|
|
BioLineRx Ltd.
|
|
|
|
|
|
|
|
|
|
By:
|
/s/ Philip A. Serlin
|
|
|
|
|
Philip A. Serlin
|
|
|
|
|
Chief Executive Officer
|
|
|
|
• |
Completed financing in January 2025 raising gross proceeds of $10 million.
|
|
|
• |
Successfully reduced operating expense run rate by over 70% beginning January 1, 2025, through the APHEXDA program transfer to Ayrmid and the resulting shutdown of the Company’s U.S. commercial operations in Q4 2024, as well as additional
headcount and other operating expense reductions.
|
|
|
• |
Reaffirms cash runway through the second half of 2026.
|
|
|
• |
APHEXDA generated sales of $1.4 million in the first quarter of 2025, providing royalty revenues to the Company of $0.3 million.
|
|
|
• |
Additional trial sites were activated for the CheMo4METPANC Phase 2b clinical trial, which is expected to have a positive impact on patient recruitment. Full enrollment in the randomized trial, which is being led by Columbia University,
and supported by both Regeneron and BioLineRx, is planned for completion in 2027, with a prespecified interim analysis planned when 40% of progression free survival (PFS) events are observed.
|
|
|
• |
An abstract featuring updated data from the pilot phase of the ongoing CheMo4METPANC clinical trial has been accepted for a poster presentation at the 2025 ASCO Annual Meeting on Saturday, May 31st. Key highlights include:
|
|
|
o |
Two patients underwent definitive treatment for metastatic pancreatic cancer: one had complete resolution of all radiologically detected liver lesions and underwent definitive radiation to the primary pancreatic tumor, and one had a
sustained partial response and underwent pancreaticoduodenectomy with pathology demonstrating a complete response.
|
|
|
o |
An analysis of pre- and on-treatment biopsies revealed that CD8+ T-cell tumor infiltration increased across all eleven patients treated with the motixafortide combination.
|
|
|
• |
Enrollment is continuing into the multi-center Phase 1 clinical trial evaluating motixafortide for the mobilization of CD34+ hematopoietic stem cells (HSCs) used in the development of gene therapies for patients with Sickle Cell Disease
(SCD). The trial is sponsored by St. Jude Children's Research Hospital.
|
|
|
• |
Reported continued progress of a Phase 1 clinical trial evaluating motixafortide as monotherapy and in combination with natalizumab for stem cell mobilization for gene therapies in sickle cell disease. The trial is sponsored by
Washington University School of Medicine in St. Louis.
|
|
|
• |
Revenues for the three-month period ended March 31, 2025 were $0.3 million, a decrease of $6.6 million, compared to revenues of $6.9 million for the three-month period ended March 31, 2024. The significant decrease in revenues from 2024 to
2025 reflects the one-time revenues recorded in 2024 relating to the out-licensing transaction with Gloria during the fourth quarter of 2023, as well as the change in the Company’s operations following the out-licensing of APHEXDA to Ayrmid
during the fourth quarter of 2024. The revenues in 2025 reflect the royalties paid by Ayrmid from the commercialization of APHEXDA in stem cell mobilization in the U.S. The revenues in 2024 primarily reflect a portion of the up-front payment
received by the Company and a milestone payment achieved under the license agreement with Gloria, which collectively amounted to $5.9 million, as well as $0.9 million of net revenues from product sales of APHEXDA in the U.S.
|
|
|
• |
Cost of revenues for the three-month period ended March 31, 2025 was immaterial, compared to cost of revenues of $1.5 million for the three-month period ended March 31, 2024. The cost of revenues in 2025 reflects sub-license fees on
royalties paid by Ayrmid from the commercialization of APHEXDA in stem cell mobilization in the U.S. The cost of revenues in 2024 primarily reflects sub-license fees on a milestone payment received under the Gloria license agreement and
royalties on net product sales of APHEXDA in the U.S., as well as amortization of intangible assets and cost of goods sold on product sales.
|
|
|
• |
Research and development expenses for the three months ended March 31, 2025 were $1.6 million, a decrease of $0.9 million, or 34.9%, compared to $2.5 million for the three months ended March 31, 2024. The decrease resulted primarily from
lower expenses related to motixafortide due to the out-licensing of U.S. rights to Ayrmid, as well as a decrease in payroll and share-based compensation, primarily due to a decrease in headcount.
|
|
|
• |
There were no sales and marketing expenses for the three months ended March 31, 2025, compared to $6.3 million for the three months ended
March 31, 2024. The decrease resulted primarily from the shutdown of U.S. commercial operations in the fourth quarter of 2024 following the Ayrmid out-licensing transaction.
|
|
|
• |
General and administrative expenses for the three months ended March 31, 2025 were $1.0 million, a decrease of $0.4 million, or 28.6%, compared to $1.4 million for the three months ended March 31, 2024. The decrease resulted primarily from
a decrease in payroll and share-based compensation, primarily due to a decrease in headcount, as well as small decreases in a number of general and administrative expenses.
|
|
|
• |
Net non-operating income for the three months ended March 31, 2025 was $7.6 million, compared to net non-operating income of $4.5 million for the three months ended March 31, 2024. Non-operating income for both periods primarily relates to
fair-value adjustments of warrant liabilities on the balance sheet, as a result of changes in the Company’s share price.
|
|
|
• |
Net financial expenses for the three months ended March 31, 2025 were $0.1 million, compared to net financial expenses of $0.4 million for the three months ended March 31, 2024. Net financial expenses for both periods primarily relate to
loan interest paid, partially offset by investment income earned on bank deposits.
|
|
|
• |
Net income for the quarter ended March 31, 2025 was $5.1 million, compared to $0.7 million for the quarter ended March 31, 2024.
|
|
|
• |
As of March 31, 2025, the Company had cash, cash equivalents, and short-term bank deposits of $26.4 million
|
|
December 31,
|
March 31,
|
|||||||
|
2024
|
2025
|
|||||||
|
in USD thousands
|
||||||||
|
Assets
|
||||||||
|
CURRENT ASSETS
|
||||||||
|
Cash and cash equivalents
|
10,436
|
9,036
|
||||||
|
Short-term bank deposits
|
9,126
|
17,333
|
||||||
|
Trade receivables
|
2,476
|
1,469
|
||||||
|
Prepaid expenses
|
443
|
312
|
||||||
|
Other receivables
|
1,478
|
452
|
||||||
|
Inventory
|
3,145
|
3,315
|
||||||
|
Total current assets
|
27,104
|
31,917
|
||||||
|
NON-CURRENT ASSETS
|
||||||||
|
Property and equipment, net
|
386
|
299
|
||||||
|
Right-of-use assets, net
|
967
|
863
|
||||||
|
Intangible assets, net
|
10,449
|
10,431
|
||||||
|
Total non-current assets
|
11,802
|
11,593
|
||||||
|
Total assets
|
38,906
|
43,510
|
||||||
|
Liabilities and equity
|
||||||||
|
CURRENT LIABILITIES
|
||||||||
|
Current maturities of long-term loan
|
4,479
|
4,684
|
||||||
|
Accounts payable and accruals:
|
||||||||
|
Trade
|
5,583
|
4,693
|
||||||
|
Other
|
3,131
|
1,751
|
||||||
|
Current maturities of lease liabilities
|
522
|
440
|
||||||
|
Warrants
|
1,691
|
2,462
|
||||||
|
Total current liabilities
|
15,406
|
14,030
|
||||||
|
NON-CURRENT LIABILITIES
|
||||||||
|
Long-term loan, net of current maturities
|
8,958
|
7,633
|
||||||
|
Lease liabilities
|
1,081
|
985
|
||||||
|
Total non-current liabilities
|
10,039
|
8,618
|
||||||
|
COMMITMENTS AND CONTINGENT LIABILITIES
|
||||||||
|
Total liabilities
|
25,445
|
22,648
|
||||||
|
EQUITY
|
||||||||
|
Ordinary shares
|
38,097
|
62,570
|
||||||
|
Share premium
|
353,693
|
333,627
|
||||||
|
Warrants
|
5,367
|
3,686
|
||||||
|
Capital reserve
|
17,547
|
17,095
|
||||||
|
Other comprehensive loss
|
(1,416
|
)
|
(1,416
|
)
|
||||
|
Accumulated deficit
|
(399,827
|
)
|
(394,700
|
)
|
||||
|
Total equity
|
13,461
|
20,862
|
||||||
|
Total liabilities and equity
|
38,906
|
43,510
|
||||||
|
Three months ended March 31,
|
||||||||
|
2024
|
2025
|
|||||||
|
in USD thousands
|
||||||||
|
REVENUES:
|
||||||||
|
License revenues
|
5,931
|
255
|
||||||
|
Product sales, net
|
924
|
-
|
||||||
|
Total revenues
|
6,855
|
255
|
||||||
|
COST OF REVENUES
|
(1,455
|
)
|
(34
|
)
|
||||
|
GROSS PROFIT
|
5,400
|
221
|
||||||
|
RESEARCH AND DEVELOPMENT EXPENSES
|
(2,494
|
)
|
(1,623
|
)
|
||||
|
SALES AND MARKETING EXPENSES
|
(6,342
|
)
|
-
|
|||||
|
GENERAL AND ADMINISTRATIVE EXPENSES
|
(1,386
|
)
|
(989
|
)
|
||||
|
OPERATING LOSS
|
(4,822
|
)
|
(2,391
|
)
|
||||
|
NON-OPERATING INCOME (EXPENSES), NET
|
4,490
|
7,644
|
||||||
|
FINANCIAL INCOME
|
565
|
294
|
||||||
|
FINANCIAL EXPENSES
|
(929
|
)
|
(420
|
)
|
||||
|
NET INCOME (LOSS) AND COMPREHENSIVE INCOME (LOSS)
|
(696
|
)
|
5,127
|
|||||
|
in USD
|
||||||||
|
EARNINGS )LOSS( PER ORDINARY SHARE - BASIC AND DILUTED
|
(0.00
|
)
|
0.00
|
|||||
|
WEIGHTED AVERAGE NUMBER OF SHARES USED IN
CALCULATION OF EARNINGS )LOSS( PER ORDINARY SHARE |
1,086,589,165
|
2,217,728,234
|
||||||
|
Ordinary shares
|
Share premium
|
Warrants
|
Capital reserve
|
Other comprehensive loss
|
Accumulated deficit
|
Total
|
||||||||||||||||||||||
|
in USD thousands
|
||||||||||||||||||||||||||||
|
BALANCE AT JANUARY 1, 2024
|
31,355
|
355,482
|
1,408
|
17,000
|
(1,416
|
)
|
(390,606
|
)
|
13,223
|
|||||||||||||||||||
|
CHANGES FOR THREE MONTHS ENDED MARCH 31, 2024:
|
||||||||||||||||||||||||||||
|
Share-based compensation
|
-
|
-
|
-
|
533
|
-
|
-
|
533
|
|||||||||||||||||||||
|
Comprehensive loss for the period
|
-
|
-
|
-
|
-
|
-
|
(696
|
)
|
(696
|
)
|
|||||||||||||||||||
|
BALANCE AT MARCH 31, 2024
|
31,355
|
355,482
|
1,408
|
17,533
|
(1,416
|
)
|
(391,302
|
)
|
13,060
|
|||||||||||||||||||
|
Ordinary shares
|
Share premium
|
Warrants
|
Capital reserve
|
Other comprehensive loss
|
Accumulated deficit
|
Total
|
||||||||||||||||||||||
|
in USD thousands
|
||||||||||||||||||||||||||||
|
BALANCE AT JANUARY 1, 2025
|
38,097
|
353,693
|
5,367
|
17,547
|
(1,416
|
)
|
(399,827
|
)
|
13,461
|
|||||||||||||||||||
|
CHANGES FOR THREE MONTHS ENDED MARCH 31, 2025:
|
||||||||||||||||||||||||||||
|
Issuance of share capital, pre-funded warrants and warrants, net
|
16,415
|
(14,836
|
)
|
501
|
-
|
-
|
-
|
2,080
|
||||||||||||||||||||
|
Pre-funded warrants exercised
|
8,058
|
(5,876
|
)
|
(2,182
|
)
|
-
|
-
|
-
|
-
|
|||||||||||||||||||
|
Employee stock options expired
|
-
|
646
|
-
|
(646
|
)
|
-
|
-
|
-
|
||||||||||||||||||||
|
Share-based compensation
|
-
|
-
|
-
|
194
|
-
|
-
|
194
|
|||||||||||||||||||||
|
Comprehensive income for the period
|
-
|
-
|
-
|
-
|
-
|
5,127
|
5,127
|
|||||||||||||||||||||
|
BALANCE AT MARCH 31, 2025
|
62,570
|
333,627
|
3,686
|
17,095
|
(1,416
|
)
|
(394,700
|
)
|
20,862
|
|||||||||||||||||||
|
Three months ended
March 31,
|
||||||||
|
2024
|
2025
|
|||||||
|
in USD thousands
|
||||||||
|
CASH FLOWS - OPERATING ACTIVITIES
|
||||||||
|
Comprehensive income (loss) for the period
|
(696
|
)
|
5,127
|
|||||
|
Adjustments required to reflect net cash used in operating activities
(see appendix below)
|
(13,413
|
)
|
(7,718
|
)
|
||||
|
Net cash used in operating activities
|
(14,109
|
)
|
(2,591
|
)
|
||||
|
CASH FLOWS - INVESTING ACTIVITIES
|
||||||||
|
Investments in short-term deposits
|
-
|
(12,307
|
)
|
|||||
|
Maturities of short-term deposits
|
16,719
|
4,130
|
||||||
|
Purchase of property and equipment
|
(32
|
)
|
-
|
|||||
|
Net cash provided by (used in) investing activities
|
16,687
|
(8,177
|
)
|
|||||
|
CASH FLOWS - FINANCING ACTIVITIES
|
||||||||
|
Issuance of share capital, pre-funded warrants and warrants,
net of issuance costs
|
-
|
10,697
|
||||||
|
Repayments of loan
|
(765
|
)
|
(1,120
|
)
|
||||
|
Repayments of lease liabilities
|
(129
|
)
|
(127
|
)
|
||||
|
Net cash provided by (used in) financing activities
|
(894
|
)
|
9,450
|
|||||
|
INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS
|
1,684
|
(1,318
|
)
|
|||||
|
CASH AND CASH EQUIVALENTS – BEGINNING OF PERIOD
|
4,255
|
10,436
|
||||||
|
EXCHANGE DIFFERENCES ON CASH AND CASH EQUIVALENTS
|
51
|
(82
|
)
|
|||||
|
CASH AND CASH EQUIVALENTS - END OF PERIOD
|
5,990
|
9,036
|
||||||
|
Three months ended
March 31,
|
||||||||
|
2024
|
2025
|
|||||||
|
in USD thousands
|
||||||||
|
Adjustments required to reflect net cash used in operating activities:
|
||||||||
|
Income and expenses not involving cash flows:
|
||||||||
|
Depreciation and amortization
|
897
|
165
|
||||||
|
Exchange differences on cash and cash equivalents
|
(51
|
)
|
82
|
|||||
|
Fair value adjustments of warrants
|
(4,444
|
)
|
(8,311
|
)
|
||||
|
Warrant issuance costs
|
-
|
702
|
||||||
|
Share-based compensation
|
533
|
194
|
||||||
|
Interest on short-term deposits
|
(163
|
)
|
(30
|
)
|
||||
|
Interest on loan
|
610
|
-
|
||||||
|
Exchange differences on lease liabilities
|
(25
|
)
|
(7
|
)
|
||||
|
(2,643
|
)
|
(7,205
|
)
|
|||||
|
Changes in operating asset and liability items:
|
||||||||
|
Decrease (increase) in trade receivables
|
(2,474
|
)
|
1,007
|
|||||
|
Increase in inventory
|
(936
|
)
|
(170
|
)
|
||||
|
Decrease in prepaid expenses and other receivables
|
81
|
1,157
|
||||||
|
Decrease in accounts payable and accruals
|
(3,511
|
)
|
(2,507
|
)
|
||||
|
Decrease in contract liabilities
|
(3,930
|
)
|
-
|
|||||
|
(10,770
|
)
|
(513
|
)
|
|||||
|
(13,413
|
)
|
(7,718
|
)
|
|||||
|
Supplemental information on interest received in cash
|
357
|
236
|
||||||
|
Supplemental information on interest paid in cash
|
255
|
361
|
||||||
|
Supplemental information on non-cash transactions:
|
||||||||
|
Changes in right-of-use asset and lease liabilities
|
32
|
44
|
||||||
|
Warrant issuance costs
|
-
|
237
|
||||||
|
Page
|
||
|
2
|
||
|
3
|
||
|
4
|
||
|
5-6
|
||
|
7-16
|
|
December 31,
|
March 31,
|
|||||||
|
2024
|
2025
|
|||||||
|
in USD thousands
|
||||||||
|
Assets
|
||||||||
|
CURRENT ASSETS
|
||||||||
|
Cash and cash equivalents
|
10,436
|
9,036
|
||||||
|
Short-term bank deposits
|
9,126
|
17,333
|
||||||
|
Trade receivables
|
2,476
|
1,469
|
||||||
|
Prepaid expenses
|
443
|
312
|
||||||
|
Other receivables
|
1,478
|
452
|
||||||
|
Inventory
|
3,145
|
3,315
|
||||||
|
Total current assets
|
27,104
|
31,917
|
||||||
|
NON-CURRENT ASSETS
|
||||||||
|
Property and equipment, net
|
386
|
299
|
||||||
|
Right-of-use assets, net
|
967
|
863
|
||||||
|
Intangible assets, net
|
10,449
|
10,431
|
||||||
|
Total non-current assets
|
11,802
|
11,593
|
||||||
|
Total assets
|
38,906
|
43,510
|
||||||
|
Liabilities and equity
|
||||||||
|
CURRENT LIABILITIES
|
||||||||
|
Current maturities of long-term loan
|
4,479
|
4,684
|
||||||
|
Accounts payable and accruals:
|
||||||||
|
Trade
|
5,583
|
4,693
|
||||||
|
Other
|
3,131
|
1,751
|
||||||
|
Current maturities of lease liabilities
|
522
|
440
|
||||||
|
Warrants
|
1,691
|
2,462
|
||||||
|
Total current liabilities
|
15,406
|
14,030
|
||||||
|
NON-CURRENT LIABILITIES
|
||||||||
|
Long-term loan, net of current maturities
|
8,958
|
7,633
|
||||||
|
Lease liabilities
|
1,081
|
985
|
||||||
|
Total non-current liabilities
|
10,039
|
8,618
|
||||||
|
COMMITMENTS AND CONTINGENT LIABILITIES
|
||||||||
|
Total liabilities
|
25,445
|
22,648
|
||||||
|
EQUITY
|
||||||||
|
Ordinary shares
|
38,097
|
62,570
|
||||||
|
Share premium
|
353,693
|
333,627
|
||||||
|
Warrants
|
5,367
|
3,686
|
||||||
|
Capital reserve
|
17,547
|
17,095
|
||||||
|
Other comprehensive loss
|
(1,416
|
)
|
(1,416
|
)
|
||||
|
Accumulated deficit
|
(399,827
|
)
|
(394,700
|
)
|
||||
|
Total equity
|
13,461
|
20,862
|
||||||
|
Total liabilities and equity
|
38,906
|
43,510
|
||||||
|
Three months ended March 31,
|
||||||||
|
2024
|
2025
|
|||||||
|
in USD thousands
|
||||||||
|
REVENUES:
|
||||||||
|
License revenues
|
5,931
|
255
|
||||||
|
Product sales, net
|
924
|
-
|
||||||
|
Total revenues
|
6,855
|
255
|
||||||
|
COST OF REVENUES
|
(1,455
|
)
|
(34
|
)
|
||||
|
GROSS PROFIT
|
5,400
|
221
|
||||||
|
RESEARCH AND DEVELOPMENT EXPENSES
|
(2,494
|
)
|
(1,623
|
)
|
||||
|
SALES AND MARKETING EXPENSES
|
(6,342
|
)
|
-
|
|||||
|
GENERAL AND ADMINISTRATIVE EXPENSES
|
(1,386
|
)
|
(989
|
)
|
||||
|
OPERATING LOSS
|
(4,822
|
)
|
(2,391
|
)
|
||||
|
NON-OPERATING INCOME (EXPENSES), NET
|
4,490
|
7,644
|
||||||
|
FINANCIAL INCOME
|
565
|
294
|
||||||
|
FINANCIAL EXPENSES
|
(929
|
)
|
(420
|
)
|
||||
|
NET INCOME (LOSS) AND COMPREHENSIVE INCOME (LOSS)
|
(696
|
)
|
5,127
|
|||||
|
in USD
|
||||||||
|
EARNINGS (LOSS) PER ORDINARY SHARE - BASIC AND DILUTED
|
(0.00
|
)
|
0.00
|
|||||
|
WEIGHTED AVERAGE NUMBER OF SHARES USED IN
CALCULATION OF EARNINGS (LOSS) PER ORDINARY SHARE |
1,086,589,165
|
2,217,728,234
|
||||||
|
Ordinary shares
|
Share premium
|
Warrants
|
Capital reserve
|
Other comprehensive loss
|
Accumulated deficit
|
Total
|
||||||||||||||||||||||
|
in USD thousands
|
||||||||||||||||||||||||||||
|
BALANCE AT JANUARY 1, 2024
|
31,355
|
355,482
|
1,408
|
17,000
|
(1,416
|
)
|
(390,606
|
)
|
13,223
|
|||||||||||||||||||
|
CHANGES FOR THREE MONTHS ENDED MARCH 31, 2024:
|
||||||||||||||||||||||||||||
|
Share-based compensation
|
-
|
-
|
-
|
533
|
-
|
-
|
533
|
|||||||||||||||||||||
|
Comprehensive loss for the period
|
-
|
-
|
-
|
-
|
-
|
(696
|
)
|
(696
|
)
|
|||||||||||||||||||
|
BALANCE AT MARCH 31, 2024
|
31,355
|
355,482
|
1,408
|
17,533
|
(1,416
|
)
|
(391,302
|
)
|
13,060
|
|||||||||||||||||||
|
Ordinary shares
|
Share premium
|
Warrants
|
Capital reserve
|
Other comprehensive loss
|
Accumulated deficit
|
Total
|
||||||||||||||||||||||
|
in USD thousands
|
||||||||||||||||||||||||||||
|
BALANCE AT JANUARY 1, 2025
|
38,097
|
353,693
|
5,367
|
17,547
|
(1,416
|
)
|
(399,827
|
)
|
13,461
|
|||||||||||||||||||
|
CHANGES FOR THREE MONTHS ENDED MARCH 31, 2025:
|
||||||||||||||||||||||||||||
|
Issuance of share
capital, pre-funded warrants and warrants, net
|
16,415
|
(14,836
|
)
|
501
|
-
|
-
|
-
|
2,080
|
||||||||||||||||||||
|
Pre-funded warrants exercised
|
8,058
|
(5,876
|
)
|
(2,182
|
)
|
-
|
-
|
-
|
-
|
|||||||||||||||||||
|
Employee stock options expired
|
-
|
646
|
-
|
(646
|
)
|
-
|
-
|
-
|
||||||||||||||||||||
|
Share-based compensation
|
-
|
-
|
-
|
194
|
-
|
-
|
194
|
|||||||||||||||||||||
|
Comprehensive income for the period
|
-
|
-
|
-
|
-
|
-
|
5,127
|
5,127
|
|||||||||||||||||||||
|
BALANCE AT MARCH 31, 2025
|
62,570
|
333,627
|
3,686
|
17,095
|
(1,416
|
)
|
(394,700
|
)
|
20,862
|
|||||||||||||||||||
|
Three months ended
March 31,
|
||||||||
|
2024
|
2025
|
|||||||
|
in USD thousands
|
||||||||
|
CASH FLOWS - OPERATING ACTIVITIES
|
||||||||
|
Comprehensive income (loss) for the period
|
(696
|
)
|
5,127
|
|||||
|
Adjustments required to reflect net cash used in operating activities (see appendix below)
|
(13,413
|
)
|
(7,718
|
)
|
||||
|
Net cash used in operating activities
|
(14,109
|
)
|
(2,591
|
)
|
||||
|
CASH FLOWS - INVESTING ACTIVITIES
|
||||||||
|
Investments in short-term deposits
|
-
|
(12,307
|
)
|
|||||
|
Maturities of short-term deposits
|
16,719
|
4,130
|
||||||
|
Purchase of property and equipment
|
(32
|
)
|
-
|
|||||
|
Net cash provided by (used in) investing activities
|
16,687
|
(8,177
|
)
|
|||||
|
CASH FLOWS - FINANCING ACTIVITIES
|
||||||||
|
Issuance of share capital, pre-funded warrants and warrants, net of issuance costs
|
-
|
10,697
|
||||||
|
Repayments of loan
|
(765
|
)
|
(1,120
|
)
|
||||
|
Repayments of lease liabilities
|
(129
|
)
|
(127
|
)
|
||||
|
Net cash provided by (used in) financing activities
|
(894
|
)
|
9,450
|
|||||
|
INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS
|
1,684
|
(1,318
|
)
|
|||||
|
CASH AND CASH EQUIVALENTS – BEGINNING OF PERIOD
|
4,255
|
10,436
|
||||||
|
EXCHANGE DIFFERENCES ON CASH AND CASH EQUIVALENTS
|
51
|
(82
|
)
|
|||||
|
CASH AND CASH EQUIVALENTS - END OF PERIOD
|
5,990
|
9,036
|
||||||
|
Three months ended
March 31,
|
||||||||
|
2024
|
2025
|
|||||||
|
in USD thousands
|
||||||||
|
Adjustments required to reflect net cash used in operating activities:
|
||||||||
|
Income and expenses not involving cash flows:
|
||||||||
|
Depreciation and amortization
|
897
|
165
|
||||||
|
Exchange differences on cash and cash equivalents
|
(51
|
)
|
82
|
|||||
|
Fair value adjustments of warrants
|
(4,444
|
)
|
(8,311
|
)
|
||||
|
Warrant issuance costs
|
-
|
702
|
||||||
|
Share-based compensation
|
533
|
194
|
||||||
|
Interest on short-term deposits
|
(163
|
)
|
(30
|
)
|
||||
|
Interest on loan
|
610
|
-
|
||||||
|
Exchange differences on lease liabilities
|
(25
|
)
|
(7
|
)
|
||||
|
(2,643
|
)
|
(7,205
|
)
|
|||||
|
Changes in operating asset and liability items:
|
||||||||
|
Decrease (increase) in trade receivables
|
(2,474
|
)
|
1,007
|
|||||
|
Increase in inventory
|
(936
|
)
|
(170
|
)
|
||||
|
Decrease in prepaid expenses and other receivables
|
81
|
1,157
|
||||||
|
Decrease in accounts payable and accruals
|
(3,511
|
)
|
(2,507
|
)
|
||||
|
Decrease in contract liabilities
|
(3,930
|
)
|
-
|
|||||
|
(10,770
|
)
|
(513
|
)
|
|||||
|
(13,413
|
)
|
(7,718
|
)
|
|||||
|
Supplemental information on interest received in cash
|
357
|
236
|
||||||
|
Supplemental information on interest paid in cash
|
255
|
361
|
||||||
|
Supplemental information on non-cash transactions:
|
||||||||
|
Changes in right-of-use asset and lease liabilities
|
32
|
44
|
||||||
|
Warrant issuance costs
|
-
|
237
|
||||||
|
|
a. |
General
|
|
|
b. |
War in Israel
|
|
|
c. |
Going concern
|
|
|
d. |
Approval of financial statements
|
|
|
a. |
General
|
|
|
b. |
New international financial reporting standards, amendments to standards and new interpretations
|
|
|
a. |
Securities purchase agreement – Highbridge
|
|
|
a. |
Securities purchase agreement – Highbridge (cont.)
|
|
|
b. |
January 2025 offering
|
|
|
b. |
January 2025 offering (cont.)
|
|
Warrants
|
||||
|
in USD thousands
|
||||
|
Balance as of December 31, 2024
|
1,691
|
|||
|
Changes during 2025:
|
||||
|
Issuances
|
10,465
|
|||
|
Day one loss
|
(1,383
|
)
|
||
|
Changes in fair value through profit and loss
|
(8,311
|
)
|
||
|
Balance as of March 31, 2025
|
2,462
|
|||
|
Number of ordinary shares
|
||||||||
|
December 31,
|
March 31,
|
|||||||
|
2024
|
2025
|
|||||||
|
Authorized share capital
|
5,000,000,000
|
5,000,000,000
|
||||||
|
Issued and paid-up share capital
|
1,336,670,575
|
2,232,601,990
|
||||||
|
In USD and NIS
|
||||||||
|
December 31,
|
March 31,
|
|||||||
|
2024
|
2025
|
|||||||
|
Authorized share capital (in NIS)
|
500,000,000
|
500,000,000
|
||||||
|
Issued and paid-up share capital (in NIS)
|
133,667,057
|
223,260,199
|
||||||
|
Issued and paid-up share capital (in USD)
|
38,096,940
|
62,569,738
|
||||||
|
|
a. |
Revenues
|
|
Three months ended
March 31,
|
||||||||
|
2024
|
2025
|
|||||||
|
in USD thousands
|
||||||||
|
License revenues
|
5,931
|
255
|
||||||
|
Product sales, net
|
924
|
-
|
||||||
|
6,855
|
255
|
|||||||
|
|
b. |
Cost of revenues
|
|
Three months ended
March 31,
|
||||||||
|
2024
|
2025
|
|||||||
|
in USD thousands
|
||||||||
|
Cost related to license revenues
|
741
|
34
|
||||||
|
Amortization of intangible asset in respect of license revenues
|
646
|
-
|
||||||
|
Cost of product sales
|
68
|
-
|
||||||
|
1,455
|
34
|
|||||||
|
|
• |
the clinical development, commercialization and market acceptance of our therapeutic candidates, including the degree and pace of market uptake of APHEXDA for the mobilization of hematopoietic stem cells for
autologous transplantation in multiple myeloma patients;
|
|
|
• |
the initiation, timing, progress and results of our preclinical studies, clinical trials and other therapeutic candidate development efforts;
|
|
|
• |
our ability to advance our therapeutic candidates into clinical trials or to successfully complete our preclinical studies or clinical trials;
|
|
|
• |
whether the clinical trial results for APHEXDA will be predictive of real-world results;
|
|
|
• |
our receipt of regulatory approvals for our therapeutic candidates, and the timing of other regulatory filings and approvals;
|
|
|
• |
whether access to APHEXDA is achieved in a commercially viable manner and whether APHEXDA receives adequate reimbursement from third-party payors;
|
|
|
• |
our ability to establish, manage, and maintain corporate collaborations, as well as the ability of our collaborators to execute on their development and commercialization plans;
|
|
|
• |
our ability to integrate new therapeutic candidates and new personnel, as well as new collaborations;
|
|
|
• |
the interpretation of the properties and characteristics of our therapeutic candidates and of the results obtained with our therapeutic candidates in preclinical studies or clinical trials;
|
|
|
• |
the implementation of our business model and strategic plans for our business and therapeutic candidates;
|
|
|
• |
the scope of protection that we are able to establish and maintain for intellectual property rights covering our therapeutic candidates and our ability to operate our business without infringing the
intellectual property rights of others;
|
|
|
• |
estimates of our expenses, future revenues, capital requirements and our need for and ability to access sufficient additional financing;
|
|
|
• |
risks related to changes in healthcare laws, rules and regulations in the United States or elsewhere;
|
|
|
• |
competitive companies, technologies and our industry;
|
|
|
• |
our ability to maintain the listing of our ADSs on Nasdaq;
|
|
|
• |
statements as to the impact of the political and security situation in Israel on our business, including the impact of Israel’s war with Hamas and other militant groups, which may exacerbate the magnitude of
the factors discussed above; and
|
|
|
• |
those factors referred to in “Risk Factors” in our Annual Report.
|
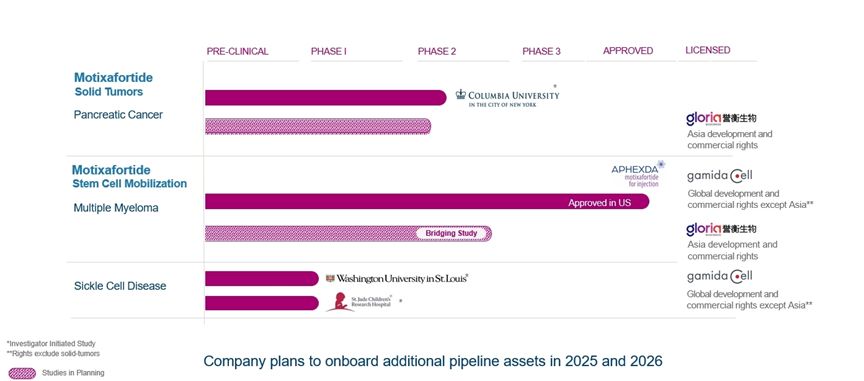
|
Project
|
Status
|
Expected Near Term Milestones
|
||
|
motixafortide
|
1.
|
FDA approval received on September 8, 2023 for stem-cell mobilization in multiple myeloma patients.
|
1.
|
Out-licensed to Ayrmid in November 2024; five-year long-term follow-up of GENESIS patients ongoing
|
|
2.
|
Reported data from single-arm pilot phase of the investigator-initiated Phase 2 combination trial in first-line PDAC. Of 11 patients with metastatic pancreatic cancer enrolled, 7 patients
(64%) experienced partial response (PR), of which 6 (55%) were confirmed PRs with one patient experiencing resolution of the hepatic (liver) metastatic lesion. 3 patients (27%) experienced stable disease, resulting in a disease control rate
of 91%. Based on these encouraging results, study was substantially revised to a multi-institution, randomized Phase 2b trial of 108 patients
|
2.
|
First patient dosed in February 2024. Interim data expected in 2026 and full enrollment projected for 2027*
|
|
|
3.
|
Phase 1 study for gene therapies in SCD (with Washington University School of Medicine in St. Louis)**
|
3.
|
First patient dosed in December 2023 and initial data from the study released in November 2024. Final data planned in 2025*
|
|
|
4.
|
Phase 1 study for gene therapies in SCD (with St. Jude Children’s Research Hospital, Inc.)**
|
4.
|
First patient dosed in February 2025, with data planned in 2025/2026*
|
|
|
5.
|
IND approved in China for initiation of pivotal bridging study in SCM under license agreement with Gloria
|
5.
|
Initiation of the study is currently delayed***
|
|
|
6.
|
Phase 2b randomized study in first-line PDAC in China under license agreement with Gloria
|
6.
|
IND submission and protocol finalization is currently delayed***
|
|
| * |
These studies are investigator-initiated studies; therefore, the timelines are ultimately controlled by the independent investigators and are subject to change.
|
| ** |
Study to be continued under the Ayrmid License Agreement
|
| *** |
Under the Gloria License Agreement, Gloria is late in the payment of $2.4 million to us for the achievement of a specific milestone under the Gloria License Agreement and for certain product supply, which was
due during 2024. In addition, the planned studies of motixafortide in China under the Gloria License Agreement are currently not advancing according to schedule and it is unclear when such studies will be initiated, if at all. There can be
no assurance that Gloria will meet its payment obligations or any other obligations under the Gloria License Agreement.
|
|
|
•
|
the number of sites included in the clinical trials;
|
|
|
•
|
the length of time required to enroll suitable patients;
|
|
|
|
|
|
|
•
|
the number of patients that participate, and are eligible to participate, in the clinical trials;
|
|
|
•
|
the duration of patient follow-up;
|
|
|
|
|
|
|
•
|
whether the patients require hospitalization or can be treated on an outpatient basis;
|
|
|
•
|
the development stage of the therapeutic candidate; and
|
|
|
•
|
the efficacy and safety profile of the therapeutic candidate.
|
|
Three months ended
March 31,
|
||||||||||||
|
2024
|
2025
|
Increase
(decrease)
|
||||||||||
|
(in thousands of U.S. dollars)
|
||||||||||||
|
License revenues
|
5,931
|
255
|
(5,676
|
)
|
||||||||
|
Product sales, net
|
924
|
-
|
(924
|
)
|
||||||||
|
Total revenues
|
6,855
|
255
|
(6,600
|
)
|
||||||||
|
Three months ended
March 31, |
||||||||||||
|
2024
|
2025
|
Increase
(decrease)
|
||||||||||
|
(in thousands of U.S. dollars)
|
||||||||||||
|
Cost related to license revenues
|
741
|
-
|
(741
|
)
|
||||||||
|
Amortization of intangible asset in respect of license revenues
|
646
|
-
|
(646
|
)
|
||||||||
|
Cost of product sales
|
68
|
34
|
(34
|
)
|
||||||||
|
Total cost of revenues
|
1,455
|
34
|
(1,421
|
)
|
||||||||
|
Three months ended
March 31,
|
||||||||||||
|
2024
|
2025
|
Increase
(decrease)
|
||||||||||
|
(in thousands of U.S. dollars)
|
||||||||||||
|
Research and development expenses
|
2,494
|
1,623
|
(871
|
)
|
||||||||
|
Three months ended
March 31,
|
||||||||||||
|
2024
|
2025
|
Increase
(decrease)
|
||||||||||
|
(in thousands of U.S. dollars)
|
||||||||||||
|
Sales and marketing expenses
|
6,342
|
-
|
(6,342
|
)
|
||||||||
|
Three months ended
March 31,
|
||||||||||||
|
2024
|
2025
|
Increase
(decrease)
|
||||||||||
|
(in thousands of U.S. dollars)
|
||||||||||||
|
General and administrative expenses
|
1,386
|
989
|
(397
|
)
|
||||||||
|
Three months ended
March 31,
|
||||||||||||
|
2024
|
2025
|
Increase
(decrease)
|
||||||||||
|
(in thousands of U.S. dollars)
|
||||||||||||
|
Non-operating income (expenses), net
|
4,490
|
7,644
|
(3,154
|
)
|
||||||||
|
Three months ended
March 31,
|
||||||||||||
|
2024
|
2025
|
Increase
(decrease)
|
||||||||||
|
(in thousands of U.S. dollars)
|
||||||||||||
|
Financial income
|
565
|
294
|
(271
|
)
|
||||||||
|
Financial expenses
|
(929
|
)
|
(420
|
)
|
509
|
|||||||
|
Net financial income (expenses)
|
(364
|
)
|
(126
|
)
|
238
|
|||||||
|
|
• |
the progress and costs of our preclinical studies, clinical trials and other research and development activities;
|
|
|
• |
the scope, prioritization and number of our clinical trials and other research and development programs;
|
|
|
• |
the amount of revenues we receive, if any, under our collaboration or licensing arrangements;
|
|
|
• |
the costs of the development and expansion of our operational infrastructure;
|
|
|
• |
the costs and timing of obtaining regulatory approval of our therapeutic candidates;
|
|
|
• |
our success in effecting out-licensing arrangements with third parties;
|
|
|
• |
the ability of our collaborators and licensees to achieve development milestones, marketing approval and other events or developments under our collaboration and out-licensing agreements;
|
|
|
• |
the costs of filing, prosecuting, enforcing and defending patent claims and other intellectual property rights;
|
|
|
• |
the costs and timing of securing manufacturing arrangements for clinical or commercial production;
|
|
|
• |
the costs of establishing sales and marketing capabilities or contracting with third parties to provide these capabilities for us;
|
|
|
• |
the costs of acquiring or undertaking development and commercialization efforts for any future therapeutic candidates;
|
|
|
• |
the magnitude of our general and administrative expenses;
|
|
|
• |
interest and principal payments on the loan from BlackRock;
|
|
|
• |
any cost that we may incur under current and future licensing arrangements relating to our therapeutic candidates; and
|
|
|
• |
market conditions.
|
|
Three months ended
March 31,
|
||||||||
|
2024
|
2025
|
|||||||
|
(in U.S. dollars)
|
||||||||
|
Earnings (loss) per ADS – basic and diluted
|
(0.38
|
)
|
1.40
|
|||||
|
Loss per ordinary share – basic and diluted
|
(0.00
|
)
|
0.00
|
|||||
|
December 31,
2024
|
March 31,
2025
|
|||||||
|
(in number of ADSs)
|
||||||||
|
Authorized share capital
|
8,333,333
|
8,333,333
|
||||||
|
Issued and paid-up capital
|
2,227,784
|
3,721,003
|
||||||