☐
|
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
☒
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
☐
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
☐
|
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
||
American Depositary Shares, each representing ten Ordinary Shares* |
KZIA |
The Nasdaq Stock Market |
* |
Not for trading, but only in connection with the registration of American Depositary Shares. |
U.S. GAAP ☐ |
International Financial Reporting Standards as issued by the International Accounting Standards Board ☒ |
Other |
☐ |
TABLE OF CONTENTS
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 20-F includes forward-looking statements, which involve a number of risks and uncertainties. These forward-looking statements can generally be identified as such because the context of the statement will include words such as “may,” “will,” “intend,” “plan,” “believe,” “anticipate,” “expect,” “estimate,” “predict,” “potential,” “continue,” “likely,” or “opportunity,” the negative of these words or other similar words. Similarly, statements that describe our future plans, strategies, intentions, expectations, objectives, goals or prospects and other statements that are not historical facts are also forward-looking statements. Discussions containing these forward-looking statements may be found, among other places, in “Business Overview” and “Operating and Financial Review and Prospects” in this Annual Report on Form 20-F. For such statements, we claim the protection of the Private Securities Litigation Reform Act of 1995 and section 27A of the Securities Act and Section 21E of the Exchange Act. Readers of this Annual Report on Form 20-F are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the time this Annual Report on Form 20-F was filed with the Securities and Exchange Commission, or SEC. These forward-looking statements are based largely on our expectations and projections about future events and future trends affecting our business and are subject to risks and uncertainties that could cause actual results to differ materially from those anticipated in the forward-looking statements. These risks and uncertainties include, without limitation, those discussed in “Risk Factors” and in “Operating and Financial Review and Prospects” of this Annual Report on Form 20-F. In addition, past financial or operating performance is not necessarily a reliable indicator of future performance, and you should not use our historical performance to anticipate results or future period trends. We can give no assurances that any of the events anticipated by the forward-looking statements will occur or, if any of them do, what impact they will have on our results of operations and financial condition. Except as required by law, we undertake no obligation to update publicly or revise our forward-looking statements to reflect events or circumstances that arise after the filing of this Annual Report on Form 20-F.
In this Annual Report on Form 20-F, “Kazia,” “Company,” “we,” “us” and “our” refer to Kazia Therapeutics Limited and its wholly owned subsidiaries on a consolidated basis, unless the context otherwise provides.
PART I
| Item 1. | Identity of Directors, Senior Management and Advisors |
Item 1 details are not required to be disclosed as part of the Annual Report.
| Item 2. | Offer Statistics and Expected Timetable |
Item 2 details are not required to be disclosed as part of the Annual Report.
| Item 3. | Key Information |
A. Reserved
B. Capitalization and Indebtedness.
Not applicable.
C. Reasons for the Offer and Use of Proceeds.
Not applicable.
D. Risk factors
Investment in our securities involves a high degree of risk. You should consider carefully the risks described below, together with other information in this Annual Report on Form 20-F and our other public filings, before making investment decisions regarding our securities. If any of the following events actually occur, our business, operating results, prospects or financial condition could be materially and adversely affected. This could cause the trading price of our common stock to decline and you may lose all or part of your investment. Moreover, the risks described below are not the only ones that we face. Additional risks not presently known to us or that we currently deem immaterial may also affect our business, operating results, prospects or financial condition.
1
Risks Related to Our Financial Condition and Capital Requirement
We have incurred significant net losses since our inception. We expect to incur significant net losses for the foreseeable future and may never achieve or maintain profitability.
We have incurred significant net losses. We anticipate that we will continue to incur significant net losses for the foreseeable future and we may never achieve or maintain profitability. We are a biotechnology company and have not yet generated significant revenue. We have incurred losses of A$8,419,484, A$25,014,055 and A$20,465,180 for the fiscal years ended June 30, 2021 (restated), 2022 (restated) and 2023, respectively. We have not generated any revenues from sales of any of our product candidates in prior financial years, however in the fiscal year ended June 30, 2021 we did generate revenues of A$15.2 million from the licensing of our development stage drug candidates.
As of 30 June 2023, we had accumulated losses of A$89,082,571. We have devoted most of our financial resources to research and development, including our clinical development activities. To date, we have financed our operations primarily through the issuance of equity securities, research and development grants from the Australian government and payments from our collaboration partners. While we have generated significant revenue in recent fiscal years from license transactions, the nature of such revenue is irregular and unpredictable, and is based upon achievement of milestones over which we have limited or no control. As a consequence, we expect to continue to incur significant operating losses for the foreseeable future due to the cost of research and development including clinical trials and the regulatory approval process for product candidates. The amount of our future net losses is uncertain and will depend, in part, on the rate of our future expenditures. Our ability to continue operations will depend on, among other things, our ability to obtain funding through equity or debt financings, strategic collaborations or grants.
We anticipate that our expenses will increase substantially if and as we:
| • | continue our research and clinical development of our product candidates; |
| • | expand the scope of our current clinical studies for our product candidates or initiate additional clinical or other studies for product candidates; |
| • | seek regulatory and marketing approvals for any of our product candidates that successfully complete clinical trials; |
| • | further develop the manufacturing process for our product candidates; |
| • | change or add additional manufacturers or suppliers; |
| • | seek to identify and validate additional product candidates; |
| • | acquire or in-license other product candidates and technologies; |
| • | maintain, protect and expand our intellectual property portfolio; |
| • | create additional infrastructure to support our operations as a public company in the United States and our product development and future commercialization efforts; and |
| • | experience any delays or encounter issues with any of the above. |
The net losses we incur may fluctuate significantly from year to year, such that a period-to-period comparison of our results of operations may not be a good indication of our future performance.
We have never generated any revenue from product sales and may never be profitable.
Our ability to generate significant revenue and achieve profitability depends on our ability, alone or with strategic collaboration partners, to successfully complete the development of and obtain the regulatory approvals for our product candidates, to manufacture sufficient supply of our product candidates, to establish a sales and marketing organization or suitable third-party alternative for the marketing of any approved products and to successfully commercialize any approved products on commercially reasonable terms. All of these activities will require us to raise sufficient funds to finance business activities. In addition, we do not anticipate generating revenue from commercializing product candidates for the foreseeable future, if ever. Our ability to generate future revenues from commercializing product candidates depends heavily on:
| • | successfully initiating and completing clinical trials of our product candidates; |
| • | the timing of the initiation and completion of preclinical studies and clinical trials; |
| • | the timing of patient enrollment and dosing in any future clinical trials; |
| • | the timing of the availability of data from clinical trials; |
| • | expectations about the successful completion of clinical trials; |
| • | obtaining regulatory and marketing approvals for product candidates for which we complete clinical trials; |
| • | the timing of expected regulatory filings; |
| • | expectations about approval by regulatory authorities of our drug candidates; |
2
| • | the clinical utility and potential attributes and benefits of our product candidates, including the potential duration of treatment effects; |
| • | potential licenses of intellectual property and collaborations; |
| • | the commercialization of our product candidates, if approved; |
| • | expectations regarding expenses, ongoing losses, future revenue and capital needs; |
| • | our financial performance; |
| • | the length of time over which we expect our cash and cash equivalents to be sufficient; |
| • | our intellectual property position and the duration of our patent portfolio; |
| • | maintaining, protecting and expanding our intellectual property portfolio, and avoiding infringing on intellectual property of third parties; |
| • | establishing and maintaining successful licenses, collaborations and alliances with third parties; |
| • | developing a sustainable, scalable, reproducible and transferable manufacturing process for our product candidates; |
| • | establishing and maintaining supply and manufacturing relationships with third parties that can provide products and services adequate, in amount and quality, to support clinical development and commercialization of our product candidates, if approved; |
| • | launching and commercializing any product candidates for which we obtain regulatory and marketing approval, either by collaborating with a partner or, if launched independently, by establishing a sales, marketing and distribution infrastructure; |
| • | obtaining market acceptance of any product candidates that receive regulatory approval as viable treatment options; |
| • | the outcome of corresponding endeavors in respect of competitive or potentially competitive product candidates by other drug development companies; |
| • | obtaining favorable coverage and reimbursement rates for our products from third-party payers; |
| • | addressing any competing technological and market developments; |
| • | identifying and validating new product candidates; and |
| • | negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter. |
Even if one or more of our product candidates is approved for commercial sale, we may incur significant costs associated with commercializing any approved product candidate. As one example, our expenses could increase beyond expectations if we are required by the U.S. Food and Drug Administration (“FDA”) or other regulatory agencies, domestic or foreign, to perform clinical and other studies in addition to those that we currently anticipate. Even if we are able to generate revenues from the sale of any approved products, we may not become profitable and may need to obtain additional funding to continue operations, which could have an adverse effect on our business, financial condition, results of operations and prospects.
The Company has two product candidates currently in clinical trials. Failure of one or both of these product candidates to show benefit to patients could materially and adversely affect the continuity of our business and our financial condition.
The Company’s lead programs include paxalisib (formerly GDC-0084), a small molecule inhibitor of the PI3K/Akt/mTOR pathway, and EVT801, a small molecule selective inhibitor of vascular endothelial growth factor receptor 3 (VEGFR3). However, even though progress has been made, such as the clinical validation of the PI3K/Akt/mTOR pathway as a target for oncology therapies, development of our product candidates may prove unsuccessful, after completion of clinical trials, due to any failure to provide adequate beneficial effect to cancer patients. It is possible that either or both product candidates may fail to show sufficient benefit as an intended treatment for the specific cancer indication to become commercially viable products, which could materially and adversely affect the continuity of our business and our financial condition.
The Company has ongoing clinical trials in which experimental therapies are administered to human subjects. If profound and unexpected safety concerns are encountered in clinical trials, it may materially and adversely affect the continuity of our business and our financial condition.
Despite all applicable efforts to characterize the safety profile of our drug development candidates through animal studies and other mechanisms, the possibility of unexpected safety concerns remains. If one or both of our clinical stage candidates were found to be associated with profound and unexpected toxicity or other safety concerns, the Company may be required to cease development of one or both candidates, and may additionally incur other impairments to the business including reputational damage, which may materially and adversely affect the continuity of our business and our financial condition.
The Company relies on third-party contract manufacturing organizations to manufacture its drug product candidates. If one or more of these vendors were unable to meet the Company’s needs, it may materially and adversely impact our business.
Manufacture of pharmaceutical material for human administration is technically complex and highly regulated. If one or more of the Company’s vendors failed to produce drug product to the requisite standard, the continuity of the Company’s operations may be severely disrupted. Even if a vendor was found deficient in respect of another product, it may impair the confidence of regulatory agencies in our product candidates, thereby disrupting our operations.
Global contract manufacturing capacity is limited, and the manufacturing process is not readily portable. As a result, the Company’s ability to manufacture its product candidates in a timely manner is dependent on the availability of suitable capacity at its vendors.
The manufactured drug products, and their intermediaries, are of significant financial value. Loss, damage, or theft of this material, for example while in storage or transit, may result in significant detriment to the Company, which may be incompletely cured by insurance.
3
There is substantial doubt about our ability to continue as a going concern, which may hinder our ability to obtain future financing.
The Company has limited cash resources and will periodically need additional funds to maintain the planned level of R&D activity. We expect to consume cash and incur operating losses for the foreseeable future as the Company continues developing its oncology drug candidates. The impact on cash resources and results from operations will vary with the extent and timing of future clinical trial programs. While it is not possible to make accurate predictions of future operating results, we expect existing cash and cash equivalents, including the capital raised in July 2023, will be sufficient to enable us to continue our research and development activities until approximately November 2023.
As at 30 June 2023, we had cash on hand at the bank of A$5.2 million. The financial statements have been prepared on a going concern basis, which contemplates continuity of normal activities and realization of assets and settlement of liabilities in the normal course of business. As is often the case with drug development companies, our ability to continue as a going concern is dependent upon our ability to derive sufficient cash from investors, from licensing and partnering and collaboration activities and from other sources of revenue such as grant funding.
Furthermore, we are limited by General Instruction I.B.5 to Form F-3 (the “Baby Shelf Rule”) as of the filing of this Annual Report, until such time as our non-affiliate public float exceeds $75 million. The amount of funds we can raise through primary non-affiliate public offerings of securities in any 12-month period using our registration statement on Form F-3 is limited to one-third of the aggregate market value of the ordinary shares held by non-affiliates of the Company, which limitation may change over time based on our stock price, number of ordinary shares outstanding and the percentage of ordinary shares held by non-affiliates. These factors raise substantial doubt about our ability to continue as a going concern within one year after the date that the financial statements are issued. The independent auditor’s report for the fiscal year ended 30 June 2023 included an explanatory paragraph in relation to the going concern uncertainty.
If the Company is unable to obtain additional funds on favorable terms or at all, it may be required to cease or reduce its operations. Our future success is dependent upon our ability to obtain additional funding. There can be no assurance, however, that we will be successful in obtaining such funding in sufficient amounts, on terms acceptable to us, or at all. Also, if the Company raises more funds by selling additional securities, the ownership interests of holders of its securities will be diluted.
Global economic uncertainty caused by rising inflation, political instability, and conflicts and other events of geopolitical significance, such as the conflict between Russia and Ukraine, and the recent conflict between Israel and Gaza, could adversely affect our business and financial performance.
Negative global economic conditions may pose challenges to the Company’s business strategy, which relies on access to capital from financial markets and/or investment by other companies. Failure to obtain sufficient funding on acceptable terms could have a material adverse effect on our business, results of operations and financial condition. Negative conditions in the global economy, including credit markets and the financial services industry, have generally made equity and debt financing more difficult to obtain, and may negatively impact the Company’s ability to complete financing transactions. We are currently operating in a period of economic uncertainty and capital markets disruption, which has been significantly impacted by the geopolitical instability due to the ongoing military conflict between Russia and Ukraine and the recently erupted conflict between Israel and Gaza. Our business, financial condition, and results of operations may be materially adversely affected by the negative impact on the global economy and capital markets resulting from the conflict in Ukraine or any other geopolitical tensions. U.S. and global markets are experiencing volatility and disruption following the escalation of geopolitical tensions, including the military conflict between Russia and Ukraine and the recent conflict between Israel and Gaza as well as any additional escalations that may develop in the Middle East region. Although the length and impact of these ongoing military conflicts are highly unpredictable, the conflict in Ukraine and the recent conflict between Israel and Gaza have led to market disruptions, including significant volatility in commodity prices, credit and capital markets, as well as supply chain disruptions.
Additionally, various of Russia’s actions have led to sanctions and other penalties being levied by the U.S., Australia, the European Union, and other countries, as well as other public and private actors and companies, against Russia and certain other geographic areas, including agreement to remove certain Russian financial institutions from the Society for Worldwide Interbank Financial Telecommunication payment system and restrictions on imports of Russian oil, liquified natural gas and coal. Additional potential sanctions and penalties have also been proposed and/or threatened. Russian military actions and the resulting sanctions could further adversely affect the global economy and financial markets and lead to instability and lack of liquidity in capital markets, potentially making it more difficult for us to obtain additional funds.
The duration and severity of these conditions is uncertain, as is the extent to which they may adversely affect the Company’s business and the business of current and prospective vendors and collaborators. If negative global economic conditions persist or worsen, the Company may be unable to secure additional funding to sustain its operations or to find suitable collaborators to advance its internal programs, even if positive results are achieved from research and development efforts.
Any of the above-mentioned factors could affect our business, prospects, financial condition, and operating results. The extent and duration of the military action, sanctions, and resulting market disruptions are impossible to predict, but could be substantial.
If we are unable to raise sufficient funding on acceptable terms due to these or other factors, we may be unable to continue to operate. There is no assurance that we will be successful in obtaining sufficient financing on acceptable terms and conditions to fund continuing operations, if at all. Our failure to obtain sufficient funds on acceptable terms when needed could have a material adverse effect on our business, results of operations and financial condition.
4
Risks Related to Our Business Operations
We may not successfully engage in strategic transactions or enter into new collaborations, which could adversely affect our ability to develop and commercialize product candidates, impact our cash position, increase our expenses and present significant distractions to our management.
From time to time, we may consider additional strategic transactions, such as collaborations, acquisitions, asset purchases or sales
and out- or in-licensing of product candidates or technologies. In particular we will evaluate and, if strategically attractive, seek to enter into additional collaborations, including with major biotechnology or pharmaceutical companies. The competition for collaborators is significant, and the negotiation process is time-consuming and complex. Any new collaboration may be on terms that are not optimal for us, and we may not be able to maintain any new or existing collaboration if, for example, development or approval of a product candidate is delayed, sales of an approved product candidate do not meet expectations or the collaborator discontinues the collaboration. Any such collaboration, or other strategic transaction, may require us to
incur non-recurring or other charges, increase our expenditures, pose significant integration or implementation challenges or disrupt our management or business.
These transactions would entail numerous operational and financial risks, including exposure to unknown liabilities, incurrence of substantial debt or dilutive issuances of equity securities to pay transaction consideration or costs, higher than expected collaboration, acquisition or integration costs, write-downs of assets or goodwill or impairment charges, increased amortization expenses, difficulty and cost in facilitating the collaboration or combining the operations and personnel of any acquired business, impairment of relationships with key suppliers, manufacturers or customers of any acquired business due to changes in management and ownership and the inability to retain key employees of any acquired business.
Accordingly, although there can be no assurance that we will undertake or successfully complete any additional transactions of the nature described above, any transactions that we do complete may be subject to the foregoing or other risks and have a material adverse effect on our business, results of operations, financial condition and prospects. Conversely, any failure to enter any collaboration or other strategic transaction that would be beneficial to us could delay and make more expensive the development and potential commercialization of our product candidates and have a negative impact on the competitiveness of any product candidate that reaches market.
Any inability to attract and retain qualified key management and technical personnel would impair our ability to implement our business plan.
Our success largely depends on the continued service of key management and other specialized personnel. The loss of one or more members of our management team or other key employees or advisors could delay or increase the cost of our research and development programs and materially harm our business, financial condition, results of operations and prospects. The relationships that our key managers have cultivated within our industry make us particularly dependent upon their continued employment with us. We are dependent on the continued service of our technical personnel because of the highly technical nature of our product candidates and the specialized nature of the regulatory approval process for our product candidates. Because our management team and key employees are not obligated to provide us with continued service, they could terminate their employment with us at any time without penalty. We do not maintain key person life insurance policies on any of our management team members or key employees. Our future success will depend in large part on our continued ability to attract and retain other highly qualified scientific, technical and management personnel, as well as personnel with expertise in clinical testing, manufacturing, governmental regulation and commercialization. We face competition for personnel from other companies, universities, public and private research institutions, government entities and other organizations.
Our collaborations with outside scientists and consultants may be subject to restriction and change.
We work with medical experts, chemists, biologists and other scientists at academic and other institutions, and consultants who assist us in our research, development and regulatory efforts, including the members of our scientific advisory board. In addition, these scientists and consultants have provided, and we expect that they will continue to provide, valuable advice regarding our programs and regulatory approval processes. These scientists and consultants are not our employees and may have other commitments that would limit their future availability to us. If a conflict of interest arises between their work for us and their work for another entity, we may lose their services. In addition, we are limited in our ability to prevent them from establishing competing businesses or developing competing products. For example, if a key scientist acting as a principal investigator in any of our future clinical trials identifies a potential product or compound that is more scientifically interesting to professional interests, their availability to remain involved in any future clinical trials could be restricted or eliminated.
5
We face potential product liability claims, and, if successful claims are brought against us, we may incur substantial liability and costs. If the use of our product candidates harms patients, or is perceived to harm patients even when such harm is unrelated to our product candidates, our regulatory approvals could be revoked or otherwise negatively impacted and we could be subject to costly and damaging product liability claims.
The use of our product candidates in clinical trials and the sale of any products for which we may in the future obtain marketing approval exposes us to the risk of product liability claims. Product liability claims might be brought against us by consumers, healthcare providers, pharmaceutical companies or others selling or otherwise coming into contact with our product candidates. There is a risk that our product candidates may induce adverse events. If we cannot successfully defend against product liability claims, we could incur substantial liability and costs. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| • | impairment of our business reputation; |
| • | withdrawal of clinical trial participants; |
| • | costs due to related litigation; |
| • | distraction of management’s attention from our primary business; |
| • | substantial monetary awards to patients or other claimants; |
| • | the inability to commercialize our product candidates; |
| • | decreased demand for our product candidates, if approved for commercial sale; and |
| • | increased cost, or impairment of our ability, to obtain or maintain product liability insurance coverage. |
We may use our limited financial and human resources to pursue a particular research program or product candidate and fail to capitalize on programs or product candidates that may be more profitable or for which there is a greater likelihood of success.
Because we have limited resources, we may forego or delay pursuit of opportunities with certain programs or product candidates or for indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs for product candidates may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through strategic collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate, or we may allocate internal resources to a product candidate in a therapeutic area in which it would have been more advantageous to enter into a collaboration arrangement.
Our internal computer and information technology systems, or those of our collaborators and other development partners, third-party Contract Research Organizations (CROs) or other contractors or consultants, may fail or suffer security breaches, which could result in a disruption of our product development programs.
Despite the implementation of security measures, our internal computer and information technology systems and those of our current and any future CROs and other contractors, consultants and collaborators are vulnerable to damage from computer viruses, cyber-attacks, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Such events could cause interruptions of our operations. While we have not experienced any material system failure, accident or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a disruption of our development programs and our business operations, whether due to a loss of our trade secrets or other similar disruptions. One of our major suppliers did experience a cyber attack in April 2023 but it did not result in any material system failure and had no long term impact on our business. For example, the loss of clinical trial data from ongoing or future clinical trials or data from preclinical studies could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Likewise, we rely on third parties to manufacture our product candidates and will rely on third parties to conduct future clinical trials, and similar events relating to their computer systems could also have similar consequences to our business. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development and commercialization of our product candidates could be delayed and become more expensive.
Our ability to utilize our net operating losses and certain other tax attributes may be limited.
We have substantial carried forward tax losses which may not be available to offset any future assessable income. In order for an Australian corporate taxpayer to carry forward and utilize tax losses, the taxpayer must pass either the continuity of ownership test, or, if it fails the COT, the same business test (“SBT”), or similar business test, in respect of relevant tax losses.
We have not carried out any formal analysis as to whether we have met the COT or, failing the COT, the SBT or similar business test over relevant periods. In addition, future shareholding changes may result in a significant ownership change for us. It is therefore uncertain as to whether any of our tax losses carried forward as of 30 June 2023 will be available to be carried forward and available to offset our assessable income, if any, in future periods.
6
Risks Related to the Product Development and Regulatory Approval of Our Product Candidates
We may not be able to obtain orphan drug exclusivity, where relevant, in all markets for our product candidates.
Regulatory authorities in some jurisdictions, including the United States, may designate drugs for relatively small patient populations as orphan drugs. Under the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is a product intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals annually in the United States. The FDA may also designate a product as an orphan drug if it is intended to treat a disease or condition of more than 200,000 individuals in the United States and there is no reasonable expectation that the cost of developing and making a drug or biological product available in the United States for this type of disease or condition will be recovered from sales of the product candidate.
Generally, if a product with an orphan drug designation subsequently receives the first marketing approval for the indication for which it has such designation, the product is entitled to a period of marketing exclusivity, which precludes the FDA from approving another marketing application for the same drug for such indication for that time period. The applicable period is seven years in the United States. Orphan drug exclusivity may be lost if the FDA determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition.
Paxalisib (formerly GDC-0084) was granted orphan drug designation by the FDA in February 2018 for the treatment of glioblastoma, in August 2020 for the treatment of malignant glioma, which includes DIPG, a rare and highly aggressive childhood brain cancer, and in June 2022 for the treatment of atypical rhabdoid / teratoid tumors (AT/RT). However, even if we obtain orphan drug exclusivity for additional products in the United States or other jurisdictions, that exclusivity may not effectively protect the product from competition because different drugs can be approved for the same condition, and the same drug could be approved for a different condition. Moreover, even after an orphan drug is approved, the FDA can subsequently approve the same drug, made by a competitor, for the same condition if the FDA concludes that the competitive product is clinically superior in that it is shown to be safer, more effective or makes a major contribution to patient care.
Positive results from preclinical studies of our product candidates are not necessarily predictive of the results of our planned clinical trials of our product candidates.
Positive results in preclinical proof of concept and animal studies of our product candidates may not result in positive results in clinical trials in humans. Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in clinical trials after achieving positive results in preclinical development or early-stage clinical trials, and we cannot be certain that we will not face similar setbacks. These setbacks have been caused by, among other things, preclinical findings made while clinical trials were underway or safety or efficacy observations made in clinical trials, including adverse events. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that believed their product candidates performed satisfactorily in preclinical studies and clinical trials nonetheless failed to obtain FDA or other regulatory authority approval. If we fail to produce positive results in our clinical trials of our product candidates, the development timeline and regulatory approval and commercialization prospects for our product candidates, and, correspondingly, our business and financial prospects, would be negatively impacted.
Even if the Company receives regulatory approval to commercialize its drug candidates, the ability to generate revenues from any resulting products will be subject to a variety of risks, many of which are out of the Company’s control.
Regardless of regulatory approval, products arising from the development process may not gain market acceptance among physicians, patients, healthcare payers or the medical community. The Company believes that the degree of market acceptance and its ability to generate revenues from such products will depend on a number of factors, including, but not limited to:
| • | advancements in the treatment of cancer that make our treatments obsolete; |
| • | market exclusivity and competitor products; |
| • | timing of market introduction of the Company’s drugs and competitive drugs; |
| • | actual and perceived efficacy and safety of the Company’s drug candidates; |
7
| • | prevalence and severity of any side effects; |
| • | potential or perceived advantages or disadvantages over alternative treatments; |
| • | strength of sales, marketing and distribution support; |
| • | price of future products, both in absolute terms and relative to alternative treatments; |
| • | the effect of current and future healthcare laws on the Company’s drug candidates; and |
| • | availability of coverage and reimbursement from government and other third-party payers. |
If any of the Company’s drugs are approved and fail to achieve market acceptance, the Company may not be able to generate significant revenue to achieve or sustain profitability.
Risks Related to Commercialization of Our Product Candidates
The Company may not be able to establish the contractual arrangements necessary to develop, market and distribute the product candidates. Our failure to do so may adversely affect our business, results of operations and financial condition.
The Company has been successful in executing contractual agreements with strategic partners. This remains a key part of the Company’s business plan and the Company must continue to partner with third parties to manufacture clinical grade drug product and conduct key pre-clinical and clinical investigations. Strategic agreements around packaging, branding, market access and distribution for its drug products will also eventually be required.
However, potential partners could be discouraged by the Company’s limited operating history. There is no assurance that the Company will be able to negotiate commercially acceptable licensing or other agreements for the future exploitation of its drug product candidates including continued clinical development, manufacture or marketing. If the Company is unable to successfully contract for these services, or if arrangements for these services are terminated, the Company may have to delay the commercialization program which will adversely affect its ability to generate operating revenues.
The Company’s commercial opportunity will be reduced or eliminated if competitors develop and market products, devices or other treatments that are more effective, have fewer side effects or are less expensive than its drug candidates.
The development of drug candidates is highly competitive and is high risk. A number of other companies have products or drug candidates in various stages of pre-clinical or clinical development that are intended for the same therapeutic indications for which the Company’s drug candidates are being developed. Some of these potential competing drugs are further advanced in development than the Company’s drug candidates and may be commercialized sooner. Even if the Company is successful in developing effective drugs, its compounds may not compete successfully with products produced by its competitors.
The Company’s competitors include pharmaceutical companies and biotechnology companies, as well as universities and public and private research institutions. In addition, companies active in different but related fields represent substantial competition. Many of the Company’s competitors developing oncology drugs have significantly greater capital resources, larger R&D staff and facilities and greater experience in drug development, regulation, manufacturing and marketing. These organizations also compete with the Company and its service providers, to recruit qualified personnel, and to attract partners for joint ventures and to license technologies. As a result, the Company’s competitors may be able to develop technologies and products that would render the Company’s technologies or its drug candidates obsolete or non-competitive.
Risks Related to Our Intellectual Property
If we are unable to protect intellectual property rights related to our product candidates, we may not be able to obtain exclusivity for our product candidates or prevent others from developing similar competitive products.
We rely upon a combination of patents, know-how, trade secret protection and confidentiality agreements to protect the intellectual property related to our product candidates. The strength of patents in the biotechnology and pharmaceutical field involves complex legal and scientific questions and can be uncertain. The patent applications that we own or in-license may fail to result in issued patents with claims that cover our product candidates in the United States or other jurisdictions. In addition, we cannot guarantee that any patents will issue from any pending or future patent applications owned by or licensed to us. There is no assurance that all of the potentially relevant prior art relating to our patents and patent applications has been found. If such prior art exists, it can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue and even if such patents cover our product candidates, third parties may initiate opposition, interference, re-examination, post-grant review, inter partes review, nullification or derivation action in court or before patent offices or similar proceedings challenging the validity, enforceability or scope of such patents, which may result in the patent claims being narrowed or invalidated. Furthermore, even if our patents and patent applications are unchallenged, they may not adequately protect our intellectual property, provide exclusivity for our product candidates or prevent others from designing around our claims. Any of these outcomes could impair our ability to prevent competition from third parties.
8
If the patent applications we hold or have in-licensed with respect to our programs or product candidates fail to issue, or are revoked, if the breadth or strength of our patent protection is threatened, or if our patent portfolio fails to provide meaningful exclusivity for our product candidates, it could dissuade companies from collaborating with us to develop product candidates and threaten our ability to commercialize future products. Any successful opposition to any patents owned by or licensed to us could deprive us of rights necessary for the successful commercialization of any product candidates that we may develop. Further, if we encounter delays in regulatory approvals, the period of time during which we could market a product candidate under patent protection could be reduced. Even where we have a valid and enforceable patent, we may not be able to exclude others from practicing our invention where the other party can show that they used the invention in commerce before our filing date or the other party benefits from a compulsory license. In addition, patents have a limited lifespan. In the United States, the natural expiration of a patent is generally 20 years after it is filed. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidates are obtained, once the patent life has expired for a product, we may be open to competition from competitive medications, including biosimilar or generic medications. This risk is material in light of the length of the development process of our products and lifespan of our current patent portfolio.
In addition to the protection afforded by patents, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent, processes for which patents are difficult to enforce and any other elements of our product candidate discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. However, trade secrets can be difficult to protect. What constitutes a trade secret and what protections are available for trade secrets varies from state to state in the United States and country by country worldwide. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. Security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. Although we expect all of our employees and consultants to assign their inventions to us, and all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information or technology to enter into confidentiality agreements, we cannot provide any assurances that all such agreements have been duly executed or that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and applications are required to be paid to the USPTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and applications. The USPTO and various corresponding governmental patent agencies outside of the United States require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process and after a patent has issued. There are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction.
Our success depends, in part, on our ability to protect our intellectual property and our technologies.
Our commercial success depends, in part, on our ability to obtain and maintain patent and trade secret protection for our technologies, our traits, and their uses, as well as our ability to operate without infringing upon the proprietary rights of others. If we do not adequately protect our intellectual property, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and ability to achieve profitability.
Filing, prosecuting and defending patents on product candidates in all countries around the world would be prohibitively expensive. In addition, we may at times in-license third-party technologies for which limited international patent protection exists and for which the time period for filing international patent applications has passed. Consequently, we may not be able to prevent third parties from practicing our inventions, or from selling or importing products made using our inventions. Potential competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection but enforcement is difficult. These products may compete with our product candidates, if approved, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
9
Many companies have encountered significant problems in protecting and defending intellectual property rights around the world. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Risks Related to Our Reliance on Third Parties
The Company relies on third parties to conduct its pre-clinical studies and clinical trials. If those parties do not successfully carry out their contractual duties or meet expected deadlines, the Company’s drug candidates may not advance in a timely manner or at all.
In the course of discovery, pre-clinical testing and clinical trials, the Company relies on third parties, including laboratories, investigators, clinical contract research organizations (“CROs”), and manufacturers, to perform critical services. For example, the Company relies on third parties to conduct all of its pre-clinical and clinical studies. These third parties may not be available when the Company needs them or, if they are available, may not comply with all regulatory and contractual requirements or may not otherwise perform their services in a timely or acceptable manner, and the Company may need to enter into new arrangements with alternative third parties and the studies may be extended, delayed or terminated. These independent third parties may also have relationships with other commercial entities, some of which may compete with the Company. As a result of the Company’s dependence on third parties, it may face delays or failures outside of its direct control. These risks also apply to the development activities of collaborators, and the Company does not control their research and development, clinical trial or regulatory activities.
The Company has no direct control over the cost of manufacturing its drug candidates. Increases in the cost of manufacturing the Company’s drug candidates would increase the costs of conducting clinical trials and could adversely affect future profitability.
The Company does not intend to manufacture the drug product candidates in-house, and it will rely on third parties for drug supplies both for clinical trials and for commercial quantities in the future. The Company has taken the strategic decision not to manufacture active pharmaceutical ingredients (“API”) for the drug candidates, as these can be more economically supplied by third parties with particular expertise in this area. The Company outsources the manufacture of its drug products and their testing to FDA requirements. The Company uses contract facilities that are registered with the FDA, have a track record of large-scale API manufacture, and have already invested in capital and equipment. The Company has no direct control over the cost of manufacturing its product candidates. If the cost of manufacturing increases, or if the cost of the materials used increases, these costs may be passed on, making the cost of conducting clinical trials more expensive. Increases in manufacturing costs could adversely affect the Company’s future profitability if it was unable to pass all of the increased costs along to its customers.
Risks Related to our Securities
Enforceability of civil liabilities under the federal securities laws against the Company or the Company’s officers and directors may be difficult.
The Company is a public company limited by shares and is registered and operates under the Australian Corporations Act 2001. Half of the Company’s directors and officers reside outside of the United States. In addition, a substantial portion of the directly owned assets of the Company are located outside of the United States. As a result, it may be difficult or impossible for investors to effect service of process within the United States against the Company or its directors and officers or to enforce against them any of the judgments, including those obtained in original actions or in actions to enforce judgments of the U.S. courts, predicated upon the civil liability provisions of the federal or state securities laws of the United States. There is doubt as to the enforceability in the Commonwealth of Australia, in original actions or in actions for enforcement of judgments of U.S. courts, of civil liabilities predicated solely upon federal or state securities laws of the U.S., especially in the case of enforcement of judgments of U.S. courts where the defendant has not been properly served in Australia.
10
Our failure to meet the continued listing requirements of Nasdaq could result in a delisting of our common stock, which could negatively impact the market price and liquidity of our common shares and our ability to access the capital markets.
Our common stock is listed on the Nasdaq Capital Market. If we fail to satisfy the continued listing requirements of Nasdaq, such as the corporate governance requirements or the minimum closing bid price requirement, Nasdaq may take steps to delist our common stock. Such a delisting would have a negative effect on the price of our common stock, impair the ability to sell or purchase our common stock when persons wish to do so, and any delisting materially adversely affect our ability to raise capital or pursue strategic restructuring, refinancing or other transactions on acceptable terms, or at all. Delisting from the Nasdaq Capital Market could also have other negative results, including the potential loss of institutional investor interest and fewer business development opportunities. In the event of a delisting, we would attempt to take actions to restore our compliance with Nasdaq’s listing requirements, but we can provide no assurance that any such action taken by us would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our common stock, prevent our common stock from dropping below the Nasdaq minimum bid price requirement or prevent future non-compliance with Nasdaq’s listing requirements.
On December 9, 2022, we received a notice from the Nasdaq Listing Qualifications Department of The Nasdaq Capital Market (“Nasdaq”) informing us that because the closing bid price of our common stock had been below $1.00 per share for 30 consecutive business days, we no longer complied with the minimum bid price requirement for continued listing on The Nasdaq Capital Market. Nasdaq Listing Rule 5550(a)(2) (the “Rule”) requires listed securities to maintain a minimum bid price of $1.00 per share, and Listing Rule 5810(c)(3)(A) provides that a failure to meet the minimum bid price requirement exists if the deficiency continues for a period of 30 consecutive business days. The notice had no immediate effect on the listing or the trading of our common stock on The Nasdaq Capital Market. Pursuant to Nasdaq Marketplace Rule 5810(c)(3)(A), the notice letter stated that we had an initial compliance period of 180 calendar days to regain compliance with the minimum bid price requirement. To regain compliance, the closing bid price of our common stock must meet or exceed $1.00 per share for a minimum of 10 consecutive business days during the 180 calendar day grace period. If at any time during this period the bid price of the company’s ADSs closes at or above US$1.00 per share for a minimum of ten consecutive business days, the company will regain compliance with the minimum bid requirement.
On April 13, 2023, we received a letter from the Listing Qualifications Staff (the “Staff”) of Nasdaq confirming the Company had regained compliance with Nasdaq’s minimum bid price requirement under Listing Rule 5550(a)(2). In the letter received on April 13, 2023, the Staff determined that, during the ten consecutive business days from March 29, 2023 to April 12, 2023, the closing bid price of the Company’s ADSs has been at $1.00 per share or greater and accordingly, the Company has regained compliance with Listing Rule 5550(a)(2). The Company also issued an ASX announcement titled, “Kazia Regains Compliance with Nasdaq Minimum Bid Price Requirement” and filed a Form 6-K with the SEC announcing that the Company had regained compliance.
The trading price of the Company’s ordinary shares and American Depositary Shares (“ADSs”) is highly volatile. Your investment could decline in value and the Company may incur significant costs from class action litigations.
The trading price of the Company’s ordinary shares and ADSs is highly volatile in response to various factors, many of which are beyond the Company’s control, including:
| • | unacceptable toxicity findings in animals and humans; |
| • | lack of efficacy in human trials at Phase II stage or beyond; |
| • | announcements of technological innovations by the Company and its competitors; |
| • | new products introduced or announced by the Company or its competitors; |
| • | changes in financial estimates by securities analysts; |
| • | actual or anticipated variations in operating results; |
| • | expiration or termination of licenses, research contracts or other collaboration agreements; |
| • | conditions or trends in the regulatory climate in the biotechnology, pharmaceutical and genomics industries; |
| • | changes in the market values of similar companies; |
| • | changes in the broader macroeconomic environment; |
| • | the liquidity of any market for the Company’s securities; and |
| • | additional sales by the Company of its shares. |
11
In addition, equity markets in general and the market for biotechnology and life sciences companies in particular, have experienced substantial price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of the companies traded in those markets. Further changes in economic conditions in Australia, the U.S., EU, or globally, could impact the Company’s ability to grow profitably. Adverse economic changes are outside the Company’s control and may result in material adverse effects on the Company’s business or results of operations. These broad market and industry factors may materially affect the market price of the Company’s ordinary shares and ADSs regardless of its development and operating performance. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has
often been instituted against that company. Such litigation, if instituted against the Company, could cause it to incur substantial costs and divert management’s attention and resources.
If the market price of the Company’s ADSs falls and remains below US$5.00 per share, under stock exchange rules, the Company’s stockholders will not be able to use such ADSs as collateral for borrowing in margin accounts. This inability to use ADSs as collateral may depress demand as certain institutional investors are restricted from investing in securities priced below US$5.00 and may lead to sales of such ADSs, creating downward pressure on and increased volatility in the market price of the Company’s ordinary shares and ADSs.
A decrease in the trading price of our ADSs could cause their delisting from Nasdaq.
Under Nasdaq rules, companies listed on the Nasdaq Capital Market are required to maintain a share price of at least US$1.00 per share to avoid delisting of their shares. If the share price declines below US$1.00 for a period of 30 consecutive business days, then that listed company would have 180 days to regain compliance with the US$1.00 per share minimum. In the event that the Company’s share price declines below US$1.00, it may be required to take action in order to comply with the Nasdaq rules that may be in effect at the time.
The delisting of our ordinary shares on the ASX may adversely affect the price, liquidity and value of the ADSs.
On 11 October 2023, we announced our intention to delist from the ASX, which is anticipated to become effective on or around 14 November 2023. If the proposed delisting is completed, our ordinary shares will no longer be quoted or traded on the ASX and only the ADSs will be listed on the Nasdaq Stock Market, and as a result, shareholders will no longer be able to trade their ordinary shares on the ASX. Following the completion of the delisting, the Company’s ordinary shares will only be capable of being traded on Nasdaq in the form of ADSs, which will require shareholders to transfer their ordinary shares to ADSs to trade on Nasdaq and engage a suitably qualified Australian broker or a U.S. based broker who is able to trade on Nasdaq, or by off-market, private transactions, which will require shareholders to identify and agree terms with potential purchasers of ordinary shares. In addition, following the completion of the delisting, the Company will no longer be subject to the ASX Listing Rules. We cannot predict the effect of the proposed delisting on the value of our ADSs, however the delisting may restrict the liquidity of these securities by providing only one market on which to trade our securities, which may impair the development or liquidity of an active trading market for the ADSs in the U.S.
You are reliant on the depositary to exercise your voting rights and to receive distributions on ADSs and, as a result, you may be unable to exercise your voting rights on a timely basis or you may not receive certain distributions.
In certain circumstances, holders of ADSs may have limited rights relative to holders of ordinary shares. The rights of holders of ADSs with respect to the voting of ordinary shares and the right to receive certain distributions may be limited in certain respects by the deposit agreement entered into by us and The Bank of New York Mellon. For example, although ADS holders are entitled under the deposit agreement, subject to any applicable provisions of Australian law and of our Constitution, to instruct the depositary as to the exercise of the voting rights pertaining to the ordinary shares represented by the ADSs, and the depositary has agreed that it will try, as far as practical, to vote the ordinary shares so represented in accordance with such instructions, ADS holders may not receive notices sent by the depositary in time to ensure that the depositary will vote the ordinary shares. This means that, from a practical point of view, the holders of ADSs may not be able to exercise their right to vote. In addition, under the deposit agreement, the depositary has the right to restrict distributions to holders of the ADSs in the event that it is unlawful or impractical to make such distributions. We have no obligation to take any action to permit distributions to holders of our ADSs. As a result, holders of ADSs may not receive distributions.
If we are, a passive foreign investment company, or PFIC, there could be adverse U.S. federal income tax consequences to U.S. investors.
Based on the composition of our assets and income, we believe that we were not a PFIC for U.S. federal income tax purposes with respect to our 2022 taxable year. However, there can be no assurance that we will not be considered a PFIC in the current year or for any future taxable year. Our treatment as a PFIC could result in a reduction in the after-tax return to the U.S. holders of our ordinary shares or ADSs and would likely cause a reduction in the value of such ordinary shares or ADSs. For U.S. federal income tax purposes, we will be classified as a PFIC for any taxable year in which either (i) 75% or more of our gross income is passive income, or (ii) at least 50% of the average quarterly value of all of our assets for the taxable year produce or are held for the production of passive income. If we are classified as a PFIC for U.S. federal income tax purposes, highly complex rules will apply to U.S. holders owning ordinary shares or ADSs. Accordingly, you are urged to consult your tax advisors regarding the application of such rules. See Item 10—Additional Information—Taxation, United States Federal Income Tax Consequences for a more complete discussion of the U.S. federal income tax risks related to owning and disposing of our ordinary shares or ADSs.
Currency fluctuations may adversely affect the price of our ordinary shares, ADSs.
Our ordinary shares are quoted in Australian dollars on the ASX and the ADSs are quoted in U.S. dollars on Nasdaq. Movements in the Australian dollar/U.S. dollar exchange rate may adversely affect the U.S. dollar price of the ADSs. In the past year the Australian dollar has generally weakened against the U.S. dollar. However, this trend may not continue and may be reversed.
We may lose our foreign private issuer status, which would then require us to comply with the Exchange Act’s domestic reporting regime and cause us to incur significant legal, accounting and other expenses.
We are a foreign private issuer. In order to maintain our current status as a foreign private issuer, at least 50% of our outstanding ordinary shares must continue to be either directly or indirectly owned of record by non-residents of the United States. If more than 50% of our outstanding ordinary shares are instead held by U.S. residents, then in order to continue to maintain our foreign private issuer status, (i) a majority of our executive officers or directors must not be U.S. citizens or residents, (ii) more than 50% of our assets must not be located in the United States, and (iii) our business must be administered principally outside the United States.
Losing our status as a foreign private issuer would require us to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act applicable to U.S. domestic issuers. We also will be required to make changes in our corporate governance practices in accordance with various SEC and Nasdaq rules. The regulatory and compliance costs to us under U.S. securities laws, if we are required to comply with the reporting requirements applicable to a U.S. domestic issuer, would be significantly higher than the cost we would incur as a foreign private issuer. As a result, we would expect that a loss of foreign private issuer status will increase our legal and financial compliance costs and will make some activities highly time consuming and costly. We also expect that if we will be required to comply with the rules and regulations applicable to U.S. domestic issuers, it will make it more difficult and expensive for us to obtain director and officer liability insurance; we may therefore be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These rules and regulations could also make it more difficult for us to attract and retain qualified members of our board of directors.
12
Australian takeover laws may discourage takeover offers being made for us or may discourage the acquisition of a significant position in our ordinary shares and ADSs.
We are incorporated in Australia and are subject to the takeover laws of Australia. Among other things, we are subject to the Australian Corporations Act 2001, or the Corporations Act. Subject to a range of exceptions, the Corporations Act prohibits the acquisition of a direct or indirect interest in our issued voting shares if the acquisition of that interest will lead to a person’s voting power in us increasing to more than 20%, or increasing from a starting point that is above 20% and below 90%. Australian takeover laws may discourage takeover offers being made for us or may discourage the acquisition of a significant position in our ordinary shares. This may have the ancillary effect of entrenching our board of directors and may deprive or limit our shareholders’ and ADS holders’ opportunity to sell their ordinary shares and ADSs and may further restrict the ability of our shareholders and ADS holders to obtain a premium from such transactions. See Item 10.B “Additional Information – Memorandum and Articles of Association.”
| Item 4. | Information on the Company |
A. History and development of the Company
Kazia Therapeutics Limited (“Kazia”), a public company limited by shares, was incorporated in March 1994 and registered in New South Wales, Australia. Kazia is registered and operates under the Australian Corporations Act 2001.
Kazia has its registered office at Three International Towers, Level 24, 300 Barangaroo Avenue, Sydney, NSW 2000, Australia. Its telephone number and other contact details are: Phone +61-2-9472 4101; email info@kaziatherapeutics.com; and website, www.kaziatherapeutics.com (the information contained in the website does not form part of the Annual Report). Our agent for service of process in the United States is Vcorp Services, LLC, 25 Robert Pitt Drive, Suite 204, Monsey, New York 10952.
The Company’s Ordinary Shares are listed on the Australian Securities Exchange (“ASX”) under the symbol ‘KZA’ and its ADSs, each representing ten Ordinary Shares, trade on the Nasdaq Capital Market under the symbol ‘KZIA’. The Depositary for the Company’s ADSs is The Bank of New York Mellon, 240 Greenwich Street, New York, NY 10286. All information we file with the SEC is available through the SEC’s Electronic Data Gathering, Analysis and Retrieval system, which may be accessed through the SEC’s website at www.sec.gov.
Resignation of Chairman
Kazia announced that Dr John Friend joined the Kazia Board as Managing Director on 1 August 2023. Kazia announced the resignation of Mr. Iain Ross as Chairman and non-executive director on 11 August 2023. The Board of Directors elected Dr John Friend as Interim Chairman on 11 August 2023.
Kazia announces voluntary delisting from ASX
On 11 October 2023 Kazia announced that it submitted a formal application to the ASX to be removed from the official list of the ASX (Official List) in accordance with ASX Listing Rule 17.11 (Delist or the Delisting). This formal request follows the receipt of in-principle advice from the ASX in relation to the proposed Delisting, subject to the satisfaction of certain conditions. The Board has ultimately determined that the costs, administrative burden and commercial disadvantages of remaining listed on ASX outweigh any benefits of a continued ASX listing. Following the Delisting, the Company will maintain its listing on the Nasdaq and the fully paid ordinary shares of the Company will no longer be quoted on the ASX.
B. Business overview
The ongoing principal business of the Company has been pharmaceutical drug development. The Company is an emerging oncology-focused biotechnology company that has a portfolio of development candidates, diversified across several distinct technologies, with the potential to yield first-in-class and best-in-class agents in a range of oncology indications.
Paxalisib
Kazia’s lead program is paxalisib, (formerly known as GDC-0084), an investigational brain-penetrant inhibitor of the PI3K / Akt / mTOR pathway, that was specifically designed to treat brain cancer.
Paxalisib was developed by Genentech, Inc (South San Francisco, California) and the company entered into a worldwide exclusive license for the asset in October 2016. Prior to this transaction, Genentech had completed an extensive preclinical development program that provided convincing validation for paxalisib as a potential drug for brain cancer. Genentech also completed a phase I clinical trial in 47 patients with advanced recurrent grade III and grade IV glioma (NCT01547546). The most common adverse events were oral mucositis and hyperglycemia. Per RANO criteria, 40% of patients exhibited a best observable response of stable disease, and 26% demonstrated a metabolic partial response on FDG-PET.
The development candidate was granted the International Non-Proprietary Name (INN) ‘paxalisib’ by the World Health Organisation in December 2019. This was confirmed as the United States Adopted Name (USAN) by the USAN Council in April 2020. Paxalisib is orally administered and is presented in a 15mg capsule formulation. The development candidate is the subject of IND 112,608 with the U.S. Food and Drug Administration (“FDA”).
Paxalisib is a potent and selective inhibitor of all four isoforms of phosphoinositide-3-kinase (PI3K) and a moderate inhibitor of the mammalian target of rapamycin (mTOR). The PI3K / Akt / mTOR signaling axis has been shown to be dysregulated in approximately 85-90% of cases of glioblastoma, per Cancer Genome Atlas, and is considered a promising target in this disease. More generally, five PI3K inhibitors have thus far been approved by FDA, for a range of hematological malignancies and solid tumors, making this a well-validated target in cancer. Paxalisib is distinguished from these products by the fact that it is the only PI3K inhibitor in mainstream clinical development which is known to cross the blood-brain barrier, a crucial prerequisite for any novel treatment in brain cancer.
13
Paxalisib’s mechanism is therefore entirely distinct from that of temozolomide, the existing FDA-approved standard of care treatment. Temozolomide functions primarily by alkylating guanine residues in DNA, thereby inhibiting cell division in the rapidly-growing tumor. Paxalisib, by contrast, inhibits a biochemical control signal, and is therefore associated with a very different resistance and toxicity profile.
Paxalisib is the subject of granted or pending composition-of-matter patents in all key territories. In general, the expiry of these patents is in December 2031. However, the company expects that it will be able to secure patent term extensions in the most substantial markets, including US, EU, China, Japan, and Korea, and that these extensions will provide effective protection until 2036. In addition, the company has recently received notice of grant for a patent protecting the manufacturing process associated with paxalisib, and this will provide an additional layer of protection in relevant territories until 2036.
Paxalisib was granted orphan drug designation (ODD) by the FDA for glioblastoma in February 2018, and for the broader indication of glioma in August 2020 and ODD for atypical rhabdoid/teratoid tumours (AT/RT), a rare highly-aggressive childhood brain cancer, in June 2022. The development candidate also received Fast Track designation (FTD) for glioblastoma in August 2020, and Rare Pediatric Disease Designation (RPDD) for diffuse midline gliomas in August 2020. Collectively, these special designations provide paxalisib with enhanced access to FDA, a waiver of PDUFA fees, a period of regulatory exclusivity and, in the specific case of RPDD, the potential to secure a pediatric Priority Review Voucher (pPRV) should paxalisib be first approved in this indication.
Brain cancers account for about 15% of pediatric cancers and are the second most common type of cancer in children whereas over 300,000 adults are diagnosed every year with primary brain cancer. We believe Paxalisib, by design, has the potential to be an integral component to precision medicine. As a targeted therapeutic, we have focused many of the ongoing trials to evaluate paxalisib in patients who have PI3K pathway mutations. Enrolling clinical trials with patients who have the potential to have the greatest response and benefits accelerates clinical trial recruitment and time to commercialization. The overall clinical development strategy for paxalisib has been crafted into three core pillars. Within the adult brain cancer pillar, we have four ongoing clinical studies across three different patient populations. There are two actively recruiting clinical studies and one recently completed study in the pediatric brain cancer pillar. Within the brain metastases pillar, there are three ongoing studies.
Paxalisib in Adult Brain Cancer
Glioblastoma (GBM) is a fast-growing and aggressive brain tumour. Paxalisib is being developed primarily for the ~65% of newly-diagnosed unmethylated GBM patients who generally do not respond to existing chemotherapy with temozolomide. The final data from a phase II study in newly diagnosed GBM patients reported promising signals of clinical activity with paxalisib and was presented at two global conferences in 2023.
GBM AGILE Pivotal study Phase II / III Clinical Trial in Glioblastoma (NCT03970447)
Paxalisib commenced recruitment to GBM AGILE a phase II / III adaptive clinical trial in glioblastoma, in January 2021. GBM AGILE (Glioblastoma Adaptive Global Innovative Learning Environment) is sponsored by the Global Coalition for Adaptive Research, a US-based 501©(3) non-profit organization dedicated to advancing the development of new therapies via the application of cutting-edge statistical methodologies. The goal is to expedite the approval of new drugs for this disease. The study is a platform study, or master protocol study, in which multiple experimental agents are evaluated in parallel, and are compared against a shared control arm. GBM AGILE uses an adaptive Bayesian statistical design to ensure that only the number of patients required to reach a definitive answer are enrolled. Three patient populations are included in the study: newly diagnosed patients with unmethylated MGMT promotor status, newly diagnosed patients with methylated MGMT promotor status, and recurrent patients. Paxalisib participated in the first and third of these groups but not patients with methylated MGMT promotor status in this study.
We announced on 1 August 2022 that the company had been advised by GCAR that the first stage of the paxalisib arm had completed recruitment. The treatment arm did not meet pre-defined criteria for continuing to a second stage, and patients enrolled in the first stage of the paxalisib arm continued on treatment as per protocol, and in follow-up, until completion of the final analysis, which we anticipate receiving in 2H CY2023. Depending on the results of the study, Kazia may use such data to support submission of a new drug application for marketing authorisation to the FDA.
LUMOS2 phase II study
Kazia is supporting the University of Sydney on a molecularly guided phase II clinical study evaluating paxalisib in adult patients with recurrent/progressive isocitrate dehydrogenase (IDH) mutant grade 2 and 3 gliomas (G2/3 gliomas). The LUMOS2 study is sponsored by the University of Sydney with a goal of investigating targeted therapeutics in these patients who have limited options. The study is expected to enroll up to 76 patients with PI3K pathway mutations and will be a multicenter study at several Australian sites, with the potential to expand internationally. We anticipate enrollment to commence in 4Q CY2023.
14
Weill Cornell Medicine Phase II Study in Glioblastoma in Combination with Ketogenesis (NCT05183204)
In June 2021, the company entered into an agreement with the Joan & Sanford I Weill Medical College of Cornell University in New York, NY, known generally as Weill Cornell Medicine, for an investigator-initiated phase II clinical trial combining paxalisib with ketogenesis in patients with newly- diagnosed and recurrent glioblastoma. In addition to the general interest in ketogenic diets as a potential adjunct to treatment for various forms of cancer, research by Professor Lew Cantley and colleagues has demonstrated the potential for insulin to antagonize PI3K inhibition. A significant and growing body of research has suggested the potential for ketogenic diets to provide benefit in a range of tumour types, including glioblastoma. Administering a PI3K inhibitor in the context of minimal insulin secretion may allow the drug to achieve its full potential, and a combination of ketogenic diet and metformin will be used in this study to achieve a hypoinsulinaemic state. Professor Cantley serves as a scientific advisor to the study, and Dr Howard Fine, a highly experienced neuro-oncologist, will serve as Principal Investigator. The study is actively enrolling in two cohorts of GBM patients, and we anticipate providing an update to this study in 4Q CY2023.
Dana Farber Cancer Institute (DFCI) Phase II Study in Primary Central Nervous System Lymphoma (PCNSL) (NCT04906096)
In September 2020, the company signed an agreement with Dana-Farber Cancer Institute in Boston, MA, for an investigator-initiated phase II clinical study of paxalisib in patients with primary CNS lymphoma (PCNSL) (NCT04906096). This study commenced recruitment in June 2021. Four of the five FDA-approved PI3K inhibitors are indicated for various forms of lymphoma, so this is considered a high-potential indication for paxalisib. The unique brain-penetrant qualities of paxalisib make it suitable for investigation in this patient group. The study is expected to recruit around 25 patients. The Principal Investigator is Professor Lakshmi Nayak, a highly experienced clinical researcher in brain cancer, with a specialist interest in PCNSL. The study commenced recruitment in June 2021 and remains ongoing. We anticipate providing a clinical update to this study in 4Q CY2023.
Paxalisib in Pediatric Brain Cancer
Brain cancer is the most common malignancy of childhood and represents about one third of all childhood cancer deaths. The PI3K/AKT/mTOR pathway is frequently upregulated in pediatric cancers and therefore therapeutics that target those pathways could lead to well long-awaited regulatory approvals. Diffuse intrinsic pontine glioma (DIPG) is the most common of a group of childhood brain cancers known as diffuse midline gliomas (DMGs). The disease has no FDA approved drug treatments and average survival from diagnosis is approximately 10 months. Kazia recognizes the critical importance and immense unmet need and is exploring paxalisib in two common forms of childhood cancer—DIPGs and Advanced Childhood Cancer with PI3K/mTOR mutations.
St Jude Children’s Hospital Phase I Study in Diffuse Intrinsic Pontine Glioma (DIPG) (NCT03696355)
In February 2020, the company’s collaborators at St Jude Children’s Research Hospital in Memphis, TN completed recruitment to a phase I investigator- initiated clinical study of paxalisib in diffuse intrinsic pontine glioma (DIPG), a rare but highly aggressive childhood brain cancer with no approved pharmacological treatments. The St Jude study (NCT03696355) sought to establish an MTD in the pediatric population before enrolling an expansion cohort to seek definitive signals of efficacy. The St Jude study is primarily funded by the hospital, with support via a financial grant from Kazia. In September 2019, the company announced that a pediatric MTD of 27 mg/m2 had been determined, which is approximately comparable to the doses used in adult clinical studies. The investigators reported interim data in an oral presentation at the SNO Annual Meeting in November 2020. The study met its primary objective and determined a maximum tolerated dose for pediatric use of 27 mg/m2. 27 patients were recruited, of whom 24 received at least one dose of paxalisib. The safety profile and pharmacokinetics were highly consistent with the adult data.
15
PNOC022 phase II Study in Diffuse Intrinsic Pontine Glioma (DIPG) (NCT05009992)
In December 2020, the company entered into a letter of intent with the Pacific Pediatric Neuro-Oncology Consortium (PNOC), an international consortium focused on the development of novel combination therapies, to execute an investigator-initiated phase II adaptive platform study of paxalisib in patients with DIPG and other DMGs, a group which collectively constitutes one of the most aggressive childhood cancers. The study will explore paxalisib in combination with ONC-201, a small-molecule investigational new drug which targets dopamine receptor D2 (DRD2), and which is manufactured by Oncoceutics, Inc, a wholly-owned subsidiary of Chimerix, Inc. The St Jude phase I study in DIPG has already provided useful information regarding dosing and safety of paxalisib in a pediatric population, but it has always been assumed that combination therapy would be required to achieve meaningful efficacy in such an aggressive tumour. The PNOC DIPG study is supported by preclinical data from an international consortium of scientists led by Associate Professor Matt Dun at the Hunter Medical Research Institute at the University of Newcastle, Australia. Dr Dun’s work has identified PI3K pathway activation as a primary resistance mechanism to ONC201 and has demonstrated synergistic activity when the two drugs are combined in preclinical models of DIPG. This work was the subject of a poster presentation at the annual International Symposium on Pediatric Neuro-Oncology (ISPNO) conference in Hamburg, Germany, in June 2022. Dr Dun also reported case studies of two patients who had received the combination therapy through compassionate access and who had demonstrated marked clinical improvement while on therapy. The study enrollment has been very robust since opening in late 2021 and the study team at PNOC and University of California, San Francisco (UCSF) are preparing data for interim analysis, which is expected in 4Q CY2023.
OPTIMISE phase II study
Kazia entered into a collaboration with the Australian and New Zealand Children’s Haematology / Oncology Group (ANZCHOG) in March 2023 for a phase II clinical study examining paxalisib as a targeted therapeutic in children with advanced solid tumours, including brain tumours. The study, named OPTIMISE, is the first Australian-led clinical trial of paxalisib and will combine the drug with chemotherapy for children with PI3K pathway mutations in their tumours. Enrollment for this study is expected to commence in 4Q CY2023.
Paxalisib in Brain Metastases
Brain metastases occur when cancer cells spread from their original site to the brain, and treatment options are very limited. Brain metastases are a common complication of many tumours, but are particularly common in breast cancer, lung cancer, and melanoma and account for 67%—89% of all cancers. Brain metastases are typically highly resistant to treatment and survival rates are generally low. Radiotherapy is a common treatment modality for brain metastases. Despite some efficacy, patients typically become resistant over time, and repeat courses of radiotherapy can be associated with significant neurological toxicity. Additionally, PI3K pathway mutations are common in brain metastasis and are frequently associated with a worse prognosis.
MSKCC phase I clinical study in Brain Metastases in Combination with Radiotherapy (NCT04192981)
Paxalisib is the subject of an ongoing phase I clinical study in patients with brain metastases and leptomeningeal metastases who harbor PI3K pathway mutations in combination with radiotherapy sponsored by Memorial Sloan Kettering Cancer Center in New York, NY. Whole brain radiotherapy (WBRT) is a ubiquitous therapeutic modality in this patient population, with an estimated 200,000 patients receiving treatment each year in the United States alone. Encouraging safety and clinical activity from this study was presented by the lead investigator, Dr. Jonathan Yang in August 2022 at the ASCO/SNO CNS meeting held in Toronto, Canada. Interim data from the first stage of the study indicated that all 9 evaluable patients experienced complete or partial response, representing an overall response rate (ORR) of 100%, according to RANO-BM criteria. The patients comprised a range of primary tumors, with breast cancer the most common, representing one third of patients. The phase I expansion cohort is currently enrolling and two well known cancer centers have joined MSKCC in this study: Miami Cancer Institute and Fred Hutchinson Cancer Center in Seattle, WA. The trial is designed in two stages: an initial exploratory stage and a confirmatory expansion stage. Recruitment to the expansion stage has already commenced, with the objective of recruiting an additional 12 patients. Preliminary data from the expansion cohort is anticipated by 1Q CY2024.
Alliance for Clinical Trials in Oncology Phase II Genomically-Guided Study in Brain Metastases (NCT03994796)
The Alliance for Clinical Trials in Oncology is sponsoring a phase II multi-drug study of multiple agents in the treatment of brain metastases from any primary tumour (NCT03994796) and substantially funded by the US National Cancer Institute. The study assigns patients to either paxalisib (PI3K mutations), abemaciclib (CDK4/6 mutations) (Eli Lilly & Co), or entrectinib (ROS/Trk mutations) (Genentech, Inc) on the basis of their tumor’s genetic characteristics. Each drug is investigated in parallel in three patient cohorts: breast cancer, lung cancer, and other tumors. In June 2022, Kazia was informed that paxalisib had graduated to an expansion stage of the study in breast cancer, with work ongoing in the other two cohorts. The enrollment is ongoing for all cohorts including the expansion stage of the study in breast cancer brain metastases patients.
16
Dana Farber Cancer Institute (DFCI) Phase II Study in HER2+ Breast Cancer Brain Metastases in Combination with Trastuzumab (NCT03765983)
Dr Jose Pablo Leone is the Principal Investigator to a phase II study in patients with HER2-positive breast cancer brain metastases, a population for which there are no approved pharmacological treatments, in which paxalisib is administered in combination with Herceptin (trastuzumab), sponsored by Dana-Farber Cancer Institute in Boston, MA. The Dana-Farber study is primarily funded by the hospital, with support via a financial grant from Kazia. Enrollment is ongoing and we anticipate providing a study update in 4Q CY2023.
Fast Track Designation
We received Fast Track Designation (FTD) by the FDA in July 2023 for paxalisib for the treatment of solid tumour brain metastases harboring PI3K pathway mutations in combination with radiation therapy, based on the promising clinical data from an interim analysis of the MSKCC phase 1 trial.
To be awarded FTD, drugs must generally be able to show some potential advantage over existing therapies, either in terms of safety or efficacy. The key benefits of FTD comprise enhanced access to FDA, with regular and more frequent opportunities for consultation and discussion. In addition, drugs with FTD may be eligible for Accelerated Approval, in which a new medicine is approved based on a surrogate endpoint, and Priority Review, in which the standard 12-month review process may be reduced to eight months. Drugs with FTD may also receive a ‘rolling review’ of their NDA submission, in which sections are submitted for review as they become available, potentially expediting the approval process.
EVT801
Kazia is also developing EVT801, a small-molecule selective inhibitor of vascular endothelial growth factor receptor 3 (VEGFR3). EVT801 was originally discovered by Sanofi SA and was licensed to Evotec SE as part of a broader transaction. Evotec conducted an extensive program of preclinical development, which showed compelling evidence of activity in broad range of animal models. The drug was licensed to Kazia in April 2021.
EVT801 Worldwide Exclusive License and Intellectual Property
The Company entered into an exclusive worldwide license agreement with Evotec SE in April 2021, under which Kazia has the right to develop and commercialize the asset in all indications. Evotec stands to receive up to €301 million in contingent milestone payments, and a royalty on net sales. Evotec has no right to direct the development of EVT801, no right of approval for Kazia to sub-license, and no right of first refusal. However, in the event of sub-licensing, Kazia may under certain circumstances share a portion of receipts from a sub-licensee with Evotec.
EVT801 is protected by granted or pending composition-of-matter patents in all key territories, with exclusivity generally through to the early 2030s.
For several decades, it has been clear that growing tumors require an extensive network of newly formed blood vessels and lymphatic vessels to satisfy their substantial nutrient requirements. Drugs which inhibit the formation of new blood vessels (angiogenesis inhibitors) have proven effective in a wide range of solid tumors, with Avastin (bevacizumab) being the best-known example of the class. However, the use of such drugs is limited by hypoxia- induced resistance mechanisms and, in the case of many small-molecule inhibitors, by toxicity. EVT801 was designed to respond to these challenges by selectively targeting lymphangiogenesis, the formation of new lymphatic vessels. Doing so, and with a high degree of selectivity, is expected to provide many of the same benefits as inhibition of angiogenesis, but without the attendant problems of resistance and toxicity.
In addition, drugs which target VEGF receptors have shown the potential to alter the population of immune cells within the tumour micro-environment, thereby potentially making ‘cold’ tumors more susceptible to immuno-oncology agents such as checkpoint inhibitors. We believe that preclinical evidence supports this hypothesis with EVT801 and may provide a second and almost entirely distinct mechanism of action through which the EVT801 may provide benefit to cancer patients.
Phase I Study in Advanced Solid Tumors (NCT05114668)
In November 2021, Kazia commenced recruitment to a phase I, first-in-human, multiple-ascending-dose, clinical trial of EVT801 in patients with advanced solid tumors which seeks to explore both of these mechanisms (inhibition of lymphangiogenesis and modulation of tumor immune micro-environment), The trial is being performed at two hospitals in France: Oncopole in Toulouse and Centre Léon Beraud in Lyons and will aim to recruit up to 96 patients with advanced cancer. In addition to the primary endpoints of safety and tolerability, the study is designed to include a rich array of biomarkers that will allow a deeper understanding of the drug’s pharmacology and may inform design of subsequent studies.
17
Preclinical data showed EVT801 to be active against a broad range of tumour types and has shown evidence of synergy with immuno-oncology agents. Over the course of FY 2023, interim data from the phase I study and preclinical EVT801 data has been presented at a number of global conferences, including AACR and ESMO. We anticipate providing additional EVT801 updates and presentations of data at medical conferences in 4Q CY2023.
Significant changes in the state of affairs
There were no significant changes in the state of affairs of the consolidated entity during the financial year.
Anticipated milestones
We anticipate that during fiscal year 2024:
| • | Final data will be reported from the phase II/III GBM AGILE clinical study of paxalisib in glioblastoma |
| • | Interim results will be reported from the phase II PNOC clinical trial of paxalisib in combination with ONC201; |
| • | Interim results will be reported from the phase II clinical trial of paxalisib in combination with trastuzumab in breast cancer metastases; |
| • | Interim results will be reported from the phase II genomically-guided study of paxalisib in brain metastases; |
| • | Interim results will be reported from the phase I study of paxalisib in combination with radiotherapy in brain metastases; and |
| • | Final data will be reported from the phase I study of paxalisib in children with DIPG. |
R&D Pipeline
Paxalisib in metastatic melanoma
Data from an ongoing research collaboration with the Huntsman Cancer Institute at the University of Utah in Salt Lake City, UT has shown paxalisib to be active in vitro and in vivo against a range of preclinical models of metastatic melanoma, the most aggressive form of skin cancer. The data suggested substantial activity for paxalisib as monotherapy in preclinical mouse models and was presented at the 19th International Congress of the Society for Melanoma Research, held in Edinburgh, Scotland.
Paxalisib in solid tumours
Kazia’s collaboration with QIMR Berghofer Medical Research Institute, one of Australia’s foremost cancer research centers, is currently exploring novel uses of paxalisib in solid tumours. The collaboration is based on research that identified an entirely separate effect of PI3K inhibition: as a modulator of the immune microenvironment within and around the tumour. Administration of PI3K inhibitors such as paxalisib, at doses and frequencies different to those conventionally used, may activate the immune system in the tumour, making it more susceptive to immunotherapy. This could therefore open up an important opportunity for paxalisib in combination with other drugs for the treatment of diseases such as breast cancer and lung cancer. The collaboration is ongoing and will build on initial research that has already led to the filing of a provisional patent in 2022, including the use of paxalisib as an immune modulator in the treatment of diseases such as breast cancer.
18
Broad Clinical Program Ongoing
| Sponsor |
Phase | Indication | Registration | |||
| PAXALISIB | ||||||
| Global Coalition for Adaptive Research | II / III | Glioblastoma | NCT03970447 | |||
| Weill Cornell Medicine | II | Glioblastoma (with ketogenesis) | NCT05183204 | |||
| Alliance for Clinical Trials in Oncology | II | Brain metastases | NCT03994796 | |||
| Dana-Farber Cancer Institute | II | Breast cancer brain metastases (with Herceptin) | NCT03765983 | |||
| Dana-Farber Cancer Institute | II | Primary CNS lymphoma | NCT04906096 | |||
| University of Sydney | I/II | Grade 2/3 IDH-mutant adult gliomas | TBD | |||
| Pacific Pediatric Neuro-Oncology Consortium | II | DIPG (childhood brain cancer) | NCT05009992 | |||
| Aus. & NZ Children’s Oncology Group | II | Advanced solid tumours in children | TBD | |||
| St Jude Children’s Research Hospital | I | DIPG | NCT03696355 | |||
| Memorial Sloan Kettering Cancer Center | I | Brain metastases (with radiotherapy) | NCT04192981 | |||
| EVT801 |
||||||
| Kazia Therapeutics | I | Advanced solid tumours | NCT05114668 | |||
Clinical Development Overview
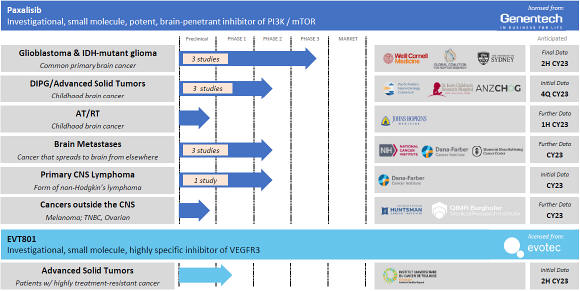
IDH: Isocitrate dehydrogenase, DIPG: Diffuse Intrinsic Pontine Glioma, AT/RT: Atypical Teratoid Rhabdoid Tumor, CNS: central nervous system, TNBC: triple negative breast cancer, VEGFR3: vascular endothelial growth factor receptor 3
Patent Protection
The Company has an aggressive global Intellectual Property (“IP”) strategy to protect its key assets and we have partnered with a large Australian law firm to lodge patents that seek to provide protection for our assets. The patent strategy is adapted for each technology platform and the principle mode of protection is through the patenting procedure, seeking to obtain exclusive licenses for all its key inventions and drug pipeline. The overarching strategy in the IP portfolio is to cover the three critical corner stones of pharmaceutical patent: composition of matter (the breadth structures covered in the patent), method of manufacture (the chemical processes used to manufacture the compounds disclosed in the patent) and method of use. Patents are submitted initially as provisional applications and after 12 months’ progress through to a Patent Cooperation Treaty (“PCT”) application.
We are continuing to expand our preclinical work on paxalisib and EVT801 through collaborations with research institutions. Where the research programs result in the generation of further patentable subject matter, the Company will pursue an aggressive patent filing strategy based on multiple jurisdictions with a focus on those member countries offering the most significant market opportunities for future development.
19
Regulatory requirements
Australian Regulatory Requirements
The Therapeutic Goods Act 1989 (“1989 Act”), sets out the legal requirements for the import, export, manufacture and supply of pharmaceutical products in Australia. The 1989 Act requires that all pharmaceutical products to be imported into, supplied in, manufactured in or exported from Australia be included in the Australian Register of Therapeutic Goods (“ARTG”), unless specifically exempted under the Act.
Medicines with a higher level of risk (prescription medicines, some non-prescription medicines) are evaluated for quality, safety and efficacy and are registered on the ARTG. Medicines with a lower risk (many over the counter medicines including vitamins) are assessed only for quality and safety. Medicines included in the ARTG can be identified by the AUST R number (for registered medicines) or an AUST L number (for listed medicines) which appears on the packaging of the medicine.
In order to ensure that a product can be included in the ARTG, a sponsoring company must make an application to the Therapeutic Goods Administration (“TGA”). The application usually consists of a form accompanied by data (based on the EU requirements) to support the quality, safety and efficacy of the product for its intended use and payment of a fee. Application details are available on the TGA website www.tga.gov.au.
The first phase of evaluation, known as the Application Entry Process, is usually a short period during which an application is assessed at an administrative level to ensure that it complies with the basic guidelines. The TGA may request further details from the applicant and may agree with sponsors that additional data (which while not actually required by the application, could enhance the assessment outcome) may be submitted later at an agreed time. The TGA must decide within at least 40 working days whether it will accept the application for evaluation.
Once an application is accepted for evaluation, aspects of the data provided are allocated to evaluators within the different relevant sections, who prepare clinical evaluation reports. Following evaluation, the chemistry, quality control bioavailability and pharmacokinetics aspects of a product may be referred to a Pharmaceutical Sub-Committee (“PSC”), which is a sub-committee of the TGA prescription medicine expert advisory committee, the Advisory Committee on Prescriptive Medicines (“ACPM”) to review the relevant clinical evaluation reports.
The clinical evaluation reports (along with any resolutions of the ACPM sub-committee) are sent to the sponsoring company who then has the opportunity to comment on the views expressed within the evaluation report, provide corrections and to submit supplementary data to address any issues raised in the evaluation reports.
Once the evaluations are complete, the TGA prepares a summary document on the key issues on which advice will be sought from either the ACPM (for new medicines) or from the Peer Review Committee (“PRC”) for extensions to products which are already registered. This summary is sent to the sponsoring company, which is able to submit a response to the ACPM or PRC dealing with issues raised in the summary and those not previously addressed in the evaluation report. The ACPM/PRC provide independent advice on the quality, risk/benefit, effectiveness and access of the product and conduct medical and scientific evaluations of the application. The ACPM meets every two months to examine the applications referred by the TGA and its resolutions are provided to the sponsoring company within five working days after the ACPM meeting.
The TGA takes into account the advice of the ACPM or PRC in reaching a decision to approve or reject a product. Any approval for registration on the ARTG may have conditions associated with it.
From the time that the TGA accepts the initial application for evaluation, the TGA must complete the evaluation and make a decision on the registration of the product within at least 255 working days. If not completed within 255 working days, the TGA forfeits 25% of the evaluation fee otherwise payable by the sponsor, but any time spent waiting for a response from the sponsor is not included in the 255 working days. The TGA also has a system of priority evaluation for products that meet certain criteria, including where the product is a new chemical entity that it is not otherwise available on the market as an approved product, and is for the treatment of a serious, life-threatening illness for which other therapies are either ineffective or not available.
U.S. Regulatory Requirements
The FDA regulates and imposes substantial requirements upon the research, development, pre-clinical and clinical testing, labelling, manufacture, quality control, storage, approval, advertising, promotion, marketing, distribution, import and export of pharmaceutical products including drugs and biologics, as well as significant reporting and record-keeping obligations. State governments may also impose obligations in some of these areas.
20
In the United States, pharmaceutical products are primarily regulated by the FDA under the Federal Food, Drug, and Cosmetic Act (“FDCA”) and its implementing regulations, and in the case of biologics, the Public Health Service Act and its implementing regulations. The Company believes that the FDA will regulate its products as drugs. The process required by the FDA before drugs may be marketed in the United States generally involves the following:
| • | pre-clinical laboratory evaluations, including formulation and stability testing, and animal tests performed under the FDA’s Good Laboratory Practices regulations to assess pharmacological activity and toxicity potential; |
| • | submission and review of an IND Application, including results of pre-clinical studies, clinical experience (if any), manufacturing information, and protocols for clinical trials, which must become effective before clinical trials may begin in the United States; |
| • | obtaining approval of Institutional Review Boards (“IRBs”), to administer the products to human subjects in clinical trials; |
| • | adequate and well-controlled human clinical trials to establish the safety and efficacy of the product for the product’s intended use; |
| • | development of manufacturing processes which conform to FDA current Good Manufacturing Practices (“cGMPs”), as confirmed by FDA inspection; |
| • | submission of results for pre-clinical and clinical studies, and chemistry, manufacture and control information on the product to the FDA in a New Drug Approval (“NDA”) Application; and |
| • | FDA review and approval of an NDA, prior to any commercial sale, promotion or shipment of a product. |
The testing and approval process requires substantial time, effort, and financial resources, and the Company cannot be certain that any approval will be granted on a timely basis, if at all.
The results of the pre-clinical studies, clinical experience together with initial specified manufacturing information, the proposed clinical trial protocol, and information about the participating investigators are submitted to the FDA as part of an IND, which must become effective before the Company may begin human clinical trials in the U.S. Additionally, an independent IRB must review and approve each study protocol and oversee conduct of the trial. An IND becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day period, raises concerns or questions about the conduct of the trials as outlined in the IND and imposes a clinical hold. If the FDA imposes a clinical hold, the IND sponsor must resolve the FDA’s concerns before clinical trials can begin. Pre-clinical tests and studies can take several years to complete, and there is no guarantee that an IND submitted, based on such tests and studies, will become effective within any specific time period, if at all.
Human clinical trials are typically conducted in three sequential phases that may overlap, which are:
| • | Phase I: The drug is initially introduced into healthy human subjects or patients and tested for safety and dosage tolerance. For oncology medicines, patients with the target disease are usually enrolled rather than healthy patients. Absorption, metabolism, distribution, and excretion testing, among other tests, are generally performed at this stage. These studies may also provide early evidence of effectiveness. The maximum tolerated dose of the drug may be calculated from phase I studies; |
| • | Phase II: The drug is studied in controlled, exploratory therapeutic trials in a limited number of subjects with the disease or medical condition for which the new drug is intended to be used in order to identify possible adverse effects and safety risks, to determine the preliminary or potential efficacy of the product for specific targeted diseases or medical conditions, and to determine dosage tolerance and the optimal effective dose; and |
| • | Phase III: While phase II studies help demonstrate that a specific dosage range of the drug may be effective and the drug has an acceptable safety profile, controlled, large-scale therapeutic, phase III trials are undertaken at multiple study sites to demonstrate clinical efficacy and to further test for safety in an expanded patient population. These studies are used to evaluate the overall benefit – risk relationship of the drug and provide a basis for physician labelling. |
The Company cannot be certain that it will successfully complete phase I, phase II or phase III testing of its products within any specific time period, if at all. Furthermore, the FDA, the IRB or the Company may suspend or terminate clinical trials at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk.
21
Results of pre-clinical studies and clinical trials, as well as detailed information about the manufacturing process, quality control methods, and product composition, among other things, are submitted to the FDA as part of an NDA seeking approval to market and commercially distribute the product on the basis of a determination that the product is safe and effective for its intended use. Before approving an NDA, the FDA will inspect the facilities at which the product is manufactured and will not approve the product unless GMP compliance is satisfactory. If applicable regulatory criteria are not satisfied, the FDA may deny the NDA or require additional testing or information. As a condition of approval, the FDA also may require post-marketing testing or surveillance to monitor the product’s safety or efficacy. Even after an NDA is approved, the FDA may impose additional obligations or restrictions (such as labelling changes), or even suspend or withdraw a product approval on the basis of data that arise after the product reaches the market, or if compliance with regulatory standards is not maintained. The Company cannot be certain that the FDA on a timely basis, if at all will approve any NDA it submits. Also, any such approval may limit the indicated uses for which the product may be marketed. Any refusal to approve, delay in approval, suspension or withdrawal of approval, or restrictions on indicated uses could have a material adverse impact on the Company’s business prospects.
A user fee, pursuant to the requirements of the Prescription Drug User Fee Act (“PDUFA”), and its amendments, applies to NDAs, unless exempted. The FDA adjusts the PDUFA user fees on an annual basis. PDUFA also imposes an annual product fee for prescription drugs and biologics, and an annual establishment fee on facilities used to manufacture prescription drugs and biologics. A written request can be submitted for a waiver under certain circumstances. Waivers may be possible for the application fee for the first human drug application that is filed by a small business, as defined by the FDCA, but there are no small business waivers for product or establishment fees. Waivers may also be possible for one or more fees, upon written request, when a waiver or reduction is necessary to protect the public health, the user fees would present a significant barrier to innovation, or the fees are anticipated to exceed the present or future costs incurred by FDA. Applications for products designated as orphan drugs are not subject to the application fee unless the application includes an indication for other than a rare disease or condition. The Company is not at the stage of development with its products where it is subject to these fees, but they are significant expenditures that may be incurred in the future and must be paid at the time of application submissions to FDA, as applicable.
Satisfaction of FDA requirements typically takes several years. The actual time required varies substantially, based upon the type, complexity, and novelty of the pharmaceutical product, among other things. Government regulation imposes costly and time-consuming requirements and restrictions throughout the product life cycle and may delay product marketing for a considerable period of time, limit product marketing, or prevent marketing altogether. Success in pre-clinical or early stage clinical trials does not ensure success in later stage clinical trials. Data obtained from pre-clinical and clinical activities are not always conclusive and may be susceptible to varying interpretations that could delay, limit, or prevent marketing approval. Even if a product receives marketing approval, the approval is limited to specific clinical indications. Further, even after marketing approval is obtained,
the discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market.
After product approval, there are continuing significant regulatory requirements imposed by the FDA, including record-keeping requirements, obligations to report adverse events in patients using the products, and restrictions on advertising and promotional activities. Quality control and manufacturing procedures must continue to conform to GMPs, and the FDA periodically inspects facilities to assess GMP compliance. Additionally, post-approval changes in ingredient composition, manufacturing processes or facilities, product labelling, or other areas may require submission of an NDA Supplement to the FDA for review and approval. New indications will require additional clinical studies and submission of an NDA Supplement. Failure to comply with FDA regulatory requirements may result in an enforcement action by the FDA, including warning letters, product recalls, suspension or revocation of product approval, seizure of product to prevent distribution, impositions of injunctions prohibiting product manufacture or distribution, and civil and criminal penalties. Maintaining compliance is costly and time-consuming. The Company cannot be certain that it, or its present or future suppliers or third-party manufacturers, will be able to comply with all FDA regulatory requirements, and potential consequences of noncompliance could have a material adverse impact on its business prospects.
The FDA’s policies may change, and additional governmental regulations may be enacted that could delay, limit, or prevent regulatory approval of the Company’s products or affect its ability to manufacture, market, or distribute its products after approval. Moreover, increased attention to the containment of healthcare costs in the U.S. and in foreign markets could result in new government regulations that could have a material adverse effect on the business. The Company’s failure to obtain coverage, an adequate level of reimbursement, or acceptable prices for future products could diminish any revenues the Company may be able to generate. The Company’s ability to commercialize future products will depend in part on the extent to which coverage and reimbursement for the products will be available from government and health administration authorities, private health insurers, and other third-party payers. EU member states and U.S. government and other third-party payers increasingly are attempting to contain healthcare costs by consideration of new laws and regulations limiting both coverage and the level of reimbursement for new drugs. The Company cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative or administrative action, either in the U.S. or abroad.
22
The Company’s activities may also be subject to state laws and regulations that affect its ability to develop and sell products. The Company is also subject to numerous federal, state, and local laws relating to such matters as safe working conditions, clinical, laboratory, and manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances. The Company may incur significant costs to comply with such laws and regulations now or in the future, and the failure to comply may have a material adverse impact on the Company.
FDA provides programs intended to facilitate and expedite development and review of new products that are intended to address an unmet medical need in the treatment of a serious or life-threatening disease or condition. These programs are referred to as fast track designation, breakthrough therapy designation and priority review designation. These designations are not mutually exclusive, and a product candidate may qualify for one or more of these programs. While these programs are intended to expedite product development and approval, they do not alter the standards for FDA approval. The FDA may designate a product for fast-track designation if it is intended, whether alone or in combination with one or more other products, for the treatment of a serious or life-threatening disease or condition, and it demonstrates the potential to address unmet medical needs for such a disease or condition. For products with fast-track designation, sponsors may have more frequent interactions with the FDA, the product is potentially eligible for accelerated approval and priority review, if relevant criteria are met. A product may be designated as a breakthrough therapy if it is intended, either alone or in combination with one or more other products, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The FDA may take certain actions with respect to breakthrough therapies, including holding meetings with the sponsor throughout the development process; providing timely advice to the product sponsor regarding development and approval; involving more senior staff managers in the review process; assigning a cross-disciplinary lead for the review team; and taking other steps to design the clinical trials in an efficient manner. The FDA may designate a product for priority review if it is a product that treats a serious condition and, if approved, would provide a significant improvement in safety or effectiveness when compared with other available therapies. Additionally, drug approval under the accelerated approval pathway may be based on evidence of clinical effect on a surrogate endpoint or intermediate clinical endpoint that is reasonably likely to predict clinical benefit. A post-marketing clinical study will be required to verify clinical benefit, and other restrictions to assure safe use may be imposed.
Under the Drug Price Competition and Patent Term Restoration Act of 1984, a sponsor may obtain marketing exclusivity for a period of time following FDA approval of certain drug applications, regardless of patent status, if the drug is a new chemical entity or if new clinical studies were required to support the marketing application for the drug. This marketing exclusivity prevents a third party from obtaining FDA approval for an identical or nearly identical drug under an Abbreviated New Drug Application. The statute also allows a patent owner to obtain an extension of applicable patent terms for a period equal to one-half the period of time elapsed between the filing of an IND and the filing of the corresponding NDA plus the period of time between the filing of the NDA and FDA approval, with reductions taken for any time an applicant did not act with due diligence. There is a five-year maximum patent extension and a maximum of 14 years protection from product approval. The Company cannot be certain that it will be able to take advantage of either the patent term extension or marketing exclusivity provisions of these laws.
23
European Union Regulatory Requirements
Outside the United States, the Company’s ability to market its products will also be contingent upon receiving marketing authorizations from the appropriate regulatory authorities and compliance with applicable post-approval regulatory requirements. Although the specific requirements and restrictions vary from country to country, as a general matter, foreign regulatory systems include risks similar to those associated with FDA regulation, described above. In the EU, a marketing authorization application may be submitted either under a centralized or a national procedure. Under the centralized procedure, a single application to the European Medicines Agency (“EMA”) leads to an approval granted by the European Commission that permits the marketing of the product throughout the EU and in the additional member states of the European Economic Area (Norway, Iceland and Liechtenstein. The centralized procedure is mandatory for certain classes of medicinal products, but optional for others. For example, all medicinal products developed by certain biotechnological means, and those containing a new active substance which is not yet authorized in the EU and which are intended for the treatment of cancer and other specified diseases and disorders, must be authorized via the centralized procedure. The Company considers that the centralized procedure will apply to its products that are developed by means of a biotechnology process. The centralized procedure is optional for any other products containing new active substances not authorized in the EU or for products which constitute a significant therapeutic, scientific, or technical innovation or for which a centralized authorization is in the interests of patients at EU level. Under the centralized procedure the maximum timeframe for the evaluation of a marketing authorization application by the EMA is 210 days, excluding clock stops, when additional written or oral information is to be provided by the applicant in response to questions asked by the EMA’s Committee for Medicinal Products for Human Use (CHMP). Clock stops may extend the timeframe of evaluation of a marketing authorization application considerably beyond 210 days. Where the CHMP gives a positive opinion, it provides the opinion together with supporting documentation to the European Commission, who make the final decision to grant a marketing authorization, which is issued within 67 days of receipt of the EMA’s recommendation. Accelerated assessment might be granted by the CHMP in exceptional cases, when a medicinal product is expected to be of major public health interest, particularly from the point of view of therapeutic innovation. The timeframe for the evaluation of a marketing authorization application under the accelerated assessment procedure is 150 days, excluding clock stops, but it is possible that the CHMP may revert to the standard time limit for the centralized procedure if it determines that the application is no longer appropriate to conduct an accelerated assessment.
As with FDA approval, the Company may not be able to secure regulatory approvals in the EU in a timely manner, if at all. Additionally, as in the U.S., post-approval regulatory requirements, such as those regarding product manufacture, marketing, or distribution, would apply to any product that is approved in the EU, and failure to comply with such obligations could have a material adverse effect on the Company’s ability to successfully commercialize any product.
The conduct of clinical trials in the EU is governed by the Clinical Trials Regulation (EU) No 536/2014 (CTR), which replaced the previous Clinical Trials Directive 2001/20/EC on January 31, 2022. No clinical trial may be commenced in the EU without a clinical trial authorization from the applicable national competent authorities and favourable ethics approval. Under the CTR, a single application can be made through the Clinical Trials Information System (CTIS) for authorization of a clinical trial in up to 30 EU/EEA countries at the same time and with a single set of documentation.
The assessment of applications for clinical trials is divided into two parts (Part I contains scientific and medicinal product documentation and Part II contains the national and patient-level documentation). Part I is assessed by a coordinated review by the competent authorities of all EU member states States in which an application for authorization of a clinical trial has been submitted (Member States Concerned) of a draft report prepared by a Reference Member State. Part II is assessed separately by each Member State Concerned. The role of the relevant ethics committees in the assessment procedure continues to be governed by the national law of the Member State Concerned, however overall related timelines are defined by the CTR. The new CTR also provides for simplified reporting procedures for clinical trial sponsors.
Environmental, social and governance (ESG) report
Environmental
The consolidated entity is not subject to any significant environmental regulation under Australian Commonwealth or State law. We are considering ways in which environmental impacts can be monitored however we do not foresee a material impact.
Sustainability
Kazia’s head office is located in a sustainable carbon neutral commercial precinct. The serviced office is located in a building with a five star NABERS energy rating.
24
Climate Change
Kazia is mindful of its impact on the environment and strives to reduce its carbon footprint. The Kazia business model is based on outsourcing, and we are working with major partners who are focused on reducing climate change and enhancing climate protection.
Society
Community Contribution
Compassionate Use Program
In rare circumstances, after careful discussion with the treating clinician, Kazia is sometimes able to provide its drug candidates for compassionate use on an individual named patient basis.
Our compassionate use program has treated over 40 patients in 7 countries since its inception in 2018.
Countries we treat compassionate patients in: Australia, USA, Israel, Spain, Switzerland, England and Ireland
Social and Governance
Social and governance matters cover a vast range of potential issues including responsible business policies. Our policies set out our commitment to high social standards.
The following policies are in place and available on our website:
| • | Anti-Corruption Compliance |
| • | Continuous Disclosure |
| • | Corporate Governance |
| • | Expanded Access |
| • | Shareholder Communications |
| • | Whistleblower |
Employees
The consolidated entity aims to ensure that it has a safe operating environment with an inclusive and diverse culture and the best talent and skills for our future success. The following employee policies are in place:
| • | Code of Business Conduct & Ethics |
| • | Recruitment and retention |
| • | Inclusion and diversity |
| • | Parents returning to work |
| • | Education and training |
| • | Employee Share Option Plan |
| • | Health and safety |
| • | Whistleblowing |
| • | Equal Employment Opportunity and Diversity |
| • | Harassment and Discrimination |
| • | Anti-corruption and anti-bribery policies |
| • | Public disclosures |
| • | Securities trading |
| • | Scientific integrity |
25
Product and Corporate Developments during Fiscal Year 2023
The Company continued to pursue its strategy of focusing resources on clinical programs, being specifically those most likely to provide a return to shareholders.
Paxalisib is involved in ten clinical trials, all being conducted by world renowned research organizations and principally funded by parties other than the Company, giving us multiple opportunities to realise value from this product candidate.
EVT801’s phase I clinical trial continues and we believe that we are on track to have initial phase I data in the 4Q CY2023.
At-The-Market (ATM) Facility
Kazia established an ‘at-the-market’ equity program (the “ATM facility”) with Oppenheimer & Co. Inc. (“Oppenheimer”), as sales agent, in April 2022. Under the ATM facility, Kazia may offer and sell through Oppenheimer up to an aggregate amount of US$35 million of its ordinary shares, in the form of American Depositary Shares (the “ADSs”), with each ADS representing ten ordinary shares. During the fiscal year ended 30 June 2023, Kazia sold an aggregate amount of US$4,203,221 (2022 US$2,956,036) of ADSs under the ATM facility. The ATM facility allows Kazia to raise capital dynamically in the open market, with no discount, no warrant coverage, and modest banking fees, allowing it to fund operations with minimal dilution to existing shareholders.
C. Organizational structure
Kazia Therapeutics Limited is incorporated in Australia and has the following wholly-owned subsidiaries:
| Name |
Country of incorporation |
|
| Kazia Laboratories Pty Ltd | Australia | |
| Kazia Research Pty Ltd | Australia | |
| Kazia Therapeutics Inc. | United States (Delaware) | |
| Glioblast Pty Ltd | Australia |
Kazia Therapeutics (Hong Kong) Limited, registered in Hong Kong, was formally deregistered and dissolved on 10 March 2023.
26
D. Property, plant and equipment
During fiscal year 2023, the Company continued to work out of a serviced office in Sydney that is subject to a renewable one-year workspace license agreement. In April 2022 an office membership agreement was signed with Deerfield Management for one year in The Cure, Deerfield’s innovation campus at 345 Park Avenue South, New York, NY, and was extended for an additional 12 months in April 2023.
| Item 4A. | Unresolved Staff Comments |
None.
| Item 5. | Operating and Financial Review and Prospects |
Critical accounting policies
We prepare our financial statements in accordance with International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board (IASB). As such, we are required to make certain estimates, judgments, and assumptions that management believes are reasonable based upon the information available. These estimates, judgments and assumptions affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the periods presented. The critical accounting policies are summarized in Item 18. “Financial Statements—Note 3 – Critical Accounting Policies”.
The following discussion and analysis should be read in conjunction with Item 18. “Financial Statements” included below. Operating results are not necessarily indicative of results that may occur in future periods. This discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. The actual results may differ materially from those anticipated in the forward-looking statements as a result of many factors including, but not limited to, those set forth under “Forward-Looking Statements” and “Risk Factors” in Item 3 “Key Information” included above in this Annual Report on Form 20-F. All forward-looking statements included in this document are based on the information available to the Company on the date of this document and the Company assumes no obligation to update any forward-looking statements contained in this Annual Report on Form 20-F.
A. Operating results
The following discussion relates to our consolidated results of operations, financial condition and capital resources. You should read this discussion in conjunction with our consolidated financial statements and the notes thereto contained elsewhere in this report.
The following tables provide a summary of Revenue, Finance income and Other income for the past three fiscal years:
| For the fiscal year ended 30 June, | ||||||||||||
| 2023 | 2022 | 2021 | ||||||||||
| A$’000 | A$’000 | A$’000 | ||||||||||
| Revenue |
— | — | 15,183 | |||||||||
| Finance income |
22 | 2 | 42 | |||||||||
| Other income: |
||||||||||||
| Payroll tax rebate |
— | — | 2 | |||||||||
| Bad debt recovery |
— | 15 | — | |||||||||
| Other sundry income |
1 | — | — | |||||||||
| Subsidies and grants |
— | 10 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Revenue, Finance and Other income |
23 | 27 | 15,227 | |||||||||
|
|
|
|
|
|
|
|||||||
27
Fiscal year 2023 compared to fiscal year 2022 and fiscal year 2022 compared to fiscal year 2021
Revenue, finance income and other income
In fiscal years 2023 and 2022, the Company did not generate any revenue from contracts with customers. The revenue recognized during fiscal year 2021, A$15.2 million, was the result of up-front license fees received from partnering transactions pertaining to the out-license agreements entered into for two of the Company’s assets, Cantrixil and paxalisib.
The Company earns interest income derived from interest bearing bank accounts, which is directly linked with the amounts held on deposit. The amount of finance income earned increased as a result of increased cash balances as well as higher interest rates in effect during the year.
The Company did not recognize any research and development rebate in fiscal years 2023, 2022 or 2021. In fiscal year 2023, the amount of qualifying expenditure in Australia was not sufficiently large to warrant making a claim for the R&D rebate, and we do not anticipate applying for the rebate in future fiscal years as this trend is set to continue.
Expenses
Research and development expenses decreased from A$20.2 million in fiscal year 2022 to A$15.6 million in fiscal year 2023 (-23%). The decrease was mainly a result of a full year of salary and benefit expenses were recognized for the US based scientific and clinical staff hired in FY22. Research and development expenses increased from A$14.5 million in fiscal year 2021 to A$20.2 million in fiscal year 2022 (39%). The increase was mainly a result of the expenditures incurred for the Company’s lead asset, paxalisib’s continuing participation in the registrational GCAR GBM AGILE trial. Funds were also spent on the expenditures incurred for the EVT801 Phase I trial, which opened and initiated enrollment in late 2021. Additional salary and benefit expenses were recognized for the US based scientific and clinical staff hired, including the Chief Medical Officer. Amortisation of the EVT801 asset also contributed to the increase during fiscal 2022.
General and administrative costs were increased from A$5.1 million (restated) in fiscal year 2022 to A$8.6 million in fiscal year 2023 (68%), due in part to the A$1.6 million additional expense due to increased directors and officers liability and business insurance costs, a full year of salary and benefits for the US based Chief Financial Officer, a full year of US office rent and the termination payment for former CEO James Garner. General and administrative costs decreased from A$7.0 million to A$5.1 million (restated) in fiscal year 2022 (27%), due in part to the A$1.46 million expense recognized resulting from a Chinese withholding tax incurred on the Company’s receipt of the up-front licencing transaction payment from Simcere Pharmaceutical in fiscal year 2021. Excluding the unrealised FX gain of A$1.8 million, corporate expenses were reduced by A$613K. The reduced costs were somewhat offset by the increased payroll related costs incurred for the US based Chief Financial Officer hired during the period.
Net loss
The Company’s loss after income tax was A$20.4 million in fiscal year 2023 compared to A$25.0 million (restated) in fiscal year 2022. The change was mainly a result of the reduced R&D expenditures a reduction in the expenditures incurred for the Company’s lead asset, paxalisib’s continuing participation in the registrational GCAR GBM AGILE trial as paxalisib arm did not meet pre-defined criteria for continuing to a second stage of the trial. Funds were also spent on the expenditures incurred for the continuing EVT801 Phase I trial including A$2.1M for manufacture of EVT-801.
The Company’s loss after income tax was A$25.0 million in fiscal year 2022 compared to A$8.4 million in fiscal year 2021. The change was mainly a result of the increased R&D expenditures incurred for the ongoing clinical trials as well as the initiation and enrolment of the EVT801 Phase 1 trial along with our collaborations in the investigator-initiated trials, as described above, during fiscal year 2022. Additionally, no revenue was recognized from partnership collaborations in fiscal year 2022.
B. Liquidity and capital resources
We have incurred cumulative losses and negative cash flows from operations since our inception and, as of 30 June 2023, we had accumulated losses of A$89.1 million. We anticipate that we will continue to incur losses for at least the next several years. We expect that our research and development expenditure will continue to increase and, as a result, we will need additional capital to fund our operations, which we may raise through a combination of equity offerings, other third-party funding, and other collaborations, strategic alliances and licensing arrangements.
There is a premium funding facility in place in fiscal year 2022 and 2023 for the insurance program.
28
As at 30 June 2023, we had cash and cash equivalents of A$5.2 million, held in both Australian dollars and U.S. dollars. Cash in excess of immediate requirements is invested in accordance with our investment policy, primarily with a view to liquidity and capital preservation. Currently, our cash and cash equivalents are held in bank accounts, with a 44.4% of funds being held in U.S. dollars.
Going Concern
We expect to consume cash and incur operating losses for the foreseeable future as the Company continues developing its oncology drug candidates. The impact on cash resources and results from operations will vary with the extent and timing of the future clinical trial programs. The financial statements have been prepared on a going concern basis, which contemplates continuity of normal activities and realization of assets and settlement of liabilities in the normal course of business. As is often the case with drug development companies, the Company’s ability to continue its development activities as a going concern is dependent upon deriving sufficient cash from investors, from licensing and partnering activities and from other sources of revenue such as grant funding. We may be required to delay, scale-back, or eliminate certain of our activities and other aspects of our operations until such time as we are successful in securing additional funding. We are exploring various dilutive and non-dilutive sources of funding, including equity and debt financings, strategic alliances, business development and other sources. Our future success is dependent upon our ability to obtain additional funding. There can be no assurance, however, that we will be successful in obtaining such funding in sufficient amounts, on terms acceptable to us, or at all. Furthermore, under General Instruction I.B.5 to Form F-3 (the “Baby Shelf Rule”) the amount of funds we can raise through primary public offerings of securities in any 12-month period using our registration statement on Form F-3 is limited to one-third of the aggregate market value of the ordinary shares held by non-affiliates of our company, which limitation may change over time based on our stock price, number of ordinary shares outstanding and the percentage of ordinary shares held by non-affiliates. We therefore are limited by the Baby Shelf Rule as of the filing of this Annual Report, until such time as our non-affiliate public float exceeds $75 million. Our independent auditor’s report for the fiscal year ended 30 June 2023 included an explanatory paragraph regarding going concern uncertainty. There is substantial doubt about our ability to continue as a going concern within one year after the date that the financial statements for the fiscal year ended 30 June 2023 are issued.
As of the date of this filing, we currently anticipate that current cash and cash equivalents, excluding any potential proceeds from our ATM facility, will be sufficient to meet our anticipated cash requirements through the end of November 2023.
Cash flows
The following table set forth the sources and uses of cash for the past three fiscal years:
| (in A$ thousands) | 2023 | 2022 | 2021 | |||||||||
| Net cash used in operating activities |
(15,156 | ) | (22,763 | ) | (9,111 | ) | ||||||
| Net cash used in investing activities |
— | (2,365 | ) | — | ||||||||
| Net cash from financing activities |
12,972 | 3,726 | 28,109 | |||||||||
Operating activities. Net cash used in operating activities for the three fiscal years primarily represents net outflows for the cost of the R&D programs and the general and administrative costs of running the business. This amount is heavily impacted by the cost of the clinical programs, as well as cost containment measures adopted to manage the general and administrative costs of the business.
Investing activities. Net cash from investing activities in fiscal year 2022 represents the payment of a development milestone for EVT801.
Financing activities. Net cash from financing activities in fiscal years 2023 arose as a result of professional placements of the Company’s ordinary shares to institutional investors in certain countries as well as Share Purchase Plans to shareholders in Australia. In addition, net cash from financing activities in fiscal years 2022 & 2023 was the result of the funds received from our sale of ordinary shares in the form of ADSs, using our ATM facility. In 2021 cash for financing activities was raised as a result of a retail entitlement offer to institutional and retail investors.
As at 30 June 2023, the Company did not hold any derivative financial instruments for managing its foreign currency; however, the Company may from time to time enter into hedging arrangements where circumstances are deemed appropriate.
The Company believes that its future ability to fund its operations will depend on deriving sufficient cash from investors through successful capital raisings, from licensing and partnering activities and government grants.
The Company had no commitments for capital expenditures or material contractual obligations at the end of fiscal year 2023. The Company continuously pursues opportunities for non-dilutive funding, such as grant applications.
The Company cannot provide assurance that it or its subsidiaries will be able to raise the funds necessary to complete the planned clinical trial programs or find appropriate collaboration or licensing opportunities.
The Company does not have any off-balance sheet arrangements.
Financing activities
Equity issues
The Company has historically financed its operations primarily from issuing equity capital.
During fiscal year 2022 the Company issued 6,743,167 ordinary shares. The details of those share issues are as follows:
| • | In December 2021 the Company issued 25,000 shares upon the exercise of options, raising a total of A$16,700. |
| • | In May 2022 the Company issued 1,855,357 shares due to the conversion of the Triaxial convertible note triggered by completion of phase II paxalisib trial announced to ASX on 21 April 2022. |
| • | In May and June 2022, the Company issued 4,862,810 shares under our ATM facility raising A$4.2 million before transaction costs. |
29
During fiscal year 2023 the Company issued 89,273,738 ordinary shares. The details of those share issues are as follows:
| • | In July 2022, the Company issued 573,370 shares under our ATM facility raising A$407,201 before transaction costs. |
| • | In August 2022, the Company issued 10,307,910 shares under our ATM facility raising A$3,268,936 before transaction costs. |
| • | In September 2022, the Company issued 679,380 shares under our ATM facility raising A$150,164 before transaction costs. |
| • | In September 2022, the Company issued 60,000 to the Scientific Advisory Board at A$12,600 before transaction costs. |
| • | In October 2022, the Company issued 13,032,940 shares under our ATM facility raising A$2,425,085 before transaction costs. |
| • | In January 2023, the Company issued 20,000 shares under our ATM facility raising A$2,761 before transaction costs. |
| • | In January 2023, the Company issued 25,387,018 shares in a professional and sophisticated investors placement raising A$2,792,572 before transaction costs. |
| • | In February 2023, the Company issued 15,522,075 shares in a professional and sophisticated investors placement raising A$1,707,428 before transaction costs. |
| • | In March 2023, the Company issued 23,691,045 shares in a share placement plan to existing eligible shareholders raising A$2,606,000 before transaction costs. |
In fiscal year 2023, the Company held a significant proportion of its cash balances in U.S. dollars and there were no conversion losses. During fiscal year 2022, the Company held the majority of its cash balances in U.S. dollars and there were no conversion losses. See Item 18. “Financial Statements – Note 24 – Financial Instruments” for disclosures about financial risk management including interest rate risk, foreign currency risk and liquidity risk.
Convertible note (Triaxial) carrying value of A$464,000
During the fiscal year ended 30 June 2013 the Company issued Convertible Notes with a face value of A$1,500,000 to Triaxial in consideration of the acquisition of patents and intellectual property assets. The terms of these Convertible Notes were amended on 4 December 2014. The amended terms allow the conversion of debt into ordinary shares, provided that the Company achieves certain milestones. Accordingly, the Convertible Note has been reclassified as an equity instrument rather than debt instrument.
During fiscal year 2017, the Company reached two milestones that triggered the conversion of a portion of its Convertible Notes. On 14 September 2016 the directors approved the issue of 20,000,000 ordinary shares as a consequence of a conversion of A$500,000 of the Convertible Notes, and on 1 November 2016 a further 16,000,000 ordinary shares were issued as a result of the conversion of a further portion of the Convertible Notes. During fiscal year 2018, one of the noteholders waived his rights to the remaining tranche of convertible notes, resulting in the reduction of the convertible note carrying value by a further A$136,000. On 21 April 2022 the completion of the phase II study of paxalisib in glioblastoma (NCT03522298) was announced and on 5 May 2022 the remaining portion of the convertible note with a carrying value of A$464,000 was extinguished and converted to 1,855,357 ordinary shares.
C. Research and development, Patents and Licenses, etc.
Expenditures during the research phase of a project are recognized as an expense when incurred. Development costs are capitalized only when technical feasibility studies identify that the project will deliver future economic benefits and these benefits can be measured reliably.
Research and development expenses consist primarily of costs incurred for the development of our product candidates, which include:
| • | expenses incurred under agreements with academic research centers, clinical research organizations and investigative sites that conduct our clinical trials; and |
| • | the cost of acquiring, developing, and manufacturing clinical trial materials. |
30
We cannot determine with certainty the duration and completion costs of the current or future product development, preclinical studies or clinical trials of our product candidates. The duration, costs, and timing of clinical trials and development of our product candidates will depend on a variety of factors, including:
| • | the scope, rate of progress, and expense of our ongoing as well as any additional clinical trials and other research and development activities; |
| • | the countries in which trials are conducted; |
| • | future clinical trial results; |
| • | uncertainties in clinical trial enrollment rates or drop-out or discontinuation rates of patients; |
| • | potential additional safety monitoring or other studies requested by regulatory agencies; |
| • | significant and changing government regulation; and |
| • | the timing and receipt of any regulatory approvals. |
A change in the outcome of any of these variables with respect to the development of a product candidate could mean a significant change in the costs and timing associated with the development of that product candidate. For example, if the FDA, or another regulatory authority were to require us to conduct clinical trials beyond those that we anticipate will be required to complete clinical development of a product candidate or if we experience significant delays in enrollment in any of our clinical trials, we could be required to expend significant additional financial resources and time on the completion of clinical development.
In fiscal years 2023, 2022 and 2021 we spent, respectively, a total of A$15.6 million, A$20.2 million (restated) and A$14.5 million (restated) on company-sponsored research and development activities.
31
D. Trend Information
Further to the risk factors discussed in Item 3D, we note that the financial information disclosed in the SEC Form 20-F may not be indicative of future results in the following areas:
| • | While we anticipate that funds will continue to be spent on research and development of our drug candidates, the amounts expended in recent years may not be indicative of the amounts to be expended in future years, because we may have more or fewer drug candidates, they may be at different stages of their lifecycle and the trials deemed suitable for their development may be more or less costly; |
| • | We did not generate revenue from licensing transactions in fiscal year 2023 and 2022 and we may not generate any revenue in future years. Should the Company generate revenues in future years, the amounts generated in fiscal year 2021 may not be representative of any such revenues in future years. This could be as a result of whether any further licensing transactions are entered into, as well as whether any milestones are met in relation to license agreements already in place; and |
| • | The quantum of general and administrative expenditures in recent years may not be indicative of the expenditures required in future years. |
E. Critical Accounting Estimates – see Note 2. Significant accounting policies
| Item 6. | Directors, Senior Management and Employees |
A. Directors and Senior Management
The names and details of the Company’s Directors and senior management at the date of this report are as follows:
| Iain Ross | Chairman, Non-Executive Director (resigned 11 August 2023) | |
| John Friend | Chief Executive Officer (Appointed 1 August 2023 Managing Director (Appointed 11 August 2023 – Interim Chairman) | |
| Bryce Carmine | Non-Executive Director | |
| Steven Coffey | Non-Executive Director | |
| Ebru Davidson | Non-Executive Director (Appointed 5 June 2023) | |
| Karen Krumeich | Chief Financial Officer | |
| Anna Sandham | Company Secretary (Appointed 28 February 2023) |
32
Directors were in office for the entire period unless otherwise stated.
Names, titles, experience and expertise
| Name: | Iain Ross | |
| Title: | Chairman, Non-Executive Director | |
| (Resigned 11 August 2023) | ||
| Qualifications: | B.Sc. (Hons). C Dir. | |
| Experience and expertise: | Iain, based in the UK, is an experienced Director and has served on a number of Australian company boards. He is Chairman of Silence Therapeutics plc (Nasdaq:SLN), Executive Chairman of ReNeuron Group plc (LSE:RENE) and a Non-executive Director of BiVitctriX Therapeutics plc (LSE:BVX). In his career he has held senior positions in Sandoz AG, Fisons Plc, Hoffmann-La Roche AG and Celltech Group Plc and also undertaken a number of start-ups and turnarounds on behalf of banks and private equity groups. His track record includes multiple financing transactions having raised in excess of £600 million, both publicly and privately, as well as extensive experience of divestments and strategic restructurings and has over 25 years in cross-border management as a Chairman and CEO. He has led and participated in 8 Initial Public Offerings, (5 LSE, 1 ASX, 2 Nasdaq) and has direct experience of mergers and acquisitions transactions in Europe, USA and the Pacific Rim | |
| Other current directorships: | Silence Therapeutics plc (LSE:SLN), ReNeuron Group plc (LSE:RENE) and BiVictriX Therapeutics plc (LSE:BVX) | |
| Former directorships (last 3 years): | Redx Pharma plc (LSE:REDX) and Palla Pharma Limited (ASX:PAL) | |
| Special responsibilities: | Former Member of Remuneration and Nomination Committee, Former Member of the Audit, Risk and Governance Committee. | |
| Name: | Bryce Carmine | |
| Title: | Non-Executive Director | |
| Qualifications: | B.Sc., Biochemistry, Microbiology & Genetics | |
| Experience and expertise: | Bryce spent 36 years working for Eli Lilly & Co. and retired as Executive Vice President for Eli Lilly & Co, and President, Lilly Bio-Medicines. Prior to this he led the Global Pharmaceutical Sales and Marketing and was a member of the Company’s Executive Committee. Bryce previously held a series of product development portfolio leadership roles culminating when he was named President, Global Pharmaceutical Product Development, with responsibility for the entire late-phase pipeline development across all therapeutic areas for Eli Lilly. During his career with Lilly, Bryce held several country leadership positions including President Eli Lilly Japan, Managing Dir. Australia/NZ & General Manager of a JV for Lilly in Seoul, Korea. Bryce is currently Chairman and CEO of HaemaLogiX Pty Ltd, a Sydney based privately owned biotech. | |
| Other current directorships: | None | |
| Former directorships (last 3 years): | None | |
| Special responsibilities: | Chair of Remuneration and Nomination Committee, member of Audit, Risk and Governance Committee. | |
| Name: | Steven Coffey | |
| Title: | Non-Executive Director | |
| Qualifications: | B. Comm, CA | |
| Experience and expertise: | Steven is a Chartered Accountant and registered company auditor and has over 35 years experience in the accounting and finance industry. He has been a partner with the chartered accounting firm Watkins Coffey Martin which recently merged with Charternet Chartered Accountants and Steven is a consultant to that group. Steven sits on the board of a number of large private family companies and audits a number of large private companies and not-for-profit entities. | |
| Other current directorships: | None | |
| Former directorships (last 3 years): | Ansarada Group Limited (ASX: AND) formerly The Docyard Limited (ASX:TDY) | |
| Special responsibilities: | Chair of Audit, Risk and Governance Committee, member of Remuneration and Nomination Committee. | |
33
| Name: | Dr James Garner | |
| Title: | Managing Director and Chief Executive Officer Resigned 30 April 2023 Terminated 30 June 2023 |
|
| Qualifications: | MA, MBA, MBBS, BSc (Hons), MAICD | |
| Experience and expertise: | Dr Garner is an experienced life sciences executive who has previously worked with companies ranging from small biotechs to multinational pharmaceutical companies such as Biogen and Takeda. His career has focused on regional and global development of new medicines from preclinical to commercialisation. | |
| Dr Garner is a physician by training and holds an MBA from the University of Queensland. He began his career in hospital medicine and worked for a number of years as a corporate strategy consultant with Bain & Company before entering the pharmaceutical industry. Prior to joining Kazia in 2016, he led R&D strategy for Sanofi in Asia-Pacific and was based in Singapore. | ||
| Other current directorships: | Antisense Therapeutics Limited (ASX:ANP) | |
| Former directorships (last 3 years): | None | |
| Special responsibilities: | None | |
| Name: | Ebru Davidson | |
| Title: | Non-Executive Director - from 5 June 2023 | |
| Qualifications: | BSc, JD (Hons), AGIA, GAICD | |
| Experience and expertise: | Ms Davidson is a highly experienced corporate lawyer and is currently the General Counsel for QBiotics Group Limited, an unlisted public Australian life sciences company. Prior to this, Ms Davidson was a partner at national law firm Thomson Geer Lawyers and has over 14 years’ experience in equity capital markets, private and public mergers and acquisitions, corporate transactions and corporate governance. Ms Davidson also has extensive experience in advising listed and unlisted entities on compliance and regulatory matters working closely with the Australian Securities and Investment Commission and Australian Securities Exchange. | |
| Other current directorships: | None | |
| Former directorships (last 3 years): | None | |
| Special responsibilities: | None | |
| Name: | Dr John Friend | |
| Title: | Chief Executive Officer (appointed 1 May 2023) | |
| Managing Director (appointed 1 August 2023) | ||
| Interim Chairman of the Board (appointed 11 August 2023) | ||
| Chief Medical Officer to 30 April 2023 | ||
| Qualifications: | B.A., M.D | |
| Experience and expertise: | Dr Friend is a highly experienced physician executive who has previously worked with companies ranging from start-up biotechnology companies to multinational pharmaceutical companies. Over the past 15 years, his focus has been in the oncology and hematology therapeutic space. Dr. Friend is a US-trained physician who practiced medicine in North Carolina before transitioning to drug development. Before joining Kazia Therapeutics, he was Chief Medical Officer and member of the executive management team at Cellectar Biosciences, Inc, a US publicly traded biopharmaceutical company. | |
| Other current directorships: | None | |
| Former directorships (last 3 years): | None | |
| Special responsibilities: | None | |
| Name: | Karen Krumeich | |
| Title: | Chief Financial Officer | |
| Qualifications: | BSc., | |
| Experience and expertise: | Karen has more than thirty years of experience in corporate finance, focused almost entirely on the life sciences sector. She has been responsible for driving the growth of numerous private and public biotech companies. For most of the last twenty years, she served as Chief Financial Officer to growth-stage biotech companies, both public and private, including Soligenix, Inc, and Theravectys, Inc. In addition to her accounting qualifications, Karen is a qualified pharmacist and a graduate of the University of Toledo School of Pharmacy. | |
34
| Name: | Kate Hill | |
| Title: | Company Secretary (resigned 28 February 2023) | |
| Qualifications: | CA, GAICD, BSc (Hons) | |
| Experience and expertise: | Kate has over 20 years’ experience as an audit partner with Deloitte Touche Tohmatsu, working with ASX listed and privately-owned clients. She has worked extensively in regulated environments including assisting with Initial Public Offerings, capital raising and general compliance, as well as operating in an audit environment. She is a Non-Executive Director of CountPlus Limited (ASX:CUP) and Elmo Software Limited (ASX:ELO) as well as Chair of the Audit and Risk Committee for both of these companies. She is also Chair of Seeing Machines Limited (LSE:SEE). Kate is a member of the Institute of Chartered Accountants in Australia and New Zealand, and a graduate of the Australian Institute of Company Directors. | |
| Name: | Anna Sandham | |
| Title: | Company Secretary (appointed 28 February 2023) | |
| Qualifications: | BEc., Grad.Dip. AppCorpGov., FGIA, | |
| Experience and expertise: | Anna has more than 25 years’ experience as a company secretary and governance professional, working with ASX listed and privately owned companies. Anna is employed by Company Matters Pty Ltd (part of the Link Group). Prior to joining Company Matters in 2012, Anna was a Company Secretary at AMP Financial Services and prior to that was Company Secretary at Westpac Banking Corporation where she also led the secretariat function for the BT Financial Group. Anna has also held company secretarial roles within the private and public sectors, including NRMA Limited. Anna holds a Bachelor of Economics (University of Sydney) and a Graduate Diploma of Applied Corporate Governance (Governance Institute of Australia). She is a Chartered Governance Professional, a Fellow of the Governance Institute of Australia and a member of its Legislative Review Committee. | |
35
B. Compensation
Principles used to determine the nature and amount of remuneration
Remuneration philosophy
Remuneration for Directors and Senior Executives is based on the overall objective of attracting and retaining people of high quality who will make a worthwhile contribution to Kazia in the short, medium and long term, and thereby contribute to long term shareholder value. The Board and its Remuneration and Nomination Committee take a balanced position between the need to pay market rates to attract talent, and the financial resources of Kazia, in determining remuneration.
Non-Executive Directors remuneration
The Constitution of Kazia and the ASX listing rules specify that the aggregate remuneration of Non-Executive Directors shall be determined from time to time by General Meeting. The last determination for the consolidated entity was at the Annual General Meeting held on 16 November 2022 when the shareholders approved the new constitution with an aggregate remuneration of $560,000.
Non-Executive Directors’ fees are reviewed periodically by the Board and are regularly compared with those of companies of comparable market capitalization and stage of development. The Chairman’s fees are determined independently to the fees of other non-executive Directors based on comparative roles in the external market.
The Chairman’s fees increased to GBP12,000 per month for April, May and June 2023 to recognise the additional time the Chair spent securing the support of, and funds from major investors in the January 2023 fundraising through his personal and direct relationships; leading all discussions and negotiations with the current Chief Executive Officer and the incoming Chief Executive Officer about the position of Chief Executive Officer; and assisting the incoming Chief Executive Officer during the next short term period to secure additional funding for the business and to participate in 3rd party discussions as necessary.
The Non-Executive Directors fee structure is a fixed fee model and includes superannuation for Australian based directors.
Executive Directors and other Key Management Personnel (“KMP”)
The Board and the Remuneration and Nomination Committee, in consultation with the Managing Director, have put in place a remuneration structure which provides incentive for employees to drive the activities of the company forward. These arrangements are reviewed annually at the end of the calendar year.
The Board determines an appropriate level of fixed remuneration for the CEO and Group Executives, as well as the proportion of performance-based remuneration.
The executive remuneration and reward framework has three components:
| • | fixed remuneration |
| • | short-term performance incentives - cash bonus |
| • | share-based payments - award of options through the ESOP |
Fixed remuneration is reviewed annually by the Remuneration and Nomination Committee based on individual performance, the overall performance of Kazia and comparable market remunerations. The Remuneration and Nomination Committee approved increases in fixed remuneration during fiscal year 2023.
The short-term incentives program is designed to align the targets of Kazia with the performance hurdles of executives. Short-term incentive payments are granted to executives based on specific annual performance objectives, metrics and performance appraisals. Annual performance reviews are conducted at the end of each calendar year and bonuses are paid shortly after the performance reviews are completed. Annual performance objectives cover matters such as progress in clinical trials, and management of the Company’s financial resources.
The Board or the Remuneration and Nomination Committee may, at its discretion, award bonuses for exceptional performance.
36
The long-term incentive comprises equity-based payments. The consolidated entity aims to attract and retain high calibre executives, and align their interests with those of the shareholders, by granting equity-based payments which are issued at the share price on date of issue and vest in tranches based on tenure. The share-options issued to executives are governed by the ESOP.
Employee share option plan
The Employee Share Option Plan (‘ESOP’) was most recently approved by shareholders on 10 November 2021.
The ESOP provides for the issue of options to eligible individuals, being employees, Officers and Non-executive directors of Kazia.
Each option issued under the ESOP entitles its holder to acquire one fully paid ordinary share and is exercisable at a price based on a formula, which includes the weighted average price of such shares at the close of trading on the Australian Securities Exchange for the five days prior to the date of issue. The number of options offered, the amount payable, the vesting period, the option period, the conditions of exercise or any other factors are at the discretion of the Board of Directors.
Kazia issued 7,930,000 share options under the ESOP during the financial year ended 30 June 2023, of which 6,000,000 were issued to KMP.
Any change to the ESOP will require approval by shareholders.
Use of remuneration consultants
During fiscal year 2023, the Company did not engage remuneration consultants to assist with the determination of remuneration levels.
Details of remuneration
Amounts of remuneration
Details of the remuneration of key management personnel of Kazia are set out in the following tables, reported in Australian dollars, the functional currency of the Company, unless otherwise noted.
The KMP of Kazia consisted of the following directors of Kazia Therapeutics Limited:
| • | Iain Ross - Non-Executive Director, Chairman (resigned 11 August 2023) |
| • | Bryce Carmine - Non-Executive Director |
| • | Steven Coffey - Non-Executive Director |
| • | Dr James Garner - Managing Director, CEO - resigned 30 April 2023, terminated 30 June 2023 |
| • | Ebru Davidson - Non-Executive Director - from 5 June 2023 |
And the following persons:
| • | Dr John Friend - Chief Medical Officer (from 15 November 2021 to 30 April 2023) |
| • | Dr John Friend - Chief Executive Officer - from 1 May 2023 |
| • | Karen Krumeich - Chief Financial Officer |
| • | Kate Hill - Company Secretary - resigned 28 February 2023 |
37
| Short-term benefits |
Short-term benefits |
Short-term benefits |
Short-term benefits |
Short-term benefits |
Post- employment benefits |
Share-based payments |
||||||||||||||||||||||||||
| Salary & fees Cash |
Bonus Cash |
Movements Non- monetary |
Movements in long service leave Non- monetary |
Healthcare & Insurance Cash |
Super- annuation |
Options Equity- settled |
Total | |||||||||||||||||||||||||
| 2023 | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| Non-Executive Directors: |
||||||||||||||||||||||||||||||||
| I Ross* |
175,016 | — | — | — | — | — | 74 | 175,090 | ||||||||||||||||||||||||
| B Carmine |
85,000 | — | — | — | — | 8,925 | 74 | 93,999 | ||||||||||||||||||||||||
| S Coffey |
85,000 | — | — | — | — | 8,925 | 74 | 93,999 | ||||||||||||||||||||||||
| E Davidson |
6,440 | — | — | — | — | 676 | — | 7,116 | ||||||||||||||||||||||||
| J Garner** |
543,750 | 365,838 | — | — | — | 77,022 | 263,945 | 1,250,555 | ||||||||||||||||||||||||
| Other Key Management Personnel: |
||||||||||||||||||||||||||||||||
| J Friend ** |
742,685 | 248,869 | 52,065 | — | 28,448 | 31,669 | 473,177 | 1,576,913 | ||||||||||||||||||||||||
| K Krumeich *** |
592,168 | 120,664 | 6,961 | — | 13,797 | 22,409 | 295,150 | 1,051,149 | ||||||||||||||||||||||||
| K Hill |
81,813 | — | — | — | — | — | 13,366 | 95,179 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| 2,311,872 | 735,371 | 59,026 | — | 42,245 | 149,626 | 1,045,860 | 4,344,000 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| * | Salary paid in GB pounds, but disclosed in Australian dollars using conversion rate of 0.5571 |
| ** | Amounts shown are for the full year, not just to resignation date of 30 April 2023. Termination payment includes annual leave owing at 30 June 2023, 4 months’ salary and superannuation in lieu of notice. |
| *** | Salary paid in USD, but disclosed in Australian dollars using conversion rate of 0.6755. Guaranteed bonus accrued at year end rate. |
Service agreements
Under Remuneration and Nomination Committee policy, employment contracts are entered into with each of the executives who is considered to be KMP. Under the terms of the contracts, remuneration is reviewed at least annually. The employment contracts of KMPs include a termination clause whereby a party can terminate the agreement on notice. Notice required is 6 months. Under the terms of each contract, payment in lieu can be made by the consolidated entity to substitute the notice period. The consolidated entity may terminate the contracts at any time without cause if serious misconduct has occurred. In the event that employment is terminated for cause, no severance pay or other benefits are payable by the consolidated entity.
38
Remuneration and other terms of employment for key management personnel are formalized in service agreements. Details of these agreements are as follows:
| Name: Title: Agreement commenced: Term of agreement: Details: |
Dr John Friend Chief Executive Officer May 1 2023 Full time employment Base salary for the year ending 30 June 2023 of USD550,000 and healthcare and insurance benefits to be reviewed annually by the Remuneration and Nomination Committee. A minimum payout of 50% of bonus target agreed for 2023 of USD165,000. John’s employment with the consolidated entity is at-will, and if terminated, it must pay any outstanding entitlements due to him. |
|
| Name: Title: Agreement commenced: Term of agreement: Details: |
Karen Krumeich Chief Financial Officer January 3, 2022 Full time employment Base salary for the year ending 30 June 2023 of USD400,000 and healthcare and insurance benefits to be reviewed annually by the Remuneration and Nomination Committee. A minimum payout of 50% of bonus target agreed for 2023 of USD80,000. Karen’s employment with the consolidated entity is at-will, and if terminated, it must pay any outstanding entitlements due to her. |
|
Key management personnel have no entitlement to termination payments in the event of removal for misconduct.
39
Share-based compensation
Issue of options
The options issued on 3 March 2023 were to John Friend and Karen Krumeich 1,000,000 options each with an exercise price set at the volume weighted average (VWAP) of shares during the 5 trading days immediately prior to the issue date with a value of $101,376. The options issued on 3 May 2023 were 3,000,000 options to John Friend and 1,000,000 options to Karen Krumeich, with an exercise price set at the volume weighted average (VWAP) of shares during the 5 trading days immediately prior to the issue date, with values of $333,300 and $111,100 respectively. Service conditions are that any unvested options are forfeited on cessation of employment. There are no performance conditions, consistent with the Company’s Employee Share Option Plan rules, as reapproved by shareholders on 6 November 2021.
The terms and conditions of each grant of options over ordinary shares granted as remuneration to Directors or other Key Management Personnel in this financial year or future financial years are set out below.
Issue of options
| Grant date | Vesting date and exercisable date |
Expiry date | Exercise price | Fair value per option at grant date |
||||||
| 3 March 2023 |
3 July 2023 | 3 March 2027 | $ | 0.1500 | $ 0.10138 | |||||
| 3 March 2023 |
3 January 2024 | 3 March 2027 | $ | 0.1500 | $ 0.10138 | |||||
| 3 March 2023 |
3 July 2024 | 3 March 2027 | $ | 0.1500 | $ 0.10138 | |||||
| 3 March 2023 |
3 January 2025 | 3 March 2027 | $ | 0.1500 | $ 0.10138 | |||||
| 3 May 2023 |
3 May 2023 | 3 May 2027 | $ | 0.1870 | $ 0.1111 | |||||
| 3 May 2023 |
3 May 2024 | 3 May 2027 | $ | 0.1870 | $ 0.1111 | |||||
| 3 May 2023 |
3 May 2025 | 3 May 2027 | $ | 0.1870 | $ 0.1111 | |||||
Options granted carry no dividend or voting rights. Each option is convertible to one ordinary share upon exercise. Approval for the issue was obtained under ASX listing rule 10.14.
Pension benefits
The Company paid A$149,626 during fiscal year 2023 for employee superannuation benefits and pension benefits related to KMPs.
C. Board Practices
The role of the Board is as follows:
| • | representing and serving the interests of shareholders by overseeing and appraising the strategies, policies and performance of the Company. This includes overviewing the financial and human resources the Company has in place to meet its objectives and the review of management performance; |
| • | protecting and optimizing Company performance and building sustainable value for shareholders in accordance with any duties and obligations imposed on the Board by law and the Company’s Constitution and within a framework of prudent and effective controls that enable risk to be assessed and managed; responsible for the overall Corporate Governance of Kazia Therapeutics Limited and its subsidiaries, including monitoring the strategic direction of the Company and those entities, formulating goals for management and monitoring the achievement of those goals; |
40
| • | setting, reviewing and ensuring compliance with the Company’s values (including the establishment and observance of high ethical standards); and |
| • | ensuring shareholders are kept informed of the Company’s performance and major developments affecting its state of affairs. |
Responsibilities/functions of the Board include:
| • | selecting, appointing and evaluating from time to time the performance of, determining the remuneration of, and planning for the successor of, the CEO; |
| • | reviewing procedures in place for appointment of senior management and monitoring of its performance, and for succession planning. This includes ratifying the appointment and the removal of the Company Secretary; |
| • | overseeing the Company, including its control and accountability systems; |
| • | input into and final approval of management development of corporate strategy, including setting performance objectives and approving operating budgets; |
| • | reviewing and guiding systems of risk management and internal control and ethical and legal compliance. This includes reviewing procedures in place to identify the main risks associated with the Company’s businesses and the implementation of appropriate systems to manage these risks; |
| • | overseeing and monitoring compliance with the Code of Conduct and other corporate governance policies; |
| • | monitoring corporate performance and implementation of strategy and policy; |
| • | approving major capital expenditure, acquisitions and divestitures, and monitoring capital management; |
| • | monitoring and reviewing management processes in place aimed at ensuring the integrity of financial and other reporting; |
| • | monitoring and reviewing policies and processes in place relating to occupational health and safety, compliance with laws, and the maintenance of high ethical standards; and |
| • | performing such other functions as are prescribed by law or are assigned to the Board. |
In carrying out its responsibilities and functions, the Board may delegate any of its powers to a Board committee, a director, employee or other person subject to ultimate responsibility of the directors under the Australian Corporations Act 2001.
Matters which are specifically reserved for the Board or its committees include the following:
| • | appointment of a Chair; |
| • | appointment and removal of the CEO; |
| • | appointment of directors to fill a vacancy or as additional directors; |
| • | establishment of Board committees, their membership and delegated authorities; |
| • | approval of dividends; |
| • | development and review of corporate governance principles and policies; |
| • | approval of major capital expenditure, acquisitions and divestitures in excess of authority levels delegated to management; |
| • | calling of meetings of shareholders; and |
| • | any other specific matters nominated by the Board from time to time. |
Structure of the Board
The Company’s Constitution governs the regulation of meetings and proceedings of the Board. The Board determines its size and composition, subject to the terms of the Constitution. The Board does not believe that it should establish a limit on tenure other than stipulated in the Company Constitution (refer to ‘Term of Directors’ below).
41
While tenure limits can help to ensure that there are fresh ideas and viewpoints available to the Board, they hold the disadvantage of losing the contribution of directors who have been able to develop, over a period of time, increasing insight in the Company and its operation and, therefore, an increasing contribution to the Board as a whole. It is intended that the Board should comprise a majority of independent non-executive directors and comprise directors with a broad range of skills, expertise and experience from a diverse range of backgrounds. The Board regularly reviews the independence of each director in light of the interests disclosed to the Board.
The Board only considers directors to be independent where they are independent of management and free of any business or other relationship that could materially interfere with, or could reasonably be perceived to interfere with, the exercise of their unfettered and independent judgment. The Board has adopted a definition of independence based on that set out in Principle 2.3 of the ASX Corporate Governance Principles and Recommendations (4th edition). The Board will review the independence of each director in light of interests disclosed to the Board from time to time. In accordance with the definition of independence above, and the materiality thresholds set, the Board considers Bryce Carmine, Steven Coffey and Ebru Davidson to be independent directors.
There are procedures in place, agreed by the Board, to enable directors in furtherance of their duties to seek independent professional advice at the Company’s expense. The appointment and expiration dates of each director in office at the date of this report is as follows:
| Name | Position | Year First Appointed | Current term expires | |||
| Dr John Friend |
Managing Director, CEO | 2023 | N/A* | |||
| Bryce Carmine |
Non-executive Director | 2015 | Nov-23 | |||
| Iain Ross (resigned 11 August 2023) |
Non-executive Director, Chairman | 2014 | Nov-24 | |||
| Steven Coffey |
Non-executive Director | 2012 | Nov-25 | |||
| Dr James Garner (resigned 30 April 2023) |
Managing Director, CEO | 2016 | Terminated 30 June 2023 |
|||
| Ebru Davidson (appointed 5 June 2023) |
Non-executive Director | 2023 | Nov-26 | |||
| * | The managing director is exempt from standing for re-election under the Company’s constitution and Australian corporate law. |
Further details on each director can be found in “Names, titles, experience and expertise” above.
Term of Directors
The Company’s Constitution requires that at each Annual General Meeting of the Company, one third (or the number nearest to but not exceeding one third) of the directors, (excluding a director who is the Managing Director, and a director appointed to fill a casual vacancy) must retire from office provided that no director may retain office for more than three years without offering himself/herself for re-election even though such submission results in more than one third of the directors retiring from office.
The Board of Directors has the power to appoint any person to be a director either to fill a casual vacancy or as an additional director (up to a maximum of 10). Any director so appointed may hold office only until the next Annual General Meeting when he or she shall be eligible for election by the Company shareholders.
Board of Directors
The Board of Kazia Limited is elected by and accountable to shareholders. The Board monitors and directs the business and is responsible for the corporate governance of the Company. As at 30 June 2023, the Board comprised of four directors, all of whom were non-executive directors.
We do have a ‘diverse’ board of directors as defined in NASDAQ Rule 5605(f). Kazia is a small company with four Directors. As noted in the Board Diversity Matrix Part II Demographic Background section, it is the opinion of the company that we do meet the requirements of a diverse Board.
Board Diversity as at 26 October 2023
| Female | Male | Non-Binary | Did Not Disclose Gender |
|||||||||||||
| Country of Principal Executive Offices: Australia |
||||||||||||||||
| Foreign Private Issuer: Yes |
||||||||||||||||
| Disclosure Prohibited Under Home Country Law: No |
||||||||||||||||
| Total Number of Directors: 4 |
||||||||||||||||
| Part I: Gender Identity |
||||||||||||||||
| Directors |
1 | 3 | ||||||||||||||
| Part II Demographic Background |
||||||||||||||||
| African American or Black |
||||||||||||||||
| Alaskan Native or Native American |
||||||||||||||||
| Asian |
||||||||||||||||
| Hispanic or Latinx |
||||||||||||||||
| Native Hawaiian or Pacific Islander |
||||||||||||||||
| White |
3 | |||||||||||||||
| Two or More Races or Ethnicities |
1 | |||||||||||||||
| LGBTQ+ |
||||||||||||||||
| Did Not Disclose Demographic Background |
||||||||||||||||
42
Committees
The Board has established an Audit, Risk and Governance Committee and a Remuneration and Nomination Committee.
Audit, Risk and Governance Committee
The Board has established an Audit, Risk and Governance Committee which operates under a Charter approved by the Board, which is available on the Company’s website. It is the Board’s responsibility to ensure that an effective internal control framework exists within the entity. This includes internal controls to deal with both the effectiveness and efficiency of significant business processes, the safeguarding of assets, the maintenance of proper accounting records, and the reliability of financial information as well as non-financial considerations such as the benchmarking of operational key performance indicators. The Board has delegated responsibility for establishing and maintaining a framework of internal control and ethical standards to the Audit, Risk and Governance Committee.
The Committee also provides the Board with additional assurance regarding the reliability of financial information for inclusion in the financial reports.
Members of the Audit, Risk and Governance Committee are Steven Coffey (Chairman) and Bryce Carmine, each of whom is an independent director.
Remuneration and Nomination Committee
The purpose of the Remuneration and Nomination Committee is to assist and advise the Board to develop, implement and, from time to time, update policies in relation to:
| • | the selection, nomination and appointment processes for directors; and |
| • | the remuneration of key management personnel and directors. |
This committee is accountable to the Board for its performance and is subject to an annual review by the Board. Members of the Remuneration and Nomination Committee are Bryce Carmine (Chairman) and Steven Coffey each of whom is an independent director.
Performance
The performance of the Board and key executives is reviewed regularly using both measurable and qualitative indicators.
On at least a bi-annual basis, directors will provide written feedback in relation to the performance of the Board and its Committees against a set of agreed criteria:
| • | each Committee of the Board will also be required to provide feedback in terms of a review of its own performance; |
| • | feedback will be collected by the chair of the Board, or an external facilitator, and discussed by the Board, with consideration being given as to whether any steps should be taken to improve performance of the Board or its Committees; |
| • | the Chief Executive Officer will also provide feedback from senior management in connection with any issues that may be relevant in the context of Board performance review; and |
| • | where appropriate to facilitate the review process, assistance may be obtained from third party advisors. |
Remuneration
It is the Company’s objective to provide maximum shareholder benefit from the retention of a high-quality Board and executive team by remunerating directors and key executives fairly and appropriately with reference to relevant employment market conditions. To assist in achieving this objective, the Board, in assuming the responsibilities of assessing remuneration to employees, links the nature and amount of executive directors’ and officers’ remuneration to the Company and Company’s financial and operational performance.
The expected outcomes of the remuneration structure are:
| • | retention and motivation of key executives; |
| • | attraction of high-quality management to the Company; and |
| • | performance incentives that allow executives to share in the success of Kazia Therapeutics Limited. |
For a more comprehensive explanation of the Company’s remuneration framework and the remuneration received by directors and key executives in the current period, please refer to the section “Compensation” above.
There is no plan to provide retirement benefits to executive or non-executive directors, except for the Australian Government Superannuation Guarantee.
The Remuneration and Nomination Committee is responsible for determining and reviewing compensation arrangements for the directors themselves and the Chief Executive Officer and executive team.
43
D. Employees
As of the end of each of the last three fiscal years, the Company employed the following number of people—FTEs:
| Category of Activity | 2023 | 2022 | 2021 | |||||||||
| Research and Development |
6.8 | 6.8 | 4.6 | |||||||||
| Finance and Administration |
2.0 | 2.2 | 1.7 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
8.8 | 9.0 | 6.3 | |||||||||
|
|
|
|
|
|
|
|||||||
| Geographic Location | 2023 | 2022 | 2021 | |||||||||
| Australia |
4.8 | 5.0 | 5.3 | |||||||||
| United States |
4.0 | 4.0 | 1 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
8.8 | 9.0 | 6.3 | |||||||||
|
|
|
|
|
|
|
|||||||
E. Share Ownership
Directors’ and KMP interests in the shares and options of the Company for fiscal year 2023:
Shareholding
The number of shares in the company held during the financial year by each director and other members of Key Management Personnel of the consolidated entity, including their personally related parties, is set out below:
| Balance at the start of the year |
Purchased on market |
Share Purchase Plan |
Off market additions/ disposals |
Disposed* purposes only) |
Balance at the end of the year |
|||||||||||||||||||
| Ordinary shares |
||||||||||||||||||||||||
| B Carmine |
419,862 | 120,000 | 181,819 | 135,000 | 856,681 | |||||||||||||||||||
| S Coffey |
484,265 | 100,000 | 272,728 | 135,000 | 991,993 | |||||||||||||||||||
| I Ross |
1,075,001 | 175,000 | 272,728 | — | 1,522,729 | |||||||||||||||||||
| J Garner |
500,000 | 100,000 | 181,819 | — | (781,819 | ) | — | |||||||||||||||||
| K Hill |
320,000 | — | — | (270,000 | ) | (50,000 | ) | — | ||||||||||||||||
| J Friend |
— | — | — | — | — | — | ||||||||||||||||||
| K Krumeich |
— | — | — | — | — | — | ||||||||||||||||||
| E Davidson |
— | — | — | — | — | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| 2,799,128 | 495,000 | 909,094 | — | (831,819 | ) | 3,371,403 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
Each Director and Key Management Personnel owns less than 1% of shareholding.
Other transactions with key management personnel and their related parties
Iain Ross (from his company Gladstone Consultancy Partnership) was reimbursed for travel and accommodation expenses of $114,684.
James Garner was reimbursed travel and accommodation expenses of $149,382.
Kate Hill (from her company Sabio Solutions Pty Ltd) was reimbursed for expenses of $74.
John Friend was for reimbursed travel and accommodation expenses of $67,440.
Karen Krumeich was reimbursed for travel and accommodation expenses of $63,157.
44
Option holding
The number of options over ordinary shares in the company held during the financial year by each Director and other members of Key Management Personnel of Kazia, including their personally related parties, is set out below:
| Balance at the start of the year |
Granted as remuneration |
Forfeited | Disposed (for KMP reporting |
Balance at the end of the year |
||||||||||||||||
| Options over ordinary shares |
||||||||||||||||||||
| J Garner * |
4,500,000 | — | (1,450,000 | ) | (3,050,000 | ) | — | |||||||||||||
| K Hill ** |
200,000 | — | (100,000 | ) | (100,000 | ) | — | |||||||||||||
| I Ross |
400,000 | — | — | — | 400,000 | |||||||||||||||
| B Carmine |
400,000 | — | — | — | 400,000 | |||||||||||||||
| S Coffey |
400,000 | — | — | — | 400,000 | |||||||||||||||
| J Friend |
800,000 | 4,000,000 | — | — | 4,800,000 | |||||||||||||||
| K Krumeich |
800,000 | 2,000,000 | — | — | 2,800,000 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| 7,500,000 | 6,000,000 | (1,550,000 | ) | (3,150,000 | ) | 8,800,000 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| * | Disposal for KMP reporting purposes only. J Garner still holds 3,050,000 options |
| ** | Disposal for KMP reporting purposes only. K Hill still holds 100,000 options. |
Share-based compensation
There were no shares issued to Directors or other KMP as part of compensation during fiscal year 2023.
| Item 7. | Major Shareholders and Related Party Transactions |
A. Major shareholders
The following table present certain information regarding the beneficial ownership of our ordinary shares based on 236,349,374 ordinary shares outstanding at 17 October, 2023, by each person known by us to be the beneficial owner of more than 5% of our ordinary shares, as well as their holdings on 7 October 2022 and 30 September 2021.
| Ordinary shares beneficially owned | ||||||||||||||||||||||||
| 5% or greater shareholders |
17 October 2023 | 7 October 2022 | 30 September 2021 | |||||||||||||||||||||
| No. | % | No. | % | No. | % | |||||||||||||||||||
| Hyecorp and associates |
34,890,910 | 14.76 | % | 19,220,000 | 12.7 | % | 18,715,000 | 14.44 | % | |||||||||||||||
| Platinum Investment Management Limited |
23,083,022 | 9.77 | % | 7,084,856 | 5.47 | % | ||||||||||||||||||
| Quest Asset Partners Pty Ltd |
11,101,710 | 8.4 | % | |||||||||||||||||||||
At 17 October 2023, there were 8,321,373 of the Company’s ADSs outstanding, representing 83,213,736 ordinary shares (or 35.21%) of the then outstanding ordinary shares. At 17 October 2023, there were 34 registered holders of the Company’s ADSs.. On that same date, 1,692,131 ordinary shares were held directly by U.S. holders. As of 17 October 2023 the total holdings for Quest Asset Partners Pty Ltd did not meet the 5% beneficial ownership required for reporting. The holdings included in the above table are as notified to the ASX by that holder.
There have been no other significant shareholders in the last three fiscal years. All shareholders have the same voting rights.
B. Related party transactions
During fiscal year 2023, and up to the date of this report, we did not enter into any transactions or loans with any: (i) enterprises that directly or indirectly, through one or more intermediaries, control, are controlled by or are under common control with us; (ii) associates; (iii) individuals owning, directly or indirectly, an interest in our voting power that gives them significant influence over us, and close members of any such individual’s family; (iv) executive officers and close members of such individuals’ families; or (v) enterprises in which a substantial interest in our voting power is owned, directly or indirectly, by any person described in (iii) or (iv) or over which such person is able to exercise significant influence.
Transactions between related parties, when they occur, are on normal commercial terms and the conditions no more favorable than those available to other non-related parties.
C. Interests of Experts and Counsel
Not applicable.
45
| Item 8. | Financial Information |
A. Consolidated Statements and Other Financial Information
Consolidated financial statements are included in Item 18. “Financial Statements” commencing on page F-1.
Legal proceedings
Dividends
There were no dividends paid, recommended or declared during fiscal years 2023, 2022 or 2021.
B. Significant Changes
On 11 October 2023 Kazia announced that it submitted a formal application to the ASX to be removed from the official list of the ASX (Official List) in accordance with ASX Listing Rule 17.11 (Delist or the Delisting). This formal request follows the receipt of in-principle advice from the ASX in relation to the proposed Delisting, subject to the satisfaction of certain conditions. Kazia’s board of directors has ultimately determined that the costs, administrative burden and commercial disadvantages of remaining listed on ASX outweighed any benefits of a continued ASX listing. Following the Delisting, the Company will maintain its listing on the Nasdaq and the fully paid ordinary shares in the Company will no longer be quoted on the ASX.
| Item 9. | The Offer and Listing |
A. Offer and listing details
See Item 9C for more information.
B. Plan of Distribution
Not applicable.
C. Markets
Kazia’s principal listing exchange and the exchange upon which its ordinary shares are quoted is the Australian Securities Exchange (“ASX”). The trading symbol on ASX is ‘KZA’.
Kazia’s ordinary shares trade in the U.S. in the form of ADSs on the Nasdaq Capital Market. Each ADS represents 10 ordinary shares of Kazia. The trading symbol on the Nasdaq Capital Market is ‘KZIA’. Kazia has entered into a Deposit Agreement with The Bank of New York Mellon under which the Bank of New York, acting as depositary, issues the ADSs.
Kazia has submitted a formal application to the ASX to be removed from the official list of the ASX (Official List) in accordance with ASX Listing Rule 17.11 (Delist or the Delisting). This formal request follows the receipt of in-principle advice from the ASX in relation to the proposed Delisting, subject to the satisfaction of the conditions set out below.
The Board has ultimately determined that the costs, administrative burden and commercial disadvantages of remaining listed on ASX outweigh any benefits of a continued ASX listing.
Following the Delisting, the Company will maintain its listing on the Nasdaq and the fully paid ordinary shares in the Company (Shares) will no longer be quoted on the ASX. Further details regarding the reasons for and consequences of the Delisting are set out below.
Further details regarding the reasons for and consequences of the Delisting are set out below.
The Board considers that it is in the best interests of the Company and its shareholders for the Company to Delist for the following reasons:
| (a) | Costs: The continued listing of Kazia on ASX requires it to incur considerable corporate and administrative costs, including listing fees. Kazia is seeking to minimise its expenditure and would cease incurring such additional costs if it is removed from the official list of ASX. The Company considers the financial, administrative and compliance obligations and costs associated with managing an ASX listing and a Nasdaq listing, including the higher level of regulatory compliance costs associated with a dual listing, noting that there are a number of material differences between the Nasdaq listing rules and the ASX listing rules, unjustifiable and not in the best interests of the Company’s securityholders. |
| (b) | Access to larger equity markets with biotechnology focus: The board of Kazia believes that delisting from ASX whilst retaining the Nasdaq listing will enable Kazia to have access to a deeper market that better understands, and values, biotechnology businesses, thereby allowing it to more readily raise more capital on better terms, from a wider investor base. This access is pivotal in assisting Kazia to raise appropriate growth capital to pursue its plans. |
| (c) | Capital raisings: The delisting from ASX and retention of a primary listing on Nasdaq is expected to improve the Company’s access to its institutional investor base and other financing options in the USA that currently has the most active biotechnology ecosystem on a global basis. Being dual listed on ASX and Nasdaq is currently limiting fundraising options for Kazia. |
| (d) | Location of directors and management: With the exception of two non-executive directors, the Company’s remaining non-executive board member, Karen Krumeich, the Company’s Chief Financial Officer and Dr John Friend, the Company’s CEO, Managing Director and Interim Chairman, are now based in the USA, reflecting the Company’s focus on international rather than domestic markets. |
| (e) | Arrangements for sale of Shares: The Company will notify shareholders whose securities are held on the Company’s Australian principal share register of the time and date at which the Company will be removed from the Official List has informed those shareholders that if they wish to sell their securities on ASX they will need to do so before that date and if they don’t do so, they will only be able to sell their securities on-market on Nasdaq. The Company will also inform those shareholders generally what they will need to do if they wish to sell their securities on Nasdaq. No change will occur to the quotation and trading of the Company’s securities on Nasdaq because of the removal from the Official List. |
46
Consequences for the Company and its shareholders
The consequences for the Company and its shareholders if the Company is removed from the Official List of the ASX are set out below:
| (a) | shareholders will no longer be able to trade their Shares on the ASX; |
| (b) | the Company’s Shares will only be capable of being traded on Nasdaq in the form of ADSs, which will require shareholders to transfer their Shares to ADSs to trade on Nasdaq and engage a suitably qualified Australian broker or a US based broker who is able to trade on Nasdaq, or by off-market, private transactions, which will require shareholders to identify and agree terms with potential purchasers of Shares; |
| (c) | following Delisting, the Company will not be subject to the ASX Listing Rules. In particular, the following ASX Listing Rule requirements will no longer apply: |
| (i) | continuous disclosure and other periodic reporting requirements (although the Company’s reporting requirements (including continuous disclosure – see below) will still be governed by the Corporations Act 2001 (Cth) (Corporations Act), the applicable rules of Nasdaq and its reporting obligations under US securities laws, as described in section (e) below); |
| (ii) | disclosure of certain information under the ASX Listing Rules (including changes of capital or information related to directors and the auditor of the Company); |
| (iii) | restrictions on the issue of new capital (such as the inability of the Company to issue in excess of 15% of its capital in any 12-month period without shareholder approval) and certain restrictions on transactions with related parties (although these will still be governed by the Corporations Act and the applicable rules of Nasdaq); |
| (iv) | requirements relating to significant changes to the Company’s activities; and |
| (v) | the requirement to report against the ASX Corporate Governance Principles and Recommendations; |
| (d) | if, following removal from the Official List, the Company has 100 or more shareholders, it will be an “unlisted disclosing entity” under the Corporations Act. As an unlisted disclosing entity, the Company will still be required to give continuous disclosure of material matters in accordance with the Corporations Act by filing notices with ASIC under section 675 of the Corporations Act and the Company will still be required to lodge annual audited and half-yearly financial statements in accordance with the requirements of the Corporations Act. However, if the Company ceases to be an unlisted disclosing entity there will be no ongoing requirement for the Company to give continuous disclosure of material matters under section 675, or lodge half-yearly financial statements reviewed by an auditor, but as a public company it will continue to be required to lodge annual audited financial statements. In addition, the Company notes that it will also be required to fulfill its public reporting obligations under US securities laws as a US public company and, while its securities are listed on Nasdaq, its disclosure obligations under applicable Nasdaq listing rules; |
| (e) | the related party transaction provisions of the Corporations Act will continue to apply to the Company as an Australian public company; and |
| (f) | directors will continue to be subject to directors’ duties under the Corporations Act, including to act in good faith and in the best interests of the Company. |
Some shareholders may consider that the reduction of obligations associated with an ASX listing is a disadvantage, including minority shareholders. While there will be differences in the regulatory regimes before and after the Delisting, minority shareholders will continue to benefit from the protections in the Corporations Act, such as in relation to the alteration of shareholder rights, financial reporting obligations and holding annual meetings of shareholders. Shareholders will also have protections because of US securities laws and applicable Nasdaq listing rules.
Conditions
ASX’s in-principle decision to approve the Delisting is subject to the Company’s compliance with the following conditions imposed by ASX under Listing Rule 17.11 and Guidance Note 33:
| (a) | The Company sends written or electronic communications to all shareholders whose Shares are held on the Company’s Australian principal share register, in form and substance satisfactory to ASX (Notice), setting out: |
| (i) | the nominated time and date at which the entity will be removed from the ASX and that: |
| (A) | if they wish to sell their Shares on ASX, they will need to do so before then; and |
| (B) | if they don’t, thereafter they will only be able to sell the underlying securities on-market on Nasdaq in the form of ADSs; and |
| (ii) | generally what they need to do if they wish to sell their securities on Nasdaq. |
| (b) | The removal shall not take place any earlier than one month after the date the information in the Notice has been sent to shareholders. |
| (c) | The Company releases the full terms of this decision to the market upon making a formal application to ASX to remove the Company from the official list of ASX. Importantly, Kazia shareholder approval is not required for the Delisting. |
Arrangements to enable shareholders to sell their Shares or convert them to ADSs
In relation to the Delisting, the Company has established a voluntary ADS conversion facility pursuant to which shareholders may elect to convert their Shares to ADSs. The company will bear the fees associated with the ADS conversion. Kazia will release additional documents that provide more information about the Delisting.
47
Remedies available to shareholders
If a shareholder of the Company considers the removal from the Official List to be contrary to the interests of the shareholders of the Company as a whole or oppressive to, unfairly prejudicial to, or unfairly discriminatory against a shareholder or shareholders, it may apply to the court for an order under Part 2F.1 of the Corporations Act. Under section 233 of the Corporations Act, the court can make any order that it considers appropriate in relation to the Company, including an order that the Company be wound up or an order regulating the conduct of the Company’s affairs in the future.
If a shareholder of the Company considers that the removal form the Official List involves “unacceptable circumstances”, it may apply to the Takeovers Panel for a declaration of unacceptable circumstances and other orders under Part 6.10 Division 2 Subdivision B of the Corporations Act (refer also to Guidance Note 1: Unacceptable Circumstances issued by the Takeovers Panel). Under section 657D of the Corporations Act, if the Takeovers Panel has declared circumstances to be unacceptable, it may make any order that it thinks appropriate to protect the rights or interests of any person or group of persons, where the Takeovers Panel is satisfied that those rights or interests are being affected, or will be or are likely to be affected, by the circumstances.
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
F. Expenses of the issue
Not applicable.
| Item 10. | Additional Information |
A. Share Capital
Not applicable.
B. Memorandum and Articles of Association
Our Constitution is similar in nature to the bylaws of a U.S. corporation. It does not provide for or prescribe any specific objectives or purposes of Kazia. Our Constitution is subject to the terms of the ASX Listing Rules and the Corporations Act. It may be amended or repealed and replaced by special resolution of shareholders, passed by at least 75% of the votes cast by shareholders entitled to vote on the resolution.
48
Under Australian law, a company has the legal capacity and powers of an individual both within and outside Australia. The material provisions of our Constitution are summarized below. This summary is not intended to be complete nor to constitute a definitive statement of the rights and liabilities of our shareholders, and is qualified in its entirety by reference to the complete text of our Constitution, a copy of which is incorporated by reference as Exhibit 1.1 to this Annual Report.
Interested Directors
Subject to the Corporations Act and the ASX Listing Rules, neither a director nor that director’s alternate may vote in respect of any contract or arrangement in which the director has, directly or indirectly, any material interest according to our Constitution. However, that director may execute or otherwise act in respect of that contract or arrangement notwithstanding any material personal interest. Unless a relevant exception applies, the Corporations Act requires our directors to provide disclosure of any material personal interest, and prohibits directors from voting on matters in which they have a material personal interest or being present while such matter is being considered at the board meeting. In addition, the Corporations Act and the ASX Listing Rules require shareholder approval of any provision of related party benefits to our directors.
Directors compensation
Our directors are paid remuneration for their services as directors (but excluding any remuneration payable to a director under any executive services contract with us or one of our related bodies corporate) which is determined in a general meeting of shareholders. The aggregate, fixed sum for directors’ remuneration is to be divided among the directors in such proportion as the directors themselves agree and in accordance with our Constitution. The fixed sum remuneration for directors may not be increased except at a general meeting of shareholders and the particulars of the proposed increase are required to have been provided to shareholders in the notice convening the meeting. In addition, executive directors may be paid remuneration as determined by the directors from time to time and, subject to the ASX Listing Rules, including as a salary, commission or participation in profits and/or by the issue of shares, options to acquire shares or performance rights or other incentives (or a combination of any of these methods of remuneration).
Fees payable to our non-executive directors must be by way of a fixed sum and not by way of a commission on or a percentage of profits. Remuneration paid to our executive directors must also not include a commission or percentage of operating revenue.
Pursuant to our Constitution, if, at our board’s request, any director performs extra services or makes special exertions, Kazia may remunerate that director by paying for those services and exertions.
In addition to other remuneration provided in our Constitution, all of our directors are entitled to be paid by us for all other travelling, accommodation and other expenses incurred by the directors in attending and returning from general meetings, board meetings, committee meetings or otherwise in connection with our business.
Borrowing powers exercisable by Directors
Pursuant to our Constitution, the management and control of our business affairs are vested in our board of directors. Our board of directors has the power to raise or borrow money or obtain other financial accommodation for Company purposes, and may grant security for the repayment of that sum or sums or the payment, performance or fulfilment of any debts, liabilities, contracts or obligations incurred or undertaken by the Company in any manner and on any terms and conditions as our board thinks fit.
Retirement of Directors
Pursuant to our Constitution and the ASX Listing Rules, at least one director, other than the Managing Director, must retire from office at every annual general meeting unless there has been an election of directors earlier that year. A director, other than the director who is the Managing Director, must retire from office at the conclusion of three years or following the third annual general meeting after which the director was elected, whichever is longer. If no director is required to retire at an annual general meeting, then the director to retire will be the director who has been longest in office since last being elected. Retired directors are eligible for a re-election to the board of directors unless disqualified from acting as a director under the Corporations Act or our Constitution.
Rights and restrictions on classes of shares
The rights attaching to our ordinary shares are detailed in our Constitution. Our Constitution provides that our directors may issue shares with any preferential, deferred or special rights, privileges or conditions or with any restriction (whether in relation to dividends, voting, return of share capital or otherwise) as our board of directors may determine. Subject to any approval which is required from our shareholders under the Corporations Act and the ASX Listing Rules, we may issue further shares on such terms and conditions as our board of directors resolves.
49
Dividend rights
Our board of directors may from time to time determine to pay and declare dividends to shareholders. All dividends unclaimed for one year after having been declared may be invested or otherwise made use of by our board of directors for our benefit until claimed or otherwise disposed of in accordance with our Constitution.
Voting rights
Under our Constitution, and subject to any voting exclusions imposed under the ASX Listing Rules (which typically exclude parties from voting on resolutions in which they have an interest), the rights and restrictions attaching to a class of shares, each shareholder has one vote on a show of hands at a meeting of the shareholders unless a poll is demanded under the Constitution or the Corporations Act. On a poll vote, each shareholder shall have one vote for each fully paid share and a fractional vote for each share held by that shareholder that is not fully paid, such fraction being equivalent to the proportion of the amount that has been paid to such date on that share. Shareholders may vote in person or by proxy, attorney or representative. Under Australian law, shareholders of a public company are generally not permitted to approve corporate matters by written consent. Our Constitution does not provide for cumulative voting. Note that ADS holders may not directly vote at a meeting of the shareholders but may instruct the depositary to vote the number of deposited ordinary shares their ADSs represent.
Right to share in our profits
Pursuant to our Constitution, our shareholders are entitled to participate in our profits only by payment of dividends. Our board of directors may from time to time determine to pay dividends to the shareholders; however, no dividend is payable except in accordance with the thresholds set out in the Corporations Act.
Rights to share in the surplus in the event of winding up
Our Constitution provides for the right of shareholders to participate in a surplus in the event of our winding up, subject to the rights attaching to a class of shares, the Constitution, the Corporations Act and the ASX Listing Rules.
No redemption provision for ordinary shares
There are no redemption provisions in our Constitution in relation to ordinary shares. Under our Constitution, any preference shares may be issued on the terms that they are, or may at the option of Kazia or the holder be, liable to be redeemed or converted into ordinary shares.
Variation or cancellation of share rights
Subject to the Corporations Act, the ASX Listing Rules and the terms of issue of shares of that class, the rights attached to shares in a class of shares may only be varied or cancelled by either:
| • | a special resolution passed at a meeting of members holding shares in that class; or |
| • | the written consent of members with at least 75% of the shares in that class. |
Directors may make calls
Our Constitution provides that our directors may make calls on a shareholder for all monies unpaid on shares held by that shareholder, other than monies payable at fixed times under the conditions of allotment.
General Meetings of Shareholders
General meetings of shareholders may be called by our board of directors. Except as permitted under the Corporations Act, shareholders may not convene a meeting. The Corporations Act requires the directors to call and arrange to hold a general meeting on the request of shareholders with at least 5% of the votes that may be cast at a general meeting. Notice of the proposed meeting of our shareholders is required at least 28 days prior to such meeting under the Corporations Act.
50
Foreign Ownership Regulation
Our Constitution does not impose specific limitations on the rights of non-residents to own securities. However, acquisitions and proposed acquisitions of securities in Australian companies may be subject to review and approval by the Australian Federal Treasurer under the Foreign Acquisitions and Takeovers Act 1975 (Cth) (the “Foreign Takeovers Act”), which generally applies to acquisitions or proposed acquisitions:
| • | by a foreign person (as defined in the Foreign Takeovers Act) or associated foreign persons that would result in such persons having an interest in 20% or more of the issued shares of, or control of 20% or more of the voting power in, an Australian company; and |
| • | by non-associated foreign persons that would result in such foreign persons having an aggregate interest in 40% or more of the issued shares of, or control of 40% or more of the voting power in, an Australian company, where the Australian company is valued above the monetary threshold prescribed by Foreign Takeovers Act. |
However, in general terms, no such review or approval under the Foreign Takeovers Act is required if the foreign acquirer is a U.S. entity or an entity from certain other countries and the value of the target is less than A$1,250 million, unless the company operates in certain sensitive industries. Exemptions do not apply to investments by foreign governments and their associated entities.
The Australian Federal Treasurer may prevent a proposed acquisition in the above categories or impose conditions on such acquisition if the Treasurer is satisfied that the acquisition would be contrary to the national interest. If a foreign person acquires shares or an interest in shares in an Australian company in contravention of the Foreign Takeovers Act, the Australian Federal Treasurer may make a range of orders including an order the divestiture of such person’s shares or interest in shares in that Australian company.
Ownership Threshold
There are no specific provisions in our Constitution that require a shareholder to disclose ownership above a certain threshold. The Corporations Act, however, requires a shareholder to notify us and the ASX once it, together with its associates, acquires a 5% interest in our ordinary shares, at which point the shareholder will be considered to be a “substantial” shareholder. Further, once a shareholder owns (alone or together with associates) a 5% interest in us, such shareholder must notify us and the ASX of any increase or decrease of 1% or more in its holding of our ordinary shares, and must also notify us and the ASX on its ceasing to be a “substantial” shareholder. As we are also a U.S. public company, our shareholders are also subject to disclosure requirements under U.S. securities laws.
Issues of Shares and Change in Capital
Subject to our Constitution, the Corporations Act, the ASX Listing Rules and any other applicable law, we may at any time issue shares and give any person a call or option over any shares on any terms, with preferential, deferred or special rights, privileges or conditions or with any restrictions and for the consideration and other terms that the directors determine.
Subject to the requirements of our Constitution, the Corporations Act, the ASX Listing Rules and any other applicable law, including relevant shareholder approvals, we may consolidate or divide our share capital into a larger or smaller number by resolution, reduce our share capital in any manner (provided that the reduction is fair and reasonable to our shareholders as a whole, does not materially prejudice our ability to pay creditors and obtains the necessary shareholder approval) or buy back our ordinary shares whether under an equal access buy-back or on a selective basis.
Change of Control
Takeovers of listed Australian public companies, such as Kazia, are regulated by the Corporations Act, which prohibits the acquisition of a “relevant interest” in issued voting shares in a listed company if the acquisition will lead to that person’s or someone else’s “voting power” (being the person’s relevant interests plus those of its associates) in Kazia’s issued shares increasing from 20% or below to more than 20% or increasing from a starting point that is above 20% and below 90% (“Takeovers Prohibition”), subject to a range of exceptions.
Generally, a person will have a relevant interest in securities if the person:
| • | is the holder of the securities or the holder of an ADS over the shares; |
| • | has power to exercise, or control the exercise of, a right to vote attached to the securities; or |
| • | has the power to dispose of, or control the exercise of a power to dispose of, the securities, including any indirect or direct power or control. |
51
If, at a particular time:
| • | a person has a relevant interest in issued securities; and |
| • | the person has: |
| • | entered or enters into an agreement with another person with respect to the securities; |
| • | given or gives another person an enforceable right, or has been or is given an enforceable right by another person, in relation to the securities (whether the right is enforceable presently or in the future and whether or not on the fulfillment of a condition); or |
| • | granted or grants an option to, or has been or is granted an option by, another person with respect to the securities; and |
| • | the other person would have a relevant interest in the securities if the agreement were performed, the right enforced or the option exercised. |
then the other person is taken to already have a relevant interest in the securities.
There are a number of exceptions to the Takeovers Prohibition on acquiring a relevant interest in issued voting shares above 20%. In general terms, some of the more significant exceptions include:
| • | when the acquisition results from the acceptance of an offer under a formal takeover bid; |
| • | when the acquisition is conducted on market by or on behalf of the bidder during the bid period for a full takeover bid that is unconditional or only conditional on certain ‘prescribed’ matters set out in the Corporations Act; |
| • | when the acquisition has been previously approved by shareholders of Kazia by resolution passed at general meeting; |
| • | an acquisition by a person if, throughout the six months before the acquisition, that person or any other person has had voting power in Kazia of at least 19% and, as a result of the acquisition, none of the relevant persons would have voting power in Kazia more than three percentage points higher than they had six months before the acquisition; |
| • | when the acquisition results from the issue of securities under a pro rata rights issue; |
| • | when the acquisition results from the issue of securities under a dividend reinvestment scheme or bonus share plan; |
| • | when the acquisition results from the issue of securities under certain underwriting arrangements; |
| • | when the acquisition results from the issue of securities through a will or through operation of law; |
| • | an acquisition that arises through the acquisition of a relevant interest in another listed company which is listed on a prescribed financial market or a foreign market approved by ASIC; |
| • | an acquisition arising from an auction of forfeited shares conducted on-market; or |
| • | an acquisition arising through a compromise, arrangement, liquidation or buy-back. |
Breaches of the takeovers provisions of the Corporations Act are criminal offenses. The Australian Securities and Investments Commission, or ASIC, and the Australian Takeover Panel have a wide range of powers relating to breaches of takeover provisions or other circumstances deemed to be unacceptable (whether or not they involve a breach of the takeover provisions), including the ability to make orders canceling contracts, freezing transfers of, and rights attached to, securities, and forcing a party to dispose of securities. There are certain defenses to breaches of the takeover provisions provided in the Corporations Act.
Access to and Inspection of Documents
Inspection of our records is governed by the Corporations Act. Any member of the public has the right to inspect or obtain copies of our registers on the payment of a prescribed fee. Shareholders are not required to pay a fee for inspection of our registers or minute books of the meetings of shareholders. Other corporate records, including minutes of directors’ meetings, financial records and other documents, are not open for inspection by shareholders. Where a shareholder is acting in good faith and an inspection is deemed to be made for a proper purpose, a shareholder may apply to the court to make an order for inspection of our books.
52
C. Material contracts
License Agreement with Genentech Inc.
In October 2016, the Company entered into a worldwide licensing agreement with Genentech, a member of the Roche Group, to develop and commercialize GDC-0084, a small molecule inhibitor of the phosphoinositide-3-kinase (PI3K) pathway. Under the terms of the agreement, the Company paid Genentech an upfront payment of US$5 million. In addition, the terms of the agreement call for performance-related consideration linked to regulatory and commercial outcomes and royalty payments in-line with industry benchmarks.
Acquisition of Glioblast Pty Ltd—Share Sale Agreement with Kilinwata Investments Pty. Ltd., Mi Ok Chong and Paul Hopper
In October 2016, the Company acquired 100% of the issued shares of Glioblast Pty Ltd, a privately-held, neuro-oncology-focused Australian biotechnology company. The transaction included an upfront payment of A$2.1 million, comprising A$600,000 in cash and ordinary fully-paid shares valued at A$1.5 million, with the actual number of shares determined on the basis of the volume-weighted average price of the Company’s shares on the ASX in the seven days prior to this announcement. The shareholders of Glioblast will be eligible for further payments in cash or equity on the achievement of performance related milestones. The first two of these milestones provide for the issue of ordinary fully-paid shares valued at
A$1.25 million respectively on commencement and successful completion of a phase II clinical trial of GDC-0084, with the actual number of shares determined on the basis of the volume-weighted average price of the Company’s shares on the ASX in the seven days prior to satisfaction of the relevant milestone being announced. A further two milestones may trigger payments in cash or equity at the Company’s sole discretion. Any issue of equity in the Company will be subject to a minimum six-month escrow period.
At the date of this report, one milestone has lapsed and two have been settled in shares. The remaining milestone relates to the successful completion of a phase II trial in GDC-0084.
Convertible Note Deed Poll and Amendment
On 4 December 2014, we and Triaxial signed a Convertible Note Deed Poll (‘Deed’) which superseded a Loan Agreement. The Deed extinguishes the liability created by the Loan Agreement, which previously allowed for a cash settlement and now allows Triaxial to convert their debt into ordinary shares, provided that the Company achieves defined milestones established in the schedule of the Deed. Accordingly, the convertible note has been reclassified as an equity instrument rather than debt instrument.
During the fiscal 2017, the Company reached two milestones triggering the conversion of a portion of its convertible note as follows;
| • | On 11 August 2016, the Company announced the submission of an IND application. On 10 September 2016, the Company received a letter from the FDA advising the study may proceed. This triggered the conversion of Convertible Notes with a face value of A$500,000 into 20,000,000 ordinary shares. |
| • | On 31 October 2016, the Company announced it had licensed a phase II ready molecule. This triggered the conversion of Convertible Notes with a face value of A$400,000 into 16,000,000 ordinary shares. |
During fiscal 2018, A$136,000 of the Convertible Notes was extinguished. The remaining Convertible Notes with a face value of A$464,000 at year end may be converted into 1,856,000 ordinary shares of the Company (post share consolidation).
On 21 April 2022 the completion of the phase II study of paxalisib in glioblastoma (NCT03522298) was announced and on 5 May 2022 the remaining portion of the convertible note was extinguished and converted to 1,855,357 ordinary shares.
Clinical Trial Collaboration and Supply Agreement with the Global Coalition for Adaptive Research
In October 2020, the Company entered into a Clinical Trial Collaboration and Supply Agreement with the Global Coalition for Adaptive Research (GCAR), a US-based 501(C)(3) non-profit organisation. The agreement relates to the inclusion of Kazia’s investigational new drug, paxalisib (GDC-0084) in a phase II/III adaptive clinical trial known as GBM AGILE (NCT03970447), which is expected to serve as the pivotal study for registration of paxalisib in glioblastoma by the US Food and Drug Administration (FDA). Under the terms of the agreement, the Company paid GCAR an upfront payment of US$5 million on execution, and will make further payments to GCAR throughout the course of the study, as defined milestones are met, with the total cost of the study capped at a pre-defined amount under the terms of the agreement. GCAR will serve as the sponsor of GBM AGILE and the company will supply investigational product for conduct of the study at its sole expense. It is expected that paxalisib’s participation in GBM AGILE will be approximately three to four years in duration.
Kazia announced on 1 August 2022 that the company had been advised by GCAR that the first stage of the paxalisib arm had completed recruitment. The treatment arm did not meet pre-defined criteria for continuing to a second stage, and patients enrolled in the first stage of the paxalisib arm will therefore continue on treatment as per protocol, and in follow-up, until completion of the final analysis, which we anticipate receiving in 2H CY2023. Depending on the results of the study, Kazia may use such data to support submission of a new drug application for marketing authorisation to the FDA.
53
License Agreement with Vivesto AB ( formerly Oasmia Pharmaceutical AB)
In March 2021, the Company entered into an exclusive worldwide license agreement with Vivesto AB, (formerly Oasmia Pharmaceutical AB), an innovation-focused specialty pharmaceutical company, for Cantrixil (TRX-E-002-1), a clinical-stage, first-in-class drug candidate under development for the treatment of ovarian cancer. Under the terms of the agreement, Vivesto assumed worldwide exclusive rights to develop and commercialize Cantrixil for all indications, with an initial focus on ovarian cancer. During fiscal 2021, Vivesto made an up-front payment of US$4 million, with contingent milestones of up to US$42 million and double-digit royalties on commercial sales.
License Agreement with Simcere Pharmaceutical Group Ltd
In March 2021, the Company entered into a licensing agreement with Simcere Pharmaceutical Group Ltd (“Simcere”) to develop and commercialize the Company’s investigational new drug, paxalisib, in Greater China. Under the terms of the agreement, Simcere assumed responsibility for the development, registration and commercialization of paxalisib in Greater China (a territory which includes Mainland China, Hong Kong, Macau and Taiwan). The Company received an upfront payment of US$11 million comprising US$7 million in cash and a US$4 million equity investment, priced at a 20% premium to recent trading. The Company will also receive contingent milestone payments of up to US$281 million for glioblastoma, with further milestones payable for indications beyond glioblastoma. Simcere will additionally pay mid-teen percentage royalties on commercial sales.
License Agreement with Evotec SE
In April 2021, the Company entered into a worldwide exclusive licensing agreement with Evotec SE, a leading European drug discovery and development company, for EVT801, a small-molecule, first-in-class oncology drug candidate. Under the terms of the agreement, Evotec has granted Kazia an exclusive license to develop, manufacture, and commercialize EVT801 in all territories and indications. The Company paid an up-front amount of €1 million (approximately A$1.6 million). In addition, the terms of the agreement call for performance-related consideration linked to regulatory and commercial outcomes up to a maximum of €308 million (approximately A$480 million) and tiered single-digit royalty payments.
D. Exchange controls
Australia has largely abolished exchange controls on investment transactions. The Australian dollar is freely convertible into U.S. dollars. In addition, (other than as specified under “taxation” below and certain restrictions imposed under Australian law in relation to dealings with the assets of and transactions with, designated countries, entities and persons specified by the Australian Government Department of Foreign Affairs and Trade from time to time, including, persons connected with terrorism) there are currently no specific rules or limitations regarding the export from Australia of profits, dividends, capital, or similar funds belonging to foreign investors, except that certain payments to non-residents must be reported to the Australian Transaction Reports and Analysis Centre, which monitors such transactions.
Under Australian law, foreign persons may require the approval from the Australian Treasurer to acquire more than a limited percentage of the interests in an Australian company. These limitations are set forth in the Australian Foreign Acquisitions and Takeovers Act 1975 (Cth) (the “Foreign Takeovers Act”).
Under the Foreign Takeovers Act, in general terms, the approval of the Australian Treasurer is required for any foreign person (either alone or together with any one or more of its associates) to acquire an interest of 20% or more of the voting power (including potential voting power) or issued shares (including rights to issued shares) (“Substantial Interest”) in an Australian entity, whose total issued securities value or total asset value (whichever is higher) exceed A$310 million. If the person is a U.S. investor, the A$310 million threshold applies only for investments in prescribed sensitive sectors, otherwise a threshold of A$1,339 million rather than A$310 million applies. Certain types of investments, such as all direct investment by foreign governments and their related entities regardless of the value of the investment, including proposals to establish new businesses, must be notified to the Australian Treasurer. Where an acquisition is made in breach of these requirements, the Australian Treasurer may make a range of orders including an order requiring the acquirer to dispose of its Substantial Interest within a specified period of time.
In addition, if a foreign person acquires a Substantial Interest in Kazia in circumstances where the above monetary thresholds would be exceeded and as a result the total holdings of all foreign persons and their associates exceeds 40% in aggregate without the approval of the Australian Treasurer, then the Australian Treasurer may make a range of orders including an order requiring the acquirer to dispose of its Substantial Interest within a specified period of time. The same rule applies if the total holdings of all foreign persons and their associates already exceeds 40% and a foreign person (or its associate) acquires any further interests, including in the course of trading in the secondary market of the ADSs.
54
Under the current Australian foreign investment policy, the Australian Treasurer has the power to make such an order in relation to an acquisition that contravenes the Foreign Takeovers Act where the level of foreign ownership exceeds 40% in the ordinary course of trading, if the Australian Treasurer is satisfied that the acquisition is contrary to the national interest. The Foreign Takeovers Act allows foreign persons to seek prior approval of acquisitions of Kazia interests which could otherwise result in the Australian Treasurer making an order requiring the foreign person to dispose of any Substantial Interest.
If a foreign person holds more than 20% of the interests of Kazia or if the level of aggregate foreign ownership of Kazia exceeds 40% at any time, Kazia would be considered a foreign person under the Foreign Takeovers Act. In such event, Kazia would be required to obtain the approval of the Australian Treasurer for Kazia, together with its associates, to acquire: (i) more than 20% of an Australian company or business with a total issued securities value or total asset value (whichever is higher) totaling over A$289 million; or (ii) any direct or indirect ownership interest in Australian land. However, as mentioned above, in general terms, proposals by U.S. investors for investment in non-sensitive sectors do not require notification to the Australian Treasurer or the Australian Treasurer’s approval unless the value of the target Australian company or business exceeds A$1,250 million.
The percentage of foreign ownership of Kazia would also be included in determining the foreign ownership of any Australian company or business in which it may choose to invest. Kazia has no current plans for any such acquisitions. The Company’s Constitution does not impose specific limitations on a non-resident’s right to hold or vote the Company’s securities.
E. Taxation
U.S. Taxation
This section describes certain material U.S. federal income tax consequences to a U.S. holder (as defined below) of owning ordinary shares or ADSs. It applies only to ordinary shares or ADSs that are held as capital assets for tax purposes. This section does not apply to a holder of ordinary shares or ADSs that is a member of a class of holders subject to special rules, including a financial institution, a dealer or trader in securities, a regulated investment company, a real estate investment trust, a grantor trust, a U.S. expatriate, a tax-exempt organization, an insurance company, a person liable for alternative minimum tax, a person who actually or constructively owns 10% or more of the stock of the Company, a person that holds ordinary shares or ADSs as part of a straddle or a hedging or conversion transaction, a person that purchases or sells ordinary shares or ADSs as part of a wash sale for tax purposes, or a person whose functional currency is not the U.S. dollar. Further, this description does not address state, local, non-U.S, or other tax laws, nor does it address the 3.8% U.S. federal Medicare tax on net investment income, the alternative minimum tax or the U.S. federal gift and estate tax consequences of owning and disposing of ordinary shares or ADSs.
For purposes of this description, a “U.S. holder” is a beneficial owner of ordinary shares or ADSs who holds such ordinary shares or ADSs as capital assets within the meaning of the Code and is, for U.S. federal income tax purposes: (i) an individual citizen or resident of the United States; (ii) a corporation created or organized in or under the laws of the United States or any state thereof, including the District of Columbia; (iii) an estate the income of which is subject to U.S. federal income taxation regardless of its source; or (iv) a trust that either (a) is subject to the supervision of a court within the United States and has one or more U.S. persons with authority to control all substantial decisions or (b) has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person.
If a partnership holds the ordinary shares or ADSs, the U.S. federal income tax treatment of a partner generally will depend on the status of the partner and the tax treatment of the partnership. A partner in a partnership holding the ordinary shares or ADSs should consult its tax advisor with regard to the U.S. federal income tax treatment of an investment in the ordinary shares or ADSs.
The discussion is based on the Code, administrative pronouncements, judicial decisions, and final, temporary and proposed Treasury Regulations, all as of the date hereof, changes to any of which may affect the tax consequences described herein — possibly with retroactive effect. There can be no assurances that the Internal Revenue Service (the “IRS”) will not take a contrary or different position concerning the tax consequences of the ownership and disposition of our ordinary shares or ADSs or that such a position would not be sustained by a court. We have not obtained, nor do we intend to obtain, a ruling with respect to the U.S. federal income tax considerations relating to the purchase, ownership or disposition of our ordinary shares or ADSs. Holders should consult their tax advisers concerning the U.S. federal, state, local and non-U.S. tax consequences of owning and disposing of our ordinary shares or ADSs in their particular circumstances.
This section is in part based on the representations of the Depositary and the assumption that each obligation in the deposit agreement and any related agreement will be performed in accordance with its terms. In general, for U.S. federal income tax purposes, a holder of ADSs will be treated as the owner of the ordinary shares represented by those ADSs. Exchanges of ordinary shares for ADSs, and ADSs for ordinary shares generally will not be subject to U.S. federal income tax.
55
Distributions
Subject to the Passive Foreign Investment Company (“PFIC”) rules discussed below, U.S. holders generally will include as dividend income the U.S. dollar value of the gross amount of any distributions of cash or property (without deduction for any withholding tax), other than certain pro rata distributions of ordinary shares, with respect to ordinary shares or ADSs to the extent the distributions are made from our current or accumulated earnings and profits, as determined for U.S. federal income tax purposes. A U.S. holder will include the dividend income on the day actually or constructively received (i) by the holder, in the case of ordinary shares, or (ii) by the depositary, in the case of ADSs. We do not intend to maintain calculations of earnings and profits, as determined for U.S. federal income tax purposes. Consequently, any distributions generally will be treated as dividend income.
Dividends paid to a non-corporate U.S. holder on shares or ADSs will generally be taxable at the preferential rates applicable to long-term capital gains provided (a) that certain holding period requirements are satisfied, (b) (i) the U.S.-Australia income tax treaty (“the Treaty”) is a qualified treaty and we are eligible for benefits under the Treaty or (ii) our ordinary shares or ADSs are readily tradable on a U.S. securities market, and (c) provided that we were not, in the taxable year prior to the year in which the dividend was paid, and are not, in the taxable year in which the dividend is paid, a PFIC. The Treaty has been approved for the purposes of the qualified dividend rules and the ADSs are listed on Nasdaq. If the Company is a PFIC, any dividends paid to a noncorporate U.S. holder will not qualify for the preferential tax rates ordinarily applicable to “qualified dividends.” In the case of a corporate U.S. holder, dividends on shares and ADSs are taxed as ordinary income and will not be eligible for the dividends received deduction generally allowed to U.S. corporations in respect of dividends received from other U.S. corporations.
The amount of any cash distribution paid in any foreign currency will be equal to the U.S. dollar value of such currency, calculated by reference to the spot rate in effect on the date such distribution is received by the U.S. holder or, in the case of ADSs, by the Depositary, regardless of whether and when the foreign currency is in fact converted into U.S. dollars. If the foreign currency is converted into U.S. dollars on the date received, the U.S. holder generally should not recognize foreign currency gain or loss on such conversion. If the foreign currency is not converted into U.S. dollars on the date received, the U.S. holder will have a basis in the foreign currency equal to its U.S. dollar value on the date received, and generally will recognize foreign currency gain or loss on a subsequent conversion or other disposal of such currency. Such foreign currency gain or loss generally will be treated as U.S. source ordinary income or loss for foreign tax credit limitation purposes.
Dividends will be income from sources outside the United States, and generally will be “passive category” income or, for certain taxpayers, “general category” income, which are treated separately from each other for the purpose of computing the foreign tax credit allowable to a U.S. holder. The availability of the foreign tax credit and the application of the limitations on its availability are fact specific and are subject to complex rules. In general, a taxpayer’s ability to use foreign tax credits may be limited and is dependent on the particular circumstances. U.S. holders should consult their own tax advisors with respect to these matters.
Sale, Exchange or other Disposition of Ordinary Shares or ADSs
Subject to the PFIC rules discussed below, a U.S. holder who sells or otherwise disposes of ordinary shares or ADSs will recognize a capital gain or loss for U.S. federal income tax purposes equal to the difference between the U.S. dollar value of the amount realized and the holder’s tax basis, determined in U.S. dollars, in those ordinary shares or ADSs. The gain or loss will generally be income or loss from sources within the United States for foreign tax credit limitation purposes. The capital gain of a non-corporate U.S. holder is generally taxed at preferential rates where the holder has a holding period greater than 12 months in the shares or ADSs sold. There are limitations on the deductibility of capital losses.
The U.S. dollar value of any foreign currency received upon a sale or other disposition of ordinary shares or ADSs will be calculated by reference to the spot rate in effect on the date of sale or other disposal (or, in the case of a cash basis or electing accrual basis taxpayer, at the spot rate of exchange on the settlement date). A U.S. holder will have a tax basis in the foreign currency received equal to that U.S. dollar amount, and generally will recognize foreign currency gain or loss on a subsequent conversion or other disposal of the foreign currency. This foreign currency gain or loss generally will be treated as U.S. source ordinary income or loss for foreign tax credit limitation purposes. If such foreign currency is converted into U.S. dollars on the date received by the U.S. holder, a cash basis or electing accrual basis U.S. holder should not recognize any gain or loss on such conversion.
Passive Foreign Investment Company
A non-U.S. corporation will be a PFIC for U.S. federal income tax purposes for any taxable year if either:
| • | 75% or more of its gross income for such year is “passive income” which for this purpose generally includes dividends, interest, royalties, rents and gains from commodities and securities transactions and gains from assets that produce passive income; or |
| • | 50% or more of the value of its gross assets (based on an average of the quarterly values of the gross assets) during such year is attributable to assets that produce passive income or are held for the production of passive income. |
56
Passive income does not include rents and royalties derived from the active conduct of a trade or business. If the stock of a non-U.S. corporation is publicly traded for the taxable year, the asset test is applied using the fair market value of the assets for purposes of measuring such corporation’s assets. If we own at least 25% (by value) of the stock of another corporation, we will be treated, for purposes of the PFIC tests, as owning our proportionate share of the other corporation’s assets and receiving our proportionate share of the other corporation’s income for purposes of the PFIC income and asset tests. If the stock of a non-U.S. corporation is publicly-traded for the taxable year, the asset test is applied using the fair market value of the assets for purposes of measuring such corporation’s assets. If we were a PFIC in any year during a U.S. holder’s holding period for our ordinary shares or ADSs, we would ordinarily continue to be treated as a PFIC for each subsequent year during which the U.S. holder owned the ordinary shares or ADSs, regardless of whether we continue to meet the tests described above unless (a) we ceased to be a PFIC and (b) the U.S. holder has made a deemed sale election under the PFIC rules which may result in recognition of gain (but not loss), taxable under the PFIC rules described below, without the receipt of any corresponding cash. Based on the composition of our assets and income, we believe that we were not a PFIC for U.S. federal income tax purposes with respect to our 2022 taxable year. However, the determination of PFIC status is a fact-intensive determination that must be made annually at the close of each taxable year applying principles and methodologies that in some circumstances are unclear and subject to varying interpretation. As a result, there can be no assurance that we will not be treated as a PFIC for the current or any future taxable year. Changes in the nature of our income or assets or a decrease in the trading price of our ordinary shares or ADSs may cause us to be considered a PFIC in the current or any subsequent year.
If we are a PFIC, and you are a U.S. holder, then unless you make one of the elections described below, a special tax regime will apply to both (a) any “excess distribution” by us to you (generally, your ratable portion of distributions in any year which are greater than 125% of the average annual distribution received by you in the shorter of the three preceding years or your holding period for our ordinary shares) and (b) any gain realized on the sale or other disposition of the ordinary shares. Under this regime, any excess distribution and realized gain will be treated as ordinary income and will be subject to tax as if (a) the excess distribution or gain had been realized ratably over your holding period, (b) the amount deemed realized in each year had been subject to tax in each year of that holding period at the highest marginal rate for such year (other than income allocated to the current period or any taxable period before we became a PFIC, which would be subject to tax at the U.S. holder’s regular ordinary income rate for the current year and would not be subject to the interest charge discussed below), and (c) the interest charge generally applicable to underpayments of tax had been imposed on the taxes deemed to have been payable in those years. In addition, dividend distributions made to you will not qualify for the lower rates of taxation applicable to long-term capital gains discussed above under “Distributions.”
Certain elections may potentially be used to reduce the adverse impact of the PFIC rules on U.S. Holders (“qualifying electing fund”, or QEF) , and “mark-to-market” elections), but these elections may accelerate the recognition of taxable income and may result in the recognition of ordinary income.
The rules described above for excess distributions would not apply to a U.S. holder if the U.S. holder makes a timely QEF election for the first taxable year of the U.S. holding period for ordinary shares and we comply with specified reporting requirements. A timely QEF election for a taxable year generally must be made on or before the due date (as may be extended) for filing the taxpayer’s U.S. federal income tax return for the year. A U.S. holder who makes a QEF election generally must report on a current year basis a pro rata share of our ordinary earnings and net capital gain for any taxable year in which we are a PFIC, whether or not those earnings or gains are distributed. A U.S. holder who makes a QEF election must file a Form 8621 with its annual income tax return. If we determine we are a PFIC for any taxable year, we intend to make available an information statement that will contain the necessary information required for a U.S. holder to make a QEF election with respect to our ordinary shares. We may choose to provide such information on our website.
If a U.S. holder does not make a QEF election for the first taxable year of the U.S. holder’s holding period for ordinary shares during which we are a PFIC, the QEF election will not be treated as timely and the adverse tax regime described above would apply to dispositions of or excess distributions on the ordinary shares. In such case, a U.S. holder may make a deemed sale election whereby the U.S. holder would be treated as if the U.S. holder had sold the ordinary shares in a fully taxable sale at fair market value on the first day of such taxable year in which the QEF election takes effect. Such U.S. holder would be required to recognize any gain on the deemed sale as an excess distribution and pay any tax and interest due on the excess distribution when making the deemed sale election. The effect of such further election would be to restart the U.S. holder’s holding period in the ordinary shares, subject to the QEF regime, and to purge the PFIC status of such ordinary shares going forward.
57
If a U.S. holder makes the mark-to-market election with respect to ordinary shares, the U.S. holder generally will recognize as ordinary income any excess of the fair market value of the ordinary shares at the end of each taxable year over their adjusted tax basis, and will recognize an ordinary loss in respect of any excess of the adjusted tax basis of the ordinary shares over their fair market value at the end of the taxable year (but only to the extent of the net amount of income previously included as a result of the mark-to-market election). If a U.S. holder makes the election, the U.S. holder’s tax basis in the ordinary shares will be adjusted to reflect these income or loss amounts. Any gain recognized on the sale or other disposition of ordinary shares in a year when we are a PFIC will be treated as ordinary income and any loss will be treated as an ordinary loss (but only to the extent of the net amount of income previously included as a result of the mark-to-market election). The mark-to-market election is available only if we are a PFIC and our ordinary shares are “regularly traded” on a “qualified exchange”. Our ordinary shares will be treated as “regularly traded” in any calendar year in which more than a de minimis quantity of the ordinary shares are traded on a qualified exchange on at least 15 days during each calendar quarter (subject to the rule that trades that have as one of their principle purposes the meeting of the trading requirement as disregarded). The Nasdaq is a qualified exchange for this purpose and consequently, if the ordinary shares are regularly traded, the mark-to-market election should be available to a U.S. holder.
U.S. holders should consult their tax advisors to determine whether any of these elections would be available and if so, what the consequences of the alternative treatments would be in their particular circumstances.
If we are a PFIC, the general tax treatment for U.S. holders described in this section would apply to indirect distributions and gains deemed to be realized by U.S. holders in respect of any of our subsidiaries that also may be determined to be PFICs.
If a U.S. holder owns ordinary shares during any year in which we are a PFIC and the U.S. holder recognizes gain on a disposition of our ordinary shares or receives distributions with respect to our ordinary shares, the U.S. Holder generally will be required to file an IRS Form 8621 (Information Return by a Shareholder of a Passive Foreign Investment Company or Qualified Electing Fund) with respect to the company, generally with the U.S. holder’s federal income tax return for that year. If our company were a PFIC for a given taxable year, then you should consult your tax advisor concerning your annual filing requirements.
The U.S. federal income tax rules relating to PFICs are complex. Prospective U.S. investors are urged to consult their tax advisers with respect to the ownership and disposition of our ordinary shares or ADSs, the consequences to them of an investment in a PFIC, any elections available with respect to our ordinary shares and the IRS information reporting obligations with respect to the ownership and disposition of our ordinary shares or ADSs.
U.S. Information Reporting and Back-up Withholding
Dividend payments with respect to our ordinary shares or ADSs and proceeds from the sale or other disposition of our ordinary shares or ADSs may be subject to information reporting to the IRS and possible U.S. backup withholding. Back-up withholding will not apply, however, to a U.S. holder who furnishes a correct taxpayer identification number and makes any other required certification or who is otherwise exempt from back-up withholding. U.S. holders who are required to establish their exempt status may be required to provide such certification on Internal Revenue Service (“IRS”) Form W-9. U.S. holders should consult their tax advisors regarding the application of the U.S. information reporting and back-up withholding rules.
Back-up withholding is not an additional tax. Amounts withheld as back-up withholding may be credited against a U.S. holder’s U.S. federal income tax liability, and such holder may obtain a refund of any excess amounts withheld under the back-up withholding rules by timely filing the appropriate claim for refund with the IRS and furnishing any required information.
Information With Respect to Foreign Financial Assets
Certain U.S. holders that own “specified foreign financial assets” with an aggregate value in excess of $50,000 are generally required to file an information statement along with their U.S. federal tax returns, currently on IRS Form 8938, with respect to such assets. “Specified foreign financial assets” include any financial accounts held at a non-U.S. financial institution, as well as securities issued by a non-U.S. issuer that are not held in accounts maintained by financial institutions. If a U.S. holder does not include in such holder’s gross income an amount relating to one or more specified foreign financial assets, and the amount such U.S. holder omits is more than $5,000, any tax such U.S. holder owes for the tax year can be assessed at any time within 6 years after the filing of such U.S. holder’s federal tax return. U.S. holders who fail to report the required information could be subject to substantial penalties. U.S. holders are encouraged to consult with their own tax advisors regarding the possible application of the foregoing to our ordinary shares or ADSs in light of their particular circumstances.
58
Australian Tax Considerations
In this section, we discuss the material Australian income tax, stamp duty and goods and services tax considerations related to the acquisition, ownership and disposal by the absolute beneficial owners of the ordinary shares or ADSs.
It is based upon existing Australian tax law as of the date of this registration statement, which is subject to change, possibly retrospectively. This discussion does not address all aspects of Australian tax law which may be important to particular investors in light of their individual investment circumstances, such as shares held by investors subject to special tax rules (for example, financial institutions, insurance companies, superannuation funds, trusts or tax-exempt organizations). In addition, this summary does not discuss any foreign or state tax considerations, other than stamp duty.
Prospective investors are urged to consult their tax advisors regarding the Australian and foreign income and other tax considerations of the acquisition, ownership and disposition of the shares. Unless otherwise mentioned, this summary is based upon the premise that the holder is not an Australian tax resident holds their shares on capital account for Australian tax purposes, and is not carrying on business in Australia through a permanent establishment (referred to as a “Non-Australian Shareholder” in this summary).
Australian Income Tax
Nature of ADSs for Australian Taxation Purposes
Ordinary shares represented by ADSs held by a U.S. holder will be treated for Australian taxation purposes as held under a “bare trust” for such holder. Consequently, the underlying ordinary shares will be regarded as owned by the ADS holder for Australian income tax and capital gains tax purposes. Dividends paid on the underlying ordinary shares will also be treated as dividends paid to the ADS holder, as the person beneficially entitled to those dividends. Therefore, in the following analysis we discuss the tax consequences to Non-Australian Shareholders which, for Australian taxation purposes, will be the same as to U.S. holders of ADSs.
Taxation of Dividends
Australia operates a dividend imputation system under which dividends may be declared to be “franked” to the extent of tax paid on company profits. Fully franked dividends are not subject to dividend withholding tax. Dividends payable to Non-Australian Shareholders will be subject to dividend withholding tax, to the extent the dividends are not declared to be conduit foreign income, or CFI, and are unfranked. Dividend withholding tax will be imposed at 30%, unless a shareholder is a resident of a country with which Australia has a double taxation agreement and qualifies for the benefits of the treaty. In accordance with the provisions of the Double Taxation Convention between Australia and the United States, the maximum rate of Australian withholding tax on any unfranked portion of a dividend to which a tax resident of the United States is beneficially entitled may be reduced to 15%, with a potential further reduction to 5% where the U.S. resident beneficially entitled to the dividends is a company which holds directly 10% or more of the voting power in our company. To rely on the Double Taxation Convention a U.S. tax resident must also be a “qualified person” within the meaning of the Double Taxation Convention. Shareholders seeking to rely on the Double Taxation Convention should obtain specialist taxation advice.
Tax on Sales or other Dispositions of Shares—Capital Gains Tax
Non-Australian Shareholders may disregard the whole of the capital gain or capital loss made on a sale or other disposal of ordinary shares, unless they, together with any associates (as defined in Australian tax law), hold 10% or more of our issued capital at the time of disposal or throughout a 12 months period during the 24 months prior to disposal.
Non-Australian Shareholders who own a 10% or more interest in the company, either alone or together with their associates, should be subject to Australian capital gains tax if more than 50% of the company’s assets held directly or indirectly, determined by reference to market value of the assets at the time of sale, consists of Australian real property (which includes land and leasehold interests) or Australian mining, quarrying or prospecting rights. The Double Taxation Convention between the United States and Australia is unlikely to limit the amount of this taxable gain. Australian capital gains tax applies to net capital gains of foreign shareholders at the Australian tax rates for non-Australian residents, which start at a marginal rate of 32.5% for individuals & 25%-30% for companies, depending on the size of the company. Net capital gains of foreign shareholders are included in the taxpayer’s assessable income and subject to income tax at the taxpayer’s marginal tax rate. The marginal tax rates for non-Australian residents, start at 32.5% for individuals. The company tax rate is 30% which may be reduced to 25% for the year ended 30 June 2023 onwards for certain small businesses. Net capital gains are calculated by reducing the taxpayer’s capital gains for the income year by its capital losses, which may only be offset against capital gains. Net capital losses may be carried forward to offset against capital gains derived in future income years. Specific loss recoupment rules apply to companies and trusts. These rules may, among other things, limit the ability to offset or obtain capital losses in a current or future income year. Shareholders should obtain specialist tax advice as to how these rules apply.
The 50% capital gains tax discount is not available to Non-Australian Shareholders. Companies are not entitled to a capital gains tax discount.
59
Broadly, where there is a disposal of certain taxable Australian property, the purchaser will be required to withhold and remit to the Australian Taxation Office (“ATO”) 12.5% of the proceeds from the sale. A transaction is excluded from the withholding requirements in certain circumstances, including where the market value of the taxable Australian property is less than A$750,000, the transaction is an on-market transaction conducted on an approved stock exchange, the transaction is in a category of certain securities lending arrangements, or the transaction is conducted using an eligible broker operated crossing system. There is also an exception to the requirement to withhold where the entity selling the shares provides the purchaser a declaration covering a certain period specifying either that they are an Australian tax resident or that the shares are not taxable Australian property (specifically, not ‘indirect Australian real property interests’). The Non-Australian Shareholder may be entitled to receive a tax credit for the tax withheld by the purchaser which they may claim in their Australian income tax return.
Tax on Sales or other Dispositions of Shares—Shareholders Holding Shares on Revenue Account
Some Non-Australian Shareholders may hold ordinary shares on revenue rather than on capital account for example, share traders, or those who hold their shares with a view to deriving a short term profit by selling their shares. These shareholders may have the gains made on the sale or other disposal of the ordinary shares and/or warrants included in their assessable income under the ordinary income provisions of the income tax law, if the income is derived directly or indirectly from Australian sources (which is a question of facts and circumstances generally requiring specialist tax advice).
Non-Australian Shareholders assessable under these ordinary income provisions should be subject to income tax in Australia starting at a marginal rate of 32.5% for individuals. The company tax rate is 30% which may be reduced to 25% for the year ended 30 June 2023 onwards for certain small businesses. Some relief from Australian income tax may be available to Non-Australian Shareholders under the Double Taxation Convention between the United States and Australia.
To the extent an amount would be included in a Non-Australian Shareholder’s assessable income under both the capital gains tax provisions and the ordinary income provisions, the capital gain amount may be reduced, so that the shareholder may not be subject to double tax on any part of the income gain or capital gain.
Non-Australian Shareholders holding shares on revenue account should obtain advice on the application of the Australian income tax law and the
Double Taxation Convention in determining the tax consequences of the disposal of their shares.
The comments above in “Tax on Sales or Other Dispositions of Shares—Capital Gains Tax” regarding a purchaser being required to withhold 12.5% tax on the acquisition of certain taxable Australian property equally applies where the disposal of the Australian real property asset by a foreign resident is likely to generate gains on revenue account, rather than a capital gain.
Dual Residency
If a shareholder is a resident of both Australia and the United States under those countries’ domestic taxation laws, that shareholder may be subject to tax as an Australian resident. If, however, the shareholder is determined to be a U.S. resident for the purposes of the Double Taxation Convention between the United States and Australia, the Australian tax may be subject to limitation by the Double Taxation Convention (albeit the tie-breaker rules only apply for individuals). Shareholders should obtain specialist taxation advice in these circumstances.
Stamp Duty
No Australian stamp duty is payable by Australian residents or non-Australian residents on the issue, transfer and/or surrender of the ADSs or the ordinary shares in Kazia, provided that the shares issued, transferred and/or surrendered do not represent 90% or more of the issued shares in Kazia.
Australian Death Duty
Australia does not have estate or death duties. As a general rule, no capital gains tax liability is realized upon the inheritance of a deceased person’s shares. The disposal of inherited shares by beneficiaries may, however, give rise to a capital gains tax liability if the gain falls within the scope of Australia’s jurisdiction to tax.
Goods and Services Tax
The supply of ADSs or ordinary shares in Kazia will not be subject to Australian goods and services tax.
F. Dividends and paying agents
Not applicable.
G. Statement by experts
Not applicable.
60
H. Documents on Display
The Company is subject to the reporting requirements of the Exchange Act that are applicable to a foreign private issuer. Under the Exchange Act, the Company is required to file periodic reports and other information with the SEC. These materials, including this Annual Report and the exhibits hereto, may be inspected without charge and copied at established rates at the public reference facilities maintained by the SEC at 100 F Street, N.E., Washington, D.C., 20549. Please call the SEC at 1-800-SEC-0330 to obtain information on the operation of the public reference room. Such materials can also be obtained at the SEC’s website at www.sec.gov.
I. Subsidiary Information
Not applicable.
| Item 11. | Quantitative and Qualitative Disclosures about Market Risk |
Interest rate risk
The Company’s exposure to market interest rates relate primarily to the investments of cash balances. The Company has cash reserves held in both Australian dollars and U.S. dollars, and places funds on deposit with financial institutions for periods generally not exceeding three months.
Credit risk
The Company places its deposits with high credit quality financial institutions, and, by policy, limits the amount of credit exposure to any single counter-party. The Company is averse to principal loss and ensures the safety and preservation of its invested funds by limiting default risk, market risk and reinvestment risk. The Company mitigates default risk by depositing funds with only the safest and highest credit quality financial institutions and by constantly positioning its portfolio to respond appropriately to a significant reduction in a credit rating of any financial institution.
The Company has no interest rate exposure due to rate changes for long-term debt obligations. The Company primarily enters into debt obligations to support general corporate purposes, including capital expenditures and working capital needs. The Company does not consider the effects of interest rate movements to be a material risk to its financial condition.
For additional disclosure regarding interest rate risk see Item 18. “Financial Statements – Note 24 – Financial Instruments”.
Foreign currency risk
The Company operates internationally and is exposed to foreign exchange risk arising from various currency exposures, primarily with respect to the U.S. dollar. Foreign exchange risk arises from future transactions and recognized assets and liabilities denominated in a currency that is not the entity’s functional currency and net investments in foreign operations.
As of 30 June 2023, the Company did not hold derivative financial instruments in managing its foreign currency, however, the Company may from time to time enter into hedging arrangements where circumstances are deemed appropriate. The Company used natural hedging to reduce the foreign currency risk, which involved processing USD payments from cash held in USD. Foreign subsidiaries with a functional currency of Australian Dollar (“AUD”) have exposure to the local currency of these subsidiaries and any other currency these subsidiaries trade in.
For additional disclosure regarding market risk see Item 18. “Financial Statements – Note 24 – Financial Instruments”.
61
| Item 12. | Description of Securities Other than Equity Securities |
A. Debt Securities
Not applicable.
B. Warrants and Rights
Not applicable.
C. Other Securities
Not applicable.
D. American Depositary Shares
The depositary for the Company’s American Depositary Shares (“ADS”) is the Bank of New York Mellon, located at 240 Greenwich Street, New York, NY 10286. The depositary collects its fees for delivery and surrender of American Depositary Shares (“ADSs”) directly from investors depositing shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deductions from cash distributions or by directly billing investors or by charging the book-entry system accounts of participants acting for them. The depositary may generally refuse to provide fee-attracting services until its fees for those services are paid. The depositary may collect any of its fees by deduction from any cash distribution payable to you that are obligated to pay those fees.
From time to time, the depositary may make payments to us to reimburse or share revenue from the fees collected from you, or waive fees and expenses for services provided, generally relating to costs and expenses arising out of establishment and maintenance of the ADS program. In performing its duties under the deposit agreement, the depositary may use brokers, dealers or other service providers that are affiliates of the depositary and that may earn or share fees or commissions.
| Persons depositing or withdrawing shares must pay: |
For: |
|
| US$5.00 (or less) per 100 ADSs (or portion of 100 ADSs) | • Issuance of ADSs, including issuances resulting from a distribution of shares or rights or other property |
|
| • Cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement terminates |
||
| US$.05 (or less) per ADS | • Any cash distribution to ADS registered holders |
|
| A fee equivalent to the fee that would be payable if securities distributed to you had been shares and the shares had been deposited for issuance of ADSs | • Distribution of securities distributed to holders of deposited securities which are distributed by the depositary to ADS registered holders |
|
| US$.05 (or less) per ADSs per calendar year | • Depositary services |
|
| Registration or transfer fees | • Transfer and registration of shares on the Company’s share register to or from the name of the depositary or its agent when you deposit or withdraw shares |
|
| Expenses of the depositary | • Cable, telex and facsimile transmissions (when expressly provided in the deposit agreement) |
|
| • Converting foreign currency to U.S. dollars |
||
| Taxes and other governmental charges the depositary or the custodian have to pay on any ADS or share underlying an ADS, for example, stock transfer taxes, stamp duty or withholding taxes | • As necessary |
|
| Any charges incurred by the depositary or its agents for servicing the deposited securities | • As necessary |
|
The Depositary may collect any of the fees by deduction from any cash distribution payable, or by selling a portion of any securities to be distributed, to holders that are obligated to pay those fees.
62
PART II
| Item 13. | Defaults, Dividend Arrearages and Delinquencies |
This item is not applicable.
| Item 14. | Material Modifications to the Rights of Security Holders and the Use of Proceeds |
This item is not applicable.
| Item 15. | Controls and Procedures |
(a) Disclosure controls and procedures
We maintain “disclosure controls and procedures,” as this term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, that are designed to provide reasonable assurance that information required to be disclosed by us in reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the Securities and Exchange Commission’s (“SEC”) rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer, Vice President of Finance and Administration and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. Our Chief Executive Officer, Vice President of Finance and Administration and Chief Financial Officer recognize that these controls, no matter how well designed and operated, cannot provide absolute assurance that the objectives of these controls will be met.
Our management, with the participation of our Chief Executive Officer, Vice President of Finance and Administration and Chief Financial Officer, has evaluated the effectiveness of the design and operation of our disclosure controls and procedures as of June 30, 2023. Based upon that evaluation, our Chief Executive Officer, Vice President of Finance and Administration and Chief Financial Officer concluded that, as a result of the material weakness in our internal control over financial reporting described below, the design and operation of our disclosure controls and procedures were not effective as of June 30, 2023.
(b) Management’s annual report on internal control over financial reporting
The management of Kazia Therapeutics Limited is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) under the Exchange Act. Under the supervision and with the participation of our management, including our Chief Executive Officer, Vice President of Finance and Administration, and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of June 30, 2023, based on the criteria set forth in Internal Control Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO 2013). Based on our evaluation under the criteria set forth in Internal Control Integrated Framework, our management concluded that our internal control over financial reporting was not effective as at June 30, 2023 because of the material weakness described below. Kazia Therapeutics Limited’s internal control over financial reporting was designed to provide reasonable assurance to the Company’s management and Board of Directors regarding the preparation and fair presentation of published financial statements. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim consolidated financial statements will not be presented or detected on a timely basis.
Specifically, during the fiscal year ended June 30, 2023 we identified a material weakness related to the incorrect application of accounting standards in relation to the acquisition of the EVT-801 intangible asset and the related contingent consideration. The calculation was found to contain errors as discounting for the time value of money was not considered on initial recognition. This was the result of a lack of personnel with specialist accounting knowledge. We are taking and will continue to take steps to remediate the material weakness, including seeking external professional accounting advice about the initial accounting of new significant transactions. The material weakness resulted in the restatement of our 2022 and 2021 consolidated financial statements. The impact of this restatement is more fully described in note 4 to the financial statements.
Notwithstanding such material weakness in internal control over financial reporting, our CEO and CFO have concluded that our consolidated financial statements included in this Annual Report on Form 20-F present fairly, in all material respects, our financial position, results of operations and cashflows at and for the periods presented in conformity with IFRS as issued by the IASB.
63
Remediation Plan for Material Weaknesses
We are taking and will continue to take steps to remediate the material weakness, including seeking external professional accounting advice about the initial accounting of new significant transactions.
The process of implementing and operating an effective financial reporting system is a continuous effort that requires us to anticipate and react to changes in business and the economic and regulatory environment and to expend significant resources to maintain a financial reporting system that is adequate to satisfy our reporting obligations. Additional time is required to complete implementation as well as to assist and ensure the sustainability of these procedures. We believe that the actions we take will be effective in remediating the material weakness described above and we will continue to devote significant time and attention to these remediation efforts. As we continue to evaluate and take actions to improve our internal control over financial reporting, we may take additional actions to address control deficiencies or modify certain of the remediation measures described above. There can be no assurance that the actions we are taking and will take will be sufficient to remediate the material weakness.
(c) Attestation Report of the Registered Public Accounting Firm
Not applicable.
(d) Changes in Internal Control over Financial Reporting
Other than the changes intended to remediate the material weakness noted above, no change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) occurred during the year ended June 30, 2023 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| Item 16. | [Reserved] |
| Item 16A. | Audit Committee Financial Expert |
| (1) | The board of directors has determined that the Company: |
| (i) | Has one audit committee financial expert serving on its audit committee |
| (2) | Steven Coffey is the audit committee expert and is independent, as that term is defined in the listing standards applicable to the registrant if the registrant is a listed issuer, as defined in 17 CFR 240.10A-3. Steven Coffey meets the independence requirements of the Nasdaq Capital Market and SEC’s rules and regulations as he is a qualified Chartered Accountant and has spent over 30 years in public practice. He is also a registered company auditor. |
| Item 16B. | Code of Ethics |
The Company has adopted a Code of Ethics and Business Conduct (the “Code”). The Code establishes a clear set of values that emphasise a culture encompassing strong corporate governance, sound business practices and good ethical conduct. The Code confirms the Company’s belief in treating all individuals with respect and recognises that different skills and diversity are essential to enrich the Company’s perspective, improve corporate performance, increase shareholder value and maximise the achievement and goals of the Company. The Code applies to all Company employees, including management and Directors. The Code is available on the Company’s website www.kaziatherapeutics.com.
| Item 16C. | Principal Accounting Fees and Services |
BDO Audit Pty Ltd (BDO) has audited the Company’s annual financial statements acting as the independent registered public accounting firm for the fiscal year ended 30 June 2023. Grant Thornton Audit Pty Ltd (“GT”) has audited the Company’s annual financial statements acting as the independent registered public accounting firm for the fiscal year ended 30 June 2022.
The table below set forth the total fees for services performed by BDO & GT in fiscal years 2023 and 2022 and summarizes these amounts by the category of service.
| 2023 A$’000 |
2022 A$’000 |
|||||||
| Grant Thornton Audit Pty Ltd |
||||||||
| - Audit or review fees |
155 | |||||||
| - Comfort letter ATM |
26 | |||||||
|
|
|
|||||||
| Total fees |
181 | |||||||
|
|
|
|||||||
| BDO Audit Pty Ltd |
||||||||
| - Audit or review fees |
292 | |||||||
| - Comfort letter ATM |
18 | |||||||
|
|
|
|||||||
| Total fees |
310 | |||||||
|
|
|
|||||||
64
Audit fees
The audit fees include the aggregate fees incurred in fiscal years 2023 and 2022 for professional services rendered in connection with the audit of the Company’s annual financial statements and for related services that are reasonably related to the performance of the audit or services that are normally provided by the auditor in connection with regulatory filings of engagements for those financial years (including review of the Company’s Annual Report on Form 20-F, consents and other services related to SEC matters).
Pre-approval policies and procedures
The Audit Committee Charter sets forth the Company’s policy regarding the appointment of independent auditors. The Audit Committee Charter also requires the Audit Committee to review and approve in advance the appointment of the independent auditors for the performance of 100% of all audit services and, after taking into account the opinion of management, 100% of lawfully permitted non-audit services. The Audit Committee may delegate authority to one or more members of the Audit Committee where appropriate, but no such delegation is permitted if the authority is required by law, regulation or listing standard to be exercised by the Audit Committee as a whole.
| Item 16D. | Exemptions from the Listing Standards for Audit Committees |
This item is not applicable.
| Item 16E. | Purchases of Equity Securities by the Issuer and Affiliated Purchasers |
This item is not applicable.
| Item 16F. | Changes in registrant’s Certifying Accountant |
Grant Thornton Audit Pty Ltd (GT) who had been engaged as the Company’s auditors since 2012 to audit the registrant’s financial statements, was not reappointed as auditors and resigned in accordance with section 329(5) of the Corporations Act 2001 (Cth) (Corporations Act) and ASIC’s consent to the resignation.
The appointment of the new auditor of the Company was approved by the Board on 6 December 2022 subject to ASIC consent.
The Company appointed BDO Audit Pty Ltd (BDO) as the new auditor in accordance with ASX Listing Rule 3.16.3, effective from 24 February 2023. GT had held the position as auditor since 2012 and accordingly it was deemed appropriate to conduct a tender process to seek a new auditor. The Company confirms that it has received a letter of resignation from GT and has also received the consent of ASIC to their resignation.
GT’s report on the Company’s financial statements for fiscal year ended June 30, 2021 did not contain an adverse opinion or a disclaimer of opinion or was qualified or modified as to uncertainty, audit scope, or accounting principles. The audit opinion for fiscal year ended June 30, 2022 was modified in respect of going concern.
During the Company’s two most recent fiscal years and any subsequent interim period preceding the resignation, there were no disagreements with Grant Thornton on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure.
The Company authorized GT to communicate with BDO as part of the hand over of the appointment as the new company auditor.
We provided GT with a copy of this annual report and requested a letter addressed to the SEC indicating whether or not it agrees with the above disclosures. A copy of GT’s letter dated 26 October 2023 is attached as Exhibit 15.3 to this annual report.
During the two fiscal years ended June 30, 2022 and 2021, and in the subsequent interim period through the date of appointment of BDO, neither the Company nor anyone on its behalf consulted BDO with respect to either (i) the application of accounting principles to a specified transaction, either completed or proposed; or the type of audit opinion that might be rendered on the Company’s consolidated financial statements, and neither a written report nor oral advice was provided to the Company that BDO concluded was an important factor considered by the Company in reaching a decision as to any accounting, auditing or financial reporting issue; or (ii) any matter that was either the subject of a disagreement (as described in Item 16F(a)(1)(iv) of Form 20-F and the related instructions to Item 16F) or a reportable event (as described in Item 16F(a)(1)(v) of Form 20-F).
| Item 16G. | Corporate Governance |
Implications of Being a Foreign Private Issuer
We are also considered a “foreign private issuer.” In our capacity as a foreign private issuer, we are exempt from certain rules under the U.S. Securities Exchange Act of 1934, as amended (the “Exchange Act”), that impose certain disclosure obligations and procedural requirements for proxy solicitations under Section 14 of the Exchange Act. In addition, our officers, directors and principal shareholders are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act and the rules under the Exchange Act with respect to their purchases and sales of our ordinary shares. Moreover, we are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. In addition, we are not required to comply with Regulation FD, which restricts the selective disclosure of material information.
Exemptions from Certain Corporate Governance Rules of the Nasdaq Stock Market, LLC
Exemptions from the corporate governance standards of the Nasdaq Stock Market, LLC (“Nasdaq”) are available to foreign private issuers such as Kazia when those standards are contrary to a law, rule or regulation of any public authority exercising jurisdiction over such issuer or contrary to generally accepted business practices in the issuer’s country of domicile. In connection with Kazia’s National Market Listing Application, Nasdaq granted Kazia exemptions from certain corporate governance standards that were contrary to the laws, rules, regulations or generally accepted business practices of Australia. These exemptions and the practices followed by Kazia are described below:
| • | Kazia is exempt from Nasdaq’s requirement that each Nasdaq issuer shall require shareholder approval of a plan or arrangement in connection with the acquisition of the stock or assets of another company if “any director, officer or substantial shareholder of the issuer has a 5 percent or greater interest (or such persons collectively have a 10 percent or greater interest), directly or indirectly, in the company or assets to be acquired or in the consideration to be paid in the transaction or series of related transactions and the present or potential issuance of common stock, or securities convertible into or exercisable for common stock, could result in an increase in outstanding common shares or voting power of 5 percent or more”. Kazia is subject to Chapter 10 of the ASX listing rules, which requires shareholder approval for an acquisition from or disposal to a “related party” (including a director) or “substantial shareholder” (who is entitled to at least 10% of the voting securities) of “substantial assets”. The Australian Corporations Act to which Kazia is also subject generally requires shareholder approval for a transaction with a director or director-controlled entity unless on arm’s length terms. |
65
| • | Nasdaq requirement under Rule 5620(c) that a quorum consist of holders of 33 1/3% of the outstanding ordinary shares — The ASX Listing Rules do not have an express requirement that each issuer listed on ASX have a quorum of any particular number of the outstanding ordinary shares, but instead allow a listed issuer to establish its own quorum requirements. Our quorum is currently three shareholders. We believe this quorum requirement is consistent with the requirements of the ASX and is appropriate and typical of generally accepted business practices in Australia. |
| • | Nasdaq requirements under Rules 5605(b)(1) and (2) relating to director independence, including the requirements that a majority of the board of directors must be comprised of independent directors and that independent directors must have regularly scheduled meetings at which only independent directors are present — The Nasdaq and ASX definitions of what constitute an independent director are not identical and the requirements relating to the roles and obligations of independent directors are not identical. The ASX, unlike Nasdaq, permits an issuer to establish its own materiality threshold for determining whether a transaction between a director and an issuer affects the director’s status as independent and it does not require that a majority of the issuer’s board of directors be independent, as long as the issuer publicly discloses this fact. In addition, the ASX does not require that the independent directors have regularly scheduled meeting at which only independent directors are present. We believe that our Board composition is consistent with the requirements of the ASX and that it is appropriate and typical of generally accepted business practices in Australia. |
| • | The requirement that our independent directors meet regularly in executive sessions under Nasdaq Listing Rules. The ASX Listing Rules and the Corporations Act do not require the independent directors of an Australian company to have such executive sessions. |
| • | The Nasdaq requirements under Rules 5605(d) and 5605(e) that compensation of an issuer’s officers must be determined, or recommended to the Board for determination, either by a majority of the independent directors, or a compensation committee comprised solely of independent directors, and that director nominees must either be selected, or recommended for the Board’s selection, either by a majority of the independent directors, or a nominations committee comprised solely of independent directors. The Nasdaq compensation committee requirements are not identical to the ASX remuneration and nomination committee requirements. Issuers listed on the ASX are recommended under applicable listing standards to establish a remuneration committee consisting of a majority of independent directors and an independent chairperson, or publicly disclose that it has not done so. Kazia has, and expects to continue to have, a Remuneration and Nomination Committee consisting of three non-executive directors. |
| • | The requirement prescribed by Nasdaq Listing Rules that issuers obtain shareholder approval prior to the issuance of securities in connection with certain acquisitions, private placements of securities, or the establishment or amendment of certain share option, purchase or other compensation plans. Applicable Australian law and the ASX Listing Rules differ from Nasdaq requirements, with the ASX Listing Rules providing generally for prior shareholder approval in numerous circumstances, including (i) issuance of equity securities exceeding 15% (or 25% under certain circumstances) of our issued share capital in any 12-month period (but, in determining the 15% limit, securities issued under an exception to the rule or with shareholder approval are not counted), (ii) issuance of equity securities to related parties (as defined in the ASX Listing Rules) and (iii) issuances of securities to directors or their associates under an employee incentive plan. |
| Item 16H. | Mine Safety Disclosure |
This item is not applicable.
| Item 16I. | Disclosure Regarding Foreign Jurisdictions that Prevent Inspections |
Not Applicable.
| Item 16J. | Insider trading policies |
Not Applicable.
| Item 16K. | Cybersecurity |
Not Applicable.
PART III
| Item 17. | Financial Statements |
Refer to “Item 18 – Financial Statements” below.
| Item 18. | Financial Statements |
The financial statements filed as part of this Annual Report commencing on page F-1.
66
| Item 19. | Exhibits |
(a) Exhibits
67
| * | Filed herewith. |
| ✓ | Certain confidential information in this exhibit was omitted by means of marking such information with brackets (“[***]”) because the identified confidential information is not material and is the type that the registrant treats as private or confidential. |
68
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this Annual Report on its behalf.
| KAZIA THERAPEUTICS LIMITED |
| /s/ John Friend |
| Dr John Friend |
| Managing Director and Chief Executive Officer |
| Date: October 26, 2023 |
69
Page |
||||
Consolidated Financial Statements for 30 June 2023, 2023 and 2021 and the years then ended: |
||||
F-2 |
||||
F-3 |
||||
F-4 |
||||
F-6 |
||||
F-7 |
||||
F-8 |
||||
F-9 |
||||
• |
Obtaining and assessing managements’ assessment of the existence of impairment indicators, including their assessment on the progress and findings of ongoing clinical trials. |
• |
Assessing the completeness, accuracy and reasonability of managements’ assessment of the progress of the clinical trials from enquiries of management and their experts and external data. |
• |
Enquiring of management and managements’ internal experts in relation to the science and potential for existence of impairment indicators relating to the science. |
• |
Obtaining and critically evaluating management’s assessment and calculation of the contingent consideration liability. |
• |
Obtaining and inspecting significant contracts. |
• |
Holding discussions with management to understand managements’ key assumptions in arriving at the timing and probability of milestone payments as well as the discount rate applied. |
• |
Critically evaluating the assumptions applied on timing and probability of milestone payments against publicly available information and published clinical trial updates and results. |
• |
Engaging valuation experts to assess the reasonability of the discount rates applied by management in determining the contingent consideration. |
• |
Assessing the impact of the EVT801 prior period error on the financial statements. |
• |
Reviewing board minutes to ensure all the new options granted during the year have been accounted for. |
• |
Reviewing relevant supporting documentation to obtain an understanding of the contractual nature and terms and conditions of the share-based payment arrangements. |
• |
Engaging valuation specialists to assess the reasonability of the estimated fair value of the options using a relevant option valuation methodology and assessing the valuations inputs. |
/s/ Grant Thornton Audit Pty Ltd |
GRANT THORNTON AUDIT PTY LTD |
Note |
2023 A$’000 |
2022 restated A$’000 |
2021 restated A$’000 |
|||||||||||||
| Revenue from continuing operations |
6 | — | — | 15,183 | ||||||||||||
| Other income |
8
|
1 | 25 | 2 | ||||||||||||
| Finance income — bank interest |
22 | 2 | 42 | |||||||||||||
| Expenses |
||||||||||||||||
| Research and development expense |
9 | (15,564 | ) | (20,169 | ) | (14,541 | ) | |||||||||
| General and administrative expense |
9 | (8,583 | ) | (5,113 | ) | (7,020 | ) | |||||||||
| Gain/(loss) on remeasurement of contingent consideration |
19 | 3,388 | — | (2,570 | ) | |||||||||||
| Commercialisation |
— | (127 | ) | — | ||||||||||||
| |
|
|
|
|
|
|||||||||||
| Loss before income tax benefit from continuing operations |
(20,736 | ) | (25,382 | ) | (8,904 | ) | ||||||||||
| Income tax benefit |
10 | 271 | 368 | 484 | ||||||||||||
| |
|
|
|
|
|
|||||||||||
| Loss after income tax benefit for the year |
(20,465 | ) | (25,014 | ) | (8,420 | ) | ||||||||||
| Other comprehensive income |
||||||||||||||||
| Items that may be reclassified subsequently to profit or loss |
||||||||||||||||
| Net exchange difference on translation of financial statements of foreign controlled entities, net of tax |
110 | 35 | 2 | |||||||||||||
| |
|
|
|
|
|
|||||||||||
| Other comprehensive income for the year, net of tax |
110 | 35 | 2 | |||||||||||||
| Total comprehensive income for the year |
(20,355 |
) |
(24,979 |
) |
(8,418 |
) | ||||||||||
| Loss for the year is attributable to: |
||||||||||||||||
| Owners of Kazia Therapeutics Limited |
(20,465 | ) | (25,014 | ) | (8,420 | ) | ||||||||||
| |
|
|
|
|
|
|||||||||||
| Total loss for the year |
(20,465 | ) | (25,014 | ) | (8,420 | ) | ||||||||||
| Total comprehensive income for the year is attributable to: |
||||||||||||||||
| Owners of Kazia Therapeutics Limited |
(20,355 | ) | (24,979 | ) | (8,418 | ) | ||||||||||
| |
|
|
|
|
|
|||||||||||
| Total comprehensive income for the year |
(20,355 |
) |
(24,979 |
) |
(8,418 |
) | ||||||||||
| |
|
|
|
|
|
|||||||||||
Note |
2023 A$ Cents |
2022 restated A$ Cents |
2021 A$ Cents |
|||||||||||||
Earnings per share for loss from continuing operations attributable to the owners of Kazia Therapeutics Limited |
||||||||||||||||
Basic earnings per share |
3 2
|
(11.23 | ) | (18.88 | ) | (7.16 | ) | |||||||||
Diluted earnings per share |
3 2
|
(11.23 | ) | (18.88 | ) | (7.16 | ) | |||||||||
|
2023 A$ Cents |
2022 restated A$ Cents |
2021 A$ Cents |
||||||||||||||
Earnings per share for loss attributable to the owners of Kazia Therapeutics Limited |
||||||||||||||||
Basic earnings per share |
3 2
|
(11.23 | ) | (18.88 | ) | (7.16 | ) | |||||||||
Diluted earnings per share |
3 2
|
(11.23 | ) | (18.88 | ) | (7.16 | ) | |||||||||
Note |
2023 A$’000 |
2022 restated A$’000 |
1 July 2021 restated A$’000 |
|||||||||||||
Assets |
||||||||||||||||
Current assets |
||||||||||||||||
Cash and cash equivalents |
11 |
5,241 |
7,361 |
27,587 |
||||||||||||
Trade and other receivables |
12 |
3,899 |
91 |
84 |
||||||||||||
Other assets |
14 |
1,632 |
1,997 |
1,720 |
||||||||||||
Total current assets |
10,772 |
9,449 |
29,391 |
|||||||||||||
Non-current assets |
||||||||||||||||
Trade and other receivables |
13 |
43 |
7,301 |
6,694 |
||||||||||||
Intangibles |
15 |
17,269 |
19,139 |
21,008 |
||||||||||||
Total non-current assets |
17,312 |
26,440 |
27,702 |
|||||||||||||
Total assets |
28,084 |
35,889 |
57,093 |
|||||||||||||
Liabilities |
||||||||||||||||
Current liabilities |
||||||||||||||||
Trade and other payables |
16 |
4,329 |
3,759 |
4,933 |
||||||||||||
Borrowings |
17 |
1,796 |
1,841 |
— |
||||||||||||
Employee benefits |
18 |
690 |
369 |
229 |
||||||||||||
Contingent consideration |
19 |
750 |
759 |
791 |
||||||||||||
Total current liabilities |
7,565 |
6,728 |
5,953 |
|||||||||||||
Non-Current liabilities |
||||||||||||||||
Deferred tax |
20 |
2,289 |
2,560 |
2,928 |
||||||||||||
Employee benefits |
18 |
59 |
117 |
55 |
||||||||||||
Contingent consideration |
19 |
6,121 |
8,209 |
10,304 |
||||||||||||
Total non-current liabilities |
8,469 |
10,886 |
13,287 |
|||||||||||||
Total liabilities |
16,034 |
17,614 |
19,240 |
|||||||||||||
Net assets |
12,050 |
18,275 |
37,853 |
|||||||||||||
Equity |
||||||||||||||||
Contributed equity |
21 |
97,452 |
84,480 |
80,290 |
||||||||||||
Other contributed equity |
— |
— |
464 |
|||||||||||||
Reserves |
22 |
3,681 |
2,412 |
1,301 |
||||||||||||
Accumulated losses |
(89,083 |
) |
(68,617 |
) |
(44,202 |
) |
||||||||||
Equity attributable to the owners of Kazia Therapeutics Limited |
12,050 |
18,275 |
37,853 |
|||||||||||||
Total equity |
12,050 |
18,275 |
37,853 |
|||||||||||||
| Contributed equity A$’000 |
Other Contributed equity A$’000 |
Reserves A$’000 |
Accumulated Losses A$’000 |
Non- controlling Interest A$’000 |
Total equity A$’000 |
|||||||||||||||||||
| Balance at 1 July 2020 |
48,781 |
464 |
1,066 |
(36,186 |
) |
— |
14,125 |
|||||||||||||||||
| Loss after income tax expense for the year (restated) |
— |
— |
— |
(8,420 |
) |
— |
(8,420 |
) | ||||||||||||||||
| Other comprehensive income for the year, net of tax |
— |
— |
2 |
— |
— |
2 |
||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total comprehensive income for the year (restated) |
— |
— |
2 |
(8,420 |
) |
— |
(8,418 |
) | ||||||||||||||||
| Transactions with owners in their capacity as owners: |
||||||||||||||||||||||||
| Contributions of equity |
32,909 |
— |
— |
— |
— |
32,909 |
||||||||||||||||||
| Share issue costs |
(1,673 |
) |
— |
— |
— |
— |
(1,673 |
) | ||||||||||||||||
| Share based payment |
— |
— |
637 |
— |
— |
637 |
||||||||||||||||||
| Issue of shares |
273 |
— |
(80 |
) |
80 |
— |
273 |
|||||||||||||||||
| Expired options |
— |
— |
(324 |
) |
324 |
— |
— |
|||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at 30 June 2021 (restated) |
80,290 |
464 |
1,301 |
(44,202 |
) |
— |
37,853 |
|||||||||||||||||
| Contributed equity A$’000 |
Other Contributed equity A$’000 |
Reserves A$’000 |
Accumulated Losses A$’000 |
Non- controlling Interest A$’000 |
Total equity A$’000 |
|||||||||||||||||||
| Balance at 1 July 2021 (restated) |
80,290 |
464 |
1,301 |
(44,202 |
) |
— |
37,853 |
|||||||||||||||||
| Loss after income tax expense for the year (restated) |
— |
— |
— |
(25,014 |
) |
— |
(25,014 |
) | ||||||||||||||||
| Other comprehensive income for the year, net of tax |
— |
— |
35 |
— |
— |
35 |
||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total comprehensive income for the year (restated) |
— |
— |
35 |
(25,014 |
) |
— |
(24,979 |
) | ||||||||||||||||
| Transactions with owners in their capacity as owners: |
||||||||||||||||||||||||
| Shares issued (note 21) |
4,202 |
— |
— |
— |
— |
4,202 |
||||||||||||||||||
| Share issue costs (note 21) |
(493 |
) |
— |
— |
— |
— |
(493 |
) | ||||||||||||||||
| Immaterial reclassification |
— |
— |
(434 |
) |
434 |
— |
— |
|||||||||||||||||
| Share based payment (note 33) |
— |
— |
1,674 |
— |
— |
1,674 |
||||||||||||||||||
| Issue of shares on exercise of options |
17 |
— |
(6 |
) |
6 |
— |
17 |
|||||||||||||||||
| Conversion of convertible note |
464 |
(464 |
) |
— |
— |
— |
— |
|||||||||||||||||
| Expired options |
— |
— |
(159 |
) |
159 |
— |
— |
|||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at 30 June 2022 (restated) |
84,480 |
— |
2,412 |
(68,617 |
) |
— |
18,275 |
|||||||||||||||||
| Contributed equity A$’000 |
Other Contributed equity A$’000 |
Reserves A$’000 |
Accumulated Losses A$’000 |
Non- controlling Interest A$’000 |
Total equity A$’000 |
|||||||||||||||||||
| Balance at 1 July 2022 (restated) |
84,480 |
— |
2,412 |
(68,617 |
) |
— |
18,275 |
|||||||||||||||||
| Loss after income tax expense for the year |
— |
— |
— |
(20,465 |
) |
— |
(20,465 |
) | ||||||||||||||||
| Other comprehensive income for the year, net of tax |
— |
— |
110 |
— |
— |
110 |
||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total comprehensive income for the year |
— |
— |
110 |
(20,465 |
) |
— |
(20,355 |
) | ||||||||||||||||
| Transactions with owners in their capacity as owners: |
||||||||||||||||||||||||
| Shares issued (note 21) |
13,373 |
— |
— |
— |
— |
13,373 |
||||||||||||||||||
| Share issue costs (note 21) |
(401 |
) |
— |
— |
— |
— |
(401 |
) | ||||||||||||||||
| Share based payment (note 33) |
— |
— |
1,159 |
— |
— |
1,159 |
||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at 30 June 2023 |
97,452 |
— |
3,681 |
(89,083 |
) |
— |
12,050 |
|||||||||||||||||
Note |
2023 A$’000 |
2022 restated A$’000 |
2021 restated A$’000 |
|||||||||||||
| Cash flows from operating activities |
||||||||||||||||
| Loss after income tax expense for the year |
(20,465 | ) | (25,014 | ) | (8,420 | ) | ||||||||||
| Adjustments for: |
||||||||||||||||
| Depreciation and amortisation |
9
|
1,869 | 1,869 | 1,265 | ||||||||||||
| Share-based payments |
1,159 | 1,675 | 637 | |||||||||||||
| Foreign exchange differences |
46 | (2,154 | ) | 428 | ||||||||||||
| Gain on remeasurement of contingent consideration |
1 9
|
(2,097 | ) | — | 2,570 | |||||||||||
| |
|
|
|
|
|
|||||||||||
(19,488 |
) |
(23,624 |
) |
(3,520 |
) | |||||||||||
| Change in operating assets and liabilities: | ||||||||||||||||
| Increase/(decrease) in trade and other receivables |
3,450 | (7 | ) | (5,027 | ) | |||||||||||
| Increase/(decrease) in prepayments |
365 | (277 | ) | (1,182 | ) | |||||||||||
| Increase/(decrease) in trade and other payables |
569 | (528 | ) | 1,010 | ||||||||||||
| Increase in other provisions |
264 | 201 | 92 | |||||||||||||
| Decrease in deferred tax liability |
(271 | ) | (368 | ) | (484 | ) | ||||||||||
| (Decrease)/increase in borrowings |
(45 | ) | 1,841 | — | ||||||||||||
| |
|
|
|
|
|
|||||||||||
| Net cash used in operating activities |
3 1
|
(15,156 |
) |
(22,762 |
) |
(9,111 |
) | |||||||||
| |
|
|
|
|
|
|||||||||||
| Cash flows from investing activities |
||||||||||||||||
| Payment of milestone relating to contingent consideration |
— | (2,365 | ) | — | ||||||||||||
| |
|
|
|
|
|
|||||||||||
| Net cash used in investing activities |
— | (2,365 | ) | — | ||||||||||||
| |
|
|
|
|
|
|||||||||||
| Cash flows from financing activities |
||||||||||||||||
| Proceeds from issue of shares |
2 1
|
12,972 | 3,726 | 28,109 | ||||||||||||
| |
|
|
|
|
|
|||||||||||
| Net cash from financing activities |
12,972 |
3,726 |
28,109 |
|||||||||||||
| |
|
|
|
|
|
|||||||||||
| Net (decrease)/increase in cash and cash equivalents |
(2,184 | ) | (21,401 | ) | 18,998 | |||||||||||
| Cash and cash equivalents at the beginning of the financial year |
7,361 | 27,587 | 8,764 | |||||||||||||
| Effects of exchange rate changes on cash |
64 | 1,175 | (175 | ) | ||||||||||||
| |
|
|
|
|
|
|||||||||||
| Cash and cash equivalents at the end of the financial year |
1 1
|
5,241 |
7,361 |
27,587 |
||||||||||||
| |
|
|
|
|
|
|||||||||||
| • | financial assets at amortised cost |
| • | financial assets at fair value through profit or loss (FVPL) |
| • | the entity’s business model for managing the financial asset |
| • | the contractual cash flow characteristics of the financial assets |
| • | they are held within a business model whose objective is to hold the financial assets and collect its contractual cash flows; and |
| • | the contractual terms of the financial assets give rise to cash flows that are solely payments of principal and interest on the principal amount outstanding. |
| • | When the deferred income tax asset or liability arises from the initial recognition of goodwill or an asset or liability in a transaction that is not a business combination and that, at the time of the transaction, affects neither the accounting nor taxable profits; or |
| • | When the taxable temporary difference is associated with interests in subsidiaries, associates or joint ventures, and the timing of the reversal can be controlled and it is probable that the temporary difference will not reverse in the foreseeable future. |
| • | during the vesting period, the liability at each reporting date is the fair value of the award at that date multiplied by the expired portion of the vesting period. |
| • | from the end of the vesting period until settlement of the award, the liability is the full fair value of the liability at the reporting date. |
30 June 2021 Reported |
Increase/ (decrease) |
1 July 2021 Restated |
30 June 2022 Reported |
Increase/ (decrease) |
30 June 2022 Restated |
|||||||||||||||||||
A$’000 |
A$’000 |
A$’000 |
A$’000 |
A$’000 |
A$’000 |
|||||||||||||||||||
Consolidated statement of financial position |
||||||||||||||||||||||||
Intangibles - licensing agreement |
27,265 | (1,044 | ) | 26,221 | 27,266 | (1,045 | ) | 26,221 | ||||||||||||||||
Less Accumulated amortisation |
(5,263 | ) | 50 | (5,213 | ) | (7,216 | ) | 134 | (7,082 | ) | ||||||||||||||
| 22,002 | (994 | ) | 21,008 | 20,050 | (911 | ) | 19,139 | |||||||||||||||||
Current contingent consideration |
(3,164 | ) | 2,373 | (791 | ) | (759 | ) | — | (759 | ) | ||||||||||||||
Non-Current contingent consideration |
(8,926 | ) | (1,377 | ) | (10,303 | ) | (8,756 | ) | 547 | (8,209 | ) | |||||||||||||
| (12,091 | ) | 997 | (11,094 | ) | (9,515 | ) | 547 | (8,968 | ) | |||||||||||||||
Net Assets |
37,851 | 2 | 37,853 | 18,638 | (364 | ) | 18,274 | |||||||||||||||||
Accumulated losses |
(44,205 | ) | 2 | (44,203 | ) | (68,253 | ) | (364 | ) | (68,617 | ) | |||||||||||||
Total equity |
37,851 | 2 | 37,853 | 18,638 | (364 | ) | 18,274 | |||||||||||||||||
30 June 2022 |
Increase/ (decrease) |
30 June 2022 Restated |
30 June 2021 |
Increase/ (decrease) |
30 June 2021 Restated |
|||||||||||||||||||
Consolidated statement of profit and loss |
||||||||||||||||||||||||
Research and development expense (Amortisation) |
(20,252 | ) | 83 | (20,169 | ) | (14,541 |
) |
(14,541 |
) |
|||||||||||||||
General and administrative expense (foreign exchange impact) |
(4,511 | ) | (450 | ) | (4,961 | ) | (7,022 |
) |
2 |
(7,020 |
) |
|||||||||||||
Loss on revaluation of contingent consideration |
(152 | ) | — | (152 | ) | (2,570 |
) |
(2,570 |
) |
|||||||||||||||
Commercialisation expense |
(127 | ) | — | (127 | ) | |||||||||||||||||||
Loss before tax |
(25,016 | ) | (366 | ) | (25,382 | ) | (8,906 |
) |
2 |
(8,904 |
) |
|||||||||||||
Income tax benefit |
368 | — | 368 | 484 |
484 |
|||||||||||||||||||
Loss after tax |
(24,648 | ) | (366 | ) | (25,014 | ) | (8,422 |
) |
2
|
(8,420 |
) |
|||||||||||||
| Impact on basic and diluted earnings per share increase/ (decrease) in earning per share | ||||||||||||||||||||||||
Basic loss for the year attributable to equity holders |
(18.61 | ) | (0.27 | ) | (18.88 | ) | (7.16 |
) |
(7.16 |
) |
||||||||||||||
Diluted loss for the year attributable to equity holders |
(18.61 | ) | (0.27 | ) | (18.88 | ) | (7.16 |
) |
(7.16 |
) |
||||||||||||||
30 June 2022 Restated |
Reclassification |
30 June 2022 Reclassified |
||||||||||
Other assets |
156 | 1,841 | 1,997 | |||||||||
Borrowings |
— | (1,841 | ) | (1,841 | ) | |||||||
Current employee benefits |
166 | 203 | 369 | |||||||||
Non-current employee benefits |
319 | (202 | ) | 117 | ||||||||
Loss on remeasurement of contingent consideration |
153 | 153 | — | |||||||||
General and administrative expense |
(4,961 | ) | (153 | ) | (5,114 | ) | ||||||
|
2023 A$’000 |
2022 A$’000 |
2021 A$’000 |
||||||||||
Licensing revenue |
— |
— |
15,183 |
|||||||||
|
2023 A$’000 |
2022 A$’000 |
2021 A$’000 |
||||||||||
Geographical regions |
||||||||||||
China |
— |
— |
10,006 |
|||||||||
Sweden |
— |
— |
5,177 |
|||||||||
— |
— |
15,183 |
||||||||||
Timing of revenue recognition |
||||||||||||
Licensing revenue at a point in time |
— |
— |
15,183 |
|||||||||
|
2023 A$’000 |
2022 A$’000 |
2021 A$’000 |
||||||||||
Payroll tax rebate |
— | — | 2 | |||||||||
Subsidies and grants |
— | 10 | — | |||||||||
Bad debt recovery |
— | 15 | ||||||||||
Other sundry income |
1 | |||||||||||
| 1 | 25 | 2 | ||||||||||
|
2023 A$’000 |
2022 restated A$’000 |
2021 restated A$’000 |
||||||||||
Loss before income tax includes the following specific |
||||||||||||
Research and development |
||||||||||||
EVT-801 program costs |
5,060 | 2,520 | 1,073 | |||||||||
Cantrixil program costs |
5 | 12 | 429 | |||||||||
Paxalisib program costs |
5,618 | 13,713 | 11,404 | |||||||||
Scientific Advisory Board costs |
31 | — | — | |||||||||
|
Employee benefits expense
- salaries & wages and staff benefits
|
2,250 | 1,665 | 336 | |||||||||
- superannuation |
29 | 25 | 26 | |||||||||
- share based payments |
702 | 365 | 8 | |||||||||
Total research & development (excluding amortisation) |
13,695 | 18,300 | 13,276 | |||||||||
Amortisation |
||||||||||||
Paxalisib licensing agreement |
1,084 | 1,084 | 1,084 | |||||||||
Evotech licensing agreement |
785 | 785 | 181 | |||||||||
Total amortisation |
1,869 | 1,869 | 1,265 | |||||||||
Total research & development |
15,564 | 20,169 | 14,541 | |||||||||
Net foreign exchange loss |
||||||||||||
Net foreign exchange loss |
46
|
(2,154 |
) |
409 | ||||||||
Rental expense relating to operating leases |
||||||||||||
Minimum lease payments |
152 | 73 | 93 | |||||||||
Superannuation expense |
||||||||||||
Defined contribution superannuation expense |
131 | 138 | 138 | |||||||||
Employee benefits expense G&A |
||||||||||||
- salaries & wages and staff benefits |
1,467 | 1,674 | 1,011 | |||||||||
- superannuation |
102 | 129 | 112 | |||||||||
- share based payments |
457 | 1,310 | 552 | |||||||||
Total employee benefits expense G&A |
2,026 | 3,113 | 1,675 | |||||||||
Other Expenses |
||||||||||||
Chinese With-Holding Tax incurred on license transaction |
— | — | 931 | |||||||||
Chinese Value Added Tax incurred on license transaction |
— | — | 538 | |||||||||
| — | — | 1,469 | ||||||||||
2022 |
2021 |
|||||||||||
2023 |
restated |
restated |
||||||||||
A$’000 |
A$’000 |
A$’000 |
||||||||||
Numerical reconciliation of income tax benefit and tax at the statutory rate |
||||||||||||
Loss before income tax benefit |
(20,736 | ) | (25,382 | ) | (8,904 | ) | ||||||
Tax at the statutory tax rate of 25% (2022 25% 202 1 26%) |
(5,184 | ) | (6,345 | ) | (2,316 | ) | ||||||
Tax effect amounts which are not deductible/(taxable) in calculating taxable income: |
||||||||||||
Research and Development claim |
— | — | — | |||||||||
Amortisation of intangibles |
467 | 467 | 348 | |||||||||
Employee option plan |
289 | 419 | 175 | |||||||||
Gain/loss on revaluation of contingent consideration |
(847 | ) | 38 | 707 | ||||||||
| (5,275 | ) | (5,421 | ) | (1,086 | ) | |||||||
Adjustment recognized for prior periods |
— | 16 | — | |||||||||
Adjustment to deferred tax balances as a result of change in statutory tax rate |
— | (113 | ) | (186 | ) | |||||||
Tax losses and timing differences not recognized |
5,004 | 5,150 | 788 | |||||||||
Income tax benefit |
(271 | ) | (368 | ) | (484 | ) | ||||||
2023 A$’000 |
2022 restated A$’000 |
2021 restated A$’000 |
||||||||||
Tax losses not recognized |
||||||||||||
Unused tax losses for which no deferred tax asset has been recognized-Australia
|
120,412 | 96,519 | 70,896 | |||||||||
Potential tax benefit @ 25.0% (202 2 25% 2021 26%) |
30,103 | 24,130 | 17,724 | |||||||||
Unused tax losses for which no deferred tax asset has been recognized US |
4,305 | 2,380 | 2,038 | |||||||||
Potential tax benefit at statutory tax rates@21%-US
|
904 | 500 | 428 | |||||||||
2023 |
2022 |
|||||||
A$’000 |
A$’000 |
|||||||
Cash at bank and on hand |
5,241 | 7,361 | ||||||
| 5,241 | 7,361 | |||||||
2023 |
2022 |
|||||||
A$’000 |
A$’000 |
|||||||
Current assets |
||||||||
Trade receivables |
1 | — | ||||||
GBM Agile deposit |
3,753 | — | ||||||
BAS receivables |
104 | 51 | ||||||
Deposits held |
41 | 40 | ||||||
| 3,899 | 91 | |||||||
2023 |
2022 |
|||||||
A$’000 |
A$’000 |
|||||||
Non-current assets |
||||||||
GBM Agile deposit |
— | 7,258 | ||||||
Corporate credit card deposit |
43 | 43 | ||||||
| 43 | 7,301 | |||||||
2022 |
||||||||
2023 |
Reclassified |
|||||||
A$’000 |
A$’000 |
|||||||
Prepayments |
1,632 | 1,997 | ||||||
Consolidated |
||||||||
2022 |
||||||||
|
2023 A$’000 |
restated A$’000 |
|||||||
Licensing agreement - Paxalisib |
16,408 | 16,408 | ||||||
Less: Accumulated amortisation |
(7,251 | ) | (6,166 | ) | ||||
| 9,157 | 10,242 | |||||||
Licensing agreement - EVT-801 |
9,813 | 9,813 | ||||||
Less: Accumulated amortisation |
(1,701 | ) | (916 | ) | ||||
| 8,112 | 8,897 | |||||||
| 17,269 | 19,139 | |||||||
|
EVT801 licensing agreement A$’000 |
Paxalisib licensing agreement A$’000 |
Total A$’000 |
||||||||||
Balance at 1 July 2021 as restated |
9,682 | 11,326 | 21,008 | |||||||||
Amortisation expense |
(785 | ) | (1,084 | ) | (1,869 | ) | ||||||
Balance at 30 June 2022 restated |
8,897 | 10,242 | 19,139 | |||||||||
Amortisation expense |
(785 | ) | (1,085 | ) | (1,870 | ) | ||||||
Balance at 30 June 2023 |
8,112 | 9,157 | 17,269 | |||||||||
2023 |
2022 |
|||||||
A$’000 |
A$’000 |
|||||||
Trade payables |
857 | 1,524 | ||||||
Accrued payables |
3,472 | 2,235 | ||||||
| 4,329 | 3,759 | |||||||
2023 |
2022 Reclassified |
|||||||
A$’000 |
A$’000 |
|||||||
Insurance premium funding |
1,796 | 1,841 | ||||||
2023 A$’000 |
2022 reclassified A$’000 |
|||||||
Current Liabilities |
||||||||
Employee benefits |
201 | — | ||||||
Annual leave |
489 | 369 | ||||||
| 690 | 369 | |||||||
Non-Current Liabilities |
||||||||
Long service leave |
59 | 117 | ||||||
| 749 | 486 | |||||||
2023 A$’000 |
2022 restated A$’000 |
|||||||
Current Liabilities |
||||||||
Contingent consideration – Paxalisib |
750 | — |
||||||
Contingent consideration – EVT801 |
— | 759 | ||||||
| 750 | 759 | |||||||
Non-current Liabilities |
||||||||
Contingent consideration - paxalisib |
654 | 1,168 | ||||||
Contingent consideration – EVT801 |
5,467 | 7,041 | ||||||
| 6,121 | 8,209 | |||||||
| 6,871 | 8,968 | |||||||
Consolidated |
||||||||
2022 |
||||||||
2023 |
restated |
|||||||
$ |
$ |
|||||||
Contingent consideration at start of period (current and non-current)
|
8,968 | 11,094 | ||||||
Payment of EVT801 milestone |
— | (2,364 | ) | |||||
Interest on unwinding of discount |
594 | 567 | ||||||
Effect of exchange rates on contingent consideration |
697 | (329 | ) | |||||
Gain on remeasurement of contingent consideration |
(3,388 | ) | — | |||||
| 6,871 | 8,968 | |||||||
2023 |
2022 |
|||||||
A$’000 |
A$’000 |
|||||||
Non-current Liabilities |
||||||||
Deferred tax liability associated with Licensing Agreement |
2,289 | 2,560 | ||||||
Consolidated |
||||||||||||||||
2023 |
2022 |
2023 |
2022 |
|||||||||||||
Shares |
Shares |
$ |
$ |
|||||||||||||
Ordinary shares - fully paid |
228,029,114 | 138,755,376 | 97,452,246 | 84,480,249 | ||||||||||||
Details |
Date |
Shares |
Issue price |
$ |
||||||||||||
Balance |
1 July 2021 | 132,012,209 | 80,290,062 | |||||||||||||
Issued on conversion of options |
15 December 2021 | 25,000 | $ | 0.6680 | 16,700 | |||||||||||
Conversion of Triaxial Convertible Note |
5 May 2022 | 1,855,357 | $ | 0.2500 | 464,000 | |||||||||||
ATM issue of shares No. 1 |
24 May 2022 | 10,000 | $ | 0.8260 | 8,256 | |||||||||||
ATM issue of shares No. 2 |
2 June 2022 | 10,000 | $ | 0.8020 | 8,025 | |||||||||||
ATM issue of shares No. 3 |
6 June 2022 | 88,710 | $ | 0.8370 | 74,258 | |||||||||||
ATM issue of shares No. 4 |
9 June 2022 | 603,500 | $ | 0.8400 | 507,035 | |||||||||||
ATM issue of shares No. 5 |
14 June 2022 | 75,940 | $ | 0.8240 | 62,583 | |||||||||||
ATM issue of shares No. 6 |
15 June 2022 | 2,000 | $ | 0.8300 | 1,661 | |||||||||||
ATM issue of shares No. 7 |
20 June 2022 | 4,072,660 | $ | 0.8690 | 3,540,403 | |||||||||||
Less: share issue transaction costs |
— | $ | 0.0000 | (492,735 | ) | |||||||||||
Balance |
30 June 2022 | 138,755,376 | 84,480,249 | |||||||||||||
ATM issue of shares No. 8 |
7 July 2022 | 573,370 | $ | 0.7102 | 407,201 | |||||||||||
ATM issue of shares No. 9 |
8 August 2022 | 8,561,490 | $ | 0.3316 | 2,839,346 | |||||||||||
ATM issue of shares No. 10 |
9 August 2022 | 10,000 | $ | 0.2723 | 2,723 | |||||||||||
ATM issue of shares No. 11 |
10 August 2022 | 158,020 | $ | 0.2465 | 38,949 | |||||||||||
ATM issue of shares No. 12 |
11 August 2022 | 330,960 | $ | 0.2413 | 79,868 | |||||||||||
ATM issue of shares No. 13 |
12 August 2022 | 1,247,440 | $ | 0.2469 | 308,050 | |||||||||||
ATM issue of shares No. 14 |
12 September 2022 | 651,030 | $ | 0.2211 | 143,964 | |||||||||||
ATM issue of shares No. 15 |
13 September 2022 | 28,350 | $ | 0.2187 | 6,200 | |||||||||||
Shares issued to Scientific Advisory Board |
14 September 2022 | 60,000 | $ | 0.2100 | 12,600 | |||||||||||
ATM issue of shares No. 16 |
7 October 2022 | 736,760 | $ | 0.1789 | 131,797 | |||||||||||
ATM issue of shares No. 17 |
28 October 2022 | 12,296,180 | $ | 0.1865 | 2,293,288 | |||||||||||
ATM issue of shares No. 18 |
11 January 2023 | 20,000 | $ | 0.1380 | 2,761 | |||||||||||
|
Professional and sophisticated investors placement
– 1st tranche
|
16 January 2023 | 25,387,018 | $ | 0.1100 | 2,792,572 | |||||||||||
|
Professional and sophisticated investors placement
– 2nd tranche
|
2 8 February 2023 |
15,522,075 | $ | 0.1100 | 1,707,428 | |||||||||||
Share Placement Plan |
3 March 2023 | 23,691,045 | $ | 0.1100 | 2,606,000 | |||||||||||
Less: share issue transaction costs |
— | $ | 0.0000 | (400,750 | ) | |||||||||||
Balance |
228,029,114 | 97,452,246 | ||||||||||||||
2023 |
2022 |
|||||||
A$’000 |
A$’000 |
|||||||
| Foreign currency reserve |
(742 | ) | (852 | ) | ||||
| Share-based payments reserve |
4,423 | 3,264 | ||||||
| |
|
|
|
|||||
| 3,681 | 2,412 | |||||||
| |
|
|
|
|||||
Assets |
Liabilities |
|||||||||||||||
| 2023 A$’000 |
2022 restated A$’000 |
2023 A$’000 |
2022 A$’000 |
|||||||||||||
| US dollars |
2,326 | 7,276 | 1,135 | 3,071 | ||||||||||||
| Euros |
— | — | 2,710 | 205 | ||||||||||||
| Singapore dollars |
— | — | 1 | — | ||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| 2,326 | 7,276 | 3,846 | 3,276 | |||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
AUD strengthened |
AUD weakened |
|||||||||||||||||||||||
Consolidated - 2023 |
% change |
Effect on profit before tax A$’000 |
Effect on equity A$’000 |
% change |
Effect on profit before tax A$’000 |
Effect on equity A$’000 |
||||||||||||||||||
| US dollars |
10 | % | (494 | ) | (494 | ) | (10 | %) | 494 | 494 | ||||||||||||||
| Euros |
10 | % | 271 | 271 | (10 | %) | (271 | ) | (271 | ) | ||||||||||||||
| |
|
|
|
|
|
|
|
|||||||||||||||||
| (223 | ) | (223 | ) | 223 | 223 | |||||||||||||||||||
| |
|
|
|
|
|
|
|
|||||||||||||||||
AUD strengthened |
AUD weakened |
|||||||||||||||||||||||
Consolidated – 2022 (restated) |
% change |
Effect on profit before tax A$’000 |
Effect on equity A$’000 |
% change |
Effect on profit before tax A$’000 |
Effect on equity A$’000 |
||||||||||||||||||
| US dollars |
10 | % | (420 | ) | (420 | ) | (10 | %) | 420 | 420 | ||||||||||||||
| Euros |
10 | % | 20 | 20 | (10 | %) | (20 | ) | (20 | ) | ||||||||||||||
| |
|
|
|
|
|
|
|
|||||||||||||||||
| (400 | ) | (400 | ) | 400 | 400 | |||||||||||||||||||
| |
|
|
|
|
|
|
|
|||||||||||||||||
2023 |
2022 |
|||||||||||||||
| Weighted average interest rate % |
Balance A$’000 |
Weighted average interest rate % |
Balance A$’000 |
|||||||||||||
| Cash at bank and in hand |
0.40 | 5,241 | — | 7,361 | ||||||||||||
| |
|
|
|
|||||||||||||
| Net exposure to cash flow interest rate risk |
5,241 | 7,361 | ||||||||||||||
| |
|
|
|
|||||||||||||
2023 |
Weighted average interest rate % |
1 year or less A$’000 |
Between 1 and 2 years A$’000 |
Between 2 and 5 years A$’000 |
Over 5 years A$’000 |
Remaining contractual maturities A$’000 |
||||||||||||||||||
| Non-derivatives |
||||||||||||||||||||||||
| Trade payables |
— | 857 | — | — | — | 857 | ||||||||||||||||||
| Accrued payables |
— | 3,472 | — | — | — | 3,472 | ||||||||||||||||||
| Contingent consideration |
— | 750 | 4,303 | 3,099 | — | 8,152 | ||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total non-derivatives
|
5,079 | 4,303 | 3,099 | — | 12,481 | |||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|||||||||||||||
2022 restated |
Weighted average interest rate % |
1 year or less A$’000 |
Between 1 and 2 years A$’000 |
Between 2 and 5 years A$’000 |
Over 5 years A$’000 |
Remaining contractual maturities A$’000 |
||||||||||||||||||
| Non-derivatives |
||||||||||||||||||||||||
| Trade payables |
— | 1,524 | — | — | — | 1,524 | ||||||||||||||||||
| Accrued payables |
— | 2,236 | — | — | — | 2,236 | ||||||||||||||||||
| Contingent consideration |
— | 759 | 8,338 | 795 | — | 9,892 | ||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total non-derivatives
|
4,519 | 8,338 | 795 | — | 13,652 | |||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|||||||||||||||
Consolidated - 2023 |
Level 1 A$’000 |
Level 2 A$’000 |
Level 3 A$’000 |
Total A$’000 |
||||||||||||
| Liabilities |
||||||||||||||||
| Contingent Consideration |
— | — | 1,404 | 1,404 | ||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Total liabilities |
— | — | 1,404 | 1,404 | ||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
Consolidated - 2022 |
||||||||||||||||
| Liabilities |
||||||||||||||||
| Contingent Consideration |
— | — | 1,168 | 1,168 | ||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Total liabilities |
— | — | 1,168 | 1,168 | ||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Level 3 A$’000 |
Total A$’000 |
|||||||
| Consolidated |
||||||||
| Balance at 1 July 2021 |
1,015 | 1,015 | ||||||
| Losses recognized in profit or loss |
153 | 153 | ||||||
| |
|
|
|
|
|
|
|
|
| Balance at 30 June 2022 |
1,168 | 1,168 | ||||||
| |
|
|
|
|||||
| Losses recognized in profit or loss |
236 | 236 | ||||||
| |
|
|
|
|||||
| Balance at 30 June 2023 |
1,404 | 1,404 | ||||||
| 2023 A$’000 |
2022 A$’000 |
2021 A$’000 |
||||||||||
| Short-term employee benefits |
3,148 | 2,589 | 1,574 | |||||||||
| Post-employment benefits |
150 | 116 | 112 | |||||||||
| Share-based payments |
1,046 | 1,560 | 617 | |||||||||
| |
|
|
|
|
|
|||||||
| 4,344 | 4,265 | 2,303 | ||||||||||
| |
|
|
|
|
|
|||||||
2023 A$’000 |
2022 A$’000 |
2021 A$’000 |
||||||||||
| Audit services – BDO Audit Pty Ltd |
||||||||||||
| Audit or review of the financial statements |
292 | — | — | |||||||||
| Other services BDO Audit Pty Ltd |
||||||||||||
| Comfort letter – ATM |
18 | — | — | |||||||||
| |
|
|
|
|
|
|||||||
| 310 | — | — | ||||||||||
| |
|
|
|
|
|
|||||||
| Audit services – Grant Thornton Audit Pty Ltd |
||||||||||||
| Audit or review of the financial statements |
— | 155 | 151 | |||||||||
| Other services - Grant Thornton Audit Pty Ltd |
||||||||||||
| Comfort letter – ATM |
— | 26 | — | |||||||||
| |
|
|
|
|
|
|||||||
| — | 181 | 151 | ||||||||||
| |
|
|
|
|
|
|||||||
Parent |
||||||||
2023 A$’000 |
2022 restated A$’000 |
|||||||
| Statement of profit or loss and other comprehensive income |
||||||||
| Loss after income tax |
(20,863 | ) | (24,241 | ) | ||||
| |
|
|
|
|||||
| Total comprehensive income |
(20,863 | ) | (24,241 | ) | ||||
| |
|
|
|
|||||
2023 A$’000 |
2022 restated A$’000 |
|||||||
| Statement of financial position |
||||||||
| Total current assets |
4,645 | 7,736 | ||||||
| Total assets |
21,915 | 26,875 | ||||||
| Total current liabilities |
7,003 | 2,931 | ||||||
| Total liabilities |
15,472 | 13,701 | ||||||
| Equity |
||||||||
| Contributed equity |
97,452 | 84,480 | ||||||
| Reserves |
4,423 | 3,264 | ||||||
| Accumulated losses |
(95,432 | ) | (74,570 | ) | ||||
| |
|
|
|
|||||
| Total equity |
6,443 | 13,174 | ||||||
| |
|
|
|
|||||
| • | Investments in subsidiaries are accounted for at cost, less any impairment, in the parent entity. |
| • | Dividends received from subsidiaries are recognized as other income by the parent entity and its receipt may be an indicator of an impairment of the investment. |
Ownership interest |
||||||||||
Name |
Principal place of business / Country of incorporation |
2023 % |
2022 % |
|||||||
Kazia Laboratories Pty Ltd |
Australia | 100.00 | % | 100.00 | % | |||||
Kazia Research Pty Ltd |
Australia | 100.00 | % | 100.00 | % | |||||
Kazia Therapeutics Inc. |
United States of America | 100.00 | % | 100.00 | % | |||||
Glioblast Pty Ltd |
Australia | 100.00 | % | 100.00 | % | |||||
*Kazia Therapeutics (Hong Kong) Limited |
Hong Kong | — | 100.00 | % | ||||||
2023 |
2022 restated |
2021 restated |
||||||||||
A$’000 |
A$’000 |
A$’000 |
||||||||||
Loss after income tax expense from continuing operations |
(20,465 | ) | (25,014 | ) | (8,420 | ) | ||||||
Adjustments for: |
||||||||||||
Depreciation & amortisation |
1,869 | 1,869 | 1,265 | |||||||||
Net fair value loss on financial assets |
— | — | — | |||||||||
Share based payments |
1,159 | 1,675 | 637 | |||||||||
Foreign exchange differences |
46 | (2,154 | ) | 428 | ||||||||
(Gain)/loss on remeasurement of contingent consideration |
(2,097 | ) | — | 2,570 | ||||||||
Change in operating assets & liabilities: |
(19,488 | ) | (23,624 | ) | (3,520 | ) | ||||||
Decrease/(increase) in trade and other receivables |
3,450 | (7 | ) | (5,027 | ) | |||||||
Decrease/(increase) in prepayments |
365 | (277 | ) | (1,182 | ) | |||||||
Decrease/(increase) in trade and other payables |
569 | (528 | ) | 1,010 | ||||||||
Decrease in deferred tax liability |
(271 | ) | (368 | ) | (484 | ) | ||||||
Increase in other provisions |
264 | 201 | 92 | |||||||||
(Decrease)/increase in borrowings |
(45 | ) | 1,841 | — | ||||||||
Net cash used in operating activities |
(15,156 | ) | (22,762 | ) | (9,111 | ) | ||||||
|
2023 A$’000 |
2022 restated A$’000 |
2021 restated A$’000 |
||||||||||
Earnings per share for loss from continuing operations |
||||||||||||
Loss after income tax attributable to the owners of Kazia Therapeutics Limited |
(20,465 | ) | (25,014 | ) | (8,420 | ) | ||||||
Loss after income tax attributable to the owners of Kazia Therapeutics Limited |
(20,465 | ) | (25,014 | ) | (8,420 | ) | ||||||
Number |
Number |
Number |
||||||||||
Weighted average number of ordinary shares used in calculating basic earnings per share |
184,284,350 | 132,467,686 | 117,674,543 | |||||||||
Weighted average number of ordinary shares used in calculating diluted earnings per share |
184,284,350 | 132,467,686 | 117,674,543 | |||||||||
Cents |
Cents |
Cents |
||||||||||
Basic earnings per share |
(11.23 | ) | (18.88 | ) | (7.16 | ) | ||||||
Diluted earnings per share |
(11.23 | ) | (18.88 | ) | (7.16 | ) | ||||||
|
Number of options 2023 |
Weighted average exercise price 2023 |
Number of options 2022 |
Weighted average exercise price 2022 |
|||||||||||||
Outstanding at the beginning of the financial year |
8,655,500 | $ | 1.2826 | 4,219,000 | $ | 0.8911 | ||||||||||
Granted |
7,930,000 | $ | 0.1785 | 4,800,000 | $ | 1.6115 | ||||||||||
Forfeited |
(1,550,000 | ) | $ | 1.8977 | — | $ | 0.0000 | |||||||||
Exercised |
— | $ | 0.0000 | (25,000 | ) | $ | 0.6700 | |||||||||
Expired |
(255,500 | ) | $ | 1.8977 | (338,500 | ) | $ | 1.1123 | ||||||||
Outstanding at the end of the financial year |
14,780,000 | $ | 0.6292 | 8,655,500 | $ | 1.2826 | ||||||||||
Exercisable at the end of the financial year |
6,483,333 | $ | 0.9572 | 3,180,500 | $ | 0.8770 | ||||||||||
Tranche |
Grant date |
Expiry date |
Exercise price |
Balance at the start of the year |
Granted |
Exercised |
Expired / lapsed on termination of employment |
Balance at the end of the year |
||||||||||||||||||||||||
1 |
07/08/2017 | 07/08/2022 | $ | 0.6700 | 15,500 | — | — | (15,500 | ) | — | ||||||||||||||||||||||
2 |
05/02/2018 | 05/02/2023 | $ | 0.7802 | 240,000 | — | — | (240,000 | ) | — | ||||||||||||||||||||||
3 |
13/11/2019 | 04/01/2024 | $ | 0.4925 | 1,200,000 | — | — | — | 1,200,000 | |||||||||||||||||||||||
4 |
13/01/2020 | 13/01/2025 | $ | 0.8812 | 200,000 | — | — | (12,500 | ) | 187,500 | ||||||||||||||||||||||
5 |
09/11/2020 | 09/11/2024 | $ | 1.1320 | 1,200,000 | — | — | — | 1,200,000 | |||||||||||||||||||||||
6 |
09/11/2020 | 13/01/2025 | $ | 0.8812 | 800,000 | — | — | (200,000 | ) | 600,000 | ||||||||||||||||||||||
7 |
04/01/2021 | 04/01/2025 | $ | 1.6900 | 200,000 | — | — | (12,500 | ) | 187,500 | ||||||||||||||||||||||
8 |
09/09/2021 | 26/06/2026 | $ | 1.3650 | 100,000 | — | — | — | 100,000 | |||||||||||||||||||||||
9 |
16/11/2021 | 16/11/2025 | $ | 1.6900 | 1,000,000 | — | — | (250,000 | ) | 750,000 | ||||||||||||||||||||||
10 |
16/11/2021 | 16/11/2025 | $ | 2.2400 | 1,500,000 | — | — | (1,000,000 | ) | 500,000 | ||||||||||||||||||||||
11 |
16/11/2021 | 16/11/2026 | $ | 1.5600 | 800,000 | — | — | — | 800,000 | |||||||||||||||||||||||
12 |
01/02/2022 | 01/02/2027 | $ | 0.9400 | 800,000 | — | — | — | 800,000 | |||||||||||||||||||||||
13 |
01/02/2022 | 01/02/2027 | $ | 0.9400 | 500,000 | — | — | (75,000 | ) | 425,000 | ||||||||||||||||||||||
14 |
24/05/2022 | 24/05/2027 | $ | 0.7800 | 100,000 | — | — | — | 100,000 | |||||||||||||||||||||||
15 |
03/03/2023 | 03/03/2027 | $ | 0.1500 | — | 3,930,000 | — | — | 3,930,000 | |||||||||||||||||||||||
16 |
03/05/2023 | 03/05/2027 | $ | 0.1870 | — | 4,000,000 | — | — | 4,000,000 | |||||||||||||||||||||||
| 8,655,500 | 7,930,000 | — | (1,805,500 | ) | 14,780,000 | |||||||||||||||||||||||||||
Weighted average exercise price |
$ | 1.2826 | $ | 0.1785 | $ | 0.0000 | $ | 1.8977 | $ | 0.6292 | ||||||||||||||||||||||
Tranche |
Grant date |
Expiry date |
Exercise price |
Balance at the start of the year |
Granted |
Exercised |
Expired / lapsed on termination of employment |
Balance at the end of the year |
||||||||||||||||||||||||
1 |
05/09/2016 | 05/09/2021 | $ | 1.6300 | 50,000 | — | — | (50,000 | ) | — | ||||||||||||||||||||||
2 |
12/10/2016 | 17/10/2021 | $ | 1.5600 | 62,000 | — | — | (62,000 | ) | — | ||||||||||||||||||||||
3 |
31/10/2016 | 01/11/2021 | $ | 1.3800 | 12,500 | — | — | (12,500 | ) | — | ||||||||||||||||||||||
4 |
21/11/2016 | 23/11/2021 |
$ | 1.3800 | 50,000 | — | — | (50,000 | ) | — | ||||||||||||||||||||||
5 |
07/08/2017 | 07/08/2022 | $ | 0.6700 | 87,000 | — | (25,000 | ) | (46,500 | ) | 15,500 | |||||||||||||||||||||
6 |
05/02/2018 | 05/02/2023 | $ | 0.7800 | 320,000 | — | — | (80,000 | ) | 240,000 | ||||||||||||||||||||||
7 |
04/01/2019 | 04/01/2024 | $ | 0.4925 | 37,500 | — | — | (37,500 | ) | — | ||||||||||||||||||||||
8 |
13/11/2019 | 13/11/2023 |
$ | 0.4925 | 1,200,000 | — | — | — | 1,200,000 | |||||||||||||||||||||||
9 |
13/01/2020 | 13/01/2025 |
$ | 0.8812 | 200,000 | — | — | — | 200,000 | |||||||||||||||||||||||
10 |
09/11/2020 | 09/11/2024 | $ | 1.1320 | 1,200,000 | — | — | — | 1,200,000 | |||||||||||||||||||||||
11 |
09/11/2020 | 09/11/2024 | $ | 0.8812 | 800,000 | — | — | — | 800,000 | |||||||||||||||||||||||
12 |
04/01/2021 | 04/01/2026 | $ | 1.6900 | 200,000 | — | — | 200,000 | ||||||||||||||||||||||||
13 |
09/09/2021 | 26/06/2026 |
$ | 1.3700 | — | 100,000 | — | — | 100,000 | |||||||||||||||||||||||
14 |
16/11/2021 | 16/11/2025 |
$ | 1.6900 | — | 1,000,000 | — | — | 1,000,000 | |||||||||||||||||||||||
15 |
16/11/2021 | 16/11/2025 |
$ | 2.2400 | — | 1,500,000 | — | — | 1,500,000 | |||||||||||||||||||||||
16 |
16/11/2021 | 16/11/2025 |
$ | 1.5600 | — | 800,000 | — | — | 800,000 | |||||||||||||||||||||||
17 |
01/02/2022 | 01/02/2027 | $ | 0.9400 | — | 800,000 | — | — | 800,000 | |||||||||||||||||||||||
18 |
01/02/2022 | 01/02/2027 | $ | 0.9400 | — | 500,000 | — | — | 500,000 | |||||||||||||||||||||||
19 |
24/05/2022 | 24/05/2027 |
$ | 0.7800 | — | 100,000 | — | — | 100,000 | |||||||||||||||||||||||
| 4,219,000 | 4,800,000 | (25,000 | ) | (338,500 | ) | 8,655,500 | ||||||||||||||||||||||||||
Weighted average exercise price |
$ | 0.8911 | $ | 1.6110 | $ | 0.6700 | $ | 1.1123 | $ | 1.2826 | ||||||||||||||||||||||
| • | Tranches 12 & 13 vests 25% 6 months from issue date and then in 3 amounts at 6 monthly intervals from the date of issue. |
| • | Tranches 14 vests 33% immediately then in two equal amounts annually from the date of grant. |
• |
Tranches 15 vests 6 months from issue date then in two equal amounts annually from the date of grant. |
• |
Tranches 16 vests 33% immediately then in two equal amounts annually from the date of grant. |
| • | The option must have vested; |
| • | Option holder must have provided the Company with an Exercise Notice and have paid the Exercise Price for the option; |
| • | The Exercise Notice must be for the exercise of at least the Minimum Number of Options; and |
| • | The Exercise Notice must have been provided to the Company and Exercise Price paid before the expiry of 4 years from the date the Option is issued. |
Grant date |
Expiry date |
Share price at Grant Date |
Exercise price |
Volatility (%) |
Dividend yield (%) |
Risk free Rate (%) |
Fair value per option |
|||||||||
| 13/11/2019 | 04/01/2024 | $0.4100 | $0.4925 | 74.50% | — | 1.95% | $ | 0.180 | ||||||||
| 13/01/2020 | 13/01/2025 | $0.6200 | $0.8812 | 74.50% | — | 1.95% | $ | 0.340 | ||||||||
| 09/11/2020 | 13/11/2024 | $0.8900 | $1.1320 | 90.00% | — | 0.10% | $ | 0.413 | ||||||||
| 09/11/2020 | 13/01/2025 | $0.8900 | $0.8812 | 90.00% | — | 0.10% | $ | 0.503 | ||||||||
| 04/01/2021 | 04/01/2025 | $1.1850 | $1.6900 | 90.00% | — | 0.19% | $ | 0.600 | ||||||||
| 09/09/2021 | 21/06/2026 | $1.4200 | $1.3700 | 76.00% | — | 1.50% | $ | 0.880 | ||||||||
| 16/11/2021 | 16/11/2025 | $1.5700 | $1.6900 | 76.00% | — | 1.50% | $ | 0.850 | ||||||||
| 16/11/2021 | 16/11/2025 | $1.5700 | $2.2400 | 76.00% | — | 1.50% | $ | 0.750 | ||||||||
| 16/11/2021 | 16/11/2026 | $1.5700 | $1.5600 | 76.00% | — | 1.50% | $ | 0.970 | ||||||||
| 01/02/2022 | 01/02/2027 | $0.9600 | $0.9400 | 79.00% | — | 1.50% | $ | 0.590 | ||||||||
| 24/05/2022 | 24/05/2027 | $0.8000 | $0.7800 | 44.00% | — | 2.95% | $ | 0.630 | ||||||||
| 03/01/2023 | 03/03/2027 | $0.1700 | $0.1500 | 80.00% | — | 3.64% | $ | 0.10137 | ||||||||
| 03/03/2023 | 03/03/2027 | $0.1700 | $0.1500 | 80.00% | — | 3.64% | $ | 0.10137 | ||||||||
| 03/05/2023 | 03/05/2027 | $0.1900 | $0.1870 | 80.00% | — | 3.22% | $ | 0.11110 | ||||||||
(a) |
The Company sends written or electronic communications to all shareholders whose Shares are held on the Company’s Australian principal share register, in form and substance satisfactory to ASX (Notice), setting out: |
(i) |
the nominated time and date at which the entity will be removed from the ASX and that: |
(A) |
if they wish to sell their Shares on ASX, they will need to do so before then; and |
(B) |
if they don’t, thereafter they will only be able to sell the underlying securities on-market on Nasdaq in the form of ADSs; and |
(ii) |
generally what they need to do if they wish to sell their securities on Nasdaq. |
(b) |
The removal shall not take place any earlier than one month after the date the information in the Notice has been sent to shareholders. |
(c) |
The Company releases the full terms of this decision to the market upon making a formal application to ASX to remove the Company from the official list of ASX. |
Exhibit 4.23

Dr John Friend
23 August 2023
| By email: | [***] |
Dear John
I refer to your appointment as Chief Executive Officer of Kazia Therapeutics Limited effective on 1 May 2023.
This letter sets out the amendments to your existing contract of employment as Chief Medical Officer (Existing Contract) dated 20 September 2021, specifically in relation to:
| • | your roles and responsibilities in your role of Chief Executive Officer; and |
| • | your remuneration. |
Unless as otherwise set out in this letter, all terms set out in your Existing Contract remain applicable.
The amended terms of your employment are set out below:
Position
Your role as Chief Executive Officer (CEO) of Kazia Therapeutics Limited involves the following roles and responsibilities:
Role Purpose
| • | The CEO drives the strategy for the company and leads the executive team in driving development and growth plans through prioritization of current and future assets in development as well as support the Board. |
| • | The CEO reports to the Board of Directors and plays a pivotal role in defining and advancing the company’s strategy, providing leadership that enables Kazia Therapeutics to leverage its outstanding product pipeline and existing external team of clinical trialists. |
| • | Important areas of focus include ensuring appropriate investment decisions are made around the portfolio, ensuring the company has sufficient long term financial runway for supporting its ambitions, delivering a focused and professional business plan, and building the already excellent reputation of the science, medicine portfolio, and team into a world-class biotechnology company. |
Key Responsibilities
Provide overall strategic leadership and management of Kazia Therapeutics covering:
| • | Leading the Executive Team and delivering the key performance indicators of the business. |
| • | Communicate the compelling story to the investor community on the strategy of the company, as well as nurturing existing and new investors. |
| • | Growing the value of the pipeline, advancing its novel medicines towards the market through delivery of pipeline advances and shifts to the next stage of development of the portfolio. |
Kazia Therapeutics Limited ABN 37 063 259 754
Three International Towers, Level 24, 300 Barangaroo Avenue, Sydney NSW 2000 Australia
T +61 2 9472 4101 E info@kaziatherapeutics.com
www.kaziatherapeutics.com
| • | Developing and leading a high-performance management team and creating an environment in which all employees will reach their potential. |
| • | Developing and maintaining strong relationships with employees from US and Australian locations. |
| • | Creating and communicating a vision for the business and winning support for that vision from all stakeholders and investors. |
| • | Developing and leading all finance raising activities and efforts in the event of another fundraising round and/or public offering. |
| • | Representing the company as the principal spokesperson to all external stakeholders and continuing to build the profile of the business internationally. |
| • | Enrich the visibility of the company through personal involvement with key industry constituents and active participation in appropriate meetings and conferences. |
| • | Generating a solid financial position through managing potential revenue and royalty growth, cost structure and retaining a solid cash and financial position. |
| • | Strategic and operational leadership in Kazia Therapeutics setting the future direction and strategy as it enters its next exciting stage of evolution and growth, specifically prioritizing Kazia Therapeutics’ assets for further development. |
| • | Reporting on all financial and operational progress in accordance with the standard required by a publicly listed company. |
Salary
Your updated compensation as CEO is set out in Attachment 1 of this letter.
| Yours sincerely |
| /s/ Steven Coffey |
| Steven Coffey |
On behalf of the Board of Kazia Therapeutics Limited
Accepted and agreed by:
| /s/ John E. Friend II |
| John E. Friend II |
Date: 23 August 2023
Attachment 1 (updated compensation for role of CEO):
|
Base Salary |
US$ 555,000 per annum.
|
|
|
Short Term Incentives |
Up to 60% of base salary per annum, subject to attainment of key performance objectives.
|
|
|
Long Term Incentives |
3,000,000 options under Kazia’s Employee Share Option Plan (as set out in your Option Letter dated 1 May 2023).
|
|
|
Notice Period |
Your Employment is ‘at will’ and you may resign your employment with the Company at any time.
In the event of termination by the company, change of control, or resignation for good reason, you are entitled to severance in the amount of six to twelve months’ compensation.
|
Exhibit 12.1
Certification of the Chief Executive Officer as required by
Rule 13a-14(a) of the Securities Exchange Act of 1934
I, John Friend, certify that:
| 1. | I have reviewed this Annual Report on Form 20-F for the fiscal year ended June 30, 2023 (‘Report’) of Kazia Therapeutics Limited (the ‘Company’); |
| 2. | Based on my knowledge, this Report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this Report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this Report, fairly present in all material respects the financial condition, results of operations and cash flows of the Company as of, and for, the periods presented in this Report; |
| 4. | The Company’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e) ) and internal control over financial reporting (as defined in the Exchange Act Rules 13a-15(f) and 15d-15(f) for the Company and have: |
| (a) | designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the Company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| (b) | designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| (c) | evaluated the effectiveness of the Company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| (d) | disclosed in this report any change in the Company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect the Company’s internal control over financial reporting; and |
| 5. | The Company’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the Company’s auditors and the audit committee of the Company’s Board of Directors (or persons performing the equivalent functions). |
| (a) | all significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the Company’s ability to record, process, summarize and report financial information; and |
| (b) | any fraud, whether or not material, that involves management or other employees who have a significant role in the Company’s internal control over financial reporting. |
| /s/ John Friend |
| John Friend |
| Chief Executive Officer |
| Date: October 26, 2023 |
Exhibit 12.2
Certification of the Director of Chief Financial Officer as required by
Rule 13a-14(a) of the Securities Exchange Act of 1934
I, Karen Krumeich, certify that:
| 1. | I have reviewed this Annual Report on Form 20-F for the fiscal year ended June 30, 2023 (‘Report’) of Kazia Therapeutics Limited (the ‘Company’); |
| 2. | Based on my knowledge, this Report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this Report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this Report, fairly present in all material respects the financial condition, results of operations and cash flows of the Company as of, and for, the periods presented in this Report; |
| 4. | The Company’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e) ) and internal control over financial reporting (as defined in the Exchange Act Rules 13a-15(f) and 15d-15(f)) for the Company and have: |
| (a) | designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the Company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared; |
| (b) | designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| (c) | evaluated the effectiveness of the Company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| (d) | disclosed in this report any change in the Company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect the Company’s internal control over financial reporting; and |
| 5. | The Company’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the Company’s auditors and the audit committee of the Company’s Board of Directors (or persons performing the equivalent functions). |
| (a) | all significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the Company’s ability to record, process, summarize and report financial information; and |
| (b) | any fraud, whether or not material, that involves management or other employees who have a significant role in the Company’s internal control over financial reporting. |
| /s/ Karen Krumeich |
| Karen Krumeich |
| Chief Financial Officer |
Date: October 26, 2023
Exhibit 13.1
Certification of the Chief Executive Officer and the Chief Financial Officer as required by Rule 13a-14(b) of
the Securities Exchange Act of 1934
Pursuant to the requirement set forth in Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. §1350), John Friend, Chief Executive Officer, and Karen Krumeich, Chief Financial Officer, of Kazia Therapeutics Limited, an Australian corporation (the ‘Company’), hereby certifies that:
| (1) | The Company’s periodic report on Form 20-F for the period ended June 30, 2023 (the ‘Form 20-F’) fully complies with the requirements of section 13(a) or Section 15(d) of the Securities Exchange Act of 1934 as amended; and |
| (2) | The information contained in the Form 20-F fairly presents, in all material respects, the financial condition and results of operations of the Company. |
* * *
| Chief Executive Officer | Chief Financial Officer | |||
| /s/ John Friend |
/s/ Karen Krumeich |
|||
| John Friend | Karen Krumeich | |||
| Date: October 26, 2023 | Date: October 26, 2023 | |||
This certification accompanies the Form 20-F to which it relates, is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference into any filing of Kazia Therapeutics Limited under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of the Form 20-F), irrespective of any general incorporation language contained in such filing.
A signed original of this written statement required by Section 906 has been provided to the Company and will be retained by the Company and furnished to the Securities and Exchange Commission or its staff upon request.
Exhibit 15.1
Consent of Independent Registered Public Accounting Firm
We hereby consent to the incorporation by reference in the Registration Statement on Form F-3 (No.333-259224) of Kazia Therapeutics Limited of our report dated October 26, 2023, related to the consolidated financial statements which appears in the Annual Report to Shareholders on Form 20-F. Our report contains an explanatory paragraph regarding the Company’s ability to continue as a going concern.
| /s/ BDO Audit Pty Ltd |
| BDO AUDIT PTY LTD |
| Sydney, Australia |
| October 26, 2023 |
Exhibit 15.2
Consent of Independent Registered Public Accounting Firm
We have issued our report dated October 14, 2022 with respect to the consolidated financial statements included in the Annual Report of Kazia Therapeutics Limited on Form 20-F, prior to the restatement due to the correction of an error, as described in Note 4, and a reclassification as described in Note 5, for the year ended June 30, 2022.
We consent to the incorporation by reference of the said report in the Registration Statement of Kazia Therapeutics Limited on Form F-3 (File No. 333-259224).
| /s/ Grant Thornton Audit Pty Ltd |
| GRANT THORNTON AUDIT PTY LTD |
| Sydney, Australia |
| October 26, 2023 |
Exhibit 15.3

|
U.S. Securities and Exchange Commission Office of the Chief Accountant 100 F Street, NE Washington, DC 20549 |
Grant Thornton Audit Pty Ltd Level 17 383 Kent Street Sydney NSW 2000 Locked Bag Q800 Queen Victoria Building NSW 1230
T +61 2 8297 2400 |
|
| 26 October 2023 |
Re: Kazia Therapeutics Limited
File No. 0-29962
Dear Sir or Madam:
We have read the statements made by Kazia Therapeutics Limited under Item 16F in its Form 20-F dated October 26, 2023, and agree with the statements concerning our Firm contained therein.
Very truly yours,
| /s/ Grant Thornton Audit Pty Ltd |
| GRANT THORNTON AUDIT PTY LTD |
www.grantthornton.com.au
ACN-130 913 594
Grant Thornton Audit Pty Ltd ACN 130 913 594 a subsidiary or related entity of Grant Thornton Australia Limited ABN 41 127 556 389 ACN 127 556 389. ‘Grant Thornton’ refers to the brand under which the Grant Thornton member firms provide assurance, tax and advisory services to their clients and/or refers to one or more member firms, as the context requires. Grant Thornton Australia Limited is a member firm of Grant Thornton International Ltd (GTIL). GTIL and the member firms are not a worldwide partnership. GTIL and each member firm is a separate legal entity. Services are delivered by the member firms. GTIL does not provide services to clients. GTIL and its member firms are not agents of, and do not obligate one another and are not liable for one another’s acts or omissions. In the Australian context only, the use of the term ‘Grant Thornton’ may refer to Grant Thornton Australia Limited ABN 41 127 556 389 ACN 127 556 389 and its Australian subsidiaries and related entities.