the Netherlands, we have benefited from certain research and development incentives. If the Belgian, the French or the Dutch governments decide to eliminate, or reduce the scope or the rate of, the research and development incentive benefits, either of which they could decide to do at any time, our results of operations could be adversely affected. As a company active in research and development in Belgium, we also expect to benefit from the “innovation income deduction” in Belgium. The innovation income deduction regime allows net profits attributable to revenue from among others patented products (or products for which the patent application is pending) to be taxed at a lower effective rate than other revenues. The effective tax rate can thus be reduced down to 3.75%. At 31 December 2023 we had €390.3 million of carry-forward innovation income deduction in Belgium. Our inability to qualify for the abovementioned advantageous tax regimes, as well as the introduction of the minimum taxable base and any other future adverse changes of Belgian tax legislation, may adversely affect our business, results of operations and financial condition. We have received several technological innovation grants to date from an agency of the Flemish government to support various research programs and technological innovation in Flanders. If we fail to comply with our contractual obligations under the applicable technological innovation grant agreements, we could be forced to repay all or part of the grants received, which could adversely affect our ability to finance our research and development projects. (In)accurate budget and performance We annually establish a detailed budget that is submitted to the Board of Directors for review and approval. Our performance compared to the budget is continuously monitored by our Executive Committee, and is discussed with the Board of Directors at least once per quarter. For the establishment of our financial information, we have

processes and methods in place that enable the preparation of non-consolidated and consolidated financial statements for our annual and quarterly reporting. Our management reporting systems – which include an advanced integrated Enterprise Resource Planning (ERP system) – secure the generation of consistent financial and operational information, allowing management to follow-up our performance on a daily basis. Natural disruptive disasters effects and geopolitical events and their The occurrence of unforeseen or catastrophic events, including extreme weather events and other acts of god or natural disasters, man-made disasters, electricity or telecommunication interruption, geopolitical and other economic and political events or conditions (such as the armed conflict between Russia and Ukraine or the conflict between Israel and Gaza), or the emergence of epidemics or diseases, depending on their scale, may cause different degrees of damage to the national and local economies, and could cause a disruption in our operations and have a material adverse effect on our financial condition and results of operations. Man-made disasters, epidemics or diseases, and other events connected with the regions in which we operate could have similar effects. Further, continuing uncertainty around these and related issues could lead to adverse effects on the economy of the United States and other economies, which could impact our ability to develop and commercialize our products and raise capital going forward. Market shares risks relating to the Galapagos We have identified the following major market risks: Possible volatility of share price The market price of the shares might be affected by a variety of factors outside management’s control, such as, without limitation, the global economic situation, the business development of competitors, and sector mergers and acquisitions; it is difficult to mitigate these risk. Economic risk due to failure in confidence General public confidence about future economic conditions or performance of us, our business, or our suppliers or customers may impact the ability or willingness of others to trade with us. Dilution through capital increases Raising additional capital may cause dilution to our existing shareholders. By raising additional capital through capital increases with cancellation of the preferential subscription rights of our existing shareholders, these shareholders would be diluted.

Dilution through exercise of subscription right plans The exercise of existing subscription rights can significantly increase the number of outstanding Galapagos shares. Inability to distribute dividends We have a limited operating history, and future profitability cannot be guaranteed. Galapagos NV has significant losses carried-forward, and will thus not be able to distribute dividends in the near future. This can cause people to refrain from investing in Galapagos’ shares. Reputational damage High ethical standards are maintained throughout the entire organization at all levels. Laws and guidelines are complied with. Our suppliers are required to adhere to contractual terms which include anti-bribery and anti-corruption provisions. In addition, our external consultants are required to comply with our Code of Conduct and our Anti-Bribery and Anti-Corruption Policy. Belgian law provisions There are several provisions of Belgian company law and certain other provisions of Belgian law, such as, without limitation, the obligation to disclose important shareholdings and merger control, that may apply to us, and which may make an unfriendly tender offer, merger, change in management or other change in control, more difficult. These provisions could discourage potential takeover attempts that third parties may consider, and thus deprive the shareholders of the opportunity to sell their shares at a premium (which is typically offered in the framework of a takeover bid). General statement about Galapagos’ risks According to our current assessment and knowledge, we consider the major risks to be manageable, and our going concern not to be endangered at the time of the current report. Assuming no further deterioration of the global business, financial, and regulatory environment, we consider ourselves prepared to meet future challenges.

Sustainability report Our commitment to society: Forward, Sustainably Pioneering science to transform patient outcomes

SUSTAINABILITY REPORT Our Sustainably Sustainability Commitment – Forward, Since our founding more than two decades ago, we have worked to discover, develop, and commercialize life-changing medicines to add years of life and improve quality of life for people around the world. Our focus on, and commitment to, patients will always remain at the center of everything we do. We strongly believe that our patient focus is supported by our commitment to the health of our planet and the wellbeing of our employees. In line with this, we are extending our commitment to patients by evolving the way we pursue breakthroughs in science and the development of innovative medicines by adopting new strategies and performance metrics to improve the health of our environment, the wellbeing and engagement of our employees, and the ethical and transparent management of our operations. Our approach to Sustainability is encapsulated in the principle “Forward, Sustainably,” our strategy designed to bring the values of ethical, responsible innovation into everything we do, from how we develop patient therapies to how we collaborate with our colleagues, partners and other stakeholders. We know that acting as a responsible and sustainable business is key to our success as we continue to focus on the needs of patients.
SUSTAINABILITY REPORT Our Ambition Informed by the results of our materiality assessment, in 2023, we worked on identifying KPIs and defining targets to reach our 2028 call for action, as depicted in the graph below. Our commitment to our call for action by 2028 remains unchanged, and we are reviewing the underlying targets and action plans to reflect the impact of the Jyseleca® transfer transaction that was completed at the end of January 2024. The updated targets will provide the basis for our sustainability reporting as of 2024. Our call for action by 2028 Develop transformational Add more years of life and therapies for patients, Provide patient quality of life for patients with patients and the access globally healthcare community Be a diverse, equitable and inclusive, Be climate neutral and trusted organization

Our Sustainability Governance In 2022, supported by the members of our Executive Committee, we established a Sustainability Steering Committee, composed of cross-functional representatives and leaders from within our organization. The Sustainability Steering Committee ensures that environmental, social, and governance considerations are fully integrated into our decision-making processes, including those related to our business strategy, key investments, and performance. The Committee consists of members of senior management and subject matter experts covering key areas of our operations, including Compliance, Patient Advocacy, Legal, Finance, Environment, Health & Safety (EHS), Procurement, Human Resources, Site Operations, Investor Relations, and Communications. The Executive Committee oversees the Sustainability Steering Committee and approves both the measures and operational structure related to Sustainability. In addition, our Board of Directors, supported by the Audit Committee, oversees the Sustainability oversight structure as well as the strategy for public disclosure with respect to ESG (Environmental, Social and Governance) matters. Our Double Materiality Assessment Driven by our purpose to transform patient outcomes through life-changing science and innovation, we understand that our business actions impact both society and our financial performance. To determine our key goals and priorities, we conducted an impact materiality assessment in 2022, which enabled us to identify the topics most relevant to our internal and external stakeholders. The analysis provided insights into our potential impact on society and the world, allowing us to better monitor emerging business challenges and opportunities. To enhance the value of the 2022 materiality assessment, we updated the methodology we applied for the 2018 assessment and significantly increased the number of stakeholders involved. Externally, we engaged with representatives from patient organizations, patient experts, healthcare providers, supply chain partners, our collaboration partners, and investors. Internally, in addition to the members of our Executive Management and our Sustainability Steering Committee, all employees were given the opportunity to provide input on the materiality of certain topics through a company-wide survey. Internal and external stakeholders were invited to review a list of 35 potential material topics and to identify the five topics they found most relevant, five topics they found
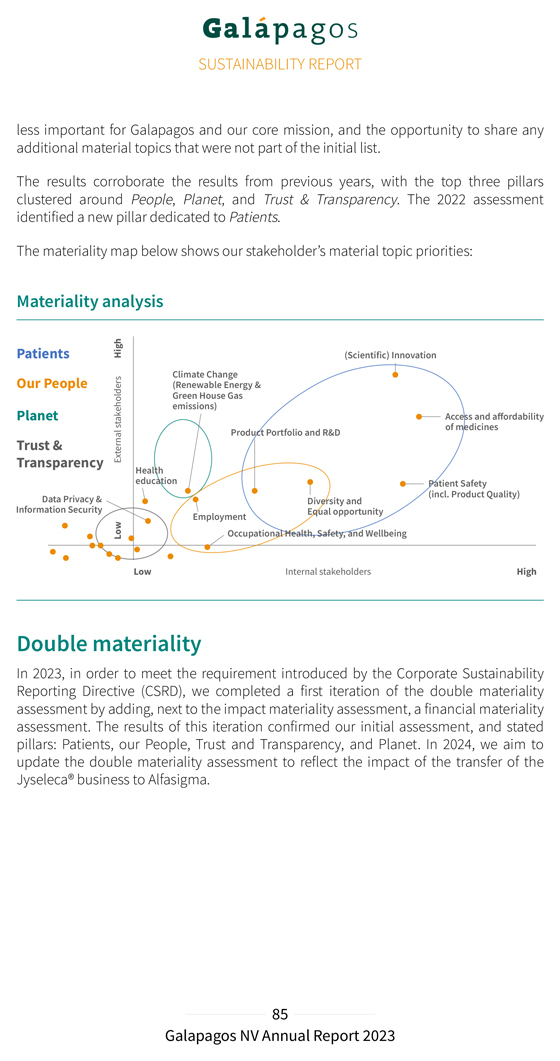
less important for Galapagos and our core mission, and the opportunity to share any additional material topics that were not part of the initial list. The results corroborate the results from previous years, with the top three pillars clustered around People, Planet, and Trust & Transparency. The 2022 assessment identified a new pillar dedicated to Patients. The materiality map below shows our stakeholder’s material topic priorities: Materiality analysis Double materiality In 2023, in order to meet the requirement introduced by the Corporate Sustainability Reporting Directive (CSRD), we completed a first iteration of the double materiality assessment by adding, next to the impact materiality assessment, a financial materiality assessment. The results of this iteration confirmed our initial assessment, and stated pillars: Patients, our People, Trust and Transparency, and Planet. In 2024, we aim to update the double materiality assessment to reflect the impact of the transfer of the Jyseleca® business to Alfasigma.
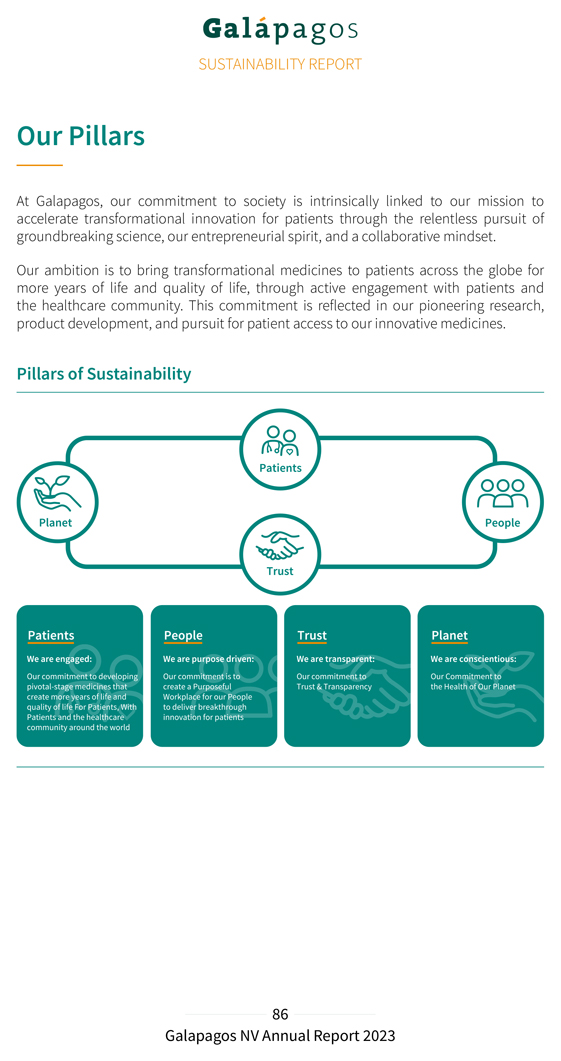
Our Pillars At Galapagos, our commitment to society is intrinsically linked to our mission to accelerate transformational innovation for patients through the relentless pursuit of groundbreaking science, our entrepreneurial spirit, and a collaborative mindset. Our ambition is to bring transformational medicines to patients across the globe for more years of life and quality of life, through active engagement with patients and the healthcare community. This commitment is reflected in our pioneering research, product development, and pursuit for patient access to our innovative medicines. Pillars of Sustainability

Patients Our Commitment to Patients We are engaged. Our commitment to developing transformational medicines that create more years of life and quality of life For Patients, With Patients and the healthcare community around the world We value continuous improvement in our approach to research, development, and healthcare access, with an unwavering focus on outcomes that deliver the greatest value to patients. We embrace change and support disruptive innovation, strive to build a culture of responsible innovation throughout a medicine’s entire lifecycle and are committed to ensuring the safe and appropriate use of our medicines, if approved, as may be prescribed by physicians and used by patients in medical practice. We focus our development efforts on areas where we have deep expertise and map out the shortest path to market with the objective of reducing the time it takes to bring new medicines to patients. At every stage of the patient journey, we aim to collaborate closely with patient organizations, beginning in the clinical study design. Through these efforts, we aim to maintain a clear line of communication with patients, having a significant positive impact on their experiences with our studies. In 2021 we co-developed our Patient Partnership Charter with the patient community to formalize our commitment to patient engagement. Using the Charter as a guideline, we defined our roadmap to integrate patient engagement systematically throughout the medicine lifecycle. We believe our research and development efforts can help advance science beyond the patients we serve. Our plain-language summaries of our data make them easy to understand, and our commitment to Open Access publishing enables us to communicate clearly and effectively with all our stakeholders.

Furthermore, since 2020, we have been an active member of the Open Pharma initiative, a first-of-its-kind collaborative, multi-sponsor, non-profit project. We believe that publications are the route to credible, compliant pharma communications. Open Pharma’s long-term goal is to secure the same terms for authors who publish company-funded research as those for authors who publish research funded through other means. As such, all research findings are freely available to read and reuse, from the date of publication. Actions 2023 We have Invested in building up early relationships with patient organizations in relevant therapeutic areas; Co-created the following internal and external, patient focused resources with the Galapagos Patient Engagement Council (PEC) : A standardized list of questions on patient-relevant endpoints; The patients & caregivers page on our corporate website; A dedicated Galapagos job aid focused on assisting colleagues who work with patients; Template letters for patient organizations and sites to communicate time-sensitive information; Systematically embedded the patient and the clinical site expectations into our late phase immunology studies; Rolled out an internal guidance document on how to share study treatments systematically with participants; Written and tested lay summaries of clinical trials’ results with patients for all studies started after June 2022; Maintained strong engagement with relevant patient organizations at key congresses; Involved patient representatives in the steering committees of all our Phase 4 real world evidence studies; Supported the initiatives led by patient organizations during relevant disease awareness days; Held trainings for in-field personnel with patients; Organized the very first Galapagos Patient Partnership Day on 15 November 2023; Incorporated the health literacy principles in our key documents for clinical trial participants; Published plain language summaries in Galapagos driven scientific manuscripts disclosing data from clinical-stage trials; Enabled continued access to Jyseleca® for patients across Europe following the transfer of Jyseleca® and the related commercial activities to Alfasigma; and Begun our efforts to increase patient access through the decentralized CAR-T manufacturing network.

Our People Our Commitment to our People We are purpose driven. Our commitment is to create a Purposeful Workplace for our People to deliver breakthrough innovation for patients 2023 was a year of significant change and continued transformation for Galapagos, and we are well aware of the impact this has on our employees and the challenges that it poses to our organization. First, to continue building our company around two core therapeutic areas, oncology and immunology, we completed the integration of the CellPoint and AboundBio teams into Galapagos with a robust onboarding program. In late June, we also completed the transfer of the drug discovery and research activities, including our research colleagues in Romainville, France, to NovAliX. Next, after carefully evaluating the strategic options to maintain a sustainable commercial business model for Jyseleca®, our Jyseleca® dedicated teams, and the patients who benefit from the medicine, we signed a letter of intent with Alfasigma S.p.A. to transfer the entire Jyseleca® business to Alfasigma, including the European and UK Marketing Authorizations, as well as the commercial, medical affairs and development activities for Jyseleca® and approximately 400 Galapagos positions in 14 European countries. The transaction was completed at the end of January 2024. Finally, to further streamline our remaining operations and implement a lean organization focused on R&D growth areas, we announced and implemented a restructuring affecting 100 positions across our European sites. At the same time, we continue to strengthen critical strategic capabilities to achieve our ambitions in oncology. To ensure we were hearing our employee’s thoughts during this time of change, we conducted a company-wide survey to better understand the impact of these changes on our company’s values and culture. More than half of our employees participated in
the survey and we are committed to taking the feedback to further evolve our corporate culture and values in 2024. We are pleased to share the efforts we have taken to foster an inspiring and engaging workplace for our people. We strive to nurture a purpose-driven culture that values diversity, equality, transparency, trust, empowerment, and leadership. We continue to build an inclusive and entrepreneurial work environment, where people can be themselves, realize their full potential and grow in their career, feel recognized for their contributions, and perform to the best of their abilities, individually and together as one team. HIGHLIGHTS 2023 Diversity, equality, and inclusion As part of our Sustainability strategy, we kicked off a program to create more awareness for diversity, equality and inclusion within Galapagos. We are proud to share that, for the fourth year in a row, we are included in the 2023 Bloomberg Gender-Equality Index. This list encompasses 484 companies headquartered in 45 countries and regions. It is an objective measure that tracks gender equality across five pillars: leadership & talent pipeline, inclusive culture, anti-sexual harassment policies, external brand, and equal pay & gender pay parity. As part of our effort to foster equal pay and gender pay parity, we perform equity checks during our promotion and end-of-year review processes across genders to mitigate potential bias. In 2023, among other initiatives to promote the culture of inclusion across our organization, we held a plenary panel talk with Pips Bunce, inspirational advocate for Diversity, Equality and Inclusion. The widely attended session generated a fruitful discussion and exploration of how a more diverse and inclusive organization can lead to a greater success.
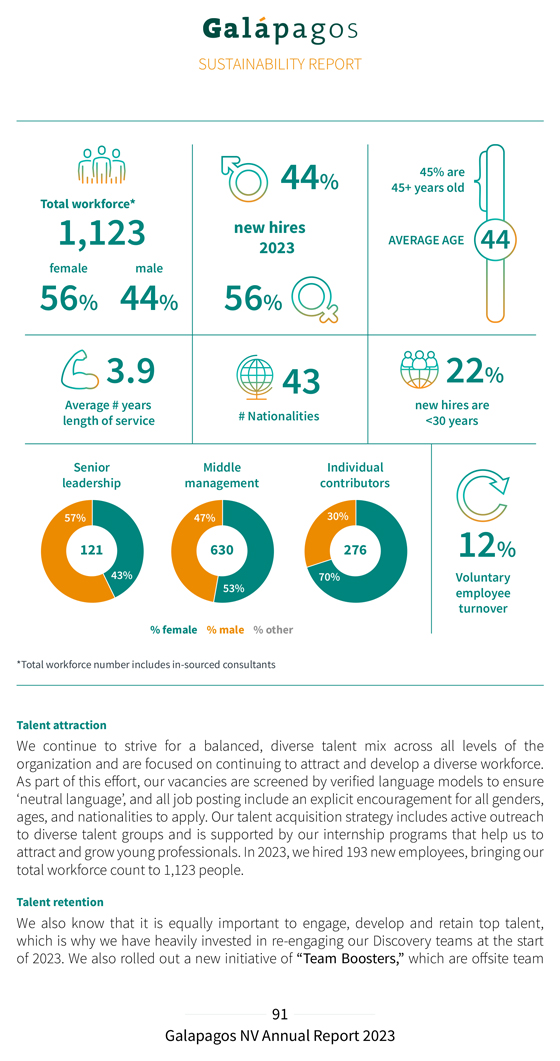
*Total workforce number includes in-sourced consultants Talent attraction We continue to strive for a balanced, diverse talent mix across all levels of the organization and are focused on continuing to attract and develop a diverse workforce. As part of this effort, our vacancies are screened by verified language models to ensure ‘neutral language’, and all job posting include an explicit encouragement for all genders, ages, and nationalities to apply. Our talent acquisition strategy includes active outreach to diverse talent groups and is supported by our internship programs that help us to attract and grow young professionals. In 2023, we hired 193 new employees, bringing our total workforce count to 1,123 people. Talent retention We also know that it is equally important to engage, develop and retain top talent, which is why we have heavily invested in re-engaging our Discovery teams at the start of 2023. We also rolled out a new initiative of “Team Boosters,” which are offsite team

development sessions for complete teams and their leader, with the objective to boost engagement and belonging, guiding all leadership and functional teams within the Discovery organization on a targeted development journey. Talent cycle is our annual cycle of specific conversations between managers and their direct reports on topics like objectives, feedback, development; this fosters an open dialogue between managers and employees. We hold regular “Hay! (How Are You) conversations” between managers and their team members to support ongoing feedback and discuss topics such as goal alignment, engagement, feedback, career, performance, and development. In addition to facilitating these individual conversations, we broadened the “Talent Talks” to the departmental level to focus on identifying personal development opportunities for employees. We believe that by proactively offering learning opportunities to all employees, we invest in employees’ career growth at Galapagos. In 2023, Galapagos employees and leaders attended more than 4,500 Galapagos learning and development events (and this number excludes Compliance training, conferences and on-the-job learning). Additionally, almost 50 managers spent in total over 2,500 hours on individual coaching conducted by an external consultant. Compensation Another critical element to retain employees is our competitive compensation offering, which is designed to recognize and reward employee performance in alignment with the company’s strategy and culture. We believe that performance bonuses and stock-based incentive opportunities help drive sustainable performance and stronger commitment to Galapagos, while appropriately rewarding employees for their contributions to our success. The benefits we offer vary from country to country, based on local standards and statutory requirements. In 2023, we enhanced our employee offerings at both the international and country level with the following changes: Expanded information about total rewards, including benefits offerings, available to employees on our intranet portal; Provided stock-based awards for our colleagues to foster ownership culture; Conducted extensive benchmarking to assess the competitiveness of our local benefits offerings; Improved various local benefits offerings ranging from additional time off and cash allowances to improved meal vouchers and new wellbeing programs; and Strengthened our commuter and transportation programs offerings to further incentivize environmentally sound transportation choices. Wellbeing We prioritize the health of our workforce as it is essential for both individual success and overall organizational prosperity. Our cross-functional initiative known as “Make It Happiness” brings together employee ambassadors who continuously design and improve our global wellbeing program. This project is centered around three key pillars:
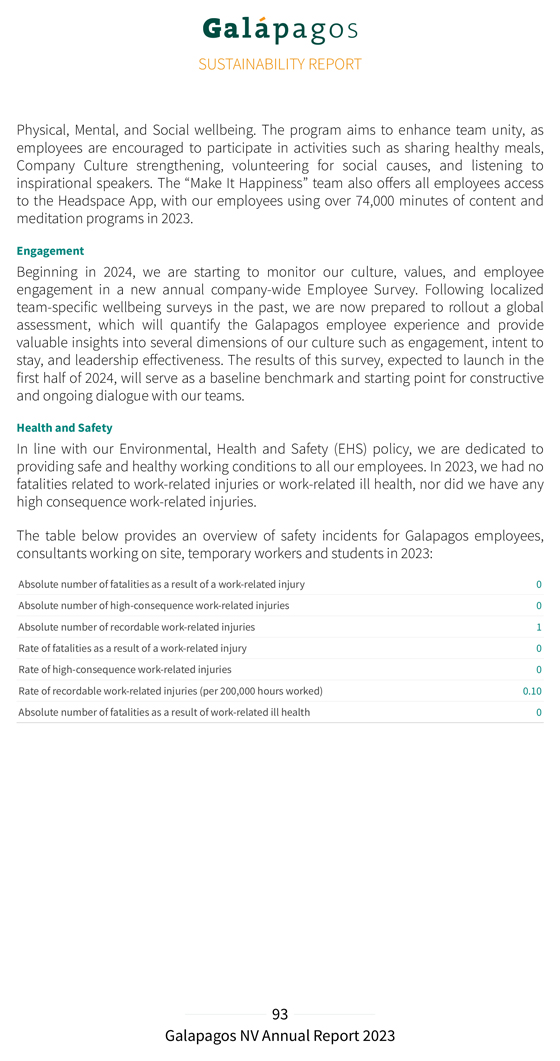
Physical, Mental, and Social wellbeing. The program aims to enhance team unity, as employees are encouraged to participate in activities such as sharing healthy meals, Company Culture strengthening, volunteering for social causes, and listening to inspirational speakers. The “Make It Happiness” team also offers all employees access to the Headspace App, with our employees using over 74,000 minutes of content and meditation programs in 2023. Engagement Beginning in 2024, we are starting to monitor our culture, values, and employee engagement in a new annual company-wide Employee Survey. Following localized team-specific wellbeing surveys in the past, we are now prepared to rollout a global assessment, which will quantify the Galapagos employee experience and provide valuable insights into several dimensions of our culture such as engagement, intent to stay, and leadership effectiveness. The results of this survey, expected to launch in the first half of 2024, will serve as a baseline benchmark and starting point for constructive and ongoing dialogue with our teams. Health and Safety In line with our Environmental, Health and Safety (EHS) policy, we are dedicated to providing safe and healthy working conditions to all our employees. In 2023, we had no fatalities related to work-related injuries or work-related ill health, nor did we have any high consequence work-related injuries. The table below provides an overview of safety incidents for Galapagos employees, consultants working on site, temporary workers and students in 2023: Absolute number of fatalities as a result of a work-related injury 0 Absolute number of high-consequence work-related injuries 0 Absolute number of recordable work-related injuries 1 Rate of fatalities as a result of a work-related injury 0 Rate of high-consequence work-related injuries 0 Rate of recordable work-related injuries (per 200,000 hours worked) 0.10 Absolute number of fatalities as a result of work-related ill health 0

Planet Our Commitment to the Planet We are conscientious. Our Commitment to the Health of Our Planet The planet health and the health and wellbeing of (our) people are interconnected. As climate change was specifically identified as a material topic to Galapagos, we set a clear aspiration to support our environmental ambitions and become climate neutral by 2028. We defined a 5-year roadmap to achieve this goal, applying a sound and realistic mix of carbon reduction and carbon compensation projects. In addition, we are embracing the circular economy by reducing waste and reusing or recycling materials where and when we can. As the reduction of green-house gas emissions is a crucial success factor in our approach, our reduction roadmap entails three pathways. Systematically replace any fossil fuels by renewable energy sources used in our buildings and car fleet; Improve energy efficiency of our operations; and Drive behavioral change by raising environmental awareness among our employees. Our Environmental, Health and Safety oversight group has developed and maintains an EHS management system based on the international ISO 140019 and ISO 4500110 standards to ensure that our approach is planned, consistent, transparent, compliant and measurable. Actions 2023 To detail our path towards being climate neutral, we took the following actions in 2023: 9 International Standard Organization 14001: Environmental management systems (EMS) 10 International Standard Organization 45001: Occupational health and safety (OH&S) management

We quantified the carbon footprint of Galapagos’ value chain (including Scope 111, 212 and 313 CO e emissions), in accordance with the Green House Gas Protocol (see Appendix table) 2 We defined and quantified energy mix and energy consumption related to our Scope 1 and Scope 2 CO2e emissions (see Appendix table). Energy generated from renewable sources currently covers 25% of our total energy needs. Progressed on our three pathways to reducing greenhouse gas emissions as defined in multi-year reduction roadmap : Systematically replacing any fossil fuels by renewable energy sources: in 2023 we launched a new mobility strategy aimed at accelerating the electrification of our car fleet and offering alternative transportation modes. In Belgium, home of our biggest company car fleet, we piloted this effort through the implementation of a new requirement that all new fleet vehicles must be fully electric as of 1 May 2024. Improving energy efficiency of our operations. we defined our expectations for BREEAM14 and WELL15 performance levels, for future consideration in selection criteria for new Galapagos facilities, an effort to improve our energy efficiency performance. in 2023, 23% of the heated surface used by Galapagos was BREEAM or WELL certified. Driving behavioral change and raising environmental awareness among our staff: we continued the work of the Green Teams in our research sites. These teams of volunteers identify opportunities to reduce Galapagos’ footprint in our day-to-day operations. we celebrated the United Nations’ World Environment Day on June 5th, by organizing site-specific activities such as a bikers’ lunch, bike repair and waste recycling workshops. We also organized a global webinar featuring Bertrand Piccard, who shared his experience as a psychiatrist, green pioneer, and founder of the Solar Impulse Foundation. We completed a Life Cycle Assessment for Jyseleca®, defining the environmental footprint of one year of treatment. As indicated by our ambitious goal of being climate neutral by 2028, we are committed to doing our part to support a healthy planet and will continue to monitor our performance to ensure we remain on track on our five-year roadmap. 11 Direct GHG (Gases that contribute to the greenhouse effect by absorbing infrared radiation) emissions resulting from sources that are 12 owned or controlled by an organization. Energy indirect GHG emissions that result from the generation of purchased or acquired electricity, heating, cooling, and steam 13 consumed by an organization. Other indirect GHG emissions not included in Scope 2 GHG emissions, that occur outside of the organization, including both upstream 14 and downstream emissions. BREEAM—Building Research Establishment Environmental Assessment Methodology is a sustainability assessment for master planning projects, infrastructure, and buildings. It recognizes and reflects the value in higher performing assets across the built environment 15 lifecycle, from new construction to in-use and refurbishment. The WELL Building Standard takes a holistic approach to health in the built environment addressing behavior, operations, and design, and is a performance-based system for measuring, certifying, and monitoring features of the built environment that impact human health and well-being, through air, water, nourishment, light, fitness, comfort and mind.

Trust & transparency Our Commitment to Trust & Transparency We are transparent. Our commitment to Trust & Transparency Doing business ethically is about being a responsible corporate citizen. The standards we apply and decisions that we make every day are thoughtful and work to ensure that we act in the best interest of patients, people, and the planet. We build trust with our stakeholders by setting measurable goals, communicating them clearly, and being open and transparent about the progress we are making to deliver on them – both when we are doing well and when we need to make improvements. We prioritize ethical management of our supply chain, vendors, and partners. Just as we seek partners and suppliers who share our commitment to the planet, we also ensure that they share our commitment to quality and ethical business practices. We refined our third-party onboarding through an enhanced risk assessment framework and due diligence on quality, IT security, data protection and privacy, compliance and ethics, and environment. Additionally, we continuously evaluate our supply chain to ensure continuity and optimization of costs, and we provide a consistent framework for partners and employees that outlines clear and comprehensive guidance for ethical and transparent behavior expectations across our company. To ascertain that our products meet the highest quality standards, we work with qualified and certified (GMP-licensed) distributors that ensure that all processes related to receipt, storage, handling and final distribution to customers comply with the regulations. We regularly audit our GxP manufacturers and distributors. We work to protect our people, patients, our planet, and our business by taking every reasonable measure to ensure that we all operate in accordance with the applicable regulations and standards and maintain compliance with all applicable laws. We nurture a speak-up culture that encourages every one of our employees to share ideas, while also supporting our managers and leaders to embody a “Listen Up” culture. We implemented a “Speak Up” web-based reporting system to enable employees and

third parties to raise any concerns regarding activities related to Galapagos. The reporting mechanism meets the requirements of the EU Whistleblowing directive and enables anonymous reporting when allowable. Individuals who wish to report a concern can choose from the local language that works best for them and can submit their report anonymously. We believe that this helps our employees and third parties to feel heard and also protected, while alerting Galapagos about any potential issues and enabling early corrective action as needed. All matters are fully reviewed and investigated as needed in accordance with our internal procedure which is managed by our Global Head of Compliance & Ethics. This framework is overseen by a Speak Up, Listen Up Committee, members of which are heads of Legal, HR, Internal Control and Compliance & Ethics, and, in the event of serious or material matters, escalated to the Chair of the Audit Committee. We also nurture a culture of integrity, with the aim that our employees, partners and suppliers value and take accountability for upholding our standards. We operate in an environment where the safety of patients is paramount. Until the transfer of the Marketing Authorisation for Jyseleca® to Alfasigma, we are responsible for the marketing of one medicine, Jyseleca®, in Europe. We implemented a pharmacovigilance system designed to monitor the safety of Jyseleca® and to detect any change to the benefit/risk profile. Animal welfare From a scientific perspective, it is not yet possible to examine all the complex interactions that a potential treatment triggers in a living organism without animal testing. Additionally, the regulatory and legal framework for drug development requires new medicines to be evaluated in animals to ensure the quality, safety, and efficacy of these product candidates. We continue to implement the 3Rs (Replacement, Reduction, and Refinement) principles as set out in our Animal Welfare Policy. Animal welfare is reinforced through several key actions at the vendor level, including a surveillance process of incidents and their remediation and a communication plan on preemptive and reactive measures taken in animal studies. Monitoring compliance of our vendors with Galapagos standards and animal welfare guidelines is part of the Animal Welfare Community mission. Although Galapagos uses vendors with state-of-the-art approaches, incidents might still happen occasionally. In such instances, we actively support investigation of the root cause, propose effective remediation solutions and diligently follow-up on any corrective actions. 100% of our vendors adhere to our Animal Welfare Policy. In 2023, we identified 3 incidents with regard to animal welfare, in our own organization and also in one single vendor facility. We investigated the identified incidents and took appropriate measures to remediate them. All incidents were adequately handled. On-site visits will be conducted to verify the effectiveness of the implemented actions in 2024. Monitoring of the ongoing remediation actions is planned.

Actions 2023 We launched our Speak up, Listen Up program in 2022, continued the roll out in 2023, and we aim to make this integral to our routine new employee onboarding in 2024. As encouraged by our Speak Up, Listen Up program, we received a number of reports for potential non-compliance. One breach of our Code of Conduct was escalated to the Audit Committee in 2023. Appropriate measures were taken to address this breach. We launched an anti-harassment and anti-discrimination policy in 2023, and further communication and training on this topic is planned for 2024 94% of our employees completed our Code of Conduct training Since late 2022, the Third-Party Risk Assessment (TPRA) process for the onboarding of new vendors is mandatory, and going forward, we aim to use the TPRA as a KPI to measure compliance and ethics in our supply chain We executed an external screening of our 100 preferred suppliers. We are currently checking different risks factors, including ESG indicators; and The animal welfare remediation plan for addressing the identified gaps was completed in 2023. We implemented more than 14 major 3R initiatives, and 100% of our trusted suppliers adhere to our Animal Welfare Policy, reinforcing our expectations when working with third parties. The communication and crisis management plan were released, and the Animal Welfare webpage was created for increasing employee’s awareness. Multiple and regular communication on the 3Rs principles, as well as a participation in a major international initiative, were associated with a decrease in the number of animals used in 2023. Close follow-up on animal welfare incidents, with issue management as a full part of the study oversight, supported fast resolution of the incidents. All KPIs were met at the end of the year, indicating that our ethical values were understood, internally and externally.

Reporting Reporting framework We are preparing detailed reporting on our material aspects according to the Corporate Sustainability Reporting Directive (CSRD) by setting up a dedicated reporting team, evaluating policies and procedures, and defining scope, targets, metrics and action plans as needed. Additionally, we are evaluating reporting systems to ensure a robust data collection process. We expect to report in line with EU Sustainability Reporting Standards (ESRS) for the 2024 fiscal year. The current sustainability report provides the non-financial information required by articles 3:6 § 4 and 3:32 § 2 of the Belgian Companies Code. For a discussion on risks, please see the section on Risk factors in the Annual Report. To standardize our 2023 data collection, we use the United Nations Sustainable Development Goals (SDGs), also known as the Global Goals, as our reference framework to link our material aspects to areas of engagement. The SDGs were adopted by all United Nations Member States in 2015 as a universal call to action to end poverty, protect the planet, and strive to ensure that all people enjoy peace and prosperity by 2030.

Advancing UN SDGs In 2023, we signed up for the Ten Principles of the United Nations Global Compact in the areas of Human Rights, Labour, Environment, and Anti-Corruption. In the annual Communication on Progress, which can be found on Galapagos’ participation profile on the UN Global Compact website, we disclose our continuous efforts to integrate the Ten Principles into our business strategy, culture, and daily operations, and contribute to United Nations goals, particularly the Sustainable Development Goals (SDG). We identified two core SDG goals where we believe we can make a difference, as well as six enabling SDG goals. Together they will help us to execute on our commitment to our four Sustainability pillars. The table below links our material aspects and engagement areas to select components of the SDG framework: CORE SDG Good health and well-being More years of life and quality of life, by transforming patient outcomes through accelerating life changing science and innovation, are at the core of what we do. Partnerships for the goals We embrace internal and external partnerships to work towards our mission to bringing much needed innovation to patients.

SUSTAINABILITY REPORT ENABLING SDG Quality education We invest in our employees and foster an inclusive, open and supportive work environment across our locations in Europe and the U.S. Gender equality We cultivate a corporate culture where we strive for gender equality. Decent work and economic growth We are a global biotechnology company with operations in Europe and the US. Industry, innovation and infrastructure Our mission is to accelerate transformational innovation through the relentless pursuit of groundbreaking science, our entrepreneurial spirit, and a collaborative mindset. Reduced inequalities We aim to develop a balanced workforce across a number of criteria, including gender, nationality, ethnicity, experience and disability. Climate action We value our planet and take initiatives to safeguard the environment and incorporate greener practices across our organization. 101 Galapagos NV Annual Report 2023

SUSTAINABILITY REPORT Reporting on EU Taxonomy EU Taxonomy 2023 statement The European Commission’s action plan on financing sustainable growth led to the creation of an EU classification system for sustainable activities, also known as the EU taxonomy. As a listed company with more than 500 employees, Galapagos is in scope of the EU Taxonomy Regulation16. As indicated in the Delegated Regulation of (EU) 2021/ 2178, non-financial undertakings shall disclose the proportion of Taxonomy-eligible and alignment of economic activities in their total turnover, capital expenditure (“CapEx”), operational expenditure (“OpEx”) and the qualitative information starting from reporting year 2022, including comparative figures for eligibility related to climate change mitigation and adaptation. Starting in reporting year 2023, the proportion of Taxonomy eligibility shall be disclosed for all remaining objectives. The EU Taxonomy introduces a classification system for environmentally sustainable activities, and an activity is deemed environmentally sustainable if it meets all of the following overarching criteria: substantially contributing to at least one of the six environmental objectives of the EU Taxonomy Regulation: (i) climate change mitigation; (ii) climate change adaptation; (iii) sustainable use and protection of water and marine resources; (iv) transition to a circular economy, (v) pollution prevention and control; and (vi) protection and restoration of biodiversity and ecosystems; not significantly harming any of these environmental objectives; complying with minimum safeguards; and complying with certain scientifically based technical screening criteria (‘TSCs’) established by the EU Commission. The EU published a catalog of economic activities that can be considered as Taxonomy-eligible activities; the determination of eligibility happens on the basis of the description of activities. An eligible activity becomes Taxonomy-aligned when it meets all of the aforementioned overarching criteria, which includes that such activity should substantially contribute to at least one of the six environmental objectives. Following a thorough analysis of the EU Taxonomy legal framework17, which was initiated by reviewing the company’s NACE codes in light of the EU Taxonomy identified activities, we do not consider our core business activities of discovering, developing and commercializing innovative medicines to be in scope of the Climate Delegated Act. Additionally, the newly added EU Taxonomy activities were screened (such as manufacturing of medicinal products), but not considered as within our control as Jyseleca® is manufactured by a third party. 16 Commission Delegated Regulation (EU) 2023/2485 of 27 June 2023 amending Delegated Regulation (EU) 2021/2139 establishing additional technical screening criteria for determining the conditions under which certain economic activities qualify as contributing substantially to climate change mitigation or climate change adaptation and for determining whether those activities cause no 17 significant harm to any of the other environmental objectives. Commission Delegated Regulation (EU) 2023/2486 of 27 June 2023 supplementing Regulation (EU) 2020/852 of the European Parliament and of the Council by establishing the technical screening criteria for determining the conditions under which an economic activity qualifies as contributing substantially to the sustainable use and protection of water and marine resources, to the transition to a circular economy, to pollution prevention and control, or to the protection and restoration of biodiversity and ecosystems and for determining whether that economic activity causes no significant harm to any of the other environmental objectives and amending Commission Delegated Regulation (EU) 2021/2178 as regards specific public disclosures for those economic activities. 102 Galapagos NV Annual Report 2023
SUSTAINABILITY REPORT Within the context of our ambition to become climate neutral by 2028, we screened the related activities and identified the following activities included in the EU Taxonomy: Acquisition and ownership of buildings Consultancy for physical climate risk management and adaptation Installation and operation of electric heat pumps Transport by motorbike, passenger cars and light commercial vehicles For the determination of turnover, CapEx and OpEx during this analysis, we use the reported data in the 2023 consolidated financial statements included in this report: Turnover covers all continuing activities of Galapagos as of 31 December 2023 and the denominator can be reconciled with the 2023 IFRS total net revenues of €239.7 million as disclosed in note 7, being the revenues collaboration activities. CapEx consists of additions to tangible and intangible assets during the financial year 2023 considered before depreciation, amortization and any re-measurements recognized by Galapagos pursuant to IAS 38. The denominator (total CapEx) can be reconciled with the sum of the lines “Additions” disclosed in notes 14 and 15 (total €20.8 million) of the consolidated financial statements. The majority of CapEx is associated with software and databases, and property, plant and equipment (covering fully-owned and leased). OpEx, according to the EU Taxonomy, is determined by the direct non-capitalized costs of research and development, building renovation measures, short-term leases, maintenance and repair and any other direct expenditures relating to the day-to-day servicing of assets of property, plant and equipment by the undertaking or third-party outsources that are necessary to ensure the continued and effective functioning of such assets. These costs are for the majority associated with our R&D expenditure, as disclosed in note 8 (total €375.3 million). Based on available data and the assessment of requirements, we report 0% Taxonomy eligible Turnover, and therefore 0% Taxonomy aligned. As a result of our climate neutral ambition by 2028 and the related investments we report 13.59% Taxonomy eligible CapEx, with 2.77% Taxonomy aligned, and 0.06% Taxonomy eligible and aligned OpEx (as presented in the tables in Appendix). Please refer to the Appendix to this Annual Report for the disclosure on KPIs of non-financial undertakings as required by Annexes II of the Climate Delegated Act. The limited “eligibility” under the EU Taxonomy refers to the fact that our core activities currently remain outside of the scope of the economic activities for which TSCs have been developed under the Delegated Regulations. We note that the required disclosures under the EU Taxonomy Regulation will keep evolving and that we will continue to consider its impact as well as future reporting obligations. 103 Galapagos NV Annual Report 2023
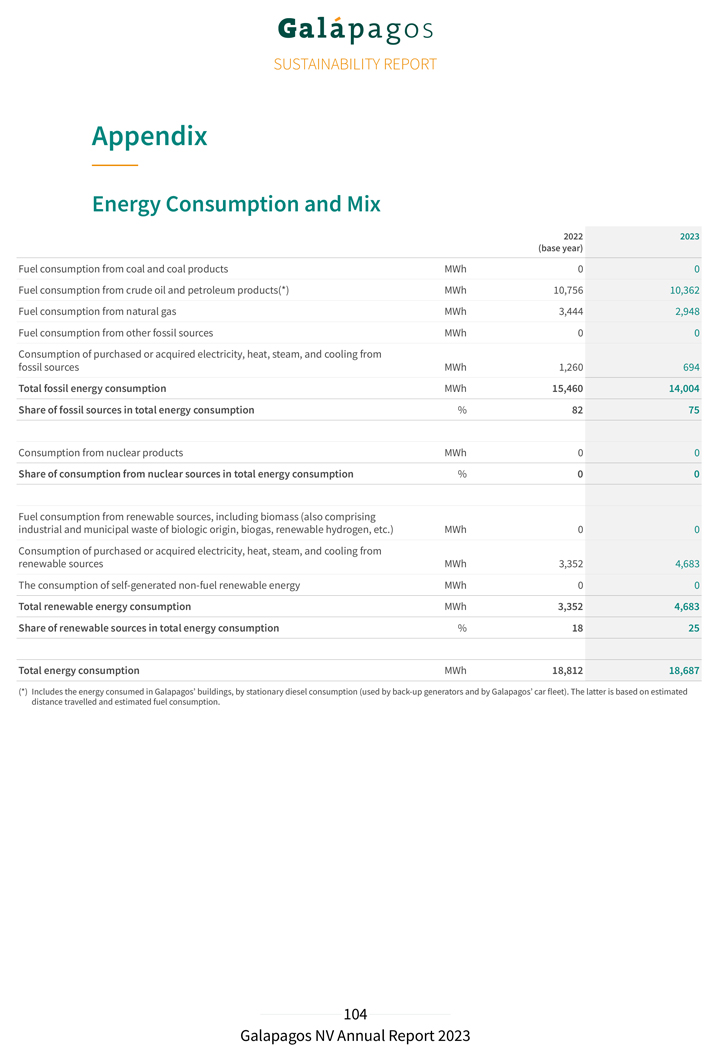
SUSTAINABILITY REPORT Appendix Energy Consumption and Mix 2022 2023 (base year) Fuel consumption from coal and coal products MWh 0 0 Fuel consumption from crude oil and petroleum products(*) MWh 10,756 10,362 Fuel consumption from natural gas MWh 3,444 2,948 Fuel consumption from other fossil sources MWh 0 0 Consumption of purchased or acquired electricity, heat, steam, and cooling from fossil sources MWh 1,260 694 Total fossil energy consumption MWh 15,460 14,004 Share of fossil sources in total energy consumption % 82 75 Consumption from nuclear products MWh 0 0 Share of consumption from nuclear sources in total energy consumption % 0 0 Fuel consumption from renewable sources, including biomass (also comprising industrial and municipal waste of biologic origin, biogas, renewable hydrogen, etc.) MWh 0 0 Consumption of purchased or acquired electricity, heat, steam, and cooling from renewable sources MWh 3,352 4,683 The consumption of self-generated non-fuel renewable energy MWh 0 0 Total renewable energy consumption MWh 3,352 4,683 Share of renewable sources in total energy consumption % 18 25 Total energy consumption MWh 18,812 18,687 (*) Includes the energy consumed in Galapagos’ buildings, by stationary diesel consumption (used by back-up generators and by Galapagos’ car fleet). The latter is based on estimated distance travelled and estimated fuel consumption. 104 Galapagos NV Annual Report 2023
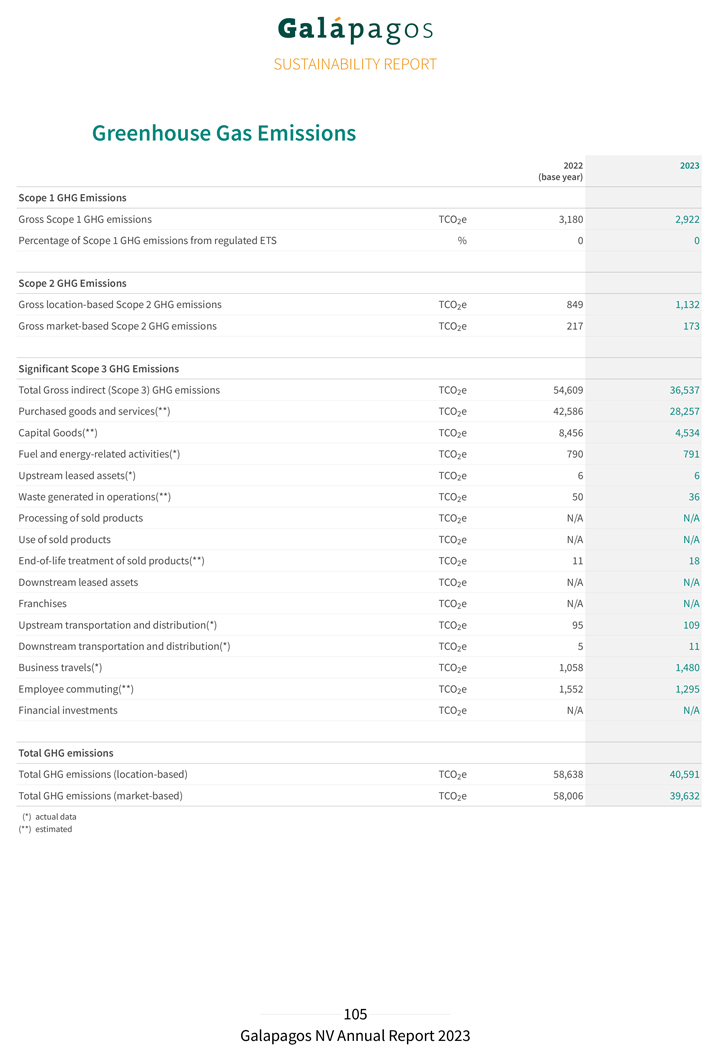
SUSTAINABILITY REPORT Greenhouse Gas Emissions 2022 2023 (base year) Scope 1 GHG Emissions Gross Scope 1 GHG emissions TCO2e 3,180 2,922 Percentage of Scope 1 GHG emissions from regulated ETS % 0 0 Scope 2 GHG Emissions Gross location-based Scope 2 GHG emissions TCO2e 849 1,132 Gross market-based Scope 2 GHG emissions TCO2e 217 173 Significant Scope 3 GHG Emissions Total Gross indirect (Scope 3) GHG emissions TCO2e 54,609 36,537 Purchased goods and services(**) TCO2e 42,586 28,257 Capital Goods(**) TCO2e 8,456 4,534 Fuel and energy-related activities(*) TCO2e 790 791 Upstream leased assets(*) TCO2e 6 6 Waste generated in operations(**) TCO2e 50 36 Processing of sold products TCO2e N/A N/A Use of sold products TCO2e N/A N/A End-of-life treatment of sold products(**) TCO2e 11 18 Downstream leased assets TCO2e N/A N/A Franchises TCO2e N/A N/A Upstream transportation and distribution(*) TCO2e 95 109 Downstream transportation and distribution(*) TCO2e 5 11 Business travels(*) TCO2e 1,058 1,480 Employee commuting(**) TCO2e 1,552 1,295 Financial investments TCO2e N/A N/A Total GHG emissions Total GHG emissions (location-based) TCO2e 58,638 40,591 Total GHG emissions (market-based) TCO2e 58,006 39,632 (*) actual data (**) estimated 105 Galapagos NV Annual Report 2023
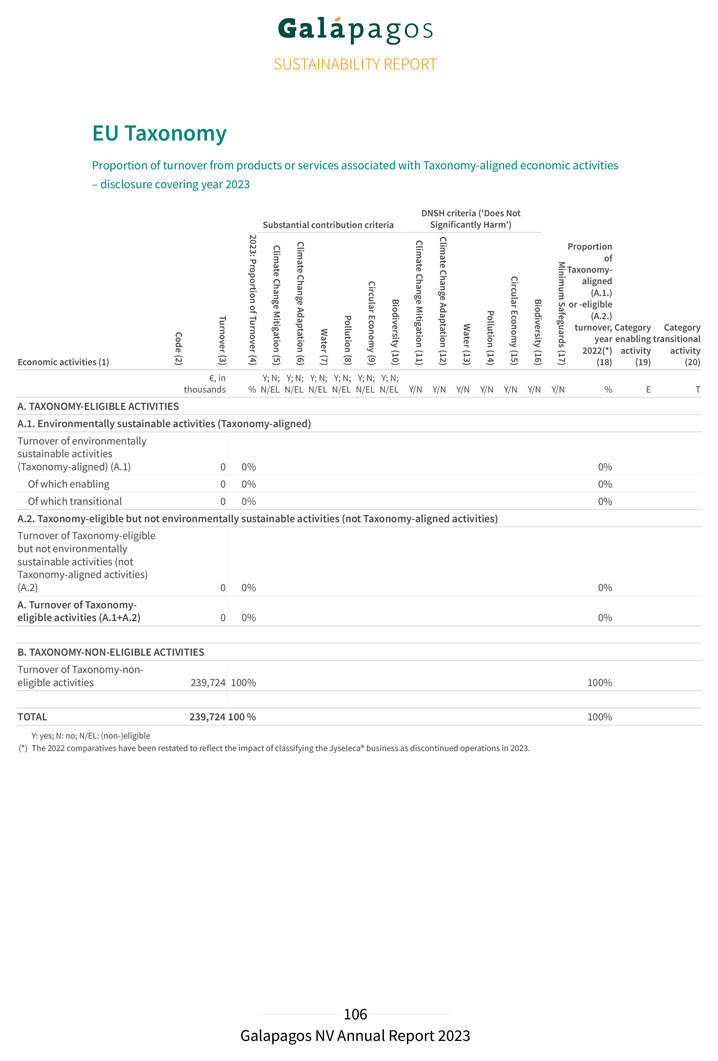
SUSTAINABILITY REPORT EU Taxonomy Proportion of turnover from products or services associated with Taxonomy-aligned economic activities – disclosure covering year 2023 DNSH criteria (‘Does Not Substantial contribution criteria Significantly Harm’) 2023: Proportion Climate of Climate Climate Taxonomy-Climate aligned Change Minimum (A.1.) Proportion Change ChangeChange Circular or -eligible of Circular (A.2.) turnover, Category Category Biodiversity Mitigation Adaptation Water Pollution Economy Biodiversity Safeguards year enabling transitional Code Turnover Turnover Mitigation Adaptation Water Pollution Economy 2022(*) activity activity Economic activities (1) (2) (3) (4) (5) (6) (7) (8) (9) (10) (11) (12) (13) (14) (15) (16) (17) (18) (19) (20) €, in Y; N; Y; N; Y; N; Y; N; Y; N; Y; N; thousands % N/EL N/EL N/EL N/EL N/EL N/EL Y/N Y/N Y/N Y/N Y/N Y/N Y/N % E T A. TAXONOMY-ELIGIBLE ACTIVITIES A.1. Environmentally sustainable activities (Taxonomy-aligned) Turnover of environmentally sustainable activities (Taxonomy-aligned) (A.1) 0 0% 0% Of which enabling 0 0% 0% Of which transitional 0 0% 0% A.2. Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) Turnover of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) 0 0% 0% A. Turnover of Taxonomy-eligible activities (A.1+A.2) 0 0% 0% B. TAXONOMY-NON-ELIGIBLE ACTIVITIES Turnover of Taxonomy-non-eligible activities 239,724 100% 100% TOTAL 239,724 100 % 100% Y: yes; N: no; N/EL: (non-)eligible (*) The 2022 comparatives have been restated to reflect the impact of classifying the Jyseleca® business as discontinued operations in 2023. 106 Galapagos NV Annual Report 2023
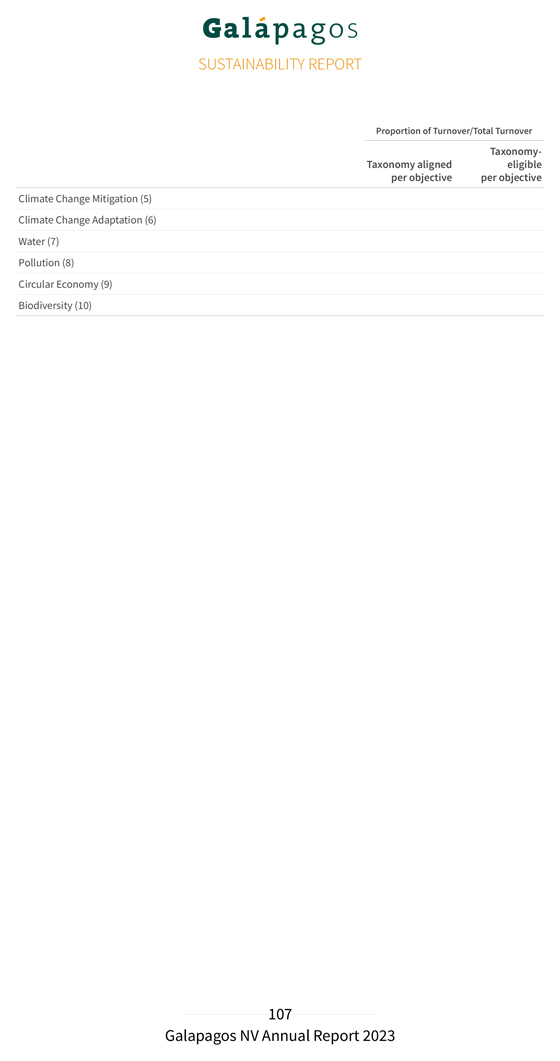
SUSTAINABILITY REPORT Proportion of Turnover/Total Turnover Taxonomy-Taxonomy aligned eligible per objective per objective Climate Change Mitigation (5) Climate Change Adaptation (6) Water (7) Pollution (8) Circular Economy (9) Biodiversity (10) 107 Galapagos NV Annual Report 2023
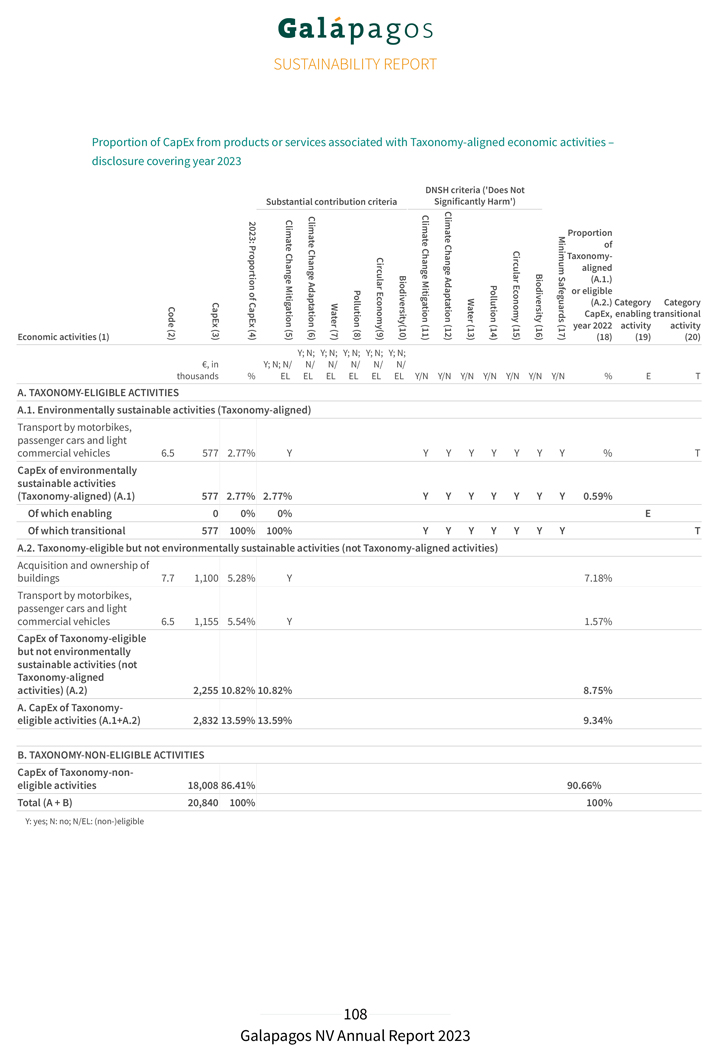
SUSTAINABILITY REPORT Proportion of CapEx from products or services associated with Taxonomy-aligned economic activities –disclosure covering year 2023 DNSH criteria (‘Does Not Substantial contribution criteria Significantly Harm’) 2023: Climate Proportion Climate Climate of Climate Taxonomy-Change Minimum aligned Change Change Change Circular (A.1.) Proportion Circular of or eligible (A.2.) Category Category Mitigation Adaptation Water Pollution Economy Biodiversity Safeguards CapEx, enabling transitional Code CapEx CapEx Mitigation Adaptation Water Pollution year 2022 activity activity Economic activities (1) (2) (3) (4) (5) (6) (7) (8) Economy(9) Biodiversity(10) (11) (12) (13) (14) (15) (16) (17) (18) (19) (20) Y; N; Y; N; Y; N; Y; N; Y; N; €, in Y; N; N/ N/ N/ N/ N/ N/ thousands % EL EL EL EL EL EL Y/N Y/N Y/N Y/N Y/N Y/N Y/N % E T A. TAXONOMY-ELIGIBLE ACTIVITIES A.1. Environmentally sustainable activities (Taxonomy-aligned) Transport by motorbikes, passenger cars and light commercial vehicles 6.5 577 2.77% Y Y Y Y Y Y Y Y % T CapEx of environmentally sustainable activities (Taxonomy-aligned) (A.1) 577 2.77% 2.77% Y Y Y Y Y Y Y 0.59% Of which enabling 0 0% 0% E Of which transitional 577 100% 100% Y Y Y Y Y Y Y T A.2. Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) Acquisition and ownership of buildings 7.7 1,100 5.28% Y 7.18% Transport by motorbikes, passenger cars and light commercial vehicles 6.5 1,155 5.54% Y 1.57% CapEx of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) 2,255 10.82% 10.82% 8.75% A. CapEx of Taxonomy-eligible activities (A.1+A.2) 2,832 13.59% 13.59% 9.34% B. TAXONOMY-NON-ELIGIBLE ACTIVITIES CapEx of Taxonomy-non-eligible activities 18,008 86.41% 90.66% Total (A + B) 20,840 100% 100% Y: yes; N: no; N/EL: (non-)eligible 108 Galapagos NV Annual Report 2023
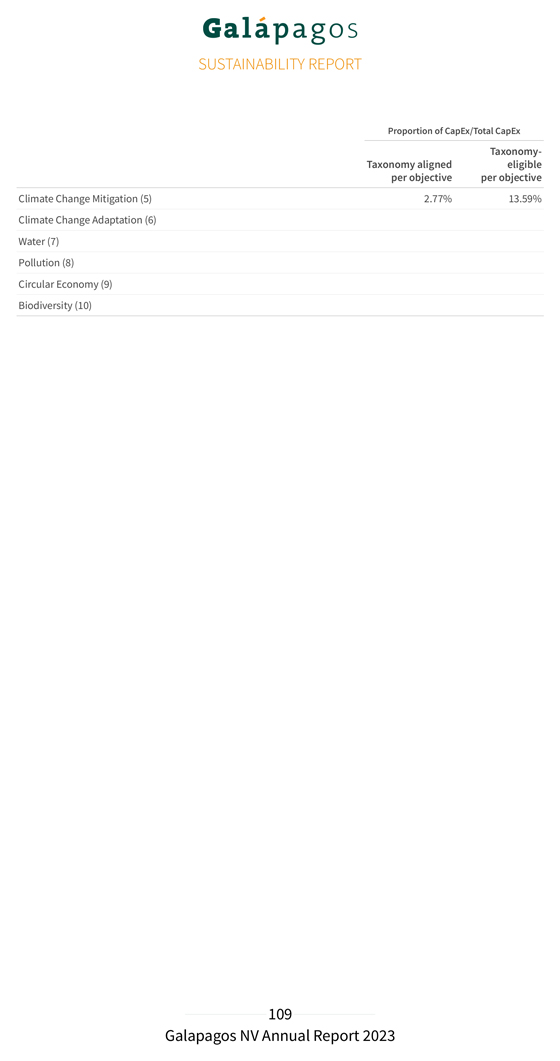
SUSTAINABILITY REPORT Proportion of CapEx/Total CapEx Taxonomy-Taxonomy aligned eligible per objective per objective Climate Change Mitigation (5) 2.77% 13.59% Climate Change Adaptation (6) Water (7) Pollution (8) Circular Economy (9) Biodiversity (10) 109 Galapagos NV Annual Report 2023
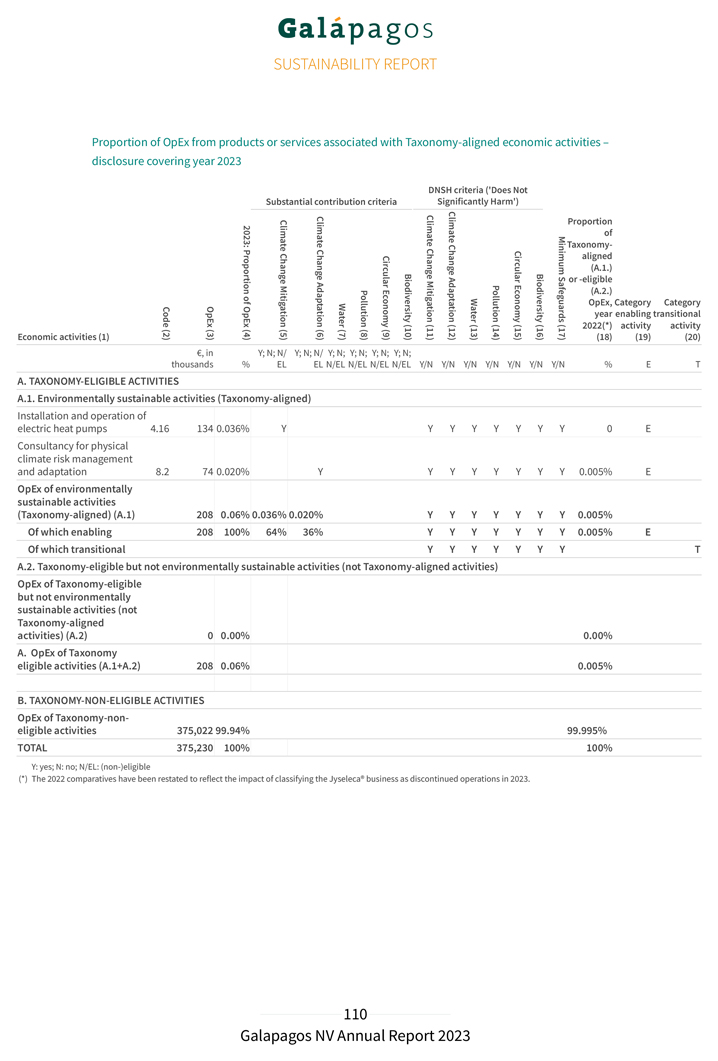
Proportion of OpEx from products or services associated with Taxonomy-aligned economic activities –disclosure covering year 2023 DNSH criteria (‘Does Not Substantial contribution criteria Significantly Harm’) Proportion Climate of 2023: Climate Climate Taxonomy- Climate aligned Change Minimum (A.1.) Change Change Change Circular or -eligible Circular Proportion (A.2.) of OpEx, Category Category Biodiversity Mitigation Adaptation Water Pollution Economy Biodiversity Safeguards year enabling transitional Code OpEx OpEx Mitigation Adaptation Water Pollution Economy 2022(*) activity activity Economic activities (1) (2) (3) (4) (5) (6) (7) (8) (9) (10) (11) (12) (13) (14) (15) (16) (17) (18) (19) (20) €, in Y; N; N/ Y; N; N/ Y; N; Y; N; Y; N; Y; N; thousands % EL EL N/EL N/EL N/EL N/EL Y/N Y/N Y/N Y/N Y/N Y/N Y/N % E T A. TAXONOMY-ELIGIBLE ACTIVITIES A.1. Environmentally sustainable activities (Taxonomy-aligned) Installation and operation of electric heat pumps 4.16 134 0.036% Y Y Y Y Y Y Y Y 0 E Consultancy for physical climate risk management and adaptation 8.2 74 0.020% Y Y Y Y Y Y Y Y 0.005% E OpEx of environmentally sustainable activities (Taxonomy-aligned) (A.1) 208 0.06% 0.036% 0.020% Y Y Y Y Y Y Y 0.005% Of which enabling 208 100% 64% 36% Y Y Y Y Y Y Y 0.005% E Of which transitional Y Y Y Y Y Y Y T A.2. Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) OpEx of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) 0 0.00% 0.00% A. OpEx of Taxonomy eligible activities (A.1+A.2) 208 0.06% 0.005% B. TAXONOMY-NON-ELIGIBLE ACTIVITIES OpEx of Taxonomy-non-eligible activities 375,022 99.94% 99.995% TOTAL 375,230 100% 100% Y: yes; N: no; N/EL: (non-)eligible (*) The 2022 comparatives have been restated to reflect the impact of classifying the Jyseleca® business as discontinued operations in 2023. 110 Galapagos NV Annual Report 2023
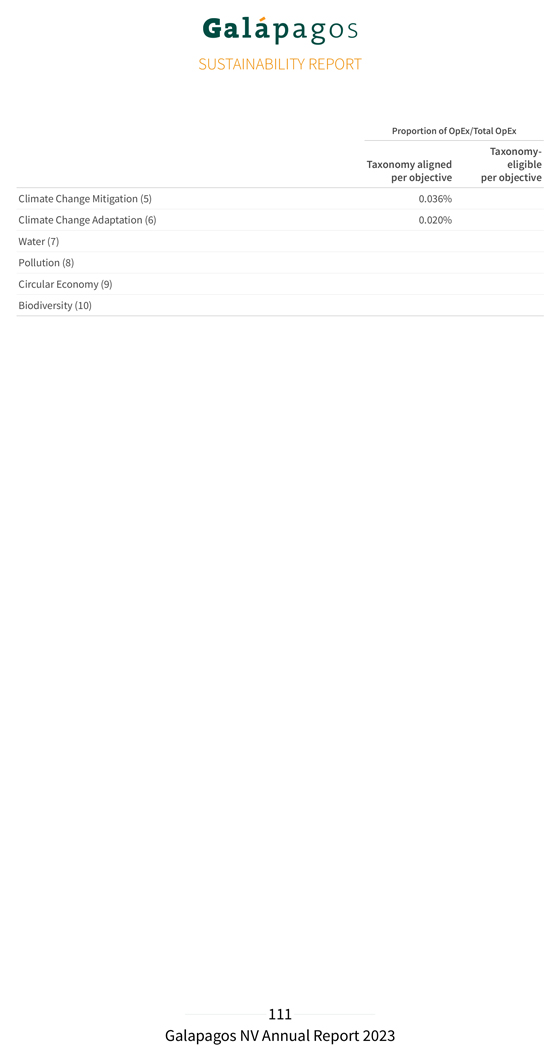
SUSTAINABILITY REPORT Proportion of OpEx/Total OpEx Taxonomy-Taxonomy aligned eligible per objective per objective Climate Change Mitigation (5) 0.036% Climate Change Adaptation (6) 0.020% Water (7) Pollution (8) Circular Economy (9) Biodiversity (10) 111 Galapagos NV Annual Report 2023

SUSTAINABILITY REPORT Nuclear and fossil gas related activities Row Nuclear energy related activities The undertaking carries out, funds or has exposures to research, development, demonstration and deployment 1. of innovative electricity generation facilities that produce energy from nuclear processes with minimal waste from the fuel cycle. NO The undertaking carries out, funds or has exposures to construction and safe operation of new nuclear 2. installations to produce electricity or process heat, including for the purposes of district heating or industrial processes such as hydrogen production, as well as their safety upgrades, using best available technologies. NO The undertaking carries out, funds or has exposures to safe operation of existing nuclear installations that 3. produce electricity or process heat, including for the purposes of district heating or industrial processes such as hydrogen production from nuclear energy, as well as their safety upgrades. NO Fossil gas related activities The undertaking carries out, funds or has exposures to construction or operation of electricity generation 4. facilities that produce electricity using fossil gaseous fuels. NO The undertaking carries out, funds or has exposures to construction, refurbishment, and operation of combined 5. heat/cool and power generation facilities using fossil gaseous fuels. NO The undertaking carries out, funds or has exposures to construction, refurbishment and operation of heat 6. generation facilities that produce heat/cool using fossil gaseous fuels. NO 112 Galapagos NV Annual Report 2023

Corporate governance Our governance in 2023 Pioneering science to transform patient outcomes

CORPORATE GOVERNANCE Galapagos’ corporate governance policies As a listed company with its registered office in Mechelen (Belgium), Galapagos NV (hereinafter “Galapagos NV” or the “Company”) is required to apply the Belgian Code of Companies and Associations (the “Belgian Companies Code”) and the 2020 Belgian Corporate Governance Code (the “2020 Code”), both of which entered into force on 1 January 2020 and as amended from time to time. For the reporting year beginning on 1 January 2023, the 2020 Code was our reference code. On 21 March 2023, as a consequence of the establishment of the Management Committee, i.e., an informal committee providing advice and assistance to the Executive Committee, the Board of Directors approved an amendment to the Company’s Corporate Governance Charter. On 19 September 2023, the Board of Directors approved another amendment that refers to the establishment of the Science and Development Committee as a specialized Board Committee to provide advice on certain matters to the Board of Directors, and that provides that non-executive Directors may only be natural persons. On 11 December 2023, the Board of Directors approved a further amendment to describe the responsibilities of the Audit Committee and management for overseeing and managing cybersecurity risks. Galapagos NV’s Corporate Governance Charter is available on our website (www.glpg.com). This Corporate Governance Charter applies in addition to the applicable laws and regulations (including, without limitation, the Belgian Companies Code and the 2020 Code) and Galapagos NV’s Articles of Association. The Company’s Corporate Governance Charter describes the main aspects of corporate governance at Galapagos NV, including its governance structure, the terms and functioning of the Board of Directors (including its Board Committees), the Executive Committee and the rules of conduct. For the reporting year beginning on 1 January 2023, the Board of Directors strove to comply with the rules and recommendations of the 2020 Code. At the same time, the Board of Directors is of the opinion that certain deviations from the rules and recommendations of the 2020 Code were justified, in view of our activities, our size, and the specific circumstances in which we operate. In such cases, which are mentioned in this corporate governance statement, we apply the “comply or explain” principle as set forth in the 2020 Code. Reference is made to the About the Board of Directors and Nomination Committee sections below. 114 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Our governance structure The 2020 Code requires companies to make an explicit choice for one of the governance structures provided for in the Belgian Companies Code. Since 26 April 2022, Galapagos NV has adopted a one-tier governance structure as provided by the Belgian Companies Code, with the Board of Directors as the ultimate decision-making body, who has delegated certain powers to manage the Company to the Executive Committee. One-tier governance structure Board of Directors executive and non-executive directors Delegation of powers Executive Committee (chaired by CEO) executive management and running of the Company Board of Directors Executive Committee Responsible for general policy and strategy Management of the Galapagos group Supervision of Executive Committee Day-to-day management by CEO Approval of the annual budget Reporting to the Board of Directors on the Powers reserved to Board of Directors pursuant to implementation of strategic guidelines, etc. Belgian Companies Code Research, identification, and development of strategic possibilities and proposals The role of the Board of Directors is to pursue a sustainable value creation by the Company, by setting the Company’s strategy, putting in place effective, responsible and ethical leadership and monitoring the Company’s performance. The Board of Directors is the ultimate decision-making body, with the overall responsibility for the management and control of the Company and is authorized to carry out all actions that are necessary or useful for the realization of the Company’s object with the exception of those reserved to the Shareholders’ Meeting by applicable law. The Board also supervises the Executive Committee. The Board acts as a collegiate body. 115 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE The Board of Directors has delegated certain powers to manage the Company to the Executive Committee, led by the Chief Executive Officer (the “CEO”). The Executive Committee is responsible and accountable to the Board of Directors for the discharge of its responsibilities. Furthermore, the Board of Directors has delegated the day-to-day management of the Company to one Executive Committee member, i.e., our CEO. In order to efficiently fulfill its tasks and in view of the size and activities of the Company, the Board of Directors has established an Audit Committee, a Remuneration Committee, a Nomination Committee, and a Scientific and Development Committee. These Board Committees serve in an advisory capacity to the Board of Directors on the matters delegated to them respectively as set forth in the applicable laws and the Company’s Corporate Governance Charter. In addition to the information set out below, we refer to the Risk management and Risk factors sections of this report for a description of the most important characteristics of our internal control and risk management systems. These Risk management and Risk factors sections are deemed fully incorporated by simple reference into this corporate governance statement. 116 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Board of Directors of Galapagos NV Composition of the Board of Directors Per 31 December 2023, our Board of Directors consists of the following members: Paul Stoffels* joined Galapagos as Chief Executive Officer in April 2022, and is an executive member and the Chairman of our Board of Directors since 26 April 2022. He also is member of the Executive Committee at Galapagos. Prior to that, he was Vice Chairman of the Executive Committee and Chief Scientific Officer of Johnson & Johnson where he set the company’s wide innovation agenda and led its pharmaceutical R&D-pipeline, as well as other external initiatives. Before that, he was worldwide Chairman of Pharmaceuticals of Johnson & Johnson which, under his leadership, significantly rejuvenated its product pipeline and adopted a transformational R&D-operating model, which resulted in the launch of 25 innovative medicines across the globe. Dr. Stoffels joined Johnson & Johnson in 2002, following the acquisition of Virco and Tibotec, where he was Chief Executive Officer and Chairman respectively, and where he led the development of several breakthrough products for the treatment of HIV. Dr. Stoffels also is a member of the Supervisory Board of Philips Healthcare in the Netherlands. *StoffelsIMC BV, permanently represented by Dr. Paul Stoffels 117 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Peter Guenter is a non-executive independent member of our Board of Directors since 30 April 2019. Mr. Guenter is a member of the Executive Board of Merck and Chief Executive Officer of Merck Healthcare since January 2021. Before joining Merck, he served as Chief Executive Officer at Almirall from 2017 to 2020. Prior to joining Almirall, he worked at Sanofi for 22 years, most recently as Executive Vice President Diabetes and Cardiovascular Global Business Unit. During his tenure at Sanofi, he held many senior positions including Vice President Eastern Europe and Northern Europe, Vice President Business Management and Support, General Manager Germany, Senior Vice President Europe, Executive Vice President Global Commercial Operations, and Executive Vice President General Medicine and Emerging Markets. He was a member of Sanofi’s Executive Committee from 2013 until August 2017. Before joining Sanofi, he held different positions in sales and marketing at Smith Kline and Ciba Geigy. Mr. Guenter also is a member of the Board of the European Federation of Pharmaceutical Industries and Associations (EFPIA). He holds a Master’s Degree in Physical Education from the Faculty of Medicine and Health Sciences, University of Ghent. 118 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Daniel O’Day is a non-executive member of our Board of Directors since 22 October 2019*. Mr. O’Day is the Chairman of the Board of Directors and Chief Executive Officer of Gilead Sciences, which employs more than 17,000 people worldwide. Prior to joining Gilead in 2019, Mr. O’Day served as the Chief Executive Officer of Roche Pharmaceuticals. His career at Roche spanned more than three decades, during which he held several executive positions in the company’s pharmaceutical and diagnostics divisions in North America, Europe and Asia. He served as a member of Roche’s Corporate Executive Committee, as well as on a number of public and private Boards, including Genentech, Flatiron Health and Foundation Medicine. Mr. O’Day also serves on the Board of Directors for the Pharmaceutical Research and Manufacturers of America Organization and Georgetown University. Mr. O’Day holds a Bachelor’s Degree in Biology from Georgetown University and a MBA from Columbia University in New York. *On 26 March 2024, Mr. O’Day resigned as member of Galapagos’ Board of Directors. He was replaced by Gilead’s CFO, Mr. Andrew Dickinson, as communicated in the press release of 26 March 2024 119 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Linda Higgins is a non-executive member of our Board of Directors since 22 October 2019. Linda Slanec Higgins, PhD, joined Gilead Sciences, Inc. in 2010 and is currently Sr. Vice President Research Strategy, Innovation & Portfolio. In her first ten years at Gilead, she led the Biology division, significantly expanding the therapeutic area scope and capabilities of the department. She founded External Innovation as integral component for Research. She previously served as President & Chief Executive Officer of InteKrin Therapeutics, and as Head of Research at Scios, a Johnson & Johnson company, where she provided leadership for drug discovery, preclinical development and translational medicine. Dr. Higgins is passionate about biopharmaceutical discovery and development, and has been dedicated to excellence in applied scientific research since 1991. She has led projects and departments in multiple therapeutic areas including central nervous system, fibrosis, inflammation, cardiovascular, virology and oncology. Dr. Higgins built many of these as new areas at Scios and Gilead. Dr. Higgins earned an A.B. in Behavioral Physiology from Kenyon College, a Ph.D. in Neurosciences from the University of California, San Diego School of Medicine, and completed Post-Doctoral training in Molecular Genetics at the Howard Hughes Medical Institute of the University of California, Berkeley. She has authored over 50 original peer reviewed scientific papers and invited articles, and is an inventor of over a dozen patents. Dr. Higgins also serves as a Non-Executive Director on the Board of Arcus Biosciences. 120 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Elisabeth Svanberg is a non-executive independent member of our Board of Directors since 28 April 2020. Dr. Svanberg received her MD and PhD from the University of Gothenburg (Sweden), and is a Board-Certified General Surgeon and Associate Professor of Surgery. Dr. Svanberg joined Serono International in 2000, initially in the field of metabolism, and subsequently held roles of increasing responsibilities before joining Bristol Myers Squibb in the United States in 2007. At BMS, Dr. Svanberg served as Development Leader for a first-in-class novel diabetes medicine, and subsequently as Head of Medical Affairs for the Intercontinental region. In 2014, Dr. Svanberg joined Janssen Pharmaceuticals (a Johnson & Johnson company) as Vice President, Head of the Established Products group where she was managing a portfolio of 90 products, used by an estimated 150 million patients globally. Dr. Svanberg subsequently served as Chief Development Officer at Ixaltis, and as Chief Medical Officer at Kuste Biopharma, specialty pharmaceutical companies developing proprietary therapeutics to treat genitourinary (GU) disorders with unmet medical need. Dr. Svanberg is a partner at Ventac Partners (since 2023) and also serves as a Non-Executive Director on the Boards of Egetis (formerly PledPharma) (since 2017), Amolyt Pharma (since 2021), LEO Pharma (since 2022), and EPICS Therapeutics (since 2022). 121 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Jérôme Contamine is a non-executive independent member of our Board of Directors since 26 April 2022. Mr. Contamine served as Chief Financial Officer of Sanofi for more than nine years from 2009 until 2018. Prior to joining Sanofi, he was Chief Financial Officer of Veolia from 2000 to 2009. He previously held various operating functions at Total, and served four years as an auditor at the Cour des Comptes (the supreme body responsible for auditing the use of public funds in France). Mr. Contamine is a graduate of France’s École Polytechnique, ENSAE (École Nationale de la Statistique et de l’Administration Économique) and École Nationale d’Administration. He held the position of non-executive director at Valeo from 2006 to 2017 and at Total Energies from 2020 to 2023. Mr. Contamine also serves as a non-executive director on the Boards of Société Générale. 122 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Dan Baker is a non-executive independent member of our Board of Directors since 26 April 2022. Dr. Baker joined Janssen/Centocor in 2000 and as Vice President Immunology R&D his responsibilities included the clinical development of Remicade, Simponi and Stelara, as well as other programs in rheumatology and dermatology. He supervised many Phase I-III trials in multiple disease areas, and oversaw more than 15 regulatory approvals in the U.S., Europe and Japan. Throughout his time at Janssen, he was responsible for evaluating business development opportunities in the immunology space. In 2015 he took on a new role as Disease Area Stronghold Leader at Janssen where he was responsible for Phase II & III clinical development plans for rheumatology products and the overall portfolio strategy in rheumatology and immunology. This included the early research strategy for immunology discovery, managing the early portfolio development and approving all late-stage efforts. Since his retirement from Janssen in 2019, he has continued to be involved in bringing therapies to patients. He raised capital (>$20MM) to fund and start an immunology company, KiRA Biotech, where he now acts as Chief Executive Officer and as Executive Director. Dr. Baker received his B.A. in Biology from Gettysburg College and his Medical Degree from the University of Pennsylvania. He completed his Medical Residency at Hershey Medical Center and Fellowship in Rheumatology and Immunology at the University of Pennsylvania, followed by a Research Fellowship in Rheumatology at Mass General Hospital. He continued on as part of the faculty of the University of Pennsylvania for 18 years before taking on industry roles. 123 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Susanne Schaffert is a non-executive independent member of our Board of Directors since 12 June 2023, and is the former Global President, Novartis Oncology, and a member of the Executive Committee of Novartis. For more than 25 years, Dr. Schaffert has dedicated her career at Novartis to helping patients live longer, better lives. Before assuming her role as President of Novartis Oncology, Dr. Schaffert served as Chairperson and President of Advanced Accelerator Applications since its acquisition by Novartis in January 2018. Prior to this, Dr. Schaffert was the Head of Region Europe at Novartis Oncology, where she was responsible for leading Novartis’ Oncology Business Unit in the European Region, marketing key products in lung, breast and renal cancer, as well as hematology and coordinating the entire Oncology operations for EU countries. From 2010 to 2012, Dr. Schaffert served as the Head of Investor Relations for Novartis Group and prior thereto, she served as the Novartis Global Franchise Head for Immunology and Infectious Diseases. Dr. Schaffert first joined Novartis Germany in 1995 as a sales representative, and she has held a series of positions in Sales & Marketing with increasing responsibilities in both national and global functions. Dr. Schaffert has experience from various Boards and Committees, and beyond serving Galapagos NV as non-executive independent Board member, she is also an independent non-executive Director on the Board of Incyte Corporation, a Board member and member of the Advisory Group at Novo Holdings in Denmark and serves as independent Board Director on the boards of ARTBio, US and Vetter Pharma, Germany. She is also a member of the Board of Partners of E. Merck KG. Dr. Schaffert holds an M.Sc. in Chemistry and a Ph.D. with honors in Organic Chemistry from University of Erlangen (Germany). 124 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Simon Sturge is a non-executive independent member of our Board of Directors since 19 September 2023, and was the former CEO of Kymab, a biotech company focused on immune-mediated diseases and immuno-oncology therapeutics, until its acquisition by Sanofi in 2021. Mr. Sturge brings over 40 years of global experience in the pharmaceutical industry, including manufacturing expertise from decades of leadership roles at Celltech Biologics (now Lonza), Boehringer Ingelheim and Merck KGgA. He is currently chairing three biotechnology companies in Switzerland, Belgium, and the United States. He also runs his family investment fund and consultancy company and is a Trustee of Weizmann UK. Mr. Sturge joined Kymab as CEO in 2019 before selling it to Sanofi two years later. Before Kymab, he spent six years at Merck Group, based at their corporate headquarters in Darmstadt, Germany, as Executive Vice President Global Strategy, Business Development & Global Operations and previously as Chief Operating Officer of Merck Healthcare, responsible for the company’s global commercial and manufacturing operations. In this capacity, he was responsible for the continued growth in global sales at Merck KGaA, as well as the commercial launches of Bavencio® (anti-PD-L1 antibody, avelumab) in solid tumors and Mavenclad® (cladribine) for relapsing multiple sclerosis. Prior to that, Mr. Sturge served as Corporate Senior Vice President, Biopharmaceuticals at Boehringer Ingelheim, where he was responsible for the company’s global biopharmaceuticals manufacturing business as well as its biosimilars portfolio. Mr. Sturge was also founder and CEO of Ribotargets (now Vernalis), which was acquired by British Biotech. Mr. Sturge holds a BSc degree in Biology from Sussex University. 125 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Changes to our Board of Directors The tenure of Dr. Rajesh Parekh and Dr. Mary Kerr as members of our Board of Directors came to an end during the financial year ended on 31 December 2023. We thank Dr. Rajesh Parekh and Dr. Mary Kerr for their contributions and commitment to the Company over the years. During its meeting of 12 June 2023, the Board of Directors appointed Dr. Susanne Schaffert by cooptation as a non-executive independent Director, replacing Dr. Rajesh Parekh who stepped down on 10 June 2023. During its meeting of 19 September 2023, the Board of Directors appointed Mr. Simon Sturge by cooptation as a non-executive independent Director, replacing Dr. Mary Kerr who stepped down on 18 September 2023. Dr. Susanne Schaffert’s and Mr. Simon Sturge’s appointments will be submitted to the confirmation by the Company’s Annual Shareholders’ Meeting which will be held on 30 April 2024. About the Board of Directors Galapagos NV’s Board of Directors consists of at least five and no more than nine members. At least three members of our Board of Directors are independent. On 31 December 2023, the Board of Directors consisted of nine members, six of whom are independent within the meaning of article 7:87 of the Belgian Companies Code and provision 3.5 of the 2020 Code. In 2023, the Board of Directors was therefore composed of a majority of independent Directors. Except for Stoffels IMC BV (permanently represented by Dr. Paul Stoffels), all members of the Board of Directors are non-executive Directors. The members of our Board of Directors are appointed at the Shareholders’ Meeting upon the proposal of the Board of Directors, for a renewable term of up to four years. Members of the Board of Directors whose mandate has come to an end may be reappointed. When a position on the Board of Directors becomes vacant, the remaining members may temporarily fill the mandate by cooptation and until appointment of a new Board member at the next Shareholders’ Meeting. Each member of the Board of Directors appointed as such by the Shareholders’ Meeting shall complete the tenure of the member of the Board of Directors he/she replaces, unless the Shareholders’ Meeting decides otherwise. The Nomination Committee nominates, for approval by the Board of Directors, candidates to fill vacancies as they arise, and advises on proposals for appointment originating from shareholders, in each case taking into account the Company’s needs and the selection criteria determined by the Board of Directors. In proposing candidates, particular consideration will be given to gender diversity and diversity in general, as well as complementary skills, knowledge and experience. Provision 3.12 of the 2020 Code recommends that, in case of a one-tier governance structure, (a) there should be a clear division of responsibilities between the person 126 Galapagos NV Annual Report 2023
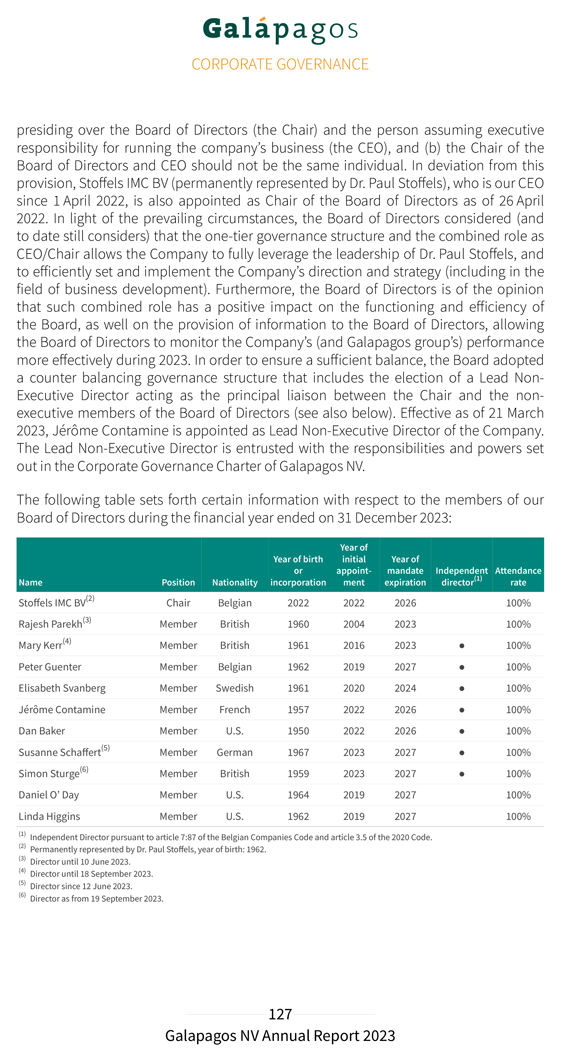
CORPORATE GOVERNANCE presiding over the Board of Directors (the Chair) and the person assuming executive responsibility for running the company’s business (the CEO), and (b) the Chair of the Board of Directors and CEO should not be the same individual. In deviation from this provision, Stoffels IMC BV (permanently represented by Dr. Paul Stoffels), who is our CEO since 1 April 2022, is also appointed as Chair of the Board of Directors as of 26 April 2022. In light of the prevailing circumstances, the Board of Directors considered (and to date still considers) that the one-tier governance structure and the combined role as CEO/Chair allows the Company to fully leverage the leadership of Dr. Paul Stoffels, and to efficiently set and implement the Company’s direction and strategy (including in the field of business development). Furthermore, the Board of Directors is of the opinion that such combined role has a positive impact on the functioning and efficiency of the Board, as well on the provision of information to the Board of Directors, allowing the Board of Directors to monitor the Company’s (and Galapagos group’s) performance more effectively during 2023. In order to ensure a sufficient balance, the Board adopted a counter balancing governance structure that includes the election of a Lead Non-Executive Director acting as the principal liaison between the Chair and the non-executive members of the Board of Directors (see also below). Effective as of 21 March 2023, Jérôme Contamine is appointed as Lead Non-Executive Director of the Company. The Lead Non-Executive Director is entrusted with the responsibilities and powers set out in the Corporate Governance Charter of Galapagos NV. The following table sets forth certain information with respect to the members of our Board of Directors during the financial year ended on 31 December 2023: Year of Year of birth initial Year of or appoint- mandate Independent (1) Attendance Name Position Nationality incorporation ment expiration director rate Stoffels IMC BV(2) Chair Belgian 2022 2022 2026 100% Rajesh Parekh(3) Member British 1960 2004 2023 100% Mary Kerr(4) Member British 1961 2016 2023 • 100% Peter Guenter Member Belgian 1962 2019 2027 • 100% Elisabeth Svanberg Member Swedish 1961 2020 2024 • 100% Jérôme Contamine Member French 1957 2022 2026 • 100% Dan Baker Member U.S. 1950 2022 2026 • 100% Susanne Schaffert(5) Member German 1967 2023 2027 • 100% Simon Sturge(6) Member British 1959 2023 2027 • 100% Daniel O’ Day Member U.S. 1964 2019 2027 100% Linda Higgins Member U.S. 1962 2019 2027 100% (1) Independent Director pursuant to article 7:87 of the Belgian Companies Code and article 3.5 of the 2020 Code. (2) Permanently represented by Dr. Paul Stoffels, year of birth: 1962. (3) Director until 10 June 2023. (4) Director until 18 September 2023. (5) Director since 12 June 2023. (6) Director as from 19 September 2023. 127 Galapagos NV Annual Report 2023
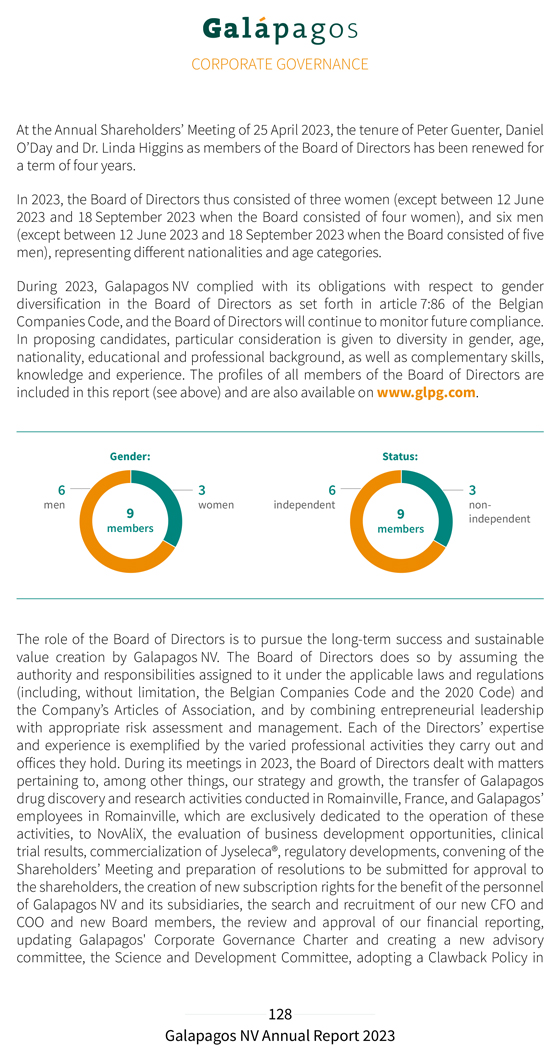
CORPORATE GOVERNANCE At the Annual Shareholders’ Meeting of 25 April 2023, the tenure of Peter Guenter, Daniel O’Day and Dr. Linda Higgins as members of the Board of Directors has been renewed for a term of four years. In 2023, the Board of Directors thus consisted of three women (except between 12 June 2023 and 18 September 2023 when the Board consisted of four women), and six men (except between 12 June 2023 and 18 September 2023 when the Board consisted of five men), representing different nationalities and age categories. During 2023, Galapagos NV complied with its obligations with respect to gender diversification in the Board of Directors as set forth in article 7:86 of the Belgian Companies Code, and the Board of Directors will continue to monitor future compliance. In proposing candidates, particular consideration is given to diversity in gender, age, nationality, educational and professional background, as well as complementary skills, knowledge and experience. The profiles of all members of the Board of Directors are included in this report (see above) and are also available on www.glpg.com. Gender: Status: 6 3 6 3 men women independent non- 9 9 independent members members The role of the Board of Directors is to pursue the long-term success and sustainable value creation by Galapagos NV. The Board of Directors does so by assuming the authority and responsibilities assigned to it under the applicable laws and regulations (including, without limitation, the Belgian Companies Code and the 2020 Code) and the Company’s Articles of Association, and by combining entrepreneurial leadership with appropriate risk assessment and management. Each of the Directors’ expertise and experience is exemplified by the varied professional activities they carry out and offices they hold. During its meetings in 2023, the Board of Directors dealt with matters pertaining to, among other things, our strategy and growth, the transfer of Galapagos drug discovery and research activities conducted in Romainville, France, and Galapagos’ employees in Romainville, which are exclusively dedicated to the operation of these activities, to NovAliX, the evaluation of business development opportunities, clinical trial results, commercialization of Jyseleca®, regulatory developments, convening of the Shareholders’ Meeting and preparation of resolutions to be submitted for approval to the shareholders, the creation of new subscription rights for the benefit of the personnel of Galapagos NV and its subsidiaries, the search and recruitment of our new CFO and COO and new Board members, the review and approval of our financial reporting, updating Galapagos’ Corporate Governance Charter and creating a new advisory committee, the Science and Development Committee, adopting a Clawback Policy in 128 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE accordance with applicable SEC rules, and the transfer of the Jyseleca® business to Alfasigma. In 2023, twelve meetings of the Board of Directors took place physically or through calls to discuss specific matters, including one meeting in the presence of a notary public (relating to the issuance of Subscription Right Plan 2023 BE, Subscription Right Plan 2023 RMV and Subscription Right Plan 2023 ROW). The meeting in the presence of a notary public was attended by Peter Guenter and Dr. Elisabeth Svanberg via call. Stoffels IMC BV (permanently represented by Dr. Paul Stoffels) was not present or represented at the Board meeting in the presence of a notary public. All other Directors were represented by proxy at the Board meeting in the presence of a notary public. The attendance rate for the other Board meetings is identified in the above table. Except for the meeting in the presence of a notary public, the overall attendance rate for Board meetings was 100%. Stoffels IMC BV (permanently represented by Dr. Paul Stoffels) recused itself from deliberation and decision-making on three agenda items because of a conflict of interests, in accordance with article 7:96 of the Belgian Companies Code, as set forth in further detail in the section titled Conflict of interests and related parties. The Board of Directors acts as a collegial body. A formal evaluation of the Board of Directors (formerly Supervisory Board) and its Board Committees was carried out in September 2021. Each member of the Board of Directors provided feedback through individual assessment forms. The results were presented on an aggregate basis by the Secretary ad interim of the (former) Supervisory Board (currently Board of Directors) and served as a basis for discussion by the full (former) Supervisory Board. This evaluation specifically addressed the functioning of the (former) Supervisory Board, the size and composition of the (former) Supervisory Board, the interaction between the (former) Supervisory Board and the (former) Management Board (currently the Executive Committee), and the functioning of the Board Committees. A new Board evaluation exercise was performed in the second half of 2022. As part of this exercise, the Board of Directors’ composition was reviewed, a composition matrix was created, and interviews were held with Board members on the functioning and composition of the Board of Directors. Board member profiles were established, which served the Board in the search for Director candidates to fill open positions by cooptation. Pursuant to the Company’s Corporate Governance Charter and as a counter balancing governance structure for the current combined CEO & Chair role within the Board, the Board of Directors has appointed a Lead Non-Executive Director. The Lead Non-Executive Director is also automatically the Vice-Chair of the Board of Directors. The Lead Non-Executive Director is entrusted with the responsibilities and powers set out in Galapagos NV’s Corporate Governance Charter, including, but not limited to, serving as principal liaison between the Non-Executive Directors and the Chair of the Board. Effective as of March 21, 2023, Jérôme Contamine was appointed as the Lead Non-Executive Director of Galapagos NV. The Board of Directors has appointed a Secretary entrusted with the functions set out in Galapagos NV’s Corporate Governance Charter, including, but not limited to, to advise the Board of Directors and its individual members on all corporate governance matters. 129 Galapagos NV Annual Report 2023
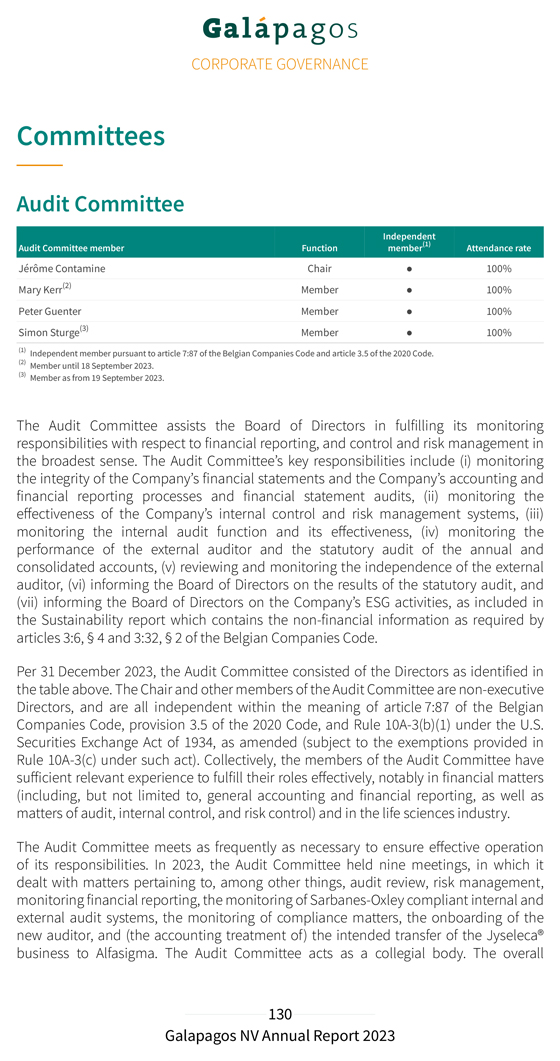
CORPORATE GOVERNANCE Committees Audit Committee Independent (1) Audit Committee member Function member Attendance rate Jérôme Contamine Chair • 100% Mary Kerr(2) Member • 100% Peter Guenter Member • 100% Simon Sturge(3) Member • 100% (1) Independent member pursuant to article 7:87 of the Belgian Companies Code and article 3.5 of the 2020 Code. (2) Member until 18 September 2023. (3) Member as from 19 September 2023. The Audit Committee assists the Board of Directors in fulfilling its monitoring responsibilities with respect to financial reporting, and control and risk management in the broadest sense. The Audit Committee’s key responsibilities include (i) monitoring the integrity of the Company’s financial statements and the Company’s accounting and financial reporting processes and financial statement audits, (ii) monitoring the effectiveness of the Company’s internal control and risk management systems, (iii) monitoring the internal audit function and its effectiveness, (iv) monitoring the performance of the external auditor and the statutory audit of the annual and consolidated accounts, (v) reviewing and monitoring the independence of the external auditor, (vi) informing the Board of Directors on the results of the statutory audit, and (vii) informing the Board of Directors on the Company’s ESG activities, as included in the Sustainability report which contains the non-financial information as required by articles 3:6, § 4 and 3:32, § 2 of the Belgian Companies Code. Per 31 December 2023, the Audit Committee consisted of the Directors as identified in the table above. The Chair and other members of the Audit Committee are non-executive Directors, and are all independent within the meaning of article 7:87 of the Belgian Companies Code, provision 3.5 of the 2020 Code, and Rule 10A-3(b)(1) under the U.S. Securities Exchange Act of 1934, as amended (subject to the exemptions provided in Rule 10A-3(c) under such act). Collectively, the members of the Audit Committee have sufficient relevant experience to fulfill their roles effectively, notably in financial matters (including, but not limited to, general accounting and financial reporting, as well as matters of audit, internal control, and risk control) and in the life sciences industry. The Audit Committee meets as frequently as necessary to ensure effective operation of its responsibilities. In 2023, the Audit Committee held nine meetings, in which it dealt with matters pertaining to, among other things, audit review, risk management, monitoring financial reporting, the monitoring of Sarbanes-Oxley compliant internal and external audit systems, the monitoring of compliance matters, the onboarding of the new auditor, and (the accounting treatment of) the intended transfer of the Jyseleca® business to Alfasigma. The Audit Committee acts as a collegial body. The overall 130 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE attendance at the Audit Committee meetings in 2023 was 100%. The attendance rate at the Audit Committee meetings in 2023 for each of its members is set forth in the table above. Some of the meetings were attended by the statutory auditor of the Company. Nomination Committee Independent (1) Nomination Committee members Function member Attendance rate Rajesh Parekh(2) Member 100% Stoffels IMC BV(3) Member 100% Jérôme Contamine Member • 100% Elisabeth Svanberg(4) Chair • 100% (1) Independent member pursuant to article 7:87 of the Belgian Companies Code and article 3.5 of the 2020 Code. (2) Chair and member until 20 March 2023. (3) Permanently represented by Dr. Paul Stoffels. (4) Chair and member as of 21 March 2023. The Nomination Committee makes recommendations to the Board of Directors with regard to the appointment of the members of the Board of Directors, the CEO, and the members of the Executive Committee. Per 31 December 2023, the Nomination Committee consisted of the Directors as identified in the table above. The majority of its members are non-executive independent Directors. The Chair of the Nomination Committee is a non-executive independent Director. Collectively, the Nomination Committee members have sufficient relevant experience to fulfill their roles effectively. Provision 4.19 of the 2020 Code recommends that the Board of Directors should set up a Nomination Committee with the majority of its members comprising independent non-executive Directors. In deviation from this provision, the Nomination Committee consisted until 20 March 2023 of one executive Director, one independent non-executive Director and one non-executive Director. The latter (Dr. Rajesh Parekh) no longer qualifies as independent pursuant to article 7:87 of the Belgian Companies Code and article 3.5 of the 2020 Code given his long tenure at Galapagos NV. The Board felt it was appropriate to appoint him as a member and Chair of the Nomination Committee in view of his experience as former Chair of the Board and to ensure a smooth transition to the new Chair. Effective as of 21 March 2023, Dr. Elisabeth Svanberg was appointed as member and Chair of the Nomination Committee, replacing Dr. Rajesh Parekh. The Nomination Committee meets as frequently as necessary to ensure effective operation of its responsibilities. In 2023, the Nomination Committee held seven meetings, dealing with, among other things, matters pertaining to the search for new Directors and Executive Officers, the proposal to reappoint certain Directors at our Shareholders’ Meeting on 25 April 2023, and succession planning. The Nomination Committee acts as a collegial body. The overall attendance at the Nomination Committee meetings in 2023 was 100%. The attendance rate at the Nomination Committee meetings in 2023 for each of its members is set forth in the table above. 131 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Remuneration Committee Independent (1) Remuneration Committee members Function member Attendance rate Rajesh Parekh(2) Chair 100% Jérôme Contamine Member • 100% Elisabeth Svanberg(3) Chair • 100% Dan Baker(4) Member • 100% (1) Independent member pursuant to article 7:87 of the Belgian Companies Code and article 3.5 of the 2020 Code. (2) Chair and member until 20 March 2023. (3) Chair as of 21 March 2023. (4) Member as of 21 March 2023. The Remuneration Committee makes recommendations to the Board of Directors with regard to the remuneration of the members of the Board of Directors, the CEO, and the members of the Executive Committee, including variable remuneration and long-term incentives, whether or not stock-related, in each case insofar as allowed by applicable laws and regulations. Per 31 December 2023, the Remuneration Committee consisted of the Directors as identified in the table above. The Chair and other members of the Remuneration Committee are non-executive Directors and are all independent within the meaning of article 7:87 of the Belgian Companies Code and provision 3.5 of the 2020 Code. Collectively, the Remuneration Committee members have sufficient relevant experience to fulfill their roles effectively. The Remuneration Committee meets as frequently as necessary to ensure effective operation of its responsibilities. In 2023, the Remuneration Committee held ten meetings, dealing with, among other things, matters pertaining to the remuneration of our new Executive Committee member, grants of subscriptions rights, restricted stock units (RSUs) and bonuses, the packages of our retiring President, Chief Operating Officer and Chief Financial Officer, and Chief Commercial Officer, the review of the Remuneration Policy and Remuneration Report, and salary increases. The Remuneration Committee acts as a collegial body. The overall attendance at the Remuneration Committee meetings in 2023 was 100%. The attendance rate at the Remuneration Committee meetings in 2023 for each of its members is set forth in the table above. The CEO participated in those meetings where the remuneration of the Executive Committee members (other than the CEO) was discussed. 132 Galapagos NV Annual Report 2023
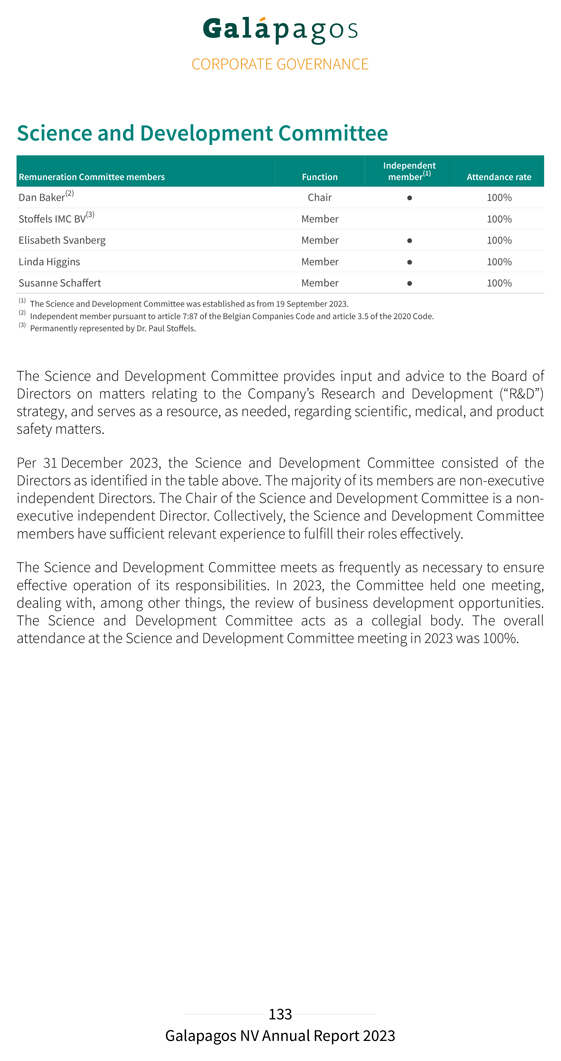
CORPORATE GOVERNANCE Science and Development Committee Independent (1) Remuneration Committee members Function member Attendance rate Dan Baker(2) Chair • 100% Stoffels IMC BV(3) Member 100% Elisabeth Svanberg Member • 100% Linda Higgins Member • 100% Susanne Schaffert Member • 100% (1) The Science and Development Committee was established as from 19 September 2023. (2) Independent member pursuant to article 7:87 of the Belgian Companies Code and article 3.5 of the 2020 Code. (3) Permanently represented by Dr. Paul Stoffels. The Science and Development Committee provides input and advice to the Board of Directors on matters relating to the Company’s Research and Development (“R&D”) strategy, and serves as a resource, as needed, regarding scientific, medical, and product safety matters. Per 31 December 2023, the Science and Development Committee consisted of the Directors as identified in the table above. The majority of its members are non-executive independent Directors. The Chair of the Science and Development Committee is a non-executive independent Director. Collectively, the Science and Development Committee members have sufficient relevant experience to fulfill their roles effectively. The Science and Development Committee meets as frequently as necessary to ensure effective operation of its responsibilities. In 2023, the Committee held one meeting, dealing with, among other things, the review of business development opportunities. The Science and Development Committee acts as a collegial body. The overall attendance at the Science and Development Committee meeting in 2023 was 100%. 133 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Executive Committee of Galapagos NV Composition of the Executive Committee Per 31 December 2023, our Executive Committee consists of the following members: Stoffels IMC BV, permanently represented by Dr. Paul Stoffels – Please refer to the Composition of the Board of Directors for a biography. Thad Huston was appointed as Chief Financial Officer and Chief Operating Officer as per July 2023, and is a member of the Executive Committee at Galapagos. He previously served a Senior Vice President, Finance and Corporate Operations of Kite Pharma, a Gilead Company, where he was responsible for all financial aspects of the market leading cell therapy business worldwide. He was also a member of the Kite Leadership Team, the Gilead CFO Leadership Teams and the Fosun-Kite Board. Before joining Kite in 2021, Thad served as Chief Financial Officer at LivaNova PLC, a medical device company specializing in cardiovascular and neuromodulation products, where he played a key role in external R&D innovation and M&A and led the global, cross-functional teams across the group. Prior to LivaNova, he spent over 25 years in leadership positions at Johnson & Johnson (J&J), which included roles as Chief Financial Officer and Chief Operating Officer of J&J Pharmaceutical Research and Development, Chief Financial Officer of J&J’s Global Surgery and Medical Devices groups managing up to $21 billion in annual revenue, and President of Xian-Janssen, leading J&J’s pharmaceutical division in China. Before that, he held senior financial roles at various J&J locations in the U.S., Belgium, Russia, and Hungary. Thad is passionate about delivering results by transforming businesses to accelerate internal and external innovation to make a real difference for patients around the world. 134 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Michele Manto* was appointed as Chief Commercial Officer in January 2020, and member of the Executive Committee at Galapagos. He joined Galapagos in September 2017 as Senior Vice President Commercial Operations to build and lead Galapagos’ commercial organization and capabilities. Previously, Mr. Manto held various commercial leadership roles at AbbVie, most recently as General Manager, Global Marketing Rheumatology and General Manager in the Netherlands. Prior to this, he led AbbVie’s commercial activities and launches in rheumatology, gastroenterology and dermatology in Germany and other European countries. He started his professional career as a management and strategy consultant at McKinsey & Company. Mr. Manto holds an MBA from INSEAD and a Degree in Engineering from the Politecnico of Milan. *On 31 December 2023, the mandate of Mr. Manto as Chief Commercial Offier and member of the Executive Committee ended. 135 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Annelies Missotten was appointed as Chief Human Resources Officer and member of the Executive Committee at Galapagos. She joined Galapagos as Vice President Human Resources in February 2018 to transform and build an expert HR team to enable business growth, and leading the transformation of Galapagos into an integrated biopharmaceutical company with an international setup. In 2020, she was appointed Senior Vice President Human Resources and strategic advisor to the CEO and Executive Committee. Before joining Galapagos, she held various senior global HR positions at GSK. She started her career at Proximus, and acquired deep expertise over time in key HR Centres of Expertise, including Training & Development, Talent Acquisition and Reward, and HR Business partnership roles. Ms. Missotten holds a Master’s Degree in Roman Philology from KU Leuven, a DEA in Italian Culture and Linguistics from the Paris IV Sorbonne (France) and L’Universitŕ Cattolica di Milano. Over the years, she completed her education with several systemic psychology and coaching certifications and business courses, amongst others, from INSEAD, Fontainebleau (France). 136 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Valeria Cnossen was appointed as General Counsel, responsible for Compliance & Ethics, the Corporate Secretary Office and Intellectual Property, and member of the Executive Committee at Galapagos. Ms. Cnossen joined Galapagos on 1 August 2022. She previously was General Counsel of the Consumer Health Group at Johnson & Johnson where she was a strategic partner and key advisor on laws and regulations, transactions and emerging areas, impacting the business such as digital, transparency, sustainability and public policy. Prior to that, she held leadership roles within the Medical Devices and Pharmaceutical Sectors of Johnson & Johnson. Ms. Cnossen joined Johnson & Johnson in 2011 through the acquisition of Crucell, where she was Head of Legal and Compliance. Prior to joining Crucell, Ms. Cnossen was in private legal practice at De Brauw Blackstone Westbroek in the Netherlands, and Cravath, Swaine & Moore in New York City. Ms. Cnossen is a purpose-driven leader, known for her ability to develop high-performing teams and the careers of others, especially as a mentor for women. 137 Galapagos NV Annual Report 2023
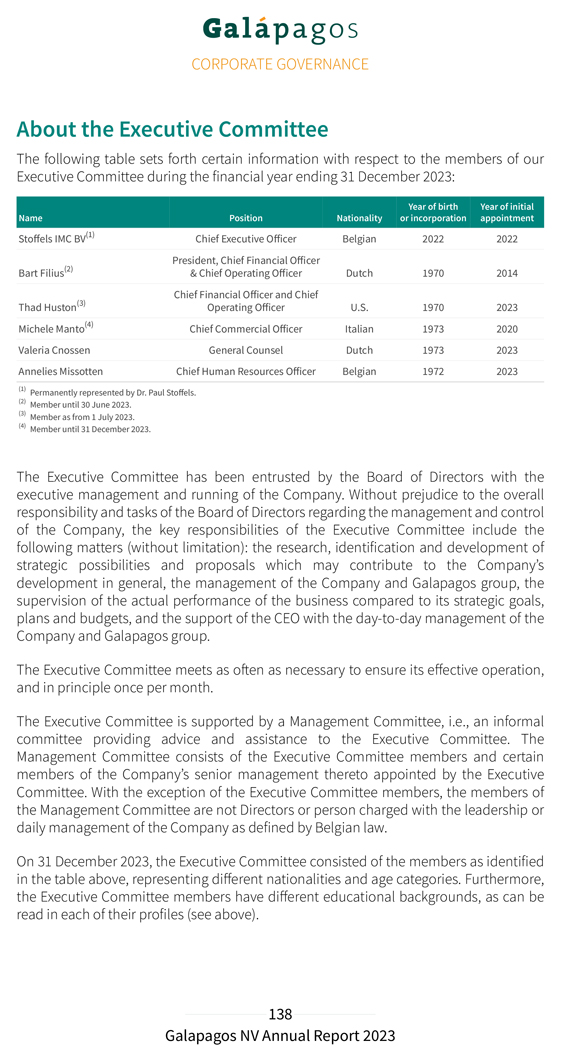
CORPORATE GOVERNANCE About the Executive Committee The following table sets forth certain information with respect to the members of our Executive Committee during the financial year ending 31 December 2023: Year of birth Year of initial Name Position Nationality or incorporation appointment Stoffels IMC BV(1) Chief Executive Officer Belgian 2022 2022 (2) President, Chief Financial Officer Bart Filius & Chief Operating Officer Dutch 1970 2014 (3) Chief Financial Officer and Chief Thad Huston Operating Officer U.S. 1970 2023 Michele Manto(4) Chief Commercial Officer Italian 1973 2020 Valeria Cnossen General Counsel Dutch 1973 2023 Annelies Missotten Chief Human Resources Officer Belgian 1972 2023 (1) Permanently represented by Dr. Paul Stoffels. (2) Member until 30 June 2023. (3) Member as from 1 July 2023. (4) Member until 31 December 2023. The Executive Committee has been entrusted by the Board of Directors with the executive management and running of the Company. Without prejudice to the overall responsibility and tasks of the Board of Directors regarding the management and control of the Company, the key responsibilities of the Executive Committee include the following matters (without limitation): the research, identification and development of strategic possibilities and proposals which may contribute to the Company’s development in general, the management of the Company and Galapagos group, the supervision of the actual performance of the business compared to its strategic goals, plans and budgets, and the support of the CEO with the day-to-day management of the Company and Galapagos group. The Executive Committee meets as often as necessary to ensure its effective operation, and in principle once per month. The Executive Committee is supported by a Management Committee, i.e., an informal committee providing advice and assistance to the Executive Committee. The Management Committee consists of the Executive Committee members and certain members of the Company’s senior management thereto appointed by the Executive Committee. With the exception of the Executive Committee members, the members of the Management Committee are not Directors or person charged with the leadership or daily management of the Company as defined by Belgian law. On 31 December 2023, the Executive Committee consisted of the members as identified in the table above, representing different nationalities and age categories. Furthermore, the Executive Committee members have different educational backgrounds, as can be read in each of their profiles (see above). 138 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Bart Filius’ mandate as President, CFO, COO and Executive Committee member ended per 30 June 2023. Michele Manto’s mandate as CCO and Executive Committee member ended per 31 December 2023. The members of the Executive Committee are appointed by the Board of Directors upon recommendation of the Nomination Committee. In proposing candidates for the Executive Committee, particular consideration is given to educational and professional background, complementary skills, knowledge and experience, as well as to diversity in age, gender and nationality. 139 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Galapagos NV’s share capital and shares Galapagos Share capital NV increases in 2023 and issue of shares by On 1 January 2023, the share capital of Galapagos NV amounted to €356,111,899.01 represented by 65,835,511 shares. In the course of 2023, there was one capital increase resulting from the exercise of subscription rights under subscription right plans, resulting in the issuance of 61,560 new shares, an increase of the share capital by €333,039.60 and an increase of the issuance premium account by €1,436,810.40. At the end of 2023, the share capital of Galapagos NV amounted to €356,444,938.61 represented by 65,897,071 shares. During 2023, the Board of Directors issued subscription rights under three subscription right plans: On 5 May 2023, the Board of Directors issued 1,538,400 subscription rights, after acceptance by the beneficiaries, within the framework of the authorized capital, for the benefit of Executive Committee members and certain employees of the Galapagos group under new subscription right plans: “Subscription Right Plan 2023 BE”, “Subscription Right Plan 2023 RMV” and “Subscription Right Plan 2023 ROW”. The subscription rights issued under Subscription Right Plan 2023 BE, Subscription Right Plan 2023 RMV and Subscription Right Plan 2023 ROW have an exercise term of eight years as of the date of the offer, and subscription rights issued under the first offer have an exercise price of €35.11 (the average closing price of the Galapagos share on Euronext Amsterdam and Brussels during the 30 calendar days preceding the date of the first offer), under the subsequent offer of €38.58 (the closing price of the share on Euronext Amsterdam and Brussels during the 30 calendar days preceding the date of the second offer), and under the second subsequent offer of €32.99 (the closing price of the share on Euronext Amsterdam and Brussels during the 30 calendar days preceding the date of the third offer). Number and form of Galapagos shares Of the 65,897,071 shares of Galapagos NV outstanding at the end of 2023, 5,846 were registered shares and 65,891,225 shares were dematerialized shares. All issued shares are fully paid up and are of the same class. Rights attached to Galapagos shares Each share (i) entitles its holder to one vote at the Shareholders’ Meetings of Galapagos NV; (ii) represents an identical fraction of the Company’s share capital and has the same rights and obligations and shares equally in the profit of Galapagos NV; and (iii) 140 Galapagos NV Annual Report 2023
CORPORATE GOVERNANCE gives its holder a preferential subscription right to subscribe to new shares, convertible bonds or subscription rights in proportion to the part of the share capital represented by the shares already held. The preferential subscription right can be restricted or cancelled by a resolution approved by the Shareholders’ Meeting, or, within the framework of the Company’s authorized capital, by the Board of Directors subject to an authorization of the Shareholders’ Meeting, in accordance with the provisions of the Belgian Companies Code and Galapagos NV’s Articles of Association. Galapagos NV’s authorized capital In accordance with the provisions of the Belgian Companies Code and the Company’s Articles of Association, the Extraordinary Shareholders’ Meeting of Galapagos NV authorized the Board of Directors to increase the share capital of Galapagos NV, in one or several times, and under certain conditions set forth in extenso in the Articles of Association of Galapagos NV. This authorization consists of two parts: A general authorization for capital increases up to 20% of the share capital at the time of convening the Shareholders’ Meeting of 22 October 2019 (i.e., €67,022,402.04) was renewed and is valid for a period of five years from the date of publication of this renewal in the Annexes to the Belgian State Gazette, i.e., 13 November 2019. This general authorization will expire on 12 November 2024; and A specific authorization for capital increases of more than 20% and up to 33% of the share capital at the time of the convening the Shareholders’ Meeting of 25 April 2017 (i.e., € 82,561,764.93), was renewed and was valid for a period of five years from the date of publication of this renewal in the Annexes to the Belgian State Gazette, i.e., 31 May 2017. This specific part of the authorized capital could, however, only be used in specific circumstances and upon a resolution of the Board of Directors that all independent Directors (within the meaning of article 7:87 of the Belgian Companies Code and provision 3.5 of the 2020 Code) approve. This specific authorization expired on 30 May 2022. In 2023, Galapagos NV’s Board of Directors made use of the right to increase the capital in the framework of the authorized capital on one occasion: On 5 May 2023, in connection with the issuance of Subscription Right Plan 2023 BE, Subscription Right Plan 2023 RMV and Subscription Right Plan 2023 ROW, under which a maximum of 1,975,000 new shares could be issued for a total maximum capital increase of €10,684,750 (plus issuance premium). On 31 December 2023, an amount of €16,566,540.17 still remained available under the general part of the authorized capital. When increasing the share capital within the limits of the authorized capital, the Board of Directors may, if in Galapagos NV’s interest, restrict or cancel the shareholders’ 141 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE preferential subscription rights, even if such restriction or cancellation is made for the benefit of one or more specific persons other than the employees of the group. Procedure capital for changes in Galapagos NV’s share In accordance with the Belgian Companies Code, Galapagos NV may increase (and issue new shares) or decrease its share capital by decision of the Extraordinary Shareholders’ Meeting approved by a qualified majority of 75% of the votes cast, at a meeting where at least 50% of the share capital of Galapagos NV is present or represented. If the attendance quorum of 50% is not met, a new Extraordinary Shareholders’ Meeting must be convened at which the shareholders may decide on the agenda items, irrespective of the percentage of share capital present or represented at such meeting. In this respect, there are no conditions imposed by Galapagos NV’s Articles of Association that are more stringent than those required by law. Within the framework of the powers granted to it under the authorized capital, the Board of Directors may also increase Galapagos NV’s share capital (and issue new shares) as specified in its Articles of Association. Purchase and sale of Galapagos NV treasury shares In accordance with the Belgian Companies Code and the Articles of Association of the Company, Galapagos NV may purchase, subject to the provisions of the Belgian Companies Code, Galapagos NV’s own shares if authorized by a prior decision of the Extraordinary Shareholders’ Meeting approved by a qualified majority of 75% of the votes cast, at a meeting where at least 50% of the share capital of Galapagos NV is present or represented. If the attendance quorum of 50% is not met, a new Extraordinary Shareholders’ Meeting must be convened at which the shareholders may decide on the agenda items, irrespective of the percentage of share capital present or represented at such meeting. The sale of Galapagos NV treasury shares is also subject to the provisions of the Belgian Companies Code. The aforementioned rules are also applicable to the acquisition of shares of Galapagos NV by its subsidiaries. The Board of Directors of Galapagos NV has currently not been authorized by an Extraordinary Shareholders’ Meeting to purchase or sell its own shares. On 31 December 2023, neither Galapagos NV nor any subsidiary of Galapagos NV held any shares in Galapagos NV, nor did any third party hold any shares in Galapagos NV on behalf of Galapagos NV or any of its subsidiaries. Anti Association -takeover provisions in Galapagos NV’s Articles of Galapagos NV’s Articles of Association currently do not contain any anti-takeover provisions. 142 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Anti-takeover provisions under Belgian law Under Belgian law, public takeover bids for all outstanding voting securities of the issuer are subject to the supervision of the FSMA. If the latter determines that a takeover violates Belgian law, it may lead to suspension of the exercise of the rights attached to any shares that were acquired in connection with the envisaged takeover. Pursuant to the Belgian Law of 1 April 2007 on public takeovers, a mandatory takeover bid must be made when, as a result of its own acquisition or the acquisition by persons acting in concert with it, a person owns, directly or indirectly, more than 30% of the securities with voting rights in a company with registered office in Belgium whose securities are admitted to trading on a regulated or recognized market. The acquirer must offer to all other shareholders the opportunity to sell their shares at the higher of (i) the highest price offered by the acquirer for shares of the issuer during the 12 months preceding the announcement of the bid or (ii) the weighted average price of the shares on the most liquid market of the last 30 calendar days prior to the date on which it became mandatory for the acquirer to launch a mandatory takeover bid for the shares of all other shareholders. Material contracts containing change of control clauses The second amended and restated collaboration agreement between Galapagos NV and AbbVie S.à.r.l. (“AbbVie”) dated 24 October 2018 contains provisions granting certain rights to AbbVie upon the occurrence of a public takeover bid on our shares or a change of control in respect of Galapagos NV, including, but not limited to clause 11.2 of the agreement (Change in Control of Galapagos), entitling AbbVie, to oblige Galapagos NV to take appropriate measures to avoid the disclosure of confidential information, to limit AbbVie’s reporting obligations to Galapagos NV, or, depending on the stage in which the change of control occurs, to terminate the agreement. Procedure for amendments to Galapagos NV’s Articles of Association Pursuant to the Belgian Companies Code, amendments to the Articles of Association of Galapagos NV, such as an increase or decrease in the share capital, the approval of the dissolution, merger or de-merger of Galapagos NV, but excluding an amendment of the Company’s purpose, may only be authorized with the approval of at least 75% (or, in case of an amendment of the Company’s purpose, 80%) of the votes validly cast at an Extraordinary Shareholders’ Meeting where at least 50% of Galapagos NV’s share capital is present or represented. If the attendance quorum of 50% is not met, a new Extraordinary Shareholders’ Meeting must be convened at which the shareholders may decide on the agenda items, irrespective of the percentage of share capital present or represented at such meeting. 143 Galapagos NV Annual Report 2023
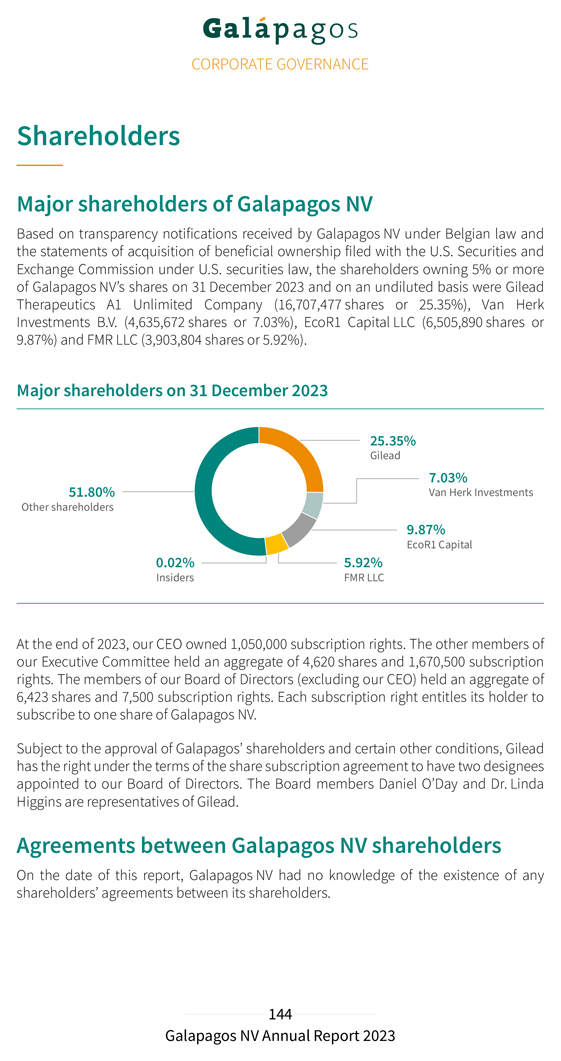
CORPORATE GOVERNANCE Shareholders Major shareholders of Galapagos NV Based on transparency notifications received by Galapagos NV under Belgian law and the statements of acquisition of beneficial ownership filed with the U.S. Securities and Exchange Commission under U.S. securities law, the shareholders owning 5% or more of Galapagos NV’s shares on 31 December 2023 and on an undiluted basis were Gilead Therapeutics A1 Unlimited Company (16,707,477 shares or 25.35%), Van Herk Investments B.V. (4,635,672 shares or 7.03%), EcoR1 Capital LLC (6,505,890 shares or 9.87%) and FMR LLC (3,903,804 shares or 5.92%). Major shareholders on 31 December 2023 25.35% Gilead 7.03% 51.80% Van Herk Investments Other shareholders 9.87% EcoR1 Capital 0.02% 5.92% Insiders FMR LLC At the end of 2023, our CEO owned 1,050,000 subscription rights. The other members of our Executive Committee held an aggregate of 4,620 shares and 1,670,500 subscription rights. The members of our Board of Directors (excluding our CEO) held an aggregate of 6,423 shares and 7,500 subscription rights. Each subscription right entitles its holder to subscribe to one share of Galapagos NV. Subject to the approval of Galapagos’ shareholders and certain other conditions, Gilead has the right under the terms of the share subscription agreement to have two designees appointed to our Board of Directors. The Board members Daniel O’Day and Dr. Linda Higgins are representatives of Gilead. Agreements between Galapagos NV shareholders On the date of this report, Galapagos NV had no knowledge of the existence of any shareholders’ agreements between its shareholders. 144 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Agreements with major Galapagos NV shareholders On 14 July 2019, we and Gilead Sciences, Inc. and its affiliated companies (hereinafter “Gilead”) announced that we entered into a 10-year global research and development collaboration. In the context of the transaction, Gilead also made an equity investment in Galapagos. We also amended and restated the license agreement for filgotinib that we originally entered into with Gilead on 16 December 2015. On 23 August 2019, the closing of the transaction took place and we received an upfront payment of €3,569.8 million ($3.95 billion) and a €960.1 million ($1.1 billion) equity investment from Gilead. On 15 December 2020 and on 30 October 2023, we and Gilead announced that we agreed to amend our existing arrangement for the commercialization and development of filgotinib again. Terms of the equity investment As part of the research and development collaboration, Gilead entered into a share subscription agreement with us. On 23 August 2019, Gilead subscribed to 6,828,985 new Galapagos shares at a price of €140.59 per share, which included an issuance premium. Subject to the approval of Galapagos’ Shareholders’ Meeting and certain other conditions, Gilead has the right under the terms of the share subscription agreement to have two designees appointed to our Board of Directors. The Special Shareholders’ Meeting of 22 October 2019 approved the appointment of Daniel O’Day and Dr. Linda Higgins as Directors of Galapagos NV, both of whom are still Directors of Galapagos NV today. On 22 October 2019, our Extraordinary Shareholders’ Meeting approved the issuance of a warrant to Gilead, known as Warrant A, that confers the right to subscribe for a number of new shares sufficient to bring the number of shares owned by Gilead and its affiliates to 25.1% of the issued and outstanding shares of the Company. Warrant A expires one year after the issue date and the exercise price per share is €140.59. On 6 November 2019, Gilead exercised Warrant A and increased its ownership in Galapagos to 25.10% of the then outstanding shares. On 22 October 2019, Gilead was also issued another warrant, known as the initial Warrant B, that confers the right to subscribe for a number of new shares sufficient to bring the number of shares owned by Gilead and its affiliates to 29.9% of the issued and outstanding shares of the Company. The initial Warrant B will expire on 23 August 2024. The exercise price per share will be the greater of (i) 120% multiplied by the arithmetic mean of the 30-day daily volume weighted average trading price of the Galapagos shares preceding the date of the exercise notice with respect to such exercise, and (ii) €140.59. Between 57 and 59 months from 23 August 2019, subject to and upon approval by the Company’s Shareholders’ Meeting, Gilead will be issued a warrant with substantially similar terms, including exercise price, to the initial Warrant B. This subsequent Warrant B will expire on the earlier of (i) the date that is five years after the fifth anniversary of the closing and (ii) five years after the date that the warrant is issued. The issuance of this warrant is on the agenda of the Extraordinary Shareholders’ Meeting of 30 April 2024. 145 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Gilead is subject to certain standstill restrictions until 10 years following the closing, which occurred on 23 August 2019. Among other things, during this time Gilead and its affiliates and any party acting in concert with them may not, without our consent, acquire voting securities of Galapagos exceeding more than 29.9% of the then issued and outstanding voting securities, and Gilead may not propose a business combination with or acquisition of Galapagos. The standstill restrictions are subject to certain exceptions as provided in the share subscription agreement. Pursuant to the terms of the share subscription agreement, Gilead also agreed to certain lock-up provisions. They shall not, and shall cause their affiliates not to, without our prior consent, dispose of any equity securities of Galapagos prior to the second anniversary of the closing (23 August 2019). During the period beginning on the date that is two years following the closing until the date that is five years following the closing, Gilead and its affiliates shall not, without our prior consent, dispose of any equity securities of Galapagos if after such disposal they would own less than 20.1% of the then issued and outstanding voting securities of Galapagos. The lock-up restrictions are subject to certain exceptions as provided in the share subscription agreement and may terminate upon certain events. In April 2021, Gilead and Galapagos agreed to amend the share subscription agreement to extend the full lock-up of all of Gilead’s securities of Galapagos for a period of five years until 22 August 2024. In 2022, Gilead and Galapagos agreed to amend the share subscription agreement for conformity with the change from a two-tier to a one-tier governance system by Galapagos. Terms collaboration of the global research and development We will fund and lead all discovery and development autonomously until the end of Phase 2. After the completion of a qualifying Phase 2 study (or, in certain circumstances, the first Phase 3 study), Gilead will have the option to acquire a license to the compound outside Europe. If the option is exercised, we and Gilead will co-develop the compound and share costs equally. Gilead will maintain option rights to our programs through the 10-year term of the collaboration. This term can be extended, at the discretion of Gilead, for up to an additional three years thereafter for those programs, if any, that have entered clinical development prior to the end of the collaboration term. On top, a final term extension can be granted in certain circumstances. For all programs resulting from the collaboration (other than GLPG1972 and GLPG1690), Gilead will make a $150 million opt-in payment per program and will owe no subsequent milestones. We will receive tiered royalties ranging from 20 – 24% on net sales of all our products licensed by Gilead in countries outside Europe as part of the agreement. For GLPG1972, Gilead declined to exercise its option under the collaboration agreement in November 2020. In February 2021, the development of GLPG1690 (ziritaxestat) was discontinued. 146 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Revised filgotinib collaboration Under the terms of the new arrangement agreed in December 2020, we assumed all development, manufacturing, commercialization and certain other rights for filgotinib in Europe. Gilead retains commercial rights and remains the marketing authorization holder for filgotinib outside of Europe, including in Japan, where filgotinib is co-marketed with Eisai. The transfer was subject to applicable local legal, regulatory and consultation requirements. Most activities transferred to Galapagos by 31 December 2021 and we completed the transition during 2022. The new arrangement was formalized in (1) the Transition and Amendment Agreement of 3 April 2021 pursuant to which Gilead transitioned the exploitation of filgotinib in Europe to Galapagos by the end of 2021, (2) the DIVERSITY Letter Agreement of 6 September 2021 pursuant to which we and Gilead agreed to transfer the sponsorship of and operational and financial responsibility for the ongoing DIVERSITY study and its long-term extension study (LTE) study from Gilead to Galapagos, and (3) the Second Amended and Restated License and Collaboration Agreement of 24 December 2021, amending and restating the existing collaboration agreement, which went into effect as of 1 January 2022. In March 2022, Gilead and Galapagos agreed to transfer the sponsorship of and the operational responsibility for the MANTA study, a safety study in men with moderately to severely active UC and CD to assess semen parameters while taking filgotinib, and its long-term extension, from Gilead to Galapagos. Since 1 January 2021, we bear the future development costs for certain studies, in lieu of the equal cost split contemplated by the previous agreement. These studies include the DARWIN3, FINCH4, FILOSOPHY, and Phase 4 studies and registries in RA, MANTA and MANTA-Ray, the PENGUIN1 and 2 and EQUATOR2 studies in PsA, the SEALION1 and 2 studies in AS, the HUMBOLDT study in uveitis in addition to other clinical and non-clinical expenses supporting these studies and support for any investigator sponsored trials in non-IBD conditions and non-clinical costs on all current trials. The existing 50/50 global development cost sharing arrangement continued for the following studies: SELECTION and its long-term extension study (LTE) in UC, DIVERSITY and its LTE, DIVERGENCE 1 and 2 and their LTEs and support for Phase 4 studies and registries in Crohn’s disease, pediatric studies and their LTEs in RA, UC and CD, and support for investigator sponsored trials in IBD. In September 2021, we and Gilead agreed to transfer the sponsorship of the DIVERSITY study and its LTE study from Gilead to Galapagos. The transfer was intended to be completed by 30 June 2022 and was completed by March 2023. From 1 April 2022, Galapagos is solely responsible for all development costs for the DIVERSITY study and its LTE study. In March 2022, we and Gilead agreed to transfer the sponsorship of the MANTA study and its LTE from Gilead to Galapagos, which transfer was largely completed by 31 December 2022. All commercial economics on filgotinib in Europe transferred to us as of 1 January 2022, subject to payment of tiered royalties of 8 to 15 percent of net sales in Europe to Gilead, starting in 2024. In connection with the amendments to the existing arrangement for the commercialization and development of filgotinib, Gilead has agreed to irrevocably 147 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE pay Galapagos €160 million, subject to certain adjustments for higher than budgeted development costs. Gilead paid €35 million in January 2021, an additional €75 million in April 2021 and €50 million in 2022. Furthermore, Gilead made a one-time payment of $15 million to Galapagos in 2022 in consideration for Galapagos assuming responsibility for the DIVERSITY study. In addition, we will no longer be eligible to receive any future milestone payments relating to filgotinib in Europe. However, we will remain eligible to receive tiered royalty percentages ranging from 20% to 30% on Gilead’s global net sales of filgotinib outside of Europe and future development and regulatory milestone-based payments of up to $275 million and sales-based milestone payments of up to $600 million. On 28 March 2022 filgotinib was approved by the Japanese Ministry of Health, Labour and Welfare for UC, for which we received a $20.0 million (€18.2 million) regulatory milestone payment from Gilead in May 2022. In March 2022, Gilead and Galapagos agreed to further amend the collaboration by adding the following countries to the Galapagos territory: Andorra, San Marino, Monaco, and Vatican City. In October 2023, Gilead and Galapagos agreed to further amend the collaboration. Gilead and Galapagos agreed to terminate the existing 50/50 global development cost sharing arrangement, with Galapagos bearing the costs going forward, and to terminate Galapagos’ obligation to pay tiered royalties to Gilead on net sales of Jyseleca® in Europe, in addition to other amendments. Our Remuneration Policy A revised remuneration policy will apply as from 1 January 2024, subject to its approval by the Shareholders’ Meeting to be held on 30 April 2024. Such document is available on our website. 148 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Remuneration Report Introduction At Galapagos, we are united around a single purpose: to transform patient outcomes through life-changing science and innovation to deliver more years of life and a better quality of life. We are committed to improving patients’ lives worldwide by targeting diseases with high unmet needs. Our objectives are to develop best-in-class therapeutic options. Our R&D capabilities comprise multiple drug modalities, including small molecules and cell therapies. Our portfolio comprises discovery and development programs in immunology and oncology. The objective of our Remuneration Policy is to attract, engage, and retain the diverse qualified and expert individuals that we need to pursue our strategic and operational objectives, whilst reinforcing our culture and sustainability ambitions for the benefit of patients, our people and planet. Our specific goals for remuneration are: to offer competitive opportunities for talented employees by benchmarking against appropriate peer groups; to incentivize exceptional and sustainable performance, aligned with corporate achievements; to provide differential rewards based on individual performance; to avoid differentiation on any grounds except for performance and other proper factors; and to reinforce an open, and equitable culture. Galapagos’ current Remuneration Policy was prepared in accordance with the Belgian Companies Code and approved by Galapagos’ shareholders at the 2022 annual Shareholders’ Meeting with 64.62% of shareholder votes. The policy became effective as from 1 January 2022 and continued to apply for the reporting year beginning on 1 January 2023. This Remuneration Report must be read together with the Remuneration Policy which, to the extent necessary, should be regarded as forming part of this Remuneration Report. The remuneration granted to the members of the Board and the Executive Committee with respect to financial year 2023 is in line with the Remuneration Policy unless otherwise stated. Galapagos encourages an open and constructive dialogue with its shareholders to discuss its approach to governance, including remuneration, and to understand what they consider best practices. We have carefully considered the feedback received and have reviewed our remuneration practices. The results of these efforts have led to a greater level of detail in this Remuneration Report compared to prior years. In addition, a proposed, revised Remuneration Policy is being presented to Galapagos’ shareholders at the 2024 annual Shareholders’ Meeting which, if approved, will be effective from 1 January 2024. We are committed to continually reviewing and improving our Remuneration Policy and reporting practices. 149 Galapagos NV Annual Report 2023
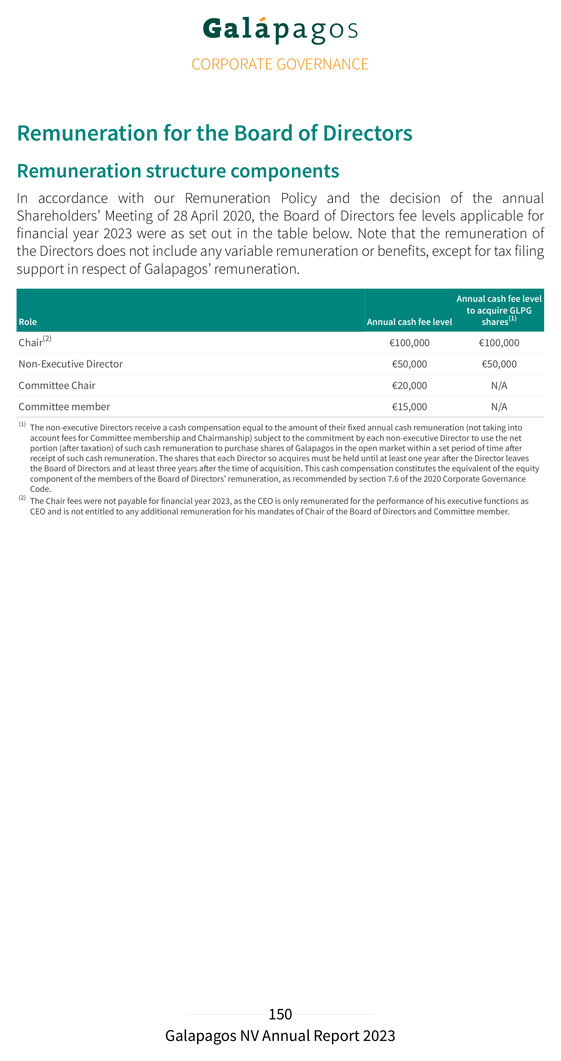
CORPORATE GOVERNANCE Remuneration for the Board of Directors Remuneration structure components In accordance with our Remuneration Policy and the decision of the annual Shareholders’ Meeting of 28 April 2020, the Board of Directors fee levels applicable for financial year 2023 were as set out in the table below. Note that the remuneration of the Directors does not include any variable remuneration or benefits, except for tax filing support in respect of Galapagos’ remuneration. Annual cash fee level to acquire (1) GLPG Role Annual cash fee level shares Chair(2) €100,000 €100,000 Non-Executive Director €50,000 €50,000 Committee Chair €20,000 N/A Committee member €15,000 N/A (1) The non-executive Directors receive a cash compensation equal to the amount of their fixed annual cash remuneration (not taking into account fees for Committee membership and Chairmanship) subject to the commitment by each non-executive Director to use the net portion (after taxation) of such cash remuneration to purchase shares of Galapagos in the open market within a set period of time after receipt of such cash remuneration. The shares that each Director so acquires must be held until at least one year after the Director leaves the Board of Directors and at least three years after the time of acquisition. This cash compensation constitutes the equivalent of the equity component of the members of the Board of Directors’ remuneration, as recommended by section 7.6 of the 2020 Corporate Governance Code. (2) The Chair fees were not payable for financial year 2023, as the CEO is only remunerated for the performance of his executive functions as CEO and is not entitled to any additional remuneration for his mandates of Chair of the Board of Directors and Committee member. 150 Galapagos NV Annual Report 2023
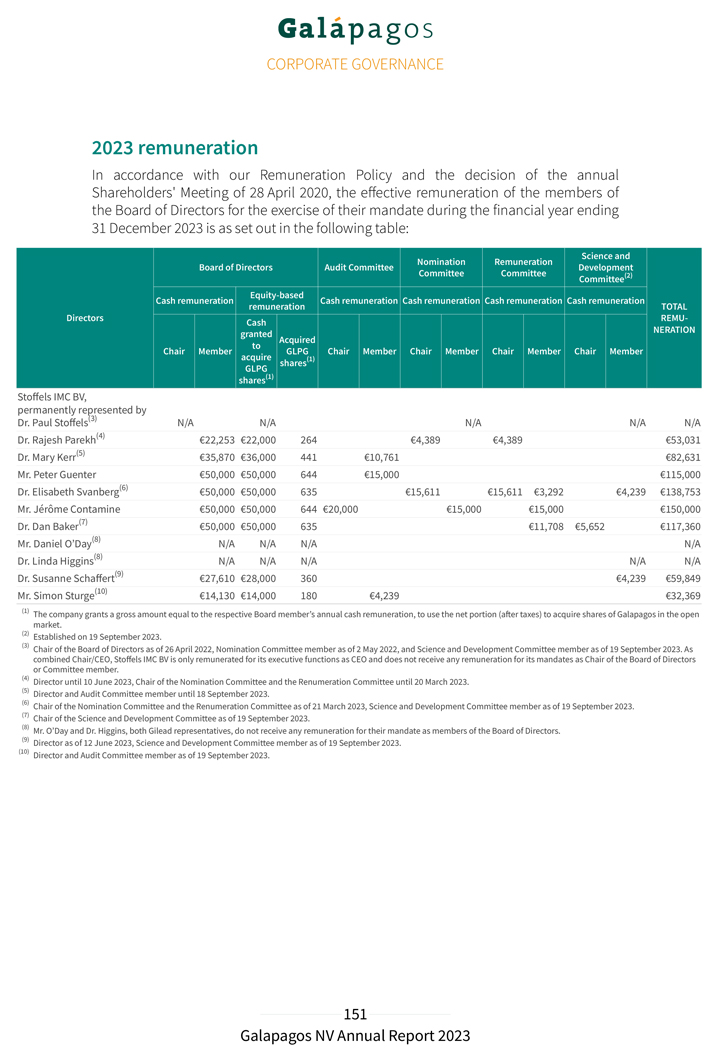
CORPORATE GOVERNANCE 2023 remuneration In accordance with our Remuneration Policy and the decision of the annual Shareholders’ Meeting of 28 April 2020, the effective remuneration of the members of the Board of Directors for the exercise of their mandate during the financial year ending 31 December 2023 is as set out in the following table: Science and Nomination Remuneration Board of Directors Audit Committee Development Committee Committee (2) Committee Equity-based Cash remuneration Cash remuneration Cash remuneration Cash remuneration Cash remuneration remuneration TOTAL Directors Cash REMU-granted NERATION Acquired to Chair Member GLPG Chair Member Chair Member Chair Member Chair Member acquire (1) shares GLPG(1) shares Stoffels IMC BV, permanently represented (3) by Dr. Paul Stoffels N/A N/A N/A N/A N/A Dr. Rajesh Parekh(4) €22,253 €22,000 264 €4,389 €4,389 €53,031 Dr. Mary Kerr(5) €35,870 €36,000 441 €10,761 €82,631 Mr. Peter Guenter €50,000 €50,000 644 €15,000 €115,000 Dr. Elisabeth Svanberg(6) €50,000 €50,000 635 €15,611 €15,611 €3,292 €4,239 €138,753 Mr. Jérôme Contamine €50,000 €50,000 644 €20,000 €15,000 €15,000 €150,000 Dr. Dan Baker(7) €50,000 €50,000 635 €11,708 €5,652 €117,360 Mr. Daniel O’Day(8) N/A N/A N/A N/A Dr. Linda Higgins(8) N/A N/A N/A N/A N/A Dr. Susanne Schaffert(9) €27,610 €28,000 360 €4,239 €59,849 Mr. Simon Sturge(10) €14,130 €14,000 180 €4,239 €32,369 (1) The company grants a gross amount equal to the respective Board member’s annual cash remuneration, to use the net portion (after taxes) to acquire shares of Galapagos in the open market. (2) Established on 19 September 2023. (3) Chair of the Board of Directors as of 26 April 2022, Nomination Committee member as of 2 May 2022, and Science and Development Committee member as of 19 September 2023. As combined Chair/CEO, Stoffels IMC BV is only remunerated for its executive functions as CEO and does not receive any remuneration for its mandates as Chair of the Board of Directors or Committee member. (4) Director until 10 June 2023, Chair of the Nomination Committee and the Renumeration Committee until 20 March 2023. (5) Director and Audit Committee member until 18 September 2023. (6) Chair of the Nomination Committee and the Renumeration Committee as of 21 March 2023, Science and Development Committee member as of 19 September 2023. (7) Chair of the Science and Development Committee as of 19 September 2023. (8) Mr. O’Day and Dr. Higgins, both Gilead representatives, do not receive any remuneration for their mandate as members of the Board of Directors. (9) Director as of 12 June 2023, Science and Development Committee member as of 19 September 2023. (10) Director and Audit Committee member as of 19 September 2023. 151 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Remuneration for Executive Committee members Peer groups A peer group and benchmarking exercise for Executive Committee roles was completed between late 2022 and early 2023 in light of our strategic transformation and revised R&D strategy, focused on immunology and oncology with the aim to transform patient outcomes through life-changing science and innovation. Galapagos is at a pivotal juncture resetting its strategic path and building an oncology franchise where attracting and retaining highly specialized expertise in an international labor market is essential to succeed. Both European and U.S. peer groups were found appropriate given the talent pool for the Executive Committee extends to both Europe and the U.S., with the majority of our competitors based in the U.S. The peer groups listed below consist of publicly listed biotechnology and pharmaceutical companies, selected considering size, international growth ambitions and, to the extent possible, business model, lifecycle stage and therapeutic areas. These benchmarks support the Board, upon recommendation of the Remuneration Committee, in its decision-making, also taking into account Galapagos’ strategic context and requirements, company performance, individual performance and skills as well as broader workforce considerations. The Remuneration Committee looks at each Executive Committee member’s home market as the primary reference point with consideration also given the internal talent market in which they operate, have operated or could operate. The Remuneration Committee strives to take a balanced and responsible approach, in particular with long-term incentives where competitive practice on quantum and structure can vary significantly between the U.S. and elsewhere. European peers U.S. peers Genmab A/S United Therapeutics Corp Argenx SE Neurocrine Biosciences Inc Jazz Pharmaceuticals PLC Sarepta Therapeutics Inc Ipsen SA Exelixis Inc Swedish Orphan Biovitrum AB Ionis Pharmaceuticals Inc Ascendis Pharma A/S Vir Biotechnology Inc Alkermes Plc Amicus Therapeutics Inc Idorsia Ltd SAGE Therapeutics Inc Immunocore Holdings PLC Ligand Pharmaceuticals Inc MorphoSys AG Kymera Therapeutics Inc Uniqure NV Ironwood Pharmaceuticals Inc Agios Pharmaceuticals Inc Nektar Therapeutics FibroGen Inc 152 Galapagos NV Annual Report 2023
CORPORATE GOVERNANCE Finally, the BEL20 (the benchmark stock market index of Euronext Brussels) general industry peer group (excluding financial services companies) is considered to ensure there is an understanding of the local Belgian listed market given the location of our headquarters. However, given the international nature of our executive leadership and specific sector considerations, it is not the only reference to inform our pay policy. 2023 remuneration summary In accordance with our Remuneration Policy, the remuneration of the members of the Executive Committee for the exercise of their mandate during the financial year ending 31 December 2023 was as set out in the following table: Fixed remuneration Variable remuneration Proportion of fixed Other Multi-year variable TOTAL REMU-Executive Committee Annual and variable Base salary compo- Pension (2) Vested Granted NERATION (1) bonus remuneration nents RSUs(3) SRs(4) Stoffels IMC BV, permanently represented Fixed: 38% by Dr. Paul Stoffels €750,000 €0.00 €0.00 €506,250 €647,536 €67,500 €1,971,286 Variable: 62% (5) Fixed: 42% Other ExCom members €1,605,839 €304,633 €208,804 €735,000 €2,127,645 €101,250 €5,083,171 Variable: 58% (1) Other components are the value of the benefits and perquisites awarded, such as a company car, tax advisory services, and health and disability insurance. (2) The one-year variable is the annual cash bonus awarded to each Executive Committee member in respect of 2023 and paid in April 2024. (3) During financial year 2023, RSUs vested under RSU plans 2019.II, 2020.I, 2020.II, 2021.I, 2021.II, 2021.IV, 2022.I and 2022.II and pay-outs occurred accordingly. (4) The value of the subscription rights (“SRs”) granted during the financial year 2023 is calculated by comparing the exercise price with the average share price of the share as quoted on Euronext Brussels and Amsterdam during the financial year 2023. (5) Pursuant to the applicable Belgian legislation for the one-tier governance system, we hereby disclose the remuneration of the other Executive Committee members on an aggregated basis. This includes remuneration paid to Bart Filius until 30 June 2023 and to Thad Huston as of 1 July 2023. Fixed remuneration Base salaries Base salary is set to reflect responsibilities, relevant experience and competence, and market rates for equivalent positions. The Board, upon recommendation of the Remuneration Committee, decided that for the financial year 2023 each member of the Executive Committee received the base salary, identified individually for the CEO and in aggregate for other members of the Executive Committee in the total remuneration table above. In particular, the base salary for the CEO remained unchanged in 2023. Pension and other components In addition, the members of the Executive Committee are provided with various benefits in line with our Remuneration Policy such as a retirement plan, insurance programs (including life insurance, disability and health), company cars and the provision of certain tax services. The pension and other components of the remuneration of each Executive Committee member are summarized in the total remuneration table above. Short-term variable remuneration Upon recommendation of the Remuneration Committee, the Board of Directors determined an overall achievement of 90% (out of a maximum of 100%) against the 2023 corporate objectives. In arriving at this determination, the Board considered performance against objectives set (highlights of which are set out in the table below), management of unforeseen developments as well as achievements towards the long- 153 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE term strategic goals of Galapagos’ transformation into a pure-play R&D biotechnology company, entering into the new therapeutic area of oncology with a streamlined organization set up for future growth. As part of this transformation, Galapagos’ research and discovery capabilities and employees based in Romainville, France, were transferred to NovAliX, and the Company signed a Share and Asset Purchase Agreement with Alfasigma for the transfer of the Jyseleca® business. The latter transaction closed in January 2024. The Jyseleca® divestiture, streamlining of the operations and transitioning of Galapagos into a pure-play R&D biotechnology company were considered key value-drivers and accomplishments for the Company. 2023 Corporate Objectives (each equally weighted) Corporate Cash Burn Deliver on cash burn guidance announced in FY results Quality & compliance Being inspection ready related to file submission Maintaining active operating licences without critical observations Achieving training compliance targets No material weakness SOx People Hire and retain senior leadership capabilities in core therapeutic areas of oncology and immunology Support the growth in oncology per strategic workforce plan Set-up U.S. organization footprint including clinical development & regulatory capabilities ESG Confirm the ESG strategy & start executing on this Prepare for regulatory requirements (CSRD) We remained disciplined in our spending and delivered on our cash burn guidance of €380-420 million for 2023 (full year 2023 cash burn: €415 million). We also laid the foundation for a sustainable and R&D focused capital allocation through the Jyseleca® divestiture and streamlining of the remaining organization. Quality and Compliance objectives were met across the board, as we continue to further strengthen our capabilities and systems. From a people perspective, we made good progress in staffing our oncology therapeutic area, attracting expertise talent. The expansion of our U.S. footprint proceeded as planned, which is an important step to attract future talent. We significantly ramped up our ESG activities by defining an overall ESG strategy for Galapagos together with an implementation plan. We are on track to meet regulatory requirements for the Corporate Sustainability Reporting Directive (EU). Commercial Maximize Jyseleca® €140-160 million net sales guidance Reliable supply We successfully managed the supply of Jyseleca® to patients across Europe, and over 21,000 patients across Europe currently benefit from the drug. However, Jyseleca® revenues did not meet expectations. This was driven mainly by a general JAKi class slowdown in the European market following the Article 20 label update. Full year 2023 Jyseleca® revenues came in at €112 million, below our original guidance and within the restated guidance at H1 2023 of €100-120 million. As a consequence of the market dynamics, we took deliberate action to conduct a strategic review of Jyseleca® mid 2023 and pivoted to a divestiture of the business in H2 2023, with an agreement being signed before year-end and closing in January 2024 (see more under Business Development). 154 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE 2023 Corporate Objectives (each equally weighted) Immunology Advance our immunology portfolio Conduct multiple Phase 1/2 trials Conduct multiple discovery programs and deliver preclinical leads Advance CAR-T programs in immunology We started recruitment into the DM and SLE Phase 2 trials with our TYK2 inhibitor GLPG3667 and are progressing both studies successfully . While we have not yet nominated preclinical product candidates, we have several preclinical immunology programs running in discovery. We decided not to advance our CAR-T program with GLPG5101 in rSLE (or other immunology indications) into the clinic. [Graphic Appears Here] Build a pipeline of best-in-class oncology therapies Start CAR-T NHL & CLL expansion cohorts, start MM CAR-T Phase 1/2 trial in MM in Europe File INDs for CD19 and MM Regulatory progress for the oncology programs Build out point-of-care manufacturing network in EU Start-up & roll-out of US sites for point-of-care manufacturing network Initiate multiple other discovery programs and deliver preclinical leads We progressed our Phase 1/2 CAR-T studies with GLPG5101 in NHL and with GLPG5201 in CLL/RT and reported encouraging preliminary safety and efficacy data at ASH in December. We also started a Phase 1/2 CAR-T study with GLPG5301 in MM. As a result, we now have three clinical studies running on our point-of-care manufacturing platform. We made regulatory progress for our oncology programs. However, as the FDA requires that the data from the ongoing tech transfer to a first U.S. site are part of the filing package, we did not yet file the IND submissions in the U.S. In 2023, we have been working on building out our point-of-care manufacturing network in Europe as well as the U.S. We currently have five centers open in three European countries (Belgium, The Netherlands, and France), and in 2023, we signed a contract with Landmark Bio out of Boston, our first U.S. site. We are in different stages of negotiations with a number of parties to open additional sites in the U.S. and in Europe. While we have not yet nominated preclinical product candidates, we have multiple preclinical oncology programs running in Discovery, across modalities. Business Development Execute Business Development transactions Execute an acquisition (in-licensing or M&A), in line with Board-approved strategy Accelerating innovation and building our pipeline through strategic partnerships and M&A remains our focus. Throughout 2023, multiple clinical stage assets were reviewed in detail, and as we remain selective, disciplined, and science-driven in our pursuit of transformational medicines with best-in-class potential, we have not executed on any. Several research collaborations were set up to accelerate our early-stage pipeline. We executed an agreement to transfer the entire Jyseleca® business to Alfasigma, including the European and UK Marketing Authorizations, and the commercial, medical affairs, and development activities for Jyseleca®, as well as 400 positions in 14 European countries. This transaction secured continued access to the product to over 21,000 patients in Europe. We move forward with a streamlined portfolio and enhanced focus, enabling more R&D investment. 155 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE The Board considered a 90% corporate funding level for 2023 achievements. This is applicable to the wider Galapagos workforce for the corporate component of their bonus funding. The Board considered this level of funding for the CEO, upon recommendation of the Remuneration Committee, and for the other Executive Committee members, upon proposal of the CEO, together with the individual performance of Executive Committee members, in order to determine the individual annual bonus outcomes for 2023 set out in the total remuneration table above. These 2023 annual bonuses will be paid in April 2024. In addition, a number of RSUs corresponding to these bonuses will be granted to the current members of the Executive Committee as part of a 2024 RSU grant. Please see the Section “Long-term variable remuneration” for more information on RSUs. Long-term variable remuneration The total remuneration table above under Section “2023 remuneration summary” sets forth the following: The value of the RSUs vested and paid out in 2023 for each member of the Executive Committee. During 2023, there were RSU vestings under eight different RSU plans: Plan 2019.II, Plan 2020.I, Plan 2020.II, Plan 2021.I, Plan 2021.II, Plan 2021.IV, Plan 2022.I, and Plan 2022.II. The pay-outs to the Executive Committee members occurred accordingly and the aggregate amounts are set forth in the total remuneration table above. The value of the subscription rights granted during the financial year 2023 calculated by comparing the exercise price with the average share price of the share as quoted on Euronext Brussels and Amsterdam during the financial year 2023. In determining the annual equity awards made to Executive Committee members in the financial year 2023, the Board considered a number of factors, including company performance, individual performance and ability to drive future value creation in the context of the current business transformation, the overall retention value of past equity awards and competitive levels of equity compensation for similarly positioned executives based on analysis of data from our disclosed peer groups. As a result, the following equity awards were made to Executive Committee members in financial year 2023: 325,000 Subscription rights under Subscription Right Plan 2023 BE, of which 50,000 were granted to the CEO 21,970 RSUs under Plan 2023.I, of which 9,695 were granted to the CEO 309,096 RSUs under Plan 2023.II, of which 129,276 were granted to the CEO Further reference is made to the Equity components of the remuneration section, which contains, among others, a description of the 2023 grant of subscription rights and RSUs. 156 Galapagos NV Annual Report 2023
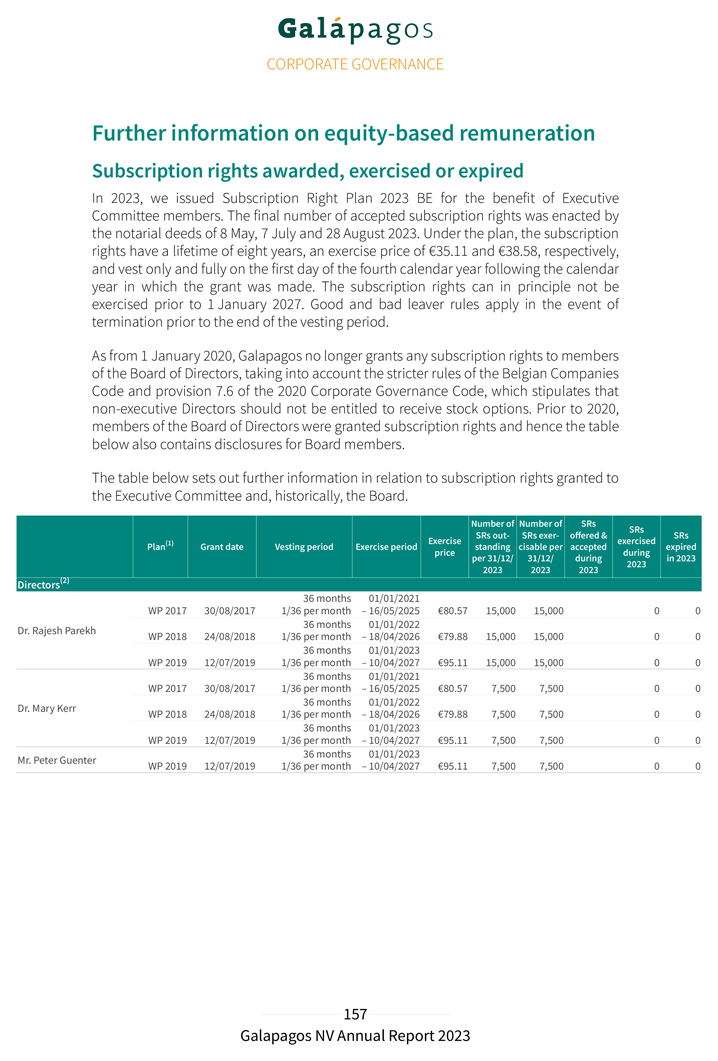
CORPORATE GOVERNANCE Further information on equity-based remuneration Subscription rights awarded, exercised or expired In 2023, we issued Subscription Right Plan 2023 BE for the benefit of Executive Committee members. The final number of accepted subscription rights was enacted by the notarial deeds of 8 May, 7 July and 28 August 2023. Under the plan, the subscription rights have a lifetime of eight years, an exercise price of €35.11 and €38.58, respectively, and vest only and fully on the first day of the fourth calendar year following the calendar year in which the grant was made. The subscription rights can in principle not be exercised prior to 1 January 2027. Good and bad leaver rules apply in the event of termination prior to the end of the vesting period. As from 1 January 2020, Galapagos no longer grants any subscription rights to members of the Board of Directors, taking into account the stricter rules of the Belgian Companies Code and provision 7.6 of the 2020 Corporate Governance Code, which stipulates that non-executive Directors should not be entitled to receive stock options. Prior to 2020, members of the Board of Directors were granted subscription rights and hence the table below also contains disclosures for Board members. The table below sets out further information in relation to subscription rights granted to the Executive Committee and, historically, the Board. Number of Number of SRs SRs SRs out- SRs exer- offered & SRs (1) Exercise exercised Plan Grant date Vesting period Exercise period standing cisable per accepted expired price during per 31/12/ 31/12/ during in 2023 2023 2023 2023 2023 Directors(2) 36 months 01/01/2021 WP 2017 30/08/2017 1/36 per month – 16/05/2025 €80.57 15,000 15,000 0 0 36 months 01/01/2022 Dr. Rajesh Parekh WP 2018 24/08/2018 1/36 per month – 18/04/2026 €79.88 15,000 15,000 0 0 36 months 01/01/2023 WP 2019 12/07/2019 1/36 per month – 10/04/2027 €95.11 15,000 15,000 0 0 36 months 01/01/2021 WP 2017 30/08/2017 1/36 per month – 16/05/2025 €80.57 7,500 7,500 0 0 36 months 01/01/2022 Dr. Mary Kerr WP 2018 24/08/2018 1/36 per month – 18/04/2026 €79.88 7,500 7,500 0 0 36 months 01/01/2023 WP 2019 12/07/2019 1/36 per month – 10/04/2027 €95.11 7,500 7,500 0 0 36 months 01/01/2023 Mr. Peter Guenter WP 2019 12/07/2019 1/36 per month – 10/04/2027 €95.11 7,500 7,500 0 0 157 Galapagos NV Annual Report 2023
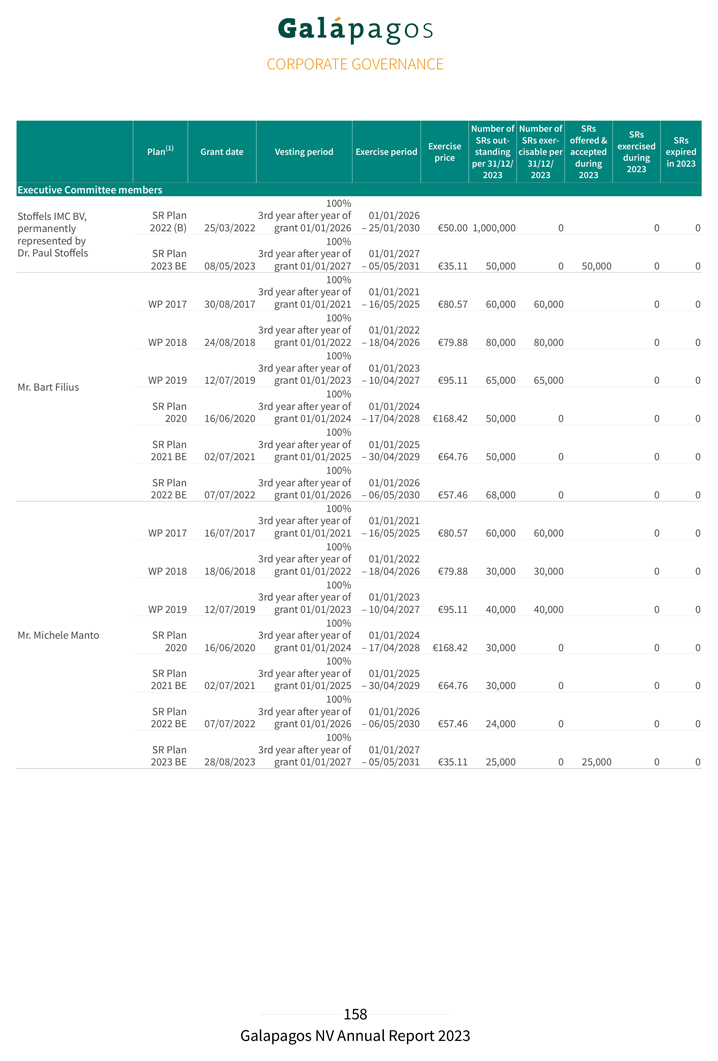
CORPORATE GOVERNANCE Number of Number of SRs SRs SRs out- SRs exer- offered & SRs (1) Exercise exercised Plan Grant date Vesting period Exercise period standing cisable per accepted expired price during per 31/12/ 31/12/ during in 2023 2023 2023 2023 2023 Executive Committee members 100% Stoffels IMC BV, SR Plan 3rd year after year of 01/01/2026 permanently 2022 (B) 25/03/2022 grant 01/01/2026 – 25/01/2030 €50.00 1,000,000 0 0 0 represented by 100% Dr. Paul Stoffels SR Plan 3rd year after year of 01/01/2027 2023 BE 08/05/2023 grant 01/01/2027 – 05/05/2031 €35.11 50,000 0 50,000 0 0 100% 3rd year after year of 01/01/2021 WP 2017 30/08/2017 grant 01/01/2021 – 16/05/2025 €80.57 60,000 60,000 0 0 100% 3rd year after year of 01/01/2022 WP 2018 24/08/2018 grant 01/01/2022 – 18/04/2026 €79.88 80,000 80,000 0 0 100% 3rd year after year of 01/01/2023 WP 2019 12/07/2019 grant 01/01/2023 – 10/04/2027 €95.11 65,000 65,000 0 0 Mr. Bart Filius 100% SR Plan 3rd year after year of 01/01/2024 2020 16/06/2020 grant 01/01/2024 – 17/04/2028 €168.42 50,000 0 0 0 100% SR Plan 3rd year after year of 01/01/2025 2021 BE 02/07/2021 grant 01/01/2025 – 30/04/2029 €64.76 50,000 0 0 0 100% SR Plan 3rd year after year of 01/01/2026 2022 BE 07/07/2022 grant 01/01/2026 – 06/05/2030 €57.46 68,000 0 0 0 100% 3rd year after year of 01/01/2021 WP 2017 16/07/2017 grant 01/01/2021 – 16/05/2025 €80.57 60,000 60,000 0 0 100% 3rd year after year of 01/01/2022 WP 2018 18/06/2018 grant 01/01/2022 – 18/04/2026 €79.88 30,000 30,000 0 0 100% 3rd year after year of 01/01/2023 WP 2019 12/07/2019 grant 01/01/2023 – 10/04/2027 €95.11 40,000 40,000 0 0 100% Mr. Michele Manto SR Plan 3rd year after year of 01/01/2024 2020 16/06/2020 grant 01/01/2024 – 17/04/2028 €168.42 30,000 0 0 0 100% SR Plan 3rd year after year of 01/01/2025 2021 BE 02/07/2021 grant 01/01/2025 – 30/04/2029 €64.76 30,000 0 0 0 100% SR Plan 3rd year after year of 01/01/2026 2022 BE 07/07/2022 grant 01/01/2026 – 06/05/2030 €57.46 24,000 0 0 0 100% SR Plan 3rd year after year of 01/01/2027 2023 BE 28/08/2023 grant 01/01/2027 – 05/05/2031 €35.11 25,000 0 25,000 0 0 158 Galapagos NV Annual Report 2023
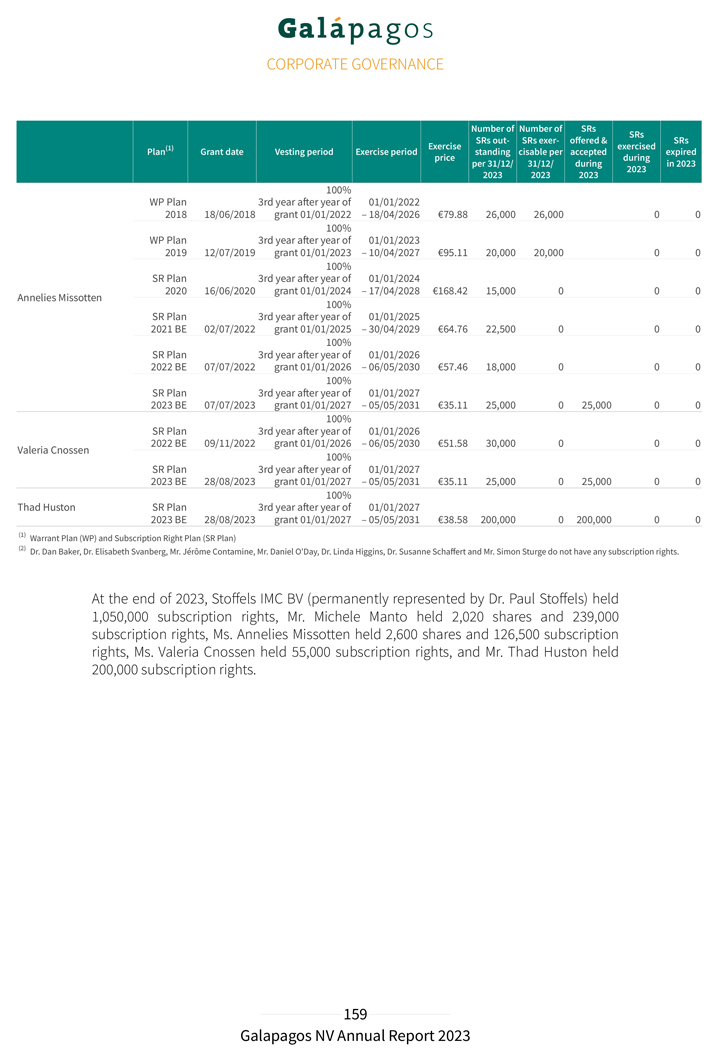
CORPORATE GOVERNANCE Number of Number of SRs SRs SRs out- SRs exer- offered & SRs (1) Exercise exercised Plan Grant date Vesting period Exercise period standing cisable per accepted expired price during per 31/12/ 31/12/ during in 2023 2023 2023 2023 2023 100% WP Plan 3rd year after year of 01/01/2022 2018 18/06/2018 grant 01/01/2022 – 18/04/2026 €79.88 26,000 26,000 0 0 100% WP Plan 3rd year after year of 01/01/2023 2019 12/07/2019 grant 01/01/2023 – 10/04/2027 €95.11 20,000 20,000 0 0 100% SR Plan 3rd year after year of 01/01/2024 2020 16/06/2020 grant 01/01/2024 – 17/04/2028 €168.42 15,000 0 0 0 Annelies Missotten 100% SR Plan 3rd year after year of 01/01/2025 2021 BE 02/07/2022 grant 01/01/2025 – 30/04/2029 €64.76 22,500 0 0 0 100% SR Plan 3rd year after year of 01/01/2026 2022 BE 07/07/2022 grant 01/01/2026 – 06/05/2030 €57.46 18,000 0 0 0 100% SR Plan 3rd year after year of 01/01/2027 2023 BE 07/07/2023 grant 01/01/2027 – 05/05/2031 €35.11 25,000 0 25,000 0 0 100% SR Plan 3rd year after year of 01/01/2026 2022 BE 09/11/2022 grant 01/01/2026 – 06/05/2030 €51.58 30,000 0 0 0 Valeria Cnossen 100% SR Plan 3rd year after year of 01/01/2027 2023 BE 28/08/2023 grant 01/01/2027 – 05/05/2031 €35.11 25,000 0 25,000 0 0 100% Thad Huston SR Plan 3rd year after year of 01/01/2027 2023 BE 28/08/2023 grant 01/01/2027 – 05/05/2031 €38.58 200,000 0 200,000 0 0 (1) Warrant Plan (WP) and Subscription Right Plan (SR Plan) (2) Dr. Dan Baker, Dr. Elisabeth Svanberg, Mr. Jérôme Contamine, Mr. Daniel O’Day, Dr. Linda Higgins, Dr. Susanne Schaffert and Mr. Simon Sturge do not have any subscription rights. At the end of 2023, Stoffels IMC BV (permanently represented by Dr. Paul Stoffels) held 1,050,000 subscription rights, Mr. Michele Manto held 2,020 shares and 239,000 subscription rights, Ms. Annelies Missotten held 2,600 shares and 126,500 subscription rights, Ms. Valeria Cnossen held 55,000 subscription rights, and Mr. Thad Huston held 200,000 subscription rights. 159 Galapagos NV Annual Report 2023
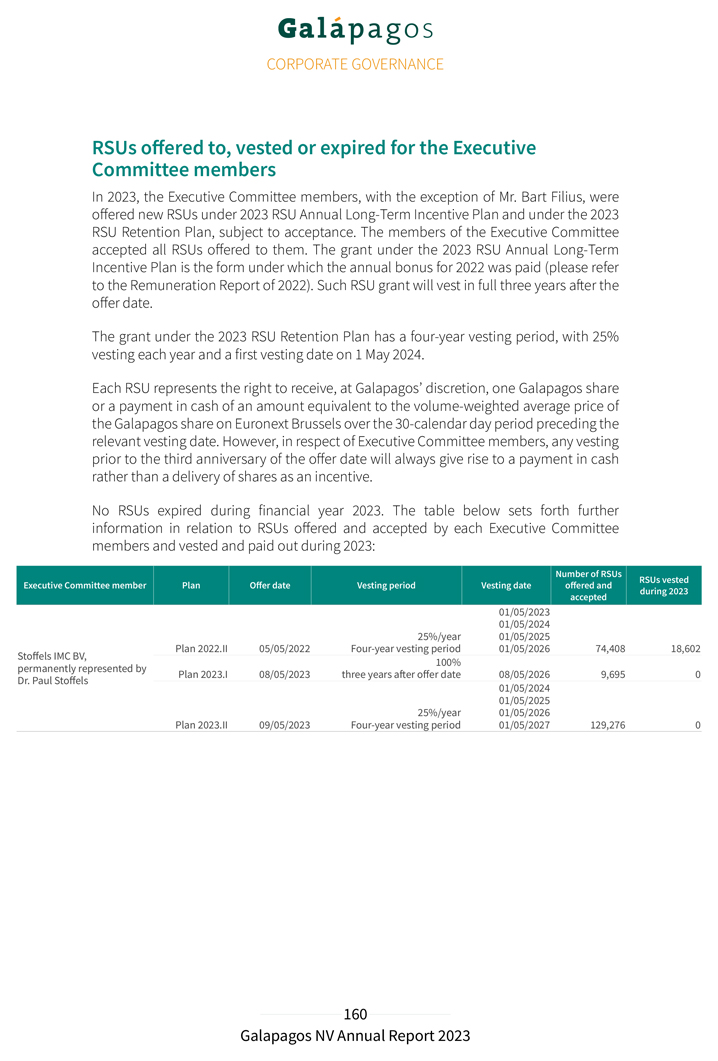
CORPORATE GOVERNANCE RSUs offered to, vested or expired for the Executive Committee members In 2023, the Executive Committee members, with the exception of Mr. Bart Filius, were offered new RSUs under 2023 RSU Annual Long-Term Incentive Plan and under the 2023 RSU Retention Plan, subject to acceptance. The members of the Executive Committee accepted all RSUs offered to them. The grant under the 2023 RSU Annual Long-Term Incentive Plan is the form under which the annual bonus for 2022 was paid (please refer to the Remuneration Report of 2022). Such RSU grant will vest in full three years after the offer date. The grant under the 2023 RSU Retention Plan has a four-year vesting period, with 25% vesting each year and a first vesting date on 1 May 2024. Each RSU represents the right to receive, at Galapagos’ discretion, one Galapagos share or a payment in cash of an amount equivalent to the volume-weighted average price of the Galapagos share on Euronext Brussels over the 30-calendar day period preceding the relevant vesting date. However, in respect of Executive Committee members, any vesting prior to the third anniversary of the offer date will always give rise to a payment in cash rather than a delivery of shares as an incentive. No RSUs expired during financial year 2023. The table below sets forth further information in relation to RSUs offered and accepted by each Executive Committee members and vested and paid out during 2023: Number of RSUs RSUs vested Executive Committee member Plan Offer date Vesting period Vesting date offered and during 2023 accepted 01/05/2023 01/05/2024 25%/year 01/05/2025 Plan 2022.II 05/05/2022 Four-year vesting period 01/05/2026 74,408 18,602 Stoffels IMC BV, 100% permanently represented by Plan 2023.I 08/05/2023 three years after offer date 08/05/2026 9,695 0 Dr. Paul Stoffels 01/05/2024 01/05/2025 25%/year 01/05/2026 Plan 2023.II 09/05/2023 Four-year vesting period 01/05/2027 129,276 0 160 Galapagos NV Annual Report 2023
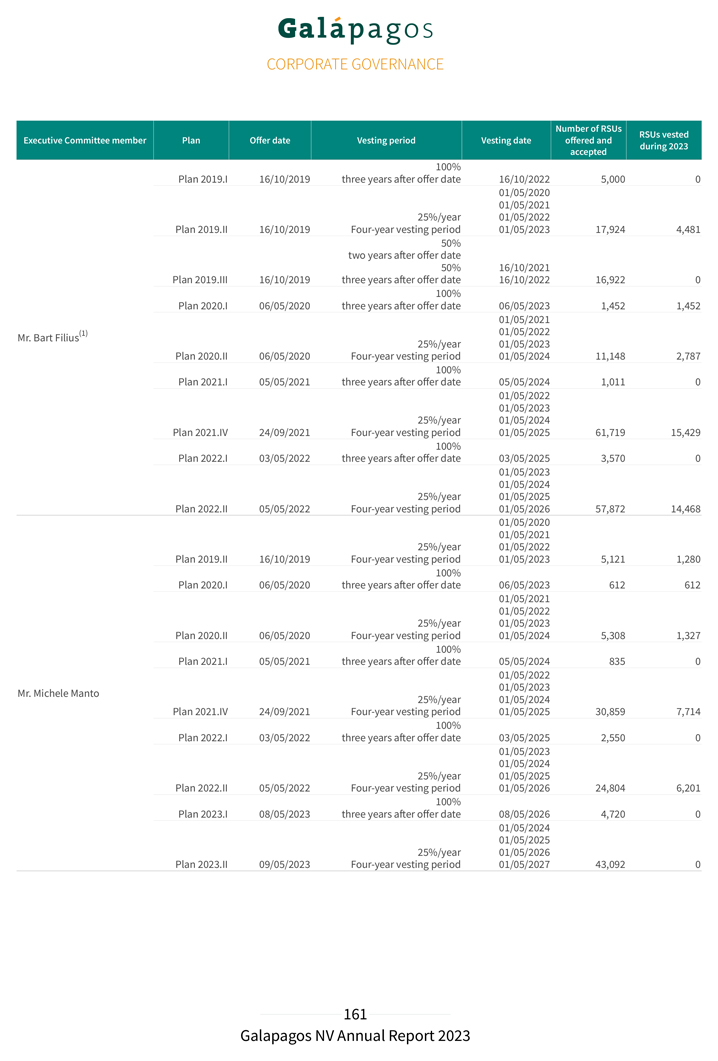
Number of RSUs RSUs vested Executive Committee member Plan Offer date Vesting period Vesting date offered and during 2023 accepted 100% Plan 2019.I 16/10/2019 three years after offer date 16/10/2022 5,000 0 01/05/2020 01/05/2021 25%/year 01/05/2022 Plan 2019.II 16/10/2019 Four-year vesting period 01/05/2023 17,924 4,481 50% two years after offer date 50% 16/10/2021 Plan 2019.III 16/10/2019 three years after offer date 16/10/2022 16,922 0 100% Plan 2020.I 06/05/2020 three years after offer date 06/05/2023 1,452 1,452 01/05/2021 (1) 01/05/2022 Mr. Bart Filius 25%/year 01/05/2023 Plan 2020.II 06/05/2020 Four-year vesting period 01/05/2024 11,148 2,787 100% Plan 2021.I 05/05/2021 three years after offer date 05/05/2024 1,011 0 01/05/2022 01/05/2023 25%/year 01/05/2024 Plan 2021.IV 24/09/2021 Four-year vesting period 01/05/2025 61,719 15,429 100% Plan 2022.I 03/05/2022 three years after offer date 03/05/2025 3,570 0 01/05/2023 01/05/2024 25%/year 01/05/2025 Plan 2022.II 05/05/2022 Four-year vesting period 01/05/2026 57,872 14,468 01/05/2020 01/05/2021 25%/year 01/05/2022 Plan 2019.II 16/10/2019 Four-year vesting period 01/05/2023 5,121 1,280 100% Plan 2020.I 06/05/2020 three years after offer date 06/05/2023 612 612 01/05/2021 01/05/2022 25%/year 01/05/2023 Plan 2020.II 06/05/2020 Four-year vesting period 01/05/2024 5,308 1,327 100% Plan 2021.I 05/05/2021 three years after offer date 05/05/2024 835 0 01/05/2022 01/05/2023 Mr. Michele Manto 25%/year 01/05/2024 Plan 2021.IV 24/09/2021 Four-year vesting period 01/05/2025 30,859 7,714 100% Plan 2022.I 03/05/2022 three years after offer date 03/05/2025 2,550 0 01/05/2023 01/05/2024 25%/year 01/05/2025 Plan 2022.II 05/05/2022 Four-year vesting period 01/05/2026 24,804 6,201 100% Plan 2023.I 08/05/2023 three years after offer date 08/05/2026 4,720 0 01/05/2024 01/05/2025 25%/year 01/05/2026 Plan 2023.II 09/05/2023 Four-year vesting period 01/05/2027 43,092 0 161 Galapagos NV Annual Report 2023
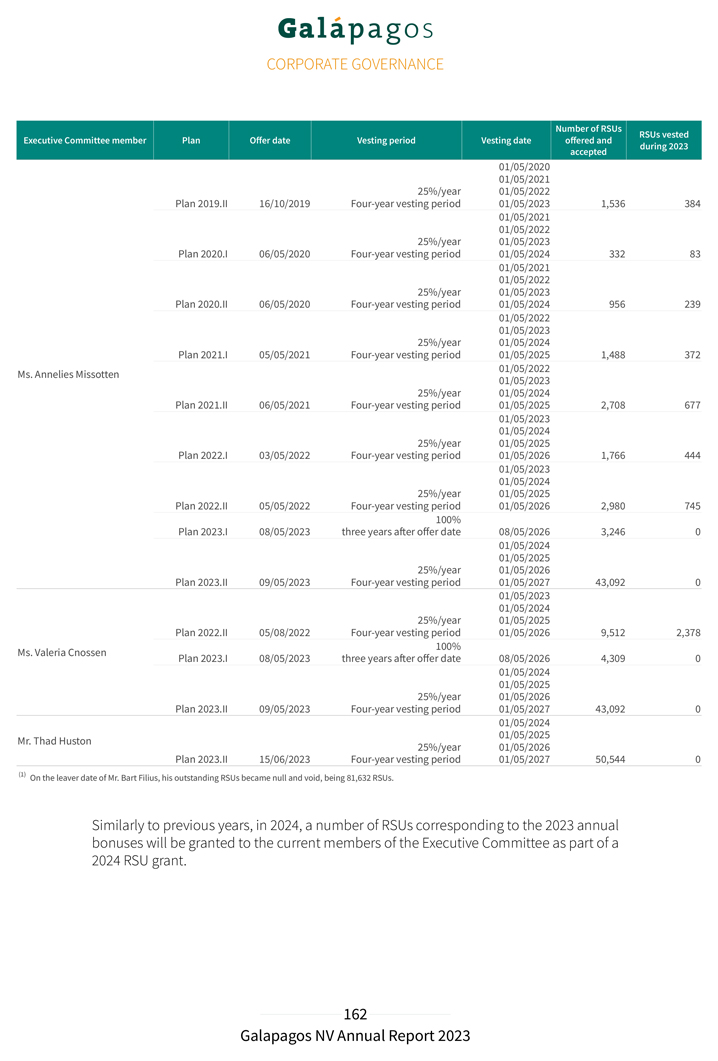
CORPORATE GOVERNANCE Number of RSUs RSUs vested Executive Committee member Plan Offer date Vesting period Vesting date offered and during 2023 accepted 01/05/2020 01/05/2021 25%/year 01/05/2022 Plan 2019.II 16/10/2019 Four-year vesting period 01/05/2023 1,536 384 01/05/2021 01/05/2022 25%/year 01/05/2023 Plan 2020.I 06/05/2020 Four-year vesting period 01/05/2024 332 83 01/05/2021 01/05/2022 25%/year 01/05/2023 Plan 2020.II 06/05/2020 Four-year vesting period 01/05/2024 956 239 01/05/2022 01/05/2023 25%/year 01/05/2024 Plan 2021.I 05/05/2021 Four-year vesting period 01/05/2025 1,488 372 01/05/2022 Ms. Annelies Missotten 01/05/2023 25%/year 01/05/2024 Plan 2021.II 06/05/2021 Four-year vesting period 01/05/2025 2,708 677 01/05/2023 01/05/2024 25%/year 01/05/2025 Plan 2022.I 03/05/2022 Four-year vesting period 01/05/2026 1,766 444 01/05/2023 01/05/2024 25%/year 01/05/2025 Plan 2022.II 05/05/2022 Four-year vesting period 01/05/2026 2,980 745 100% Plan 2023.I 08/05/2023 three years after offer date 08/05/2026 3,246 0 01/05/2024 01/05/2025 25%/year 01/05/2026 Plan 2023.II 09/05/2023 Four-year vesting period 01/05/2027 43,092 0 01/05/2023 01/05/2024 25%/year 01/05/2025 Plan 2022.II 05/08/2022 Four-year vesting period 01/05/2026 9,512 2,378 100% Ms. Valeria Cnossen Plan 2023.I 08/05/2023 three years after offer date 08/05/2026 4,309 0 01/05/2024 01/05/2025 25%/year 01/05/2026 Plan 2023.II 09/05/2023 Four-year vesting period 01/05/2027 43,092 0 01/05/2024 01/05/2025 Mr. Thad Huston 25%/year 01/05/2026 Plan 2023.II 15/06/2023 Four-year vesting period 01/05/2027 50,544 0 (1) On the leaver date of Mr. Bart Filius, his outstanding RSUs became null and void, being 81,632 RSUs. Similarly to previous years, in 2024, a number of RSUs corresponding to the 2023 annual bonuses will be granted to the current members of the Executive Committee as part of a 2024 RSU grant. 162 Galapagos NV Annual Report 2023
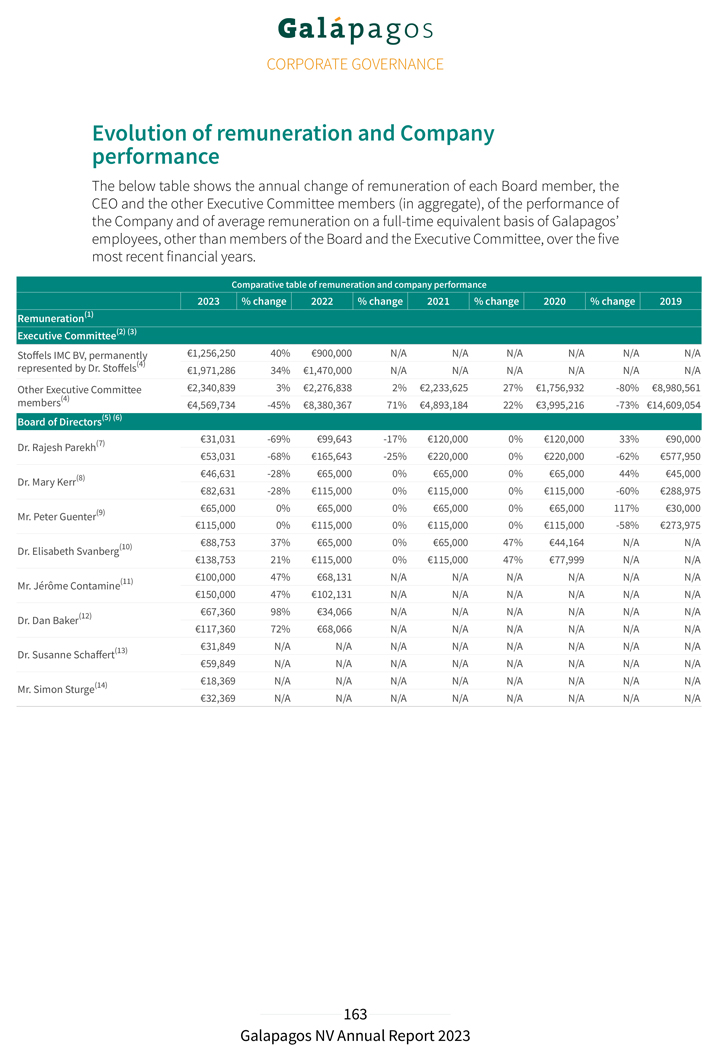
CORPORATE GOVERNANCE Evolution performance of remuneration and Company The below table shows the annual change of remuneration of each Board member, the CEO and the other Executive Committee members (in aggregate), of the performance of the Company and of average remuneration on a full-time equivalent basis of Galapagos’ employees, other than members of the Board and the Executive Committee, over the five most recent financial years. Comparative table of remuneration and company performance 2023 % change 2022 % change 2021 % change 2020 % change 2019 Remuneration(1) Executive Committee(2) (3) Stoffels IMC BV, permanently €1,256,250 40% €900,000 N/A N/A N/A N/A N/A N/A represented by Dr. Stoffels(4) €1,971,286 34% €1,470,000 N/A N/A N/A N/A N/A N/A Other Executive Committee €2,340,839 3% €2,276,838 2% €2,233,625 27% €1,756,932 -80% €8,980,561 members(4) €4,569,734 -45% €8,380,367 71% €4,893,184 22% €3,995,216 -73% €14,609,054 Board of Directors(5) (6) (7) €31,031 -69% €99,643 -17% €120,000 0% €120,000 33% €90,000 Dr. Rajesh Parekh €53,031 -68% €165,643 -25% €220,000 0% €220,000 -62% €577,950 (8) €46,631 -28% €65,000 0% €65,000 0% €65,000 44% €45,000 Dr. Mary Kerr €82,631 -28% €115,000 0% €115,000 0% €115,000 -60% €288,975 (9) €65,000 0% €65,000 0% €65,000 0% €65,000 117% €30,000 Mr. Peter Guenter €115,000 0% €115,000 0% €115,000 0% €115,000 -58% €273,975 (10) €88,753 37% €65,000 0% €65,000 47% €44,164 N/A N/A Dr. Elisabeth Svanberg €138,753 21% €115,000 0% €115,000 47% €77,999 N/A N/A (11) €100,000 47% €68,131 N/A N/A N/A N/A N/A N/A Mr. Jérôme Contamine €150,000 47% €102,131 N/A N/A N/A N/A N/A N/A (12) €67,360 98% €34,066 N/A N/A N/A N/A N/A N/A Dr. Dan Baker €117,360 72% €68,066 N/A N/A N/A N/A N/A N/A (13) €31,849 N/A N/A N/A N/A N/A N/A N/A N/A Dr. Susanne Schaffert €59,849 N/A N/A N/A N/A N/A N/A N/A N/A (14) €18,369 N/A N/A N/A N/A N/A N/A N/A N/A Mr. Simon Sturge €32,369 N/A N/A N/A N/A N/A N/A N/A N/A 163 Galapagos NV Annual Report 2023
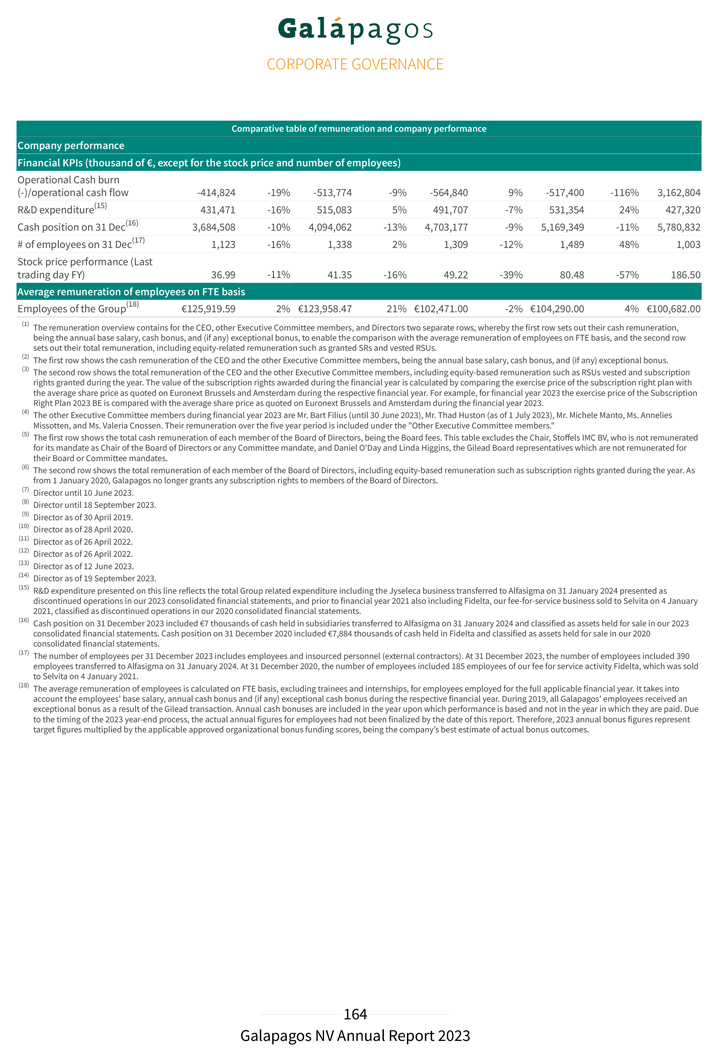
CORPORATE GOVERNANCE Comparative table of remuneration and company performance Company performance Financial KPIs (thousand of €, except for the stock price and number of employees) Operational Cash burn (-)/operational cash flow -414,824 -19% -513,774 -9% -564,840 9% -517,400 -116% 3,162,804 R&D expenditure(15) 431,471 -16% 515,083 5% 491,707 -7% 531,354 24% 427,320 Cash position on 31 Dec(16) 3,684,508 -10% 4,094,062 -13% 4,703,177 -9% 5,169,349 -11% 5,780,832 # of employees on 31 Dec(17) 1,123 -16% 1,338 2% 1,309 -12% 1,489 48% 1,003 Stock price performance (Last trading day FY) 36.99 -11% 41.35 -16% 49.22 -39% 80.48 -57% 186.50 Average remuneration of employees on FTE basis Employees of the Group(18) €125,919.59 2% €123,958.47 21% €102,471.00 -2% €104,290.00 4% €100,682.00 (1) (1) The remuneration overview contains for the CEO, other Executive Committee members, and Directors two separate rows, whereby the first row sets out their cash remuneration, being the annual base salary, cash bonus, and (if any) exceptional bonus, to enable the comparison with the average remuneration of employees on FTE basis, and the second row sets out their total remuneration, including equity-related remuneration such as granted SRs and vested RSUs. (2) The first row shows the cash remuneration of the CEO and the other Executive Committee members, being the annual base salary, cash bonus, and (if any) exceptional bonus. (3) The second row shows the total remuneration of the CEO and the other Executive Committee members, including equity-based remuneration such as RSUs vested and subscription rights granted during the year. The value of the subscription rights awarded during the financial year is calculated by comparing the exercise price of the subscription right plan with the average share price as quoted on Euronext Brussels and Amsterdam during the respective financial year. For example, for financial year 2023 the exercise price of the Subscription Right Plan 2023 BE is compared with the average share price as quoted on Euronext Brussels and Amsterdam during the financial year 2023. (4) The other Executive Committee members during financial year 2023 are Mr. Bart Filius (until 30 June 2023), Mr. Thad Huston (as of 1 July 2023), Mr. Michele Manto, Ms. Annelies Missotten, and Ms. Valeria Cnossen. Their remuneration over the five year period is included under the “Other Executive Committee members.” (5) The first row shows the total cash remuneration of each member of the Board of Directors, being the Board fees. This table excludes the Chair, Stoffels IMC BV, who is not remunerated for its mandate as Chair of the Board of Directors or any Committee mandate, and Daniel O’Day and Linda Higgins, the Gilead Board representatives which are not remunerated for their Board or Committee mandates. (6) The second row shows the total remuneration of each member of the Board of Directors, including equity-based remuneration such as subscription rights granted during the year. As from 1 January 2020, Galapagos no longer grants any subscription rights to members of the Board of Directors. (7) Director until 10 June 2023. (8) Director until 18 September 2023. (9) Director as of 30 April 2019. (10) Director as of 28 April 2020. (11) Director as of 26 April 2022. (12) Director as of 26 April 2022. (13) Director as of 12 June 2023. (14) Director as of 19 September 2023. (15) R&D expenditure presented on this line reflects the total Group related expenditure including the Jyseleca business transferred to Alfasigma on 31 January 2024 presented as discontinued operations in our 2023 consolidated financial statements, and prior to financial year 2021 also including Fidelta, our fee-for-service business sold to Selvita on 4 January 2021, classified as discontinued operations in our 2020 consolidated financial statements. (16) Cash position on 31 December 2023 included €7 thousands of cash held in subsidiaries transferred to Alfasigma on 31 January 2024 and classified as assets held for sale in our 2023 consolidated financial statements. Cash position on 31 December 2020 included €7,884 thousands of cash held in Fidelta and classified as assets held for sale in our 2020 consolidated financial statements. (17) The number of employees per 31 December 2023 includes employees and insourced personnel (external contractors). At 31 December 2023, the number of employees included 390 employees transferred to Alfasigma on 31 January 2024. At 31 December 2020, the number of employees included 185 employees of our fee for service activity Fidelta, which was sold to Selvita on 4 January 2021. (18) The average remuneration of employees is calculated on FTE basis, excluding trainees and internships, for employees employed for the full applicable financial year. It takes into account the employees’ base salary, annual cash bonus and (if any) exceptional cash bonus during the respective financial year. During 2019, all Galapagos’ employees received an exceptional bonus as a result of the Gilead transaction. Annual cash bonuses are included in the year upon which performance is based and not in the year in which they are paid. Due to the timing of the 2023 year-end process, the actual annual figures for employees had not been finalized by the date of this report. Therefore, 2023 annual bonus figures represent target figures multiplied by the applicable approved organizational bonus funding scores, being the company’s best estimate of actual bonus outcomes. 164 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Ratio between the highest and lowest remuneration The ratio between the highest and lowest remuneration at Galapagos during financial year 2023 is: 29:1. The ratio is calculated on the basis of the lowest FTE pay per 31 December 2023, excluding trainees and internships. The remuneration which has been taken into account in this exercise includes the annual base salary, annual cash bonus and (if any) exceptional bonus; annual cash bonus is included in the year upon which performance is based and not in the year in which it is paid. Due to the timing of the 2023 year-end process, the actual annual bonus figures for employees below the Executive Committee level had not been finalized by the date of this report. Therefore, target figures for these employees were used, multiplied by the applicable approved organizational bonus funding scores, being the Company’s best estimate of 2023 actual bonus outcomes. Minimum share ownership From the financial year 2020, our Remuneration Policy has set a minimum threshold of shares to be held at any time by the CEO to be equivalent to one year of the CEO’s annual base salary and by the other members of the Executive Committee to be equivalent to six months of the relevant Executive Committee member’s annual base salary. Thresholds are re-calculated on an annual basis and need to be reached within four years. At this stage all Executive Committee members (in office since 2022 and 2023 respectively) are building their shareholding. severance Contractual for provisions Executive regarding Committee compensation members for In 2023, all Executive Committee members have provided their services under agreements with the Galapagos Group, with a notice period, or indemnity in lieu of notice period, of nine months for the CEO and six months for the other Executive Committee members. The agreements do not provide for severance payments. In the event of termination, Galapagos may enter into non-competition undertakings with the CEO and the other Executive Committee members providing for non-competition indemnities. In the event their contract with the group is terminated as a result of a change of control of Galapagos, the CEO and the other Executive Committee members would be entitled to the immediate vesting of subscription rights and severance compensation of (i) 12 months’ base salary for the CEO and (ii) nine months’ base salary for the other Executive Committee members. 165 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Severance payments On 2 May 2023, Galapagos announced the departure of Mr. Bart Filius, President, COO and CFO and Executive Committee member per 30 June 2023. Upon substantiated recommendation of the Remuneration Committee, the Board approved a termination compensation of €1,650,000, consisting of compensation for a non-compete obligation for 12 months in an amount of €545,000, and a termination amount of €1,105,000 taking into account loss of 2023 bonus and loss of unvested RSUs. Effective 1 July 2023, Mr. Filius was no longer a member of the Executive Committee. He exercised an advisory role until 31 December 2023, for a total consultancy fee of €330,000. The Board determined this arrangement would best serve the interests of Galapagos, in particular given the critical role played by the outgoing President, COO and CFO in onboarding the then new CEO as well as remaining with Galapagos to support business continuity as a successor was found. Mr. Bart Filius was not eligible for any equity grants (RSUs and subscription rights) in 2023. He qualifies as a good leaver under the terms and conditions of the relevant subscription right plans and this is not part of his termination package. On 2 January 2024, Galapagos announced the departure of Mr. Michele Manto, CCO and Executive Committee member per 31 December 2023. No termination compensation was awarded. From 1 January 2024 until 31 May 2024, Mr. Manto will execute an advisory role to support the Jyseleca® transition to Alfasigma, for which he will receive a total fee of €191,900 and remain entitled to RSU pay-outs during this period. Mr. Manto will be entitled to his annual cash bonus for 2023, but will not be eligible for any equity grants (RSUs and subscription rights) in 2024. He qualifies as a good leaver under the terms and conditions of the relevant subscription right plans. Claw-back and malus As from financial year 2020, contractual provisions apply to each member of the Executive Committee to ensure that Galapagos has the right to have each Executive Committee member forfeit any unvested RSUs, deferred portions of previous cash bonuses or unvested subscription rights in the event of a restatement of the financial statements that has a material negative effect on Galapagos or a material breach of our Code of Conduct. In addition, from 1 December 2023, clawback undertakings have been in place to comply with the new SEC rules to recover erroneously awarded incentive-based compensation if Galapagos is required to prepare an accounting restatement due to material non-compliance with any financial reporting requirement. During the financial year 2023 no claw-back events occurred. The RSU and subscription right plans also contain bad leaver provisions that can result in forfeiture of any unvested RSU and/or subscription right grants in case the beneficiary leaves Galapagos prior to the relevant vesting date. 166 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Deviations from the Remuneration Policy Galapagos’ Remuneration Policy sets out that the Board may decide to deviate from any items of the policy if necessary to serve the long-term interests and sustainability of Galapagos. Any such deviation must be discussed at the Remuneration Committee, which will provide a substantiated recommendation to the Board. During the financial year 2023, the Board decided to deviate from the Remuneration Policy, considering exceptional circumstances and upon substantiated recommendation of the Remuneration Committee, with the intention of serving the long-term interests and sustainability of Galapagos and in view of a successful and thorough implementation of the leadership transition whilst guaranteeing continuity, on one occasion: As indicated above, on 2 May 2023, a termination package for Mr. Bart Filius was approved, being a termination compensation of €1,650,000, including compensation for a non-compete obligation for 12 months. This total termination package did not exceed his total annual remuneration for the financial year 2022. As a result, no shareholder approval for Mr. Filius’ termination package is required in accordance with Belgian law. 167 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Conflict of interests and related parties We consider that Gilead became a related party of Galapagos NV in 2019 because of (i) Gilead’s then 25.84% shareholding (25.35% on 31 December 2023) in Galapagos NV, and (ii) the fact that Gilead is entitled to propose two candidates to be appointed to the Board of Directors of Galapagos NV under the share subscription agreement dated 14 July 2019, as amended. On 30 October 2023, we entered into a related party transaction with Gilead within the meaning of article 7:97 of the Belgian Companies Code, by agreeing to further amend the collaboration agreement. Gilead and Galapagos agreed to terminate the existing 50/50 global development cost sharing arrangement, with Galapagos bearing the costs going forward, and to terminate Galapagos’ obligation to pay tiered royalties to Gilead on net sales of Jyseleca® in Europe, in addition to other amendments. The Board of Directors applied the related party transaction approval procedure as set forth in article 7:97 of the Belgian Companies Code. Within the context of this procedure, a committee of three independent members of the Board of Directors of Galapagos (the “Committee”) issued an advice to the Board of Directors in which the Committee assessed the amended terms of the collaboration agreement. In its advice to the Board of Directors, the Committee concluded the following: “The Committee believes that, under the circumstances, the proposed amendments to the filgotinib collaboration between Gilead and Galapagos are reasonable and fair from the point of view of Galapagos and its shareholders, and in line with the strategy of the Company. The proposed amendments offer an important opportunity to have autonomy on development and commercial activities in Europe in its ongoing collaboration with Gilead. The proposed amendments also come with a number of challenges and risks, but these are not unreasonable and can be managed going forward. The Committee therefore believes that the proposed amendments to the collaboration with Gilead in relation to filgotinib are in the interest of Galapagos, and in any event not manifestly abusive. In view hereof, the Committee issues a favourable and unqualified opinion to the Board of Directors of Galapagos.” The Board of Directors did not deviate from the Committee’s advice. The assessment by the Statutory Auditor of Galapagos of the advice of the Committee and the minutes of the Board of Directors is as follows: “Based on our assessment, nothing has come to our attention that causes us to believe that the financial and accounting data reported in the advice of the Ad hoc committee of the independent members of the Board of Directors dated 30 October 2023 and in the minutes of the Board of Directors dated 30 October 2023, which justify the proposed transaction, are not consistent, in all material respects, compared to the information we possess in the context of our assignment.” A more detailed explanation of some of our transactions with Gilead can be found in the section titled Agreements with major Galapagos NV shareholders. We further refer to note 32. 168 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE In the event of a transaction where a member of the Board of Directors has a conflict of interests within the meaning of article 7:96 of the Belgian Companies Code, such Board member shall notify the Board of Directors in advance of the respective conflict, and will act in accordance with the relevant rules as set out in the Belgian Companies Code. Pursuant to our Corporate Governance Charter, if a member of the Executive Committee has a direct or indirect interest of a monetary nature that conflicts with the interests of the Company in respect of a decision or an act falling within the scope of the responsibilities of the Executive Committee, the Executive Committee shall refrain from making any decision. The Executive Committee shall instead escalate the matter to the Board of Directors. The Board of Directors shall decide whether or not to approve such decision or act, and shall apply the conflict of interests procedure set out in article 7:96 of the Belgian Companies Code. In the event a conflict of interest exists within the Executive Committee that falls outside of the scope of article 7:96 of the Belgian Companies Code, the existence of such conflict shall be reported by the relevant Executive Committee member, its existence shall be included in the minutes (but shall not be published) and the relevant Executive Committee member shall not vote on the matter. In addition to the above, the Company’s Corporate Governance Charter and Related Person Transaction Policy contain certain procedures for transactions between Galapagos NV (including its affiliated and associated companies within the meaning of articles 1:20 and 1:21 of the Belgian Companies Code) and its Board members, Executive Committee members, major shareholders, or any of their immediate family members and affiliates. Without prejudice to the procedures as set out in the applicable laws, these policies provide (among others) that all transactions between Galapagos NV (including its affiliated and associated companies within the meaning of articles 1:20 and 1:21 of the Belgian Companies Code) and any of its Board members or Executive Committee members, need the approval of the Audit Committee and the Board of Directors, which approval can only be provided for transactions at arm’s length. Moreover, conflicts of interests, even if they are not a conflict of interests within the meaning of article 7:96 of the Belgian Companies Code, are enacted in the Board of Directors’ meeting minutes (but shall not be published), and the relevant Board member cannot participate in the deliberation or voting on the concerned item on the agenda. In 2023, the following conflicts of interests between Galapagos NV and a Director within the meaning of article 7:96 of the Belgian Companies Code were noted: In a meeting of the Board of Directors held on 20 February 2023, the following was reported in connection with the proposed compensation of the CEO (cash bonus and RSUs): the Chair informed the Board of Directors of a conflict of interest, concerning the proposed compensation of the CEO. The Board considered that said compensation was a justified reward for the results achieved by the CEO in 2022. The Board shared the opinion of the Remuneration Committee that the proposed compensation is justified and reasonable. The Chair did not take part in the deliberation and vote concerning this decision. 169 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE In a meeting of the Board of Directors held on 2 May 2023, the following was reported in accordance with article 7:96 of the Belgian Companies Code in connection with the proposed grants of subscription rights and RSUs to the CEO under the 2023 plans: the Chair informed the Board of Directors of a conflict of interest, concerning the proposed grants of subscription rights and RSUs to the CEO under the 2023 plans. The Board considered that said compensation was a justified reward for the results achieved by the CEO in 2022, in line with the contractual arrangement with the CEO executed in 2022 and with the Company’s Remuneration Policy. Furthermore, the Board deemed the proposed grants to be an important tool in the retention of Stoffels IMC BV as CEO of the Company and considered that these grants have no material impact on the financial position of the Company. The Board shared the opinion of the Remuneration Committee that the proposed compensation is justified and reasonable. The Chair did not take part in the deliberation and the vote concerning this decision. In a meeting of the Board of Directors held on 5 May 2023, the following was reported in accordance with article 7:96 of the Belgian Companies Code in connection with the proposed issuance of the 2023 subscription right plans: The CEO and also Chair of the Board of Directors, Stoffels IMC BV, reported prior to this meeting that he had a conflict of interest within the meaning of article 7:96 of the Belgian Companies Code in connection with the issuance of the number of subscription rights under the Subscription Right Plan 2023 BE, Subscription Right Plan 2023 RMV, and Subscription Right Plan 2023 ROW, for the benefit of employees of the Company and its subsidiaries, with cancellation of the preferential subscription right of the existing shareholders in the framework of the issuance of these subscription rights and the related possible future capital increase, as the CEO will be a beneficiary under Subscription Right Plan 2023 BE. The Board of Directors, upon the recommendation of the Remuneration Committee, is of the opinion that the proposed agenda items and the proposed grant of subscription rights to the CEO are consistent with the Company’s Remuneration Policy and are justified and reasonable. The nature of the proposed decision and the financial impact on the Company are described in more detail in the above-mentioned special report of the Board of Directors. In accordance with the procedure provided for in article 7:96 of the Belgian Companies Code, the CEO and also Chair of the Board of Directors, Stoffels IMC BV, does not attend this meeting and will not take part in the deliberation and the vote. In a meeting of the Board of Directors held on 30 October 2023, the following was reported in accordance with article 7:96 of the Belgian Companies Code in connection with the proposed amendment to the Galapagos-Gilead filgotinib agreement: The Chair reported that, prior to this meeting, Daniel O’Day and Dr. Linda Higgins had informed him that, since they are representatives of Gilead, they might have a conflict of interest in relation to the resolutions to be passed by the Board of Directors in relation to this agenda topic. Accordingly, Daniel and Linda had recused themselves for this part of the meeting, and did not take part in the deliberation and resolutions in relation to this agenda topic. 170 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Code of Conduct We have established a Code of Conduct to ensure that our members of the Board of Directors and Executive Committee and employees are making ethical and compliant decisions and acting with integrity, ethics and respect for human rights when conducting Galapagos’ business and performing their day-to-day duties. We expect any conflicts of interest to be addressed appropriately, and corruption and fraud prevented. To this end, we give various trainings, including on our Code of Conduct to all our employees and consultants. This year, 94% of our employees completed the Code of Conduct training and we measure against all employees, including those that may be on long term leave or ill. Our Code of Conduct is available on our website (www.glpg.com). At the beginning of 2023, we made some updates to our Code of Conduct to ensure that it continues to reflect who we are as an organization, including an explicit applicability of our Code of Conduct to our suppliers and business partners and more ESG related provisions. One breach of our Code of Conduct was escalated to the Audit Committee in 2023. Appropriate measures were taken to address this breach. 171 Galapagos NV Annual Report 2023

CORPORATE GOVERNANCE Statement by the Board of Directors The Board of Directors of Galapagos NV, represented by all its members, declares that, as far as it is aware, the non-consolidated and consolidated financial statements, both prepared in conformity with the applicable standards for financial statements, give a true and fair view of the equity, the financial position, and the results of Galapagos NV and the companies included in the consolidation as of 31 December 2023. The Board of Directors of Galapagos NV, represented by all its members, further declares that, as far as it is aware, this annual report related to the financial year ended on 31 December 2023, gives a true and fair view of the development, the results, and the position of Galapagos NV and the companies included in the consolidation, as well a description of the most important risks and uncertainties with which Galapagos NV and the companies included in the consolidation are confronted. The Board of Directors of Galapagos NV will submit proposed resolutions to its shareholders at its Annual Shareholders’ Meeting (to be held on 30 April 2024) to approve the non-consolidated annual accounts of the Company for the financial year ended on 31 December 2023 (including the allocation of the annual result as proposed by the Board of Directors), and to release from liability, by separate vote, the members of the Board of Directors, each of the former Directors who was in office during the financial year ended on 31 December 2023, and the statutory auditor for the performance of their respective mandates during the financial year ended on 31 December 2023. Mechelen, 26 March 2024 On behalf of the Board of Directors Jérôme Contamine Chair of the Audit Committee and member of the Board of Directors Stoffels IMC BV permanently represented by Dr. Paul Stoffels Chair of the Board of Directors 172 Galapagos NV Annual Report 2023

Financial statements 2023 consolidated and non-consolidated financial statements Pioneering science to transform patient outcomes
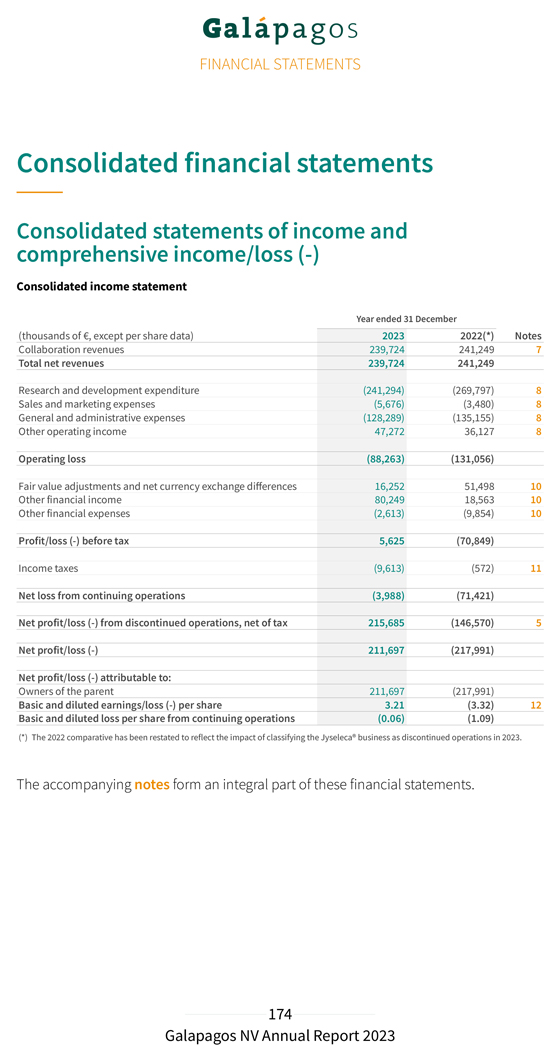
FINANCIAL STATEMENTS Consolidated financial statements comprehensive Consolidated statements income/loss of income (-) and Consolidated income statement Year ended 31 December (thousands of €, except per share data) 2023 2022(*) Notes Collaboration revenues 239,724 241,249 7 Total net revenues 239,724 241,249 Research and development expenditure (241,294) (269,797) 8 Sales and marketing expenses (5,676) (3,480) 8 General and administrative expenses (128,289) (135,155) 8 Other operating income 47,272 36,127 8 Operating loss (88,263) (131,056) Fair value adjustments and net currency exchange differences 16,252 51,498 10 Other financial income 80,249 18,563 10 Other financial expenses (2,613) (9,854) 10 Profit/loss (-) before tax 5,625 (70,849) Income taxes (9,613) (572) 11 Net loss from continuing operations (3,988) (71,421) Net profit/loss (-) from discontinued operations, net of tax 215,685 (146,570) 5 Net profit/loss (-) 211,697 (217,991) Net profit/loss (-) attributable to: Owners of the parent 211,697 (217,991) Basic and diluted earnings/loss (-) per share 3.21 (3.32) 12 Basic and diluted loss per share from continuing operations (0.06) (1.09) (*) The 2022 comparative has been restated to reflect the impact of classifying the Jyseleca® business as discontinued operations in 2023. The accompanying notes form an integral part of these financial statements. 174 Galapagos NV Annual Report 2023
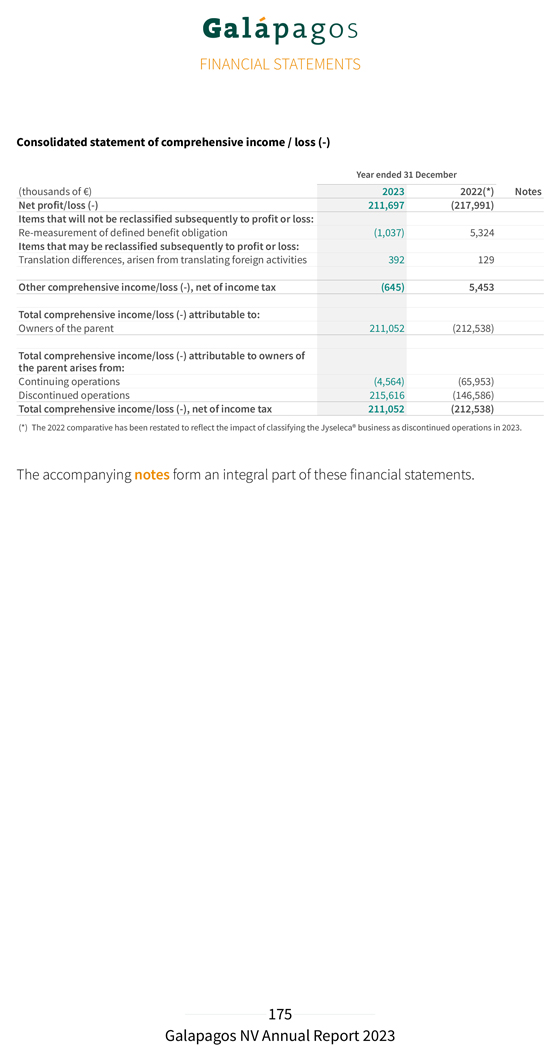
FINANCIAL STATEMENTS Consolidated statement of comprehensive income / loss (-) Year ended 31 December (thousands of €) 2023 2022(*) Notes Net profit/loss (-) 211,697 (217,991) Items that will not be reclassified subsequently to profit or loss: Re-measurement of defined benefit obligation (1,037) 5,324 Items that may be reclassified subsequently to profit or loss: Translation differences, arisen from translating foreign activities 392 129 Other comprehensive income/loss (-), net of income tax (645) 5,453 Total comprehensive income/loss (-) attributable to: Owners of the parent 211,052 (212,538) Total comprehensive income/loss (-) attributable to owners of the parent arises from: Continuing operations (4,564) (65,953) Discontinued operations 215,616 (146,586) Total comprehensive income/loss (-), net of income tax 211,052 (212,538) (*) The 2022 comparative has been restated to reflect the impact of classifying the Jyseleca® business as discontinued operations in 2023. The accompanying notes form an integral part of these financial statements. 175 Galapagos NV Annual Report 2023
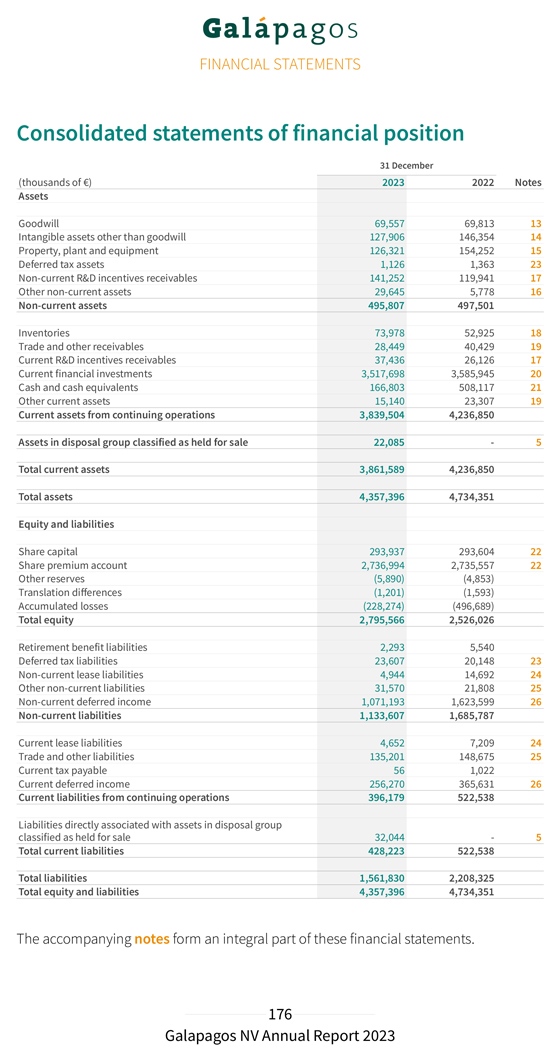
FINANCIAL STATEMENTS Consolidated statements of financial position 31 December (thousands of €) 2023 2022 Notes Assets Goodwill 69,557 69,813 13 Intangible assets other than goodwill 127,906 146,354 14 Property, plant and equipment 126,321 154,252 15 Deferred tax assets 1,126 1,363 23 Non-current R&D incentives receivables 141,252 119,941 17 Other non-current assets 29,645 5,778 16 Non-current assets 495,807 497,501 Inventories 73,978 52,925 18 Trade and other receivables 28,449 40,429 19 Current R&D incentives receivables 37,436 26,126 17 Current financial investments 3,517,698 3,585,945 20 Cash and cash equivalents 166,803 508,117 21 Other current assets 15,140 23,307 19 Current assets from continuing operations 3,839,504 4,236,850 Assets in disposal group classified as held for sale 22,085—5 Total current assets 3,861,589 4,236,850 Total assets 4,357,396 4,734,351 Equity and liabilities Share capital 293,937 293,604 22 Share premium account 2,736,994 2,735,557 22 Other reserves (5,890) (4,853) Translation differences (1,201) (1,593) Accumulated losses (228,274) (496,689) Total equity 2,795,566 2,526,026 Retirement benefit liabilities 2,293 5,540 Deferred tax liabilities 23,607 20,148 23 Non-current lease liabilities 4,944 14,692 24 Other non-current liabilities 31,570 21,808 25 Non-current deferred income 1,071,193 1,623,599 26 Non-current liabilities 1,133,607 1,685,787 Current lease liabilities 4,652 7,209 24 Trade and other liabilities 135,201 148,675 25 Current tax payable 56 1,022 Current deferred income 256,270 365,631 26 Current liabilities from continuing operations 396,179 522,538 Liabilities directly associated with assets in disposal group classified as held for sale 32,044—5 Total current liabilities 428,223 522,538 Total liabilities 1,561,830 2,208,325 Total equity and liabilities 4,357,396 4,734,351 The accompanying notes form an integral part of these financial statements. 176 Galapagos NV Annual Report 2023
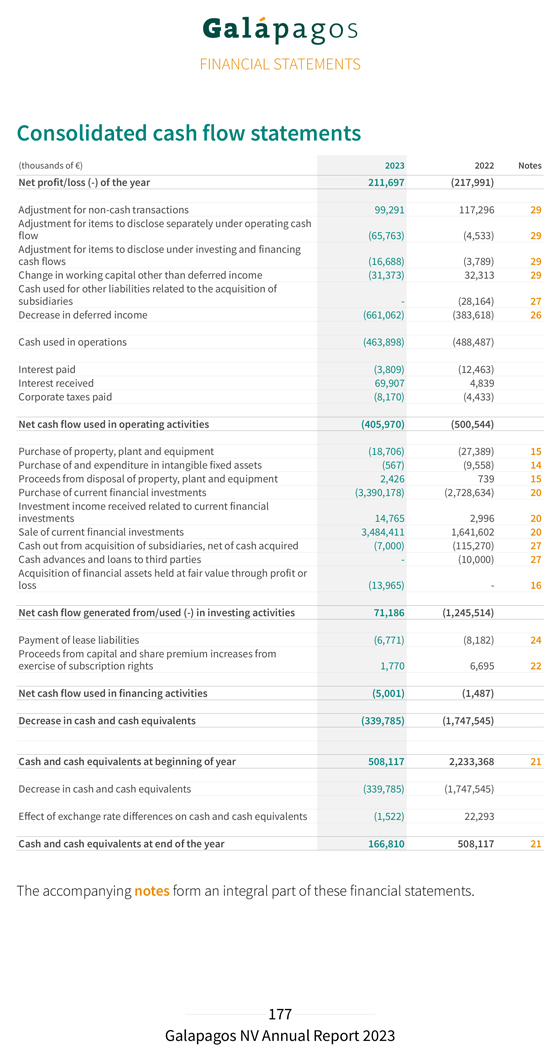
FINANCIAL STATEMENTS Consolidated cash flow statements (thousands of €) 2023 2022 Notes Net profit/loss (-) of the year 211,697 (217,991) Adjustment for non-cash transactions 99,291 117,296 29 Adjustment for items to disclose separately under operating cash flow (65,763) (4,533) 29 Adjustment for items to disclose under investing and financing cash flows (16,688) (3,789) 29 Change in working capital other than deferred income (31,373) 32,313 29 Cash used for other liabilities related to the acquisition of subsidiaries—(28,164) 27 Decrease in deferred income (661,062) (383,618) 26 Cash used in operations (463,898) (488,487) Interest paid (3,809) (12,463) Interest received 69,907 4,839 Corporate taxes paid (8,170) (4,433) Net cash flow used in operating activities (405,970) (500,544) Purchase of property, plant and equipment (18,706) (27,389) 15 Purchase of and expenditure in intangible fixed assets (567) (9,558) 14 Proceeds from disposal of property, plant and equipment 2,426 739 15 Purchase of current financial investments (3,390,178) (2,728,634) 20 Investment income received related to current financial investments 14,765 2,996 20 Sale of current financial investments 3,484,411 1,641,602 20 Cash out from acquisition of subsidiaries, net of cash acquired (7,000) (115,270) 27 Cash advances and loans to third parties—(10,000) 27 Acquisition of financial assets held at fair value through profit or loss (13,965)—16 Net cash flow generated from/used (-) in investing activities 71,186 (1,245,514) Payment of lease liabilities (6,771) (8,182) 24 Proceeds from capital and share premium increases from exercise of subscription rights 1,770 6,695 22 Net cash flow used in financing activities (5,001) (1,487) Decrease in cash and cash equivalents (339,785) (1,747,545) Cash and cash equivalents at beginning of year 508,117 2,233,368 21 Decrease in cash and cash equivalents (339,785) (1,747,545) Effect of exchange rate differences on cash and cash equivalents (1,522) 22,293 Cash and cash equivalents at end of the year 166,810 508,117 21 The accompanying notes form an integral part of these financial statements. 177 Galapagos NV Annual Report 2023
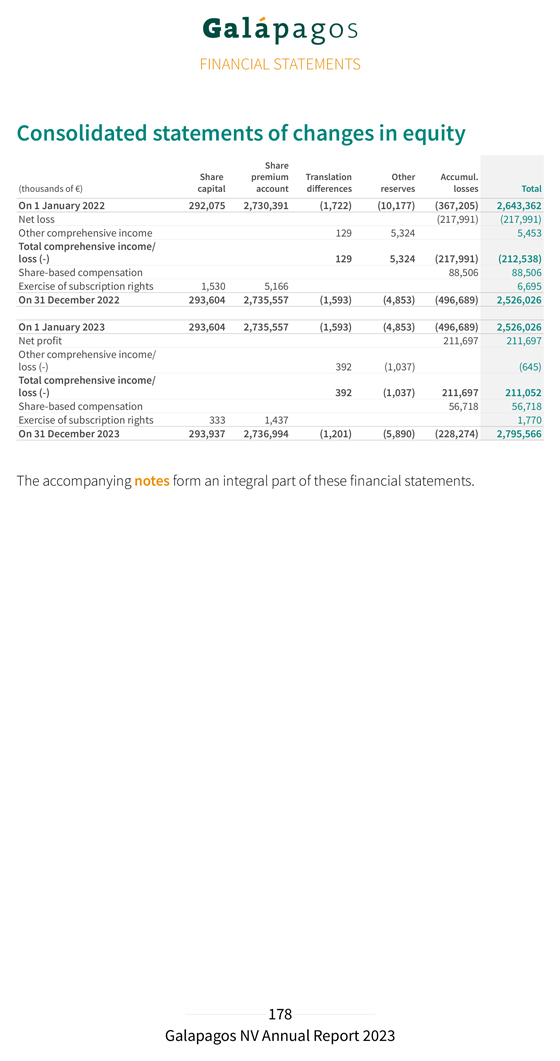
FINANCIAL STATEMENTS Consolidated statements of changes in equity Share Share premium Translation Other Accumul. (thousands of €) capital account differences reserves losses Total On 1 January 2022 292,075 2,730,391 (1,722) (10,177) (367,205) 2,643,362 Net loss (217,991) (217,991) Other comprehensive income 129 5,324 5,453 Total comprehensive income/ loss (-) 129 5,324 (217,991) (212,538) Share-based compensation 88,506 88,506 Exercise of subscription rights 1,530 5,166 6,695 On 31 December 2022 293,604 2,735,557 (1,593) (4,853) (496,689) 2,526,026 On 1 January 2023 293,604 2,735,557 (1,593) (4,853) (496,689) 2,526,026 Net profit 211,697 211,697 Other comprehensive income/ loss (-) 392 (1,037) (645) Total comprehensive income/ loss (-) 392 (1,037) 211,697 211,052 Share-based compensation 56,718 56,718 Exercise of subscription rights 333 1,437 1,770 On 31 December 2023 293,937 2,736,994 (1,201) (5,890) (228,274) 2,795,566 The accompanying notes form an integral part of these financial statements. 178 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS Notes statements to the consolidated financial 1. General information Galapagos NV is a limited liability company incorporated in Belgium and has its registered office at Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium. In the notes to the consolidated financial statements, references to “we”, “us,” “the group” or “Galapagos” include Galapagos NV together with its subsidiaries. We refer to note 33 for a list of consolidated companies. We are a global biotechnology company with operations in Europe and the US dedicated to developing medicines focusing on oncology and immunology. The components of the result presented in the financial statements include the results of the companies mentioned in note 33 Consolidated companies as of 31 December 2023. Our operations had 1,123 employees on 31 December 2023 (as compared to 1,338 employees on 31 December 2022) mainly working in our operating facilities in Mechelen (the Belgian headquarters), the Netherlands, France, Switzerland, Germany, Italy, Spain and the United Kingdom. Effective as from 1 July 2023 we transferred our drug discovery and research activities in Romainville, France, and 121 employees exclusively dedicated to the operation of these activities to NovAliX, who assumes all ongoing research and discovery activities in Romainville. On 31 January 2024 we announced that we successfully completed the transfer of the Jyseleca® business to Alfasigma, including the European and UK Marketing Authorizations, the commercial, medical and development activities for Jyseleca® and approximately 400 positions in 14 European countries. The transfer of our Jyseleca® business has been determined to meet the criteria to be classified as held for sale and discontinued operations in our financial statements for the year ended 31 December 2023. We also presented all income statement items fully related to the Jyseleca® business to be transferred on a separate line “Net profit/loss (–) from discontinued operations, net of tax” in our consolidated income statement. The consolidated income statements for all comparative periods reported in these consolidated financial statements were restated as well to show the discontinued operations on a separate line. Our continuing operations had 646 employees on 31 December 2023 (as compared to 724 employees on 31 December 2022) mainly working in our operating facilities in Mechelen (the Belgian headquarters), the Netherlands, France, Switzerland, and the United States. We refer to note 33 for a list of the entities included in discontinued operations and to note 5 for more details on the discontinued operations. 179 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS 2. Summary of significant transactions Transfer of Jyseleca® business to Alfasigma On 30 October 2023, we signed a letter of intent contemplating a transfer of the Jyseleca® business to Alfasigma S.p.A. (Alfasigma). The final share and asset purchase agreement was signed on 30 December 2023 and the transaction was closed on 31 January 2024. The transfer includes the European and UK Marketing Authorizations, and the commercial, medical affairs and development activities for Jyseleca®. In connection with the completion of the transaction, approximately 400 of our positions in 14 European countries transferred to Alfasigma to support business continuity and ongoing patient access for the Jyseleca® business. We received a €50 million upfront payment in connection with the transfer, at closing of the transaction in 2024, and are entitled to receive potential milestone payments totaling €120 million and mid-single to mid-double-digit royalties on European sales. We will contribute up to €40 million by June 2025 to Alfasigma for Jyseleca® related development activities. In addition, we plan to streamline our remaining operations and further build efficiencies, with an envisaged reduction of approximately 100 positions across the organization. Effective 31 January 2024, following the closing of the transaction between us and Alfasigma for the transfer the Jyseleca® business, we assigned our rights and obligations under the Gilead filgotinib collaboration to Alfasigma, except for our right to receive royalties from Gilead on net sales in the Gilead Territory under a separate agreement between Gilead and us entered into in October 2023. On 31 January 2024, we also signed a transition agreement with Alfasigma enacting the responsibilities and services that will be provided by the parties during a transition period for the transfer of the business. The gradual transfer of our remaining inventories to Alfasigma is also governed by this agreement. We refer to note 5 for more details on the discontinued operations. Gilead collaboration agreement On 14 July 2019 we and Gilead announced that we entered into a 10-year global research and development collaboration. Through this agreement, Gilead gained exclusive access to our innovative portfolio of compounds, including clinical and preclinical programs and a proven drug discovery platform. At inception of this collaboration in 2019, we received an upfront payment of €3,569.8 million ($3.95 billion) and a €960.1 million ($1.1 billion) equity investment from Gilead. We identified the following three performance obligations as part of this collaboration: (i) the transfer of an extended license on ziritaxestat (GLPG1690) (this performance obligation was satisfied completely in 2019), (ii) the granting of exclusive access to our drug discovery platform (i.e. the IP, technology, expertise and capabilities) during the collaboration period and exclusive option rights on our current and future clinical programs after Phase 2 (or, in certain circumstances, the first Phase 3 study) outside Europe and (iii) an increased cost share from 20/80 to 50/50 on the global development activities of filgotinib, as a result of the revised license and collaboration agreement. 180 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS In the years thereafter (2020-2023), the collaboration agreement relating to filgotinib was restated several times (see further in this chapter). We however retain the following performance obligations: (i) the granting of exclusive access to our drug discovery platform (i.e. the IP, technology, expertise and capabilities) during the collaboration period and exclusive option rights on our current and future clinical programs after Phase 2 (or, in certain circumstances, the first Phase 3 study) outside Europe and (ii) an increased cost share from 20/80 to 50/50 to 100/0 (for certain agreed activities (“Group A activities”, as defined below)) until the end of the third quarter of 2023, and to 100/0 since then of the costs of the global development activities of filgotinib going forward. This second performance obligation was transferred to Alfasigma on 31 January 2024, when we closed the transaction for the transfer of the Jyseleca® business to Alfasigma and the (amended and restated) collaboration agreement relating to filgotinib was assigned to Alfasigma as a consequence thereof. Terms of the collaboration relating to our drug discovery platform We will fund and lead all discovery and development autonomously until the end of Phase 2. After the completion of a qualifying Phase 2 study (or, in certain circumstances, the first Phase 3 study), Gilead will have the option to acquire a license to the compound outside Europe. If the option is exercised, we and Gilead will co-develop the compound and share costs equally. Gilead will maintain option rights to our programs through the 10-year term of the collaboration. This term can be extended for up to an additional three years thereafter for those programs, if any, that have entered clinical development prior to the end of the collaboration term. In addition, a final term extension can be granted in certain circumstances. Gilead will make a $150 million opt-in payment per program and will owe no subsequent milestones. We will receive tiered royalties ranging from 20% –24% on net sales of all our products licensed by Gilead in all countries outside Europe as part of the agreement. Revised filgotinib collaboration Since the revised agreement of December 2020, we assumed all development, manufacturing, commercialization and certain other rights for filgotinib in Europe. Since 1 January 2021, we bear the full future development costs for certain studies (defined as “Group A activities”), in lieu of the equal cost split contemplated by the previous agreement. The 50/50 global development cost sharing arrangement continued for certain other studies. All commercial economics on filgotinib in Europe were transferred to us as of 1 January 2022, subject to payment of tiered royalties of 8% to 15% of net sales in Europe to Gilead, starting in 2024. In connection with all the amendments to the existing arrangement for the commercialization and development of filgotinib, Gilead paid us €172.6 million in total in previous years. Since the amendment of December 2020, we are also no longer eligible to receive any future milestone payments relating to filgotinib in Europe. Other terms of the original license agreement remained in effect. 181 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS On 30 October 2023, we and Gilead agreed to amend the filgotinib agreement by terminating the existing 50/50 global development cost sharing arrangement with us bearing the costs going forward, and to terminate our obligation to pay tiered royalties to Gilead on net sales of Jyseleca® in Europe, in addition to other amendments. Effective 31 January 2024, following the closing of the transaction between us and Alfasigma for the transfer of the Jyseleca® business, we assigned our rights and obligations under the filgotinib collaboration to Alfasigma, except for our right to receive royalties from Gilead on net sales in the Gilead Territory under a separate agreement between Gilead and us entered into in October 2023. Gilead remains responsible for commercial activities outside of Europe. Terms of the equity investment As part of the research and development collaboration of 2019 Gilead also entered into a share subscription agreement with us. Gilead’s equity investment consisted of a subscription for new Galapagos shares. This equity subscription took place at closing of the transaction, on 23 August 2019 and increased Gilead’s stake in Galapagos from approximately 12.3% to 22.04% of the then issued and outstanding shares in Galapagos. In addition, the Extraordinary General Meeting of Shareholders of 22 October 2019 approved the issuance of warrant A and initial warrant B allowing Gilead to further increase its ownership of Galapagos to up to 29.9% of the company’s issued and outstanding shares. On 6 November 2019, Gilead exercised warrant A and increased its ownership in Galapagos to 25.10% of the then outstanding shares. The initial warrant B has a term of five years and an exercise price per share equal to the greater of (i) 120% multiplied by the arithmetic mean of the 30-day daily volume weighted average trading price of Galapagos’ shares as traded on Euronext Brussels and Euronext Amsterdam, and (ii) €140.59. Subsequent warrant B is still subject to approval by an Extraordinary General Meeting of Shareholders. This Extraordinary General Meeting of Shareholders shall take place between 57 and 59 months after the closing of the subscription agreement (23 August 2019) and this warrant will have substantially similar terms, including as to exercise price, to the initial warrant B. On 31 December 2023 the value of the subsequent Warrant B decreased to €0.05 million, driven by the decrease of our share price, and of the implied volatility in 2023. The agreement also includes a 10-year standstill restricting Gilead’s ability to propose a business combination with or acquisition of Galapagos or increase its stake in Galapagos beyond 29.9% of the company’s issued and outstanding shares, subject to limited exceptions. Gilead’s ownership amounted to 25.35% at 31 December 2023. Since 22 October 2019, Gilead has two representatives on the Board of Directors of Galapagos (Daniel O’Day and Linda Higgins). Evolution of the total transaction price The transaction price is currently composed of a fixed part, being non-refundable upfront and license fees and a variable part, being milestone payments, sales based milestones and sales based royalties, and cost reimbursements for R&D activities 182 Galapagos NV Annual Report 2023
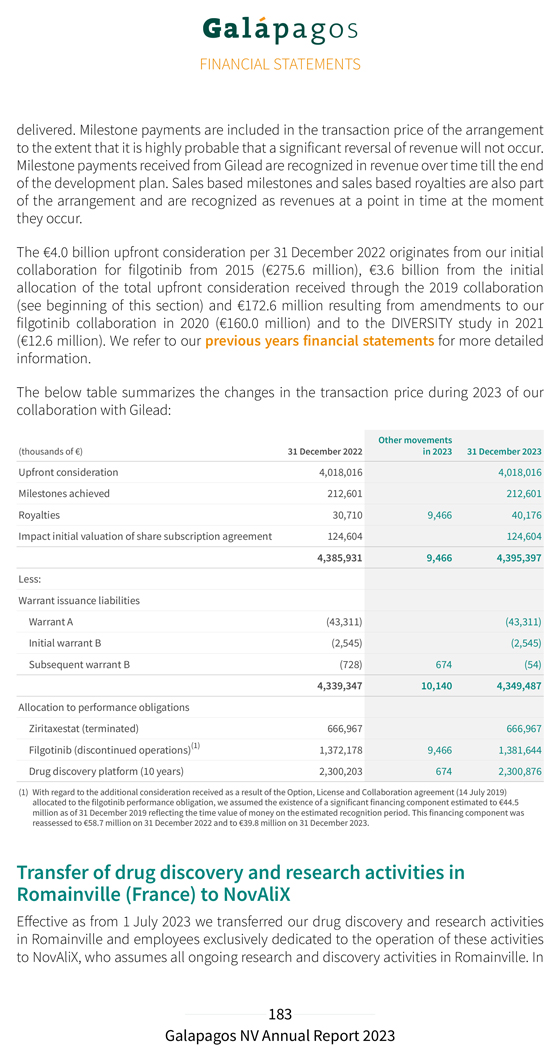
FINANCIAL STATEMENTS delivered. Milestone payments are included in the transaction price of the arrangement to the extent that it is highly probable that a significant reversal of revenue will not occur. Milestone payments received from Gilead are recognized in revenue over time till the end of the development plan. Sales based milestones and sales based royalties are also part of the arrangement and are recognized as revenues at a point in time at the moment they occur. The €4.0 billion upfront consideration per 31 December 2022 originates from our initial collaboration for filgotinib from 2015 (€275.6 million), €3.6 billion from the initial allocation of the total upfront consideration received through the 2019 collaboration (see beginning of this section) and €172.6 million resulting from amendments to our filgotinib collaboration in 2020 (€160.0 million) and to the DIVERSITY study in 2021 (€12.6 million). We refer to our previous years financial statements for more detailed information. The below table summarizes the changes in the transaction price during 2023 of our collaboration with Gilead: Other movements (thousands of €) 31 December 2022 in 2023 31 December 2023 Upfront consideration 4,018,016 4,018,016 Milestones achieved 212,601 212,601 Royalties 30,710 9,466 40,176 Impact initial valuation of share subscription agreement 124,604 124,604 4,385,931 9,466 4,395,397 Less: Warrant issuance liabilities Warrant A (43,311) (43,311) Initial warrant B (2,545) (2,545) Subsequent warrant B (728) 674 (54) 4,339,347 10,140 4,349,487 Allocation to performance obligations Ziritaxestat (terminated) 666,967 666,967 Filgotinib (discontinued operations)(1) 1,372,178 9,466 1,381,644 Drug discovery platform (10 years) 2,300,203 674 2,300,876 (1) With regard to the additional consideration received as a result of the Option, License and Collaboration agreement (14 July 2019) allocated to the filgotinib performance obligation, we assumed the existence of a significant financing component estimated to €44.5 million as of 31 December 2019 reflecting the time value of money on the estimated recognition period. This financing component was reassessed to €58.7 million on 31 December 2022 and to €39.8 million on 31 December 2023. Transfer of drug discovery and research activities in Romainville (France) to NovAliX Effective as from 1 July 2023 we transferred our drug discovery and research activities in Romainville and employees exclusively dedicated to the operation of these activities to NovAliX, who assumes all ongoing research and discovery activities in Romainville. In 183 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS return, we are committed to utilizing the research capabilities and expertise of NovAliX through a five year-collaboration and within the context of our R&D portfolio during which we are committed to purchase for a total of €73.8 million services from NovAliX. We made an upfront payment amounting to €8.3 million at closing of the transaction which will be released over the five year-period. The loss realized on disposal, on closing of the transaction, was capitalized as an advance and will gradually be released through our income statement in accordance with the progress of our future purchase commitment. We refer to note 28 for more details on this transaction. 3. Material accounting policies Our material accounting policies are summarized below. Basis of preparation and going concern assumption The consolidated financial statements are prepared in accordance with the International Financing Reporting Standards (IFRS), as adopted by the EU. The consolidated financial statements provide a general overview of our activities and the results achieved. They give a true and fair view of our financial position, our financial performance and cash flows, on a going concern basis. The consolidated financial statements are presented in Euros, which is also our functional currency. Amounts are rounded to the nearest thousand, unless otherwise stated. Reclassification of accrued interests on financial investments – comparative periods not restated Accrued interests on financial investments were in the past recorded on the “current financial investments” line for treasury bills while reported on a separate current assets/ current liabilities line for the other financial investments measured at amortized cost (mainly term deposits). During the third quarter of 2023 we decided to align the presentation of all our financial investments at amortized cost and present all accrued interests as part of the amortized cost measurement on the “current financial investments” line / “cash and cash equivalents” line. As the impact of this classification misstatement on the prior period comparative numbers was deemed immaterial for all comparative periods presented, we did not restate the prior period financial statements. This is in accordance with IAS 8.42 that requires to only adjust the comparatives for material prior period errors. The impact of this error on 31 December 2022 amounted to €9.9 million net positive accrued interests mainly related to accrued positive interests on current financial investments that would have increased our “current financial investments” and “cash and cash equivalents” line and decreased our “other current assets” line by the same amount. 184 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS Discontinued operations presentation – comparative periods restated Our income statements and statements of comprehensive income of the comparative periods presented in these financial statements have been restated to present the activities related to the Jyseleca® business on a separate line “Net income/loss (-) from discontinued operations, net of tax”. We refer to note 5 for more detailed information on these discontinued operations. New standards and interpretations applicable for the annual period beginning on 1 January 2023 New standards and interpretations applicable for the annual period beginning on 1 January 2023 did not have a material impact on our consolidated financial statements except for the application of the amendments made to IAS 1 Disclosure of Accounting Policies. As a result of this amendment, we reassessed our accounting policies in order to remain with only the material accounting policies. Standards and interpretations published, but not yet applicable for the annual period beginning on 1 January 2023 A number of new standards are effective for annual periods beginning on or after 1 January 2024 with earlier adoption permitted. However we have not early adopted new or amended standards in preparing our consolidated financial statements. We are currently still assessing the impact of these new accounting standards and amendments that are not yet effective but we expect no standard to have a material impact on our financial statements in the period of initial application. The following amendments are effective for the period beginning 1 January 2024: Liability in a Sale and leaseback (Amendments to IFRS 16); Classification of liabilities as current or non-current (Amendment to IAS 1); Non-current liabilities with covenants (Amendment to IAS 1); Supplier Finance Arrangements (Amendments to IAS 7 and IFRS 7). The following amendments are effective for the period beginning 1 January 2025: Amendments to IAS 21 The Effects of Changes in Foreign Exchange Rates: Lack of Exchangeability Business combinations Business combinations are accounted for using the acquisition method. In the statement of financial position, all identifiable assets, liabilities and contingent liabilities are initially recognized at their fair value at the acquisition date. The results of acquired operations are included in our consolidated income statement from the date on which 185 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS control is obtained. Any contingent consideration to be transferred by us is recognized at fair value at the acquisition date. Subsequent changes to the fair value of the contingent consideration, which is deemed to be an asset or liability, will be recognized in profit or loss. The excess of the fair value of the total purchase consideration transferred over the fair value of the acquired assets and assumed liabilities is recognized as goodwill. The valuations in support of fair value determinations are based on information available at the acquisition date. Acquisition related costs are expensed as incurred. Any contingent consideration to be transferred by us in relation to businesses acquired are linked to milestone payments are initially recognized at fair value as a financial liability. They are adjusted for the probability of their likelihood of payment and are appropriately discounted to reflect the impact of time. Changes in the fair value of these contingent consideration liabilities in subsequent periods are recognized in our consolidated income statement on the line “other operating income/expense”. The effect of unwinding the discount over time is recognized on the line “other financial expenses”. Contingent amounts payable or paid by us to former shareholders of acquired companies, who continue to be employed by us, but which would be automatically forfeited (or become repayable) upon termination of employment before a specific date, are classified as remuneration for post-combination services in our consolidated income statement. These cash-settled contingent amounts are recognized in accordance with IAS 19 and are recorded on the balance sheet on the lines “other (non-) current assets” and “other non-current/trade and other liabilities” depending on the timing of the payment by us. Goodwill Goodwill is initially measured as the excess of the total purchase consideration transferred and the fair value of the acquired assets and assumed liabilities. Subsequently, goodwill is stated at cost less impairments. As goodwill is considered to have an indefinite life, it is tested for impairment at least once a year (at each year-end), and whenever there is an indication that it may be impaired, by comparing its carrying amount with its recoverable amount. Any impairment costs are recorded in our consolidated income statement on the line “Other operating income/expense”. Intangible assets other than goodwill Expenditure on research activities is recognized as an expense in the period in which it is incurred. An internally generated intangible asset arising from our development activities is recognized only if all of the following conditions are met: Technically feasible to complete the intangible asset so that it will be available for use or sale 186 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS We have the intention to complete the intangible assets and use or sell it We have the ability to use or sell the intangible assets The intangible asset will generate probable future economic benefits, or indicate the existence of a market Adequate technical, financial and other resources to complete the development are available We are able to measure reliably the expenditure attributable to the intangible asset during its development. (i) Internally generated intangible assets The amount capitalized as internally generated intangible assets is the sum of the development costs incurred as of the date that the asset meets the conditions described above. Because of risks and uncertainties inherent to the regulatory authorizations and to the development process itself, management estimates that the conditions for capitalization are not met until we obtain regulatory approval from the competent authorities. Currently we recognize all development costs as an expense in the period in which they are incurred, even for approved products because they do not generate separately identifiable incremental future economic benefits that can be reliably measured. (ii) Licenses, rights, technology and in-process research and development Acquired in-process research and development obtained through in-licensing agreements, business combinations, collaboration agreements or separate acquisitions are capitalized as an intangible asset provided that they are separately identifiable, controlled by us and expected to provide economic benefits. As the probability criterion in IAS 38 is always considered to be satisfied for separately acquired research and development assets, upfront and milestone payments to third parties for products or compounds for which regulatory approval has not yet been obtained are recognized as intangible assets. We consider such intangible assets as not yet available for use until the moment that the underlying asset is approved and commercially launched. Amortization will commence when the underlying asset is approved for commercialization and the asset will be amortized over its useful life. Intangible assets may also consist of upfront fees paid to third party institutions in exchange for an option to negotiate a license to any of the third party’s rights in technology resulting from the collaboration. The upfront fee paid in exchange for this option is capitalized as intangible asset and amortized over the expected duration of the option. Exclusivity contracts and technology acquired through business combinations are valued independently as part of the fair value of the businesses acquired and are amortized over their estimated useful lives. The estimated useful life is based on the lower of the contract life or the economic useful life. 187 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS In the event an asset has an indefinite life, this fact is disclosed along with the reasons for being deemed to have an indefinite life. Intangible assets with an indefinite useful life and intangible assets which are not yet available for use are tested for impairment annually, and whenever there is an indication that the asset might be impaired. (iii) Software and databases Acquired software is recognized at cost less accumulated amortization and any impairment loss. Amortization is recognized so as to write off the cost of assets over their useful lives (generally between 3 and 5 years), using the straight-line method. (iv) Contract costs Contract costs only include success fees that were capitalized in relation to the Gilead agreement of 2019. These costs are currently amortized on a straight-line basis over a period of 10 years, reflecting the term of our collaboration with Gilead. We review at each balance sheet date the carrying amount of our intangible assets to determine whether there is any indication that those assets have suffered an impairment loss. If any such indication exists, the recoverable amount of the asset is estimated in order to determine the extent of the impairment loss (if any). Where the asset does not generate cash flows that are independent from other assets, we estimate the recoverable amount of the cash-generating unit to which the asset belongs. If the recoverable amount of an asset or cash generating unit is estimated to be less than the carrying amount, the carrying amount of the asset is reduced to its recoverable amount. An impairment loss is recognized as an expense immediately. Property, plant and equipment Property, plant and equipment are recognized at cost less accumulated depreciation and any impairment loss. Depreciation of an asset begins when it is available for use, ie when it is in the location and condition necessary for it to be capable of operating in the manner intended by management. Depreciation is recognized so as to write off the cost of assets over their useful lives, using the straight-line method, on the following bases: Buildings: 33 years Installation & machinery: 3 – 15 years Furniture, fixtures & vehicles: 4 – 10 years Leasehold improvements are depreciated over 3 – 10 years, being the term of the lease, unless a shorter useful life is expected. The other tangible assets category mainly consists of assets under construction. Assets under construction are not depreciated. 188 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS Any gain or loss incurred at the disposal of an asset is determined as the difference between the sale proceeds and the carrying amount of the asset and is recognized in profit or loss. We review at each balance sheet date the carrying amount of our property, plant and equipment to determine whether there is any indication that those assets have suffered an impairment loss. If any such indication exists, the recoverable amount of the asset is estimated in order to determine the extent of the impairment loss (if any). Leases All leases are accounted for by recognizing a right-of-use asset and a corresponding lease liability except for: Leases of low value assets; and Leases with a duration of 12 months or less. Liabilities arising from a lease are initially measured on a present value basis. Lease liabilities include the net present value of the lease payments that are not paid at the commencement date, discounted using the incremental borrowing rate . Our lease payments generally only include fixed payments and extension option payments if we are reasonably certain to exercise this option. After initial recognition, the lease liability is measured at amortized cost using the discount rate determined at commencement and will be re-measured (with a corresponding adjustment to the related right-of-use asset) when there is a change in future lease payments, generally in case of reassessment of options. At the commencement date, the right-of-use assets are measured at cost, comprising the amount of the initial lease liability, less any lease incentives received from the lessors. After initial recognition, the right-of-use assets are measured at cost and generally depreciated over the lease term on a straight-line basis. The right-of-use assets will be adjusted for any re-measurements of the lease liability as a result of lease modifications. The right-of-use assets are subject to impairment testing if there is an indicator for impairment, as for property, plant and equipment. The right-of-use assets are presented in the statement of financial position under the caption “Property, plant and equipment” and the lease liabilities are presented as current and non-current lease liabilities. We only include extension options (or periods after termination options) in the lease term if the lease is reasonably certain to be extended (or not terminated). The assessment is reviewed if a significant event or a significant change in circumstances occurs which affects this assessment and that is within our control. Inventories Inventories consist of raw materials, semi-finished products and finished products. These inventories are initially recognized at cost, and subsequently at the lower of cost 189 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS and net realizable value. Cost comprises all costs of purchase, conversion costs and transportation costs, and is determined using the FIFO-method. Financial instruments Financial assets and financial liabilities are recognized on our balance sheet when we become a party to the contractual provisions of the instrument. (i) Financial assets Financial assets are initially recognized either at fair value or at their transaction price. All recognized financial assets are subsequently measured at either amortized cost or fair value under IFRS 9 on the basis of both our business model for managing the financial assets and the contractual cash flow characteristics of the financial asset. a financial asset that (i) is held within a business model whose objective is to collect the contractual cash flows and (ii) has contractual cash flows that are solely payments of principal and interest on the principal amount outstanding is measured at amortized cost (net of any write down for impairment), unless the asset is designated at fair value through profit or loss (FVTPL) under the fair value option; all other financial assets are measured at FVTPL. A financial asset is classified as current when the cash flows expected to flow from the instrument mature within one year. We derecognize a financial asset when the contractual rights to the cash flows from the asset expire, or we transfer the rights to receive the contractual cash flows on the financial asset in a transaction in which substantially all the risks and rewards of ownership of the financial asset are transferred. (a) Financial assets at fair value through profit or loss Financial assets are designated at fair value through profit or loss if we manage such investments and make purchase and sale decisions based on their fair value in accordance with the investment strategy. Attributable transaction costs are recognized in profit or loss as incurred. Financial assets at fair value through profit or loss are measured at fair value, and changes therein, which take into account any dividend income, are recognized in profit or loss. Equity instruments We hold investments in equity instruments, which based on IFRS 9, are designated as financial assets at fair value through profit or loss. The fair value of listed investments is based upon the closing price of such securities on Euronext at each reporting date. If there is no active market for an equity instrument, we establish the fair value by using valuation techniques. Current financial investments measured at fair value through profit or loss Current financial investments include financial assets measured at fair value through profit or loss and may comprise short term bond funds that have a maturity equal or less than 12 months, and money market funds. 190 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS Cash equivalents measured at fair value through profit or loss Cash equivalents measured at fair value through profit or loss may comprise bonds and money market funds that are readily convertible to cash and are subject to an insignificant risk of changes in value. (b) Financial assets at amortized cost Receivables Receivables are designated as financial assets measured at amortized cost. They are initially measured either at fair value or at transaction price, in the absence of a significant financing component. All receivables are subsequently measured in the balance sheet at amortized cost, which generally corresponds to nominal value less expected credit loss provision. Receivables mainly comprise trade and other receivables and current/non-current R&D incentives receivables. The R&D incentives receivables relate to refunds resulting from R&D incentives on research and development expenses in France and Belgium. This is a grant receivable that is based on annual declarations and is only refunded in case it cannot be offset by a tax payable. Research and development incentives receivables are discounted over the period until maturity date according to the appropriate discount rates. We refer to the accounting policy on grants and R&D incentives. Current financial investments measured at amortized cost Current financial investments measured at amortized cost include treasury bills that have a maturity equal to or less than 12 months. We apply settlement date accounting for the recognition and de-recognition of current financial investments measured at amortized cost. Current financial investments measured at amortized cost also include term deposits with maturities exceeding three months from the acquisition date. Cash and cash equivalents measured at amortized cost Cash and cash equivalents measured at amortized cost mainly comprise of notice accounts and term deposits that are readily convertible to cash within three months or less, that are subject to an insignificant risk of changes in their value and that are held for the purpose of meeting short-term cash commitments. Cash and cash equivalents exclude restricted cash, which is presented in the line other non-current assets in the statement of financial position. Impairment The impairment loss of a financial asset measured at amortized cost is calculated based on the expected loss model. For trade receivables, in the absence of a significant financing component, the loss allowance is measured at an amount equal to lifetime expected credit losses. Those are 191 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS the expected credit losses that result from all possible default events over the expected life of those trade receivables. Impairment losses are recognized in the consolidated income statement. (ii) Financial liabilities Financial liabilities are initially measured either at fair value or at their transaction price. Subsequent to initial recognition, financial liabilities are measured at amortized cost or at fair value. Financial liabilities measured at amortized cost mainly comprise trade and other liabilities. Trade and other liabilities are comprised of liabilities that are due less than one year from the balance sheet date and are in general not interest bearing and settled on an ongoing basis during the financial year. They also include accrued expenses related to our research and development project costs. We derecognize a financial liability when our contractual obligations are discharged, cancelled or expire. Taxation Income tax in the profit or loss accounts represents the sum of the current tax and deferred tax. Current tax is the expected tax payable on the taxable profit of the year. The taxable profit of the year differs from the profit as reported in the financial statements as it excludes items of income or expense that are taxable or deductible in other years and it further excludes items that are never taxable or deductible. Our liability for current tax is calculated using tax rates that have been enacted or substantively enacted by the balance sheet date. Deferred income tax is provided in full, using the liability-method, on temporary differences arising between the tax bases of assets and liabilities and their carrying amounts in the financial statements. However, the deferred income tax is not accounted for if it arises from the initial recognition of an asset or liability in a transaction other than a business combination that at the time of the transaction affects neither accounting nor taxable profit nor loss. Deferred income tax is determined using tax rates (and laws) that have been enacted or substantively enacted by the balance sheet date and are expected to apply when the related deferred income tax asset is realized or the deferred income tax liability is settled. Deferred tax assets are recognized to the extent that it is probable that future taxable profit will be available against which the temporary differences can be utilized. As such, a deferred tax asset for the carry forward of unused tax losses will be recognized to the extent that is probable that future taxable profits will be available. 192 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS Revenue recognition Revenues to date have consisted principally of collaboration revenues, which consist of milestones, license fees, non-refundable upfront fees and royalties received in connection with collaboration and license agreements. Starting in 2021 we also have commercial revenues from the sales of Jyseleca, which are reported as “Product net sales” on the discontinued operations line in our consolidated income statement. The revenue recognition policies can be summarized as follows: We recognize revenue when our customer obtains control of promised goods or services, in an amount that reflects the consideration that we expect to receive in exchange for those goods or services. To determine revenue recognition for agreements that we determine are within the scope of IFRS 15, we perform the following five steps: Collaboration revenues (i) identify the contract In our agreements with customers we are mainly transferring licenses on our IP and in some cases this is combined with access rights and/or providing research and development services and/or cost sharing mechanisms. In some cases our collaborations also include an equity subscription component. If this is the case, we analyze if the criteria to combine contracts, as set out by IFRS 15, are met. (ii) identify the performance obligations in the contract Depending on the type of the agreement, there can be one or more distinct performance obligations under IFRS 15. This is based on an assessment of whether the promises in an agreement are capable of being distinct and are distinct from the other promises to transfer goods and/or services in the context of the contract. For some of our agreements we combine the transfer of the license with the performance of research and development activities because we consider that the license is not capable of being distinct and is not distinct in the context of the contract. (iii) determine the transaction price Collaboration and license agreements with our commercial partners for research and development activities generally include non-refundable upfront fees; milestone payments, the receipt of which is dependent upon the achievement of certain clinical, regulatory or commercial milestones; license fees, royalties on sales and sometimes reimbursement income or profits sharing arrangements. (a) License fees or upfront payments If the license to our intellectual property is determined to be distinct from the other performance obligations identified in the arrangement, we recognize revenues from non-refundable upfront fees allocated to the license at the point in time the license is transferred to the customer and the customer has the right to use the license. 193 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS For licenses that are bundled with other promises, we utilize judgment to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time. If the performance obligation is satisfied over time, revenue is recognized based on a pattern that best reflects the transfer of control of the service to the customer. (b) Milestone payments other than sales based milestones A milestone payment is only included in the transaction price to the extent that it is highly probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved (which is generally only when the milestone is achieved). Where milestone payments are included in the transaction price we estimate the amount to be included in the transaction price using the most likely amount method. The transaction price is allocated to each performance obligation on a stand-alone selling price basis. We recognize revenue as or when the performance obligations under the contract are satisfied. At the end of each subsequent reporting period, we re-evaluate the probability of achievement of relevant milestones and any related constraint. If necessary we adjust our estimate of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis, which would affect revenue and earnings in the period of adjustment. (c) Reimbursement income for R&D services Collaboration and license agreements may include reimbursement or cost sharing for research and development services: such as outsourcing costs and payment for full-time equivalents at contractual rates. R&D services are performed and satisfied over time given that the customer simultaneously receives and consumes the benefits provided by us. Such costs reimbursements received are recognized in revenues when costs are incurred and agreed by the parties when we are acting as a principal in the scope of our stake of the R&D activities. If the later condition is not fulfilled, costs reimbursements are accounted for as a decrease of the related expenses. (d) Sales based milestone payments and royalties License and collaboration agreements include sales-based royalties, including commercial milestone payments based on the level of sales, and the license has been deemed to be the predominant item to which the royalties relate. Related revenue is recognized as the subsequent underlying sales occur. (iv) allocate the transaction price to the performance obligations in the contract We allocate the transaction price to each performance obligation identified in the contract based upon stand-alone selling price. The stand-alone selling price of each performance obligation is estimated by using one of the following methods: adjusted market assessment approach, the expected cost plus a margin approach or the residual approach. If management assesses that there is only one single performance obligation, the entire transaction price would be allocated to this performance obligation. 194 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS (v) recognize revenue when (or as) the entity satisfies a performance obligation Revenue is recognized when our customer obtains control of the goods and/or services foreseen in the contracts. The control can be transferred over time or at a point in time –which results in recognition of revenue over time or at a point in time. In case of revenue recognition over time, we use an input model that considers estimates of the percentage of total research and development costs that are completed each period compared to the total estimated costs (percentage of completion method) to measure the progress of the satisfaction of the underlying performance obligation (which is the applied method for the filgotinib performance obligation). In other cases, depending on specific circumstances, we recognize revenue on a straight-line basis over the estimated term of the performance obligation (which is the applied method for the performance obligation related to our drug discovery platform). Product net sales Revenue on the sale of Jyseleca® is recorded as “Product net sales” on the discontinued operations line in our consolidated income statement. Product net sales is the net amount of revenue recognized resulting from transferring control over our products to our customer (for example wholesalers and hospitals). Product sales revenue is recognized at a point in time when control of the goods has transferred to the customer. This is generally when the goods are delivered to the customer depending on the specific incoterms in the contract with a customer. The amount of revenue recognized is the amount allocated to the satisfied performance obligation taking into account variable consideration. The estimated amount of variable consideration is included in the transaction price only to the extent that it is highly probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. Variable consideration that is included in the transaction price is primarily composed of rebates, discounts, cash discounts and chargebacks granted to various customers that are part of commercial and governmental contractual arrangements or other reimbursement programs. Shelf stock adjustments are granted to some of our customers to cover the inventory held by them at the time of a price decrease becomes effective. A liability is recognized for expected rebates, cash discounts, chargebacks or other reimbursements payable directly or indirectly to customers in relation to sales made until the end of the reporting period. The amount of variable consideration is estimated using several elements such as third-party market data, product pricing, the specific terms in the individual agreements, estimated inventory levels and the shelf life of our product. If actual results differ, these estimates will be adjusted. Net sales are presented net of value added tax and other sales related taxes. 195 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS Cost of sales Our cost of sales includes primarily the purchase cost of the goods sold and transportation costs. Other operating income Grants and R&D incentives As we carry out extensive research and development activities, we benefit from various grants and R&D incentives from certain governmental agencies. These grants and R&D incentives generally aim to partly reimburse (approved) expenditures incurred in our research and development efforts and are credited to the income statement, under other income, when the relevant expenditure has been incurred and there is reasonable assurance that the grants or R&D incentives are receivable. Share-based payments (i) Equity-settled share-based payments We grant equity-settled incentives to certain employees, members of the Executive Committee and consultants in the form of subscription rights. Equity-settled subscription rights are measured at fair value at the date of acceptance. The fair value determined at the acceptance date of the subscription rights is expensed over time until the end of the vesting period, based on our estimate of subscription rights that are expected to be exercised. Fair value is measured by use of the Black & Scholes model. The expected life used in the model has been adjusted, based on management’s best estimate, for the effects of non-transferability, exercise restrictions, and behavioral considerations. (ii) Long-term incentive plans in RSUs (Restricted Stock Units) Members of the Executive Committee and other employees are granted RSUs. An RSU is a grant that takes the form of a promise that employees will receive Galapagos stock in the future and it will be payable, at the company’s discretion in cash or in shares, upon completion of a certain vesting period. Each RSU reflects the value of one Galapagos share. The RSUs are measured based on the volume weighted average share price over the 30-calendar day period preceding the measurement date. We recognize the corresponding expense and liability over the vesting period. The fair value of the liability is re-measured at each reporting date because currently it is management’s intention to settle the RSUs in cash. Segment reporting We currently have one operating and reportable segment. 196 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS Assets held for sale and discontinued operations A discontinued operation is a component of an entity that either has been disposed of, or that is classified as held for sale. It must either: represent a major separate line of business or geographical area of operations; be part of a single coordinated disposal plan; or be a subsidiary acquired exclusively with a view to resale. Intercompany transactions between continuing and discontinued operations are eliminated against discontinuing operations. Non-current assets and disposal groups are classified as assets held for sale if their carrying amount is to be recovered principally through a sale transaction rather than through continuing use. This condition is regarded as met only when the sale is highly probable and the asset (or disposal group) is available for immediate sale in its present condition. A transaction is assumed to be highly probable if there are no significant risks of completion of the transaction, which depends on the specific circumstances but usually required at least an agreed binding term sheet. They are stated at the lower of carrying amount and fair value less costs to sell with any resulting impairment recognized. Assets related to discontinued operations and assets of disposal group held for sale are not depreciated. On 30 October 2023, we signed a letter of intent to transfer our Jyseleca® business to Alfasigma and the final agreement was signed on 30 December 2023. We classified the assets and the associated liabilities of the Jyseleca® business as held for sale in our financial statements for the year ended 31 December 2023. The transaction was closed on 31 January 2024. Where applicable and in accordance with IFRS 5, we have restated the 2022 comparatives in the consolidated income statement and in the notes to reflect the impact of classifying the Jyseleca® business as discontinued operations in 2023. We refer to note 5 of our consolidated financial statements. 197 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS estimation 4. Critical accounting uncertainty judgments and key sources of In the application of the accounting policies, we are required to make judgments, estimates and assumptions about the carrying amounts of assets and liabilities that are not readily apparent from other sources. The estimates and associated assumptions are based on historical experience and other factors that are considered to be relevant. Actual results may differ from these estimates. Our estimates and assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revisions and future periods if the revision affects both current and future periods. The following are the critical judgments that we have made in the process of applying the accounting policies and the key sources of estimation uncertainty that have the most significant effect on the amounts recognized in the consolidated financial statements presented elsewhere in this annual report. Critical judgments in applying accounting policies IFRS 15 – Revenue recognition of the collaboration with Gilead for the development of filgotinib (reported within the results from discontinued operations) Our critical judgments were as follows: Identification of the contract Despite the recent additional amendment to the collaboration with Gilead for the development of filgotinib (reference is made to note 2), management judged that all activities are still beneficial for the further development of filgotinib, for which Gilead still owns the ex-Europe rights. All contract modifications have thus been analyzed following the requirements of IFRS 15 as we concluded that Gilead is still to be considered as a customer. This is also supported by the fact that we concluded that there continues to be only one performance obligation with respect to filgotinib. Identification of the performance obligation The recent modifications to the collaboration with Gilead (reference is made to note 2) did not give rise to new performance obligations. There was only a change in scope and price of the existing filgotinib performance obligation, which was only partly satisfied at the time of the modification. Based on this, the contract modification has been treated on a cumulative catch-up basis under IFRS 15. 198 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS Allocation of the total transaction price We assessed that the contract modification only changes the scope of the filgotinib performance obligation and the change in both fixed and variable consideration is reflective of the updated stand-alone selling price for the remaining activities of this performance obligation. If we would have concluded that the increased consideration was not, or only partially, related to the filgotinib performance obligation, the consideration would have been potentially allocated to other performance obligations in the contract, which would alter the timing of revenue recognition. The denominator used in the calculation of the percentage of completion reflects our best estimate of our total costs to complete the filgotinib performance obligation. These costs were assessed considering management’s best estimate of the design and duration of ongoing and planned clinical trials and the expected closing of the transaction with Alfasigma. As a result of this transaction, the contract with Gilead relating to filgotinib will be transferred to Alfasigma and we will be released from our performance obligation. The remaining costs per 31 December 2023 mainly reflect the costs that we still estimate to incur before the transfer to Alfasigma. IFRS 5 – Classification of group of assets/liabilities held for sale (disposal group) and discontinued operations Management determined that selling the Jyseleca® business represents a “discontinued operation” in accordance with IFRS 5. We assessed that the Jyseleca® business represents a component of the group for which the related operations and cashflows could be distinguished from the rest of the entity. Jyseleca® is our only commercialized product and represents a major line of business. Management assessed that, at the reporting date, the sale of the Jyseleca® business to Alfasigma was highly probable. A letter of intent was signed on 30 October 2023 and included a customary break-up fee in the event that the parties would not proceed with definitive agreements (share and asset purchase agreement and transition agreement). These definitive agreements were signed on 30 December 2023 and only included usual and customary closing conditions. Based on this, we assessed that the sale was highly probable and classified the disposal group as held for sale per 31 December 2023. Our inventories were not considered to be part of the disposal group held for sale. The inventories will not transfer to Alfasigma on closing of the sale transaction but will gradually be transferred to Alfasigma over the coming years. In the meantime, we will bear all risks related to these inventories. We refer to note 5 for more information about the discontinued operations and disposal group held for sale. Key sources of estimation uncertainty The following are the key sources of estimation uncertainty that have the most significant effect on the amounts recognized in our consolidated financial statements for the year ended 31 December 2023. 199 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS Costs to complete the filgotinib performance obligation The denominator used in the calculation of the percentage of completion reflects our best estimate of the total costs to complete the filgotinib performance obligation (which is composed of the actual costs already incurred at reporting date and our best estimate of the remaining costs to complete the performance obligation). As our estimate of the costs is depending on the evolution of the development activities and the expected closing date of the transfer of the Jyseleca® business to Alfasigma, it may be subject to change in the future. If the outcome of certain activities would be different from the assumptions that we made, it could lead to a material adjustment to the total estimated costs, resulting in a reallocation of revenue between current and future periods. Our total deferred income balance related to this filgotinib performance obligation amounts to €26.3 million on 31 December 2023 and will mainly be released to revenue from discontinued operations in the first quarter of 2024 as a result of the completion of the sale of the Jyseleca® business to Alfasigma on 31 January 2024. The sale to Alfasigma includes the transfer of the amended filgotinib agreement, and by consequence marks the end of our performance obligation towards Gilead. At reporting date, had our best estimate of the remaining cost to complete the filgotinib performance obligation been increased by 10%, this would have resulted in a decrease in revenue recognition in 2023 of €2.6 million and a corresponding increase in current deferred income. Had our best estimate of the remaining cost to complete the filgotinib performance obligation been decreased by 10%, this would have resulted in an increase in revenue recognition in 2023 of €2.6 million and a corresponding decrease in current deferred income. We refer to note 5 for more information about the results from discontinued operations. Goodwill impairment Determining whether goodwill is subject to impairment requires an estimate of the recoverable amount of the cash-generating unit to which the goodwill has been allocated. The calculation of this recoverable amount includes forecasts of future cash flows of the cash-generating unit (highly dependent upon the probability of success linked to the progress of our clinical programs) that cover a period of 17 years and an appropriate discount rate is required to calculate present values, a process which involves estimates. Given that the calculation contains cashflows that go beyond the 5-years horizon it becomes less verifiable and more assumptions are used. Unexpected events, inherent in the business, can cause that results are completely different than the ones predicted. These estimates are constantly monitored, and an impairment test will be performed as soon as there is an impairment indicator and at least annually. The carrying value of goodwill at 31 December 2023 is €69.6 million. We refer to note 13 for more information about the goodwill and impairment of goodwill. Contingent consideration The contingent consideration included in the consideration payable for the acquisition of CellPoint was recorded at fair value at the date of acquisition and is updated at each reporting date. The carrying amount at 31 December 2023 amounts to €21.0 million. These fair values were mainly based on our best estimate of probabilities of reaching the underlying milestones and by applying an appropriate discount rate. The fair values 200 Galapagos NV Annual Report 2023

INANCIAL STATEMENTS are reviewed at each reporting date and any changes are reflected in our consolidated income statement. We refer to note 25 for more information about the contingent consideration payable for the acquisition of CellPoint. 5. Discontinued operations and assets held for sale On 30 October 2023 we announced that we had signed a letter of intent contemplating a transfer of the Jyseleca® business to Alfasigma, including the European and UK Marketing Authorizations, the commercial, medical and development activities for Jyseleca® and approximately 400 positions in 14 European countries. On 30 December 2023 we signed a final share and asset purchase agreement with Alfasigma. On 31 December 2023, the transaction was still subject to certain closing conditions such as the finalization of the consultation process with the workers councils and FDI clearance in Italy, France and Denmark. The transaction was closed on 31 January 2024, upon obtaining all necessary approvals. We received a €50.0 million upfront payment in 2024, and are entitled to potential sales-based milestone payments totalling €120.0 million and mid-single to mid-double-digit royalties on European sales. We will contribute up to €40.0 million to Alfasigma by June 2025 for Jyseleca® related development activities. On 31 January 2024, we also signed a transition agreement with Alfasigma enacting the responsibilities and services that will be provided by the parties during a transition period for the transfer of the business. The gradual transfer of our remaining inventories to Alfasigma is also governed by this contract. The transfer of our Jyseleca® business has been determined to meet the criteria to be classified as held for sale and discontinued operations in our financial statements for the year ended 31 December 2023. 201 Galapagos NV Annual Report 2023
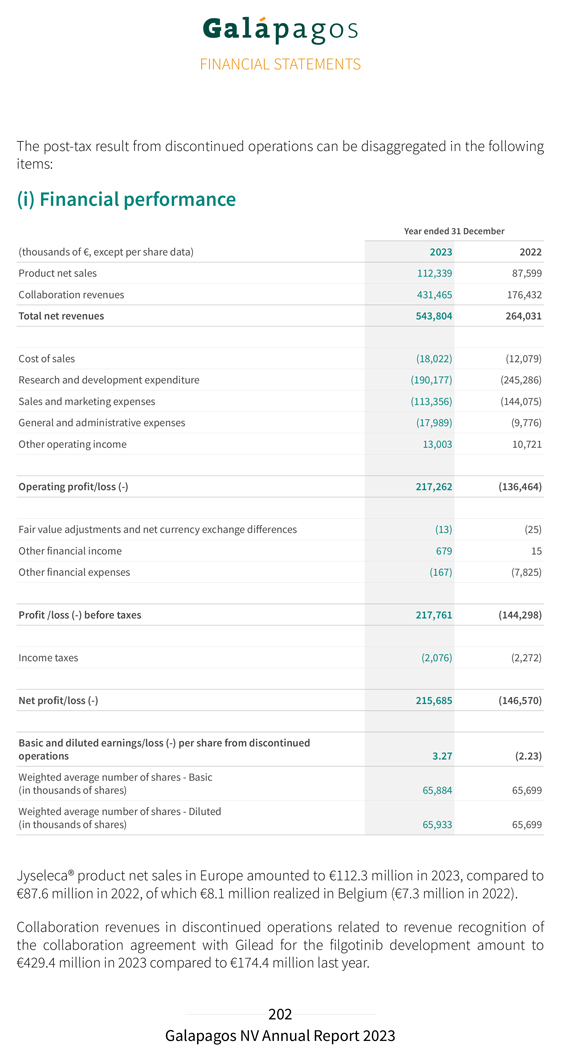
FINANCIAL STATEMENTS The post-tax result from discontinued operations can be disaggregated in the following items: (i) Financial performance Year ended 31 December (thousands of €, except per share data) 2023 2022 Product net sales 112,339 87,599 Collaboration revenues 431,465 176,432 Total net revenues 543,804 264,031 Cost of sales (18,022) (12,079) Research and development expenditure (190,177) (245,286) Sales and marketing expenses (113,356) (144,075) General and administrative expenses (17,989) (9,776) Other operating income 13,003 10,721 Operating profit/loss (-) 217,262 (136,464) Fair value adjustments and net currency exchange differences (13) (25) Other financial income 679 15 Other financial expenses (167) (7,825) Profit /loss (-) before taxes 217,761 (144,298) Income taxes (2,076) (2,272) Net profit/loss (-) 215,685 (146,570) Basic and diluted earnings/loss (-) per share from discontinued operations 3.27 (2.23) Weighted average number of shares—Basic (in thousands of shares) 65,884 65,699 Weighted average number of shares—Diluted (in thousands of shares) 65,933 65,699 Jyseleca® product net sales in Europe amounted to €112.3 million in 2023, compared to €87.6 million in 2022, of which €8.1 million realized in Belgium (€7.3 million in 2022). Collaboration revenues in discontinued operations related to revenue recognition of the collaboration agreement with Gilead for the filgotinib development amount to €429.4 million in 2023 compared to €174.4 million last year. 202 Galapagos NV Annual Report 2023
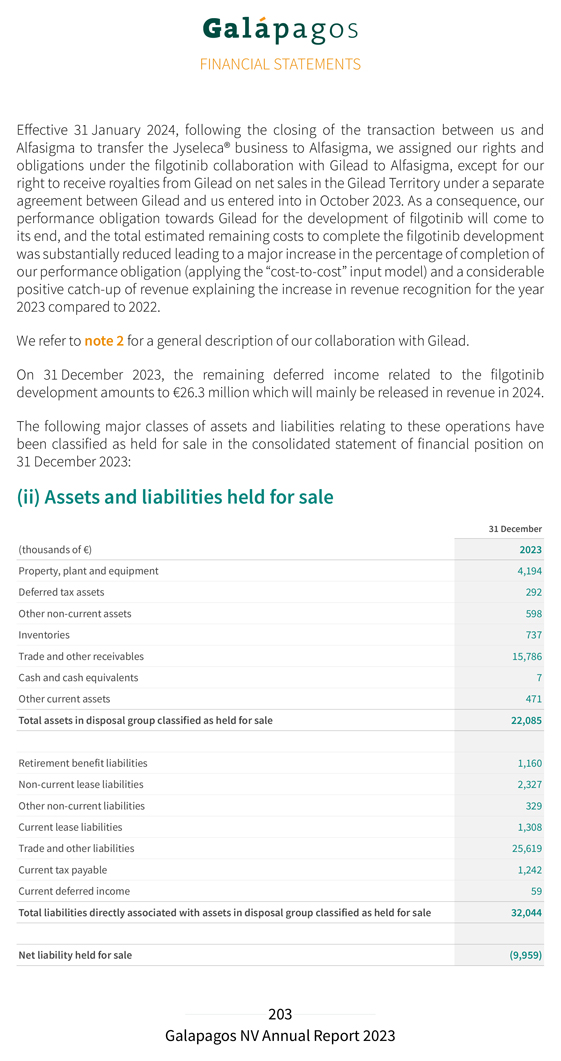
FINANCIAL STATEMENTS Effective 31 January 2024, following the closing of the transaction between us and Alfasigma to transfer the Jyseleca® business to Alfasigma, we assigned our rights and obligations under the filgotinib collaboration with Gilead to Alfasigma, except for our right to receive royalties from Gilead on net sales in the Gilead Territory under a separate agreement between Gilead and us entered into in October 2023. As a consequence, our performance obligation towards Gilead for the development of filgotinib will come to its end, and the total estimated remaining costs to complete the filgotinib development was substantially reduced leading to a major increase in the percentage of completion of our performance obligation (applying the “cost-to-cost” input model) and a considerable positive catch-up of revenue explaining the increase in revenue recognition for the year 2023 compared to 2022. We refer to note 2 for a general description of our collaboration with Gilead. On 31 December 2023, the remaining deferred income related to the filgotinib development amounts to €26.3 million which will mainly be released in revenue in 2024. The following major classes of assets and liabilities relating to these operations have been classified as held for sale in the consolidated statement of financial position on 31 December 2023: (ii) Assets and liabilities held for sale 31 December (thousands of €) 2023 Property, plant and equipment 4,194 Deferred tax assets 292 Other non-current assets 598 Inventories 737 Trade and other receivables 15,786 Cash and cash equivalents 7 Other current assets 471 Total assets in disposal group classified as held for sale 22,085 Retirement benefit liabilities 1,160 Non-current lease liabilities 2,327 Other non-current liabilities 329 Current lease liabilities 1,308 Trade and other liabilities 25,619 Current tax payable 1,242 Current deferred income 59 Total liabilities directly associated with assets in disposal group classified as held for sale 32,044 Net liability held for sale (9,959) 203 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS This disposal group mainly contains all assets and liabilities of the Galapagos subsidiaries that were fully dedicated to the Jyseleca® business and that will be transferred to Alfasigma in the current transaction. The divestiture includes 100% of the shares of the following subsidiaries, including most of the employees: Galapagos Biotech Limited (UK), Galapagos Biopharma Belgium BV, Galapagos Biopharma GmbH, Galapagos Biopharma Italy S.r.l., Galapagos Biopharma Netherlands B.V., Galapagos Biopharma Spain S.L.U., Galapagos Biopharma Denmark ApS, Galapagos Biopharma Sweden AB, Galapagos Biopharma Finland Oy, Galapagos Biopharma Ireland Ltd., Galapagos Biopharma Norway AS, Galapagos Biopharma Austria GmbH. In addition, and as part of the same transaction, we will transfer all assets, liabilities and employees directly related to the Jyseleca® business but belonging to Galapagos NV or other Galapagos subsidiaries, of which the main asset is the worldwide IP relating to Jyseleca®. Our inventories were not considered as part of the disposal group as these did not transfer to Alfasigma on closing of the transaction on 31 January 2024 but will gradually transfer to Alfasigma during the coming years and we will bear the risks associated with it as long as it is not transferred. Held for sale assets are stated at their carrying amount, which is lower than the fair value less costs to sell. We concluded that the expected present value of the purchase price to be obtained from Alfasigma for the sale of the Jyseleca® business approximates the fair value less costs to sell of the disposal group. (iii) Cash flow from discontinued operations (thousands of €) 2023 2022 Net cash flow used in operating activities (175,627) (191,095) Net cash flow used in investing activities (105) (136) Net cash flow used in financing activities (1,928) (1,841) Net cash flow used in discontinued operations (177,660) (193,072) 204 Galapagos NV Annual Report 2023
FINANCIAL STATEMENTS 6. Segment information We are currently operating as a single operating segment. Geographical information In 2022 and 2023 our continuing operations were mainly located in Belgium, France, the Netherlands, Switzerland and the United States. The revenues from our collaboration partner Gilead represented nearly 100% of our total net revenues from continuing operations in 2023 (99.9% in 2022). Following table summarizes our net revenues by destination of customer: Year ended 31 December (thousands of €) 2023 2022 United States of America 665,174 414,129 Europe 118,354 91,151 Total net revenues 783,528 505,280 minus: United States of America 425,466 172,980 Europe 118,338 91,051 Total net revenues from discontinued operations 543,804 264,031 United States of America 239,708 241,149 Europe 16 100 Total net revenues from continuing operations 239,724 241,249 On 31 December 2023, we held €323.8 million (€370.4 million in 2022) of property, plant and equipment, intangible assets and goodwill distributed as follows: 31 December (thousands of €) 2023 2022 Belgium 56,209 72,087 France 1,438 20,397 The Netherlands 251,230 255,461 Switzerland 3,247 4,962 Spain—3,037 United States of America 11,660 12,729 Other—1,747 Total 323,784 370,420 205 Galapagos NV Annual Report 2023
FINANCIAL STATEMENTS 7. Total net revenues from our continuing operations Collaboration revenues The following table summarizes our collaboration revenues for the years ended 31 December 2023 and 2022 by collaboration and by category of revenue: upfront payments and license fees, reimbursement income and royalties. Year ended 31 December Point in (thousands of €) Over time time 2023 2022(*) Recognition of non-refundable upfront payments and license fees 230,242 230,423 Gilead collaboration agreement for drug discovery platform 230,242 230,423 Reimbursement income—56 Novartis collaboration agreement for MOR106—56 Royalties 9,482 10,770 Gilead royalties on Jyseleca® 9,466 10,726 Other royalties 16 44 Total collaboration revenues 239,724 241,249 (*) The 2022 comparative has been restated to reflect the impact of classifying the Jyseleca® business as discontinued operations in 2023. We recognize the consideration from Gilead allocated to the drug discovery platform on a linear basis over the 10-year period of our collaboration, of which we recognized €230.2 million in revenue in 2023. We expect to recognize the same amount in the coming years, until the end of the 10-year period. Since signing of the letter of intent with Alfasigma in October 2023, we classified all activities that were directly related to the Jyseleca® business, including the revenue recognition related to the filgotinib performance obligation, as discontinued operations in accordance with IFRS 5. We refer to note 5 “Discontinued Operations” for additional information. For the year ended 31 December 2023 we also recognized in revenue €9.5 million of royalties from Gilead on filgotinib. The royalties on sales of Jyseleca® performed by Gilead in Japan were not reported as discontinued operations as we still have the right to receive those royalties on future sales made by Gilead and its commercialization partners (this right is not subject to transfer to Alfasigma as part of the transfer of the Jyseleca® business to them). 206 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS Collaboration with Gilead We refer to note 2 of this financial report for a general description of our collaboration with Gilead. In addition, we concluded as follows for the remaining performance obligations: Access rights to the drug discovery platform, option rights and R&D activities The revenue allocated to the drug discovery platform is recognized over time as Gilead receives exclusive access to our drug discovery platform and option rights on our current and future pipeline as well as R&D activities during the collaboration term. Management concluded that an equal spread over the collaboration period is the most reliable and appropriate recognition method. At inception of the collaboration (July 2019) we assessed the appropriate period over which to recognize the drug discovery platform revenue to be 10 years. This is because we granted exclusive rights over a 10-year period. However, if at the end of the 10-year period, some programs in existence as of this time would have reached the clinic (i.e. IND filed with regulatory authorities), the rights for those specific programs may be extended, for a maximum of three years. This critical estimate is reassessed at each year-end based on the evolution of our pipeline and is still valid per 31 December 2023. 207 Galapagos NV Annual Report 2023
FINANCIAL STATEMENTS 8. Operating costs and other operating income Operating costs Research and development expenditure The following table summarizes research and development expenditure for the years ended 31 December 2023 and 2022. Year ended 31 December (thousands of €) 2023 2022(*) Personnel costs (95,788) (115,484) Subcontracting (82,997) (61,192) Disposables and lab fees and premises costs (18,083) (19,529) Depreciation and impairment (22,254) (51,493) Professional fees (9,272) (9,316) Other operating expenses (12,900) (12,783) Total research and development expenditure (241,294) (269,797) (*) The 2022 comparative has been restated to reflect the impact of classifying the Jyseleca® business as discontinued operations in 2023. The table below summarizes our research and development expenditure for the years ended 31 December 2023 and 2022, broken down by program: Year ended 31 December (thousands of €) 2023 2022(*) SIKi program (18,900) (47,727) TYK2 program on GLPG3667 (31,289) (24,467) CAR-T programs in oncology (82,218) (29,999) Other programs (108,887) (167,603) Total research and development expenditure (241,294) (269,797) (*) The 2022 comparative has been restated to reflect the impact of classifying the Jyseleca® business as discontinued operations in 2023. 208 Galapagos NV Annual Report 2023
FINANCIAL STATEMENTS Sales and marketing expenses The following table summarizes the sales and marketing expenses of our continuing operations for the years ended 31 December 2023 and 2022. Year ended 31 December (thousands of €) 2023 2022(*) Personnel costs (2,997) (1,693) Depreciation (113) (59) External outsourcing costs (1,776) (1,267) Professional fees (131) (99) Other operating expenses (659) (363) Total sales and marketing expenses (5,676) (3,480) (*) The 2022 comparative has been restated to reflect the impact of classifying the Jyseleca® business as discontinued operations in 2023. General and administrative expenses The following table summarizes the general and administrative expenses for the years ended 31 December 2023 and 2022. Year ended 31 December (thousands of €) 2023 2022(*) Personnel costs (66,098) (76,536) Depreciation and impairment (15,978) (8,529) Legal and professional fees (23,250) (23,715) Other operating expenses (22,963) (26,375) Total general and administrative expenses (128,289) (135,155) (*) The 2022 comparative has been restated to reflect the impact of classifying the Jyseleca® business as discontinued operations in 2023. Other operating income The following table summarizes other operating income for the years ended 31 December 2023 and 2022. Year ended 31 December (thousands of €) 2023 2022(*) Grant income 6,618 1,873 R&D incentives income 32,968 29,104 Other 7,686 5,150 Total other operating income 47,272 36,127 (*) The 2022 comparative has been restated to reflect the impact of classifying the Jyseleca® business as discontinued operations in 2023. 209 Galapagos NV Annual Report 2023
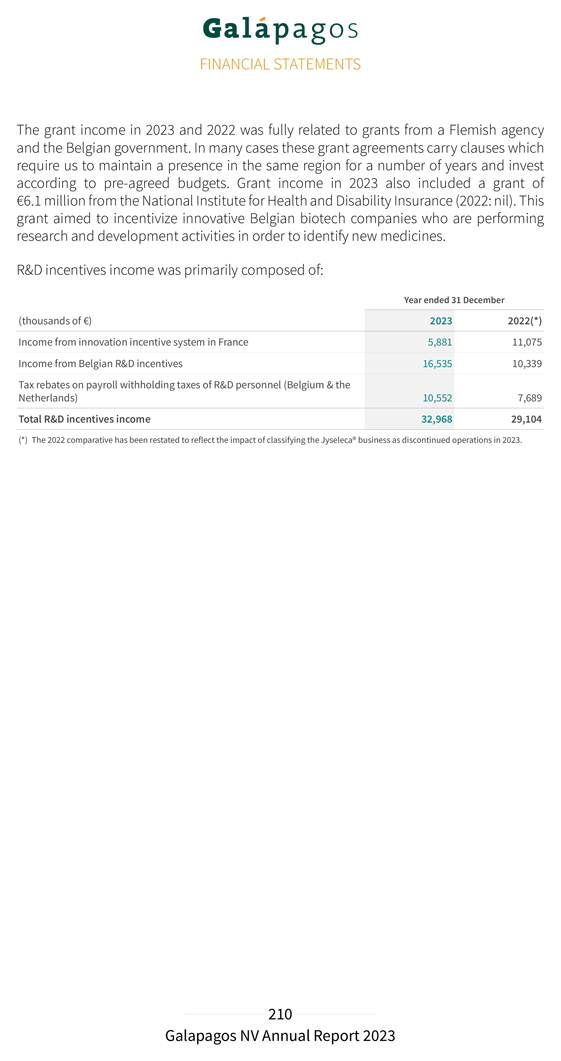
FINANCIAL STATEMENTS The grant income in 2023 and 2022 was fully related to grants from a Flemish agency and the Belgian government. In many cases these grant agreements carry clauses which require us to maintain a presence in the same region for a number of years and invest according to pre-agreed budgets. Grant income in 2023 also included a grant of €6.1 million from the National Institute for Health and Disability Insurance (2022: nil). This grant aimed to incentivize innovative Belgian biotech companies who are performing research and development activities in order to identify new medicines. R&D incentives income was primarily composed of: Year ended 31 December (thousands of €) 2023 2022(*) Income from innovation incentive system in France 5,881 11,075 Income from Belgian R&D incentives 16,535 10,339 Tax rebates on payroll withholding taxes of R&D personnel (Belgium & the Netherlands) 10,552 7,689 Total R&D incentives income 32,968 29,104 (*) The 2022 comparative has been restated to reflect the impact of classifying the Jyseleca® business as discontinued operations in 2023. 210 Galapagos NV Annual Report 2023
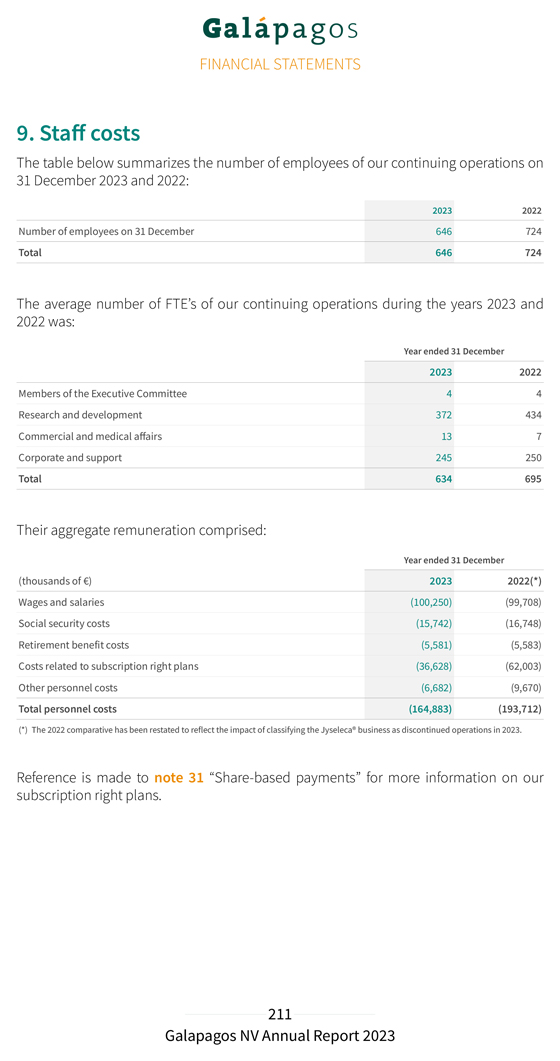
FINANCIAL STATEMENTS 9. Staff costs The table below summarizes the number of employees of our continuing operations on 31 December 2023 and 2022: 2023 2022 Number of employees on 31 December 646 724 Total 646 724 The average number of FTE’s of our continuing operations during the years 2023 and 2022 was: Year ended 31 December 2023 2022 Members of the Executive Committee 4 4 Research and development 372 434 Commercial and medical affairs 13 7 Corporate and support 245 250 Total 634 695 Their aggregate remuneration comprised: Year ended 31 December (thousands of €) 2023 2022(*) Wages and salaries (100,250) (99,708) Social security costs (15,742) (16,748) Retirement benefit costs (5,581) (5,583) Costs related to subscription right plans (36,628) (62,003) Other personnel costs (6,682) (9,670) Total personnel costs (164,883) (193,712) (*) The 2022 comparative has been restated to reflect the impact of classifying the Jyseleca® business as discontinued operations in 2023. Reference is made to note 31 “Share-based payments” for more information on our subscription right plans. 211 Galapagos NV Annual Report 2023
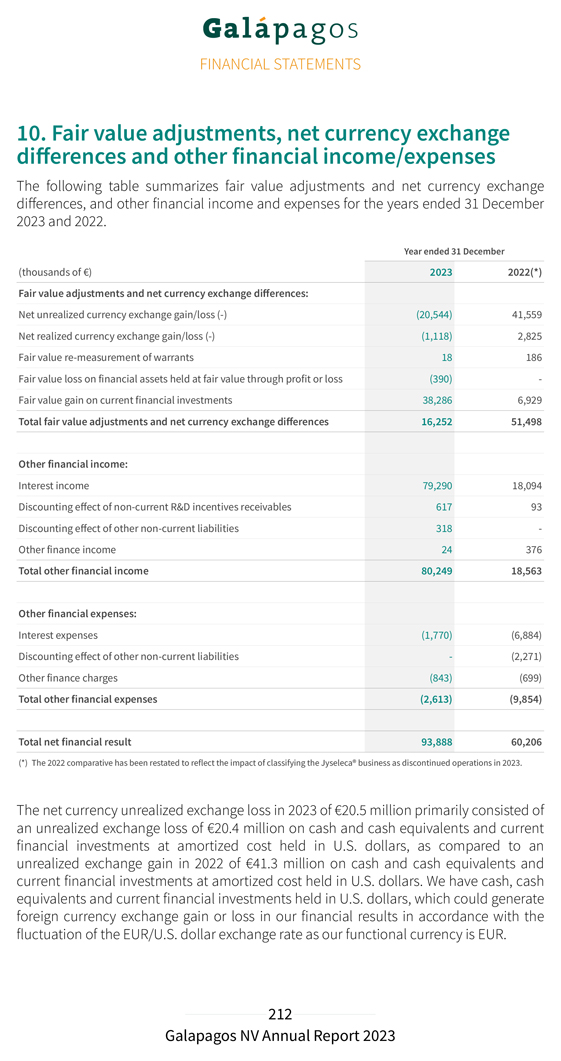
FINANCIAL STATEMENTS differences 10. Fair value and adjustments, other financial net income/expenses currency exchange The following table summarizes fair value adjustments and net currency exchange differences, and other financial income and expenses for the years ended 31 December 2023 and 2022. Year ended 31 December (thousands of €) 2023 2022(*) Fair value adjustments and net currency exchange differences: Net unrealized currency exchange gain/loss (-) (20,544) 41,559 Net realized currency exchange gain/loss (-) (1,118) 2,825 Fair value re-measurement of warrants 18 186 Fair value loss on financial assets held at fair value through profit or loss (390) -Fair value gain on current financial investments 38,286 6,929 Total fair value adjustments and net currency exchange differences 16,252 51,498 Other financial income: Interest income 79,290 18,094 Discounting effect of non-current R&D incentives receivables 617 93 Discounting effect of other non-current liabilities 318 -Other finance income 24 376 Total other financial income 80,249 18,563 Other financial expenses: Interest expenses (1,770) (6,884) Discounting effect of other non-current liabilities—(2,271) Other finance charges (843) (699) Total other financial expenses (2,613) (9,854) Total net financial result 93,888 60,206 (*) The 2022 comparative has been restated to reflect the impact of classifying the Jyseleca® business as discontinued operations in 2023. The net currency unrealized exchange loss in 2023 of €20.5 million primarily consisted of an unrealized exchange loss of €20.4 million on cash and cash equivalents and current financial investments at amortized cost held in U.S. dollars, as compared to an unrealized exchange gain in 2022 of €41.3 million on cash and cash equivalents and current financial investments at amortized cost held in U.S. dollars. We have cash, cash equivalents and current financial investments held in U.S. dollars, which could generate foreign currency exchange gain or loss in our financial results in accordance with the fluctuation of the EUR/U.S. dollar exchange rate as our functional currency is EUR. 212 Galapagos NV Annual Report 2023
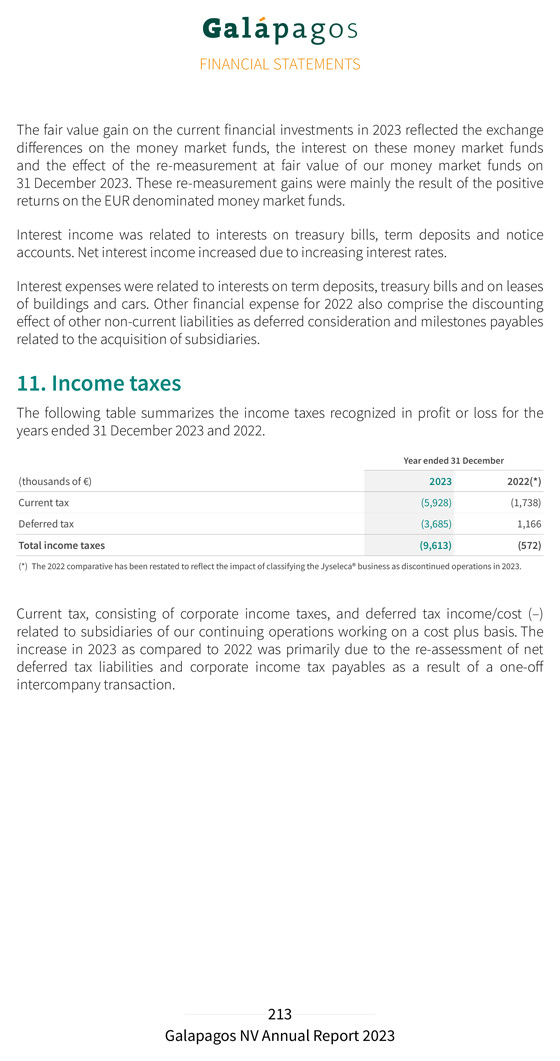
FINANCIAL STATEMENTS The fair value gain on the current financial investments in 2023 reflected the exchange differences on the money market funds, the interest on these money market funds and the effect of the re-measurement at fair value of our money market funds on 31 December 2023. These re-measurement gains were mainly the result of the positive returns on the EUR denominated money market funds. Interest income was related to interests on treasury bills, term deposits and notice accounts. Net interest income increased due to increasing interest rates. Interest expenses were related to interests on term deposits, treasury bills and on leases of buildings and cars. Other financial expense for 2022 also comprise the discounting effect of other non-current liabilities as deferred consideration and milestones payables related to the acquisition of subsidiaries. 11. Income taxes The following table summarizes the income taxes recognized in profit or loss for the years ended 31 December 2023 and 2022. Year ended 31 December (thousands of €) 2023 2022(*) Current tax (5,928) (1,738) Deferred tax (3,685) 1,166 Total income taxes (9,613) (572) (*) The 2022 comparative has been restated to reflect the impact of classifying the Jyseleca® business as discontinued operations in 2023. Current tax, consisting of corporate income taxes, and deferred tax income/cost (–) related to subsidiaries of our continuing operations working on a cost plus basis. The increase in 2023 as compared to 2022 was primarily due to the re-assessment of net deferred tax liabilities and corporate income tax payables as a result of a one-off intercompany transaction. 213 Galapagos NV Annual Report 2023
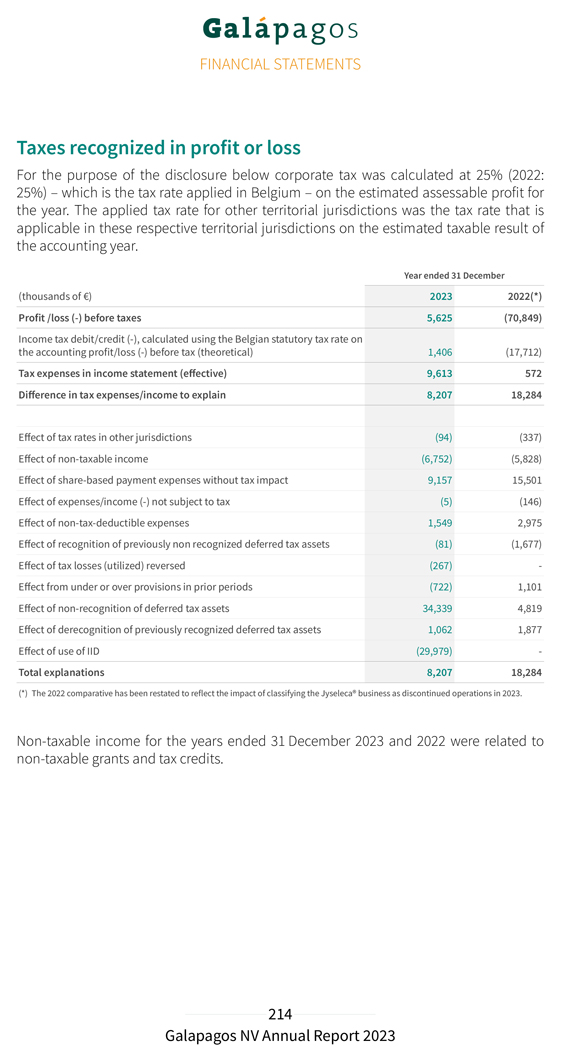
FINANCIAL STATEMENTS Taxes recognized in profit or loss For the purpose of the disclosure below corporate tax was calculated at 25% (2022: 25%) – which is the tax rate applied in Belgium – on the estimated assessable profit for the year. The applied tax rate for other territorial jurisdictions was the tax rate that is applicable in these respective territorial jurisdictions on the estimated taxable result of the accounting year. Year ended 31 December (thousands of €) 2023 2022(*) Profit /loss (-) before taxes 5,625 (70,849) Income tax debit/credit (-), calculated using the Belgian statutory tax rate on the accounting profit/loss (-) before tax (theoretical) 1,406 (17,712) Tax expenses in income statement (effective) 9,613 572 Difference in tax expenses/income to explain 8,207 18,284 Effect of tax rates in other jurisdictions (94) (337) Effect of non-taxable income (6,752) (5,828) Effect of share-based payment expenses without tax impact 9,157 15,501 Effect of expenses/income (-) not subject to tax (5) (146) Effect of non-tax-deductible expenses 1,549 2,975 Effect of recognition of previously non recognized deferred tax assets (81) (1,677) Effect of tax losses (utilized) reversed (267) -Effect from under or over provisions in prior periods (722) 1,101 Effect of non-recognition of deferred tax assets 34,339 4,819 Effect of derecognition of previously recognized deferred tax assets 1,062 1,877 Effect of use of IID (29,979)—Total explanations 8,207 18,284 (*) The 2022 comparative has been restated to reflect the impact of classifying the Jyseleca® business as discontinued operations in 2023. Non-taxable income for the years ended 31 December 2023 and 2022 were related to non-taxable grants and tax credits. 214 Galapagos NV Annual Report 2023
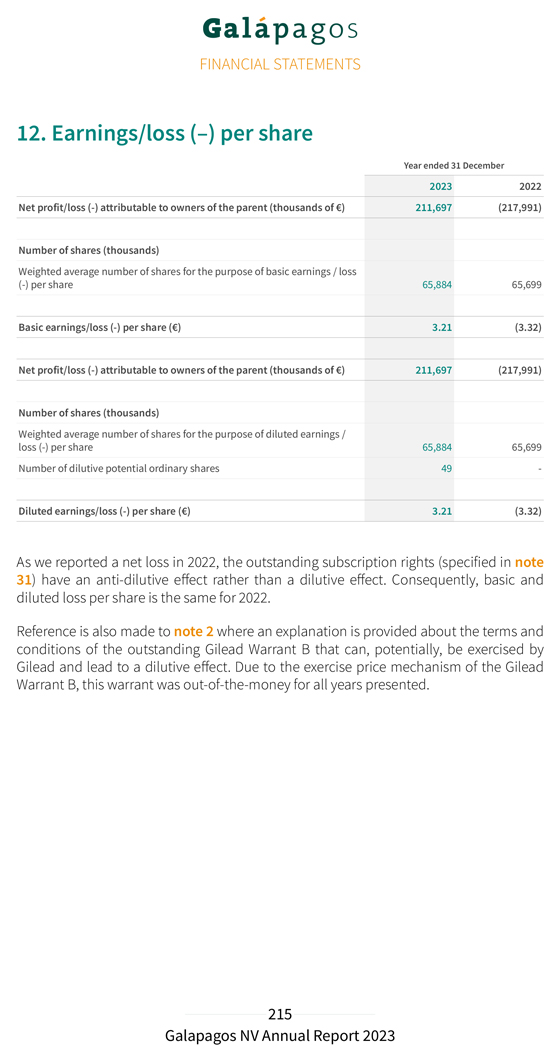
FINANCIAL STATEMENTS 12. Earnings/loss (–) per share Year ended 31 December 2023 2022 Net profit/loss (-) attributable to owners of the parent (thousands of €) 211,697 (217,991) Number of shares (thousands) Weighted average number of shares for the purpose of basic earnings / loss (-) per share 65,884 65,699 Basic earnings/loss (-) per share (€) 3.21 (3.32) Net profit/loss (-) attributable to owners of the parent (thousands of €) 211,697 (217,991) Number of shares (thousands) Weighted average number of shares for the purpose of diluted earnings / loss (-) per share 65,884 65,699 Number of dilutive potential ordinary shares 49—Diluted earnings/loss (-) per share (€) 3.21 (3.32) As we reported a net loss in 2022, the outstanding subscription rights (specified in note 31) have an anti-dilutive effect rather than a dilutive effect. Consequently, basic and diluted loss per share is the same for 2022. Reference is also made to note 2 where an explanation is provided about the terms and conditions of the outstanding Gilead Warrant B that can, potentially, be exercised by Gilead and lead to a dilutive effect. Due to the exercise price mechanism of the Gilead Warrant B, this warrant was out-of-the-money for all years presented. 215 Galapagos NV Annual Report 2023
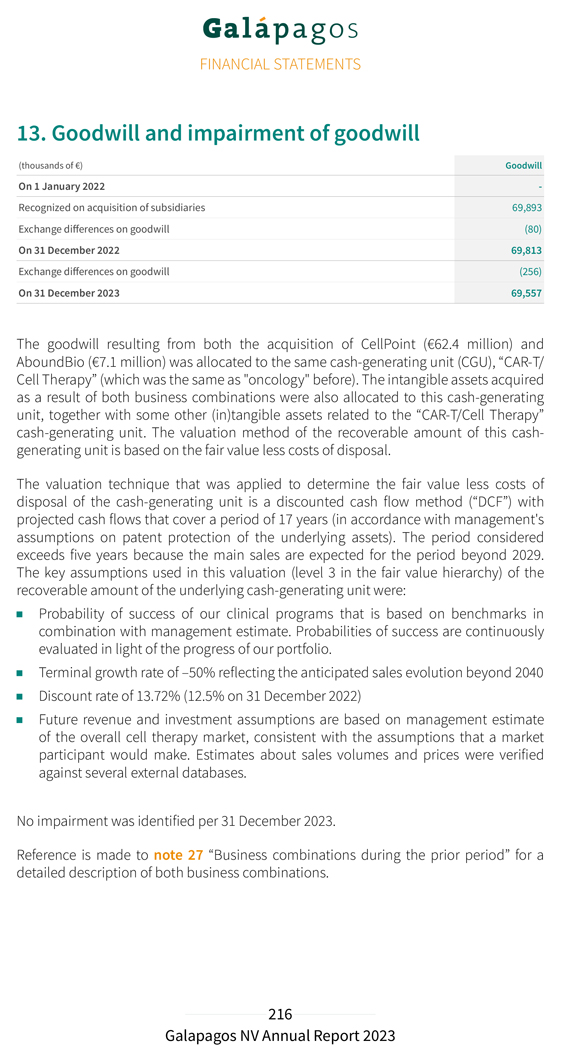
FINANCIAL STATEMENTS 13. Goodwill and impairment of goodwill (thousands of €) Goodwill On 1 January 2022—Recognized on acquisition of subsidiaries 69,893 Exchange differences on goodwill (80) On 31 December 2022 69,813 Exchange differences on goodwill (256) On 31 December 2023 69,557 The goodwill resulting from both the acquisition of CellPoint (€62.4 million) and AboundBio (€7.1 million) was allocated to the same cash-generating unit (CGU), “CAR-T/ Cell Therapy” (which was the same as “oncology” before). The intangible assets acquired as a result of both business combinations were also allocated to this cash-generating unit, together with some other (in)tangible assets related to the “CAR-T/Cell Therapy” cash-generating unit. The valuation method of the recoverable amount of this cash-generating unit is based on the fair value less costs of disposal. The valuation technique that was applied to determine the fair value less costs of disposal of the cash-generating unit is a discounted cash flow method (“DCF”) with projected cash flows that cover a period of 17 years (in accordance with management’s assumptions on patent protection of the underlying assets). The period considered exceeds five years because the main sales are expected for the period beyond 2029. The key assumptions used in this valuation (level 3 in the fair value hierarchy) of the recoverable amount of the underlying cash-generating unit were: Probability of success of our clinical programs that is based on benchmarks in combination with management estimate. Probabilities of success are continuously evaluated in light of the progress of our portfolio. Terminal growth rate of –50% reflecting the anticipated sales evolution beyond 2040 Discount rate of 13.72% (12.5% on 31 December 2022) Future revenue and investment assumptions are based on management estimate of the overall cell therapy market, consistent with the assumptions that a market participant would make. Estimates about sales volumes and prices were verified against several external databases. No impairment was identified per 31 December 2023. Reference is made to note 27 “Business combinations during the prior period” for a detailed description of both business combinations. 216 Galapagos NV Annual Report 2023
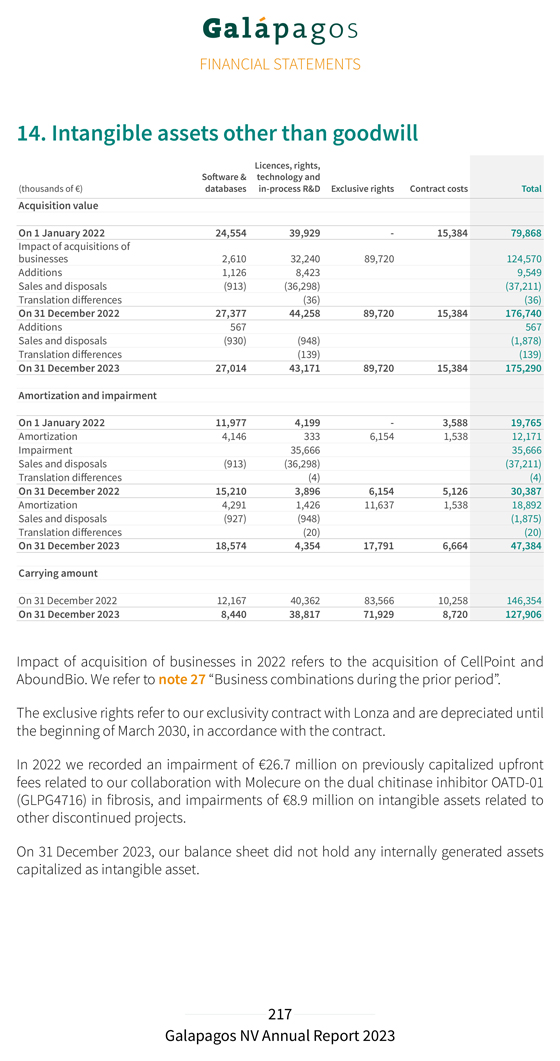
FINANCIAL STATEMENTS 14. Intangible assets other than goodwill Licences, rights, Software & technology and (thousands of €) databases in-process R&D Exclusive rights Contract costs Total Acquisition value On 1 January 2022 24,554 39,929—15,384 79,868 Impact of acquisitions of businesses 2,610 32,240 89,720 124,570 Additions 1,126 8,423 9,549 Sales and disposals (913) (36,298) (37,211) Translation differences (36) (36) On 31 December 2022 27,377 44,258 89,720 15,384 176,740 Additions 567 567 Sales and disposals (930) (948) (1,878) Translation differences (139) (139) On 31 December 2023 27,014 43,171 89,720 15,384 175,290 Amortization and impairment On 1 January 2022 11,977 4,199—3,588 19,765 Amortization 4,146 333 6,154 1,538 12,171 Impairment 35,666 35,666 Sales and disposals (913) (36,298) (37,211) Translation differences (4) (4) On 31 December 2022 15,210 3,896 6,154 5,126 30,387 Amortization 4,291 1,426 11,637 1,538 18,892 Sales and disposals (927) (948) (1,875) Translation differences (20) (20) On 31 December 2023 18,574 4,354 17,791 6,664 47,384 Carrying amount On 31 December 2022 12,167 40,362 83,566 10,258 146,354 On 31 December 2023 8,440 38,817 71,929 8,720 127,906 Impact of acquisition of businesses in 2022 refers to the acquisition of CellPoint and AboundBio. We refer to note 27 “Business combinations during the prior period”. The exclusive rights refer to our exclusivity contract with Lonza and are depreciated until the beginning of March 2030, in accordance with the contract. In 2022 we recorded an impairment of €26.7 million on previously capitalized upfront fees related to our collaboration with Molecure on the dual chitinase inhibitor OATD-01 (GLPG4716) in fibrosis, and impairments of €8.9 million on intangible assets related to other discontinued projects. On 31 December 2023, our balance sheet did not hold any internally generated assets capitalized as intangible asset. 217 Galapagos NV Annual Report 2023
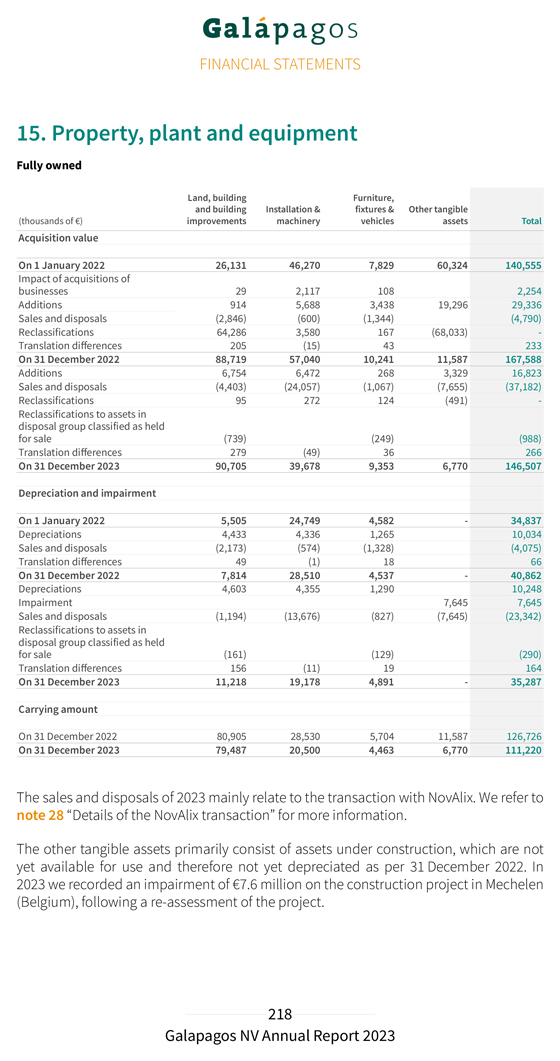
FINANCIAL STATEMENTS 15. Property, plant and equipment Fully owned Land, building Furniture, and building Installation & fixtures & Other tangible (thousands of €) improvements machinery vehicles assets Total Acquisition value On 1 January 2022 26,131 46,270 7,829 60,324 140,555 Impact of acquisitions of businesses 29 2,117 108 2,254 Additions 914 5,688 3,438 19,296 29,336 Sales and disposals (2,846) (600) (1,344) (4,790) Reclassifications 64,286 3,580 167 (68,033) -Translation differences 205 (15) 43 233 On 31 December 2022 88,719 57,040 10,241 11,587 167,588 Additions 6,754 6,472 268 3,329 16,823 Sales and disposals (4,403) (24,057) (1,067) (7,655) (37,182) Reclassifications 95 272 124 (491) -Reclassifications to assets in disposal group classified as held for sale (739) (249) (988) Translation differences 279 (49) 36 266 On 31 December 2023 90,705 39,678 9,353 6,770 146,507 Depreciation and impairment On 1 January 2022 5,505 24,749 4,582—34,837 Depreciations 4,433 4,336 1,265 10,034 Sales and disposals (2,173) (574) (1,328) (4,075) Translation differences 49 (1) 18 66 On 31 December 2022 7,814 28,510 4,537—40,862 Depreciations 4,603 4,355 1,290 10,248 Impairment 7,645 7,645 Sales and disposals (1,194) (13,676) (827) (7,645) (23,342) Reclassifications to assets in disposal group classified as held for sale (161) (129) (290) Translation differences 156 (11) 19 164 On 31 December 2023 11,218 19,178 4,891—35,287 Carrying amount On 31 December 2022 80,905 28,530 5,704 11,587 126,726 On 31 December 2023 79,487 20,500 4,463 6,770 111,220 The sales and disposals of 2023 mainly relate to the transaction with NovAlix. We refer to note 28 “Details of the NovAlix transaction” for more information. The other tangible assets primarily consist of assets under construction, which are not yet available for use and therefore not yet depreciated as per 31 December 2022. In 2023 we recorded an impairment of €7.6 million on the construction project in Mechelen (Belgium), following a re-assessment of the project. 218 Galapagos NV Annual Report 2023
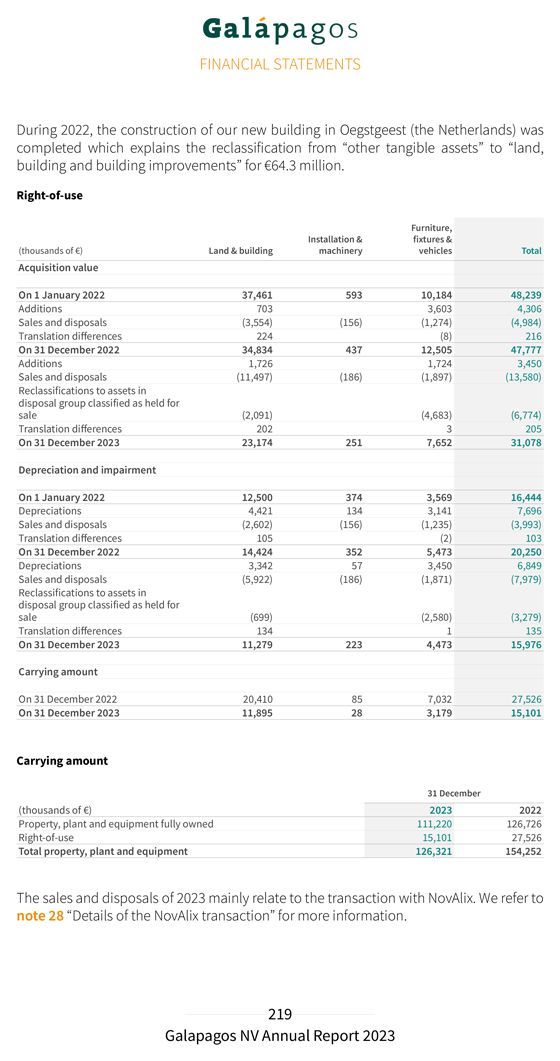
FINANCIAL STATEMENTS During 2022, the construction of our new building in Oegstgeest (the Netherlands) was completed which explains the reclassification from “other tangible assets” to “land, building and building improvements” for €64.3 million. Right-of-use Furniture, Installation & fixtures & (thousands of €) Land & building machinery vehicles Total Acquisition value On 1 January 2022 37,461 593 10,184 48,239 Additions 703 3,603 4,306 Sales and disposals (3,554) (156) (1,274) (4,984) Translation differences 224 (8) 216 On 31 December 2022 34,834 437 12,505 47,777 Additions 1,726 1,724 3,450 Sales and disposals (11,497) (186) (1,897) (13,580) Reclassifications to assets in disposal group classified as held for sale (2,091) (4,683) (6,774) Translation differences 202 3 205 On 31 December 2023 23,174 251 7,652 31,078 Depreciation and impairment On 1 January 2022 12,500 374 3,569 16,444 Depreciations 4,421 134 3,141 7,696 Sales and disposals (2,602) (156) (1,235) (3,993) Translation differences 105 (2) 103 On 31 December 2022 14,424 352 5,473 20,250 Depreciations 3,342 57 3,450 6,849 Sales and disposals (5,922) (186) (1,871) (7,979) Reclassifications to assets in disposal group classified as held for sale (699) (2,580) (3,279) Translation differences 134 1 135 On 31 December 2023 11,279 223 4,473 15,976 Carrying amount On 31 December 2022 20,410 85 7,032 27,526 On 31 December 2023 11,895 28 3,179 15,101 Carrying amount 31 December (thousands of €) 2023 2022 Property, plant and equipment fully owned 111,220 126,726 Right-of-use 15,101 27,526 Total property, plant and equipment 126,321 154,252 The sales and disposals of 2023 mainly relate to the transaction with NovAlix. We refer to note 28 “Details of the NovAlix transaction” for more information. 219 Galapagos NV Annual Report 2023
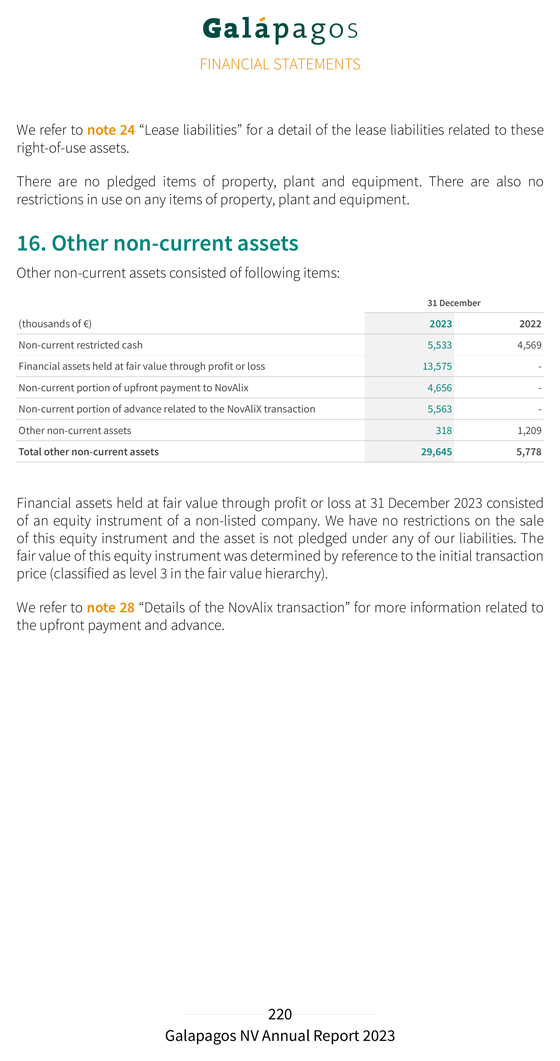
FINANCIAL STATEMENTS We refer to note 24 “Lease liabilities” for a detail of the lease liabilities related to these right-of-use assets. There are no pledged items of property, plant and equipment. There are also no restrictions in use on any items of property, plant and equipment. 16. Other non-current assets Other non-current assets consisted of following items: 31 December (thousands of €) 2023 2022 Non-current restricted cash 5,533 4,569 Financial assets held at fair value through profit or loss 13,575 -Non-current portion of upfront payment to NovAlix 4,656 -Non-current portion of advance related to the NovAliX transaction 5,563 -Other non-current assets 318 1,209 Total other non-current assets 29,645 5,778 Financial assets held at fair value through profit or loss at 31 December 2023 consisted of an equity instrument of a non-listed company. We have no restrictions on the sale of this equity instrument and the asset is not pledged under any of our liabilities. The fair value of this equity instrument was determined by reference to the initial transaction price (classified as level 3 in the fair value hierarchy). We refer to note 28 “Details of the NovAlix transaction” for more information related to the upfront payment and advance. 220 Galapagos NV Annual Report 2023
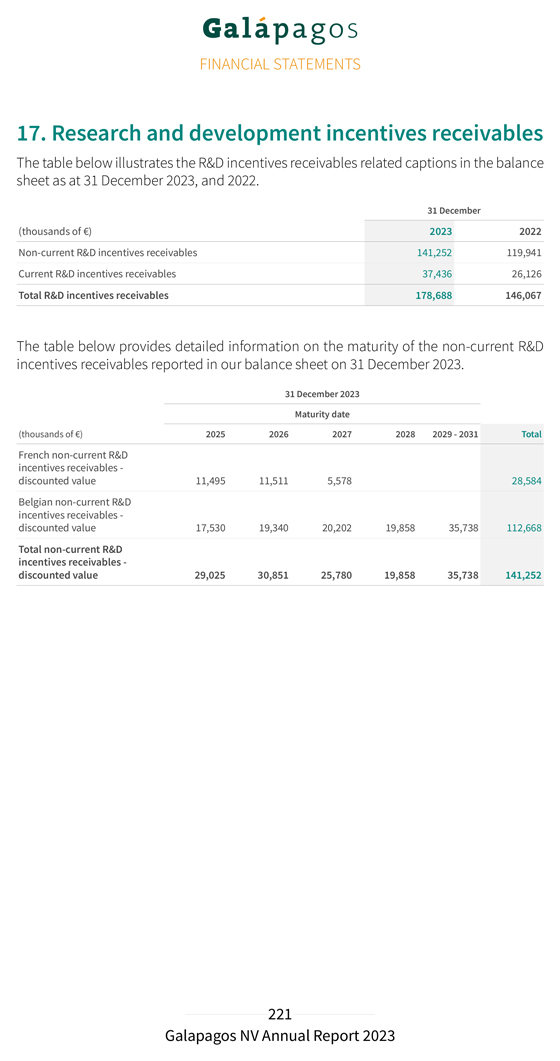
FINANCIAL STATEMENTS 17. Research and development incentives receivables The table below illustrates the R&D incentives receivables related captions in the balance sheet as at 31 December 2023, and 2022. 31 December (thousands of €) 2023 2022 Non-current R&D incentives receivables 141,252 119,941 Current R&D incentives receivables 37,436 26,126 Total R&D incentives receivables 178,688 146,067 The table below provides detailed information on the maturity of the non-current R&D incentives receivables reported in our balance sheet on 31 December 2023. 31 December 2023 Maturity date (thousands of €) 2025 2026 2027 2028 2029—2031 Total French non-current R&D incentives receivables -discounted value 11,495 11,511 5,578 28,584 Belgian non-current R&D incentives receivables -discounted value 17,530 19,340 20,202 19,858 35,738 112,668 Total non-current R&D incentives receivables -discounted value 29,025 30,851 25,780 19,858 35,738 141,252 221 Galapagos NV Annual Report 2023
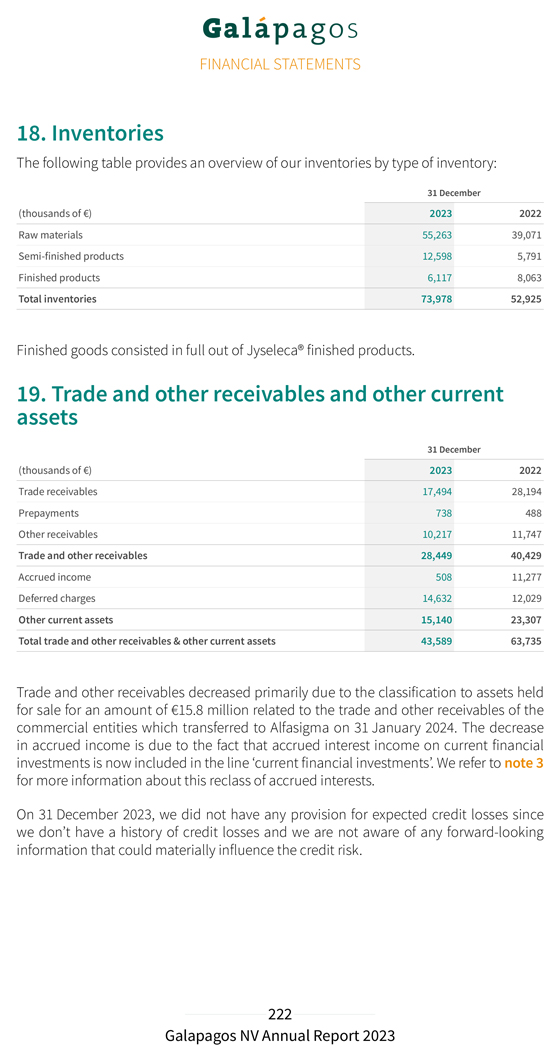
FINANCIAL STATEMENTS 18. Inventories The following table provides an overview of our inventories by type of inventory: 31 December (thousands of €) 2023 2022 Raw materials 55,263 39,071 Semi-finished products 12,598 5,791 Finished products 6,117 8,063 Total inventories 73,978 52,925 Finished goods consisted in full out of Jyseleca® finished products. 19 assets . Trade and other receivables and other current 31 December (thousands of €) 2023 2022 Trade receivables 17,494 28,194 Prepayments 738 488 Other receivables 10,217 11,747 Trade and other receivables 28,449 40,429 Accrued income 508 11,277 Deferred charges 14,632 12,029 Other current assets 15,140 23,307 Total trade and other receivables & other current assets 43,589 63,735 Trade and other receivables decreased primarily due to the classification to assets held for sale for an amount of €15.8 million related to the trade and other receivables of the commercial entities which transferred to Alfasigma on 31 January 2024. The decrease in accrued income is due to the fact that accrued interest income on current financial investments is now included in the line ‘current financial investments’. We refer to note 3 for more information about this reclass of accrued interests. On 31 December 2023, we did not have any provision for expected credit losses since we don’t have a history of credit losses and we are not aware of any forward-looking information that could materially influence the credit risk. 222 Galapagos NV Annual Report 2023
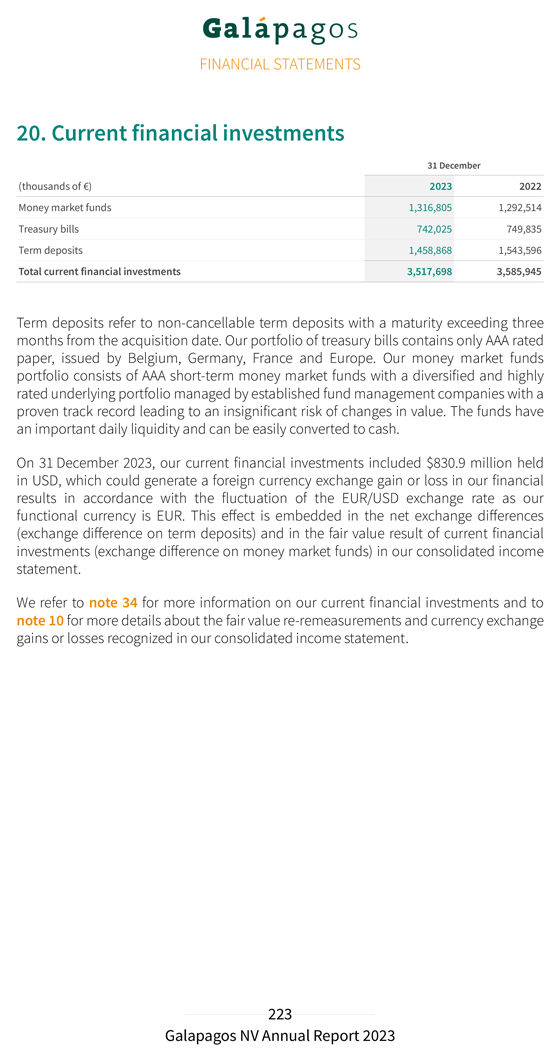
FINANCIAL STATEMENTS 20. Current financial investments 31 December (thousands of €) 2023 2022 Money market funds 1,316,805 1,292,514 Treasury bills 742,025 749,835 Term deposits 1,458,868 1,543,596 Total current financial investments 3,517,698 3,585,945 Term deposits refer to non-cancellable term deposits with a maturity exceeding three months from the acquisition date. Our portfolio of treasury bills contains only AAA rated paper, issued by Belgium, Germany, France and Europe. Our money market funds portfolio consists of AAA short-term money market funds with a diversified and highly rated underlying portfolio managed by established fund management companies with a proven track record leading to an insignificant risk of changes in value. The funds have an important daily liquidity and can be easily converted to cash. On 31 December 2023, our current financial investments included $830.9 million held in USD, which could generate a foreign currency exchange gain or loss in our financial results in accordance with the fluctuation of the EUR/USD exchange rate as our functional currency is EUR. This effect is embedded in the net exchange differences (exchange difference on term deposits) and in the fair value result of current financial investments (exchange difference on money market funds) in our consolidated income statement. We refer to note 34 for more information on our current financial investments and to note 10 for more details about the fair value re-remeasurements and currency exchange gains or losses recognized in our consolidated income statement. 223 Galapagos NV Annual Report 2023
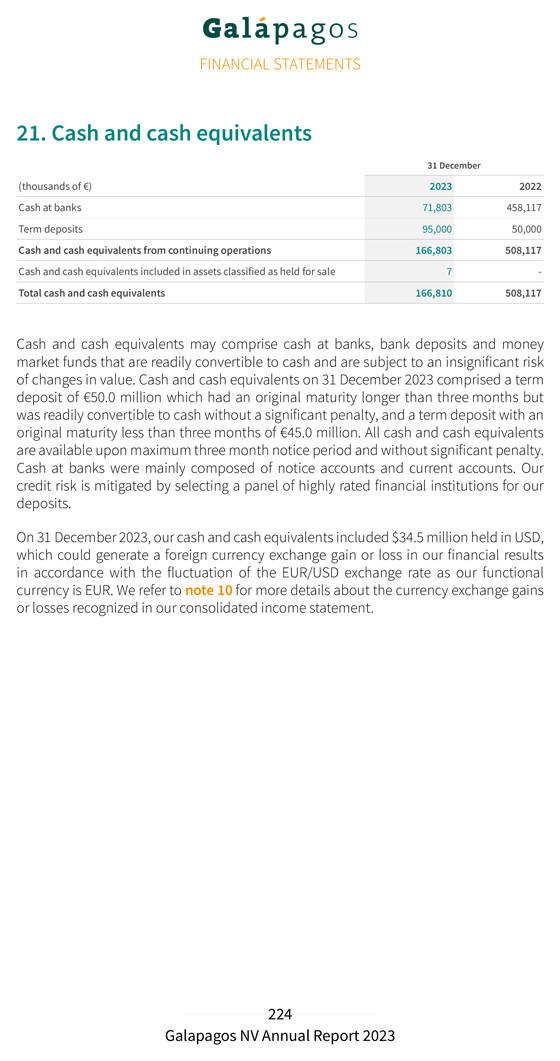
FINANCIAL STATEMENTS 21. Cash and cash equivalents 31 December (thousands of €) 2023 2022 Cash at banks 71,803 458,117 Term deposits 95,000 50,000 Cash and cash equivalents from continuing operations 166,803 508,117 Cash and cash equivalents included in assets classified as held for sale 7—Total cash and cash equivalents 166,810 508,117 Cash and cash equivalents may comprise cash at banks, bank deposits and money market funds that are readily convertible to cash and are subject to an insignificant risk of changes in value. Cash and cash equivalents on 31 December 2023 comprised a term deposit of €50.0 million which had an original maturity longer than three months but was readily convertible to cash without a significant penalty, and a term deposit with an original maturity less than three months of €45.0 million. All cash and cash equivalents are available upon maximum three month notice period and without significant penalty. Cash at banks were mainly composed of notice accounts and current accounts. Our credit risk is mitigated by selecting a panel of highly rated financial institutions for our deposits. On 31 December 2023, our cash and cash equivalents included $34.5 million held in USD, which could generate a foreign currency exchange gain or loss in our financial results in accordance with the fluctuation of the EUR/USD exchange rate as our functional currency is EUR. We refer to note 10 for more details about the currency exchange gains or losses recognized in our consolidated income statement. 224 Galapagos NV Annual Report 2023
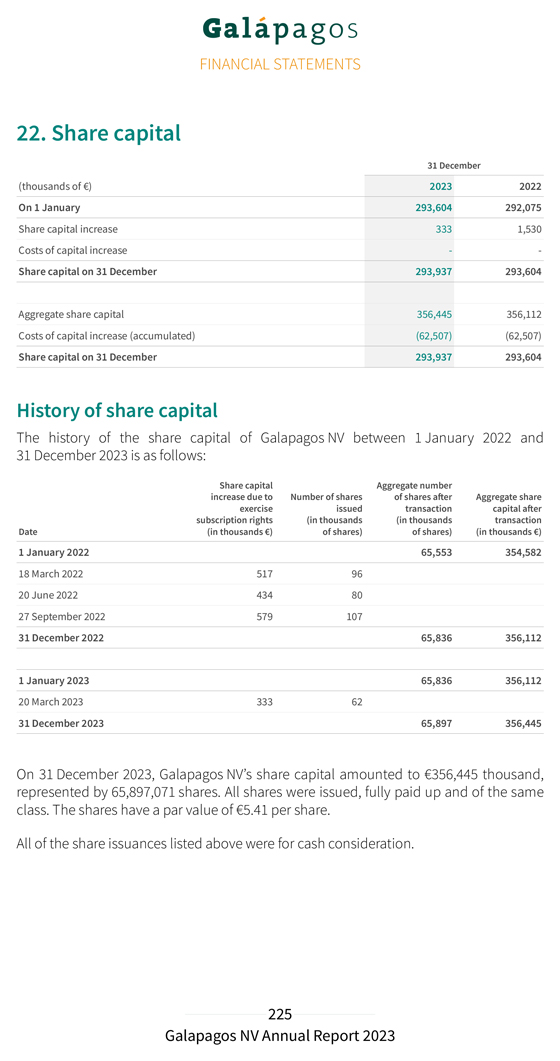
FINANCIAL STATEMENTS 22. Share capital 31 December (thousands of €) 2023 2022 On 1 January 293,604 292,075 Share capital increase 333 1,530 Costs of capital increase — Share capital on 31 December 293,937 293,604 Aggregate share capital 356,445 356,112 Costs of capital increase (accumulated) (62,507) (62,507) Share capital on 31 December 293,937 293,604 History of share capital The history of the share capital of Galapagos NV between 1 January 2022 and 31 December 2023 is as follows: Share capital Aggregate number increase due to Number of shares of shares after Aggregate share exercise issued transaction capital after subscription rights (in thousands (in thousands transaction Date (in thousands €) of shares) of shares) (in thousands €) 1 January 2022 65,553 354,582 18 March 2022 517 96 20 June 2022 434 80 27 September 2022 579 107 31 December 2022 65,836 356,112 1 January 2023 65,836 356,112 20 March 2023 333 62 31 December 2023 65,897 356,445 On 31 December 2023, Galapagos NV’s share capital amounted to €356,445 thousand, represented by 65,897,071 shares. All shares were issued, fully paid up and of the same class. The shares have a par value of €5.41 per share. All of the share issuances listed above were for cash consideration. 225 Galapagos NV Annual Report 2023
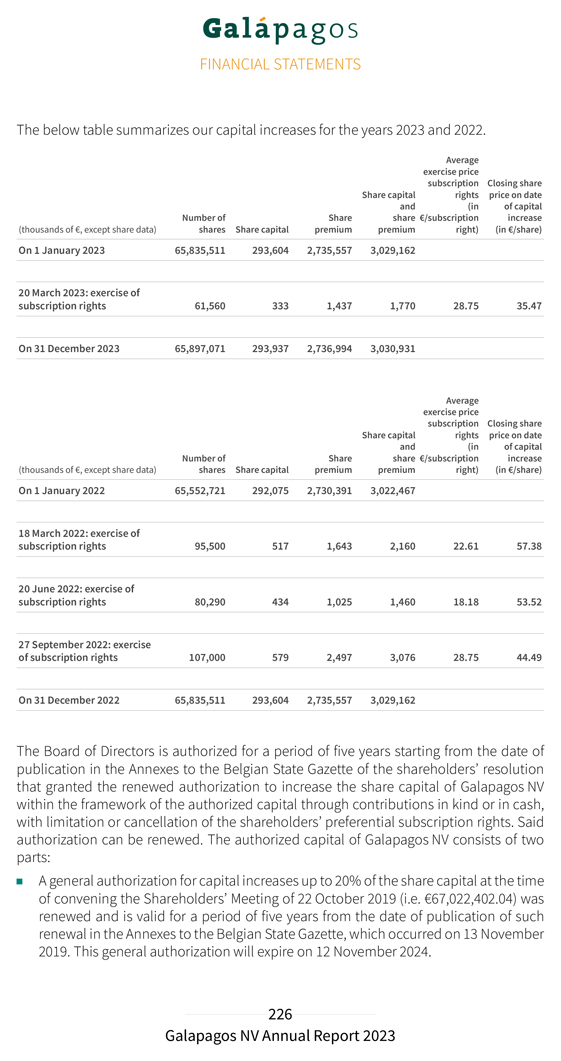
FINANCIAL STATEMENTS The below table summarizes our capital increases for the years 2023 and 2022. Average exercise price subscription Closing share Share capital rights price on date and (in of capital Number of Share share €/subscription increase (thousands of €, except share data) shares Share capital premium premium right) (in €/share) On 1 January 2023 65,835,511 293,604 2,735,557 3,029,162 20 March 2023: exercise of subscription rights 61,560 333 1,437 1,770 28.75 35.47 On 31 December 2023 65,897,071 293,937 2,736,994 3,030,931 Average exercise price subscription Closing share Share capital rights price on date and (in of capital Number of Share share €/subscription increase (thousands of €, except share data) shares Share capital premium premium right) (in €/share) On 1 January 2022 65,552,721 292,075 2,730,391 3,022,467 18 March 2022: exercise of subscription rights 95,500 517 1,643 2,160 22.61 57.38 20 June 2022: exercise of subscription rights 80,290 434 1,025 1,460 18.18 53.52 27 September 2022: exercise of subscription rights 107,000 579 2,497 3,076 28.75 44.49 On 31 December 2022 65,835,511 293,604 2,735,557 3,029,162 The Board of Directors is authorized for a period of five years starting from the date of publication in the Annexes to the Belgian State Gazette of the shareholders’ resolution that granted the renewed authorization to increase the share capital of Galapagos NV within the framework of the authorized capital through contributions in kind or in cash, with limitation or cancellation of the shareholders’ preferential subscription rights. Said authorization can be renewed. The authorized capital of Galapagos NV consists of two parts: A general authorization for capital increases up to 20% of the share capital at the time of convening the Shareholders’ Meeting of 22 October 2019 (i.e. €67,022,402.04) was renewed and is valid for a period of five years from the date of publication of such renewal in the Annexes to the Belgian State Gazette, which occurred on 13 November 2019. This general authorization will expire on 12 November 2024. 226 Galapagos NV Annual Report 2023
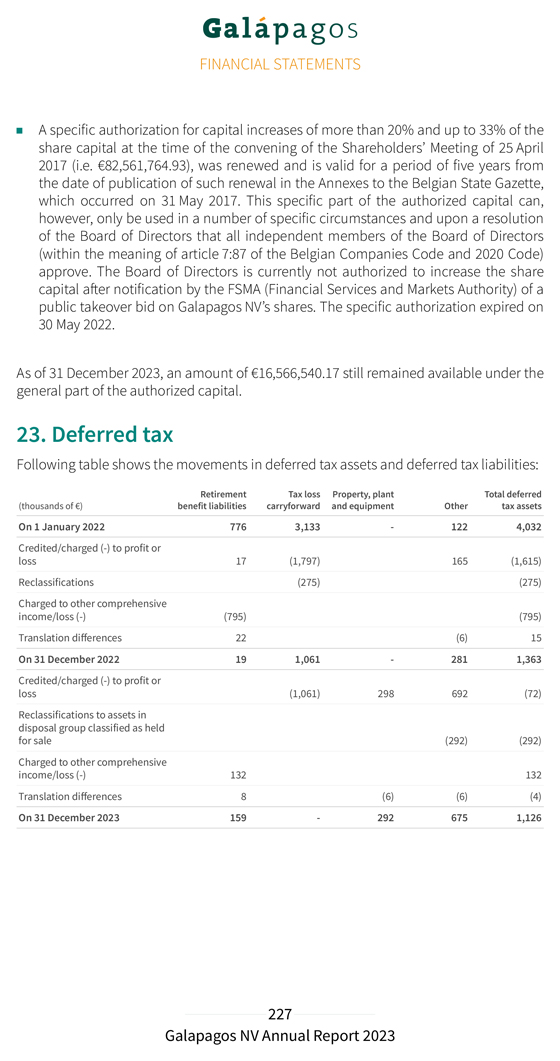
FINANCIAL STATEMENTS A specific authorization for capital increases of more than 20% and up to 33% of the share capital at the time of the convening of the Shareholders’ Meeting of 25 April 2017 (i.e. €82,561,764.93), was renewed and is valid for a period of five years from the date of publication of such renewal in the Annexes to the Belgian State Gazette, which occurred on 31 May 2017. This specific part of the authorized capital can, however, only be used in a number of specific circumstances and upon a resolution of the Board of Directors that all independent members of the Board of Directors (within the meaning of article 7:87 of the Belgian Companies Code and 2020 Code) approve. The Board of Directors is currently not authorized to increase the share capital after notification by the FSMA (Financial Services and Markets Authority) of a public takeover bid on Galapagos NV’s shares. The specific authorization expired on 30 May 2022. As of 31 December 2023, an amount of €16,566,540.17 still remained available under the general part of the authorized capital. 23. Deferred tax Following table shows the movements in deferred tax assets and deferred tax liabilities: Retirement Tax loss Property, plant Total deferred (thousands of €) benefit liabilities carryforward and equipment Other tax assets On 1 January 2022 776 3,133—122 4,032 Credited/charged (-) to profit or loss 17 (1,797) 165 (1,615) Reclassifications (275) (275) Charged to other comprehensive income/loss (-) (795) (795) Translation differences 22 (6) 15 On 31 December 2022 19 1,061—281 1,363 Credited/charged (-) to profit or loss (1,061) 298 692 (72) Reclassifications to assets in disposal group classified as held for sale (292) (292) Charged to other comprehensive income/loss (-) 132 132 Translation differences 8 (6) (6) (4) On 31 December 2023 159—292 675 1,126 227 Galapagos NV Annual Report 2023
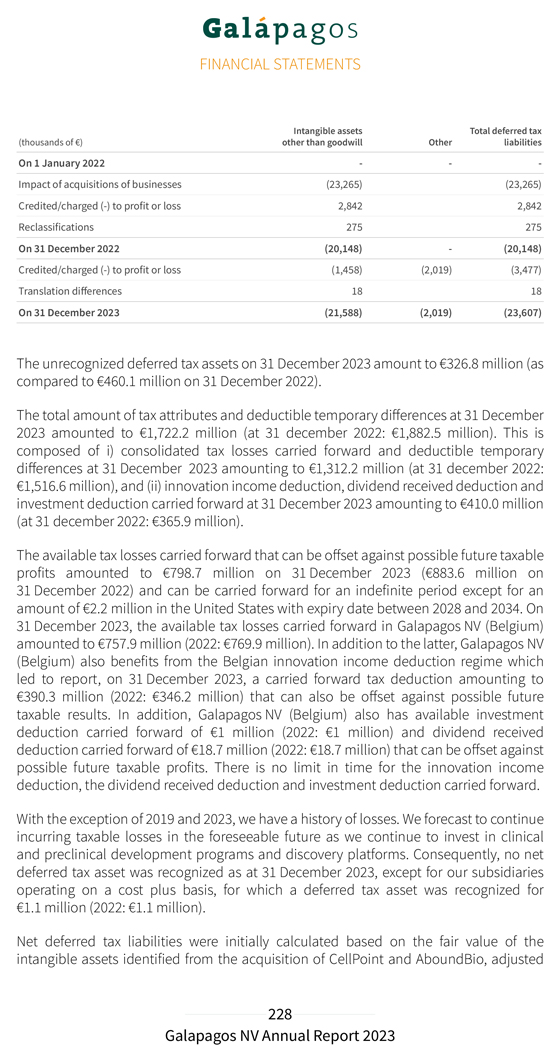
FINANCIAL STATEMENTS Intangible assets Total deferred tax (thousands of €) other than goodwill Other liabilities On 1 January 2022 ——Impact of acquisitions of businesses (23,265) (23,265) Credited/charged (-) to profit or loss 2,842 2,842 Reclassifications 275 275 On 31 December 2022 (20,148)—(20,148) Credited/charged (-) to profit or loss (1,458) (2,019) (3,477) Translation differences 18 18 On 31 December 2023 (21,588) (2,019) (23,607) The unrecognized deferred tax assets on 31 December 2023 amount to €326.8 million (as compared to €460.1 million on 31 December 2022). The total amount of tax attributes and deductible temporary differences at 31 December 2023 amounted to €1,722.2 million (at 31 december 2022: €1,882.5 million). This is composed of i) consolidated tax losses carried forward and deductible temporary differences at 31 December 2023 amounting to €1,312.2 million (at 31 december 2022: €1,516.6 million), and (ii) innovation income deduction, dividend received deduction and investment deduction carried forward at 31 December 2023 amounting to €410.0 million (at 31 december 2022: €365.9 million). The available tax losses carried forward that can be offset against possible future taxable profits amounted to €798.7 million on 31 December 2023 (€883.6 million on 31 December 2022) and can be carried forward for an indefinite period except for an amount of €2.2 million in the United States with expiry date between 2028 and 2034. On 31 December 2023, the available tax losses carried forward in Galapagos NV (Belgium) amounted to €757.9 million (2022: €769.9 million). In addition to the latter, Galapagos NV (Belgium) also benefits from the Belgian innovation income deduction regime which led to report, on 31 December 2023, a carried forward tax deduction amounting to €390.3 million (2022: €346.2 million) that can also be offset against possible future taxable results. In addition, Galapagos NV (Belgium) also has available investment deduction carried forward of €1 million (2022: €1 million) and dividend received deduction carried forward of €18.7 million (2022: €18.7 million) that can be offset against possible future taxable profits. There is no limit in time for the innovation income deduction, the dividend received deduction and investment deduction carried forward. With the exception of 2019 and 2023, we have a history of losses. We forecast to continue incurring taxable losses in the foreseeable future as we continue to invest in clinical and preclinical development programs and discovery platforms. Consequently, no net deferred tax asset was recognized as at 31 December 2023, except for our subsidiaries operating on a cost plus basis, for which a deferred tax asset was recognized for €1.1 million (2022: €1.1 million). Net deferred tax liabilities were initially calculated based on the fair value of the intangible assets identified from the acquisition of CellPoint and AboundBio, adjusted 228 Galapagos NV Annual Report 2023
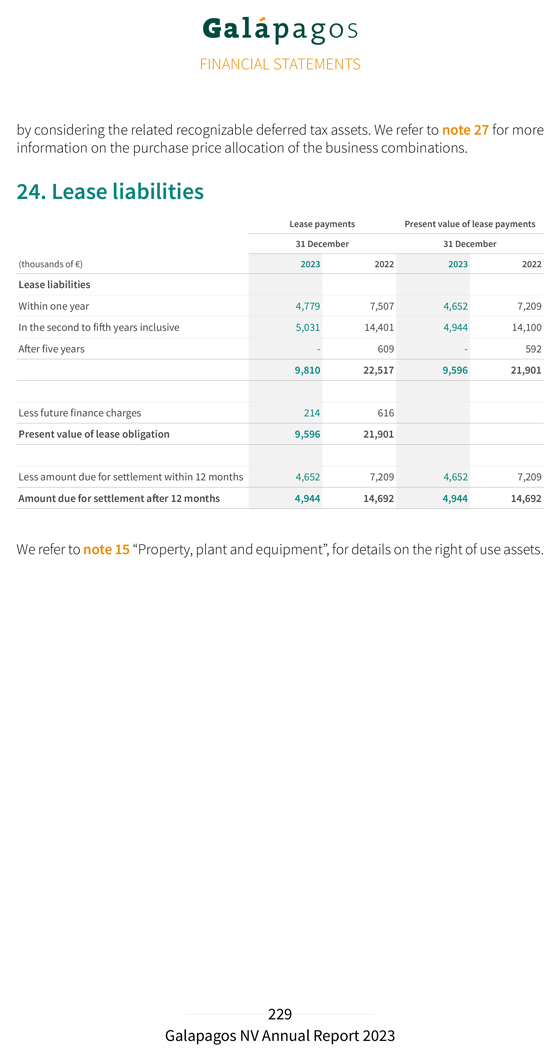
FINANCIAL STATEMENTS by considering the related recognizable deferred tax assets. We refer to note 27 for more information on the purchase price allocation of the business combinations. 24. Lease liabilities Lease payments Present value of lease payments 31 December 31 December (thousands of €) 2023 2022 2023 2022 Lease liabilities Within one year 4,779 7,507 4,652 7,209 In the second to fifth years inclusive 5,031 14,401 4,944 14,100 After five years—609—592 9,810 22,517 9,596 21,901 Less future finance charges 214 616 Present value of lease obligation 9,596 21,901 Less amount due for settlement within 12 months 4,652 7,209 4,652 7,209 Amount due for settlement after 12 months 4,944 14,692 4,944 14,692 We refer to note 15 “Property, plant and equipment”, for details on the right of use assets. 229 Galapagos NV Annual Report 2023
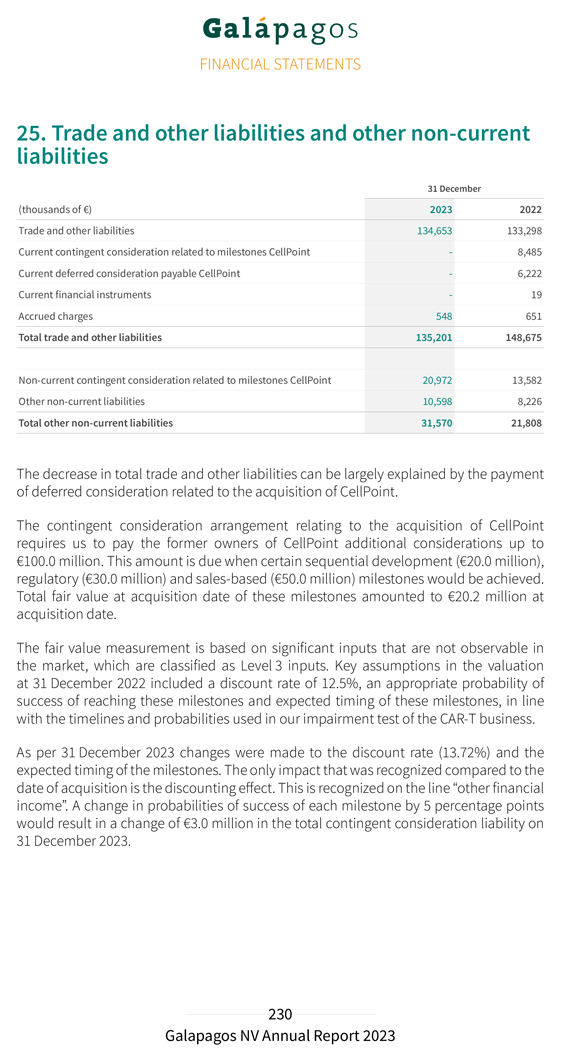
FINANCIAL STATEMENTS 25 liabilities . Trade and other liabilities and other non-current 31 December (thousands of €) 2023 2022 Trade and other liabilities 134,653 133,298 Current contingent consideration related to milestones CellPoint—8,485 Current deferred consideration payable CellPoint—6,222 Current financial instruments—19 Accrued charges 548 651 Total trade and other liabilities 135,201 148,675 Non-current contingent consideration related to milestones CellPoint 20,972 13,582 Other non-current liabilities 10,598 8,226 Total other non-current liabilities 31,570 21,808 The decrease in total trade and other liabilities can be largely explained by the payment of deferred consideration related to the acquisition of CellPoint. The contingent consideration arrangement relating to the acquisition of CellPoint requires us to pay the former owners of CellPoint additional considerations up to €100.0 million. This amount is due when certain sequential development (€20.0 million), regulatory (€30.0 million) and sales-based (€50.0 million) milestones would be achieved. Total fair value at acquisition date of these milestones amounted to €20.2 million at acquisition date. The fair value measurement is based on significant inputs that are not observable in the market, which are classified as Level 3 inputs. Key assumptions in the valuation at 31 December 2022 included a discount rate of 12.5%, an appropriate probability of success of reaching these milestones and expected timing of these milestones, in line with the timelines and probabilities used in our impairment test of the CAR-T business. As per 31 December 2023 changes were made to the discount rate (13.72%) and the expected timing of the milestones. The only impact that was recognized compared to the date of acquisition is the discounting effect. This is recognized on the line “other financial income”. A change in probabilities of success of each milestone by 5 percentage points would result in a change of €3.0 million in the total contingent consideration liability on 31 December 2023. 230 Galapagos NV Annual Report 2023
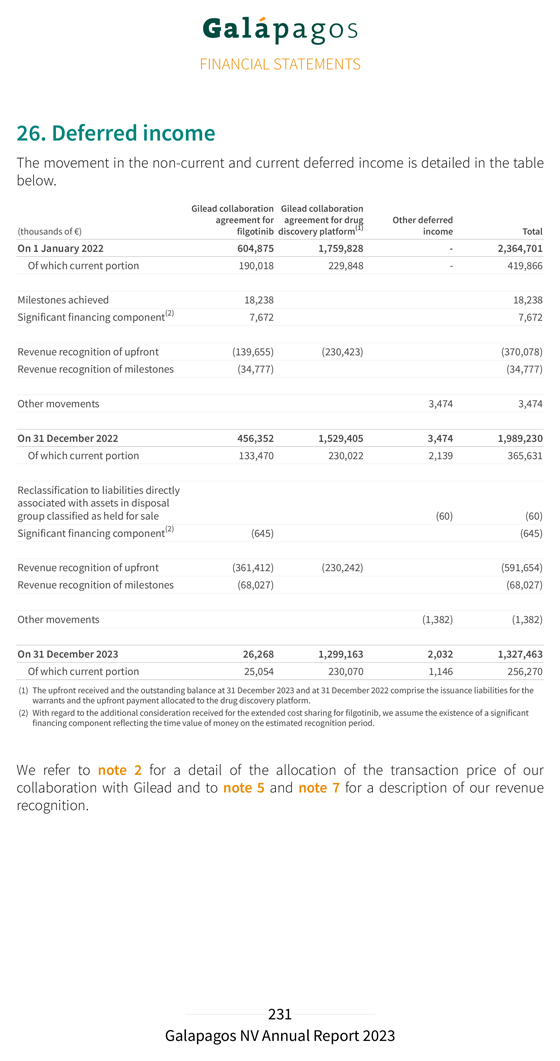
FINANCIAL STATEMENTS 26. Deferred income The movement in the non-current and current deferred income is detailed in the table below. Gilead collaboration Gilead collaboration agreement for agreement for drug (1) Other deferred (thousands of €) filgotinib discovery platform income Total On 1 January 2022 604,875 1,759,828—2,364,701 Of which current portion 190,018 229,848—419,866 Milestones achieved 18,238 18,238 Significant financing component(2) 7,672 7,672 Revenue recognition of upfront (139,655) (230,423) (370,078) Revenue recognition of milestones (34,777) (34,777) Other movements 3,474 3,474 On 31 December 2022 456,352 1,529,405 3,474 1,989,230 Of which current portion 133,470 230,022 2,139 365,631 Reclassification to liabilities directly associated with assets in disposal group classified as held for sale (60) (60) Significant financing component(2) (645) (645) Revenue recognition of upfront (361,412) (230,242) (591,654) Revenue recognition of milestones (68,027) (68,027) Other movements (1,382) (1,382) On 31 December 2023 26,268 1,299,163 2,032 1,327,463 Of which current portion 25,054 230,070 1,146 256,270 (1) The upfront received and the outstanding balance at 31 December 2023 and at 31 December 2022 comprise the issuance liabilities for the warrants and the upfront payment allocated to the drug discovery platform. (2) With regard to the additional consideration received for the extended cost sharing for filgotinib, we assume the existence of a significant financing component reflecting the time value of money on the estimated recognition period. We refer to note 2 for a detail of the allocation of the transaction price of our collaboration with Gilead and to note 5 and note 7 for a description of our revenue recognition. 231 Galapagos NV Annual Report 2023
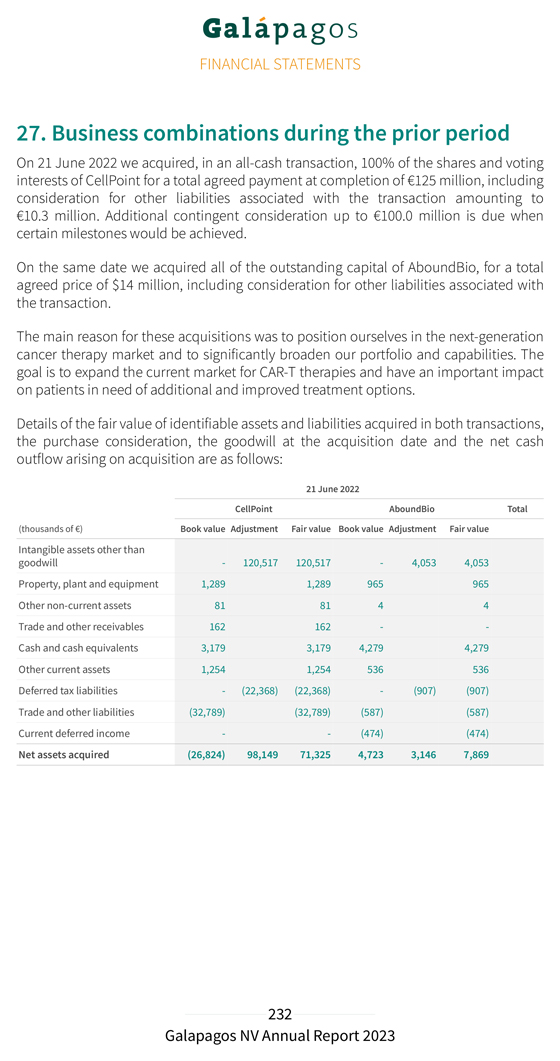
FINANCIAL STATEMENTS 27. Business combinations during the prior period On 21 June 2022 we acquired, in an all-cash transaction, 100% of the shares and voting interests of CellPoint for a total agreed payment at completion of €125 million, including consideration for other liabilities associated with the transaction amounting to €10.3 million. Additional contingent consideration up to €100.0 million is due when certain milestones would be achieved. On the same date we acquired all of the outstanding capital of AboundBio, for a total agreed price of $14 million, including consideration for other liabilities associated with the transaction. The main reason for these acquisitions was to position ourselves in the next-generation cancer therapy market and to significantly broaden our portfolio and capabilities. The goal is to expand the current market for CAR-T therapies and have an important impact on patients in need of additional and improved treatment options. Details of the fair value of identifiable assets and liabilities acquired in both transactions, the purchase consideration, the goodwill at the acquisition date and the net cash outflow arising on acquisition are as follows: 21 June 2022 CellPoint AboundBio Total (thousands of €) Book value Adjustment Fair value Book value Adjustment Fair value Intangible assets other than goodwill—120,517 120,517—4,053 4,053 Property, plant and equipment 1,289 1,289 965 965 Other non-current assets 81 81 4 4 Trade and other receivables 162 162 —Cash and cash equivalents 3,179 3,179 4,279 4,279 Other current assets 1,254 1,254 536 536 Deferred tax liabilities—(22,368) (22,368)—(907) (907) Trade and other liabilities (32,789) (32,789) (587) (587) Current deferred income — (474) (474) Net assets acquired (26,824) 98,149 71,325 4,723 3,146 7,869 232 Galapagos NV Annual Report 2023
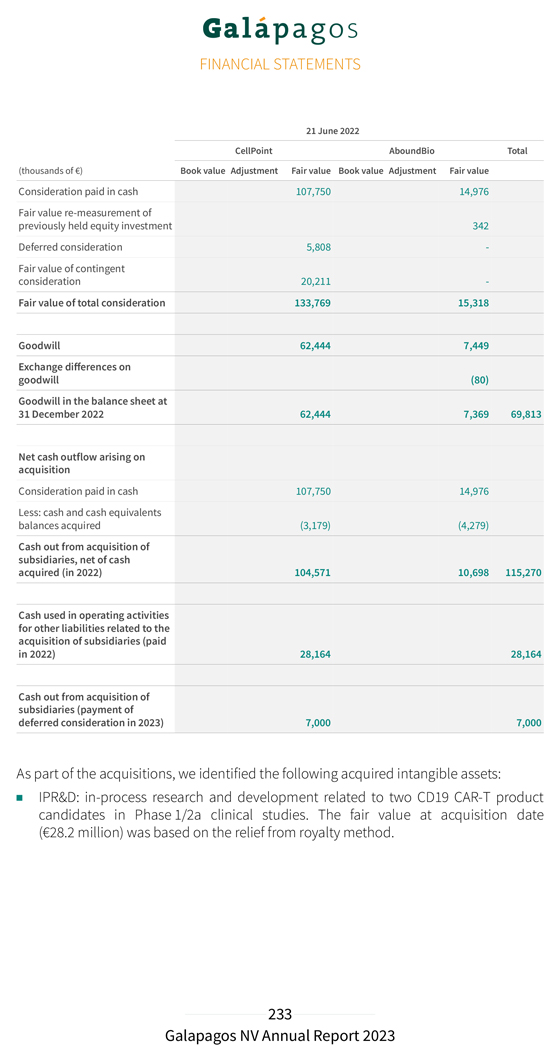
FINANCIAL STATEMENTS 21 June 2022 CellPoint AboundBio Total (thousands of €) Book value Adjustment Fair value Book value Adjustment Fair value Consideration paid in cash 107,750 14,976 Fair value re-measurement of previously held equity investment 342 Deferred consideration 5,808 -Fair value of contingent consideration 20,211—Fair value of total consideration 133,769 15,318 Goodwill 62,444 7,449 Exchange differences on goodwill (80) Goodwill in the balance sheet at 31 December 2022 62,444 7,369 69,813 Net cash outflow arising on acquisition Consideration paid in cash 107,750 14,976 Less: cash and cash equivalents balances acquired (3,179) (4,279) Cash out from acquisition of subsidiaries, net of cash acquired (in 2022) 104,571 10,698 115,270 Cash used in operating activities for other liabilities related to the acquisition of subsidiaries (paid in 2022) 28,164 28,164 Cash out from acquisition of subsidiaries (payment of deferred consideration in 2023) 7,000 7,000 As part of the acquisitions, we identified the following acquired intangible assets: IPR&D: in-process research and development related to two CD19 CAR-T product candidates in Phase 1/2a clinical studies. The fair value at acquisition date (€28.2 million) was based on the relief from royalty method. 233 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS Exclusive rights: through the acquisition of CellPoint we acquired on the one hand a collaboration agreement between CellPoint and Lonza providing the exclusive right to use the automated Lonza Cocoon® Platform in the development and commercialization of CAR-T cell products, and secondly, a collaboration agreement between CellPoint and Hypertrust providing exclusivity to use the jointly developed XCellit® software for workflow management and monitoring for the manufacturing of the CAR-T cells using the Lonza Cocoon® Platform. The fair values at acquisition date amounted to €89.7 million and €2.6 million respectively. A with and without method was retained to value the exclusivity with Lonza and the XCellit® software was valued based on the applicable royalty rate in the contract. Technology: through the acquisition of AboundBio, we acquired a fully human antibody-based therapeutics platform which was valued at €4.1 million at the time of acquisition. We assessed that the carrying value of all other acquired assets and assumed liabilities approximate their fair value at acquisition date. The goodwill arising from both transactions totaling €69.8 million was attributable to buyer specific synergies, the value of the assembled workforce and the accounting for net deferred tax liabilities for a total amount of €23.3 million, consisting of deferred tax liabilities on the acquired intangible assets of €32.3 million less recognized deferred tax assets of €9.0 million. The acquisition costs related to both transactions were considered not to be material and were recognized in our consolidated income statement on the line “general & administrative expenses”. 234 Galapagos NV Annual Report 2023
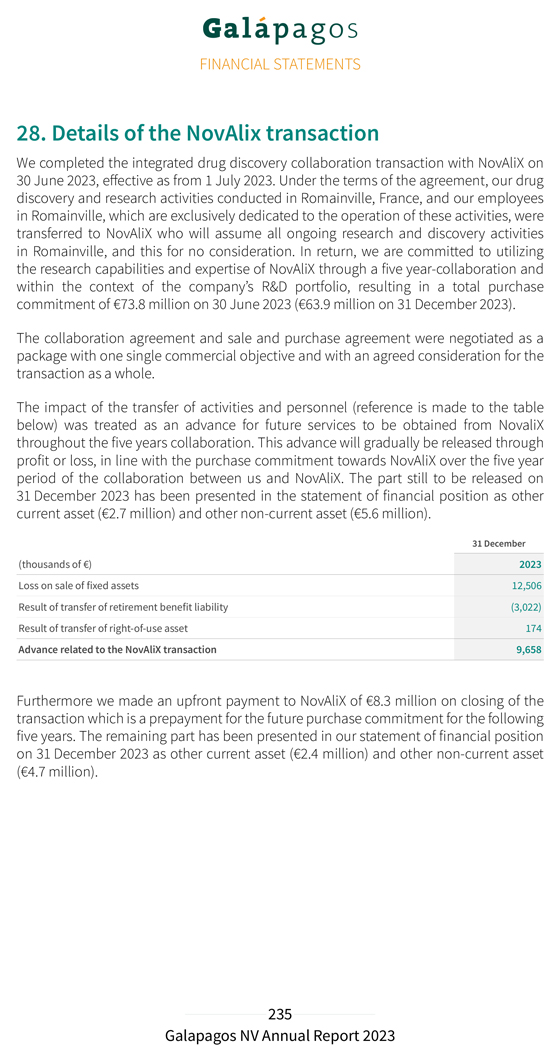
FINANCIAL STATEMENTS 28. Details of the NovAlix transaction We completed the integrated drug discovery collaboration transaction with NovAliX on 30 June 2023, effective as from 1 July 2023. Under the terms of the agreement, our drug discovery and research activities conducted in Romainville, France, and our employees in Romainville, which are exclusively dedicated to the operation of these activities, were transferred to NovAliX who will assume all ongoing research and discovery activities in Romainville, and this for no consideration. In return, we are committed to utilizing the research capabilities and expertise of NovAliX through a five year-collaboration and within the context of the company’s R&D portfolio, resulting in a total purchase commitment of €73.8 million on 30 June 2023 (€63.9 million on 31 December 2023). The collaboration agreement and sale and purchase agreement were negotiated as a package with one single commercial objective and with an agreed consideration for the transaction as a whole. The impact of the transfer of activities and personnel (reference is made to the table below) was treated as an advance for future services to be obtained from NovaliX throughout the five years collaboration. This advance will gradually be released through profit or loss, in line with the purchase commitment towards NovAliX over the five year period of the collaboration between us and NovAliX. The part still to be released on 31 December 2023 has been presented in the statement of financial position as other current asset (€2.7 million) and other non-current asset (€5.6 million). 31 December (thousands of €) 2023 Loss on sale of fixed assets 12,506 Result of transfer of retirement benefit liability (3,022) Result of transfer of right-of-use asset 174 Advance related to the NovAliX transaction 9,658 Furthermore we made an upfront payment to NovAliX of €8.3 million on closing of the transaction which is a prepayment for the future purchase commitment for the following five years. The remaining part has been presented in our statement of financial position on 31 December 2023 as other current asset (€2.4 million) and other non-current asset (€4.7 million). 235 Galapagos NV Annual Report 2023
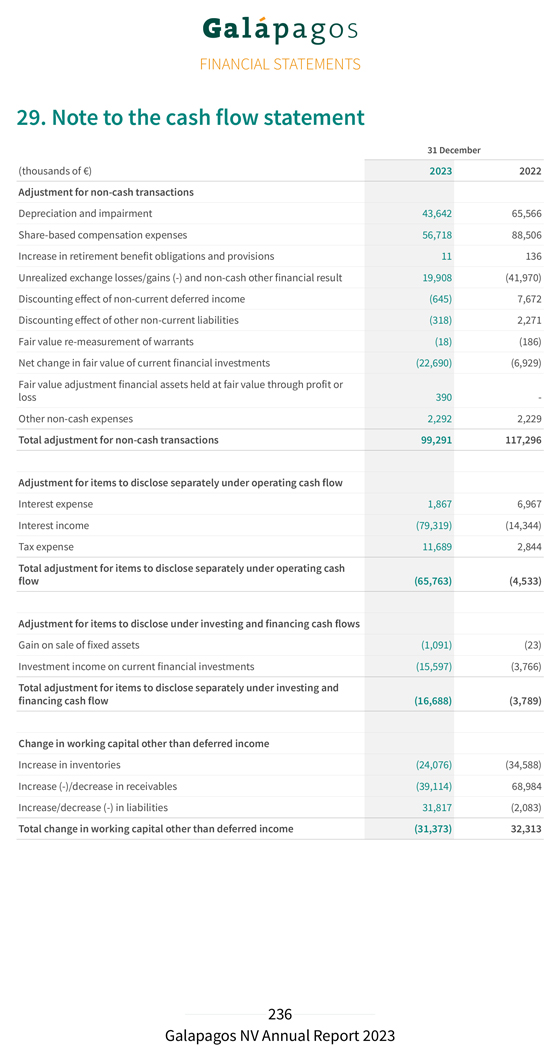
FINANCIAL STATEMENTS 29. Note to the cash flow statement 31 December (thousands of €) 2023 2022 Adjustment for non-cash transactions Depreciation and impairment 43,642 65,566 Share-based compensation expenses 56,718 88,506 Increase in retirement benefit obligations and provisions 11 136 Unrealized exchange losses/gains (-) and non-cash other financial result 19,908 (41,970) Discounting effect of non-current deferred income (645) 7,672 Discounting effect of other non-current liabilities (318) 2,271 Fair value re-measurement of warrants (18) (186) Net change in fair value of current financial investments (22,690) (6,929) Fair value adjustment financial assets held at fair value through profit or loss 390 -Other non-cash expenses 2,292 2,229 Total adjustment for non-cash transactions 99,291 117,296 Adjustment for items to disclose separately under operating cash flow Interest expense 1,867 6,967 Interest income (79,319) (14,344) Tax expense 11,689 2,844 Total adjustment for items to disclose separately under operating cash flow (65,763) (4,533) Adjustment for items to disclose under investing and financing cash flows Gain on sale of fixed assets (1,091) (23) Investment income on current financial investments (15,597) (3,766) Total adjustment for items to disclose separately under investing and financing cash flow (16,688) (3,789) Change in working capital other than deferred income Increase in inventories (24,076) (34,588) Increase (-)/decrease in receivables (39,114) 68,984 Increase/decrease (-) in liabilities 31,817 (2,083) Total change in working capital other than deferred income (31,373) 32,313 236 Galapagos NV Annual Report 2023
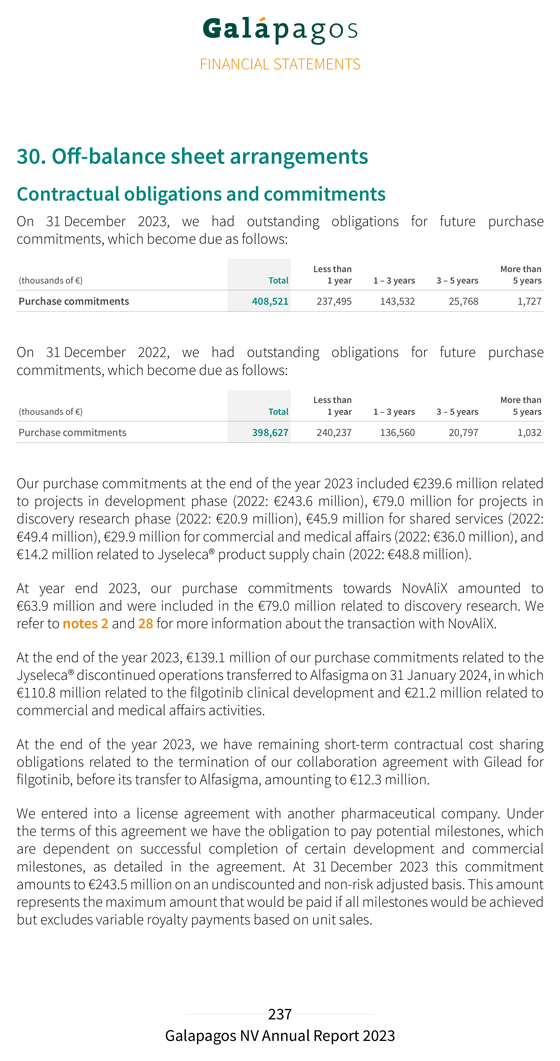
FINANCIAL STATEMENTS 30. Off-balance sheet arrangements Contractual obligations and commitments On 31 December 2023, we had outstanding obligations for future purchase commitments, which become due as follows: Less than More than (thousands of €) Total 1 year 1 – 3 years 3 – 5 years 5 years Purchase commitments 408,521 237,495 143,532 25,768 1,727 On 31 December 2022, we had outstanding obligations for future purchase commitments, which become due as follows: Less than More than (thousands of €) Total 1 year 1 – 3 years 3 – 5 years 5 years Purchase commitments 398,627 240,237 136,560 20,797 1,032 Our purchase commitments at the end of the year 2023 included €239.6 million related to projects in development phase (2022: €243.6 million), €79.0 million for projects in discovery research phase (2022: €20.9 million), €45.9 million for shared services (2022: €49.4 million), €29.9 million for commercial and medical affairs (2022: €36.0 million), and €14.2 million related to Jyseleca® product supply chain (2022: €48.8 million). At year end 2023, our purchase commitments towards NovAliX amounted to €63.9 million and were included in the €79.0 million related to discovery research. We refer to notes 2 and 28 for more information about the transaction with NovAliX. At the end of the year 2023, €139.1 million of our purchase commitments related to the Jyseleca® discontinued operations transferred to Alfasigma on 31 January 2024, in which €110.8 million related to the filgotinib clinical development and €21.2 million related to commercial and medical affairs activities. At the end of the year 2023, we have remaining short-term contractual cost sharing obligations related to the termination of our collaboration agreement with Gilead for filgotinib, before its transfer to Alfasigma, amounting to €12.3 million. We entered into a license agreement with another pharmaceutical company. Under the terms of this agreement we have the obligation to pay potential milestones, which are dependent on successful completion of certain development and commercial milestones, as detailed in the agreement. At 31 December 2023 this commitment amounts to €243.5 million on an undiscounted and non-risk adjusted basis. This amount represents the maximum amount that would be paid if all milestones would be achieved but excludes variable royalty payments based on unit sales. 237 Galapagos NV Annual Report 2023
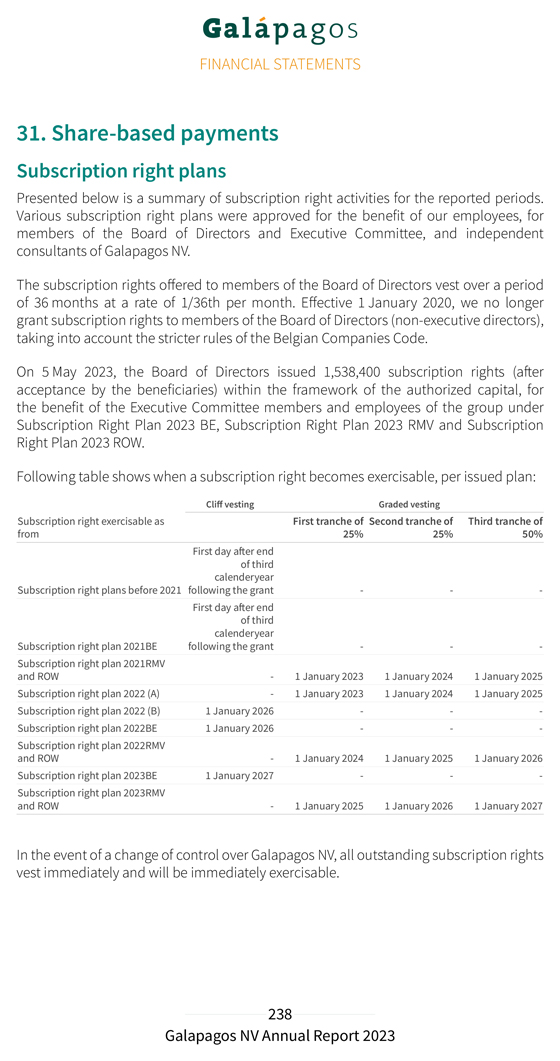
FINANCIAL STATEMENTS 31. Share-based payments Subscription right plans Presented below is a summary of subscription right activities for the reported periods. Various subscription right plans were approved for the benefit of our employees, for members of the Board of Directors and Executive Committee, and independent consultants of Galapagos NV. The subscription rights offered to members of the Board of Directors vest over a period of 36 months at a rate of 1/36th per month. Effective 1 January 2020, we no longer grant subscription rights to members of the Board of Directors (non-executive directors), taking into account the stricter rules of the Belgian Companies Code. On 5 May 2023, the Board of Directors issued 1,538,400 subscription rights (after acceptance by the beneficiaries) within the framework of the authorized capital, for the benefit of the Executive Committee members and employees of the group under Subscription Right Plan 2023 BE, Subscription Right Plan 2023 RMV and Subscription Right Plan 2023 ROW. Following table shows when a subscription right becomes exercisable, per issued plan: Cliff vesting Graded vesting Subscription right exercisable as First tranche of Second tranche of Third tranche of from 25% 25% 50% First day after end of third calenderyear Subscription right plans before 2021 following the grant — -First day after end of third calenderyear Subscription right plan 2021BE following the grant — -Subscription right plan 2021RMV and ROW—1 January 2023 1 January 2024 1 January 2025 Subscription right plan 2022 (A)—1 January 2023 1 January 2024 1 January 2025 Subscription right plan 2022 (B) 1 January 2026 — -Subscription right plan 2022BE 1 January 2026 — -Subscription right plan 2022RMV and ROW—1 January 2024 1 January 2025 1 January 2026 Subscription right plan 2023BE 1 January 2027 — -Subscription right plan 2023RMV and ROW—1 January 2025 1 January 2026 1 January 2027 In the event of a change of control over Galapagos NV, all outstanding subscription rights vest immediately and will be immediately exercisable. 238 Galapagos NV Annual Report 2023
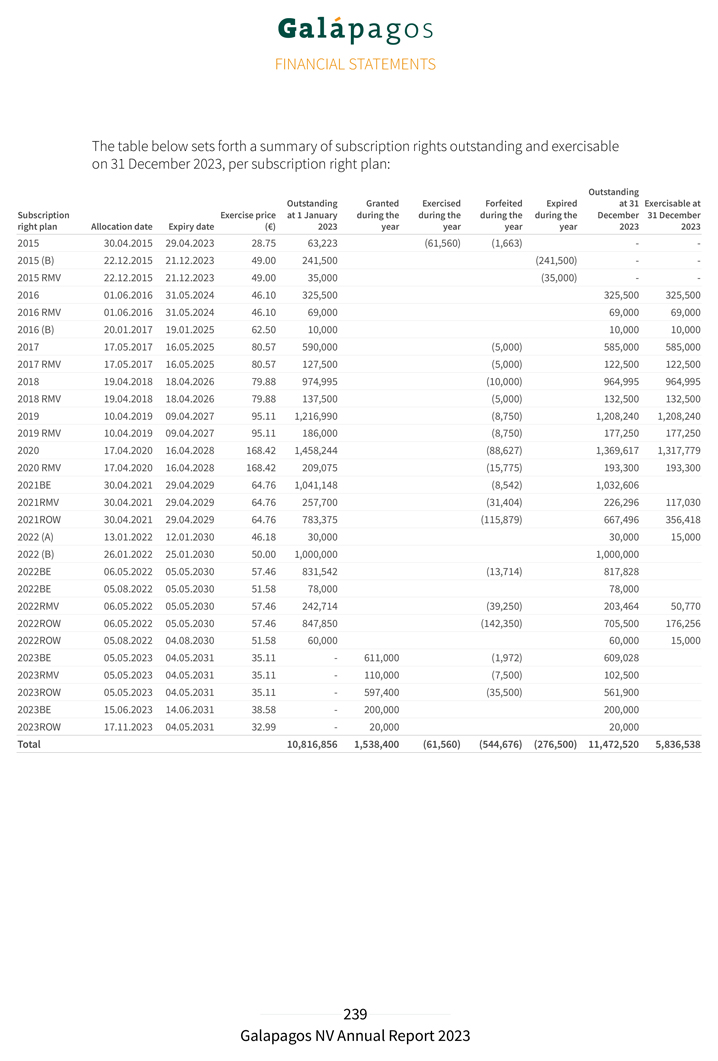
FINANCIAL STATEMENTS The table below sets forth a summary of subscription rights outstanding and exercisable on 31 December 2023, per subscription right plan: Outstanding Outstanding Granted Exercised Forfeited Expired at 31 Exercisable at Subscription Exercise price at 1 January during the during the during the during the December 31 December right plan Allocation date Expiry date (€) 2023 year year year year 2023 2023 2015 30.04.2015 29.04.2023 28.75 63,223 (61,560) (1,663) —2015 (B) 22.12.2015 21.12.2023 49.00 241,500 (241,500) —2015 RMV 22.12.2015 21.12.2023 49.00 35,000 (35,000) —2016 01.06.2016 31.05.2024 46.10 325,500 325,500 325,500 2016 RMV 01.06.2016 31.05.2024 46.10 69,000 69,000 69,000 2016 (B) 20.01.2017 19.01.2025 62.50 10,000 10,000 10,000 2017 17.05.2017 16.05.2025 80.57 590,000 (5,000) 585,000 585,000 2017 RMV 17.05.2017 16.05.2025 80.57 127,500 (5,000) 122,500 122,500 2018 19.04.2018 18.04.2026 79.88 974,995 (10,000) 964,995 964,995 2018 RMV 19.04.2018 18.04.2026 79.88 137,500 (5,000) 132,500 132,500 2019 10.04.2019 09.04.2027 95.11 1,216,990 (8,750) 1,208,240 1,208,240 2019 RMV 10.04.2019 09.04.2027 95.11 186,000 (8,750) 177,250 177,250 2020 17.04.2020 16.04.2028 168.42 1,458,244 (88,627) 1,369,617 1,317,779 2020 RMV 17.04.2020 16.04.2028 168.42 209,075 (15,775) 193,300 193,300 2021BE 30.04.2021 29.04.2029 64.76 1,041,148 (8,542) 1,032,606 2021RMV 30.04.2021 29.04.2029 64.76 257,700 (31,404) 226,296 117,030 2021ROW 30.04.2021 29.04.2029 64.76 783,375 (115,879) 667,496 356,418 2022 (A) 13.01.2022 12.01.2030 46.18 30,000 30,000 15,000 2022 (B) 26.01.2022 25.01.2030 50.00 1,000,000 1,000,000 2022BE 06.05.2022 05.05.2030 57.46 831,542 (13,714) 817,828 2022BE 05.08.2022 05.05.2030 51.58 78,000 78,000 2022RMV 06.05.2022 05.05.2030 57.46 242,714 (39,250) 203,464 50,770 2022ROW 06.05.2022 05.05.2030 57.46 847,850 (142,350) 705,500 176,256 2022ROW 05.08.2022 04.08.2030 51.58 60,000 60,000 15,000 2023BE 05.05.2023 04.05.2031 35.11—611,000 (1,972) 609,028 2023RMV 05.05.2023 04.05.2031 35.11—110,000 (7,500) 102,500 2023ROW 05.05.2023 04.05.2031 35.11—597,400 (35,500) 561,900 2023BE 15.06.2023 14.06.2031 38.58—200,000 200,000 2023ROW 17.11.2023 04.05.2031 32.99—20,000 20,000 Total 10,816,856 1,538,400 (61,560) (544,676) (276,500) 11,472,520 5,836,538 239 Galapagos NV Annual Report 2023
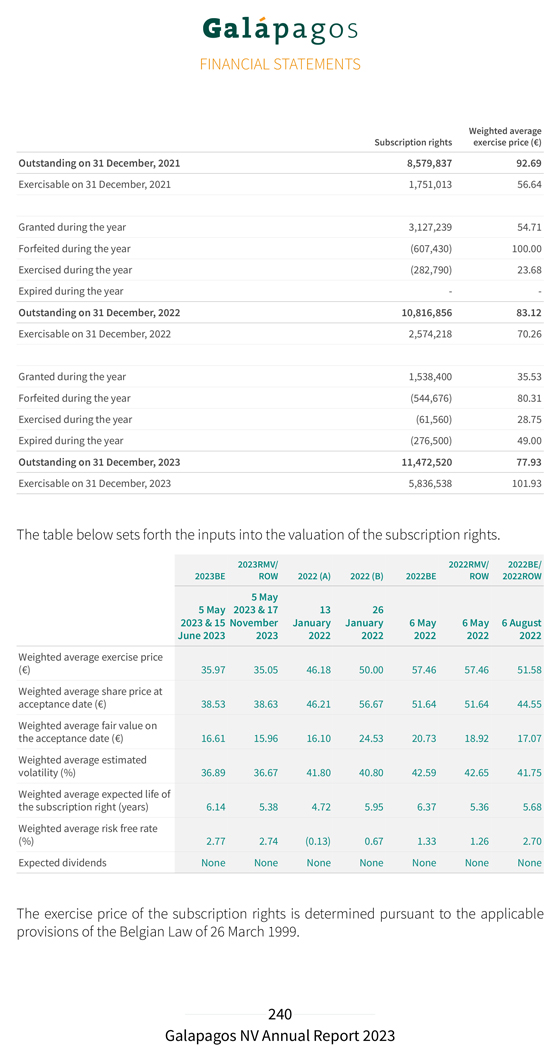
FINANCIAL STATEMENTS Weighted average Subscription rights exercise price (€) Outstanding on 31 December, 2021 8,579,837 92.69 Exercisable on 31 December, 2021 1,751,013 56.64 Granted during the year 3,127,239 54.71 Forfeited during the year (607,430) 100.00 Exercised during the year (282,790) 23.68 Expired during the year — Outstanding on 31 December, 2022 10,816,856 83.12 Exercisable on 31 December, 2022 2,574,218 70.26 Granted during the year 1,538,400 35.53 Forfeited during the year (544,676) 80.31 Exercised during the year (61,560) 28.75 Expired during the year (276,500) 49.00 Outstanding on 31 December, 2023 11,472,520 77.93 Exercisable on 31 December, 2023 5,836,538 101.93 The table below sets forth the inputs into the valuation of the subscription rights. 2023RMV/ 2022RMV/ 2022BE/ 2023BE ROW 2022 (A) 2022 (B) 2022BE ROW 2022ROW 5 May 5 May 2023 & 17 13 26 2023 & 15 November January January 6 May 6 May 6 August June 2023 2023 2022 2022 2022 2022 2022 Weighted average exercise price (€) 35.97 35.05 46.18 50.00 57.46 57.46 51.58 Weighted average share price at acceptance date (€) 38.53 38.63 46.21 56.67 51.64 51.64 44.55 Weighted average fair value on the acceptance date (€) 16.61 15.96 16.10 24.53 20.73 18.92 17.07 Weighted average estimated volatility (%) 36.89 36.67 41.80 40.80 42.59 42.65 41.75 Weighted average expected life of the subscription right (years) 6.14 5.38 4.72 5.95 6.37 5.36 5.68 Weighted average risk free rate (%) 2.77 2.74 (0.13) 0.67 1.33 1.26 2.70 Expected dividends None None None None None None None The exercise price of the subscription rights is determined pursuant to the applicable provisions of the Belgian Law of 26 March 1999. 240 Galapagos NV Annual Report 2023
FINANCIAL STATEMENTS The weighted average estimated volatility is calculated on the basis of the implied volatility of the share price over the weighted average expected life of the subscription rights. The weighted average expected life of the subscription right is calculated as the estimated duration until exercise, taking into account the specific features of the plans. Our share-based compensation expense in 2023 in relation to subscription right plans amounted to €56,718 thousand (2022: €88,506 thousand), of which €36,628 thousand (€62,003 thousand) from continuing operations and €20,090 thousand (2022: €26,503 thousand) from discontinued operations. The following table provides an overview of the outstanding subscription rights per category of subscription right holders on 31 December 2023 and 31 December 2022: 31 December Category 2023 2022 Members of the Board of Directors 7,500 75,000 Executive Committee members 1,670,500 1,864,000 Personnel 9,794,520 8,877,856 Total subscription rights outstanding 11,472,520 10,816,856 The outstanding subscription rights at the end of the accounting period have a weighted average exercise price of €77.93 (2022: €83.10) and a weighted average remaining life of 1,728 days (2022: 1,914 days). Restricted stock units (RSUs) Each RSU represents the right to receive, at Galapagos’ discretion, one Galapagos share or a payment in cash of an amount equivalent to the volume-weighted average price of the Galapagos share on Euronext Brussels over the 30-calendar day period preceding the relevant vesting date, in accordance with the terms and conditions of the relevant RSU program. We currently have the following RSU programs: Plan 2020.I, Plan 2021.I, Plan 2022.I and Plan 2023.I: these plans are intended to provide a long-term incentive to certain of our employees and Executive Committee members; Plan 2020.II, Plan 2021.II, Plan 2021.IV, Plan 2022.II and Plan 2023.II: these plans are designed with the aim to retain a specific group of our key employees and Executive Committee members whose retention is considered so important for our future performance that an additional incentive is desirable. The beneficiaries are nominated by the Remuneration committee and the Board of Directors approves this list of beneficiaries. The four-year vesting period is designed to be aligned with long-term shareholder interests; 241 Galapagos NV Annual Report 2023
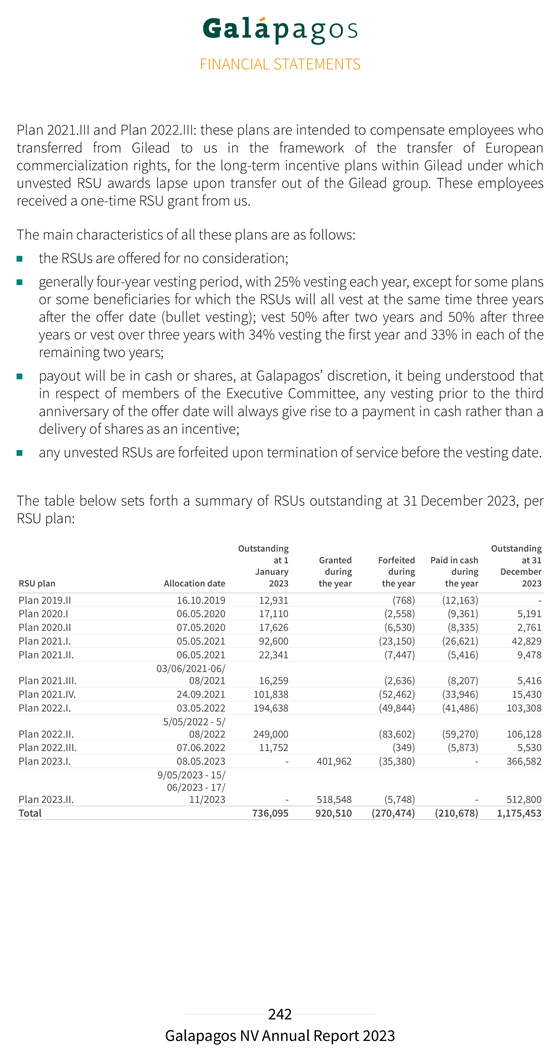
FINANCIAL STATEMENTS Plan 2021.III and Plan 2022.III: these plans are intended to compensate employees who transferred from Gilead to us in the framework of the transfer of European commercialization rights, for the long-term incentive plans within Gilead under which unvested RSU awards lapse upon transfer out of the Gilead group. These employees received a one-time RSU grant from us. The main characteristics of all these plans are as follows: the RSUs are offered for no consideration; generally four-year vesting period, with 25% vesting each year, except for some plans or some beneficiaries for which the RSUs will all vest at the same time three years after the offer date (bullet vesting); vest 50% after two years and 50% after three years or vest over three years with 34% vesting the first year and 33% in each of the remaining two years; payout will be in cash or shares, at Galapagos’ discretion, it being understood that in respect of members of the Executive Committee, any vesting prior to the third anniversary of the offer date will always give rise to a payment in cash rather than a delivery of shares as an incentive; any unvested RSUs are forfeited upon termination of service before the vesting date. The table below sets forth a summary of RSUs outstanding at 31 December 2023, per RSU plan: Outstanding Outstanding at 1 Granted Forfeited Paid in cash at 31 January during during during December RSU plan Allocation date 2023 the year the year the year 2023 Plan 2019.II 16.10.2019 12,931 (768) (12,163) -Plan 2020.I 06.05.2020 17,110 (2,558) (9,361) 5,191 Plan 2020.II 07.05.2020 17,626 (6,530) (8,335) 2,761 Plan 2021.I. 05.05.2021 92,600 (23,150) (26,621) 42,829 Plan 2021.II. 06.05.2021 22,341 (7,447) (5,416) 9,478 03/06/2021-06/ Plan 2021.III. 08/2021 16,259 (2,636) (8,207) 5,416 Plan 2021.IV. 24.09.2021 101,838 (52,462) (33,946) 15,430 Plan 2022.I. 03.05.2022 194,638 (49,844) (41,486) 103,308 5/05/2022—5/ Plan 2022.II. 08/2022 249,000 (83,602) (59,270) 106,128 Plan 2022.III. 07.06.2022 11,752 (349) (5,873) 5,530 Plan 2023.I. 08.05.2023—401,962 (35,380)—366,582 9/05/2023—15/ 06/2023—17/ Plan 2023.II. 11/2023—518,548 (5,748)—512,800 Total 736,095 920,510 (270,474) (210,678) 1,175,453 242 Galapagos NV Annual Report 2023
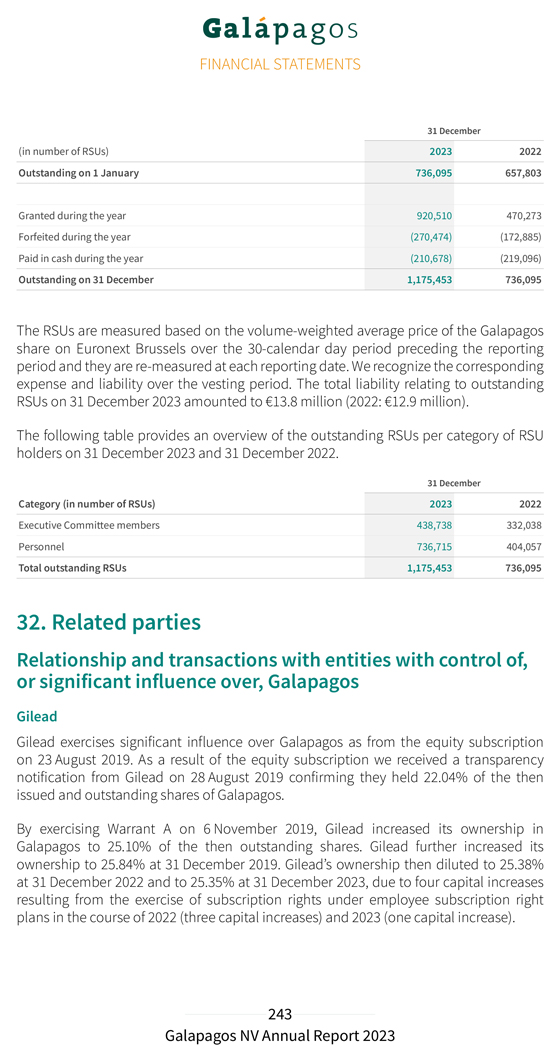
FINANCIAL STATEMENTS 31 December (in number of RSUs) 2023 2022 Outstanding on 1 January 736,095 657,803 Granted during the year 920,510 470,273 Forfeited during the year (270,474) (172,885) Paid in cash during the year (210,678) (219,096) Outstanding on 31 December 1,175,453 736,095 share on Euronext Brussels over the 30-calendar day period preceding the reporting period and they are re-measured at each reporting date. We recognize the corresponding expense and liability over the vesting period. The total liability relating to outstanding RSUs on 31 December 2023 amounted to €13.8 million (2022: €12.9 million). The following table provides an overview of the outstanding RSUs per category of RSU holders on 31 December 2023 and 31 December 2022. 31 December Category (in number of RSUs) 2023 2022 Executive Committee members 438,738 332,038 Personnel 736,715 404,057 Total outstanding RSUs 1,175,453 736,095 32. Related parties Relationship and transactions with entities with control of, or significant influence over, Galapagos Gilead Gilead exercises significant influence over Galapagos as from the equity subscription on 23 August 2019. As a result of the equity subscription we received a transparency notification from Gilead on 28 August 2019 confirming they held 22.04% of the then issued and outstanding shares of Galapagos. By exercising Warrant A on 6 November 2019, Gilead increased its ownership in Galapagos to 25.10% of the then outstanding shares. Gilead further increased its ownership to 25.84% at 31 December 2019. Gilead’s ownership then diluted to 25.38% at 31 December 2022 and to 25.35% at 31 December 2023, due to four capital increases resulting from the exercise of subscription rights under employee subscription right plans in the course of 2022 (three capital increases) and 2023 (one capital increase). 243 Galapagos NV Annual Report 2023
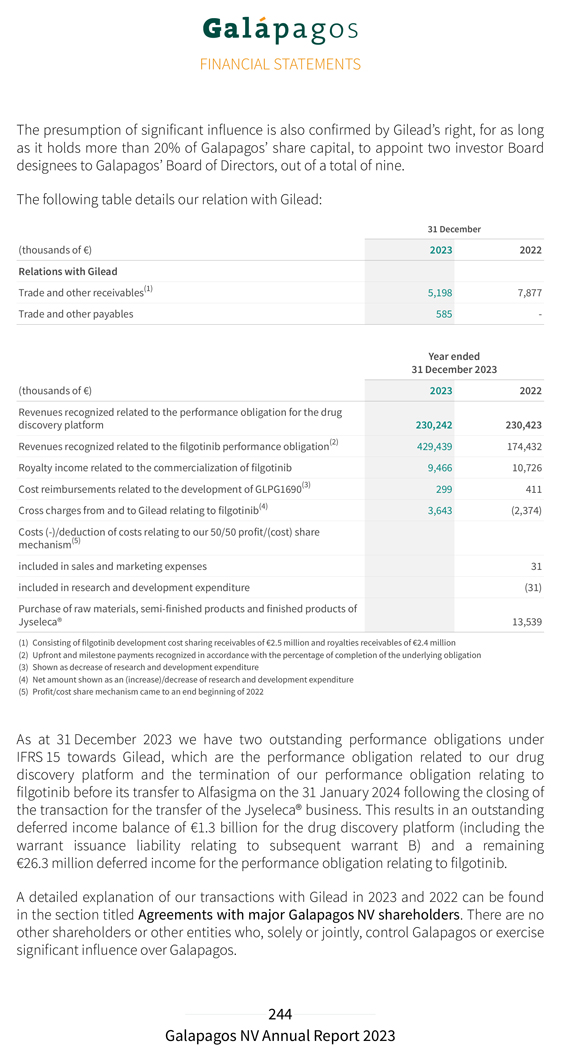
FINANCIAL STATEMENTS The presumption of significant influence is also confirmed by Gilead’s right, for as long as it holds more than 20% of Galapagos’ share capital, to appoint two investor Board designees to Galapagos’ Board of Directors, out of a total of nine. The following table details our relation with Gilead: 31 December (thousands of €) 2023 2022 Relations with Gilead Trade and other receivables(1) 5,198 7,877 Trade and other payables 585—Year ended 31 December 2023 (thousands of €) 2023 2022 Revenues recognized related to the performance obligation for the drug discovery platform 230,242 230,423 Revenues recognized related to the filgotinib performance obligation(2) 429,439 174,432 Royalty income related to the commercialization of filgotinib 9,466 10,726 Cost reimbursements related to the development of GLPG1690(3) 299 411 Cross charges from and to Gilead relating to filgotinib(4) 3,643 (2,374) Costs (-)/deduction (5) of costs relating to our 50/50 profit/(cost) share mechanism included in sales and marketing expenses 31 included in research and development expenditure (31) Purchase of raw materials, semi-finished products and finished products of Jyseleca® 13,539 (1) Consisting of filgotinib development cost sharing receivables of €2.5 million and royalties receivables of €2.4 million (2) Upfront and milestone payments recognized in accordance with the percentage of completion of the underlying obligation (3) Shown as decrease of research and development expenditure (4) Net amount shown as an (increase)/decrease of research and development expenditure (5) Profit/cost share mechanism came to an end beginning of 2022 As at 31 December 2023 we have two outstanding performance obligations under IFRS 15 towards Gilead, which are the performance obligation related to our drug discovery platform and the termination of our performance obligation relating to filgotinib before its transfer to Alfasigma on the 31 January 2024 following the closing of the transaction for the transfer of the Jyseleca® business. This results in an outstanding deferred income balance of €1.3 billion for the drug discovery platform (including the warrant issuance liability relating to subsequent warrant B) and a remaining €26.3 million deferred income for the performance obligation relating to filgotinib. A detailed explanation of our transactions with Gilead in 2023 and 2022 can be found in the section titled Agreements with major Galapagos NV shareholders. There are no other shareholders or other entities who, solely or jointly, control Galapagos or exercise significant influence over Galapagos. 244 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS Relationship and transactions with subsidiaries Please see note 33 for an overview of the consolidated companies of the group, which are all wholly-owned subsidiaries of Galapagos NV. Relationship and transactions with key management personnel Our key management personnel consists of the members of the Executive Committee and members of the Board of Directors. All amounts mentioned in this section are based on expenses recognized in the financial statements for the relevant financial year. Remuneration of key management personnel On 31 December 2023, our Executive Committee had five members: Stoffels IMC BV (permanently represented by Dr. Paul Stoffels), Mr. Thad Huston, Mr. Michele Manto, Ms. Valeria Cnossen and Ms. Annelies Missotten. They provide their services to us on a full-time basis. Mr. Michele Manto’s mandate as a member of the Executive Committee ended on 31 December 2023. On 31 December 2023, our Board of Directors consisted of nine members: Stoffels IMC BV (permanently represented by Dr. Paul Stoffels), Mr. Peter Guenter, Mr. Daniel O’Day, Dr. Linda Higgins, Dr. Elisabeth Svanberg, Mr. Jérôme Contamine, Dr. Dan Baker, Dr. Susanne Schaffert and Mr. Simon Sturge. During its meeting of 12 June 2023, the Board of Directors appointed Dr. Susanne Schaffert by cooptation as a non-executive independent Director, replacing Dr. Rajesh Parekh who stepped down on 10 June 2023. During its meeting of 19 September 2023, the Board of Directors appointed Mr. Simon Sturge by cooptation as a non-executive independent Director, replacing Dr. Mary Kerr who stepped down on 18 September 2023. Dr. Susanne Schaffert’s and Mr. Simon Sturge’s appointments will be submitted to the confirmation of the Company’s Annual Shareholders’ Meeting which will be held on 30 April 2024. Effective from 1 January 2020, Galapagos no longer grants any subscription rights to members of the Board of Directors, taking into account the stricter rules of the Belgian Companies Code. Prior to 2020, Board members were granted subscription rights. Effective from 26 April 2022, our CEO, Stoffels IMC BV, permanently represented by Dr. Paul Stoffels, has been appointed as the Chair of the Board of Directors of Galapagos. The CEO will only be remunerated for the performance of its executive functions as CEO and is not entitled to any additional remuneration for its mandates of Chair of the Board of Directors or of any Committee. Reference is made to the Remuneration Report, which discloses the remuneration awarded to each member of the Board of Directors and Executive Committee during 2023. 245 Galapagos NV Annual Report 2023
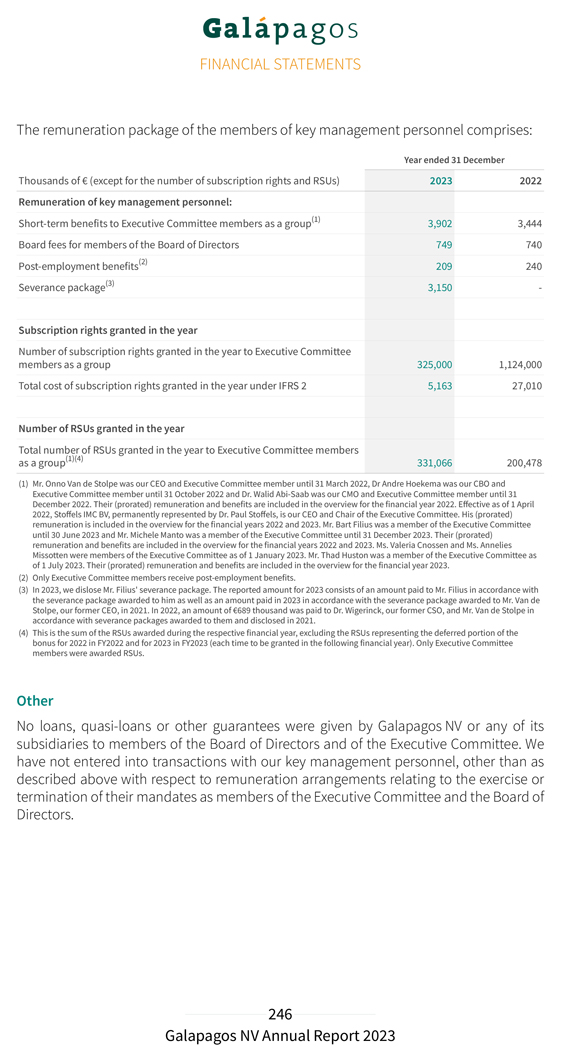
FINANCIAL STATEMENTS The remuneration package of the members of key management personnel comprises: Year ended 31 December Thousands of € (except for the number of subscription rights and RSUs) 2023 2022 Remuneration of key management personnel: Short-term benefits to Executive Committee members as a group(1) 3,902 3,444 Board fees for members of the Board of Directors 749 740 Post-employment benefits(2) 209 240 Severance package(3) 3,150—Subscription rights granted in the year Number of subscription rights granted in the year to Executive Committee members as a group 325,000 1,124,000 Total cost of subscription rights granted in the year under IFRS 2 5,163 27,010 Number of RSUs granted in the year Total number (1)(4) of RSUs granted in the year to Executive Committee members as a group 331,066 200,478 (1) Mr. Onno Van de Stolpe was our CEO and Executive Committee member until 31 March 2022, Dr Andre Hoekema was our CBO and Executive Committee member until 31 October 2022 and Dr. Walid Abi-Saab was our CMO and Executive Committee member until 31 December 2022. Their (prorated) remuneration and benefits are included in the overview for the financial year 2022. Effective as of 1 April 2022, Stoffels IMC BV, permanently represented by Dr. Paul Stoffels, is our CEO and Chair of the Executive Committee. His (prorated) remuneration is included in the overview for the financial years 2022 and 2023. Mr. Bart Filius was a member of the Executive Committee until 30 June 2023 and Mr. Michele Manto was a member of the Executive Committee until 31 December 2023. Their (prorated) remuneration and benefits are included in the overview for the financial years 2022 and 2023. Ms. Valeria Cnossen and Ms. Annelies Missotten were members of the Executive Committee as of 1 January 2023. Mr. Thad Huston was a member of the Executive Committee as of 1 July 2023. Their (prorated) remuneration and benefits are included in the overview for the financial year 2023. (2) Only Executive Committee members receive post-employment benefits. (3) In 2023, we dislose Mr. Filius’ severance package. The reported amount for 2023 consists of an amount paid to Mr. Filius in accordance with the severance package awarded to him as well as an amount paid in 2023 in accordance with the severance package awarded to Mr. Van de Stolpe, our former CEO, in 2021. In 2022, an amount of €689 thousand was paid to Dr. Wigerinck, our former CSO, and Mr. Van de Stolpe in accordance with severance packages awarded to them and disclosed in 2021. (4) This is the sum of the RSUs awarded during the respective financial year, excluding the RSUs representing the deferred portion of the bonus for 2022 in FY2022 and for 2023 in FY2023 (each time to be granted in the following financial year). Only Executive Committee members were awarded RSUs. Other No loans, quasi-loans or other guarantees were given by Galapagos NV or any of its subsidiaries to members of the Board of Directors and of the Executive Committee. We have not entered into transactions with our key management personnel, other than as described above with respect to remuneration arrangements relating to the exercise or termination of their mandates as members of the Executive Committee and the Board of Directors. 246 Galapagos NV Annual Report 2023
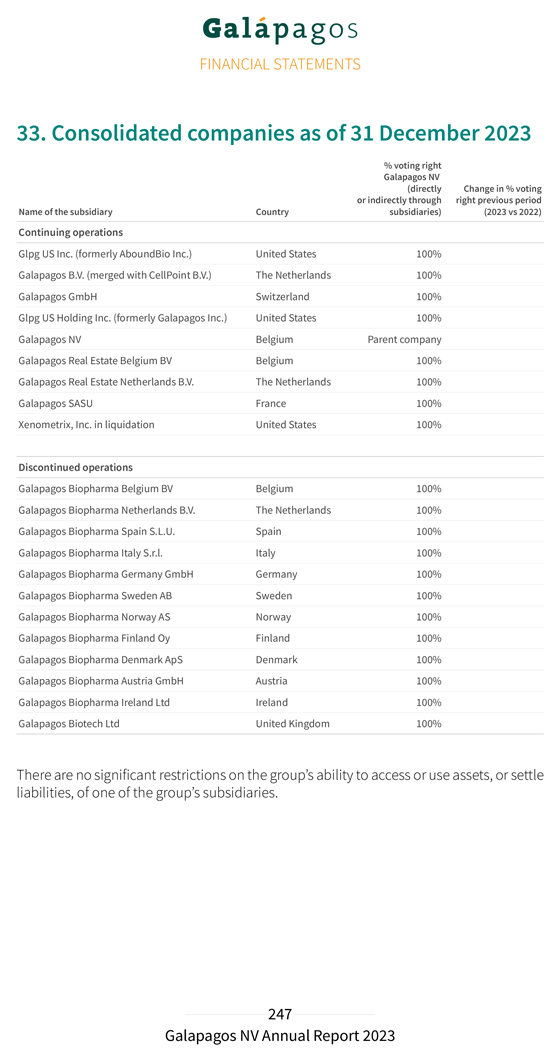
FINANCIAL STATEMENTS 33. Consolidated companies as of 31 December 2023 % voting right Galapagos NV (directly Change in % voting or indirectly through right previous period Name of the subsidiary Country subsidiaries) (2023 vs 2022) Continuing operations Glpg US Inc. (formerly AboundBio Inc.) United States 100% Galapagos B.V. (merged with CellPoint B.V.) The Netherlands 100% Galapagos GmbH Switzerland 100% Glpg US Holding Inc. (formerly Galapagos Inc.) United States 100% Galapagos NV Belgium Parent company Galapagos Real Estate Belgium BV Belgium 100% Galapagos Real Estate Netherlands B.V. The Netherlands 100% Galapagos SASU France 100% Xenometrix, Inc. in liquidation United States 100% Discontinued operations Galapagos Biopharma Belgium BV Belgium 100% Galapagos Biopharma Netherlands B.V. The Netherlands 100% Galapagos Biopharma Spain S.L.U. Spain 100% Galapagos Biopharma Italy S.r.l. Italy 100% Galapagos Biopharma Germany GmbH Germany 100% Galapagos Biopharma Sweden AB Sweden 100% Galapagos Biopharma Norway AS Norway 100% Galapagos Biopharma Finland Oy Finland 100% Galapagos Biopharma Denmark ApS Denmark 100% Galapagos Biopharma Austria GmbH Austria 100% Galapagos Biopharma Ireland Ltd Ireland 100% Galapagos Biotech Ltd United Kingdom 100% There are no significant restrictions on the group’s ability to access or use assets, or settle liabilities, of one of the group’s subsidiaries. 247 Galapagos NV Annual Report 2023
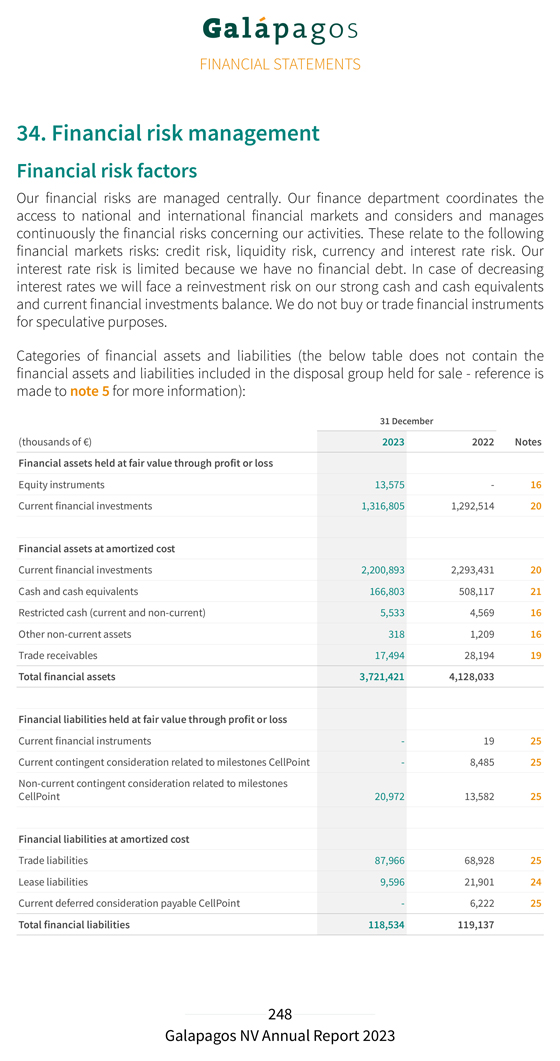
FINANCIAL STATEMENTS 34. Financial risk management Financial risk factors Our financial risks are managed centrally. Our finance department coordinates the access to national and international financial markets and considers and manages continuously the financial risks concerning our activities. These relate to the following financial markets risks: credit risk, liquidity risk, currency and interest rate risk. Our interest rate risk is limited because we have no financial debt. In case of decreasing interest rates we will face a reinvestment risk on our strong cash and cash equivalents and current financial investments balance. We do not buy or trade financial instruments for speculative purposes. Categories of financial assets and liabilities (the below table does not contain the financial assets and liabilities included in the disposal group held for sale—reference is made to note 5 for more information): 31 December (thousands of €) 2023 2022 Notes Financial assets held at fair value through profit or loss Equity instruments 13,575—16 Current financial investments 1,316,805 1,292,514 20 Financial assets at amortized cost Current financial investments 2,200,893 2,293,431 20 Cash and cash equivalents 166,803 508,117 21 Restricted cash (current and non-current) 5,533 4,569 16 Other non-current assets 318 1,209 16 Trade receivables 17,494 28,194 19 Total financial assets 3,721,421 4,128,033 Financial liabilities held at fair value through profit or loss Current financial instruments—19 25 Current contingent consideration related to milestones CellPoint—8,485 25 Non-current contingent consideration related to milestones CellPoint 20,972 13,582 25 Financial liabilities at amortized cost Trade liabilities 87,966 68,928 25 Lease liabilities 9,596 21,901 24 Current deferred consideration payable CellPoint—6,222 25 Total financial liabilities 118,534 119,137 248 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS The carrying amounts of trade payables and trade receivables are considered to be the same as their fair values, due to their short-term nature. Financial assets held at fair value through profit or loss Financial assets held at fair value through profit or loss consisted of an equity instrument of a non-listed company and current financial investments. We have no restrictions on the sale of this equity instrument and the asset is not pledged under any of our liabilities. The fair value of the equity instrument in the non-listed company has been determined mainly by reference to the initial transaction price (classified as level 3 in the fair value hierarchy). Current financial investments include money market funds in EUR and USD, which all classify for level 1 fair value measurement. Liquidity risk Current financial investments and cash and cash equivalents amounted to €3,684.5 million on 31 December 2023. Management forecasts our liquidity requirements to ensure that we have sufficient cash to meet operational needs. We have no credit lines. Such forecasting is based on realistic assumptions with regards to royalties, milestone and upfront payments to be received, taking into account our past track record, including the assumption that not all new projects that are being planned will be realized. All our cash and cash equivalents have only an insignificant liquidity risk as they are all convertible upon a maximum three month notice period and without incurring a significant penalty in normal market circumstances. Credit risk The term “credit risk” refers to the risk that counterparty will default on its contractual obligations resulting in financial loss for us. We grant credit to our clients in the framework of our normal business activities. Usually, we require no pledge or other collateral to cover the amounts due. All our receivables are considered collectable. We did not account for a provision for expected credit losses relating to our trade and other receivables given that there is no history of material credit losses, nor does forward looking information reveals any potential risk and due to the high-quality nature of our customers. 249 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS Aging balance of receivables that are due, but that are still considered collectable: 31 December (thousands of €) 2023 2022 60 – 90 days 3 424 90 – 120 days 3 208 more than 120 days 117 473 Our cash and cash equivalents are invested primarily in current, notice and term accounts. For banks and financial institutions, only independently rated parties with a minimum rating of ‘A’ are accepted at the beginning of the term. Our current financial investments are also kept within different financial institutions and include term deposits, money market funds and treasury bills with an AAA rating. The money market funds are invested in a well-diversified portfolio of highly rated assets. Interest rate risk The only variable interest-bearing financial instruments are cash and cash equivalents and current financial investments. Changes in interest rates may cause variations in interest income and expenses resulting from short-term interest-bearing assets. Management does not expect the short-term interest rates to decrease significantly in the immediate foreseeable future, which limits the interest exposure on our cash and cash equivalents and current financial investments. Effect of interest rate fluctuation A 100 basis points increase in interest rates at balance sheet date would have increased profit or loss, and equity, by approximately €36.8 million (2022: €40.9 million); a 100 basis points decrease in interest rates would have decreased profit or loss, and equity, by approximately €36.8 million (2022: €40.9 million). These scenarios assume our entire cash portfolio would immediately reprice at the new interest rates. Foreign exchange risk We are exposed to foreign exchange risk arising from various currency exposures. Our principal functional currency is euro, but we receive payments from our main collaboration partner Gilead in U.S. dollars and acquire some consumables and materials in U.S. dollars, Swiss francs, and GB pounds. To limit this risk, we attempt to align incoming and outgoing cash flows in currencies other than EUR. In addition, contracts closed by our different entities are mainly in the functional currencies of that entity, except for the collaboration agreement signed with Gilead for which payments are denominated in U.S. dollars. 250 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS The exchange rate risk in case of a 10% change in the exchange rate amounts to: 31 December Net book value (thousands of €) 2023 2022 Increase in Euros—U.S. Dollars (78,013) (85,140) Increase in Euros—GB Pounds 666 960 Increase in Euros—CH Francs 385 557 The exchange rate risk on the U.S. dollar is primarily related to our cash and cash equivalents and current financial investments held in U.S. dollars. Capital risk factors We manage our capital to safeguard that we will be able to continue as a going concern. At the same time, we want to ensure the return to our shareholders through the results from our research and development activities. Our capital structure consists of current financial investments, cash and cash equivalents, and equity attributed to the holders of our equity instruments, such as capital, reserves and results carried forward, as mentioned in the consolidated statement of changes in equity. We manage our capital structure and make the necessary adjustments in the light of changes of economic circumstances, the risk characteristics of underlying assets and the projected cash needs of the current research and development activities. The adequacy of the capital structure will depend on many factors, including scientific progress in the research and development programs, the magnitude of those programs, the commitments to existing and new clinical CROs, the ability to establish new alliance or collaboration agreements, the capital expenditures, the new commercial activities, market developments and any future acquisition. Neither Galapagos NV nor any of its subsidiaries are subject to any externally imposed capital requirements, other than those imposed by generally applicable company law requirements. 251 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS 35. Statutory auditor’s remuneration BDO Bedrijfsrevisoren BV (BDO) was appointed as statutory auditor by the Shareholders’ Meeting held on 25 April 2023, for a term of three years expiring immediately after the Annual Shareholders’ meeting to be held in 2026 which will have decided upon the annual accounts for the financial year to be ended on 31 December 2025. Deloitte Bedrijfsrevisoren BV (Deloitte) ceased to be our statutory auditor as of the date of the Annual Shareholders’ Meeting held on 25 April 2023. Our principal accountants billed the following fees to us for professional services rendered in 2023 (BDO) and 2022 (Deloitte). The statutory auditor’s fees for carrying out its mandate at group level amounted to €1,124.0 thousand in 2023 (2022: €1,127.1 thousand). The 2023 audit fee was €191.3 thousand higher compared to the fee that was approved by the Shareholders’ Meeting held on 25 April 2023 due to exceptional audit activities and special assignments performed by the statutory auditor. Audit-related fees, which generally the auditor provides, amounted to €20.2 thousand in 2023 (2022: €26.9 thousand). Other fees related to non-audit services executed by the statutory auditor amounted to €6.6 thousand in 2023 (2022: €nil) and related to ESG reporting. Other fees related to non-audit services executed by persons related to the statutory auditor amounted to €nil in 2023 (2022: €429.5 thousand and related to advisory services in relation to IT and quality management). Tax fees amounted to €68.0 thousand in 2023 (2022: €nil) and related to tax assistance relating to personal payroll taxes related to prior year filings. The Audit Committee and the Board of Directors are of the opinion that these non-audit services do not affect the independence of the statutory auditor in the performance of his audit. The abovementioned additional fees were fully approved by the Audit Committee in accordance with article 3:64 of the Belgian Code of Companies and Associations. 36. Events after balance sheet date On 3 January 2024, we signed a strategic collaboration and license agreement with BridGene Biosciences to further strengthen our growing early-stage oncology precision medicine pipeline. Under the terms of the agreement, BridGene will receive from us up to $27 million in upfront and preclinical research milestone payments and potentially over $700 million in clinical and commercial milestones, assuming success of the programs. In addition, BridGene will be entitled to receive single-digit tiered royalties on net sales of each product resulting from the collaboration. On 4 January 2024, we announced that we entered into a strategic collaboration agreement with Thermo Fisher Scientific for CAR-T manufacturing and kitting services for our point-of-care CAR-T product candidate in the San Francisco area. Under the terms of the agreement, Thermo Fisher will provide GMP manufacturing as well as BioServices and Specialty Logistics for our CAR-T hemato-oncology clinical program in the San Francisco area, effective January 2024. We will initiate the technology transfer to enable Thermo Fisher’s manufacturing activities. 252 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS On 31 January 2024, we successfully completed the transaction with Alfasigma for the transfer of the Jyseleca® business after having met all closing conditions. As part of the transaction, the amended Filgotinib Agreement between us and Gilead has been assigned by us to Alfasigma. Alfasigma paid us an upfront payment of €50.0 million plus €13.2 million for cash and working capital subject to final settlement based on completion accounts. We are entitled to potential future sales-based milestone payments totaling €120 million and mid-single to mid-double-digit royalties on European sales. We will contribute up to €40 million to Alfasigma by June 2025 for Jyseleca® related development activities. On 31 January 2024, we participated for $40.0 million in Series C financing round of Frontier Medicines, a pioneer in precision oncology with a unique technology platform and a pipeline of potential best-in-class assets that fit with our precision oncology R&D approach. The investment aligns with our innovation acceleration strategy to bring transformational medicines to patients around the world. On 26 March 2024, our consolidated financial statements were approved by the Board of Directors and authorized for publication. They were signed on behalf of the Board of Directors by: (signed) Stoffels IMC BV permanently represented by Dr. Paul Stoffels Chairman of the Board of Directors Jérôme Contamine Chairman of the Audit Committee and member of the Board of Directors 26 March 2024 253 Galapagos NV Annual Report 2023
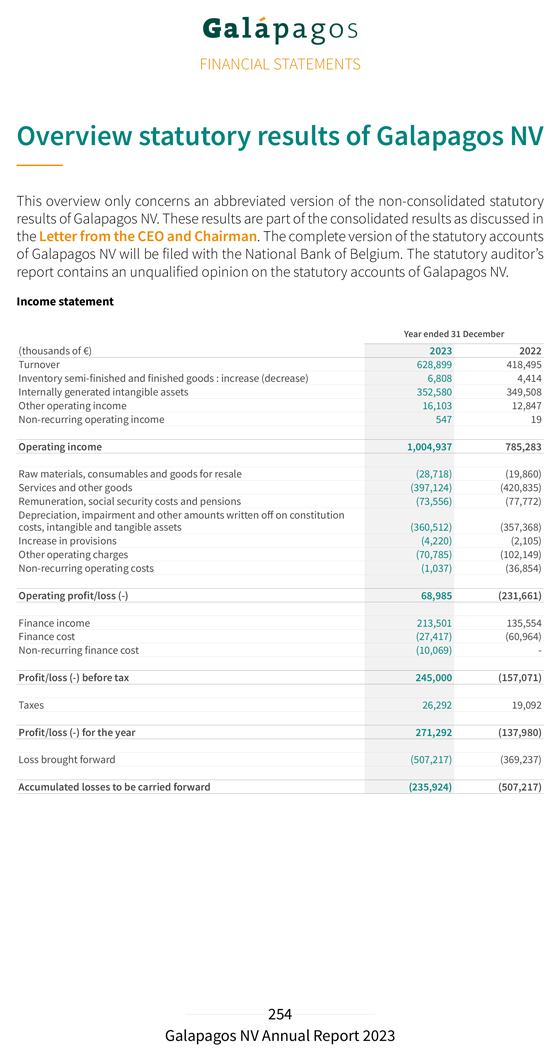
FINANCIAL STATEMENTS Overview statutory results of Galapagos NV This overview only concerns an abbreviated version of the non-consolidated statutory results of Galapagos NV. These results are part of the consolidated results as discussed in the Letter from the CEO and Chairman. The complete version of the statutory accounts of Galapagos NV will be filed with the National Bank of Belgium. The statutory auditor’s report contains an unqualified opinion on the statutory accounts of Galapagos NV. Income statement Year ended 31 December (thousands of €) 2023 2022 Turnover 628,899 418,495 Inventory semi-finished and finished goods : increase (decrease) 6,808 4,414 Internally generated intangible assets 352,580 349,508 Other operating income 16,103 12,847 Non-recurring operating income 547 19 Operating income 1,004,937 785,283 Raw materials, consumables and goods for resale (28,718) (19,860) Services and other goods (397,124) (420,835) Remuneration, social security costs and pensions (73,556) (77,772) Depreciation, impairment and other amounts written off on constitution costs, intangible and tangible assets (360,512) (357,368) Increase in provisions (4,220) (2,105) Other operating charges (70,785) (102,149) Non-recurring operating costs (1,037) (36,854) Operating profit/loss (-) 68,985 (231,661) Finance income 213,501 135,554 Finance cost (27,417) (60,964) Non-recurring finance cost (10,069)—Profit/loss (-) before tax 245,000 (157,071) Taxes 26,292 19,092 Profit/loss (-) for the year 271,292 (137,980) Loss brought forward (507,217) (369,237) Accumulated losses to be carried forward (235,924) (507,217) 254 Galapagos NV Annual Report 2023
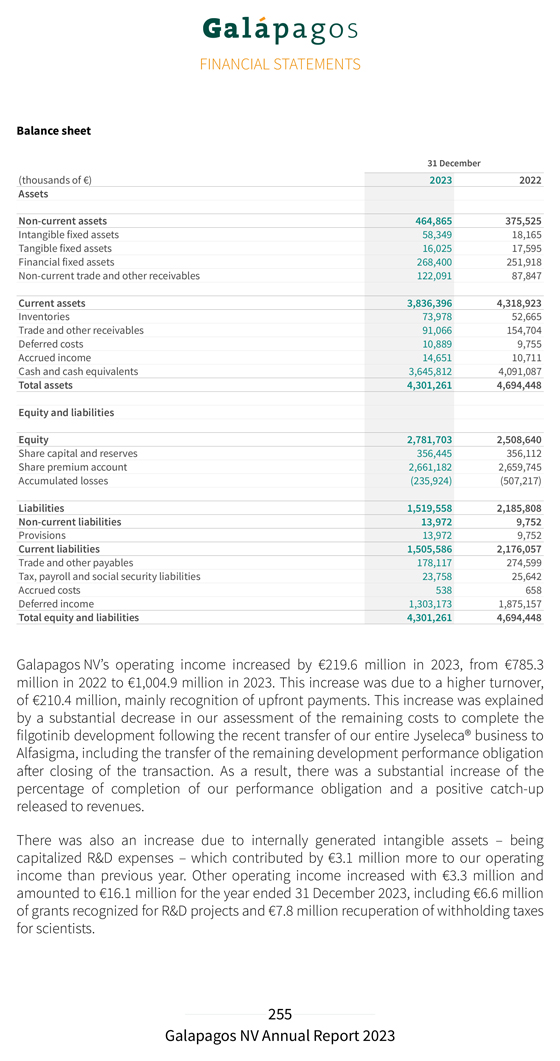
Balance sheet 31 December (thousands of €) 2023 2022 Assets Non-current assets 464,865 375,525 Intangible fixed assets 58,349 18,165 Tangible fixed assets 16,025 17,595 Financial fixed assets 268,400 251,918 Non-current trade and other receivables 122,091 87,847 Current assets 3,836,396 4,318,923 Inventories 73,978 52,665 Trade and other receivables 91,066 154,704 Deferred costs 10,889 9,755 Accrued income 14,651 10,711 Cash and cash equivalents 3,645,812 4,091,087 Total assets 4,301,261 4,694,448 Equity and liabilities Equity 2,781,703 2,508,640 Share capital and reserves 356,445 356,112 Share premium account 2,661,182 2,659,745 Accumulated losses (235,924) (507,217) Liabilities 1,519,558 2,185,808 Non-current liabilities 13,972 9,752 Provisions 13,972 9,752 Current liabilities 1,505,586 2,176,057 Trade and other payables 178,117 274,599 Tax, payroll and social security liabilities 23,758 25,642 Accrued costs 538 658 Deferred income 1,303,173 1,875,157 Total equity and liabilities 4,301,261 4,694,448 Galapagos NV’s operating income increased by €219.6 million in 2023, from €785.3 million in 2022 to €1,004.9 million in 2023. This increase was due to a higher turnover, of €210.4 million, mainly recognition of upfront payments. This increase was explained by a substantial decrease in our assessment of the remaining costs to complete the filgotinib development following the recent transfer of our entire Jyseleca® business to Alfasigma, including the transfer of the remaining development performance obligation after closing of the transaction. As a result, there was a substantial increase of the percentage of completion of our performance obligation and a positive catch-up released to revenues. There was also an increase due to internally generated intangible assets – being capitalized R&D expenses – which contributed by €3.1 million more to our operating income than previous year. Other operating income increased with €3.3 million and amounted to €16.1 million for the year ended 31 December 2023, including €6.6 million of grants recognized for R&D projects and €7.8 million recuperation of withholding taxes for scientists. 255 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS The operating costs of 2023 amounted to €935.9 million compared to €1,016.9 million in 2022. Material purchases increased from €19.9 million in 2022 to €28.7 million in 2023. Services and other goods decreased substantially to €397.1 million compared to €420.8 million in 2022, primarily due to decreased external subcontracting for our preclinical studies and clinical trials. Personnel costs in 2023 decreased to €73.6 million compared to €77.8 million in 2022. The number of employees at Galapagos NV at the end of 2023 amounted to 367 as compared to 442 at the end of 2022, excluding insourced personnel. The average number of FTE in 2023 decreased to 369, compared to 433 in 2022. Depreciation increased to €360.5 million in 2023, compared to €357.4 million in 2022, and related primarily to amortization of capitalized R&D expenses. Galapagos NV capitalizes its incurred R&D expenses and fully amortizes them in the same year. Other operating charges decreased from €102.1 million in 2022 to €70.8 million in 2023 caused by a reduction in transferpricing management fees. Non-recurring operating costs in 2022 consisted of impairments of intangible fixed assets related to discontinued projects. Galapagos NV’s 2023 financial income increased to €213.5 million compared to €135.6 million in 2022, financial costs decreased to €27.4 million compared to €61.0 million in 2022. Non-recurring finance cost in 2023 consisted of impairment on financial assets. The net exchange gain decreased from €54.9 million in 2022 to a net exchange loss of €29.3 million in 2023 and consisted mainly of non-realized currency exchange losses on U.S. dollar. The net interest income in 2023 amounted to €97.9 million as compared to a net interest income of €10.8 million in 2022. Financial income also included dividend income of €109.5 million. Tax income recorded in 2023 of €26.3 million as compared to €19.1 million tax income in 2022, related to tax incentives for investments in intangible fixed assets. Investments in fixed assets in 2023 amounted to €47.6 million, excluding the internally generated assets. They consisted mainly of investments in intangible assets, being a license payment and software, as well of costs for building improvements, new laboratory and IT equipment. Non-current and current other receivables amounted to respectively €122.1 million and €64.1 million and included the receivable for tax incentives amounting to respectively €117.4 million and €13.8 million in 2023, compared to other receivables for tax incentives of €87.8 million and €14.2 million in 2022. Galapagos NV’s cash position at the end of 2023 amounted to €3,645.8 million. The non-consolidated annual accounts of Galapagos NV which we submit for your approval were prepared in accordance with Belgian accounting rules as well as with the 256 Galapagos NV Annual Report 2023

FINANCIAL STATEMENTS legal and regulatory requirements. They show a positive result. The financial year 2023 closed with a profit of €271.3 million compared to a loss of €138.0 million in 2022. The non-consolidated annual accounts of Galapagos NV show accumulated losses of €235.9 million as at 31 December 2023; we refer to the Going concern statement for justification for the application of the valuation rules under the going concern assumption. In 2023, Galapagos NV did not make use of financial instruments. Following common practice, Galapagos NV has given customary representations and warranties which are capped and limited in time. 257 Galapagos NV Annual Report 2023

REPORT OF THE STATUTORY AUDITOR Report of the statutory auditor Galapagos Statutory auditor’s NV for the report year ended to the general 31 December meeting 2023 of (Consolidated Financial Statements) In the context of the statutory audit of the consolidated financial statements of Galapagos NV (‘the Company’) and its subsidiaries (together referred to as ‘the Group’), we hereby present our statutory auditor’s report. It includes our report of the consolidated financial statements and the other legal and regulatory requirements. This report is an integrated whole and is indivisible. We have been appointed as statutory auditor by the general meeting of 25 April 2023, following the proposal formulated by the administrative body, issued upon recommendation of the Audit Committee and upon presentation by the works’ council. Our statutory auditor’s mandate expires on the date of the General Meeting deliberating on the financial statements closed on 31 December 2025. We have performed the statutory audit of the consolidated financial statements of the Group for one year. Report on the consolidated financial statements Unqualified opinion We have performed the statutory audit of the Group’s consolidated financial statements, which comprise the consolidated statement of financial position as at 31 December 2023, the consolidated income statement, consolidated statement of comprehensive income/loss, changes in equity and cash flows for the year then ended, and notes to the consolidated financial statements, including a summary of material accounting policies and other explanatory information, and which is characterised by a consolidated statement of financial position total of 4,357,396 thousand EUR and for which the consolidated income statement shows a profit for the year of 211,697 thousand EUR. In our opinion, the consolidated financial statements give a true and fair view of the Group’s net equity and financial position as at 31 December 2023, as well as of its consolidated financial performance and its consolidated cash flows for the year then ended, in accordance with International Financial Reporting Standards (IFRS) as adopted by the European Union and with the legal and regulatory requirements applicable in Belgium. Basis for unqualified opinion We conducted our audit in accordance with International Standards on Auditing (ISA) as applicable in Belgium. 258 Galapagos NV Annual Report 2023

REPORT OF THE STATUTORY AUDITOR Our responsibilities under those standards are further described in the ‘Statutory auditor’s responsibilities for the audit of the consolidated financial statements’ section in this report. We have complied with all the ethical requirements that are relevant to the audit of consolidated financial statements in Belgium, including those concerning independence. We have obtained from the administrative body and company officials the explanations and information necessary for performing our audit. We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our opinion Key audit matters Key audit matters are those matters that, in our professional judgment, were of most significance in our audit of the consolidated financial statements of the current year. These matters were addressed in the context of our audit of the consolidated financial statements as a whole, and in forming our opinion thereon, and we do not provide a separate opinion on these matters. Determination for revenue recognition of the percentage related to of the completion filgotinib used collaboration performance obligation agreement under with Gilead, the license reported and within the results from discontinued operations Critical Audit Matter Description As described in notes 2, 4, 5 and 26 to the consolidated financial statements, the Company recognized collaboration revenues of 429.4 million EUR in 2023 from upfront payments and milestone payments related to the filgotinib performance obligation under the license and collaboration agreement with Gilead (the “agreement”). The Company recognized revenue using the cost-to-cost input method, which management believes best depicts the transfer of control to the customer. The extent of progress towards completion is measured based on the ratio of actual costs incurred to date, to the total estimated costs expected upon satisfying the filgotinib performance obligation. Significant management judgment is required in determining the total estimated costs still to incur and the period over which the Company is expected to complete its performance obligation, impacting the revenue recognition. This significant estimate is the principal consideration for our conclusion that procedures relating to the determination of the estimated costs to complete the performance obligation, impacting the revenue recognition for the filgotinib performance obligation, is a critical audit matter. This increased level of judgment by management led to a high degree of auditor judgment, complexity, and effort in performing procedures and in evaluating audit 259 Galapagos NV Annual Report 2023

REPORT OF THE STATUTORY AUDITOR evidence related to management’s assumptions of the estimation of total costs to complete. How the Critical Audit Matter Was Addressed in the Audit The primary procedures we performed to address this critical audit matter included: Testing the design and operating effectiveness of controls over management’s assessment on determining the estimate of total costs to complete the performance obligation, which included evaluating the reasonableness of significant assumptions related to the estimate; Testing the accuracy and completeness of actual costs incurred to date based on a sample; Evaluating management’s ability to reasonably estimate the costs to complete the performance obligation, including: Evaluating the appropriateness of changes made during the period to management’s estimates of total costs to complete. Performing a comparison of management’s prior period cost estimates to actual costs incurred and approved. Evaluating the period over which management is expecting the Company to complete its performance obligation. Comparing certain costs to third-party supporting evidence. Considering the impact of any subsequent events on management’s assumptions. Discontinued held for sale related operations to the and transfer assets of and Jyseleca® liabilities business to Alfasigma Critical Audit Matter Description As described in note 2 and 5 to the consolidated financial statements, the Company announced on October 30, 2023, the signing of a letter of intent contemplating the transfer of the Jyseleca® business to Alfasigma. On December 30, 2023 the Share and Asset Purchase Agreement (‘SAPA’) was signed with the final closing of the transaction being subject to closing conditions. On January 31, 2024 these conditions, including the signing of the Transition agreement, were fulfilled and the transaction was closed. Significant management judgement was used in the determination and classification of the expenses incurred directly in relation to the Jyseleca® business as discontinued operations, which involved certain judgments in allocating expenses and in the identification of the assets and liabilities that form part of the disposal group relating to the transfer of the Jyseleca® business, considering the transition period and conditions agreed with Alfasigma. This significant judgment is the principal consideration for our conclusion that procedures relating to auditing management’s judgements applied to the determination and classification of the expenses and in the identification of the 260 Galapagos NV Annual Report 2023

REPORT OF THE STATUTORY AUDITOR assets and liabilities is a critical audit matter. Significant audit effort was involved in performing these procedures. How the Critical Audit Matter Was Addressed in the Audit The primary procedures we performed to address this critical audit matter included: Testing the design and operating effectiveness of controls over management’s accounting treatment for discontinued operations, including those over the determination of the assets and liabilities allocated to the disposal group; Evaluating management’s judgements over the identification of all assets and liabilities belonging to the disposal group by reading relevant agreements and assessing the Company’s ongoing involvement during the transition period agreed with Alfasigma; Assessing the reasonableness of the judgements applied by management in allocating expenses to the discontinued operations by inspecting supporting documentation, and inquiring management regarding specific assumptions made. Responsibilities of the administrative body for the drafting of the consolidated financial statements The administrative body is responsible for the preparation of consolidated financial statements that give a true and fair view in accordance with the International Financial Reporting Standards (IFRS) as adopted by the European Union and with the legal and regulatory provisions applicable in Belgium, and for such internal control as the administrative body determines is necessary to enable the preparation of consolidated financial statements that are free from material misstatements, whether due to fraud or error. In preparing the consolidated financial statements, the administrative body is responsible for assessing the Group’s ability to continue as a going concern, disclosing, as applicable, matters related to going concern and using the going concern basis of accounting unless the administrative body either intends to liquidate the Group or to cease operations, or has no realistic alternative but to do so. Statutory auditor’s responsibilities for the audit of the consolidated financial statements Our objectives are to obtain reasonable assurance about whether the consolidated financial statements as a whole are free from material misstatement, whether due to fraud or error, and to issue a statutory auditor’s report that includes our opinion. Reasonable assurance is a high level of assurance, but it is not a guarantee that an audit conducted in accordance with ISAs will always detect a material misstatement when it exists. Misstatements can arise from fraud or error and are considered material if, individually or in the aggregate, they could reasonably be expected to influence the economic decisions of users taken on the basis of these consolidated financial statements. 261 Galapagos NV Annual Report 2023

REPORT OF THE STATUTORY AUDITOR When executing our audit, we respect the legal, regulatory and normative framework applicable for the audit of the consolidated financial statements in Belgium. However, a statutory audit does not guarantee the future viability of the Group, neither the efficiency and effectiveness of the management of the Group by the administrative body. Our responsibilities regarding the continuity assumption applied by the administrative body are described below. As part of an audit in accordance with ISAs, we exercise professional judgment and maintain professional skepticism throughout the audit. We also: Identify and assess the risks of material misstatement of the consolidated financial statements, whether due to fraud or error, design and perform audit procedures responsive to those risks, and obtain audit evidence that is sufficient and appropriate to provide a basis for our opinion. The risk of not detecting a material misstatement resulting from fraud is higher than for one resulting from error, as fraud may involve collusion, forgery, intentional omissions, misrepresentations, or the override of internal control; Obtain an understanding of internal control relevant to the audit in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Group’s internal control; Evaluate the appropriateness of accounting policies used and the reasonableness of accounting estimates and related disclosures made by the administrative body; Conclude on the appropriateness of the administrative body’s use of the going concern basis of accounting and, based on the audit evidence obtained, whether a material uncertainty exists related to events or conditions that may cast significant doubt on the Group’s ability to continue as a going concern. If we conclude that a material uncertainty exists, we are required to draw attention in our statutory auditor’s report to the related disclosures in the consolidated financial statements or, if such disclosures are inadequate, to modify our opinion. Our conclusions are based on the audit evidence obtained up to the date of our statutory auditor’s report. However, future events or conditions may cause the Group to cease to continue as a going concern; Evaluate the overall presentation, structure and content of the consolidated financial statements and whether the consolidated financial statements represent the underlying transactions and events in a manner that achieves fair presentation; Obtain sufficient appropriate audit evidence regarding the financial information of the entities or business activities within the Group to express an opinion on the consolidated financial statements. We are responsible for the management, the supervision and the performance of the Group audit. We assume full responsibility for the auditor’s opinion. We communicate with the Audit Committee regarding, among other matters, the planned scope and timing of the audit and significant audit findings, including any significant deficiencies in internal control identified during the audit. 262 Galapagos NV Annual Report 2023

REPORT OF THE STATUTORY AUDITOR We also provide the Audit Committee with a statement that we respected the relevant ethical requirements relating to independence, and we communicate with them about all relationships and other issues which may influence our independence, and, if applicable, about the related measures to guarantee our independence. From the matters communicated with the Audit Committee, we determine those matters that were of most significance in the audit of the consolidated financial statements of the current year, and are therefore the key audit matters. We describe these matters in our statutory auditor’s report, unless law or regulation precludes public disclosure about the matter. Other legal and regulatory requirements Responsibilities of the administrative body The administrative body is responsible for the preparation and the contents of the director’s report on the consolidated financial statements, the statement of non-financial information attached to the director’s report on the consolidated financial statements and for the other information included in the annual report on the consolidated financial statements. Responsibilities of the statutory auditor In the context of our mission and in accordance with the Belgian standard (version revised 2020) which is complementary to the International Standards on Auditing (ISA) as applicable in Belgium, it is our responsibility to verify, in all material aspects, the director’s report on the consolidated financial statements, the statement of non-financial information attached to the director’s report on the consolidated financial statements and the other information included in the annual report on the consolidated financial statements, as well as to report on these elements. Aspects relating to the director’s report on the consolidated financial statements and to the other information included in the annual report on the consolidated financial statements In our opinion, after having performed specific procedures in relation to the director’s report, this director’s report is consistent with the consolidated financial statements for the same financial year, and it is prepared in accordance with article 3:32 of the Code of companies and associations. In the context of our audit of the consolidated financial statements, we are also responsible for considering, in particular based on the knowledge we have obtained during the audit, whether the director’s report on the consolidated financial statements and the other information included in the annual report on the consolidated financial statements, contain a material misstatement, i.e. information which is inadequately disclosed or otherwise misleading. Based on the procedures we have performed, there are no material misstatements we have to report to you. 263 Galapagos NV Annual Report 2023

REPORT OF THE STATUTORY AUDITOR The non-financial information, as required by article 3:32, §2 of the Code of companies and associations, has been included in a separate report attached to the director’s report on the consolidated annual accounts, which is part of the section on sustainability in the annual report. This report of non-financial information contains the information required by article 3:32, §2 of the Code of companies and associations and is consistent with the consolidated annual accounts for the same financial year. In preparing this non-financial information, the Company has based itself on the United Nations’ Sustainable Development Goals (“SDG’s”). In accordance with article 3:80, §1, first paragraph, 5° of the Code of companies and associations, we do not express an opinion on the question whether this non-financial information has been prepared in accordance with the information contained in the director’s report on the consolidated annual accounts in accordance with the SDG’s. Statement concerning independence Our audit firm and our network did not provide services which are incompatible with the statutory audit of the consolidated financial statements and our audit firm remained independent of the Group during the terms of our mandate. The fees related to additional services which are compatible with the statutory audit as referred to in article 3:65 of the Code of companies and associations were duly itemised and valued in the notes to the consolidated financial statements. European Single Electronic Format (ESEF) In accordance with the draft standard of the Institute of Bedrijfsrevisoren concerning the standard on auditing the conformity of financial statements with the European Single Electronic Format (hereinafter “ESEF”), we also audited the conformity of the ESEF format with the regulatory technical standards established by Commission Delegated Regulation (EU) 2019/815 of 17 December 2018 (hereinafter: “Delegated Regulation”). The administrative body is responsible for preparing, in accordance with ESEF requirements, the consolidated financial statements in the form of an electronic file in ESEF format (hereinafter “digital consolidated financial statements”) included in the annual financial report. It is our responsibility to obtain sufficient and appropriate supporting information to conclude that the format and mark-up language of the digital consolidated financial statements comply in all material aspects with the ESEF requirements under the Delegated Regulation. Based on our work, we believe that the format and the mark-up of information in the official Dutch version of the digital consolidated financial statements included in the annual financial report of Galapagos NV as at 31 December 2023 comply in all material aspects with the ESEF requirements under the Delegated Regulation. 264 Galapagos NV Annual Report 2023

REPORT OF THE STATUTORY AUDITOR Other statements This report is in compliance with the contents of our additional report to the Audit Committee as referred to in article 11 of regulation (EU) No 537/2014. Zaventem, 28 March 2024 BDO Bedrijfsrevisoren BV Statutory auditor Represented by Ellen Lombaerts* Auditor *Acting for a company 265 Galapagos NV Annual Report 2023
OTHER INFORMATION Glossary ADS American Depositary Share; Galapagos has a Level 3 ADS listed on Nasdaq with ticker symbol GLPG and CUSIP number 36315X101. One ADS is equivalent to one ordinary share in Galapagos NV Antibody A blood protein produced in response to and counteracting a specific antigen. Antibodies combine chemically with substances which the body recognizes as alien, such as bacteria, viruses, and foreign substances Antigen-binding fragment (Fab) The fragment antigen-binding (Fab fragment) is a region on an antibody that binds to antigens. It is composed of one constant and one variable domain of each of the heavy and the light chain Assays Laboratory tests to determine characteristics ATALANTA-1 ATALANTA-1 Phase 1/2 study with point-of-care manufactured CD19 CAR-T candidate, GLPG5101, in patients with replapsed/ refractory non-Hodgkin lymphoma (rrNHL) Axial spondyloarthritis (AxSpA) Axial spondyloarthritis (axSpA) is a type of arthritis. It mostly causes pain and swelling in the spine and the joints that connect the bottom of the spine to the pelvis (sacroiliac joint). Other joints can be affected as well. It is a systemic disease, which means it may affect other body parts and organs. The disease tends to run in families BCMA B cell maturation antigen (BCMA) is a member of the tumor necrosis factor receptor superfamily that plays an important role in regulating B-cell proliferation and survival. BCMA is central to the survival of multiple myeloma cells Biologics Biologics, also referred to as Biologicals, are those class of medicines which are grown and then purified from large-scale cell cultures of bacteria or yeast, or plant or animal cells. Biologicals are a diverse group of medicines which includes vaccines, growth factors, immune modulators, monoclonal antibodies, as well as products derived from 266 Galapagos NV Annual Report 2023

OTHER INFORMATION human blood and plasma. What distinguishes biologics from other medicines is that these are generally proteins purified from living culture systems or from blood, whereas other medicines are considered as ‘small molecules’ and are either made synthetically or purified from plants Black & Scholes model A mathematical description of financial markets and derivative investment instruments that is widely used in the pricing of European options and subscription rights CAR-T Chimeric antigen receptor T cells (also known as CAR-T cells) are T cells that have been genetically engineered to produce an artificial T cell receptor for use in immunotherapy Cash position Current financial investments and cash and cash equivalents CD19 CD19 is a protein found on the surface of B-cells, a type of white blood cell. Since CD19 is a hallmark of B-cells, the protein has been used to diagnose cancers that arise from this type of cell, notably B-cell lymphomas Cell therapy Cell therapy aims to treat diseases by restoring or altering certain sets of cells or by using cells to carry a therapy through the body. With cell therapy, cells are cultivated or modified outside the body before being injected into the patient. The cells may originate from the patient (autologous cells) or a donor (allogeneic cells) CHMP Committee for Medicinal Products for Human Use is the European Medicines Agency’s (EMA) committee responsible for human medicines and plays a vital role in the authorization of medicines in the European Union (EU) Chronic Lymphocytic Leukemia (CLL) Chronic lymphocytic leukemia is the most common leukemia in adults. It is a type of cancer that starts in cells that become certain white blood cells (called lymphocytes) in the bone marrow. The cancer (leukemia) cells originate in the bone marrow and migrate to the bloodstream Complete Response Rate (CRR) Term used for the absence of all detectable cancer after the treatment is completed Compound A chemical substance, often a small molecule with drug-like properties 267 Galapagos NV Annual Report 2023
OTHER INFORMATION Contract research organization (CRO) Organization which provides drug discovery and development services to the pharmaceutical, biotechnology and medical devices industry Crohn’s disease (CD) An IBD involving inflammation of the small and large intestines, leading to pain, bleeding, and ultimately in some cases surgical removal of parts of the bowel Cryopreservation Process where biological material—cells, tissues, or organs—are frozen to preserve the material for an extended period of time Cytokine A category of small proteins which play important roles in signaling in processes in the body Cytokine release syndrome (CRS) Condition that develops when your immune system responds too aggressively to infection or after certain types of immunotherapy, such as CAR-T-cell therapy DARWIN Phase 2 program for filgotinib in RA. DARWIN 1 explored three doses, in twice-daily and once-daily administration, for up to 24 weeks in RA patients with insufficient response to methotrexate (MTX) and who remained on their stable background treatment with MTX. DARWIN 2 explored three once-daily doses for up to 24 weeks in RA patients with insufficient response to methotrexate (MTX) and who washed out of their treatment with MTX. DARWIN 1 and 2 were double-blind, placebo-controlled trials which recruited approximately 900 patients globally and for which results were reported in 2015. DARWIN 3 is a long term extension trial in which all patients are on 200mg filgotinib, except for U.S. males who are on 100mg. The Week 156 results from DARWIN 3 were reported in 2019 Dermatomyositis (DM) Dermatomyositis is a rare inflammatory disease. Common symptoms include distinctive skin rash, and inflammatory myopathy, or inflamed muscles, causing muscle weakness Development All activities required to bring a new drug to the market. This includes preclinical and clinical development research, chemical and pharmaceutical development and regulatory filings of product candidates 268 Galapagos NV Annual Report 2023

OTHER INFORMATION Discovery Process by which new medicines are discovered and/or designed. At Galapagos, this is the department that oversees target and drug discovery research through to nomination of preclinical candidates DIVERSITY Phase 3 program evaluating filgotinib in CD Dose-range finding study Phase 2 clinical study exploring the balance between efficacy and safety among various doses of treatment in patients. Results are used to determine doses for later studies Double-blind Term to characterize a clinical trial in which neither the physician nor the patient knows if the patient is taking placebo or the treatment being evaluated EC European Commission Efficacy Effectiveness for intended use EMA European Medicines Agency, in charge of European market authorization of new medications End-to-end A process that takes a system or service from beginning to end and delivers a complete functional solution, usually without strong reliance on third parties EUPLAGIA-1 EUPLAGIA-1 Phase 1/2 study with point-of-care manufactured CD19 CAR-T candidate, GLPG5201, in patients with replapsed/ refractory chronic lymphocytic leukemia (rrCLL) and small lymphocytic lymphoma (rrSLL), with or without Richter transformation (RT) FDA The U.S. Food and Drug Administration is an agency responsible for protecting and promoting public health and in charge of American market approval of new medications 269 Galapagos NV Annual Report 2023

OTHER INFORMATION Filgotinib Formerly known as GLPG0634, commercial name is Jyseleca®. Small molecule preferential JAK1 inhibitor, approved in RA and UC in the European Union, Great-Britain and Japan. Phase 4 studies in both RA and UC are ongoing FILOSOPHY Phase 4 program evaluating filgotinib in RA FINCH Phase 3 program evaluating filgotinib in RA FORM 20-F Form 20-F is an SEC filing submitted to the US Securities and Exchange Commission FSMA The Belgian market authority: Financial Services and Markets Authority, or Autoriteit voor Financiële Diensten en Markten FTE Full-time equivalent; a way to measure an employee’s involvement in a project. For example, an FTE of 1.0 means that the equivalent work of one full-time worker was used on the project G&A expenses General & administrative expenses GALACELA Phase 2 study with GLPG3667 in patients with systemic lupus erythematous GALARISSO Phase 2 study with GLPG3667 in patients with dermatomyositis GLPG0634 Molecule number currently known as filgotinib and Jyseleca® GLPG3667 A TYK2 kinase inhibitor discovered by us, topline results from the Phase 1b in psoriasis reported in July 2021 270 Galapagos NV Annual Report 2023

OTHER INFORMATION GLPG5101 A second generation anti-CD19/4-1BB CAR-T product candidate currently in Phase 1/2 study in rrNHL GLPG5201 A second generation anti-CD19/4-1BB CAR-T product candidate currently in Phase 1/2 study in rrCLL/SLL with or wthout RT GLPG5301 A BCMA CAR-T product candidate IBD Inflammatory Bowel Disease. This is a general term for an autoimmune disease affecting the bowel, including CD and UC. CD affects the small and large intestine, while UC affects the large intestine. Both diseases involve inflammation of the intestinal wall, leading to pain, bleeding, and ultimately, in some cases, surgical removal of part of the bowel Immune effector cell-associated neurotoxicity syndrome (ICAN) Clinical and neuropsychiatric syndrome that can occur in the days to weeks following administration of certain types of immunotherapy, especially immune effector cell (IEC) and T cell engaging therapy Immunology The study of the immune system and is a very important branch of the medical and biological sciences. The immune system protects humans from infection through various lines of defence. If the immune system is not functioning as it should, it can result in disease, such as autoimmunity, allergy, and cancer In-/out-licensing Receiving/granting permission from/to another company or institution to use a brand name, patent, or other proprietary right, in exchange for a fee and/or royalty Intellectual property Creations of the mind that have commercial value and are protected or protectable, including by patents, trademarks or copyrights Investigational New Drug (IND) Application United States Federal law requires a pharmaceutical company to obtain an exemption to ship an experimental drug across state lines, usually to clinical investigators, before a marketing application for the drug has been approved. The IND is the means by which the sponsor obtains this exemption, allowing them to perform clinical studies 271 Galapagos NV Annual Report 2023

OTHER INFORMATION In vitro Studies performed with cells outside their natural context, for example in a laboratory In vivo Studies performed with animals in a laboratory setting JAK Janus kinases (JAK) are critical components of signaling mechanisms utilized by a number of cytokines and growth factors, including those that are elevated in RA. Filgotinib is a preferential JAK1 inhibitor Jyseleca® Jyseleca® is the brand name for filgotinib Leukapheresis Laboratory procedure in which white blood cells are separated from a sample of blood Lymphocyte Type of white blood cell that is part of the immune system MACE Major adverse cardiovascular events; a composite endpoint frequently used in cardiovascular research MANTA A Phase 2 semen parameter trial with filgotinib in male patients with CD or UC MANTA-RAy Phase 2 semen parameter trial with filgotinib in male patients with RA, PsA, or AS MHLW Japanese Ministry of Health, Labor and Welfare (MHLW), in charge of Japanese market authorization of new medications MHRA Medicines and Healthcare products Regulatory Agency in Great Britain Milestone Major achievement in a project or program; in our alliances, this is usually associated with a payment 272 Galapagos NV Annual Report 2023

OTHER INFORMATION Multiple myeloma (MM) Multiple myeloma (MM) is typically characterized by the neoplastic proliferation of plasma cells producing a monoclonal immunoglobulin. The plasma cells proliferate in the bone marrow and can result in extensive skeletal destruction with osteolytic lesions, osteopenia, and/or pathologic fractures. NDA A new drug application (NDA) is a request to the FDA for a license to market a new drug in the U.S. A NDA must show the chemical and pharmacologic description of the drug, the results of clinical trials, and the proposed drug label Non-Hodgkin’s lymphoma (NHL) Non-Hodgkin’s lymphoma is a type of cancer that begins in the lymphatic system, which is part of the body’s germ-fighting immune system. In non-Hodgkin’s lymphoma, white blood cells called lymphocytes grow abnormally and form tumors throughout the body Objective Response Rate (ORR) The response rate is the percentage of patients on whom a therapy has some defined effect; for example, the cancer shrinks or disappears after treatment. When used as a clinical endpoint for trials of cancer treatments, this is often called the objective response rate OLINGUITO Phase 3 study with filgotinib in patients with axial spondyloarthritis Oncology Field of medicine that deal with the diagnosis, treatment, prevention, and early detection of cancer Oral dosing Administration of medicine by the mouth, either as a solution or solid (capsule, pill) form Outsourcing Contracting work to a third party PAPILIO-1 Phase 1/2 study with GLPG5301 in patients with relapsed/refractory multiple myeloma Pharmacokinetics (PK) Study of what a body does to a drug; the fate of a substance delivered to a body. This includes absorption, distribution to the tissues, metabolism and excretion. These 273 Galapagos NV Annual Report 2023

OTHER INFORMATION processes determine the blood concentration of the drug and its metabolite(s) as a function of time from dosing Phase 1 First stage of clinical testing of an investigational drug designed to assess the safety and tolerability, pharmacokinetics of a drug, usually performed in a small number of healthy human volunteers Phase 2 Second stage of clinical testing, usually performed in no more than several hundred patients, in order to determine efficacy, tolerability and the dose to use Phase 3 Large clinical trials, usually conducted in several hundred to several thousand patients to gain a definitive understanding of the efficacy and tolerability of the candidate treatment; serves as the principal basis for regulatory approval Pivotal trials Registrational clinical trials Placebo A substance having no pharmacological effect but administered as a control in testing a biologically active preparation Point-of-care Drug treatment is provided close to or near the patient PRAC Pharmacovigilance Risk Assessment Committee of the European Medicines Agency, responsible for assessing all aspects of risk management of human medicines Preclinical Stage of drug research development, undertaken prior to the administration of the drug to humans. Consists of in vitro and in vivo screening, pharmacokinetics, toxicology, and chemical upscaling Preclinical candidate (PCC) A new molecule and potential drug that meets chemical and biological criteria to begin the development process 274 Galapagos NV Annual Report 2023

OTHER INFORMATION Product candidate Substance that has satisfied the requirements of early preclinical testing and has been selected for development, starting with formal preclinical safety evaluation followed by clinical testing for the treatment of a certain disorder in humans R&D operations Research and development operations; unit responsible for discovery and developing new product candidates for internal pipeline or as part of risk/reward sharing alliances with partners Refractory “Refractory” refers to a patient with cancer that is/has become resistant to, or does not respond to, treatment Relapsed “Relapsed” refers to a patient with cancer that develops cancer again after a period of improvement Rheumatoid arthritis (RA) A chronic, systemic inflammatory disease that causes joint inflammation, and usually leads to cartilage destruction, bone erosion and disability Richter transformation Richter transformation (RT) is an uncommon clinicopathological condition observed in patients with CLL. It is characterized by the sudden transformation of the CLL into a significantly more aggressive form of large cell lymphoma, and occurs in approximately 2-10% of all CLL patients. S&M expenses Sales and marketing expenses SEC Securities and Exchange Commission in the US SELECTION Phase 3 program evaluating filgotinib in UC patients. Full results were published in The Lancet in 2021 SIK Salt-inducible kinase 275 Galapagos NV Annual Report 2023

OTHER INFORMATION Single-chain variable fragments (scFv) Single-chain variable fragments (scFvs) are small-sized artificial constructs composed of the immunoglobulin heavy and light chain variable regions connected by a peptide linker Small cell lymphocyte leukemia (SLL) Small cell lymphocyte leukemia is a type of B-cell non-Hodgkin lymphoma, where the SLL cancer is located in lymp nodes and/or the spleen Systemic lupus erythematosus (SLE) An autoimmune disease, with systemic manifestations including skin rash, erosion of joints or even kidney failure Target Protein that has been shown to play a role in a disease process and that forms the basis of a therapeutic intervention or discovery of a medicine Target discovery Identification and validation of proteins that have been shown to play a role in a disease process TEAE Treatment Emergent Adverse Event, is any event not present prior to the initiation of the treatments or any event already present that worsens in either intensity or frequency following exposure to the treatments TYK Tyrosine kinase is an enzyme that can transfer a phosphate group from ATP to the tyrosine residues of specific proteins inside a cell. It functions as an “on” or “off” switch in many cellular functions. Tyrosine kinases belong to a larger class of enzymes known as protein kinases which also attach phosphates to other amino acids such as serine and threonine. GLPG3667 is a reversible and selective TYK2 kinase domain inhibitor Ulcerative colitis (UC) UC is an IBD causing chronic inflammation of the lining of the colon and rectum (unlike CD with inflammation throughout the gastrointestinal tract) Variable heavy (VH) domain The variable domain of an immunoglobulin heavy chain is a part of an antibody that binds to a specific antigen 276 Galapagos NV Annual Report 2023
OTHER INFORMATION Financial calendar 30 April 2024 Annual Shareholders’ Meeting in Mechelen, Belgium 2 May 2024 First quarter 2024 results 1 August 2024 First half year 2024 results 30 October 2024 Third quarter 2024 results 277 Galapagos NV Annual Report 2023

OTHER INFORMATION Colophon Concept, design and online programming nexxar GmbH, Vienna – Online annual reports and online sustainability reports www.nexxar.com Photography Frank van Delft Cocoon® images courtesy of Lonza Group AG Private photographs Video Videofactory BV Drawify – Axelle Vanquaillie Copy deadline: 26 March 2024 This report is also available in Dutch and available for download in the Downloads section of this report or on the Galapagos website. 278 Galapagos NV Annual Report 2023

OTHER INFORMATION Contact Sofie Van Gijsel Head of Investor Relations Galapagos NV Generaal De Wittelaan L11 A3 2800 Mechelen, Belgium Tel. +1 781 296 1143 Email: ir@glpg.com Sandra Cauwenberghs Director of Investor Relations Galapagos NV Generaal De Wittelaan L11 A3 2800 Mechelen, Belgium Tel. +32 15 34 29 00 Email: ir@glpg.com Marieke Vermeersch Head of Corporate Communication Galapagos NV Generaal De Wittelaan L11 A3 2800 Mechelen, Belgium Tel. +32 479 49 06 03 Email: communications@glpg.com 279 Galapagos NV Annual Report 2023
Exhibit 99.3

Free translation from Dutch
For information purposes only
GALAPAGOS NV
Limited liability company
Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium
Company Number: 0466.460.429
RLE Antwerp - division Mechelen
Invitation to the Annual and Extraordinary Shareholders’ Meetings to be held on 30 April 2024
The Board of Directors of Galapagos NV (hereinafter the “Company”, or “Galapagos”) has the honor to invite the shareholders, holders of subscription rights, directors, and statutory auditor of the Company to the annual shareholders’ meeting (hereinafter the “Annual Shareholders’ Meeting”). After the agenda of the Annual Shareholders’ Meeting has been treated, the meeting will be shortly suspended in order to be continued as an extraordinary general shareholders’ meeting before a notary public (the “Extraordinary Shareholders’ Meeting”). The Annual Shareholders’ Meeting and the Extraordinary Shareholders’ Meeting will be held sequentially on Tuesday 30 April 2024, as from 2:00 p.m. (Belgian time) at the registered office of the Company (Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium) (together, the “Shareholders’ Meetings”).
There is no attendance quorum requirement for the deliberation and voting on the agenda items referred to in the below agenda of the Annual Shareholders’ Meeting. There is, however, an attendance quorum requirement for the items on the agenda of the Extraordinary Shareholders’ Meeting (see also below under “2. Extraordinary Shareholders’ Meeting”). If the attendance quorum for the items on the agenda of the Extraordinary Shareholders’ Meeting were not to be reached, a second extraordinary general shareholders’ meeting will be held for these items before a notary public at the Company’s registered office on Tuesday 25 June 2024 at 2:00 p.m. (Belgian time), unless, as the case may be, decided otherwise on behalf of the Board of Directors. The attendance quorum will not apply to this second meeting.
1. Annual Shareholders’ Meeting
The Annual Shareholders’ Meeting will be held on Tuesday, 30 April 2024, at 2 p.m. (Belgian time) at the Company’s registered office.
Details of the applicable registration and voting formalities relating to the Annual Shareholders’ Meeting are set forth below.
Agenda and proposed resolutions
The agenda and the proposed resolutions of the Annual Shareholders’ Meeting of the Company which, as the case may be, can be amended at the meeting on behalf of the Board of Directors, are as follows:
| 1. | Acknowledgement and discussion of (a) the annual report of the Board of Directors in relation to the non-consolidated and consolidated annual accounts of the Company for the financial year ended on 31 December 2023, and (b) the report of the statutory auditor in relation to the non-consolidated annual accounts of the Company for the financial year ended on 31 December 2023. |
| 2. | Acknowledgement and approval of the non-consolidated annual accounts of the Company for the financial year ended on 31 December 2023, and approval of the allocation of the annual result as proposed by the Board of Directors. |
Proposed resolution: The shareholders’ meeting resolves to approve the non-consolidated annual accounts of the Company for the financial year ended on 31 December 2023, and the allocation of the annual result as proposed by the Board of Directors.
| Galapagos NV | AGM/EGM 30 April 2024 | Convening Notice | Page 1 of 12 | |

Free translation from Dutch
For information purposes only
| 3. | Acknowledgement and discussion of the report of the statutory auditor relating to the consolidated annual accounts of the Company for the financial year ended on 31 December 2023. |
| 4. | Acknowledgement and discussion of the consolidated annual accounts of the Company for the financial year ended on 31 December 2023. |
| 5. | Acknowledgement and approval of the remuneration report. |
Proposed resolution: The shareholders’ meeting resolves to approve the remuneration report included in the annual report of the Board of Directors for the financial year ended on 31 December 2023.
| 6. | Acknowledgement and approval of the amended remuneration policy. |
Proposed resolution: The shareholders’ meeting resolves to approve the amended remuneration policy.
| 7. | Release from liability to be granted to the (current and former) members of the Board of Directors, and the (current and former) statutory auditor for the performance of their respective mandates during the financial year ended on 31 December 2023. |
Proposed resolution: The shareholders’ meeting resolves, by a separate vote, to release each (current and former) member of the Board of Directors, and the (current and former statutory) auditor from any liability arising from the performance of their respective mandates during the financial year ended on 31 December 2023.
| 8. | Remuneration of directors. |
Proposed resolution: Upon recommendation and advice of the Remuneration Committee of the Company, the shareholders’ meeting resolves that the annual compensation (excluding expenses) of the non-executive directors, other than the non-executive directors representing a shareholder, for the exercise of their mandate shall consist of a cash remuneration and an equity-based remuneration as follows:
(a) cash remuneration: (i) Chairperson of the Board of Directors: EUR 110,000; (ii) Lead Non-Executive Director: EUR 75,000, (iii) other non-executive directors: EUR 55,000 each; (iv) additional annual compensation for the chairpersonship of a committee within the Board of Directors: EUR 20,000; and (v) additional annual compensation for the membership of a committee within the Board of Directors: EUR 15,000;
(b) equity-based remuneration: (i) Chairperson of the Board of Directors: EUR 110,000; (ii) Lead Non-Executive Director: EUR 75,000; (iii) other non-executive directors: EUR 55,000 each; in each case (i), (ii) and (iii) subject to the requirement to use the net amount (after taxes) to acquire Galapagos shares. These latter payments make up the equivalent of an equity component of the directors’ remuneration and the resulting shares are to be held until at least one year after the non-executive director leaves the Board of Directors and at least three years after the time of acquisition.
The abovementioned remuneration for the Chairperson of the Board of Directors will only be payable if such Chairperson is not simultaneously Chief Executive Officer (CEO) of the Company. The abovementioned remuneration for the Lead Non-Executive Director will only be payable to the extent appointed in accordance with Galapagos’ Corporate Governance Charter.
The shareholders’ meeting resolves that the mandate of a non-executive director representing a shareholder will not be remunerated.
| Galapagos NV | AGM/EGM 30 April 2024 | Convening Notice | Page 2 of 12 | |

Free translation from Dutch
For information purposes only
The rules set out above shall apply as per 1 May 2024.
| 9. | Re-appointment of Dr. Elisabeth Svanberg as independent non-executive director. |
Proposed resolution: Upon proposal of the Board of Directors, and in accordance with the recommendation and advice of the Nomination Committee, the shareholders’ meeting resolves (a) to re-appoint Dr. Elisabeth Svanberg as an independent non-executive member of the Board of Directors of the Company, for a period of four years, effective as of today, ending immediately after the annual shareholders’ meeting to be held in 2028, and (b) to confirm her mandate as an independent member of the Board of Directors since (i) Dr. Elisabeth Svanberg meets the independence criteria set forth in article 7:87 of the Belgian Companies and Associations Code, and article 3.5 of the Belgian Corporate Governance Code 2020, (ii) Dr. Elisabeth Svanberg has explicitly declared not to have (and the Board of Directors is not aware of) any connections with the Company or a major shareholder, which would interfere with her independence, and (iii) the Board of Directors has no indication of any element that might call Dr. Elisabeth Svanberg’s independence into question. The shareholders’ meeting also resolves that the mandate of Dr. Elisabeth Svanberg is remunerated as provided for non-executive members of the Board of Directors in the Company’s remuneration policy and as approved by the shareholders’ meeting from time to time.
| 10. | Appointment of Dr. Susanne Schaffert as independent non-executive director. |
Taking into account the recommendation and advice of the Nomination Committee, the Board of Directors recommends that the appointment of Dr. Susanne Schaffert as independent non-executive director of the Company be confirmed and continued for an additional term of four years. For further information regarding Dr. Susanne Schaffert, reference is made to the corporate governance statement included in the the annual report of the Board of Directors on the (non-consolidated) statutory financial statements of the Company for the financial year ended on 31 December 2023. Notably, on 12 June 2023, the Board of Directors appointed Dr. Susanne Schaffert as independent non-executive director of the Company by co-optation following the resignation on 10 June 2023 of Dr. Rajesh Parekh as non-executive director, who was appointed for a term up to and including the closing of the annual shareholders’ meeting to be held in 2025 which will have decided upon the financial statements for the financial year ended on 31 December 2024.
Proposed resolution: Upon proposal of the Board of Directors, and in accordance with the recommendation and advice of the Nomination Committee, the shareholders’ meeting resolves (a) to confirm the appointment by co-optation on 12 June 2023, following the resignation of Dr. Rajesh Parekh on 10 June 2023, and to appoint Dr. Susanne Schaffert as an independent non-executive member of the Board of Directors of the Company, for an additional period of four years, up to and including the closing of the annual shareholders’ meeting to be held in 2028 which will have decided upon the financial statements for the financial year ended on 31 December 2027, and (b) to confirm her mandate as an independent member of the Board of Directors since (i) Dr. Susanne Schaffert meets the independence criteria set forth in article 7:87 of the Belgian Companies and Associations Code, and article 3.5 of the Belgian Corporate Governance Code 2020, (ii) Dr. Susanne Schaffert has explicitly declared not to have (and the Board of Directors is not aware of) any connections with the Company or a major shareholder, which would interfere with her independence, and (iii) the Board of Directors has no indication of any element that might call Dr. Susanne Schaffert’s independence into question. The shareholders’ meeting also resolves that the mandate of Dr. Susanne Schaffert is remunerated as provided for non-executive members of the Board of Directors in the Company’s remuneration policy and as approved by the shareholders’ meeting from time to time.
| Galapagos NV | AGM/EGM 30 April 2024 | Convening Notice | Page 3 of 12 | |

Free translation from Dutch
For information purposes only
| 11. | Appointment of Mr. Simon Sturge as independent non-executive director. |
Taking into account the recommendation and advice of the Nomination Committee, the Board of Directors recommends that the appointment of Mr. Simon Sturge as independent non-executive director of the Company be confirmed and continued for an additional term of four years. For further information regarding Mr. Simon Sturge, reference is made to the corporate governance statement included in the the annual report of the Board of Directors on the (non-consolidated) statutory financial statements of the Company for the financial year ended on 31 December 2023. Notably, on 19 September 2023, the Board of Directors appointed Mr. Simon Sturge as independent non-executive director of the Company by co-optation following the resignation on 18 September 2023 of Dr. Mary Kerr as non-executive director, who was appointed for a term up to and including the closing of this annual shareholders’ meeting.
Proposed resolution: Upon proposal of the Board of Directors, and in accordance with the recommendation and advice of the Nomination Committee, the shareholders’ meeting resolves (a) to confirm the appointment by co-optation on 19 September 2023, following the resignation of Dr. Mary Kerr on 18 September 2023, and to appoint Mr. Simon Sturge as an independent non-executive member of the Board of Directors of the Company, for an additional period of four years, up to and including the closing of the annual shareholders’ meeting to be held in 2028 which will have decided upon the financial statements for the financial year ended on 31 December 2027, and (b) to confirm his mandate as an independent member of the Board of Directors since (i) Mr. Simon Sturge meets the independence criteria set forth in article 7:87 of the Belgian Companies and Associations Code, and article 3.5 of the Belgian Corporate Governance Code 2020, (ii) Mr. Simon Sturge has explicitly declared not to have (and the Board of Directors is not aware of) any connections with the Company or a major shareholder, which would interfere with his independence, and (iii) the Board of Directors has no indication of any element that might call Mr. Simon Sturge’s independence into question. The shareholders’ meeting also resolves that the mandate of Mr. Simon Sturge is remunerated as provided for non-executive members of the Board of Directors in the Company’s remuneration policy and as approved by the shareholders’ meeting from time to time.
| 12. | Appointment of Mr. Andrew Dickinson as non-executive director. |
Taking into account the recommendation and advice of the Nomination Committee, the Board of Directors recommends that the appointment of Mr. Andrew Dickinson as non-executive director of the Company be confirmed and continued for an additional term of four years. Notably, on 26 March 2024, and with effect as per 27 March 2024, the Board of Directors appointed Mr. Andrew Dickinson as non-executive director of the Company by co-optation following the resignation on 26 March 2024 of Mr. Daniel O’Day, as non-executive director, who was appointed for a term up to and including the closing of the annual shareholders’ meeting to be held in 2027 which will have decided upon the financial statements for the financial year ended on 31 December 2026.
Proposed resolution: Upon proposal of the Board of Directors, and in accordance with the recommendation and advice of the Nomination Committee, the shareholders’ meeting resolves (a) to confirm the appointment by co-optation on 26 March 2024, and with effect as per 27 March 2024, following the resignation of Mr. Daniel O’Day on 26 March 2024, and to appoint Mr. Andrew Dickinson as a non-executive member of the Board of Directors of the Company, for an additional period of four years, up to and including the closing of the annual shareholders’ meeting to be held in 2028 which will have decided upon the financial statements for the financial year ended on 31 December 2027. The shareholders’ meeting also resolves that the mandate of Andrew Dickinson will not be remunerated.
| 13. | Charging of the statutory auditor with respect to the “assurance” of the CSRD sustainability reporting |
Proposed resolution: In accordance with the recommendation of the Audit Committee and upon proposal of the Board of Directors, and taking into account the pending transposition of the Corporate Sustainability Reporting Directive 2022/2464/EU (“CSRD”) into Belgian law, the shareholders’
| Galapagos NV | AGM/EGM 30 April 2024 | Convening Notice | Page 4 of 12 | |

Free translation from Dutch
For information purposes only
meeting resolves (a) to charge the Company’s statutory auditor, being BDO Bedrijfsrevisoren BV, with its registered office at Da Vincilaan 9/E.6, 1930 Zaventem, and registered with the Crossroads Enterprise Database (RPR Brussels, Dutch-speaking division) under the number 0431.088.289, permanently represented by Ellen Lombaerts, for a period of one year, with the assurance of the sustainability reporting of the Company, as referred to in the CSRD, for the financial year ending on 31 December 2024 in accordance with applicable law, and (b) to determine the remuneration of BDO Bedrijfsrevisoren BV for such assurance at EUR 120,000.00 (if any, VAT exclusive). The charging of the statutory auditor with respect to the aforementioned assurance will expire immediately after the annual shareholders’ meeting to be held in 2025.
No attendance quorum: There is no attendance quorum requirement for the deliberation and voting on the agenda items referred to in the aforementioned agenda of the Annual Shareholders’ Meeting.
Voting and majority: Subject to applicable legal provisions, each share shall have one vote. In accordance with applicable law, the proposed resolutions referred to in the aforementioned agenda of the Annual Shareholders’ Meeting shall be passed if they are approved by a simple majority of the votes validly cast by the shareholders.
2. Extraordinary Shareholders’ Meeting
The Extraordinary Shareholders’ Meeting will be held in the presence of a notary public, and will take place on Tuesday, 30 April 2024, at 3 p.m. (Belgian time) at the Company’s registered office.
Details of the applicable registration and voting formalities relating to the Extraordinary General Meeting are set forth below.
Agenda and proposed resolutions:
The agenda and the proposed resolutions of the Extraordinary Shareholders’ Meeting of the Company which, as the case may be, can be amended at the meeting on behalf of the Board of Directors, are as follows:
| 1. | Consideration and discussion of the report of the Board of Directors of the Company prepared in accordance with articles 7:180, 7:191 and 7:193 of the Belgian Companies and Associations Code in connection with the proposed issuance of one subscription right (in the form of a warrant) for the benefit of Gilead Therapeutics A1 Unlimited Company (“Gilead Therapeutics”), called the “Subsequent Gilead Warrant B”, and the proposal to cancel, in the interest of the Company, the statutory preferential subscription right of the Company’s existing shareholders for the benefit of Gilead Therapeutics. |
| 2. | Consideration and discussion of the report of the statutory auditor of the Company prepared in accordance with articles 7:180, 7:191 and 7:193 of the Belgian Companies and Associations Code in connection with the proposed issuance of one subscription right (in the form of a warrant) for the benefit of Gilead Therapeutics, called the ‘Subsequent Gilead Warrant B’, and the proposal to cancel, in the interest of the Company, the statutory preferential subscription right of the Company’s existing shareholders for the benefit of Gilead Therapeutics. |
| 3. | Approval of the issuance of one (1) new subscription right (in the form of a warrant) for the benefit of Gilead Therapeutics and related cancellation of the statutory preferential subscription right. |
This proposal is made in accordance with the terms of the subscription agreement entered into between the Company and Gilead Therapeutics on 14 July 2019, as amended from time to time (the “Subscription Agreement”), which included a commitment to make a proposal to the general meeting to issue the ‘Subsequent Gilead Warrant B’ that would enable Gilead Therapeutics to further increase its shareholding in the Company, as described in the report of the Board of Directors as referred to in item 1 of the agenda.
| Galapagos NV | AGM/EGM 30 April 2024 | Convening Notice | Page 5 of 12 | |

Free translation from Dutch
For information purposes only
Proposed resolution: The shareholders’ meeting of the Company resolves to approve the issuance of one (1) new subscription right (in the form of a warrant) for the benefit of Gilead Therapeutics, called the ‘Subsequent Gilead Warrant B’ (the “Warrant”), and to cancel, in the interest of the Company, the statutory preferential subscription right of the existing shareholders of the Company (and, insofar as required, of the Company’s existing holders of subscription rights (stock options)) for the benefit of Gilead Therapeutics, in accordance with the report of the Board of Directors prepared in accordance with articles 7:180, 7:191 and 7:193 of the Belgian Companies and Associations Code, as referred to in item 1 of the agenda.
In view thereof, the shareholders’ meeting of the Company resolves to approve the terms and conditions (the “Conditions”) of the Warrant as set forth in Annex 1 to the report of the Board of Directors referred to in item 1 of the agenda, a copy of which shall remain attached to the minutes reflecting the present resolution. The main Conditions of the Warrant can, for informational purposes, be summarized as follows:
| a) | Issuer of the Warrant: The Company. |
| b) | Term: The Warrant has a term starting as of the date of this resolution and ending on 11:59 p.m. on the calendar day before the fifth anniversary of the date of this resolution. The Warrant can be exercised at one or several occasions as from 23 August 2024 (on 11:59 p.m.) during the entire term of the Warrant, but not more than once per period of three (3) months. As set out in the Conditions, this limitation does not apply in case of material development regarding the Company or the trading of the Company’s shares, or in case of certain (requests for) convocations of shareholders’ meetings of the Company. |
| c) | Issue Price: The Warrant will be issued without any additional consideration being due by Gilead Therapeutics or any of its affiliates. |
| d) | Exercise Price: The Exercise Price (as defined in the Conditions) of the Warrant shall, per share that shall be subscribed for upon an exercise of the Warrant in relation to such shares, be equal to the greater of (i) 120% multiplied by the arithmetic mean of the daily volume weighted average trading price of the Company’s shares as traded on Euronext Brussels and Euronext Amsterdam (or such other regulated markets on which the Company’s shares will be trading at that time) on each of the trading days during the period of 30 calendar days ending on the calendar day immediately preceding the date of the Exercise Notice (as defined in the Conditions) with respect to such exercise, and (ii) EUR 140.59. The Exercise Price is subject to adjustments set out in the Conditions. |
| e) | Number of shares issuable upon an exercise of the Warrant: Subject to the Conditions, the Warrant entitles the holder thereof to subscribe, during the entire term of the Warrant, upon each exercise of the Warrant, for a maximum number of shares that is sufficient to bring the number of shares owned by Gilead Therapeutics, Gilead Sciences and any of their affiliates and any other party Acting in Concert (as defined in the Conditions) with Gilead Therapeutics, Gilead Sciences or any of their affiliates to 29.9% of the actually issued and outstanding shares immediately after the issue of the shares that are to be issued upon the relevant exercise of the Warrant (rounded down to the nearest whole share) (the “Warrant Limit”). The Warrant remains outstanding for the remaining duration of its term even if exercised for a number of shares that is equal to the then applicable Warrant Limit. For clarity, the overall shareholding resulting from the full exercise of the Warrant shall in aggregate not exceed 29.9%. |
| f) | Nature of the Warrant: The Warrant will confer the right (but not the obligation) to subscribe, upon any exercise of the Warrant, for a number of new shares to be issued by the Company, as aforementioned. Except as otherwise provided for under Belgian law, the holder of the Warrant will be no shareholder of the Company solely by virtue of holding the Warrant, and |
| Galapagos NV | AGM/EGM 30 April 2024 | Convening Notice | Page 6 of 12 | |

Free translation from Dutch
For information purposes only
| therefore does not have the rights of a shareholder in relation to the shares to be issued or delivered to the holder of the Warrant upon an exercise of the Warrant until the exercise of the Warrant and the issue or delivery of the relevant shares. |
| g) | Form of the Warrant: The Warrant will be in registered form. |
| h) | No listing of the Warrant: The Warrant shall not be listed at any time on a securities exchange, regulated market or similar securities market. |
| i) | Allocation and subscription: The Warrant will be allocated to Gilead Therapeutics, and can only be subscribed for by Gilead Therapeutics. |
| j) | Underlying shares: Each new share to be issued by the Company upon each exercise of the Warrant shall be fully paid up and shall have the same rights and benefits as, and rank pari passu in all respects including as to entitlement to dividends and other distributions, with the existing and outstanding shares of the Company at the moment of their issue and will be entitled to dividends and other distributions in respect of which the relevant record date or due date falls on or after the date of their issue. |
The shareholders’ meeting resolves, subject to, and to the extent of, each exercise of Warrant, to increase the Company’s share capital and to issue the relevant number of new shares issuable upon such exercise as provided for in the relevant Conditions of the Warrant.
The shareholders’ meeting resolves that any issue premium that will be booked in connection with the exercise of the Warrant and the issuance of new shares, as applicable, shall be accounted for on the liabilities side of the Company’s balance sheet as net equity. The account on which the issue premium shall be booked shall, like the share capital, serve as the guarantee for third parties and, save for the possibility of a capitalization of those reserves, can only be reduced on the basis of a valid resolution of the general shareholders’ meeting passed in the manner required for an amendment to the Company’s articles of association.
The shareholders’ meeting of the Company resolves to authorize the Board of Directors to implement and execute the resolutions passed by the shareholders’ meeting of the Company in connection with the Warrant, and to take all steps and carry out all formalities that shall be required by virtue of the Conditions of the Warrant, the Company’s articles of association and applicable law in order to issue or transfer shares upon an exercise of Warrant. Furthermore, each of the General Counsel of the Company and the directors of the Company (each such person, a “Special Proxy Holder”), acting individually and with possibility of sub-delegation and the power of subrogation, shall have the power, upon each exercise of the Warrant, to proceed with the recording of (i) the capital increase and issue of new shares resulting from such exercise, (ii) the allocation of the issue price to the share capital and (as applicable) the issue premium in accordance with the relevant Conditions of the Warrant, (iii) the amendment of the Company’s articles of association in order to reflect the new share capital and number of outstanding shares following the exercise of the Warrant and the issuance of new shares. Finally, each Special Proxy Holder, acting individually and with possibility of sub-delegation and the power of subrogation, shall also have the power, upon an exercise of a Warrant, (a) to sign and deliver, on behalf of the Company, the relevant Euroclear, Euronext, and bank documentation, the share register and all necessary documents in connection with the issuance and delivery of the shares (acquired as a result of the exercise of the Warrant) to the beneficiary and (b) to do whatever may be necessary or useful (including but not limited to the preparation and execution of all documents and forms) for the admission of the shares issued upon an exercise of the Warrant to trading on the regulated markets of Euronext Brussels and Euronext Amsterdam (and such other regulated markets on which the Company’s shares will be trading at that time).
| 4. | Consideration and discussion of the report of the Board of Directors in accordance with article 7:199 of the Belgian Companies and Associations Code relating to the renewal of its authorization with respect to, and the increase of, the authorized capital, and the specific circumstances and purposes for the use of the renewed authorized capital. |
| Galapagos NV | AGM/EGM 30 April 2024 | Convening Notice | Page 7 of 12 | |

Free translation from Dutch
For information purposes only
| 5. | Renewal of the authorization to the Board of Directors to increase the share capital within the framework of the authorized capital by up to 20% of the share capital. |
This proposal is made in accordance with the terms of the aforementioned Subscription Agreement, which included a commitment to make a proposal to the shareholders’ meeting to authorize the Board of Directors to increase the share capital of the Company in one or several times with an amount up to 20% of the share capital at the time of the convening of the shareholders’ meeting. The current authorisation was approved by the Company’s extraordinary shareholders’ meeting on 22 October 2019 and will expire on 12 November 2024. The proposed resolution will renew the aforementioned authorisation (as agreed in the Subscription Agreement).
Proposed resolution: The shareholders’ meeting of the Company resolves to renew the authorization to the Board of Directors to increase the share capital on one or more occasions, during a period of five (5) years as of the publication in the Annexes to the Belgian State Gazette of this authorization, with an aggregate amount equal to up to 20% of the current amount of the share capital of the Company, and this in accordance with the terms and conditions set forth in the report of the Board of Directors prepared in accordance with article 7:199 of the Belgian Companies and Associations Code, as referred to in item 4 of the agenda of the Extraordinary Shareholders’ Meeting. Consequently, the shareholders’ meeting resolves to delete the section “Authorized Capital” of the temporary provisions of the articles of association of the Company entirely and to replace it with the following text (whereby the amount of 20 percent of the subscribed capital referred to below between square brackets shall be determined on the basis of the outstanding subscribed capital at that time):
“Authorized capital
The Board of Directors has been granted the authority to increase the subscribed capital of the company, in accordance with applicable law, in one or several times, to the extent set forth hereafter. This authorization is valid for a period of five years from the date of publication of this authorization in the Annexes to the Belgian State Gazette.
Without prejudice to more restrictive rules set forth by law, the Board of Directors can increase the subscribed capital of the company in one or several times with an amount of up to EUR [•], i.e. 20 percent of the subscribed capital at the time of the convening of the shareholders’ meeting granting this authorization. In accordance with applicable law, the Board of Directors cannot use the aforementioned authorization after the Financial Services and Markets Authority (FSMA) has notified the company of a public takeover bid for the company’s shares.
The capital increases within the framework of the authorized capital may be achieved by the issuance of shares (below, above or at the fractional value of the existing shares, with or without voting rights, and as the case may be in the context of a subscription rights plan for the company’s or its subsidiaries’ members of the personnel within the meaning of article 1:27 of the Belgian Companies and Associations Code (including members of the Board of Directors and/or independent consultants)), convertible bonds and/or subscription rights exercisable by contributions in cash or in kind, with or without issuance premium, and also by the conversion of reserves, issuance premiums, profits carried forward or other equity components. Aforementioned subscription rights plans can provide that, in exceptional circumstances (among others in the event of a change in control of the company or decease), subscription rights can be exercised before the third anniversary of their award, even if the beneficiary of such subscription right is a member of the Board of Directors or a person entrusted with the day-to-day management.
When increasing the subscribed capital within the limits of the authorized capital, the Board of Directors may, in the company’s interest, restrict or cancel the shareholders’ statutory preferential subscription rights, even if such restriction or cancellation is made for the benefit of one or more specific persons other than the company’s or its subsidiaries’ members of the personnel within the meaning of article 1:27 of the Belgian Companies and Associations Code.
| Galapagos NV | AGM/EGM 30 April 2024 | Convening Notice | Page 8 of 12 | |

Free translation from Dutch
For information purposes only
The Board of Directors can ask for an issuance premium when issuing new shares in the framework of the authorized capital. If the Board of Directors decides to do so, such issuance premium is to be booked on a non-available reserve account that can only be reduced or transferred by a decision of the shareholders’ meeting adopted in the manner required for amending the articles of association.
The Board of Directors is authorized to bring the company’s articles of association in line with the capital increases which have been decided upon within the framework of the authorized capital, or to instruct a notary public to do so.”.
| 6. | Proxy for coordination. |
Proposed resolution: The shareholders’ meeting resolves to authorize each collaborator of undersigned notary or notary Matthieu Derynck to draw up, sign and file the coordinated text of the Company’s articles of association in the electronic database provided for that purpose under the applicable laws.
| 7. | Authorization to the Board of Directors. |
Proposed resolution: The shareholders’ meeting resolves to grant all powers to the Company’s Board of Directors to execute the decisions taken.
| 8. | Proxy for the Crossroad Bank for Enterprises, counters for enterprises, registers of the enterprise court, administrative agencies and fiscal administrations. |
Proposed resolution: The shareholders’ meeting resolves to grant a special power of attorney to any member of the Board of Directors and/or Mrs. Valeria Cnossen, Mrs. Annelies Denecker, Mrs. Elien van Mol and Mr. Stefan Mees, who – for the execution of this proxy – are all electing domicile at Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium, each acting separately and each with individual power of substitution and sub-delegation, to fulfill all formalities and/or sign all documents that must be fulfilled or signed in the name of or on behalf of the Company pursuant to or in the framework of the foregoing, including, but not limited to, the completion of all necessary formalities with the Crossroad Bank for Enterprises, counters for enterprises, registers of the enterprise court, administrative agencies and fiscal administrations with respect to the decisions taken at the present meeting.
Attendance quorum: According to the Belgian Companies and Associations Code, at least 50% of the outstanding shares must be present or represented at the Extraordinary Shareholders’ Meeting for the deliberation and voting on the agenda items of the aforementioned agenda of the Extraordinary Shareholders’ Meeting. If such attendance quorum is not reached, a second Extraordinary Shareholders’ Meeting will be convened for these agenda items, unless, as the case may be, decided otherwise on behalf of the Board of Directors, and the attendance quorum requirement will not apply to such second meeting.
Voting and majority: Subject to applicable legal provisions, each share shall have one vote. In accordance with applicable law, the proposed resolutions referred to in the aforementioned agenda of the Extraordinary Shareholders’ Meeting shall be passed if they are approved by a majority of 75% of the votes validly cast by the shareholders.
Registration and admission formalities
To be admitted to the Shareholders’ Meetings, the holders of securities issued by the Company must comply with (a) article 7:134 of the Belgian Companies and Associations Code, and (b) article 23 of the Company’s articles of association, as well as complete the formalities and make the notifications further described below.
Only shareholders are entitled to vote at the Shareholders’ Meetings. The holders of subscription rights issued by the Company can, in accordance with article 7:135 of the Belgian Companies and Associations Code, only attend the Shareholders’ Meetings with an advisory vote. Article 23 of the Company’s articles of association determines the formalities that these security holders must complete to be admitted to the Shareholder’ Meetings.
| Galapagos NV | AGM/EGM 30 April 2024 | Convening Notice | Page 9 of 12 | |

Free translation from Dutch
For information purposes only
In order to be able to participate to the Shareholders’ Meetings, a holder of shares or subscription rights issued by the Company must satisfy two conditions: (i) be registered as holder of such shares or subscription rights on the Record Date (as defined below), and (ii) notify the Company thereof, as described below:
| 1 | Registration: Firstly, the right for a holder of shares or subscription rights issued by the Company to participate to and, as applicable, to vote at the Shareholders’ Meetings is only granted on the basis of the registration of the securities concerned on 16 April 2024 at midnight (24:00) (Belgian time) (hereinafter the “Record Date”), via registration, in the applicable register book for the securities concerned (for registered securities) or in the accounts of a certified account holder or the relevant central securities depository for the securities concerned (for dematerialised securities), and this irrespective of the number of shares or subscription rights that they hold on the date of the Shareholders’ Meetings. |
| 2 | Notification: Secondly, in order to be admitted to the Shareholders’ Meetings, the holders of shares or subscription rights issued by the Company must notify the Company in writing that they have the intention to participate to the meeting and must do so prior to or at the latest on at the latest on 24 April 2024. The holders of securities that wish to make such notification can make use of the registration notice form that can be obtained at the Company’s registered office and on the Company’s website (www.glpg.com/shareholders-meetings). The notice must reach the Company by e-mail at shareholders@glpg.com or by mail at its registered office (Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium, Attention: Ms. Annelies Denecker) at the latest on the sixth calendar day prior to the Shareholders’ Meetings, i.e., on or before 24 April 2024 at the latest. For the holders of dematerialised shares, the notification should include a certificate confirming the number of shares that have been registered in their name on the Record Date. The certificate can be obtained by the holders of the dematerialised shares with the certified account holder, the relevant central securities depository, or the relevant financial intermediary for the shares concerned. |
Only persons who are a holder of shares or subscription rights of the Company on the Record Date and have indicated their intention to participate in the Shareholders’ Meetings as set out above, will be entitled to participate in, and, in the case of shares, vote at, the Shareholders’ meetings.
Proxy
In accordance with article 24 of the Company’s articles of association, shareholders having complied with the registration and admission formalities set out above, may be represented at the Shareholders’ Meetings by the General Counsel of the Company, with power of substitution, in her capacity as proxy holder, provided that the proxy does contain specific voting instructions for each item on the agenda of the Shareholders’ Meetings.
Shareholders who so wish to be represented by proxy, should use the proxy form (with voting instructions) made available on the Company’s website (www.glpg.com/shareholders-meetings). The signed proxy form must be submitted to Galapagos by e-mail (shareholders@glpg.com) or by mail (Galapagos NV, attn.: Ms. Annelies Denecker, Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium), and must reach Galapagos by no later than 24 April 2024.
Voting by letter
In accordance with article 7:146 of Belgian Companies and Associations Code, and article 24 of the Company’s articles of association, shareholders having complied with the registration and admission formalities set out above, are allowed to vote by letter - prior to the Shareholders’ Meetings - by means of a form made available on the Company’s website (www.glpg.com/shareholders-meetings).
| Galapagos NV | AGM/EGM 30 April 2024 | Convening Notice | Page 10 of 12 | |

Free translation from Dutch
For information purposes only
The signed form must be submitted to Galapagos by e-mail (shareholders@glpg.com) or by mail (Galapagos NV, attn.: Ms. Annelies Denecker, Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium), and must reach Galapagos by no later than 24 April 2024.
Right to ask questions
In accordance with article 7:139 of the Belgian Companies and Associations Code, and article 28 of the articles of association of the Company, shareholders and holders of subscription rights issued by the Company, are entitled, whether during the Shareholders’ Meetings or in writing before the meeting, to ask questions to (a) the members of the Board of Directors with respect to its report, or the agenda items, and (b) the statutory auditor with respect to its report.
Questions asked in writing, will only be answered if the relevant shareholder, or holder of subscription rights, has fulfilled the registration and admission formalities set out above, and if the written question has been received by the Company at the latest on 24 April 2024. Any written questions must be submitted to Galapagos by e-mail (shareholders@glpg.com) or by mail (Galapagos NV, attn.: Ms. Annelies Denecker, Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium).
Right to add agenda items
In accordance with article 7:130 of the Belgian Companies and Associations Code, and article 22 of the articles of association of the Company, one or more shareholders who alone or together possess at least 3% of the share capital of the Company, and provided that the applicable legal restrictions have been complied with, may request for additional items to be added to the agenda of the Shareholders’ Meetings, and submit proposed resolutions in relation to existing agenda items or new items to be added to the agenda. Such request, along with proof of ownership of the required participation, and, as the case may be, the text of the items to be dealt with and related proposed resolutions, must be submitted to Galapagos by e-mail (shareholders@glpg.com) or by mail (Galapagos NV, attn.: Ms. Annelies Denecker, Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium), and must reach Galapagos by no later than 8 April 2024. As the case may be, the Company will publish a modified agenda for the Shareholders’ Meetings at the latest on 15 April 2024.
Availability of documents
The documentation relating to the Shareholders’ Meetings, or that must be made available pursuant to the applicable laws, as well as the total number of shares and voting rights at the date hereof, are made available on the Company’s website (www.glpg.com/shareholders-meetings). Any hard copies of this documentation can be obtained at no cost by e-mail (shareholders@glpg.com) or by mail (Galapagos NV, attn.: Ms. Annelies Denecker, Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium).
Please address any requests for more information to the Legal department of Galapagos (+32 15 342 900). Correspondence can be sent by e-mail (shareholders@glpg.com) or by mail (Galapagos NV, attn.: Ms. Annelies Denecker, Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium).
| Galapagos NV | AGM/EGM 30 April 2024 | Convening Notice | Page 11 of 12 | |

Free translation from Dutch
For information purposes only
Miscellaneous
To facilitate an expedient registration, the participants are requested to be present at least 15 minutes prior to the start of the Shareholders’ Meetings.
The convening notice for the Shareholders’ Meetings is drafted in Dutch and English, it being however understood that (i) the English version of the convening notice is only a free translation for information purposes, and (ii) in case of an inconsistency between the Dutch and English version of the convening notice, the Dutch version will at all times prevail.
The natural persons who intend to attend the Shareholders’ Meetings in their capacity as holder of securities, proxy holders, or representatives of a legal entity, must provide evidence of their identity in order to be granted access to the meeting. In addition, the representatives of legal entities must hand over all documents establishing their capacity as corporate representative or attorney-in-fact. These documents will be verified immediately before the start of the Shareholders’ Meetings.
Data protection
The Company is responsible for the processing of personal data it receives from, or collects about, holders of securities issued by the Company and proxy holders in the context of shareholders’ meetings. The processing of such data will be carried out for the purposes of the organization and conduct of the relevant shareholders’ meeting, including (without limitation) the convening notices, registrations, attendance and voting, as well as for maintaining lists or registers of security holders, and the analysis of the investor and security holder base of the Company. These data include, amongst others, identification data, the number and nature of securities of any holder of securities issued by the Company, proxies and voting instructions. These data may also be transferred to third parties for the purposes of assistance or services to the Company in connection with the foregoing. The processing of the above data will be carried out, mutatis mutandis, in accordance with the Company’s Privacy Statement, available on the Company’s website (www.glpg.com/privacy-notice). The Company draws the attention of the holders of securities issued by the Company and proxy holders to the description of the rights they may have as data subjects, such as, among others, the right to access, the right to rectify and the right to object to processing, which are further outlined in the section ‘Your rights as a data subject’ of the aforementioned Privacy Statement. All this does not affect the rules that apply in connection with the registration and participation to the shareholders’ meeting. To exercise rights as a data subject, as well as for all other information regarding the processing of personal data by or on behalf of the Company, the Company can be contacted by e-mail at dpo@glpg.com.
On behalf of the Board of Directors
| Galapagos NV | AGM/EGM 30 April 2024 | Convening Notice | Page 12 of 12 | |
Exhibit 99.4

Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 Volmacht Proxy (enkel te gebruiken door aandeelhouders) (to be used by shareholders only) De ondergetekende: The undersigned: (Naam en adres / Name and address) hierin optredend als lastgever, herein acting as grantor of a proxy, eigenaar van het volgende aantal aandelen in Galapagos NV owner of the following number of shares in Galapagos NV (public (naamloze vennootschap naar Belgisch recht, met zetel te limited liability company organized under the laws of Belgium, Generaal De Wittelaan L11 A3, 2800 Mechelen, België en with registered office at Generaal De Wittelaan L11 A3, 2800 ingeschreven in het Rechtspersonenregister (RPR Antwerpen, Mechelen, Belgium and registered with the Register of Legal afdeling Mechelen) onder het nummer 0466.460.429) (de Entities (RLE Antwerp, division Mechelen) under the number “Vennootschap”): 0466.460.429) (the “Company”): (Aantal aandelen / Number of shares) stelt hiermee aan tot zijn / haar bijzondere volmachtdrager, met herewith appoints as his / her / its special proxy holder, with recht van indeplaatsstelling: power of substitution: Valeria Cnossen, General Counsel van/of Galapagos NV, p/a Generaal De Wittelaan L11 A3, 2800 Mechelen, België/Belgium of de volgende persoon: / or the following person: (Naam en adres van de gevolmachtigde / Name and address of the proxy holder) hierna de “volmachtdrager”, hereinafter the “proxy holder”, aan wie hij / zij volmacht geeft tot bijwoning van, en uitoefening van de stemrechten op, de Gewone en Buitengewone Algemene Vergaderingen van de Vennootschap, die zullen worden gehouden op dinsdag 30 april 2024, om 14.00 uur (Belgische tijd) en op de zetel van de Vennootschap, alsook elke andere Algemene Vergadering met dezelfde agenda die daarna zou worden bijeengeroepen als gevolg van verdaging of uitstel, in naam en voor rekening van de ondergetekende, met als bedoeling om de hierna uiteengezette agenda in overweging te nemen en, voor dit doel, deel te nemen aan de beraadslaging, te stemmen ofwel zich te onthouden, alle aanwezigheidslijsten, notulen of enig andere stukken te ondertekenen, woonplaats te kiezen, subdelegatie te geven, en meer in het algemeen te doen wat nuttig of nodig wordt geacht door de volmachtdrager voor de uitvoering van deze volmacht. Ingeval dat, overeenkomstig artikel 7:130 van het Wetboek van Vennootschappen en Verenigingen nieuw te behandelen onderwerpen op de agenda zijn opgenomen nadat onderhavige volmacht ter kennis van de Vennootschap is gebracht, zal de volmachtdrager ook over deze nieuwe agendapunten kunnen stemmen voor zover de volmachtdrager daarbij geen ander belang dan het belang van ondergetekende aandeelhouder nastreeft. De volmachtformulieren die de Vennootschap bereiken voorafgaand aan de publicatie van de gewijzigde agenda of voorstellen van besluit, blijven geldig voor de agendapunten to whom he / she / it gives power of attorney to attend, as well as to exercise voting rights, at the Annual and Extraordinary Shareholders’ Meeting of the Company, to be held on Tuesday 30 April 2024, at 2.00 p.m (Belgian time) and at the registered office of the Company, as well as at any other Shareholders’ Meeting with the same agenda that may be convened subsequently as a result of adjournment or delay, in the name of and on behalf of the undersigned, for the purpose of considering the agenda set forth below and, for this purpose, to participate in the deliberation, to vote or abstain, to sign all attendance lists, minutes or any other documents, to elect domicile, to sub-delegate authority, and more generally to do anything the proxy holder deems useful or necessary for the exercise of this proxy. If, pursuant to article 7:130 of the Belgian Companies and Associations Code, new items to be dealt with are included in the agenda after the present proxy form has been submitted to the Company, the proxy holder shall be entitled to vote on such new agenda items insofar as the proxy holder, by doing so, does not pursue another interest than the interest of the undersigned shareholder. Proxies that reach the Company prior to the publication of the amended agenda or proposed resolutions remains valid for the agenda items to which the proxies apply, subject, however, to applicable law.

Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 waarop de volmachten betrekking hebben, doch evenwel onder voorbehoud van de toepasselijke wetgeving. Deze volmacht is geen verzoek tot verlening van volmacht This proxy does not constitute a proxy solicitation in accordance overeenkomstig de bepalingen van artikelen 7:144 of 7:145 van het with the provisions of articles 7:144 or 7:145 of the Belgian Wetboek van Vennootschappen en Verenigingen. Companies and Associations Code. Deze volmacht dient handgeschreven of elektronisch te worden This proxy should be signed either in handwriting or electronically. ondertekend. Indien gebruik wordt gemaakt van de mogelijkheid If the opportunity to sign this proxy form electronically is made use om dit formulier elektronisch te tekenen, dient het te gaan om een of, it must be an electronic signature within the meaning of article elektronische handtekening in de zin van artikel 3.10 van 3.10 of Regulation (EU) No 910/2014 of the European Parliament Verordening (EU) Nr. 910/2014 van het Europees Parlement en de and of the Council of 23 July 2014 on electronic identification and Raad van 23 juli 2014 betreffende elektronische identificatie en trust services for electronic transactions in the internal market and vertrouwensdiensten voor elektronische transacties in de interne repealing Directive 1999/93/EC, as amended, or a qualified markt en tot intrekking van Richtlijn 1999/93/EG, zoals gewijzigd, electronic signature within the meaning of article 3.12 of the same hetzij een gekwalificeerde elektronische handtekening in de zin Regulation. van artikel 3.12 van dezelfde Verordening. Agenda Gewone Algemene Vergadering Annual Shareholders’ Meeting 1. Kennisname en bespreking van (a) het jaarverslag van de Raad 1. Acknowledgement and discussion of (a) the annual report of van Bestuur betreffende de statutaire en geconsolideerde the Board of Directors in relation to the non-consolidated and jaarrekening van de Vennootschap over het boekjaar afgesloten consolidated annual accounts of the Company for the financial op 31 december 2023, en van (b) het verslag van de commissaris year ended on 31 December 2023, and (b) the report of the over de statutaire jaarrekening van de Vennootschap over het statutory auditor in relation to the non-consolidated annual boekjaar afgesloten op 31 december 2023. accounts of the Company for the financial year ended on 31 December 2023. 2. Kennisname en goedkeuring van de statutaire jaarrekening 2. Acknowledgement and approval of the non-consolidated van de Vennootschap over het boekjaar afgesloten op 31 annual accounts of the Company for the financial year ended on december 2023 en goedkeuring van de door de Raad van Bestuur 31 December 2023, and approval of the allocation of the annual voorgestelde bestemming van het jaarresultaat. result as proposed by the Board of Directors. Voorstel van besluit: De algemene vergadering besluit de Proposed resolution: The shareholders’ meeting resolves to statutaire jaarrekening van de Vennootschap over het boekjaar approve the non-consolidated annual accounts of the Company for afgesloten op 31 december 2023, goed te keuren, alsmede de door the financial year ended on 31 December 2023, and the allocation de Raad van Bestuur voorgestelde bestemming van het of the annual result as proposed by the Board of Directors. jaarresultaat goed te keuren. Agendapunt 2—Steminstructie: Agenda item 2—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention 3. Kennisname en bespreking van het verslag van de commissaris 3. Acknowledgement and discussion of the report of the statutory betreffende de geconsolideerde jaarrekening van de auditor relating to the consolidated annual accounts of the Vennootschap over het boekjaar afgesloten op 31 december Company for the financial year ended on 31 December 2023. 2023. 4. Kennisname en bespreking van de geconsolideerde 4. Acknowledgement and discussion of the consolidated annual jaarrekening van de Vennootschap over het boekjaar afgesloten accounts of the Company for the financial year ended on 31 op 31 december 2023. December 2023. 5. Kennisname en goedkeuring van het remuneratieverslag. 5. Acknowledgement and approval of the remuneration report. Voorstel van besluit: De algemene vergadering besluit om het Proposed resolution: The shareholders’ meeting resolves to remuneratieverslag, opgenomen in het jaarverslag van de Raad approve the remuneration report included in the annual report of van Bestuur over het boekjaar afgesloten op 31 december 2023, the Board of Directors for the financial year ended on 31 December goed te keuren. 2023. Agendapunt 5—Steminstructie: Agenda item 5—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention
Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 6. Kennisname en goedkeuring van het gewijzigde 6. Acknowledgement and approval of the amended remuneratiebeleid. remuneration policy. Voorstel van besluit: De algemene vergadering besluit om het Proposed resolution: The shareholders’ meeting resolves to gewijzigde remuneratiebeleid goed te keuren. approve the amended remuneration policy. Agendapunt 6—Steminstructie: Agenda item 6—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention 7. Kwijting aan de (huidige en voormalige) leden van de Raad van 7. Release from liability to be granted to the (current and former) Bestuur en de (huidige en voormalige) commissaris voor de members of the Board of Directors, and the (current and former) uitoefening van hun respectievelijk mandaat uitgeoefend tijdens statutory auditor for the performance of their respective het boekjaar afgesloten op 31 december 2023. mandates during the financial year ended on 31 December 2023. Voorstel van besluit: De algemene vergadering besluit, bij Proposed resolution: The shareholders’ meeting resolves, by a afzonderlijke stemming, om kwijting te geven aan elk (huidig en separate vote, to release each (current and former) member of the voormalig) lid van de Raad van Bestuur, en aan de (huidige en Board of Directors, and the (current and former statutory) auditor voormalige) commissaris voor alle aansprakelijkheid from any liability arising from the performance of their respective voortvloeiend uit de uitoefening van hun respectieve mandaten mandates during the financial year ended on 31 December 2023. gedurende het boekjaar afgesloten op 31 december 2023. Agendapunt 7—Steminstructie: Agenda item 7—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention 8. Bezoldiging van bestuurders. 8. Remuneration of directors. Voorstel van besluit: Op aanbeveling en advies van het Proposed resolution: Upon recommendation and advice of the Remuneratiecomité van de Vennootschap, besluit de algemene Remuneration Committee of the Company, the shareholders’ vergadering dat de jaarlijkse bezoldiging (exclusief meeting resolves that the annual compensation (excluding onkostenvergoeding) van de niet-uitvoerende bestuurders, andere expenses) of the non-executive directors, other than the non-dan de niet-uitvoerende bestuurders die een aandeelhouder executive directors representing a shareholder, for the exercise of vertegenwoordigen, voor de uitoefening van hun mandaat zal their mandate shall consist of a cash remuneration and an bestaan uit een bezoldiging in contanten en een bezoldiging op equity-based remuneration as follows: basis van aandelen als volgt vast te stellen: (a) cash remuneration: (i) Chairperson of the Board of Directors: (a) vergoeding in contanten: (i) Voorzitter van de Raad van EUR 110,000; (ii) Lead Non-Executive Director: EUR 75,000, (iii) Bestuur: EUR 110.000; (ii) Lead Non-Executive Director: EUR 75.000; other non-executive directors: EUR 55,000 each; (iv) additional (iii) andere niet-uitvoerende bestuurders: elkeen EUR 55.000, (iv) annual compensation for the chairpersonship of a committee jaarlijkse bijkomende bezoldiging voor het voorzitterschap van within the Board of Directors: EUR 20,000; and (v) additional een comité binnen de Raad van Bestuur: EUR 20.000; en (v) annual compensation for the membership of a committee within jaarlijkse bijkomende bezoldiging voor lidmaatschap van een the Board of Directors: EUR 15,000; comité binnen de Raad van Bestuur: EUR 15.000; (b) equity-based remuneration: (i) Chairperson of the Board of (b) vergoeding op basis van aandelen: (i) Voorzitter van de Raad Directors: EUR 110,000; (ii) Lead Non-Executive Director: EUR van Bestuur: EUR 110.000; (ii) Lead Non-Executive Director: EUR 75,000; (iii) other non-executive directors: EUR 55,000 each; in 75.000; (iii) andere niet-uitvoerende bestuurders: elkeen EUR each case (i), (ii) and (iii) subject to the requirement to use the net 55.000; in elk van (i), (ii) en (iii), onder de verplichting om het amount (after taxes) to acquire Galapagos shares. These latter nettobedrag (na belastingen) te gebruiken voor de verwerving van payments make up the equivalent of an equity component of the Galapagos aandelen. Deze laatste betalingen vormen het directors’ remuneration and the resulting shares are to be held equivalent van een bestuurdersbezoldiging in aandelen en de until at least one year after the non-executive director leaves the resulterende aandelen dienen te worden aangehouden tot ten Board of Directors and at least three years after the time of minste één jaar nadat de niet-uitvoerende bestuurder de Raad van acquisition. Bestuur heeft verlaten en ten minste drie jaar na het tijdstip van de The abovementioned remuneration for the Chairperson of the verwerving. Board of Directors will only be payable if such Chairperson is not De bovenvermelde bezoldiging voor de Voorzitter van de Raad van simultaneously Chief Executive Officer (CEO) of the Company. The Bestuur is enkel betaalbaar indien deze Voorzitter niet tegelijk abovementioned remuneration for the Lead Non-Executive Chief Executive Officer (CEO) is van de Vennootschap. De Director will only be payable to the extent appointed in bovenvermelde vergoeding voor de Lead Non-Executive Director is accordance with Galapagos’ Corporate Governance Charter. enkel betaalbaar voor zover geldig benoemd overeenkomstig The shareholders’ meeting resolves that the mandate of a non-

Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 Galapagos’ Corporate Governance Charter. executive director representing a shareholder will not be De algemene vergadering besluit dat het mandaat van een niet- remunerated. uitvoerend bestuurder die een aandeelhouder vertegenwoordigt, The rules set out above shall apply as per 1 May 2024. niet zal worden bezoldigd. De hierboven uiteengezette regels zijn van toepassing vanaf 1 mei 2024. Agendapunt 8—Steminstructie: Agenda item 8—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention 9. Herbenoeming van Dr. Elisabeth Svanberg als onafhankelijk 9. Re-appointment of Dr. Elisabeth Svanberg as independent non-niet-uitvoerend bestuurder. executive director. Voorstel van besluit: Op voorstel van de Raad van Bestuur, en in Proposed resolution: Upon proposal of the Board of Directors, and overeenstemming met de aanbeveling en het advies van het in accordance with the recommendation and advice of the Benoemingscomité, besluit de algemene vergadering (a) om Dr. Nomination Committee, the shareholders’ meeting resolves (a) to Elisabeth Svanberg te herbenoemen als onafhankelijk niet- re-appoint Dr. Elisabeth Svanberg as an independent non-uitvoerend lid van de Raad van Bestuur van de Vennootschap, voor executive member of the Board of Directors of the Company, for a een termijn van vier jaar vanaf heden, die een einde zal nemen period of four years, effective as of today, ending immediately after onmiddellijk na de gewone algemene vergadering te houden in the annual shareholders’ meeting to be held in 2028, and (b) to 2028, en (b) haar mandaat te bevestigen in diens hoedanigheid als confirm her mandate as an independent member of the Board of onafhankelijk lid van de Raad van Bestuur doordat (i) Dr. Elisabeth Directors since (i) Dr. Elisabeth Svanberg meets the independence Svanberg beantwoordt aan de onafhankelijkheidscriteria van het criteria set forth in article 7:87 of the Belgian Companies and artikel 7:87 van het Wetboek van Vennootschappen en Associations Code, and article 3.5 of the Belgian Corporate Verenigingen en bepaling 3.5 van de Belgische Corporate Governance Code 2020, (ii) Dr. Elisabeth Svanberg has explicitly Governance Code 2020, (ii) Dr. Elisabeth Svanberg uitdrukkelijk declared not to have (and the Board of Directors is not aware of) heeft verklaard geen (en de Raad van Bestuur evenmin op de any connections with the Company or a major shareholder, which hoogte is van) banden met de Vennootschap of een belangrijke would interfere with her independence, and (iii) the Board of aandeelhouder te hebben, die diens onafhankelijkheid in het Directors has no indication of any element that might call Dr. gedrang zou brengen, en (iii) de Raad van Bestuur geen indicatie Elisabeth Svanberg’s independence into question. The heeft van enig element dat de onafhankelijkheid van Dr. Elisabeth shareholders’ meeting also resolves that the mandate of Dr. Svanberg in twijfel zou kunnen trekken. De algemene vergadering Elisabeth Svanberg is remunerated as provided for non-executive besluit dat het mandaat van Dr. Elisabeth Svanberg wordt vergoed members of the Board of Directors in the Company’s remuneration zoals voorzien voor niet-uitvoerende leden van de Raad van policy and as approved by the shareholders’ meeting from time to Bestuur in het remuneratiebeleid van de Vennootschap en zoals time. goedgekeurd door de algemene vergadering van tijd tot tijd. Agendapunt 9—Steminstructie: Agenda item 9—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention 10. Benoeming van Dr. Susanne Schaffert als 10. Appointment of Dr. Susanne Schaffert as independent onafhankelijk niet-uitvoerend bestuurder. non-executive director. Rekening houdend met de aanbeveling en het advies van het Taking into account the recommendation and advice of the Benoemingscomité, beveelt de Raad van Bestuur aan om de Nomination Committee, the Board of Directors recommends that benoeming van Dr. Susanne Schaffert als onafhankelijk niet- the appointment of Dr. Susanne Schaffert as independent non-uitvoerende bestuurder van de Vennootschap te bevestigen en executive director of the Company be confirmed and continued voort te zetten voor een bijkomende termijn van vier jaar. Voor for an additional term of four years. For further information meer informatie over Dr. Susanne Schaffert wordt verwezen naar regarding Dr. Susanne Schaffert, reference is made to the de verklaring inzake corporate governance opgenomen in het corporate governance statement included in the the annual jaarverslag van de Raad van Bestuur over de (niet- report of the Board of Directors on the (non-consolidated) geconsolideerde) statutaire jaarrekening van de Vennootschap statutory financial statements of the Company for the financial voor het boekjaar afgesloten op 31 december 2023. In het year ended on 31 December 2023. Notably, on 12 June 2023, the bijzonder, heeft de Raad van Bestuur op 12 juni 2023 Dr. Susanne Board of Directors appointed Dr. Susanne Schaffert as Schaffert benoemd tot on af hankelijk niet-uitvoerend bestuurder independent non-executive director of the Company by co-van de Vennootschap door coöptatie na het aftreden op 10 juni optation following the resignation on 10 June 2023 of Dr. Rajesh 2023 van Dr. Rajesh Parekh als niet-uitvoerend bestuurder, die Parekh as non-executive director, who was appointed for a term werd benoemd voor een termijn tot en met de sluiting van de up to and including the closing of the annual shareholders’

Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 jaarlijkse aandeelhoudersvergadering te houden in 2025 die zal meeting to be held in 2025 which will have decided upon the hebben besloten over de jaarrekening voor het boekjaar financial statements for the financial year ended on 31 December eindigend op 31 december 2024. 2024. Voorstel van besluit: Op voorstel van de Raad van Bestuur, en in Proposed resolution: Upon proposal of the Board of Directors, and overeenstemming met de aanbeveling en het advies van het in accordance with the recommendation and advice of the Benoemingscomité van de Vennootschap, besluit de algemene Nomination Committee, the shareholders’ meeting resolves (a) to vergadering (a) om de benoeming door coöptatie op 12 juni 2023 confirm the appointment by co-optation on 12 June 2023, following te bevestigen, volgend op het aftreden van Dr. Rajesh Parekh op 10 the resignation of Dr. Rajesh Parekh on 10 June 2023, and to juni 2023, en om Dr. Susanne Schaffert te benoemen als appoint Dr. Susanne Schaffert as an independent non-executive onafhankelijk niet-uitvoerend lid van de Raad van Bestuur van de member of the Board of Directors of the Company, for an additional Vennootschap, voor een bijkomende termijn van vier jaar vanaf period of four years, up to and including the closing of the annual heden, die een einde zal nemen onmiddellijk na de gewone shareholders’ meeting to be held in 2028 which will have decided algemene vergadering te houden in 2028, dewelke zal hebben upon the financial statements for the financial year ended on 31 besloten over de jaarrekening voor het boekjaar eindigend op 31 December 2027, and (b) to confirm her mandate as an independent december 2027, en om (b) haar mandaat in diens hoedanigheid als member of the Board of Directors since (i) Dr. Susanne Schaffert onafhankelijk lid van de Raad van Bestuur te bevestigen doordat meets the independence criteria set forth in article 7:87 of the (i) Dr. Susanne Schaffert beantwoordt aan de Belgian Companies and Associations Code, and article 3.5 of the onafhankelijkheidscriteria van het artikel 7:87 van het Wetboek Belgian Corporate Governance Code 2020, (ii) Dr. Susanne van Vennootschappen en Verenigingen en bepaling 3.5 van de Schaffert has explicitly declared not to have (and the Board of Belgische Corporate Governance Code 2020, (ii) Dr. Susanne Directors is not aware of) any connections with the Company or a Schaffert uitdrukkelijk heeft verklaard geen (en de Raad van major shareholder, which would interfere with her independence, Bestuur evenmin op de hoogte is van) banden met de and (iii) the Board of Directors has no indication of any element that Vennootschap of een belangrijke aandeelhouder te hebben, die might call Dr. Susanne Schaffert’s independence into question. The diens onafhankelijkheid in het gedrang zou brengen, en (iii) de shareholders’ meeting also resolves that the mandate of Dr. Raad van Bestuur geen indicatie heeft van enig element dat de Susanne Schaffert is remunerated as provided for non-executive onafhankelijkheid van Dr. Susanne Schaffert in twijfel zou kunnen members of the Board of Directors in the Company’s remuneration trekken. De algemene vergadering beslist tevens dat het mandaat policy and as approved by the shareholders’ meeting from time to van Dr. Susanne Schaffert wordt vergoed zoals voorzien voor niet- time. uitvoerende leden van de Raad van Bestuur in het remuneratiebeleid van de Vennootschap en zoals goedgekeurd door de algemene vergadering van tijd tot tijd. Agendapunt 10—Steminstructie: Agenda item 10—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention 11. Benoeming van dhr. Simon Sturge als onafhankelijk niet- 11. Appointment of Mr. Simon Sturge as independent non-uitvoerend bestuurder. executive director. Rekening houdend met de aanbeveling en het advies van het Taking into account the recommendation and advice of the Benoemingscomité, beveelt de Raad van Bestuur aan om de Nomination Committee, the Board of Directors recommends that benoeming van dhr. Simon Sturge als onafhankelijk niet- the appointment of Mr. Simon Sturge as independent non-uitvoerende bestuurder van de Vennootschap te bevestigen en executive director of the Company be confirmed and continued voort te zetten voor een bijkomende termijn van vier jaar. Voor for an additional term of four years. For further information meer informatie over de heer Simon Sturge wordt verwezen naar regarding Mr. Simon Sturge, reference is made to the corporate de verklaring inzake corporate governance opgenomen in het governance statement included in the the annual report of the jaarverslag van de Raad van Bestuur over de (niet- Board of Directors on the (non-consolidated) statutory financial geconsolideerde) statutaire jaarrekening van de Vennootschap statements of the Company for the financial year ended on 31 voor het boekjaar afgesloten op 31 december 2023. In het December 2023. Notably, on 19 September 2023, the Board of bijzonder, heeft de Raad van Bestuur op 19 september 2023 de Directors appointed Mr. Simon Sturge as independent non-heer Simon Sturge benoemd tot onafhankelijk niet-uitvoerend executive director of the Company by co-optation following the bestuurder van de Vennootschap door coöptatie na het aftreden resignation on 18 September 2023 of Dr. Mary Kerr as non-op 18 september 2023 van Dr. Mary Kerr als niet-uitvoerend executive director, who was appointed for a term up to and bestuurder, die werd benoemd voor een termijn tot en met de including the closing of this annual shareholders’ meeting. sluiting van deze jaarlijkse aandeelhoudersvergadering. Voorstel van besluit: Op voorstel van de Raad van Bestuur, en in Proposed resolution: Upon proposal of the Board of Directors, and overeenstemming met de aanbeveling en het advies van het in accordance with the recommendation and advice of the Benoemingscomité van de Vennootschap, besluit de algemene Nomination Committee, the shareholders’ meeting resolves (a) to vergadering (a) om de benoeming door coöptatie op 19 september confirm the appointment by co-optation on 19 September 2023, 2023 te bevestigen, volgend op het aftreden van Dr. Mary Kerr op following the resignation of Dr. Mary Kerr on 18 September 2023, 18 september 2023, en om dhr. Simon Sturge te benoemen als and to appoint Mr. Simon Sturge as an independent non-executive onafhankelijk niet-uitvoerend lid van de Raad van Bestuur van de member of the Board of Directors of the Company, for an additional Vennootschap, voor een bijkomende termijn van vier jaar vanaf period of four years, up to and including the closing of the annual
Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 heden, die een einde zal nemen onmiddellijk na de gewone shareholders’ meeting to be held in 2028 which will have decided algemene vergadering te houden in 2028, dewelke zal hebben upon the financial statements for the financial year ended on 31 besloten over de jaarrekening voor het boekjaar eindigend op 31 December 2027, and (b) to confirm his mandate as an independent december 2027, en om (b) zijn mandaat in diens hoedanigheid als member of the Board of Directors since (i) Mr. Simon Sturge meets onafhankelijk lid van de Raad van Bestuur te bevestigen doordat the independence criteria set forth in article 7:87 of the Belgian (i) dhr. Simon Sturge beantwoordt aan de Companies and Associations Code, and article 3.5 of the Belgian onafhankelijkheidscriteria van het artikel 7:87 van het Wetboek Corporate Governance Code 2020, (ii) Mr. Simon Sturge has van Vennootschappen en Verenigingen en bepaling 3.5 van de explicitly declared not to have (and the Board of Directors is not Corporate Governance Code 2020, (ii) dhr. Simon Sturge aware of) any connections with the Company or a major uitdrukkelijk heeft verklaard geen (en de Raad van Bestuur shareholder, which would interfere with his independence, and (iii) evenmin op de hoogte is van) banden met de Vennootschap of een the Board of Directors has no indication of any element that might belangrijke aandeelhouder te hebben, die diens onafhankelijkheid call Mr. Simon Sturge’s independence into question. The in het gedrang zou brengen, en (iii) de Raad van Bestuur geen shareholders’ meeting also resolves that the mandate of Mr. Simon indicatie heeft van enig element dat de onafhankelijkheid van dhr. Sturge is remunerated as provided for non-executive members of Simon Sturge in twijfel zou kunnen trekken. De algemene the Board of Directors in the Company’s remuneration policy and vergadering beslist tevens dat het mandaat van dhr. Simon Sturge as approved by the shareholders’ meeting from time to time. wordt vergoed zoals voorzien voor niet-uitvoerende leden van de Raad van Bestuur in het remuneratiebeleid van de Vennootschap en zoals goedgekeurd door de algemene vergadering van tijd tot tijd. Agendapunt 11—Steminstructie: Agenda item 11—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention 12. Benoeming van de heer Andrew Dickinson tot niet-uitvoerend 12. Appointment of Mr. Andrew Dickinson as non-executive bestuurder. director. Rekening houdend met de aanbeveling en het advies van het Taking into account the recommendation and advice of the Benoemingscomité, beveelt de Raad van Bestuur aan de Nomination Committee, the Board of Directors recommends that benoeming van de heer Andrew Dickinson als niet-uitvoerend the appointment of Mr. Andrew Dickinson as non-executive bestuurder van de Vennootschap te bevestigen en voort te zetten director of the Company be confirmed and continued for an voor een bijkomende termijn van vier jaar. In het bijzonder, heeft additional term of four years. Notably, on 26 March 2024, and with de Raad van Bestuur, op 26 maart 2024, en met ingang vanaf 27 effect as per 27 March 2024, the Board of Directors appointed Mr. maart 2024, de heer Andrew Dickinson benoemd tot niet- Andrew Dickinson as non-executive director of the Company by uitvoerend bestuurder van de Vennootschap door coöptatie naar co-optation following the resignation on 26 March 2024 of Mr. aanleiding van het aftreden op 26 maart 2024 van de heer Daniel Daniel O’Day, as non-executive director, who was appointed for a O’Day, als niet-uitvoerend bestuurder, die werd benoemd voor term up to and including the closing of the annual shareholders’ een termijn tot en met de afsluiting van de jaarlijkse meeting to be held in 2027 which will have decided upon the aandeelhoudersvergadering die zal worden gehouden in 2027 en financial statements for the financial year ended on 31 December die zal hebben beslist over de jaarrekening voor het boekjaar 2026. afgesloten op 31 december 2026. Voorstel van besluit: Op voorstel van de Raad van Bestuur, en in Proposed resolution: Upon proposal of the Board of Directors, and overeenstemming met de aanbeveling en het advies van het in accordance with the recommendation and advice of the Benoemingscomité, besluit de algemene vergadering (a) de Nomination Committee, the shareholders’ meeting resolves (a) to benoeming door coöptatie op 26 maart 2024, en met ingang vanaf confirm the appointment by co-optation on 26 March 2024, and 27 maart 2024, te bevestigen, volgend op het aftreden op 26 maart with effect as per 27 March 2024, following the resignation of Mr. 2024 van de heer Daniel O’Day, Dhr. Andrew Dickinson te Daniel O’Day on 26 March 2024, and to appoint Mr. Andrew benoemen tot niet-uitvoerend lid van de Raad van Bestuur van de Dickinson as a non-executive member of the Board of Directors of Vennootschap, voor een bijkomende termijn van vier jaar, tot en the Company, for an additional period of four years, up to and met de afsluiting van de gewone algemene vergadering van including the closing of the annual shareholders’ meeting to be aandeelhouders die zal worden gehouden in 2028, en die zal held in 2028 which will have decided upon the financial statements hebben besloten over de jaarrekening voor het boekjaar eindigend for the financial year ended on 31 December 2027. The op 31 december 2027. De algemene vergadering besluit tevens dat shareholders’ meeting also resolves that the mandate of Andrew het mandaat van Andrew Dickinson onbezoldigd zal zijn. Dickinson will not be remunerated. Agendapunt 12—Steminstructie: Agenda item 12—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention

Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 13. Benoeming van de commissaris met betrekking tot de 13. Charging of the statutory auditor with respect to the “waarborging” van de CSRD duurzaamheidsverslaggeving. “assurance” of the CSRD sustainability reporting. Voorstel van besluit: In overeenstemming met de aanbeveling Proposed resolution: In accordance with the recommendation of van het Auditcomité en op voorstel van de Raad van Bestuur, en the Audit Committee and upon proposal of the Board of Directors, rekening houdend met de hangende omzetting van de Corporate and taking into account the pending transposition of the Corporate Sustainability Reporting Directive 2022/2464/EU (“CSRD”) in het Sustainability Reporting Directive 2022/2464/EU (“CSRD”) into Belgisch recht, besluit de algemene vergadering (a) de Belgian law, the shareholders’ meeting resolves (a) to charge the commissaris van de Vennootschap, zijnde BDO Bedrijfsrevisoren Company’s statutory auditor, being BDO Bedrijfsrevisoren BV, with BV, met maatschappelijke zetel te Da Vincilaan 9/E.6. 1930 its registered office at Da Vincilaan 9/E.6, 1930 Zaventem, and Zaventem, en ingeschreven in de Kruispuntbank Ondernemingen registered with the Crossroads Enterprise Database (RPR Brussels, (RPR Brussel, Nederlandstalige afdeling) onder het nummer Dutch-speaking division) under the number 0431.088.289, 0431.088.289, vast vertegenwoordigd door Ellen Lombaerts, voor permanently represented by Ellen Lombaerts, for a period of one een termijn van één jaar, te belasten met de waarborging van de year, with the assurance of the sustainability reporting of the duurzaamheidsrapportering van de Vennootschap, zoals bedoeld Company, as referred to in the CSRD, for the financial year ending in de CSRD, voor het boekjaar dat afgesloten wordt op 31 on 31 December 2024 in accordance with applicable law, and (b) to december 2024 in overeenstemming met de toepasselijke determine the remuneration of BDO Bedrijfsrevisoren BV for such wetgeving, en (b) de vergoeding van BDO Bedrijfsrevisoren BV voor assurance at EUR 120,000.00 (if any, VAT exclusive). The charging of deze waarborging vast te stellen op EUR 120.000 (indien enige, the statutory auditor with respect to the aforementioned exclusief BTW). De aanstelling van de commissaris met betrekking assurance will expire immediately after the annual shareholders’ tot de voormelde waarborging zal vervallen onmiddellijk na de meeting to be held in 2025. gewone algemene vergadering van aandeelhouders die zal worden gehouden in 2025. Agendapunt 13—Steminstructie: Agenda item 13—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention Buitengewone Algemene Vergadering Extraordinary Shareholders’ Meeting 1. Kennisname en bespreking van het verslag van de Raad van 1. Consideration and discussion of the report of the Board of Bestuur van de Vennootschap opgesteld overeenkomstig de Directors of the Company prepared in accordance with articles artikelen 7:180, 7:191 en 7:193 van het Wetboek van 7:180, 7:191 and 7:193 of the Belgian Companies and Associations Vennootschappen en Verenigingen in verband met de Code in connection with the proposed issuance of one voorgestelde uitgifte van één inschrijvingsrecht (in de vorm van subscription right (in the form of a warrant) for the benefit of een warrant) aan Gilead Therapeutics A1 Unlimited Company Gilead Therapeutics A1 Unlimited Company (“Gilead (“Gilead Therapeutics”), de “Subsequent Gilead Warrant B” Therapeutics”), called the “Subsequent Gilead Warrant B”, and genaamd, en het voorstel om, in het belang van de the proposal to cancel, in the interest of the Company, the Vennootschap, het wettelijk voorkeurrecht van de bestaande statutory preferential subscription right of the Company’s aandeelhouders van de Vennootschap op te heffen ten gunste existing shareholders for the benefit of Gilead Therapeutics. van Gilead Therapeutics. 2. Kennisname en bespreking van het verslag van de commissaris 2. Consideration and discussion of the report of the statutory van de Vennootschap opgesteld overeenkomstig de artikelen auditor of the Company prepared in accordance with articles 7:180, 7:191 en 7:193 van het Wetboek van Vennootschappen en 7:180, 7:191 and 7:193 of the Belgian Companies and Associations Verenigingen in verband met de voorgestelde uitgifte van één Code in connection with the proposed issuance of one inschrijvingsrecht (in de vorm van een warrant) aan Gilead subscription right (in the form of a warrant) for the benefit of Therapeutics, de ‘Subsequent Gilead Warrant B’ genaamd, en het Gilead Therapeutics, called the ‘Subsequent Gilead Warrant B’, voorstel om, in het belang van de Vennootschap, het wettelijk and the proposal to cancel, in the interest of the Company, the voorkeurrecht van de bestaande aandeelhouders van de statutory preferential subscription right of the Company’s Vennootschap op te heffen ten gunste van Gilead Therapeutics. existing shareholders for the benefit of Gilead Therapeutics. 3. Goedkeuring van de uitgifte van één (1) nieuw 3. Approval of the issuance of one (1) new subscription right (in inschrijvingsrecht (in de vorm van een warrant) aan Gilead the form of a warrant) for the benefit of Gilead Therapeutics and Therapeutics en de daarmee verband houdende opheffing van related cancellation of the statutory preferential subscription het wettelijk voorkeurrecht. right. Dit voorstel wordt gedaan overeenkomstig de voorwaarden van This proposal is made in accordance with the terms of the de inschrijvingsovereenkomst gesloten tussen de Vennootschap subscription agreement entered into between the Company and en Gilead Therapeutics op 14 juli 2019, zoals van tijd tot tijd Gilead Therapeutics on 14 July 2019, as amended from time to gewijzigd (de “Inschrijvingsovereenkomst”), die een verbintenis time (the “Subscription Agreement”), which included a bevatte om een voorstel te doen aan de algemene vergadering commitment to make a proposal to the general meeting to issue om de ‘Subsequent Gilead Warrant B’ uit te geven die Gilead the ‘Subsequent Gilead Warrant B’ that would enable Gilead Therapeutics in staat zou stellen om haar deelneming in de Therapeutics to further increase its shareholding in the Company, Vennootschap verder te vergroten, zoals beschreven in het

verslag van de Raad van Bestuur als bedoeld in punt 1 van de as described in the report of the Board of Directors as referred to agenda. in item 1 of the agenda. Voorstel van besluit: De algemene vergadering van de Proposed resolution: The shareholders’ meeting of the Company Vennootschap besluit om de uitgifte goed te keuren van één (1) resolves to approve the issuance of one (1) new subscription right nieuw inschrijvingsrecht (in de vorm van een warrant) aan Gilead (in the form of a warrant) for the benefit of Gilead Therapeutics, Therapeutics, de ‘Subsequent Gilead Warrant B’ genaamd (de called the ‘Subsequent Gilead Warrant B’ (the “Warrant”), and to “Warrant”), en in het belang van de Vennootschap, het wettelijk cancel, in the interest of the Company, the statutory preferential voorkeurrecht van de bestaande aandeelhouders van de subscription right of the existing shareholders of the Company Vennootschap op te heffen (en, voor zover vereist, van de (and, insofar as required, of the Company’s existing holders of bestaande houders van inschrijvingsrechten van de subscription rights (stock options)) for the benefit of Gilead Vennootschap) ten gunste van Gilead Therapeutics, Therapeutics, in accordance with the report of the Board of overeenkomstig het verslag van de Raad van Bestuur opgesteld Directors prepared in accordance with articles 7:180, 7:191 and overeenkomstig artikelen 7:180, 7:191 en 7:193 van het Wetboek 7:193 of the Belgian Companies and Associations Code, as referred van Vennootschappen en Verenigingen, waarnaar wordt verwezen to in item 1 of the agenda. in punt 1 van de agenda. In view thereof, the shareholders’ meeting of the Company resolves Met het oog hierop, beslist de algemene vergadering van de to approve the terms and conditions (the “Conditions”) of the Vennootschap om de bepalingen en voorwaarden (de Warrant as set forth in Annex 1 to the report of the Board of “Voorwaarden”) van de Warrant goed te keuren zoals uiteengezet Directors referred to in item 1 of the agenda, a copy of which shall in Bijlage 1 van het verslag van de Raad van Bestuur waarnaar remain attached to the minutes reflecting the present resolution. wordt verwezen in punt 1 van de agenda, waarvan een kopie aan The main Conditions of the Warrant can, for informational de notulen van dit besluit wordt gehecht. De belangrijkste purposes, be summarized as follows: Voorwaarden van de Warrant kunnen, voor informatiedoeleinden, a) Issuer of the Warrant: The Company. als volgt worden samengevat: b) Term: The Warrant has a term starting as of the date of this a) Emittent van de Warrant: De Vennootschap. resolution and ending on 11:59 p.m. on the calendar day before b) Duurtijd: De Warrant heeft een duurtijd die aanvangt op de the fifth anniversary of the date of this resolution. The Warrant datum van dit besluit en die eindigt om 23:59 uur op de can be exercised at one or several occasions as from 23 August kalenderdag voor de vijfde verjaardag van de datum van dit 2024 (on 11:59 p.m.) during the entire term of the Warrant, but besluit. De Warrant kan tijdens haar gehele duurtijd van de not more than once per period of three (3) months. As set out in Warrant één of meerdere keren vanaf 23 augustus 2024 (om the Conditions, this limitation does not apply in case of material 23:59 uur) worden uitgeoefend, maar niet meer dan éénmaal development regarding the Company or the trading of the per periode van drie (3) maanden. Zoals uiteengezet in de Company’s shares, or in case of certain (requests for) Voorwaarden, is deze beperking niet van toepassing in geval convocations of shareholders’ meetings of the Company. van een materiële ontwikkeling met betrekking tot de c) Issue Price: The Warrant will be issued without any additional Vennootschap of de verhandeling van de aandelen van de consideration being due by Gilead Therapeutics or any of its Vennootschap, of in geval van bepaalde (verzoeken tot) affiliates. oproepingen tot algemene vergaderingen van de d) Exercise Price: The Exercise Price (as defined in the Conditions) Vennootschap. of the Warrant shall, per share that shall be subscribed for upon c) Uitgifteprijs: De Warrant zal worden uitgegeven zonder dat an exercise of the Warrant in relation to such shares, be equal enige bijkomende vergoeding door Gilead Therapeutics of to the greater of (i) 120% multiplied by the arithmetic mean of enige met haar verbonden persoon verschuldigd is. the daily volume weighted average trading price of the d) Uitoefeningsprijs: De Uitoefeningsprijs (zoals gedefinieerd in Company’s shares as traded on Euronext Brussels and de Voorwaarden) van de Warrant zal, per aandeel waarop Euronext Amsterdam (or such other regulated markets on ingeschreven wordt bij een uitoefening van de Warrant met which the Company’s shares will be trading at that time) on betrekking tot dergelijke aandelen, gelijk zijn aan het hoogste each of the trading days during the period of 30 calendar days van (i) 120% vermenigvuldigd met het rekenkundig ending on the calendar day immediately preceding the date of gemiddelde van de dagelijks volume-gewogen gemiddelde the Exercise Notice (as defined in the Conditions) with respect koers van de aandelen van de Vennootschap verhandeld op to such exercise, and (ii) EUR 140.59. The Exercise Price is Euronext Brussels en Euronext Amsterdam (of dergelijke subject to adjustments set out in the Conditions. andere gereglementeerde markten waarop de aandelen van e) Number of shares issuable upon an exercise of the Warrant: de Vennootschap verhandeld zullen worden op dat tijdstip) op Subject to the Conditions, the Warrant entitles the holder elk van de beursdagen gedurende de periode van 30 thereof to subscribe, during the entire term of the Warrant, kalenderdagen eindigend op de kalenderdag die onmiddellijk upon each exercise of the Warrant, for a maximum number of voorafgaat aan de datum van het Bericht van Uitoefening shares that is sufficient to bring the number of shares owned by (zoals gedefinieerd in de Voorwaarden) met betrekking tot die Gilead Therapeutics, Gilead Sciences and any of their affiliates uitoefening, en (ii) EUR 140,59. De Uitoefeningsprijs is and any other party Acting in Concert (as defined in the onderhevig aan gebruikelijke aanpassingen uiteengezet in de Conditions) with Gilead Therapeutics, Gilead Sciences or any of Voorwaarden. their affiliates to 29.9% of the actually issued and outstanding e) Aantal aandelen uit te geven bij een uitoefening van de shares immediately after the issue of the shares that are to be Warrant: Overeenkomstig de Voorwaarden geeft de Warrant issued upon the relevant exercise of the Warrant (rounded het recht aan de houder daarvan om in te schrijven, gedurende down to the nearest whole share) (the “Warrant Limit”). The de gehele duurtijd van de Warrant, bij elke uitoefening van de Warrant remains outstanding for the remaining duration of its Warrant, op een maximum aantal aandelen dat voldoende is term even if exercised for a number of shares that is equal to om het aantal aandelen in eigendom van Gilead Therapeutics, the then applicable Warrant Limit. For clarity, the overall Gilead Sciences en enige met hen verbonden persoon en, elke shareholding resulting from the full exercise of the Warrant andere partij die in Onderling Overleg Handelt (zoals shall in aggregate not exceed 29.9%.

gedefinieerd in de Voorwaarden) met Gilead Therapeutics, Gilead Sciences of enige met hen verbonden persoon te brengen tot 29,9% van de daadwerkelijk uitgegeven en uitstaande aandelen onmiddellijk na de uitgifte van de aandelen die moeten worden uitgegeven bij de relevante uitoefening van de Warrant (naar beneden afgerond tot het dichtstbijzijnde gehele aandeel) (de “Warrantlimiet”). De Warrant blijft uitstaan voor de resterende duur van zijn duurtijd, zelfs indien deze wordt uitgeoefend voor een aantal aandelen dat gelijk is aan de op dat moment geldende Warranlimiet. Voor alle duidelijkheid, de totale deelneming die voortvloeit uit de volledige uitoefening van de Warrant zal in totaal niet meer bedragen dan 29,9%. f) Aard van de Warrant: De Warrant geeft het recht (maar niet de verplichting) om in te schrijven, bij enige uitoefening van de Warrant, op een aantal nieuwe aandelen uit te geven door de Vennootschap zoals hiervoor vermeld. Behalve indien anders bepaald door Belgisch recht, kwalificeert de houder van de Warrant door het loutere houden van de Warrant niet als aandeelhouder van de Vennootschap, en beschikt hij bijgevolg niet over de rechten van een aandeelhouder met betrekking tot de aandelen uit te geven of te leveren aan de houder van de Warrant bij een uitoefening van de Warrant tot aan de uitoefening van de Warrant en de uitgifte of levering van de relevante aandelen. g) Vorm van de Warrant: De Warrant zal op naam zijn. h) Geen notering van de Warrant: De Warrant zal op geen enkel moment genoteerd worden op een effectenbeurs, gereglementeerde markt of soortgelijke effectenmarkt. i) Toekenning en inschrijving: De Warrant zal worden toegekend aan Gilead Therapeutics, en er kan enkel door Gilead Therapeutics op worden ingeschreven. j) Onderliggende aandelen: De nieuwe door de Vennootschap bij elke uitoefening van de Warrant uit te geven aandelen zullen volledig volgestort zijn en dezelfde rechten en voordelen hebben als, en in alle opzichten, inclusief voor wat betreft de gerechtigheid op dividenden en andere uitkeringen, pari passu rangschikken met de bestaande en uitstaande aandelen van de Vennootschap op het ogenblik van hun uitgifte en zullen gerechtigd zijn op dividenden en andere uitkeringen waarvoor de relevante registratiedatum of vervaldatum valt op of na de datum van hun uitgifte. De algemene vergadering besluit, onder voorbehoud van, en in de mate van, elke uitoefening van de Warrant, om het kapitaal van de Vennootschap te verhogen en bij een dergelijke uitoefening het toepasselijke aantal nieuwe aandelen uit te geven zoals voorzien in de toepasselijke Voorwaarden van de Warrant. De algemene vergadering besluit dat enige uitgiftepremie die geboekt zal worden in verband met de uitoefening van de Warrant en de uitgifte van nieuwe aandelen, zoals toepasselijk, zal worden geboekt op de passiefzijde van de balans van de Vennootschap als eigen vermogen. De rekening waarop de uitgiftepremie zal worden geboekt zal, net als het kapitaal, een waarborg vormen voor derden en kan, behoudens bij incorporatie ervan in kapitaal, enkel worden verminderd op grond van een geldig besluit van de algemene vergadering van aandeelhouders genomen op de wijze vereist voor een wijziging van de statuten van de Vennootschap. De algemene vergadering van de Vennootschap besluit de Raad van Bestuur te machtigen om de besluiten van de algemene vergadering van de Vennootschap in verband met de Warrant te implementeren en uit te voeren, en om alle stappen te ondernemen en alle formaliteiten te vervullen die krachtens de Voorwaarden van de Warrant, de statuten van de Vennootschap en de toepasselijke wetgeving vereist zijn om aandelen uit te geven of over te dragen bij uitoefening van de Warrant. Bovendien zal elk f) Nature of the Warrant: The Warrant will confer the right (but not the obligation) to subscribe, upon any exercise of the Warrant, for a number of new shares to be issued by the Company, as aforementioned. Except as otherwise provided for under Belgian law, the holder of the Warrant will be no shareholder of the Company solely by virtue of holding the Warrant, and therefore does not have the rights of a shareholder in relation to the shares to be issued or delivered to the holder of the Warrant upon an exercise of the Warrant until the exercise of the Warrant and the issue or delivery of the relevant shares. g) Form of the Warrant: The Warrant will be in registered form. h) No listing of the Warrant: The Warrant shall not be listed at any time on a securities exchange, regulated market or similar securities market. i) Allocation and subscription: The Warrant will be allocated to Gilead Therapeutics, and can only be subscribed for by Gilead Therapeutics. j) Underlying shares: Each new share to be issued by the Company upon each exercise of the Warrant shall be fully paid up and shall have the same rights and benefits as, and rank pari passu in all respects including as to entitlement to dividends and other distributions, with the existing and outstanding shares of the Company at the moment of their issue and will be entitled to dividends and other distributions in respect of which the relevant record date or due date falls on or after the date of their issue. The shareholders’ meeting resolves, subject to, and to the extent of, each exercise of Warrant, to increase the Company’s share capital and to issue the relevant number of new shares issuable upon such exercise as provided for in the relevant Conditions of the Warrant. The shareholders’ meeting resolves that any issue premium that will be booked in connection with the exercise of the Warrant and the issuance of new shares, as applicable, shall be accounted for on the liabilities side of the Company’s balance sheet as net equity. The account on which the issue premium shall be booked shall, like the share capital, serve as the guarantee for third parties and, save for the possibility of a capitalization of those reserves, can only be reduced on the basis of a valid resolution of the general shareholders’ meeting passed in the manner required for an amendment to the Company’s articles of association. The shareholders’ meeting of the Company resolves to authorize the Board of Directors to implement and execute the resolutions passed by the shareholders’ meeting of the Company in connection with the Warrant, and to take all steps and carry out all formalities that shall be required by virtue of the Conditions of the Warrant, the Company’s articles of association and applicable law in order to issue or transfer shares upon an exercise of Warrant. Furthermore, each of the General Counsel of the Company and the directors of the Company (each such person, a “Special Proxy Holder”), acting individually and with possibility of sub-delegation and the power of subrogation, shall have the power, upon each exercise of the Warrant, to proceed with the recording of (i) the capital increase and issue of new shares resulting from such exercise, (ii) the allocation of the issue price to the share capital and (as applicable) the issue premium in accordance with the relevant Conditions of the Warrant, (iii) the amendment of the Company’s articles of association in order to reflect the new share capital and number of outstanding shares following the exercise of the Warrant and the issuance of new shares. Finally, each Special Proxy Holder, acting individually and with possibility of sub-delegation and the power of subrogation, shall also have the power, upon an exercise of a Warrant, (a) to sign and deliver, on behalf of the Company, the relevant Euroclear, Euronext, and bank documentation, the share register and all necessary documents in connection with the issuance and delivery of the shares (acquired as a result of the
van de General Counsel van de Vennootschap en de bestuurders van de Vennootschap (elk van deze personen, een “Bijzondere Gevolmachtigde”), individueel handelend en met mogelijkheid tot subdelegatie en de bevoegdheid tot subrogatie, de bevoegdheid hebben om bij elke uitoefening van de Warrant over te gaan tot de vaststelling van (i) de kapitaalverhoging en de uitgifte van nieuwe aandelen die het gevolg zijn van deze uitoefening, (ii) de toewijzing van de uitgifteprijs aan het kapitaal en (in voorkomend geval) de uitgiftepremie overeenkomstig de toepasselijke Voorwaarden van de Warrant, (iii) de wijziging van de statuten van de Vennootschap om het nieuwe kapitaal en het aantal uitstaande aandelen na de uitoefening van de Warrant en de uitgifte van nieuwe aandelen weer te geven. Tot slot, hebben elk van de Bijzondere Gevolmachtigden, individueel handelend en met de mogelijkheid tot subdelegatie en de bevoegdheid van subrogatie, ook de bevoegdheid om, bij een uitoefening van een Warrant, (a) in naam van de Vennootschap, de relevante Euroclear-, Euronext en bankdocumentatie, het aandelenregister en alle noodzakelijke documenten in verband met de uitgifte en levering van de aandelen (verworven als gevolg van de uitoefening van de Warrant) aan de begunstigde te ondertekenen en af te leveren, en (b) alles te doen wat nodig of nuttig kan zijn (met inbegrip van maar niet beperkt tot de voorbereiding en uitvoering van alle documenten en formulieren) voor de toelating van de aandelen uitgegeven naar aanleiding van een uitoefening van de Warrant tot de verhandeling op de gereglementeerde markten van Euronext Brussels en Euronext Amsterdam (en dergelijke andere gereglementeerde markten waarop de aandelen van de Vennootschap op dat moment worden verhandeld). exercise of the Warrant) to the beneficiary and (b) to do whatever may be necessary or useful (including but not limited to the preparation and execution of all documents and forms) for the admission of the shares issued upon an exercise of the Warrant to trading on the regulated markets of Euronext Brussels and Euronext Amsterdam (and such other regulated markets on which the Company’s shares will be trading at that time). 4. Kennisname en bespreking van het verslag van de Raad van Bestuur van de Vennootschap opgesteld overeenkomstig het artikel 7:199 van het Wetboek van Vennootschappen en Verenigingen in verband met de hernieuwing van zijn machtiging met betrekking tot, en de verhoging van, het toegestaan kapitaal, en de specifieke omstandigheden en doeleinden voor het gebruik van het hernieuwd toegestaan kapitaal. 5. Hernieuwing van de machtiging van de Raad van Bestuur om het kapitaal binnen het kader van het toegestaan kapitaal te verhogen met een bedrag tot 20% van het kapitaal. Dit voorstel wordt gedaan overeenkomstig de voorwaarden van de Inschrijvingsovereenkomst, die een verbintenis bevatte om een voorstel te doen aan de algemene vergadering om de Raad van Bestuur te machtigen om het kapitaal van de Vennootschap in één of meerdere malen te verhogen met een bedrag tot 20% van het kapitaal op het ogenblik van de oproeping tot de algemene vergadering. De huidige machtiging werd goedgekeurd door de buitengewone algemene vergadering van de Vennootschap op 22 oktober 2019 en vervalt op 12 november 2024. Het voorgestelde besluit zal de bovengenoemde machtiging (zoals overeengekomen in de Inschrijvingsovereenkomst) verlengen. Voorstel van besluit: De algemene vergadering van de Vennootschap besluit om de machtiging aan de Raad van Bestuur te hernieuwen om het kapitaal in één of meerdere malen te verhogen, gedurende een periode van vijf (5) jaar vanaf de bekendmaking van deze machtiging in de Bijlagen bij het Belgisch Staatsblad, met een totaalbedrag tot 20% van het huidige bedrag 4. Consideration and discussion of the report of the Board of Directors in accordance with article 7:199 of the Belgian Companies and Associations Code relating to the renewal of its authorization with respect to, and the increase of, the authorized capital, and the specific circumstances and purposes for the use of the renewed authorized capital. 5. Renewal of the authorization to the Board of Directors to increase the share capital within the framework of the authorized capital by up to 20% of the share capital. This proposal is made in accordance with the terms of the aforementioned Subscription Agreement, which included a commitment to make a proposal to the shareholders’ meeting to authorize the Board of Directors to increase the share capital of the Company in one or several times with an amount up to 20% of the share capital at the time of the convening of the shareholders’ meeting. The current authorisation was approved by the Company’s extraordinary shareholders’ meeting on 22 October 2019 and will expire on 12 November 2024. The proposed resolution will renew the aforementioned authorisation (as agreed in the Subscription Agreement). Proposed resolution: The shareholders’ meeting of the Company resolves to renew the authorization to the Board of Directors to increase the share capital on one or more occasions, during a period of five (5) years as of the publication in the Annexes to the Belgian State Gazette of this authorization, with an aggregate amount equal to up to 20% of the current amount of the share

van het kapitaal van de Vennootschap, en dit in overeenstemming met de voorwaarden van het verslag van de Raad van Bestuur opgesteld overeenkomstig artikel 7:199 van het Wetboek van Vennootschappen en Verenigingen, zoals vermeld in punt 4 van de agenda van de buitengewone algemene vergadering. Bijgevolg besluit de algemene vergadering om de rubriek “Toegestaan kapitaal” van de tijdelijke bepalingen van de statuten van de Vennootschap volledig te schrappen en te vervangen door de volgende tekst (waarbij het bedrag van 20 procent van het geplaatste kapitaal hieronder opgenomen tussen vierkante haken wordt bepaald op basis van het op dat moment uitstaande geplaatste kapitaal): “Toegestaan kapitaal Aan de Raad van Bestuur werd de machtiging verleend om in overeenstemming met de terzake geldende wettelijke bepalingen, in één of meerdere malen, het geplaatste kapitaal van de vennootschap te verhogen in de hierna bepaalde mate. Deze machtiging geldt voor een periode van vijf jaar te rekenen vanaf de datum van bekendmaking van deze machtiging in de Bijlagen bij het Belgisch Staatsblad. Onverminderd strengere wettelijke bepalingen, kan de Raad van Bestuur het geplaatste kapitaal van de vennootschap in één of meerdere malen verhogen met een bedrag van maximaal EUR [ ], zijnde 20 procent van het geplaatst kapitaal op het ogenblik van de oproeping tot de algemene vergadering die deze machtiging heeft verleend. Overeenkomstig de terzake geldende wettelijke bepalingen, kan de Raad van Bestuur deze machtiging niet gebruiken nadat de Autoriteit voor Financiële Diensten en Markten (FSMA) de vennootschap kennis heeft gegeven van een openbaar overnamebod op de aandelen van de vennootschap. De kapitaalverhogingen in het kader van het toegestaan kapitaal kunnen worden gerealiseerd door de uitgifte van aandelen (onder, boven of tegen de fractiewaarde van de bestaande aandelen, met of zonder stemrecht, en desgevallend in het kader van een inschrijvingsrechtenplan voor de leden van het personeel van de vennootschap of haar dochtervennootschappen in de zin van artikel 1:27 van het Wetboek van Vennootschappen en Verenigingen (met inbegrip van leden van de raad van bestuur, en/of zelfstandige consulenten)), converteerbare obligaties en/of inschrijvingsrechten uitoefenbaar door inbreng in speciën of in natura, met of zonder uitgiftepremie, en ook door de omzetting van reserves, uitgiftepremies, overgedragen winst of andere vermogenscomponenten. Bovenvermelde inschrijvingsrechtenplannen mogen voorzien dat, in uitzonderlijke gevallen (onder meer in geval van wijziging in de controle van de vennootschap of overlijden), inschrijvingsrechten kunnen worden uitgeoefend vóór de derde verjaardag van de toekenning ervan, zelfs indien de begunstigde van deze inschrijvingsrechten een lid van de Raad van Bestuur of een persoon belast met het dagelijks bestuur is. De Raad van Bestuur kan bij een verhoging van het geplaatste kapitaal binnen de grenzen van het toegestaan kapitaal, in het belang van de vennootschap, het wettelijk voorkeurrecht van de aandeelhouders beperken of opheffen, zelfs indien deze beperking of opheffing gedaan wordt ten gunste van één of meerdere bepaalde personen andere dan leden van het personeel van de vennootschap of haar dochtervennootschappen in de zin van artikel 1:27 van het Wetboek van Vennootschappen en Verenigingen. De Raad van Bestuur kan een uitgiftepremie vragen bij de uitgifte van nieuwe aandelen in het kader van het toegestaan kapitaal. Indien de Raad van Bestuur hiertoe beslist, dient deze uitgiftepremie op een onbeschikbare reserverekening te worden geboekt die slechts kan worden verminderd of overgeboekt door capital of the Company, and this in accordance with the terms and conditions set forth in the report of the Board of Directors prepared in accordance with article 7:199 of the Belgian Companies and Associations Code, as referred to in item 4 of the agenda of the Extraordinary Shareholders’ Meeting. Consequently, the shareholders’ meeting resolves to delete the section “Authorized Capital” of the temporary provisions of the articles of association of the Company entirely and to replace it with the following text (whereby the amount of 20 percent of the subscribed capital referred to below between square brackets shall be determined on the basis of the outstanding subscribed capital at that time): “Authorized capital The Board of Directors has been granted the authority to increase the subscribed capital of the company, in accordance with applicable law, in one or several times, to the extent set forth hereafter. This authorization is valid for a period of five years from the date of publication of this authorization in the Annexes to the Belgian State Gazette. Without prejudice to more restrictive rules set forth by law, the Board of Directors can increase the subscribed capital of the company in one or several times with an amount of up to EUR [•], i.e. 20 percent of the subscribed capital at the time of the convening of the shareholders’ meeting granting this authorization. In accordance with applicable law, the Board of Directors cannot use the aforementioned authorization after the Financial Services and Markets Authority (FSMA) has notified the company of a public takeover bid for the company’s shares. The capital increases within the framework of the authorized capital may be achieved by the issuance of shares (below, above or at the fractional value of the existing shares, with or without voting rights, and as the case may be in the context of a subscription rights plan for the company’s or its subsidiaries’ members of the personnel within the meaning of article 1:27 of the Belgian Companies and Associations Code (including members of the Board of Directors and/or independent consultants)), convertible bonds and/or subscription rights exercisable by contributions in cash or in kind, with or without issuance premium, and also by the conversion of reserves, issuance premiums, profits carried forward or other equity components. Aforementioned subscription rights plans can provide that, in exceptional circumstances (among others in the event of a change in control of the company or decease), subscription rights can be exercised before the third anniversary of their award, even if the beneficiary of such subscription right is a member of the Board of Directors or a person entrusted with the day-to-day management. When increasing the subscribed capital within the limits of the authorized capital, the Board of Directors may, in the company’s interest, restrict or cancel the shareholders’ statutory preferential subscription rights, even if such restriction or cancellation is made for the benefit of one or more specific persons other than the company’s or its subsidiaries’ members of the personnel within the meaning of article 1:27 of the Belgian Companies and Associations Code. The Board of Directors can ask for an issuance premium when issuing new shares in the framework of the authorized capital. If the Board of Directors decides to do so, such issuance premium is to be booked on a non-available reserve account that can only be reduced or transferred by a decision of the shareholders’ meeting adopted in the manner required for amending the articles of association. The Board of Directors is authorized to bring the company’s articles of association in line with the capital increases which have been decided upon within the framework of the authorized capital, or to instruct a notary public to do so.”.

een besluit van de algemene vergadering genomen op de wijze die vereist is voor de wijziging van statuten. De Raad van Bestuur is gemachtigd om de statuten van de vennootschap in overeenstemming te brengen met de kapitaalverhogingen waartoe binnen het kader van het toegestaan kapitaal werd beslist, of om een notaris hiertoe opdracht te geven.” 6. Volmacht voor de coördinatie. Voorstel van besluit: De algemene vergadering besluit om machtiging te geven aan iedere medewerker van ondergetekende notaris of notaris Matthieu Derynck teneinde de gecoördineerde tekst van de statuten van de Vennootschap op te stellen, te ondertekenen en neer te leggen in de daartoe voorziene elektronische databank overeenkomstig de toepasselijke wettelijke bepalingen. 6. Proxy for coordination. Proposed resolution: The shareholders’ meeting resolves to authorize each collaborator of undersigned notary or notary Matthieu Derynck to draw up, sign and file the coordinated text of the Company’s articles of association in the electronic database provided for that purpose under the applicable laws. 7. Machtiging aan de Raad van Bestuur. Voorstel van besluit: De algemene vergadering besluit om alle machten aan de Raad van Bestuur van de Vennootschap te verlenen tot uitvoering van de genomen besluiten. 7. Authorization to the Board of Directors. Proposed resolution: The shareholders’ meeting resolves to grant all powers to the Company’s Board of Directors to execute the decisions taken. 8. Volmacht Kruispuntbank voor Ondernemingen, ondernemingsloket, griffies van de Ondernemingsrechtbank, administraties en fiscale diensten. Voorstel van besluit: De algemene vergadering besluit aan elk lid van de Raad van Bestuur en/of aan, mevrouw Valeria Cnossen, mevrouw Annelies Denecker, mevrouw Elien Van Mol en de heer Stefan Mees, die – voor de uitvoering van deze volmacht – elk woonstkeuze doen te Generaal De Wittelaan L11 A3, 2800 Mechelen, België, een bijzondere volmacht te geven, om alleen optredend en met individueel recht van indeplaatsstelling en sub-delegatie, teneinde in naam en voor rekening van de Vennootschap alle formaliteiten te vervullen en/of documenten te ondertekenen die namens de Vennootschap moeten vervuld en/of ondertekend worden ter uitvoering van de hoger genomen besluiten, inclusief, doch niet beperkt tot, de uitvoering van alle formaliteiten die zijn vereist met betrekking tot alle door huidige vergadering genomen besluiten ten aanzien van de Kruispuntbank voor Ondernemingen, het ondernemingsloket, de griffies van de Ondernemingsrechtbank, de administraties en de fiscale diensten. 8. Proxy for the Crossroad Bank for Enterprises, counters for enterprises, registers of the enterprise court, administrative agencies and fiscal administrations. Proposed resolution: The shareholders’ meeting resolves to grant a special power of attorney to any member of the Board of Directors and/or Mrs. Valeria Cnossen, Mrs. Annelies Denecker, Mrs. Elien van Mol and Mr. Stefan Mees, who – for the execution of this proxy – are all electing domicile at Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium, each acting separately and each with individual power of substitution and sub-delegation, to fulfill all formalities and/or sign all documents that must be fulfilled or signed in the name of or on behalf of the Company pursuant to or in the framework of the foregoing, including, but not limited to, the completion of all necessary formalities with the Crossroad Bank for Enterprises, counters for enterprises, registers of the enterprise court, administrative agencies and fiscal administrations with respect to the decisions taken at the present meeting.

De ondergetekende is er uitdrukkelijk mee akkoord dat: (i) bij afwezigheid van steminstructies voor enig agendapunt, ofwel in het geval dat er, om welke reden dan ook, enige onduidelijkheid inzake de steminstructies zou ontstaan, de volmachtdrager altijd “voor” het voorstel tot besluit zal stemmen voor deze punten waarvoor geen of een onduidelijke steminstructie is gegeven, en dat dit een specifieke steminstructie geacht zal zijn in de zin van artikel 7:143 §4 2° van het Wetboek van Vennootschappen en Verenigingen; (ii) voor zover de General Counsel wordt aangesteld als volmachtdrager zoals voormeld, zulke volmachtdrager, als lid van het personeel van Galapagos NV (of een met haar verbonden vennootschap), een potentieel belangenconflict zoals bepaald in artikel 7:143, §4 van het Wetboek van Vennootschappen en Verenigingen zal hebben; en (iii) de Engelse versie van deze volmacht slechts een vrije vertaling voor informatiedoeleinden betreft, en dat de Nederlandstalige versie altijd voorrang heeft op de Engelse versie. De onderhavige volmacht zal eveneens gelden als aanmelding in de zin van artikel 7:134 van het Wetboek van Vennootschappen en Verenigingen. The undersigned expressly agrees that: (i) in the absence of voting instructions for any agenda item, or if, for any reason whatsoever, any uncertainty would arise with regards to the voting instructions, the proxy holder will always vote “in favor” of the proposal for such items for which no or an unclear voting instruction is given, and that this will be deemed to be a specific voting instruction in the sense of article 7:143 §4 2° of the Belgian Companies and Associations Code; (ii) to the extent that the General Counsel is appointed as proxyholder as aforementioned, such proxyholder will, as a member of the personnel of Galapagos NV (or any of its affiliated companies), have a potential conflict of interest as provided for in article 7:143, §4 of the Belgian Companies and Associations Code; and (iii) the English version of this proxy is a free translation for information purposes only, and that the Dutch version shall at all times prevail over the English translation. This proxy shall also serve as notification within the meaning of article 7:134 of the Belgian Companies and Associations Code. Het ondertekend volmachtformulier dient Galapagos NV te bereiken uiterlijk op 24 april 2024. Deze moet worden bezorgd per e-mail (shareholders@glpg.com) of met de post (Galapagos NV, t.a.v. mw. Annelies Denecker, Generaal De Wittelaan L11 A3, 2800 Mechelen, België). Aandeelhouders die zich wensen te laten vertegenwoordigen, moeten ook aan de registratie- en toelatingsvoorwaarden voldoen, zoals beschreven in de oproeping tot de Gewone en Buitengewone Algemene Vergaderingen. The signed proxy form must reach Galapagos NV on 24 April 2024 at the latest. It should be submitted by e-mail (shareholders@glpg.com) or by mail (Galapagos NV, attn. Ms. Annelies Denecker, Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium). Shareholders who wish to be represented by proxy, must also comply with the registration and admission formalities set out in the convening notice to the Annual and Extraordinary Shareholders’ Meetings.
Exhibit 99.5
| F-cap 1 |
ANNUAL ACCOUNTS AND OTHER DOCUMENTS TO BE FILED IN
ACCORDANCE WITH THE BELGIAN COMPANIES AND ASSOCIATIONS
CODE
IDENTIFICATION DETAILS (at the filing date)
NAME: Galapagos
| Legal form 1: Public limited company | ||||||
| Address: Generaal De Wittelaan | ||||||
| Postal code: 2800 | Town: Mechelen | N°. L11 , box A3 | ||||
| Country: Belgium | ||||||
| Register of legal persons - commercial court: Antwerp, division Mechelen | ||||||
| Website 2: | ||||||
| E-mail address 2: | ||||||
| Company registration number | 0466.460.429 | |||||
DATE 21-03-2023 of filing the most recent document mentioning the date of publication of the deed of incorporation and of the deed of amendment of the articles of association.
This filing concerns 3:
☒ the ANNUAL ACCOUNTS in EURO4 approved by the general meeting of 30-04-2024
☒ the OTHER DOCUMENTS
regarding
the financial year covering the period from 01-01-2023 to 31-12-2023
the preceding period of the annual accounts from 01-01-2022 to 31-12-2022
The amounts for the preceding period are / 5 identical to the ones previously published.
Total number of pages filed: 57 Numbers of the sections of the standard model form not filed because they serve no useful purpose: 6.1, 6.2.1, 6.2.4, 6.17, 7, 8, 9, 11, 12, 13, 14, 15
| Signature (name and position) Stoffels IMC BV, represented by Dr. Paul Stoffels Chairman of the board of directors |
Signature (name and position) Jérôme Contamine Director |
| 1 | Where appropriate, “in liquidation” is stated after the legal form. |
| 2 | Optional mention. |
| 3 | Tick the appropriate box(es). |
| 4 | If necessary, change to currency in which the amounts are expressed. |
| 5 | Strike out what does not apply. |
1/57
| N°. | 0466.460.429 | F-cap 2.1 |
LIST OF DIRECTORS, BUSINESS MANAGERS AND AUDITORS AND
DECLARATION REGARDING A COMPLIMENTARY REVIEW OR
CORRECTION ASSIGNMENT
LIST OF DIRECTORS, BUSINESS MANAGERS AND AUDITORS
COMPLETE LIST with surname, first names, profession, place of residence (address, number, postal code and town) and position within the company
Stoffels IMC BV 0780.918.294
Generaal De Wittelaan L11, box A3, 2800 Mechelen, Belgium
Mandate: Chairman of the board of directors, start: 26 04-2022, end: 28-04-2026
Represented by:
1 Stoffels Paul
Parekh Raj
Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium
Mandate: Director, start: 28-04-2021, end: 10-06-2023
Kerr Mary
Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium
Mandate: Director, start: 28-04-2020, end: 18-09-2023
Guenter Peter
Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium
Mandate: Director, start: 25-04-2023, end: 27-04-2027
O’Day Daniel
Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium
Mandate: Director, start: 25-04-2023, end: 27-04-2027
Higgins Linda
Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium
Mandate: Director, start: 25-04-2023, end: 27-04-2027
Svanberg Elisabeth
Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium
Mandate: Director, start: 28-04-2020, end: 30-04-2024
Baker Dan
Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium
Mandate: Director, start: 26-04-2022, end: 28-04-2026
Contamine Jérôme
Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium
Mandate: Director, start: 26-04-2022, end: 28-04-2026
2/57
| Nr. | 0466.460.429 | F-cap 2.1 |
LIST OF DIRECTORS, BUSINESS MANAGERS AND AUDITORS (continued from previous page)
Schaffert Susanne
Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium
Mandate: Director, start: 12-06-2023, end: 30-04-2028
Sturge Simon
Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium
Mandate: Director, start: 19-09-2023, end: 30-04-2028
BDO Bedrijfsrevisoren BV 0431.088.289
Elsinore Building - Da Vincilaan 9, box E6, 1930 Zaventem, Belgium
Membership number: B00023
Mandate: Auditor, start: 25 04-2023, end: 28-04-2026
Represented by:
| 1 | Lombaerts Ellen |
Elsinore Building, Da Vincilaan 9 , box E6 1930 Zaventem Belgium
Bedrijfsrevisor, Membership number : A02372
3/57
| N°. | 0466.460.429 | F-cap 2.2 |
DECLARATION REGARDING A COMPLIMENTARY REVIEW OR CORRECTION ASSIGNMENT
The managing board declares that not a single audit or correction assignment has been given to a person not authorized to do so by law, pursuant to article 5 of the law of 17 March 2019 concerning the professions of accountant and tax advisor.
The annual accounts / were not * audited or corrected by a certified accountant or by a company auditor who is not the statutory auditor.
If affirmative, should be mentioned hereafter: surname, first names, profession and address of each certified accountant or company auditor and their membership number at their Institute, as well as the nature of their assignment:
| A. | Bookkeeping of the company **, |
| B. | Preparing the annual accounts **, |
| C. | Auditing the annual accounts and/or |
| D. | Correcting the annual accounts. |
If the tasks mentioned under A or B are executed by accountants or fiscal accountants, the following information can be mentioned hereafter: surname, first names, profession and address of each accountant or fiscal accountant and their membership number at the Institute of Accountants and Tax advisors, as well as the nature of their assignment.
| Surname, first names, profession and address | Membership number | Nature of the assignment (A, B, C and/or D) |
| * | Strike out what does not apply. |
| ** | Optional mention. |
4/57
| N°. | 0466.460.429 | F-cap 3.1 |
ANNUAL ACCOUNTS
BALANCE SHEET AFTER APPROPRIATION
| Notes | Codes | Period | Preceding period | |||||||||||||
| ASSETS |
||||||||||||||||
| FORMATION EXPENSES |
6.1 | 20 | ||||||||||||||
|
|
|
|
|
|||||||||||||
| FIXED ASSETS |
21/28 | 342.773.663 | 287.678.343 | |||||||||||||
|
|
|
|
|
|||||||||||||
| Intangible fixed assets |
6.2 | 21 | 58.349.404 | 18.165.369 | ||||||||||||
| Tangible fixed assets |
6.3 | 22/27 | 16.024.739 | 17.595.161 | ||||||||||||
| Land and buildings |
22 | 871.570 | 1.795.864 | |||||||||||||
| Plant, machinery and equipment |
23 | 11.668.614 | 13.524.237 | |||||||||||||
| Furniture and vehicles |
24 | 1.231.151 | 1.761.885 | |||||||||||||
| Leasing and other similar rights |
25 | 0 | 0 | |||||||||||||
| Other tangible fixed assets |
26 | 0 | 0 | |||||||||||||
| Assets under construction and advance payments |
27 | 2.253.405 | 513.176 | |||||||||||||
| Financial fixed assets |
6.4 / 6.5.1 | 28 | 268.399.521 | 251.917.813 | ||||||||||||
| Affiliated Companies |
6.15 | 280/1 | 249.427.250 | 247.851.399 | ||||||||||||
| Participating interests |
280 | 204.127.249 | 154.642.558 | |||||||||||||
| Amounts receivable |
281 | 45.300.001 | 93.208.841 | |||||||||||||
| Other companies linked by participating interests |
6.15 | 282/3 | 1 | 1 | ||||||||||||
| Participating interests |
282 | 1 | 1 | |||||||||||||
| Amounts receivable |
283 | |||||||||||||||
| Other financial fixed assets |
284/8 | 18.972.270 | 4.066.414 | |||||||||||||
| Shares |
284 | 13.965.180 | ||||||||||||||
| Amounts receivable and cash guarantees |
285/8 | 5.007.090 | 4.066.414 | |||||||||||||
5/57
| N°. | 0466.460.429 | F-cap 3.1 |
| Notes | Codes | Period | Preceding period | |||||||||||
| CURRENT ASSETS |
29/58 | 3.958.487.436 | 4.406.769.756 | |||||||||||
|
|
|
|
|
|||||||||||
| Amounts receivable after more than one year |
|
29 | 122.091.450 | 87.847.046 | ||||||||||
| Trade debtors |
290 | 0 | 0 | |||||||||||
| Other amounts receivable |
291 | 122.091.450 | 87.847.046 | |||||||||||
| Stocks and contracts in progress |
3 | 73.978.238 | 52.665.395 | |||||||||||
| Stocks |
30/36 | 73.978.238 | 52.665.395 | |||||||||||
| Raw materials and consumables |
|
30/31 | 55.263.353 | 39.071.496 | ||||||||||
| Work in progress |
32 | 12.598.378 | 5.790.624 | |||||||||||
| Finished goods |
33 | |||||||||||||
| Goods purchased for resale |
34 | 6.116.507 | 7.803.275 | |||||||||||
| Immovable property intended for sale |
|
35 | ||||||||||||
| Advance payments |
36 | |||||||||||||
| Contracts in progress |
37 | |||||||||||||
| Amounts receivable within one year |
40/41 | 91.065.773 | 154.703.979 | |||||||||||
| Trade debtors |
40 | 26.961.771 | 72.294.845 | |||||||||||
| Other amounts receivable |
41 | 64.104.002 | 82.409.134 | |||||||||||
| Current investments |
6.5.1 /6.6 | 50/53 | 3.576.430.159 | 3.635.944.614 | ||||||||||
| Own shares |
50 | |||||||||||||
| Other investments |
51/53 | 3.576.430.159 | 3.635.944.614 | |||||||||||
| Cash at bank and in hand |
54/58 | 69.381.431 | 455.142.600 | |||||||||||
| Accruals and deferred income |
6.6 | 490/1 | 25.540.385 | 20.466.121 | ||||||||||
|
|
|
|
|
|||||||||||
| TOTAL ASSETS |
20/58 | 4.301.261.099 | 4.694.448.099 | |||||||||||
|
|
|
|
|
|||||||||||
6/57
| N°. | 0466.460.429 | F-cap 3.2 |
| Notes | Codes | Period | Preceding period | |||||||||||||||||
| EQUITY AND LIABILITIES |
||||||||||||||||||||
| EQUITY |
10/15 | 2.781.701.891 | 2.508.639.934 | |||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Contributions |
6.7.1 | 10/11 | 3.017.626.375 | 3.015.856.525 | ||||||||||||||||
| Capital |
10 | 356.444.939 | 356.111.899 | |||||||||||||||||
| Issued capital |
100 | 356.444.939 | 356.111.899 | |||||||||||||||||
| Uncalled capital 6 |
101 | |||||||||||||||||||
| Beyond capital |
11 | 2.661.181.436 | 2.659.744.625 | |||||||||||||||||
| Share premium account |
1100/10 | 2.661.181.436 | 2.659.744.625 | |||||||||||||||||
| Other |
1109/19 | |||||||||||||||||||
| Revaluation surpluses |
12 | |||||||||||||||||||
| Reserves |
13 | |||||||||||||||||||
| Reserves not available |
130/1 | |||||||||||||||||||
| Legal reserve |
130 | |||||||||||||||||||
| Reserves not available statutorily |
1311 | |||||||||||||||||||
| Purchase of own shares |
1312 | |||||||||||||||||||
| Financial support |
1313 | |||||||||||||||||||
| Other |
1319 | |||||||||||||||||||
| Untaxed reserves |
132 | |||||||||||||||||||
| Available reserves |
133 | |||||||||||||||||||
| Accumulated profits (losses) |
(+)/(-) | 14 | -235.924.484 | -507.216.591 | ||||||||||||||||
| Capital subsidies |
15 | |||||||||||||||||||
| Advance to shareholders on the distribution of net assets 7 |
|
19 | ||||||||||||||||||
| PROVISIONS AND DEFERRED TAXES |
|
16 | 13.972.066 | 9.751.647 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Provisions for liabilities and charges |
160/5 | 13.972.066 | 9.751.647 | |||||||||||||||||
| Pensions and similar obligations |
160 | |||||||||||||||||||
| Taxes |
161 | |||||||||||||||||||
| Major repairs and maintenance |
162 | |||||||||||||||||||
| Environmental obligations |
163 | |||||||||||||||||||
| Other liabilities and charges |
6.8 | 164/5 | 13.972.066 | 9.751.647 | ||||||||||||||||
| Deferred taxes |
168 | |||||||||||||||||||
| 6 | Amount to be deducted from the issued capital. |
| 7 | Amount to be deducted from the other components of equity. |
7/57
| N°. | 0466.460.429 | F-cap 3.2 |
| Notes | Codes | Period | Preceding period | |||||||||||
| AMOUNTS PAYABLE |
17/49 | 1.505.587.142 | 2.176.056.519 | |||||||||||
|
|
|
|
|
|||||||||||
| Amounts payable after more than one year |
6.9 | 17 | ||||||||||||
| Financial debts |
170/4 | |||||||||||||
| Subordinated loans |
170 | |||||||||||||
| Unsubordinated debentures |
171 | |||||||||||||
| Leasing and other similar obligations |
172 | |||||||||||||
| Credit institutions |
173 | |||||||||||||
| Other loans |
174 | |||||||||||||
| Trade debts |
175 | |||||||||||||
| Suppliers |
1750 | |||||||||||||
| Bills of exchange payable |
1751 | |||||||||||||
| Advance payments on contracts in progress |
176 | |||||||||||||
| Other amounts payable |
178/9 | |||||||||||||
| Amounts payable within one year |
6.9 | 42/48 | 201.874.356 | 300.241.235 | ||||||||||
| Current portion of amounts payable after more than one year falling due within one year |
42 | |||||||||||||
| Financial debts |
43 | |||||||||||||
| Credit institutions |
430/8 | |||||||||||||
| Other loans |
439 | |||||||||||||
| Trade debts |
44 | 129.248.124 | 67.168.086 | |||||||||||
| Suppliers |
440/4 | 129.248.124 | 67.168.086 | |||||||||||
| Bills of exchange payable |
441 | |||||||||||||
| Advance payments on contracts in progress |
46 | |||||||||||||
| Taxes, remuneration and social security |
6.9 | 45 | 23.757.581 | 25.642.149 | ||||||||||
| Taxes |
450/3 | 2.310.567 | 2.045.672 | |||||||||||
| Remuneration and social security |
454/9 | 21.447.014 | 23.596.477 | |||||||||||
| Other amounts payable |
47/48 | 48.868.651 | 207.431.000 | |||||||||||
| Accruals and deferred income |
6.9 | 492/3 | 1.303.712.786 | 1.875.815.283 | ||||||||||
|
|
|
|
|
|||||||||||
| TOTAL LIABILITIES |
10/49 | 4.301.261.099 | 4.694.448.099 | |||||||||||
|
|
|
|
|
|||||||||||
| 6 | Amount to be deducted from the issued capital. |
| 7 | Amount to be deducted from the other components of equity. |
8/57
| N°. | 0466.460.429 | F-cap 4 |
PROFIT AND LOSS ACCOUNT
| Notes | Codes | Period | Preceding period | |||||||||||||
| Operating income |
70/76A | 1.004.937.475 | 785.283.037 | |||||||||||||
| Turnover |
6.10 | 70 | 628.899.063 | 418.495.174 | ||||||||||||
| Stocks of finished goods and work and contracts in progress: increase (decrease) |
(+)/(-) | 71 | 6.807.754 | 4.414.416 | ||||||||||||
| Produced fixed assets |
72 | 352.580.235 | 349.507.630 | |||||||||||||
| Other operating income |
6.10 | 74 | 16.102.765 | 12.846.816 | ||||||||||||
| Non-recurring operating income |
6.12 | 76A | 547.659 | 19.000 | ||||||||||||
| Operating charges |
60/66A | 935.952.364 | 1.016.944.470 | |||||||||||||
| Goods for resale, raw materials and consumables |
60 | 28.718.045 | 19.860.033 | |||||||||||||
| Purchases |
600/8 | 43.223.135 | 47.750.441 | |||||||||||||
| Stocks: decrease (increase) |
(+)/(-) | 609 | -14.505.090 | -27.890.408 | ||||||||||||
| Services and other goods |
61 | 397.123.712 | 420.835.395 | |||||||||||||
| Remuneration, social security and pensions |
(+)/(-) | 6.10 | 62 | 73.555.658 | 77.772.120 | |||||||||||
| Amortisations of and other amounts written down on formation expenses, intangible and tangible fixed assets |
630 | 360.512.079 | 357.368.024 | |||||||||||||
| Amounts written down on stocks, contracts in progress and trade debtors: additions (write-backs) |
(+)/(-) | 6.10 | 631/4 | 1.239.142 | ||||||||||||
| Provisions for liabilities and charges: appropriations (uses and write-backs) |
(+)/(-) | 6.10 | 635/8 | 4.220.419 | 866.214 | |||||||||||
| Other operating charges |
6.10 | 640/8 | 70.785.043 | 102.149.120 | ||||||||||||
| Operating charges reported as assets under restructuring costs |
(-) | 649 | ||||||||||||||
| Non-recurring operating charges |
6.12 | 66A | 1.037.409 | 36.854.421 | ||||||||||||
| Operating profit (loss) |
(+)/(-) | 9901 | 68.985.111 | -231.661.433 | ||||||||||||
9/57
| N°. | 0466.460.429 | F-cap 4 |
| Notes | Codes | Period | Preceding period | |||||||||||||||
| Financial income |
75/76B | 213.501.352 | 135.553.985 | |||||||||||||||
| Recurring financial income |
75 | 213.501.352 | 135.553.985 | |||||||||||||||
| Income from financial fixed assets |
750 | 109.515.173 | 10.500.604 | |||||||||||||||
| Income from current assets |
751 | 101.978.360 | 22.459.253 | |||||||||||||||
| Other financial income |
6.11 | 752/9 | 2.007.818 | 102.594.128 | ||||||||||||||
| Non-recurring financial income |
6.12 | 76B | 0 | |||||||||||||||
| Financial charges |
6.11 | 65/66B | 37.486.367 | 60.963.868 | ||||||||||||||
| Recurring financial charges |
65 | 27.417.452 | 60.963.868 | |||||||||||||||
| Debt charges |
650 | 4.053.120 | 11.085.226 | |||||||||||||||
| Amounts written down on current assets other than stocks, contracts in progress and trade debtors: additions (write-backs) |
(+)/(-) | 651 | -9.102.765 | 0 | ||||||||||||||
| Other financial charges |
652/9 | 32.467.098 | 49.878.642 | |||||||||||||||
| Non-recurring financial charges |
6.12 | 66B | 10.068.915 | 0 | ||||||||||||||
| Profit (Loss) for the period before taxes |
(+)/(-) | 9903 | 245.000.095 | -157.071.315 | ||||||||||||||
| Transfer from deferred taxes |
780 | |||||||||||||||||
| Transfer to deferred taxes |
680 | |||||||||||||||||
| Income taxes on the result |
(+)/(-) | 6.13 | 67/77 | -26.292.013 | -19.091.591 | |||||||||||||
| Taxes |
670/3 | 72.259 | 196.797 | |||||||||||||||
| Adjustment of income taxes and write-back of tax provisions |
77 | 26.364.272 | 19.288.388 | |||||||||||||||
| Profit (Loss) of the period |
(+)/(-) | 9904 | 271.292.107 | -137.979.724 | ||||||||||||||
| Transfer from untaxed reserves |
789 | |||||||||||||||||
| Transfer to untaxed reserves |
689 | |||||||||||||||||
| Profit (Loss) of the period available for appropriation |
(+)/(-) | 9905 | 271.292.107 | -137.979.724 | ||||||||||||||
10/57
| N°. | 0466.460.429 | F-cap 5 |
APPROPRIATION ACCOUNT
| Codes | Period | Preceding period | ||||||||||||
| Profit (Loss) to be appropriated |
(+)/(-) | 9906 | -235.924.484 | -507.216.591 | ||||||||||
| Profit (Loss) of the period available for appropriation |
(+)/(-) | (9905) | 271.292.107 | -137.979.724 | ||||||||||
| Profit (Loss) of the preceding period brought forward |
(+)/(-) | 14P | -507.216.591 | -369.236.867 | ||||||||||
| Transfers from equity |
791/2 | |||||||||||||
| from contributions |
791 | |||||||||||||
| from reserves |
792 | |||||||||||||
| Appropriations to equity |
691/2 | |||||||||||||
| to contributions |
691 | |||||||||||||
| to legal reserve |
6920 | |||||||||||||
| to other reserves |
6921 | |||||||||||||
| Profit (loss) to be carried forward |
(+)/(-) | (14) | -235.924.484 | -507.216.591 | ||||||||||
| Shareholders’ contribution in respect of losses |
794 | |||||||||||||
| Profit to be distributed |
694/7 | |||||||||||||
| Compensation for contributions |
694 | |||||||||||||
| Directors or managers |
695 | |||||||||||||
| Employees |
696 | |||||||||||||
| Other beneficiaries |
697 | |||||||||||||
11/57
| N°. | 0466.460.429 | F-cap 6.2.2 |
| Codes | Period | Preceding period | ||||||||||||||
| RESEARCH COSTS MADE IN A PERIOD THAT STARTED BEFORE 1 JANUARY 2016 |
||||||||||||||||
| Acquisition value at the end of the period |
8055P | xxxxxxxxxxxxxxx | 392.970.251 | |||||||||||||
| Movements during the period |
||||||||||||||||
| Acquisitions, including produced fixed assets |
8025 | |||||||||||||||
| Sales and disposals |
8035 | |||||||||||||||
| Transfers from one heading to another |
(+ | )/(-) | 8045 | |||||||||||||
| Acquisition value at the end of the period |
8055 | 392.970.251 | ||||||||||||||
| Amortisations and amounts written down at the end of the period |
8125P | xxxxxxxxxxxxxxx | 392.970.251 | |||||||||||||
| Movements during the period |
||||||||||||||||
| Recorded |
8075 | |||||||||||||||
| Written back |
8085 | |||||||||||||||
| Acquisitions from third parties |
8095 | |||||||||||||||
| Cancelled owing to sales and disposals |
8105 | |||||||||||||||
| Transferred from one heading to another |
(+ | )/(-) | 8115 | |||||||||||||
| Amortisations and amounts written down at the end of the period |
8125 | 392.970.251 | ||||||||||||||
| NET BOOK VALUE AT THE END OF THE PERIOD |
81312 | 0 | ||||||||||||||
|
|
|
|||||||||||||||
| Codes | Period | |||||||
| RESEARCH COSTS MADE IN A PERIOD THAT STARTED AFTER 31 DECEMBER 2015 |
||||||||
| Acquisition value at the end of the period |
8056 | 2.563.955.263 | ||||||
| Amortisations and amounts written down at the end of the period |
8126 | 2.563.955.263 | ||||||
| NET BOOK VALUE AT THE END OF THE PERIOD |
81313 | 0 | ||||||
|
|
|
|||||||
12/57
| N°. | 0466.460.429 | F-cap 6.2.3 |
| Codes | Period | Preceding period | ||||||||||||||
| CONCESSIONS, PATENTS LICENSES, KNOW-HOW, BRANDS AND SIMILAR RIGHTS |
||||||||||||||||
| Acquisition value at the end of the period |
8052P | xxxxxxxxxxxxxxx | 1.253.307.639 | |||||||||||||
| Movements during the period |
||||||||||||||||
| Acquisitions, including produced fixed assets |
8022 | 397.647.633 | ||||||||||||||
| Sales and disposals |
8032 | 1.081.047 | ||||||||||||||
| Transfers from one heading to another |
(+ | )/(-) | 8042 | |||||||||||||
| Acquisition value at the end of the period |
8052 | 1.649.874.224 | ||||||||||||||
| Amortisations and amounts written down at the end of the period |
8122P | xxxxxxxxxxxxxxx | 1.235.142.270 | |||||||||||||
| Movements during the period |
||||||||||||||||
| Recorded |
8072 | 357.463.598 | ||||||||||||||
| Written back |
8082 | |||||||||||||||
| Acquisitions from third parties |
8092 | |||||||||||||||
| Cancelled owing to sales and disposals |
8102 | 1.081.047 | ||||||||||||||
| Transfers from one heading to another |
(+ | )/(-) | 8112 | |||||||||||||
| Amortisations and amounts written down at the end of the period |
8122 | 1.591.524.821 | ||||||||||||||
| NET BOOK VALUE AT THE END OF THE PERIOD |
211 | 58.349.404 | ||||||||||||||
|
|
|
|||||||||||||||
13/57
| N°. | 0466.460.429 | F-cap 6.2.5 |
| Codes | Period | Preceding period | ||||||||||||||
| ADVANCE PAYMENTS |
||||||||||||||||
| Acquisition value at the end of the period |
8054P | xxxxxxxxxxxxxxx | 0 | |||||||||||||
| Movements during the period |
||||||||||||||||
| Acquisitions, including produced fixed assets |
8024 | |||||||||||||||
| Sales and disposals |
8034 | |||||||||||||||
| Transfers from one heading to another |
(+ | )/(-) | 8044 | |||||||||||||
| Acquisition value at the end of the period |
8054 | 0 | ||||||||||||||
| Amortisations and amounts written down at the end of the period |
8124P | xxxxxxxxxxxxxxx | ||||||||||||||
| Movements during the period |
||||||||||||||||
| Recorded |
8074 | |||||||||||||||
| Written back |
8084 | |||||||||||||||
| Acquisitions from third parties |
8094 | |||||||||||||||
| Cancelled owing to sales and disposals |
8104 | |||||||||||||||
| Transferred from one heading to another |
(+ | )/(-) | 8114 | |||||||||||||
| Amortisations and amounts written down at the end of the period |
8124 | |||||||||||||||
| NET BOOK VALUE AT THE END OF THE PERIOD |
213 | 0 | ||||||||||||||
|
|
|
|||||||||||||||
14/57
| N°. | 0466.460.429 | F-cap 6.3.1 |
STATEMENT OF TANGIBLE FIXED ASSETS
| Codes | Period | Preceding period | ||||||||||||
| LAND AND BUILDINGS |
||||||||||||||
| Acquisition value at the end of the period |
8191 | P | xxxxxxxxxxxxxxx | 5.406.558 | ||||||||||
| Movements during the period |
||||||||||||||
| Acquisitions, including produced fixed assets |
8161 | 210.688 | ||||||||||||
| Sales and disposals |
8171 | 166.476 | ||||||||||||
| Transfers from one heading to another |
(+)/(-) | 8181 | ||||||||||||
| Acquisition value at the end of the period |
8191 | 5.450.770 | ||||||||||||
| Revaluation surpluses at the end of the period |
8251 | P | xxxxxxxxxxxxxxx | |||||||||||
| Movements during the period |
||||||||||||||
| Recorded |
8211 | |||||||||||||
| Acquisitions from third parties |
8221 | |||||||||||||
| Cancelled |
8231 | |||||||||||||
| Transferred from one heading to another |
(+)/(-) | 8241 | ||||||||||||
| Revaluation surpluses at the end of the period |
8251 | |||||||||||||
| Amortisations and amounts written down at the end of the period |
8321 | P | xxxxxxxxxxxxxxx | 3.610.694 | ||||||||||
| Movements during the period |
||||||||||||||
| Recorded |
8271 | 1.130.928 | ||||||||||||
| Written back |
8281 | |||||||||||||
| Acquisitions from third parties |
8291 | |||||||||||||
| Cancelled owing to sales and disposals |
8301 | 162.422 | ||||||||||||
| Transferred from one heading to another |
(+)/(-) | 8311 | ||||||||||||
| Amortisations and amounts written down at the end of the period |
8321 | 4.579.201 | ||||||||||||
| NET BOOK VALUE AT THE END OF THE PERIOD |
(22 | ) | 871.570 | |||||||||||
|
|
|
|||||||||||||
15/57
| N°. | 0466.460.429 | F-cap 6.3.2 |
| Codes | Period | Preceding period | ||||||||||||
| PLANT, MACHINERY AND EQUIPMENT |
||||||||||||||
| Acquisition value at the end of the period |
8192 | P | xxxxxxxxxxxxxxx | 27.002.896 | ||||||||||
| Movements during the period |
||||||||||||||
| Acquisitions, including produced fixed assets |
8162 | 548.407 | ||||||||||||
| Sales and disposals |
8172 | 747.916 | ||||||||||||
| Transfers from one heading to another |
(+)/(-) | 8182 | ||||||||||||
| Acquisition value at the end of the period |
8192 | 26.803.387 | ||||||||||||
| Revaluation surpluses at the end of the period |
8252 | P | xxxxxxxxxxxxxxx | |||||||||||
| Movements during the period |
||||||||||||||
| Recorded |
8212 | |||||||||||||
| Acquisitions from third parties |
8222 | |||||||||||||
| Cancelled |
8232 | |||||||||||||
| Transferred from one heading to another |
(+)/(-) | 8242 | ||||||||||||
| Revaluation surpluses at the end of the period |
8252 | |||||||||||||
| Amortisations and amounts written down at the end of the period |
8322 | P | xxxxxxxxxxxxxxx | 13.478.660 | ||||||||||
| Movements during the period |
||||||||||||||
| Recorded |
8272 | 2.320.135 | ||||||||||||
| Written back |
8282 | |||||||||||||
| Acquisitions from third parties |
8292 | |||||||||||||
| Cancelled owing to sales and disposals |
8302 | 664.022 | ||||||||||||
| Transferred from one heading to another |
(+)/(-) | 8312 | ||||||||||||
| Amortisations and amounts written down at the end of the period |
8322 | 15.134.773 | ||||||||||||
| NET BOOK VALUE AT THE END OF THE PERIOD |
(23 | ) | 11.668.614 | |||||||||||
|
|
|
|||||||||||||
16/57
| N°. | 0466.460.429 | F-cap 6.3.3 |
| Codes | Period | Preceding period | ||||||||||||
| FURNITURE AND VEHICLES |
||||||||||||||
| Acquisition value at the end of the period |
8193 | P | xxxxxxxxxxxxxxx | 3.457.445 | ||||||||||
| Movements during the period |
||||||||||||||
| Acquisitions, including produced fixed assets |
8163 | 21.610 | ||||||||||||
| Sales and disposals |
8173 | 240.308 | ||||||||||||
| Transfers from one heading to another |
(+)/(-) | 8183 | ||||||||||||
| Acquisition value at the end of the period |
8193 | 3.238.747 | ||||||||||||
| Revaluation surpluses at the end of the period |
8253 | P | xxxxxxxxxxxxxxx | |||||||||||
| Movements during the period |
||||||||||||||
| Recorded |
8213 | |||||||||||||
| Acquisitions from third parties |
8223 | |||||||||||||
| Cancelled |
8233 | |||||||||||||
| Transfers from one heading to another |
(+)/(-) | 8243 | ||||||||||||
| Revaluation surpluses at the end of the period |
8253 | |||||||||||||
| Amortisations and amounts written down at the end of the period |
8323 | P | xxxxxxxxxxxxxxx | 1.695.560 | ||||||||||
| Movements during the period |
||||||||||||||
| Recorded |
8273 | 530.079 | ||||||||||||
| Written back |
8283 | |||||||||||||
| Acquisitions from third parties |
8293 | |||||||||||||
| Cancelled owing to sales and disposals |
8303 | 218.043 | ||||||||||||
| Transfers from one heading to another |
(+)/(-) | 8313 | ||||||||||||
| Amortisations and amounts written down at the end of the period |
8323 | 2.007.596 | ||||||||||||
| NET BOOK VALUE AT THE END OF THE PERIOD |
(24 | ) | 1.231.151 | |||||||||||
|
|
|
|||||||||||||
17/57
| N°. | 0466.460.429 | F-cap 6.3.4 |
| Codes | Period | Preceding period | ||||||||||||
| LEASING AND OTHER SIMILAR RIGHTS |
||||||||||||||
| Acquisition value at the end of the period |
8194 | P | xxxxxxxxxxxxxxx | 0 | ||||||||||
| Movements during the period |
||||||||||||||
| Acquisitions, including produced fixed assets |
8164 | |||||||||||||
| Sales and disposals |
8174 | |||||||||||||
| Transfers from one heading to another |
(+)/(-) | 8184 | ||||||||||||
| Acquisition value at the end of the period |
8194 | 0 | ||||||||||||
| Revaluation surpluses at the end of the period |
8254 | P | xxxxxxxxxxxxxxx | |||||||||||
| Movements during the period |
||||||||||||||
| Recorded |
8214 | |||||||||||||
| Acquisitions from third parties |
8224 | |||||||||||||
| Cancelled |
8234 | |||||||||||||
| Transfers from one heading to another |
(+)/(-) | 8244 | ||||||||||||
| Revaluation surpluses at the end of the period |
8254 | |||||||||||||
| Amortisations and amounts written down at the end of the period |
8324 | P | xxxxxxxxxxxxxxx | 0 | ||||||||||
| Movements during the period |
||||||||||||||
| Recorded |
8274 | |||||||||||||
| Written back |
8284 | |||||||||||||
| Acquisitions from third parties |
8294 | |||||||||||||
| Cancelled owing to sales and disposals |
8304 | |||||||||||||
| Transferred from one heading to another |
(+)/(-) | 8314 | ||||||||||||
| Amortisations and amounts written down at the end of the period |
8324 | 0 | ||||||||||||
| NET BOOK VALUE AT THE END OF THE PERIOD |
(25 | ) | 0 | |||||||||||
|
|
|
|||||||||||||
| Of which |
||||||||||||||
| Land and buildings |
250 | |||||||||||||
| Plant, machinery and equipment |
251 | |||||||||||||
| Furniture and vehicles |
252 | |||||||||||||
18/57
| N°. | 0466.460.429 | F-cap 6.3.5 |
| Codes | Period | Preceding period | ||||||||||||
| OTHER TANGIBLE FIXED ASSETS |
||||||||||||||
| Acquisition value at the end of the period |
8195 | P | xxxxxxxxxxxxxxx | |||||||||||
| Movements during the period |
||||||||||||||
| Acquisitions, including produced fixed assets |
8165 | |||||||||||||
| Sales and disposals |
8175 | |||||||||||||
| Transfers from one heading to another |
(+)/(-) | 8185 | ||||||||||||
| Acquisition value at the end of the period |
8195 | |||||||||||||
| Revaluation surpluses at the end of the period |
8255 | P | xxxxxxxxxxxxxxx | |||||||||||
| Movements during the period |
||||||||||||||
| Recorded |
8215 | |||||||||||||
| Acquisitions from third parties |
8225 | |||||||||||||
| Cancelled |
8235 | |||||||||||||
| Transferred from one heading to another |
(+)/(-) | 8245 | ||||||||||||
| Revaluation surpluses at the end of the period |
8255 | |||||||||||||
| Amortisations and amounts written down at the end of the period |
8325 | P | xxxxxxxxxxxxxxx | |||||||||||
| Movements during the period |
||||||||||||||
| Recorded |
8275 | |||||||||||||
| Written back |
8285 | |||||||||||||
| Acquisitions from third parties |
8295 | |||||||||||||
| Cancelled owing to sales and disposals |
8305 | |||||||||||||
| Transferred from one heading to another |
(+)/(-) | 8315 | ||||||||||||
| Amortisations and amounts written down at the end of the period |
8325 | |||||||||||||
| NET BOOK VALUE AT THE END OF THE PERIOD |
(26 | ) | 0 | |||||||||||
|
|
|
|||||||||||||
19/57
| N°. | 0466.460.429 | F-cap 6.3.6 |
| Codes | Period | Preceding period | ||||||||||||
| ASSETS UNDER CONSTRUCTION AND ADVANCE PAYMENTS |
||||||||||||||
| Acquisition value at the end of the period |
8196 | P | xxxxxxxxxxxxxxx | 513.176 | ||||||||||
| Movements during the period |
||||||||||||||
| Acquisitions, including produced fixed assets |
8166 | 1.740.229 | ||||||||||||
| Sales and disposals |
8176 | |||||||||||||
| Transfers from one heading to another |
(+)/(-) | 8186 | ||||||||||||
| Acquisition value at the end of the period |
8196 | 2.253.405 | ||||||||||||
| Revaluation surpluses at the end of the period |
8256 | P | xxxxxxxxxxxxxxx | |||||||||||
| Movements during the period |
||||||||||||||
| Recorded |
8216 | |||||||||||||
| Acquisitions from third parties |
8226 | |||||||||||||
| Cancelled |
8236 | |||||||||||||
| Transferred from one heading to another |
(+)/(-) | 8246 | ||||||||||||
| Revaluation surpluses at the end of the period |
8256 | |||||||||||||
| Amortisations and amounts written down at the end of the period |
8326 | P | xxxxxxxxxxxxxxx | |||||||||||
| Movements during the period |
||||||||||||||
| Recorded |
8276 | |||||||||||||
| Written back |
8286 | |||||||||||||
| Acquisitions from third parties |
8296 | |||||||||||||
| Cancelled owing to sales and disposals |
8306 | |||||||||||||
| Transferred from one heading to another |
(+)/(-) | 8316 | ||||||||||||
| Amortisations and amounts written down at the end of the period |
8326 | |||||||||||||
| NET BOOK VALUE AT THE END OF THE PERIOD |
(27 | ) | 2.253.405 | |||||||||||
|
|
|
|||||||||||||
20/57
| N°. | 0466.460.429 | F-cap 6.4.1 |
STATEMENT OF FINANCIAL FIXED ASSETS
| Codes | Period | Preceding period | ||||||||||||
| AFFILIATED COMPANIES - PARTICIPATING INTERESTS AND SHARES |
||||||||||||||
| Acquisition value at the end of the period |
8391 | P | xxxxxxxxxxxxxxx | 168.701.235 | ||||||||||
| Movements during the period |
||||||||||||||
| Acquisitions |
8361 | 50.125.013 | ||||||||||||
| Sales and disposals |
8371 | |||||||||||||
| Transfers from one heading to another |
(+)/(-) | 8381 | ||||||||||||
| Acquisition value at the end of the period |
8391 | 218.826.248 | ||||||||||||
| Revaluation surpluses at the end of the period |
8451 | P | xxxxxxxxxxxxxxx | |||||||||||
| Movements during the period |
||||||||||||||
| Recorded |
8411 | |||||||||||||
| Acquisitions from third parties |
8421 | |||||||||||||
| Cancelled |
8431 | |||||||||||||
| Transferred from one heading to another |
(+)/(-) | 8441 | ||||||||||||
| Revaluation surpluses at the end of the period |
8451 | |||||||||||||
| Amounts written down at the end of the period |
8521 | P | xxxxxxxxxxxxxxx | 14.058.677 | ||||||||||
| Movements during the period |
||||||||||||||
| Recorded |
8471 | 640.322 | ||||||||||||
| Written back |
8481 | |||||||||||||
| Acquisitions from third parties |
8491 | |||||||||||||
| Cancelled owing to sales and disposals |
8501 | |||||||||||||
| Transferred from one heading to another |
(+)/(-) | 8511 | ||||||||||||
| Amounts written down at the end of the period |
8521 | 14.698.999 | ||||||||||||
| Uncalled amounts at the end of the period |
8551 | P | xxxxxxxxxxxxxxx | |||||||||||
| Movements during the period |
(+)/(-) | 8541 | ||||||||||||
| Uncalled amounts at the end of the period |
8551 | |||||||||||||
| NET BOOK VALUE AT THE END OF THE PERIOD |
(280 | ) | 204.127.249 | |||||||||||
|
|
|
|||||||||||||
| AFFILIATED COMPANIES - AMOUNTS RECEIVABLE |
||||||||||||||
| NET BOOK VALUE AT THE END OF THE PERIOD |
281 | P | xxxxxxxxxxxxxxx | 93.208.841 | ||||||||||
|
|
|
|
|
|||||||||||
| Movements during the period |
||||||||||||||
| Appropriations |
8581 | 1.774.695 | ||||||||||||
| Repayments |
8591 | 40.254.942 | ||||||||||||
| Amounts written down |
8601 | 9.428.593 | ||||||||||||
| Amounts written back |
8611 | |||||||||||||
| Exchange differences |
(+)/(-) | 8621 | ||||||||||||
| Other movements |
(+)/(-) | 8631 | ||||||||||||
| NET BOOK VALUE AT THE END OF THE PERIOD |
(281 | ) | 45.300.001 | |||||||||||
|
|
|
|||||||||||||
| ACCUMULATED AMOUNTS WRITTEN DOWN ON AMOUNTS RECEIVABLE AT END OF THE PERIOD |
8651 | 9.428.593 | ||||||||||||
|
|
|
|||||||||||||
21/57
| N°. | 0466.460.429 | F-cap 6.4.2 |
| Codes | Period | Preceding period | ||||||||||||
| COMPANIES LINKED BY PARTICIPATING INTERESTS - PARTICIPATING INTERESTS AND SHARES |
||||||||||||||
| Acquisition value at the end of the period |
8392 | P | xxxxxxxxxxxxxxx | 1 | ||||||||||
| Movements during the period |
||||||||||||||
| Acquisitions |
8362 | |||||||||||||
| Sales and disposals |
8372 | |||||||||||||
| Transfers from one heading to another |
(+)/(-) | 8382 | ||||||||||||
| Acquisition value at the end of the period |
8392 | 1 | ||||||||||||
| Revaluation surpluses at the end of the period |
8452 | P | xxxxxxxxxxxxxxx | |||||||||||
| Movements during the period |
||||||||||||||
| Recorded |
8412 | |||||||||||||
| Acquisitions from third parties |
8422 | |||||||||||||
| Cancelled |
8432 | |||||||||||||
| Transferred from one heading to another |
(+)/(-) | 8442 | ||||||||||||
| Revaluation surpluses at the end of the period |
8452 | |||||||||||||
| Amounts written down at the end of the period |
8522 | P | xxxxxxxxxxxxxxx | |||||||||||
| Movements during the period |
||||||||||||||
| Recorded |
8472 | |||||||||||||
| Written back |
8482 | |||||||||||||
| Acquisitions from third parties |
8492 | |||||||||||||
| Cancelled owing to sales and disposals |
8502 | |||||||||||||
| Transferred from one heading to another |
(+)/(-) | 8512 | ||||||||||||
| Amounts written down at the end of the period |
8522 | |||||||||||||
| Uncalled amounts at the end of the period |
8552 | P | xxxxxxxxxxxxxxx | |||||||||||
| Movements during the period |
(+)/(-) | 8542 | ||||||||||||
| Uncalled amounts at the end of the period |
8552 | |||||||||||||
| NET BOOK VALUE AT THE END OF THE PERIOD |
(282 | ) | 1 | |||||||||||
|
|
|
|||||||||||||
| COMPANIES LINKED BY PARTICIPATING INTERESTS - AMOUNTS RECEIVABLE |
||||||||||||||
| NET BOOK VALUE AT THE END OF THE PERIOD |
283 | P | xxxxxxxxxxxxxxx | |||||||||||
|
|
|
|||||||||||||
| Movements during the period |
||||||||||||||
| Appropriations |
8582 | |||||||||||||
| Repayments |
8592 | |||||||||||||
| Amounts written down |
8602 | |||||||||||||
| Amounts written back |
8612 | |||||||||||||
| Exchange differences |
(+)/(-) | 8622 | ||||||||||||
| Other movements |
(+)/(-) | 8632 | ||||||||||||
| NET BOOK VALUE AT THE END OF THE PERIOD |
(283 | ) | ||||||||||||
|
|
|
|||||||||||||
| ACCUMULATED AMOUNTS WRITTEN DOWN ON AMOUNTS RECEIVABLE AT END OF THE PERIOD |
8652 | |||||||||||||
|
|
|
|||||||||||||
22/57
| N°. | 0466.460.429 | F-cap 6.4.3 |
| Codes | Period | Preceding period | ||||||||||||
| OTHER COMPANIES - PARTICIPATING INTERESTS AND SHARES |
||||||||||||||
| Acquisition value at the end of the period |
8393 | P | xxxxxxxxxxxxxxx | 0 | ||||||||||
| Movements during the period |
||||||||||||||
| Acquisitions |
8363 | 13.965.180 | ||||||||||||
| Sales and disposals |
8373 | |||||||||||||
| Transfers from one heading to another |
(+)/(-) | 8383 | ||||||||||||
| Acquisition value at the end of the period |
8393 | 13.965.180 | ||||||||||||
| Revaluation surpluses at the end of the period |
8453 | P | xxxxxxxxxxxxxxx | |||||||||||
| Movements during the period |
||||||||||||||
| Recorded |
8413 | |||||||||||||
| Acquisitions from third parties |
8423 | |||||||||||||
| Cancelled |
8433 | |||||||||||||
| Transferred from one heading to another |
(+)/(-) | 8443 | ||||||||||||
| Revaluation surpluses at the end of the period |
8453 | |||||||||||||
| Amounts written down at the end of the period |
8523 | P | xxxxxxxxxxxxxxx | 0 | ||||||||||
| Movements during the period |
||||||||||||||
| Recorded |
8473 | |||||||||||||
| Written back |
8483 | |||||||||||||
| Acquisitions from third parties |
8493 | |||||||||||||
| Cancelled owing to sales and disposals |
8503 | |||||||||||||
| Transferred from one heading to another |
(+)/(-) | 8513 | ||||||||||||
| Amounts written down at the end of the period |
8523 | 0 | ||||||||||||
| Uncalled amounts at the end of the period |
8553 | P | xxxxxxxxxxxxxxx | |||||||||||
| Movements during the period |
(+)/(-) | 8543 | ||||||||||||
| Uncalled amounts at the end of the period |
8553 | |||||||||||||
| NET BOOK VALUE AT THE END OF THE PERIOD |
(284 | ) | 13.965.180 | |||||||||||
|
|
|
|||||||||||||
| OTHER COMPANIES - AMOUNTS RECEIVABLE |
||||||||||||||
| NET BOOK VALUE AT THE END OF THE PERIOD |
285/8 | P | xxxxxxxxxxxxxxx | 4.066.414 | ||||||||||
|
|
|
|
|
|||||||||||
| Movements during the period |
||||||||||||||
| Appropriations |
8583 | 1.008.750 | ||||||||||||
| Repayments |
8593 | 68.074 | ||||||||||||
| Amounts written down |
8603 | |||||||||||||
| Amounts written back |
8613 | |||||||||||||
| Exchange differences |
(+)/(-) | 8623 | ||||||||||||
| Other movements |
(+)/(-) | 8633 | ||||||||||||
| NET BOOK VALUE AT THE END OF THE PERIOD |
(285/8 | ) | 5.007.090 | |||||||||||
|
|
|
|||||||||||||
| ACCUMULATED AMOUNTS WRITTEN DOWN ON AMOUNTS RECEIVABLE AT END OF THE PERIOD |
8653 | |||||||||||||
|
|
|
|||||||||||||
23/57
| N°. | 0466.460.429 | F-cap 6.5.1 |
PARTICIPATING INTERESTS INFORMATION
PARTICIPATING INTERESTS AND OTHER RIGHTS IN OTHER COMPANIES
The following list mentions the companies in which the company holds a participating interest (recorded in headings 280 and 282 of assets), as well as the companies in which the company holds rights (recorded in headings 284 and 51/53 of assets) for an amount of at least 10% of the capital, the equity or a class of shares of the company.
| Rights held |
Data extracted from the most recent annual accounts | |||||||||||||||||||||||||||
|
NAME, full address of the REGISTERED OFFICE and, for an entity governed by Belgian law, the COMPANY REGISTRATION NUMBER |
Directly |
Subsidiaries | Annual accounts as per |
Currency code |
Equity | Net result | ||||||||||||||||||||||
| Nature |
Number |
% | % | (+) or (-) (in units) |
||||||||||||||||||||||||
| Galapagos BV |
31-12-2023 | EUR | 3.211.184 | -2.024.862 | ||||||||||||||||||||||||
| Foreign company |
||||||||||||||||||||||||||||
| Willem Einthovenstraat 13 |
||||||||||||||||||||||||||||
| 2342 BH Oegstgeest |
||||||||||||||||||||||||||||
| Netherlands |
||||||||||||||||||||||||||||
| 807938397B01 |
||||||||||||||||||||||||||||
| Registered shares | 200 | 100,00 | 0,00 | |||||||||||||||||||||||||
| Galapagos Biotech Limited |
31-12-2023 | GBP | 4.748.401 | 2.198.252 | ||||||||||||||||||||||||
| Foreign company |
||||||||||||||||||||||||||||
| Salisbury House, Station |
||||||||||||||||||||||||||||
| CB1 2LA Cambridgeshire |
||||||||||||||||||||||||||||
| United Kingdom |
||||||||||||||||||||||||||||
| 203017668 |
||||||||||||||||||||||||||||
| Registered shares | 84.700.014 | 100,00 | 0,00 | |||||||||||||||||||||||||
| Galapagos SASU |
31-12-2023 | EUR | 74.691.265 | 12.333.065 | ||||||||||||||||||||||||
| Foreign company |
||||||||||||||||||||||||||||
| Avenue Gaston Roussel 102 |
||||||||||||||||||||||||||||
| 93230 Romainville |
||||||||||||||||||||||||||||
| France |
||||||||||||||||||||||||||||
| 27440348480 |
||||||||||||||||||||||||||||
| Registered shares | 51.199 | 100,00 | 0,00 | |||||||||||||||||||||||||
| Galapagos GmbH |
31-12-2023 | CHF | 2.394.452 | 1.916.959 | ||||||||||||||||||||||||
| Foreign company |
||||||||||||||||||||||||||||
| Aeschengraben 27 |
||||||||||||||||||||||||||||
| 4051 Basel |
||||||||||||||||||||||||||||
| Switzerland |
||||||||||||||||||||||||||||
| CHE388581566 |
||||||||||||||||||||||||||||
| Registered shares | 200 | 100,00 | 0,00 | |||||||||||||||||||||||||
| Galapagos Real Estate Belgium |
31-12-2023 | EUR | -8.575.421 | -8.765.899 | ||||||||||||||||||||||||
| Private limited company |
||||||||||||||||||||||||||||
| Generaal De Wittelaan L11 , box A3 |
||||||||||||||||||||||||||||
| 2800 Mechelen |
||||||||||||||||||||||||||||
| Belgium |
||||||||||||||||||||||||||||
| 0714.965.620 |
||||||||||||||||||||||||||||
| Registered shares | 1.120 | 100,00 | 0,00 | |||||||||||||||||||||||||
| Galapagos Biopharma Belgium BV |
31-12-2023 | EUR | 1.149.553 | 445.453 | ||||||||||||||||||||||||
| Private limited company |
||||||||||||||||||||||||||||
| Generaal De Wittelaan L11 , box A3 |
||||||||||||||||||||||||||||
| 2800 Mechelen |
||||||||||||||||||||||||||||
| Belgium |
||||||||||||||||||||||||||||
| 0727.786.248 |
||||||||||||||||||||||||||||
| Registered shares | 500.000 | 100,00 | 0,00 | |||||||||||||||||||||||||
24/57
| N°. | 0466.460.429 | F-cap 6.5.1 |
PARTICIPATING INTERESTS INFORMATION
PARTICIPATING INTERESTS AND OTHER RIGHTS IN OTHER COMPANIES
The following list mentions the companies in which the company holds a participating interest (recorded in headings 280 and 282 of assets), as well as the companies in which the company holds rights (recorded in headings 284 and 51/53 of assets) for an amount of at least 10% of the capital, the equity or a class of shares of the company.
| Rights held |
Data extracted from the most recent annual accounts | |||||||||||||||||||||||||||
| NAME, full address of the REGISTERED OFFICE and, for an entity governed by Belgian law, the COMPANY REGISTRATION NUMBER |
Directly |
Subsidiaries | Annual accounts as per |
Currency code |
Equity | Net result | ||||||||||||||||||||||
| Nature |
Number |
% | % | (+) or (-) (in units) |
||||||||||||||||||||||||
| Galapagos Biopharma Netherlands BV |
31-12-2023 | EUR | 932.382 | 271.695 | ||||||||||||||||||||||||
| Foreign company |
||||||||||||||||||||||||||||
| Willem Einthovenstraat 13 |
||||||||||||||||||||||||||||
| 2342 BH Oestgeest |
||||||||||||||||||||||||||||
| Netherlands |
||||||||||||||||||||||||||||
| NL860020368B |
||||||||||||||||||||||||||||
| Registered shares | 500.000 | 100,00 | 0,00 | |||||||||||||||||||||||||
| Galapagos Biopharma Spain S.L.U |
31-12-2023 | EUR | 1.910.557 | 421.685 | ||||||||||||||||||||||||
| Foreign company |
||||||||||||||||||||||||||||
| Cuatro Torres - Paseo de la Castellana |
||||||||||||||||||||||||||||
| 28046 Madrid |
||||||||||||||||||||||||||||
| Spain |
||||||||||||||||||||||||||||
| ESB67445429 |
||||||||||||||||||||||||||||
| Registered shares | 3.000 | 100,00 | 0,00 | |||||||||||||||||||||||||
| Galapagos Biopharma Italy S.r.l. |
31-12-2023 | EUR | 269.943 | 252.931 | ||||||||||||||||||||||||
| Foreign company |
||||||||||||||||||||||||||||
| Via Ceresio 7 |
||||||||||||||||||||||||||||
| 20154 Milano |
||||||||||||||||||||||||||||
| Italy |
||||||||||||||||||||||||||||
| 1097290096 |
||||||||||||||||||||||||||||
| Registered shares | 50.000 | 100,00 | 0,00 | |||||||||||||||||||||||||
| Galapagos Biopharma Germany GmbH |
31-12-2023 | EUR | 3.117.463 | 1.415.625 | ||||||||||||||||||||||||
| Foreign company |
||||||||||||||||||||||||||||
| Luise-Ulrich strasse 8 |
||||||||||||||||||||||||||||
| 80636 Munchen |
||||||||||||||||||||||||||||
| Germany |
||||||||||||||||||||||||||||
| DE326847734 |
||||||||||||||||||||||||||||
| Registered shares | 25.000 | 100,00 | 0,00 | |||||||||||||||||||||||||
| Galapagos Real Estate Netherlands B.V. |
31-12-2023 | EUR | 31.217.607 | -3.098.918 | ||||||||||||||||||||||||
| Foreign company |
||||||||||||||||||||||||||||
| Willem Einthovenstraat 13 |
||||||||||||||||||||||||||||
| 2342 BH Oestgeest |
||||||||||||||||||||||||||||
| Netherlands |
||||||||||||||||||||||||||||
| 860584859B01 |
||||||||||||||||||||||||||||
| Registered shares | 10.000.000 | 100,00 | 0,00 | |||||||||||||||||||||||||
| Galapagos Biopharma Denmark ApS |
31-12-2023 | DKK | 3.275.222 | 1.391.584 | ||||||||||||||||||||||||
| Foreign company |
||||||||||||||||||||||||||||
| Sundkrogsgade , Harbour House 21 |
||||||||||||||||||||||||||||
| 2100 Copenhagen |
||||||||||||||||||||||||||||
| Denmark |
||||||||||||||||||||||||||||
| 42447242 |
||||||||||||||||||||||||||||
25/57
| N°. | 0466.460.429 | F-cap 6.5.1 |
PARTICIPATING INTERESTS INFORMATION
PARTICIPATING INTERESTS AND OTHER RIGHTS IN OTHER COMPANIES
The following list mentions the companies in which the company holds a participating interest (recorded in headings 280 and 282 of assets), as well as the companies in which the company holds rights (recorded in headings 284 and 51/53 of assets) for an amount of at least 10% of the capital, the equity or a class of shares of the company.
| Rights held |
Data extracted from the most recent annual accounts | |||||||||||||||||||||||||||
| NAME, full address of the REGISTERED OFFICE and, for an entity governed by Belgian law, the COMPANY REGISTRATION NUMBER |
Directly |
Subsidiaries | Annual accounts as per |
Currency code |
Equity | Net result | ||||||||||||||||||||||
| Nature |
Number |
% | % | (+) or (-) (in units) |
||||||||||||||||||||||||
| Registered shares | 40.000 | 100,00 | 0,00 | |||||||||||||||||||||||||
| Galapagos Biopharma Sweden AB |
31-12-2023 | SEK | 5.315.134 | 2.576.327 | ||||||||||||||||||||||||
| Foreign company |
||||||||||||||||||||||||||||
| Vasagatan 7 |
||||||||||||||||||||||||||||
| 10123 Stockholm |
||||||||||||||||||||||||||||
| Sweden |
||||||||||||||||||||||||||||
| 559311454801 |
||||||||||||||||||||||||||||
| Registered shares | 25.000 | 100,00 | 0,00 | |||||||||||||||||||||||||
| Galapagos Biopharma Finland Oy |
31-12-2023 | EUR | 115.740 | 24.118 | ||||||||||||||||||||||||
| Foreign company |
||||||||||||||||||||||||||||
| Uudenmaankatu 1-5 |
||||||||||||||||||||||||||||
| 00120 Helsinki |
||||||||||||||||||||||||||||
| Finland |
||||||||||||||||||||||||||||
| 32201288 |
||||||||||||||||||||||||||||
| Registered shares | 100,00 | 0,00 | ||||||||||||||||||||||||||
| Galapagos Biopharma Ireland Ltd |
31-12-2023 | EUR | 67.994 | 39.460 | ||||||||||||||||||||||||
| Foreign company |
||||||||||||||||||||||||||||
| Block 3 Harcourt Centre, Harcourt Road |
||||||||||||||||||||||||||||
| D02A339 Dublin |
||||||||||||||||||||||||||||
| Ireland |
||||||||||||||||||||||||||||
| 3791298BH |
||||||||||||||||||||||||||||
| Registered shares | 100,00 | 0,00 | ||||||||||||||||||||||||||
| Galapagos Biopharma Norway AS |
31-12-2023 | NOK | 762.776 | 293.119 | ||||||||||||||||||||||||
| Foreign company |
||||||||||||||||||||||||||||
| Wergelansveien 7 |
||||||||||||||||||||||||||||
| 0167 Oslo |
||||||||||||||||||||||||||||
| Norway |
||||||||||||||||||||||||||||
| 927480654 |
||||||||||||||||||||||||||||
| Registered shares | 30.000 | 100,00 | 0,00 | |||||||||||||||||||||||||
| Galapagos Biopharma Austria Gmbh |
31-12-2023 | EUR | 754.438 | 319.094 | ||||||||||||||||||||||||
| Foreign company |
||||||||||||||||||||||||||||
| Schottenring 25 |
||||||||||||||||||||||||||||
| 1010 Vienna |
||||||||||||||||||||||||||||
| Austria |
||||||||||||||||||||||||||||
| U77339907 |
||||||||||||||||||||||||||||
| Registered shares | 100,00 | 0,00 | ||||||||||||||||||||||||||
| Galapagos US Holding Inc |
31-12-2023 | USD | 8.395.558 | -295.935 | ||||||||||||||||||||||||
| Foreign company |
||||||||||||||||||||||||||||
| North State Street 800 , box 304 |
||||||||||||||||||||||||||||
| 19901 Dover, Delaware |
||||||||||||||||||||||||||||
| United States |
||||||||||||||||||||||||||||
26/57
| N°. | 0466.460.429 | F-cap 6.5.1 |
PARTICIPATING INTERESTS INFORMATION
PARTICIPATING INTERESTS AND OTHER RIGHTS IN OTHER COMPANIES
The following list mentions the companies in which the company holds a participating interest (recorded in headings 280 and 282 of assets), as well as the companies in which the company holds rights (recorded in headings 284 and 51/53 of assets) for an amount of at least 10% of the capital, the equity or a class of shares of the company.
| Rights held |
Data extracted from the most recent annual accounts | |||||||||||||||||||||||||||
| NAME, full address of the REGISTERED OFFICE and, for an entity governed by Belgian law, the COMPANY REGISTRATION NUMBER |
Directly |
Subsidiaries | Annual accounts as per |
Currency code |
Equity | Net result | ||||||||||||||||||||||
| Nature |
Number |
% | % | (+) or (-) (in units) |
||||||||||||||||||||||||
| Aandelen op naam | 100 | 100,00 | 0,00 | |||||||||||||||||||||||||
27/57
| N°. | 0466.460.429 | F-cap 6.5.2 |
LIST OF COMPANIES FOR WHICH THE COMPANY HAS UNLIMITED LIABILITY IN THE CAPACITY OF UNLIMITED LIABLE PARTNER OR MEMBER
The annual accounts of each company for which the company has unlimited liability, are being enclosed to and published with these annual accounts, unless the second column contains the reason why this is not the case; this mention is made by referring to the applicable code (A,B, C or D) defined below.
The annual accounts of the mentioned company:
| A. | are published by the company by filing them at the National Bank of Belgium; |
| B. | are actually published by the company in a different member country of the European Union, pursuant to article 16 of directive (EU) 2017/1132; |
| C. | are being inserted by complete or proportional consolidation into the consolidated annual accounts of the company, drawn up, verified and published in accordance with the stipulations of the Belgian Companies and Associations Code concerning the consolidated annual accounts; |
| D. | concern a limited partnership (société simple/maatschap). |
| NAME, full address of the REGISTERED OFFICE, LEGAL FORM and, for an entity governed by Belgian law, the COMPANY REGISTRATION NUMBER |
Potential code |
|||
| Galapagos BV |
C | |||
| Foreign company |
||||
| Willem Einthovenstraat 13 2342 Oestgeest |
||||
| Netherlands |
||||
| Galapagos Biopharma Netherlands B.V. |
C | |||
| Foreign company |
||||
| Willem Einthovenstraat 13 2342 Oestgeest |
||||
| Netherlands |
||||
| Galapagos Real Estate Netherlands B.V. |
C | |||
| Foreign company |
||||
| Willem Einthovenstraat 13 2342 Oestgeest |
||||
| Netherlands |
||||
28/57
| N°. | 0466.460.429 | F-cap 6.6 |
CURRENT INVESTMENTS AND ACCRUALS AND DEFERRED INCOME
| Codes | Period | Preceding period | ||||||||||
| CURRENT INVESTMENTS - OTHER INVESTMENTS |
||||||||||||
| Shares and investments other than fixed income investments |
51 | |||||||||||
| Shares – Book value increased with the uncalled amount |
8681 | |||||||||||
| Shares – Uncalled amount |
8682 | |||||||||||
| Precious metals and works of art |
8683 | |||||||||||
| Fixed-income securities |
52 | |||||||||||
| Fixed income securities issued by credit institutions |
8684 | |||||||||||
| Term accounts with credit institutions |
53 | 1.540.936.652 | 1.593.596.194 | |||||||||
| With a remaining term or notice up to one month |
8686 | 95.000.000 | 55.000.000 | |||||||||
| between one month and one year |
8687 | 1.445.936.652 | 1.488.596.194 | |||||||||
| over one year |
8688 | 50.000.000 | ||||||||||
| Other investments not mentioned above |
8689 | 2.035.493.507 | 2.042.348.421 | |||||||||
| Period | ||||
| ACCRUALS AND DEFERRED INCOME |
||||
| Allocation of account 490/1 of assets if the amount is significant |
||||
| Deferred charges |
10.889.216 | |||
| Accrued income |
14.607.692 | |||
| Accrued subsidies |
43.477 | |||
29/57
| N°. | 0466.460.429 | F-cap 6.7.1 |
STATEMENT OF CAPITAL AND SHAREHOLDERS’ STURCTURE
| Codes | Period | Preceding period | ||||||||||
| STATEMENT OF CAPITAL |
||||||||||||
| Capital |
||||||||||||
| Issued capital at the end of the period |
100P | XXXXXXXXXXXXXX | 356.111.899 | |||||||||
| Issued capital at the end of the period |
(100 | ) | 356.444.939 | |||||||||
| Codes | Period | Number of shares | ||||||||||
| Modifications during the period |
||||||||||||
| Uitoefening warranten 20/03/2023 |
333.040 | 61.560 | ||||||||||
| Composition of the capital |
||||||||||||
| Share types |
||||||||||||
| Aandelen zonder nominale waarde |
356.444.939 | 65.897.071 | ||||||||||
| Registered shares |
8702 | XXXXXXXXXXXXXX | 5.846 | |||||||||
| Shares dematerialized |
8703 | XXXXXXXXXXXXXX | 65.891.225 | |||||||||
| Codes | Uncalled amount | Called up amount, unpaid | ||||||||||
| Unpaid capital |
||||||||||||
| Uncalled capital |
(101 | ) | XXXXXXXXXXXXXX | |||||||||
| Called up capital, unpaid |
8712 | XXXXXXXXXXXXXX | ||||||||||
| Shareholders that still need to pay up in full |
||||||||||||
| Codes | Period | |||||||||||
| Own shares |
||||||||||||
| Held by the company itself |
||||||||||||
| Amount of capital held |
8721 | |||||||||||
| Number of shares |
8722 | |||||||||||
| Held by a subsidiary |
||||||||||||
| Amount of capital held |
8731 | |||||||||||
| Number of shares |
8732 | |||||||||||
| Commitments to issuing shares |
||||||||||||
| Owing to the exercise of conversion rights |
||||||||||||
| Amount of outstanding convertible loans |
8740 | |||||||||||
| Amount of capital to be subscribed |
8741 | |||||||||||
| Corresponding maximum number of shares to be issued |
8742 | |||||||||||
| Owing to the exercise of subscription rights |
||||||||||||
| Number of outstanding subscription rights |
8745 | 11.472.521 | ||||||||||
| Amount of capital to be subscribed |
8746 | 62.056.203 | ||||||||||
| Corresponding maximum number of shares to be issued |
8747 | 11.472.521 | ||||||||||
| Authorised capital not issued |
8751 | 16.566.540 | ||||||||||
30/57
| N°. | 0466.460.429 | F-cap 6.7.1 |
| Codes | Period | |||||||
| Shares issued, non-representing capital |
||||||||
| Distribution |
||||||||
| Number of shares |
8761 | |||||||
| Number of voting rights attached thereto |
8762 | |||||||
| Allocation by shareholder |
||||||||
| Number of shares held by the company itself |
8771 | |||||||
| Number of shares held by its subsidiaries |
8781 | |||||||
| Period | ||||||||
| ADDITIONAL NOTES REGARDING CONTRIBUTIONS (INCLUDING CONTRIBUTIONS IN THE FORM OF SERVICES OR KNOW-HOW) |
||||||||
31/57
| N°. | 0466.460.429 | F-cap 6.7.2 |
SHAREHOLDERS’ STRUCTURE OF THE COMPANY AT YEAR-END CLOSING DATE
As reflected in the notifications received by the company pursuant to article 7:225 of the Belgian Companies and Associations Code, article 14 fourth paragraph of the law of 2 May 2007 on the publication of major holdings and article 5 of the Royal Decree of 21 August 2008 on further rules for certain multilateral trading facilities.
| NAME of the person(s) who hold rights of the company, together with the ADDRESS (of the registered office, in the case of a legal person) and the COMPANY REGISTRATION NUMBER, in the case of a company governed by Belgian law |
Rights held | |||||||||||||||
| Number of voting rights | ||||||||||||||||
| Nature | Attached to securities |
Not attached to securities |
% | |||||||||||||
| Gilead Therapeutics A1 Unlimited Company 70 Sir John Rogerson’s Quay, Dublin 2, Ireland |
|
Dematerialized shares |
|
16.707.477 | 25,35 | % | ||||||||||
| Van Herk Investments B.V. Lichtenauerlaan 30, 3062 ME Rotterdam, NL |
|
Dematerialized shares |
|
4.635.672 | 7,03 | % | ||||||||||
| EcoR1 Capital LLC 357 Tehema Street, Suite 3, San Francisco, CA 94103, United States of America |
|
Dematerialized shares |
|
6.505.890 | 9,87 | % | ||||||||||
| FMR LLC The Corporation Trust Center, 1209 Orange Street, Wilmington, New Castle County, Delaware, 19801 |
|
Dematerialized shares |
|
3.903.804 | 5,92 | % | ||||||||||
| Total shares | 65.897.071,00 | |||||||||||||||
32/57
| N°. | 0466.460.429 | F-cap 6.8 |
PROVISIONS FOR OTHER LIABILITIES AND CHARGES
| Period | ||||
| ALLOCATION OF ACCOUNT 164/5 OF LIABILITIES IF THE AMOUNT IS SIGNIFICANT |
||||
| Provision Restricted Stock Units |
10.594.912 | |||
| Tax recovery mechanism |
3.377.154 | |||
33/57
| N°. | 0466.460.429 | F-cap 6.9 |
STATEMENT OF AMOUNTS PAYABLE AND ACCRUALS AND DEFERRED INCOME (LIABILITIES)
| Codes | Period | |||||||
| BREAKDOWN OF AMOUNTS PAYABLE WITH AN ORIGINAL TERM OF MORE THAN ONE YEAR, ACCORDING TO THEIR RESIDUAL MATURITY |
||||||||
| Current portion of amounts payable after more than one year falling due within one year |
||||||||
| Financial debts |
8801 | |||||||
| Subordinated loans |
8811 | |||||||
| Unsubordinated debentures |
8821 | |||||||
| Leasing and other similar obligations |
8831 | |||||||
| Credit institutions |
8841 | |||||||
| Other loans |
8851 | |||||||
| Trade debts |
8861 | |||||||
| Suppliers |
8871 | |||||||
| Bills of exchange payable |
8881 | |||||||
| Advance payments on contracts in progress |
8891 | |||||||
| Other amounts payable |
8901 | |||||||
| Total current portion of amounts payable after more than one year falling due within one year |
(42 | ) | ||||||
| Amounts payable with a remaining term of more than one year, yet less than 5 years |
||||||||
| Financial debts |
8802 | |||||||
| Subordinated loans |
8812 | |||||||
| Unsubordinated debentures |
8822 | |||||||
| Leasing and other similar obligations |
8832 | |||||||
| Credit institutions |
8842 | |||||||
| Other loans |
8852 | |||||||
| Trade debts |
8862 | |||||||
| Suppliers |
8872 | |||||||
| Bills of exchange payable |
8882 | |||||||
| Advance payments on contracts in progress |
8892 | |||||||
| Other amounts payable |
8902 | |||||||
| Total amounts payable with a remaining term of more than one year, yet less than 5 years |
8912 | |||||||
| Amounts payable with a remaining term of more than 5 years |
||||||||
| Financial debts |
8803 | |||||||
| Subordinated loans |
8813 | |||||||
| Unsubordinated debentures |
8823 | |||||||
| Leasing and other similar obligations |
8833 | |||||||
| Credit institutions |
8843 | |||||||
| Other loans |
8853 | |||||||
| Trade debts |
8863 | |||||||
| Suppliers |
8873 | |||||||
| Bills of exchange payable |
8883 | |||||||
| Advance payments on contracts in progress |
8893 | |||||||
| Other amounts payable |
8903 | |||||||
| Amounts payable with a remaining term of more than 5 years |
8913 | |||||||
34/57
| N°. | 0466.460.429 | F-cap 6.9 |
| Codes | Period | |||||||
| AMOUNTS PAYABLE GUARANTEED (included in accounts 17 and 42/48 of liabilities) |
||||||||
| Amounts payable guaranteed by the Belgian government agencies |
||||||||
| Financial debts |
8921 | |||||||
| Subordinated loans |
8931 | |||||||
| Unsubordinated debentures |
8941 | |||||||
| Leasing and other similar obligations |
8951 | |||||||
| Credit institutions |
8961 | |||||||
| Other loans |
8971 | |||||||
| Trade debts |
8981 | |||||||
| Suppliers |
8991 | |||||||
| Bills of exchange payable |
9001 | |||||||
| Advance payments on contracts in progress |
9011 | |||||||
| Remuneration and social security |
9021 | |||||||
| Other amounts payable |
9051 | |||||||
| Total of the amounts payable guaranteed by the Belgian government agencies |
9061 | |||||||
| Amounts payable guaranteed by real securities given or irrevocably promised by the company on its own assets |
||||||||
| Financial debts |
8922 | |||||||
| Subordinated loans |
8932 | |||||||
| Unsubordinated debentures |
8942 | |||||||
| Leasing and other similar obligations |
8952 | |||||||
| Credit institutions |
8962 | |||||||
| Other loans |
8972 | |||||||
| Trade debts |
8982 | |||||||
| Suppliers |
8992 | |||||||
| Bills of exchange payable |
9002 | |||||||
| Advance payments on contracts in progress |
9012 | |||||||
| Taxes, remuneration and social security |
9022 | |||||||
| Taxes |
9032 | |||||||
| Remuneration and social security |
9042 | |||||||
| Other amounts payable |
9052 | |||||||
| Total amounts payable guaranteed by real securities given or irrevocably promised by the company on its own assets |
9062 | |||||||
| Codes | Period | |||||||
| TAXES, REMUNERATION AND SOCIAL SECURITY |
||||||||
| Taxes (headings 450/3 and 178/9 of liabilities) |
||||||||
| Outstanding tax debts |
9072 | |||||||
| Accruing taxes payable |
9073 | 2.310.567 | ||||||
| Estimated taxes payable |
450 | |||||||
| Remuneration and social security (headings 454/9 and 178/9 of liabilities) |
||||||||
| Amounts due to the National Social Security Office |
9076 | |||||||
| Other amounts payable in respect of remuneration and social security |
9077 | 21.447.014 | ||||||
35/57
| N°. | 0466.460.429 | F-cap 6.9 |
| Period | ||||
| ACCRUALS AND DEFERRED INCOME |
||||
| Allocation of heading 492/3 of liabilities if the amount is significant |
||||
| Accrued charges |
537.523 | |||
| Deferred income |
1.297.596.976 | |||
| Deferred subsidies |
809.861 | |||
| Deferred tax credit |
4.768.426 | |||
36/57
| N°. | 0466.460.429 | F-cap 6.10 |
OPERATING RESULTS
| Codes | Period | Preceding period | ||||||||||||||
| OPERATING INCOME |
||||||||||||||||
| Net turnover |
||||||||||||||||
| Allocation by categories of activity |
||||||||||||||||
| Research & Development |
581.275.559 | 418.495.174 | ||||||||||||||
| Royalties |
9.465.229 | |||||||||||||||
| Sales of trading products |
38.158.275 | |||||||||||||||
| Allocation by geographical market |
||||||||||||||||
| US - United States |
493.768.671 | 355.933.790 | ||||||||||||||
| FR - France |
1.412.714 | 1.061.117 | ||||||||||||||
| DE - Germany |
23.703.679 | 0 | ||||||||||||||
| LU - Luxemburg |
0 | |||||||||||||||
| BS - Bahama’s |
88.171.352 | 32.218.166 | ||||||||||||||
| BE - Belgium |
2.850.918 | 17.377.287 | ||||||||||||||
| NL - The Netherlands |
1.427.094 | 497.512 | ||||||||||||||
| ES - Spain |
1.126.435 | 513.166 | ||||||||||||||
| GB - United Kingdom |
3.069.118 | 1.475.413 | ||||||||||||||
| IE - Ireland |
544.095 | 437.798 | ||||||||||||||
| IT - Italy |
1.806.386 | 1.246.643 | ||||||||||||||
| AT - Austria |
3.794.280 | 2.471.940 | ||||||||||||||
| CH - Switserland |
55.839 | |||||||||||||||
| Fi - Finland |
764.067 | 264.843 | ||||||||||||||
| SE - Sweden |
3.960.278 | 2.745.974 | ||||||||||||||
| No - Norway |
2.474.127 | 2.195.686 | ||||||||||||||
| DK - Denmark |
25.850 | |||||||||||||||
| Other operating income |
||||||||||||||||
| Operating subsidies and compensatory amounts received from public authorities |
740 | 6.618.032 | 1.804.327 | |||||||||||||
| OPERATING CHARGES |
||||||||||||||||
| Employees for whom the company submitted a DIMONA declaration or who are recorded in the general personnel register |
||||||||||||||||
| Total number at the closing date |
9086 | 367 | 442 | |||||||||||||
| Average number of employees calculated in full-time equivalents |
9087 | 368,6 | 432,6 | |||||||||||||
| Number of actual hours worked |
9088 | 622.373 | 720.120 | |||||||||||||
| Personnel costs |
||||||||||||||||
| Remuneration and direct social benefits |
620 | 55.931.319 | 59.050.615 | |||||||||||||
| Employers’ contribution for social security |
621 | 12.942.717 | 12.402.668 | |||||||||||||
| Employers’ premiums for extra statutory insurance |
622 | 2.724.157 | 2.558.186 | |||||||||||||
| Other personnel costs |
623 | 1.957.465 | 3.760.652 | |||||||||||||
| Retirement and survivors’ pensions |
624 | |||||||||||||||
37/57
| N°. | 0466.460.429 | F-cap 6.10 |
| Codes | Period | Preceding period | ||||||||||||||
| Provisions for pensions and similar obligations |
||||||||||||||||
| Appropriations (uses and write-backs) |
(+ | )/(-) | 635 | |||||||||||||
| Depreciations |
||||||||||||||||
| On stock and contracts in progress |
||||||||||||||||
| Recorded |
9110 | 1.239.142 | ||||||||||||||
| Written back |
9111 | |||||||||||||||
| On trade debtors |
||||||||||||||||
| Recorded |
9112 | |||||||||||||||
| Written back |
9113 | |||||||||||||||
| Provisions for liabilities and charges |
||||||||||||||||
| Appropriations |
9115 | 4.220.419 | 866.214 | |||||||||||||
| Uses and write-backs |
9116 | |||||||||||||||
| Other operating charges |
||||||||||||||||
| Taxes related to operation |
640 | 3.731.217 | 4.041.673 | |||||||||||||
| Other |
641/8 | 67.053.825 | 98.107.447 | |||||||||||||
| Hired temporary staff and personnel placed at the company’s disposal |
||||||||||||||||
| Total number at the closing date |
9096 | |||||||||||||||
| Average number calculated in full-time equivalents |
9097 | 0,7 | 6,5 | |||||||||||||
| Number of actual hours worked |
9098 | 1.440 | 3.238 | |||||||||||||
| Costs to the company |
617 | 34.068 | 84.787 | |||||||||||||
38/57
| N°. | 0466.460.429 | F-cap 6.11 |
FINANCIAL RESULTS
| Codes | Period | Preceding period | ||||||||||||||
| RECURRING FINANCIAL INCOME |
||||||||||||||||
| Other financial income |
||||||||||||||||
| Subsidies paid by public authorities, added to the profit and loss account |
||||||||||||||||
| Capital subsidies |
9125 | |||||||||||||||
| Interest subsidies |
9126 | |||||||||||||||
| Allocation of other financial income |
||||||||||||||||
| Exchange differences realized |
754 | 1.620.821 | 13.265.513 | |||||||||||||
| Other |
||||||||||||||||
| Other financial gains |
22.666 | 31.623 | ||||||||||||||
| Unrealized exchange gains |
0 | 89.296.991 | ||||||||||||||
| RECURRING FINANCIAL CHARGES |
||||||||||||||||
| Depreciation of loan issue expenses |
6501 | |||||||||||||||
| Capitalised interests |
6502 | 4.053.120 | 2.197.496 | |||||||||||||
| Depreciations on current assets |
||||||||||||||||
| Recorded |
6510 | |||||||||||||||
| Written back |
6511 | 9.102.765 | ||||||||||||||
| Other financial charges |
||||||||||||||||
| Amount of the discount borne by the company, as a result of negotiating amounts receivable |
653 | 125 | ||||||||||||||
| Provisions of a financial nature |
||||||||||||||||
| Appropriations |
6560 | |||||||||||||||
| Uses and write-backs |
6561 | |||||||||||||||
| Allocation of other financial costs |
||||||||||||||||
| Exchange differences realized |
654 | 0 | 9.927.654 | |||||||||||||
| Results from the conversion of foreign currencies |
655 | |||||||||||||||
| Other |
||||||||||||||||
| Unrealized exchange losses |
30.873.435 | 37.714.315 | ||||||||||||||
| Bank charges |
714.971 | 615.692 | ||||||||||||||
| Payment differences |
3.497 | 1.703 | ||||||||||||||
| Other financial charges |
875.195 | 1.619.153 | ||||||||||||||
39/57
| N°. | 0466.460.429 | F-cap 6.12 |
INCOME AND CHARGES OF EXCEPTIONAL SIZE OR FREQUENCY
| Codes | Period | Preceding period | ||||||||||||||
| NON-RECURRING INCOME |
76 | 547.659 | 19.000 | |||||||||||||
| Non-recurring operating income |
(76A | ) | 547.659 | 19.000 | ||||||||||||
| Write-back of depreciation and of amounts written off intangible and tangible fixed assets |
760 | |||||||||||||||
| Write-back of provisions for extraordinary operating liabilities and charges |
7620 | |||||||||||||||
| Capital profits on disposal of intangible and tangible fixed assets |
7630 | 3.614 | 19.000 | |||||||||||||
| Other non-recurring operating income |
764/8 | 544.045 | ||||||||||||||
| Non-recurring financial income |
(76B | ) | 0 | |||||||||||||
| Write-back of amounts written down financial fixed assets |
761 | |||||||||||||||
| Write-back of provisions for extraordinary financial liabilities and charges |
7621 | |||||||||||||||
| Capital profits on disposal of financial fixed assets |
7631 | |||||||||||||||
| Other non-recurring financial income |
769 | |||||||||||||||
| NON-RECURRING CHARGES |
66 | 11.106.324 | 36.854.421 | |||||||||||||
| Non-recurring operating charges |
(66A | ) | 1.037.409 | 36.854.421 | ||||||||||||
| Non-recurring depreciation of and amounts written off formation expenses, intangible and tangible fixed assets |
660 | 932.662 | 35.566.302 | |||||||||||||
| Provisions for extraordinary operating liabilities and charges: |
(+ | )/(-) | 6620 | |||||||||||||
| Capital losses on disposal of intangible and tangible fixed assets |
6630 | 104.748 | 288.120 | |||||||||||||
| Other non-recurring operating charges |
664/7 | 1.000.000 | ||||||||||||||
| Non-recurring operating charges carried to assets as restructuring costs |
(- | ) | 6690 | |||||||||||||
| Non-recurring financial charges |
(66B | ) | 10.068.915 | 0 | ||||||||||||
| Amounts written off financial fixed assets |
661 | 10.068.915 | ||||||||||||||
| Provisions for extraordinary financial liabilities and charges -appropriations (uses) |
(+ | )/(-) | 6621 | |||||||||||||
| Capital losses on disposal of financial fixed assets |
6631 | |||||||||||||||
| Other non-recurring financial charges |
668 | |||||||||||||||
| Non-recurring financial charges carried to assets as restructuring costs |
(- | ) | 6691 | |||||||||||||
40/57
| N°. | 0466.460.429 | F-cap 6.13 |
TAXES
| Codes | Period | |||||||
| INCOME TAXES |
||||||||
| Income taxes on the result of the period |
9134 | 72.259 | ||||||
| Income taxes paid and withholding taxes due or paid |
9135 | 304.654 | ||||||
| Excess of income tax prepayments and withholding taxes paid recorded under assets |
9136 | 304.654 | ||||||
| Estimated additional taxes |
9137 | 72.259 | ||||||
| Income taxes on the result of prior periods |
9138 | |||||||
| Additional income taxes due or paid |
9139 | |||||||
| Additional income taxes estimated or provided for |
9140 | |||||||
| Major reasons for the differences between pre-tax profit, as it results from the annual accounts, and estimated taxable profit |
||||||||
| Non-deductible expenses |
5.193.861 | |||||||
| Tax credit |
-26.711.807 | |||||||
| Excess depreciations |
-30.400.753 | |||||||
| Exemption regional contributions |
-538.407 | |||||||
| Period | ||||
| Influence of non-recurring results on income taxes on the result of the period |
| Codes | Period | |||||||
| Sources of deferred taxes |
||||||||
| Deferred taxes representing assets |
9141 | 1.701.941.806 | ||||||
| Accumulated tax losses deductible from future taxable profits |
9142 | 757.947.489 | ||||||
| Other deferred taxes representing assets |
||||||||
| Investment deduction |
966.348 | |||||||
| Deduction for innovation income |
390.310.849 | |||||||
| Excess depreciations |
534.028.071 | |||||||
| DBI |
18.689.049 | |||||||
| Deferred taxes representing liabilities |
9144 | |||||||
| Allocation of deferred taxes representing liabilities |
||||||||
| Codes | Period | Preceding period | ||||||||||
| VALUE-ADDED TAXES AND TAXES BORNE BY THIRD PARTIES |
||||||||||||
| Value-added taxes charged |
||||||||||||
| To the company (deductible) |
9145 | 27.244.542 | 29.346.606 | |||||||||
| By the company |
9146 | 12.736.222 | 11.975.602 | |||||||||
| Amounts withheld on behalf of third party by way of |
||||||||||||
| Payroll withholding taxes |
9147 | 23.134.701 | 29.561.849 | |||||||||
| Withholding taxes on investment income |
9148 | |||||||||||
41/57
| N°. | 0466.460.429 | F-cap 6.14 |
RIGHTS AND COMMITMENTS NOT REFLECTED IN THE BALANCE SHEET
| Codes | Period | |||||||
| PERSONAL GUARANTEES PROVIDED OR IRREVOCABLY PROMISED BY THE COMPANY AS SECURITY FOR DEBTS AND COMMITMENTS OF THIRD PARTIES |
9149 | 4.998.336 | ||||||
|
|
|
|||||||
| Of which |
||||||||
| Bills of exchange in circulation endorsed by the company |
9150 | |||||||
| Bills of exchange in circulation drawn or guaranteed by the company |
9151 | |||||||
| Maximum amount for which other debts or commitments of third parties are guaranteed by the company |
9153 | 4.998.336 | ||||||
| REAL GUARANTEES |
||||||||
| Real guarantees provided or irrevocably promised by the company on its own assets as security of debts and commitments of the company |
||||||||
| Mortgages |
||||||||
| Book value of the immovable properties mortgaged |
91611 | |||||||
| Amount of registration |
91621 | |||||||
| For irrevocable mortgage mandates, the amount for which the agent can take registration |
91631 | |||||||
| Pledging of goodwill |
||||||||
| Maximum amount up to which the debt is secured and which is the subject of registration |
91711 | |||||||
| For irrevocable mandates to pledge goodwill, the amount for which the agent can take the inscription |
91721 | |||||||
| Pledging of other assets or irrevocable mandates to pledge other assets |
||||||||
| Book value of the immovable properties mortgaged |
91811 | |||||||
| Maximum amount up to which the debt is secured |
91821 | |||||||
| Guarantees provided or irrevocably promised on future assets |
||||||||
| Amount of assets in question |
91911 | |||||||
| Maximum amount up to which the debt is secured |
91921 | |||||||
| Vendor’s privilege |
||||||||
| Book value of sold goods |
92011 | |||||||
| Amount of the unpaid price |
92021 | |||||||
42/57
| N°. | 0466.460.429 | F-cap 6.14 |
| Codes | Period | |||||||
| Real guarantees provided or irrevocably promised by the company on its own assets as security of debts and commitments of third parties |
||||||||
| Mortgages |
||||||||
| Book value of the immovable properties mortgaged |
91612 | |||||||
| Amount of registration |
91622 | |||||||
| For irrevocable mortgage mandates, the amount for which the agent can take registration |
91632 | |||||||
| Pledging of goodwill |
||||||||
| Maximum amount up to which the debt is secured and which is the subject of registration |
91712 | |||||||
| For irrevocable mandates to pledge goodwill, the amount for which the agent can take the inscription |
91722 | |||||||
| Pledging of other assets or irrevocable mandates to pledge other assets |
||||||||
| Book value of the immovable properties mortgaged |
91812 | |||||||
| Maximum amount up to which the debt is secured |
91822 | |||||||
| Guarantees provided or irrevocably promised on future assets |
||||||||
| Amount of assets in question |
91912 | |||||||
| Maximum amount up to which the debt is secured |
91922 | |||||||
| Vendor’s privilege |
||||||||
| Book value of sold goods |
92012 | |||||||
| Amount of the unpaid price |
92022 | |||||||
| Codes | Period | |||||||
| GOODS AND VALUES, NOT REFLECTED IN THE BALANCE SHEET, HELD BY THIRD PARTIES IN THEIR OWN NAME BUT FOR THE BENEFIT AND AT THE RISK OF THE COMPANY |
||||||||
| SUBSTANTIAL COMMITMENTS TO ACQUIRE FIXED ASSETS |
||||||||
| Commitments to the purchase of tangible and intangible assets |
684.785 | |||||||
| SUBSTANTIAL COMMITMENTS TO DISPOSE OF FIXED ASSETS |
||||||||
| FORWARD TRANSACTIONS |
||||||||
| Goods purchased (to be received) |
9213 | |||||||
| Goods sold (to be delivered) |
9214 | |||||||
| Currencies purchased (to be received) |
9215 | |||||||
| Currencies sold (to be delivered) |
9216 | |||||||
| Period | ||||||||
| COMMITMENTS RELATING TO TECHNICAL GUARANTEES IN RESPECT OF SALES OR SERVICES |
||||||||
| Period | ||||||||
| AMOUNT, NATURE AND FORM CONCERNING LITIGATION AND OTHER IMPORTANT COMMITMENTS |
||||||||
43/57
| N°. | 0466.460.429 | F-cap 6.14 |
SETTLEMENT REGARDING THE COMPLEMENTARY RETIREMENT OR SURVIVORS’ PENSION FOR PERSONNEL AND BOARD MEMBERS
Brief description
Galapagos NV has a group insurance and guaranteed income insurance in favor of their personnel and directors. Galapagos NV carries all costs related to these insurances. The amount of the premium contribution was 2.757K EUR in 2023.
Measures taken to cover the related charges
| Code | Period | |||||||
| PENSIONS FUNDED BY THE COMPANY ITSELF |
||||||||
| Estimated amount of the commitments resulting from past services |
9220 | |||||||
| Methods of estimation |
||||||||
| Galapagos NV has a group insurance and guaranteed income insurance in favor of their personnel and directors. Galapagos NV carries all costs related to these insurances. The amount of the premium contribution was 2.757K EUR in 2023. |
||||||||
| Period | ||||||||
| NATURE AND FINANCIAL IMPACT OF SIGNIFICANT EVENTS AFTER THE CLOSING DATE not reflected in the balance sheet or income statement |
||||||||
| On January 3, 2024, a strategic collaboration and licensing agreement was signed with BridGene Biosciences. BridGene has received an upfront payment of USD 27 million. |
24.434.389 | |||||||
| On January 31, 2024, the transaction regarding the Jyseleca activities was successfully completed with Alfasigma. For this Alfasigma made an upfront payment of EUR 50 million, plus EUR 13.2 million for the cash position and net working capital. |
63.200.000 | |||||||
| On January 31, 2024, Galapagos NV participated in a Series C financing round of Frontier Medicines for an amount of USD 40 million. |
36.199.095 | |||||||
| Period | ||||||||
| COMMITMENTS TO PURCHASE OR SALE AVAILABLE TO THE COMPANY AS ISSUER OF OPTIONS FOR SALE OR PURCHASE |
||||||||
| Period | ||||||||
| NATURE, COMMERCIAL OBJECTIVE AND FINANCIAL CONSEQUENCES OF TRANSACTIONS NOT REFLECTED IN THE BALANCE SHEET |
||||||||
| If the risks and benefits resulting from such transactions are of any meaning and if publishing such risks and benefits is necessary to appreciate the financial situation of the company |
||||||||
| Period | ||||||||
| OTHER RIGHTS AND COMMITMENTS NOT REFLECTED IN THE BALANCE SHEET (including those that cannot be calculated) |
||||||||
44/57
| N°. | 0466.460.429 | F-cap 6.15 |
RELATIONSHIPS WITH AFFILIATED COMPANIES, ASSOCIATED COMPANIES AND OTHER COMPANIES LINKED BY PARTICIPATING INTERESTS
| Codes | Period | Preceding period | ||||||||||
| AFFILIATED COMPANIES |
||||||||||||
| Financial fixed assets |
(280/1 | ) | 249.427.250 | 247.851.399 | ||||||||
| Participating interests |
(280 | ) | 204.127.249 | 154.642.558 | ||||||||
| Subordinated amounts receivable |
9271 | 45.300.001 | 93.208.841 | |||||||||
| Other amounts receivable |
9281 | |||||||||||
| Amounts receivable |
9291 | 43.820.046 | 64.503.786 | |||||||||
| Over one year |
9301 | |||||||||||
| Within one year |
9311 | 43.820.046 | 64.503.786 | |||||||||
| Current investments |
9321 | |||||||||||
| Shares |
9331 | |||||||||||
| Amounts receivable |
9341 | |||||||||||
| Amounts payable |
9351 | 48.868.651 | 230.595.113 | |||||||||
| Over one year |
9361 | |||||||||||
| Within one year |
9371 | 48.868.651 | 230.595.113 | |||||||||
| Personal and real guarantees |
||||||||||||
| Provided or irrevocably promised by the company as security for debts or commitments of affiliated companies |
9381 | 4.162.896 | 1.950.737 | |||||||||
| Provided or irrevocably promised by affiliated companies as security for debts or commitments of the company |
9391 | |||||||||||
| Other significant financial commitments |
9401 | |||||||||||
| Financial results |
||||||||||||
| Income from financial fixed assets |
9421 | 109.515.173 | 10.500.000 | |||||||||
| Income from current assets |
9431 | 7.591.058 | ||||||||||
| Other financial income |
9441 | 3.591.036 | ||||||||||
| Debt charges |
9461 | 3.019.431 | ||||||||||
| Other financial charges |
9471 | |||||||||||
| Disposal of fixed assets |
||||||||||||
| Capital profits realised |
9481 | |||||||||||
| Capital losses realised |
9491 | |||||||||||
45/57
| N°. | 0466.460.429 | F-cap 6.15 |
RELATIONSHIPS WITH AFFILIATED COMPANIES, ASSOCIATED COMPANIES AND OTHER COMPANIES LINKED BY PARTICIPATING INTERESTS
| Codes | Period | Preceding period | ||||||||||
| ASSOCIATED COMPANIES |
||||||||||||
| Financial fixed assets |
9253 | |||||||||||
| Participating interests |
9263 | |||||||||||
| Subordinated amounts receivable |
9273 | |||||||||||
| Other amounts receivable |
9283 | |||||||||||
| Amounts receivable |
9293 | |||||||||||
| Over one year |
9303 | |||||||||||
| Within one year |
9313 | |||||||||||
| Amounts payable |
9353 | |||||||||||
| Over one year |
9363 | |||||||||||
| Within one year |
9373 | |||||||||||
| Personal and real guarantees |
||||||||||||
| Provided or irrevocably promised by the company as security for debts or commitments of affiliated companies |
9383 | |||||||||||
| Provided or irrevocably promised by affiliated companies as security for debts or commitments of the company |
9393 | |||||||||||
| Other significant financial commitments |
9403 | |||||||||||
| COMPANIES LINKED BY PARTICIPATING INTERESTS |
||||||||||||
| Financial fixed assets |
9252 | 1 | 1 | |||||||||
| Participating interests |
9262 | 1 | 1 | |||||||||
| Subordinated amounts receivable |
9272 | |||||||||||
| Other amounts receivable |
9282 | |||||||||||
| Amounts receivable |
9292 | |||||||||||
| Over one year |
9302 | |||||||||||
| Within one year |
9312 | |||||||||||
| Amounts payable |
9352 | |||||||||||
| Over one year |
9362 | |||||||||||
| Within one year |
9372 | |||||||||||
46/57
| N°. | 0466.460.429 | F-cap 6.15 |
RELATIONSHIPS WITH AFFILIATED COMPANIES, ASSOCIATED COMPANIES AND OTHER COMPANIES LINKED BY PARTICIPATING INTERESTS
| Period | ||||
| TRANSACTIONS WITH AFFILIATED PARTIES BEYOND NORMAL MARKET CONDITIONS |
||||
| Mention of these transactions if they are significant, including the amount of the transactions, the nature of the link, and all information about the transactions that should be necessary to get a better understanding of the financial situation of the company |
||||
| Due to the absence of legal criteria that allow transactions with affiliated parties beyond normal market conditions to be enumerated, no transactions were included in F-cap 6.15. |
||||
47/57
| N°. | 0466.460.429 | F-cap 6.16 |
FINANCIAL RELATIONSHIPS WITH
| Codes | Period | |||||||
| DIRECTORS AND MANAGERS, INDIVIDUALS OR LEGAL PERSONS WHO CONTROL THE COMPANY DIRECTLY OR INDIRECTLY WITHOUT BEING ASSOCIATED THEREWITH, OR OTHER COMPANIES CONTROLLED DIRECTLY OR INDIRECTLY BY THESE PERSONS |
||||||||
| Amounts receivable from these persons |
9500 | |||||||
| Principal conditions regarding amounts receivable, rate of interest, duration, any amounts repaid, cancelled or written off |
||||||||
| Guarantees provided in their favour |
9501 | |||||||
| Other significant commitments undertaken in their favour |
9502 | |||||||
| Amount of direct and indirect remunerations and pensions, reflected in the income statement, as long as this disclosure does not concern exclusively or mainly, the situation of a single identifiable person |
||||||||
| To directors and managers |
9503 | 748.993 | ||||||
| To former directors and former managers |
9504 | |||||||
| Codes | Period | |||||||
| THE AUDITOR(S) AND THE PERSONS WHOM HE (THEY) IS (ARE) COLLABORATING WITH |
||||||||
| Auditors’ fees |
9505 | 1.043.258 | ||||||
| Fees for exceptional services or special assignments executed within the company by the auditor |
||||||||
| Other audit assignments |
95061 | 20.209 | ||||||
| Tax consultancy assignments |
95062 | |||||||
| Other assignments beyond the audit |
95063 | 6.605 | ||||||
| Fees for exceptional services or special assignments executed within the company by people the auditor(s) is (are collaborating with |
||||||||
| Other audit assignments |
95081 | |||||||
| Tax consultancy assignments |
95082 | 67.964 | ||||||
| Other assignments beyond the audit |
95083 | |||||||
Mentions related to article 3:64, § 2 and § 4 of the Belgian Companies and Associations Code
48/57
| N°. | 0466.460.429 | F-cap 6.18.1 |
DECLARATION WITH REGARD TO THE CONSOLIDATED ANNUAL ACCOUNTS
INFORMATION TO DISCLOSE BY EACH COMPANY GOVERNED BY THE BELGIAN COMPANIES AND ASSOCIATIONS CODE ON THE CONSOLIDATED ANNUAL ACCOUNTS
The company has prepared and published consolidated annual accounts and a consolidated annual report*
f
Name, full address of the registered office and, if it concerns companies under Belgian law, the company registration number of the parent company(ies) and the indication if this (these) parent company(ies) prepares (prepare) and publishes (publish) consolidated annual accounts, in which the annual accounts are included by means of consolidation**:
If the parent company(ies) is (are) (a) company(ies) governed by foreign law, the location where the abovementioned annual accounts are available**:
| * | Strike out what does not apply. |
| ** | Where the annual accounts of the company are consolidated at different levels, the information should be given, on the one hand at the highest and on the other at the lowest level of companies of which the company is a subsidiary and for which consolidated accounts are prepared and published. |
49/57
| N°. | 0466.460.429 | F-cap 6.18.2 |
FINANCIAL RELATIONSHIPS OF THE GROUP THE COMPANY IS IN CHARGE OF IN BELGIUM WITH THE AUDITOR(S) AND THE PERSONS WITH WHOM HE (THEY) IS (ARE) LINKED
| Codes | Period | |||||||
| Mentions related to article 3:65, § 4 and § 5 of the Belgian Companies and Associations Code |
||||||||
| Fees to auditors according to the mandate at the group level led by the company publishing the information |
9507 | 1.124.038 | ||||||
| Fees for exceptional services or special missions executed by the auditor(s) at this group |
||||||||
| Other audit assignments |
95071 | 20.209 | ||||||
| Tax consultancy assignments |
95072 | |||||||
| Other assignments beyond the audit |
95073 | 6.605 | ||||||
| Fees to people auditors are linked to according to the mandate at the group level led by the company publishing the information |
9509 | |||||||
| Fees for exceptional services or special assignments executed at this group by people the auditor(s) is (are) linked to |
||||||||
| Other audit assignments |
95091 | |||||||
| Tax consultancy assignments |
95092 | 67.964 | ||||||
| Other assignments beyond the audit |
95093 | |||||||
Mentions related to article 3:64, § 2 and § 4 of the Belgian Companies and Associations Code
50/57
| N°. | 0466.460.429 | F-cap 6.19 |
VALUATION RULES
The valuation rules were prepared in accordance with the provisions of Chapter II of the Royal Decree of April 29, 2019 related to corporate financial statements and are valid for evaluating all assets, receivables, payables and obligations of the company.
Any changes will be submitted in advance to the Board of Directors for approval.
The current rules have been established and the evaluation rules are being carried in view of the continuation of the company. Summary of Valuation Rules:
1. Fixed assets
(a) Intangible assets
The company invests in research and development projects. Research and development expenses are, as of fiscal year 2010, recorded on the assets only to the extent that their cost does not exceed a prudent estimate of their value in use or their future return for the company and amortized over a 3-year period through fiscal year 2015. As from fiscal year 2016 research and development costs that do not qualify as part of a development phase are recorded on the assets and fully amortized in the same fiscal year (according to CBN Opinions 2016/16 and 2016/27).
Research and development in progress acquired through licensing agreements, business combinations, collaboration agreements or separate acquisitions are recognized as intangible assets if they are separately identifiable, controlled by us and can generate economic benefit. Since there is a consideration that for separately acquired research and development assets the probability criterion is met, upfront and success payments to third parties for products or drug candidates for which approval has not yet been received have been recognized as intangible assets. We consider these intangible fixed assets not yet available for use until the underlying asset is approved and commercially launched.
As from approval for commercialization of the underlying asset, depreciation is recorded and the asset will be depreciated over its useful life.
Licenses, patents and know-how are amortized on a straight-line basis over the useful life (usually between 5 and 20 years). Other intangible assets, including acquired intellectual property, are recorded at acquisition cost. These assets are depreciated on a straight-line basis over their estimated useful lives as soon as they are ready for their intended use. They are included in the assets to the extent that their net book value does not exceed a prudent estimate of their value in use or their future returns for the company.
(b) Tangible fixed assets
Property, plant and equipment are recorded at cost. Depreciation is on a straight-line basis, taking into account the economic life of the assets.
| • | Lab material: 5-10 years |
| • | IT hardware and software: 3-5 years |
| • | Furniture and rolling stock: 5-10 years |
2. Trade receivables
Trade receivables are recorded at face value. Foreign currency receivables are translated at the exchange rate valid at the balance sheet date. Exchange differences are recognized in the income statement.
When collection becomes doubtful, a provision is made for doubtful debtors.
3. Stocks
Raw materials, auxiliary materials and trade goods are valued at acquisition cost.
Work in progress and finished goods are valued at cost.
Cost includes, in addition to direct production and material costs, a proportionate share of depreciation and amortization of assets that were used in the production process used.
Inventories are valued using the FIFO method. If the acquisition cost or cost exceeds the net realizable value, valuation at the lower net realizable value is applied. Net realizable value is equal to the estimated normal sales price, less estimated completion costs and estimated costs required to make the sale.
4. Cash investments and liquid assets
Deposits with financial institutions are valued at nominal value. Securities are valued at acquisition cost.
Additional costs are immediately charged to earnings. Write-downs are recorded if the realization value at the balance sheet date is less than the amount previously recorded.
Foreign currency balances are translated at the exchange rate valid at the balance sheet date. Exchange differences are recorded in the income statement.
5. Provisions for other risks and costs.
This included this year’s provisions for restricted stock units following the 2018/16 CBN opinion and this reasoning was upheld for the deferred management bonus.
6. Revenue
Revenue to date consists primarily of success payments, license fees, and prepayments obtained from collaboration agreements. The Company also generates revenues from various research and development incentives and grants.
Cooperation agreements with the Company’s commercial partners for activities related to research and development generally include non-refundable prepayments received; success payments, whose receipt depends on the achievement of certain clinical, regulatory or commercial milestones; license fees and royalties on selling.
In case of revenue recognition staggered over time, the unrecognized portion is recorded as revenue carried forward.
The revenue recognition policy can be summarized as follows:
(a) Prepayments received
Non-refundable prepayments received in connection with cooperative research agreements and development are spread and recognized over the relevant and desired period of the company’s performance commitment. Payments and company involvement are contractually defined by phase. At the outset, Management makes a estimate of the duration of the company’s involvement, as well as the costs related to the project. Prepayments are recognized over the expected period of engagement, either on a straight-line basis or based on costs incurred in the framework of the project if these costs can be reliably estimated. Periodically, the Company reviews the estimated time and cost for the project and adjusts the period over which revenue is spread.
51/57
| N°. | 0466.460.429 | F-cap 6.19 |
VALUATION RULES
(b) Success payments
A success payment is included in the transaction price only when there is a substantial likelihood that a material reversal of cumulative recognized revenue will not occur.
Success payments that are not irrevocable, substantial or proportionate, are recognized as revenue to be carried forward. Revenue from these activities can vary greatly from period to period due to the timing of the success payment.
(c) Licenses
Revenues arising from licenses limited in time are spread over the period covered by the license, which increases the obligation of the license reflects over that term, to update the content and ensure continuous updating.
Proceeds from perpetual licenses are recognized immediately upon sale to the extent that there are no further obligations.
(d) Royalty agreements
Royalty income is recognized when the Company can estimate the amount with reasonable certainty and a reasonable assurance of collectability exists. As a result, the company is generally going to recognize royalties in the period that the licensee reported the royalties to the company through royalty reports, i.e., the royalty income is normally recognized only retrospectively, after the period in which sales by the licensee have occurred. Under this accounting rule, revenues arising from royalties included in the reporting of the company are not based on an estimate by the company but are typically reported in the same period as the obtained payment from the licensee.
(e) Grants and R&D support measures.
Because the company participates extensively in research and development activities, the company also benefits from multiple subsidies and R&D support measures from certain government agencies. These subsidies and R&D support measures are generally used to partially reimburse approved research and development costs. They are therefore credited to the earnings, under other income (excluding tax credit for research and development costs), when the relevant expenditures have been made and reasonable certainty exists about the subsidies (to be) received and R&D support measures.
f) Proceeds recognition from collaboration with Gilead to develop filgotinib
This is included in the result due to the termination of activities for a value of EUR 348.728.994.
| • | Identification of the contract |
Despite the recent addition to the collaboration agreement with Gilead for the development of filgotinib, management assessed that all activities remain beneficial for the development of filgotinib, of which Gilead still retains the rights outside Europe. All contract changes were analyzed as we believe that Gilead should still be a customer to be considered as. This is also supported by the fact that we concluded that there is only one results commitment remains related to filgotinib.
| • | Identification of the result obligation |
The recent changes to the collaboration agreement with Gilead did not give rise to new results obligations. There was only an adjustment in the scope and price of the existing filgotinib result obligation, which was only partially fulfilled at the time of the change.
| • | Allocation of the total transaction price |
We assessed that the contract change only affects the scope of the filgotinib performance commitment and that the change in both fixed and variable compensation reflects the adjusted stand-alone sales price of the remaining activities of this result obligation. If we had assessed that the higher reimbursement was not or only partially related to the filgotinib result obligation, then the compensation might have been allocated to other result obligations in the contract, leading to a change in the moment of revenue recognition.
The denominator used in the percentage of completion calculation reflects our best estimate of our total costs required to meet the filgotinib performance commitment. These costs were estimated taking into account management’s best estimate of the design and duration of ongoing and planned clinical studies and the timing of the closing of the transaction with Alfasigma.
As a result of this transaction, the agreement with Gilead regarding filgotinib will be transferred to Alfasigma and our performance obligation will be terminated. Reflect the remaining costs as of December 31, 2023 mainly the costs we expect to incur for the transfer to Alfasigma.
The increase in revenue is mainly the result of higher recognition of revenue from upfront payments. This increase is explained by a substantial decrease in our estimated remaining cost to complete the development of filgotinib as a result of the recent sale of all our Jyseleca® activities to Alfasigma including the transfer of the remaining development performance obligation after the closing of the transaction. This led to a significant increase in the percentage of completion of our result commitment and a significant positive catch-up effect in our revenues.
7. Continuity
To date, we have incurred significant operating losses, which is reflected in the balance sheet. However, we believe that our existing short-term financial investments, cash and cash equivalents at December 31, 2023 will enable us to fund our operating costs and capital expenditures for at least the next 12 months. The Board of Directors is also of the opinion that additional funding—if needed—can be obtained. Taking this into account, as well as the potential developments in our drug discovery and development activities, the Board of Directors believes that they can present the financial statements on a going concern basis. Although our short-term financial investments, cash and cash equivalents are sufficiently high for at least the next 12 months, the Board of Directors points out that if R&D activities go well, we will seek additional funding to support the ongoing development of our products or to support other business opportunities.
52/57
| N°. | 0466.460.429 | F-cap 6.20 |
OTHER INFORMATION TO DISCLOSE
A comfort letter was provided to Galapagos Real Estate Belgium B.V.
53/57
| N°. | 0466.460.429 | F-cap 10 |
SOCIAL BALANCE SHEET
Numbers of the joint industrial committees competent for the company: 207
STATEMENT OF THE PERSONS EMPLOYED
EMPLOYEES FOR WHOM THE COMPANY SUBMITTED A DIMONA DECLARATION OR WHO ARE RECORDED IN THE GENERAL PERSONNEL REGISTER
| During the period | Codes | Total | 1. Men | 2. Women | ||||||||||||
| Average number of employees |
||||||||||||||||
| Full-time |
1001 | 316,9 | 136,8 | 180,1 | ||||||||||||
| Part-time |
1002 | 63,0 | 11,5 | 51,5 | ||||||||||||
| Total in full-time equivalents (FTE) |
1003 | 368,6 | 146,2 | 222,4 | ||||||||||||
| Number of actual hours worked |
||||||||||||||||
| Full-time |
1011 | 539.184 | 239.584 | 299.600 | ||||||||||||
| Part-time |
1012 | 83.189 | 15.665 | 67.524 | ||||||||||||
| Total |
1013 | 622.373 | 255.249 | 367.124 | ||||||||||||
| Personnel costs |
||||||||||||||||
| Full-time |
1021 | 63.890.106 | 31.198.026 | 32.692.080 | ||||||||||||
| Part-time |
1022 | 9.665.552 | 2.190.556 | 7.474.996 | ||||||||||||
| Total |
1023 | 73.555.658 | 33.388.582 | 40.167.076 | ||||||||||||
| Benefits in addition to wages |
1033 | |||||||||||||||
| During the preceding period | Codes | P. Total | 1P. Men | 2P. Women | ||||||||||||
| Average number of employees in FTE |
1003 | 432,6 | 176,6 | 256,0 | ||||||||||||
| Number of actual hours worked |
1013 | 720.120 | 297.566 | 422.554 | ||||||||||||
| Personnel costs |
1023 | 77.772.120 | 36.721.873 | 41.050.247 | ||||||||||||
| Benefits in addition to wages |
1033 | 567.933 | 232.742 | 335.192 | ||||||||||||
54/57
| N°. | 0466.460.429 | F-cap 10 |
EMPLOYEES FOR WHOM THE COMPANY SUBMITTED A DIMONA DECLARATION OR WHO ARE RECORDED IN THE GENERAL PERSONNEL REGISTER (continuation)
| At the closing date of the period | Codes | 1. Full-time | 2. Part-time | 3. Total in full-time equivalents |
||||||||||||
| Number of employees |
105 | 312 | 55 | 357,2 | ||||||||||||
| By nature of the employment contract |
||||||||||||||||
| Contract for an indefinite period |
110 | 312 | 55 | 357,2 | ||||||||||||
| Contract for a definite period |
111 | |||||||||||||||
| Contract for the execution of a specifically assigned work |
112 | |||||||||||||||
| Replacement contract |
113 | |||||||||||||||
| According to gender and study level |
||||||||||||||||
| Men |
120 | 137 | 7 | 142,7 | ||||||||||||
| primary education |
1200 | 5 | 5,0 | |||||||||||||
| secondary education |
1201 | 9 | 9,0 | |||||||||||||
| higher non-university education |
1202 | 18 | 2 | 19,7 | ||||||||||||
| university education |
1203 | 105 | 5 | 109,0 | ||||||||||||
| Women |
121 | 175 | 48 | 214,5 | ||||||||||||
| primary education |
1210 | 5 | 5,0 | |||||||||||||
| secondary education |
1211 | 3 | 2 | 4,6 | ||||||||||||
| higher non-university education |
1212 | 40 | 16 | 53,0 | ||||||||||||
| university education |
1213 | 127 | 30 | 151,9 | ||||||||||||
| By professional category |
||||||||||||||||
| Management staff |
130 | |||||||||||||||
| Salaried employees |
134 | 312 | 55 | 357,2 | ||||||||||||
| Hourly employees |
132 | |||||||||||||||
| Other |
133 | |||||||||||||||
HIRED TEMPORARY STAFF AND PERSONNEL PLACED AT THE DISPOSAL OF THE COMPANY
| During the period | Codes | 1. Hired temporary staff |
2. Hired temporary staff and personnel placed at the company’s disposal |
|||||||||
| Average number of persons employed |
150 | 0,7 | ||||||||||
| Number of actual hours worked |
151 | 1.440 | ||||||||||
| Costs to the company |
152 | 34.068 | ||||||||||
55/57
| N°. | 0466.460.429 | F-cap 10 |
LIST OF PERSONNEL MOVEMENTS DURING THE PERIOD
| ENTRIES | Codes | 1. Full-time | 2. Part-time | 3. Total in full-time equivalents |
||||||||||||
| Number of employees for whom the company submitted a DIMONA declaration or who have been recorded in the general personnel register during the period |
205 | 58 | 58,0 | |||||||||||||
| By nature of the employment contract |
||||||||||||||||
| Contract for an indefinite period |
210 | 58 | 58,0 | |||||||||||||
| Contract for a definite period |
211 | |||||||||||||||
| Contract for the execution of a specifically assigned work |
212 | |||||||||||||||
| Replacement contract |
213 | |||||||||||||||
| DEPARTURES | Codes | 1. Full-time | 2. Part-time | 3. Total in full-time equivalents |
||||||||||||
| Number of employees whose contract-termination date has been included in the DIMONA declaration or in the general personnel register during the period |
305 | 111 | 22 | 128,8 | ||||||||||||
| By nature of the employment contract |
||||||||||||||||
| Contract for an indefinite period |
310 | 111 | 22 | 128,8 | ||||||||||||
| Contract for a definite period |
311 | |||||||||||||||
| Contract for the execution of a specifically assigned work |
312 | |||||||||||||||
| Replacement contract |
313 | |||||||||||||||
| By reason of termination of contract |
||||||||||||||||
| Retirement |
340 | |||||||||||||||
| Unemployment with extra allowance from enterprise |
341 | |||||||||||||||
| Dismissal |
342 | 74 | 17 | 87,7 | ||||||||||||
| Other reason |
343 | 37 | 5 | 41,1 | ||||||||||||
| Of which: the number of persons who continue to render services to the company at least half-time on a self-employment basis |
350 | |||||||||||||||
56/57
| N°. | 0466.460.429 | F-cap 10 |
INFORMATION ON TRAINING PROVIDED TO EMPLOYEES DURING THE PERIOD
| Total of initiatives of formal professional training at the expense of the employer | Codes | Men | Codes | Women | ||||||||||||
| Number of employees involved |
5801 | 5811 | ||||||||||||||
| Number of actual training hours |
5802 | 5812 | ||||||||||||||
| Net costs for the company |
5803 | 5813 | ||||||||||||||
| of which gross costs directly linked to training |
58031 | 58131 | ||||||||||||||
| of which contributions paid and payments to collective funds |
58032 | 58132 | ||||||||||||||
| of which grants and other financial advantages received (to deduct) |
58033 | 58133 | ||||||||||||||
| Total of initiatives of less formal or informal professional training at the expense of the employer |
||||||||||||||||
| Number of employees involved |
5821 | 181 | 5831 | 277 | ||||||||||||
| Number of actual training hours |
5822 | 3.522 | 5832 | 5.758 | ||||||||||||
| Net costs for the company |
5823 | 960.504 | 5833 | 1.465.063 | ||||||||||||
| Total of initial initiatives of professional training at the expense of the employer |
||||||||||||||||
| Number of employees involved |
5841 | 5851 | ||||||||||||||
| Number of actual training hours |
5842 | 5852 | ||||||||||||||
| Net costs for the company |
5843 | 5853 | ||||||||||||||
57/57
Exhibit 99.6

|
Phone: +32 (0)2 778 01 00 www.bdo.be |
The Corporate Village Da Vincilaan 9, Box E.6 Elsinore Building B-1930 Zaventem |
GALAPAGOS NV
Statutory auditor’s report
to the general meeting
for the year ended 31 December 2023
Free translation
BDO Bedrijfsrevisoren BV / BTW BE 0431.088.289 / RPR Brussel
BDO Réviseurs d’Entreprises SRL / TVA BE 0431.088.289 / RPM Bruxelles
BDO Bedrijfsrevisoren - BDO Réviseurs d’Entreprises BV/SRL, a company under Belgian law in the form of a private limited liability company, is a member of BDO International Limited, a UK company limited by guarantee, and forms part of the international BDO network of independent member firms.
BDO is the brand name for the BDO network and for each of the BDO Member Firms.

|
Phone: +32 (0)2 778 01 00 www.bdo.be |
The Corporate Village Da Vincilaan 9, Box E.6 Elsinore Building B-1930 Zaventem |
Free translation
STATUTORY AUDITOR’S REPORT TO THE GENERAL MEETING OF GALAPAGOS NV FOR THE YEAR ENDED 31 DECEMBER 2023
In the context of the statutory audit of the annual accounts of Galapagos NV (“the Company”), we hereby present our statutory auditor’s report. It includes our report of the annual accounts and the other legal and regulatory requirements. This report is an integrated whole and is indivisible.
We have been appointed as statutory auditor by the general meeting of 25 April 2023, following the proposal formulated by the administrative body issued upon recommendation of the Audit Committee and upon presentation by the works’ council. Our statutory auditor’s mandate expires on the date of the general meeting deliberating on the annual accounts closed on 31 December 2025. This is the first year of our statutory audit of the Company’s annual accounts.
REPORT ON THE ANNUAL ACCOUNTS
Unqualified opinion
We have audited the annual accounts of the Company, which comprise the balance sheet as at 31 December 2023, the profit and loss account for the year then ended and the notes to the annual accounts, characterised by a balance sheet total of 4.301.261.099 EUR and a profit and loss account showing a profit for the year of 271.292.107 EUR.
In our opinion, the annual accounts give a true and fair view of the Company’s net equity and financial position as at 31 December 2023, as well as of its results for the year then ended, in accordance with the financial reporting framework applicable in Belgium.
Basis for unqualified opinion
We conducted our audit in accordance with International Standards on Auditing (ISAs) as applicable in Belgium. Our responsibilities under those standards are further described in the ‘Statutory auditor’s responsibilities for the audit of the annual accounts’ section in this report. We have complied with all the ethical requirements that are relevant to the audit of annual accounts in Belgium, including those concerning independence.
We have obtained from the administrative body and the officials of the Company the explanations and information necessary for performing our audit.
We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our opinion.
BDO Bedrijfsrevisoren BV / BTW BE 0431.088.289 / RPR Brussel
BDO Réviseurs d’Entreprises SRL / TVA BE 0431.088.289 / RPM Bruxelles Key audit matters are those matters that, in our professional judgment, were of most significance in our audit of the annual accounts of the current year.
BDO Bedrijfsrevisoren - BDO Réviseurs d’Entreprises BV/SRL, a company under Belgian law in the form of a private limited liability company, is a member of BDO International Limited, a UK company limited by guarantee, and forms part of the international BDO network of independent member firms.
BDO is the brand name for the BDO network and for each of the BDO Member Firms.

Key audit matters
These matters were addressed in the context of our audit of the annual accounts as a whole, and in forming our opinion thereon, and we do not provide a separate opinion on these matters.
Determination of the percentage of completion used for revenue recognition related to the filgotinib performance obligation under the license and collaboration agreement with Gilead
Critical Audit Matter Description
The Company recognized collaboration revenues of 348,7 million EUR in 2023 from upfront payments related to the filgotinib performance obligation under the license and collaboration agreement with Gilead (the “agreement”). The Company recognized revenue using the cost-to-cost input method, which management believes best depicts the transfer of control to the customer. The extent of progress towards completion is measured based on the ratio of actual costs incurred to date, to the total estimated costs expected upon satisfying the filgotinib performance obligation.
Significant management judgment is required in determining the total estimated costs still to incur and the period over which the Company is expected to complete its performance obligation, impacting the revenue recognition. This significant estimate is the principal consideration for our conclusion that procedures relating to the determination of the estimated costs to complete the performance obligation, impacting the revenue recognition for the filgotinib performance obligation, is a critical audit matter. This increased level
of judgment by management led to a high degree of auditor judgment, complexity, and effort in performing procedures and in evaluating audit evidence related to management’s assumptions of the estimation of total costs to complete.
The disclosure related to the revenue recognition are included in VOL-kap 6.19 of the annual accounts.
How the Critical Audit Matter Was Addressed in the Audit
The primary procedures we performed to address this critical audit matter included:
| • | Testing the design and operating effectiveness of controls over management’s assessment on determining the estimate of total costs to complete the performance obligation, which included evaluating the reasonableness of significant assumptions related to the estimate; |
| • | Testing the accuracy and completeness of actual costs incurred to date based on a sample; |
| • | Evaluating management’s ability to reasonably estimate the costs to complete the performance obligation, including: |
| • | Evaluating the appropriateness of changes made during the period to management’s estimates of total costs to complete; |
| • | Performing a comparison of management’s prior period cost estimates to actual costs incurred and approved; |
| • | Evaluating the period over which management is expecting the Company to complete its performance obligation; |
| • | Comparing certain costs to third-party supporting evidence; |
| GALAPAGOS NV: Statutory auditor’s report to the general meeting of the company on the annual accounts for the year ended 31 December 2023 |
3. |

| • | Considering the impact of any subsequent events on management’s assumptions. |
Responsibilities of administrative body for the drafting of the annual accounts
The administrative body is responsible for the preparation of annual accounts that give a true and fair view in accordance with the financial reporting framework applicable in Belgium, and for such internal control as the administrative body determines is necessary to enable the preparation of annual accounts that are free from material misstatement, whether due to fraud or error.
In preparing the annual accounts, the administrative body is responsible for assessing the Company’s ability to continue as a going concern, disclosing, as applicable, matters related to going concern and using the going concern basis of accounting unless the administrative body either intends to liquidate the Company or to cease operations, or has no realistic alternative but to do so.
Statutory auditor’s responsibilities for the audit of the annual accounts
Our objectives are to obtain reasonable assurance about whether the annual accounts as a whole are free from material misstatement, whether due to fraud or error, and to issue a statutory auditor’s report that includes our opinion. Reasonable assurance is a high level of assurance, but is not a guarantee that an audit conducted in accordance with ISAs will always detect a material misstatement when it exists.
Misstatements can arise from fraud or error and are considered material if, individually or in the aggregate, they could reasonably be expected to influence the
economic decisions of users taken on the basis of these annual accounts.
When executing our audit, we respect the legal, regulatory and normative framework applicable for the audit of annual accounts in Belgium. However, a statutory audit does not guarantee the future viability of the Company, neither the efficiency and effectiveness of the management of the Company by the administrative body. Our responsibilities with respect to the administrative body’s use of the going concern basis of accounting are described below.
As part of an audit in accordance with ISAs, we exercise professional judgment and maintain professional skepticism throughout the audit. We also:
| • | Identify and assess the risks of material misstatement of the annual accounts, whether due to fraud or error, design and perform audit procedures responsive to those risks, and obtain audit evidence that is sufficient and appropriate to provide a basis for our opinion. The risk of not detecting a material misstatement resulting from fraud is higher than for one resulting from error, as fraud may involve collusion, forgery, intentional omissions, misrepresentations, or the override of internal control; |
| • | Obtain an understanding of internal control relevant to the audit in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control; |
| GALAPAGOS NV: Statutory auditor’s report to the general meeting of the company on the annual accounts for the year ended 31 December 2023 |
4. |

| • | Evaluate the appropriateness of accounting policies used and the reasonableness of accounting estimates and related disclosures made by the administrative body; |
| • | Conclude on the appropriateness of the administrative body’s use of the going concern basis of accounting and, based on the audit evidence obtained, whether a material uncertainty exists related to events or conditions that may cast significant doubt on the Company’s ability to continue as a going concern. If we conclude that a material uncertainty exists, we are required to draw attention in our statutory auditor’s report to the related disclosures in the annual accounts or, if such disclosures are inadequate, to modify our opinion. Our conclusions are based on the audit evidence obtained up to the date of our statutory auditor’s report. However, future events or conditions may cause the Company to cease to continue as a going concern; |
| • | Evaluate the overall presentation, structure and content of the annual accounts and whether the annual accounts represent the underlying transactions and events in a manner that achieves fair presentation. |
We communicate with the Audit Committee regarding, among other matters, the planned scope and timing of the audit and significant audit findings, including any significant deficiencies in internal control that we identified during our audit.
We also provide the Audit Committee with a statement that we have complied with relevant ethical requirements regarding independence, and to communicate with them all relationships and other matters that may reasonably be thought to bear on our independence and, where applicable, related safeguards.
From the matters communicated with the Audit Committee, we determine those matters that were of most significance in the audit of the consolidated financial statements of the current year, and are therefore the key audit matters. We describe these matters in our statutory auditor’s report, unless law or regulation precludes public disclosure about the matter.
OTHER LEGAL AND REGULATORY REQUIREMENTS
Responsibilities of the administrative body
The administrative body is responsible for the preparation and the content of the director’s report the statement of non-financial information included in the director’s report and of the other information included in the annual report, for the documents to be deposited in accordance with the legal and regulatory requirements, as well as for the compliance with the legal and regulatory requirements regarding bookkeeping, with the Code of companies and associations and with the Company’s by-laws.
Responsibilities of the statutory auditor
In the context of our mission and in accordance with the Belgian standard (version revised 2020) which is complementary to the International Standards on Auditing (ISAs) as applicable in Belgium, it is our responsibility to verify, in all material aspects, the director’s report the statement of non-financial information attached to the director’s report and the other information included in the annual report, certain documents to be deposited in accordance with the legal and regulatory requirements, and compliance with certain provisions of the Code of companies and associations and of the Company’s by-laws, as well as to report on these elements.
| GALAPAGOS NV: Statutory auditor’s report to the general meeting of the company on the annual accounts for the year ended 31 December 2023 |
5. |

Aspects related to the director’s report and to the other information included in the annual report
In our opinion, after having performed specific procedures in relation to the director’s report, the director’s report is consistent with the annual accounts for the same financial year, and it is prepared in accordance with articles 3:5 and 3:6 of the Code of companies and associations.
In the context of our audit of the annual accounts, we are also responsible for considering, in particular based on the knowledge we have obtained during the audit, whether the director’s report and the other information included in the annual report contain a material misstatement, i.e. information which is inadequately disclosed or otherwise misleading. Based on the procedures we have performed, there are no material misstatements we have to report to you.
The non-financial information, as required by article 3:6, §4 of the Code of companies and associations, has been included in a separate report, which is part of section related to sustainability of the annual report. This report of non-financial information contains the information required by article 3:6, §4 of the Code of companies and associations and is in accordance with the annual accounts for the same financial year. In preparing this non-financial information, the Company has based itself on the United Nations’ Sustainable Development Goals (SDG’s). In accordance with article 3:75, §1, first paragraph, 6° of the Code of companies and associations, we do not express an opinion on the question whether
this non-financial information has been prepared in accordance with the information stated in the director’s report in accordance with the SDG’s.
Statement related to the social balance sheet
The social balance sheet, to be deposited at the National Bank of Belgium in accordance with article 3:12, §1, 8° of the Code of companies and associations, includes, both in terms of form and content, the information required by the said Code, including that relating to information on wages and training and does not present any material inconsistencies with the information that we have at our disposition during the performance of our mission.
Statement related to independence
| • | Our audit firm and our network did not provide services which are incompatible with the statutory audit of annual accounts and our audit firm remained independent of the Company during the terms of our mandate. |
| • | The fees related to additional services which are compatible with the statutory audit of annual accounts as referred to in article 3:65 of the Code of companies and associations, were duly itemised and valued in the notes to the annual accounts. |
Other statements
| • | Without prejudice to certain formal aspects of minor importance, the accounting records are maintained in accordance with the legal and regulatory requirements applicable in Belgium. |
| GALAPAGOS NV: Statutory auditor’s report to the general meeting of the company on the annual accounts for the year ended 31 December 2023 |
6. |

| • | The appropriation of results proposed to the general meeting complies with the legal provisions and the Company’s by-laws. |
| • | We do not have to report to you any transactions undertaken or decisions taken in breach of the by-laws or the Code of companies and associations. |
| • | This report is in compliance with the contents of our additional report to the Audit Committee as referred to in article 11 of regulation (EU) No 537/2014. |
| • | We have assessed the pecuniary consequences for the Company of the decisions related to the conflict of interests as described in the conclusions of the administrative body and having nothing to report to you. |
Zaventem, 28 March 2024
/s/ BDO Bedrijfsrevisoren BV
Statutory auditor
Represented by Ellen Lombaerts*
Auditor
*Acting for a company
| GALAPAGOS NV: Statutory auditor’s report to the general meeting of the company on the annual accounts for the year ended 31 December 2023 |
7. |
| English translation for information purposes | Exhibit 99.7
|
GALAPAGOS
Limited Liability Company
Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium
Company number: 0466.460.429
RLE Antwerp, division Mechelen
(the “Company”)
Report of the Board of Directors in accordance with
Articles 7:180, 7:191 and 7:193 of the Belgian Companies and Associations Code
Cancellation of the preferential subscription rights of the existing shareholders in favor of
Gilead Therapeutics A1 Unlimited Company in the framework of the proposed issuance of
the ‘Subsequent Gilead Warrant B’
| 1 | Introduction |
On 14 July 2019, the Company announced that it entered into a 10-year global research and development collaboration with Gilead Sciences, Inc. (“Gilead Sciences”). Within the framework of this collaboration, Gilead Therapeutics A1 Unlimited Company (“Gilead Therapeutics”, and together with Gilead Sciences, “Gilead”), a subsidiary of Gilead Sciences, entered into a Subscription Agreement (as further defined below) on 14 July 2019 pursuant to which Gilead Therapeutics agreed to invest in the share capital of the Company in consideration of new shares. This investment was effected on 23 August 2019.
The Subscription Agreement also provided, amongst other things, for the issuance to Gilead Therapeutics of the so-called ‘Initial Gilead Warrant A’ and the ‘Initial Gilead Warrant B’, which issuance was approved by the extraordinary shareholders’ meeting of the Company on 22 October 2019. The Initial Gilead Warrant A was exercised on 6 November 2019. The Initial Gilead Warrant B is still outstanding on the date of this report and will expire on 23 August 2024. The Company also agreed in the Subscription Agreement to issue to Gilead Therapeutics an additional subscription right (in the form of a warrant), the so-called Subsequent Warrant B (as defined below), having terms and conditions that (mutatis mutandis) are the same as the terms and conditions of the Initial Gilead Warrant B.
In view hereof, and as the Company’s annual shareholders’ meeting (the “AGM”) will be held on 30 April 2024, the proposed issuance of the Subsequent Warrant B to Gilead Therapeutics, together with the related proposal to cancel, in the interest of the Company, the preferential subscription rights of the existing shareholders for the benefit of Gilead Therapeutics, will be submitted to an extraordinary shareholders’ meeting to be convened by the Company on 30 April 2024, which is when also the AGM will be held (or on 25 June 2024 should the required quorum for the extraordinary shareholders’ meeting not be achieved at the first meeting) (the “EGM”).
It is noted that the Subsequent Warrant B is merely the continuation of a subscription right (in the form of a warrant) that was already approved, issued and granted in the past (namely, the aforementioned ‘Initial Gilead Warrant B’), and which is to expire on 23 August 2024. The potential dilutive effects of the Subsequent Warrant B are therefore similar to those related to the Initial Gilead Warrant B. It should further be noted that the Company has a contractual obligation to endeavor to bring about the continuation of such a subscription right.
| Board Report (Art. 7:180-7:191-7:193 BCAC) | Page 1 of 13 |
| English translation for information purposes |

|
This report has been established on 28 March 2024 by the Board of Directors of the Company pursuant to articles 7:180, 7:191 and 7:193 of the Belgian Companies and Associations Code of 23 March 2019, as amended from time to time (the “Belgian Companies and Associations Code”) and has been drawn up in connection with the proposed issuance of the Subsequent Warrant B for the benefit of Gilead Therapeutics. It provides an explanation and justification for the proposed issuance of the Subsequent Warrant B and the cancellation of the preferential subscription rights of the existing shareholders for the benefit of Gilead Therapeutics (with notably a justification of the proposed exercise price of the Subsequent Warrant B and a description of the consequences for the financial and shareholder rights of the shareholders of the Company and specifically taking into account the financial situation of the Company, the identity of Gilead Therapeutics, and the nature and importance of the contribution of Gilead Therapeutics). It must be read together with the report prepared by the statutory auditor of the Company in accordance with articles 7:180, 7:191 and 7:193 of the Belgian Companies and Associations Code, which will also be submitted to the EGM.
| 2 | Summary of the Agreements with Gilead of 14 July 2019 (as amended) |
| 2.1 | Option, License and Collaboration Agreement with Gilead Sciences |
On 14 July 2019, the Company and Gilead Sciences entered into a 10-year global research and development collaboration agreement (the “Option, License and Collaboration Agreement”). Under such agreement, (i) the Company received a USD 3.95 billion upfront payment from Gilead Sciences, and (ii) Gilead Sciences gained exclusive access and certain rights to the Company’s innovative portfolio of compounds, including clinical and preclinical programs and a proven drug discovery platform, as well as certain exclusive product license and option rights.
Under the Option, License and Collaboration Agreement, the Company funds and leads all discovery and development autonomously until the end of the relevant Phase 2 clinical study. After the completion of the Phase 2 clinical study (or, in certain circumstances, the first Phase 3 study), Gilead Sciences has the option to acquire an exclusive commercial license to that program in all countries outside of Europe. If the option is exercised, the Company and Gilead Sciences will co-develop the compound and share costs equally.
Gilead Sciences maintains option rights to the Company’s programs through the 10-year term of the collaboration. This term can be extended for up to an additional three years thereafter for those programs, if any, that have entered clinical development prior to the end of the collaboration term of the Option, License and Collaboration Agreement.
The Option, License and Collaboration Agreement also provided that if GLPG1690 would be approved in the United States, Gilead Sciences would pay the Company an additional USD 325 million milestone fee. However, in February 2021, the development of GLPG1690 was discontinued.
For GLPG1972, Gilead Sciences received the option to in-license the compound in the United States after the completion of the ongoing Phase 2b study in osteoarthritis; the exercise of this option would trigger the payment by Gilead Sciences to the Company of a license fee of USD 250 million. However, in November 2020, Gilead Sciences declined to exercise its option for GLPG1972.
For all other programs resulting from the collaboration, Gilead Sciences will make a USD 150 million opt-in payment per program if it exercises its option, and will owe no subsequent milestones.
The Company will receive tiered royalties ranging from 20% to 24% on net sales of all of the Company’s products licensed by Gilead Sciences in all countries outside Europe as part of the Option, License and Collaboration Agreement.
The Option, License and Collaboration Agreement received clearance from the U.S. Federal Trade Commission under the Hart-Scott-Rodino Antitrust Improvements Acts of 1976 and merger control approval from the Austrian Federal Competition Authority, and the transaction was subsequently closed on 23 August 2019.
| Board Report (Art. 7:180-7:191-7:193 BCAC) | Page 2 of 13 |
| English translation for information purposes |

|
| 2.2 | Subscription Agreement with Gilead Therapeutics |
On 14 July 2019, the Company and Gilead Therapeutics also entered into a subscription agreement (the “Subscription Agreement”) pursuant to which:
| (a) | the Company agreed to proceed with a capital increase within the framework of the authorized capital, with the cancellation of the preferential subscription rights of the Company’s existing shareholders for the benefit of Gilead Therapeutics; |
| (b) | the Company agreed to seek shareholder approval to issue a number of subscription rights (namely, the ‘Initial Gilead Warrant A’, the ‘Initial Gilead Warrant B’, and the Subsequent Warrant B), allowing Gilead Therapeutics to further increase its ownership in the Company. Subject to certain limitations, the Company also agreed that Gilead Therapeutics may acquire additional shares via open market purchases; |
| (c) | Gilead Therapeutics committed to a standstill and lock-up in relation to (the shares of) the Company; |
| (d) | Gilead Therapeutics is entitled to nominate two directors of the Company; |
| (e) | the Company agreed to seek shareholder approval to renew the authorization of the Board of Directors to increase the share capital of the Company with an amount up to 20% of the share capital at the time of the convening of the relevant extraordinary shareholders’ meeting. |
The capital increase referred to in paragraph (a) above was effected on 23 August 2019 by the Board of Directors of the Company within the framework of the authorized capital, through the issuance of 6,828,985 new shares to Gilead Therapeutics. The new shares were admitted to trading on the regulated markets of Euronext Brussels and Amsterdam on 27 August 2019. For further information on this transaction, reference is made to section 3 of the special report of the Board of Directors dated 23 August 2019 in accordance with (then applicable) article 596 and 598 of the Belgian Companies Code of 7 May 1999, available on the Company’s website (https://www.glpg.com/special-reports).
The issuance of the ‘Initial Gilead Warrant A’ and the ‘Initial Gilead Warrant B’ referred to in paragraph (b) above, the proposed election of directors referred to in paragraph (d) above, and the proposed renewal of the authorized capital referred to in paragraph (e) above, were submitted to, and approved by, an extraordinary shareholders’ meeting on 22 October 2019. The Initial Gilead Warrant A conferred the right to subscribe for a number of new shares sufficient to bring the number of shares owned by Gilead and its affiliates to 25.1% of the issued and outstanding shares of the Company. The Initial Gilead Warrant A was exercised on 6 November 2019 into 2,617,791 shares at EUR 140.59 per share for a total of EUR 368,035,236.69. The Initial Gilead Warrant B confers the right to subscribe for a number of new shares sufficient to bring the number of shares owned by Gilead and its affiliates to 29.9% of the issued and outstanding shares of the Company. The Initial Gilead Warrant B is still outstanding on the date of this report. For further information on the issuance of the ‘Initial Gilead Warrant A’ and the ‘Initial Gilead Warrant B’, reference is made to the report of the Board of Directors dated 19 September 2019 prepared in accordance with articles 583, 596 and 598 of the Belgian Companies Code of 7 May 1999, available on the Company’s website (Shareholder Information - Galapagos (glpg.com)).
As the Initial Gilead Warrant B will expire on 23 August 2024, and as the Company initially agreed to provide Gilead a right to subscribe for shares for a term of 10 years, the Subscription Agreement provided that between the 57 month and 59 month anniversary of the capital increase referred to in paragraph (a) above (i.e., between 23 May 2024 and 23 July 2024), the Board of Directors agreed to convene an extraordinary general meeting of the shareholders of the Company at which the shareholders of the Company will be asked to approve:
| (a) | the issuance to Gilead Therapeutics of an additional new subscription right (in the form of a warrant), the so-called ‘Subsequent Gilead Warrant B’, having terms and conditions that (mutatis mutandis) are the same as the terms and conditions of the Initial Gilead Warrant B, including notably as to the Exercise Price (on a per share basis) and the maximum number of shares issuable (the “Subsequent Warrant B”); |
| Board Report (Art. 7:180-7:191-7:193 BCAC) | Page 3 of 13 |
| English translation for information purposes |

|
| (b) | the authorization to the Board of Directors, valid for a period of five (5) years from the date of publication of the authorization in the Annexes to the Belgian Official Gazette, to increase the share capital of the Company in one or several times with an amount up to 20% of the share capital at the time of the convening of the relevant extraordinary general meeting, which capital increases may be achieved by the issuance of shares, convertible bonds and/or subscription rights (warrants) exercisable by contributions in cash or in kind, with or without issuance premium, and which authorization will explicitly authorize the Board of Directors to restrict or cancel the shareholders’ preferential subscription rights, even if such restriction or cancellation is made for the benefit of one or more specific persons other than the employees of the Company or its subsidiaries (including Gilead and its affiliates). For further information on the proposed renewal of the authorized capital, reference is made to the report of the Board of Directors prepared in accordance with article 7:199 of the Belgian Companies and Associations Code (which will also be submitted to the EGM). |
As the Company’s AGM will be held on 30 April 2024, the Company will submit the aforementioned proposals to the EGM on 30 April 2024, which is when also the AGM will be held.
The Company and Gilead agreed that the issuance of certain subscription rights (including the Subsequent Warrant B) to the benefit of Gilead Therapeutics was an essential part of the consideration offered to Gilead for entering into the aforementioned agreements. The terms of the contractual arrangements between the Company and Gilead (of which the issuance of certain subscription rights (including the Subsequent Warrant B) formed an integral part) had been determined during at arm’s length negotiations between the Company and Gilead at the time.
For the sake of completeness, the Board of Directors notes that in April 2021, the Company and Gilead Therapeutics agreed to amend the Subscription Agreement to extend the lock-up in relation to Gilead’s equity securities in the Company until 22 August 2024.
In 2022, the Company and Gilead Therapeutics also agreed to amend the Subscription Agreement for conformity with the change from a two-tier to a one-tier governance system by Galapagos.
| 3 | Proposed issuance of the Subsequent Warrant B |
In accordance with what was agreed in the Subscription Agreement, the Board of Directors of the Company proposes to the EGM to issue the Subsequent Warrant B, being one subscription right (in the form of a warrant) named the ‘Subsequent Gilead Warrant B’, with cancellation of the preferential subscription rights of the existing shareholders for the benefit of Gilead Therapeutics.
| 3.1 | Terms and Conditions of the Subsequent Warrant B |
The proposed terms and conditions (the “Conditions”) of the Subsequent Warrant B are set out in Annex 1 to this report. The main Conditions can, for information purposes, be summarized as follows and are, mutatis mutandis, equivalent to the previously approved terms and conditions of the ‘Initial Gilead Warrant B’:
| (a) | Issuer: The Company (Galapagos NV). |
| (b) | Number of subscription rights issued: One (1) subscription right of the Company (in the form of one (1) warrant). |
| (c) | Term: The Subsequent Warrant B will have a term starting as of the Issue Date (as defined in the Conditions, which is the date of the EGM approving the issuance of the Subsequent Warrant B) and ending on 11:59 p.m. on the calendar day before the fifth anniversary of the Issue Date. The Subsequent Warrant B can be exercised at one or several occasions as from 23 August 2024 |
| Board Report (Art. 7:180-7:191-7:193 BCAC) | Page 4 of 13 |
| English translation for information purposes |

|
| (on 11:59 p.m.) during the entire term of the Subsequent Warrant B, but not more than once per period of three (3) months. As set out in the Conditions, this limitation does not apply in case of material development regarding the Company or the trading of the Company’s shares, or in case of certain (requests for) convocations of shareholders’ meetings of the Company. |
| (d) | Number of shares issuable upon exercise: Subject to the Conditions, the Subsequent Warrant B entitles the holder thereof to subscribe, during the entire term of the Subsequent Warrant B, upon each exercise of the Subsequent Warrant B, for a maximum number of shares that is sufficient to bring the number of shares owned by Gilead Therapeutics, Gilead Sciences and any of their affiliates and any other party Acting in Concert (as defined in the Conditions) with Gilead Therapeutics, Gilead Sciences or any of their affiliates to 29.9% of the actually issued and outstanding shares immediately after the issue of the shares that are to be issued upon the relevant exercise of the Subsequent Warrant B (rounded down to the nearest whole share) (the “Warrant Limit”). The Subsequent Warrant B remains outstanding for the remaining duration of its term even if exercised for a number of shares that is equal to the then applicable Warrant Limit. |
| (e) | Exercise Price: The Exercise Price (as defined in the Conditions) of the Subsequent Warrant B shall, per share that shall be subscribed for upon an exercise of the Subsequent Warrant B in relation to such shares, be equal to the greater of (i) 120% multiplied by the arithmetic mean of the daily volume weighted average trading price of the Company’s shares as traded on Euronext Brussels and Euronext Amsterdam (or such other regulated markets on which the Company’s shares will be trading at that time) on each of the trading days during the period of 30 calendar days ending on the calendar day immediately preceding the date of the Exercise Notice (as defined in the Conditions) with respect to such exercise, and (ii) EUR 140.59 (which is the same as the issue price of the 6,828,985 new shares that were issued to Gilead Therapeutics on 23 August 2019). The Exercise Price is subject to customary adjustments set out in the Conditions. |
| (f) | Nature of the shares issuable upon exercise: Each new share to be issued by the Company upon each exercise of the Subsequent Warrant B shall have the same rights and benefits as, and rank pari passu in all respects including as to entitlement to dividends and other distributions, with the existing and outstanding shares of the Company at the moment of their issue and will be entitled to dividends and other distributions in respect of which the relevant record date or due date falls on or after the date of their issue. |
| (g) | Capital increase and allocation of the Exercise Price: Upon each exercise of the Subsequent Warrant B and the resulting issue of new shares, the Company’s share capital will be increased, as further specified in the Conditions. Each time upon an exercise of the Subsequent Warrant B and the issue of new shares pursuant to the Conditions, the applicable aggregate Exercise Price shall be allocated to the share capital of the Company. If the applicable Exercise Price per share issued is greater than the fractional value of the existing shares immediately prior to the capital increase, then the applicable aggregate Exercise Price shall be allocated in such a manner that per share issued (i) a part of the applicable aggregate Exercise Price equal to the fractional value of the existing shares immediately prior to the capital increase shall be booked as share capital, and (ii) the balance of the applicable aggregate Exercise Price shall be booked as issue premium. Such issue premium shall be accounted for on the liabilities side of the Company’s balance sheet as net equity. The account on which the issue premium shall be booked shall, like the share capital, serve as the guarantee for third parties and, save for the possibility of a capitalization of those reserves, can only be reduced on the basis of a valid resolution of the general shareholders’ meeting passed in the manner required for an amendment to the Company’s articles of association. |
| Board Report (Art. 7:180-7:191-7:193 BCAC) | Page 5 of 13 |
| English translation for information purposes |

|
| (h) | Listing of the underlying shares: If the admission of the shares that are to be issued upon an exercise of the Subsequent Warrant B to trading on the regulated markets of Euronext Brussels and Euronext Amsterdam (and such other regulated markets on which the Company’s shares will be trading at that time) legally requires a listing prospectus, the Company shall use reasonable efforts to obtain such admission within ninety (90) days following the issue of such shares. In such event, the effective admission to listing will be subject to regulatory approval of the listing prospectus. If the admission of the shares that are to be issued upon an exercise of the Subsequent Warrant B to trading on the regulated markets of Euronext Brussels and Euronext Amsterdam (and such other regulated markets on which the Company’s shares will be trading at that time) does not legally require a listing prospectus, the Company shall cause such admission as soon as practicable after the issue of such shares, and in any event no later than five (5) Business Days (as defined in the Conditions) after the issue of such shares. |
| (i) | Nature of the Subsequent Warrant B: The Subsequent Warrant B will confer the right (but not the obligation) to subscribe, upon any exercise of the Subsequent Warrant B, for a number of new shares to be issued by the Company, as aforementioned. Except as otherwise provided for under Belgian law, the holder of the Subsequent Warrant B will be no shareholder of the Company solely by virtue of holding the Subsequent Warrant B, and therefore does not have the rights of a shareholder in relation to the shares to be issued or delivered to the holder of the Subsequent Warrant B upon an exercise of the Subsequent Warrant B until the exercise of the Subsequent Warrant B and the issue or delivery of the relevant shares. |
| (j) | Form of the Subsequent Warrant B: The Subsequent Warrant B will be in registered form. The Subsequent Warrant B cannot be converted into a bearer instrument or in dematerialized form. |
| (k) | No listing of the Subsequent Warrant B: The Subsequent Warrant B shall not be listed at any time on a securities exchange, regulated market or similar securities market. |
| (l) | Issue Price of the Subsequent Warrant B: The Subsequent Warrant B will be issued, without any additional consideration being due by Gilead Therapeutics or any of its affiliates. |
| 3.2 | Exercise Price of the proposed Subsequent Warrant B |
In the Subscription Agreement, the Exercise Price of the proposed Subsequent Warrant B has been determined at the greater of (i) 120% multiplied by the arithmetic mean of the daily volume weighted average trading price of the Company’s shares as traded on Euronext Brussels and Euronext Amsterdam (or such other regulated markets on which the Company’s shares will be trading at that time) on each of the trading days during the period of 30 calendar days ending on the calendar day immediately preceding the date of the Exercise Notice (as defined in the Conditions) with respect to such exercise, and (ii) EUR 140.59, (on a per share basis) (see section 3.1(e) above) (which represents a 20% premium as compared to the average of the volume weighted average prices of the Company’s shares on the regulated market of Euronext (Brussels and Amsterdam) during the thirty calendar days preceding the date of signing the Subscription Agreement).
| 4 | Reasons for the proposed issuance of the Subsequent Warrant B with cancellation of the preferential subscription rights for the benefit of Gilead Therapeutics |
The issuance of subscription rights to Gilead (including the proposed issuance of the Subsequent Warrant B) was one of the elements that has been agreed to by the Company with Gilead within the framework of the aforementioned transaction that was announced on 14 July 2019.
The Board of Directors is of the opinion that the strategic collaboration with Gilead, of which the issuance of the subscription rights (including the proposed issuance of the Subsequent Warrant B) form an integral part, has benefited, and is still benefiting, the Company as it, among other things, allowed the Company to obtain additional significant cash resources and to significantly strengthen its net equity position through a USD 3.95 billion upfront payment and an aggregate EUR 1.33 billion equity contribution by Gilead, and allows access to Gilead’s chemistry and development expertise, its commercial infrastructure and operations, and its speed to the relevant markets.
| Board Report (Art. 7:180-7:191-7:193 BCAC) | Page 6 of 13 |
| English translation for information purposes |

|
Furthermore, while it cannot be guaranteed that the Subsequent Warrant B will ultimately be exercised (in particular as the minimum Exercise Price of the Subsequent Warrant B is substantially higher than the trading price of the Company’s shares on the regulated market of Euronext Brussels and Euronext Amsterdam on the date of this report), the exercise of the proposed Subsequent Warrant B by Gilead Therapeutics, and the payment of the relevant exercise price of the Subsequent Warrant B by Gilead Therapeutics, if any, will enable the Company to obtain additional cash resources and to strengthen its net equity position.
Finally, the Subsequent Warrant B is merely the continuation of a subscription right (in the form of a warrant) that has already been approved by the extraordinary shareholders’ meeting of the Company on 22 October 2019. Pursuant to Belgian company law, the ‘Initial Gilead Warrant B’ could not be issued for a term of 10 years. In line with what was agreed in the Subscription Agreement, the ‘Initial Gilead Warrant B’ will need to be renewed.
For the sake of completeness, it should be noted that the Subscription Agreement provides for a number of consequences if the EGM were not to approve the proposed issuance of the Subsequent Warrant B. Notably, in such situation:
| (a) | the Company and Gilead Therapeutics shall collaborate to structure and organize the right to subscribe for shares as reflected by the Subsequent Warrant B via a contractual subscription or option agreement or otherwise, allowing, to the greatest extent legally possible, Gilead Therapeutics to subscribe for or acquire the number of shares of the Company it would otherwise be able to subscribe for or acquire upon exercise of the Subsequent Warrant B, whether through the Company’s authorized capital or otherwise; |
| (b) | as long as the Board of Directors shall have the power to issue shares within the framework of the authorized capital, the authorized capital shall to the greatest extent legally possible be used with priority for the purpose of the issuance of shares to Gilead Therapeutics, without prejudice to the right of the Company to use the authorized capital for equity-based incentives or compensation for current or future employees, consultants, directors and/or officers of the Company or its affiliates; and |
| (c) | the Company will submit the proposed issuance of the Subsequent Warrant B as soon as possible to a subsequent general shareholders’ meeting of the Company and, if such subsequent meeting were not to approve the proposed issuance of the Subsequent Warrant B, the Company will be obliged to re-submit the proposed issuance of the Subsequent Warrant B to another extraordinary general shareholders’ meeting at the occasion of the annual general shareholders’ meeting of the Company (and this until the Subsequent Warrant B has been issued). |
For all of the abovementioned reasons, the Board of Directors recommends that the EGM approves the proposed issuance of the Subsequent Warrant B with cancellation of the preferential subscription rights for the benefit of Gilead Therapeutics.
| 5 | Consequences for the Company’s existing shareholders |
The dilution and effect on the equity value of the Company that will result from the issuance and exercise of the proposed Subsequent Warrant B is indicatively set out in the tables below.
Ultimately, whether or not a Subsequent Warrant B will be exercised depends on the (sole) decision of Gilead Therapeutics. Such decision may depend in part on the price of the share of the Company at the moment of exercise as compared with the relevant Exercise Price. As mentioned in section 7 below, the current trading price of the Company’s shares on Euronext Brussels and Euronext Amsterdam is currently substantially lower than the minimum Exercise Price of the Subsequent Warrant B.
The Board of Directors also notes that the Subsequent Warrant B is merely the continuation of the Initial Gilead Warrant B (which is to expire on 23 August 2024).
| Board Report (Art. 7:180-7:191-7:193 BCAC) | Page 7 of 13 |
| English translation for information purposes |

|
| 5.1 | Introductory comments and assumptions |
Certain key parameters of the exercise of the proposed Subsequent Warrant B (such as the applicable Exercise Price(1) or the maximum number of new shares to be issued in connection with the proposed Subsequent Warrant B(2)), are unknown on the date of this report. Hence, the actual effects of the exercise of the proposed Subsequent Warrant B can only be illustrated, and cannot yet be determined with certainty.
| Accordingly, the discussion herein of the financial consequences of the exercise of the proposed Subsequent Warrant B is purely illustrative, and based on purely hypothetical financial parameters. The actual Exercise Price of the Subsequent Warrant B and the number of new shares to be issued in connection with the proposed Subsequent Warrant B may vary significantly from the hypothetical values used in this report. The hypothetical financial parameters used in the simulations below by no means anticipate the final financial parameters that will be used for an actual exercise of the Subsequent Warrant B. |
Subject to the foregoing reservations, for the purposes of the illustration of some of the financial consequences of an exercise in full of the proposed Subsequent Warrant B and notably the dilution for the shareholders, the following assumptions were used:
| • | The Company has a registered share capital of EUR 356,444,938.61, represented by 65,897,071 shares, with a fractional value of (rounded up) EUR 5.41 per share. |
| • | On 31 December 2023, there were 11,472,520 subscription rights outstanding under the different share option plans for members of the personnel of the Company and its subsidiaries described in the Company’s annual report for financial year 2023 (which is also submitted to the AGM), each giving the right to subscribe for one new share in the Company (subject to certain conditions). In this report, when reference is made to any “outstanding” subscription rights, this refers to subscription rights that have already been granted and accepted, and (depending on the terms and conditions of such subscription rights) have not yet been exercised and have not yet expired. For the purpose of the full-dilution scenario calculations further below, it is assumed that all of the 11,472,520 outstanding subscription rights have vested, are immediately exercisable (regardless of their terms and conditions), and have been fully exercised prior to the completion of the issuance and exercise of the Subsequent Warrant B. The calculations below will not take into account any shares to be issued upon exercise of the Initial Gilead Warrant B (as the Subsequent Warrant B is merely a continuation of such Initial Gilead Warrant B). |
| • | Gilead and affiliates hold 16,707,477 shares of the Company. |
| • | As provided for in section 3.1(d) of this report, the maximum number of shares issuable upon each exercise of the Subsequent Warrant B is equal to the number of shares that is sufficient to bring the number of shares owned by Gilead Therapeutics, Gilead Sciences and any of their affiliates and any other party Acting in Concert with Gilead Therapeutics, Gilead Sciences or any of their affiliates to 29.9% of the actually issued and outstanding shares immediately after the issue of the shares that are to be issued upon the relevant exercise of the Subsequent Warrant B (rounded down to the nearest whole share). Hence, for the purposes of the illustrations below, the maximum number of shares to be issued to Gilead Therapeutics upon an exercise in full of the Subsequent Warrant B at the date of this report is equal to 4,273,533 shares (assuming that the Company does not issue any new shares during the term of the Subsequent Warrant B). Assuming that Gilead and affiliates hold 16,707,477 shares before the exercise of the Subsequent Warrant B, the exercise of the Subsequent Warrant B (and the subsequent issuance of the 4,273,533 shares) would result in an aggregate shareholding of 20,981,010 shares, being 29.9% |
| 1 | For more information, see section 3.1(i)(e) of this report. |
| 2 | For more information, see section 3.1(i)(d) of this report. |
| Board Report (Art. 7:180-7:191-7:193 BCAC) | Page 8 of 13 |
| English translation for information purposes |

|
| of the 70,170,604 outstanding shares of the Company (after the exercise of the Subsequent Warrant B). It should be noted that the Subsequent Warrant B can be exercised at one or several occasions during its entire term3 and that the actual number of shares issuable upon an exercise of the Subsequent Warrant B could be higher, depending on the number of outstanding shares of the Company at the time of that exercise of the Subsequent Warrant B. In any event, the number of shares issuable cannot be in excess of the then applicable Warrant Limit (as defined in section 3.1(d) of this report). |
| • | As provided for in section 3.1(e) of this report, the Exercise Price of the Subsequent Warrant B, per share that shall be subscribed for upon an exercise of the Subsequent Warrant B in relation to such shares, is equal to the greater of (i) 120% multiplied by the arithmetic mean of the daily volume weighted average trading price of the Company’s shares as traded on Euronext Brussels and Euronext Amsterdam (or such other regulated markets on which the Company’s shares will be trading at that time) on each of the trading days during the period of 30 calendar days ending on the calendar day immediately preceding the date of the Exercise Notice (as defined in the Conditions) with respect to such exercise, and (ii) EUR 140.59. For the purpose of the illustrations attached to this report in Annex 2, the hypothetical Exercise Price for the Subsequent Warrant B that has been used in the simulations has been set at the floor price of EUR 140.59. |
| • | On the basis of the audited consolidated annual financial statements of the Company for the financial year ended on 31 December 2023 (which have been prepared in accordance with the International Financial Reporting Standards, as adopted by the European Union (“IFRS”)) and which will be submitted to the AGM.4 The consolidated net equity of the Company as at 31 December 2023 amounted to EUR 2,795,566,256.00. This number does not take into account changes in the consolidated net equity since 31 December 2023. |
| • | As referred to above, the Company’s consolidated financial statements are prepared in accordance with IFRS. While on the date of this report the accounting treatment of the Subsequent Warrant B is still under review by the Company’s management and not yet validated by the Company’s auditor, it is currently expected that, within the framework of the Company’s consolidated financial statements, the Subsequent Warrant B will be accounted for in accordance with (amongst others) IFRS 9 (Financial Instruments). As aforementioned, the actual application of the reporting standard, the initial recognition moment and valuation of the Subsequent Warrant B is still being determined and assessed. |
For further information regarding the Company’s consolidated net equity position on 31 December 2023, reference is made to the consolidated financial statements of the Company, which are available on the Company’s website and which will be submitted to the AGM.
| 5.2 | Dilution resulting from the issuance and exercise of the Subsequent Warrant B |
Each share in the Company currently represents an equal part of the share capital of the Company and carries the right to one vote. The issuance of the Subsequent Warrant B to Gilead Therapeutics will, upon exercise of the relevant Subsequent Warrant B, lead to a dilution of the existing shareholders of the Company and of the relative voting power of each share in the Company.
The dilution relating to the voting right also applies, mutatis mutandis, to the participation of each share in the profit and liquidation proceeds and other rights attached to the shares of the Company, such as the statutory preferential subscription right in the case of a capital increase in cash through the issuance of shares.
| 3 | The Subsequent Warrant B shall remain outstanding for the remaining duration of its term even if exercised for a number of shares that is equal to the then applicable Warrant Limit (as defined in section 3.1(i)(d) of this report). |
| 4 | For further information regarding the Company’s net equity position on the aforementioned date, reference is made to the financial statements of the Company, which are available on the Company’s website and which will be submitted to the AGM. |
| Board Report (Art. 7:180-7:191-7:193 BCAC) | Page 9 of 13 |
| English translation for information purposes |

|
Specifically, prior to the exercise of the Subsequent Warrant B, each share participates equally in the profit and liquidation proceeds of the Company and each shareholder has a statutory preferential subscription right in the case of a capital increase in cash. Each new share to be issued by the Company upon exercise of the relevant Subsequent Warrant B shall have the same rights and benefits as, and rank pari passu in all respects including as to entitlement to dividends and other distributions, with the existing and outstanding shares at the moment of their issue and will be entitled to dividends and other distributions in respect of which the relevant record date or due date falls on or after the date of their issue. As a result (and to the extent the Subsequent Warrant B will be issued and exercised), the participation by the existing shares in the profit and liquidation proceeds of the Company and their holder’s statutory preferential subscription right in the case of a capital increase in cash, shall be diluted accordingly.
Based on the methodology and assumptions referred to in section 5.1 above, the tables below show the dilution of voting power and liquidation and dividend rights that would result from the issuance and exercise of the Subsequent Warrant B in a scenario in which none of the outstanding subscription rights are exercised (see section 5.2.1) and in a scenario in which all of the outstanding subscription rights are exercised in full (see section 5.2.2).
5.2.1 Non-diluted basis
| (A) Number of currently existing and outstanding shares on a non-diluted basis | 65,897,071 | |||
| (B) Number of shares to be issued as a result of the issuance and full exercise of the Subsequent Warrant B (on a non-diluted basis) (5) | 4,273,533 | |||
| (C) Number of shares after the issuance and full exercise of the Subsequent Warrant B on a non-diluted basis | 70,170,604 | |||
| (D) Dilution of existing shareholders on the basis of the number of currently existing and outstanding shares on a non-diluted basis | 6.09 | % |
The table above demonstrates that, assuming that the Subsequent Warrant B is issued and fully exercised on the basis of the methodology and assumptions set out in section 5.1 of this report, the currently existing and outstanding shares would no longer represent 1/65,897,071 of the share capital, but 1/70,170,604 of the resulting share capital, which represents a dilution of the participation in the share capital and the results of the Company of 6.09%.
5.2.2 Fully diluted basis(6)
| (A) Number of currently existing and outstanding shares on a fully diluted basis | 77,369,591 | |||
| (B) Number of shares to be issued as a result of the issuance and full exercise of the Subsequent Warrant B (on a fully diluted basis) (5) | 9,166,948 | |||
| (C) Number of shares after the issuance and full exercise of the Subsequent Warrant B on a fully diluted basis | 86,536,539 | |||
| (D) Dilution of existing shareholders on the basis of the number of currently existing and outstanding shares on a fully diluted basis | 10.59 | % |
| 5 | For further information on the number of shares to be issued as a result of the issuance and exercise of the Subsequent Warrant B, reference is made to section 5.1 of this report. |
| 6 | Assuming that all 11,472,520 outstanding subscription rights are exercised, resulting in the issuance of 11,472,520 new shares, as a result of which the Company’s share capital would be represented by 77,369,591 shares. |
| Board Report (Art. 7:180-7:191-7:193 BCAC) | Page 10 of 13 |
| English translation for information purposes |

|
The table above demonstrates that, assuming that the Subsequent Warrant B is issued and fully exercised on the basis of the methodology and assumptions set out in section 5.1 of this report and assuming that all outstanding subscription rights are exercised and new shares would be issued as a result thereof, the previously existing shares, would no longer represent 1/77,369,591 of the share capital, but 1/86,536,539 of the resulting share capital, which represents a dilution of the participation in the share capital and the results of the Company of 10.59%.
| 5.3 | Effect of the issuance and exercise of the Subsequent Warrant B on the equity of the Company |
On the basis of the methodology and assumptions set out in section 5.1 of this report, a number of simulations have been prepared. These simulations are attached to this report as Annex 2 and reflect the impact of the exercise of the proposed Subsequent Warrant B on the Company’s equity.
In the event that the proposed Subsequent Warrant B would be exercised in full on the basis of the methodology and assumptions set out in section 5.1 of this report, there would be an increase of the equity of the Company for an amount equal to the product of the number of shares issuable upon exercise of the Subsequent Warrant B and the relevant Exercise Price (on a per share basis). If the relevant Exercise Price is higher than the equity value per share and the relevant Subsequent Warrant B is de facto exercised, there would be a positive effect on the equity value per share for the existing shareholders.
In any event, it should be noted that the accounting treatment of the Subsequent Warrant B is still under review, as indicated in section 5.1 of this report.
| 5.4 | Effect of the issuance and exercise of the Subsequent Warrant B on the shareholding of Gilead Therapeutics |
Following the issuance and exercise of the Subsequent Warrant B, on the basis of the methodology and assumptions set out in section 5.1 of this report, Gilead Therapeutics would hold 20,981,010 shares in the Company, which is equal to an ownership percentage of 29.90% on a non-diluted basis.(7)
As mentioned in section 5.1 of this report, it should be noted that the Subsequent Warrant B can be exercised at one or several occasions during their entire term8 and that the actual number of shares issuable upon an exercise of the respective Subsequent Warrant B could be higher, depending on the number of outstanding shares of the Company at the time of that exercise of the Subsequent Warrant B. In any event, the number of shares issuable cannot be in excess of the then applicable Warrant Limit.
| 6 | Individuals, other than employees, for whose benefit the preferential subscription rights are cancelled |
Within the framework of the proposed issuance of the Subsequent Warrant B (as agreed in the Subscription Agreement), the Board of Directors proposes, in accordance with Article 7:191 and 7:193 of the Belgian Companies and Associations Code, to cancel the preferential subscription rights of the existing shareholders for the benefit of Gilead Therapeutics A1 Unlimited Company, an unlimited liability company incorporated and validly existing under Irish law, with registered seat at 70 Sir John Rogerson’s Quay, Dublin 2 (Ireland) and registered with Ireland’s Companies Registration Office under number 615395.
| 7 | Justification of the Exercise Price of the Subsequent Warrant B |
The terms of the Conditions (including the mechanisms pursuant to which the Exercise Price of the Subsequent Warrant B has been determined) are the result of arm’s length negotiations that occurred
| 7 | This number 20,981,010 is equal to the assumed shareholding of Gilead Therapeutics on the date of this report and the 4,273,533 shares assumed to be issued to Gilead Therapeutics upon exercise of the Subsequent Warrant B on the basis of the methodology and assumptions as set out in section 5.1 of this report. |
| 8 | The Subsequent Warrant B shall remain outstanding for the remaining duration of their term even if exercised for a number of shares that is equal to the then applicable Warrant Limit (as defined in section 3.1(i)(d) of this report). |
| Board Report (Art. 7:180-7:191-7:193 BCAC) | Page 11 of 13 |
| English translation for information purposes |

|
between the Company and Gilead at that time (by taking into account the trading price of the Company’s shares on Euronext Brussels and Euronext Amsterdam at that time).
The Exercise Price of the Subsequent Warrant B is subject to customary adjustments set out in the Conditions.
The Board of Directors notes that the minimum Exercise Price of the Subsequent Warrant B is approx. 4.5x higher than the trading price of the Company’s shares on the regulated market of Euronext Brussels and Euronext Amsterdam on 22 March 2024. Without exercise, there would also be no dilution for the shareholders.
Hence, in view of all of the foregoing, and the fact that (the Exercise Price of) the Subsequent Warrant B is a continuation of (the same Exercise Price of) the Initial Gilead Warrant B, the Board of Directors believes that the Exercise Price of the Subsequent Warrant B is sufficiently justified.
| 8 | Justification of the cancellation of the preferential subscription rights of the existing shareholders for the benefit of Gilead Therapeutics |
The cancellation of the preferential subscription rights of the existing shareholders for the benefit of Gilead Therapeutics is required in order to allow the Company to issue the Subsequent Warrant B to Gilead Therapeutics. This issuance is an integral part of aforementioned transaction that was announced on 14 July 2019 and allowed the Company to secure a strong and strategic collaboration with Gilead, which was, and still is, in the Company’s interest. Accordingly, the Board of Directors recommends that the EGM approves the proposed issuance of the Subsequent Warrant B.
| 9 | Statutory Auditor’s report |
The statutory auditor of the Company has been requested to issue a report in accordance with Articles 7:180, 7:191 and 7:193 of the Belgian Companies and Associations Code.
| 10 | Conclusion |
Taking into account the abovementioned considerations, the Board of Directors is of the opinion that the proposed issuance of the Subsequent Warrant B with cancellation of the preferential subscription rights of the existing shareholders for the benefit of Gilead Therapeutics is in the Company’s interest.
The issuance of the Subsequent Warrant B is subject to the approval by the EGM to be held on or around 30 April 2024 (or on 25 June 2024 should the required quorum not be achieved at the first meeting).
Done on 28 March 2024.
[Signature page follows]
| Board Report (Art. 7:180-7:191-7:193 BCAC) | Page 12 of 13 |
| English translation for information purposes |

|
For the Board of Directors of the Company,
| [Signed] |
[Signed] |
|
| Stoffels IMC BV, permanently represented by | Jérôme Contamine | |
| Dr. Paul Stoffels | Director | |
| Chair |
| [signature page to special board report 7:180-7:191-7:193 BCAC] | Page 13 of 13 |
| English translation for information purposes |

|
Annex 1
Terms and Conditions of the Subsequent Warrant B
| Annex to Board Report (Art. 7:180-7:191-7:193 BCAC) |
SUBSEQUENT GILEAD WARRANT B
– issued by –
GALAPAGOS NV
– dated –
[date] 2024
TABLE OF CONTENT
| Articles | Page | |||
| 1. Certain Definitions and Interpretation |
1 | |||
| 1.1. Certain definitions |
1 | |||
| 1.2. Headings |
3 | |||
| 1.3. Meaning of references |
4 | |||
| 1.4. Fractional value of Shares |
4 | |||
| 1.5. Language |
4 | |||
| 2. Issuance, Nature and Form of the Warrant |
5 | |||
| 2.1. Issuance and nature |
5 | |||
| 2.2. Registered form |
5 | |||
| 2.3. Transferability of the Warrant |
5 | |||
| 2.4. No listing of the Warrant |
5 | |||
| 3. Term of the Warrant |
5 | |||
| 4. Shares |
6 | |||
| 4.1. Number of Shares issuable upon an exercise of the Warrant |
6 | |||
| 4.2. Nature and form of the Shares |
6 | |||
| 4.3. Listing of the Shares |
7 | |||
| 5. Exercise Price |
7 | |||
| 6. Specific Conditions |
7 | |||
| 7. Exercise of the Warrant |
8 | |||
| 7.1. Exercise Notice |
8 | |||
| 7.2. Payment of the Exercise Price |
8 | |||
| 7.3. Issue and delivery of the Shares |
9 | |||
| 7.4. Allocation of the Exercise Price |
9 | |||
| 8. Adjustments |
9 | |||
| 8.1. General |
9 | |||
| 8.2. Adjustments for Share Reorganisations |
10 | |||
| 8.3. Adjustments for mergers and de-mergers |
10 | |||
| 9. Representations and Warranties |
11 | |||
| 9.1. Representations and Warranties of the Company |
11 | |||
| 9.2. Representations and Warranties of the holder of the Warrant |
12 | |||
| 10. Miscellaneous |
12 | |||
| 10.1. Binding nature of the Conditions |
12 | |||
| 10.2. Severability |
13 | |||
| 10.3. Specific Enforcement |
13 | |||
| 10.4. Costs and expenses |
13 | |||
| 10.5. Governing law and jurisdiction |
13 | |||
| 10.6. Notices |
14 | |||
i
GALAPAGOS NV
Limited Liability Company
Generaal De Wittelaan L11 A3, 2800 Mechelen (Belgium)
Register of Legal Persons VAT BE 0466.460.429 (Antwerp, division Mechelen)
SUBSEQUENT GILEAD WARRANT B
RECITALS
On 14 July 2019, Galapagos NV (hereafter further referred to as the “Company”), entered into a Subscription Agreement (as defined below) with Gilead Therapeutics A1 Unlimited Company (hereafter further referred to as the “Investor”), which in the meantime has been amended at several occasions. The Investor is an indirect wholly-owned subsidiary of Gilead Sciences, Inc. (hereafter further referred to as the “Parent Investor”), a U.S. corporation listed on the NASDAQ Stock Market and a research-based biopharmaceutical company focused on developing innovative medicines for life-threatening diseases. Simultaneously with the execution of the Subscription Agreement, the Company and the Parent Investor also entered into an Option, License and Collaboration Agreement (as defined below). Pursuant to the Option, License and Collaboration Agreement, the Company agreed to discover, research, and develop molecules and products, and Parent Investor agreed to have an option to participate in the development and commercialisation of molecules and products, in each case, on the terms and conditions set forth in such agreement. Pursuant to the Subscription Agreement, the Investor agreed to make an investment into the share capital of the Company. The investment was effected on 23 August 2019. As part of the overall agreement between the Company, the Investor and the Parent Investor, the Subscription Agreement also provided for the issuance to the Investor of a number of warrants (namely, the Initial Gilead Warrants (as defined below) and the Subsequent Gilead Warrant B (as defined below)). On 22 October 2019, the extraordinary general shareholders’ meeting of the Company decided to approve to the issuance of the Initial Gilead Warrants, as contemplated by the Subscription Agreement. The present terms and conditions (hereinafter referred to as the “Conditions”) contain the issue and exercise conditions of the Subsequent Gilead Warrant B issued by the Company, as contemplated by the Subscription Agreement. The issue of the Warrant is exclusively reserved to the Investor, in consideration of the subscription for shares and entry into the collaboration contemplated by the agreements referred to above.
| 1. | CERTAIN DEFINITIONS AND INTERPRETATION |
| 1.1. | Certain definitions |
In these Conditions, the following words and expressions that are not defined elsewhere in these Conditions shall have the following meanings, save where the context requires otherwise:
“Acting in Concert” means, when used in relation to a person or entity, acting in concert (in onderling overleg handelende personen / personnes agissant de concert) in the sense of Article 3, §1, 5° of the Belgian Act of 1 April 2007 regarding public takeover bids, or Article 1, §2, 5° of the Belgian Royal Decree of 27 April 2007 regarding public takeover bids.
“Affiliate” means, when used with respect to a person or entity, any person, corporation, partnership, or other entity that controls, is controlled by or is under common control with such person or entity, for so long as such control exists, regardless of whether such person or entity is or becomes an Affiliate on or after the date of the Subscription Agreement.
1
For the purposes of this definition, the word “control” (including, with correlative meaning, the terms “controlled by” or “under the common control with”) means the actual power, either directly or indirectly through one or more intermediaries, to direct or cause the direction of the management and policies of such entity, whether by the ownership of more than fifty percent (50%) of the voting stock of such entity, or by contract or otherwise.
“Belgian Companies and Associations Code” means the Belgian Companies and Associations Code of 23 March 2019, as amended from time to time, and the rules and regulations promulgated thereunder.
“Business Day” means a day other than (a) a Saturday or a Sunday, (b) a bank or other public holiday in California, United States, (c) a bank or other public holiday in Brussels, Belgium, (d) a bank or other public holiday in Ireland or (e) the period commencing on December 25th and ending on January 1st (inclusive).
“Company” means Galapagos NV/SA, a corporation (naamloze vennootschap / société anonyme) organized and existing under the laws of Belgium, with registered office at Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium, and registered with the Register of Legal Persons under enterprise number 0466.460.429 (Antwerp, division Mechelen).
“Conditions” means the present terms and conditions of the Warrant.
“Equity Security” means (a) any Share, and (b) any other security, financial instrument, certificate and other right (including options, futures, swaps and other derivatives) issued or, with respect to options, futures, swaps and other derivatives, contracted by the Company and representing, being exercisable, convertible or exchangeable into or for, or otherwise providing a right to acquire, directly or indirectly, any of the Equity Securities referred to in (a).
“Exercise Account” has the meaning as defined in Article 7.2(b).
“Exercise Date” has the meaning as defined in Article 7.1(b).
“Exercise Notice” has the meaning as defined in Article 7.1(a).
“Exercise Price” means the exercise price of the Warrant, per Share that shall be subscribed for upon an exercise of the Warrant in relation to such Shares, as determined pursuant to Article 5.
“Expiry Date” has the meaning as defined in Article 3(a).
“Gilead Warrant” means each of the Initial Gilead Warrants and Subsequent Gilead Warrant B.
“Initial Gilead Warrant” means each of the warrants (inschrijvingsrechten or warrants / droits de souscription) issued by the Company on 22 October 2019 to the Investor, and named, respectively, the “Initial Gilead Warrant A” and “Initial Gilead Warrant B”.
“Investor” means Gilead Therapeutics A1 Unlimited Company, an unlimited liability company formed under the laws of Ireland, registered with Ireland’s Companies Registration Office under number 615395.
“Issue Date” means the date on which the Warrant has been issued by the extraordinary general shareholders’ meeting of the Company, i.e. [date] 2024.
“Notice” has the meaning given to it in Article 10.6.
2
“Option, License and Collaboration Agreement” means the option, license and collaboration agreement, dated 14 July 2019, by and between the Company and the Parent Investor, as amended from time to time.
“Parent Investor” means Gilead Sciences, Inc., a corporation incorporated under the laws of Delaware.
“Reference Date” means 23 August 2019, being the date on which the Subscription Shares were issued by the Company and were subscribed for by the Investor pursuant to the Subscription Agreement.
“Reference Exercise Price” means a price, per Share subscribed for upon an exercise of the Warrant in relation to such Shares, that shall be equal to the greater of (i) 120% multiplied by the arithmetic mean of the daily volume weighted average trading price of the Company’s Shares as traded on Euronext Brussels and Euronext Amsterdam (or such other regulated markets on which the Company’s Shares will be trading at that time) on each of the trading days during the period of 30 calendar days ending on the calendar day immediately preceding the date of the Exercise Notice with respect to such exercise, and (ii) EUR 140.59, being the issue price, on a per Share basis, that was paid by the Investor with respect to the Subscription Shares that were issued to the Investor on the Reference Date pursuant to the Subscription Agreement.
“Share” means any share (aandeel / action) outstanding from time to time representing the Company’s share capital.
“Share Reorganisation” has the meaning given to it in Article 8.2(a).
“Subscription Agreement” means the subscription agreement, dated 14 July 2019, by and between the Company and the Investor, in relation to the subscription by the Investor for the Subscription Shares and the Initial Gilead Warrants and Subsequent Gilead Warrant B, as amended from time to time.
“Subscription Shares” means the 6,828,985 Shares that were issued by the Company and subscribed for by the Investor on the Reference Date pursuant to the Subscription Agreement.
“Subsequent Gilead Warrant B” means the warrant (inschrijvingsrecht or warrant / droit de souscription), named “Subsequent Gilead Warrant B” issued by the Company on the Issue Date to the Investor.
“Succession Transaction” has the meaning given to it in Article 8.3(a).
“Successor Company” has the meaning given to it in Article 8.3(a).
“Term” means the term of the Warrant as referred to in Article 3.
“Warrant” means the Subsequent Gilead Warrant B.
“Warrant Limit” has the meaning given to it in Article 4.1(a).
| 1.2. | Headings |
Headings and the table of contents used in these Conditions are for convenience purposes only and shall not affect the construction or interpretation of these Conditions.
3
| 1.3. | Meaning of references |
Unless the context does not so permit, or save where specifically indicated otherwise:
| (a) | references to Articles are to Articles in these Conditions, and references to sub-Articles or paragraphs are to sub-Articles or paragraphs of the Article in which such references appear; |
| (b) | the words “herein”, “hereof”, “hereunder”, “hereby”, “hereto”, “herewith” and words of similar import shall refer to these Conditions as a whole and not to any particular Article, paragraph or other subdivision; |
| (c) | references to the word “include” or “including” (or any similar term) are not to be construed as implying any limitation, and general words introduced by the word “other” (or any similar term) shall not be given a restrictive meaning by reason of the fact that they are preceded by words indicating a particular class of acts, matters or things; |
| (d) | any reference to “writing” or “written” includes any method of reproducing words or text in a legible and non-transitory form but, for the avoidance of doubt, shall not include e-mail; |
| (e) | references to any statute, regulation or statutory provision shall be deemed to include reference to any statute, regulation or statutory instrument which amends, extends, consolidates or replaces the same (or shall have done so) and to any other regulation, statutory instrument or other subordinate legislation made thereunder or pursuant thereto, provided that no such reference shall include any amendment, extension or replacement of the same with retrospective effect; |
| (f) | all periods of time set out herein shall be calculated from midnight to midnight local time in Brussels, Belgium. They shall start on the day following the day on which the event triggering the relevant period of time has occurred. The expiration date shall be included in the period of time. If the expiration date is not a Business Day, it shall be postponed until the next Business Day. Unless otherwise provided herein, all periods of time shall be calculated in calendar days. All periods of time consisting of a number of months (or years) shall be calculated from the day in the month (or year) when the triggering event has occurred until the eve of the same day in the following month(s) (or year(s)) (“van de zoveelste tot de dag vóór de zoveelste” / “de quantième à veille de quantième”). |
| 1.4. | Fractional value of Shares |
For the purpose of these Conditions, the fractional value (fractiewaarde / pair comptable) of the Company’s Shares from time to time shall be determined as a fraction, (a) the numerator of which is the amount of the Company’s share capital at that time, and (b) the denominator of which is the aggregate number of actually issued and outstanding Shares of the Company at that time.
| 1.5. | Language |
The Conditions have been prepared in English and a Dutch translation was prepared. In the case of discrepancies between the English and the Dutch version, the English version shall prevail between the parties hereto to the fullest extent possible and permitted by Belgian law. Notwithstanding the foregoing, Belgian legal concepts which are expressed in English language terms, are to be interpreted in accordance with the Belgian legal terms to which they refer, and the use herein of Dutch and/or French words in these Conditions as translation for certain words or concepts shall be conclusive in the determination of the relevant legal concept under Belgian law of the words or concepts that are so translated herein.
4
| 2. | ISSUANCE, NATURE AND FORM OF THE WARRANT |
| 2.1. | Issuance and nature |
| (a) | The Warrant has been issued, without any additional consideration being due by the Investor or any of its Affiliates, pursuant to a resolution of the extraordinary general shareholders’ meeting of the Company held on the Issue Date, with dis-application of the statutory preferential subscription rights of the shareholders of the Company for the benefit of the Investor. |
| (b) | Subject to, and in accordance with, the terms and conditions set forth in these Conditions, the Warrant confers the right (but not the obligation) on the holder thereof to subscribe, upon any exercise of the Warrant, for a number of new Shares to be issued by the Company. |
| (c) | Except as otherwise provided under Belgian law, the holder of the Warrant is no shareholder of the Company solely by virtue of holding the Warrant, and therefore does not have the rights of a shareholder in relation to the Shares to be issued or delivered to the holder of the Warrant upon an exercise of the Warrant until the exercise of the Warrant and the issue or delivery of the relevant Shares. |
| 2.2. | Registered form |
The Warrant is in registered form. In accordance with applicable law, the Warrant is recorded in a warrant register book, which is kept at the registered office of the Company. The Warrant cannot be converted into a bearer instrument or in dematerialized form.
| 2.3. | Transferability of the Warrant |
The Warrant shall be transferrable in the same manner and in accordance with the same rules as those that apply (mutatis mutandis) to the Subscription Shares pursuant to the Subscription Agreement. Transfers of the Warrant that do not comply with this Article 2.3 are not enforceable vis-à-vis the Company.
| 2.4. | No listing of the Warrant |
The Warrant shall not be listed at any time on a securities exchange, regulated market or similar securities market.
| 3. | TERM OF THE WARRANT |
| (a) | The Warrant has a term (the “Term”) starting as of the Issue Date and ending on 11:59 p.m. on the calendar day before the fifth anniversary of the Issue Date (the “Expiry Date”). |
| (b) | The Warrant automatically lapses and becomes invalid (vervallen) by operation of law on 11:59 p.m. of the Expiry Date. |
| (c) | Subject to and in accordance with the terms and conditions set forth in these Conditions, the Warrant can be exercised at one or several occasions at any time as from 23 August 2024 (on 11:59 p.m.) during the Term. |
5
| 4. | SHARES |
| 4.1. | Number of Shares issuable upon an exercise of the Warrant |
| (a) | Subject to the terms and conditions set forth in these Conditions, the Warrant entitles the holder thereof to subscribe, during the entire Term of the Warrant, upon each exercise of the Warrant, for a maximum number of Shares (the “Warrant Limit”) that is, in the aggregate with respect to each exercise of the Warrant, sufficient to bring the number of Shares owned by the Investor, the Parent Investor and any of their Affiliates and any other party Acting in Concert with the Investor, the Parent Investor or any of their Affiliates to 29.9% of the actually issued and outstanding Shares immediately after the issue of the Shares that are to be issued upon the relevant exercise of the Warrant (rounded down to the nearest whole Share). |
| (b) | Notwithstanding the provisions of paragraph (a), if and as long as the person or entity exercising the Warrant is not the Investor, the Parent Investor or an Affiliate of the Investor or Parent Investor, the Warrant Limit shall be equal to one (1) Share. The provisions of paragraph (a) of this Article 4.1 will again apply when the person or entity exercising the Warrant is the Investor, the Parent Investor or any Affiliate of the Investor and the Parent Investor. |
| (c) | The Warrant can be exercised at one or several occasions during the entire Term, but not more than once per period of three (3) months; provided that such limitation on the frequency of exercising the Warrant shall not apply within a given three (3) month period in which there has been already an exercise of the Warrant to the extent that within such three (3) month period there has been a material development regarding the Company or the trading of Shares or if the Company or any other person or entity (other than the Investor, the Parent Investor, any of the Affiliates of the Investor or Parent Investor, or any party Acting in Concert with the Investor, the Parent Investor or any Affiliate of the Investor or the Parent Investor) provides notice that it intends to convene, requests to convene, or convenes, a meeting of shareholders. On each occasion the Warrant is exercised, the number of Shares that the holder of the Warrant will be entitled to subscribe for, will, in the aggregate with respect to such exercise of the Warrant, be limited to the then applicable Warrant Limit. The Warrant shall remain outstanding for the remaining duration of the Term even if exercised for a number of Shares that is equal to the then applicable Warrant Limit. |
| (d) | The Warrant can only be exercised for a whole number of Shares, and not with respect to fractions of Shares. |
| 4.2. | Nature and form of the Shares |
| (a) | Each new Share to be issued by the Company upon each exercise of the Warrant shall have the same rights and benefits as, and rank pari passu in all respects including as to entitlement to dividends and other distributions, with the existing and outstanding Shares at the moment of their issue and will be entitled to dividends and other distributions in respect of which the relevant record date or due date falls on or after the date of their issue. |
| (b) | Notwithstanding any other provision of these Conditions, upon each exercise of the Warrant, the Company shall have the right, to be determined by it in its discretion, to deliver a number of existing Shares in lieu of (all or a portion of) the new Shares that would otherwise need to be issued upon such exercise of the Warrant, provided that (i) such existing Shares confer the rights referred to in paragraph (a) and (ii) the delivery of such number of existing Shares (together with any new Shares issued upon such exercise of the Warrant) results in the Investor, the Parent Investor and any of their |
6
| Affiliates and any other party Acting in Concert with the Investor, the Parent Investor or any of their Affiliates owning the same percentage of Shares of the actually issued and outstanding Shares of the Company immediately after the issue of the Shares that had to be issued upon such exercise of the Warrant, rounded down to the nearest whole Share, that they would otherwise own if only new Shares were to have been issued upon such exercise of the Warrant. The holder of the Warrant cannot be obliged to pay a price per Share for the delivery of existing Shares that is higher than the applicable Exercise Price. |
| (c) | The Shares to be delivered upon each exercise of the Warrant shall be delivered in registered form. |
| 4.3. | Listing of the Shares |
If the admission of the Shares that are to be issued upon an exercise of the Warrant to trading on the regulated markets of Euronext Brussels and Euronext Amsterdam (and such other regulated markets on which the Company’s Shares will be trading at that time) legally requires a listing prospectus, the Company shall use reasonable efforts to obtain such admission within ninety (90) days following the issue of such Shares. In such event, the effective admission to listing will be subject to regulatory approval of the listing prospectus.
If the admission of the Shares that are to be issued upon an exercise of the Warrant to trading on the regulated markets of Euronext Brussels and Euronext Amsterdam (and such other regulated markets on which the Company’s Shares will be trading at that time) does not legally require a listing prospectus, the Company shall cause such admission as soon as practicable after the issue of such Shares, and in any event no later than five (5) Business Days after the issue of such Shares.
| 5. | EXERCISE PRICE |
The Exercise Price of the Warrant shall, per Share that shall be subscribed for upon an exercise of the Warrant in relation to such Shares, be equal to the Reference Exercise Price.
| 6. | SPECIFIC CONDITIONS |
| (a) | Upon subscription for the Warrant and when exercising the Warrant, the holder of the Warrant will comply with (i) the Belgian Act of 2 May 2007 regarding the disclosure of important participations in issuers of which shares are admitted to trading on a regulated market and miscellaneous provisions as amended from time to time, and the rules and regulations promulgated thereunder, and (ii) insider trading and/or dealings or transactions in the securities of the Company, including notably Regulation (EU) No 596/2014 of the European Parliament and of the Council of 16 April 2014 on market abuse (market abuse regulation) and repealing Directive 2003/6/EC of the European Parliament and of the Council and Commission Directives 2003/124/EC, 2003/125/EC and 2004/72/EC, and the relevant regulations, directives and other rules promulgated thereunder, as well as similar rules and regulations elsewhere in other jurisdictions. |
| (b) | During the Term, the Investor shall, upon written request from the Company, provide the Company within five (5) Business Days, the aggregate number of Equity Securities owned by the Investor, the Parent Investor and their Affiliates to the extent calculable (it being understood that the Warrants can be described without calculating a number of shares issuable thereunder). During the Term, the Company shall provide the Investor with notice of any exercise or conversion of any options, warrants, convertible securities or other right to acquire, directly or indirectly, any of the new shares of the Company by any person or entity (other than the Investor, the Parent Investor and their Affiliates) within five (5) Business Days after such exercise or conversion. |
7
| 7. | EXERCISE OF THE WARRANT |
| 7.1. | Exercise Notice |
| (a) | The Warrant can only be exercised by means of a written notice to the Company (the “Exercise Notice”). The Exercise Notice must be served on the Company in accordance with the provisions of Article 10.6. |
| (b) | The date on which the Exercise Notice with respect to a specific exercise of the Warrant shall have been served (or be deemed served) on the Company pursuant to Article 10.6 shall be the exercise date of the Warrant with respect to that exercise (the “Exercise Date”). |
| (c) | The Exercise Notice must state (i) the number of Shares with respect to which the Warrant is exercised at that occasion, and (ii) the applicable aggregate Exercise Price, as determined in accordance with the provisions of these Conditions. The Exercise Date should fall within the Term. |
| (d) | If the number of Shares with respect to which the Warrant is exercised as indicated in the Exercise Notice exceeds the then applicable Warrant Limit, the Warrant shall be deemed exercised with respect to the number of Shares just below the then applicable Warrant Limit only. The Company shall as soon as practical, and in any event before the expiry of the term within which the relevant Shares are to be issued to the holder of the Warrant pursuant to Article 7.3 of these Conditions, notify the holder of the Warrant thereof in accordance with the provisions of Article 10.6, which notification shall include the reasoned calculation of the then applicable Warrant Limit. |
| (e) | Upon receipt of the Exercise Notice, the Company may request the holder of the Warrant in writing to provide to the Company with such further declarations and documents, which are necessary to comply with all applicable legal and regulatory provisions in connection with the exercise of the Warrant and the issue or delivery of the Shares resulting therefrom, including pursuant to Article 6(b) of these Conditions. |
| 7.2. | Payment of the Exercise Price |
| (a) | Upon each exercise of the Warrant, the applicable aggregate Exercise Price must be paid by means of a payment in cash. |
| (b) | Following the receipt of the Exercise Notice, the Company shall, as promptly as practicable and in any event no later than one (1) Business Day after the Exercise Date, notify the holder of the Warrant of the account onto which the applicable aggregate Exercise Price must be paid (the “Exercise Account”). |
| (c) | The amount of the applicable aggregate Exercise Price must be paid by means of a wire transfer of such amount in immediately available funds in euro to the Exercise Account. |
| (d) | If the applicable aggregate Exercise Price is not paid in accordance with paragraph (c) within a term of ten (10) Business Days following the date on which the Company shall have notified the holder of the Warrant of the details of the Exercise Account in accordance with paragraph (b), the Warrant shall be deemed not to have been exercised, without prejudice to the right of the holder of the Warrant to exercise the Warrant at later occasions until the Expiry Date subject to and in accordance with the terms and conditions set forth in these Conditions. |
8
| 7.3. | Issue and delivery of the Shares |
| (a) | The Company shall only be obliged to issue Shares upon an exercise of the Warrant provided that (i) the relevant Exercise Notice has been made in accordance with Article 7.1, and (ii) the applicable aggregate Exercise Price has been paid in accordance with the provisions of Article 7.2. Subject to the foregoing, the Company shall issue or deliver the relevant Shares as soon as practicable, but in any event no later than the later of (x) six (6) calendar days after the Exercise Date, and (y) the day other than (a) a Saturday or a Sunday or (b) a bank or other public holiday in Belgium following the receipt of the applicable aggregate Exercise Price on the Exercise Account. |
| (b) | The Company shall take all steps and carry out all formalities that shall be required by virtue of these Conditions, the Company’s articles of association and applicable law in order to issue the new Shares upon an exercise of the Warrant (without prejudice, however, to the right of the Company to deliver existing Shares in accordance with the provisions of Article 4.2(b)). |
| (c) | In accordance with applicable law, upon each exercise of the Warrant, the capital increase and issue of new Shares resulting therefrom (as relevant) shall be formally recorded before a notary public by one authorised representative of the Company. |
| 7.4. | Allocation of the Exercise Price |
| (a) | Each time upon an exercise of the Warrant and the issue of new Shares pursuant to these Conditions, the applicable aggregate Exercise Price shall be allocated to the share capital of the Company. If the applicable Exercise Price per Share issued is greater than the fractional value of the existing Shares immediately prior to the capital increase, then the applicable aggregate Exercise Price shall be allocated in such a manner that per Share issued (i) a part of the applicable aggregate Exercise Price equal to the fractional value of the existing Shares immediately prior to the capital increase shall be booked as share capital, and (ii) the balance of the applicable aggregate Exercise Price shall be booked as issue premium. Such issue premium shall be accounted for on the liabilities side of the Company’s balance sheet as net equity. The account on which the issue premium shall be booked shall, like the share capital, serve as the guarantee for third parties and, save for the possibility of a capitalisation of those reserves, can only be reduced on the basis of a valid resolution of the general shareholders’ meeting passed in the manner required for an amendment to the Company’s articles of association. |
| (b) | Following the issue of new Shares and the capital increase resulting therefrom, each of the Shares (existing and new) shall represent the same fraction of the Company’s share capital. |
| 8. | ADJUSTMENTS |
| 8.1. | General |
| (a) | Notwithstanding Article 7:71, §1 under the Belgian Companies and Associations Code (or its successor provision), and with the exception of the issue of any Shares with a different fractional value (fractiewaarde) than the then existing Shares or with a par value (nominale waarde), of any securities with voting rights other than Shares or of any Equity Securities in relation to any Shares with a different fractional value (fractiewaarde) than the then existing Shares or with a par value (nominale waarde) or |
9
| any securities with voting rights other than Shares and any reclassification of existing Shares (which shall not be permitted pursuant to this Article 8.1(a)), the Company may proceed with all actions that it deems appropriate in relation to its capital, its articles of association, its financial condition, even if such actions lead to a reduction of the benefits allocated to the Warrant, including but not limited to, mergers or acquisitions, capital increases or reductions (including those subject to conditions precedent), the incorporation of reserves into the capital with or without the issue of new Shares, the issue of dividends or other distributions, the issue of other Equity Securities and the amendment of arrangements or provisions relating to the distribution of profits or liquidation proceeds, provided, however, that (i) the terms of the Warrant may not be amended without Investor’s written consent, and (ii) that Shares issued or issuable under the Warrant shall not be treated differently (had they already been issued at that time) than other Shares already issued. If the rights of the holder of the Warrant are affected by an action or transaction permitted by the immediately preceding sentence, the holder of the Warrant will not be entitled to a change of the Exercise Price, the Reference Exercise Price or the Warrant Limit, an amendment to the Conditions or any other form of compensation (financial or otherwise) unless (i) specifically provided for in Articles 8.2 and 8.3 of these Conditions and/or (ii) such action or transaction was undertaken with the primary purpose of adversely affecting the rights or value of the Warrant. |
| (b) | The provisions of this Article 8 are without prejudice to the provisions of the Subscription Agreement (such as the anti-dilution protection). |
| 8.2. | Adjustments for Share Reorganisations |
| (a) | If at any time as of the Issue Date up to the Expiry Date there is a change of the fractional value of the Shares as a result of a consolidation (or reverse stock split), a subdivision (or stock split) or otherwise, in each such case without increase or reduction of the Company’s share capital (each such transaction a “Share Reorganisation”), the Warrant Limit shall not be affected (since it is expressed as a percentage of the actually issued and outstanding Shares). In addition, the Exercise Price of the Warrant (i.e. on a per Share basis) shall be divided by a fraction, (A) the numerator of which is equal to the fractional value of the outstanding Shares of the Company immediately before to the Share Reorganisation, and (B) the denominator of which is equal to the fractional value of the outstanding Shares of the Company immediately after the Share Reorganisation. |
| (b) | Any adjustment made pursuant to paragraph (a) of this Article 8.2 shall become effective immediately after the effective date of the relevant Share Reorganisation that gives rise to such adjustment. The Company shall notify the holder of the Warrant of such adjustment by written notice as soon as practicable after the effective date of the Share Reorganisation concerned. |
| 8.3. | Adjustments for mergers and de-mergers |
| (a) | If at any time as of the Issue Date up to the Expiry Date there is a merger (fusie / fusion) of the Company with or into another legal person or entity whereby the Company is not the surviving entity or a de-merger (splitsing / scission) in whole or in part, whereby in each of these cases the Shares of the Company are exchanged into, or the shareholders of the Company receive, shares, other securities, cash or other property of one or more other legal persons or entities (each such legal person or entity a “Successor Company”) as a result of such merger or de-merger (each such transaction a “Succession Transaction”), then the Warrant shall be replaced by (or, if the |
10
| Company survives, to the holder of the Warrant shall be issued) one or more warrants to be issued by each of the respective Successor Companies that give right to such respective shares, other securities, cash or other property that the holder of the Warrant concerned had been entitled to receive if the Warrant had been exercised in full up to the Warrant Limit immediately before to the occurrence of the Succession Transaction concerned but after giving effect to dilution upon the exercise or conversion of all rights to acquire Shares that are exercised or converted in connection with such transaction. The number of warrants to be so issued in replacement of the Warrant (or, if the Company survives, to be so issued), as well as the terms and conditions of such warrants (including the Exercise Price and the Warrant Limit) will need to be, as a whole, equivalent to the terms and conditions of the Warrant and have economically substantially the same effect for the holder of the Warrant concerned. |
| (b) | An adjustment made pursuant to paragraph (a) of this Article 8.3 shall become effective upon, and subject to, the completion of the Succession Transaction that gives rise to such adjustment. The Company (or the respective Successor Companies, as the case may be) shall notify the holder of the Warrant of such adjustment as soon as practicable after the completion of the Succession Transaction concerned. |
| (c) | In the case of any merger or de-merger of the Company as contemplated by paragraph (a) of this Article 8.3, the Company must procure that the successor or acquiring persons or entities shall expressly assume the due observance and performance of the covenants and obligations set out in the Conditions. |
| 9. | REPRESENTATIONS AND WARRANTIES |
| 9.1. | Representations and Warranties of the Company |
Upon each exercise of the Warrant, the Company shall be deemed to represent and warrant to the holder of the Warrant on the date of the issue or delivery of the Shares to be issued or delivered by the Company upon such exercise of the Warrant:
| (a) | Incorporation. It is duly incorporated and validly existing and in good standing under the laws of Belgium, with full power and authority to conduct its business and is not in violation of any of the provisions of its organisational documents. |
| (b) | Validity of Shares and Absence of Breach. Each Share to be issued or delivered by the Company upon such exercise of the Warrant, and, with respect to new Shares only, as of when issued and paid for in accordance with these Conditions, are validly and duly issued and fully paid ordinary shares of the Company in accordance with the applicable provisions of the Company’s organisational documents and Belgian law, having the same fractional value (fractiewaarde) as the then existing Shares, and free and clear of all liens, pledges, encumbrances, mortgages, security interests, or easement or transfer restrictions of any nature whatsoever (other than those that find their origin solely with the holder of the Warrant and save for the transfer restrictions referred to in the Subscription Agreement, as the case may be). The issue or delivery of the Shares to be issued or delivered by the Company upon such exercise of the Warrant will not result in a breach of, default under any material agreement to which the Company is a party or the Company’s organisational documents or any law, regulation or stock exchange rule, or give rise to the activation of any material rights of third parties under any agreement, law, rule or regulation binding on the Company or any of its subsidiaries. |
11
| (c) | Consents. All necessary consents, authorisations, notification, actions or things required to be taken, fulfilled or done under Belgian law or any of the Antitrust Laws (as defined in the Option, License and Collaboration Agreement) (including, without limitation, the obtaining of any consent or license or the making of any filing or registration) for the issue or delivery of the Shares to be issued or delivered by the Company upon such exercise of the Warrant, the actions contemplated by these Conditions and the Warrant or the compliance by the Company with the terms of these Conditions and the Warrant will, save as otherwise set forth in these Conditions in Article 4.3, be in full force and effect. |
| (d) | Brokers and Finders. No person will have, as a result of the exercise of the Warrant, any right, interest or claim against or upon the holder of the Warrant for any commission, fee or other compensation relating to the Shares to be issued or delivered by the Company upon such exercise of the Warrant. |
| 9.2. | Representations and Warranties of the holder of the Warrant |
Upon each exercise of the Warrant, the holder of the Warrant shall be deemed to represent and warrant to the Company on the date of the issue or delivery of the Shares to be issued or delivered by the Company upon such exercise of the Warrant:
| (a) | Incorporation. It is duly incorporated and validly existing and in good standing under the laws of its jurisdiction of incorporation, with full power and authority to conduct its business and is not in violation of any of the provisions of its organisational documents. |
| (b) | Consents. All necessary consents, authorisations, notification, actions or things required to be taken, fulfilled or done under the law applicable to its jurisdiction of organisation or incorporation, or any of the Antitrust Laws (as defined in the Option, License and Collaboration Agreement) (including, without limitation, the obtaining of any consent or license or the making of any filing or registration) for the exercise of the Warrant, the actions contemplated by these Conditions and the Warrant or the compliance by the Warrant holder with the terms of these Conditions and the Warrant will, save as otherwise set forth in those Conditions in Article 4.3, be in full force and effect. |
| (c) | Information. Without taking into account the Warrants or the shares issuable (but not yet issued) thereunder, except to give effect to the issue and delivery of the Shares to be issued or delivered by the Company to the Investor upon the particular exercise of the Warrant that occurs on the date that this representation and warranty is made, the Investor, the Parent Investor, and their respective Affiliates do not, directly or indirectly, own, or have the right to acquire, voting securities of the Company in excess of the Warrant Limit (assuming the exercise, conversion or exchange of any Equity Securities (other than the Warrants except as described in this Article 9.2(c)) held by any of them at that time that are exercisable, convertible or exchangeable into or for shares of the Company at that time) (the resulting number of securities rounded down). |
| 10. | MISCELLANEOUS |
| 10.1. | Binding nature of the Conditions |
In the case of subscription for the Warrant, the subscriber shall be bound by, and deemed to have accepted, the present Conditions. In the event of a transfer of the Warrant (or any right thereto), the acquirer or transferee shall be bound by, and deemed to have accepted, the present Conditions.
12
| 10.2. | Severability |
Whenever possible, the provisions of the Conditions shall be interpreted in such a manner that they are valid and enforceable under the applicable legislation.
If any provision in these Conditions is held to be illegal, invalid or unenforceable, in whole or in part, under any applicable law, then such provision or part of it shall be deemed not to form part of these Conditions, and the legality, validity or enforceability of the remainder of these Conditions shall not be affected.
In that event, the illegal, invalid or non-enforceable provision or part thereof is automatically replaced with the legal, valid and enforceable provision that is the closest to the original provision or part thereof as regards content, bearing and intention.
| 10.3. | Specific Enforcement |
Notwithstanding anything in these Conditions to the contrary, nothing in these Conditions shall in any way limit the ability of the Company and the holder of the Warrant to seek or obtain from any court of competent jurisdiction any remedies available at law or in equity (including injunctive relief) to enforce any covenant or agreement of the holder of the Warrant respectively the Company hereunder.
| 10.4. | Costs and expenses |
The Company shall pay any stamp, issue, registration, documentary or other taxes and duties, including interest and penalties, payable in Belgium on or in connection with the issue or delivery of the Shares upon each exercise of the Warrant. The Company shall also pay all costs associated with the listing of the relevant Shares on the regulated markets of Euronext Brussels and Amsterdam (and such other regulated markets on which the Company’s Shares will be trading at that time).
| 10.5. | Governing law and jurisdiction |
The Conditions and the Warrant and any non-contractual obligations arising out of or in connection with each of them are governed by, and are to be construed in accordance with, Belgian law.
Any dispute, controversy, difference or claim which may arise between the Company and the holder of the Warrant out of or in relation to or in connection with the Conditions or the Warrant (including arising out of or relating to the validity, construction, interpretation, enforceability, breach, performance, application, exercise, expiry or termination of the Conditions or the Warrant), shall be settled by binding arbitration in accordance with the applicable rules of the International Chamber of Commerce (“ICC Rules”) by three (3) arbitrators, one chosen by the holder of the Warrant, one chosen by the Company and the third chosen by mutual agreement of the first two, and otherwise in accordance with the ICC Rules. The arbitrators shall have significant experience and shall have expertise in Belgian corporate law. Each of the Company and the holder of the Warrant may refer such dispute to arbitration by submitting a written notice of such request to the holder of the Warrant respectively the Company. The place of arbitration shall be New York and the language (including all testimony, evidence and written documentation) shall be English. The arbitrators shall establish procedures to facilitate and complete such arbitration as soon and efficiently as practicable. Unless the arbitrators expressly determine otherwise, neither the Company nor the holder of the Warrant shall be required to give general discovery of documents, but may be required only to produce specific, identified documents that are relevant to the dispute. Each of the Company and the holder of the Warrant shall have the right to be represented by counsel. Any judgment or award rendered by the arbitrators shall be final and binding on the Company and the holder of the Warrant, and shall be governed by the terms and conditions hereof and the limitation on damages set forth in Article 13 of the Subscription Agreement.
13
The parties hereto agree that such a judgment or award may be enforced in any court of competent jurisdiction. The statute of limitations of Belgian law applicable to the commencement of a lawsuit shall apply to the commencement of arbitration. The arbitrators shall determine the allocation of costs and expenses and attorneys’ fees in the arbitration to be borne by each of the Company and the holder of the Warrant. All proceedings and decisions of the arbitrators shall be deemed Confidential Information (as set out in the Subscription Agreement) of each of the Company and the holder of the Warrant, and shall be subject to Article 13 of the Option, License and Collaboration Agreement.
| 10.6. | Notices |
Any notice, notification, demand or other communication (“notice”) to be given under these Conditions shall be in writing, shall specifically refer to these Conditions, and shall be addressed to the appropriate party at the address specified below or such other address as may be specified by such party in writing in accordance with this Article 10.6, and shall be deemed to have been given for all purposes (i) when received, if hand-delivered, sent by a reputable international expedited delivery service or sent by facsimile (with transmission confirmed), or (ii) five (5) Business Days after mailing, if mailed by first class certified or registered mail, postage prepaid, return receipt requested. Any notice delivered by facsimile shall be confirmed by a hard copy delivered by a reputable international expedited delivery service as soon as practicable thereafter. The current details for notices are:
| (a) | if to the Company: the address of the Company’s registered office, with the notice made for the attention of the General Counsel of the Company, or the address for notices to the Company pursuant to the Subscription Agreement. |
| (b) | if to the holder of the Warrant: to such holder’s address as set out in the warrant register book, or the address for notices to such party (as the case may be) pursuant to the Subscription Agreement. |
* * *
14
| English translation for information purposes |

|
Annex 2
Simulations
The simulations below are based on the methodology and assumptions set out in section 5.1 of the report to which this annex is attached.
| A Current situation, before issuance and exercise of the Subsequent Warrant B - Basic |
||
| Equity (in EUR) | ||
| Amount represented by 1 share(1) |
42.42 | |
| Total Equity |
2,795,566,256.00 | |
| B Situation before issuance and exercise of the Subsequent Warrant B - Fully diluted (2) |
||
| Equity (in EUR) | ||
| Amount represented by 1 share(1) |
47.69 | |
| Total Equity |
3,689,613,617.00 | |
| C Situation after issuance and exercise in full of the Subsequent Warrant B with an Exercise Price of EUR 140.59 - Fully diluted (3) |
||
| Equity (in EUR) | ||
| Amount represented by 1 share(1) |
57.53 | |
| Total Equity |
4,978,394,836.00 | |
| 1 | Rounded up. |
| 2 | Assuming that all 11,472,520 outstanding subscription rights are exercised, resulting in the issuance of 11,472,520 new shares, as a result of which the share capital of the Company would be represented by 77,369,591 shares (being the sum of (i) the 65,897,071 shares outstanding as at the date of the report to which this annex is attached and (ii) the relevant 11,472,520 new shares). |
| 3 | Assuming that (i) all 11,472,520 outstanding subscription rights are exercised (resulting in the issuance of 11,472,520 new shares) and (ii) the Subsequent Warrant B is issued and exercised in full on the basis of the methodology and assumptions as set out in section 5.1 and 5.2.2. of the report to which this annex is attached. |
| Annex to Board Report (Art. 7:180-7:191-7:193 BCAC) |
Exhibit 99.8

|
Phone : +32 (0)2 778 01 00 www.bdo.be |
The Corporate Village Da Vincilaan 9, Box E.6 Elsinore Building B-1930 Zaventem |
GALAPAGOS NV
Report addressed to the general meeting concerning
the financial and accounting data included in the
report of the administrative body in accordance with
of articles 7:180 CCA (issuance of subscription rights)
and 7:191 (cancellation of the preferential
subscription right) and 7:193 of the CCA (cancellation
of the preferential subscription right to the benefit of
certain persons who are not part of the staff).
Free translation
BDO Bedrijfsrevisoren BV / VAT BE 0431.088.289 / RPR Brussels
BDO Réviseurs d’Entreprises SRL / VAT BE 0431.088.289 / RPM Brussels
BDO Bedrijfsrevisoren BV / BDO Réviseurs d’Entreprises SRL, a private limited liability company under Belgian law, is a member of BDO International Limited, a UK company limited by guarantee, and forms part of the international BDO network of independent member firms.
BDO is the brand name for the BDO network and for each of the BDO Member Firms.

Report addressed to the general meeting of Galapagos NV concerning the financial and accounting data included in the report of the administrative body in accordance with 7:180 CCA (issuance subscription rights) and 7:191 CCA (cancellation of the preferential subscription right) and 7:193 of the CCA (cancellation of the preferential subscription right to the benefit of certain persons who are not part of the staff).
Mission
In accordance with articles 7:180 and 7:191 and 7:193 of the Code of companies and associations (hereinafter ‘CCA’), in our capacity as statutory auditor, we issue a report addressed to the general meeting of Galapagos NV (hereinafter ‘the Company’) on the financial and accounting data included in the report of the administrative body.
Conclusion of the report
Unmodified conclusion
Based on our review of the financial and accounting data included in the report of the administrative body, nothing has come to our attention that causes us to believe that the data, which includes the justification of the issuance price and the consequences for the shareholders’ capital and membership rights, is not, in all material respects, fair and sufficient to inform the general meeting, which is to vote on the proposed transaction.
In the context of articles 7:193 CCA, we have reviewed the justification of the issuance price.
Basis for the conclusion
We carried out our mission in accordance with the normative framework applicable in Belgium.
For the purpose of this mission, we must determine whether we have established facts that
cause us to believe that the financial and accounting data as a whole - included in the report of the administrative body - are not, in all material respects, sufficient and fair to inform the general meeting, which is to vote on the proposed transaction.
We have complied with all the ethical requirements that are relevant to the mission
We believe that the supporting information we have obtained is sufficient and appropriate as a basis for our conclusion.
| GALAPAGOS NV Report OF the statutory auditor in accordance with Articles 7:180 and 7:191 and 7:193 CCA |
2/4 |

Responsibilities of the administrative body with regard to preparing a report including the financial and accounting data
The administrative body is responsible for:
| • | drawing up a report that: |
| • | in accordance with article 7:180 CCA justifies the proposed transaction. This report also justifies the issuance price and describes the consequences of the transaction on the shareholders’ capital and membership rights; |
| • | in accordance with article 7:191 CCA, explicitly justifies the reasons for the limitation or cancellation of the preferential subscription right and describes the consequences on the shareholders’ capital and membership rights; |
| • | in accordance with article 7:193 CCA justifies the transaction and the issuance price in the Company’s interest, taking into account in particular the financial position of the Company, the identity of the beneficiaries, the nature and size of their contribution; |
| • | drawing up the financial and accounting data included in its report; |
| • | the fairness and sufficiency, in all material respects, of the information provided, to enable the general assembly to take a decision with informed consent . |
Responsibilities of the statutory auditor
Our responsibility is to express a conclusion with limited assurance as to the financial and accounting data included in the report of the administrative body in accordance with articles 7:180 and 7:191 and 7:193 CCA, based on our review.
The review of the financial and accounting data included in the report of the administrative body consists of making inquiries, primarily to persons responsible for financial and accounting matters, as well as performing analytical and other review procedures. The scope of a review is substantially less than an audit obtaining reasonable assurance. For this reason, our review does not enable us to obtain reasonable assurance that we will become aware of all significant matters that may be identified as a result of an audit.
Accordingly, we do not express an audit opinion on the financial and accounting data.
The scope of a review assignment does not provide assurance regarding the future
viability of the Company, nor its efficiency or effectiveness with which the administrative body has managed or will manage the Company.
Our mission is not to comment on the appropriateness or opportunity of the transaction, nor on the legality and fairness of the transaction (“no fairness opinion”).
| GALAPAGOS NV Report OF the statutory auditor in accordance with Articles 7:180 and 7:191 and 7:193 CCA |
3/4 |

Limitation on the use of our report
This report has solely been prepared in accordance with articles 7:180 and 7:191 and 7:193 CCA within the context of “Cancellation of the preferential subscription rights of the existing shareholders in favor of Gilead Therapeutics A1 Unlimited Company in the framework of the proposed issuance of the Subsequent Gilead Warrant B”, proposed to the general meeting and may not be used for any other purpose.
Zaventem, 28 March 2024
BDO Bedrijfsrevisoren BV
Statutory Auditor
Represented by Ellen Lombaerts *
Auditor
*Acting for a company
Annex: report of the administrative body in accordance with articles 7:180 and 7:191 and 7:193 CCA
| GALAPAGOS NV Report OF the statutory auditor in accordance with Articles 7:180 and 7:191 and 7:193 CCA |
4/4 |
Exhibit 99.9
| English translation for information purposes |

|
GALAPAGOS
Limited Liability Company
Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium
Company number: 0466.460.429
RLE Antwerp (division Mechelen)
Report of the Board of Directors in accordance with
Article 7:199 of the Belgian Companies and Associations Code
Dear shareholders,
In accordance with the provisions of article 7:199 of the Belgian Companies and Associations Code of 23 March 2019, as amended from time to time (the “Belgian Companies and Associations Code”), the Board of Directors of Galapagos NV (the “Company” or “Galapagos”) has the honor to report, in this special report, on the proposal that shall be made to the extraordinary shareholders’ meeting to be convened on 30 April 2024 (or on 25 June 2024 should the required quorum not be achieved at the first meeting) (the “EGM”) to renew the authorization to the Board of Directors to increase the share capital of Galapagos with an amount up to 20% in the framework of the authorized capital.
| 1 | Background |
The extraordinary shareholders’ meeting of 29 March 2005 has authorized the Board of Directors to increase the share capital of the Company. This authorization was granted for a period of 5 years. It has been renewed (and amended and increased) by the extraordinary shareholders’ meetings of 6 January 2006, 24 April 2007, 2 June 2009, 23 May 2011, 26 April 2016, 25 April 2017, and, most recently, on 22 October 2019.
Through the authorization of 22 October 2019 the Company’s Board of Directors has been authorized to increase the share capital in one or more times with an amount of EUR 67,022,402.04 (excluding issue premium). The aforementioned authorization can be used by the Board of Directors by a simple majority resolution for capital increases up to 20% of the share capital at the time of the convening of the extraordinary shareholders’ meeting of 22 October 2019. The authorisation is valid for a period of five years as from 13 November 2019, and is still valid on the date of this special report. On the date of this special report, the authorized capital has been used at six occasions:
| • | On 17 April 2020, in connection with the issuance of the so-called ‘Subscription Right Plan 2020’ and ‘Subscription Right Plan 2020 RMV’, under which a maximum of 2,173,335 new shares could be issued (upon exercise of granted and accepted subscription rights) for a total maximum capital increase of EUR 11,757,742.35 (excluding issuance premium); |
| • | On 30 April 2021, in connection with the issuance of the so-called ‘Subscription Right Plan 2021 BE’, the ‘Subscription Right Plan 2021 RMV’ and the ‘Subscription Right Plan 2021 ROW’ under which a maximum of 2,493,433 new shares could be issued (upon exercise of granted and accepted subscription rights) for a total maximum capital increase of EUR 13,489,472.53 (excluding issuance premium); |
| • | On 13 January 2022, in connection with the issuance of the so-called ‘Subscription Right Plan 2022 (A)’ under which a maximum of 30,000 new shares could be issued (upon exercise of granted and accepted subscription rights) for a total maximum capital increase of EUR 162,300.00 (excluding issuance premium); |
| • | On 26 January 2022, in connection with the issuance of the so-called ‘Subscription Right Plan 2022 (B)’ under which a maximum of 1,000,000 new shares could be issued (upon exercise of granted and accepted subscription rights) for a total maximum capital increase of EUR 5,410,000.00 (excluding issuance premium); |
1
| English translation for information purposes |

|
| • | On 6 May 2022, in connection with the issuance of the so-called ‘Subscription Right Plan 2022 BE’, ‘Subscription Right Plan 2022 RMV’ and ‘Subscription Right Plan 2022 ROW’, under which a maximum of 2,091,239 new shares could be issued (upon exercise of granted and accepted subscription rights) for a total maximum capital increase of EUR 11,313,602.99 (excluding issuance premium); and |
| • | On 5 May 2023, in connection with the issuance of the so-called ‘Subscription Right Plan 2023 BE’, ‘Subscription Right Plan 2023 RMV’ and ‘Subscription Right Plan 2023 ROW’, under which a maximum of 1,538,400 new shares could be issued (upon exercise of granted and accepted subscription rights) for a total maximum capital increase of EUR 8,322,744.00 (excluding issuance premium). |
On the date of this special report an aggregate amount of EUR 50,455,861.87 of the authorized capital has been used, as a result of which EUR 16,566,540.17 of the authorized capital remains available.
| 2 | Proposal to renew the authorized capital |
| 2.1 | Summary of proposal |
On 14 July 2019, the Company announced that it entered into a 10-year global research and development collaboration with Gilead Sciences, Inc. Within the framework of such collaboration, Gilead Therapeutics A1 Unlimited Company entered into a subscription agreement on 14 July 2019 pursuant to which it agreed to invest in the share capital of the Company in consideration of new shares (the “Subscription Agreement”). This capital increase was effected on 23 August 2019. The Subscription Agreement also included, amongst other things, a commitment to make a proposal to an extraordinary shareholders’ meeting of the Company to renew the authorization of the Board of Directors to increase the share capital of the Company in one or several times with an amount up to 20% of the share capital at the time of the convening of the aforementioned extraordinary shareholders’ meeting. Such authorisation was approved on 22 October 2019 and was published in the annexes to the Belgian Official Gazette on 13 November 2019.
The Subscription Agreement provides that between the 57 month and 59 month anniversary of the capital increase referred to above (i.e., between 23 May 2024 and 23 July 2024), the Board of Directors agrees to convene an extraordinary general meeting of the shareholders of the Company at which the shareholders of the Company would be asked to approve:
| • | the issuance to Gilead Therapeutics A1 Unlimited Company of an additional new subscription right (in the form of a warrant), the so-called ‘Subsequent Gilead Warrant B’, having terms and conditions that (mutatis mutandis) are the same as the terms and conditions of the outstanding ‘Initial Gilead Warrant B’. For further information on the proposed issuance of the ‘Subsequent Gilead Warrant B’, reference is made to the report of the Board of Directors prepared in accordance with article 7:180, 7:191 and 7:193 of the Belgian Companies and Associations Code (which will also be submitted to the EGM). |
| • | the authorization to the Board of Directors, valid for a period of five (5) years from the date of publication of the authorization in the Annexes to the Belgian Official Gazette, to increase the share capital of the Company in one or several times with an amount up to 20% of the share capital at the time of the convening of the relevant extraordinary general meeting, which capital increases may be achieved by the issuance of shares, convertible bonds and/or subscription rights (warrants) exercisable by contributions in cash or in kind, with or without issuance premium, and which authorization will explicitly authorize the Board of Directors to restrict or cancel the shareholders’ preferential subscription rights, even if such restriction or cancellation is made for the benefit of one or more specific persons other than the employees of the Company or its subsidiaries (including Gilead Therapeutics A1 Unlimited Company and its affiliates). |
2
| English translation for information purposes |

|
In view hereof, and as the Company’s annual shareholders’ meeting will be held on 30 April 2024, the proposed renewal of the authorized capital, will be submitted to the EGM on 30 April 2024, (or on 25 June 2024 should the required quorum for the EGM not be achieved at the first meeting).
The renewed authorization is to be valid for a 5-year term from the date of publication of the renewed authorization in the Annexes to the Belgian State Gazette and can only be used if no public takeover bid is ongoing (i.e. non-defensive use only).
For the sake of completeness, it should be noted that the Subscription Agreement provides for certain consequences in case the EGM were not to approve the proposed renewal of the aforementioned authorisation. Notably, in such situation the Company will have to submit the proposed renewal of the authorisation as soon as possible to a subsequent general shareholders’ meeting of the Company and, if such subsequent meeting were not to approve the proposed renewal of the authorization, the Company will be obliged to re-submit the proposed renewal of the authorisation to another extraordinary general shareholders’ meeting at the occasion of the annual general shareholders’ meeting of the Company (and this until the proposed renewal of the authorization has been approved).
| 2.2 | Renewal of authorized capital up to 20% of share capital |
The Board of Directors of the Company proposes to the extraordinary shareholders’ meeting to be authorized for a period of five years to increase the share capital in one or several times with an amount of up to 20% of the current share capital, provided that the Company has not been notified of a public takeover bid for its shares at the time of such increase. In particular, the Board of Directors proposes to delete the section “Authorized Capital” of the temporary provisions of the articles of association of the Company entirely and to replace it with the following text (whereby the amount of 20 percent of the subscribed capital referred to below between square brackets shall be determined on the basis of the outstanding subscribed capital at that time):
“Authorized capital
The Board of Directors has been granted the authority to increase the subscribed capital of the company, in accordance with applicable law, in one or several times, to the extent set forth hereafter. This authorization is valid for a period of five years from the date of publication of this authorization in the Annexes to the Belgian State Gazette.
Without prejudice to more restrictive rules set forth by law, the Board of Directors can increase the subscribed capital of the company in one or several times with an amount of up to EUR [•], i.e. 20 percent of the subscribed capital at the time of the convening of the shareholders’ meeting granting this authorization. In accordance with applicable law, the Board of Directors cannot use the aforementioned authorization after the Financial Services and Markets Authority (FSMA) has notified the company of a public takeover bid for the company’s shares.
The capital increases within the framework of the authorized capital may be achieved by the issuance of shares (below, above or at the fractional value of the existing shares, with or without voting rights, and as the case may be in the context of a subscription rights plan for the company’s or its subsidiaries’ members of the personnel within the meaning of article 1:27 of the Belgian Companies and Associations Code (including members of the Board of Directors and/or independent consultants)), convertible bonds and/or subscription rights exercisable by contributions in cash or in kind, with or without issuance premium, and also by the conversion of reserves, issuance premiums, profits carried forward or other equity components. Aforementioned subscription rights plans can provide that, in exceptional circumstances (among others in the event of a change in control of the company or decease), subscription rights can be exercised before the third anniversary of their award, even if the beneficiary of such subscription right is a member of the Board of Directors or a person entrusted with the day-to-day management.
When increasing the subscribed capital within the limits of the authorized capital, the Board of Directors may, in the company’s interest, restrict or cancel the shareholders’ statutory preferential subscription rights, even if such restriction or cancellation is made for the benefit of one or more specific persons other than the company’s or its subsidiaries’ members of the personnel within the meaning of article 1:27 of the Belgian Companies and Associations Code.
The Board of Directors can ask for an issuance premium when issuing new shares in the framework of the authorized capital. If the Board of Directors decides to do so, such issuance premium is to be booked on a non-available reserve account that can only be reduced or transferred by a decision of the shareholders’ meeting adopted in the manner required for amending the articles of association.
3
| English translation for information purposes |

|
The Board of Directors is authorized to bring the company’s articles of association in line with the capital increases which have been decided upon within the framework of the authorized capital, or to instruct a notary public to do so.”
| 3 | Specific circumstances and purposes for use of authorized capital |
The Company’s Board of Directors is of the opinion that the renewal of the authorized capital is necessary to meet the needs of the Company as a listed company.
Especially with the Company’s shares being publicly listed on Euronext Brussels and Euronext Amsterdam and the Company’s American Depositary Shares being publicly listed on NASDAQ, the procedure of convening an extraordinary shareholders’ meeting for a capital increase is complex, expensive, and time consuming. This procedure can thus be incompatible with the fluctuations on the capital markets or with certain business opportunities that the Company would otherwise be able to take advantage of. The proposed authorizations would allow the Board of Directors to increase the Company’s share capital in those circumstances in which it would be undesirable, impossible or inopportune to convene an extraordinary shareholders’ meeting.
Such situation could occur when the Board of Directors determines that the cash position of the Company needs to be strengthened within a relatively short timeframe to finance and support its research and development programs or to enable the Company to react to new research and development opportunities in a quick and flexible way. In addition, the Company may wish to finance certain transactions, such as acquisitions (of companies, business or assets), corporate partnerships, in-licensing deals or other types of mergers, partnerships or strategic alliances, wholly or partially with the issue of new shares or to permit one or more specific new shareholders to participate in its share capital. The convening of a shareholders’ meeting could in such circumstances, for example, lead to a premature announcement of the relevant transaction. With such (and other) transactions the Board of Directors shall aim for the continuous growth of the Company, where appropriate and/or possible by means of equity financing, and to strengthen the capital base of the Company by attracting strategic shareholders where possible.
Furthermore, the renewal of the authorized capital would enable the Company’s Board of Directors to comply with its commitments under the aforementioned Subscription Agreement. In particular, it should be noted that the aforementioned Subscription Agreement provides that, if the extraordinary general meeting were not to approve the proposed issuance of the aforementioned ‘Subsequent Gilead Warrant B’, amongst other things:
| • | the Company and Gilead Therapeutics A1 Unlimited Company shall collaborate to structure and organize the right to subscribe for shares as reflected by the ‘Subsequent Gilead Warrant B’ via a contractual subscription or option agreement or otherwise, allowing, to the greatest extent legally possible, Gilead Therapeutics A1 Unlimited Company to subscribe for or acquire the number of shares of the Company it would otherwise be able to subscribe for or acquire upon exercise of the ‘Subsequent Gilead Warrant B’, whether through the Company’s authorized capital or otherwise; and |
| • | as long as the Board of Directors shall have the power to issue shares within the framework of the authorized capital, the authorized capital shall to the greatest extent legally possible be used with priority for the purpose of the issuance of shares to Gilead Therapeutics A1 Unlimited Company, without prejudice to the right of the Company to use the authorized capital for equity-based incentives or compensation for current or future employees, consultants, directors and/or officers of the Company or its affiliates. |
The Board of Directors can also use the authorized capital to issue subscription rights (as the case may be, in the form of warrants) in connection with the Company’s remuneration policy for its and its subsidiaries’ employees, directors, independent consultants and other members of the personnel within the meaning of article 1:27 of the Belgian Companies and Associations Code.
4
| English translation for information purposes |

|
Finally, the Board of Directors notes that the proposed authorization cannot be used after the Belgian Financial Services and Markets Authority (FSMA) has notified the Company of a public takeover bid for the shares of the Company.
Done on 28 March 2024.
[Signature page follows]
5
| English translation for information purposes |

|
For the Board of Directors of the Company,
| [Signed] |
[Signed] |
|||
| Stoffels IMC BV, permanently represented by Dr. Paul Stoffels |
Jérôme Contamine Director |
6
Exhibit 99.10
| Time Sensitive Materials |
Depositary’s Notice of Shareholders’ Meetings’ of Galapagos NV
| ADSs: |
American Depositary Shares (“ADSs”). | |
| ADS CUSIP No.: |
36315X101. | |
| Company: |
Galapagos NV, a company organized and existing under the laws of the Kingdom of Belgium (the “Company”). | |
| ADS Record Date: |
March 25, 2024 (close of business in New York). Date used to determine ADS Holders who are to receive these materials and who are eligible to give voting instructions to the Depositary upon the terms described herein. | |
| Share Record Date: |
April 16, 2024 (Midnight CEST). Date on which ADS Holders are required under Belgian law to hold their interests in the shares of the Company in order to be eligible to vote at the Shareholders’ Meetings |
|
| Meeting Specifics: |
Annual Shareholders’ Meeting and Extraordinary Shareholders’ Meeting to be held sequentially at 2:00 P.M. (CEST) on Tuesday, April 30, 2024, at the registered office of the Company located at Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium (together, the “Shareholders’ Meetings”). | |
| Meeting Agendas: |
Documents related to the Shareholders’ Meetings Agenda items will be available on the Company’s website at http://www.glpg.com/shareholders-meetings. | |
| ADS Voting Instructions Deadline: |
On or before 10:00 A.M. (New York City time) on April 22, 2024. | |
| Deposited Securities: |
Ordinary shares of the Company. | |
| ADS Ratio: |
One (1) Share to one (1) ADS. | |
| Depositary: |
Citibank, N.A. | |
| Custodian of Deposited Securities: |
Citibank Europe plc. | |
| Deposit Agreement: |
Amended and Restated Deposit Agreement, dated as of May 4, 2015, by and among the Company, the Depositary and all Holders and Beneficial Owners of ADSs issued thereunder. | |
To be counted, your Voting Instructions for ADSs need to be received by the Depositary prior
to
10:00 A.M. (New York City time) on
April 22, 2024 and your Voting Instructions for ordinary shares need to be received by the
Depositary prior to Midnight Central European Time on April 24, 2024.
Only those holders of record of the ADS on March 25, 2024 and for ordinary shares on
April 16, 2024 are entitled to vote in the Shareholders’ Meetings.
The Company has announced that the Shareholders’ Meetings will be held at the date, time and location identified above. Documents related to the Shareholders’ Meetings Agenda items will be available on the Company’s website at http://www.glpg.com/shareholders-meetings. The information with respect to the Shareholders’ Meetings and the Voting Instructions contained herein and in any related materials may change after the date hereof as a result of a change in circumstances (e.g. an adjournment or cancellation of the Shareholders’ Meetings, and change in manner of holding the Shareholders’ Meetings). The Company intends to announce any changes and updates on its website at http://www.glpg.com/shareholders-meetings. We encourage you to check the referenced Company website for any updates to the information with respect to the Shareholders’ Meetings and the Voting Instructions as it is not expected that any additional information will be distributed to you via mail or email.
Holders of ADSs wishing to give voting instructions to the Depositary must sign, complete and return the enclosed Voting Instructions prior to the ADS Voting Instructions Deadline in the enclosed pre-addressed envelope.
Subject to Belgian law, the Articles of Association of the Company, the provisions of or governing the Deposited Securities, the terms of the Deposit Agreement, Registered Holders (as defined below) and DTC Holders (as defined below), in each case as of the close of business on the ADS Record Date, will be entitled to instruct the Depositary as to the exercise of voting rights pertaining to the Deposited Securities represented by their ADSs. However, as mentioned above, the voting instructions of any such holder will be disregarded if the Depositary is unable to confirm such holder’s continued ownership of the ADSs as of the Share Record Date.
DTC Holders
In order to vote their ADSs, owners of ADSs (“DTC Holders”) holding their ADSs in a brokerage or custodian account through The Depository Trust Company (“DTC”) as of the ADS Record Date must continue to own their ADSs as of the Share Record Date and must instruct their broker or custodian to give voting instructions to the Depositary and to confirm ownership of the ADSs to the Depositary. On the Share Record Date, the Depositary will verify the continued ownership of the ADSs by the instructing DTC Holders with the applicable brokers or custodians (through which the instructing DTC Holders provided voting instructions to the Depositary). Failure to confirm continued ownership of ADSs as of the Share Record Date will invalidate the voting instructions previously delivered.
Registered Holders
In order to vote their ADSs, Holders of ADSs registered in their name on the books of the Depositary (“Registered Holders”) must timely deliver a Voting Instruction Form to the Depositary and continue to be the Registered Holders of their ADSs as of the Share Record Date. If a Registered Holder transfers or cancels ADSs at any time before the Share Record Date, any voting instructions delivered to the Depositary will be invalidated. On the Share Record Date, the Depositary will verify the continued registration on its books of the ADSs in the name of the instructing Registered Holders (who also held the ADSs as of the ADS Record Date) and will recognize as valid only the voting instructions that were timely received from Registered Holders as of the ADS Record Date who continue to be the Registered Holders as of the Share Record Date.
Voting instructions may be given only in respect of a number of ADSs representing an integral number of Deposited Securities. Upon the timely receipt from a Holder of ADSs, as of the ADS Record Date and as of the Share Record Date, of voting instructions in the manner specified by the Depositary, the Depositary shall endeavor, insofar as practicable and permitted under applicable law, the provisions of the Deposit Agreement, Articles of Association of the Company and the provisions of the Deposited Securities, to vote, or cause the Custodian to vote, the Deposited Securities (in person or by proxy) represented by such Holder’s ADSs in accordance with such voting instructions. The Depositary agrees not to vote, cause to be voted or attempt to exercise the right to vote that attaches to any Deposited Securities, other than in accordance with valid voting instructions given or deemed given in compliance with the Deposit Agreement.
Deposited Securities represented by ADSs for which no timely voting instructions are received by the Depositary from the Holder shall not be voted (except as otherwise described herein). Neither the Depositary nor the Custodian shall under any circumstances exercise any discretion as to voting and neither the Depositary nor the Custodian shall vote, attempt to exercise the right to vote, or in any way make use of, for purposes of establishing a quorum or otherwise, the Deposited Securities represented by ADSs, except pursuant to and in accordance with the voting instructions timely received from Holders or as otherwise contemplated herein. If the Depositary timely receives voting instructions from a Holder which fail to specify the manner in which the Depositary is to vote the Deposited Securities represented by such Holder’s ADSs, the Depositary will deem such Holder to have instructed the Depositary to vote in favor of the items set forth in such voting instructions.
The right of any Holder of ADSs to give instructions to the Depositary as to the exercise of voting rights or the right of any Holder of ADSs to vote withdrawn ordinary shares in person or by proxy may be limited if such ADS holder fails to (i) comply with the information requests, (ii) comply with ownership restrictions, (iii) meet reporting obligations, (iv) obtain regulatory approvals (if any), or (v) disclose their interest held in the Company, in each case as described in the Deposit Agreement.
Holders of ADSs who have delivered voting instructions agree that such voting instructions may, at the request of the Company, be disclosed by the Company, for purposes of compliance with Belgian law, in connection with the Shareholders’ Meetings, whether prior, during or after such Shareholders’ Meetings.
The information contained herein with respect to the Shareholders’ Meetings has been provided by the Company. Citibank, N.A. is forwarding this information to you solely as Depositary and in accordance with the terms of the Deposit Agreement and disclaims any responsibility with respect to the accuracy of such information. Citibank, N.A. does not, and should not be deemed to, express any opinion with respect to the proposals to be considered at the Shareholders’ Meetings. The rights and obligations of Holders and Beneficial Owners of ADSs, the Company and the Depositary are set forth in their entirety in the Deposit Agreement and summarized in the ADRs. If you wish to receive a copy of the Deposit Agreement, please contact the Depositary at the number set forth below.
If you have any questions about the way in which Voting Instructions may be delivered to the Depositary, please contact Citibank, N.A. - ADR Shareholder Services at 1-877-CITI-ADR (1-877-248-4237).
Citibank, N.A., as Depositary
Appendix A
Annual Shareholders’ Meeting Agenda and Proposed Resolutions
| 1. | Acknowledgement and discussion of (a) the annual report of the Board of Directors in relation to the non-consolidated and consolidated annual accounts of the Company for the financial year ended on 31 December 2023, and (b) the report of the statutory auditor in relation to the non-consolidated annual accounts of the Company for the financial year ended on 31 December 2023. (*) |
| 2. | Acknowledgement and approval of the non-consolidated annual accounts of the Company for the financial year ended on 31 December 2023, and approval of the allocation of the annual result as proposed by the Board of Directors. |
| 3. | Acknowledgement and discussion of the report of the statutory auditor relating to the consolidated annual accounts of the Company for the financial year ended on 31 December 2023. (*) |
| 4. | Acknowledgement and discussion of the report of the statutory auditor relating to the consolidated annual accounts of the Company for the financial year ended on 31 December 2023. (*) |
| 5. | Acknowledgement and approval of the remuneration report. |
| 6. | Acknowledgement and approval of the amended remuneration policy. |
| 7. | Release from liability to be granted to the (current and former) members of the Board of Directors, and the (current and former) statutory auditor for the performance of their respective mandates during the financial year ended on 31 December 2023. |
| 8. | Remuneration of the directors. |
| 9. | Re-appointment of Dr. Elisabeth Svanberg as independent non-executive director. |
| 10. | Appointment of Dr. Susanne Schaffert as independent non-executive director. |
| 11. | Appointment of Mr. Simon Sturge as independent non-executive director. |
| 12. | Appointment of Mr. Andrew Dickinson as non-executive director. |
| 13. | Charging of the statutory auditor with respect to the “assurance” of the CSRD sustainability reporting. |
Extraordinary Shareholders’ Meeting Agenda and Proposed Resolutions
| 1. | Consideration and discussion of the report of the Board of Directors of the Company prepared in accordance with articles 7:180, 7:191 and 7:193 of the Belgian Companies and Associations Code in connection with the proposed issuance of one subscription right (in the form of a warrant) for the benefit of Gilead Therapeutics A1 Unlimited Company (“Gilead Therapeutics”), called the ‘Subsequent Gilead Warrant B’, and the proposal to cancel, in the interest of the Company, the statutory preferential subscription right of the Company’s existing shareholders for the benefit of Gilead Therapeutics. (*) |
| 2. | Consideration and discussion of the report of the statutory auditor of the Company prepared in accordance with articles 7:180, 7:191 and 7:193 of the Belgian Companies and Associations Code in connection with the proposed issuance of one subscription right (in the form of a warrant) for the benefit of Gilead Therapeutics, called the ‘Subsequent Gilead Warrant B’, and the proposal to cancel, in the interest of the Company, the statutory preferential subscription right of the Company’s existing shareholders for the benefit of Gilead Therapeutics. (*) |
| 3. | Approval of the issuance of one (1) new subscription right (in the form of a warrant) for the benefit of Gilead Therapeutics and related cancellation of the statutory preferential subscription right. |
| 4. | Consideration and discussion of the report of the Board of Directors in accordance with article 7:199 of the Belgian Companies and Associations Code relating to the renewal of its authorization with respect to, and the increase of, the authorized capital, and the specific circumstances and purposes for the use of the renewed authorized capital. (*) |
| 5. | Renewal of the authorization to the Board of Directors to increase the share capital within the framework of the authorized capital by up to 20% of the share capital. |
| 6. | Proxy for coordination. |
| 7. | Authorization to the Board of Directors. |
| 8. | Proxy for the Crossroad Bank for Enterprises, counters for enterprises, registers of the enterprise court, administrative agencies and fiscal administrations. |
(*) These items will not be voted upon.
Please refer to the enclosed Convening Notice to read the full resolutions.
Exhibit 99.11
Annual and Extraordinary Shareholders’ Meetings
The Voting Instructions must be signed, completed and received at the indicated address prior to
10:00 A.M. (New York City time) on April 22, 2024 for action to be taken.
| 2024 VOTING INSTRUCTIONS | AMERICAN DEPOSITARY SHARES |
Galapagos NV (the “Company”)
| ADS CUSIP No.: | 36315X101. | |
| ADS Record Date: | March 25, 2024 (close of business in New York). | |
| Share Record Date: | April 16, 2024 (Midnight CEST). | |
| Meeting Specifics: | Annual Shareholders’ Meeting and Extraordinary Shareholders’ Meeting to be held sequentially at 2:00 P.M. | |
| (CEST) on Tuesday, April 30, 2024, at the registered office of the Company located at Generaal De Wittelaan L11 | ||
| A3, 2800 Mechelen, Belgium (together, the “Shareholders’ Meetings”). | ||
| Meeting Agenda: | Documents related to the Shareholders’ Meetings Agenda items will be available on the Company’s website at http://www.glpg.com/shareholders-meetings. | |
| Depositary: | Citibank, N.A. | |
| Deposit Agreement: | Amended and Restated Deposit Agreement, dated as of May 4, 2015, by and among the Company, the Depositary | |
| and all Holders and Beneficial Owners of American Depositary Shares (“ADSs”) issued thereunder. | ||
| Deposited Securities: | Ordinary shares of the Company. | |
| Custodian: | Citibank Europe plc. | |
The undersigned Holder of the ADSs identified above, as of the ADS Record Date, hereby authorizes and directs the Depositary to cause to be voted at the Shareholders’ Meetings (and any adjournment or postponement thereof) the Deposited Securities represented by the ADSs in the manner indicated on the reverse side hereof. The undersigned recognizes that any sale, transfer or cancellation of ADSs before the Share Record Date will invalidate these voting instructions if the Depositary is unable to verify the continued ownership of ADSs as of the Share Record Date.
Voting instructions may be given only in respect of a number of ADSs representing an integral number of Deposited Securities. Upon the timely receipt from a Holder of ADSs, as of the ADS Record Date and as of the Share Record Date, of voting instructions in the manner specified by the Depositary, the Depositary shall endeavor, insofar as practicable and permitted under applicable law, the provisions of the Deposit Agreement, Articles of Association of the Company and the provisions of the Deposited Securities, to vote, or cause the Custodian to vote, the Deposited Securities (in person or by proxy) represented by such Holder’s ADSs in accordance with such voting instructions. The Depositary agrees not to vote, cause to be voted or attempt to exercise the right to vote that attaches to any Deposited Securities, other than in accordance with valid voting instructions given or deemed given in compliance with the Deposit Agreement.
Deposited Securities represented by ADSs for which no timely voting instructions are received by the Depositary from the Holder shall not be voted (except as otherwise described herein). Neither the Depositary nor the Custodian shall under any circumstances exercise any discretion as to voting and neither the Depositary nor the Custodian shall vote, attempt to exercise the right to vote, or in any way make use of, for purposes of establishing a quorum or otherwise, the Deposited Securities represented by ADSs, except pursuant to and in accordance with the voting instructions timely received from Holders or as otherwise contemplated herein. If the Depositary timely receives voting instructions from a Holder which fail to specify the manner in which the Depositary is to vote the Deposited Securities represented by such Holder’s ADSs, the Depositary will deem such Holder to have instructed the Depositary to vote in favor of the items set forth in such voting instructions.
The right of any holders of ADSs to give instructions to the Depositary as to the exercise of voting rights may be limited if such holder fails to comply with the requirements under Belgian law (which are summarized in Sections 3.4, 3.5, 3.6 and 3.7 of the Deposit Agreement).
In order to exercise voting rights, an owner who is not the registered holder of ADSs on the books of the Depositary will be required, subject to applicable provisions of the laws of Belgium, the Articles of Association of the Company and the Deposit Agreement, to have such ownership of ADSs, verified by the Depositary as of the Share Record Date.
Please indicate on the reverse side hereof how the Deposited Securities are to be voted.
The Voting Instructions must be marked, signed and returned on time in order to be counted.
By signing on the reverse side hereof, the undersigned represents to the Depositary and the Company that the undersigned is duly authorized to give the Voting Instructions contained therein.
Please refer to Appendix A of the Depositary Notice for the Shareholders’ Meetings Agenda and Proposed Resolutions.
| A |
Resolutions |
|||||||||||||
| Annual Shareholders’ Meeting |
Extraordinary Shareholders’ Meeting | |||||||||||||||||
| For | Against | Abstain | For | Against | Abstain | |||||||||||||
| Resolution 2 |
☐ | ☐ | ☐ | Resolution 3 | ☐ | ☐ | ☐ | |||||||||||
| Resolution 5 |
☐ | ☐ | ☐ | Resolution 5 | ☐ | ☐ | ☐ | |||||||||||
| Resolution 6 |
☐ | ☐ | ☐ | Resolution 6 | ☐ | ☐ | ☐ | |||||||||||
| Resolution 7 |
☐ | ☐ | ☐ | Resolution 7 | ☐ | ☐ | ☐ | |||||||||||
| Resolution 8 |
☐ | ☐ | ☐ | Resolution 8 | ☐ | ☐ | ☐ | |||||||||||
| Resolution 9 |
☐ | ☐ | ☐ | |||||||||||||||
| Resolution 10 |
☐ | ☐ | ☐ | |||||||||||||||
| Resolution 11 |
☐ | ☐ | ☐ | |||||||||||||||
| Resolution 12 |
☐ | ☐ | ☐ | |||||||||||||||
| Resolution 13 |
☐ | ☐ | ☐ | |||||||||||||||
| B |
Authorized Signatures - Sign Here - This section must be completed for your instructions to be executed. |
|||||||||||||
If these Voting Instructions are signed and timely returned to the Depositary but no specific direction as to voting is marked above as to an issue, the undersigned shall be deemed to have directed the Depositary to vote in favor of such issue.
If these Voting Instructions are signed and timely returned to the Depositary but multiple specific directions as to voting are marked above as to an issue, the undersigned shall be deemed to have directed the Depositary to give an “ABSTAIN” Voting Instruction for such issue.
Please be sure to sign and date this Voting Instructions Card.
Please sign your name to the Voting Instructions exactly as printed. When signing in a fiduciary or representative capacity, give full title as such. Where more than one owner, each MUST sign. Voting Instructions executed by a corporation should be in full name by a duly authorized officer with full title as such.
| Signature 1 - Please keep signature within the line |
Signature 2 - Please keep signature within the line | Date (mm/dd/yyyy) | ||
|
|
|
/ / |
Exhibit 99.12
| Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 |

|
| Kennisgeving van deelname (te gebruiken door houders van aandelen of inschrijvingsrechten) |
Notification of participation (to be used by holders of shares or subscription rights) |
|||||
| De ondergetekende: | The undersigned: | |||||
| (Naam en adres / Name and address) | ||||||
| heeft kennisgenomen van de agenda van de Gewone en Buitengewone Algemene Vergaderingen van Galapagos NV (een naamloze vennootschap naar Belgisch recht, met zetel te Generaal De Wittelaan L11 A3, 2800 Mechelen, België en ingeschreven in het Rechtspersonenregister (Antwerpen, afdeling Mechelen) onder nummer 0466.460.429) (de “Vennootschap”), | has taken note of the agenda of the Annual and Extraordinary Shareholders’ Meetings of Galapagos NV (a limited liability company organized under the laws of Belgium, with registered office at Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium and registered with the Register of Legal Entities (Antwerp, division Mechelen) under number 0466.460.429) (the “Company”), | |||||
| die zullen worden gehouden op dinsdag 30 april 2024, om 14.00 uur (Belgische tijd) en op de zetel van de Vennootschap, | to be held on Tuesday 30 April 2024, at 2.00 p.m. (Belgian time) and at the registered office of the Company, | |||||
| en brengt de Vennootschap hierbij op de hoogte van zijn / haar voornemen om deel te nemen aan de bovenvermelde Gewone en Buitengewone Algemene Vergaderingen, | and hereby notifies the Company of his / her intention to attend the aforementioned Annual and Extraordinary Shareholders’ Meetings, | |||||
| met de volgende effecten: | with the following securities: | |||||
|
|
aandelen en/of shares and/or | |||||
|
|
inschrijvingsrechten subscription rights | |||||
| (Aantal) | (Number) | |||||
| (Datum / Date)
|
||||||
| (Naam / Name)
|
||||||
| (Handtekening / Signature) | ||||||
| De ondertekende kennisgeving dient uiterlijk op 24 april 2024 bij Galapagos NV toe te komen. Deze moet worden bezorgd per e-mail (shareholders@glpg.com) of met de post (Galapagos NV, t.a.v. mw. Annelies Denecker, Generaal De Wittelaan L11 A3, 2800 Mechelen, België).
|
The signed notification must be received by Galapagos NV on 24 April 2024 at the latest. It should be submitted by e-mail (shareholders@glpg.com) or by mail (Galapagos NV, attn. Ms. Annelies Denecker, Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium).
|
|||||
| Voor de houders van gedematerialiseerde aandelen dient de kennisgeving een attest te bevatten dat het aantal aandelen bevestigt dat op hun naam geregistreerd is op 16 april 2024 om middernacht (24:00 uur) (Belgische tijd). Het attest kan door de houders van de gedematerialiseerde aandelen worden verkregen bij de erkende rekeninghouder, de relevante centrale effectenbewaarinstelling of de relevante financiële tussenpersoon voor de betreffende aandelen. | For the holders of dematerialised shares, the notification should include a certificate confirming the number of shares that have been registered in their name on 16 April 2024 at midnight (24:00) (Belgian time). The certificate can be obtained by the holders of the dematerialised shares with the certified account holder, the relevant central securities depository, or the relevant financial intermediary for the shares concerned. | |||||
| De ondergetekende gaat ermee akkoord dat de Engelse versie van deze kennisgeving slechts een vrije vertaling voor informatiedoeleinden betreft, en dat de Nederlandstalige versie altijd voorrang heeft op de Engelse versie. | The undersigned agrees that the English version of this notification is a free translation for information purposes only, and that the Dutch version shall at all times prevail over the English version. | |||||
Galapagos NV | Kennisgeving van deelname JAV/BAV 30 april 2024 | Attendance notification AGM/EGM 30 April 2024
P. 1 | 2
| Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 |

|
| Aandeelhouders die willen stemmen per brief, of zich willen laten vertegenwoordigen, moeten eveneens voldoen aan de relevante voorwaarden zoals beschreven in de oproeping tot de Gewone en Buitengewone Algemene Vergaderingen. | Shareholders who wish to vote by letter, or who wish to be represented by proxy, need also to comply with the relevant conditions as described in the convening notice to the Annual and Extraordinary Shareholders’ Meetings. |
Galapagos NV | Kennisgeving van deelname JAV/BAV 30 april 2024 | Attendance notification AGM/EGM 30 April 2024
P. 2 | 2
Exhibit 99.13

GALAPAGOS
Naamloze Vennootschap / Limited Liability Company
Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium
RPR / RLE Antwerp, division Mechelen 0466.460.429
Op 28 maart 2024 bedraagt het totaal aantal aandelen en stemrechten van Galapagos NV
65.897.071
On 28 March 2024, the total number of shares and voting rights of Galapagos NV amounts to
65,897,071
Exhibit 99.14

Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 Galapagos NV | Formulier stemmen per brief JAV/BAV 30 april 2024 | Voting by letter form AGM/EGM 30 April 2024 P. 1 | 13 Stemmen per brief (enkel te gebruiken door aandeelhouders) Voting by letter (to be used by shareholders only) De ondergetekende: The undersigned: (Naam en adres / Name and address) eigenaar van het volgende aantal aandelen in Galapagos NV (naamloze vennootschap naar Belgisch recht, met zetel te Generaal De Wittelaan L11 A3, 2800 Mechelen, België en ingeschreven in het Rechtspersonenregister (RPR Antwerpen, afdeling Mechelen) onder het nummer 0466.460.429) (de “Vennootschap”): owner of the following number of shares in Galapagos NV (public limited liability company organized under the laws of Belgium, with registered office at Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium and registered with the Register of Legal Entities (RLE Antwerp, division Mechelen) under the number 0466.460.429) (the “Company”): (Aantal aandelen op naam / Number of registered shares) (Aantal gedematerialiseerde aandelen / Number of dematerialized shares) heeft kennisgenomen van de Gewone en Buitengewone Algemene Vergaderingen van de Vennootschap, die zullen worden gehouden op dinsdag 30 april 2024, om 14.00 uur (Belgische tijd) en op de zetel van de Vennootschap, has taken note of the Annual and Extraordinary Shareholders’ Meetings of the Company, to be held on Tuesday 30 April 2024 at 2.00 p.m. (Belgian time) and at the registered office of the Company, en brengt de Vennootschap hierbij op de hoogte van zijn / haar wens om aan deze Gewone en Buitengewone Algemene Vergaderingen, alsmede aan elke andere Algemene Vergadering met dezelfde agenda die daarna zou worden bijeengeroepen als gevolg van uitstel of verdaging, deel te nemen door gebruik te maken van zijn / haar mogelijkheid om te stemmen per brief conform artikel 7:146 van het Wetboek van Vennootschappen en Verenigingen en artikel 24 van de Statuten van de Vennootschap, and hereby notifies the Company of his / her intention to attend this Annual and Extraordinary Shareholders’ Meetings, as well as at any other Shareholders’ Meeting with the same agenda that may be convened subsequently as a result of delay or adjournment, by use of his / her / its possibility to vote by letter in accordance with article 7:146 of the Code of Companies and Associations and article 24 of the Articles of Association of the Company, waarbij hij / zij verklaart als volgt op onherroepelijke wijze te stemmen over de voorstellen tot besluit geplaatst op de agenda van de bovenvermelde Gewone en Buitengewone Algemene Vergaderingen: by which he / she / it declares that he / she / it irrevocably votes as follows on the proposed resolutions placed on the agenda of the aforementioned Annual and Extraordinary Shareholders’ Meetings: Indien, overeenkomstig artikel 7:130 van het Wetboek van Vennootschappen en Verenigingen nieuw te behandelen onderwerpen op de agenda zijn opgenomen nadat het onderhavig stemformulier ter kennis van de Vennootschap is gebracht, zal de Vennootschap gewijzigde formulieren voor stemming per brief ter beschikking stellen. De stemmen per brief die de Vennootschap bereiken vooraf aan de publicatie van een gewijzigde agenda of voorstellen van besluit, blijven geldig voor de agendapunten waarop de stemmen per brief betrekking hebben, doch evenwel onder voorbehoud van de toepasselijke wetgeving. If, in accordance with Article 7:130 of the Companies and Associations Code, new subjects to be dealt with are included in the agenda after this voting form has been notified to the Company, the Company shall provide revised forms for voting by letter. The votes by letter that reach the Company prior to the publication of the amended agenda or proposed resolutions, remain valid for the agenda items to which the votes by letter apply, subject, however, to applicable law. Bij gebreke aan een specifieke stemwijze voor een bepaald agendapunt, of indien, om welke reden ook, er onduidelijkheid zou bestaan over de meegedeelde stemwijze, zal de ondergetekende geacht worden geselecteerd te hebben “Voor”. If no specific manner of voting is given for a specific item on the agenda, or if, for whatever reason, there is a lack of clarity with regard to the indicated manner of voting, the undersigned shall be deemed to have selected “In favor”. Dit formulier dient handgeschreven of elektronisch te worden ondertekend. Indien gebruik wordt gemaakt van de mogelijkheid om dit formulier elektronisch te tekenen, dient het te gaan om een elektronische handtekening in de zin van artikel 3.10 van Verordening (EU) Nr. 910/2014 van het Europees Parlement en de Raad van 23 juli 2014 betreffende elektronische identificatie en This form should be signed either in handwriting or electronically. If the opportunity to sign this proxy form electronically is made use of, it must be an electronic signature within the meaning of article 3.10 of Regulation (EU) No 910/2014 of the European Parliament and of the Council of 23 July 2014 on electronic identification and trust services for electronic transactions in the internal market and

Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 Galapagos NV | Formulier stemmen per brief JAV/BAV 30 april 2024 | Voting by letter form AGM/EGM 30 April 2024 P. 2 | 13 vertrouwensdiensten voor elektronische transacties in de interne markt en tot intrekking van Richtlijn 1999/93/EG, zoals gewijzigd, hetzij een gekwalificeerde elektronische handtekening in de zin van artikel 3.12 van dezelfde Verordening. repealing Directive 1999/93/EC, as amended, or a qualified electronic signature within the meaning of article 3.12 of the same Regulation. Agenda Gewone Algemene Vergadering Annual Shareholders’ Meeting 1. Kennisname en bespreking van (a) het jaarverslag van de Raad van Bestuur betreffende de statutaire en geconsolideerde jaarrekening van de Vennootschap over het boekjaar afgesloten op 31 december 2023, en van (b) het verslag van de commissaris over de statutaire jaarrekening van de Vennootschap over het boekjaar afgesloten op 31 december 2023. 1. Acknowledgement and discussion of (a) the annual report of the Board of Directors in relation to the non-consolidated and consolidated annual accounts of the Company for the financial year ended on 31 December 2023, and (b) the report of the statutory auditor in relation to the non-consolidated annual accounts of the Company for the financial year ended on 31 December 2023. 2. Kennisname en goedkeuring van de statutaire jaarrekening van de Vennootschap over het boekjaar afgesloten op 31 december 2023 en goedkeuring van de door de Raad van Bestuur voorgestelde bestemming van het jaarresultaat. 2. Acknowledgement and approval of the non-consolidated annual accounts of the Company for the financial year ended on 31 December 2023, and approval of the allocation of the annual result as proposed by the Board of Directors. Voorstel van besluit: De algemene vergadering besluit de statutaire jaarrekening van de Vennootschap over het boekjaar afgesloten op 31 december 2023, goed te keuren, alsmede de door de Raad van Bestuur voorgestelde bestemming van het jaarresultaat goed te keuren. Proposed resolution: The shareholders’ meeting resolves to approve the non-consolidated annual accounts of the Company for the financial year ended on 31 December 2023, and the allocation of the annual result as proposed by the Board of Directors. Agendapunt 2—Steminstructie: Agenda item 2—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention 3. Kennisname en bespreking van het verslag van de commissaris betreffende de geconsolideerde jaarrekening van de Vennootschap over het boekjaar afgesloten op 31 december 2023. 3. Acknowledgement and discussion of the report of the statutory auditor relating to the consolidated annual accounts of the Company for the financial year ended on 31 December 2023. 4. Kennisname en bespreking van de geconsolideerde jaarrekening van de Vennootschap over het boekjaar afgesloten op 31 december 2023. 4. Acknowledgement and discussion of the consolidated annual accounts of the Company for the financial year ended on 31 December 2023. 5. Kennisname en goedkeuring van het remuneratieverslag. 5. Acknowledgement and approval of the remuneration report. Voorstel van besluit: De algemene vergadering besluit om het remuneratieverslag, opgenomen in het jaarverslag van de Raad van Bestuur over het boekjaar afgesloten op 31 december 2023, goed te keuren. Proposed resolution: The shareholders’ meeting resolves to approve the remuneration report included in the annual report of the Board of Directors for the financial year ended on 31 December 2023. Agendapunt 5—Steminstructie: Agenda item 5—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention 6. Kennisname en goedkeuring van het gewijzigde remuneratiebeleid. 6. Acknowledgement and approval of the amended remuneration policy. Voorstel van besluit: De algemene vergadering besluit om het gewijzigde remuneratiebeleid goed te keuren. Proposed resolution: The shareholders’ meeting resolves to approve the amended remuneration policy. Agendapunt 6—Steminstructie: Agenda item 6—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention

Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 Galapagos NV | Formulier stemmen per brief JAV/BAV 30 april 2024 | Voting by letter form AGM/EGM 30 April 2024 P. 3 | 13 7. Kwijting aan de (huidige en voormalige) leden van de Raad van Bestuur en de (huidige en voormalige) commissaris voor de uitoefening van hun respectievelijk mandaat uitgeoefend tijdens het boekjaar afgesloten op 31 december 2023. 7. Release from liability to be granted to the (current and former) members of the Board of Directors, and the (current and former) statutory auditor for the performance of their respective mandates during the financial year ended on 31 December 2023. Voorstel van besluit: De algemene vergadering besluit, bij afzonderlijke stemming, om kwijting te geven aan elk (huidig en voormalig) lid van de Raad van Bestuur, en aan de (huidige en voormalige) commissaris voor alle aansprakelijkheid voortvloeiend uit de uitoefening van hun respectieve mandaten gedurende het boekjaar afgesloten op 31 december 2023. Proposed resolution: The shareholders’ meeting resolves, by a separate vote, to release each (current and former) member of the Board of Directors, and the (current and former statutory) auditor from any liability arising from the performance of their respective mandates during the financial year ended on 31 December 2023. Agendapunt 7—Steminstructie: Agenda item 7—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention 8. Bezoldiging van bestuurders. 8. Remuneration of directors. Voorstel van besluit: Op aanbeveling en advies van het Remuneratiecomité van de Vennootschap, besluit de algemene vergadering dat de jaarlijkse bezoldiging (exclusief onkostenvergoeding) van de niet-uitvoerende bestuurders, andere dan de niet-uitvoerende bestuurders die een aandeelhouder vertegenwoordigen, voor de uitoefening van hun mandaat zal bestaan uit een bezoldiging in contanten en een bezoldiging op basis van aandelen als volgt vast te stellen: (a) vergoeding in contanten: (i) Voorzitter van de Raad van Bestuur: EUR 110.000; (ii) Lead Non-Executive Director: EUR 75.000; (iii) andere niet-uitvoerende bestuurders: elkeen EUR 55.000, (iv) jaarlijkse bijkomende bezoldiging voor het voorzitterschap van een comité binnen de Raad van Bestuur: EUR 20.000; en (v) jaarlijkse bijkomende bezoldiging voor lidmaatschap van een comité binnen de Raad van Bestuur: EUR 15.000; (b) vergoeding op basis van aandelen: (i) Voorzitter van de Raad van Bestuur: EUR 110.000; (ii) Lead Non-Executive Director: EUR 75.000; (iii) andere niet-uitvoerende bestuurders: elkeen EUR 55.000; in elk van (i), (ii) en (iii), onder de verplichting om het nettobedrag (na belastingen) te gebruiken voor de verwerving van Galapagos aandelen. Deze laatste betalingen vormen het equivalent van een bestuurdersbezoldiging in aandelen en de resulterende aandelen dienen te worden aangehouden tot ten minste één jaar nadat de niet-uitvoerende bestuurder de Raad van Bestuur heeft verlaten en ten minste drie jaar na het tijdstip van de verwerving. De bovenvermelde bezoldiging voor de Voorzitter van de Raad van Bestuur is enkel betaalbaar indien deze Voorzitter niet tegelijk Chief Executive Officer (CEO) is van de Vennootschap. De bovenvermelde vergoeding voor de Lead Non-Executive Director is enkel betaalbaar voor zover geldig benoemd overeenkomstig Galapagos’ Corporate Governance Charter. De algemene vergadering besluit dat het mandaat van een niet-uitvoerend bestuurder die een aandeelhouder vertegenwoordigt, niet zal worden bezoldigd. De hierboven uiteengezette regels zijn van toepassing vanaf 1 mei 2024. Proposed resolution: Upon recommendation and advice of the Remuneration Committee of the Company, the shareholders’ meeting resolves that the annual compensation (excluding expenses) of the non-executive directors, other than the non-executive directors representing a shareholder, for the exercise of their mandate shall consist of a cash remuneration and an equity-based remuneration as follows: (a) cash remuneration: (i) Chairperson of the Board of Directors: EUR 110,000; (ii) Lead Non-Executive Director: EUR 75,000, (iii) other non-executive directors: EUR 55,000 each; (iv) additional annual compensation for the chairpersonship of a committee within the Board of Directors: EUR 20,000; and (v) additional annual compensation for the membership of a committee within the Board of Directors: EUR 15,000; (b) equity-based remuneration: (i) Chairperson of the Board of Directors: EUR 110,000; (ii) Lead Non-Executive Director: EUR 75,000; (iii) other non-executive directors: EUR 55,000 each; in each case (i), (ii) and (iii) subject to the requirement to use the net amount (after taxes) to acquire Galapagos shares. These latter payments make up the equivalent of an equity component of the directors’ remuneration and the resulting shares are to be held until at least one year after the non-executive director leaves the Board of Directors and at least three years after the time of acquisition. The abovementioned remuneration for the Chairperson of the Board of Directors will only be payable if such Chairperson is not simultaneously Chief Executive Officer (CEO) of the Company. The abovementioned remuneration for the Lead Non-Executive Director will only be payable to the extent appointed in accordance with Galapagos’ Corporate Governance Charter. The shareholders’ meeting resolves that the mandate of a non-executive director representing a shareholder will not be remunerated. The rules set out above shall apply as per 1 May 2024. Agendapunt 8—Steminstructie: Agenda item 8—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention

Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 Galapagos NV | Formulier stemmen per brief JAV/BAV 30 april 2024 | Voting by letter form AGM/EGM 30 April 2024 P. 4 | 13 9. Herbenoeming van Dr. Elisabeth Svanberg als onafhankelijk niet-uitvoerend bestuurder. 9. Re-appointment of Dr. Elisabeth Svanberg as independent non-executive director. Voorstel van besluit: Op voorstel van de Raad van Bestuur, en in overeenstemming met de aanbeveling en het advies van het Benoemingscomité, besluit de algemene vergadering (a) om Dr. Elisabeth Svanberg te herbenoemen als onafhankelijk niet-uitvoerend lid van de Raad van Bestuur van de Vennootschap, voor een termijn van vier jaar vanaf heden, die een einde zal nemen onmiddellijk na de gewone algemene vergadering te houden in 2028, en (b) haar mandaat te bevestigen in diens hoedanigheid als onafhankelijk lid van de Raad van Bestuur doordat (i) Dr. Elisabeth Svanberg beantwoordt aan de onafhankelijkheidscriteria van het artikel 7:87 van het Wetboek van Vennootschappen en Verenigingen en bepaling 3.5 van de Belgische Corporate Governance Code 2020, (ii) Dr. Elisabeth Svanberg uitdrukkelijk heeft verklaard geen (en de Raad van Bestuur evenmin op de hoogte is van) banden met de Vennootschap of een belangrijke aandeelhouder te hebben, die diens onafhankelijkheid in het gedrang zou brengen, en (iii) de Raad van Bestuur geen indicatie heeft van enig element dat de onafhankelijkheid van Dr. Elisabeth Svanberg in twijfel zou kunnen trekken. De algemene vergadering besluit dat het mandaat van Dr. Elisabeth Svanberg wordt vergoed zoals voorzien voor niet-uitvoerende leden van de Raad van Bestuur in het remuneratiebeleid van de Vennootschap en zoals goedgekeurd door de algemene vergadering van tijd tot tijd. Proposed resolution: Upon proposal of the Board of Directors, and in accordance with the recommendation and advice of the Nomination Committee, the shareholders’ meeting resolves (a) to re-appoint Dr. Elisabeth Svanberg as an independent non-executive member of the Board of Directors of the Company, for a period of four years, effective as of today, ending immediately after the annual shareholders’ meeting to be held in 2028, and (b) to confirm her mandate as an independent member of the Board of Directors since (i) Dr. Elisabeth Svanberg meets the independence criteria set forth in article 7:87 of the Belgian Companies and Associations Code, and article 3.5 of the Belgian Corporate Governance Code 2020, (ii) Dr. Elisabeth Svanberg has explicitly declared not to have (and the Board of Directors is not aware of) any connections with the Company or a major shareholder, which would interfere with her independence, and (iii) the Board of Directors has no indication of any element that might call Dr. Elisabeth Svanberg’s independence into question. The shareholders’ meeting also resolves that the mandate of Dr. Elisabeth Svanberg is remunerated as provided for non-executive members of the Board of Directors in the Company’s remuneration policy and as approved by the shareholders’ meeting from time to time. Agendapunt 9—Steminstructie: Agenda item 9—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention 10. Benoeming van Dr. Susanne Schaffert als onafhankelijk niet-uitvoerend bestuurder. 10. Appointment of Dr. Susanne Schaffert as independent non-executive director. Rekening houdend met de aanbeveling en het advies van het Benoemingscomité, beveelt de Raad van Bestuur aan om de benoeming van Dr. Susanne Schaffert als onafhankelijk niet-uitvoerende bestuurder van de Vennootschap te bevestigen en voort te zetten voor een bijkomende termijn van vier jaar. Voor meer informatie over Dr. Susanne Schaffert wordt verwezen naar de verklaring inzake corporate governance opgenomen in het jaarverslag van de Raad van Bestuur over de (niet-geconsolideerde) statutaire jaarrekening van de Vennootschap voor het boekjaar afgesloten op 31 december 2023. In het bijzonder, heeft de Raad van Bestuur op 12 juni 2023 Dr. Susanne Schaffert benoemd tot onafhankelijk niet-uitvoerend bestuurder van de Vennootschap door coöptatie na het aftreden op 10 juni 2023 van Dr. Rajesh Parekh als niet-uitvoerend bestuurder, die werd benoemd voor een termijn tot en met de sluiting van de jaarlijkse aandeelhoudersvergadering te houden in 2025 die zal hebben besloten over de jaarrekening voor het boekjaar eindigend op 31 december 2024. Taking into account the recommendation and advice of the Nomination Committee, the Board of Directors recommends that the appointment of Dr. Susanne Schaffert as independent non-executive director of the Company be confirmed and continued for an additional term of four years. For further information regarding Dr. Susanne Schaffert, reference is made to the corporate governance statement included in the the annual report of the Board of Directors on the (non-consolidated) statutory financial statements of the Company for the financial year ended on 31 December 2023. Notably, on 12 June 2023, the Board of Directors appointed Dr. Susanne Schaffert as independent non-executive director of the Company by co-optation following the resignation on 10 June 2023 of Dr. Rajesh Parekh as non-executive director, who was appointed for a term up to and including the closing of the annual shareholders’ meeting to be held in 2025 which will have decided upon the financial statements for the financial year ended on 31 December 2024. Voorstel van besluit: Op voorstel van de Raad van Bestuur, en in overeenstemming met de aanbeveling en het advies van het Benoemingscomité van de Vennootschap, besluit de algemene vergadering (a) om de benoeming door coöptatie op 12 juni 2023 te bevestigen, volgend op het aftreden van Dr. Rajesh Parekh op 10 juni 2023, en om Dr. Susanne Schaffert te benoemen als onafhankelijk niet-uitvoerend lid van de Raad van Bestuur van de Vennootschap, voor een bijkomende termijn van vier jaar vanaf heden, die een einde zal nemen onmiddellijk na de gewone algemene vergadering te houden in 2028, dewelke zal hebben besloten over de jaarrekening voor het boekjaar eindigend op 31 december 2027, en om (b) haar mandaat in diens hoedanigheid als Proposed resolution: Upon proposal of the Board of Directors, and in accordance with the recommendation and advice of the Nomination Committee, the shareholders’ meeting resolves (a) to confirm the appointment by co-optation on 12 June 2023, following the resignation of Dr. Rajesh Parekh on 10 June 2023, and to appoint Dr. Susanne Schaffert as an independent non-executive member of the Board of Directors of the Company, for an additional period of four years, up to and including the closing of the annual shareholders’ meeting to be held in 2028 which will have decided upon the financial statements for the financial year ended on 31 December 2027, and (b) to confirm her mandate as an independent member of the Board of Directors since (i) Dr. Susanne Schaffert

Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 Galapagos NV | Formulier stemmen per brief JAV/BAV 30 april 2024 | Voting by letter form AGM/EGM 30 April 2024 P. 5 | 13 onafhankelijk lid van de Raad van Bestuur te bevestigen doordat (i) Dr. Susanne Schaffert beantwoordt aan de onafhankelijkheidscriteria van het artikel 7:87 van het Wetboek van Vennootschappen en Verenigingen en bepaling 3.5 van de Belgische Corporate Governance Code 2020, (ii) Dr. Susanne Schaffert uitdrukkelijk heeft verklaard geen (en de Raad van Bestuur evenmin op de hoogte is van) banden met de Vennootschap of een belangrijke aandeelhouder te hebben, die diens onafhankelijkheid in het gedrang zou brengen, en (iii) de Raad van Bestuur geen indicatie heeft van enig element dat de onafhankelijkheid van Dr. Susanne Schaffert in twijfel zou kunnen trekken. De algemene vergadering beslist tevens dat het mandaat van Dr. Susanne Schaffert wordt vergoed zoals voorzien voor niet-uitvoerende leden van de Raad van Bestuur in het remuneratiebeleid van de Vennootschap en zoals goedgekeurd door de algemene vergadering van tijd tot tijd. meets the independence criteria set forth in article 7:87 of the Belgian Companies and Associations Code, and article 3.5 of the Belgian Corporate Governance Code 2020, (ii) Dr. Susanne Schaffert has explicitly declared not to have (and the Board of Directors is not aware of) any connections with the Company or a major shareholder, which would interfere with her independence, and (iii) the Board of Directors has no indication of any element that might call Dr. Susanne Schaffert’s independence into question. The shareholders’ meeting also resolves that the mandate of Dr. Susanne Schaffert is remunerated as provided for non-executive members of the Board of Directors in the Company’s remuneration policy and as approved by the shareholders’ meeting from time to time. Agendapunt 10—Steminstructie: Agenda item 10—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention 11. Benoeming van dhr. Simon Sturge als onafhankelijk niet-uitvoerend bestuurder. 11. Appointment of Mr. Simon Sturge as independent non-executive director. Rekening houdend met de aanbeveling en het advies van het Benoemingscomité, beveelt de Raad van Bestuur aan om de benoeming van dhr. Simon Sturge als onafhankelijk niet-uitvoerende bestuurder van de Vennootschap te bevestigen en voort te zetten voor een bijkomende termijn van vier jaar. Voor meer informatie over de heer Simon Sturge wordt verwezen naar de verklaring inzake corporate governance opgenomen in het jaarverslag van de Raad van Bestuur over de (niet-geconsolideerde) statutaire jaarrekening van de Vennootschap voor het boekjaar afgesloten op 31 december 2023. In het bijzonder, heeft de Raad van Bestuur op 19 september 2023 de heer Simon Sturge benoemd tot onafhankelijk niet-uitvoerend bestuurder van de Vennootschap door coöptatie na het aftreden op 18 september 2023 van Dr. Mary Kerr als niet-uitvoerend bestuurder, die werd benoemd voor een termijn tot en met de sluiting van deze jaarlijkse aandeelhoudersvergadering. Taking into account the recommendation and advice of the Nomination Committee, the Board of Directors recommends that the appointment of Mr. Simon Sturge as independent non-executive director of the Company be confirmed and continued for an additional term of four years. For further information regarding Mr. Simon Sturge, reference is made to the corporate governance statement included in the the annual report of the Board of Directors on the (non-consolidated) statutory financial statements of the Company for the financial year ended on 31 December 2023. Notably, on 19 September 2023, the Board of Directors appointed Mr. Simon Sturge as independent non-executive director of the Company by co-optation following the resignation on 18 September 2023 of Dr. Mary Kerr as non-executive director, who was appointed for a term up to and including the closing of this annual shareholders’ meeting. Voorstel van besluit: Op voorstel van de Raad van Bestuur, en in overeenstemming met de aanbeveling en het advies van het Benoemingscomité van de Vennootschap, besluit de algemene vergadering (a) om de benoeming door coöptatie op 19 september 2023 te bevestigen, volgend op het aftreden van Dr. Mary Kerr op 18 september 2023, en om dhr. Simon Sturge te benoemen als onafhankelijk niet-uitvoerend lid van de Raad van Bestuur van de Vennootschap, voor een bijkomende termijn van vier jaar vanaf heden, die een einde zal nemen onmiddellijk na de gewone algemene vergadering te houden in 2028, dewelke zal hebben besloten over de jaarrekening voor het boekjaar eindigend op 31 december 2027, en om (b) zijn mandaat in diens hoedanigheid als onafhankelijk lid van de Raad van Bestuur te bevestigen doordat (i) dhr. Simon Sturge beantwoordt aan de onafhankelijkheidscriteria van het artikel 7:87 van het Wetboek van Vennootschappen en Verenigingen en bepaling 3.5 van de Corporate Governance Code 2020, (ii) dhr. Simon Sturge uitdrukkelijk heeft verklaard geen (en de Raad van Bestuur evenmin op de hoogte is van) banden met de Vennootschap of een belangrijke aandeelhouder te hebben, die diens onafhankelijkheid in het gedrang zou brengen, en (iii) de Raad van Bestuur geen indicatie heeft van enig element dat de onafhankelijkheid van dhr. Simon Sturge in twijfel zou kunnen trekken. De algemene vergadering beslist tevens dat het mandaat van dhr. Simon Sturge Proposed resolution: Upon proposal of the Board of Directors, and in accordance with the recommendation and advice of the Nomination Committee, the shareholders’ meeting resolves (a) to confirm the appointment by co-optation on 19 September 2023, following the resignation of Dr. Mary Kerr on 18 September 2023, and to appoint Mr. Simon Sturge as an independent non-executive member of the Board of Directors of the Company, for an additional period of four years, up to and including the closing of the annual shareholders’ meeting to be held in 2028 which will have decided upon the financial statements for the financial year ended on 31 December 2027, and (b) to confirm his mandate as an independent member of the Board of Directors since (i) Mr. Simon Sturge meets the independence criteria set forth in article 7:87 of the Belgian Companies and Associations Code, and article 3.5 of the Belgian Corporate Governance Code 2020, (ii) Mr. Simon Sturge has explicitly declared not to have (and the Board of Directors is not aware of) any connections with the Company or a major shareholder, which would interfere with his independence, and (iii) the Board of Directors has no indication of any element that might call Mr. Simon Sturge’s independence into question. The shareholders’ meeting also resolves that the mandate of Mr. Simon Sturge is remunerated as provided for non-executive members of the Board of Directors in the Company’s remuneration policy and as approved by the shareholders’ meeting from time to time.

Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 Galapagos NV | Formulier stemmen per brief JAV/BAV 30 april 2024 | Voting by letter form AGM/EGM 30 April 2024 P. 6 | 13 wordt vergoed zoals voorzien voor niet-uitvoerende leden van de Raad van Bestuur in het remuneratiebeleid van de Vennootschap en zoals goedgekeurd door de algemene vergadering van tijd tot tijd. Agendapunt 11—Steminstructie: Agenda item 11—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention 12. Benoeming van de heer Andrew Dickinson tot niet-uitvoerend bestuurder. 12. Appointment of Mr. Andrew Dickinson as non-executive director. Rekening houdend met de aanbeveling en het advies van het Benoemingscomité, beveelt de Raad van Bestuur aan de benoeming van de heer Andrew Dickinson als niet-uitvoerend bestuurder van de Vennootschap te bevestigen en voort te zetten voor een bijkomende termijn van vier jaar. In het bijzonder, heeft de Raad van Bestuur, op 26 maart 2024, en met ingang vanaf 27 maart 2024, de heer Andrew Dickinson benoemd tot niet-uitvoerend bestuurder van de Vennootschap door coöptatie naar aanleiding van het aftreden op 26 maart 2024 van de heer Daniel O’Day, als niet-uitvoerend bestuurder, die werd benoemd voor een termijn tot en met de afsluiting van de jaarlijkse aandeelhoudersvergadering die zal worden gehouden in 2027 en die zal hebben beslist over de jaarrekening voor het boekjaar afgesloten op 31 december 2026. Taking into account the recommendation and advice of the Nomination Committee, the Board of Directors recommends that the appointment of Mr. Andrew Dickinson as non-executive director of the Company be confirmed and continued for an additional term of four years. Notably, on 26 March 2024, and with effect as per 27 March 2024, the Board of Directors appointed Mr. Andrew Dickinson as non-executive director of the Company by co-optation following the resignation on 26 March 2024 of Mr. Daniel O’Day, as non-executive director, who was appointed for a term up to and including the closing of the annual shareholders’ meeting to be held in 2027 which will have decided upon the financial statements for the financial year ended on 31 December 2026. Voorstel van besluit: Op voorstel van de Raad van Bestuur, en in overeenstemming met de aanbeveling en het advies van het Benoemingscomité, besluit de algemene vergadering (a) de benoeming door coöptatie op 26 maart 2024, en met ingang vanaf 27 maart 2024, te bevestigen, volgend op het aftreden op 26 maart 2024 van de heer Daniel O’Day, Dhr. Andrew Dickinson te benoemen tot niet-uitvoerend lid van de Raad van Bestuur van de Vennootschap, voor een bijkomende termijn van vier jaar, tot en met de afsluiting van de gewone algemene vergadering van aandeelhouders die zal worden gehouden in 2028, en die zal hebben besloten over de jaarrekening voor het boekjaar eindigend op 31 december 2027. De algemene vergadering besluit tevens dat het mandaat van Andrew Dickinson onbezoldigd zal zijn. Proposed resolution: Upon proposal of the Board of Directors, and in accordance with the recommendation and advice of the Nomination Committee, the shareholders’ meeting resolves (a) to confirm the appointment by co-optation on 26 March 2024, and with effect as per 27 March 2024, following the resignation of Mr. Daniel O’Day on 26 March 2024, and to appoint Mr. Andrew Dickinson as a non-executive member of the Board of Directors of the Company, for an additional period of four years, up to and including the closing of the annual shareholders’ meeting to be held in 2028 which will have decided upon the financial statements for the financial year ended on 31 December 2027. The shareholders’ meeting also resolves that the mandate of Andrew Dickinson will not be remunerated. Agendapunt 12—Steminstructie: Agenda item 12—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention 13. Benoeming van de commissaris met betrekking tot de “waarborging” van de CSRD duurzaamheidsverslaggeving. 13. Charging of the statutory auditor with respect to the “assurance” of the CSRD sustainability reporting. Voorstel van besluit: In overeenstemming met de aanbeveling van het Auditcomité en op voorstel van de Raad van Bestuur, en rekening houdend met de hangende omzetting van de Corporate Sustainability Reporting Directive 2022/2464/EU (“CSRD”) in het Belgisch recht, besluit de algemene vergadering (a) de commissaris van de Vennootschap, zijnde BDO Bedrijfsrevisoren BV, met maatschappelijke zetel te Da Vincilaan 9/E.6. 1930 Zaventem, en ingeschreven in de Kruispuntbank Ondernemingen (RPR Brussel, Nederlandstalige afdeling) onder het nummer 0431.088.289, vast vertegenwoordigd door Ellen Lombaerts, voor een termijn van één jaar, te belasten met de waarborging van de duurzaamheidsrapportering van de Vennootschap, zoals bedoeld in de CSRD, voor het boekjaar dat afgesloten wordt op 31 december 2024 in overeenstemming met de toepasselijke wetgeving, en (b) Proposed resolution: In accordance with the recommendation of the Audit Committee and upon proposal of the Board of Directors, and taking into account the pending transposition of the Corporate Sustainability Reporting Directive 2022/2464/EU (“CSRD”) into Belgian law, the shareholders’ meeting resolves (a) to charge the Company’s statutory auditor, being BDO Bedrijfsrevisoren BV, with its registered office at Da Vincilaan 9/E.6, 1930 Zaventem, and registered with the Crossroads Enterprise Database (RPR Brussels, Dutch-speaking division) under the number 0431.088.289, permanently represented by Ellen Lombaerts, for a period of one year, with the assurance of the sustainability reporting of the Company, as referred to in the CSRD, for the financial year ending on 31 December 2024 in accordance with applicable law, and (b) to determine the remuneration of BDO Bedrijfsrevisoren BV for such

Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 Galapagos NV | Formulier stemmen per brief JAV/BAV 30 april 2024 | Voting by letter form AGM/EGM 30 April 2024 P. 7 | 13 de vergoeding van BDO Bedrijfsrevisoren BV voor deze waarborging vast te stellen op EUR 120.000 (indien enige, exclusief BTW). De aanstelling van de commissaris met betrekking tot de voormelde waarborging zal vervallen onmiddellijk na de gewone algemene vergadering van aandeelhouders die zal worden gehouden in 2025. assurance at EUR 120,000.00 (if any, VAT exclusive). The charging of the statutory auditor with respect to the aforementioned assurance will expire immediately after the annual shareholders’ meeting to be held in 2025. Agendapunt 13—Steminstructie: Agenda item 13—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention Buitengewone Algemene Vergadering Extraordinary Shareholders’ Meeting 1. Kennisname en bespreking van het verslag van de Raad van Bestuur van de Vennootschap opgesteld overeenkomstig de artikelen 7:180, 7:191 en 7:193 van het Wetboek van Vennootschappen en Verenigingen in verband met de voorgestelde uitgifte van één inschrijvingsrecht (in de vorm van een warrant) aan Gilead Therapeutics A1 Unlimited Company (“Gilead Therapeutics”), de “Subsequent Gilead Warrant B” genaamd, en het voorstel om, in het belang van de Vennootschap, het wettelijk voorkeurrecht van de bestaande aandeelhouders van de Vennootschap op te heffen ten gunste van Gilead Therapeutics. 1. Consideration and discussion of the report of the Board of Directors of the Company prepared in accordance with articles 7:180, 7:191 and 7:193 of the Belgian Companies and Associations Code in connection with the proposed issuance of one subscription right (in the form of a warrant) for the benefit of Gilead Therapeutics A1 Unlimited Company (“Gilead Therapeutics”), called the “Subsequent Gilead Warrant B”, and the proposal to cancel, in the interest of the Company, the statutory preferential subscription right of the Company’s existing shareholders for the benefit of Gilead Therapeutics. 2. Kennisname en bespreking van het verslag van de commissaris van de Vennootschap opgesteld overeenkomstig de artikelen 7:180, 7:191 en 7:193 van het Wetboek van Vennootschappen en Verenigingen in verband met de voorgestelde uitgifte van één inschrijvingsrecht (in de vorm van een warrant) aan Gilead Therapeutics, de ‘Subsequent Gilead Warrant B’ genaamd, en het voorstel om, in het belang van de Vennootschap, het wettelijk voorkeurrecht van de bestaande aandeelhouders van de Vennootschap op te heffen ten gunste van Gilead Therapeutics. 2. Consideration and discussion of the report of the statutory auditor of the Company prepared in accordance with articles 7:180, 7:191 and 7:193 of the Belgian Companies and Associations Code in connection with the proposed issuance of one subscription right (in the form of a warrant) for the benefit of Gilead Therapeutics, called the ‘Subsequent Gilead Warrant B’, and the proposal to cancel, in the interest of the Company, the statutory preferential subscription right of the Company’s existing shareholders for the benefit of Gilead Therapeutics. 3. Goedkeuring van de uitgifte van één (1) nieuw inschrijvingsrecht (in de vorm van een warrant) aan Gilead Therapeutics en de daarmee verband houdende opheffing van het wettelijk voorkeurrecht. 3. Approval of the issuance of one (1) new subscription right (in the form of a warrant) for the benefit of Gilead Therapeutics and related cancellation of the statutory preferential subscription right. Dit voorstel wordt gedaan overeenkomstig de voorwaarden van de inschrijvingsovereenkomst gesloten tussen de Vennootschap en Gilead Therapeutics op 14 juli 2019, zoals van tijd tot tijd gewijzigd (de “Inschrijvingsovereenkomst”), die een verbintenis bevatte om een voorstel te doen aan de algemene vergadering om de ‘Subsequent Gilead Warrant B’ uit te geven die Gilead Therapeutics in staat zou stellen om haar deelneming in de Vennootschap verder te vergroten, zoals beschreven in het verslag van de Raad van Bestuur als bedoeld in punt 1 van de agenda. This proposal is made in accordance with the terms of the subscription agreement entered into between the Company and Gilead Therapeutics on 14 July 2019, as amended from time to time (the “Subscription Agreement”), which included a commitment to make a proposal to the general meeting to issue the ‘Subsequent Gilead Warrant B’ that would enable Gilead Therapeutics to further increase its shareholding in the Company, as described in the report of the Board of Directors as referred to in item 1 of the agenda. Voorstel van besluit: De algemene vergadering van de Vennootschap besluit om de uitgifte goed te keuren van één (1) nieuw inschrijvingsrecht (in de vorm van een warrant) aan Gilead Therapeutics, de ‘Subsequent Gilead Warrant B’ genaamd (de “Warrant”), en in het belang van de Vennootschap, het wettelijk voorkeurrecht van de bestaande aandeelhouders van de Vennootschap op te heffen (en, voor zover vereist, van de bestaande houders van inschrijvingsrechten van de Vennootschap) ten gunste van Gilead Therapeutics, overeenkomstig het verslag van de Raad van Bestuur opgesteld overeenkomstig artikelen 7:180, 7:191 en 7:193 van het Wetboek van Vennootschappen en Verenigingen, waarnaar wordt verwezen in punt 1 van de agenda. Met het oog hierop, beslist de algemene vergadering van de Vennootschap om de bepalingen en voorwaarden (de Proposed resolution: The shareholders’ meeting of the Company resolves to approve the issuance of one (1) new subscription right (in the form of a warrant) for the benefit of Gilead Therapeutics, called the ‘Subsequent Gilead Warrant B’ (the “Warrant”), and to cancel, in the interest of the Company, the statutory preferential subscription right of the existing shareholders of the Company (and, insofar as required, of the Company’s existing holders of subscription rights (stock options)) for the benefit of Gilead Therapeutics, in accordance with the report of the Board of Directors prepared in accordance with articles 7:180, 7:191 and 7:193 of the Belgian Companies and Associations Code, as referred to in item 1 of the agenda. In view thereof, the shareholders’ meeting of the Company resolves to approve the terms and conditions (the “Conditions”) of the

Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 Galapagos NV | Formulier stemmen per brief JAV/BAV 30 april 2024 | Voting by letter form AGM/EGM 30 April 2024 P. 8 | 13 “Voorwaarden”) van de Warrant goed te keuren zoals uiteengezet in Bijlage 1 van het verslag van de Raad van Bestuur waarnaar wordt verwezen in punt 1 van de agenda, waarvan een kopie aan de notulen van dit besluit wordt gehecht. De belangrijkste Voorwaarden van de Warrant kunnen, voor informatiedoeleinden, als volgt worden samengevat: a) Emittent van de Warrant: De Vennootschap. b) Duurtijd: De Warrant heeft een duurtijd die aanvangt op de datum van dit besluit en die eindigt om 23:59 uur op de kalenderdag voor de vijfde verjaardag van de datum van dit besluit. De Warrant kan tijdens haar gehele duurtijd van de Warrant één of meerdere keren vanaf 23 augustus 2024 (om 23:59 uur) worden uitgeoefend, maar niet meer dan éénmaal per periode van drie (3) maanden. Zoals uiteengezet in de Voorwaarden, is deze beperking niet van toepassing in geval van een materiële ontwikkeling met betrekking tot de Vennootschap of de verhandeling van de aandelen van de Vennootschap, of in geval van bepaalde (verzoeken tot) oproepingen tot algemene vergaderingen van de Vennootschap. c) Uitgifteprijs: De Warrant zal worden uitgegeven zonder dat enige bijkomende vergoeding door Gilead Therapeutics of enige met haar verbonden persoon verschuldigd is. d) Uitoefeningsprijs: De Uitoefeningsprijs (zoals gedefinieerd in de Voorwaarden) van de Warrant zal, per aandeel waarop ingeschreven wordt bij een uitoefening van de Warrant met betrekking tot dergelijke aandelen, gelijk zijn aan het hoogste van (i) 120% vermenigvuldigd met het rekenkundig gemiddelde van de dagelijks volume-gewogen gemiddelde koers van de aandelen van de Vennootschap verhandeld op Euronext Brussels en Euronext Amsterdam (of dergelijke andere gereglementeerde markten waarop de aandelen van de Vennootschap verhandeld zullen worden op dat tijdstip) op elk van de beursdagen gedurende de periode van 30 kalenderdagen eindigend op de kalenderdag die onmiddellijk voorafgaat aan de datum van het Bericht van Uitoefening (zoals gedefinieerd in de Voorwaarden) met betrekking tot die uitoefening, en (ii) EUR 140,59. De Uitoefeningsprijs is onderhevig aan gebruikelijke aanpassingen uiteengezet in de Voorwaarden. e) Aantal aandelen uit te geven bij een uitoefening van de Warrant: Overeenkomstig de Voorwaarden geeft de Warrant het recht aan de houder daarvan om in te schrijven, gedurende de gehele duurtijd van de Warrant, bij elke uitoefening van de Warrant, op een maximum aantal aandelen dat voldoende is om het aantal aandelen in eigendom van Gilead Therapeutics, Gilead Sciences en enige met hen verbonden persoon en, elke andere partij die in Onderling Overleg Handelt (zoals gedefinieerd in de Voorwaarden) met Gilead Therapeutics, Gilead Sciences of enige met hen verbonden persoon te brengen tot 29,9% van de daadwerkelijk uitgegeven en uitstaande aandelen onmiddellijk na de uitgifte van de aandelen die moeten worden uitgegeven bij de relevante uitoefening van de Warrant (naar beneden afgerond tot het dichtstbijzijnde gehele aandeel) (de “Warrantlimiet”). De Warrant blijft uitstaan voor de resterende duur van zijn duurtijd, zelfs indien deze wordt uitgeoefend voor een aantal aandelen dat gelijk is aan de op dat moment geldende Warranlimiet. Voor alle duidelijkheid, de totale deelneming die voortvloeit uit de volledige uitoefening van de Warrant zal in totaal niet meer bedragen dan 29,9%. f) Aard van de Warrant: De Warrant geeft het recht (maar niet de verplichting) om in te schrijven, bij enige uitoefening van de Warrant, op een aantal nieuwe aandelen uit te geven door de Vennootschap zoals hiervoor vermeld. Behalve indien anders bepaald door Belgisch recht, kwalificeert de houder van de Warrant as set forth in Annex 1 to the report of the Board of Directors referred to in item 1 of the agenda, a copy of which shall remain attached to the minutes reflecting the present resolution. The main Conditions of the Warrant can, for informational purposes, be summarized as follows: a) Issuer of the Warrant: The Company. b) Term: The Warrant has a term starting as of the date of this resolution and ending on 11:59 p.m. on the calendar day before the fifth anniversary of the date of this resolution. The Warrant can be exercised at one or several occasions as from 23 August 2024 (on 11:59 p.m.) during the entire term of the Warrant, but not more than once per period of three (3) months. As set out in the Conditions, this limitation does not apply in case of material development regarding the Company or the trading of the Company’s shares, or in case of certain (requests for) convocations of shareholders’ meetings of the Company. c) Issue Price: The Warrant will be issued without any additional consideration being due by Gilead Therapeutics or any of its affiliates. d) Exercise Price: The Exercise Price (as defined in the Conditions) of the Warrant shall, per share that shall be subscribed for upon an exercise of the Warrant in relation to such shares, be equal to the greater of (i) 120% multiplied by the arithmetic mean of the daily volume weighted average trading price of the Company’s shares as traded on Euronext Brussels and Euronext Amsterdam (or such other regulated markets on which the Company’s shares will be trading at that time) on each of the trading days during the period of 30 calendar days ending on the calendar day immediately preceding the date of the Exercise Notice (as defined in the Conditions) with respect to such exercise, and (ii) EUR 140.59. The Exercise Price is subject to adjustments set out in the Conditions. e) Number of shares issuable upon an exercise of the Warrant: Subject to the Conditions, the Warrant entitles the holder thereof to subscribe, during the entire term of the Warrant, upon each exercise of the Warrant, for a maximum number of shares that is sufficient to bring the number of shares owned by Gilead Therapeutics, Gilead Sciences and any of their affiliates and any other party Acting in Concert (as defined in the Conditions) with Gilead Therapeutics, Gilead Sciences or any of their affiliates to 29.9% of the actually issued and outstanding shares immediately after the issue of the shares that are to be issued upon the relevant exercise of the Warrant (rounded down to the nearest whole share) (the “Warrant Limit”). The Warrant remains outstanding for the remaining duration of its term even if exercised for a number of shares that is equal to the then applicable Warrant Limit. For clarity, the overall shareholding resulting from the full exercise of the Warrant shall in aggregate not exceed 29.9%. f) Nature of the Warrant: The Warrant will confer the right (but not the obligation) to subscribe, upon any exercise of the Warrant, for a number of new shares to be issued by the Company, as aforementioned. Except as otherwise provided for under Belgian law, the holder of the Warrant will be no shareholder of the Company solely by virtue of holding the Warrant, and therefore does not have the rights of a shareholder in relation to the shares to be issued or delivered to the holder of the Warrant upon an exercise of the Warrant until the exercise of the Warrant and the issue or delivery of the relevant shares. g) Form of the Warrant: The Warrant will be in registered form. h) No listing of the Warrant: The Warrant shall not be listed at any time on a securities exchange, regulated market or similar securities market.

Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 Galapagos NV | Formulier stemmen per brief JAV/BAV 30 april 2024 | Voting by letter form AGM/EGM 30 April 2024 P. 9 | 13 Warrant door het loutere houden van de Warrant niet als aandeelhouder van de Vennootschap, en beschikt hij bijgevolg niet over de rechten van een aandeelhouder met betrekking tot de aandelen uit te geven of te leveren aan de houder van de Warrant bij een uitoefening van de Warrant tot aan de uitoefening van de Warrant en de uitgifte of levering van de relevante aandelen. g) Vorm van de Warrant: De Warrant zal op naam zijn. h) Geen notering van de Warrant: De Warrant zal op geen enkel moment genoteerd worden op een effectenbeurs, gereglementeerde markt of soortgelijke effectenmarkt. i) Toekenning en inschrijving: De Warrant zal worden toegekend aan Gilead Therapeutics, en er kan enkel door Gilead Therapeutics op worden ingeschreven. j) Onderliggende aandelen: De nieuwe door de Vennootschap bij elke uitoefening van de Warrant uit te geven aandelen zullen volledig volgestort zijn en dezelfde rechten en voordelen hebben als, en in alle opzichten, inclusief voor wat betreft de gerechtigheid op dividenden en andere uitkeringen, pari passu rangschikken met de bestaande en uitstaande aandelen van de Vennootschap op het ogenblik van hun uitgifte en zullen gerechtigd zijn op dividenden en andere uitkeringen waarvoor de relevante registratiedatum of vervaldatum valt op of na de datum van hun uitgifte. De algemene vergadering besluit, onder voorbehoud van, en in de mate van, elke uitoefening van de Warrant, om het kapitaal van de Vennootschap te verhogen en bij een dergelijke uitoefening het toepasselijke aantal nieuwe aandelen uit te geven zoals voorzien in de toepasselijke Voorwaarden van de Warrant. De algemene vergadering besluit dat enige uitgiftepremie die geboekt zal worden in verband met de uitoefening van de Warrant en de uitgifte van nieuwe aandelen, zoals toepasselijk, zal worden geboekt op de passiefzijde van de balans van de Vennootschap als eigen vermogen. De rekening waarop de uitgiftepremie zal worden geboekt zal, net als het kapitaal, een waarborg vormen voor derden en kan, behoudens bij incorporatie ervan in kapitaal, enkel worden verminderd op grond van een geldig besluit van de algemene vergadering van aandeelhouders genomen op de wijze vereist voor een wijziging van de statuten van de Vennootschap. De algemene vergadering van de Vennootschap besluit de Raad van Bestuur te machtigen om de besluiten van de algemene vergadering van de Vennootschap in verband met de Warrant te implementeren en uit te voeren, en om alle stappen te ondernemen en alle formaliteiten te vervullen die krachtens de Voorwaarden van de Warrant, de statuten van de Vennootschap en de toepasselijke wetgeving vereist zijn om aandelen uit te geven of over te dragen bij uitoefening van de Warrant. Bovendien zal elk van de General Counsel van de Vennootschap en de bestuurders van de Vennootschap (elk van deze personen, een “Bijzondere Gevolmachtigde”), individueel handelend en met mogelijkheid tot subdelegatie en de bevoegdheid tot subrogatie, de bevoegdheid hebben om bij elke uitoefening van de Warrant over te gaan tot de vaststelling van (i) de kapitaalverhoging en de uitgifte van nieuwe aandelen die het gevolg zijn van deze uitoefening, (ii) de toewijzing van de uitgifteprijs aan het kapitaal en (in voorkomend geval) de uitgiftepremie overeenkomstig de toepasselijke Voorwaarden van de Warrant, (iii) de wijziging van de statuten van de Vennootschap om het nieuwe kapitaal en het aantal uitstaande aandelen na de uitoefening van de Warrant en de uitgifte van nieuwe aandelen weer te geven. Tot slot, hebben elk van de Bijzondere Gevolmachtigden, individueel handelend en met de mogelijkheid tot subdelegatie en de bevoegdheid van subrogatie, ook de bevoegdheid om, bij een uitoefening van een Warrant, (a) in naam van de Vennootschap, de relevante Euroclear-, Euronext en bankdocumentatie, het aandelenregister en alle noodzakelijke i) Allocation and subscription: The Warrant will be allocated to Gilead Therapeutics, and can only be subscribed for by Gilead Therapeutics. j) Underlying shares: Each new share to be issued by the Company upon each exercise of the Warrant shall be fully paid up and shall have the same rights and benefits as, and rank pari passu in all respects including as to entitlement to dividends and other distributions, with the existing and outstanding shares of the Company at the moment of their issue and will be entitled to dividends and other distributions in respect of which the relevant record date or due date falls on or after the date of their issue. The shareholders’ meeting resolves, subject to, and to the extent of, each exercise of Warrant, to increase the Company’s share capital and to issue the relevant number of new shares issuable upon such exercise as provided for in the relevant Conditions of the Warrant. The shareholders’ meeting resolves that any issue premium that will be booked in connection with the exercise of the Warrant and the issuance of new shares, as applicable, shall be accounted for on the liabilities side of the Company’s balance sheet as net equity. The account on which the issue premium shall be booked shall, like the share capital, serve as the guarantee for third parties and, save for the possibility of a capitalization of those reserves, can only be reduced on the basis of a valid resolution of the general shareholders’ meeting passed in the manner required for an amendment to the Company’s articles of association. The shareholders’ meeting of the Company resolves to authorize the Board of Directors to implement and execute the resolutions passed by the shareholders’ meeting of the Company in connection with the Warrant, and to take all steps and carry out all formalities that shall be required by virtue of the Conditions of the Warrant, the Company’s articles of association and applicable law in order to issue or transfer shares upon an exercise of Warrant. Furthermore, each of the General Counsel of the Company and the directors of the Company (each such person, a “Special Proxy Holder”), acting individually and with possibility of sub-delegation and the power of subrogation, shall have the power, upon each exercise of the Warrant, to proceed with the recording of (i) the capital increase and issue of new shares resulting from such exercise, (ii) the allocation of the issue price to the share capital and (as applicable) the issue premium in accordance with the relevant Conditions of the Warrant, (iii) the amendment of the Company’s articles of association in order to reflect the new share capital and number of outstanding shares following the exercise of the Warrant and the issuance of new shares. Finally, each Special Proxy Holder, acting individually and with possibility of sub-delegation and the power of subrogation, shall also have the power, upon an exercise of a Warrant, (a) to sign and deliver, on behalf of the Company, the relevant Euroclear, Euronext, and bank documentation, the share register and all necessary documents in connection with the issuance and delivery of the shares (acquired as a result of the exercise of the Warrant) to the beneficiary and (b) to do whatever may be necessary or useful (including but not limited to the preparation and execution of all documents and forms) for the admission of the shares issued upon an exercise of the Warrant to trading on the regulated markets of Euronext Brussels and Euronext Amsterdam (and such other regulated markets on which the Company’s shares will be trading at that time).

Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 Galapagos NV | Formulier stemmen per brief JAV/BAV 30 april 2024 | Voting by letter form AGM/EGM 30 April 2024 P. 10 | 13 documenten in verband met de uitgifte en levering van de aandelen (verworven als gevolg van de uitoefening van de Warrant) aan de begunstigde te ondertekenen en af te leveren, en (b) alles te doen wat nodig of nuttig kan zijn (met inbegrip van maar niet beperkt tot de voorbereiding en uitvoering van alle documenten en formulieren) voor de toelating van de aandelen uitgegeven naar aanleiding van een uitoefening van de Warrant tot de verhandeling op de gereglementeerde markten van Euronext Brussels en Euronext Amsterdam (en dergelijke andere gereglementeerde markten waarop de aandelen van de Vennootschap op dat moment worden verhandeld). Agendapunt 3—Steminstructie: Agenda item 3—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention 4. Kennisname en bespreking van het verslag van de Raad van Bestuur van de Vennootschap opgesteld overeenkomstig het artikel 7:199 van het Wetboek van Vennootschappen en Verenigingen in verband met de hernieuwing van zijn machtiging met betrekking tot, en de verhoging van, het toegestaan kapitaal, en de specifieke omstandigheden en doeleinden voor het gebruik van het hernieuwd toegestaan kapitaal. 4. Consideration and discussion of the report of the Board of Directors in accordance with article 7:199 of the Belgian Companies and Associations Code relating to the renewal of its authorization with respect to, and the increase of, the authorized capital, and the specific circumstances and purposes for the use of the renewed authorized capital. 5. Hernieuwing van de machtiging van de Raad van Bestuur om het kapitaal binnen het kader van het toegestaan kapitaal te verhogen met een bedrag tot 20% van het kapitaal. 5. Renewal of the authorization to the Board of Directors to increase the share capital within the framework of the authorized capital by up to 20% of the share capital. Dit voorstel wordt gedaan overeenkomstig de voorwaarden van de Inschrijvingsovereenkomst, die een verbintenis bevatte om een voorstel te doen aan de algemene vergadering om de Raad van Bestuur te machtigen om het kapitaal van de Vennootschap in één of meerdere malen te verhogen met een bedrag tot 20% van het kapitaal op het ogenblik van de oproeping tot de algemene vergadering. De huidige machtiging werd goedgekeurd door de buitengewone algemene vergadering van de Vennootschap op 22 oktober 2019 en vervalt op 12 november 2024. Het voorgestelde besluit zal de bovengenoemde machtiging (zoals overeengekomen in de Inschrijvingsovereenkomst) verlengen. This proposal is made in accordance with the terms of the aforementioned Subscription Agreement, which included a commitment to make a proposal to the shareholders’ meeting to authorize the Board of Directors to increase the share capital of the Company in one or several times with an amount up to 20% of the share capital at the time of the convening of the shareholders’ meeting. The current authorisation was approved by the Company’s extraordinary shareholders’ meeting on 22 October 2019 and will expire on 12 November 2024. The proposed resolution will renew the aforementioned authorisation (as agreed in the Subscription Agreement). Voorstel van besluit: De algemene vergadering van de Vennootschap besluit om de machtiging aan de Raad van Bestuur te hernieuwen om het kapitaal in één of meerdere malen te verhogen, gedurende een periode van vijf (5) jaar vanaf de bekendmaking van deze machtiging in de Bijlagen bij het Belgisch Staatsblad, met een totaalbedrag tot 20% van het huidige bedrag van het kapitaal van de Vennootschap, en dit in overeenstemming met de voorwaarden van het verslag van de Raad van Bestuur opgesteld overeenkomstig artikel 7:199 van het Wetboek van Vennootschappen en Verenigingen, zoals vermeld in punt 4 van de agenda van de buitengewone algemene vergadering. Bijgevolg besluit de algemene vergadering om de rubriek “Toegestaan kapitaal” van de tijdelijke bepalingen van de statuten van de Vennootschap volledig te schrappen en te vervangen door de volgende tekst (waarbij het bedrag van 20 procent van het geplaatste kapitaal hieronder opgenomen tussen vierkante haken wordt bepaald op basis van het op dat moment uitstaande geplaatste kapitaal): Proposed resolution: The shareholders’ meeting of the Company resolves to renew the authorization to the Board of Directors to increase the share capital on one or more occasions, during a period of five (5) years as of the publication in the Annexes to the Belgian State Gazette of this authorization, with an aggregate amount equal to up to 20% of the current amount of the share capital of the Company, and this in accordance with the terms and conditions set forth in the report of the Board of Directors prepared in accordance with article 7:199 of the Belgian Companies and Associations Code, as referred to in item 4 of the agenda of the Extraordinary Shareholders’ Meeting. Consequently, the shareholders’ meeting resolves to delete the section “Authorized Capital” of the temporary provisions of the articles of association of the Company entirely and to replace it with the following text (whereby the amount of 20 percent of the subscribed capital referred to below between square brackets shall be determined on the basis of the outstanding subscribed capital at that time): “Toegestaan kapitaal Aan de Raad van Bestuur werd de machtiging verleend om in overeenstemming met de terzake geldende wettelijke bepalingen, in één of meerdere malen, het geplaatste kapitaal van de vennootschap te verhogen in de hierna bepaalde mate. Deze “Authorized capital The Board of Directors has been granted the authority to increase the subscribed capital of the company, in accordance with applicable law, in one or several times, to the extent set forth hereafter. This authorization is valid for a period of five years from

Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 Galapagos NV | Formulier stemmen per brief JAV/BAV 30 april 2024 | Voting by letter form AGM/EGM 30 April 2024 P. 11 | 13 machtiging geldt voor een periode van vijf jaar te rekenen vanaf de datum van bekendmaking van deze machtiging in de Bijlagen bij het Belgisch Staatsblad. Onverminderd strengere wettelijke bepalingen, kan de Raad van Bestuur het geplaatste kapitaal van de vennootschap in één of meerdere malen verhogen met een bedrag van maximaal EUR [•], zijnde 20 procent van het geplaatst kapitaal op het ogenblik van de oproeping tot de algemene vergadering die deze machtiging heeft verleend. Overeenkomstig de terzake geldende wettelijke bepalingen, kan de Raad van Bestuur deze machtiging niet gebruiken nadat de Autoriteit voor Financiële Diensten en Markten (FSMA) de vennootschap kennis heeft gegeven van een openbaar overnamebod op de aandelen van de vennootschap. De kapitaalverhogingen in het kader van het toegestaan kapitaal kunnen worden gerealiseerd door de uitgifte van aandelen (onder, boven of tegen de fractiewaarde van de bestaande aandelen, met of zonder stemrecht, en desgevallend in het kader van een inschrijvingsrechtenplan voor de leden van het personeel van de vennootschap of haar dochtervennootschappen in de zin van artikel 1:27 van het Wetboek van Vennootschappen en Verenigingen (met inbegrip van leden van de raad van bestuur, en/of zelfstandige consulenten)), converteerbare obligaties en/of inschrijvingsrechten uitoefenbaar door inbreng in speciën of in natura, met of zonder uitgiftepremie, en ook door de omzetting van reserves, uitgiftepremies, overgedragen winst of andere vermogenscomponenten. Bovenvermelde inschrijvingsrechtenplannen mogen voorzien dat, in uitzonderlijke gevallen (onder meer in geval van wijziging in de controle van de vennootschap of overlijden), inschrijvingsrechten kunnen worden uitgeoefend vóór de derde verjaardag van de toekenning ervan, zelfs indien de begunstigde van deze inschrijvingsrechten een lid van de Raad van Bestuur of een persoon belast met het dagelijks bestuur is. De Raad van Bestuur kan bij een verhoging van het geplaatste kapitaal binnen de grenzen van het toegestaan kapitaal, in het belang van de vennootschap, het wettelijk voorkeurrecht van de aandeelhouders beperken of opheffen, zelfs indien deze beperking of opheffing gedaan wordt ten gunste van één of meerdere bepaalde personen andere dan leden van het personeel van de vennootschap of haar dochtervennootschappen in de zin van artikel 1:27 van het Wetboek van Vennootschappen en Verenigingen. De Raad van Bestuur kan een uitgiftepremie vragen bij de uitgifte van nieuwe aandelen in het kader van het toegestaan kapitaal. Indien de Raad van Bestuur hiertoe beslist, dient deze uitgiftepremie op een onbeschikbare reserverekening te worden geboekt die slechts kan worden verminderd of overgeboekt door een besluit van de algemene vergadering genomen op de wijze die vereist is voor de wijziging van statuten. De Raad van Bestuur is gemachtigd om de statuten van de vennootschap in overeenstemming te brengen met de kapitaalverhogingen waartoe binnen het kader van het toegestaan kapitaal werd beslist, of om een notaris hiertoe opdracht te geven.” the date of publication of this authorization in the Annexes to the Belgian State Gazette. Without prejudice to more restrictive rules set forth by law, the Board of Directors can increase the subscribed capital of the company in one or several times with an amount of up to EUR [•], i.e. 20 percent of the subscribed capital at the time of the convening of the shareholders’ meeting granting this authorization. In accordance with applicable law, the Board of Directors cannot use the aforementioned authorization after the Financial Services and Markets Authority (FSMA) has notified the company of a public takeover bid for the company’s shares. The capital increases within the framework of the authorized capital may be achieved by the issuance of shares (below, above or at the fractional value of the existing shares, with or without voting rights, and as the case may be in the context of a subscription rights plan for the company’s or its subsidiaries’ members of the personnel within the meaning of article 1:27 of the Belgian Companies and Associations Code (including members of the Board of Directors and/or independent consultants)), convertible bonds and/or subscription rights exercisable by contributions in cash or in kind, with or without issuance premium, and also by the conversion of reserves, issuance premiums, profits carried forward or other equity components. Aforementioned subscription rights plans can provide that, in exceptional circumstances (among others in the event of a change in control of the company or decease), subscription rights can be exercised before the third anniversary of their award, even if the beneficiary of such subscription right is a member of the Board of Directors or a person entrusted with the day-to-day management. When increasing the subscribed capital within the limits of the authorized capital, the Board of Directors may, in the company’s interest, restrict or cancel the shareholders’ statutory preferential subscription rights, even if such restriction or cancellation is made for the benefit of one or more specific persons other than the company’s or its subsidiaries’ members of the personnel within the meaning of article 1:27 of the Belgian Companies and Associations Code. The Board of Directors can ask for an issuance premium when issuing new shares in the framework of the authorized capital. If the Board of Directors decides to do so, such issuance premium is to be booked on a non-available reserve account that can only be reduced or transferred by a decision of the shareholders’ meeting adopted in the manner required for amending the articles of association. The Board of Directors is authorized to bring the company’s articles of association in line with the capital increases which have been decided upon within the framework of the authorized capital, or to instruct a notary public to do so.”. Agendapunt 5—Steminstructie: Agenda item 5—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention 6. Volmacht voor de coördinatie. 6. Proxy for coordination. Voorstel van besluit: De algemene vergadering besluit om machtiging te geven aan iedere medewerker van ondergetekende Proposed resolution: The shareholders’ meeting resolves to authorize each collaborator of undersigned notary or notary

Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 Galapagos NV | Formulier stemmen per brief JAV/BAV 30 april 2024 | Voting by letter form AGM/EGM 30 April 2024 P. 12 | 13 notaris of notaris Matthieu Derynck teneinde de gecoördineerde tekst van de statuten van de Vennootschap op te stellen, te ondertekenen en neer te leggen in de daartoe voorziene elektronische databank overeenkomstig de toepasselijke wettelijke bepalingen. Matthieu Derynck to draw up, sign and file the coordinated text of the Company’s articles of association in the electronic database provided for that purpose under the applicable laws. Agendapunt 6—Steminstructie: Agenda item 6—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention 7. Machtiging aan de Raad van Bestuur. 7. Authorization to the Board of Directors. Voorstel van besluit: De algemene vergadering besluit om alle machten aan de Raad van Bestuur van de Vennootschap te verlenen tot uitvoering van de genomen besluiten. Proposed resolution: The shareholders’ meeting resolves to grant all powers to the Company’s Board of Directors to execute the decisions taken. Agendapunt 7—Steminstructie: Agenda item 7—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention 8. Volmacht Kruispuntbank voor Ondernemingen, ondernemingsloket, griffies van de Ondernemingsrechtbank, administraties en fiscale diensten. 8. Proxy for the Crossroad Bank for Enterprises, counters for enterprises, registers of the enterprise court, administrative agencies and fiscal administrations. Voorstel van besluit: De algemene vergadering besluit aan elk lid van de Raad van Bestuur en/of aan, mevrouw Valeria Cnossen, mevrouw Annelies Denecker, mevrouw Elien Van Mol en de heer Stefan Mees, die – voor de uitvoering van deze volmacht – elk woonstkeuze doen te Generaal De Wittelaan L11 A3, 2800 Mechelen, België, een bijzondere volmacht te geven, om alleen optredend en met individueel recht van indeplaatsstelling en sub-delegatie, teneinde in naam en voor rekening van de Vennootschap alle formaliteiten te vervullen en/of documenten te ondertekenen die namens de Vennootschap moeten vervuld en/of ondertekend worden ter uitvoering van de hoger genomen besluiten, inclusief, doch niet beperkt tot, de uitvoering van alle formaliteiten die zijn vereist met betrekking tot alle door huidige vergadering genomen besluiten ten aanzien van de Kruispuntbank voor Ondernemingen, het ondernemingsloket, de griffies van de Ondernemingsrechtbank, de administraties en de fiscale diensten. Proposed resolution: The shareholders’ meeting resolves to grant a special power of attorney to any member of the Board of Directors and/or Mrs. Valeria Cnossen, Mrs. Annelies Denecker, Mrs. Elien van Mol and Mr. Stefan Mees, who – for the execution of this proxy – are all electing domicile at Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium, each acting separately and each with individual power of substitution and sub-delegation, to fulfill all formalities and/or sign all documents that must be fulfilled or signed in the name of or on behalf of the Company pursuant to or in the framework of the foregoing, including, but not limited to, the completion of all necessary formalities with the Crossroad Bank for Enterprises, counters for enterprises, registers of the enterprise court, administrative agencies and fiscal administrations with respect to the decisions taken at the present meeting. Agendapunt 8—Steminstructie: Agenda item 8—Voting instruction: Voor / In favor Tegen / Against Onthouding / Abstention Onderhavig stemformulier geldt tevens als aanmelding in de zin van het artikel 7:134 van het Wetboek van Vennootschappen en Verenigingen. The present voting form shall also serve as notification within the meaning of the article 7:134 of the Belgian Companies and Associations Code. De ondergetekende gaat ermee akkoord dat de Engelse versie van het onderhavige formulier slechts een vrije vertaling voor informatiedoeleinden betreft, en dat de Nederlandstalige versie altijd voorrang heeft op de Engelse versie. The undersigned agrees that the English version of the present form is a free translation for information purposes only, and that the Dutch version shall always prevail over the English version.

Gewone en Buitengewone Algemene Vergaderingen van 30 april 2024 Annual and Extraordinary Shareholders’ Meetings of 30 April 2024 Galapagos NV | Formulier stemmen per brief JAV/BAV 30 april 2024 | Voting by letter form AGM/EGM 30 April 2024 P. 13 | 13 (Datum / Date) (Naam / Name) (Handtekening / Signature) Het ondertekende formulier dient uiterlijk op 24 april 2024 Galapagos NV te bereiken. Deze dient te worden bezorgd per e-mail (shareholders@glpg.com) of met de post (Galapagos NV, t.a.v. mw. Annelies Denecker, Generaal De Wittelaan L11 A3, 2800 Mechelen, België). The signed form must reach Galapagos NV on 24 April 2024 at the latest. It should be submitted by e-mail (shareholders@glpg.com) or by mail (Galapagos NV, attn. Ms. Annelies Denecker, Generaal De Wittelaan L11 A3, 2800 Mechelen, Belgium). Aandeelhouders die wensen te stemmen per brief, moeten ook voldoen aan de registratie- en toelatingsvoorwaarden zoals beschreven in de oproeping tot de Gewone en Buitengewone Algemene Vergaderingen. Shareholders who wish to vote per letter, must also comply with the registration and admission formalities set out in the convening notice to the Annual and Extraordinary Shareholders’ Meetings.
Exhibit 99.15

Remuneration Policy

2 WWW.GLPG.COM #PioneeringForPatients This revised Remuneration Policy (‘Remuneration Policy’) of Galapagos NV (‘Galapagos’) is established in accordance with Article 7:89/1 of the Belgian Code of Companies and Associations and the 2020 Belgian Code of Corporate Governance (‘2020 Code’) and is applicable to Galapagos’ non-executive Directors and Executive Committee members. The Remuneration Policy was approved by the Board of Directors (‘Board’ or ‘Board of Directors’) on 26 March 2024, upon recommendation of the Remuneration Committee. The Remuneration Policy is to be submitted to the Annual General Meeting of Shareholders (‘AGM’) on 30 April 2024 and, if approved, will be applicable as from the financial year starting on 1 January 2024. It will be made available on Galapagos’ website for as long as it is applicable. Galapagos proactively engages with key stakeholders to understand the spectrum of views held and consider how and when to improve remuneration policies and practices. The Remuneration Policy is intended to be applicable for four years in accordance with Belgian law, unless the Board of Directors seeks approval for material changes to this Remuneration Policy at an earlier point if appropriate. Information you can find in this document Purpose and objectives 3 Remuneration Policy review 3 Remuneration Policy for members of the Board 4 Summary of remuneration elements 4 Remuneration Policy for Executive Committee members 6 Benchmarking approach 6 Summary of remuneration elements 6 Minimum share ownership 12 Clawback and malus provisions 12 Joining arrangements 13 Contracts and termination 13 Remuneration arrangements for employees 13 Modification of, or temporary deviation from the Remuneration Policy 14

3 WWW.GLPG.COM #PioneeringForPatients Purpose and objectives At Galapagos, we are united around a single purpose: to transform patient outcomes through life-changing science and innovation to deliver more years of life and quality of life. We are committed to improving patients’ lives worldwide by targeting diseases with high unmet needs. Our R&D objectives are to develop best-in-class therapeutic options. Our capabilities comprise multiple drug modalities, including small molecules and cell therapies. Our portfolio comprises discovery and development programs in immunology and oncology. The objective of our Remuneration Policy is intended to attract, engage, develop and retain the diverse qualified and expert individuals that we need to pursue our strategic and operational objectives, whilst reinforcing our culture and sustainability ambitions for the benefit of patients, our people and planet. Our specific goals for remuneration are: • to offer competitive opportunities for talented individuals by benchmarking against appropriate peer groups; • to incentivize exceptional and sustainable performance aligned with corporate achievements; • to provide differential rewards based on individual performance; • to avoid differentiation on any grounds except for performance and other proper factors; and • to reinforce an open and equitable culture. Remuneration Policy review As communicated to shareholders in the message from our Board of 23 March 2023 related to our 2023 AGM, the Remuneration Committee undertook a review of the Remuneration Policy adopted in 2022. The feedback obtained, together with the renewed strategic drivers at Galapagos, provided the following guiding principles for revision: • to provide simplicity, clarity and transparency in the design and communication of our remuneration policies and practices; • to align shareholder and management’s interests as far as practicable; • to enable the Remuneration Policy to support our international business footprint and potential changes over time; and • to emphasize strategic corporate performance and reinforce the link between individual remuneration and company performance. Key highlights and significant changes in comparison to the Remuneration Policy adopted in 2022 are summarized below: 1. Further transparency in the rationale and characteristics of the peer groups referenced. The Remuneration Committee acknowledges that this better aligns with market practice and views from shareholders gathered during our review process. Details of the actual constituent companies will be included in the relevant Remuneration Reports. 2. For transparency and simplicity the number of Restricted Stock Unit (‘RSU’) plans operated for the Executive Committee members will be reduced from two (previously the Annual Long-Term Incentive Plan and the RSU Retention Plan) to one (the Annual RSU Plan). Grants will be made annually at the determination of the Board. The RSUs will have a four-year vesting period, with 25% vesting each year, in line with market practice for such long-term incentive vehicles across both our U.S. and European peer groups. The Remuneration Committee and the Board considered this was an appropriate vesting schedule taking into account our need to attract and retain executives, including those from the U.S. through our current business transformation, and taking into account the strengthened share ownership guidelines and the introduction of Performance Share Units with three-year cliff vesting.

4 WWW.GLPG.COM #PioneeringForPatients 3. The Remuneration Committee determined that Performance Share Units (‘PSUs’) will be introduced as part of the broader long-term incentive mix starting in financial year 2025 as our business transformation progresses. The Remuneration Committee believes this demonstrates a clear commitment to better align with market practice and views from shareholders gathered during our review process, as well as reinforce the pay for performance linkages in our remuneration arrangements. 4. To further align financial interests of the Executive Committee members with those of shareholders and demonstrate their commitment, the existing share ownership requirements will be doubled. 5. To align the interests of the Executive Committee members with the long-term success and sustainability of Galapagos, the current malus provisions covering long-term incentives will be made more robust, including an extension to cover annual bonus as well as clawback situations. 6. To align with market practices, drive exceptional performance and strengthen the link between pay and performance, the maximum annual bonus funding opportunity for the Executive Committee members has been set at 125% of target for truly exceptional performance; while retaining the possibility of zero payout in case of unacceptable levels of performance. Previously, there was no opportunity for funding in excess of target. Remuneration Policy for members of the Board The remuneration structure and fee levels for the Directors are reviewed periodically by the Board. When reviewing fees, reference is made to the structure and fee levels payable in the same or similar peer groups used for the Executive Committee. Attention is given to ensuring our remuneration approach helps attract and retain Board members with the appropriate caliber, taking into account expertise, geography, gender and diversity requirements, the extent of duties performed and expected time commitment of the role. Revision of Director fees is proposed by the Board to the shareholders for approval, in accordance with Belgian law. Board members are elected by the shareholders, for a maximum term of four years, that can be renewed. Their agreements do not provide for any severance indemnities. Summary of remuneration elements
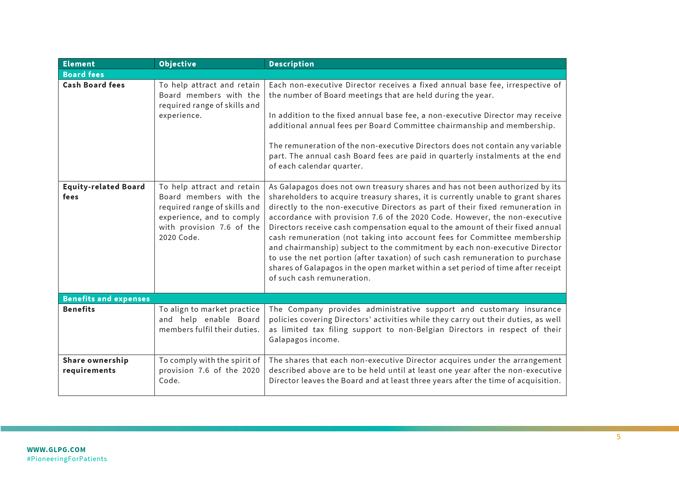
5 WWW.GLP G.COM #PioneeringForPatients Element Objective Description Board fees Cash Board fees To help attract and retain Board members with the required range of skills and experience. Each non-executive Director receives a fixed annual base fee, irrespective of the number of Board meetings that are held during the year. In addition to the fixed annual base fee, a non-executive Director may receive additional annual fees per Board Committee chairmanship and membership. The remuneration of the non-executive Directors does not contain any variable part. The annual cash Board fees are paid in quarterly instalments at the end of each calendar quarter. Equity-related Board fees To help attract and retain Board members with the required range of skills and experience, and to comply with provision 7.6 of the 2020 Code. As Galapagos does not own treasury shares and has not been authorized by its shareholders to acquire treasury shares, it is currently unable to grant shares directly to the non-executive Directors as part of their fixed remuneration in accordance with provision 7.6 of the 2020 Code. However, the non-executive Directors receive cash compensation equal to the amount of their fixed annual cash remuneration (not taking into account fees for Committee membership and chairmanship) subject to the commitment by each non-executive Director to use the net portion (after taxation) of such cash remuneration to purchase shares of Galapagos in the open market within a set period of time after receipt of such cash remuneration. Benefits and expenses Benefits To align to market practice and help enable Board members fulfil their duties. The Company provides administrative support and customary insurance policies covering Directors’ activities while they carry out their duties, as well as limited tax filing support to non-Belgian Directors in respect of their Galapagos income. Share ownership requirements To comply with the spirit of provision 7.6 of the 2020 Code. The shares that each non-executive Director acquires under the arrangement described above are to be held until at least one year after the non-executive Director leaves the Board and at least three years after the time of acquisition.

6 WWW.GLPG.COM #PioneeringForPatients Effective from 1 April 2022, Galapagos appointed a new CEO who was also appointed as member and Chair of the Board of Galapagos. The CEO is only remunerated for the performance of his executive functions as CEO and is not entitled to any additional remuneration for his mandate of member and Chair of the Board of Directors or any of the Board Committees. As long as the CEO serves as the Chair, a Lead Non-Executive Director will act as the principal liaison between the non-executive Directors and the Chair. A separate category of fees specific to this role is being submitted for shareholder approval. Remuneration Policy for Executive Committee members Benchmarking approach Galapagos’ Remuneration Policy strives to take into account relevant benchmarks and trends across appropriate peer companies to help ensure that our Remuneration Policy is appropriate and competitive in the markets where we compete for executive talent. Periodic peer group and benchmarking exercises are supported by independent external advisors. A peer group and benchmarking exercise for Executive Committee roles was completed between late 2022 and early 2023 in light of our strategic transformation and revised R&D strategy focused on immunology and oncology with the aim to transform patient outcomes through life-changing science and innovation. Galapagos is at a pivotal juncture resetting its strategic path and building an oncology franchise where attracting and retaining highly specialized expertise in an international labor market is essential to succeed. Both European and U.S. peer groups were found appropriate given the talent pool for the Executive Committee extends to both Europe and the U.S., with the majority of our competitors based in the U.S. The peer groups consist of publicly listed biotechnology and pharmaceutical companies, selected considering size, international growth ambitions and, to the extent possible, business model, lifecycle stage and therapeutic areas. These benchmarks support the Board, upon recommendation of the Remuneration Committee in its decision-making, also taking into account Galapagos’ strategic context and requirements, company performance, individual performance and skills as well as broader workforce considerations. The Remuneration Committee looks at each Executive Committee member’s home market as the primary reference point with consideration also given to the international talent market in which they have operated or could operate. The Remuneration Committee strives to take a balanced and responsible approach, in particular with long-term incentives where competitive practice on quantum and structure can vary significantly between the U.S. and elsewhere. Finally, the BEL20 (the benchmark stock market index of Euronext Brussels) general industry peer group (excluding financial services companies) is considered to ensure there is understanding of the Belgian listed market given the location of our headquarters. However, given the international nature of our executive leadership and specific sector considerations, it is not the only reference to inform our pay policy. Summary of remuneration elements The remuneration of the Executive Committee members is set out in the below summary whereby, in accordance with Belgian law, the grant of all variable remuneration is dependent on the achievement of certain criteria and at least 50% of the variable remuneration consists of long-term incentives. The vesting schemes of the long-term incentive instruments take into account that, in accordance with Belgian law, at least one-fourth of the variable remuneration is determined on the basis of objective criteria measured over at least two years and at least one-fourth of the variable remuneration is determined on the basis of objective criteria measured over at least three years.
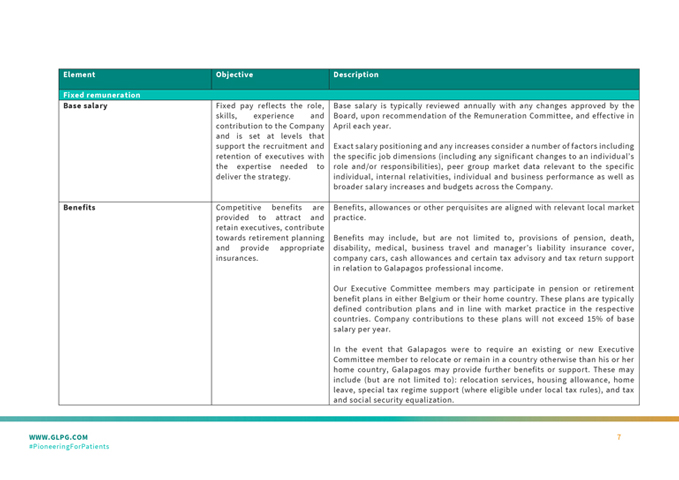
7 WWW.GLPG.COM #PioneeringForPatients Element Objective Description Fixed remuneration Base salary Fixed pay reflects the role, skills, experience and contribution to the Company and is set at levels that support the recruitment and retention of executives with the expertise needed to deliver the strategy. Base salary is typically reviewed annually with any changes approved by the Board, upon recommendation of the Remuneration Committee, and effective in April each year. Exact salary positioning and any increases consider a number of factors including the specific job dimensions (including any significant changes to an individual’s role and/or responsibilities), peer group market data relevant to the specific individual, internal relativities, individual and business performance as well as broader salary increases and budgets across the Company. Benefits Competitive benefits are provided to attract and retain executives, contribute towards retirement planning and provide appropriate insurances. Benefits, allowances or other perquisites are aligned with relevant local market practice. Benefits may include, but are not limited to, provisions of pension, death, disability, medical, business travel and manager’s liability insurance cover, company cars, cash allowances and certain tax advisory and tax return support in relation to Galapagos professional income. Our Executive Committee members may participate in pension or retirement benefit plans in either Belgium or their home country. These plans are typically defined contribution plans and in line with market practice in the respective countries. Company contributions to these plans will not exceed 15% of base salary per year. In the event that Galapagos were to require an existing or new Executive Committee member to relocate or remain in a country otherwise than his or her home country, Galapagos may provide further benefits or support. These may include (but are not limited to): relocation services, housing allowance, home leave, special tax regime support (where eligible under local tax rules), and tax and social security equalization.

8 WWW.GLPG.COM #PioneeringForPatients Variable remuneration Short-term incentive (annual bonus) The annual bonus is designed to incentivize and reward executives for the delivery of short-term corporate objectives and progress towards longer-term strategic objectives as well as the individual’s contribution to these over a time horizon of one year. The Board, upon recommendation of the Remuneration Committee, determines the corporate objectives annually. These objectives are designed to be challenging to achieve. The category of objectives and their relative importance may vary from year to year considering the strategic priorities of Galapagos. Further, the Remuneration Committee believes that company performance needs to be measured considering short-term delivery against corporate objectives set, as well as sustainable progress towards long-term strategic goals, taking into account the management of unforeseen developments and broader achievements. By way of example, corporate objectives may include elements of research progress, clinical trial progression, cash position, corporate development and sustainability. On the latter, the Remuneration Committee strives to include specific aspects of organizational health and broader sustainability in the corporate objectives, through stand-alone objectives and/or through the overarching mission of improving patients’ lives worldwide by targeting diseases with high unmet needs. The annual bonus target for the CEO is 75% of annual gross base salary and 50% for the other members of the Executive Committee. The level of available bonus funding achieved is established annually by the Board upon recommendation of the Remuneration Committee, ranging from 0% to a maximum of 125% of target for truly exceptional performance. The Board upon recommendation of the Remuneration Committee determines the individual pay-outs for the Executive Committee members after the end of the performance period, with the proposal for the CEO being made by the Chair of the Board (unless the Chair is the CEO in which case the proposal is made by the Lead Non-Executive Director) and the proposal for the other members of the Executive Committee being made by the CEO. These pay-out recommendations consider company performance against objectives as well as individual contribution and behaviours.
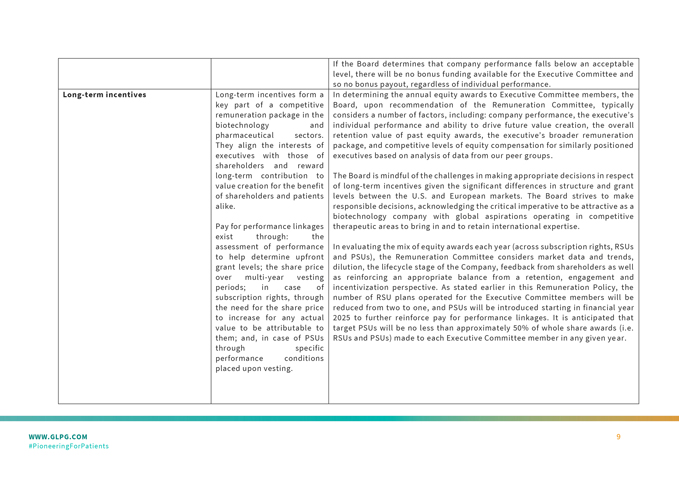
9 WWW.GLPG.COM #PioneeringForPatients If the Board determines that company performance falls below an acceptable level, there will be no bonus funding available for the Executive Committee and so no bonus payout, regardless of individual performance. Long-term incentives Long-term incentives form a key part of a competitive remuneration package in the biotechnology and pharmaceutical sectors. They align the interests of executives with those of shareholders and reward long-term contribution to value creation for the benefit of shareholders and patients alike. Pay for performance linkages exist through: the assessment of performance to help determine upfront grant levels; the share price over multi-year vesting periods; in case of subscription rights, through the need for the share price to increase for any actual value to be attributable to them; and, in case of PSUs through specific performance conditions placed upon vesting. In determining the annual equity awards to Executive Committee members, the Board, upon recommendation of the Remuneration Committee, typically considers a number of factors, including: company performance, the executive’s individual performance and ability to drive future value creation, the overall retention value of past equity awards, the executive’s broader remuneration package, and competitive levels of equity compensation for similarly positioned executives based on analysis of data from our peer groups. The Board is mindful of the challenges in making appropriate decisions in respect of long-term incentives given the significant differences in structure and grant levels between the U.S. and European markets. The Board strives to make responsible decisions, acknowledging the critical imperative to be attractive as a biotechnology company with global aspirations operating in competitive therapeutic areas to bring in and to retain international expertise. In evaluating the mix of equity awards each year (across subscription rights, RSUs and PSUs), the Remuneration Committee considers market data and trends, dilution, the lifecycle stage of the Company, feedback from shareholders as well as reinforcing an appropriate balance from a retention, engagement and incentivization perspective. As stated earlier in this Remuneration Policy, the number of RSU plans operated for the Executive Committee members will be reduced from two to one, and PSUs will be introduced starting in financial year 2025 to further reinforce pay for performance linkages. It is anticipated that target PSUs will be no less than approximately 50% of whole share awards (i.e. RSUs and PSUs) made to each Executive Committee member in any given year.
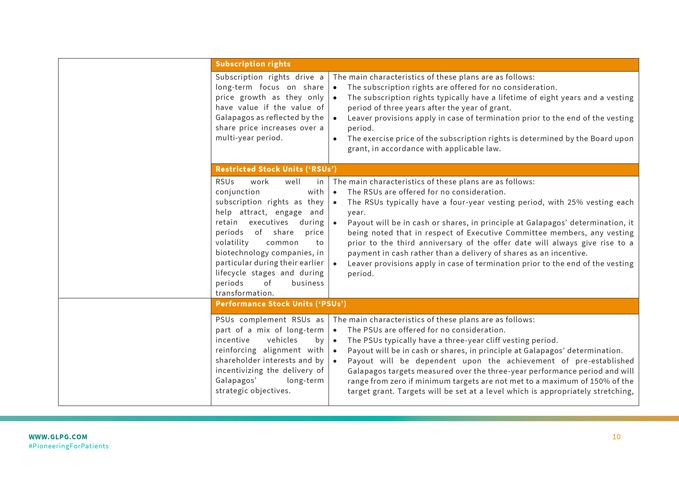
10 WWW.GLPG.COM #PioneeringForPatients Subscription rights Subscription rights drive a long-term focus on share price growth as they only have value if the value of Galapagos as reflected by the share price increases over a multi-year period. The main characteristics of these plans are as follows: • The subscription rights are offered for no consideration. • The subscription rights typically have a lifetime of eight years and a vesting period of three years after the year of grant. • Leaver provisions apply in case of termination prior to the end of the vesting period. • The exercise price of the subscription rights is determined by the Board upon grant, in accordance with applicable law. Restricted Stock Units (‘RSUs’) RSUs work well in conjunction with subscription rights as they help attract, engage and retain executives during periods of share price volatility common to biotechnology companies, in particular during their earlier lifecycle stages and during periods of business transformation. The main characteristics of these plans are as follows: • The RSUs are offered for no consideration. • The RSUs typically have a four-year vesting period, with 25% vesting each year. • Payout will be in cash or shares, in principle at Galapagos’ determination, it being noted that in respect of Executive Committee members, any vesting prior to the third anniversary of the offer date will always give rise to a payment in cash rather than a delivery of shares as an incentive. • Leaver provisions apply in case of termination prior to the end of the vesting period. Performance Stock Units (‘PSUs’) PSUs complement RSUs as part of a mix of long-term incentive vehicles by reinforcing alignment with shareholder interests and by incentivizing the delivery of Galapagos’ long-term strategic objectives. The main characteristics of these plans are as follows: • The PSUs are offered for no consideration. • The PSUs typically have a three-year cliff vesting period. • Payout will be in cash or shares, in principle at Galapagos’ determination. • Payout will be dependent upon the achievement of pre-established Galapagos targets measured over the three-year performance period and will range from zero if minimum targets are not met to a maximum of 150% of the target grant. Targets will be set at a level which is appropriately stretching,

11 WWW.GLPG.COM #PioneeringForPatients and the maximum will be linked to performance that would be considered exceptional. • The performance criteria and targets are defined by the Board upon proposal of the Remuneration Committee at the time of grant with a focus on achievements that would drive and sustain shareholder value creation. Such performance criteria may include but will not be limited to one or more of the following criteria: entering into new or extended collaboration arrangements; progression of Galapagos’ R&D pipeline in clinical and pre-clinical phases and filings of INDs and/or CTAs in accordance with or exceeding projected expectations; and sustainability milestones. • Subject to commercial or strategic sensitivity considerations, Galapagos will disclose further information in the Remuneration Reports at the beginning and end of the relevant vesting periods. • Leaver provisions apply in case of termination prior to the end of the vesting period.

12 WWW.GLPG.COM #PioneeringForPatients Minimum share ownership Minimum share ownership requirements are intended to further align Executive Committee members’ decision-making and financial interests with sustained, long-term shareholder value creation. The applicable Executive Committee member shall be required to hold a number of Galapagos shares corresponding to the value of such member’s annual gross base salary, as follows, during their tenure as Executive Committee member: • Chief Executive Officer: two times annual gross base salary; and • Other Executive Committee members: one time annual gross base salary. The Board expects RSU plan vesting (in case of cash-settled RSUs, using the net cash to acquire shares, subject to compliance with applicable securities laws) over time to be used to reach the minimum share ownership requirement. In particular, it is encouraged that Executive Committee members use at least 50% of vesting RSUs, net of taxes, social security and any other necessary deductions until this share ownership requirement is met. Given the grant levels and vesting schedules of our RSU plans, we expect Executive Committee members should reach these minimum share ownership requirements within five years of this Remuneration Policy taking effect (or as from a later Executive Committee appointment). The fulfilment of the minimum share ownership requirement is periodically reviewed. The Board may, on a reasonable and fair basis, allow divergence from the aforementioned requirement in exceptional circumstances. Clawback and malus provisions The Board may determine, in its sole discretion, to reduce or cancel any portion of unvested or unpaid variable remuneration (malus) and reclaim in full or in part, variable components of compensation that were paid, transferred or granted to Executive Committee members (clawback), in the event of a material breach of our Code of Conduct by such Executive Committee member, such as fraud or serious misconduct. In addition, as a public company listed on the NASDAQ Exchange, Galapagos is required to recover certain variable remuneration received by Executive Committee members in the event that Galapagos elects to, or is required to, restate one or more financial statements filed during the preceding three-year period due to an accounting error that is material to an applicable financial statement or would result in a material misstatement in the current financial statements if the error remains uncorrected. In accordance with NASDAQ Rule 5608(d), the Remuneration Committee shall recalculate the variable remuneration granted to such Executive Committee member, considering the restated financial results, and seek to reduce, cancel, seek repayment for or otherwise reclaim the amount of variable remuneration paid in excess of the newly calculated amount without regard to any taxes paid. This clawback shall be applied to annual bonus amounts paid or transferred and long-term incentives (subscription rights, RSUs and PSUs) granted to Executive Committee members beginning when such person became an Executive Committee member and to individuals who previously served as Executive Committee member for achieving financial results for a period of up to three years preceding the event triggering the clawback. Galapagos shall not indemnify any Executive Committee member against losses due to this clawback.

13 WWW.GLPG.COM #PioneeringForPatients Joining arrangements In determining the remuneration for a new Executive Committee member appointment, the Remuneration Committee would consider elements such as skills and experience of the individual, market benchmarks and competitive hiring context, and the existing remuneration of other executives. To help enable Galapagos attract, engage and retain the necessary caliber of executive, the Remuneration Committee can make appropriate and balanced recommendations on a case-by-case basis to provide a sign-on and/or buyout in the form of cash, subscription rights, RSUs and/or PSUs which may deviate from the remuneration arrangements otherwise set out in this policy. The Remuneration Committee would consider various factors including the nature and scale of losses that the candidate would otherwise incur or negative cashflow impacts. The Remuneration Committee would ensure any arrangements would be in the best interests of Galapagos and its shareholders, not seeking to pay more than appropriate to recruit, engage and retain the right candidate. Any such sign-on or buyout would be approved by the Board based on a recommendation from the Remuneration Committee and would be disclosed in the relevant Remuneration Report. Contracts and termination Providing appropriate termination-related benefits helps to attract, engage and retain experienced executives by mitigating the risks associated with leaving a previous employer and accepting a new position with Galapagos, and by providing income continuity following an unexpected termination of employment. • As of the date of this Remuneration Policy, all members of the Executive Committee have provided their services under self-employed agreements for an indefinite period of time with the Galapagos group, with a notice period, or indemnity in lieu of notice period, of nine months for the CEO and six months for the other members of the Executive Committee. The agreements do not provide for severance payments. Galapagos reserves the possibility of concluding employment agreements with members of the Executive Committee in the future, in accordance with applicable law. • In the event of termination, Galapagos may enter into non-competition undertakings with the CEO and the other members of the Executive Committee providing for non-competition indemnities. • In the event their contract with the group is terminated as a result of a change of control of Galapagos, the CEO and the other members of the Executive Committee would be entitled to the immediate vesting of subscription rights and severance compensation of (i) 12 months’ base salary for the CEO and (ii) nine months’ base salary for the other members of the Executive Committee. • For any Executive Committee member, the total value of a severance payment cannot exceed 12 months of total remuneration, including all components of remuneration, without prior approval of the Company’s shareholders’ meeting. Remuneration arrangements for employees This Remuneration Policy applicable to members of the Board and members of the Executive Committee is embedded in the general remuneration framework of the group. Remuneration arrangements for employees below the Executive Committee are set out in separate policies, but driven by the same business context and purpose, values and overall remuneration approach. To the extent practicable, our remuneration approach is cascaded throughout the organization considering local practices where appropriate. • All employees receive salary and benefits which are benchmarked to the local markets and countries in which they work. Base salaries are reviewed annually. • All employees participate in a bonus plan; the maximum opportunity available is based on role, responsibility and location, with the amount that can be earned based on the same corporate scorecard that is used for the Executive Committee members.

14 WWW.GLPG.COM #PioneeringForPatients • Finally, employees may be eligible for long-term incentive awards in the form of similar vehicles as the Executive Committee members, namely subscription rights, restricted stock units and/or performance stock units depending on their specific role, level and market. The long-term incentives help drive alignment with strategic goals and reward employees for their contributions. Modification of, or temporary deviation from the Remuneration Policy In accordance with Belgian law, the Board of Directors is allowed to modify any item of this Remuneration Policy, without having to seek the approval of the shareholders’ meeting, provided that the change is not material. Any such change will be explained and disclosed in the applicable Remuneration Report. Furthermore, in accordance with Belgian law, the Board may, but only in exceptional circumstances, temporarily deviate from any item of the Remuneration Policy involving a material change. Deviations may apply in the form of one-off bonuses, benefits and buyouts as well as exit conditions and shall be decided by the Board based on a recommendation from the Remuneration Committee. Any such temporary deviations involving a material change from the approved Remuneration Policy will be explained and disclosed in the applicable Remuneration Report.