| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
France |
||
|
State or other jurisdiction of incorporation or organization |
(I.R.S. Employer Identification No.) |
|
|
177-181 avenue Pierre BrossoletteMontrouge 92120 France |
||
(Address of principal executive offices) |
(Zip Code) |
|
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
||
American Depositary Shares, each representing one-half of one ordinary share, nominal value €0.10 per share |
DBVT |
The Nasdaq Stock Market LLC |
||
|
share* |
n/a |
The Nasdaq Stock Market LLC |
| * | Not for trading, but only in connection with the registration of the American Depositary Shares. |
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ | |||
| Emerging growth company | ☐ | |||||
SPECIAL NOTE REGARDING FORWARD LOOKING STATEMENTS.
This Annual Report on Form 10-K contains forward-looking statements which are made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). These statements may be identified by such forward-looking terminology as “may,” “should,” “expects,” “intends,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue” or variations of these words or similar expressions that are intended to identify forward-looking statements, although not all forward-looking statements contain these words. Any forward-looking statement involves known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to differ materially from any future results, levels of activity, performance or achievements expressed or implied by such forward-looking statement. Forward-looking statements include statements, other than statements of historical fact, about, among other things:
| • | our expectations regarding the timing or likelihood of regulatory filings and approvals, including with respect to our anticipated re-submission of a Biologics License Application, or a BLA, for Viaskin™ Peanut to the U.S. Food and Drug Administration, or the FDA; |
| • | the timing and anticipated results of interactions with regulatory agencies, |
| • | the initiation, timing, progress, results and success of our pre-clinical studies and clinical trials, and our research and development programs; |
| • | the sufficiency of existing capital resources; |
| • | our business model and our other strategic plans for our business, product candidates and technology; |
| • | our ability to manufacture clinical and commercial supplies of our product candidates and comply with regulatory requirements related to the manufacturing of our product candidates; |
| • | our ability to build our own sales and marketing capabilities, or seek collaborative partners, to commercialize Viaskin Peanut and/or our other product candidates, if approved; |
| • | the commercialization of our product candidates, if approved; |
| • | our expectations regarding the potential market size and the size of the patient populations for Viaskin Peanut and/or our other product candidates, if approved, and our ability to serve such markets; |
| • | the pricing and reimbursement of our product candidates, if approved; |
| • | the rate and degree of market acceptance of Viaskin Peanut and/or our other product candidates, if approved, by physicians, patients, third-party payors and others in the medical community; |
| • | our ability to advance product candidates into, and successfully complete, clinical trials; |
| • | the scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates and technology; |
| • | estimates of our expenses, future revenues, capital requirements and our needs for additional financing; |
| • | the potential benefits of strategic collaboration agreements and our ability to enter into strategic arrangements; |
| • | our ability to maintain and establish collaborations or obtain additional funding; |
| • | our financial performance; |
| • | developments relating to our competitors and our industry, including competing therapies; and |
| • | other risks and uncertainties, including those listed under the caption “Risk Factors.” |
i
Although we believe that we have a reasonable basis for each forward-looking statement contained in this Annual Report on Form 10-K, these statements are based on our estimates or projections of the future that are subject to known and unknown risks and uncertainties and other important factors that may cause our actual results, level of activity, performance, experience or achievements to differ materially from those expressed or implied by any forward-looking statement. These risks, uncertainties and other factors are described in greater detail under the caption “Risk Factors” in Part I. Item 1A and elsewhere in this Annual Report on Form 10-K. As a result of the risks and uncertainties, the results or events indicated by the forward-looking statements may not occur. Undue reliance should not be placed on any forward-looking statement.
In addition, any forward-looking statement in this Annual Report represents our views only as of the date of this annual report and should not be relied upon as representing our views as of any subsequent date. We anticipate that subsequent events and developments may cause our views to change. Although we may elect to update these forward-looking statements publicly at some point in the future, we specifically disclaim any obligation to do so, except as required by applicable law. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make.
ii
RISK FACTOR SUMMARY
The below summary risk factors provide an overview of certain of the risks we are exposed to in the normal course of our business activities. The below summary risk factors do not contain all of the information that may be important to investors, and investors should read the summary risk factors together with the more detailed discussion of risks set forth in Part I, Item 1A, “Risk Factors,” of this Annual Report.
| • | We have incurred significant losses since our inception and anticipate that we will continue to incur significant losses for the foreseeable future. |
| • | We will require substantial additional funding, which may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit, or terminate our product development efforts or other operations. |
| • | We are limited in our ability to raise additional share capital, which may make it difficult for us to raise capital to fund our operations. |
| • | We are obligated to develop and maintain a system of effective internal controls over financial reporting. These internal controls may be determined to be not effective, which may adversely affect investor confidence in our company and, as a result, the value of our ordinary shares and ADSs. |
| • | We depend almost entirely on the successful development of our novel Viaskin technology. We cannot be certain that we will be able to obtain regulatory approval for, or successfully commercialize, Viaskin products. |
| • | Our product candidates have undergone and/or will be required to undergo clinical trials that are time- consuming and expensive, the outcomes of which are unpredictable, and for which there is a high risk of failure. If clinical trials of our product candidates fail to satisfactorily demonstrate safety and efficacy to the FDA and other regulators, we, or our collaborators, may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of these product candidates. |
| • | In most of our clinical trials, we utilize an oral food challenge procedure intentionally designed to trigger an allergic reaction, which could be severe or life-threatening. |
| • | Delays, suspensions and terminations in our clinical trials could result in increased costs to us and delay or prevent our ability to generate revenues. |
| • | If our product candidates are not approved by the FDA, or comparable foreign regulatory authorities, we will be unable to commercialize them in the United States or foreign countries. |
| • | The approval process outside the United States varies among countries and may limit our ability to develop, manufacture and sell our products internationally. Failure to obtain regulatory approval in foreign countries would prevent our product candidates from being marketed abroad. |
| • | Even if we, or our collaborators, obtain regulatory approvals for our product candidates, the terms of approvals and ongoing regulation of our products may limit how we or they market our products, which could materially impair our ability to generate revenue. |
| • | Any of our product candidates for which we, or our collaborators, obtain regulatory approval in the future could be subject to post-marketing restrictions or withdrawal from the market and we, and our collaborators, may be subject to substantial penalties if we, or they, fail to comply with regulatory requirements or if we, or they, experience unanticipated problems with our products following approval. |
| • | If we do not achieve our projected development and commercialization goals in the timeframes we announce and expect, the commercialization of our product candidates may be delayed, and our business will be harmed. |
iii
| • | Access to raw materials and products necessary for the conduct of clinical trials, for commercialization, if approved, and manufacturing of our product candidates and product, if any, is not guaranteed. |
| • | Relying on third-party manufacturers may result in delays in our clinical development or commercialization efforts. |
| • | We rely, and will rely in the future, on third parties to conduct our clinical trials and perform data collection and analysis, which may result in costs and delays that prevent us from successfully commercializing product candidates. |
| • | Even if collaborators with which we contract in the future successfully complete clinical trials of our product candidates, those candidates may not be commercialized successfully for other reasons. |
| • | Currently, we do not have commercial-ready marketing and sales infrastructure. If we are unable to establish effective sales or marketing capabilities or enter into agreements with third parties to sell or market our product candidates, we may not be able to effectively sell or market our product candidates, if approved, or generate product revenues. |
| • | Our product candidates are regulated as biological products, or biologics, which may subject them to competition sooner than anticipated. |
| • | Our product candidates may cause undesirable side effects that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following regulatory approval, if any. |
| • | Changes in regulatory requirements, or guidance from the FDA and foreign regulatory authorities or unanticipated events during our clinical trials of Viaskin patch products may occur, which may result in changes to clinical trial protocols or additional clinical trial requirements, and could result in increased costs to us and could delay our development timeline. |
| • | If we do not secure collaborations with strategic partners to test, commercialize and manufacture certain product candidates outside of food allergies, we may not be able to successfully develop products and generate meaningful revenues. |
| • | Our ability to compete may decline if we do not adequately protect our proprietary rights. |
| • | Biopharmaceutical patents and patent applications involve highly complex legal and factual questions, which, if determined adversely to us, could negatively impact our patent position. |
| • | We will not seek to protect our intellectual property rights in all jurisdictions throughout the world and we may not be able to adequately enforce our intellectual property rights even in the jurisdictions where we seek protection. |
| • | Failure or perceived failure to comply with existing or future laws, regulations, contracts, self- regulatory schemes, standards, and other obligations related to data privacy and security (including security incidents) could harm our business. Compliance or the actual or perceived failure to comply with such obligations could negatively affect our operating results and business. |
| • | Our failure to maintain certain tax benefits applicable to French technology companies may adversely affect our results of operations. |
| • | We may be forced to repay conditional advances prematurely if we fail to comply with our contractual obligations under the applicable innovation grant agreements. |
| • | We will need to develop and implement sales, marketing and distribution capabilities before we are able to bring any product candidate to market, if approved, and as a result, we may encounter difficulties in managing this development and expansion, which could disrupt our operations. |
| • | If we are not able to comply with the applicable continued listing requirements or standards of the Nasdaq Global Select Market, or Nasdaq, our ADSs could be delisted. |
| • | The dual listing of our ordinary shares and our ADSs may adversely affect the liquidity and value of the ADSs. |
iv
TABLE OF CONTENTS
| i | ||||
| iii | ||||
| PART I | ||||
| 1 | ||||
| 43 | ||||
| 98 | ||||
| 98 | ||||
| 100 | ||||
| 100 | ||||
| 100 | ||||
| PART II | ||||
| 100 | ||||
| 101 | ||||
| Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations |
101 | |||
| Item 7A. Quantitative and Qualitative Disclosures About Market Risk |
116 | |||
| 116 | ||||
| Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
116 | |||
| 116 | ||||
| 117 | ||||
| Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections |
117 | |||
| PART III | ||||
| Item 10. Directors, Executive Officers and Corporate Governance |
117 | |||
| 118 | ||||
| 118 | ||||
| Item 13. Certain Relationships and Related Transactions, and Director Independence |
118 | |||
| 118 | ||||
| PART IV | ||||
| 118 | ||||
| 122 |
Unless the context otherwise requires, we use the terms “DBV,” “DBV Technologies,” the “Company,” “we,” “us” and “our” in this Annual Report on Form 10-K, or Annual Report, to refer to DBV Technologies S.A. and, where appropriate, its consolidated subsidiaries. “Viaskin™”, “EPIT™” and our other registered and common law trade names, trademarks and service marks are the property of DBV Technologies S.A. or our subsidiaries. All other trademarks, trade names and service marks appearing in this Annual Report on Form 10-K are the property of their respective owners. Solely for convenience, the trademarks and trade names in this Annual Report on Form 10-K may be referred to without the ® andTM symbols, but such references should not be construed as any indicator that their respective owners will not assert their rights thereto.
v
PART I
Item 1. Business.
Overview
DBV Technologies is a clinical-stage specialty biopharmaceutical company focused on changing the field of immunotherapy by developing a novel technology platform called Viaskin. Our therapeutic approach is based on epicutaneous immunotherapy, or EPIT, our proprietary method of delivering biologically active compounds to the immune system through intact skin using Viaskin, an epicutaneous patch (i.e., a skin patch). We have generated significant data demonstrating that Viaskin’s mechanism of action is novel and differentiated. Viaskin targets specific antigen-presenting immune cells in the skin, called Langerhans cells, that capture the antigen and migrate to the lymph node in order to activate the immune system without passage of the antigen into the bloodstream, minimizing systemic exposure in the body. We are advancing this unique technology to treat children suffering from food allergies for whom safety is paramount since the introduction of the offending allergen into their bloodstream can cause severe or life-threatening allergic reactions, such as anaphylactic shock. We believe Viaskin may offer convenient, self-administered, non-invasive immunotherapy to patients.
Our most advanced product candidate is Viaskin Peanut, which has been evaluated as a potential therapy for children with peanut allergy in eleven clinical trials, including four Phase 2 trials and four completed Phase 3 trials. We also have an ongoing Phase 3 trial of Viaskin Peanut in children ages four to seven with peanut allergy, as well as two planned Phase 3 supplementary safety studies, one in peanut-allergic children ages four through seven, and one in peanut-allergic toddlers, ages one through three.
We have earlier-stage food allergy programs including Viaskin Milk, which is in Phase 2 of clinical development for Cow’s Milk Allergy and Eosinophilic Esophagitis, or EoE.
Our Strategy
Our goal is to change the field of immunotherapy by developing and commercializing safe, effective, and convenient therapies for patients with food allergies and other immunological conditions. Key elements of our strategy are:
| • | Pursue the continued development of Viaskin Peanut for toddlers and children with peanut allergy. |
| • | Seek regulatory approval for Viaskin Peanut in the United States and the European Union. |
| • | Advance the clinical development of additional Viaskin product candidates in the United States and other major markets. |
| • | Build a broad immunotherapy product pipeline with our innovative Viaskin technology platform. |
Peanut Allergy
Unmet Medical Need
Peanut allergy is one of the most common food allergies globally with an overall prevalence across all age groups of approximately 1%, which increases up to 2% in the pediatric population. Based on a 2018 publication, an estimated 2.2% of the pediatric population in the United States, approximately 1.6 million children, is allergic to peanuts. This reflects an increasing prevalence, as has been shown by several epidemiologic studies, including a cross-sectional survey-based study in the United States in which the prevalence of peanut allergy more than tripled between 1997 and 2008 from 0.4% to 1.4%. Studies indicate that most children do not outgrow their peanut allergy, with resolution occurring in only about 20% of young children, making this allergy a life-long affliction in most cases.
1
Clinically, peanut allergy is characterized by rapid onset of symptoms which are triggered by the release of mediators from mast cells and basophils and typically involves one or more target organs. Presentation and severity of allergic reactions are unpredictable and may vary from mild to severe (anaphylaxis) within populations and within individuals over time. In the case of peanut allergy, all individuals are therefore considered at risk for severe allergic reactions, irrespective of their past history.
Current Challenges in the Management of Peanut Allergy Patients
The standard of care for the management of peanut allergy is strict allergen avoidance and the use of epinephrine in case of an allergic reaction. However, since peanut is a common ingredient in many foods, strict avoidance is difficult to achieve, and accidental exposures in peanut-allergic children remains a common issue. The estimated rate of accidental peanut exposure in peanut-allergic children is estimated to be 12.4% per year, with approximately 40% of children experiencing an accidental exposure within 3 years of diagnosis. In addition, the constant vigilance required to avoid allergen exposure can affect the quality of life of peanut-allergic children and their parents/caregivers. Daily family activities and social events are negatively impacted by the anxiety and fear of accidental peanut ingestion. According to a 2020 publication, a recent survey conducted across eight European countries reported high rates of frustration, stress and isolation in peanut-allergic individuals and their caregivers. The current management of peanut allergy has significant limitations and highlights the need for safe and effective treatments that can induce clinical desensitization (i.e., increased tolerance to peanut allergen), thus minimizing the risk of reaction due to accidental ingestion.
Current and Emerging Peanut Allergy Treatments
Several non-specific and allergen-specific treatment approaches are in various stages of clinical development for the treatment of peanut allergy. Food allergen-specific approaches include epicutaneous immunotherapy, or EPIT, oral immunotherapy, or OIT, (both with and without adjunctive therapies), and sublingual immunotherapy, or SLIT. EPIT is an emerging therapeutic approach to food allergy that utilizes the unique immune properties of the skin to deliver allergen directly to antigen-presenting cells in the epidermis and dermis to initiate desensitization. Although efficacious, peanut OIT may not be suitable or a preferred option for all children with peanut allergy because of its relatively high rate of systemic side effects and the limitations the treatment places on activities of daily living, including exercise, and unpredictability of tolerance in the setting of intercurrent illness. A proprietary form of OIT, Palforzia®, is approved in the US and the European Union for the treatment of peanut allergy in children aged 4–17 years. Xolair® (omalizumab), an anti-immunoglobulin E (IgE) antibody was recently approved by the FDA for the reduction of allergic reactions, including anaphylaxis, that may occur with accidental exposure to one or more foods in adult and pediatric patients aged 1 year and older with IgE-mediated food allergy. SLIT for peanut allergy has demonstrated evidence of clinical success, with a more satisfactory side effect profile compared to OIT. Despite the evident interest of clinicians to further evaluate these treatment procedures, OIT and SLIT may not be applicable across all ages and risk categories of peanut-allergic children and adults.
There remains an unmet need for additional therapies for patients with peanut allergy. In most other therapeutic areas, healthcare providers, patients and their families have several treatment options, and they are able to choose the treatment that best fits their needs. For example, in the case of respiratory allergies, symptomatic and maintenance allergy treatments, such as antihistamines, bronchodilators and corticosteroids, are available and all among the most widely used treatments in the world.
Our Viaskin Technology Platform
Over the last decade, we have developed an innovative immunotherapy technology platform, with the potential for sustained therapeutic effect, by delivering biologically active compounds, including antigens, via intact skin. Epicutaneous, also known as on the skin, immunotherapy, or EPIT, exposes tolerance-promoting immune cells in the skin to an adhesive dermal patch containing a small (micrograms) dose of antigen, such as food protein. This technology platform, which we call Viaskin, is an innovative approach to potentially treating immunological disorders, with a primary focus on food allergy.
2
In EPIT, intact skin is exposed to allergen via the Viaskin technology using a patch that contains microgram amounts of food protein. Allergen applied via EPIT is captured in the superficial layers of the skin by specialized antigen presenting cells (Langerhans cells within the epidermis), as well as dermal dendritic cells, thus limiting exposure to the bloodstream. In experimental models, EPIT induced a population of regulatory T cells, or Tregs, with specific properties that resulted in suppression of allergic. symptoms and protection against further sensitizations. EPIT-induced epigenetic modifications favored a Treg-mediated immune response and a downregulated Th2 response and may play a role in the sustainability of effect. Based on our trials and research, we believe that EPIT has the potential to provide all of the intended benefits of a disease-modifying treatment in allergy, while avoiding severe or life-threatening allergic reactions.
The key elements of the Viaskin patch mechanism of action, which are illustrated below, are the following:
| • | Containing a dry layer of allergen in its center, the patch is positioned on intact skin, without prior preparation. |
| • | The condensation chamber formed between the skin and the center of the patch creates hyperhydration of the skin and an accumulation of water. |
| • | The accumulation of water solubilizes the allergen. Due to this condensation chamber, the epidermis becomes more permeable allowing passage of the allergen into the epidermis. |
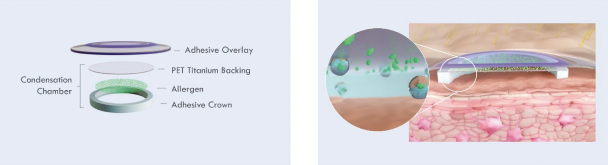
Once in the epidermis, the allergen is captured by a population of highly specialized cells: Langerhans cells. These cells can capture the protein at the surface of the skin, process it and present its epitopes to the T-lymphocytes in the lymph nodes.
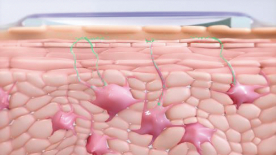
Langerhans cells in the epidermis capturing peanut allergen (depicted in green) within the stratum corneum (the outermost layer of the skin) following solubilization of allergen and permeation into the skin after Viaskin patch application.
3
Our Product Candidates
Our product development strategy is based on leveraging Viaskin’s clinical potential. We select our target product candidates with the aim to address allergies that have high unmet medical needs. The following table summarizes the current development status of our product candidates:
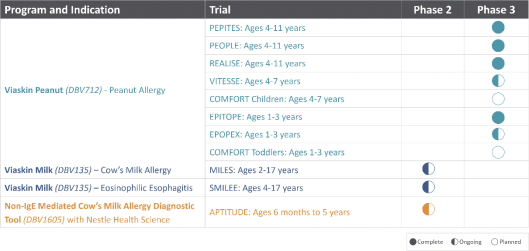
Viaskin Peanut for children ages 4-11
Our lead product candidate, Viaskin Peanut, has completed a global Phase 3 development program for the treatment of peanut allergic patients four to 11 years of age. The program comprised of the following clinical trials:
| • | PEPITES (Peanut EPIT Efficacy and Safety Study), a randomized, placebo-controlled pivotal Phase 3 trial investigating the safety and efficacy of Viaskin Peanut 250 µg in 356 patients after 12 months of treatment. |
| • | REALISE (REAL Life Use and Safety of EPIT), a randomized, placebo-controlled Phase 3 trial designed to generate safety data after six months of blinded treatment, as well as to evaluate the use of Viaskin Peanut 250 µg in routine clinical practice. |
| • | PEOPLE (PEPITES OPen Label Extension Study), a long-term, open-label extension trial of Viaskin Peanut 250 µg. In the PEOPLE trial, patients who were randomized and received active treatment during PEPITES received Viaskin Peanut 250 µg for up to four additional years, while patients who received placebo during PEPITES were treated with Viaskin Peanut 250 µg for up to five years. |
The results from PEPITES and REALISE formed the basis for our 2019 regulatory submission in the United States, a Biologics License Application, or BLA, for the use of Viaskin Peanut in peanut-allergic patients four to 11 years of age. The results from PEPITES, REALISE and PEOPLE formed the basis for our 2020 regulatory submission in the European Union, a Marketing Authorization Application, or MAA, for the use of Viaskin Peanut in peanut- allergic patients four to 11 years of age.
United States Regulatory History
Viaskin Peanut obtained fast track designation and breakthrough therapy designation in children from the FDA, which are regulatory designations intended to expedite or facilitate the process of reviewing new drugs and biological products that are intended to treat a serious or life-threatening disease or condition and demonstrate the potential to address unmet medical needs for the disease or condition.
4
In August 2019, we announced the submission of a BLA to the FDA for Viaskin Peanut for the treatment of peanut allergy in children four to 11 years of age.
In October 2019, we announced the FDA’s acceptance for review of our BLA for Viaskin Peanut, with a target action date, provided by the FDA, of August 5, 2020.
In February 2020, the FDA announced an Allergenic Products Advisory Committee meeting to be held on May 15, 2020 to discuss the BLA for Viaskin Peanut. On March 16, 2020, we announced that the FDA had informed us that during its ongoing review of our BLA for Viaskin Peanut, it had identified questions regarding efficacy, including the impact of patch-site adhesion. Therefore, the Advisory Committee meeting to discuss the BLA originally scheduled on May 15, 2020 was cancelled.
In August 2020, we received a Complete Response Letter, or CRL, in which the FDA indicated it could not approve the Viaskin Peanut BLA in its current form. The FDA identified concerns regarding the impact of patch- site adhesion on efficacy and indicated the need for patch modifications, and subsequently a new human factor study. The FDA also indicated that supplementary clinical data would need to be generated to support the modified patch. In addition, the FDA requested additional Chemistry, Manufacturing and Controls, or CMC, data. The FDA did not raise any safety concerns related to Viaskin Peanut.
In January 2021, we received written responses from the FDA to questions provided in the Type A meeting request we submitted in October 2020 following the CRL. The FDA agreed with our position that a modified Viaskin Peanut patch should not be considered as a new product entity provided the occlusion chamber of the current Viaskin Peanut patch and the peanut protein dose of 250 µg (approximately 1/1,000 of one peanut) remains unchanged and performs in the same way it has performed previously. In order to confirm the consistency of efficacy data between the existing and a modified patch, FDA requested an assessment comparing the uptake of allergen (peanut protein) between the patches in peanut allergic children ages 4-11. We named that assessment EQUAL, which stands for Equivalence in Uptake of Allergen. The FDA also recommended conducting a 6-month, well-controlled safety and adhesion trial to assess a modified Viaskin Peanut patch in the intended patient population. We later named this clinical trial STAMP, which stands for Safety, Tolerability, and Adhesion of Modified Patches.
Based on the January 2021 FDA feedback, we defined three parallel workstreams:
| 1. | Identify a modified Viaskin patch (which we call mVP). |
| 2. | Generate the 6-month safety and adhesion clinical data FDA requested via STAMP, which we expected to be the longest component of the mVP clinical plan. We prioritized the STAMP protocol submission so we could begin the clinical trial as soon as possible. |
| 3. | Demonstrate the equivalence in allergen uptake between the current and modified patches in the intended patient population via EQUAL. The complexity of EQUAL hinged on the lack of established clinical and regulatory criteria to characterize allergen uptake via an epicutaneous patch. To support those exchanges, we outlined our proposed approach to demonstrate allergen uptake equivalence between the two patches, and allotted time to generate informative data through two additional Phase 1 clinical trials in healthy adult volunteers: |
| a. | PREQUAL, a Phase 1 trial with adult healthy volunteers to optimize the allergen sample collection methodologies and validate the assays we intend to use in EQUAL. The data collection phase of the trial is complete, and the data analysis phase is ongoing. |
| b. | ‘EQUAL in adults,’ a second Phase 1 trial with adult healthy volunteers to compare the allergen uptake of the original patch (which we call cVP) and mVP. |
5
In March 2021, we commenced CHAMP (Comparison of adHesion Among Modified Patches), a Phase 1 trial in healthy adult volunteers to evaluate the adhesion of five modified Viaskin Peanut patches. We completed CHAMP in the second quarter of 2021. All modified Viaskin Peanut patches demonstrated better adhesion performance as compared to the then-current Viaskin Peanut patch (cVP), and based on the results of CHAMP, we then selected two modified patches that performed best out of the five modified patches studied for further development. We then selected the circular patch for further development, which is approximately 50% larger in size (in terms of the surface area in contact with the skin) relative to cVP and circular in shape.
In May 2021, we submitted our proposed STAMP protocol to the FDA, and on October 14, 2021, we received an Advice/Information Request letter from the FDA. In this letter, the FDA requested a stepwise approach to the modified Viaskin patch development program and provided partial feedback on the STAMP protocol. Specifically, the FDA requested that we conduct allergen uptake comparison trials (i.e., ‘PREQUAL in Adults,’ PREQUAL (a Phase 1 study in healthy volunteers to optimize allergen sample collection methodologies and validate the assays DBV intended to be used in EQUAL, a second Phase 1 study that was planned (but not initiated) comparing allergen uptake following application of mVP and cVP), and submit the allergen uptake comparison data for FDA review and feedback prior to starting the STAMP study. The FDA’s explanation was that the results from the allergen uptake trials might affect the design of the STAMP study.
After careful review of the FDA’s information requests, in December 2021, we decided not to pursue the sequential approach to the development plans for Viaskin Peanut as requested by the FDA in the October 2021 feedback. We estimated that the FDA’s newly proposed sequential approach would require at least five rounds of exchanges that necessitate FDA alignment prior to initiating STAMP, the 6-month safety and adhesion study. As such, in December 2021, we announced our plan to initiate a pivotal Phase 3 placebo-controlled efficacy trial for a modified Viaskin Peanut patch (mVP) in children in the intended patient population. We consider this approach the most straightforward to potentially demonstrate effectiveness, safety, and improved in vivo adhesion of the modified Viaskin Peanut system. The FDA confirmed our change in strategy was agreeable via oral and written exchanges. In 2022, we announced the new Phase 3 pivotal study of the modified Viaskin Peanut (mVP) patch would be in younger (4-7 years old) and more sensitive children with peanut allergy.
European Union Regulatory History
In November 2020, we announced that our Marketing Authorization Application, or MAA, for Viaskin Peanut, submitted under the name “Abylqis®”, had been validated by the European Medicines Agency, or EMA. The validation of the MAA confirmed that the submission was sufficiently complete to begin the formal review process for Viaskin Peanut to treat peanut allergies in children ages four to 11 years. Following the MAA validation, the EMA’s Committee for Medicinal Products for Human Use, or CHMP, reviews the application and provides a recommendation to the European Commission, on whether to grant a marketing authorization. On March 11, 2021, we announced that we had received the EMA’s Day 120 questions, which were consistent with both our expectations and pre- filing conversations with the EMA. We did not receive questions about the impact of adhesion on efficacy.
On August 2, 2021, we announced we had received from the EMA the Day 180 list of outstanding issues, which is an established part of the prescribed EMA review process. It is a letter that is meant to include any remaining questions or objections at that stage in the process. The EMA indicated many of their objections and major objections from the Day 120 list of questions had been answered. One major objection remained at Day 180. The Major Objection questioned the limitations of the data, for example, the clinical relevance and effect size supported by a single pivotal study.
On December 17, 2021, we announced we had withdrawn the MAA for Viaskin Peanut, submitted under the name “Abylqis”, and formally notified the EMA of our decision. The initial filing was supported by data from a single, placebo-controlled Phase 3 pivotal trial known as PEPITES (V712-301). The decision to withdraw was based on the view of CHMP that the data available to date from a single pivotal clinical trial were not sufficient to preclude a Major Objection at Day 180 in the review cycle.
6
We believe data from a second Viaskin Peanut pivotal clinical trial will support a more robust path for licensure of Viaskin Peanut in the EU. We intend to resubmit the MAA when that data set is available.
PEPITES (Peanut EPIT Efficacy and Safety Study)
In December 2015, we initiated a pivotal Phase 3 trial designed to evaluate the safety and efficacy of Viaskin Peanut 250 µg in children four to 11 years of age suffering from peanut allergy. PEPITES was a global, randomized 2:1, double-blind, placebo-controlled Phase 3 trial, in which 356 pediatric peanut-allergic patients were treated with Viaskin Peanut 250 µg or placebo for 12 months. A new patch was applied each day, and after 2 weeks, each patch was worn for 24 hours, plus-or-minus 4 hours. During the trial, patients’ sensitivity to peanut protein was assessed using a double-blind, placebo-controlled food challenge, or DBPCFC, at baseline and again after 12 months of treatment. The DBPCFC was halted once the patient exhibited an objective symptom, as described on a pre- specified scale, thus establishing a subject’s peanut reactivity level, also known as the patient’s eliciting dose, or ED. The median baseline reactive dose in PEPITES was 100 mg at baseline.
The primary responder analysis was conducted after 12 months of treatment. For patients with a baseline peanut protein ED equal to or less than 10 mg, a responder was defined as a patient with a peanut protein ED equal to or greater than 300 mg of peanut protein after 12 months of treatment. For patients with a baseline ED greater than 10 mg but less than or equal to 300 mg, a responder was defined as a patient with a peanut protein ED equal to or greater than 1,000 mg of peanut protein after 12 months of treatment. Secondary endpoints included the change from baseline of mean and median cumulative reactive dose of peanut protein, or CRD, which is used to establish the total quantity of peanut protein consumed during the DBPCFC. Serological markers were also measured at baseline, three, six and 12 months to characterize the immunological changes observed in patients.
Results of PEPITES Trial
In October 2017, we announced topline results from PEPITES, in which we observed a statistically significant response with a favorable tolerability profile, with (based on “responder” definitions above) 35.3% of patients responding to Viaskin Peanut 250 µg after 12 months of treatment as compared to 13.6% of patients in the placebo arm (difference in response rates = 21.7%; p=0.00001; 95% CI = 12.4%—29.8%). However, the primary endpoint, which evaluated the 95% CI in the difference in response rates between the active and placebo arms, did not reach the 15% lower bound of the CI that was proposed in the study’s Statistical Analysis Plan submitted to the FDA. The clinical relevance of this is not known. Detailed results were published in The Journal of the American Medical Association (JAMA) in February 2019.
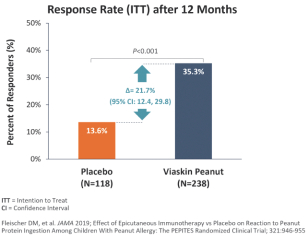
7
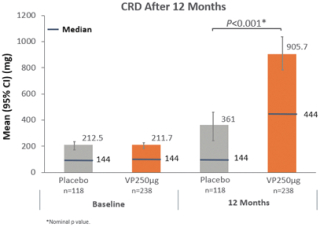
With respect to CRD, a key secondary endpoint which measures threshold reactivity during the DBPCFC, we observed that at month 12, patients treated with Viaskin Peanut 250 µg or placebo reached a mean CRD of 906 mg (median 444 mg) and 361 mg (median 144 mg) of peanut protein, respectively. Patients in the active and placebo arms entered the trial at similar sensitivity levels; mean CRD at baseline was 211.7 mg (median 144 mg) in the Viaskin Peanut arm and 212.5 mg (median 144 mg) in the placebo arm. A difference in the CRD was observed between Viaskin Peanut and placebo (nominal p-value < 0.001) following 12 months of treatment.
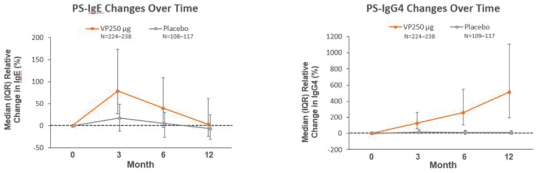
Exploratory analyses showed that changes in peanut-specific biomarkers, including immunoglobulin E(IgE), and immunoglobulin G4(IgG4), support the immunomodulatory effect of Viaskin Peanut. The median observed increase from baseline in peanut-specific IgE was greater in the Viaskin Peanut group vs placebo group, respectively, at month 3 (70.1 kilounits of antibody per liter, or kUA/L vs. 9.8 kUA/L) and month 6 (27.4 kUA/L vs. 1.32 kUA/L). However, at month 12, peanut-specific IgE levels were observed to return to near baseline in both groups (1.1 kUA/L vs. -1.1 kUA/L). Median peanut-specific IgG4 were observed to increase over time in the Viaskin Peanut group (change from baseline at month 3: 0.81 mg/L; month 6: 1.79 mg/L; month 12: 3.27 mg/ L), while levels remained unchanged from baseline in the placebo group. The change from baseline in peanut-specific IgG4 was greater at all time points with Viaskin Peanut vs placebo, and the groups were observed to be highly distinguished by this marker, given a flat trend in the placebo arm. These changes are consistent with trends that have been observed with other forms of immunotherapy such as for venom and inhalant allergies.
PEPITES Immunological Responses
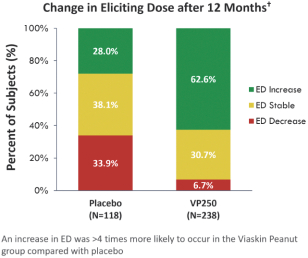
In a post-hoc analysis, the majority of subjects on Viaskin Peanut exhibited an increased ED compared to the placebo group (62.6% in active vs. 28% in placebo) at 12 months. An additional post-hoc analysis showed that 53.1% of subjects treated with Viaskin Peanut increased their baseline ED from 100 mg or less to 300 mg or more, compared to 19% in the placebo group.
8
Based on this analysis, we believe that increasing the ED should translate to a reduction in the risk of reaction to accidental peanut exposures, as it will take a higher ingestion quantity to trigger a reaction. Indeed, based on quantitative risk analysis, or QRA, modeling from Baumert et al using national databases of consumption and contamination amounts, this improvement in ED from ≤100 mg to ≥300 mg is predicted to reduce the risk of an allergic reaction due to accidental peanut exposure through a group of common contaminated packaged foods by over 95%.
A favorable safety and tolerability profile was observed with Viaskin Peanut. Treatment adherence was high (98.5%), and similar discontinuation rates between treatment groups were reported, with 89.9% of subjects completing the trial. There was a low discontinuation rate due to treatment-emergent adverse events, or TEAEs, (1.7%), and the overall rate of TEAEs, regardless of relatedness to the treatment, was comparable between treatment and placebo groups, at 95.4% and 89.0%, respectively. The most commonly reported TEAEs were mild to moderate application-site reactions that decreased after month one in both frequency and severity. There were no treatment-related gastrointestinal adverse events or cases of eosinophilic esophagitis in this trial.
There were no cases of severe anaphylaxis in the trial. SAEs were balanced between the Viaskin Peanut and placebo group, at 4.2% vs. 5.1%, respectively. Four SAEs reported in three Viaskin Peanut patients (1.3%) were determined by the investigator as possibly or probably related to treatment. A low rate of treatment-related epinephrine use was reported (2.9% treatment group vs. 0.8% placebo group). Ten cases in eight Viaskin Peanut subjects (3.4%) of possibly or probably treatment-related anaphylaxis occurred, and all were classified as mild or moderate without evidence of cardiovascular, neurologic, or respiratory compromise. Six of these ten cases were treated with epinephrine, and five of the eight subjects continued on Viaskin Peanut in the trial.
Following the completion of PEPITES, all eligible subjects were invited to enroll in PEOPLE (Open-Label Follow-Up Study of the PEPITES Study to Evaluate the Long-term Efficacy and Safety of Viaskin Peanut), a long-term, open-label extension trial of Peanut 250 µg in children. In the PEOPLE trial, subjects who were randomized and received active treatment during PEPITES received Viaskin Peanut 250 µg for two additional years, while subjects who previously received placebo during PEPITES were treated with Viaskin Peanut 250 µg for three years. In August 2017, we announced the completion of enrollment of the PEOPLE trial, with 298 (92%) subjects who completed PEPITES enrolling in this follow-up trial.
PEOPLE (PEPITES Open Label Extension Study)
The PEOPLE trial, which was completed in October 2022, is an open-label extension study that evaluated the long-term safety, tolerability and efficacy of Viaskin Peanut 250 µg in patients who have completed the Phase 3 PEPITES trial. The last patient visit of the PEOPLE trial occurred on October 12, 2022.
9
In January 2020, we announced positive topline results up to Year 3 from the open-label extension of our Phase 3 PEPITES trial, or PEOPLE trial, evaluating the long-term efficacy and safety of investigational Viaskin Peanut in peanut-allergic children ages four to 11 years. The results demonstrated long-term clinical benefit as shown by an increase in eliciting dose, or ED, which may decrease the chance of reacting to an accidental peanut exposure. Results of the PEOPLE trial for participants receiving 3 years of active treatment were published in the Journal of Allergy and Clinical Immunology in October 2020.
Of the 356 participants who were enrolled in PEPITES, 298 eligible participants opted to enroll in PEOPLE. Of the 213 patients who were randomized in the active treatment arm of PEPITES and completed the 12-month trial, 198 patients opted to enter the PEOPLE clinical trial (safety population). Of these patients, 148 were considered completers after 36 months and 141 subjects completed all treatment according to the clinical trial protocol without major deviations. Efficacy data were analyzed from these 141 subjects(per protocol).
Topline results from Year 3 of PEOPLE support the long-term tolerability and clinical benefit of Viaskin Peanut, demonstrating desensitization over 36 months of treatment, with 75.9% (107/141) of patients increasing their ED from baseline. After 36 months, 51.8% (73/141) of subjects reached an ED of at least 1,000 mg peanut protein, an increase of 40.4% (57/141) relative to Month 12. In addition, 13.5% (19/141) of subjects completed the food challenge without meeting stopping criteria at 36 months (cumulative dose of 5,444 mg). At Month 36, the mean cumulative reactive dose (CRD) was 1,768.8 mg (median 944 mg) compared to 223.8 mg (median 144 mg) at baseline.
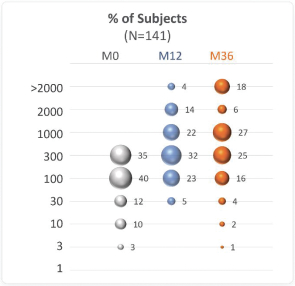
Changes in ED were maintained or improved over 3 years in the majority of subjects in the Open-label extension study (Fleischer DM, et al. J Allergy Clin Immunol. 2020;146:863-874).
The safety profile of Viaskin Peanut was consistent with that observed in the clinical program to date in over 1,000 study participants aged 4-11 years old. During the PEOPLE trial, the most common adverse events were mild to moderate skin reactions localized to the administration site, and there was no epinephrine use deemed related to treatment. No treatment related serious adverse events were reported. One subject experienced one case of mild anaphylaxis that was determined by the investigator to be possibly related to treatment and resolved without treatment. Treatment compliance remained high throughout the trial at a mean of 98% over three years of treatment. Low discontinuations due to adverse events were observed, with two children discontinuing the trial due to treatment- related TEAEs during PEOPLE.
10
Exploratory analyses suggest Viaskin Peanut may offer sustained effect even after a period without treatment. All participants who reached an ED ≥1,000 mg at Month 36 were eligible to continue the trial for two additional months without treatment while maintaining a peanut-free diet. A further double-blind placebo-controlled food challenge to determine ED was administered at the end of this period (Month 38). The analysis showed that 77.8% (14/18) of the children who completed the oral food challenge at Month 38 maintained desensitization with an ED ≥ 1,000 mg.
REALISE (REAL Life Use and Safety of EPIT)
In November 2016, we initiated a Phase 3 trial in peanut-allergic children four to 11 years of age designed to assess the use and safety of Viaskin Peanut 250 µg in routine clinical practice. REALISE was a multicenter, randomized 3:1, double-blind, placebo-controlled Phase 3 trial, in which pediatric peanut allergic subjects were treated with Viaskin Peanut 250 µg or placebo for six months, followed by an open-label extension period in which all participants were offered up to 36 months total of active treatment. Treatment course with Viaskin Peanut consists of a daily application of the patch on the backs of the patients.
No DBPCFCs were required for entry or during the trial, in order to replicate routine clinical practice. Subjects in the clinical trial were selected, as per clinical practice, based on a well-documented medical history of IgE- mediated reactions to peanut, including children with a history of severe anaphylaxis, along with skin and serum test results highly predictive of peanut allergy. As no DBPCFCs were required, the primary endpoint of the clinical trial was safety as measured by adverse events, treatment-emergent adverse events and serious adverse events after six months of blinded treatment. Secondary endpoints included evolution of peanut-specific serological markers over time, including IgE, IgG and skin prick test wheal. Exploratory criteria also included scores from subjects’ Food Allergy Quality of Life Questionnaire, or FAQLQ, and the Food Allergy Independent Measure, FAIM.
In March 2017, we announced the completion of enrollment in REALISE, which randomized 393 subjects in 32 centers across North America.
After the initial blinded six-month period, 97.5% of subjects in both the placebo and active arms opted into an open-label portion of the study, which continued monitoring subjects for a total of 36 months of active treatment.
Results of REALISE Trial
Results from the 6-month blinded portion of this trial were comparable with outcomes from previous trials of Viaskin Peanut 250 µg. The most commonly reported adverse events were local application site reactions, which were mostly mild and moderate in nature. No imbalance in SAEs was observed in the trial, with three cases in three patients in the active arm (1.0%) and two cases in two subjects in the placebo arm (2.0%). One case in one subject in the active arm was qualified by the investigator as moderate anaphylaxis probably related to treatment. The subject responded to standard outpatient therapy. In the six-month blinded period, the discontinuation rate was 2.5%, with a 1.0% dropout related to adverse events. The mean participant compliance was above 95%.
In November 2021, long-term results of from REALISE, including the safety of Viaskin Peanut over three years and potential impact on health-related quality of life (HRQL), were presented at the American College of Allergy, Asthma & Immunology (ACAAI) Annual Scientific Meeting.
Viaskin Peanut for Children ages 1-3
We are also developing Viaskin Peanut for the treatment of peanut allergy in toddlers one to three years of age, given the high unmet need and absence of approved treatments for this population. This program is independent from the Viaskin Peanut Program in 4–7-year-olds and uses the cVP (original patch).
11
The Viaskin Peanut program for toddlers comprises three Phase 3 clinical trials, with the intent for the trials to support a future BLA submission in this age group:
| • | EPITOPE (EPIT in Toddlers with Peanut Allergy), a randomized, two-part, pivotal Phase 3 clinical trial assessing the safety and efficacy of Viaskin Peanut for the treatment of peanut-allergic toddlers one to three years of age. |
| • | COMFORT Toddlers (Characterization of the Optimal Management of Food allergy Relief and Treatment), a supplemental safety study to bring the (total) number of subjects on active therapy close to 600 in total when combined with EPITOPE. |
| • | EPOPEX (Phase 3 Open-Label Extension to the EPITOPE Trial), a follow-up of the EPITOPE study to evaluate the long-term efficacy and safety of Viaskin Peanut in very young children, |
In August 2017, we initiated Part A of the EPITOPE (EPIT in Toddlers with Peanut Allergy) trial of Viaskin Peanut. EPITOPE is a two-part, pivotal Phase 3 clinical trial assessing the safety and efficacy of Viaskin Peanut for the treatment of peanut-allergic toddlers one to three years of age.
In September 2018, we announced that the independent data safety and monitoring board, or DSMB, completed its review of Part A of EPITOPE and recommended that the dose of Viaskin Peanut 250 µg be evaluated in Part B. On October 26, 2018, we announced that the first subject was enrolled in Part B of EPITOPE.
On June 26, 2020, we announced that in Part A, subjects in both treatment arms showed consistent treatment effect after 12 months of therapy, as assessed by a double-blind placebo-controlled food challenge and biomarker results. Part A subjects were not included in Part B and the efficacy analyses from Part A were not statistically powered to demonstrate superiority of either dose versus placebo. These results validate the ongoing investigation of the 250 µg dose in this age group, which is the dose that was studied in Part B of the study. Enrollment of Part B of EPITOPE was completed in the first quarter of 2021.
In June 2022, we announced positive topline results from Part B of EPITOPE, which enrolled 362 subjects ages 1 to 3 years, of which 244 and 118 were in the active and placebo arms, respectively. Enrollment was balanced for age and baseline disease characteristics between the active and placebo treatment arms. The median subject baseline eliciting dose (ED) was 100 mg in each treatment arm. A double-blind, placebo-controlled food challenge (DBPCFC) was administered at baseline and month 12 to determine a subject’s ED at each timepoint. A treatment responder was defined as either a subject with a baseline ED ≤10 mg who reached an ED ≥300 mg of peanut protein at month 12, or a subject with a baseline ED >10 mg and ≤300 mg who reached an ED ≥1,000 mg of peanut protein at month 12.
Viaskin Peanut demonstrated a statistically significant treatment effect (p<0.001), with 67.0% of subjects in the Viaskin Peanut arm meeting the treatment responder criteria after 12 months, as compared to 33.5% of subjects in the placebo arm (difference in response rates = 33.4%; 95% the lower bound of the 95% confidence interval (CI) for the difference in response rates between the active and placebo groups was 22.4%, exceeding the predefined threshold of 15%); left hand side chart. In addition, the proportion of subjects achieving an ED of ≥1000 mg (equivalent to approximately three peanuts) after one year of treatment with Viaskin Peanut 250 µg (VP250) was significantly increased relative to placebo (64.2% versus 29.6%; p<0.001, right hand side chart)
12
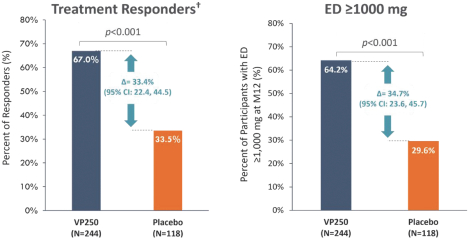
†Responder definition = If eliciting dose (ED) ≤10 mg at baseline, a subject is deemed a responder if ED ≥300 mg at M12. Alternatively, if ED >10 mg and <300 mg at baseline, a subject was deemed a responder if ED ≥1000 mg at M12.
The EPITOPE safety results were generally consistent with the safety profile of Viaskin Peanut 250 µg observed in children with peanut allergy ages 4 years and older in prior clinical trials. No imbalance in the overall adverse event (AE) rate was observed in the trial between the active and placebo arms.
Overall, 21 subjects (8.6%) in the Viaskin Peanut arm and 3 subjects (2.5%) in the placebo arm experienced a serious adverse event (SAE). Only 1 of the SAEs (0.4%), which was mild periorbital edema (swelling around the eye) in the Viaskin Peanut arm, was deemed related to treatment. The most commonly reported adverse events were skin reactions localized to the administration site, the majority of which were mild to moderate in nature.
Fifty-five subjects (22.5%) in the Viaskin Peanut arm experienced an application site reaction that was assessed as severe by an investigator compared with 10 subjects (8.5%) in the placebo arm. Based on investigators’ reported observations from examinations of the skin at each study visit, using the skin grading systems defined in the protocol, the severity of administration site skin reactions following patch application decreased throughout the course of the 12-month treatment period. Four (1.6%) subjects in the Viaskin Peanut arm experienced an anaphylactic reaction determined to be related to, or possibly related to, treatment. Among these anaphylactic reactions, 3 resolved with a single dose of epinephrine and 1 resolved without epinephrine. All anaphylactic reactions were mild to moderate in severity and were characterized mainly by skin and respiratory symptoms.
Eight subjects (3.3%) in the Viaskin Peanut arm discontinued due to adverse events. In the 12-month treatment period, the trial completion rate was 84.8% and was balanced between the Viaskin Peanut and placebo arms. Mean subject compliance to daily patch treatment was above 95% in both the active and placebo arms.
In May 2023, the EPITOPE trial results were published in the New England Journal of Medicine with an accompanying editorial article from Alkis Togias titled “Good News for Toddlers with Peanut Allergies.” The EPITOPE primary data were also presented as an oral presentation at the American College of Allergy, Asthma and Immunology (ACAAI) in November 2022 . We anticipate to perform additional analyses of the data collected from EPITOPE for further potential publication opportunities.
Supplemental Safety Study in Toddlers (COMFORT Toddlers)
In April 2023, we received pre-BLA Type B Meeting Written Responses from the FDA related to the Viaskin Peanut program in toddlers. The FDA did not request an additional efficacy study in 1-3-year-olds (i.e., the Agency agreed that the primary endpoint was satisfactorily met in DBV’s Phase 3 trial EPITOPE).
13
There was agreement with the FDA to conduct a supplemental safety study (COMFORT Toddlers) using the original square (cVP) Viaskin™ Peanut patch to augment the safety data collected from EPITOPE and have close to 600 total subjects on active treatment in the controlled safety database.
In July 2023, we received Type C Meeting Written Responses from the FDA regarding key study design elements for the COMFORT Toddlers supplemental safety study. In summary, COMFORT Toddlers will be a 6-month Double-Blind, Placebo-Controlled (DBPC) study involving approximately 400 toddlers, aged 1 through 3 years, randomized at a 3:1 ratio (active to placebo) with a 12-month open-label extension. Subsequently, in October 2023, we received feedback from the FDA addressing the remaining protocol design elements for COMFORT Toddlers. This feedback included language simplification for how the product should be used (i.e., where each epicutaneous system is intended to be worn for a full day (24 hours)). Furthermore, the key inclusion criteria for the COMFORT Toddlers study will be based on a Double-Blind, Placebo-Controlled Food Challenge (DBPCFC) performed at entry. Recruiting a study population close to EPITOPE is critical for the future BLA and aligning with the intended patient population if Viaskin Peanut is approved. We believe that an entry DBPCFC represents the best way to ensure that the optimal study population (i.e., as close to EPITOPE as possible) is enrolled. The revised protocol design of the safety study was submitted to the FDA in Q4 2023.
Interim Results from Open-label Extension to EPITOPE Study (EPOPEX)
Following the 12-month treatment period of EPITOPE, eligible subjects could opt to enroll in the open-label, extension (“OLE”) study for up to three years of active total treatment. This ongoing, open label extension to EPITOPE is known as EPOPEX and is evaluating the long-term clinical benefit of Viaskin Peanut in subjects who completed the Phase 3 EPITOPE trial. Subjects randomized to active treatment in EPITOPE could receive an additional 2-years of treatment in the OLE and subjects randomized to placebo in EPITOPE cross-over to receive 3 years of active treatment with annual double-blind placebo-controlled food challenges (DBPCFC) and safety assessments.
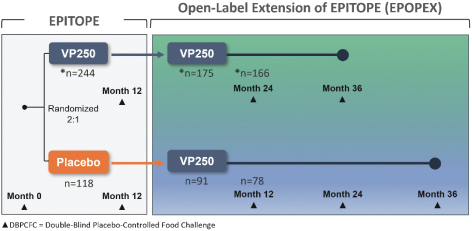
266 eligible EPITOPE participants enrolled in EPOPEX; 244 underwent the Month-24 DBPCFC (n=166 subjects treated with Viaskin Peanut 250 µg for 24 months); 78 subjects originally randomized to the placebo arm of EPITOPE who crossed-over and received active treatment with Viaskin Peanut for 1 year in the OLE. In November 2023, we announced the interim analyses from the first year of the open-label extension of EPITOPE. These data were presented at the annual American College of Allergy, Asthma, and Immunology (ACAAI) in November 2023. Using the same primary endpoint definition that was used in EPITOPE, 83.9% of subjects who completed the DBPCFC met the responder criteria after 24 months. This compares to 67% of subjects after one year of therapy. 81.3% of Viaskin Peanut subjects reached an eliciting dose (ED) of ≥1000 mg (equivalent to approximately 3 peanuts; central chart), relative to 64% after 1-year of treatment observed in EPITOPE.
14
Furthermore, following an additional year of treatment, 55.9% completed the food challenge without meeting the stopping criteria (i.e., consumed the equivalent of about 12-14 peanuts).
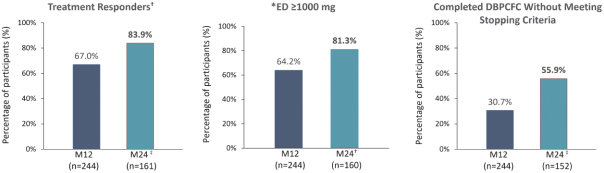
Greenhawt et al. EPOPEX, Efficacy and Safety of Epicutaneous Immunotherapy in Peanut-allergic Toddlers: 1-year Open-Label Extension to EPITOPE. Oral Presentation at ACAAI Meeting Nov 2023.
| † | Responder definition = If eliciting dose (ED) ≤10 mg at baseline, a subject was deemed a responder if ED ≥300 mg at M12. Alternatively, if ED >10 mg and <300 mg at baseline, subject was deemed a responder if ED ≥1000 mg at M12. |
| * | 100 mg = Median ED at Baseline (Month 0); *125 mg = Median dose consumed at accidental consumption of peanut (Deschildre A, et al. Clin Exp Allergy 2015; Peanut-allergic patients in the MIRABEL survey: characteristics, allergists’ dietary advice and lessons from real life. 46:610-620). |
| ‡ | Number of subjects with non-missing food challenge endpoint. |
Regarding safety and tolerability findings, no new safety signals were observed, and findings were generally similar to what was reported during the first year of treatment with Viaskin Peanut in EPITOPE. Local application site reactions continued to be the most reported adverse event, with frequency decreasing during the 2nd year of treatment. The frequency of treatment related TEAEs also decreased in year 2 relative to year 1. There were no treatment related serious TEAEs reported during the 2nd year of treatment (versus 1% in EPITOPE). As observed during the first year of treatment with Viaskin Peanut, no TEAEs led to permanent study treatment discontinuation. Finally, no treatment-related anaphylactic events were observed in the second year of treatment (compared with 1.7% of participants during the first year of treatment with Viaskin Peanut in EPITOPE). In summary, two years of VP250 in 1-3-year-old peanut-allergic toddlers resulted in continued increases in treatment effect, beyond those observed after one year, without any new safety signals.
In placebo-treated EPITOPE participants, outcomes after 12 months of cross-over to Viaskin Peanut in EPOPEX were consistent with EPITOPE treatment results: 68.0% were responders (compared to 67% of subjects on active treatment in the first year of EPITOPE); 62.7% of subjects reached an ED ≥1000 mg (relative to 64.2% in EPITOPE); 36.5% reached an ED ≥2000 mg (relative to 37% in EPITOPE); 28.4% completed the DBPCFC without meeting stopping criteria (relative to 30.7% in EPITOPE). There was 1 event of treatment-related anaphylaxis in Year 2.
We anticipate communicating the results of the Year Two results as a manuscript that is currently in preparation. In addition, We anticipate that Month 36 results will become available in the second half of 2024 and we anticipate additional analyses of that data will be performed.
Viaskin Peanut for Children ages 4-7
We will evaluate the modified (circular) Viaskin Peanut patch in children ages 4-7 years with peanut allergy in two Phase 3 clinical trials with the intent for the trials to support a future BLA submission in this age group.
15
VITESSE (Viaskin Peanut Immunotherapy Trial to Evaluate Safety, Simplicity and Efficacy)
On September 7, 2022, we announced the initiation of VITESSE, a new Phase 3 pivotal study of the modified Viaskin Peanut (mVP) patch in children ages 4-7 years with peanut allergy. We defined initiation as the submission of the trial protocol to selected study sites for subsequent Institutional Review Board (IRB) approval and Ethics Committee (EC) opinion.
On September 21, 2022, we announced we had received feedback from the FDA in the form of a partial clinical hold on VITESSE. In the partial clinical hold letter, the FDA specified changes to elements of the VITESSE protocol, acknowledging the intent for the trial to support a future BLA submission. In the following months, we engaged with the FDA to address the feedback provided in the partial clinical hold letter and to finalize the VITESSE protocol. In addition, we continued internal preparations for VITESSE and conducted certain site assessment and start-up activities for prompt study launch once the partial clinical hold was lifted.
On December 23, 2022, we announced the FDA lifted the partial clinical hold and confirmed we satisfactorily addressed all clinical hold issues. The FDA stated that VITESSE may proceed with the revised trial protocol.
On March 7, 2023, the Company announced screening of the first subject in VITESSE. Screening of the last subject was anticipated in the first half of 2024, and topline results are anticipated in the first half of 2025.
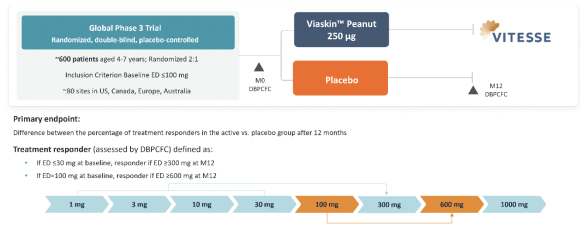
We expect to enroll 600 subjects for participation in the VITESSE study, randomized 2:1 active to placebo. The primary efficacy endpoint is the percentage of treatment responders in the active versus placebo arms at month 12. The primary efficacy analysis includes the success criterion of the lower bound of the confidence interval of the difference in responder rates between active and placebo groups being greater than or equal to 15%.
A treatment responder is defined as either a subject with a baseline eliciting dose (ED) ≤30 mg who reaches an ED ≥300 mg of peanut protein at month 12, or a subject with a baseline ED = 100 mg who reaches an ED ≥600 mg of peanut protein at month 12. A double-blind, placebo-controlled food challenge (DBPCFC) will be administered at baseline and month 12 to determine a subject’s ED at both timepoints. We defined the peanut protein sensitivity inclusion criteria to align with peanut allergy patients at the greatest risk of experiencing reactions to accidental peanut ingestion and with the highest unmet need. We added a 600 mg dose of peanut protein to the month 12 DBPCFC to increase the sensitivity of the efficacy assessment.
Participants will apply the modified patch (either Viaskin Peanut 250 µg or a placebo) daily for a period of 12 months. The maximum study duration per subject is 58 weeks: a four-week screening period, a 12-month treatment period and a two-week follow-up period. During the screening period, subjects will undergo an initial screening visit with assessment for eligibility according to peanut skin prick test (SPT) and serum peanut IgE.
16
Those meeting these criteria will proceed to a peanut DBPCFC to confirm their peanut allergy and establish an entry peanut ED. The entry DBPCFC will be 1 mg peanut protein, and will escalate up to a highest single dose of 100 mg peanut protein. Subjects who react with an ED at or below the dose of 100 mg peanut protein are considered eligible. At month 12, a post-treatment DBPCFC will be performed, with a starting dose of 3 mg peanut protein, escalating to a highest dose of 1,000 mg peanut protein according to the following schedule: 3, 10, 30, 100, 300, 600, 1,000 mg. Secondary efficacy endpoints include changes in Cumulative Reactive Dose, ED and severity of allergic reaction at baseline and month 12 food challenge. VITESSE will also evaluate the safety of the modified Viaskin Peanut patch based on overall adverse events, local site reactions and systemic allergic reactions.
The VITESSE Instructions for Use (IFU) will direct caregivers to apply one patch at approximately the same time each day, following removal of the previous day’s patch. The updated IFU now outlines that Viaskin Peanut 250 µg is to be worn for as close to a full day as possible (i.e., 24 hours) with a minimum daily wear time of 20 hours each day.
Patch adhesion will be assessed in VITESSE to affirm the modified Viaskin Peanut patch performs adequately, which aligns with existing regulatory requirements for patch-based therapies. In post-PCH discussions, we agreed with the FDA that a statistical test of adhesion will be included in the VITESSE statistical analysis plan and further considered patch adhesion data collection and interpretation in the context of the novel nature of the Viaskin patch platform.
We initiated subject screening for VITESSE in Q1 2023 (the first subject was screened in February 2023 and randomized in March) and anticipate that the last patient will be screened by Q3 2024.
Supplemental Safety Study in children ages 4-7 years with peanut allergy
In 2024, we plan to initiate a supplemental safety study (COMFORT Children) in peanut-allergic children aged 4-7 years. COMFORT Children comprises a 6-month, randomized, double-blind, placebo-controlled period followed by a 12-month, open-label, single-arm active treatment period. The additional safety data generated by the 6-month DBPC study will supplement the safety data generated by the VITESSE trial, resulting in a controlled safety database close to 600 children (total) aged 4 to 7 years treated with Viaskin Peanut.
In July 2023, we received Type C Meeting Written Responses from the FDA regarding key study design elements for COMFORT Children. In summary, there was an agreement with the Agency that COMFORT Children will be a Double-Blind, Placebo-Controlled study involving approximately 270 children, randomized at a 3:1 ratio (active to placebo). Participation will not necessitate a food challenge, and patch adhesion data will be generated using the same approach as previously agreed upon with the FDA for the VITESSE phase 3 study.
Subsequently, in October 2023, we received feedback from the FDA addressing the remaining protocol design elements for COMFORT Children. This feedback included language simplification for how Viaskin should be used. Furthermore, the key inclusion criteria for the COMFORT Children study will be based on a physician-diagnosed peanut allergy, peanut-specific IgE and a Skin Prick Test (with no requirement for a DBPCFC). The revised protocol design of the safety study was submitted to the FDA in Q4 2023. COMFORT Children is anticipated to be initiated towards the end of VITESSE enrollment. We intend that enrollment of the COMFORT Children safety study will be strategically timed to avoid competition with the VITESSE study for the same subjects.
Viaskin Milk
Our second product candidate, Viaskin Milk, is in development for the treatment of cow’s milk protein allergy, (IgE-mediated) or CMPA, in children two to 17 years of age, and received fast track designation from the FDA in September 2016.
17
In November 2014, we initiated a multi-center, double-blind, placebo-controlled, randomized Phase 1/2 dose-finding trial to study the safety and efficacy of Viaskin Milk in 198 subjects with Immunoglobulin E, or IgE, mediated CMPA, which we refer to as the Milk Efficacy and Safety, or MILES, trial. The MILES (Milk Efficacy and Safety) clinical trial was designed to determine a safe and effective dose in two age groups: children ages two to 11 and adolescents ages 12 to 17. In June 2015, we announced completion of Part A of the MILES study, or Phase 1, for which the DSMB recommended to continue the trial as planned and did not raise any safety concerns, and we launched Part B, or Phase 2, in October 2015.
In February 2018, we announced topline results from Part B of the MILES study. Following analyses of the data, the 300 µg dose of Viaskin Milk was identified as the dose with the greatest observed clinical activity for children (intent-to-treat, or ITT, p=0.042). We believe these results support further advancement of the Viaskin Milk program, and we intend to discuss findings with regulatory authorities to determine the design of future clinical trial.
Other Applications for the Viaskin Platform
In addition to our development programs in food allergies, we have also explored the use of our Viaskin technology for the treatment of inflammatory and autoimmune diseases with high unmet medical need. Human proof-of-concept trials have been conducted with Viaskin in EoE and as a booster vaccination against Bordetella pertussis, or whooping cough, in healthy adults. Our other earlier stage research programs have included vaccination for respiratory syncytial virus (RSV), as well as potential treatments for inflammatory bowel disease (IBD), celiac disease and type I diabetes.
Diagnostic Tool Development
In May 2016, we entered into a Development Collaboration and License Agreement (the “Collaboration Agreement”) with Société des Produits Nestlé S.A. (formerly NESTEC S.A.) (“NESTEC”). The Collaboration Agreement related to an exclusive global collaboration with Nestlé Health Science for the development and, if approved, commercialization of MAG1C, a ready-to-use and standardized atopy patch test tool for the diagnosis of CMPA (non-mediated IgE) in infants.
Under the terms of the Collaboration Agreement, the Company was responsible for leading the development activities of MAG1C up through a pivotal Phase 3 clinical program, and if the appropriate regulatory approvals were received, Nestlé Health Science would support the commercialization of MAG1C globally. The Company was eligible to receive up to €100.0 million in potential development, clinical, regulatory and commercial milestones, including an upfront payment of €10.0 million received in July 2016.
On October 30, 2023, the Company and NESTEC entered into a Mutual Termination Letter Agreement terminating the Collaboration Agreement. Each party remains responsible for its own costs and expenses related to its respective wind-down activities. Any and all licenses and sublicenses, granted by either party to the other party under the Collaboration Agreement, including, without limitation, any licenses to intellectual property, were revoked and terminated.
We may explore selective collaborations with parties who have relevant clinical and commercial expertise in other geographies, including certain European countries, and indications outside of food allergies.
Potential Biomarker Applications
We are continuing to explore other cellular mechanisms modulated by EPIT, such as biomarkers, in collaboration with external companies and academic institutions in both the United States and EU. We believe that with improved knowledge about the evolution of immunological biomarkers and epigenetic modulation, we may be able to determine the level of patient response earlier during treatment, ensure follow-up and measure tolerance maintained once treatment is completed.
18
At the 2016 EAACI meeting in Vienna, Austria, we presented initial findings from some of these collaborations, which suggest that proprietary biomarker modeling may be used to help monitor patient responses to Viaskin Peanut. Additional research is planned to further strengthen the results of these early findings.
Manufacturing and Supply
Our Proprietary Viaskin Technology
We have engineered a proprietary manufacturing technology for Viaskin patch, which is designed to comply with the most stringent pharmaceutical production standards, including those promulgated by the FDA, in order to enable Viaskin to deliver proteins via intact skin. This novel pharmaceutical process, which was fully developed by us, uses an electrospray to spray homogeneous, thin, dry protein layers onto the Viaskin patch.
This process sprays a liquid solution of electrically charged proteins onto the patch’s backing, which is then turned into dry solid charged layers, which remain stuck onto the patch’s backing. It deposits very small and precise quantities of the active substance, devoid of adjuvants. The patch can then be stored at room temperature. We believe this patented technology is highly scalable and complies with cGMP requirements.
The principles of the Viaskin electrospray technology are the following:
| • | A constant flow of liquid in a capillary is subjected to a high voltage electric field. |
| • | With our electrospray machine, we can transform these electrically charged liquid droplets into dry solid layers, deposited onto the patch’s backing. |
| • | The electric field directs particles precisely toward the Viaskin patch’s backing. |
With Viaskin manufacturing technology, we believe we can achieve:
| • | a homogeneous layer of protein on the Viaskin patch; |
| • | a specific mass of active substance per Viaskin patch; |
| • | an adjustable active substance dosage for clinical trials; |
| • | instant drying of the active substance; |
| • | a high solubility of the active substance; and |
| • | the possibility of spraying on the Viaskin patch both biological and chemical substances. |
Viaskin is a Highly Scalable Manufacturing Technology
We currently rely on a single contract manufacturer to manufacture and supply the active pharmaceutical ingredients (“API”) used in our Viaskin product candidates. On February 1, 2018, we entered into a Master API Supply Agreement with Sanofi which sets forth the terms and conditions governing the manufacture and supply of peanut, milk and egg API to be used in our Viaskin product candidates. The agreement expires on a Viaskin product basis five years after the first date of regulatory approval in any jurisdiction of the applicable Viaskin product candidate and requires us to purchase at least 75% of our required API from Sanofi.
19
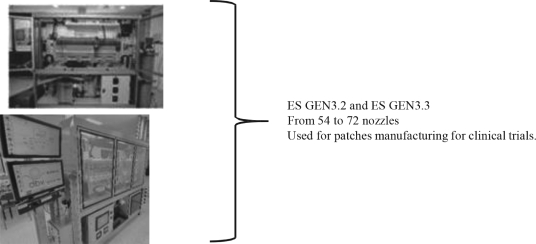
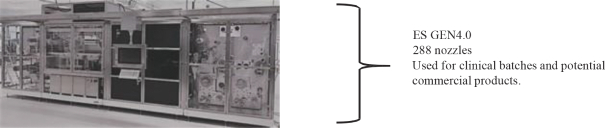
We believe our proprietary Viaskin manufacturing technology creates high barriers to entry to our line of business, particularly in the engineering and manufacturing of our Viaskin product candidates. We have designed, developed, and built our manufacturing tools, and contract third- party manufacturers to operate it.
We currently rely on a single contract manufacturer, FAREVA Amboise (“FAREVA”), to manufacture and supply clinical and commercial batches of Viaskin Peanut patches. We have entered into a Development Services Agreement, dated August 1, 2015, as amended (the “Development Agreement”), with FAREVA setting forth the terms and conditions whereby DBV selected FAREVA as its contract manufacturing organization to implement the Viaskin production process and to manufacture and supply to DBV batches of finished product for validation and clinical purposes. We have also entered into a Commercial Supply Agreement, dated January 13, 2020, as amended (the “Commercial Supply Agreement”), with FAREVA setting forth the terms and conditions for the manufacture and supply of commercial batches of Viaskin Peanut by FAREVA. We have agreed with FAREVA to delay implementation of the Commercial Supply Agreement through December 31, 2024, unless we, at our option, decide to reinstate the Commercial Supply Agreement sooner.
Intellectual Property
Our patent portfolio includes pending patent applications and issued patents in the United States and in foreign countries. To date, patents directed to the Viaskin electrostatic patch, as well as allergen desensitization methods, have been issued in the major markets, including in particular the United States, Europe, Canada and Australia. We also have extensive know-how and trade secrets covering part of the Viaskin patch manufacturing method using electrospray technology.
These patents and applications generally fall into five broad categories:
| • | two U.S. patents, which we own, relating to the Viaskin electrostatic patch and its use, which expired in 2022; |
20
| • | patents and patent applications which we own relating to our electrospray method of manufacturing the Viaskin electrostatic patch, which may expire as early as 2029; |
| • | patents and patent applications we co-own with Assistance-Publique-Hôpitaux de Paris, or AP-HP, and the Université Paris Cité (formerly Université de Paris-Descartes, prior to merger and name change) relating to the treatment of peanut, milk, egg, and other allergies using our Viaskin patch technology, which may expire as early as 2028; |
| • | design patents and patent applications, which we own relating to various components of the Viaskin patch, which may expire as early as 2038; and |
| • | a variety of other patent applications that we own or co-own relating, for example, to prophylactic uses of the Viaskin patch technology and to treatment of other indications using the Viaskin patch technology. |
U.S. Patent Term Extension and Marketing Exclusivity
Depending upon the timing, duration, and specifics of the FDA approval of our product candidates, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent extension of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. Accordingly, if the remaining patent term has fourteen (14) or more years after the FDA approval date, the patent would not be eligible for any patent extension.
The amount of time by which a patent term may be extended is generally one-half the time between the effective date of an IND submission and the submission date of a BLA plus the time between the submission date of a BLA and the FDA’s approval of that application, except that the review period is reduced by any time during which the applicant failed to exercise due diligence. Only one patent applicable to an approved drug is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent, and within 60 days of the FDA’s approval of the product. The U.S. Patent and Trademark Office, or USPTO, in consultation with the FDA, reviews and approves the application for any patent term extension. In the future, we may apply for extension of patent term for our currently owned or licensed patents to add patent life beyond its current expiration date, depending on the expected length of the clinical trials and other factors involved in the filing of the relevant BLA. Some foreign jurisdictions have analogous patent term extension provisions that allow for extension of the term of a patent that covers a device approved by the applicable foreign regulatory agency. In the future, if a Viaskin patch receives FDA approval, we expect to apply for a patent term extension on the patent that we believe will provide the best exclusivity position if extended.
An abbreviated approval pathway for biological products shown to be similar to, or interchangeable with, an FDA-licensed reference biological product was created by the Biologics Price Competition and Innovation Act of 2009, or BPCIA. Biosimilarity, which requires that the biological product be highly similar to the reference product notwithstanding minor differences in clinically inactive components and that there be no clinically meaningful differences between the product and the reference product in terms of safety, purity, and potency, which can be shown through analytical studies, animal studies, and a clinical trial or trials. Interchangeability requires that a biological product be biosimilar to the reference product and the product can be expected to produce the same clinical results as the reference product in any given patient and, for products administered multiple times, the product and the reference product may be alternated or switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biological product. A reference biological product is granted twelve years of exclusivity from the time of first licensure of the product, and the FDA will not accept an application for a biosimilar or interchangeable product based on the reference biological product until four years after the date of first licensure. “First licensure” typically means the initial date the particular product at issue was licensed in the United States.
21
This does not include a supplement for the biological product or a subsequent application by the same sponsor or manufacturer of the biological product (or licensor, predecessor in interest, or other related entity) for a change that results in a new indication, route of administration, dosing schedule, dosage form, delivery system, delivery device, or strength, unless that change is a modification to the structure of the biological product and such modification changes its safety, purity, or potency. Whether a subsequent application, if approved, warrants exclusivity as the “first licensure” of a biological product is determined on a case-by-case basis with data submitted by the sponsor.
Pediatric exclusivity is another type of regulatory market exclusivity in the United States. Pediatric exclusivity, if granted, adds six months to existing exclusivity periods and patent terms. This six-month exclusivity, which runs from the end of other exclusivity protection or patent term, may be granted based on the voluntary completion of a pediatric trial in accordance with an FDA-issued “Written Request” for such a trial.
Co-Ownership Agreement
AP-HP and Université Paris Cité (formerly known as Université de Paris-Descartes)
In December 2008, we entered into an assignment, development and co-ownership agreement with AP-HP and Université Paris-Descartes, which through a merger and a name change became Université Paris Cité, by which we agreed to terms of co-ownership with AP-HP and Université Paris Cité of certain U.S. and foreign patents and patent applications, referred to herein as the shared patents. We, and any licensees or sublicensees that we designate, have the exclusive right to commercial uses of the shared patents. AP-HP and Université Paris Cité agreed to use the shared patents only for internal research purposes and not to license the shared patents to any third party. Upon commercialization of any product covered by the shared patents, which we expect would include our Viaskin product candidates, we will be obligated to pay AP-HP and Université Paris Cité a percentage of net sales as a royalty. This royalty is in the low single digits and varies depending on the particular patent used in the product. Additionally, if we license any of the shared patents to a third party and a licensee commercializes products covered by such shared patents, we will be obligated to pay AP-HP and Université Paris Cité a percentage in the low single digits of the money that we receive from our licensee.
If we do not sell any of our product candidates covered by the shared patents within 30 months from the date we first market such product candidates, AP-HP may, upon six months’ notice and subject to certain exceptions, convert our exclusive right to the commercial use of the shared patents to a non-exclusive right.
Any party may terminate the assignment, development and co-ownership agreement in the event of another party’s substantial breach which remains uncured after six months of receiving written notice of such breach. The agreement will also terminate in the event we cease operations or are subject to a dissolution or bankruptcy proceedings.
Absent early termination, the assignment, development and co-ownership agreement will automatically terminate upon the expiration of the last shared patent. In the event the agreement is terminated, we would no longer have the exclusive right to commercial use of the shared patents, though we would retain our shared ownership rights. In addition, our ownership stake in certain jointly made improvements covered by the shared patents would survive termination of the agreement. The longest-lived patent rights under the agreement are currently expected to expire in 2031, absent patent term extension.
Competition
The biotechnology and pharmaceutical industries are highly competitive and subject to significant and rapid change as researchers learn more about diseases and develop new technologies and treatments.
22
Differentiating competitive factors in the pharmaceutical industry include product efficacy and safety; quality and breadth of an organization’s technology; skill of an organization’s employees and its ability to recruit and retain key employees; timing and scope of regulatory approvals; government reimbursement rates for, and the average selling price of, products; the availability of raw materials and qualified manufacturing capacity; manufacturing and distribution costs; intellectual property and patent rights and their protection; and sales and marketing capabilities.
Our competitors may succeed in obtaining FDA or other regulatory approvals for their product candidates more rapidly than we are able to do, which could place us at a significant competitive disadvantage. Market acceptance of our product candidates will depend on a number of factors, including: (1) potential advantages over existing or alternative therapies or tests; (2) the actual or perceived safety and efficacy of similar classes of products; (3) the effectiveness of selling, marketing, and distribution capabilities; and (4) the scope of any approval provided by the FDA or comparable foreign regulatory authorities.
Although we believe our product candidates possess attractive attributes, we cannot assure you that our product candidate will achieve regulatory or market acceptance, or that we will be able to compete effectively in the biopharmaceutical drug markets. If our product candidates fail to gain regulatory approvals and acceptance in their intended markets, we may not generate meaningful revenues or achieve profitability.
Numerous pharmaceutical and biotechnology companies, universities and other research entities are actively involved in the discovery, development and commercialization of therapeutic options to treat allergies. There are competitors in the food allergy space that have greater resources and experience than we do.
We are aware of several food allergy studies and pharmaceutical developmental efforts connected with such studies that are currently being conducted in major medical centers and hospitals worldwide. These studies are evaluating forms of allergen desensitization treatments such as oral, or OIT; sublingual, or SLIT; subcutaneous, or SCIT; oral mucosal, or OMIT; and cutaneuos or intranasal immunotherapy, synthetic, denatured allergens, small molecule inhibitors, or combinations of medicines or methods.
Studies combining methods of allergen immunotherapy, such as OIT, with monoclonal antibodies also are being conducted currently. These types of co-administrations may significantly improve the safety of specific allergen immunotherapies administered orally or subcutaneously. In addition, the use of monoclonal antibodies as monotherapy for certain food allergies, including peanut allergy, is being studied in clinical trials. Monoclonal antibodies, used alone or in combination with allergen immunotherapy, may become significant competitors to our products.
There is one treatment for peanut allergy approved by the FDA and the European Commission: Palforzia, a formulation of peanut flour developed by Aimmune Therapeutics, Inc., or Aimmune. Nestlé S.A. acquired Aimmune in October 2020 and divested the Palforzia business to Stallergenes Greer in September 2023. In addition, Xolair (omalizumab) is approved by the FDA for the reduction of allergic reactions including reducing the risk of anaphylaxis, that may occur with accidental exposure to one or more food allergens, including peanut. Omalizumab is an anti-immunoglobulin E (IgE) monoclonal antibody that is administered via subcutaneous injection. The prescribing information for both Palforzia and Xolair indicate patients should continue to avoid all foods to which they are allergic.
Government Regulation
Government authorities in the United States at the federal, state and local level and in other countries extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing and export and import of drug and biological products, or biologics, such as our product candidates. Generally, before a new drug or biologic can be marketed, considerable data demonstrating its quality, safety and efficacy must be obtained, organized into a format specific to each regulatory authority, submitted for review and approved by the regulatory authority.
23
U.S. Biological Product Development
In the United States, the FDA regulates biologics under the Federal Food, Drug, and Cosmetic Act, or FDCA, and the Public Health Service Act, or PHSA, and their implementing regulations. Biologics are also subject to other federal, state and local statutes and regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to administrative or judicial sanctions. These sanctions could include, among other actions, the FDA’s refusal to approve pending applications, withdrawal of an approval, a clinical hold, untitled or warning letters, product recalls or withdrawals from the market, product seizures, total or partial suspension of production or distribution injunctions, fines, refusals of government contracts, restitution, disgorgement, or civil or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on us.
Our product candidates must be approved by the FDA through the BLA process before they may be legally marketed in the United States. The process required by the FDA before a biologic may be marketed in the United States generally involves the following:
| • | completion of extensive nonclinical, sometimes referred to as pre-clinical laboratory tests, pre-clinical animal studies and formulation studies in accordance with applicable regulations, including the FDA’s Good Laboratory Practice, or GLP, regulations; |
| • | submission to the FDA of an IND, which must become effective before human clinical trials may begin; |
| • | performance of adequate and well-controlled human clinical trials in accordance with applicable IND and other clinical trial-related regulations, sometimes referred to as good clinical practices, or GCPs, to establish the safety and efficacy of the proposed product candidate for its proposed indication; |
| • | submission to the FDA of a BLA; |
| • | satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities where the product is produced to assess compliance with the FDA’s current good manufacturing practice, or cGMP, requirements to assure that the facilities, methods and controls are adequate to preserve the product’s identity, strength, quality, purity and potency; |
| • | potential FDA audit of the pre-clinical and/or clinical trial sites that generated the data in support of the BLA; and |
| • | FDA review and approval of the BLA prior to any commercial marketing or sale of the product in the United States. |
The data required to support a BLA is generated in two distinct development stages: pre-clinical and clinical. The pre-clinical development stage generally involves laboratory evaluations of drug chemistry, formulation and stability, as well as studies to evaluate toxicity in animals, which support subsequent clinical testing. The conduct of the pre-clinical studies must comply with federal regulations, including GLPs. The sponsor must submit the results of the pre-clinical studies, together with manufacturing information, analytical data, any available clinical data or literature and a proposed clinical protocol, to the FDA as part of the IND. An IND is a request for authorization from the FDA to administer an investigational drug product to humans. The central focus of an IND submission is on the general investigational plan and the protocol(s) for human trials. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA raises concerns or questions regarding the proposed clinical trials and places the IND on clinical hold within that 30-day time period. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. The FDA may also impose clinical holds on a product candidate at any time before or during clinical trials due to safety concerns or regulatory non-compliance. Accordingly, we cannot be sure that submission of an IND will result in the FDA allowing clinical trials to begin, or that, once begun, issues will not arise that could cause the trial to be suspended or terminated.
24
The clinical stage of development involves the administration of the product candidate to healthy volunteers or disease-affected subjects under the supervision of qualified investigators, generally physicians not employed by or under the trial sponsor’s control, in accordance with GCPs, which include the requirement that all research subjects provide their informed consent for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria, and the parameters to be used to monitor subject safety and assess efficacy. Each protocol, and any subsequent amendments to such protocol, must be submitted to the FDA as part of the IND. Further, each clinical trial must be reviewed and approved by an independent institutional review board, or IRB, at or servicing each institution at which the clinical trial will be conducted. An IRB is charged with protecting the welfare and rights of trial participants and considers such items as whether the risks to participants in a clinical trial are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the informed consent form that must be provided to each clinical trial subject or his or her legal representative and must monitor the clinical trial until completed. There are also requirements governing the reporting of ongoing clinical trials and completed clinical trial results to public registries. Sponsors of certain clinical trials of FDA-regulated products, including biologics, are required to register and publicly disclose specified clinical trial information on www.clinicaltrials.gov. Information related to the product candidate, patient population, phase of investigation, study sites and investigators, and other aspects of the clinical trial is then made public as part of the registration. Sponsors are also obligated to disclose the results of their clinical trials after completion.
Clinical trials are generally conducted in three sequential phases that may overlap, known as Phase 1, Phase 2 and Phase 3 clinical trials. Phase 1 clinical trials generally involve a small number of healthy volunteers who are initially exposed to a single dose and then multiple doses of the product candidate. The primary purpose of these clinical trials is to assess the metabolism, pharmacologic action, side effect tolerability and safety of the product candidate and, if possible, to gain early evidence on effectiveness. Phase 2 clinical trials, if Phase 1 trials do not reveal unacceptable toxicity, typically involve studies in disease-affected patients to determine the dose required to produce the desired benefits. At the same time, safety and further pharmacokinetic and pharmacodynamic information is collected, as well as identification of possible adverse effects and safety risks and preliminary evaluation of efficacy. Phase 3 clinical trials generally involve large numbers of subjects at multiple sites, in multiple countries (from several hundred to several thousand subjects) and are designed to provide the data necessary to demonstrate the efficacy of the product candidate for its intended use, its safety in use, and to establish the overall benefit/risk relationship of the product candidate and provide an adequate basis for product approval. Phase 3 clinical trials may include comparisons with placebo and/or other comparator treatments. The duration of treatment is often extended to mimic the actual use of a product during marketing. Generally, the FDA requires two adequate and well-controlled Phase 3 clinical trials for approval of a BLA.
Post-approval trials, sometimes referred to as Phase 4 clinical trials, may be conducted after initial marketing approval. These trials are used to gather information about a product candidate’s safety, efficacy, and optimal use from the treatment of subjects in the intended therapeutic indication. In certain instances, FDA may condition approval of a BLA on the sponsor’s agreement to conduct additional clinical trials to further assess the biologic’s safety and effectiveness after BLA approval.
Progress reports detailing the results of a clinical trial must be submitted periodically to the FDA. Written IND safety reports must be submitted to the FDA and the investigators for serious and unexpected suspected adverse, findings from other studies suggesting a significant risk to humans exposed to the drug, findings from animal or in vitro testing suggesting a significant risk to humans, and any clinically important rate increase of a serious suspected adverse reaction over that listed in the protocol or investigator brochure. Phase 1, Phase 2 and Phase 3 clinical trials may not be completed successfully within any specified period, if at all. The FDA, the IRB, or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk.
25
Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the product has been associated with unexpected serious harm to subjects or patients. Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group provides authorization for whether or not a trial may move forward at designated intervals based on access to certain data from the trial. A sponsor may also suspend or terminate a clinical trial based on evolving business objectives and/or competitive climate.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the product candidate as well as finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, must develop methods for testing the identity, strength, quality and purity of the final product. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
BLA and FDA Review Process
Following completion of a clinical trial, data generated from such trial is analyzed to assess safety and efficacy. The results of pre-clinical studies and clinical trials are then submitted to the FDA as part of a BLA, along with proposed labeling for the product candidate and information about the manufacturing process and facilities that will be used to ensure product quality, results of analytical testing conducted on the chemistry of the product candidate, and other relevant information. The BLA is a request for approval to market a biologic product for one or more specified indications and must contain proof of safety, purity, potency and efficacy, which is demonstrated by extensive pre-clinical and clinical testing. The application includes both negative or ambiguous results of pre-clinical and clinical trials and positive findings. Data may come from company-sponsored clinical trials intended to test the safety and efficacy of a use of a product, or from a number of alternative sources, including studies initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and efficacy of the investigational product to the satisfaction of the FDA. FDA approval of a BLA must be obtained before a biologic product may be marketed in the United States.
Under the Prescription Drug User Fee Act, or PDUFA, as amended, each BLA must be accompanied by a significant user fee, which is adjusted on an annual basis. PDUFA also imposes an annual program fee for approved drugs. Fee waivers or reductions may be available in certain circumstances, including a waiver of the application fee for the first application filed by a small business.
Once a BLA has been accepted for filing, which occurs, if at all, sixty days after the BLA’s submission, the FDA’s goal is to review such BLA within ten months of the filing date for standard review or six months of the filing date for priority review (if granted by the FDA), if the application is for a product intended for a serious or life-threatening condition and the product, if approved, would provide a significant improvement in safety or effectiveness. The review process is often significantly extended by FDA requests for additional information or clarification. If not accepted for filing, the sponsor must resubmit the BLA and begin the FDA’s review process again, including the initial sixty-day review to determine if the application is sufficiently complete to permit substantive review.
After the BLA submission is accepted for filing, the FDA reviews the BLA to determine, among other things, whether the proposed product candidate is safe and effective for its intended use, and whether the product candidate is being manufactured in accordance with cGMP to assure and preserve the product candidate’s identity, strength, quality, purity and potency. The FDA may refer applications for novel drug product candidates or drug product candidates which present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and under what conditions.
26
The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions. The FDA will likely re-analyze the clinical trial data, which could result in extensive discussions between the FDA and the sponsor during the review process. The review and evaluation of a BLA by the FDA is extensive and time consuming and may take longer than originally planned to complete, and the sponsor may not receive a timely approval, if at all.
Before approving a BLA, the FDA will conduct a pre-approval inspection of the manufacturing facilities for the product candidate to determine whether they comply with cGMPs. The FDA will not approve the product candidate unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and are adequate to assure consistent production of the product candidate within required specifications. In addition, before approving a BLA, the FDA may also audit data from clinical trials to ensure compliance with GCP requirements. After the FDA evaluates the application, manufacturing process and manufacturing facilities, it may issue an approval letter or a Complete Response Letter. An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications. A Complete Response Letter indicates that the review cycle of the application is complete and the application will not be approved in its present form. A Complete Response Letter usually describes the specific deficiencies in the BLA identified by the FDA. The Complete Response Letter may require additional clinical data and/or an additional pivotal Phase 3 clinical trial(s), and/or other significant and time-consuming requirements related to clinical trials, pre-clinical studies or manufacturing. If a Complete Response Letter is issued, the applicant may either resubmit the BLA, addressing all of the deficiencies identified in the letter, or withdraw the application. Even if such data and information is submitted, the FDA may ultimately decide that the BLA does not satisfy the criteria for approval. Data obtained from clinical trials are not always conclusive and the FDA may interpret data differently than a sponsor interprets the same data.
There is no assurance that the FDA will ultimately approve a product for marketing in the United States and a sponsor may encounter significant difficulties or costs during the review process. If a product receives marketing approval, the approval may be significantly limited to specific populations, severities of allergies, and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings or precautions be included in the product labeling or may condition the approval of the BLA on other changes to the proposed labeling, development of adequate controls and specifications, or a commitment to conduct post-market testing or clinical trials and surveillance to monitor the effects of approved products. For example, the FDA may require Phase 4 testing which involves clinical trials designed to further assess the product’s safety and effectiveness and may require testing and surveillance programs to monitor the safety of approved products that have been commercialized. The FDA may also place other conditions on approvals including the requirement for a Risk Evaluation and Mitigation Strategy, or REMS, to assure the safe use of the product. If the FDA concludes a REMS is needed, the sponsor of the BLA must submit a proposed REMS. The FDA will not approve the BLA without an approved REMS, if required. A REMS could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. Any of these limitations on approval or marketing could restrict the commercial promotion, distribution, prescription or dispensing of products. Product approvals may be withdrawn for non-compliance with regulatory standards or if problems occur following initial marketing.
Review and Approval of Combination Products in the United States
Certain products may be comprised of components that would normally be regulated under different types of regulatory authorities, and frequently by different centers at the FDA. These products are known as combination products. Specifically, under regulations issued by the FDA, a combination product may be:
| • | a product comprised of two or more regulated components that are physically, chemically, or otherwise combined or mixed and produced as a single entity; |
27
| • | two or more separate products packaged together in a single package or as a unit and comprised of drug and device products; |
| • | a drug, device, or biological product packaged separately that according to its investigational plan or proposed labeling is intended for use only with an approved individually specified drug, device or biological where both are required to achieve the intended use, indication, or effect and where upon approval of the proposed product the labeling of the approved product would need to be changed, e.g., to reflect a change in intended use, dosage form, strength, route of administration, or significant change in dose; or |
| • | any investigational drug, device, or biological packaged separately that according to its proposed labeling is for use only with another individually specified investigational drug, device, or biological product where both are required to achieve the intended use, indication, or effect. |
Our Viaskin product candidates are combination products comprising a device for delivery of a biologic product. Under the FDCA, the FDA is charged with assigning a center with primary jurisdiction, or a lead center, for review of a combination product. That determination is based on the “primary mode of action” of the combination product, which means the mode of action expected to make the greatest contribution to the overall intended therapeutic effects. Thus, if the primary mode of action of a device-biologic combination product is attributable to the biologic product, that is, if it acts by means of a virus, therapeutic serum, toxin, antitoxin, vaccine, blood, blood component or derivative, allergenic product, or analogous product, the FDA center responsible for premarket review of the biologic product would have primary jurisdiction for the combination product.
Expedited Development and Review Programs
The FDA has a fast track program that is intended to expedite or facilitate the process for reviewing new drugs and biological products that meet certain criteria. Specifically, new drugs and biological products are eligible for fast track designation if they are intended to treat a serious or life-threatening condition and nonclinical or clinical data demonstrate the potential to address an unmet medical need. Fast track designation applies to the combination of the product and the specific indication for which it is being studied. The sponsor of a new drug or biologic may request the FDA to designate the drug or biologic as a fast track product concurrently with the submission of an IND or at any time before a pre-NDA meeting, and the FDA must determine if the product qualifies for fast track designation within 60 days of receipt of the sponsor’s request. Unique to a fast track product, the FDA may consider for review sections of the marketing application on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the application, the FDA agrees to accept sections of the application and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the application.
Any product submitted to the FDA for marketing, including under a fast track program, may be eligible for other types of FDA programs intended to expedite development and review, such as priority review and accelerated approval. Any product is eligible for priority review, or review within a six-month timeframe from the date a complete BLA is accepted for filing, if it treats a serious condition and has the potential to provide a significant improvement in safety or effectiveness. The FDA will attempt to direct additional resources to the evaluation of an application for a new drug or biological product designated for priority review in an effort to facilitate the review.
Additionally, a product may be eligible for accelerated approval. Drug or biological products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval, which means that they may be approved on the basis of adequate and well-controlled clinical trials establishing that the product has an effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit, or on the basis of an effect on a clinical endpoint
other than survival or irreversible morbidity. As a condition of approval, the FDA may require that a sponsor of a drug or biological product receiving accelerated approval perform adequate and well-controlled post-marketing clinical trials.
28
If the FDA concludes that a drug shown to be effective can be safely used only if distribution or use is restricted, it will require such post-marketing restrictions as it deems necessary to assure safe use of the drug, such as:
| • | distribution restricted to certain facilities or physicians with special training or experience; or |
| • | distribution conditioned on the performance of specified medical procedures. |
The limitations imposed would be commensurate with the specific safety concerns presented by the product. In addition, the FDA currently requires pre-approval of promotional materials as a condition for accelerated approval, which could adversely impact the timing of the commercial launch of the product. Fast track designation, priority review and accelerated approval do not change the standards for approval but may expedite the development or approval process.
Breakthrough Designation
The Food and Drug Administration Safety and Innovation Act, or FDASIA, amended the FDCA to require the FDA to expedite the development and review of a breakthrough therapy. A product can be designated as a breakthrough therapy if it is intended to treat a serious or life-threatening condition and preliminary clinical evidence indicates that it may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. A sponsor may request that a product candidate be designated as a breakthrough therapy concurrently with the submission of an IND or any time before an end-of-Phase-II meeting, and the FDA must determine if the product candidate qualifies for breakthrough therapy designation within 60 days of receipt of the sponsor’s request. If so designated, the FDA shall act to expedite the development and review of the product’s marketing application, including by meeting with the sponsor throughout the product’s development, providing timely advice to the sponsor to ensure that the development program to gather pre-clinical and clinical data is as efficient as practicable, involving senior managers and experienced review staff in a cross-disciplinary review, assigning a cross-disciplinary project lead for the FDA review team to facilitate an efficient review of the development program and to serve as a scientific liaison between the review team and the sponsor, and taking steps to ensure that the design of the clinical trials is as efficient as practicable.
Pediatric Trials
Under the Pediatric Research Equity Act, or PREA, a BLA or supplement to a BLA must contain data to assess the safety and efficacy of the product for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. FDASIA requires that a sponsor who is planning to submit a marketing application for a drug or biological product that includes a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration submit an initial Pediatric Study Plan, or PSP, within sixty days of an end-of-Phase 2 meeting or as may be agreed between the sponsor and FDA. The initial PSP must include an outline of the pediatric study or studies that the sponsor plans to conduct, including study objectives and design, age groups, relevant endpoints and statistical approach, or a justification for not including such detailed information, and any request for a deferral of pediatric assessments or a full or partial waiver of the requirement to provide data from pediatric studies along with supporting information. FDA and the sponsor must reach agreement on the PSP. A sponsor can submit amendments to an agreed-upon initial PSP at any time if changes to the pediatric plan need to be considered based on data collected from nonclinical studies, early phase clinical trials, and/or other clinical development programs. The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of data or full or partial waivers.
29
Post-Marketing Requirements
Following approval of a new product, a manufacturer and the approved product are subject to continuing regulation by the FDA, including, among other things, monitoring and recordkeeping activities, reporting to the applicable regulatory authorities of adverse experiences with the product, providing the regulatory authorities with updated safety and efficacy information, product sampling and distribution requirements, and complying with promotion and advertising requirements, which include, among others, standards for direct-to-consumer advertising, restrictions on promoting products for uses or in patient populations that are not described in the product’s approved labeling, also known as off-label use, limitations on industry-sponsored scientific and educational activities, and requirements for promotional activities involving the internet. Although physicians may prescribe legally available drugs and biologics for off-label uses, manufacturers may not market or promote such off-label uses. Modifications or enhancements to the product or its labeling or changes of the site of manufacture are often subject to the approval of the FDA and other regulators, which may or may not be received or may result in a lengthy review process. Prescription drug promotional materials must be submitted to the FDA in conjunction with their first use. Any distribution of prescription drug products and pharmaceutical samples must comply with the U.S. Prescription Drug Marketing Act, or the PDMA, a part of the FDCA.
In the United States, once a product is approved, its manufacture is subject to comprehensive and continuing regulation by the FDA. The FDA regulations require that products be manufactured in specific approved facilities and in accordance with cGMP. Moreover, the constituent parts of a combination product retain their regulatory status, for example, as a biologic or device, and as such, we may be subject to additional requirements in the Quality System Regulation, or QSR, applicable to medical devices, such as design controls, purchasing controls, and corrective and preventive action. We rely, and expect to continue to rely, on third parties for the production of clinical and commercial quantities of our products in accordance with cGMP regulations. cGMP regulations require, among other things, quality control and quality assurance as well as the corresponding maintenance of records and documentation and the obligation to investigate and correct any deviations from cGMP. Manufacturers and other entities involved in the manufacture and distribution of approved products are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance. These regulations also impose certain organizational, procedural and documentation requirements with respect to manufacturing and quality assurance activities. BLA holders using contract manufacturers, laboratories or packagers are responsible for the selection and monitoring of qualified firms, and, in certain circumstances, qualified suppliers to these firms. These firms and, where applicable, their suppliers are subject to inspections by the FDA at any time, and the discovery of violative conditions, including failure to conform to cGMP, could result in enforcement actions that interrupt the operation of any such facilities or the ability to distribute products manufactured, processed or tested by them. Discovery of problems with a product after approval may result in restrictions on a product, manufacturer, or holder of an approved BLA, including, among other things, recall or withdrawal of the product from the market.
The FDA also may require post-approval testing, sometimes referred to as Phase 4 testing, REMS and post-marketing surveillance to monitor the effects of an approved product or place conditions on an approval that could restrict the distribution or use of the product. Discovery of previously unknown problems with a product or the failure to comply with applicable FDA requirements can have negative consequences, including adverse publicity, judicial or administrative enforcement, warning letters from the FDA, mandated corrective advertising or communications with doctors, and civil or criminal penalties, among others. Newly discovered or developed safety or effectiveness data may require changes to a product’s approved labeling, including the addition of new warnings and contraindications, and also may require the implementation of other risk management measures. Also, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our products under development.
30
Other Regulatory Matters
Manufacturing, sales, promotion and other activities following product approval are also subject to regulation by numerous regulatory authorities in addition to the FDA, including the Centers for Medicare & Medicaid Services, or CMS, other divisions of the Department of Health and Human Services, the Drug Enforcement Administration, the Consumer Product Safety Commission, the Federal Trade Commission, the Occupational Safety & Health Administration, the Environmental Protection Agency and state and local governments. In the United States, sales, marketing and scientific/educational programs, among other activities, must also comply with state and federal fraud and abuse laws, data privacy and security laws, transparency laws, and pricing and reimbursement requirements in connection with governmental payor programs, among others. The handling of any controlled substances must comply with the U.S. Controlled Substances Act and Controlled Substances Import and Export Act. Products must meet applicable child-resistant packaging requirements under the U.S. Poison Prevention Packaging Act. Manufacturing, sales, promotion and other activities are also potentially subject to federal and state consumer protection and unfair competition laws.
The distribution of pharmaceutical products is subject to additional requirements and regulations, including extensive record-keeping, licensing, storage and security requirements intended to prevent the unauthorized sale of pharmaceutical products.
The failure to comply with regulatory requirements subjects firms to possible legal or regulatory action. Depending on the circumstances, failure to meet applicable regulatory requirements can result in civil, criminal and administrative penalties, damages, fines, disgorgement, injunctions, recall or seizure of products, total or partial suspension of production, denial or withdrawal of product approvals, the exclusion from participation in federal and state healthcare programs or refusal to allow a firm to enter into supply contracts, including government contracts, integrity obligations and individual imprisonment. In addition, even if a firm complies with FDA and other requirements, new information regarding the safety or efficacy of a product could lead the FDA to modify or withdraw product approval. Prohibitions or restrictions on sales or withdrawal of future products marketed by us could materially affect our business in an adverse way. Changes in regulations, statutes or the interpretation of existing regulations could impact our business in the future by requiring, for example: (i) changes to our manufacturing arrangements; (ii) additions or modifications to product labeling; (iii) the recall or discontinuation of our products; or (iv) additional record-keeping requirements. If any such changes were to be imposed, they could adversely affect the operation of our business.
European Union Drug Development
In the European Union, or the EU, product candidates may also be subject to extensive regulatory requirements. Approval from the competent authorities of EU Member States must be obtained before commencing clinical trials. In addition, as in the United States, medicinal products can only be marketed if a marketing authorization from the competent regulatory authorities has been obtained.
Clinical Trials in the EU
Similar to the United States, the various phases of pre-clinical and clinical research in the EU are subject to significant regulatory controls. Certain preclinical (also termed “non-clinical”) data is required in order to enable clinical trials and later to be used in a dossier for a marketing authorization application. The requisite amount of preclinical data enables the design of a clinical trial, from Phase 1 (first-in-human clinical trials) through to Phases 2 and 3, which are quality, safety and efficacy studies. During all phases of clinical development, national competent authorities of EU Member States and other comparable regulatory authorities require extensive monitoring and auditing of all clinical activities, clinical data and clinical trial investigators.
In the EU, clinical trials are governed by the Clinical Trials Regulation (EU) No 536/2014, or CTR, which entered into application on January 31, 2022, repealing and replacing the Clinical Trials Directive 2001/20, or CTD. Clinical trials of medicinal products in the EU must be conducted in accordance with EU and national regulations governing clinical trials, including the Good Clinical Practice Directive 2005/28. The CTR is intended to harmonize and streamline clinical trial authorizations, simplify adverse-event reporting procedures, improve the supervision of clinical trials and increase transparency.
31
Prior to commencing a clinical trial, the sponsor must obtain a clinical trial authorization from competent authorities of EU Member States in which the sponsor intends on carrying out clinical trials, and a positive opinion from an independent Ethics Committee. The CTR, which is directly applicable in all EU Member States, introduces a streamlined application procedure through a single-entry point, the “EU portal”, the Clinical Trials Information System, or CTIS. Since January 31, 2023, the use of CTIS has become mandatory for all clinical trial sponsors submitting initial applications for the approval of their clinical trials in the EU. The CTR also establishes a single set of documents to be prepared and submitted for the application including, among other things, a copy of the trial protocol and an investigational medicinal product dossier containing information about the manufacture and quality of the medicinal product under investigation, as well as simplified reporting procedures for clinical trial sponsors.
A harmonized procedure for the assessment of applications for clinical trials has been introduced and is divided into two parts. Part I assessment is led by the competent authorities of a reporting EU Member State selected by the trial sponsor and relates to clinical trial aspects that are considered to be scientifically harmonized across EU Member States. This assessment is then submitted to the competent authorities of all the concerned EU Member States in which the trial is to be conducted for their review. Part II is assessed separately by the competent authorities and Ethics Committees in each concerned EU Member State. Each concerned EU Member State will issue a single decision on the authorization of the clinical trial including input from the national competent authority and Ethics Committee. Individual EU Member States, therefore, retain the power to authorize the conduct of clinical trials in their territory.
The CTR establishes a general principle according to which information contained in CTIS shall be made publicly accessible unless confidentiality is justified on grounds of necessary to protect personal data, or commercially confidential information, necessary to protect confidential communications between EU Member States in relation to the preparation of an assessment report, or necessary to ensure effective supervision of the conduct of a clinical trial by EU Member States. The confidentiality exception may be overruled if there is an overriding public interest in disclosure. The publication of data and documents in relation to the conduct of a clinical trial will take place in accordance with specific timelines. The timelines are established by the European Medicines Agency, or EMA, and are determined based on the documents and the categorization of the clinical trial.
The CTR includes a three-year transition period. The extent to which on-going clinical trials will be governed by the CTR varies. For clinical trials in relation to which an application for approval was made on the basis of the CTD before January 31, 2023, the CTD will continue to apply on a transitional basis until January 31, 2025. By that date, all ongoing trials will become subject to the provisions of the CTR. The CTR will apply to clinical trials from an earlier date if the related clinical trial application was made on the basis of the CTR or if the clinical trial has already transitioned to the CTR framework before January 31, 2025.
In all cases, clinical trials must be conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki. Medicines used in clinical trials, including advanced therapy medicinal products, or ATMPs, must be manufactured in accordance with the guidelines on cGMP and in a GMP licensed facility, which can be subject to GMP inspections.
European Union Drug Review and Approval
In the European Economic Area, or EEA, which is comprised of the 27 Member States of the EU plus Norway, Iceland and Liechtenstein, medicinal products can only be commercialized after obtaining a Marketing Authorization, or MA.
32
To obtain a MA for a product in the EEA, an applicant must submit a Marketing Authorization Application, or MAA either under a centralized procedure administered by the EMA or one of the procedures administered by competent authorities in the EU Member States (decentralized procedure, national procedure or mutual recognition procedure). An MA may be granted only to an applicant established in the EU.
The centralized procedure provides for the grant of a single MA by the European Commission that is valid for all EU Member States. Pursuant to Regulation (EC) No 726/2004, the centralized procedure is compulsory for specific products, including for (i) medicinal products derived from biotechnological processes, (ii) products designated as orphan medicinal products, (iii) ATMPs, and (iv) products with a new active substance indicated for the treatment of HIV/AIDS, cancer, neurodegenerative diseases, diabetes, auto immune and other immune dysfunctions and viral diseases. For products with a new active substance indicated for the treatment of other diseases and products that are highly innovative or for which a centralized process is in the interest of patients, authorization through the centralized procedure is optional on related approval.
Under the centralized procedure, the EMA’s Committee for Medicinal Products for Human Use, or CHMP, conducts the initial assessment of a product. The CHMP is also responsible for several post-authorization and maintenance activities, such as the assessment of modifications or extensions to an existing MA.
Under the centralized procedure in the EU, the maximum timeframe for the evaluation of an MAA is 210 days, excluding clock stops when additional information or written or oral explanation is to be provided by the applicant in response to questions of the CHMP. Accelerated assessment may be granted by the CHMP in exceptional cases, when a medicinal product targeting an unmet medical need is expected to be of major interest from the point of view of public health and, in particular, from the viewpoint of therapeutic innovation. If the CHMP accepts a request for accelerated assessment, the time limit of 210 days will be reduced to 150 days (excluding clock stops). The CHMP can, however, revert to the standard time limit for the centralized procedure if it considers that it is no longer appropriate to conduct an accelerated assessment.
Unlike the centralized authorization procedure, the decentralized MA procedure requires a separate application to, and leads to separate approval by, the competent authorities of each EU Member State in which the product is to be marketed. This application is identical to the application that would be submitted to the EMA for authorization through the centralized procedure. The reference EU Member State prepares a draft assessment and drafts of the related materials within 120 days after receipt of a valid application. The resulting assessment report is submitted to the concerned EU Member States who, within 90 days of receipt, must decide whether to approve the assessment report and related materials. If a concerned EU Member State cannot approve the assessment report and related materials due to concerns relating to a potential serious risk to public health, disputed elements may be referred to the Heads of Medicines Agencies’ Coordination Group for Mutual Recognition and Decentralised Procedures – Human, or CMDh, for review. The subsequent decision of the European Commission is binding on all EU Member States.
The mutual recognition procedure allows companies that have a medicinal product already authorized in one EU Member State to apply for this authorization to be recognized by the competent authorities in other EU Member States. Like the decentralized procedure, the mutual recognition procedure is based on the acceptance by the competent authorities of the EU Member States of the MA of a medicinal product by the competent authorities of other EU Member States. The holder of a national MA may submit an application to the competent authority of an EU Member State requesting that this authority recognize the MA delivered by the competent authority of another EU Member State.
An MA has, in principle, an initial validity of five years. The MA may be renewed after five years on the basis of a re-evaluation of the risk-benefit balance by the EMA or by the competent authority of the EU Member State in which the original MA was granted. To support the application, the MA holder must provide the EMA or the competent authority with a consolidated version of the eCTD (Common Technical Document) providing up-to-date data concerning the quality, safety and efficacy of the product, including all variations introduced since the MA was granted, at least nine months before the MA ceases to be valid.
33
The European Commission or the competent authorities of the EU Member States may decide on justified grounds relating to pharmacovigilance, to proceed with one further five-year renewal period for the MA. Once subsequently definitively renewed, the MA shall be valid for an unlimited period. Any authorization which is not followed by the actual placing of the medicinal product on the EU market (for a centralized MA) or on the market of the authorizing EU Member State within three years after authorization ceases to be valid (the so-called sunset clause).
Innovative products that target an unmet medical need and are expected to be of major public health interest may be eligible for a number of expedited development and review programs, such as the Priority Medicines, or PRIME, scheme, which provides incentives similar to the breakthrough therapy designation in the U.S. PRIME is a voluntary scheme aimed at enhancing the EMA’s support for the development of medicinal products that target unmet medical needs. Eligible products must target conditions for which there is an unmet medical need (there is no satisfactory method of diagnosis, prevention or treatment in the EU or, if there is, the new medicinal product will bring a major therapeutic advantage) and they must demonstrate the potential to address the unmet medical need by introducing new methods of therapy or improving existing ones. Benefits accrue to sponsors of product candidates with PRIME designation, including but not limited to, early and proactive regulatory dialogue with the EMA, frequent discussions on clinical trial designs and other development program elements, and potentially accelerated MAA assessment once a dossier has been submitted.
In the EU, a “conditional” MA may be granted in cases where all the required safety and efficacy data are not yet available. The European Commission may grant a conditional MA for a medicinal product if it is demonstrated that all of the following criteria are met: (i) the benefit-risk balance of the medicinal product is positive; (ii) it is likely that the applicant will be able to provide comprehensive data post-authorization; (iii) the medicinal product fulfils an unmet medical need; and (iv) the benefit of the immediate availability to patients of the medicinal product is greater than the risk inherent in the fact that additional data are still required. The conditional MA is subject to conditions to be fulfilled for generating the missing data or ensuring increased safety measures. It is valid for one year and must be renewed annually until all related conditions have been fulfilled. Once any pending studies are provided, the conditional MA can be converted into a traditional MA. However, if the conditions are not fulfilled within the timeframe set by the EMA and approved by the European Commission, the MA will cease to be renewed.
An MA may also be granted “under exceptional circumstances” where the applicant can show that it is unable to provide comprehensive data on efficacy and safety under normal conditions of use even after the product has been authorized and subject to specific procedures being introduced. These circumstances may arise in particular when the intended indications are very rare and, in the state of scientific knowledge at that time, it is not possible to provide comprehensive information, or when generating data may be contrary to generally accepted ethical principles. Like a conditional MA, an MA granted in exceptional circumstances is reserved to medicinal products intended to be authorized for treatment of rare diseases or unmet medical needs for which the applicant does not hold a complete data set that is required for the grant of a standard MA. However, unlike the conditional MA, an applicant for authorization in exceptional circumstances is not subsequently required to provide the missing data. Although the MA “under exceptional circumstances” is granted definitively, the risk-benefit balance of the medicinal product is reviewed annually, and the MA will be withdrawn if the risk-benefit ratio is no longer favorable.
Pediatric Development
In the EU, Regulation (EC) No 1901/2006 provides that all MAAs for new medicinal products have to include the results of trials conducted in the pediatric population, in compliance with a pediatric investigation plan, or PIP, agreed with the EMA’s Pediatric Committee, or PDCO. The PIP sets out the timing and measures proposed to generate data to support a pediatric indication of the medicinal product for which the MA is being sought. The PDCO can grant a deferral of the obligation to implement some or all of the measures provided in the PIP until there are sufficient data to demonstrate the efficacy and safety of the product in adults.
34
Further, the obligation to provide pediatric clinical trial data can be waived by the PDCO when these data are not needed or appropriate because the product is likely to be ineffective or unsafe in children, the disease or condition for which the product is intended occurs only in adult populations, or when the product does not represent a significant therapeutic benefit over existing treatments for pediatric patients. Once the MA is obtained in all EU Member States and study results are included in the product information, even when negative, the product is eligible for a six-month extension to the Supplementary Protection Certificate, or SPC, if any is in effect at the time of authorization or, in the case of orphan medicinal products, a two-year extension of orphan market exclusivity.
Data and Market Exclusivity
The EU provides opportunities for data and market exclusivity related to MAs. Upon receiving an MA, innovative medicinal products are generally entitled to receive eight years of data exclusivity and 10 years of market exclusivity. Data exclusivity prevents regulatory authorities in the EU from referencing the innovator’s data to assess a generic application or biosimilar application for eight years from the date of authorization of the innovative product, after which a generic or biosimilar MAA can be submitted, and the innovator’s data may be referenced. The market exclusivity period prevents a successful generic or biosimilar applicant from commercializing its product in the EU until 10 years have elapsed from the initial MA of the reference product in the EU. The overall ten-year period may, occasionally, be extended for a further year to a maximum of 11 years if, during the first eight years of those ten years, the MA holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. However, there is no guarantee that a product will be considered by the EU’s regulatory authorities to be a new chemical/biological entity, and products may not qualify for data exclusivity.
In the EU, there is a special regime for biosimilars, or biological medicinal products that are similar to a reference medicinal product but that do not meet the definition of a generic medicinal product. For such products, the results of appropriate preclinical or clinical trials must be provided in support of an application for MA. Guidelines from the EMA detail the type of quantity of supplementary data to be provided for different types of biological product.
Post-approval Requirements
Where an MA is granted in relation to a medicinal product in the EU, the holder of the MA is required to comply with a range of regulatory requirements applicable to the manufacturing, marketing, promotion and sale of medicinal products. Similar to the United States, both MA holders and manufacturers of medicinal products are subject to comprehensive regulatory oversight by the EMA, the European Commission and/or the competent regulatory authorities of the individual EU Member States. The holder of an MA must establish and maintain a pharmacovigilance system and appoint an individual qualified person for pharmacovigilance who is responsible for oversight of that system. Key obligations include expedited reporting of suspected serious adverse reactions and submission of periodic safety update reports, or PSURs.
All new MAAs must include a risk management plan, or RMP, describing the risk management system that the company will put in place and documenting measures to prevent or minimize the risks associated with the product. The regulatory authorities may also impose specific obligations as a condition of the MA. Such risk- minimization measures or post-authorization obligations may include additional safety monitoring, more frequent submission of PSURs, or the conduct of additional clinical trials or post-authorization safety studies.
In the EU, the advertising and promotion of medicinal products are subject to both EU and EU Member States’ laws governing promotion of medicinal products, interactions with physicians and other healthcare professionals, misleading and comparative advertising and unfair commercial practices. Although general requirements for advertising and promotion of medicinal products are established under EU legislation, the details are governed by regulations in individual EU Member States and can differ from one country to another.
35
For example, applicable laws require that promotional materials and advertising in relation to medicinal products comply with the product’s Summary of Product Characteristics, or SmPC, as approved by the competent authorities in connection with an MA. The SmPC is the document that provides information to physicians concerning the safe and effective use of the product. Promotional activity that does not comply with the SmPC is considered off-label and is prohibited in the EU. Direct-to-consumer advertising of prescription medicinal products is also prohibited in the EU.
Combination Products
The EU regulates medical devices and medicinal products separately, and through different legislative instruments. Products that are a combination of a medicinal product and a medical device may be regulated as either a medicinal product, a medical device or, subject to certain requirements, on the basis of both sets of rules. The applicable requirements governing placing a drug-device combination on the EU market will vary depending on the type of drug-device combination product and on which of the components of the combination has the primary mode of action.
Drug-device combination products that form a single integral product that is not reusable and for which the action of the medicinal product is principal to that of the medical device are governed by the regulatory framework applicable to medicinal products. However, the General Safety and Performance Requirements, or GSPRs, of Annex I to Regulation (EU) 2017/745 on Medical Devices, or MDR, will be applicable to the safety and performance of the medical device part of the product in the context of its use with the medicinal product. In these circumstances, an MAA must be submitted to the competent authorities responsible for evaluating the safety and effectiveness of medicinal products. As part of the MAA, the applicant must also submit, where available, the results of the assessment of the conformity of the medical device part of the product with the MDR contained in the manufacturer’s EU Declaration of Conformity of the device or the relevant Certificate of Conformity issued by a Notified Body. If the MAA does not include the results of the conformity assessment, and where the conformity assessment of the device, if used separately, requires the involvement of a Notified Body, the competent authorities must require the applicant to provide a Notified Body Opinion on the conformity of the device with the relevant GSPRs. Based on this approach, the competent authorities responsible for medicinal products will review the specific aspects of the medical devices part of the product which are relevant to the safety and efficacy of the medicinal product and the Notified Body – where applicable – will evaluate the relevant GSPRs of the device.
Drug-device combination products that form a single integral product that is not reusable and for which the action of the medicinal products is ancillary to that of the medical device are governed by the regulatory framework applicable to MDR. However, the quality, safety and usefulness of the medicinal product must also be verified as part of the device and a scientific opinion from a national competent authority of an EU Member State or from the EMA, depending on its nature and therapeutic intention, must be sought regarding the quality and safety of the medicinal product, including the benefit or risk of its incorporation into the medical device. Where the primary mode of action of the combined product comes from the medicinal product , it is regulated as a medicinal product. In this case, the medicinal product should also be compliant with regulation (EU) 2017/745 and particularly the article 117. This article requires a Notified Body opinion on the conformity of the device part to the relevant General Safety and Performance Requirements, or GSPRs of the MDR.
Other Regulatory Matters
UK Regulations
The United Kingdom’s, or UK, withdrawal from the EU on January 31, 2020, commonly referred to as Brexit, has changed the regulatory relationship between the UK and the EU. The Medicines and Healthcare products Regulatory Agency, or MHRA, is now the UK’s standalone regulator for medicinal products and medical devices.
36
Great Britain (England, Scotland and Wales) is now a third country to the EU. Northern Ireland will, with regard to EU regulations, continue to follow the EU regulatory rules for now.
The UK regulatory framework in relation to clinical trials is governed by the Medicines for Human Use (Clinical Trials) Regulations 2004, as amended, which is derived from the CTD, as implemented into UK national law through secondary legislation. On January 17, 2022, the MHRA launched an eight-week consultation on reframing the UK legislation for clinical trials, and which aimed to streamline clinical trials approvals, enable innovation, enhance clinical trials transparency, enable greater risk proportionality, and promote patient and public involvement in clinical trials. The UK Government published its response to the consultation on March 21, 2023 confirming that it would bring forward changes to the legislation. These resulting legislative amendments will determine how closely the UK regulations will align with the CTR. In October 2023, the MHRA announced a new Notification Scheme for clinical trials which enables a more streamlined and risk-proportionate approach to initial clinical trial applications for Phase 4 and low-risk Phase 3 clinical trial applications.
Marketing authorizations in the UK are governed by the Human Medicines Regulations (SI 2012/1916), as amended. Since January 1, 2021, an applicant for the EU centralized procedure marketing authorization can no longer be established in the UK. As a result, since this date, companies established in the UK cannot use the EU centralized procedure and instead must follow one of the UK national authorization procedures or one of the remaining post-Brexit international cooperation procedures to obtain an marketing authorization to market products in the UK. All existing EU marketing authorizations for centrally authorized products were automatically converted or grandfathered into UK marketing authorization, effective in Great Britain only, free of charge on January 1, 2021, unless the marketing authorization holder opted-out of this possibility. Northern Ireland currently remains within the scope of EU authorizations in relation to centrally authorized medicinal products. Accordingly, until the Windsor Framework is implemented in Northern Ireland on January 1, 2025, products falling within the scope of the EU centralized procedure can only be authorized through UK national authorization procedures in Great Britain.
The MHRA has also introduced changes to national marketing authorization procedures. This includes introduction of procedures to prioritize access to new medicines that will benefit patients, including a 150-day assessment route, a rolling review procedure and the International Recognition Procedures which entered into application on January 1, 2024. Since January 1, 2024, the MHRA may also rely on the International Recognition Procedure, or IRP, when reviewing certain types of marketing authorization applications. This procedure is available for applicants for marketing authorization who have already received an authorization for the same product from a reference regulator. These include the FDA, the EMA, and national competent authorities of individual EEA countries. A positive opinion from the EMA and CHMP, or a positive end of procedure outcome from the mutual recognition or decentralized procedures are considered to be authorizations for the purposes of the IRP.
There is no pre-marketing authorization orphan designation for medicinal products in the UK. Instead, the MHRA reviews applications for orphan designation in parallel to the corresponding marketing authorization application. The criteria are essentially the same as those in the EU, but have been tailored for the market. This includes the criterion that prevalence of the condition in Great Britain, rather than the EU, must not be more than five in 10,000. Upon the grant of a marketing authorization with orphan status, the medicinal product will benefit from up to 10 years of market exclusivity from similar products in the approved orphan indication. The start of this market exclusivity period will be set from the date of first approval of the product in Great Britain.
Reimbursement and Reform
Significant uncertainty exists as to the coverage and reimbursement status of any product candidates for which we or our collaborators obtain regulatory approval. Sales of our products will depend, in part, on the extent to which our products, once approved, will be covered and reimbursed by third-party payors, such as government health programs, commercial insurance and managed healthcare organizations.
37
These third-party payors are increasingly reducing reimbursements for medical products and services. The process for determining whether a third-party payor will provide coverage for a drug product typically is separate from the process for setting the price of a drug product or for establishing the reimbursement rate that a payor will pay for the drug product once coverage is approved. Third-party payors may limit coverage to specific drug products on an approved list, also known as a formulary, which might not include all of the FDA approved drugs for a particular indication.
In order to secure coverage and reimbursement for any product candidate that might be approved for sale, sponsors may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost- effectiveness of the product candidate, in addition to the costs required to obtain FDA or other comparable regulatory approvals. Whether or not we conduct such studies, our product candidates may not be considered medically necessary or cost-effective. A third-party payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Further, one payor’s determination to provide coverage for a product does not assure that other payors will also provide coverage, and adequate reimbursement, for the product. Third party reimbursement may not be sufficient to enable us to maintain price levels high enough to realize an appropriate return on our investment in product development.
The containment of healthcare costs has become a priority of federal and state governments, and the prices of drugs have been a focus in this effort. The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost- containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. Decreases in third-party reimbursement for our product candidate or a decision by a third-party payor to not cover our product candidate could reduce physician usage of the product candidate and have a material adverse effect on our sales, results of operations and financial condition.
For example, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or, collectively, the ACA, was enacted in March 2010 and continues to significantly impact the health care industry. The ACA was expansive health reform legislation designed to expand coverage for the uninsured while at the same time containing overall healthcare costs enhance remedies against fraud and abuse, add new transparency requirements for health care and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms, and other changes. With regard to biopharmaceutical products, among other things, the ACA expanded and increased industry rebates for drugs covered under Medicaid programs and made changes to the coverage requirements under the Medicare Part D program. However, there have been executive, judicial and Congressional challenges to certain aspects of the ACA. For example, on June 17, 2021 the U.S. Supreme Court dismissed a challenge on procedural grounds that argued the ACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress. On August 16, 2022, President Biden signed the Inflation Reduction Act of 2022, or IRA, into law, which, among other things, extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025. The IRA also eliminates the “donut hole” under the Medicare Part D program beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost and creating a new manufacturer discount program. It is possible that the ACA will be subject to judicial or Congressional challenges in the future. It is unclear how such challenges and the healthcare reform measures of the Biden administration will impact the ACA.
In addition, other legislative changes have been proposed and adopted in the United States since the ACA was enacted. On August 2, 2011, the Budget Control Act of 2011 among other things, created measures for spending reductions by Congress. The Joint Select Committee on Deficit Reduction was tasked with recommending to Congress proposals in spending reductions. Because they did not achieve a targeted deficit reduction of at least $1.2 trillion for fiscal years 2012 through 2021, it triggered the legislation’s automatic reduction to several government programs.
38
This includes aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, which went into effect in April 2013 and, due to subsequent legislative amendments, including the Infrastructure Investment and Jobs Act, will stay in effect until 2032,unless additional Congressional action is taken. On January 2, 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, or the ATRA, which among other things, also reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. We expect that additional federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, and in turn could significantly reduce the projected value of certain development projects and reduce our profitability.
Additionally, in the United States, there have been several recent Congressional inquiries and federal and state legislative activity designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. At the federal level, in July 2021, the Biden administration released an executive order “Promoting Competition in the American Economy,” with multiple provisions aimed at prescription drugs. In response to Biden’s executive order, on September 9, 2021, the U.S. Department of Health and Human Services, or HHS, released a Comprehensive Plan for Addressing High Drug Prices that outlines principles for drug pricing reform and sets out a variety of potential legislative policies that Congress could pursue as well as potential administrative actions HHS can take to advance these principles. In addition, the IRA, among other things, (i) directs HHS to negotiate the price of certain high-expenditure, single-source drugs and biologics covered under Medicare, and subject drug manufacturers to civil monetary penalties and a potential excise tax by offering a price that is not equal to or less than the negotiated “maximum fair price” for such drugs and biologics under the law, and (ii) imposes rebates with respect to certain drugs and biologics covered under Medicare Part B or Medicare Part D to penalize price increases that outpace inflation. These provisions take effect progressively starting in fiscal year 2023. On August 29, 2023, HHS announced the list of the first ten drugs that will be subject to price negotiations, although the Medicare drug price negotiation program is currently subject to legal challenges. It is currently unclear how the IRA will be implemented but is likely to have a significant impact on the pharmaceutical industry. In response to the Biden administration’s October 2022 executive order, on February 14, 2023, HHS released a report outlining three new models for testing by the CMS Innovation Center which will be evaluated on their ability to lower the cost of drugs, promote accessibility, and improve quality of care. It is unclear whether the models will be utilized in any health reform measures in the future. Further, on December 7, 2023, the Biden administration announced an initiative to control the price of prescription drugs through the use of march-in rights under the Bayh-Dole Act. On December 8, 2023, the National Institute of Standards and Technology published for comment a Draft Interagency Guidance Framework for Considering the Exercise of March-In Rights which for the first time includes the price of a product as one factor an agency can use when deciding to exercise march-in rights. While march-in rights have not previously been exercised, it isuncertain if that will continue under the new framework. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
Additional legislative proposals to reform healthcare and government insurance programs, along with the trend toward managed healthcare in the United States, could influence the purchase of medicines and reduce demand and prices for our products, if approved. This could harm our or our collaborators’ ability to market any products and generate revenues. Cost containment measures that healthcare payors and providers are instituting and the effect of further healthcare reform could significantly reduce potential revenues from the sale of any of our product candidates approved in the future, and could cause an increase in our compliance, manufacturing, or other operating expenses.
39
In addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, the European Union provides options for its Member States to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. An EU Member State may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. In France, for example, effective access to the market can be achieved either at a free price, decided by the pharmaceutical company, or with a system of cover/reimbursement with a price regulated by the authorities. In this case, the future products must be included, for coverage by hospitals, on the list of proprietary medicinal products approved for use by local authorities and various public services (known as the “Liste Collectivités”) (Article L. 5123-2 of the Public Health Code) or included on the list of proprietary medicinal products reimbursable to insured persons (known as the “Liste Sécurité Sociale”) for reimbursement by the Social Security system (Article L. 162-17 of the Social Security Code).
Indeed, in France, the manufacturer’s price excluding tax of medicines reimbursable to insured persons (registered on the Social Security List) is the subject of a multi-year agreement negotiated between each pharmaceutical company and the Economic Committee for Health Products, or CEPS (failing this, by unilateral decision of the CEPS). A framework agreement has been concluded between LEEM (the trade union representing the pharmaceutical industries) and CEPS. The last framework agreement was signed on March 5, 2021 and has a three-year term. In addition, the transfer prices of medicines on the Sus List and the Retrocession List are also set by agreement between the operating laboratory and the CEPS.
There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our product candidates. Historically, products launched in the European Union do not follow price structures of the United States and generally tend to be significantly lower.
Other Healthcare Laws and Compliance Requirements
Our business operations in the United States and our arrangements with clinical investigators, healthcare providers, consultants, third-party payors and patients may expose us to broadly applicable federal, state, and foreign fraud and abuse and other healthcare laws. These laws may impact, among other things, our research, proposed sales, marketing and education programs of our product candidates that obtain regulatory approval. The healthcare laws and regulations that may affect our ability to operate include, among others:
| • | the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration (including any kickback, bribe or rebate), directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, lease, order, or recommendation of, an item, good, facility or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs. Although there are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution, the exceptions and safe harbors are drawn narrowly. Practices that involve remuneration that may be alleged to be intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exception or safe harbor. The intent standard under the federal Anti-Kickback Statute was amended by the ACA to a stricter standard such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. Moreover, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act; |
| • | federal civil and criminal false claims laws, including the federal civil False Claims Act, which impose penalties and provide for civil whistleblower or qui tam actions, and civil monetary penalty laws, which prohibit, among other things, knowingly presenting, or causing to be presented, claims for |
40
| payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent, or making a false statement or record material to payment of a false claim or avoiding, decreasing, or concealing an obligation to pay money to the federal government, including for example, providing inaccurate billing or coding information to customers or promoting a product off-label; |
| • | HIPAA, which created additional federal criminal statutes that prohibit knowingly and willfully executing or attempting to execute a scheme to defraud any healthcare benefit program, knowingly and willfully falsifying, concealing or covering up a material fact or making false statements relating to healthcare matters, knowingly and willfully embezzling or stealing from a healthcare benefit program, or willfully obstructing a criminal investigation of a healthcare offense. Similar to the federal Anti- Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation; |
| • | the federal Physician Payments Sunshine Act, enacted as part of the ACA, which requires applicable manufacturers of covered drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to track and annually report to CMS payments and other transfers of value provided to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), other healthcare professionals (such as physician assistants and nurse practitioners), and teaching hospitals and information regarding certain ownership and investment interests held by physicians or their immediate family members; |
| • | federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and its implementing regulations, which imposes certain requirements on covered entities and their business associates, and their covered subcontractors, relating to the privacy, security and transmission of individually identifiable health information; and |
| • | state, local and foreign law equivalents of each of the above federal laws, such as state anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers; state and local marketing and/or transparency laws applicable to manufacturers that may be broader in scope than the federal requirements; state laws that require biopharmaceutical companies to comply with the biopharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government; state and local laws that require licensure or registration by pharmaceutical sales representatives; state laws that require disclosure of information related to drug pricing; and state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect as HIPAA, thus complicating compliance efforts. |
Outside the United States, interactions between pharmaceutical companies and healthcare professionals are also governed by strict laws, such as national anti-bribery laws of EU Member States, national sunshine rules and regulations, industry self-regulation codes of conduct and physicians’ codes of professional conduct. Failure to comply with these requirements could result in administrative penalties, fines or imprisonment, reputational risk and public reprimands.
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant administrative, civil, and/or criminal penalties, damages, fines, disgorgement, individual imprisonment, exclusion from government funded healthcare programs, such as Medicare and Medicaid, or comparable foreign programs, integrity obligations, contractual damages, reputational harm, diminished profits and future earnings, and the curtailment or restructuring of our operations.
41
If the physicians or other healthcare providers or entities with whom we expect to do business are found to be not in compliance with applicable laws, they may be subject to significant administrative, civil, and/or criminal sanctions, including individual imprisonment and exclusion from government funded healthcare programs.
Data Privacy and Security
We are subject to stringent and evolving United States and foreign laws, regulations, rules, contractual obligations, policies and other obligations related to data privacy and security, including the EU’s General Data Protection Regulation ((EU) 2016/679), or GDPR, and the UK’s General Data Protection Regulation, or UK GDPR. New privacy rules are being enacted in the United States and globally, and existing ones are being expanded, updated and strengthened.
The collection and use of personal health data in the EEA is governed by the GDPR, which became effective on May 25, 2018. The GDPR applies to any company established in the EEA and to companies established outside the EEA that process personal data in connection with the offering of goods or services to data subjects in the EU or the monitoring of the behavior of data subjects in the EU. The GDPR enhances data protection obligations for controllers and processors of personal data, including stringent requirements relating to the consent of data subjects, expanded disclosures about how personal data is used, requirements to conduct privacy impact assessments for high-risk processing, limitations on retention of personal data and mandatory data breach notification and privacy by design requirements, and creates direct obligations on service providers acting as data processors. The GDPR also imposes strict rules on the transfer of personal data outside of the EEA to countries that do not ensure an adequate level of protection, such as the U.S. Failure to comply with the requirements of the GDPR and the related national data protection laws of the EEA Member States may result in fines up to 20 million Euros or 4% of a company’s global annual revenues for the preceding financial year, whichever is higher. Moreover, the GDPR grants data subjects the right to claim compensation for damages resulting from infringement of the GDPR.
Following the UK’s withdrawal and the expiration of the transition period, from January 31, 2020, companies doing business in the EU and the UK will be obliged to comply with both the GDPR and the UK GDPR. On June 28, 2021, the European Commission adopted an adequacy decision permitting flows of personal data between the EU and the UK to continue without additional requirements. However, the UK adequacy decision will automatically expire in June 2025 unless the European Commission re-assesses and renews or extends that decision and remains under review by the European Commission during this period. The relationship between the UK and the EU in relation to certain aspects of data protection law remains unclear, and it is unclear how UK data protection laws and regulations will develop in the medium to longer term, and how data transfers to and from the UK will be regulated in the long term.
Employees and Human Capital Resources
As of December 31, 2023, we had 104 full-time employees, including approximately 23 with M.D. or Ph.D. degrees, and 1 part-time employee. Most of these employees, are engaged in research and development, clinical development and operations, medical affairs, and biostatistics activities. Our human capital resources objectives include, as applicable, identifying, recruiting, retaining, incentivizing and integrating our existing and additional employees. The principal purposes of our equity incentive plans are to attract, retain and motivate selected employees, consultants and directors through the granting of equity-based compensation awards.
Corporate Information
Our legal and commercial name is DBV Technologies S.A. We were incorporated as a société par actions simplifiée (S.A.S.) under the laws of the French Republic on March 29, 2002 for a period of 99 years and subsequently converted on March 13, 2003 into a société anonyme. We are registered at the Nanterre Commerce
42
and Companies Register under the number 441 772 522. Our principal executive offices are located at 177-181 avenue Pierre Brossolette, 92120 Montrouge, France, and our telephone number is +33 1 55 42 78 78. Our agent for service of process in the United States is Cogency Global Inc.
Available Information
Our website address is http://www.dbv-technologies.com. We make available on our website, free of charge, our Annual Reports on Form 10-K, our Quarterly Reports on Form 10-Q and our Current Reports on Form 8-K and any amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission, or the SEC. The SEC maintains a website that contains reports, proxy and information statements and other information regarding our filings at www.sec.gov. Information contained on or accessible through our website is not a part of our Annual Report on Form 10-K, and the inclusion of our website address in this Annual Report on Form 10-K is an inactive textual reference only. The information found on our website is not incorporated by reference into this Annual Report on Form 10-K or any other report we file with or furnish to the SEC.
| Item 1A. | Risk Factors. |
Investing in our securities involves a high degree of risk. The following information about these risks, together with the other information appearing elsewhere in this Annual Report on form 10-K, including our consolidated financial statements and related notes thereto and management’s discussion and analysis of financial condition and results of operation, should be carefully considered before a decision to invest in our securities. The occurrence of any of the following risks could have a material adverse effect on our business, financial condition, results of operations and future growth prospects or cause our actual results to differ materially from those contained in forward-looking statements we have made in this report and those we may make from time to time. Additional risks that are currently unknown to us or that we currently believe to be immaterial may also impair our business. In these circumstances, the market price of our securities could decline, and holders of our securities may lose all or part of their investment. We cannot provide assurance that any of the events discussed below will not occur.
Risks Related to Our Financial Condition and Capital Requirements
We have incurred significant losses since our inception and anticipate that we will continue to incur significant losses for the foreseeable future.
We are a clinical-stage biopharmaceutical company, and we have not yet generated significant income from operating activities. We have incurred net losses in each year since our inception in 2002, including net losses of $72.7million and $96.3 million for the years ended December 31, 2023 and 2022 respectively. As of December 31, 2023, we had an accumulated deficit of $238.9 million. We have devoted most of our financial resources to research and development, including our clinical and pre-clinical development activities. To date, we have financed our operations primarily through the sale of equity securities, obtaining public assistance in support of innovation, such as conditional advances from OSEO Innovation, or OSEO, reimbursements of research tax credit claims and strategic collaborations. The amount of our future net losses will depend, in part, on the pace and amount of our future expenditures and our ability to obtain funding through equity or debt financings, strategic collaborations, or additional grants or tax credits. To date, we have not generated any product revenue and we continue to advance the clinical and regulatory development of Viaskin Peanut in the United States and European Union. Even if we obtain regulatory approval to market Viaskin Peanut or any other product candidate, our future revenues will depend upon the size of any markets in which our product candidates have received approval, and our ability to achieve sufficient market acceptance, reimbursement from third-party payors and adequate market share for any approved products in those markets.
43
Our near-term prospects, including our ability to finance our company and generate revenue, will depend heavily on the successful development, regulatory approval and commercialization of Viaskin Peanut. We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future. We anticipate that our expenses will increase substantially if and as we:
| • | seek regulatory approvals and pursue commercial activities for Viaskin Peanut, and for which we continue to seek regulatory approvals in the United States; |
| • | continue our research, pre-clinical and clinical development of our product candidates, including additional trials related to our pursuit of regulatory approval of Viaskin Peanut in the United States; |
| • | seek regulatory approvals for our other product candidates that successfully complete clinical trials; |
| • | establish a sales, marketing and distribution infrastructure to commercialize Viaskin Peanut, if approved, and any other products for which we may obtain regulatory approval, especially in North America; |
| • | further develop the manufacturing process for our product candidates, including any modifications to our patch technology; |
| • | change or add additional manufacturers or suppliers; |
| • | expand the scope of our current clinical trials for our product candidates; |
| • | initiate and conduct any post-approval clinical trials, if required by the FDA or comparable foreign regulatory authorities, for our approved products, if any; |
| • | initiate additional pre-clinical, clinical or other studies for our other product candidates; |
| • | seek to identify and validate additional product candidates; |
| • | acquire or in-license other product candidates and technologies; |
| • | make milestone or other payments under any in-license agreements; |
| • | maintain, protect and expand our intellectual property portfolio; |
| • | attract and retain new and existing skilled personnel; |
| • | add operational, financial and management information systems and personnel, including personnel to support our product development and commercialization efforts, as well as a company listed on both the U.S. and French stock markets; and |
| • | experience any delays or encounter issues with any of the above. |
The net losses we incur may fluctuate significantly from year to year, such that a period-to-period comparison of our results of operations may not be a good indication of our future performance. In any particular period or periods, our operating results could be below the expectations of securities analysts or investors, which could cause the price of our ADSs or ordinary shares to decline.
Based on our current operations, as well as our plans and assumptions, we expect that our balance of cash and cash equivalents of $141.4 million as of December 31, 2023 will be sufficient to fund our operations until December 31, 2024.
The company has incurred operating losses and negative cash flows from operations since inception.
As of the date of the filing, our available cash is not projected to be sufficient to support our operating plan for at least the next 12 months. As such, there is substantial doubt regarding our ability to continue as a going concern. We intend to seek additional capital as we prepare for the launch of Viaskin Peanut, if approved, and continue other research and development efforts. The Company will require substantial additional capital to fund its research and development and ongoing operating expenses.
44
The Company will seek to fund these capital requirements through debt and public or private equity before December 31, 2024.
We intend to seek additional capital as we prepare for the launch of Viaskin Peanut, if approved, and continue other research and development efforts. We may seek to finance our future cash needs through a combination of public or private equity or debt financings, collaborations, license and development agreements and other forms of non-dilutive financings.
We cannot guarantee that we will be able to obtain the necessary financing to meet our needs or to obtain funds at attractive terms and conditions, including as a result of disruptions to the global financial markets . A severe or prolonged economic downturn could result in a variety of risks to us, including reduced ability to raise additional capital when needed or on acceptable terms, if at all.
If we are not successful in our financing objectives, we could have to scale back our operations, notably by delaying or reducing the scope of our research and development efforts or obtain financing through arrangements with collaborators or others that may require us to relinquish rights to our product candidates that we might otherwise seek to develop or commercialize independently.
If we are unable to continue as a going concern, we may have to liquidate our assets and may receive less than the value at which those assets are carried on our financial statements, and it is likely that investors will lose all or a part of their investment. Further, the perception that we may be unable to continue as a going concern may impede our ability to pursue strategic opportunities or operate our business due to concerns regarding our ability to discharge our contractual obligations.
We will require substantial additional funding, which may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit, or terminate our product development efforts or other operations.
We are currently advancing our product candidates through pre-clinical and clinical development. Developing product candidates is expensive, lengthy and risky, and we expect our research and development expenses to increase substantially in connection with our ongoing activities, particularly as we seek regulatory approval for Viaskin Peanut. Furthermore, if we obtain regulatory approval for Viaskin Peanut or any other product candidate that we may develop, we expect our commercialization expenses related to product sales, marketing, distribution and manufacturing to increase significantly as we develop the appropriate infrastructure to commercialize. In addition, our expenses could increase beyond expectations if the FDA requires us to perform nonclinical studies, clinical trials or post-approval clinical trials for our approved products, if any, in addition to those that we currently anticipate.
As of December 31, 2023, our cash and cash equivalents were $141.4 million. Since our inception, we have primarily funded our operations with equity financings, and, to a lesser extent, public assistance aimed at supporting innovation and payments associated with research tax credits (Crédit d’Impôt Recherche). We do not generate product revenue and continue to prepare for the potential launch of our first product in the United States and in the European Union, if approved.
Based on our current operations, as well as our plans and assumptions, we expect that our balance of cash and cash equivalents of $141,4 million as of December 31, 2023 will be sufficient to fund our operations until December 31, 2024.
We expect that we will need to raise substantial additional capital as we prepare for the launch of Viaskin Peanut, if approved, and continue other research and development efforts. We may seek to finance our future cash needs through a combination of public or private equity or debt financings, collaborations, license and development agreements and other forms of non-dilutive financings.
45
We cannot guarantee that we will be able to obtain the necessary financing to meet our needs or to obtain funds at attractive terms and conditions, including as a result of disruptions to the global financial markets . A severe or prolonged economic downturn could result in a variety of risks to us, including reduced ability to raise additional capital when needed or on acceptable terms, if at all.
If we cannot conduct necessary operations or otherwise capitalize on our business opportunities because we lack sufficient capital, our business, financial condition and results of operations could be materially adversely affected.
Additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our product candidates. Moreover, the terms of any financing may adversely affect the holdings or the rights of our shareholders and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our ADSs or ordinary shares to decline. The sale of additional equity or convertible securities would dilute all of our shareholders. The incurrence of indebtedness would result in increased fixed payment obligations and we may be required to agree to certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. We could also be required to seek funds through arrangements with collaborative partners or otherwise at an earlier stage than otherwise would be desirable and we may be required to relinquish rights to some of our technologies or product candidates or otherwise agree to terms unfavorable to us, any of which may have a material adverse effect on our business, operating results and prospects.
If we are unable to obtain sufficient funding on a timely basis, we may be required to scale back our operating plan, significantly curtail, delay or discontinue one or more of our research or development programs or the commercialization of any product candidate, or be unable to expand our operations or otherwise capitalize on our business opportunities, as desired, which could materially affect our business, financial condition and results of operations.
We are limited in our ability to raise additional share capital, which may make it difficult for us to raise capital to fund our operations.
Under French law, our share capital may be increased only with shareholders’ approval at an extraordinary general shareholders’ meeting following the recommendation of our board of directors. The shareholders may delegate to our board of directors either the authority (délégation de compétence) or the power (délégation de pouvoir) to carry out any increase in share capital.
In addition, the French Commercial Code imposes certain limitations on our ability to price any offering of our share capital without preferential subscription right (sans droit préférentiel de souscription), which limitation may prevent us from successfully completing any such offering. Specifically, under the French Commercial Code, unless the offering is less than 10% of issued share capital, securities cannot be sold in an offering at a price that is more than a 10% discount to the volume weighted average trading price on Euronext Paris over the last three trading days preceding the commencement of the marketing of the transaction. In addition, the combined shareholders’ meeting dated April 12, 2023 granted authority to our board of directors to increase our share capital up to 100% of issued share capital, if the investors in such offering fit within categories of persons meeting certain characteristics. In this case securities cannot be sold in such an offering at a price that is more than a 15% discount to (i) the last closing price of the Company’s shares on the regulated market Euronext Paris prior to the date on which the issue price is set, (ii) the volume-weighted average price of the share of the Company on the regulated market of Euronext Paris over a period determined by the Board of Directors of between one to five consecutive trading days chosen from the last thirty trading days prior to the date on which the issue price is set.
46
Our business could be adversely affected by economic downturns, inflation, increases in interest rates, natural disasters, public health crises such as the COVID-19 pandemic, political crises, geopolitical events, such as the crisis in Ukraine and the Israel-Hamas war, or other macroeconomic conditions, which have in the past and may in the future negatively impact our business and financial performance.
The global economy, including credit and financial markets, has experienced extreme volatility and disruptions, including, among other things, severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, supply chain shortages, increases in inflation rates, higher interest rates and uncertainty about economic stability.
For example, the COVID-19 pandemic resulted in widespread unemployment, economic slowdown and extreme volatility in the capital markets. As a result of the COVID-19 pandemic, our ability to conduct clinical trials was affected. Future pandemics, epidemics or other public health crises (collectively, “public health crises”) could have an impact on our ability to conduct clinical trials, and clinical site initiation, subject enrollment and subject visits (including food challenges) in any of our clinical trials may be suspended or delayed due to prioritization of hospital resources toward responding to such public health crises. Some participants may not be able to comply with clinical trial protocols if quarantines impede patient movement or interrupt healthcare services. Similarly, our ability to recruit and retain subjects and principal investigators and site staff who, as healthcare providers may adversely impact our future clinical trial operations. The COVID-19 pandemic and related government and private sector responsive actions affected, and any future public health crises could affect, the broader economies and financial markets, triggering an economic downturn, which at points adversely affected or could adversely affect, our ability to access capital, which could negatively affect our business. In addition, the recession or resulting adverse impacts on the capital markets resulting from the COVID-19 pandemic, and any future public health crises, could materially affect our business.
The U.S. Federal Reserve recently raised interest rates multiple times in response to concerns about inflation and it may raise them again. Higher interest rates, coupled with reduced government spending and volatility in financial markets may increase economic uncertainty and affect consumer spending. If the equity and credit markets deteriorate, including as a result of political unrest or war, it may make any necessary debt or equity financing more difficult to obtain in a timely manner or on favorable terms, more costly or more dilutive. Increased inflation rates can adversely affect us by increasing our costs, including labor and employee benefit costs.
Our business could be materially and adversely affected by the effects of any future public health crises in regions where we or third parties on which we rely have significant manufacturing facilities, concentrations of clinical trial sites or other business operations. Any future public health crises could materially affect our operations as well as cause significant disruption in the operations and business of third-party manufacturers, CROs, other services providers, and collaborators with whom we conduct business.
It is impossible to predict all effects and the ultimate impact of any public health crises, including the COVID-19 pandemic. The full extent the impact of any future public health crises on our clinical development and other operations and financial performance depends on continuing developments that are uncertain and unpredictable, including the timing of any future vaccine development and rollouts and herd immunity, virus mutations and variants, and any new information that may emerge concerning future virus, vaccines, and containment, all of which may vary across regions. Any of these factors could have a material adverse impact on our business, financial condition, operating results, and ability to execute and capitalize on our strategies.
On February 24, 2022, Russian forces launched significant military action against Ukraine, and sustained conflict and disruption in the region is possible. The impact to Ukraine as well as actions taken by other countries, including new and stricter sanctions imposed by Canada, the United Kingdom, the European Union, the United States and other countries and companies and organizations against officials, individuals, regions, and industries in Russia and Ukraine, and actions taken by Russia in response to such sanctions, and responses of countries and political bodies to such sanctions, tensions, and military actions and the potential for more widespread conflict, have resulted in supply chain disruptions, and resulting increases in inflation, financial market volatility and capital markets disruption, potentially increasing in magnitude, and such effects on the global economy and financial markets could affect our business, operations, operating results and financial condition as well as the price of our common stock and our ability to raise additional capital when needed on acceptable terms.
47
Separately, in early October 2023, Hamas, a militant group in control of Gaza, and Israel began an armed conflict in Israel, the Gaza Strip, and surrounding areas, which threatens to spread to other Middle Eastern countries, including Lebanon, Syria, and Iran. The Hamas-Israel military conflict is ongoing, and its length and outcome are highly unpredictable. Any or all of the effects of these conflicts could disrupt our and our collaborators’ supply chains and adversely affect our and our collaborators’ ability to conduct ongoing and future clinical trials of our product candidates. The extent and duration of the military action, sanctions and resulting economic, market and other disruptions are impossible to predict, but could be substantial. Any such disruptions may magnify the impact of the other risks described in this report.
We are obligated to develop and maintain a system of effective internal controls over financial reporting. These internal controls may be determined to be not effective, which may adversely affect investor confidence in our company and, as a result, the value of our ordinary shares and ADSs.
We have been and are required, pursuant to Section 404 of the Sarbanes-Oxley Act, to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting on an annual basis. This assessment includes disclosure of any material weaknesses identified by our management in our internal control over financial reporting. During the evaluation and testing process, if we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal controls are effective and would be required to disclose any material weaknesses identified in Management’s Report on Internal Control over Financial Reporting. While we have established certain procedures and control over our financial reporting processes, we cannot assure you that these efforts will prevent restatements of our financial statements in the future.
Depending on our future filer status with the SEC, our independent registered public accounting firm may also require, pursuant to Section 404 of the Sarbanes-Oxley Act, to report on the effectiveness of our internal control over financial reporting. This assessment will include disclosure of any material weaknesses identified by our management in our internal control over financial reporting. For future reporting periods, our independent registered public accounting firm may issue a report that is adverse in the event it is not satisfied with the level at which our controls are documented, designed or operating. We may not be able to remediate any future material weaknesses, or to complete our evaluation, testing and any required remediation in a timely fashion.
If we are unable to conclude that our internal control over financial reporting is effective, or if our independent registered public accounting firm is unable to express an opinion that our internal controls over financial reporting are effective if and when a report from such accounting firm is required, investors could lose confidence in the accuracy and completeness of our financial reports, which could cause the price of our ordinary shares and ADSs to decline, and we could be subject to sanctions or investigations by regulatory authorities, including the SEC and Nasdaq. Failure to remediate any material weakness in our internal control over financial reporting, or to maintain other effective control systems required of public companies, could also restrict our future access to the capital markets.
48
If we do not obtain the capital necessary to fund our operations, we will be unable to successfully commercialize, develop or pursue regulatory approval for our biopharmaceutical products.
The development of biopharmaceutical products is capital-intensive. We anticipate that we will require additional financing to continue to fund our operations. Our future capital requirements will depend on, and could increase significantly as a result of, many factors including:
| • | the scope, progress in, results and the costs of, our pre-clinical studies and clinical trials and other research and development programs, particularly as we seek regulatory and marketing approvals for our product candidates that successfully complete clinical trials; |
| • | the approval of Viaskin Peanut by the FDA, European Commission, or other comparable regulatory authorities; |
| • | the costs of commercialization activities, including product sales, marketing, manufacturing and distribution, for any of our product candidates for which we receive regulatory approval, especially in North America; |
| • | the costs of securing manufacturing arrangements for commercial production; |
| • | revenue, if any, received from commercial sales of our product candidates, should any of our product candidates receive regulatory approval; |
| • | the scope, prioritization and number of our research and development programs; |
| • | the costs, timing and outcome of regulatory review of our product candidates; |
| • | the achievement of milestones or occurrence of other developments that trigger payments under our existing collaboration agreements, and any additional collaboration agreements we may enter into; |
| • | the extent to which we are obligated to reimburse, or entitled to reimbursement of, clinical trial costs under our existing collaboration agreements and future collaboration agreements, if any; and |
| • | the costs involved in filing, prosecuting, enforcing and defending patent claims and other intellectual property rights. |
Until we can generate significant continuing revenues, we expect to satisfy our future cash needs through a combination of public or private equity or debt financings, collaborations, license and development agreements and other forms of non-dilutive financings. Uncertainty and dislocations in the financial markets have generally made equity and debt financing more difficult to obtain, and may have a material adverse effect on our ability to meet our future fundraising needs. We cannot be certain that additional funding will be available to us on acceptable terms, if at all. If funds are not available, we may be required to delay, reduce the scope of, or eliminate one or more of our research or development programs or our commercialization efforts. Additional funding, if obtained, may significantly dilute existing shareholders if that financing is obtained through issuing equity or instruments convertible into equity. We could also be required to seek funds through collaborations or licensing arrangements with third parties, and we could be required to do so at an earlier stage than otherwise would be desirable. In connection with any such collaborations or licensing arrangements, we may be required to relinquish valuable rights to our intellectual property, future revenue streams, research programs or product candidates, grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves, or otherwise agree to terms unfavorable to us, any of which may have a material adverse effect on our business, operating results and prospects.
The requirements of being a U.S. public company may strain our resources, divert management’s attention and affect our ability to attract and retain executive management and qualified board members.
As a U.S. public company, we have incurred and will continue to incur significant legal, accounting and other expenses that we did not previously incur. We are subject to the reporting requirements of the Securities Exchange Act of 1934, or the Exchange Act, the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the Nasdaq listing requirements and other applicable securities rules and regulations.
49
Compliance with these rules and regulations will continue to increase our legal and financial compliance costs, make some activities more difficult, time-consuming or costly and increase demand on our systems and resources, particularly as we now qualify as a domestic filer. The Exchange Act requires that, as a public company that no longer qualifies as a foreign private issuer, we file annual, quarterly and current reports with respect to our business, financial condition and result of operations. Because we are no longer a foreign private issuer, we will also be required to file proxy statements in connection with any meetings of our shareholders. As a result of being a U.S. public company, management’s attention may be diverted from other business concerns, which could adversely affect our business and results of operations. The Sarbanes-Oxley Act requires, among other things, that we maintain effective internal controls for financial reporting and disclosure controls and procedures. In particular, we must perform system and process evaluations and testing of our internal controls over financial reporting to allow management to report on the effectiveness of our internal controls over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act. Compliance with Section 404 may require that we incur substantial accounting expenses and expend significant management efforts. Our independent registered public accounting firm may also be required, pursuant to Section 404 of the Sarbanes-Oxley Act, to report on the effectiveness of our internal control over financial reporting.
Our testing may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses. In the event we identify significant deficiencies or material weaknesses in our internal controls that we cannot remediate in a timely manner, or if our independent registered public accounting firm is unable to express an opinion that our internal controls over financial reporting are effective, the market price of our ordinary shares and ADSs could decline if investors and others lose confidence in the reliability of our financial statements, we could be subject to sanctions or investigations by the SEC or other applicable regulatory authorities and our business could be harmed.
As a U.S. public company that is subject to these rules and regulations, we may find it is more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified members of our board of directors, particularly to serve on our audit committee and compensation committee, and qualified executive officers.
As a result of disclosure of information in filings required of a U.S. public company, particularly as we are no longer a foreign private issuer, our business and financial condition will become more visible than they would be if we were a privately-owned company or if our securities were listed only on Euronext Paris, which we believe may result in threatened or actual litigation, including by competitors and other third parties. If such claims are successful, our business and results of operations could be adversely affected, and even if the claims do not result in litigation or are resolved in our favor, these claims, and the time and resources necessary to resolve them, could divert the resources of our management and adversely affect our business and results of operations.
Further, being both a U.S. public company and a French public company has an impact on disclosure of information and compliance with two sets of applicable rules. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
Risks Related to Product Development, Regulatory Approval and Commercialization
We depend almost entirely on the successful development of our novel Viaskin technology. We cannot be certain that we will be able to obtain regulatory approval for, or successfully commercialize, Viaskin products.
We currently have no drug or biological product approved for sale and may never be able to develop a marketable drug or biological product.
50
We may not be successful in developing and commercializing Viaskin Peanut and our other product candidates, including, without limitation, Viaskin Milk, and our commercial opportunities may be limited.
We are currently conducting VITESSE, a Phase 3 pivotal study in children aged 4 through 7 years of age with confirmed diagnosis of peanut allergy with Type V Viaskin Peanut System, or the modified Viaskin Peanut system. Additionally, we intend to carry out two additional Phase 3 safety studies in response to the FDA’s request regarding the size of the controlled safety database. One study will be conducted in peanut allergic children 4 through 7 years of age using the Type Viaskin Peanut System (mVP), and the other will focus on peanut allergic children 1 through 3 years of age with the Type IV Viaskin Peanut System (the original Viaskin Peanut system, or cVP). Positive results in all these studies are imperative for us to seek regulatory approval before we are permitted to commence commercialization, if ever. Viaskin Milk will also require substantial additional clinical development, testing, and regulatory approval before we are permitted to commence its commercialization, if ever. Many of our other product candidates are still in pre-clinical or early proof-of-concept phase development. The clinical trials of our product candidates are, and the manufacturing and marketing of our product candidates will be, subject to extensive and rigorous review and regulation by numerous government authorities in the United States and in other countries where we intend to test and, if approved, market any product candidate. Before obtaining regulatory approvals for the commercial sale of any product candidate, we must demonstrate through preclinical testing and clinical trials that, among other things, the product candidate is safe and effective for use in each target indication. This process can take many years and may include post-marketing requirements and surveillance, including the completion of pediatric clinical trials to satisfy both U.S. and EU requirements, which will require the expenditure of substantial resources. Of the large number of drugs in development in the United States, only a small percentage successfully completes the FDA regulatory approval process and are commercialized. Accordingly, even if we are able to obtain the requisite financing to continue to fund our development and clinical programs, we cannot assure you that any of our product candidates will be approved by relevant regulators or will be successfully developed or commercialized.
In addition, in some jurisdictions such as the EU, initiating Phase 3 clinical trials, including clinical trials in the pediatric population, is subject to a requirement to obtain approval or a waiver from the competent authorities of the EU Member States and/or the EMA. If we do not obtain such approval our ability to conduct clinical trials and obtain marketing authorizations may be severely impaired and our business may be adversely impacted.
We are not permitted to market any of our product candidates in the United States or in any other country until we receive the requisite approval from the applicable regulators. Obtaining requisite regulatory approval in any country is a complex, lengthy, expensive and uncertain process, and the FDA or the applicable foreign regulatory authority may delay, limit or deny approval of a Viaskin product, for many reasons, including, among others:
| • | we may not be able to demonstrate that a product candidate is a safe and effective treatment, to the satisfaction of the FDA or the applicable foreign regulatory authority; |
| • | the results of our clinical trials or the clinical trials conducted by third party academic institutions and included in our application package may not meet the level of statistical or clinical significance required by the FDA or the applicable foreign regulatory authority for regulatory approval; |
| • | the FDA or the applicable foreign regulatory authority may disagree with the number, design, size, conduct or implementation of our clinical trials; |
| • | the FDA or the applicable foreign regulatory authority may require that we conduct additional clinical trials; |
| • | the FDA or the applicable foreign regulatory authority may not approve the formulation, labeling or specifications of a product candidate; |
| • | the clinical research organizations, or CROs, that we retain to conduct our clinical trials may take actions outside of our control that materially adversely impact our clinical trials; |
51
| • | the FDA or the applicable foreign regulatory authority may find the data from pre-clinical studies and clinical trials from a product candidate insufficient to demonstrate that the clinical or other benefits of such product candidate outweighs its respective safety risks; |
| • | the FDA or the applicable foreign regulatory authority may disagree with our analysis or interpretation of data from our pre-clinical studies and clinical trials; |
| • | the FDA or the applicable foreign regulatory authority may not accept data generated at our clinical trial sites; |
| • | an advisory committee, or similar body, may recommend against approval of our application or may recommend that the FDA or the applicable foreign regulatory authority require, as a condition of approval, additional pre-clinical studies or clinical trials, limitations on approved labeling or distribution and use restrictions; |
| • | the FDA or the applicable foreign regulatory authority may require development or implementation of a Risk Evaluation and Mitigation Strategy(or REMS), or comparable foreign requirements, as a condition of approval or post-approval; |
| • | the FDA or the applicable foreign regulatory authority may restrict the use of our products to a narrow population; |
| • | the FDA or the applicable foreign regulatory authority may not approve the manufacturing processes or facilities of our own or of third-party manufacturers with which we contract, or may issue inspectional findings that require significant expense and time to address; or |
| • | the FDA or the applicable foreign regulatory authority may change their approval policies or new legislation governing the approval processes. |
Any of these factors, many of which are beyond our control, could jeopardize our ability to obtain regulatory approval for and successfully market any of our product candidates based on our Viaskin technology platform. Moreover, because our business is almost entirely dependent upon our Viaskin technology, any such setback in our pursuit of regulatory approval would have a material adverse effect on our business and prospects.
Our product candidates have undergone and/or will be required to undergo clinical trials that are time- consuming and expensive, the outcomes of which are unpredictable, and for which there is a high risk of failure. If clinical trials of our product candidates fail to satisfactorily demonstrate safety and efficacy to the FDA and other comparable foreign regulatory authorities, we, or our collaborators, may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of these product candidates.
Pre-clinical testing and clinical trials are long, expensive and unpredictable processes that can be subject to extensive delays. We cannot guarantee that any clinical trials will be conducted as planned or completed on schedule, if at all. It may take several years to complete the pre-clinical testing and clinical development necessary to commercialize a drug or biologic, and delays or failure can occur at any stage. Interim results of clinical trials do not necessarily predict final results, and success in pre-clinical testing and early clinical trials does not ensure that later clinical trials will be successful. A number of companies in the pharmaceutical, biopharmaceutical and biotechnology industries have suffered significant setbacks in advanced clinical trials even after positive results in earlier trials, and we cannot be certain that we will not face similar setbacks. The design of a clinical trial can determine whether its results will support regulatory approval of a product, and flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced. An unfavorable outcome in one or more trials would be a major setback for our product candidates and for us. Due to our limited financial resources, an unfavorable outcome in one or more trials may require us to delay, reduce the scope of, or eliminate one or more product development programs, which could have a material adverse effect on our business and financial condition and on the value of our ADSs and ordinary shares.
52
In connection with clinical testing and trials, we face a number of risks, including, but not limited to:
| • | a product candidate is ineffective, inferior to existing approved medicines or treatment options, unacceptably toxic, or has unacceptable side effects; |
| • | patients may die or suffer other adverse effects for reasons that may or may not be related to the product candidate being tested, especially during the double-blind, placebo-controlled food challenges; |
| • | extension studies on long-term tolerance could invalidate the use of our product, showing Viaskin does not generate a sustained protective effect; |
| • | any positive results of earlier testing or trials may not be confirmed by results of subsequent trials; and |
| • | the results may not meet the level of statistical significance required by the FDA or other comparable regulatory authorities to establish the safety and efficacy of our product candidates. |
The results of pre-clinical studies do not necessarily predict clinical success, and larger and later-stage clinical trials may not produce the same results as earlier-stage clinical trials. As a result, we may not observe a similarly favorable safety and efficacy profile as our prior clinical trials. For example, in August 2020, we received a Complete Response Letter, or CRL, in which the FDA indicated it could not approve the Viaskin Peanut BLA in its then-current form. The FDA identified concerns regarding the impact of system adhesion on efficacy and indicated the need for modifications, and new human factors studies. The FDA also indicated that supplementary clinical data would need to be generated to support applications for both the Type IV Viaskin Peanut System (the original Viaskin Peanut system), or cVP, and the Type V Viaskin Peanut System (the modified Viaskin Peanut System), or mVP, and requested additional Chemistry, Manufacturing and Controls, or CMC, data. Further, in September 2022, we announced that FDA had imposed a partial clinical hold on the VITESSE trial, which was lifted in December 2022 after we made additional revisions to the protocol in order to address FDA concerns. In addition, we cannot assure you that in the course of potential widespread use in future, some drawbacks would not appear in maintaining production quality, protein stability or allergenic strength. Frequently, product candidates developed by pharmaceutical, biopharmaceutical and biotechnology companies have shown positive results in early pre-clinical studies or clinical trials, but have subsequently suffered significant setbacks or failed in later clinical trials. In addition, clinical trials of potential products sometimes reveal that it is not possible or practical to continue development efforts for these product candidates.
If we do not successfully complete pre-clinical and clinical development, we will be unable to market and sell our product candidates and generate revenues. Even if we do successfully complete clinical trials, those results are not necessarily predictive of results of additional trials that may be needed before an application for regulatory approval may be submitted to the FDA or a comparable foreign regulatory authority. Although there are a large number of drugs and biologics in development in the United States and other countries, only a small percentage result in the submission of an application for regulatory approval to a regulatory authority, such as an NDA or a BLA to the FDA, or comparable foreign regulatory authorities, even fewer are approved for commercialization, and only a small number achieve widespread physician and consumer acceptance following regulatory approval. If our clinical trials are substantially delayed or fail to prove the safety and effectiveness of our product candidates in development, we may not receive regulatory approval of any of these product candidates and our business and financial condition will be materially harmed.
In many of clinical trials, we utilize an oral food challenge procedure intentionally designed to trigger an allergic reaction, which could be severe or life-threatening.
In accordance with our food allergy clinical trial protocols, we utilize a double-blind, placebo-controlled food challenge procedure at various points in our clinical trials. This consists of giving the offending food protein to subjects to assess the sensitivity of their food allergy to determine eligibility to participate and to evaluate the efficacy of our product candidates versus placebo. The food challenge protocol is meant to induce objective symptoms of an allergic reaction. These oral food challenge procedures can potentially trigger anaphylaxis or potentially life-threatening systemic allergic reactions.
53
Even though these procedures are well-controlled, standardized and performed in highly specialized centers with intensive care units, there are inherent risks in conducting a trial of this nature. An uncontrolled allergic reaction could potentially lead to serious or even fatal reactions. Any such serious clinical event could potentially adversely affect our clinical development timelines, including a complete clinical hold on our food allergy clinical trials. We may also become liable to subjects who participate in our clinical trials and experience any such serious or fatal reactions. Any of the foregoing could have a material adverse effect on our business, prospects, stock price or financial condition.
Delays, suspensions and terminations in our clinical trials could result in increased costs to us and delay or prevent our ability to generate revenues.
Human clinical trials are very expensive, time-consuming, and difficult to design, implement and complete. The completion of trials for Viaskin Peanut and our other product candidates may be delayed for a variety of reasons, including, but not limited to, delays in:
| • | demonstrating sufficient safety and efficacy to obtain regulatory approval to commence a clinical trial; |
| • | reaching agreement on acceptable terms with prospective CROs, and clinical trial sites; |
| • | validating test methods to support quality testing of the drug substance and drug product; |
| • | obtaining sufficient quantities of the drug substance or other materials necessary to conduct clinical trials; |
| • | manufacturing sufficient quantities of a product candidate; |
| • | obtaining timely responses from and permission to proceed from the FDA under an investigational new drug, or IND, application, or foreign equivalent approval from regulatory authorities outside the United States; |
| • | obtaining institutional review board, or IRB, approval or positive Ethics Committee opinions as part of the single decision on the authorization of a clinical trial issued by EU Member States including input from the national competent authority and Ethics Committee, to conduct a clinical trial at a prospective clinical trial site; |
| • | determining dosing and clinical design and making related adjustments; and |
| • | subject enrollment, which is a function of many factors, including the size of the population, the nature of the protocol, the proximity of participants to clinical trial sites, the availability of effective treatments for the relevant disease and the eligibility criteria for the clinical trial. |
The commencement and completion of clinical trials for our product candidates may be delayed, suspended or terminated due to a number of factors, including:
| • | lack of effectiveness of product candidates during clinical trials; |
| • | adverse events, safety issues or side effects relating to the product candidates or their formulation; |
| • | serious adverse events relating to the double-blind, placebo-controlled food challenge procedure when testing participants for the sensitivity of their allergies; |
| • | inability to raise additional capital in sufficient amounts to continue clinical trials or development programs, which are very expensive; |
| • | the need to sequence clinical trials as opposed to conducting them concomitantly in order to conserve resources; |
| • | our inability to enter into collaborations relating to the development and commercialization of our product candidates; |
| • | failure by us or our collaborators to conduct clinical trials in accordance with regulatory requirements; |
54
| • | our inability or the inability of our collaborators to manufacture or obtain from third parties materials sufficient for use in pre-clinical studies and clinical trials; |
| • | governmental or regulatory delays, changes by regulatory agencies, including, without limitation, unexpected changes, unrelated to new developments of the science, in prior guidance and instruction provided to us, changes in regulatory requirements, policy and guidelines, and mandated changes in the scope or design of clinical trials or requests for supplemental information with respect to clinical trial results; |
| • | failure of our collaborators to advance our product candidates through clinical development; |
| • | delays in enrollment, variability in the number and types of subjects available for clinical trials, and lower-than anticipated retention rates for subjects in clinical trials; |
| • | difficulty in subject monitoring and data collection due to failure of subjects to maintain contact after treatment; |
| • | a regional disturbance where we or our collaborative partners are enrolling patients in our clinical trials, such as the COVID-19 pandemic or any other pandemics, epidemics, or global health crises, terrorist activities or war, or a natural disaster; and |
| • | varying interpretations of our data, and regulatory commitments and requirements by the FDA and similar foreign regulatory authorities. |
For example, we announced in September 2022 that FDA had imposed a partial clinical hold on the VITESSE trial, which was lifted in December 2022, resulting in a delay in initiation and conduct of the VITESSE trial.
Many of these factors may also ultimately lead to denial of our applications for regulatory approval for our product candidates. If we experience delay, suspensions or terminations of a clinical trial, the commercial prospects for the related product candidate will be harmed, and our ability to generate product revenues will be delayed or such revenues could be reduced or fail to materialize.
In addition, we may encounter delays or product candidate rejections based on new governmental regulations, future legislative or administrative actions, resource constraints or changes in resources at the regulatory agencies tasked with reviewing our submissions, resulting in delays in receiving timely and consistent guidance, or changes in FDA or other similar foreign regulatory authority policy or interpretation during the period of product development. If we obtain required regulatory approvals, such approvals may later be withdrawn, varied or suspended. Delays or failures in obtaining regulatory approvals may result in:
| • | varying interpretations of data and commitments by the FDA and similar foreign regulatory authorities; and |
| • | diminishment of any competitive advantages that such product candidates may have or attain. |
Furthermore, if we fail to comply with applicable FDA and other regulatory requirements at any stage during this regulatory process, we may encounter or be subject to:
| • | issuance of warning letters, show cause notices or untitled letters describing alleged violations, which may be publicly available; |
| • | diminishment of any competitive advantages that such product candidates may have or attain; |
| • | suspension, delays or termination in clinical trials or commercialization; |
| • | delays or refusal by the FDA or similar foreign regulatory authorities to review pending applications for regulatory approval or supplements to approved applications; |
| • | voluntary or mandatory product recalls or seizures; |
| • | refusal to permit the import or export of medicinal products or intermediary chemicals; |
| • | suspension, restrictions or additional requirements on operations, including of manufacturing or revocation of necessary licenses; |
55
| • | withdrawals, variations or suspensions of regulatory approvals; and |
| • | fines, civil penalties, and criminal prosecutions. |
If our product candidates are not approved by the FDA, or comparable foreign regulatory authorities, we will be unable to commercialize them in the United States or in other countries.
The FDA must approve any new drug or biologic before it can be commercialized, marketed, promoted or sold in the United States. We must provide the FDA with data from pre-clinical studies and clinical trials that demonstrate that, among other things, our product candidates are safe and effective for a defined indication before they can be approved for commercial distribution. Clinical testing is expensive, difficult to design and implement, can take many years to complete and is inherently uncertain as to outcome. There is significant competition to secure clinical trial support resources, including CROs. Clinical sites are resource constrained with the availability of these sites further limited due to, in certain instances, participation in multiple clinical trials. In addition, there are various opportunities for subjects eligible to participate in our clinical trials to participate in other food allergy clinical trials or allergy related trials. We must provide data to ensure the identity, strength, quality and purity of the drug substance and drug product. Also, we must assure the FDA that the characteristics and performance of the clinical batches will be replicated consistently in the commercial batches. We will not obtain approval for a product candidate unless and until the FDA approves a BLA, if at all.
The processes by which regulatory approvals are obtained from the FDA to market and sell a new or repositioned product are complex, require a number of years and involve the expenditure of substantial resources. We have already experienced several setbacks and delays in our previously anticipated ability to obtain approval of Viaskin Peanut from the FDA and the European Commission, and we may experience additional delays in the future. We cannot assure you that any of our product candidates will receive FDA approval, or regulatory approval from a comparable foreign regulatory authority, in the future, and the time for receipt of any such approval is currently incapable of estimation.
A Fast Track designation by the FDA, or equivalent foreign programs, may not actually lead to a faster development or regulatory review or approval process, and it does not increase the likelihood that our product candidates will receive regulatory approval.
We have obtained Fast Track designation from the FDA for the development of Viaskin Peanut and Viaskin Milk, and we may apply for that designation for other product candidates as well. If a product is intended for the treatment of a serious condition and nonclinical or clinical data demonstrate the potential to address unmet medical needs for this condition, the sponsor may apply for FDA Fast Track designation. The FDA has broad discretion to grant this designation, and even if we believe our product candidates are eligible for this designation, we cannot be sure that the FDA would decide to grant it. Even if we do have Fast Track designation, we may not experience a faster development process, review or approval compared to conventional FDA procedures. Generally, a Fast Track designation affords the possibility of rolling review, enabling the FDA to review portions of our marketing application before submission of a complete application. The FDA may withdraw fast track designation if it believes that the designation is no longer supported by data from our clinical development program.
The regulatory approval process outside the United States varies among countries and may limit our ability to develop, manufacture and sell our products internationally. Failure to obtain regulatory approval in international jurisdictions would prevent our product candidates from being marketed abroad.
In order to market and sell our product candidates in the European Union and many other jurisdictions, we, and our collaborators, must obtain separate regulatory approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and may involve additional testing.
We may, in the future, conduct clinical trials for, and seek regulatory approval to market, product candidates in countries other than the United States. Depending on the results of clinical trials and the process for obtaining regulatory approvals in other countries, we may decide to first seek regulatory approvals of a product candidate in countries other than the United States, or we may simultaneously seek regulatory approvals in the United States and other countries.
56
If we or our collaborators seek marketing approvals for a product candidate outside the United States, we will be subject to the regulatory requirements of health authorities in each country in which we seek approvals. With respect to marketing authorizations in the European Union, we will be required to submit an MAA to the EMA which conducts a validation and scientific review process in evaluating a product for safety and efficacy. The regulatory approval procedures vary among countries and may involve additional testing, and the time required to obtain approvals may differ from that required to obtain FDA approval.
Pursuing regulatory approvals from regulatory authorities in countries outside the United States is likely to subject us to all of the risks associated with pursuing FDA approval described above. In addition, regulatory approval by the FDA does not ensure approval by the regulatory authorities of any other country, and approval by foreign regulatory authorities does not ensure regulatory approval by the FDA.
Even if we, or our collaborators, obtain regulatory approvals for our product candidates, the terms of approvals and ongoing regulation of our products may limit how we or they market our products, which could materially impair our ability to generate revenue.
Even if we receive regulatory approval for Viaskin Peanut or any of our other product candidates, this approval may carry conditions that limit the market for the product or put the product at a competitive disadvantage relative to alternative therapies. For instance, a regulatory approval may limit the indicated uses for which we can market a product or limit the patient population that may utilize the product or require a product to carry a warning in its labeling and on its packaging. Products with boxed warnings are subject to more restrictive advertising regulations than products without such warnings. These restrictions could make it more difficult to market any product candidate effectively. Accordingly, assuming we, or our collaborators, receive regulatory approval for Viaskin Peanut or any of our other product candidates, we and our collaborators will continue to expend time, money and effort in all areas of regulatory compliance.
Any of our product candidates for which we, or our collaborators, obtain regulatory approval in the future could be subject to post-marketing requirements, post-marketing commitments or withdrawal from the market and we, and our collaborators, may be subject to substantial penalties if we, or they, fail to comply with regulatory requirements or if we, or they, experience unanticipated problems with our products following approval.
Any of our product candidates for which we, or our collaborators, obtain regulatory approval in the future, as well as the manufacturing processes, post-marketing requirements and commitments, labeling, advertising and promotional activities for such products, among other things, will be subject to continual requirements of and review by the FDA and other foreign regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, registration and listing requirements, requirements relating to manufacturing, quality control, quality assurance and corresponding maintenance of records and documents and requirements regarding the distribution of samples to physicians and recordkeeping. Even if regulatory approval of a product candidate is granted, the approval will be subject to limitations on the indicated uses for which the product may be marketed or may be subject to other conditions of approval, including the FDA requirement to implement a REMS, or comparable foreign requirements to ensure that the benefits of a drug or biological product outweigh its risks.
The FDA or comparable foreign regulatory authorities may also impose requirements for costly post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of a product, such as long-term observational studies on natural exposure. The FDA and other agencies, including, without limitation, the U.S. Department of Justice, and comparable foreign regulatory authorities closely regulate and monitor the post-approval marketing and promotion of products to ensure that they are manufactured, marketed, and distributed only for the approved indications and in accordance with the provisions of the approved labeling.
57
The FDA and comparable foreign regulatory authorities impose stringent restrictions on manufacturers’ communications regarding off-label use and if we, or our collaborators, market any of our product candidates for which we, or they, receive regulatory approval for treatment other than their approved indications, we, or they, may be subject to warnings or enforcement action for off-label marketing. Violation of the Federal Food, Drug, and Cosmetic Act, or FDCA, and other statutes, including the False Claims Act, relating to the promotion and advertising of prescription drugs may lead to investigations or allegations of violations of federal and state health care fraud and abuse laws and state consumer protection laws.
Failure to comply with EU and EU Member State laws that apply to the conduct of clinical trials, manufacturing approval, marketing authorization of medicinal products and marketing of such products, both before and after grant of the marketing authorization, or with other applicable regulatory requirements may result in administrative, civil or criminal penalties. These penalties could include delays or refusal to authorize the conduct of clinical trials, or to grant marketing authorization, product withdrawals and recalls, product seizures, suspension, withdrawal or variation of the marketing authorization, total or partial suspension of production, distribution, manufacturing or clinical trials, operating restrictions, injunctions, suspension of licenses, fines and criminal penalties.
If we do not achieve our projected development and commercialization goals in the timeframes we announce and expect, the commercialization of our product candidates may be delayed, and our business will be harmed.
We sometimes estimate the timing of the accomplishment of various scientific, clinical, regulatory, and other product development objectives or milestones for planning purposes. These milestones may include our expectations regarding the commencement or completion of scientific studies and clinical trials, the submission of regulatory filings, or commercialization objectives. From time to time, we may publicly announce the expected timing of some of these milestones, such as the completion of an ongoing clinical trial, the initiation of other clinical programs, receipt of regulatory approval, or a commercial launch of a product. The achievement of many of these milestones may be outside of our control. All of these milestones are based on a variety of assumptions which may cause the timing of achievement of the milestones to vary considerably from our estimates, including:
| • | our available capital resources or capital constraints we experience; |
| • | our receipt of approvals, if any, by the FDA and other comparable foreign regulatory authorities and the timing thereof; |
| • | the rate of progress, costs and results of our clinical trials and research and development activities, including the extent of scheduling conflicts with participating clinicians and collaborators, and our ability to identify and enroll patients who meet clinical trial eligibility criteria; |
| • | other actions, decisions or rules issued by regulators; |
| • | our ability to access sufficient, reliable and affordable supplies of compounds used in the manufacture of our product candidates; |
| • | the efforts of our collaborators with respect to the commercialization of our products; and |
| • | the securing of, costs related to, and timing issues associated with, product manufacturing, as well as sales and marketing activities. |
If we fail to achieve announced milestones in the timeframes we expect, the commercialization of our product candidates may be delayed, our business and results of operations may be harmed, the trading price of the ADSs or ordinary shares may decline.
58
Access to raw materials and products necessary for the conduct of clinical trials, for commercialization, if approved, and manufacturing of our product candidates and product, if any, is not guaranteed.
We are dependent on third parties for the supply of various materials, chemical or biological products that are necessary to produce Viaskin patches for our clinical trials, and will need to depend on third parties to produce patches for our commercial supply, if Viaskin Peanut is approved. The supply of these materials could be reduced or interrupted at any time, including, without limitation, as a result of impacts due to pandemics, epidemics or other global health crises, natural disasters, new laws or regulations applicable to us or our suppliers, or other unfavorable global economic conditions, including as a result of the ongoing conflict between Russia-Ukraine, Irael-Hamas and other global political or military conflicts. In such case, we may not be able to find other suppliers of acceptable materials in appropriate quantities at an acceptable cost. If key suppliers or manufacturers are lost or the supply of materials is diminished or discontinued, we may not be able to continue to develop, manufacture and market our product candidates or products, if any, in a timely and competitive manner. In addition, these materials are subject to stringent manufacturing processes and rigorous testing. Delays in the completion and validation of facilities and manufacturing processes of these materials could adversely affect our ability to complete trials and commercialize our products, if any, in a cost- effective and timely manner. To prevent such situations, we intend to diversify our supply sources by identifying a second source of supply for critical raw materials and materials, such as natural protein . If we encounter difficulties in the supply of these materials, chemicals or biological products, if we were not able to maintain our supply agreements or establish new agreements to develop and manufacture our products in the future, our business, prospects, financial condition, results and development could be significantly affected.
Relying on third-party manufacturers may result in delays in our clinical development or commercialization efforts.
Developing and commercializing new medicines entails significant risks and expenses. Our clinical trials may be delayed if third-party manufacturers are unable to assure a sufficient quantity of the drug product to meet our study needs. Currently, we have only one manufacturer, Sanofi S.A., or Sanofi, of the active pharmaceutical ingredients, or API, used in our Viaskin product candidates, including Viaskin Peanut, such as peanut protein extract and unmodified allergen milk extract. In February 2020, Sanofi announced that it plans to create a new company dedicated to the production and marketing to third parties of API. Subsequently, Sanofi consolidated its API commercial and development activities conducted in six of its European API production sites. While those API sites do not include the site in which the API used in our Viaskin product candidates is produced, there can be no assurances that this transition will not adversely impact our supply of API from Sanofi. If Sanofi does not continue to manufacture the API as required by us in a timely manner, we may not be able to find a substitute manufacturer on a timely basis and our commercialization efforts and clinical trials may be delayed. Further, Sanofi’s strategic alliance partner, Regeneron, entered into a clinical collaboration with Aimmune Therapeutics, to evaluate treatment with Palforzia in combination with Dupilumab in peanut allergic patients. Regeneron commenced a Phase 2 clinical trial in October 2018 under this collaboration. This potential competitive dynamic may make Sanofi less inclined to continue or renew their manufacturing arrangement with us on commercially reasonable terms or at all and, notwithstanding contractual protections, Sanofi may be able to utilize knowledge gained through their relationship with us in furtherance of their development of competitive therapies.
We also expect to rely on Sanofi and on FAREVA for the manufacturing of the patch and on other third-party manufacturers for the manufacturing of commercial supply of Viaskin Peanut, if approved, and any other product for which we obtain regulatory approval. Sanofi may not be able to effectively scale its manufacturing capacity of our API to meet our commercialization needs and we may be unable to establish any agreements with other third-party manufacturers or to do so on acceptable terms. Even if Sanofi is able to meet our commercialization needs or if we are able to establish agreements with other third-party manufacturers, reliance on third-party manufacturers entails additional risks, including:
| • | reliance on the third party for regulatory compliance and quality assurance; |
| • | the possible breach of the manufacturing agreement by the third party; |
59
| • | the possible misappropriation of our proprietary information, including our trade secrets and know- how; and |
| • | the possible termination or non-renewal of the agreement by the third party at a time that is costly or inconvenient for us. |
Once regulatory approval is obtained, a marketed product and its manufacturer are subject to continual review. The discovery of previously unknown problems with a product or manufacturer may result in restrictions on the product, manufacturer or manufacturing facility, including withdrawal of the product from the market. Manufacturers of products with which we contract are required to operate in accordance with FDA-mandated current good manufacturing practices, or cGMPs, or comparable GMP requirements in foreign countries. A failure of any of our contract manufacturers to establish and follow cGMPs and to document their adherence to such practices may lead to significant delays in the launch or availability of products based on our product candidates into the market. Moreover, the constituent parts of a combination product retain their regulatory status (as a biologic or medical device, for example) and, as such, we or our contract manufacturers may be subject to additional requirements in the Quality System Regulation, or QSR, or comparable quality management systems in foreign countries, applicable to medical devices, such as design controls, purchasing controls, and corrective and preventive action. We, our contract manufacturers, any future collaborators and their contract manufacturers could be subject to periodic unannounced inspections by the FDA or other comparable foreign regulatory authorities, to monitor and ensure compliance with cGMP. Despite our efforts to audit and verify regulatory compliance, one or more of our third-party manufacturing vendors may be found on regulatory inspection by the FDA or other comparable foreign regulatory authorities to be noncompliant with cGMP regulations. Failure by third-party manufacturers to comply with applicable regulations could result in sanctions being imposed on us, including shutdown of the third-party vendor, fines, injunctions, civil penalties, revocation or suspension of regulatory approval for any products granted pre-market approvals, invalidation of drug product lots or processes, seizures or recalls of products, operating restrictions, and criminal prosecutions.
Our current and anticipated future dependence upon others for the manufacture of our product candidates or products, if approved, may adversely affect our future profit margins and our ability to commercialize any products that receive regulatory approval on a timely and competitive basis.
Our Viaskin product candidates may not be able to be manufactured profitably on a large enough scale to support commercialization.
To date, our Viaskin product candidates have only been manufactured at a scale which is adequate to supply our research activities and clinical trials. There can be no assurance that the procedures currently used to manufacture our product candidates will work at a scale which is adequate for commercial needs and we may encounter difficulties in the production of Viaskin patches due to our or our partners’ manufacturing capabilities. For example, in large-scale use, there is a possibility that our electrospray manufacturing tool, ES GEN4.0, may have issues related to maintenance of production quality, protein stability, and allergenicity. Additionally, during production, the containment of the electrospray function and the use of the allergen in liquid form keep the environment from being contaminated by the allergens. However, if there is a malfunction in the handling or storage phases or during the production phases, allergens could be released into the atmosphere and sensitize anyone present in the environment. We have not built commercial-scale manufacturing facilities, and we have limited manufacturing experience with Viaskin patches.
Additionally, while the production process was developed in strict compliance with current regulations, due to the originality of the product, we cannot predict if European or U.S. regulatory authorities will make new regulations applicable to our production process, or if we will have any future disagreements with such regulatory authorities regarding our interpretation of the regulatory requirements.
We rely on a single supplier to produce, or contract for the production of, active ingredients and we rely on a single manufacturer to produce patches for our clinical trials and for our commercial supplies of any future approved products.
60
Even if we were to obtain access to quantities of active ingredients sufficient to allow us otherwise to expand our Viaskin manufacturing capabilities, we may not be able to produce sufficient quantities of the product at an acceptable cost, or at all. In the event our Viaskin product candidates cannot be manufactured in sufficient quantities for commercialization, our future prospects could be significantly impacted and our financial prospects would be materially harmed.
We, or the third parties upon whom we depend, may be adversely affected by earthquakes, other natural disasters or outbreaks of contagious diseases and our business continuity and disaster recovery plans may not adequately protect us from a serious disaster.
Earthquakes, other natural disasters or an outbreak of a contagious disease, such as COVID-19, could severely disrupt our operations, and have a material adverse effect on our business, results of operations, financial condition and prospects. If a natural disaster, power outage or other event occurred that prevented us from using all or a significant portion of our facilities or infrastructure, that damaged critical infrastructure, such as the manufacturing facilities of our third-party contract manufacturers, or that otherwise disrupted operations, it may be difficult or, in certain cases, impossible for us to continue our business for a substantial period of time. The disaster recovery and business continuity plans we have in place may prove inadequate in the event of a serious disaster or similar event. We may incur substantial expenses as a result of the limited nature of our disaster recovery and business continuity plans, which, particularly when taken together with our lack of earthquake insurance, could have a material adverse effect on our business.
We rely, and will rely in the future, on third parties to conduct our clinical trials and perform data collection and analysis, which may result in costs and delays that prevent us from successfully commercializing product candidates.
We rely, and will rely in the future, on medical institutions, clinical investigators, CROs, contract laboratories and collaborators to perform data collection and analysis and others to carry out our clinical trials. Our development activities or clinical trials conducted in reliance on third parties may be delayed, suspended, or terminated if:
| • | the third parties do not successfully carry out their contractual duties or fail to meet regulatory obligations or expected deadlines; |
| • | we replace a third party; or |
| • | the quality or accuracy of the data obtained by third parties is compromised due to their failure to adhere to clinical protocols, regulatory requirements, or for other reasons. |
Third party performance failures may increase our development costs, delay our ability to obtain regulatory approval, and delay or prevent the commercialization of our product candidates. While we believe that there are numerous alternative sources to provide these services, in the event that we seek such alternative sources, we may not be able to enter into replacement arrangements without incurring delays or additional costs.
Even if collaborators with which we contract in the future successfully complete clinical trials of our product candidates, those candidates may not be commercialized successfully for other reasons.
Even if we contract with collaborators that successfully complete clinical trials for one or more of our product candidates, those candidates may not be commercialized for other reasons, including:
| • | failing to receive regulatory approval to market them as drugs; |
| • | being subject to proprietary rights held by others; |
| • | failing to obtain approval from regulatory authorities on the manufacturing of our products; |
| • | being difficult or expensive to manufacture on a commercial scale; |
61
| • | having adverse side effects that make their use less desirable; |
| • | failing to compete effectively with products or treatments commercialized by competitors; or |
| • | failing to show long-term risk/benefit ratio of our products. |
Currently, we do not have commercial-ready marketing and sales infrastructure. If we are unable to establish effective sales or marketing capabilities or enter into agreements with third parties to sell or market our product candidates, we may not be able to effectively sell or market our product candidates, if approved, or generate product revenues.
We currently have a limited commercial infrastructure. To achieve commercial success for any approved product candidate for which we retain sales and marketing responsibilities, we must build our sales, marketing, managerial, and other non-technical capabilities or make arrangements with third parties to perform these services. For example, we are planning to hire sales representatives for the marketing of Viaskin Peanut in the United States, if approved. There are risks involved with both establishing our own sales and marketing capabilities and entering into arrangements with third parties to perform these services. For example, recruiting and training a sales force is expensive and time consuming and could delay any product launch. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel.
Factors that may inhibit our efforts to commercialize our product candidates on our own include:
| • | our inability to recruit, hire, retain and incentivize adequate numbers of effective sales and marketing personnel; |
| • | the inability of sales personnel to obtain access to physicians or educate adequate numbers of physicians on the benefits of prescribing any future products; |
| • | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and |
| • | unforeseen costs and expenses associated with establishing an independent sales and marketing organization. |
If we enter into arrangements with third parties to perform sales, marketing and distribution services for the commercialization of Viaskin Peanut in the United States or the European Union, if approved, our product revenues or the profitability of these product revenues to us are likely to be lower than if we were to market and sell any product candidates that we develop ourselves. In addition, we may not be successful in entering into arrangements with third parties to sell and market Viaskin Peanut or any of our other product candidates or may be unable to do so when needed or on terms that are favorable to us. We likely will have more limited control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our product candidates effectively, or they may fail to comply with promotional requirements for prescription products that could render our products misbranded in violation of government regulations and thus potentially subject to enforcement. If we do not establish sales and marketing capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing Viaskin Peanut or any of our other product candidates that receive regulatory approval, or any such commercialization may experience delays or limitations. If we are not successful in commercializing Viaskin Peanut or any of our other product candidates, either on our own or through collaborations with one or more third parties, our business, results of operations, financial condition and prospects will be materially and adversely affected.
62
Our product candidates are regulated as biological products, or biologics, which may subject them to competition sooner than anticipated.
The Biologics Price Competition and Innovation Act, or BPCIA, established an abbreviated licensure pathway for biological products shown to be biosimilar to, or interchangeable with, an FDA-licensed biological reference product. “Biosimilarity” means that the biological product is highly similar to the reference product notwithstanding minor differences in clinically inactive components and there are no clinically meaningful differences between the biological product and the reference product in terms of safety, purity, and potency of the product. To meet the higher standard of “interchangeability,” an applicant must provide sufficient information to show biosimilarity and demonstrate that the biological product can be expected to produce the same clinical result as the reference product in any given patient and, if the biological product is administrated more than once to an individual, the risk in terms of safety or diminished efficacy of alternating or switching between the use of the biological product and the reference product is not greater than the risk of using the reference product without such alternation or switch.
Under the BPCIA, an application for a biosimilar or interchangeable product cannot be approved by the FDA until 12 years after the reference product was first licensed, and the FDA will not even accept an application for review until four years after the date of first licensure. The law is evolving, complex and is still being interpreted and implemented by the FDA. As a result, its ultimate impact, implementation, and meaning are subject to uncertainty and could have a material adverse effect on the future commercial prospects for our biological products.
We believe that any of our product candidates approved as a biological product under a BLA should qualify for the 12-year period of exclusivity. However, there is a risk that this exclusivity could be shortened due to congressional action or otherwise, potentially creating the opportunity for biosimilar or interchangeable competition sooner than anticipated. Moreover, the process by which an interchangeable product, once approved, will be substituted for any one of our reference products in a way that is similar to traditional generic substitution for non-biological products (i.e., drugs) is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing and subject to interpretation.
The European Union provides opportunities for data and market exclusivity related to marketing authorizations. Upon receiving a marketing authorization, innovative medicinal products are generally entitled to receive eight years of data exclusivity and 10 years of market exclusivity, which run in parallel. Data exclusivity, if granted, prevents regulatory authorities in the European Union from referencing the innovator’s data to assess a generic application or biosimilar application for eight years from the date of authorization of the innovative product, after which a generic or biosimilar marketing authorization application can be submitted, and the innovator’s data may be referenced. The market exclusivity period prevents a successful generic or biosimilar applicant from commercializing its product in the European Union until 10 years have elapsed from the initial marketing authorization of the reference product in the European Union. The overall ten-year period may, occasionally, be extended for a further year to a maximum of 11 years if, during the first eight years of those ten years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. However, there is no guarantee that a product will be considered by the European Union’s regulatory authorities to be a new chemical/biological entity, and products may not qualify for data exclusivity.
In the European Union, there is a special regime for biosimilars, or biological medicinal products that are similar to a reference medicinal product but that do not meet the definition of a generic medicinal product. For such products, the results of appropriate preclinical or clinical trials must be provided in support of an application for Marketing Authorization. Guidelines from the EMA detail the type of quantity of supplementary data to be provided for different types of biological product.
63
We also believe that our product candidates in the European Union should benefit from data and market exclusivity. As with the U.S., however, if competitors obtain marketing authorization for their biosimilar products, our products may become subject to competition from these biosimilars, with the attendant competitive pressure and consequences.
Even if any of our product candidates are commercialized, they may not be accepted by physicians, patients, or the medical community in general. Even if we, or our collaborators, are able to commercialize our product candidates, the products may become subject to market conditions that could harm our business.
Even if the medical community accepts a product as safe and efficacious for its indicated use, prescribers may choose to restrict the use of the product if we are,or any collaborator is, unable to demonstrate that, based on experience, clinical data, side-effect profiles and other factors, our product is preferable to any existing drugs or treatments. We cannot predict the degree of market acceptance of any product candidate that receives regulatory approval, which will depend on a number of factors, including, but not limited to:
| • | the demonstration of the clinical efficacy and safety of the product; |
| • | the approved labeling for the product and any required warnings; |
| • | the advantages and disadvantages of the product compared to alternative treatments; |
| • | our and any collaborator’s ability to educate the medical community about the safety and effectiveness of the product; |
| • | the coverage and reimbursement policies of government and commercial third-party payors pertaining to the product; |
| • | the market price of our product relative to competing treatments; and |
| • | our ability to effectively implement a scientific publication strategy. |
We face substantial competition from companies with considerably more resources and experience than we have, which may result in others discovering, developing, receiving approval for, or commercializing products before or more successfully than us.
The biopharmaceuticals industry is highly competitive. Numerous biopharmaceutical and biotechnology companies, universities and other research entities are actively involved in the discovery, development and commercialization of therapeutic options to treat allergies, making it a highly competitive field. We have competitors in several jurisdictions, many of which have substantially greater name recognition, commercial infrastructures and financial, technical and personnel resources than we have. Although we believe we are currently in a unique position with respect to the testing and treatment of food allergies in children, established competitors may invest heavily to quickly discover and develop novel compounds that could make any of our product candidates obsolete or uneconomical. Any new product that competes with an approved product may need to demonstrate compelling advantages in efficacy, convenience, tolerability and safety to be commercially successful. Other competitive factors, including generic competition, could force us to lower prices or could result in reduced sales. In addition, new products developed by others could emerge as competitors to any of our product candidates. If we are not able to compete effectively against our current and future competitors, our business will not grow and our financial condition and operations will suffer.
In the case of food allergies, we are aware of several food allergy academic studies and pharmaceutical developmental efforts connected with such studies that are currently being conducted in major medical centers and hospitals worldwide. These studies are evaluating forms of allergen desensitization treatments such as oral (OIT), sublingual (SLIT), subcutaneous (SCIT), or oral mucosal (OMIT), and cutaneous (CIT) immunotherapy, or products using synthetic allergens, denatured allergens, small molecule inhibitors, or combinations of medicines or methods, or medicines using traditional methods such as Chinese herbs.
64
Studies combining other methods of allergen immunotherapy, such as OIT, with monoclonal antibodies (anti-IgE and anti-IL-4Rα) as adjunct therapy are being conducted currently. These types of co-administrations may significantly improve the safety of specific allergen immunotherapies administered orally or subcutaneously. Monoclonal antibodies, used alone as monotherapy or in combination with allergen immunotherapy, may become significant competitors to our products. On February 16 2024, the FDA approved Xolair® (omalizumab) for the reduction of allergic reactions, including anaphylaxis, that may occur with accidental exposure to one or more foods in adult and pediatric patients aged 1 year and older with IgE-mediated food allergy.
There is one treatment that is specific for peanut allergy, a proprietary form of OIT which was approved by the FDA and the European Commission: Palforzia, formulation of peanut flour developed by Aimmune Therapeutics, Inc., or Aimmune. Nestlé S.A. acquired Aimmune in October 2020, and divested the Palforzia business to Stallergenes Greer in September 2023.
Government restrictions on pricing and reimbursement, as well as other healthcare payor cost-containment initiatives, may negatively impact our ability to generate revenues if we obtain regulatory approval to market a product.
The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare costs to contain or reduce costs of healthcare may adversely affect one or more of the following:
| • | our ability or our collaborators’ ability to set a price we believe is fair for our products, if approved; |
| • | our ability or our collaborators’ ability to obtain and maintain market acceptance by the medical community and patients; |
| • | our ability to generate revenues and achieve profitability; and |
| • | the availability of capital. |
Sales of our products, when and if approved for marketing, will depend, in part, on the extent to which our products will be covered by third-party payors, such as federal, state, and foreign government health care programs, commercial insurance and managed healthcare organizations. There may be significant delays in obtaining coverage and reimbursement for newly approved products, and coverage may be more limited than the purposes for which the product is approved by the FDA or comparable foreign regulatory authorities. Moreover, eligibility for coverage and reimbursement does not imply that a product will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sales and distribution. Third-party payors are increasingly reducing reimbursements for medical products, drugs and services. In addition, the U.S. government, state legislatures and foreign governments have continued implementing cost containment programs, including price controls, restrictions on coverage and reimbursement and requirements for substitution of generic products. Adoption of price controls and cost containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. Limited third-party reimbursement for our product candidates or a decision by a third-party payor not to cover our product candidates could reduce physician usage of our products once approved and have a material adverse effect on our sales, results of operations and financial condition.
Various provisions of the Patient Protection and Affordable Care Act as amended by the Health Care and Education Reconciliation Act, or collectively, the ACA, were designed to impact the provision of, or payment for, health care in the United States, including expanded Medicaid eligibility, subsidized insurance premiums, provided incentives for businesses to provide health care benefits, prohibited denials of coverage due to pre-existing conditions, established health insurance exchanges, and provided additional support for medical research. With regard to biopharmaceutical products, among other things, the ACA expanded and increased industry rebates for drugs covered under Medicaid programs and made changes to the coverage requirements under the Medicare prescription drug benefit.
65
However, there have been executive, judicial and Congressional challenges to certain aspects of the ACA. For example, on June 17, 2021 the U.S. Supreme Court dismissed a challenge on procedural grounds that argued the ACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress. On August 16, 2022, President Biden signed the Inflation Reduction Act of 2022, or IRA, into law, which, among other things, extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025. The IRA also eliminates the “donut hole” under the Medicare Part D program beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost and creating a new manufacturer discount program. It is possible that the ACA will be subject to judicial or Congressional challenges in the future. It is unclear how such challenges and the healthcare reform measures of the Biden administration will impact the ACA or operations.
Following ACA, both the Budget Control Act of 2011 and the American Taxpayer Relief Act of 2012, or the ATRA, include, among other things, mandatory reductions in Medicare payments to certain providers. Additionally, in the United States, there have been several recent Congressional inquiries and federal and state legislative activity designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. At the federal level, in July 2021, the Biden administration released an executive order, “Promoting Competition in the American Economy,” with multiple provisions aimed at prescription drugs. In response to the Biden administration’s executive order, on September 9, 2021, the U.S. Department of Health and Human Services, or HHS, released a Comprehensive Plan for Addressing High Drug Prices that outlines principles for drug pricing reform and sets out a variety of potential legislative policies that Congress could pursue as well as potential administrative actions HHS can take to advance these principles. In addition, the IRA, among other things, (i) directs HHS to negotiate the price of certain high-expenditure, single-source drugs and biologics covered under Medicare, and subject drug manufacturers to civil monetary penalties and a potential excise tax by offering a price that is not equal to or less than the negotiated “maximum fair price” for such drugs and biologics under the law, and (ii) imposes rebates with respect to certain drugs and biologics covered under Medicare Part B or Medicare Part D to penalize price increases that outpace inflation. These provisions take effect progressively starting in fiscal year 2023. On August 29, 2023, HHS announced the list of the first ten drugs that will be subject to price negotiations, although the Medicare drug price negotiation program is currently subject to legal challenge. It is currently unclear how the IRA will be implemented but is likely to have a significant impact on the pharmaceutical industry. In response to the Biden administration’s October 2022 executive order, on February 14, 2023, HHS released a report outlining three new models for testing by the CMS Innovation Center which will be evaluated on their ability to lower the cost of drugs, promote accessibility, and improve quality of care. It is unclear whether the models will be utilized in any health reform measures in the future. Further, on December 7, 2023, the Biden administration announced an initiative to control the price of prescription drugs through the use of march-in rights under the Bayh-Dole Act. On December 8, 2023, the National Institute of Standards and Technology published for comment a Draft Interagency Guidance Framework for Considering the Exercise of March-In Rights which for the first time includes the price of a product as one factor an agency can use when deciding to exercise march-in rights. While march-in rights have not previously been exercised, it is uncertain if that will continue under the new framework. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
Additional legislative proposals to reform healthcare and government insurance programs, along with the trend toward managed healthcare in the United States, could influence the purchase of medicines and reduce demand and prices for our products, if approved. This could harm our or our collaborators’ ability to market any products and generate revenues. Cost containment measures that healthcare payors and providers are instituting and the effect of further healthcare reform could significantly reduce potential revenues from the sale of any of our product candidates approved in the future, and could cause an increase in our compliance, manufacturing, or other operating expenses.
66
In some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. In addition, in certain foreign markets, the pricing of prescription drugs is subject to government control and reimbursement may in some cases be unavailable. The requirements governing drug pricing vary widely from country to country. For example, the European Union provides options for its Member States to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. An EU Member State may approve a specific price for a medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market.
Many EU Member States periodically review their reimbursement procedures for medicinal products, which could have an adverse impact on reimbursement status. We expect that legislators, policymakers and healthcare insurance funds in the EU Member States will continue to propose and implement cost-containing measures, such as lower maximum prices, lower or lack of reimbursement coverage and incentives to use cheaper, usually generic, products as an alternative to branded products, and/or branded products available through parallel import to keep healthcare costs down. Moreover, in order to obtain reimbursement for our products in some European countries, including some EU Member States, we may be required to compile additional data comparing the cost- effectiveness of our products to other available therapies. Health Technology Assessment, or HTA, of medicinal products is becoming an increasingly common part of the pricing and reimbursement procedures in some EU Member States, including those representing the larger markets. The HTA process is the procedure to assess therapeutic, economic and societal impact of a given medicinal product in the national healthcare systems of the individual country. The outcome of an HTA will often influence the pricing and reimbursement status granted to these medicinal products by the competent authorities of individual EU Member States. The extent to which pricing and reimbursement decisions are influenced by the HTA of the specific medicinal product currently varies between EU Member States. In December 2021, Regulation No 2021/2282 on Health Technology Assessment, amending Directive 2011/24/EU, was adopted in the EU. This Regulation, which entered into force in January 2022 and will apply as of January 2025, is intended to boost cooperation among EU Member States in assessing health technologies, including new medicinal products, and providing the basis for cooperation at EU level for joint clinical assessments in these areas. The Regulation foresees a three-year transitional period and will permit EU Member States to use common HTA tools, methodologies, and procedures across the EU, working together in four main areas, including joint clinical assessment of the innovative health technologies with the most potential impact for patients, joint scientific consultations whereby developers can seek advice from HTA authorities, identification of emerging health technologies to identify promising technologies early, and continuing voluntary cooperation in other areas. Individual EU Member States will continue to be responsible for assessing non-clinical (e.g., economic, social, ethical) aspects of health technologies, and making decisions on pricing and reimbursement. If we are unable to maintain favorable pricing and reimbursement status in EU Member States for product candidates that we may successfully develop and for which we may obtain regulatory approval, any anticipated revenue from and growth prospects for those products in the EU could be negatively affected.
Legislators, policymakers and healthcare insurance funds in the EU may continue to propose and implement cost-containing measures to keep healthcare costs down; particularly due to the financial strain that the COVID-19 pandemic placed on national healthcare systems of the EU Member States. These measures could include limitations on the prices we would be able to charge for product candidates that we may successfully develop and for which we may obtain regulatory approval or the level of reimbursement available for these products from governmental authorities or third-party payors. Further, an increasing number of EU and other foreign countries use prices for medicinal products established in other countries as “reference prices” to help determine the price of the product in their own territory. Consequently, a downward trend in prices of medicinal products in some countries could contribute to similar downward trends elsewhere.
There can be no assurance that any country that has price controls or reimbursement limitations for biopharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products. Historically, biopharmaceutical products launched in the European Union do not follow price structures of the United States and generally tend to have significantly lower prices.
67
We believe that pricing pressures at the federal and state levels in the United States, as well as internationally, will continue and may increase, which may make it difficult for us to sell our potential products that may be approved in the future at a price acceptable to us or any of our future collaborators.
Guidelines and recommendations published by various organizations may impact the use or reimbursement of Viaskin Peanut, if approved.
Government authorities promulgate regulations and guidelines that may be directly applicable to us and any approved products. However, professional societies, practice management groups, insurance carriers, physicians’ groups, private health and science foundations and organizations involved in various diseases also publish guidelines and recommendations to healthcare providers, administrators and payors, as well as patient communities.
Recommendations by government authorities or other groups and organizations may relate to such matters as usage, dosage, route of administration and use of related therapies, and a growing number of organizations are providing assessments of the value and pricing of pharmaceutical products. These assessments may come from private organizations, such as the Institute for Clinical and Economic Review, or ICER, which publish their findings and offer recommendations relating to the products’ reimbursement by government and private payors. In July 2019, ICER published its final report assessing the comparative clinical effectiveness and value of treatments for peanut allergy, including Viaskin Peanut and a competitor product candidate. The results of this or any future ICER report or any similar recommendations or guidelines may affect our reputation, and any recommendations or guidelines that result in decreased use or reimbursement of Viaskin Peanut, if approved, could have a material adverse effect on our results of operations and financial condition. In addition, the occurrence of any of the foregoing, or the perception by the investment community or shareholders that such recommendations or guidelines will result in decreased use or reimbursement of Viaskin Peanut, if approved, could adversely affect the market price of our securities.
Our product candidates may cause undesirable side effects that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following regulatory approval, if any.
Our product candidates are being developed to address the needs of allergic patients, for some of whom they can have a profound and life-threatening adverse reaction if exposed to even minute amounts of an allergen. Accordingly, safety is of paramount importance in developing these product candidates. To date, more than twelve clinical trials of Viaskin Peanut and Viaskin Milk product candidates have been conducted both outside and inside of the United States in over 1,000 human subjects to evaluate the safety and efficacy of these product candidates for the treatment of peanut allergies and milk allergies, respectively. Adverse events observed in these clinical trials have primarily involved general disorders such skin and subcutaneous tissue, immune system and administration site conditions, such as erythema, pruritus, edema and urticaria. However, in clinical trials to date, one case of mild to moderate anaphylaxis has been reported, and it is possible that anaphylaxis or other systemic reactions may occur in the future. It is worth noting that, as a desensitization patch bringing the allergen into contact with the skin, reactions, which are a source of itching and discomfort for subjects, are common. This reaction is typically temporary in duration and fades after a few weeks of use. In addition, during daily administration of the patches during treatments, depending on the severity of the allergies and subject response to treatment, precautionary measures are necessary when handling the patches after use due to risk of contamination.
Undesirable side effects caused by our product candidates could cause us or regulatory authorities to interrupt, delay, halt or terminate clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other regulatory authorities. Further, if our Viaskin patch product candidates receive regulatory approval and we or others identify undesirable side effects caused by the products (or any other similar products) after approval, a number of potentially significant negative consequences could result, including:
68
| • | regulatory authorities may withdraw or limit their approval of the products; |
| • | regulatory authorities may require the addition of labeling statements, such as a “boxed” warning or a contraindication; |
| • | we may be required to change the way the products are distributed or administered, conduct additional clinical trials or change the labeling of the products; |
| • | we may decide to remove the products from the marketplace; |
| • | we could be sued and held liable for injury caused to individuals exposed to or taking our products; and |
| • | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of the affected products and could substantially increase the costs of commercializing our products and significantly impact our ability to successfully commercialize our products and generate revenues.
Our future growth depends, in part, on our ability to penetrate foreign markets, where we would be subject to additional regulatory burdens and other risks and uncertainties.
Our future profitability will depend, in part, on our ability to commercialize product candidates based on our Viaskin technology platform in multiple markets, including but not limited to those within the United States and Europe. If we commercialize product candidates based on our Viaskin technology platform in foreign markets, we would be subject to additional risks and uncertainties, including:
| • | the burden of complying with complex and changing foreign regulatory, tax, accounting and legal requirements; |
| • | different medical practices and customs in foreign countries affecting acceptance in the marketplace; |
| • | import or export licensing requirements; |
| • | longer accounts receivable collection times; |
| • | longer lead times for shipping; |
| • | language barriers for technical training; |
| • | reduced protection of intellectual property rights in some foreign countries, and related prevalence of generic alternatives to therapeutics; |
| • | foreign currency exchange rate fluctuations; |
| • | patients’ ability to obtain reimbursement for Viaskin patch products in foreign markets; and |
| • | the interpretation of contractual provisions governed by foreign laws in the event of a contract dispute. |
Foreign sales of Viaskin patch products could also be adversely affected by the imposition of governmental controls, political and economic instability, trade restrictions and changes in tariffs.
The United Kingdom’s withdrawal from the EU may have a negative effect on global economic conditions, financial markets and our business, which could reduce the price of our common shares.
Following Brexit, the UK and the EU signed a EU-UK Trade and Cooperation Agreement, or TCA, which became provisionally applicable on January 1, 2021 and entered into force on May 1, 2021. This agreement provides details on how some aspects of the UK and EU’s relationship will operate going forwards however there are still uncertainties.
69
The TCA primarily focuses on ensuring free trade between the EU and the UK in relation to goods, including medicinal products. Among the changes that have occurred are that Great Britain (England, Scotland and Wales) is treated as a “third country,” a country that is not a member of the EU and whose citizens do not enjoy the EU right to free movement. Northern Ireland continues to follow many aspects of the EU regulatory rules, particularly in relation to trade in goods. As part of the TCA, the EU and the UK recognize GMP inspections carried out by the other party and the acceptance of official GMP documents issued by the other party. The TCA also encourages, although it does not oblige, the parties to consult one another on proposals to introduce significant changes to technical regulations or inspection procedures. Among the areas of absence of mutual recognition are batch testing and batch release. The UK has unilaterally agreed to accept EU batch testing and batch release. However, the EU continues to apply EU laws that require batch testing and batch release to take place in the EU territory. This means that medicinal products that are tested and released in the UK must be retested and re-released when entering the EU market for commercial use.
As it relates to marketing authorizations, Great Britain has a separate regulatory submission process, approval process and a separate national marketing authorization. Northern Ireland continues, however, to be covered by the marketing authorizations granted by the European Commission. For example, the scope of a marketing authorization for a medicinal product granted by the European Commission or by the competent authorities of EU Member States no longer encompasses Great Britain (England, Scotland and Wales). In these circumstances, a separate marketing authorization granted by the UK competent authorities is required to place medicinal products on the market in Great Britain. Northern Ireland continues, however, to be covered by the marketing authorizations granted by the European Commission.
On February 27, 2023, the UK Government and the European Commission reached a political agreement on the so-called “Windsor Framework”. The Framework is intended to revise the Northern Ireland Protocol to address some of the perceived shortcomings in its operation. The agreement was adopted at the Withdrawal Agreement Joint Committee on March 24, 2023. If the changes are adopted in the form proposed, medicinal products to be placed on the market in the UK will be authorized solely in accordance with UK laws. Northern Ireland would be reintegrated back into a UK-only regulatory environment under the authority of the MHRA with respect to all medicinal products. The implementation of the Windsor Framework would occur in stages, with new arrangements relating to the supply of medicinal products into Northern Ireland anticipated to take effect in 2025.
A significant proportion of the regulatory framework in the UK applicable to medicinal products is currently derived from EU Directives and Regulations. The potential for UK legislation to diverge from EU legislation following Brexit could materially impact the regulatory regime with respect to the development, manufacture, import, approval, and commercialization of our product candidates in the UK or the EU. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies governing clinical trials, our development plans may be impacted.
All of these changes could increase our costs and otherwise adversely affect our business. Any delay in obtaining, or an inability to obtain, any regulatory approvals, as a result of Brexit or otherwise, would prevent us from commercializing our product candidates in the UK or the EU and restrict our ability to generate revenue and achieve and sustain profitability. In addition, we may be required to pay taxes or duties or be subjected to other hurdles in connection with the importation of our product candidates into the EU. If any of these outcomes occur, we may be forced to restrict or delay efforts to seek regulatory approval in the UK or the EU for our product candidates, or incur significant additional expenses to operate our business, which could significantly and materially harm or delay our ability to generate revenues or achieve profitability of our business. Any further changes in international trade, tariff and import/export regulations as a result of Brexit or otherwise may impose unexpected duty costs or other non-tariff barriers on us. These developments, or the perception that any of them could occur, may significantly reduce global trade and, in particular, trade between the impacted nations and the UK. It is also possible that Brexit may negatively affect our ability to attract and retain employees, particularly those from the EU.
70
We are subject to healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, integrity obligations, exclusion from government healthcare programs, individual imprisonment, contractual damages, reputational harm and diminished profits and future earnings, among other consequences.
Healthcare providers and others will play a primary role in the recommendation and prescription of Viaskin patch products, if approved. Our arrangements with such persons and third-party payors will expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we research, market, sell and distribute Viaskin patch products, if we obtain regulatory approval. Restrictions under applicable federal, state and foreign healthcare laws and regulations include but are not limited to the following:
| • | The federal Anti-Kickback Statute prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration (including any kickback, bribe or rebate), directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for or the purchase, lease, order or recommendation of any item, good, facility or service for which payment may be made under federal healthcare programs such as Medicare and Medicaid. Although there are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution, the exceptions and safe harbors are drawn narrowly. Practices that involve remuneration that may be alleged to be intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exception or safe harbor. The intent standard under the federal Anti-Kickback Statute was amended by the ACA to a stricter standard such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. Moreover, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act. |
| • | The federal civil and criminal false claims laws, including the civil False Claims Act, impose criminal and civil penalties, including those from civil whistleblower or qui tam actions, and civil monetary penalties laws, which prohibit, among other things, knowingly presenting, or causing to be presented, claims for payment that are false or fraudulent or making a false statement to avoid, decrease, or conceal an obligation to pay money to the federal government. |
| • | The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created federal criminal and civil liability for, among other things, executing a scheme to defraud any healthcare benefit program or knowingly and willingly falsifying, concealing or covering up a material fact or making false statements relating to healthcare matters. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation. |
| • | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and its implementing regulations, which impose certain requirements on covered entities and their business associates, and their covered subcontractors, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information. |
| • | The federal transparency requirements under the Physician Payments Sunshine Act, enacted as part of the ACA, that require applicable manufacturers of covered drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program, with specific exceptions, to track and annually report to CMS payments and other transfers of value provided to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), other healthcare professionals (such as physician assistants and nurse practitioners), and teaching hospitals and certain ownership and investment interests held by physicians or their immediate family members in the applicable manufacturer, and disclosure of such information will be made by CMS on a publicly available website. |
71
| • | Analogous state, local or foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to items or services reimbursed by any third-party payor, including commercial insurers; state and local marketing and/or transparency laws applicable to manufacturers that may be broader in scope than the federal requirements, state laws that require biopharmaceutical companies to comply with the biopharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, state and local laws that require licensure or registration of pharmaceutical sales representatives; state laws that require disclosure of information related to drug pricing; and state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect as HIPAA. |
Outside the United States, interactions between pharmaceutical companies and health care professionals are also governed by strict laws, such as national anti-bribery laws of European countries, national sunshine rules, regulations, industry self-regulation codes of conduct and physicians’ codes of professional conduct. Failure to comply with these requirements could result in reputational risk, public reprimands, administrative penalties, fines or imprisonment.
Ensuring that our business arrangements with third parties comply with applicable healthcare laws and regulations could be costly. Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our current and/or future business activities could be subject to challenge under one or more of these laws. If our operations were found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from government funded healthcare programs, such as Medicare and Medicaid, or comparable foreign programs, integrity obligations, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of operations, any of which could substantially disrupt our operations. Defending against any such actions can be costly, time- consuming and may require significant financial and personnel resources. Even if we are successful in defending against any such actions that may be brought against us, our business may be impaired. If the physicians or other providers or entities with whom we expect to do business are found not to be in compliance with applicable laws, they may be subject to significant criminal, civil or administrative sanctions, including exclusion from government funded healthcare programs.
Changes in regulatory requirements, or guidance from the FDA or comparable foreign regulatory authorities or unanticipated events during our clinical trials of Viaskin products may occur, which may result in changes to clinical trial protocols or additional clinical trial requirements, which could result in increased costs to us and could delay our development timeline.
Changes in regulatory requirements, or guidance from the FDA or comparable foreign regulatory authorities or unanticipated events during our clinical trials may force us to amend clinical trial protocols or the FDA or certain foreign regulatory authorities may impose additional clinical trial requirements. Discussions with regulatory authorities have caused us to adjust certain trial protocols. Amendments to our clinical trial protocols would require resubmission to the FDA and IRBs or competent foreign regulatory authorities, for review and approval, as applicable, which may adversely impact the cost, timing or successful completion of a clinical trial. If we experience delays completing, or if we terminate, any of our clinical trials, or if we are required to conduct additional clinical trials, the commercial prospects for the Viaskin patch product candidates, or any other product candidates, may be harmed and our ability to generate product revenue will be delayed.
In addition, the policies of the FDA, the competent authorities of the EU Member States, the EMA, the European Commission and other comparable regulatory authorities responsible for clinical trials may change and additional government regulations may be enacted. For instance, the regulatory landscape related to clinical trials in the EU recently evolved. The EU Clinical Trials Regulation, or CTR, which was adopted in April 2014 and repeals the EU Clinical Trials Directive, became applicable on January 31, 2022. The CTR allows sponsors to make a single submission to both the competent authority and an ethics committee in each EU Member State, leading to a single decision for each EU Member State.
72
The assessment procedure for the authorization of clinical trials has been harmonized as well, including a joint assessment by all EU Member States concerned, and a separate assessment by each EU Member State with respect to specific requirements related to its own territory, including ethics rules. Each EU Member State’s decision is communicated to the sponsor via the centralized EU portal. Once the clinical trial approved, clinical study development may proceed. The CTR foresees a three-year transition period. The extent to which ongoing and new clinical trials will be governed by the CTR varies. For clinical trials in relation to which application for approval was made on the basis of the Clinical Trials Directive before January 31, 2023. By that date, all ongoing trials has become subject to the provisions of the CTR. The CTR will apply to clinical trials from an earlier date if the related clinical trial application was made on the basis of the CTR or if the clinical trial has already transitioned to the CTR framework before January 31, 2025. Compliance with the CTR requirements by us and our third-party service providers, such as CROs, may impact our developments plans.
It is currently unclear to what extent the UK will seek to align its regulations with the EU in the future. The UK regulatory framework in relation to clinical trials is derived from existing EU legislation (as implemented into UK law, through secondary legislation).
On January 17, 2022, the UK Medicines and Healthcare products Regulatory Agency, or MHRA, launched an eight-week consultation on reframing the UK legislation for clinical trials. The UK Government published its response to the consultation on March 21, 2023 confirming that it would bring forward changes to the legislation. These resulting legislative amendments will determine how closely the UK regulations will align with the CTR. Failure of the UK to closely align its regulations with the EU may have an effect on the cost of conducting clinical trials in the UK as opposed to other countries and/or make it harder to seek a marketing authorization for our product candidates on the basis of clinical trials conducted in the UK.
If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies governing clinical trials, our development plans may be impacted.
The FDA and other comparable foreign regulatory authorities actively enforce the laws and regulations prohibiting the promotion of off-label uses. If we are found to have improperly promoted off-label uses, we may become subject to significant liability.
The FDA and other comparable foreign regulatory authorities strictly regulate the promotional claims that may be made about prescription products, such as Viaskin patch products, if approved. In particular, a product may not be promoted for uses that are not approved by the FDA or such other comparable foreign regulatory authorities, as reflected in the product’s approved labeling. If we receive regulatory approval for Viaskin patch products as a treatment for a particular allergy, physicians, in their independent professional medical judgment, may nevertheless prescribe Viaskin patch products to their patients in a manner that is inconsistent with the approved label. Additionally, it is permissible to share in certain circumstances and in accordance with applicable FDA, and comparable regulatory authorities’, guidance and regulations truthful and non-misleading information that is consistent with, but not contained in, the product’s approved labeling. If we are found to have promoted off-label uses or promoted our product before approval, we may become subject to significant liability under the FDCA and other statutory authorities, such as laws prohibiting false claims for reimbursement. The federal government has levied large civil and criminal fines against companies for alleged improper promotion and has enjoined several companies from engaging in off-label promotion. The FDA and other U.S. government agencies has also requested that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed. If we cannot successfully manage the marketing of Viaskin patch products, if approved, by restricting off-label promotion, we could become subject to significant liability, which would materially adversely affect our business and financial condition. Similar limitations and penalties are provided in the EU both at EU level and at national level in individual EU Member States.
73
Our product development programs may require substantial financial resources and may ultimately be unsuccessful.
The success of our business depends primarily upon our ability to identify, develop and commercialize products to treat food allergies. In addition to Viaskin Peanut, we may pursue development of our other development programs, including Viaskin Milk. None of our other product candidates and potential product candidates has commenced any clinical trials since we scaled down our research and clinical development efforts in 2020 and 2021 to focus on Viaskin Peanut. There are a number of FDA or foreign requirements that we must satisfy before we can commence clinical trials. Satisfaction of these requirements will entail substantial time, effort and financial resources. We may never satisfy these requirements. We may never commence clinical trials of such development programs despite expending significant resources in pursuit of their development. If we do commence clinical trials of our other potential product candidates, such product candidates may never be approved by the FDA or comparable foreign regulatory authorities. If any of these events occur, we may be forced to abandon our development efforts for a program or programs, which would have a material adverse effect on our business and could potentially cause us to cease operations.
If we do not secure collaborations with strategic partners to test, commercialize and manufacture certain product candidates outside of food allergies, we may not be able to successfully develop products and generate meaningful revenues.
A key aspect of our current strategy is to selectively enter into collaborations with third parties to conduct clinical testing. Our ability to generate revenues from these arrangements will depend on our collaborators’ abilities to successfully perform the functions assigned to them in these arrangements. We currently have multiple collaboration agreements in effect, including collaborations for the development of applications in the field of respiratory allergies or autoimmune disease, as well as other therapeutic domains, such as vaccines. Collaboration agreements typically call for milestone payments that depend on successful demonstration of efficacy and safety, obtaining regulatory approvals and clinical trial results. Collaboration revenues are not guaranteed, even when efficacy and safety are demonstrated. The current economic environment may result in potential collaborators electing to reduce their external spending, which may prevent us from developing our product candidates.
Even if we succeed in securing collaborators, the collaborators may fail to develop or effectively commercialize products using our product candidates. Collaborations involving our product candidates pose a number of risks, including the following:
| • | collaborators may not have sufficient resources or decide not to devote the necessary resources due to internal constraints such as budget limitations, lack of human resources, or a change in strategic focus; |
| • | collaborators may believe our intellectual property is not valid, is not infringed by potential competitors or is unenforceable or the product candidate infringes on the intellectual property rights of others; |
| • | collaborators may dispute their responsibility to conduct development and commercialization activities pursuant to the applicable collaboration, including the payment of related costs or the division of any revenues; |
| • | collaborators may decide to pursue a competitive product developed outside of the collaboration arrangement; |
| • | collaborators may not be able to obtain, or believe they cannot obtain, the necessary regulatory approvals; or |
| • | collaborators may delay the development or commercialization of our product candidates in favor of developing or commercializing another party’s product candidate. |
74
Thus, collaboration agreements may not lead to development or commercialization of product candidates in the most efficient manner or at all.
Collaboration agreements are generally terminable without cause on short notice. Once a collaboration agreement is signed, it may not lead to commercialization of a product candidate. We also face competition in seeking out collaborators. If we are unable to secure new collaborations that achieve the collaborator’s objectives and meet our expectations, we may be unable to advance our product candidates and may not generate meaningful revenues.
Intellectual Property Risks Related to Our Business
Our ability to compete may decline if we do not adequately protect our proprietary rights.
Our commercial success depends on obtaining and maintaining proprietary rights to our product candidates for the treatment of common food or other allergies, as well as successfully defending these rights against third-party challenges. We will only be able to protect our product candidates, and their uses from unauthorized use by third parties to the extent that valid and enforceable patents, or effectively protected trade secrets, cover them. Our ability to obtain patent protection for our product candidates is uncertain due to a number of factors, including:
| • | we may not have been the first to make the inventions covered by pending patent applications or issued patents; |
| • | we may not have been the first to file patent applications for our product candidates or the compositions we developed or for their uses; |
| • | others may independently develop identical, similar or alternative products or compositions and uses thereof; |
| • | our disclosures in patent applications may not be sufficient to meet the statutory requirements for patentability; |
| • | any or all of our pending patent applications may not result in issued patents; |
| • | we may not seek or obtain patent protection in countries that may eventually provide us a significant business opportunity; |
| • | any patents issued to us may not protect, encompass, or embody commercially viable products, may not provide any competitive advantages, or may be successfully challenged by third parties; |
| • | our compositions and methods may not be patentable; |
| • | others may design around our patent claims to produce competitive products which fall outside of the scope of our patents; or |
| • | others may identify prior art or other bases which could invalidate our patents. |
Even if we have or obtain patents covering our product candidates or compositions, we may still be barred from making, using and selling our product candidates or technologies because of the patent rights of others. Others may have filed, and in the future may file, patent applications covering compositions or products that are similar or identical to our compositions or products. There are many issued U.S. and foreign patents relating to biological or chemical compounds and therapeutic products, and some of these relate to compounds we intend to commercialize. Numerous U.S. and foreign issued patents and pending patent applications owned by others exist in the allergy treatment field in which we are developing products. Any or all of these could materially affect our ability to develop our product candidates or sell our products if approved. Because patent applications can take many years to issue as patents, and because there can be procedures to keep some applications secret, there may be currently pending applications unknown to us that may later result in issued patents that our product candidates or compositions may infringe. These patent applications may have priority over patent applications filed by us.
75
Obtaining and maintaining a patent portfolio entails significant expense and resources. Part of the expense includes periodic maintenance fees, renewal fees, annuity fees, various other governmental fees on patents and/or applications due in several stages over the lifetime of patents and/or applications, as well as the cost associated with complying with numerous procedural provisions during the patent application process and after a patent grants. There may also be significant expenses associated with enforcing and/or defending various patents in a patent portfolio. We may or may not choose to pursue or maintain protection for particular inventions. In addition, there are situations in which failure to make certain payments or noncompliance with certain requirements in the patent process can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction (perhaps irrevocably). If we choose to forgo patent protection or allow a patent application or patent to lapse purposefully or inadvertently, our competitive position could suffer.
Legal actions to enforce and/or defend our patent rights can be expensive and may involve the diversion of significant management time. In addition, these legal actions could be unsuccessful and could also result in the invalidation of our patents and/or a finding that they are unenforceable. We may or may not choose to pursue litigation or other actions against those that have infringed on our patents, or used them without authorization, due to the associated expense and time commitment of monitoring these activities. If we fail to protect or to enforce our intellectual property rights successfully, our competitive position could suffer, which could harm our results of operations. If we develop a reputation of failing to attempt to protect or to enforce our intellectual property rights, our competitive position could suffer, which could harm results of operation.
Biopharmaceutical patents and patent applications involve highly complex legal and factual questions, which, if determined adversely to us, could negatively impact our patent position.
The patent positions of biopharmaceutical companies can be highly uncertain and involve complex legal and factual questions. The interpretation and breadth of claims allowed in some patents covering biopharmaceutical compositions may be uncertain and difficult to determine, and are often affected materially by the facts and circumstances that pertain to the patented compositions and the related patent claims. The standards of the United States Patent and Trademark Office, or USPTO, are sometimes uncertain and could change in the future. Consequently, the issuance and scope of patents cannot be predicted with certainty. Patents, if issued, may be challenged, invalidated or circumvented. U.S. patents and patent applications may also be subject to interference proceedings; U.S. patents may be subject to reexamination proceedings, post-grant review and/or inter partes review in the USPTO (collectively, “post-grant proceedings”). Foreign patents may be subject also to opposition or comparable proceedings in the corresponding foreign patent office, which could result in either loss of the patent or denial of the patent application or loss or reduction in the scope of one or more of the claims of the patent or patent application. In addition, such interference, reexamination, post-grant review, inter partes review and opposition proceedings may be costly. Accordingly, rights under any issued patents may not provide us with sufficient protection against competitive products or processes.
In addition, changes in or different interpretations of patent laws in the United States and foreign countries may permit others to use our discoveries or to develop and commercialize our technology and products without providing any compensation to us, or may limit the number of patents or claims we can obtain. The laws of some countries do not protect intellectual property rights to the same extent as U.S. laws and those countries may lack adequate rules and procedures for defending our intellectual property rights.
If we fail to obtain and maintain patent protection and trade secret protection of our product candidates, we could lose our competitive advantage and competition we face would increase, reducing any potential revenues and adversely affecting our ability to attain or maintain profitability.
Developments in patent law could have a negative impact on our business.
From time to time, the United States Supreme Court, the United States Court of Appeals for the Federal Circuit, other federal courts, the United States Congress, the USPTO or similar foreign authorities may change the standards of patentability and any such changes could have a negative impact on our business.
76
For example, recently the federal courts and the United States Supreme Court have issued (and may issue additional) rules generally related to standards for upholding the validity of biological and chemical “genus” claims. Any rulings that make it more difficult to uphold the validity of biological or chemical “genus” claims could potentially negatively impact our patent portfolio and negatively impact our business.
In addition, the Leahy-Smith America Invents Act, or the America Invents Act, which was signed into law in 2011, includes a number of significant changes to U.S. patent law. These changes include a transition from a “first-to-invent” system to a “first-to-file” system, changes to the way issued patents are challenged, and changes to the way patent applications are disputed and prosecuted during the examination process. These changes may favor larger and more established companies that have greater resources to devote to patent application filing and prosecution. The USPTO has developed (and continues to develop) new and untested, or relatively lightly tested, regulations and procedures to govern the full implementation of the America Invents Act, and many of the substantive changes to patent law associated with the America Invents Act, and, in particular, the first-to-file provisions, became effective on March 16, 2013. Substantive changes to patent law associated with the America Invents Act may affect our ability to obtain patents, and if obtained, to enforce or defend them. Accordingly, it is not clear what, if any, impact the America Invents Act will have on the cost of prosecuting our patent applications, our ability to obtain patents based on our discoveries and our ability to enforce or defend any patents that may issue from our patent applications, all of which could have a material adverse effect on our business.
In addition, over the past few years, bills in the U.S. Congress have bene proposed that, if passed, would make changes to the America Invents Act. For example, bills have been introduced that would reduce the discretion of the Patent Trial and Appeal Board (PTAB) to deny some or all post-grant proceedings. In addition, bills, rules and/or regulations have been introduced that would provide the director of the U.S.P.T.O more authority to set aside PTAB decisions. If these bills are eventually passed by the U.S. Congress and become law, they could impact our ability to enforce/defend patents.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to patent protection, because we operate in the highly technical field of development of therapies, we rely in part on trade secret protection in order to protect our proprietary technology and processes. However, trade secrets are difficult to protect. We do, and expect to, enter into confidentiality and intellectual property assignment agreements with our employees, consultants, outside scientific collaborators, sponsored researchers, and other advisors. These agreements generally require that the other party keep confidential and not disclose to third parties all confidential information developed by the party or made known to the party by us during the course of the party’s relationship with us. These agreements also generally provide that inventions conceived by the party in the course of rendering services to us will be our exclusive property. However, these agreements may not be honored and may not effectively assign intellectual property rights to us.
In addition to contractual measures, we try to protect the confidential nature of our proprietary information using physical and technological security measures. Such measures may not, for example, in the case of misappropriation of a trade secret by an employee or third party with authorized access, provide adequate protection for our proprietary information. Our security measures may not prevent an employee or consultant from misappropriating our trade secrets and providing them to a competitor, and recourse we take against such misconduct may not provide an adequate remedy to protect our interests fully. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret can be difficult, expensive, and time-consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets. Trade secrets may be independently developed by others in a manner that could prevent legal recourse by us. If any of our confidential or proprietary information, such as our trade secrets, were to be disclosed or misappropriated, or if any such information was independently developed by a competitor, our competitive position could be harmed.
77
We will not seek to protect our intellectual property rights in all jurisdictions throughout the world and we may not be able to adequately enforce our intellectual property rights even in the jurisdictions where we seek protection.
Filing, prosecuting, defending, and maintaining patents, and defending other intellectual property rights such as trade secrets, on our product candidates in all countries and jurisdictions throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States could be less extensive than those in the United States, assuming that rights are obtained in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. The statutory deadlines for pursuing patent protection in individual foreign jurisdictions are generally based on the priority dates of each of our patent applications.
Competitors may use our technologies in jurisdictions where we do not pursue and obtain patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing. Even if we pursue and obtain issued patents in particular jurisdictions, our patent claims or other intellectual property rights may not be effective or sufficient to prevent third parties from so competing in these or other jurisdictions.
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of some countries, particularly developing countries, do not favor the enforcement of patents and other intellectual property protection, especially those relating to biopharmaceuticals or biotechnologies. This could make it difficult for us to stop the infringement of our patents, if obtained, or the misappropriation of our other intellectual property rights. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties under certain circumstances. In addition, many countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, patents may provide limited or no benefit. Patent protection must ultimately be sought on a country-by-country basis, which is an expensive and time-consuming process with uncertain outcomes. Accordingly, we may choose not to seek patent protection in certain countries, and we will not have the benefit of patent protection in such countries.
Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly, could put our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. In addition, changes in the law and legal decisions by courts in the United States and foreign countries may affect our ability to obtain adequate protection for our technology and the enforcement of intellectual property. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Third parties may assert ownership or commercial rights to inventions we develop.
Third parties may in the future make claims challenging the inventorship or ownership of our intellectual property. We have written agreements with collaborators that provide for the ownership of intellectual property arising from our collaborations.
78
These agreements provide that we may have to negotiate certain commercial rights with collaborators with respect to joint inventions or inventions made by our collaborators that arise from the results of the collaboration. In some instances, there may not be adequate written provisions to address clearly the resolution of intellectual property rights that may arise from a collaboration. If we cannot successfully negotiate sufficient ownership and commercial rights to the inventions that result from our use of a third-party collaborator’s materials where required, or if disputes otherwise arise with respect to the intellectual property developed with the use of a collaborator’s samples, we may be limited in our ability to capitalize on the market potential of these inventions. In addition, we may face claims by third parties that our agreements with employees, contractors, or consultants obligating them to assign intellectual property to us are ineffective, or in conflict with prior or competing contractual obligations of assignment, which could result in ownership disputes regarding intellectual property we have developed or will develop and interfere with our ability to capture the commercial value of such inventions. Litigation may be necessary to resolve an ownership dispute, and if we are not successful, we may be precluded from using certain intellectual property, or may lose our exclusive rights in that intellectual property. Either outcome could have an adverse impact on our business.
Third parties may assert that our employees or consultants have wrongfully used or disclosed confidential information or misappropriated trade secrets.
We employ individuals who were previously employed at universities or other biopharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees and consultants do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of a former employer or other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
A dispute concerning the infringement or misappropriation of our proprietary rights or the proprietary rights of others could be time consuming and costly, and an unfavorable outcome could harm our business.
There is significant litigation in the biopharmaceutical industry regarding patent and other intellectual property rights. While we are not currently subject to any pending intellectual property litigation, and are not aware of any such threatened litigation, we may be exposed to future litigation by third parties based on claims that our product candidates, technologies or activities infringe the intellectual property rights of others. If our development activities are found to infringe any such patents or other intellectual property rights, we may have to pay significant damages or seek licenses to such patents. A patentee could prevent us from using the patented drugs or compositions. We may need to resort to litigation to enforce a patent issued to us, to protect our trade secrets, or to determine the scope and validity of third-party proprietary rights. From time to time, we may hire scientific personnel or consultants formerly employed by other companies involved in one or more areas similar to the activities conducted by us.
Either we or these individuals may be subject to allegations of trade secret misappropriation or other similar claims as a result of prior affiliations.
If we become involved in litigation, it could consume a substantial portion of our managerial and financial resources, regardless of whether we win or lose. We may not be able to afford the costs of litigation. Any adverse ruling or perception of an adverse ruling in defending ourselves against these claims could have a material adverse impact on our cash position and the price of the ADSs. Any legal action against us or our collaborators could lead to:
| • | payment of damages, potentially treble damages, if we are found to have willfully infringed a party’s patent rights; |
79
| • | injunctive or other equitable relief that may effectively block our ability to further develop, commercialize, and sell products; or |
| • | us or our collaborators having to enter into license arrangements that may not be available on commercially acceptable terms, if at all, all of which could have a material adverse impact on our cash position and business and financial condition. As a result, we could be prevented from commercializing current or future product candidates. |
We may infringe the intellectual property rights of others, which may prevent or delay our product development efforts and stop us from commercializing or increase the costs of commercializing our product candidates, if approved.
Our success will depend in part on our ability to operate without infringing the intellectual property and proprietary rights of third parties. We cannot assure you that our business, products and methods do not or will not infringe the patents or other intellectual property rights of third parties.
The biopharmaceutical industry is characterized by extensive litigation regarding patents and other intellectual property rights. Other parties may allege that our product candidates or the use of our technologies infringes patent claims or other intellectual property rights held by them or that we are employing their proprietary technology without authorization. Patent and other types of intellectual property litigation can involve complex factual and legal questions, and their outcome is uncertain. Any claim relating to intellectual property infringement that is successfully asserted against us may require us to pay substantial damages, including treble damages and attorney’s fees if we are found to be willfully infringing another party’s patents, for past use of the asserted intellectual property and royalties and other consideration going forward if we are forced to take a license. In addition, if any such claim were successfully asserted against us and we could not obtain such a license, we may be forced to stop or delay developing, manufacturing, selling or otherwise commercializing Viaskin™ patch products.
Even if we are successful in these proceedings, we may incur substantial costs and divert management time and attention in pursuing these proceedings, which could have a material adverse effect on us. If we are unable to avoid infringing the patent rights of others, we may be required to seek a license, defend an infringement action or challenge the validity of the patents in court, or redesign our products. Patent litigation is costly and time consuming. We may not have sufficient resources to bring these actions to a successful conclusion. In addition, intellectual property litigation or claims could force us to do one or more of the following:
| • | cease developing, selling or otherwise commercializing our product candidates; |
| • | pay substantial damages for past use of the asserted intellectual property; |
| • | obtain a license from the holder of the asserted intellectual property, which license may not be available on reasonable terms, if at all; and |
| • | in the case of trademark claims, redesign, or rename, Viaskin™ or other trademarks we may own, to avoid infringing the intellectual property rights of third parties, which may not be possible and, even if possible, could be costly and time-consuming. |
Any of these risks coming to fruition could have a material adverse effect on our business, results of operations, financial condition and prospects.
Issued patents covering our product candidates could be found invalid or unenforceable if challenged in court.
If we or one of our licensing partners initiated legal proceedings against a third party to enforce a patent covering our product candidate, the defendant could counterclaim that the patent covering our product candidate is invalid and/or unenforceable.
80
In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge include alleged failures to meet any of several statutory requirements, including lack of novelty, obviousness or non-enablement. Grounds for unenforceability assertions include allegations of lack of candor or good faith in dealing with USPTO, that someone connected with prosecution of the patent withheld relevant and/or materials information from the USPTO, or made a misleading statement, during prosecution. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, post grant review, inter partes review, and equivalent proceedings in foreign jurisdictions, e.g., opposition proceedings. Such proceedings could result in revocation or amendment of our patents in such a way that they no longer cover, encompass, or protect our product candidates or competitive products. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to validity, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our product candidates. If a party were to prevail on a legal assertion of unenforceability, such a holding could also affect other related patents. Such a loss of patent protection would have a material adverse impact on our business.
Risks Related to Our Organization, Structure and Operations
We depend on key personnel and attracting qualified management personnel and our business could be harmed if we lose key personnel and cannot attract new personnel.
Our success depends to a significant degree upon the technical and management skills of our officers and key personnel. The loss of the services of any of these individuals would likely have an adverse effect on us. Our success also will depend upon our ability to attract and retain additional qualified management. Recruiting and retaining qualified scientific, clinical, manufacturing, sales and marketing personnel will also be critical to our success. The loss of the services of our key executives could impede the achievement of our research, development and commercialization objectives and seriously harm our ability to successfully implement our business strategy. Furthermore, replacing executive officers and key personnel may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to successfully develop, obtain marketing approval of and commercialize products.
Competition to hire from this limited pool is intense, and we may be unable to hire, train, retain or motivate these key personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We compete for such personnel against numerous companies, including larger, more established companies with significantly greater financial resources than we possess. There can be no assurance that we will be successful in attracting or retaining such personnel and the failure to do so could have a material adverse effect on our business, financial condition, and results of operations.
Our employees may engage in misconduct or other improper activities, including violating applicable regulatory standards and requirements or engaging in insider trading, which could significantly harm our business.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to: comply with the regulations of the FDA and applicable foreign regulatory authorities, provide accurate information to the FDA and applicable foreign regulatory authorities, comply with fraud and abuse and other healthcare laws and regulations in the United States and abroad, report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of, including trading on, information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation.
81
We have adopted a code of conduct, but it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may be ineffective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions.
Product liability and other lawsuits could divert our resources, result in substantial liabilities and reduce the commercial potential of our product candidates.
The risk that we may be sued on product liability claims is inherent in the development and commercialization of biopharmaceutical products. Side effects of, or manufacturing defects in, products that we develop could result in the deterioration of a patient’s condition, injury or even death. For example, product liability claims may be brought by subjects participating in our clinical trials as a result of unexpected side effects from our product candidates. Once a product is approved for sale and commercialized, the likelihood of product liability lawsuits increases. Criminal or civil proceedings might be filed against us by patients, regulatory authorities, other biopharmaceutical companies and any other third party using or marketing our products. These actions could include claims resulting from acts by our partners, licensees and subcontractors, over which we have little or no control. These lawsuits may divert our management from pursuing our business strategy and may be costly to defend. In addition, if we are held liable in any of these lawsuits, we may incur substantial liabilities and we may be forced to limit or forgo further commercialization of the affected products.
We may incur significant costs from class action litigation.
The market price for our ordinary shares or ADSs recently has and may continue to fluctuate for many reasons, including as a result of public announcements regarding the progress of our development and commercialization efforts or the development and commercialization efforts of our collaborators and/or competitors, the addition or departure of our key personnel, variations in our operating results and changes in market valuations of pharmaceutical and biotechnology companies. When the market price of a security has been volatile as the market price for our ordinary shares and ADSs has been, holders of that security have occasionally brought securities class action litigation against the company that issued the security.
For example, in December 2018, we announced that we voluntarily withdrew our BLA for Viaskin Peanut following correspondence with the FDA regarding additional data needs on manufacturing procedures and quality controls, and our ADS price declined significantly as a result. Following this announcement, a class action complaint was filed on January 15, 2019 in the United States District Court for the District of New Jersey alleging that we and our former Chief Executive Officer, our current Chief Executive Officer, our former Deputy Chief Executive Officer, and our former Chief Business Officer violated certain federal securities laws, specifically under Sections 10(b) and 20(a) of the Exchange Act, and Rule 10b-5 promulgated thereunder. The plaintiffs sought unspecified damages on behalf of a purported class of persons that purchased our securities between February 14, 2018 and August 4, 2020 and also held our securities on December 20, 2018 and/or March 16, 2020 and/or August 4, 2020. The complaint, as amended, was dismissed with prejudice on July 29, 2022, and the matter was resolved with finality thirty days thereafter.
Whether or not a plaintiff’s claims are successful, this type of litigation is often expensive and diverts management’s attention and resources, which could adversely affect the operation of our business. If we are ultimately required to pay significant defense costs, damages or settlement amounts, such payments could adversely affect our operations.
We may be the target of similar litigation in the future. Any future litigation could result in substantial costs and divert our management’s attention and resources, which could cause serious harm to our business, operating results and financial condition.
82
We maintain liability insurance; however, if any costs or expenses associated with this or any other litigation exceed our insurance coverage, we may be forced to bear some or all of these costs and expenses directly, which could be substantial.
We may be subject to legal or administrative proceedings and litigation other than product liability lawsuits which may be costly to defend and could materially harm our business, financial condition and operations.
Our inability to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of product candidates we develop. We currently carry product liability insurance coverage for our clinical trials. Although we maintain such insurance, our insurance coverage may be insufficient to reimburse us for any expenses or losses we may suffer. In addition, in the future, we may not be able to obtain or maintain sufficient insurance coverage at an acceptable cost or to otherwise protect against potential product or other legal or administrative liability claims by us or our partners, licensees or subcontractors, which could prevent or inhibit the commercial production and sale of any of our product candidates that receive regulatory approval, which could adversely affect our business. Product liability claims could also harm our reputation, which may adversely affect our collaborators’ ability to commercialize our products successfully.
Our failure to maintain certain tax benefits applicable to French technology companies may adversely affect our results of operations.
As a French technology company, we have benefited from certain tax advantages, including, for example, the French research tax credit (credit d’impôt recherche), or CIR. The CIR is a French tax credit aimed at stimulating research and development. Beginning in the fiscal year ending December 31, 2021, the Company recovered its Small and Medium-sized Enterprises, or SMEs, status under EU law, and became therefore eligible again for the immediate reimbursement of the Research Tax Credit. During the fiscal year ending December 31, 2022, the Company received the reimbursement of the 2019, 2020 and 2021 fiscal year research tax credit for a total amount of $26.1 million. The CIR is calculated based on our claimed amount of eligible research and development expenditures in France and represented $8.9 millions and $5.7 millions, as of December 31, 2023 and 2022 respectively. The French tax authority with the assistance of the Research and Technology Ministry may audit each research and development program in respect of which a CIR benefit has been claimed and assess whether such program qualifies in its view for the CIR benefit. The French tax authorities may challenge our eligibility to, or our calculation of certain tax reductions and/or deductions in respect of our research and development activities and, should the French tax authorities be successful, we may be liable to additional corporate income tax, and penalties and interest related thereto, which could have a significant impact on our results of operations and future cash flows. Furthermore, if the French Parliament decides to eliminate, or reduce the scope or the rate of, the CIR benefit, either of which it could decide to do at any time, our results of operations could be adversely affected.
We may be exposed to significant foreign exchange risk. Exchange rate fluctuations may adversely affect the foreign currency value of our ADSs.
We incur portions of our expenses, and may in the future derive revenues, in currencies other than the euro, in particular, the U.S. dollar. As a result, we are exposed to foreign currency exchange risk as our results of operations and cash flows are subject to fluctuations in foreign currency exchange rates. We currently do not engage in hedging transactions to protect against uncertainty in future exchange rates between particular foreign currencies and the euro. Therefore, for example, an increase in the value of the euro against the U.S. dollar could be expected to have a negative impact on our revenue and earnings growth as U.S. dollar revenue and earnings, if any, would be translated into euros at a reduced value. We cannot predict the impact of foreign currency fluctuations, and foreign currency fluctuations in the future may adversely affect our financial condition, results of operations and cash flows. The ADSs are quoted in U.S. dollars on the Nasdaq Global Select Market and our ordinary shares are trading in euros on Euronext Paris. Our financial statements are prepared in euros.
83
Fluctuations in the exchange rate between euros and the U.S. dollar will affect, among other matters, the U.S. dollar value and the euro value of our ordinary shares and ADSs.
We may use hazardous chemicals and biological materials in our business. Any claims relating to improper handling, storage or disposal of these materials could be time consuming and costly.
Our research and development processes may involve the controlled use of hazardous materials, including chemicals and biological materials. We cannot eliminate the risk of accidental contamination or discharge and any resultant injury from these materials. For example, in production, the confinement of the electrospray function and the use of the allergen in liquid form make it possible to prevent the allergens from contaminating the environment. However, we cannot assure you that in case of malfunction during the handling, storage or production process, allergen would not be released into the atmosphere and sensitize the persons present in the environment. We may be sued for any injury or contamination that results from our use or the use by third parties of these materials, and our liability may exceed any insurance coverage and our total assets. Federal, state, local or foreign laws and regulations govern the use, manufacture, storage, handling and disposal of these hazardous materials and specified waste products, as well as the discharge of pollutants into the environment and human health and safety matters. An allegation of noncompliance by applicable regulatory authorities with environmental laws and regulations may be expensive and may impair our research and development efforts. If we fail to comply with these requirements, we could incur substantial costs, including civil or criminal fines and penalties, clean-up costs or capital expenditures for control equipment or operational changes necessary to achieve and maintain compliance. In addition, we cannot predict the impact on our business of new or amended environmental laws or regulations or any changes in the way existing and future laws and regulations are interpreted and enforced.
We are subject to stringent and changing obligations related to data privacy and security. Our actual or perceived failure to comply with such obligations could lead to regulatory investigations or actions, litigation, fines and penalties, disruptions of our business operations, reputational harm, loss of revenue or profits, and other adverse business consequences.
In the ordinary course of business, we collect, receive, store, process, generate, use, transfer, disclose, make accessible, protect, secure, dispose of, transmit, and share (collectively, processing) personal data and other sensitive information, including proprietary and confidential business data, trade secrets, intellectual property, data we collect about trial participants in connection with clinical trials, and sensitive third-party data. Our data processing activities subject us to numerous data privacy and security obligations, such as various laws, regulations, guidance, industry standards, external and internal privacy and security policies, contractual requirements, and other obligations that govern the processing of personal data by us and on our behalf.
In the United States, federal, state, and local governments have enacted numerous data privacy and security laws, including data breach notification laws, personal data privacy laws, and consumer protection laws. For example, HIPAA, as amended by HITECH, imposes specific requirements relating to the privacy, security, and transmission of individually identifiable health information. In addition, the California Consumer Privacy Act of 2018, or CCPA, imposes obligations on covered businesses. These obligations include, but are not limited to, providing specific disclosures in privacy notices and affording California residents certain rights related to their personal data. The CCPA allows for statutory fines for noncompliance (up to $7,500 per violation). Although the CCPA exempts some data processed in the context of clinical trials, the CCPA may increase compliance costs and potential liability with respect to other personal data we maintain about California residents. In addition, it is anticipated that the California Privacy Rights Act of 2020, CPRA, effective January 1, 2023, has expanded the CCPA. The CPRA establishes a new California Privacy Protection Agency to implement and enforce the CPRA, which could increase the risk of enforcement. Other states have enacted data privacy laws. For example, Virginia passed the Consumer Data Protection Act, and Colorado passed the Colorado Privacy Act, both of which become effective in 2023. In addition, data privacy and security laws have been proposed at the federal, state, and local levels in recent years, which could further complicate compliance efforts.
84
Outside the United States, an increasing number of laws, regulations, and industry standards apply to data privacy and security. For example, the European Union’s General Data Protection Regulation, or EU GDPR, and the United Kingdom’s GDPR, or UK GDPR, impose strict requirements for processing personal data. For example, under the EU GDPR, government regulators may impose temporary or definitive bans on data processing, as well as fines of up to 20 million euros or 4% of the total annual global revenue of the preceding year, whichever is greater. Furthermore, companies may face private litigation related to processing of personal data brought by data subjects, classes of data subjects or consumer protection organizations authorized at law to represent their interests.
Certain jurisdictions have enacted data localization laws and cross-border personal data transfer laws, which could make it more difficult to transfer information across jurisdictions (such as transferring or receiving personal data that originates in the European Economic Area, or EEA, or in other foreign jurisdictions). Although there are currently various mechanisms that may be used to transfer personal data from the EEA and UK to the United States in compliance with law, these mechanisms are subject to legal challenges, and there is no assurance that we can satisfy or rely on these measures to lawfully transfer personal data to the United States on a long-term basis. The EU GDPR generally restricts the transfer of personal data to countries outside of the European Economic Area, or EEA, such as the United States. The European Commission adopted an adequacy decision for the EU-US Data Privacy Framework, or DPF, on July 10, 2023, further to the Biden Administration’s Executive Order dated October 7, 2022 which provides that entities in the EEA may transfer personal data to entities in the United States that adhere and comply with the DPF without having to implement additional safeguards. However, the DPF is may not provide adequate protection given the previous successive invalidations of previously EU-US adequacy mechanisms and the periodic review of the DPF by the European Commission. The European Commission released a set of “Standard Contractual Clauses,” or SCCs, that are designed to be a valid mechanism to facilitate personal data transfers out of the EEA to these jurisdictions. These SCCs can be a valid mechanism to transfer personal data outside of the EEA. However, only entering into SCCs may not be sufficiency and additional compliance burdens are required, such as conducting transfer impact assessments to determine whether additional security measures are necessary to protect the at-issue personal data. In addition, Switzerland and the UK similarly restrict personal data transfers outside of those jurisdictions to countries, such as the United States, and certain countries outside Europe (e.g., Russia, China, Brazil) have also passed or are considering laws requiring local data residency or otherwise impeding the transfer of personal data across borders, any of which could increase the cost and complexity of doing business.
If we cannot transfer personal data from the EEA, the UK or other jurisdictions to the United States in a lawful manner, or if the costs for such lawful transfers of personal data are too high, we may face increased exposure to regulatory actions, substantial fines and penalties, and injunctions against processing or transferring personal data from Europe or other foreign jurisdictions. The inability to import personal data to the United States could significantly and negatively impact our business operations; limiting our ability to collaborate with parties that are subject to such cross-border data transfer or localization laws; or requiring us to increase our personal data processing capabilities and infrastructure in foreign jurisdictions at significant expense; or interrupting or adversely impacting our operations.
Our obligations related to data privacy and security are quickly changing in an increasingly stringent fashion, creating some uncertainty as to the effective future legal framework. Additionally, these obligations may be subject to differing applications and interpretations, which may be inconsistent or conflict among jurisdictions. Preparing for and complying with these obligations requires significant resources and may necessitate changes to our information technologies, systems, and practices and to those of any third parties that process personal data on our behalf. Although we endeavor to comply with all applicable data privacy and security obligations, we may at times fail (or be perceived to have failed) to do so. Moreover, despite our efforts, our personnel or third parties upon whom we rely may fail to comply with such obligations, which could negatively impact our business operations and compliance posture. For example, any failure by a third-party processor to comply with applicable law, regulations, or contractual obligations including, providing appropriate notice to data subjects, obtaining necessary consents, or establishing a legal basis for the transfer and processing of the data by us, could result in adverse effects, including inability to or interruption in our ability to operate our business and proceedings against us by governmental entities or others.
85
If we fail, or are perceived to have failed, to address or comply with data privacy and security obligations, we could face significant consequences. These consequences may include, but are not limited to, government enforcement actions (e.g., investigations, fines, penalties, audits, inspections, and similar); litigation (including class-related claims); additional reporting requirements and/or oversight; payment of damages; bans on processing personal data; and orders to destroy or not use personal data.
Any of these events could have a material adverse effect on our reputation, business, or financial condition, including but not limited to: loss of customers; interruptions in our business operations (including, as relevant, clinical trials); inability to process personal data or to operate in certain jurisdictions; limited ability to develop or commercialize our products; expenditure of time and resources to defend any claim or inquiry; adverse publicity; revision or restructuring of our operations; or loss of revenue or profits; and other adverse business consequences.
If our information technology systems or sensitive information, or those of third parties upon which we rely, are or were compromised, we could experience adverse consequences resulting from such compromise, including, but not limited to, regulatory investigations or actions, litigation, fines and penalties, disruptions of our business operations, reputational harm, loss of revenue or profits, and other adverse consequences.
In the ordinary course of our business, we and the third parties upon which we rely, may process proprietary, confidential, and sensitive data, including personal data (such as health-related data), intellectual property, and trade secrets (collectively, sensitive information). We may rely upon third-party service providers and technologies to operate critical business systems to process sensitive information in a variety of contexts, including, without limitation, third-party providers of cloud-based infrastructure, encryption and authentication technology, employee email, and other functions. Our ability to monitor these third parties’ information security practices is limited, and these third parties may not have adequate information security measures in place. If our third-party service providers experience a security incident or other interruption, we could experience adverse consequences. While we may be entitled to damages if our third-party service providers fail to satisfy their privacy or security-related obligations to us, any award may be insufficient to cover our damages, or we may be unable to recover such award.
Cyberattacks, malicious internet-based activity, and online and offline fraud threaten the confidentiality, integrity, and availability of our sensitive information and information technology systems, and those of the third parties upon which we rely. These threats are prevalent and continue to increase. These threats come from a variety of sources, including traditional computer “hackers,” threat actors, “hacktivists”, organized criminal threat actors, personnel (such as through theft or misuse), sophisticated nation states, and nation-state-supported actors. Some actors now engage and are expected to continue to engage in cyber-attacks, including, without limitation, nation-state actors for geopolitical reasons and in conjunction with military conflicts and defense activities. During times of war and other major conflicts, we and the third parties upon which we rely may be vulnerable to a heightened risk of these attacks, including cyber-attacks that could materially disrupt our systems and operations, supply chain, and ability to produce, sell and distribute our products. We and the third parties upon which we rely may be subject to a variety of evolving threats, including, but not limited to, social-engineering attacks (including through phishing attacks), malicious code (such as viruses and worms), malware (including as a result of advanced persistent threat intrusions), denial-of-service attacks (such as credential stuffing), credential harvesting, personnel misconduct or error, ransomware attacks, supply-chain attacks, software bugs, server malfunctions, software or hardware failures, loss of data or other information technology assets, adware, telecommunications failures, earthquakes, fires, flood and other similar threats.
Severe ransomware attacks, including by organized criminal threat actors, nation-states, and nation-state-supported actors, are becoming increasingly prevalent and severe and can lead to significant interruptions in our operations, loss of data and income, reputational harm, and diversion of funds.
86
Extortion payments may alleviate the negative impact of a ransomware attack, but we may be unwilling or unable to make such payments due to, for example, applicable laws or regulations prohibiting such payments. Similarly, supply-chain attacks have increased in frequency and severity, and we cannot guarantee that third parties and infrastructure in our supply chain or our third-party partners’ supply chains have not been compromised or that they do not contain exploitable defects or bugs that could result in a breach of or disruption to our information technology systems or the third-party information technology systems that support us and our services. Additionally, our workforce’s use of network connections, computers, and devices outside our premises or networks, including working remotely from home, while in transit, and in public locations, poses increased risks to our information technology systems and data. Future or past business transactions (such as acquisitions or integrations) could also expose us to additional cybersecurity risks and vulnerabilities, as our systems could be negatively affected by vulnerabilities present in acquired or integrated entities’ systems and technologies. Furthermore, we may discover security issues that were not previously identified while conducting due diligence acquired or integrated entities and it may be difficult to integrate companies into our information technology environment and security program.
Any of the previously identified or similar threats could cause a security incident or other interruption that could result in unauthorized, unlawful, or accidental acquisition, modification, destruction, loss, alteration, encryption, disclosure of, or access to sensitive information held by us or our information technology systems, or those of the third parties upon whom we rely. A security incident or other interruption disrupt our ability (and that of third parties upon whom we rely) to conduct our business operations.
We may expend significant resources or modify our business activities to try to protect against security incidents. Certain data privacy and security obligations may require us to implement and maintain specific security measures, industry-standard or reasonable security measures to protect our information technology systems and sensitive information. While we have implemented security measures designed to protect against security incidents, there can be no assurance that these measures will be effective. We take steps to detect and remediate vulnerabilities but we may not be able to detect and remediate all vulnerabilities because threats and techniques used to exploit the vulnerability change frequently, are often sophisticated in nature. Therefore, such vulnerabilities could be exploited but may not be detected until after a security incident has occurred. These vulnerabilities pose material risks to our business. Further, we may experience delays in developing and deploying remedial measures designed to address any such identified vulnerabilities.
Applicable data privacy and security obligations may require us to notify relevant stakeholders of security incidents. Such disclosures are costly, and the disclosures or the failure to comply with such requirements could lead to adverse consequences. If we (or a third party upon whom we rely) experience a security incident or are perceived to have experienced a security incident, we may experience adverse consequences. These consequences may include: government enforcement actions (for example, investigations, fines, penalties, audits, and inspections); additional reporting requirements and/or oversight; restrictions on processing sensitive information (including personal data); litigation (including class claims); indemnification obligations; negative publicity; reputational harm; monetary fund diversions; interruptions in our operations (including availability of data); financial loss; and other similar harms. Security incidents and attendant consequences may cause interruptions in our operations and could result in a material disruption of our programs. For example, the loss of clinical trial data for our product candidates could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data.
Our contracts may not contain limitations of liability, and even where they do, there can be no assurance that limitations of liability in our contracts are sufficient to protect us from liabilities, damages, or claims related to our data privacy and security obligations. We cannot be sure that our insurance coverage will be adequate or sufficient to protect us from or to mitigate liabilities arising out of our privacy and security practices, that such coverage will continue to be available on commercially reasonable terms or at all, or that such coverage will pay future claims.
87
We may acquire businesses or products, or form strategic alliances, in the future, and we may not realize the benefits of such acquisitions.
At this stage, our strategy does not involve plans to acquire companies or technologies facilitating or enabling us to access to new medicines, new research projects or new geographical areas, or enabling us to express synergies with our existing operations. However, if our strategy changes or if such acquisitions were to become necessary in the future, we may not be able to identify appropriate targets or make acquisitions under satisfactory conditions, in particular, satisfactory price conditions. In addition, we may be unable to obtain the financing for these acquisitions under favorable conditions, and could be led to finance these acquisitions using cash that could be allocated to other purposes in the context of existing operations. If we acquire businesses with promising markets or technologies, we may not be able to realize the benefit of acquiring such businesses if we are unable to successfully integrate them with our existing operations and company culture. We may encounter numerous difficulties in developing, manufacturing and marketing any new products resulting from a strategic alliance or acquisition that delay or prevent us from realizing their expected benefits or enhancing our business. We cannot assure you that, following any such acquisition, we will achieve the expected synergies to justify the transaction, which could have a material adverse effect on our business, financial conditions, earnings and prospects.
We are subject to U.S. and certain foreign export and import controls, sanctions, embargoes, anti-corruption laws, and anti-money laundering laws and regulations. Compliance with these legal standards could impair our ability to compete in domestic and international markets. We can face criminal liability and other serious consequences for violations, which can harm our business.
We are subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations, various economic and trade sanctions regulations administered by the U.S. Treasury Department’s Office of Foreign Assets Controls, the U.S. Foreign Corrupt Practices Act of 1977, as amended, or FCPA, the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the USA PATRIOT Act, and other state and national anti-bribery and anti-money laundering laws in the countries in which we conduct activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, contractors, and other collaborators from authorizing, promising, offering, or providing, directly or indirectly, improper payments or anything else of value to recipients in the public or private sector. We may engage third parties to sell our products sell our products outside the United States, to conduct clinical trials, and/or to obtain necessary permits, licenses, patent registrations, and other regulatory approvals. We have direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities, and other organizations. We can be held liable for the corrupt or other illegal activities of our employees, agents, contractors, and other collaborators, even if we do not explicitly authorize or have actual knowledge of such activities. Any violations of the laws and regulations described above may result in substantial civil and criminal fines and penalties, imprisonment, the loss of export or import privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm, and other consequences.
We will need to develop and implement sales, marketing and distribution capabilities before we are able to bring any product candidate to market, and as a result, we may encounter difficulties in managing this development and expansion, which could disrupt our operations.
As of December 31, 2023, we had 104 full-time employees. Before we can commercialize Viaskin Peanut, if approved, and any of our other product candidates in North America, we will need to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Also, our management may need to divert a disproportionate amount of its attention away from our day-to-day activities and devote a substantial amount of time to managing any such development activities we may pursue. Due to our limited resources, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. This may result in weaknesses in our infrastructure, give rise to operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Any physical expansion of our operations may lead to significant costs and may divert financial resources from other projects, such as the development of our product candidates.
88
If our management is unable to effectively manage our expected development and expansion, our expenses may increase more than expected, our ability to generate or increase our revenue could be reduced and we may not be able to implement our business strategy. Our future financial performance and our ability to commercialize our product candidates, if approved, and compete effectively will depend, in part, on our ability to effectively manage the future development and expansion of our company.
Risks Related to Ownership of Our Ordinary Shares and ADSs
The market price for our ordinary shares and ADSs may be volatile or may decline regardless of our operating performance.
The trading price of our ADSs and ordinary shares has fluctuated, and is likely to continue to fluctuate, substantially. The trading price of our securities depends on a number of factors, including those described in this “Risk Factors” section, many of which are beyond our control and may not be related to our operating performance.
Our ADSs were sold in our initial public offering on Nasdaq in October 2014 at a price of $21.64 per share, and the price per ADS has ranged from as low as $0.70 and as high as $2.23 during 2023. During this same period, our ordinary share prices have ranged from as low as €1.40 to as high as €4.07. The market price of our securities may fluctuate significantly in response to numerous factors, many of which are beyond our control, including:
| • | actual or anticipated fluctuations in our financial condition and operating results; |
| • | actual or anticipated changes in our growth rate relative to our competitors; |
| • | competition from existing products or new products that may emerge; |
| • | regulatory actions with respect to our products or our competitors’ products, including the potential resubmission to the FDA of a BLA for Viaskin Peanut; |
| • | announcements by us, our partners or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations, or capital commitments; |
| • | failure to meet or exceed financial estimates and projections of the investment community or that we provide to the public; |
| • | issuance of new or updated research or reports by securities analysts; |
| • | fluctuations in the valuation of companies perceived by investors to be comparable to us; |
| • | price and volume fluctuations attributable to inconsistent trading volume levels of the ADSs and/or ordinary shares; |
| • | additions or departures of key management or scientific personnel; |
| • | disputes or other developments related to proprietary rights, including patents, litigation matters, and our ability to obtain patent protection for our technologies; |
| • | changes in the structure of healthcare payment systems; |
| • | changes to coverage policies or reimbursement levels by commercial third-party payors and government payors and any announcements relating to coverage policies or reimbursement levels; |
| • | announcement or expectation of additional debt or equity financing efforts; |
| • | sales of our ordinary shares or ADSs by us, our insiders or our other shareholders; and |
| • | general economic and market conditions. These and other market and industry factors may cause the market price and demand for our securities to fluctuate substantially, regardless of our actual operating |
89
| performance, which may limit or prevent investors from readily selling their ADSs or ordinary shares and may otherwise negatively affect the liquidity of our ADSs and ordinary shares. In addition, the stock market in general, and biopharmaceutical companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. |
Share ownership is concentrated in the hands of our principal shareholders and management, who will continue to be able to exercise a direct or indirect controlling influence on us.
As of December 31, 2023, our executive officers, directors, current 5% or greater shareholders and affiliated entities, including entities affiliated with Baker Bros. Advisors LP, entities affiliated with Braidwell, L.P., entities affiliated with VR Adviser, LLC, and entities affiliated with Bpifrance Participations S.A., together beneficially own approximately 47% of our ordinary shares. As a result, these shareholders, acting together, will have significant influence over all matters that require approval by our shareholders, including the election of directors and approval of significant corporate transactions. Corporate action might be taken even if other shareholders oppose them. This concentration of ownership might also have the effect of delaying or preventing a change of control of our company that other shareholders may view as beneficial.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, the price of the ADSs and trading volume could decline.
The trading market for our ADSs and ordinary shares depends in part on the research and reports that securities or industry analysts publish about us or our business. If no or few securities or industry analysts cover our company, the trading price for our ADSs and ordinary shares would be negatively impacted. If one or more of the analysts who covers us downgrades our ADSs or ordinary shares or publishes incorrect or unfavorable research about our business, the price of our ADSs and ordinary shares would likely decline. If one or more of these analysts ceases coverage of our company or fails to publish reports on us regularly, or downgrades our ADSs or ordinary shares, demand for our ADSs and ordinary shares could decrease, which could cause the price of our ADSs or ordinary shares or trading volume to decline.
If we are not able to comply with the applicable continued listing requirements or standards of Nasdaq, our ADSs could be delisted.
Our ADSs are currently listed on The Nasdaq Global Market. In order to maintain that listing, we must satisfy certain continued listing requirements and standards, including, among others, minimum stockholders’ equity, minimum share price, director independence and independent committee requirements, and certain corporate governance requirements. There can be no assurances that we will be able to comply with the applicable listing standards.
For instance, on January 14, 2021, we received a notice from Nasdaq indicating that we did not meet Nasdaq’s quorum requirement under Listing Rule 5620(c)(i), or the Nasdaq Quorum Requirement, because our bylaws do not require a quorum for shareholders’ meetings of at least 33 1/3% of the outstanding shares of our voting ordinary shares. While our ADSs are listed on Nasdaq, our ordinary shares are listed on Euronext Paris. Applicable French laws and regulations prohibit French listed companies from having a quorum requirement for shareholders’ meetings that is higher than the minimums set by French law. The minimum quorum requirements under French law are lower than the Nasdaq Quorum Requirement. In April 2021 following our discussions with Nasdaq, Nasdaq modified the Nasdaq Quorum Requirement, such that Nasdaq will accept a quorum requirement of the home country of a non-U.S. company that is lower than that required by Nasdaq, provided the company fulfill certain requirements. In April 2021, in accordance with the amended Nasdaq Quorum Requirement, we fulfilled such requirements, including filing with the SEC a Current Report on Form 8-K disclosing that we had submitted a letter from our independent French counsel to Nasdaq stating that the laws of France mandate a lower quorum for shareholders’ meetings than that required by the Nasdaq Quorum Requirement, and that we cannot obtain an exemption or waiver from such requirements.
90
We also posted a statement regarding our reliance on the exception from the Nasdaq Quorum Requirement on our website. On April 26, 2021, Nasdaq notified us that we regained compliance with the Nasdaq Quorum Requirement.
On December 20, 2023, we received a letter from the Listing Qualifications Staff of Nasdaq notifying the us that for the last 30 consecutive business days, the bid price of our ADSs had closed below $1.00 per share, the minimum closing bid price required by the continued listing requirements of Nasdaq Listing Rule 5550(a)(2). In accordance with Nasdaq Listing Rule 5810(c)(3)(A), we have 180 calendar days, or until June 17, 2024, to regain compliance with the minimum bid price requirement. To regain compliance, the closing bid price of our ADSs must be at least $1.00 per share for a minimum of 10 consecutive business days before the expiration of the 180-day period. To regain compliance, during the 180 day period the minimum bid price of our ADSs must close at $1.00 per share or more for a minimum of 10 consecutive business days. If we do not regain compliance with the Nasdaq Listing Rules prior to the expiration of the 180-day compliance period, we may be eligible for additional time to regain compliance pursuant to Nasdaq Listing Rule 5810(c)(3)(A)(ii).
Notwithstanding our ability to regain compliance with the Nasdaq Listing Rules, we may fail to satisfy one or more Nasdaq requirements for continued listing of our ADSs in the future. In the event that our ADSs are delisted from Nasdaq and are not eligible for quotation or listing on another market or exchange, trading of our ADSs could be conducted only in the over-the-counter market or on an electronic bulletin board established for unlisted securities such as the Pink Sheets or the OTC Bulletin Board. In such event, it could become more difficult to dispose of, or obtain accurate price quotations for, our ADSs, and there would likely also be a reduction in our coverage by securities analysts and the news media, which could cause the price of our ADSs to decline further. Also, it may be difficult for us to raise additional capital if we are not listed on a major exchange.
Delisting would also likely have a negative effect on the price of our ADSs, would affect our ability to raise additional capital through the public or private sale of equity securities, and would impair your ability to sell or purchase our ADSs when you wish to do so. Delisting could also have other negative results, including the potential loss of confidence by employees, the loss of institutional interest and fewer business development opportunities. In the event of a delisting, we may take actions to restore our compliance with Nasdaq’s listing requirements, but we can provide no assurance that any such action taken by us would allow our ADSs to become listed again, stabilize the market price or improve the liquidity of our ADSs, prevent our ADSs from dropping below Nasdaq minimum bid price requirement or prevent future non-compliance with Nasdaq’s listing requirements.
We do not currently intend to pay dividends on our securities and, consequently, your ability to achieve a return on your investment, if any, will depend on appreciation in the price of the ADSs. In addition, French law may limit the amount of dividends we are able to distribute.
We have never declared or paid any cash dividends on our ordinary shares and do not currently intend to do so for the foreseeable future. We currently intend to invest our future earnings, if any, to fund our growth.
Therefore, you are not likely to receive any dividends on your ADSs for the foreseeable future and the success of an investment in ADSs will depend upon any future appreciation in its value. Consequently, investors may need to sell all or part of their holdings of ADSs after price appreciation, which may never occur, as the only way to realize any future gains on their investment. There is no guarantee that the ADSs will appreciate in value or even maintain the price at which our shareholders have purchased the ADSs. Investors seeking cash dividends should not purchase the ADSs. Further, under French law, the determination of whether we have been sufficiently profitable to pay dividends is made on the basis of our annual financial statements. Therefore, we may be more restricted in our ability to declare dividends than companies not based in France.
91
In addition, exchange rate fluctuations may affect the amount of euros that we are able to distribute, and the amount in U.S. dollars that our shareholders receive upon the payment of cash dividends or other distributions we declare and pay in euros, if any. These factors could harm the value of the ADSs, and, in turn, the U.S. dollar proceeds that holders receive from the sale of the ADSs.
Future sales of ordinary shares or ADSs by existing shareholders could depress the market price of the ADSs.
As of December 31, 2023, 96,431,770 ordinary shares were issued and outstanding. Sales of a substantial number of shares of our ordinary shares or ADSs in the public market, or the perception that these sales might occur, could depress the market price of our securities and could impair our ability to raise capital through the sale of additional equity securities. A substantial number of our shares are now generally freely tradable, subject, in the case of sales by our affiliates, to the volume limitations and other provisions of Rule 144 under the Securities Act. If holders of these shares sell, or indicate an intent to sell, substantial amounts of our securities in the public market, the trading price of our securities could decline significantly.
In June 2022, we completed a $194 million PIPE financing (the “June 2022 PIPE”) from the sale of (i) 32,855,669 Ordinary Shares, nominal value €0.10 per share at a price per Ordinary Share of €3.00 (corresponding to $3.22 on the basis of an exchange rate of $1.0739 = €1.00 published by the European Central Bank on June 8, 2022), and (ii) pre-funded warrants to purchase an aggregate of 28,276,331 Ordinary Shares (the “Warrant Shares”) at a pre-funded price per pre-funded warrant of €2.90 (corresponding to $3.11), which equals the per share price of the Ordinary Shares less the exercise price of €0.10 per pre-funded warrant. Each pre-funded warrant has an exercise price of €0.10 per Warrant Share. Pursuant to a registration rights agreement (the “Registration Rights Agreement”) with the investors, the Company filed a registration statement with the SEC registering the resale of 59,269,629 ordinary shares issued in the June 2022 PIPE, including ordinary shares underlying the pre-funded warrants. The Company also filed a registration statement with the SEC registering the resale of 11,593,170 ordinary shares by Entities affiliated with Baker Bros. Advisors, issued in the June 2022 PIPE, including ordinary shares underlying the pre-funded warrants. As a result, subject to certain beneficial ownership limitations contained in the pre- funded warrants, these shares are freely tradable, without restriction, in the public market. In addition, the exercise of some or all of the pre-funded warrants will increase the number of our outstanding ordinary shares, which may dilute the ownership percentage or voting power of our shareholders.
In addition, we have filed a registration statement with the SEC to register the ordinary shares that may be issued under our equity incentive plans. The ordinary shares subject to outstanding options under our equity incentive plans, ordinary shares reserved for future issuance under our equity incentive plans and ordinary shares subject to outstanding warrants will become eligible for sale in the public market in the future, subject to certain legal and contractual limitations. Sales of a large number of the shares issued under these plans in the public market could have an adverse effect on the market price of our securities.
The dual listing of our ordinary shares and our ADSs may adversely affect the liquidity and value of the ADSs.
Our ADSs are traded on the Nasdaq Global Select Market, and our ordinary shares are listed on Euronext Paris. The dual listing of our ordinary shares and our ADSs may dilute the liquidity of these securities in one or both markets and may adversely affect the maintenance of an active trading market for our ADSs in the United States. The price of our ADSs could also be adversely affected by trading in our ordinary shares on Euronext Paris, and vice versa. In addition, currency fluctuations as between the euro and U.S. dollar may have an adverse impact on the value of our ADSs.
92
Our by-laws and French corporate law contain provisions that may delay or discourage a takeover attempt.
Provisions contained in our by-laws and the corporate laws of France, the country in which we are incorporated, could make it more difficult for a third-party to acquire us, even if doing so might be beneficial to our shareholders. In addition, provisions of our by-laws impose various procedural and other requirements, which could make it more difficult for shareholders to effect certain corporate actions. These provisions include the following:
| • | under French law, a non-French resident as well as any French entity controlled by non-French residents may have to file a declaration for statistical purposes with the Banque de France, within 20 working days following the date of certain direct foreign investments in us, including any purchase of our ADSs. In particular, such filings are required in connection with investments exceeding €15,000,000 that lead to the acquisition of at least 10% of our share capital or voting rights or cross such 10% threshold; |
| • | under French law, certain investments in a French company relating to certain strategic industries by individuals or entities not established in a Member State of the EU are subject to prior authorization of the Ministry of Economy; |
| • | the owner of 90% of the share capital and voting rights of a public company listed on a regulated market in an EEA country, including from the main French Stock Exchange, has the right to force out minority shareholders following a tender offer made to all shareholders; |
| • | a merger (i.e., in a French law context, a share for share exchange following which our company would be dissolved into the acquiring entity and our shareholders would become shareholders of the acquiring entity) of our company into a company incorporated in the European Union would require the approval of our board of directors as well as a two-thirds majority of the votes held by the shareholders present, represented by proxy or voting by mail at the relevant meeting; |
| • | under French law, a cash merger is treated as a share purchase and would require the consent of each participating shareholder; |
| • | our shareholders have granted and may grant in the future our board of directors’ broad authorizations to increase our share capital or to issue additional ordinary shares or other securities (for example, warrants) to our shareholders, the public or qualified investors, including as a possible defense following the launching of a tender offer for our shares; |
| • | our shareholders have preferential subscription rights on a pro rata basis on the issuance by us of any additional securities for cash or a set-off of cash debts, which rights may only be waived by the extraordinary general meeting (by a two-thirds majority vote) of our shareholders or on an individual basis by each shareholder; |
| • | our board of directors has the right to appoint directors to fill a vacancy created by the resignation or death of a director, subject to the approval by the shareholders of such appointment at the next shareholders’ meeting, which prevents shareholders from having the sole right to fill vacancies on our board of directors; |
| • | our board of directors can only be convened by our chairman or our managing director, if any, or, when no board meeting has been held for more than two consecutive months, by directors representing at least one-third of the total number of directors; |
| • | our board of directors meetings can only be regularly held if at least half of the directors attend either physically or by way of videoconference or teleconference enabling the directors’ identification and ensuring their effective participation in the board’s decisions; however, this mode of participation (by way of videoconference or teleconference) does not apply to the adoption of decisions taken for the closing of the accounts for the fiscal year, including the consolidated financial statements; |
| • | our shares are nominative or bearer, if the legislation so permits, according to the shareholder’s choice. Shares issued are registered in individual accounts opened by us or any authorized intermediary, in the |
93
| name of each shareholder and kept according to the terms and conditions laid down by the legal and regulatory provisions; |
| • | approval of at least a majority of the votes held by shareholders present, represented by a proxy, or voting by mail at the relevant ordinary shareholders’ general meeting is required to remove directors with or without cause; |
| • | advance notice is required for nominations to the board of directors or for proposing matters to be acted upon at a shareholders’ meeting, except that a vote to remove and replace a director can be proposed at any shareholders’ meeting without notice; |
| • | our by-laws can be changed in accordance with applicable laws; |
| • | the crossing of certain thresholds has to be disclosed and can impose certain obligations; |
| • | transfers of shares shall comply with applicable insider trading rules and regulations and in particular with the Market Abuse Directive and Regulation dated April 16, 2014; and |
| • | pursuant to French law, the sections of the by-laws relating to the number of directors and election and removal of a director from office may only be modified by a resolution adopted by at least a two thirds majority vote of our shareholders present, represented by a proxy or voting by mail at the meeting. |
You may not be able to exercise your right to vote the ordinary shares underlying your ADSs.
Holders of ADSs may exercise voting rights with respect to the ordinary shares represented by the ADSs only in accordance with the provisions of the deposit agreement. The deposit agreement provides that, upon receipt of notice of any meeting of holders of our ordinary shares, the depositary will fix a record date for the determination of ADS holders who shall be entitled to give instructions for the exercise of voting rights. Upon timely receipt of notice from us, if we so request, the depositary shall distribute to the holders as of the record date (1) the notice of the meeting or solicitation of consent or proxy sent by us and (2) a statement as to the manner in which instructions may be given by the holders.
You may instruct the depositary of your ADSs to vote the ordinary shares underlying your ADSs. If the depositary timely receives voting instructions from you, it will endeavor to vote the securities (in person or by proxy) represented by the ADSs in accordance with such voting instructions. If the depositary receives voting instructions which fail to specify the manner in which the depositary is to vote the deposited securities, you will be deemed to have instructed the depositary to vote in favor of all resolutions endorsed by our board of directors. Otherwise, you will not be able to exercise your right to vote, unless you withdraw the ordinary shares underlying the ADSs you hold. However, you may not know about the meeting far enough in advance to withdraw those ordinary shares. If we ask for your instructions, the depositary, upon timely notice from us, will notify you of the upcoming vote and arrange to deliver our voting materials to you. We cannot guarantee you that you will receive the voting materials in time to ensure that you can instruct the depositary to vote your ordinary shares or to withdraw your ordinary shares so that you can vote them yourself. If the depositary does not receive timely voting instructions from you, it may give a proxy to a person designated by us to vote the ordinary shares underlying your ADSs. In addition, the depositary and its agents are not responsible for failing to carry out voting instructions or for the manner of carrying out voting instructions. This means that you may not be able to exercise your right to vote, and there may be nothing you can do if the ordinary shares underlying your ADSs are not voted as you requested.
Your right as a holder of ADSs to participate in any future preferential subscription rights or to elect to receive dividends in shares may be limited, which may cause dilution to your holdings.
According to French law, if we issue additional securities for cash, current shareholders will have preferential subscription rights for these securities on a pro rata basis, transferable during a period starting two days prior to the opening of the subscription period or, if that day is not a trading day, the preceding trading day; and ending two days prior to the closing of the subscription period or, of that day is not a trading day, the preceding trading day, unless they waive those rights at an extraordinary meeting of our shareholders (by a two-thirds majority vote) or individually by each shareholder.
94
However, the ADS holders in the United States will not be entitled to exercise or sell such rights unless we register the rights and the securities to which the rights relate under the Securities Act or an exemption from the registration requirements is available. In addition, the deposit agreement provides that the depositary will not make rights available to you unless the distribution to ADS holders of both the rights and any related securities are either registered under the Securities Act or exempted from registration under the Securities Act. Further, if we offer holders of our ordinary shares the option to receive dividends in either cash or shares, under the deposit agreement the depositary may require satisfactory assurances from us that extending the offer to holders of ADSs does not require registration of any securities under the Securities Act before making the option available to holders of ADSs. We are under no obligation to file a registration statement with respect to any such rights or securities or to endeavor to cause such a registration statement to be declared effective. Moreover, we may not be able to establish an exemption from registration under the Securities Act. Accordingly, ADS holders may be unable to participate in our rights offerings or to elect to receive dividends in shares and may experience dilution in their holdings. In addition, if the depositary is unable to sell rights that are not exercised or not distributed or if the sale is not lawful or reasonably practicable, it will allow the rights to lapse, in which case you will receive no value for these rights.
According to French law, as of December 31, 2023 we have issued :
| - | 28,276,331 pre-funded warrants at a pre-funded price per pre-funded warrant of €2.90 (corresponding to $3.11), Each pre-funded warrant bears an exercise price of €0.10 per Warrant Share; |
| - | Restricted Stock Units (“RSU”), stock options plan (“SO”), and non-employee warrants (Bons de Souscription d’Actions i.e. “BSA”) representing globally 9,458,901 outstanding shares as of December 31, 2023. |
The exercise of some or all pre-funded warrants, RSU, SO and non-employee warrants will increase the number of outstanding ordinary shares, which may dilute the ownership percentage or voting power of shareholders by 9,8% (without pre-funded warrants exercise, and 39,1% should all pre-funded warrants be exercised).
You may be subject to limitations on the transfer of your ADSs and the withdrawal of the underlying ordinary shares.
Your ADSs, which may be evidenced by ADRs, are transferable on the books of the depositary. However, the depositary may close its books at any time or from time to time when it deems expedient in connection with the performance of its duties. The depositary may refuse to deliver, transfer or register transfers of your ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary think it is advisable to do so because of any requirement of law, government or governmental body, or under any provision of the deposit agreement, or for any other reason subject to your right to cancel your ADSs and withdraw the underlying ordinary shares. Temporary delays in the cancellation of your ADSs and withdrawal of the underlying ordinary shares may arise because the depositary has closed its transfer books or we have closed our transfer books, the transfer of ordinary shares is blocked to permit voting at a shareholders’ meeting or we are paying a dividend on our ordinary shares. In addition, you may not be able to cancel your ADSs and withdraw the underlying ordinary shares when you owe money for fees, taxes and similar charges and when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to ADSs or to the withdrawal of ordinary shares or other deposited securities.
95
The biotechnology industry has been included in the list of critical technologies subject to foreign investment control procedure in France, which may limit the ability to certain non-French investors to participate in this or any other offering of our securities.
The completion of any investment (i) by (a) an individual of foreign nationality, (b) any individual of French nationality not domiciled in France within the meaning of article 4B of the French General Tax Code (Code Général des Impôts), (c) any entity governed by foreign law, and (d) any entity governed by French law controlled by one or more of the entities referred to in (a) to (c), (ii) which would result in (a) the acquisition of control—within the meaning of article L. 233-3 of the French Commercial Code (Code de Commerce)—of a French company, (b) the acquisition of all or part of a branch of activity of a French company, or (c) for individuals who are not nationals of a Member State of the European Union or of a State party to the agreement on the European Economic Area that has entered into an administrative assistance agreement with France and/or are not domiciled in one of these States, or for legal entities of which at least one of the members of the control chain is not governed by the law of one of these States or is not a national and/or is not domiciled there, to cross the threshold of 25% of the voting rights of a French company, or (d) for individuals who are not nationals of a Member State of the EU or of a State party to the agreement on the EEA that has entered into an administrative assistance agreement with France and/or are not domiciled in one of these State, or for legal entities of which at least one of the members of the control chain is not governed by the law of one of these States or is not a national and/or is not domiciled there, to cross the threshold of 10% of the voting rights of a French company whose shares are admitted to trading on a regulated market and (iii) whose activities concern, even occasionally, the research and development of so-called critical technologies, such as biotechnologies, and considered essential to the protection of public health, is subject to prior authorization by the French Minister of the Economy (Ministère de l’Economie). The French Decree No. 2023-1293 of December 28, 2023 has made permanent the temporary regime under French Decree No. 2022-1622 of December 23, 2022, which expired on December 31, 2023.
The crossing of the threshold of 10% of the voting rights of French companies whose shares are admitted to trading on a regulated market is subject to a fast track review procedure (filing of a simplified form, delay for the Minister to respond limited to 10 days, transaction deemed authorized in the absence of a response at the end of the delay).
If an investment in the Company requiring the prior authorization of the Minister of the Economy is made without such authorization having been granted, the Minister of the Economy may cancel the transaction or order (possibly under financial penalty) the investor concerned (i) to submit an application for authorization, (ii) to have the previous situation restored at its own expense or (iii) to modify the investment. In addition, the Minister may impose undertakings and conditions on the investor (including regular reporting commitments). The investor concerned could also be declared criminally liable and be sanctioned, in particular, by exclusion from any public contract or by a fine which may not exceed the highest of the following three amounts: (i) twice the amount of the investment concerned, (ii) 10% of the Company’s annual pre-tax revenues and (iii) 5 million euros (for a company) or 1 million euros (for an individual). The application of these regulations is likely to constitute a potential barrier to investments made by investors located outside the European Economic Area and could therefore limit the Company’s access to sources of financing.
U.S. Investors may have difficulty enforcing civil liabilities against our company and directors and senior management.
Certain members of our board of directors and senior management, and those of our subsidiaries, are non-residents of the United States, and all or a substantial portion of our assets and the assets of such persons are located outside the United States. As a result, it may not be possible to serve process on such persons or us in the United States or to enforce judgments obtained in U.S. courts against them or us based on civil liability provisions of the securities laws of the United States. Additionally, it may be difficult to assert U.S. securities law claims in actions originally instituted outside of the United States. Foreign courts may refuse to hear a U.S. securities law claim because foreign courts may not be the most appropriate forums in which to bring such a claim.
96
Even if a foreign court agrees to hear a claim, it may determine that the law of the jurisdiction in which the foreign court resides, and not U.S. law, is applicable to the claim. Further, if U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact, which can be a time-consuming and costly process, and certain matters of procedure would still be governed by the law of the jurisdiction in which the foreign court resides. In particular, there is some doubt as to whether French courts would recognize and enforce certain civil liabilities under U.S. securities laws in original actions or judgments of U.S. courts based upon these civil liability provisions. In addition, awards of punitive damages in actions brought in the United States or elsewhere may be unenforceable in France. An award for monetary damages under the U.S. securities laws would be considered punitive if it does not seek to compensate the claimant for loss or damage suffered but is intended to punish the defendant. The enforceability of any judgment in France will depend on the particular facts of the case as well as the laws and treaties in effect at the time. The United States and France do not currently have a treaty providing for recognition and enforcement of judgments (other than arbitration awards) in civil and commercial matters.
The rights of shareholders in companies subject to French corporate law differ in material respects from the rights of shareholders of corporations incorporated in the United States.
We are a French company with limited liability. Our corporate affairs are governed by our by-laws and by the laws governing companies incorporated in France. The rights of shareholders and the responsibilities of members of our board of directors are in many ways different from the rights and obligations of shareholders in companies governed by the laws of U.S. jurisdictions. For example, in the performance of its duties, our board of directors is required by French law to consider the interests of our company, our shareholders, employees and other stakeholders, rather than solely our shareholders and/or creditors. It is possible that some of these parties will have interests that are different from, or are in addition to, your interests as a shareholder.
We are a “smaller reporting company,” and the reduced disclosure requirements applicable to smaller reporting companies may make our ADSs less attractive to investors.
We are currently a “smaller reporting company” as defined in the Securities Exchange Act of 1934, as amended, or the Exchange Act. We may continue to be a smaller reporting company even after we are no longer an emerging growth company. We will be a smaller reporting company and may take advantage of the scaled disclosures available to smaller reporting companies for so long as (i) the market value of our voting and non- voting ordinary shares held by non-affiliates is less than $250.0 million measured on the last business day of our second fiscal quarter or (ii) (a) our annual revenue is less than $100.0 million during the most recently completed fiscal year and (b) the market value of our voting and non-voting ordinary shares held by non-affiliates is less than $700.0 million measured on the last business day of our second fiscal quarter.
We are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not smaller reporting companies. These scaled disclosure requirements include, but are not limited to, the following:
| • | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes- Oxley Act, or Section 404; |
| • | reduced disclosure obligations regarding financial information; and |
| • | reduced disclosure obligations regarding executive compensation. |
We may choose to take advantage of some, but not all, of the available exemptions. We cannot predict whether investors will find our ADSs less attractive if we rely on certain or all of these exemptions. If some investors find our ADSs less attractive as a result, there may be a less active trading market for our ADSs and our ADS price may be more volatile.
97
U.S. holders may suffer adverse tax consequences if we are characterized as a passive foreign investment company.
Under the U.S. Internal Revenue Code of 1986, as amended, or the Code, we will be a passive foreign investment company, or PFIC, for any taxable year in which, after the application of certain “look-through” rules with respect to subsidiaries, either (i) 75% or more of our gross income consists of “passive income,” or (ii) 50% or more of the average quarterly value of our assets, including cash, consists of assets that produce, or are held for the production of, “passive income.” Passive income generally includes interest, dividends, rents, certain non-active royalties and capital gains. Whether we will be a PFIC in any year depends on the composition of our income and the nature and composition of our assets, which we expect may vary substantially over time. Based on the composition of our gross income and the nature and composition of our gross assets, we believe that we may have been a PFIC for the taxable year ending December 31, 2023. Because the determination of our PFIC status is based on complicated provisions of the Code and applicable administrative authorities, there can be no assurance that our conclusions concerning our PFIC status for the taxable year ending December 31, 2022 are correct and will not be successfully challenged by applicable tax authorities, and we cannot provide any assurance regarding our PFIC status for the current taxable year or any future taxable year.
If you are a shareholder that is a United States person for U.S. federal income tax purposes, or a U.S. holder during a taxable year when the Company is considered a PFIC, then regardless of whether we continue to be characterized as a PFIC in subsequent taxable years, you may suffer adverse tax consequences, including the treatment of gains realized on the sale of our ADSs as ordinary income, rather than as capital gain, the inapplicability of the preferential rate that otherwise would be applicable to dividends received on our ADSs by individual U.S. holders, the addition of interest charges to the tax on such gains and certain distributions, and additional reporting requirements.
A U.S. holder in certain circumstances may mitigate the adverse tax consequences of the PFIC rules by filing an election to treat the PFIC “qualified electing fund”, or as a QEF, or, if shares of the PFIC are “marketable stock” for purposes of the PFIC rules, by making a mark-to-market election with respect to the shares of the PFIC. For any taxable year in which we are a PFIC, we will determine whether we will provide to U.S. holders the information required to make a QEF election; for the taxable year ending December 31, 2023, we have provided that information. However, there is no assurance that such information will be provided in future taxable years, and prospective investors should not assume that a QEF election will be available.
U.S. Holders are strongly urged to consult with, and rely solely upon, their personal tax advisors regarding the implications of the tax provisions applicable to U.S. persons who own, directly or indirectly, interests in a foreign corporation that is or may become a PFIC.
Item 1B. Unresolved Staff Comments.
Not applicable.
Item 1C. Cybersecurity.
Risk management and strategy
We have implemented and maintain various information security processes designed to identify, assess and manage material risks from cybersecurity threats to our critical computer networks, third party hosted services, communications systems, hardware and software, and our critical data, including intellectual property, confidential information that is proprietary, strategic or competitive in nature, and data related to our clinical trials and technology platform (“Information Systems and Data”).
Our Information Systems function, led by our Vice President for Information Systems and Director of Information Systems, helps identify, assess and manage the Company’s cybersecurity threats and risks.
98
Our Information Systems function identifies and assesses risks from cybersecurity threats by monitoring and evaluating our threat environment using various methods including, for example automated tools, subscribing to and analyzing reports and services that identify cybersecurity threats, conducting scans of the Company’s threat environment, evaluating threats that are reported to us, conducting internal audits, internal threat assessments, and conducting vulnerability assessments.
Depending on the environment and system, we implement and maintain various technical, physical, and organizational measures, processes, standards and policies designed to manage and mitigate material risks from cybersecurity threats to our Information Systems and Data, including, for example: an information systems security policy; incident management; disaster recovery procedures; periodic backup recovery tests; risk assessments; encryption of certain data; network security controls; data segregation for certain systems and environments; access controls; physical security; asset management, tracking, and disposal; systems monitoring; employee training and phishing simulations; penetration tests; outsourced managed detection and response services; maintaining cybersecurity insurance; and having dedicated cybersecurity staff.
Our assessment and management of material risks from cybersecurity threats are integrated into the Company’s overall risk management processes. For example, cybersecurity risk is addressed as a component of the Company’s enterprise risk management program and identified in the Company’s risk mapping and management documentation. The cybersecurity component of the Company’s risk mapping and management documentation is updated annually, and our Information Systems function, led by our Vice President for Information Systems and Director of Information Systems, prepares cybersecurity roadmaps designed to prioritize our risk management processes and mitigate cybersecurity threats that are more likely to lead to a material impact to our business.
We use third-party service providers to assist us from time to time to identify, assess, and manage material risks from cybersecurity threats, including for example, cybersecurity software providers, managed cybersecurity service providers, and penetration testing firms.
We use third-party service providers to perform a variety of functions throughout our business, such as contract research organizations (CROs), contract manufacturing organizations (CMOs), cloud hosting and other SaaS providers. Depending on the nature of the services provided, the sensitivity of the Information Systems and Data at issue, and the identity of the provider, we take various measures designed to help manage risk associated with our use of certain of these providers. These measures include, for example, obtaining confirmation of certain cybersecurity certifications, information security questionnaires, and imposition of certain contractual obligations.
For a description of the risks from cybersecurity threats that may materially affect the Company and how they may do so, see our risk factors under Part 1. Item 1A. Risk Factors in this Annual Report on Form 10-K, including If our information technology systems or sensitive information, or those of third parties upon which we rely, are or were compromised, we could experience adverse consequences resulting from such compromise, including, but not limited to, regulatory investigations or actions, litigation, fines and penalties, disruptions of our business operations, reputational harm, loss of revenue or profits, and other adverse consequences.
Governance
Our board of directors addresses the Company’s cybersecurity risk management as part of its general oversight function. The board of directors’ audit committee is responsible for overseeing the Company’s cybersecurity risk management processes, including oversight and mitigation of risks from cybersecurity threats.
Our cybersecurity risk assessment and management processes are implemented and maintained by certain Company management, including Cyril Guyardeau, our Director of Information Systems Security, who had previously spent 15 years as an IT infrastructure engineer in the healthcare industry, and Cecile Delansorne, our Vice President for Information Systems, who has held similar executive positions overseeing information systems in other pharmaceutical companies for over 7years.
99
Our Vice President for Information Systems reports to our CFO.
The Vice President for Information Systems is responsible for hiring appropriate personnel, helping to integrate cybersecurity risk considerations into the Company’s overall risk management strategy, and communicating key priorities to relevant personnel. The Vice President for Information Systems is also responsible for approving budgets, helping prepare for cybersecurity incidents, approving cybersecurity processes, and reviewing security assessments and other security-related reports.
Our cybersecurity incident response and vulnerability management procedures are designed to escalate certain cybersecurity incidents to members of management depending on the impact of the incident, including the CFO, Data Privacy Officer, Company Legal department, and Executive Committee, who work with the Company’s incident response team to help the Company mitigate and remediate cybersecurity incidents of which they are notified.
The audit committee may receive periodic reports from our CFO concerning the Company’s significant threats and risk, including, if applicable, those related to cybersecurity threats, and the processes the Company has implemented to address them. The audit committee also has access to various reports, summaries or presentations related to cybersecurity threats, risk and mitigation.
Item 2. Properties.
Our corporate headquarters are located in Montrouge, France. Our principal office occupies a 4,470 square meter facility, pursuant to a lease agreement, signed on March 3, 2015, with an effective date of August 1, 2015 and which expires on July 31, 2024.
The company leased a commercial facility in Chatillon, France with early availability of the premises from November , 2023 and commencement of the lease on April 16, 2024. Our principal offices will represent 2 446,7 square meter facility.
Our primary U.S office is located in Basking Ridge, New Jersey. On March 28, 2022, we entered into a lease agreement, commencing on April 1, 2022 and effective for 38 months, for an office of 5,799 square feet in Basking Ridge, New Jersey.
We consider our facilities to be suitable and adequate for the management and operation of our business.
Item 3. Legal Proceedings.
From time to time, we may become subject to various legal proceedings and claims that arise in the ordinary course of our business activities. We are not currently subject to any material legal proceedings.
Item 4. Mine Safety Disclosures.
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our ADSs have been listed on the Nasdaq Global Select Market under the symbol “DBVT” since October 22, 2014. Prior to that date, there was no public trading market for our ADSs. Our ordinary shares have been trading on Euronext Paris under the symbol “DBV” since March 28, 2012. Prior to that date, there was no public trading market for our ADSs or our ordinary shares.
100
Holders of Ordinary Shares
As of March 6, 2024, there were approximately 402 holders of record of our ordinary shares and 65 holders of record of our ADSs. The actual number of holders is greater than these numbers of record holders, and includes beneficial owners whose ordinary shares or ADSs are held in street name by brokers and other nominees. This number of holders of record also does not include holders whose shares may be held in trust by other entities. The number of beneficial owners of the ADSs in the United States is likely to be much larger than the number of record holders of our ordinary shares in the United States.
Dividend Policy
We have never paid cash dividends on any of our share capital and currently intend to retain our future earnings, if any, to fund the development and growth of our business.
Recent Sales of Unregistered Equity Securities
During the year ended December 31, 2023, we issued the following unregistered securities:
| • | On March 23, 2023, the issuance of an aggregate of 10,174 ordinary shares to U.S. and on-U.S. employees upon settlement of RSUs; |
| • | On May 29, 2023, the issuance of an aggregate of 2,500 ordinary shares to a non-U.S. employee upon settlement of RSUs; |
| • | On May 22, 2023, the issuance of an aggregate of 14,364 ordinary shares to non-U.S. employees upon settlement of RSUs; |
| • | On May 24, 2023, the issuance of an aggregate of 34,321 ordinary shares to U.S. and non-U.S. employees upon settlement of RSUs; |
| • | On September 23, 2023, the issuance of an aggregate of 2,599 ordinary shares to U.S. and non-U.S. employees upon settlement of RSUs |
None of the foregoing transactions involved any underwriters, underwriting discounts or commissions, or any public offering. Unless otherwise specified above, we believe these transactions were exempt from registration under the Securities Act in reliance on Section 4(a)(2) of the Securities Act, Regulation S, Regulation D or Rule 701 promulgated under Section 3(b) of the Securities Act as transactions by an issuer not involving any public offering or under benefit plans and contracts relating to compensation as provided under Rule 701. The recipients of the securities in each of these transactions represented their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof. All recipients had adequate access, through their relationships with us, to information about us. The sales of these securities were made without any general solicitation or advertising.
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
None.
Item 6. [Reserved].
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You should read this discussion and analysis of our financial condition and consolidated results of operations together with the consolidated financial statements, related notes and other financial information included in this Annual Report on Form 10-K. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report on Form 10-K, including statements of our plans, objectives, expectations and intentions, contain forward-looking statements that involve risks and uncertainties.
101
As a result of many factors, including those factors set forth in the “Risk Factors” section of this Annual Report on Form 10-K, our actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis. Please also see the section titled “Forward-Looking Statements.”
Overview
We are a clinical-stage specialty biopharmaceutical company focused on changing the field of immunotherapy by developing a novel technology platform called Viaskin. Our therapeutic approach is based on epicutaneous immunotherapy, or EPITTM, our proprietary method of delivering biologically active compounds to the immune system through intact skin using Viaskin, an epicutaneous patch (i.e., a skin patch). We have generated significant data demonstrating that Viaskin’s mechanism of action is novel and differentiated. Viaskin targets specific antigen-presenting immune cells in the skin, called Langerhans cells, that capture the antigen and migrate to the lymph node in order to activate the immune system without passage of the antigen into the bloodstream, minimizing systemic exposure in the body. We are advancing this unique technology to treat children suffering from food allergies, for whom safety is paramount, since the introduction of the offending allergen into their bloodstream can cause severe or life-threatening allergic reactions, such as anaphylactic shock. We believe Viaskin may offer convenient, self-administered, non-invasive immunotherapy to patients, if approved.
Our most advanced product candidate is Viaskin Peanut, which has been evaluated as a potential therapy for children with peanut allergy in twelve clinical trials, including three Phase 2 trials and four completed Phase 3 trials. We have two ongoing Phase 3 trial of Viaskin Peanut in children ages one to three and ages four to seven with peanut allergy.
Financial Overview
Since our inception, we have primarily funded our operations with equity financings, and, to a lesser extent, public assistance aimed at supporting innovation and payments associated with research tax credits (Crédit d’Impôt Recherche). We do not generate product revenue and continue to prepare for the potential launch of our first product in the United States and in the European Union, if approved.
Based on its current operations, the Company expects that its balance of cash and cash equivalents of $141.4 million as of December 31, 2023 will be sufficient to fund its operations until December 31, 2024.
The company has incurred operating losses and negative cash flows from operations since inception.
As of the date of the filing, our available cash is not projected to be sufficient to support our operating plan for at least the next 12 months. As such, there is substantial doubt regarding our ability to continue as a going concern. We intend to seek additional capital as we prepare for the launch of Viaskin Peanut, if approved, and continue other research and development efforts. The Company will require substantial additional capital to fund its research and development and ongoing operating expenses. These capital requirements are expected to be funded through debt and equity offerings before December 31, 2024.
We intend to seek additional capital as we prepare for the launch of Viaskin Peanut, if approved, and continue other research and development efforts. We may seek to finance our future cash needs through a combination of public or private equity or debt financings, collaborations, license and development agreements and other forms of non-dilutive financings.
We cannot guarantee that we will be able to obtain the necessary financing to meet our needs or to obtain funds at attractive terms and conditions, including as a result of disruptions to the global financial markets resulting from geopolitical instability, macroeconomic conditions, global health crises, or other factors.
102
If we are not successful in our financing objectives, we could have to scale back our operations, notably by delaying or reducing the scope of our research and development efforts or obtain financing through arrangements with collaborators or others that may require us to relinquish rights to our product candidates that we might otherwise seek to develop or commercialize independently.
We anticipate that our expenses will increase substantially in connection with our ongoing activities, as we:
| • | continue our research, pre-clinical and clinical development of our product candidates, in particular expanding the scope of our trials for Viaskin Peanut; |
| • | seek regulatory and marketing approvals and pursue commercial activities for Viaskin Peanut, primarily in North America and in the European Union; |
| • | seek regulatory and marketing approvals for our other product candidates that successfully complete clinical trials; |
| • | establish a sales, marketing and distribution infrastructure to commercialize Viaskin Peanut, if approved, and any other products for which we may obtain marketing approval, especially in North America and in the European Union; |
| • | further develop the manufacturing process for our product candidates; |
| • | change or add additional manufacturers or suppliers; |
| • | initiate and conduct any post-approval clinical trials, if required by the FDA or by the EMA, for our approved products, if any; |
| • | initiate additional pre-clinical, clinical or other studies for our product candidates; |
| • | seek to identify and validate additional product candidates; |
| • | acquire or in-license other product candidates and technologies; |
| • | make milestone or meet other payments deadlines under any in-license agreements; |
| • | maintain, protect and expand our intellectual property portfolio; |
| • | attract and retain new and existing skilled personnel; |
| • | add operational, financial and management information systems and personnel, including personnel to support our product development and commercialization efforts, as well as a company listed on both the U.S. and French stock markets; |
| • | experience any delays or encounter issues with any of the above. |
Our financial statements have been prepared on a going concern basis assuming that we will be successful in our financing objectives. As such, no adjustments have been made to the financial statements relating to the recoverability and classification of the asset carrying amounts or classification of liabilities that might be necessary should we not be able to continue as a going concern.
Business Trends
We engage in substantial research and development efforts to develop innovative pharmaceutical product candidates. Research and development expense consists primarily of:
| • | cost of third-party contractors such as contract research organizations, or CROs, that conduct our non- clinical studies and clinical trials; |
| • | personnel costs, including salaries, related benefits and share-based compensation, for our employees engaged in scientific research and development functions; |
103
| • | purchases, real-estate leasing costs, as well as conferences and travel costs; and |
| • | depreciation, amortization and provisions. |
Our direct research and development expenses consist principally of external costs, such as startup fees paid to investigators, consultants, central laboratories, and CROs in connection with our clinical trials, and costs related to acquiring and manufacturing clinical study materials. We do not allocate personnel-related costs, costs associated with our general platform improvements, depreciation or other indirect costs to specific programs, as they are deployed across multiple projects under development and, as such, are separately classified as personnel and other expenses.
Research and Development activities are central to our business. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We expect that our Research and Development expenses will continue to increase in the foreseeable future as we initiate clinical trials for certain product candidates and pursue later stages of clinical development of our product candidates.
In the year ended December 31, 2023, we spent $60.2 million in research and development expenses to advance the development of our product candidates. The following table provides a breakdown of our direct research and development expenses for our two lead development programs, as well as expenses not allocated to the programs and share-based compensation expenses included in research and development expenses, for the years ended December 31, 2023 and 2022, respectively:
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| (thousands of U.S. Dollars) | ||||||||
| Research and development expenses related to Viaskin Peanut(1) |
$ | 60,329 | $ |
47,766 |
|
|||
| As a percentage of research and development expenses, excluding share-based compensation Expense(2) |
105 | % | 65 | % | ||||
| Research and development expenses related to Viaskin Milk(1) |
$ | 6,019 | $ | 8,180 | ||||
| As a percentage of research and development expenses excluding share-based compensation Expense(3) |
10 | % | 11 | % | ||||
| Other research and development expenses(1) |
$ | (8,621 | ) | $ | 17,295 | |||
| Total research and development expenses, excluding share-based compensation expense |
$ | 57,727 | $ | 73,241 | ||||
| Share-based compensation expenses included in research and development expenses |
$ | 2,496 | $ | 2,303 | ||||
| Total research and development expenses |
$ | 60,223 | $ | 75,543 | ||||
| (1) | Excludes employee share-based compensation expense after $19,9 millions loss on completion accrual reversal as of December 2023. |
| (2) | If we exclude Mag1c impact the percentage of research and development expenses related to Viaskin Peanut in 2023 would be 84% |
| (3) | If we exclude Mag1c impact the percentage of research and development expenses related to Viaskin Milk in 2023 would be 8% |
We cannot determine with certainty the duration and completion costs of the current or future clinical trials of our product candidates or if, when, or to what extent we will generate revenue from the commercialization and sale of any of our product candidates that obtain regulatory approval.
104
We may never succeed in achieving regulatory approval for any of our product candidates. The duration, costs and timing of clinical trials and development of our product candidates will depend on a variety of factors, many of which are outside of our control including:
| • | the FDA’s approval of our BLA for Viaskin Peanut; |
| • | the costs of future commercialization activities, including product sales, marketing, manufacturing and distribution, for any of our product candidates for which we receive marketing approval, especially in North America; |
| • | the costs of securing manufacturing arrangements for commercial production; |
| • | revenue, if any, received from commercial sales of our product candidates, should any of our product candidates receive marketing approval; |
| • | the scope, progress in, results and the costs of, our pre-clinical studies and clinical trials and other research and development programs, particularly as we seek regulatory and marketing approvals for our product candidates that successfully complete clinical trials; |
| • | the scope, prioritization and number of our research and development programs; |
| • | the costs, timing and outcome of regulatory review of our product candidates; |
| • | the achievement of milestones or occurrence of other developments that trigger payments under our existing collaboration agreements, and any additional collaboration agreements we may enter into; |
| • | the extent to which we are obligated to reimburse, or entitled to reimbursement of, clinical trial costs under any collaboration agreements or future collaborations, if any; and |
| • | the costs involved in filing, prosecuting, enforcing and defending patent claims and other intellectual property rights. |
A change in the outcome of any of these variables with respect to the development and commercialization of Viaskin Peanut, if approved, or any other product candidate that we are developing could mean a significant change in the costs and timing associated with the development and commercialization of Viaskin Peanut, if approved, or such other product candidate. For example, if the FDA or other regulatory authority were to require us to conduct pre-clinical and clinical trials beyond those which we currently anticipate will be required for the completion of clinical development, if we experience significant delays in enrollment in any clinical trials or if the FDA or other regulatory authority were to require us to conduct post-approval clinical trials, we could be required to spend significant additional financial resources and time on the completion of the clinical development and potential launch of commercialization.
Components of Our Results of Operations
Operating Income
Our operating income consists of other operating income, as described below, as we generated no revenue from our operating activities in 2023 or 2022.
Other Operating Income
Research Tax Credits
The Research Tax Credit (Crédit d’Impôt Recherche, or CIR) is granted to companies by the French tax authorities in order to encourage them to conduct technical and scientific research. Companies that prove that they have expenditures that meet the required criteria receive a tax credit that can be used for the payment of the corporate tax due for the fiscal year in which the expenditures were made and the next three fiscal years, or, as applicable, can be reimbursed for the excess portion. The expenditures taken into account for the calculation of the research tax credit involve only research expenses.
105
If a company meets certain criteria in terms of sales, headcount or assets to be considered a Small and Medium- sized Enterprises, or SMEs, under EU law, immediate payment of the CIR can be requested. We no longer benefited from the immediate reimbursement of the CIR due to the loss of the SME status under EU law for the fiscal year ending December 31, 2019 and 2020. The CIRs were to be refunded three years after the tax declaration in the event we could not offset it against corporate income tax due.
Beginning in the fiscal year ending December 31, 2021, we recovered our SME status, and became therefore eligible again for the immediate reimbursement of the CIR. During the fiscal year ending December 31, 2022, the Company received the reimbursement of the 2019, 2020 and 2021 fiscal year research tax credits for a total amount of $26.1 million. During the fiscal year ending December 31, 2023, the Company received the reimbursement of the 2022 fiscal year research tax credits for a total amount of $5.9 million.
Collaboration Agreement with Nestlé Health Science
In May 2016, we entered into a Development Collaboration and License Agreement (the “Collaboration Agreement”) with Société des Produits Nestlé S.A. (formerly NESTEC S.A.) (“NESTEC”). The Collaboration Agreement related to an exclusive global collaboration with Nestlé Health Science for the development and, if approved, commercialization of MAG1C, a ready-to-use and standardized atopy patch test tool for the diagnosis of CMPA (non-mediated IgE) in infants.
Under the terms of the Collaboration Agreement, the Company was responsible for leading the development activities of MAG1C up through a pivotal Phase 3 clinical program, and if the appropriate regulatory approvals were received, Nestlé Health Science would support the commercialization of MAG1C globally. The Company was eligible to receive up to €100.0 millions ($105.0 millions at December 31, 2023 closing exchange rate) in potential development, clinical, regulatory and commercial milestones, including an upfront payment of €10.0 millions received in July 2016.
On October 30, 2023, the Company and NESTEC entered into a Mutual Termination Letter Agreement terminating the Collaboration Agreement. Each party remains responsible for its own costs and expenses related to its respective wind-down activities. Any and all licenses and sublicenses, granted by either party to the other party under the Collaboration Agreement, including, without limitation, any licenses to intellectual property, were revoked and terminated.
Consequently, since signing the Mutual Termination Letter Agreement and as of December 31, 2023, we recorded the following :
| • | Loss on completion accrual reversal $19,9 millions (Other Operating Income); |
| • | Deferred revenue accrual reversal $6.9 millions (Operating Expenses); |
| • | Accrual for ongoing Clinical study completion $2.3 millions (Operating Expenses). This accrual represents our best estimate of the remainder expenses related to the ongoing clinical study which will be incurred after December 31, 2023 and until the end of the study. |
Operating Expenses
Since our inception, our operating expenses have consisted primarily of Research and Development activities, General and Administration costs and to lesser extent sales and marketing costs.
Research and Development Expenses
Research and Development expenses comprise clinical trials direct costs as well as salaries, share-based payments and benefits for internal Research and Development personnel.
106
Consultants, costs of clinical trials costs related to manufacturing clinical study materials, sponsored research, clinical trials insurance, other external costs, depreciation (of Research and Development equipments and other depreciation related to Research and Development like loss on completion on MAG1C study), and facility costs related to the development of drug candidates. The Company records upfront, non-refundable payments made to outside vendors, or other payments made in advance of services performed or goods being delivered, as prepaid expenses, which are expensed as services are performed or the goods are delivered.
Certain Research and Development projects are, or have been, partially funded by collaboration agreements, The Company records the related reimbursement of research and development costs under these agreements as income in the period in which such costs are incurred.
Sales and Marketing
Sales and marketing expense consists primarily of personnel costs, consultant fees and share-based compensation for sales and marketing employees, as well as fees related to pre-commercialization activities for Viaskin Peanut in North America and in the European Union. We anticipate that our sales and marketing expenses will increase significantly in the future as we prepare for the potential launch and commercialization of Viaskin Peanut in North America and in the European Union, if approved.
General and Administrative
General and Administrative expenses consist primarily of personnel costs including share-based compensation for Finance, Legal, IT, Human Resources and other Administrative employees. General and Administrative expense also consists of Information Systems architecture, software licenses, IT equipment and, to obtaining a directors and officers liability insurance policy and fees for professional services, mainly related to audit, tax and legal services, real-estate leasing costs, insurance costs, consulting costs, investor relations costs and corporate communication and travel costs.
We anticipate that our General and Administrative expenses will increase in the future to support the expected growth in our Research and Development activities and the potential launch and commercialization of Viaskin Peanut in North America and in European Union, if approved. We also anticipate continued increased expenses associated with being a public company in the United States.
Finance Income (Expense)
Our cash and cash equivalents have been deposited primarily in savings and deposit accounts with a short term remaining maturity at the date of purchase or less, refundable within one month, for which the risk of changes in value is considered to be insignificant. Savings and deposit accounts generate a limited amount of interest income, with very low counterparty risks. We expect to continue this investment strategy.
107
Results of Operations
Comparison of the Years Ended December 31, 2023 and 2022
The following table summarizes the results of our operations, derived from our consolidated financial statements, prepared in compliance with generally accepted accounting principles in the United States, or U.S. GAAP, for the years ended December 31, 2023 and 2022:
| December 31, | $ change | % change | ||||||||||||||
| (Dollar amounts presented in thousands, except per share amounts) | 2023 | 2022 | ||||||||||||||
| Operating income |
$ | 15,728 | $ | 4,844 | 10,885 | 225 | % | |||||||||
| Operating expenses |
||||||||||||||||
| Research and development expenses |
(60,223 | ) | (75,543 | ) | 15,320 | (20 | %) | |||||||||
| Sales and marketing expenses |
(2,438 | ) | (1,608 | ) | (830 | ) | 52 | % | ||||||||
| General and administrative expenses |
(29,500 | ) | (24,324 | ) | (5,176 | ) | 21 | % | ||||||||
| Restructuring income (expenses) |
— | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total Operating expenses |
(92,161 | ) | (101,475 | ) | 9,314 | (9 | %) | |||||||||
|
|
|
|
|
|
|
|||||||||||
| Financial income (expense) |
3,714 | 427 | 3,286 | 769 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Income tax |
(7 | ) | (70 | ) | 63 | (90 | %) | |||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (72,726 | ) | $ | (96,274 | ) | 23,548 | (24 | %) | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Basic/diluted Net loss per share attributable to shareholders |
(0.76 | ) | (1.24 | ) | ||||||||||||
Operating Income
The following table summarizes our operating income for the years presented:
| December 31, | $ change | % change | ||||||||||||||
| (Dollar amounts presented in thousands) | 2023 | 2022 | ||||||||||||||
| Sales |
— | — | ||||||||||||||
| Other income |
15,728 | 4,844 | 10,884 | 225 | % | |||||||||||
| Research tax credit |
8,766 | 5,718 | 3,048 | 53 | % | |||||||||||
| Other operating (loss) income |
6,962 | (874 | ) | 7,836 | (896 | %) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total operating income |
15,728 | 4,844 | 10,884 | 225 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
We generated operating income of $15.7 millions for the year ended December 31, 2023 compared to $4.8 millions for the year ended December 31, 2022. The increase in operating income is due to the increase in research tax credit and the revenue recognition of $6.9 millions related to the deferred revenue following the termination of the Collaboration Agreement with Nestlé.
Research tax credit increased by $3.0 millions for the year ended December 31, 2023 compared to the year ended December 31, 2022 as a result of the extension of the eligible expense base to include clinical supplies. A corrective Research tax credit was filed by the Company for $2.9 millions for 2020, 2021 and 2022 fiscal year research tax credit during the year ended December 31, 2023.
Other operating income increased by $7.8 millions for the year ended December 31, 2023 compared to the year ended December 31, 2022 mainly due the reversal of deferred revenue following the Mutual Termination Letter Agreement, effective October 30, 2023, of the Collaboration Agreement between the Company and Nestlé Health Science.
108
Operating Expenses
Research and Development Expenses
The following table summarizes our research and development expenses for the years presented:
| December 31, | $ change | % change | ||||||||||||||
| (Dollar amounts presented in thousands) | 2023 | 2022 | ||||||||||||||
| Research and development expenses |
||||||||||||||||
| External clinical-related expenses |
49,044 | 42,248 | 6,796 | 16 | % | |||||||||||
| Employee-related costs excl. share-based payment expenses |
14,401 | 10,752 | 3,649 | 34 | % | |||||||||||
| Share-based payment expenses |
2,496 | 2,303 | 193 | 8 | % | |||||||||||
| Depreciation and amortization |
(13,658 | ) | 12,965 | (26,623 | ) | (205 | %) | |||||||||
| Other costs |
7,940 | 7,276 | 664 | 9 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total Research and development expenses |
60,223 | 75,443 | (15,320 | ) | (20 | %) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Our research and development expenses consisted primarily of external costs, such as startup fees paid to investigators, consultants, central laboratories and CROs in connection with our clinical trials, and costs related to acquiring and manufacturing clinical study materials.
Research and Development expenses decreased by $15.3 millions for the year ended December 31, 2023 compared to the year ended December 31, 2022 mainly as a result of :
| • | loss on completion accrual net reversal $17,6 millions (compared to a $10.4 millions depreciation as of December 31, 2022) resulting from Nestlé Collaboration Agreement termination, that offset; |
| • | the global increase of $11.3 million in research and development expenses. |
External clinical-related expenses increased by $6.8 millions for the year ended December 31, 2023 compared to the year ended December 31, 2022, reflecting intensified Research and Development activities (1) after the initiation of the VITESSE trial with the first patient screened in March 2023, and (2) as part of the new safety study for toddlers and children after the FDA confirmed additional safety data is required for BLA.
Employee-related costs, excluding share-based payment expenses, increased by $3.6 million for the year ended December 31, 2023 compared to the year ended December 31, 2022 due to the workforce increase to support research and development activities on VITESSE trial and the new safety study for toddlers and children.
Sales and Marketing Expenses
The following table summarizes our sales and marketing expenses for the years presented:
| December 31, | $ change | % change | ||||||||||||||
| (Dollar amounts presented in thousands) | 2023 | 2022 | ||||||||||||||
| Sales and marketing expenses |
||||||||||||||||
| Employee-related costs incl. share-based payment expenses |
754 | 914 | (160 | ) | (18 | %) | ||||||||||
| External professional services and other costs |
1,784 | 694 | 990 | 143 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total Sales and marketing expenses |
2,438 | 1,608 | 830 | 52 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
Sales and marketing expenses primarily included payroll for the U.S. and European employees as well as fees related to pre- commercialization activities for Viaskin Peanut in North America.
Sales and Marketing expenses increased by $0.8 million for the year ended December 31, 2023 compared to the year ended December 31, 2022, primarily due to an increase in fees related to pre-commercialization activities for Viaskin Peanut in North America.
109
Employee-related costs (including share-based payments expenses) related to payroll for the U.S. and European employees, decreased by $0.2 million for the year ended December 31, 2023 compared to the year ended December 31, 2022, due to employee departure in the US.
External professional services and other costs increased by $1.0 million for the year ended December 31, 2023 compared to the year ended December 31, 2022, mainly due to an increase in fees related to pre-commercialization activities for Viaskin Peanut in North America.
General and Administrative Expenses
The following table summarizes our general and administrative expenses for the years presented:
| December 31, | $ change | % change | ||||||||||||||
| (Dollar amounts presented in thousands) | 2023 | 2022 | ||||||||||||||
| General and administrative expenses |
||||||||||||||||
| External professional services fees |
8,750 | 5,947 | 2,803 | 47 | % | |||||||||||
| Employee-related costs excl. share-based payment expenses |
8,200 | 7,320 | 881 | 12 | % | |||||||||||
| Share-based payment expenses |
3,389 | 2,688 | 701 | 26 | % | |||||||||||
| Depreciation, amortization and other costs |
9,161 | 8,369 | 2,523 | 30 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total General and administrative expenses |
29,500 | 24,324 | 5,176 | 21 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
General and administrative expenses increased by $5.2 millions for the year ended December 31, 2023, compared to the year ended December 31, 2022. The source of this increase is threefold (1) an increase by $2.8 millions of external professional services fees incurred in our financing activities, (2) an increase by $0.9 million in employee-related costs to support General and Administrative activities, and (3) an increase by $0.8 million in depreciation, amortization and other costs mainly due to Montrouge office revamping which will be departed for a new location in Q2 of 2024.
The workforce dedicated to general and administrative activities increased from 27 employees in 2022 to 34 employees in 2023.
Financial income (loss)
Our financial income was $3.7 millions in 2023 and $0.4 million in 2022, and primarily includes the financial income on our financial assets and foreign exchange gains.
Income tax
Our income tax expense was $7,000 for the year ended December 31, 2023, compared to a US Tax income of $70,000 for the year ended December 31, 2022.
Net loss
Net loss was $72.7 million for the year ended December 31, 2023, compared to $96.3 million for the year ended December 31, 2022. Net loss per share (based on the weighted average number of shares outstanding over the period) was $0.76 and $1.24 for the year ended December 31, 2023 and 2022, respectively.
Liquidity and Capital Resources
Financial Condition
On December 31, 2023, we held $141.4 millions in cash and cash equivalents compared to $209.2 millions of cash and cash equivalents on December 31, 2022.. Net cash used for operating activities was $79.6 and $55.7 million for the years ended December 31, 2023 and 2022, respectively.
110
As of December 31, 2023, we recorded a net loss of $72.7 million. Our net cash flows provided by financing activities totaled $7.1 million in 2023, mainly consisting of the proceeds from our ATM program.
Sources of Liquidity and Material Cash Requirements
Based on its current operations, plans and assumptions as revised pursuant to 2023 announcements related to EPITOPE Phase 3 study topline results and VITESSE Phase 3 partial clinical hold lift, the Company expects that its balance of cash and cash equivalents of $141.4 million as of December 31, 2023 will be sufficient to fund its operations until December 31, 2024.
As of the date of the filing, our available cash is not projected to be sufficient to support our operating plan for at least the next 12 months. As such, there is substantial doubt regarding our ability to continue as a going concern.
We fund short-term cash requirements primarily from payments associated with research tax credits (Crédit d’Impôt Recherche).
In May 2022, we established an At-The-Market (“ATM”) program to offer and sell, including with unsolicited investors who have expressed an interest, a total gross amount of up to $100 million of American Depositary Shares (“ADSs”), each ADS representing one-half of one ordinary share of the Company The ATM program is intended to be effective through the expiration of the Company’s existing registration statement registering the ADSs to be issued under the ATM program, i.e. until July 16, 2024, unless terminated prior to such date in accordance with the sales agreement or the maximum amount of the program has been reached. The Company intent is to use the net proceeds, if any, of sales of ADSs issued under the program, together with its existing cash and cash equivalents, primarily for activities associated with potential approval and launch of Viaskin Peanut, as well as to advance the development of the Company’s product candidates using its Viaskin Platform and for working capital and other general corporate purposes.
Pursuant to the ATM program, the Company issued and completed sales of new Ordinary Shares in the form of ADSs for a total gross amount of $15.3 million on May 4, 2022, and of $7.8 million on June 14, 2023. Respectively, 6,036,238 and 2,052,450 new Ordinary Shares in the form of ADSs were issued through a capital increase without preferential subscription rights of the shareholders reserved to specific categories of persons fulfilling certain characteristics (the “ATM issuance”), at a unit subscription price of $1.27 and $1.90 per ADS, each ADS giving the right to receive one-half of one ordinary share of the Company.
During the years ended December 31, 2023 and 2022, we obtained the following financing on the public markets by issuance of securities, net of commissions and estimated offering expenses:
| Equity capital | Bank Loans | Other debt | Total | |||||||||||||
| (Amounts in thousands of U.S. Dollars) | ||||||||||||||||
| 2022 |
194,446 | — | — | 194,446 | ||||||||||||
| 2023 |
6,921 | — | — | 6,921 | ||||||||||||
| Total |
201,367 | — | — | 201,367 | ||||||||||||
We have incurred net losses each year since our inception. Substantially all of our net losses resulted from costs incurred in connection with our development programs and from general and administrative expenses associated with our operations. We have not incurred any bank debt.
We intend to seek additional capital as we prepare for the launch of Viaskin Peanut, if approved, and continue other research and development efforts. We may seek to finance our future cash needs through a combination of public or private equity or debt financings, collaborations, license and development agreements and other forms of non-dilutive financings.
We cannot guarantee that we will be able to obtain the necessary financing to meet our needs or to obtain funds at attractive terms and conditions, including as a result of disruptions to the global financial markets due any future pandemics, epidemics or global health crises and conflict in Ukraine or other global political or military crises.
111
The COVID-19 pandemic and the conflict in Ukraine caused extreme volatility and disruptions in the capital and credit markets. A severe or prolonged economic downturn could result in a variety of risks to us, including reduced ability to raise additional capital when needed or on acceptable terms, if at all. If we are not successful in our financing objectives, we could have to scale back its operations, notably by delaying or reducing the scope of our research and development efforts or obtain financing through arrangements with collaborators or others that may require us to relinquish rights to our product candidates that we might otherwise seek to develop or commercialize independently.
The following table presents our material expenses commitments for future periods:
| Material expenses Commitments Due by the Year Ended December 31, |
||||||||||||||||||||
| 2024 | 2025 | 2026 | Thereafter | Total | ||||||||||||||||
| (Amounts in thousands) | ||||||||||||||||||||
| Operating leases |
1,205 | 65 | 421 | 5,514 | 7,205 | |||||||||||||||
| Purchase obligations—Obligations Under the Terms of CRO commitments |
22,732 | 11,006 | 1,406 | 1,831 | 36,974 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
23,937 | 11,071 | 1,827 | 7,345 | 44,179 | |||||||||||||||
The commitment amounts in the table above are associated with contracts that are enforceable and legally binding and that specify all significant terms, including interest on long-term debt, fixed or minimum services to be used, fixed, minimum or variable price provisions, and the approximate timing of the actions under the contracts. The table does not include obligations under agreements that we can cancel without a significant penalty.
Future events could cause actual payments to differ from these estimates.
Operating leases
Our corporate headquarters are located in Montrouge, France. Our principal offices occupy a 4,470 square meter facility, pursuant to a lease agreement dated March 3, 2015 and represents a $1.1 million cash requirement as of December 31, 2023 until July, 2024.
In November, 2023, the Company entered into new agreements to relocate its headquarters in Chatillon, France:
| • | a short term lease agreement for the fitting works of the new offices, |
| • | a lease agreement starting April 16, 2024 with a minimum duration of six years. |
Our primary U.S. office is located in Basking Ridge, New Jersey. In March 2022, we entered into a lease agreement, commencing on April 1, 2022 and effective for 38 months, for an office of 5,799 square feet in Basking Ridge, New Jersey. The Basking Ridge office represent a $0.4 million cash requirement as of December 31, 2022 which expires June 1, 2025.
In light of the current stage of regulatory interactions regarding Viaskin Peanut, we achieved the resizing of our facility use in North America that was initially intended to support our U.S. subsidiary as well as future commercialization needs, explaining partially operating leases costs as of December 31, 2023 and December 31, 2022 :
| • | In January 2022, we concluded a termination agreement for our 21,548 square feet commercial facility in Summit, New Jersey. A one-time lump sum early termination fee of $1.5 million was paid in 2022 and offset by the recognition of an income of $1.2 million due to the early termination of this lease. |
| • | In June 2021, we entered into a sublease agreement of our 3,780 square feet office space in Tower 49, New York, New York that both expire in the first quarter of 2023, simultaneously with the lease term. |
112
Purchase obligations—Obligations Under the Terms of CRO Agreements
In connection with the launch of our clinical trials for Viaskin Peanut and Viaskin Milk, we signed agreements with several contract research organizations. As of December 31, 2023, expenses associated with the ongoing trials amounted globally to $114.4 million, and we had non-cancellable contractual obligations with CRO until year ended 2025 amounting to $44.2 million.
Cash flows
The table below summarizes our sources and uses of cash for the years ended December 31, 2023 and 2022.
| December 31, | $ change | % change | ||||||||||||||
| (Amounts in thousands of U.S. Dollars) | 2023 | 2022 | ||||||||||||||
| Net cash flows used in operating activities |
(79,653 | ) | (55,666 | ) | (23,982 | ) | 43 | % | ||||||||
| Net cash flows used in investing activities |
(808 | ) | (100 | ) | (1,017 | ) | 1016 | % | ||||||||
| Net cash flows provided by financing activities |
6,767 | 194,120 | (187,045 | ) | (96 | %) | ||||||||||
| Effect of exchange rate changes on cash and cash equivalents |
5,867 | (6,461 | ) | 12,328 | (191 | %) | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net (decrease) increase in cash and cash equivalents |
(67,827 | ) | 131,893 | (199,716 | ) | * | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| * | Percentage not meaningful |
Operating Activities
Our net cash flows used in operating activities were $79.7 millions and $55.7 millions in 2023 and 2022 respectively. Our net cash flows used in operating activities increased by $24.0 millions, or 43%, mainly due to the collection in 2022 of the research tax credit receivable relating to fiscal years 2019 to 2021 for €24.8 millions (corresponding to $28.1 millions on the basis of 2021 closing exchange rate).
Investing Activities
Our net cash flows used in investing activities were $0.8 million and $0.1 million in 2023 and 2022 respectively.
Financing Activities
Our net cash flows resulting from financing activities decreased to $6.8 millions in 2023 from $194.1 millions in 2022. For the year ended December 31, 2023, financing activities are primarily composed of the ATM in June 2023 compared to $194.4 millions during for the year ended December 31, 2022 (that consisted of our May 2022 ATM and June 2022 PIPE offering in second quarter of 2022).
Consistent with customary practice in the French securities market, we entered into a liquidity agreement (contrat de liquidité) with Natixis on April 13, 2012. The liquidity agreement complies with applicable laws and regulations in France. The liquidity agreement authorizes Natixis to carry out market purchases and sales of our shares on Euronext Paris. The amount is classified in other non-current financial assets in our statement of financial position. At December 31, 2023, 222,988 shares and $0.2 million were in the liquidity account. The liquidity agreement has a term of one year and will renew automatically unless otherwise terminated by either party.
Critical Accounting Policies and Significant Judgments and Estimates
Our financial statements are prepared in accordance with U.S. GAAP. Some of the accounting methods and policies used in preparing our financial statements under U.S. GAAP are based on complex and subjective assessments by our management or on estimates based on past experience and assumptions deemed realistic and reasonable based on the facts and circumstances concerned.
113
The actual value of our assets, liabilities and shareholders’ equity and of our earnings could differ from the value derived from these estimates if conditions changed and these changes had an impact on the assumptions adopted. We believe that the most significant management judgments and assumptions in the preparation of our financial statements are described below. See Note 1 to our financial statements for a description of our other significant accounting policies.
Revenue Recognition—Collaboration Agreement with Nestlé Health Science
On May 31, 2016, we announced our entry into an exclusive global collaboration with Nestlé Health Science to develop MAG1C, a ready-to-use and standardized atopy patch test tool for the diagnosis of cow’s milk protein allergy in infants and toddlers. Under the terms of the exclusive collaboration, we are responsible for leading the development activities of MAG1C up through a pivotal Phase 3 clinical program, and if appropriate regulatory approvals are received, Nestlé Health Science will support the commercialization of MAG1C globally, while prioritizing certain agreed-upon countries. We entered into an amendment with Nestlé Health Science on July 12, 2018. We are eligible to receive up to €100.0 million in potential development, clinical, regulatory and commercial milestones, inclusive of a non-refundable upfront payment of €10.0 million that we received in July 2016.
Effective October 30, 2023 ,the Company and Nestlé Health Science signed an agreement, terminating the collaboration agreement between the two parties and the PII clinical study by which upfront and milestones 1 to 3 are definitively acquired by DBV.
Consequently, as of the signing of the Mutual Termination Letter Agreement and as of December 31 2023, we recorded the following :
| • | Loss on completion accrual reversal $19,9 millions; |
| • | Deferred revenue accrual reversal $6.9 millions; |
| • | Accrual for ongoing Clinical study completion $2.3 millions. This accrual represents our best estimate of the remainder expenses related to the ongoing clinical study which will be incurred after December 31, 2023 and until the end of the study. |
Clinical studies costs committed beyond December 31 2023 are to be settled by DBV. Our estimation of costs yet to be incurred for the completion of the study contains uncertainties as they require management to make assumptions and to apply judgment to estimate future cost and timelines to finish the study. These estimates are subjective and our ability to achieve current best estimates may be affected by factors. A $2.3 millions provision representing our current best estimates of costs yet to be incurred for the completion of the study was booked as of December 31, 2023.
Share-Based Compensation
We have several share-based compensation plans for employees and non-employees. We account for share-based compensation in accordance with the authoritative guidance on share-based compensation. Under the fair value recognition provisions of this guidance, share-based compensation is measured at the grant date based on the fair value of the award and is recognized as expense, net of estimated forfeitures, over the requisite service period, which is generally the vesting period of the respective award.
Determining the fair value of the share-based payments at the grant date requires judgment. We calculated the fair value of stock options on the grant date using the Black-Scholes option pricing model. The Black-Scholes model requires the input of highly subjective assumptions, including the expected volatility, expected term, risk- free interest rate and dividend yield.
114
Exercise price
The exercise price of our stock options is based on the fair market value of our ordinary shares.
Risk-free interest rate
The risk-free interest rate is based on French government bonds (GFRN) with a maturity corresponding to the maturity of the share options.
Expected term
We determine the expected term based on the average period the stock options are expected to remain outstanding.
Expected volatility
We determine the expected volatility based on the historical data period corresponding to the stock options expected maturity.
Expected dividend yield
We have never declared or paid any cash dividends and we do not presently plan to pay cash dividends in the foreseeable future. Consequently, we use an expected dividend yield of zero.
In the following table, the weighted average fair value of underlying shares are provided in euros, as we are incorporated in France and the euro is the currency used for the grants. We estimated the following assumptions for the calculation of the fair value of our stock options:
| Assumptions per year ended December 31, |
||||||||
| Stock options per grant date |
2023 | 2022 | ||||||
| Weighted average shares price at grant date in € |
2,08 | 2,33 | ||||||
| Weighted average expected volatility |
97,02 | % | 98,90 | % | ||||
| Weighted average risk-free interest rate |
2,99 | % | 2,20 | % | ||||
| Weighted average expected term (in years) |
6 | 6 | ||||||
| Dividend yield |
— | — | ||||||
| Weighted average fair value of stock-options in € |
1,33 | 2,23 | ||||||
| * | The weighted average fair value of underlying shares is presented in euros, as we are incorporated in France and the euro is the currency used for the grants. |
Pre-funded warrants
The Company has assessed the pre-funded warrants for appropriate equity or liability classification. During this assessment, the Company determined the pre-funded warrants are freestanding instruments that do not meet the definition of a liability pursuant to ASC 480 and do not meet the definition of a derivative pursuant to ASC 815.
The 2022 Warrants are classified as a component of permanent equity because they are freestanding financial instruments that are legally detachable and separately exercisable from the shares of common stock with which they were issued, are immediately exercisable, do not embody an obligation for the Company to repurchase its shares, and permit the holders to receive a fixed number of shares of common stock upon exercise. In addition, the 2022 Warrants do not provide any guarantee of value or return.
115
Accordingly, the pre-funded warrants are classified as equity and accounted for as a component of additional paid-in capital at the time of issuance.
Smaller Reporting Company Status
We are a smaller reporting company as defined in the Securities Exchange Act of 1934, as amended. We may, and intend to, take advantage of certain of the scaled disclosures available to smaller reporting companies and will be able to take advantage of these scaled disclosures for so long as we are a smaller reporting company. We may be a smaller reporting company in any year in which (i) the market value of our voting and non-voting ordinary shares held by non-affiliates is less than $250.0 million measured on the last business day of our second fiscal quarter or (ii) (a) our annual revenue is less than $100.0 million during the most recently completed fiscal year and (b) the market value of our voting and non-voting ordinary shares held by non-affiliates is less than $700.0 million measured on the last business day of our second fiscal quarter.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide the information required under this item.
Item 8. Financial Statements and Supplementary Data.
The financial statements required by this item are set forth beginning on page F-1 of this Annual Report on Form 10-K.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures.
We maintain “disclosure controls and procedures,” as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, that are designed to ensure that information required to be disclosed in the reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the rules and forms of the Securities and Exchange Commission. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is accumulated and communicated to management, including our chief executive officer (principal executive officer) and chief financial officer (principal financial officer), as appropriate, to allow timely decisions regarding required disclosure.
Our principal executive officer and principal financial officer evaluated the effectiveness of these disclosure controls and procedures and concluded that as of December 31, 2023, our disclosure controls and procedures were effective.
Management’s Report on Internal Control Over Financial Reporting.
Our management is responsible for establishing and maintaining adequate internal controls over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) and for the assessment of the effectiveness of our internal control over financial reporting.
116
Item 11. Executive Compensation.
The information required by this Item 11 will be included in the sections titled “Executive Compensation” (excluding the information under the subheading “Pay Versus Performance”) and “Board of Directors and Corporate Governance” in our Proxy Statement and is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information required by this Item 12 will be included in the sections titled “Security Ownership of Certain Beneficial Owners and Management” and “Executive Compensation” in our Proxy Statement and is incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The information required by this Item 13 will be included in the sections titled “Board of Directors and Corporate Governance” and “Certain Relationships and Related Person Transactions” in our Proxy Statement and is incorporated herein by reference.
Item 14. Principal Accountant Fees and Services.
The information required by this Item 14 will be included in Proposal 5 in the section titled “Audit Fees and Services” in our Proxy Statement and is incorporated herein by reference.
PART IV
Item 15. Exhibits and Financial Statement Schedules.
The financial statements schedules and exhibits filed as part of this Annual Report on Form 10-K are as follows:
(a)(1) Financial Statements
Reference is made to the financial statements included in Item 8 of Part II hereof.
(a)(2) Financial Statement Schedules
All other schedules are omitted because they are not required or the required information is included in the financial statements or notes thereto.
(a)(3) Exhibits
118
EXHIBIT INDEX
119
120
| * | Filed herewith. |
| ** | Furnished herewith and not deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and shall not be deemed to be incorporated by reference into any filing under the Securities Act .of 1933, as amended, or the Exchange Act (whether made before or after the date of the Form 10-K), irrespective of any general incorporation language contained in such filing. |
| † | Indicates a management contract or any compensatory plan, contract or arrangement. |
| # | Confidential treatment has been granted from the Securities and Exchange Commission as to certain portions of this document |
121
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| DBV Technologies S.A. | ||
| /s/ Daniel Tassé | ||
| Name: Daniel Tassé | ||
| Title: Chief Executive Officer | ||
| (Principal Executive Officer) | ||
Date: March 7, 2024
Each person whose individual signature appears below hereby authorizes and appoints Daniel Tassé and Virginie Boucinha, and each of them, with full power of substitution and resubstitution and full power to act without the other, as his or her true and lawful attorney-in-fact and agent to act in his or her name, place and stead and to execute in the name and on behalf of each person, individually and in each capacity stated below, and to file any and all amendments to this report on Form 10-K, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing, ratifying and confirming all that said attorneys-in-fact and agents or any of them or their or his substitute or substitutes may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report on Form 10-K has been signed below by the following persons on behalf of the Registrant in the capacities indicated on March 7, 2024.
| Signature |
Title |
|
| /s/ Daniel Tassé Daniel Tassé |
Chief Executive Officer and Director (Principal Executive Officer) |
|
| /s/ Virginie Boucinha Virginie Boucinha |
Chief Financial Officer (Principal Financial and Accounting Officer) |
|
| /s/ Michel de Rosen Michel de Rosen |
Director |
|
| /s/ Mailys Ferrere Mailys Ferrere |
Director |
|
| /s/ Michael J. Goller Michael J. Goller |
Director |
|
| /s/ Danièle Guyot-Caparros Danièle Guyot-Caparros |
Director |
|
| /s/ Timothy E. Morris Timothy E. Morris |
Director |
|
122
| Signature |
Title |
|
| /s/ Ador Ndu Adora Ndu |
Director |
|
| /s/ Julie O’Neill Julie O’Neill |
Director |
|
| /s/ Ravi Madduri Rao Ravi Madduri Rao |
Director |
|
| /s/ Daniel Soland Daniel Soland |
Director |
|
123
Page |
||||
| F-2 | ||||
| F- 4
|
||||
| F- 5
|
||||
| F- 6
|
||||
| F- 7
|
||||
| F- 8
|
||||
• |
We evaluated the design of the internal control related to the Company’s going concern assessment; |
• |
We evaluated the reasonableness of the Company’s forecasted operating expenses by inquiring of senior management to gain an understanding of the Company’s operations, strategy, and research and development activities, compared the forecasted operating expenses to historical operating expenses and challenged expected costs, especially those costs that relate to future clinical trials; |
• |
We assessed management’s ability to forecast operating expenses and cash flows by comparing prior year forecasts to actual financial results; |
• |
We assessed the adequacy of the consolidated financial statements’ disclosure related to the going concern assessment by comparing it to the audit evidence obtained. |
/s/ Deloitte & Associés |
KPMG S.A. | |
/s/ Cédric Adens | ||
Partner | ||
We have served as the Company’s auditor since 2011. |
We have served as the Company’s auditor since 2020. | |
|
Paris-La Défense, France |
||
| March 7, 2024 | ||
Year ended December 31, |
||||||||||||
Note |
2023 |
2022 |
||||||||||
| Assets |
||||||||||||
| Current assets: |
||||||||||||
| Cash and cash equivalents |
3 |
$ | 141,367 | $ | 209,194 | |||||||
| Trade receivables |
— | — | ||||||||||
| Other current assets |
4 |
17,548 | 13,880 | |||||||||
| |
|
|
|
|||||||||
| Total current assets |
158,915 |
223,074 |
||||||||||
| Property, plant, and equipment, net |
5 |
12,623 | 15,096 | |||||||||
| Right-of-use assets related to operating leases |
6 |
5,247 | 2,513 | |||||||||
| Intangible assets |
58 | 10 | ||||||||||
| Other non-current assets |
7 |
6,144 | 5,824 | |||||||||
| |
|
|
|
|||||||||
| Total non-current assets |
24,071 |
23,444 |
||||||||||
| |
|
|
|
|||||||||
| Total Assets |
$ |
182,986 |
$ |
246,518 |
||||||||
| |
|
|
|
|||||||||
| Liabilities and shareholders’ equity |
||||||||||||
| Current liabilities |
||||||||||||
| Trade payables |
8 |
$ | 23,302 | $ | 14,473 | |||||||
| Short-term operating leases |
6 |
1,144 | 1,894 | |||||||||
| Short-term financial debt |
9 |
— | — | |||||||||
| Current contingencies |
13 |
3,959 | 3,944 | |||||||||
| Other current liabilities |
8/9 |
8,934 | 9,210 | |||||||||
| |
|
|
|
|||||||||
| Total current liabilities |
37,339 |
29,521 |
||||||||||
| Long-term operating leases |
6 |
4,526 | 1,127 | |||||||||
| Long-term financial debt |
— | — | ||||||||||
| Non-current contingencies |
13 |
935 | 16,680 | |||||||||
| Other non-current liabilities |
9 |
— | 4,735 | |||||||||
| |
|
|
|
|||||||||
| Total non-current liabilities |
5,461 |
22,543 |
||||||||||
| |
|
|
|
|||||||||
| Total liabilities |
$ |
42,799 |
$ |
52,064 |
||||||||
| |
|
|
|
|||||||||
| Shareholders’ equity: |
||||||||||||
| Ordinary shares, €0.10 par value; 96,431,770 and 94,137,145 shares authorized, and issued as at December 31,
2023 and 2022, respectively |
$ | 10,972 | $ | 10,720 | ||||||||
| Additional paid-in capital |
377,468 | 458,221 | ||||||||||
| Treasury stock, 222,988 and 149,793 ordinary shares as of December 31, 2023 and 2022, respectively, at cost
|
(1,263 | ) | (1,109 | ) | ||||||||
| Accumulated deficit |
(238,862 | ) | (259,578 | ) | ||||||||
| Accumulated other comprehensive income |
742 | 781 | ||||||||||
| Accumulated currency translation effect |
(8,871 | ) | (14,581 | ) | ||||||||
| |
|
|
|
|||||||||
| Total shareholders’ equity |
11 |
$ |
140,187 |
$ |
194,453 |
|||||||
| |
|
|
|
|||||||||
| Total liabilities and shareholders’ equity |
$ |
182,986 |
$ |
246,518 |
||||||||
| |
|
|
|
|||||||||
Year ended December 31, |
||||||||||||
Note |
2023 |
2022 |
||||||||||
| Operating income |
14 |
$ |
15,728 |
$ |
4,844 |
|||||||
| Operating expenses |
||||||||||||
| Research and development expenses |
15 |
(60,223 | ) | (75,543 | ) | |||||||
| Sales & marketing expenses |
15 |
(2,438 | ) | (1,608 | ) | |||||||
| General & administrative expenses |
15 |
(29,500 | ) | (24,324 | ) | |||||||
| Total Operating expenses |
(92,161 | ) | (101,475 | ) | ||||||||
| |
|
|
|
|||||||||
| Loss from operations |
(76,432 |
) |
(96,631 |
) | ||||||||
| |
|
|
|
|||||||||
| Financial income (expenses) |
3,714 | 427 | ||||||||||
| |
|
|
|
|||||||||
| Loss before taxes |
(72,719 |
) |
(96,204 |
) | ||||||||
| |
|
|
|
|||||||||
| Income tax |
16 |
(7 | ) | (70 | ) | |||||||
| |
|
|
|
|||||||||
| Net loss |
$ |
(72,726 |
) |
$ |
(96,274 |
) | ||||||
| |
|
|
|
|||||||||
| Foreign currency translation differences, net of taxes |
5,710 | (8,429 | ) | |||||||||
| Actuarial gains on employee benefits, net of taxes |
(38 | ) | 262 | |||||||||
| |
|
|
|
|||||||||
| Total comprehensive loss |
$ |
(67,054 |
) |
$ |
(104,441 |
) | ||||||
| |
|
|
|
|||||||||
| Basic/diluted Net loss per share attributable to shareholders |
19 |
$ |
(0.76 |
) |
$ |
(1.24 |
) | |||||
| Weighted average number of shares outstanding used in computing per share amounts: |
19 |
95,121,390 | 77,384,133 | |||||||||
Year ended December 31, |
||||||||||||
Notes |
2023 |
2022 |
||||||||||
| Net loss for the period |
$ |
(72,726 |
) |
$ |
(96,274 |
) | ||||||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation, amortization and accrued contingencies |
(13,998 | ) | 13,162 | |||||||||
| Retirement pension obligations |
76 | 105 | ||||||||||
| Expenses related to share-based payments |
6,019 | 5,026 | ||||||||||
| Other elements |
23 | (7 | ) | |||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Decrease (increase) in inventories and work in progress |
— | — | ||||||||||
| Decrease (increase) in trade receivables |
— | — | ||||||||||
| Decrease (increase) in other current assets |
(3,795 | ) | 20,961 | |||||||||
| (Decrease) increase in trade payables |
8,420 | 3,456 | ||||||||||
| (Decrease) increase in other current and non-current liabilities |
(5,334 | ) | 152 | |||||||||
| Change in operating lease liabilities and right of use assets |
1,662 | (2,249 | ) | |||||||||
| Net cash flow used in operating activities |
(79,653 |
) |
(55,666 |
) | ||||||||
| |
|
|
|
|||||||||
| Cash flows used in investing activities: |
||||||||||||
| Acquisitions of property, plant, and equipment |
(677 | ) | (754 | ) | ||||||||
| Proceeds from property, plant, and equipment dispositions |
— | 8 | ||||||||||
| Acquisitions of intangible assets |
— | — | ||||||||||
| Acquisitions of non-current financial assets |
(285 | ) | (123 | ) | ||||||||
| Proceeds from non-current financial assets dispositions |
154 | 770 | ||||||||||
| |
|
|
|
|||||||||
| Net cash flows used in investing activities |
(808 |
) |
(100 |
) | ||||||||
| |
|
|
|
|||||||||
| Cash flows provided by financing activities: |
||||||||||||
| (Decrease) increase in conditional advances |
— | (474 | ) | |||||||||
| Treasury shares |
(154 | ) | 123 | |||||||||
| Capital increases, net of transaction costs |
6,921 | 194,471 | ||||||||||
| Other cash flows related to financing activities |
— | — | ||||||||||
| |
|
|
|
|||||||||
| Net cash flows provided by financing activities |
6,767 |
194,120 |
||||||||||
| |
|
|
|
|||||||||
| Effect of exchange rate changes on cash and cash equivalents |
5,867 | (6,461 | ) | |||||||||
| |
|
|
|
|||||||||
| Net (decrease) / increase in cash and cash equivalents |
(67,827 |
) |
131,893 |
|||||||||
| |
|
|
|
|||||||||
| Net cash and cash equivalents at the beginning of the period |
209,194 | 77,301 | ||||||||||
| |
|
|
|
|||||||||
| Net cash and cash equivalents at the end of the period |
3 |
$ |
141,367 |
$ |
209,194 |
|||||||
| |
|
|
|
|||||||||
Ordinary shares |
Acc. other comprehensive income |
Acc. currency translation effect |
||||||||||||||||||||||||||||||
Number of Shares Note 11 |
Amount |
Additional paid-in
capital |
Treasury stock |
Acc. deficit |
Total Equity |
|||||||||||||||||||||||||||
| Balance at December 31, 2021 |
55,095,762 |
$ |
6,538 |
$ |
358,115 |
$ |
(1,232 |
) |
$ |
(258,528 |
) |
$ |
519 |
$ |
(6,137 |
) |
$ |
99,274 |
||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net (loss) |
(96,274 |
) |
(96,274 |
) | ||||||||||||||||||||||||||||
| Other comprehensive (loss) |
262 |
(8,429 |
) |
(8,167 |
) | |||||||||||||||||||||||||||
| Issuance of ordinary shares |
39,041,383 |
4,182 |
102,194 |
106,377 |
||||||||||||||||||||||||||||
| Issuance of share warrants |
— |
— |
88,094 |
— |
— |
— |
— |
88,074 |
||||||||||||||||||||||||
| Treasury shares |
123 |
123 |
||||||||||||||||||||||||||||||
| Share-based payments (income) expenses |
5,026 |
5,026 |
||||||||||||||||||||||||||||||
| Allocation of accumulated net losses |
— |
— |
(95,209 |
) |
— |
95,209 |
— |
— |
— |
|||||||||||||||||||||||
| Other change in equity |
— |
— |
— |
— |
15 |
(15 |
) |
— |
||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2022 |
94,137,145 |
$ |
10,720 |
$ |
458,221 |
$ |
(1,109 |
) |
$ |
(259,578 |
) |
$ |
781 |
$ |
(14,581 |
) |
$ |
194,453 |
||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net (loss) |
(72,726 |
) |
(72,726 |
) | ||||||||||||||||||||||||||||
| Other comprehensive income (loss) |
(38 |
) |
5,710 |
5,672 |
||||||||||||||||||||||||||||
| Issuance of ordinary shares |
2 294 625 |
252 |
6,670 |
6,921 |
||||||||||||||||||||||||||||
| Issuance of share warrants |
— |
— |
— |
— |
— |
— |
— |
— |
||||||||||||||||||||||||
| Treasury shares |
(154 |
) |
(154 |
) | ||||||||||||||||||||||||||||
| Share-based payments (income) expenses |
6,019 |
6,019 |
||||||||||||||||||||||||||||||
| Allocation of accumulated net losses |
(93,441 |
) |
93,441 |
— |
||||||||||||||||||||||||||||
| Other change in equity |
— |
— |
— |
— |
— |
— |
— |
— |
||||||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance at December 31, 2023 |
96,431,770 |
$ |
10,972 |
$ |
377,468 |
$ |
(1,263 |
) |
$ |
(238,862 |
) |
$ |
742 |
$ |
(8,871 |
) |
$ |
140,187 |
||||||||||||||
| • | DBV Technologies Inc. was incorporated in Delaware on April 7, 2014 (the “US subsidiary”). The share capital of this US subsidiary is 100% owned by DBV Technologies S.A. |
| • | DBV Australia Pty Ltd. was incorporated in New South Wales, Australia on July 3, 2018 (the “Australian subsidiary”). The share capital of this Australian subsidiary is 100% owned by DBV Technologies S.A. (“DBV Technologies”). |
| • | DBV Pharma was incorporated in Paris on December 21, 2018 (the “French subsidiary”). The share capital of this French subsidiary is 100% owned by DBV Technologies S.A. |
PROPERTY, PLANT, AND EQUIPMENT ITEM PERIOD |
DEPRECIATION |
|||
| Laboratory equipment and technical facilities |
3 to 10 years | |||
| Building fixtures and leasehold improvements |
5 to 9 years | |||
| Office equipment and furniture |
5 years | |||
| Computer equipment |
3 years | |||
| • | Level 1—Quoted market prices (unadjusted) in active markets for identical assets or liabilities that the reporting entity has the ability to access at the measurement date. |
| • | Level 2—Quoted market prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, or other inputs that are observable, either directly or indirectly. Fair value determined through the use of models or other valuation methodologies. |
| • | Level 3—Significant unobservable inputs for assets or liabilities that cannot be corroborated by market data. Fair value is determined by the reporting entity’s own assumptions utilizing the best information available and includes situations where there is little market activity for the asset or liability. |
| • | discount rate; |
| • | future salary increases; |
| • | employee turnover; and |
| • | mortality tables. |
| • | information available before the consolidated financial statements are issued indicates that it is probable that an asset had been impaired or a liability had been incurred at the date of the consolidated financial statements; and |
| • | the amount of loss can be reasonably estimated. |
| 1. | Identify a modified Viaskin patch (which the Company calls mVP). |
| 2. | Generate the 6-month safety and adhesion clinical data FDA requested via STAMP, which the Company expected to be the longest component of the mVP clinical plan. The Company prioritized the STAMP protocol submission so the Company could begin the clinical trial as soon as possible. |
| 3. | Demonstrate the equivalence in allergen uptake between the current and modified patches in the intended patient population via EQUAL. The complexity of EQUAL hinged on the lack of established clinical and regulatory criteria to characterize allergen uptake via an epicutaneous patch. To support those exchanges, the Company outlined its proposed approach to demonstrate allergen uptake equivalence between the two patches, and allotted time to generate informative data through two additional Phase 1 clinical trials in healthy adult volunteers: |
| a. | PREQUAL, a Phase 1 trial with adult healthy volunteers to optimize the allergen sample collection methodologies and validate the assays we intend to use in EQUAL. The data collection phase of the trial is complete, and the data analysis phase is ongoing. |
| b. | ‘EQUAL in adults’—a second Phase 1 trial with adult healthy volunteers to compare the allergen uptake of cVP and mVP. |
• |
Loss on completion accrual reversal $19,9 millions (Other Operating Income); |
• |
Deferred revenue accrual reversal $6.9 millions (Operating Expenses); |
• |
Accrual for ongoing Clinical study completion $2.3 millions (Operating Expenses). This accrual represents our best estimate of the remainder expenses related to the ongoing clinical study which will be incurred after December 31, 2023 and until the end of the study. |
December 31, |
||||||||
2023 |
2022 |
|||||||
| Cash |
10,530 | 30,104 | ||||||
| Cash equivalents |
130,826 | 179,090 | ||||||
| |
|
|
|
|||||
| Total cash and cash equivalents as reported in statement of financial position |
141,367 |
209,194 |
||||||
| |
|
|
|
|
|
|
|
|
| Bank overdrafts |
— | — | ||||||
| |
|
|
|
|||||
| Total net cash and cash equivalents as reported in the statement of cash flow |
141,367 |
209,194 |
||||||
| |
|
|
|
|
|
|
|
|
December 31, |
||||||||
2023 |
2022 |
|||||||
| Research tax credit |
8,857 | 5,792 | ||||||
| Other tax claims |
5,236 | 3,903 | ||||||
| Prepaid expenses |
2,103 | 2,680 | ||||||
| Other receivables |
1,353 | 1,504 | ||||||
| |
|
|
|
|||||
| Total |
17,548 |
13,880 |
||||||
| |
|
|
|
|
|
|
|
|
• |
received the reimbursement of $5.9 millions of the 2022 fiscal year research tax credit ; |
• |
made a complementary statement for 2020, 2021 and 2022 fiscal year research tax credit. A complementary research tax credit has been booked for $2.9 millions . |
Amount in thousands of US Dollars |
||||
Opening balance sheet receivable as of January 1, 2022 |
28,092 | |||
+ 2022 fiscal year research tax credit |
5,718 | |||
- Payment received |
(26,117 | ) | ||
- Adjustment and currency translation effect |
(1,901 | ) | ||
Closing balance sheet receivable as of December 31, 2022 |
5,792 |
|||
Of which—Non-current portion |
— | |||
Of which—Current portion |
5,792 | |||
Amount in thousands of US Dollars |
||||
Opening balance sheet receivable as of January 1, 2023 |
5,792 | |||
+ 2023 fiscal year research tax credit (1) |
8,766 | |||
- Payment received |
(5,971 | ) | ||
- Adjustment and currency translation effect |
271 | |||
Closing balance sheet receivable as of December 31, 2023 |
8,857 |
|||
Of which—Non-current portion |
— | |||
Of which—Current portion |
8,857 | |||
| (1) | Included 2020, 2021 and 2022 complementary research tax credit made during the fiscal year ended December 31, 2023 |
1/1/2022 |
Currency translation effect |
Increase |
Decrease |
Reclassification |
12/31/2022 |
|||||||||||||||||||
Laboratory equipment |
21,434 | (1,246 | ) | — | — | 270 | 20,459 | |||||||||||||||||
Building fixtures |
3,958 | (196 | ) | 55 | (604 | ) | — | 3,214 | ||||||||||||||||
Office equipment |
864 | (25 | ) | 74 | (428 | ) | — | 485 | ||||||||||||||||
Computer equipment |
1,299 | (65 | ) | 16 | — | 8 | 1,258 | |||||||||||||||||
Property, plant, and equipment in progress |
4,390 | (252 | ) | 608 | — | (278 | ) | 4,468 | ||||||||||||||||
Total, gross |
31,945 |
(1,783 |
) |
754 |
(1,032 |
) |
— |
29,884 |
||||||||||||||||
Less accumulated amort. and deprec. |
(13,799 | ) | 703 | (2,723 | ) | 1,031 | — | (14,788 | ) | |||||||||||||||
Total, net |
18,146 |
(1,080 |
) |
(1,968 |
) |
(1 |
) |
— |
15,096 |
|||||||||||||||
1/1/2023 |
Currency translation effect |
Increase |
Decrease |
Reclassification |
12/31/2023 |
|||||||||||||||||||
Laboratory equipment |
20,459 | 815 | — | — | 3,565 | 24,839 | ||||||||||||||||||
Building fixtures |
3,214 | 114 | — | — | 3,327 | |||||||||||||||||||
Office equipment |
485 | 5 | 53 | — | 552 | |||||||||||||||||||
Computer equipment |
1,258 | 40 | — | 126 | 1,425 | |||||||||||||||||||
Property, plant, and equipment in progress |
4,468 | 91 | 625 | — | (3,750 | ) | 1,433 | |||||||||||||||||
Total, gross |
29,884 |
1,074 |
677 |
— |
(59 |
) |
31,577 |
|||||||||||||||||
Less accumulated amortization and depreciation |
(14,788 | ) | (60 | ) | (3,566 | ) | — | — | (18,954 | ) | ||||||||||||||
Total, net |
15,096 |
474 |
(2,889 |
) |
— |
(59 |
) |
12,623 |
||||||||||||||||
December 31, 2023 |
December 31, 2022 |
|||||||||||||||||||||||
Real estate |
Other assets |
Total |
Real estate |
Other assets |
Total |
|||||||||||||||||||
Current portion |
1,205 | 71 | 1,275 | 1,972 | 79 | 2,051 | ||||||||||||||||||
Year 2 |
65 | 11 | 75 | 1,168 | 74 | 1,243 | ||||||||||||||||||
Year 3 |
421 | — | 412 | 65 | 6 | 71 | ||||||||||||||||||
Year 4 |
919 | — | 919 | — | — | — | ||||||||||||||||||
Year 5 |
919 | — | 919 | — | — | — | ||||||||||||||||||
Thereafter |
3,677 | — | 3,677 | — | — | — | ||||||||||||||||||
Total minimum lease payments |
7,205 |
81 |
7,286 |
3,204 |
160 |
3,364 |
||||||||||||||||||
Less: Effects of discounting |
(1,617 | ) | (9 | ) | (1,626 | ) | (325 | ) | (17 | ) | (343 | ) | ||||||||||||
Present value of operating lease |
5,588 |
73 |
5,661 |
2,879 |
143 |
3,021 |
||||||||||||||||||
Less: current portion |
(1,072 | ) | (68 | ) | (1,144 | ) | (1,823 | ) | (71 | ) | (1,894 | ) | ||||||||||||
Long-term operating lease |
4,516 |
5 |
4,526 |
1,055 |
72 |
1,127 |
||||||||||||||||||
Weighted average remaining lease term (years) |
7.954 | — | 1.40 | — | ||||||||||||||||||||
Weighted average discount rate |
4.53 | % | 2.50 | % | 3.00 | % | 2.45 | % | ||||||||||||||||
December 31, |
||||||||
2023 |
2022 |
|||||||
Operating lease expense |
1,776 | 1,800 | ||||||
Refurbishing impact |
1,750 | — | ||||||
Net termination impact |
(92 | ) | (1,657 | ) | ||||
| • | a short term lease agreement in order to fit the new offices, |
| • | A lease agreement starting on April 16, 2024 |
December 31, |
||||||||
2023 |
2022 |
|||||||
| Cash paid for amounts included in the measurement of lease liabilities |
||||||||
| Operating cash flows from operating leases |
1,956 | 2,195 | ||||||
December 31, |
||||||||
2023 |
2022 |
|||||||
| FX facility collateral account |
3,904 | 3,739 | ||||||
| Deposits, pledged securities and other non-current financial assets |
2,104 | 1,773 | ||||||
| Liquidity contract |
166 | 312 | ||||||
| |
|
|
|
|||||
| Total other non-current assets |
6,144 |
5,824 |
||||||
| |
|
|
|
|||||
December 31, |
||||||||
2023 |
2022 |
|||||||
| Social debt |
7,828 | 5,872 | ||||||
| Deferred income |
— | 2,137 | ||||||
| Tax liabilities |
223 | 69 | ||||||
| Other debts |
883 | 1,131 | ||||||
| |
|
|
|
|||||
| Total |
8,934 |
9,210 |
||||||
| |
|
|
|
|||||
Total |
2024 |
2025 |
Thereafter |
|||||||||||||
| Other current liabilities |
8,934 | 8,934 | — | — | ||||||||||||
| Supplier accounts payable and related payables |
23,302 | 23,302 | — | — | ||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Total liabilities |
32,236 |
32,236 |
— |
— |
||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
• |
Deferred revenue accrual reversal $6.9 millions (including $4.7 millions recorded in Other non-current liabilities as of December 31, 2022). |
| • | decided, within the framework of the June 2022 PIPE the principle of a capital increase in cash with cancellation of preferential subscription rights, reserved for categories of persons meeting the characteristics set out in the 18th nd |
| • | granted a number of authorizations for the purpose of carrying out the issuance; |
| • | sub-delegated its authority to the Chief Executive Officer for the purpose of implementing the financing. |
| • | decided, making use of the 18 th |
| • | decided to set the maximum nominal amount of the capital increase resulting from the full exercise of the prefunded warrants at € 2,827,633.10, by issuing a maximum of 28,276,331 ordinary shares, with a value of € 0.10 to be subscribed in cash at the price of € 0.10 euro (without share premium), and to be fully paid up at the time of subscription, i.e. a capital increase of a maximum nominal amount of € 2,827,633.10 (and a share premium corresponding to the amount of the pre-financed price released in advance at the time of the subscription of the prefunded warrants ), being specified that this amount does not take into account the nominal value of the ordinary shares to be issued in order to preserve the rights of the holders of securities giving access to the capital issued or to be issued, in accordance with the legal and regulatory provisions and the contractual stipulations providing for other cases of adjustment if necessary; |
| • | determined the list of beneficiaries (designated within each of the categories of persons defined in the 18 th resolution of the Board General Meeting) and the number of New Ordinary Shares and warrants allocated to each of them under the conditions defined in the 18th resolution of the Board General Meeting beneficiaries under the conditions defined in section 5 of the offering circular relating to the June 2022 PIPE. |
Prefunded warrants |
||||
| Balance as of December 31, 2022 |
28,276,331 |
|||
| Granted during the period |
— | |||
| Forfeited during the period |
— | |||
| Exercised/released during the period |
— | |||
| Expired during the period |
— | |||
| |
|
|||
| Balance as of December 31, 2023 |
28,276,331 |
|||
| |
|
|||
Date |
Nature of the transactions |
Share capital* |
Additional paid-in
capital |
Number of shares |
||||||||||
| Balance as of December 31, 2021 |
6,538 |
358,115 |
55,095,762 |
|||||||||||
| |
|
|
|
|
|
|||||||||
03/23/2022 |
Capital increase by ordinary shares |
0 |
(0 |
) |
775 |
|||||||||
05/10/2022 |
Capital increase by ATM program |
637 |
13,442 |
6,036,238 |
||||||||||
05/12/2022 |
Retained earnings charged on share premium |
(95,209 |
) |
|||||||||||
05/19/2022 |
Capital increase by employee warrants |
1 |
(1 |
) |
5,000 |
|||||||||
05/24/2022 |
Capital increase by employee warrants |
3 |
(3 |
) |
26,135 |
|||||||||
06/09/2022 |
Capital increase by ordinary shares |
3,530 |
88,743 |
32,855,669 |
||||||||||
06/09/2022 |
Capital increase by share warrants |
88,094 |
||||||||||||
06/10/2022 |
Capital increase by employee warrants |
0 |
13 |
3,100 |
||||||||||
07/08/2022 |
Capital increase by employee warrants |
0 |
10 |
2,513 |
||||||||||
09/23/2022 |
Capital increase by ordinary shares |
0 |
(0 |
) |
249 |
|||||||||
11/19/2022 |
Capital increase by ordinary shares |
0 |
(0 |
) |
2,500 |
|||||||||
11/22/2022 |
Capital increase by ordinary shares |
3 |
(3 |
) |
30,625 |
|||||||||
11/24/2022 |
Capital increase by ordinary shares |
8 |
(8 |
) |
78,579 |
|||||||||
12/31/2021 |
Share-based payments |
5,026 |
||||||||||||
| |
|
|
|
|
|
|||||||||
| Balance as of December 31, 2022 |
10,720 |
458,220 |
94,137,145 |
|||||||||||
| |
|
|
|
|
|
|||||||||
| 03/23/2023 |
Capital increase by employee warrants |
1 |
(1 |
) |
10,174 |
|||||||||
| 04/12/2023 |
Retained earnings charged on share premium |
(93,441 |
) |
|||||||||||
| 05/19/2023 |
Capital increase by ordinary shares |
0 |
(0 |
) |
2,500 |
|||||||||
| 05/22/2023 |
Capital increase by ordinary shares |
2 |
(2 |
) |
14,374 |
|||||||||
| 05/24/2023 |
Capital increase by ordinary shares |
4 |
(4 |
) |
34,321 |
|||||||||
| 06/16/2023 |
Capital increase by ATM program |
225 |
6,696 |
2,052,450 |
||||||||||
| 09/23/2023 |
Capital increase by ordinary shares |
0 |
(0 |
) |
2,599 |
|||||||||
| 10/25/2023 |
Capital increase by ordinary shares |
4 |
(4 |
) |
35,000 |
|||||||||
| 11/19/2023 |
Capital increase by ordinary shares |
0 |
(0 |
) |
2,500 |
|||||||||
| 11/21/2023 |
Capital increase by ordinary shares |
6 |
(6 |
) |
57,775 |
|||||||||
| 11/22/2023 |
Capital increase by ordinary shares . . . . . . . . . . . |
6 |
(6 |
) |
50,058 |
|||||||||
| 11/24/2023 |
Capital increase by ordinary shares . . . . . . . . . . . |
4 |
(4 |
) |
32,884 |
|||||||||
| 12/31/2023 |
|
Share-based payments |
|
|
|
|
|
|
6,020
|
|
|
|
|
|
| |
|
|
|
|
|
|||||||||
| Balance as of December 31, 2023 |
10,972 |
377,468 |
96,431,770 |
|||||||||||
| |
|
|
|
|
|
|||||||||
| Share-based
payments instrument |
General meeting of shareholders |
Board of directors meeting |
Grant date |
Number granted |
||||||
| BSA |
12/9/11 |
9/25/12 |
9/25/12 |
30,000 |
||||||
| BSA |
6/4/13 |
7/25/13 |
7/25/13 |
73,000 |
||||||
| SO |
12/9/11 |
9/18/13 |
9/18/13 |
518,000 |
||||||
| BSA |
6/3/14 |
3/24/15 |
3/24/15 |
10,000 |
||||||
| SO |
6/3/14 |
6/23/15 |
6/23/15 |
120,000 |
||||||
| BSA |
6/23/15 |
11/19/15 |
11/19/15 |
22,500 |
||||||
| BSA |
6/23/15 |
12/15/15 |
2/15/16 |
90,000 |
||||||
| SO |
6/3/14 |
4/6/16 |
4/21/16 |
33,000 |
||||||
| SO |
6/3/14 |
6/21/16 |
6/21/16 |
110,000 |
||||||
| BSA |
6/21/16 |
6/21/16 |
8/21/16 |
20,000 |
||||||
| SO |
6/3/14 |
6/21/16 |
9/15/16 |
9,300 |
||||||
| SO |
6/3/14 |
6/21/16 |
10/17/16 |
16,500 |
||||||
| BSA |
6/21/16 |
12/9/16 |
2/9/16 |
59,000 |
||||||
| SO |
6/3/14 |
6/21/16 |
12/9/16 |
74,960 |
||||||
| RSU |
9/21/15 |
3/14/17 |
3/14/17 |
22,500 |
||||||
| RSU |
9/21/15 |
4/20/17 |
4/20/17 |
24,000 |
||||||
| BSA |
6/15/17 |
6/15/17 |
8/15/17 |
9,000 |
||||||
| SO |
6/3/14 |
6/15/17 |
6/15/17 |
126,000 |
||||||
| SO |
6/15/17 |
6/15/17 |
6/15/17 |
111,600 |
||||||
| SO |
6/15/17 |
6/15/17 |
9/15/17 |
52,600 |
||||||
| SO |
6/15/17 |
11/17/17 |
12/5/17 |
625,200 |
||||||
| BSA |
6/15/17 |
5/2/18 |
7/2/18 |
44,000 |
||||||
| RSU |
6/22/18 |
6/22/18 |
6/22/18 |
486,153 |
||||||
| RSU |
6/22/18 |
9/6/18 |
9/6/18 |
450 |
||||||
| SO |
6/22/18 |
9/6/18 |
9/6/18 |
65,000 |
||||||
| SO |
6/22/18 |
6/22/18 |
10/15/18 |
76,700 |
||||||
| RSU |
6/22/18 |
11/1/18 |
11/1/18 |
57,000 |
||||||
| SO |
6/22/18 |
11/29/18 |
11/29/18 |
350,000 |
||||||
| RSU |
6/22/18 |
12/12/18 |
12/12/18 |
16,250 |
||||||
| RSU |
6/22/18 |
12/12/18 |
12/17/18 |
3,000 |
||||||
| SO |
6/22/18 |
3/4/19 |
3/20/19 |
547,100 |
||||||
| RSU |
6/22/18 |
5/10/19 |
5/10/19 |
100,000 |
||||||
| SO |
5/24/19 |
5/24/19 |
5/24/19 |
150,000 |
||||||
| SO |
5/24/19 |
7/1/19 |
7/1/19 |
403,400 |
||||||
| SO |
5/24/19 |
7/1/19 |
7/22/19 |
75,000 |
||||||
| RSU |
5/24/19 |
10/11/19 |
10/11/19 |
40,000 |
||||||
| SO |
5/24/19 |
10/11/19 |
1/15/20 |
94,500 |
||||||
| RSU |
5/24/19 |
10/11/19 |
3/16/20 |
5,000 |
||||||
| RSU |
4/20/20 |
4/20/20 |
4/29/20 |
20,000 |
||||||
| RSU |
4/20/20 |
11/24/20 |
11/24/20 |
475,000 |
||||||
| SO |
4/20/20 |
11/24/20 |
11/24/20 |
1,216,200 |
||||||
| RSU |
4/20/20 |
3/23/21 |
3/23/21 |
24,900 |
||||||
| SO |
4/20/20 |
3/23/21 |
3/23/21 |
75,200 |
||||||
| RSU |
5/19/21 |
5/19/21 |
5/19/21 |
20,000 |
||||||
| Share-based
payments instrument |
General meeting of shareholders |
Board of directors meeting |
Grant date |
Number granted |
||||||
| BSA |
5/19/21 |
5/19/21 |
6/3/21 |
39,185 |
||||||
| RSU |
5/19/21 |
11/22/21 |
11/22/21 |
257,300 |
||||||
| SO |
5/19/21 |
11/22/21 |
11/22/21 |
1,107,300 |
||||||
| RSU |
5/19/21 |
5/12/22 |
5/12/22 |
3,200 |
||||||
| SO |
5/19/21 |
5/12/22 |
5/12/22 |
19,000 |
||||||
| RSU |
5/12/22 |
7/29/22 |
7/29/22 |
66,700 |
||||||
| SO |
5/12/22 |
7/29/22 |
7/29/22 |
135,500 |
||||||
| RSU |
5/12/22 |
11/21/22 |
11/21/22 |
519,650 |
||||||
| SO |
5/12/22 |
11/21/22 |
11/21/22 |
1,771,786 |
||||||
| RSU |
4/12/23 |
1/09/23 |
1/9/23 |
35,800 |
||||||
| SO |
4/12/23 |
1/09/23 |
1/9/23 |
59,200 |
||||||
| RSU |
4/12/23 |
11/20/23 |
11/20/23 |
912,650 |
||||||
| SO |
4/12/23 |
11/20/23 |
11/20/23 |
2,290,722 |
||||||
| Weighted average share price at grant date (in €) |
10.75 | |||
| Weighted average expected volatility |
90.0 | % | ||
| Weighted average risk-free interest rate |
(0.53 | )% | ||
| Weighted average expected term (in years) |
3.21 | |||
| Dividend yield |
— | |||
| Weighted average fair value of warrants (in €) |
— |
Number of warrants outstanding |
Weighted- average exercise price (in Euros) |
Weighted- average remaining contractual term (in years) |
Aggregate intrinsic value (in thousands of Euros) |
|||||||||||||
| Balance as of December 31, 2021 |
256,693 |
47.51 |
4.35 |
— |
||||||||||||
| Granted during the period |
— | — | — | — | ||||||||||||
| Forfeited during the period |
— | — | — | — | ||||||||||||
| Exercised during the period |
— | — | — | — | ||||||||||||
| Expired during the period |
(5,000 | ) | 8.59 | — | — | |||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Balance as of December 31, 2022 |
251,693 |
48.29 |
4.36 |
— |
||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Warrants exercisable as of December 31, 2022 |
251,693 | 48.29 | 4.36 | — | ||||||||||||
Number of warrants outstanding |
Weighted- average exercise price (in Euros) |
Weighted- average remaining contractual term (in years) |
Aggregate intrinsic value (in thousands of Euros) |
|||||||||||||
| Balance as of December 31, 2022 |
251,693 |
48.29 |
4.35 |
— |
||||||||||||
| Granted during the period |
— | — | — | — |
||||||||||||
| Forfeited during the period |
— | — | — | — | ||||||||||||
| Exercised during the period |
— | — | — | — | ||||||||||||
| Expired during the period |
(7,000 | ) |
— | — | — | |||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Balance as of December 31, 2023 |
244,693 |
48.29 |
4.35 |
— |
||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Warrants exercisable as of December 31, 2023 |
244,693 | 48.29 | 4.35 | — | ||||||||||||
| • | Before June 22, 2018 and from January 15, 2020 to November 22, 2021, SO granted mainly vest over four years at a rate of 25% upon the first anniversary of the issuance date and 12.5% every 6 months thereafter, subject to the beneficiary being still employed by the Company (except in specific contractual clause or board of directors’ decisions), |
• |
Between June 22, 2018 and January 15, 2020, SO may be exercised by the beneficiary once both of the following conditions have been met: |
| • | Service condition: 25% upon the first anniversary of the issuance date and 12.5% every 6 months thereafter, subject to the beneficiary being still employed by the Company (except in specific contractual clause or board of directors’ decisions), and, |
| • | Performance condition: approval of Viaskin ™ Peanut by the US Food and Drug Administration, |
| • | Since November 22, 2021, SO granted mainly vest over four years at a rate of 25% upon the first anniversary of the issuance date and 25% every 12 months thereafter, subject to the beneficiary being still employed by the Company (except in specific contractual clause or board of directors’ decisions), |
Number of SO outstanding |
Weighted- average exercise price in Euros |
Weighted- average remaining contractual term (in years) |
Aggregate intrinsic value in thousands of Euros |
|||||||||||||
| Balance as of December 31, 2021 |
3,631,210 |
15.25 |
8.67 |
|||||||||||||
| Granted during the period |
1,926,286 | 3.12 | — | — | ||||||||||||
| Forfeited during the period |
(245,314 | ) |
12.22 | — | — | |||||||||||
| Exercised during the period |
(5,613 | ) | 4.16 | — | — | |||||||||||
| Expired during the period |
— | — | — | — | ||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Balance as of December 31, 2022 |
5,306,569 |
11.00 |
8.41 |
|||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Options exercisable as of December 31, 2022 |
1,331,508 |
20.20 |
6.69 |
— |
||||||||||||
Number of SO outstanding |
Weighted- average exercise price in Euros |
Weighted- average remaining contractual term (in years) |
Aggregate intrinsic value in thousands of Euros |
|||||||||||||
| Balance as of December 31, 2022 |
5,306,569 |
11.00 |
8.41 |
|||||||||||||
| Granted during the period |
1,926,286 |
2.03 |
— |
— |
||||||||||||
| Forfeited during the period |
(369,800 |
) |
3.76 |
— |
— |
|||||||||||
| Exercised during the period |
— |
— |
— |
— |
||||||||||||
| Expired during the period |
(168,000 |
) |
— |
— |
— |
|||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Balance as of December 31, 2023 |
7,118,691 |
8.55 |
9.10 |
|||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Options exercisable as of December 31, 2023 |
602,995 |
38.70 |
5.35 |
— |
||||||||||||
Assumptions per year ended, December 31, |
||||||||||||||||||||||||||||
| Stock options per grant date |
2017 |
2018 |
2019 |
2020 |
2021 |
2022 |
2023 |
|||||||||||||||||||||
| Weighted average shares price at grant date in € |
45.49 | 31.86 | 15.26 | 5.54 | 5.71 | 2.33 | 2.03 | |||||||||||||||||||||
| Weighted average expected volatility |
41,80 | % | 47,10 | % | 70,80 | % | 87,30 | % | 90,20 | % | 98,90 | % | 93,70 | % | ||||||||||||||
| Weighted average risk-free interest rate |
-0,10 | % | 0,30 | % | -0,10 | % | -0,50 | % | -0,06 | % | 2,20 | % | 2,95 | % | ||||||||||||||
| Weighted average expected term (in years) |
6 | 6 | 6 | 6 | 6 | 6 | ||||||||||||||||||||||
| Dividend yield |
0 | 0 | 0 | 0 | 0 | — | — | |||||||||||||||||||||
| Weighted average fair value of stock-options in € |
17.16 | 13.67 | 9.65 | 3.9 | 4.17 | 2.23 | 1.67 | |||||||||||||||||||||
| • | Before May 31, 2019, the vesting of RSUs granted is subject to the expiration of the presence condition of one (1) or two (2) years (except in specific board of directors’ decisions). The release of RSUs for |
| these plans is subject to the achievement of performance conditions (submission of a BLA to U.S. FDA for Viaskin ™ Peanut, approval of Viaskin™ Peanut by the U.S. FDA, first sale of Viaskin™ Peanut in the United States); |
| • | Between May 31, 2019 and November 23, 2020, the vesting of RSUs is subject either to the expiration of the presence condition of two (2) years only, or to the dual condition of expiration of the presence condition and achievement of the performance condition (date of approval of Viaskin ™ Peanut by the U.S. FDA); |
| • | Between November 24, 2020 and November 20, 2023, RSUs vest over four years at a rate of 25% upon the first anniversary of the issuance date and 12.5% every 6 months thereafter, subject to the beneficiary being still employed by the Company (except in specific board of directors’ decisions). |
| • | Since November 20, 2023, RSUs vest over four years at a rate of 25% upon the first anniversary of the issuance date and 25% every126 months thereafter, subject to the beneficiary being still employed by the Company (except in specific board of directors’ decisions). |
Number of RSU outstanding |
Weighted average grant date fair value in Euros |
|||||||
| Balance as of December 31, 2021 |
1,240,520 |
18.77 |
||||||
| Granted during the period |
589,550 | 2.67 | ||||||
| Forfeited during the period |
) | 4.96 | ||||||
| Released during the period |
(143,863 | ) |
5.15 | |||||
| Expired during the period |
— | — | ||||||
| |
|
|
|
|||||
| Balance as of December 31, 2022 |
1,589,081 |
14.69 |
||||||
| |
|
|
|
|||||
Number of RSU outstanding |
Weighted average grant date fair value in Euros |
|||||||
| Balance as of December 31, 2022 |
1,589,081 |
14.69 |
||||||
| Granted during the period |
589,550 | 1.69 | ||||||
| Forfeited during the period |
(191,659 | ) | 4.04 | |||||
| Released during the period |
(250,355 | ) | 6.77 | |||||
| Expired during the period |
— | — | ||||||
| |
|
|
|
|||||
| Balance as of December 31, 2023 |
2,095,517 |
10.73 |
||||||
| |
|
|
|
|||||
December 31, |
||||||||||||
2023 |
2022 |
|||||||||||
| Research and development |
SO | (1,661 | ) | (1,462 | ) | |||||||
| RSU | (835 | ) | (841 | ) | ||||||||
| Sales and marketing |
SO | (102 | ) | (31 | ) | |||||||
| RSU | (33 | ) | (4 | ) | ||||||||
| General and administrative |
SO | (2,985 | ) | (2,374 | ) | |||||||
| RSU | (403 | ) | (315 | ) | ||||||||
| |
|
|
|
|||||||||
| Total share-based compensation (expense) income |
(6,019 |
) |
(5,026 |
) | ||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
||||||||
2023 |
2022 |
|||||||
| Current contingencies |
3,959 | 3,944 | ||||||
| Non-current contingencies |
935 | 16,680 | ||||||
| |
|
|
|
|||||
| Total contingencies |
4,894 |
20,625 |
||||||
| |
|
|
|
|
|
|
|
|
Pension retirement obligations |
Collaboration agreement—Loss at completion |
Other contingencies |
Total |
|||||||||||||
| At January 1, 2022 |
1,008 |
9,800 |
45 |
10,853 |
||||||||||||
| Increases in liabilities |
105 |
12,455 |
— |
12,560 |
||||||||||||
| Used liabilities |
— |
— |
(42 |
) |
(42 |
) | ||||||||||
| Reversals of unused liabilities |
— |
(1,984 |
) |
— |
(1,984 |
) | ||||||||||
| Net interest related to employee benefits, an unwinding of discount |
— |
— |
— |
— |
||||||||||||
| Actuarial gains and losses on defined-benefit plans |
(262 |
) |
— |
— |
(262 |
) | ||||||||||
| Currency translation effect |
(61 |
) |
(436 |
) |
(3 |
) |
(500 |
) | ||||||||
| |
|
|
|
|
|
|
|
|||||||||
| At December 31, 2022 |
790 |
19,835 |
— |
20,625 |
||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Of which Current |
— |
3,944 |
— |
3,944 |
||||||||||||
| Of which Non-current
|
790 |
15,891 |
— |
16,680 |
||||||||||||
| At January 1, 2023 |
790 |
19,835 |
— |
20,625 |
||||||||||||
| Increases in liabilities |
76 |
— |
3,874 |
3,950 |
||||||||||||
| Used liabilities |
— |
— |
— |
— |
||||||||||||
| Reversals of unused liabilities |
— |
(20,108 |
) |
— |
(20,108 |
) | ||||||||||
| Net interest related to employee benefits, and unwinding of discount |
— |
— |
— |
— |
||||||||||||
| Actuarial gains and losses on defined-benefit plans |
38 |
— |
— |
38 |
||||||||||||
| Currency translation effect |
31 |
273 |
85 |
389 |
||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| At December 31, 2023 |
935 |
— |
3,959 |
4,893 |
||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Of which Current |
— |
— |
3,959 |
3,959 |
||||||||||||
| Of which Non-current
|
935 |
— |
— |
935 |
||||||||||||
• |
Loss on completion accrual reversal of $19,9 millions ; |
• |
Accrual for ongoing Clinical study completion of $2.3 millions. This accrual represents our best estimate of the remainder expenses related to the ongoing clinical study which will be incurred after December 31, 2023 and until the end of the study. |
December 31, |
||||||||
2023 |
2022 |
|||||||
| % Social security contributions |
50.0 | % | 50.0 | % | ||||
| Salary increases |
2.0 | % | 2.0 | % | ||||
| Discount rate—Iboxx Corporates AA 10+ |
3.17 | % | 3.77 | % | ||||
| Expected staff turnover |
10.0 | % | 10.0 | % | ||||
| Estimated retirement age |
67 | 65 | ||||||
| Life table |
TGH05-TGF05 |
|||||||
| Collective agreement |
National Collective Agreement of the pharmaceutical industry |
| ||||||
December 31, |
||||||||
2023 |
2022 |
|||||||
| Research tax credit |
8,766 | 5,718 | ||||||
| Other operating income . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
6,962 | (874 | ) | |||||
| |
|
|
|
|||||
| Total |
15,728 |
4,844 |
||||||
| |
|
|
|
|
|
|
|
|
December 31, |
|
|
||||||||||||||
(Dollar amounts presented in thousands) |
2023 |
2022 |
$ change |
% change |
||||||||||||
| Research and development expenses |
||||||||||||||||
| External clinical-related expenses |
49,044 |
42,248 |
6,796 |
16 |
% | |||||||||||
| Employee-related costs excl. share-based payment expenses |
14,401 |
10,752 |
3,649 |
34 |
% | |||||||||||
| Share-based payment expenses |
2,496 |
2,303 |
193 |
8 |
% | |||||||||||
| Depreciation and amortization |
(13,658 |
) |
12,965 |
(26,623 |
) |
(205 |
%) | |||||||||
| Other costs |
7,940 |
7,276 |
664 |
9 |
% | |||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Total Research and development expenses |
60,223 |
75,543 |
(15,320 |
) |
(20 |
%) | ||||||||||
| |
|
|
|
|
|
|
|
|||||||||
• |
loss on completion accrual net reversal $17,6 millions (compared to a $10.4 millions depreciation as of December 31, 2022) resulting from Nestlé Collaboration Agreement termination, that offset; |
• |
the global increase of $11.3 million in research and development expenses. |
December 31, |
||||||||||||||||
(Dollar amounts presented in thousands) |
2023 |
2022 |
$ change |
% change |
||||||||||||
| Sales and marketing expenses |
||||||||||||||||
| Employee-related costs incl. share-based payment expenses |
754 |
914 |
(160 |
) |
(18 |
%) | ||||||||||
| External professional services and other costs |
1,784 |
694 |
990 |
143 |
% | |||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Total Sales and marketing expenses |
2,438 |
1,608 |
830 |
52 |
% | |||||||||||
| |
|
|
|
|
|
|
|
|||||||||
December 31, |
||||||||||||||||
(Dollar amounts presented in thousands) |
2023 |
2022 |
$ change |
% change |
||||||||||||
| General and administrative expenses |
||||||||||||||||
| External professional services fees |
8,750 |
5,947 |
2,803 |
47 |
% | |||||||||||
| Employee-related costs excl. share-based payment expenses |
8,200 |
7,320 |
881 |
12 |
% | |||||||||||
| Share-based payment expenses |
3,389 |
2,688 |
701 |
26 |
% | |||||||||||
| Depreciation, amortization and other costs |
9,161 |
8,369 |
2,523 |
30 |
% | |||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Total General and administrative expenses |
29,500 |
24,324 |
5,176 |
21 |
% | |||||||||||
| |
|
|
|
|
|
|
|
|||||||||
December 31, |
||||||||
2023 |
2022 |
|||||||
| Research and Development expenses |
16,897 | 13,055 | ||||||
| Sales and Marketing expenses |
754 | 914 | ||||||
| General and Administrative expenses |
11,589 | 10,008 | ||||||
| |
|
|
|
|||||
| Total personnel expenses |
29,240 |
23,977 |
||||||
| |
|
|
|
|||||
December 31, |
||||||||
2023 |
2022 |
|||||||
| Wages and salaries |
18,108 | 14,802 | ||||||
| Social security contributions |
4,176 | 3,206 | ||||||
| Expenses for pension commitments |
935 | 943 | ||||||
| Share-based payments |
6,019 | 5,026 | ||||||
| |
|
|
|
|||||
| Total |
29,240 |
23,977 |
||||||
| |
|
|
|
|||||
December 31, |
||||||||
2023 |
2022 |
|||||||
| (Loss) before taxes |
(72,709 | ) | (96,204 | ) | ||||
| Theoretical company tax rate |
25.00 | % | 25.00 | % | ||||
| Nominal tax expense |
18,179 | 24,051 | ||||||
| Increase/decrease in tax expense arising from: |
||||||||
| Research tax credit |
2,192 | 1,430 | ||||||
| Share-based compensation |
(1,852 | ) | (784 | ) | ||||
| Other permanent differences |
(110 | ) | (100 | ) | ||||
| Non recognition of deferred tax assets mainly related to tax losses |
(18,802 | ) | (24,746 | ) | ||||
| Other differences |
386 | 79 | ||||||
| Effective tax expenses—current |
(7 | ) | (70 | ) | ||||
| Effective tax expenses—deferred |
— | — | ||||||
| Effective tax rate |
(0.01 | )% |
(0.07 | )% | ||||
December 31, |
||||||||
2023 |
2022 |
|||||||
| Deferred tax assets: |
||||||||
| Net operating loss carryforwards |
307,300 | 273,964 | ||||||
| Share-based compensation |
509 | 1,102 | ||||||
| Personnel-related accruals |
422 | 389 | ||||||
| Pension retirement obligations |
509 | 197 | ||||||
| Leases |
32 | 6 | ||||||
| Other |
1,205 | 5,248 | ||||||
| |
|
|
|
|||||
| Total deferred tax assets |
309,702 |
280,907 |
||||||
| |
|
|
|
|||||
| Less: Valuation allowance |
(309,702 | ) |
(280,907 | ) | ||||
| |
|
|
|
|||||
| Net deferred tax assets |
— |
— |
||||||
December 31, |
||||||||
2023 |
2022 |
|||||||
| Short-term benefits |
4,864 | 4,625 | ||||||
| Post-employment benefits |
29 | 33 | ||||||
| Termination benefits |
— | 24 | ||||||
| Share-based payments |
3,792 | 3,355 | ||||||
| |
|
|
|
|||||
| Total |
8,685 |
8,037 |
||||||
December 31, |
||||||||
202 3
|
2022 |
|||||||
| Compensation |
2,112 | 2,009 | ||||||
| Pension obligations |
107 | 83 | ||||||
| |
|
|
|
|||||
| Total |
2,219 |
2,092 |
||||||
December 31, |
||||||||
2023 |
2022 |
|||||||
| Net loss |
(72,726 | ) | (96,274 | ) | ||||
| Weighted average number of ordinary shares |
95,121,390 | 77,384,133 | ||||||
| |
|
|
|
|||||
| Basic and diluted net loss per share attributable to ordinary shareholders ($/share) |
(0.76 |
) |
(1.24 |
) | ||||
| |
|
|
|
|||||
December 31, |
||||||||
2023 |
2022 |
|||||||
| Non-employee warrants |
244,693 | 251,693 | ||||||
| Employee warrants |
— | — | ||||||
| Stock-options |
7,118,691 | 5,306,569 | ||||||
| Restricted stock units |
2,095,518 | 1,618,778 | ||||||
| Prefunded warrants |
28,276,331 | 28,276,331 | ||||||
Exhibit 3.1
BY-LAWS
(updated by decision of the CEO on November 24, 2023)
DBV Technologies
Limited Company with share capital of € 9,643,177
177-181 avenue Pierre Brossolette - 92120 Montrouge, France
Nanterre Trade and Companies Register No. 441 772 522
1
I. - CHARACTERISTIC FEATURES OF THE COMPANY
Article 1 - Form
The Company was incorporated in the form of a French Limited Company (Société Anonyme) with a Board of Directors.
Article 2 - Name
The name of the Company is: “DBV Technologies”
Article 3 - Registered office
The registered office is located at: 177-181 avenue Pierre Brossolette - 92120 Montrouge, France
Article 4 - Corporate Purpose
The Company’s corporate purpose in France and in all countries is:
| • | the development of any innovative medical products, including any drugs, or diagnostic or treatment products; |
| • | the study, research, development, industrial manufacturing, and marketing of said products; |
| • | the use and development of any patents or licenses relating to these products, and generally speaking any commercial, investment or real estate, financial or other transactions that are directly or indirectly related to the corporate purpose in whole or in part, or to any other similar or related purpose, and that may promote the operation and commercial development of the Company. |
Article 5 - Term
The Company’s term is ninety-nine years as from its registration in the Trade and Companies Register.
Article 6 - Share capital
The share capital has been set at € 9,643,177.
It is divided into 96,431,770 ordinary shares with a par value of 10-euro cents (€0.10) each. All of the shares have been fully subscribed, and their full amount paid up in cash.
Article 7 - Changes to the share capital
I. The share capital may be increased either via the issue of new shares, or by increasing the par value of the existing shares.
2
The new shares will be paid for in cash, or via a contribution in kind, offset against liquid and due receivables, or via the incorporation of profits, reserves, or share premiums into the share capital, either as the result of a merger or demerger, or following the exercise of a right attached to transferable securities granting entitlement to the share capital, including payment of the corresponding amounts, where applicable.
The new equity securities will be issued either at their par value, or at that amount plus a share premium.
Only the Extraordinary General Meeting of Shareholders has the power to decide on increasing the share capital, based on a report from the Board of Directors containing the disclosures required by law.
However, the Extraordinary General Meeting of Shareholders may delegate this power to the Board of Directors under the conditions determined by law. The Board of Directors has the requisite powers to perform a capital increase in one or several installments, to determine its terms and conditions, to record its completion, and to amend the By-Laws accordingly within the limits of the powers so granted by the Extraordinary General Meeting of Shareholders.
If the General Meeting of Shareholders decides to increase the share capital, it may delegate the powers required to perform the transaction to the Board of Directors.
If a delegation of power or of authority is used, the Board of Directors will draw up a supplementary report at the next Ordinary General Meeting of Shareholders.
If the capital increase is performed via the incorporation of profits, reserves, or share premiums, the Extraordinary General Meeting of Shareholders will take decisions under the quorum and majority conditions provided for Ordinary General Meetings of Shareholders. In this case, it may decide that rights amounting to fractional shares may neither be traded nor transferred, and that the corresponding equity securities must be sold. The proceeds from the sale will be allocated to the holders in proportion to their rights.
A capital increase by increasing the par value of the shares can only be decided with the shareholders’ unanimous consent, except if it results from the incorporation of profits, reserves, or share premiums into the share capital.
Shareholders will have a preferential right to subscribe to the cash shares issued in order to perform a capital increase, in proportion to the number of shares that they hold. The shares purchased as a result of exercising this right will be shares in the same class as the one for the shares giving rise to said right, together with the shares resulting from the purchase of other transferable securities than shares.
The shareholders may sell all or some of their subscription rights throughout the subscription period. These rights will be tradable if they are stripped from shares that are themselves tradable. Otherwise, they may be sold under the same conditions as the actual shares.
Shareholders may waive their preferential subscription right on an individual basis.
The Extraordinary General Meeting of Shareholders that decides on the capital increase may waive the preferential subscription right under the conditions and limits determined by law, and rule to that effect on the reports prepared by the Board of Directors and the Statutory Auditors under the conditions determined by the laws and regulations in effect.
If the Extraordinary General Meeting of Shareholders, or the Board of Directors in the event of a delegation of authority, has expressly decided to do so, any shares that have not been subscribed on an irrevocable basis will be allotted to shareholders who subscribed to a higher number of shares on a revocable basis than the number to which they were able to subscribe on a preferential basis, in proportion to the subscription rights that they hold, and within the limits of their request, in any event.
If, for any reason, subscriptions have not absorbed the full amount of the capital increase, the Board of Directors may use the options provided for below, or only some of them, in the order that it determines:
3
| (i) | limiting the capital increase to the amount of the subscriptions, subject to the general condition that it amounts to at least three quarters of the increase decided upon, and that this option was not expressly excluded by the Extraordinary General Meeting of Shareholders at the time of issue; |
| (ii) | allocating the balance of the shares if the Extraordinary General Meeting of Shareholders has not decided otherwise; |
| (iii) | opening the subscription process to the public if the Extraordinary General Meeting of Shareholders has expressly authorized it. |
If subscriptions have not absorbed the entire capital increase following the exercise of these options, or three-quarters of the increase in the case provided for under (i) above, the capital increase will not be performed.
However, the Board of Directors may automatically limit the capital increase to the amount raised in all cases where the unsubscribed shares account for less than 3% of the capital increase.
In the event of a capital increase with or without preferential subscription rights, the Extraordinary General Meeting of Shareholders may provide that the number of securities may be increased by up to 15% of the initial issue, at the same price as the one used for the initial issue within a period of thirty days following the close of the subscription period.
If the capital increase creates fractions of shares, shareholders who have an insufficient number of subscription or allotment rights must make arrangements to purchase or sell the rights required to obtain the delivery of a whole number of new shares.
II. The Extraordinary General Meeting of Shareholders (or the Board of Directors in the event of a delegation of authority) may also authorize or decide on a capital decrease, subject to the rights of creditors, where applicable.
Decreasing the share capital below the legal limit can only be decided under the condition precedent of a capital increase intended to return the share capital to an amount that is at least equal to the minimum legal threshold, unless the Company turns itself into a company with another legal form. Otherwise, any interested party may apply to the courts to have the Company wound up. The court may not order the Company to be wound up if the amount of the share capital has been restored to the statutory minimum by the day when it rules on the substance of the case.
Article 8 - Financial year
The financial year runs from January 1 to December 31.
II. - ADMINISTRATION OF THE COMPANY
Article 9 - Executive Management exercise method
The executive management of the Company is the responsibility either of the Chairman of the Board of Directors or of another individual appointed by the Board of Directors bearing the title of Chief Executive Officer.
The Board of Directors chooses between the two Executive Management exercise methods based on the unanimous vote of all of its members.
Where responsibility for the Company’s Executive Management is held by the Chairman of the Board of Directors, the following provisions concerning the role of Chief Executive Officer apply.
4
A. The Board of Directors
Article 10 - Composition of the Board of Directors
The Company is governed by a Board of Directors that consists of between 3 and 18 directors.
The Directors are appointed by the General Meeting of Shareholders, deliberating under the quorum and majority conditions for Ordinary General Meetings of Shareholders.
The term of office for the Directors appointed during the term of the company is three (3) years. This term expires at the end of the meeting convened to approve the financial statements for the year just ended, and which is held in the year during which their term of office expires.
By way of exception and in order to allow exclusively for the implementation or maintenance of the staggered terms of office of Directors, the ordinary General Meeting of Shareholders may appoint one or more members of the Board for a term of two (2) years or one (1) year.
The Directors may be dismissed at any time and without any good reason by the General Meeting of Shareholders, deliberating under the quorum and majority conditions for Ordinary General Meetings of Shareholders.
The number of Directors aged over eighty cannot exceed one third of the Board members.
Article 11 - Board Discussions
The Board of Directors meets as often as is required by the Company’s interests at the invitation of the Chairman of the Board of Directors, at the registered office or the place specified in the notice of meeting. The invitation may be issued by any means five days in advance: it may also be issued orally and immediately if all of the Directors and non-voting Board members agree.
The Board of Directors may also make decisions by written consultation of the directors under the conditions provided by law.
The Board of Directors may also, at the discretion of its Chairman, make the following decisions by written consultation:
| • | cooptation following (i) a death, (ii) a resignation, (iii) when the number of directors has fallen below the statutory minimum, or (iv) when the gender balance is no longer respected; |
| • | authorization of sureties, endorsements and guarantees given by the Company; |
| • | transfer of the registered office in the same department; |
| • | amendment of the articles of association to bring them into line with the conditions laid down by law; |
| • | convening of the General Meeting. |
In the event of a written consultation, the Chairman sends to each director, alternatively (i) by registered letter with acknowledgement of receipt, (ii) by e-mail with acknowledgement of receipt, the text of the proposed decisions as well as all documents useful for his information.
The directors have a period of five calendar days (ending at 11:59 p.m., Paris time, on the last day of this period) from the date of dispatch of the draft decisions to express their vote in writing. The reply is sent alternatively (i) by registered letter with acknowledgement of receipt, (ii) by e-mail with acknowledgement of receipt, to the attention of the Chairman of the Board of Directors, at the Company’s registered office, if any.
The Board of Directors may only validly deliberate on a written consultation if at least half of its members have replied within the time limit indicated above.
Decisions are taken by a majority of the votes of the members who have replied, each member having one vote.
5
If it has not met for over two months, at least one quarter of the members of the Board of Directors may ask the Chairman to convene the Board based on a determined agenda. The Chief Executive Officer or a Director may also ask the Chairman to convene the Board of Directors based on a determined agenda. The Chairman will be bound by any such requests.
An attendance register will be kept, and minutes will be drawn up following each meeting. The Board may only validly take decisions if at least half of its members are present.
Except where the choice of the method for exercising Executive Management is concerned, decisions will be taken based on a majority vote of the Directors present or represented. The Chairman will have a casting vote in the event that the vote is split.
The Directors and any individuals asked to attend the Board of Directors’ meetings are required to exercise discretion with respect to information of a confidential nature, and which is provided as such by the Chairman of the Board of Directors.
Article 12 - The Board’s powers
The Board of Directors determines the Company’s guidelines, and ensures their implementation. Subject to the powers specifically assigned to General Meetings of Shareholders, and within the limits of the corporate purpose, the Board will deal with any matter involving the proper operation of the Company, and settle any matters concerning it through its discussions.
The Board of Directors carries out the controls and verifications that it considers appropriate. Every Director will receive all of the information required to fulfill their assignment, and may ask for the disclosure of any documents that they consider useful.
Article 13 - The Chairman of the Board of Directors
The Board of Directors elects a Chairman, who must be a private individual, from among its members, and determines their remuneration, in accordance with applicable law. The Chairman is appointed for a period that may not exceed the length of their term of office as a Director. They are eligible for reelection. The Board of Directors may dismiss the Chairman at any time. Any provisions to the contrary will be considered void.
No one aged 75 or over may be appointed as Chairman. If the incumbent Chairman reaches this age during a financial year, their duties will automatically end following the Ordinary General Meeting of Shareholders convened to approve the financial statements for that financial year.
The Chairman organizes and directs the work undertaken by the Board, and accounts for it at the General Meeting of Shareholders. They ensure that the Company’s bodies operate properly, and especially that the Directors are in a position to fulfill their assignment.
Article 14 - Non-Voting Board Members
The General Meeting of Shareholders may appoint one or two non-voting Board members for the Company who are private individuals, regardless of whether they are shareholders; they will be aged 65 at most on the day of their appointment.
Non-voting Board members are appointed for a period of two (2) years. Their assignment ends after the General Meeting of Shareholders that has approved the financial statements for the year just ended, and held in the year during which their term of office expires.
Non-voting Board members do not receive any remuneration. They may receive allowances determined by the Board of Directors in order to reimburse the expenses that they are required to incur as part of the normal performance of their duties. If the Board delegates a specific assignment to the non-voting Board members or to one of them, they may allocate them an allowance in proportion to the importance of the assignment entrusted to them, as well as a budget for performing said assignment. Non-voting Board members are invited to all of the Board of Directors’ meetings and to all of the General Meeting of Shareholders, and take part in the discussions in an advisory capacity. Non-voting Board members perform a general and permanent advisory and supervisory role at the Company. However, they may not interfere in the management of the Company under any circumstances, or, in general, replace its legal bodies.
6
B. The Executive Management
Article 15 - Chief Executive Officers and Deputy Chief Executive Officers
The executive management of the Company is the responsibility of a private individual appointed by the Board of Directors bearing the title of Chief Executive Officer, under the Company’s responsibility.
The Board of Directors may appoint one or more private individuals responsible for assisting the Chief Executive Officer, who will bear the title of Deputy Chief Executive Officer, on the recommendation of the Chief Executive Officer. The number of Deputy Chief Executive Officers cannot exceed five.
The Chief Executive Officer may be dismissed by the Board of Directors at any time. The same applies to the Deputy Chief Executive Officers, on the recommendation of the Chief Executive Officer. If the dismissal is not on justified grounds, it may result in the payment of damages and interest.
Where the Chief Executive Officer ceases, or is otherwise prevented from performing their duties, the Deputy Chief Executive Officers will retain their positions and their assignments until a new Chief Executive Officer is appointed, unless the Board decides otherwise.
The Board of Directors determines the compensation paid to the Chief Executive Officer and the Deputy Chief Executive Officers, in accordance with applicable law.
Article 16 - Powers of the Chief Executive Officer and Deputy Chief Executive Officers
The Chief Executive Officer is granted very extensive powers to act in the Company’s name in all circumstances. They exercise the powers within the limit of the corporate purpose, and subject to those that the law and these By-Laws expressly assign to General Meeting of Shareholders and to the Board of Directors.
They represent the Company in its dealings with third parties. The Company will be committed even by the Chief Executive Officer’s actions that do not relate to the corporate purpose, unless it proves that the third party was aware that the action exceeded that purpose, or could not ignore this fact in view of the circumstances. The sole publication of the By-Laws does not amount to sufficient proof.
The Board of Directors determines the scope and term of the powers granted to the Deputy Chief Executive Officers, with the Chief Executive Officer’s consent. The Deputy Chief Executive Officers have the same powers as the Chief Executive Officer where third parties are concerned.
III. - GENERAL MEETING OF SHAREHOLDERS
Article 17 - General Meeting of Shareholders
The duly constituted General Meeting of Shareholders represents the entire body of shareholders.
Its decisions, which are taken in accordance with the law and the By-Laws, are binding on all of the shareholders, even if they are absent, disagree, or are incapable.
There are three forms of meetings, depending on the purpose of the resolutions put forward:
| • | Ordinary General Meetings; |
7
| • | Extraordinary General Meetings; |
| • | Special Meetings that bring together the holders of shares in a given class. |
Article 18 - Invitations
The Meetings are convened by the Board of Directors. They may also be convened by the Statutory Auditor or by a court representative, under the conditions and in accordance with the procedures provided for by law.
Meetings are convened by the liquidator(s) during the liquidation period.
The Meetings are held at the registered office or at any other location specified in the notice of meeting.
A notice of meeting is published in the Bulletin des Annonces Légales Obligatoires (French Official Gazette, or BALO) at least thirty-five days before a Meeting is held. In addition to the information relating to the Company, the notice specifies the agenda for the Meeting, and the wording of the draft resolutions that will be put forward. Requests to enter points or draft resolutions on the agenda must be addressed to the Company under the conditions provided for by the regulations in effect.
The Meetings are held at the registered office or at any other location specified in the notice of meeting.
Subject to specific legal provisions, the invitation is issued at least fifteen days before the date of the Meeting by a notice inserted in a legal gazette published in the Department where the registered office is located, as well as in the BALO.
The holders of registered shares must be convened under the conditions provided for by the regulations in force.
The notice of meeting must also specify the conditions under which shareholders may vote by post, and the places where, and terms and conditions according to which, they may obtain postal vote forms.
The notice of meeting may be sent, where applicable, with a proxy form and a postal voting form, under the conditions specified in Article 21 of these Articles of Association, or with a postal voting form only, under the conditions specified in Article 21 of these Articles of Association.
Where a Meeting has been unable to take decisions as a result of failing to achieve the quorum required, a second Meeting will be convened, subject to specific legal provisions, at least ten days in advance, in the forms provided for by the regulations in effect.
Article 19 - Agenda
The agenda for Meetings will be prepared by the person convening the meeting.
One or several shareholders, who represent at least the percentage of the share capital specified by law, and acting in accordance with the legal conditions and timeframes, have the option to request the inclusion of points or draft resolutions on the agenda for the Meeting, via registered letter with a request for acknowledgment of receipt.
The Meeting may not discuss an issue that has not been entered on the agenda, which cannot be altered at the time of the second invitation. However, it may dismiss one or several members of the Board of Directors, and replace them in all circumstances.
Article 20 - Participation of Shareholders in Meetings
Any shareholder may participate, personally or by proxy, in the meetings upon proof of identity and ownership of his or her shares, in accordance with the procedures provided for by the laws and regulations in force.
8
Article 21 - Postal and proxy voting
Postal voting is carried out in accordance with the terms and conditions laid down by the legal and regulatory provisions. In particular, any shareholder may send postal voting forms either in paper form or, if the Board of Directors decides to do so and publishes the decision in the notice of meeting, by electronic means, before the meetings. Proxy forms may be sent either in paper form or by electronic means before the meetings.
If the Board of Directors decides at the time of convening the meeting to allow the transmission of voting or proxy forms by electronic means, the electronic signature of these forms may result from a reliable process for identifying the shareholder, guaranteeing its link with the remote form to which its signature is attached. The vote thus expressed before the meeting by this electronic means, as well as the acknowledgement of receipt given, will be considered as non-revocable writings and opposable to all. The proxy is however revocable in the same way as those required for the appointment of the proxy. In the event of a transfer of ownership of securities occurring before midnight (Paris time) on the second business day preceding the meeting, the Company will invalidate or modify accordingly, as the case may be, the proxy or the vote cast before the meeting by this electronic means.
Article 22 - Attendance sheet
An attendance sheet containing the information specified by law will be kept at each Meeting.
This attendance sheet, duly initialed by the shareholders present and the proxies, and the shareholders attending via video-conference or another means of telecommunication, in accordance with the legal and regulatory requirements, and to which the powers granted to each representative are appended, together with the postal voting forms, will be certified as accurate by the Meeting Bureau.
The Meetings will be chaired by the Chairman of the Board of Directors. Otherwise, the Meeting will elect its own Chairman.
The tellers’ duties will be performed by two shareholders who are present and agree to do so, and who represent the highest number of votes, both on their own behalf and as proxies.
The Bureau formed in this way will appoint a secretary, who may be chosen from outside the shareholders.
Article 23 - Voting rights attached to shares
The voting right attached to the shares is proportional to the percentage of the total share capital that they represent. Each equity share or dividend share will grant entitlement to one vote. Fully paid-up shares for which proof can be provided that they have been registered in the name of the same shareholder for at least two years do not benefit from double voting rights.
Article 24 - Minutes
The decisions taken at the Meetings will be recorded in minutes that are drawn up in a special ledger held at the registered office, and signed by the members of the Bureau.
Copies or excerpts of the minutes of the decisions will be certified either by the Chairman of the Board of Directors or by the Meeting Secretary. They will be validly certified by the liquidator(s) in the event of liquidation proceedings.
Article 25 - Disclosure of documents
Any shareholder has the right to obtain disclosure of, and the Board of Directors is required to send or make available to them, the documents required to enable them to form an opinion in full knowledge of the facts, and to make an informed judgment on the Company’s management and operations.
The nature of these documents, and the conditions for sending them or making them available to the shareholders are determined by the regulations in effect.
9
Every shareholder or their representative may seek the assistance of an expert registered on one of the lists drawn up by the courts, in order to exercise their right of disclosure.
The exercise of the right of disclosure entails the right to take copies, except where records are concerned.
Article 26 - Ordinary General Meeting of Shareholders
The Ordinary General Meeting of Shareholders takes all of the decisions that exceed the powers of the Board of Directors and which do not fall within the remit of the Extraordinary General Meeting of Shareholders.
The Meeting is convened at least once a year, within a period of six months following the end of each financial year, in order to approve the financial statements for that year, subject to this period being extended by an order from the Presiding Judge of the Commercial Court ruling at the request of the Board of Directors.
The Meeting is convened on an extraordinary basis every time that this appears to be in the Company’s interests.
When convened for the first time, the Ordinary General Meeting of Shareholders may only validly deliberate if the shareholders present, represented, or who have voted by post hold at least one fifth of the shares to which voting rights are attached.
No quorum is required if the meeting is convened for a second time and the original agenda has not been amended.
The Ordinary General Meeting of Shareholders decides by a majority of the votes expressed by the shareholders present, represented or voting by mail. The expressed votes do not include those attached to shares for which the shareholder has not taken part in the vote, has abstained or has voted blank or null.
Article 27 - Extraordinary General Meeting of Shareholders
Only the Extraordinary General Meeting of Shareholders is authorized to amend all of the provisions of the By-Laws, and to specifically decide on turning the Company into a company with another legal form. It cannot, however increase the shareholders’ undertakings, except in the case of transactions resulting from a duly executed reverse share split.
The Extraordinary General Meeting of Shareholders may only validly deliberate if the shareholders present, represented or who have voted by post hold at least one quarter of the shares with voting rights at the time of the first invitation, and one fifth of the shares with voting rights at the time of the second invitation. If the second quorum is not achieved, the second Meeting may be postponed to a date no later than two months after the date on which it was convened.
The Meeting passes resolutions based on a two-thirds majority vote expressed by the shareholders who are present, represented, or have voted by post, or who are attending the Meeting via video-conference or another means of telecommunication, in accordance with the legal and regulatory provisions.
As a legal exemption to the above provisions, a General Meeting of Shareholders that decides on a capital increase via the capitalization of reserves, profits, or share premiums may pass resolutions under the same quorum and majority conditions as an Ordinary General Meeting of Shareholders.
Furthermore, where the Extraordinary General Meeting of Shareholders is required to discuss the approval of a contribution in kind or the granting of a particular benefit, the shares held by the individual making the contribution or the beneficial owner will not be taken into account to calculate the majority. The individual making the contribution or the beneficial owner will not have a vote, either on their own behalf, or as a proxy.
10
Article 28 - Special Meeting
If there are several share classes, no change may be made to the rights attached to shares in one of these classes without a due vote at an Extraordinary General Meeting of Shareholders open to all shareholders and, furthermore, without an equally compliant vote at a Special Meeting open only to the holders of shares in the class in question.
Special Meetings may only validly discuss matters if the shareholders present, represented, who have voted by post, or who are attending the Meeting via video-conference or via another means of telecommunication in accordance with the legal and regulatory provisions, hold at least one third of the shares with voting rights, where an amendment to those rights is planned, on the first invitation, and one fifth of the shares on the second invitation. Otherwise, the second Special Meeting may be postponed to a date no later than two months after the date on which it was convened.
Special Meetings pass resolutions based on a two-thirds majority of the expressed votes of the shareholders present or represented.
IV. - THE COMPANY’S SECURITIES
Article 29 - Payment for the shares
At least 25% of the par value of shares subscribed in cash must be paid at the time of subscription, together with the full share premium, where applicable.
The balance must be paid in one or several installments, as called by the Board of Directors, and within a period of five years from the date on which the capital increase was finalized.
Calls for funds are made known to the shareholders via a notice published in the BALO fifteen (15) days in advance.
If the shareholder does not make the required payments on the amount of the shares to which they have subscribed at the times determined by the Board of Directors, these payments will automatically bear interest payable to the Company at the legal rate determined in Article L. 313-2 of the French Monetary and Financial Code, as from the end of the month following the date when they are due, without any requirement for a court application or letter of notice. Furthermore, shares for which the required payments have not been made at the end of a period of 30 days as from the sending of a letter of notice to the defaulting shareholder, to which no reply has been received, will no longer grant the right to attend General Meetings of Shareholders and to vote at those Meetings, and will be deducted from the quorum calculation. The right to dividends, and the preferential right to subscribe to capital increases attached to the shares will be suspended. These rights will be recovered once the capital and interest amounts due have been paid. The shareholder may then request the payment of dividends that have not expired, and exercise their preferential subscription right, if the determined timeframe for exercising that right has not expired.
The share capital must be fully paid up before any issue of new shares to be paid for in cash.
Article 30 - Form of the shares - Management of the securities accounts
The shares may be in registered or bearer form, if the legislation allows, depending on the shareholder’s choice.
Issued shares give rise to a registration in individual accounts in the name of each shareholder opened by the Company or any authorized intermediary. These accounts are held under the conditions and in accordance with the procedures provided for by the legal and regulatory provisions.
In order to identify the owners of bearer shares, the company may, under the conditions provided for by the legal and regulatory provisions in force, request, at any time, information concerning the owners of its shares and securities conferring immediate or future voting rights at its own General Meetings of Shareholders.
11
Article 31 - Transfer of the shares
Shares registered on an account are transferred from account to account.
Cash shares are freely tradable as from the completion of the capital increase. Shares resulting from contributions are freely tradable as from the completion of the capital increase, i.e. the date of the Meeting or of the meeting of the Board of Directors acting on a delegation of authority, which approved the contributions, in the event of a contribution in kind during the term of the company.
The transfer of ownership will result from their registration on the purchaser’s account, on the date and under the conditions determined by law and the applicable regulations, where applicable.
The shares will be freely tradable, subject to the provisions provided for by law.
Article 32 - Crossing of thresholds
Any private individual or legal entity referred to in Articles L. 233-7, L. 233-9, and L. 223-10 of the French Commercial Code who comes to directly or indirectly hold a number of shares representing a percentage of the Company’s share capital or voting rights higher than or equal to 2.5% or a multiple of that percentage, either on a stand-alone basis or in concert, must inform the Company of the total number of shares, voting rights, and securities granting access to the share capital or to voting rights immediately or in the future that they hold, via registered letter with a request for an acknowledgment of receipt sent to the registered office within a period of four trading days, prior to the market close as from the point when they crossed said percentage threshold(s).
The disclosure obligation provided for above also applies under the same conditions when each threshold mentioned above is crossed downwards.
If they have not been reported under the conditions specified above, shares or voting rights that exceed the percentage that should have been reported will be stripped of their voting rights at General Meetings of Shareholders at any Meeting that may be held until the expiry of a two-year period following the date when the notice of interest was made compliant, in accordance with Article L. 233-14 of the French Commercial Code, if a failure to report has been observed, and if one or several shareholders holding an interest of at least 2.5% have made a request recorded in the minutes of the General Meeting of Shareholders.
The above reports will apply notwithstanding the reports on the crossing of thresholds provided for by the legal or regulatory provisions in effect.
Article 33 - Rights and obligations attached to the shares
Each share entitles the holder to a share in the Company’s profits and assets, in proportion to the amount of capital that it represents.
Furthermore, each share entitles the holder to vote and be represented at General Meetings of Shareholders under legal and statutory provisions.
Shareholders will only be liable up to the amount of the par value of the shares that they hold; any calls for funds above that amount are prohibited.
Ownership of a share automatically entails adherence to the Company’s By-Laws and to the decisions of the General Meeting of Shareholders.
Heirs, creditors, assigns, or other representatives of a shareholder will not be entitled to request seizure of the Company’s assets or securities, or ask for them to be shared out or sold at auction, nor interfere in administrative acts relating to the Company in order to exercise their rights; they must refer to the company records and to the resolutions of the General Meeting of Shareholders.
12
Whenever it is necessary to hold several shares in order to exercise a given right, such as in the case of an exchange, reverse share split or allotment of shares, or an increase or decrease in the share capital, or a merger or other corporate transaction, the holders of single shares, or of a lower number of shares than required, may only exercise these rights if they personally arrange for the consolidation, and potentially the purchase or sale of the shares required.
However, in the event of the exchange of securities following a merger or demerger transaction, a capital decrease, a reverse share split or share split, and the mandatory conversion of bearer shares to registered shares, or of the distribution of securities charged to the reserves relating to a capital decrease, or the distribution or allotment of bonus shares, based solely on a decision by the Board of Directors, the Company may sell securities that the beneficiaries have requested to be delivered to them, as long as it has carried out the publication formalities provided for in the regulations at least two years beforehand.
As from the sale, the old securities or the old rights to distributions or allotments will be canceled, as and when required, and their holders will only be able to claim the cash allocation of the net proceeds of the sale of the unclaimed securities.
Article 34 - Beneficial & Bare ownership
The shares are indivisible as regards the Company.
Joint owners of shares are required to have themselves represented to the Company by just one of them, who will be considered as the sole owner, or by a single proxy; in the event of disagreement, the single proxy may be appointed by a court at the request of the first joint owner to do so.
Unless the Company has been notified of an agreement to the contrary, the beneficial owners of shares will validly represent the bare owners with the Company. Voting rights will be held by the beneficial owner at Ordinary General Meetings of Shareholders and by the bare owner at Extraordinary General Meetings of Shareholders.
Unless otherwise agreed between the parties, the preferential subscription right attached to securities belongs to the bare owner where the shares are encumbered by a usufruct interest.
V. - COMPANY FINANCIAL STATEMENTS
Article 35. - Preparation and approval of the company financial statements
| a) | The Board of Directors will draw up an inventory and the annual financial statements at the end of each financial year, and will then prepare the management report. |
Where applicable, the Board of Directors will prepare and publish the consolidated financial statements, together with the report regarding the management of the Group.
| b) | The Ordinary General Meeting of Shareholders will approve the annual company financial statements within a period of six months following the financial year-end, after familiarizing itself with the management report and the report prepared by the Statutory Auditors; the consolidated financial statements and the report regarding the management of the Group will be presented at that Meeting, if required. |
All information measures will be taken in compliance with the law and the regulations.
Article 36 - Audit of the financial statements
The financial statements will be audited by one or several incumbent, and, where applicable, alternate Statutory Auditors, under the conditions determined by Articles L. 225-218 of the French Commercial Code.
13
Article 37 - Allocation of the amounts available for distribution
Following the approval of the financial statements, and the recording of the existence of amounts available for distribution, the Ordinary General Meeting of Shareholders will determine the share of these amounts allotted to the shareholders in the form of a dividend; this dividend will be charged to the distributable profit for the year as a priority.
The procedures for paying the dividends or interim dividends are determined by the General Meeting of Shareholders.
Write-down differences are not available for distribution.
If required, the Meeting will allocate the non-distributed portion of the profit for the financial year available for distribution in the proportions that it determines, either to one or several reserves, which may be general or special, which remain at its disposal, or to the “retained earnings” account.
Any losses will be carried forward, unless the Meeting decides to offset them against existing reserves.
VI. - LIQUIDATION OF THE COMPANY
Article 38 - Liquidation
Once it has been wound up, the Company will be liquidated under the conditions determined by the French Commercial Code.
Unless the Ordinary General Meeting of Shareholders decides otherwise, the liquidator or liquidators will pursue any ongoing business until it is completed.
The net proceeds of the liquidation, following the settlement of the liabilities and payroll expenses, and repayment to the shareholders of the non-amortized par value of their shares, will be divided between the shareholders, taking the rights of the different share categories into account, where applicable.
Vll - MISCELLANEOUS ITEMS
Article 39 - Powers
All powers will be granted to the bearers of original copies of these By-Laws, or of copies or excerpts certified as original, in order to carry out all formalities.
14
Exhibit 10.2
COMMERCIAL LEASE
BETWEEN:
SCI DANTON MALAKOFF, a French non-trading real estate company with share capital of 1,000 euros, registered in the Paris Trade and Companies Register under number 838 690 071, whose registered office is located at 30 avenue Kleber 75116 PARIS,
Represented by Céline Leonardi, Marketing Director, duly empowered for the purposes hereof,
Hereinafter referred to as the LESSOR,
party of the first part,
AND,
DBV TECHNOLOGIES, a French public limited company with share capital of 9,625,355.30 euros, registered in the Nanterre Trade and Companies Register under number 441 772 522, with its registered office at 177-181 avenue Pierre Brossolette in MONTROUGE (92120),
Represented by Caroline Danière acting in her capacity as Director of Human Resources and Chief of Staff, duly empowered for the purposes hereof,
Hereinafter referred to as the LESSEE,
party of the second part,
The LESSOR and the LESSEE being hereinafter referred to collectively as the Parties and individually as a Party.
TABLE OF CONTENTS
| PRELIMINARY STATEMENT |
| I - GENERAL CONTRACTUAL PROVISIONS |
| ARTICLE I - PURPOSE |
| ARTICLE II - DESIGNATION |
| ARTICLE III - PURPOSE OF THE LEASED PREMISES |
| ARTICLE IV - DURATION |
| ARTICLE V - TERMS AND CONDITIONS AND USE |
| V.1 - ENTERING THE LEASED PREMISES |
| V.2 - LESSOR’S RIGHT OF ACCESS |
| V.3 - MAINTENANCE - REPAIRS |
| V.4 - FURNISHINGS - OPERATION |
| V.5 - WORKS |
| V.6 - LESSOR’S RIGHT OF ACCESSION / RESTORATION TO ORIGINAL CONDITION |
| V.7 - ASBESTOS TECHNICAL FILE (DTA) |
| V.8 1- STATEMENT OF RISKS |
| V.8 2 - ENERGY PERFORMANCE |
| V.8 3 - ENVIRONMENTAL PERFORMANCE |
| V.8 4 - “TERTIARY DECREE” |
| V.9 - CONTRIBUTIONS - TAXES |
| V.10 - LIABILITY - RECOURSE - INSURANCE |
| V.10.1 Liability and recourse |
| V.10.2 Insurance |
| V.10.2.1 Insurance taken out by the LESSOR |
| V.10.2.2 Insurance taken out by the LESSEE |
| V.10.3 Reciprocal waivers of recourse |
| V.11 - ASSIGNMENT |
| V.12 - SUBLEASING - LEASE MANAGEMENT |
| V.13 - CUSTODY OF LEASED PREMISES |
| V.14 - DESTRUCTION OF LEASED PREMISES |
| V.15 - EXPENSES |
| V.16 - SAFETY |
| V.17 - LIEN |
| V.18 - EXPROPRIATION |
| V.19 - INFORMATION |
| V.20 - RECEIVERSHIP / SUPERVISED LIQUIDATION |
| V.21 - ASSIGNMENT OF CLAIMS |
| V.22 - TRANSFER OF RIGHTS |
| V.23 - RETURN OF PREMISES |
| V.24 - OCCUPANCY INDEMNITY |
| ARTICLE VI - FINANCIAL CONDITIONS |
| VI.1 - RENT |
| VI.2 - PAYMENT TERMS |
| VI.3 - RENT INDEXATION |
| VI.4 - SECURITY DEPOSIT - BANK GUARANTEE |
| VI.5 - RENEWAL RENT |
| VI.5.1. Rental value |
| VI.5.2. Determination of rental value |
| VI.5.3 Right of option |
| ARTICLE VII - TERMINATION - INTEREST |
| ARTICLE VIII - JURISDICTION |
| ARTICLE IX - ADDRESS FOR SERVICE |
| ARTICLE X - FEES |
| ARTICLE XI - DISPOSALS |
| II - SPECIFIC CONTRACTUAL PROVISIONS |
| ARTICLE XII - DESIGNATION OF LEASED PROPERTY |
| ARTICLE XIII - PURPOSE |
| ARTICLE XIV - DURATION |
| ARTICLE XV - REFERENCE DATE |
| ARTICLE XVI - ANNUAL BASE RENT |
| ARTICLE XVII - REFERENCE INDEX |
| ARTICLE XVIII - SECURITY DEPOSIT |
| ARTICLE XIX - CLAIMS DECLARATION |
| ARTICLE XX - INTER-COMPANY RESTAURANT |
| ARTICLE XXI - LESSEE’S FITTING-OUT WORK |
| ARTICLE XXII - EARLY AVAILABILITY OF LEASED PREMISES |
| ARTICLE XXIII - PREFERENTIAL RIGHT |
| ARTICLE XXIV - GOVERNING LANGUAGE |
PRELIMINARY STATEMENT
SCI DANTON MALAKOFF owns an office building (hereinafter referred to as the “Building”) with a floor area of 25,559 m2 TUFA, located in CHÂTILLON (Hauts-de-Seine) 82, 84, 86, 88 and 90, rue Pierre Semard, 35, 37 and 39, rue Etienne Deforges and 107, 109, 111, 113 and 115, avenue de la République, acquired before building work was completed from SNC CHÂTILLON IRO on December 12, 2018.
The Building was completed on September 15, 2020.
DBV TECHNOLOGIES has expressed an interest in taking a lease on the 2nd floor of the Building on Lot A, representing a surface area of 2,446.7 m2, TUFA (share of common areas and inter-company restaurant included).
The LESSEE declares that it has visited the Leased Premises (as this term is defined hereinafter) and consulted the documents listed in the Appendix (Appendix 0) as well as the various appendices to the Lease in order to enable it to assess the situation and the extent of the Leased Premises.
The LESSEE declares that it is satisfied with the condition of the Leased Premises.
As the LESSEE was looking for office space corresponding to the characteristics of the Leased Premises, the Parties approached each other and together defined the conditions under which the LESSEE would lease part of the premises in the Building.
The Parties, having discussed the matter, have decided to sign this lease (the “Lease”) on the terms and conditions set out below.
The Parties declare that all the clauses of this Lease have been negotiated between them taking into consideration the mutual obligations subscribed to in the Lease as a whole.
The Parties have taken care to avoid any significant imbalance as mentioned in Article 1171 of the French Civil Code.
This preliminary statement forms an integral part of the Lease.
IT HAS BEEN AGREED AS FOLLOWS:
I - GENERAL CONTRACTUAL PROVISIONS
ARTICLE I - PURPOSE
The LESSOR hereby leases to the LESSEE who accepts the rights and property hereinafter designated in accordance with the provisions of Articles L 145-1 et seq. and Articles R145-1 et seq. of the French Commercial Code, the uncodified provisions of Decree 53-960 of September 30, 1953, and the conditions hereinafter.
ARTICLE II - DESIGNATION
This Lease covers a set of premises more fully described in Article XII, as well as the rights pertaining thereto (the “Leased Premises”).
The said premises are leased as they stand, without any exception or reservation, without the need for any further description, the LESSEE declaring that it is well aware of what the said property consists of, having visited it before today.
Any difference between the surface areas quoted and the actual dimensions of the aforementioned premises will not justify any reduction or increase in rent, the Parties will refer to the nature of the premises as they stand.
ARTICLE Ill - USE OF LEASED PREMISES
The LESSEE shall occupy the Leased Premises peacefully, in accordance with Articles 1728 and 1729 of the French Civil Code, and shall carry on exclusively and continuously the activity defined in Article XII of the special provisions.
The LESSEE expressly undertakes to use the Leased Premises in accordance with their intended purpose, it being specified that any other use or assignment is expressly prohibited.
The LESSEE declares that it is personally responsible for obtaining all the authorizations required for the exercise of its activity, as prescribed by current or future legislation.
To this end, where necessary, it is expressly stipulated that the LESSEE shall transfer to the LESSOR, if the latter sees fit, the benefit of all administrative authorizations to which the Building has been or will be subject and which will have been applied for and obtained by the LESSEE.
The LESSEE undertakes to comply with regulations, to carry out at its own expense, risk and peril, throughout the duration of the Lease, all work incumbent upon it in this respect, provided that the compliance work is related to its specific activity (in particular, its laboratory and workshop activities within the R&D and storage technical premises), so as to save and hold the LESSOR harmless in this respect, and to pay all sums, fees, taxes, dues of any kind, relating to the activity carried out in the Leased Premises and to the use of the premises.
The Parties expressly agree that the Leased Premises form a single, indivisible whole.
ARTICLE IV - DURATION
This Lease is granted and accepted for a duration fixed in Article XIV.
Unless otherwise stipulated below, the LESSEE will have the option of giving notice of six (6) months for the expiry of each three-year period, in accordance with the regulations in force.
On expiry of this duration, the Parties will have the option of giving notice of six (6) months to terminate the initial duration set out in Article XIV; failing this, the Lease will be extended by tacit renewal in accordance with the provisions of Article L. 145-9 of the French Commercial Code.
In the event that the LESSEE gives notice as provided for in this Article, the LESSOR will have the right to inspect the Leased Premises during the notice period, and if it so wishes, in the presence of the LESSEE. Any visit must take place during the week and be subject to at least one working day’s notice, with the LESSOR undertaking not to interfere with the LESSEE’s activity in the Leased Premises. During this same period, the LESSOR will also have the option of affixing a sign or billboard to the front of the Building, with a view to attracting a new lessee.
If, due to the LESSEE’s fault, the LESSOR is unable to visit the Leased Premises, rent them out, deliver them to a new lessee or occupy them itself, if this was the LESSOR’s intention at the time set for the end of the Lease, the LESSOR will be entitled to compensation at least equal to two months’ rent, without prejudice to any damages and interest.
If this Lease is renewed, it will be for a duration of 9 years, as will be subsequent renewals, with the option for the LESSEE to terminate every three years, in accordance with Article L. 145-9 of the French Commercial Code.
ARTICLE V - TERMS AND CONDITIONS AND USE
This Lease is entered into subject to the usual lawful terms and conditions, and in particular to those set out below, which the LESSEE undertakes to execute and strictly comply with, under penalty of damages and even termination of this Lease, if the LESSOR sees fit.
In view of the negotiations which preceded the conclusion of this agreement, each of the Parties declares that it has made its commitment with full knowledge of the facts. Consequently, in the event of an unforeseeable change in circumstances as referred to in Article 1195 of the French Civil Code, the Parties expressly agree to waive the application of these provisions and thus refrain from seeking any judicial review of this Lease.
In any event, notwithstanding Article 1223 of the French Civil Code, the LESSEE may not unilaterally apply any reduction in rent, charges or any other consideration in the event that the LESSEE alleges imperfect performance of its obligations by the LESSOR.
V.1 - ENTERING THE LEASED PREMISES
The LESSEE shall take the Leased Premises, the subject of this Lease, in the condition in which they will be on the Date of Early Availability (as this term is defined below), without being able to require the LESSOR to carry out any work.
In order to comply with the provisions of Article L. 145-40-1 of the French Commercial Code, two copies of an inventory of fixtures will be drawn up on the Date of Early Availability, by mutual agreement between the Parties or by a third party appointed by them. Failing this, it will be drawn up by a bailiff, at the initiative of the most diligent Party, at a cost shared equally between the LESSOR and the LESSEE.
V.2 - LESSOR’S RIGHT OF ACCESS
The LESSOR will have the right to visit the Leased Premises, giving 48 hours’ notice except in emergencies, in order to ensure compliance with the various clauses of the Lease and, in particular, the proper maintenance of the Leased Premises and the performance by the LESSEE of all work for which it is responsible.
The LESSEE shall notify the LESSOR of any degradation or deterioration of the Leased Premises as soon as it is aware of it, failing which it shall bear the possible consequences of its failure to do so.
The LESSOR will also have the right to have the Leased Premises visited under the conditions set forth in Article IV if it intends to sell the premises which are the subject of this Lease.
V.3 - MAINTENANCE - REPAIRS
The LESSEE shall enjoy the use of the Leased Premises in a responsible manner, shall maintain them in a perfect state of repair and shall, moreover, carry out throughout the term of this Lease, and at its own expense, any repairs that may be necessary, with the exception of:
| • | compliance work not related to the LESSEE’s specific activity, |
| • | complete replacement of major equipment, |
| • | major repairs covered by Article 606 of the French Civil Code, and |
| • | work resulting from obsolescence. |
The LESSEE shall be responsible for any compliance work related to its specific activity (in particular, work related to its laboratory and workshop activities within the R&D and storage premises).
The LESSEE shall also bear the costs associated with the maintenance and proper operation of the equipment.
The LESSEE shall be liable for any damage caused to the Leased Premises and/or the Building due to overloading of the floors and elevators.
The LESSEE undertakes to have the installations and equipment of the Leased Premises, and in particular the emergency equipment (fire extinguishers), regularly checked for proper operation and compliance with regulatory standards.
This inspection will be carried out at the LESSEE’s expense by a body approved by the plenary assembly of fire insurance companies [assemblée plénière des sociétés d’assurances contre l’incendie] (C.E.P., VERITAS, etc.).
The LESSEE shall provide proof of its contracts at the LESSOR’s request, comply with the suggestions contained in the inspection authority’s report and carry out any compliance work that may be necessary, provided that the compliance work is related to its specific activity (in particular that related to its laboratory and workshop activities within the R&D and storage premises).
V.4 - FURNISHINGS - OPERATION
V.4.1 The LESSEE shall furnish the said Leased Premises and keep them constantly furnished throughout the term of the Lease, with furniture and equipment, as security in the event of non-payment of rent or breach of the terms and conditions of this Lease.
V.4.2 Throughout the term of the Lease, the LESSEE shall maintain the Leased Premises for the use set forth in Article XIII and in a state of effective and normal operation, without being able, under any pretext, to use the Leased Premises even temporarily for another purpose, either by addition or substitution of activities.
V.5 - WORK
V.5.1
The LESSEE may not carry out any demolition, construction or installation, fitting out, piercing of walls or change of distribution in the Leased Premises, and generally may not make any modification whatsoever to them or to the installations, without having first obtained the prior written authorization of the LESSOR, it being specified that the work must be carried out under the supervision of the LESSOR’s architect, whose fees will be paid by the LESSEE.
To this end, the LESSEE shall send the LESSOR a works file (“Works File”) including the following:
| • | A description of the work and equipment planned (written and graphic documents), including in particular: |
| • | Plans, cross-sections and sketches required for a clear understanding of the project, |
| • | A detailed description of the equipment and materials used, |
| • | The execution file in accordance with good professional practice, |
| • | The work schedule, |
| • | The draft application for administrative authorization (if necessary), |
| • | The copies of insurance policies (if applicable). |
If necessary, the documents in the Works File must be drawn up by a project manager and validated by a technical inspector (RICT) (rapport initial du contrôleur technique [initial technical inspection report]).
V.5.2
However, the LESSEE may freely carry out, without the prior agreement of the LESSOR, and if applicable by way of derogation from the above, all routine fitting-out work, i.e. interior painting, wall and floor coverings, wiring, as well as light partitioning work, provided that such work does not modify or alter the HVAC (heating - ventilation - air conditioning) systems and other technical installations of the Building and the Leased Premises, which the LESSEE shall justify to the LESSOR in advance by sending the Works File.
V.5.3
All work shall be carried out in compliance with all applicable regulations and the rights of third parties, after taking out all necessary insurance.
In the event that the work involves a modification of the partitioning inside the premises, the LESSEE undertakes to send the LESSOR the plans of the modifications as soon as possible.
All work shall comply with the LESSEE specifications in Appendix 2.
All HVAC work shall be entrusted to the facility manager who manages the Building, and to the company in charge of the BMS.
The facility manager will be responsible for setting the air-conditioning and lighting parameters.
Work may not commence until written authorization has been obtained from the LESSOR.
Deliveries shall be made via the staircase or the freight elevator, which must be protected.
Common areas:
| • | Piercing of stone or wood panels in lobbies and story landings is not permitted. |
| • | No work is permitted in common areas. |
No holes may be made in facade profiles in private areas.
The opening of firefighting vents, smoke extraction vents, smoke extraction shutters, technical closets and hatches shall not be impeded by the presence of furniture and/or partitions.
Noise generated by drilling and other activities must be limited to before 9:00 am, between 12:00 and 2:00 pm and after 6:00 pm.
For all work of any nature whatsoever carried out by the LESSEE, the LESSEE assumes all liability that may arise, particularly in the event of accidents and/or incidents that may occur as a result of the execution and existence of construction, installations and modifications carried out by the LESSEE, as well as the operations to which they may give rise. In particular, the LESSEE guarantees the LESSOR against any claims that may be brought against it as a result of the said accidents and/or incidents and their consequences.
The LESSEE shall cover or have covered all risks incurred, by insurance policies taken out with a solvent insurance company.
The LESSEE shall ensure that no damage is caused by its workers or employees. The LESSEE shall be liable for any damage or loss affecting the Leased Premises or the Building and its installations.
The LESSEE shall put an end to justified complaints and carry out all work necessary so as to save and hold the LESSOR harmless in this respect.
One month at the latest after acceptance of the work, the LESSEE shall provide the LESSOR with:
| • | The complete as-built file (DOE) and the updated advisory maintenance file (DIUO), |
| • | The inspection authority’s report, with no comments. |
The LESSEE may not erect any signs, billboards or awnings, or carry out any installation whatsoever concerning the external appearance of the Building, without the prior written consent of the LESSOR, who will, where necessary, seek the authorizations provided for in the co-ownership regulations or any other regulations, specifications or articles of association to which it may be subject. However, the LESSEE will be personally responsible for obtaining the required administrative authorizations and for paying any taxes that may be due as a result, so as to save and hold the LESSOR harmless in this respect.
V.5.4 The LESSEE accepts, for the entire duration of the Lease, the execution in the Building or in the Leased Premises of all work, including reconstruction work and repairs of any kind that the LESSOR may deem necessary, without being able to claim any indemnity or reduction in the rent hereinafter indicated, even if such work lasts more than twenty-one days.
The LESSOR undertakes to use its best efforts to carry out this work diligently and without interruption, with a view to minimizing the disturbance to the LESSEE’s use of the premises. The LESSOR also undertakes in any event to maintain access to the Leased Premises. Work inside the Leased Premises expected to last more than eight days will be scheduled by mutual agreement with the LESSEE, except in the case of an emergency or force majeure.
The LESSEE shall also accept all work carried out on the public highway, or in the buildings adjacent to the one to which the Leased Premises belong, regardless of the inconvenience this may cause to the operation of its activity, without prejudice to its right of recourse against the administration, the contractor for the work, and the neighboring owners, and shall always hold the LESSOR harmless.
Notwithstanding the provisions of Article 1723 of the French Civil Code, the LESSEE shall accept any modifications that the LESSOR deems necessary to the exterior appearance of the Building and its accessibility, subject to maintaining access to the Leased Premises and minimizing any disturbance to the LESSEE’s use of the Leased Premises.
V.6 - LESSOR’S RIGHT OF ACCESSION / RESTORATION TO ORIGINAL CONDITION
V.6.1 All constructions and installations, all modifications and generally all improvements or embellishments carried out by the LESSEE, including those which may have been imposed by legislative or regulatory provisions, shall become the property of the LESSOR without compensation at the end of the term of the Lease, subject to the option available to the LESSOR under Article V.6.2.
V.6.2 The LESSOR may require the LESSEE to return the Leased Premises, in whole or in part, to their original condition on the Date of Early Availability, at the exclusive expense of the LESSEE, except if the LESSEE’s leaves the premises 6 years or more after the Effective Date of the Lease or beyond. In the latter case, in the event of the LESSEE’s departure 6 years or more after the Effective Date of the Lease, the LESSOR can only require the LESSEE to remove its laboratory and workshop fittings at the LESSEE’s expense.
V.7 - ASBESTOS TECHNICAL FILE (DTA)
As the building permit for the Building was issued after July 1, 1997, the “Asbestos” technical file defined in Article R. 1334-29-4 of the French Public Health Code is not applicable.
V.8 1- STATEMENT OF RISKS
In accordance with Article L. 125-5 of the French Environment Code, if the Leased Premises are located in an area covered by a prescribed or approved risk prevention plan, a statement of risks less than 6 months old is appended hereto, together with information from the LESSOR on any losses of which it is aware that have been the subject of compensation for natural, mining or technological disasters.
The LESSEE acknowledges that it has been given access to the information mentioned in Article L. 125-5 of the French Environment Code and that a statement of risks has been provided to it in accordance with the provisions of Article L. 125-5 of the French Environment Code.
In addition, Article L. 125-7 of the French Environment Code stipulates that, when a plot of land located in the soil hazard information sector (secteur d’information sur les sols) referred to in Article L. 125-6 of the Environment Code is the subject of a sale or lease agreement, the seller or lessor of the land shall inform the purchaser or lessee in writing. The seller or lessor shall provide the information made public by the French government, in accordance with Article L. 125-6 of the Environment Code. The deed of sale or lease attests to the completion of this formality.
The statement of risks appended to the Lease (Appendix 3) includes this information and indicates that the land is not located in a soil hazard information sector (SIS).
V.8 2- ENERGY PERFORMANCE
In accordance with the provisions of Article L. 126-29 of the French Construction and Housing Code, an energy performance diagnosis is attached to this Lease (Appendix 4).
In accordance with the above provisions, the LESSEE shall not make use of the recommendations contained in the energy performance diagnosis against the LESSOR, which are for information purposes only.
V.8 3- ENVIRONMENTAL PERFORMANCE
The Building is HQE Bâtiments Tertiaires certified (high environmental quality of commercial buildings) as Excellent and BREEAM certified with a rating of Excellent.
The Parties undertake to use their best efforts to maintain the environmental performance of the Building within the framework of the labeling or certification obtained. The Parties undertake, each insofar as it is concerned, to use their best efforts to obtain or maintain certification for the operation. To this end, the Parties undertake to comply with the terms of the Environmental Performance Appendix attached hereto (Appendix 5).
The Environmental Performance Appendix will be updated at each renewal of the Lease, or at any earlier date if the Parties agree otherwise.
However, in the event that a regulation imposes quantified targets for the reduction of energy, water and/or waste consumption, the LESSEE undertakes to comply with them in the context of its operation of the Leased Premises.
The LESSEE undertakes to bear the cost of all measures required for the operation and technical management of the Building and its equipment, in accordance with the provisions relating to the allocation of expenses described in Article V.15.
These measures will be implemented at a level of quality that ensures the proper conservation of the Building and its equipment in accordance with the terms of the Environmental Performance Appendix, the maintenance of the legal warranties applicable to builders and/or manufacturers, and any future operating certification.
It is hereby specified that all work resulting from existing or future labeling or certification of the Building will remain the responsibility of the LESSOR.
V.8 4- “TERTIARY DECREE”
Under the terms of Article L. 174.1 of the French Construction and Housing Code, “actions to reduce final energy consumption are implemented in existing buildings, parts of buildings or groups of buildings for tertiary use, defined by decree in the Council of State, in order to achieve a reduction in final energy consumption for all buildings subject to the obligation of at least 40% in 2030, 50% in 2040 and 60% in 2050, compared with 2010 [....] All buildings, parts of buildings or groups of buildings subject to the obligation must achieve the following targets for each of the years 2030, 2040 and 2050:
1/ Either a level of final energy consumption reduced by 40%, 50% and 60% respectively compared with a reference energy consumption that cannot be prior to 2010;
2/ Or a level of final energy consumption set in absolute terms, based on the energy consumption of new buildings in their category.”
In this respect, the Parties undertake to cooperate and use their best efforts to meet the energy consumption reduction targets set by the above provisions, and where appropriate, to carry out any work necessary to this end.
In accordance with Article R. 174-27 of the French Construction and Housing Code, various data relating to the previous year (tertiary use, surface areas, annual energy consumption by type of energy, etc.) must be transmitted each year by deadlines set by a joint order of the ministers for construction and energy; the order of September 29, 2021, sets this date at September 30 of each year from 2022 onwards.
The LESSEE undertakes:
| • | To make an annual declaration of its energy consumption for the Leased Premises (parts of the Building restricted to private use) or for which it is billed, on OPERAT, the digital platform provided for in Article L. 174-1 of the French Construction and Housing Code, within the legal and/or regulatory deadlines. |
| • | To acknowledge having been informed and being fully aware of the administrative and financial penalties associated with non-compliance with end-use energy reduction targets and failure to transmit the required data to the OPERAT digital platform in good time. |
| • | To indemnify the LESSOR against any penalty imposed on the LESSOR as a result of the LESSEE’s failure to transmit its energy consumption data to the OPERAT digital platform in a timely manner. |
| • | To inform the LESSOR of any modification to the equipment of the Leased Premises which would have consequences on energy consumption or lead to a deterioration in the level of greenhouse gas emissions. |
The LESSOR undertakes:
| • | To draw up an action plan which will be presented to all lessees; it will determine the work and actions to be undertaken to enable the objectives set to be achieved; it being specified that this work will be carried out and borne in accordance with the rules for financial responsibility set out in this Lease between the LESSOR and the LESSEE. |
| • | To open or have opened an account on the OPERAT platform for the Building, and enter the surface areas for tertiary use. |
| • | To enter the identities of lessees who will have access to the platform. |
| • | To declare energy consumption in communal areas of the Building. |
V.9 - CONTRIBUTIONS - TAXES
The LESSEE shall pay its personal contributions, property taxes, and the French business property tax (CFE) of the French territorial economic contribution (CET) for which it is liable, and shall meet all sweeping, lighting and other expenses to which a lessee is ordinarily liable, so as to save and hold the LESSOR harmless in this regard.
It will also reimburse the LESSOR for all taxes, dues and fees related to property, including property taxes and additional taxes to the property tax, as well as all taxes, dues and fees related to the use of the Leased Premises or the Building in which the Leased Premises are located, or a service from which the LESSEE benefits directly or indirectly, including household waste tax and taxes on offices, warehouses, shops and parking spaces, present or future, normally payable by the owner, so that the rent received by the LESSOR is net of all taxes.
V.10 - LIABILITY - RECOURSE - INSURANCE
V.10.1 Liability and recourse
The LESSEE hereby declares that it waives all liability claims against the LESSOR in the following cases:
| • | In the event of theft or any other criminal act of which the LESSEE could be victim in the Leased Premises, the LESSOR having no obligation to monitor the Leased Premises and the Building. |
| • | In the event of damage to the Leased Premises, to movable objects or goods located in the Leased Premises, as a result of leaks, infiltration, damp or other circumstances, the LESSEE being responsible for protection against these risks, without recourse against the LESSOR. |
The LESSEE also undertakes not to claim any compensation from the LESSOR, nor any reduction in rent or charges:
| • | in the event of a stoppage in the distribution of water, electricity or other fluids, and in the event of a stoppage of operation for any reason whatsoever of the Building’s technical installations (air conditioning, district heating, elevators, etc.) as a result of maintenance, repair, replacement, lack of supply, strikes or any other causes beyond the LESSOR’s control; |
| • | in the event of liability-generating actions by other lessees, their staff, suppliers or customers; |
| • | in the event of changes or modifications made by any person whatsoever, and in particular by the LESSOR, to the common areas of the Building. |
V.10.2 Insurance
Real estate and personal property must be insured with companies known to be solvent, as follows:
V.10.2.1 Insurance taken out by the LESSOR
The LESSOR shall insure the Building, including real property and permanent fixtures, installations permanently fixed within the meaning of Article 525 of the French Civil Code, equipment and installations in place on the day the Lease is signed, against the risks of fire, lightning, explosions, damage caused by electricity, water damage, glass breakage and more generally against all risks related to the nature of the Building, its quality and use, for the replacement value of the Building.
The LESSOR will also take out any civil liability insurance that the LESSOR may incur in its capacity as owner.
The insurance premiums thus paid by the LESSOR will be reimbursed in full by the LESSEE.
V.10.2.2 Insurance taken out by the LESSEE
The LESSEE undertakes to insure, for the entire term of the Lease, with a solvent insurance company authorized to insure on French territory, the risks listed below:
| • | Material damage to works and embellishments (fittings and fixtures), whether or not carried out at the LESSEE’s expense, and to all objects, equipment or other movable property belonging to the LESSEE or in the LESSEE’s custody, guaranteeing the Leased Premises for damage resulting from events such as fire, lightning, explosion, water damage, costs of excavation, demolition, glass breakage, sprinkler or other liquid leaks, electrical damage, falling aircraft and aerial objects, malicious damage, sabotage, impact of land vehicles, natural disasters, hurricanes, cyclones, tornadoes, storms, and hail on roofs, smoke, riots and civil commotion, expert fees, as well as all expenses incurred in restoring the Building to its original condition; |
| • | Loss of use or deprivation of use for up to 24 months; |
| • | Any civil liability it may incur under Articles 1240 to 1242 of the French Civil Code for bodily injury or material or immaterial damage caused to third parties and arising directly or indirectly from its activity, from the goods referred to in the above paragraph, and from its employees. |
The LESSEE undertakes to:
| • | not contravene in any way whatsoever one or more of its insurance policies, which could result in the cancellation of such policy or policies; |
| • | pay the premiums for its insurance policy(ies) on time; |
| • | provide annual proof of compliance with the foregoing clauses at the LESSOR’s first request, by producing the insurance policy(ies) and the related premium receipts; |
| • | notify the LESSOR of any fact making it necessary to issue an endorsement to the LESSOR’s insurance policies; |
| • | notify the LESSOR of any damage, within five days of becoming aware of it, and of any repairs under its control that may become necessary during the term of the Lease, failing which it will remain personally liable for the damage. |
The LESSEE undertakes to change the insurance policy only after giving fifteen days’ notice to the LESSOR.
Mention shall be made in the LESSEE’s insurance policy(ies) that the cancellation thereof may only take effect at the end of a period of fifteen days after notification made to the LESSOR by the LESSEE’s insurer.
Should the LESSEE fail to take out, renew the policies or pay the premiums relating thereto as provided for above, the LESSOR reserves the right to do so and claim reimbursement of the premiums thus advanced from the LESSEE.
V.10.3 Reciprocal waivers of recourse
The LESSEE and its insurers waive all recourse against the LESSOR and its insurers, and by way of reciprocity, the LESSOR and its insurers waive all recourse against the LESSEE and its insurers.
V.11 - ASSIGNMENT
The LESSEE may assign or contribute its right to this Lease, including during any tacit extension, only to the purchaser of its business.
The LESSEE may assign its leasehold rights to any company in its group within the meaning of Article 1233-3 of the French Commercial Code, subject to the prior written approval of the LESSOR, who may refuse to do so for any legitimate reason, in particular in the event of the assignee’s lack of solvency.
Any assignment will be subject to the following mandatory conditions of validity:
| • | Prior settlement of all arrears in principal, charges and incidentals, |
| • | Stipulation of a joint and several guarantee by the assignor and all successive assignees for a period of three years from the date of assignment for the payment of rents, charges and incidentals and the performance of the clauses of the Lease, the assignees also being jointly and severally liable without being able to invoke the benefit of division and discussion, |
| • | Delivery of an enforceable copy or an original copy of the deed of assignment, if applicable in the form of an extract, within one month of signature at the LESSEE’s expense, failing which the Lease will be automatically terminated, if the LESSOR sees fit, |
| • | And restoration of the premises by the assignor in accordance with the provisions of Article V.23. |
In order to comply with the provisions of Article L. 145-40-1 of the French Commercial Code, two copies of an inventory of fixtures will be drawn up as soon as possible on the date of assignment, by mutual agreement between the LESSOR and the assignee, or by a third party appointed by them. It is hereby specified that the inventory of fixtures drawn up at the time of the assignment will, for the LESSOR, be deemed to be a simple statement of the existing condition on the day it is drawn up, without the LESSOR waiving any rights it may have under the Lease and the history of the rental relationship.
No assignment of the right to this Lease may take place less than one month after prior notification sent by the LESSEE to the LESSOR by registered letter with acknowledgment of receipt or extrajudicial act inviting the LESSOR to participate in the planned assignment, including full disclosure of the planned assignment and specifying the place, day and time scheduled for the final completion of this assignment.
In the absence of intervention, or even in the event of pure and simple intervention, the sale must not in any way prejudice the prior rights and actions of the LESSOR, and any clause in the assignment that is contrary to or inconsistent with the clauses and conditions of the Lease will be deemed unwritten by operation of law.
In the event of supervised liquidation or receivership of the LESSEE, the assignment of the Lease by the administrator or liquidator may be made only under the conditions stipulated above.
In the event that the assignment is made to a company that is not a joint stock company, the manager(s) or corporate officer(s) of said company will be jointly and severally liable for the payment of rent and the performance of the Lease’s terms and conditions.
V.12 - SUBLEASING - LEASE MANAGEMENT
Total or partial sublease is prohibited. The same applies to all lease management.
Notwithstanding the foregoing, the LESSEE is authorized to partially sublease the Leased Premises and any company in its group within the meaning of Articles L. 233-1 and L. 233-3 of the French Commercial Code. Total sublease is prohibited.
Sublessee companies must belong to the LESSEE’s group as defined above for the entire duration of the sublease. Failing this, the sublease will be immediately terminated by operation of law, and the authorization granted by the LESSOR will lapse.
Provisions common to all authorized subleases:
| • | In all cases of authorized sublease, the occupancy of the sublessee must be consistent with the purpose of the Leased Premises and its activity must be compatible with that purpose. |
| • | The LESSEE will remain liable for all rents, charges and incidentals and will continue to be bound by all obligations due under the Lease. |
| • | The sublease terms and conditions must be compatible with all those stipulated in the main Lease. In the event of incompatibility, the clauses of the main Lease will prevail. |
| • | Under no circumstances may a sublease be granted for a duration longer than the remaining term of the main Lease. |
| • | The sublease agreement must contain a clause in which the sublessee declares that it is fully aware and acknowledges that the fate of the sublease follows that of the main Lease, and that the expiry or termination of the latter will automatically result in the termination of the former. |
| • | All sublease agreements must include a map showing the location of the sublease premises. |
| • | In the event of authorized sublease, the LESSEE will remain jointly and severally liable for the obligations of its sublessee(s). |
| • | As the Leased Premises form an indivisible whole in fact and by the joint intent of the Parties, the sublease(s) will not be enforceable against the LESSOR and will entail an express waiver by the sublessee(s) of any action, in particular with a view to their continued occupancy of the premises, and of any right to renew the sublease against the LESSOR. |
| • | This Article must be reproduced in all sublease contracts, which must be sent to the LESSOR within 30 (thirty) days of signature, by any means, with proof that the sublessee belongs to the LESSEE’s group. |
V.13 - CUSTODY OF LEASED PREMISES
The LESSEE will be responsible for the surveillance and guarding of the Leased Premises and its equipment.
The LESSEE shall not exercise any recourse or claim against the LESSOR for any disturbance and/or deprivation of use originating from third parties and will be personally responsible for any recourse against the author of the damage, the LESSOR subrogating it with respect to its rights to this effect.
V.14 - DESTRUCTION OF LEASED PREMISES
1. In the event that the Leased Premises are completely destroyed as a result of any damage whatsoever, regardless of its origin, and unless otherwise agreed by the Parties, the Lease will be terminated ipso jure.
In the event that the Leased Premises are partially destroyed and this destruction:
| • | prevents the use of the non-destroyed Leased Premises under normal office conditions for more than eighteen (18) months, particularly in view of the duration of the restoration work, or |
| • | concerns more than 50% of the surface area of the Leased Premises, |
then the Lease may be terminated by either the LESSEE or the LESSOR, without compensation on either side.
2. In the event that the Leased Premises are partially destroyed and this destruction:
| • | does not prevent the use of the non-destroyed Leased Premises under normal office conditions for more than eighteen (18) months, particularly in view of the duration of the restoration work, and |
| • | concerns no more than 50% of the surface area of the Leased Premises, then the Lease will not be terminated. |
The LESSOR will then be obliged to rebuild the damaged part of the Leased Premises, as soon as the loss in question is covered by the insurance company or companies and subject to the LESSOR obtaining the necessary administrative authorizations for reconstruction. In such a case, the LESSEE will be exempted during this period from payment of the rent for the destroyed part of the Leased Premises of which it will be deprived. The LESSOR will retain the full benefit of any insurance indemnities.
3. In any event, the LESSEE may not, by express agreement, claim any compensation other than that awarded to it by the insurance company or companies for the damage caused to it.
In the event of disagreement as to the proportion of the Leased Premises destroyed or not usable in accordance with their intended purpose following such destruction, as to the duration of the restoration of the Leased Premises, as to the rent reduction and the duration of application of the said rent reduction, the Parties agree to respect the opinions of the expert chosen by mutual agreement between the Parties or, failing that, appointed by the President of the Court of Justice ruling in summary proceedings, the costs being borne by the requesting Party.
V.15 - EXPENSES
The LESSEE shall comply with the provisions of any contractual or regulatory document applicable to the Building and shall pay to the LESSOR all charges and provisions, in particular those of co-ownership or co-use, which defines the allocation of charges between the various lessees of the Building, including those usually borne by the owner, including property management fees of 1.5% of the annual rent excluding VAT, technical management fees, as well as all charges to which the LESSOR will be liable in its capacity as owner, all in accordance with the inventory of categories of charges, taxes and fees appended hereto (Appendix 6), so that the rent received by the LESSOR is net of all charges, subject only to those charges expressly mentioned as being borne by the LESSOR.
An advance on charges will be called with each rental term and will be paid by the LESSEE in accordance with the rental payment conditions.
The LESSOR will close the accounts annually, applying the usual accounting rules and practices. Consequently, it undertakes to provide the LESSEE with an exact breakdown of rental charges by category and basis of allocation, with an indication of the debit or credit balance for the past year. This breakdown will be sent to it within nine (9) months of the expiry of the said year, or three (3) months of the presentation of charges if the Building is placed under the co-ownership system.
The accounting documents will be made available to the LESSEE at the LESSOR’s head office.
The LESSEE may inspect the LESSOR’s receipts for expenses and allocation accounts.
If, at the end of the year, the provisions paid turn out to be less than the actual expenses, the
LESSEE undertakes to reimburse, upon a first call from the LESSOR, all sums that may be necessary to offset the total amount.
In the event that the provisions paid exceed the actual expenses for the expired year, the amounts overpaid will be deducted from the provisions for the current year.
In the event of the LESSEE’s departure, the sums due will be claimed or the overpaid provisions will be reimbursed.
If applicable, the LESSEE shall pay the LESSOR, on the Effective Date of the Lease, the working capital relating to the leased property.
Working capital and provisions for charges will be periodically revalued.
A summary statement of work carried out by the LESSOR over the last three years and a statement of projected work it plans to carry out over the next three years are appended (Appendix 7).
These summaries are issued by the LESSOR for information purposes only, and the LESSEE may not use the information contained in said summaries against the LESSOR; in particular, the statement of projected work for the next three years does not constitute any commitment on the part of the LESSOR to carry out said work, as the LESSOR remains free to carry it out in full or in part, to refrain from carrying it out, or to carry out other work.
The LESSEE shall scrupulously comply with all current and future regulations, rules and orders, in particular those relating to roads, health, hygiene, safety, police, labor inspection, the plenary assembly of damage insurance companies [Assemblée Plénière des Sociétés d’Assurances Dommages] (APSAD), environmental protection - including environmental regulations governing asbestos, legionella, lead and energy performance - and bear, where applicable, the cost of compliance with said regulations, as well as any work, modifications or improvements ordered by the administrative authorities, insofar as said compliance relates to its specific activity (in particular laboratory and workshop activities within the R&D technical and storage premises), so as to save and hold the LESSOR harmless in this respect.
The LESSEE shall provide the LESSOR, at the latter’s first request, with all maintenance contracts entered into in respect of the obligations resulting from this Lease.
V.16 - SAFETY
The current capacity of the Leased Premises, which the LESSEE undertakes to respect, is specified in the safety notice appended hereto (Appendix 8).
The LESSEE is responsible for the safety of persons and property in connection with the Leased Premises and their use.
In order to prevent the risk of fire or panic in the Leased Premises covered by this Lease, when these are used as part of an establishment subject to the regulations governing establishments open to the public or classified establishments, the LESSEE, in addition to complying with the legal and regulatory obligations incumbent upon it, shall set up a general safety control system for the said premises.
To this end, acting both on its own behalf and on behalf of the LESSOR, it shall take out a subscription for periodic inspection visits with an approved organization. The checks carried out must cover all buildings, facilities, installations and equipment subject in some way to regulations on the safety of people and property.
The LESSOR may ask the LESSEE for a copy of each inspection report drawn up by the inspection body.
In the absence of a response from the LESSEE and in order to verify the safety measures implemented by the LESSEE, the LESSOR may, at any time during the term of the Lease, subject to observing, except in emergencies, a 48-hour notice period, have an approved inspection body carry out a safety inspection of the Leased Premises and their fittings against the risks of fire or panic.
In application of these principles, the cost of intervention by the inspection bodies will always be borne by the LESSEE.
V.17 - LIEN
In the event that this Lease becomes encumbered by a charge or lien, the LESSOR must immediately be notified by extrajudicial act by the LESSEE, and at the latest within fifteen days of said registration.
V.18 - EXPROPRIATION
In the event of expropriation for public utility, the LESSEE may not claim anything from the LESSOR, all the LESSEE’s rights being reserved against the expropriating Party.
V.19 - INFORMATION
The LESSEE undertakes to notify the LESSOR of any major change in its economic, legal or financial situation, in particular by merger, transformation, modification or extension of activity, within one month of the event.
V.20 - RECEIVERSHIP / SUPERVISED LIQUIDATION
In the event of receivership or supervised liquidation, or in the event of the LESSEE’s death if the LESSEE is an individual, the heirs, assigns or representatives shall be jointly and severally liable for the payment of rent, charges and incidentals and for the performance of the terms of this Lease, without being able to invoke the benefit of discussion.
In addition, they will bear the costs of service provided for under Article 877 of the French Civil Code, under the same conditions.
V.21 - ASSIGNMENT OF CLAIMS
The LESSEE acknowledges and expressly accepts that the LESSOR’s claims against the LESSEE hereunder may be assigned or delegated by way of security to any credit institution that has granted the LESSOR a loan for the purpose of financing the acquisition of the Leased Premises.
V.22 - TRANSFER OF RIGHTS
The LESSOR may freely assign or contribute its rights and obligations under this Lease (and any renewals thereof) at any time, without the need to comply with the formalities set out in Article 1690 of the French Civil Code.
V.23 - RETURN OF PREMISES
The LESSEE shall return the Leased Premises:
| • | In a perfect state of maintenance, cleanliness and repair if the Leased Premises are returned prior to the expiry of 4.5 years from the Effective Date of the Lease. |
| • | In a very good state of maintenance, cleanliness and repair if the Leased Premises are returned at the end of 4.5 years from the Effective Date of the Lease or before the end of 9 years from the Effective Date of the Lease. |
| • | In a good state of maintenance, cleanliness and repair if the Leased Premises are returned at the end of a period of 9 years from the Effective Date of the Lease or beyond. |
As mentioned in Article V.6.2, the LESSOR may, under certain conditions, ask the LESSEE to remove its fixtures and fittings and works at its own expense before its departure.
No later than five (5) months prior to the LESSEE’s departure date, a joint inspection of the Leased Premises will be carried out in the presence of any technician, architect or manager appointed by either of the Parties to draw up a preliminary inventory of fixtures.
At the end of this visit, the LESSOR will send a statement of the repairs and work to be carried out by the LESSEE and of the rework following removal in order to comply with the return conditions defined in this Lease, without prejudice to any reservations that may be formulated at the time of the inventory of fixtures drawn up on return of the Leased Premises.
The LESSEE shall then, at its own expense, carry out all repairs and work/removals noted in this preliminary inventory of fixtures by the date of its departure at the latest.
However, at the LESSEE’s request, which shall be made no later than thirty (30) days following the drawing up of the aforementioned preliminary inventory of fixtures, the LESSOR will have the cost of the repairs and work/removals incumbent upon the LESSEE identified in the preliminary inventory of fixtures quantified by at least two companies, and will forward the estimates to the LESSEE at least three (3) months prior to the LESSEE’s departure date.
The LESSEE will be authorized to carry out its own costing of repairs and work/removals incumbent on the LESSEE identified in the preliminary inventory of fixtures within the same period. If the LESSEE’s estimate differs significantly from those of the LESSOR, it may be presented to the LESSOR and the Parties will then enter into a discussion, in good faith, on the cost of repairs and work/removals to be borne by the LESSEE on the basis of their estimates. The Parties shall use their best efforts to reach a mutually satisfactory agreement no later than two (2) months prior to the departure of the LESSEE.
The LESSEE may then dispense with carrying out the repairs and work/removals incumbent upon it by paying the amount of the estimate retained under the above conditions, provided that the LESSEE has made this position known to the LESSOR at least two (2) months prior to its departure.
In the event that the LESSEE simply refuses the estimates presented by the LESSOR, or in the event that the Parties have not reached agreement on the amount of the estimate selected at least two (2) months prior to the LESSEE’s departure, or the LESSEE has not made its position known at least two (2) months prior to its departure, the LESSEE will be deemed to have opted to carry out all repairs and work/removals incumbent upon it as identified in the preliminary inventory of fixtures, at its own expense, by the date of its departure at the latest.
A joint inventory of fixtures will be drawn up by the Parties in duplicate, signed or initialed by each Party, on the date of return of the Leased Premises.
At the request of either Party, this report may be drawn up by a bailiff appointed for this purpose by the LESSOR at a cost shared equally between the Parties:
In the latter case, the bailiff will notify the LESSEE of the report by registered letter with acknowledgment of receipt, at the address provided by the LESSEE when the Leased Premises are returned.
If, for any reason whatsoever, the LESSEE does not appear on the date on which it has been summoned by the LESSOR, the inventory of fixtures drawn up by the bailiff will be deemed to have been drawn up jointly.
In the event that the LESSEE decides to carry out the repairs and work/removals incumbent upon it by virtue of the foregoing, but that these have not been carried out or completed by the date of its departure, as in the event that reservations are noted when the inventory of fixtures is carried out at the time of departure, the LESSEE shall pay, in addition to the cost of repairs/work/removals not carried out or reservations, the amount of which will be defined in good faith by mutual agreement between the Parties and, failing that, set by the courts, a daily indemnity calculated on the basis of the current daily rent plus 75%, plus current charges and taxes, for the entire period required to prepare and carry out the restoration work.
V.24 - OCCUPANCY INDEMNITY
In the event that the LESSEE, deprived of all occupancy rights, does not completely vacate the Leased Premises of all occupants and/or all furniture and movable objects, resists an eviction order, or obtains a court-ordered delay in its departure, it shall owe the LESSOR, ipso jure and without notice, per day of delay, in addition to the charges and without prejudice to any rights to damages in favor of the LESSOR, an irreducible contractual occupancy indemnity equal to the daily rent increased by 75%, until complete removal and return of the keys, the said indemnity being intended to compensate the LESSOR for the prejudice caused by the occupation of the Leased Premises.
ARTICLE VI - FINANCIAL CONDITIONS
VI. 1 - RENT
This Lease is granted and accepted in consideration of an annual base rent, excluding charges and taxes, as indicated in Article XVI of the special provisions.
As the LESSOR has opted to make this Lease subject to value added tax, the LESSEE will reimburse the LESSOR for the amount of the said tax applicable to the rent and charges on the occasion of the payment of each term of rent.
The LESSEE undertakes to pay the rent to the LESSOR quarterly in advance on the 1st day of January, April, July and October of each year.
Should the Lease commence on a date other than the first day of the quarter, the rent for the current term will be calculated on a time-apportioned basis.
The LESSEE will be liable for all duties and taxes (including any variation in rates) which may be payable on the said rent, charges and other payments provided for in this Lease.
VI. 2- PAYMENT TERMS
Payments will be made to the LESSOR or its agent by bank transfer to the account whose RIB is shown in the appendix (Appendix 9).
VI. 3- RENT INDEXATION
The rent will be readjusted at the end of each annual period, upwards or downwards, ipso jure and without any formality or request, according to annual variations in the French index of rents for tertiary activities (ILAT) published by INSEE (base 100: last quarter 2010).
For the first indexation, the annual indexation rate will be calculated on the basis of the base index referred to in Article XVII, and the revision index will be the index for the same quarter of the following year. For subsequent indexations, the base index will be the previous revision index, and the revision index, the strictly corresponding quarterly index for each subsequent year.
If this index is not known on the anniversary date of the Lease, the LESSOR may proceed with a provisional indexation based on the last known index.
Should the chosen index disappear, or be inapplicable for any reason whatsoever, it will be replaced by the replacement index or, failing that, by any similar index to be determined or, if necessary, reconstituted by an expert appointed to act for both Parties (hereinafter the “Expert”) who will be appointed either by agreement of the Parties, or, failing this, by an order issued at the request of the most diligent Party by the President of the Court of Justice, who, in the event of refusal, departure or impediment of any kind whatsoever, will be replaced in the same manner.
In all cases, the Expert will have all the powers of a representative appointed to act for both Parties and its decision will be binding on the Parties and will therefore be final and without recourse. Pending the Expert’s decision, the LESSEE may not defer payment, and shall make a provisional payment upon presentation of the receipt, equal to the amount previously paid, the readjustment taking effect retroactively to the effective date of the revision.
The LESSEE expressly acknowledges that the above indexation clause constitutes an essential and determining condition of this Lease, without which it would not have been concluded.
VI. 4 - SECURITY DEPOSIT - BANK GUARANTEE
VL.4.1 Security deposit
As security for the performance of the LESSEE’s obligations of all kinds under this Lease, the LESSEE shall pay to the LESSOR, upon signature of this Lease, a sum as referred to in Article XVIII, equal to three months’ rent excluding taxes and charges, as a security deposit.
RECEIPT OF WHICH IS HEREBY ACKNOWLEDGED SUBJECT TO COLLECTION
This sum will be maintained or reconstituted during the term of the Lease so as to always correspond to three months’ rent excluding taxes and charges; in particular, it will be increased or decreased at the same time and in the same proportion as the rent, each time the latter is modified, the difference being paid with the first modified term.
This sum will be retained by the LESSOR for the entire term of the Lease, and will be reimbursed to the LESSEE at the end of the Lease, after the LESSEE has moved out and the LESSOR has received the keys, less any sums due to the LESSOR for any reason whatsoever.
It will not generate any interest. It is delivered to the LESSOR in full ownership, the LESSEE being able to bring a restitution claim against the LESSOR under the terms and conditions of this Article.
However, in the event of the opening of receivership or supervised liquidation proceedings against the LESSEE, the LESSOR may request, if it sees fit, and even in the event of continuation of the Lease, compensation with the sums due under the liabilities.
In the event of an assignment of the Lease, the security deposit shall be reconstituted by the assignee in such a way that it is always equal to one quarter of the annual rent.
VL4.2 Bank guarantee
The LESSEE may, at any time during the term of the Lease, substitute for the security deposit an independent bank guarantee on first demand for the same amount corresponding to three (3) months’ rent excluding taxes and charges (the “Guaranteed Amount”) issued by a first-tier bank with a branch in France (the “Bank Guarantee”).
The Guaranteed Amount will be adjusted at the same time and in the same proportion as the rent, each time the latter is modified, so as to always remain equal to a sum equivalent to three (3) months’ rent excluding taxes and charges.
In such a case, the LESSOR will return the security deposit to the LESSEE within 30 days of the delivery of the Bank Guarantee.
The Bank Guarantee will conform to the model shown in Appendix 10, it being specified that the Bank Guarantee may include changes with respect to the model, provided that such changes have been approved in advance by the LESSOR. The Bank Guarantee must be granted for the entire term of the Lease plus six (6) months.
In the event that the Bank Guarantee is invoked in whole or in part by the LESSOR due to the LESSEE’s failure to pay a sum due and payable under the Lease, the LESSEE will be required to provide the LESSOR within eight (8) working days with a new Bank Guarantee for an amount corresponding to that called by the LESSOR from the bank concerned, or failing that, pay the LESSOR a security deposit of an identical amount, so that the LESSOR has, in any event, a guarantee equal to three (3) months’ rent excluding taxes and charges.
In the event of assignment or contribution of the Lease, the assignee or successor shall provide the LESSOR with a Bank Guarantee equivalent to that referred to above or a cash security deposit equal to 3 months’ rent excluding taxes and charges, as a condition of validity of the assignment or contribution.
In the event of sale of the Building, the new owner will automatically benefit from this Bank Guarantee. The same applies to all successive owners.
It is specified that in the event of continuation of the Lease beyond its expiry or in the event of renewal, the LESSEE shall provide the LESSOR, in exchange for the initial Bank Guarantee, with a new Bank Guarantee, under identical terms and conditions no later than the expiry date of the initial Bank Guarantee, so that the Lease cannot be continued or renewed without a guarantee, for a duration corresponding to the term of the renewed Lease plus six (6) months.
VI. 5- RENEWAL RENT
It is agreed that in the event of renewal of the Lease, the rent for the renewed Lease will be set at the rental value of the Leased Premises, as determined under the conditions of Articles VI.5.1 and VI.5.2 below, the LESSEE irrevocably waiving, insofar as necessary, the right to invoke the capping rule of Article L. 145-34 of the French Commercial Code.
The LESSEE also expressly waives its right to invoke the provisions of Article L. 145-34 of the French Commercial Code, including its last paragraph.
Unless expressly stated otherwise, all other clauses and conditions of the Lease will be maintained and applied under the renewed Lease.
VL5.1. Rental value
For the determination of the rental value, the Parties declare that they are bound by the following stipulations:
Will be taken into account:
| • | the rents in force on the day of renewal of leases freely debated between a landlord and lessee, either when the new premises are leased, or on the occasion of Lease modifications, or on the occasion of amicable or judicial lease renewals, in respect of the two years preceding renewal; |
| • | properties comparable to the Leased Premises, i.e. |
| • | of the same nature as the Leased Premises; and |
| • | having the same characteristics as those of the Leased Premises (standard of quality, construction, integrated services, technical equipment, functionality), preferably with reference to equivalent surface areas, or to other reference criteria if this information is lacking, on condition that they are comparable. |
The surface areas contractually determined by the Parties shall be binding on any expert required to give an opinion on the rental value of the Leased Premises. They may in no way be weighted or assigned any coefficient whatsoever by the said expert.
This Article is a decisive factor in the undertaking of the Parties, without which they would not have entered into the Lease, and will be binding on any expert having to give an opinion on the amount of the rental value of the Leased Premises.
VI.5.2. Determination of rental value
The rental value of the Leased Premises will be established in accordance with the following stipulations, which the Parties may not depart from under any circumstances:
Each Party will choose an expert from the list of real estate appraisers appointed by the Versailles Court of Appeal. Each Party shall bear the fees and expenses of its expert. The experts appointed by the Parties will work together or separately to determine the rental value of the Leased Premises, on the basis of the definition set out above in Article VI.5.1.
At the end of their work, if they reach agreement on the said rental value, it will be binding on the Parties.
Should the experts chosen by the Parties be unable to agree on the rental value, they will appoint a third expert to determine the rental value as defined above. This third expert must not have worked for one of the Parties in the eighteen (18) months preceding their appointment. If the two experts do not agree on the third expert to be appointed, the most diligent Party will refer the matter to the President of the Court of Justice of the location of the Building, who will appoint the said expert.
The experts will act under a common interest mandate, in the same way as the third party responsible for determining the price in sales law (Article 1592 of the French Civil Code).
They must render their decision within two (2) months of their referral. The decision taken by mutual agreement between the two experts appointed by the Parties or that taken by the third expert chosen by the said experts or appointed by the court will be irrevocably binding on the Parties and will not be subject to appeal.
This procedure does not affect the LESSOR’s right to refuse renewal of the Lease or the LESSEE’s right to terminate its Lease under the conditions set out in Article V1.5.3 below.
VIL.5.3 Right of option
By express agreement between the Parties, they will, no later than at the expiry of a period of two (2) months following notification (which will be made by the most diligent Party) of the decision of the expert required to give their opinion on the amount of the rental value of the Leased Premises under the conditions of Article VI.5.2 above, draw up a new lease under the conditions set by the said decision, unless the LESSEE waives renewal or the LESSOR refuses renewal.
| i. | If, within this two (2) month period, the LESSEE waives renewal of the Lease, it shall notify the LESSOR of its decision by extrajudicial act. In this case, the LESSEE undertakes to pay the LESSOR the rent referred to in Article XVI below, indexed annually in accordance with the conditions of the Lease, for a period of six months (6 months) from the date of notification to the LESSOR of its waiver of renewal, even if the LESSEE vacates the Leased Premises before the expiry of the aforementioned period of six months (6 months). |
| ii. | If, within this two (2) month period, the LESSOR refuses to renew the Lease, it will notify its decision to the LESSEE by extrajudicial act. In this case, the provisions of the French Commercial Code will apply (except for the option exercise period, which is extended to two (2) months in accordance with this Article). |
ARTICLE VII - TERMINATION - INTEREST
VII.1 - TERMINATION CLAUSE
Should the LESSEE fail to pay a single term of rent when due, or to perform any of the provisions set out in this Lease, and after one month has elapsed since a summons to pay or to perform the outstanding provision has remained without effect, containing a declaration by the LESSOR of its intention to avail itself of this clause, this Lease will be terminated immediately and by operation of law if the LESSOR sees fit, without the need to comply with any formality, the interim relief judge being competent, if necessary, to order the eviction of the LESSEE, subject to damages.
VII.2 - TERMINATION
The Parties hereby waive their right to unilaterally terminate the Lease pursuant to Article 1226 of the French Civil Code, but retain the right to request termination of the Lease in accordance with the provisions of Article 1227 of the French Civil Code.
VII.3 - INTEREST ON ARREARS
In addition, in the event of non-payment of the rent due by the LESSEE on the due date following a formal notice that has remained unanswered for a period of fifteen days, the LESSOR will receive late payment interest at the rate of 1% per month from the due date, each month started being considered as a full month.
ARTICLE VIII - JURISDICTION
For any dispute arising in connection with the interpretation or performance of this Lease, the Parties agree to bring their dispute before the courts of the place where the Leased Premises are located.
ARTICLE IX - ADDRESS FOR SERVICE
For the performance of this Lease, the Parties elect domicile at their registered offices.
ARTICLE X - FEES
Each Party will bear its own costs and fees for drafting and negotiating this Lease.
The LESSEE shall bear all expenses incurred by the LESSOR in connection with actions validly brought against the LESSEE to obtain enforcement of the clauses and conditions of this Lease, once the LESSEE has been recognized as at fault under a court decision.
ARTICLE XI - MISCELLANEOUS PROVISIONS
1) Any modification of this Lease may be made only by an express written document in the form of a bilateral act or an exchange of letters recording this agreement.
It is formally agreed that any tolerances on the part of the LESSOR relating to the clauses of this Lease, however frequent or long they may last, may not be considered as constituting a novation or bringing about a modification or deletion of the clauses and conditions of this Lease, nor as generating any right whatsoever; the LESSOR may always terminate them without notice.
2) The preliminary statement forms an integral part of the contractual provisions herein.
3) The nullity of any one of the stipulations herein will not entail the nullity of the whole Lease, and the Parties hereby undertake to negotiate in good faith to replace the stipulation in question with a stipulation having an equivalent effect.
4) Notwithstanding the provisions of Articles 1219 and 1220 of the French Civil Code, the Parties expressly waive their right to refuse or suspend performance of their obligations in the event of non-performance by the co-contracting Party or in the event that it is clear that the co-contracting Party will not perform when due, without prejudice to the right of the aggrieved Party to take legal action.
5) Processing of personal data
In the context of the signature of this Lease, each of the Parties may receive or have access to personal data protected by regulations relating to the protection of personal data including the provisions of the French Act No. 78-17 of January 6, 1978, on Data Processing, Files and Freedoms amended by Act No. 2018-493 of June 20, 2018 (the Data Protection Act), as well as Regulation (EU) 2016/679 of the European Parliament and of the Council of April 27, 2016 (the GDPR), hereinafter referred to together as the Regulations.
The terms defined in the GDPR and listed below have the meaning given to them by the GDPR.
Contacts:
The LESSOR’s Data Protection Officer can be contacted at the following address: dpo@covivio.fr
The LESSEE’s Data Protection Officer can be contacted at the following address: pauline.parant@dbv-technologies.com
With regard to the personal data of each Party processed by the other Party in the context of their contractual relationship
In the context of this Lease and in order to ensure the proper performance of this Lease and to pursue their legitimate interest, each Party, acting in its capacity as data controller, is likely to process personal data of employees, collaborators, agents, representatives, in particular corporate officers, legal representatives, where applicable beneficial owners or any natural person responsible for representing each of the Parties in its communications with the other Party, all of these persons being hereinafter referred to as the “Collaborators”.
Information concerning the LESSOR acting as Data Controller:
The Data Controller is the LESSOR acting jointly with the entities of the Covivio Group (Covivio SA, a French public limited company, registered in the Metz Trade and Companies Register under number RCS B 364 800 060, whose registered office is located at 18 Avenue François Mitterrand, 57000 METZ as well as its controlled subsidiaries within the meaning of Articles L. 233-3-I and II of the French Commercial Code) (together hereinafter referred to as the “Data Controller”).
Information concerning the LESSEE acting as Data Controller:
The Data Controller is the LESSEE acting jointly with DBV Technologies, a French public limited company, registered in the Nanterre Trade and Companies Register under number 441 772 522, whose registered office is located at 177-181 avenue Pierre Brossolette in MONTROUGE (92120).
The personal data relating to the Collaborators of the Parties processed for the conclusion of the Lease or its performance concern surnames, first names, addresses, e-mails, telephone numbers, where applicable, copies of proof of identity or data appearing on invoices (hereinafter the “Personal Data”).
In the context of this Lease, the Collaborators of the Parties are informed that the information collected by the other Party may be subject to processing, whether automated or not, for which each Party acts in the capacity of “Data Controller”.
The purpose of this processing is to manage the contractual relationship and the performance of the Lease between the Parties.
The legal basis for data processing is the performance of this Lease and the legitimate interest of each Party.
The Personal Data is used only by the internal departments of each Party involved in the performance of the Lease and their technical service providers, and each Party undertakes to ensure or have ensured the security and confidentiality of such data in accordance with the Regulations.
Personal Data is kept by each Party for the duration of the Lease, plus any statutory limitation periods.
By way of exception, certain “contact data” type Personal Data of the employees of each Party (in particular surnames, first names, business addresses, business e-mail addresses, business telephone numbers, functions, name of employer) may be kept for a longer period insofar as the data subject does not object, for the purposes of continuing the business relations between each Party.
In the event that Personal Data is transferred outside the European Union or the European Economic Area, each Party undertakes to comply with the Regulations specific to this type of transfer as specified in Articles 44 to 49 of the GDPR.
In accordance with the Regulations, individuals concerned by the processing of their Personal Data have a right of access, rectification and deletion.
The Personal Data relating to the Collaborators of the Parties processed for the conclusion of the Lease or its performance concerns surnames, first names, addresses, e-mails, telephone numbers, where applicable, copies of proof of identity or data appearing on invoices (hereinafter the “Personal Data”).
In the context of this Lease, the Collaborators of the Parties are informed that the information collected by the other Party may be subject to processing, whether automated or not, for which each Party acts in the capacity of “Data Controller”.
The purpose of this processing is to manage the contractual relationship and the performance of the Lease between the Parties.
The legal basis for data processing is the performance of this Lease and the legitimate interest of each Party.
The Personal Data is used only by the internal departments of each Party involved in the performance of the Lease and their technical service providers, and each Party undertakes to ensure or have ensured the security and confidentiality of such data in accordance with the Regulations.
Personal Data is kept by each Party for the duration of the Lease, plus any statutory limitation periods.
By way of exception, certain “contact data” type Personal Data of the employees of each Party (in particular surnames, first names, business addresses, business e-mail addresses, business telephone numbers, functions, name of employer) may be kept for a longer period insofar as the data subject does not object, for the purposes of continuing the business relations between each Party.
In the event that Personal Data is transferred outside the European Union or the European Economic Area, each Party undertakes to comply with the Regulations specific to this type of transfer as specified in Articles 44 to 49 of the GDPR.
In accordance with the Regulations, individuals concerned by the processing of their Personal Data have the right to access, rectify and delete their Personal Data, insofar as the data is not necessary for the performance of the Lease, as well as the right to request a restriction on the processing of their Personal Data and, where applicable, a right to request the portability of their Personal Data.
These rights may be exercised by writing to the registered offices of the Parties as indicated at the top of this Lease or by writing to the above-mentioned contacts.
In all cases, data subjects may lodge a complaint with the competent data protection authority.
Each Party will use its best efforts to inform its Collaborators concerned by the processing of their Personal Data of the provisions set forth in this Article.
6) Fight against money laundering and corruption
The LESSEE undertakes, on its own behalf and on behalf of the companies in its group (i.e. any entity held directly or indirectly within the meaning of Article L. 233-3 I and II of the French Commercial Code), to comply with the laws and regulations applicable to the fight against (i) corruption, (ii) money laundering and (iii) the financing of terrorism (“AML/CFT”), (including the provisions of the French Penal Code punishing acts of corruption and French law no. 2016-1691 of December 9, 2016, and, where applicable, foreign laws and regulations of extraterritorial scope insofar as the latter are applicable), to implement any appropriate procedure for this purpose, and to justify this to the LESSOR upon the latter’s request.
The LESSEE shall behave in a professional and ethical manner in its business dealings and, in particular, shall not engage in influence peddling or corruption of any kind (active or passive, financial or otherwise, directly or indirectly through a third party).
Corruption includes, but is not limited to, any behavior by which a person grants, requests or accepts benefits in kind or in money, including excessive remuneration for services rendered, undue advantages, gifts or anything else of value, insider influence, extortion, embezzlement, in order to obtain, retain or grant business in the context of national or international activities.
Identification of Parties to the transaction
The LESSOR hereby informs the LESSEE that it is subject to the rules set forth in the applicable legislation concerning the fight against money laundering and the financing of terrorism, as set forth in Articles L. 561-1 et seq. of the French Monetary and Financial Code, and that, as such, the LESSOR has a duty of vigilance during the business relationship it maintains with its counterparty, resulting, in particular, in the collection of the information mentioned in the KYC form in Appendix 11.
The LESSEE undertakes to provide the information and supporting documents mentioned in the “KYC form” in Appendix 11 at each renewal of the Lease. The LESSEE therefore acknowledges that the LESSOR may terminate this Lease without recourse or compensation on either side if it does not receive the said information and supporting documents, which the LESSEE expressly accepts.
II - SPECIFIC CONTRACTUAL PROVISIONS
Having freely negotiated this Lease, the Parties agree to the following special provisions, which are intended to supplement and, where applicable, derogate from the general provisions.
ARTICLE XII - DESIGNATION OF LEASED PROPERTY
The Leased Premises are located in CHÂTILLON (Hauts-de-Seine) 82, 84, 86, 88 and 90, rue Pierre Semard, 35, 37 and 39, rue Etienne Deforges and 107, 109, 111, 113 and 115, avenue de la République, and consist of:
| • | Lot A of R+2, identified on the attached plan (Appendix 1), representing for information purposes a gross leasable area of 2,446.7 m2 TUFA of office space (including the share of common areas and use of the inter-company restaurant) broken down as follows: |
| • | private areas: 1,815.9 m2 TUFA |
| • | share of common areas excluding inter-company restaurant: 381.9 m2 TUFA |
| • | share of inter-company restaurant: 248.9 m2 TUFA |
| • | 30 underground parking spaces for cars, including three equipped with electric charging stations |
| • | 5 underground parking spaces for two-wheeled motorcycles. |
The TUFA corresponds to the total useful floor area as defined in the appended statement (Appendix 1).
ARTICLE XIII - PURPOSE
The Leased Premises will be used exclusively for offices, with the LESSEE allocating between 180 and 200 m2 to R&D technical premises and ancillary storage for small materials.
ARTICLE XIV - DURATION
This Lease is granted and accepted for a term of ten full and consecutive years, commencing on the Effective Date of the Lease referred to in Article XV below.
Notwithstanding the provisions of Article IV, the LESSEE waives the right to give notice at the end of the first three-year period, so that the Lease is concluded for a firm term of six years.
However, the LESSEE will have the option of terminating the Lease:
| • | at the end of the 3rd year from the Effective Date of the Lease, subject to the payment of an indemnity to the LESSOR of €900,731 exclusive of tax (nine hundred thousand seven hundred and thirty-one euros exclusive of tax), subject to giving the LESSOR 6 months’ notice of termination; |
| • | at the end of 4.5 years from the Effective Date of the Lease, in return for payment of an indemnity to the LESSOR of €450,365.50 exclusive of tax (four hundred and fifty thousand three hundred and sixty-five euros and fifty cents exclusive of tax), subject to giving the LESSOR 6 months’ notice of termination. |
The amount of the applicable indemnity shall then be paid by the LESSEE to the LESSOR no later than 15 days before the effective date of the notice, failing which the notice will be null and void.
ARTICLE XV - REFERENCE DATE
| • | Effective Date of the Lease: April 16, 2024. |
The Effective Date of the Lease may be brought forward depending on the progress of the LESSEE’s fitting-out work. In such a case, the Parties will enter into an amendment to the Lease in order to record the new Effective Date of the Lease.
ARTICLE XVI - ANNUAL BASE RENT
This Lease is granted and accepted in consideration of an annual base rent of 831,444 euros excluding VAT (eight hundred and thirty-one thousand four hundred and forty-four euros excluding VAT), plus VAT payable by the LESSEE.
For information, the rent is allocated as follows:
| • | €782,944/year excl. VAT and charges for offices (including the share of common areas and inter-company restaurant), i.e. €320/m 2/year excl. and charges, |
| • | €45,000/year excl. VAT and charges for parking spaces for cars, i.e. €1,500/space/year excl. VAT and charges, |
| • | €3,500/year excl. VAT and charges for parking spaces for two-wheeled motorcycles, i.e. €700/space/year excl. VAT and charges. |
Exceptionally, the LESSOR grants the LESSEE a rent-free allowance of €1,247,166 (one million two hundred and forty-seven thousand one hundred and sixty-six euros), corresponding to 18 months of the annual base rent excluding VAT and charges (the Deductible).
The Deductible applies exclusively to the rent, so that all taxes, charges, fees and dues remain payable by the LESSEE from the Effective Date of the Lease. The Deductible will therefore apply from the Effective Date of the Lease, to the first rent payments and until the above amount has been paid.
ARTICLE XVII - REFERENCE INDEX
Base index: Latest French index of rents for tertiary activities (ILAT) published on the Effective Date of the Lease.
Indexation date on the anniversary of the Effective Date of the Lease and for the first time 12 months after that date.
ARTICLE XVIII - SECURITY DEPOSIT
In accordance with the provisions of Article VI.4.1, the LESSEE hereby pays the LESSOR a security deposit of €207,861 (two hundred and seven thousand eight hundred and sixty-one euros), which will be revised in accordance with the provisions of this Article.
The LESSOR has the option of replacing this security deposit with a Bank
Guarantee under the conditions set out in Article VI.4.2.
ARTICLE XIX - CLAIMS DECLARATION
In application of Article L. 125-5 IV of the French Environment Code, the LESSOR declares that during the period of its ownership, the property concerned by this Lease has not suffered any loss giving rise to the payment of an indemnity in application of Article L. 125-2 or Article L. 128-2 of the French Insurance Code and that, furthermore, it has not itself been informed of any such loss in application of this provisions.
ARTICLE XX - INTER-COMPANY RESTAURANT
The Building on which the Leased Premises depend includes an inter-company restaurant, made available to the lessees for the supply of meals to be consumed on the premises by their staff, to the exclusion of any other use.
The LESSEE wishes to become a member of the group that manages the inter-company restaurant (in Appendix 12).
Consequently, the LESSEE irrevocably undertakes to perform all the obligations, in particular the financial obligations, relating to the inter-company restaurant and mentioned below; it will also comply with any special provisions laid down for the inter-company restaurant by the Building’s internal regulations (Appendix 13) as of the Effective Date of the Lease.
The LESSEE expressly undertakes from the Effective Date of the Lease and throughout the term of the Lease, both personally and, where applicable, as a member of the association or group to:
| • | use the inter-company restaurant for its intended purpose as defined above. |
| • | be personally responsible for the operation of the inter-company restaurant; consequently, the LESSEE shall comply with the internal regulations defining the terms of use of the inter-company restaurant by its staff, and shall take out all necessary contracts, in particular the contract with the restaurant owner if this is necessary, as well as all appropriate insurance; in any event, the LESSEE alone shall assume full responsibility for any damage that may be caused to persons or property as a result of or in connection with the use of the inter-company restaurant, so as to save and hold the LESSOR harmless in this respect, the LESSEE expressly waiving any recourse against the LESSOR. |
| • | bear all costs relating to the materials and equipment of all kinds required to operate the inter-company restaurant, in accordance with the inter-company restaurant group regulations and the inter-company restaurant provision agreement in Appendix 12. |
| • | assume responsibility for the cost of the upkeep of the inter-company restaurant premises and for repairs of all kinds, in accordance with the inter-company restaurant group regulations and the inter-company restaurant provision agreement in Appendix 12. |
| • | bear the share of the charges for the premises housing the inter-company restaurant and all related taxes, expenses and costs, including property tax. |
ARTICLE XXI - LESSEE’S FITTING-OUT WORK
The LESSEE wishes to carry out, at its own expense and under its own responsibility, in the Leased Premises and as from the Date of Early Availability as defined in Article XXII below, fitting-out work, which must be validated prior to its implementation by the LESSOR in accordance with the terms and conditions described in Article V.5.1 of the Lease.
The LESSEE is responsible for obtaining any administrative authorizations required for its work.
The LESSOR grants the LESSEE, on an exceptional and commercial basis, support in the form of a financial contribution toward the completion of the LESSEE’s fitting-out work for a maximum overall amount of 554,296 euros (five hundred and fifty-four thousand two hundred and ninety-six euros), corresponding to 8 months’ base rent excluding VAT and charges, it being specified that the LESSOR will be responsible only for work of a real estate nature from an accounting standpoint.
The LESSOR undertakes to reimburse the LESSEE, within the overall budget of €554,296 exclusive of tax, on presentation of invoices issued by the LESSEE to which must be attached the receipted invoices of the companies that carried out the LESSEE’s work in the Leased Premises. The LESSOR will pay each invoice within forty-five (45) days of receipt of the invoice.
This financial contribution by the LESSOR will be made only if the LESSEE has sent the invoices for the work with supporting documents before December 31, 2024. After this deadline, invoices received by the LESSOR will no longer be accepted under this clause.
ARTICLE XXII - EARLY AVAILABILITY OF LEASED PREMISES
The LESSEE has expressed the wish to have access to the Leased Premises prior to the Effective Date of the Lease in order to carry out its fitting-out work. Subject to the signature of an early availability agreement between the Parties (Early Availability Agreement) entered into today between the Parties under a separate act, the LESSOR agrees to make the Leased Premises available to the LESSEE in advance from November 20, 2023 (hereinafter the “Date of Early Availability”) until April 15, 2024.
This early availability is granted for the sole purpose of enabling the LESSEE to carry out its fitting-out work, any operation of the Leased Premises during this period being prohibited.
The Parties agree that this early availability is granted free of charge and on the express condition that the LESSEE complies with the Early Availability Agreement in its entirety. It will terminate automatically on the Effective Date of the Lease, at which time all the stipulations of the Lease, in particular those relating to the payment of the rent, charges and taxes, will apply immediately and automatically.
However, the LESSEE will bear the costs associated with the completion of the LESSEE’s fitting-out work (fluids, insurance, etc.) from the Date of Early Availability.
ARTICLE XXIII - PREFERENTIAL RIGHT
| 1. | The LESSEE may be interested in leasing Lot B on the second floor, with a surface area of approximately 1,357.1 m2 TUFA (including the share of common areas and inter-company restaurant) (Lot B), which is currently vacant, but is unable to take a position at the date of signature of this Lease. The LESSOR hereby grants the LESSEE, under this Lease and for its entire term, a preferential right to lease the premises of Lot B under the following conditions (the Preferential Right). |
| 2. | The LESSOR will notify the LESSEE by registered letter with acknowledgment of receipt (with a copy by e-mail) of any rental offer sent or received and which the LESSOR intends to accept concerning Lot B (the Notification of Offer). The LESSEE will have a period of 7 (seven) working days from receipt of the Notification of Offer e-mail to notify the LESSOR by registered letter with acknowledgment of receipt (with a copy by e-mail) of its wish to lease the said Lot B. |
The LESSEE’s response must be made under the same conditions, in particular financial conditions, as the Notification of Offer.
It is specified that in the absence of a response from the LESSEE within 7 (seven) working days or if no lease is signed between the Parties within 30 (thirty) days of the LESSEE’s response, this Preferential Right will be deemed to have been exercised in respect of Lot B, and the LESSOR will regain full freedom to proceed with the lease of Lot B to any third party in return for (i) an annual headline rent at least equal to 95% of the annual headline rent proposed in the Notification of Offer and (ii) support measures per binding lease year which may not exceed by more than 15% the support measures per binding lease year proposed to the LESSEE in the Notification of Offer.
In the event that the LESSOR is prepared to enter into a lease with a third party on more favorable terms in return for (i) an annual headline rent of less than 95% of the annual headline rent proposed to the LESSEE and/or (ii) support measures per binding lease year exceeding by more than 15% the support measures per binding lease year proposed to the LESSEE, it will notify the LESSEE and propose this new headline rent and/or these new support measures, and the deadlines of 7 (seven) working days for the LESSEE’s response and 30 (thirty) days for the signing of the Lease will then apply.
| 3. | In the event that, after waiver of the Preferential Right under the conditions set out in point 2 above, Lot B is leased to a third party and this third party gives notice to vacate, the LESSOR will notify the LESSEE by registered letter with acknowledgment of receipt (with a copy by e-mail) of a rental offer concerning Lot B (the Post-Notification to Vacate). |
The LESSEE will have a period of 7 (seven) working days from receipt of the e-mail of the Post-Notification to Vacate to notify the LESSOR by registered letter with acknowledgment of receipt (with a copy by e-mail) of its wish to lease the said Lot B.
The LESSEE’s response must be made under the same conditions, particularly financial, as the Post-Notification to Vacate. It is specified that in the absence of a response from the LESSEE within 7 (seven) working days or if no lease is signed between the Parties within 30 (thirty) days of the LESSEE’s response, this Preferential Right will be deemed to have been exercised in respect of Lot B, and the LESSOR will regain full freedom to proceed with the lease of Lot B to any third party in return for (i) an annual headline rent at least equal to 95% of the annual headline rent proposed in the Post-Notification to Vacate and (ii) support measures per binding lease year which may not exceed by more than 15% the support measures per binding lease year proposed to the LESSEE in the Post-Notification to Vacate.
In the event that the LESSOR is prepared to enter into a lease with a third party on more favorable terms in return for (i) an annual headline rent of less than 95% of the annual headline rent proposed to the LESSEE and/or (ii) support measures per binding lease year exceeding by more than 15% the support measures per binding lease year proposed to the LESSEE, it will notify the LESSEE and propose this new headline rent and/or these new support measures, and the deadlines of 7 (seven) working days for the LESSEE’s response and 30 (thirty) days for the signing of the Lease will then apply.
ARTICLE XXIV – GOVERNING LANGUAGE
This Lease Agreement is drafted in and shall be governed by the French language. The French version of this Lease Agreement is the sole legally binding version. In the event of any dispute arising from the interpretation of this Lease Agreement, or any conflict in meaning between the English and French versions, the French version shall prevail in all respects. The English translation provided herewith is for informational purposes only and shall not be accorded any legal weight.
Paris, October 2, 2023, in two counterparts
| SCI DANTON MALAKOFF
By Céline Leonardi
[SIGNATURE] |
DBV TECHNOLOGIES
By Caroline Danière
[SIGNATURE] |
APPENDICES
| • | Appendix 0 - List of documents with which the LESSEE has been acquainted prior to signing the Lease |
| • | Appendix 1 - Plans and surface surveys |
| • | Appendix 2 - Lessee specifications |
| • | Appendix 3 - Statement of risks |
| • | Appendix 4 - Energy performance diagnosis |
| • | Appendix 5 - Environmental performance appendix |
| • | Appendix 6 - Inventory of LESSOR/LESSEE categories of charges, taxes, fees and dues |
| • | Appendix 7 - Summary statement of work carried out in the three years preceding the lease and its cost and statement of projected work for the three years following the lease and projected budget |
| • | Appendix 8 - Safety instructions |
| • | Appendix 9 - Bank details of the LESSOR |
| • | Appendix 10 - Security deposit model |
| • | Appendix 11 - Know your customer “KYC form” |
| • | Appendix 12 - Signed IRO inter-company restaurant group regulations |
| • | Appendix 13 - Internal regulations |
Exhibit 10.3
LEASE AGREEMENT
This LEASE AGREEMENT (this “Lease”) is dated MARCH 28, 2022, and is between SIG 106 LLC, a New Jersey limited liability company (“Landlord”), and DBV TECHNOLOGIES, INC., a Delaware corporation (“Tenant”).
Landlord is the owner of certain real property located at 106 Allen Road, Basking Ridge, Bernards Township, New Jersey (“Real Property”) and that certain multi-level office building located thereon deemed to contain one hundred thirty-three thousand, six hundred eighty-four (133,684) square feet of rentable office area (“Building”). The Building and the Real Property are collectively referred to as the “Property.”
Landlord desires to lease to Tenant, and Tenant desires to lease from Landlord, that certain space on the fourth (4th) floor of the Building identified as Suite 400 and containing an agreed-upon five thousand, seven hundred ninety-nine (5,799) rentable square feet, as depicted on Exhibit A attached hereto and made a part hereof (the “Premises”), for the term and subject to the terms, covenants, agreements, and conditions in this Lease.
The parties therefore agree as follows:
BASIC PROVISIONS
(1) Base Rent: the annual amount payable as set forth in the following table:
| Period of the Lease Term: |
Annual Base Rent Per RSF: |
Annual Base Rent Rate: |
Monthly Base Rent Payment: |
|||||||||
| Lease Months 1 through 12 |
$ | 25.50 | $ | 147,874.50 | $ | 12,322.88 | ||||||
| Lease Months 13 through 24 |
$ | 26.00 | $ | 150,774.00 | $ | 12,564.50 | ||||||
| Lease Months 25 through 36 |
$ | 26.50 | $ | 153,673.50 | $ | 12,806.13 | ||||||
| Lease Months 37 through 38 |
$ | 27.00 | $ | 156,573.00 | $ | 13,047.75 | ||||||
(2) Base Rent Credit: a credit against Tenant’s obligation to pay Base Rent in the amount of twenty-four thousand, six hundred forty-five and 76/100 dollars ($24,645.76).
(3) Broker(s): Cushman & Wakefield (representing Landlord), and Jones Lang LaSalle (representing Tenant).
(4) Building Hours: 8:00 a.m. to 6:00 p.m. Monday through Friday (excluding Holidays).
Page 1 of 26
(5) Commencement Date: the later of (a) the date on which the Premises are delivered to Tenant in “AS-IS, WHERE-IS” condition, or (b) April 1, 2022.
(6) Electric Charge: the amount of one and 75/100 dollars ($1.75) per rentable square foot of the Premises per year.
(7) Expiration Date: the last day of the thirty-eighth (38th ) Lease Month.
(8) Holidays: New Year’s Day, Memorial Day, Independence Day, Labor Day, Thanksgiving Day and Christmas Day and any additional holidays commonly recognized by the U.S. Federal Government.
(9) [intentionally omitted].
(10) Landlord Notice Address: c/o Signature Acquisitions, LLC, Attn: Richard J. Travaglini, 20 Commerce Drive, Suite 140, Cranford, NJ 07016.
(11) Landlord Payment Address: c/o Signature Acquisitions, LLC, 20 Commerce Drive, Suite 140, Cranford, NJ 07016. At Landlord’s option upon at least thirty (30) days’ prior written notice, Tenant shall make all payments by means of electronic transfer of funds.
(12) Lease Month: each calendar month during the Term commencing (a) on the Commencement Date if the Commencement Date falls on the first day of a calendar month, or (b) if the Commencement Date is not the first day of a calendar month, on the first day of the month following the Commencement Date, with the first Lease Month to include the initial partial calendar month in which the Commencement Date occurs.
(13) [intentionally omitted] .
(14) Operating Charges Base Year: calendar year 2022.
(15) Parking Allotment: a ratio, rounded down to the nearest whole number, equal to four (4) parking spaces for every 1,000 rentable square feet of the Premises, which as of the date of this Lease equals twenty-three (23) parking spaces.
(16) Real Estate Taxes Base Year: calendar year 2022.
(17) Security Deposit Amount: the amount of twenty-four thousand, six hundred forty-five and 76/100 dollars ($24,645.76).
Page 2 of 26
(18) Tenant Notice Address:
| Prior to Commencement Date: | Sebastien.Robitaille@dbv-technologies.com | |
| On and After Commencement | DBV Technologies | |
| Date: | 106 Allen Road, Suite 400 | |
| Basking Ridge, Bernards Township, NJ 07920 | ||
| Attention: Chief Legal Officer | ||
| Email: michele.robertson@dbv-technologies.com | ||
| With a copy to: | Sebastien.Robitaille@dbv-technologies.com | |
(19) Tenant’s Proportionate Share: four and 34/100 percent (4.34%) (i.e., a fraction, expressed as a percentage, the numerator of which is the number of rentable square feet in the Premises, and the denominator of which is the number of rentable square feet in the Building).
(20) Term: the period commencing on the Commencement Date and ending on the Expiration Date, consisting of thirty-eight (38) Lease Months.
LEASE TERMS
1. Premises; Term; FF&E.
1.1. Demise. Landlord hereby leases to Tenant, and Tenant leases from Landlord the Premises for the Term. If Landlord is unable to deliver possession of the Premises or any portion thereof on any specified date because of the holding-over or retention of possession of any tenant or other occupant thereof, or for any other reason, except as set forth in Section 1.4, Landlord shall have no liability to Tenant for failure to give possession on said date and the validity of this Lease shall not be impaired under such circumstances. Subject to Landlord’s maintenance obligations set forth in Section 6.2 hereof, upon accepting possession of the Premises, Tenant will conclusively be deemed to have accepted the Premises in its then “AS IS, WHERE IS” condition.
1.2. Certificate Affirming the Commencement Date. Promptly after the Commencement Date is ascertained, Landlord and Tenant shall execute the certificate attached to this Lease as Exhibit E. Failure to execute said certificate shall not affect the Commencement Date or Expiration Date of the Term.
1.3. FF&E. Tenant and Landlord acknowledge and agree that certain items of furniture, fixtures, and equipment owned by Landlord are located within the Premises as of the date of this Lease (such items, the “FF&E”). An inventory list of the FF&E is attached hereto as Exhibit C and made a part hereof. Landlord hereby grants to Tenant a license to use the FF&E during the Te.rm. Tenant acknowledges and agrees that the license granted herein does not grant Tenant any property or ownership rights in the FF&E, and this license is personal to Tenant and may not be assigned by Tenant in whole or in part except in connection with a Permitted Transfer (defined in Section 8.5). Notwithstanding Landlord’s continuing ownership of the FF&E, during the Term the parties shall deem the FF&E to be Tenant’s personal property for all purposes arising from or related to the Lease, and Tenant shall maintain the FF&E in good condition. Tenant represents that it has examined the FF&E and is fully-satisfied with the condition thereof. Tenant shall accept the FF&E on the Commencement Date strictly in its “AS IS, WHERE IS” condition, WITH ALL DEFECTS, including, without limitation, the nature, condition and usability thereof, and will be deemed to have assumed all risk, if any, resulting from any latent defects.
Page 3 of 26
Landlord makes no representations or warranties, express or implied, by operation of law or otherwise, with respect to the FF&E, including but not limited representations or warranties as to suitability, merchantability, or fitness for a particular purpose. None of the Rent payable by Tenant hereunder is applicable to the FF&E, and Tenant shall be permitted to use the FF&E at no cost to Tenant. Notwithstanding anything herein to the contrary, if a Default occurs at any time during the Term and the Lease is terminated, the parties shall deem Tenant’s license to use the FF&E irrevocably rescinded, null, and void.
1.4. Anticipated Commencement Date. It is presently anticipated that the Premises will be delivered to Tenant on or about April 1, 2022 (“Anticipated Commencement Date”); provided, however, that if Landlord does not deliver possession of the Premises by such date, Landlord shall not have any liability whatsoever, and this Lease shall not be rendered void or voidable, as a result thereof. If the Commencement Date does not occur on or before the ninetieth (90th) day after the Anticipated Commencement Date, then, provided no Default exists under this Lease, Tenant shall have the right, as its sole and exclusive remedy, to terminate this Lease by delivering written notice of the exercise of such right to Landlord. Such right of termination may be exercised by Tenant only during the period commencing on the ninety-first (91st) day after the Anticipated Commencement Date and continuing through the tenth (10th) business day thereafter, and if such right is not exercised by Tenant by said tenth (10th) business day, such right shall thereafter lapse and be of no further force or effect. If this Lease is terminated pursuant to this subsection, then neither party shall have any further obligations or liability hereunder to the other party; provided, however, that Landlord shall promptly refund any and all security deposits or advance rent previously deposited by Tenant with Landlord in accordance with the provisions of this Lease. Notwithstanding the foregoing, the Anticipated Commencement Date shall be extended on a day-for-day basis for each day the Commencement Date is delayed as a result of any of the factors or causes described in Section 21.11.
2. Common Areas. Subject to the terms of this Lease, Landlord grants to Tenant, for the benefit of Tenant and its employees, agents, suppliers, guests, and invitees, the non-exclusive right to use during the Term, in common with others entitled to such use, areas and facilities outside the Premises within the Property that are provided and designated by Landlord, from time to time (“Common Areas”). Common Areas include lobbies, plazas, corridors, stairways, bathrooms, lounges, and the parking facilities and the driveways on the Real Property (collectively, the “Parking Facility”). Landlord reserves the right to make alterations to the Common Areas, and to impose reasonable rules and regulations or restrictions governing the use of the Common Areas pursuant to Exhibit D. provided such rules are not inconsistent with the provisions of this Lease. Notwithstanding the foregoing, except as may otherwise be expressly provided in this Lease, the lease of the Premises does not include the right to use the roof, mechanical rooms, electrical closets, janitorial closets, telephone rooms, non-common or non-public areas of any portion of the Building, whether or not any such areas are located within the Premises.
3. Rent.
3.1. Base Rent. From and after the Commencement Date, Tenant shall pay Base Rent in equal monthly installments in advance on the first day of each month during the Term. Concurrently with Tenant’s execution of this Lease, Tenant shall pay an amount equal to one (1) monthly installment of Base Rent, which amount shall be credited toward the monthly installment of Base Rent payable for the first full calendar month of the Term after application of the Base Rent Credit.
Page 4 of 26
If the Commencement Date is not the first day of a month, then the Base Rent from the Commencement Date until the first day of the following month shall be prorated on a per diem basis at the rate of one-thirtieth (1/30th) of the monthly installment of the Base Rent then-payable, and Tenant shall pay such prorated installment of the Base Rent on the Commencement Date.
3.2. Payment. All sums payable by Tenant under this Lease shall be paid to Landlord in legal tender of the United States, without setoff, deduction, or demand (except as may otherwise be expressly provided in this Lease), at the Landlord Payment Address, or to such other party or such other address as Landlord may designate in writing. Landlord’s acceptance of rent after it shall have become due and payable shall not excuse a delay upon any subsequent occasion or constitute a waiver of any of Landlord’s rights hereunder. Base Rent and all other monetary obligations of Tenant to Landlord hereunder, if any, are collectively referred to as “Rent.”
3.3. Operating Charges and Real Estate Taxes. From and after the Commencement Date, Tenant shall pay additional rent pursuant to the terms of Exhibit B attached hereto and made a part hereof.
3.4. Late Payment. If any Rent is not paid to Landlord within five (5) days of its due date, Tenant shall pay to Landlord, as additional Rent, a late charge of eight percent (8%) of the then-late payment. In addition, Tenant shall pay interest on any payments of Rent not received by Landlord when due, as additional Rent, at the rate of 9%, from the date five (5) days after such payment was due until the date full payment (including accrued interest) is received by Landlord. Tenant shall also pay a fee of $150.00 as additional rent for any dishonored check.
3.5. Base Rent Credit and Termination. Landlord shall apply a credit against Tenant’s obligation to pay Base Rent in the amount of the Base Rent Credit on the condition that no Default (defined in Section 15.1) occurs during the Term. Landlord shall apply the Base Rent Credit in two (2) equal installments of $12,322.88 against, respectively, each of the Base Rent payments due for the Lease Months 1 and 2. If a Default occurs, then : (a) Landlord shall cease applying the Base Rent Credit to the extent unapplied, and (b) Landlord and Tenant shall deem any unamortized portion of the Base Rent Credit null and void and Tenant shall immediately pay to Landlord, without further notice or demand, as additional Rent, an amount equal to unamortized Base Rent Credit at the time of such Default.
4. Permitted Use. Tenant shall use and occupy the Premises for general office use and any other use allowed by law and consistent with the first-class image of the Building (the “Permitted Use”), and for no other use or purpose. Tenant shall use the Premises in a careful, safe, and lawful manner, and shall not use the Premises in such a manner as to cause loss, waste or destruction thereto, reasonable wear and tear and casualty damage excepted. Tenant shall comply with all laws concerning the use, occupancy, and condition of the Premises, and all machinery, equipment, furnishings, fixtures, and improvements therein, all in a timely manner at Tenant’s sole expense. If any law requires a use permit or license for the operation of Tenant’s business conducted therein, then Tenant shall obtain and keep current such use permit or license at Tenant’s expense and shall promptly deliver a copy thereof to Landlord following Landlord’s written request therefor. Following the Commencement Date, Landlord shall obtain from the local municipality any certificate of occupancy or use permit which may be required for Tenant’s lawful use of the Premises.
Page 5 of 26
5. Utilities and Services.
5.1. From and after the Commencement Date, Landlord will provide to the Premises: air-conditioning and heating during Building Hours as required in Landlord’s reasonable judgment based upon the applicable season; janitorial service after 5:30 p.m. on Monday through Friday only (excluding Holidays); electric power from the utility provider sufficient for customary lighting purposes and normal office use; standard hot and cold water in Building bathrooms and chilled water in Building drinking fountains; elevator service (with at least one (1) elevator in operation at all times, except in the event of an emergency); and landscaping and snow removal during the seasons they are required. If Tenant requires air-conditioning or heat beyond the Building Hours, then Landlord will furnish the same provided Tenant gives Landlord advance notice of such requirement (by 3:00 p.m. of the same day for extra service needed Monday through Friday, and by 3:00 p.m. on Friday for extra service needed on Saturday or Sunday) (which notice may be provided by telephone). Tenant shall pay for such extra service at the rate of $85.00 per hour per zone. To the extent Tenant provides or contracts for any services relating to any Building structure or system or any service or utility being provided by Landlord to the Premises directly from the supplier (which Tenant shall not be permitted to do without Landlord’s prior written consent, which consent shall not be unreasonably withheld conditioned or delayed), Tenant shall enter into and maintain a service contract therefor with a contractor licensed to do business in the jurisdiction in which the Building is located and otherwise approved by Landlord. Tenant shall have access to the Building twenty-four (24) hours per day each day of the year (except in the event of an emergency). Landlord shall provide a card key (or similar type of) access system to provide access to the Building at all times. A reasonable number of access cards or other means of access shall be provided to Tenant at no cost to Tenant (except that Landlord may charge Tenant for replacement cards). Such access cards shall be issued by Landlord to the specific individuals that are designated by Tenant. Tenant shall not permit anyone, except for Tenant’s employees, permitted subtenants and assigns and authorized guests, agents or invitees, to enter the Building at times other than the Building Hours. All persons entering or exiting the Building at times other than the Building Hours shall, at Landlord’s discretion, be required to sign in and out.
5.2. Tenant shall pay the Electric Charge to Landlord, as additional Rent, in advance of or on the first day of each month during the Term. Landlord shall not be required to furnish, and Tenant shall not install a connected load (including all of Tenant’s equipment and systems, but excluding the Building systems) or otherwise draw, in excess of six (6) watts per rentable square foot of Premises; and, in any event, Tenant’s use of electric energy shall never exceed the capacity of the then existing feeders, risers or wiring installations serving the Premises. If any tax is imposed upon Landlord’s receipts from the sale or resale of electric energy to Tenant (directly or indirectly through a general tax on such receipts) by any federal, state or municipal authority, then Tenant shall pay, or reimburse Landlord, such taxes (or its share thereof) in addition to the other charges for electricity described in this Section 5. Tenant will at all times comply with all reasonable rules and regulations of the utility company that are provided to Tenant by Landlord in writing, to the extent the same are applicable to Tenant’s use of electric energy in the Premises. Landlord shall not in any way be liable or responsible to Tenant for any loss, damage or expense which Tenant may sustain or incur if (a) the supply of electric energy to the Premises is temporarily interrupted, or (b) the quantity or character of electric service is changed or is no longer available or suitable for Tenant’s requirements.
Page 6 of 26
5.3. Notwithstanding the provisions of Section 5.2, Landlord, at Landlord’s expense, may, at any time during the Term, install and maintain one or more electrical submeters to measure Tenant’s demand and consumption with respect to the electricity furnished by Landlord (such submeter(s) being herein called “Tenant’s Submeter”). In the event Tenant’s Submeter is so installed, then from and after the date of such installation Tenant, throughout the remainder of the Term, in lieu of the charges described in Section 5.2 above, shall pay Landlord for such electricity as measured by Tenant’s Submeter as follows: Tenant, for any billing period, shall pay Landlord an amount determined by applying (i) Tenant’s electrical demand (measured in KWs) and consumption (measured in KWHRs) for such period, as measured by Tenant’s Submeter, to (ii) the rate schedule (inclusive of all taxes, surcharges and other charges payable thereunder or in connection therewith) of the utility company serving the Building (herein called the “Utility Company”) which would then be applicable to Tenant if it purchased electricity directly from the Utility Company for such period. Tenant shall pay the amount due for any billing period within thirty (30) days after being billed therefor, which bills Landlord may render from time to time (but no more frequently than monthly). Tenant shall also pay to Landlord an amount equal to the actual costs incurred by Landlord to a meter company or otherwise in respect of having Tenant’s Submeter read and having bills prepared and delivered based upon such readings.
5.4. Tenant shall arrange for the provision of internet and telephone service to the Premises, as Tenant deems necessary in its discretion.
6. Maintenance.
6.1. Tenant’s Maintenance Obligations. Subject to Landlord’s maintenance obligations set forth in Section 6.2, Tenant, at Tenant’s sole cost and expense, shall promptly make all repairs and replacements, and perform all maintenance, in and to the Premises to keep the Premises in good operating condition and repair, in a clean, safe and tenantable condition, and otherwise in accordance with all applicable laws and the requirements of this Lease. Tenant shall likewise maintain all fixtures, furnishings and equipment located in and exclusively serving the Premises and make all required repairs and replacements thereto. Tenant shall also maintain, repair and replace, at Tenant’s sole cost and expense, the Tenant Items, if any, and shall keep in force customary maintenance and service contracts therefor. “Tenant Items” means all non-Building standard supplemental heating, ventilation and air conditioning equipment and systems serving exclusively the Premises and any special tenant areas, facilities and finishes, any special fire protection equipment installed by Tenant, any telecommunications, security, data, computer and similar equipment, cabling and wiring, kitchen/galley equipment and fixtures, all other furniture, furnishings, equipment and systems of Tenant and all Alterations, if any. Tenant shall give Landlord prompt written notice of any defects or damage to the structure of, or equipment or fixtures in, the Premises or any part thereof, or any mold or moisture condition, of which Tenant has knowledge. Tenant shall suffer no waste or injury to any part of the Premises, and shall, at the expiration or earlier termination of the Term, surrender the Premises in an order and condition equal to or better than that on the Commencement Date, except for ordinary wear and tear and as otherwise provided in Section 13. Except as otherwise provided in Section 13, all injury, breakage and damage to the Premises and to any other part of the Building or the Real Property caused by any negligent or willful act or omission of Tenant or any agent of Tenant, shall be repaired by Tenant at Tenant’s expense.
Page 7 of 26
If either an emergency condition exists or the Term has expired or Tenant fails to commence and diligently prosecute to completion repa ir of any such injury, breakage or damage within a reasonable period (not to exceed ten (10) days) following Tenant’s receipt of notice from Landlord, then Landlord shall have the right at Landlord’s option to make any such repair and to charge Tenant for all costs and expenses incurred in connection therewith. Landlord, at Tenant’s expense, shall provide and install replacement lighting, tubes, lamps, bulbs and ballasts for Building standard light fixtures.
6.2. Landlord’s Maintenance Obligations. Landlord, at Landlord’s cost (subject to reimbursement pursuant to Exhibit B, if and to the extent permitted thereby), shall keep clean and in good operating condition the Common Areas, the exterior and common area walls, main lobby in the Building, slab floors, exterior windows, load bearing elements, foundations, roof, and the Building-standard mechanical, electrical, HVAC and plumbing systems, pipes and conduits that are provided by Landlord in the operation of the Building as a whole. Notwithstanding any of the foregoing to the contrary, Landlord shall have no obligation to make any repairs whatsoever brought about by any act or omission of Tenant.
7. Alterations.
7.1. Except for Minor Alterations (as that term is defined herein), Tenant shall not make or permit anyone to make any structural or other alterations, decorations, additions, installations, demolitions, improvements or other changes (“Alterations”) in or to the Premises or the Building without the prior written consent of Landlord, which consent may be withheld or granted in Landlord’s sole and absolute discretion with respect to (1) any Alteration that will or may necessitate any changes, replacements or additions to the load-bearing or exterior walls, non-drop ceilings, partitions (load-bearing or non-demising), columns or floor, or to the fire protection, water, sewer, electrical, mechanical, plumbing, HVAC or other base building systems, of the Premises or the Building (“Structural and System Alterations”), and (2) any Alterations which are visible from the exterior of the Premises; and which consent shall not be unreasonably withheld, conditioned, or delayed with respect to all other Alterations. Notwithstanding the forgoing, provided Tenant delivers reasonable prior notice to landlord, landlord’s consent shall not be required for any Alteration that satisfies all of the following criteria (a “Minor Alteration”): (a) is of a cosmetic nature such as painting, wallpapering, hanging pictures, installing carpeting and installing trade fixtures; (b) is not visible from outside the Premises or Building; (c) will not affect the systems or structure of the Building; (d) does not require a permit; and (e) does not exceed $100,000 in hard construction costs. All Alterations made by Tenant shall be made: (i) in a good, workmanlike, and prompt manner (subject to Force Majeure delays); (ii) using new or comparable materials only; (iii) by a contractor reasonably approved in writing by Landlord; (iv) on days and at times reasonably approved in writing by Landlord; (v) under the supervision of an architect reasonably approved in writing by Landlord, if applicable; (vi) in accordance with plans and specifications reasonably acceptable to landlord; and (vii) in accordance with all applicable laws. Tenant acknowledges that, except with respect to the landlord Work and landlord’s continuing maintenance obligations set forth in this lease, any Alterations are accomplished for Tenant’s account, landlord having no obligation or responsibility in respect thereof. landlord’s approval of any plans and drawings (and changes thereto) regarding any Alterations or any contractor or subcontractor performing such Alterations shall not constitute landlord’s representation that such approved plans, drawings, changes or Alterations comply with applicable laws.
Page 8 of 26
Any deficiency in design or construction, although same had prior approval of Landlord, shall be solely the responsibility of Tenant. All Alterations involving structural, electrical, mechanical or plumbing work, the heating, ventilation and air conditioning system of the Premises or the Building, fire and life safety system, the roof of the Building, or any areas outside of the Premises shall, at Landlord’s election, be performed by Landlord’s designated contractor or subcontractor at Tenant’s expense (provided the cost therefor is competitive). In connection with any Alteration other than a Minor Alteration, Landlord shall be paid a construction supervision fee in an amount equal to three percent (3%) of the total cost of such Alteration, and Tenant shall also reimburse Landlord upon demand for Landlord’s reasonable out-of-pocket costs and expenses for reviewing the plans and specifications therefor. After the completion of an Alteration, and following written request from Landlord, Tenant at its expense shall promptly deliver to Landlord three (3) sets of accurate as-built (or record) drawings and CAD drawings showing such Alteration in place.
7.2. If any Alterations that require Landlord’s consent are made without the prior written consent of Landlord, then Landlord shall have the right, at Tenant’s expense, to so remove and correct such Alterations and restore the Premises and the Building. All Alterations to the Premises or the Building made by either party shall immediately become the property of Landlord and shall remain upon and be surrendered with the Premises as a part thereof at the expiration or earlier termination of the Term; provided, however, that (i) if Tenant is not in default under this Lease, then Tenant shall have the right to remove, prior to the expiration or earlier termination of the Term, all movable furniture, furnishings and equipment installed in the Premises solely at the expense of Tenant, and (ii) Tenant shall remove at its expense all Alterations and other items (including any telecommunications, security, data, computer and similar equipment, cabling and wiring) in the Premises or the Building which Landlord designates in writing for removal at the time Tenant requests Landlord’s consent to make such Alteration. If Tenant fails to return the Premises to Landlord as required by this Section, then Tenant shall pay to Landlord, all reasonable and actual costs (including a construction supervision fee in the amount set forth in Section 7.1) incurred by Landlord in effectuating such return.
7.3. Landlord, at its expense, using Building-standard materials and colors selected by Tenant, shall paint the interior walls and install new carpet in the Premises (the “Landlord Work”). Landlord is under no obligation to make any Alterations in or to the Premises or the Building except for the Landlord Work and as may be otherwise expressly provided in this Lease. Landlord shall use commercially reasonable efforts to complete Landlord’s Work as soon as possible after the Commencement Date. Tenant shall fully and promptly cooperate with Landlord and Landlord’s contractor in order to ensure timely selection of any specifications required for Landlord’s Work. Landlord shall perform Landlord’s Work only once, it being understood that Landlord’s obligation to perform Landlord’s Work is a single, non-recurring obligation. Tenant acknowledges and agrees that, in connection with the performance of Landlord’s Work, Landlord will have access through and across the Premises, and the performance of Landlord’s Work may temporarily and negatively impact Tenant’s use of the Premises. Landlord shall use reasonable construction practices in an effort to minimize any disruption of Tenant’s business operations during the performance of Landlord’s Work, but Landlord shall not be required to incur any extra costs with respect thereto, such as overtime and/or evening or weekend work. Tenant shall not make any claim, and is not entitled to any abatement or reduction of rent, by reason of any interruption caused by the performance of Landlord’s Work.
Page 9 of 26
8. Assignment and Subletting.
8.1. Subject to the terms of Section 8.5 below, Tenant shall not assign, transfer or otherwise encumber (collectively, “assign”) this Lease or all or any of Tenant’s rights hereunder or interest herein, or sublet or permit anyone to use or occupy (collectively, “sublet”) the Premises or any part thereof, without obtaining the prior written consent of Landlord, which consent may be withheld or granted in Landlord’s sole and absolute discretion (subject to the remainder of this Section 8). Notwithstanding the foregoing, provided no Default exists, and subject to Landlord’s rights and Tenant’s obligations pursuant to this Section, Landlord shall not unreasonably withhold, condition or delay its consent to any proposed subletting of the entire or any portion of the Premises or assignment of the Lease in its entirety. For purposes of the immediately preceding sentence, it shall be reasonable for Landlord to withhold its consent if, for example: (i) the proposed subtenant or assignee is engaged in a business, or the Premises will be used in a manner, that is inconsistent with the first-class image of the Building; or (ii) Landlord is not reasonably satisfied with the financial condition of the proposed subtenant or assignee; or (iii) the proposed use of the Premises is not in compliance with Section 4, or is not compatible with the other uses within, and the terms of other leases with respect to, the Building; or (iv) [intentionally omitted]; or (v) the initial Tenant does not remain fully liable as a primary obligor for the payment of all rent and other charges payable by Tenant under this Lease and for the performance of all other obligations of Tenant under this Lease; or (vi) the proposed subtenant or assignee is a governmental or quasi-governmental agency; or (vii) the holders of any mortgages encumbering the Building shall fail to consent (Landlord hereby agreeing to use commercially reasonable efforts to obtain such consent if Landlord approves such transaction); or (viii) the proposed subtenant or assignee is either (A) an existing tenant of the Building (or any parent, subsidiary or affiliate thereof) if Landlord has adequate space available in the Building for a comparable term, or (B) for a period of forty-five (45) days following the submission of a written proposal for the lease of space (and thereafter if a mutual agreement such as a letter of intent is executed within such period), any other person or entity with which Landlord is in the process of negotiating for the rental of space in the Building. If at any time during the Term Tenant desires to assign, sublet or mortgage all or part of this Lease or the Premises, then in connection with Tenant’s request to Landlord for Landlord’s consent where required, Tenant shall give to Landlord a notice containing: the identity of a proposed assignee, subtenant or other party and its business; the terms of the proposed assignment, subletting, or other transaction (including a copy of the proposed document for same); the proposed sublease commencement date; the proposed sublet space; financial statements for the prior two (2) years certified by an authorized officer of the proposed assignee, subtenant, or other party, or a certified public accounting firm, or other evidence of financial responsibility of such proposed assignee, subtenant to other party; and a certification executed by Tenant and such party stating whether or not any premium or other consideration is being paid for the assignment, sublease or other transaction.
8.2. No assignment or right of occupancy hereunder may be effectuated by operation of law or otherwise without the prior written consent of Landlord. Any attempted assignment, transfer or other encumbrance of this Lease or all or any of Tenant’s rights hereunder or interest herein, and any sublet or permission to use or occupy the Premises or any part thereof not in accordance with this Section 8, shall be void and of no force or effect.
Page 10 of 26
Any assignment or subletting, Landlord’s consent thereto, the listing or posting of any name other than Tenant’s, or Landlord’s collection or acceptance of rent from any assignee or subtenant shall not be construed either as waiving or releasing Tenant from any of its liabilities or obligations under this Lease as a principal and not as a guarantor or surety, or as relieving Tenant or any assignee or subtenant from the obligation of obtaining Landlord’s prior written consent to any subsequent assignment or subletting. As security for this Lease, Tenant hereby assigns to Landlord the rent due from any assignee or subtenant of Tenant. During any period that there exists an uncured Default under this Lease, Tenant hereby authorizes each such assignee or subtenant to pay said rent directly to Landlord upon receipt of notice from Landlord specifying same. Landlord’s collection of such rent shall not be construed as an acceptance of such assignee or subtenant as a tenant. Tenant shall pay to Landlord an administrative fee equal to one thousand five hundred and No/100 dollars ($1,500.00) plus all other reasonable, out-of-pocket, third party expenses (including reasonable attorneys’ fees and accounting costs) incurred by Landlord in connection with Tenant’s request for Landlord to give its consent to any assignment, subletting, or mortgage, not to exceed an aggregate amount of five thousand and No/100 dollars ($5,000.00) (including the administrative fee), and Landlord’s receipt of such sum shall be a condition to Landlord providing such consent. Any sublease, assignment or mortgage shall, at Landlord’s option, be effected on forms reasonably approved by Landlord. Tenant shall deliver to Landlord a fully-executed copy of each agreement evidencing a sublease, assignment or mortgage, and Landlord’s consent thereto, within ten (10) days after execution thereof.
8.3. Subject to the terms of Section 8.5 below, if the proposed term with respect to a proposed sublet space is either: (a) longer than seventy-five percent (75%) of the then remaining Term, or (b) to extend (including any renewal or extension options) beyond the first (1st) day of the twelfth (12th) calendar month before the then scheduled expiration of the Term; or (c) if the proposed sublet space is (or, when aggregated with other space being sublet or assigned by Tenant, will be) more than seventy-five percent (75%) of the total number of rentable square feet in the Premises, then, in either such event, Landlord shall have the right in its sole and absolute discretion to terminate this Lease with respect to the proposed sublet space by sending Tenant written notice of such termination within thirty (30) days after Landlord’s receipt of Tenant’s written notice requesting Landlord’s consent. If the proposed sublet space does not constitute the entire Premises and Landlord so terminates, then: (i) Tenant shall tender the proposed sublet space to Landlord on the proposed sublease commencement date and such space shall thereafter be deleted from the Premises, and (ii) as to that portion of the Premises which is not part of the proposed sublet space, this Lease shall remain in full force and effect except that Base Rent and additional rent shall be reduced pro rata . The cost of any construction required to permit the operation of the proposed sublet space separate from the balance of the Premises shall be paid by Tenant as additional Rent. If the proposed sublet space constitutes the entire Premises and Landlord so terminates, then Tenant shall tender the proposed sublet space to Landlord, and this Lease shall terminate on the proposed sublease commencement date.
8.4.
Page 11 of 26
If any sublease or assignment provides that the subtenant or assignee thereunder is to pay any amount in excess of the sum of: (a) the rent and other charges due under this Lease, plus (b) the reasonable out-of-pocket expenses (excluding, however, any costs attributable to vacancy periods or “downtime”) reasonably incurred by Tenant in connection with the procurement of such sublease, assignment or other transfer (which expenses shall be amortized on a straight-line basis over the initial sublease term for the purposes hereof) (the “Sublease Profit” or “Assignment Profit”, as applicable), then, whether such net excess be in the form of an increased monthly or annual rental, a lump sum payment, payment for the sale, transfer or lease of Tenant’s fixtures, leasehold improvements, furniture and other personal property, or any other form of payment having the effect of a “disguised” rental payment (and if the subleased or assigned space does not constitute the entire Premises, the existence of such excess shall be determined on a pro-rata basis), Tenant shall pay to Landlord, along with Base Rent, fifty percent (50%) of any such Sublease or Assignment Profit, which amount shall be calculated and paid by Tenant to Landlord on a monthly basis as additional Rent, except that the terms of this sentence shall not apply to any Permitted Transfer pursuant to Section 8.5 below. As used in the foregoing sentence, “reasonable out-of-pocket expenses” means (i) in the event of a sale (or contribution) of Tenant’s personal property, the then unamortized or undepreciated cost thereof determined on the basis of Tenant’s federal income tax returns, (ii) the reasonable out of pocket costs and expenses of Tenant in making such sublease or assignment, such as brokers’ fees, attorneys’ fees, and advertising fees paid to unrelated third parties (iii) any payments required to be made by Tenant in connection with the assignment of its interest in this Lease pursuant to any real property transfer taxes or applicable laws, (iv) any sums paid by Tenant to Landlord pursuant to Section 8.2, (v) the cost of improvements or alterations made by Tenant expressly and solely for the purpose of preparing the Premises for such assignment, including the cost of any construction set forth in Section 8.3; (vi) the unamortized or undepreciated cost of any Tenant’s property leased to and used by such assignee, and (viii) the then unamortized or undepreciated cost of any Alterations determined on the basis of Tenant’s federal income tax returns. Notwithstanding the foregoing, Landlord is not intending to receive any amounts considered to be based on the net income or profits of Tenant or any subtenant. Acceptance by Landlord of any payments due under this Section shall not be deemed to constitute approval by Landlord of any sublease or assignment, nor shall such acceptance waive any rights of Landlord hereunder. Landlord shall have the right to request, and Tenant shall provide, Tenant’s books and records relating to the calculation of any Sublease Profit.
8.5. Notwithstanding anything contained in this Section 8 to the contrary, provided no Default exists hereunder, Tenant may, upon not more than ten (10) days’ written prior notice to Landlord (which notice shall contain a written certificate from Tenant stating the legal and beneficial relationship of Tenant and the proposed assignee, transferee or subtenant) but without Landlord’s prior written consent and without being subject to Landlord’s rights and Tenant’s obligations set forth in Sections 8.1, 8.2, 8.3, and 8.4, assign or transfer its entire interest in this Lease or sublease the entire or any portion of the Premises (each a “ Permitted Transfer”) to: (a) a corporation or other business entity (a “successor corporation”) into or with which Tenant shall be merged or consolidated, or to which substantially all of the assets or stock of Tenant may be transferred or sold, provided that such successor corporation shall have a net worth and liquidity factor of at least $25,000,000.00 or otherwise reasonably acceptable to Landlord taking into account the fact that the original Tenant under this Lease is not being released, and provided that the successor corporation shall assume in writing all of the obligations and liabilities of Tenant under this Lease and the proposed use of the Premises is in compliance with this Lease; or (b) a corporation or other business entity (a “related corporation”) which shall control, be controlled by or be under common control with Tenant, shall have a net worth and liquidity factor at least equal to the net worth and liquidity factor of at least $25,000,000.00 or otherwise reasonably acceptable to Landlord taking into account the fact that the original Tenant under this Lease is not being released, and provided that such related corporation shall assume in writing all of the obligations and liabilities of Tenant under this Lease (without relieving Tenant therefrom) and the proposed use of the Premises is in compliance with this Lease.
Page 12 of 26
For purposes of clause (b) above, “control” shall be deemed to be ownership of more than fifty percent (50%) of the stock or other voting interest of the controlled corporation or other business entity. In the event of any such assignment or subletting, Tenant shall remain fully liable as a primary obligor for the payment of all Rent and other charges required hereunder and for the performance of all obligations to be performed by Tenant hereunder. Notwithstanding the foregoing, if Tenant structures an assignment or sublease to an entity that meets the definition of a successor corporation or a related corporation for the purpose of circumventing the restrictions on subleases and assignments provided elsewhere in this Section 8 then such subtenant or assignee shall conclusively be deemed not to be a successor corporation or a related corporation and subject to all such restrictions.
9. Parking. During the Term, Tenant shall have the right to use the Parking Facility for the parking of standard-sized passenger automobiles, such use to be on a non-exclusive and unreserved basis with other tenants of the Building, upon the rules and regulations implemented in compliance with Section 11; provided, however, that Tenant shall not at any time simultaneously utilize more spaces than Tenant’s Parking Allotment. Landlord reserves the right in its absolute discretion to determine whether the Parking Facility is becoming crowded and to allocate and assign parking spaces among Tenant and the other tenants; provided, however, no such allocation shall reduce the parking spaces Tenant is entitled to use to below Tenant’s Parking Allotment. If Landlord, in its sole and absolute discretion, grants to any other tenant of the Building the exclusive right to use any particular parking spaces, then neither Tenant nor its employees or visitors shall use such spaces. Tenant shall not use parking areas for the servicing or overnight storage of vehicles. Except in connection with an assignment of this Lease or sublet of the Premises, Tenant shall not assign, sublet or transfer any rights with respect to the Parking Facility. It is understood and agreed that Landlord assumes no responsibility, and shall not be held liable, for any damage or loss to any automobiles parked in the Parking Facility or to any personal property located therein, or for any injury sustained by any person in or about the Parking Facility. Landlord reserves the right to close the Parking Facility during periods of unusually inclement weather or for repairs, provided Landlord shall use commercially reasonably efforts to minimize any such closures and to provide substitute parking. Landlord shall not be liable to Tenant and this Lease shall not be affected if any parking rights hereunder are impaired by any law imposed after the Commencement Date.
10. Security Deposit. Simultaneously with Tenant’s execution of this Agreement, Tenant shall deposit with Landlord a sum equal to the Security Deposit Amount as a security deposit for the performance by Tenant of all of Tenant’s obligations, covenants, conditions and agreements under this Lease. Landlord shall not be required to maintain such security deposit in a separate account, and Tenant shall not be entitled to interest on the security deposit. Within ninety (90) days of the end of the Term or earlier termination of this Lease, Landlord shall return such security deposit to Tenant, less such portion thereof as Landlord shall have appropriated to satisfy any of Tenant’s obligations under this Lease or to satisfy a Default, pursuant to the terms of this Lease. If there shall be any Default under this Lease, then Landlord shall have the right, but shall not be obligated, to use, apply or retain all or any portion of the security deposit for the payment of any Rent or any other sum applicable to such Default, or any amount Landlord may spend or become obligated to spend, or for the compensation of Landlord for any losses incurred by reason of such Default.
Page 13 of 26
11. Rules. Tenant shall at all times abide by and observe the rules specified in Exhibit D. Tenant shall also abide by and observe any other rule that Landlord may reasonably promulgate from time to time for the operation and maintenance of the Building, provided that written notice thereof is given and such rule is not inconsistent with the provisions of this Lease. All rules shall be binding upon Tenant and enforceable by Landlord as if they were contained herein. Nothing contained in this Lease shall be construed as imposing upon Landlord any duty or obligation to enforce such rules, or the terms, conditions or covenants contained in any other lease, as against any other tenant, and Landlord shall not be liable to Tenant for the violation of such rules by any other tenant or its employees, agents, assignees, subtenants, invitees or licensees, provided Landlord shall not enforce any rule or regulation in a manner which unreasonably discriminates among similarly situated tenants.
12. Indemnity and Insurance.
12.1. Tenant’s Insurance. Tenant shall maintain during the Term commercial general liability insurance, with limits of not less than one million dollars ($1,000,000) per occurrence, three million dollars ($3,000,000) general aggregate (on a per location basis) for personal injury, bodily injury or death, or property damage or destruction for any one occurrence. Landlord shall be covered under each policy as an additional insured as it pertains to the commercial general liability. Tenant shall also maintain during the Term worker compensation insurance as required by statute, and primary, noncontributory, “all-risk” property damage insurance covering Tenant’s personal property, business records, fixtures, and equipment, for damage or other loss caused by fire or other casualty or cause including, but not limited to, vandalism and malicious mischief, theft, water damage of any type, including sprinkler leakage, bursting or stoppage of pipes, explosion, business interruption, and other insurable risks in amounts not less than the full insurable replacement value of such property and full insurable value of such other interests of Tenant (subject to reasonable deductible amounts). Landlord reserves the right from time to time to reasonably require higher minimum limits or different types of insurance. Tenant shall deliver to Landlord an ACORD certificate or its equivalent with respect to all liability and personal property insurance on or before the date Tenant takes possession of the Premises and at least annually thereafter. Tenant shall deliver to Landlord upon request copies of all required insurance policies, including endorsements and declarations.
12.2. Waiver of Subrogation. Every insurance policy carried by either Tenant or Landlord shall include provisions denying to the insurer subrogation rights against the other party. Each party hereby waives any and all rights of recovery against the other for loss or damage occurring to the Premises, or to any property contained therein or elsewhere on the Property, regardless of the cause of such loss or damage to the extent that the loss or damage is or could be covered by insurance (without regard to any deductible provision in any policy). This Section 12.2 shall not apply to (a) any claim for willful misconduct or intentional acts that are not covered by the required insurance or to the deductible portion of any insured loss sustained by Landlord; (b) claims for damages of less than $1,000; and (c) claims for personal injury or wrongful death.
12.3. Indemnity.
Page 14 of 26
Tenant shall defend with counsel reasonably approved by Landlord (and any counsel selected by Tenant’s insurer is deemed approved by Landlord), indemnify, and hold harmless and provide a waiver of subrogation and all rights of recovery in favor of, the Landlord, managing agent, all employees, officers, directors, partners, members, managers and shareholders of Landlord, Landlord’s property manager, and any other party having an interest therein from and against any and all liabilities, losses, damages, costs, expenses (including reasonable attorneys’ fees and expenses), causes of action, suits, claims, demands, judgments or penalties of any nature (collectively, “Claims”) arising from or with respect to (a) any injury to or death of any person or damage to or loss of property in, on or about the Premises, to the extent caused by Tenant’s negligent act or omission, or (b) any construction or other work by Tenant on or about the Premises. Notwithstanding the foregoing, Tenant’s indemnity obligation contained herein shall not apply to the extent of any Claim arising out of Landlord’s negligence or willful misconduct or covered under any insurance policy required to be maintained by Landlord pursuant to Section 12.1.
12.4. Non-Liability. Landlord shall not be liable to Tenant or any other person or entity for any damage, injury, loss, or claim based on or arising out of any cause whatsoever, including the following: repair to any portion of the Premises or the Building; interruption in the use of the Premises or the Building or any equipment therein; any accident or damage resulting from any use or operation (by Landlord, Tenant, or any other person or entity) of elevators or heating, cooling, electrical, sewage or plumbing equipment or apparatus; termination of this Lease by reason of damage to the Premises or the Building; any fire, robbery, theft, vandalism, mysterious disappearance or any other casualty; actions of any other tenant of the Building or of any other person or entity; failure or inability to furnish any service specified in this Lease; and leakage in any part of the Premises or the Building from water, rain, ice or snow that may leak into, or flow from, any part of the Premises or the Building, or from drains, pipes or plumbing fixtures in the Premises or the Building. If any condition exists which may be the basis of a claim of constructive eviction, then Tenant shall give Landlord written notice thereof and a reasonable opportunity to correct such condition, and in the interim Tenant shall not claim that it has been constructively evicted or is entitled to a rent abatement. Any property placed by Tenant or any agent of Tenant in or about the Premises or the Building shall be at the sole risk of Tenant, and Landlord shall not in any manner be held responsible therefor. Any person receiving an article delivered for Tenant shall be acting as Tenant’s agent for such purpose and not as Landlord’s agent. For purposes of this Section, the term “Building” shall be deemed to include the Real Property. Notwithstanding the foregoing provisions of this Section, Landlord shall not be released from liability to Tenant for any physical injury to any natural person caused by the negligence or willful misconduct of Landlord or Landlord’s representatives to the extent such injury is not covered by insurance either carried by Tenant (or such person) or required by this Lease to be carried by Tenant.
12.5. Consequential Damages Waiver. Except to the extent arising from a Tenant holdover in the Premises, neither party nor any party’s representatives (nor any past, present or future board member, partner, trustee, director, member, officer, employee, agent, representative or advisor of any of them), shall under any circumstances be liable for any exemplary, punitive, consequential or indirect damages (or for any interruption of or loss to business) in connection with or relating to this Lease.
12.6. Landlord’s Insurance. During the Term, Landlord will keep in force the following coverage: (i) Commercial general liability insurance; (ii) Causes of loss – special form commercial property insurance (including standard extended coverage endorsement perils, leakage from fire protective devices and other water damage) covering the full replacement cost of the Building (excluding Alterations made by Tenant in the Premises); (iii) Boiler and machinery or equipment breakdown insurance; and (iv) Other insurance that Landlord elects to maintain.
Page 15 of 26
13. Casualty and Condemnation.
13.1. Casualty. If the Premises or the Building are totally or partially damaged or destroyed thereby rendering the Premises totally or partially inaccessible or unusable, then Landlord shall repair and restore the Premises and the Building to substantially the same condition they were in prior to such damage or destruction; provided, however, that if in Landlord’s reasonable judgment such repair and restoration cannot be completed within two hundred seventy (270) days after the occurrence of such damage or destruction (taking into account the time needed for effecting a satisfactory settlement with any insurance company involved, removal of debris, preparation of plans and issuance of all required governmental permits), then Landlord shall have the right to terminate this Lease by giving written notice of termination within forty-five (45) days after the occurrence of such damage or destruction. If this Lease is terminated pursuant to this Section 13.1, then rent shall be apportioned (based on the portion of the Premises which is usable or used after such damage or destruction) and paid to the earlier of the date of termination or the date Tenant completely vacates and abandons the Premises on account of such damage and Landlord shall be entitled to any insurance proceeds received by Tenant that are attributable to improvements insured or required to be insured by Tenant that would remain in the Premises at the end of the Term. If this Lease is not terminated as a result of such damage or destruction, then until such repair and restoration of the Premises are substantially complete, Tenant shall be required to pay rent only for the portion of the Premises that is usable while such repair and restoration are being made; provided, however, that (x) if such damage or destruction was caused by the gross negligence or willful misconduct of Tenant, then Tenant shall not be entitled to any such rent reduction, and (y) if Tenant fails to promptly pay over to Landlord insurance proceeds when received from Tenant’s insurance any such rent abatement shall end on the date when Landlord would have been able to substantially complete repair and restoration of the Premises had Tenant timely paid Landlord such insurance proceeds. After receipt of all insurance proceeds (including proceeds of insurance maintained by Tenant), Landlord shall proceed with and bear the expenses of such repair and restoration of the Premises and the Building; provided, however, that (a) if such damage or destruction was caused by the act or omission of Tenant, then Tenant shall pay Landlord’s deductible and the amount by which such expenses exceed the insurance proceeds, if any, actually received by Landlord on account of such damage or destruction, (b) Tenant shall pay the amount by which the cost of restoring any item which Landlord is required to restore and Tenant is required to insure exceeds the insurance proceeds received with respect thereto, and (c) Landlord shall not be required to repair or restore any tenant improvements installed in the Premises (except to the extent Landlord receives proceeds therefor from Tenant’s insurance), any Alterations or any other contents of the Premises (including Tenant’s trade fixtures, decorations, furnishings, equipment or personal property). Notwithstanding anything herein to the contrary, Landlord shall have the right to terminate this Lease if (1) insurance proceeds plus deductibles are insufficient to pay the full cost of such repair and restoration, (2) the holder of any mortgage fails or refuses to make such insurance proceeds available for such repair and restoration, (3) zoning or other applicable Laws or regulations do not permit such repair and restoration, or (4) the damage to the Building exceeds thirty-five percent (35%) of the replacement value of the Building.
Page 16 of 26
13.2. Condemnation. In the event of any condemnation of all or any portion of the Premises, this Lease shall terminate as to the part so taken as of the date the condemning authority takes title or possession, whichever occurs first. If as a result of a partial condemnation of the Building or Premises, Tenant, in its reasonable discretion, is unable to use the Premises for the purposes intended hereunder, Tenant may, at Tenant’s option, to be exercised in writing within fifteen (15) days after Landlord shall have given Tenant written notice of such taking (or in the absence of such notice, within fifteen (15) days after the condemning authority shall have taken possession) terminate this Lease as of the date the condemning authority takes such possession. Any such notice of termination shall cause this Lease to expire with the same force and effect as though the date set forth in such notice were the date originally set as the expiration date of this Lease and the parties shall make an appropriate adjustment as of such termination date with respect to payments due to the other under this Agreement. All awards, damages and other compensation paid on account of such condemnation shall belong to Landlord, and Tenant assigns to Landlord all rights to such awards, damages and compensation. Tenant shall not make any claim against Landlord or any condemning authority for any portion of any award, damages, or compensation attributable to damage to the Premises, value of the unexpired portion of the Term, loss of profits or goodwill, leasehold improvements, or severance damages.
14. Holding Over. Tenant acknowledges that it is extremely important that Landlord have substantial advance notice of the date on which Tenant will vacate the Premises, and that if Tenant fails to surrender the Premises or any portion thereof at the expiration or earlier termination of the Term, then it will be conclusively presumed that the value to Tenant of remaining in possession, and the loss that will be suffered by Landlord as a result thereof, far exceed the Base Rent and additional rent that would have been payable had the Term continued during such holdover period. Therefore, if Tenant (or anyone claiming through Tenant) does not immediately surrender the Premises or any portion thereof upon the expiration or earlier termination of the Term, then, during the first month of Tenant’s holdover, the Base Rent payable by Tenant hereunder shall be increased to equal one hundred fifty percent (150%) of the Base Rent payable under this Lease during the last month of the Term. After such initial month of holdover by Tenant, the Base Rent payable by Tenant hereunder shall be increased to equal two hundred percent (200%) of the Base Rent payable under this Lease during the last month of the Term. Such Base Rent and all additional Rent shall be payable on the first day of such holdover period and the first day of each calendar month thereafter during such holdover period until the Premises have been vacated. Notwithstanding any other provision of this Lease, Landlord’s acceptance of such rent shall not in any manner adversely affect Landlord’s other rights and remedies, including Landlord’s right to evict Tenant and to recover all damages. Any such holdover shall be deemed to be a tenancy-at-sufferance and not a tenancy-at-will or tenancy from month-to-month. In no event shall any holdover be deemed a permitted extension or renewal of the Term, and nothing contained herein shall be construed to constitute Landlord’s consent to any holdover or to give Tenant any right with respect thereto.
Page 17 of 26
15. Defaults by Tenant; Remedies.
15.1. Each of the following constitutes a default (“Default”): (a) Tenant’s failure to make when due any payment of the Base Rent, additional rent or other sum, which failure shall continue for a period of five (5) days after Landlord sends Tenant written notice thereof; (b) Tenant’s failure to perform or observe any other covenant or condition of this Lease, which failure shall continue for a period of thirty (30) days after Landlord sends Tenant written notice thereof, provided, however, that if the continuance of failure to perform or observe for the period required for cure will not (i) subject Landlord or any mortgagee to prosecution for a crime or any other fine or charge, (ii) subject the Premises or any part thereof or the Property or any part thereof, to being condemned or vacated, (iii) subject the Building or Property, or any part thereof, to any lien or encumbrance which is not removed or bonded within the time period required under this Lease, or (iv) result in the foreclosure of any mortgage, and such the cure for which cannot reasonably be effected within such thirty (30) day period, and Tenant begins such cure promptly within such thirty (30) day period and is pursuing such cure in good faith and with diligence and continuity during such thirty (30) day period, then, except in the event of an emergency, Tenant shall have such additional time (not to exceed ninety (90) days in total) as is reasonably necessary to effect such cure; (c) bankruptcy which is not discharged within thirty (30) days of filing; or (d) Tenant’s failure to pay any sum or perform or observe any covenant or condition of this Lease when required under this Lease (without regard to any grace period otherwise allowed) more than twice during any twelve month period during the Term.
15.2. In addition to any other remedies available at law or equity, if a Default remains uncured after the applicable notice and cure period provided in Section 15.1, Landlord may, upon five (5) days’ prior written notice to Tenant pursue any of the following: (a) terminate Tenant’s right to possession of the Premises by any lawful means, in which case this Lease shall terminate and Tenant shall surrender possession to Landlord; (b) enter and take possession of the Premises, and remove Tenant, with or without having terminated the Lease; or (c) alter locks and other security devices at the Premises, as permitted by applicable law.
15.3. If Landlord terminates this Lease or ends Tenant’s right to possess the Premises due to a Default, Landlord may hold Tenant liable for Rent, and other indebtedness accrued to the Expiration Date. Tenant shall also be liable for the Rent and other indebtedness that otherwise would have been payable by Tenant during the remainder of the Term had there been no Default, reduced by any sums Landlord receives by reletting the Premises during the Term or by taking other mitigation measures. If Tenant is in Default and has vacated the Premises, and if Landlord has terminated this Lease as a result of such Default, then Landlord shall thereafter use reasonable efforts to relet the Premises; provided, however, that Tenant understands and agrees that Landlord’s main priority will be the leasing of other space in the Building and the reletting of the Premises will be of lower priority.
15.4. All rights and remedies of Landlord set forth in this Lease are cumulative and in addition to all other rights and remedies available to Landlord at law or in equity. The exercise by Landlord of any such right or remedy shall not prevent the concurrent or subsequent exercise of any other right or remedy. No delay or failure by Landlord or Tenant to exercise or enforce any of its respective rights or remedies or the other party’s obligations (except to the extent a time period is specified in this Lease therefor) shall constitute a waiver of any such or subsequent rights, remedies or obligations.
Page 18 of 26
Neither party shall be deemed to have waived any default by the other party unless such waiver expressly is set forth in a written instrument signed by the party against whom such waiver is asserted. If Landlord waives in writing any default by Tenant, such waiver shall not be construed as a waiver of any covenant, condition or agreement set forth in this Lease except as to the specific circumstances described in such written waiver.
16. Environmental. Tenant shall not allow, cause, or permit any Hazardous Materials to be generated, used, treated, released, stored or disposed of in or about the Premises in violation of applicable Environmental Law, except cleaning supplies, copier toner or other similar type products commonly found in commercial office space. “Hazardous Materials” means (a) asbestos and any asbestos containing material and any substance that is then defined or listed in, or otherwise classified pursuant to, any Environmental Law or any other applicable Law as a “hazardous substance,” “hazardous material,” “hazardous waste,” “infectious waste,” “toxic substance,” “toxic pollutant” or any other formulation intended to define, list, or classify substances by reason of deleterious properties such as ignitability, corrosivity, reactivity, carcinogenicity, toxicity, reproductive toxicity, or Toxicity Characteristic Leaching Procedure (TCLP) toxicity, (b) any petroleum and drilling fluids, produced waters, and other wastes associated with the exploration, development or production of crude oil, natural gas, or geothermal resources, (c) toxic mold, mildew or any substance that reasonably can be expected to give rise to toxic mold or mildew, or (d) any petroleum product, polychlorinated biphenyls, urea formaldehyde, radon gas, radioactive material (including any source, special nuclear, or by-product material), medical waste, chlorofluorocarbon, lead or lead-based product, and any other substance whose presence could be detrimental to the Premises. At the expiration or earlier termination of this Lease, with respect to conditions existing on account of Tenant’s use or occupancy of the Premises or any action or inaction of Tenant or any agent of Tenant, Tenant shall surrender the Premises to Landlord free of Hazardous Materials and in compliance with all Environmental Laws. “Environmental Law” means any present and future law and any amendments (whether common law, statute, rule, order, regulation or otherwise), permits and other requirements or guidelines of governmental authorities applicable to the Premises and relating to the environment and environmental conditions or to any Hazardous Material (including CERCLA, 42 U.S.C. § 9601 et seq., the Resource Conservation and Recovery Act of 1976, 42 U.S.C. § 6901 et seq., the Hazardous Materials Transportation Act, 49 U.S.C. § 1801 et seq., the Federal Water Pollution Control Act, 33 U.S.C. § 1251 et seq., the Clean Air Act, 42 U.S.C. § 7401 et seq., the Toxic Substances Control Act, 15 U.S.C. § 2601 et seq., the Safe Drinking Water Act, 42 U.S.C. § 300f et seq., the Emergency Planning and Community Right-To-Know Act, 42 U.S.C. § 1101 et seq., the Occupational Safety and Health Act, 29 U.S.C. § 651 et seq., and any so-called “Super Fund” or “Super Lien” law, any Law requiring the filing of reports and notices relating to hazardous substances, environmental laws administered by the Environmental Protection Agency, and any similar state and local Laws, all amendments thereto and all regulations, orders, decisions, and decrees now or hereafter promulgated thereunder concerning the environment, industrial hygiene or public health or safety). Tenant shall promptly deliver to Landlord copies of any notices or other items received from or submitted to any governmental or quasi-governmental agency, or any claim instituted or threatened by any third party, concerning the Premises, Tenant’s occupancy or use thereof, or the existence or potential existence of Hazardous Materials therein.
Page 19 of 26
Upon any violation of an Environmental Law, or any release, spill or discharge of a Hazardous Material on or from the Premises, or any environmental condition requiring responsive action (provided none of the foregoing is caused by Landlord’s negligence or willful misconduct), in addition to all other rights available to Landlord under this Lease, at law or in equity, Landlord shall have the right but not the obligation to enter the Premises following reasonable notice thereof to Tenant, to supervise and approve any actions taken by Tenant to address same, and, if Tenant fails to promptly address same in accordance with this Lease, to perform, with respect to conditions existing on account of Tenant’s use or occupancy of the Premises or any action or inaction of Tenant or any agent of Tenant, at Tenant’s sole cost and expense, any lawful action necessary to address same. Landlord represents that, as of the date of this Lease, (i) Landlord has not received any notices of any violations of Environmental Laws concerning the Premises, and (ii) to the best of Landlord’s actual knowledge, there has been no discharge of Hazardous Materials in, on, under or at the Premises and no Hazardous Materials, including but not limited to asbestos, currently exist or have previously existed in, on, under or at the Premises in violation of Environmental Laws.
17. Quiet Enjoyment. Landlord hereby covenants and agrees that Tenant, upon paying the Rent and keeping the covenants of this Lease, shall have the right to lawfully and quietly hold and occupy the Premises and enjoy the Premises during the Term without any interference, ejection or molestation.
18. Signs. Landlord shall list, at Landlord’s expense, the name of Tenant in the Building directory and will provide a Building-standard sign on or adjacent to one (1) suite entry door. Tenant shall not place, inscribe, paint, affix or otherwise display any sign, advertisement, picture, lettering, or notice of any kind on any part of the exterior or interior of the Building outside the Premises (including windows and doors), or on any part of the interior of the Premises which can be seen from outside the Premises, without the prior written approval of Landlord, which may be granted or withheld in Landlord’s sole and absolute discretion. If any such item that has not been approved by Landlord is so displayed, then Landlord shall have the right to remove such item at Tenant’s expense. Landlord reserves the right to install and display signs, advertisements and notices on any part of the exterior or interior of the Building.
19. Subordination.
19.1. Subordination. This Lease shall be subordinate to any ground lease, mortgage, deed of trust or any other hypothecation for security now or hereafter placed upon the real property of which the Premises are a part and to all renewals, modifications, consolidations, replacements and extensions thereof. If any mortgagee, trustee or ground lessor shall elect to have this Lease be prior to the lien of its mortgage, deed of trust or ground lease, and shall give written notice thereof to Tenant, this Lease shall be deemed prior to such mortgage, deed of trust or ground lease, whether this Lease is dated prior or subsequent to the date of said mortgage, deed of trust or ground lease or the date of recording thereof. This section is self-operating, provided, that Tenant agrees to promptly execute any document, in form reasonably acceptable to Tenant, necessary to effectuate the foregoing subordination or to make this Lease prior to the lien of any mortgage, deed of trust or ground lease.
19.2. Attornment. If the holder of any ground lease, mortgage, deed of trust or security described above (or its successor-in-interest), enforces its remedies provided by law or under the pertinent mortgage, deed of trust or security instrument and succeeds to Landlord’s interest in the Leased Premises, Tenant agrees, upon written request of any such holder or any purchaser at foreclosure sale, to attorn and pay Rent to such party and to execute and deliver any instruments necessary or appropriate to evidence or effectuate such attornment (provided such holder or purchaser shall agree to accept this Lease and not disturb Tenant’s occupancy, so long as Tenant does not default and fail to cure within the time(s) permitted hereunder).
Page 20 of 26
20. Prohibition of Liens. Tenant shall not suffer, create, or permit any mechanics’ liens or other liens to be filed against the Property or against Tenant’s leasehold interest, by reason of any work, labor, services, or materials supplied or claimed to have been supplied to Tenant or anyone holding the Premises or any part thereof through or under Tenant. If such a lien is filed, then Tenant shall, within ten (10) after receiving written notice from Landlord, shall cause such lien to be bonded or discharged.
21. Miscellaneous.
21.1. Brokers. Landlord represents to Tenant, and Tenant represents to Landlord, that no broker or finder has been engaged by it, respectively, in connection with this Lease, other than the Brokers. In the event of a claim for broker’s or finder’s fee or commissions in connection with this Lease, then Landlord shall indemnify, defend and hold harmless Tenant from the same if it shall be based upon any statement or agreement alleged to have been made by Landlord, and Tenant shall indemnify, defend and hold harmless Landlord from the same if it shall be based upon any statement or agreement alleged to have been made by Tenant. Landlord shall pay any and all commissions owed to the Brokers pursuant to a separate written agreement.
21.2. Statement. At any time and from time to time, upon not less than ten (10) days’ prior written notice, Tenant and each subtenant, assignee, licensee or concessionaire or occupant of Tenant shall execute, acknowledge and deliver to Landlord and/or any other person or entity designated by Landlord, a written statement in a form reasonably acceptable to Tenant (or shall provide to Landlord Tenant’s comments to the form of statement provided by Landlord) certifying: (a) that this Lease is unmodified and in full force and effect (or if there have been modifications, that this Lease is in full force and effect as modified and stating the modifications); (b) the dates to which the Rent and any other charges have been paid; (c) to Tenant’s knowledge, whether or not Landlord is in default in the performance of any obligation, and if so, specifying the nature of such default; (d) the address to which notices to Tenant are to be sent; (e) that this Lease is subject and subordinate to all mortgages encumbering the Premises; (f) that Tenant has accepted the Premises; and (g) such other matters as Landlord may reasonably request. Any such statement may be relied upon by Landlord, any prospective purchaser of the Premises, or any holder or prospective holder of a mortgage or any other person or entity. Tenant acknowledges that time is of the essence to the delivery of such statements.
21.3. Successors. All rights, remedies and liabilities herein recorded or imposed upon either of the parties hereto shall extend to their heirs, executors, administrators, successors and assigns.
21.4. Severability. If any term or provision of this Lease or any amendments hereto shall be found to be invalid or unenforceable to any extent, the remainder of this Lease shall not be affected thereby, and each term and provision of this Lease shall be valid and enforced to the fullest extent permitted by law.
Page 21 of 26
21.5. Incorporation. This Lease, upon full execution, supersedes and revokes any and all previous leases governing the Premises, lease negotiations, arrangements, letters of intent, offers to lease, lease proposals or drafts, brochures, representations, and information conveyed, whether oral or written, between the parties hereto or their respective representatives or any other person purported to represent Landlord or Tenant.
21.6. Notices. All notices or other communications required under this Lease shall be in writing and shall be deemed duly given and received when delivered in person (with receipt therefor), on the next business day after deposit with a recognized overnight delivery service, or on the second day after being sent by certified or registered mail, return receipt requested, postage prepaid, to the following addresses: (a) if to Landlord, at the Landlord Notice Address specified in the Basic Provisions; (b) if to Tenant, at the Tenant Notice Address specified in the Basic Provisions. Notice is not effectively transmitted by facsimile, email, or other electronic transmission. Either party may change its address for the giving of notices by written notice given in accordance with this Section. If Landlord or the holder of any mortgage notifies Tenant in writing that a copy of any notice to Landlord shall be sent to such holder at a specified address, then Tenant shall send (in the manner specified in this Section and at the same time such notice is sent to Landlord) a copy of each such notice to such holder. Any such holder shall have thirty (30) days after receipt of such notice to cure any Landlord default before Tenant may exercise any remedy (provided that in the case of a Landlord default arising from an act or omission which cannot be reasonably remedied within said thirty (30) day period, then the holder of any mortgage shall have as long as reasonably necessary to remedy such act or omission provided that (i) such holder commences such remedy and notifies Tenant within said thirty (30) day period of holder’s desire to remedy, and (ii) holder pursues completion of such remedy with due diligence following such giving of notice and following the time when holder should have become entitled under the mortgage to remedy the same). Any cure of Landlord’s default by such holder shall be treated as performance by Landlord.
21.7. Interpretation. Section headings shall not be used in interpreting this Lease. Each party acknowledges that such party and its counsel, after negotiation and consultation, have reviewed and revised this Lease. As such, the terms of this Lease shall be fairly construed and the usual rule of construction, to the effect that any ambiguities herein should be resolved against the drafting party, shall not be employed in the interpretation of this Lease, or any amendments, modifications or exhibits hereto or thereto. Whenever the words “including”, “include” or “includes” are used in this Lease, they shall be interpreted in a non-exclusive manner. Except as otherwise indicated, all Exhibit and Section references in this Lease shall be deemed to refer to the Exhibits and Sections in this Lease.
21.8. Governing Law. This Lease shall be construed and enforced in accordance with the laws of the State of New Jersey, without regard to conflicts of laws principles.
21.9. Attorneys’ Fees. If on account of any default by Tenant of its obligations under this Lease it becomes necessary or appropriate for Landlord to employ attorneys or other persons to enforce any of Landlord’s rights or remedies hereunder, Tenant shall pay upon demand all fees of such attorneys and other persons and all other costs of any kind so incurred.
Page 22 of 26
21.10. Representations. Landlord and Tenant each represent to each other that the person(s) executing and delivering this Lease on their behalf are duly authorized to so act; that Landlord and Tenant are duly organized, are in good standing under the laws of the state of its organization and the laws of the State of New Jersey, and have the power and authority to enter into this Lease; that Landlord and Tenant are not, and the entities or individuals constituting Landlord and Tenant or which may own or control Landlord or Tenant, among the individuals or entities identified on any list compiled by the U.S. Government for the purpose of identifying suspected terrorists, and Landlord and Tenant are not engaging in this transaction on behalf of any such individual or entity; that Landlord and Tenant are not in violation of any anti-money laundering Law; and that all action required to authorize Landlord and Tenant and such person to enter into this Lease have been duly taken.
21.11. Force Majeure. If Landlord or Tenant is in any way delayed or prevented from performing any obligation (except, with respect to Tenant, its obligations to pay Rent under this Lease, any obligation with respect to insurance, any obligation to give notice with respect to extensions, expansions or otherwise, and any holdover) due to fire, act of God, governmental act or failure to act, strike, labor dispute, inability to procure materials, order of governmental authority, pandemic, epidemic, virus, disease, or other public health emergency, or any cause beyond Landlord’s or Tenant’s (as applicable) reasonable control (whether similar or dissimilar to the foregoing events) (collectively, “Force Majeure”), then the time for performance of such obligation shall be excused for the period of such delay or prevention and extended for a period equal to the period of such delay or prevention; provided, however, that no such force majeure event shall excuse the timely payment of all items of Rent by Tenant. Financial disability or hardship shall never constitute a Force Majeure event.
21.12. Relocation. Landlord shall have the right to change the location and configuration of the Premises no more than once during the Term and subject to the following terms and conditions: (a) Landlord shall provide Tenant not less than sixty (60) days’ advance written notice of the date Tenant must vacate the Premises; (b) Landlord shall provide Tenant with substitute space of similar nature, size, functionality, and quality as the Premises elsewhere in the Building or in a building within one (1) mile of the Building which is owned by Landlord or one of its affiliates (the “Substitute Premises”); (c) Landlord shall at Landlord’s expense (1) remove Tenant’s equipment, furniture and personal property from the Premises and reinstall or relocate those items, as applicable, in the Substitute Premises, (2) redecorate the Substitute Premises in a manner substantially similar to the manner in which the Premises were decorated, and (3) pay reasonable costs associated with any replacement letterhead, and (d) such relocation may not occur within the last six (6) months of the Term. Within thirty (30) days after the date Landlord submits an amendment of this Lease indicating the location and configuration of the Substitute Premises, Tenant shall execute such amendment; provided, however, if the Substitute Premises is larger than the Premises, neither Rent nor Tenant’s Proportionate Share shall be increased.
21.13. Entry. Upon at least 24 hours’ prior written notice to Tenant (except in the case of an emergency, when no notice shall be required), Tenant shall permit Landlord, its agents and representatives, and the holder of any mortgage, to enter the Premises, without charge therefor and without diminution of the Rent payable by Tenant, in order to examine, inspect or protect the Premises and the Building, to make such alterations and/or repairs as in the sole but reasonable judgment of Landlord may be deemed necessary or desirable, or to exhibit the same to brokers, prospective tenants (during the last twelve (12) months of the Term), lenders, purchasers and others.
Page 23 of 26
21.14. Financial Statement. At any time during the Term, but no more than once during any calendar year, Tenant shall provide landlord with the most current financial statement for Tenant, and financial statements for the two (2) years prior to the current financial statement year. Such statements are to be certified by Tenant to be true, correct and complete, prepared in accordance with generally accepted accounting principles and, if it is the normal practice of Tenant, audited by any independent certified public accountant. landlord and any party would be provided with a copy of such financial statements shall execute Tenant’s required confidentiality agreement and Tenant shall deliver such financial statements within five (5) business days following receipt of such confidentiality agreement.
21.15. Electronic Signatures. The parties agree that this lease may be transmitted between them by email, and intend that electronic signatures (such as, without limitation, DocuSign signatures or scanned signatures in .pdf format) constitute original signatures, and that an electronic Lease containing the signatures (original or scanned) of all the parties is binding on the parties.
21.16. Entire Agreement. It is understood and agreed by the parties hereto that this Lease represents the entire agreement of the parties with respect to the subject matter of the lease and that any additions, variations or modifications to this Lease shall be void and ineffective unless in writing signed by the parties hereto and made a part hereof.
22. Renewal Option.
22.1. Landlord hereby grants to Tenant an option to extend the Term for one (1) period of five (5) years (a “Renewal Term”) upon the following conditions: (i) Tenant has not previously sublet any part or all of the Premises or assigned this lease; (ii) Tenant has delivered to Landlord written notice of its intention to exercise the option (“Renewal Notice”) not less than two hundred seventy (270) days prior to the expiration of the Term of the lease; and (iii) all lease terms for the Renewal Term shall be the same as in the lease, except that Tenant shall pay Base Rent for the Renewal Term in an amount equal to the greater of either: (A) the Base Rent applicable to the last month of the then-expiring term, or (B) 95% of the Fair Market Rent (defined herein) determined as of the date of Tenant’s Renewal Notice; it being the intention of the parties that, notwithstanding the Fair Market Rent, Tenant will in no event pay an amount less than the Base Rent applicable to the last month of the then-expiring term.
22.2. In addition to the conditions set forth in Section 22.1, Tenant’s right to exercise its option for a Renewal Term shall be suspended at the election of Landlord during any period in which a Default has occurred and is continuing, but the period of time within which such option may be exercised shall not be extended. Notwithstanding Tenant’s due and timely exercise of its option, if, after such exercise and prior to the commencement of the Renewal Term, a Default occurs and is continuing, then landlord shall have the right in its sole and absolute discretion to cancel Tenant’s exercise of the option by delivery of written notice to Tenant prior to commencement of the applicable Renewal Term.
Page 24 of 26
22.3. In the event Tenant exercises its option for a Renewal Term pursuant to the terms of this Section 22, landlord and Tenant shall determine the Fair Market Rent for the Premises during the Renewal Term as follows:
| (i) | Within twenty (20) calendar days after Landlord receives the Renewal Notice, Landlord shall deliv!;!r to Tenant a notice of the proposed Base Rent for the Renewal Term (“Landlord’s Rent Notice” ). |
| (ii) | Within twenty (20) calendar days after receipt of Landlord’s Rent Notice, Tenant may, at its option, deliver to Landlord a notice of Tenant’s proposed Base Rent for the Renewal Term (“Tenant’s Rent Notice”). Landlord shall deem receipt of Tenant’s Rent Notice a rejection of Landlord’s Rent Notice. If Landlord does not receive Tenant’s Rent Notice within the aforementioned twenty (20) calendar days, then the parties shall deem the Base Rent set out in Landlord’s Rent Notice accepted by both parties as the Fair Market Rent for the Premises. |
| (iii) | Within fifteen (15) calendar days after receipt of Tenant’s Rent Notice, Landlord shall deliver to Tenant a notice of its acceptance or rejection of Tenant’s proposed Base Rent. If Landlord does not deliver notice of its acceptance or rejection of the Tenant’s proposed Base Rent within the aforementioned thirty (30) days, then Tenant’s proposed Base Rent shall be deemed rejected by Landlord. |
| (iv) | If Landlord rejects Tenant’s proposed Base Rent, the parties shall obtain an appraisal of the Fair Market Rent for the Premises by a member of the American Institute of Real Estate Appraisers mutually acceptable to both Landlord and Tenant (“Appraiser”). Landlord and Tenant shall share equally the cost of the appraisal. The parties hereby agree that the Appraiser’s determination of the Fair Market Rent for the Renewal Term will be final and binding on both parties. “Fair Market Rent” as used in this Section 22 means the annual base rent at which tenants comparable to Tenant, as of the date of Tenant’s Renewal Notice, are renewing leases for a comparable term and space comparable to the Premises, from a willing, comparable landlord, at arm’s length, which comparable space is located in “Comparable Buildings” in the vicinity of the Building (i.e., of a similar age and quality, considering any recent renovations or modernization, and floor plate size or, if such Comparable Buildings, or comparable space within Comparable Buildings, is not available, adjustments shall be made in determination of Fair Market Rent to reflect the age and quality of the Building and the Premises as contrasted to other buildings used for comparison purposes), with similar amenities, taking into consideration size, location, floor level, proposed term of the lease, extent of services to be provided, the time that the particular rate under consideration became or is to become effective, annual escalations, the improvements to the Premises, as well as all tenant concessions and inducements and the creditworthiness of the tenant (including Tenant). Additionally, in considering comparable space within Comparable Buildings, appropriate adjustments shall be made to the standard measurement by which the rentable square footage is measured, the ratio of rentable square feet to usable square feet, the type of escalation clause (e.g., whether increases in rent and additional rent are determined on a net or gross basis, and if gross, whether such increases are determined according to a base year or a base dollar amount expense stop), the extent of tenant’s liability under the lease, abatement provisions reflecting free rent and/or no rent during the period of construction or any other period during the Term. |
Page 25 of 26
22.4. Upon determination of the Base Rent for the Renewal Term, Landlord shall prepare, and Tenant shall execute within ten (10) calendar days of its receipt, an amendment to the Lease confirming the terms of the Renewal Term.
The parties are signing this Lease as of the date stated in the introductory clause.
| SIG 106 LLC, | DBV TECHNOLOGIES, INC., | |||||||
| a New Jersey limited liability company | a Delaware corporation | |||||||
| By: | /s/ Rich Travaglini |
By: | /s/ Caroline Danière |
|||||
| Name: | Rich Travaglini | Name: | Caroline Danière | |||||
| Title: | SVP-Director of Leasing | Title: | Secretary | |||||
Page 26 of 26
Exhibit 10.7
26 October 2023
Société des Produits Nestlé S.A. (formerly named NESTEC S.A.)
55, Avenue Nestlé
CH-1800 Vevey
Switzerland
| Attn: | Claudio Kuoni |
General Counsel of Nestlé Health Science
| Re: | Termination of the Development Collaboration and License Agreement |
Dear Mr. Kuoni:
Reference is made herein to that certain Development Collaboration and License Agreement, dated 27 May 2016, as amended (the “Agreement”), by and between NESTEC S.A. (“NESTEC”) and DBV TECHNOLOGIES S.A. (“DBV”). Capitalized terms used in herein not otherwise defined shall have the meanings given to them in the Agreement.
NESTEC and DBV entered into the Agreement to establish a collaboration whereby DBV would develop a diagnostic test for CMPA using DBV’s proprietary Viaskin™ Technology, and NESTEC would receive a license to commercialize such diagnostic product. Through no fault of either party, and after both parties having used Commercially Reasonable Efforts to pursue the development and commercialization of a diagnostic test for CMPA, the parties desire to cease all activities under the Agreement, including, without limitation, all Development activities, and the parties desire to terminate the Agreement in its entirety in accordance with the terms of this letter (this “Letter”), effective as of the last date of signature hereof, (the “Effective Date”).
Notwithstanding anything to the contrary in Section 15, NESTEC and DBV hereby agree to terminate the Agreement effective as of the Effective Date, subject to the orderly completion of any necessary wind-down activities related to the MAG1C study. Each party shall remain responsible for its own costs and expenses related to its respective wind-down activities. For the sake of clarity, any and all licenses and sublicenses, granted by either party to the other party under the Agreement, including, without limitation, any licenses to intellectual property, are hereby immediately revoked and terminated in accordance with Section 15.3.3(a) of the Agreement. Furthermore, no intellectual property of either party, including, without limitation, either party’s Know-How and Patents, is, or is intended to be, transferred, assigned, or conveyed by operation of this Letter.
NESTEC acknowledges that DBV deems the Agreement to be a “material definitive agreement” for purposes of appliable U.S. securities laws and that DBV is required to publicly disclose (through filings with the U.S. Securities and Exchange Commission (“SEC”), press release, or as otherwise determined necessary by DBV) the termination of the Agreement in compliance with applicable U.S. securities laws. Notwithstanding anything to the contrary in the Agreement, NESTEC hereby consents to DBV’s public disclosure of the termination of this Agreement, as of the Effective Date of this Letter, through SEC filing, press release, or as otherwise determined necessary by DBV to comply with applicable U.S. securities laws. DBV will consult with NESTEC before making any such public disclosure.
1
NESTEC and DBV each hereby knowingly, voluntarily, irrevocably, unconditionally, and absolutely fully, finally, and forever generally release, waive, quitclaim, and discharge the other party, and any parent, subsidiary, affiliate, predecessor, successor, and their respective directors, managers, officers, principals, employees, agents, contractors, shareholders, owners, partners and representatives, whether past or present (the “Released Parties”), from or for any and all claims, causes of action, demands, suits, liabilities, damages, costs, expenses, attorneys’ fees, and losses of every kind, nature, and description, whether known or unknown, actual or potential, suspected or unsuspected, fixed or contingent, at law or in equity, which NESTEC or DBV has or may hereafter have or claim to have had arising out of or related in any way to the Agreement or any other business dealing between NESTEC and DBV arising from or in connection with the Agreement (collectively, the “Claims”). NESTEC and DBV each further agree not to commence or participate in, and to take all actions necessary to opt out of, any Claims against the Released Parties. NESTEC and DBV each further declare and represent that they intend this release to be complete and not subject to any claim of mistake, and that such release herein expresses the full and complete release and NESTEC and DBV each intend such release to be final and complete. The release of the Claims set forth in this Letter may be pled by either party as a full and complete defense to, and may be used as a basis for injunctive relief against, any action that may be prosecuted, instituted, or attempted by the other party in breach hereof. The release set forth herein shall be effective upon the last date of signature of this Letter. This Letter shall in no event be construed as or deemed to be evidence of an admission or concession on the part of either party of any claim or any fault or liability for damages whatsoever. This Letter may not be introduced into evidence in any proceeding by any person or entity, nor may it be used in support of or for the prosecution of any cause of against either NESTEC or DBV, except solely for the purpose of enforcing the terms and conditions hereof.
Each party represents and warrants to the other party other that the individual signing for and on behalf of it has complete and full authority to act on such party’s behalf and has the authority to bind all other persons or entities with any right, title, or interest in that party’s Claims.
This Letter, together with the Agreement, constitutes the entire agreement and understanding of the parties with respect to the subject matter herein, and shall supersede all oral negotiations with prior writings with respect thereto. This Letter may be amended, modified, or supplemented only by a written instrument signed by both parties. None of the rights or obligations of either party arising hereunder may be assigned without the prior written consent of the other party and any such assignment absent such prior written consent shall be void ab initio and shall be of no force or effect. This Letter shall be binding upon, and shall inure to the benefit of, the parties, their heirs, executors, administrators, successors and permitted assigns.
IN WITNESS WHEREOF, NESTEC and DBV have executed this Letter as of the last date of signature hereof.
| Société des Produits Nestlé S.A. | DBV TECHNOLOGIES S.A. | |||||||
| By: | /s/ Claudio Kuoni |
By: | /s/ Michele Robertson |
|||||
| Name: | Claudio Kuoni | Name: | Michele Robertson | |||||
| Title: | General Counsel | Title: | Chief Legal Officer | |||||
| Date: | 27 October 2023 | Date: | 30 October 2023 | |||||
2
Exhibit 10.36
English Summary Translation of
Separation Agreement and Release between Sébastien Robitaille and DBV Technologies S.A.
Date: October 30, 2023
Parties: DBV Technologies S.A. (the “Company”) and Sébastien Robitaille
In connection with Mr. Robitaille’s departure from his position as Chief Financial Officer of the Company, among other things:
| • | Mr. Robitaille will continue to serve as an executive officer of the Company until November 17, 2023; |
| • | Certain equity incentive awards that were granted to Mr. Robitaille, and that would have otherwise been forfeited due to his departure from the Company, will vest on October 13, 2023 in accordance with the Separation Agreement. These equity awards consist of 35,000 restricted stock units. |
| • | Mr. Robitaille will be entitled to a severance payment of $425,527.45, a portion of his 2023 bonus in the amount of $99,479.60 and continuation of medical benefit coverage for a period of 3 months following his departure. |
| • | Mutual releases, subject to customer exceptions, and mutual covenants not to disparage. |
The Company is prohibited under applicable French law from disclosing any further details of Mr. Robitaille’s departure and this summary does not purport to be complete.
Exhibit 10.37
BETWEEN THE UNDERSIGNED:
DBV Technologies, a French public limited company (société anonyme) with share capital of 9,625,355.30 euros, registered in the Nanterre Trade Register under number 441 772 522, with its head office at 171-181, avenue Pierre Brossolette 92120 Montrouge, represented by Caroline Danière, in her capacity as Group Human Resources Director, duly authorized to act on behalf of DBV Technologies.
Hereinafter referred to as “the Company”,
ON THE ONE HAND,
AND
Virginie Boucinha, residing at 12 rue du Berger, Montreuil, born October 18, 1969, French nationality, social security number 2 69 10 91 326 047 12
Hereinafter referred to as Virginie
Boucinha or “the Employee”.
ON THE OTHER HAND,
IT HAS BEEN AGREED AS FOLLOWS:
ARTICLE 1 – COMMITMENT
The present employment contract is concluded for an indefinite period, commencing on November 6, 2023, it being specified that Virginie Boucinha will benefit from an informational and preventative care visit to a health professional within three months of her hiring.
Virginie Boucinha acknowledges that she is free from any commitments or non-competition clauses.
Virginie Boucinha’s pre-employment declaration has been filed with the URSSAF des Hauts-de-Seine. Virginie Boucinha may exercise her right of access and rectification under French Law no. 78.17 of January 6, 1978.
DBV Technologies
Head office: 177-181 Avenue Pierre Brossolette – 92120 Montrouge
No. 441 772 522 Nanterre Trade Register
Tel: 01 55 42 78 78; Fax: 01 43 26 10 83
1/9

In addition, and in accordance with Article D8223-1 et seq. of the French Labor Code, the Employee may obtain any information relating to the employer’s completion of this declaration of employment, by sending a written request to the relevant services, in particular the labor inspectorate or the Employee’s social security fund.
ARTICLE 2 – EMPLOYMENT AND QUALIFICATIONS
The Company has appointed Virginie Boucinha as Chief Financial Officer (Directeur Financier), with executive status.
In this capacity, Virginie Boucinha will serve on the Company’s Executive Committee. However, it is expressly agreed between the Parties that membership of the Company’s Executive Committee is not a determining factor in employee consent, and that any subsequent departure from this Committee would therefore not constitute a modification of the employment contract, but merely a change in working conditions.
This position falls under group XI of the French National Collective Bargaining Agreement for the Pharmaceutical Industry (La Convention collective nationale de l’industrie pharmaceutique), currently applicable in the Company.
Within the scope of her duties as defined by the collective bargaining agreement, the Employee will participate in the performance of work falling within the competencies for this position, as defined in the job classification.
In view of the need to adapt skills to changes in the Company, Virginie Boucinha’s duties may be adapted to the Company’s requirements at an equivalent group and level, without this constituting a change in her employment contract. Virginie Boucinha hereby undertakes to follow any adaptation training offered as part of her employment contract.
In the performance of her duties, Virginie Boucinha will be placed under the operational and functional authority of Daniel Tassé, Chief Executive Officer (Directeur Général), or any other person at an equivalent level.
ARTICLE 3 – TRIAL PERIOD
The Employee’s appointment can only be confirmed after a trial period of 4 months’ actual work, in accordance with the applicable legal and collective bargaining provisions.
During the trial period, each Party is free to withdraw from the contract by sending a registered letter with acknowledgment of receipt, respecting the legal notice period.
This period may be increased by periods of suspension of the employment contract during the trial period, in particular in the event of sick leave or workplace accident.
2/9

This trial period cannot be extended on account of the notice period.
During the trial period, the Employee undertakes to provide all the information required to draw up her dossier.
ARTICLE 4 – COMPENSATION AND WORKING HOURS
In view of the responsibilities entrusted to her, which involve a high degree of independence in the organization of her schedule, her authorization to make independent decisions, and her remuneration, which is among the highest in the Company, Virginie Boucinha qualifies as a senior executive as defined by the French Labor Code.
As a result, Virginie Boucinha is not subject to the legal and collective bargaining rules governing working hours, and in particular is not required to keep a record of her working hours (Article L.3111-2 of the French Labor Code).
Virginie Boucinha receives flat-rate compensation for her work, on the understanding that there is no link between the amount of this compensation and actual working hours.
This annual compensation consists of the following elements:
| • | A fixed portion amounting to 295,000 euros gross per year, payable in twelve equal monthly installments. |
| • | A variable portion equivalent to 40% of her gross annual base salary. This payment will be conditional on the achievement of individual and collective annual targets, in accordance with the Company’s own terms and conditions. |
It has also been agreed to grant her, as part of the collective share allocation plan taking place at the end of November 2023, subject to approval by the Board of Directors on November 20, 2023: 113,000 stock options and 19,000 free shares subject to an attendance requirement, as defined in the detailed plan regulations, which Virginie Boucinha will receive following approval.
ARTICLE 5 – PLACE OF WORK
For information purposes and subject to the provisions below, Virginie Boucinha will perform her duties at the Company’s premises at 177-181 avenue Pierre Brossolette, Montrouge (92).
In view of the nature of her duties, Virginie Boucinha undertakes to accept any change in her place of work that may be justified by changes in the Company’s business, organization or, more generally, the smooth running of the Company (in the context of the conclusion of partnership contracts, or the creation or relocation of a Company-owned establishment). This mobility may be exercised throughout the Paris region, in the following French departments: 75, 77, 78, 91, 92, 93 and 94.
3/9

ARTICLE 6 – OCCASIONAL TRAVEL AND COMPENSATION
Virginie Boucinha may be required to make occasional business trips, in the course of her duties, in France or abroad, in particular to any research and/or manufacturing establishment working in collaboration with the Company. To do so, she must have a valid passport that can be used for travel abroad. The Employee agrees to undertake all business travel necessary for the performance of her duties.
Business expenses incurred by Virginie Boucinha in the performance of her duties and in accordance with the Company’s instructions will be reimbursed on presentation of receipts at the end of each month, in accordance with the reimbursement terms and conditions set by the Company on the date the expenses were incurred.
ARTICLE 7 – OBLIGATIONS OF VIRGINIE BOUCINHA
| ➣ | Discretion |
In view of her duties and responsibilities, Virginie Boucinha is bound by an obligation of absolute discretion with regard to all matters concerning the performance of her duties, to secrets or special processes relating to research, manufacturing, trade or the organization of the Company, without this list being considered exhaustive.
Virginie Boucinha acknowledges that all non-public information and knowledge that she may acquire in the performance of this contract, including in particular, the results of the Company’s research work, all information concerning the products, equipment and processes used or developed by the Company, and all details concerning the Company’s contracts, customers or finances, are and must remain strictly confidential.
Consequently, they may not be used for purposes other than those provided for in this contract, and may under no circumstances be communicated, made visible or accessible to third parties, nor may they be made public without the Company’s prior agreement. Virginie Boucinha undertakes to take every precaution to preserve the utmost confidentiality, both during the term of this contract and after its expiry or termination for any reason whatsoever.
In addition, Virginie Boucinha acknowledges that she may receive certain non-public information from third parties in the course of collaborations between the Company and those third parties, which is subject to confidentiality and limited use obligations. Virginie Boucinha undertakes to use (and not to copy, reproduce or duplicate) this information solely for the purposes for which it was communicated, and to disclose it only to those Company employees who need it for these purposes.
This obligation applies both to third parties and to Company employees.
4/9

The Company reserves the right, in the event of infringement on her part, to claim damages for the harm caused, independently of any criminal sanctions it may incur.
| ➣ | Company Equipment |
Virginie Boucinha expressly agrees not to use the various items, equipment and documents made available to her for any purpose other than that authorized by the company.
All documents of any kind whatsoever, correspondence, circulars, memos and instructions, given by the Company to Virginie Boucinha or of which she may become aware in the course of her duties, and without the above list being considered exhaustive, as well as any equipment Virginie Boucinha may have in her possession for the performance of her duties, are confidential and remain the exclusive property of the Company. They must be returned to the Company immediately upon request and in the event of departure.
| ➣ | Compliance with Instructions |
Throughout her contract, Virginie Boucinha undertakes to comply with any instructions given to her by her employer and to abide by the rules governing the Company’s internal operations.
| ➣ | Loyalty |
In application of the principle of loyalty in the performance of the employment contract, throughout the term of her employment contract, the Employee undertakes not to engage directly or indirectly in any activity in competition with the Company.
5/9

ARTICLE 8 – SUPPLEMENTARY PENSION AND PROVIDENT SCHEME
Virginie Boucinha is covered by the Régime de Prévoyance de l’Industrie Pharmaceutique (RPC) for provident benefits (insured by AXA—313, Terrasses de l’Arche—92727 NANTERRE Cedex) and medical expenses (insured by APGIS and managed by Aon – Service Frais Médicaux, 28 Allée de Bellevue – CS 70000, 16918 Angoulême – Cedex 9), as well as by supplementary schemes set up by unilateral decision of the employer. Information leaflets on the benefits in force have been given to the Employee.
The Employee will be affiliated to the AGIRC-ARRCO KLESIA supplementary pension fund (4 rue Marie-Georges Picquart 75017 Paris) to which DBV Technologies is affiliated.
ARTICLE 9 – ELECTRONIC SIGNATURE AND ELECTRONIC REGISTERED MAIL
Pursuant to the provisions of the French Civil Code and the French Postal and Electronic Communications Code currently in force, Virginie Boucinha:
| • | Acknowledges that her electronic signature has the same legal value as her handwritten signature, and accepts this, in accordance with the provisions of Article 1367 of the aforementioned Code. |
| • | Confirms that the data relating to this signature (username and password) are of a personal and confidential nature and undertakes not to pass them on to any third party whatsoever. Any breach of this commitment may lead the Company to consider disciplinary action. |
| • | Declares that she accepts the electronic delivery of registered mail from DBV Technologies concerning the conclusion or performance of her employment contract. |
Virginie Boucinha must inform the Personnel Department as soon as possible of her personal e-mail address to which these messages may be sent. If her e-mail address changes in the future, she must inform the department immediately.
6/9

ARTICLE 10 – INTELLECTUAL AND/OR INDUSTRIAL PROPERTY RIGHTS
Virginie Boucinha recognizes the particular importance to the Company of protecting its industrial and intellectual property rights over all creations, and in particular all products, formulas, sequences, processes, applications and inventions, that the Employee creates, discovers or develops, whatever the legal nature of these creations (in particular trade secrets, patents, copyrights, software).
Consequently, if in the course of her duties, Virginie Boucinha creates any inventions whatsoever, whether patentable or not, or creates designs, models, methods, programs, formulas or processes relating to the Company’s activities, studies or research, whether or not they are likely to be protected, the resulting intellectual or industrial property rights will automatically belong to the Company as and when they are realized.
It is also noted that, in accordance with legal provisions, the economic rights to the software and documentation created by Virginie Boucinha in the course of her duties or on the Company’s instructions will automatically be vested in the Company, which alone will be entitled to exercise them.
Consequently, Virginie Boucinha undertakes not to profit from these creations, directly or indirectly, for her own account or for that of any third party, either during the term of this contract or after its expiry or termination for any reason whatsoever.
In particular, the Employee acknowledges that the Company has the right:
| • | To apply for and obtain protection in its own name by all appropriate means, in particular by filing patent applications, both in France and abroad, and possibly by registering models and trademarks, it being understood that the inventors’ names will be mentioned in the patents, in accordance with the applicable rules in force. |
| • | To assign and/or license the industrial and intellectual property rights it holds to any other person or company in accordance with this Article. |
| • | To carry out any industrial, commercial or financial operations in which the rights held by it in accordance with this Article may be included. |
In addition, Virginie Boucinha undertakes, both during the term of this contract and after its expiry or termination for any reason whatsoever, to make all declarations, complete all formalities and take all steps that may be necessary for the Company (or any other company that it may designate for this purpose) to assert the aforementioned rights and to enable the Company to obtain, maintain and defend the exploitation rights of the creations produced, the inventions made or the software developed in the performance of this Agreement.
The results obtained by the Employee in the performance of the present Agreement may be used for the preparation of scientific communications or publications, but may only be presented after the Company’s commercial interests have been protected, and in particular not before the Company has obtained the appropriate industrial protection by filing patent applications.
7/9

Consequently, the Employee will have to submit a proposal to the Company and obtain its authorization before proceeding with any publication or other public communication concerning these results.
By signing this contract, Virginie Boucinha undertakes to refrain from any concealment and to declare, in particular, all accomplishments and inventions of which she is the author or co-author by communicating to the Company all information, studies, drawings, plans or documents in her possession relating to inventions or creations made by her or with her assistance.
ARTICLE 11 – INFORMATION
This employment relationship is governed by the following texts and provisions to which the employment contract sometimes refers, within the limits and according to the terms of their own application:
| • | The legal, regulatory and collective bargaining provisions concerning the acquisition and taking of paid leave. |
| • | The French national collective bargaining agreement for the pharmaceutical industry currently applicable in the Company, which governs, in particular, the notice period in the event of termination of the employment contract. |
| • | Employer practices and decisions applicable by category. |
The Parties undertake to comply with the legal, regulatory and collective bargaining provisions in force in the Company, as well as all the Company’s internal procedures and regulations.
Virginie Boucinha declares in particular that she has read the internal regulations and appendices.
ARTICLE 12 – GENERAL DATA PROTECTION REGULATION
In the course of managing its personnel, the Company collects personal data on employees, in particular their first and last names, addresses, telephone numbers, etc.
These personal data will only be processed or used insofar as this is necessary for:
| • | Executing the employment contract (use of bank details, use of postal address for sending various correspondence, etc.) |
8/9

And
| • | Meeting legal and/or regulatory obligations (use of last name, first name, date and place of birth, social security number for the DPAE (pre-employment declaration), and DSN (nominative social security declaration) with the URSSAF, |
| • | Using marital status and number of children to enroll in provident and health insurance plans. |
Personal data will be kept for as long as is necessary for the performance of the contract, the fulfillment of the Company’s legal and regulatory obligations, and the exercise of the prerogatives conferred upon it by law and case law.
Throughout the period of storage of personal data, the Company takes all necessary steps to ensure the confidentiality and security of such data, in order to prevent damage, deletion or access by unauthorized third parties.
Access to personal data is strictly limited to Company employees authorized to process such data on the basis of their duties. The information collected may be communicated to third parties contractually bound to the Company for the performance of subcontracted tasks necessary for the management of the Employee’s contract. It is specified that, in the context of the performance of their services, third parties have only limited access to the data and are obliged to use it in compliance with the provisions of the applicable legislation on the protection of personal data.
In accordance with the applicable legal and regulatory provisions, the Employee has the right to access, rectify, transfer and request the deletion of her data, or to limit the processing of her data. The Employee may also, for legitimate reasons, object to the processing of data concerning her.
Drawn up in two original copies, one of which will be kept by Virginie Boucinha and the other by the Company, duly initialed, dated, and signed, preceded by the words “lu et approuvé, bon pour accord” (“read and approved, agreed”).
| Lu et approuvé, bon pour accord | Lu et approuvé, bon pour accord | |
| For the Company Caroline Danière Group Human Resources Director |
The Employee Virginie Boucinha |
|
| /s/ Caroline Danière November 1, 2023 | /s/ Virginie Boucinha November 1, 2023 | |
9/9
Exhibit 21.1
Subsidiaries
| Name of Subsidiary |
State or Other Jurisdiction of Incorporation |
|
| DBV Technologies Inc. | Delaware, United State of America | |
| DBV Technologies Australia PTY Ltd. | New South Wales, Australia | |
| DBV Pharma S.A.S. | France | |
Exhibit 23.1
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We consent to the incorporation by reference in the Registration Statement No. 333-257569 on Form S-3, the Registration Statement No. 333-266449 on Form S-3, the Registration Statement No. 333-266448 on Form S-3, the Registration Statement No. 333-271166 on Form S-3, the Registration Statement No. 333-199513 on Form S-8, and the Registration Statement No. 333-275662 on Form S-8 of our report dated March 7, 2024, relating to the consolidated financial statements of DBV Technologies S.A. appearing in this Annual Report on Form 10-K for the year ended December 31, 2023.
/s/ Deloitte & Associés
Paris-La Défense, France
March 7, 2024
Exhibit 23.2

KPMG SA
Tour EQHO
2 Avenue Gambetta
CS 60055
92066 Paris La Défense Cedex
Consent of Independent Registered Public Accounting Firm
We consent to the incorporation by reference in the registration statements Nos. 333-257569, 333-266448, 333-266449 and 333-271166 on Form S-3 and the registration statements No. 333-199513 and 333-275662 on Form S-8 of our report dated March 7, 2024, with respect to the consolidated financial statements of DBV Technologies S.A. and subsidiaries.
KPMG S.A.
Cédric Adens
Partner
Paris La Défense, France
March 7, 2024
| KPMG SA société française membre du réseau KPMG constitué de cabinets indépendants adhérents de KPMG International Limited, une société de droit anglais (“a private company limited by guarantee”). |
SA Société de commissariat aux comptes Siège social : Tour EQHO 2 Avenue Gambetta CS 60055 92066 Paris La Défense Cedex 775726417 RCS NANTERRE |
Exhibit 31.1
Certification by the Principal Executive Officer pursuant to
Securities Exchange Act Rules 13a-14(a) and 15d-14(a)
as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
I, Daniel Tassé, certify that:
| 1. | I have reviewed this Annual Report on Form 10-K of DBV Technologies S.A.; |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report; |
| 4. | The company’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the company and have: |
| (a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| (b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| (c) | Evaluated the effectiveness of the company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| (d) | Disclosed in this report any change in the company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company’s internal control over financial reporting; and |
| 5. | The company’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company’s auditors and the audit committee of the company’s board of directors (or persons performing the equivalent functions): |
| (a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company’s ability to record, process, summarize and report financial information; and |
| (b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the company’s internal control over financial reporting. |
Date: March 7, 2024
| /s/ Daniel Tassé |
| Name: Daniel Tassé |
| Title: Chief Executive Officer |
| (Principal Executive Officer) |
Exhibit 31.2
Certification by the Principal Financial Officer pursuant to
Securities Exchange Act Rules 13a-14(a) and 15d-14(a)
as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
I, Virginie Boucinha, certify that:
| 1. | I have reviewed this Annual Report on Form 10-K of DBV Technologies S.A.; |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report; |
| 4. | The company’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the company and have: |
| (a) | Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| (b) | Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| (c) | Evaluated the effectiveness of the company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| (d) | Disclosed in this report any change in the company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company’s internal control over financial reporting; and |
| 5. | The company’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company’s auditors and the audit committee of the company’s board of directors (or persons performing the equivalent functions): |
| (a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company’s ability to record, process, summarize and report financial information; and |
| (b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the company’s internal control over financial reporting. |
| Date: March 7, 2024 | ||
| /s/ Virginie Boucinha |
||
| Name: | Virginie Boucinha | |
| Title: | Chief Financial Officer | |
| (Principal Financial Officer) | ||
Exhibit 32.1
CERTIFICATION
Pursuant to the requirement set forth in Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended, (the “Exchange Act”) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. §1350), Daniel Tassé, Chief Executive Officer of DBV Technologies S.A. (the “Company”), and Virginie Boucinha, Chief Financial Officer of the Company, each hereby certifies that, to the best of his knowledge:
| 1. | The Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, to which this Certification is attached as Exhibit 32.1 (the “Annual Report”), fully complies with the requirements of Section 13(a) or Section 15(d) of the Exchange Act; and |
| 2. | The information contained in the Annual Report fairly presents, in all material respects, the financial condition and results of operations of the Company. |
Dated: March 7, 2024
IN WITNESS WHEREOF, the undersigned have set their hands hereto as of the 7th day of March, 2024.
| /s/ Daniel Tassé Daniel Tassé Chief Executive Officer |
/s/ Virginie Boucinha Virginie Boucinha Chief Financial Officer |
This certification accompanies the Form 10-K to which it relates, is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference into any filing of DBV Technologies S.A. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of the Form 10-K), irrespective of any general incorporation language contained in such filing.
Exhibit 97.1
DBV TECHNOLOGIES S.A.
INCENTIVE COMPENSATION RECOUPMENT POLICY
| 1. | INTRODUCTION |
The Board of Directors (the “Board”) of DBV Technologies S.A., a société anonyme organized under the laws of France (the “Company”), upon recommendation of the Compensation Committee of the Board (the “Compensation Committee”), has determined that it is in the best interests of the Company and its shareholders to adopt this Incentive Compensation Recoupment Policy (this “Policy”) providing for the Company’s recoupment of Recoverable Incentive Compensation that is received by Covered Officers of the Company under certain circumstances. Certain capitalized terms used in this Policy have the meanings given to such terms in Section 3 below.
This Policy is designed to comply with, and shall be interpreted to be consistent with, Section 10D of the Exchange Act, Rule 10D-1 promulgated thereunder (“Rule 10D-1”) and Nasdaq Listing Rule 5608 (the “Listing Standards”).
| 2. | EFFECTIVE DATE |
This Policy shall apply to all Incentive Compensation that is received by a Covered Officer on or after October 2, 2023 (the “Effective Date”). Incentive Compensation is deemed “received” in the Company’s fiscal period in which the Financial Reporting Measure specified in the Incentive Compensation award is attained, even if the payment or grant of such Incentive Compensation occurs after the end of that period.
| 3. | DEFINITIONS |
“Accounting Restatement” means an accounting restatement that the Company is required to prepare due to the material noncompliance of the Company with any financial reporting requirement under the U.S. securities laws, including any required accounting restatement to correct an error in previously issued financial statements that is material to the previously issued financial statements, or that would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period.
“Accounting Restatement Date” means the earlier to occur of (a) the date that the Board, a committee of the Board authorized to take such action, or the officer or officers of the Company authorized to take such action if Board action is not required, concludes, or reasonably should have concluded, that the Company is required to prepare an Accounting Restatement, or (b) the date that a court, regulator or other legally authorized body directs the Company to prepare an Accounting Restatement.
“Code” means the U.S. Internal Revenue Code of 1986, as amended, and the regulations promulgated thereunder.
“Covered Officer” means each current and former Executive Officer.
“Exchange” means the Nasdaq Stock Market.
“Exchange Act” means the U.S. Securities Exchange Act of 1934, as amended.
“Executive Officer” means the Company’s president, principal financial officer, principal accounting officer (or if there is no such accounting officer, the controller), any vice-president of the
Company in charge of a principal business unit, division, or function (such as sales, administration, or finance), any other officer who performs a policy-making function, or any other person who performs similar policy-making functions for the Company. Executive officers of the Company’s parent(s) or subsidiaries are deemed executive officers of the Company if they perform such policy-making functions for the Company. Policy-making function is not intended to include policy-making functions that are not significant. Identification of an executive officer for purposes of this Policy would include at a minimum executive officers identified pursuant to Item 401(b) of Regulation S-K promulgated under the Exchange Act.
“Financial Reporting Measures” means measures that are determined and presented in accordance with the accounting principles used in preparing the Company’s financial statements, and any measures derived wholly or in part from such measures, including Company share price and total shareholder return
(“TSR”). A measure need not be presented in the Company’s financial statements or included in a filing with the SEC in order to be a Financial Reporting Measure.
“Incentive Compensation” means any compensation that is granted, earned or vested based wholly or in part upon the attainment of a Financial Reporting Measure.
“Lookback Period” means the three (3) completed fiscal years immediately preceding the Accounting Restatement Date, as well as any transition period (resulting from a change in the Company’s fiscal year) within or immediately following those three completed fiscal years (except that a transition period of at least nine months shall count as a completed fiscal year). Notwithstanding the foregoing, the Lookback Period shall not include fiscal years completed prior to the Effective Date.
“Recoverable Incentive Compensation” means Incentive Compensation received by a Covered Officer during the Lookback Period that exceeds the amount of Incentive Compensation that would have been received had such amount been determined based on the Accounting Restatement, computed without regard to any taxes paid (i.e., on a gross basis without regard to tax withholdings and other deductions). For any compensation plans or programs that take into account Incentive Compensation, the amount of Recoverable Incentive Compensation for purposes of this Policy shall include, without limitation, the amount contributed to any notional account based on Recoverable Incentive Compensation and any earnings to date on that notional amount. For any Incentive Compensation that is based on share price or TSR, where the Recoverable Incentive Compensation is not subject to mathematical recalculation directly from the information in an Accounting Restatement, the Compensation Committee will determine the amount of Recoverable Incentive Compensation based on a reasonable estimate of the effect of the Accounting Restatement on the share price or TSR upon which the Incentive Compensation was received. The Company shall maintain documentation of the determination of that reasonable estimate and provide such documentation to the Exchange in accordance with the Listing Standards.
“SEC” means the U.S. Securities and Exchange Commission.
| 4. | RECOUPMENT |
(a) Applicability of Policy. This Policy applies to Incentive Compensation received by a Covered Officer (i) after beginning services as an Executive Officer, (ii) who served as an Executive Officer at any time during the performance period for such Incentive Compensation, (iii) while the Company had a class of securities listed on a U.S. national securities exchange or a national securities association, and (iv) during the Lookback Period.
(b) Recoupment Generally. Pursuant to the provisions of this Policy, if there is an Accounting Restatement, the Company must reasonably promptly recoup the full amount of the Recoverable Incentive Compensation, unless the conditions of one or more subsections of Section 4(c) of this Policy are met and the Compensation Committee, or, if the Compensation Committee does not consist solely of independent directors, a majority of the independent directors serving on the Board, has made a determination that recoupment would be impracticable. Recoupment is required regardless of whether the Covered Officer engaged in any misconduct and regardless of fault, and the Company’s obligation to recoup Recoverable Incentive Compensation is not dependent on whether or when any restated financial statements are filed.
(c) Impracticability of Recovery. Recoupment may be determined to be impracticable if, and only if:
(i) the direct expense paid to a third party to assist in enforcing this Policy would exceed the amount of the applicable Recoverable Incentive Compensation; provided that, before concluding that it would be impracticable to recover any amount of Recoverable Incentive Compensation based on expense of enforcement, the Company shall make a reasonable attempt to recover such Recoverable Incentive Compensation, document such reasonable attempt(s) to recover, and provide that documentation to the Exchange in accordance with the Listing Standards; or
(ii) recoupment of the applicable Recoverable Incentive Compensation would likely cause an otherwise tax-qualified retirement plan, under which benefits are broadly available to employees of the Company, to fail to meet the requirements of Code Section 401(a)(13) or Code Section 411(a) and regulations thereunder.
(d) Sources of Recoupment. To the extent permitted by applicable law, the Compensation Committee shall, in its sole discretion, determine the timing and method for recouping Recoverable Incentive Compensation hereunder, provided that such recoupment is undertaken reasonably promptly. The Compensation Committee may, in its discretion, seek recoupment from a Covered Officer from any of the following sources or a combination thereof, whether the applicable compensation was approved, awarded, granted, payable or paid to the Covered Officer prior to, on or after the Effective Date: (i) direct repayment of Recoverable Incentive Compensation previously paid to the Covered Officer; (ii) cancelling prior cash or equity-based awards (whether vested or unvested and whether paid or unpaid); (iii) cancelling or offsetting against any planned future cash or equity-based awards; (iv) forfeiture of deferred compensation, subject to compliance with Code Section 409A; and (v) any other method authorized by applicable law or contract. Subject to compliance with any applicable law, the Compensation Committee may effectuate recoupment under this Policy from any amount otherwise payable to the Covered Officer, including amounts payable to such individual under any otherwise applicable Company plan or program, e.g., base salary, bonuses or commissions and compensation previously deferred by the Covered Officer. The Compensation Committee need not utilize the same method of recovery for all Covered Officers or with respect to all types of Recoverable Incentive Compensation.
(e) No Indemnification of Covered Officers. Notwithstanding any indemnification agreement, applicable insurance policy or any other agreement or provision of the Company’s articles of association or bylaws to the contrary, no Covered Officer shall be entitled to indemnification or advancement of expenses in connection with any enforcement of this Policy by the Company, including paying or reimbursing such Covered Officer for insurance premiums to cover potential obligations to the Company under this Policy.
(f) Indemnification of Compensation Committee. Any members of the Compensation Committee, and any other members of the Board who assist in the administration of this Policy, shall not be personally liable for any action, determination or interpretation made with respect to this Policy and shall be indemnified by the Company to the fullest extent under applicable law and Company policy with respect to any such action, determination or interpretation. The foregoing sentence shall not limit any other rights to indemnification of the members of the Board under applicable law or Company policy.
(g) No “Good Reason” for Covered Officers. Any action by the Company to recoup or any recoupment of Recoverable Incentive Compensation under this Policy from a Covered Officer shall not be deemed (i) “good reason” for resignation or to serve as a basis for a claim of constructive termination under any benefits or compensation arrangement applicable to such Covered Officer, or (ii) to constitute a breach of a contract or other arrangement to which such Covered Officer is party.
| 5. | ADMINISTRATION |
Except as specifically set forth herein, this Policy shall be administered by the Compensation Committee. The Compensation Committee shall have full and final authority to make any and all determinations required under this Policy. Any determination by the Compensation Committee with respect to this Policy shall be final, conclusive and binding on all interested parties and need not be uniform with respect to each individual covered by this Policy. In carrying out the administration of this Policy, the Compensation Committee is authorized and directed to consult with the full Board or such other committees of the Board as may be necessary or appropriate as to matters within the scope of such other committee’s responsibility and authority. Subject to applicable law, the Compensation Committee may authorize and empower any officer or employee of the Company to take any and all actions that the Compensation Committee, in its sole discretion, deems necessary or appropriate to carry out the purpose and intent of this Policy (other than with respect to any recovery under this Policy involving such officer or employee).
| 6. | SEVERABILITY |
If any provision of this Policy or the application of any such provision to a Covered Officer shall be adjudicated to be invalid, illegal or unenforceable in any respect, such invalidity, illegality or unenforceability shall not affect any other provisions of this Policy, and the invalid, illegal or unenforceable provisions shall be deemed amended to the minimum extent necessary to render any such provision or application enforceable.
| 7. | NO IMPAIRMENT OF OTHER REMEDIES |
Nothing contained in this Policy, and no recoupment or recovery as contemplated herein, shall limit any claims, damages or other legal remedies the Company or any of its affiliates may have against a Covered Officer arising out of or resulting from any actions or omissions by the Covered Officer. This Policy does not preclude the Company from taking any other action to enforce a Covered Officer’s obligations to the Company, including, without limitation, termination of employment and/or institution of civil proceedings. This Policy is in addition to the requirements of Section 304 of the Sarbanes-Oxley Act of 2002 (“SOX 304”) that are applicable to the Company’s Chief Executive Officer and Chief Financial Officer and to any other compensation recoupment policy and/or similar provisions in any employment, equity plan, equity award, or other individual agreement, to which the Company is a party or which the Company has adopted or may adopt and maintain from time to time; provided, however, that compensation recouped pursuant to this Policy shall not be duplicative of compensation recouped pursuant to SOX 304 or any such compensation recoupment policy and/or similar provisions in any such employment, equity plan, equity award, or other individual agreement except as may be required by law.
| 8. | AMENDMENT; TERMINATION |
The Compensation Committee may amend, terminate or replace this Policy or any portion of this Policy at any time and from time to time in its sole discretion. The Compensation Committee shall amend this Policy as it deems necessary to comply with applicable law or any Listing Standard.
| 9. | SUCCESSORS |
This Policy shall be binding and enforceable against all Covered Officers and, to the extent required by Rule 10D-1 and/or the applicable Listing Standards, their beneficiaries, heirs, executors, administrators or other legal representatives.
| 10. | REQUIRED FILINGS |
The Company shall make any disclosures and filings with respect to this Policy that are required by law, including as required by the SEC.
* * * * *
DBV TECHNOLOGIES S.A.
INCENTIVE COMPENSATION RECOUPMENT POLICY
FORM OF EXECUTIVE ACKNOWLEDGMENT
I, the undersigned, agree and acknowledge that I am bound by, and subject to, the DBV Technologies S.A. Incentive Compensation Recoupment Policy, as may be amended, restated, supplemented or otherwise modified from time to time (the “Policy”). In the event of any inconsistency between the Policy and the terms of any employment agreement, offer letter or other individual agreement with DBV Technologies S.A. (the “Company”) to which I am a party, or the terms of any compensation plan, program or agreement, whether or not written, under which any compensation has been granted, awarded, earned or paid to me, the terms of the Policy shall govern.
In the event that the Compensation Committee (as defined in the Policy) determines that any compensation granted, awarded, earned or paid to me must be forfeited or reimbursed to the Company pursuant to the Policy, I will promptly take any action necessary to effectuate such forfeiture and/or reimbursement. I further agree and acknowledge that I am not entitled to indemnification, and hereby waive any right to advancement of expenses, in connection with any enforcement of the Policy by the Company.
Agreed and Acknowledged:
|
|
||
| Name: |
|
|
| Title: |
|
|
| Date: |
|
|