UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported)
June 8, 2023
SAVARA INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-32157 | 84-1318182 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
6836 Bee Cave Road, Building I, Suite 205
Austin, TX 78746
(Address of principal executive offices, including zip code)
(512) 614-1848
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange on which registered |
||
| Common Stock, par value $0.001 per share | SVRA | The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01. | Regulation FD Disclosure. |
Savara has updated its corporate presentation, which is available on the Investor Relations page of Savara’s website at https://savarapharma.com/investors/events-presentations/. A copy of the presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K. Savara undertakes no duty or obligation to update or revise the information contained in this presentation, although it may do so from time to time. Any such updates may be made through the Investor Relations page of the Savara website, the filing of other reports or documents with the U.S. Securities and Exchange Commission (the “SEC”), press releases, or other public disclosure.
The information in Item 7.01 in this Current Report on Form 8-K shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that Section, nor shall it be incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
| Item 9.01. | Financial Statements and Exhibits. |
(d) Exhibits.
| Exhibit No. |
Description | |
| 99.1 | Savara Corporate Presentation | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document). | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: June 8, 2023 |
SAVARA INC. |
|||||
| By: | /s/ Dave Lowrance |
|||||
| Dave Lowrance Chief Financial & Administrative Officer |
||||||

Corporate Overview Developing New Therapies for Rare Respiratory Diseases June 2023 Exhibit 99.1

© Savara Inc. All Rights Reserved. Safe Harbor Statement Savara Inc. (“Savara” or the “Company”) cautions you that statements in this presentation that are not a description of historical fact are forward-looking statements which may be identified by the use of words such as “expect,” “intend,” “plan,” “anticipate,” “believe,” and “will,” among others. Such statements include, but are not limited to, statements regarding the nature, strategy and focus of Savara; the Savara investment thesis; the timing, design and other matters related to clinical trials of our product candidate; the safety, efficacy and projected development timeline of our product candidate; the potential health benefits of our product candidate; our anticipated corporate milestones; the potential market size, commercial opportunity, and competitive landscape for our product; Savara’s plans regarding disease awareness and anti-GM-CSF antibody testing, and the potential impact of those programs; and the sufficiency of our resources to fund the advancement of our development program and potential sources of additional capital. Savara may not actually achieve any of its plans or product development goals in a timely manner, if at all, or otherwise carry out its current intentions or meet the expectations or projections disclosed in its forward-looking statements, and you should not place undue reliance on these forward-looking statements. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. These forward-looking statements are based upon Savara's current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, the risks and uncertainties related to the impact of widespread health concerns impacting healthcare providers or patients and geopolitical conditions on our business and operations; risks and uncertainties associated with the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations; the availability of sufficient resources for our operations and to conduct or continue planned clinical development programs; the timing and ability of Savara to raise additional capital as needed to fund continued operations; the ability to successfully conduct clinical trials for our product candidate; the ability to successfully develop our product candidate; and the risks associated with the process of developing, obtaining regulatory approval for and commercializing drug candidates that are safe and effective for use as human therapeutics. The risks and uncertainties facing Savara are described more fully in Savara's filings with the Securities and Exchange Commission including our filings on Form 8-K, our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, and our Quarterly Report on Form 10-Q for the quarter ended March 31, 2023. You are cautioned not to place undue reliance on our forward-looking statements, which speak only as of the date on which they were made. Savara undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as may be required by law. Third-party information included herein has been obtained from sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, and should not be construed as a representation by, the Company. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products.

© Savara Inc. All Rights Reserved. Executive Leadership Team Matthew Pauls, J.D., M.B.A. Chair & Chief Executive Officer Dave Lowrance Chief Financial & Administrative Officer Peter Clarke, Ph.D. EVP, Global Technical Operations Kate McCabe, J.D. SVP, General Counsel Anne Erickson SVP, Head of Global Business Operations Charles LaPree SVP, Global Regulatory Affairs and Quality Assurance Ray Pratt, M.D. FACP Chief Medical Officer Scott Wilhoit EVP, Global Commercial Rob Lutz, M.B.A. Chief Operating Officer

Pursuing Transformative Therapies for Rare Respiratory Diseases © Savara Inc. All Rights Reserved. Focused on single Phase 3 program: molgramostim nebulizer solution (molgramostim) in autoimmune pulmonary alveolar proteinosis (aPAP) Recombinant form of human granulocyte-macrophage colony-stimulating factor (GM-CSF) Favorable efficacy and safety data generated from the first IMPALA trial Pivotal Phase 3 trial underway – builds on key learnings from IMPALA Seasoned management team Deep experience in the development and commercialization of rare respiratory therapeutics and pulmonary medicines Capitalized through major clinical and regulatory milestones ~$115M* in cash expected to fund company ~18-months beyond Phase 3 data read-out, beyond BLA filing, and through potential approval Quality investor base *As of 3/31/23
Investment Thesis © Savara Inc. All Rights Reserved. The molgramostim in aPAP clinical program has a high probability of success Significant global commercial opportunity As a novel inhaled biologic, molgramostim has the potential for a long-term, durable revenue stream Strong balance sheet – funded through 2025
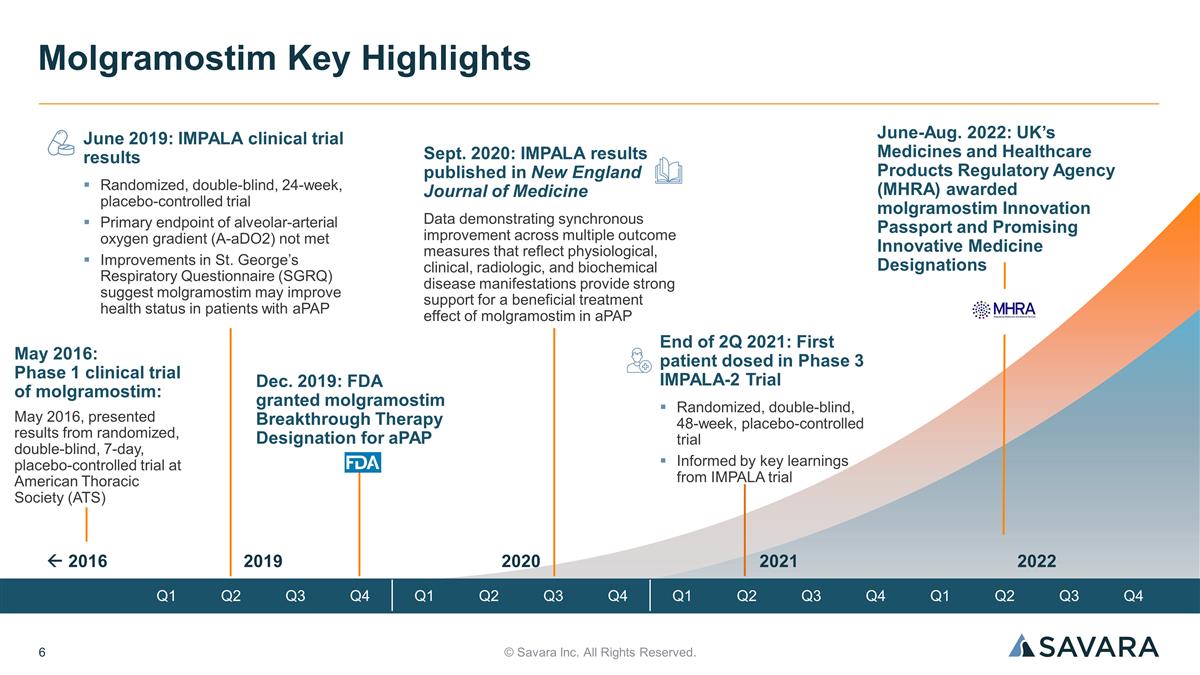
© Savara Inc. All Rights Reserved. Molgramostim Key Highlights June 2019: IMPALA clinical trial results Randomized, double-blind, 24-week, placebo-controlled trial Primary endpoint of alveolar-arterial oxygen gradient (A-aDO2) not met Improvements in St. George’s Respiratory Questionnaire (SGRQ) suggest molgramostim may improve health status in patients with aPAP Dec. 2019: FDA granted molgramostim Breakthrough Therapy Designation for aPAP End of 2Q 2021: First patient dosed in Phase 3 IMPALA-2 Trial Randomized, double-blind, 48-week, placebo-controlled trial Informed by key learnings from IMPALA trial Sept. 2020: IMPALA results published in New England Journal of Medicine Data demonstrating synchronous improvement across multiple outcome measures that reflect physiological, clinical, radiologic, and biochemical disease manifestations provide strong support for a beneficial treatment effect of molgramostim in aPAP May 2016: Phase 1 clinical trial of molgramostim: May 2016, presented results from randomized, double-blind, 7-day, placebo-controlled trial at American Thoracic Society (ATS) 2019 2020 2021 2022 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 ß 2016 June-Aug. 2022: UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) awarded molgramostim Innovation Passport and Promising Innovative Medicine Designations
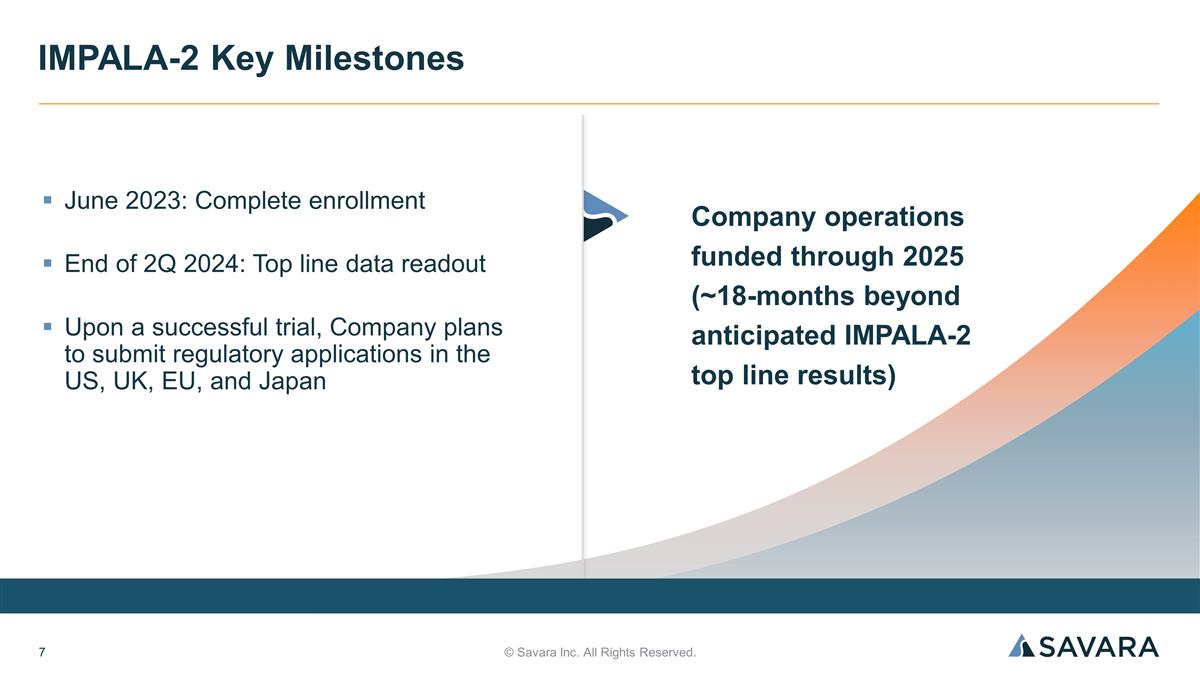
June 2023: Complete enrollment End of 2Q 2024: Top line data readout Upon a successful trial, Company plans to submit regulatory applications in the US, UK, EU, and Japan © Savara Inc. All Rights Reserved. IMPALA-2 Key Milestones Company operations funded through 2025 (~18-months beyond anticipated IMPALA-2 top line results)

Molgramostim Molgramostim for Autoimmune Pulmonary Alveolar Proteinosis (aPAP)
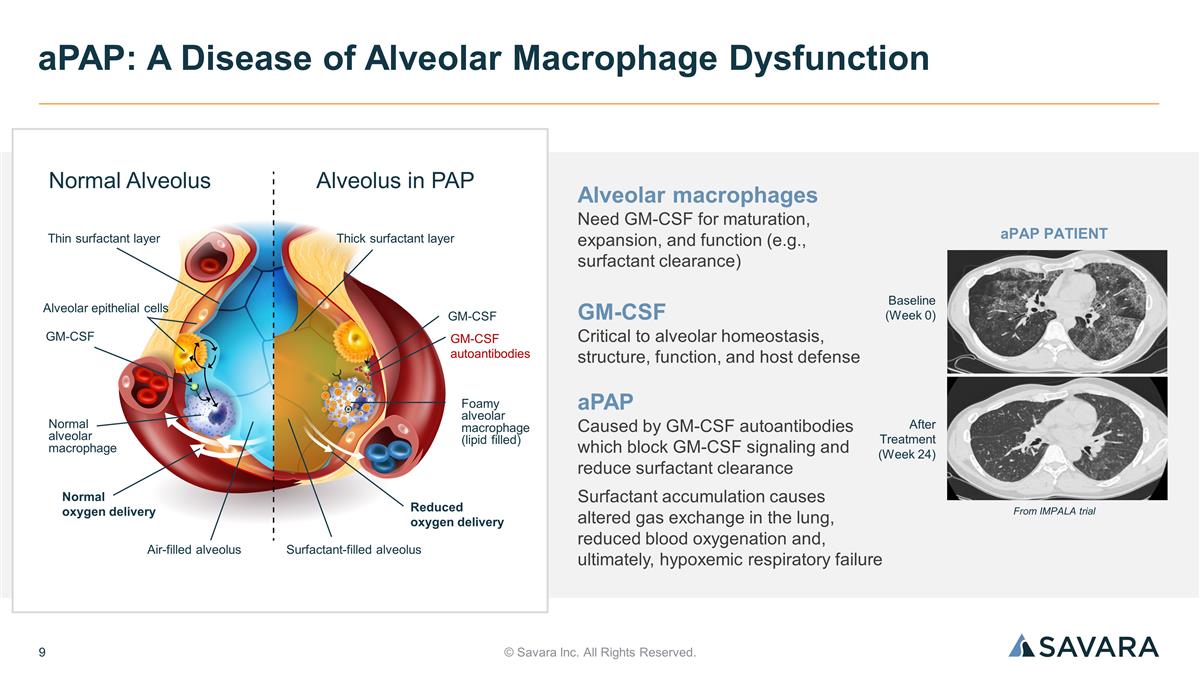
© Savara Inc. All Rights Reserved. aPAP: A Disease of Alveolar Macrophage Dysfunction Alveolar macrophages Need GM-CSF for maturation, expansion, and function (e.g., surfactant clearance) GM-CSF Critical to alveolar homeostasis, structure, function, and host defense aPAP Caused by GM-CSF autoantibodies which block GM-CSF signaling and reduce surfactant clearance Surfactant accumulation causes altered gas exchange in the lung, reduced blood oxygenation and, ultimately, hypoxemic respiratory failure Baseline (Week 0) After Treatment (Week 24) From IMPALA trial aPAP PATIENT GM-CSF autoantibodies Alveolus in PAP Normal Alveolus Thin surfactant layer Thick surfactant layer Alveolar epithelial cells GM-CSF GM-CSF Normal oxygen delivery Reduced oxygen delivery Foamy alveolar macrophage (lipid filled) Normal alveolar macrophage Air-filled alveolus Surfactant-filled alveolus
© Savara Inc. All Rights Reserved. aPAP is a Rare, Long-Term, Chronic Disease Progressive Shortness of Breath Gas exchange in the lungs is impaired and patients may experience shortness of breath At first it occurs upon exertion, but as disease progresses, it can occur even when a person is at rest Fatigue, Decreased Exercise Tolerance Fatigue and significantly reduced exercise capacity can dramatically impact the simplest of daily activities, e.g., getting winded walking up a flight of stairs Cough and Episodes of Fever Cough, sputum production, and episodes of fever, especially if secondary lung infection develops Fibrosis and Lung Transplant In the long-term, the disease can lead to serious complications, including fibrosis, often leading to the need for lung transplantation There are no approved drugs for the treatment of aPAP. Only option is whole lung lavage, an invasive procedure.
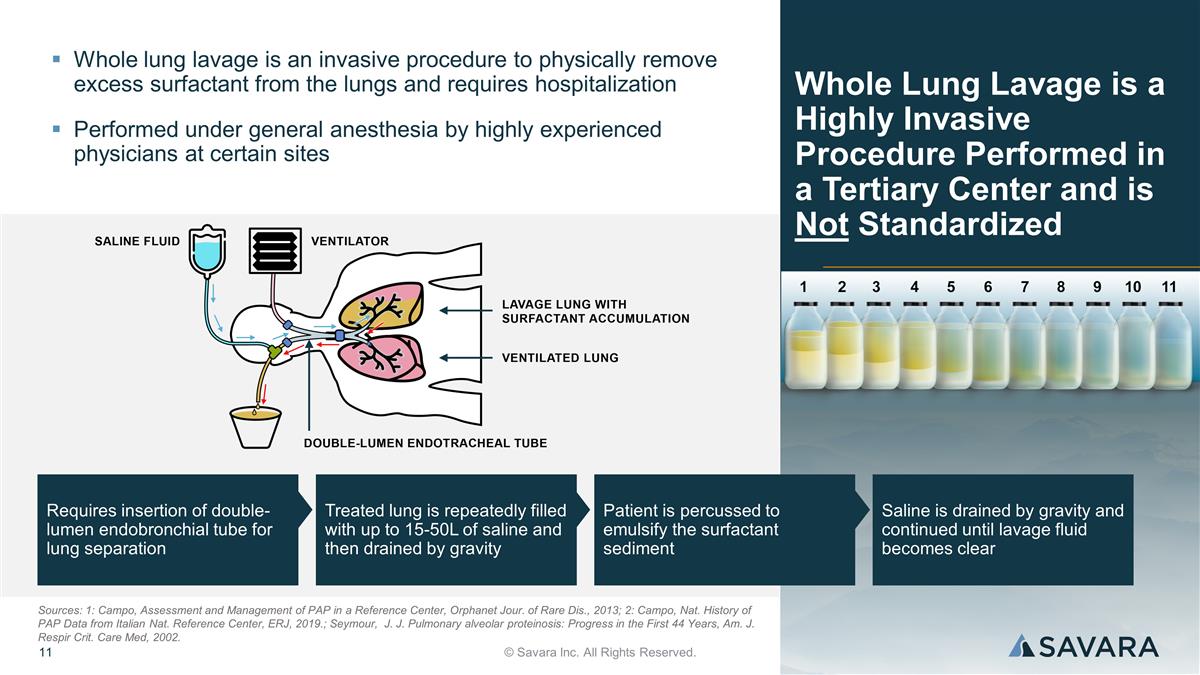
Requires insertion of double-lumen endobronchial tube for lung separation Treated lung is repeatedly filled with up to 15-50L of saline and then drained by gravity Patient is percussed to emulsify the surfactant sediment Saline is drained by gravity and continued until lavage fluid becomes clear Whole Lung Lavage is a Highly Invasive Procedure Performed in a Tertiary Center and is Not Standardized © Savara Inc. All Rights Reserved. Whole lung lavage is an invasive procedure to physically remove excess surfactant from the lungs and requires hospitalization Performed under general anesthesia by highly experienced physicians at certain sites Sources: 1: Campo, Assessment and Management of PAP in a Reference Center, Orphanet Jour. of Rare Dis., 2013; 2: Campo, Nat. History of PAP Data from Italian Nat. Reference Center, ERJ, 2019.; Seymour, J. J. Pulmonary alveolar proteinosis: Progress in the First 44 Years, Am. J. Respir Crit. Care Med, 2002.
© Savara Inc. All Rights Reserved. Complications and Short-Comings of Whole Lung Lavage Rib fracture Hypoxia Pneumothorax (collapsed lung) Hydrothorax (fluid in pleural cavity) Superimposed infection Acute Respiratory Distress Syndrome (ARDS) Short Comings Treatment fails to address pathophysiology of disease Patients continue to experience symptomatic deterioration between procedures – and can require more than one whole lung lavage Rollercoaster ride of improvement and decline The procedure, performed under general anesthesia, is not standardized and remains highly operator-dependent Potential Complications
Due to aPAP’s rarity and associated non-specific symptoms, patients are often misdiagnosed with more common pulmonary illnesses (e.g., recurrent pneumonia, chronic bronchitis, COPD, asthma) © Savara Inc. All Rights Reserved. Journey to Diagnosis Can Be Long and Misdiagnosis is Common Diagnostic tests typically conducted to rule-out other more common pulmonary diseases: Transbronchial biopsy and cytological analysis of bronchoalveolar lavage fluid Pulmonary function tests Imaging Secondary PAP testing
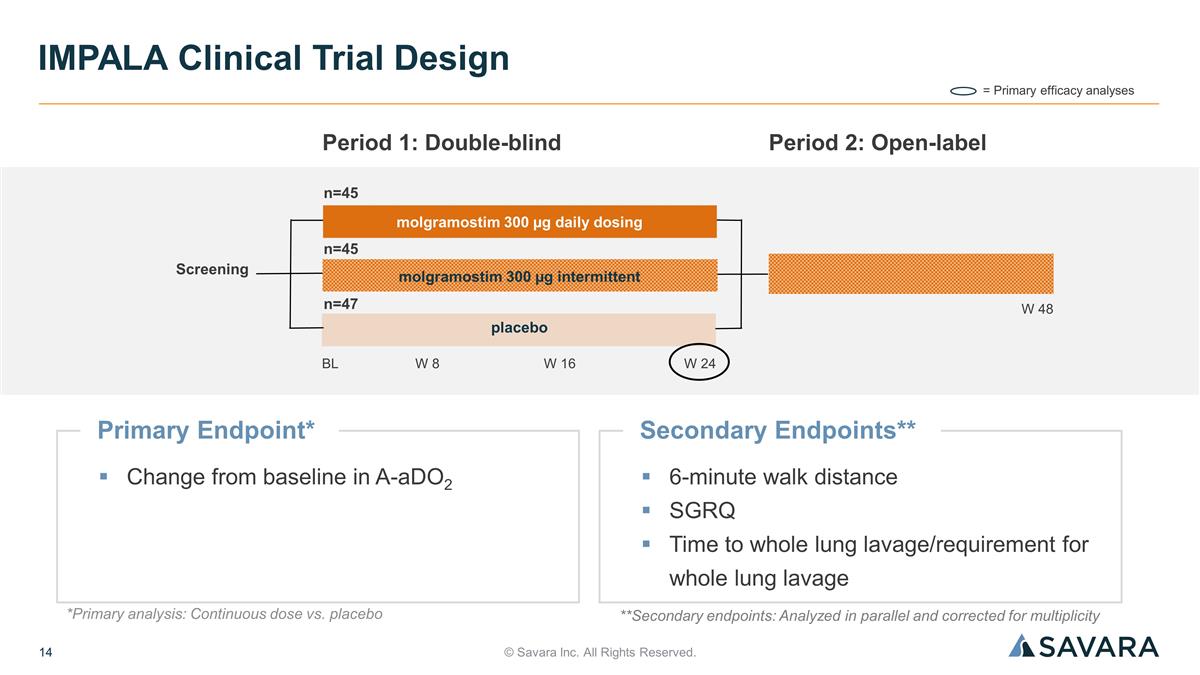
© Savara Inc. All Rights Reserved. IMPALA Clinical Trial Design **Secondary endpoints: Analyzed in parallel and corrected for multiplicity Primary Endpoint* Change from baseline in A-aDO2 = Primary efficacy analyses Screening BL n=45 Period 1: Double-blind W 8 W 24 W 16 Period 2: Open-label n=45 n=47 W 48 molgramostim 300 µg daily dosing molgramostim 300 µg intermittent placebo Secondary Endpoints** 6-minute walk distance SGRQ Time to whole lung lavage/requirement for whole lung lavage *Primary analysis: Continuous dose vs. placebo
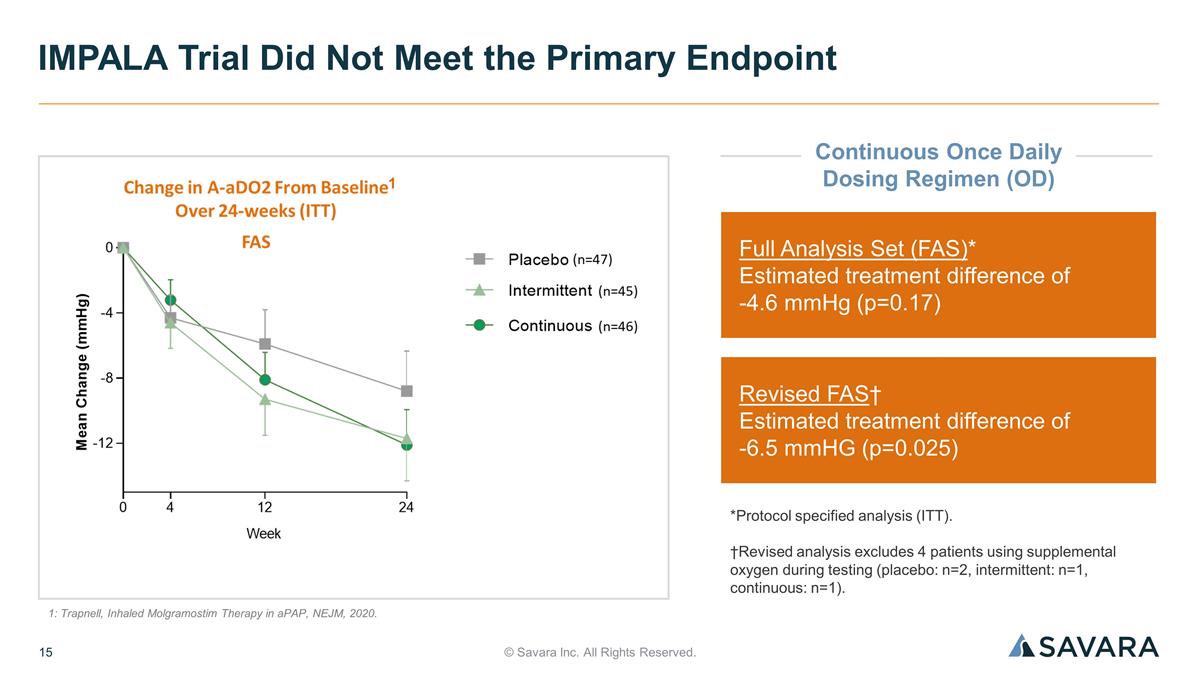
© Savara Inc. All Rights Reserved. IMPALA Trial Did Not Meet the Primary Endpoint Full Analysis Set (FAS)* Estimated treatment difference of -4.6 mmHg (p=0.17) Revised FAS† Estimated treatment difference of -6.5 mmHG (p=0.025) *Protocol specified analysis (ITT). †Revised analysis excludes 4 patients using supplemental oxygen during testing (placebo: n=2, intermittent: n=1, continuous: n=1). 1: Trapnell, Inhaled Molgramostim Therapy in aPAP, NEJM, 2020. 1 Continuous Once Daily Dosing Regimen (OD)
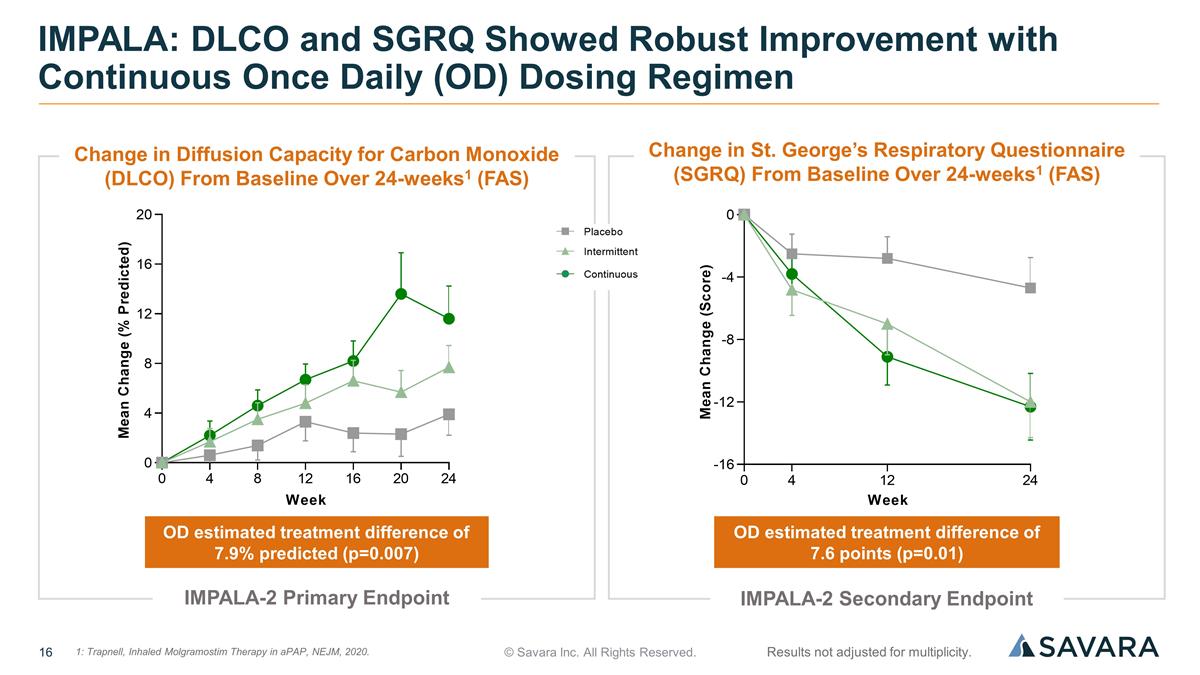
© Savara Inc. All Rights Reserved. IMPALA: DLCO and SGRQ Showed Robust Improvement with Continuous Once Daily (OD) Dosing Regimen OD estimated treatment difference of 7.9% predicted (p=0.007) OD estimated treatment difference of 7.6 points (p=0.01) Results not adjusted for multiplicity. IMPALA-2 Primary Endpoint IMPALA-2 Secondary Endpoint 1: Trapnell, Inhaled Molgramostim Therapy in aPAP, NEJM, 2020. 1 Change in Diffusion Capacity for Carbon Monoxide (DLCO) From Baseline Over 24-weeks1 (FAS) Change in St. George’s Respiratory Questionnaire (SGRQ) From Baseline Over 24-weeks1 (FAS)
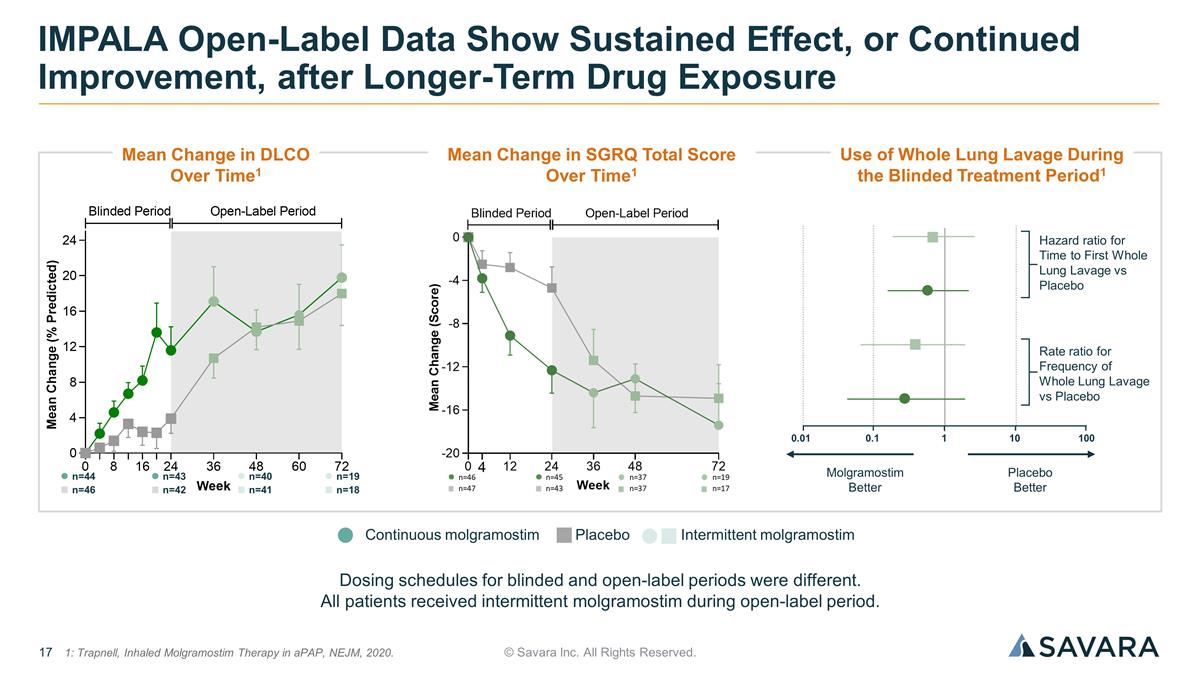
© Savara Inc. All Rights Reserved. IMPALA Open-Label Data Show Sustained Effect, or Continued Improvement, after Longer-Term Drug Exposure Placebo Continuous molgramostim Intermittent molgramostim Dosing schedules for blinded and open-label periods were different. All patients received intermittent molgramostim during open-label period. n=44 n=46 n=43 n=42 n=40 n=41 n=19 n=18 1: Trapnell, Inhaled Molgramostim Therapy in aPAP, NEJM, 2020. Mean Change in DLCO Over Time1 Mean Change in SGRQ Total Score Over Time1 Use of Whole Lung Lavage During the Blinded Treatment Period1 Hazard ratio for Time to First Whole Lung Lavage vs Placebo Rate ratio for Frequency of Whole Lung Lavage vs Placebo Placebo Better Molgramostim Better 100 10 1 0.1 0.01
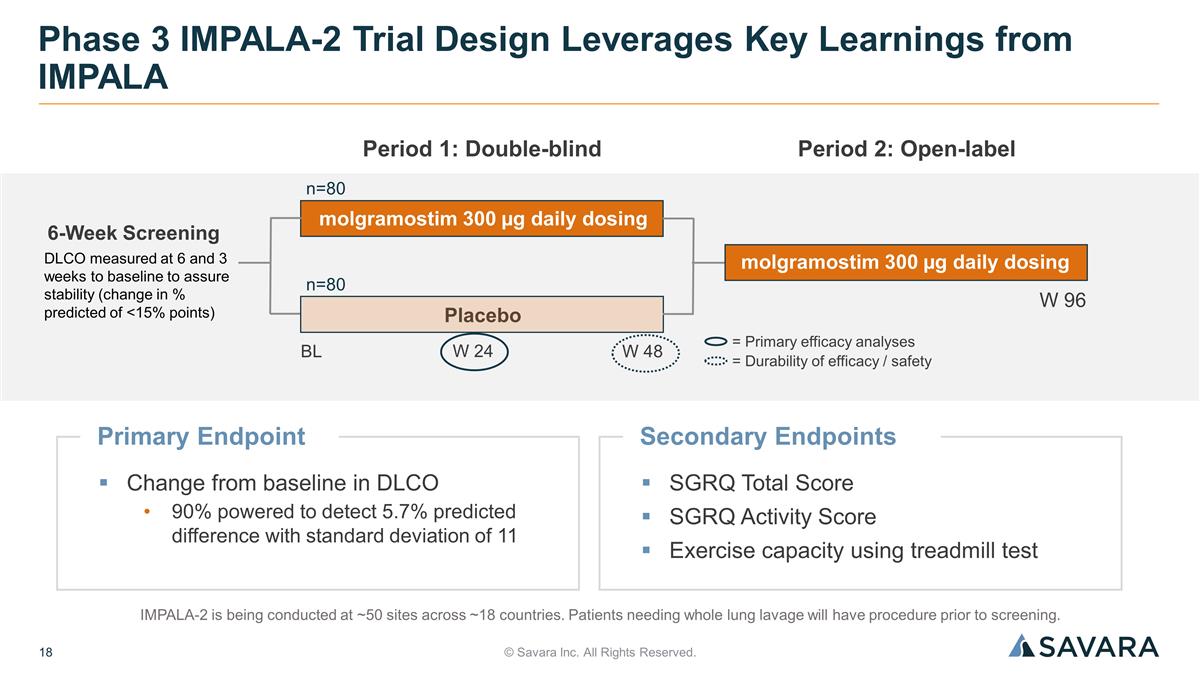
Primary Endpoint Change from baseline in DLCO 90% powered to detect 5.7% predicted difference with standard deviation of 11 © Savara Inc. All Rights Reserved. Phase 3 IMPALA-2 Trial Design Leverages Key Learnings from IMPALA IMPALA-2 is being conducted at ~50 sites across ~18 countries. Patients needing whole lung lavage will have procedure prior to screening. = Primary efficacy analyses = Durability of efficacy / safety 6-Week Screening BL molgramostim 300 µg daily dosing Period 1: Double-blind W 24 W 48 Period 2: Open-label W 96 Placebo molgramostim 300 µg daily dosing n=80 n=80 DLCO measured at 6 and 3 weeks to baseline to assure stability (change in % predicted of <15% points) Secondary Endpoints SGRQ Total Score SGRQ Activity Score Exercise capacity using treadmill test
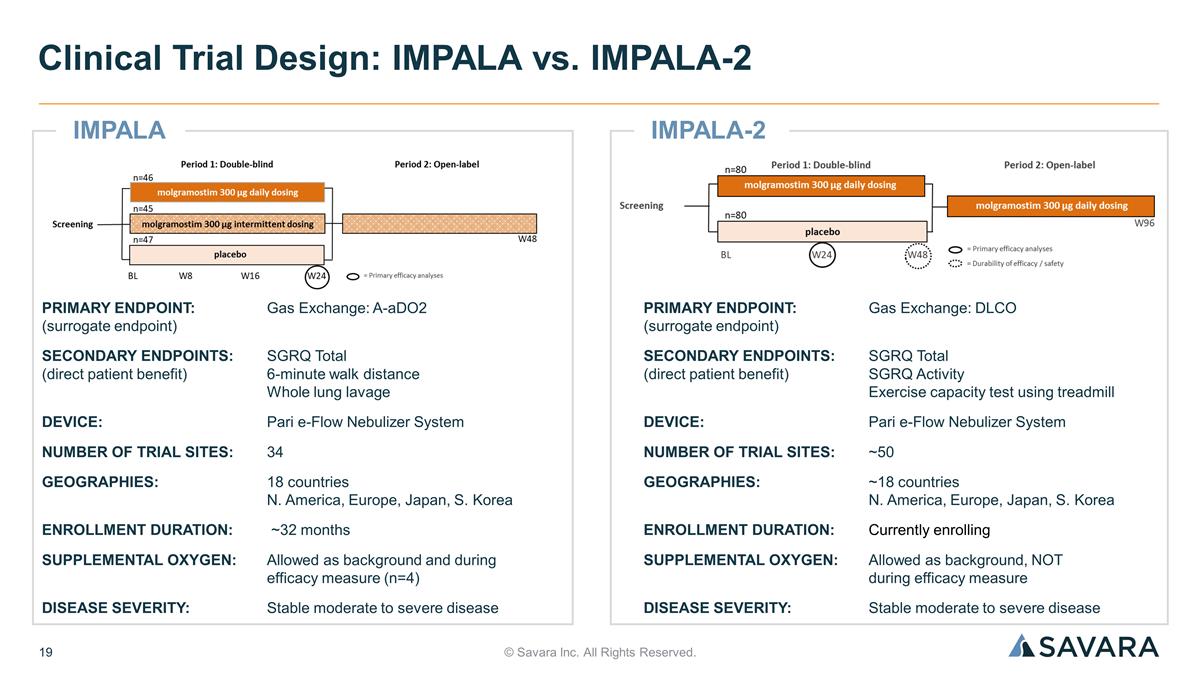
IMPALA-2 © Savara Inc. All Rights Reserved. Clinical Trial Design: IMPALA vs. IMPALA-2 PRIMARY ENDPOINT: Gas Exchange: A-aDO2 (surrogate endpoint) SECONDARY ENDPOINTS:SGRQ Total (direct patient benefit) 6-minute walk distance Whole lung lavage DEVICE:Pari e-Flow Nebulizer System NUMBER OF TRIAL SITES:34 GEOGRAPHIES:18 countries N. America, Europe, Japan, S. Korea ENROLLMENT DURATION: ~32 months SUPPLEMENTAL OXYGEN:Allowed as background and during efficacy measure (n=4) DISEASE SEVERITY:Stable moderate to severe disease PRIMARY ENDPOINT: Gas Exchange: DLCO (surrogate endpoint) SECONDARY ENDPOINTS: SGRQ Total (direct patient benefit) SGRQ Activity Exercise capacity test using treadmill DEVICE: Pari e-Flow Nebulizer System NUMBER OF TRIAL SITES: ~50 GEOGRAPHIES:~18 countries N. America, Europe, Japan, S. Korea ENROLLMENT DURATION: Currently enrolling SUPPLEMENTAL OXYGEN: Allowed as background, NOT during efficacy measure DISEASE SEVERITY: Stable moderate to severe disease IMPALA
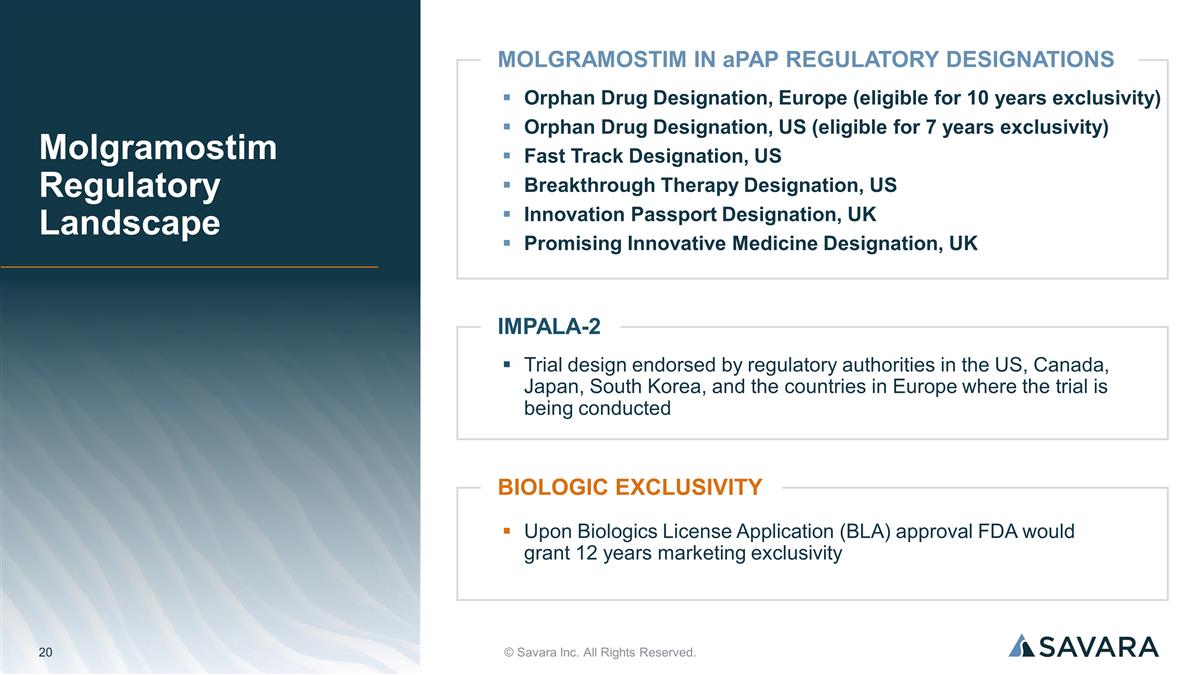
Upon Biologics License Application (BLA) approval FDA would grant 12 years marketing exclusivity BIOLOGIC EXCLUSIVITY Trial design endorsed by regulatory authorities in the US, Canada, Japan, South Korea, and the countries in Europe where the trial is being conducted IMPALA-2 Molgramostim Regulatory Landscape © Savara Inc. All Rights Reserved. Orphan Drug Designation, Europe (eligible for 10 years exclusivity) Orphan Drug Designation, US (eligible for 7 years exclusivity) Fast Track Designation, US Breakthrough Therapy Designation, US Innovation Passport Designation, UK Promising Innovative Medicine Designation, UK MOLGRAMOSTIM IN aPAP REGULATORY DESIGNATIONS

Commercial Outlook
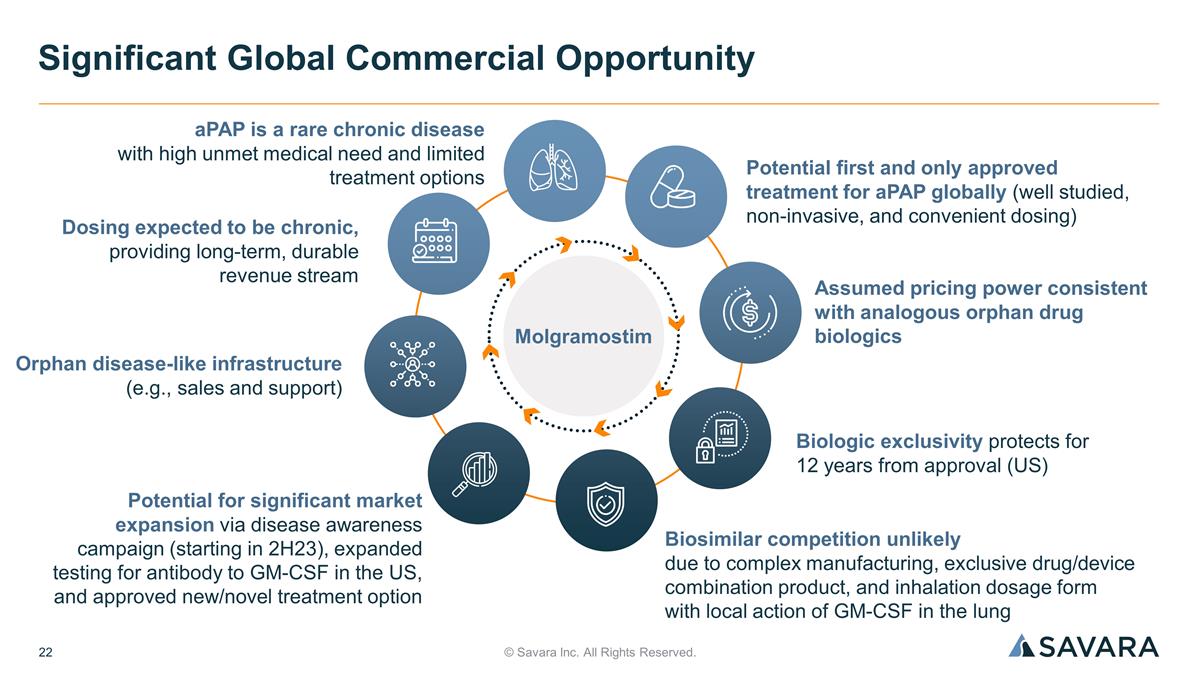
© Savara Inc. All Rights Reserved. Significant Global Commercial Opportunity aPAP is a rare chronic disease with high unmet medical need and limited treatment options Potential first and only approved treatment for aPAP globally (well studied, non-invasive, and convenient dosing) Dosing expected to be chronic, providing long-term, durable revenue stream Assumed pricing power consistent with analogous orphan drug biologics Potential for significant market expansion via disease awareness campaign (starting in 2H23), expanded testing for antibody to GM-CSF in the US, and approved new/novel treatment option Biologic exclusivity protects for 12 years from approval (US) Orphan disease-like infrastructure (e.g., sales and support) Biosimilar competition unlikely due to complex manufacturing, exclusive drug/device combination product, and inhalation dosage form with local action of GM-CSF in the lung Molgramostim
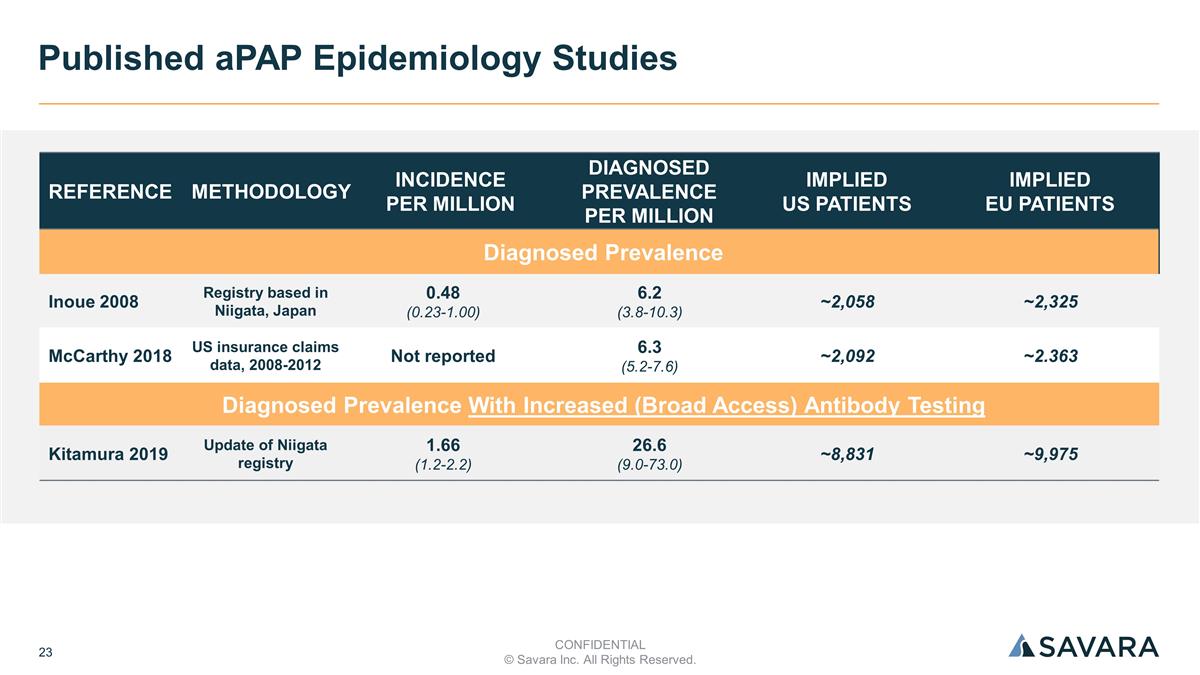
REFERENCE METHODOLOGY INCIDENCE PER M INCIDENCE PER MILLION DIAGNOSED PREVALENCE PER MILLION IMPLIED US PATIENTS IMPLIED EU PATIENTS Diagnosed Prevalence Inoue 2008 Registry based in Niigata, Japan 0.48 (0.23-1.00) 6.2 (3.8-10.3) ~2,058 ~2,325 McCarthy 2018 US insurance claims data, 2008-2012 Not reported 6.3 (5.2-7.6) ~2,092 ~2.363 Diagnosed Prevalence With Increased (Broad Access) Antibody Testing Kitamura 2019 Update of Niigata registry 1.66 (1.2-2.2) 26.6 (9.0-73.0) ~8,831 ~9,975 Published aPAP Epidemiology Studies CONFIDENTIAL © Savara Inc. All Rights Reserved.
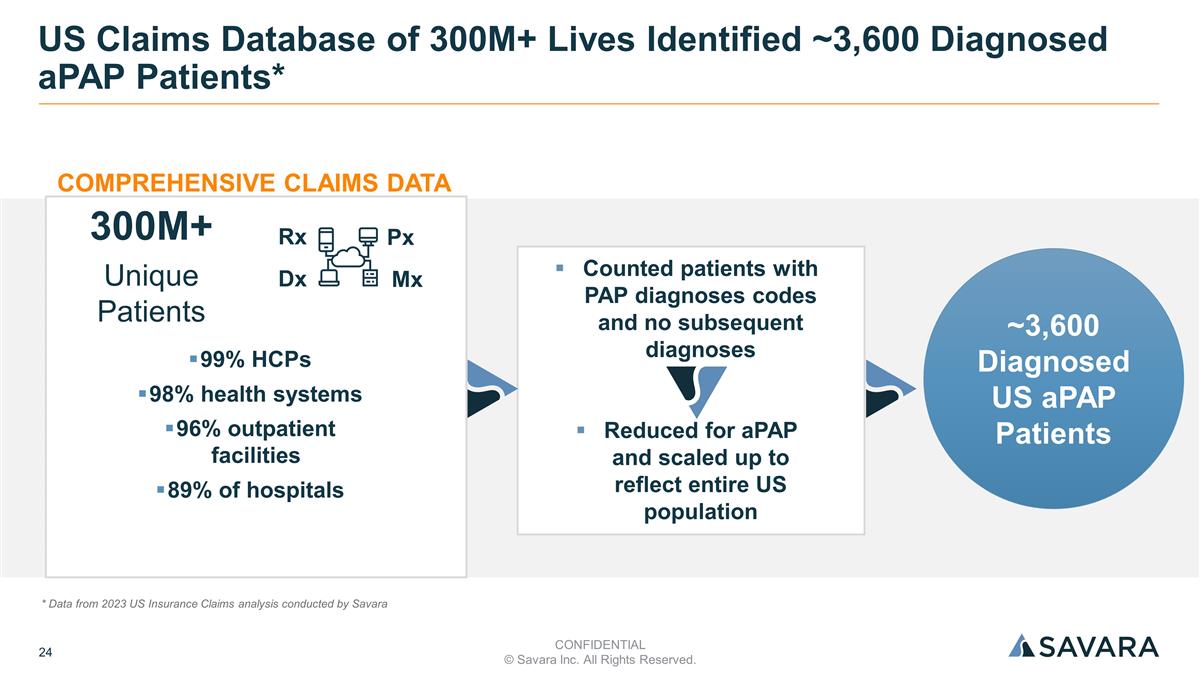
US Claims Database of 300M+ Lives Identified ~3,600 Diagnosed aPAP Patients* ~3,600 Diagnosed US aPAP Patients 300M+ Unique Patients 99% HCPs 98% health systems 96% outpatient facilities 89% of hospitals Rx Px Dx Mx COMPREHENSIVE CLAIMS DATA Counted patients with PAP diagnoses codes and no subsequent diagnoses Reduced for aPAP and scaled up to reflect entire US population CONFIDENTIAL © Savara Inc. All Rights Reserved. * Data from 2023 US Insurance Claims analysis conducted by Savara
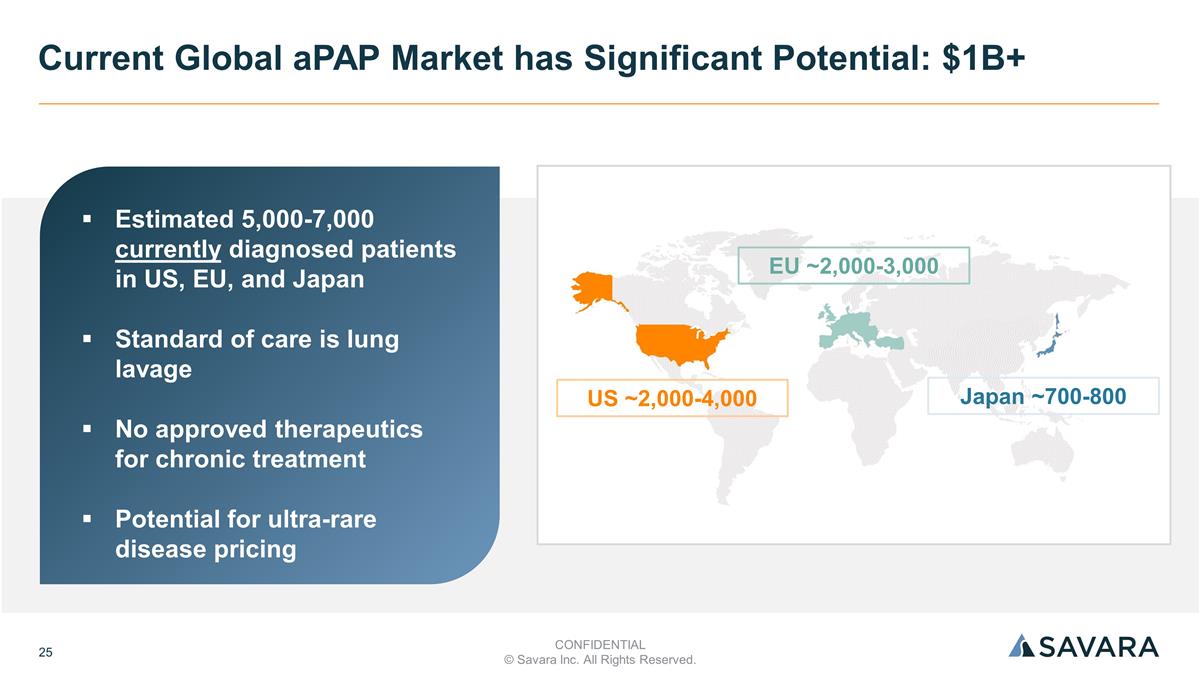
Current Global aPAP Market has Significant Potential: $1B+ Estimated 5,000-7,000 currently diagnosed patients in US, EU, and Japan Standard of care is lung lavage No approved therapeutics for chronic treatment Potential for ultra-rare disease pricing US ~2,000-4,000 EU ~2,000-3,000 Japan ~700-800 CONFIDENTIAL © Savara Inc. All Rights Reserved.
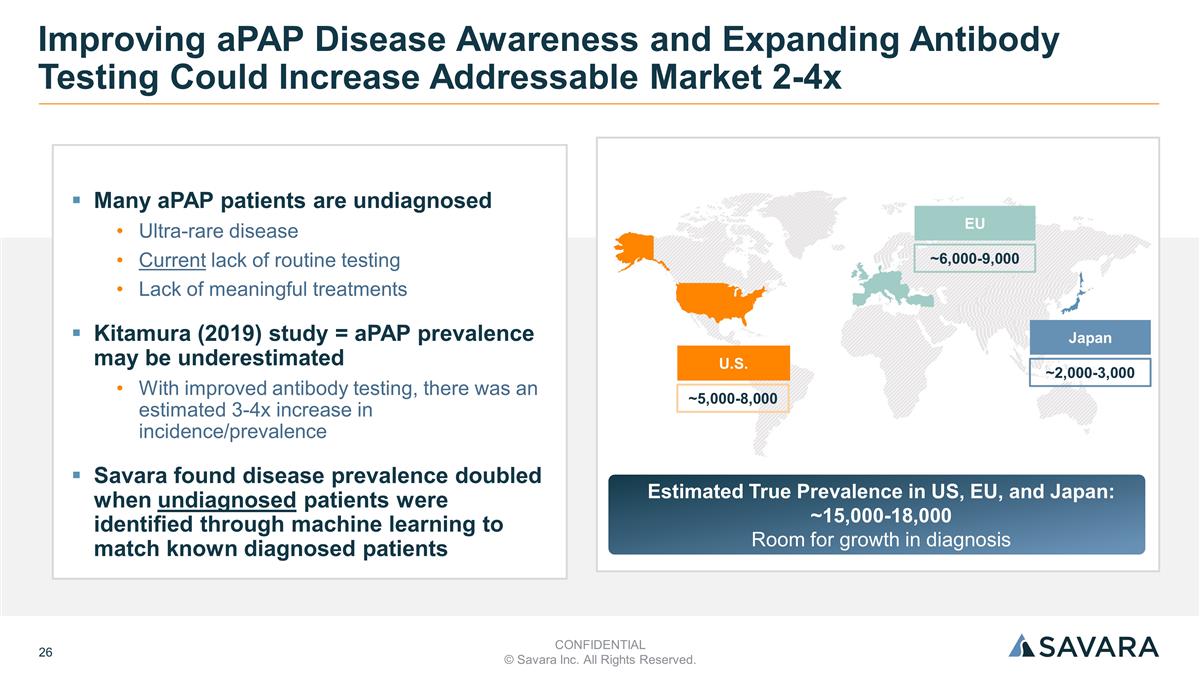
Many aPAP patients are undiagnosed Ultra-rare disease Current lack of routine testing Lack of meaningful treatments Kitamura (2019) study = aPAP prevalence may be underestimated With improved antibody testing, there was an estimated 3-4x increase in incidence/prevalence Savara found disease prevalence doubled when undiagnosed patients were identified through machine learning to match known diagnosed patients Improving aPAP Disease Awareness and Expanding Antibody Testing Could Increase Addressable Market 2-4x Estimated True Prevalence in US, EU, and Japan: ~15,000-18,000 Room for growth in diagnosis U.S. ~5,000-8,000 EU ~6,000-9,000 Japan ~2,000-3,000 CONFIDENTIAL © Savara Inc. All Rights Reserved.
SAVARA PLANS TO Launch a Disease Awareness and Education Campaign: US Healthcare Provider (HCP) Website Launch in 2023 Increase HCP awareness of aPAP, including hallmark symptoms of the disease Educate HCPs on the need for routine antibody testing Seek to change clinical diagnostic guidelines to accelerate testing Offer No-Cost Antibody Testing Savara plans to offer a simple, accurate, no-cost, antibody blood test: US: 2023 EU: 2024 Disease Awareness and Antibody Testing Campaigns CONFIDENTIAL © Savara Inc. All Rights Reserved.
Analog: Pulmozyme® (dornase alpha) © Savara Inc. All Rights Reserved. Prototype inhaled biologic Approved by the FDA in 1993 No biosimilar available Pulmozyme® Pulmozyme is a registered trademark of Genentech

Financials

Financial Highlights © Savara Inc. All Rights Reserved. Well capitalized ~$115M in cash (as of 3/31/23) Cash runway extends ~18-months beyond anticipated IMPALA-2 top line results Strong investor support with coverage from 6 equity research analysts Evercore ISI Liisa Bayko, MSC, MBA H.C. Wainwright Andrew Fein Jefferies Andrew Tsai Ladenburg Thalmann & Co. Michael Higgins Oppenheimer Francois Brisebois Piper Sandler Yasmeen Rahimi, PhD ANALYST COVERAGE

Investment Thesis © Savara Inc. All Rights Reserved. The molgramostim in aPAP clinical program has a high probability of success Significant global commercial opportunity As a novel inhaled biologic, molgramostim has the potential for a long-term, durable revenue stream Strong balance sheet – funded through 2025

Thank You