UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant
to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of report (Date of earliest event reported): March 5, 2025
Silexion Therapeutics Corp
(Exact name of registrant as specified in its charter)
|
Cayman Islands
|
|
001-42253
|
|
N/A |
|
(State or other jurisdiction
|
|
(Commission File Number)
|
|
(I.R.S. Employer
|
|
of incorporation)
|
|
|
|
Identification No.)
|
|
12 Abba Hillel Road Ramat-Gan, Israel |
|
5250606
|
|
(Address of principal executive offices)
|
|
(Zip Code)
|
Check the appropriate box below if the Form 8-K is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
|
Trading Symbol(s)
|
|
Name of each exchange on which
registered |
|
Ordinary Shares, par value $0.0009 per share
|
|
SLXN |
|
The Nasdaq Stock Market LLC
|
|
Warrants exercisable for Ordinary Shares at an exercise price of $103.50 per share
|
|
SLXNW
|
|
The Nasdaq Stock Market LLC
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
|
Item 7.01
|
Regulation FD Disclosure.
|
|
Item 9.01
|
Financial Statements and Exhibits
|
|
|
|
|
|
|
||
|
|
|
|
|
104
|
|
Cover Page Interactive Data File (formatted in Inline XBRL)
|
|
|
SILEXION THERAPEUTICS CORP
|
|
|
|
|
|
|
Date: March 5, 2025
|
/s/ Ilan Hadar
|
|
|
|
Name:
|
Ilan Hadar
|
|
|
Title:
|
Chief Executive Officer
|
|
|
• |
Initial validation using orthotopic models: SIL204 administered
subcutaneously (systemically) showed significant efficacy in orthotopic xenograft models where tumors grow in their native pancreatic environment, representing a more clinically relevant setting than our previous subcutaneous xenograft
models.
|
|
|
• |
Cell line-specific efficacy profiles: SIL204 showed robust
activity across multiple pancreatic cancer cell lines with different KRAS mutation profiles:
|
|
|
o |
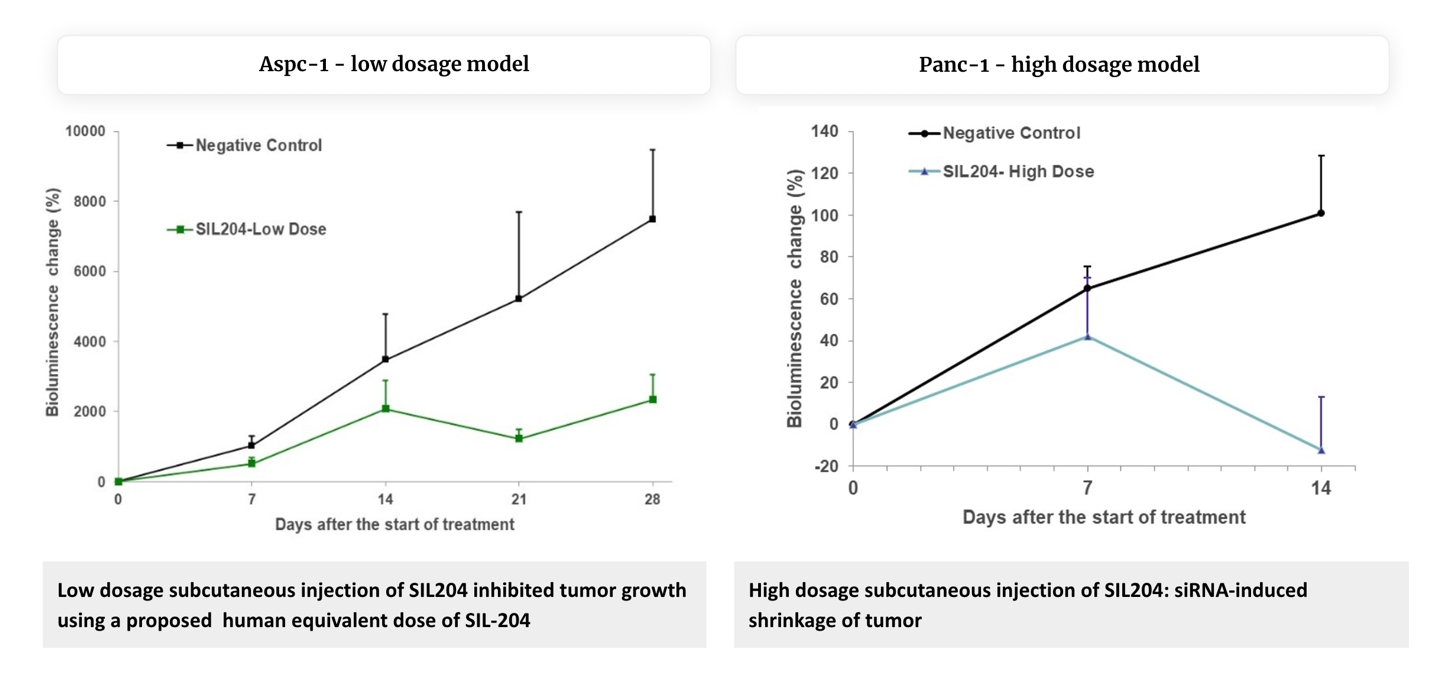
In AsPC-1 (harboring KRAS G12D mutation): ~70% reduction in overall bioluminescence (an indication of tumor cell number) as compared to the control group, by day 28.
|
|
|
o |
In Panc-1 (harbouring KRAS G12D mutation): Bioluminescence or tumor cell numbers decreased dramatically in a dose-dependent manner, with the highest-dose group showing
the most significant effect. Bioluminescence in the control group increased by approximately more than 100% while in the SIL204 treated group it decreased by 12% compared to baseline, a substantial reduction by day 14 compared with the
control group (P-value < 0.05), demonstrating the significant therapeutic impact of SIL204.
|
|
|
o |
In BxPC-3 (additional KRAS wild-type model): ~80% reduction in overall bioluminescence, as compared to the control group, by day 28.
|
|
|
• |
Metastasis reduction demonstrated for the first time: SIL204
treatment significantly reduced metastatic spread to secondary organs; substantially lowering metastatic burden across the liver, intestine, spleen and stomach in the two models checked for the various organs, Panc-1 and BxPC-3 models.
|
|
|
• |
Initial validation for systemic delivery efficacy: Subcutaneous
administration of SIL204 proved effective in reaching and treating pancreatic tumors and their metastases, confirming systemic delivery as a viable administration route.
|