UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): November 2, 2023
DISC MEDICINE, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-39438 | 85-1612845 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 321 Arsenal Street, Suite 101, Watertown, MA 02472 | 02472 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (617) 674-9274
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol |
Name of each exchange on which registered |
||
| Common Stock, par value $0.0001 per share | IRON | The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On November 2, 2023, Disc Medicine, Inc. (the “Company”) issued a press release announcing that the Company will present data from multiple programs in its hematology portfolio at the upcoming 65th American Society of Hematology (“ASH”) Annual Meeting and Exposition, which will be held in San Diego, CA on December 9, 2023 through December 12, 2023. The Company will host a conference call on December 11 at 9:30 p.m. ET to review such data and the Company’s operational plans. An archived webcast will be available following the call for 30 days on the Events & Presentations section of the Company’s website. A copy of the press release and the abstracts related to the Company’s presentation are attached as Exhibits 99.1, 99.2 and 99.3 to this Current Report on Form 8-K.
The information contained in Item 7.01 of this Current Report on Form 8-K, including Exhibits 99.1, 99.2 and 99.3, is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly provided by specific reference in such filing. The Company undertakes no obligation to update, supplement or amend the material attached hereto as Exhibits 99.1, 99.2 and 99.3.
| Item 8.01 | Other Events. |
On November 2, 2023, the Company announced updates from its ongoing Phase 2 BEACON and AURORA studies of evaluating bitopertin in patients with erythropoietic protoporphyria (“EPP”) and its ongoing Phase 1b/2 study of DISC-0974 in patients with myelofibrosis (“MF”) and anemia.
Bitopertin
| • | Enrollment for phase 2 BEACON (n=22 adults) and AURORA (n=75 adults) studies of bitopertin in EPP is complete; BEACON has been expanded to enroll adolescents (age 12-18) |
| • | Interim analyses of the BEACON trial (July 5, 2023 data cutoff) demonstrated significant and consistent improvements in light tolerance across patients: |
| • | Observed magnitude of effects were comparable to previously reported improvements in sunlight tolerance |
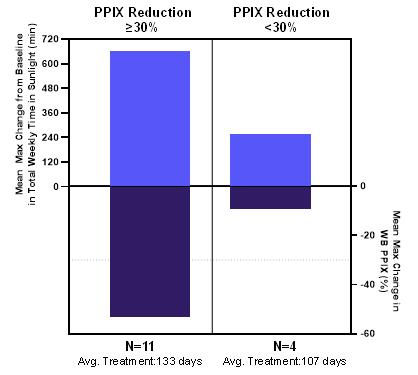
| • | An initial sub-group analysis indicated that greater suppression of protoporphyrin IX (“PPIX”) was associated with increased maximal weekly sunlight tolerance: >10 hours of improvement on average in patients with a mean maximal PPIX reduction >30% (n=11) and >4 hours of improvement on average in patients with mean maximal PPIX reduction <30% (n=4) |
| • | Updated BEACON data from all adult patients and with longer duration of therapy will be presented as an oral presentation at ASH, including: |
| • | Measures of PPIX, photosensitivity, quality of life, safety and tolerability |
| • | Preliminary analysis of the precedented pivotal endpoint, cumulative time in light over 6 months on days without pain |
| • | Topline AURORA data is expected to be presented in early 2024 |
DISC-0974
| • | Dose escalation is ongoing for both phase 1b/2 studies of DISC-0974 in MF and anemia, and non-dialysis dependent chronic kidney disease (“NDD-CKD”) |
| • | Initial data from the phase 1b/2 study of DISC-0974 in patients with MF and anemia will be presented at ASH |
| • | Data from 10-20 patients in the dose-escalation phase |
| • | Safety and changes in hepcidin, iron, and hemoglobin levels |
| • | Data from the 28 mg dose cohort of the phase 1b/2 study in NDD-CKD patients with anemia will be presented as part of the management call |
Cautionary Statement Regarding Forward-Looking Statements
This Current Report on Form 8-K contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, express or implied statements regarding the Company’s expectations with respect to its AURORA Phase 2 and BEACON Phase 2 clinical studies of bitopertin and the results thereof, and its Phase 1b/2 study of bitopertin in Diamond-Blackfan Anemia, its Phase 1b/2 clinical studies of DISC-0974 in patients with MF and NDD-CKD patients with anemia, projected timelines for the initiation and completion of its clinical trials, anticipated timing of release of data, and other clinical activities; and the Company’s business plans and objectives. The use of words such as, but not limited to, “believe,” “expect,” “estimate,” “project,” “intend,” “future,” “potential,” “continue,” “may,” “might,” “plan,” “will,” “should,” “seek,” “anticipate,” or “could” or the negative of these terms and other similar words or expressions that are intended to identify forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on the Company’s current beliefs, expectations and assumptions regarding the future of the Company’s business, future plans and strategies, clinical results and other future conditions. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements.
The Company may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements, and investors should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements as a result of a number of material risks and uncertainties including but not limited to: the nature, strategy and focus of the Company; the difficulty in predicting the time and cost of development of the Company’s product candidates; the Company’s plans to research, develop and commercialize its current and future product candidates; that enrollment timelines of both the BEACON and AURORA studies may not necessarily be predictive of future enrollment timelines; the timing of initiation of the Company’s planned clinical trials; the timing of the availability of data from the Company’s clinical trials; the Company’s ability to identify additional product candidates with significant commercial potential and to expand its pipeline in hematological diseases; the timing and anticipated results of the Company’s preclinical studies and clinical trials and the risk that the results of the Company’s clinical trials may not be predictive of future results in connection with future studies or clinical trials and may not support further development and marketing approval; the other risks and uncertainties described in the “Risk Factors” section of the Company’s Annual Report on Form 10-K for the year ended December 31, 2022, Quarterly Reports on Form 10-Q for the quarters ended March 31, 2023 and June 30, 2023, and other documents filed by the Company from time to time with the SEC, as well as discussions of potential risks, uncertainties, and other important factors in the Company’s subsequent filings with the SEC. Any forward-looking statement speaks only as of the date on which it was made. None of the Company, nor its affiliates, advisors or representatives, undertake any obligation to publicly update or revise any forward-looking statement, whether as result of new information, future events or otherwise, except as required by law.
| Item 9.01. | Exhibits. |
(d) Exhibits
| 99.1 | Press release issued by Disc Medicine, Inc. on November 2, 2023, furnished herewith. | |
| 99.2 | “Interim Analyses from the BEACON Trial: A Phase 2, Randomized, Open-Label Trial of Bitopertin in Erythropoietic Protoporphyria,” furnished herewith. | |
| 99.3 | “A Phase 1b Trial of DISC-0974, an Anti-Hemojuvelin Antibody, in Patients with Myelofibrosis and Anemia,” furnished herewith. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document). | |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| DISC MEDICINE, INC. | ||||||
| Date: November 2, 2023 | By: | /s/ John Quisel |
||||
| Name: | John Quisel, J.D. Ph.D. | |||||
| Title: | Chief Executive Officer | |||||
Exhibit 99.1

Disc Medicine Announces Multiple Presentations Across Portfolio at the 65th American Society of
Hematology Annual Meeting and Key Program Updates
| • | Completion of enrollment for phase 2 BEACON and AURORA studies of bitopertin in erythropoietic protoporphyria (EPP) |
| • | Oral presentation at ASH meeting of updated interim data from BEACON, including a preliminary analysis of the precedented pivotal endpoint, cumulative time in sunlight over 6 months on days without pain |
| • | Company will present preliminary data on pharmacodynamic activity from initial cohorts of phase 1b study of DISC-0974 in myelofibrosis (MF) patients with anemia, including changes in hemoglobin |
| • | Management will host a conference call on December 11th at 9:30 pm ET / 6:30 pm PT |
WATERTOWN, Mass. (November 2, 2023) – Disc Medicine, Inc. (NASDAQ:IRON), a clinical-stage biopharmaceutical company focused on the discovery, development, and commercialization of novel treatments for patients suffering from serious hematologic diseases, today announced several key updates and that it will present data from multiple programs in its hematology portfolio at the upcoming 65th American Society of Hematology (ASH) Annual Meeting and Exposition, which will be held in San Diego, CA on December 9-12, 2023.
“We look forward to presenting updated interim data from all patients enrolled in the BEACON study at the upcoming ASH meeting, as well as preliminary clinical data from initial cohorts of both DISC-0974 anemia studies.” said John Quisel, J.D., Ph.D., President and Chief Executive Officer of Disc. “In addition, we’re pleased to announce the full enrollment of both the BEACON and AURORA studies of bitopertin in patients with EPP. We were able to enroll both trials in under a year, and I want to express my gratitude to our team, collaborators and the EPP community for helping us reach this important milestone.”
Key Program Updates:
Bitopertin
| • | Enrollment for phase 2 BEACON (n=22 adults) and AURORA (n=75 adults) studies of bitopertin in EPP is complete; BEACON has been expanded to enroll adolescents (age 12-18) |
| • | Interim analyses of the BEACON trial (July 5, 2023 data cutoff) demonstrated significant and consistent improvements in light tolerance across patients: |
| • | Observed magnitude of effects were comparable to previously reported improvements in sunlight tolerance |
| • | An initial sub-group analysis indicated that greater suppression of protoporphyrin IX (PPIX) was associated with increased maximal weekly sunlight tolerance: >10 hours of improvement on average in patients with a mean maximal PPIX reduction >30% (n=11) and >4 hours of improvement on average in patients with mean maximal PPIX reduction <30% (n=4) |
| • | Updated BEACON data from all adult patients and with longer duration of therapy will be presented as an oral presentation at ASH, including: |
| • | Measures of PPIX, photosensitivity, QOL, safety and tolerability |
| • | Preliminary analysis of the precedented pivotal endpoint, cumulative time in light over 6 months on days without pain |
| • | Topline AURORA data is expected to be presented in early 2024 |

DISC-0974
| • | Dose escalation is ongoing for both phase 1b/2 studies of DISC-0974 in MF and anemia, and non-dialysis dependent chronic kidney disease (NDD-CKD) |
| • | Initial data from the phase 1b/2 study of DISC-0974 in patients with MF and anemia will be presented at ASH |
| • | Data from 10-20 patients in the dose-escalation phase |
| • | Safety and changes in hepcidin, iron, and hemoglobin levels |
| • | Data from the 28 mg dose cohort of the phase 1b/2 study in NDD-CKD patients with anemia will be presented as part of the management call |
Bitopertin and DISC-0974 are investigational agents and are not approved for use as therapies in any jurisdiction worldwide.
Details of Presentations and Abstracts
The full abstracts are now available through the ASH conference website.
Abstract Number: 923
Title: Interim Analyses from the BEACON Trial: A Phase 2, Randomized, Open-Label Trial of Bitopertin in Erythropoietic Protoporphyria
Date / Time: Monday, December 11, 5:30 PM PT
Session: 102. Iron Homeostasis and Biology: Exploring Molecular Mechanisms and Therapeutic Options in Iron Homeostasis (Oral Presentation) Presenter: Gayle Ross, M.D.
Abstract Number: 4564
Title: A Phase 1b Trial of DISC-0974, an Anti-Hemojuvelin Antibody, in Patients with Myelofibrosis and Anemia
Date / Time: Monday, December 11, 6:00-8:00 PM PT
Session: 634. Myeloproliferative Syndromes: Clinical and Epidemiological: Poster III
Presenter: William Savage, M.D., Ph.D.
Other abstracts available on the ASH conference website:
Abstract Number: 5224 (Online abstract only)
Title: Anti-Hemojuvelin Monoclonal Antibody DISC-0974 Elicits a Durable and Consistent Response with Repeated Dosing in Cynomolgus Monkeys
Abstract Number: 5236 (Online abstract only)
Title: A Phase 1b Double-Blind, Placebo-Controlled Study of DISC-0974, an Anti-Hemojuvelin Antibody, in Patients with Non-Dialysis Dependent Chronic Kidney Disease and Anemia
Abstract Number: 5228 (Online abstract only)
Title: Application of a Validated Method for Quantifying Circulating Protoporphyrin IX to the Beacon Trial of Bitopertin in Erythropoietic Protoporphyria
Webcast Conference Call Information
Management will host a call on Monday, December 11th at 9:30 pm ET / 6:30 pm PT to review data and operational plans. Please register for management’s webcast on the Events and Presentations page of Disc’s website (https://ir.discmedicine.com/).

About Disc Medicine
Disc Medicine (NASDAQ:IRON) is a clinical-stage biopharmaceutical company committed to discovering, developing, and commercializing novel treatments for patients who suffer from serious hematologic diseases. We are building a portfolio of innovative, potentially first-in-class therapeutic candidates that aim to address a wide spectrum of hematologic diseases by targeting fundamental biological pathways of red blood cell biology, specifically heme biosynthesis and iron homeostasis. For more information, please visit www.discmedicine.com.
Disc Cautionary Statement Regarding Forward-Looking Statements
This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, express or implied statements regarding Disc’s expectations with respect to its AURORA Phase 2 and BEACON Phase 2 clinical studies of bitopertin and the results thereof, and its Phase 1b/2 study of bitopertin in Diamond-Blackfan Anemia, its Phase 1b/2 clinical studies of DISC-0974 in patients with MF and NDD-CKD patients with anemia, projected timelines for the initiation and completion of its clinical trials, anticipated timing of release of data, and other clinical activities; and Disc’s business plans and objectives. The use of words such as, but not limited to, “believe,” “expect,” “estimate,” “project,” “intend,” “future,” “potential,” “continue,” “may,” “might,” “plan,” “will,” “should,” “seek,” “anticipate,” or “could” or the negative of these terms and other similar words or expressions that are intended to identify forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on Disc’s current beliefs, expectations and assumptions regarding the future of Disc’s business, future plans and strategies, clinical results and other future conditions. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements.
Disc may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements, and investors should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements as a result of a number of material risks and uncertainties including but not limited to: the nature, strategy and focus of Disc; the difficulty in predicting the time and cost of development of Disc’s product candidates; Disc’s plans to research, develop and commercialize its current and future product candidates; that enrollment timelines of both the BEACON and AURORA studies may not necessarily be predictive of future enrollment timelines; the timing of initiation of Disc’s planned clinical trials; the timing of the availability of data from Disc’s clinical trials; Disc’s ability to identify additional product candidates with significant commercial potential and to expand its pipeline in hematological diseases; the timing and anticipated results of Disc’s preclinical studies and clinical trials and the risk that the results of Disc’s clinical trials may not be predictive of future results in connection with future studies or clinical trials and may not support further development and marketing approval; the other risks and uncertainties described in the “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2022, Quarterly Reports on Form 10-Q for the quarters ended March 31, 2023 and June 30, 2023, and other documents filed by Disc from time to time with the SEC, as well as discussions of potential risks, uncertainties, and other important factors in Disc’s subsequent filings with the SEC. Any forward-looking statement speaks only as of the date on which it was made. None of Disc, nor its affiliates, advisors or representatives, undertake any obligation to publicly update or revise any forward-looking statement, whether as result of new information, future events or otherwise, except as required by law.

Media Contact
Peg Rusconi
Verge Scientific Communications
prusconi@vergescientific.com
Investor Relations Contact
Christina Tartaglia
Stern Investor Relations
christina.tartaglia@sternir.com
Exhibit 99.2
Interim Analyses from the BEACON Trial: A Phase 2, Randomized, Open-label Trial of Bitopertin in Erythropoietic Protoporphyria
Gayle Rossa; Peter Stewartb, George Mensingc; Melanie Chinc; Haley Howellc; Heidi Mangusc; Will Savagec
| a | Royal Melbourne Hospital, Melbourne, Australia |
| b | Royal Prince Alfred Hospital, Sydney, Australia |
| c | Disc Medicine, Watertown, MA |
Introduction: Erythropoietic protoporphyria (EPP) is associated with accumulation of photoreactive protoporphyrin IX (PPIX) in the skin and other organs, causing debilitating phototoxic skin reactions following exposure to sunlight, and potentially life-threatening protoporphyric hepatopathy in some patients. Reduction of PPIX is associated with amelioration of disease in the settings of hematopoietic stem cell transplant, pregnancy, and extracorporeal photoinactivation.1-3
Glycine transporter 1 (GlyT1) supplies extracellular glycine for the initial step of heme biosynthesis in erythroid cells.4 Bitopertin is an investigational, orally-administered inhibitor of GlyT1. It is hypothesized that GlyT1 inhibition leads to a decrease in heme pathway intermediates, including PPIX, and can improve light tolerance.5
Methods: BEACON is a Phase 2, randomized, open-label, parallel-arm trial (ACTRN12622000799752) of 22 participants who will receive oral, once-daily administration of 20 mg or 60 mg of bitopertin for 24 weeks. The trial is being conducted at 2 sites in Australia and includes participants ≥18 years of age with a confirmed diagnosis of EPP. The primary efficacy endpoint is percent change in whole-blood metal-free PPIX. Additional endpoints include daily patient-reported outcomes (PRO) of light tolerance and quality of life, as well as safety and tolerability.
Results: As of data cutoff (05 July 2023), a total of 17 subjects had been enrolled. Treatment with bitopertin resulted in mean (SD) decreases in PPIX of -39% ± 17% by Day 43 (n=12), with more pronounced decreases observed with 60 mg compared to 20 mg (Figure 1). Bitopertin also improved multiple measures of light tolerance. A participant randomized to 20 mg bitopertin reported a >80-fold increase in sunlight tolerance on Day 88 of treatment, increasing from 4.5 minutes at baseline to over 6 hours; no prodromes were reported during any sunlight challenge after Day 20. A participant randomized to 60 mg bitopertin reported a >200-fold increase in sunlight tolerance on Day 74 of treatment, increasing from 1.3 minutes at baseline to over 4 hours, and did not report a prodrome during any sunlight challenge after Day 120. Aggregate measures of light tolerance also improved over time, including time to prodrome averaged over a 2-week period and weekly averages of total time in sunlight. The proportion of prodrome-free sunlight challenges increased from 2% during screening to 49% while receiving bitopertin (n=17), and the proportion of days without symptoms (with sun exposure) increased from 26% during screening to 74% while receiving bitopertin (n=17). Improvements in weekly averages of total time in sunlight were consistent and observed in 15/15 participants with post-baseline PPIX values.
The greatest increases in weekly averages of total time in sunlight were observed in participants who experienced reductions in PPIX of at least 30% (Figure 2), for which total time in sunlight increased from a weekly average of 339 minutes at baseline to a maximum of 999 minutes with bitopertin (n=11; mean duration of treatment: 133 days [range: 76 to ≥169 days]). Patient-reported phototoxic reactions decreased by 93% while on treatment compared to baseline (n=17). By Day 43, 15/16 participants reported in the Patient Global Impression of Change (PGIC) their EPP was much better or a little better, and 15/16 reported in the Patient Global Impression of Severity (PGIS) their EPP was mild or not at all severe. A majority (13/16) of participants responded in a novel PRO, the EPP Impact Questionnaire (EPIQ), that EPP had little or no impact on their quality of life while receiving bitopertin.
No serious adverse events, discontinuations due to adverse events, or dose reductions have been reported. A total of 12 (71%) participants reported treatment-emergent adverse events; only dizziness and headache were reported in more than one subject. No meaningful changes in hemoglobin have been observed.
Conclusion: By reducing whole-blood PPIX levels, bitopertin targets the underlying pathophysiology of EPP, resulting in consistent improvements in multiple measures of light tolerance and quality of life. Bitopertin has been well tolerated to date with no changes in hemoglobin, and its safety profile in EPP is consistent with prior studies that enrolled more than 4000 participants. Updated results will be presented at the meeting.
Figure 1. Percent Changes in Whole-Blood (WB) Protoporphyrin IX (PPIX) with Bitopertin
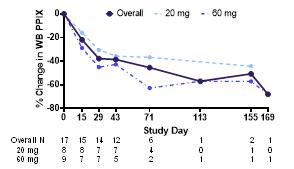
Figure 2. Maximum Changes from Baseline in Weekly Total Time in Sunlight and Corresponding Changes in PPIX with Bitopertin
References
1. Heerfordt IM, Wulf HC. Protoporphyrin IX in the skin measured noninvasively predicts photosensitivity in patients with erythropoietic protoporphyria. Br J Dermatol. 2016;175(6):1284-1289.
2. Wulf HC, Nissen CV, Philipsen PA. Inactivation of protoporphyrin IX in erythrocytes in patients with erythropoietic protoporphyria: a new treatment modality. Photodiagnosis Photodyn Ther. 2020;29:101582.
3. Poh-Fitzpatrick MB. Human protoporphyria: reduced cutaneous photosensitivity and lower erythrocyte porphyrin levels during pregnancy. J Am Acad Dermatol. 1997;36(1):40-43.
4.Garcia-Santos D, Schranzhofer M, Bergeron R, et al. Extracellular glycine is necessary for optimal hemoglobinization of erythroid cells. Haematologica. 2017;102(8):1314-1323.
5. Halloy F, Iyer P, Ghidini A, et al. Repurposing of glycine transport inhibitors for the treatment of erythropoietic protoporphyria. Cell Chem Biol. 2021;28(8):1221-1234.
Exhibit 99.3
A Phase 1b Trial of DISC-0974, an Anti-Hemojuvelin Antibody, in Patients with Myelofibrosis and Anemia
NASEEMA GANGAT1, JAMES FORAN2, ANNA HALPERN3,4, RAAJIT RAMPAL5, NATASHA NOVIKOV6, AKSHAY BUCH6, OLIVIA PELLETIER6, WILLIAM SAVAGE6, AYALEW TEFFERI1
| 1 | Division of Hematology, Department of Internal Medicine, Mayo Clinic, Rochester, MN |
| 2 | Division of Hematology, Department of Medicine, Mayo Clinic, Jacksonville, FL |
| 3 | Department of Medicine, University of Washington, Seattle, WA |
| 4 | Clinical Research Division, Fred Hutchinson Cancer Center, Seattle, WA |
| 5 | Memorial Sloan-Kettering Cancer Center, New York, NY |
| 6 | Disc Medicine, Watertown, MA |
Hepcidin, a central regulator of iron homeostasis, is pathologically elevated in patients with myelofibrosis (MF) and anemia. Chronic elevations in hepcidin contribute to the onset and severity of anemia. DISC-0974 is an investigational, first-in-class, monoclonal antibody that blocks hemojuvelin, a co-receptor in the bone morphogenetic protein-signaling pathway driving hepcidin expression. Preclinical studies have shown that DISC-0974 suppresses hepcidin and increases serum iron. A healthy volunteer study has demonstrated dose-dependent reductions in serum hepcidin, increases in serum iron with doses up to 56 mg administered subcutaneously, and increasing trends in reticulocyte count, reticulocyte hemoglobin, mean corpuscular hemoglobin, total hemoglobin (Hgb), and red blood cell count.
In this Phase 1b/2a, open-label, multiple-ascending dose study (NCT05320198), we are assessing the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of DISC-0974 in patients with MF and anemia.
Eligible participants include patients over 18 years of age with intermediate-2 or higher-risk MF and anemia. MF is required to be confirmed using the World Health Organization 2016 criteria. Anemia is defined per protocol as Hgb < 10 g/dL or transfusion dependence, as defined by the International Working Group for Myelofibrosis Research and Treatment (IWG-MRT). A stable dose of hydroxyurea and/or Janus kinase (JAK) inhibitor is allowed on trial. Major exclusion criteria include: liver iron concentration ≥ 7 mg/g dry weight; splenectomy; nutritional, genetic, infectious, or autoimmune causes of anemia. Dose escalation is based on a Bayesian optimal interval design with accelerated titration. Safety Review Committee assessments gate escalation decisions. Dosing occurs monthly for a total of 6 doses.
Primary endpoints include safety and tolerability assessments of adverse events (AEs), clinical laboratory assessments, vital signs, physical examinations, and electrocardiograms. Secondary endpoints include anemia response as defined by the IWG-MRT, transfusion response as defined by a decrease of 50% in transfusion requirement over any 8-week period, standard PK parameters and PD metrics including serum hepcidin-25, iron, transferrin saturation, ferritin, and hematology biomarkers. All data are summarized using descriptive statistics.
Initial data show a dose-related increase in pharmacokinetic parameters. DISC-0974 treatment led to dose-dependent reductions in serum hepcidin-25 and increases in serum iron levels. There have been no serious AEs or AEs leading to study withdrawal. Most AEs were mild, transient, and unrelated to DISC-0974.
As of August 1, 2023, enrollment and dose escalation are ongoing. Safety, PK and PD data from at least the first two cohorts will be presented.