|
|
IM CANNABIS CORP.
|
|
|
|
(Registrant)
|
|
|
|
|
|
|
Date: November 13, 2025
|
By:
|
/s/ Oren Shuster
|
|
|
Name:
|
Oren Shuster
|
|
|
Title:
|
Chief Executive Officer and Director
|
|
|
• |
Consistent Revenue for Q3 2025 and 2024 of $13.9 million.
|
|
|
• |
13% Gross profit decrease vs. Q3 2024 of $2.7 million vs. $3.1 million.
|
|
|
• |
13% Gross Margin decrease vs. Q3 2024 of 20% vs. 23%.
|
|
|
• |
$3.1 million One time goodwill and intangible asset impairment in Q3 2025 Operating expenses.
|
|
|
• |
Non IFRS Adjusted EBITDA loss of $0.6 million in Q3 2025 vs. $0.2 million in Q3 2024.
|
|
|
• |
Net loss in Q3 2025 was $3.9 million, compared to net loss of $1.1 million in Q3 2024. Net loss increase mainly due to goodwill and intangible asset impairment recorded at Q3 2025.
|
|
|
• |
Revenue for the third quarter of 2025 amounted to $13.9 million, similar to Q3 2024, while revenues for the first 9 months ended September 30, 2025, amounted to $39 million vs. $40.7
million in the same period of 2024.
|
|
|
• |
Gross profit for the third quarter of 2025 was $2.7 million, compared to $3.1 million in Q3 2024, a decrease of 13%.
|
|
|
• |
Gross margin for the third quarter of 2025 was 20%, compared to 23% in Q3 2024, a decrease of 13%.
|
|
|
• |
Total operating expenses in Q3 2025 were $6.9 million compared to $4.1 million in Q3 2024, an increase of 68%. The increase is mainly due to goodwill
and intangible asset impairment recorded at Q3 2025.
|
|
|
• |
Net loss in Q3 2025 was $0.8 million, compared to net loss of $1.1 million in Q3 2024, excluding the one-time goodwill and intangible asset impairment. In total, net loss in Q3 2025 was
$3.9 million, compared to net loss of $1.1 million in Q3 2024.
|
|
|
• |
G&A Expenses in Q3 2025 were $2.4 million, similar to Q3 2024.
|
|
|
• |
Selling and Marketing Expenses in Q3 2025 were $1.4 million, compared to $1.5 million in Q3 2024, a decrease of 7%.
|
|
|
• |
Basic and diluted Loss per Share in Q3 2025 was $0.75 million, compared to a loss of $0.41 million per Share in Q3 2024.
|
|
|
• |
Non-IFRS Adjusted EBITDA Loss in Q3 2025 was $0.6 million, compared to a Non-IFRS adjusted EBITDA loss of $0.2 million in Q3 2024, a decline of
143%.
|
|
|
• |
Cash and Restricted Cash on hand as of September 30, 2025, were $2.3 million compared to $0.9 million on December 31, 2024.
|
|
|
• |
Total Assets as of September 30, 2025, were $44.3 million, compared to $39.2 million on December 31, 2024, an increase of 13%. The increase is mainly attributed to an increase of $2.4
million in advances to suppliers and $6.8 million in inventory, offset by decreases of $2.5 million in trade receivables and $3.1 million in accordance with goodwill and intangible assets impairment.
|
|
|
• |
Total Liabilities as of September 30, 2025, were $40 million, compared to $36 million on December 31, 2024,
an increase of 11%. The increase is mainly due to $8.9 million in other accounts payable and $0.9 million due to increase in short and long term credit from banks. This is offset by a $4 million decrease in trade payables and $1.4
million decrease in convertible debentures.
|
|
September 30, 2025
|
December 31, 2024
|
|||||||||||
|
Note
|
(Unaudited)
|
|||||||||||
|
ASSETS
|
||||||||||||
|
CURRENT ASSETS:
|
||||||||||||
|
Cash
|
$
|
1,182
|
$
|
863
|
||||||||
|
Restricted cash
|
1,134
|
64
|
||||||||||
|
Trade receivables
|
11,255
|
13,803
|
||||||||||
|
Other current assets
|
8,073
|
5,419
|
||||||||||
|
Inventory
|
10,023
|
3,215
|
||||||||||
|
31,667
|
23,364
|
|||||||||||
|
NON-CURRENT ASSETS:
|
||||||||||||
|
Investments in affiliate
|
4
|
1,742
|
1,631
|
|||||||||
|
Property, plant and equipment, net
|
|
3,819
|
3,730
|
|||||||||
|
Intangible assets, net
|
3I
|
|
1,586
|
3,333
|
||||||||
|
Goodwill
|
3I
|
|
5,005
|
6,679
|
||||||||
|
Right-of-use assets, net
|
|
513
|
451
|
|||||||||
|
12,665
|
15,824
|
|||||||||||
|
Total assets
|
$
|
44,332
|
$
|
39,188
|
||||||||
|
September 30,
2025
|
December 31, 2024
|
|||||||||||
|
Note
|
(Unaudited)
|
|||||||||||
|
LIABILITIES AND SHAREHOLDERS' EQUITY
|
||||||||||||
|
CURRENT LIABILITIES:
|
||||||||||||
|
Current maturities of operating lease liabilities
|
$
|
379
|
$
|
262
|
||||||||
|
Trade payables
|
7,147
|
11,159
|
||||||||||
|
Other current liabilities
|
13,917
|
5,001
|
||||||||||
|
Loans and credit from bank institution and others
|
15,417
|
15,145
|
||||||||||
|
Convertible debentures
|
3D
|
|
597
|
1,968
|
||||||||
|
Derivative warrants liabilities and prefunded warrants
|
3C, 4
|
1,002
|
1,383
|
|||||||||
|
38,459
|
34,918
|
|||||||||||
|
NON-CURRENT LIABILITIES:
|
||||||||||||
|
Operating lease liabilities
|
92
|
171
|
||||||||||
|
Loans and credit from bank institution and others
|
1,078
|
466
|
||||||||||
|
Deferred tax liabilities
|
400
|
487
|
||||||||||
|
1,570
|
1,124
|
|||||||||||
|
Total liabilities
|
40,029
|
36,042
|
||||||||||
|
EQUITY ATTRIBUTABLE TO SHAREHOLDERS OF THE COMPANY:
|
5
|
|||||||||||
|
Share capital and premium
|
269,574
|
265,000
|
||||||||||
|
Capital reserve from share-based payment transactions
|
475
|
150
|
||||||||||
|
Amount received on account of financial instruments and other
|
3,112
|
297
|
||||||||||
|
Capital reserve from translation differences of foreign operations
|
(3,783
|
)
|
(1,265
|
)
|
||||||||
|
Capital reserve from transaction with non-controlling interests
|
(2,872
|
)
|
-
|
|||||||||
|
Capital reserve from transaction with controlling shareholder
|
33
|
-
|
||||||||||
|
Accumulated deficit
|
(262,576
|
)
|
(258,939
|
)
|
||||||||
|
Total equity attributable to shareholders of the Company
|
3,963
|
5,243
|
||||||||||
|
Non-controlling interests
|
340
|
(2,097
|
)
|
|||||||||
|
Total equity
|
4,303
|
3,146
|
||||||||||
|
Total liabilities and equity
|
$
|
44,332
|
$
|
39,188
|
||||||||
|
Nine months ended
September 30,
|
Three months ended
September 30,
|
|||||||||||||||||||
|
Note
|
2025
|
2024
|
2025
|
2024
|
||||||||||||||||
|
Revenue
|
$
|
39,047
|
$
|
40,696
|
$
|
13,851
|
$
|
13,883
|
||||||||||||
|
Cost of revenue
|
29,443
|
34,925
|
11,120
|
10,735
|
||||||||||||||||
|
Gross profit
|
9,604
|
5,771
|
2,731
|
3,148
|
||||||||||||||||
|
Selling and marketing expenses
|
3,935
|
5,279
|
1,373
|
1,506
|
||||||||||||||||
|
General and administrative expenses
|
6,924
|
6,846
|
2,433
|
2,351
|
||||||||||||||||
|
Share-based compensation
|
14
|
364
|
2
|
244
|
||||||||||||||||
|
Other expenses
|
3I
|
|
3,076
|
2,734
|
3,076
|
-
|
||||||||||||||
|
Total operating expenses
|
13,949
|
15,223
|
6,884
|
4,101
|
||||||||||||||||
|
Operating loss
|
(4,345
|
)
|
(9,452
|
)
|
(4,153
|
)
|
(953
|
)
|
||||||||||||
|
Finance income
|
3,181
|
495
|
1,111
|
1
|
||||||||||||||||
|
Finance expenses
|
(2,634
|
)
|
(2,577
|
)
|
(682
|
)
|
(156
|
)
|
||||||||||||
|
Finance income (expenses), net
|
547
|
(2,082
|
)
|
429
|
(155
|
)
|
||||||||||||||
|
Loss before tax benefit
|
(3,798
|
)
|
(11,534
|
)
|
(3,724
|
)
|
(1,108
|
)
|
||||||||||||
|
Taxes on income (tax benefit)
|
86
|
(976
|
)
|
141
|
(26
|
)
|
||||||||||||||
|
Net loss
|
$
|
(3,884
|
)
|
$
|
(10,558
|
)
|
$
|
(3,865
|
)
|
$
|
(1,082
|
)
|
||||||||
|
Nine months ended
September 30,
|
Three months ended
September 30,
|
|||||||||||||||||||
|
Note
|
2025
|
2024 (*)
|
|
2025
|
2024 (*)
|
|
||||||||||||||
|
Other comprehensive income that will not be reclassified to profit or loss in subsequent periods:
|
||||||||||||||||||||
|
Remeasurement gain on defined benefit plan
|
48
|
1,633
|
-
|
49
|
||||||||||||||||
|
Other comprehensive income (loss) that will be reclassified to profit or loss in subsequent periods:
|
||||||||||||||||||||
|
Adjustments arising from translating financial statements of foreign operations
|
(2,440
|
)
|
(508
|
)
|
(961
|
)
|
(482
|
)
|
||||||||||||
|
Total other comprehensive income (loss)
|
(2,392
|
)
|
1,125
|
(961
|
)
|
(433
|
)
|
|||||||||||||
|
Total comprehensive loss
|
$
|
(6,276
|
)
|
$
|
(9,433
|
)
|
$
|
(4,826
|
)
|
$
|
(1,515
|
)
|
||||||||
|
Net income (loss) attributable to:
|
||||||||||||||||||||
|
Shareholders of the Company
|
|
|
$
|
(3,685
|
)
|
$
|
(9,574
|
)
|
$
|
(3,651
|
)
|
$
|
(922
|
)
|
||||||
|
Non-controlling interests
|
(199
|
)
|
(984
|
)
|
(214
|
)
|
(160
|
)
|
||||||||||||
|
$
|
(3,884
|
)
|
$
|
(10,558
|
)
|
$
|
(3,865
|
)
|
$
|
(1,082
|
)
|
|||||||||
|
Total comprehensive income (loss) attributable to:
|
||||||||||||||||||||
|
Shareholders of the Company
|
$
|
(6,155
|
)
|
$
|
(8,458
|
)
|
$
|
(4,627
|
)
|
$
|
(1,357
|
)
|
||||||||
|
Non-controlling interests
|
(121
|
)
|
(975
|
)
|
(199
|
)
|
(158
|
)
|
||||||||||||
|
$
|
(6,276
|
)
|
$
|
(9,433
|
)
|
$
|
(4,826
|
)
|
$
|
(1,515
|
)
|
|||||||||
|
Net loss per share attributable to shareholders of the Company:
|
6
|
|
||||||||||||||||||
|
Basic net loss per share (in CAD)
|
$
|
(0.98
|
)
|
$
|
(4.29
|
)
|
$
|
(0.75
|
)
|
$
|
(0.41
|
)
|
||||||||
|
Diluted net loss per share (in CAD)
|
$
|
(1.00
|
)
|
$
|
(4.29
|
)
|
$
|
(0.75
|
)
|
$
|
(0.41
|
)
|
||||||||
| (*) |
Loss per share includes the effect of Reverse Share Split (see also Note 5A below).
|
|
Share capital and premium
|
Capital reserve from share-based payment transactions
|
Amount received on account of financial instruments and other
|
Capital reserve from translation difference of foreign operations
|
Capital reserve from transaction with non-controlling interests
|
Capital reserve from transaction with controlling shareholder
|
Accumulated deficit
|
Total
|
Non-controlling interests
|
Total
equity
|
|||||||||||||||||||||||||||||||
|
Balance as of January 1, 2025
|
$
|
265,000
|
$
|
150
|
$
|
297
|
$
|
(1,265
|
)
|
$
|
-
|
$
|
-
|
$
|
(258,939
|
)
|
$
|
5,243
|
$
|
(2,097
|
)
|
$
|
3,146
|
|||||||||||||||||
|
Net loss
|
-
|
-
|
-
|
-
|
-
|
-
|
(3,685
|
)
|
(3,685
|
)
|
(199
|
)
|
(3,884
|
)
|
||||||||||||||||||||||||||
|
Total other comprehensive income (loss)
|
-
|
-
|
-
|
(2,518
|
)
|
-
|
-
|
48
|
(2,470
|
)
|
78
|
(2,392
|
)
|
|||||||||||||||||||||||||||
|
Total comprehensive income (loss)
|
-
|
-
|
-
|
(2,518
|
)
|
-
|
-
|
(3,637
|
)
|
(6,155
|
)
|
(121
|
)
|
(6,276
|
)
|
|||||||||||||||||||||||||
|
Recognition of capital contribution from a controlling shareholder (Note 3B6)
|
-
|
-
|
-
|
-
|
-
|
33
|
-
|
33
|
-
|
33
|
||||||||||||||||||||||||||||||
|
Common shares issued upon exercise of pre-funded warrants (Note 3C)
|
372
|
-
|
-
|
-
|
-
|
-
|
-
|
372
|
-
|
372
|
||||||||||||||||||||||||||||||
|
Expiration of conversion feature related to convertible debentures (Note 3D)
|
297
|
-
|
(297
|
)
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|||||||||||||||||||||||||||||
|
Recognition of conversion feature related to convertible debentures (Note 3D)
|
-
|
-
|
364
|
-
|
-
|
-
|
-
|
364
|
-
|
364
|
||||||||||||||||||||||||||||||
|
Common shares issued upon partial conversion of convertible debentures (Note 3D)
|
1,651
|
-
|
(256
|
)
|
-
|
-
|
-
|
-
|
1,395
|
-
|
1,395
|
|||||||||||||||||||||||||||||
|
Common shares issued as consideration upon acquisition on non-controlling interest (Note 3E)
|
314
|
-
|
-
|
-
|
(2,872
|
)
|
-
|
-
|
(2,558
|
)
|
2,558
|
-
|
||||||||||||||||||||||||||||
|
Common shares issued upon debt settlement (Note 3F)
|
190
|
-
|
-
|
-
|
-
|
-
|
-
|
190
|
-
|
190
|
||||||||||||||||||||||||||||||
|
Net proceeds received upon completion of private placement transaction (Note 3G)
|
1,750
|
311
|
3,004
|
-
|
-
|
-
|
-
|
5,065
|
-
|
5,065
|
||||||||||||||||||||||||||||||
|
Share-based compensation
|
-
|
14
|
-
|
-
|
-
|
-
|
-
|
14
|
-
|
14
|
||||||||||||||||||||||||||||||
|
Balance as of September 30, 2025
|
$
|
269,574
|
$
|
475
|
$
|
3,112
|
$
|
(3,783
|
)
|
$
|
(2,872
|
)
|
$
|
33
|
$
|
(262,576
|
)
|
$
|
3,963
|
$
|
340
|
$
|
4,303
|
|||||||||||||||||
|
Share
Capital and premium
|
Capital reserve from share-based payment transactions
|
Amount received on account of financial instruments and other
|
Translation reserve
|
Accumulated deficit
|
Total
|
Non-controlling interests
|
Total
equity
|
|||||||||||||||||||||||||
|
Balance as of January 1, 2024
|
$
|
253,882
|
$
|
9,637
|
$
|
-
|
$
|
95
|
$
|
(249,145
|
)
|
$
|
14,469
|
$
|
(769
|
)
|
$
|
13,700
|
||||||||||||||
|
Net loss
|
-
|
-
|
-
|
-
|
(9,574
|
)
|
(9,574
|
)
|
(984
|
)
|
(10,558
|
)
|
||||||||||||||||||||
|
Total other comprehensive income
|
-
|
-
|
-
|
1,049
|
67
|
1,116
|
9
|
1,125
|
||||||||||||||||||||||||
|
Total comprehensive loss
|
-
|
-
|
-
|
1,049
|
(9,507
|
)
|
(8,458
|
)
|
(975
|
)
|
(9,433
|
)
|
||||||||||||||||||||
|
Other comprehensive loss classification
|
-
|
-
|
-
|
-
|
(748
|
)
|
(748
|
)
|
-
|
(748
|
)
|
|||||||||||||||||||||
|
Recognition of conversion feature related to convertible debentures
|
-
|
-
|
327
|
-
|
-
|
327
|
-
|
327
|
||||||||||||||||||||||||
|
Share-based compensation
|
-
|
364
|
-
|
-
|
-
|
364
|
-
|
364
|
||||||||||||||||||||||||
|
Forfeited options
|
2,803
|
(2,803
|
)
|
-
|
-
|
-
|
-
|
-
|
-
|
|||||||||||||||||||||||
|
Balance as of September 30, 2024
|
$
|
256,685
|
$
|
7,198
|
$
|
327
|
$
|
1,144
|
$
|
(259,400
|
)
|
$
|
5,954
|
$
|
(1,744
|
)
|
$
|
4,210
|
||||||||||||||
|
Nine months ended
September 30,
|
||||||||
|
2025
|
2024
|
|||||||
|
Cash flow from operating activities:
|
||||||||
|
Net loss
|
$
|
(3,884
|
)
|
$
|
(10,558
|
)
|
||
|
Adjustments for non-cash items:
|
||||||||
|
Revaluation of financial instruments
|
(9
|
)
|
(24
|
)
|
||||
|
Discount expenses in respect of convertible debentures
|
178
|
197
|
||||||
|
Depreciation of property, plant and equipment
|
212
|
332
|
||||||
|
Amortization of intangible assets
|
991
|
1,036
|
||||||
|
Depreciation of right-of-use assets
|
232
|
274
|
||||||
|
Impairment of Goodwill
|
3,076
|
-
|
||||||
|
Impairment of property, plant and equipment
|
-
|
10
|
||||||
|
Loss from deconsolidation of subsidiary
|
-
|
2,734
|
||||||
|
Recognition of extension fee related to debentures
|
209
|
-
|
||||||
|
Finance expenses, net
|
(716
|
)
|
2,268
|
|||||
|
Deferred tax liability, net
|
(111
|
)
|
(138
|
)
|
||||
|
Share-based payments
|
14
|
364
|
||||||
|
Changes in employe benefit liabilities, net
|
-
|
(71
|
)
|
|||||
|
Discount expenses in respect of loans and credit received
|
141
|
-
|
||||||
|
4,217
|
6,982
|
|||||||
|
Changes in working capital:
|
||||||||
|
Decrease (increase) in trade receivables
|
3,306
|
(8,184
|
)
|
|||||
|
Increase in other current assets
|
(2,494
|
)
|
(2,775
|
)
|
||||
|
Decrease (increase) in inventory
|
(6,222
|
)
|
4,864
|
|||||
|
Increase (decrease) in trade payables
|
(3,896
|
)
|
10,595
|
|||||
|
Increase in other current liabilities
|
9,442
|
2,420
|
||||||
|
136
|
6,920
|
|||||||
|
Taxes (paid) received
|
22
|
(222
|
)
|
|||||
|
Net cash provided by operating activities
|
491
|
3,122
|
||||||
|
Cash flows from investing activities:
|
||||||||
|
Purchase of property, plant and equipment
|
(8
|
)
|
(126
|
)
|
||||
|
Deconsolidation of subsidiary
|
-
|
(346
|
)
|
|||||
|
Change in restricted cash
|
(1,070
|
)
|
-
|
|||||
|
Net cash used in investing activities
|
$
|
(1,078
|
)
|
$
|
(472
|
)
|
||
|
Nine months ended
September 30,
|
||||||||
|
2025
|
2024
|
|||||||
|
Cash flow from financing activities:
|
||||||||
|
Net proceeds received upon completion of private placement transaction
|
5,064
|
-
|
||||||
|
Repayment of lease liabilities
|
(230
|
)
|
(265
|
)
|
||||
|
Payment of interest on lease liabilities
|
(26
|
)
|
(44
|
)
|
||||
|
Proceeds from loans and credit received
|
4,634
|
1,803
|
||||||
|
Repayment of loans and credit
|
(2,573
|
)
|
(4,427
|
)
|
||||
|
Interest paid
|
(1,954
|
)
|
(1,572
|
)
|
||||
|
Proceeds from (repayment of) discounted checks
|
(1,647
|
)
|
4,483
|
|||||
|
Net cash provided by (used in) financing activities
|
3,268
|
(22
|
)
|
|||||
|
Effect of foreign exchange on cash
|
(2,362
|
)
|
(2,483
|
)
|
||||
|
Change in cash
|
319
|
145
|
||||||
|
Cash at the beginning of the period
|
863
|
1,813
|
||||||
|
Cash at end of the period
|
$
|
1,182
|
$
|
1,958
|
||||
|
Supplemental disclosure of non-cash activities:
|
||||||||
|
Right-of-use assets recognized with corresponding lease liabilities
|
$
|
272
|
$
|
40
|
||||
|
Issuance of convertible debentures in exchange for loans (principal and interest) received (Note 3C)
|
$
|
-
|
$
|
2,092
|
||||
|
Common shares issued upon exercise of pre-funded warrants (Note 3C)
|
$
|
372
|
$
|
-
|
||||
|
Common shares issued upon partial conversion of convertible debentures (Note 3D)
|
$
|
1,395
|
$
|
-
|
||||
|
Common shares issued as debt settlement (Note 3F)
|
$
|
190
|
$
|
-
|
||||

|
|
Page
|
|
|
|
||
|
|
F-2 - F-3
|
|
|
|
||
|
|
F-4 - F-5
|
|
|
|
||
|
|
F-6 - F-7
|
|
|
|
||
|
|
F-8 - F-9
|
|
|
|
||
|
|
F-10 - F-25
|
|
September 30, 2025
|
December 31, 2024
|
||||||||||
|
Note
|
(Unaudited)
|
||||||||||
|
ASSETS
|
|||||||||||
|
CURRENT ASSETS:
|
|||||||||||
|
Cash
|
$
|
1,182
|
$
|
863
|
|||||||
|
Restricted cash
|
1,134
|
64
|
|||||||||
|
Trade receivables
|
11,255
|
13,803
|
|||||||||
|
Other current assets
|
8,073
|
5,419
|
|||||||||
|
Inventory
|
10,023
|
3,215
|
|||||||||
|
31,667
|
23,364
|
||||||||||
|
NON-CURRENT ASSETS:
|
|||||||||||
|
Investments in affiliate
|
4
|
1,742
|
1,631
|
||||||||
|
Property, plant and equipment, net
|
3,819
|
3,730
|
|||||||||
|
Intangible assets, net
|
3I
|
|
1,586
|
3,333
|
|||||||
|
Goodwill
|
3I
|
|
5,005
|
6,679
|
|||||||
|
Right-of-use assets, net
|
513
|
451
|
|||||||||
|
12,665
|
15,824
|
||||||||||
|
Total assets
|
$
|
44,332
|
$
|
39,188
|
|||||||
|
September 30,
2025
|
December 31,
2024
|
||||||||||
|
Note
|
(Unaudited)
|
||||||||||
|
LIABILITIES AND SHAREHOLDERS' EQUITY
|
|||||||||||
|
CURRENT LIABILITIES:
|
|||||||||||
|
Current maturities of operating lease liabilities
|
$
|
379
|
$
|
262
|
|||||||
|
Trade payables
|
7,147
|
11,159
|
|||||||||
|
Other current liabilities
|
13,917
|
5,001
|
|||||||||
|
Loans and credit from bank institution and others
|
15,417
|
15,145
|
|||||||||
|
Convertible debentures
|
3D
|
|
597
|
1,968
|
|||||||
|
Derivative warrants liabilities and prefunded warrants
|
3C, 4
|
1,002
|
1,383
|
||||||||
|
38,459
|
34,918
|
||||||||||
|
NON-CURRENT LIABILITIES:
|
|||||||||||
|
Operating lease liabilities
|
92
|
171
|
|||||||||
|
Loans and credit from bank institution and others
|
1,078
|
466
|
|||||||||
|
Deferred tax liabilities
|
400
|
487
|
|||||||||
|
1,570
|
1,124
|
||||||||||
|
Total liabilities
|
40,029
|
36,042
|
|||||||||
|
EQUITY ATTRIBUTABLE TO SHAREHOLDERS OF THE COMPANY:
|
5
|
||||||||||
|
Share capital and premium
|
269,574
|
265,000
|
|||||||||
|
Capital reserve from share-based payment transactions
|
475
|
150
|
|||||||||
|
Amount received on account of financial instruments and other
|
3,112
|
297
|
|||||||||
|
Capital reserve from translation differences of foreign operations
|
(3,783
|
)
|
(1,265
|
)
|
|||||||
|
Capital reserve from transaction with non-controlling interests
|
(2,872
|
)
|
-
|
||||||||
|
Capital reserve from transaction with controlling shareholder
|
33
|
-
|
|||||||||
|
Accumulated deficit
|
(262,576
|
)
|
(258,939
|
)
|
|||||||
|
Total equity attributable to shareholders of the Company
|
3,963
|
5,243
|
|||||||||
|
Non-controlling interests
|
340
|
(2,097
|
)
|
||||||||
|
Total equity
|
4,303
|
3,146
|
|||||||||
|
Total liabilities and equity
|
$
|
44,332
|
$
|
39,188
|
|||||||
|
Nine months ended
September 30,
|
Three months ended
September 30,
|
||||||||||||||||||
|
Note
|
2025
|
2024
|
2025
|
2024
|
|||||||||||||||
|
Revenue
|
$
|
39,047
|
$
|
40,696
|
$
|
13,851
|
$
|
13,883
|
|||||||||||
|
Cost of revenue
|
29,443
|
34,925
|
11,120
|
10,735
|
|||||||||||||||
|
Gross profit
|
9,604
|
5,771
|
2,731
|
3,148
|
|||||||||||||||
|
Selling and marketing expenses
|
3,935
|
5,279
|
1,373
|
1,506
|
|||||||||||||||
|
General and administrative expenses
|
6,924
|
6,846
|
2,433
|
2,351
|
|||||||||||||||
|
Share-based compensation
|
14
|
364
|
2
|
244
|
|||||||||||||||
|
Other expenses
|
3I
|
|
3,076
|
2,734
|
3,076
|
-
|
|||||||||||||
|
Total operating expenses
|
13,949
|
15,223
|
6,884
|
4,101
|
|||||||||||||||
|
Operating loss
|
(4,345
|
)
|
(9,452
|
)
|
(4,153
|
)
|
(953
|
)
|
|||||||||||
|
Finance income
|
3,181
|
495
|
1,111
|
1
|
|||||||||||||||
|
Finance expenses
|
(2,634
|
)
|
(2,577
|
)
|
(682
|
)
|
(156
|
)
|
|||||||||||
|
Finance income (expenses), net
|
547
|
(2,082
|
)
|
429
|
(155
|
)
|
|||||||||||||
|
Loss before tax benefit
|
(3,798
|
)
|
(11,534
|
)
|
(3,724
|
)
|
(1,108
|
)
|
|||||||||||
|
Taxes on income (tax benefit)
|
86
|
(976
|
)
|
141
|
(26
|
)
|
|||||||||||||
|
Net loss
|
$
|
(3,884
|
)
|
$
|
(10,558
|
)
|
$
|
(3,865
|
)
|
$
|
(1,082
|
)
|
|||||||
|
Nine months ended
September 30,
|
Three months ended
September 30,
|
||||||||||||||||||
|
Note
|
2025
|
2024 (*)
|
2025
|
2024 (*)
|
|||||||||||||||
|
Other comprehensive income that will not be reclassified to profit or loss in subsequent periods:
|
|||||||||||||||||||
|
Remeasurement gain on defined benefit plan
|
48
|
1,633
|
-
|
49
|
|||||||||||||||
|
Other comprehensive income (loss) that will be reclassified to profit or loss in subsequent periods:
|
|||||||||||||||||||
|
Adjustments arising from translating financial statements of foreign operations
|
|
(2,440
|
)
|
(508
|
)
|
(961
|
)
|
(482
|
)
|
||||||||||
|
|
|||||||||||||||||||
|
Total other comprehensive income (loss)
|
|
(2,392
|
)
|
1,125
|
(961
|
)
|
(433
|
)
|
|||||||||||
|
|
|||||||||||||||||||
|
Total comprehensive loss
|
$
|
(6,276
|
)
|
$
|
(9,433
|
)
|
$
|
(4,826
|
)
|
$
|
(1,515
|
)
|
|||||||
|
Net income (loss) attributable to:
|
|||||||||||||||||||
|
Shareholders of the Company
|
$
|
(3,685
|
)
|
$
|
(9,574
|
)
|
$
|
(3,651
|
)
|
$
|
(922
|
)
|
|||||||
|
Non-controlling interests
|
(199
|
)
|
(984
|
)
|
(214
|
)
|
(160
|
)
|
|||||||||||
|
$
|
(3,884
|
)
|
$
|
(10,558
|
)
|
$
|
(3,865
|
)
|
$
|
(1,082
|
)
|
||||||||
|
Total comprehensive income (loss) attributable to:
|
|||||||||||||||||||
|
Shareholders of the Company
|
$
|
(6,155
|
)
|
$
|
(8,458
|
)
|
$
|
(4,627
|
)
|
$
|
(1,357
|
)
|
|||||||
|
Non-controlling interests
|
(121
|
)
|
(975
|
)
|
(199
|
)
|
(158
|
)
|
|||||||||||
|
$
|
(6,276
|
)
|
$
|
(9,433
|
)
|
$
|
(4,826
|
)
|
$
|
(1,515
|
)
|
||||||||
|
Net loss per share attributable to shareholders of the Company:
|
6
|
||||||||||||||||||
|
Basic net loss per share (in CAD)
|
$
|
(0.98
|
)
|
$
|
(4.29
|
)
|
$
|
(0.75
|
)
|
$
|
(0.41
|
)
|
|||||||
|
Diluted net loss per share (in CAD)
|
$
|
(1.00
|
)
|
$
|
(4.29
|
)
|
$
|
(0.75
|
)
|
$
|
(0.41
|
)
|
|||||||
| (*) |
Loss per share includes the effect of Reverse Share Split (see also Note 5A below).
|
|
Share capital and premium
|
Capital reserve from share-based payment transactions
|
Amount received on account of financial instruments and other
|
Capital reserve from translation difference of foreign operations
|
Capital reserve from transaction with non-controlling interests
|
Capital reserve from transaction with controlling shareholder
|
Accumulated deficit
|
Total
|
Non-controlling interests
|
Total
equity
|
|||||||||||||||||||||||||||||||
|
Balance as of January 1, 2025
|
$
|
265,000
|
$
|
150
|
$
|
297
|
$
|
(1,265
|
)
|
$
|
-
|
$
|
-
|
$
|
(258,939
|
)
|
$
|
5,243
|
$
|
(2,097
|
)
|
$
|
3,146
|
|||||||||||||||||
|
Net loss
|
-
|
-
|
-
|
-
|
-
|
-
|
(3,685
|
)
|
(3,685
|
)
|
(199
|
)
|
(3,884
|
)
|
||||||||||||||||||||||||||
|
Total other comprehensive income (loss)
|
-
|
-
|
-
|
(2,518
|
)
|
-
|
-
|
48
|
(2,470
|
)
|
78
|
(2,392
|
)
|
|||||||||||||||||||||||||||
|
Total comprehensive income (loss)
|
-
|
-
|
-
|
(2,518
|
)
|
-
|
-
|
(3,637
|
)
|
(6,155
|
)
|
(121
|
)
|
(6,276
|
)
|
|||||||||||||||||||||||||
|
Recognition of capital contribution from a controlling shareholder (Note 3B6)
|
-
|
-
|
-
|
-
|
-
|
33
|
-
|
33
|
-
|
33
|
||||||||||||||||||||||||||||||
|
Common shares issued upon exercise of pre-funded warrants (Note 3C)
|
372
|
-
|
-
|
-
|
-
|
-
|
-
|
372
|
-
|
372
|
||||||||||||||||||||||||||||||
|
Expiration of conversion feature related to convertible debentures (Note 3D)
|
297
|
-
|
(297
|
)
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|||||||||||||||||||||||||||||
|
Recognition of conversion feature related to convertible debentures (Note 3D)
|
-
|
-
|
364
|
-
|
-
|
-
|
-
|
364
|
-
|
364
|
||||||||||||||||||||||||||||||
|
Common shares issued upon partial conversion of convertible debentures (Note 3D)
|
1,651
|
-
|
(256
|
)
|
-
|
-
|
-
|
-
|
1,395
|
-
|
1,395
|
|||||||||||||||||||||||||||||
|
Common shares issued as consideration upon acquisition on non-controlling interest (Note 3E)
|
314
|
-
|
-
|
-
|
(2,872
|
)
|
-
|
-
|
(2,558
|
)
|
2,558
|
-
|
||||||||||||||||||||||||||||
|
Common shares issued upon debt settlement (Note 3F)
|
190
|
-
|
-
|
-
|
-
|
-
|
-
|
190
|
-
|
190
|
||||||||||||||||||||||||||||||
|
Net proceeds received upon completion of private placement transaction (Note 3G)
|
1,750
|
311
|
3,004
|
-
|
-
|
-
|
-
|
5,065
|
-
|
5,065
|
||||||||||||||||||||||||||||||
|
Share-based compensation
|
-
|
14
|
-
|
-
|
-
|
-
|
-
|
14
|
-
|
14
|
||||||||||||||||||||||||||||||
|
Balance as of September 30, 2025
|
$
|
269,574
|
$
|
475
|
$
|
3,112
|
$
|
(3,783
|
)
|
$
|
(2,872
|
)
|
$
|
33
|
$
|
(262,576
|
)
|
$
|
3,963
|
$
|
340
|
$
|
4,303
|
|||||||||||||||||
|
Share
Capital and premium
|
Capital reserve from share-based payment transactions
|
Amount received on account of financial instruments and other
|
Translation reserve
|
Accumulated deficit
|
Total
|
Non-controlling interests
|
Total
equity
|
|||||||||||||||||||||||||
|
Balance as of January 1, 2024
|
$
|
253,882
|
$
|
9,637
|
$
|
-
|
$
|
95
|
$
|
(249,145
|
)
|
$
|
14,469
|
$
|
(769
|
)
|
$
|
13,700
|
||||||||||||||
|
Net loss
|
-
|
-
|
-
|
-
|
(9,574
|
)
|
(9,574
|
)
|
(984
|
)
|
(10,558
|
)
|
||||||||||||||||||||
|
Total other comprehensive income
|
-
|
-
|
-
|
1,049
|
67
|
1,116
|
9
|
1,125
|
||||||||||||||||||||||||
|
Total comprehensive loss
|
-
|
-
|
-
|
1,049
|
(9,507
|
)
|
(8,458
|
)
|
(975
|
)
|
(9,433
|
)
|
||||||||||||||||||||
|
Other comprehensive loss classification
|
-
|
-
|
-
|
-
|
(748
|
)
|
(748
|
)
|
-
|
(748
|
)
|
|||||||||||||||||||||
|
Recognition of conversion feature related to convertible debentures
|
-
|
-
|
327
|
-
|
-
|
327
|
-
|
327
|
||||||||||||||||||||||||
|
Share-based compensation
|
-
|
364
|
-
|
-
|
-
|
364
|
-
|
364
|
||||||||||||||||||||||||
|
Forfeited options
|
2,803
|
(2,803
|
)
|
-
|
-
|
-
|
-
|
-
|
-
|
|||||||||||||||||||||||
|
Balance as of September 30, 2024
|
$
|
256,685
|
$
|
7,198
|
$
|
327
|
$
|
1,144
|
$
|
(259,400
|
)
|
$
|
5,954
|
$
|
(1,744
|
)
|
$
|
4,210
|
||||||||||||||
|
Nine months ended
September 30,
|
||||||||
|
2025
|
2024
|
|||||||
|
Cash flow from operating activities:
|
||||||||
|
Net loss
|
$
|
(3,884
|
)
|
$
|
(10,558
|
)
|
||
|
Adjustments for non-cash items:
|
||||||||
|
Revaluation of financial instruments
|
(9
|
)
|
(24
|
)
|
||||
|
Discount expenses in respect of convertible debentures
|
178
|
197
|
||||||
|
Depreciation of property, plant and equipment
|
212
|
332
|
||||||
|
Amortization of intangible assets
|
991
|
1,036
|
||||||
|
Depreciation of right-of-use assets
|
232
|
274
|
||||||
|
Impairment of intangible assets and goodwill (Note 3I)
|
3,076
|
-
|
||||||
|
Impairment of property, plant and equipment
|
-
|
10
|
||||||
|
Loss from deconsolidation of subsidiary
|
-
|
2,734
|
||||||
|
Recognition of extension fee related to debentures (Note 3D)
|
209
|
-
|
||||||
|
Finance expenses, net
|
(716
|
)
|
2,268
|
|||||
|
Deferred tax liability, net
|
(111
|
)
|
(138
|
)
|
||||
|
Share-based payments
|
14
|
364
|
||||||
|
Changes in employe benefit liabilities, net
|
-
|
(71
|
)
|
|||||
|
Discount expenses in respect of loans and credit received
|
141
|
-
|
||||||
|
4,217
|
6,982
|
|||||||
|
Changes in working capital:
|
||||||||
|
Decrease (increase) in trade receivables
|
3,306
|
(8,184
|
)
|
|||||
|
Increase in other current assets
|
(2,494
|
)
|
(2,775
|
)
|
||||
|
Decrease (increase) in inventory
|
(6,222
|
)
|
4,864
|
|||||
|
Increase (decrease) in trade payables
|
(3,896
|
)
|
10,595
|
|||||
|
Increase in other current liabilities
|
9,442
|
2,420
|
||||||
|
136
|
6,920
|
|||||||
|
Taxes (paid) received
|
22
|
(222
|
)
|
|||||
|
Net cash provided by operating activities
|
491
|
3,122
|
||||||
|
Cash flows from investing activities:
|
||||||||
|
Purchase of property, plant and equipment
|
(8
|
)
|
(126
|
)
|
||||
|
Deconsolidation of subsidiary
|
-
|
(346
|
)
|
|||||
|
Change in restricted cash
|
(1,070
|
)
|
-
|
|||||
|
Net cash used in investing activities
|
$
|
(1,078
|
)
|
$
|
(472
|
)
|
||
|
Nine months ended
September 30,
|
||||||||
|
2025
|
2024
|
|||||||
|
Cash flow from financing activities:
|
||||||||
|
Net proceeds received upon completion of private placement transaction (Note 3G)
|
5,064
|
-
|
||||||
|
Repayment of lease liabilities
|
(230
|
)
|
(265
|
)
|
||||
|
Payment of interest on lease liabilities
|
(26
|
)
|
(44
|
)
|
||||
|
Proceeds from loans and credit received
|
4,634
|
1,803
|
||||||
|
Repayment of loans and credit
|
(2,573
|
)
|
(4,427
|
)
|
||||
|
Interest paid
|
(1,954
|
)
|
(1,572
|
)
|
||||
|
Proceeds from (repayment of) discounted checks
|
(1,647
|
)
|
4,483
|
|||||
|
Net cash provided by (used in) financing activities
|
3,268
|
(22
|
)
|
|||||
|
Effect of foreign exchange on cash
|
(2,362
|
)
|
(2,483
|
)
|
||||
|
Change in cash
|
319
|
145
|
||||||
|
Cash at the beginning of the period
|
863
|
1,813
|
||||||
|
Cash at end of the period
|
$
|
1,182
|
$
|
1,958
|
||||
|
Supplemental disclosure of non-cash activities:
|
||||||||
|
Right-of-use assets recognized with corresponding lease liabilities
|
$
|
272
|
$
|
40
|
||||
|
Issuance of convertible debentures in exchange for loans (principal and interest) received (Note 3C)
|
$
|
-
|
$
|
2,092
|
||||
|
Common shares issued upon exercise of pre-funded warrant (Note 3C)
|
$
|
372
|
$
|
-
|
||||
|
Common shares issued upon partial conversion of convertible debentures (Note 3D)
|
$
|
1,395
|
$
|
-
|
||||
|
Common shares issued as debt settlement (Note 3F)
|
$
|
190
|
$
|
-
|
||||
| NOTE 1 - |
GENERAL
|
| A. |
Corporate information
|
| B. |
Definitions
|
|
The Company, or IMCC
|
-
|
IM Cannabis Corp.
|
|
The Group
|
-
|
IM Cannabis Corp., its Subsidiaries
|
|
Subsidiaries
|
-
|
Companies that are controlled by the Company (as defined in IFRS 10) and whose accounts are consolidated with those of the Company
|
|
CAD or $
|
-
|
Canadian Dollar
|
|
US$
|
-
|
United States dollar
|
|
NIS
|
-
|
New Israeli Shekel
|
| NOTE 1 - |
GENERAL (Cont.)
|
| C. |
Liquidity and capital resources and going concern
|
| D. |
Impact of continued interest rate on the Group's business activity
|
| NOTE 1 - |
GENERAL (Cont.)
|
| E. |
Impact of potential Germany's legalization of cannabis
|
| F. |
Impact of the security situation on the Group's business activity
|
| G. |
Approval of consolidated financial statements
|
| NOTE 2 - |
MATERIAL ACCOUNTING POLICIES
|
| A. |
Basis of presentation
|
| B. |
Use of estimates in the preparation of financial statements
|
| C. |
Disclosure of new standards amendments and interpretations to existing standards that are effective and relevant to the Group's business activity
|
| NOTE 2 - |
MATERIAL ACCOUNTING POLICIES (Cont.)
|
| D. |
Disclosure of new standards in the period prior to their adoption
|
|
■
|
IFRS 18 changes the structure of the profit or loss report and includes three new defined categories: operating, investment and financing and adds two new interim summaries: operating profit and profit before financing and income
taxes.
|
|
■
|
IFRS 18 includes guidelines for providing disclosure on performance indicators defined by management (Management-defined performance measures).
|
|
■
|
IFRS 18 provides guidelines regarding the aggregation and disaggregation of the information in the financial statements in relation to the question of whether information should be included in the main reports or in
explanations and disclosures regarding items defined as "other".
|
|
■
|
IFRS 18 includes amendments to other standards, including limited amendments to International Accounting Standard 7, Statement of Cash Flows.
|
| NOTE 3 - |
SIGNIFICANT EVENTS DURING THE REPORTING PERIOD
|
| A. |
Telecana Agreements
|
| B. |
Credit facilities
|
|
|
1. |
On January 16, 2025, IMC Holdings entered into a second amendment to an agreement with a non-financial institution for the extension of maturity date of a loan principal amounted NIS 1,800 thousand (approximately $729) until May 16,
2025. In addition, IMC Holdings paid an additional fee of NIS 150 thousand (approximately $61).
|
|
|
2. |
On January 30, 2025, Yarok Pharm entered into an amendments to an agreement with a non-financial institution under which loan principal of NIS 1,000 thousand (approximately $393) was paid and the maturity date of the remaining loan
principal of NIS 2,000 thousand (approximately $844) was extended until June 30, 2026.
|
|
|
3. |
On June 12, 2025, IMC Holdings entered into a fifth amendment to an agreement with a non-financial institution for the extension of maturity date of a loan received amounted to NIS 4,500 thousand (approximately $1,822) until September
30, 2025. Currently, the parties are discussing to amend the commercial terms.
|
|
|
4. |
On March 20, 2025, the Company and commercial bank signed an agreement under which a short-term loan of NIS 5 million (approximately $1,950) received from the bank was refinanced by the way that (i) an outstanding principal loan of NIS
4 million (approximately $1,619) was extended as a loan with 5-month grace period, after which repayment will be made in 31 monthly installments commencing September 21, 2025. The principal loan will bear an annual interest rate of P+2.9%
to be paid monthly commencing April 20, 2025 and (ii) remaining amount of NIS 1 million (approximately $405) was extended as a credit line from March 19, 2025 to March 12, 2026.
|
| NOTE 3 - |
SIGNIFICANT EVENTS DURING THE REPORTING PERIOD (Cont.)
|
|
|
B. |
Credit facilities (Cont.)
|
|
|
5. |
On April 29, 2025, IMC Holdings entered into a loan agreement with a non-financial institution in the amount of NIS 1,000 thousand (approximately $375) which bears interest at an annual rate of 17% and shall be matured 12 months from
the signing date of the loan agreement.
|
|
|
6. |
During the period of nine months ended September 30, 2025, IMC Holdings entered in a loan agreement with the Company's then chairman of the Board, Chief Executive Officer and main shareholder (the “Main Shareholder”), in the amount of
NIS 1,450 thousand (approximately $612) which bear fixed annual interest at the rate prescribed by the Income Tax Regulations for determining the interest rate under Section 3(i) of the Income Tax Ordinance and shall be repaid up to April
30, 2026.
|
|
|
7. |
On May 25, 2025, IMC Holdings entered into a loan agreement with a non-financial institution in the amount of NIS 350 thousand (approximately $131) which bears interest at an annual rate of 17% and shall be matured 12 months from the
signing date of the loan agreement.
|
|
|
8. |
On July 6, 2025, the Company entered into a loan agreement with a non-financial institution in the amount of US$1,000 thousand (approximately $1,364) which bears interest at an annual rate of 8% and shall be matured by June 30, 2026.
In the event of non-repayment by June 30, 2026, a default interest at a rate of 15% per annum on the unpaid balance will apply. The Loan is secured by a pledge over the issued and outstanding shares of IMC Holdings with the pledged shares
held directly by the Company. The pledge was registered on July 7, 2025. Pursuant to the Loan Agreement, the Company will not issue new shares or commit to issue new shares, except for under certain circumstances as defined under the loan
agreement.
|
|
|
9. |
On July 16, 2025, Rosen entered into a loan agreement with a non-financial institution in the amount of NIS 500 thousand (approximately $202) which bears interest at an annual rate of 17% and shall be matured 12 months from the signing
date of the loan agreement. Through the approval date of these interim condensed consolidated financial statements, the principal amount and the accrued interest were fully paid.
|
| NOTE 3 - |
SIGNIFICANT EVENTS DURING THE REPORTING PERIOD (Cont.)
|
| C. |
Exercise of Pre-Funded Warrants
|
|
|
D. |
Closing of Secured Debenture Offering
|
| NOTE 3 - |
SIGNIFICANT EVENTS DURING THE REPORTING PERIOD (Cont.)
|
|
|
D. |
Closing of Secured Debenture Offering (Cont.)
|
|
May 26, 2025
|
||||
|
Debentures (host instrument) (*)
|
$
|
571
|
||
|
Embedded conversion feature
|
107
|
|||
|
Total fair value of the convertible debentures
|
$
|
678
|
||
|
|
(*) |
At the initial date, the Debentures' fair value was measured by management using the assistance of an external appraiser taking into account a debt discount rate of 18.79%.
|
|
Debentures
|
||||
|
Balance at January 1, 2025
|
$
|
1,968
|
||
|
Amortization of discount expenses
|
178
|
|||
|
Recognition of extension fee
|
209
|
|||
|
Recognition of discount equal to embedded conversion feature
|
(363
|
)
|
||
|
Partial conversion into common shares (*)
|
(1,395
|
)
|
||
|
Balance at September 30, 2025
|
$
|
597
|
||
|
|
(*) |
During the period commencing May 26, 2025 through September 30, 2025, total debentures at carrying amount of approximately $1,395 have been converted into 625,461 common shares of the Company.
|
|
|
E. |
Closing of Focus Transaction
|
| NOTE 3 - |
SIGNIFICANT EVENTS DURING THE REPORTING PERIOD (Cont.)
|
|
|
F. |
Debt Settlement
|
|
|
G. |
Private placement offering
|
| NOTE 3 - |
SIGNIFICANT EVENTS DURING THE REPORTING PERIOD (Cont.)
|
|
|
G. |
Private placement offering (Cont.)
|
|
|
H. |
Amendment to Common Share purchase warrants
|
|
|
I. |
Intangible assets and goodwill impairment
|
| NOTE 4 - |
FINANCIAL INSTRUMENTS
|
|
Financial Instruments Measured at Fair Value
|
|
Fair Value Method
|
|
Liability for warrants and pre-funded warrants (*)
Investment in Xinteza (**)
|
|
Black & Scholes model (Level 3 category)
Market comparable (Level 3 category)
|
|
|
(*) |
Finance income from revaluation of warrants and prefunded warrants measured at fair value for the period of nine months ended September 30, 2025 and 2024, amounted to $9 and $24, respectively.
|
|
|
(**) |
No quantitative or qualitative indicators have been identified during the period of nine months ended September 30, 2025, indicating a significant change in fair value of Investment in Xinteza from December 31, 2024.
|
|
September 30, 2025
|
||||||||||||
|
Series 2024
|
Series 2023
|
Series 2021
|
||||||||||
|
Expected volatility
|
66.19
|
%
|
59.32
|
%
|
66.1
|
%
|
||||||
|
Share price (Canadian Dollar)
|
2.67
|
2.67
|
2.67
|
|||||||||
|
Expected life (in years)
|
4.83
|
0.37
|
0.616
|
|||||||||
|
Risk-free interest rate
|
2.67
|
%
|
3.98
|
%
|
3.83
|
%
|
||||||
|
Expected dividend yield
|
0
|
%
|
0
|
%
|
0
|
%
|
||||||
|
Per Warrant (Canadian Dollar)
|
$
|
1.35
|
$
|
-
|
$
|
-
|
||||||
|
Total Warrants (Canadian Dollar in thousands)
|
$
|
1,002
|
$
|
-
|
$
|
-
|
||||||
| NOTE 5 - |
EQUITY
|
|
|
A. |
Composition of share capital:
|
|
September 30, 2025
|
December 31, 2024
|
|||||||||||||||
|
Authorized
|
Issued and outstanding
|
Authorized
|
Issued and outstanding
|
|||||||||||||
|
Unaudited
|
Audited
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
Common shares without par value
|
Unlimited
|
5,246,812
|
Unlimited
|
3,085,452
|
||||||||||||
|
|
B. |
Changes in issued and outstanding share capital:
|
|
Nine months period ended
September 30, 2025
|
||||
|
Unaudited
|
||||
|
Balance as of January 1, 2025
|
3,085,452
|
|||
|
Common shares issued upon pre-funded warrants exercised (Note 3C)
|
152,701
|
|||
|
Common shares issued upon debentures converted (Note 3D)
|
625,461
|
|||
|
Common shares issued as consideration upon acquisition of non-controlling interest (Note 3E)
|
128,818
|
|||
|
Common shares issued upon debt settlement (Note 3F)
|
52,380
|
|||
|
Common shares issued through private placement offering (Note 3G)
|
1,202,000
|
|||
|
Balance as of September 30, 2025
|
5,246,812
|
|||
| NOTE 6 - |
NET LOSS PER SHARE
|
|
Nine months ended
September 30,
|
Three months ended
September 30,
|
|||||||||||||||
|
2025
|
2024 (
|
*)
|
2025
|
2024 (
|
*)
|
|||||||||||
|
(Unaudited)
|
||||||||||||||||
|
Numerator:
|
||||||||||||||||
|
Net basic loss attributable to shareholders of the Company
|
$
|
(3,685
|
)
|
$
|
(9,574
|
)
|
$
|
(3,651
|
)
|
$
|
(922
|
)
|
||||
|
Change in fair value of derivative pre-funded warrant liability
|
$
|
(124
|
)
|
$
|
-
|
$
|
-
|
$
|
-
|
|||||||
|
Net diluted loss
|
$
|
(3,809
|
)
|
$
|
(9,574
|
)
|
$
|
(3,651
|
)
|
$
|
(922
|
)
|
||||
|
Denominator:
|
||||||||||||||||
|
Common shares used in computing basic net loss per share
|
3,742
|
2,232
|
4,864
|
2,232
|
||||||||||||
|
Common shares to be issued upon exercise of derivative pre-funded warrant liability
|
81
|
-
|
-
|
-
|
||||||||||||
|
Common shares used in computing diluted net loss per share
|
3,823
|
2,232
|
4,864
|
2,232
|
||||||||||||
|
Basic net loss per common share
|
$
|
(0.98
|
)
|
$
|
(4.29
|
)
|
$
|
(0.75
|
)
|
$
|
(0.41
|
)
|
||||
|
Diluted net loss per common share
|
$
|
(1.00
|
)
|
$
|
(4.29
|
)
|
$
|
(0.75
|
)
|
$
|
(0.41
|
)
|
||||
|
|
(*) |
Include the effect of Reverse Share Split (see also Note 5A above).
|
| NOTE 7 - |
OPERATING SEGMENTS
|
|
Israel
|
Germany
|
Adjustments
|
Total
|
|||||||||||||
|
Revenue
|
$
|
15,772
|
$
|
23,275
|
$
|
-
|
$
|
39,047
|
||||||||
|
Segment loss
|
$
|
(1,392
|
)
|
$
|
(677
|
)
|
$
|
-
|
$
|
(2,069
|
)
|
|||||
|
Unallocated corporate expenses
|
$
|
(2,276
|
)
|
$
|
(2,276
|
)
|
||||||||||
|
Total operating loss
|
$
|
(4,345
|
)
|
|||||||||||||
|
Depreciation and amortization
|
$
|
1,314
|
$
|
121
|
$
|
-
|
$
|
1,435
|
||||||||
|
Israel
|
Germany
|
Adjustments
|
Total
|
|||||||||||||
|
Revenue
|
$
|
30,219
|
$
|
10,477
|
$
|
-
|
$
|
40,696
|
||||||||
|
Segment profit (loss)
|
$
|
(8,261
|
)
|
$
|
993
|
$
|
-
|
$
|
(7,268
|
)
|
||||||
|
Unallocated corporate expenses
|
$
|
(2,184
|
)
|
$
|
(2,184
|
)
|
||||||||||
|
Total operating loss
|
$
|
(9,452
|
)
|
|||||||||||||
|
Depreciation and amortization
|
$
|
1,520
|
$
|
122
|
$
|
-
|
$
|
1,642
|
||||||||
| NOTE 8 - |
SUBSEQUENT EVENTS
|
|
|
A. |
Execution of non-binding term sheet
|
|
|
B. |
Credit facilities
|
|
|
1. |
On October 5, 2025, IMC Holdings entered into a loan agreement with the Company's then chairman of the Board, Chief Executive Officer and main shareholder, in the amount of NIS 300 thousand (approximately $127) which bears fixed annual
interest at the rate prescribed by the Income Tax Regulations for determining the interest rate under Section 3(i) of the Income Tax Ordinance and shall be repaid by January 5, 2026.
|
|
|
2. |
On October 5, 2025, IMC Holdings entered into a loan agreement with a non-financial institution in the amount of NIS 500 thousand (approximately $211) which bears an interest at an annual rate of 17% and shall be matured on November
13, 2025.
|
|
|
C. |
Exercise of Pre-Funded Warrants
|

| 4 | |
| 7 | |
| 7 | |
| 9 | |
| 11 | |
| 11 | |
| 12 | |
| 17 | |
| 55 | |
| 66 | |
| 73 |
|
Legal Entity
|
Jurisdiction
|
Relationship with the Company
|
|
I.M.C. Holdings Ltd. (“IMC Holdings”)
|
Israel
|
Wholly-owned subsidiary
|
|
I.M.C. Pharma Ltd. (“IMC Pharma”)
|
Israel
|
Wholly-owned subsidiary of IMC Holdings
|
|
Focus Medical Herbs Ltd. (“Focus")(1)
|
Israel
|
Wholly-owned subsidiary of IMC Holdings
|
|
R.A. Yarok Pharm Ltd. (“Pharm Yarok”)
|
Israel
|
Wholly-owned subsidiary of IMC Holdings
|
|
Rosen High Way Ltd. (“Rosen High Way”)
|
Israel
|
Wholly-owned subsidiary of IMC Holdings
|
|
Rivoly Trading and Marketing Ltd. d/b/a Vironna Pharm (“Vironna”)”
|
Israel
|
Subsidiary of IMC Holdings
|
|
Adjupharm GmbH (“Adjupharm”)
|
Germany
|
Wholly-owned subsidiary of IMC Holdings
|
|
Trichome Financial Corp. (“Trichome") (2)
|
Canada
|
Former wholly-owned subsidiary
|
|
Xinteza API Ltd (“Xinteza”)
|
Israel
|
Subsidiary of IMC Holdings
|
|
Shiran Societe Anonyme (“Greece”)
|
Greece
|
Subsidiary of IMC Holdings
|
|
IM Cannabis Holding NL B.V Netherlands (“IMC Holdings NL”)
|
Netherlands
|
Wholly-owned subsidiary of IMC Holdings
|
|
|
(1) |
Effective February 26, 2024, IMC Holdings exercised its option to acquire a 74% ownership stake in Focus, and effective May 26, 2025, IMC Holdings acquired the remaining 26% from Ewave Group Ltd.
|
|
|
(2) |
Discontinued operations. Please see note 21 in the 2024 Annual Financial Statements.
|
| • |
Develop and execute a long-term growth plan in Germany, based on the strong sourcing infrastructure in Israel, which is powered by advanced product knowledge and regulatory expertise.
|
| • |
Optimize inventory to meet demand while managing INCB/BfArM import-estimate constraints and aligning products to Ph. Eur. 11.5 specifications. Diversify EU-GMP suppliers (Israel and other countries) to support availability.
|
| • |
Properly position brands with respect to target-market, price, potency and quality, such as our IMC brand in Israel and Germany.
|
| • |
Strong focus on efficiencies and synergies with domestic expertise in Israel and Germany.
|
| • |
High-quality, reliable supply to our customers and patients, leading to recurring sales.
|
| • |
Ongoing introduction of new Stock Keeping Units (“SKU”) to keep consumers and patients engaged.
|
| • |
Anticipate potential limits on telemedicine and mail-order by broadening local-pharmacy coverage, using pharmacy couriers where allowed, and supporting in-person prescribing with key physicians.
|
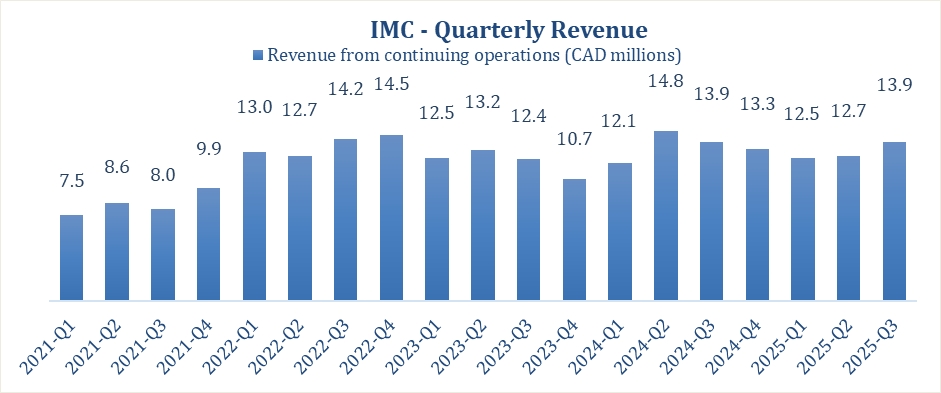


|
For the nine months
ended September 30,
|
For the three months
ended September 30,
|
For the Year ended
December 31, |
||||||||||||||||||
|
2025
|
2024
|
2025
|
2024
|
2024
|
||||||||||||||||
|
Net Revenues
|
$
|
39,047
|
$
|
40,696
|
$
|
13,851
|
$
|
13,883
|
$
|
54,031
|
||||||||||
|
Gross profit
|
$
|
9,604
|
$
|
5,771
|
$
|
2,731
|
$
|
3,148
|
$
|
8,451
|
||||||||||
|
Gross margin (%)
|
25
|
%
|
14
|
%
|
20
|
%
|
23
|
%
|
16
|
%
|
||||||||||
|
Operating Loss
|
$
|
(4,345
|
)
|
$
|
(9,452
|
)
|
$
|
(4,153
|
)
|
$
|
(953
|
)
|
$
|
(10,234
|
)
|
|||||
|
Loss
|
$
|
(3,884
|
)
|
$
|
(10,558
|
)
|
$
|
(3,865
|
)
|
$
|
(1,082
|
)
|
$
|
(11,771
|
)
|
|||||
|
Loss per share attributable to equity holders of the Company –
Basic (in CAD) |
$
|
(0.98
|
)
|
$
|
(4.29
|
)
|
$
|
(0.75
|
) |
$
|
(0.41
|
) |
$
|
(4.51
|
)
|
|||||
|
Loss per share attributable to equity holders of the Company -
Diluted (in CAD) |
$
|
(1
|
)
|
$
|
(4.29
|
)
|
$
|
(0.75
|
) |
$
|
(0.41
|
)
|
$
|
(4.51
|
)
|
|||||
|
Germany Region Revenue for the three months ended
|
||||||||||||||||||||||||||||
|
2025
|
2024
|
|||||||||||||||||||||||||||
|
September 30,
|
June 30,
|
March 31,
|
December 31,
|
September 30,
|
June 30,
|
March 31,
|
||||||||||||||||||||||
|
Revenue for
the period
|
$
|
8,768
|
$
|
6,802
|
$
|
7,705
|
$
|
5,031
|
$
|
5,817
|
$
|
3,508
|
$
|
1,152
|
||||||||||||||
|
Q vs Q change%
|
29
|
%
|
-12
|
%
|
53
|
%
|
-14
|
%
|
66
|
%
|
205
|
%
|
-
|
|||||||||||||||
|
|
● |
Revenues from the Israeli operation were attributed to the sale of medical cannabis through the Company’s subsidiaries and revenues from the Israeli Pharmacies the Company owns, mostly from cannabis products.
|
|
|
● |
Revenues from the German operation were attributed to the sale of medical cannabis through Adjupharm.
|
|
Israel
|
Germany
|
Adjustments
|
Total
|
|||||||||||||||||||||||||||||
|
2025
|
2024
|
2025
|
2024
|
2025
|
2024
|
2025
|
2024
|
|||||||||||||||||||||||||
|
Revenues
|
$
|
15,772
|
$
|
30,219
|
$
|
23,275
|
$
|
10,477
|
$
|
-
|
$
|
-
|
$
|
39,047
|
$
|
40,696
|
||||||||||||||||
|
Segment income (loss)
|
$
|
(1,392
|
)
|
$
|
(8,261
|
)
|
$
|
(677
|
)
|
$
|
993
|
$
|
-
|
$
|
-
|
$
|
(2,069
|
)
|
$
|
(7,268
|
)
|
|||||||||||
|
Unallocated corporate expenses
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
(2,276
|
)
|
$
|
(2,184
|
)
|
$
|
(2,276
|
)
|
$
|
(2,184
|
)
|
||||||||||||
|
Total operating income (loss)
|
$
|
(1,392
|
)
|
$
|
(8,261
|
)
|
$
|
(677
|
)
|
$
|
993
|
$
|
(2,276
|
)
|
$
|
(2,184
|
)
|
$
|
(4,345
|
)
|
$
|
(9,452
|
)
|
|||||||||
|
Depreciation& amortization
|
$
|
1,314
|
$
|
1,520
|
$
|
121
|
$
|
122
|
$
|
-
|
$
|
-
|
$
|
1,435
|
$
|
1,642
|
||||||||||||||||
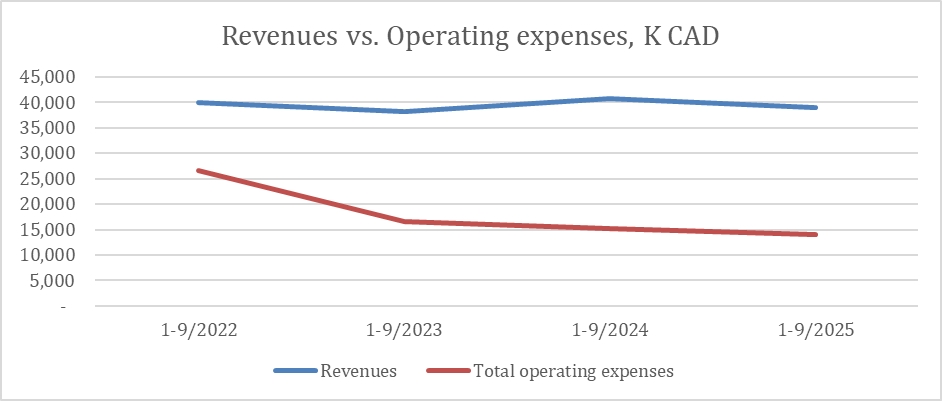
|
September 30,
|
December 31,
|
|||||||
|
2025
|
2024
|
|||||||
|
Credit from Bank institutions
|
$
|
1,033
|
$
|
2,586
|
||||
|
Credit from non-financial institutions
|
10,129
|
6,384
|
||||||
|
Check receivables
|
5,333
|
6,641
|
||||||
|
$
|
16,495
|
$
|
15,611
|
|||||
|
Less than one year
|
1 to 5 years
|
6 to 10 years
|
> 10 years
|
|||||||||||||
|
Contractual Obligations
|
$
|
15,811
|
$
|
1,175
|
-
|
-
|
||||||||||
|
Payments Due by Period
|
||||||||||||||||||||
|
Contractual Obligations
|
Total
|
Less than one year
|
1 to 3 years
|
4 to 5 years
|
After 5 years
|
|||||||||||||||
|
Debt
|
$
|
16,495
|
$
|
15,417
|
$
|
1,078
|
$
|
-
|
$
|
-
|
||||||||||
|
Finance Lease Obligations
|
$
|
491
|
$
|
394
|
$
|
97
|
$
|
-
|
$
|
-
|
||||||||||
|
Total Contractual Obligations
|
$
|
16,986
|
$
|
15,811
|
$
|
1,175
|
$
|
-
|
$
|
-
|
||||||||||
|
|
• |
$1,560 (NIS 4 million) will be extended as a loan with a five-month grace period, after which repayment will be made in 31 monthly installments commencing September 10, 2025. The principal loan will not require a personal guarantee and
will bear an interest at a rate of P+2.9% to be paid monthly, commencing April 20, 2025.
|
|
|
• |
The remaining $390 (NIS 1 million) will be extended as a credit line from March 19, 2025, to March 12, 2026.
|
|
For the nine months
ended September 30,
|
For the three months
ended September 30,
|
For the Year ended December 31,
|
||||||||||||||||||
|
Net cash provided by (used in):
|
2025
|
2024
|
2025
|
2024
|
2024
|
|||||||||||||||
|
Operating activities
|
$
|
491
|
$
|
3,122
|
$
|
(3,498
|
)
|
$
|
2,754
|
$
|
(1,077
|
)
|
||||||||
|
Investing activities
|
$
|
(1,078
|
)
|
$
|
(472
|
)
|
(1,097
|
)
|
$
|
(74
|
)
|
$
|
(470
|
)
|
||||||
|
Financing activities
|
$
|
3,268
|
$
|
(22
|
)
|
$
|
6,095
|
$
|
(665
|
)
|
$
|
3,825
|
||||||||
|
Effect of foreign exchange
|
$
|
(2,362
|
)
|
$
|
(2,483
|
)
|
$
|
(1,112
|
)
|
$
|
(757
|
)
|
$
|
(3,228
|
)
|
|||||
|
Increase (Decrease) in cash
|
$
|
319
|
$
|
145
|
$
|
388
|
$
|
1,258
|
$
|
(950
|
)
|
|||||||||
|
For the year ended
|
December 31,
2024 |
December 31,
2023
|
December 31,
2022
|
|||||||||
|
Revenues
|
$
|
54,031
|
$
|
48,804
|
$
|
54,335
|
||||||
|
Net Loss
|
$
|
(11,771
|
)
|
$
|
(10,228
|
)
|
$
|
(24,922
|
)
|
|||
|
Basic net income (Loss) per share:
|
$
|
(4.51
|
)
|
$
|
(4.45
|
)
|
$
|
(18.81
|
)
|
|||
|
Diluted net income (Loss) per share:
|
$
|
(4.51
|
)
|
$
|
(4.45
|
)
|
$
|
(22.87
|
)
|
|||
|
Total assets
|
$
|
39,188
|
$
|
48,813
|
$
|
60,676
|
||||||
|
Total non-current liabilities
|
$
|
1,124
|
$
|
2,267
|
$
|
3,060
|
||||||
|
For the three months ended
|
September 30,
2025 |
June 30,
2025 |
March 31,
2025 |
December 31,
2024 |
||||||||||||
|
Revenues
|
$
|
13,851
|
$
|
12,696
|
$
|
12,500
|
$
|
13,335
|
||||||||
|
Net Profit (loss)
|
$
|
(3,865
|
)
|
$
|
(194
|
)
|
$
|
175
|
$
|
(1,213
|
)
|
|||||
|
Basic net income (loss) per share:
|
$
|
(0.75
|
)
|
$
|
(0.09
|
)
|
$
|
0.09
|
$
|
(0.32
|
)
|
|||||
|
Diluted net income (loss) per share:
|
$
|
(0.75
|
)
|
$
|
(0.09
|
)
|
$
|
0.09
|
$
|
(0.32
|
)
|
|||||
|
For the three months ended
|
September 30,
2024 |
June 30,
2024 |
March 31,
2024 |
December 31,
2023
|
||||||||||||
|
Revenues
|
$
|
13,883
|
$
|
14,750
|
$
|
12,063
|
$
|
10,698
|
||||||||
|
Net income (loss)
|
$
|
(1,082
|
)
|
$
|
(3,456
|
)
|
$
|
(6,020
|
)
|
$
|
(3,520
|
)
|
||||
|
Basic net income (loss) per share:
|
$
|
(0.41
|
)
|
$
|
(1.36
|
)
|
$
|
(2.52
|
)
|
$
|
(1.47
|
)
|
||||
|
Diluted net income (loss) per share:
|
$
|
(0.41
|
)
|
$
|
(1.36
|
)
|
$
|
(2.52
|
)
|
$
|
(1.47
|
)
|
||||
|
For the nine months
ended September 30,
|
For the three months
ended September 30,
|
For the Year ended December 31,
|
||||||||||||||||||
|
|
2025
|
2024
|
2025
|
2024
|
2024
|
|||||||||||||||
|
Net Revenue
|
$
|
39,047
|
$
|
40,696
|
$
|
13,851
|
$
|
13,883
|
$
|
54,031
|
||||||||||
|
Cost of sales
|
$
|
(29,443
|
)
|
$
|
(34,925
|
)
|
$
|
(11,120
|
)
|
$
|
(10,735
|
)
|
$
|
(45,580
|
)
|
|||||
|
Gross profit before FV adjustments
|
$
|
9,604
|
$
|
5,771
|
$
|
2,731
|
$
|
3,148
|
$
|
8,451
|
||||||||||
|
Gross margin before FV adjustments (non-IFRS)
|
25
|
%
|
14
|
%
|
20
|
%
|
23
|
%
|
16
|
%
|
||||||||||
|
For the Nine Months ended
September 30,
|
For the Three Months ended
September 30,
|
For the Year ended December 31,
|
||||||||||||||||||
|
2025
|
2024
|
2025
|
2024
|
2024
|
||||||||||||||||
|
Operating Loss
|
$
|
(4,345
|
)
|
$
|
(9,405
|
)
|
$
|
(4,153
|
)
|
$
|
(931
|
)
|
$
|
(10,234
|
)
|
|||||
|
Depreciation & Amortization
|
$
|
1,435
|
$
|
1,642
|
$
|
501
|
$
|
451
|
$
|
2,184
|
||||||||||
|
EBITDA
|
$
|
(2,910
|
)
|
$
|
(7,763
|
)
|
$
|
(3,652
|
)
|
$
|
(480
|
)
|
$
|
(8,050
|
)
|
|||||
|
Share-based payments
|
$
|
14
|
$
|
364
|
$
|
2
|
$
|
244
|
$
|
369
|
||||||||||
|
Other non-recurring costs 1
|
$
|
3,076
|
$
|
2,734
|
$
|
3,076
|
$
|
-
|
$
|
6,612
|
||||||||||
|
Adjusted EBITDA (Non-IFRS)
|
$
|
180
|
$
|
(4,665
|
)
|
$
|
(574
|
)
|
$
|
(236
|
)
|
$
|
(1,069
|
)
|
||||||
| 1. |
Due to goodwill impairment for the nine and three months ended September 30,2025, and the revocation of the Oranim transaction dated April 16, 2024.
|
|
Less than one year
|
1 to 5 years
|
6 to 10 years
|
>10 years
|
|||||||||||||
|
Lease liabilities
|
$
|
394
|
$
|
97
|
-
|
-
|
||||||||||
|
Less than one year
|
1 to 5 years
|
6 to 10 years
|
>10 years
|
|||||||||||||
|
Lease liabilities
|
$
|
276
|
$
|
280
|
-
|
-
|
||||||||||
|
|
1. |
The contractual party of the company was not Stroakmont. The contract with Stroakmont was only concluded as a sham transaction to cover up a contract with a company named Uniclaro GmbH (“Uniclaro”).
Therefore, Stroakmont is not the real purchaser rather than Uniclaro.
|
|
|
2. |
The company allegedly placed an order with Uniclaro for a total of 4.3 million Clongene COVID-19 tests, of which Uniclaro claims to have a payment claim against the company for a partial delivery of 380,400 Clongene COVID-19 tests in
the total amount of EUR 942 thousand. Uniclaro has assigned this alleged claim against the company to Stroakmont Trading GmbH, and Stroakmont Trading GmbH has precautionary declared a set-off against the company’s claim.
|
| 1. |
Adjupharm was not sentenced. Uniclaro's lawsuit for payment of approximately EUR 1,046 thousand in exchange for delivery of 300,000 Clungene tests was dismissed.
|
| 2. |
Uniclaro is sentenced to pay Adjupharm approximately EUR 54 thousand plus interest at 5 percentage points above the German basis rate since 17.01.2023.
|
| 3. |
Uniclaro shall bear the procedural costs.
|
| • |
On April 2, 2019, IMC Holdings and Focus entered into an option agreement pursuant to which IMC Holdings acquired an option to purchase, at its sole discretion and in compliance with Israeli cannabis regulation, all the ordinary shares
held by Messrs. Shuster and Gabay held in Focus at a price equal to NIS 765.67 per ordinary share until April 2029 (the “Focus Agreement”). On November 30, 2023, IMC Holdings sent a request letter
to approve IMC Holding’s exercise of the option and on February 26, 2024, IMCA's approval was obtained. Effective February 26, 2024, IMC Holdings acquired 74% of the ordinary shares of Focus.
|
| • |
The Company is a party to Indemnification Agreement with certain directors and officers of the Company and Trichome to cover certain tax liabilities, interest and penalties arising from the Trichome Transaction. See “Risk Factors - Tax Remittance” section of the MD&A.
|
| • |
On April 17, 2024, Pharm Yarok entered into the April 2024 Loan. The April 2024 Loan is secured by the following collaterals and guarantees: (a) a first-ranking floating charge over the assets of Pharm Yarok, (b) a first-ranking fixed
charge over the holdings (23.3%) of its subsidiary, IMC Holdings, of Xinteza, (c) a personal guarantee by Mr. Oren Shuster, the Company’s Chief Executive Officer and (D) a guarantee by the Company.
|
| • |
On October 12, 2023, Oren Shuster, the CEO, loaned an amount of NIS 500 thousand (approximately $170) to IMC Holdings. The participation of the CEO constituted a “related party transaction”, as such term is defined in Multilateral
Instrument 61-101 – Protection of Minority Shareholders in Special Transactions (“MI 61-101”) and would require the Company to receive minority shareholder
approval for and obtain a formal valuation for the subject matter of, the transaction in accordance with MI 61-101, prior to the completion of such transaction. However, in completing the loan, the Company has relied on exemptions from
the formal valuation and minority shareholder approval requirements of MI 61-101, in each case on the basis that the fair market value of the CEO’s loan did not exceed 25% of the market capitalization of the Company, as determined in
accordance with MI 61-101.
|
| • |
On May 29, 2024, the Company completed the May 2024 Private Placement. Mr. Shuster had subscribed for an aggregate of approximately $237, of May 2024 Debentures. Mr. Shuster’s participation constituted a “related party transaction”
pursuant to MI 61-101.
|
| • |
On November 12, 2024, the Company completed the November 2024 Offering. Oren Shuster, the CEO, Shmulik Arbel, a director of the Company, and Rafael Gabay, an insider of the Company, each participated in the November 2024 Offering and
Mr. Shuster participated in the November 2024 Debt Settlement. The foregoing individuals’ participation in the November 2024 Offering constitutes a “related party transaction”, as such term is defined in MI 61-101 and would require the
Company to receive minority shareholder approval for and obtain a formal valuation for the subject matter of, the transaction in accordance with MI 61-101, prior to the completion of such transaction. However, in completing the November
2024 Offering, the Company relied on exemptions from the formal valuation and minority shareholder approval requirements of MI 61-101, on the basis of subsections 5.5(g) and 5.7(g) – Financial Hardship of MI 61-101, as the Company is (i)
in a situation of serious financial difficulty; (ii) the November 2024 Offering and November 2024 Debt Settlement was designed to improve the financial position of the Company as (x) the Company would be unable to repay the ADI Loan, and
(y) would have been unable to obtain Loans without Mr. Shuster personal guaranteeing them; (iii) the circumstances described in Section 5.5(f) of MI 61-101 are not applicable, and (iv) the Board and independent directors (as such term is
defined in MI 61-101) have, acting in good faith, determined that (i) and (ii) apply and the terms of the Transactions are reasonable in the circumstances of the Company.
|
| • |
On April 29, 2025, Oren Shuster, the CEO, loaned an amount of NIS 1,000 (approximately $384) to IMC Holdings. The loan shall bear fixed annual interest at the rate prescribed by the Income Tax Regulations for determining the interest
rate under Section 3(i) of the Income Tax Ordinance, from the date the loan is provided until the repayment date, and shall be paid together with applicable VAT as required by law. The participation of the Mr. Shuster constituted a
“related party transaction”, as such term is defined in MI 61-101 and would require the Company to receive minority shareholder approval for and obtain a formal valuation for the subject matter of, the transaction in accordance with MI
61-101, prior to the completion of such transaction. However, in completing the loan, the Company has relied on exemptions from the formal valuation and minority shareholder approval requirements of MI 61-101, in each case on the basis
that the fair market value of the CEO’s loan did not exceed 25% of the market capitalization of the Company, as determined in accordance with MI 61-101.
|
| • |
On May 2026, the Company has closed a non brokered private placement (the "Secured Debenture Offering") of secured convertible debentures of the Company (each, a "Debenture")
for aggregate proceeds of C$2,301,174.70. The Debentures were issued to holders of debentures that matured on May 26, 2025, subject to a 10% extension fee. The Debentures will mature on May 26, 2026, and will not incur interest except in
the event of default. The Debentures may be converted into Common Shares at a conversion price of C$2.61 per Common Share. Oren Shuster, a director, officer and Control Person of the Company, and Rafael Gabay, an insider of the Company,
(together, the "Participating Insiders") each participated in the Secured Debenture Offering. Mr. Shuster subscribed for a Debenture in the principal amount of C$260,935.40 and Mr. Gabay subscribed
for a Debenture in the principal amount of C$260,278.70. The participation of the Participating Insiders in the Secured Debenture Offering constituted a "related party transaction", as such term is defined in MI 61-101 and as such would
have required the Company to receive minority shareholder approval for, and obtain a formal valuation for the subject matter of, the transaction in accordance with MI 61-101, prior to the completion of such transactions, unless the
Company was able to rely on exemptions from the formal valuation and minority shareholder approval requirements of MI 61-101. The Participating Insiders' participation in the Secured Debenture Offering was exempt from the formal valuation
and minority shareholder approval requirements of MI 61-101 pursuant to sections 5.5(a) and 5.7(1)(a) of MI 61- 101, as neither the fair market value of the subject matter of the transactions, nor the consideration payable under the
transactions, exceeded 25% of the Company's market capitalization insofar as it involves interested parties.
|
| • |
The Company, through I.M.C. Holdings acquired from Ewave Group Ltd.'s ("Ewave") the remaining 26% interest in Focus (the "Focus Transaction") following the
approval of the Focus Transaction at the annual general and special meeting held on May 2023, 2025 (the "Meeting"). The Focus Transaction was closed effective May 26, 2025, following receipt of
disinterested shareholder approval at the Meeting. Ewave is a privately held entity jointly owned by Messrs. Shuster and Gabay, related parties to the Company. The Focus Transaction constitutes a "related party transaction", as such term
is defined in Multilateral Instrument 61-101 – Protection of Minority Shareholders in Special Transactions ("MI 61-101"), due to the involvement of Ewave, a privately-held entity jointly owned by
Messrs. Shuster and Gabay, related parties to the Company, and as such requires the Company to receive minority Shareholder approval for, and obtain a formal valuation for the subject matter of, the transaction in accordance with MI
61-101, prior to the completion of such transaction, unless the Company is able to rely on exemptions from the formal valuation and minority Shareholder approval requirements of MI 61-101. Notwithstanding the fact that the Focus
Transaction is exempt from the formal valuation and minority shareholder approval requirements of MI 61-101 pursuant to sections 5.5(a) and 5.7(1)(a) of MI 61-101, as neither the fair market value of the subject matter of the transaction,
nor the consideration payable under the transaction, exceeds 25% of the Company's market capitalization insofar as it involves interested parties, the Board commissioned an arm's length third-party to prepare a report to determine the
Focus Purchase Price and the Company is seeking disinterested shareholders' approval of the Focus Transaction at the upcoming Meeting as a means of good governance.
|
|
|
■ |
IFRS 18 changes the structure of the profit or loss report and includes three new defined categories: operating, investment and financing and adds two new interim summaries: operating profit and profit before financing and income
taxes.
|
|
|
■ |
IFRS 18 includes guidelines for providing disclosure on performance indicators defined by management (Management-defined performance measures).
|
|
|
■ |
IFRS 18 provides guidelines regarding the aggregation and disaggregation of the information in the financial statements in relation to the question of whether information should be included in the main reports or in explanations and
disclosures regarding items defined as "other".
|
|
|
■ |
IFRS 18 includes amendments to other standards, including limited amendments to International Accounting Standard 7, Statement of Cash Flows.
|
|
|
● |
maintenance of records in reasonable detail, that accurately and fairly reflect the transactions and dispositions of assets.
|
|
|
● |
reasonable assurance that transactions are recorded as necessary to permit the preparation of financial statements in accordance with applicable IFRS.
|
|
|
● |
receipts and expenditures are only being made in accordance with authorizations of management or the Board; and
|
|
|
● |
reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial instruments.
|

|
|
(a) |
the Company receiving economic benefits from Focus (and the terms of the contractual agreements between the Company and Focus cannot be changed without the approval of IMC Holdings);
|
|
|
(b) |
IMC Holdings holds 74% interest in Focus;
|
|
|
(c) |
Messrs. Shuster and Gabay each being a director of Focus (while Mr. Shuster concurrently being a CEO, director, and substantial shareholder of the Company and Mr. Gabay concurrently being a substantial shareholder of the Company); and
|
|
|
(d) |
the Company providing management and support activities to Focus through a services agreement.
|
|
|
• |
the Company’s business objectives and milestones and the anticipated timing of execution;
|
|
|
• |
the performance of the Company’s business, strategies and operations;
|
|
|
• |
the Company’s intentions to expand its business, operations and potential activities;
|
|
|
• |
the Company’s plans to expand sales channels, distribution, delivery, storage capacity, and reach to medical cannabis patients;
|
|
|
• |
the competitive conditions of the cannabis industry and the growth of medical or adult-use recreational cannabis markets in the jurisdictions in which the Company operates;
|
|
|
• |
the Company’s ability to maintain or grow market share and maintain its competitive advantages;
|
|
|
• |
statements relating to the Company’s commitment to responsible growth and compliance with the strictest regulatory environments;
|
|
|
• |
the Company’s focus on providing premium cannabis products to medical patients in the jurisdictions in which the Company conducts business and any other jurisdiction in which the Company may conduct business in the future;
|
|
|
• |
the Company’s plans to amplify its commercial and brand power to become a global high-quality cannabis player;
|
|
|
• |
the Company’s primary goal of sustainably increasing revenue in its core markets;
|
|
|
• |
the demand and momentum in the Company’s Israeli and Germany operations;
|
|
|
• |
how the Company intends to position its brands;
|
|
|
• |
the efficiencies and synergies of the Company as a global organization with domestic expertise in Israel and Germany;
|
|
|
• |
expectations that providing high-quality, reliable supply to the Company’s customers and patients will lead to recurring sales;
|
|
|
• |
expectations related to the Company’s introduction of new SKUs
|
|
|
• |
anticipated cost savings from the reorganization of the Company and the completion thereof upon the timelines disclosed herein;
|
|
|
• |
geographic diversification and brand recognition and the growth of the Company’s brands in the jurisdictions that the Company operates in or may expand to;
|
|
|
• |
expectations related to the Company’s ability to address the ongoing needs and preferences of medical cannabis patients;
|
|
|
• |
the Company’s retail presence, distribution capabilities and data-driven insights;
|
|
|
• |
the future impact of the Regulations Amendment (as defined herein) regarding the transition from licenses to prescriptions for medical treatment of cannabis in Israel;
|
|
|
• |
the Company’s continued partnerships with third party suppliers and partners and the benefits thereof;
|
|
|
• |
the Company’s ability to achieve profitability in 2025;
|
|
|
• |
the number of patients in Israel licensed by the Israeli Ministry of Health (“MOH”) to consume medical cannabis;
|
|
|
• |
expectations relating to the number of patients paying out-of-pocket for medical cannabis products in Germany;
|
|
|
• |
the anticipated decriminalization or legalization of adult-use recreational cannabis in Israel and Germany;
|
|
|
• |
expectations related to the demand and the ability of the Company to source premium and ultra-premium cannabis products exclusively and competition in this product segment;
|
|
|
• |
the anticipated impact of inflation and liquidity on the Company’s performance;
|
|
|
• |
expectations with respect to the Company’s operating budget and the assumptions related thereto;
|
|
|
• |
expectations relating to the Company as a going concern and its ability to conduct business under the ordinary course of operations;
|
|
|
• |
expectations related to the collection the payment awarded in the Judgment and the chances of the claim advancing or the potential outcome of the Test Kits Appeal (as defined herein);
|
|
|
• |
the continued listing of the Common Shares on Nasdaq;
|
|
|
• |
cannabis licensing in the jurisdictions in which the Company operates;
|
|
|
• |
the renewal and/or extension of the Company’s licenses;
|
|
|
• |
the Company’s anticipated operating cash requirements and future financing needs;
|
|
|
• |
the Company’s expectations regarding its Gross Margins, EBITDA, Adjusted EBITDA, revenue, expenses, profit margins and operations;
|
|
|
• |
the expected increase in revenue and margins in its Israeli medical cannabis market activities arising from its acquisitions;
|
|
|
• |
future opportunities for the Company in Israel, particularly in the retail and distribution segments of the cannabis market;
|
|
|
• |
future expansion and growth opportunities for the Company in Germany and Europe and the timing of such; and
|
|
|
• |
contractual obligations and commitments; and
|
|
|
• |
the Company’s ability to maintain compliance with Nasdaq’s continued listing standards, including regaining and sustaining the minimum stockholders’ equity requirements or satisfying alternative criteria;
|
|
|
• |
the potential impact of a delisting from Nasdaq on the Company’s operations, financing ability, and shareholder value;
|
|
|
• |
the Company’s plans to access the capital markets under the Shelf Registration Statement filed on Form F-3 and the use of proceeds from any such offerings;
|
|
|
• |
expectations relating to the Company’s ability to complete future private placements or loan agreements to meet liquidity needs;
|
|
|
• |
the Company’s ability to successfully implement its cost-reduction and operational efficiency plans to support its going concern assumptions;
|
|
|
• |
expectations relating to potential acceleration rights or repayment terms under outstanding loan agreements;
|
|
|
• |
the expected resolution of delays in Canadian export permits and the impact on Israeli operations and supply chain continuity;
|
|
|
• |
the anticipated benefits of the acquisition of the remaining interest in Focus Medical Herbs Ltd. and the expected synergies;
|
|
|
• |
the Company’s ability to achieve sales growth through the introduction of new cannabis strains and product lines in Israel and Germany;
|
|
|
• |
expectations related to ongoing tax obligations, debenture conversions, or settlement agreements.
|
|
|
• |
the Company has the ability to achieve its business objectives and milestones under the stated timelines;
|
|
|
• |
the Company will succeed in carrying out its business, strategies and operations;
|
|
|
• |
the Company will realize upon its intentions to expand the business, operations and potential activities of the Company;
|
|
|
• |
the Company will expand its sales channels, distribution, delivery and storage capacity, and reach to medical cannabis patients;
|
|
|
• |
the competitive conditions of the cannabis industry and the growth of medical or adult-use recreational cannabis in the jurisdictions in which the Company operates;
|
|
|
• |
the competitive conditions of the industry will be favorable to the Company, and the Company has the ability to maintain or grow its market share and maintain its competitive advantages;
|
|
|
• |
the Company will commit to responsible growth and compliance with the strictest regulatory environments;
|
|
|
• |
the Company will remain focused on providing premium cannabis products to medical patients in the jurisdictions in which the Company conducts business and any other jurisdiction in which the Company may conduct business in the future;
|
|
|
• |
the Company has the ability to amplify its commercial and brand power to become a global high-quality cannabis player;
|
|
|
• |
the Company will maintain its primary goal of sustainably increasing revenue in its core markets;
|
|
|
• |
the demand and momentum in the Company’s Israeli and Germany operations will be favorable to the Company;
|
|
|
• |
the Company will carry out its plans to position its brands as stated;
|
|
|
• |
the Company’s Company has the ability to realize upon the stated efficiencies and synergies the Company as a global organization with domestic expertise in Israel and Germany;
|
|
|
• |
providing a high-quality, reliable supply to the Company’s customers and patients will lead to recurring sales;
|
|
|
• |
the Company will introduce new SKUs;
|
|
|
• |
the Company will realize the anticipated cost savings from its reorganization;
|
|
|
• |
the Company has the ability to achieve geographic diversification and brand recognition and the growth of the Company’s brands in the jurisdictions that the Company operates in or may expand to;
|
|
|
• |
the Company’s has the ability to address the ongoing needs and preferences of medical cannabis patients;
|
|
|
• |
the Company has the ability to realize upon its retail presence, distribution capabilities and data-driven insights;
|
|
|
• |
the future impact of the Regulations Amendment will be favorable to the Company;
|
|
|
• |
the Company will maintain its partnerships with third parties, suppliers and partners;
|
|
|
• |
the Company has the ability to achieve profitability in 2025;
|
|
|
• |
the accuracy of number of patients in Israel licensed by the MOH to consume medical cannabis;
|
|
|
• |
the accuracy of the number of patients paying out-of-pocket medical cannabis products in Germany;
|
|
|
• |
the anticipated decriminalization or legalization of adult-use recreational cannabis in Israel and Germany will occur;
|
|
|
• |
the Company has the ability to source premium and ultra-premium cannabis products exclusively and competition in this product segment;
|
|
|
• |
the anticipated impact of inflation and liquidity on the Company’s performance will be as forecasted;
|
|
|
• |
the accuracy with respect to the Company’s operating budget and the assumptions related thereto;
|
|
|
• |
the Company will remain as going concern;
|
|
|
• |
a favorable outcome with respect to the collection of the awards in successful judgements, and the success of other ongoing claims the Company is involved in;
|
|
|
• |
the Company’s Common Shares will remain listed on the Nasdaq;
|
|
|
• |
the Company’s ability to maintain cannabis licensing in the jurisdictions in which the Company operates;
|
|
|
• |
the Company has the ability to obtain the renewal and/or extension of the Company’s licenses;
|
|
|
• |
the Company has the ability to meet operating cash requirements and future financing needs;
|
|
|
• |
the Company will meet or surpass its expectations regarding its Gross Margins, EBITDA, Adjusted EBITDA, revenue, expenses, profit margins and operations;
|
|
|
• |
the Company will increase its revenue and margins in its Israeli medical cannabis market activities arising from its acquisitions;
|
|
|
• |
the Company has the ability to capitalize on future opportunities for the Company in Israel, particularly in the retail and distribution segments of the cannabis market;
|
|
|
• |
the Company will carry out its future expansion and growth opportunities for the Company in Germany and Europe and the timing of such; and
|
|
|
• |
the Company will fulfill its contractual obligations and commitments;
|
|
|
• |
the Company’s plan submitted to Nasdaq to regain compliance will be accepted and effectively executed; and
|
|
|
• |
the Company’s continued access to debt and equity financing under acceptable terms through private placements, loan facilities, or under its Shelf Registration; and
|
|
|
• |
the ability of the Company to comply with covenants and repayment terms under existing and future loan agreements; and
|
|
|
• |
the Company will overcome export permit delays and secure a reliable supply chain to support its sales channels in Israel; and
|
|
|
• |
the acquisition of the remaining stake in Focus Medical Herbs will contribute positively to the Company’s financial results and operational structure; and
|
|
|
• |
that recent and future board and management appointments will support improved governance and financial performance; and
|
|
|
• |
the proposed “Quantum” transaction progressing from the signed non-binding term sheet to definitive agreements and closing, on terms substantially consistent with the term sheet and within anticipated timelines, subject to customary
conditions;
|
|
|
• |
there will be no material adverse changes to the macroeconomic environment (e.g., inflation, interest rates, currency exchange) that impact financing or operations.
|
|
|
• |
the Company’s inability to achieve its business objectives and milestones under the stated timelines;
|
|
|
• |
the Company inability to carry out its business, strategies and operations;
|
|
|
• |
the Company’s inability to realize upon its intentions to expand the business, operations and potential activities of the Company;
|
|
|
• |
the Company will not expand its sales channels, distribution, delivery and storage capacity, and reach to medical cannabis patients;
|
|
|
• |
the competitive conditions of the cannabis industry and the growth of medical or adult-use recreational cannabis markets will be unfavorable to the Company in the jurisdictions in which the Company operates;
|
|
|
• |
the competitive conditions of the industry will be unfavorable to the Company, and the Company’s inability to maintain or grow its market share and maintain its competitive advantages;
|
|
|
• |
the Company will not commit to responsible growth and compliance with the strictest regulatory environments;
|
|
|
• |
the Company’s inability to remain focused on providing premium cannabis products to medical patients in the jurisdictions in which the Company conducts business and any other jurisdiction in which the Company may conduct business in
the future;
|
|
|
• |
the Company inability to amplify its commercial and brand power to become a global high-quality cannabis player;
|
|
|
• |
the Company will not maintain its primary goal of sustainably increasing revenue in its core markets;
|
|
|
• |
the demand and momentum in the Company’s Israeli and Germany operations will be unfavorable to the Company;
|
|
|
• |
the Company will not carry out its plans to position its brands as stated;
|
|
|
• |
the Company’s inability to realize upon the stated efficiencies and synergies of the Company as a global organization with domestic expertise in Israel and Germany;
|
|
|
• |
providing a high-quality, reliable supply to the Company’s customers and patients will not lead to recurring sales;
|
|
|
• |
the Company will not introduce new SKUs;
|
|
|
• |
the Company’s inability to realize upon the anticipated cost savings from the reorganization;
|
|
|
• |
the Company’s inability to achieve geographic diversification and brand recognition and the growth of the Company’s brands in the jurisdictions that the Company operates in or may expand to;
|
|
|
• |
the Company’s inability to address the ongoing needs and preferences of medical cannabis patients;
|
|
|
• |
the Company’s inability to realize upon its retail presence, distribution capabilities and data-driven insights;
|
|
|
• |
the future impact of the Regulations Amendment will be unfavorable to the Company;
|
|
|
• |
the Company will not maintain its partnerships with third party suppliers and partners;
|
|
|
• |
the Company’s inability to achieve profitability in the next quarters of 2025;
|
|
|
• |
the inaccuracy of number of patients in Israel licensed by the MOH to consume medical cannabis;
|
|
|
• |
the inaccuracy of the number of patients paying out-of-pocket for medical cannabis products in Germany;
|
|
|
• |
the anticipated decriminalization or legalization of adult-use recreational cannabis in Israel and Germany will not occur;
|
|
|
• |
the Company’s ability to source premium and ultra-premium cannabis products exclusively and competition in this product segment;
|
|
|
• |
the anticipated impact of inflation and liquidity on the Company’s performance will not be as forecasted;
|
|
|
• |
the inaccuracy with respect to the Company’s operating budget and the assumptions related thereto;
|
|
|
• |
the Company will not remain as going concern;
|
|
|
• |
an unfavorable outcome of legal proceedings the Company is involved in;
|
|
|
• |
an unfavorable outcome with respect to the collection of the award in the Judgment of the Test Kits Appeal and the Company being unsuccessful in other ongoing claims the Company is involved in;
|
|
|
• |
the Company’s Common Shares will not remain listed on the Nasdaq;
|
|
|
• |
the Company’s inability to maintain cannabis licensing in the jurisdictions in which the Company operates;
|
|
|
• |
the Company’s inability to obtain the renewal and/or extension of the Company’s licenses;
|
|
|
• |
the Company’s inability to meet operating cash requirements and future financing needs;
|
|
|
• |
the Company will not meet or surpass its expectations regarding its Gross Margins, EBITDA, Adjusted EBITDA, revenue, expenses, profit margins, and operations;
|
|
|
• |
the Company will not increase its revenue and margins in its Israeli medical cannabis market activities arising from its acquisitions;
|
|
|
• |
the Company’s ability to capitalize on future opportunities for the Company in Israel, particularly in the retail and distribution segments of the cannabis market;
|
|
|
• |
the Company will not carry out its future expansion and growth opportunities for the Company in Germany and Europe and the timing of such;
|
|
|
• |
the Company will not fulfill its contractual obligations and commitments;
|
|
|
• |
the Company may fail to regain or maintain compliance with Nasdaq listing standards, resulting in delisting and reduced access to capital markets;
|
|
|
• |
the Company may not be able to raise sufficient capital under its Shelf Registration or other financing initiatives, leading to liquidity constraints;
|
|
|
• |
the risk of default or acceleration under loan agreements, including those subject to pledges or performance conditions;
|
|
|
• |
continued supply chain disruptions, including export permit delays from Canada, may impact revenue and inventory availability;
|
|
|
• |
the acquisition of Focus Medical Herbs may not yield the anticipated benefits or operational synergies;
|
|
|
• |
the Company may not be able to progress any proposed transaction from term sheet to definitive agreements and closing and, even if the Company is able to sign
definitive agreements, the transactions may not have the intended benefits to the Company; and
|
|
|
• |
the Company may be unable to collect amounts owed from prior judgments or settlements, affecting its financial position.
|
| 1. |
Review: I have reviewed the interim financial report and interim MD&A (together, the “Interim
Filings”) of IM Cannabis Corp. (the “issuer”) for the interim period ended September 30, 2025.
|
| 2. |
No misrepresentations: Based on my knowledge, having exercised reasonable diligence, the interim filings do not contain any untrue
statement of a material fact or omit to state a material fact required to be stated or that is necessary to make a statement not misleading in light of the circumstances under which it was made, with respect to the period covered by the
interim filings.
|
| 3. |
Fair presentation: Based on my knowledge, having exercised reasonable diligence, the interim financial report together with the other
financial information included in the interim filings fairly present in all material respects the financial condition, financial performance and cash flows of the issuer, as of the date of and for the periods presented in the interim
filings.
|
| 4. |
Responsibility: The issuer’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (DC&P) and internal control
over financial reporting (ICFR), as those terms are defined in National Instrument 52-109 Certification of Disclosure in Issuers’ Annual and Interim Filings, for the issuer.
|
| 5. |
Design: Subject to the limitations, if any, described in paragraphs 5.2 and 5.3, the issuer’s other certifying officer and I have, as at the end of the period covered by the
interim filings:
|
|
|
(a) |
designed DC&P, or caused it to be designed under our supervision, to provide reasonable assurance that
|
|
|
(i) |
material information relating to the issuer is made known to us by others, particularly during the period in which the interim filings are being prepared; and
|
|
|
(ii) |
information required to be disclosed by the issuer in its annual filings, interim filings or other reports filed or submitted by it under securities legislation is recorded, processed, summarized and reported within the time periods
specified in securities legislation; and
|
|
|
(b) |
designed ICFR, or caused it to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with the
issuer’s GAAP.
|
| 5.1 |
Control framework: The control framework the issuer’s other certifying officer(s) and I used to design the issuer’s ICFR is Internal Control – Integrated Framework (COSO
Framework 2013) published by The Committee of Sponsoring Organization of the Treadway Commission (COSO).
|
| 5.2 |
N/A
|
| 5.3 | Limitation on scope of design: The issuer has disclosed in its interim MD&A |
|
|
(a) |
he fact that the issuer’s other certifying officer(s) and I have limited the scope of our design of DC&P and ICFR to exclude controls, policies and procedures of
|
|
|
(i) |
N/A
|
|
|
(ii) |
N/A
|
|
|
(iii) |
a business that the issuer acquired not more than 365 days before the last day of the period covered by the interim filings; and
|
|
|
(b) |
summary financial information about the proportionately consolidated entity, special purpose entity or business that the issuer acquired that has been proportionately consolidated or consolidated in the issuer’s financial statements.
|
| 6. |
Reporting changes in ICFR: The issuer has disclosed in its interim MD&A any change in the issuer’s ICFR that occurred during the
period beginning on April 1, 2025 and ended on September 30, 2025 that has materially affected, or is reasonably likely to materially affect, the issuer’s ICFR.
|
| ”Uri Birenberg” |
|
|
| 1. |
Review: : I have reviewed the interim financial report and interim MD&A (together, the “interim filings”) of IM Cannabis Corp. (the
“issuer”) for the interim period ended September 30, 2025.
|
| 2. |
No misrepresentations: Based on my knowledge, having exercised reasonable diligence, the interim filings do not contain any untrue statement of a material fact or omit to state
a material fact required to be stated or that is necessary to make a statement not misleading in light of the circumstances under which it was made, with respect to the period covered by the interim filings.
|
| 3. |
Fair presentation: Based on my knowledge, having exercised reasonable diligence, the interim financial report together with the other
financial information included in the interim filings fairly present in all material respects the financial condition, financial performance and cash flows of the issuer, as of the date of and for the periods presented in the interim
filings.
|
| 4. |
Responsibility: The issuer’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (DC&P) and internal control
over financial reporting (ICFR), as those terms are defined in National Instrument 52-109 Certification of Disclosure in Issuers’ Annual and Interim Filings, for the issuer.
|
| 5. |
Design: Subject to the limitations, if any, described in paragraphs 5.2 and 5.3, the issuer’s other certifying officer and I have, as at the end of the period covered by the
interim filings
|
|
|
(a) |
designed DC&P, or caused it to be designed under our supervision, to provide reasonable assurance that
|
|
|
(i) |
material information relating to the issuer is made known to us by others, particularly during the period in which the interim filings are being prepared; and
|
|
|
(ii) |
information required to be disclosed by the issuer in its annual filings, interim filings or other reports filed or submitted by it under securities legislation is recorded, processed, summarized and reported within the time periods
specified in securities legislation; and
|
|
|
(b) |
designed ICFR, or caused it to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with the
issuer’s GAAPP.
|
| 5.1 |
Control framework: The control framework the issuer’s other certifying officer(s) and I used to design the issuer’s ICFR is Internal Control – Integrated Framework (COSO
Framework 2013) published by The Committee of Sponsoring Organization of the Treadway Commission (COSO).
|
| 5.2 |
N/A
|
| 5.3 |
Limitation on scope of design: The issuer has disclosed in its interim MD&A
|
|
|
(a) |
the fact that the issuer’s other certifying officer(s) and I have limited the scope of our design of DC&P and ICFR to exclude controls, policies and procedures of
|
|
|
(i) |
N/A
|
|
|
(ii) |
N/A
|
|
|
(b) |
summary financial information about the proportionately consolidated entity, special purpose entity or business that the issuer acquired that has been proportionately consolidated or consolidated in the issuer’s financial statements
|
| 7. |
Reporting changes in ICFR: The issuer has disclosed in its interim MD&A any change in the issuer’s ICFR that occurred during the
period beginning on April 1, 2025 and ended on September 30, 2025 that has materially affected, or is reasonably likely to materially affect, the issuer’s ICFR.
|
| ”Oren Shuster” |
|
|