|
FORM 20-F
|
||
|
|
|
|
|
☐
|
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
☒
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
☐
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
☐
|
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Title of each class
|
Trading symbol(s)
|
Name of each exchange on which registered
|
|
Ordinary shares, par value NIS 0.02 per share
|
EVGN
|
Nasdaq Global Market
|
|
Large accelerated filer ☐
|
Accelerated filer ☐
|
|
Non-accelerated filer ☒
|
Emerging Growth Company ☐
|
|
U.S. GAAP ☐
|
International Financial Reporting Standards as issued by the International Accounting Standards Board ☒
|
Other ☐
|
| 4 | ||
| 6 | ||
| 7 | ||
|
PART I |
||
| 9 | ||
| 9 | ||
| 9 | ||
| 38 | ||
| 65 | ||
| 65 | ||
| 81 | ||
| 98 | ||
| 101 | ||
| 102 | ||
| 103 | ||
| 112 | ||
| 113 | ||
|
|
|
|
|
PART II |
|
|
| 113 | ||
| 113 | ||
| 114 | ||
| 114 | ||
| 114 | ||
| 115 | ||
| 115 | ||
| 115 | ||
| 115 | ||
| 116 | ||
| 116 | ||
| 116 | ||
| Disclosure Regarding Foreign Jurisdictions that Prevent Inspections | 116 | |
| Insider Trading Plans | 116 | |
| Cybersecurity | 117 | |
|
PART III |
||
| 117 | ||
| 117 | ||
| 118 | ||
|
|
119 | |
| F-1 | ||
|
|
◾ |
references to “Evogene,” “we,” “us,” “our,” “our company” and “the
company” refer to Evogene Ltd. and its consolidated subsidiaries, consisting of AgPlenus Ltd., or AgPlenus, Biomica Ltd., or Biomica,
Canonic Ltd., or Canonic, Casterra Ag Ltd., or Casterra, Evogene Inc., Lavie Bio Ltd., or Lavie Bio, and their consolidated subsidiaries;
|
|
|
◾ |
references to “U.S. dollars,” “USD,” “$” or “dollars” are to United States dollars;
|
|
|
◾ |
references to “NIS” or “shekels” are to New Israeli Shekels; |
|
|
◾ |
references to the “U.S.” are to the United States; |
|
|
◾ |
references to “ordinary shares,” “our shares” and similar expressions refer to our Ordinary Shares, par value
NIS 0.02 per share; |
|
|
◾ |
references to the “articles of association” are to our Amended and Restated Articles of Association, which became effective
upon the closing of the U.S. initial public offering, as subsequently amended; |
|
|
◾ |
references to the “Companies Law” are to the Israeli Companies Law, 5759-1999, as amended; |
|
|
◾ |
references to the “Securities Act” are to the Securities Act of 1933, as amended; |
|
|
◾ |
references to the “Exchange Act” are to the Securities Exchange Act of 1934, as amended; |
|
|
◾ |
references to the “NYSE” are to the New York Stock Exchange; |
|
|
◾ |
references to the “Nasdaq” are to the Nasdaq Stock Market LLC or the Nasdaq Global Market; |
|
|
◾ |
references to the “TASE” are to the Tel Aviv Stock Exchange; and |
|
|
◾ |
references to the “SEC” are to the United States Securities and Exchange Commission. |
|
|
◾ |
our expectations regarding our revenue, expenses and other operating results; |
|
|
◾ |
whether we or our subsidiaries are able to raise capital on commercially reasonable terms to sustain the financial condition of each
respective entity; |
|
|
◾ |
our potential strategic alternatives with respect to Canonic, including a potential transfer of Canonic
to a third party; |
|
|
◾ |
the extent to which we continue to maintain our holdings in our subsidiary companies; |
|
|
◾ |
the extent to which our discoveries and product candidates will have the desired effect so as to reach the stage of commercialization;
|
|
|
◾ |
whether we are able to achieve commercialization of our product candidates; |
|
|
◾ |
whether we and our collaborators are able to allocate the resources needed to develop commercial products from our discoveries and
product candidates; |
|
|
◾ |
the length and degree of complexity of the process of our developing commercial products based on our discoveries and product candidates
and the probability of our success, and the success of our collaborators, in developing such products; |
|
|
◾ |
whether we are able to efficiently produce and scale up the production of our products, whether ourselves or through third party
contractors, to achieve our commercialization targets; |
|
|
◾ |
the degree of success of third parties upon whom we rely to conduct certain activities, such as field-trials and pre-clinical studies;
|
|
|
◾ |
whether we and our subsidiaries are able to comply with applicable law and the associated regulatory requirements that currently
apply or become applicable to each respective business; |
|
|
◾ |
the extent of the future growth of the agriculture, human health and industrial application industries in which we operate;
|
|
|
◾ |
whether we can maintain our current business models; |
|
|
◾ |
the actual commercial value of our key product candidates; |
|
|
◾ |
whether we or our collaborators receive regulatory approvals for the product candidates developed by us or our collaborators;
|
|
|
◾ |
whether milestones are met by us or by our collaborators with respect to our product candidates that generate revenues and whether
products containing or based on our discoveries are commercialized and generate revenues or royalties; |
|
|
◾ |
whether we are able to recruit, retain and develop knowledgeable or specialized personnel to perform our research and development
work; |
|
|
◾ |
the degree of our success at adapting to the continuous technological changes in our industries; |
|
|
◾ |
whether we can maintain our collaboration agreements with our current collaborators or enter into new collaboration agreements and
expand our research and development to new fields; |
|
|
◾ |
whether we can improve our existing, or develop and launch new, computational technologies and screening and validation systems;
|
|
|
◾ |
whether we can patent our discoveries and protect our trade secrets and proprietary know-how; and |
|
|
◾ |
the current war between Israel and Hamas and any worsening of the situation in Israel such as further mobilizations or escalation
in the northern border of Israel. |
|
|
◾ |
We have a history of operating losses and negative cash flow, and may never achieve or maintain profitability. Various factors may
delay, hinder, or prevent achievement of research and development milestones and commercialization of our product candidates. Moreover,
we may experience difficulties in collecting royalties or never receive them, potentially resulting in costly litigation and loss of reputation.
|
|
|
◾ |
We may need substantial additional capital in the future which may dilute our shareholders. Additionally, subsidiary financings have
diluted, and may continue to dilute, our equity holdings in our subsidiary companies, which will likely negatively impact and/or decrease
our results of operations, including revenues, and the benefits of the value that may be created in such subsidiary companies. Additionally,
we may need to finance the cost of the development of our independent product candidates ourselves. |
|
|
◾ |
Our discoveries and product candidates may not result in commercially viable products. In addition, our product development cycle
is lengthy and uncertain and various factors may delay or prevent commercialization of our product candidates. We may never sell or earn
royalties on the sale of commercial products based on our discoveries. |
|
|
◾ |
If we are unable to maintain our Computational Predictive Biology, or CPB, platform and its technological engines, our research and
development activities may be substantially reduced. |
|
|
◾ |
Failure to efficiently produce and scale our products, whether in-house or through contractors, could hinder our commercialization
goals. Furthermore, we or our collaborators may fail to meet obligations under the collaboration agreements. |
|
|
◾ |
We depend on a few collaborators to develop and commercialize product candidates. A reduction in research spending by key companies
in our target markets could threaten our collaborations, affecting their continuation or expansion and hindering our ability to form new
collaborations. |
|
|
◾ |
We are operating in multiple industries, each of which consists of multiple companies with much greater resources than us. If we
are unable to compete effectively, our financial resources will be diluted and our financial results will suffer. |
|
|
◾ |
Our efforts to develop and commercialize any of our products may be unsuccessful. |
|
|
◾ |
If Lavie Bio is unable to establish successful marketing distribution and/or retail channels for the commercialization of its products,
it will not be able to meet its commercialization plans. |
|
|
◾ |
We may fail to attract, recruit, retain and develop qualified employees, which could materially and adversely impact our business,
financial condition and results of operations. |
|
|
◾ |
Our business is regulated by government agencies. Failure to obtain necessary approvals could halt our operations. Changes in laws
and regulations may raise costs, reduce revenues, and disrupt operations. Dual reporting requirements in Israel and the U.S. may increase
compliance costs and distract management. |
|
|
◾ |
Disruption to our information technology and systems could adversely affect our reputation and future demand for our products or
collaborative relationships. |
|
|
◾ |
We currently need, and in the future we may need, to obtain licenses of third-party technology that may not be available to us or
are available only on commercially unreasonable terms. |
|
|
◾ |
Our licenses granted to our collaborators may limit our opportunities to enter into additional licensing or other arrangements.
|
|
|
◾ |
We might face significant liabilities from product liability, warrant liability or personal injury claims and litigation. Our involvement
in the medical cannabis sector brings legal and reputational risks. Additionally, our operations involve health and environmental hazards
due to handling toxic materials. |
|
|
◾ |
Ending leases, altering terms, or being locked into long-term leases may threaten our operations and significantly impact our financial
status or performance. |
|
|
◾ |
Lavie Bio’s research and development, or R&D, facility in the U.S., our contracts with foreign businesses and any other
current or future operations outside of Israel expose us to additional market and operational risks. |
|
|
◾ |
Growing cycles and adverse weather conditions may decrease our results from operations. |
|
|
◾ |
Our success depends on our ability to protect our intellectual property and our proprietary technologies. Any change to the patent
laws in applicable jurisdictions may impair our ability to protect our product candidates. |
|
|
◾ |
If we or one of our collaborators are sued for infringing the intellectual property rights of a third party, such litigation could
be costly and time consuming and could prevent us or our collaborators from developing or commercializing our product candidates.
|
|
|
◾ |
We may be required to pay royalties to employees who develop inventions that have been or will be commercialized by us. |
|
|
◾ |
Our agreements with our employees and with third parties may not adequately prevent disclosure of trade secrets, know-how and other
proprietary information. In addition, we may not be able to fully enforce covenants not to compete with our key employees. |
|
|
◾ |
Conditions in Israel could adversely affect our business, including (i) the recent attack by Hamas and other terrorist organizations
from the Gaza Strip and elsewhere in the region and Israel’s war against them as well as the war’s potential impact on our
business and operations and (ii) the military hostilities with the Hezbollah organization in the Northern border of Israel. |
|
|
◾ |
Exchange rate fluctuations between the U.S. dollar and the NIS may negatively affect our financial results and interest rate fluctuations
may negatively affect our financial results, financial condition, or investments. |
|
|
◾ |
The terms of our Israeli government grants may require us to satisfy specified conditions in order to manufacture products and transfer
technologies supported by such grants outside of Israel. |
|
|
◾ |
Your rights and responsibilities as a shareholder are under Israeli law, potentially differing from those of U.S. corporations. Israeli
law might hinder or discourage acquisitions of our shares or assets. |
|
|
◾ |
The price of our ordinary shares may fluctuate significantly. Further, there is no guarantee of a continuing public market to resell
our ordinary shares. In addition, our ordinary shares are traded on more than one market and this may result in price variations.
|
|
|
◾ |
The requirements of being a public company in the U.S. and Israel may strain our resources and distract our management, which could
make it difficult to manage our business. |
|
|
◾ |
U.S. shareholders owning at least 10% of our ordinary shares may face adverse federal income tax consequences. We were a passive
foreign investment company, or PFIC, for U.S. tax purposes in 2023, and there is a risk of being classified as a PFIC in 2024, potentially
leading to adverse tax consequences for U.S. shareholders. |
|
|
◾ |
Any inability to meet the Nasdaq listing requirements may have an adverse effect on our share price and lead to our delisting from
Nasdaq. |
|
|
◾ |
If we fail to maintain effective internal control over financial reporting, the price of our ordinary shares may be adversely affected.
|
|
ITEM 1. |
IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS |
|
ITEM 2. |
OFFER STATISTICS AND EXPECTED TIMETABLE |
|
ITEM 3. |
KEY INFORMATION |
|
A. |
[Reserved] |
|
B. |
Capitalization and Indebtedness |
|
C. |
Reasons for the Offer and Use of Proceeds |
|
D. |
Risk Factors |
|
|
• |
delay, scale back or discontinue the development, manufacturing scale-up or commercialization of our or our subsidiaries’ product
candidate; |
|
|
• |
accept for one or more of our or our subsidiaries’ product candidates terms that are less favorable than might otherwise be
available; or |
|
|
• |
relinquish or license to additional parties, on unfavorable terms, our rights to our or our subsidiaries’ product candidates
that we or our subsidiaries otherwise would seek to develop or commercialize ourselves. |
|
|
◾ |
our discoveries and product candidates may not be successfully validated or may not have the desired effect required in order to
become, or to be incorporated into, commercial products; |
|
|
◾ |
the process of developing product candidates based on our discoveries is lengthy and expensive, and we or our collaborators may not
be able to allocate the resources needed to complete such development within the desired timeline; |
|
|
◾ |
we or our collaborators may decide to discontinue, pause, reduce, or alter the scope of the development efforts for our product candidates;
|
|
|
◾ |
we may fail to satisfy, in a timely manner or at all, relevant milestones under our agreements with our collaborators; |
|
|
◾ |
regulatory conditions related to our product candidates may change in different territories, thus negatively affecting the relevant
development processes and extending their length or limiting the commercialization of such product candidates; |
|
|
◾ |
we or our collaborators may be unable to obtain the requisite regulatory approvals for product candidates based on our discoveries;
|
|
|
◾ |
our competitors may launch competing or more effective products; |
|
|
◾ |
we or our collaborators may be unable to fully develop and commercialize product candidates containing our discoveries or may decide,
for whatever reason, not to commercialize, or to delay the commercialization of, such product candidates; |
|
|
◾ |
a market may not exist for products containing our discoveries or such products may not be commercially successful or relevant;
|
|
|
◾ |
we may be unable to protect the intellectual property underlying our discoveries in the necessary jurisdictions; and |
|
|
◾ |
we may encounter production and scale-up challenges with respect to our product candidates that hinder their commercialization.
|
|
|
◾ |
we or our collaborators may not be able to allocate the resources needed to develop product candidates based on our discoveries;
|
|
|
◾ |
we or our collaborators may revise the process of product development or make other decisions regarding the product development pipelines
that may extend the development period; |
|
|
◾ |
we or our collaborators may prioritize other development activities ahead of development activities with respect to the product candidates
on which we collaborate; |
|
|
◾ |
our discoveries may not be successfully validated or may not have the desired effect sought by us or by our collaborators; and
|
|
|
◾ |
we or our collaborators may be unable to obtain the requisite regulatory approvals for the product candidates based on our discoveries
within expected timelines or at all. |
|
|
◾ |
failure to establish the requisite infrastructure to enable the discovery and development of microbial bio-stimulants; |
|
|
◾ |
failure to identify and develop microbial candidates that enhance plant performance at the desired efficacy and stability;
|
|
|
◾ |
failure to successfully complete development of microorganisms to achieve cost-effective and commercially viable products;
|
|
|
◾ |
failure to obtain and maintain patent and trade secret protection for its product candidates; |
|
|
◾ |
failure to operate without infringing or violating the valid and enforceable patents or other intellectual property rights of third
parties; |
|
|
◾ |
inability to obtain sufficient funding to fully execute its business plan; |
|
|
◾ |
failure to meet regulatory requirements; |
|
|
◾ |
failure to establish efficient and reliable production and scale up capabilities of Lavie Bio’s products through third party
contractors; and |
|
|
◾ |
failure to establish cost-effective go-to-market models for selling its products. |
|
|
◾ |
failure of its relatively novel target-based approach to lead to an effective product candidate or failure to identify chemical compounds
that will display required level of performance; |
|
|
◾ |
failure to establish cost-effective production of AgPlenus’ product candidates; |
|
|
◾ |
failure to obtain and maintain patent and trade secret protection for its product candidates; |
|
|
◾ |
failure to operate without infringing or violating the valid and enforceable patents or other intellectual property rights of third
parties; |
|
|
◾ |
inability to obtain sufficient funding to fully execute its ag-chemical business plan; |
|
|
◾ |
one of our main research molecules suppliers is located in Ukraine, and has had, and may have in the future, limitations in access
to molecules since the war in Ukraine, although such supplier has an alternative production site, and it is not our only supplier for
research molecules; |
|
|
◾ |
failure to meet regulatory requirements; and |
|
|
◾ |
increase in regulatory requirements and limitations of use in various geographies on the use of ag-chemicals might decrease the potential
market size for AgPlenus ag-chemical candidates for products. |
|
|
◾ |
failure to identify and develop candidate genomic elements having the desired effect on the target trait in the plant of interest;
|
|
|
◾ |
failure to identify and develop toxin candidates having the desired effect on the target insects when inserted into the plants of
interest; |
|
|
◾ |
failure to obtain and maintain patent and trade secret protection for our product candidates; |
|
|
◾ |
failure to operate without infringing or violating the valid and enforceable patents or other intellectual property rights of third
parties; |
|
|
◾ |
inability to obtain sufficient funding to fully execute the business plan; |
|
|
◾ |
failure to successfully complete development of our seed trait product candidates; and |
|
|
◾ |
our failure to meet regulatory requirements for seed trait and pest control product candidates. |
|
|
◾ |
failure to complete pre-clinical studies and clinical trials with positive results in which the FDA agrees with the design,
endpoints or implementation; |
|
|
◾ |
failure to receive regulatory approvals or authorizations for conducting our planned clinical trials or future clinical trials;
|
|
|
◾ |
failure to obtain sufficient financing for the development and commercialization of its product candidates; |
|
|
◾ |
failure to obtain and maintain patent and trade secret protection and regulatory exclusivity for its product candidates; |
|
|
◾ |
interruption of, or delays in receiving, supplies of our product candidates from our contract manufacturing organizations due to
staffing shortages, production slowdowns or stoppages and disruptions in delivery systems; |
|
|
◾ |
failure to launch commercial sales of its products, if and when approved, whether alone or in collaboration with others; |
|
|
◾ |
failure to enter into new collaborations throughout the development process as appropriate, from pre-clinical studies through to
commercialization; |
|
|
◾ |
failure to achieve acceptance of its products, if and when approved, by patients, the medical community and third-party payors;
|
|
|
◾ |
failure to effectively compete with companies developing and commercializing other therapies for the indications that Biomica’s
product candidates target; |
|
|
◾ |
failure to obtain and maintain coverage and adequate reimbursement by third-party payors, including government payors, for its products,
if approved; |
|
|
◾ |
failure to protect its rights in its intellectual property portfolio; |
|
|
◾ |
failure to operate without infringing or violating the valid and enforceable patents or other intellectual property rights of third
parties; |
|
|
◾ |
failure to maintain a continued acceptable safety profile of the products following approval; and |
|
|
◾ |
failure to maintain and develop an organization of scientists and business people who can develop and commercialize its products
and technology. |
|
|
◾ |
failure to reach desired yields of its castor seed varieties on a commercial scale to secure economic viability as bio-based oil
feedstock; |
|
|
◾ |
failure to establish an efficient mechanical harvest solution; |
|
|
◾ |
failure to establish a cost-effective production of castor bean grains, allowing grower profitability; |
|
|
◾ |
failure to reach large scale adoption of castor by growers, including the successful management of diseases and pests; |
|
|
◾ |
failure to address the health and environmental risks posed by castor bean seeds, which contain ricin, a naturally occurring poison;
|
|
|
◾ |
failure to comply with any regulatory requirement related to sales of castor beans, and in particular those related to the import
of such beans and the potential effects of ricin; |
|
|
◾ |
Our cultivation and agro-technical support activities in Africa and South America may be materially and adversely affected by an
economic slowdown, uncertainties with respect to the legal system and violent crime or terrorism in these regions; and |
|
|
◾ |
failure to establish efficient and reliable production and scale up capabilities of castor seeds, independently or through third
party contractors. |
|
|
◾ |
enhanced regulation in Israel with respect to our cannabis-related activities. In particular, Israeli regulation requires that we
obtain a specific permit for each of the following activities: research, propagation, cultivation, production, marketing and distribution
and use; |
|
|
◾ |
a lack of clarity with respect to the regulation of cannabis products for medical use purposes in Israel. The current regulatory
requirements in Israel may be subject to differing interpretations. This creates a risk of differing enforcement policies by the Israeli
authorities, which may change with or without notice. If the Israeli authorities begin to enforce laws relating to cannabis in a manner
that differs from the current interpretation of such laws, the Company’s business, results of operations, financial condition and
prospects and the Company generally may be materially adversely affected. Similarly, any changes in laws, regulations, guidelines, enforcement
policies, and/or interpretations by the relevant government authorities in jurisdictions where the Company may market and/or sell its
cannabis products in the future, may likewise result in significant additional compliance costs for us or limit our ability to operate
in certain jurisdictions; |
|
|
◾ |
certain banks will not accept deposits from or provide other bank services to businesses involved with cannabis; |
|
|
◾ |
third parties with whom we do business may perceive that they are exposed to reputational risk as a result of our cannabis-related
business activities and may ultimately elect not to do business with us; |
|
|
◾ |
certain investors or investment banks are reluctant to work with companies affiliated with activity in the cannabis industry;
|
|
|
◾ |
future sales of medical cannabis products may expose us to consumer complaints or legal claims with respect to product quality or
activity; and |
|
|
◾ |
increased premiums under our D&O liability insurance policies. |
|
|
◾ |
fluctuations in foreign currency exchange rates and rising global inflation; |
|
|
◾ |
potentially adverse tax consequences; |
|
|
◾ |
difficulties in staffing and managing foreign operations; |
|
|
◾ |
hiring and retention of employees and/or consultants under foreign employment laws which are not familiar to us; |
|
|
◾ |
laws and business practices that sometimes favor local business; |
|
|
◾ |
compliance with foreign legislation, being subject to laws, regulations and the court systems of multiple jurisdictions; and
|
|
|
◾ |
tariffs, trade barriers and other regulatory or contractual limitations on our ability to develop (and, when applicable in the future,
sell) our solutions in certain foreign markets. |
|
|
◾ |
impair or eliminate our ability to research and develop our product candidates, including validating our product candidates through
lab, greenhouse, field or clinical trials; |
|
|
◾ |
increase our compliance and other costs of doing business through increases in the cost to patent or otherwise protect our intellectual
property or increases in the cost to our collaborators to obtain the necessary regulatory approvals to commercialize and market the product
candidates we develop with them; |
|
|
◾ |
require significant product redesign or systems redevelopment; |
|
|
◾ |
render our product candidates less profitable, obsolete or less attractive compared to competing products; |
|
|
◾ |
affect our collaborators’ willingness to do business with us; |
|
|
◾ |
jeopardize import or export of raw material or end products, such as with respect to medical cannabis seeds, seedlings and products;
|
|
|
◾ |
reduce the amount of revenues we receive from our collaborators through milestone payments or royalties; and |
|
|
◾ |
discourage our collaborators from offering, and consumers from purchasing, products that incorporate our discoveries. |
|
|
◾ |
our inability to obtain additional funding; |
|
|
◾ |
any delay in filing a regulatory submission for any of our product or product candidates and any adverse development or perceived
adverse development with respect to the review of that regulatory submission by the applicable regulatory body; |
|
|
◾ |
actual or anticipated fluctuations in our results of operations; |
|
|
◾ |
variance in our financial performance from the expectations of market analysts; |
|
|
◾ |
announcements by us or our competitors of significant business developments, changes in relationships with our collaborators, acquisitions
or expansion plans; |
|
|
◾ |
our involvement in litigation; |
|
|
◾ |
our sale, or the sale by our significant shareholders, of ordinary shares or other securities in the future; |
|
|
◾ |
failure to publish research or the publishing of inaccurate or unfavorable research; |
|
|
◾ |
market conditions in our industry and changes in estimates of the future size and growth rate of our markets; |
|
|
◾ |
changes in key personnel; |
|
|
◾ |
the trading volume of our ordinary shares; and |
|
|
◾ |
general economic and market conditions, including as a result of the scope and duration of the war in Israel. |
| ITEM 4. |
INFORMATION ON THE COMPANY |
|
A. |
History and Development of the Company |
|
B. |
Business Overview |
|
|
1. |
Licensing: we grant time-limited licenses to third parties, our subsidiaries, or related entities, allowing them to leverage our
tech-engines for product development within specified commercial domains. |
|
|
• |
License fee and R&D reimbursement; |
|
|
• |
Dividends to Evogene as a shareholder; and |
|
|
• |
Significant one-time payment upon an exit event (in case Evogene is a main shareholder). |
|
|
2. |
Collaborations: we engage in collaborative ventures with industry leaders, pooling resources to drive joint product development.
Typically, our partners take the lead in later-stage development and commercialization, leveraging our unique tech-engines to identify
the product candidate and optimize it towards a commercial product. |
|
|
• |
Upfront payments; |
|
|
• |
R&D fees; and |
|
|
• |
Royalties from sales of end-products. |
|
|
◾ |
Horizon grant (May 2023) – Evogene’s Ag-Seed Division was granted €1.2 million through a Horizon grant to develop
high carbon dioxide, or CO2, assimilation and drought-tolerant
oil-seed crops. |
|
|
◾ |
Registered Direct Offering (July 2023) – On July 17, 2023 Evogene entered into a securities purchase agreement with certain
institutional investors pursuant to which we sold to such investors, 8,500,000 ordinary shares at $1.00 per share, generating $8,500,000
in gross proceeds. |
|
|
◾ |
Management change (March 2023) – Mr. Amit Noam assumed the role of Chief Executive Officer of Lavie Bio |
|
|
◾ |
Licensing agreement (July 2023) – Lavie Bio entered a licensing agreement with Corteva Agriscience LLC, or Corteva, for bio
fungicide lead candidates, granting Corteva exclusive rights to further develop and commercialize these candidates targeting fruit rots
and powdery mildew. The agreement includes an initial payment of approximately $5 million, potential future milestone payments, and royalties
from Corteva’s sales of the products. |
|
|
◾ |
Advancement (November 2023) – Lavie Bio reported advancement in its bio-fungicide program against downy mildew with 2023 field
trial results. |
|
|
◾ |
Financing round (April 2023) – Biomica closed a $20 million financing round led by Shanghai Healthcare Capital. |
|
|
◾ |
Commercial agreement (January 2023) – Casterra entered into a commercial-scale cultivation agreement with a leading energy
company. |
|
|
◾ |
Framework agreement (June 2023) – Casterra signed a framework agreement with a ENI Kenya B.V., or ENI, for the sale of castor
seeds for sustainable biofuel production, involving initial purchase orders of $9.1 million. |
|
|
◾ |
Purchase orders (June 2023) – Casterra received an additional $2.2 million of purchase orders to supply castor seeds for new
African territories. |
|
|
◾ |
Management change (December 2023) – Mr. Yoash Zohar will be appointed as Chief Executive Officer of Casterra effective as of
January 1, 2024. |
|
|
◾ |
Management change (July 2023) –Dr. Adrian Percy was appointed to AgPlenus’ Board of Directors. |
|
|
◾ |
Licensing and collaboration agreement (February 2024) – AgPlenus entered into a licensing and collaboration agreement with
Bayer AG, for the development of a new sustainable weed control solution. |
|
|
◾ |
Management change (February 2024) – Mr. Dan Jacob Gelvan was appointed as Chief Executive Officer effective as of February
19, 2024. |
|
|
◾ |
New Products (February 2023) – Canonic launched six second-generation cannabis products with higher THC and rich terpene profiles.
|
|
|
◾ |
(May 2023) – Plantis Agro Ltd. licensed two of Canonic’s proprietary cannabis varieties to expand its offerings in the
Israeli market. |
|
|
(i) |
Direct sales model – in fragmented markets Lavie Bio expects to complete product development
of its products independently, while establishing a tailored market access strategy per specific product and territory, such as commercialization
through distribution channels. Under this model, the production of Lavie Bio’s products is achieved through third party toll manufacturers.
Revenues may include sales to distributors. Under the direct sales model, Lavie Bio has sold its inoculant Yalos™ (formerly known
as Thrivus™) in the U.S. for spring wheat growers. |
|
|
(ii) |
Collaboration model – Lavie Bio offers tailored
solutions to potential partners. In this model, Lavie Bio’s partner produces and commercializes the products being developed. Lavie
Bio’s revenues in such engagements may include research and development payments, payments upon achievement of development milestones
and royalties. The scope of collaboration may differ. The typical model is that Lavie Bio develops a product until it is ready for commercialization,
and the partner is responsible for the production and commercialization of the product. This model was used in the agreement with Corteva
that was signed in July 2023, where Corteva received a license to Lavie Bio’s bio-fungicide product targeting fruit rots in grapes
and other high value crops. Another model is when the collaboration starts in a much earlier development phase, where Lavie Bio would
typically commence with candidate strains discovery and development, followed by co-development with the partner towards commercialization.
Lavie Bio’s collaboration with ICL is an example of this broader collaboration model. |
|
|
◾ |
Discovery: The identification of a candidate microbial strain, or microbial
strain teams, having the potential to improve the target trait and the potential to achieve other product requirements such as consistency
and commercial viability. A collection of selected microbial candidates is typically tested on the crop(s) of choice in greenhouse screens
or limited field experiments for various efficacy, consistency and commercial viability criteria. Candidates that meet the testing criteria
are referred to as “Hits”. Typically, based on Lavie Bio’s experience, the duration of the discovery phase is approximately
12-18 months. |
|
|
◾ |
Pre-development: Promising Hits are advanced to pre-development phase,
in order to further assess and optimize performance criteria such as shelf life stability, efficacy and consistency. Successfully performing
microbial candidates are referred to as “Advanced Hits”. Typically, based on Lavie Bio’s experience, the duration of
this phase is approximately 12-18 months. |
|
|
◾ |
Development: This phase is usually divided into Development Stage 1, resulting
with a “Lead”, and Development Stage 2, resulting with a “Pre-Product”. In this phase, the fermentation and formulation
procedures are further optimized to allow for further testing and validation of efficacy and consistency in the field as well as for commercial
viability at the scale production, addressing cost of goods targets and compatibility with other agricultural inputs. Based on industry
benchmarks and its experience, Lavie Bio estimates the duration of this stage to be approximately 24 months. |
|
|
◾ |
Pre-commercialization: In this phase, extensive field tests are undertaken
to demonstrate the effectiveness of product candidates in enhancing the target trait, including production of data to support product
positioning. Additional activities towards launch are performed, including packaging development, upscale manufacturing protocol, registration
and regulation. Based on industry benchmarks and its experience, Lavie Bio estimates the duration of this stage in the U.S. to be approximately
24 months for bio-stimulants and 36-48 months for bio-pesticides due to longer regulation processes. |
|
◾ |
Commercial:
After initial commercialization of a product, different scale-up activities are undertaken, such as production under toll-manufacturing
agreements and deployment of end-product at point of sale. Toll manufacturing involves development of production protocols for large fermentation
vessels and down-stream-process protocol with the toll manufacturer. In addition, the product is examined for potential market expansion
to new crops and against additional diseases. |

|
|
◾ |
Identification of Targets – identification and validation of vital targets or proteins that when inhibited (for instance by
a chemical), lead to weed, insect or fungi death. |
|
|
◾ |
Identification of Hits – screening of chemical compounds for the identification of candidate Hits that potentially inhibit
identified vital targets and are capable of achieving the desired impact on the weeds, insects or fungi of interest. The discovery process
includes in-silico as well as biological screening and validation activities. |
|
|
◾ |
Hit-to-Lead process – Hits displaying confirmed activity in the initial validation screens will enter the Hit-to-Lead process,
including several optimization cycles, each constructed of compound design (in our case, focusing on computational optimization), synthesis
of compounds and validation experiments. This stage ends with a ‘Lead’ compound, which is a validated Hit that has confirmed
activity in advanced validation screens proving field translation in initial trials. |
|
|
◾ |
In this stage, multiple field trials are conducted in diverse geographies, as well as greenhouse experiments on resistant weed biotypes
and on commercial crops, and the compound structure and formulation are finalized. Lead optimization also entails initial toxicology tests,
process engineering on the molecule and a significantly detailed cost of goods analysis. |
|
|
◾ |
In this stage, field trials to validate all commercial cases are conducted, including testing product mixtures, as well as additional
safety trials. This stage ends with a ‘Pre-Development’ compound. |
|
|
◾ |
In the final development phases, new chemical products are registered with the proper regulatory authorities in relevant territories
and then launched for commercialization. We expect that these last stages of development will be conducted by our collaboration partners
or licensees of our product candidates. |

|
|
◾ |
Discovery: The identification of target genetic elements for enhancing
specified plant traits. We test these elements in different validation systems to determine their ability to enhance the specified trait.
In our experience, the Discovery phase takes approximately 6-18 months. The target genetic elements may be applicable to product development
through different technological approaches (i.e. genome editing, GM or advanced breeding). In our collaborations, we typically undertake
this phase. |
|
|
◾ |
Phase I, or “Proof of Concept”: Validated candidate genetic
elements are advanced to Phase I. In this phase, they are tested in target plants through greenhouse trials, field trials, or both, for
their efficacy in improving plant performance. Phase I may be conducted by us or by our collaborators, and in our experience, may last
between two and five years for a GM product or, three years for a genome editing or advanced breeding product. For products developed
through genome editing, deregulation process for classifying a product as non-GM is typically initiated during Phase I. |
|
|
◾ |
Phase II, or “Early Development”: In this phase, the field
tests are expanded, and our collaborators evaluate the genetic elements on multiple geographical locations and varieties, to reach commercially
viable success rates. We estimate the duration of Phase II is between two to four years. For a GM product, by the end of this phase, a
specific product candidate will be selected to advance to Phase III. For genome editing and advanced breeding products, the end of this
phase will lead straight to Phase IV (Pre-Launch). |
|
|
◾ |
Phase III, or “Advanced Development and Regulation”: This
phase is relevant only for the development of GM products. Extensive field trials are performed to test the effectiveness of the selected
product candidate across locations, and regulatory approvals are obtained, including potential environmental impact assessments, toxicity
and allergenicity. We estimate the duration of Phase III is between one to two years. |
|
|
◾ |
Phase IV, or “Pre-Launch”: This phase involves preparation
for commercial launch. The range of activities here includes preparing the seeds for commercial sales, formulation of a marketing strategy
and preparation of marketing materials. We estimate the duration of Phase IV is between one to two years. |
|
Program |
Crop |
Technology |
Collaborator |
Development Phase |
|
1 |
Corn |
GM |
Bayer |
Phase I – at collaborator under license. |
|
2 |
Canola and rapeseed |
GM |
As part of Crop4Clima consortium |
Phase I |
|
Program |
Crop |
Trait |
Technology |
Collaborator |
Development Phase |
|
1 |
Corn |
Fusarium |
GM & genome editing |
Bayer |
Undisclosed. At collaborator under license. |
|
2 |
Soybean |
Nematodes |
Genome editing |
TMG |
Discovery |
|
|
◾ |
Irritable Bowel Syndrome (IBS) is a common disorder that affects the large intestine.
Signs and symptoms include cramping, abdominal pain, bloating, gas, and diarrhea or constipation, or both. It is estimated that the total
market for IBS reached $1.5 billion in 2018, with 45 million patients in the U.S. alone and is expected to reach $3.3 billion in 2026,
according to a report titled “Irritable Bowel Syndrome Treatment Market Size, Share & Analysis Report By Type,” the 2019-2026
segment and an article in PR Newswire, dated July 23, 20197,
both of which are not incorporated by reference herein. Existing drugs for IBS mainly treat the symptoms of the condition, leaving patients
exposed to cycles of remission and relapse that characterize this chronic condition. |
|
|
◾ |
IBD is a group of GI diseases, mainly comprised of Ulcerative colitis and Crohn’s disease.
IBDs cause long term chronic as well as severe inflammation in the gastrointestinal tract without any known cause. According to the Centers
for Disease Control and Prevention, or CDC, in 2015 an estimated 3.1 million people (1.3% of the entire population) in the United States
were diagnosed either with Crohn’s disease or with Ulcerative Colitis. According to a report published by Allied Market Research
in July 2022, which is not incorporated by reference herein, the global IBD drug market size was valued at $21 billion in 2021, and is
projected to reach $34.5 billion by 2031, growing at a compound annual growth rate, or CAGR, of 5.1% from 2022 to 20318.
|
|
|
◾ |
Clostridium Difficile Infection (CDI) – The U.S. Centers for Disease Control and Prevention
in a report titled “Antibiotic Resistance Threats In The United States” published in December 20199,
which is not incorporated by reference herein, has identified CDI as one of the most urgent antibiotic-resistant bacterial threats in
the United States. CDI is most often caused by the use of broad-spectrum antibiotics which induce dysbiosis of the microbiome causing
susceptibility to infection by C. difficile, a spore forming bacterium. It is the most common cause of hospital acquired infection in
the United States. |
|
|
◾ |
Methicillin-Resistant Staphylococcus Aureus (MRSA) – One of the most common Staphylococcus
aureus infections is caused by MRSA, which is a multi-drug resistant bacterium, responsible for several difficult-to-treat infections
in humans, leading to tens of thousands of annual cases of mortality in the U.S. MRSA is the leading causative agent for hospital acquired
infections and has recently been documented as community-acquired as well as livestock-acquired. Current medical treatments include broad
spectrum antibiotics that are becoming increasingly ineffective. Bloomberg estimates according to a report published on September 24,
2019, which is not incorporated by reference herein, that the current MRSA market was valued at approximately $922 million in 2018 and
is projected to reach over $1.3 billion by 202611.
|
|
|
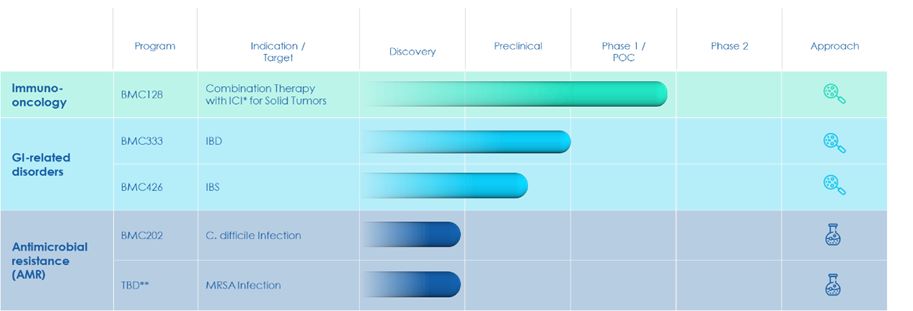
◾ |
At the taxonomic level Biomica's analysis allows strain-level resolution and relies on an extensive proprietary strain database.
|
|
|
◾ |
At the functional level, Biomica's proprietary resources rely on a comprehensive catalog of microbial genes enabling mapping of an
average of 90% of the functions of the human gut microbiome obtained through metagenomics sequencing. |
|
|
• |
Synergy - marketed under the T20/C4 category (16%-24% THC 0%-7% CBD), as defined by the Israeli Ministry of Health;
|
|
|
• |
Combo - marketed under the T20/C4 category (16%-24% THC 0%-7% CBD), as defined by the Israeli Ministry of Health;
|
|
|
• |
Mash Kush - marketed under the T20/C4 category (16%-24% THC 0%-7% CBD), as defined by the Israeli Ministry of Health;
|
|
|
• |
Mosaic - marketed under the T20/C4 category (16%-24% THC 0%-7% CBD), as defined by the Israeli Ministry of Health;
|
|
|
• |
Two Stars - marketed under the T20/C4 category (16%-24% THC 0%-7% CBD), as defined by the Israeli Ministry of Health;
and |
|
|
• |
Blend Kush - marketed under the T20/C4 category (16%-24% THC 0%-7% CBD), as defined by the Israeli Ministry of Health.
|
|
|
• |
Two Aces - marketed under the T20/C4 category (16%-24% THC 0%-7% CBD), as defined by the Israeli Ministry of Health;
|
|
|
• |
Southside - marketed under the T20/C4 category (16%-24% THC 0%-7% CBD), as defined by the Israeli Ministry
of Health. |
|
|
• |
Tango – marketed under the T20/C4 category (16%-24% THC 0%-7% CBD), as defined by the Israeli Ministry of Health.
|
|
C. |
Organizational Structure |
|
Name
of Subsidiary |
Jurisdiction
|
Ownership
Interest | ||
|
AgPlenus Ltd. |
Israel |
98.3% (1)
| ||
|
Biomica Ltd. |
Israel |
75.8% (2)
| ||
|
Canonic Ltd. |
Israel |
100% | ||
|
Casterra Ag Ltd. (formerly
known as Evofuel Ltd.). |
Israel |
100% | ||
|
Lavie Bio Ltd. |
Israel |
70.7% (3)(4)
|
|
|
(1) |
The remaining 1.7% of AgPlenus Ltd.’s issued and outstanding share capital is held by AgPlenus’ former Chief Executive
Officer and current director as a result of exercise of options. |
|
|
(2) |
The remaining 24.2% of Biomica Ltd.’s issued and outstanding share capital is held by: (i) SHC, who holds 22.7%, and (ii) Biomica's
Chief Technology Officer, who holds 1.5%. For more information see “Item 4.B. Information on the Company—Business Overview—Market
Segments—Human Health—Biomica Ltd.—Overview”. |
|
|
(3) |
The remaining 29.3% of Lavie Bio Ltd.’s issued and outstanding share capital is held by (i) Pioneer Hi-Bred International,
Inc. (also known by the name Corteva), who holds 27.3%, and (ii) Lavie Bio’s former employees, who hold 2.0% as a result of exercise
of options. |
|
|
(4) |
ICL (through an affiliate company) has an outstanding convertible amount of $10 million invested in Lavie Bio Ltd. under a SAFE agreement,
which is convertible into shares of Lavie Bio Ltd. pursuant to the terms thereof. For more information see “Item 4.B. Information
on the Company—Business Overview—Market Segments—Agriculture—Lavie Bio Ltd.—Overview”. |
|
D. |
Property, Plants and Equipment |
|
ITEM 4A. |
Unresolved Staff Comments |
| ITEM 5. |
OPERATING AND FINANCIAL REVIEW AND PROSPECTS |
|
Operating Segment: |
2023 |
2022 |
2021 |
|||||||||
|
(U.S. dollars, in thousands) |
||||||||||||
|
Agriculture |
$ |
3,791 |
$ |
876 |
$ |
628 |
||||||
|
Industry |
1,075 |
72 |
40 |
|||||||||
|
Human |
487 |
513 |
183 |
|||||||||
|
Unallocated |
287 |
214 |
79 |
|||||||||
|
Total |
$ |
5,640 |
$ |
1,675 |
$ |
930 |
||||||
|
Geographical Region: |
2023 |
2022 |
2021 |
|||||||||
|
United States |
65 |
% |
51 |
% |
56 |
% | ||||||
|
Israel |
16 |
% |
45 |
% |
38 |
% | ||||||
|
Brazil |
- |
- |
2 |
% | ||||||||
|
Other |
19 |
% |
4 |
% |
4 |
% | ||||||
|
Total |
100 |
% |
100 |
% |
100 |
% | ||||||
|
|
◾ |
Agriculture: our agriculture segment includes our division and subsidiaries engaged in agricultural
activities, including seed traits activity, ag-chemicals activity (now through our subsidiary AgPlenus) and ag-biologicals activity (now
through our subsidiary Lavie Bio). |
|
|
◾ |
Human Health: our human health segment focuses mainly on discovery
and development of human microbiome-based therapeutics (through our subsidiary Biomica). |
|
|
◾ |
Industrial Applications: our industrial applications segment focuses on the development and
commercialization of improved castor bean seeds for industrial uses (through our subsidiary Casterra). |
|
Agriculture |
Industrial Applications |
Human Health |
Unallocated |
Total |
||||||||||||||||
|
(in thousands) |
||||||||||||||||||||
|
Year ended December 31, 2023 |
||||||||||||||||||||
|
Revenues |
$ |
3,791 |
$ |
1,075 |
$ |
487 |
$ |
287 |
$ |
5,640 |
||||||||||
|
Operating loss |
$ |
(11,100 |
) |
$ |
(39 |
) |
$ |
(10,349 |
) |
$ |
(5,020 |
) |
$ |
(26,508 |
) | |||||
|
Year ended December 31, 2022 |
||||||||||||||||||||
|
Revenues |
$ |
876 |
$ |
72 |
$ |
513 |
$ |
214 |
$ |
1,675 |
||||||||||
|
Operating loss |
$ |
(12,256 |
) |
$ |
(220 |
) |
$ |
(8,875 |
) |
$ |
(5,590 |
) |
$ |
(26,941 |
) | |||||
|
Year ended December 31, 2021 |
||||||||||||||||||||
|
Revenues |
$ |
628 |
$ |
40 |
$ |
183 |
$ |
79 |
$ |
930 |
||||||||||
|
Operating loss |
$ |
(12,248 |
) |
$ |
(169 |
) |
$ |
(10,087 |
) |
$ |
(8,449 |
) |
$ |
(30,953 |
) | |||||
|
A. |
Operating Results |
|
2023 |
2022 |
2021 |
||||||||||
|
Consolidated Statements of Comprehensive loss: |
||||||||||||
|
(U.S. dollars, in thousands) |
||||||||||||
|
Revenues |
$ |
5,640 |
$ |
1,675 |
$ |
930 |
||||||
|
Cost of revenues |
1,692 |
909 |
767 |
|||||||||
|
Gross profit |
3,948 |
766 |
163 |
|||||||||
|
Operating expenses (income): |
||||||||||||
|
Research and development, net
|
20,777 |
20,792 |
21,125 |
|||||||||
|
Sales and marketing
|
3,611 |
3,933 |
2,738 |
|||||||||
|
General and administrative
|
6,068 |
6,482 |
7,253 |
|||||||||
|
Other income |
- |
(3,500 |
) |
- |
||||||||
|
Total operating expenses, net |
30,456 |
27,707 |
31,116 |
|||||||||
|
Operating loss |
(26,508 |
) |
(26,941 |
) |
(30,953 |
) | ||||||
|
Financing income |
1,486 |
516 |
1,935 |
|||||||||
|
Financing expenses |
(965 |
) |
(3,329 |
) |
(1,414 |
) | ||||||
|
Loss before taxes on income |
(25,987 |
) |
(29,754 |
) |
(30,432 |
) | ||||||
|
Taxes on income (tax benefit) |
(33 |
) |
90 |
13 |
||||||||
|
Loss |
$ |
(25,954 |
) |
$ |
(29,844 |
) |
$ |
(30,445 |
) | |||
|
B. |
Liquidity and Capital Resources |
|
2023 |
2022 |
2021 |
||||||||||
|
(U.S. dollars, in thousands) |
||||||||||||
|
Net cash used in operating activities |
$ |
(21,577 |
) |
$ |
(23,678 |
) |
$ |
(24,716 |
) | |||
|
Net cash provided by (used in) investing activities |
(4,538 |
) |
13,274 |
(20,566 |
) | |||||||
|
Net cash provided by financing activities |
18,152 |
9,343 |
30,276 |
|||||||||
|
Exchange rate differences - cash and cash equivalents balances |
(245 |
) |
(2,284 |
) |
1,102 |
|||||||
|
Decrease in cash and cash equivalents |
$ |
(8,208 |
) |
$ |
(3,345 |
) |
$ |
(13,904 |
) | |||
|
C. |
Research and Development, Patents and Licenses, etc. |
|
D. |
Trend Information |
|
E. |
Critical Accounting Estimates |
|
|
◾ |
amortization over an eight-year period of the cost of purchased know-how and patents and rights to use a patent and know-how which
were purchased in good faith and are used for the development or advancement of the Industrial Enterprise, commencing in the year in which
such rights were first exercised; |
|
|
◾ |
under limited conditions, an election to file consolidated tax returns together with Israeli Industrial Companies controlled by it;
and |
|
|
◾ |
expenses related to a public offering are deductible in equal amounts over a three-year period, commencing in the year of the offering.
|
| ITEM 6. |
DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES |
|
A. |
Directors and Senior Management |
|
Name |
Age |
Position | ||
|
Executive officers |
||||
|
Mr. Ofer Haviv |
57 |
President and Chief Executive Officer, Chief Executive Officer of Canonic Ltd. | ||
|
Dr. Nir Arbel |
44 |
Chief Product Officer | ||
|
Mr. Yaron Eldad |
58 |
Chief Financial Officer | ||
|
Dr. Elran Haber |
43 |
Chief Executive Officer of Biomica Ltd. | ||
|
Mr. Mark Kapel |
47 |
Chief Technology Officer | ||
|
Mr. Sassi Masliah |
45 |
Vice President Corporate Development | ||
|
Mr. Eyal Ronen |
53 |
Executive Vice President of Business Development | ||
|
Mr. Yoash Zohar |
57 |
Chief Executive Officer of Casterra Ag Ltd. | ||
|
Mr. Amit Noam |
42 |
Chief Executive Officer of Lavie Bio Ltd. | ||
|
Mr. Dan Jacob Gelvan |
59 |
Chief Executive Officer of Ag Plenus Ltd. | ||
|
Directors |
||||
|
Ms. Sarit Firon(3)(4)
|
57 |
Chairperson of the Board | ||
|
Mr. Dan Falk(1)(2)(4)
|
78 |
Director | ||
|
Mr. Nir Nimrodi(1)(2)(3)(4)
|
55 |
Director | ||
|
Dr. Adrian Percy(4)
|
58 |
Director | ||
|
Mr. Leon Y. Recanati(3)(4)
|
75 |
Director | ||
|
Dr. Oded Shoseyov(1)(2)(4)
|
67 |
Director |
|
|
(1) |
Member of our Audit Committee. |
|
|
(2) |
Member of our Compensation and Nominating Committee. |
|
|
(3) |
Member of our Pricing/Investment Committee. |
|
|
(4) |
Independent director under the Nasdaq Listing Rules. |
|
Country of Principal Executive Offices |
Israel | |||
|
Foreign Private Issuer |
Yes | |||
|
Disclosure Prohibited under Home Country Law |
No | |||
|
Total Number of Directors |
6 | |||
|
Part I: Gender Identity |
Female |
Male |
Non-Binary |
Did Not Disclose
Gender |
|
Directors |
1 |
5 |
|
|
|
Part II: Demographic Background |
| |||
|
Underrepresented Individual in Home Country Jurisdiction |
0 | |||
|
LGBTQ+ |
0 | |||
|
Did Not Disclose Demographic Background |
0 | |||
|
B. |
Compensation |
|
(in thousands, US$)(1) |
||||||||||||||||
|
Name and Position |
Salary and related benefits |
Bonus(2) |
Value of Options Granted(3)
|
Total
|
||||||||||||
|
Ofer Haviv
President and Chief Executive Officer |
390 |
- |
11 |
401 |
||||||||||||
|
Amit Noam
CEO of Lavie Bio |
136 |
- |
470 |
606 |
||||||||||||
|
Brian Ember
Former CEO of AgPlenus |
277 |
10 |
169 |
456 |
||||||||||||
|
Elran Haber
CEO of Biomica |
260 |
42 |
37 |
339 |
||||||||||||
|
Nir Arbel
Chief Product Officer |
228 |
- |
64 |
292 |
||||||||||||
|
|
(1) |
All amounts reported in the table are in terms of cost to the Company, as recorded in our financial statements. |
|
|
(2) |
Bonus amounts shown in this table reflect bonuses that were paid in 2023 relating to the office holders’ service in our Company
in 2022, as approved by our Compensation and Nominating Committee and Board of Directors, and, to the extent required, also by our shareholders.
|
|
|
(3) |
Consists of amounts recognized as non-cash expenses in our statement of profit or loss for the year ended December 31, 2023 in respect
of option grants. |
|
|
◾ |
Annual fees in an amount of approximately $24,000 for directors classified as experts; and |
|
|
◾ |
Per-meeting fees in an amount of approximately $1,300 for directors classified as experts; 60% of such amounts for participation
in meetings via telecommunication and 50% of such amounts for resolutions adopted in writing. |
|
Subsidiary |
Percentage of Subsidiary's Equity Issuable as Equity Incentives
|
Percentage of Equity Granted as of March 17, 2024
as Equity Incentives |
||||||
|
AgPlenus |
13.8 |
% |
12.2 |
% | ||||
|
Biomica |
12 |
% |
7.62 |
% | ||||
|
Casterra |
8.0 |
% |
4.1 |
% | ||||
|
Canonic |
10.8 |
% |
6.4 |
% | ||||
|
Lavie Bio |
10.5 |
% |
9.2 |
% | ||||
|
C. |
Board Practices |
|
|
◾ |
such majority includes at least 2/3 of the shares held by all shareholders who are not controlling shareholders and do not have a
personal interest in such appointment, present and voting at such meeting; or |
|
|
◾ |
the total number of shares of non-controlling shareholders who do not have a personal interest in such appointment voting against
such appointment does not exceed two percent of the aggregate voting rights in the company. |
|
|
◾ |
retaining and terminating the services of our independent auditors, subject to the approval of the board of directors and shareholders;
|
|
|
◾ |
pre-approval of audit and non-audit services to be provided by the independent auditors; |
|
|
◾ |
reviewing with management and our independent directors our financial reports prior to their submission to the SEC; and |
|
|
◾ |
approval of certain transactions with office holders and other related-party transactions. |
|
|
◾ |
reviewing and recommending an overall compensation policy with respect to our Chief Executive Officer and other executive officers,
as described above under “Item 6. Directors, Senior Management and Employees—B. Compensation—Compensation Policy”;
|
|
|
◾ |
reviewing and approving corporate goals and objectives relevant to the compensation of our Chief Executive Officer and other executive
officers, including evaluating their performance in light of such goals and objectives; |
|
|
◾ |
reviewing and recommending to our board of directors to approve the granting of options and other incentive awards; |
|
|
◾ |
overseeing our company’s policy for recovery of erroneously awarded compensation; |
|
|
◾ |
reviewing, evaluating and making recommendations regarding the compensation and benefits for our non-employee directors; and
|
|
|
◾ |
advising our board of directors in selecting individuals who are best able to fulfill the responsibilities of a director or executive
officer of our company. |
|
|
• |
such majority includes at least a majority of the shares held by shareholders who are not controlling shareholders and shareholders
who do not have a personal interest in such compensation policy; or |
|
|
• |
the total number of shares of non-controlling shareholders and shareholders who do not have a personal interest in the compensation
policy and who vote against the policy, does not exceed two percent (2%) of the aggregate voting rights in the Company. |
|
|
• |
the education, skills, experience, expertise and accomplishments of the relevant office holder; |
|
|
• |
the office holder’s position and responsibilities; |
|
|
• |
prior compensation agreements with the office holder; |
|
|
• |
the ratio between the cost of the terms of employment of an office holder and the cost of the employment of other employees of the
company, including employees employed through contractors who provide services to the company, in particular the ratio between such cost
to the average and median salary of such employees of the company, as well as the impact of disparities between them on the work;
|
|
|
• |
relationships in the company; |
|
|
• |
if the terms of employment include variable components — the possibility of reducing variable components at the discretion
of the board of directors and the possibility of setting a limit on the value of non-cash variable equity-based components; and
|
|
|
• |
if the terms of employment include severance compensation — the term of employment or office of the office holder, the terms
of the office holder’s compensation during such period, the company’s performance during such period, the office holder’s
individual contribution to the achievement of the company’s goals and the maximization of its profits and the circumstances under
which he or she is leaving the company. |
|
|
• |
with regard to variable components: |
|
|
o |
with the exception of office holders who report to the chief executive officer, a means of determining the variable components on
the basis of long-term performance and measurable criteria; provided that the company may determine that an immaterial part of the variable
components of the compensation package of an office holder shall be awarded based on non-measurable criteria, or if such amount is not
higher than three months’ salary per annum, taking into account such office holder’s contribution to the company; and
|
|
|
o |
the ratio between variable and fixed components, as well as the limit of the values of variable components at the time of their payment,
or in the case of equity-based compensation, at the time of grant; |
|
|
• |
a condition under which the office holder will return to the company, according to conditions to be set forth in the compensation
policy, any amounts paid as part of the office holder’s terms of employment, if such amounts were paid based on information later
to be discovered to be wrong, and such information was restated in the company’s financial statements; |
|
|
• |
the minimum holding or vesting period of variable equity-based components, while taking into consideration long-term incentives;
and |
|
|
• |
a limit to retirement grants. |
|
|
◾ |
at least a majority of the voting rights in the company held by non-controlling shareholders who have no conflict of interest (referred
to under the Companies Law as a “personal interest”) in the transaction or arrangement and who are present and voting (in
person or by proxy) at the general meeting, must be voted in favor of approving the transaction or arrangement (for this purpose, abstentions
are disregarded); or |
|
|
◾ |
the voting rights held by non-controlling, non-conflicted shareholders (as described in the previous bullet point) who are present
and voting (in person or by proxy) at the general meeting, and who vote against the transaction, do not exceed two percent of the voting
rights in the company. |
|
|
◾ |
financial liability imposed on him or her in favor of another person pursuant to a judgment, settlement or arbitrator’s award
approved by a court. However, if an undertaking to indemnify an office holder with respect to such liability is provided in advance, then
such an undertaking must be limited to events which, in the opinion of the board of directors, can be foreseen based on the company’s
activities when the undertaking to indemnify is given, and to an amount or according to criteria determined by the board of directors
as reasonable under the circumstances, and such undertaking shall detail the abovementioned events and amount or criteria; |
|
|
◾ |
reasonable litigation expenses, including attorneys’ fees, incurred by the office holder as a result of an investigation or
proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that (i) no
indictment was filed against such office holder as a result of such investigation or proceeding; and (ii) no financial liability,
such as a criminal penalty, was imposed upon him or her as a substitute for the criminal proceeding as a result of such investigation
or proceeding or, if such financial liability was imposed, it was imposed with respect to an offense that does not require proof of criminal
intent; and |
|
|
◾ |
reasonable litigation expenses, including attorneys’ fees, incurred by the office holder or imposed by a court in proceedings
instituted against him or her by the company, on its behalf or by a third party or in connection with criminal proceedings in which the
office holder was acquitted or as a result of a conviction for an offense that does not require proof of criminal intent. |
|
D. |
Employees |
|
Company |
Female |
Male |
Total |
|
Evogene |
55% |
45% |
75 |
|
AgPlenus |
67% |
33% |
12 |
|
Lavie Bio |
46% |
54% |
26 |
|
Canonic |
14% |
86% |
7 |
|
Biomica |
61% |
39% |
18 |
|
Casterra |
0% |
100% |
4 |
|
Total |
51% |
49% |
142 |
|
Company |
Female |
Male |
|
Evogene |
50% |
50% |
|
AgPlenus |
80% |
20% |
|
Lavie Bio |
30% |
70% |
|
Canonic |
33% |
67% |
|
Biomica |
67% |
33% |
|
Casterra |
0% |
100% |
|
As of December 31, 2021 |
As of December 31, 2022 |
As of December 31, 2023 |
||||||||||||||||||||||||||||||||||
|
Israel |
U.S. |
Total |
Israel |
U.S. |
Total |
Israel |
U.S. |
Total |
||||||||||||||||||||||||||||
|
Executive management |
5 |
- |
5 |
6 |
- |
6 |
5 |
- |
5 |
|||||||||||||||||||||||||||
|
General and administrative |
33 |
- |
33 |
25 |
- |
25 |
31 |
- |
31 |
|||||||||||||||||||||||||||
|
Technology platform |
41 |
- |
41 |
44 |
- |
44 |
39 |
- |
39 |
|||||||||||||||||||||||||||
|
Ag-Seeds division |
2 |
- |
2 |
- |
- |
- |
- |
- |
- |
|||||||||||||||||||||||||||
|
Lavie Bio Ltd. |
17 |
7 |
24 |
21 |
6 |
27 |
21 |
5 |
26 |
|||||||||||||||||||||||||||
|
AgPlenus Ltd. |
11 |
1 |
12 |
11 |
1 |
12 |
11 |
1 |
12 |
|||||||||||||||||||||||||||
|
Casterra Ag Ltd. |
1 |
- |
1 |
1 |
- |
1 |
4 |
- |
4 |
|||||||||||||||||||||||||||
|
Biomica Ltd. |
13 |
- |
13 |
13 |
- |
13 |
18 |
- |
18 |
|||||||||||||||||||||||||||
|
Canonic Ltd. |
10 |
- |
10 |
9 |
- |
9 |
7 |
- |
7 |
|||||||||||||||||||||||||||
|
Total |
133 |
8 |
141 |
130 |
7 |
137 |
136 |
6 |
142 |
|||||||||||||||||||||||||||
|
E. |
Share Ownership |
|
F. |
Disclosure of a Registrant’s Action to Recover Erroneously
Awarded Compensation |
| ITEM 7. |
MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS |
|
A. |
Major Shareholders |
|
Shares Beneficially Held
|
||||||||
|
Name of Beneficial Owner |
|
Number
|
|
|
Percentage of Class
|
|||
|
Principal Shareholders |
||||||||
|
SilverArc Capital Management, LLC |
3,100,000 |
(1) |
6.1 |
% | ||||
|
Executive Officers and Directors |
||||||||
|
Mr. Ofer Haviv |
895,000 |
**(2) |
1.7 |
% | ||||
|
Dr. Nir Arbel |
108,747 |
(3) |
* |
|||||
|
Mr. Yaron Eldad |
85,250 |
(4) |
* |
|||||
|
Dr. Brian N. Ember |
0 |
*** |
* |
|||||
|
Dr. Dan Jacob Gelvan |
0 |
* |
||||||
|
Dr. Elran Haber |
25,000 |
(5) |
* |
|||||
|
Mr. Mark Kapel |
203,950 |
(6) |
* |
|||||
|
Mr. Sassi Masliah |
185,250 |
(7) |
* |
|||||
|
Mr. Amit Noam |
0 |
* |
||||||
|
Mr. Eyal Ronen |
84,375 |
(8) |
* |
|||||
|
Mr. Yoash Zohar |
0 |
* |
||||||
|
Ms. Sarit Firon |
127,375 |
(9) |
* |
|||||
|
Mr. Dan Falk |
36,000 |
(10) |
* |
|||||
|
Mr. Nir Nimrodi |
61,000 |
(11) |
* |
|||||
|
Dr. Adrian Percy |
67,125 |
(12) |
* |
|||||
|
Mr. Leon Y. Recanati |
909,734 |
(13) |
1.8 |
% | ||||
|
Prof. Oded Shoseyov |
67,750 |
(14) |
* |
|||||
|
All directors and executive officers as a group (16 persons***) |
2,935,919 |
5.5 |
% | |||||
|
|
* |
Less than 1%. |
|
|
** |
On August 16, 2023, our board of directors approved a grant of options to purchase 500,000 ordinary shares to Mr. Haviv, which, under
the Companies Law, is subject to the approval at the general meeting of shareholders of the Company, and therefore are not reflected herein.
|
|
|
*** |
The engagement of Dr. Ember as executive officer ended during 2024. |
|
|
(1) |
This information is based upon a Schedule 13G filed by SilverArc Capital Management, LLC, or SilverArc, with the SEC on February
14, 2024. SilverArc is a Delaware limited liability company and possesses shared voting and dispositive power over these ordinary shares
with Devesh Gandhi, a U.S. citizen. The principal address for SilverArc is 20 Park Plaza, 4th
Floor, Boston, MA 02116. |
|
|
(2) |
Consists of 895,000 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days
of March 17, 2024, of which options to purchase the following number of shares expire on the following dates, respectively: 170,000 on
March 22, 2025, 225,000 on August 8, 2027 and 500,000 on April 21, 2030. The weighted average exercise price of these options is NIS 14.31
per ordinary share. |
|
|
(3) |
Consists of 94,372 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days
of March 17, 2024, of which options to purchase the following number of shares expire on the following dates, respectively: 78,122 on
September 1, 2031 and 16,250 on March 8, 2033. The weighted average exercise price of these options is NIS 8.07 per ordinary share. Also
includes 14,375 shares issuable upon vesting of RSUs that are currently vested or will become vested within 60 days of March 17, 2024,
all of which expire on March 8, 2033, and with no exercise price. |
|
|
(4) |
Consists of 75,000 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days
of March 17, 2024, which expire on March 30, 2032. The exercise price of these options is NIS 4.09 per ordinary share. Also includes 10,250
shares issuable upon vesting of RSUs that are currently vested or will become vested within 60 days of March 17, 2024, all of which expire
on March 8, 2033, and with no exercise price. |
|
|
(5) |
Consists of 25,000 ordinary shares of Evogene issuable upon exercise of options that are currently exercisable or exercisable within
60 days of March 17, 2024, which expire on September 1, 2031. The exercise price of these options is NIS 9.17 per ordinary share. Elran
Haber serves as the CEO of our subsidiary company Biomica, and as such, also holds options to purchase shares of Biomica. For a description
of our subsidiaries’ equity incentive plans, please see “Item 6. Directors, Senior Management and Employees—B. Compensation—Share
Option and Incentive Plans—Subsidiary Equity Incentive Plans”. |
|
|
(6) |
Consists of 203,950 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days
of March 17, 2024, of which options to purchase the following number of shares expire on the following dates, respectively: 12,000 on
March 22, 2025, 23,200 on August 8, 2027, 60,000 on February 26, 2028, 30,000 on February 4, 2029, 35,000 on July 30, 2029, 31,250 on
November 16, 2031 and 12,500 on March 8, 2033. The weighted average exercise price of these options is NIS 12.14 per ordinary share.
|
|
|
(7) |
Includes 185,250 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days of
March 17, 2024, of which options to purchase the following number of shares expire on the following dates, respectively: 17,000 on March
22, 2025, 18,000 on August 8, 2027, 3,750 on May 28, 2028, 10,000 on February 4, 2029, 24,000 on July 30, 2029, 43,750 on September 22,
2030, 62,500 on November 16, 2031 and 6,250 on March 8, 2033. The weighted average exercise price of these options is NIS 11.04 per ordinary
share. |
|
|
(8) |
Includes 84,375 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days of
March 17, 2024, of which options to purchase the following number of shares expire on the following dates, respectively: 65,625 on August
2, 2032 and 18,750 on August 16, 2033. The weighted average exercise price of these options is NIS 3.08 per ordinary share. |
|
|
(9) |
Consists of 127,375 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days
of March 17, 2024, of which options to purchase the following number of shares expire on the following dates, respectively: 10,000 on
August 10, 2026, 2,500 on August 8, 2027,2,500 on August 6, 2028, 2,500 on September 23, 2029, 1,875 on September 22, 2030, 36,000 on
September 1, 2031, 36,000 on September 15, 2032 and 36,000 on May 11, 2033. The weighted average exercise price of these options is NIS
7.10 per ordinary share. |
|
|
(10) |
Consists of 36,000 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days
of March 17, 2024, of which options to purchase the following number of shares expire on the following dates, respectively: 18,000 on
September 15, 2032 and 18,000 on May 11, 2033. The weighted average exercise price of these options is NIS 2.91 per ordinary share.
|
|
|
(11) |
Consists of 61,000 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days
of March 17, 2024, of which options to purchase the following number of shares expire on the following dates, respectively: 25,000 on
April 20, 2030, 18,000 on September 15, 2032 and 18,000 on May 11, 2033. The weighted average exercise price of these options is $0.94
per ordinary share. |
|
|
(12) |
Consists of 67,125 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days
of March 17, 2024, of which options to purchase the following number of shares expire on the following dates, respectively: 10,000 on
December 23, 2028, 2,500 on February 1, 2030, 625 on February 1, 2031, 18,000 on August 10, 2031, 18,000 on September 15, 2032 and 18,000
on May 11, 2033. The weighted average exercise price of these options is $ 1.74 per ordinary share. |
|
|
(13) |
Includes 838,859 ordinary shares held by Mr. Recanati. Also includes 70,875 ordinary shares issuable upon exercise of options that
are currently exercisable or exercisable within 60 days of March 17, 2024, of which options to purchase the following number of shares
expire on the following dates, respectively: 2,500 on August 17, 2024, 2,500 on July 2, 2025, 2,500 on May 18, 2026, 2,500 on May 16,
2027, 2,500 on June 25, 2028, 2,500 on July 30, 2029, 1,875 on November 17, 2030, 18,000 on September 1, 2031, 18,000 on September 15,
2032 and 18,000 on May 11, 2033. The weighted average exercise price of these options is NIS 9.77 per ordinary share. |
|
|
(14) |
Consists of 67,750 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days
of March 17, 2024, of which options to purchase the following number of shares expire on the following dates, respectively: 10,000 on
November 13, 2028, 2,500 on December 19, 2029, 1,250 on November 13, 2030, 18,000 on September 1, 2031, 18,000 on September 15, 2032 and
18,000 on May 11, 2033. The exercise price of these options is NIS 6.00 per ordinary share. |
|
B. |
Related Party Transactions |
|
C. |
Interests of Experts and Counsel |
| ITEM 8. |
FINANCIAL INFORMATION |
|
A. |
Consolidated Statements and Other Financial Information |
|
B. |
Significant Changes |
| ITEM 9. |
THE OFFER AND LISTING |
|
A. |
Offer and Listing Details |
|
B. |
Plan of Distribution |
|
C. |
Markets |
|
D. |
Selling Shareholders |
|
E. |
Dilution |
|
F. |
Expenses of the Issue |
| ITEM 10. |
ADDITIONAL INFORMATION |
|
A. |
Share Capital |
|
B. |
Memorandum and Articles of Association |
|
C. |
Material Contracts |
|
|
◾ |
Evogene Ltd. Officers Compensation Policy. See “Item 6. Directors, Senior Management and Employees” for more information
about this document. |
|
|
◾ |
Evogene Ltd. Officers Clawback Policy. See “Item 6. Directors, Senior Management and Employees” for more information
about this document. |
|
|
◾ |
Evogene Share Option Plan (2002). See “Item 6. Directors, Senior Management and Employees” for more information about
this document. |
|
|
◾ |
Evogene Ltd. Key Employee Share Incentive Plan, 2003. See “Item 6. Directors, Senior Management and Employees” for more
information about this document. |
|
|
◾ |
Evogene Ltd. 2013 Share Option Plan. See “Item 6. Directors, Senior Management and Employees” for more information about
this document. |
|
|
◾ |
Evogene 2021 Share Incentive Plan. See “Item 6. Directors, Senior Management and Employees” for more information about
this document. |
|
D. |
Exchange Controls |
|
E. |
Taxation |
|
|
◾ |
banks, financial institutions or insurance companies; |
|
|
◾ |
real estate investment trusts, regulated investment companies or grantor trusts; |
|
|
◾ |
dealers or traders in securities, commodities or currencies; |
|
|
◾ |
tax-exempt entities; |
|
|
◾ |
certain former citizens or long-term residents of the United States; |
|
|
◾ |
persons that received our shares as compensation for the performance of services; |
|
|
◾ |
persons that will hold our shares as part of a “hedging,” “integrated” or “conversion” transaction
or as a position in a “straddle” for United States federal income tax purposes; |
|
|
◾ |
partnerships (including entities classified as partnerships for United States federal income tax purposes) or other pass-through
entities, or holders that will hold our shares through such an entity; |
|
|
◾ |
persons subject to special tax accounting rules as a result of any item of gross income with respect to the ordinary shares being
taken into account in an “applicable financial statement” pursuant to Section 451(b) of the Code; |
|
|
◾ |
U.S. Holders (as defined below) whose “functional currency” is not the U.S. dollar; or |
|
|
◾ |
holders that own directly, indirectly or through attribution 10.0% or more of the voting power or value of our shares. |
|
|
◾ |
a citizen or resident of the United States; |
|
|
◾ |
a corporation (or other entity treated as a corporation for United States federal income tax purposes) created or organized in or
under the laws of the United States or any state thereof, including the District of Columbia; |
|
|
◾ |
an estate the income of which is subject to United States federal income taxation regardless of its source; or |
|
|
◾ |
a trust if such trust has validly elected to be treated as a United States person for United States federal income tax purposes or
if (1) a court within the United States is able to exercise primary supervision over its administration and (2) one or more
United States persons have the authority to control all of the substantial decisions of such trust. |
|
|
◾ |
at least 75% of its gross income is “passive income”; or |
|
|
◾ |
at least 50% of the average quarterly value of its gross assets (which may be determined in part by the market value of our ordinary
shares, which is subject to change) is attributable to assets that produce “passive income” or are held for the production
of passive income. |
|
F. |
Dividends and Paying Agents |
|
G. |
Statement by Experts |
|
H. |
Documents on Display |
|
I. |
Subsidiary Information |
|
J. |
Annual Report to Security Holders |
|
ITEM 11. |
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
| ITEM 12. |
DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES |
| ITEM 13. |
DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES |
| ITEM 14. |
MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS |
|
ITEM 15. |
CONTROLS AND PROCEDURES |
|
|
(a) |
Disclosure Controls and Procedures |
|
|
(b) |
Management’s Annual Report on Internal Control Over Financial Reporting |
|
|
(c) |
Attestation Report of Registered Public Accounting Firm |
|
|
(d) |
Changes in internal control over financial reporting |
| ITEM 16. |
[RESERVED] |
| ITEM 16A. |
AUDIT COMMITTEE FINANCIAL EXPERT |
| ITEM 16B. |
CODE OF ETHICS |
| ITEM 16C. |
PRINCIPAL ACCOUNTANT FEES AND SERVICES |
|
2022 |
2023 |
|||||||
|
Audit Fees |
$ |
190,000 |
$ |
190,000 |
||||
| Audit Related Fees | - | - | ||||||
|
Tax Fees |
20,000 |
20,000 |
||||||
|
All other fees |
- |
10,000 |
||||||
|
Total |
$ |
210,000 |
$ |
220,000 |
||||
| ITEM 16D. |
EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES |
| ITEM 16E. |
PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS |
| ITEM 16F. |
CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT |
| ITEM 16G. |
CORPORATE GOVERNANCE |
|
|
◾ |
Quorum. As permitted under the Companies Law, pursuant to our articles of association, the
quorum required for an ordinary meeting of shareholders consists of at least two shareholders present in person, by proxy or by other
voting instrument in accordance with the Companies Law, who hold at least 25% of the voting power of our shares (and in an adjourned meeting,
with some exceptions, at least two shareholders), instead of 33 1/3% of the issued share capital, as required under the Nasdaq Listing
Rules. |
|
|
◾ |
Executive sessions of independent directors. Israeli law does not require executive sessions
of independent directors. Although all of our current directors are “independent directors” under the applicable Nasdaq criteria,
we do not intend to comply with this requirement if we have directors who are not independent. |
|
|
◾ |
Shareholder approval. We seek shareholder approval for all corporate actions requiring such
approval under the Companies Law, which include (i) transactions with directors concerning the terms of their service or indemnification,
exemption and insurance for their service (or for any other position that they may hold at our company), (ii) transactions concerning
the compensation, indemnification, exculpation and insurance of the chief executive officer; (iii) the compensation policy recommended
by the compensation committee of our board of directors and approved by our board of directors (and any amendments thereto); (iv) extraordinary
transactions with, and the terms of employment or other engagement of, a controlling shareholder (if and when this becomes relevant to
our company), (v) amendments to our articles of association, and (vi) certain non-public issuances of securities. In addition,
under the Companies Law, a merger requires approval of the shareholders of each of the merging companies. We are not required, however,
to seek shareholder approval for any of the following events described in the Nasdaq Listing Rules: |
|
|
◾ |
certain issuances of shares in excess of 20% of the outstanding shares of the Company; |
|
|
◾ |
an issuance that will result in a change of control of our company; and |
|
|
◾ |
adoption of, or material changes to, our equity compensation plans. |
| ITEM 16H. |
MINE SAFETY DISCLOSURE |
|
ITEM 16I. |
DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS |
|
ITEM 16J. |
INSIDER TRADING PLANS (10B5-1) |
|
ITEM 16K. |
Cybersecurity |
| ITEM 17. |
FINANCIAL STATEMENTS |
| ITEM 18. |
FINANCIAL STATEMENTS |
| ITEM 19. |
EXHIBITS |
|
ANNUAL REPORT ON FORM 20-F
INDEX OF EXHIBITS
| |
|
Exhibit No. |
Description |
|
101 |
The following financial information from Evogene Ltd.’s Annual Report on Form
20-F for the year ended December 31, 2023 formatted in inline XBRL (eXtensible Business Reporting Language): (i) Consolidated Statements
of Financial Position at December 31, 2023 and 2022; (ii) Consolidated Statements of Profit or Loss for the years ended December 31, 2023,
2022 and 2021; (iii) Consolidated Statements of Changes in Equity for the years ended December 31, 2023, 2022 and 2021; (iv) Consolidated
Statements of Cash Flows for the years ended December 31, 2023, 2022 and 2021; and (v) Notes to Consolidated Financial Statements, tagged
as blocks of text.† |
|
104 |
Cover Page Interactive Data File 101 |
| † |
Filed herewith. |
| ^ |
Furnished herewith. |
| * |
In accordance with the rules of the SEC certain confidential information contained in this exhibit, has been omitted because it (i)
is not material and (ii) is the type that the Company treats as private or confidential. |
|
Evogene Ltd. | |
|
Date: March 28, 2024 |
By: /s/ Ofer Haviv Name: Ofer Haviv Title: President and Chief Executive Officer |
|
Page
|
|
|
Report of Independent Registered Public Accounting Firm (PCAOB ID: 1281)
|
F-2 - F-3
|
|
F-4
|
|
|
F-5
|
|
|
F-6 - F-7
|
|
|
F-8 - F-9
|
|
|
F-10 - F-54
|
 |
Kost Forer Gabbay & Kasierer
144 Menachem Begin Road, Building A
Tel-Aviv 6492102, Israel
|
Tel: +972-3-6232525
Fax: +972-3-5622555
ey.com
|
|
December 31,
|
|||||||||||
|
Note
|
2023
|
2022
|
|||||||||
|
CURRENT ASSETS:
|
|||||||||||
|
Cash and cash equivalents
|
6
|
$
|
20,772
|
$
|
28,980
|
||||||
|
Short-term bank deposits
|
10,291
|
-
|
|||||||||
|
Marketable securities
|
7
|
-
|
6,375
|
||||||||
|
Trade receivables
|
357
|
348
|
|||||||||
|
Other receivables and prepaid expenses
|
8
|
2,973
|
1,482
|
||||||||
|
Inventories
|
76
|
566
|
|||||||||
|
34,469
|
37,751
|
||||||||||
|
LONG-TERM ASSETS:
|
|||||||||||
|
Long-term deposits and other receivables
|
28
|
74
|
|||||||||
|
Deferred taxes
|
17d
|
-
|
94
|
||||||||
|
Right-of-use-assets
|
9
|
980
|
1,568
|
||||||||
|
Property, plant and equipment, net
|
10
|
2,455
|
2,499
|
||||||||
|
Intangible assets, net
|
11
|
13,169
|
14,140
|
||||||||
|
16,632
|
18,375
|
||||||||||
|
$
|
51,101
|
$
|
56,126
|
||||||||
|
CURRENT LIABILITIES:
|
|||||||||||
|
Trade payables
|
$
|
1,785
|
$
|
1,036
|
|||||||
|
Employees and payroll accruals
|
2,537
|
1,987
|
|||||||||
|
Lease liability
|
9
|
853
|
884
|
||||||||
|
Liabilities in respect of government grants
|
12
|
388
|
79
|
||||||||
|
Deferred revenues and other advances
|
362
|
22
|
|||||||||
|
Other payables
|
1,019
|
1,617
|
|||||||||
|
6,944
|
5,625
|
||||||||||
|
LONG-TERM LIABILITIES:
|
|||||||||||
|
Lease liability
|
9
|
285
|
932
|
||||||||
|
Liabilities in respect of government grants
|
12
|
4,426
|
4,665
|
||||||||
|
Other advances
|
393
|
-
|
|||||||||
|
Convertible SAFE
|
5f, 13
|
10,368
|
10,114
|
||||||||
|
15,472
|
15,711
|
||||||||||
|
SHAREHOLDERS’ EQUITY:
|
18
|
||||||||||
|
Ordinary shares of NIS 0.02 par value:
|
|||||||||||
|
Authorized – 150,000,000 ordinary shares; Issued and outstanding –50,584,888 ordinary shares on December 31, 2023 and 41,260,439 ordinary shares on December 31, 2022
|
286
|
235
|
|||||||||
|
Share premium and other capital reserves
|
269,353
|
261,402
|
|||||||||
|
Accumulated deficit
|
(257,586
|
)
|
(233,707
|
)
|
|||||||
|
Equity attributable to equity holders of the Company
|
12,053
|
27,930
|
|||||||||
|
Non-controlling interests
|
16,632
|
6,860
|
|||||||||
|
Total equity
|
28,685
|
34,790
|
|||||||||
|
$
|
51,101
|
$
|
56,126
|
||||||||
|
March 28, 2024
|
||||||
|
Date of approval of the
|
Sarit Firon
|
Ofer Haviv
|
Yaron Eldad
|
|||
|
financial statements
|
Chairperson of the board
|
Chief Executive Officer
|
Chief Financial Officer
|
F - 4
|
Year ended December 31,
|
|||||||||||||||
|
Note
|
2023
|
2022
|
2021
|
||||||||||||
|
Revenues
|
22b
|
$
|
5,640
|
$
|
1,675
|
$
|
930
|
||||||||
|
Cost of revenues
|
20a
|
|
1,692
|
909
|
767
|
||||||||||
|
Gross profit
|
3,948
|
766
|
163
|
||||||||||||
|
Operating expenses (income):
|
|||||||||||||||
|
Research and development, net
|
20b
|
|
20,777
|
20,792
|
21,125
|
||||||||||
|
Sales and marketing
|
20c
|
|
3,611
|
3,933
|
2,738
|
||||||||||
|
General and administrative
|
20d
|
|
6,068
|
6,482
|
7,253
|
||||||||||
|
Other income
|
20e
|
|
-
|
(3,500
|
)
|
-
|
|||||||||
|
Total operating expenses, net
|
30,456
|
27,707
|
31,116
|
||||||||||||
|
Operating loss
|
(26,508
|
)
|
(26,941
|
)
|
(30,953
|
)
|
|||||||||
|
Financing income
|
20f
|
|
1,486
|
516
|
1,935
|
||||||||||
|
Financing expenses
|
20f
|
|
(965
|
)
|
(3,329
|
)
|
(1,414
|
)
|
|||||||
|
Financing income (expenses), net
|
521
|
(2,813
|
)
|
521
|
|||||||||||
|
Loss before taxes on income
|
(25,987
|
)
|
(29,754
|
)
|
(30,432
|
)
|
|||||||||
|
Taxes on income (tax benefit)
|
17
|
(33
|
)
|
90
|
13
|
||||||||||
|
Loss
|
$
|
(25,954
|
)
|
$
|
(29,844
|
)
|
$
|
(30,445
|
)
|
||||||
|
Attributable to:
|
|||||||||||||||
|
Equity holders of the Company
|
(23,879
|
)
|
(26,638
|
)
|
(27,793
|
)
|
|||||||||
|
Non-controlling interests
|
(2,075
|
)
|
(3,206
|
)
|
(2,652
|
)
|
|||||||||
|
$
|
(25,954
|
)
|
$
|
(29,844
|
)
|
$
|
(30,445
|
)
|
|||||||
|
Basic and diluted loss per share, attributable to equity holders of the Company
|
21
|
$
|
(0.52
|
)
|
$
|
(0.65
|
)
|
$
|
(0.69
|
)
|
|||||
|
Weighted average number of shares used in computing basic and diluted loss per share
|
45,685,619
|
41,210,184
|
40,433,303
|
||||||||||||
F - 5
|
Attributable to equity holders of the Company
|
||||||||||||||||||||||||
|
Share
capital
|
Share premium and other capital reserves
|
Accumulated deficit
|
Total
|
Non-controlling interests
|
Total equity
|
|||||||||||||||||||
|
Balance as of January 1, 2021
|
$
|
200
|
$
|
225,121
|
$
|
(179,276
|
)
|
$
|
46,045
|
$
|
10,837
|
$
|
56,882
|
|||||||||||
|
Loss
|
-
|
-
|
(27,793
|
)
|
(27,793
|
)
|
(2,652
|
)
|
(30,445
|
)
|
||||||||||||||
|
Issuance of ordinary shares, net
|
27
|
29,555
|
-
|
29,582
|
-
|
29,582
|
||||||||||||||||||
|
Forfeiture of non-controlling interests regarding share-based compensation
|
-
|
536
|
-
|
536
|
(536
|
)
|
-
|
|||||||||||||||||
|
Benefit to non-controlling interests regarding share-based compensation
|
-
|
(23
|
)
|
-
|
(23
|
)
|
23
|
-
|
||||||||||||||||
|
Exercise of subsidiary options
|
-
|
(378
|
)
|
-
|
(378
|
)
|
378
|
-
|
||||||||||||||||
|
Exercise of pre-funded warrants
|
6
|
4,359
|
-
|
4,365
|
-
|
4,365
|
||||||||||||||||||
|
Exercise of options
|
1
|
426
|
-
|
427
|
-
|
427
|
||||||||||||||||||
|
Restricted stock units (“RSUs”) vested
|
*
|
)
|
*
|
)
|
-
|
-
|
-
|
-
|
||||||||||||||||
|
Share-based compensation and RSUs
|
-
|
892
|
-
|
892
|
1,717
|
2,609
|
||||||||||||||||||
|
Balance as of December 31, 2021
|
$
|
234
|
$
|
260,488
|
$
|
(207,069
|
)
|
$
|
53,653
|
$
|
9,767
|
$
|
63,420
|
|||||||||||
|
Loss
|
-
|
-
|
(26,638
|
)
|
(26,638
|
)
|
(3,206
|
)
|
(29,844
|
)
|
||||||||||||||
|
Issuance of ordinary shares, net
|
1
|
20
|
-
|
21
|
-
|
21
|
||||||||||||||||||
|
Forfeiture of non-controlling interests regarding share-based compensation
|
-
|
272
|
-
|
272
|
(272
|
)
|
-
|
|||||||||||||||||
|
Benefit to non-controlling interests regarding share-based compensation
|
-
|
(2
|
)
|
-
|
(2
|
)
|
2
|
-
|
||||||||||||||||
|
Exercise of subsidiary options
|
-
|
*
|
)
|
-
|
*
|
)
|
-
|
*
|
)
|
|||||||||||||||
|
Exercise of options
|
*
|
)
|
7
|
-
|
7
|
-
|
7
|
|||||||||||||||||
|
RSUs vested
|
*
|
)
|
*
|
)
|
-
|
*
|
)
|
-
|
*
|
)
|
||||||||||||||
|
Share-based compensation and RSUs
|
-
|
617
|
-
|
617
|
569
|
1,186
|
||||||||||||||||||
|
Balance as of December 31, 2022
|
$
|
235
|
$
|
261,402
|
$
|
(233,707
|
)
|
$
|
27,930
|
$
|
6,860
|
$
|
34,790
|
|||||||||||
F - 6
|
Attributable to equity holders of the Company
|
||||||||||||||||||||||||
|
Share
capital
|
Share premium and other capital reserves
|
Accumulated deficit
|
Total
|
Non-controlling interests
|
Total equity
|
|||||||||||||||||||
|
Balance as of December 31, 2022
|
$
|
235
|
$
|
261,402
|
$
|
(233,707
|
)
|
$
|
27,930
|
$
|
6,860
|
$
|
34,790
|
|||||||||||
|
Loss
|
-
|
-
|
(23,879
|
)
|
(23,879
|
)
|
(2,075
|
)
|
(25,954
|
)
|
||||||||||||||
|
Issuance of ordinary shares, net
|
51
|
8,398
|
-
|
8,449
|
-
|
8,449
|
||||||||||||||||||
|
Forfeiture of non-controlling interests regarding share-based compensation
|
-
|
71
|
-
|
71
|
(71
|
)
|
-
|
|||||||||||||||||
|
Benefit to non-controlling interests regarding share-based compensation
|
-
|
3
|
-
|
3
|
(3
|
)
|
-
|
|||||||||||||||||
|
Issuance of a subsidiary ordinary shares to the company
|
-
|
(809
|
)
|
-
|
(809
|
)
|
809
|
-
|
||||||||||||||||
|
Issuance of a subsidiary preferred shares to non-controlling interests
|
-
|
(238
|
)
|
-
|
(238
|
)
|
9,761
|
9,523
|
||||||||||||||||
|
RSUs vested
|
*
|
)
|
*
|
)
|
-
|
*
|
)
|
-
|
*
|
)
|
||||||||||||||
|
Share-based compensation and RSUs
|
-
|
526
|
-
|
526
|
1,351
|
1,877
|
||||||||||||||||||
|
Balance as of December 31, 2023
|
$
|
286
|
$
|
269,353
|
$
|
(257,586
|
)
|
$
|
12,053
|
$
|
16,632
|
$
|
28,685
|
|||||||||||
F - 7
|
Year ended
December 31,
|
||||||||||||
|
2023
|
2022
|
2021
|
||||||||||
|
Cash flows from operating activities:
|
||||||||||||
|
Loss
|
$
|
(25,954
|
)
|
$
|
(29,844
|
)
|
$
|
(30,445
|
)
|
|||
|
Adjustments to reconcile loss to net cash used in operating activities:
|
||||||||||||
|
Adjustments to the profit or loss items:
|
||||||||||||
|
Depreciation
|
1,641
|
1,513
|
1,302
|
|||||||||
|
Amortization of intangible assets
|
971
|
1,067
|
932
|
|||||||||
|
Share-based compensation
|
1,877
|
1,186
|
2,609
|
|||||||||
|
Revaluation of convertible SAFE
|
254
|
114
|
-
|
|||||||||
|
Net financing expenses (income)
|
(666
|
)
|
2,979
|
(884
|
)
|
|||||||
|
Decrease in accrued bank interest
|
-
|
7
|
11
|
|||||||||
|
Loss (gain) from sale of property, plant and equipment
|
(26
|
)
|
-
|
121
|
||||||||
|
Taxes on income (tax benefit)
|
(33
|
)
|
90
|
13
|
||||||||
|
4,018
|
6,956
|
4,104
|
||||||||||
|
Changes in asset and liability items:
|
||||||||||||
|
Increase in trade receivables
|
(9
|
)
|
(67
|
)
|
(59
|
)
|
||||||
|
Decrease (increase) in other receivables
|
(1,445
|
)
|
1,113
|
637
|
||||||||
|
Decrease (increase) in inventories
|
490
|
(474
|
)
|
(92
|
)
|
|||||||
|
Decrease (increase) in deferred taxes
|
94
|
(94
|
)
|
-
|
||||||||
|
Increase (decrease) in trade payables
|
742
|
(469
|
)
|
625
|
||||||||
|
Increase (decrease) in employees and payroll accruals
|
550
|
(675
|
)
|
127
|
||||||||
|
Increase (decrease) in other payables
|
(534
|
)
|
48
|
290
|
||||||||
|
Increase (decrease) in deferred revenues and other advances
|
(288
|
)
|
(153
|
)
|
128
|
|||||||
|
(400
|
)
|
(771
|
)
|
1,656
|
||||||||
|
Cash received (paid) during the year for:
|
||||||||||||
|
Interest received
|
905
|
186
|
297
|
|||||||||
|
Interest paid
|
(115
|
)
|
(165
|
)
|
(315
|
)
|
||||||
|
Taxes paid
|
(31
|
)
|
(40
|
)
|
(13
|
)
|
||||||
|
Net cash used in operating activities
|
$
|
(21,577
|
)
|
$
|
(23,678
|
)
|
$
|
(24,716
|
)
|
|||
F - 8
|
Year ended
December 31,
|
||||||||||||
|
2023
|
2022
|
2021
|
||||||||||
|
Cash flows from investing activities:
|
||||||||||||
|
Purchase of property, plant and equipment
|
$
|
(785
|
)
|
$
|
(1,171
|
)
|
$
|
(847
|
)
|
|||
|
Proceeds from sale of marketable securities
|
6,924
|
12,356
|
4,395
|
|||||||||
|
Purchase of marketable securities
|
(503
|
)
|
(911
|
)
|
(23,114
|
)
|
||||||
|
Proceeds from sale of property, plant and equipment
|
26
|
-
|
-
|
|||||||||
|
Withdrawal from (investment in) bank deposits, net
|
(10,200
|
)
|
3,000
|
(1,000
|
)
|
|||||||
|
Net cash provided by (used in) investing activities
|
(4,538
|
)
|
13,274
|
(20,566
|
)
|
|||||||
|
Cash flows from financing activities:
|
||||||||||||
|
Issuance of a subsidiary preferred shares to non-controlling interests
|
9,523
|
-
|
-
|
|||||||||
|
Proceeds from issuance of ordinary shares, net of issuance expenses
|
8,449
|
21
|
29,582
|
|||||||||
|
Proceeds from issuance of convertible SAFE
|
-
|
10,000
|
-
|
|||||||||
|
Proceeds from exercise of options
|
-
|
7
|
484
|
|||||||||
|
Repayment of lease liability
|
(836
|
)
|
(803
|
)
|
(580
|
)
|
||||||
|
Proceeds from government grants
|
1,089
|
149
|
824
|
|||||||||
|
Repayment of government grants
|
(73
|
)
|
(31
|
)
|
(34
|
)
|
||||||
|
Net cash provided by financing activities
|
18,152
|
9,343
|
30,276
|
|||||||||
|
Exchange rate differences - cash and cash equivalent balances
|
(245
|
)
|
(2,284
|
)
|
1,102
|
|||||||
|
Decrease in cash and cash equivalents
|
(8,208
|
)
|
(3,345
|
)
|
(13,904
|
)
|
||||||
|
Cash and cash equivalents beginning of the year
|
28,980
|
32,325
|
46,229
|
|||||||||
|
Cash and cash equivalents end of the year
|
$
|
20,772
|
$
|
28,980
|
$
|
32,325
|
||||||
|
Significant non-cash activities
|
||||||||||||
|
Acquisition of property, plant and equipment
|
$
|
81
|
$
|
74
|
$
|
32
|
||||||
|
Increase of right-of-use asset recognized with corresponding lease liability
|
$
|
194
|
$
|
90
|
$
|
841
|
||||||
|
Exercise of pre-funded warrants
|
$
|
-
|
$
|
-
|
$
|
4,365
|
||||||
F - 9
| a. |
The Company principally derives its revenues from collaboration and licensing agreements, sales from castor seeds and sales of medical cannabis products in Israel (see Note 5). As to major customers, see Note 22c.
|
| b. |
The Company has the following direct and indirect subsidiaries: Casterra Ag Ltd. (formerly Evofuel Ltd.), Evogene Inc., Biomica Ltd., AgPlenus Ltd., AgPlenus Inc., Lavie Bio Ltd., Lavie Bio Inc., Lavie Tech Inc., Taxon Biosciences, Inc. and Canonic Ltd.
|
F - 10
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTE 1: - GENERAL (Cont.)
| c. |
On August 6, 2019, Corteva Inc. (“Corteva”), through its subsidiary Pioneer Hi-Bred International, Inc., made an investment in the Company’s agriculture biologicals subsidiary, Lavie Bio Ltd., which included a cash investment of $10,000 and the contribution of all shares of Corteva’s wholly owned subsidiary Taxon Biosciences, Inc. in consideration for 27.84% of Lavie Bio Ltd.’s shares. As part of the foregoing transaction, the parties entered into a commercial arrangement, including the grant to Corteva of certain commercialization rights with respect to Lavie Bio Ltd.’s products, mainly in corn and soybean (see also Note 11 and Note 18f(1)).
|
| d. |
In August 2022, an affiliate company of ICL and Lavie Bio Ltd. entered a multi-year collaboration agreement for developing novel bio-stimulant products to enrich fertilizer efficiency. As part of the collaboration, ICL invested through an affiliate company in Lavie Bio Ltd. $10,000 under a SAFE agreement (simple agreement for future equity) (see also Note 13).
|
| e. |
On December 21, 2022, Biomica, signed a definitive agreement for a $20,000 financing round, led by Shanghai Healthcare Capital (“SHC”), out of which $10,000 shall be invested by the Company in Biomica preferred shares. Following the closing of the transaction on April 27, 2023, the Company was diluted to approximately 67% of the share capital of Biomica, on a fully diluted basis, while SHC is holding approximately 20%, on a fully diluted basis.
|
| f. |
In January 2021, the Company entered into a Controlled Equity Offering Sales Agreement, pursuant to which the Company issued 3,803,594 ordinary shares during January and February 2021, in an at-the-market (“ATM”) offering, with a weighted average selling price of $7.36 per share, resulting in gross proceeds of approximately $28,000.
|
F - 11
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTE 1: - GENERAL (Cont.)
| g. |
On February 19, 2021, the Company entered into a new Controlled Equity Offering Sales Agreement, having an aggregate offering price of up to $50,000 (subsequently reduced to approximately $19,500), pursuant to which the Company issued 726,832 ordinary shares during April through September 2021, in an ATM offering, with a weighted average selling price of $3.64 per share, resulting in gross proceeds of approximately $2,600. During December 2022, 28,507 ordinary shares were issued through the ATM offering, with a weighted selling price of $0.77 per share, resulting in gross proceeds of approximately $22. During 2023, 720,221 ordinary shares were issued through the ATM offering, with a weighted selling price of $0.96 per share, resulting in gross proceeds of approximately $695. See also Note 24e.
|
| h. |
On July 17, 2023, the Company entered into securities purchase agreements with certain institutional investors for the sale of 8,500,000 ordinary shares in a registered direct offering at a purchase price of $1.00 per ordinary share (the “offering”). The gross proceeds from the offering amounted to approximately $8,500, before deducting placement agent fees and other offering expenses.
|
| i. |
The Company’s subsidiaries and divisions are split into three operating segments: (1) Agriculture – Evogene seed traits division, Lavie Bio Ltd. and AgPlenus Ltd.; (2) Human health – Biomica Ltd. and Canonic Ltd.; and (3) Industrial – Casterra Ag Ltd. (see also Note 22).
|
| j. |
Definitions
|
| Subsidiary |
- A company that is controlled by the Company (as defined in International Financial Reporting Standards (“IFRS”) 10- Consolidated Financial Statements) and whose accounts are consolidated with those of the Company.
|
| Related parties |
- As defined in International Accounting Standard (“IAS”) 24- Related Party Disclosures.
|
F - 12
EVOGENE LTD. AND ITS SUBSIDIARIES
|
NOTE 2: - SIGNIFICANT ACCOUNTING POLICIES |
| a. |
Basis of presentation of the financial statements:
|
| b. |
Functional currency, presentation currency and foreign currency:
|
| 1. |
Functional currency and presentation currency:
|
| 2. |
Transactions, assets and liabilities in foreign currency:
|
| c. |
Cash equivalents:
|
F - 13
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 2: - |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| d. |
Short-term deposits:
|
| e. |
Inventories:
|
| f. |
Government grants:
|
F - 14
EVOGENE LTD. AND ITS SUBSIDIARIES
| g. |
Leases:
|
|
1.
|
Right-of-use assets
|
|
Years
|
Mainly
|
|||
|
Office space
|
2-8
|
6
|
||
|
Laboratory space
|
2-8
|
6
|
||
|
Motor vehicles
|
3
|
3
|
| 2. |
Lease liabilities
|
F - 15
EVOGENE LTD. AND ITS SUBSIDIARIES
| 3. |
Short-term leases and leases of low-value assets
|
| h. |
Property, plant and equipment:
|
|
%
|
Mainly %
|
|||
|
Laboratory equipment
|
9-30
|
15
|
||
|
Computers and peripheral equipment
|
15-33.33
|
33.33
|
||
|
Office equipment and furniture
|
6-20
|
6
|
||
|
Leasehold improvements
|
see below
|
F - 16
EVOGENE LTD. AND ITS SUBSIDIARIES
| i. |
Intangible assets:
|
|
Years
|
||
|
Pipeline Products
|
17
|
|
|
Potential Products
|
19
|
|
|
Microorganisms Collection
|
20
|
| j. |
Impairment of non-financial assets:
|
F - 17
EVOGENE LTD. AND ITS SUBSIDIARIES
| k. |
Revenue recognition:
|
F - 18
EVOGENE LTD. AND ITS SUBSIDIARIES
| l. |
Taxes on income:
|
| 1. |
Current taxes:
|
| 2. |
Deferred taxes:
|
| m. |
Financial instruments:
|
| 1. |
Financial assets:
|
F - 19
EVOGENE LTD. AND ITS SUBSIDIARIES
| 2. |
Impairment of financial assets:
The Company evaluates at the end of each reporting period the loss allowance for financial debt instruments which are not measured at fair value through profit or loss. The Company has short-term financial assets such as trade receivables in respect of which the Company applies a simplified approach and measures the loss allowance in an amount equal to the lifetime expected credit losses. An impairment loss on debt instruments measured at amortized cost is recognized in profit or loss with a corresponding loss allowance that is offset from the carrying amount of the financial asset.
|
| 3. |
Financial liabilities:
|
| a) |
Financial liabilities measured at amortized cost:
|
| b) |
Financial liabilities measured at fair value through profit or loss:
|
F - 20
EVOGENE LTD. AND ITS SUBSIDIARIES
| 4. |
De-recognition of financial instruments:
|
| a. |
Financial assets:
|
| b. |
Financial liabilities:
|
|
n.
|
Fair value measurement:
|
F - 21
EVOGENE LTD. AND ITS SUBSIDIARIES
|
Level 1
|
-
|
Quoted prices (unadjusted) in active markets for identical assets or liabilities.
|
|
Level 2
|
-
|
Inputs other than quoted prices included within Level 1 that are observable directly or indirectly.
|
|
Level 3
|
-
|
Inputs that are not based on observable market data (valuation techniques which use inputs that are not based on observable market data).
|
| o. |
Employee benefit liabilities:
|
| 1. |
Short-term employee benefits:
|
| 2. |
Post-employment benefits:
|
F - 22
EVOGENE LTD. AND ITS SUBSIDIARIES
NOTE 2: - SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
p.
|
Share-based payment transactions:
|
|
q.
|
Non-controlling interests measurement:
|
F - 23
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 3: - |
SIGNIFICANT ACCOUNTING JUDGMENTS, ESTIMATES AND ASSUMPTIONS USED IN THE PREPARATION OF THE FINANCIAL STATEMENTS
|
| a. |
Judgments:
|
| - |
Determining the timing of satisfaction of performance obligations:
|
| - |
Discount rate for a lease liability:
|
| b. |
Estimates and assumptions:
|
| - |
Government grants:
|
F - 24
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 3: - |
SIGNIFICANT ACCOUNTING JUDGMENTS, ESTIMATES AND ASSUMPTIONS USED IN THE PREPARATION OF THE FINANCIAL STATEMENTS (Cont.)
|
| - |
Legal claims:
|
| - |
Determining the fair value of share-based payment transactions:
|
| - |
Determining the fair value of convertible SAFE:
|
| - |
Leases - Estimating the IBR:
|
F - 25
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 3: - |
SIGNIFICANT ACCOUNTING JUDGMENTS, ESTIMATES AND ASSUMPTIONS USED IN THE PREPARATION OF THE FINANCIAL STATEMENTS (Cont.)
|
| - |
Lease extension and/or termination options:
|
| - |
Intangible assets - Estimating the fair value:
|
F - 26
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 4: - |
DISCLOSURE OF NEW STANDARDS IN THE PERIOD PRIOR TO THEIR ADOPTION
|
| a. |
Amendment to IAS 1, "Presentation of Financial Statements":
|
| • |
Only financial covenants with which an entity must comply on or before the reporting date will affect a liability's classification as current or non-current.
|
| • |
In respect of a liability for which compliance with financial covenants is to be evaluated within twelve months from the reporting date, disclosure is required to enable users of the financial statements to assess the risks related to that liability. The Subsequent Amendment requires disclosure of the carrying amount of the liability, information about the financial covenants, and the facts and circumstances at the end of the reporting period that could result in the conclusion that the entity may have difficulty in complying with the financial covenants.
|
| NOTE 5: - |
COLLABORATION, RESEARCH AND DISTRIBUTION AGREEMENTS-
|
| a. |
In March 2020, AgPlenus Ltd. entered into a multi-year collaboration with Corteva for the discovery and development of novel herbicides. Under the terms of the collaboration agreement, AgPlenus Ltd. and Corteva work together to optimize herbicide product candidates originating from the Company’s pipeline. Successful candidates from this collaboration are expected to be further developed by Corteva (see also Note 23e and Customer A in Note 22c).
|
|
F - 27
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 5: - |
COLLABORATION, RESEARCH AND DISTRIBUTION AGREEMENTS (Cont.)
|
| b. |
In August 2021, Canonic Ltd. entered into an agreement with customer C (see Note 22c) for the distribution in Israel of Canonic Ltd.’s medical cannabis products, through its distribution channels, on a consignment basis to licensed pharmacies, under the Canonic brand. The initial term of the agreement is 36 months.
|
| c. |
In November 2022, Casterra Ag Ltd. entered into an agreement with a customer, under which Casterra Ag Ltd. would sell to the customer castor seeds, equipment, machinery and materials. In November 2023, the agreement was extended until November 1, 2024.
|
| d. |
In June 2023, Casterra Ag Ltd. signed a framework agreement with a leading oil and gas energy company (Customer D, see Note 22c) for the sale of castor varieties at a commercial scale for biofuel production. Under the framework of the agreement, during June 2023, Casterra Ag Ltd. received an order totaling $9,100. In addition, during June 2023 Casterra Ag Ltd. received an additional order totaling approximately $2,200 to supply castor seeds.
|
| e. |
During July 2023, Lavie Bio entered a licensing agreement with Corteva, conferring exclusive rights to Corteva for advancing and commercializing Lavie Bio's lead bio-fungicides, LAV311 and LAV312. Lavie Bio received an initial payment of $5,000, in two installments, a first payment of $2,500 was received during September 2023 (see also Note 24d). In addition, Lavie Bio will also be eligible for additional future milestone payments and royalties from Corteva's sales of the products.
|
| f. |
In August 2022, an affiliate company of ICL and Lavie Bio Ltd. entered a multi-year collaboration agreement for developing novel bio-stimulant products to enrich fertilizer efficiency. Under the Agreement, Lavie Bio Ltd. carries out dedicated product development programs, and Lavie Bio Ltd. and ICL will enter a licensing agreement that will define, among other aspects, Lavie Bio Ltd.’s consideration for commercialization of resulting products by ICL. As part of the collaboration, ICL invested through an affiliate company in Lavie Bio Ltd. $10,000 under a SAFE (see also Note 13).
|
| g. |
In May 2023 Evogene signed an agreement for an EU Horizon grant of approximately €1.2 million to support the creation of oil-seed crops that have high carbon-dioxide assimilation and enhanced drought tolerance. The project is expected to be executed over 32 months. In May 2023 Evogene received a pre-financing payment of approximately €0.9 million (approximately $1,023) from the grant mentioned above.
|
F - 28
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 6: - |
CASH AND CASH EQUIVALENTS |
|
December 31,
|
||||||||
|
2023
|
2022
|
|||||||
|
Cash for immediate withdrawal in USD
|
$
|
19,067
|
$
|
22,315
|
||||
|
Cash for immediate withdrawal in New Israeli Shekels (“NIS”)
|
1,642
|
6,204
|
||||||
|
Cash for immediate withdrawal in Euro and other currencies
|
63
|
461
|
||||||
|
$
|
20,772
|
$
|
28,980
|
|||||
| NOTE 7: - |
MARKETABLE SECURITIES |
|
December 31,
|
||||||||
|
2023
|
2022
|
|||||||
|
Corporate bonds and government treasury notes
|
$
|
-
|
$
|
6,375
|
||||
| NOTE 8: - |
OTHER RECEIVABLES AND PREPAID EXPENSES |
|
December 31,
|
||||||||
|
2023
|
2022
|
|||||||
|
Government authorities
|
$
|
226
|
$
|
284
|
||||
|
Grant receivables
|
88
|
63
|
||||||
|
Patent cost reimbursement
|
-
|
6
|
||||||
|
Prepaid expenses
|
909
|
995
|
||||||
|
Restricted cash
|
-
|
32
|
||||||
|
Suppliers advances
|
1,617
|
-
|
||||||
|
Other
|
133
|
102
|
||||||
|
$
|
2,973
|
$
|
1,482
|
|||||
| NOTE 9: - |
LEASES |
F - 29
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 9: - |
LEASES (Cont.) |
| a. |
Information on leases in which the Company is a lessee:
|
|
Year ended December 31
|
||||||||
|
2023
|
2022
|
|||||||
|
Interest expense on lease liabilities
|
$
|
115
|
$
|
165
|
||||
|
Exchange rate differences
|
(57
|
)
|
(237
|
)
|
||||
|
CPI recognized on lease liabilities and right-of-use assets
|
39
|
95
|
||||||
|
Depreciation expenses on right-of-use assets
|
805
|
726
|
||||||
|
Expense due to removal of lease liabilities and right-of-use assets
|
-
|
(1
|
)
|
|||||
| b. |
Lease extension and cancelation options:
|
F - 30
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 9: - |
LEASES (Cont.) |
| c. |
Disclosures of right-of-use assets:
|
|
Leasehold
|
Motor vehicles
|
Total
|
||||||||||
|
Cost:
|
||||||||||||
|
Balance as of January 1, 2023
|
$
|
3,522
|
$
|
747
|
$
|
4,269
|
||||||
|
Additions during the year:
|
||||||||||||
|
Additions to right-of-use assets for new leases in the period
|
-
|
194
|
194
|
|||||||||
|
Revaluation recognized in CPI
|
31
|
8
|
39
|
|||||||||
|
Disposals during the year:
|
||||||||||||
|
Disposals of right-of-use assets for leases terminated in the period
|
-
|
(75
|
)
|
(75
|
)
|
|||||||
|
Balance as of December 31, 2023
|
3,553
|
874
|
4,427
|
|||||||||
|
Accumulated depreciation:
|
||||||||||||
|
Balance as of January 1, 2023
|
2,276
|
425
|
2,701
|
|||||||||
|
Additions during the year:
|
||||||||||||
|
Depreciation
|
592
|
213
|
805
|
|||||||||
|
Disposals during the year:
|
||||||||||||
|
Disposals of right-of-use assets
|
-
|
(59
|
)
|
(59
|
)
|
|||||||
|
Balance as of December 31, 2023
|
2,868
|
579
|
3,447
|
|||||||||
|
Depreciated cost on December 31, 2023
|
$
|
685
|
$
|
295
|
$
|
980
|
||||||
|
Leasehold
|
Motor vehicles
|
Total
|
||||||||||
|
Cost:
|
||||||||||||
|
Balance as of January 1, 2022
|
$
|
3,441
|
$
|
744
|
$
|
4,185
|
||||||
|
Additions during the year:
|
||||||||||||
|
Additions to right-of-use assets for new leases in the period
|
-
|
102
|
102
|
|||||||||
|
Revaluation recognized in CPI
|
81
|
14
|
95
|
|||||||||
|
Disposals during the year:
|
||||||||||||
|
Disposals of right-of-use assets for leases terminated in the period
|
-
|
(113
|
)
|
(113
|
)
|
|||||||
|
Balance as of December 31, 2022
|
3,522
|
747
|
4,269
|
|||||||||
|
Accumulated depreciation:
|
||||||||||||
|
Balance as of January 1, 2022
|
1,718
|
358
|
2,076
|
|||||||||
|
Additions during the year:
|
||||||||||||
|
Depreciation
|
558
|
168
|
726
|
|||||||||
|
Disposals during the year:
|
||||||||||||
|
Disposals of right-of-use assets
|
-
|
(101
|
)
|
(101
|
)
|
|||||||
|
Balance as of December 31, 2022
|
2,276
|
425
|
2,701
|
|||||||||
|
Depreciated cost on December 31, 2022
|
$
|
1,246
|
$
|
322
|
$
|
1,568
|
||||||
F - 31
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 9: - |
LEASES (Cont.) |
| d. |
Disclosures of lease liability:
|
|
Leasehold
|
Motor vehicles
|
Total
|
||||||||||
|
Balance as of January 1, 2023
|
$
|
1,536
|
$
|
280
|
$
|
1,816
|
||||||
|
Lease payments
|
(744
|
)
|
(207
|
)
|
(951
|
)
|
||||||
|
Lease deposits
|
-
|
(2
|
)
|
(2
|
)
|
|||||||
|
Interest expense
|
90
|
25
|
115
|
|||||||||
|
Exchange rate differences
|
(45
|
)
|
(12
|
)
|
(57
|
)
|
||||||
|
Additions to lease liability for new leases in the period
|
-
|
194
|
194
|
|||||||||
|
Reduction of lease liability for leases terminated in the period
|
-
|
(16
|
)
|
(16
|
)
|
|||||||
|
Revaluation recognized in CPI
|
31
|
8
|
39
|
|||||||||
|
Balance as of December 31, 2023
|
$
|
868
|
$
|
270
|
$
|
1,138
|
||||||
|
Leasehold
|
Motor vehicles
|
Total
|
||||||||||
|
Balance as of January 1, 2022
|
$
|
2,286
|
$
|
383
|
$
|
2,669
|
||||||
|
Lease payments
|
(770
|
)
|
(187
|
)
|
(957
|
)
|
||||||
|
Lease deposits
|
-
|
(10
|
)
|
(10
|
)
|
|||||||
|
Interest expense
|
143
|
22
|
165
|
|||||||||
|
Exchange rate differences
|
(204
|
)
|
(33
|
)
|
(237
|
)
|
||||||
|
Additions to lease liability for new leases in the period
|
-
|
102
|
102
|
|||||||||
|
Reduction of lease liability for leases terminated in the period
|
-
|
(11
|
)
|
(11
|
)
|
|||||||
|
Revaluation recognized in CPI
|
81
|
14
|
95
|
|||||||||
|
Balance as of December 31, 2022
|
$
|
1,536
|
$
|
280
|
$
|
1,816
|
||||||
|
Leasehold
|
Motor vehicles
|
Total
|
||||||||||
|
2024
|
$
|
727
|
$
|
194
|
$
|
921
|
||||||
|
2025
|
110
|
101
|
211
|
|||||||||
|
2026
|
70
|
33
|
103
|
|||||||||
|
2027
|
41
|
-
|
41
|
|||||||||
|
2028
|
21
|
-
|
21
|
|||||||||
|
Total lease payments
|
$
|
969
|
$
|
328
|
$
|
1,297
|
||||||
|
Less: imputed interest
|
(101
|
)
|
(58
|
)
|
(159
|
)
|
||||||
|
Present value of lease liabilities
|
$
|
868
|
$
|
270
|
$
|
1,138
|
||||||
F - 32
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 10: - |
PROPERTY, PLANT AND EQUIPMENT, NET
|
|
Laboratory equipment
|
Computers and peripheral equipment
|
Office equipment and furniture
|
Leasehold improvements
|
Total
|
||||||||||||||||
|
Cost:
|
||||||||||||||||||||
|
Balance on January 1, 2023
|
$
|
4,655
|
$
|
2,040
|
$
|
253
|
$
|
13,866
|
$
|
20,814
|
||||||||||
|
Additions
|
380
|
179
|
73
|
166
|
798
|
|||||||||||||||
|
Deductions
|
(41
|
)
|
-
|
-
|
-
|
(41
|
)
|
|||||||||||||
|
Balance on December 31, 2023
|
4,994
|
2,219
|
326
|
14,032
|
21,571
|
|||||||||||||||
|
Accumulated Depreciation:
|
||||||||||||||||||||
|
Balance on January 1, 2023
|
3,998
|
1,553
|
189
|
12,575
|
18,315
|
|||||||||||||||
|
Additions
|
325
|
261
|
14
|
242
|
842
|
|||||||||||||||
|
Deductions
|
(41
|
)
|
-
|
-
|
-
|
(41
|
)
|
|||||||||||||
|
Balance on December 31, 2023
|
4,282
|
1,814
|
203
|
12,817
|
19,116
|
|||||||||||||||
|
Depreciated cost on December 31, 2023
|
$
|
712
|
$
|
405
|
$
|
123
|
$
|
1,215
|
$
|
2,455
|
||||||||||
|
Laboratory equipment
|
Computers and peripheral equipment
|
Office equipment and furniture
|
Leasehold improvements
|
Total
|
||||||||||||||||
|
Cost:
|
||||||||||||||||||||
|
Balance on January 1, 2022
|
$
|
4,256
|
$
|
1,608
|
$
|
253
|
$
|
13,512
|
$
|
19,629
|
||||||||||
|
Additions
|
399
|
432
|
-
|
354
|
1,185
|
|||||||||||||||
|
Balance on December 31, 2022
|
4,655
|
2,040
|
253
|
13,866
|
20,814
|
|||||||||||||||
|
Accumulated Depreciation:
|
||||||||||||||||||||
|
Balance on January 1, 2022
|
3,672
|
1,347
|
176
|
12,361
|
17,556
|
|||||||||||||||
|
Additions
|
326
|
206
|
13
|
214
|
759
|
|||||||||||||||
|
Balance on December 31, 2022
|
3,998
|
1,553
|
189
|
12,575
|
18,315
|
|||||||||||||||
|
Depreciated cost on December 31, 2022
|
$
|
657
|
$
|
487
|
$
|
64
|
$
|
1,291
|
$
|
2,499
|
||||||||||
F - 33
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 11: - |
INTANGIBLE ASSETS, NET |
|
Pipeline Products
|
Potential Products
|
Microorganisms Collection
|
Total
|
|||||||||||||
|
Cost:
|
||||||||||||||||
|
Balance on January 1, 2023
|
$
|
7,028
|
$
|
4,920
|
$
|
5,500
|
$
|
17,448
|
||||||||
|
Additions
|
-
|
-
|
-
|
-
|
||||||||||||
|
Balance on December 31, 2023
|
$
|
7,028
|
$
|
4,920
|
$
|
5,500
|
$
|
17,448
|
||||||||
|
Accumulated Depreciation:
|
||||||||||||||||
|
Balance on January 1, 2023
|
$
|
1,374
|
$
|
862
|
$
|
1,072
|
$
|
3,308
|
||||||||
|
Additions
|
403
|
253
|
315
|
971
|
||||||||||||
|
Balance on December 31, 2023
|
1,777
|
1,115
|
1,387
|
4,279
|
||||||||||||
|
Amortized cost on December 31, 2023
|
$
|
5,251
|
$
|
3,805
|
$
|
4,113
|
$
|
13,169
|
||||||||
|
Pipeline Products
|
Potential Products
|
Microorganisms Collection
|
Total
|
|||||||||||||
|
Cost:
|
||||||||||||||||
|
Balance on January 1, 2022
|
$
|
7,028
|
$
|
4,920
|
$
|
5,500
|
$
|
17,448
|
||||||||
|
Additions
|
-
|
-
|
-
|
-
|
||||||||||||
|
Balance on December 31, 2022
|
$
|
7,028
|
$
|
4,920
|
$
|
5,500
|
$
|
17,448
|
||||||||
|
Accumulated Depreciation:
|
||||||||||||||||
|
Balance on January 1, 2022
|
$
|
971
|
$
|
609
|
$
|
661
|
$
|
2,241
|
||||||||
|
Additions
|
403
|
253
|
411
|
1,067
|
||||||||||||
|
Balance on December 31, 2022
|
1,374
|
862
|
1,072
|
3,308
|
||||||||||||
|
Amortized cost on December 31, 2022
|
$
|
5,654
|
$
|
4,058
|
$
|
4,428
|
$
|
14,140
|
||||||||
| NOTE 12: - |
LIABILITIES IN RESPECT OF GOVERNMENT GRANTS
|
|
2023
|
2022
|
|||||||
|
Balance on January 1,
|
$
|
4,744
|
$
|
4,396
|
||||
|
Grants received *)
|
66
|
212
|
||||||
|
Royalties paid
|
(73
|
)
|
(31
|
)
|
||||
|
Amounts recorded in profit or loss
|
77
|
167
|
||||||
|
Balance on December 31,
|
$
|
4,814
|
$
|
4,744
|
||||
F - 34
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 12: - |
LIABILITIES IN RESPECT OF GOVERNMENT GRANTS (Cont.)
|
| NOTE 13: - |
CONVERTIBLE SAFE
|
F - 35
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 14: - |
FINANCIAL INSTRUMENTS
|
| a. |
Classification of financial instruments by fair value hierarchy:
|
|
December 31,
|
||||||||
|
2023
|
2022
|
|||||||
|
Financial assets:
|
||||||||
|
Marketable securities – Level 1
|
$
|
-
|
$
|
6,375
|
||||
|
$
|
-
|
$
|
6,375
|
|||||
| b. |
Financial risk factors:
|
|
1.
|
Market Risk:
|
| a. |
Foreign currency risk:
|
| b. |
Price risk:
|
| 2. |
Credit Risk:
|
F - 36
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 14: - |
FINANCIAL INSTRUMENTS (Cont.)
|
| 3. |
Liquidity Risk:
|
|
Up to 1 year
|
1 year to 2 years
|
2 years
to 3 years
|
3 years to 4 years
|
4 years to 5 years
|
Over 5 years
|
Total
|
||||||||||||||||||||||
|
Trade payables
|
$
|
1,785
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
1,785
|
||||||||||||||
|
Employees and payroll accruals
|
2,537
|
- |
-
|
-
|
- |
-
|
2,537
|
|||||||||||||||||||||
|
Other payables
|
1,019
|
- |
-
|
-
|
- |
-
|
1,019
|
|||||||||||||||||||||
|
Leases liability
|
921
|
211
|
103
|
41
|
21
|
-
|
1,297
|
|||||||||||||||||||||
|
Liabilities in respect of government grants
|
388
|
676
|
778
|
1,123
|
1,566
|
1,315
|
5,846
|
|||||||||||||||||||||
|
$
|
6,650
|
$
|
887
|
$
|
881
|
$
|
1,164
|
$
|
1,587
|
$
|
1,315
|
$
|
12,484
|
|||||||||||||||
|
Up to 1 year
|
1 year to 2 years
|
2 years
to 3 years
|
3 years to 4 years
|
4 years to 5 years
|
Over 5 years
|
Total
|
||||||||||||||||||||||
|
Trade payables
|
$
|
1,036
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
1, 036
|
||||||||||||||
|
Employees and payroll accruals
|
1,987
|
-
|
-
|
-
|
-
|
-
|
1,987
|
|||||||||||||||||||||
|
Other payables
|
1,617
|
-
|
-
|
-
|
-
|
-
|
1,617
|
|||||||||||||||||||||
|
Leases liability
|
943
|
819
|
131
|
70
|
41
|
21
|
2,025
|
|||||||||||||||||||||
|
Liabilities in respect of government grants
|
79
|
251
|
573
|
1,085
|
1,841
|
1,962
|
5,791
|
|||||||||||||||||||||
|
$
|
5,662
|
$
|
1,070
|
$
|
704
|
$
|
1,155
|
$
|
1,882
|
$
|
1,983
|
$
|
12,456
|
|||||||||||||||
| c. |
Fair Value:
|
F - 37
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 14: - |
FINANCIAL INSTRUMENTS (Cont.)
|
| d. |
Sensitivity tests relating to changes in market factors:
|
|
December 31,
|
||||||||
|
2023
|
2022
|
|||||||
|
Sensitivity test to changes in the NIS/USD exchange rate:
|
||||||||
|
Gain (loss) from the change:
|
||||||||
|
Decrease of 5% in the U.S. dollar relative to the NIS
|
$
|
(377
|
)
|
$
|
(69
|
)
|
||
|
Increase of 5% in the U.S. dollar relative to the NIS
|
$
|
377
|
$
|
69
|
||||
|
Sensitivity test to changes in the market price of listed securities:
|
||||||||
|
Gain (loss) from the change:
|
||||||||
|
Increase of 5% in market price
|
$
|
-
|
$
|
319
|
||||
|
Decrease of 5% in market price
|
$
|
-
|
$
|
(319
|
)
|
|||
| NOTE 15: - |
COMMITMENTS AND CONTINGENT LIABILITIES |
| a. |
Claims:
|
| b. |
Government grants:
|
F - 38
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 16: - |
SEVERANCE PAY LIABILITY
|
|
Year ended December 31,
|
||||||||||||
|
2023
|
2022
|
2021
|
||||||||||
|
Expenses – defined contribution plan
|
$
|
816
|
$
|
877
|
$
|
837
|
||||||
| NOTE 17: - |
TAXES ON INCOME
|
|
a.
|
Tax rates applicable to the Company and its subsidiaries:
|
| 1. |
The Israeli corporate income tax rate was 23% for all years presented.
|
| 2. |
The Company’s U.S. subsidiaries, Evogene Inc., Lavie Bio Inc., Lavie Tech Inc., Taxon Biosciences, Inc., and AgPlenus Inc., are subject to U.S. income taxes. During the years 2021 through 2023, the tax rates applicable to those companies, based on the main state where the companies had the most presence, were 21% (federal tax applicable for the years 2021, 2022 and 2023), approximately 3.41% (state tax applicable for 2023) and approximately 6.5% (state tax applicable for the years 2021and 2022).
|
|
b.
|
Tax assessments:
|
| c. |
Carryforward losses for tax purposes and other temporary differences:
|
F - 39
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 17: - |
TAXES ON INCOME (Cont.)
|
| d. |
Deferred taxes:
|
| e. |
Theoretical tax:
|
| NOTE 18: - |
SHAREHOLDERS' EQUITY |
| a. |
Share capital:
|
|
December 31,
|
||||||||||||||||
|
2023
|
2022
|
|||||||||||||||
|
Authorized
|
Issued and Outstanding
|
Authorized
|
Issued and Outstanding
|
|||||||||||||
|
Number of shares
|
||||||||||||||||
|
Ordinary shares of NIS 0.02 par value each
|
150,000,000
|
50,584,888
|
150,000,000
|
41,260,439
|
||||||||||||
| b. |
Changes in share capital:
|
|
Number of shares
|
NIS par value
|
|||||||
|
Outstanding on January 1, 2022
|
41,170,168
|
823,404
|
||||||
|
Exercise of options and vesting of RSUs
|
61,764
|
1,235
|
||||||
|
Issuance of ordinary shares
|
28,507
|
570
|
||||||
|
Outstanding on December 31, 2022
|
41,260,439
|
825,209
|
||||||
|
Exercise of options and vesting of RSUs
|
104,228
|
2,085
|
||||||
|
Issuance of ordinary shares
|
9,220,221
|
184,404
|
||||||
|
Outstanding on December 31, 2023
|
50,584,888
|
1,011,698
|
||||||
F - 40
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 18: - |
SHAREHOLDERS' EQUITY (Cont.) |
| 1. |
On January 14, 2021, the Company entered a Controlled Equity Offering Sales Agreement, pursuant to which it issued 3,803,594 ordinary shares during January and February 2021, in an ATM offering, with a weighted average selling price of $7.36 per share, resulting in gross proceeds of approximately $28,000.
|
| 2. |
On February 19, 2021, the Company entered a new Controlled Equity Offering Sales Agreement. In accordance with the terms of the sales agreement, from time to time the Company may offer and sell its ordinary shares in an ATM offering having an aggregate offering price of up to $50,000, which was subsequently reduced to approximately $19,500. During April through September 2021, 726,832 ordinary shares were issued through the ATM offering, with a weighted average selling price of $3.64 per share, resulting in gross proceeds of approximately $2,600.
|
| 3. |
During December 2022, 28,507 ordinary shares were issued through the ATM offering, with a weighted selling price of $0.77 per share, resulting in gross proceeds of approximately $22.
|
| 4. |
During 2023, 720,221 ordinary shares were issued through the ATM offering, with a weighted selling price of $0.96 per share, resulting in gross proceeds of approximately $695.
|
| 5. |
On July 17, 2023, Evogene Ltd. entered into securities purchase agreements with certain institutional investors for the sale of 8,500,000 ordinary shares in a registered direct offering at a purchase price of $1.00 per ordinary share. The gross proceeds from the offering amounted to approximately $8,500, before deducting placement agent fees and other offering expenses.
|
| c. |
Rights attached to shares:
|
| d. |
Rights attached to pre-funded warrants:
|
F - 41
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 18: - |
SHAREHOLDERS' EQUITY (Cont.) |
| e. |
Capital management in the Company:
|
| f. |
Composition of non-controlling interests in the statement of financial position:
|
|
December 31,
|
||||||||
|
2023
|
2022
|
|||||||
|
Balance on January 1,
|
$
|
6,860
|
$
|
9,767
|
||||
|
Forfeiture of non-controlling interests regarding share-based compensation
|
(71
|
)
|
(272
|
)
|
||||
|
Share-based compensation
|
1,351
|
569
|
||||||
|
Issuance of a subsidiary ordinary shares to the company
|
809
|
-
|
||||||
|
Issuance of a subsidiary preferred shares to non-controlling interests
|
9,761
|
-
|
||||||
|
Benefit to non-controlling interests regarding share-based compensation
|
(3
|
)
|
2
|
|||||
|
Loss attributed to non-controlling interests
|
(2,075
|
)
|
(3,206
|
)
|
||||
|
Balance on December 31,
|
$
|
16,632
|
$
|
6,860
|
||||
| 1. |
On August 6, 2019, Corteva, through its subsidiary Pioneer Hi-Bred International, Inc., made an investment in the Company's agriculture biologicals subsidiary, Lavie Bio Ltd., which included the contribution of all Corteva’s holdings in its wholly owned subsidiary Taxon Biosciences, Inc. along with an amount of $10,000. Upon consummation of the foregoing transactions, Corteva was issued 27.84% of Lavie Bio Ltd.’s equity while Evogene Ltd. held 72.16% of Lavie Bio Ltd.’s equity following such investment. As a result, the Company recorded a share premium and a non-controlling interest in the amounts of $17,406 and $10,042, respectively.
On November 16, 2021, 203,826 options were exercised in Lavie Bio Ltd. into its ordinary shares. Upon the exercise of options, the non-controlling interest was issued 1.99% of Lavie Bio Ltd.'s equity. As a result, the Company recorded an increase in non-controlling interest in the amount of $378.
On January 31, 2022, 8,270 options were exercised in Lavie Bio Ltd. into its ordinary shares. Upon the exercise of options, the non-controlling interest was issued 0.08% of Lavie Bio Ltd.'s equity. As a result, the Company recorded an increase in non-controlling interest in the amount less than $1.
|
F - 42
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 18: - |
SHAREHOLDERS' EQUITY (Cont.)
|
| 2. |
On July 24, 2020, 36,520 options were exercised in AgPlenus Ltd. into its ordinary shares. Upon the exercise of options, the non-controlling interest was issued 1.66% of AgPlenus Ltd.'s equity. As a result, the Company recorded an increase in non-controlling interest in the amount $82.
|
| 3. |
On December 21, 2022, Biomica, signed a definitive agreement for a $20,000 financing round, led by SHC, out of which $10,000 shall be invested by the Company in Biomica preferred shares. As a result, the Company recorded a negative capital reserve and an increase of non-controlling interest in the amounts of $238 and $9,761, respectively. In addition, certain convertible loans in total amount of $10,000 were converted by the Company to Biomica’s ordinary shares. As a result, the Company recorded an adjustment to capital reserve and non-controlling interest in the amount of $809. Following the closing of the transaction on April 27, 2023, the Company was diluted to approximately 67% of the share capital of Biomica, on a fully diluted basis, while SHC is holding approximately 20%, on a fully diluted basis.
|
| NOTE 19: - |
SHARE- BASED COMPENSATION |
| a. |
Expenses recognized in the financial statements:
|
|
Year ended December 31,
|
||||||||||||
|
2023
|
2022
|
2021
|
||||||||||
|
Share-based compensation – Attributable to equity holders of the Company
|
$
|
526
|
$
|
617
|
$
|
892
|
||||||
|
Share-based compensation – Attributable to non-controlling interests (see Note 18f)
|
1,351
|
569
|
1,717
|
|||||||||
|
$
|
1,877
|
$
|
1,186
|
$
|
2,609
|
|||||||
| b. |
The Company maintains four share option and incentive plans: Evogene Ltd. 2002 Share Option Plan, Evogene Ltd. 2003 Key Employee Share Incentive Plan, Evogene Ltd. 2013 Share Option Plan and Evogene Ltd. 2021 Share Incentive Plan (the “2021 Plan”). All such option and incentive plans provide for the grant of options to purchase the Company's ordinary shares that generally expire 10 years from the grant date.
|
|
F - 43
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 19: - |
SHARE- BASED COMPENSATION (Cont.) |
| c. |
Evogene Ltd. Share-based payment plan for employees, directors and consultants:
|
| d. |
Evogene Ltd. Share options activity:
|
|
2023
|
2022
|
2021
|
||||||||||||||||||||||
|
Number of options
|
Weighted average exercise prices ($)
|
Number of options
|
Weighted average exercise prices ($)
|
Number of options
|
Weighted average exercise prices ($)
|
|||||||||||||||||||
|
Outstanding on January 1,
|
4,036,024
|
4.17
|
4,233,950
|
5.54
|
4,030,702
|
6.24
|
||||||||||||||||||
|
Granted
|
626,000
|
0.80
|
605,500
|
1.04
|
987,750
|
3.69
|
||||||||||||||||||
|
Exercised
|
-
|
-
|
(5,624
|
)
|
1.09
|
(151,995
|
)
|
2.81
|
||||||||||||||||
|
Forfeited
|
(687,506
|
)
|
7.81
|
(797,802
|
)
|
5.75
|
(632,507
|
)
|
9.06
|
|||||||||||||||
|
Outstanding at December 31,
|
3,974,518
|
2.88
|
4,036,024
|
4.17
|
4,233,950
|
5.54
|
||||||||||||||||||
|
Exercisable at December 31,
|
2,841,828
|
3.48
|
2,755,280
|
5.32
|
2,558,643
|
7.31
|
||||||||||||||||||
|
Options outstanding
|
||||||||||||
|
Range of exercise prices ($)
|
Number outstanding
|
Average
remaining
contractual
life
|
Weighted
average
exercise
price
|
|||||||||
|
0.47 – 1.00
|
761,000
|
8.99
|
0.78
|
|||||||||
|
1.02 – 1.79
|
1,320,068
|
7.03
|
1.19
|
|||||||||
|
2.24 – 4.91
|
1,220,450
|
6.02
|
3.24
|
|||||||||
|
5.15 -7.66
|
419,500
|
3.76
|
5.83
|
|||||||||
|
10.41 – 17.80
|
253,500
|
1.14
|
11.49
|
|||||||||
|
Total
|
3,974,518
|
6.37
|
2.88
|
|||||||||
F - 44
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 19: - |
SHARE- BASED COMPENSATION (Cont.) |
|
2023
|
2022
|
2021
|
||||
|
Dividend yield (%)
|
-
|
-
|
-
|
|||
|
Expected volatility of the share prices (%)
|
51-53
|
48-50
|
43-47
|
|||
|
Risk-free interest rate (%)
|
3.4-4.4
|
1.5-3.5
|
0.9-1.9
|
|||
|
Suboptimal factor
|
1.8-2
|
1.8-2
|
1.8-2
|
|||
|
Post-vesting forfeiture rate (%)
|
5-20
|
5-20
|
5-10
|
| e. |
Evogene Ltd. RSUs activity:
|
| 2023 |
2022
|
|||||||||||||||
|
Number of RSUs
|
Weighted average grant date fair value
|
Number of RSUs
|
Weighted average grant date fair value
|
|||||||||||||
|
Outstanding on January 1
|
196,580
|
2.55
|
247,775
|
2.28
|
||||||||||||
|
Granted
|
352,600
|
0.75
|
58,200
|
1.23
|
||||||||||||
|
Vested
|
(104,228
|
)
|
1.87
|
(56,140
|
)
|
3.06
|
||||||||||
|
Forfeited
|
(30,750
|
)
|
2.01
|
(53,255
|
)
|
2.54
|
||||||||||
|
Outstanding at December 31
|
414,202
|
1.24
|
196,580
|
2.55
|
||||||||||||
F - 45
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 19: - |
SHARE- BASED COMPENSATION (Cont.) |
|
|
f. |
The Company's subsidiaries maintain share option and incentive plans with similar terms and conditions.
|
|
2023
|
2022
|
|||||||||||||||
|
Number of options
|
Weighted average exercise prices ($)
|
Number of options
|
Weighted average exercise prices ($)
|
|||||||||||||
|
Outstanding on January 1,
|
2,273,489
|
1.72
|
1,901,992
|
1.39
|
||||||||||||
|
Granted
|
854,139
|
2.10
|
877,689
|
2.74
|
||||||||||||
|
Exercised
|
-
|
-
|
(8,270
|
)
|
0.20
|
|||||||||||
|
Forfeited
|
(596,494
|
)
|
2.68
|
(497,922
|
)
|
4.05
|
||||||||||
|
Outstanding on December 31,
|
2,531,134
|
1.63
|
2,273,489
|
1.72
|
||||||||||||
|
Exercisable on December 31,
|
1,530,420
|
1.09
|
1,194,122
|
0.58
|
||||||||||||
| g. |
The fair value of Company's subsidiaries’ share options granted to employees, directors and consultants for the years ended December 31, 2023 and 2022 was estimated using the binomial model with the following assumptions:
|
|
2023
|
2022
|
||||
|
Dividend yield (%)
|
-
|
-
|
|||
|
Expected volatility of the share prices (%)
|
61-84
|
65-90
|
|||
|
Risk-free interest rate (%)
|
3.52-5.05
|
0.44-4.75
|
|||
|
Suboptimal factor
|
1.8-2.0
|
1.8-2.0
|
|||
|
Post-vesting forfeiture rate (%)
|
5-10
|
5-10
|
| NOTE 20: - |
STATEMENTS OF PROFIT OR LOSS – ADDITIONAL INFORMATION |
| a. |
Cost of revenues:
|
|
Year ended December 31,
|
||||||||||||
|
2023
|
2022
|
2021
|
||||||||||
|
Salaries and benefits
|
$
|
432
|
$
|
238
|
$
|
514
|
||||||
|
Materials and sub-contractors
|
1,260
|
671
|
253
|
|||||||||
|
$
|
1,692
|
$
|
909
|
$
|
767
|
|||||||
F - 46
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 20: - |
STATEMENTS OF COMPREHENSIVE LOSS – ADDITIONAL INFORMATION (Cont.) |
| b. |
Research and development, net:
|
|
Year ended December 31,
|
||||||||||||
|
2023
|
2022
|
2021
|
||||||||||
|
Salaries and benefits
|
$
|
9,862
|
$
|
11,545
|
$
|
10,841
|
||||||
|
Share-based compensation
|
764
|
593
|
1,348
|
|||||||||
|
Materials and sub-contractors
|
6,349
|
5,514
|
5,709
|
|||||||||
|
Plant growth and greenhouse maintenance
|
744
|
839
|
802
|
|||||||||
|
Office maintenance
|
639
|
437
|
682
|
|||||||||
|
Depreciation and amortization
|
2,549
|
2,540
|
2,234
|
|||||||||
|
Loss (Gain) from derecognition of property, plant and equipment
|
(26
|
)
|
-
|
121
|
||||||||
|
Participation in respect of government grants
|
(143
|
)
|
(726
|
)
|
(658
|
)
|
||||||
|
Other
|
39
|
50
|
46
|
|||||||||
|
$
|
20,777
|
$
|
20,792
|
$
|
21,125
|
|||||||
| c. |
Sales and marketing:
|
|
Year ended December 31,
|
||||||||||||
|
2023
|
2022
|
2021
|
||||||||||
|
Salaries and benefits
|
$
|
1,996
|
$
|
2,475
|
$
|
1,424
|
||||||
|
Share-based compensation
|
595
|
323
|
574
|
|||||||||
|
Subcontractors and professional fees
|
855
|
883
|
554
|
|||||||||
|
Travel
|
142
|
76
|
39
|
|||||||||
|
Legal
|
10
|
120
|
87
|
|||||||||
|
Other
|
13
|
56
|
60
|
|||||||||
|
$
|
3,611
|
$
|
3,933
|
$
|
2,738
|
|||||||
| d. |
General and administrative:
|
|
Year ended December 31,
|
||||||||||||
|
2023
|
2022
|
2021
|
||||||||||
|
Salaries and benefits
|
$
|
2,902
|
$
|
2,929
|
$
|
2,866
|
||||||
|
Share-based compensation
|
518
|
270
|
687
|
|||||||||
|
Professional fees
|
2,281
|
2,876
|
3,484
|
|||||||||
|
Other
|
367
|
407
|
216
|
|||||||||
|
$
|
6,068
|
$
|
6,482
|
$
|
7,253
|
|||||||
| e. |
Other income:
|
F - 47
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 20: - |
STATEMENTS OF COMPREHENSIVE LOSS – ADDITIONAL INFORMATION (Cont.) |
| f. |
Financing income and expenses
|
|
Year ended December 31,
|
||||||||||||
|
2023
|
2022
|
2021
|
||||||||||
|
Exchange differences
|
$
|
167
|
$
|
319
|
$
|
1,525
|
||||||
|
Interest income
|
1,248
|
182
|
291
|
|||||||||
|
Financial income in respect of government grants
|
26
|
15
|
-
|
|||||||||
|
Change in the fair value of marketable securities
|
45
|
-
|
119
|
|||||||||
|
$
|
1,486
|
$
|
516
|
$
|
1,935
|
|||||||
|
Year ended December 31,
|
||||||||||||
|
2023
|
2022
|
2021
|
||||||||||
|
Bank expenses and commissions
|
$
|
56
|
$
|
76
|
$
|
88
|
||||||
|
Exchange differences
|
412
|
2,060
|
460
|
|||||||||
|
Change in the fair value of marketable securities
|
-
|
721
|
181
|
|||||||||
|
Revaluation of pre-funded warrants
|
-
|
-
|
212
|
|||||||||
|
Lease liability interest
|
115
|
165
|
315
|
|||||||||
|
Revaluation of Convertible SAFE
|
254
|
114
|
-
|
|||||||||
|
Financial expenses in respect of government grants
|
128
|
193
|
158
|
|||||||||
|
$
|
965
|
$
|
3,329
|
$
|
1,414
|
|||||||
| NOTE 21: - |
LOSS PER SHARE |
|
Year ended December 31,
|
||||||||||||||||||||||||
|
2023
|
2022
|
2021
|
||||||||||||||||||||||
|
Weighted number of shares *)
|
Loss attributable to equity holders of the Company
|
Weighted number of shares *)
|
Loss attributable to equity holders of the Company
|
Weighted number of shares *)
|
Loss attributable to equity holders of the Company
|
|||||||||||||||||||
|
Number of shares and loss
|
45,685,619
|
(23,879
|
)
|
41,210,184
|
(26,638
|
)
|
40,433,303
|
(27,793
|
)
|
|||||||||||||||
| *) |
To compute diluted loss per share, potential ordinary shares have not been taken into account due to their anti-dilutive effect. See Notes 19(d) and Note 19(e) for number of outstanding options and RSUs.
|
F - 48
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 22: - |
OPERATING SEGMENTS |
| a. |
General:
|
|
Agriculture segment
|
-
|
Develops seed traits, ag-chemical products, and ag-biological products to improve plant performance.
|
|
Industrial applications segment
|
-
|
Develops improved castor bean seeds to serve as a feedstock source for other industrial uses.
|
|
Human health segment
|
-
|
Discovers and develops human microbiome-based therapeutics and cannabis activity.
|
|
Unallocated
|
-
|
Other corporate expenses and general development of enabling technologies discovery and optimization.
|
|
b.
|
The following table presents our revenues and operating loss by segments:
|
|
Agriculture
|
Industrial
application
|
Human
health
|
Unallocated
|
Total
|
||||||||||||||||
|
For the Year Ended December 31, 2023
|
||||||||||||||||||||
|
Revenues
|
$
|
3,791
|
$
|
1,075
|
$
|
487
|
$
|
287
|
$
|
5,640
|
||||||||||
|
Operating loss
|
$
|
(11,100
|
)
|
$
|
(39
|
)
|
$
|
(10,349
|
)
|
$
|
(5,020
|
)
|
$
|
(26,508
|
)
|
|||||
|
Net financing income
|
$
|
521
|
||||||||||||||||||
|
Loss before taxes on income
|
$
|
(25,987
|
)
|
|||||||||||||||||
F - 49
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 22: - |
OPERATING SEGMENTS (Cont.)
|
|
Agriculture
|
Industrial
application
|
Human
health
|
Unallocated
|
Total
|
||||||||||||||||
|
For the Year Ended December 31, 2022
|
||||||||||||||||||||
|
Revenues
|
$
|
876
|
$
|
72
|
$
|
513
|
$
|
214
|
$
|
1,675
|
||||||||||
|
Operating loss
|
$
|
(12,256
|
)
|
$
|
(220
|
)
|
$
|
(8,875
|
)
|
$
|
(5,590
|
)
|
$
|
(26,941
|
)
|
|||||
|
Net financing income
|
$
|
(2,813
|
)
|
|||||||||||||||||
|
Loss before taxes on income
|
$
|
(29,754
|
)
|
|||||||||||||||||
|
Agriculture
|
Industrial
application
|
Human
health
|
Unallocated
|
Total
|
||||||||||||||||
|
For the Year Ended December 31, 2021
|
||||||||||||||||||||
|
Revenues
|
$
|
628
|
$
|
40
|
$
|
183
|
$
|
79
|
$
|
930
|
||||||||||
|
Operating loss
|
$
|
(12,248
|
)
|
$
|
(169
|
)
|
$
|
(10,087
|
)
|
$
|
(8,449
|
)
|
$
|
(30,953
|
)
|
|||||
|
Net financing expenses
|
$
|
521
|
||||||||||||||||||
|
Loss before taxes on income
|
$
|
(30,432
|
)
|
|||||||||||||||||
| c. |
Major customers:
|
|
Year ended December 31,
|
||||||||||||
|
2023
|
2022
|
2021
|
||||||||||
|
Customer A (subsidiary shareholder)
|
62
|
%
|
48
|
%
|
35
|
%
|
||||||
|
Customer B
|
-
|
-
|
20
|
%
|
||||||||
|
Customer C
|
-
|
26
|
%
|
17
|
%
|
|||||||
|
Customer D
|
17
|
%
|
-
|
-
|
||||||||
F - 50
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 22: - |
OPERATING SEGMENTS (Cont.)
|
| d. |
Geographical information:
|
|
Year ended December 31,
|
||||||||||||
|
2023
|
2022
|
2021
|
||||||||||
|
United States
|
65
|
%
|
51
|
%
|
56
|
%
|
||||||
|
Israel
|
16
|
%
|
45
|
%
|
38
|
%
|
||||||
|
Brazil
|
-
|
-
|
2
|
%
|
||||||||
|
Other
|
19
|
%
|
4
|
%
|
4
|
%
|
||||||
|
100
|
%
|
100
|
%
|
100
|
%
|
|||||||
|
December 31,
|
||||||||||||
|
2023
|
2022
|
2021
|
||||||||||
|
United States
|
80
|
%
|
79
|
%
|
81
|
%
|
||||||
|
Israel
|
20
|
%
|
21
|
%
|
19
|
%
|
||||||
|
100
|
%
|
100
|
%
|
100
|
%
|
|||||||
| NOTE 23: - |
BALANCES AND TRANSACTIONS WITH EXECUTIVE OFFICERS AND CERTAIN SHAREHOLDERS
|
| a. |
As reported by the shareholders, and based on publicly available information, the Company believes that as of December 31, 2023, Corteva (through its subsidiary Pioneer Hi-Bred International, Inc.) holds 27.26% of the Company's subsidiary shares )Lavie Bio Ltd.’s(. In addition, Corteva is a major customer (see Note 22c, customer A).
|
| b. |
Balances:
|
|
Executive officers
|
Certain shareholders
|
|||||||
|
Receivables
|
$
|
-
|
$
|
186
|
||||
|
Other payables
|
$
|
557
|
$
|
-
|
||||
|
Executive officers
|
Certain shareholders
|
|||||||
|
Receivables
|
$
|
-
|
$
|
6
|
||||
|
Other payables
|
$
|
331
|
$
|
47
|
||||
F - 51
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 23: - |
BALANCES AND TRANSACTIONS WITH EXECUTIVE OFFICERS AND CERTAIN SHAREHOLDERS (Cont.)
|
| c. |
Benefits to directors:
|
|
Year ended December 31,
|
||||||||||||
|
2023
|
2022
|
2021
|
||||||||||
|
Compensation to directors not employed by the Company or on its behalf
|
$
|
246
|
$
|
262
|
$
|
279
|
||||||
|
Share-based compensation to directors not employed by the Company or on its behalf
|
64
|
83
|
106
|
|||||||||
|
$
|
310
|
$
|
345
|
$
|
385
|
|||||||
|
Number of directors that received the above compensation by the Company
|
6
|
7
|
6
|
|||||||||
| d. |
Salary and Benefits to Executive officers:
|
|
Year ended December 31,
|
||||||||||||
|
2023
|
2022
|
2021
|
||||||||||
|
Salary and related benefits
|
$
|
2,441
|
$
|
2,543
|
$
|
2,429
|
||||||
|
Share-based compensation
|
869
|
231
|
731
|
|||||||||
|
$
|
3,310
|
$
|
2,774
|
$
|
3,160
|
|||||||
|
Number of people that received salary and benefits
|
10
|
12
|
11
|
|||||||||
| e. |
Transactions:
|
|
Executive officers
|
Certain shareholders
|
|||||||
|
Revenues (see Note 5)
|
$
|
-
|
$
|
3,475
|
||||
|
Participation in research and development expenses
|
-
|
115
|
||||||
|
Research and development expenses
|
756
|
125
|
||||||
|
Sales and marketing expenses
|
1,185
|
-
|
||||||
|
General and administrative expenses
|
$
|
1,369
|
$
|
-
|
||||
F - 52
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 23: - |
BALANCES AND TRANSACTIONS WITH EXECUTIVE OFFICERS AND CERTAIN SHAREHOLDERS (Cont.)
|
|
Executive officers
|
Certain shareholders
|
|||||||
|
Revenues
|
$
|
-
|
$
|
811
|
||||
|
Other income
|
-
|
3,500
|
||||||
|
Participation in research and development expenses
|
-
|
1,898
|
||||||
|
Research and development expenses
|
570
|
297
|
||||||
|
Sales and marketing expenses
|
1,098
|
-
|
||||||
|
General and administrative expenses
|
$
|
1,111
|
$
|
-
|
||||
|
Executive officers
|
Certain shareholder
|
|||||||
|
Revenues
|
$
|
-
|
$
|
329
|
||||
|
Participation in research and development expenses
|
-
|
1,946
|
||||||
|
Research and development expenses
|
541
|
54
|
||||||
|
Sales and marketing expenses
|
1,210
|
-
|
||||||
|
General and administrative expenses
|
1,409
|
-
|
||||||
|
Financing expenses
|
$
|
-
|
$
|
212
|
||||
| NOTE 24: - |
SUBSEQUENT EVENTS
|
| a. |
On February 16, 2024, AgPlenus entered into a Licensing and Collaboration Agreement with Bayer AG (“Bayer”) for the development of a new sustainable weed control solution. This agreement grants Bayer an exclusive license for the development and commercialization of products developed within the collaboration. AgPlenus will be entitled to receive an upfront payment, ongoing research funding, milestone payments, and royalties based on future product sales, subject to certain conditions as stipulated in the agreement.
|
| b. |
In February 2024, Lavie Bio Ltd. received the Israeli Ministry of Economy approval to be included in “Smart money” grant program for initial exporting to Canada. The maximum grant amount from this program is approximately $83. Lavie Bio Ltd. undertook to pay royalties of 3% of yearly revenues above approximately $276 derived from the operation in Canada, up to 100% of the grants received (linked to the CPI) and can choose to apply the program retroactively from August 2023.
|
| c. |
In February 2024, Lavie Bio Ltd. and Syngenta Crop Protection, a leader in agricultural innovation, entered an agreement for the discovery and development of new biological insecticidal solutions. According to the agreement Syngenta shall pay Lavie Bio an upfront research fee and additional fees upon achievement of certain milestones.
|
F - 53
EVOGENE LTD. AND ITS SUBSIDIARIES
| NOTE 24: - |
SUBSEQUENT EVENTS (Cont.)
|
| d. |
In March 2024, Lavie Bio Ltd. received the second payment of $2,500 as part of the licensing agreement with Corteva conferring exclusive rights to Corteva for advancing and commercializing Lavie Bio's lead bio-fungicides, LAV311 and LAV312 (see also Note 5e).
|
| e. |
In March 2024, the Company entered a new At-The-Market Issuance Sales Agreement (the “Sales Agreement”), with Lake Street Capital Markets, LLC as selling agent. In accordance with the terms of the Sales Agreement, from time to time the Company may offer and sell its ordinary shares in an ATM offering having an aggregate offering price of up to $7,300.
|
| f. |
The Company is in advanced discussions regarding the potential transfer of Canonic's operations to a third party. The completion and terms of such a transfer are uncertain.
|
|
Corteva Agriscience LLC
By:_________________________________
Name: Vidyadhar Hegde Title: VP, Crop Protection Discovery & Development |
Lavie Bio Ltd.
By:__________________________________ Name: Ofer Haviv Title: Chairman of the Board
Lavie Bio Ltd.
By:__________________________________ Name: Amit Noam Title: Chief Executive Officer
|
| 1.1 |
Definitions
|
|
|
• |
bill of lading
|
|
|
• |
phytosanitary certificate
|
|
|
• |
certificate of fumigation, to the extent applicable.
|
|
|
• |
ISTA orange certificate
|
|
|
• |
commercial invoice
|
|
|
• |
packing list
|
|
|
• |
certificate of origin and / or certificate of conformity
|
|
|
• |
any other relevant delivery documentation which may be required under the Kenyan applicable laws and regulations.
|
| 1.2 |
Interpretation
In this AGREEMENT:
|
|
|
(a) |
reference to an article, clause, paragraph or schedule is a reference to an article, clause, paragraph or schedule of or to this AGREEMENT, unless the context requires otherwise.
|
|
|
(b) |
reference to one gender includes a reference to the other gender, and words in the singular include the plural and, in the plural, include the singular.
|
|
|
(c) |
reference to a statute or statutory provision is a reference to it as it is in force for the time being taking account of any amendment, extension, or re-enactment and includes any subordinate legislation for the time being in force made
under it.
|
|
|
(d) |
Unless otherwise specifically provided for, any monetary sum payable by either PARTY under or pursuant to this AGREEMENT shall be made in USA Dollars (USD).
|
|
|
(e) |
References to month means the period beginning on the first day of a calendar month and ending on the last day of such calendar month and monthly shall be
construed accordingly.
|
|
|
(f) |
reference to writing includes typing, printing, lithography and photography and facsimile but excludes email or any other form of electronic communication except for notification of a FORCE MAJEURE EVENT.
|
|
|
(g) |
reference to a document is to that document as amended, varied or novated from time to time otherwise than in breach of this AGREEMENT or that document.
|
|
|
(h) |
unless the context otherwise requires, the words including and include and words of similar effect shall not be deemed to limit the general effect of the
words which precede them.
|
|
|
(i) |
the headings in this AGREEMENT are for ease of reference only and shall not affect its construction or interpretation.
|
|
|
(j) |
references to a person shall be construed so as to include any individual, firm, company, government, state or agency of a state or any joint venture, association, partnership, works council or
employee representative body (whether or not having separate legal personality).
|
|
|
(k) |
if a definition of a particular term or expression in this AGREEMENT imposes substantive rights and obligations on a PARTY such rights and obligations shall be given effect to and shall be enforceable notwithstanding that they are
contained in a definition.
|
|
|
(l) |
where there is any inconsistency between the definitions set out in Clause 1.1 and the definitions set out in any other clause or a schedule, then for the purposes of construing such clause or schedule, the definitions set out in such
other clause or schedule shall prevail.
|
| 2.1 |
On the terms and conditions set out herein, SELLER shall sell and deliver to BUYER, and BUYER shall take and purchase from SELLER, the type and quantity of PRODUCTS specified in each agreed CALL OFF ORDER in accordance with the provisions
of Article 3. BUYER undertakes to purchase the PRODUCTS for the purpose of:
|
|
|
(i) |
growing Seed Beans, directly or through its AUTHORISED RECEPIENTS for its own internal use in the TERRITORY;
|
|
|
(ii) |
reselling of any non-used PRODUCTS (excess) to its AFFILIATES for the purpose of growing Seed Beans, directly or through the AFFILIATES’ AUTHORISED RECEPIENTS for the AFFILIATE’s own internal use in the TERRITORY, provided that any resale
to an AFFILIATE shall be subject to the prior written consent of the SELLER.
|
|
|
In any event, the BUYER or its AFFILIATE shall not resale or transfer to any third party, excluding resale or transfer to AUTHORIZED RECIPIENT or to an AFFILIATE as set forth above. For the avoidance of doubt, BUYER, its AFFILIATE or
AUTHORIZED RECIPIENTS will not use the SEED BEANS for re-sowing.
|
| 2.2 |
Neither the general terms and conditions of sale of SELLER, nor the general terms and conditions of purchase of BUYER, shall apply to this AGREEMENT or to the sale and purchase of the PRODUCTS (whether or not they are provided to the
BUYER). The terms and conditions of sale and purchase applicable to the sale and purchase of PRODUCTS shall be exclusively those expressly included in this AGREEMENT and the CALL OFF ORDER and any changes to these terms and conditions of sale
and purchase shall be negotiated and agreed in writing between the PARTIES.
|
| 2.3 |
The SELLER has and shall maintain in force for the term of this AGREEMENT all licenses, permits, authorizations and consents needed to supply the PRODUCTS in accordance with this AGREEMENT as well as with any applicable laws.
|
| 3.1 |
Not later than [***] month prior to the beginning of each CALENDAR YEAR, the PARTIES shall agree on the ANNUAL PLAN for the next following [***] SEASONS.
|
| 3.2 |
Unless otherwise agreed, not later than [***] months before the beginning of each SEASON, the BUYER shall submit to SELLER a proposed CALL OFF ORDER specifying the type and quantity of PRODUCTS that BUYER would be ready to take and
purchase from SELLER at the DELIVERY PLACE in respect of such SEASON.
|
| 3.3 |
In the event that a CALL OFF ORDER requires multiple deliveries, then as soon as practical after agreement of a CALL OFF ORDER, the BUYER and the SELLER shall agree upon a delivery schedule applicable to such CALL OFF ORDER setting out the
date, quantity and DELIVERY PLACE of each individual delivery of PRODUCTS pertaining to such CALL OFF ORDER (“DELIVERY SCHEDULE”). As a general rule, there shall be no less than 2 and no more than [***]
deliveries per [***], unless the PARTIES agree otherwise.
|
| 3.4 |
Any request by a PARTY to change the date of a delivery of PRODUCTS agreed under the DELIVERY SCHEDULE shall be notified by such PARTY to the other PARTY no later than [***] calendar [***] prior to the date in question. In such event, the
date of such delivery shall be re-scheduled by PARTIES to a date acceptable to both PARTIES which is as close as possible to the originally agreed date. Any request for change of the date of DELIVERY SCHEDULE will not be made more than [***]
with respect to each CALL OFF ORDER. In case the DELIVRY SCHEDULE is delayed by more than [***], the BUYER shall be required to pay the SELLER the storge costs of the SEEDS during such term.
|
| 3.5 |
It is understood and agreed that, by virtue of this AGREEMENT, BUYER shall have no obligation to purchase, and SELLER shall have no obligation to sell, if and to the extent an agreement on a CALL OFF ORDER is not reached by the Parties in
accordance with this ARTICLE 3.
|
| 4.1 |
PRODUCTS shall be delivered by the SELLER on an [***] basis at the DELIVERY PLACE.
|
| 4.2 |
SELLER shall ensure, with respect to each delivery, that the PRODUCTS are packaged and loaded in such a way as to prevent losses of, and damage to, PRODUCTS. SELLER shall also ensure that its own employees and/or contractors and/or
employees of such contractors handling, packaging and loading the PRODUCTS pursuant to this AGREEMENT: (i) are provided with all properly working machinery and with the individual and collective protection devices necessary for the proper and
safe performance of their respective duties; (ii) properly use the machineries, devices, and safety garments provided to them; (iii) comply with the safety and health regulations in force and applicable laws.
|
| 4.3 |
Each delivery of PRODUCTS shall be accompanied by its related DELIVERY DOCUMENTS.
|
| 4.4 |
The PRODUCTS’ quality determinations shall be carried out by SELLER through an accredited independent laboratory and in accordance with the ISTA standard procedures as required by Kenya's regulations at the time of delivery, and verified
by BUYER’s representative or an inspector appointed by the BUYER. The final quality certificate issued shall demonstrate that the PRODUCTS meet the specifications set forth in Exhibit A, and upon acceptance by the PARTIES’ representatives,
shall be final and binding as to the quality of PRODUCTS of a given delivery, save in case of fraud or manifest error (“ISTA ORANGE CERTIFICATE”). For the avoidance of doubt all quality and quantity
tests shall be made before packaging and shipment of the Products to BUYER.
|
| 4.5 |
The Parties agree that if the PRODUCTS’ quality does not meet the specifications set forth in Exhibit B, then the PARTIES shall meet and agree appropriate measures.
|
| 4.6 |
Title to, risk of loss of or damage to the PRODUCTS shall pass from SELLER to BUYER, at the DELIVERY PLACE at the time both PARTIES shall have signed the DELIVERY DOCUMENTS.
|
| 4.7 |
With regard to all other matters concerning delivery, taxes, risk, and obligations of each PARTY concerning delivery of PRODUCTS, the commercial terms of [***] as set out in INCOTERMS 2020 shall apply to the extent that these are not
inconsistent with the terms of this AGREEMENT.
|
| 5.1 |
Following the quality and quantity verifications set forth in Clause 4.4, and delivery of the PRODUCTS, the BUYER shall not be entitled to any cancellation, return, or any claims related to the PRODUCTS’ quality or quantity delivered,
except in case of fraud or manifest error (including typographical error).
|
| 5.2 |
The SELLER shall also comply with applicable laws, enactments, orders, regulations and other instruments relating to the packaging, storage, and handling of PRODUCTS.
|
| 5.3 |
If the SELLER fails to deliver PRODUCTS complying with the QUALITY SPECIFICATIONS for [***] or more deliveries under an agreed DELIVERY SCHEDULE, [***].
|
| 6.1 |
In the event that at the end of any SEASON as evidenced by the applicable DELIVERY DOCUMENTS:
|
|
|
(i) |
BUYER shall have failed to take delivery and purchase from the SELLER the quantity of PRODUCTS specified in the agreed CALL OFF ORDER, or
|
|
|
(ii) |
SELLER shall have failed to deliver and sell to the BUYER the quantity of PRODUCTS specified in the agreed CALL OFF ORDER,
|
| 6.2 |
If any such SHORTFALL persists for the next [***], such occurrence shall be considered a material breach by the BREACHING PARTY, and [***].
|
| 7.1 |
During the term of this AGREEMENT, the per-KG unit price of PRODUCTS (the “UNIT PRICE”) shall be agreed by the PARTIES within, and as part of, each applicable CALL OFF ORDER, and the UNIT PRICE so
agreed shall be and remain valid for any and all deliveries of PRODUCTS in the applicable SEASON, including any quantity carried-over under Article 6.
|
| 7.2 |
The UNIT PRICE shall be:
|
|
|
(i) |
expressed in USD and invoiced; and
|
|
|
(ii) |
net of Value Added Tax.
|
| 7.3 |
[***]
|
| 8.1 |
SELLER shall issue an invoice to BUYER for each delivery of PRODUCTS. The amount of the invoice shall be equal to the then applicable UNIT PRICE multiplied by the quantity of PRODUCTS delivered to the BUYER,
as evidenced in the relevant DELIVERY DOCUMENTS.
|
| 8.2 |
Each invoice issued by the SELLER shall include the following:
|
|
|
(a) |
the reference numbers: (i) of the CALL OFF ORDER; and (ii) of each of the deliveries made in the month in question as shown in the relevant DELIVERY DOCUMENTS;
|
|
|
(b) |
the total quantity expressed in KG of the PRODUCTS delivered and accepted by BUYER.
|
|
|
(c) |
the DELIVERY PLACE named in the CALL OFF ORDER;
|
|
|
(d) |
the UNIT PRICE;
|
|
|
(e) |
the relevant DELIVERY DOCUMENTS;
|
|
|
(f) |
the total amount due, equal to the quantity in (b) multiplied by the applicable UNIT PRICE;
|
|
|
(g) |
any applicable Value Added Tax.
|
|
|
(h) |
the total payable, equal to the sum of the values in (f) plus (g).
|
| 8.3 |
Each undisputed invoice shall be paid in [***] instalments, being [***] of the total amount due paid by BUYER upon [***], together with the other documents and as set forth in clause 8.2, and the remaining [***] upon [***], but in any
event no later than [***] days from [***] (each, a “PAYMENT DUE DATE”). In the event that the PAYMENT DUE DATE falls on a non-BUSINESS DAY, then payment shall be made on the next BUSINESS DAY. In case
of late payment, a late interest payment will apply, at the rate of [***].
|
| 8.4 |
Payment shall be made, by bank transfer to the following SELLER’s bank account opened and maintained in the name and to the benefit of the SELLER and in a jurisdiction where the SELLER is incorporated or has its centralized cash management
unit:
|
|
|
• | Account Name: | [***] |
|
|
• | Bank: | [***] |
|
|
• | Account Number: | [***] |
|
|
• | Swift Number: | [***] |
|
|
• | Bank Code: | [***] |
|
|
• | Branch Code: | [***] |
|
|
• | Currency: | [***] |
|
|
• | Address of Bank: | [***] |
|
|
• |
Corresponding bank:
|
| 8.5 |
To the extent permitted by [***] applicable laws, any PARTY may, without limiting any other rights or remedies it may have, set-off any amounts owed by it to the other PARTY under this AGREEMENT against any amounts owed to it by the other
PARTY under this AGREEMENT.
|
| 9.1 |
If a PARTY (the “AFFECTED PARTY”) is prevented, hindered or delayed from or in performing its contractual obligations by Force Majeure (the “FORCE MAJEURE EVENT”):
|
|
|
(a) |
the AFFECTED PARTY's obligations under this AGREEMENT shall be suspended while the FORCE MAJEURE EVENT continues to the extent that they are prevented, hindered or delayed;
|
|
|
(b) |
as soon as reasonably possible after the start of the Force Majeure Event, the AFFECTED PARTY shall notify the other PARTY in writing of the occurrence of the Force Majeure Event, the date on which the FORCE MAJEURE EVENT started and the
effects of the FORCE MAJEURE EVENT on its ability to perform its obligations under this AGREEMENT;
|
|
|
(c) |
the AFFECTED PARTY shall make all reasonable efforts to mitigate the effects of the FORCE MAJEURE EVENT on the performance of its obligations under this AGREEMENT;
|
|
|
(d) |
if the Affected PARTY does not comply with Clause 9.1 (b) and (c) it shall forfeit its rights under this Clause 9.1; and
|
|
|
(e) |
as soon as reasonably possible after the end of the FORCE MAJEURE EVENT, the AFFECTED PARTY shall notify the other PARTY that the FORCE MAJEURE EVENT has ended and resume performance of its obligations under this AGREEMENT.
|
| 9.2 |
If the FORCE MAJEURE EVENT continues for more than [***] starting on the day the Force Majeure starts, a PARTY may terminate this AGREEMENT by giving not less than [***] prior written notice to the other PARTY.
|
| 9.3 |
In this Clause 9, FORCE MAJEURE EVENT means an event beyond the reasonable control of the AFFECTED PARTY including, without limitation, dry or extreme weather conditions, act of God, pandemics, war, riot, civil commotion, malicious damage,
embargos, explosion, accident, breakdown or shutdown of plant or machinery, fire, flood and storm, occurred during the performance of this AGREEMENT, provided that the Affected PARTY cannot reasonably foresee the occurrence of the event at
the time of execution of this AGREEMENT.
|
| 11.1 |
This AGREEMENT and any dispute or claim (including non-contractual disputes or claims) arising out of or in connection with it or its subject matter or formation shall be governed by and construed in accordance with the law of England.
|
| 11.2 |
Arbitration Clause
|
|
|
(a) |
Each PARTY shall settle amicably any dispute arising out of or in connection with this AGREEMENT or in its validity, interpretation or termination.
|
|
|
(b) |
Save as herein otherwise specifically provided, any dispute (including as to the interpretation validity enforceability or termination of this AGREEMENT) between the PARTIES as to matters arising under or pursuant to this AGREEMENT as
aforesaid which cannot be settled amicably within fifteen (15) days after receipt by one PARTY of the other PARTY’s request for such amicable settlement may be submitted by either PARTY to arbitration in accordance with the provisions of
Clauses 11.2(c) to 11.2(f) (both inclusive).
|
|
|
(c) |
If the PARTIES so agree, the dispute shall be referred to a single arbitrator or if they are unable to agree upon the person to be appointed as arbitrator within sixty (60) days from the date of the notice requesting arbitration, the
arbitrator shall, at the request of either PARTY, be appointed by the Chairman of the Chartered Institute of Arbitrators of the United Kingdom, London Branch. The venue and seat of the arbitration shall be London.
|
|
|
(d) |
Arbitration proceedings shall be conducted in accordance with the rules or procedures for arbitration of England, which rules are deemed to be incorporated by reference into this clause.
|
|
|
(e) |
If for any reason an arbitrator is unable to perform his/her function, a substitute shall be appointed in the same manner as the original arbitrator.
|
|
|
(f) |
The decision of the arbitrator shall be final and binding on the PARTIES.
|
| 11.3 |
Exclusion of Vienna Convention: the terms of the United Nations Convention on Contracts for the International Sale of Goods will not apply to this AGREEMENT.
|
| 12.1 |
Each PARTY shall keep confidential and shall not disclose to any other third party any business secrets or other confidential information it acquires in connection with or as a consequence of the performance of this AGREEMENT. Confidential
information shall mean any and all confidential and/or proprietary knowledge, data, or information that was disclosed in the past or which may be disclosed at any time after the signing of this AGREEMENT to a PARTY or any of its associated
companies by or on behalf of the other PARTY, either directly or indirectly, in writing, graphically, electronically, orally or by inspection. By way of illustration but not limitation, confidential information includes trade secrets,
inventions, germplasm (including but not limited to the Casterra’s castor varieties, all progenies and parts thereof, and all substances isolated therefrom), seeds, cultivation methods, machinery, biological materials, genes, data including
data generated from field trials, ideas, processes, formulas, protocols, including without limitation, growth protocols, programs, other works of authorship, know-how, improvements, discoveries, developments, designs and
techniques, information regarding plans for research or development, new products, marketing and selling, business plans, budgets and unpublished financial statements, licenses, prices and costs, suppliers and customers.
|
| 12.2 |
The PARTEIS’ obligations under this Article 12 shall survive expiration or termination of this AGREEMENT for three (3) years.
|
| 12.3 |
The obligations of this Article 12 shall not apply, however, to confidential information which:
|
|
|
a) |
prior to the transmission to the receiving PARTY was of general public knowledge or becomes, subsequent to the time of transmission to the other PARTY, a matter of general public knowledge, otherwise than as a consequence of a breach by
the receiving PARTY or its employees of its obligations hereunder;
|
|
|
b) |
was in the possession of the receiving PARTY in documentary form prior to the time of disclosure by the disclosing PARTY, and was not acquired, directly or indirectly from the disclosing PARTY and is held by the receiving PARTY free of any
obligation of confidence to the disclosing PARTY or any third PARTY;
|
|
|
c) |
is received in good faith from a third PARTY having the right to disclose it, and who, to the best of the receiving PARTY’s knowledge, did not obtain the same from the disclosing PARTY and who imposes no obligation of secrecy on the
receiving PARTY with respect to such information; or
|
|
|
d) |
Confidential information which any PARTY, as the case may be, is required to disclose by any authority, law or regulation, or on the basis of any applicable judgment, order or decree of any court or governmental body or agency having
jurisdiction over the respective PARTY, provided that such PARTY shall give to the other one reasonable prior notice of the requirement to disclose such confidential information.
|
| 12.4 |
BUYER acknowledges that the SELLER is a subsidiary of Evogene Ltd., which is a publicly-traded company listed on the Nasdaq and Tel-Aviv Stock Exchange, and therefore BUYER is required to comply with the restrictions set-forth in the
Israeli and US Securities Law with respect to the prohibition on use and disclosure of any “Inside Information” (as defined in the Israeli and US Securities Law) pertaining to Evogene Ltd. of which it may become aware.
|
| 12.5 |
Any public disclosure or issue of any press release, and their content, in connection with this Agreement or any transactions contemplated by this Agreement shall be agreed in advance by the PARTIES. No PARTY shall use the name, trade
name, trademarks, or other distinctive sign of the other PARTY without its prior written consent.
|
| 13.1 |
The SELLER declares to have reviewed and acknowledged of: (a) the Eni's Code of Ethics, adopted by the BUYER, (b) the Eni's "Anti-Corruption Management System Guideline" and (c) the Eni's Statement on Respect for Human Rights. The SELLER
acknowledges that the documents referred to in (a), (b) and (c) above are available on the website www.eni.com and agrees to respect their principles.
|
| 13.2 |
With reference to the performance of the activities covered by this AGREEMENT, the SELLER undertakes to respect and ensure that its directors and employees - as well as any collaborators (consultants, agents, intermediaries,
sub-contractors and any third PARTIES involved in the performance of this AGREEMENT) - comply with the applicable regulations aimed at combating and punishing the phenomenon of corruption, such as (i) Legislative Decree 231/200112, (ii) the
FCPA, (iii) the UK Bribery Act 2010, (iv) other applicable anti-corruption laws in force worldwide and (v) international anti-corruption treaties such as the Organisation for Economic Cooperation and Development Convention on Combating
Bribery of Foreign Public Officials in International Business Transactions and the United Nations Convention against Corruption (hereinafter "anti-corruption laws").
|
| 13.3 |
With reference to the performance of the activities covered by this AGREEMENT, the SELLER declares and guarantees that it has given and implemented instructions to its directors, employees and/or any collaborators, aimed at preventing the
commission, even attempted, of the conduct contrary to the anti-corruption laws, and undertakes with respect to the BUYER to ensure the full implementation of these provisions for the entire duration of the AGREEMENT.
|
| 13.4 |
The SELLER declares that it has no conflict of interest with respect to the performance of this AGREEMENT and agrees to promptly inform the BUYER in the event that such a situation should arise during execution thereof. For the purposes of
this AGREEMENT, by conflict of interest is meant any situation referring to the SELLER that can interfere with the ability of the directors, employees and collaborators of the BUYER to make impartial decisions in the latter's interest.
|
| 13.5 |
With regard to the performance of the activities covered by this AGREEMENT, the SELLER agrees to:
|
|
|
a) |
record every amount received or paid in relation to this AGREEMENT in its accounting books, in a clear and transparent manner;
|
|
|
b) |
promptly inform the BUYER of any information relating to pending investigations, proceedings, sanctions or decisions against it and its owners (for the purpose of this clause, owners mean each direct shareholder of the SELLER, each member
of the Board of Directors, Chief Operating Officer or equivalent figure), even if not definitive, related to conduct contrary to anti-corruption laws and anti-mafia regulations;
|
|
|
c) |
timely inform the BUYER of any request or demand relative to any undue payment of money or other advantage received in relation to the performance of this AGREEMENT;
|
|
|
d) |
not to subject its workers to working conditions, methods of surveillance or degrading housing situations in violation of applicable legislation. The BUYER reserves the right to carry out inspections and audits in the event that it becomes
aware of circumstantial information that reasonably infers the violation of the provisions contained in this letter. For this purpose, the SELLER agrees to provide the BUYER with all the information related to the performance of the contract
in the manner agreed to by the PARTIES.
|
| 13.6 |
The PARTIES agree that even partial failure by the SELLER to comply with the declarations, guarantees and obligations referred to in this article that could reasonably determine negative consequences for the BUYER will constitute a serious
breach and will give the BUYER the right to terminate this AGREEMENT in accordance with law, as this express termination clause.
|
| 13.7 |
In the presence of formal documents of the judicial authority, learned also from any media, from which such a breach may be inferred, while awaiting investigations or the final decisions of the law the BUYER will have the right to suspend
the performance of the AGREEMENT. In any case, the SELLER shall indemnify the BUYER from any direct loss and/or damage suffered by the BUYER and hold the BUYER harmless from any third PARTY action arising from to such a breach.
|
| 14.1 |
The SELLER warrants to the BUYER that, at the time of the delivery of the PRODUCTS:
|
|
|
(i) |
it has the full title and right over the PRODUCTS and that it has the right to transfer the title to the PRODUCTS to the BUYER;
|
|
|
(ii) |
the PRODUCTS are free from any liens, charges and/or encumbrances of any kind;
|
|
|
(iii) |
the PRODUCTS are compliant with any applicable laws, regulations and with the QUALITY SPECIFICATIONS; and
|
|
|
(iv) |
the packages containing the PRODUCTS are fit for the purpose of safely carrying, holding, and transporting the PRODUCTS and comply with any applicable laws and regulations.
|
| 14.2 |
The SELLER shall not be liable or responsible for any act or omission of the BUYER’s AUTHORIZED RECIPIENTS.
|
|
14.3
|
Each PARTY represents and warrants to the other PARTY that:
|
|
|
(i) |
it is duly organized and validly existing under the laws of the jurisdiction of its organization or incorporation and, if relevant under such laws, it is in good standing;
|
|
|
(ii) |
it has the power to execute and perform the AGREEMENT and has taken all necessary action to authorize the execution and the performance;
|
|
|
(iii) |
the execution and the performance of this AGREEMENT do not violate or conflict with any law applicable to it, any provision of its constitutional documents, any order or judgment of any court or other agency of government applicable to it
or any of its assets or any contractual restriction binding on or affecting it or any of its assets;
|
|
|
(iv) |
all governmental and other consents or permits or license, which are required to have been obtained by it with respect to the AGREEMENT, have been obtained and are in full force and effect and all conditions of any such consents have been
complied with; and
|
|
|
(v) |
its obligations under the AGREEMENT constitute its legal, valid and binding obligations, enforceable in accordance with its respective terms (subject to applicable bankruptcy, re-organization, insolvency, moratorium or similar laws
affecting creditors’ rights generally and subject, as to the enforceability, to equitable principles of general application (regardless of whether enforcement is sought in a proceeding in equity or at law).
|
| 14.4 |
Each PARTY shall indemnify and hold harmless the other PARTY from and against any liability suffered by, brought or threatened against the other PARTY as a result of and to the extent caused by any breach of the warranties set forth in
clauses 14.1 and 14.3 by the indemnifying PARTY. Nothing in this AGREEMENT shall operate to exclude or restrict the SELLER’s liability for breach of its warranties in article 14.1(i) and article 14.1(ii).
|
| 14.5 |
EXCEPT AS EXPRESSLY PROVIDED IN SECTION 14 ABOVE, NEITHER PARTY MAKES ANY WARRANTY OF ANY KIND, EXPRESS OR IMPLIED, AND HEREBY DISCLAIMS ANY WARRANTY OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE AND NONINFRINGEMENT. EXCEPT AS
EXPRESSLY SET OUT HEREIN THE SEEDS ARE PROVIDED "AS-IS" AND "AS-AVAILABLE", AND SELLER DOES NOT WARRANT THAT: (I) BUYER’S USE OF THE SEEDS WILL SECURE ANY SUCCESS OR GENERATE ANY REVENUE.
|
| 14.4 |
The SELLER will be responsible for and will indemnify, save, defend and hold harmless the BUYER and its personnel against all LIABILITIES arising out of or in connection with:
|
|
|
(i) |
any claim made against the BUYER or its personnel for actual or alleged infringement of a third PARTY's intellectual property rights arising out of, or in connection with, the supply, receipt, possession or use of the PRODUCTS; and
|
| (ii) |
any environmental contamination or pollution caused by or attributable to the packaging and loading of any PRODUCTS at the DELIVERY PLACE, except to the extent such contamination or pollution is attributable to the fault of the BUYER or
its personnel.
|
|
14.5
|
Without prejudice to the above, SELLER shall not be held liable for the infringement of a third PARTY's intellectual property rights caused:
|
|
|
(i) |
by the use by BUYER of PRODUCTS in combination with goods and/or services not supplied by the SELLER provided such use is not set in this AGREEMENT;
|
|
|
(ii) |
when the PRODUCTS have been modified, designed and/or produced on the basis of specific requests of BUYER;
|
|
|
(iii) |
by unauthorized additions or modifications by BUYER to the PRODUCTS; and
|
|
|
(iv) |
where the use by BUYER of the PRODUCTS does not correspond to the SELLER’S standards and specifications provided under this AGREEMENT.
|
| 14.6 |
IN NO EVENT WILL EITHER PARTY BE LIABLE TO THE OTHER PARTY OR TO ANY THIRD PARTY FOR ANY INDIRECT, SPECIAL, INCIDENTAL, PUNITIVE OR CONSEQUENTIAL DAMAGES OR LOSSES, LOSS OF USE, DATA, BUSINESS OR PROFITS, NOR COSTS OF PROCURING SUBSTITUTE
SERVICES OR PRODUCTS, ARISING OUT OF OR IN CONNECTION WITH THIS AGREEMENT OR THE SERVICES OR ANY DELIVERABLES PROVIDED HEREUNDER, EVEN IF A PARTY HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES. EXCLUDING LIABILITY FOR BREACHES OF
CONFIDENTIALITY OR INTELLECTUAL PROPERTY RIGHTS INFRINGEMENT CLAIMS, EACH PARTY'S TOTAL LIABILITY TO THE OTHER WILL BE LIMITED TO AND WILL NOT EXCEED THE AMOUNTS PAID TO SELLER BY BUYER FOR THE SEEDS UNDER THE APPLICABLE CALL OFF ORDER [***]
PRIOR TO THE EVENT GIVING RISE TO SUCH LIABILITY. Nothing in this AGREEMENT shall operate to exclude or restrict a PARTY’s liability for:
|
|
|
(i) |
death or personal injury resulting from negligence; and
|
|
|
(ii) |
fraud.
|
| 14.7 |
Each PARTY must indemnify the other PARTY and the other PARTY’s personnel from and against any claim or loss that any of them may suffer or incur in connection with:
|
|
|
in each case, directly in connection with the performance of its obligations under this AGREEMENT.
|
|
14.8
|
BUYER undertakes to (i) promptly notify the SELLER in writing of any third party claim arising from this AGREEMENT and cooperate with the SELLER in the defense of such claim; and (ii)
refrain from admitting liability or otherwise compromising the claim in whole or in part without the express prior written permission of SELLER.
|
| 15.1 |
Each PARTY shall have the right, without prejudice to its other rights or remedies, to terminate this AGREEMENT, with effect from the date of the termination notice, by giving a written notice to the other PARTY, if such other PARTY:
|
|
|
(a) |
is in material breach of this AGREEMENT and either such breach is incapable of remedy or the PARTY in breach shall have failed to remedy such breach within [***] BUSINESS DAYS after receiving written notice requiring it to remedy that
breach;
|
|
|
(b) |
is unable or is deemed unable or admits inability to pay its debts as they fall due or is liable to be wound up by a court of competent jurisdiction;
|
|
|
(d) |
enters into a composition or arrangement with its creditors or a moratorium is declared in respect of any of its indebtedness or any creditor action;
|
|
|
(e) |
takes any action to appoint, to request the appointment of, or suffers the appointment of, a receiver, administrative receiver, administrator, trustee or similar officer over all or a material part of its assets or undertaking;
|
|
|
(f) |
has a winding-up or administration petition presented in relation to it or has documents filed with a court for an administration in relation to it provided that, in the case of a winding up petition, if the other PARTY is contesting the
winding up petition in good faith and with due diligence it shall not be in default until a period of five BUSINESS DAYS has expired since the presentation of the winding up petition without it having been either discharged or struck out.
|
| 15.2 |
Party may also, upon a [***] prior written notice to the other Party, terminate the AGREEMENT, provided however, that any CALL OF ORDER, which was agreed upon by the parties shall remain in effect for its term.
|
| 15.3 |
Any termination or expiry of this AGREEMENT shall not affect any accrued rights or LIABILITIES of either PARTY (including LIABILITIES accrued under an agreed CALL OFF ORDER), nor shall it affect clauses which shall survive such termination
or expiry.
|
| 16.1 |
The PARTIES pursue excellence in health, safety and environment protection management.
|
| 16.2 |
SELLER’s Obligations in respect of health and safety.
|
|
|
a) |
respect and comply with all procedures, laws, directives and regulations in respect of health, safety and environment protection as well as with any instructions provided by the BUYER and procedures and discharge practices applicable at
the DELIVERY PLACE;
|
|
|
b) |
carry out the activities hereunder with its own employees and/or contractors and/or with third PARTY’s employees and/or contractors, qualified for the performance of such activities;
|
|
|
c) |
warrant and ensure that its own employees and/or contractors and/or third PARTY’s employees and/or contractors carrying out the activities under this AGREEMENT: (i) are provided with all working machinery and with the individual and
collective protection devices, necessary for the proper and safe performance of the loading/unloading activities in respect of the specific risks connected to the performance of such activities, environmental risks, and the risks connected to
the use of the machinery in the DELIVERY PLACE, (ii) properly use the machineries, devices, and safety garments attributed to them, (iii) perform the activities in compliance with the safety and health regulations in force at each DELIVERY
PLACE and in compliance with the regulations set out is the present article; and
|
|
|
d) |
organize for all its own employees and for third PARTY’s employees carrying out the delivery activities at the DELIVERY PLACE, professional training and information courses.
|
| 17.1 |
The PARTIES declare that they comply with the principles contained in the applicable norms, laws, regulations, conventions and guidelines, whether national or international, aimed at preventing and contrasting violations in relation to
human rights, in particular in relation to slavery, forced labour, child labour and human trafficking, including the UN Guiding Principles on Business and Human Rights, the OECD Guidelines for Multinational Enterprises and the ILO Declaration
on Fundamental Principles and Rights at Work and relative fundamental Conventions (hereinafter “Human Rights”). In this regard, the SELLER declares that it has reviewed and has knowledge of Eni’s
Statement on Respect for Human Rights and its Slavery and Human Trafficking Statement, available on the website www.eni.com. The SELLER also declares that it operates according to principles in line with those expressed in said documents.
|
| 17.2 |
With reference to the performance of the activities under this AGREEMENT, SELLER shall, in any case:
|
|
|
(i) |
respect Human Rights and make the utmost effort to avoid violating and/or contributing to violating Human Rights;
|
|
|
(ii) |
ensure working conditions through the entire supply chain in line with applicable standards, laws and conventions, in particular those regarding pay, hours, discrimination, slavery, forced labour, child labour and human trafficking or the
like;
|
|
|
(iii) |
monitor its supply chain to ensure it does not, in performing its obligations under this AGREEMENT, procure or use any resources, materials, goods or services from suppliers or subcontractors who violate Human Rights or use forced labour
or the like per Article 18.2(ii) above;
|
|
|
(iv) |
negotiate contractual provisions containing the same warranties, declarations and obligations as contained in this clause in any agreements entered into with third PARTIES – including suppliers and subcontractors – involved in carrying out
the activities referred to in this Contract, in order to ensure their respect for Human Rights;
|
|
|
(v) |
notify the BUYER immediately in writing in case of any suspected or actual violations of Human Rights by it or any of its directors, employees, agents, suppliers or subcontractors, of which it becomes aware and, in any case, make itself
available for any verifications.
|
| 17.3 |
The SELLER warrants that neither it, nor to the best of its knowledge, its legal representatives are involved in investigations or proceedings concerning Human Rights violations.
|
| 17.4 |
With reference to the performance of the activities, including those performed by suppliers or subcontractors, under this AGREEMENT, SELLER shall also:
|
|
|
(i) |
ensure the rationale use of natural resources and the environmentally responsible production to protect soil, water and air;
|
|
|
(ii) |
preserve the ecosystem and the biodiversity, and conserve forest and wildlife, including the protection of threatened or vulnerable species, or which are of other ecological or cultural importance;
|
|
|
(iii) |
guarantee the sustainable exploitation of the land, and in any case the protection of land with high biodiversity value or high carbon stock;
|
|
|
(iv) |
in the event the PRODUCTS sold by the SELLER under this AGREEMENT are not produced by the SELLER but acquired by the SELLER from third parties suppliers: (i) recognize them a fair price and timely pay a fair compensation for the PRODUCTS;
(ii) ensure a fair treatment for its third parties suppliers and duly comply with and fulfil its undertakings towards them;
|
|
|
(v) |
take all actions necessary to recognize safe working conditions to any and all employees through the entire supply chain, as well as compliance with human and labour laws and international treaties and comply with and/or ensure
compliance with all applicable national and international laws, treaties and regulations on on land rights;
|
| 17.5 |
The PARTIES agree that even partial failure by the SELLER to comply with the declarations, guarantees and obligations referred to in this Article that could reasonably determine negative consequences – even if only reputational – for the
BUYER, will constitute a material breach and grant the BUYER right to terminate the AGREEMENT in accordance with law.
|
| 18.1 |
Nothing in this AGREEMENT and no action taken by the PARTIES pursuant to this AGREEMENT shall constitute, or be deemed to constitute, a partnership, association, joint venture or other co-operative entity between the PARTIES.
|
| 18.2 |
This AGREEMENT constitutes the whole and only agreement between the PARTIES concerning the subject matter of this AGREEMENT and supersedes and extinguishes any prior drafts, agreements, undertakings, representations, warranties and
arrangements of any nature whatsoever, whether or not in writing, concerning the subject matter of this AGREEMENT.
|
| 18.3 |
Each PARTY acknowledges that, in entering into this AGREEMENT on the terms set out in this AGREEMENT, it is not relying upon any representation, warranty, promise or assurance made or given by the other PARTY or any other person, whether
or not in writing, at any time prior to the execution of this AGREEMENT, which is not expressly set out herein.
|
| 18.4 |
The failure or delay of a PARTY to exercise or enforce any right or privilege under this AGREEMENT shall not be deemed a waiver of that right nor shall a partial exercise thereof preclude any other or future exercise of any such right.
|
| 18.5 |
Any amendment to, or variation of, this AGREEMENT must be in writing and signed by the duly authorized representatives of the PARTIES. No amendment to this AGREEMENT shall be effected by the acknowledgement or acceptance by any of the
PARTIES of Orders, invoices, shipping instructions, forms or other similar documents which contain terms at variance with or in addition to those set forth in this AGREEMENT, unless such acknowledgement or acceptance specifically states that
it is intended to amend this AGREEMENT and it is accepted by the other PARTY.
|
| 18.6 |
Failure by a PARTY at any time to require performance by another PARTY or to claim a breach of any provision of this AGREEMENT shall not be construed as a waiver of any right accruing hereunder, nor shall it affect any subsequent breach or
the effectiveness of this AGREEMENT or any part of this AGREEMENT, or prejudice either PARTY as regards any subsequent action. The rights and remedies contained in this AGREEMENT are cumulative and not exclusive of any rights or remedies
provided by applicable laws.
|
| 18.7 |
All notices, reports or other communications between the PARTIES given pursuant to this AGREEMENT shall be in writing, in the English language, delivered by courier, registered mail, certified e-mail or fax (with a copy anticipated via
e-mail) to the PARTY for whom it is intended, at the address of such PARTY set forth below or to such different address as such PARTY may hereafter notify in writing to the other. Notice shall be deemed given on the date of actual receipt by
the addressee. The addresses of the PARTIES hereto are as follows:
|
| 18.8 |
This AGREEMENT is personal to the PARTIES and may not be assigned, by a PARTY, in whole or in part, without the prior written consent of the other PARTY, except that BUYER may, upon prior written notice to the SELLER, assign this AGREEMENT
to any member of BUYER’s group.
|
| 18.9 |
This AGREEMENT may be executed in counterparts, each of which shall be deemed to be an original and each of which together shall constitute one and the same document.
|
| 18.10 |
In the event any provision of this AGREEMENT is declared illegal or unenforceable in any respect under any law of any jurisdiction, it shall automatically be severed here from and neither the legality, validity or enforceability of the
remaining provisions nor the legality, validity or enforceability of such provision under the law of any other jurisdiction (as applicable) will in any way be affected or impaired; provided, however, that should such invalidity substantially
injure the rights of either PARTY, the PARTIES shall promptly renegotiate the relevant terms of this AGREEMENT.
|
| 18.11 |
A Person who is not a PARTY to this AGREEMENT has no rights under the Contracts (Rights of Third Parties) Act 1999 to enforce any term of this AGREEMENT.
|
| 21.1 |
As between the parties, all right, title and interest in and to: (a) the PRODUCTS, the castor varieties, all progenies and parts thereof, all substances isolated therefrom, and all data, inventions, know how, growth protocols and results
relating to any of the foregoing, and (b) all patents, plant variety protection rights, plant breeders rights, trade secrets, copyrights, trademarks or other intellectual property rights in respect of any of the foregoing, whether now
existing or hereafter created, developed, arising or otherwise coming into being, and whether patentable or not, vest and shall vest solely in SELLER (all the foregoing, collectively, “Casterra IP”).
|
| 21.2 |
If BUYER becomes aware of any third-party breach of any of Casterra IP rights relating to the Casterra castor varieties, any progenies or parts thereof or any substances isolated therefrom, it shall promptly notify Casterra in writing
thereof.
|
| 21.3 |
BUYER shall not (i) reverse engineer, disassemble, decompile, modify or alter the PRODUCTS, (ii) use the PRODUCTS for any unlawful purpose or any purpose other than the purposes set forth in this Agreement. BUYER agrees that any breach of
the provisions in this Section will constitute a material breach of this AGREEMENT and shall grant SELLER the right (without derogating from any other remedy available to it) to terminate this Agreement with immediate effect upon written
notice to BUYER.
|
|
For Eni Kenya B.V.:
Signature: __________________
|
For CASTERRA AG LTD.
Signature:__________________
|
|
Name: Enrico Tavolini
Title: Managing Director
|
Name: Eyal Ronen
Signature:__________________
Name: Ofer Haviv
Title: Chairman of the Board
|
|
PRODUCTS
|
SPECIFICATION
|
|
Castor Seeds:
|
[***]
|
|
[***]
|
|
|
[***]
|
|
|
[***]
|
|
|
[***]
|
|
|
[***]
|
|
|
[***]
|
|
|
[***]
|
|
|
[***]
|
|
|
[***]
|
|
Date
|
xxxxxx
|
To:
|
||||
|
CALL-OFF ORDER No.
|
xxxxxx
|
Contractor’s Name
|
xxxx
|
|||
|
CONTRACT No.
|
xxxxx
|
Contractor’s Address
|
xxx
|
|||
|
COMPANY Department
|
AGRI DEV
|
|||||
|
SAP Call-Off No.
|
||||||
|
Start Date of SERVICES
|
xxxxx
|
|||||
|
End Date of SERVICES
|
xxxx
|
|||||
|
In accordance to the Terms and Conditions of the above-mentioned CONTRACT, this document constitutes a specific request for purchase of castor
seeds.
|
||||||
|
List of requested material/service
|
||||||
|
Item Number
|
Description
|
Quantity
|
Rate
|
Duration of Service
|
Notes
|
Estimated Amount in USD exc. of VAT
|
|
1.
|
xxx
|
xxx tons
|
xxxx
|
xxx
|
xxx
|
|
|
Total
|
xxx
|
|||||
|
This CALL-OFF ORDER is issued on behalf of Eni Kenya B.V
|
||||
|
Prepared By;
|
||||
|
Name
|
xxxxx
|
xxxxxxx
|
||
|
Title
|
AGRICULTURE DEVELOPMENT MANAGER
|
Signature
|
||
|
Date
|
xxx
|
|||
|
Approved By:
|
(COMPANY’s Authorized Signatory)
|
|||
|
Name:
|
Xxxxxx
|
xxxxxx
|
||
|
Title:
|
MANAGING DIRECTOR
|
|||
|
Date:
|
||||
|
For information concerning this CALL-OFF ORDER, please, contact:
Contract Admin Name: xxxxxxx
Contract Admin Tel: xxxxx
Contract Admin Email: xxxxxxxx
|
||||
|
s
|
Please, confirm acceptance of this CALL-OFF ORDER by signing and returning the enclosed duplicate copy of this CALL-OFF ORDER:
|
|||
|
By:
|
(CONTRACTOR’s Authorized Signatory)
|
|||
|
Name:
|
||||
|
Title:
|
||||
|
Date:
|
||||
|
|
/s/ KOST FORER GABBAY & KASIERER |
|
|
|
| Tel-Aviv, Israel |
KOST FORER GABBAY & KASIERER |
| March 28, 2024 |
A Member of Ernst & Young Global |
| 1. |
Persons Subject to Policy
|
| 2. |
Compensation Subject to Policy
|
| 3. |
Recovery of Compensation
|
| 4. |
Manner of Recovery; Limitation on Duplicative Recovery
|
| 5. |
Administration
|
| 6. |
Interpretation
|
| 7. |
No Indemnification; No Liability
|
| 8. |
Application; Enforceability
|
| 9. |
Severability
|
| 10. |
Amendment and Termination
|
| 11. |
Definitions
|
|
___________________
Date
|
________________________________________
Signature
|
|
________________________________________
Name
|
|
|
________________________________________
Title
|