UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): October 9, 2023
Reneo Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 001-40315 | 47-2309515 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
| 18575 Jamboree Road, Suite 275-S | ||
| Irvine, California | 92612 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (858) 283-0280
N/A
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange on which registered |
||
| Common stock, par value $0.0001 per share | RPHM | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On October 9, 2023, Reneo Pharmaceuticals, Inc. (the “Company”) hosted a Key Opinion Leader (“KOL”) meeting utilizing the slide presentation furnished as Exhibit 99.1 to this Current Report on Form 8-K. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.1.
The information in this Item 7.01 of this Current Report on Form 8-K and Exhibit 99.1 shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section, nor shall it be deemed to be incorporated by reference into any filing of the Company under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
| Item 8.01 | Other Events. |
On October 9, 2023, the Company announced last patient last visit in the pivotal STRIDE study of mavodelpar in primary mitochondrial myopathies. Topline data results from the STRIDE study are expected in December 2023. In addition, the Company has enrolled over 85% of eligible patients in the Company’s ongoing STRIDE AHEAD study.
The Company anticipates completing the final steps in the clinical process for the STRIDE study in the coming months. Subsequently, the Company plans to share the results of data analysis with the United States Food and Drug Administration (“FDA”) in the first quarter of 2024. The Company expects that the STRIDE and STRIDE AHEAD studies will form the basis of a New Drug Application (“NDA”) to the FDA which is planned for submission in the first half of 2024 and thereafter to additional regulatory agencies.
Forward-Looking Statements
Statements contained in this Current Report on Form 8-K regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include statements regarding, among other things, the timing of topline data from the STRIDE study, the timing of the final steps in the clinical process for the STRIDE study and for sharing the results of data analysis with the FDA, the prospects of the STRIDE AHEAD study, and the potential filing and timing of an NDA to the FDA and thereafter to additional regulatory agencies. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “plans,” “will,” “believes,” “anticipates,” “expects,” “intends,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon the Company’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks and uncertainties associated with the Company’s business in general, and the other risks described in the Company’s filings with the Securities and Exchange Commission. All forward-looking statements contained in this Current Report on Form 8-K speak only as of the date on which they were made. The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits.
| Exhibit No. |
Description |
|
| 99.1 | Reneo Pharmaceuticals, Inc. KOL Event Presentation, dated October 9, 2023 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Reneo Pharmaceuticals, Inc. | ||||||
| Date: October 10, 2023 | By: | /s/ Gregory J. Flesher |
||||
| Gregory J. Flesher Chief Executive Officer |
||||||

Exhibit 99.1 Focused on Improving the Lives of Patients with Rare Genetic Mitochondrial Diseases K Ke ey y O Opi pin niio on n L Le ead ade er r an and d Man Manag age em me en nt t U Updat pdate e October 9, 2023

Forward-Looking Statements This presentation contains forward-looking statements that are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our business strategy, objectives and opportunities. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors that may cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied by our forward-looking statements, including, but not limited to, those related to our dependence on third-party clinical research organizations, manufacturers, suppliers and distributors; our ability to obtain necessary additional capital; our ability to obtain necessary regulatory approvals for our products and, if and when approved, market acceptance of our products; the commercialization plans and expectations for commercializing mavodelpar (REN001) in the United States and rest of world, estimates of the number of patients impacted by PMM or LC-FAOD and who are appropriate for treatment with mavodelpar, the potential benefits of mavodelpar, the financial impact or revenues from any commercialization we undertake, the impact of competitive products and therapies; our ability to attract and retain key employees; the costs of our commercialization plans and development programs; the design, implementation, timing and outcomes of our clinical trials; our ability to manage the growth and complexity of our organization; our ability to maintain, protect and enhance our intellectual property; and our ability to continue to stay in compliance with applicable laws and regulations. You should refer to the section entitled “Risk Factors” set forth in our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and other filings we make with the Securities and Exchange Commission (SEC) from time to time for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to update any forward-looking statements after the date of this presentation except as may be required by law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Projections, assumptions and estimates of the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Mavodelpar is an investigational drug product candidate that is under clinical investigation, and which has not yet been approved for marketing by the U.S. Food and Drug Administration, European Medicines Agency, or any other global regulatory agency. No representation is made as to the safety or effectiveness of this product candidate for the use for which it is being studied. We use our website (https://www.reneopharma.com) and LinkedIn page (https://www.linkedin.com/company/reneo-pharmaceuticals) as channels of distribution of information about our company, product candidates, planned announcements, attendance at upcoming conferences and other matters. Such information may be deemed material information, and we may use these channels to comply with our disclosure obligations under Regulation Fair Disclosure. Therefore, investors should monitor our website and LinkedIn page in addition to following our SEC filings, press releases, public conference calls and webcasts. 2
Today’s Speakers Welcome & Opening Remarks Gregory J. Flesher President & CEO Reneo Pharmaceuticals, Inc. Overview of Mitochondrial Disease Amel Karaa, MD Director of the Mito Clinic Massachusetts General Hospital Harvard Medical School Mavodelpar Development Program Alejandro Dorenbaum, MD Chief Medical Officer Reneo Pharmaceuticals, Inc. Addressable Patients (US) Michael Cruse Chief Operating Officer Reneo Pharmaceuticals, Inc. 3
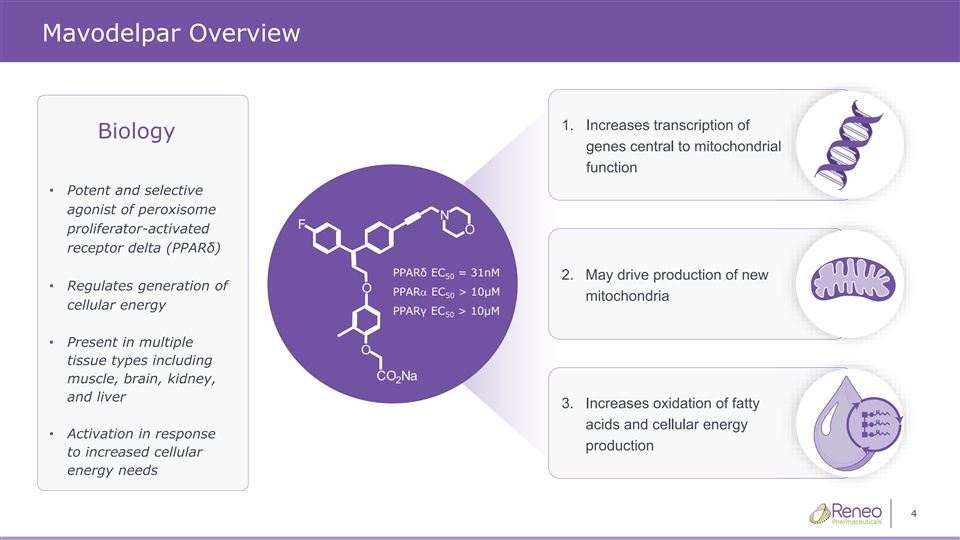
Mavodelpar Overview 1. Increases transcription of Biology gen genes es ce centra ntral l to to mito mitoch chond ondria rial l function • Potent and selective agonist of peroxisome proliferator-activated receptor delta (PPARδ) PPARδ EC = 31nM 50 2. May drive production of new • Regulates generation of PPARa EC > 10μM 50 mitochondria cellular energy PPARγ EC > 10μM 50 • Present in multiple tissue types including muscle, brain, kidney, and liver 3. Increases oxidation of fatty acids and cellular energy • Activation in response production to increased cellular energy needs 4
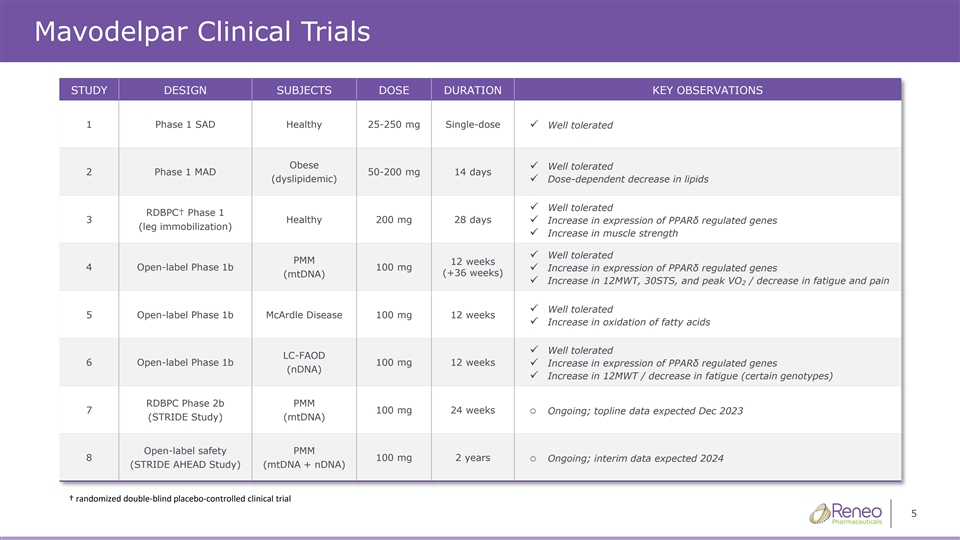
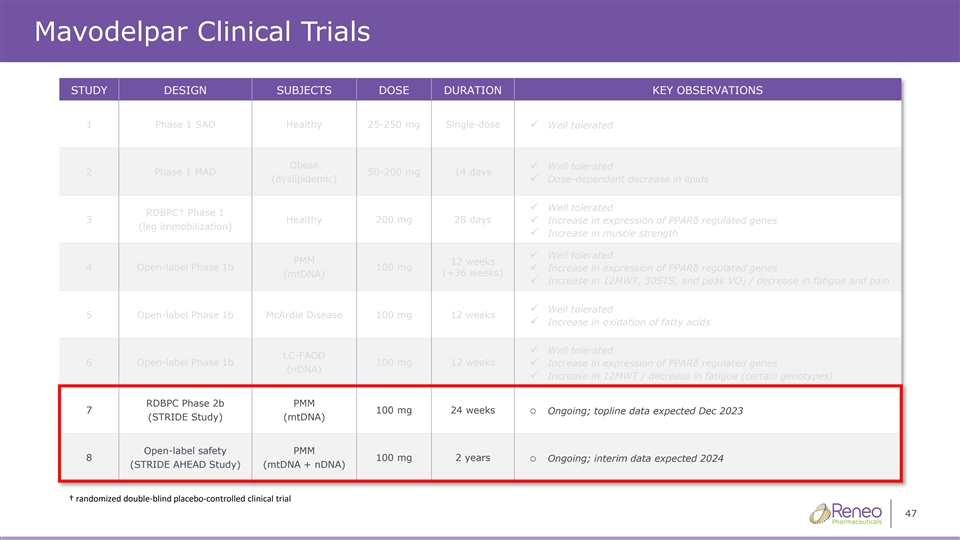
Mavodelpar Clinical Trials STUDY DESIGN SUBJECTS DOSE DURATION KEY OBSERVATIONS 1 Phase 1 SAD Healthy 25-250 mg Single-dose ✓ Well tolerated Obese Well tolerated ✓ 2 Phase 1 MAD 50-200 mg 14 days (dyslipidemic)✓ Dose-dependent decrease in lipids Well tolerated ✓ RDBPC† Phase 1 3 Healthy 200 mg 28 days ✓ Increase in expression of PPARδ regulated genes (leg immobilization) ✓ Increase in muscle strength ✓ Well tolerated PMM 12 weeks 4 Open-label Phase 1b 100 mg ✓ Increase in expression of PPARδ regulated genes (+36 weeks) (mtDNA) ✓ Increase in 12MWT, 30STS, and peak VO / decrease in fatigue and pain 2 ✓ Well tolerated 5 Open-label Phase 1b McArdle Disease 100 mg 12 weeks ✓ Increase in oxidation of fatty acids ✓ Well tolerated LC-FAOD 6 Open-label Phase 1b 100 mg 12 weeks✓ Increase in expression of PPARδ regulated genes (nDNA) ✓ Increase in 12MWT / decrease in fatigue (certain genotypes) RDBPC Phase 2b PMM 7 100 mg 24 weekso Ongoing; topline data expected Dec 2023 (STRIDE Study) (mtDNA) Open-label safety PMM 8 100 mg 2 yearso Ongoing; interim data expected 2024 (STRIDE AHEAD Study) (mtDNA + nDNA) † randomized doubleͲŽďĂŝĚŶŽŶƚƌŽůůͲĞĚĐĂůůŝŶŝĐĐƚƌŝĂů 5 ďůƉůĐĞ
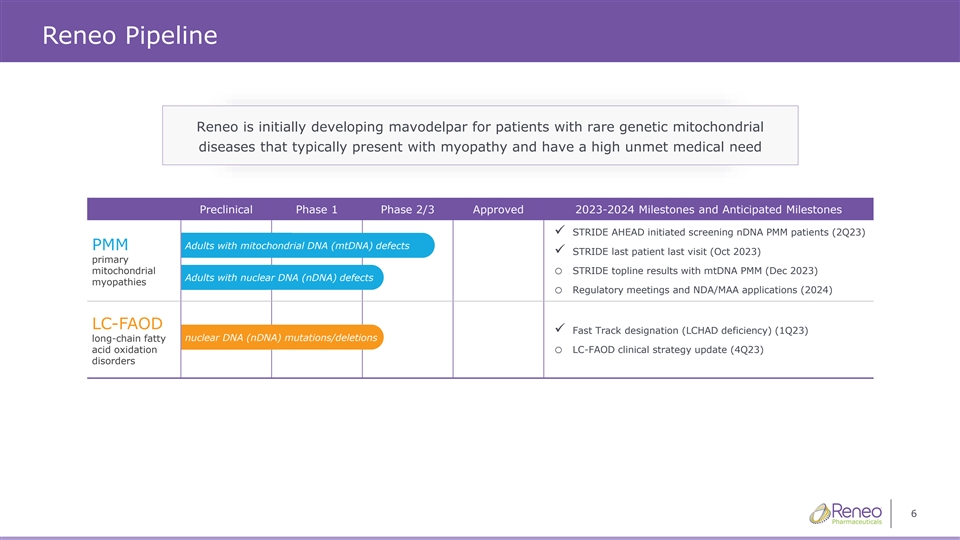
Reneo Pipeline Reneo is initially developing mavodelpar for patients with rare genetic mitochondrial diseases that typically present with myopathy and have a high unmet medical need Preclinical Phase 1 Phase 2/3 Approved 2023-2024 Milestones and Anticipated Milestones ✓ STRIDE AHEAD initiated screening nDNA PMM patients (2Q23) Adults with mitochondrial DNA (mtDNA) defects PMM ✓ STRIDE last patient last visit (Oct 2023) primary mitochondrialo STRIDE topline results with mtDNA PMM (Dec 2023) Adults with nuclear DNA (nDNA) defects myopathies o Regulatory meetings and NDA/MAA applications (2024) LC-FAOD ✓ Fast Track designation (LCHAD deficiency) (1Q23) nuclear DNA (nDNA) mutations/deletions long-chain fatty acid oxidationo LC-FAOD clinical strategy update (4Q23) disorders 6
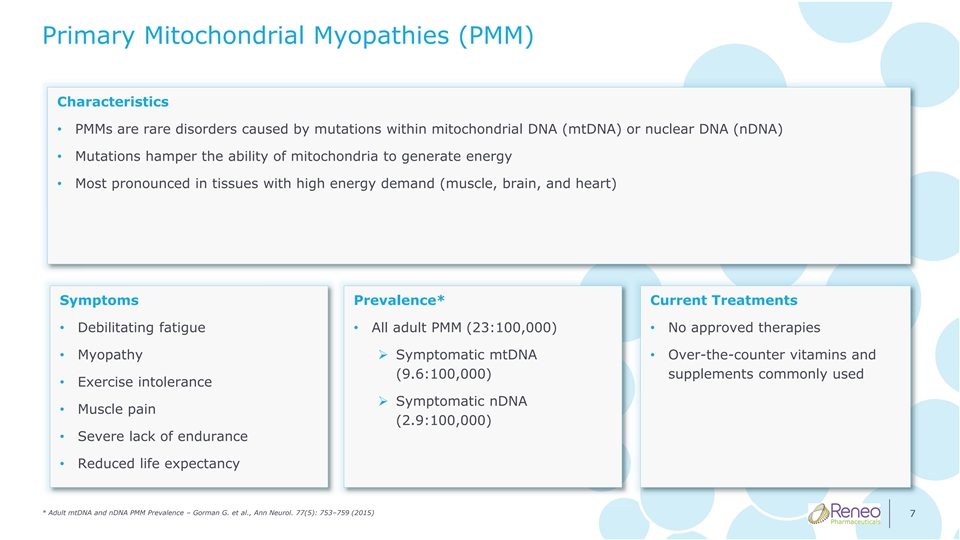
Primary Mitochondrial Myopathies (PMM) Characteristics • PMMs are rare disorders caused by mutations within mitochondrial DNA (mtDNA) or nuclear DNA (nDNA) • Mutations hamper the ability of mitochondria to generate energy • Most pronounced in tissues with high energy demand (muscle, brain, and heart) Symptoms Prevalence* Current Treatments • Debilitating fatigue • All adult PMM (23:100,000) • No approved therapies • Myopathy➢ Symptomatic mtDNA • Over-the-counter vitamins and (9.6:100,000) supplements commonly used • Exercise intolerance ➢ Symptomatic nDNA • Muscle pain (2.9:100,000) • Severe lack of endurance • Reduced life expectancy * Adult mtDNA and nDNA PMM Prevalence – Gorman G. et al., Ann Neurol. 77(5): 753–759 (2015) 7
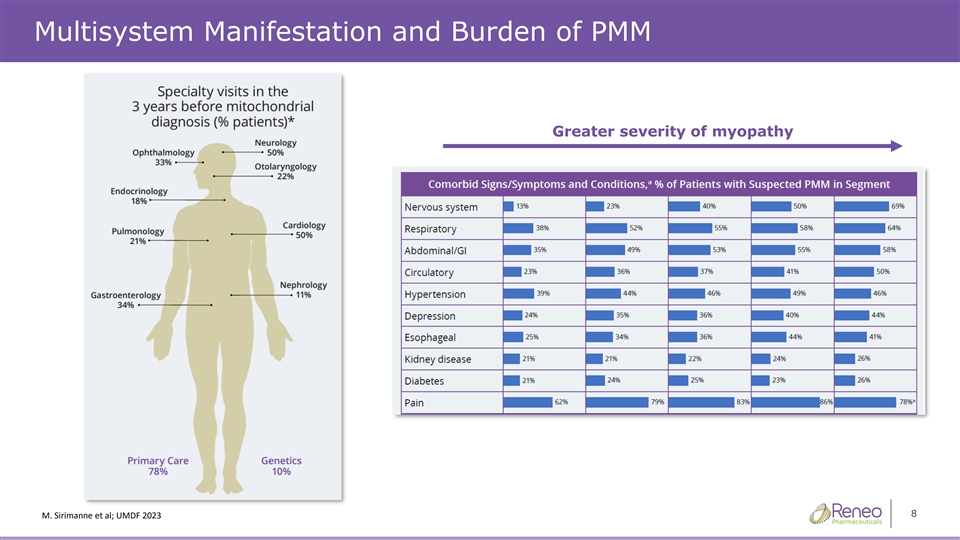
Multisystem Manifestation and Burden of PMM Greater severity of myopathy 8 ĞŶ^ŝD͘ƌŝŵĂŶĞƚϮϯĂůϮϬ͖hD&

Overview of Mitochondrial Disease Amel Karaa, MD Director of the Mito Clinic Massachusetts General Hospital Harvard Medical School STRIDE Principal Investigator

Mitochondrial Myopathy Primer AMEL KARAA, MD DIRECTOR OF THE MITO CLINIC MASSACHUSET TS GENERAL HOSPITAL HARVARD MEDICAL SCHOOL

Disclosures & Disclaimers UMDF: fellow, SMAB Chair-Elect, Planning committee. Mitochondrial clinical network founder and governance board member. North American Mitochondrial Disease Consortium site PI Immediate past President of the Mitochondrial Medicine Society and MitoAction medical board. Grants and research support from Stealth BioTherapeutics, Reata pharmaceuticles, Astellas, MitoBridge, Reneo, Cyclerion, Sanofi Genzyme, Shire, Portalix, Idorsia… Consulting for Sanofi Genzyme, Stealth Biotherapeutics, Alexion, Lumleian, Homology, MitoBridge, Akros, Astellas, Neurovive, Mivovia, Reneo, Zogenix, Cyclerion, UCB, Pretzels Therapeutics, Nanna Therapeutics ….

Disclaimer: The information presented are my own professional and not that of my employer, organization, committee or other group or individual I work with.

Overview of mitochondrial disease
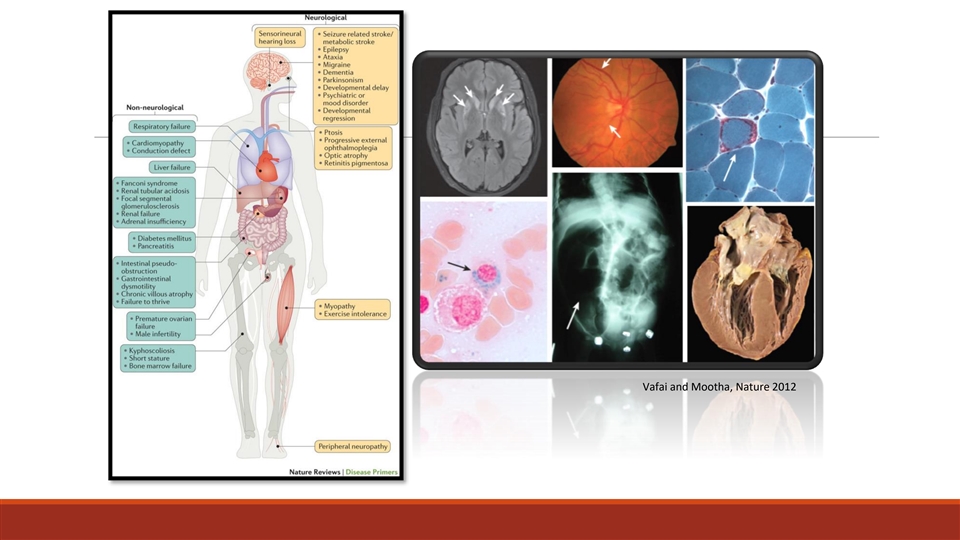
Vafai and Mootha, Nature 2012
Primary Mitochondrial Myopathy (Consortium of International Experts in Mitochondrial Disease) • PMM refers to a subset of primary mitochondrial disease that predominantly but not exclusively affect skeletal muscles. Neuromuscul Disord. 2017 December ; 27(12): 1126–1137. doi:10.1016/j.nmd.2017.08.006.
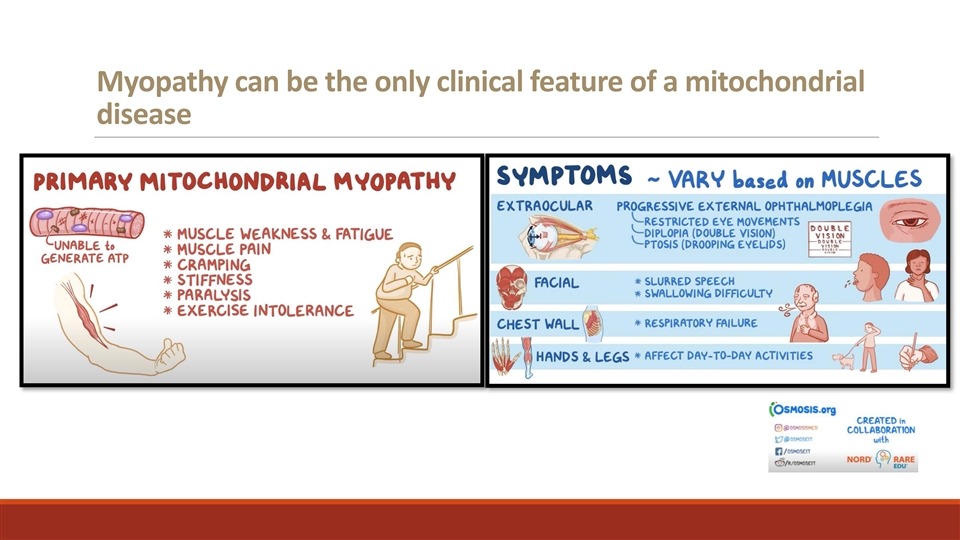
Myopathy can be the only clinical feature of a mitochondrial disease
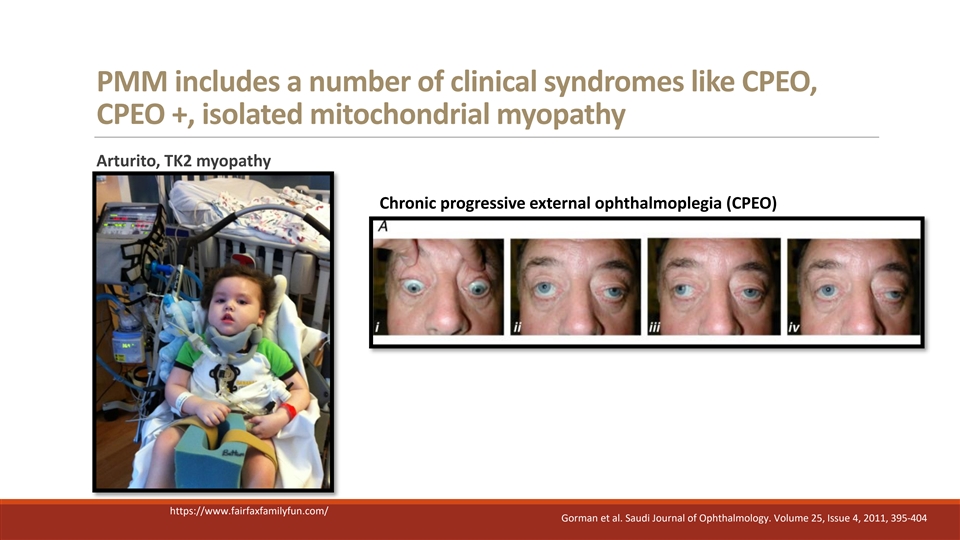
PMM includes a number of clinical syndromes like CPEO, CPEO +, isolated mitochondrial myopathy Arturito, TK2 myopathy Chronic progressive external ophthalmoplegia (CPEO) https://www.fairfaxfamilyfun.com/ Gorman et al. Saudi Journal of Ophthalmology. Volume 25, Issue 4, 2011, 395-404
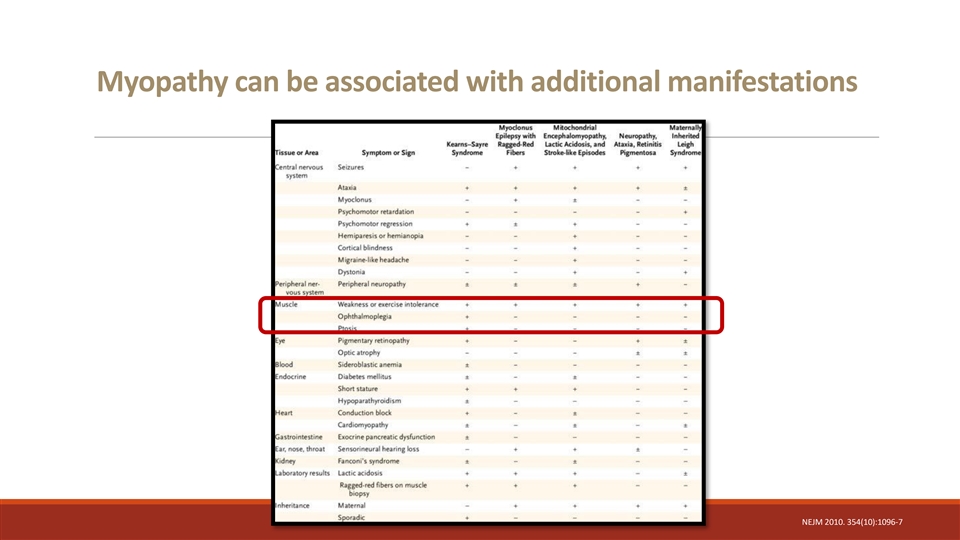
Myopathy can be associated with additional manifestations NEJM 2010. 354(10):1096-7
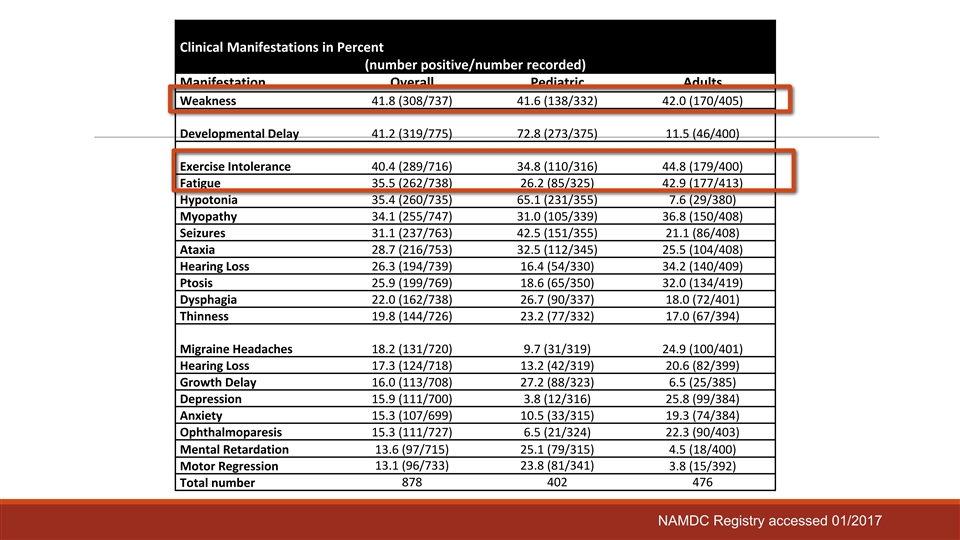
Clinical Manifestations in Percent (number positive/number recorded) Manifestation Overall Pediatric Adults Weakness 41.8 (308/737) 41.6 (138/332) 42.0 (170/405) Developmental Delay 41.2 (319/775) 72.8 (273/375) 11.5 (46/400) Exercise Intolerance 40.4 (289/716) 34.8 (110/316) 44.8 (179/400) Fatigue 35.5 (262/738) 26.2 (85/325) 42.9 (177/413) Hypotonia 35.4 (260/735) 65.1 (231/355) 7.6 (29/380) Myopathy 34.1 (255/747) 31.0 (105/339) 36.8 (150/408) Seizures 31.1 (237/763) 42.5 (151/355) 21.1 (86/408) Ataxia 28.7 (216/753) 32.5 (112/345) 25.5 (104/408) Hearing Loss 26.3 (194/739) 16.4 (54/330) 34.2 (140/409) Ptosis 25.9 (199/769) 18.6 (65/350) 32.0 (134/419) Dysphagia 22.0 (162/738) 26.7 (90/337) 18.0 (72/401) Thinness 19.8 (144/726) 23.2 (77/332) 17.0 (67/394) Migraine Headaches 18.2 (131/720) 9.7 (31/319) 24.9 (100/401) Hearing Loss 17.3 (124/718) 13.2 (42/319) 20.6 (82/399) Growth Delay 16.0 (113/708) 27.2 (88/323) 6.5 (25/385) Depression 15.9 (111/700) 3.8 (12/316) 25.8 (99/384) Anxiety 15.3 (107/699) 10.5 (33/315) 19.3 (74/384) Ophthalmoparesis 15.3 (111/727) 6.5 (21/324) 22.3 (90/403) Mental Retardation 13.6 (97/715) 25.1 (79/315) 4.5 (18/400) 13.1 (96/733) 23.8 (81/341) Motor Regression 3.8 (15/392) Total number 878 402 476 NAMDC Registry accessed 01/2017
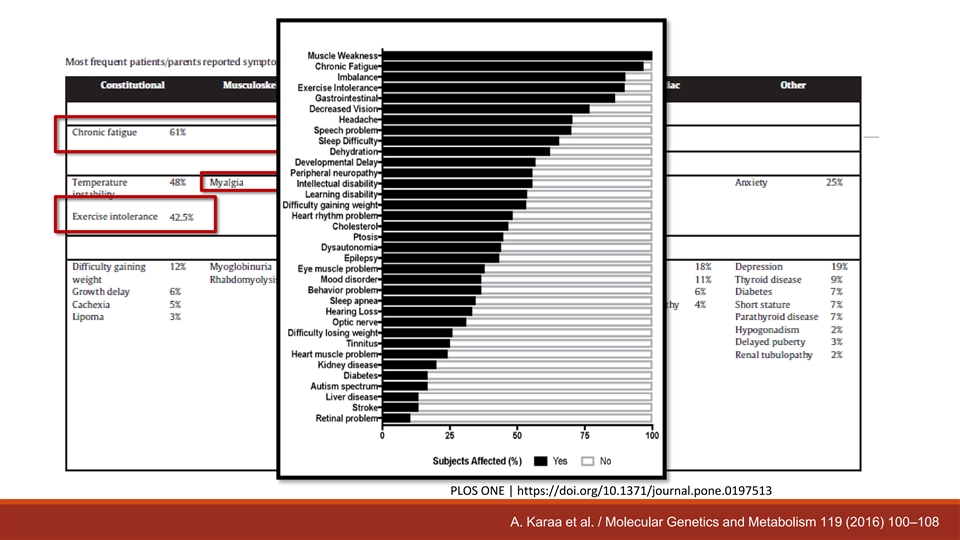
PLOS ONE | https://doi.org/10.1371/journal.pone.0197513 A. Karaa et al. / Molecular Genetics and Metabolism 119 (2016) 100–108
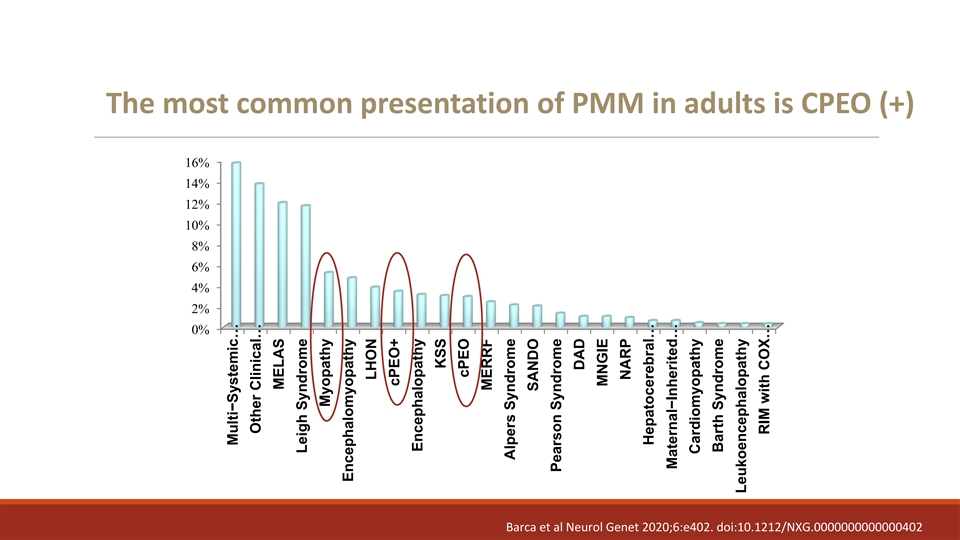
The most common presentation of PMM in adults is CPEO (+) 16% 14% 12% 10% 8% 6% 4% 2% 0% Barca et al Neurol Genet 2020;6:e402. doi:10.1212/NXG.0000000000000402 Multi−Systemic … Other Clinical… MELAS Leigh Syndrome Myopathy Encephalomyopathy LHON cPEO+ Encephalopathy KSS cPEO MERRF Alpers Syndrome SANDO Pearson Syndrome DAD MNGIE NARP Hepatocerebral… Maternal−Inherited … Cardiomyopathy Barth Syndrome Leukoencephalopathy RIM with COX…
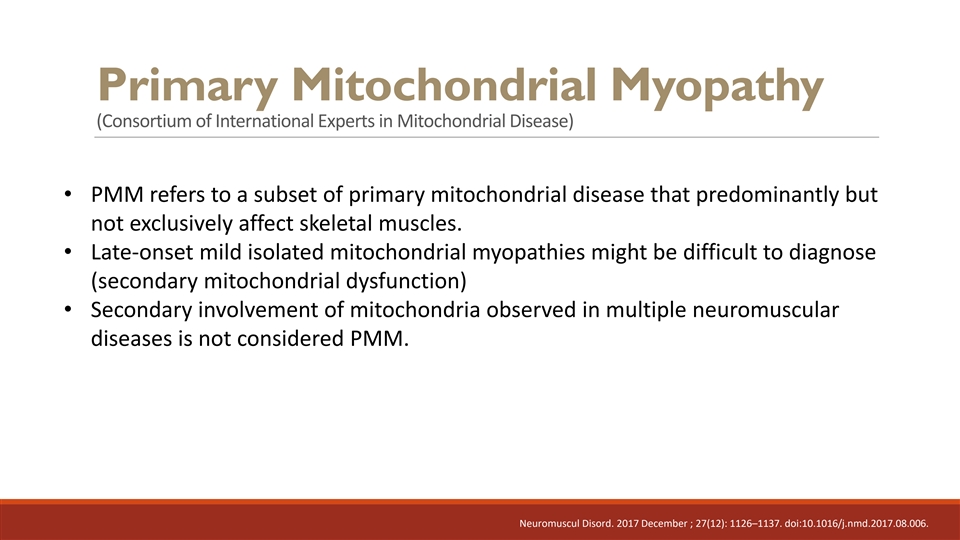
Primary Mitochondrial Myopathy (Consortium of International Experts in Mitochondrial Disease) • PMM refers to a subset of primary mitochondrial disease that predominantly but not exclusively affect skeletal muscles. • Late-onset mild isolated mitochondrial myopathies might be difficult to diagnose (secondary mitochondrial dysfunction) • Secondary involvement of mitochondria observed in multiple neuromuscular diseases is not considered PMM. Neuromuscul Disord. 2017 December ; 27(12): 1126–1137. doi:10.1016/j.nmd.2017.08.006.
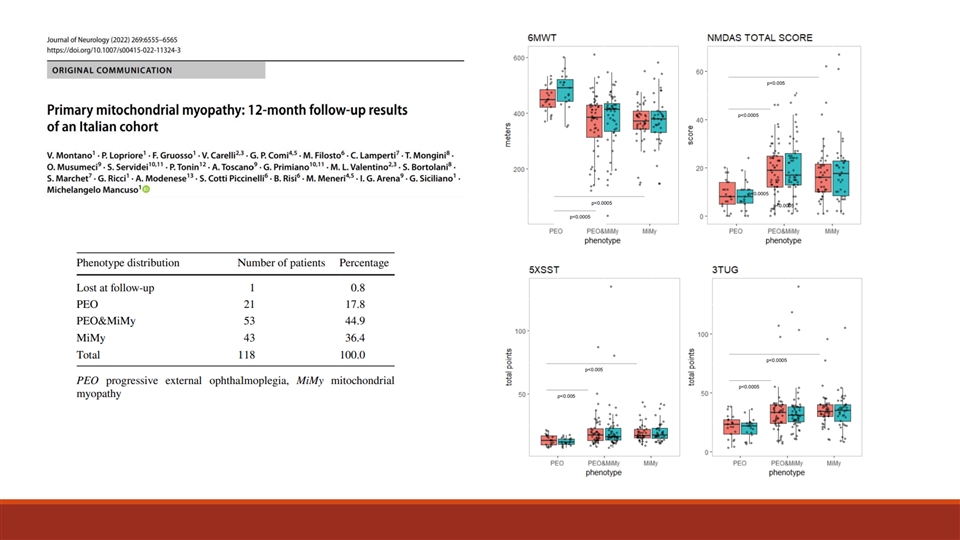
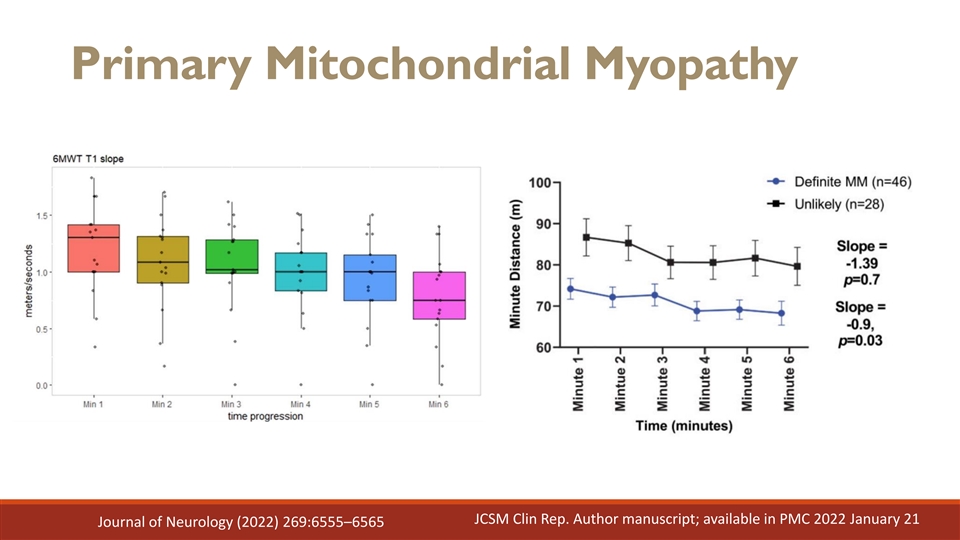
Primary Mitochondrial Myopathy JCSM Clin Rep. Author manuscript; available in PMC 2022 January 21 Journal of Neurology (2022) 269:6555–6565
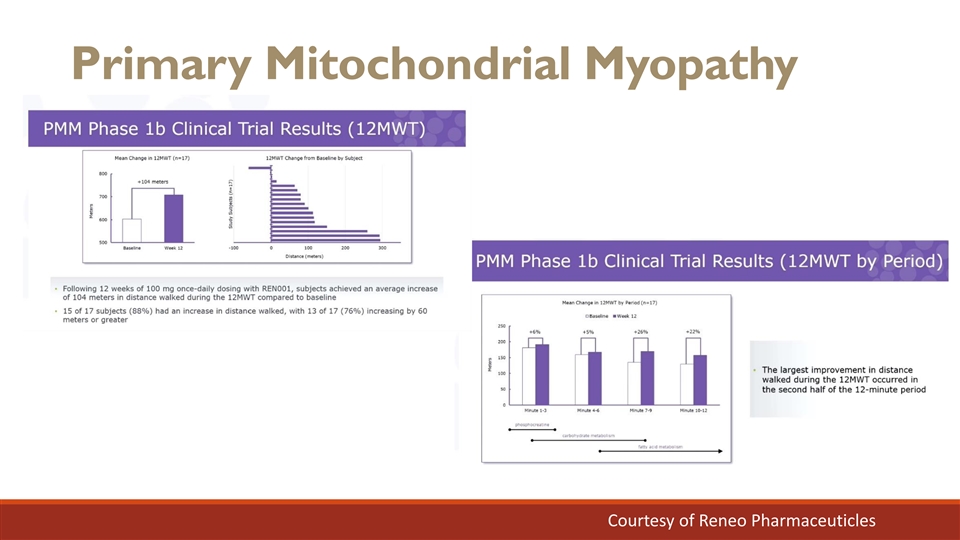
Primary Mitochondrial Myopathy Courtesy of Reneo Pharmaceuticles

Overview of the unmet needs Mitochondrial diseases remain difficult to diagnose - Lack of diagnostic consensus - No gold standard diagnostic method
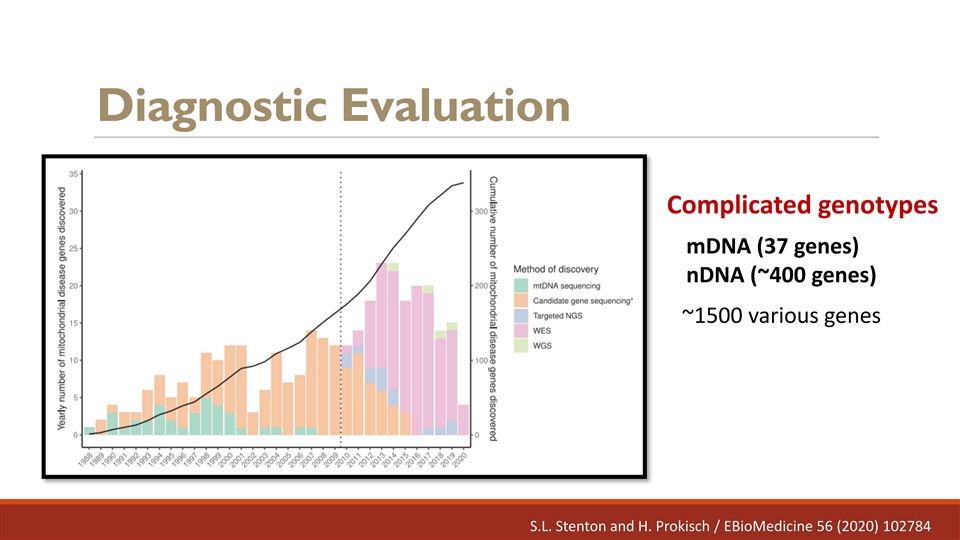
Diagnostic Evaluation Complicated genotypes mDNA (37 genes) nDNA (~400 genes) ~1500 various genes S.L. Stenton and H. Prokisch / EBioMedicine 56 (2020) 102784
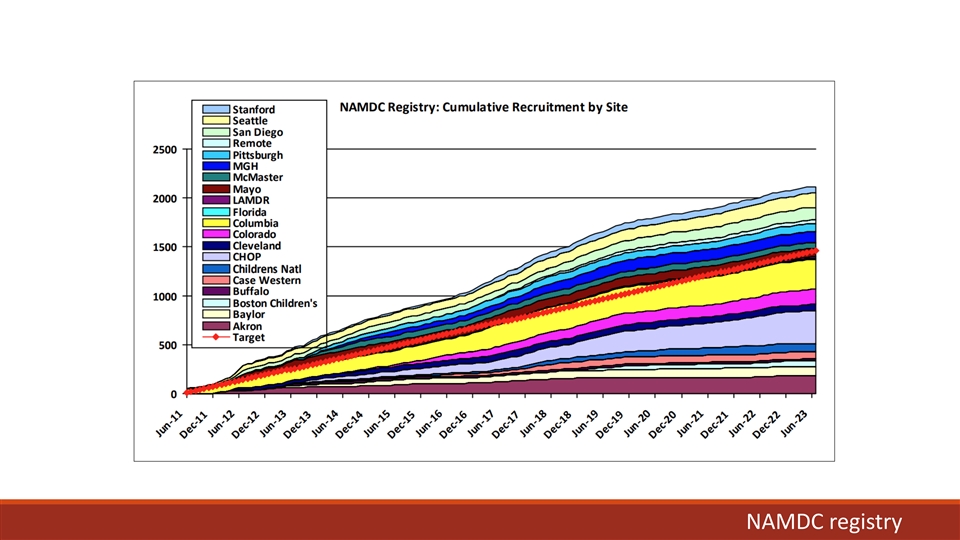
NAMDC registry
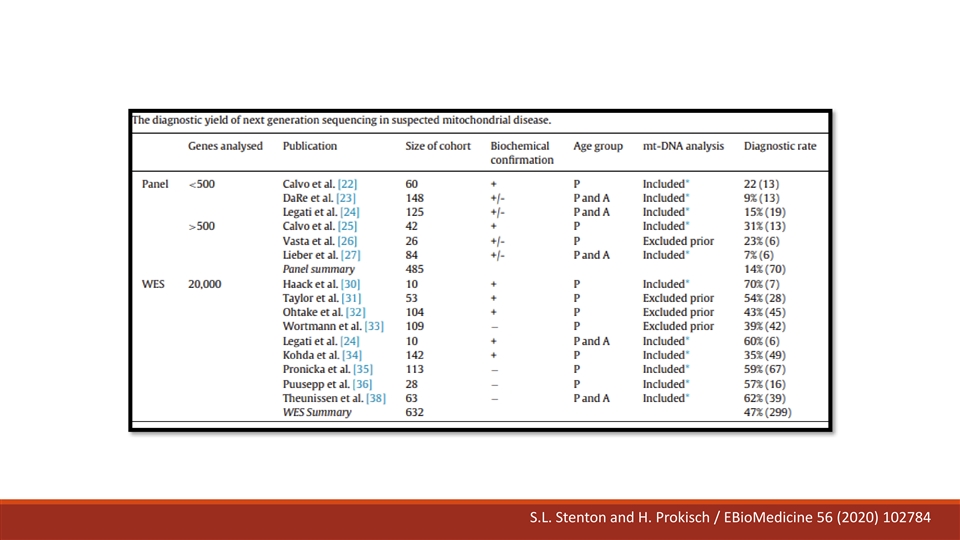
S.L. Stenton and H. Prokisch / EBioMedicine 56 (2020) 102784
Diagnostic Evaluation Symptoms Family history Testing:
Diagnostic Evaluation Evaluating organ involvement
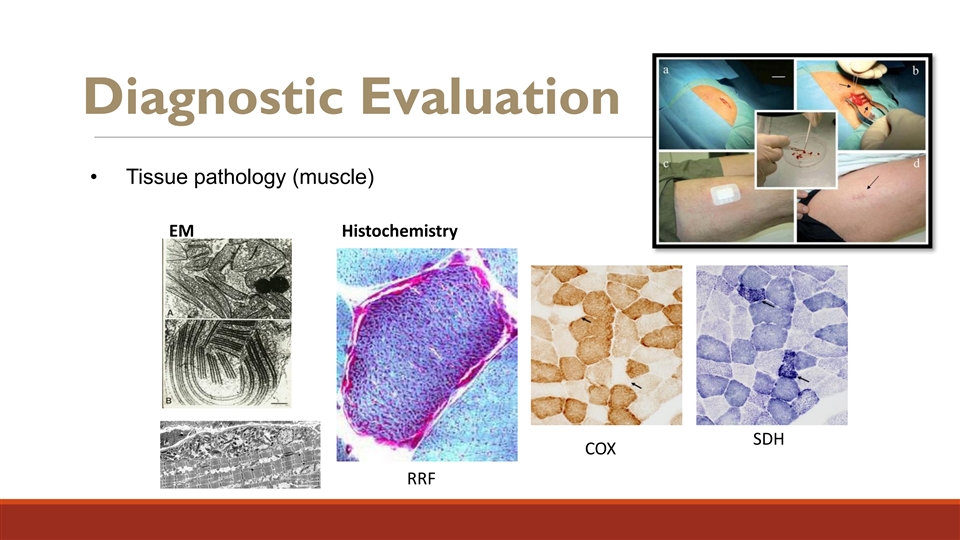
Diagnostic Evaluation • Tissue pathology (muscle) EM Histochemistry SDH COX RRF
Diagnostic Evaluation • Tissue pathology (muscle) • Functional assays (EMG, PFT, Swallow, sniff test…) • Biochemical tests (lactate, CPK, UOA, …) Parikh S, Karaa A, et al. J Med Genet. 2019 Mar;56(3):123-130.
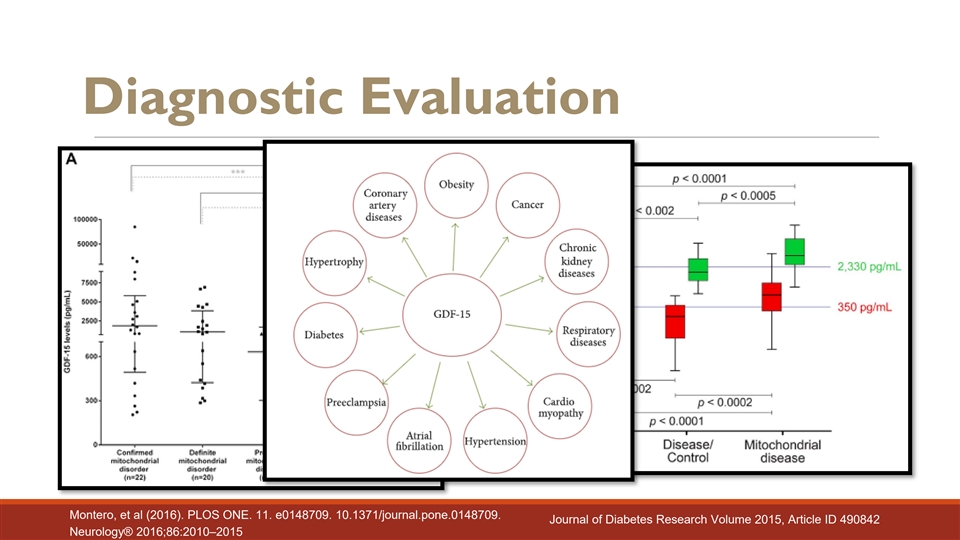
Diagnostic Evaluation Montero, et al (2016). PLOS ONE. 11. e0148709. 10.1371/journal.pone.0148709. Journal of Diabetes Research Volume 2015, Article ID 490842 Neurology® 2016;86:2010–2015
Other diagnostic barriers Mitochondrial Care Network - Disease complexity - Lack of awareness - Lack of education - Insurance
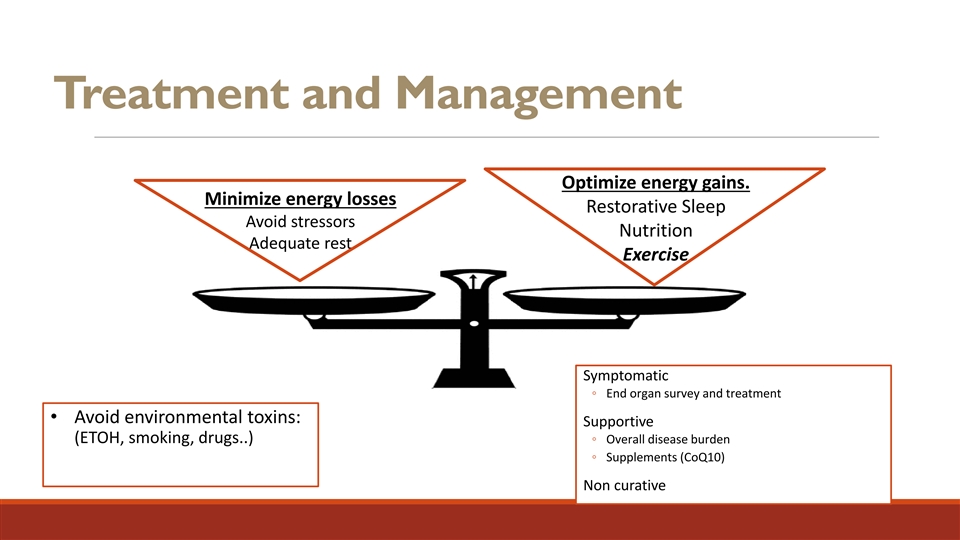
Treatment and Management Optimize energy gains. Minimize energy losses Restorative Sleep Avoid stressors Nutrition Adequate rest Exercise Symptomatic ◦ End organ survey and treatment • Avoid environmental toxins: Supportive (ETOH, smoking, drugs..) ◦ Overall disease burden ◦ Supplements (CoQ10) Non curative
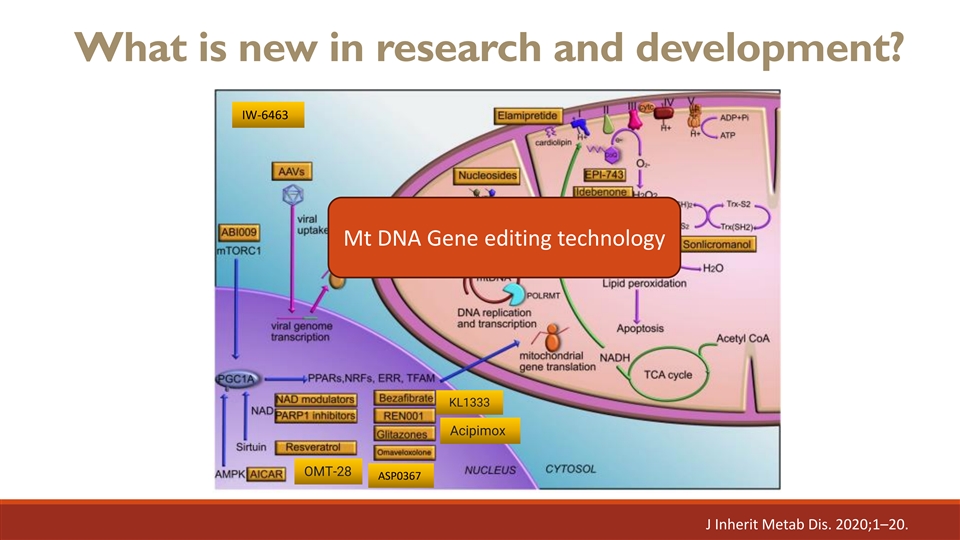
What is new in research and development? IW-6463 Mt DNA Gene editing technology KL1333 Acipimox OMT-28 ASP0367 J Inherit Metab Dis. 2020;1–20.

Thank you, and any questions? For any questions: Amel Karaa, MD Massachusetts General Hospital Harvard Medical School akaraa@mgh.Harvard.edu

Mavodelpar Development Program Alejandro Dorenbaum, MD Chief Medical Officer Reneo Pharmaceuticals, Inc.
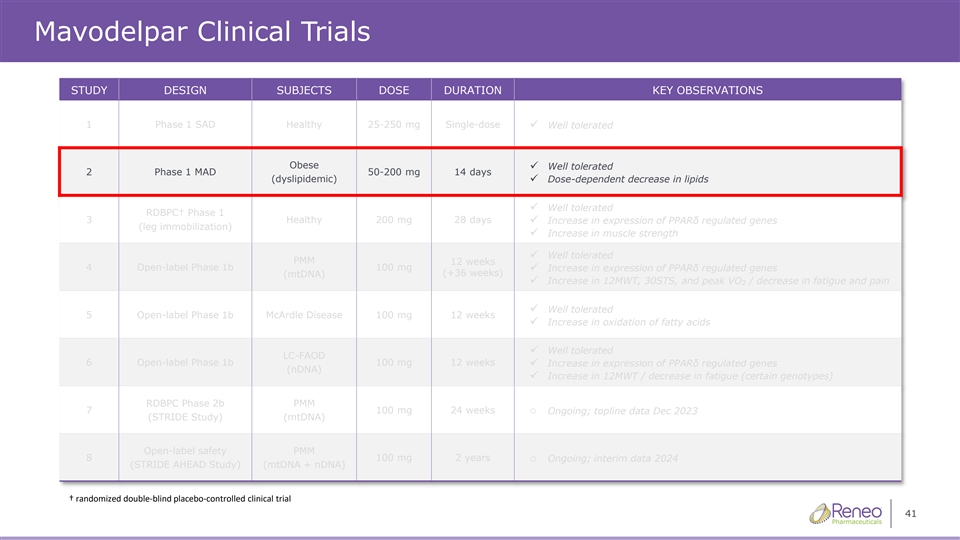
Mavodelpar Clinical Trials STUDY DESIGN SUBJECTS DOSE DURATION KEY OBSERVATIONS 1 Phase 1 SAD Healthy 25-250 mg Single-dose ✓ Well tolerated Obese Well tolerated ✓ 2 Phase 1 MAD 50-200 mg 14 days (dyslipidemic)✓ Dose-dependent decrease in lipids Well tolerated ✓ RDBPC† Phase 1 3 Healthy 200 mg 28 days ✓ Increase in expression of PPARδ regulated genes (leg immobilization) ✓ Increase in muscle strength ✓ Well tolerated PMM 12 weeks 4 Open-label Phase 1b 100 mg ✓ Increase in expression of PPARδ regulated genes (+36 weeks) (mtDNA) ✓ Increase in 12MWT, 30STS, and peak VO / decrease in fatigue and pain 2 ✓ Well tolerated 5 Open-label Phase 1b McArdle Disease 100 mg 12 weeks ✓ Increase in oxidation of fatty acids ✓ Well tolerated LC-FAOD 6 Open-label Phase 1b 100 mg 12 weeks✓ Increase in expression of PPARδ regulated genes (nDNA) ✓ Increase in 12MWT / decrease in fatigue (certain genotypes) RDBPC Phase 2b PMM 7 100 mg 24 weekso Ongoing; topline data Dec 2023 (STRIDE Study) (mtDNA) Open-label safety PMM 8 100 mg 2 yearso Ongoing; interim data 2024 (STRIDE AHEAD Study) (mtDNA + nDNA) † randomized doubleͲŽďĂŝŶĚŽŶƚƌŽůůͲĐĞĚĂůůŝŶŝĐĐƚƌŝĂů 41 ďůƉůĐĞ
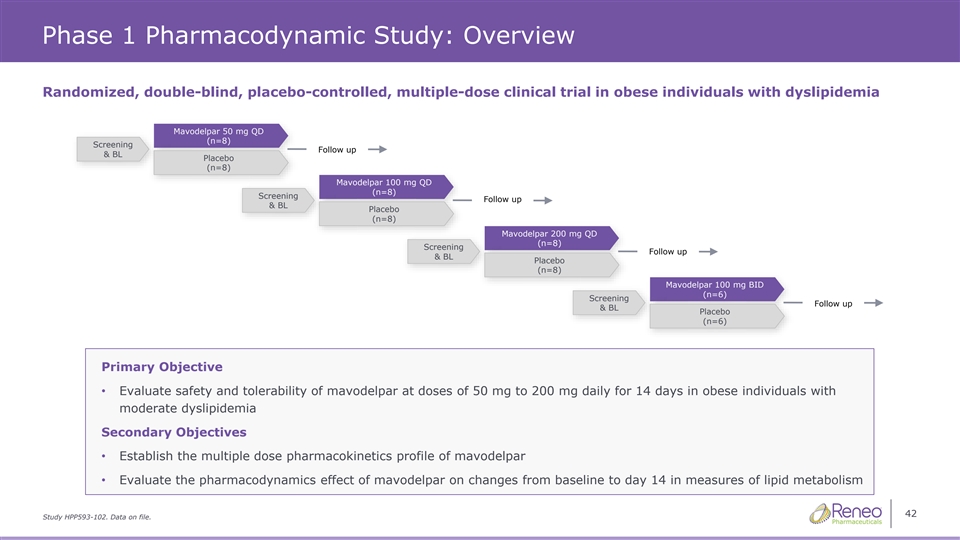
Phase 1 Pharmacodynamic Study: Overview Randomized, double-blind, placebo-controlled, multiple-dose clinical trial in obese individuals with dyslipidemia Mavodelpar 50 mg QD (n (n (n= = =8 8 8) ) ) Screening Follow up & BL Placebo (n=8) Mavodelpar 100 mg QD (n= (n= (n=8 8 8) ) ) Screening Follow up & BL Placebo (n=8) Mavodelpar 200 mg QD (n (n (n= = =8 8 8) ) ) Screening Follow up & BL Placebo (n=8) Mavodelpar 100 mg BID (n (n (n= = =6 6 6) ) ) Screening Follow up & BL Placebo (n=6) Primary Objective • Evaluate safety and tolerability of mavodelpar at doses of 50 mg to 200 mg daily for 14 days in obese individuals with moderate dyslipidemia Secondary Objectives • Establish the multiple dose pharmacokinetics profile of mavodelpar • Evaluate the pharmacodynamics effect of mavodelpar on changes from baseline to day 14 in measures of lipid metabolism 42 Study HPP593-102. Data on file.
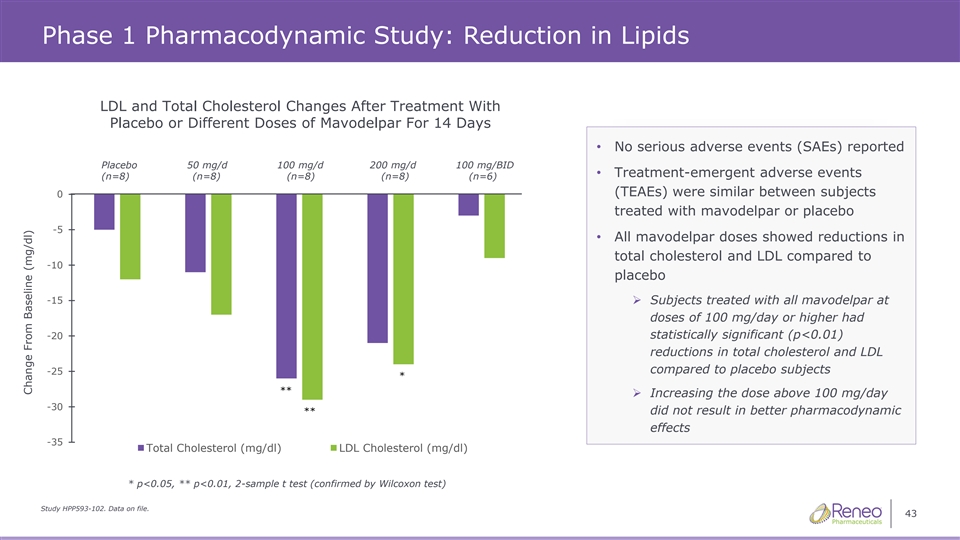
Phase 1 Pharmacodynamic Study: Reduction in Lipids LDL and Total Cholesterol Changes After Treatment With Placebo or Different Doses of Mavodelpar For 14 Days • No serious adverse events (SAEs) reported Placebo 50 mg/d 100 mg/d 200 mg/d 100 mg/BID • Treatment-emergent adverse events (n=8) (n=8) (n=8) (n=8) (n=8) (n=8) (n=8) (n=8) (n=6) (n=6) (TEAEs) were similar between subjects 0 treated with mavodelpar or placebo -5 • All mavodelpar doses showed reductions in total cholesterol and LDL compared to -10 placebo -15➢ Subjects treated with all mavodelpar at doses of 100 mg/day or higher had statistically significant (p<0.01) -20 reductions in total cholesterol and LDL compared to placebo subjects -25 * ** ➢ Increasing the dose above 100 mg/day -30 ** did not result in better pharmacodynamic effects -35 Total Cholesterol (mg/dl) LDL Cholesterol (mg/dl) * p<0.05, ** p<0.01, 2-sample t test (confirmed by Wilcoxon test) Study HPP593-102. Data on file. 43 Change From Baseline (mg/dl)
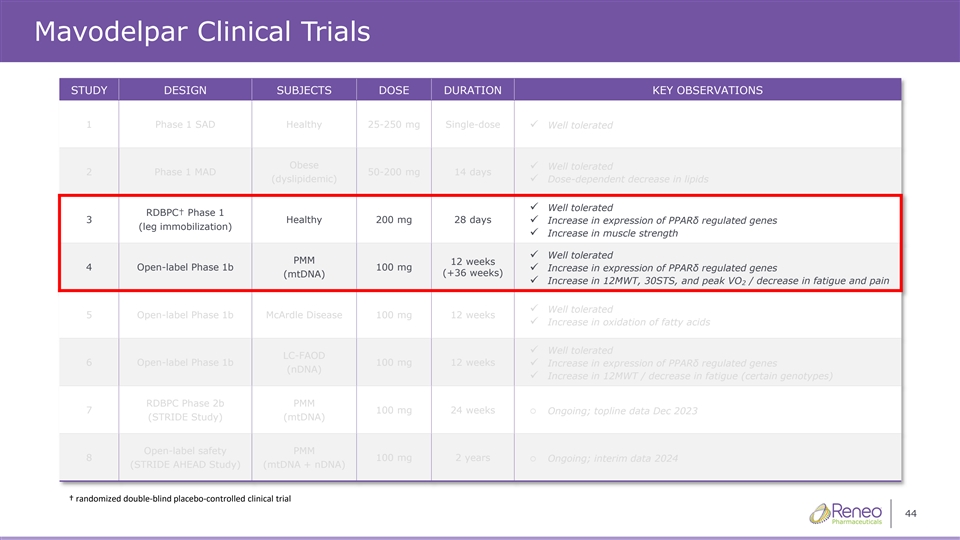
Mavodelpar Clinical Trials STUDY DESIGN SUBJECTS DOSE DURATION KEY OBSERVATIONS 1 Phase 1 SAD Healthy 25-250 mg Single-dose ✓ Well tolerated Obese Well tolerated ✓ 2 Phase 1 MAD 50-200 mg 14 days (dyslipidemic)✓ Dose-dependent decrease in lipids Well tolerated ✓ RDBPC† Phase 1 3 Healthy 200 mg 28 days ✓ Increase in expression of PPARδ regulated genes (leg immobilization) ✓ Increase in muscle strength ✓ Well tolerated PMM 12 weeks 4 Open-label Phase 1b 100 mg ✓ Increase in expression of PPARδ regulated genes (+36 weeks) (mtDNA) ✓ Increase in 12MWT, 30STS, and peak VO / decrease in fatigue and pain 2 ✓ Well tolerated 5 Open-label Phase 1b McArdle Disease 100 mg 12 weeks ✓ Increase in oxidation of fatty acids ✓ Well tolerated LC-FAOD 6 Open-label Phase 1b 100 mg 12 weeks✓ Increase in expression of PPARδ regulated genes (nDNA) ✓ Increase in 12MWT / decrease in fatigue (certain genotypes) RDBPC Phase 2b PMM 7 100 mg 24 weekso Ongoing; topline data Dec 2023 (STRIDE Study) (mtDNA) Open-label safety PMM 8 100 mg 2 yearso Ongoing; interim data 2024 (STRIDE AHEAD Study) (mtDNA + nDNA) † randomized doubleͲŽďĂŝŶĚŽŶƚƌŽůůͲĐĞĚĂůůŝŶŝĐĐƚƌŝĂů 44 ďůƉůĐĞ
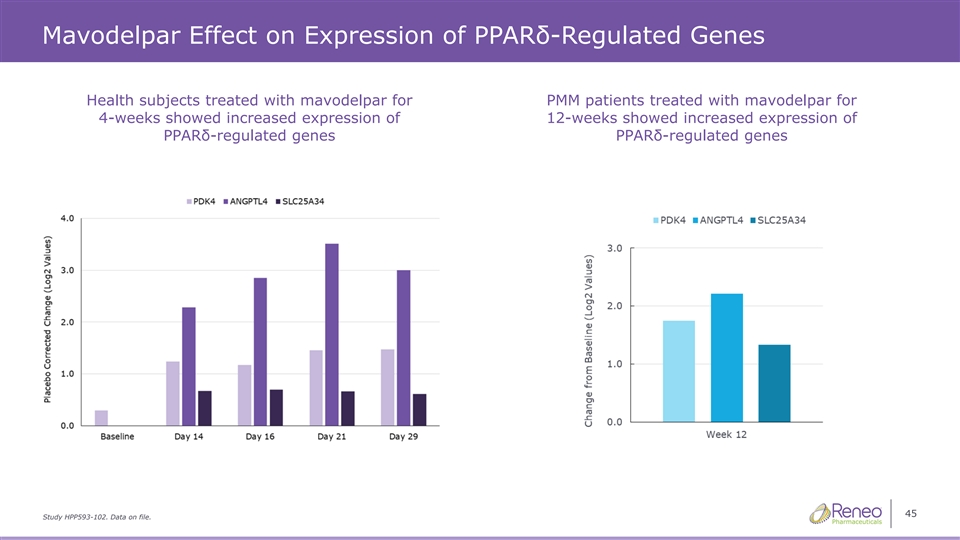
Mavodelpar Effect on Expression of PPARδ-Regulated Genes Health subjects treated with mavodelpar for PMM patients treated with mavodelpar for 4-weeks showed increased expression of 12-weeks showed increased expression of PPARδ-regulated genes PPARδ-regulated genes 45 Study HPP593-102. Data on file.
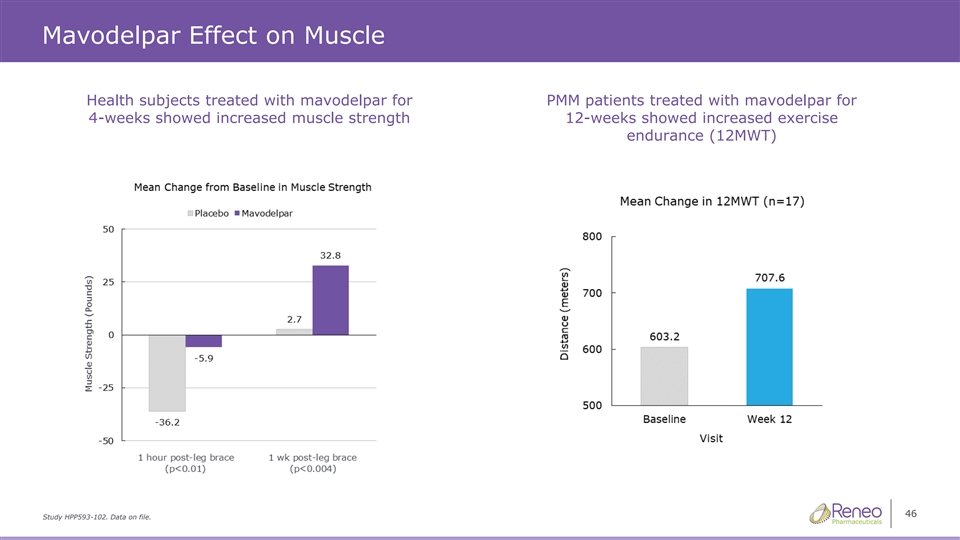
Mavodelpar Effect on Muscle Health subjects treated with mavodelpar for PMM patients treated with mavodelpar for 4-weeks showed increased muscle strength 12-weeks showed increased exercise endurance (12MWT) 46 Study HPP593-102. Data on file.
Mavodelpar Clinical Trials STUDY DESIGN SUBJECTS DOSE DURATION KEY OBSERVATIONS 1 Phase 1 SAD Healthy 25-250 mg Single-dose ✓ Well tolerated Obese Well tolerated ✓ 2 Phase 1 MAD 50-200 mg 14 days (dyslipidemic)✓ Dose-dependent decrease in lipids Well tolerated ✓ RDBPC† Phase 1 3 Healthy 200 mg 28 days ✓ Increase in expression of PPARδ regulated genes (leg immobilization) ✓ Increase in muscle strength ✓ Well tolerated PMM 12 weeks 4 Open-label Phase 1b 100 mg ✓ Increase in expression of PPARδ regulated genes (+36 weeks) (mtDNA) ✓ Increase in 12MWT, 30STS, and peak VO / decrease in fatigue and pain 2 ✓ Well tolerated 5 Open-label Phase 1b McArdle Disease 100 mg 12 weeks ✓ Increase in oxidation of fatty acids ✓ Well tolerated LC-FAOD 6 Open-label Phase 1b 100 mg 12 weeks✓ Increase in expression of PPARδ regulated genes (nDNA) ✓ Increase in 12MWT / decrease in fatigue (certain genotypes) RDBPC Phase 2b PMM 7 100 mg 24 weekso Ongoing; topline data expected Dec 2023 (STRIDE Study) (mtDNA) Open-label safety PMM 8 100 mg 2 yearso Ongoing; interim data expected 2024 (STRIDE AHEAD Study) (mtDNA + nDNA) † randomized doubleͲŽďĂŝŶĚŽŶƚƌŽůůͲĞĚĐĂůůŝŶŝĐĐƚƌŝĂů 47 ďůƉůĐĞ
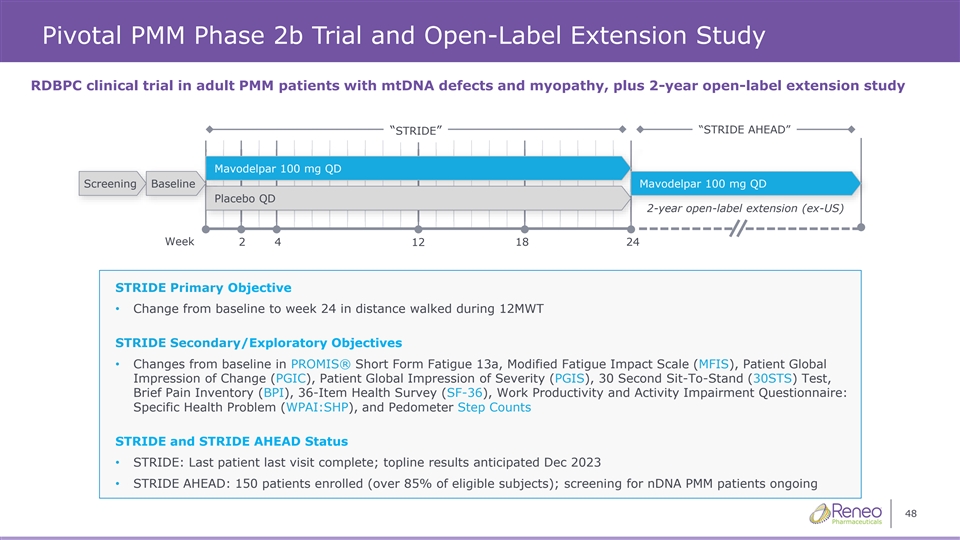
Pivotal PMM Phase 2b Trial and Open-Label Extension Study RDBPC clinical trial in adult PMM patients with mtDNA defects and myopathy, plus 2-year open-label extension study “STRIDE AHEAD” “STRIDE” Mavo Mavodel delpar 1 par 10 00 0 m mg g QD QD Screening Baseline Mavodelpar 100 mg QD Pl Plac acebo QD ebo QD 2-year open-label extension (ex-US) Week 2 4 12 18 24 STRIDE Primary Objective • Change from baseline to week 24 in distance walked during 12MWT STRIDE Secondary/Exploratory Objectives • Changes from baseline in PROMIS® Short Form Fatigue 13a, Modified Fatigue Impact Scale (MFIS), Patient Global Impression of Change (PGIC), Patient Global Impression of Severity (PGIS), 30 Second Sit-To-Stand (30STS) Test, Brief Pain Inventory (BPI), 36-Item Health Survey (SF-36), Work Productivity and Activity Impairment Questionnaire: Specific Health Problem (WPAI:SHP), and Pedometer Step Counts STRIDE and STRIDE AHEAD Status • STRIDE: Last patient last visit complete; topline results anticipated Dec 2023 • STRIDE AHEAD: 150 patients enrolled (over 85% of eligible subjects); screening for nDNA PMM patients ongoing 48
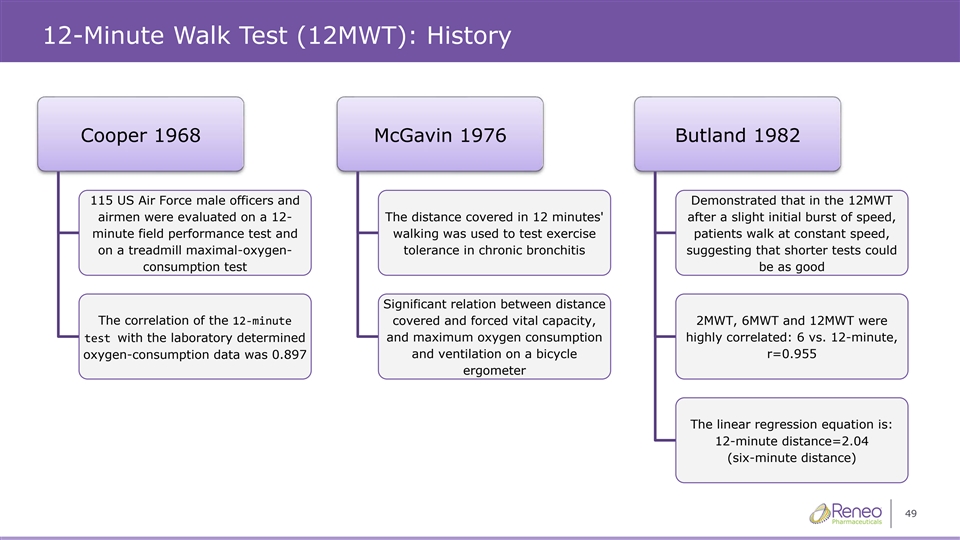
12-Minute Walk Test (12MWT): History Cooper 1968 McGavin 1976 Butland 1982 115 US Air Force male officers and Demonstrated that in the 12MWT airmen were evaluated on a 12- The distance covered in 12 minutes' after a slight initial burst of speed, minute field performance test and walking was used to test exercise patients walk at constant speed, on a treadmill maximal-oxygen- tolerance in chronic bronchitis suggesting that shorter tests could consumption test be as good Significant relation between distance The correlation of the 12-minute covered and forced vital capacity, 2MWT, 6MWT and 12MWT were and maximum oxygen consumption highly correlated: 6 vs. 12-minute, test with the laboratory determined oxygen-consumption data was 0.897 and ventilation on a bicycle r=0.955 ergometer The linear regression equation is: 12-minute distance=2.04 (six-minute distance) 49
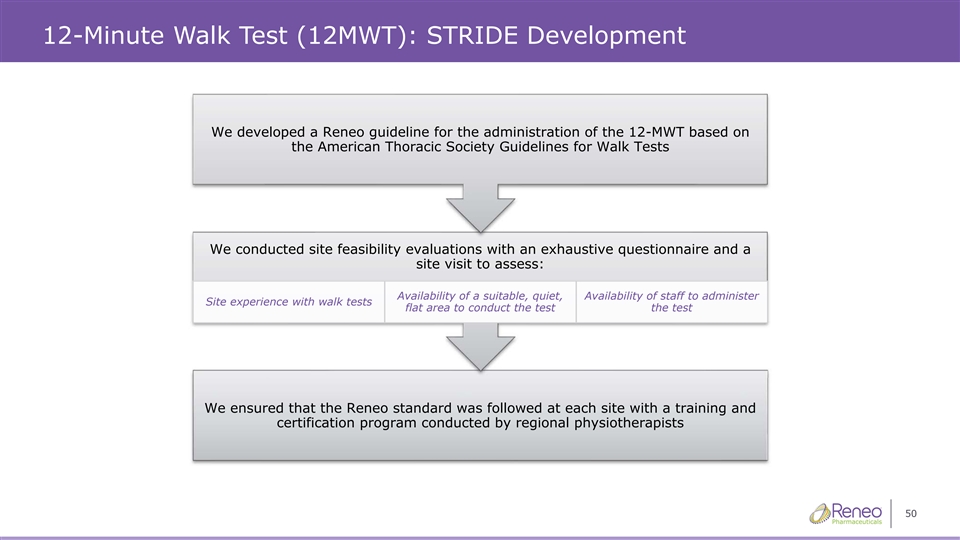
12-Minute Walk Test (12MWT): STRIDE Development We developed a Reneo guideline for the administration of the 12-MWT based on the American Thoracic Society Guidelines for Walk Tests We conducted site feasibility evaluations with an exhaustive questionnaire and a site visit to assess: Availability of a suitable, quiet, Availability of staff to administer Site experience with walk tests flat area to conduct the test the test We ensured that the Reneo standard was followed at each site with a training and certification program conducted by regional physiotherapists 50
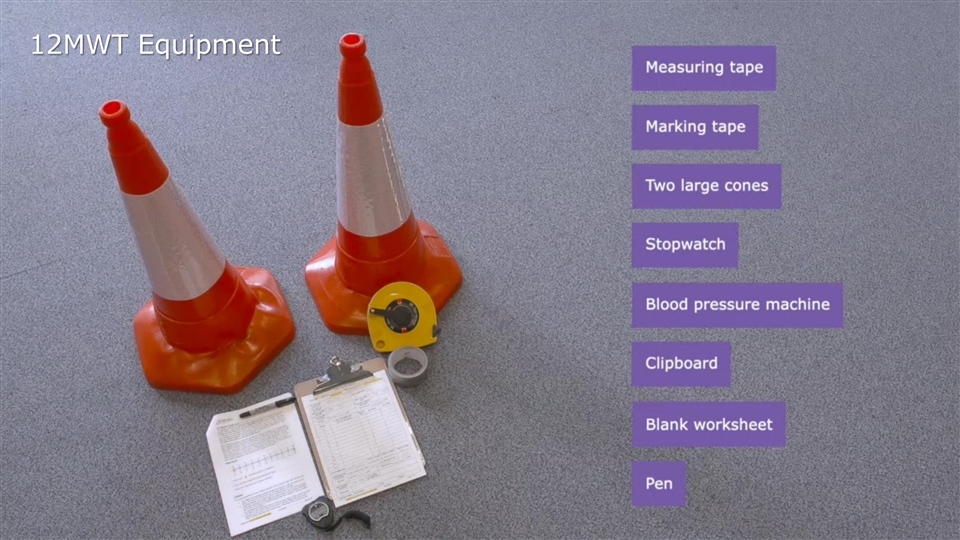
12-Minute Walk Test (12MWT): STRIDE Study Equipment 12MWT Equipment 51

12-Minute Walk Test (12MWT): STRIDE Training & Certification • Detailed exercise manual (v1.0) provided to each site • Standardized tools, equipment, and script for performing the 12MWT • Required training video developed with an expert physiotherapist • All sites underwent training and certification during a site visit by a regional physiotherapist ➢ Visits were conducted face to face (preferred) or remotely ➢ Site performed a 12MWT that was reviewed by the regional physiotherapist ➢ The regional physiotherapist approved the physical area as suitable for conducting the test and the ability of staff to perform the test ➢ Refresher 12MWT training was conducted by regional physiotherapists ➢ Training records were collected and retained ➢ Regional physiotherapists review all subjects screening 12MWT worksheets for errors or issues 52

12-Minute Walk Test (12MWT): STRIDE Study Equipment 12MWT Course 53
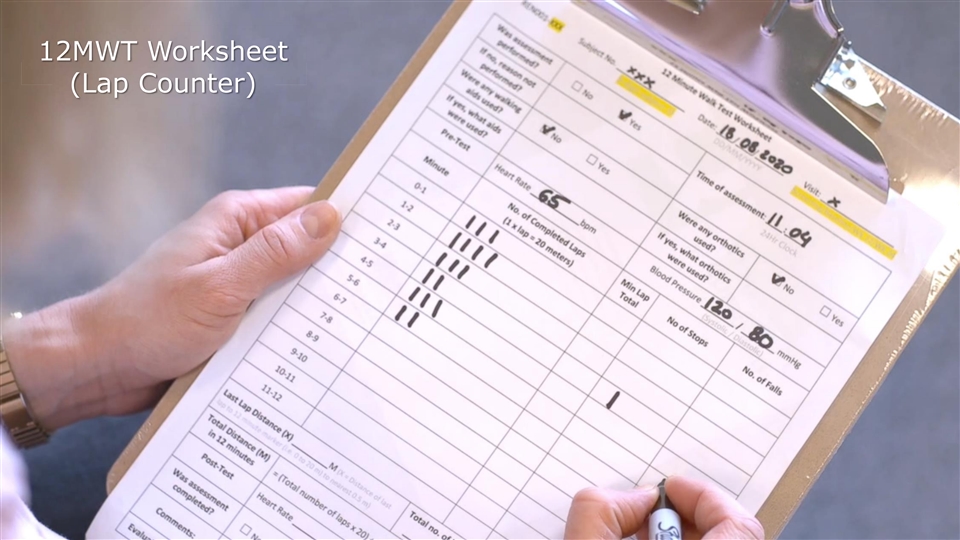
12-Minute Walk Test (12MWT): STRIDE Study Equipment 12MWT 12MWT W Workshe orksheet et (Lap Counter) 54

12-Minute Walk Test (12MWT): STRIDE Study Equipment Compl Completed eted 12MWT 12MWT (Marked Final Step) 55

12-Minute Walk Test (12MWT): STRIDE Study Equipment 56
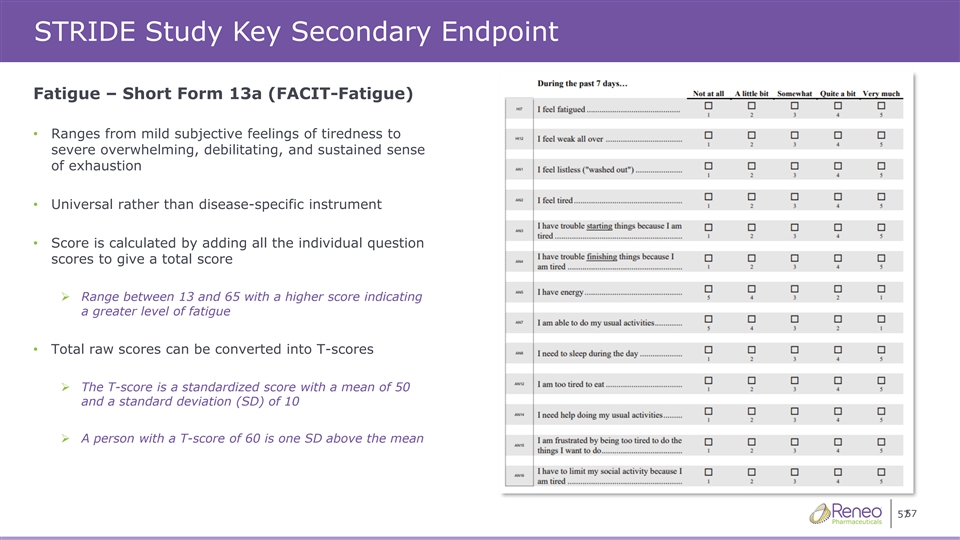
STRIDE Study Key Secondary Endpoint Fatigue – Short Form 13a (FACIT-Fatigue) • Ranges from mild subjective feelings of tiredness to severe overwhelming, debilitating, and sustained sense of exhaustion • Universal rather than disease-specific instrument • Score is calculated by adding all the individual question scores to give a total score ➢ Range between 13 and 65 with a higher score indicating a greater level of fatigue • Total raw scores can be converted into T-scores ➢ The T-score is a standardized score with a mean of 50 and a standard deviation (SD) of 10 ➢ A person with a T-score of 60 is one SD above the mean 57 57

Addressable Patients (US) Michael Cruse Chief Operating Officer Reneo Pharmaceuticals, Inc.
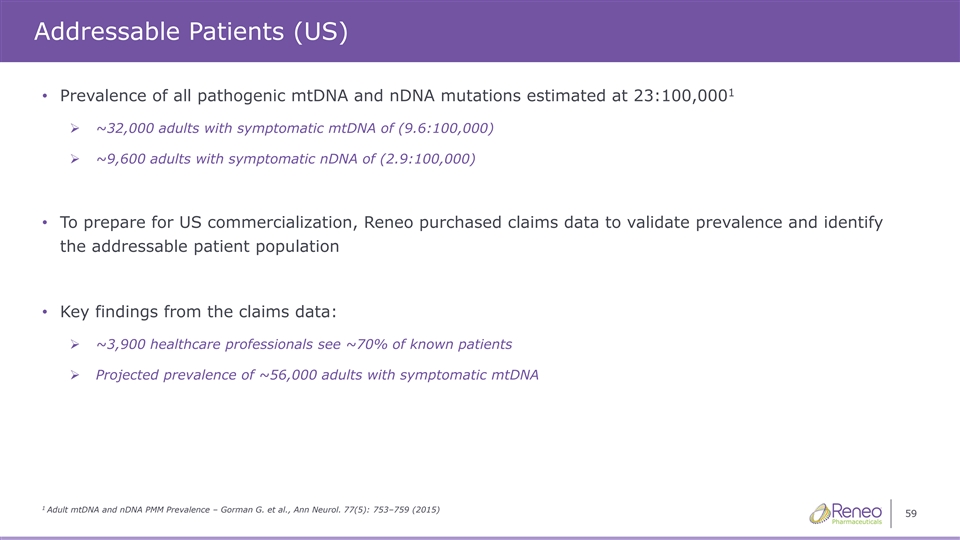
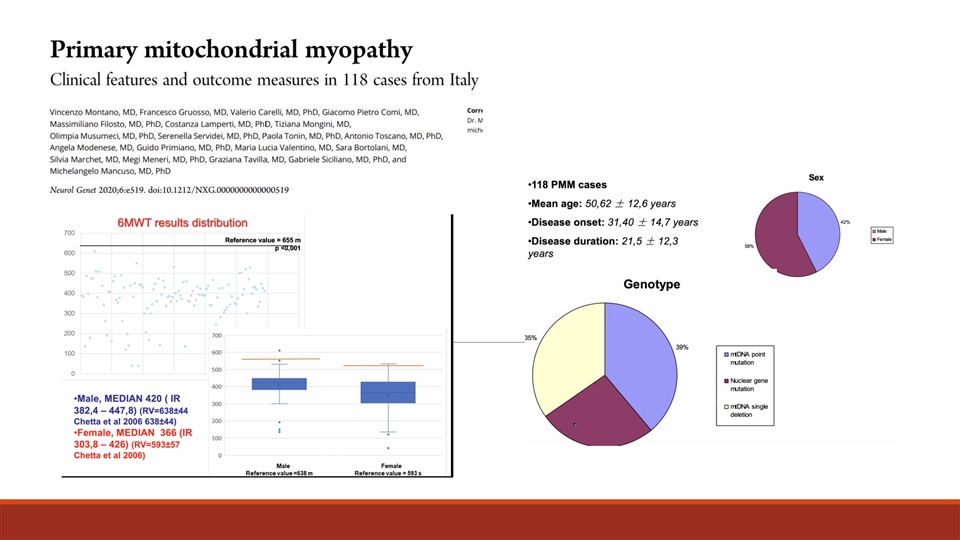
Addressable Patients (US) 1 • Prevalence of all pathogenic mtDNA and nDNA mutations estimated at 23:100,000 ➢ ~32,000 adults with symptomatic mtDNA of (9.6:100,000) ➢ ~9,600 adults with symptomatic nDNA of (2.9:100,000) • To prepare for US commercialization, Reneo purchased claims data to validate prevalence and identify the addressable patient population • Key findings from the claims data: ➢ ~3,900 healthcare professionals see ~70% of known patients ➢ Projected prevalence of ~56,000 adults with symptomatic mtDNA 1 Adult mtDNA and nDNA PMM Prevalence – Gorman G. et al., Ann Neurol. 77(5): 753–759 (2015) 59
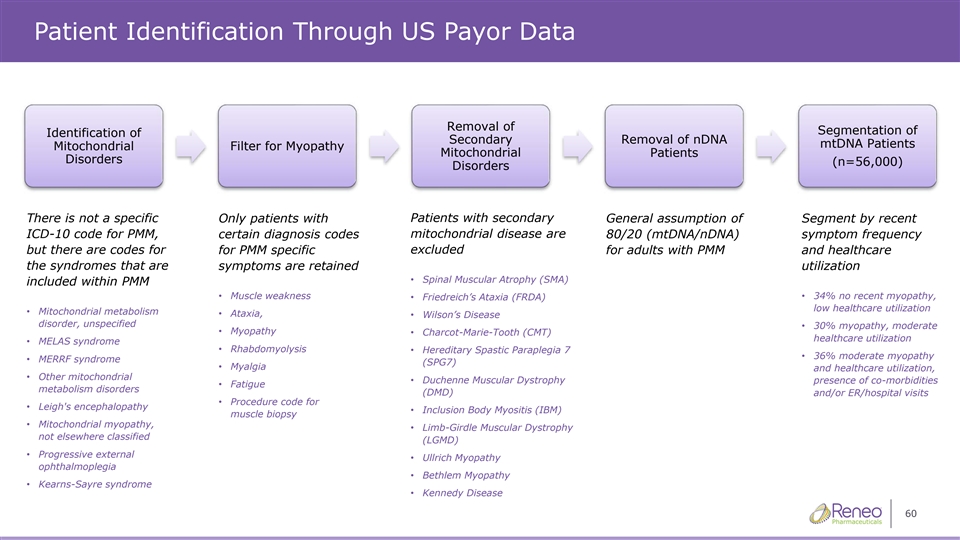
Patient Identification Through US Payor Data Removal of Segmentation of Identification of Secondary Removal of nDNA mtDNA Patients Mitochondrial Filter for Myopathy Mitochondrial Patients Disorders (n=56,000) Disorders There is not a specific Only patients with Patients with secondary General assumption of Segment by recent ICD-10 code for PMM, certain diagnosis codes mitochondrial disease are 80/20 (mtDNA/nDNA) symptom frequency excluded but there are codes for for PMM specific for adults with PMM and healthcare the syndromes that are symptoms are retained utilization • Spinal Muscular Atrophy (SMA) included within PMM • Muscle weakness • Friedreich’s Ataxia (FRDA) • 34% no recent myopathy, low healthcare utilization • Mitochondrial metabolism • Ataxia, • Wilson’s Disease disorder, unspecified • 30% myopathy, moderate • Myopathy • Charcot-Marie-Tooth (CMT) healthcare utilization • MELAS syndrome • Rhabdomyolysis • Hereditary Spastic Paraplegia 7 • 36% moderate myopathy • MERRF syndrome (SPG7) • Myalgia and healthcare utilization, • Other mitochondrial • Duchenne Muscular Dystrophy presence of co-morbidities • Fatigue metabolism disorders (DMD) and/or ER/hospital visits • Procedure code for • Leigh's encephalopathy • Inclusion Body Myositis (IBM) muscle biopsy • Mitochondrial myopathy, • Limb-Girdle Muscular Dystrophy not elsewhere classified (LGMD) • Progressive external • Ullrich Myopathy ophthalmoplegia • Bethlem Myopathy • Kearns-Sayre syndrome • Kennedy Disease 60
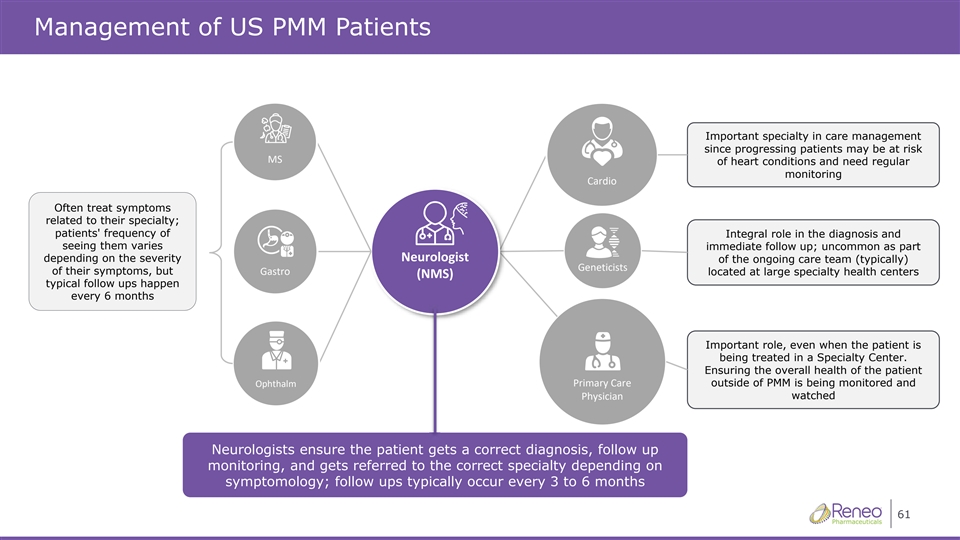
Management of US PMM Patients Important specialty in care management since progressing patients may be at risk D^ of heart conditions and need regular monitoring ĂƌĚŝŽ Often treat symptoms related to their specialty; patients' frequency of Integral role in the diagnosis and seeing them varies immediate follow up; uncommon as part ůŽŐŝƐƚEĞƵƌŽ depending on the severity of the ongoing care team (typically) 'ĞŶĞƚŝĐŝƐƚƐ of their symptoms, but ŽƌƚƐĂ' located at large specialty health centers ;ED^Ϳ typical follow ups happen every 6 months Important role, even when the patient is being treated in a Specialty Center. Ensuring the overall health of the patient KƉŚƚŚĂůŵŝŵĂƌLJĂƌĞWƌ outside of PMM is being monitored and WŚLJƐŝĐŝĂŶ watched Neurologists ensure the patient gets a correct diagnosis, follow up monitoring, and gets referred to the correct specialty depending on symptomology; follow ups typically occur every 3 to 6 months 61
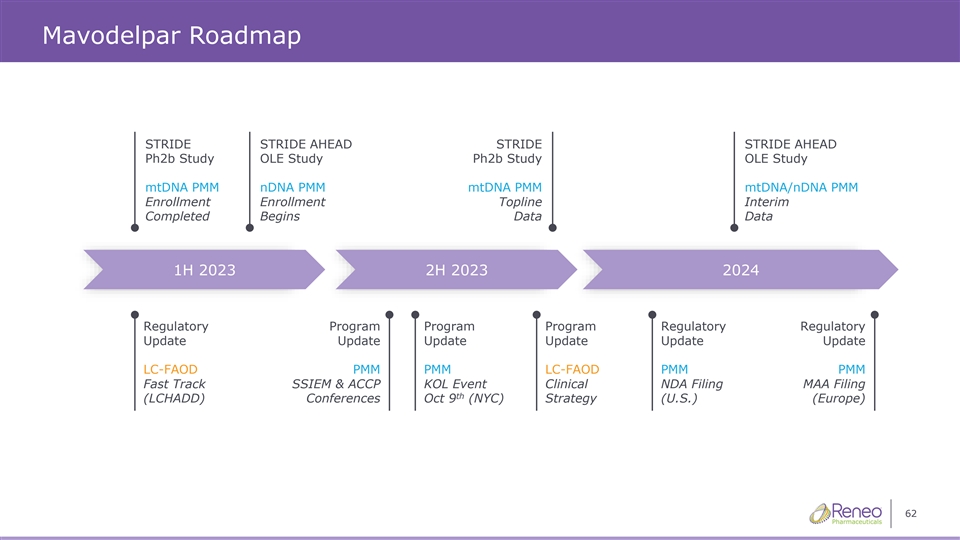
Mavodelpar Roadmap STRIDE STRIDE AHEAD STRIDE STRIDE AHEAD Ph2b Study OLE Study Ph2b Study OLE Study mtDNA PMM nDNA PMM mtDNA PMM mtDNA/nDNA PMM Enrollment Enrollment Topline Interim Completed Begins Data Data 1H 2023 2H 2023 2024 Regulatory Program Program Program Regulatory Regulatory Update Update Update Update Update Update LC-FAOD PMM PMM LC-FAOD PMM PMM Fast Track SSIEM & ACCP KOL Event Clinical NDA Filing MAA Filing th (LCHADD) Conferences Oct 9 (NYC) Strategy (U.S.) (Europe) 62

Thank You!