UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant
to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of report (Date of earliest event reported): September 11, 2025
Silexion Therapeutics Corp
(Exact name of registrant as specified in its charter)
|
Cayman Islands
|
|
001-42253
|
|
N/A |
|
(State or other jurisdiction
|
|
(Commission File Number)
|
|
(I.R.S. Employer
|
|
of incorporation)
|
|
|
|
Identification No.)
|
|
12 Abba Hillel Road Ramat-Gan, Israel |
|
5250606
|
|
(Address of principal executive offices)
|
|
(Zip Code)
|
Check the appropriate box below if the Form 8-K is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
|
Trading Symbol(s)
|
|
Name of each exchange on which registered
|
| Ordinary Shares, par value $0.0135 per share |
|
SLXN |
|
The Nasdaq Stock Market LLC |
| Warrants exercisable for Ordinary Shares at an exercise price of $1,552.50 per share |
|
SLXNW |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
|
|
|
|
104
|
Cover Page Interactive Data File (formatted in Inline XBRL)
|
|
|
SILEXION THERAPEUTICS CORP
|
|
|
|
|
|
|
Date: September 11, 2025
|
By: /s/ Ilan Hadar
|
|
|
|
Name:
|
Ilan Hadar
|
|
|
Title:
|
Chief Executive Officer
|

|
|
• |
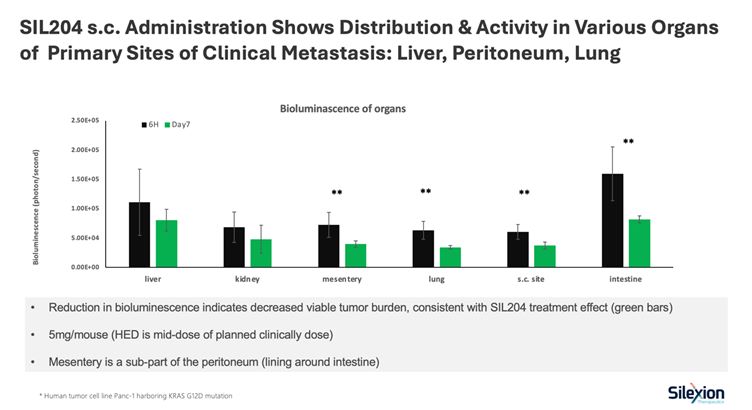
SIL204 successfully distributed to all major metastatic sites following a single subcutaneous injection at 5mg/mouse (mid-range human equivalent dose for planned clinical trials)
|
|
|
• |
Reductions in bioluminescent signal, indicating decreased tumor burden, were observed at day 7 across all evaluated organs
|
|
|
• |
Statistically significant reductions (p<0.01) were achieved in the peritoneum (mesentery), lung, and intestine
|
|
|
• |
The liver, the most common site of pancreatic cancer metastasis, showed measurable reduction in tumor burden
|
|
|
• |
Studies utilized human pancreatic cancer cells (Panc-1) harboring the KRAS G12D mutation
|
|
|
• |
The use of human equivalent dosing demonstrates that these results were achieved at drug concentrations directly relevant to planned clinical use, providing important validation for the transition from preclinical to human studies
|