UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 4, 2024
KYMERA THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-39460 | 81-2992166 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
Kymera Therapeutics, Inc.
| 200 Arsenal Yards Blvd., Suite 230 |
| Watertown, Massachusetts 02472 |
| (Address of principal executive offices, including zip code) |
(857) 285-5300
(Registrant’s telephone number, including area code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trade Symbol(s) |
Name of each exchange on which registered |
||
| Common Stock, $0.0001 par value per share | KYMR | The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On January 4, 2024, Kymera Therapeutics, Inc. (the “Company”) held a virtual immunology research and development day event to provide an overview of the Company’s emerging pipeline of high-value immunology programs, including new target disclosures, supporting preclinical data and timing to clinical study initiation. A form of the slide presentation is being furnished as Exhibit 99.1 to this Current Report on Form 8-K. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.1.
The information in this Item 7.01, including Exhibit 99.1 attached hereto, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, except as expressly set forth by specific reference in such filing.
| Item 9.01. | Exhibits |
(d) Exhibits
| Exhibit No. |
Description | |
| 99.1 | Kymera Therapeutics, Inc. R&D Day Presentation, dated January 4, 2024, furnished herewith. | |
| 104 | Cover Page Interactive Data (embedded within the Inline XBRL document). | |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Kymera Therapeutics, Inc. | ||||||
| Date: January 4, 2024 | By: | /s/ Nello Mainolfi |
||||
| Nello Mainolfi, Ph.D. | ||||||
| Founder, President and Chief Executive Officer | ||||||

Exhibit 99.1 2024 IMMUNOLOGY R&D DAY January 4, 2024 ©2023 KYMERA THERAPEUTICS, INC. PAGE 1

Welcome Justine Koenigsberg Vice President, Investor Relations 2

Forward Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. These statements include, but are not limited to, implied and express statements about our strategy, business plans and objectives for our programs; plans and timelines for the preclinical and clinical development of our product candidates, including the therapeutic potential, clinical benefits and safety profiles of such product candidates; expectations regarding timing, success and data announcements of ongoing preclinical studies and clinical trials; our ability to initiate new clinical programs, including plans to submit investigational new drug (IND) applications; the initiation, timing, progress and results of our current and future preclinical studies and clinical trials of our current and prospective product candidates; our plans to develop and commercialize our current and any future product candidates and the implementation of our business model and strategic plans for our business, current and any future product candidates. All statements other than statements of historical facts contained in this presentation, including express or implied statements regarding our strategy, future financial condition, future operations, projected costs, prospects, plans, objectives of management and expected market growth, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as ‘‘anticipate,’’ ‘‘assume,’’ ‘‘believe,’’ ‘‘could,’’ ‘‘estimate,’’ ‘‘expect,’’ ‘‘goal,’’ ‘‘intend,’’ ‘‘may,’’ “milestones,” ‘‘objective,’’ ‘‘plan,’’ ‘‘predict,’’ ‘‘potential,’’ ‘‘seek,’’ ‘‘should,’’ ‘‘target,’’ ‘‘will,’’ ‘‘would’’ and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. You should not rely upon forward-looking statements as predictions of future events and actual results or events could differ materially from the plans, intentions and expectations disclosed herein. Any forward-looking statements are based on management's current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements including, without limitation, risks associated with: the timing and anticipated results of our current and future preclinical studies and clinical trials, supply chain, strategy and future operations; the delay of any current and future preclinical studies or clinical trials or the development of our drug candidates; the risk that the results of prior preclinical studies and clinical trials may not be predictive of future results in connection with current or future preclinical studies and clinical trials, including those for KT-474, KT-333, KT-253, KT-621, and KT-294; our ability to successfully demonstrate the safety and efficacy of our drug candidates; the timing and outcome of any interactions with regulatory authorities; obtaining, maintaining and protecting our intellectual property; our relationships with existing and future collaboration partners; the impacts of current macroeconomic and geopolitical events. In addition, any forward-looking statements represent Kymera’s views only as of today and should not be relied upon as representing its views as of any subsequent date. Kymera explicitly disclaims any obligation to update any forward-looking statements, except as required by law. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. As a result of these risks and others, including those set forth in our filings with the SEC, actual results could vary significantly from those anticipated in this presentation, and our financial condition and results of operations could be materially adversely affected. Certain information contained in this presentation and statements made orally during this presentation relate to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party studies, publications, surveys and other data to be reliable as of the date of the presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent sources have evaluated the reasonableness or accuracy of the Company’s internal estimates or research, and no reliance should be made on any information or statements made in this presentation relating to or based on such internal estimates and research. This presentation contains trademarks, trade names and service marks of other companies, which are the property of their respective owners. 3

Agenda Welcome 10:00 - 10:05 Justine Koenigsberg, Vice President, Investor Relations Revolutionizing Immunology with Small Molecule Oral Degraders 10:05 - 10:25 Nello Mainolfi, Ph.D., Founder, President and CEO Paving the Way: KT-474, a First-in-Class Oral IRAK4 Degrader 10:25 - 10:35 Jared Gollob, M.D., Chief Medical Officer Dupilumab-like Activity in a Pill: KT-621, a First-in-Class Oral STAT6 Degrader 10:35 - 10:55 Amy Wang, Ph.D., Senior Director and Program Lead, Immunology Degrading a Proven Target: KT-294, a First-in-Class Oral TYK2 Degrader 10:55 - 11:15 Juliet Williams, Ph.D., Head of Research Closing Remarks: Solving Big Problems with Small Molecules 11:15 - 11:20 Nello Mainolfi, Ph.D., Founder, President and CEO Q&A 11:20 - 12:00 4

Revolutionizing Immunology with Small Molecule Oral Degraders Nello Mainolfi, Ph.D., Founder, President and CEO 5

Mission Build a global medicines company that harnesses novel modalities to revolutionize healthcare

What I Will Cover • Targeted Protein Degradation: Harnessing a Game-Changing Novel Modality • Demonstrating Reproducible and Scalable Clinical Innovation • Our Target Selection Strategy • Why Oral Degraders in Immunology • Our Two New Programs 7
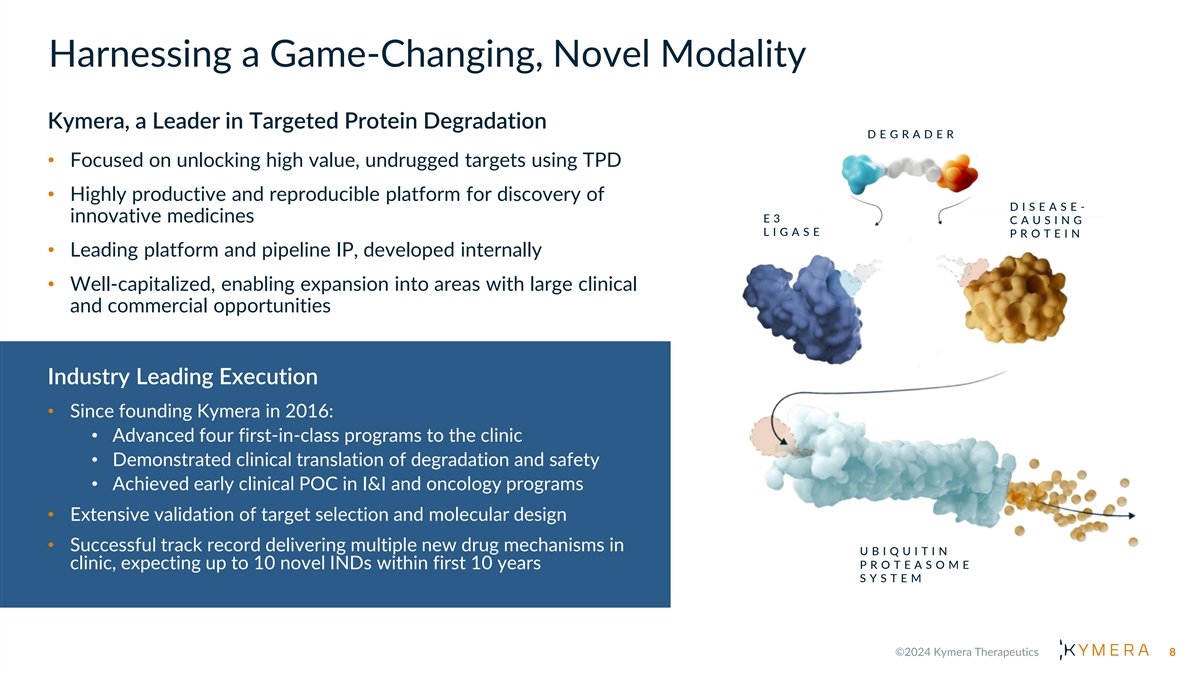
Harnessing a Game-Changing, Novel Modality Kymera, a Leader in Targeted Protein Degradation D E G R A D E R • Focused on unlocking high value, undrugged targets using TPD • Highly productive and reproducible platform for discovery of D I S E A S E - innovative medicines E3 C A U S I N G L I G A S E P R O T E I N • Leading platform and pipeline IP, developed internally • Well-capitalized, enabling expansion into areas with large clinical and commercial opportunities Industry Leading Execution • Since founding Kymera in 2016: • Advanced four first-in-class programs to the clinic • Demonstrated clinical translation of degradation and safety • Achieved early clinical POC in I&I and oncology programs • Extensive validation of target selection and molecular design • Successful track record delivering multiple new drug mechanisms in U B I Q U I T I N P R O T E A S O M E clinic, expecting up to 10 novel INDs within first 10 years S Y S T E M 8

Target Selection Strategy Focus on First- or Best-in-Class Opportunities Undrugged or Inadequately Strong Genetic/Pathway Clear Path to Early Clinical Large Clinical/Commercial Drugged targets Validation Differentiation Opportunities Small Molecule Inhibitor Degrader T R A N S C R I P T I O N A R E A S O F A P P R O V E D D R U G S I N S U P E R I O R I T Y V S F A C T O R S & S I G N I F I C A N T V A L U E S A M E P A T H W A Y P A T H W A Y D R U G S S C A F F O L D I N G P R O T E I N S C R E A T I O N 9

Demonstrating Reproducible and Scalable Clinical Innovation IRAK4 Degradation leads to Early POC STAT3 Degradation Leads to Major Response I R A K 4 S T A T 3 KT - 474 in HS and AD in cHL Patient KT - 333 STAT3 Levels 700 Bone Lesion Size Reduction in cHL Patient Reduction of AN Counts in HS Patients IRAK4 Levels 0 600 -10 500 -19.9 -20 400 -27.4 -31.6 -30 300 -40.6 -40 200 -46.1 -45.4 100 -50 -49.6 -50.7 0 -60 0 7 14 21 28 35 42 Screening Cycle 5 Day 1 Day Degradation of IRAK4 and Ikaros/Aiolos MDM2 Degradation Leads to I R A K I M I D M D M 2 in Humans was Well Tolerated Major Response in MCC Patient with no Heme-tox KT - 413 KT - 253 GDF15 Levels Lesion Size Reduction in MCC Patient IRAK4 Ikaros Aiolos 10 Mean % Change (+/- S.E. of Mean)
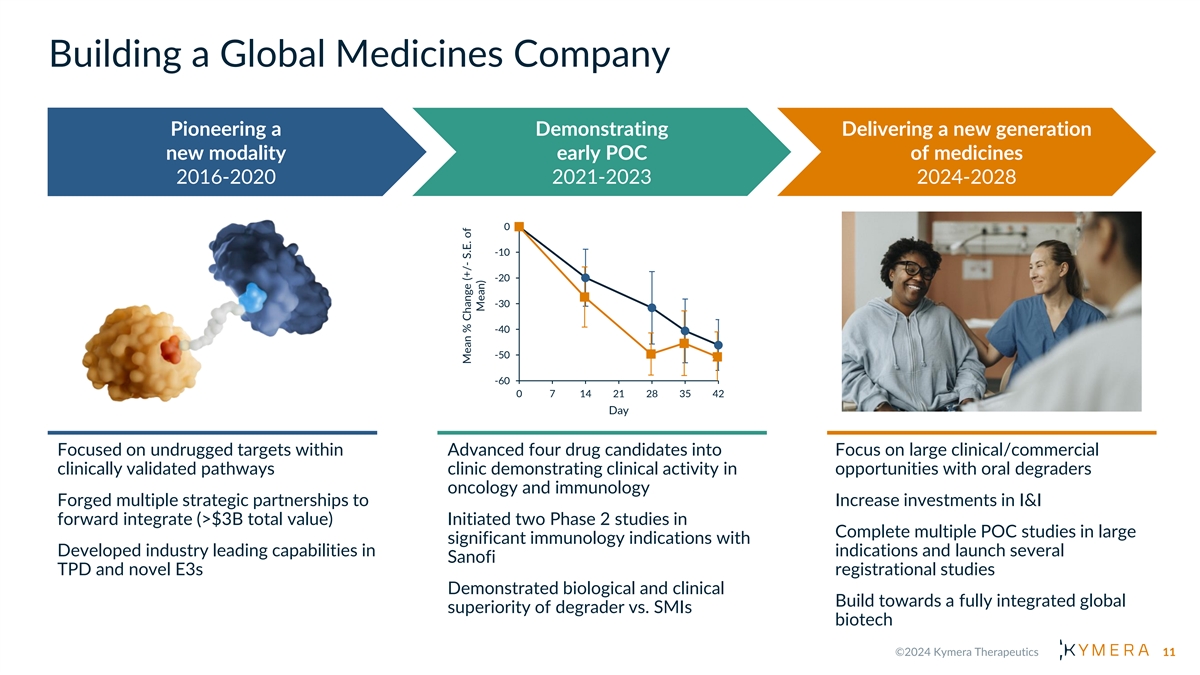
Building a Global Medicines Company Pioneering a Demonstrating Delivering a new generation new modality early POC of medicines 2016-2020 2021-2023 2024-2028 0 -10 -19.9 -20 -45.4 -27.4 -31.6 -30 -40.6 -40 -46.1 -50 -49.6 -50.7 -60 0 7 14 21 28 35 42 Day Focused on undrugged targets within Advanced four drug candidates into Focus on large clinical/commercial clinically validated pathways clinic demonstrating clinical activity in opportunities with oral degraders oncology and immunology Forged multiple strategic partnerships to Increase investments in I&I forward integrate (>$3B total value) Initiated two Phase 2 studies in Complete multiple POC studies in large significant immunology indications with Developed industry leading capabilities in indications and launch several Sanofi TPD and novel E3s registrational studies Demonstrated biological and clinical Build towards a fully integrated global superiority of degrader vs. SMIs biotech 11 Mean % Change (+/- S.E. of Mean)

The Opportunity in Immunology Immune- Orals inflammation is a $250B WW Oncology 1 market spanning multiple $171B therapeutic areas. $250B Other $81B Immune- Immunology $156B inflammation $40B Infectious Injectables $72B Disease $131B dominate, comprising >75% of CNS Cardio- the established Metabolic market. Immune-inflammatory mechanisms = outside of traditional immunology 12

Why Small Molecule Oral Degraders in Immunology Key pathways/cytokines Oral Degraders Can Offer validated as drivers of many Biologic-like Efficacy in a Pill diseases in I&I Biologics blocking these pathways/cytokines have Patients on Biologics that 1 Would Switch to Orals revolutionized treatment Biologics are injected, can be 75% inconvenient for patients and costly to manufacture Degraders can provide comparable 2 IL-23 Biologics vs TYK2 SMI pathway inhibition to biologics, PASI 75 in PsO 100 Traditional small molecule 80 convenience of oral dosing, ease of 60 inhibitors insufficiently block manufacturing and potentially 91% 40 these pathways, limiting efficacy 56% access broader populations 20 0 Guselkumab Deucravacitinib (IL-23 Ab) (TYK2 SMI) 13 PASI 75 (%)* @ Wk 10 - 16
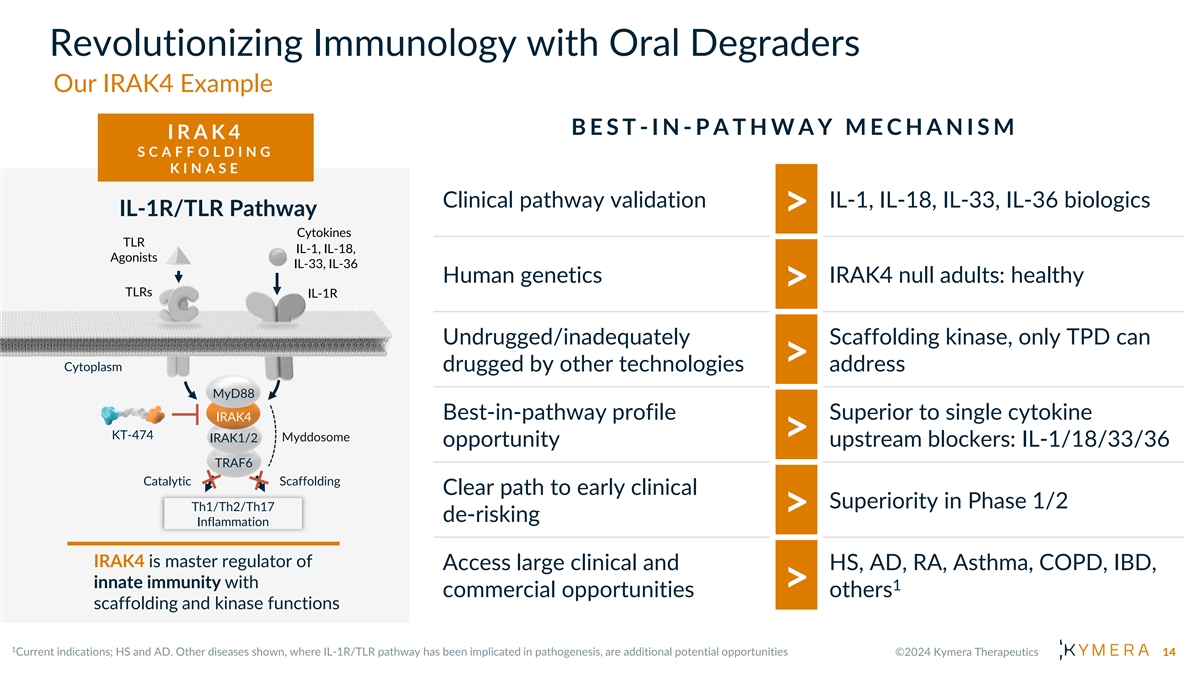
Revolutionizing Immunology with Oral Degraders Our IRAK4 Example B E S T -IN - P A T H W A Y M E C H A N I S M I R A K 4 S C A F F O L D I N G K I N A S E Clinical pathway validation IL-1, IL-18, IL-33, IL-36 biologics IL-1R/TLR Pathway > Cytokines TLR IL-1, IL-18, Agonists IL-33, IL-36 Human genetics IRAK4 null adults: healthy > TLRs IL-1R Undrugged/inadequately Scaffolding kinase, only TPD can > Cytoplasm drugged by other technologies address MyD88 Best-in-pathway profile Superior to single cytokine IRAK4 KT-474 Myddosome IRAK1/2 > opportunity upstream blockers: IL-1/18/33/36 TRAF6 Catalytic Scaffolding Clear path to early clinical Superiority in Phase 1/2 Th1/Th2/Th17 > de-risking Inflammation IRAK4 is master regulator of Access large clinical and HS, AD, RA, Asthma, COPD, IBD, innate immunity with 1 > commercial opportunities others scaffolding and kinase functions 14
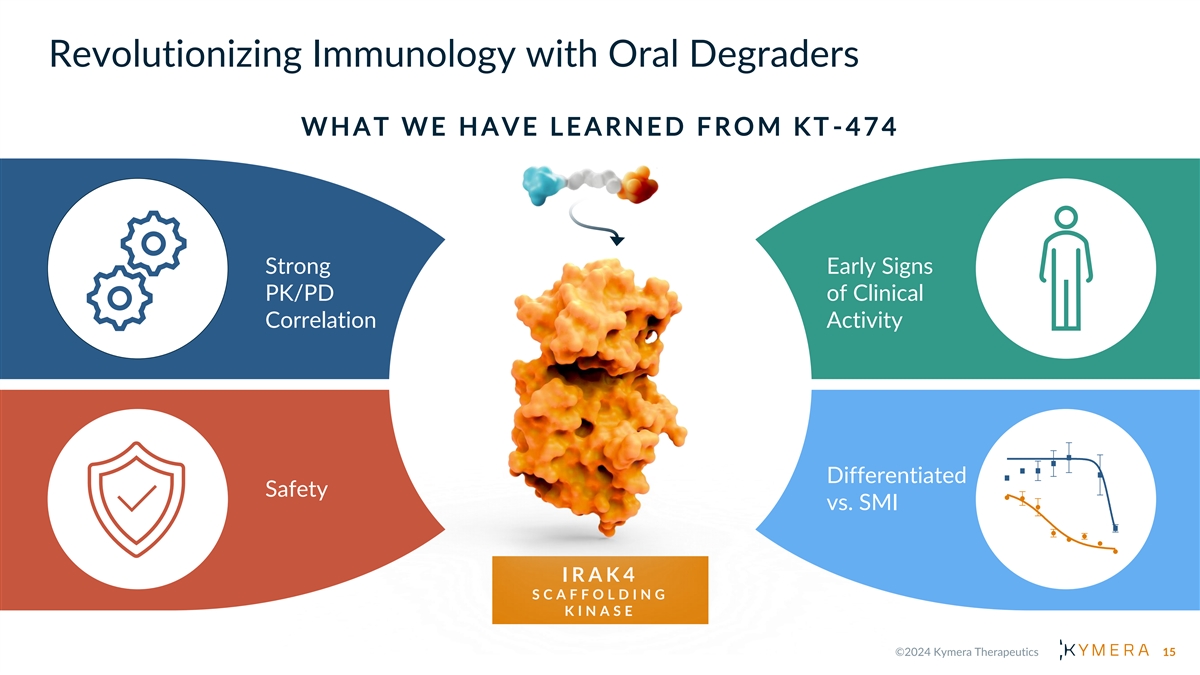
Revolutionizing Immunology with Oral Degraders W H A T W E H A V E L E A R N E D F R O M K T -474 Strong Early Signs PK/PD of Clinical Correlation Activity Differentiated Safety vs. SMI I R A K 4 S C A F F O L D I N G K I N A S E 15

KT-474: Fidelity of Translation from Preclinical to Clinical Profile Human PBMC After Oral Dosing QD 0 –20 –40 –60 –80 –92% DC = 3 ng/mL –95% –98% 85 –96% –100 Placebo 25 mg 50 mg 100 mg 200 mg (n = 9) (n = 9) (n = 9) (n = 12) (n = 9) Plasma KT-474 C (ng/mL) trough C (ng/mL) 16.7 4.4 7.8 11.2 trough PK/PD modeling of observed data predicts comparable DC (~3 ng/mL) in dog and human 85 showing excellent preclinical to clinical translation and predictability of IRAK4 degradation 16 Predicted % IRAK4 Reduction from Baseline Percent IRAK4 Change from Baseline in PBMC + on Day 14 (mean ( s.e.))
Revolutionizing Immunology with Small Molecule Oral Degraders Our Two New Programs S P E C I F I C I L - 4 / 1 3 S P E C I F I C I L - 2 3 / I F N T R A N S C R I P T I O N F A C T O R S C A F F O L D I N G K I N A S E S T A T 6 T Y K 2 17

STAT6 Degrader: Dupilumab-like Activity in a Pill S T A T 6 B E S T -IN - P A T H W A Y M E C H A N I S M T R A N S C R I P T I O N F A C T O R IL-4/13 Pathway Clinical pathway validation Dupilumab > Type I IL-4 Type II IL-4 Receptor Receptor IL-4 IL-4 IL-13 Gain of function variants cause Human genetics severe allergic diseases; IL-4Rα γc IL-4Rα IL-13Rα1 > KO phenotype (mouse) normal Undrugged/inadequately Transcription factor, TPD can fully > Cytoplasm drugged by other technologies block target/pathway STAT6 STAT6 Best-in-pathway profile Dupilumab-like activity with oral KT-621 > opportunity small molecule profile Allergic Th2 Inflammation Clear path to early clinical Phase 1/2 efficacy > de-risking STAT6 is the only specific transcription factor responsible Dupilumab indications (AD, Asthma, for IL-4/13 signaling Access large clinical and COPD, CRSwNP, EoE, PN, others), > commercial opportunities mega-blockbuster potential 18
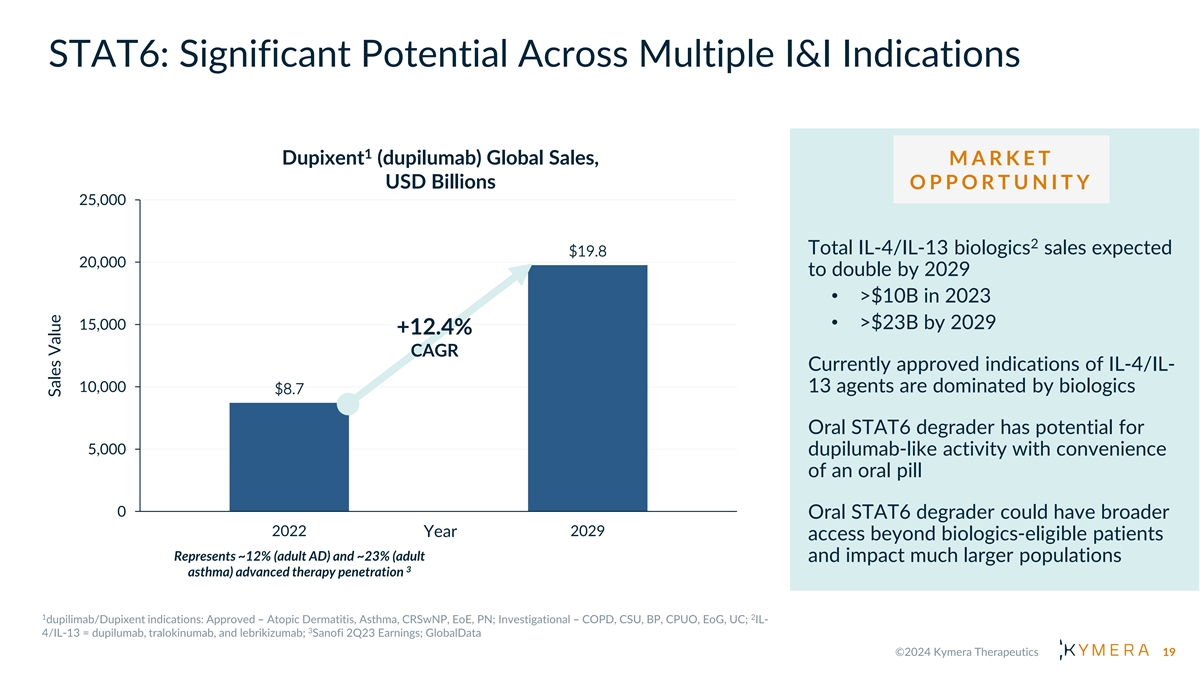
STAT6: Significant Potential Across Multiple I&I Indications 1 Dupixent (dupilumab) Global Sales, M A R K E T USD Billions O P P O R T U N I T Y 25,000 2 Total IL-4/IL-13 biologics sales expected $19.8 20,000 to double by 2029 • >$10B in 2023 • >$23B by 2029 15,000 +12.4% CAGR Currently approved indications of IL-4/IL- 10,000 13 agents are dominated by biologics $8.7 Oral STAT6 degrader has potential for 5,000 dupilumab-like activity with convenience of an oral pill 0 Oral STAT6 degrader could have broader 2022 2029 Year access beyond biologics-eligible patients Represents ~12% (adult AD) and ~23% (adult and impact much larger populations 3 asthma) advanced therapy penetration 19 Sales Value

TYK2 Degrader: Degrading a Proven Target for a Best-in-Class Profile T Y K 2 B E S T -IN - P A T H W A Y M E C H A N I S M S C A F F O L D I N G K I N A S E IL-23 biologics (ustekinumab), IL-12/23/IFN Pathways Clinical pathway validation TYK2 inhibitor (deucravacitinib) > approved, others Type I IL-23 IL-12 interferons LOF variant is protective in Human genetics immunological diseases and > generally normal TYK2 JAK1 TYK2 JAK2 TYK2 JAK2 Undrugged/inadequately drugged Scaffolding kinase, SMIs do not > by other technologies fully block pathway Scaffolding Scaffolding Scaffolding KT-294 TYK2 degrader recapitulates LOF Best-in-pathway profile phenotype: biologic-like activity Type I IFN & IL-23 inflammation > opportunity and convenience of oral pill Clear path to early clinical TYK2 is a JAK family scaffolding Phase 1 differentiation > de-risking kinase required for Type I IFN, IL- 12 and IL-23 cytokine signaling Access large clinical and IL-23, IFN indications, beyond: > commercial opportunities IBD, PsO, PsA, Lupus, others 20

TYK2: Significant Potential Across Multiple I&I Indications M A R K E T IL-23 and Type I IFN Annual Market O P P O R T U N I T Y USD Billions 30,000 $26.9 Total IL-23 and Type I IFN 1 biologics sales expected to grow 25,000 >6% by over $9B by 2029 CAGR • ~$18B in 2022 20,000 $17.7 • ~$27B by 2029 15,000 Currently approved indications dominated by biologics, with oral options challenged by efficacy 10,000 and/or safety 5,000 TYK2 degrader has potential for biologic-like efficacy with 0 convenience of oral pill 2022 2029 Year 21 Sales Value

Kymera Immunology Oral Degrader Portfolio Complementary, First-in-class Mechanisms I R A K 4 S T A T 6 T Y K 2 S C A F F O L D I N G T R A N S C R I P T I O N S C A F F O L D I N G K I N A S E F A C T O R K I N A S E IL-1R/TLR Pathway IL-4/13 Pathway IL-12/23/IFN Pathways Cytokines Type I IL-4 Type II IL-4 Type I TLR IL-1, IL-18, Receptor Receptor interferons: Agonists IL-23 IL-12 IL-33, IL-36 IL-4 IL-4 IL-13 IFNα and IFNβ IL-4Rα γc IL-4Rα IL-13Rα1 TLRs IL-1R Cytoplasm JAK1 JAK2 JAK2 TYK2 TYK2 TYK2 MyD88 Scaffolding Scaffolding Scaffolding IRAK4 STAT6 STAT6 KT-474 KT-621 Myddosome IRAK1/2 KT-294 TRAF6 Catalytic Scaffolding Type I IFN & IL-23 inflammation Allergic Th2 Inflammation Th1/Th2/Th17 Inflammation IRAK4 is master regulator of STAT6 is the only specific TYK2 is a JAK family scaffolding innate immunity with transcription factor responsible kinase required for Type I IFN, IL- 12 and IL-23 cytokine signaling scaffolding and kinase functions for IL-4/13 signaling 22

Industry-leading Oral Immunology Pipeline Three Fundamental Immune-inflammatory Pathways with Large Market Potential High value undrugged/ Next generation oral Building the industry- inadequately drugged drugs with potential leading oral targets best-in-class profiles immunology portfolio I R A K 4 ( K T -474) S T A T 6 ( K T -621) T Y K 2 ( K T -294) S C A F F O L D I N G K I N A S E T R A N S C R I P T I O N F A C T O R S C A F F O L D I N G K I N A S E Potential • AD, Asthma, COPD, • IBD, PsO, PsA, Lupus, • HS, AD, RA, Asthma, COPD, 1 CRSwNP, EoE, PN, others Indications others IBD, others • Biologic-like activity in • Dupilumab-like activity • First-in-class broad anti- Opportunity a pill in a pill inflammatory oral degrader Commercial • Wholly owned • Wholly owned • Up to 50% US with Sanofi, 2 Rights tiered royalties in ROW 23

W H A T T O E X P E CT T O D A Y Vision for our I/I portfolio and Kymera’s unique ability to capitalize on power of TPD Update on IRAK4 program Detailed overview of STAT6 and TYK2 programs • Clinical opportunities • Degrader advantage • Compelling preclinical data package • Timelines to clinic

Paving the Way: KT-474, a First- in-Class IRAK4 Oral Degrader Jared Gollob, M.D., Chief Medical Officer 25

IRAK4: What We Will Cover • Pathway Biology and Validation • Clinical Development/Commercial Opportunities • Degrader Profile and Advantage • Our Clinical Data and Fidelity of Translation • Our Ongoing Phase 2 Studies 26
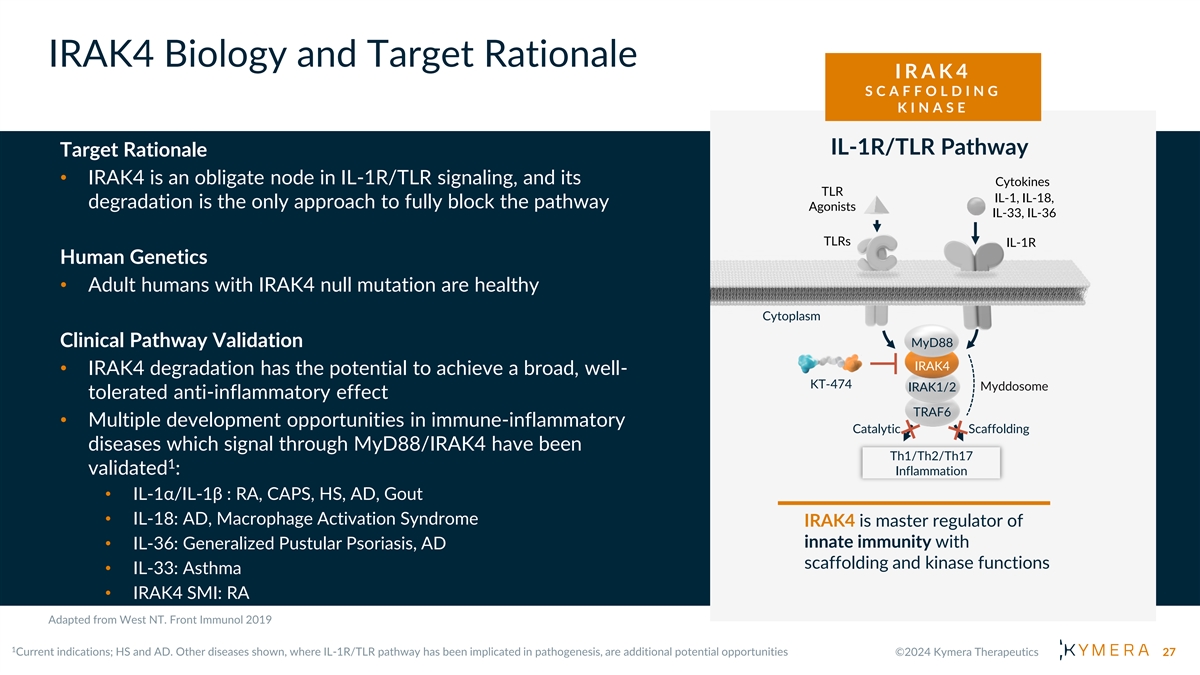
IRAK4 Biology and Target Rationale I R A K 4 S C A F F O L D I N G K I N A S E IL-1R/TLR Pathway Target Rationale • IRAK4 is an obligate node in IL-1R/TLR signaling, and its Cytokines TLR IL-1, IL-18, degradation is the only approach to fully block the pathway Agonists IL-33, IL-36 TLRs IL-1R Human Genetics • Adult humans with IRAK4 null mutation are healthy Cytoplasm Clinical Pathway Validation MyD88 IRAK4 • IRAK4 degradation has the potential to achieve a broad, well- KT-474 IRAK1/2 Myddosome tolerated anti-inflammatory effect TRAF6 • Multiple development opportunities in immune-inflammatory Catalytic Scaffolding diseases which signal through MyD88/IRAK4 have been Th1/Th2/Th17 1 validated : Inflammation • IL-1α/IL-1β : RA, CAPS, HS, AD, Gout • IL-18: AD, Macrophage Activation Syndrome IRAK4 is master regulator of innate immunity with • IL-36: Generalized Pustular Psoriasis, AD scaffolding and kinase functions • IL-33: Asthma • IRAK4 SMI: RA 27

IL-1R/TLR Pathway Potential Impact Across Multiple Immune- Inflammatory Diseases D E R M A T O L O G Y R E S P I R A T O R Y GI R H E U M A T O L O G Y RA Atopic Asthma CD Dermatitis COPD HS UC SLE 1 Total Potential Patient Impact : >150M patients Numerous indication IRAK4 degradation leading to Oral degrader medicines opportunities across multiple full pathway inhibition has the offer opportunity to reach therapeutic areas validated by potential to deliver superior broader patient sub-optimal pathway inhibitors profile to upstream biologics populations 28

IRAK4 Degrader Advantage Only Degrader Can Fully Block Inflammation Preclinical Data (Kymera IRAK4 Backgrounder) • IRAK4 KO is able to block TLR activation LPS + IL-1b → IL-6 unlike the kinase dead rescue Cytokines • IRAK4 scaffolding function is critical in TLR IL-1, IL-18, Agonists Myddosome formation and pathway IL-33, IL-36 signaling Inhibitor TLRs IL-1R • IRAK4 degradation, but not kinase inhibition, can block TLR induced NF-κB translocation and IL1R+TLR activation Cytoplasm Degrader • IRAK4 degradation is superior to kinase MyD88 inhibition at blocking downstream IRAK4 phosphoproteome KT-474 IRAK1/2 Myddosome • IRAK4 degradation is superior to inhibition TRAF6 Log[Compound] (nM) in a variety of preclinical efficacy models Catalytic Scaffolding Th1/Th2/Th17 Inflammation Clinical Data (Nature Medicine*) • IRAK4 degradation reduces signs and symptoms of HS and AD, while IRAK4 SMI inactive in IRAK4 caps the oligomer size of Phase 2 HS trial MYD88 to trigger myddosome formation • IRAK4 blocks inflammation in blood and skin of HS and AD patients 29

KT-474: Selective and Potent IRAK4 Degrader Active in Multiple Cell Types Selectivity in PBMC Potency in Blood and Skin Cells KT-474 selectively degrades IRAK4 in human immune KT-474 Degradation Across Immune Cell Types cells at concentration 10-fold above the DC 90 Potent degradation in PBMC subsets and skin cells including fibroblasts, with single-digit nM DC 50 Associated with functional inhibition of TLR- and IL-1b- Cell type KT-474 stimulated cytokine Source (Human) DC (nM) 50 production Monocytes Blood 2.6 B cells Blood 2.7 Comprehensive CD4 T cells Blood 1.5 understanding of CD8 T cells Blood 1.5 degradation kinetics across NK cells Blood 1.8 cell types to enable human Fibroblasts Skin 1.5 translation Keratinocytes Skin 7.8 30

Initial Clinical Focus for KT-474: Moderate to Severe HS and AD Hidradenitis Suppurativa (HS) Atopic Dermatitis (AD) Chronic and debilitating skin disease with painful nodules, Chronic inflammatory skin disease with scaly, dry, erythematous lesions; intense itching/scratching, predisposition to infections abscesses and draining fistulae/tunnels Major QoL impact: Pain, itching, depression, social isolation Major QoL impact: Itching, pain, sleep disturbance Many diagnosed in their 20s/30s; more common in females Onset usually in early childhood; affects an estimated 98 1 (~3:1); prevalence estimated to be up to 1-3% of population in million adults in US/EU5/JP US and EU Lesions characterized by pleotropic inflammation with Th2 Lesions characterized by pleotropic inflammation with skewing; bacterial infection and skin barrier breakdown Th1/Th17 skewing; bacterial infection and tissue destruction leading to TLR activation; IL-33 and IL-1 production leading to TLR activation; IL-1 and IL-36 production Active agents approved or in development target IL-4/IL-13, Active agents approved or in development target TNF-a, IL-17 JAK/STAT and OX40/OX40-L pathways and JAK/STAT pathways KT-474 Opportunity: Potential for broad anti-inflammatory effect, competitive efficacy vs. pathway biologics and convenience of once-daily oral dosing 31
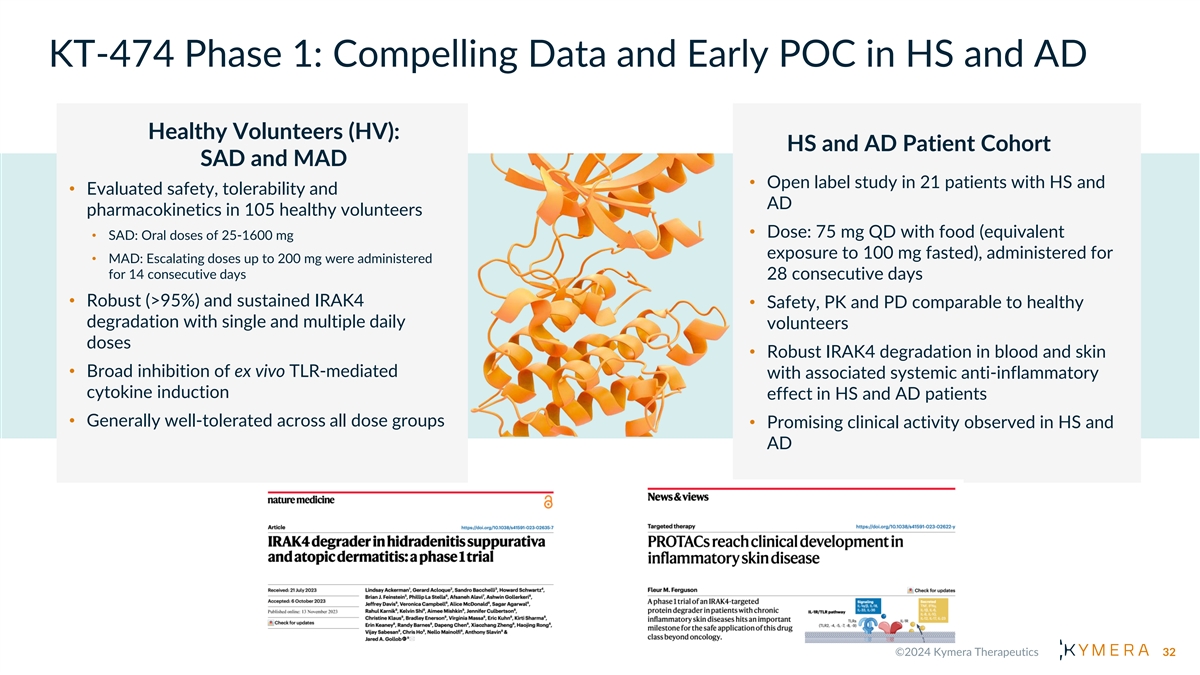
KT-474 Phase 1: Compelling Data and Early POC in HS and AD Healthy Volunteers (HV): HS and AD Patient Cohort SAD and MAD • Open label study in 21 patients with HS and • Evaluated safety, tolerability and AD pharmacokinetics in 105 healthy volunteers • Dose: 75 mg QD with food (equivalent • SAD: Oral doses of 25-1600 mg exposure to 100 mg fasted), administered for • MAD: Escalating doses up to 200 mg were administered for 14 consecutive days 28 consecutive days • Robust (>95%) and sustained IRAK4 • Safety, PK and PD comparable to healthy degradation with single and multiple daily volunteers doses • Robust IRAK4 degradation in blood and skin • Broad inhibition of ex vivo TLR-mediated with associated systemic anti-inflammatory cytokine induction effect in HS and AD patients • Generally well-tolerated across all dose groups • Promising clinical activity observed in HS and AD 32

Near-Complete Degradation and Broad Cytokine Impact in Healthy Volunteers Mean % Reduction of IRAK4 Ex Vivo Inhibition of 9 Disease-Relevant (Daily oral doses for 14 days) Cytokines, Day 7-14 R848 (TLR7/8) Stimulation IFNγ IL1β IL6 IL8 IL10 IL12 IL17 TNFα Pbo >500% 15% 35% -5% -23% 3% 107% -7% 50 mg QD -62% -59% -18% -28% 2% -58% -24% -21% 100 mg QD -85% -68% -53% -32% -50% -72% -46% -59% • High fidelity of PKPD translation from preclinical species to humans. • Human efficacious concentrations (C 3 ng/mL) and doses (50-200 mg) were correctly predicted trough 33

High Skin Exposure and Degradation in Skin of HS and AD Patients High KT-474 Exposure in HS and AD Patients Skin Reduced IRAK4 in Skin Lesions of AD and HS Patients MAD Healthy AD Patients HS Patients AD Patients HS Patients Plasma Day 28 N=21 Subjects (Baseline) (Baseline) (Day 28) (Day 28) (Baseline) Skin Day 28 N=12 N 46 7 11 6 9 Mean 0.12 0.22 0.24 0.1 0.11 34
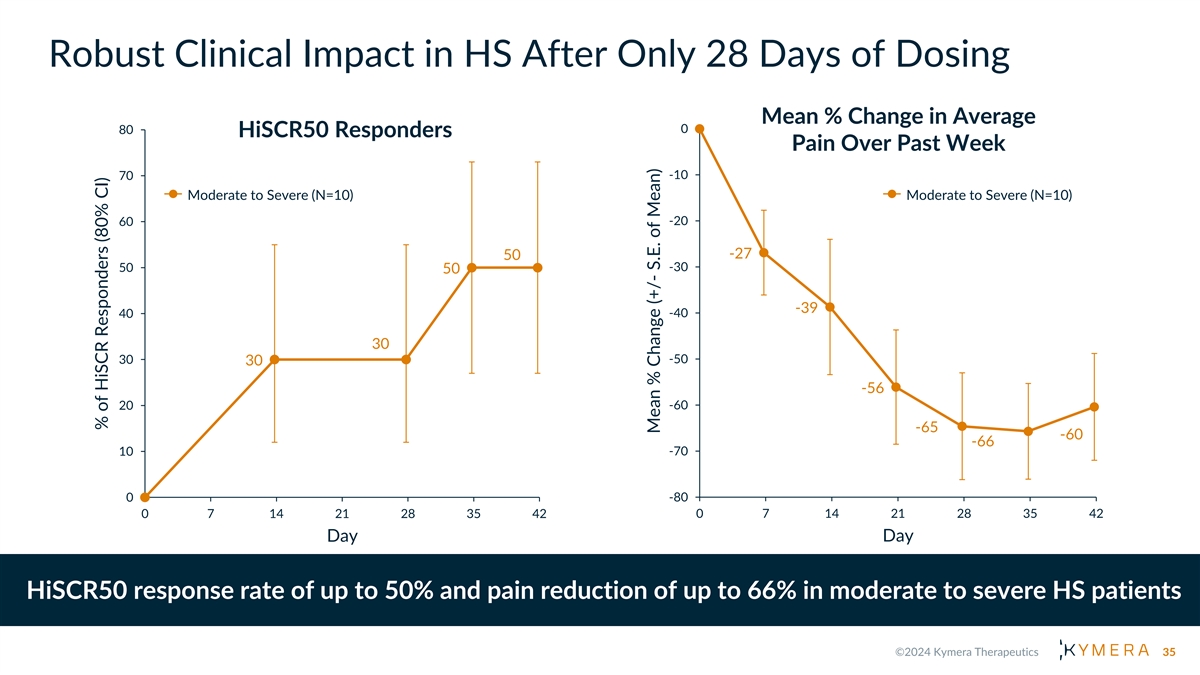
Robust Clinical Impact in HS After Only 28 Days of Dosing Mean % Change in Average 80 0 HiSCR50 Responders Pain Over Past Week -10 70 Moderate to Severe (N=10) Moderate to Severe (N=10) -20 60 -27 50 -30 50 50 -39 40 -40 30 30 -50 30 -56 20 -60 -65 -60 -66 10 -70 -80 0 0 7 14 21 28 35 42 0 7 14 21 28 35 42 Day Day HiSCR50 response rate of up to 50% and pain reduction of up to 66% in moderate to severe HS patients 35 % of HiSCR Responders (80% CI) Mean % Change (+/- S.E. of Mean)

Robust Clinical Impact in AD After Only 28 Days of Dosing Mean % Change in EASI Mean % Change of Worst Pruritus 0 0 10 Score Over Time (N=7) Over Time (N=7) 0 -10 -7 -6 -10 -18 -20 -20 -28 -30 -33 -32 -37 -36 -30 -39 -40 -43 -45 -52 -45 -36 -50 -40 -51 -60 -50 Past Week -63 Past 24 Hours -70 -80 -60 0 7 14 21 28 35 42 0 7 14 21 28 35 42 Day Day EASI score reduction of up to 36% and pruritus reduction of up to 63% in moderate to severe AD patients 36 Mean % Change (+/-S.E. of Mean) Mean % Change (+/-S.E. of Mean)

KT-474/SAR444656: Positioned for Clinical Success Phase 2 HS Trial Phase 2 AD Trial (ZEN) (ADVANTA) • Double-blind, placebo-controlled • Double-blind, placebo-controlled • Up to 99 patients, dosed for 16 • Up to 115 patients, dosed for 16 weeks weeks • 1 KT-474 dose arm, 1 placebo arm • 2 KT-474 dose arms, 1 placebo arm • Primary endpoint: % Change in AN • Primary endpoint: % Change in EASI Count • Additional endpoints (select): • Additional endpoints (select): • EASI 50/75/90, vIGA-AD, PP-NRS • HiSCR50, IHS4, HS-Skin Pain-NRS30 • Primary completion (est.): January • Primary completion (est.): February 2025 2025 Topline data expected 1H 2025 37

Oral IRAK4 Degrader: KT-474 A best-in-pathway broad oral anti-inflammatory agent for multiple inflammatory diseases Validated Biology Competitive Profile KT-474 Progress/Next Steps Mediates signaling through IL-1 Potential for Broad Activity Phase 1 complete: • Robust IRAK4 degradation and toll-like receptors Across Th1-Th17 and Th2 • Favorable safety profile Diseases Upstream cytokine blockers • Systemic suppression of proinflammatory cytokines and with proven clinical activity >$50B in combined global drug 1 chemokines across many diseases sales opportunity • Early signs of strong clinical activity Scaffolding kinase at the Large potential for oral Partner Sanofi conducting interface of innate and adaptive degraders with best in pathway Phase 2 trials in HS and AD immune responses with a efficacy variety of functions Phase 2 data expected in 1H 2025 Activity and fidelity of translation of TPD platform in KT-474 Phase 1 trial informs probability of success with STAT6 and TYK2 immunology programs 38
Dupilumab-like activity in a pill: KT-621, a First-in-class Oral STAT6 Degrader Amy Wang, Ph.D., Senior Director, Biology Project Lead 39

What We Will Cover • STAT6 Biology and Target Rationale • Clinical Development/Commercial Opportunities • Degrader Advantage • Preclinical Data • Next Steps 40

STAT6 Biology and Target Rationale S T A T 6 T R A N S C R I P T I O N F A C T O R Target Biology and rationale • STAT6 is the specific transcription factor required for IL-4 and IL-13 Type I IL-4 Receptor Type II IL-4 Receptor cytokine signaling • STAT6 regulated cytokines are clinically validated targets for allergic Tralokinumab Dupilumab Dupilumab Lebrikizumab diseases IL-4 IL-13 IL-4 Human and Mouse Genetics IL-4Rα γc IL-4Rα IL-13Rα1 • Gain of function (GOF) mutations of STAT6 cause severe allergic diseases in human • STAT6 KO mice develop normally, are viable and fertile JAK JAK JAK JAK Clinical Pathway Validation • Dupilumab, an IL-4Rα monoclonal Ab has been approved in: Atopic JAK Inhibitors dermatitis, Asthma, CRSwNP, Eosinophilic Esophagitis, Prurigo STAT6 STAT6 KT-621 Nodularis, has positive Phase 3 data in COPD and is in development for multiple additional indications Allergic Th2 Inflammation • STAT6 degradation can achieve dupilumab–like pathway inhibition 41

Oral STAT6 Degraders Can Transform Treatment Paradigm in Multiple Indications De-risked by Dupilumab D E R M A T O L O G Y R E S P I R A T O R Y ENT GI Atopic Asthma Dermatitis CRSwNP EoE COPD PN 1 Total Potential Patient Impact : >150M patients STAT6 degradation leading to Oral degrader medicines Numerous indication full pathway inhibition has the offer opportunity to opportunities across multiple potential to deliver dupilumab- reach broader patient therapeutic areas de-risked by like activity populations dupilumab 42

STAT6 Degrader Advantage Type I IL-4 Receptor Type II IL-4 Receptor • STAT6 is the specific and Tralokinumab Dupilumab Dupilumab Lebrikizumab essential transcription factor in the IL-4/13 pathway IL-4 IL-13 IL-4 Degrader • Occupancy based approaches IL-4Rα γc IL-4Rα IL-13Rα1 STAT6 (e.g., SMI) unlikely to block pathway fully in a pharmacologically relevant manner JAK JAK JAK JAK Proteasome • Only degradation of STAT6 can block its activity fully and JAK Inhibitors STAT6 STAT6 match dupilumab pathway KT-621 blockade in vitro and in vivo Allergic Th2 Inflammation 43

KT-621: A Picomolar Degrader of STAT6 Consistent Degradation Across All Disease Relevant Cell Types Evaluated STAT6 Degradation in Hematopoietic Cells Human Primary Cell Type KT-621, DC (pM) 50 Hematopoietic cell (all TH2 diseases) Human PBMC 13 Human CD3 T cell 36 Blood Human CD14 monocyte 60 Human CD19 B cell 86 Human eosinophil 99 Epithelial cell (AD, CPG, CU, asthma, COPD) Human keratinocyte (adult) 22 Skin STAT6 Degradation in Tissue Cells Human keratinocyte (neonatal) 18 Human bronchial tracheal epithelial cell 33 Lungs Human small airway epithelial cell 35 Smooth muscle cell (asthma, COPD, EoE) Human bronchial smooth muscle cell 25 Throat/ Airway Human esophageal smooth muscle cell 33 Endothelial cell (all TH2 diseases) Blood Human vascular endothelial cell 46 Vessels 44

KT-621: Exquisite Degradation Selectivity for STAT6 Complete STAT6 degradation selectivity in human PBMC proteome at 100 x DC 90 No other STATs are degraded to any extent 45
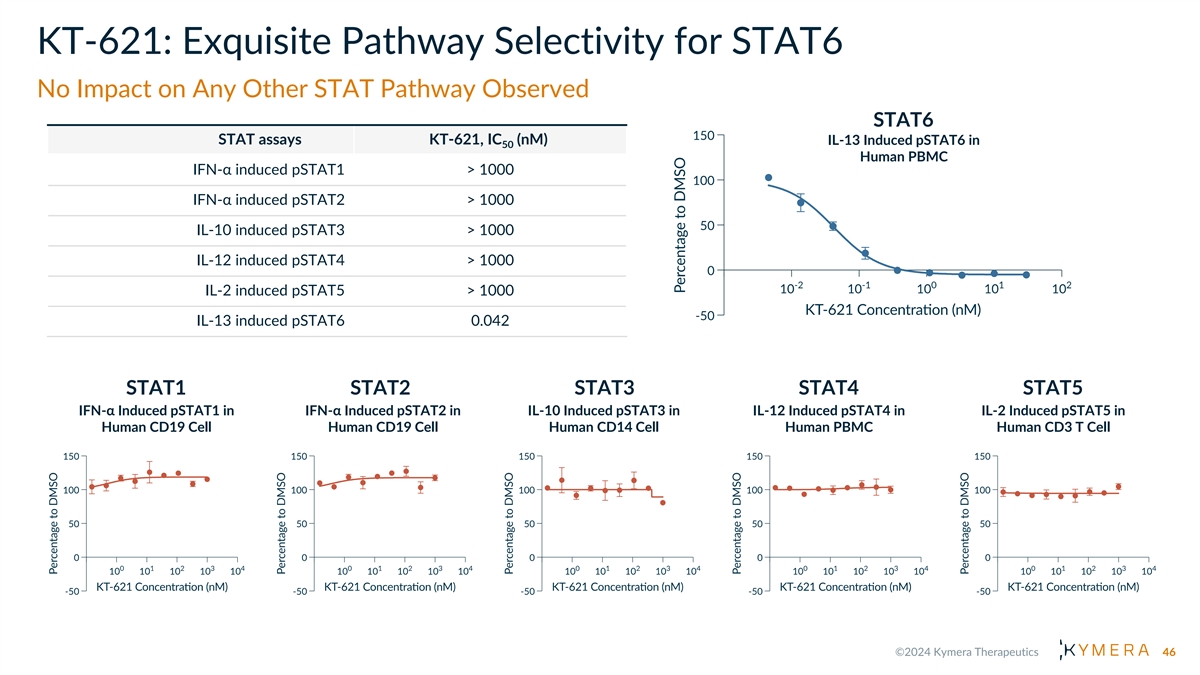
KT-621: Exquisite Pathway Selectivity for STAT6 No Impact on Any Other STAT Pathway Observed STAT6 STAT assays KT-621, IC (nM) IL-13 Induced pSTAT6 in 50 Human PBMC IFN-α induced pSTAT1 > 1000 IFN-α induced pSTAT2 > 1000 IL-10 induced pSTAT3 > 1000 IL-12 induced pSTAT4 > 1000 IL-2 induced pSTAT5 > 1000 IL-13 induced pSTAT6 0.042 STAT1 STAT2 STAT3 STAT4 STAT5 IFN-α Induced pSTAT1 in IFN-α Induced pSTAT2 in IL-10 Induced pSTAT3 in IL-12 Induced pSTAT4 in IL-2 Induced pSTAT5 in Human CD19 Cell Human CD19 Cell Human CD14 Cell Human PBMC Human CD3 T Cell 46

KT-621 Fully Blocks IL-4/13 Pathway, More Potently than Dupilumab KT-621 Dupilumab Cellular Functional Assay IC (pM) IC (pM) 50 50 IL-4 TARC release in human PBMC 62 194 Serum Th2 biomarker, chemoattractant for Th2 TARC cell IL-13 TARC release in human PBMC 43 113 IL-4 CD23 expression in human CD19 B cell 125 354 B cell activation marker, correlates with IgE class CD23 switch IL-13 CD23 expression in human CD19 B cell 98 1070 IL-13 Periostin release in human bronchial smooth muscle cell 24 637 Serum Th2 biomarker and ECM protein associated PERIOSTIN with tissue remodeling in atopic diseases IL-13 Periostin release in human esophageal smooth muscle cell 39 431 IL-13 Induced Periostin Release in IL-13 Induced CD23 Expression in IL-4 Induced TARC Release in Human Bronchial Smooth Muscle Cell Human CD19 B Cell Human PBMC 47

KT-621 Achieves Dose Dependent Deep Degradation of STAT6 in vivo with Low Oral Doses STAT6 Degradation in Dog Blood post 7 days of KT-621 QD Oral Dosing 0 KT-621 potently degrades STAT6 across multiple preclinical species -50 KT-621 can degrade STAT6 to -66 depletion with low oral doses -85 -94 -98 -100 Pre dose 0.2 mpk 0.8 mpk 3.2 mpk 12.8 mpk 48 STAT6 % Change from Baseline
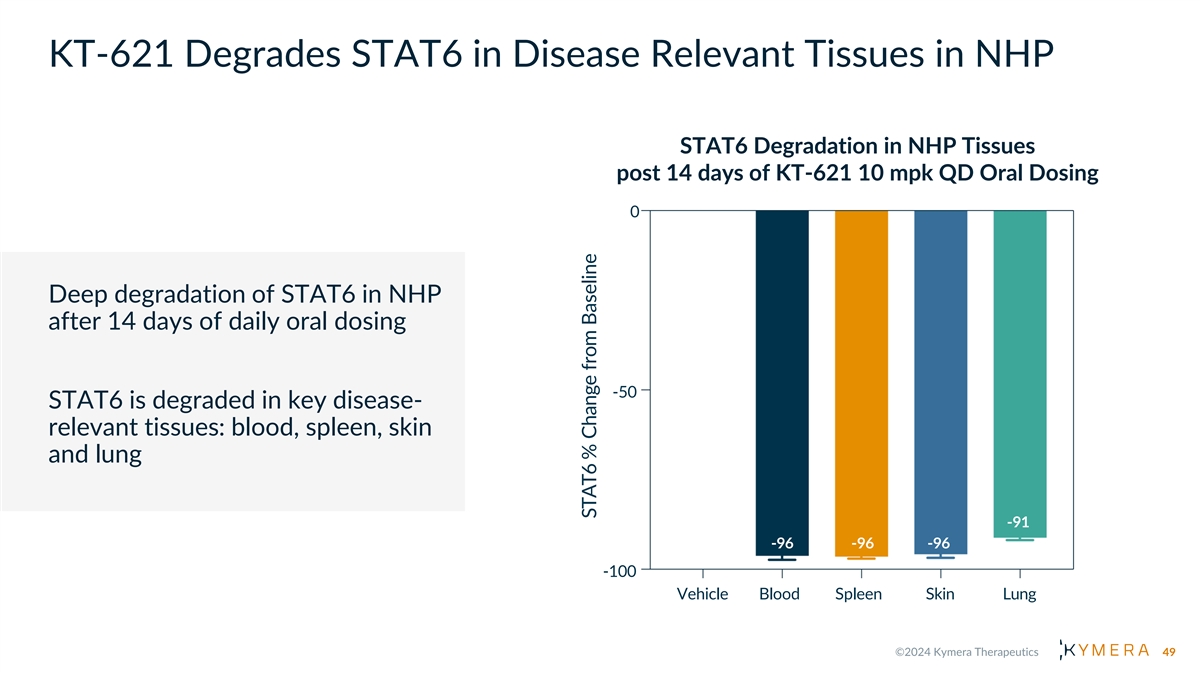
KT-621 Degrades STAT6 in Disease Relevant Tissues in NHP STAT6 Degradation in NHP Tissues post 14 days of KT-621 10 mpk QD Oral Dosing 0 Deep degradation of STAT6 in NHP after 14 days of daily oral dosing -50 STAT6 is degraded in key disease- relevant tissues: blood, spleen, skin and lung -91 -96 -96 -96 -100 Vehicle Blood Spleen Skin Lung 49 STAT6 % Change from Baseline

KT-621 Has Comparable in vivo Efficacy to IL-4Rα Saturating Dose of Dupilumab in the MC903 Atopic Dermatitis Model STAT6 Degradation in Mouse Spleen Total Serum IgE An atopic dermatitis model induced by topical application of low- calcemic vitamin D3 analog MC903 with prominent Th2 inflammation in the IL4/IL4RA humanized mice: • KT-621 dosed QD orally for 11 days • Dupilumab dosed 4 times subcutaneously, 25 mpk twice a week (IL-4Rα saturating dose); effect equivalent to 300 mg every other week in human 50

KT-621 Has Comparable or Superior in vivo Efficacy to IL-4Rα Saturating Dose of Dupilumab in the Intranasal HDM Asthma Model A lung inflammation model induced by intranasal house dust mite administration with dominant Th2 inflammation in the IL4/IL4RA humanized mice (Le Floc’h et al. Allergy. 2020) • KT-621 dosed QD orally for 31 days. 2/8/32 mpk doses showed 72/85/91% STAT6 degradation respectively in mouse spleen • Dupilumab dosed 9 times subcutaneously, 25 mpk BIW (IL-4Rα saturating dose), effect equivalent to 300 mg every other week in human TARC in Periostin in Eosinophil in Total Serum IgE Bronchoalveolar Lavage Bronchoalveolar Lavage Bronchoalveolar Lavage 51
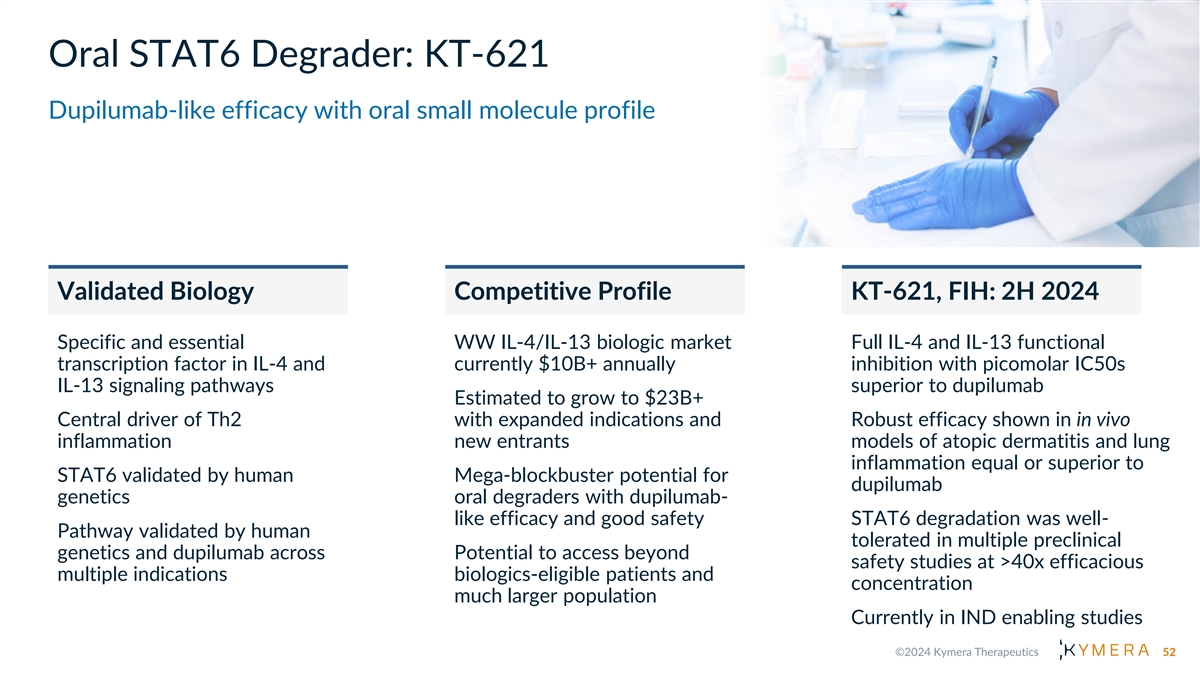
Oral STAT6 Degrader: KT-621 Dupilumab-like efficacy with oral small molecule profile Validated Biology Competitive Profile KT-621, FIH: 2H 2024 Specific and essential WW IL-4/IL-13 biologic market Full IL-4 and IL-13 functional transcription factor in IL-4 and currently $10B+ annually inhibition with picomolar IC50s IL-13 signaling pathways superior to dupilumab Estimated to grow to $23B+ Central driver of Th2 with expanded indications and Robust efficacy shown in in vivo inflammation new entrants models of atopic dermatitis and lung inflammation equal or superior to STAT6 validated by human Mega-blockbuster potential for dupilumab genetics oral degraders with dupilumab- like efficacy and good safety STAT6 degradation was well- Pathway validated by human tolerated in multiple preclinical genetics and dupilumab across Potential to access beyond safety studies at >40x efficacious multiple indications biologics-eligible patients and concentration much larger population Currently in IND enabling studies 52

Degrading a Proven Target: KT-294, a First-in-Class Oral TYK2 Degrader Juliet Williams, Ph.D., Head of Research 53

What We Will Cover • TYK2 Biology and Target Rationale • Clinical Development/Commercial Opportunities • Degrader Advantage • Preclinical Data • Next Steps 54

TYK2 Biology and Target Rationale T Y K 2 S C A F F O L D I N G K I N A S E Target Biology and Rationale Ustekinumab Anifrolumab • TYK2 is a member of the JAK family required for Type I IFN, IL- Risankizumab 12 and IL-23 cytokine signaling • TYK2 regulated cytokines are clinically validated targets for autoimmune and inflammatory diseases Type I interferons: IL-23 IL-12 IFNα and IFNβ Human Genetics • Loss-of-function variant of TYK2 is protective in autoimmune and inflammatory diseases Clinical Pathway Validation TYK2 TYK2 TYK2 JAK1 JAK2 JAK2 • IL-23 (± IL-12)-targeting agents include ustekinumab, KT-294 Scaffolding Scaffolding Scaffolding risankizumab, guselkumab, and tildrakizumab, with approvals in PsO, PsA, CD, UC Deucravacitinib • Type I IFN-targeting agents include anifrolumab with approval in SLE Type I IFN IL-12/23 Inflammation • TYK2 SMI deucravacitinib recently approved in PsO Inflammation 55
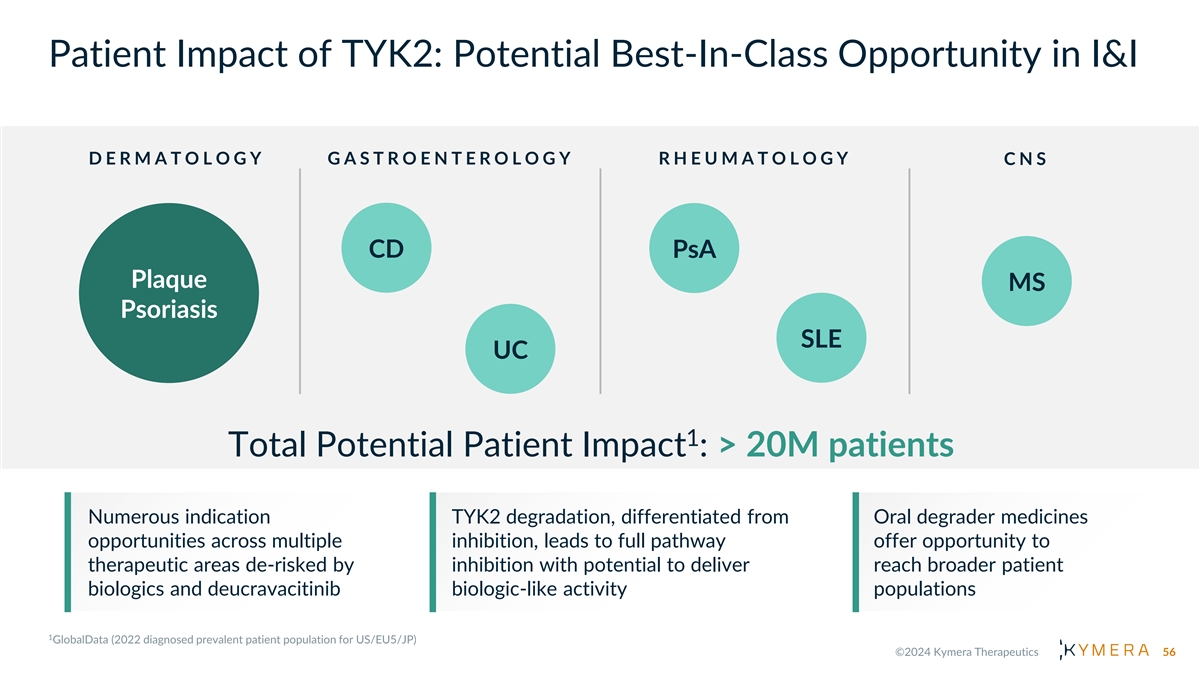
Patient Impact of TYK2: Potential Best-In-Class Opportunity in I&I D E R M A T O L O G Y G A S T R O E N T E R O L O G Y R H E U M A T O L O G Y C N S CD PsA Plaque MS Psoriasis SLE UC 1 Total Potential Patient Impact : > 20M patients Numerous indication TYK2 degradation, differentiated from Oral degrader medicines opportunities across multiple inhibition, leads to full pathway offer opportunity to therapeutic areas de-risked by inhibition with potential to deliver reach broader patient biologics and deucravacitinib biologic-like activity populations 56

TYK2 Degrader Advantage Only TYK2 Degraders Can Reach Biologics-like Activity • TYK2 has a well-established scaffolding function that is responsible for cytokine Ustekinumab Anifrolumab Risankizumab receptor surface expression and Degrader activation • Unlike SMIs, only TYK2 degradation IL-23/ Type I TYK2 recapitulates the human LOF phenotype IL-10 IL-12 interferons of full pathway inhibition of Type I IFN, IL- 12 and IL-23 and sparing of IL-10 • Unlike deucravacitinib, which inhibits IL-10 through JAK1, KT-294 does not inhibit IL- 10, which is important in IBD Proteasome TYK2 JAK1 TYK2 JAK2 TYK2 TYK2 • Compared to TAK-279, KT-294 fully KT-294 JAK1 JAK1 Scaffolding Scaffolding inhibits Type I IFN Deucravacitinib Deucravacitinib • Full TYK2 degradation demonstrated by IL-10, KT-294 leads to superior pathway Type I IFN IL-12/23 Immunomodulation inhibition to existing SMIs and potentially Inflammation Inflammation and Intestinal Homeostasis reach biologic-like activity 57

TYK2 Has Well-Established Scaffolding Function • TYK2 complete deficiency severely impairs IL-23, Type I IFN, and IL-12 signaling but spares IL-10 in humans • TYK2 scaffolding functions are demonstrated by differential pathway inhibitions in complete TYK2 deficiency vs a kinase dead variant in humans • TYK2 deficient humans are generally healthy with only increased risk of some mycobacteria and viral infections that are relatively mild, curable and tend not to recur, de-risking safety for TYK2 degradation Cytokine Pathway IL-23 Type I IFN IL-12 IL-10 WT TYK2 ++++ ++++ ++++ ++++ Complete deficiency + + + +++ TYK2 -/- TYK2 Kinase dead + ++++ ++++ ++++ P1104A/P1104A Degrading TYK2 is the only small molecule approach to eliminate all scaffolding and catalytic functions of TYK2, fully recapitulating the human TYK2-/- biology 58

KT-294, a Highly Selective Picomolar TYK2 Degrader, Recapitulates TYK2 Human Deficiency Biology Fully Inhibits of Type I IFN and IL-12/23 and Spares IL-10/22 KT-294 Cellular Degradation/Functional Assay DC /IC (nM) Selective TYK2 Degradation by KT-294 in 50 50 hPBMC Proteome at 10x DC Human PBMC degradation 0.08 90 Human keratinocyte (neonatal and adult) 0.07 IL-23 pathway IL-23 pSTAT4 in human PBMC 0.7 IL-23 pSTAT3 in human CD3+CD161high TH17 cell 2.1 IL-23/IL-1β IFN-γ release in human PBMC 2.4 Type I IFN pathway IFN-α pSTAT1 in human CD19 B cell 13 IFN-α pSTAT2 in human CD19 B cell 15 IFN-α IP10 release in human PBMC 4.9 IL-12 pathway IL-12/IL-18 pSTAT4 in human PBMC 1.3 IL-12/IL-18 IFN-γ release in human PBMC 10 IL-10 and IL-22 pathways IL-10 pSTAT3 in human CD14 monocyte > 1000 IL-22 pSTAT1 in HT29 cell > 1000 IL-22 pSTAT3 in HT29 cell > 1000 59

KT-294, Unlike Allosteric TYK2 Inhibitor Deucravacitinib, Does not Inhibit IL-10 IL-10 has essential roles in intestinal homeostasis • Loss of function mutations of the IL-10 pathway cause early onset refractory colitis in humans Deucravacitinib inhibits IL-10 through JAK1 • Deucra JAK1 Ki = 0.33 nM (Burke et al. Sci Transl Med. 2019) • KT-294 JAK1 Ki = > 1000 nM Deucra Inhibited IL-10 Deucra Inhibited IL-10 Deucra Inhibits IL-10’s Function of induced pSTAT3 Induced pSTAT3 Suppressing LPS Induced TNF-α Release in TYK2 KO EBV B Cell in Human CD14 Monocyte in Human CD14 Monocyte KT-294 KT-294 TYK2 KO TYK2 WT IC = > 1000 nM IC = > 1000 nM 50 50 Deucravacitinib Deucravacitinib Deucra 0.1 1 0.1 1 IC = 64 nM IC = 89 nM 50 50 (μM) IL10 - + + + - + + + pSTAT3 TYK2 60

Superior Inhibition of Type I IFN Pathway and Innate Immunity by KT-294 vs TAK-279 12-gene Innate Signature Score 21-gene IFN⍺ Signature Score was Measured by RNAseq was Measured by RNAseq SG15 IFI6 IFI27 SPATS2L LY6E Mean inhibition DEFB1 IFI44L by KT-294 ISG15 OAS3 Mean inhibition DSP OAS1 by KT-294 HPSE USP18 ZBP1 SIGLEC1 HMOX1 IFIT1 HSPA1A IFI44 BST2 HERC5 CYSTM1 RTP4 DHX58 OAS2 PSMD9 MX1 ARL8A EPSTI1 RSAD2 IFIT3 LAMP3 PLSCR1 Doses Used: • TAK-279 = 422nM (IFN⍺ stimulated pSTAT2 IC ). Clinical exposure Cmax (free) at 35mg = ~ 77 nM 95 • KT-294 = 56nM (IFN⍺ stimulated pSTAT2 IC ) 95 At concentrations where TAK-279 and KT-294 block pathway 95%, degrader demonstrates superior biological effect. (TAK-279 does not reach these exposures in clinic) 61

KT-294 Achieved Dose Dependent Deep Degradation of TYK2 in vivo with Low Oral Doses TYK2 Degradation in NHP Blood Post 7 days of KT-294 QD Oral Dosing 0 KT-294 potently degrades TYK2 across multiple preclinical species In NHP, KT-294 can degrade TYK2 -50 to depletion with low oral doses -79 -94 -96 -100 Pre dose 0.3 mpk 1 mpk 3 mpk 62 TYK2 % Change from Baseline
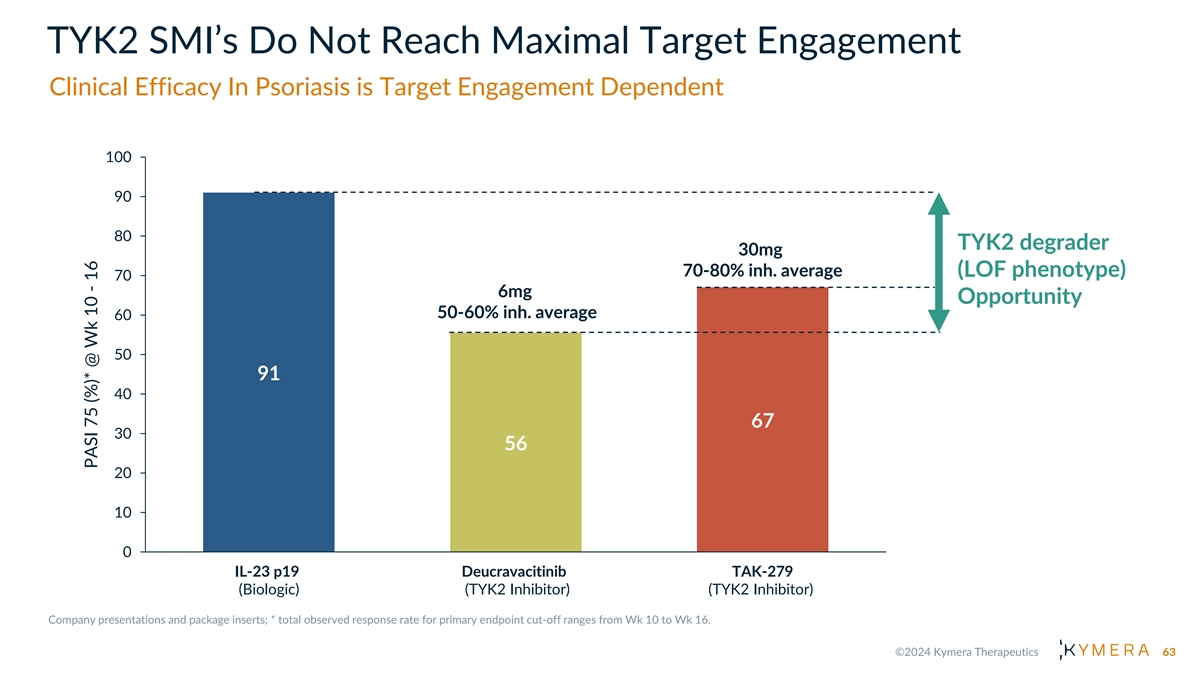
TYK2 SMI’s Do Not Reach Maximal Target Engagement Clinical Efficacy In Psoriasis is Target Engagement Dependent 100 90 80 TYK2 degrader 30mg 70-80% inh. average (LOF phenotype) 70 6mg Opportunity 50-60% inh. average 60 50 91 40 67 30 56 20 10 0 IL-23 p19 Deucravacitinib TAK-279 (Biologic) (TYK2 Inhibitor) (TYK2 Inhibitor) 63 PASI 75 (%)* @ Wk 10 - 16
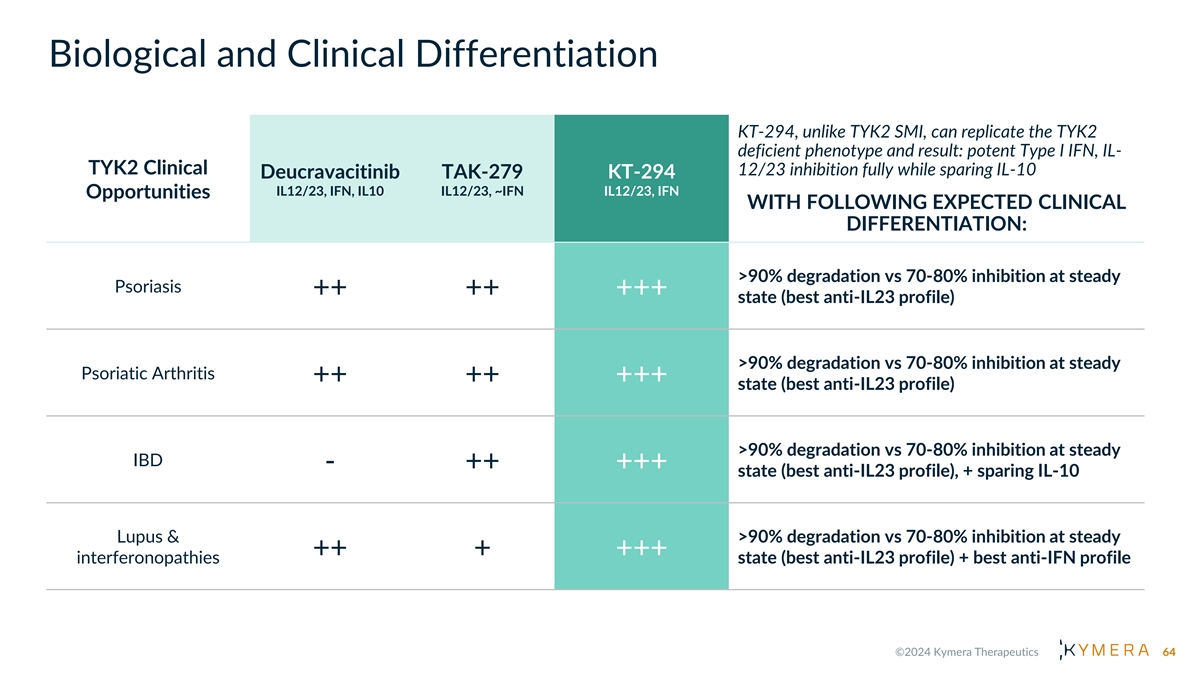
Biological and Clinical Differentiation KT-294, unlike TYK2 SMI, can replicate the TYK2 deficient phenotype and result: potent Type I IFN, IL- TYK2 Clinical 12/23 inhibition fully while sparing IL-10 Deucravacitinib TAK-279 KT-294 IL12/23, IFN, IL10 IL12/23, ~IFN IL12/23, IFN Opportunities WITH FOLLOWING EXPECTED CLINICAL DIFFERENTIATION: >90% degradation vs 70-80% inhibition at steady Psoriasis ++ ++ +++ state (best anti-IL23 profile) >90% degradation vs 70-80% inhibition at steady Psoriatic Arthritis ++ ++ +++ state (best anti-IL23 profile) >90% degradation vs 70-80% inhibition at steady IBD - ++ +++ state (best anti-IL23 profile), + sparing IL-10 Lupus & >90% degradation vs 70-80% inhibition at steady ++ + +++ interferonopathies state (best anti-IL23 profile) + best anti-IFN profile 64

Oral TYK2 Degrader: KT-294 Potential Best-in-Class Opportunity with Biologic-like Profile Validated Biology Competitive Profile KT-294, FIH: 1H 2025 Degrades TYK2 in human cells with IL-23 and Type 1 IFN-based biologic TYK2 is a member of the JAK family pM potency market currently ~$18B annually required for Type I IFN, IL-12 and IL-23 cytokine signaling Recapitulates the phenotype of Estimated to grow to ~$27B with TYK2 human deficiency showing expanded indications and new Pathway validated by upstream potent IFN-α, IL-12 and IL-23 entrants biologics (i.e. ustekinumab) and inhibition and sparing IL-10 TYK2 SMI across many diseases TYK2 SM inhibitors have limitations due to selectivity (deucravacitinib) Dosed orally, shows complete TYK2 TYK2 validated by human genetics or lack of potent IFN-α activity degradation in NHP providing a path (TAK-279) and limited clinical target to full target engagement in clinic, engagement (both) unlike current SMI Mega-blockbuster potential for oral Currently in IND enabling studies degrader with biologic-like activity that is superior to TYK2 SMI 65

Solving Big Problems with Small Molecules Nello Mainolfi, Ph.D., Founder, President and CEO 66

A Powerful Strategy That Puts Patients First We focus on genetically validated targets/pathways that are either undrugged or inadequately drugged, where TPD is the best or the only solution With a growing understanding of the underlying biology and many key pathways/targets validated by biologics, I&I offers large clinical/commercial opportunities TPD can provide a unique solution with biologic-like specificity and efficacy, with the flexibility of oral small molecules Our unique approach and capabilities have generated a best-in-industry oral I&I pipeline of first-in-class, highly valuable programs 67

Revolutionizing Immunology with Small Molecule Oral Degraders I R A K 4 ( K T -474) S T A T 6 ( K T -621) T Y K 2 ( K T -294) S C A F F O L D I N G T R A N S C R I P T I O N S C A F F O L D I N G K I N A S E F A C T O R K I N A S E • IND-Enabling • IND-Enabling • Phase 2 Trials in HS and AD Status with Sanofi Potential • IBD, PsO, PsA, Lupus, others • AD, Asthma, COPD, CRSwNP, • HS, AD, RA, Asthma, COPD, Indications 1 EoE, PN, others IBD, others Next • FIH: 2H 2024• FIH: 1H 2025 • HS and AD Ph2 data: 1H 2025 Milestone • Biologic-like activity in a pill • First-in-class broad anti-• Dupilumab-like activity Opportunity inflammatory oral degrader in a pill Commercial • Up to 50% US with Sanofi, • Wholly owned• Wholly owned 2 tiered royalties in ROW Rights 68

Kymera Immunology Oral Degrader Portfolio Complementary Mechanisms Each with Mega-blockbuster Potential Market Opportunity (2022 Sales) $1B $8B $2B $10B $16B $12B 1 IL-1R/TLR pathway $28B IRAK4 : HS $19B Th1/17/Th2 biology $16B SLE IL-4/13 pathway STAT6: AD Th2 biology Asthma IL-23/IFN pathway TYK2: COPD PsO IBD Potential Indications MS RA 69

Immunology Pipeline with Clear Line of Sight to Large Value Creation Potential 2024 2025 2026+ Upcoming Milestones Rights Indications Oral QD Small Molecule Degraders HS, AD, RA, HS Ph2 HS Late Development Ph2 HS & AD Data IRAK4* Asthma, 1H 2025 KT-474 1 AD Ph2 AD Late Development IBD, other 50/50 US AD, Asthma, Ph1 Start STAT6 COPD, PN Ph1 Mid-Late Development IND 2H 2024 KT-621 CRSwNP, EoE Psoriasis, TYK2 Ph1 Start Mid-Late IND Mid-Late Development IBD, PsA, Ph1 1H 2025 KT-294 Development Lupus, other Our Discovery Engine in Immunology Undrugged or Clear Path to Early ➢ Humoral Immunity➢ Respiratory Inflammation Inadequately Clinical Areas Drugged Targets Differentiation of ➢ Rheumatology➢ GI Inflammation Large Focus: Strong Genetic/ Clinical/Commercial ➢ Plasma Cell Biology➢ Local Inflammation Pathway Validation Opportunities = key data readout 70
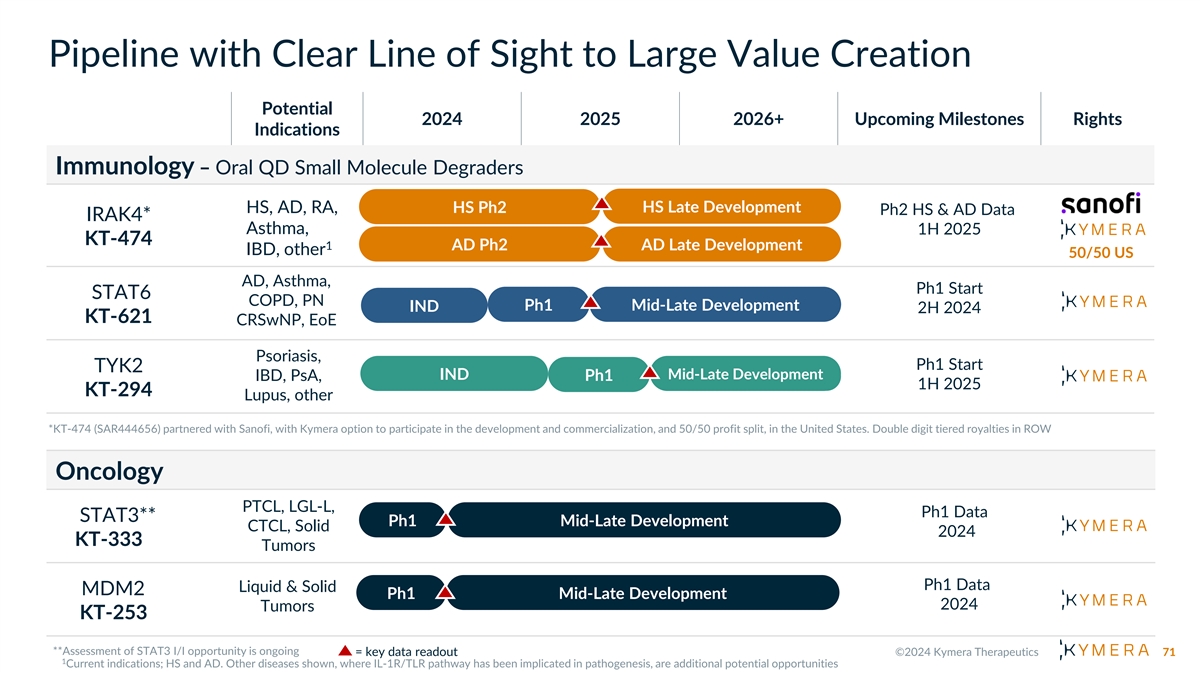
Pipeline with Clear Line of Sight to Large Value Creation Potential 2024 2025 2026+ Upcoming Milestones Rights Indications Immunology – Oral QD Small Molecule Degraders HS Late Development HS, AD, RA, HS Ph2 Ph2 HS & AD Data IRAK4* Asthma, 1H 2025 KT-474 1 AD Ph2 AD Late Development IBD, other 50/50 US AD, Asthma, Ph1 Start STAT6 COPD, PN Ph1 Mid-Late Development IND 2H 2024 KT-621 CRSwNP, EoE Psoriasis, Ph1 Start TYK2 Mid-Late IND Mid-Late Development IBD, PsA, Ph1 1H 2025 Development KT-294 Lupus, other Oncology PTCL, LGL-L, Ph1 Data STAT3** Ph1 Mid-Late Development CTCL, Solid 2024 KT-333 Tumors Ph1 Data Liquid & Solid MDM2 Ph1 Mid-Late Development 2024 Tumors KT-253 = key data readout 71

2 0 2 4 P R I O R I T I E S • Completion of enrollment in IRAK4 oral degrader KT-474 Phase 2 trials in HS and AD by partner Sanofi (topline data 1H 2025) • Initiation of oral STAT6 degrader KT-621 Phase 1 • IND ready for oral TYK2 degrader KT-294 • Additional oncology proof-of-concept data for the STAT3 degrader KT-333 and MDM2 degrader KT- 253

Thank You Q&A investors@kymeratx.com media@kymeratx.com KYMERA THERAPEUTICS 200 Arsenal Yards Blvd., Suite 230 Watertown, MA 02472
Abbreviations KO Knockout Ab Antibody FIH First-in-Human AD Atopic Dermatitis LGL-L Large Granular Lymphocytic Leukemia GDF15 Growth Differentiation Factor 15 LOF Loss of Function AN Count Abscess and Inflammatory Nodule Count GI Gastrointestinal CAGR Compound Annual Growth Rate LPS Lipopolysaccharide Solution GOF Gain of Function CAPS Cryopyrin-Associated Periodic Syndrome MAD Multiple Ascending Dose Study HDM House Dust Mite CD Crohn’s Disease MCC Merkel Cell Carcinoma HiSCR Hidradenitis Suppurativa Clinical Response cHL Classic Hodgkin’s Lymphoma MDM2 Mouse Double Minute 2 hPBMC Human Peripheral Blood Mononuclear Cells MS Multiple Sclerosis CNS Central Nervous System HS Hidradenitis Suppurativa COPD Chronic Obstructive Pulmonary Disease Myeloid Differentiation Primary Response HV Healthy Volunteers MYD88 Protein 88 CRSwNP Chronic Rhinosinusitis with Nasal Polyps I&I Immunology and Inflammation NF-kB Nuclear Factor Kappa B CTCL Cutaneous T-Cell Lymphoma IBD Inflammatory Bowel Disease nM Nanomolar Ctrl Control IC Inhibitory Concentration # NRS Numerical Rating Scale C Trough Concentration trough IFN Interferon PASI Psoriasis Area and Severity Index CU Chronic Urticaria International Hidradenitis Suppurativa Severity IHS4 Score PBMC Peripheral Blood Mononuclear Cells DC Degradation Concentration # IL Interleukin DMSO Dimethyl Sulfoxide Pbo Placebo IND Investigational New Drug Application EASI Eczema Area and Severity Index Ph Phase IP Intellectual Property EBV Epstein-Barr Virus PK/PD Pharmacokinetics/Pharmacodynamics IRAK4 Interleukin 1 Receptor Associated Kinase 4 ECM Extracellular Matrix PN Prurigo Nodularis ENT Ear Nose Throat IRAKIMiD IRAK4 and IMiD substrates POC Proof-of-Concept JAK Janus Kinase EoE Eosinophilic Esophagitis PP-NRS Peak Pruritus Numerical Rating Scale EU European Union JP Japan PsA Psoriatic Arthritis 74

Abbreviations PsO Psoriasis UC Ulcerative Colitis pSTAT Signal Transducer and Activator of Transcription US United States vIGA Validated Investigator Global Assessment for AD PTCL Peripheral T-Cell Lymphoma QD Once a day WT Wildtype QoL Quality of Life WW Worldwide R&D Research and Development RA Rheumatoid Arthritis RNAseq Ribonucleic Acid Sequencing ROW Rest of World SAD Single Ascending Dose study SLE Systemic Lupus Erythematosus SMI Small Molecule Inhibitor STAT Signal Transducer and Activator of Transcription STAT3 Signal Transducer and Activator of Transcription 3 STAT6 Signal Transducer and Activator of Transcription 6 TARC Thymus and Activation-Regulated Chemokine Th1 Type 1 Th2 Type 2 Th17 Type 17 TLR Toll-like Receptors TPD Targeted Protein Degradation TYK2 Tyrosine Kinase 2 75