false2023--12-31Q300011786700.003494140036500011786702023-01-012023-09-3000011786702023-10-27xbrli:shares00011786702023-09-30iso4217:USD00011786702022-12-31iso4217:USDxbrli:shares0001178670us-gaap:ProductMember2023-07-012023-09-300001178670us-gaap:ProductMember2022-07-012022-09-300001178670us-gaap:ProductMember2023-01-012023-09-300001178670us-gaap:ProductMember2022-01-012022-09-300001178670alny:CollaborationsMember2023-07-012023-09-300001178670alny:CollaborationsMember2022-07-012022-09-300001178670alny:CollaborationsMember2023-01-012023-09-300001178670alny:CollaborationsMember2022-01-012022-09-300001178670us-gaap:RoyaltyMember2023-07-012023-09-300001178670us-gaap:RoyaltyMember2022-07-012022-09-300001178670us-gaap:RoyaltyMember2023-01-012023-09-300001178670us-gaap:RoyaltyMember2022-01-012022-09-3000011786702023-07-012023-09-3000011786702022-07-012022-09-3000011786702022-01-012022-09-300001178670us-gaap:CommonStockMember2022-12-310001178670us-gaap:AdditionalPaidInCapitalMember2022-12-310001178670us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-12-310001178670us-gaap:RetainedEarningsMember2022-12-310001178670us-gaap:CommonStockMember2023-01-012023-03-310001178670us-gaap:AdditionalPaidInCapitalMember2023-01-012023-03-3100011786702023-01-012023-03-310001178670us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-03-310001178670us-gaap:RetainedEarningsMember2023-01-012023-03-310001178670us-gaap:CommonStockMember2023-03-310001178670us-gaap:AdditionalPaidInCapitalMember2023-03-310001178670us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-03-310001178670us-gaap:RetainedEarningsMember2023-03-3100011786702023-03-310001178670us-gaap:CommonStockMember2023-04-012023-06-300001178670us-gaap:AdditionalPaidInCapitalMember2023-04-012023-06-3000011786702023-04-012023-06-300001178670us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-04-012023-06-300001178670us-gaap:RetainedEarningsMember2023-04-012023-06-300001178670us-gaap:CommonStockMember2023-06-300001178670us-gaap:AdditionalPaidInCapitalMember2023-06-300001178670us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-06-300001178670us-gaap:RetainedEarningsMember2023-06-3000011786702023-06-300001178670us-gaap:CommonStockMember2023-07-012023-09-300001178670us-gaap:AdditionalPaidInCapitalMember2023-07-012023-09-300001178670us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-07-012023-09-300001178670us-gaap:RetainedEarningsMember2023-07-012023-09-300001178670us-gaap:CommonStockMember2023-09-300001178670us-gaap:AdditionalPaidInCapitalMember2023-09-300001178670us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-09-300001178670us-gaap:RetainedEarningsMember2023-09-300001178670us-gaap:CommonStockMember2021-12-310001178670us-gaap:AdditionalPaidInCapitalMember2021-12-310001178670us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-12-310001178670us-gaap:RetainedEarningsMember2021-12-3100011786702021-12-310001178670us-gaap:CommonStockMember2022-01-012022-03-310001178670us-gaap:AdditionalPaidInCapitalMember2022-01-012022-03-3100011786702022-01-012022-03-310001178670us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-01-012022-03-310001178670us-gaap:RetainedEarningsMember2022-01-012022-03-310001178670us-gaap:CommonStockMember2022-03-310001178670us-gaap:AdditionalPaidInCapitalMember2022-03-310001178670us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-03-310001178670us-gaap:RetainedEarningsMember2022-03-3100011786702022-03-310001178670us-gaap:CommonStockMember2022-04-012022-06-300001178670us-gaap:AdditionalPaidInCapitalMember2022-04-012022-06-3000011786702022-04-012022-06-300001178670us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-04-012022-06-300001178670us-gaap:RetainedEarningsMember2022-04-012022-06-300001178670us-gaap:CommonStockMember2022-06-300001178670us-gaap:AdditionalPaidInCapitalMember2022-06-300001178670us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-06-300001178670us-gaap:RetainedEarningsMember2022-06-3000011786702022-06-300001178670us-gaap:CommonStockMember2022-07-012022-09-300001178670us-gaap:AdditionalPaidInCapitalMember2022-07-012022-09-300001178670us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-07-012022-09-300001178670us-gaap:RetainedEarningsMember2022-07-012022-09-300001178670us-gaap:CommonStockMember2022-09-300001178670us-gaap:AdditionalPaidInCapitalMember2022-09-300001178670us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-09-300001178670us-gaap:RetainedEarningsMember2022-09-3000011786702022-09-30alny:product0001178670country:USalny:ONPATTROMember2023-07-012023-09-300001178670country:USalny:ONPATTROMember2022-07-012022-09-300001178670country:USalny:ONPATTROMember2023-01-012023-09-300001178670country:USalny:ONPATTROMember2022-01-012022-09-300001178670srt:EuropeMemberalny:ONPATTROMember2023-07-012023-09-300001178670srt:EuropeMemberalny:ONPATTROMember2022-07-012022-09-300001178670srt:EuropeMemberalny:ONPATTROMember2023-01-012023-09-300001178670srt:EuropeMemberalny:ONPATTROMember2022-01-012022-09-300001178670alny:ONPATTROMemberalny:NonUSOrEuropeMember2023-07-012023-09-300001178670alny:ONPATTROMemberalny:NonUSOrEuropeMember2022-07-012022-09-300001178670alny:ONPATTROMemberalny:NonUSOrEuropeMember2023-01-012023-09-300001178670alny:ONPATTROMemberalny:NonUSOrEuropeMember2022-01-012022-09-300001178670alny:ONPATTROMember2023-07-012023-09-300001178670alny:ONPATTROMember2022-07-012022-09-300001178670alny:ONPATTROMember2023-01-012023-09-300001178670alny:ONPATTROMember2022-01-012022-09-300001178670country:USalny:AmvuttraMember2023-07-012023-09-300001178670country:USalny:AmvuttraMember2022-07-012022-09-300001178670country:USalny:AmvuttraMember2023-01-012023-09-300001178670country:USalny:AmvuttraMember2022-01-012022-09-300001178670alny:AmvuttraMembersrt:EuropeMember2023-07-012023-09-300001178670alny:AmvuttraMembersrt:EuropeMember2022-07-012022-09-300001178670alny:AmvuttraMembersrt:EuropeMember2023-01-012023-09-300001178670alny:AmvuttraMembersrt:EuropeMember2022-01-012022-09-300001178670alny:AmvuttraMemberalny:NonUSOrEuropeMember2023-07-012023-09-300001178670alny:AmvuttraMemberalny:NonUSOrEuropeMember2022-07-012022-09-300001178670alny:AmvuttraMemberalny:NonUSOrEuropeMember2023-01-012023-09-300001178670alny:AmvuttraMemberalny:NonUSOrEuropeMember2022-01-012022-09-300001178670alny:AmvuttraMember2023-07-012023-09-300001178670alny:AmvuttraMember2022-07-012022-09-300001178670alny:AmvuttraMember2023-01-012023-09-300001178670alny:AmvuttraMember2022-01-012022-09-300001178670alny:GIVLAARIMembercountry:US2023-07-012023-09-300001178670alny:GIVLAARIMembercountry:US2022-07-012022-09-300001178670alny:GIVLAARIMembercountry:US2023-01-012023-09-300001178670alny:GIVLAARIMembercountry:US2022-01-012022-09-300001178670alny:GIVLAARIMembersrt:EuropeMember2023-07-012023-09-300001178670alny:GIVLAARIMembersrt:EuropeMember2022-07-012022-09-300001178670alny:GIVLAARIMembersrt:EuropeMember2023-01-012023-09-300001178670alny:GIVLAARIMembersrt:EuropeMember2022-01-012022-09-300001178670alny:GIVLAARIMemberalny:NonUSOrEuropeMember2023-07-012023-09-300001178670alny:GIVLAARIMemberalny:NonUSOrEuropeMember2022-07-012022-09-300001178670alny:GIVLAARIMemberalny:NonUSOrEuropeMember2023-01-012023-09-300001178670alny:GIVLAARIMemberalny:NonUSOrEuropeMember2022-01-012022-09-300001178670alny:GIVLAARIMember2023-07-012023-09-300001178670alny:GIVLAARIMember2022-07-012022-09-300001178670alny:GIVLAARIMember2023-01-012023-09-300001178670alny:GIVLAARIMember2022-01-012022-09-300001178670country:USalny:OXLUMOMember2023-07-012023-09-300001178670country:USalny:OXLUMOMember2022-07-012022-09-300001178670country:USalny:OXLUMOMember2023-01-012023-09-300001178670country:USalny:OXLUMOMember2022-01-012022-09-300001178670alny:OXLUMOMembersrt:EuropeMember2023-07-012023-09-300001178670alny:OXLUMOMembersrt:EuropeMember2022-07-012022-09-300001178670alny:OXLUMOMembersrt:EuropeMember2023-01-012023-09-300001178670alny:OXLUMOMembersrt:EuropeMember2022-01-012022-09-300001178670alny:OXLUMOMemberalny:NonUSOrEuropeMember2023-07-012023-09-300001178670alny:OXLUMOMemberalny:NonUSOrEuropeMember2022-07-012022-09-300001178670alny:OXLUMOMemberalny:NonUSOrEuropeMember2023-01-012023-09-300001178670alny:OXLUMOMemberalny:NonUSOrEuropeMember2022-01-012022-09-300001178670alny:OXLUMOMember2023-07-012023-09-300001178670alny:OXLUMOMember2022-07-012022-09-300001178670alny:OXLUMOMember2023-01-012023-09-300001178670alny:OXLUMOMember2022-01-012022-09-300001178670us-gaap:ProductMember2023-09-300001178670us-gaap:ProductMember2022-12-310001178670alny:RocheMemberus-gaap:CollaborativeArrangementMember2023-07-012023-09-300001178670alny:RocheMemberus-gaap:CollaborativeArrangementMember2022-07-012022-09-300001178670alny:RocheMemberus-gaap:CollaborativeArrangementMember2023-01-012023-09-300001178670alny:RocheMemberus-gaap:CollaborativeArrangementMember2022-01-012022-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberus-gaap:CollaborativeArrangementMember2023-07-012023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberus-gaap:CollaborativeArrangementMember2022-07-012022-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberus-gaap:CollaborativeArrangementMember2023-01-012023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberus-gaap:CollaborativeArrangementMember2022-01-012022-09-300001178670us-gaap:CollaborativeArrangementMemberalny:NovartisMember2023-07-012023-09-300001178670us-gaap:CollaborativeArrangementMemberalny:NovartisMember2022-07-012022-09-300001178670us-gaap:CollaborativeArrangementMemberalny:NovartisMember2023-01-012023-09-300001178670us-gaap:CollaborativeArrangementMemberalny:NovartisMember2022-01-012022-09-300001178670us-gaap:CollaborativeArrangementMemberalny:OtherCollaborationsMember2023-07-012023-09-300001178670us-gaap:CollaborativeArrangementMemberalny:OtherCollaborationsMember2022-07-012022-09-300001178670us-gaap:CollaborativeArrangementMemberalny:OtherCollaborationsMember2023-01-012023-09-300001178670us-gaap:CollaborativeArrangementMemberalny:OtherCollaborationsMember2022-01-012022-09-300001178670us-gaap:CollaborativeArrangementMember2023-07-012023-09-300001178670us-gaap:CollaborativeArrangementMember2022-07-012022-09-300001178670us-gaap:CollaborativeArrangementMember2023-01-012023-09-300001178670us-gaap:CollaborativeArrangementMember2022-01-012022-09-300001178670us-gaap:CollaborativeArrangementMember2023-09-300001178670us-gaap:CollaborativeArrangementMember2022-12-310001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:ClinicalTrialAndManufacturingMember2023-07-012023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:ExternalServicesMember2023-07-012023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:OtherMember2023-07-012023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:ClinicalTrialAndManufacturingMember2022-07-012022-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:ExternalServicesMember2022-07-012022-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:OtherMember2022-07-012022-09-300001178670alny:ClinicalTrialAndManufacturingMemberalny:OtherCollaborationsMember2023-07-012023-09-300001178670alny:ExternalServicesMemberalny:OtherCollaborationsMember2023-07-012023-09-300001178670alny:OtherMemberalny:OtherCollaborationsMember2023-07-012023-09-300001178670alny:ClinicalTrialAndManufacturingMemberalny:OtherCollaborationsMember2022-07-012022-09-300001178670alny:ExternalServicesMemberalny:OtherCollaborationsMember2022-07-012022-09-300001178670alny:OtherMemberalny:OtherCollaborationsMember2022-07-012022-09-300001178670alny:ClinicalTrialAndManufacturingMember2023-07-012023-09-300001178670alny:ExternalServicesMember2023-07-012023-09-300001178670alny:OtherMember2023-07-012023-09-300001178670alny:ClinicalTrialAndManufacturingMember2022-07-012022-09-300001178670alny:ExternalServicesMember2022-07-012022-09-300001178670alny:OtherMember2022-07-012022-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:ClinicalTrialAndManufacturingMember2023-01-012023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:ExternalServicesMember2023-01-012023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:OtherMember2023-01-012023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:ClinicalTrialAndManufacturingMember2022-01-012022-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:ExternalServicesMember2022-01-012022-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:OtherMember2022-01-012022-09-300001178670alny:ClinicalTrialAndManufacturingMemberalny:OtherCollaborationsMember2023-01-012023-09-300001178670alny:ExternalServicesMemberalny:OtherCollaborationsMember2023-01-012023-09-300001178670alny:OtherMemberalny:OtherCollaborationsMember2023-01-012023-09-300001178670alny:ClinicalTrialAndManufacturingMemberalny:OtherCollaborationsMember2022-01-012022-09-300001178670alny:ExternalServicesMemberalny:OtherCollaborationsMember2022-01-012022-09-300001178670alny:OtherMemberalny:OtherCollaborationsMember2022-01-012022-09-300001178670alny:ClinicalTrialAndManufacturingMember2023-01-012023-09-300001178670alny:ExternalServicesMember2023-01-012023-09-300001178670alny:OtherMember2023-01-012023-09-300001178670alny:ClinicalTrialAndManufacturingMember2022-01-012022-09-300001178670alny:ExternalServicesMember2022-01-012022-09-300001178670alny:OtherMember2022-01-012022-09-300001178670alny:RocheMemberalny:RocheCollaborationAndLicenseAgreementMember2023-07-212023-07-210001178670alny:RocheMemberalny:RocheCollaborationAndLicenseAgreementMemberalny:SpecifiedDevelopmentAndRegulatoryMilestonesMember2023-07-212023-07-210001178670alny:RocheMemberalny:SalesBasedMilestonesMemberalny:RocheCollaborationAndLicenseAgreementMember2023-07-212023-07-210001178670alny:RocheCollaborationAndLicenseAgreementMember2023-07-212023-07-21xbrli:pure0001178670alny:RocheCollaborationAndLicenseAgreementMember2023-07-21alny:performance_obligation0001178670alny:RocheMemberalny:RocheCollaborationAndLicenseAgreementMemberalny:LicenseObligationMember2023-09-300001178670alny:RocheMemberalny:RocheCollaborationAndLicenseAgreementMemberalny:LicenseObligationMember2023-07-012023-09-300001178670alny:RocheMemberalny:RocheCollaborationAndLicenseAgreementMemberalny:DevelopmentServicesObligationMember2023-09-300001178670alny:RocheMemberalny:RocheCollaborationAndLicenseAgreementMemberalny:DevelopmentServicesObligationMember2023-07-012023-09-300001178670alny:RocheMemberalny:RocheCollaborationAndLicenseAgreementMemberalny:TechnologyTransferObligationMember2023-09-300001178670alny:RocheMemberalny:RocheCollaborationAndLicenseAgreementMemberalny:TechnologyTransferObligationMember2023-07-012023-09-300001178670alny:RocheMemberalny:RocheCollaborationAndLicenseAgreementMember2023-09-300001178670alny:RocheMemberalny:RocheCollaborationAndLicenseAgreementMember2023-07-012023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMember2019-04-012019-04-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMembersrt:MaximumMember2019-04-012019-04-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:MilestoneMemberalny:GlobalStrategicCollaborationMember2023-07-012023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMember2023-07-012023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:ContingentMilestoneMemberalny:GlobalStrategicCollaborationMember2023-07-012023-09-30alny:target0001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMemberalny:FundingAtProgramInitiationMember2023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMemberalny:FundingAtLeadCandidateIdentificationMember2023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMemberalny:FundingAnAnnualDiscoveryMember2019-04-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMembersrt:MaximumMember2019-04-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMemberalny:ResearchServicesObligationMember2019-04-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:C5LicenseObligationMemberalny:GlobalStrategicCollaborationMember2019-04-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMemberalny:C5CoCoObligationMember2019-04-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMember2019-04-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMember2023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMember2023-01-012023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMemberalny:ResearchServicesObligationMember2023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMemberalny:ResearchServicesObligationMember2022-12-310001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:C5LicenseObligationMemberalny:GlobalStrategicCollaborationMember2023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:C5LicenseObligationMemberalny:GlobalStrategicCollaborationMember2022-12-310001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMemberalny:C5CoCoObligationMember2023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMemberalny:C5CoCoObligationMember2022-12-310001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMember2022-12-310001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMemberalny:ResearchServicesObligationMember2023-07-012023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMemberalny:ResearchServicesObligationMember2022-07-012022-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMemberalny:ResearchServicesObligationMember2023-01-012023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMemberalny:ResearchServicesObligationMember2022-01-012022-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:C5LicenseObligationMemberalny:GlobalStrategicCollaborationMember2023-07-012023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:C5LicenseObligationMemberalny:GlobalStrategicCollaborationMember2022-07-012022-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:C5LicenseObligationMemberalny:GlobalStrategicCollaborationMember2023-01-012023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:C5LicenseObligationMemberalny:GlobalStrategicCollaborationMember2022-01-012022-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMemberalny:C5CoCoObligationMember2023-07-012023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMemberalny:C5CoCoObligationMember2022-07-012022-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMemberalny:C5CoCoObligationMember2023-01-012023-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMemberalny:C5CoCoObligationMember2022-01-012022-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMember2022-07-012022-09-300001178670alny:RegeneronPharmaceuticalsIncorporationMemberalny:GlobalStrategicCollaborationMember2022-01-012022-09-300001178670us-gaap:CollaborativeArrangementMemberalny:BlackstoneGroupIncMember2020-04-300001178670us-gaap:CollaborativeArrangementMemberalny:BlackstoneGroupIncMember2020-04-012020-04-300001178670srt:ScenarioForecastMemberus-gaap:CollaborativeArrangementMemberalny:BlackstoneGroupIncMember2030-01-010001178670us-gaap:CollaborativeArrangementMemberalny:BlackstoneGroupIncMember2023-09-300001178670us-gaap:CollaborativeArrangementMemberalny:BlackstoneGroupIncMember2022-12-310001178670us-gaap:CollaborativeArrangementMemberalny:BlackstoneGroupIncMember2023-01-012023-09-300001178670us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2023-09-300001178670us-gaap:FairValueInputsLevel1Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2023-09-300001178670us-gaap:FairValueInputsLevel2Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2023-09-300001178670us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2023-09-300001178670us-gaap:FairValueMeasurementsRecurringMemberus-gaap:CommercialPaperMember2023-09-300001178670us-gaap:FairValueInputsLevel1Memberus-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMember2023-09-300001178670us-gaap:FairValueInputsLevel2Memberus-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMember2023-09-300001178670us-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2023-09-300001178670us-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMember2023-09-300001178670us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMember2023-09-300001178670us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMember2023-09-300001178670us-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMemberus-gaap:FairValueInputsLevel3Member2023-09-300001178670us-gaap:USGovernmentSponsoredEnterprisesDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2023-09-300001178670us-gaap:FairValueInputsLevel1Memberus-gaap:USGovernmentSponsoredEnterprisesDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2023-09-300001178670us-gaap:FairValueInputsLevel2Memberus-gaap:USGovernmentSponsoredEnterprisesDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2023-09-300001178670us-gaap:USGovernmentSponsoredEnterprisesDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2023-09-300001178670us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2023-09-300001178670us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2023-09-300001178670us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2023-09-300001178670us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2023-09-300001178670us-gaap:FairValueMeasurementsRecurringMemberus-gaap:CertificatesOfDepositMember2023-09-300001178670us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CertificatesOfDepositMember2023-09-300001178670us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CertificatesOfDepositMember2023-09-300001178670us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Memberus-gaap:CertificatesOfDepositMember2023-09-300001178670us-gaap:FairValueMeasurementsRecurringMember2023-09-300001178670us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2023-09-300001178670us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2023-09-300001178670us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2023-09-300001178670us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001178670us-gaap:FairValueInputsLevel1Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001178670us-gaap:FairValueInputsLevel2Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001178670us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2022-12-310001178670us-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMember2022-12-310001178670us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMember2022-12-310001178670us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMember2022-12-310001178670us-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMemberus-gaap:FairValueInputsLevel3Member2022-12-310001178670us-gaap:USGovernmentSponsoredEnterprisesDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001178670us-gaap:FairValueInputsLevel1Memberus-gaap:USGovernmentSponsoredEnterprisesDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001178670us-gaap:FairValueInputsLevel2Memberus-gaap:USGovernmentSponsoredEnterprisesDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001178670us-gaap:USGovernmentSponsoredEnterprisesDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2022-12-310001178670us-gaap:FairValueMeasurementsRecurringMemberus-gaap:CommercialPaperMember2022-12-310001178670us-gaap:FairValueInputsLevel1Memberus-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001178670us-gaap:FairValueInputsLevel2Memberus-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001178670us-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2022-12-310001178670us-gaap:FairValueMeasurementsRecurringMemberus-gaap:CertificatesOfDepositMember2022-12-310001178670us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CertificatesOfDepositMember2022-12-310001178670us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CertificatesOfDepositMember2022-12-310001178670us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Memberus-gaap:CertificatesOfDepositMember2022-12-310001178670us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001178670us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001178670us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001178670us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2022-12-310001178670us-gaap:FairValueMeasurementsRecurringMember2022-12-310001178670us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001178670us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001178670us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2022-12-310001178670us-gaap:USTreasurySecuritiesMember2023-09-300001178670us-gaap:USGovernmentSponsoredEnterprisesDebtSecuritiesMember2023-09-300001178670us-gaap:CorporateDebtSecuritiesMember2023-09-300001178670us-gaap:CommercialPaperMember2023-09-300001178670us-gaap:CertificatesOfDepositMember2023-09-300001178670us-gaap:USTreasurySecuritiesMember2022-12-310001178670us-gaap:USGovernmentSponsoredEnterprisesDebtSecuritiesMember2022-12-310001178670us-gaap:CorporateDebtSecuritiesMember2022-12-310001178670us-gaap:CommercialPaperMember2022-12-310001178670us-gaap:CertificatesOfDepositMember2022-12-310001178670us-gaap:OtherAssetsMember2023-09-300001178670us-gaap:OtherAssetsMember2022-12-310001178670alny:AccumulatedLossOnInvestmentInJointVentureMember2022-12-310001178670us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2022-12-310001178670us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2022-12-310001178670us-gaap:AccumulatedTranslationAdjustmentMember2022-12-310001178670alny:AccumulatedLossOnInvestmentInJointVentureMember2023-01-012023-09-300001178670us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2023-01-012023-09-300001178670us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2023-01-012023-09-300001178670us-gaap:AccumulatedTranslationAdjustmentMember2023-01-012023-09-300001178670alny:AccumulatedLossOnInvestmentInJointVentureMember2023-09-300001178670us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2023-09-300001178670us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2023-09-300001178670us-gaap:AccumulatedTranslationAdjustmentMember2023-09-300001178670alny:AccumulatedLossOnInvestmentInJointVentureMember2021-12-310001178670us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2021-12-310001178670us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2021-12-310001178670us-gaap:AccumulatedTranslationAdjustmentMember2021-12-310001178670alny:AccumulatedLossOnInvestmentInJointVentureMember2022-01-012022-09-300001178670us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2022-01-012022-09-300001178670us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2022-01-012022-09-300001178670us-gaap:AccumulatedTranslationAdjustmentMember2022-01-012022-09-300001178670alny:AccumulatedLossOnInvestmentInJointVentureMember2022-09-300001178670us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2022-09-300001178670us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2022-09-300001178670us-gaap:AccumulatedTranslationAdjustmentMember2022-09-300001178670us-gaap:ConvertibleDebtMemberalny:ConvertibleSeniorNotesDue2027InitialAmountMember2022-09-120001178670alny:ConvertibleSeniorNotesDue2027Memberus-gaap:ConvertibleDebtMember2022-09-122022-09-120001178670alny:ConvertibleSeniorNotesDue2027AdditionalAmountMemberus-gaap:ConvertibleDebtMember2022-09-130001178670alny:ConvertibleSeniorNotesDue2027Memberus-gaap:ConvertibleDebtMember2022-09-132022-09-130001178670alny:ConvertibleSeniorNotesDue2027Memberus-gaap:ConvertibleDebtMember2022-09-130001178670alny:ConvertibleSeniorNotesDue2027Memberus-gaap:ConvertibleDebtMember2022-09-152022-09-150001178670alny:DebtConversionTermsOneMemberalny:ConvertibleSeniorNotesDue2027Memberus-gaap:ConvertibleDebtMember2022-09-152022-09-15alny:day0001178670alny:ConvertibleSeniorNotesDue2027Memberalny:DebtConversionTermsTwoMemberus-gaap:ConvertibleDebtMember2022-09-152022-09-150001178670alny:ConvertibleSeniorNotesDue2027Memberus-gaap:ConvertibleDebtMember2022-09-120001178670alny:ConvertibleSeniorNotesDue2025Memberus-gaap:ConvertibleDebtMember2022-09-152022-09-150001178670alny:ConvertibleSeniorNotesDue2027Memberus-gaap:ConvertibleDebtMember2023-09-300001178670alny:ConvertibleSeniorNotesDue2027Memberus-gaap:ConvertibleDebtMember2023-01-012023-09-300001178670us-gaap:CallOptionMemberus-gaap:ConvertibleDebtMember2023-09-30iso4217:USDalny:Unit0001178670us-gaap:CallOptionMemberus-gaap:ConvertibleDebtMember2022-09-120001178670us-gaap:CallOptionMemberus-gaap:ConvertibleDebtMember2023-01-012023-09-300001178670us-gaap:CollaborativeArrangementMemberalny:VutrisiranAndZilebesiranMemberalny:BlackstoneLifeSciencesMember2020-08-012020-08-310001178670alny:HELIOSBPhase3ClinicalTrialMemberus-gaap:CollaborativeArrangementMemberalny:BlackstoneLifeSciencesMember2020-08-012020-08-310001178670us-gaap:CollaborativeArrangementMemberalny:ALNAGTPhase2ClinicalTrialMemberalny:BlackstoneLifeSciencesMember2020-08-012020-08-310001178670alny:ALNAGTPhase3ClinicalTrialMemberus-gaap:CollaborativeArrangementMemberalny:BlackstoneLifeSciencesMember2020-08-012020-08-310001178670alny:VutrisiranMemberus-gaap:CollaborativeArrangementMemberalny:BlackstoneLifeSciencesMember2020-08-012020-08-310001178670alny:DevelopmentMilestoneMemberalny:BlackstoneLifeSciencesMember2023-09-012023-09-300001178670us-gaap:CollaborativeArrangementMemberalny:ALNAGTPhase2ClinicalTrialMemberalny:BlackstoneLifeSciencesMember2023-09-012023-09-300001178670alny:BlackstoneLifeSciencesMember2023-09-300001178670us-gaap:DerivativeMember2022-12-310001178670us-gaap:DerivativeMember2023-01-012023-09-300001178670us-gaap:DerivativeMember2023-09-300001178670us-gaap:ResearchAndDevelopmentExpenseMember2023-07-012023-09-300001178670us-gaap:ResearchAndDevelopmentExpenseMember2022-07-012022-09-300001178670us-gaap:ResearchAndDevelopmentExpenseMember2023-01-012023-09-300001178670us-gaap:ResearchAndDevelopmentExpenseMember2022-01-012022-09-300001178670us-gaap:SellingGeneralAndAdministrativeExpensesMember2023-07-012023-09-300001178670us-gaap:SellingGeneralAndAdministrativeExpensesMember2022-07-012022-09-300001178670us-gaap:SellingGeneralAndAdministrativeExpensesMember2023-01-012023-09-300001178670us-gaap:SellingGeneralAndAdministrativeExpensesMember2022-01-012022-09-300001178670us-gaap:EmployeeStockOptionMember2023-07-012023-09-300001178670us-gaap:EmployeeStockOptionMember2022-07-012022-09-300001178670us-gaap:EmployeeStockOptionMember2023-01-012023-09-300001178670us-gaap:EmployeeStockOptionMember2022-01-012022-09-300001178670us-gaap:RestrictedStockMember2023-07-012023-09-300001178670us-gaap:RestrictedStockMember2022-07-012022-09-300001178670us-gaap:RestrictedStockMember2023-01-012023-09-300001178670us-gaap:RestrictedStockMember2022-01-012022-09-300001178670us-gaap:ConvertibleDebtMember2023-07-012023-09-300001178670us-gaap:ConvertibleDebtMember2022-07-012022-09-300001178670us-gaap:ConvertibleDebtMember2023-01-012023-09-300001178670us-gaap:ConvertibleDebtMember2022-01-012022-09-300001178670us-gaap:EmployeeStockOptionMember2023-07-012023-09-300001178670us-gaap:EmployeeStockOptionMember2022-07-012022-09-300001178670us-gaap:EmployeeStockOptionMember2023-01-012023-09-300001178670us-gaap:EmployeeStockOptionMember2022-01-012022-09-300001178670us-gaap:RestrictedStockUnitsRSUMember2023-07-012023-09-300001178670us-gaap:RestrictedStockUnitsRSUMember2022-07-012022-09-300001178670us-gaap:RestrictedStockUnitsRSUMember2023-01-012023-09-300001178670us-gaap:RestrictedStockUnitsRSUMember2022-01-012022-09-300001178670us-gaap:EmployeeStockMember2023-07-012023-09-300001178670us-gaap:EmployeeStockMember2022-07-012022-09-300001178670us-gaap:EmployeeStockMember2023-01-012023-09-300001178670us-gaap:EmployeeStockMember2022-01-012022-09-300001178670alny:MichaelWBonneyMember2023-07-012023-09-300001178670alny:MichaelWBonneyMember2023-01-012023-09-300001178670alny:MichaelWBonneyMember2023-09-300001178670alny:DavidEIPyottMember2023-07-012023-09-300001178670alny:DavidEIPyottMember2023-01-012023-09-300001178670alny:DavidEIPyottMember2023-09-30
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
____________________________________________
FORM 10-Q
____________________________________________
|
|
|
|
|
|
| ☒ |
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended September 30, 2023
OR
|
|
|
|
|
|
| ☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ___________ to ___________
Commission File Number 001-36407
__________________________________________
ALNYLAM PHARMACEUTICALS, INC.
(Exact Name of Registrant as Specified in Its Charter)
__________________________________________
|
|
|
|
|
|
|
Delaware
(State or Other Jurisdiction of
Incorporation or Organization)
|
77-0602661
(I.R.S. Employer
Identification No.)
|
|
|
|
|
|
|
|
675 West Kendall Street,
Henri A. Termeer Square
Cambridge, MA
(Address of Principal Executive Offices)
|
02142
(Zip Code)
|
(617) 551-8200
(Registrant’s Telephone Number, Including Area Code)
__________________________________________
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Title of Each Class |
|
Trading Symbol(s) |
|
Name of Each Exchange on Which Registered |
|
|
Common Stock, $0.01 par value per share |
|
ALNY |
|
The Nasdaq Stock Market LLC |
|
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes x No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
|
|
|
|
|
|
|
|
|
|
|
| Large accelerated filer |
x |
Accelerated filer |
☐ |
| Non-accelerated filer |
☐ |
Smaller reporting company |
☐ |
| Emerging growth company |
☐ |
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No x
At October 27, 2023, the registrant had 125,492,927 shares of Common Stock, $0.01 par value per share, outstanding.
ALNYLAM PHARMACEUTICALS, INC.
QUARTERLY REPORT ON FORM 10-Q
“Alnylam,” ONPATTRO®, AMVUTTRA®, GIVLAARI®, OXLUMO®, Alnylam Act®, GEMINI™ and IKARIA™ are trademarks and registered trademarks of Alnylam Pharmaceuticals, Inc. Our logo, trademarks and service marks are property of Alnylam. All other trademarks or service marks appearing in this Quarterly Report on Form 10-Q are the property of their respective holders.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q contains forward-looking statements within the meaning of the federal securities laws, Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995 and are including this statement for purposes of complying with those safe harbor provisions. All statements other than statements of historical facts contained in this Quarterly Report on Form 10-Q are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “could,” “expects,” “plans,” “intends,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology. These forward-looking statements include, but are not limited to, statements about:
•our views with respect to the potential for approved and investigational RNAi therapeutics, including ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO, Leqvio® (inclisiran), fitusiran and zilebesiran;
•our plans for additional global regulatory filings and the continuing product launches of ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO and our partner's plans with respect to Leqvio;
•our future ability to successfully expand the indication for AMVUTTRA;
•our expectations regarding potential market size for, and the successful commercialization of, ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO, Leqvio or any future products;
•our ability to obtain and maintain regulatory approvals and pricing and reimbursement for ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO or any future products, and our partners' ability with respect to Leqvio and fitusiran;
•the progress of our research and development programs, including programs in both rare and prevalent diseases;
•the potential for improved product profiles to emerge from our new technologies, including our IKARIA and GEMINI platforms and our ability to expand our product engine to include extrahepatic tissues;
•our current and anticipated clinical trials and expectations regarding the reporting of data from these trials;
•risks related to the direct or indirect impact of the novel coronavirus, or COVID-19, global pandemic, emerging or future variants of COVID-19 or any future pandemic, or public health emergency, on, among other things, our financial performance, business and operations, including manufacturing, supply chain, research and development activities and pipeline programs, and other potential impacts to our business;
•any impact of the ongoing conflicts in Ukraine and Israel, including disruptions to our clinical trials;
•the timing of regulatory filings and interactions with, or actions or advice of, regulatory authorities, which may affect the design, initiation, timing, continuation and/or progress of clinical trials, or result in the need for additional pre-clinical and/or clinical testing or the timing or likelihood of regulatory approvals;
•the status of our manufacturing operations and any delays, interruptions or failures in the manufacture and supply of ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO or any of our product candidates (or other products or product candidates being developed and commercialized by our partners), by our or their contract manufacturers or by us or our partners;
•our progress continuing to build and leverage our global commercial infrastructure;
•the possible impact of any competing products on the commercial success of ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO and Leqvio, as well as our product candidates, and, our, or with respect to Leqvio or fitusiran, our partners', ability to compete against such products;
•our ability to manage our growth and operating expenses;
•our views and plans with respect to our 5-year Alnylam P5x25 strategy and our intentions to achieve the metrics associated with this strategy, including to become a top-tier biotech company by the end of 2025;
•our belief that our current cash balance should enable us to achieve a self-sustainable profile without the need for future equity financing;
•our expectations regarding the length of time our current cash, cash equivalents and marketable equity and debt securities will support our operations based on our current operating plan;
•our dependence on third parties for development, manufacture and distribution of products;
•our expectations regarding our corporate collaborations, including potential future licensing fees and milestone and royalty payments under existing or future agreements;
•our ability to obtain, maintain and protect our intellectual property;
•our ability to attract and retain qualified key management and scientists, development, medical and commercial staff, consultants and advisors and to successfully execute on our Alnylam P5x25 strategy;
•the outcome of litigation, including our patent infringement suits against Pfizer, Inc., BioNTech SE and Moderna, Inc., or of other legal proceedings or government investigations;
•regulatory developments in the United States, or U.S., and foreign countries;
•the impact of laws and regulations;
•developments relating to our competitors and our industry;
•our ability to satisfy our payment obligations, and to service the interest on, or to refinance our indebtedness, including our convertible notes, or to make cash payments in connection with any conversion of our convertible notes, to the extent required;
•our expectations regarding the effect of the capped call transactions and the anticipated market activities of the option counterparties and/or their respective affiliates; and
•other risks and uncertainties, including those listed under the caption Part II, Item 1A, "Risk Factors" of this Quarterly Report on Form 10-Q.
The risks set forth above are not exhaustive. Other sections of this Quarterly Report on Form 10-Q may include additional factors that could adversely affect our business and financial performance. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to time and it is not possible for management to predict all risk factors, nor can we assess the impact of all risk factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. Any forward-looking statements in this Quarterly Report on Form 10-Q reflect our current views with respect to future events and with respect to our business and future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by these forward-looking statements. Factors that may cause actual results to differ materially from current expectations include, among other things, those described under Part II, Item 1A, "Risk Factors" and elsewhere in this Quarterly Report on Form 10-Q. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Except as required by law, we assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future. You are advised, however, to consult any further disclosure we make in our reports filed with the SEC.
PART I. FINANCIAL INFORMATION
ALNYLAM PHARMACEUTICALS, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(In thousands, except per share amounts)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
September 30, 2023 |
|
December 31, 2022 |
| ASSETS |
|
|
|
| Current assets: |
|
|
|
| Cash and cash equivalents |
$ |
1,033,024 |
|
|
$ |
866,394 |
|
| Marketable debt securities |
1,362,843 |
|
|
1,297,890 |
|
| Marketable equity securities |
10,411 |
|
|
28,122 |
|
| Accounts receivable, net |
325,445 |
|
|
237,963 |
|
| Inventory |
95,771 |
|
|
128,962 |
|
| Prepaid expenses and other current assets |
157,958 |
|
|
132,916 |
|
|
|
|
|
| Total current assets |
2,985,452 |
|
|
2,692,247 |
|
| Property, plant and equipment, net |
525,591 |
|
|
523,494 |
|
| Operating lease right-of-use assets |
203,485 |
|
|
215,136 |
|
| Restricted investments |
49,390 |
|
|
49,390 |
|
| Other assets |
75,155 |
|
|
66,092 |
|
| Total assets |
$ |
3,839,073 |
|
|
$ |
3,546,359 |
|
| LIABILITIES AND STOCKHOLDERS’ DEFICIT |
|
|
|
| Current liabilities: |
|
|
|
| Accounts payable |
$ |
73,840 |
|
|
$ |
98,094 |
|
| Accrued expenses |
713,094 |
|
|
545,460 |
|
| Operating lease liability |
41,516 |
|
|
41,967 |
|
| Deferred revenue |
77,140 |
|
|
42,105 |
|
| Liability related to the sale of future royalties |
44,195 |
|
|
40,289 |
|
| Total current liabilities |
949,785 |
|
|
767,915 |
|
Operating lease liability, net of current portion |
247,711 |
|
|
261,339 |
|
| Deferred revenue, net of current portion |
196,086 |
|
|
193,791 |
|
| Convertible debt |
1,019,809 |
|
|
1,016,942 |
|
| Liability related to the sale of future royalties, net of current portion |
1,310,814 |
|
|
1,252,015 |
|
| Other liabilities |
280,734 |
|
|
212,580 |
|
| Total liabilities |
4,004,939 |
|
|
3,704,582 |
|
| Commitments and contingencies (Note 13) |
|
|
|
| Stockholders’ deficit: |
|
|
|
Preferred stock, $0.01 par value per share, 5,000 shares authorized and no shares issued and outstanding as of September 30, 2023 and December 31, 2022 |
— |
|
|
— |
|
Common stock, $0.01 par value per share, 250,000 shares authorized; 125,454 shares issued and outstanding as of September 30, 2023; 123,925 shares issued and outstanding as of December 31, 2022 |
1,255 |
|
|
1,240 |
|
| Additional paid-in capital |
6,736,939 |
|
|
6,454,540 |
|
| Accumulated other comprehensive loss |
(32,339) |
|
|
(44,654) |
|
| Accumulated deficit |
(6,871,721) |
|
|
(6,569,349) |
|
| Total stockholders’ deficit |
(165,866) |
|
|
(158,223) |
|
| Total liabilities and stockholders’ deficit |
$ |
3,839,073 |
|
|
$ |
3,546,359 |
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
ALNYLAM PHARMACEUTICALS, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
AND COMPREHENSIVE INCOME (LOSS)
(In thousands, except per share amounts)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
September 30, |
|
Nine Months Ended
September 30, |
|
2023 |
|
2022 |
|
2023 |
|
2022 |
| Statements of Operations |
|
|
|
|
|
|
|
| Revenues: |
|
|
|
|
|
|
|
| Net product revenues |
$ |
313,153 |
|
|
$ |
232,267 |
|
|
$ |
895,186 |
|
|
$ |
632,654 |
|
| Net revenues from collaborations |
427,472 |
|
|
29,297 |
|
|
469,778 |
|
|
64,267 |
|
| Royalty revenue |
9,905 |
|
|
2,742 |
|
|
23,610 |
|
|
5,462 |
|
| Total revenues |
750,530 |
|
|
264,306 |
|
|
1,388,574 |
|
|
702,383 |
|
| Operating costs and expenses: |
|
|
|
|
|
|
|
| Cost of goods sold |
79,473 |
|
|
36,507 |
|
|
196,241 |
|
|
94,002 |
|
| Cost of collaborations and royalties |
4,836 |
|
|
4,609 |
|
|
28,307 |
|
|
23,549 |
|
| Research and development |
253,179 |
|
|
245,371 |
|
|
732,274 |
|
|
620,976 |
|
| Selling, general and administrative |
199,175 |
|
|
235,859 |
|
|
597,523 |
|
|
560,314 |
|
| Total operating costs and expenses |
536,663 |
|
|
522,346 |
|
|
1,554,345 |
|
|
1,298,841 |
|
Income (loss) from operations |
213,867 |
|
|
(258,040) |
|
|
(165,771) |
|
|
(596,458) |
|
| Other (expense) income: |
|
|
|
|
|
|
|
| Interest expense |
(30,893) |
|
|
(41,084) |
|
|
(89,883) |
|
|
(126,055) |
|
| Interest income |
25,425 |
|
|
7,820 |
|
|
65,155 |
|
|
10,731 |
|
| Other expense, net |
(57,658) |
|
|
(38,053) |
|
|
(105,331) |
|
|
(131,604) |
|
| Loss on the extinguishment of debt |
— |
|
|
(76,586) |
|
|
— |
|
|
(76,586) |
|
| Total other expense, net |
(63,126) |
|
|
(147,903) |
|
|
(130,059) |
|
|
(323,514) |
|
Income (loss) before income taxes |
150,741 |
|
|
(405,943) |
|
|
(295,830) |
|
|
(919,972) |
|
(Provision for) benefit from income taxes |
(2,988) |
|
|
23 |
|
|
(6,542) |
|
|
(3,691) |
|
Net income (loss) |
$ |
147,753 |
|
|
$ |
(405,920) |
|
|
$ |
(302,372) |
|
|
$ |
(923,663) |
|
|
|
|
|
|
|
|
|
Net income (loss) per common share - basic |
$ |
1.18 |
|
|
$ |
(3.32) |
|
|
$ |
(2.43) |
|
|
$ |
(7.62) |
|
Net income (loss) per common share - diluted |
$ |
1.15 |
|
|
$ |
(3.32) |
|
|
$ |
(2.43) |
|
|
$ |
(7.62) |
|
|
|
|
|
|
|
|
|
Weighted-average common shares- basic |
125,220 |
|
|
122,166 |
|
|
124,667 |
|
|
121,158 |
|
Weighted-average common shares- diluted |
131,337 |
|
|
122,166 |
|
|
124,667 |
|
|
121,158 |
|
|
|
|
|
|
|
|
|
Statements of Comprehensive Income (Loss) |
|
|
|
|
|
|
|
Net income (loss) |
$ |
147,753 |
|
|
$ |
(405,920) |
|
|
$ |
(302,372) |
|
|
$ |
(923,663) |
|
| Other comprehensive income (loss): |
|
|
|
|
|
|
|
Unrealized gain (loss) on marketable securities |
1,878 |
|
|
(2,053) |
|
|
3,978 |
|
|
(11,004) |
|
Foreign currency translation gain |
2,873 |
|
|
460 |
|
|
8,356 |
|
|
377 |
|
| Defined benefit pension plans, net of tax |
(10) |
|
|
34 |
|
|
(19) |
|
|
103 |
|
| Total other comprehensive income (loss) |
4,741 |
|
|
(1,559) |
|
|
12,315 |
|
|
(10,524) |
|
Comprehensive income (loss) |
$ |
152,494 |
|
|
$ |
(407,479) |
|
|
$ |
(290,057) |
|
|
$ |
(934,187) |
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
ALNYLAM PHARMACEUTICALS, INC.
CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ (DEFICIT) EQUITY
(In thousands)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock |
|
Additional
Paid-in
Capital |
|
Accumulated
Other
Comprehensive
Loss |
|
Accumulated
Deficit |
|
Total
Stockholders’
Deficit |
|
Shares |
|
Amount |
|
|
|
|
| Balance as of December 31, 2022 |
123,925 |
|
|
$ |
1,240 |
|
|
$ |
6,454,540 |
|
|
$ |
(44,654) |
|
|
$ |
(6,569,349) |
|
|
$ |
(158,223) |
|
| Exercise of common stock options, net of tax withholdings |
269 |
|
|
3 |
|
|
26,415 |
|
|
— |
|
|
— |
|
|
26,418 |
|
| Issuance of common stock under equity plans |
47 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
| Stock-based compensation expense |
— |
|
|
— |
|
|
41,136 |
|
|
— |
|
|
— |
|
|
41,136 |
|
| Other comprehensive income |
— |
|
|
— |
|
|
— |
|
|
5,530 |
|
|
— |
|
|
5,530 |
|
| Net loss |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(174,101) |
|
|
(174,101) |
|
| Balance as of March 31, 2023 |
124,241 |
|
|
1,243 |
|
|
6,522,091 |
|
|
(39,124) |
|
|
(6,743,450) |
|
|
(259,240) |
|
| Exercise of common stock options, net of tax withholdings |
372 |
|
|
4 |
|
|
38,111 |
|
|
— |
|
|
— |
|
|
38,115 |
|
| Issuance of common stock under equity plans |
288 |
|
|
3 |
|
|
9,981 |
|
|
— |
|
|
— |
|
|
9,984 |
|
| Stock-based compensation expense |
— |
|
|
— |
|
|
76,990 |
|
|
— |
|
|
— |
|
|
76,990 |
|
| Other comprehensive income |
— |
|
|
— |
|
|
— |
|
|
2,044 |
|
|
— |
|
|
2,044 |
|
| Net loss |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(276,024) |
|
|
(276,024) |
|
| Balance as of June 30, 2023 |
124,901 |
|
|
1,250 |
|
|
6,647,173 |
|
|
(37,080) |
|
|
(7,019,474) |
|
|
(408,131) |
|
| Exercise of common stock options, net of tax withholdings |
246 |
|
|
2 |
|
|
24,896 |
|
|
— |
|
|
— |
|
|
24,898 |
|
| Issuance of common stock under equity plans |
307 |
|
|
3 |
|
|
(3) |
|
|
— |
|
|
— |
|
|
— |
|
| Stock-based compensation expense |
— |
|
|
— |
|
|
64,873 |
|
|
— |
|
|
— |
|
|
64,873 |
|
| Other comprehensive income |
— |
|
|
— |
|
|
— |
|
|
4,741 |
|
|
— |
|
|
4,741 |
|
Net income |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
147,753 |
|
|
147,753 |
|
| Balance as of September 30, 2023 |
125,454 |
|
|
$ |
1,255 |
|
|
$ |
6,736,939 |
|
|
$ |
(32,339) |
|
|
$ |
(6,871,721) |
|
|
$ |
(165,866) |
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
ALNYLAM PHARMACEUTICALS, INC.
CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ (DEFICIT) EQUITY
(In thousands)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock |
|
Additional
Paid-in
Capital |
|
Accumulated
Other
Comprehensive
Loss
|
|
Accumulated
Deficit |
|
Total
Stockholders’
(Deficit) Equity
|
|
Shares |
|
Amount |
|
|
|
|
| Balance as of December 31, 2021 |
120,182 |
|
|
$ |
1,202 |
|
|
$ |
6,058,453 |
|
|
$ |
(33,259) |
|
|
$ |
(5,438,193) |
|
|
$ |
588,203 |
|
| Exercise of common stock options, net of tax withholdings |
524 |
|
|
5 |
|
|
28,054 |
|
|
— |
|
|
— |
|
|
28,059 |
|
| Issuance of common stock under equity plans |
23 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
| Stock-based compensation expense |
— |
|
|
— |
|
|
30,051 |
|
|
— |
|
|
— |
|
|
30,051 |
|
| Other comprehensive loss |
— |
|
|
— |
|
|
— |
|
|
(4,806) |
|
|
— |
|
|
(4,806) |
|
| Net loss |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(240,341) |
|
|
(240,341) |
|
| Balance as of March 31, 2022 |
120,729 |
|
|
1,207 |
|
|
6,116,558 |
|
|
(38,065) |
|
|
(5,678,534) |
|
|
401,166 |
|
| Exercise of common stock options, net of tax withholdings |
192 |
|
|
2 |
|
|
13,890 |
|
|
— |
|
|
— |
|
|
13,892 |
|
| Issuance of common stock under equity plans |
71 |
|
|
1 |
|
|
8,089 |
|
|
— |
|
|
— |
|
|
8,090 |
|
| Stock-based compensation expense |
— |
|
|
— |
|
|
34,453 |
|
|
— |
|
|
— |
|
|
34,453 |
|
| Other comprehensive loss |
— |
|
|
— |
|
|
— |
|
|
(4,159) |
|
|
— |
|
|
(4,159) |
|
| Net loss |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(277,402) |
|
|
(277,402) |
|
| Balance as of June 30, 2022 |
120,992 |
|
|
1,210 |
|
|
6,172,990 |
|
|
(42,224) |
|
|
(5,955,936) |
|
|
176,040 |
|
| Exercise of common stock options, net of tax withholdings |
1,657 |
|
|
17 |
|
|
153,259 |
|
|
— |
|
|
— |
|
|
153,276 |
|
| Issuance of common stock under equity plans |
342 |
|
|
3 |
|
|
— |
|
|
— |
|
|
— |
|
|
3 |
|
| Stock-based compensation expense |
— |
|
|
— |
|
|
129,133 |
|
|
— |
|
|
— |
|
|
129,133 |
|
| Purchase of capped calls related to convertible debt |
— |
|
|
— |
|
|
(118,611) |
|
|
— |
|
|
— |
|
|
(118,611) |
|
| Other comprehensive loss |
— |
|
|
— |
|
|
— |
|
|
(1,559) |
|
|
— |
|
|
(1,559) |
|
| Net loss |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(405,920) |
|
|
(405,920) |
|
| Balance as of September 30, 2022 |
122,991 |
|
|
$ |
1,230 |
|
|
$ |
6,336,771 |
|
|
$ |
(43,783) |
|
|
$ |
(6,361,856) |
|
|
$ |
(67,638) |
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
ALNYLAM PHARMACEUTICALS, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended September 30, |
|
2023 |
|
2022 |
| Cash flows from operating activities: |
|
|
|
| Net loss |
$ |
(302,372) |
|
|
$ |
(923,663) |
|
| Non-cash adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
| Depreciation and amortization |
40,572 |
|
|
30,157 |
|
| Amortization and interest accretion related to operating leases |
32,486 |
|
|
30,508 |
|
| Non-cash interest expense on liability related to the sale of future royalties |
78,918 |
|
|
79,621 |
|
| Stock-based compensation |
179,686 |
|
|
187,882 |
|
| Realized and unrealized loss on marketable equity securities |
17,711 |
|
|
40,108 |
|
| Loss on extinguishment of debt |
— |
|
|
76,586 |
|
| Change in fair value of development derivative liability |
67,895 |
|
|
70,776 |
|
|
|
|
|
| Other |
15,088 |
|
|
20,607 |
|
| Changes in operating assets and liabilities: |
|
|
|
| Accounts receivable, net |
(91,808) |
|
|
7,672 |
|
| Inventory |
6,377 |
|
|
(15,158) |
|
| Prepaid expenses and other assets |
(32,427) |
|
|
(47,194) |
|
| Accounts payable, accrued expenses and other liabilities |
119,506 |
|
|
89,152 |
|
| Operating lease liability |
(35,014) |
|
|
(31,718) |
|
| Deferred revenue |
37,333 |
|
|
(24,632) |
|
Net cash provided by (used in) operating activities |
133,951 |
|
|
(409,296) |
|
| Cash flows from investing activities: |
|
|
|
| Purchases of property, plant and equipment |
(46,902) |
|
|
(50,424) |
|
| Purchases of marketable securities |
(1,234,344) |
|
|
(1,253,584) |
|
| Sales and maturities of marketable securities |
1,189,990 |
|
|
1,626,848 |
|
| Proceeds from maturity of restricted investments |
58,475 |
|
|
89,951 |
|
| Purchases of restricted investments |
(58,475) |
|
|
(98,451) |
|
| Other investing activities |
(4,438) |
|
|
(5,075) |
|
Net cash (used in) provided by investing activities |
(95,694) |
|
|
309,265 |
|
| Cash flows from financing activities: |
|
|
|
| Proceeds from exercise of stock options and other types of equity, net |
116,570 |
|
|
202,646 |
|
| Proceeds from convertible debt, net |
— |
|
|
1,016,888 |
|
| Repayment of term loan |
— |
|
|
(762,107) |
|
| Purchases of capped calls related to convertible debt |
— |
|
|
(118,611) |
|
|
|
|
|
| Proceeds from development derivative |
16,333 |
|
|
23,500 |
|
|
|
|
|
|
|
|
|
| Net cash provided by financing activities |
132,903 |
|
|
362,316 |
|
| Effect of exchange rate changes on cash, cash equivalents and restricted cash |
(4,506) |
|
|
(9,049) |
|
Net increase in cash, cash equivalents and restricted cash |
166,654 |
|
|
253,236 |
|
| Cash, cash equivalents and restricted cash, beginning of period |
868,556 |
|
|
822,153 |
|
| Cash, cash equivalents and restricted cash, end of period |
$ |
1,035,210 |
|
|
$ |
1,075,389 |
|
| Supplemental disclosure of cash flows: |
|
|
|
| Cash paid for interest |
$ |
26,675 |
|
|
$ |
43,932 |
|
|
|
|
|
| Supplemental disclosure of noncash investing activities: |
|
|
|
| Capital expenditures included in accounts payable and accrued expenses |
$ |
5,154 |
|
|
$ |
6,156 |
|
|
|
|
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
1. NATURE OF BUSINESS
Alnylam Pharmaceuticals, Inc. (also referred to as Alnylam, we, our or us) commenced operations on June 14, 2002 as a biopharmaceutical company seeking to develop and commercialize novel therapeutics based on ribonucleic acid interference, or RNAi. We are committed to the advancement of our company strategy of building a multi-product, global, commercial biopharmaceutical company with a deep and sustainable clinical pipeline of RNAi therapeutics for future growth and a robust, organic research engine for sustainable innovation and great potential for patient impact. Since inception, we have focused on discovering, developing and commercializing RNAi therapeutics by establishing and maintaining a strong intellectual property position in the RNAi field, establishing strategic alliances with leading pharmaceutical and life sciences companies, generating revenues through licensing agreements, and ultimately developing and commercializing RNAi therapeutics globally, either independently or with our strategic partners. We have devoted substantially all of our efforts to business planning, research, development, manufacturing and commercial efforts, acquiring, filing and expanding intellectual property rights, recruiting management and technical staff, and raising capital.
In early 2021, we launched our Alnylam P5x25 strategy, which focuses on our planned transition to a top-tier biotech company by the end of 2025. With Alnylam P5x25, we aim to deliver transformative rare and prevalent disease medicines for patients around the world through sustainable innovation, while delivering exceptional financial performance.
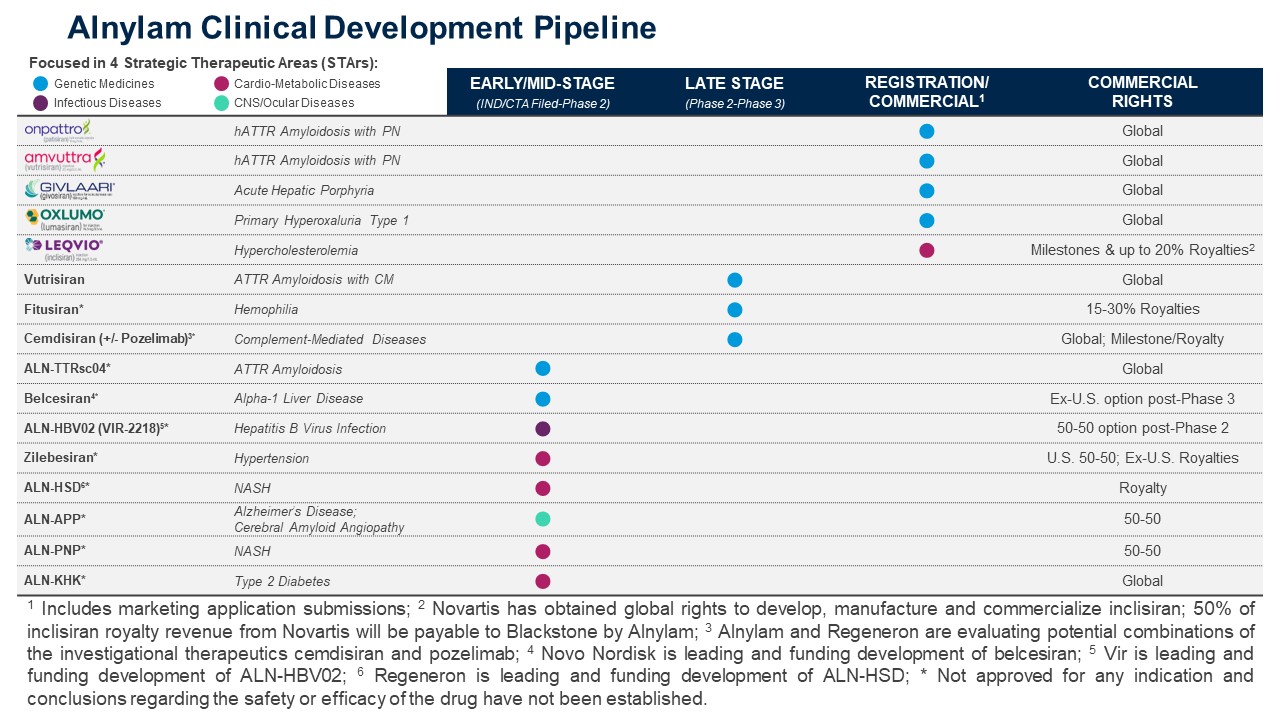
As of September 30, 2023, we have five marketed products, including one partnered product, and multiple late-stage investigational programs advancing towards potential commercialization. We currently generate worldwide product revenues from four commercialized products, ONPATTRO, AMVUTTRA, GIVLAARI and OXLUMO, primarily in the United States, or U.S., Europe and Japan.
2. BASIS OF PRESENTATION AND PRINCIPLES OF CONSOLIDATION
The accompanying condensed consolidated financial statements of Alnylam are unaudited and have been prepared in accordance with accounting principles generally accepted in the United States of America, or GAAP, applicable to interim periods and, in the opinion of management, include all normal and recurring adjustments that are necessary to state fairly the results of operations for the reported periods. Our condensed consolidated financial statements have also been prepared on a basis substantially consistent with, and should be read in conjunction with, our audited consolidated financial statements for the year ended December 31, 2022, which were included in our Annual Report on Form 10-K that was filed with the Securities and Exchange Commission on February 23, 2023. The year-end condensed consolidated balance sheet data was derived from our audited financial statements but does not include all disclosures required by GAAP. The results of our operations for any interim period are not necessarily indicative of the results of our operations for any other interim period or for a full fiscal year.
The accompanying condensed consolidated financial statements reflect the operations of Alnylam and our wholly-owned subsidiaries. All intercompany accounts and transactions have been eliminated.
Our significant accounting policies are described in Note 2 of the Notes to Consolidated Financial Statements included in our Annual Report on Form 10-K for the year ended December 31, 2022.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America, or GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the condensed consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. In our condensed consolidated financial statements, we use estimates and assumptions related to our inventory valuation and related reserves, liability related to the sale of future royalties, development derivative liability, income taxes, revenue recognition, research and development expenses, and stock-based compensation. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable. Actual results could differ from those estimates. Changes in estimates are reflected in reported results in the period in which they become known.
Liquidity
Based on our current operating plan, we believe that our cash, cash equivalents and marketable securities as of September 30, 2023, together with the cash we expect to generate from product sales and under our current alliances, will be sufficient to enable us to advance our Alnylam P5x25 strategy for at least the next 12 months from the filing of this Quarterly Report on Form 10-Q.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
3. NET PRODUCT REVENUES
Net product revenues consist of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
September 30, |
|
Nine Months Ended
September 30, |
| (In thousands) |
2023 |
|
2022 |
|
2023 |
|
2022 |
| ONPATTRO |
|
|
|
|
|
|
|
| United States |
$ |
21,869 |
|
|
$ |
67,196 |
|
|
$ |
77,246 |
|
|
$ |
200,588 |
|
| Europe |
50,371 |
|
|
57,217 |
|
|
166,442 |
|
|
167,185 |
|
| Rest of World |
9,349 |
|
|
20,537 |
|
|
31,852 |
|
|
67,614 |
|
| Total |
81,589 |
|
|
144,950 |
|
|
275,540 |
|
|
435,387 |
|
|
|
|
|
|
|
|
|
| AMVUTTRA |
|
|
|
|
|
|
|
| United States |
113,508 |
|
|
25,060 |
|
|
288,990 |
|
|
25,060 |
|
| Europe |
19,417 |
|
|
169 |
|
|
40,590 |
|
|
169 |
|
| Rest of World |
15,755 |
|
|
— |
|
|
53,004 |
|
|
— |
|
| Total |
148,680 |
|
|
25,229 |
|
|
382,584 |
|
|
25,229 |
|
|
|
|
|
|
|
|
|
| GIVLAARI |
|
|
|
|
|
|
|
| United States |
37,009 |
|
|
31,169 |
|
|
102,496 |
|
|
84,505 |
|
| Europe |
12,430 |
|
|
12,477 |
|
|
40,952 |
|
|
36,059 |
|
| Rest of World |
4,709 |
|
|
2,013 |
|
|
16,505 |
|
|
5,522 |
|
| Total |
54,148 |
|
|
45,659 |
|
|
159,953 |
|
|
126,086 |
|
|
|
|
|
|
|
|
|
| OXLUMO |
|
|
|
|
|
|
|
| United States |
9,713 |
|
|
6,383 |
|
|
27,564 |
|
|
18,916 |
|
| Europe |
15,086 |
|
|
9,348 |
|
|
40,611 |
|
|
25,099 |
|
| Rest of World |
3,937 |
|
|
698 |
|
|
8,934 |
|
|
1,937 |
|
| Total |
28,736 |
|
|
16,429 |
|
|
77,109 |
|
|
45,952 |
|
|
|
|
|
|
|
|
|
| Total net product revenues |
$ |
313,153 |
|
|
$ |
232,267 |
|
|
$ |
895,186 |
|
|
$ |
632,654 |
|
The following table presents the balance of our receivables related to our net product revenues:
|
|
|
|
|
|
|
|
|
|
|
|
| (In thousands) |
As of September 30,
2023 |
|
As of December 31,
2022 |
| Receivables included in “Accounts receivable, net” |
$ |
198,487 |
|
|
$ |
203,844 |
|
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
4. NET REVENUES FROM COLLABORATIONS
Net revenues from collaborations consist of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
| (In thousands) |
2023 |
|
2022 |
|
2023 |
|
2022 |
Roche |
$ |
311,328 |
|
|
$ |
— |
|
|
$ |
311,328 |
|
|
$ |
— |
|
| Regeneron Pharmaceuticals |
80,254 |
|
|
21,979 |
|
|
97,407 |
|
|
34,405 |
|
| Novartis AG |
18,381 |
|
|
5,803 |
|
|
41,941 |
|
|
27,472 |
|
| Other |
17,509 |
|
|
1,515 |
|
|
19,102 |
|
|
2,390 |
|
| Total |
$ |
427,472 |
|
|
$ |
29,297 |
|
|
$ |
469,778 |
|
|
$ |
64,267 |
|
The following table presents the balance of our receivables and contract liabilities related to our collaboration agreements:
|
|
|
|
|
|
|
|
|
|
|
|
| (In thousands) |
As of September 30, 2023 |
|
As of December 31, 2022 |
| Receivables included in “Accounts receivable, net” |
$ |
116,447 |
|
|
$ |
32,342 |
|
| Contract liabilities included in “Deferred revenue” |
$ |
272,770 |
|
|
$ |
235,528 |
|
We recognized revenue of $50.5 million and $42.0 million in the three and nine months ended September 30, 2023, respectively, and revenue of $11.7 million and $17.0 million in the three and nine months ended September 30, 2022, respectively, that was included in the contract liability balance at the beginning of the period.
To determine revenue recognized in the period from contract liabilities, we first allocate revenue to the individual contract liability balance outstanding at the beginning of the period until the revenue exceeds that balance. If additional consideration is received on those contracts in subsequent periods, we assume all revenue recognized in the reporting period first applies to the beginning contract liability as opposed to a portion applying to the new consideration for the period.
The following table provides research and development expenses incurred by type, for which we recognize net revenue, that are directly attributable to our collaboration agreements, by collaboration partner:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
2023 |
|
2022 |
| (In thousands) |
Clinical Trial and Manufacturing |
|
External Services |
|
Other |
|
Clinical Trial and Manufacturing |
|
External Services |
|
Other |
| Regeneron Pharmaceuticals |
$ |
8,672 |
|
|
$ |
1,954 |
|
|
$ |
8,313 |
|
|
$ |
9,812 |
|
|
$ |
1,154 |
|
|
$ |
8,982 |
|
| Other |
1,047 |
|
|
1,139 |
|
|
1,179 |
|
|
— |
|
|
357 |
|
|
— |
|
| Total |
$ |
9,719 |
|
|
$ |
3,093 |
|
|
$ |
9,492 |
|
|
$ |
9,812 |
|
|
$ |
1,511 |
|
|
$ |
8,982 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended September 30, |
|
2023 |
|
2022 |
| (In thousands) |
Clinical Trial and Manufacturing |
|
External Services |
|
Other |
|
Clinical Trial and Manufacturing |
|
External Services |
|
Other |
| Regeneron Pharmaceuticals |
$ |
27,144 |
|
|
$ |
4,104 |
|
|
$ |
27,006 |
|
|
$ |
12,926 |
|
|
$ |
2,141 |
|
|
$ |
27,935 |
|
| Other |
1,444 |
|
|
1,323 |
|
|
1,944 |
|
|
156 |
|
|
679 |
|
|
337 |
|
| Total |
$ |
28,588 |
|
|
$ |
5,427 |
|
|
$ |
28,950 |
|
|
$ |
13,082 |
|
|
$ |
2,820 |
|
|
$ |
28,272 |
|
The research and development expenses incurred for the agreements included in the table above consist of costs incurred for (i) clinical expenses, including manufacturing of clinical product, (ii) external services including consulting services and lab supplies and services, and (iii) other expenses, including professional services, facilities and overhead allocations, and a reasonable estimate of compensation and related costs as billed to our counterparties, for which we recognize net revenues from collaborations. For the three and nine months ended September 30, 2023 and 2022, we did not incur material selling, general and administrative expenses related to our collaboration agreements.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
In addition, we recognized a reduction to our research and development expenses of $4.5 million and $14.7 million for the three and nine months ended September 30, 2023, respectively, and $2.3 million and $9.7 million for the three and nine months ended September 30, 2022, respectively, from cost reimbursement due under certain of our collaboration agreements with Regeneron Pharmaceuticals, Inc., or Regeneron, accounted for under Accounting Standards Codification, or ASC, Topic 808, Collaborative Arrangements, or ASC 808.
Product Alliances
Roche
On July 21, 2023, or the Effective Date, we entered into a Collaboration and License Agreement, or the Roche Agreement, with F. Hoffmann-La Roche Ltd. and Genentech, Inc., or, collectively, Roche, pursuant to which we and Roche established a worldwide, strategic collaboration for the joint development of pharmaceutical products containing zilebesiran. Zilebesiran is our investigational siRNA therapeutic targeting liver-expressed angiotensinogen, which is in Phase 2 clinical trials for the treatment of hypertension.
Under the Roche Agreement, we granted to Roche (i) co-exclusive rights to develop zilebesiran worldwide and commercialize zilebesiran in the U.S., referred to as the Co-Commercialization Territory, (ii) exclusive rights to commercialize zilebesiran outside of the U.S., referred to as the Roche Territory, and (iii) non-exclusive rights to manufacture zilebesiran for the development and commercialization of zilebesiran in the Roche Territory. In connection with the Roche Agreement, Roche made an upfront, non-refundable payment of $310.0 million. In addition, we will be eligible to receive up to $1.24 billion in contingent payments based on the achievement of specified development and regulatory milestones and up to $1.28 billion in sales-based milestones.
We will lead the global clinical development for zilebesiran. We will be responsible for forty percent (40%) and Roche will be responsible for the remaining sixty percent (60%) of development costs incurred in the conduct of development activities that support regulatory approval of zilebesiran globally. We and Roche will share equally (50/50) all costs incurred in connection with development activities that are conducted to support regulatory approval of zilebesiran solely in the Co-Commercialization Territory if incremental development activities are needed. Roche will be solely responsible for all costs incurred in the conduct of development activities primarily to support regulatory approval in the Roche Territory if incremental development activities are needed. Upon regulatory approval Roche has the exclusive right to commercialize zilebesiran in the Roche Territory and will pay us tiered, low double-digit royalties based on net sales of zilebesiran on a country-by-country and product-by-product basis during the applicable royalty term. We and Roche will co-commercialize zilebesiran in the Co-Commercialization Territory and share equally (50/50) profits and losses (including commercialization costs).
Roche has the right to terminate the Roche Agreement for any or no reason at all upon prior written notice, however, if the termination notice occurs after the achievement of the first development milestone and before the achievement of the third development milestone, Roche is required to pay us a termination fee of $50.0 million. In addition, either party may terminate the Roche Agreement for a material breach by, or insolvency of, the other party, subject to a cure period. Unless earlier terminated pursuant to its terms, the Roche Agreement commences on the Effective Date and will remain in effect until expiration on a country-by-country and product-by-product basis (a) in the Roche Territory, upon expiration of the applicable royalty term for such product in the applicable country and (b) in the Co-Commercialization Territory, upon expiration of the term of the co-commercialization efforts for the applicable product.
Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, we may not receive any milestone, royalty or profit share payments from Roche under the Roche Agreement.
We evaluated the Roche Agreement and concluded that the Roche Agreement had elements that were within the scope of ASC 606, Revenue from Contracts with Customers and ASC 808, Collaborative Arrangements.
As of the Effective Date, we identified the following promises in the Roche Agreement that were evaluated under the scope of ASC 606: delivery of (i) a co-exclusive license to develop zilebesiran worldwide and commercialize zilebesiran within the Co-Commercialization Territory, a non-exclusive license to manufacture zilebesiran in the Roche Territory solely for purposes of developing and commercializing zilebesiran in the Roche Territory, and an exclusive license to commercialize zilebesiran in the Roche Territory, collectively referred to as Roche License Obligation, (ii) development services, including the manufacture of clinical supply, that support regulatory approval of zilebesiran, referred to as the Roche Development Services Obligation, and (iii) technology transfer of the existing manufacturing process for zilebesiran, referred to as the Roche Technology Transfer Obligation. The three performance obligations under the Roche Agreement are collectively referred to as the Roche Performance Obligations.
We also evaluated whether certain options outlined within the Roche Agreement represented material rights that would give rise to a performance obligation and concluded that none of the options convey a material right to Roche and therefore are not considered separate performance obligations within the Roche Agreement.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
We assessed the above promises and determined that the Roche License Obligation, Roche Development Services Obligation and Roche Technology Transfer Obligation are reflective of a vendor-customer relationship and therefore represent performance obligations within the scope of ASC 606. The Roche License Obligation is considered functional intellectual property and distinct from other promises under the contract as Roche can benefit from the licenses on its own or together with other readily available resources. As the licenses are delivered at the same time, they are considered one performance obligation at contract inception. The Roche Development Services Obligation is considered distinct as Roche can benefit from the development services together with the licenses transferred by us at the inception of the agreement. The development services are not expected to significantly modify or customize the initial intellectual property as zilebesiran is in the second phase of clinical development. The Roche Technology Transfer Obligation is distinct as Roche can benefit from the manufacturing license transferred by us at the inception of the agreement given the advancements of our RNAi platform and our utilization of third-party contract manufacturing organizations to manufacture zilebesiran. Therefore, each represents a separate performance obligation within the contract with a customer under the scope of ASC 606 at contract inception.
We consider the collaborative activities associated with the co-commercialization of zilebesiran in the U.S. to be a separate unit of account within the scope of ASC 808 as we and Roche are both active participants in the commercialization activities and are exposed to significant risks and rewards that are dependent on the commercial success of the activities in the arrangement.
We determined the transaction price under ASC 606 at the inception of the Roche Agreement to be $857.0 million, consisting of the $310.0 million up front payment and $547.0 million additional variable consideration attributed to cost reimbursement from development and manufacturing services related to the Roche Performance Obligations. Since the related revenue would be allocated to the Roche Development Services Obligation and the Roche Technology Transfer Obligation recognized only as the costs are incurred, we determined it is not probable that a significant reversal of cumulative revenue would occur. We utilized the expected value method to determine the amount of reimbursement for these activities. We determined that any variable consideration related to development and regulatory milestones is deemed to be fully constrained and therefore excluded from the transaction price due to the high degree of uncertainty and risk associated with these potential payments as we determine that we cannot assert that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur. We also determined that royalties and sales milestones relate solely to the licenses of intellectual property and are therefore excluded from the transaction price under the sales-or-usage based royalty exception of ASC 606.
We developed the estimated standalone selling price for each of the Roche Performance Obligations with the objective of determining the price at which we would sell such an item if it were to be sold regularly on a standalone basis. We developed the estimated standalone selling price for the Roche License Obligation primarily based on the probability-weighted present value of expected future cash flows associated with each underlying license or activity. In developing such estimates, we applied judgment in determining the forecasted revenues, taking into consideration the applicable market conditions and relevant entity-specific factors, the probability of success, the time needed to develop zilebesiran and the discount rate. We developed the estimated standalone selling price for the services and clinical supply included in the Roche Development Services Obligation and the Roche Technology Transfer Obligation primarily based on the level of effort necessary to perform the service and the costs for full-time equivalent employees and expected resources to be committed plus a reasonable margin.
We allocated the variable consideration related to the estimated reimbursements for the Roche Development Services Obligation and the Roche Technology Transfer Obligation to each performance obligation as the terms of the variable payment relate specifically to our efforts to satisfy the performance obligation and allocating the variable amount of consideration entirely to the respective performance obligation is consistent with the allocation objective of ASC 606 when considering all of the performance obligations and payment terms in the contract. We allocated the fixed up-front consideration of $310.0 million entirely to the Roche License Obligation as the value of the fixed consideration together with the expected value of the development and regulatory milestones, sales-based milestones and royalties, all of which are either currently constrained or subject to the sales-and usage-based royalty exception, approximates the standalone selling price of the Roche License Obligation. Therefore, allocating the fixed up-front consideration entirely to the Roche License Obligation is consistent with the allocation objective of ASC 606 when considering all of the performance obligations and payment terms in the contract.
The Roche License Obligation was satisfied at a point in time upon transfer of the license to Roche. Control of the licenses was transferred on the Effective Date and Roche could begin to use and benefit from the licenses. For the Roche Development Services Obligation, we measure proportional performance over time using an input method based on cost incurred relative to the total estimated cost of the obligation, on a quarterly basis, by determining the proportion of effort incurred as a percentage of total effort we expect to expend. This ratio is applied to the transaction price allocated to the obligation. Management has applied significant judgment in the process of developing our estimates. We re-evaluate the transaction price as of the end of each reporting period.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
The following tables provide a summary of the transaction price allocated to each performance obligation, in addition to revenue activity during the period, in thousands:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Transaction Price Allocated |
|
|
|
Revenue Recognized During |
|
|
| Performance Obligations |
|
As of September 30, 2023 |
|
|
|
Three Months Ended
September 30, 2023 |
|
|
Roche License Obligation |
|
$ |
310,000 |
|
|
|
|
$ |
310,000 |
|
|
|
Roche Development Services Obligation |
|
545,000 |
|
|
|
|
200 |
|
|
|
Roche Technology Transfer Obligation |
|
2,000 |
|
|
|
|
— |
|
|
|
|
|
$ |
857,000 |
|
|
|
|
$ |
310,200 |
|
|
|
As of September 30, 2023, the aggregate amount of the transaction price allocated to the Roche Performance Obligations that was unsatisfied was $546.8 million, which is expected to be recognized through the term of the Roche Agreement as the services are performed.
Regeneron Pharmaceuticals, Inc.
In April 2019, we entered into a global, strategic collaboration with Regeneron to discover, develop and commercialize RNAi therapeutics for a broad range of diseases by addressing therapeutic targets expressed in the eye and central nervous system, or CNS, in addition to a select number of targets expressed in the liver, which we refer to as the Regeneron Collaboration. The Regeneron Collaboration is governed by a Master Agreement, referred to as the Regeneron Master Agreement, which became effective on May 21, 2019. In connection with the Regeneron Master Agreement, we and Regeneron entered into (i) a binding co-co collaboration term sheet covering the continued development of cemdisiran, our C5 small interfering RNA, or siRNA, currently in Phase 2 development for C5 complement-mediated diseases, as a monotherapy and (ii) a binding license term sheet to evaluate anti-C5 antibody-siRNA combinations for C5 complement-mediated diseases including evaluating the combination of Regeneron’s pozelimab (REGN3918), currently in Phase 3 development, and cemdisiran. The C5 co-co collaboration and license agreements were executed in August 2019.
Under the terms of the Regeneron Collaboration, we are working exclusively with Regeneron to discover RNAi therapeutics for eye and CNS diseases for an initial research period of approximately five years, which we refer to as the Initial Research Term. Regeneron has an option to extend the Initial Research Term (referred to as the Research Term Extension Period, and together with the Initial Research Term, the Research Term) for up to an additional five years, for a research term extension fee of $300.0 million. The Regeneron Collaboration also covers a select number of RNAi therapeutic programs designed to target genes expressed in the liver, including our previously announced collaboration with Regeneron to identify RNAi therapeutics for the chronic liver disease nonalcoholic steatohepatitis. We retain broad global rights to all of our other unpartnered liver-directed clinical and pre-clinical pipeline programs. The Regeneron Collaboration is governed by a joint steering committee that is comprised of an equal number of representatives from each party.
Regeneron leads development and commercialization for all programs targeting eye diseases (subject to limited exceptions), entitling us to certain potential milestone and royalty payments pursuant to the terms of a license agreement, the form of which has been agreed upon by the parties. We and Regeneron are alternating leadership on CNS and liver programs covered by the Regeneron Collaboration, with the lead party retaining global development and commercial responsibility. For such CNS and liver programs, both we and Regeneron have the option at lead candidate selection to enter into a co-co collaboration agreement, the form of which has been agreed upon by the parties, whereby both companies will share equally all costs of, and profits from, all development and commercialization activities under the program. If the non-lead party elects to not enter into a co-co collaboration agreement with respect to a given CNS or liver program, we and Regeneron will enter into a license agreement with respect to such program and the lead party will be the “Licensee” for the purposes of the license agreement. If the lead party for a CNS or liver program elects to not enter into the co-co collaboration agreement, then we and Regeneron will enter into a license agreement with respect to such program and leadership of the program will transfer to the other party and the former non-lead party will be the “Licensee” for the purposes of the license agreement.
With respect to the programs directed to C5 complement-mediated diseases, we retain control of cemdisiran monotherapy development, and Regeneron is leading combination product development. Pursuant to the C5 co-co collaboration agreement, Regeneron notified us in November 2022 of its decision to exercise its right to opt-out of the further development and commercialization of cemdisiran monotherapy. As a result, Regeneron no longer shares costs and potential future profits on any monotherapy program with us. We continue to perform our obligations under the agreement, including considering other strategic alternatives, and we are solely responsible for all development and commercialization costs. Regeneron will be eligible to receive tiered double-digit royalties on net sales. Under the C5 license agreement, for cemdisiran to be used as part of a combination product, Regeneron is solely responsible for all development and commercialization costs and we will receive low double-digit royalties and commercial milestones of up to $325.0 million on any potential combination product sales. The C5 co-co collaboration agreement, the C5 license agreement, and the Master Agreement have been combined for accounting purposes and treated as a single agreement.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
In connection with the Regeneron Master Agreement, Regeneron made an upfront payment of $400.0 million. In the third quarter of 2023, we earned a $100.0 million milestone as we met certain criteria during early clinical development for our CNS program, ALN-APP. We recognized a cumulative catch-up adjustment of $65.0 million in net revenues from collaborations for the satisfied portion of our performance obligation that is satisfied over time. We are also eligible to receive up to an additional $100.0 million in milestone payments upon achievement of certain criteria during early clinical development for an eye program. We and Regeneron plan to advance programs directed to up to 30 targets in the first five years under the Regeneron Collaboration during the Initial Research Term. For each program, Regeneron will provide us with $2.5 million in funding at program initiation and an additional $2.5 million at lead candidate identification, with the potential for approximately $30.0 million in annual discovery funding to us as the Regeneron Collaboration reaches steady state.
Regeneron has the right to terminate the Regeneron Master Agreement for convenience upon ninety days’ notice. The termination of the Regeneron Master Agreement does not affect the term of any license agreement or co-co collaboration agreement then in effect. In addition, either party may terminate the Regeneron Master Agreement for a material breach by, or insolvency of, the other party. Unless earlier terminated pursuant to its terms, the Regeneron Master Agreement will remain in effect with respect to each program until (a) such program becomes a terminated program or (b) the parties enter into a license agreement or co-co collaboration agreement with respect to such program. The Regeneron Master Agreement includes various representations, warranties, covenants, dispute escalation and resolution mechanisms, indemnities and other provisions customary for transactions of this nature.
For any license agreement subsequently entered into, the licensee will generally be responsible for its own costs and expenses incurred in connection with the development and commercialization of the collaboration products. The licensee will pay to the licensor certain development and/or commercialization milestone payments totaling up to $150.0 million for each collaboration product. In addition, following the first commercial sale of the applicable collaboration product under a license agreement, the licensee is required to make certain tiered royalty payments, ranging from low double-digits up to 20%, to the licensor based on the aggregate annual net sales of the collaboration product, subject to customary reductions.
For any co-co collaboration agreement subsequently entered into, we and Regeneron will share equally all costs of, and profits from, development and commercialization activities. Reimbursement of our share of costs will be recognized as a reduction to research and development expense in the condensed consolidated statements of operations and comprehensive income (loss). In the event that a party exercises its opt-out right, the lead party will be responsible for all costs and expenses incurred in connection with the development and commercialization of the collaboration products under the applicable co-co collaboration agreement, subject to continued sharing of costs through defined points. If a party exercises its opt-out right, following the first commercial sale of the applicable collaboration product under a co-co collaboration agreement, the lead party is required to make certain tiered royalty payments, ranging from low double-digits up to 20%, to the other party based on the aggregate annual net sales of the collaboration product and the timing of the exercise of the opt-out right, subject to customary reductions and a reduction for opt-out transition costs.
Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, we may not receive any additional milestone or royalty payments from Regeneron under the Regeneron Master Agreement, the C5 license agreement, or any future license agreement, or under any co-co collaboration agreement in the event we exercise our opt-out right.
Our obligations under the Regeneron Collaboration include: (i) a research license and research services, collectively referred to as the Research Services Obligation; (ii) a worldwide license to cemdisiran for combination therapies, and manufacturing and supply, and development service obligations, collectively referred to as the C5 License Obligation; and (iii) development, manufacturing and commercialization activities for cemdisiran monotherapies, referred to as the C5 Co-Co Obligation.
The research license is not distinct from the research services primarily as a result of Regeneron being unable to benefit on its own or with other resources reasonably available, as the license is providing access to specialized expertise, particularly as it relates to RNAi technology that is not available in the marketplace. Similarly, the worldwide license to cemdisiran for combination therapies is not distinct from the manufacturing and supply, and development service obligations, as Regeneron cannot benefit on its own from the value of the license without receipt of supply.
Separately, prior to Regeneron’s decision in November 2022 to exercise its right to opt-out of the further development and commercialization of cemdisiran monotherapy, the cemdisiran monotherapy co-co collaboration agreement was under the scope of ASC 808 as we and Regeneron were both active participants in the development and manufacturing activities and were exposed to significant risks and rewards that were dependent on commercial success of the activities of the arrangement. Regeneron’s decision to exercise its right to opt out of the arrangement caused a change in the role of Regeneron and its exposure to significant risks and rewards under the arrangement.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
As a result, we determined that the arrangement no longer represents a collaborative arrangement.
The arrangement now represents a vendor-customer relationship under ASC 606 as we perform our obligation to provide development and manufacturing activities under the arrangement. The transaction price allocated to the C5 Co-Co obligation unit of account will be recognized over time using an input method based on cost incurred relative to the total estimated costs for the identified performance obligation by determining the proportion of effort incurred as a percentage of total effort we expect to expend.
The total transaction price is comprised of the $400.0 million upfront payment and additional variable consideration related to research, development, manufacturing and supply activities related to the Research Services Obligation and the C5 License Obligation. We utilized the expected value method to determine the amount of reimbursement for these activities. We determined that any variable consideration related to sales-based royalties and milestones related to the worldwide license to cemdisiran for combination therapies is deemed to be constrained and therefore has been excluded from the transaction price. In addition, we are eligible to receive a future milestone upon the achievement of certain criteria during early clinical development for an eye program. We are also eligible to receive royalties on future commercial sales for certain eye, CNS or liver targets, if any; however, these amounts are excluded from variable consideration under the Regeneron Collaboration as we are only eligible to receive such amounts if, after a drug candidate is identified, the form of license agreement is subsequently executed resulting in a license that is granted to Regeneron. Any such subsequently granted license would represent a separate transaction under ASC 606.
We allocated the initial transaction price to each unit of account based on the applicable accounting guidance as follows, in thousands:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Performance Obligations |
|
Standalone Selling Price |
|
Transaction Price Allocated |
|
|
| Research Services Obligation |
|
$ |
130,700 |
|
|
$ |
183,100 |
|
|
|
| C5 License Obligation |
|
97,600 |
|
|
92,500 |
|
|
|
| C5 Co-Co Obligation |
|
364,600 |
|
|
246,000 |
|
|
|
|
|
|
|
$ |
521,600 |
|
|
|
The transaction price was allocated to the obligations based on the relative estimated standalone selling prices of each obligation, over which management has applied significant judgment. We developed the estimated standalone selling price for the licenses included in the Research Services Obligation and the C5 License Obligation primarily based on the probability-weighted present value of expected future cash flows associated with each license related to each specific program. In developing such estimate, we applied judgment in the determination of the forecasted revenues, taking into consideration the applicable market conditions and relevant entity-specific factors, the expected number of targets or indications expected to be pursued under each license, the probability of success, the time needed to develop a product candidate pursuant to the associated license and the discount rate. We developed the estimated standalone selling price for the services and/or manufacturing and supply included in each of the obligations, as applicable, primarily based on the nature of the services to be performed and/or goods to be manufactured and estimates of the associated costs. The estimated standalone selling price of the C5 Co-Co Obligation was developed by estimating the present value of expected future cash flows that Regeneron is entitled to receive. In developing such estimate, we applied judgment in determining the indications that will be pursued, the forecasted revenues for such indications, the probability of success and the discount rate.
For the Research Services Obligation, the C5 License Obligation, and the C5 Co-Co Obligation accounted for under ASC 606, we measure proportional performance over time using an input method based on cost incurred relative to the total estimated costs for each of the identified obligations, on a quarterly basis, by determining the proportion of effort incurred as a percentage of total effort we expect to expend. This ratio is applied to the transaction price allocated to each obligation. Management has applied significant judgment in the process of developing our estimates. Any changes to these estimates will be recognized in the period in which they change as a cumulative catch up. We re-evaluate the transaction price as of the end of each reporting period and as of September 30, 2023, the total transaction price was determined to be $650.1 million, an increase of $91.2 million from December 31, 2022. As of September 30, 2023, the transaction price is comprised of the upfront payment and variable consideration related to development, manufacture, and supply activities. Revenue recognized under this agreement is accounted for as collaboration revenue.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
The following tables provide a summary of the transaction price allocated to each unit of account based on the applicable accounting guidance, in addition to revenue activity during the period and deferred revenue as of the balance sheet date, in thousands:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Transaction Price Allocated |
|
Deferred Revenue |
|
|
| Performance Obligations |
|
As of September 30,
2023 |
|
As of September 30,
2023 |
|
As of December 31,
2022 |
|
|
| Research Services Obligation |
|
$ |
305,680 |
|
|
$ |
72,000 |
|
|
$ |
26,200 |
|
|
|
| C5 License Obligation |
|
98,400 |
|
|
3,800 |
|
|
7,000 |
|
|
|
| C5 Co-Co Obligation |
|
246,000 |
|
|
187,400 |
|
|
193,600 |
|
|
|
|
|
$ |
650,080 |
|
|
$ |
263,200 |
|
|
$ |
226,800 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue Recognized During |
|
|
|
|
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
|
|
| Performance Obligations |
|
2023 |
|
2022 |
|
2023 |
|
2022 |
|
|
| Research Services Obligation |
|
$ |
73,000 |
|
|
$ |
10,300 |
|
|
$ |
66,700 |
|
|
$ |
19,100 |
|
|
|
| C5 License Obligation |
|
700 |
|
|
1,400 |
|
|
6,000 |
|
|
(2,100) |
|
|
|
| C5 Co-Co Obligation |
|
2,000 |
|
|
5,400 |
|
|
6,200 |
|
|
9,400 |
|
|
|
|
|
$ |
75,700 |
|
|
$ |
17,100 |
|
|
$ |
78,900 |
|
|
$ |
26,400 |
|
|
|
As of September 30, 2023, the aggregate amount of the transaction price allocated to the remaining Research Services Obligation and the C5 License Obligation that was unsatisfied was $115.2 million, which is expected to be recognized through the term of the Regeneron Collaboration as the services are performed. The aggregate amount of the transaction price allocated to C5 Co-Co Obligation that was unsatisfied was $187.4 million, which is expected to be recognized as we perform our obligation to provide development and manufacturing activities under the arrangement, but could be recognized earlier after an assessment of strategic alternatives which could change the scope of our obligation. Deferred revenue related to the Regeneron Collaboration is classified as either current or non-current in the condensed consolidated balance sheets based on the period the revenue is expected to be recognized.
Novartis AG
2013 Collaboration with The Medicines Company
In February 2013, we and The Medicines Company, or MDCO, entered into a license and collaboration agreement pursuant to which we granted to MDCO an exclusive, worldwide license to develop, manufacture and commercialize RNAi therapeutics targeting proprotein convertase subtilisin/kexin type 9, or PCSK9, for the treatment of hypercholesterolemia and other human diseases, including inclisiran. We refer to this agreement, as amended through the date hereof, as the MDCO License Agreement. In 2020, Novartis AG, or Novartis, completed its acquisition of MDCO and assumed all rights and obligations under the MDCO License Agreement. Additional details regarding the terms, milestones earned upon the achievement of certain events and additional milestones we are entitled to receive upon the achievement of future events under the MDCO License Agreement are described in Note 4 of the Notes to Consolidated Financial Statements included in our Annual Report on Form 10-K for the year ended December 31, 2022, as filed with the Securities and Exchange Commission on February 23, 2023.
Novartis License Agreement
In December 2021, we and Novartis entered into a collaboration and license agreement, or the Novartis License Agreement, pursuant to which we granted to Novartis an exclusive, worldwide license to develop, manufacture and commercialize siRNAs targeting end-stage liver disease, or ESLD, potentially leading to the development of a treatment designed to promote the regrowth of functional liver cells and to provide an alternative to transplantation for patients with liver failure. Additional details regarding the terms and transaction price under the Novartis License Agreement are described in Note 4 of the Notes to Consolidated Financial Statements included in our Annual Report on Form 10-K for the year ended December 31, 2022, as filed with the Securities and Exchange Commission on February 23, 2023.
Other
In addition to the collaboration agreements discussed above, we have various other collaboration agreements that are not individually significant to our operating results or financial condition at this time. Pursuant to the terms of those agreements, we may be required to pay, or we may receive, additional amounts contingent upon the occurrence of various future events (e.g.,
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
upon the achievement of various development and commercial milestones) which in the aggregate could be significant. We may also incur, or be reimbursed for, significant research and development costs. In addition, if any products related to these collaborations are approved for sale, we may be required to pay, or we may receive, royalties on future sales. The payment or receipt of these amounts, however, is contingent upon the occurrence of various future events.
5. LIABILITY RELATED TO THE SALE OF FUTURE ROYALTIES
In April 2020, we entered into a purchase and sale agreement, or Purchase Agreement, with BX Bodyguard Royalties L.P. (an affiliate of The Blackstone Group Inc.), or Blackstone Royalties, under which Blackstone Royalties acquired 50% of royalties payable, or Royalty Interest, with respect to net sales by MDCO, its affiliates or sublicensees of inclisiran (or the branded drug product, Leqvio) and any other licensed products under the MDCO License Agreement, and 75% of the commercial milestone payments payable under the MDCO License Agreement, together with the Royalty Interest, the Purchased Interest. If Blackstone Royalties does not receive payments in respect of the Royalty Interest by December 31, 2029, equaling at least $1.00 billion, Blackstone Royalties will receive 55% of the Royalty Interest beginning on January 1, 2030. In consideration for the sale of the Purchased Interest, Blackstone Royalties paid us $1.00 billion.
We continue to own or control all inclisiran intellectual property rights and are responsible for certain ongoing manufacturing and supply obligations related to the generation of the Purchased Interest. Due to our continuing involvement, we will continue to account for any royalties and commercial milestones due to us under the MDCO License Agreement as revenue on our condensed consolidated statement of operations and comprehensive income (loss) and record the proceeds from this transaction as a liability, net of closing costs, on our condensed consolidated balance sheet.
In order to determine the amortization of the liability related to the sale of future royalties, we are required to estimate the total amount of future payments to Blackstone Royalties over the life of the Purchase Agreement. The $1.00 billion liability, recorded at execution of the agreement, will be accreted to the total of these royalty and commercial milestone payments as interest expense over the life of the Purchase Agreement. As of September 30, 2023, our estimate of this total interest expense resulted in an effective annual interest rate of 8%. These estimates contain assumptions that impact both the amount recorded at execution and the interest expense that will be recognized in future periods.
As payments are made to Blackstone Royalties, the balance of the liability will be effectively repaid over the life of the Purchase Agreement. The exact timing and amount of repayment is likely to change each reporting period. A significant increase or decrease in Leqvio global net revenue will materially impact the liability related to the sale of future royalties, interest expense and the time period for repayment. We will periodically assess the expected payments to Blackstone Royalties and to the extent the amount or timing of such payments is materially different than our initial estimates, we will prospectively adjust the amortization of the liability related to the sale of future royalties and the related interest expense.
As of September 30, 2023, the carrying value of the liability related to the sale of future royalties was $1.36 billion, net of closing costs of $10.2 million. The carrying value of the liability related to the sale of future royalties approximates fair value as of September 30, 2023 and is based on our current estimates of future royalties and commercial milestones expected to be paid to Blackstone Royalties over the life of the arrangement, which are considered Level 3 inputs.
The following table shows the activity with respect to the liability related to the sale of future royalties, in thousands:
|
|
|
|
|
|
|
|
|
Carrying value as of December 31, 2022 |
|
$ |
1,292,304 |
|
| Interest expense recognized |
|
78,918 |
|
| Payments |
|
(16,213) |
|
Carrying value as of September 30, 2023 |
|
$ |
1,355,009 |
|
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
6. FAIR VALUE MEASUREMENTS
The following tables present information about our financial assets and liabilities that are measured at fair value on a recurring basis and indicate the fair value hierarchy of the valuation techniques we utilized to determine such fair value:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (In thousands) |
|
As of September 30, 2023 |
|
Quoted Prices in Active Markets
(Level 1) |
|
Significant Observable Inputs
(Level 2) |
|
Significant Unobservable Inputs
(Level 3) |
| Financial assets |
|
|
|
|
|
|
|
|
| Cash equivalents: |
|
|
|
|
|
|
|
|
| Money market funds |
|
$ |
61,799 |
|
|
$ |
61,799 |
|
|
$ |
— |
|
|
$ |
— |
|
| Commercial paper |
|
9,149 |
|
|
— |
|
|
9,149 |
|
|
— |
|
| U.S. treasury securities |
|
8,959 |
|
|
— |
|
|
8,959 |
|
|
— |
|
|
|
|
|
|
|
|
|
|
| Marketable debt securities: |
|
|
|
|
|
|
|
|
| U.S. treasury securities |
|
694,376 |
|
|
— |
|
|
694,376 |
|
|
— |
|
| U.S. government-sponsored enterprise securities |
|
434,257 |
|
|
— |
|
|
434,257 |
|
|
— |
|
| Corporate notes |
|
164,773 |
|
|
— |
|
|
164,773 |
|
|
— |
|
| Commercial paper |
|
65,850 |
|
|
— |
|
|
65,850 |
|
|
— |
|
| Certificates of deposit |
|
3,587 |
|
|
— |
|
|
3,587 |
|
|
— |
|
|
|
|
|
|
|
|
|
|
| Marketable equity securities |
|
10,411 |
|
|
10,411 |
|
|
— |
|
|
— |
|
| Restricted cash (money market funds) |
|
1,207 |
|
|
1,207 |
|
|
— |
|
|
— |
|
| Total financial assets |
|
$ |
1,454,368 |
|
|
$ |
73,417 |
|
|
$ |
1,380,951 |
|
|
$ |
— |
|
| Financial liabilities |
|
|
|
|
|
|
|
|
| Development derivative liability |
|
$ |
293,505 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
293,505 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (In thousands) |
|
As of December 31, 2022 |
|
Quoted Prices in Active Markets
(Level 1) |
|
Significant Observable Inputs
(Level 2) |
|
Significant Unobservable Inputs
(Level 3) |
| Financial assets |
|
|
|
|
|
|
|
|
| Cash equivalents: |
|
|
|
|
|
|
|
|
| Money market funds |
|
$ |
270,394 |
|
|
$ |
270,394 |
|
|
$ |
— |
|
|
$ |
— |
|
| U.S. treasury securities |
|
44,817 |
|
|
— |
|
|
44,817 |
|
|
— |
|
| U.S. government-sponsored enterprise securities |
|
41,763 |
|
|
— |
|
|
41,763 |
|
|
— |
|
| Commercial paper |
|
22,350 |
|
|
— |
|
|
22,350 |
|
|
— |
|
| Certificates of deposit |
|
3,289 |
|
|
— |
|
|
3,289 |
|
|
— |
|
| Corporate notes |
|
1,024 |
|
|
— |
|
|
1,024 |
|
|
— |
|
| Marketable debt securities: |
|
|
|
|
|
|
|
|
| U.S. treasury securities |
|
820,913 |
|
|
— |
|
|
820,913 |
|
|
— |
|
| U.S. government-sponsored enterprise securities |
|
230,770 |
|
|
— |
|
|
230,770 |
|
|
— |
|
| Corporate notes |
|
208,284 |
|
|
— |
|
|
208,284 |
|
|
— |
|
| Commercial paper |
|
36,793 |
|
|
— |
|
|
36,793 |
|
|
— |
|
| Certificates of deposit |
|
1,130 |
|
|
— |
|
|
1,130 |
|
|
— |
|
|
|
|
|
|
|
|
|
|
| Marketable equity securities |
|
28,122 |
|
|
28,122 |
|
|
— |
|
|
— |
|
| Restricted cash (money market funds) |
|
1,197 |
|
|
1,197 |
|
|
— |
|
|
— |
|
| Total financial assets |
|
$ |
1,710,846 |
|
|
$ |
299,713 |
|
|
$ |
1,411,133 |
|
|
$ |
— |
|
| Financial liabilities |
|
|
|
|
|
|
|
|
| Development derivative liability |
|
$ |
209,277 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
209,277 |
|
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
The carrying amounts reflected on our condensed consolidated balance sheets for cash, accounts receivable, net, other current assets, accounts payable and accrued expenses approximate fair value due to their short-term maturities.
7. MARKETABLE DEBT SECURITIES
We invest our excess cash balances in marketable debt securities and at each balance sheet date presented, we classify all of our investments in debt securities as available-for-sale and as current assets as they represent the investment of funds available for current operations. We did not record any impairment charges related to our marketable debt securities during the nine months ended September 30, 2023 or 2022.
The following tables summarize our marketable debt securities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of September 30, 2023 |
| (In thousands) |
Amortized
Cost |
|
Gross Unrealized Gains |
|
Gross Unrealized Losses |
|
Fair Value |
| U.S. treasury securities |
$ |
705,415 |
|
|
$ |
13 |
|
|
$ |
(2,093) |
|
|
$ |
703,335 |
|
| U.S. government-sponsored enterprise securities |
436,723 |
|
|
2 |
|
|
(2,468) |
|
|
434,257 |
|
| Corporate notes |
165,719 |
|
|
1 |
|
|
(947) |
|
|
164,773 |
|
| Commercial paper |
74,999 |
|
|
— |
|
|
— |
|
|
74,999 |
|
| Certificates of deposit |
3,587 |
|
|
— |
|
|
— |
|
|
3,587 |
|
|
|
|
|
|
|
|
|
| Total |
$ |
1,386,443 |
|
|
$ |
16 |
|
|
$ |
(5,508) |
|
|
$ |
1,380,951 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of December 31, 2022 |
|
|
|
|
| (In thousands) |
Amortized
Cost |
|
Gross Unrealized Gains |
|
Gross Unrealized Losses |
|
Fair Value |
|
|
|
|
| U.S. treasury securities |
$ |
870,033 |
|
|
$ |
79 |
|
|
$ |
(4,382) |
|
|
$ |
865,730 |
|
|
|
|
|
| U.S. government-sponsored enterprise securities |
275,610 |
|
|
24 |
|
|
(3,101) |
|
|
272,533 |
|
|
|
|
|
| Corporate notes |
211,398 |
|
|
16 |
|
|
(2,106) |
|
|
209,308 |
|
|
|
|
|
| Commercial paper |
59,143 |
|
|
— |
|
|
— |
|
|
59,143 |
|
|
|
|
|
| Certificates of deposit |
4,419 |
|
|
— |
|
|
— |
|
|
4,419 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total |
$ |
1,420,603 |
|
|
$ |
119 |
|
|
$ |
(9,589) |
|
|
$ |
1,411,133 |
|
|
|
|
|
The fair values of our marketable debt securities by classification in the condensed consolidated balance sheets were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
| (In thousands) |
As of September 30, 2023 |
|
As of December 31, 2022 |
| Marketable debt securities |
$ |
1,362,843 |
|
|
$ |
1,297,890 |
|
| Cash and cash equivalents |
18,108 |
|
|
113,243 |
|
| Total |
$ |
1,380,951 |
|
|
$ |
1,411,133 |
|
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
8. OTHER BALANCE SHEET DETAILS
Inventory
The components of inventory are summarized as follows:
|
|
|
|
|
|
|
|
|
|
|
|
| (In thousands) |
As of September 30, 2023 |
|
As of December 31, 2022 |
| Raw materials |
$ |
20,121 |
|
|
$ |
22,315 |
|
| Work in progress |
88,691 |
|
|
113,783 |
|
| Finished goods |
27,658 |
|
|
25,606 |
|
| Total |
$ |
136,470 |
|
|
$ |
161,704 |
|
As of September 30, 2023 and December 31, 2022, we had $40.7 million and $32.7 million of long-term inventory, respectively, included within other assets in our condensed consolidated balance sheet as we anticipate it being consumed beyond our normal operating cycle.
Cash, Cash Equivalents and Restricted Cash
The following table provides a reconciliation of cash, cash equivalents and restricted cash reported within our condensed consolidated balance sheets that sum to the total of these amounts shown in the condensed consolidated statements of cash flows:
|
|
|
|
|
|
|
|
|
|
|
|
|
As of September 30, |
| (In thousands) |
2023 |
|
2022 |
| Cash and cash equivalents |
$ |
1,033,024 |
|
|
$ |
1,073,228 |
|
| Total restricted cash included in other assets |
2,186 |
|
|
2,161 |
|
Total cash, cash equivalents, and restricted cash shown in the condensed consolidated statements of cash flows |
$ |
1,035,210 |
|
|
$ |
1,075,389 |
|
Accumulated Other Comprehensive (Loss) Income
The following tables summarize the changes in accumulated other comprehensive (loss) income, by component:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (In thousands) |
Loss on Investment in Joint Venture |
|
Defined Benefit Pension
Plans |
|
Unrealized (Losses) Gains from Debt
Securities
|
|
Foreign Currency Translation
Adjustment |
|
Total Accumulated Other
Comprehensive Loss
|
| Balance as of December 31, 2022 |
$ |
(32,792) |
|
|
$ |
(1,092) |
|
|
$ |
(9,470) |
|
|
$ |
(1,300) |
|
|
$ |
(44,654) |
|
| Other comprehensive (loss) income before reclassifications |
— |
|
|
— |
|
|
(10) |
|
|
8,356 |
|
|
8,346 |
|
| Amounts reclassified from other comprehensive (loss) income |
— |
|
|
(19) |
|
|
3,988 |
|
|
— |
|
|
3,969 |
|
| Net other comprehensive (loss) income |
— |
|
|
(19) |
|
|
3,978 |
|
|
8,356 |
|
|
12,315 |
|
| Balance as of September 30, 2023 |
$ |
(32,792) |
|
|
$ |
(1,111) |
|
|
$ |
(5,492) |
|
|
$ |
7,056 |
|
|
$ |
(32,339) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (In thousands) |
Loss on Investment in Joint Venture |
|
Defined Benefit Pension
Plans |
|
Unrealized (Losses) Gains from Debt
Securities
|
|
Foreign Currency Translation
Adjustment |
|
Total Accumulated Other Comprehensive Loss |
| Balance as of December 31, 2021 |
$ |
(32,792) |
|
|
$ |
(2,811) |
|
|
$ |
(1,630) |
|
|
$ |
3,974 |
|
|
$ |
(33,259) |
|
Other comprehensive income before reclassifications |
— |
|
|
— |
|
|
6 |
|
|
377 |
|
|
383 |
|
| Amounts reclassified from other comprehensive income (loss) |
— |
|
|
103 |
|
|
(11,010) |
|
|
— |
|
|
(10,907) |
|
| Net other comprehensive income (loss) |
— |
|
|
103 |
|
|
(11,004) |
|
|
377 |
|
|
(10,524) |
|
| Balance as of September 30, 2022 |
$ |
(32,792) |
|
|
$ |
(2,708) |
|
|
$ |
(12,634) |
|
|
$ |
4,351 |
|
|
$ |
(43,783) |
|
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
Amounts reclassified out of accumulated other comprehensive loss relate to settlements of marketable equity securities and amortization of our pension obligation which are recorded as other income in the condensed consolidated statements of operations and comprehensive income (loss).
9. CONVERTIBLE DEBT
Convertible Senior Notes Due 2027
On September 12, 2022, we commenced a private offering of $900.0 million in aggregate principal amount of 1% Convertible Senior Notes due 2027, or the Initial Notes. On September 13, 2022, the initial purchasers in such offering exercised their option to purchase an additional $135.0 million in aggregate principal amount of our 1% Convertible Senior Notes due 2027, or the Additional Notes, and together with the Initial Notes collectively referred to as the Notes, bringing the total aggregate principal amount of the Notes to $1.04 billion. The Notes were issued pursuant to an indenture, dated September 15, 2022, or the Indenture. The Indenture includes customary covenants and sets forth certain events of default after which the Notes may be declared immediately due and payable and sets forth certain types of bankruptcy or insolvency events of default involving the Company after which the Notes become automatically due and payable.
The Notes will mature on September 15, 2027, unless earlier converted, redeemed or repurchased. The Notes will bear interest from September 15, 2022 at a rate of 1% per year payable semiannually in arrears on March 15 and September 15 of each year, beginning on March 15, 2023. The Notes are convertible at the option of the noteholder on or after June 15, 2027. Prior to June 15, 2027, the Notes are convertible only under the following circumstances: (1) During any calendar quarter commencing after the calendar quarter ending on December 31, 2022 (and only during such calendar quarter), if the last reported sale price of our common stock for at least 20 trading days (whether or not consecutive) during a period of 30 consecutive trading days ending on, and including, the last trading day of the immediately preceding calendar quarter is greater than or equal to 130% of the conversion price on each applicable trading day; (2) During the five business day period after any ten consecutive trading day period in which the trading price per $1,000 principal amount of the Notes for each trading day of that ten consecutive trading day period was less than 98% of the product of the last reported sale price of our common stock and the conversion rate of the Notes on such trading day; (3) If we call any or all of the Notes for redemption; or (4) Upon the occurrence of specific corporate events as set forth in the Indenture governing the Notes. We will settle any conversions of Notes by paying or delivering, as applicable, cash, shares of our common stock, or a combination of cash and shares of common stock, at our election.
The conversion rate for the Notes will initially be 3.4941 shares of common stock per $1,000 principal amount of Notes, which is equivalent to an initial conversion price of approximately $286.20 per share of common stock. The initial conversion price of the Notes represents a premium of approximately 35% over the $212.00 per share last reported sale price of common stock on September 12, 2022. The conversion rate is subject to adjustment under certain circumstances in accordance with the terms of the Indenture.
We may not redeem the Notes prior to September 20, 2025. We may redeem for cash equal to 100% of the principal amount of the Notes being redeemed plus accrued and unpaid interest of all or any portion of the Notes, at our option, on or after September 20, 2025, if the last reported sales price of our common stock has been at least 130% of the conversion price then in effect for at least 20 trading days (whether or not consecutive) during any 30 consecutive trading day period. No sinking fund is provided for the Notes and therefore we are not required to redeem or retire the Notes periodically.
If we undergo a fundamental change, as defined in the indenture agreement, then subject to certain conditions, holders may require us to repurchase for cash all or any portion of their Notes at a fundamental change repurchase price equal to 100% of the principal amount of the Notes to be repurchased plus accrued and unpaid interest. In addition, if specific corporate events occur prior to the maturity date or if we issue a notice of redemption, we will increase the conversion rate by pre-defined amounts for holders who elect to convert their notes in connection with such corporate event. The conditions allowing holders of the Notes to convert were not met this quarter.
As of September 30, 2023, the Notes are classified as a long-term liability, net of issuance costs of $19.2 million, on the condensed consolidated balance sheets. As of September 30, 2023, the estimated fair value of the Notes was approximately $961.8 million. The fair value was determined based on the last actively traded price per $100 of the Notes for the nine months ended September 30, 2023 (Level 2). The Notes were issued at par and costs associated with the issuance of the Notes are amortized to interest expense over the contractual term of the Notes. As of September 30, 2023, the effective interest rate of the Notes is 1%.
Capped Call Transactions
In September 2022, in connection with the pricing of the Initial Notes and the initial purchasers’ exercise of their option to purchase the Additional Notes, we entered into privately negotiated capped call transactions, or Capped Call Transactions. The Capped Call Transactions initially cover, subject to customary anti-dilution adjustments, the number of shares of common stock that underlie the Notes.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
The cap price of the Capped Call Transactions is initially $424.00 per share, which represents a premium of 100% over the last reported sale price of common stock of $212.00 per share on September 12, 2022, and is subject to certain adjustments under the terms of the Capped Call Transactions. We used approximately $118.6 million of the proceeds from the offering of Notes to pay the cost of the Capped Call Transactions.
We evaluated the Capped Call Transactions and determined that they should be accounted for separately from the Notes. The cost of $118.6 million to purchase the Capped Call Transactions was recorded as a reduction to additional paid-in capital in the condensed consolidated balance sheet as the Capped Call Transactions are indexed to our own stock and met the criteria to be classified in stockholders' deficit.
10. DEVELOPMENT DERIVATIVE LIABILITY
In August 2020, we entered into a co-development agreement, referred to as the Funding Agreement, with BXLS V Bodyguard – PCP L.P. and BXLS Family Investment Partnership V – ESC L.P., collectively referred to as Blackstone Life Sciences, pursuant to which Blackstone Life Sciences will provide up to $150.0 million in funding for the clinical development of vutrisiran and zilebesiran, two of our cardiometabolic programs. With respect to vutrisiran, Blackstone Life Sciences has committed to provide up to $70.0 million to fund development costs related to the HELIOS-B Phase 3 clinical trial. In November 2021, Blackstone Life Sciences opted in to Phase 2 clinical trial funding of zilebesiran, committing to fund, upon meeting certain patient enrollment thresholds, up to $26.0 million. Furthermore, Blackstone Life Sciences has the right, but is not obligated, to fund up to $54.0 million for development costs related to a Phase 3 clinical trial of zilebesiran. The amount of funding ultimately provided by Blackstone Life Sciences is dependent on us achieving specified development milestones with respect to each clinical trial. We retain sole responsibility for the development and commercialization of both vutrisiran and zilebesiran.
As consideration for Blackstone Life Sciences’ funding for vutrisiran clinical development costs, we have agreed to pay Blackstone Life Sciences a 1% royalty on net sales of AMVUTTRA (vutrisiran) for a 10-year term beginning upon the first commercial sale following regulatory approval of vutrisiran for ATTR-cardiomyopathy, as well as fixed payments of up to 2.5 times their investment over a two-year period upon regulatory approval of vutrisiran for ATTR-cardiomyopathy in specified countries, unless it is later withdrawn from the market following a mandatory recall. As consideration for Blackstone Life Sciences’ funding for Phase 2 clinical development costs of zilebesiran, we have agreed to pay Blackstone Life Sciences fixed payments of up to 3.25 times their Phase 2 investment over a four-year period upon the successful completion of the zilebesiran Phase 2 clinical trial, unless certain regulatory events affecting the continued development of zilebesiran occur. In September 2023, the Company announced positive topline results from KARDIA-1 Phase 2 of zilebesiran, triggering the achievement of a development milestone of $84.5 million payable to Blackstone over four years. As consideration for Blackstone Life Sciences’ funding for Phase 3 clinical development costs of zilebesiran, we have agreed to pay Blackstone Life Sciences fixed payments of up to 4.5 times their Phase 3 investment over a four-year period upon regulatory approval of zilebesiran in specified countries, unless it is later withdrawn from the market following a mandatory recall.
Our payment obligations under the Funding Agreement will be secured, subject to certain exceptions, by security interests in intellectual property owned by us relating to vutrisiran and zilebesiran, as well as in our bank account in which the funding deposits will be made.
We and Blackstone Life Sciences each have the right to terminate the Funding Agreement in its entirety in the event of the other party’s bankruptcy or similar proceedings. We and Blackstone Life Sciences may each terminate the Funding Agreement in its entirety or with respect to either product in the event of an uncured material breach by the other party, or with respect to a product for certain patient health and safety reasons, or if regulatory approval in specified major market countries is not obtained for the product following the completion of clinical trials for the product. In addition, Blackstone Life Sciences has the right to terminate the Funding Agreement in its entirety upon the occurrence of certain events affecting our ability to make payments under the agreement or to develop or commercialize the products, or upon a change of control of us. Blackstone Life Sciences may also terminate the Funding Agreement with respect to a product if the joint steering committee elects to terminate the development program for that product in its entirety, if certain clinical endpoints are not achieved for that product or, with respect to vutrisiran only, if our right to develop or commercialize vutrisiran is enjoined in a specified major market as a result of an alleged patent infringement. In certain termination circumstances, we will be obligated to pay Blackstone Life Sciences an amount that is equal to, or a multiplier of, the development funding received from Blackstone Life Sciences, and we may remain obligated under certain circumstances to make the payments to Blackstone Life Sciences described above, or the royalty described above in the case of AMVUTTRA, should we obtain regulatory approval for zilebesiran or vutrisiran for ATTR-cardiomyopathy following termination.
We account for the Funding Agreement under ASC 815, Derivatives and Hedging, as a derivative liability, measured at fair value, recorded within accrued expenses or other liabilities on our condensed consolidated balance sheets, depending on timing of our payment to Blackstone Life Sciences.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
The change in fair value due to the remeasurement of the development derivative liability is recorded as other expense on our condensed consolidated statements of operations and comprehensive income (loss).
As of September 30, 2023, the derivative liability is classified as a Level 3 financial liability in the fair value hierarchy. The valuation method incorporates certain unobservable Level 3 key inputs including (i) the probability and timing of achieving stated development milestones to receive payments from Blackstone Life Sciences, (ii) the probability and timing of achieving regulatory approval and payments to Blackstone Life Sciences, (iii) an estimate of the amount and timing of the royalty payable on net sales of AMVUTTRA, assuming regulatory approval for ATTR-cardiomyopathy, (iv) our cost of borrowing (12%), and (v) Blackstone Life Sciences' cost of borrowing (4%).
The following table presents the activity with respect to the development derivative liability, in thousands:
|
|
|
|
|
|
|
|
|
Carrying value as of December 31, 2022 |
|
$ |
209,277 |
|
| Amount received under the Funding Agreement |
|
16,333 |
|
| Change in fair value of development derivative liability |
|
67,895 |
|
Carrying value as of September 30, 2023 |
|
$ |
293,505 |
|
11. STOCK-BASED COMPENSATION
The following table summarizes stock-based compensation expenses included in operating costs and expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
| (In thousands) |
2023 |
|
2022 |
|
2023 |
|
2022 |
| Research and development |
$ |
29,155 |
|
|
$ |
52,962 |
|
|
$ |
78,188 |
|
|
$ |
75,217 |
|
| Selling, general and administrative |
34,782 |
|
|
75,156 |
|
|
101,498 |
|
|
112,665 |
|
| Total |
$ |
63,937 |
|
|
$ |
128,118 |
|
|
$ |
179,686 |
|
|
$ |
187,882 |
|
12. NET INCOME (LOSS) PER COMMON SHARE
We compute basic net income (loss) per common share by dividing net income (loss) by the weighted-average number of common shares outstanding. We compute diluted net income (loss) per common share by dividing net income (loss) by the weighted-average number of common shares and dilutive potential common share equivalents then outstanding during the period. In the diluted net income (loss) per share calculation, net income (loss) is adjusted for the elimination of interest expense on the convertible debt. Potential common shares consist of shares issuable upon the vesting of restricted stock units, the exercise of stock options (the proceeds of which are then assumed to have been used to repurchase outstanding shares using the treasury stock method) and upon conversion of the convertible debt outstanding during the period (calculated using the if-converted method assuming the conversion of the convertible debt as of the earliest period reported or at the date of issuance, if later). Because the inclusion of potential common shares would be anti-dilutive for periods presenting a net loss, diluted net loss per common share is the same as basic net loss per common share.
The following table sets forth the potential common shares (prior to consideration of the treasury stock or if-converted methods for net loss periods) excluded from the calculation of net income (loss) per common share because their inclusion would be anti-dilutive:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
September 30, |
|
Nine Months Ended
September 30, |
| (In thousands) |
2023 |
|
2022 |
|
2023 |
|
2022 |
| Options to purchase common stock, inclusive of performance-based stock options |
1,301 |
|
|
10,741 |
|
|
7,716 |
|
|
10,741 |
|
| Unvested restricted stock units, inclusive of performance-based restricted stock units |
4 |
|
|
1,617 |
|
|
1,996 |
|
|
1,617 |
|
| Convertible debt |
— |
|
|
3,616 |
|
|
3,616 |
|
|
3,616 |
|
| Total |
1,305 |
|
|
15,974 |
|
|
13,328 |
|
|
15,974 |
|
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
The following table sets forth the computation of basic and diluted net income (loss) per share:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
September 30, |
|
Nine Months Ended
September 30, |
(In thousands, except per share amounts) |
2023 |
|
2022 |
|
2023 |
|
2022 |
Net income (loss), as reported |
$ |
147,753 |
|
|
$ |
(405,920) |
|
|
$ |
(302,372) |
|
|
$ |
(923,663) |
|
Adjustment for the elimination of interest expense on the convertible debt |
3,525 |
|
|
— |
|
|
— |
|
|
— |
|
Net income (loss), for use in diluted income per share |
$ |
151,278 |
|
|
$ |
(405,920) |
|
|
$ |
(302,372) |
|
|
$ |
(923,663) |
|
|
|
|
|
|
|
|
|
| Weighted-average common shares- basic |
125,220 |
|
|
122,166 |
|
|
124,667 |
|
|
121,158 |
|
Effect of dilutive securities: |
|
|
|
|
|
|
|
| Convertible debt |
3,616 |
|
|
— |
|
|
— |
|
|
— |
|
| Options to purchase common stock, inclusive of performance-based stock options |
2,134 |
|
|
— |
|
|
— |
|
|
— |
|
Restricted stock units, inclusive of performance-based restricted stock units |
361 |
|
|
— |
|
|
— |
|
|
— |
|
Employee stock purchase program |
6 |
|
|
— |
|
|
— |
|
|
— |
|
| Weighted-average common shares- diluted |
131,337 |
|
|
122,166 |
|
|
124,667 |
|
|
121,158 |
|
|
|
|
|
|
|
|
|
| Net income (loss) per common share - basic |
$ |
1.18 |
|
|
$ |
(3.32) |
|
|
$ |
(2.43) |
|
|
$ |
(7.62) |
|
| Net income (loss) per common share - diluted |
$ |
1.15 |
|
|
$ |
(3.32) |
|
|
$ |
(2.43) |
|
|
$ |
(7.62) |
|
13. COMMITMENTS AND CONTINGENCIES
Technology License and Other Commitments
We have licensed from third parties the rights to use certain technologies and information in our research processes as well as in any other products we may develop. In accordance with the related license or technology agreements, we are required to make certain fixed payments to the licensor or a designee of the licensor over various agreement terms. Many of these agreement terms are consistent with the remaining lives of the underlying intellectual property that we have licensed. As of September 30, 2023, our commitments over the next five years to make fixed and cancellable payments under existing license agreements were not material.
Legal Matters
From time to time, we may be a party to litigation, arbitration or other legal proceedings in the course of our business, including the matters described below. The claims and legal proceedings in which we could be involved include challenges to the scope, validity or enforceability of patents relating to our products or product candidates, and challenges by us to the scope, validity or enforceability of the patents held by others. These include claims by third parties that we infringe their patents or breach our license or other agreements with such third parties. The outcome of any such legal proceedings, regardless of the merits, is inherently uncertain. In addition, litigation and related matters are costly and may divert the attention of our management and other resources that would otherwise be engaged in other activities. If we were unable to prevail in any such legal proceedings, our business, results of operations, liquidity and financial condition could be adversely affected. Our accounting policy for accrual of legal costs is to recognize such expenses as incurred.
Patent Infringement Lawsuits
In March 2022, we filed separate lawsuits in the U.S. District Court for the District of Delaware against (1) Pfizer, Inc. and its subsidiary Pharmacia & Upjohn Co. LLC, collectively referred to as Pfizer, and (2) Moderna, Inc. and its subsidiaries ModernaTX, Inc., and Moderna US, Inc., collectively referred to as Moderna. The lawsuits seek damages for infringement of U.S. Patent No. 11,246,933, or ‘933 Patent, in Pfizer’s and Moderna’s manufacture and sale of their messenger RNA, or mRNA, COVID-19 vaccines. The patent relates to the Company’s biodegradable cationic lipids that are foundational to the success of the mRNA COVID-19 vaccines.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
We are seeking judgment that each of Pfizer and Moderna is infringing the ‘933 Patent, as well as damages adequate to compensate for the infringement, but in no event less than a reasonable royalty for the unlicensed uses made of our patented lipids by Pfizer and Moderna, together with interest and costs as may be awarded by the court. As stated in the filed complaints, we are not seeking injunctive relief in these lawsuits.
On May 23, 2022, Moderna filed a partial motion to dismiss, asserting an affirmative defense under Section 1498(a). We responded on May 27, 2022, opposing their motion arguing Moderna had significant non-government sales and the government contract ended in April 2022. Moderna responded on June 13, 2022, requesting a partial motion to dismiss those claims for sales under 1498(a).
On May 27, 2022, Pfizer filed an answer to our complaint, denying the allegations, and asserting invalidity and non-infringement defenses. In addition, Pfizer added BioNTech SE to the suit and added counter-claims seeking a declaratory judgment that our patent is invalid and a second claim alleging that our patent is invalid due to patent misuse. We believe their defenses and counter-claims have no merit and responded on June 10, 2022, with substantive arguments as to the validity of our claims and the lack of merit of their patent misuse claim.
On July 12, 2022, we filed an additional lawsuit against each of Pfizer and Moderna seeking damages for infringement of U.S. Patent No. 11,382,979, or ‘979 patent, in Pfizer’s and Moderna’s manufacture and sale of their mRNA COVID-19 vaccines. The parties agreed to combine the two patents in one lawsuit, separately against each of Moderna and Pfizer/BioNTech.
On February 8, 2023, we received notification from the U.S. Patent Office that a third patent would issue on February 28, 2023, as U.S. Patent No. 11,590,229, or ‘229 patent, which we also believe Pfizer and Moderna’s COVID-19 vaccines infringe upon. On February 15, 2023, we filed a motion with the court to add this patent to the existing cases against Pfizer and Moderna, and on April 26, 2023, the court held a hearing and denied Moderna’s partial motion to dismiss those claims for sales under 1498(a), our motion to add the ‘229 patent to the then current lawsuits as well as a motion filed by Moderna to add certain invalidity arguments made by Pfizer in our case to supplement Moderna’s invalidity arguments previously made.
On May 26, 2023, we filed additional lawsuits against Pfizer and Moderna in Delaware seeking damages for infringing the ‘229 patent. In addition to this patent, we added U.S. Patent Nos. 11,633,479 and 11,633,480 in the recently filed suits against both Pfizer and Moderna and also U.S. Patent No. 11,612,657 against Pfizer only.
On August 9, 2023, a Markman hearing was held in the U.S. District Court for the District of Delaware to consider the meaning of three disputed terms as used in the ’933 and ’979 patents. On August 21, 2023, the court issued an order construing two of the three terms, and deferred a ruling on the third term pending an evidentiary hearing to be held on January 4, 2024. Subsequently, we and Moderna jointly agreed to final judgment of non-infringement of two of our patents, and such judgment was entered by the court on August 30, 2023, and on September 7, 2023, we appealed the claim construction ruling to the Court of Appeals for the Federal Circuit in the initial lawsuit against Moderna. The claim construction ruling did not affect one of the patents in the lawsuit filed against Moderna on May 26, 2023, and that case is going forward on a schedule to be set by the court.
The two separate suits against Pfizer are ongoing subject to the January 2024 claim construction hearing on the third claim term, and in September 2023, we and Pfizer agreed to consolidate the 2022 and 2023 lawsuits in one case, which will require moving the trial date from November 2024 to the first half of 2025, with the final schedule to be determined by the court.
Indemnifications
In connection with license agreements we may enter with companies to obtain rights to intellectual property, we may be required to indemnify such companies for certain damages arising in connection with the intellectual property rights licensed under the agreements. Under such agreements, we may be responsible for paying the costs of any litigation relating to the license agreements or the underlying intellectual property rights, including the costs associated with certain litigation regarding the licensed intellectual property. We are also a party to a number of agreements entered into in the ordinary course of business, which contain typical provisions that obligate us to indemnify the other parties to such agreements upon the occurrence of certain events, including litigation or other legal proceedings. In addition, we have agreed to indemnify our officers and directors for expenses, judgments, fines, penalties, excise taxes, and settlement amounts paid in connection with any threatened, pending or completed litigation proceedings, including, for example, the recently closed government investigation, in which an officer or director was, is or will be involved as a party, on account of such person’s status as an officer or director, or by reason of any action taken by the officer or director while acting in such capacity, subject to certain limitations. These indemnification costs are charged to selling, general and administrative expense.
Our maximum potential future liability under any such indemnification provisions is uncertain. We have determined that the estimated aggregate fair value of our potential liabilities under all such indemnification provisions is minimal and had not recorded any liability related to such indemnification provisions as of September 30, 2023.
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Overview
We are a global commercial-stage biopharmaceutical company developing novel therapeutics based on ribonucleic acid interference, or RNAi. RNAi is a naturally occurring biological pathway within cells for sequence-specific silencing and regulation of gene expression. By harnessing the RNAi pathway, we have developed a new class of innovative medicines, known as RNAi therapeutics. RNAi therapeutics are comprised of small interfering RNA, or siRNA, and function upstream of conventional medicines by potently silencing messenger RNA, or mRNA, that encode for proteins implicated in the cause or pathway of disease, thus preventing them from being made. We believe this is a revolutionary approach with the potential to transform the care of patients with rare and prevalent diseases. To date, our efforts to advance this revolutionary approach have yielded the approval of five first-in-class RNAi-based medicines, ONPATTRO® (patisiran), AMVUTTRA® (vutrisiran), GIVLAARI® (givosiran), OXLUMO® (lumasiran) and Leqvio® (inclisiran).
Our research and development strategy is to target genetically validated genes that have been implicated in the cause or pathway of human disease. We utilize a N-acetylgalactosamine (GalNAc) conjugate approach or lipid nanoparticle (LNP) to enable hepatic delivery of siRNAs. For delivery to the central nervous system, or CNS, and the eye (ocular delivery), we are utilizing an alternative conjugate approach based on a hexadecyl (C16) moiety as a lipophilic ligand. We are also advancing approaches for heart, skeletal muscle and adipose tissue delivery of siRNAs. Our focus is on clinical indications where there is a high unmet need, a genetically validated target, early biomarkers for the assessment of clinical activity in Phase 1 clinical studies, and a definable path for drug development, regulatory approval, patient access and commercialization.
In early 2021, we launched our Alnylam P5x25 strategy, which focuses on our planned transition to a top-tier biotech company by the end of 2025. With Alnylam P5x25, we aim to deliver transformative rare and prevalent disease medicines for patients around the world through sustainable innovation, while delivering exceptional financial performance.
We currently have five marketed products and more than ten clinical programs, including several in late-stage development, across four Strategic Therapeutic Areas, or “STArs:” Genetic Medicines; Cardio-Metabolic Diseases; Hepatic Infectious Diseases; and CNS/Ocular Diseases. Four of our marketed products are within the Genetic Medicines STAr, ONPATTRO, AMVUTTRA, GIVLAARI and OXLUMO.
ONPATTRO is approved by the United States Food and Drug Administration, or the FDA, for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis, or hATTR amyloidosis, in adults and has also been approved in the European Union, or EU, for the treatment of hATTR amyloidosis in adult patients with stage 1 or stage 2 polyneuropathy, in Japan for the treatment of transthyretin, or TTR, type familial amyloidosis with polyneuropathy, and in multiple additional countries, including Brazil. In August 2022, we reported positive results from the APOLLO-B Phase 3 study of patisiran (the non-branded name of ONPATTRO) in patients with the cardiomyopathy of transthyretin-mediated amyloidosis, or ATTR amyloidosis, and in December 2022, we submitted a supplemental New Drug Application, or sNDA, to the FDA for patisiran as a potential treatment for the cardiomyopathy of ATTR amyloidosis. In February 2023, the FDA accepted the sNDA for filing and set an action date of October 8, 2023, under the Prescription Drug User Fee Act, or PDUFA, and on September 13, 2023, the FDA’s Cardiovascular and Renal Drugs Advisory Committee, or CRDAC, met to discuss the sNDA for patisiran and voted 9:3 that its benefits outweigh its risks for the treatment of the cardiomyopathy of ATTR amyloidosis. On October 6, 2023, the FDA completed its review of the sNDA for patisiran and issued a complete response letter, or CRL, indicating that evidence of clinical meaningfulness of patisiran was not established for the cardiomyopathy of ATTR amyloidosis, and therefore, the sNDA for patisiran could not be approved in its present form. The CRL did not identify any issues with respect to clinical safety, study conduct, drug quality or manufacturing. Patisiran remains under regulatory review with Brazilian Health Regulatory Agency (ANVISA) for ONPATTRO for the treatment of the cardiomyopathy of ATTR amyloidosis.
AMVUTTRA is approved in the U.S. for the treatment of the polyneuropathy of hATTR amyloidosis in adults. In September 2022, AMVUTTRA was granted marketing authorization in Europe and the United Kingdom, or UK, for the treatment of hATTR amyloidosis in adult patients with stage 1 or stage 2 polyneuropathy, and in Japan for the treatment of TTR type familial amyloidosis with polyneuropathy. In December 2022, AMVUTTRA was approved in Brazil for the treatment of hATTR amyloidosis in adults. Regulatory filings continue in other territories with submissions currently under review or planned for the remainder of 2023 and beyond.
GIVLAARI is approved in the U.S. for the treatment of adults with acute hepatic porphyria, or AHP, in the EU for the treatment of AHP in adults and adolescents aged 12 years and older, and in several additional countries, including Brazil, Canada, Switzerland and Japan. Regulatory filings for givosiran (the non-branded drug name for GIVLAARI) in other territories are pending or planned during the remainder of 2023 and beyond.
OXLUMO is approved in the U.S. and EU for the treatment of primary hyperoxaluria type 1, or PH1, in all age groups, and in several additional countries including Brazil and Switzerland. In October 2022, we announced that the FDA approved our sNDA for lumasiran (the non-branded drug name for OXLUMO) for the treatment of PH1 to lower urinary oxalate and plasma oxalate levels in pediatric and adult patients.
Regulatory filings in other territories are pending and additional filings are planned during the remainder of 2023 and beyond.
Our fifth product, Leqvio (inclisiran), is in the Cardio-Metabolic Diseases STAr. Leqvio is being developed and commercialized by our partner Novartis AG, or Novartis, and has received marketing authorization from the European Commission, or EC, for the treatment of adults with hypercholesterolemia or mixed dyslipidemia and from the FDA as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia, or HeFH, or clinical atherosclerotic cardiovascular disease, or ASCVD, who require additional lowering of LDL-C. In July 2023, the FDA approved an expanded indication for Leqvio to include treatment of adults with high LDL-C and who are at increased risk of heart disease. As of September 30, 2023, Leqvio has been approved in more than 90 countries.
In addition to our marketed products, we have multiple late-stage investigational programs advancing toward potential commercialization. These programs include our wholly owned programs: vutrisiran (the non-branded drug name for AMVUTTRA) for the treatment of ATTR (wild-type or hereditary) amyloidosis with cardiomyopathy; as well as fitusiran for the treatment of hemophilia, which is being advanced by our partner Genzyme Corporation, a Sanofi Company, or Sanofi; and cemdisiran for the treatment of complement-mediated diseases, where our partner Regeneron Pharmaceuticals, Inc., or Regeneron, is advancing cemdisiran in combination with pozelimab in Phase 3 studies in myasthenia gravis and paroxysmal nocturnal hemoglobinuria.
As part of our Alnylam P5x25 strategy, we have multiple drivers of future growth, including the development of transformative prevalent disease medicines. In addition to Leqvio, we are advancing zilebesiran, an investigational, subcutaneously administered RNAi therapeutic targeting angiotensinogen, or AGT, in development for the treatment of hypertension. In November 2021, we reported positive interim data from the ongoing Phase 1 study of zilebesiran, and initiated the KARDIA Phase 2 clinical studies for zilebesiran. KARDIA-1 is designed to evaluate zilebesiran as a monotherapy across different doses administered quarterly and biannually. KARDIA-2 is designed to evaluate the safety and efficacy of zilebesiran administered biannually as a concomitant therapy in patients whose blood pressure is not adequately controlled by a standard of care antihypertensive medication. In January 2023, we announced that we completed enrollment of patients in the KARDIA-1 Phase 2 study and in July 2023, we announced that we completed enrollment of patients in the KARDIA-2 Phase 2 study. In September 2023, we reported positive topline results from the KARDIA-1 Phase 2 study of zilebesiran in patients at high cardiovascular risk. Topline results from the KARDIA-2 Phase 2 study are anticipated in early 2024. In July 2023, we entered into a Collaboration and License Agreement, or the Roche Collaboration and License Agreement, with F. Hoffmann-La Roche Ltd. and Genentech, Inc. or, collectively, Roche, pursuant to which we established a worldwide, strategic collaboration for the joint development of zilebesiran. A description of our collaboration with Roche is described in more detail below under the heading “Strategic Alliances.”
We are also advancing ALN-APP, an investigational RNAi therapeutic targeting amyloid precursor protein in development for the treatment of Alzheimer’s disease and cerebral amyloid angiopathy. In April 2023 and in July 2023, we reported positive interim results from the ongoing single ascending dose part of the Phase 1 study of ALN-APP in patients with early-onset Alzheimer’s disease. These results establish the first human translation of our proprietary C16-siRNA conjugate platform for CNS delivery and are the first clinical demonstration of gene silencing in the human brain using an RNAi therapeutic.
In further support of our Alnylam P5x25 strategy and in view of our evolving risk profile, we remain focused on the continued evolution of our global infrastructure, including key objectives such as optimizing our global structure for execution in key markets, enhancing performance consistent with our values, and continuing to strengthen our culture. We maintain focus on our global compliance program to drive its evolution and enhancement in view of the Alnylam P5x25 strategy. Building from our global Code of Business Conduct and Ethics, our compliance program is designed to empower our employees and those with whom we work to execute on our strategy consistent with our values and in compliance with applicable laws and regulations, and to mitigate risk. Comprised of components such as risk assessment and monitoring; policies, procedures, and guidance; training and communications; dedicated resources; and systems and processes supporting activities such as third-party relationships and investigations and remediation; our program and related controls are built to enhance our business processes, structures, and controls across our global operations, and to empower ethical decision making.
Based on our expertise in RNAi therapeutics and broad intellectual property estate, we have formed alliances with leading pharmaceutical and life sciences companies to support our development and commercialization efforts, including Regeneron, Roche, Novartis (which acquired our partner The Medicines Company, or MDCO, in 2020), Sanofi, Vir Biotechnology, Inc., or Vir, Dicerna Pharmaceuticals, Inc. (acquired by Novo Nordisk A/S, or Novo Nordisk, in December 2021), or Dicerna, and PeptiDream, Inc., or PeptiDream.
We have incurred significant losses since we commenced operations in 2002 and as of September 30, 2023, we had an accumulated deficit of $6.87 billion. Historically, we have generated losses principally from costs associated with research and development activities, acquiring, filing and expanding intellectual property rights, and selling, general and administrative costs. As a result of planned expenditures for research and development activities relating to our research platform, our drug development programs, including clinical trial and manufacturing costs, the establishment of late-stage clinical and commercial capabilities, including global commercial operations, continued management and growth of our patent portfolio, collaborations and general corporate activities, we expect to incur additional operating losses, however we expect 2019 represents our peak operating loss year as we transition towards a self-sustainable financial profile.
We anticipate that our operating results will continue to fluctuate for the foreseeable future. Therefore, period-to-period comparisons should not be relied upon as predictive of the results in future periods.
We currently have programs focused on a number of therapeutic areas and, as of September 30, 2023, we generate worldwide product revenues from four commercialized products, ONPATTRO, AMVUTTRA, GIVLAARI and OXLUMO, primarily in the U.S., Europe and Japan. However, our ongoing development efforts may not be successful and we may not be able to commence sales of any other products and/or successfully market and sell our approved products or any other products approved in the future. A portion of our total revenues in recent years has been derived from collaboration revenues from strategic alliances with Regeneron, Vir and Novartis. In addition to revenues from the commercial sales of our approved products and potentially from sales of future products, we expect our sources of potential funding for the next several years to continue to be derived in part from existing and new strategic alliances. Such alliances include, or may include in the future, license and other fees, funded research and development, milestone payments and royalties on product sales by our licensors, including royalties on sales of Leqvio made by our partner Novartis, as well as proceeds from the sale of equity or debt.
Convertible Senior Notes
In September 2022, we issued $1.04 billion aggregate principal amount of 1.00% Convertible Senior Notes due 2027, or Notes. The Notes will mature on September 15, 2027, unless earlier converted, redeemed or repurchased. Before June 15, 2027, noteholders will have the right to convert their Notes in certain circumstances and during specified periods. From and after June 15, 2027, the Notes will be convertible at the option of the noteholders at any time prior to the close of business on the second scheduled trading day immediately preceding the maturity date. We will settle any conversions of Notes by paying or delivering, as applicable, cash, shares of our common stock, or a combination of cash and shares of our common stock, at our election.
In connection with the issuance of the Notes, we paid $118.6 million, including expenses, to enter into privately negotiated capped call transactions with certain initial purchasers of the Notes or their respective affiliates and certain other financial institutions, or capped call transactions. The capped call transactions are expected generally to reduce the potential dilution upon conversion of the Notes in the event that the market price per share of our common stock, as measured under the terms of the capped call transactions, is greater than the strike price of the capped call transactions, which initially corresponds to the conversion price of the Notes, and is subject to anti-dilution adjustments generally similar to those applicable to the conversion rate of the Notes. The cap price of the capped call transactions will initially be $424.00 per share, which represents a premium of approximately 100% based on the last reported sale price of our common stock of $212.00 per share on September 12, 2022, and is subject to certain adjustments under the terms of the capped call transactions. If, however, the market price per share of our common stock, as measured under the terms of the capped call transactions, exceeds the cap price of the capped call transactions, there would nevertheless be dilution upon conversion of the Notes to the extent that such market price exceeds the cap price of the capped call transactions.
We used approximately $762.0 million of the net proceeds from the offering of the Notes to repay borrowings, inclusive of prepayment premiums, under our credit agreement with Blackstone, with the remaining net proceeds designated for general corporate purposes.
Research and Development
Since our inception, we have focused on drug discovery and development programs. Research and development expenses represent a substantial percentage of our total operating expenses, as reflected by our broad pipeline of clinical development programs, which includes multiple programs in late-stage development.
Our Product Pipeline
Our broad pipeline, including five approved products and multiple late and early-stage investigational RNAi therapeutics, is focused in four STArs: Genetic Medicines; Cardio-Metabolic Diseases; Hepatic Infectious Diseases; and CNS/Ocular Diseases.
The chart below is a summary of our commercial products and late- and early-stage development programs as of October 31, 2023. It identifies those programs for which we have received marketing approval, the stage of our programs and our commercial rights to such programs:
During the third quarter of 2023 and recent period, we reported the following updates from our commercially approved products and our late-stage clinical programs:
Commercial
Total TTR: ONPATTRO & AMVUTTRA
•We achieved global net product revenues for ONPATTRO and AMVUTTRA for the third quarter of 2023 of $81.6 million and $148.7 million, respectively.
Total Ultra-Rare: GIVLAARI & OXLUMO
•We achieved global net product revenues for GIVLAARI and OXLUMO for the third quarter of 2023 of $54.1 million and $28.7 million, respectively.
Leqvio
•Our partner, Novartis, continued the launch of Leqvio in the U.S. and in other markets, with focus on patient on-boarding, removing access hurdles and enhancing medical education.
Late-Stage Clinical Development
•We continued to advance zilebesiran, an investigational RNAi therapeutic in development to treat hypertension in patients at high cardiovascular risk:
◦Reported positive topline results from the KARDIA-1 Phase 2 dose-ranging study of zilebesiran.
•Our partner, Sanofi, reported positive data from the Phase 3 ATLAS-OLE study of fitusiran, in development for the treatment of hemophilia A or B, with or without inhibitors, and is currently in discussions with the FDA regarding filing in 2024.
There is a risk that any drug discovery or development program may not produce revenue for a variety of reasons, including the possibility that we will not be able to adequately demonstrate the safety and effectiveness of the product candidate or obtain approval or the desired labeling for the product candidate from regulatory authorities. Moreover, there are uncertainties specific to any new field of drug discovery, including RNAi. The success of ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO or any other product candidate we develop is highly uncertain.
Due to the numerous risks associated with developing drugs, we cannot reasonably estimate or know the nature, timing and estimated costs of the efforts necessary to complete the development of any potential product candidate or indication, or the period, if any, in which material net cash inflows will commence from any approved product or indication. Any failure to complete any stage of the development of any potential products or any approved product for an expanded indication in a timely manner or successfully launch, market and sell any of our commercially approved products, could have a material adverse effect on our operations, financial position and liquidity. A discussion of some of the risks and uncertainties associated with completing our research and development programs within the planned timeline, or at all, and the potential consequences of failing to do so, are set forth in Part II, Item 1A below under the heading “Risk Factors.”
Strategic Alliances
Our business strategy is to develop and commercialize a broad pipeline of RNAi therapeutic products directed towards our four STArs. As part of this strategy, we have entered into, and expect to enter into additional, collaboration and licensing agreements as a means of obtaining resources, capabilities and funding to advance our investigational RNAi therapeutic programs.
Our collaboration strategy is to form alliances that create significant value for ourselves and our collaborators in the advancement of RNAi therapeutics as a new class of innovative medicines. Specifically, with respect to our CNS/Ocular Disease pipeline, in April 2019, we entered into a global, strategic collaboration with Regeneron to discover, develop and commercialize RNAi therapeutics for a broad range of diseases by addressing disease targets expressed in the eye and CNS, in addition to a select number of targets expressed in the liver. In July 2020, Regeneron exercised its co-development/co-commercialization option on our first CNS-targeted development candidate, ALN-APP, an investigational RNAi therapeutic in development for the treatment of hereditary cerebral amyloid angiopathy and autosomal dominant Alzheimer’s Disease, which we are leading. We are also advancing multiple other programs with Regeneron.
With respect to our Cardio-Metabolic pipeline, in March 2013, we entered into an exclusive, worldwide license with MDCO (acquired by Novartis AG in January 2020) pursuant to which MDCO was granted the right to develop, manufacture and commercialize RNAi therapeutics targeting proprotein convertase subtilisin/kexin type 9 for the treatment of hypercholesterolemia and other human diseases, including inclisiran. In March 2018, we entered into a discovery collaboration with Regeneron to identify RNAi therapeutics for nonalcoholic steatohepatitis, or NASH, and potentially other related diseases, and in November 2018, we and Regeneron entered into a separate, fifty-fifty collaboration to further research, co-develop and commercialize any therapeutic product candidates that emerge from these discovery efforts. In April 2020, we entered into a development and commercialization collaboration with Dicerna to advance investigational RNAi therapeutics for the treatment of alpha-1 antitrypsin deficiency-associated liver disease, or alpha-1 liver disease.
In addition, in July 2023, we entered into the Roche Collaboration and License Agreement, pursuant to which we and Roche established a worldwide, strategic collaboration for the joint development of pharmaceutical products containing zilebesiran. Under the Roche Collaboration and License Agreement, we granted to Roche (i) co-exclusive rights to develop and commercialize zilebesiran in the U.S. and (ii) exclusive rights to develop and commercialize zilebesiran outside of the U.S. Roche will make an upfront payment of $310.0 million under the Roche Collaboration and License Agreement. In addition, we will be eligible to receive up to $2.50 billion in contingent payments based on the achievement of specified development, regulatory and sales-based milestones. We will be responsible for forty percent (40%) and Roche will be responsible for the remaining sixty percent (60%) of development costs incurred in the conduct of development activities that support regulatory approval of zilebesiran globally. We and Roche will share equally (50/50) all costs incurred in connection with development activities that are conducted primarily to support regulatory approval of zilebesiran in the U.S., and Roche will be solely responsible for costs incurred in connection with commercialization of zilebesiran outside of the U.S. and will pay us tiered, low double digit royalties based on net sales of zilebesiran on a country-by-country basis outside of the U.S. during the royalty term. We and Roche will share equally (50/50) profits and losses (including commercialization costs) of zilebesiran in the U.S.
With respect to our Hepatic Infectious Disease pipeline, in October 2017, we announced an exclusive licensing agreement with Vir for the development and commercialization of RNAi therapeutics for infectious diseases, including chronic HBV infection. In March 2020, we announced an expansion of our exclusive licensing agreement with Vir to include the development and commercialization of RNAi therapeutics targeting SARS-CoV-2, the virus that causes the disease COVID-19, which we further expanded in April 2020 to include up to three additional targets focused on host factors for SARS-CoV-2, including angiotensin converting enzyme-2, and transmembrane protease, serine 2, and potentially a third mutually selected host factor target. In July 2021, we notified Vir that we elected to discontinue ALN-COV, in development for the treatment of COVID-19, and all other COVID-19 research and development activities, based on a portfolio prioritization in view of the availability of highly effective vaccines and alternative treatment options. Following such discontinuation of COVID-19 related activities, we have no further obligations to work on the COVID-related targets and Vir has no further rights to such targets under our exclusive licensing agreement.
With respect to our Genetic Medicine pipeline, we formed a broad strategic alliance with Sanofi in 2014. In January 2018, we and Sanofi amended our 2014 collaboration and entered into the Exclusive License Agreement, referred to as the Exclusive TTR License, under which we have the exclusive right to pursue the further global development and commercialization of all TTR products, including ONPATTRO, AMVUTTRA and any back-up products, and the ALN-AT3 Global License Terms, referred to as the AT3 License Terms, under which Sanofi has the exclusive right to pursue the further global development and commercialization of fitusiran and any back-up products.
In April 2019, we and Sanofi agreed to further amend the 2014 Sanofi collaboration to conclude the research and option phase and to amend and restate the AT3 License Terms to modify certain of the business terms.
We intend to continue to evaluate and explore partnership opportunities through collaboration and licensing arrangements, and may enter into new collaborations to advance certain products or disease areas. For example, in January 2022, we announced that we and Novartis agreed to collaborate on the discovery and development of an siRNA-based targeted therapy to restore functional liver cells in patients with end-stage liver diseases.
We also have entered into license agreements to obtain rights to intellectual property in the field of RNAi. In addition, because delivery of RNAi therapeutics has historically been an important objective of our research activities, we have entered into various collaboration and licensing arrangements with other companies and academic institutions to gain access to delivery technologies, including various LNP delivery technologies, and we may enter into such agreements in the future to gain access to products or technologies. For example, in 2021, we entered into a license and collaboration agreement with PeptiDream to discover and develop peptide-siRNA conjugates leveraging PeptiDream's proprietary Peptide Discovery Platform System technology.
Critical Accounting Policies and Estimates
Our critical accounting policies are described in the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of our Annual Report on Form 10-K for the year ended December 31, 2022, which we filed with the SEC on February 23, 2023. There have been no significant changes to our critical accounting policies since the beginning of this fiscal year.
Results of Operations
The following data summarizes the results of our operations:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
| (In thousands, except percentages) |
2023 |
|
2022 |
|
$ Change |
|
% Change |
|
2023 |
|
2022 |
|
$ Change |
|
% Change |
| Total revenues |
$ |
750,530 |
|
|
$ |
264,306 |
|
|
$ |
486,224 |
|
|
184 |
% |
|
$ |
1,388,574 |
|
|
$ |
702,383 |
|
|
$ |
686,191 |
|
|
98 |
% |
Operating costs and expenses |
$ |
536,663 |
|
|
$ |
522,346 |
|
|
$ |
14,317 |
|
|
3 |
% |
|
$ |
1,554,345 |
|
|
$ |
1,298,841 |
|
|
$ |
255,504 |
|
|
20 |
% |
| Income (loss) from operations |
$ |
213,867 |
|
|
$ |
(258,040) |
|
|
$ |
471,907 |
|
|
(183) |
% |
|
$ |
(165,771) |
|
|
$ |
(596,458) |
|
|
$ |
430,687 |
|
|
(72) |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net income (loss) |
$ |
147,753 |
|
|
$ |
(405,920) |
|
|
$ |
553,673 |
|
|
(136) |
% |
|
$ |
(302,372) |
|
|
$ |
(923,663) |
|
|
$ |
621,291 |
|
|
(67) |
% |
Discussion of Results of Operations
Revenues
Total revenues consist of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
| (In thousands, except percentages) |
2023 |
|
2022 |
|
$ Change |
|
% Change |
|
2023 |
|
2022 |
|
$ Change |
|
% Change |
| Net product revenues |
$ |
313,153 |
|
|
$ |
232,267 |
|
|
$ |
80,886 |
|
|
35 |
% |
|
$ |
895,186 |
|
|
$ |
632,654 |
|
|
$ |
262,532 |
|
|
41 |
% |
Net revenues from collaborations |
427,472 |
|
|
29,297 |
|
|
398,175 |
|
|
* |
|
469,778 |
|
|
64,267 |
|
|
405,511 |
|
|
* |
| Royalty revenue |
9,905 |
|
|
2,742 |
|
|
7,163 |
|
|
261 |
% |
|
23,610 |
|
|
5,462 |
|
|
18,148 |
|
|
332 |
% |
| Total |
$ |
750,530 |
|
|
$ |
264,306 |
|
|
$ |
486,224 |
|
|
184 |
% |
|
$ |
1,388,574 |
|
|
$ |
702,383 |
|
|
$ |
686,191 |
|
|
98 |
% |
* Indicates the percentage change period over period is greater than 500%. |
Net Product Revenues
Net product revenues consist of the following, by product and region:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
| (In thousands, except percentages) |
2023 |
|
2022 |
|
$ Change |
|
% Change |
|
2023 |
|
2022 |
|
$ Change |
|
% Change |
| ONPATTRO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| United States |
$ |
21,869 |
|
|
$ |
67,196 |
|
|
$ |
(45,327) |
|
|
(67) |
% |
|
$ |
77,246 |
|
|
$ |
200,588 |
|
|
$ |
(123,342) |
|
|
(61) |
% |
| Europe |
50,371 |
|
|
57,217 |
|
|
(6,846) |
|
|
(12) |
% |
|
166,442 |
|
|
167,185 |
|
|
(743) |
|
|
— |
% |
| Rest of World |
9,349 |
|
|
20,537 |
|
|
(11,188) |
|
|
(54) |
% |
|
31,852 |
|
|
67,614 |
|
|
(35,762) |
|
|
(53) |
% |
| Total |
81,589 |
|
|
144,950 |
|
|
(63,361) |
|
|
(44) |
% |
|
275,540 |
|
|
435,387 |
|
|
(159,847) |
|
|
(37) |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| AMVUTTRA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| United States |
113,508 |
|
|
25,060 |
|
|
88,448 |
|
|
353 |
% |
|
288,990 |
|
|
25,060 |
|
|
263,930 |
|
|
* |
| Europe |
19,417 |
|
|
169 |
|
|
19,248 |
|
|
* |
|
40,590 |
|
|
169 |
|
|
40,421 |
|
|
* |
| Rest of World |
15,755 |
|
|
— |
|
|
15,755 |
|
|
N/A |
|
53,004 |
|
|
— |
|
|
53,004 |
|
|
N/A |
| Total |
148,680 |
|
|
25,229 |
|
|
123,451 |
|
|
489 |
% |
|
382,584 |
|
|
25,229 |
|
|
357,355 |
|
|
* |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| GIVLAARI |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| United States |
37,009 |
|
|
31,169 |
|
|
5,840 |
|
|
19 |
% |
|
102,496 |
|
|
84,505 |
|
|
17,991 |
|
|
21 |
% |
| Europe |
12,430 |
|
|
12,477 |
|
|
(47) |
|
|
— |
% |
|
40,952 |
|
|
36,059 |
|
|
4,893 |
|
|
14 |
% |
| Rest of World |
4,709 |
|
|
2,013 |
|
|
2,696 |
|
|
134 |
% |
|
16,505 |
|
|
5,522 |
|
|
10,983 |
|
|
199 |
% |
| Total |
54,148 |
|
|
45,659 |
|
|
8,489 |
|
|
19 |
% |
|
159,953 |
|
|
126,086 |
|
|
33,867 |
|
|
27 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| OXLUMO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| United States |
9,713 |
|
|
6,383 |
|
|
3,330 |
|
|
52 |
% |
|
27,564 |
|
|
18,916 |
|
|
8,648 |
|
|
46 |
% |
| Europe |
15,086 |
|
|
9,348 |
|
|
5,738 |
|
|
61 |
% |
|
40,611 |
|
|
25,099 |
|
|
15,512 |
|
|
62 |
% |
| Rest of World |
3,937 |
|
|
698 |
|
|
3,239 |
|
|
464 |
% |
|
8,934 |
|
|
1,937 |
|
|
6,997 |
|
|
361 |
% |
| Total |
28,736 |
|
|
16,429 |
|
|
12,307 |
|
|
75 |
% |
|
77,109 |
|
|
45,952 |
|
|
31,157 |
|
|
68 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total net product revenues |
$ |
313,153 |
|
|
$ |
232,267 |
|
|
$ |
80,886 |
|
|
35 |
% |
|
$ |
895,186 |
|
|
$ |
632,654 |
|
|
$ |
262,532 |
|
|
41 |
% |
* Indicates the percentage change period over period is greater 500%. |
Net product revenues increased during the three and nine months ended September 30, 2023, compared to the same periods in 2022, primarily due to the launch of AMVUTTRA in the third quarter of 2022, partially offset by a decrease of demand for ONPATTRO due to patient switches to AMVUTTRA. Additional growth was related to an increase in patients on GIVLAARI and OXLUMO therapies.
We expect net product revenues to continue to increase during the remainder of 2023, as compared to 2022, as we continue to add new patients onto our commercial products, as well as launch these products into additional markets.
Net Revenues from Collaborations and Royalty Revenue
Net revenues from collaborations consist of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
| (In thousands, except percentages) |
2023 |
|
2022 |
|
$ Change |
|
% Change |
|
2023 |
|
2022 |
|
$ Change |
|
% Change |
Roche |
$ |
311,328 |
|
|
$ |
— |
|
|
$ |
311,328 |
|
|
N/A |
|
$ |
311,328 |
|
|
$ |
— |
|
|
$ |
311,328 |
|
|
N/A |
| Regeneron Pharmaceuticals |
80,254 |
|
|
21,979 |
|
|
58,275 |
|
|
265 |
% |
|
97,407 |
|
|
34,405 |
|
|
63,002 |
|
|
183 |
% |
| Novartis AG |
18,381 |
|
|
5,803 |
|
|
12,578 |
|
|
217 |
% |
|
41,941 |
|
|
27,472 |
|
|
14,469 |
|
|
53 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other |
17,509 |
|
|
1,515 |
|
|
15,994 |
|
|
* |
|
19,102 |
|
|
2,390 |
|
|
16,712 |
|
|
* |
| Total |
$ |
427,472 |
|
|
$ |
29,297 |
|
|
$ |
398,175 |
|
|
* |
|
$ |
469,778 |
|
|
$ |
64,267 |
|
|
$ |
405,511 |
|
|
* |
* Indicates the percentage change period over period is greater than 500%. |
Net revenues from collaborations increased during the three and nine months ended September 30, 2023, as compared to the same period in 2022, primarily due to revenue recognized under our agreements with Roche and Regeneron. During the quarter, we recognized $310.0 million of revenue under our agreement with Roche as the Roche License Obligation was satisfied this quarter upon transfer of licenses to Roche and, in connection with the Regeneron Collaboration, we recognized a cumulative catch-up adjustment of $65.0 million of revenue from the $100.0 million milestone earned for achieving certain criteria during early clinical development for our CNS program, ALN-APP.
Royalty revenue increased during the three and nine months ended September 30, 2023, as compared to the same period in 2022, due to increased royalties earned from global net sales of Leqvio by our partner, Novartis.
Recognition of our combined net revenues from collaborations and royalty revenue is dependent on a variety of factors including the level of work reimbursed by partners, achievement of milestones under our collaboration agreements, and royalties associated with sales of Leqvio. We expect net revenues from collaboration and royalty revenue to continue to increase in the remainder of 2023, as compared to 2022, due to the timing of manufacturing activities, achievement of milestones under our collaboration agreements, and royalties associated with sales of Leqvio. The amount of revenue from collaborations that we recognize, in part, is based on estimates of total costs to be incurred. These estimates reflect our historical experiences, current contractual requirements, and forecasted plans of development or manufacturing activities. We adjust these estimates for changes in actual costs incurred, contractual terms, and further forecasts. Such changes in estimates could have a significant impact on revenue and earnings in the period of the adjustment.
Operating Costs and Expenses
Operating costs and expenses consist of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
| (In thousands, except percentages) |
2023 |
|
2022 |
|
$ Change |
|
% Change |
|
2023 |
|
2022 |
|
$ Change |
|
% Change |
| Cost of goods sold |
$ |
79,473 |
|
|
$ |
36,507 |
|
|
$ |
42,966 |
|
|
118 |
% |
|
$ |
196,241 |
|
|
$ |
94,002 |
|
|
$ |
102,239 |
|
|
109 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cost of goods sold as a percentage of net product revenues |
25.4 |
% |
|
15.7 |
% |
|
|
|
|
|
21.9 |
% |
|
14.9 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cost of collaborations and royalties |
4,836 |
|
|
4,609 |
|
|
227 |
|
|
5 |
% |
|
28,307 |
|
|
23,549 |
|
|
4,758 |
|
|
20 |
% |
| Research and development |
253,179 |
|
|
245,371 |
|
|
7,808 |
|
|
3 |
% |
|
732,274 |
|
|
620,976 |
|
|
111,298 |
|
|
18 |
% |
Selling, general and administrative |
199,175 |
|
|
235,859 |
|
|
(36,684) |
|
|
(16) |
% |
|
597,523 |
|
|
560,314 |
|
|
37,209 |
|
|
7 |
% |
| Total |
$ |
536,663 |
|
|
$ |
522,346 |
|
|
$ |
14,317 |
|
|
3 |
% |
|
$ |
1,554,345 |
|
|
$ |
1,298,841 |
|
|
$ |
255,504 |
|
|
20 |
% |
Cost of goods sold
Cost of goods sold as a percentage of net product revenues increased during the three and nine months ended September 30, 2023, as compared to the same periods in 2022, primarily due to recording an impairment of ONPATTRO inventory that had been manufactured for future demand associated with the ATTR cardiomyopathy indication for patisiran for which we did not receive regulatory approval.
We expect cost of goods sold and cost of goods sold as a percentage of net product revenues will continue to increase during the remainder of 2023, as compared to 2022, primarily as a result of an expected increase in total net product sales and increased AMVUTTRA royalties.
Cost of collaborations and royalties
Cost of collaborations and royalties increased during the three and nine months ended September 30, 2023, as compared to the same periods in 2022, primarily due to increased demand of GalNAc material supplied to our collaboration partners to support certain product manufacturing and ongoing clinical trials and increased royalties payable to third parties on the net sales of licensed products by Novartis.
We expect cost of collaborations and royalties to continue to increase during the remainder of 2023, as compared to 2022, due to the timing and demand of GalNAc material to be supplied to our collaboration partners.
Research and development
Research and development expenses consist of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
| (In thousands, except percentages) |
2023 |
|
2022 |
|
$ Change |
|
% Change |
|
2023 |
|
2022 |
|
$ Change |
|
% Change |
| Clinical research and outside services |
$ |
112,558 |
|
|
$ |
104,762 |
|
|
$ |
7,796 |
|
|
7 |
% |
|
$ |
333,853 |
|
|
$ |
288,833 |
|
|
$ |
45,020 |
|
|
16 |
% |
| Compensation and related |
68,112 |
|
|
58,377 |
|
|
9,735 |
|
|
17 |
% |
|
197,539 |
|
|
163,416 |
|
|
34,123 |
|
|
21 |
% |
| Occupancy and all other costs |
43,354 |
|
|
29,270 |
|
|
14,084 |
|
|
48 |
% |
|
122,694 |
|
|
93,510 |
|
|
29,184 |
|
|
31 |
% |
| Stock-based compensation |
29,155 |
|
|
52,962 |
|
|
(23,807) |
|
|
(45) |
% |
|
78,188 |
|
|
75,217 |
|
|
2,971 |
|
|
4 |
% |
| Total |
$ |
253,179 |
|
|
$ |
245,371 |
|
|
$ |
7,808 |
|
|
3 |
% |
|
$ |
732,274 |
|
|
$ |
620,976 |
|
|
$ |
111,298 |
|
|
18 |
% |
For the three months ended September 30, 2023, the increase in research and development expenses, as compared to the same period in 2022, was primarily due to the following:
•Increased occupancy and all other costs as a result of a technology access milestone payable to a partner, higher costs related to supplies and raw materials that will be consumed in various clinical activities, and higher costs related to IT and other professional services to support our growing clinical footprint; and
•Increased compensation and related expenses as a result of increased headcount to support our R&D pipeline and development expenses.
Offset by:
•Decreased stock-based compensation expense related to the accounting for certain performance-based awards that vested in 2022.
For the nine months ended September 30, 2023, the increase in research and development expenses, as compared to the same period in 2022, was primarily due to the following:
•Increased clinical research and outside services primarily associated with zilebesiran as enrollment activities for our KARDIA-1 and KARDIA-2 clinical studies continued and additional costs associated with manufacturing batches associated with those clinical activities. Costs associated with clinical trials of other programs such as ALN-TTRsc04 and our ongoing early development studies also were higher when compared to last year. These costs were offset by decreased costs related to preclinical activities as targets moved into the clinic during 2023;
•Increased compensation and related expenses as a result of increased headcount to support our R&D pipeline and development expenses; and
•Increased occupancy and all other costs as a result of higher costs related to supplies and raw materials that will be consumed in various clinical activities, and higher costs related to IT and other professional services to support our growing clinical footprint.
During the three and nine months ended September 30, 2023 and 2022, in connection with advancing activities under our collaboration agreements, we incurred research and development expenses, primarily related to external development and clinical services, including the manufacture of clinical product.
The following table summarizes research and development expenses incurred, for which we recognize net revenue, that are directly attributable to our collaboration agreements, by collaboration partner:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
| (In thousands) |
2023 |
|
2022 |
|
2023 |
|
2022 |
| Regeneron Pharmaceuticals |
$ |
18,939 |
|
|
$ |
19,948 |
|
|
$ |
58,254 |
|
|
$ |
43,002 |
|
| Other |
3,365 |
|
|
357 |
|
|
4,711 |
|
|
1,172 |
|
| Total |
$ |
22,304 |
|
|
$ |
20,305 |
|
|
$ |
62,965 |
|
|
$ |
44,174 |
|
Selling, general and administrative
Selling, general and administrative expenses consist of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
| (In thousands, except percentages) |
2023 |
|
2022 |
|
$ Change |
|
% Change |
|
2023 |
|
2022 |
|
$ Change |
|
% Change |
| Compensation and related |
$ |
75,199 |
|
|
$ |
70,361 |
|
|
$ |
4,838 |
|
|
7 |
% |
|
$ |
225,032 |
|
|
$ |
197,346 |
|
|
$ |
27,686 |
|
|
14 |
% |
| Consulting and professional services |
54,187 |
|
|
59,557 |
|
|
(5,370) |
|
|
(9) |
% |
|
162,723 |
|
|
155,138 |
|
|
7,585 |
|
|
5 |
% |
| Occupancy and all other costs |
35,007 |
|
|
30,785 |
|
|
4,222 |
|
|
14 |
% |
|
108,270 |
|
|
95,165 |
|
|
13,105 |
|
|
14 |
% |
| Stock-based compensation |
34,782 |
|
|
75,156 |
|
|
(40,374) |
|
|
(54) |
% |
|
101,498 |
|
|
112,665 |
|
|
(11,167) |
|
|
(10) |
% |
| Total |
$ |
199,175 |
|
|
$ |
235,859 |
|
|
$ |
(36,684) |
|
|
(16) |
% |
|
$ |
597,523 |
|
|
$ |
560,314 |
|
|
$ |
37,209 |
|
|
7 |
% |
For the three months ended September 30, 2023, the decrease in selling, general and administrative expenses, as compared to the same period in 2022, was primarily due to decreased stock-based compensation expense related to the accounting for certain performance-based awards that vested in 2022.
For the nine months ended September 30, 2023, the increase in selling, general and administrative expenses, as compared to the same period in 2022, was primarily due to increased compensation and related expenses attributed to increased headcount and increased other costs related to various investments in IT and other digital initiatives in support of the global launch of AMVUTTRA and other areas of strategic growth.
We expect that research and development expenses combined with selling, general and administrative expenses will continue to increase during the remainder of 2023, as compared to 2022, as we continue to advance and develop our platform and pipeline, advance our product candidates, including partnered programs, into later-stage development, prepare regulatory submissions and continue to build-out our global commercial and compliance infrastructure and field team to support our commercial portfolio as well as launch our commercial products into additional markets, assuming regulatory approvals. However, we expect that certain expenses will be variable depending on the timing of manufacturing batches, clinical trial enrollment and results, regulatory review of our product candidates and programs, and stock-based compensation expenses due to our determination regarding the probability of vesting for performance-based awards.
Other (Expense) Income
Other (expense) income consists of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Three Months Ended September 30, |
|
Nine Months Ended September 30, |
| (In thousands, except percentages) |
2023 |
|
2022 |
|
$ Change |
|
% Change |
|
2023 |
|
2022 |
|
$ Change |
|
% Change |
| Interest expense |
$ |
(30,893) |
|
|
$ |
(41,084) |
|
|
$ |
10,191 |
|
|
(25) |
% |
|
$ |
(89,883) |
|
|
$ |
(126,055) |
|
|
$ |
36,172 |
|
|
(29) |
% |
| Interest income |
25,425 |
|
|
7,820 |
|
|
17,605 |
|
|
225 |
% |
|
65,155 |
|
|
10,731 |
|
|
54,424 |
|
|
* |
| Other expense, net |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Realized and unrealized loss on marketable equity securities |
(16,844) |
|
|
(7,850) |
|
|
(8,994) |
|
|
115 |
% |
|
(17,711) |
|
|
(40,108) |
|
|
22,397 |
|
|
(56) |
% |
| Change in fair value of development derivative liability |
(31,209) |
|
|
(25,084) |
|
|
(6,125) |
|
|
24 |
% |
|
(67,895) |
|
|
(70,776) |
|
|
2,881 |
|
|
(4) |
% |
| Other |
(9,605) |
|
|
(5,119) |
|
|
(4,486) |
|
|
88 |
% |
|
(19,725) |
|
|
(20,720) |
|
|
995 |
|
|
(5) |
% |
| Loss on the extinguishment of debt |
— |
|
|
(76,586) |
|
|
76,586 |
|
|
(100) |
% |
|
— |
|
|
(76,586) |
|
|
76,586 |
|
|
(100) |
% |
| Total |
$ |
(63,126) |
|
|
$ |
(147,903) |
|
|
$ |
84,777 |
|
|
(57) |
% |
|
$ |
(130,059) |
|
|
$ |
(323,514) |
|
|
$ |
193,455 |
|
|
(60) |
% |
* Indicates the percentage change period over period is greater than 500%. |
Total other expense decreased during the three and nine months ended September 30, 2023, as compared to the same periods in 2022, primarily due to increased interest income driven by higher market interest rates on our marketable debt securities, decreased interest expense as a result of a more favorable interest rate under the Notes compared with the interest rate under the credit facility held with Blackstone and a $76.6 million loss on the extinguishment of the Blackstone credit agreement recognized in 2022.
Liquidity and Capital Resources
The following table summarizes our cash flow activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended September 30, |
| (In thousands) |
2023 |
|
2022 |
Net cash provided by (used in): |
|
|
|
| Operating activities |
$ |
133,951 |
|
|
$ |
(409,296) |
|
| Investing activities |
$ |
(95,694) |
|
|
$ |
309,265 |
|
| Financing activities |
$ |
132,903 |
|
|
$ |
362,316 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating activities
Net cash provided by operating activities increased during the nine months ended September 30, 2023, compared to the same period ended 2022, primarily due to the receipt of a $310.0 million up-front payment received in connection with the Roche Agreement in addition to stronger cash receipts from increased product sales.
Investing activities
Net cash used in investing activities increased during the nine months ended September 30, 2023, compared to the same period ended 2022, primarily due to net activities related to our marketable debt securities.
Financing activities
Net cash provided by financing activities decreased during the nine months ended September 30, 2023, compared to the same period ended 2022, primarily due to $136.2 million received from the issuance of convertible debt, net of repayment of the credit facility held with Blackstone and purchase of capped call transactions in September 2022, in addition to decreased net proceeds from the issuance of common stock in connection with stock option exercises and other types of equity.
Additional Capital Requirements
We currently have programs focused in many therapeutic areas and, as of September 30, 2023, have received regulatory approval and commercially launched four products. However, our ongoing development efforts may not be successful and we may not be able to commence sales of any other products or successfully expand the indications for our approved products, including AMVUTTRA in the future. In addition, we anticipate that we will continue to generate losses as a result of planned expenditures for research and development activities relating to our research platform, our drug development programs, including clinical trial and manufacturing costs, the establishment of late-stage clinical, manufacturing, commercial and compliance capabilities, including global operations, continued management and growth of our intellectual property including our patent portfolio, collaborations and general corporate activities.
Our expected working and other capital requirements are described in our 2022 Annual Report on Form 10-K in “Part II, Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.” As of September 30, 2023, other than the changes disclosed in the “Notes to Condensed Consolidated Financial Statements” and “Liquidity and Capital Resources” section in this Quarterly Report on Form 10-Q, there have been no other material changes to our expected working and other capital requirements as described in our 2022 Annual Report on Form 10-K.
Based on our current operating plan, we believe that our cash, cash equivalents and marketable securities as of September 30, 2023, together with the cash we expect to generate from product sales and under our current alliances, will be sufficient to satisfy our near-term capital and operating needs for at least the next 12 months from the filing of this Quarterly Report on Form 10-Q. However, due to numerous factors described in more detail under the caption Part II, Item 1A, "Risk Factors" of this Quarterly Report on Form 10-Q, we may require significant additional funds earlier than we currently expect in order to continue to commercialize our approved products, and to develop, conduct clinical trials for, manufacture and, if approved, commercialize additional product candidates.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Financial market risks related to interest rates are described in our Annual Report on Form 10-K for the year ended December 31, 2022. As of September 30, 2023, there have been no significant changes to the financial market risks described as of December 31, 2022. We do not currently anticipate any other near-term changes in the nature of our financial market risk exposures or in management’s objectives and strategies with respect to managing such exposures.
ITEM 4. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer (principal executive officer) and executive vice president, Chief Financial Officer (principal financial officer), evaluated the effectiveness of our disclosure controls and procedures as of September 30, 2023. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of September 30, 2023, our Chief Executive Officer and executive vice president, Chief Financial Officer concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
Changes in Internal Control
There were no changes in our internal control over financial reporting during the quarter ended September 30, 2023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART II. OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
For a discussion of material pending legal proceedings, please read Note 13, Commitments and Contingencies, to our condensed consolidated financial statements included in Part I, Item I, “Financial Statements (Unaudited),” of this Quarterly Report on Form 10-Q, which is incorporated into this item by reference.
ITEM 1A. RISK FACTORS
We operate in an environment that involves a number of significant risks and uncertainties. We caution you to read the following risk factors, which have affected, and/or in the future could affect, our business, prospects, operating results, and financial condition. The risks described below include forward-looking statements, and actual events and our actual results may differ materially from these forward-looking statements. Additional risks and uncertainties not currently known to us or that we currently deem immaterial may also impair our business, prospects, operating results, and financial condition. Furthermore, additional risks and uncertainties are described under other captions in this report and should also be considered by our investors.
SUMMARY OF MATERIAL RISKS ASSOCIATED WITH OUR BUSINESS
Our business is subject to numerous risks and uncertainties, discussed in more detail in the following section. These risks include, among others, the following key risks:
Business Related Risks – Risks Related to Our Financial Results
•The marketing and sale of our approved products or any future products may be unsuccessful or less successful than anticipated and we may be unable to expand the indication for AMVUTTRA.
•We have a history of losses and may never become and remain consistently profitable.
•We will require substantial funds to continue our research, development and commercialization activities.
•Any future outbreaks of COVID-19 and its variants, or other highly infectious or contagious diseases, may directly or indirectly adversely affect our business, results of operations and financial condition.
•Although we sold a portion of the expected royalty stream and commercial milestones related to global sales of Leqvio by Novartis, we are entitled to retain the remaining portion of such future royalties and, if certain specified thresholds are met, to the remaining portion of commercial milestone payments, and any negative developments related to Leqvio could have a material adverse effect on the timing or amount of those payments.
Risks Related to Our Dependence on Third Parties
•We may not be able to execute our business strategy if we are unable to maintain existing or enter into new alliances with other companies that can provide business and scientific capabilities and funds for the development and commercialization of certain of our product candidates.
•If any collaborator materially amends, terminates or fails to perform its obligations under agreements with us, the development and commercialization of certain of our product candidates could be delayed or terminated and we could suffer other economic harm.
•We expect to continue to grow our manufacturing capabilities and resources and we must incur significant costs to develop this expertise and/or rely on third parties to manufacture our products.
•We rely on third parties to conduct our clinical trials, and if they fail to fulfill their obligations, our development plans may be adversely affected.
Risks Related to Managing Our Operations
•If we are unable to attract and retain qualified key management and scientists, development, medical and commercial staff, consultants and advisors, our ability to implement our business plan may be adversely affected.
•We may have difficulty expanding our operations successfully as we continue our evolution from a U.S.- and Europe-based company primarily involved in discovery, pre-clinical testing and clinical development into a global company that develops and commercializes multiple drugs in multiple geographies including Asia, Latin America and the Middle East.
Industry Related Risks – Risks Related to Development, Clinical Testing and Regulatory Approval of Our Product Candidates and the Commercialization of Our Approved Products
•Any product candidates we or our partners develop may fail in development or be delayed to a point where they do not become commercially viable.
•We or our partners may be unable to obtain U.S. or foreign regulatory approval for our or our partnered product candidates, or if approved, may fail to obtain desired labeling for such products.
•Even if we or our partners obtain regulatory approvals, our marketed drugs will be subject to ongoing regulatory oversight.
•Even if we receive regulatory approval to market our product candidates, and our collaborators receive regulatory approval to market product candidates discovered by us or developed with our technology, the market may not be receptive to such product candidates upon their commercial introduction, which could prevent us from becoming profitable.
•We are a multi-product commercial company and expect to continue to invest significant financial and management resources to continue to scale our marketing, sales, market access and distribution capabilities and further establish our global commercial and compliance infrastructure, and our commercial efforts may not be successful.
•We may incur significant liability if enforcement authorities allege or determine that we are engaging in commercial activities or promoting our commercially approved products in a way that violates applicable regulations.
•Any drugs we develop may become subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives, thereby harming our business.
Risks Related to Patents, Licenses and Trade Secrets
•If we are not able to obtain and enforce patent protection for our discoveries, our ability to develop and commercialize our product candidates will be harmed.
•We license patent rights from third-party owners. If such owners do not properly or successfully obtain, maintain or enforce the patents underlying such licenses, our competitive position and business prospects may be harmed.
•Other companies or organizations may challenge our patent rights or may assert patent rights that prevent us from developing and commercializing our products.
•If we become involved in intellectual property litigation or other proceedings related to a determination of rights, including our ongoing patent infringement litigation against Pfizer, Inc., or Pfizer, and Moderna, Inc., we could incur substantial costs and expenses, and in the case of such litigation or proceedings against us, substantial liability for damages or be required to stop our product development and commercialization efforts.
•If we fail to comply with our obligations under any licenses or related agreements, we may be required to pay damages and could lose license or other rights that are necessary for developing, commercializing and protecting our RNAi technology.
Risks Related to Competition
•The pharmaceutical market is intensely competitive. If we are unable to compete effectively with existing drugs, new treatment methods and new technologies, we may be unable to commercialize successfully any drugs that we or our collaborators develop.
•We face competition from other companies that are working to develop novel drugs and technology platforms using technology similar to ours, as well as from companies utilizing emerging technologies, including gene therapy and gene editing.
Risks Related to Our Common Stock
•If our stock price fluctuates, purchasers of our common stock could incur substantial losses.
•We may incur significant costs from class action litigation.
•Future sales of shares of our common stock, including by our significant stockholders, us or our directors and officers, could cause the price of our common stock to decline.
Risks Related to Our Convertible Notes
•Servicing our debt may require a significant amount of cash. We may not have sufficient cash flow from our business to pay our indebtedness.
•We may not have the ability to raise the funds necessary to settle for cash conversions of the Notes or to repurchase the Notes for cash upon a fundamental change, and our future debt may contain limitations on our ability to pay cash upon conversion of the Notes or to repurchase the Notes.
•The conditional conversion feature of the Notes, if triggered, may adversely affect our financial condition and operating results.
Risks Related to Our Business
Risks Related to Our Financial Results
The marketing and sale of our approved products or any future products may be less successful than anticipated, and we may be unable to expand the indication for certain of our commercial products, including AMVUTTRA.
In 2018, our first commercial product, ONPATTRO, was approved by the FDA and EMA, and we have since received approval and launched ONPATTRO in several additional territories. In 2019, the FDA approved our second product, GIVLAARI, which was also approved by the EMA and has since received approval in several additional territories, and in 2020, the FDA and EMA approved our third product, OXLUMO, which received additional regulatory approvals in 2021 and 2022. In June 2022, the FDA approved AMVUTTRA, which was granted marketing authorization in Europe and the UK in September 2022 and has since received regulatory approval in Japan and Brazil. We also have multiple product candidates in late-stage clinical development. While we have commercially launched four products, we cannot predict whether we will successfully market and sell our approved products, or successfully expand the indications of certain of our approved products, including AMVUTTRA. For example, in August and September 2022, we reported positive safety and efficacy results from the APOLLO-B Phase 3 clinical trial of patisiran, which was designed and powered to evaluate the effects of patisiran on functional capacity and quality of life in patients with ATTR amyloidosis with cardiomyopathy. While we believe that the APOLLO-B results after 12 months validate the therapeutic hypothesis of RNAi therapeutics targeting TTR as potential treatment for patients with ATTR amyloidosis with cardiomyopathy, in October 2023, the FDA issued a CRL for our sNDA for patisiran for the treatment of the cardiomyopathy of ATTR amyloidosis, indicating that the clinical meaningfulness of patisiran’s treatment effects for the cardiomyopathy of ATTR amyloidosis had not been established, and therefore, the sNDA could not be approved in its present form.
To execute our business plan of building a profitable, top-tier biotech company by the end of 2025 and achieving our Alnylam P5x25 strategy and the metrics associated with such strategy, in addition to successfully marketing, selling and expanding the indications of our approved products, we will need to successfully:
•execute product development activities and continue to leverage new technologies related to both RNAi and to the delivery of siRNAs to the relevant tissues and cells, including the liver, CNS, eye, lung and muscle;
•build and maintain a strong intellectual property portfolio;
•gain regulatory acceptance for the development and commercialization of our product candidates and market success for our approved products, as well as any other products we commercialize;
•attract and retain customers for our products;
•develop and maintain successful strategic alliances; and
•manage our spending as costs and expenses increase due to clinical trials, regulatory approvals and commercialization.
If we are unsuccessful in accomplishing the objectives set forth above, we may not be able to develop product candidates, successfully commercialize our approved products or any future products, raise capital, if needed, repay our indebtedness, achieve financial self-sustainability or continue our operations.
We have a history of losses and may never become and remain consistently profitable.
We have experienced significant operating losses since our inception. As of September 30, 2023, we had an accumulated deficit of $6.87 billion. Although to date we have launched four products in the U.S., EU and various other countries globally, and expect to launch our commercially approved products in additional countries during 2023 and beyond, we may never attain profitability or positive cash flow from operations. For the three and nine months ended September 30, 2023, we recognized $313.2 million and $895.2 million, respectively, in net product revenues from sales of ONPATTRO, AMVUTTRA, GIVLAARI and OXLUMO. While we believe 2019 was our peak operating loss year, we expect to continue to incur annual operating losses, and will require substantial resources over the next several years as we expand our efforts to discover, develop and commercialize RNAi therapeutics, and aim to achieve self-sustainability by the end of 2025. While we believe our current cash, cash equivalents and marketable equity and debt securities, as well as the revenue we expect to generate from product sales and under our current alliances, including milestones and royalties on Leqvio sales, should enable us to achieve a self-sustainable profile without the need for future equity financing, we will depend on our ability to generate revenues to achieve this goal. In addition to revenues derived from sales of our current and future, if any, commercially approved products, we anticipate that a portion of any revenues we generate over the next several years will continue to be from alliances with pharmaceutical and biotechnology companies, including Roche, Novartis and Regeneron.
We cannot be certain that we will be able to maintain our existing alliances, secure and maintain new alliances, meet the obligations, or achieve any milestones that we may be required to meet or achieve to receive payments under our existing or new alliances. Moreover, we cannot be certain that our partners, including Novartis, will continue to successfully execute their obligations under our alliance agreements and generate additional revenues for us.
We believe that to become and remain consistently profitable, we must succeed in discovering, developing and commercializing novel drugs with significant market potential. This will require us to build upon the success we have had in a range of challenging activities, including continued platform innovation, pre-clinical testing and clinical trial stages of development, obtaining regulatory approval and reimbursement for these novel drugs and manufacturing, marketing and selling them. We may never generate revenues that are significant enough to achieve profitability and, even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. If we cannot become and remain consistently profitable, the market price of our common stock could decline. In addition, we may be unable to raise capital, expand our business, develop additional product candidates or continue our operations.
We will require substantial funds to continue our research, development and commercialization activities and if the funds we require are greater than what we have estimated, we may need to critically limit or significantly scale back or cease certain of our activities.
We have used substantial funds to develop our RNAi technologies and will require substantial funds to conduct further research and development, including pre-clinical testing and clinical trials of our product candidates, and to manufacture, market and sell our four approved products and any other products that are approved for commercial sale. Because the length of time or activities associated with successful development of our product candidates, including zilebesiran, may be greater than we anticipate, we are unable to estimate the actual funds we will require to develop and commercialize them.
We believe 2019 was our peak operating loss year, and believe that our current cash, cash equivalents and marketable equity and debt securities, as well as revenue we expect to generate from product sales and under our current alliances, including milestones and royalties on Leqvio sales, will enable us to achieve a self-sustainable financial profile without need for future equity financing. However, our future capital requirements and the period for which we expect our existing resources to support our operations may vary from what we expect. We have based our expectations on a number of factors, many of which are difficult to predict or are outside of our control, including:
•progress in our research and development programs, including programs in both rare and prevalent diseases as well as what may be required by regulatory bodies to advance these programs;
•the timing, receipt and amount of milestone and other payments, if any, from present and future collaborators, if any, including milestones from Roche with respect to the development or commercialization of zilebesiran, as well as milestone payments related to Leqvio, which is being commercialized by our partner, Novartis;
•our ability to maintain and establish additional collaborative arrangements and/or new business initiatives;
•the potential for improved product profiles to emerge from our new technologies and our ability to successfully advance our delivery efforts in extrahepatic tissues;
•the resources, time and costs required to successfully initiate and complete our pre-clinical and clinical studies, obtain regulatory approvals, prepare for global commercialization of our product candidates and obtain and maintain licenses to third-party intellectual property;
•our ability to establish, maintain and operate our own manufacturing facilities in a timely and cost-effective manner;
•our ability to manufacture, or contract with third parties for the manufacture of, our product candidates for clinical testing and commercial sale;
•the impact of any future pandemics or public health emergencies or the ongoing conflicts in Israel and Ukraine on the initiation or completion of pre-clinical studies or clinical trials and the supply of our products or product candidates;
•the resources, time and cost required for the preparation, filing, prosecution, maintenance and enforcement of patent claims;
•the costs associated with legal activities, including litigation and government investigations, arising in the course of our business activities and our ability to prevail or reach a satisfactory result in any such legal disputes and investigations;
•the timing, receipt and amount of sales and royalties, if any, from our approved products and our potential products, if and when approved; and
•the outcome of the regulatory review process and commercial success of drug products for which we are entitled to receive royalties, including Leqvio.
If our estimates, predictions and financial guidance relating to these factors are incorrect, we may need to modify our operating plan and may be required to seek additional funding in the future. We may do so through either collaborative arrangements, public or private equity offerings or debt financings, royalty or other monetization transactions or a combination of one or more of these funding sources. Additional funds may not be available to us on acceptable terms or at all.
The terms of any financing we may be required to pursue in the future may adversely affect the holdings or the rights of our stockholders. If we raise additional funds by issuing equity securities, further dilution to our existing stockholders will result. In addition, as a condition to providing additional funding to us, future investors may demand, and may be granted, rights superior to those of existing stockholders.
If we are unable to obtain additional funding on a timely basis, we may be required to significantly delay or curtail one or more of our research or development programs, or delay or curtail the further development of our global commercial infrastructure, and our ability to achieve our long-term strategic goals may be delayed or diminished. We also could be required to seek funds through arrangements with collaborators or others that may require us to relinquish rights to some of our technologies, product candidates or products that we would otherwise pursue on our own.
Any future outbreaks of COVID-19 and its variants, or other highly infectious or contagious diseases, may directly or indirectly adversely affect our business, results of operations and financial condition.
In the future, we may experience disruptions from COVID-19 or a future pandemic or public health emergency that could impact our business and operations, including our ability to successfully commercialize our approved products, and we may not be able to meet expectations with respect to commercial sales as a result. In addition, we may also experience decreased patient demand for our approved products if current or potential patients decide to delay treatment as a result of the COVID-19 or a future pandemic or public health emergency. Business interruptions from future pandemics or public health emergencies, including staffing shortages, raw material or other supply chain shortages, production slowdowns and disruptions in delivery systems, may also adversely impact the third parties we or our partners rely on in the U.S. and abroad to sufficiently manufacture our approved products and to produce product candidates in quantities we require, which may impair our commercialization efforts, our research and development activities and the potential commercialization of our product candidates.
Additionally, timely completion of pre-clinical activities and initiation of planned clinical trials are dependent upon the availability of, for example, pre-clinical and clinical trial sites, researchers and investigators, patients or healthy volunteer subjects available for recruitment and enrollment, and regulatory agency personnel, which may be adversely affected by global health matters, such as the COVID-19 pandemic or any future pandemic or public health emergency. We are conducting and plan to continue to conduct pre-clinical activities and clinical trials for our drug product candidates in geographies which have been and may again be affected by COVID-19, and any resurgence of the COVID-19 pandemic and its variants could have an impact on various aspects of our ongoing clinical trials and on the clinical trials and pre-clinical studies we expect to initiate during 2023.
Health regulatory agencies globally may also experience disruptions in their operations as a result of the COVID-19 pandemic or future public health emergencies, which could impact review, inspection and approval timelines. Since March 2020, when foreign and domestic inspections of facilities were largely placed on hold, the FDA has been working to resume pre-pandemic levels of inspection activities, including routine surveillance, bioresearch monitoring and pre-approval inspections. Should the FDA determine that an inspection is necessary for approval of a marketing application and an inspection cannot be completed during the review cycle due to restrictions on travel, and the agency does not determine a remote interactive evaluation to be adequate, the FDA has stated that it generally intends to issue, depending on the circumstances, a complete response letter or defer action on the application until an inspection can be completed.
While the ultimate impact of COVID-19, or any future pandemic or public health emergency, on our business is uncertain, any negative impacts of such pandemic or public health emergency, alone or in combination with others, could exacerbate other risk factors discussed herein. The full extent to which COVID-19, or any future pandemic or public health emergency, will negatively affect our operations, financial performance, and stock price will depend on future developments that are highly uncertain and cannot be predicted.
Although we sold a portion of the royalty stream and commercial milestones from the global sales of Leqvio by our collaborator, Novartis, we are entitled to retain the remaining portion of future royalties from the global sales of Leqvio and, if certain specified thresholds are met, to the remaining portion of commercial milestone payments, and any negative developments related to Leqvio could have a material adverse effect on our receipt of those payments.
In April 2020, we sold to Blackstone 50% of the royalties payable to us with respect to net sales by Novartis, its affiliates or sublicensees of Leqvio and 75% of the commercial milestone payments payable to us under the MDCO agreement. If Blackstone does not receive royalty payments in respect of global sales of Leqvio equaling at least $1.00 billion by December 31, 2029, Blackstone’s royalty interest will increase to 55% effective January 1, 2030. Our receipt of future royalty payments and a portion of commercial milestone payments may be negatively impacted if the Leqvio royalty stream and commercial milestones payments are insufficient to meet the specified thresholds. Any negative impact to future royalty payments and commercial milestone payments could affect our ability to meet the specified repayment thresholds.
Additional factors that may have an adverse effect on the Leqvio royalty stream and commercial milestones include:
•companies working to develop new therapies or alternative formulations of products for ASCVD;
•foreign currency exchange rate fluctuations, which could have a negative impact on Novartis’ sales of Leqvio, thereby reducing the royalties;
•any negative developments relating to Leqvio, such as safety, efficacy, or reimbursement issues, could reduce demand for Leqvio;
•any disputes concerning patents, proprietary rights, or license and collaboration agreements could negatively impact our receipt of commercial milestone payments or royalties; and
•adverse regulatory or legislative developments could limit or prohibit the sale of Leqvio, such as restrictions on the use of Leqvio or safety-related label changes, including enhanced risk management programs, which may significantly reduce expected royalty revenue and commercial milestone payments and could require significant expense to address the associated legal and regulatory issues.
If the revenues generated by sales of Leqvio are lower than expected, our business could be materially adversely affected.
Geopolitical risks associated with the ongoing military conflict between Russia and Ukraine could have an adverse impact on our business, financial condition and results of operations, including our clinical trials.
Russia’s invasion of Ukraine, and the global response, including the imposition of sanctions by the U.S., EU and other countries, has resulted in global business disruptions and economic volatility and may have an adverse impact on our business, including our clinical trials. The uncertain nature, magnitude, and duration of hostilities stemming from the conflict in Ukraine, including the potential effects of sanctions limitations, retaliatory cyber-attacks on the world economy and markets, have contributed to increased market volatility and uncertainty, which could continue to have an adverse impact on macroeconomic factors that might affect our business and operations.
Additionally, the ongoing conflict in Ukraine disrupted the ability of certain of our contract research organizations, or CROs, to conduct clinical trials at certain sites in Ukraine. We cannot be certain what the overall impact of this conflict will be on our ability to conduct and complete our clinical trials on schedule. However, interruptions of our clinical trials could significantly delay our clinical development plans and potential authorization or approval of our product candidates, which could increase our costs and jeopardize our ability to successfully commercialize our product candidates.
We expect our operating results to fluctuate in future periods, which may adversely affect our stock price.
Our quarterly operating results have fluctuated in the past, and may continue to do so in the future. Our operating results may fluctuate due to the level of success of our commercial efforts and resulting revenues, as well as the variable nature of our operating expenses as a result of the timing and magnitude of expenditures. For example, due to the impact of the COVID-19 pandemic, combined net product revenues in the first quarter of 2022 for our commercially approved products were negatively impacted. In addition, in one or more future periods, our results of operations may fall below the expectations of securities analysts and investors. In that event, the market price of our common stock could substantially decline.
If the estimates we make, or the assumptions on which we rely, in preparing our condensed consolidated financial statements and/or our projected guidance prove inaccurate, our actual results may vary from those reflected in our projections and accruals.
Our condensed consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America, or GAAP. The preparation of these condensed consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of our assets, liabilities, revenues and expenses, the amounts of charges accrued by us and related disclosure of contingent assets and liabilities. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. We cannot assure you, however, that our estimates, or the assumptions underlying them, will be correct.
Further, from time to time we issue financial guidance relating to our expectations regarding our combined product sales, collaboration and royalty revenues, and GAAP and non-GAAP combined research and development and selling, general and administrative expenses, which guidance is based on estimates and the judgment of management. If, for any reason, our revenues and/or expenses differ materially from our guidance, we may have to adjust our publicly announced financial guidance. For example, in April 2022, we decreased our 2022 guidance range for combined net product revenues, and in October 2022, we decreased our guidance range for our collaboration and royalty revenue. If we fail to meet, or if we are required to change or update any element of, our publicly disclosed financial guidance or other expectations about our business, our stock price could decline.
The investment of our cash, cash equivalents and marketable securities is subject to risks which may cause losses and affect the liquidity of these investments.
As of September 30, 2023, we had $2.41 billion in cash, cash equivalents and marketable securities. We historically have invested these amounts in high–grade corporate notes, commercial paper, securities issued or sponsored by the U.S. government, certificates of deposit and money market funds meeting the criteria of our investment policy, which is focused on the preservation of our capital. Corporate notes may also include foreign bonds denominated in U.S. dollars. These investments are subject to general credit, liquidity, market and interest rate risks. We may realize losses in the fair value of these investments or a complete loss of these investments, which would have a negative effect on our condensed consolidated financial statements. In addition, should our investments cease paying or reduce the amount of interest paid to us, our interest income would suffer. The market risks associated with our investment portfolio may have an adverse effect on our results of operations, liquidity and financial condition.
Volatility in foreign currency exchange rates could have a material adverse effect on our operating results.
Our revenue from outside of the U.S. is expected to increase as our products, whether commercialized by us or our collaborators, gain marketing approval in such jurisdictions. Our primary foreign currency exposure relates to movements in the Japanese yen, Euro and British pound. If the U.S. dollar weakens against a specific foreign currency, our revenues will increase, having a positive impact on net income, but our overall expenses will increase, having a negative impact. Conversely, if the U.S. dollar strengthens against a specific foreign currency, our revenues will decrease, having a negative impact on net income, but our overall expenses will decrease, having a positive impact. For example, during 2022, we experienced an unfavorable impact from foreign exchange rates on our international revenues. Continued volatility in foreign exchange rates is likely to continue to impact our operating results and financial condition.
Changes in tax law could adversely affect our business and financial condition.
Our business is subject to numerous international, federal, state, and other governmental laws, rules, and regulations that may adversely affect our operating results, including, taxation and tax policy changes, tax rate changes, new tax laws, or revised tax law interpretations, which individually or in combination may cause our effective tax rate to increase. In the U.S., the rules dealing with federal, state, and local income taxation are constantly under review by persons involved in the legislative process and by the Internal Revenue Service and the U.S. Treasury Department. Changes to tax laws (which changes may have retroactive application) could adversely affect us or holders of our common stock. In recent years, many such changes have been made and changes are likely to continue to occur in the future. Future changes in tax laws could have a material adverse effect on our business, cash flow, financial condition or results of operations.
Additionally, the Organization for Economic Co-operation and Development, or OECD, the EC, and individual taxing jurisdictions where we and our affiliates do business have recently focused on issues related to the taxation of multinational corporations. The OECD has released its comprehensive plan to create an agreed set of international rules for fighting base erosion and profit shifting. In addition, the OECD, the EC and individual countries are examining changes to how taxing rights should be allocated among countries considering the digital economy. As a result, the tax laws in the U.S. and other countries in which we and our affiliates do business could change on a prospective or retroactive basis and any such changes could materially adversely affect our business.
Risks Related to Our Dependence on Third Parties
We may not be able to execute our business strategy if we are unable to maintain existing or enter into new alliances with other companies that can provide business and scientific capabilities and funds for the development and commercialization of our product candidates. If we are unsuccessful in forming or maintaining these alliances on terms favorable to us, our business may not succeed.
We are continuing to advance our commercial capabilities, including in marketing, sales, market access and distribution, to support our wholly-owned products. We also continue to advance our growing pipeline of RNAi therapeutic opportunities. However, we may not have adequate capacity or capabilities to advance all of our therapeutic opportunities. Accordingly, we have entered into alliances with other companies and collaborators that we believe can provide such capabilities in certain territories and/or for certain product candidates, and we intend to enter into additional such alliances in the future. Our collaboration strategy is to form alliances that create significant value for us and our collaborators in the advancement of RNAi therapeutics as a new class of innovative medicines. Specifically, with respect to our Genetic Medicine pipeline, as a result of our broad strategic alliance with Sanofi formed in 2014, Sanofi has the right to develop and commercialize fitusiran globally. In addition, we formed a collaboration with MDCO (which was acquired by Novartis in January 2020) to advance inclisiran. In March 2018, we entered into a discovery collaboration with Regeneron to identify RNAi therapeutics for NASH and potentially other related diseases, and in November 2018, we and Regeneron entered into a separate, fifty-fifty collaboration to further research, co-develop and commercialize any therapeutic product candidates that emerge from these discovery efforts. In October 2017, we announced an exclusive licensing agreement with Vir for the development and commercialization of RNAi therapeutics for infectious diseases, including chronic HBV infection. In April 2020, we entered into a development and commercialization collaboration with Dicerna (which was acquired by Novo Nordisk in December 2021) to advance investigational RNAi therapeutics for the treatment of alpha-1 liver disease.
With respect to our CNS/Ocular Disease pipeline, in April 2019, we announced a global, strategic collaboration with Regeneron to discover, develop and commercialize RNAi therapeutics for a broad range of diseases by addressing therapeutic targets expressed in the eye and CNS, in addition to a select number of targets expressed in the liver. With respect to our Cardio-Metabolic pipeline, in July 2023, we entered into a Collaboration and License Agreement with Roche for the worldwide joint development of pharmaceutical products containing zilebesiran.
In such alliances, we expect our current, and may expect our future, collaborators to provide substantial capabilities in clinical development, regulatory affairs, and/or marketing, sales and distribution. Under certain of our alliances, we also may expect our collaborators to develop, market and/or sell certain of our product candidates. We may have limited or no control over the development, sales, marketing and distribution activities of these third parties. Our future revenues may depend heavily on the success of the efforts of these third parties. For example, we will rely entirely on (i) Regeneron for the development and commercialization of all programs targeting eye diseases (subject to limited exceptions), and potentially other CNS and liver programs, (ii) Novartis for all future development and commercialization of Leqvio worldwide, (iii) Sanofi for the development and commercialization of fitusiran worldwide, and (iv) Roche for the commercialization of zilebesiran outside of the U.S. In the case of each such collaboration referenced in clauses (i)-(iv) above, we are entitled to royalties on the sales of each of these products. If our collaborators are not successful in their development and/or commercialization efforts, our future revenues from RNAi therapeutics for these indications may be adversely affected. For example, while Leqvio was granted marketing authorization by the EC in Europe, in December 2020, Novartis received a complete response letter from the FDA stating that the agency could not approve the NDA by the PDUFA action date due to unresolved inspection-related conditions at a third party manufacturing facility. While Leqvio was approved by the FDA in December 2021, the resolution of the complete response letter resulted in a delay in the payment of an approval milestone and potential U.S. royalties. If the revenues generated by the royalties received by Blackstone from us with respect to Leqvio sales do not reach a certain level by the end of 2029, Blackstone will be entitled to a higher royalty percentage beginning in 2030, which would have an adverse impact on our revenues beginning in 2030.
We may not be successful in entering into future alliances on terms favorable to us due to various factors, including our ability to demonstrate improved product profiles from our new technologies, including our IKARIA and GEMINI platforms, our ability to successfully demonstrate proof-of-concept for our technology in humans in certain tissues or disease areas, our ability to demonstrate the safety and efficacy of our specific drug candidates, our ability to manufacture or have third parties manufacture RNAi therapeutics, the strength of our intellectual property and/or concerns around challenges to our intellectual property. For example, the occurrence of a fatal thrombotic serious adverse event, or SAE, in our fitusiran study in 2017 and a subsequent pause in dosing and enrollment in fitusiran clinical studies in 2020 could contribute to further concerns about the safety of specific therapeutic candidates or therapeutic candidates for specific diseases. Even when we succeed in securing such alliances, we may not be able to maintain them if, for example, development or approval of a product candidate is delayed, challenges are raised as to the validity or scope of our intellectual property, we are unable to secure adequate reimbursement from payors or sales of an approved drug are lower than we expected.
Furthermore, any delay in entering into collaboration agreements would likely either delay the development and commercialization of certain of our product candidates and reduce their competitiveness even if they reach the market, or prevent the development of certain product candidates. Any such delay related to our collaborations could adversely affect our business.
For certain product candidates, we have formed collaborations to fund all or part of the costs of drug development and commercialization, such as our collaborations with Regeneron, Roche, Novartis, Vir, Dicerna and Sanofi. We may not, however, be able to enter into additional collaborations for certain other programs, and the terms of any collaboration agreement we do secure may not be favorable to us. If we are not successful in our efforts to enter into future collaboration arrangements with respect to one or more of our product candidates, we may not have sufficient funds or other resources to develop these product candidates or other product candidates internally, or to bring our product candidates to market. If we do not have sufficient funds to develop and bring our product candidates to market, we will not be able to generate revenues from these product candidates, and this will substantially harm our business.
If any collaborator materially amends, terminates or fails to perform its obligations under agreements with us, the development and commercialization of our product candidates could be delayed or terminated.
Our dependence on collaborators for capabilities and funding means that our business could be adversely affected if any collaborator materially amends or terminates its collaboration agreement with us or fails to perform its obligations under that agreement. Our current or future collaborations, if any, may not be scientifically or commercially successful. Disputes may arise in the future with respect to the ownership of rights to technology or products developed with collaborators, which could have an adverse effect on our ability to develop and commercialize any affected product candidate. Our current collaborations allow, and we expect that any future collaborations will allow, either party to terminate the collaboration for a material breach by the other party. In addition, our collaborators may have additional termination rights for convenience with respect to the collaboration or a particular program under the collaboration, under certain circumstances.
For example, our agreement with MDCO, which was acquired by Novartis in January 2020, relating to the development and commercialization of inclisiran worldwide may be terminated by Novartis at any time upon four months’ prior written notice, provided if the agreement is terminated by Novartis for convenience, Novartis must grant a license to us under certain of our technology developed in the course of MDCO's activities under the agreement, subject to a royalty to be negotiated between the parties. Moreover, any adverse actions by Novartis with respect to the MDCO License Agreement or disputes with Novartis regarding each party's rights and obligations under the MDCO License Agreement could adversely impact our ability to comply with our obligations under our agreements with Blackstone. If we were to lose a commercialization collaborator, we would have to attract a new collaborator or develop expanded sales, distribution and marketing capabilities internally, which would require us to invest significant amounts of financial and management resources.
In addition, if we have a dispute with a collaborator over the ownership of technology or other matters, or if a collaborator terminates its collaboration with us, for breach or otherwise, or determines not to pursue the research, development and/or commercialization of RNAi therapeutics, it could delay our development of product candidates, result in the need for additional company resources to develop product candidates, require us to expend time and resources to develop expanded sales and marketing capabilities on a more expedited timeline, make it more difficult for us to attract new collaborators and could adversely affect how we are perceived in the business and financial communities.
Moreover, a collaborator, or in the event of a change in control of a collaborator or the assignment of a collaboration agreement to a third party, the successor entity or assignee, as in the case of MDCO and Novartis, could determine that it is in its interests to:
•pursue alternative technologies or develop alternative products, either on its own or jointly with others, that may be competitive with the products on which it is collaborating with us or which could affect its commitment to the collaboration with us;
•pursue higher-priority programs or change the focus of its development programs, which could affect the collaborator’s commitment to us; or
•if it has marketing rights, choose to devote fewer resources to the marketing of our product candidates, if any are approved for marketing, than it does for product candidates developed without us.
If any of these occur, the development and commercialization of one or more products or product candidates could be delayed, curtailed or terminated because we may not have sufficient financial resources or capabilities to continue such development and commercialization on our own.
We expect to continue to grow our manufacturing capabilities and resources and we must incur significant costs to develop this expertise and/or rely on third parties to manufacture our products.
We have growing manufacturing capabilities, and in order to continue to commercialize our approved products, continue to develop our current product candidates, apply for regulatory approvals and, if approved, commercialize future products, we will need to continue to develop, contract for, or otherwise arrange for any necessary external manufacturing capabilities. Historically, our internal manufacturing capabilities were limited to small-scale production of material for use in in vitro and in vivo experiments and not required to be produced under current good manufacturing practice standards, or cGMP. During 2012, we developed cGMP, capabilities, and processes for the manufacture of patisiran formulated bulk drug product for late-stage clinical trial use and commercial supply. In addition, during 2020, we completed construction and qualification of our cGMP manufacturing facility in Norton, Massachusetts where we manufacture drug substances for early-stage clinical development with the possibility to manufacture drug substances for late-stage clinical development and, to the extent our products are approved, commercial use, in the future.
At the present time, we can only manufacture limited quantities of clinical trial drug substance ourselves, and otherwise we continue to rely on third party CMOs to manufacture additional drug substance and all of our finished drug products required for clinical and commercial use. There are a limited number of CMOs and we currently rely on a limited number of North American and European CMOs to manufacture our siRNA therapeutic products. There are risks inherent in pharmaceutical manufacturing that could affect the ability of our CMOs to meet our delivery time requirements or provide adequate amounts of material to meet our needs, and ultimately delay our clinical trials and potentially put at risk commercial supply, as well as result in additional expense to us. To fulfill our future requirements, we will likely need to secure additional CMOs and such alternative suppliers may be limited, not be readily available, or we may be unable to enter into agreements with them on reasonable terms and in a timely manner. As noted above, in order to ensure long-term supply capabilities for our RNAi therapeutics, we are developing our own capabilities to manufacture synthetic siRNA drug substances.
In addition to the manufacture of the synthetic siRNAs, we may have additional manufacturing requirements related to the technology required to deliver the siRNA to the relevant cell or tissue type, such as LNPs or conjugates or other drug delivery technologies. In some cases, the delivery technology we utilize is highly specialized or proprietary, and for technical and/or legal reasons, we may have access to only one or a limited number of potential manufacturers for such delivery technology. In addition, the scale-up of our delivery technologies could be very difficult and/or take significant time. We also have limited experience in such scale-up and manufacturing, requiring us to depend on a limited number of third parties, who might not be able to deliver in a timely manner, or at all. Failure by manufacturers to properly manufacture our delivery technology and/or formulate our siRNAs for delivery could result in unusable product, supply delays and drug shortages.
Furthermore, competition for supply from our manufacturers from other companies, a breach by such manufacturers of their contractual obligations or a dispute with such manufacturers would cause delays in our discovery and development efforts, as well as additional expense to us.
In developing manufacturing capabilities by building our own manufacturing facilities, we have incurred substantial expenditures, and expect to incur significant additional expenditures in the future. Also, we have had to, and will likely need to continue to recruit, hire, and train qualified employees to staff our facilities. If we are unable to manufacture sufficient quantities of material or if we encounter problems with our facilities in the future, we may also need to secure alternative suppliers, and such alternative suppliers may not be available, or we may be unable to enter into agreements with them on reasonable terms and in a timely manner. Given our dependence on a limited number of CMOs to supply our commercial products and clinical candidates, and our growing dependence on our own facility, any delay or setback in the manufacture of our products could impede ongoing clinical and commercial supply, which could significantly impact our revenues and operating results. In addition, to the extent we or our partners rely on CMOs to supply our product candidates, any delays or disruptions in supply could have a material adverse impact on the research and development activities and potential commercialization of our or our partners' product candidates.
The manufacturing process for our approved products and any other products that we may develop is subject to the FDA and foreign regulatory authority approval process and we will need to meet, and will need to contract with CMOs who can meet, all applicable FDA and foreign regulatory authority requirements on an ongoing basis. The failure of any CMO to meet required regulatory authority requirements could result in the delayed submission of regulatory applications, or delays in receiving regulatory approval for any of our or our current or future collaborators' product candidates. For example, in April 2022, due to an amendment to our vutrisiran NDA submission to address a pending inspection classification at a third-party secondary packaging and labeling facility, the FDA extended the review timeline of the NDA. In addition, if we receive the necessary regulatory approval for any product candidate, we also expect to rely on third parties, including potentially our commercial collaborators, to produce materials required for commercial supply.
To the extent that we have existing, or enter into future, manufacturing arrangements with third parties, we depend, and will depend in the future, on these third parties to perform their obligations in a timely manner and consistent with contractual and regulatory requirements, including those related to quality control and quality assurance. The failure of any CMO to perform its obligations as expected, or, to the extent we manufacture all or a portion of our product candidates ourselves, our failure to execute on our manufacturing requirements, could adversely affect our business in a number of ways, including:
•we or our current or future collaborators may not be able to initiate or continue clinical trials of product candidates that are under development;
•we or our current or future collaborators may be delayed in submitting regulatory applications, or receiving regulatory approvals, for our product candidates;
•we may lose the cooperation of our collaborators;
•our facilities and those of our CMOs, and our products could be the subject of inspections by regulatory authorities that could have a negative outcome and result in delays in supply;
•we may be required to cease distribution or recall some or all batches of our products or take action to recover clinical trial material from clinical trial sites; and
•ultimately, we may not be able to meet the clinical and commercial demands for our products.
We rely on third parties to conduct our clinical trials and source certain materials for our pre-clinical testing and studies, and if they fail to fulfill their obligations, our development plans may be adversely affected.
We rely on independent clinical investigators, CROs, and other third-party service providers to assist us in managing, monitoring and otherwise carrying out our clinical trials. We have contracted, and we plan to continue to contract with, certain third parties to provide certain services, including site selection, enrollment, monitoring, auditing and data management services. These investigators and CROs are not our employees and we have limited control over the amount of time and resources they dedicate to our programs. These third parties may have contractual relationships with other entities, some of which may be our competitors, which may draw their time and resources away from our programs. Although we depend heavily on these parties, we control only certain aspects of their activity and therefore, we cannot be assured that these third parties will adequately perform all of their contractual obligations to us in compliance with regulatory and other legal requirements and our internal policies and procedures. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards, and our reliance on third parties does not relieve us of our regulatory responsibilities. We and our CROs are required to comply with applicable good clinical practice, or GCP, requirements, which are regulations and guidelines enforced by the FDA and comparable foreign regulatory authorities for all of our product candidates in clinical development, and to implement timely corrective action to any non-compliance. Regulatory authorities enforce these GCP requirements through periodic inspections of trial sponsors, principal investigators and trial sites, including in connection with the review of marketing applications.
If we or any of our CROs fail to comply with applicable GCP requirements, or fail to take any such corrective action, the clinical data generated in our clinical trials may be deemed unreliable and the FDA, the EMA, the PMDA in Japan or comparable foreign regulatory authorities may require us to take additional action or perform additional clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority in the future, such regulatory authority will determine that any of our clinical trials comply with GCP regulations.
If our third-party service providers cannot adequately and timely fulfill their obligations to us for any reason, or if the quality and accuracy of our clinical trial data is compromised due to failure by such third party to adhere to our protocols or regulatory requirements or if such third parties otherwise fail to meet deadlines, our development plans and/or regulatory reviews for marketing approvals may be delayed or terminated. As a result, our stock price would likely be negatively impacted, and our results of operations and the commercial prospects for our product candidates would be harmed, our costs could increase and our ability to generate additional revenues could be delayed.
Before conducting clinical trials to demonstrate the safety and efficacy of our product candidates in humans in support of Investigational New Drug, or IND, applications or similar applications in other jurisdictions, we must complete pre-clinical studies, which includes animal studies. In addition, we rely on third-party service providers to source certain materials for such pre-clinical studies. Our ability to complete our pre-clinical studies is contingent on, among other things, our ability to source animals and other supplies required for the conduct of such studies. If we are unable to obtain such supplies, we may be unable to complete such pre-clinical studies in a timely manner or at all. For example, some of our IND-enabling toxicology and other studies require certain non-human primates that have customarily been imported from the People’s Republic of China, or the PRC, and Cambodia. The supply of these non-human primates is currently constrained due to factors such as their limited worldwide availability, trade relations between the U.S. and the PRC, and heightened scrutiny of non-human primates originating from Cambodia following allegations in late 2022 that certain Cambodian businesses and government officials may have engaged in the smuggling of non-human primates. We may encounter delays in obtaining a sufficient supply of such non-human primates to enable the conduct of our pre-clinical studies. Our inability to obtain access to a sufficient supply of these non-human primates in a timely manner or at all may impair our ability to complete pre-clinical studies to support IND applications or similar applications in other jurisdictions or delay the submission of such applications.
Risks Related to Managing Our Operations
If we are unable to attract and retain qualified key management and scientists, development, medical and commercial staff, consultants and advisors, our ability to implement our business plan may be adversely affected.
We are highly dependent upon our senior management and our scientific, clinical, sales and medical staff. The loss of the service of any of the members of our senior management could significantly delay or prevent the achievement of product development and commercialization, and other business objectives, and adversely impact our stock price. Our employment arrangements with our key personnel are terminable without notice. We do not carry key person life insurance on any of our employees.
We have grown our workforce significantly over the past several years and we face intense competition for qualified individuals from numerous pharmaceutical and biotechnology companies, universities, governmental entities and other research institutions, many of which have substantially greater resources with which to attract and reward qualified individuals than we do. In addition, if we are not successful commercializing our approved products, we may be unable to attract and retain highly qualified sales and marketing professionals to support our approved products and our future products, if approved. Accordingly, we may be unable to attract and retain suitably qualified individuals in order to support our growing research, development and global commercialization efforts and initiatives, and our failure to do so could have an adverse effect on our ability to implement our future business plans.
We may have difficulty expanding our operations successfully as we continue our evolution from a U.S.- and EU-based company primarily involved in discovery, pre-clinical testing and clinical development into a global company that develops and commercializes multiple drugs.
As we continue the commercial launches of our approved products, and increase the number of product candidates we are developing, we will need to continue to expand our operations in the U.S. and further develop operations in the EU and other geographies, including Asia and Latin America. To date, we have received regulatory approval for four products, which we have launched in multiple geographies globally, and we continue to expand the reach of these products with additional regulatory filings and launches.
We have grown our workforce significantly over the last several years and anticipate additional employee growth globally in the future as we focus on the commercialization of our approved products, and achieving our Alnylam P5x25 strategy. This growth has placed a strain on our administrative and operational infrastructure and, as a result, we will need to continue to develop additional and/or new infrastructure and capabilities to support our growth and obtain additional space to conduct our global operations in the U.S., the EU, Japan, Latin America and other geographies. If we are unable to develop such additional infrastructure or obtain sufficient space to accommodate our growth in a timely manner and on commercially reasonable terms, our business could be negatively impacted.
As we continue the commercialization of our approved products, and as the product candidates we develop enter and advance through clinical trials, we will need to continue to expand our global development, regulatory, manufacturing, quality, compliance, and marketing and sales capabilities, or contract with other organizations to provide these capabilities for us. In addition, as our operations continue to expand, we will need to successfully manage additional relationships with various collaborators, suppliers, distributors and other organizations. Our ability to manage our operations and future growth will require us to continue to enhance our operational, financial and management controls and systems, reporting systems and infrastructure, ethics and compliance functions, and policies and procedures. We may not be able to implement enhancements to our management information and control systems in an efficient or timely manner and may discover deficiencies in existing systems and controls.
The use of social media presents risks and challenges.
Social media is being used to communicate about our clinical development programs and the diseases our investigational RNAi therapeutics are being developed to treat, and we are utilizing what we believe is appropriate social media in connection with our commercialization efforts for our approved products, and we intend to do the same for our future products, if approved. Social media practices in the biopharmaceutical industry continue to evolve and regulations and regulatory guidance relating to such use are evolving and not always clear. This evolution creates uncertainty and risk of noncompliance with regulations applicable to our business, resulting in potential regulatory actions against us, along with the potential for litigation related to off-label marketing or other prohibited activities. For example, for our clinical-stage candidates, patients may use social media channels to comment on their experience in an ongoing blinded clinical study or to report an alleged adverse event, or AE. When such disclosures occur, there is a risk that study enrollment may be adversely impacted, we fail to monitor and comply with applicable AE reporting obligations or that we may not be able to defend our business or the public’s legitimate interests in the face of the political and market pressures generated by social media due to restrictions on what we may say about our investigational products. There is also a risk of inappropriate disclosure of sensitive information or negative or inaccurate posts or comments about us on any online platform, including a blog on the internet, or a post on a website, that can be distributed rapidly and could negatively harm our reputation. If any of these events were to occur or we otherwise fail to comply with applicable regulations, we could incur liability, face regulatory actions or incur other harm to our business.
Our business and operations could suffer in the event of system failures or unauthorized or inappropriate use of or access to our systems.
We are increasingly dependent on our information technology systems and infrastructure for our business. We collect, store and transmit sensitive information including intellectual property, proprietary business information, including highly sensitive clinical trial data, and personal information in connection with business operations. The secure maintenance of this information is critical to our operations and business strategy. Some of this information could be an attractive target of criminal attack or unauthorized access and use by third parties with a wide range of motives and expertise, including organized criminal groups, “hacktivists,” patient groups, disgruntled current or former employees and others. Cyber-attacks are of ever-increasing levels of sophistication, and despite our security measures, our information technology and infrastructure may be vulnerable to such attacks or may be breached, including due to employee error or malfeasance.
The pervasiveness of cybersecurity incidents in general and the risks of cyber-crime are complex and continue to evolve. Although we are making significant efforts to maintain the security and integrity of our information systems and are exploring various measures to manage the risk of a security breach or disruption, there can be no assurance that our security efforts and measures will be effective or that attempted security breaches or disruptions would not be successful or damaging. Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage or interruption from computer viruses, unauthorized or inappropriate access or use, natural disasters, pandemics or public health emergencies, terrorism, war (including the ongoing conflict in Ukraine), and telecommunication and electrical failures. Such events could cause interruption of our operations. For example, the loss of pre-clinical trial data or data from completed or ongoing clinical trials for our product candidates could result in delays in our regulatory filings and development efforts, as well as delays in the commercialization of our products, and significantly increase our costs. To the extent that any disruption, security breach or unauthorized or inappropriate use or access to our systems were to result in a loss of or damage to our data, or inappropriate disclosure of confidential or proprietary information, including but not limited to patient, employee or vendor information, we could incur notification obligations to affected individuals and government agencies, liability, including potential lawsuits from patients, collaborators, employees, stockholders or other third parties and liability under foreign, federal and state laws that protect the privacy and security of personal information, and the development and potential commercialization of our product candidates could be delayed.
Risks Related to Our Industry
Risks Related to Development, Clinical Testing and Regulatory Approval of Our Product Candidates and the Commercialization of Our Approved Products
Any product candidates we or our partners develop may fail in development or be delayed to a point where they do not become commercially viable.
Before obtaining regulatory approval for the commercial distribution of our product candidates, we must conduct, at our own expense, extensive nonclinical tests and clinical trials to demonstrate the safety and/or efficacy in humans of our product candidates. Nonclinical and clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome, and the historical failure rate for product candidates is high. We currently have multiple programs in clinical development, including internal and partnered programs in Phase 3 development, as well as several earlier-stage clinical programs. However, we may not be able to further advance any of our product candidates through clinical trials and regulatory approval.
Additionally, several of our planned and ongoing clinical trials utilize an “open-label” trial design. An “open-label” clinical trial is one where both the patient and investigator know whether the patient is receiving the investigational product candidate or either an existing approved drug or placebo. Most typically, open-label clinical trials test only the investigational product candidate and sometimes may do so at different dose levels. Open-label clinical trials are subject to various limitations that may exaggerate any therapeutic effect as patients in open-label clinical trials are aware when they are receiving treatment. Open-label clinical trials may be subject to a “patient bias” where patients perceive their symptoms to have improved merely due to their awareness of receiving an experimental treatment. In addition, open-label clinical trials may be subject to an “investigator bias” where those assessing and reviewing the physiological outcomes of the clinical trials are aware of which patients have received treatment and may interpret the information of the treated group more favorably given this knowledge. The results from an open-label trial may not be predictive of future clinical trial results with any of our product candidates for which we include an open-label clinical trial when studied in a controlled environment with a placebo or active control.
If we enter into clinical trials, the results from nonclinical testing or early or late stage clinical trials of a product candidate may not predict the results that will be obtained in subsequent subjects or in subsequent human clinical trials of that product candidate or any other product candidate. For example, we are conducting the HELIOS-B Phase 3 clinical trial of vutrisiran, which is investigating the potential of vutrisiran to treat the cardiac manifestations of disease in patients with ATTR amyloidosis with cardiomyopathy. While vutrisiran has demonstrated positive results in patients with hATTR amyloidosis with polyneuropathy, we cannot be certain that the results from HELIOS-B will be positive or that the results from HELIOS-B will support approval of vutrisiran for the treatment of patients with ATTR amyloidosis with cardiomyopathy. There is a high failure rate for drugs proceeding through clinical studies. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in clinical development even after achieving promising results in earlier studies, and any such setbacks in our clinical development, including with respect to vutrisiran, could have a material adverse effect on our business and operating results. Moreover, our approved products and our current product candidates, employ novel delivery technologies that, with the exception of inclisiran, have yet to be extensively evaluated in human clinical trials and proven safe and effective.
In addition, we, the FDA or other applicable regulatory authorities, or an institutional review board, or IRB, or similar foreign review board or committee, may delay initiation of or suspend clinical trials of a product candidate at any time for various reasons, including if we or they believe the healthy volunteer subjects or patients participating in such trials are being exposed to unacceptable health risks. Among other reasons, adverse side effects of a product candidate or related product on healthy volunteer subjects or patients in a clinical trial could result in our decision, or a decision by the FDA or foreign regulatory authorities, to suspend or terminate the trial, or, in the case of regulatory agencies, a refusal to approve a particular product candidate for any or all indications of use.
Clinical trials of a new product candidate require the enrollment of a sufficient number of patients, including patients who are suffering from the disease the product candidate is intended to treat and who meet other eligibility criteria. Rates of patient enrollment are affected by many factors, including the size of the patient population, the age and condition of the patients, the stage and severity of disease, the availability of clinical trials for other investigational drugs for the same disease or condition, the nature of the protocol, the proximity of patients to clinical sites, the availability of effective treatments for the relevant disease, and the eligibility criteria for the clinical trial. For example, we or our partners may experience difficulty enrolling our clinical trials, including, but not limited to, the ongoing clinical trials for fitusiran, due to the availability of existing approved treatments, as well as other investigational treatments in development. In addition, in November 2018 we announced that due to recruitment challenges, we had discontinued a Phase 2 study of cemdisiran in atypical hemolytic uremic syndrome and are focusing our cemdisiran clinical development efforts in a different indication. Delays or difficulties in patient enrollment, or difficulties retaining trial participants, including as a result of the availability of existing or other investigational treatments or safety concerns, including the impact of public health emergencies, can result in increased costs, longer development times or termination of a clinical trial.
Although our investigational RNAi therapeutics have been generally well-tolerated in our clinical trials to date, new safety findings may emerge. For example, in September 2017, we announced that we had temporarily suspended dosing in all ongoing fitusiran studies pending further review of a fatal thrombotic serious adverse event, or SAE, that occurred in a patient with hemophilia A without inhibitors who was receiving fitusiran in our Phase 2 open label extension, or OLE, study. More recently, in October 2020, Sanofi voluntarily paused dosing in all ongoing fitusiran clinical studies to assess reports of non-fatal thrombotic events in patients participating in the ATLAS Phase 3 program. Following an assessment of available data and alignment with regulators, patients restarted on fitusiran under amended protocols in ongoing clinical studies. In October 2021, Sanofi announced that a potential filing date for fitusiran had been moved to 2024 due to the introduction of a revised dosing regimen in the ongoing phase 3 studies.
As demonstrated by the discontinuation of our revusiran program in October 2016, the temporary suspension of dosing in September 2017 in our fitusiran studies, as well as Sanofi's voluntary pause of fitusiran studies in October 2020, the occurrence of SAEs and/or AEs can result in the suspension or termination of clinical trials of a product candidate by us, our partners, or the FDA or a foreign regulatory authority. The occurrence of SAEs and/or AEs could also result in refusal by the FDA or a foreign regulatory authority to approve a particular product candidate for any or all indications of use.
Clinical trials also require the review, oversight and approval of IRBs, or, outside of the U.S., an independent ethics committee, which continually review clinical investigations and protect the rights and welfare of human subjects. Inability to obtain or delay in obtaining IRB or ethics committee approval can prevent or delay the initiation and completion of clinical trials, and the FDA or foreign regulatory authorities may decide not to consider any data or information derived from a clinical investigation not subject to initial and continuing IRB or ethics committee review and approval, as the case may be, in support of a marketing application.
Our product candidates that we develop may encounter problems during clinical trials that will cause us, an IRB, ethics committee or regulatory authorities to delay, suspend or terminate these trials, or that will delay or confound the analysis of data from these trials. If we experience any such problems, we may not have the financial resources to continue development of the product candidate that is affected, or development of any of our other product candidates. We may also lose, or be unable to enter into, collaborative arrangements for the affected product candidate and for other product candidates we are developing.
A failure of one or more of our clinical trials can occur at any stage of testing. We may experience numerous unforeseen events during, or as a result of, nonclinical testing and the clinical trial process that could delay or prevent regulatory approval or our ability to commercialize our product candidates, including:
•our nonclinical tests or clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional nonclinical testing or clinical trials, or we may abandon projects that we expect to be promising;
•delays in filing IND applications or comparable foreign applications or delays or failure in obtaining the necessary approvals from regulators or IRBs/ethics committees in order to commence a clinical trial at a prospective trial site, or their suspension or termination of a clinical trial once commenced;
•conditions imposed on us by an IRB or ethics committee, or the FDA or comparable foreign authorities regarding the scope or design of our clinical trials;
•problems in engaging IRBs or ethics committees to oversee clinical trials or problems in obtaining or maintaining IRB or ethics committee approval of trials;
•delays in enrolling patients and volunteers into clinical trials, and variability in the number and types of patients and volunteers available for clinical trials, including as a result of the COVID-19 pandemic, a future pandemic or public health emergency and the ongoing conflict in Ukraine;
•disruptions caused by man-made or natural disasters or pandemics, epidemics or public health emergencies or other business interruptions;
•high drop-out rates for patients and volunteers in clinical trials;
•negative or inconclusive results from our clinical trials or the clinical trials of others for product candidates similar to ours;
•inadequate supply or quality of product candidate materials or other materials necessary for the conduct of our clinical trials or disruption or delays in the clinical supply due to the COVID-19 or a future pandemic or public health emergency;
•greater than anticipated clinical trial costs;
•serious and unexpected drug-related side effects experienced by participants in our clinical trials or by individuals using drugs similar to our product candidates;
•poor or disappointing effectiveness of our product candidates during clinical trials;
•unfavorable FDA or other regulatory agency inspection and review of a clinical trial site or records of any clinical or nonclinical investigation;
•failure of our third-party contractors or investigators to comply with regulatory requirements, including GCP and cGMP, or otherwise meet their contractual obligations in a timely manner, or at all;
•governmental or regulatory delays and changes in regulatory requirements, policy and guidelines, including the imposition of additional regulatory oversight around clinical testing generally or with respect to our technology in particular; or
•interpretations of data by the FDA and similar foreign regulatory agencies that differ from ours.
Even if we successfully complete clinical trials of our product candidates, any given product candidate may not prove to be a safe and effective treatment for the disease for which it was being tested.
We or our partners may be unable to obtain U.S. or foreign regulatory approval for our or our partnered product candidates and, as a result, we or our partners may be unable to commercialize such product candidates.
Our and our partnered product candidates are subject to extensive governmental regulations relating to, among other things, research, testing, development, manufacturing, safety, efficacy, approval, recordkeeping, reporting, labeling, storage, pricing, marketing and distribution of drugs. Rigorous nonclinical testing and clinical trials and an extensive regulatory approval process are required to be successfully completed in the U.S. and in many foreign jurisdictions before a new drug can be marketed. Satisfaction of these and other regulatory requirements is costly, time consuming, uncertain and subject to unanticipated delays. It is possible that the product candidates we and our partners are developing will not obtain the regulatory approvals necessary for us or our collaborators to begin selling them, or, in the case of vutrisiran, will not obtain regulatory approval to be sold for broader indications than are currently approved. It is also possible that the FDA or other regulatory authorities may determine that the data generated in clinical trials for a product candidate is not sufficient to support the approval of an application for regulatory approval. For example, although we reported positive results from the APOLLO-B Phase 3 study of patisiran in patients with the cardiomyopathy of ATTR amyloidosis, and received a 9:3 vote from the FDA’s CRDAC that patisiran's benefits outweighed its risks for the treatment of the cardiomyopathy of ATTR amyloidosis, in October 2023, the FDA issued a CRL in response to our sNDA for patisiran, indicating the sNDA could not be approved in its present form.
The time required to obtain FDA and other regulatory approvals is unpredictable but typically takes many years following the commencement of clinical trials, depending upon the type, complexity and novelty of the product candidate. The standards that the FDA and its foreign counterparts use when regulating us are not always applied predictably or uniformly and can change. Any analysis we perform of data from nonclinical and clinical activities is subject to confirmation and interpretation by regulatory authorities, which could delay, limit or prevent regulatory approval. We or our partners may also encounter unexpected delays or increased costs due to new government regulations, for example, from future legislation or administrative action, or from changes in FDA policy during the period of product development, clinical trials and FDA regulatory review. It is impossible to predict whether legislative changes will be enacted, or whether FDA or foreign regulations, guidance or interpretations will be changed, or what the impact of such changes, if any, may be.
Because the drugs we or our partners are developing represent a new class of drug, the FDA and its foreign counterparts have not yet established any definitive policies, practices or guidelines in relation to these drugs. The lack of policies, practices or guidelines may hinder or slow review by the FDA of any regulatory filings that we or our partners may submit. Moreover, the FDA may respond to these submissions by defining requirements we or our partners may not have anticipated. Such responses could lead to significant delays and increased costs in the development of our or our partnered product candidates. In addition, because there may be approved treatments for some of the diseases for which we or our partners may seek approval, including vutrisiran for the treatment of ATTR amyloidosis with cardiomyopathy, or treatments in development which are approved by the time we or they apply for approval, in order to receive regulatory approval, we or they may need to demonstrate through clinical trials that the product candidates we develop to treat these diseases, if any, are not only safe and effective, but safer or more effective than existing products. Interruption or delays in the operations of the FDA, EMA and comparable foreign regulatory agencies may impact the review, inspection and approval timelines for our or our partnered product candidates. During the COVID-19 public health emergency, the FDA worked to ensure timely reviews of applications for medical products in line with its user fee performance goals and conducted mission critical domestic and foreign inspections to ensure compliance of manufacturing facilities with FDA quality standards. In addition, during the COVID-19 public health emergency, a number of companies announced receipt of complete response letters due to the FDA's inability to complete required inspections for their applications. In December 2020, the FDA issued a complete response letter regarding Novartis' NDA for inclisiran, stating that the agency could not approve the NDA by the PDUFA action date due to unresolved facility inspection-related conditions. In July 2021, Novartis announced that the resubmission to the FDA of the inclisiran NDA to address the complete response letter was filed, and the FDA approved Leqvio (which is the trade name under which inclisiran is marketed in the U.S.) in December 2021. The delay in the approval of Leqvio resulted in delayed milestone and royalty revenue to us. Any similar interruption or delay by the FDA, EMA or comparable foreign regulatory agency could have a material adverse effect on our efforts to obtain regulatory approval for our product candidates, which could have a material adverse effect on our financial results.
For instance, the FDA may request additional clinical or other data or information in connection with the regulatory review of our or our partners’ product candidates, including by issuing a complete response letter which may require that we or our partners’ submit additional clinical or other data or impose other conditions that must be met in order to secure final approval of our or our partners’ NDA applications, including potentially requiring a facility inspection. Even if such data and information are submitted, or any such inspection is completed, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval.
Any delay or failure in obtaining required approvals for our product candidates or our partnered product candidates could have a material adverse effect on our ability to generate revenues from any product candidate for which we or our partners may seek approval in the future. For example, as a result of the recent CRL from the FDA in response to our sNDA for patisiran as a potential treatment for the cardiomyopathy of ATTR amyloidosis, our ability to generate product revenues for patisiran will be negatively impacted. Furthermore, any regulatory approval to market any product may be subject to limitations on the approved uses for which we or our partners may market the product or the labeling or other restrictions, which could limit each such product’s market opportunity and have a negative impact on our results of operations and our stock price. In addition, the FDA has the authority to require a Risk Evaluation and Mitigation Strategy, or REMS, plan as part of an NDA, or after approval, which may impose further requirements or restrictions on the distribution or use of an approved drug, such as limiting prescribing to certain physicians or medical centers that have undergone specialized training, limiting treatment to patients who meet certain safe-use criteria and requiring treated patients to enroll in a registry. In the EU, we or our partners could be required to adopt a similar plan, known as a risk management plan, and our products could be subject to specific risk minimization measures, such as restrictions on prescription and supply, the conduct of post-marketing safety or efficacy studies, or the distribution of patient and/or prescriber educational materials. In either instance, these limitations and restrictions may limit the size of the market for the product and affect reimbursement by third-party payors.
We are also subject to numerous foreign regulatory requirements governing, among other things, the conduct of clinical trials, manufacturing and marketing authorization, pricing and third-party reimbursement. The foreign regulatory approval process varies among countries and includes all of the risks associated with FDA approval described above as well as risks attributable to the satisfaction of local regulations in foreign jurisdictions. Approval by the FDA does not ensure approval by regulatory authorities outside the U.S. and vice versa.
Even if we or our partners obtain regulatory approvals, our marketed drugs will be subject to ongoing regulatory oversight. If we or our partners fail to comply with continuing U.S. and foreign requirements, our approvals could be limited or withdrawn, we could be subject to other penalties, and our business would be seriously harmed.
Following any initial regulatory approval of drugs we or our partners may develop, including our four approved drugs, we will also be subject to continuing regulatory oversight, including the review of adverse drug experiences and clinical results that are reported after our drug products are made commercially available. This would include results from any post-marketing tests or surveillance to monitor the safety and efficacy of our approved drugs or other drug products required as a condition of approval or agreed to by us. The regulatory approvals that we receive for ONPATTRO, AMVUTTRA, GIVLAARI and OXLUMO, as well as any regulatory approvals we receive for any other product candidates may also be subject to limitations on the approved uses for which the product may be marketed, including any expanded label for AMVUTTRA. Other ongoing regulatory requirements include, among other things, submissions of safety and other post-marketing information and reports, registration and listing, as well as continued compliance with good practice quality guidelines and regulations, including cGMP requirements and GCP requirements for any clinical trials that we conduct post-approval. In addition, we are conducting, and intend to continue to conduct, clinical trials for our product candidates, and we intend to seek approval to market our product candidates, in jurisdictions outside of the U.S., and therefore will be subject to, and must comply with, regulatory requirements in those jurisdictions.
The FDA has significant post-market authority, including, for example, the authority to require labeling changes based on new safety information and to require post-market studies or clinical trials to evaluate serious safety risks related to the use of a drug and to require withdrawal of the product from the market. The FDA also has the authority to require a REMS plan after approval, which may impose further requirements or restrictions on the distribution or use of an approved drug. As our approved products are used commercially, we or others could identify previously unknown side effects or known side effects could be observed as being more frequent or severe than in clinical studies or earlier post-marketing periods, in which case:
•sales of our approved products may be more modest than originally anticipated;
•regulatory approvals for our approved products may be restricted or withdrawn;
•we may decide, or be required, to send product warning letters or field alerts to physicians, pharmacists and hospitals;
•additional nonclinical or clinical studies, changes in labeling, adoption of a REMS plan, or changes to manufacturing processes, specifications and/or facilities may be required; and
•government investigations or lawsuits, including class action suits, may be brought against us.
Any of the above occurrences could reduce or prevent sales of our approved products, increase our expenses and impair our ability to successfully commercialize one or more of these products.
The CMO and manufacturing facilities we use to make our approved products and certain of our current product candidates, including our Cambridge facility, our Norton facility, as well as facilities at Agilent and other CMOs, will also be subject to periodic review and inspection by the FDA and other regulatory agencies. For example, Agilent and our Cambridge-based facility were subject to regulatory inspection by the FDA and the EMA in connection with the review of our applications for regulatory approval for ONPATTRO and GIVLAARI, and may be subject to similar inspection in connection with any subsequent applications for regulatory approval of one or more of our products filed in other territories. The discovery of any new or previously unknown problems with our facilities or our CMOs, or our or their manufacturing processes or facilities, may result in restrictions on the drug or CMO or facility, including delay in approval or, in the future, withdrawal of the drug from the market. For example, due to a routine inspection by the FDA at a CMO facility that resulted in a pending inspection classification, we amended our regulatory submission for vutrisiran, which delayed our PDUFA goal date and AMVUTTRA's FDA approval. We have developed cGMP capabilities and processes for the manufacture of patisiran formulated bulk drug product for commercial use. In addition, in 2020, we completed construction of a cGMP manufacturing facility for drug substance for clinical and, eventually, commercial use. We may not have the ability or capacity to manufacture material at a broader commercial scale in the future. We may manufacture clinical trial materials, or we may contract a third party to manufacture this material for us. Reliance on CMOs entails risks to which we would not be subject if we manufactured products ourselves, including reliance on the CMO for regulatory compliance.
If we or our collaborators, CMOs or service providers fail to comply with applicable continuing regulatory requirements in the U.S. or foreign jurisdictions in which we may seek to market our products, we or they may be subject to, among other things, fines, warning letters, holds on clinical trials, refusal by the FDA or foreign regulatory authorities to approve pending applications or supplements to approved applications, suspension or withdrawal of regulatory approval, product recalls and seizures, refusal to permit the import or export of products, operating restrictions, injunction, civil penalties and criminal prosecution.
We may incur significant liability if enforcement authorities allege or determine that we are engaging in commercial activities or promoting our commercially approved products in a way that violates applicable regulations.
Physicians have the discretion to prescribe approved drug products for uses that are not described in the product’s labeling and that differ from those approved by the FDA or other applicable regulatory agencies. Off-label uses are common across medical specialties. Although the FDA and other regulatory agencies that approve drug products do not regulate a physician’s practice of medicine or choice of treatments, the FDA and other regulatory agencies regulate a manufacturer’s communications regarding off-label use and prohibit off-label promotion, as well as the dissemination of false or misleading labeling or promotional materials, including by their agents. Manufacturers and their agents may not promote drugs for off-label uses or provide off-label information in the promotion of drug products that is not consistent with the approved labeling for those products. For example, we may not promote ONPATTRO or AMVUTTRA in the U.S. for use in any indications other than the treatment of the polyneuropathy of hATTR amyloidosis in adults. The FDA and other regulatory and enforcement authorities actively enforce laws and regulations prohibiting promotion of off-label uses and the promotion of products for which marketing approval has not been obtained, and if in the future we are found to have improperly marketed or promoted any of our commercial products, we may be subject to a broad range of civil, administrative and criminal penalties, including injunctive relief related to such commercial products’ promotional activities, substantial fines or penalties, and other legal or equitable sanctions. Any adverse decision, finding, allegation, or exercise of enforcement or regulatory discretion could harm our business, prospects, operating results, and financial condition. Other internal or government investigations or legal or regulatory proceedings, including lawsuits brought by private litigants, may also follow as a consequence.
Notwithstanding regulations related to product promotion, the FDA and other regulatory authorities allow companies to engage in truthful, non-misleading and non-promotional scientific exchange concerning their products, and we intend to engage in medical education activities and communicate with healthcare providers in compliance with all applicable laws and regulatory guidance. Nonetheless, the FDA, other applicable regulatory authorities, competitors, and other third parties may take the position that we are not in compliance with such regulations, and if such non-compliance is proven, it could harm our reputation, financial condition or divert financial and management resources from our core business, and would have a material adverse effect on our business, financial condition and results of operations. Moreover, any threatened or actual government enforcement actions or lawsuits by third parties could also generate adverse publicity, which could decrease demand for our products and require that we devote substantial resources that could be used productively on other aspects of our business.
In addition to our medical education efforts, we also offer patient support services to assist patients receiving treatment with our commercially approved products. Manufacturers have increasingly become the focus of government investigation of patient support programs based on allegations that through such services illegal inducements are provided to physicians and/or patients, leading to improper utilization of government resources through Medicare, Medicaid and other government programs. Companies that are found to have violated laws such as the federal Anti-Kickback Statute and/or the federal False Claims Act, or FCA, face significant liability, including civil and administrative penalties, criminal sanctions, and potential exclusion from participation in government programs.
As described above, we remain focused on our global compliance program, which is designed to support the execution of these programs and activities in compliance with applicable laws.
Even if we receive regulatory approval to market our product candidates, the market may not be receptive to our product candidates upon their commercial introduction, which could prevent us from becoming profitable.
The product candidates that we are developing are based upon new technologies or therapeutic approaches. Key participants in pharmaceutical marketplaces, such as physicians, third-party payors and consumers, may not accept a product intended to improve therapeutic results based on RNAi technology. As a result, it may be more difficult for us to convince the medical community and third-party payors to accept and use our product, or to provide favorable reimbursement.
Other factors that we believe will materially affect market acceptance of our product candidates include:
•the timing of our receipt of any marketing approvals, the terms of any approvals and the countries in which approvals are obtained;
•the safety and efficacy of our product candidates, as demonstrated in clinical trials and as compared with alternative treatments, if any;
•relative convenience and ease of administration of our product candidates;
•the willingness of patients to accept potentially new routes of administration or new or different therapeutic approaches and mechanisms of action;
•the success of our physician education programs;
•the availability of adequate government and third-party payor reimbursement;
•the pricing of our products, particularly as compared to alternative treatments, and the market perception of such prices and any price increase that we may implement in the future; and
•availability of alternative effective treatments for the diseases that product candidates we develop are intended to treat and the relative risks, benefits and costs of those treatments.
For example, one of our two commercially approved therapeutics for the treatment of the polyneuropathy of hATTR amyloidosis in adults, ONPATTRO, utilizes an intravenous mode of administration with pre-medication that physicians and/or patients may not readily adopt, or which may not compete favorably with other available options, including inotersen, marketed by Ionis in several countries, which is administered subcutaneously, or tafamidis, marketed by Pfizer in several countries, which is in pill form. In addition, fitusiran represents a new approach to treating hemophilia which may not be readily accepted by patients and their caregivers. Assuming positive Phase 3 results, vutrisiran, if approved for the treatment of ATTR amyloidosis with cardiomyopathy, could face similar challenges in market acceptance.
We are a multi-product commercial company and expect to continue to invest significant financial and management resources to continue to build our marketing, sales, market access and distribution capabilities and further establish our global infrastructure. Even if we successfully scale our commercial capabilities, the market may not be receptive to our commercial products.
Having received our first product approval in August 2018, we have established our capabilities for marketing, sales, market access and distribution over the last several years. We currently expect to rely on third parties to launch and market certain of our product candidates in certain geographies, if approved. However, we are commercializing ONPATTRO, AMVUTTRA, GIVLAARI and OXLUMO, and intend to commercialize several of our late-stage product candidates, if approved, on our own globally in major markets. Accordingly, we have developed internal marketing, sales, market access and distribution capabilities as part of our core product strategy initially in the U.S., Europe and Japan, with expansion ongoing globally, which has, and will continue to, require significant financial and management resources. For those products for which we will perform marketing, sales, market access and distribution functions ourselves, including ONPATTRO, AMVUTTRA, GIVLAARI and OXLUMO, and for future products we successfully develop where we may retain certain product development and commercialization rights, we could face a number of additional risks, including:
•scaling and retaining our global sales, marketing and administrative infrastructure and capabilities;
•hiring, training, managing and supervising our personnel worldwide;
•the cost of further developing, or leveraging an established, marketing or sales force, which may not be justifiable in light of the revenues generated by any particular product and/or in any specific geographic region; and
•our direct sales and marketing efforts may not be successful.
If we are unable to continue to develop and scale our own global marketing, sales, market access and distribution capabilities for our current and any future products, we will not be able to successfully commercialize our products without reliance on third parties.
The patient populations suffering from hATTR amyloidosis, AHP and PH1 are small and have not been established with precision. If the actual number of patients is smaller than we estimate, or if we cannot raise awareness of these diseases and diagnosis is not improved, our revenue and ability to achieve profitability from these products may be adversely affected.
Our estimates regarding the potential market size for ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO or any future products at the time we commence commercialization, may be materially different from the actual market size, including as a result of the indication approved by regulatory authorities, which could result in significant changes in our business plan and may have a material adverse effect on our results of operations and financial condition. In addition, our efforts to raise disease awareness and improve diagnosis of our relevant disease states were impacted by the COVID-19 pandemic. For example, in 2020 and 2021, we saw a reduction in peer to peer educational opportunities, reduced physician attendance at congresses and symposia and overall opportunities for physician engagement. As is the case with most orphan diseases, if we are unable to successfully raise awareness of these diseases and improve diagnosis, it will be more difficult or impossible to achieve profitability.
Any drugs we develop may become subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives, thereby harming our business.
The regulations that govern marketing approvals, pricing and reimbursement for new drugs vary widely from country to country. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. We are actively monitoring these regulations as we market and sell our approved products and as several of our other programs move through late stages of development. However, a number of our programs are currently in the earlier stages of development, and we will not be able to assess the impact of price regulations for such programs for a number of years. We might also obtain regulatory approval for a product, including one or more of our approved products, in a particular country, but then be subject to price regulations or price controls that delay our commercial launch of the product and negatively impact the revenues we are able to generate from the sale of the product in that country and potentially in other countries due to reference pricing.
Our ability to commercialize our approved products or any future products successfully also will depend in part on the extent to which reimbursement for these products and related treatments will be available from government health administration authorities, private health insurers and other organizations. One or more of our approved products and other products for which we are able to obtain marketing approval may not be considered cost-effective, and the amount reimbursed may be insufficient to allow us to sell such product(s) or any future products on a competitive basis. Increasingly, the third-party payors who reimburse patients or healthcare providers, such as government and private insurance plans, are requiring that drug companies provide them with predetermined discounts from list prices, and are seeking to reduce the prices charged or the amounts reimbursed for drug products. In the U.S., we have entered into over 40 value-based agreements, or VBAs, and are negotiating additional VBAs with commercial health insurers. The goal of these agreements is to ensure that we are paid based on the ability of our commercially approved products to deliver results in the real world setting comparable to those demonstrated in clinical trials, and the agreements are structured to link the performance of our approved products in real-world use to financial terms. Partnering with payers on these agreements is also intended to provide more certainty to them for their investment and help accelerate coverage decisions for patients. If the payment we receive for our products, or the reimbursement provided for such products, is inadequate in light of our development and other costs, or if reimbursement is denied, our return on investment could be adversely affected. In addition, we have stated publicly that we intend to grow through continued scientific innovation rather than arbitrary price increases. Specifically, we have stated that we will not raise the price of any product for which we receive marketing approval over the rate of inflation, as determined by the consumer price index for urban consumers (approximately 3.7% currently) absent a significant value driver. Our patient access philosophy could also negatively impact the revenues we are able to generate from the sale of one or more of our products in the future.
Some of the drugs we market need to be administered under the supervision of a physician or other healthcare professional on an outpatient basis, including ONPATTRO, AMVUTTRA, GIVLAARI and OXLUMO. Under currently applicable U.S. law, certain drugs that are not usually self-administered (including injectable drugs) may be eligible for coverage under the Medicare Part B program if:
•they are incident to a physician’s services;
•they are reasonable and necessary for the diagnosis or treatment of the illness or injury for which they are administered according to accepted standards of medical practice; and
•they have been approved by the FDA and meet other requirements of the statute.
There may be significant delays in obtaining coverage for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or foreign regulatory authorities. Moreover, eligibility for coverage does not imply that any drug will be reimbursed in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution or that covers a particular provider’s cost of acquiring the drug. Interim payments for new drugs, if applicable, may also not be sufficient to cover our costs and may not be made permanent.
Reimbursement may be based on payments allowed for lower-cost drugs that are already reimbursed, may be incorporated into existing payments for other services and may reflect budgetary constraints or imperfections in Medicare data. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the U.S. In particular, governments in certain markets such as in EU, the U.K., Japan, and China, provide healthcare at low (or zero) direct costs to consumers at the point of care, and thus have significant power as large single payers to regulate prices or impose other cost control mechanisms.
President Biden signed an Executive Order on July 9, 2021 affirming the administration’s policy to (i) support legislative reforms that would lower the prices of prescription drugs, including by allowing Medicare to negotiate drug prices, by imposing inflation caps, and, by supporting the development and market entry of lower-cost generic drugs and biosimilars; and (ii) support the enactment of a public health insurance option. Among other things, the Executive Order also directs the U.S. Department of Health and Human Services, or HHS, to provide a report on actions to combat excessive pricing of prescription drugs, continue to clarify and improve the approval framework for generic drugs and identify and address any efforts to impede generic drug competition, enhance the domestic drug supply chain, reduce the price that the Federal government pays for drugs, and address price gouging in the industry; and directs the FDA to work with states and Indian Tribes that propose to develop section 804 Importation Programs in accordance with the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, and the FDA’s implementing regulations. The FDA released such implementing regulations on September 24, 2020, which went into effect on November 30, 2020, providing guidance for states to build and submit importation plans for drugs from Canada. In response, authorities in Canada have passed rules designed to safeguard the Canadian drug supply from shortages. If implemented, importation of drugs from Canada and the Most Favored Nation, or MFN, Model may materially and adversely affect the price we receive for any of our commercially approved products. Further, on November 20, 2020, the Centers for Medicare and Medicaid Services, or CMS, issued an Interim Final Rule implementing the MFN Model under which Medicare Part B reimbursement rates will be calculated for certain drugs based on the lowest price drug manufacturers receive in OECD countries with a similar gross domestic product per capita. The MFN Model regulations mandate participation by identified Part B providers and would have applied to all U.S. states and territories for a seven-year period beginning January 1, 2021, and ending December 31, 2027. However, on December 29, 2021, CMS rescinded the proposed MFN rule. Additionally, on December 2, 2020, HHS published a regulation removing safe harbor protection for price reductions from pharmaceutical manufacturers to plan sponsors under Part D, either directly or through pharmacy benefit managers, unless the price reduction is required by law. The rule also creates a new safe harbor for price reductions reflected at the point-of-sale, as well as a safe harbor for certain fixed fee arrangements between pharmacy benefit managers and manufacturers. Pursuant to court order, the removal and addition of the aforementioned safe harbors were delayed until January 1, 2023, requiring manufacturers to ensure the full value of co-pay assistance is passed on to the patient or these dollars will count toward the Average Manufacturer Price and Best Price calculation of the drug. On May 17, 2022, the U.S. District Court for the District of Columbia granted the Pharmaceutical Research and Manufacturers of America’s motion for summary judgement invalidating the accumulator adjustment rule. Although a number of these and other proposed measures may require authorization through additional legislation to become effective, and the current U.S. presidential administration may reverse or otherwise change these measures, both the current U.S. presidential administration and Congress have indicated that they will continue to seek new legislative measures to control drug costs.
We believe that the efforts of governments and third-party payors to contain or reduce the cost of healthcare and legislative and regulatory proposals to broaden the availability of healthcare will continue to affect the business and financial condition of pharmaceutical and biopharmaceutical companies. In the U.S., pharmaceutical pricing is subject to both government and public scrutiny and calls for reform, and the government has continued to focus on legislative and regulatory changes designed to control costs. Specifically, there have been several recent U.S. Congressional inquiries and proposed federal and state legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs.
A number of other legislative and regulatory changes in the healthcare system in the U.S. and other major healthcare markets have been proposed or enacted in recent months and years, and such efforts have expanded substantially in recent years. These developments could, directly or indirectly, affect our ability to sell ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO or future products, if approved, at a favorable price.
In particular, in March 2010, the Patient Protection and Affordable Care Act, also referred to as the Affordable Care Act, or the ACA, was signed into law. This legislation changed the system of healthcare insurance and benefits intended to broaden coverage and control costs. The law also contains provisions that affect companies in the pharmaceutical industry and other healthcare related industries by imposing additional costs and changes to business practices. Among the provisions affecting pharmaceutical companies are the following:
•Mandatory rebates for drugs sold into the Medicaid program were increased, and the rebate requirement was extended to drugs used in risk-based Medicaid managed care plans.
•The 340B Drug Pricing Program under the Public Health Service Act was extended to require mandatory discounts for drug products sold to certain critical access hospitals, cancer hospitals and other covered entities.
•Pharmaceutical companies are required to offer discounts on brand-name drugs to patients who fall within the Medicare Part D coverage gap, commonly referred to as the “donut hole.”
•Pharmaceutical companies are required to pay an annual non-tax deductible fee to the federal government based on each company’s market share of prior year total sales of branded products to certain federal healthcare programs, such as Medicare, Medicaid, Department of Veterans Affairs and Department of Defense. Since we expect our branded pharmaceutical sales to constitute a small portion of the total federal healthcare program pharmaceutical market, we do not expect this annual assessment to have a material impact on our financial condition.
•The law provides that approval of an application for a follow-on biologic product may not become effective until 12 years after the date on which the reference innovator biologic product was first licensed by the FDA, with a possible six-month extension for pediatric products. After this exclusivity ends, it will be easier for generic manufacturers to enter the market, which is likely to reduce the pricing for such products and could affect our profitability.
•The law creates a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected.
•The law expands eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to certain individuals with income at or below 133% of the federal poverty level, thereby potentially increasing a manufacturer’s Medicaid rebate liability.
•The law expands the entities eligible for discounts under the Public Health Service Act pharmaceutical pricing program.
•The law expands healthcare fraud and abuse laws, including the civil FCA and the federal Anti-Kickback Statute, new government investigative powers, and enhanced penalties for noncompliance.
•The law establishes new requirements to report financial arrangements with physicians and teaching hospitals and to annually report drug samples that manufacturers and distributors provide to physicians.
•The law establishes a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research.
•The law established the Center for Medicare and Medicaid Innovation within CMS to test innovative payment and service delivery methods.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. In August 2011, the Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. These changes included aggregate reductions to Medicare payments to providers of 2% per fiscal year. These reductions went into effect on April 1, 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2030, with the exception of a temporary suspension from May 1, 2020 through March 31, 2022 due to the COVID-19 pandemic. Following the suspension, a 1% payment reduction began on April 1, 2022, lasting through June 30, 2022. The 2% payment reduction resumed on July 1, 2022. The American Taxpayer Relief Act of 2012, among other things, reduced Medicare payments to several providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding and otherwise affect the prices we may obtain for our approved products or any of our product candidates for which we may obtain regulatory approval, or the frequency with which our products or any future product is prescribed or used.
Further, there have been several changes to the 340B Drug Pricing Program, which imposes ceilings on prices that drug manufacturers can charge for medications sold to certain healthcare facilities. On December 27, 2018, the District Court for the District of Columbia invalidated a reimbursement formula change under the 340B Drug Pricing Program, and CMS subsequently altered the fiscal years 2019 and 2018 reimbursement formula on specified covered outpatient drugs. The court ruled this change was not an “adjustment” which was within the Secretary’s discretion to make but was instead a fundamental change in the reimbursement calculation. However, most recently, on July 31, 2020, the U.S. Court of Appeals for the District of Columbia Circuit overturned the district court’s decision and found that the changes were within the Secretary’s authority. On September 14, 2020, the plaintiffs-appellees filed a Petition for Rehearing En Banc (i.e., before the full court), and the court denied this petition on October 16, 2020. Plaintiffs-appellees filed a petition for a writ of certiorari at the Supreme Court on February 10, 2021. On July 2, 2021, the Supreme Court granted the petition. On June 15, 2022, the Supreme Court unanimously reversed the Court of Appeals’ decision, holding that HHS’s 2018 and 2019 reimbursement rates for 340B hospitals were contrary to the statute and unlawful. It is unclear how these developments could affect covered hospitals who might purchase our future products and affect the rates we may charge such facilities for our approved products in the future, if any.
The Inflation Reduction Act of 2022, or IRA, which among other things, allows for CMS to negotiate prices for certain single-source drugs and biologics reimbursed under Medicare Part B and Part D, beginning with ten high-cost drugs paid for by Medicare Part D starting in 2026, followed by 15 Part D drugs in 2027, 15 Part B or Part D drugs in 2028, and 20 Part B or Part D drugs in 2029 and beyond. The legislation subjects drug manufacturers to civil monetary penalties and a potential excise tax for failing to comply with the legislation by offering a price that is not equal to or less than the negotiated “maximum fair price” under the law or for taking price increases that exceed inflation. The legislation also requires manufacturers to pay rebates for drugs in Medicare Part D whose price increases exceed inflation. Further, the legislation caps Medicare beneficiaries’ annual out-of-pocket drug expenses at $2,000. Under the IRA, a second FDA approval for vutrisiran for Stargardt Disease would cause us to lose the single-orphan exemption for AMVUTTRA from Medicare price negotiation. As a result, in October 2022, we announced we would not pursue a Phase 3 clinical trial to study vutrisiran in Stargardt Disease. The effect of the IRA on our business and the healthcare industry in general continues to develop and may have additional adverse impacts on our company or our industry.
The full effects of the U.S. healthcare reform legislation cannot be known until the law is fully implemented through regulations or guidance issued by CMS and other federal and state healthcare agencies. The financial impact of the U.S. healthcare reform legislation over the next few years will depend on a number of factors, including, but not limited, to the policies reflected in implementing regulations and guidance, and changes in sales volumes for products affected by the new system of rebates, discounts and fees. This legislation may also have a positive impact on our future net sales, if any, by increasing the aggregate number of persons with healthcare coverage in the U.S.
Since its enactment, there have been numerous judicial, administrative, executive, and legislative challenges to certain aspects of the ACA, and we expect there will be additional challenges and amendments to the ACA in the future. On June 17, 2021, the U.S. Supreme Court dismissed the most recent judicial challenge to the ACA brought by several states without specifically ruling on the constitutionality of the ACA. Prior to the Supreme Court's decision, President Biden issued an executive order to initiate a special enrollment period from February 15, 2021 through August 15, 2021 for the purpose of obtaining health insurance coverage through the ACA marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. It is unclear how other healthcare reform measures of the Biden administration or other efforts, if any, to challenge, repeal or replace the ACA will impact our business.
At the state level, legislatures have become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical product pricing. Some of these measures include price or patient reimbursement constraints, discounts, restrictions on certain product access, marketing cost disclosure and transparency measures, and, in some cases, measures designed to encourage importation from other countries and bulk purchasing. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. These measures could reduce the ultimate demand for our products, once approved, or put pressure on our product pricing.
We cannot predict what healthcare reform initiatives may be adopted in the future. Further federal and state legislative and regulatory developments are likely, and we expect ongoing initiatives in the U.S. to increase pressure on drug pricing. Such reforms could have an adverse effect on anticipated revenues from one or more of our approved products or other product candidates that we may successfully develop and for which we may obtain regulatory approval and may affect our overall financial condition and ability to develop drug candidates.
We are subject to U.S. and certain foreign export and import controls, sanctions, embargoes, anti-corruption laws, and anti-money laundering laws and regulations. Failure to comply with these legal standards could impair our ability to compete in domestic and international markets. We can face criminal liability and other serious consequences for violations, which can harm our business.
We are subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations, various economic and trade sanctions regulations administered by the U.S. Treasury Department’s Office of Foreign Assets Control, the Foreign Corrupt Practices Act of 1977, as amended, the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the USA PATRIOT Act, and other state and national anti-bribery and anti-money laundering laws in the countries in which we conduct activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, contractors, and other collaborators from authorizing, promising, offering, or providing, directly or indirectly, improper payments or anything else of value to recipients in the public or private sector. From time to time, we may engage third parties to conduct clinical trials outside of the U.S., to sell our products abroad, and/or to obtain necessary permits, licenses, patent registrations, and other regulatory approvals. We have direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities, and other organizations.
We can be held liable for the corrupt or other illegal activities of our employees, agents, contractors, and other collaborators, even if we do not explicitly authorize or have actual knowledge of such activities. Any violations of the laws and regulations described above may result in substantial civil and criminal fines and penalties, imprisonment, the loss of export or import privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm, and other consequences.
We remain focused on these laws and the activities they regulate and, as detailed above, maintain a global compliance program designed to empower our business to operate in compliance with their requirements.
Governments outside the U.S. may impose strict price controls, which may adversely affect our revenues.
The pricing of prescription pharmaceuticals is also subject to governmental control outside the U.S. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of regulatory approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our product candidates to other available therapies. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our ability to generate revenues and become profitable could be impaired.
In some countries, including Member States of the EU, or Japan, the pricing of prescription drugs is subject to governmental control. Additional countries may adopt similar approaches to the pricing of prescription drugs. In such countries, pricing negotiations with governmental authorities can take considerable time after receipt of regulatory approval for a product. In addition, there can be considerable pressure by governments and other stakeholders on prices and reimbursement levels, including as part of cost containment measures. Moreover, political, economic and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after coverage and reimbursement have been obtained. Reference pricing used by various countries and parallel distribution, or arbitrage between low-priced and high-priced countries, can further reduce prices. In some countries, we may be required to conduct a clinical study or other studies that compare the cost-effectiveness of a product candidate to other available therapies in order to obtain or maintain reimbursement or pricing approval, which is time-consuming and costly. We cannot be sure that such prices and reimbursement will be acceptable to us or our strategic partners. Publication of discounts by third-party payors or authorities may lead to further pressure on the prices or reimbursement levels within the country of publication and other countries. If pricing is set at unsatisfactory levels or if reimbursement of our products is unavailable or limited in scope or amount, our revenues from sales by us or our strategic partners and the potential profitability of our approved products or any future products in those countries would be negatively affected. Another impact from the tightening pricing control could be felt from greater competition from less expensive generic or biosimilar products once the exclusivity expires; the governments have adopted policies to switch prescribed products to generic versions in order to cut the medical cost.
If we or our collaborators, CMOs or service providers fail to comply with healthcare laws and regulations, or legal obligations related to privacy, data protection and information security, we or they could be subject to enforcement actions, which could affect our ability to develop, market and sell our products and may harm our reputation.
As a manufacturer of pharmaceuticals, we are subject to federal, state, and comparable foreign healthcare laws and regulations pertaining to fraud and abuse and patients’ rights, in addition to legal obligations related to privacy, data protection and information security. These laws and regulations include:
•The U.S. federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce, or in return for, the purchase, lease, order, arrangement, or recommendation of any good, facility, item or service for which payment may be made, in whole or in part, under a federal healthcare program, such as the Medicare and Medicaid programs. A person or entity does not need to have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it to have committed a violation. Violations are subject to civil and criminal fines and penalties for each violation, plus up to three times the remuneration involved, imprisonment, and exclusion from government healthcare programs. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal FCA or federal civil money penalties.
•The U.S. federal false claims laws, including the FCA, which prohibit, among other things, individuals or entities from knowingly presenting or causing to be presented, claims for payment by government-funded programs such as Medicare or Medicaid that are false or fraudulent, making, using or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government, or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government. Manufacturers can be held liable under the FCA even when they do not submit claims directly to government payors if they are deemed to “cause” the submission of false or fraudulent claims. The FCA also permits a private individual acting as a “whistleblower” to bring actions on behalf of the federal government alleging violations of the FCA and to share in any monetary recovery.
•The federal civil monetary penalties laws, which impose civil fines for, among other things, the offering or transfer of remuneration to a Medicare or state healthcare program beneficiary if the person knows or should know it is likely to influence the beneficiary’s selection of a particular provider, practitioner, or supplier of services reimbursable by Medicare or a state healthcare program, unless an exception applies.
•The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private) and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for, healthcare benefits, items or services relating to healthcare matters. Similar to the federal Anti-Kickback Statute, a person or entity can be found guilty of violating HIPAA without actual knowledge of the statute or specific intent to violate it.
•HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, which imposes requirements relating to the privacy, security, and transmission of individually identifiable health information; and requires notification to affected individuals and regulatory authorities of certain breaches of security of individually identifiable health information.
•Federal “sunshine” requirements imposed by the ACA on drug, device, biological and medical supply manufacturers when payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to HHS under the Open Payments Program, information regarding any payment or other “transfer of value” made or distributed to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain non-physician providers such as physician assistants and nurse practitioners, and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. Failure to submit required information may result in civil monetary penalties for all payments, transfers of value or ownership or investment interests that are not timely, accurately, and completely reported in an annual submission.
•Federal price reporting laws, which require manufacturers to calculate and report complex pricing metrics to government programs, where such reported prices may be used in the calculation of reimbursement and/or discounts on approved products.
•Federal statutory and regulatory requirements applicable to pricing and sales of product to Federal Government Agencies.
•Federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers.
•State and foreign laws comparable to each of the above federal laws, including in the EU laws prohibiting giving healthcare professionals any gift or benefit in kind as an inducement to prescribe our products, national transparency laws requiring the public disclosure of payments made to healthcare professionals and institutions, and data privacy laws, in addition to anti-kickback and false claims laws applicable to commercial insurers and other non-federal payors, requirements for mandatory corporate regulatory compliance programs, and laws relating to government reimbursement programs, patient data privacy and security.
•European privacy laws including Regulation 2016/679, known as the General Data Protection Regulation, or the EU GDPR, and the EU GDPR as transposed into the laws of the UK, the UK GDPR, collectively referred to as the GDPR, and the e-Privacy Directive (2002/58/EC), and the national laws implementing each of them, as well as the Public and Electronic Communications Regulations 2003 in the UK and the privacy laws of Japan and other territories. Failure to comply with our obligations under the privacy regime could expose us to significant fines and/or adverse publicity, which could have material adverse effects on our reputation and business.
•The California Consumer Privacy Act of 2018, or CCPA, effective as of January 1, 2020, that gives California residents expanded rights to access and require deletion of their personal information, opt out of certain personal information sharing, and receive detailed information about how their personal information is used. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation.
•Additionally, a new California ballot initiative, the California Privacy Rights Act of 2020, or CPRA, was passed in November 2020. Effective as of January 1, 2023, the CPRA imposes additional obligations on companies covered by the legislation and will significantly modify the CCPA, including by expanding consumers’ rights with respect to certain sensitive personal information. The CPRA also creates a new state agency that will be vested with authority to implement and enforce the CCPA and the CPRA. Furthermore, similar laws were enacted in four other states and proposed in numerous others. The effects of the CCPA and the CPRA are potentially significant and may require us to modify our data collection or processing practices and policies and to incur substantial costs and expenses in an effort to comply and increase our potential exposure to regulatory enforcement and/or litigation.
Some state laws also require pharmaceutical manufacturers to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, in addition to requiring manufacturers to report information related to payments to physicians and other healthcare provides or marketing expenditures and pricing information. State and foreign laws also govern the privacy and security of health information, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
If our operations are found to be in violation of any of the aforementioned requirements, we may be subject to penalties, including civil or criminal penalties (including individual imprisonment), criminal prosecution, monetary damages, the curtailment or restructuring of our operations, loss of eligibility to obtain approvals from the FDA, or exclusion from participation in government contracting, healthcare reimbursement or other government programs, including Medicare and Medicaid, or the imposition of a corporate integrity agreement with the Office of Inspector General of the Department of Health and Human Services, any of which could adversely affect our financial results. We remain focused on enhancing our global compliance infrastructure following the commercial launch of our four products over the last four years in the U.S., EU and multiple other geographies, and as we prepare for the launch of our products in additional countries, assuming regulatory approvals. Although effective compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, these risks cannot be entirely eliminated. For additional information, see the Risk Factor captioned “We may incur significant liability if enforcement authorities allege or determine that we are engaging in commercial activities or promoting our commercially approved products in a way that violates applicable regulations.” Any action against us for an alleged or suspected violation could cause us to incur significant legal expenses and could divert our management’s attention from the operation of our business, even if our defense is successful. In addition, achieving and sustaining compliance with applicable laws and regulations may be costly to us in terms of money, time and resources.
If we or our collaborators, CMOs or service providers fail to comply with applicable federal, state or foreign laws or regulations, we could be subject to enforcement actions, which could affect our ability to develop, market and sell our approved products, or any other future products, successfully and could harm our reputation and lead to reduced acceptance of our products by the market. These enforcement actions include, among others:
•adverse regulatory inspection findings;
•untitled letters or warning letters;
•voluntary or mandatory product recalls or public notification or medical product safety alerts to healthcare professionals;
•restrictions on, or prohibitions against, marketing our products;
•restrictions on, or prohibitions against, importation or exportation of our products;
•suspension of review or refusal to approve pending applications or supplements to approved applications;
•exclusion from participation in government-funded healthcare programs;
•exclusion from eligibility for the award of government contracts for our products;
•suspension or withdrawal of product approvals;
•product seizures;
•injunctions; and
•civil and criminal penalties, up to and including criminal prosecution resulting in fines, exclusion from healthcare reimbursement programs and imprisonment.
Moreover, federal, state or foreign laws or regulations are subject to change, and while we, our collaborators, CMOs and/or service providers currently may be compliant, that could change due to changes in interpretation, prevailing industry standards or the legal structure.
Third party patient assistance programs that receive financial support from companies have become the subject of enhanced government and regulatory scrutiny. The OIG has established guidelines that suggest that it is lawful for pharmaceutical manufacturers to make donations to charitable organizations who provide co-pay assistance to Medicare patients, provided that such organizations, among other things, are bona fide charities, are entirely independent of and not controlled by the manufacturer, provide aid to applicants on a first-come basis according to consistent financial criteria and do not link aid to use of a donor's product. However, donations to patient assistance programs have received some negative publicity and have been the subject of multiple government enforcement actions, related to allegations regarding their use to promote branded pharmaceutical products over other less costly alternatives. Specifically, in recent years, there have been multiple settlements resulting out of government claims challenging the legality of their patient assistance programs under a variety of federal and state laws. It is possible that we may make grants to independent charitable foundations that help financially needy patients with their premium, co-pay, and co-insurance obligations. If we choose to do so, and if we or our vendors or donation recipients are deemed to fail to comply with relevant laws, regulations or evolving government guidance in the operation of these programs, we could be subject to damages, fines, penalties, or other criminal, civil, or administrative sanctions or enforcement actions.
We cannot ensure that our compliance controls, policies, and procedures will be sufficient to protect against acts of our employees, business partners, or vendors that may violate the laws or regulations of the jurisdictions in which we operate. Regardless of whether we have complied with the law, a government investigation could impact our business practices, harm our reputation, divert the attention of management, increase our expenses, and reduce the availability of foundation support for our patients who need assistance.
We are subject to governmental regulation and other legal obligations, particularly related to privacy, data protection and information security, and we are subject to consumer protection laws that regulate our marketing practices and prohibit unfair or deceptive acts or practices. Our actual or perceived failure to comply with such obligations could harm our business.
The GDPR imposes strict requirements on controllers and processors of personal data, including special protections for “special category data,” which includes health, biometric and genetic information of data subjects located in the EEA and UK. Further, GDPR provides a broad right for EEA Member States to create supplemental national laws, such as laws relating to the processing of health, genetic and biometric data, which could further limit our ability to use and share such data or could cause our costs to increase, and harm our business and financial condition.
Failure to comply with the requirements of the GDPR and the related national data protection laws of the EEA Member States and the UK, which may deviate slightly from the GDPR, may result in fines of up to 4% of total global annual revenue, or €20.0 million (₤17.5 million under the UK GDPR), whichever is greater, and in addition to such fines, we may be the subject of litigation and/or adverse publicity, which could have a material adverse effect on our reputation and business. As a result of the implementation of the GDPR, we are required to put in place a number of measures to ensure compliance with the data protection regime. The GDPR requires us to inform data subjects of how we process their personal data and how they can exercise their rights, ensure we have a valid legal basis to process personal data (if this is consent, the requirements for obtaining consent carries a higher threshold), appoint a data protection officer where sensitive personal data (i.e., health data) is processed on a large scale, introduces mandatory data breach notification requirements throughout the EEA and UK, requires us to maintain records of our processing activities and to document data protection impact assessments where there is high risk processing, imposes additional obligations on us when we are contracting with service providers, requires appropriate technical and organisational measures to be put in place to safeguard personal data and requires us to adopt appropriate privacy governance including policies, procedures, training and data audit.
Significantly, the GDPR imposes strict rules on the transfer of personal data out of the EEA and UK to the U.S. or other regions that have not been deemed to offer “adequate” privacy protections. In the past, companies in the U.S. were able to rely upon the EU-U.S., UK-U.S. and the Swiss-U.S. Privacy Shield frameworks as a basis for lawful transfer of personal data from the EU and the UK to the U.S. In July 2020, the Court of Justice of the European Union, or CJEU, in Case C-311/18 (Data Protection Commissioner v Facebook Ireland and Maximillian Schrems, or Schrems II) invalidated the EU-U.S. Privacy Shield on the grounds that the Privacy Shield failed to offer adequate protections to EU personal data transferred to the U.S. The CJEU, in the same decision, deemed that the Standard Contractual Clauses, or SCCs, published by the EC are valid. However, the CJEU ruled that transfers made pursuant to the SCCs need to be assessed on a case-by-case basis to ensure the law in the recipient country provides “essentially equivalent” protections to safeguard the transferred personal data as the EU, and required businesses to adopt supplementary measures if such standard is not met. Subsequent guidance published by the European Data Protection Board, or EDPB, in June 2021 described what such supplementary measures must be, and stated that businesses should avoid or cease transfers of personal data if, in the absence of supplementary measures, equivalent protections cannot be afforded. On June 4, 2021, the EC published new versions of the SCCs, which seek to address the issues identified by the CJEU’s Schrems II decision and provide further details regarding the transfer assessments that the parties are required to conduct when implementing the new SCCs. However, there continue to be concerns about whether the SCCs and other mechanisms will face additional challenges. Similarly, in September 2020, the Swiss data protection authority determined the Swiss-U.S. Privacy Shield framework was no longer a valid mechanism for Swiss-U.S. data transfers and also raised questions about the validity of the SCCs as a mechanism for transferring personal data from Switzerland. While SCCs provide an alternative to our Privacy Shield certification for EU-U.S. data flows, the decision (and certain regulatory guidance issued in its wake) casts doubt on the legality of EU-U.S. data flows in general. Any inability to transfer or burdensome restrictions on the ability to transfer personal data from the EU to the U.S. in compliance with applicable data protection laws may impede our ability to conduct trials and may adversely affect our business and financial position. The UK is not subject to the EC’s new SCCs but has published its own transfer mechanism, the International Data Transfer Agreement or International Data Transfer Addendum, which enables transfers from the UK. On March 25, 2022, the EC and the U.S. announced a political agreement on a new “Trans-Atlantic Data Privacy Framework” to replace the invalidated Privacy Shield. The framework introduced new binding safeguards to address all concerns raised by the CJEU in Schrems II. On July 10, 2023, the EC announced that it had adopted its adequacy decision for that data privacy framework, labelled the EU-U.S. Data Privacy Framework. The adequacy decision concluded that the U.S. ensures an adequate level of protection for personal data transferred from the EU to US companies under the new framework, and the EC stated that as a result personal data can flow safely from the EU to US companies participating in the framework, without having to put in place additional data protection safeguards. The EU-U.S.
Data Privacy Framework is subject to periodic reviews, to be conducted by the EC, together with other European data protection authorities and U.S. authorities, with the first review to take place within a year of the adequacy decision.
EEA Member States have adopted implementing national laws to implement the GDPR which may partially deviate from the GDPR and the competent authorities in the EEA Member States may interpret GDPR obligations slightly differently from country to country, so that we do not expect to operate in a uniform legal landscape in the EU. In addition, the UK Government has now introduced a Data Protection and Digital Information Bill, or the UK Bill, into the UK legislative process. The aim of the UK Bill is to reform UK’s data protection regime following Brexit. If passed, the final version of the UK Bill may have the effect of further altering the similarities between the UK and EEA data protection regime.
We are subject to the supervision of local data protection authorities in those jurisdictions where we are monitoring the behavior of individuals in the EEA or UK (i.e., undertaking clinical trials). We depend on a number of third parties in relation to the provision of our services, a number of which process personal data of EU and/or UK individuals on our behalf. With each such provider we enter or intend to enter into contractual arrangements under which they are contractually obligated to only process personal data according to our instructions, and conduct or intend to conduct diligence to ensure that they have sufficient technical and organizational security measures in place.
We are also subject to evolving European privacy laws on electronic marketing and cookies. The EU is in the process of replacing the e-Privacy Directive (2002/58/EC) with a new set of rules taking the form of a regulation, which will be directly implemented in the laws of each European member state, without the need for further enactment. While the e-Privacy Regulation was originally intended to be adopted on May 25, 2018 (alongside the GDPR), it is still going through the European legislative process. Draft regulations were rejected by the Permanent Representatives Committee of the Council of EU on November 22, 2019; it is not clear when new regulations will be adopted.
Compliance with U.S. and international data protection laws and regulations could require us to take on more onerous obligations in our contracts, restrict our ability to collect, use and disclose data, or in some cases, impact our ability to operate in certain jurisdictions. Failure to comply with these laws and regulations could result in government enforcement actions (which could include civil, criminal and administrative penalties), private litigation, and/or adverse publicity and could negatively affect our operating results and business. Moreover, clinical trial subjects, employees and other individuals about whom we or our potential collaborators obtain personal information, as well as the providers who share this information with us, may limit our ability to collect, use and disclose the information. Claims that we have violated individuals’ privacy rights, failed to comply with data protection laws, or breached our contractual obligations, even if we are not found liable, could be expensive and time-consuming to defend and could result in adverse publicity that could harm our business.
Our ability to obtain services, reimbursement or funding from the federal government may be impacted by possible reductions in federal spending and services, and any inability on our part to effectively adapt to such changes could substantially affect our financial position, results of operations and cash flows.
Under the Budget Control Act of 2011, the failure of Congress to enact deficit reduction measures of at least $1.2 trillion for the years 2013 through 2021 triggered automatic cuts to most federal programs. These cuts included aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, starting in 2013. Certain of these automatic cuts have been implemented resulting in reductions in Medicare payments to physicians, hospitals, and other healthcare providers, among other things. Due to legislation amending the statute, including the Bipartisan Budget Act of 2018, these reductions will stay in effect through 2030 unless additional Congressional action is taken. Pursuant to the CARES Act, as well as subsequent legislation, these reductions were suspended from May 1, 2020 through December 31, 2021 due to the COVID-19 pandemic. The full impact on our business of these automatic cuts is uncertain.
If other federal spending is reduced, any budgetary shortfalls may also impact the ability of relevant agencies, such as the FDA or the National Institutes of Health to continue to function. Amounts allocated to federal grants and contracts may be reduced or eliminated. These reductions may also impact the ability of relevant agencies to timely review and approve drug research and development, manufacturing, and marketing activities, which may delay our ability to develop, market and sell our approved products and any other products we may develop. Further, there has been heightened governmental scrutiny recently over the manner in which drug manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products.
There is a substantial risk of product liability claims in our business. If we are unable to obtain sufficient insurance, a product liability claim against us could adversely affect our business.
Our business exposes us to significant potential product liability risks that are inherent in the development, testing, manufacturing and marketing of human therapeutic products. Product liability claims could delay or prevent completion of our clinical development programs. Following the decision to discontinue clinical development of revusiran, we conducted a comprehensive evaluation of available revusiran data. We reported the results of this evaluation in August 2017, however, our investigation did not result in a conclusive explanation regarding the cause of the mortality imbalance observed in the ENDEAVOUR Phase 3 study.
In addition, in September 2017, we announced that we had temporarily suspended dosing in all ongoing fitusiran studies pending further review of a fatal thrombotic SAE and agreement with regulatory authorities on a risk mitigation strategy. Notwithstanding the risks undertaken by all persons who participate in clinical trials, and the information on risks provided to study investigators and patients participating in our clinical trials, including the revusiran and fitusiran studies, it is possible that product liability claims will be asserted against us relating to the worsening of a patient’s condition, injury or death alleged to have been caused by one of our product candidates, including revusiran or fitusiran. Such claims might not be fully covered by product liability insurance. In addition, product liability claims could result in an FDA investigation of the safety and effectiveness of our approved products, our manufacturing processes and facilities or our marketing programs, and potentially a recall of our products or more serious enforcement action, limitations on the approved indications for which they may be used, or suspension or withdrawal of approvals. Regardless of the merits or eventual outcome, liability claims may also result in decreased demand for our products, injury to our reputation, costs to defend the related litigation, a diversion of management’s time and our resources, substantial monetary awards to trial participants or patients and a decline in our stock price. We currently have product liability insurance that we believe is appropriate for our stage of development, including the marketing and sale of our approved products. Any insurance we have or may obtain may not provide sufficient coverage against potential liabilities. Furthermore, clinical trial and product liability insurance is becoming increasingly expensive. As a result, we may be unable to obtain sufficient insurance at a reasonable cost to protect us against losses caused by product liability claims that could have a material adverse effect on our business.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements or insider trading violations, which could significantly harm our business.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with governmental regulations, comply with healthcare fraud and abuse and anti-kickback laws and regulations in the U.S. and abroad, or failure to report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of, including improper trading based upon, information obtained in the course of clinical studies, which could result in regulatory sanctions and serious harm to our reputation. We maintain a global compliance program and remain focused on its evolution and enhancement. Our program includes efforts such as risk assessment and monitoring, fostering a speak up culture encouraging employees and third parties to raise good faith questions or concerns, and defined processes and systems for reviewing and remediating allegations and identified potential concerns. It is not always possible, however, to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.
If we do not comply with laws regulating the protection of the environment and health and human safety, our business could be adversely affected.
Our research, development and manufacturing involve the use of hazardous materials, chemicals and various radioactive compounds. We maintain quantities of various flammable and toxic chemicals in our facilities in Cambridge and Norton that are required for our research, development and manufacturing activities. We are subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. We believe our procedures for storing, handling and disposing these materials in our Cambridge and Norton facilities comply with the relevant guidelines of the City of Cambridge, the town of Norton, the Commonwealth of Massachusetts and the Occupational Safety and Health Administration of the U.S. Department of Labor. Although we believe that our safety procedures for handling and disposing of these materials comply with the standards mandated by applicable regulations, the risk of accidental contamination or injury from these materials cannot be eliminated. If an accident occurs, we could be held liable for resulting damages, which could be substantial. We are also subject to numerous environmental, health and workplace safety laws and regulations, including those governing laboratory procedures, exposure to blood-borne pathogens and the handling of biohazardous materials.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of these materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials. Additional federal, state and local laws and regulations affecting our operations may be adopted in the future. We may incur substantial costs to comply with, and substantial fines or penalties if we violate, any of these laws or regulations.
Risks Related to Patents, Licenses and Trade Secrets
If we are not able to obtain and enforce patent protection for our discoveries, our ability to develop and commercialize our product candidates will be harmed.
Our success depends, in part, on our ability to protect proprietary compositions, methods and technologies that we develop under the patent and other intellectual property laws of the U.S. and other countries, so that we can prevent others from unlawfully using our inventions and proprietary information. However, we may not hold proprietary rights to some patents required for us to manufacture and commercialize our proposed products. Because certain U.S. patent applications are confidential until the patents issue, such as applications filed prior to November 29, 2000, or applications filed after such date which will not be filed in foreign countries, third parties may have filed patent applications for subject matter covered by our pending patent applications without our being aware of those applications, and our patent applications may not have priority over those applications. For this and other reasons, we may be unable to secure desired patent rights, thereby losing desired exclusivity. Further, we or our licensees may be required to obtain licenses under third-party patents to market one or more of our or our partner's approved products, or further develop and commercialize future products, or continue to develop candidates in our pipeline being developed by us or our licensees. If licenses are not available to us or not available on reasonable terms, we or our licensees may not be able to market the affected products or conduct the desired activities.
Our strategy depends on our ability to rapidly identify and seek patent protection for our discoveries. In addition, we may rely on third-party collaborators to file patent applications relating to proprietary technology that we develop jointly during certain collaborations. The process of obtaining patent protection is expensive and time-consuming. If our present or future collaborators fail to file and prosecute all necessary and desirable patent applications at a reasonable cost and in a timely manner, our business may be adversely affected. Despite our efforts and the efforts of our collaborators to protect our proprietary rights, unauthorized parties may be able to obtain and use information that we regard as proprietary. While issued patents are presumed valid, this does not guarantee that the patent will survive a validity challenge or be held enforceable. Any patents we have obtained, or obtain in the future, may be challenged, invalidated, adjudged unenforceable or circumvented by parties attempting to design around our intellectual property. Moreover, third parties or the United States Patent and Trademark Office, or USPTO, may commence interference proceedings involving our patents or patent applications. Any challenge to, finding of unenforceability or invalidation or circumvention of, our patents or patent applications, would be costly, would require significant time and attention of our management, could reduce or eliminate royalty payments to us from third party licensors and could have a material adverse effect on our business.
Our pending patent applications may not result in issued patents. The patent position of pharmaceutical or biotechnology companies, including ours, is generally uncertain and involves complex legal and factual considerations. The standards that the USPTO and its foreign counterparts use to grant patents are not always applied predictably or uniformly and can change. Similarly, the ultimate degree of protection that will be afforded to biotechnology inventions, including ours, in the U.S. and foreign countries, remains uncertain and is dependent upon the scope of the protection decided upon by patent offices, courts and lawmakers. Moreover, there are periodic discussions in the Congress of the United States and in international jurisdictions about modifying various aspects of patent law. For example, the America Invents Act, or AIA, included a number of changes to the patent laws of the U.S. If any of the enacted changes do not provide adequate protection for discoveries, including our ability to pursue infringers of our patents for substantial damages, our business could be adversely affected. One major provision of the AIA, which took effect in March 2013, changed U.S. patent practice from a first-to-invent to a first-to-file system. If we fail to file an invention before a competitor files on the same invention, we no longer have the ability to provide proof that we were in possession of the invention prior to the competitor’s filing date, and thus would not be able to obtain patent protection for our invention. There is also no uniform, worldwide policy regarding the subject matter and scope of claims granted or allowable in pharmaceutical or biotechnology patents.
Accordingly, we do not know the degree of future protection for our proprietary rights or the breadth of claims that will be allowed in any patents issued to us or to others. We also rely to a certain extent on trade secrets, know-how and technology, which are not protected by patents, to maintain our competitive position. If any trade secret, know-how or other technology not protected by a patent were to be disclosed to or independently developed by a competitor, our business and financial condition could be materially adversely affected.
Failure to obtain and maintain all available regulatory exclusivities, broad patent scope and to maximize patent term restoration or extension on patents covering our products may lead to loss of exclusivity and early generic entry resulting in a loss of market share and/or revenue.
We license patent rights from third-party owners. If such owners do not properly or successfully obtain, maintain or enforce the patents underlying such licenses, our competitive position and business prospects may be harmed.
We are a party to a number of licenses that give us rights to third-party intellectual property that is necessary or useful for our business. In particular, we have obtained licenses from, among others, Ionis, Arbutus Biopharma Corp., or Arbutus, and Dicerna. We also intend to enter into additional licenses to third-party intellectual property in the future.
Our success will depend in part on the ability of our licensors to obtain, maintain and enforce patent protection for our licensed intellectual property, in particular, those patents to which we have secured exclusive rights. Our licensors may not successfully prosecute the patent applications to which we are licensed. Even if patents issue in respect of these patent applications, our licensors may fail to maintain these patents, may determine not to pursue litigation against other companies that are infringing these patents, or may pursue such litigation less aggressively than we would. Without protection for the intellectual property we license, other companies might be able to offer substantially identical products for sale, which could adversely affect our competitive business position and harm our business prospects. In addition, we sublicense our rights under various third-party licenses to our collaborators. Any impairment of these sublicensed rights could result in reduced revenues under our collaboration agreements or result in termination of an agreement by one or more of our collaborators.
Other companies or organizations may challenge our patent rights or may assert patent rights that prevent us from developing and commercializing our products.
RNAi is a relatively new scientific field, the commercial exploitation of which has resulted in many different patents and patent applications from organizations and individuals seeking to obtain patent protection in the field. We have obtained grants and issuances of RNAi patents and have licensed many of these patents from third parties on an exclusive basis. The issued patents and pending patent applications in the U.S. and in key markets around the world that we own or license claim many different methods, compositions and processes relating to the discovery, development, manufacture and commercialization of RNAi therapeutics.
Specifically, we have a portfolio of patents, patent applications and other intellectual property covering: fundamental aspects of the structure and uses of siRNAs, including their use as therapeutics, and RNAi-related mechanisms; chemical modifications to siRNAs that improve their suitability for therapeutic and other uses; siRNAs directed to specific targets as treatments for particular diseases; delivery technologies, such as in the fields of carbohydrate conjugates and cationic liposomes; and all aspects of our specific development candidates.
As the field of RNAi therapeutics is maturing, patent applications are being fully processed by national patent offices around the world. There is uncertainty about which patents will issue, and, if they do, as to when, to whom, and with what claims. It is likely that there will be significant litigation and other proceedings, such as interference, re-examination and opposition proceedings, as well as inter partes and post-grant review proceedings introduced by provisions of the AIA, which became available to third party challengers on September 16, 2012, in various patent offices relating to patent rights in the RNAi field. In addition, third parties may challenge the validity of our patents. For example, a third party has filed an opposition in the European Patent Office, or EPO, against our owned patent EP 2723758, with claims directed to compositions and methods of ANGPTL3, arguing that the granted claims are invalid. An oral hearing was held at the EPO in February 2021, where the patent was revoked. A notice of appeal of the EPO's decision was filed in June 2021. In addition, in February 2023, a third party filed an opposition with the EPO against our owned patent EP 3366775, titled “Modified RNA Agents” seeking to revoke the patent. An oral hearing is anticipated at a time to be determined by the EPO. We expect that additional oppositions will be filed in the EPO and elsewhere, and other challenges will be raised relating to other patents and patent applications in our portfolio. In many cases, the possibility of appeal exists for either us or our opponents, and it may be years before final, unappealable rulings are made with respect to these patents in certain jurisdictions. The timing and outcome of these and other proceedings is uncertain and may adversely affect our business if we are not successful in defending the patentability and scope of our pending and issued patent claims. Even if our rights are not directly challenged, disputes could lead to the weakening of our intellectual property rights. Our defense against any attempt by third parties to circumvent or invalidate our intellectual property rights could be costly to us, could require significant time and attention of our management and could have a material adverse effect on our business and our ability to successfully compete in the field of RNAi.
There are many issued and pending patents that claim aspects of oligonucleotide chemistry and modifications that we may need for our siRNA products marketed by us or our licensees, our late-stage therapeutic candidates being developed by us or our licensees, including zilebesiran and fitusiran, as well as our other pipeline products. There are also many issued patents that claim targeting genes or portions of genes that may be relevant for siRNA drugs we wish to develop. In addition, there may be issued and pending patent applications that may be asserted against us in a court proceeding or otherwise based upon the asserting party’s belief that we may need such patents for our siRNA therapeutic candidates or marketed products, or to further develop and commercialize future products, or to continue to develop candidates in our pipeline that are being developed by us or our licensees. Thus, it is possible that one or more organizations will hold patent rights to which we may need a license, or hold patent rights which could be asserted against us. If those organizations refuse to grant us a license to such patent rights on reasonable terms and/or a court rules that we need such patent rights that have been asserted against us and we are not able to obtain a license on reasonable terms, we may be unable to market products, including ONPATTRO, AMVUTTRA, GIVLAARI or OXLUMO, or perform research and development or other activities covered by such patents. For example, during 2017 and 2018, Silence Therapeutics, plc, or Silence, filed claims in several jurisdictions, including the High Court of England and Wales, and named us and our wholly owned subsidiary Alnylam UK Ltd. as co-defendants. Silence alleged various claims, including that ONPATTRO infringed one or more Silence patents. There were also a number of related actions brought by us or Silence in connection with this intellectual property dispute. In December 2018, we entered into a Settlement and License Agreement with Silence, resolving all ongoing claims, administrative proceedings, and regulatory proceedings worldwide between us regarding, among other issues, patent infringement, patent invalidity and breach of contract.
If we become involved in intellectual property litigation or other proceedings related to a determination of rights, we could incur substantial costs and expenses, and in the case of such litigation or proceedings against us, substantial liability for damages or be required to stop our product development and commercialization efforts.
Third parties may sue us for infringing their patent rights. For example, in October 2017 Silence sued us in the UK alleging that ONPATTRO and other investigational RNAi therapeutics we or MDCO are developing infringed one or more Silence patents. In December 2018 we and Silence settled all ongoing litigation between us. A third party may also claim that we have improperly obtained or used its confidential or proprietary information.
Furthermore, third parties may challenge the inventorship of our patents or licensed patents. For example, in March 2011, The University of Utah, or Utah, filed a complaint against us, Max Planck Gesellschaft Zur Foerderung Der Wissenschaften e.V. and Max Planck Innovation, together, Max Planck, Whitehead, MIT and the University of Massachusetts, claiming that a professor of Utah was the sole inventor, or in the alternative, a joint inventor of certain of our in-licensed patents. Utah was seeking correction of inventorship of the Tuschl patents, unspecified damages and other relief. After several years of court proceedings and discovery, the court granted our motions for summary judgment, and dismissed Utah’s state law damages claims as well. During the pendency of this litigation, as well as the Dicerna litigation described above, we incurred significant costs, and in each case, the litigation diverted the attention of our management and other resources that would otherwise have been engaged in other activities.
We may need to resort to litigation to enforce a patent issued or licensed to us or to determine the scope and validity of proprietary rights of others or protect our proprietary information and trade secrets. For example, during the second quarter of 2015, we filed a trade secret misappropriation lawsuit against Dicerna to protect our rights in the RNAi assets we purchased from Merck Sharp & Dohme Corp., or Merck. We and Dicerna settled the ongoing litigation between us in April 2018. In March 2022, we announced that we separately filed suit in United States District Court for the District of Delaware against Pfizer and Moderna, Inc., seeking damages for infringement of U.S. Patent No. 11,246,933 in the parties’ manufacture and sale of their messenger RNA, or mRNA, COVID-19 vaccines. Pfizer joined BioNTech SE, or BioNTech, to the suit and filed counterclaims. In July 2022, we filed an additional lawsuit in United States District Court for the District of Delaware against each of Pfizer/BioNTech and Moderna seeking damages for infringing U.S. Patent No. 11,382,979. The court combined the two patents in a single suit for each of Pfizer/BioNTech and Moderna with trial dates set for each in November 2024. On May 26, 2023, we filed additional lawsuits against Pfizer and Moderna in Delaware seeking damages for infringing U.S. Patent No. 11,590,229 in the United States District Court for the District of Delaware. In addition to this patent, we added U.S. Patent Nos. 11,633,479 and 11,633,480 in the recently filed suits against both Pfizer and Moderna and also U.S. Patent No. 11,612,657 against Pfizer only. On August 9, 2023, a Markman hearing was held in the U.S. District Court for the District of Delaware to consider the meaning of three disputed terms as used in the ’933 and ’979 patents. On August 21, 2023, the court issued an order construing two of the three terms, and deferred a ruling on the third term pending an evidentiary hearing scheduled to be held on January 4, 2024. Subsequently, we and Moderna jointly agreed to final judgment of non-infringement of two of our patents, and that judgment was entered by the court on August 30, 2023, and on September 7, 2023, we appealed the claim construction ruling to the Court of Appeals for the Federal Circuit in the 2022 lawsuit against Moderna. The claim construction ruling did not affect one of the patents in the lawsuit filed against Moderna on May 26, 2023, and that case is going forward on a schedule to be set by the court. The two separate suits against Pfizer are ongoing subject to the January 2024 hearing on the third claim term, and in September 2023, we and Pfizer agreed to consolidate the 2022 and 2023 lawsuits in one case, which will require moving the trial date from November 2024 to the first half of 2025, with the final schedule to be determined by the court. The aforementioned patents relate to our biodegradable cationic lipids that are foundational to the success of the mRNA COVID-19 vaccines.
In protecting our intellectual patent rights through litigation or other means, a third party may claim that we have improperly asserted our rights against them. For example, in August 2017, Dicerna successfully added counterclaims against us in the above-referenced trade secret lawsuit alleging that our lawsuit represented abuse of process and claiming tortious interference with its business. In addition, in August 2017, Dicerna filed a lawsuit against us in the United States District Court of Massachusetts alleging attempted monopolization by us under the Sherman Antitrust Act. As noted above, in April 2018, we and Dicerna settled the ongoing litigation between us.
In addition, in connection with certain license and collaboration agreements, we have agreed to indemnify certain third parties for certain costs incurred in connection with litigation relating to intellectual property rights or the subject matter of the agreements. The cost to us of any litigation or other proceeding relating to intellectual property rights, even if resolved in our favor, could be substantial, and litigation would divert our management’s efforts. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Uncertainties resulting from the initiation and continuation of any litigation or legal proceeding could delay our research, development and commercialization efforts and limit our ability to continue our operations.
If any parties successfully claim that our creation or use of proprietary technologies infringes upon or otherwise violates their intellectual property rights, we might be forced to pay damages, potentially including treble damages, if we are found to have willfully infringed on such parties’ patent rights. In addition to any damages we might have to pay, a court could issue an injunction requiring us to stop the infringing activity or obtain a license. Any license required under any patent may not be made available on commercially reasonable terms, if at all. In addition, such licenses are likely to be non-exclusive and, therefore, our competitors may have access to the same technology licensed to us. If we fail to obtain a required license and are unable to design around a patent, we may be unable to effectively market some of our technology and products, which could limit our ability to generate revenues or achieve profitability and possibly prevent us from generating revenue sufficient to sustain our operations. Moreover, we expect that a number of our collaborations will provide that royalties payable to us for licenses to our intellectual property may be offset by amounts paid by our collaborators to third parties who have competing or superior intellectual property positions in the relevant fields, which could result in significant reductions in our revenues from products developed through collaborations.
If we fail to comply with our obligations under any licenses or related agreements, we may be required to pay damages and could lose license or other rights that are necessary for developing, commercializing and protecting our RNAi technology, as well as our approved products and any other product candidates that we develop, or we could lose certain rights to grant sublicenses.
Our current licenses impose, and any future licenses we enter into are likely to impose, various development, commercialization, funding, milestone, royalty, diligence, sublicensing, insurance, patent prosecution and enforcement, and other obligations on us. If we breach any of these obligations, or use the intellectual property licensed to us in an unauthorized manner, we may be required to pay damages and the licensor may have the right to terminate the license or render the license non-exclusive, which could result in us being unable to develop, manufacture, market and sell products that are covered by the licensed technology or enable a competitor to gain access to the licensed technology. Moreover, we could incur significant costs and/or disruption to our business and distraction of our management defending against any breach of such licenses alleged by the licensor. For example, in June 2018, Ionis sent us a notice claiming that it was owed payments under our second amended and restated strategic collaboration and license agreement as a result of the January 2018 amendment of our collaboration agreement with Sanofi and the related Exclusive TTR License and AT3 License Terms. Ionis claimed it was owed technology access fees, or TAFs, based on rights granted and amounts paid to us in connection with the Sanofi restructuring. Ionis later filed a Demand for Arbitration with the Boston office of the American Arbitration Association against us, asserting, among other things, breach of contract. Upon completion of the arbitration process in the second quarter of 2020, in October 2020, a partial award was issued by the arbitration panel that sought additional information from us. The arbitration panel issued its final award in December 2020, which ruled in favor of Ionis’s request for a TAF on certain rights the panel determined we received in the Sanofi restructuring (but rejecting the TAF amount sought by Ionis), and in favor of us in denying Ionis’s request for a TAF on a milestone payment received by us in the same restructuring. The panel’s final award also denied Ionis’s request for pre-judgement interest and attorney’s fees. Pursuant to the panel's final award, we paid $41.2 million to Ionis in January 2021.
Moreover, our licensors may own or control intellectual property that has not been licensed to us and, as a result, we may be subject to claims, regardless of their merit, that we are infringing or otherwise violating the licensor’s rights. In addition, while we cannot currently determine the amount of the royalty obligations we will be required to pay on sales of each of our approved products or future products, if any, the amounts may be significant. The amount of our future royalty obligations will depend on the technology and intellectual property we use in such products. Therefore, even if we successfully develop and commercialize products, we may be unable to achieve or maintain profitability.
Confidentiality agreements with employees and others may not adequately prevent disclosure of trade secrets and other proprietary information.
In order to protect our proprietary technology and processes, we rely in part on confidentiality agreements with our collaborators, employees, consultants, CMOs, outside scientific collaborators and sponsored researchers, and other advisors. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover trade secrets and proprietary information, and in such cases we could not assert any trade secret rights against such party. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
Risks Related to Competition
The pharmaceutical market is intensely competitive. If we are unable to compete effectively with existing drugs, new treatment methods and new technologies, we may be unable to commercialize successfully any drugs that we develop.
The pharmaceutical market is intensely competitive and rapidly changing. Many large pharmaceutical and biotechnology companies, academic institutions, governmental agencies and other public and private research organizations are pursuing the development of novel drugs for the same diseases that we are targeting or expect to target. Many of our competitors have:
•much greater financial, technical and human resources than we have at every stage of the discovery, development, manufacture and commercialization of products;
•more extensive experience in pre-clinical testing, conducting clinical trials, obtaining regulatory approvals, and in manufacturing, marketing and selling drug products;
•product candidates that are based on previously tested or accepted technologies;
•products that have been approved or are in late stages of development; and
•collaborative arrangements in our target markets with leading companies and research institutions.
We will face intense competition from drugs that have already been approved and accepted by the medical community for the treatment of the conditions for which we may develop drugs. For example, assuming positive Phase 3 results and regulatory approval, vutrisiran, our RNAi therapeutic in development for ATTR amyloidosis patients with cardiomyopathy, would compete with tafamidis, marketed by Pfizer, which is currently approved to treat this disease. We also expect to face competition from new drugs that enter the market. There are a number of drugs currently under development, which may become commercially available in the future, for the treatment of conditions for which we may try to develop drugs. These drugs may be more effective, safer, less expensive, or marketed and sold more effectively, than any products we develop and commercialize. For example, we developed ONPATTRO and AMVUTTRA for the treatment of hATTR amyloidosis. In August 2018, the FDA approved ONPATTRO lipid complex injection for the treatment of the polyneuropathy of hATTR amyloidosis in adults, and the EC granted marketing authorization for ONPATTRO for the treatment of hATTR amyloidosis in adults with stage 1 or stage 2 polyneuropathy. We are aware of other approved products used to treat this disease, including tafamidis, and inotersen, developed and marketed by Ionis, as well as product candidates in various stages of clinical development, including eplontersen, an additional investigational drug developed by Ionis in partnership with AstraZeneca, which met co-primary and secondary endpoints in a Phase 3 study for the polyneuropathy of hATTR amyloidosis, and is currently under regulatory review by the FDA with a PDUFA action date of December 22, 2023. Finally, BridgeBio Pharma, Inc., or BridgeBio, announced positive results from its Phase 3 clinical trial of acoramidis, a TTR stabilizer, in ATTR-CM in July 2023, and noted that they anticipate filing an NDA with the FDA by the end of 2023. While we believe that ONPATTRO and AMVUTTRA have and will continue to have a competitive product profile for the treatment of patients with hATTR amyloidosis with polyneuropathy, it is possible that ONPATTRO and/or AMVUTTRA may not compete favorably with these products and product candidates, or others, and, as a result, may not achieve commercial success. Moreover, positive or negative data and/or the commercial success or failure of competitive products could negatively impact our stock price. For example, our stock price was negatively impacted by the results of Part A of BridgeBio's Phase 3 clinical trial.
If we continue to successfully develop product candidates, and obtain approval for them, we will face competition based on many different factors, including:
•the safety and effectiveness of our products relative to alternative therapies, if any;
•the ease with which our products can be administered and the extent to which patients accept relatively new routes of administration;
•the timing and scope of regulatory approvals for these products;
•the availability and cost of manufacturing, marketing and sales capabilities;
•the price of our products relative to alternative approved therapies;
•reimbursement coverage; and
•patent position.
We are aware of product candidates in various stages of clinical development for the treatment of PH1 which would compete with OXLUMO, our RNAi therapeutic approved in the U.S. and EU for the treatment of this disease, including Oxabact®, a bacteria-based investigational therapy in development by OxThera AB and reloxaliase an investigational enzyme therapy in Phase 3 development for primary or severe secondary hyperoxaluria by Allena Pharmaceuticals, Inc. In addition, Novo Nordisk's RivflozaTM (nedosiran), a once-monthly subcutaneous RNAi therapy, to lower urinary oxalate levels in children 9 years of age and older and adults with PH1 and relatively preserved kidney function, developed by Dicerna, was approved by the FDA in October 2023, and will compete with OXLUMO. In April 2020, we and Dicerna granted each other a non-exclusive cross-license to our respective intellectual property related to lumasiran, and Dicerna’s nedosiran.
Our competitors may develop or commercialize products with significant advantages over any products we develop based on any of the factors listed above or on other factors. In addition, our competitors may develop strategic alliances with or receive funding from larger pharmaceutical or biotechnology companies, providing them with an advantage over us. Our competitors may therefore be more successful in commercializing their products than we are, which could adversely affect our competitive position and business. Competitive products may make any products we develop obsolete or noncompetitive before we can recover the expenses of developing and commercializing our product candidates. Such competitors could also recruit our employees, which could negatively impact our level of expertise and the ability to execute on our business plan. Furthermore, we also face competition from existing and new treatment methods that reduce or eliminate the need for drugs, such as the use of advanced medical devices. The development of new medical devices or other treatment methods for the diseases we are targeting could make our product candidates noncompetitive, obsolete or uneconomical.
We face competition from other companies that are working to develop novel drugs and technology platforms using technology similar to ours. If these companies develop drugs more rapidly than we do or their technologies, including delivery technologies, are more effective, our ability to successfully commercialize drugs may be adversely affected.
In addition to the competition we face from competing drugs in general, we also face competition from other companies working to develop novel drugs using technology that competes more directly with our own. We are aware of several other companies that are working to develop RNAi therapeutic products. Some of these companies are seeking, as we are, to develop chemically synthesized siRNAs as drugs. Others are following a gene therapy approach, with the goal of treating patients not with synthetic siRNAs but with synthetic, exogenously-introduced genes designed to produce siRNA-like molecules within cells. Companies working on chemically synthesized siRNAs include, but are not limited to, Takeda Pharmaceutical Company Ltd., or Takeda, Marina Biotech, Inc., Arrowhead Pharmaceuticals Inc, Inc., or Arrowhead, Quark Pharmaceuticals, Inc., or Quark, Silence, Arbutus, Sylentis, S.A.U, or Sylentis, Dicerna and its collaborators, WAVE Life Sciences Ltd., Arcturus Therapeutics Inc., and Genevant Sciences, launched by Arbutus and Roivant Sciences. In addition, we granted licenses or options for licenses to Ionis, Benitec Biopharma Ltd., or Benitec, Arrowhead, Arbutus, Quark, Sylentis and others under which these companies may independently develop RNAi therapeutics against a limited number of targets. Any one of these companies may develop its RNAi technology more rapidly and more effectively than us.
In addition, as a result of agreements that we have entered into, Takeda has obtained a non-exclusive license, and Arrowhead, as the assignee of Novartis, has obtained specific exclusive licenses for 30 gene targets, that include access to certain aspects of our technology. We also compete with companies working to develop antisense-based drugs. Like RNAi therapeutics, antisense drugs target mRNAs in order to suppress the activity of specific genes. Akcea Therapeutics, Inc. (acquired by Ionis in October 2020), has received marketing approval for an antisense drug, inotersen that was developed by Ionis, for the treatment of stage 1 or stage 2 polyneuropathy in adult patients with hATTR amyloidosis. Several antisense drugs developed by Ionis have been approved and are currently marketed, and Ionis has multiple antisense product candidates in clinical trials. Ionis is also developing antisense drugs using ligand-conjugated GalNAc technology licensed from us, and these drugs have been shown to have increased potency at lower doses in clinical and pre-clinical studies, compared with antisense drugs that do not use such licensed GalNAc technology. The development of antisense drugs and antisense technology may become the preferred technology for drugs that target mRNAs to silence specific genes.
In addition to competition with respect to RNAi and with respect to specific products, we face substantial competition to discover and develop safe and effective means to deliver siRNAs to the relevant cell and tissue types. Safe and effective means to deliver siRNAs to the relevant cell and tissue types may be developed by our competitors, and our ability to successfully commercialize a competitive product would be adversely affected. In addition, substantial resources are being expended by third parties in the effort to discover and develop a safe and effective means of delivering siRNAs into the relevant cell and tissue types, both in academic laboratories and in the corporate sector. Some of our competitors have substantially greater resources than we do, and if our competitors are able to negotiate exclusive access to those delivery solutions developed by third parties, we may be unable to successfully commercialize our product candidates.
Risks Related to Our Common Stock
If our stock price fluctuates, purchasers of our common stock could incur substantial losses.
The market price of our common stock has fluctuated significantly and may continue to fluctuate significantly in response to factors that are beyond our control. The stock market in general has from time to time experienced extreme price and volume fluctuations, and the biotechnology sector in particular has experienced extreme price and volume fluctuations. The market prices of securities of pharmaceutical and biotechnology companies have been extremely volatile, and have experienced fluctuations that often have been unrelated or disproportionate to the clinical development progress or operating performance of these companies, including as a result of adverse development events reported by other companies. For example, the trading price for our common stock and the common stock of other biopharmaceutical companies was highly volatile during the initial stages of the COVID-19 pandemic. These broad market and sector fluctuations have resulted and could in the future result in extreme fluctuations in the price of our common stock, which could cause purchasers of our common stock to incur substantial losses.
We may incur significant costs from class action litigation.
Our stock price may fluctuate for many reasons, including as a result of public announcements regarding the progress of our development and commercialization efforts or the development and commercialization efforts of our collaborators and/or competitors, the addition or departure of our key personnel, variations in our quarterly operating results and changes in market valuations of pharmaceutical and biotechnology companies. When the market price of a stock has been volatile as our stock price has been, holders of that stock have occasionally brought securities class action litigation against the company that issued the stock.
For example, on September 12, 2019, the Chester County Employees Retirement Fund, individually and on behalf of all others similarly situated, filed a purported securities class action complaint alleging violation of federal securities laws against us, certain of our current and former directors and officers, and the underwriters of our November 14, 2017 public stock offering, in the Supreme Court of the State of New York, New York County. While we believe the allegations in the New York State Securities Litigation were without merit, in August 2021, the parties reached an agreement in principle to resolve the matter. At a hearing on April 12, 2022, the Supreme Court of the State of New York granted final approval to the settlement. Proceedings in the First Department were adjourned until April 2022, pending final approval of any settlement, and were withdrawn as a result of final approval on April 18, 2022. Future litigation, including future securities litigation, could result in substantial costs and divert our management’s attention and resources, which could cause serious harm to our business, operating results and financial condition. We maintain liability insurance; however, if any costs or expenses associated with litigation exceed our insurance coverage, we may be forced to bear some or all of these costs and expenses directly, which could be substantial. In addition, we have obligations to indemnify third parties, including our officers and directors, in connection with certain litigation, and those obligations may not be covered by insurance.
Future sales of shares of our common stock, including by our significant stockholders, us or our directors and officers, could cause the price of our common stock to decline.
A small number of our stockholders beneficially own a substantial amount of our common stock. As of September 30, 2023, our seven largest stockholders beneficially owned in excess of 50% of our outstanding shares of common stock. If our significant stockholders, or we or our officers and directors, sell substantial amounts of our common stock in the public market, or there is a perception that such sales may occur, the market price of our common stock could be adversely affected. Sales of common stock by our significant stockholders might make it more difficult for us to raise funds by selling equity or equity-related securities in the future at a time and price that we deem appropriate.
Regeneron’s ownership of our common stock could delay or prevent a change in corporate control.
As of May 21, 2019, the closing date of the stock purchase in connection with the 2019 Regeneron collaboration, Regeneron held approximately 4% of our outstanding common stock and has the right to increase its ownership up to 30%. This concentration of ownership could harm the market price of our common stock in the future by:
•delaying, deferring or preventing a change in control of our company;
•impeding a merger, consolidation, takeover or other business combination involving our company; or
•discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of our company.
Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our certificate of incorporation and our bylaws may delay or prevent an acquisition of us or a change in our management. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors. Because our board of directors is responsible for appointing the members of our management team, these provisions could in turn affect any attempt by our stockholders to replace current members of our management team. These provisions include:
•a classified board of directors;
•a prohibition on actions by our stockholders by written consent;
•limitations on the removal of directors; and
•advance notice requirements for election to our board of directors and for proposing matters that can be acted upon at stockholder meetings.
In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner. These provisions would apply even if the proposed merger or acquisition could be considered beneficial by some stockholders.
Risks Related to Our Convertible Notes
Servicing our debt may require a significant amount of cash. We may not have sufficient cash flow from our business to pay our indebtedness.
On September 12, 2022, we commenced a private offering of $900.0 million in aggregate principal amount of the Initial Notes. On September 13, 2022, the initial purchasers in such offering exercised their option to purchase an additional $135.0 million in aggregate principal amount of the Additional Notes, bringing the total aggregate principal amount of the Notes to $1.04 billion. The interest rate for the Notes is fixed at 1.00% per annum and is payable semi-annually in arrears on May 15 and September 15 of each year, beginning on March 15, 2023. Our ability to make scheduled payments of the principal of, to pay interest on or to refinance our indebtedness, including the Notes, or to make cash payments in connection with any conversions of Notes, depends on our future performance, which is subject to economic, financial, competitive and other factors beyond our control. Our business may not generate cash flow from operations in the future sufficient to service our debt and make necessary capital expenditures. If we are unable to generate such cash flow, we may be required to adopt one or more alternatives, such as selling assets, restructuring debt or obtaining additional debt financing or equity capital on terms that may be onerous or highly dilutive. Our ability to refinance any future indebtedness will depend on the capital markets and our financial condition at such time. We may not be able to engage in any of these activities or engage in these activities on desirable terms, which could result in a default on our debt obligations. In addition, any of our future debt agreements may contain restrictive covenants that may prohibit us from adopting any of these alternatives. Our failure to comply with these covenants could result in an event of default which, if not cured or waived, could result in the acceleration of our debt.
We may not have the ability to raise the funds necessary to settle for cash conversions of the Notes or to repurchase the Notes for cash upon a fundamental change, and our future debt may contain limitations on our ability to pay cash upon conversion of the Notes or to repurchase the Notes.
Holders of the Notes have the right to require us to repurchase their Notes upon the occurrence of a fundamental change (as defined in the indenture governing the Notes) at a repurchase price equal to 100% of the principal amount of the Notes to be repurchased, plus accrued and unpaid interest, if any. Upon conversion of the Notes, unless we elect to deliver solely shares of our common stock to settle such conversion (other than paying cash in lieu of delivering any fractional share), we will be required to make cash payments in respect of the Notes being converted. We may not have enough available cash or be able to obtain financing at the time we are required to make repurchases of Notes surrendered or Notes being converted. In addition, our ability to repurchase the Notes or to pay cash upon conversions of the Notes may be limited by law, by regulatory authority or by agreements governing our future indebtedness. Our failure to repurchase Notes at a time when the repurchase is required by the indenture governing such notes or to pay any cash payable on future conversions of the Notes as required by such indenture would constitute a default under such indenture. A default under the indenture governing the Notes or the fundamental change itself could also lead to a default under agreements governing our future indebtedness. If the repayment of the related indebtedness were to be accelerated after any applicable notice or grace periods, we may not have sufficient funds to repay the indebtedness and repurchase the Notes or make cash payments upon conversions.
In addition, our indebtedness, combined with our other financial obligations and contractual commitments, could have other important consequences. For example, it could:
•make us more vulnerable to adverse changes in general U.S. and worldwide economic, industry and competitive conditions and adverse changes in government regulation;
•limit our flexibility in planning for, or reacting to, changes in our business and our industry;
•place us at a disadvantage compared to our competitors who have less debt;
•limit our ability to borrow additional amounts to fund acquisitions, for working capital and for other general corporate purposes; and
•make an acquisition of our company less attractive or more difficult.
Any of these factors could harm our business, results of operations and financial condition. In addition, if we incur additional indebtedness, the risks related to our business and our ability to service or repay our indebtedness would increase.
The conditional conversion feature of the Notes, if triggered, may adversely affect our financial condition and operating results.
In the event the conditional conversion feature of the Notes is triggered, holders of the Notes will be entitled to convert the Notes at any time during specified periods at their option. If one or more holders elect to convert their Notes, unless we elect to satisfy our conversion obligation by delivering solely shares of our common stock (other than paying cash in lieu of delivering any fractional share), we would be required to settle a portion or all of our conversion obligation through the payment of cash, which could adversely affect our liquidity. In addition, even if holders do not elect to convert their Notes, we could be required under applicable accounting rules to reclassify all or a portion of the outstanding principal of the Notes as a current liability, rather than long-term liability, which would result in a material reduction of our net working capital.
Transactions relating to our Notes may affect the value of our common stock.
The conversion of some or all of the Notes would dilute the ownership interests of existing stockholders to the extent we satisfy our conversion obligation by delivering shares of our common stock upon any conversion of such Notes. Our Notes may become in the future convertible at the option of their holders under certain circumstances. If holders of our Notes elect to convert their notes, we may settle our conversion obligation by delivering to them a significant number of shares of our common stock, which would cause dilution to our existing stockholders.
In addition, in connection with the issuance of the Notes, we entered into the Capped Calls with certain financial institutions, or the Option Counterparties. The Capped Calls are generally expected to reduce potential dilution to our common stock upon any conversion or settlement of the Notes and/or offset any cash payments we are required to make in excess of the principal amount of converted Notes, with such reduction and/or offset subject to a cap.
In connection with establishing their initial hedges of the Capped Calls, the Option Counterparties or their respective affiliates entered into various derivative transactions with respect to our common stock and/or purchased shares of our common stock concurrently with or shortly after the pricing of the Notes.
From time to time, the Option Counterparties or their respective affiliates may modify their hedge positions by entering into or unwinding various derivative transactions with respect to our common stock and/or purchasing or selling our common stock or other securities of ours in secondary market transactions prior to the maturity of the Notes (and are likely to do so following any conversion of the Notes, any repurchase of the Notes by us on any fundamental change repurchase date, any redemption date, or any other date on which the Notes are retired by us, in each case, if we exercise our option to terminate the relevant portion of the Capped Calls). This activity could cause a decrease and/or increased volatility in the market price of our common stock.
We do not make any representation or prediction as to the direction or magnitude of any potential effect that the transactions described above may have on the price of the Notes or our common stock. In addition, we do not make any representation that the Option Counterparties will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
We are subject to counterparty risk with respect to the Capped Calls.
The Option Counterparties are financial institutions, and we will be subject to the risk that any or all of them might default under the Capped Calls. Our exposure to the credit risk of the Option Counterparties will not be secured by any collateral. Past global economic conditions have resulted in the actual or perceived failure or financial difficulties of many financial institutions. If an Option Counterparty becomes subject to insolvency proceedings, we will become an unsecured creditor in those proceedings with a claim equal to our exposure at that time under the Capped Calls with such Option Counterparty. Our exposure will depend on many factors but, generally, an increase in our exposure will be correlated to an increase in the market price and in the volatility of our common stock. In addition, upon a default by an Option Counterparty, we may suffer adverse tax consequences and more dilution than we currently anticipate with respect to our common stock. We can provide no assurances as to the financial stability or viability of the Option Counterparties.
The accounting method for convertible debt securities that may be settled in cash, such as the Notes, could have a material effect on our reported financial results.
The accounting method for reflecting the Notes on our condensed consolidated balance sheet, accruing interest expense for the Notes and reflecting the underlying shares of our common stock in our reported diluted earnings per share may adversely affect our reported earnings and financial condition.
In August 2020, the Financial Accounting Standards Board published an Accounting Standards Update, which we refer to as ASU 2020-06, which simplified certain of the accounting standards that apply to convertible notes. ASU 2020-06 became effective for us beginning January 1, 2022.
In accordance with ASU 2020-06, the Notes will be reflected as a liability on our condensed consolidated balance sheets, with the initial carrying amount equal to the principal amount of the Notes, net of issuance costs. The issuance costs will be treated as a debt discount for accounting purposes, which will be amortized into interest expense over the term of the Notes. As a result of this amortization, the interest expense that we expect to recognize for the Notes for accounting purposes will be greater than the cash interest payments we will pay on the Notes, which will result in lower reported net income or higher reported net loss, as the case may be.
In addition, we expect that the shares of common stock underlying the Notes will be reflected in our diluted earnings per share using the “if converted” method, in accordance with ASU 2020-06. Under that method, diluted earnings per share would generally be calculated assuming that all the Notes were converted solely into shares of common stock at the beginning of the reporting period, unless the result would be anti-dilutive. The application of the if-converted method may reduce our reported diluted earnings per share to the extent we are profitable in the future, and accounting standards may change in the future in a manner that may adversely affect our diluted earnings per share.
Furthermore, if any of the conditions to the convertibility of the Notes is satisfied, then we may be required under applicable accounting standards to reclassify the liability carrying value of the Notes as a current, rather than a long-term, liability. This reclassification could be required even if no holders convert their notes and could materially reduce our reported working capital.
ITEM 5. OTHER INFORMATION
Adoption of 10b5-1 Trading Plans by Our Officers and Directors
During our fiscal quarter ended September 30, 2023, certain of our directors entered into contracts, instructions or written plans for the purchase or sale of our securities that are intended to satisfy the conditions specified in Rule 10b5-1(c) under the Exchange Act for an affirmative defense against liability for trading in securities on the basis of material nonpublic information. We refer to these contracts, instructions, and written plans as “Rule 10b5-1 trading plans” and each one as a “Rule 10b5-1 trading plan.” We describe the material terms of these Rule 10b5-1 trading plans below.
Michael W. Bonney, Director
On August 10, 2023, Michael W. Bonney, a member of our board of directors, entered into a Rule 10b5-1 trading plan that provides that Mr. Bonney, acting through a broker, may sell up to an aggregate of 30,000 shares of our common stock received upon the exercise of options granted to Mr. Bonney as director compensation, subject to adjustments for stock splits, stock combinations, stock dividends and other similar changes to our common stock. Sales of shares under the plan may only occur if the market price of our common stock is above specified prices from November 13, 2023 to December 17, 2024. The plan is scheduled to terminate on December 17, 2024, subject to earlier termination upon the sale of all shares subject to the plan, upon termination by Mr. Bonney or the broker, or as otherwise provided in the plan.
David E.I. Pyott, Director
On August 11, 2023, David E.I. Pyott, a member of our board of directors, entered into a Rule 10b5-1 trading plan that provides that Mr. Pyott, acting through a broker, may sell up to an aggregate of 32,450 shares of our common stock received upon the exercise of options granted to Mr. Pyott as director compensation, subject to adjustments for stock splits, stock combinations, stock dividends and other similar changes to our common stock. Sales of shares under the plan may only occur if the market price of our common stock is above specified prices from November 16, 2023 to November 15, 2024. The plan is scheduled to terminate on November 15, 2024, subject to earlier termination upon the sale of all shares subject to the plan upon termination by Mr. Pyott or the broker, or as otherwise provided in the plan.
ITEM 6. EXHIBITS
|
|
|
|
|
|
10.1#† |
|
| 31.1# |
|
| 31.2# |
|
| 32.1#+ |
|
| 32.2#+ |
|
| 101.SCH |
Inline XBRL Taxonomy Extension Schema Document |
| 101.CAL |
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
| 101.LAB |
Inline XBRL Taxonomy Extension Label Linkbase Document |
| 101.PRE |
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
| 101.DEF |
Inline XBRL Taxonomy Extension Definition Linkbase Document |
| 104 |
Cover Page Interactive Data File (formatted as inline XBRL with applicable taxonomy extension information contained in Exhibits 101.*) |
|
|
|
|
| # |
Filed herewith. |
| † |
Portions of this exhibit (indicated by asterisks) have been omitted in accordance with the rules of the Securities and Exchange Commission because such information (i) is not material and (ii) would likely cause competitive harm to the Registrant if publicly disclosed. |
| + |
This certification will not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, or otherwise subject to the liability of that section. Such certification will not be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, except to the extent specifically incorporated by reference into such filing. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
ALNYLAM PHARMACEUTICALS, INC. |
|
|
|
| Date: November 2, 2023 |
|
/s/ Yvonne L. Greenstreet, MBChB, MBA |
|
|
Yvonne L. Greenstreet, MBChB, MBA
Chief Executive Officer
(Principal Executive Officer) |
|
|
|
| Date: November 2, 2023 |
|
/s/ Jeffrey V. Poulton |
|
|
Jeffrey V. Poulton
Executive Vice President, Chief Financial Officer
(Principal Financial and Accounting Officer) |
EX-10.1
2
alny2023q310-qex101.htm
EX-10.1
Document
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY [****], HAS BEEN OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL.
COLLABORATION AND LICENSE AGREEMENT
by and between
ALNYLAM PHARMACEUTICALS, INC.
on the one hand
and
F. Hoffmann-La Roche Ltd.
and
Genentech, Inc.
on the other hand.
EFFECTIVE DATE: July 21, 2023
TABLE OF CONTENTS
Schedules and Exhibits
|
|
|
|
|
|
Schedule 1.72 |
Co-Commercialization Costs Cap |
Schedule 1.114 |
Development Costs Cap |
Schedule 1.134 |
Exclusivity Period |
Schedule 1.175 |
KARDIA-6 Preliminary Key Elements and Considerations |
Schedule 1.190 |
KARDIA-3 Study Design Overview |
Schedule 1.254 |
Primary Indication Target Product Profile |
Schedule 1.351 |
Zilebesiran |
| Schedule 3.7(b) |
Development Data |
| Schedule 4.2(c)(i) |
Major Regulatory Communications |
| Schedule 6.2 |
Technology Transfer Documentation |
| Schedule 6.3(b) |
Preliminary Manufacturing Budget |
| Schedule 6.13 |
Approved Roche CMOs |
| Schedule 8.2(a) |
Financial Appendix |
|
|
| Exhibit A |
Initial Development Plan |
| Exhibit B |
Co-Promotion Term Sheet |
| Exhibit C |
[Intentionally deleted] |
| Exhibit D |
Commercial Supply Term Sheet |
|
|
|
|
|
|
| Exhibit E |
Alnylam Patents |
| Exhibit F |
Press Release |
COLLABORATION AND LICENSE AGREEMENT
This COLLABORATION AND LICENSE AGREEMENT (this “Agreement”) is entered into as of July 21, 2023 (the “Effective Date”) by and between ALNYLAM PHARMACEUTICALS, INC., a Delaware corporation having its principal place of business at 675 West Kendall Street, Cambridge, Massachusetts 02142 USA (“Alnylam”), on the one hand, and F. HOFFMANN-LA ROCHE LTD., having an office and place of business at Grenzacherstrasse 124, 4070 Basel, Switzerland (“Roche Basel”) and GENENTECH, INC., with an office and place of business at 1 DNA Way, South San Francisco, California 94080, (“Genentech”; each of Roche Basel and Genentech individually and collectively, “Roche”), on the other hand. Alnylam and Roche are sometimes referred to herein individually as a “Party” and collectively as the “Parties.”
RECITALS
WHEREAS, Roche is a fully integrated pharmaceutical company with expertise in the development, manufacture and commercialization of human therapeutic products;
WHEREAS, Alnylam is a biotechnology company with expertise and experience in the development of product candidates for multiple indications, including cardiovascular indications;
WHEREAS, Alnylam is developing Zilebesiran (as defined below), an investigational, subcutaneously administered siRNA therapeutic targeting liver-expressed angiotensinogen (“AGT”), which is in Phase 2 Clinical Trials for the treatment of hypertension; and
WHEREAS, Roche and Alnylam desire to establish a broad, worldwide, strategic collaboration for the joint continued development and, if successful, regulatory approval for, and commercialization of, Zilebesiran in hypertension and additional indications.
NOW THEREFORE, in consideration of the foregoing premises and the mutual promises, covenants and conditions contained in this Agreement, the Parties agree as follows:
ARTICLE 1
DEFINITIONS
1.1General. Capitalized terms used in this Agreement, whether used in the singular or plural, shall have the meanings ascribed to such terms in this Agreement, including as specified in this Article 1.
1.2“Accounting Standards” has the meaning set forth in the Financial Appendix.
1.3“Account Management Activities” has the meaning set forth in Section 5.10.
1.4“Acquired Party” has the meaning set forth in Section 7.8(a)(i).
1.5“Acquiring Party” has the meaning set forth in Section 7.8(a)(ii).
1.6“Acquiror” has the meaning set forth in Section 7.8(a)(i).
1.7“Additional Indication” has the meaning set forth in Section 3.5(b)(i).
1.8“Additional Program Plan” has the meaning set forth in Section 3.5(b)(i).
1.9“Additional Study” has the meaning set forth in Section 3.5(b)(i).
1.10“Administration Device” has the meaning set forth in Section 3.5(c)(i).
1.11“Affiliate” means, with respect to a particular Person, another Person that directly or indirectly controls, is controlled by, or is under common control with such Person. For the purposes of this definition, the word “control” (including, with correlative meaning, the terms “controlled by” or “under the common control with”) means the possession, either directly or indirectly through one or more intermediaries, of (a) power to control or cause the control of the management or policies of such entity (whether through ownership of securities or other ownership interests, by contract or otherwise), or (b) beneficial ownership of at least fifty percent (50%) of the voting securities or other ownership interest (whether directly or pursuant to any option, warrant or other similar arrangement) or other comparable equity interests of an entity. Notwithstanding the foregoing, neither the following entities nor their respective subsidiaries shall be Affiliates of Roche hereunder, unless and until Roche provides written notice to Alnylam stating that one or more of such entities or their respective subsidiaries are Affiliates of Roche hereunder: (i) Chugai, or its subsidiaries, [****] the “Roche Excluded Affiliates”).
1.12“Affiliate Sublicense Agreement” has the meaning set forth in Section 7.3(c)(i).
1.13“Agreement” has the meaning set forth in the preamble.
1.14“AGT” has the meaning set forth in the Recitals.
1.15“AGT EMO” means [****].
1.16“AGT EMO Acquisition” has the meaning set forth in Section 7.8(a)(ii).
1.17“AGT EMO Program” has the meaning set forth in Section 7.8(a)(i).
1.18“Alliance Manager” has the meaning set forth in Section 2.6.
1.19“Allowable Commercialization Expenses” has the meaning set forth in the Financial Appendix.
1.20“Alnylam” has the meaning set forth in the preamble.
1.21“Alnylam Background Know-How” means any and all Know-How to the extent Controlled by Alnylam or any of its Affiliates as of the Effective Date or during the Term, which Know-How has been used prior to the Effective Date in, or is otherwise necessary or reasonably useful for, the Development, Manufacture or Commercialization of any Products for use in the Field in the Territory in accordance with the terms of this Agreement, other than Alnylam Collaboration Know-How and Alnylam’s interest in Joint Know-How. For clarity, “Alnylam Background Know-How” (a) includes Alnylam Core Know-How and Alnylam Product-Specific Know-How and (b) excludes any and all Patent Rights and Alnylam Excluded IP.
1.22“Alnylam Background Patents” means any and all Patents Rights to the extent Controlled by Alnylam or any of its Affiliates as of the Effective Date or during the Term that Cover Alnylam Background Know-How. For clarity, the “Alnylam Background Patents” (a) includes Alnylam Core Patents and Alnylam Product-Specific Patents and (b) excludes Alnylam Excluded IP.
1.23“Alnylam Collaboration Know-How” means any and all Know-How to the extent Controlled by Alnylam or any of its Affiliates after the Effective Date and during the Term that (a) is necessary or reasonably useful for the Development, Manufacture or Commercialization of Products for use in the Field in the Territory in accordance with the terms of this Agreement and (b) is conceived or reduced to practice (in whole or in part) or otherwise identified, developed, made, or discovered solely by or on behalf of (including by subcontractors) Alnylam or any of its Related Parties in its conduct of the Collaboration activities under this Agreement. For clarity, “Alnylam Collaboration Know-How” (i) may include Alnylam Core Know-How and Alnylam Product-Specific Know-How and (ii) excludes any and all Patent Rights and Alnylam Excluded IP.
1.24“Alnylam Collaboration Patents” means any and all Patents Rights to the extent Controlled by Alnylam or any of its Affiliates during the Term that Cover any Alnylam Collaboration Know-How. For clarity, “Alnylam Collaboration Patents” (a) excludes Alnylam’s interest in Joint Patents and Alnylam Excluded IP and (b) may include Alnylam Core Patents and Alnylam Product-Specific Patents.
1.25“Alnylam Core Improvements” has the meaning set forth in Section 1.182.
1.26“Alnylam Core Know-How” means any and all Alnylam Know-How other than (a) Alnylam Product-Specific Know-How, (b) Alnylam’s interest in Joint Know-How and (c) Alnylam Excluded IP.
1.27“Alnylam Core Patents” means any and all Alnylam Patents other than Alnylam Product-Specific Patents, excluding any (a) Alnylam Excluded IP and (b) Alnylam’s interest in Joint Patents.
1.28“Alnylam Excluded IP” means any and all [****] and (c) Know-How and Patent Rights specifically related to any active pharmaceutical ingredient, but not Zilebesiran or REVERSIR.
1.29“Alnylam Indemnitee” has the meaning set forth in Section 11.2.
1.30“Alnylam Know-How” means any and all Alnylam Background Know-How, Alnylam Collaboration Know-How and Alnylam’s interest in Joint Know-How.
1.31“Alnylam Lead Study” has the meaning set forth in Section 3.6(a).
1.32“Alnylam Licensed IP” means the Alnylam Know-How and Alnylam Patents, in each case, excluding any Alnylam Excluded IP.
1.33“Alnylam Manufacturing Change” has the meaning set forth in Section 6.10(c)(i).
1.34“Alnylam-Originated Transfer Activities” has the meaning set forth in Section 13.8(e)(iii).
1.35“Alnylam Patents” means any and all Alnylam Background Patents, Alnylam Collaboration Patents and Alnylam’s interest in Joint Patents. For clarity, Alnylam Patents include the Patent Rights disclosed in Exhibit E.
1.36“Alnylam Product-Specific Know-How” means, on a Product-by-Product basis, [****].
1.37“Alnylam Product-Specific Patents” means any and all Patent Rights to the extent Controlled by Alnylam or any of its Affiliates as of the Effective Date or during the Term that Cover Alnylam Product-Specific Know-How.
1.38“Anti-Corruption Laws” means any and all anti-bribery and anti-corruption Applicable Laws, including the U.S. Foreign Corruption Practices Act of 1977, the U.S. Travel Act, the U.K. Bribery Act 2010, and Applicable Laws implementing the OECD Convention on Combating Bribery of Foreign Public Officials in International Business Transactions.
1.39“APAC” means, collectively, [****].
1.40“API” means active pharmaceutical ingredient.
1.41“Applicable Data Protection Laws” has the meaning set forth in Section 15.16.
1.42“Applicable Laws” means, individually and collectively, all laws, statutes, ordinances, codes, regulations, rules, orders, writs, judgments, injunctions, decrees, stipulations or rulings of any kind of any Governmental Authority that may be in effect from time to time and applicable to the activities contemplated by this Agreement.
1.43[****].
1.44“Assigning Party” has the meaning set forth in Section 9.2(d).
1.45“Auditor” has the meaning set forth in Section 8.12(a).
1.46“Bankruptcy Code” has the meaning set forth in Section 13.12.
1.47[****].
1.48[****].
1.49[****].
1.50“Breaching Party” has the meaning set forth in Section 13.5(a).
1.51“Bridging Study” means any Non-Clinical Study or human clinical trial conducted in the Roche Territory for the purpose of providing country or region-specific Development Data on safety, efficacy, dosage, or dosing regimen to permit the extrapolation of other Development Data to a population in the Roche Territory or in relation to Regulatory Approvals of a Product in such country, or any other Non-Clinical Study or Clinical Trial designed to demonstrate equivalency by extrapolating results from a different study.
1.52“Business Day” means a day other than a Saturday, Sunday, or a bank or other public or federal holiday in Boston, Massachusetts; San Francisco, California; or Basel, Switzerland.
1.53“Calculated Amounts” has the meaning given such term in Section 8.12(a).
1.54“Calendar Quarter” means for each Calendar Year, each of the three (3)-month periods ending March 31, June 30, September 30 and December 31; provided that (a) the first Calendar Quarter of the Term shall begin on the Effective Date and end on the first to occur of March 31, June 30, September 30 or December 31 thereafter and the last Calendar Quarter of the Term shall end on the last day of the Term, and (b) the first Calendar Quarter of a Royalty Term for a Product in a country shall begin on the First Commercial Sale of such Product in such country and end on the first to occur of March 31, June 30, September 30 or December 31 thereafter.
1.55“Calendar Quarter Report” has the meaning set forth in Section 8.7(d).
1.56“Calendar Year” means each successive period of twelve (12) months commencing on January 1 and ending on December 31; provided that (a) the first Calendar Year of the Term shall begin on the Effective Date and end on the first December 31 thereafter and the last Calendar Year of the Term shall end on the last day of the Term, and (b) the first Calendar Year of a Royalty Term for a Product in a country shall begin on the First Commercial Sale of such Product in such country and end on the first December 31 thereafter.
1.57“Certification Notice” has the meaning set forth in Section 9.8.
1.58“Certification Notice Party” has the meaning set forth in Section 9.8.
1.59“Change of Control” means, with respect to a Party (or its ultimate parent), (a) a merger, acquisition, consolidation or reorganization of such Party (or its ultimate parent) with a Third Party that results in the voting securities of such Party (or its ultimate parent) outstanding immediately prior thereto, or any securities into which such voting securities have been converted or exchanged, ceasing to represent more than fifty percent (50%) of the combined voting power of the outstanding securities of the surviving entity or the parent of the surviving entity immediately after such transaction, or (b) a transaction or series of related transactions in which a Third Party or group, together with their respective Affiliates, becomes the “beneficial owner” (as such term is used in Section 13(d) of the Securities Exchange Act of 1934 and Rule 13d-3 thereunder, except that a Person shall be deemed to have “beneficial ownership” of all shares that any such Person or its Affiliates has the right to acquire, whether such right may be exercised immediately or only after the passage of time), directly or indirectly, of more than fifty percent (50%) of the combined voting power of the outstanding securities of such Party (or its ultimate parent), or (c) the sale or other transfer to a Third Party or group, whether directly or indirectly by a Party or an Affiliate thereof, of all or substantially all of such Party’s (or its ultimate parent’s) business to which the subject matter of this Agreement relates.
1.60“Chugai” means Chugai Pharmaceutical Co. Ltd., a Japanese corporation having an address at 1-1 Nihonbashi-Muromachi 2-chome, Chuo-ku Tokyo, 103-8324, Japan.
1.61[****].
1.62“Chugai Sublicense Agreement” has the meaning set forth in Section 7.3(b)(ii).
1.63“Clinical Supply Agreement” has the meaning set forth in Section 6.7(b).
1.64“Clinical Supply Costs” has the meaning set forth in the Financial Appendix.
1.65“Clinical Trial” means any Phase 1 Clinical Trial, Phase 2 Clinical Trial, Phase 3 Clinical Trial, Bridging Study that is not a Non-Clinical Study, or Phase 4 Clinical Trial.
1.66“CMC” means chemistry, manufacturing, and controls.
1.67“CMC Manufacturing Development” means the activities to enable development of the design of the Manufacturing process for the supply of clinical and commercial quantities of a pharmaceutical product or device, which includes, as applicable, Manufacturing process development and Manufacturing process scale-up (but excluding manufacturing facilities scale-up), test method development and stability testing, bulk production and fill/finish work associated with the supply of such pharmaceutical product (including for Non-Clinical Studies, Clinical Trials and commercial sale) and related quality assurance technical support activities as well as validation and qualification of commercial Manufacturing processes.
1.68“CMO” means a Third Party contract manufacturing organization contracted by a Party or any of its Affiliates to Manufacture Product to fulfill certain of such Party’s Manufacturing obligations under this Agreement.
1.69[****].
1.70“Co-Commercialization Budget” has the meaning set forth in Section 5.4.
1.71“Co-Commercialization Costs” has the meaning set forth in the Financial Appendix.
1.72[****].
1.73[****].
1.74“Co-Commercialization Opt-Out Notice” has the meaning set forth in Section 13.9(a).
1.75“Co-Commercialization Plan” has the meaning set forth in Section 5.3.
1.76“Co-Commercialization Term” means, with respect to the Co-Commercialization Territory, the period commencing on the start of the Launch Preparation Period and continuing until a Co-Commercialization Termination Date determined in accordance with Section 13.9.
1.77“Co-Commercialization Termination Date” has the meaning set forth in Section 13.9(a).
1.78“Co-Commercialization Territory” means the U.S.
1.79“Co-Promotion” or “Co-Promote” means, on a Product-by-Product basis, the detailing, marketing and promotional activities (including performing sales calls) for such Product for use in the Field performed by personnel of one or both Parties in the Co-Commercialization Territory in accordance with the Co-Promotion Agreement, Co-Promotion Plan and Co-Commercialization Plan.
1.80“Co-Promotion Agreement” has the meaning set forth in Section 5.10.
1.81“Co-Promotion Plan” has the meaning set forth in Section 5.10.
1.82“Co-Promotion Term Sheet” has the meaning set forth in Section 5.10.
1.83“Code” means the U.S. Internal Revenue Code of 1986.
1.84“Collaboration” means the Development, Manufacturing, and Commercialization activities undertaken by the Parties under this Agreement.
1.85“Combination Product” means a product that includes Zilebesiran or REVERSIR (for clarity, or both) as an active pharmaceutical ingredient combined with or comprised of (whether co-formulated, co-packaged or packaged separately but sold together for a single price) one (1) or more other active pharmaceutical ingredient(s) or pharmaceutical product(s).
1.86“Commercial Medical Affairs Activities” means Medical Affairs Activities for the Commercialization of a Product in the Field, including the coordination of medical information requests and field-based medical science liaisons, and activities of medical science liaisons, (a) with respect to the Co-Commercialization Territory, following the start of the Launch Preparation Period, and (b) with respect to the Roche Territory, following the First Commercial Sale of such Product in the Roche Territory.
1.87“Commercial Supply Agreement” has the meaning set forth in Section 6.9(b).
1.88“Commercial Supply Costs” has the meaning set forth in the Financial Appendix.
1.89“Commercial Supply Term Sheet” has the meaning set forth in Section 6.9(b)
1.90“Commercialization” means, with respect to a pharmaceutical product, the conduct of all activities relating to the promotion, sales, marketing and distribution (including importing, exporting, transporting, customs clearance, warehousing, invoicing, handling, and delivering products to customers) of such product, including sales force efforts, detailing, advertising, marketing, sales force training, market research and market access (including price setting and reimbursement activities); provided that, with respect to a Product, Commercialization shall refer to such activities (x) with respect to the Co-Commercialization Territory, following the start of the Launch Preparation Period for such Product and (y) with respect to the Roche Territory, following the First Commercial Sale of such Product for use in the Field in the Roche Territory, including Commercial Medical Affairs Activities. “Commercialize” has a correlative meaning.
1.91“Commercialization Records” has the meaning set forth in Section 5.15.
1.92“Commercialization Report” has the meaning set forth in Section 5.15.
1.93“Commercially Reasonable Efforts” means with respect to the obligations of a Party under this Agreement that relate to the Development, Manufacture or Commercialization of a Product, the carrying out of such obligations [****] products of similar market potential at a similar stage in development or product life, taking into account, to the extent reasonable and relevant and measured by the facts and circumstances at the time such efforts are due, all relevant factors, including issues of safety and efficacy, product labeling or anticipated labeling, competitiveness of the applicable Product and other competitive products in the marketplace or under development, the patent or other proprietary position of the applicable Product, the regulatory structure involved and the potential profitability of the applicable Product marketed or to be marketed, [****].
1.94“Committee” means the Joint Steering Committee, any Operating Committee, or the JPT, as applicable.
1.95“Compulsory Sublicense” has the meaning set forth in Section 1.96.
1.96“Compulsory Sublicense Compensation” means for a given country or region in the Territory, [****] through the order, decree or grant of a Governmental Authority having competent jurisdiction in such country or region, authorizing such Third Party to manufacture, use, sell, offer for sale, import or export a Product in the Field in such country or region.
1.97“Compulsory Sublicensee” has the meaning set forth in Section 1.96.
1.98“Confidential Information” has the meaning set forth in Section 12.1.
1.99“Confirmatory Clinical Trial” means a clinical trial, irrespective of phase, of a drug product in human subjects required to be conducted as part of a contingent Regulatory Approval of such drug product.
1.100“Continuation Election Notice” has the meaning set forth in Section 13.8(c).
1.101“Continuing Party” has the meaning set forth in Section 13.9(a).
1.102“Control” means, with respect to any intellectual property right that a Party, directly or through an Affiliate, (a) owns such intellectual property right, or (b) has a license or right to use such intellectual property right (but without taking into account any rights granted by one Party to the other Party pursuant to this Agreement), in each case with the ability to grant to the other Party access, a right to use, a license, or a sublicense (as applicable) to such intellectual property right on the terms and conditions set forth herein, without violating the terms of any agreement or other arrangement with any Third Party or any Applicable Laws.
1.103“Core Disease Awareness Materials” has the meaning set forth in Section 5.11(a)(i).
1.104“Core Promotional Materials” means core visual aids for healthcare providers, patient brochures, value proposition for payors, and direct-to-consumer healthcare provider and patient campaigns for Non-Personal Digital Promotions, in each case, with respect to Products.
1.105“Cover” means (a) as to any product and Patent Right, that, in the absence of a license granted under, or ownership of, such Patent Right, the Development, Manufacture, Commercialization or other exploitation of such pharmaceutical product would infringe a Valid Claim of such Patent Right (or, in the case of any pending claim included in such Patent Right, would infringe such Patent Right if such pending claim were to issue in an issued patent without modification), (b) as to Know-How and a Patent Right, that, in the absence of a license granted under, or ownership of, such Patent Right, the use or practice of such Know-How would infringe a Valid Claim of such Patent Right (or, in the case of any pending claim included in such Patent Right, would infringe such Patent Right if such pending claim were to issue in an issued patent without modification), and (c) as to a pharmaceutical product or technology and Know-How, that the exploitation of such pharmaceutical product or technology incorporates, uses, employs, embodies, or practices such Know-How. The determination of whether any of the foregoing, as applicable, are Covered by a particular Valid Claim shall be made on a country-by-country basis.
1.106“Covered Indications” means all Indications, provided that [****]. For clarity, the Hypertension Indication and subsets of the Hypertension Indication (e.g., primary hypertension or secondary hypertension) shall remain Covered Indications at all times during the Term.
1.107“Damages” has the meaning set forth in Section 11.1.
1.108“Data Subjects” has the meaning set forth in Section 15.16.
1.109“Derivative Disease Awareness Materials” has the meaning set forth in Section 5.11(a)(i).
1.110“Derivative Promotional Materials” has the meaning set forth in Section 5.11(a)(i).
1.111“Development” means, with respect to a pharmaceutical product or device, as applicable, all activities related to the discovery, research, preclinical development, clinical development, and Regulatory Activities with respect to such product or device, toxicology, design, compatibility testing, animal efficacy studies, formulation, quality assurance/quality control development and support activities, statistical analysis and report writing, Clinical Trials, and Development Medical Affairs Activities, preparing and submitting Regulatory Materials (including applications for Regulatory Approval), together with Regulatory Activities with respect thereto, whether before or after Regulatory Approval for such product has been obtained; provided that “Development” shall exclude Commercialization (including, for the avoidance of doubt, the conduct of Post-Approval Studies) and Manufacture (including, for the avoidance of doubt, the conduct of CMC Manufacturing Development). “Develop” has a correlative meaning.
1.112“Development Budget” has the meaning set forth in Section 3.3.
1.113“Development Costs” has the meaning set forth in the Financial Appendix.
1.114[****].
1.115[****].
1.116“Development Data” means any and all scientific, technical, test, or other data related to any Product that is generated by or on behalf of either Party or any of its Related Parties (or subcontractors acting on their behalf), under the Development Plan, including research data, clinical pharmacology data, CMC data (including analytical and quality control data and stability data), preclinical data, clinical data, clinical study reports, and all data used in connection with submissions and approvals of an IND or NDA in the Territory.
1.117“Development Lead Option” has the meaning set forth in Section 3.6(b)(ii).
1.118“Development Medical Affairs Activities” means Medical Affairs Activities for a Product conducted to support the Development of such Product in the Field in the Territory (for clarity, excluding Commercialization and Manufacturing activities for such Product), (a) with respect to the Co-Commercialization Territory prior to the start of the Launch Preparation Period, and (b) with respect to the Roche Territory prior to the First Commercial Sale of such Product in the Roche Territory.
1.119“Development Milestone” has the meaning set forth in Section 8.5(a).
1.120“Development Milestone Payment” has the meaning set forth in Section 8.5(a).
1.121“Development Plan” has the meaning set forth in Section 3.1.
1.122“Development Records” has the meaning set forth in Section 3.9.
1.123“Development Report” has the meaning set forth in Section 3.9.
1.124“Device Program Plan” has the meaning set forth in Section 3.5(c)(i).
1.125“Direct Costs” has the meaning set forth in the Financial Appendix.
1.126“Directed to” means, with respect to siRNA and a target, that such siRNA binds to and interferes with the function of any messenger RNA encoded by such target. For clarity, in the event a siRNA has been engineered to bind to and interfere with the function of any messenger RNA encoded by a particular target other than AGT (and has not be engineered to bind to and interfere with the function of any messenger RNA encoded by AGT) but such siRNA additionally binds to or interferes with the function of the messenger RNA encoded by AGT, either directly or indirectly, then such product will not be deemed to be Directed to AGT.
1.127“Discloser” has the meaning set forth in Section 12.1.
1.128“Disease Awareness Materials” means any and all disease awareness and disease education materials to be used by a Party, its Related Parties or its subcontractors acting on their behalf in connection with Commercial Medical Affairs Activities for Products for use in the Field.
1.129“Disputed Matter” has the meaning set forth in Section 14.1.
1.130“Divestment Period” has the meaning set forth in Section 7.8(c)(i).
1.131“e-Signature” has the meaning set forth in Section 15.17.
1.132“Effective Date” has the meaning set forth in the preamble.
1.133“Europe” means, collectively, [****].
1.134“Exclusivity Period” has the meaning set forth in [****].
1.135“Executive Officer” means (a) in the case of Roche, the Chief Executive Officer of the Roche Pharmaceuticals Division for Development, Manufacturing, Regulatory and Commercialization related issues, and for all other issues the Head of Pharma Partnering for the Roche Pharmaceuticals Division, or, in each case his or her designee; and (b) in the case of Alnylam, its Chief Executive Officer or his or her designee.
1.136“Existing Confidentiality Agreement” means that certain Mutual Confidential Disclosure Agreement, by and between Roche Ltd. and Alnylam, dated as of [****], as amended by that certain Amendment No. 1 to Mutual Confidential Disclosure Agreement, dated as of Effective Date.
1.137“Existing Process Technology Transfer” has the meaning set forth in Section 6.8(a).
1.138“Existing Process Technology Transfer Plan” has the meaning set forth in Section 6.8(a).
1.139“Expanded Access Program” means the use of investigational new drug products outside of Clinical Trials to treat patients with serious or immediately life-threatening disease or conditions when there are no comparable or satisfactory alternative treatment options, as described in 21 CFR Section 312.305 or comparable Applicable Laws in jurisdictions outside the U.S. “Expanded Access Program” includes so-called “treatment IND sales,” “named patient sales,” and “compassionate use sales.”
1.140“Expiration Condition” has the meaning set forth in Section 13.9(a).
1.141[****].
1.142“FD&C Act” means the U.S. Federal Food, Drug and Cosmetic Act.
1.143“FDA” means the U.S. Food and Drug Administration or its successor.
1.144“Field” means all uses; provided that “Field” shall exclude [****].
1.145“Financial Appendix” has the meaning set forth in Section 8.2(a).
1.146“First Commercial Sale” means, on a country-by-country basis, the first commercial sale of a Product in a country by or on behalf of Roche or any of its Related Parties (or subcontractors acting on their behalf) to a Third Party (other than a Related Party of Roche) or Governmental Authority following Regulatory Approval of such Product in such country. If no such Regulatory Approval or similar marketing approval is required in a country for marketing and sale of a Product, First Commercial Sale means the date upon which such Product is first commercially sold in such country to end users. Sales or transfers of reasonable quantities of a Product for research or Development, including proof of concept studies or other Clinical Trial purposes, or for compassionate or similar use, shall not be considered a First Commercial Sale; provided that [****].
1.147“First Patient In” or “FPI” means, with respect to a Clinical Trial of Product, the date that the first subject is dosed in such Clinical Trial.
1.148“Flash Sales Report” has the meaning set forth in Section 8.7(d)(i).
1.149“force majeure” has the meaning set forth in Section 15.2.
1.150“GCPs” means the then-current standards, practices and procedures promulgated or endorsed by FDA for the design, conduct, performance, monitoring, auditing, recording, analyses, and reporting of clinical trials as set forth in the guidelines titled “Guidance for Industry E6 Good Clinical Practice: Consolidated Guidance,” (which also titled by FDA as “E6(R2) Good Clinical Practice: Integrated Addendum to ICH E6(R1)”), including related regulatory requirements imposed by FDA, including 21 CFR Parts 50, 54, and 56, as well as comparable Applicable Laws in jurisdictions outside the U.S. that provide assurance that the data and reported results are credible and accurate, and that the rights, integrity, and confidentiality of trial subjects are protected.
1.151“Genentech” has the meaning set forth in the preamble.
1.152“Generic Product” means, with respect to a particular Product, any pharmaceutical product that (a) contains (i) if such Product is a Therapeutic Product, Zilebesiran, or (ii) if such Product is a REVERSIR Product, REVERSIR, in each case as an active pharmaceutical ingredient, (b) is not distributed or sold by, with respect to the Co-Commercialization Territory, either Party or any of its Related Parties (or subcontractors acting on their behalf), or with respect to the Roche Territory, Roche or any of its Related Parties (or subcontractors acting on their behalf) and (c) is distributed or sold under a separate Regulatory Approval application approved by a Regulatory Authority in reliance, in whole or in part, on a Party’s or any of its Related Party’s (or a subcontractor’s acting on their behalf) Regulatory Approval application for the applicable Product in such country (or on safety or efficacy data submitted by any such Person in support of the Regulatory Approval application for the applicable Product in such country), including any product authorized for sale (i) in the U.S. pursuant to Section 505(b)(2) or Section 505(j) of the FFDCA (21 U.S.C. § 355(b)(2) and 21 U.S.C. § 355(j), respectively), (ii) in the European Union pursuant to a provision of Articles 10, 10a or 10b of Parliament and Council Directive 2001/83/EC (including an application under Article 6.1 of Parliament and Council Regulation (EC) No. 726/2004 that relies for its content on any such provision) or (iii) in any other country or jurisdiction pursuant to comparable Applicable Laws or if no such comparable Applicable Laws exist in such country or jurisdiction, is approved by an expedited process that relies in whole or in part on safety and efficacy data generated for the first Regulatory Approval of the Product.
1.153“Generic Product Entry” means, in a given country in the Territory, if one or more Generic Products with respect to a given Product is sold in any Calendar Quarter.
1.154“Global Brand Strategy” has the meaning set forth in Section 5.2.
1.155“GLPs” means the then-current good laboratory practice standards promulgated or endorsed by FDA as defined in 21 C.F.R. Part 58, and comparable Applicable Laws in jurisdictions outside the U.S.
1.156“GMPs” means the then-current good manufacturing practices required by FDA under section 501(a) of the FD&C Act or other applicable statutes, including 21 C.F.R. Parts 210 and 211 and other Applicable Laws promulgated thereunder, for the manufacture and testing of pharmaceutical materials, and comparable Applicable Laws applicable to the manufacture and testing of pharmaceutical materials in jurisdictions outside the U.S. For clarity, GMPs shall include applicable quality guidelines promulgated under the International Conference on Harmonization.
1.157“Governmental Authority” means any multi-national, federal, state, local, municipal or other government authority of any nature (including any governmental division, subdivision, department, agency, bureau, branch, office, commission, council, court or other tribunal).
1.158“H-W Suit Notice” has the meaning set forth in Section 9.8.
1.159[****].
1.160[****].
1.161“Hypertension Indication” means any of the following Indications: (a) the Primary Indication or (b) treatment of essential (primary) or secondary hypertension to lower blood pressure [****].
1.162“ICC” has the meaning set forth in Section 14.2(a).
1.163“ICC Court” has the meaning set forth in Section 14.2(c).
1.164“ICD-11” means the Eleventh Revision of the International Statistical Classifications of Diseases and Related Health Problems, as may be revised or amended from time to time, or a successor classification.
1.165“Idle Capacity” has the meaning set forth in the Financial Appendix.
1.166“IND” means (a) an Investigational New Drug Application as defined in the FD&C Act and applicable regulations promulgated thereunder by FDA, or (b) any comparable submission to the applicable Regulatory Authority in any other regulatory jurisdiction that is required to initiate or conduct clinical testing of a pharmaceutical product in humans in such jurisdiction (including any Clinical Trial Authorizations (“CTA”) submitted to the EMA).
1.167“Indemnification Claim Notice” has the meaning set forth in Section 11.3(a).
1.168“Indemnifying Party” has the meaning set forth in Section 11.3(a).
1.169“Indemnitee” has the meaning set forth in Section 11.3(a).
1.170“Indication” means any distinct indication or patient population in the Field, determined as follows: (a) to distinguish one indication from another indication, the two indications have to be either (i) listed in two different blocks of the ICD-11 (as a way of example, any essential hypertension under BA00 is in a different block from any hypertensive disease under block BA01, whereas BA00.0 and BA00.1 belong to the same block) or (ii) Developed by the Parties (as applicable) under separate Clinical Trials, and (b) to distinguish one patient population from another patient population, the two patient populations must be investigated in separate Phase 2 Clinical Trials or Phase 3 Clinical Trials (e.g., recently diagnosed treatment naive hypertension would be distinct from high-risk patients with uncontrolled hypertension); provided that, with respect to the foregoing (i) and (ii), if a Phase 2 Clinical Trial or Phase 3 Clinical Trial is conducted with patients with two (2) or more indications or patients consisting of two (2) or more patient populations (e.g., such Clinical Trial includes both recently diagnosed treatment naïve hypertension patients with high cardiovascular risk and recently diagnosed treatment naïve hypertension patients without cardiovascular risk) and a Phase 2 Clinical Trial or Phase 3 Clinical Trial is separately conducted with only one of those indications or patient populations (e.g., treatment naïve hypertension patients with high cardiovascular risk), then such indication or patient population for which the second Phase 2 Clinical Trial or Phase 3 Clinical Trial (i.e., recently diagnosed treatment naïve hypertension patients without cardiovascular risk) shall be deemed to be a distinct indication or patient population from the first Phase 2 Clinical Trial or Phase 3 Clinical Trial for purposes of this definition. By way of example and without limiting the foregoing, each of the following shall be deemed [****].
1.171“Indirect Costs” has the meaning set forth in the Financial Appendix.
1.172“Indirect Taxes” has the meaning set forth in Section 8.8(d).
1.173“Information Security Incident” means, with respect to Confidential Information, any unauthorized use, unauthorized disclosure, corruption (including ransomware attack) or similar loss of such Confidential Information.
1.174“Initial Co-Commercialization Plan” has the meaning set forth in Section 5.5.
1.175“Initial CVOT Study” means any cardiovascular outcomes Phase 3 Clinical Trial of the Therapeutic Product as an add-on to standard of care in patients with uncontrolled hypertension and who are at high risk of cardiovascular events[****].
1.176“Initial Development Plan” has the meaning set forth in Section 3.4.
1.177“Initial Manufacturing Plan” has the meaning set forth in Section 6.4.
1.178[****].
1.179[****].
1.180“Joint Commercialization Committee” or “JCC” has the meaning set forth in Section 2.4(a).
1.181“Joint Development Committee” or “JDC” has the meaning set forth in Section 2.2(a).
1.182“Joint Know-How” means any and all Know-How first conceived, reduced to practice or otherwise identified, developed made or discovered, jointly by or on behalf of (including by subcontractors) Roche or any of its Related Parties, on the one hand, and (including by subcontractors) Alnylam or any of its Related Parties, on the other hand, in the course of conducting the Collaboration; provided, however, that notwithstanding the foregoing, any invention or discovery first conceived or reduced to practice or, with respect to any invention or discovery other than patentable inventions, otherwise identified, developed, made or discovered, jointly by or on behalf of employees or consultants of Roche or any of its Related Parties, on the one hand, and Alnylam or any of its Related Parties, on the other hand in the course of conducting the Collaboration that (a) is an improvement to, or modification of, Alnylam Core Know-How, including any Patent Rights that Cover such inventions or discoveries (the “Alnylam Core Improvements”), shall be Alnylam Collaboration Know-How or Alnylam Collaboration Patents, as applicable, and owned by Alnylam pursuant to Section 9.2(a); or (b) is an improvement to, or modification of, Roche Core Know-How, including any Patent Rights that Cover such inventions or discoveries (the “Roche Core Improvements”), shall be Roche Collaboration Know-How or Roche Collaboration Patents, as applicable, and owned by Roche pursuant to Section 9.2(b).
1.183“Joint Manufacturing Committee” or “JMC” has the meaning set forth in Section 2.3(a).
1.184“Joint MLRB” has the meaning set forth in Section 5.11(a)(ii).
1.185“Joint Patents” means any and all Patents Rights Covering Joint Know-How to the extent Controlled by the Parties. For clarity, “Joint Patents” does not include: (a) Alnylam Background Patents, (b) Alnylam Collaboration Patents, (c) Roche Background Patents or (d) Roche Collaboration Patents.
1.186“Joint Program Team” or “JPT” means the cross-functional joint program team described in Section 2.9.
1.187“Joint Steering Committee” or “JSC” has the meaning set forth in Section 2.1(a).
1.188“KARDIA-1” means the Phase 2 Clinical Trial entitled “A Study to Evaluate Efficacy and Safety of ALN-AGT01 in Patients with Mild To-Moderate Hypertension” (NCT04936035). For clarity, KARDIA-1 is an Alnylam Lead Study and is set forth in the Initial Development Plan.
1.189“KARDIA-2” means the Phase 2 Clinical Trial entitled “Zilebesiran as an Add-on Therapy in Patients With Hypertension Not Adequately Controlled by a Standard of Care Antihypertensive Medication” (NCT05103332). For clarity, KARDIA-2 is an Alnylam Lead Study and is set forth in the Initial Development Plan.
1.190“KARDIA-3” means the Phase 2 Clinical Trial of Therapeutic Product in patients with uncontrolled hypertension and at high cardiovascular risk. For clarity, KARDIA-3 is an Alnylam Lead Study and is set forth in the Initial Development Plan, and [****].
1.191“Know-How” means all technical, scientific, regulatory, business and other information, results, knowledge, techniques and data, in whatever form and whether or not patented or patentable, including inventions, invention disclosures, discoveries, plans, processes, practices, methods, knowledge, trade secrets, know-how, instructions, skill, experience, ideas, concepts, drawings, data (including biological, chemical, pharmacological, toxicological, pharmaceutical, physical and analytical, safety, quality control, and preclinical and clinical data), manufacturing documentation (including manufacturing batch records and documentation supporting cGMP), formulae, formulations, compositions, specifications, marketing, pricing, distribution, costs, sales and manufacturing data or descriptions, cells, cell lines, assays, chemical structures, chemical sequences and other physical, biological, and chemical materials, expertise, and technology that are confidential and necessary or useful in the discovery, manufacture, research, development and/or commercialization of Products, and all derivatives, modifications, and improvements of the foregoing. For clarity, “Know-How” does not include Patent Rights Covering any of the foregoing.
1.192“Launch Preparation Period” has the meaning set forth in the Financial Appendix.
1.193“Lead Development Party” has the meaning set forth in Section 3.6.
1.194“Lead Regulatory Party” has the meaning set forth in Section 4.2.
1.195“Long Term Development Budget” has the meaning set forth in Section 3.3.
1.196“Long Term Development Plan” has the meaning set forth in Section 3.2(d).
1.197“Major Markets” means [****].
1.198[****].
1.199“Major Regulatory Communication” has the meaning set forth in Section 4.2(c)(i).
1.200“Manufacture” and “Manufacturing” means all activities related to the production, manufacturing, processing, filling, finishing, packaging, labeling, assembling, shipping, and holding of any product, or any intermediate or component thereof, and any placebo, as the case may be (including any devices or other delivery technologies that are packaged or distributed with such product), including process development, process qualification and validation, scale-up (including manufacturing facility scale-up), preclinical, clinical and commercial manufacture and analytic development, product characterization, stability testing, quality assurance, and quality control, and management of any Third Parties conducting such activities.
1.201“Manufacturing Budget” has the meaning set forth in Section 6.3(a).
1.202“Manufacturing Changes” has the meaning set forth in Section 6.10.
1.203“Manufacturing Plan” has the meaning set forth in Section 6.2.
1.204“Manufacturing Records” has the meaning set forth in Section 6.12.
1.205“Manufacturing Reports” has the meaning set forth in Section 6.12.
1.206“Medical Affairs Activities” means with respect to the Product in the Field in the Territory activities of medical science liaisons, activities involving key opinion leaders and professional societies, the provision of medical information services, evidence and real world evidence generation strategy, coordination and development of content to address medical information requests, health economics and outcomes research, publication and congress planning and continuing medical education.
1.207“MicroRNA” or “miRNA” means a structurally defined functional RNA molecule usually between nineteen (19) and twenty-five (25) nucleotides in length, which is derived from an endogenous, genetically encoded RNA which is predicted to fold into a hairpin RNA structure that is a substrate for the double-stranded RNA-specific ribonuclease drosha and subsequently is predicted to serve as a substrate for the enzyme dicer, a member of the ribonuclease III enzyme family.
1.208“MicroRNA Mimic” means a single-stranded or double-stranded oligonucleotide with the same base composition and sequence (including chemically modified bases) [****] as a particular natural miRNA and which is designed to mimic the activity of such miRNA. For clarity, MicroRNA Mimic excludes a double-stranded oligonucleotide which functions or is designed to function as a siRNA.
1.209[****].
1.210“NDA” means (a) a New Drug Application or supplemental New Drug Application, as defined in the FD&C Act and applicable regulations promulgated thereunder by FDA, or (b) any comparable submission to the applicable Regulatory Authority in any other regulatory jurisdiction that is required to sell a pharmaceutical product in such jurisdiction. including any Marketing Authorization Application (“MAA”) in any jurisdiction.
1.211“NDC” means the FDA National Drug Code.
1.212“Net Profits and Net Losses” has the meaning set forth in the Financial Appendix.
1.213“Net Sales” means [****].
1.214“Net Sales Deduction” has the meaning set forth in Section 1.213.
1.215“New Manufacturing Process” has the meaning set forth in Section 6.8(b).
1.216“New Manufacturing Process Technology Transfer” has the meaning set forth in Section 6.8(b).
1.217“New Manufacturing Process Technology Transfer Plan” has the meaning set forth in Section 6.8(b).
1.218“New York Court” has the meaning set forth in Section 14.2(g).
1.219“Non-Acquiring Party” has the meaning set forth in Section 7.8(a).
1.220“Non-breaching Party” has the meaning set forth in Section 13.5(a).
1.221“Non-Clinical Studies” means all non-human studies of pharmaceutical products.
1.222“Non-Personal Digital Promotions” means any non-personal promotional materials using digital channels to promote Products including search engines, social media, email, websites, digital advertisements, and television and radio advertisements.
1.223[****].
1.224“Notice Date” has the meaning set forth in Section 13.2.
1.225“Notice Period” has the meaning set forth in Section 13.2.
1.226“Operating Committee” means the Joint Development Committee, the Joint Manufacturing Committee, the Joint Commercialization Committee, or any other subcommittee of the JSC, or any of the foregoing committees, which may be established by the JSC from time to time in accordance with the terms hereof; provided that neither the JPT nor any working group formed hereunder are “Operating Committees.”
1.227“Opt-Out Party” has the meaning set forth in Section 13.9(a).
1.228“Other Manufacturing Costs” has the meaning set forth in the Financial Appendix.
1.229“Other Outcomes Trial” means, other than the Initial CVOT Study, any Phase 3 Clinical Trial that is a cardiovascular or renal outcomes trial of a Therapeutic Product.
1.230“Party” has the meaning set forth in the preamble.
1.231“Patent Challenge” has the meaning set forth in Section 13.3.
1.232“Patent Costs” has the meaning set forth in Section 9.5.
1.233“Patent Dispute” has the meaning set forth in Section 14.1.
1.234“Patent Rights” means (a) all national, regional and international patents, priority patent filings, and patent applications, including provisional patent applications and rights to claim priority from any of these patents or applications, (b) any renewals, divisions, continuations (in whole or in part), or requests for continued examination of any of such patents and patent applications, and any and all patents issuing thereon, (c) any and all reissues, reexaminations, extensions, supplementary protection certificates, divisions, renewals, substitutions, confirmations, registrations, revalidations, revisions, and additions of or to any of the foregoing and (d) any foreign equivalents of each of the foregoing (a) through (c).
1.235“PCD Strategy” has the meaning set forth in Section 5.9.
1.236“Person” means an individual, firm, corporation, partnership, trust, incorporated or unincorporated association, joint venture, limited liability company, joint stock company, Governmental Authority or other entity of any kind.
1.237“Personal Data” has the meaning set forth in Section 15.16.
1.238“Pharmacovigilance Agreement” has the meaning set forth in Section 4.5.
1.239“Phase 1 Clinical Trial” means a clinical trial of a drug product in human subjects that is designed to satisfy the requirements for a Phase 1 study as described in 21 C.F.R. § 312.21(a), regardless of where such clinical trial is conducted.
1.240“Phase 2 Clinical Trial” means a clinical trial of a drug product in human subjects that is designed to satisfy the requirements for a Phase 2 study as described in 21 C.F.R. § 312.21(b), regardless of where such clinical trial is conducted. [****].
1.241“Phase 3 Clinical Trial” means a clinical trial of a drug product in human subjects designed to satisfy the requirements for a Phase 3 study as defined in 21 C.F.R. § 312.21(c), regardless of where such clinical trial is conducted [****].
1.242“Phase 4 Clinical Trial” means a clinical trial of a drug product in human subjects conducted after Regulatory Approval of such drug product has been obtained from an appropriate Regulatory Authority, including a Confirmatory Clinical Trial.
1.243“PhRMA Code” means the Pharmaceutical Research and Manufacturers of America Code on Interactions with Health Care Professionals.
1.244“PII/Samples” has the meaning set forth in Section 13.8(e)(ii).
1.245“PIP Trial” means any Clinical Trial of Product in pediatric patients, including any such Clinical Trial that is part of a pediatric investigation plan or pediatric study plan required by a Regulatory Authority.
1.246“Post-Approval Study” means any Phase 4 Clinical Trial, real-world evidence study, non-interventional study, investigator-initiated study, or disease registry conducted following Regulatory Approval for the applicable Product for use in the Field in the applicable country or jurisdiction.
1.247“Pre-Approval Trial” means a Phase 1 Clinical Trial, Phase 2 Clinical Trial or Phase 3 Clinical Trial.
1.248“Pre-Existing Affiliates” means (a) with respect to an Acquired Party, the Affiliates of such Acquired Party that exist immediately prior to the effective date of the applicable Change of Control (not including, for clarity, the Acquiror or any of its Affiliates that exist immediately prior to the effective date of the applicable Change of Control) and any successors thereto and (b) with respect an Acquiror Party, the Affiliates of such Acquiror Party that exist immediately prior to the effective date of the applicable Change of Control (not including, for clarity, the Acquired Party or any of its Affiliates that exist immediately prior to the applicable Change of Control) and any successors thereto.
1.249“Preliminary Manufacturing Budget” has the meaning set forth in Section 6.3(b).
1.250“Presiding Arbitrator” has the meaning set forth in Section 14.2(c).
1.251“Price Approval” means, in any country where a Governmental Authority authorizes reimbursement for, or approves or determines pricing for, pharmaceutical products, receipt (or, if required to make such authorization, approval or determination effective, publication) of such reimbursement authorization or pricing approval or determination (as the case may be).
1.252“Primary Indication” means the reduction of one or more major adverse cardiovascular events in high-risk patients with hypertension [****].
1.253“Primary Indication Program” means any and all Development activities directed to obtaining Regulatory Approval of the Therapeutic Product for the Primary Indication as contemplated in, or in furtherance of, the Primary Indication TPP and the Development Plan (including the initiation and conduct of KARDIA-3, the Initial CVOT Study and any PIP Trial).
1.254“Primary Indication Target Product Profile” or “Primary Indication TPP” has the meaning set forth in Schedule 1.254.
1.255“Primary Indication TPP Failure” means that the criteria set forth in both of the following clauses (a) and (b) are satisfied for the Therapeutic Product in [****].
1.256“Product” means any pharmaceutical product (including any Combination Product) that is comprised of or contains (a) Zilebesiran as an active pharmaceutical ingredient (the “Therapeutic Product”) or (b) a REVERSIR (a “REVERSIR Product”), in each case ((a) and (b)), in any form, presentation, dosage and formulation; provided that any such form, presentation, dosage or formulation comprises or contains either Zilebesiran or REVERSIR as an active pharmaceutical ingredient. For clarity, the Development, Manufacture and/or Commercialization of any Combination Product under this Agreement will be subject, as applicable, to Section 3.5 and [****].
1.257“Product Action Concern” has the meaning set forth in Section 4.4(a).
1.258“Product Trademarks” means any trademarks, service marks, trade names and related trade dress, designs, logos, domain names or symbols used in connection with the Commercialization of Products (a) by Roche in the Roche Territory or (b) by the Parties in the Co-Commercialization Territory, in each case (the foregoing (a) and (b)), that is not a corporate name or logo of either Party or any of its Related Parties.
1.259“Promotional Materials” means any and all promotional materials to be used in promoting the Products for use in the Field, including all forms of Non-Personal Digital Promotions, non-personal print promotions, print promotions, and any materials presented to congresses in the U.S. or any other congresses in the Territory with global reach.
1.260“Quality Agreement” has the meaning set forth in Section 6.10.
1.261“Recipient” has the meaning set forth in Section 12.1.
1.262“Redacted Agreement” has the meaning set forth in Section 12.4(c).
1.263[****].
1.264[****].
1.265[****].
1.266[****].
1.267“Region” means one or more of the following: [****].
1.268“Register” has the meaning set forth in Section 9.6.
1.269“Regulatory Activities” means preparing, obtaining, or maintaining Regulatory Materials and Regulatory Approvals for the Product or activities otherwise relating to Pre-Approval Trials or Post-Approval Studies for the Product for use in the Field in the Territory, in each case to the extent consistent with the Development Plan or Co-Commercialization Plan, as applicable.
1.270“Regulatory Approval” means all approvals (other than Price Approval) necessary for the Manufacture, Commercialization or other exploitation of a pharmaceutical product for one or more Indications in a country or regulatory jurisdiction, which may include satisfaction of all applicable regulatory and notification requirements. For avoidance of doubt, “Regulatory Approval” shall include (a) accelerated approval as such term is described in 21 C.F.R. Part 314 Subpart H or an equivalent conditional approval of a Regulatory Authority outside the United States and (b) any label expansion or an approval by the applicable Regulatory Authority to include Development Data pertaining to such Indication in the label of such pharmaceutical product.
1.271“Regulatory Authority” means, in a particular country or regulatory jurisdiction, any applicable Governmental Authority involved in granting Regulatory Approval or, to the extent required in such country or regulatory jurisdiction, Price Approval of a pharmaceutical product in such country or regulatory jurisdiction and any successor thereto.
1.272“Regulatory Exclusivity” means any exclusive marketing rights or data exclusivity rights conferred by any Regulatory Authority under Applicable Laws with respect to a Product in a country or jurisdiction in the Territory that generally have the effect of preventing or delaying all or some Third Parties from selling such Product in such country or jurisdiction during such period of exclusivity, other than Patent Rights, including rights conferred in the U.S. under the Hatch-Waxman Act or the FDA Modernization Act of 1997 (including pediatric exclusivity), or rights similar thereto outside the U.S.
1.273“Regulatory Materials” means regulatory applications, submissions, notifications, correspondences, registrations, Regulatory Approvals or other filings made to or with, or other approvals granted by, a Regulatory Authority that are necessary or reasonably desirable in order to Develop, Manufacture, Commercialize or otherwise exploit a Product in a particular country or regulatory jurisdiction. “Regulatory Materials” include INDs and NDAs.
1.274“Related Party” means, with respect to a Party, such Party’s Affiliates and Sublicensees.
1.275“Representatives” has the meaning set forth in Section 12.3(f).
1.276“Required Manufacturing Change” has the meaning set forth in Section 6.10(a).
1.277“Reversion Product” has the meaning set forth in Section 13.8(c).
1.278“REVERSIR” means ATG01-RVR or any other siRNA designed to reverse Zilebesiran-mediated AGT knockdown that is developed in accordance with and subject to the terms of this Agreement.
1.279“REVERSIR Product” has the meaning set forth in Section 1.256.
1.280“Reviewing Party” has the meaning set forth in Section 12.5.
1.281“RNA” means ribonucleic acid.
1.282“Roche” has the meaning set forth in the preamble.
1.283“Roche Background Know-How” means any and all Know-How to the extent Controlled by Roche or any of its Affiliates as of the Effective Date or during the Term, which Know-How is necessary or reasonably useful for the Development, Manufacture or Commercialization of any Products for use in the Field in the Territory in accordance with the terms of this Agreement, other than Roche Collaboration Know-How and Roche’s interest in Joint Know-How. For clarity, “Roche Background Know-How” (a) includes Roche Core Know-How and (b) excludes any and all Patent Rights and Roche Excluded IP. For purposes of this definition only, “Controlled” means Know-How to the extent actually used by Roche or any of its Affiliates as part of the Collaboration activities hereunder.
1.284“Roche Background Patents” means any and all Patents Rights to the extent Controlled by Roche or any of its Affiliates as of the Effective Date or during the Term that Cover Roche Background Know-How, other than Roche Excluded IP.
1.285“Roche Basel” has the meaning set forth in the preamble.
1.286“Roche Collaboration Know-How” means any and all Know-How to the extent Controlled by Roche or any of its Affiliates after the Effective Date and during the Term that (a) is necessary or reasonably useful for the Development, Manufacture or Commercialization of Products for use in the Field in the Territory in accordance with the terms of this Agreement and (b) is conceived or reduced to practice (in whole or in part) or otherwise identified, developed, made, or discovered solely by or on behalf of (including by subcontractors) Roche or any of its Related Parties in its conduct of Collaboration activities under this Agreement. For clarity, “Roche Collaboration Know-How” (a) includes Roche Core Know-How and Roche Product-Specific Know-How, and (b) excludes any and all Patent Rights and Roche Excluded IP.
1.287“Roche Collaboration Patents” means any and all Patents Rights to the extent Controlled by Roche or any of its Affiliates during the Term that Cover any Roche Collaboration Know-How. For clarity, “Roche Collaboration Patents” excludes Roche’s interest in Joint Patents and Roche Excluded IP.
1.288“Roche Commercialization Costs” has the meaning set forth in the Financial Appendix.
1.289“Roche Core Improvements” has the meaning set forth in Section 1.182.
1.290“Roche Core Know-How” means any and all Roche Know-How other than (a) Roche Product-Specific Know-How, (b) Roche’s interest in Joint Know-How and (c) Roche Excluded IP.
1.291“Roche Core Patents” means any and all Roche Patents other than Roche Product-Specific Patents, excluding Roche’s interest in Joint Patents and Roche Excluded IP.
1.292[****].
1.293“Roche Excluded IP” means any and all [****]; (b) Know-How and Patent Rights specifically related to any active pharmaceutical ingredient, but not Zilebesiran or REVERSIR; [****].
1.294“Roche Indemnitee” has the meaning set forth in Section 11.1.
1.295“Roche Know-How” means any and all Roche Background Know-How, Roche Collaboration Know-How and Roche’s interest in Joint Know-How.
1.296“Roche Lead Study” has the meaning set forth in Section 3.6(b)(i).
1.297“Roche Licensed IP” means the Roche Know-How and Roche Patents.
1.298“Roche Manufacturing Change” has the meaning set forth in Section 6.10(c)(ii).
1.299[****].
1.300“Roche Patents” means any and all Roche Background Patents, Roche Collaboration Patents and Roche’s interest in Joint Patents.
1.301“Roche Product-Specific Know-How” means, on a Product-by-Product basis, [****].
1.302“Roche Product-Specific Patents” means any and all Patent Rights to the extent Controlled by Roche or any of its Affiliates during the Term that Cover Roche Product-Specific Know-How.
1.303“Roche-Requested Manufacturing Change” has the meaning set forth in Section 6.10(b).
1.304“Roche Territory” means, subject to Section 7.3(b) and Section 13.10 with respect to Japan, all countries and regulatory jurisdictions throughout the world other than the Co-Commercialization Territory.
1.305“Roche Territory Compulsory Sublicense Compensation” means Compulsory Sublicense Compensation paid to Roche for the Roche Territory.
1.306“Roche Transfer Activities” has the meaning set forth in Section 13.8(c)(xii).
1.307“Royalty Payment” has the meaning set forth in Section 8.7(a).
1.308“Royalty Rate” has the meaning set forth in Section 8.7(a).
1.309“Royalty Term” has the meaning set forth in Section 8.7(b).
1.310“Rules” has the meaning set forth in Section 14.2(a).
1.311“Sales” means [****].
1.312“Sales Milestone” has the meaning set forth in Section 8.6(a).
1.313“Sales Milestone Payments” has the meaning set forth in Section 8.6(a).
1.314“Sanctioned Jurisdiction” means a country or territory that is itself the subject or target of any Sanctions and Export Control Laws (as of the date of this Agreement, Cuba, Iran, North Korea, Syria, and the Crimea, the so-called Luhansk People’s Republic, and the so-called Donetsk People’s Republic regions of Ukraine, and the non-government controlled areas of Ukraine in the oblasts of Kherson and Zaporizhzhia).
1.315“Sanctioned Person” means any Person targeted by Sanctions and Export Control Laws, including as a result of being (a) listed in any list of sanctioned Persons, including those maintained by the U.S. (including the Department of the Treasury’s Office of Foreign Assets Control and the Department of State), the United Kingdom, or the European Union; (b) located, organized, or resident in a Sanctioned Jurisdiction; (c) directly or indirectly owned fifty percent (50%) or more or controlled, individually or in the aggregate, by one or more Persons described in the foregoing clauses (a) and/or (b); or identified on the U.S. Department of Commerce’s Entity List, Denied Persons List, Unverified List or Military End User List, or the U.S. Department of State’s Debarred List.
1.316“Sanctions and Export Control Laws” means any and all Applicable Laws related to (a) import and export controls, including the U.S. Export Administration Regulations, (b) economic or financial sanctions and trade embargoes, including those administered by the Office of Foreign Assets Control of the U.S. Department of the Treasury, the U.S. Department of State, the European Union, and the United Kingdom or (c) anti-boycott measures.
1.317“SEC” means the U.S. Securities and Exchange Commission or any successor thereof.
1.318“Shared Development Costs” has the meaning set forth in the Financial Appendix.
1.319“Short Term Development Budget” has the meaning set forth in Section 3.3.
1.320“Short Term Development Plan” has the meaning set forth in Section 3.2.
1.321“siRNA” means an oligonucleotide composition of native or chemically modified RNA that targets a gene through activation of the RNA interference pathway, and that is not a MicroRNA, MicroRNA antagonist or MicroRNA Mimic.
1.322“Sublicense Agreement” has the meaning set forth in Section 7.3(c)(ii).
1.323“Sublicensee” means a Third Party that is granted a sublicense (through one or multiple tiers) by either Party under the licenses granted in Section 7.1, with respect to Roche, or Section 7.2, with respect to Alnylam, in each case other than through a Compulsory Sublicense.
1.324“Supply Agreements” has the meaning set forth in Section 6.9(b).
1.325“Tax” means any tax (including any income tax, franchise tax, capital gains tax, gross receipts tax, VAT, surtax, excise tax, ad valorem tax, transfer tax, stamp tax, sales tax, use tax, property tax, business tax, withholding tax or payroll tax), levy, assessment, tariff, duty (including any customs duty) and any related charge or amount (including any fine, addition, penalty or interest), imposed, assessed or collected by any Governmental Authority.
1.326“Tax Representative” means the “partnership representative” as such term is defined in Section 6223 of the Code.
1.327“Technology Transfer” means the Existing Process Technology Transfer and the New Manufacturing Process Technology Transfer.
1.328“Technology Transfer Completion Date” has the meaning set forth in Section 6.2.
1.329“Technology Transfer Costs” means all Direct Costs and Indirect Costs incurred by or on behalf of a Party or any of its Affiliates in conducting any (a) Technology Transfer pursuant to Section 6.8 or (b) activities to enable Roche, its Affiliates or [****] to obtain the authorization or ability to Manufacture Products.
1.330“Technology Transfer Plans” means the Existing Process Technology Transfer Plan and the New Manufacturing Process Technology Transfer Plan.
1.331“Term” has the meaning set forth in Section 13.1.
1.332“Terminated Territory” has the meaning set forth in Section 13.8(c).
1.333“Termination Effective Date” has the meaning set forth in Section 13.8(c).
1.334“Termination Notice Period” has the meaning set forth in Section 13.8(a).
1.335“Territory” means, collectively, the Co-Commercialization Territory and the Roche Territory.
1.336“Therapeutic Product” has the meaning set forth in Section 1.256.
1.337“Third Party” means any entity other than Alnylam or Roche or any of their respective Affiliates.
1.338“Third Party Claim” has the meaning set forth in Section 11.1.
1.339“Third Party Infringement” has the meaning set forth in Section 9.10(a).
1.340“Third Party License” means any agreement entered into after the Effective Date in accordance with Section 7.6 by and between a Party or its Affiliate, on the one hand, and a Third Party on the other hand, pursuant to which such Party or its Affiliates are granted a license to intellectual property rights of such Third Party or its Affiliates (excluding any such agreement which relates solely to an active pharmaceutical ingredient of a Combination Product that is not Zilebesiran or a REVERSIR).
1.341“Third Party Payment” has the meaning set forth in Section 7.6(b).
1.342“Third Party Sublicense Agreement” has the meaning set forth in Section 7.3(c)(ii).
1.343“Trademark Costs” mean those out-of-pocket costs and expenses incurred by or on behalf of a Party or any of its Affiliates for outside counsel and other Third Parties, and filing and maintenance, in each case incurred in connection with the establishment and maintenance of rights under Product Trademarks, including costs of trademark filings and registration fees, and actions to enforce or maintain the applicable trademarks.
1.344“Transition Agreement” has the meaning set forth in Section 13.8(c)(ii).
1.345“U.S.” or “United States” means the United States of America (including all possessions and territories thereof).
1.346“U.S. Territory Partnership” has the meaning set forth in Section 8.8(e).
1.347“Valid Claim” means (a) a claim (including a process, use, or composition of matter claim) of an issued and unexpired Patent Right, which has not been held invalid or unenforceable by a patent office, court or other Governmental Authority of competent jurisdiction, which holding is unappealable or unappealed within the time allowed for appeal, and which has not been admitted to be invalid by the owner through reissue, disclaimer or otherwise, or (b) a claim (including a process, use, or composition of matter claim) within a patent application that is pending for no more [****] years and that has not been revoked, cancelled, withdrawn, or affirmatively abandoned, or held invalid, unenforceable, unpatentable, or abandoned by a patent office, court or other Governmental Authority of competent jurisdiction, which holding is unappealable or unappealed within the time allowed for appeal.
1.348“VAT” has the meaning set forth in Section 8.8(b).
1.349“Withholding” has the meaning set forth in Section 8.8(c).
1.350“Withholding Action” has the meaning set forth in Section 8.8(c).
1.351“Zilebesiran” means the molecule designated by Alnylam as AD-85481 [****], and any modification thereto developed in accordance with and subject to the terms of this Agreement.
ARTICLE 2
GOVERNANCE
2.1Joint Steering Committee.
(a)Purpose; Formation. Within [****] days after the Effective Date, the Parties shall establish a joint steering committee (the “Joint Steering Committee” or “JSC”) to monitor and oversee the activities under this Agreement and facilitate communication between the Parties with respect to the Development, Manufacture and Commercialization of Products for use in the Field in the Territory under this Agreement.
(b)Composition. Each Party shall initially appoint [****] representatives to serve as JSC members. Each JSC member shall have appropriate seniority and functional expertise to make decisions arising within the JSC’s responsibilities. The JSC may change its size from time to time by mutual written agreement of its members; provided that the JSC shall consist at all times of an equal number of members of each of Alnylam and Roche. Each Party may replace its JSC members at any time upon written notice to the other Party; provided that the applicable replacement has the requisite expertise and seniority for JSC members hereunder. The JSC may invite non-members to participate in the discussions and meetings of the JSC; provided that such participants (i) are subject to confidentiality obligations (whether in writing or by operation of law) consistent with this Agreement, (ii) are participating in or supporting activities conducted under this Agreement and (iii) for clarity, have no voting rights at the JSC. Each Party shall appoint one of its JSC members as its chairperson, and the JSC shall be co-chaired by such chairpersons. Each Party may replace its chairperson at any time upon written notice to the other Party. The role of each chairperson shall be to convene and preside at meetings of the JSC, but neither chairperson shall have any additional powers or rights beyond those held by the other JSC members.
(c)Specific Responsibilities of the JSC. In addition to its overall responsibility for monitoring and providing a forum to discuss and coordinate the Parties’ activities under this Agreement, the JSC shall in particular:
(i)oversee the collaborative activities of the Parties under this Agreement;
(ii)review and approve any amendments, changes or other updates to the Development Plan, Manufacturing Plan, Co-Commercialization Plan, PCD Strategy and each Global Brand Strategy, including all budget recommendations from any Operating Committee and the adoption of, or any amendments, changes or other updates to, any Additional Program Plans or Device Program Plans proposed by a Party to be included in the Development Plan;
(iii)review and discuss the Parties’ activities under the Development Plan, the Manufacturing Plan and the Co-Commercialization Plan and set the overall strategy for coordination of activities among the Parties relating to Products for use in the Field within the Territory;
(iv)oversee the establishment, termination and activities of additional subcommittees as it deems necessary to achieve the objectives and intent of this Agreement;
(v)delegate duties to Operating Committees, direct particular projects or activities at an appropriate time and agree on operating procedures of such Operating Committees;
(vi)approve plans brought forth by the Operating Committees to the JSC;
(vii)approve termination of the JPT if so proposed by the Operating Committees;
(viii)attempt to resolve issues presented to it by, and disputes within, any Operating Committee; and
(ix)perform such other functions as may be appropriate to further the purposes of this Agreement, as mutually agreed upon and directed by the Parties in writing or as expressly specified in this Agreement.
For clarity, the JSC shall have no responsibility or authority other than that expressly set forth in this Agreement.
(d)Meetings. The Parties shall endeavor to have their first meeting of the JSC within [****] days after the establishment of the JSC; provided that such meeting shall be no later than [****] days after the Effective Date. The JSC shall meet at least [****] per Calendar Quarter during the Term (spaced at regular intervals) unless the JSC mutually agrees in writing to a different frequency for such meetings. Either Party may also call a special meeting of the JSC (by videoconference or teleconference) by providing at least [****] Business Days’ prior written notice to the other Party in the event such Party reasonably believes that a significant matter must be addressed prior to the next scheduled meeting of the JSC, and such Party shall provide the JSC no later than [****] Business Days prior to the special meeting with materials that such Party reasonably believes in good faith are sufficient to enable the JSC members to make an informed decision; provided that for time sensitive matters, a Party may call a special meeting of the JSC on less than [****] Business Days’ prior written notice if the Parties, as confirmed in writing by the Alliance Managers, agree that an issue warrants an expedited meeting. No later than [****] Business Days prior to any meeting of the JSC, the Alliance Managers (or their respective designees) shall prepare and circulate an agenda for such meeting and each Party shall be permitted to propose additional topics to be included on such agenda. The JSC may meet in person, by videoconference or by teleconference. In-person JSC meetings will be held at locations alternately selected by Alnylam and by Roche. Each Party will bear the expense of its respective JSC members’ participation in JSC meetings. Meetings of the JSC shall be effective only if at least one (1) member of each Party is present or participating in such meeting. The Alliance Managers (or their respective designees) will be responsible for the preparation of written minutes of all JSC meetings that reflect material decisions and actions made at such meetings. The Alliance Managers (or their respective designees) shall deliver draft meeting minutes to each member of the JSC for their review within [****] Business Days after each JSC meeting. Such minutes will be deemed approved unless one or more members of the JSC objects in writing to the accuracy of such minutes within [****] Business Days after receipt thereof.
(e)Decision-Making. In addition to resolving issues within the scope of the JSC’s express responsibilities hereunder, the JSC shall have the authority in accordance with Section 2.5(b) to resolve any disputes not resolved by any Operating Committee in accordance with Section 2.5. Subject to the remainder of this Section 2.1(e) and Section 2.7, the JSC shall act by consensus, with each Party having, collectively, one (1) vote on behalf of that Party. If the JSC cannot reach consensus on an issue that comes before the JSC and over which the JSC has responsibility, then such matter shall be resolved in accordance with Section 2.5(c) or Section 2.5(d), as applicable.
2.2Joint Development Committee.
(a)Purpose; Formation. Within [****] days after the Effective Date, the Parties shall establish a joint development committee to oversee and coordinate, and facilitate communication regarding, Development of Products for use in the Field in the Territory under this Agreement (the “Joint Development Committee” or “JDC”).
(b)Composition. Each Party shall initially appoint [****] representatives to serve as JDC members. Each JDC member shall have appropriate seniority and sufficient expertise in the Development of pharmaceutical products similar to the Products to make decisions arising within the scope of the JDC’s responsibilities. The JDC may change its size from time to time by mutual written agreement of its members; provided that the JDC shall consist at all times of an equal number of members of each of Alnylam and Roche and in no event shall the JDC consist of less than [****] members of each Party. Each Party may replace its JDC members at any time upon written notice to the other Party; provided that the applicable replacement has the requisite expertise and seniority for JDC members hereunder. The JDC may invite non-members to participate in the discussions and meetings of the JDC; provided that such participants (i) are subject to confidentiality obligations (whether in writing or by operation of law) consistent with this Agreement, (ii) are participating in or supporting Development activities conducted with respect to the Products under this Agreement and (iii) for clarity, have no voting rights at the JDC. Each Party shall appoint one of its JDC members as its chairperson, and the JDC shall be co-chaired by such chairpersons. Each Party may replace its chairperson at any time upon written notice to the other Party. The role of each chairperson shall be to convene and preside at meetings of the JDC, but neither chairperson shall have any additional powers or rights beyond those held by the other JDC members.
(c)Specific Responsibilities of the JDC. In addition to its general responsibilities, the JDC shall in particular:
(i)monitor, implement, and oversee the Development of the Products through Regulatory Approval in accordance with, and compare Development progress against, the Development Plan;
(ii)discuss and approve, for submission to the JSC, any proposed changes, amendments, or other updates to the Development Plan (including the Development Budget) no less than [****], including amendments regarding whether to: conduct additional Pre-Approval Trials of Products for use in the Field in the Territory; develop Products for use in the Field in combination (whether co-administered or co-formulated) with any other active pharmaceutical ingredient or other pharmaceutical product; or discuss and amend the Development Plan (including the Development Budget) to include any Additional Program Plan or Device Program Plan;
(iii)discuss and approve a template clinical trial agreement for use in connection with Clinical Trials conducted in accordance with the Development Plan or Co-Commercialization Plan;
(iv)review and discuss the Development Reports submitted by each Party;
(v)discuss and approve a regulatory strategy to meet the objectives of the target product profile for Products in the Field in the Territory;
(vi)approve the Indication, overall study design and dosing, as applicable, for any Post-Approval Study for any Product in the Territory;
(vii)prior to initiation of any Phase 3 Clinical Trial for a Product for use in the Field, [****];
(viii)approve (subject to the timing requirement set forth in the last sentence of Section 3.7(b)) the initiation of [****];
(ix)following completion of the Phase 3 Clinical Trial for a Product or Indication in the Field, review and approve the Regulatory Approval strategy (including label strategy, label negotiation strategy and any proposed changes thereto, for use in the Co-Commercialization Territory) therefor;
(x)approve the initial submission of an NDA and major labeling updates for a Product for use in the Field to the FDA;
(xi)if applicable, develop a plan for an Expanded Access Program with respect to Products for use in the Field in the Territory;
(xii)[****]; and
(xiii)perform such other functions as may be appropriate to further the purposes of this Agreement, as directed by the JSC or as expressly specified in this Agreement.
For clarity, the JDC shall have no responsibility or authority other than that expressly set forth in this Agreement.
(d)Meetings. The JDC shall meet at least [****] per Calendar Quarter during the Term (spaced at regular intervals) unless the Parties mutually agree in writing to a different frequency for such meetings. Either Party may also call a special meeting of the JDC (by videoconference or teleconference) by providing at least [****] Business Days’ prior written notice to the other Party in the event such Party reasonably believes that a significant matter must be addressed prior to the next scheduled meeting of the JDC, and such Party shall provide the JDC no later than [****] Business Days prior to the special meeting with materials that such Party reasonably believes in good faith are sufficient to enable the JDC members to make an informed decision; provided that for time sensitive matters, a Party may call a special meeting of the JDC on less than [****] Business Days’ prior written notice if the Parties, as confirmed in writing by the Alliance Managers, agree that an issue warrants an expedited meeting. No later than [****] Business Days prior to any meeting of the JDC, the Alliance Managers (or their respective designees) shall prepare and circulate an agenda for such meeting and each Party shall be permitted to propose additional topics to be included on such agenda. The JDC may meet in person, by videoconference or by teleconference. In-person JDC meetings will be held at locations alternately selected by Alnylam and by Roche. Each Party will bear the expense of its respective JDC members’ participation in JDC meetings. Meetings of the JDC shall be effective only if at least one (1) member of each Party is present or participating in such meeting. The Alliance Managers (or their respective designees) will be responsible for the preparation of written minutes of all JDC meetings that reflect material decisions and actions made at such meetings. The Alliance Managers (or their respective designees) will deliver draft meeting minutes to each member of the JDC for their review within [****] Business Days after each JDC meeting. Such minutes will be deemed approved unless one or more members of the JDC objects in writing to the accuracy of such minutes within [****] Business Days after receipt thereof.
(e)Decision-Making. Subject to the remainder of this Section 2.2(e) and Section 2.7, the JDC shall act by consensus, with each Party having, collectively, one (1) vote on behalf of that Party. If the JDC cannot reach consensus on an issue that comes before the JDC and over which the JDC has oversight within [****] days after such issue having come before the JDC, then such matter shall be resolved in accordance with Section 2.5.
2.3Joint Manufacturing Committee.
(a)Purpose; Formation. Within [****] days after the Effective Date, the Parties shall establish a joint manufacturing committee to oversee, and facilitate communication regarding, Manufacturing of clinical and commercial supplies of Products (the “Joint Manufacturing Committee” or “JMC”).
(b)Composition. Each Party shall initially appoint [****] representatives to serve as members of the JMC. Each JMC member shall have appropriate seniority and sufficient expertise in the Manufacturing of pharmaceutical products similar to the Products to make decisions within the scope of the JMC’s responsibility. The JMC may change its size from time to time by mutual written agreement of its members; provided that the JMC shall consist at all times of an equal number of members of each of Alnylam and Roche. Each Party may replace its JMC members at any time upon written notice to the other Party; provided that the applicable replacement has the requisite expertise and seniority for JMC members hereunder. The JMC may invite non-members to participate in the discussions and meetings of the JMC; provided that such participants (i) are subject to confidentiality obligations (whether in writing or by operation of law) consistent with this Agreement, (ii) are involved in or supporting Manufacturing activities related to Products under this Agreement and (iii) for clarity, have no voting rights at the JMC. Each Party shall appoint one of its JMC members as its chairperson, and the JMC shall be co-chaired by such chairpersons. Each Party may replace its chairperson at any time upon written notice to the other Party. The role of each chairperson shall be to convene and preside at meetings of the JMC, but neither chairperson shall have any additional powers or rights beyond those held by the other JMC members.
(c)Specific Responsibilities of the JMC. In addition to its general responsibilities, the Joint Manufacturing Committee shall in particular:
(i)discuss and approve, for submission to the JSC, the Manufacturing Plan (including the Manufacturing Budget) for each Product, including any amendments thereto;
(ii)oversee and coordinate implementation of the Manufacturing Plan and Supply Agreements, including [****] as further described in the Commercial Supply Term Sheet;
(iii)oversee Technology Transfers to Roche with respect to the Manufacture of Products in the Roche Territory in accordance with and subject to the terms hereof and the applicable Technology Transfer Plan;
(iv)oversee the exchange of information between the Parties relating to Manufacturing Changes or other Manufacturing improvements;
(v)[****];
(vi)discuss and approve, for submission to the JSC, amendments to the Manufacturing Plan (including the Manufacturing Budget) with respect to [****];
(vii)review and discuss Manufacturing Reports submitted by each Party;
(viii)discuss and approve, for submission to the JSC, amendments to the Development Plan (including the Development Budget) solely for any CMC Manufacturing Development activities;
(ix)discuss CMOs to be used to Manufacture Product in the Field for the Territory (including applicable contractual terms), [****];
(x)perform such other functions as may be appropriate to further the purposes of this Agreement, as directed by the JSC or as expressly specified in this Agreement.
For clarity, the JMC shall have no responsibility or authority other than that expressly set forth in this Agreement.
(d)Meetings. The JMC shall meet at least [****] time per Calendar Quarter during the Term (spaced at regular intervals) unless the Parties mutually agree in writing to a different frequency for such meetings. Either Party may also call a special meeting of the JMC (by videoconference or teleconference) by providing at least [****] Business Days’ prior written notice to the other Party in the event such Party reasonably believes that a significant matter must be addressed prior to the next scheduled meeting of the JMC, and such Party shall provide the JMC no later than [****] Business Days prior to the special meeting with materials that such Party reasonably believes in good faith are sufficient to enable the JMC members to make an informed decision; provided that for time sensitive matters, a Party may call a special meeting of the JMC on less than [****] Business Days’ prior written notice if the Parties, as confirmed in writing by the Alliance Managers, agree that an issue warrants an expedited meeting. No later than [****] Business Days prior to any meeting of the JMC, the Alliance Managers (or their respective designees) shall prepare and circulate an agenda for such meeting and each Party shall be permitted to propose additional topics to be included on such agenda. The JMC may meet in person, by videoconference or by teleconference. In-person JMC meetings will be held at locations alternately selected by Alnylam and by Roche. Each Party will bear the expense of its respective JMC members’ participation in JMC meetings. Meetings of the JMC shall be effective only if at least one (1) member of each Party is present or participating in such meeting. The Alliance Managers (or their respective designees) will be responsible for the preparation of minutes of all JMC meetings that reflect material decisions made and actions at such meetings. The Alliance Managers (or their respective designees) will deliver draft meeting minutes to each member of the JMC for their review within [****] Business Days after each JMC meeting. Such minutes will be deemed approved unless one or more members of the JMC objects in writing to the accuracy of such minutes within [****] Business Days after receipt thereof.
(e)Decision-Making. Subject to the remainder of this Section 2.3(e) and Section 2.7, the JMC shall act by consensus, with each Party having, collectively, one (1) vote on behalf of that Party. If the JMC cannot reach consensus on an issue that comes before the JMC and over which the JMC has oversight within [****] days after such issue having come before the JMC, then such matter shall be resolved in accordance with Section 2.5.
2.4Joint Commercialization Committee.
(a)Purpose; Formation. By no later than [****] years prior to the anticipated launch of the first Product under this Agreement, the Parties shall establish a joint commercialization committee to oversee and coordinate, and facilitate communication regarding, Commercialization of Products (other than commercial Manufacture of Products, which shall be overseen by the JMC) (the “Joint Commercialization Committee” or “JCC”).
(b)Composition. Each Party shall initially appoint [****] representatives to serve as members of the JCC. Each JCC member shall have appropriate seniority and sufficient expertise in the Commercialization of pharmaceutical products similar to the Products to make decisions within the scope of the JCC’s responsibilities. The JCC may change its size from time to time by mutual written agreement of its members; provided that the JCC shall consist at all times of an equal number of members of each of Alnylam and Roche. Each Party may replace its JCC members at any time upon written notice to the other Party; provided that the applicable replacement has the requisite expertise and seniority for JCC members hereunder. The JCC may invite non-members to participate in the discussions and meetings of the JCC; provided that such participants (i) are subject to confidentiality obligations (whether in writing or by operation of law) consistent with this Agreement, (ii) are involved in or supporting Manufacturing activities related to Products under this Agreement and (iii) for clarity, have no voting rights at the JCC. Each Party shall appoint one of its JCC members as its chairperson, and the JCC shall be co-chaired by such chairpersons. Each Party may replace its chairperson at any time upon written notice to the other Party. The role of each chairperson shall be to convene and preside at meetings of the JCC, but neither chairperson shall have any additional powers or rights beyond those held by the other JCC members.
(c)Specific Responsibilities of the Joint Commercialization Committee. In addition to its general responsibilities, the Joint Commercialization Committee shall in particular:
(i)monitor Commercialization of Products in the Roche Territory and coordinate Commercialization of Products in the Co-Commercialization Territory, in accordance with the Co-Commercialization Plan;
(ii)discuss and approve, for submission to the JSC, the Co-Commercialization Plan (including the Co-Commercialization Budget) sufficiently in advance so that it may be approved at least [****] years prior to the anticipated launch of the first Product for use in the Field in the Co-Commercialization Territory, and any amendments thereto, which amendments shall be discussed, prepared and approved no less than [****] in accordance with and subject to the terms hereof;
(iii)review Commercialization strategies and plans of the Parties, including with respect to the specified activities (and performance thereof) and priorities with respect to the Commercialization of Products in the Field in the Territory;
(iv)review and discuss Commercialization Reports submitted by each Party;
(v)discuss and approve, for submission to the JSC, the PCD Strategy and the Global Brand Strategy for each Product;
(vi)[****];
(vii)discuss and approve the conduct of any Post-Approval Study (subject to Section 2.2(c)(vi)) and discuss the status and results of such Post-Approval Study;
(viii)discuss whether to sell any Product [****]; and
(ix)perform such other functions as may be appropriate to further the purposes of this Agreement, as directed by the JSC or as expressly specified in this Agreement.
The JCC shall have no responsibility or authority other than that expressly set forth in this Agreement.
(d)Meetings. The JCC shall meet at least [****] per Calendar Quarter during the Term (spaced at regular intervals) unless the Parties mutually agree in writing to a different frequency for such meetings. Either Party may also call a special meeting of the JCC (by videoconference or teleconference) by providing at least [****] Business Days’ prior written notice to the other Party in the event such Party reasonably believes that a significant matter must be addressed prior to the next scheduled meeting of the JCC, and such Party shall provide the JCC no later than [****] Business Days prior to the special meeting with materials that such Party reasonably believes in good faith are sufficient to enable the JCC members to make an informed decision; provided that for time sensitive matters, a Party may call a special meeting of the JCC on less than [****] Business Days’ prior written notice if the Parties, as confirmed in writing by the Alliance Managers, agree that an issue warrants an expedited meeting. No later than [****] Business Days prior to any meeting of the JCC, the Alliance Managers (or their respective designees) shall prepare and circulate an agenda for such meeting and each Party shall be permitted to propose additional topics to be included on such agenda. The JCC may meet in person, by videoconference or by teleconference. In-person JCC meetings will be held at locations alternately selected by Alnylam and by Roche. Each Party will bear the expense of its respective JCC members’ participation in JCC meetings. Meetings of the JCC shall be effective only if at least one (1) member of each Party is present or participating in such meeting. The Alliance Managers (or their respective designees) will be responsible for the preparation of written minutes of all JCC meetings that reflect material decisions made at such meetings. The Alliance Managers (or their respective designees) will deliver draft meeting minutes to each member of the JCC for their review within [****] Business Days after each JCC meeting. Such minutes will be deemed approved unless one or more members of the JCC objects in writing to the accuracy of such minutes within [****] Business Days after receipt thereof.
(e)Decision-Making. Subject to the remainder of this Section 2.4(e) and Section 2.7, the JCC shall act by consensus, with each Party having, collectively, one (1) vote on behalf of that Party. If the JCC cannot reach consensus on an issue that comes before the JCC and over which the JCC has oversight within [****] days after such issue having come before the JCC, then such matter shall be resolved in accordance with Section 2.5.
2.5Resolution of Committee Disputes.
(a)Within Operating Committees. Subject to the exceptions specified in this Section 2.5, if any Operating Committee fails to reach consensus on a matter properly before such Operating Committee in accordance with Section 2.2(e), 2.3(e) or 2.4(e), as applicable, then any member of such Operating Committee shall have the right to refer such matter to the JSC for resolution by providing the JSC with written notice of such matter within [****] days. Such matter shall be resolved in accordance with Section 2.5(b).
(b)Within The JSC. If a matter is referred by an Operating Committee to the JSC in accordance with Section 2.5, the JSC shall use good faith efforts to promptly address such matter within [****] Business Days after the matter is first referred to the JSC in accordance with Section 2.5 with the goal to resolve such matter within [****] days after the matter is first referred to the JSC in accordance with Section 2.5. If the JSC is unable to reach consensus on (i) any matter referred to the JSC in accordance with Section 2.5 or (ii) any other matter within the scope of the JSC’s express responsibilities hereunder, in each case ((i) and (ii)) within [****] days after such matter having come before the JSC, then either Party’s JSC members shall have the right to submit (by way of such Party’s Alliance Manager) such matter for resolution to the Parties’ Executive Officers within [****] days of such matter being referred to the JSC or otherwise arising within the JSC. Such matter shall be resolved in accordance with Section 2.5(c).
(c)Referral To Executive Officers. If a matter is referred by the JSC to the Executive Officers in accordance with Section 2.5(b), each Party shall promptly submit in writing the respective positions of such Party’s JSC members to each Party’s Executive Officers. Such Executive Officers shall use good faith efforts to promptly resolve such matter, and in no event later than [****] Business Days after each Party’s submission of the respective positions of the Parties with respect to such matter to the Executive Officers (which submissions shall be provided within [****] Business Days of the matter first being referred to the Executive Officers under Section 2.5(b)). If the Executive Officers are unable to reach consensus on any matter referred to the Executive Officers in accordance with Section 2.5(b), then such matter shall be resolved in accordance with Section 2.5(d).
(d)Final Decision-Making Authority.
(i)Alnylam Final Decision-Making Authority. Subject to Section 2.5(d)(iii), in the event that the Executive Officers are unable to reach consensus on any matter referred to the Executive Officers in accordance with Section 2.5(b) for a period of [****] Business Days as described in Section 2.5(c), Alnylam shall have final decision-making authority regarding the following matters:
[****].
(ii)Roche Final Decision-Making Authority. Subject to Section 2.5(d)(iii), in the event that the Executive Officers are unable to reach consensus on any matter referred to the Executive Officers in accordance with Section 2.5(b) for a period of [****] Business Days, Roche shall have final decision-making authority regarding the following matters:
[****].
(iii)Mutual Consent. Notwithstanding anything to the contrary herein, unless otherwise agreed by the Parties in writing, in the event that the Executive Officers are unable to reach consensus on any matter referred to the Executive Officers in accordance with Section 2.5(b) for a period of [****] Business Days, no decision shall be made, and the status quo shall be maintained regarding the following matters:
[****].
2.6Appointment of Alliance Managers. Each Party shall appoint an appropriately qualified individual to serve as alliance manager under this Agreement (each such individual, an “Alliance Manager”). Such individuals shall endeavor to assure clear and responsive communication between the Parties and the effective exchange of information, and may serve as a single point of contact for any matters arising under this Agreement. The Alliance Managers shall strive to facilitate resolution of potential and pending issues and potential disputes to reach consensus and avert escalation of such issues or potential disputes. Alliance Managers may attend all Committee meetings; provided, however, that the Alliance Managers shall not be members of any Committee established pursuant to this Agreement and shall not have final decision-making authority with respect to any matter. Each Party may change its designated Alliance Manager from time to time upon written notice to the other Party; provided that the applicable replacement has the requisite expertise and seniority for Alliance Managers hereunder.
2.7General Committee Authority. Each Committee shall only have those powers expressly assigned to it in this Article 2 and elsewhere in this Agreement. Notwithstanding anything to the contrary herein, and for clarity, (a) no Committee shall have any power to amend, modify, or waive compliance with the terms of this Agreement and (b) the Parties expressly acknowledge and agree that the decision-making authority granted to Alnylam and Roche pursuant to Section 2.5 shall not be used to authorize a Party to (i) perform any function not delegated to a Committee, (ii) unilaterally modify or amend, or waive its own compliance with, the terms of this Agreement, or (iii) impose any additional material performance obligations on the other Party, other than those for which such other Party is expressly responsible hereunder. Notwithstanding anything to the contrary in this Agreement, neither Party nor any of its Affiliates shall be required to take, or shall be penalized for not taking, any action that is not in compliance with such Party’s ethical business practices and policies or that such Party reasonably believes is not in compliance with Applicable Laws.
2.8Discontinuation of Participation on a Committee. Each Committee shall continue to exist until the Parties mutually agree to disband such Committee, except that the JDC shall disband upon the conclusion of all Development activities hereunder, the JMC will disband following the Technology Transfer Completion Date, and the JCC will disband at the end of the Co-Commercialization Term. If any Committee is disbanded in accordance with the foregoing, all decisions formerly made within or by such Committee shall become a decision that shall be made by the JSC.
2.9Joint Program Team.
(a)Composition; Responsibilities. The Parties shall establish a cross-functional Joint Program Team that is composed of representatives designated by each Party. Representatives must be appropriate for the tasks then being undertaken and the stage of Development, Manufacturing or Commercialization, as the case may be, in terms of their seniority, availability, function in their respective organizations, training and experience. Each Party shall designate one of its representatives as its primary JPT contact. Each Party may replace its representatives from time to time upon written notice to the other Party; provided, however, if a Party’s representative is unable to attend a meeting, such Party may designate a knowledgeable alternate to attend such meeting and perform the functions of such representative. The JPT will, subject to the oversight of the applicable Operating Committee, undertake the following activities: (i) preparing proposed changes, amendments, or other updates to the Development Plan (including the Development Budget) for submission to the JDC (other than those changes, amendments and updates with respect to CMC Manufacturing Development activities, which are addressed below in (iii)), (ii) preparing the regulatory strategy for Products in the Field in the Territory for submission to the JDC, (iii) preparing the Manufacturing Plan and any proposed changes, amendments, or other updates to the Manufacturing Plan for submission to the JMC, (iv) preparing any amendment to the Development Plan for CMC Manufacturing Development activities for submission to the JMC, (v) preparing the Co-Commercialization Plan and any proposed changes, amendments, or other updates to the Co-Commercialization Plan for submission to the JCC, (vi) preparing the PCD Strategy and the Global Brand Strategy for each Product, (vii) any other responsibilities delegated to the JPT by an Operating Committee, and (viii) discuss and coordinate any desired or planned publications or presentations (or other public disclosure of any Development Data, or any other results of any Clinical Trial or analysis therefor) relating to Product in the Territory and coordinate publications in either Party’s clinical trials registry. The JPT shall meet at least [****] by audio or video teleconference or as otherwise agreed by such JPT. The JPT shall not have the authority to interpret or otherwise amend this Agreement.
(b)Working Groups. To facilitate the accomplishment of the JPT’s responsibilities, the JPT may agree to establish working groups composed of representatives designated by each Party to interact on a more frequent and shorter-term basis on specific projects and tasks assigned to them by the JPT; provided that the authority of such working groups shall not expand beyond the authority of the JPT. Any such working group shall have no decision-making authority, but may make recommendations to the JPT for review and as applicable, submission to the relevant Operating Committee(s) for their review and as applicable, approval. Following the Effective Date, the JPT shall assess the need to establish any working groups based on the needs of any Collaboration activities.
ARTICLE 3
DEVELOPMENT
3.1Overview. The Parties agree to collaborate with respect to the Development of Products for use in the Field in the Territory as provided in this Article 3 under the direction of the JDC and the JSC (if applicable), and pursuant to a development plan, which shall include the level of detail specified in this Article 3 (the “Development Plan”) to be prepared by the JPT for approval of the JDC and JSC.
3.2Development Plans. The Development of all Products for use in the Field for the Territory shall be conducted pursuant to the comprehensive (including all timelines, objectives and planned tasks for the conduct of Development activities hereunder), worldwide Development Plan which shall describe, for a rolling period of three (3) Calendar Years (broken down by Calendar Quarter for the first Calendar Year, and by Calendar Year for the following two (2) Calendar Years), beginning with the Effective Date (such portion of the Development Plan, the “Short Term Development Plan”):
(a)the proposed overall program of Development for Products for use in the Field in the Territory, including Non-Clinical Studies and Pre-Approval Trials [****], Product label strategy (including the timing of transition of ownership of the core data sheets to Roche), Product label negotiations strategy and proposed Product label content, and regulatory plans and other Regulatory Activities to obtain Regulatory Approval for Products for use in the Field in the Territory;
(b)the anticipated start dates and Development Data availability dates of such Non-Clinical Studies and Pre-Approval Trials, and anticipated timelines for filing applications for Regulatory Approvals in the Territory;
(c)subject to Section 3.6, the respective roles and responsibilities of each Party (including designation of a Lead Development Party), in connection with such activities; and
(d)the Development Budget.
Together with the Short Term Development Plan, the Development Plan shall include a high-level description of the activities in each Calendar Year subsequent to the period covered by the Short Term Development Plan through the expected first filing of an NDA in the Territory for Products for use in the applicable Indications in the Field (“Long Term Development Plan”).
3.3Development Budget. The Development Plan will include a budget for all Development activities for the Products for use in the Field in the Territory (the “Development Budget”), comprising a detailed rolling budget for the Development activities to be performed under the Development Plan during the [****]; provided that only [****]. Notwithstanding anything to the contrary herein, unless the Parties agree in writing (in each of their sole discretion), in no event shall the amount allocated to a Party under the Development Budget exceed such Party’s share of the [****]. For clarity, no Party shall have any obligation, to incur Development Costs in the Territory in excess of such Party’s share of the [****].
3.4Initial Development Plan. An initial high-level outline of the Development activities currently contemplated as needed to obtain Regulatory Approval in the Territory for the Therapeutic Product as described in the Primary Indication Target Product Profile and for the REVERSIR Product, an initial outline of the Short Term Development Budget, which includes the Development Costs anticipated to be incurred in the conduct of the activities outlined in the Initial Development Plan (other than KARDIA-1 and KARDIA-2) during the three (3) Calendar Year-period beginning on the Effective Date. and an initial outline of the Long Term Development Budget, including high-level, non-binding and preliminary estimates of the long term Development Costs through Regulatory Approval is attached hereto as Exhibit A (the “Initial Development Plan”). Within [****] days after the Effective Date, the JPT shall prepare, for review and approval by the JDC in accordance with Section 2.2(c)(ii), and the JSC in accordance with Section 2.1(c)(ii), a Development Plan (for clarity, including a Short Term Development Plan and a Long Term Development Plan, and a Short Term Development Budget and a Long Term Development Budget), which shall be consistent with the Initial Development Plan (including with the Primary Indication Target Product Profile, clinical development plans and Clinical Trials designs therein) and the initial draft of the Short Term Development Budget, including all timelines set forth therein, and shall set forth the objectives and planned tasks for the conduct of Development activities hereunder, including regulatory strategy. The Initial Development Plan shall be effective from the Effective Date until amended and updated by the JPT and approved by the JDC and JSC in accordance with Section 3.5.
3.5Amendments to the Development Plan.
(a)Annual Updates. On a Calendar Year basis (no later than [****] days prior to the start of the following Calendar Year), or more often as the Parties may agree upon in writing from time to time, the JSC and the JDC shall review and as it determines appropriate, update and approve any changes, amendments or other updates to the Development Plan prepared by the JPT (including the Development Budget contained therein). For clarity, such updated and amended Development Plan shall reflect any changes, re-prioritization of studies within, reallocation of resources with respect to, and additions to the then-current Development Plan and shall include a Short Term Development Plan (including a Short Term Development Budget) and a Long Term Development Plan (including a Long Term Development Budget). Once approved in accordance with Article 2, the amended Development Plan shall become effective for the applicable period commencing January 1st (or such other date that the JSC may specify) and any amended Development Plan so approved shall supersede the previous Development Plan for the applicable period. If the JSC (or, for clarity, a Party, in accordance with Article 2) decides to discontinue Developing a Product for use in one or more Indications upon recommendation by the JDC, the Development Plan shall terminate with respect to such Product for use in such Indication(s) upon such decision.
(b)Additional Program Plans.
(i)From time to time, during the Term, each Party shall have the right to propose to the JDC that the Development Plan be amended to include (1) any new Indication in the Field (including any Hypertension Indication) for the Product other than the Primary Indication (each, an “Additional Indication”), (2) any additional Non-Clinical Studies or Pre-Approval Trials needed to Develop the Products in an Additional Indication that was previously added to the Development Plan under this Section 3.5 (each, an “Additional Study”), or (3) any Combination Product or any other co-administration of the Product with another pharmaceutical product, in each case (the foregoing (1) through (3)), by submitting a written proposal to the JDC describing the proposed Additional Indication (including its proposed target product profile), Additional Study, or Combination Product (including its proposed target product profile), as applicable, and anticipated costs and timelines with respect thereto, in sufficient detail for the JDC to consider such Additional Indication, Additional Study or Combination Product for inclusion in the Development Plan (each such proposal, an “Additional Program Plan”).
(ii)Following a Party’s submission of an Additional Program Plan to the JDC in accordance with Section 3.5(b)(i), the JDC shall, at its next regularly scheduled meeting, review and determine whether to modify or amend such Additional Program Plan. In the event that the JDC accepts such Additional Program Plan (as modified or amended, as applicable), then the JDC shall recommend such Additional Program Plan (with any modifications or amendments agreed upon by the JDC) for consideration by the JSC for inclusion in the Development Plan by providing the JSC with such Additional Program Plan. Following the JDC’s submission of an Additional Program Plan to the JSC in accordance with this Section 3.5(b)(ii), the JSC shall, at its next regularly scheduled meeting, review and determine whether to approve such Additional Program Plan, either in the form provided by the JDC or with such modifications or amendments agreed upon by the JSC. If the JSC approves such Additional Program Plan (with any modifications or amendments agreed upon by the JSC), such Additional Program Plan shall be incorporated in the Development Plan. If the JSC fails to approve the applicable Additional Program Plan, then neither Party may, directly or indirectly, itself or through any Affiliate or Third Party, conduct the applicable Additional Program Plan or any activities therein.
(c)Device Program Plans.
(i)The Parties agree that, as of the Effective Date, the Development Plan for the Therapeutic Product will include Development activities [****]. From time to time, during the Term, each Party shall have the right to propose to the JDC that the Development Plan be amended to include a device, [****], for administration of any Product for use in the Field (each such device, an “Administration Device”) in the Co-Commercialization Territory or in the Roche Territory by submitting a written proposal to the JDC describing the proposed Administration Device and anticipated costs and timelines with respect thereto, in sufficient detail for such Party to consider for inclusion in the Development Plan (each such proposal, a “Device Program Plan”).
(ii)Following a Party’s submission of a Device Program Plan to the JDC in accordance with Section 3.5(c)(i), the JDC shall, at its next regularly scheduled meeting, review and determine whether to modify or amend such Device Program Plan. In the event that the JDC accepts such Device Program Plan (as modified or amended, as applicable), then the JDC shall recommend such Device Program Plan for consideration by the JSC for inclusion in the Development Plan (with any modifications or amendments agreed upon by the JDC) by providing the JSC with such Device Program Plan. Following the JDC’s submission of a Device Program Plan to the JSC in accordance with this Section 3.5(c)(ii), the JSC shall, at its next regularly scheduled meeting, review and determine whether to approve such Device Program Plan, either in the form provided by the JDC or with such modifications or amendments agreed upon by the JSC. If the JSC approves such Device Program Plan (with any modifications or amendments agreed upon by the JSC), such Device Program Plan shall be incorporated in the Development Plan. If the JSC fails to approve the Device Program Plan in accordance with Section 2.5, then (1) for the Co-Commercialization Territory, neither Party may, directly or indirectly, itself or through any Affiliate or Third Party, Develop or Commercialize the applicable Administration Device for the Co-Commercialization Territory and (2) for the Roche Territory, Roche may, at its sole cost and expense, Develop the applicable Administration Device for the administration of a Product Developed for use in the Field pursuant to the Development Plan for use solely in the Roche Territory.
3.6Lead Development Party. One Party shall have primary responsibility for the day-to-day operational activities and decision-making with respect to each Non-Clinical Study and Pre-Approval Trial in connection with Development of Products hereunder, as further described in this Section 3.6 (such Party, the “Lead Development Party”).
(a)Alnylam as Lead Development Party. Alnylam shall be the Lead Development Party and shall have primary operational responsibility for leading the conduct and execution of (i) all Non-Clinical Studies and Pre-Approval Trials of the Therapeutic Product for the Hypertension Indications (except as provided in Section 3.6(b)(i)(2) below), including KARDIA-1, KARDIA-2, KARDIA-3, the Initial CVOT Study, and any PIP Trial for the Primary Indication Program, (ii) all Non-Clinical Studies and Pre-Approval Trials of the REVERSIR Product, and (iii) all Non-Clinical Studies and Pre-Approval Trials of Products for any Additional Program Plan for an Additional Indication for which Roche does not exercise its Development Lead Option and for which Alnylam elects to do so by providing written notice to Roche (as described in Section 3.6(b)(ii) below) (each of the foregoing (i) through (iii), an “Alnylam Lead Study”).
(b)Roche as Lead Development Party.
(i)Roche shall be the Lead Development Party and shall have primary operational responsibility for (1) all Non-Clinical Studies and Pre-Approval Trials of any Product for any Additional Program Plan for which Roche exercises its Development Lead Option pursuant to Section 3.6(b)(ii) below, and (2) subject to Section 3.6(a)(i), all Non-Clinical Studies and Pre-Approval Trials of any Product that are conducted primarily to support Regulatory Approval of the applicable Product in the Roche Territory (which shall be deemed to include any Bridging Studies) (each of the foregoing (i) and (ii), a “Roche Lead Study”).
(ii)For a period of [****] Business Days after the date on which the JSC approves the first Additional Program Plan for an Additional Indication, and every Additional Program Plan for an Additional Indication thereafter, Roche shall have the right, but not the obligation, to elect to be the Lead Development Party for such Additional Program Plans (the “Development Lead Option”) by providing Alnylam with written notice of such election during such time period. For clarity, upon providing such notice in accordance with the foregoing, Roche shall become the Lead Development Party for the Non-Clinical Studies and Pre-Approval Trials set forth in the applicable Additional Program Plan. For clarity, in the event that Roche does not provide such notice in accordance with the foregoing, Alnylam shall have the right, but not the obligation, to be the Lead Development Party for the applicable Additional Program Plan by providing Roche with written notice thereof.
(c)Rights and Responsibilities. The rights and responsibilities of the Lead Development Party shall include managing operationalization of the applicable study, negotiating and entering into contracts with participating clinical sites and investigators (using a clinical trial agreement template previously agreed upon by the Parties in accordance with Section 2.2(c)(iii) that includes terms (including intellectual property ownership, assignment and confidentiality principles) mutually agreed upon that are consistent with this Agreement). The Party that is not the Lead Development Party for a specific Non-Clinical Study or Pre-Approval Trial shall perform those activities related to such study that are allocated to it under the Development Plan, shall reasonably cooperate with the Lead Development Party in the conduct of such study, and shall have the right to provide the Lead Development Party with input and feedback regarding the conduct of such study (which input and feedback the Lead Development Party will consider in good faith).
3.7Transfer of Development Data.
(a)Alnylam will provide Roche with the data included in [****], and any other Development Data generated by or on behalf of Alnylam with respect to [****] to the extent not provided to Roche pursuant to the foregoing; and
(b)without limiting the foregoing clause (a), the Lead Development Party will provide the other Party with a copy of all Development Data generated in any Clinical Trial or other Development activity conducted by the Lead Development Party under the Development Plan, including as set forth on Schedule 3.7(b). For each delivery of [****].
3.8Development Diligence and Standards of Conduct. Each Party shall use Commercially Reasonable Efforts to carry out the tasks assigned to it under the Development Plan. Without limiting the foregoing, each Party shall conduct its activities under the Development Plan in a good scientific manner and in compliance with all Applicable Laws.
3.9Development Records and Reports. Each Party shall maintain complete and accurate records (in the form of technical notebooks or electronic files where appropriate) of all work conducted by it under the Development Plan and all information and Development Data resulting from such work (such records, “Development Records”). Such Development Records, including any electronic files where such information or Development Data may also be contained, shall fully and properly reflect all work done and results achieved in the performance of the Development Plan in sufficient detail and in good scientific manner appropriate for patent and regulatory purposes. Each Party shall have the right to review and copy Development Records maintained by the other Party at reasonable times and to the extent needed for patent or regulatory purposes, to obtain access to originals. Within [****] days after the end of each Calendar Quarter, each Party shall provide the JDC with reports detailing its Development activities under the Development Plan for the immediately preceding Calendar Quarter (each such report, a “Development Report”) and the results of such activities as the JDC requests.
3.10Subcontracts. Each Party may perform any of its Development obligations under this Agreement through one or more subcontractors or consultants in accordance with, and subject to, Section 7.3.
ARTICLE 4
REGULATORY MATTERS
4.1Overview. The Parties agree to collaborate with respect to the Regulatory Materials (including Regulatory Approvals and regulatory label negotiation strategy) for Products for use in the Field in the Territory as provided in this Agreement (including this Article 4) under the direction of the JDC and the JSC, and pursuant to the Development Plan. The Development Plan shall set forth the regulatory strategy (including the label strategy, and the content of the initially submitted label, and the content of any major labeling updates) for seeking Regulatory Approvals in the Territory of all Products being Developed under the terms of this Agreement.
4.2Lead Regulatory Party. One Party shall have primary responsibility for the day-to-day operational activities and decision-making with respect to certain Regulatory Materials and interactions with the applicable Regulatory Authorities for a Product, as further described in this Section 4.2 (such Party, the “Lead Regulatory Party”).
(a)Alnylam as Lead Regulatory Party.
(i)As between the Parties, Alnylam will be the Lead Regulatory Party, and in furtherance thereof, will be responsible for, (1) preparing and submitting any IND, and any related Regulatory Materials necessary or desirable for conducting any Alnylam Lead Study (and Alnylam will be the sponsor of such Non-Clinical Trial or Clinical Trial); (2) preparing and submitting any NDA and other application for Regulatory Approval, and any other related Regulatory Materials necessary or desirable for obtaining, registering, listing and maintaining Regulatory Approval for any Product for use in the Field in the Co-Commercialization Territory; and (3) performing those day-to-day activities (including corresponding and participating in any meetings with the applicable Regulatory Authorities) required to obtain or maintain any of the foregoing. All such Regulatory Materials will be in Alnylam’s or its designee’s name and owned by Alnylam; provided that (x) Roche shall be responsible for, and hold in its or its Affiliate’s name, the NDC that is affixed to the Product packaging for Product in the Co-Commercialization Territory, and (y) to the extent Applicable Laws or Regulatory Authorities in the relevant jurisdiction require such Regulatory Materials to be in Roche’s name, such Regulatory Materials shall be in Roche’s name to such extent (with Alnylam as the responsible party or owner to the extent permitted by Applicable Laws), and following Alnylam’s reasonable written request, Roche shall cooperate with Alnylam to transfer such Regulatory Materials to Alnylam as and to the extent permitted by Applicable Laws. Notwithstanding anything to the contrary herein, for clarity, Alnylam shall also hold, in its or its Affiliate’s or other designee’s name, one or more NDCs with respect to Products.
(ii)Notwithstanding Section 4.2(b), prior to the Technology Transfer Completion Date, Alnylam will be responsible for authoring the core CMC dossier of any Regulatory Materials for the Product for use in the Field and providing Roche with a copy of such core CMC dossier for Roche to include in those Regulatory Materials for which it is responsible pursuant to the terms hereof. Roche shall use such sections [****].
(b)Roche as Lead Regulatory Party. Subject to Section 4.2(a)(ii), as between the Parties, Roche will be the Lead Regulatory Party and in furtherance thereof, will be responsible for (i) preparing and submitting any IND, and any related Regulatory Materials necessary or desirable for conducting any Roche Lead Study (and Roche will be the sponsor of such study); (ii) preparing and submitting any NDA and other application for Regulatory Approval, any related Regulatory Materials necessary or desirable for obtaining, registering, listing and maintaining such Regulatory Approval for any Product for use in the Field in the Roche Territory in accordance with the terms hereof; (iii) holding in its (or its Affiliate’s) name and maintaining the NDC that is affixed to the Product packaging and (iv) performing those day-to-day activities (including corresponding and participating in any meetings with the applicable Regulatory Authorities) required to obtain or maintain any of the foregoing. All such Regulatory Materials will be in Roche’s or its designee’s name and owned by Roche; provided that to the extent Applicable Laws or Regulatory Authorities in the relevant jurisdiction require such Regulatory Materials to be in Alnylam’s name, such Regulatory Materials shall be in Alnylam’s name to such extent (with Roche as the responsible party or owner to the extent permitted by Applicable Laws), and following Roche’s reasonable written request, Alnylam shall cooperate with Roche to transfer such Regulatory Materials to Roche as and to the extent permitted by Applicable Laws.
(c)Additional Lead Regulatory Party Responsibilities.
(i)Submissions to Regulatory Authorities. The Lead Regulatory Party will provide the other Party with a copy of any [****] and to the extent set forth on Schedule 4.2(c)(i), other significant filings or communications, in each case, for submission to any Regulatory Authority (each, a “Major Regulatory Communication”), in each case (the foregoing (1)-(3)), in English and reasonably in advance of submission of such Major Regulatory Communication to the applicable Regulatory Authority and reasonably consider (to the extent reasonably practicable) comments promptly provided by the other Party in good faith. If following such consideration, any disagreement remains between the Parties as to the content of a Major Regulatory Communication, such matter shall be [****].
(ii)Communications with Regulatory Authorities. Within [****] Business Days of receipt, the Lead Regulatory Party shall provide the other Party with copies of all written or electronic Major Regulatory Communications received by it from any Regulatory Authority. To the extent the other Party receives any communication from a Regulatory Authority that addresses, in whole or in part, a study for which it is not the Lead Regulatory Party, such other Party shall provide the Lead Regulatory Party with copies of any such written or electronic communications within [****] Business Days of receipt.
(iii)Regulatory Meetings. With respect to those matters for which a Lead Regulatory Party is responsible in accordance with the terms hereof, such Lead Regulatory Party shall provide the other Party with reasonable (but no less than [****]) prior written notice of any requested or scheduled meeting with any Regulatory Authority including engagements with OPDP, [****]. Representatives of the Lead Regulatory Party will be the primary spokespeople at any such meeting [****]. The Lead Regulatory Party shall reasonably consider any proposed input for the meeting provided by the other Party in good faith; provided that, for clarity and notwithstanding anything to the contrary herein, the Lead Regulatory Party shall have final decision-making authority in the event of a dispute with respect to the content and information to be discussed at a meeting with a Regulatory Authority.
4.3Right of Reference.
(a)Subject to the terms and conditions of this Agreement (including the licenses granted pursuant to Section 7.1), Alnylam hereby grants to Roche, its Affiliates and Sublicensees a right to cross-reference, file or incorporate by reference any Regulatory Materials (including Regulatory Approvals) for any Product for use in the Field in the Co-Commercialization Territory, and Development Data and other Know-How included or referenced therein or filed in support thereof, to the extent such Regulatory Materials, Development Data and other Know-How are Controlled by Alnylam or any of its Affiliates, solely for Roche or its Related Parties to Develop Products in accordance with the Development Plan, Manufacture Products for use in the Field for the Roche Territory after completion of Technology Transfer by Alnylam pursuant to Section 6.8 and obtain and maintain Regulatory Approvals for Products in the Roche Territory, in each case, to the extent permitted under this Agreement and to otherwise enable Roche to fulfill its obligations with respect to Products hereunder.
(b)Subject to the terms and conditions of this Agreement (including the licenses granted pursuant to Section 7.2), Roche hereby grants to Alnylam, its Affiliates and Sublicensees a right to cross-reference, file or incorporate by reference any Regulatory Materials (including Regulatory Approvals) for any Product, and Development Data and other Know-How included or referenced therein or filed in support thereof, to the extent such Regulatory Materials, Development Data and other Know-How are Controlled by Roche or any of its Affiliates, solely for Alnylam or its Related Parties (or subcontractors acting on their behalf) to Develop Products for use in the Field, Manufacture Products for use in the Territory and obtain and maintain Regulatory Approvals for Products in the Co-Commercialization Territory, in each case, to the extent permitted under this Agreement and to otherwise enable Alnylam to fulfill its obligations with respect to Products hereunder.
(c)Each Party shall duly execute and deliver, or cause to be duly executed and delivered, such instruments and shall do and cause to be done such reasonable acts and things, as may be necessary under, or as the other Party may reasonably request, to effectuate the rights granted under Section 4.3(a) and 4.3(b).
(d)Neither Party will grant any right of reference or other access to Regulatory Materials (including Regulatory Approvals) or Development Data with respect to Product in the Territory to any Third Party other than as necessary to Related Parties (or subcontractors acting on their behalf) for the Development, Manufacture or Commercialization of Product in the Field in the Territory hereunder, or as otherwise permitted herein.
4.4Product Withdrawals and Recalls.
(a)Co-Commercialization Territory. If either Party becomes aware of any Regulatory Authority that (i) has threatened, initiated or advised any action to remove any Product for use in the Field from the market or (ii) has required or advised Alnylam, Roche, or any of their Related Parties to distribute a “Dear Doctor” letter or its equivalent regarding use of such Product in the Field (collectively, a “Product Action Concern”), in each case ((i) and (ii)) with respect to, or in, the Co-Commercialization Territory, then such Party shall notify the other Party in writing of such event within [****] Business Days (or sooner if required by Applicable Laws) after such Party becomes aware of the Product Action Concern. Following receipt of such written notice, the Parties will discuss and attempt to agree upon whether to recall or withdraw the applicable Product for use in the Field in the Co-Commercialization Territory in response to such Product Action Concern; provided, however, that if the Parties fail to agree within an appropriate time period [****].
(b)Roche Territory. If either Party becomes aware of any Regulatory Authority that raises a Product Action Concern with respect to, or in, the Roche Territory, then such Party shall notify the other Party in writing of such event within [****] Business Days (or sooner if required by Applicable Laws) after such Party becomes aware of the Product Action Concern. Following receipt of such written notice, the Parties will discuss and attempt to agree upon whether to recall or withdraw the applicable Product for use in the Field in the Roche Territory; provided, however, that if the Parties fail to agree within an appropriate time period [****].
4.5Safety Reporting. As soon as practicable after the Effective Date, the Parties shall mutually agree and execute a separate agreement (a “Pharmacovigilance Agreement”) specifying the procedures and timeframes for compliance with Applicable Laws pertaining to safety reporting of the Products and their related activities. The Pharmacovigilance Agreement will set forth each Party’s responsibilities and obligations pertaining to safety collection, assessment and reporting for the Product based on Applicable Laws, including a timeline and procedures for transfer of the global safety database from Alnylam to Roche.
4.6Regulatory Standards of Conduct. Each Party shall make regulatory filings, seek Regulatory Approvals and conduct all other Regulatory Activities under this Agreement (including this Article 4) in compliance with all Applicable Laws, including any Anti-Corruption Laws.
4.7Subcontracts. Each Party may perform any of its Regulatory Activities under this Agreement through one or more Sublicensees or subcontractors in accordance with, and subject to, Section 7.3.
ARTICLE 5
COMMERCIALIZATION
5.1Overview. The Parties agree to collaborate with respect to the Commercialization of Products for use in the Field in the Co-Commercialization Territory as provided in this Agreement (including this Article 5) under the direction of the JCC and the JSC, and pursuant to the Co-Commercialization Plan and the Global Brand Strategy (each of which shall be prepared by the JPT for approval of JSC following the review and approval by the JCC). Roche shall have the sole right and responsibility for Commercializing Products for use in the Field in the Roche Territory in accordance with the Global Brand Strategy and this Agreement.
5.2Global Brand Strategy. For each Product, the JPT will prepare for review and approval of the JSC (following the review and approval by the JCC), and thereafter update from time to time, in each case with the approval of the JSC) a global brand strategy, including global positioning and global brand elements, for such Product for use in the Field in the Territory (each, a “Global Brand Strategy”).
5.3Co-Commercialization Plan. The Commercialization of all Products for use in the Field for the Co-Commercialization Territory shall be conducted pursuant to a comprehensive, worldwide co-commercialization plan that is consistent with the Global Brand Strategy(ies) (the “Co-Commercialization Plan”) that describes [****] for a rolling period of [****], beginning at [****] (as agreed by the Parties in writing):
(a)pre-launch, launch and subsequent Commercialization activities for such Product in the Co-Commercialization Territory [****];
(b)[****];
(c)the relative responsibilities of the Parties, including which Party will lead the operationalization of any Post-Approval Study (subject to Sections 2.5(d)(i)(6) and 2.5(d)(i)(7)); and
(d)the Co-Commercialization Budget.
5.4Co-Commercialization Budget. In accordance with Section 5.3(d), the Co-Commercialization Plan shall include an overall budget for the anticipated Co-Commercialization Costs for each Product in the Co-Commercialization Territory during the Co-Commercialization Term, which budget shall comprise a rolling budget for the Commercialization activities to be performed under the Co-Commercialization Plan during the following [****] (each such budget, and any revisions thereto, the “Co-Commercialization Budget”). Notwithstanding anything to the contrary hereunder, unless the Parties agree in writing (in each of their sole discretion), in no event shall the amount allocated to a Party under the Co-Commercialization Budget with respect to Therapeutic Product [****]. [****].
5.5Initial Co-Commercialization Plan. At least [****] prior to the anticipated First Commercial Sale of a Product for use in the Field in the Co-Commercialization Territory (as agreed upon by the Parties or such other time period that the Parties agree is sufficiently in advance of such anticipated First Commercial Sale to ensure approval of Co-Commercialization Plan prior to start of the Launch Preparation Period for such Product in the Co-Commercialization Territory), the JPT shall prepare, for the JSC’s review and approval (following the review and approval by the JCC), the initial Co-Commercialization Plan for such Product for use in the Field in the Co-Commercialization Territory (including the initial Co-Commercialization Budget, the “Initial Co-Commercialization Plan”). The Initial Co-Commercialization Plan shall be effective from the date it is approved by the JSC or otherwise in accordance with the terms hereof until amended and updated by the JCC and approved by the JSC in accordance with the terms hereof.
5.6Amendments to the Co-Commercialization Plan. On a [****] basis (on timing to be coordinated with Roche’s then typical internal commercial plan review and approval processes used for Roche’s other pharmaceutical products), or more often as the Parties may agree upon in writing from time to time, the JSC shall review and as it determines appropriate, update and approve, any amendments to the Co-Commercialization Plan (including the Co-Commercialization Budget contained therein) as prepared by the JPT and submitted to the JCC for review and approval for further submission to the JSC for approval. [****]. Once approved by the JSC, the amended Co-Commercialization Plan shall become effective for the applicable period and any JSC-approved amended Co-Commercialization Plan shall supersede the previous Co-Commercialization Plan for the applicable period. If the JSC decides to discontinue Commercializing a Product for use in one or more Indications upon recommendation by the JCC, the Co-Commercialization Plan shall terminate with respect to such Product for use in such Indication(s) upon such decision.
5.7Roche Territory. Roche shall at all times conduct Commercialization of Products in the Field in the Roche Territory in accordance with the Global Brand Strategy. Roche shall provide informational updates to the JCC of its plans for Commercialization activities for Products for use in the Roche Territory on an annual basis (no later than once every [****], and shall respond in a timely fashion to any reasonable requests of Alnylam with respect to such plans and Commercialization activities in the Roche Territory. Roche will consider in good faith Alnylam’s input on such plans; provided that for clarity, Roche shall have final decision making authority at the JCC with respect to Commercialization of Products in the Roche Territory in accordance with Section 2.5(d)(ii)(3).
5.8Names and Logos of the Parties. The JPT (for review and approval of the JCC) shall determine the manner in which the Parties will be presented and described in (1) any Promotional Materials, Disease Awareness Materials, Product packaging or other materials related to a particular Product, and the placement of the names and logos of the Parties (provided that all such Promotional Materials, Disease Awareness Materials and Product packaging shall include, with equal prominence, the names and logos of both Parties), in each case to the extent permitted by Applicable Laws and (2) the labeling for the applicable Product approved by the applicable Regulatory Authority.
5.9PCD Strategy. The JPT will prepare a global pricing, contracting and distribution strategy (the “PCD Strategy”) and submit it to the JCC for review and approval prior to Product Regulatory Approval in the Territory. The PCD Strategy will provide [****]. For clarity, if the JCC and JSC cannot agree, [****]. Roche will update the JCC at each regularly scheduled JCC meeting with respect to all such pricing, contracting and distribution activities which Roche shall conduct in a manner consistent with the PCD Strategy in accordance with, and subject to, Section 2.5(d). Roche shall also be solely responsible for handling all returns, recalls or withdrawals (as finally determined to be undertaken pursuant to Section 4.4(a)), order processing, invoicing and collection, and receivables using the appropriate Roche (or its Affiliate’s) NDC that is affixed to the Product packaging for Product sales and distribution in the Co-Commercialization Territory. Alnylam shall not accept orders for Products or sell Products for its own account or for Roche’s account, in the Field in the Territory, and if Alnylam receives any order for Products for use in the Field in the Territory, it shall refer such orders to Roche for acceptance or rejection. Roche shall book all sales of Product for use in the Field in the Territory.
5.10Co-Promotion of Products. Alnylam shall participate with Roche in Co-Promoting each Product in the Field in the Co-Commercialization Territory on the terms and conditions set forth in this Agreement and the Co-Promotion Agreement. Beginning upon the start of the Launch Preparation Period for the Product for use in the Field in the Co-Commercialization Territory, the Parties shall (a) negotiate in good faith and execute a co-promotion agreement (the “Co-Promotion Agreement”), which shall set forth the terms and conditions applicable to such Co-Promotion and (b) prepare a marketing and sales plan (the “Co-Promotion Plan”) consistent with the Commercialization Plan for each such Product for use in the Field in the Co-Commercialization Territory, each of which (the foregoing (a) and (b)), shall incorporate the terms set forth on Exhibit B (the “Co-Promotion Term Sheet”) and other applicable relevant co-promotion terms and conditions set forth hereunder, and such other customary or appropriate terms and conditions agreed upon in writing by the Parties. Notwithstanding the foregoing, unless otherwise agreed by the Parties, the Co-Promotion Agreement shall specify that [****]. For clarity, Roche shall not have the right to use its [****] under Section 2.5(d)(ii)(3) to modify the Co-Promotion Term Sheet or finalize or modify the Co-Promotion Agreement.
5.11Promotional Materials and Disease Awareness Materials.
(a)Co-Commercialization Territory.
(i)Creation of Promotional Materials and Disease Awareness Materials. With respect to the Co-Commercialization Territory, as may be more specifically set forth in the Co-Promotion Term Sheet and agreed in the Co-Promotion Agreement, the JPT (for clarity, including representatives of both Parties) will co-create promotional strategies, key claims, key messages and Core Promotional Materials, and either Party may create Promotional Materials consisting of derivative marketing and promotional materials from, and consistent with, the Core Promotional Materials for the Co-Commercialization Territory (“Derivative Promotional Materials”). The Parties will [****]. The JPT (for clarity, including representatives of both Parties) will co-create Disease Awareness Materials for Products for use in the Field for use in the Co-Commercialization Territory consisting of disease awareness and disease education materials to increase knowledge and understanding of a specific disease in the Field and its symptoms and causes (collectively, the “Core Disease Awareness Materials”) and either Party may create Disease Awareness Materials consisting of derivative disease awareness materials from, and consistent with, the Core Disease Awareness Materials for the Co-Commercialization Territory (“Derivative Disease Awareness Materials”). The Parties will [****]. All Promotional Materials, Disease Awareness Materials and all related promotional practices, including detailing, distribution of study reprints, interactions with healthcare practitioners, sample distribution, voucher programs, and any payments made to healthcare practitioners to serve as speakers or on advisory boards, shall comply with all Applicable Laws and be consistent with the applicable Product labeling and the Global Brand Strategy. No Promotional Material or Disease Awareness Material will be [****]. All Promotional Materials and Disease Awareness Materials will be used by the Parties [****].
(ii)Approval of Core Promotional Materials and Disease Awareness Materials. Subject to [****] each Core Promotional Material and Core Disease Awareness Material to be utilized by either Party for Products for use in the Field in the Co-Commercialization Territory must be submitted in writing to, and reviewed and approved in writing prior to use by, a joint medical, legal, regulatory review board comprised of an equal number of representatives from each Party who have sufficient expertise and seniority to fulfill their obligations as set forth herein (“Joint MLRB”). The Joint MLRB also shall be responsible for [****]. The Joint MLRB shall meet [****]. The representative members from each Party on the Joint MLRB will have, collectively, [****] vote on behalf of that Party, and all decision-making shall be [****]. If the Joint MLRB cannot [****] with respect to any Core Promotional Materials and Core Disease Awareness Materials, then the matter will be escalated to [****]; provided that the Parties shall [****].
(iii)Approval of Derivative Promotional Materials and Derivative Disease Awareness Materials. The Joint MLRB shall establish processes and procedures [****] to approve [****] Derivative Promotional Materials and Derivative Disease Awareness Materials [****]. If such lead representatives cannot [****] with respect to the approval of any Derivative Promotional Material or Derivative Disease Awareness Material, or reasonably disagree as to whether such Promotional Material is a Derivative Promotional Material or Derivative Disease Awareness Material, then the review and approval of such Promotional Material shall be [****]; provided that the Parties shall [****].
(iv)Omnichannel Infrastructure/Tools and Non-Personal Digital Promotions. Each Party’s responsibilities with respect to Omnichannel infrastructure/tools and Non-Personal Digital Promotions for Product in the Co-Commercialization Territory shall be as specified in the Co-Promotion Term Sheet.
(v)Use Only of Approved Promotional Materials. For clarity, each Party shall only utilize Promotional Materials and Disease Awareness Materials approved by, or on behalf of the, the Joint MLRB in accordance with clause (ii) or clause (iii) above, in each case in Commercializing the Products for use in the Field in the Co-Commercialization Territory. Neither Party shall, and each Party shall cause its Related Parties (and subcontractors acting on their behalf) to not, change the Promotional Materials or Disease Awareness Materials approved by the Joint MLRB in any way, including by: [****]. Notwithstanding anything to the contrary herein, neither Party shall be required to use any Promotional Materials or Disease Awareness Materials, which, in such Party’s reasonable judgment, are not compliant with Applicable Laws, the PhRMA Code or such Party’s internal compliance policies.
(vi)Discontinued Promotional Materials. If the Joint MLRB informs the Parties that a particular Promotional Material or Disease Awareness Material may no longer be used or distributed in the Co-Commercialization Territory for use with the Product in the Field, each Party shall, and shall cause its sales force and medical liaisons to, cease using and distributing such Promotional Material or Disease Awareness Materials (as applicable) after the no-use date specified by the Joint MLRB. If, as of such no-use date, either Party has any remaining inventory of the applicable Promotional Material or Disease Awareness Material, such Party shall, within [****] days after such date, destroy in accordance with Applicable Laws such Promotional Materials or Disease Awareness Materials (as applicable) in its possession, except for a reasonable, limited number of copies which may be retained for archival purposes or as required by Applicable Laws.
(vii)Submission to Regulatory Authorities. To the extent any Promotional Materials (as approved by, or on behalf of, the MLRB in accordance with this Section 5.11(a)) are required by Applicable Laws to be submitted to any Regulatory Authority in the Co-Commercialization Territory, as between the Parties, [****].
(b)Roche Territory. Roche shall be solely responsible for developing the Promotional Materials and Disease Awareness Materials for use by Roche in the Commercialization of the Products for use in the Field in the Roche Territory. Roche may develop the Promotional Materials and Disease Awareness Materials for the Products for use in the Field in the Roche Territory that are different than the Promotional Materials and Disease Awareness Materials used in the Co-Commercialization Territory; provided that such Promotional Materials, Disease Awareness Materials, and all related promotional practices, including detailing, distribution of study reprints, interactions with healthcare practitioners, sample distribution, voucher programs, and any payments made to healthcare practitioners to serve as speakers or on advisory boards (i) include, with equal prominence, the names and logos of both Parties, (ii) are consistent with the applicable Product labeling, the Global Brand Strategy and the Core Promotional Materials and Core Disease Awareness Materials, as applicable, (iii) do not have an adverse effect on the Commercialization activities with respect to any Products in the Co-Commercialization Territory and (iv) notwithstanding anything to the contrary herein, comply with all Applicable Laws.
5.12Medical Information. After the First Commercial Sale of the Product for use in the Field in the Co-Commercialization Territory, Roche shall be responsible for maintaining a medical information call center for responding to medical information requests from healthcare professionals and consumers with respect to Product in the Field in the Territory consistent with its standard practice for such activity. Alnylam and Roche shall discuss and agree upon procedures for responding via medical science liaisons in a consistent manner to medical information requests on Products for use in the Field from healthcare professionals and consumers received after the First Commercial Sale of a Product for use in the Field in the Co-Commercialization Territory.
5.13Medical Affairs Activities.
(a)Co-Commercialization Territory. Subject to the oversight of the JCC and Section 2.5(d), and as further agreed upon by the Parties in the Co-Promotion Agreement, Alnylam and Roche shall each be responsible for undertaking Commercial Medical Affairs Activities for Products for use in the Field in the Co-Commercialization Territory in accordance with the Co-Commercialization Plan upon the start of the Launch Preparation Period. Each of the Parties shall use Commercially Reasonable Efforts to perform Commercial Medical Affairs Activities in support of Products for use in the Field in the Co-Commercialization Territory, and to carry out the tasks assigned to it under the Co-Commercialization Plan in a timely and effective manner and in compliance in all material respects with all Applicable Laws and applicable industry codes, including the PhRMA Code. The number of medical affairs personnel and roles shall be set forth in the Co-Promotion Agreement (which, for clarity, shall be consistent with the terms and conditions set forth in the Co-Promotion Term Sheet).
(b)Roche Territory. Roche shall have the sole right and responsibility for Commercial Medical Affairs Activities in support of the Products for use in the Field in the Roche Territory. Roche shall use Commercially Reasonable Efforts to perform such Commercial Medical Affairs Activities in support of Products for use in the Roche Territory and shall conduct its activities in compliance in all material respects with all Applicable Laws.
5.14Commercialization Diligence and Standards of Conduct. Roche and Alnylam each shall use Commercially Reasonable Efforts to Commercialize each Product for use in the Field in the Co-Commercialization Territory upon Regulatory Approval. Roche shall use Commercially Reasonable Efforts to Commercialize each Product for use in the Field in [****], in each case, following Regulatory Approval for such Product for use in the Field for such country. Without limiting the foregoing, each Party shall use Commercially Reasonable Efforts to carry out the tasks assigned to it under the Co-Commercialization Plan in compliance with all Applicable Laws.
5.15Commercialization Records and Reports. Each Party shall maintain complete and accurate records (in the form of electronic files where appropriate) of all Commercialization activities conducted by such Party in connection with this Agreement (including the Co-Commercialization Plan, as applicable) (such records, “Commercialization Records”). Such Commercialization Records, including any electronic files where such information may also be contained, shall fully and properly reflect all work done in connection with the Commercialization of Products under this Agreement (including the Co-Commercialization Plan, applicable) in sufficient detail for regulatory purposes. To the extent needed for regulatory and patent purposes, or as otherwise reasonably necessary for purposes of either Party fulfilling its obligations under this Agreement, each Party shall have the right to review and copy Commercialization Records maintained by the other Party upon reasonable request at reasonable times and to the extent needed for regulatory and patent purposes, to obtain access to originals. Within [****] days after the end of each Calendar Quarter during which a Party performs any Commercialization activities under this Agreement in the Territory, each Party shall provide the JCC with a report or presentation summarizing (at the level of detail normally prepared internally by such Party) its Commercialization activities pursuant to this Agreement (including the Co-Commercialization Plan, as applicable) during the immediately preceding Calendar Quarter (each such report, a “Commercialization Report”) and the results of such activities as the JCC requests.
5.16Subcontracts. Each Party may perform any of its obligations under the Co-Commercialization Plan through one or more Sublicensees or subcontractors in accordance with, and subject to, Section 7.3.
ARTICLE 6
MANUFACTURE AND SUPPLY
6.1Overview. The Parties agree to collaborate with respect to the Manufacture of Products for use in the Field in the Territory as provided in this Agreement (including this Article 6) under the direction of the JMC and the JSC, and pursuant to the Manufacturing Plan.
6.2Manufacturing Plan. The Manufacture of all Products for use in the Field for the Territory shall be conducted pursuant to a comprehensive, worldwide manufacturing plan (each and any revisions thereto, the “Manufacturing Plan”) that describes, for a rolling period of three (3) Calendar Years, beginning with the Effective Date:
(a)[****];
(b)subject to the remainder of this Article 6, the respective roles and responsibilities of each Party;
(c)the Existing Process Technology Transfer Plan and any New Manufacturing Process Technology Transfer Plan, as applicable;
(d)anticipated Manufacturing Changes;
(e)the Manufacturing Budget;
(f)based upon information from then current Development Plan and Commercialization Plan, as applicable, [****]; and
(g)planned and anticipated CMO supply chain, including with respect to Product packaging and labeling;
provided that, except for any New Manufacturing Process Technology Transfer Plan or anticipated Manufacturing Changes, the Manufacturing Plan (including the Manufacturing Budget) shall only address Manufacturing for Products for use in the Field for use in the Roche Territory until the date upon which Roche is able to Manufacture the first GMP batch of Therapeutic Product and finalization of the relevant documentation related to the Technology Transfer as set forth in Schedule 6.2 (such date, the “Technology Transfer Completion Date”).
6.3Manufacturing Budget.
(a)In accordance with Section 6.2(e), the Manufacturing Plan shall include an overall budget for the anticipated Clinical Supply Costs, Commercial Supply Costs and Other Manufacturing Costs for each Product in the Co-Commercialization Territory, and prior to the Technology Transfer Completion Date, in the Roche Territory, which budget shall comprise a rolling budget for the Manufacturing activities to be performed under the Manufacturing Plan during the following three (3) Calendar Years (broken down by Calendar Quarter for the first Calendar Year), and a forecast of the annual budgets for each subsequent Calendar Year (each such budget, and any revisions thereto, the “Manufacturing Budget”)
(b)A preliminary Manufacturing Budget [****] (the “Preliminary Manufacturing Budget”) and includes an initial outline of the preliminary, non-binding Clinical Supply Costs and Other Manufacturing Costs anticipated to be incurred in the conduct of the activities outlined in the Initial Development Plan (other than KARDIA-1 and KARDIA-2) during the three (3) Calendar Year-period beginning on the Effective Date, as well as a high-level, non-binding and preliminary estimate of the long term Clinical Supply Costs and Other Manufacturing Costs through Regulatory Approval.
6.4Initial Manufacturing Plan. Within [****] days after the Effective Date, the Parties shall develop through the JPT for the JMC’s and JSC’s review and as it determines appropriate, approval of, the initial Manufacturing Plan for the Therapeutic Product for use in the Field in the Territory (including the initial Manufacturing Budget, the “Initial Manufacturing Plan”). The Initial Manufacturing Plan shall be effective from the date it is approved by the JSC or otherwise in accordance with the terms hereof until amended and updated by the JMC and approved by the JSC in accordance with the terms hereof.
6.5Amendments to the Manufacturing Plan. On a [****] basis (no later than [****] days prior to the start of the next following Calendar Year), or more often as the Parties may agree upon in writing from time to time or as necessary to incorporate any Existing Process Technology Transfer Plan, New Manufacturing Process Technology Transfer Plan, Manufacturing Change or other amendment, update or change, the JSC shall review and as it determines appropriate, update and approve, any amendments to the Manufacturing Plan (including the Manufacturing Budget contained therein) as prepared by the JPT and reviewed by the JMC. Once approved by the JSC, the amended Manufacturing Plan shall become effective for the applicable period on the date approved by the JSC (or such other date as the JSC shall specify) and any JSC-approved amended Manufacturing Plan shall supersede the previous Manufacturing Plan for the applicable period. If the JSC decides to discontinue Developing or Commercializing a Product upon recommendation by the JDC or JCC, respectively, the Manufacturing of such Product pursuant to such Manufacturing Plan and this Agreement shall terminate, except to the extent necessary to supply any then ongoing Clinical Trial.
6.6API Process Development and Scale-Up. The Parties shall collaborate through the JPT with respect to [****].
6.7Clinical Supply.
(a)Overview. Subject to the remainder of this Article 6, as between the Parties, Alnylam (itself or through an Affiliate or one or more CMOs) shall have the responsibility for Manufacture of Products (for clarity, including REVERSIR Product) for Non-Clinical Studies and Pre-Approval Trials under the Development Plan for the Co-Commercialization Territory, and, prior to the Technology Transfer Completion Date, for the Roche Territory; provided that Alnylam (itself or through an Affiliate or one or more CMOs) shall have the responsibility for Manufacture of REVERSIR Product for Non-Clinical Studies and Pre-Approval Trials under the Development Plan for the Roche Territory. Following the Technology Transfer Completion Date, Roche shall be responsible for Manufacture of Therapeutic Products for (i) Non-Clinical Studies and Pre-Approval Trials under the Development Plan that are primarily to support Regulatory Approval of a Product for use in the Field in the Roche Territory and (ii) any Post-Approval Studies conducted only for the Roche Territory, unless any such Non-Clinical Study, Pre-Approval Trial or Post-Approval Study under (i) or (ii) above have been initiated prior to the Technology Transfer Completion Date, in which case Alnylam shall continue the supply of Therapeutic Product for such Non-Clinical Study, Pre-Approval Trial or Post-Approval Study until Roche can switch to its own supply and Roche shall use Commercially Reasonable Efforts to switch to its own supply for Therapeutic Product for the Roche Territory as soon as practicable following the Technology Transfer Completion Date.
(b)Clinical Supply Agreement. Within [****] following the Effective Date (unless mutually agreed otherwise), the Parties shall negotiate in good faith and enter into a clinical supply agreement (the “Clinical Supply Agreement”) for the Manufacture and supply by Alnylam of quantities of Products for the conduct of Non-Clinical Studies and Pre-Approval Trials of Products under this Agreement in accordance with the Development Plan for the Co-Commercialization Territory and prior to the Technology Transfer Completion Date, for the Roche Territory, and for the Roche Territory following the Technology Transfer Completion Date for REVERSIR Product. The Clinical Supply Agreement shall include terms consistent with the allocation of Clinical Supply Costs as Shared Development Costs, as set forth in Section 8.2 and the Financial Appendix, and such other reasonable and customary terms (including terms identified in the Commercial Supply Term Sheet that expressly refer to applying to the Clinical Supply Agreement) agreed upon by the Parties. For clarity, the Clinical Supply Agreement will contain the terms applicable to Alnylam’s supply of Product for the Roche Lead Studies; provided that Alnylam will have no obligation to supply Product for Roche Lead Studies prior to the execution of the Clinical Supply Agreement.
6.8Technology Transfer.
(a)Existing Process Technology Transfer. The Parties will, under the oversight of the JMC, and subject to Section 6.9(a), use commercially reasonable efforts to collaborate and complete a one-time transfer by Alnylam (or any of its applicable CMOs) to Roche or [****] designated by Roche of the Know-How within the Alnylam Licensed IP that is necessary, or that Alnylam determines (in good faith) is reasonably useful, to Manufacture drug substance and drug product for the Products for use in the Field using the Manufacturing process in use at such time by Alnylam or its CMO (as applicable) in order for Roche (or such [****]) to Manufacture the Products for clinical and commercial supply in the Roche Territory in accordance with, and subject to, the terms of this Agreement (the “Existing Process Technology Transfer”). For clarity, the Existing Process Technology Transfer will include the transfer of those analytical methods included in the Alnylam Licensed IP that are necessary to ensure quality control and release of the Product. Prior to initiation of the Existing Process Technology Transfer, the Parties, through the JMC, shall submit to the JSC for approval, as part of the Manufacturing Plan, a technology transfer plan, defining the scope, timeline and conditions of the Existing Process Technology Transfer (the “Existing Process Technology Transfer Plan”), which technology transfer shall continue until the Technology Transfer Completion Date. The Parties shall use commercially reasonable efforts to (i) initiate the Existing Process Technology Transfer within [****] after the start of the Launch Preparation Period for such Product, and (ii) complete the Existing Process Technology Transfer as soon as reasonably practicable following initiation in accordance with the foregoing clause (i); provided that if, despite the Parties’ commercially reasonable efforts, the Technology Transfer Completion Date has not occurred as of the First Commercial Sale of Product in the Roche Territory, Alnylam, at Roche’s request, shall Manufacture Product for Commercialization (for clarity, including for Post-Approval Studies) in the Roche Territory until the earlier of: [****]; provided in each case, that the relevant quantities of Product are set forth in Roche’s binding forecast under the applicable Supply Agreement. Following the Technology Transfer Completion Date, Roche shall use commercially reasonable efforts to obtain and maintain the capability to Manufacture Products for use in the Field in the Roche Territory and for which Roche is otherwise responsible hereunder, either itself or through an Affiliate or one or more [****], in sufficient quantities to meet Roche’s Development and Commercialization obligations for the Roche Territory hereunder.
(b)New Manufacturing Process Technology Transfer. In the event that, after the Technology Transfer Completion Date, Alnylam develops an alternative Manufacturing process [****] for Zilebesiran drug substance for use in the Field throughout the Territory (such process, the “New Manufacturing Process”), Alnylam will, reasonably promptly following implementation of such New Manufacturing Process in Alnylam’s, or its Affiliate’s or CMO’s facilities, provide Roche with written notice thereof. Within [****] days after such written notice, Roche shall have the right to provide Alnylam with written notice electing that the Parties will, under the oversight of the JMC, use commercially reasonable efforts to collaborate and complete a one-time transfer by Alnylam (or its applicable CMOs) to Roche or [****] designated by Roche of the Know-How within the Alnylam Licensed IP that is necessary, or that Alnylam determines (in good faith) is reasonably useful, to Manufacture the Products for clinical and commercial supply in the Roche Territory using such New Manufacturing Process in accordance with, and subject to, the terms of this Agreement (the “New Manufacturing Process Technology Transfer” and, together with the Existing Process Technology Transfer, the “Technology Transfers”). Prior to initiation of the New Manufacturing Process Technology Transfer, the Parties, through the JMC, shall submit to the JSC for approval, as part of the Manufacturing Plan, a technology transfer plan, defining the scope, timeline and conditions of such technology transfer (the “New Manufacturing Process Technology Transfer Plan”). The Parties shall use commercially reasonable efforts to complete any New Manufacturing Process Technology Transfer within a reasonable time period after the approval of the New Manufacturing Process Technology Transfer Plan.
(c)Additional Studies/Clarifications. For clarity, following the Technology Transfer Completion Date, Roche shall be solely responsible for completing any additional studies or testing required to obtain and maintain any qualifications and other Regulatory Approvals (including manufacturing licenses) from any Regulatory Authorities or other Governmental Authorities necessary to Manufacture a Product for use in the Field in the Roche Territory and for which Roche is otherwise responsible hereunder. Prior to conducting any such additional studies or testing, Roche shall submit a reasonably detailed plan of such studies and testing to the JMC for approval, as part of the Manufacturing Plan. Upon completion of any such studies or testing, Roche shall promptly provide to the JMC copies of reports from any such additional studies or testing (including any clinical study reports, as applicable) in English.
6.9Commercial Supply.
(a)Roche Territory. Following the Technology Transfer Completion Date, Roche will be responsible (either itself or through an Affiliate or one or more [****]) for the Manufacture of all quantities of Therapeutic Product for Commercialization of the Product for use in the Field in the Roche Territory. Alnylam shall Manufacture and supply the REVERSIR Product for Commercialization for use in the Field in the Roche Territory which shall be included in the Commercial Supply Agreement, and the REVERSIR Product will not be part of the Technology Transfer to Roche under Section 6.8.
(b)Co-Commercialization Territory. Alnylam shall be responsible, either itself or through any of its Affiliates or CMOs, for the Manufacture of quantities of Products required for Commercialization (for clarity, including Post-Approval Studies) in the Co-Commercialization Territory pursuant to the Commercial Supply Agreement. No later than [****] years prior to the anticipated date of First Commercial Sale of the Product for use in the Field in the Co-Commercialization Territory (as agreed upon by the Parties through the JCC), the Parties shall negotiate in good faith and enter into a commercial supply agreement (the “Commercial Supply Agreement” and, together with the Clinical Supply Agreement, the “Supply Agreements”), for the Manufacture and supply by or on behalf of Alnylam or any of its Affiliates (including a CMO) to Roche of quantities of Products for Commercialization of the Products for use in the Field in the Co-Commercialization Territory. The Commercial Supply Agreement shall contain those terms set forth on Exhibit D (the “Commercial Supply Term Sheet”), terms consistent with the allocation of Commercial Supply Costs as part of shared Net Profits and Net Losses as set forth in Section 8.3 and the Financial Appendix, and such other reasonable customary terms agreed upon by the Parties in writing. For the avoidance of doubt, the terms and conditions to be included in the Commercial Supply Agreement shall not be subject to Roche’s or Alnylam’s final decision-making authority under Section 2.5(d)(i) or Section 2.5(d)(ii), as applicable.
6.10Manufacturing Changes. Changes to the specifications or the processes or methods used, for the Manufacture of any Product for use in the Field in the Territory (such changes, if any, “Manufacturing Changes”) shall be implemented in accordance with this Agreement and the relevant quality agreement to be entered into between the Parties pursuant to the Supply Agreements (each, a “Quality Agreement”).
(a)Required Manufacturing Changes. In the event that a Manufacturing Change is requested or required (i) due to a requirement of a Regulatory Authority, or (ii) as necessary to obtain or maintain Regulatory Approval for a Product for use in the Field (each, a “Required Manufacturing Change”), the Parties shall work together in good faith, and Alnylam, or, in the case of the Roche Territory following the Technology Transfer Completion Date, Roche, as applicable, shall use commercially reasonable efforts to make, or cause the applicable CMO to make, such Manufacturing Change.
(b)Manufacturing Changes Requested by Roche. Subject to Section 6.10(a), if, with respect to the Roche Territory prior to the Technology Transfer Completion Date, or with respect to the Co-Commercialization Territory, Roche requests any Manufacturing Changes (any such requested change, a “Roche-Requested Manufacturing Change”), then Alnylam shall, in consultation with the applicable Alnylam CMO (if any), consider such Roche-Requested Manufacturing Change in good faith, including meeting with Roche upon Roche’s reasonable request to discuss such Roche-Requested Manufacturing Change, and, to the extent Alnylam determines in its sole discretion to make such Roche-Requested Manufacturing Change, shall use commercially reasonable efforts to, or to cause its applicable CMO to, make such Roche-Requested Manufacturing Change.
(c)Other Manufacturing Changes.
(i)Manufacturing Changes by Alnylam. Alnylam (itself or through its Affiliates and CMOs) may make Manufacturing Changes for the Co-Commercialization Territory or Territory (but that do not apply solely to the Manufacture of Products for the Roche Territory) that Alnylam reasonably believes are needed to support the development or implementation of any (1) new dosage, form or formulation of any Product or (2) process development, process improvement, manufacturing lifecycle management, or other Manufacturing activities to improve the yield, efficiency or proprietary nature of the Manufacturing process for any Product (each, an “Alnylam Manufacturing Change”); provided that (x) to the extent an Alnylam Manufacturing Change would require a filing with a Regulatory Authority, Alnylam shall propose such Alnylam Manufacturing Changes to the JMC for its review and approval, and (y) any such proposed Alnylam Manufacturing Changes that are not covered by the foregoing clause (x) shall be discussed by the JPT (but, for clarity, shall not be subject to any escalation to, or decision-making of, the JMC or JSC). Alnylam shall not make any such Alnylam Manufacturing Changes covered by clause (x) until approved in accordance with Article 2. For clarity, and notwithstanding anything to the contrary herein, “Alnylam Manufacturing Changes” shall not include Required Manufacturing Changes.
(ii)Manufacturing Changes by Roche. Following the Technology Transfer Completion Date, Roche (itself or through its Affiliates and [****]) may make Manufacturing Changes for the Roche Territory that Roche reasonably believes are needed to support the development or implementation of any (1) new dosage, form or formulation of any Product or (2) process development, process improvement, manufacturing lifecycle management, or other Manufacturing activities to improve the yield, efficiency or proprietary nature of the Manufacturing process for any Product (each, a “Roche Manufacturing Change”); provided that (x) to the extent a Roche Manufacturing Change would require a filing with a Regulatory Authority, Roche shall propose such Roche Manufacturing Changes to the JMC for its review and approval, and (y) any such proposed Roche Manufacturing Changes that are not covered by the foregoing clause (x) shall be discussed by the JPT (but, for clarity, shall not be subject to any escalation to, or decision-making of, the JMC or JSC). For clarity, and notwithstanding anything to the contrary herein, “Roche Manufacturing Changes” shall not include Required Manufacturing Changes.
(d)Change Control Procedures. Alnylam shall have, and shall cause its CMOs to have, a change control procedure with respect to the Manufacturing of the Product in connection with this Agreement, which procedure shall be operated and maintained in accordance with the terms of the applicable Quality Agreement.
6.11Manufacturing Diligence and Standards of Conduct. Each Party shall use Commercially Reasonable Efforts to conduct its activities under the Manufacturing Plan and shall conduct such activities in a good scientific manner and in compliance with all Applicable Laws.
6.12Manufacturing Records and Reports. Each Party shall maintain complete and accurate records (in the form of technical notebooks or electronic files where appropriate) of all work conducted by or on behalf of such Party or any of its Affiliates (including any applicable CMOs) with respect to Manufacturing Products and all information resulting from such work (such records, “Manufacturing Records”). Such Manufacturing Records, including any electronic files where such information may also be contained, shall fully and properly reflect all work done and results achieved in the performance of the Manufacturing Plan in sufficient detail and in good scientific manner appropriate for patent and regulatory purposes. Each Party shall have the right to review and copy Manufacturing Records maintained by the other Party at reasonable times following reasonable notice and to the extent needed for patent or regulatory purposes, to obtain access to originals. Within [****] days after the end of each Calendar Quarter during which a Party performs any Manufacturing activities under the Manufacturing Plan, such Party shall provide the JMC with a report detailing its Manufacturing activities under the Manufacturing Plan for the immediately preceding Calendar Quarter (each such report, a “Manufacturing Report”) and the results of such activities as the JMC requests.
6.13Subcontracts. Each Party may perform any of its manufacturing and supply obligations under the Manufacturing Plan or Supply Agreements through its Affiliates or
one or more CMOs in accordance with, and subject to, Section 7.3; provided that in the case of [****].
ARTICLE 7
LICENSES AND EXCLUSIVITY
7.1License to Roche under Alnylam Licensed IP. Subject to the terms and conditions of this Agreement, Alnylam hereby grants, and shall cause its Affiliates to grant, to Roche:
(a)an exclusive (even as to Alnylam and its Affiliates), royalty-bearing, sublicensable (solely to the extent permitted in accordance with Section 7.3) license, under the Alnylam Licensed IP solely to Commercialize Products solely for use in the Field in the Roche Territory (for clarity, the Commercialization of Products under this license grant shall be subject to the Global Brand Strategy);
(b)a co-exclusive (with Alnylam and its Affiliates), sublicensable (solely to the extent permitted in accordance with Section 7.3) license under the Alnylam Licensed IP solely to (i) Develop Products solely for use in the Field in the Territory in accordance with the Development Plan and (ii) Commercialize Products solely for use in the Field in the Co-Commercialization Territory. For clarity, the Development and Commercialization of Product under this license grant shall be subject to the terms and conditions of this Agreement including the Co-Commercialization Plan, the Co-Promotion Agreement and the Global Brand Strategy; and
(c)a non-exclusive, sublicensable (solely to the extent permitted in accordance with Section 7.3) license under the Alnylam Licensed IP solely to Manufacture Products in the Territory solely for permitted Development and Commercialization of such Products for use in the Field in the Roche Territory following Technology Transfer to Roche in accordance with Section 6.8.
(d)For clarity, the licenses granted to Roche under this Section 7.1 do not include any licenses or other rights to (i) any Know-How or Patent Rights related to (1) any active pharmaceutical ingredient other than Zilebesiran and REVERSIR, or (2) any siRNA, MicroRNA, MicroRNA antagonist, MicroRNA Mimic, or single or double-stranded oligonucleotide Controlled by Alnylam or any of its Affiliates as of the Effective Date or during the Term that is Directed to, or otherwise modulates the expression of, a target other than AGT or (iii) any Alnylam Excluded IP. Notwithstanding anything to the contrary herein, Alnylam and its Related Parties retain the right to use the Alnylam Licensed IP for any research and development purpose, other than clinically Developing, Commercializing, or Manufacturing for clinical Development or Commercialization, any Products for use in the Field in the Territory.
7.2Licenses to Alnylam under Roche Licensed IP. Subject to the terms and conditions of this Agreement, Roche hereby grants, and shall cause its Affiliates to grant, to Alnylam:
(a)a co-exclusive (with Roche and its Affiliates), sublicensable (solely to the extent permitted in accordance with Section 7.3), royalty-free license, under Roche Licensed IP solely to (i) Develop Products solely for use in the Field in the Territory in accordance with the Development Plan and (ii) Commercialize Products solely for use in the Field in the Co-Commercialization Territory (for clarity, the Development and Commercialization of Product under such license shall be subject to the terms and conditions of this Agreement including the Co-Commercialization Plan, the Co-Promotion Agreement and the Global Brand Strategy);
(b)a non-exclusive, sublicensable (solely to the extent permitted in accordance with Section 7.3) royalty-free license under the Roche Licensed IP to Manufacture Products in the Territory solely for use in the Field for Alnylam to fulfill its supply obligations with respect to Product under this Agreement; and
(c)if and solely to the extent permitted under Section 7.3(b), an exclusive, sublicensable (solely to the extent permitted in accordance with Section 7.3) license under the Roche Licensed IP solely to Develop and Commercialize Products solely for use in the Field in Japan.
(d)For clarity, the foregoing licenses granted to Alnylam do not include any rights to any Know-How or Patent Rights related to any (i) active pharmaceutical ingredient other than Zilebesiran and REVERSIR, (ii) any siRNA, MicroRNA, Micro RNA antagonist, MicroRNA Mimic, or single or double-stranded oligonucleotide Controlled by Roche or any of its Affiliates as of the Effective Date or during the Term that is Directed to, or otherwise modulates the expression of, a target other than AGT or (iii) any Roche Excluded IP. Notwithstanding anything to the contrary herein, Roche and its Related Parties retain the right to use the Roche Licensed IP for any research and development purpose, other than clinically Developing, Commercializing, or Manufacturing for clinical Development or Commercialization, any Products for use in the Field in the Territory.
7.3Sublicensing and Subcontracting.
(a)Scope of Permissible Sublicensing.
(i)By Roche. The licenses granted by Alnylam to Roche under Section 7.1 may be sublicensed by Roche solely following receipt of Alnylam’s prior written consent (not to be unreasonably withheld, conditioned or delayed); provided that such consent shall not be required for sublicenses granted by Roche to: [****].
(ii)By Alnylam. The licenses granted by Roche to Alnylam under Section 7.2 may be sublicensed by Alnylam solely following receipt of Roche’s prior written consent (not to be unreasonably withheld, conditioned or delayed); provided that (1) such consent shall not be required for sublicenses granted by Alnylam to [****].
(b)Sublicense to Chugai for Japan.
(i)Roche shall use commercially reasonable efforts to notify and engage Chugai as a Sublicensee with respect to performance of Roche’s obligations hereunder in Japan as soon as reasonably practicable following the Effective Date.
(ii)Without limiting the foregoing Section 7.3(b)(i), in the event that Chugai [****], then Japan shall cease to be included in the Roche Territory as of the earlier of [****].
(iii)Notwithstanding anything to the contrary herein, and for clarity, commencing on the earlier of [****], in each case only if there is not a Chugai Sublicense Agreement prior to such time period, (A) Alnylam shall be permitted, itself or through a Related Party or subcontractor, to Develop, Manufacture, and Commercialize Products in Japan, (B) Alnylam shall have final decision-making authority with respect to the Development, Manufacture, Commercialization and other exploitation of Products for Japan, (C) Alnylam shall make Royalty Payments to Roche on Net Sales of Products in Japan [****], (D) Japan shall cease to be included in the Roche Territory for purposes of this Agreement, and (E) Roche shall not be obligated to make any Royalty Payments, Development Milestone Payments or Sales Milestone Payments related to Japan to Alnylam, and Alnylam shall be responsible for all costs associated with the Development, Manufacture and Commercialization of Product for Japan. In addition, the Parties agree that during the period prior to the earlier of the preceding clauses (1) and (2), Alnylam shall have the right to conduct Development activities for the Therapeutic Product in Japan as needed to meet the timelines in the Initial Development Plan and during such time any costs associated with such Development activities in Japan shall be Alnylam’s sole responsibility; provided that if Chugai shall enter into a Chugai Sublicense Agreement with Roche [****], then Roche shall reimburse Alnylam for such costs.
(iv)Subject in all cases to Section 7.3(b)(iii), and without limiting the Parties’ rights and obligations hereunder, if Japan is no longer part of the Roche Territory in accordance with this Section 7.3, then the Parties shall reasonably cooperate in good faith to establish necessary and appropriate procedures to coordinate the Development, Manufacturing and Commercialization of Product in the Field in Japan with the Development, Manufacturing and Commercialization of Product in the Field in the Co-Commercialization Territory and the Roche Territory.
(c)Sublicenses.
(i)Sublicense to an Affiliate. With respect to an agreement under which a Party grants a sublicense to an Affiliate (or, in the case of Roche to Chugai under a Chugai Sublicense Agreement pursuant to Section 7.3(b)) under any license granted to such Party pursuant to Section 7.1 or Section 7.2 (each, an “Affiliate Sublicense Agreement”), such Party shall ensure that all of the applicable terms and conditions of this Agreement shall apply to such Affiliate (or Chugai as applicable) to the same extent as they apply to such Party for all applicable purposes; provided that in accordance with [****]. The grant of any sublicense in compliance with this Section 7.3 shall not relieve the granting Party of its obligations under this Agreement, and as between Alnylam and Roche, the Party granting such sublicense assumes full responsibility for the performance of all obligations to be performed by, and observance of all terms so imposed on, such Affiliate (or Chugai as applicable). For clarity, if a Sublicensee ceases to be an Affiliate, then any Affiliate Sublicense Agreements with such sublicensee shall automatically become, and shall be subject to the terms of this Agreement applicable to, Third Party Sublicense Agreements as set forth in Section 7.3(c)(ii), and shall thereafter not constitute an Affiliate Sublicense Agreement; provided that such Sublicensee and the corresponding Sublicense Agreement comply with the terms and conditions set forth herein with respect to Sublicensees that are Third Parties and Third Party Sublicense Agreements (including that any consent required to be obtained in connection with such Third Party Sublicensee and Third Party Sublicense Agreement has been granted by the other Party). For clarity, if such Sublicensee (as a Third Party Sublicensee) or the corresponding Sublicense Agreement does not comply with the terms hereof as described in the foregoing sentence, such Sublicense Agreement shall automatically terminate upon the applicable Sublicensee ceasing to be an Affiliate.
(ii)Sublicenses to a Third Party. Each Party shall, in each agreement under which it grants a sublicense to a Third Party under a license granted to such Party pursuant to Section 7.1 or 7.2 (each, a “Third Party Sublicense Agreement” and, together with any Affiliate Sublicense Agreement, a “Sublicense Agreement”):
(1)ensure that all of the terms and conditions applicable to a Sublicensee of this Agreement apply to the Third Party in the applicable Third Party Sublicense Agreement; provided that [****]; and
(2)require such Sublicensee to provide the following to such Party: (A) the assignment and transfer of sponsorship and ownership and all Regulatory Materials held or possessed by such Sublicensee with respect to a Product, as applicable; (B) the assignment of, or a freely sublicensable exclusive license to, all intellectual property Controlled by such Sublicensee, as the case may be, that Covers a Product or its respective Development, Manufacture, Commercialization or other exploitation that was created by or on behalf of such Sublicensee during the exercise of its rights or fulfillment of its obligations pursuant to such Sublicense Agreement, (C) the assignment and transfer of ownership and possession of any Product Trademarks created during the exercise of such Sublicensee’s rights or fulfillment of its obligations pursuant to such Sublicense Agreement and all goodwill associated therewith, and (D) confidentiality and non-use obligations regarding Confidential Information, that are at least as protective as those undertaken by the Parties pursuant to Article 12 hereof.
(iii)Each Sublicense Agreement shall be subject to the applicable terms and conditions of this Agreement and any applicable Third Party License sublicensed to the Sublicensee. Any Sublicense Agreement entered into by a Party with a Third Party shall be in writing. A copy of any Sublicense Agreement executed by a Party with a Third Party shall be provided to the other Party within [****] days after its execution; provided that the terms of any such Sublicense Agreement may be redacted to the extent not pertinent to an understanding of a Party’s obligations or benefits under this Agreement. Each Party shall remain responsible for the work allocated to, and payment to, its Sublicensees to the same extent it would if it were doing such work itself.
(d)Subcontractors.
(i)Each Party shall have the right to subcontract the work performed by it under this Agreement without the prior approval of the other Party; provided that (i) Roche shall only be able to subcontract its Manufacturing obligations under this Agreement with respect to Product for the Roche Territory solely following receipt of Alnylam’s prior written consent (not to be unreasonably withheld, conditioned or delayed) or [****], and (ii) (A) each Party shall obtain the prior written consent of the other Party to [****]; and (B) the Parties shall discuss though the JPT, any of its planned subcontracting out of any of its field force obligations under the Co-Commercialization Plan. Any subcontract entered into by either Party shall be consistent with the applicable terms and conditions of this Agreement, and such Party shall be responsible for the actions or omissions of its subcontractors in performing work hereunder and the compliance of its subcontractors with the terms and conditions of this Agreement. Each Party shall remain responsible for the work allocated to, and payment to, its subcontractors to the same extent it would if it were doing such work itself.
(ii)If Roche engages the services of any Roche Excluded Affiliate to conduct Collaboration activities on behalf of Roche under the Agreement, such Roche Excluded Affiliate will be considered a subcontractor for the purposes of this Section 7.3(d) [****].
7.4[****].
7.5No Implied Licenses. Except to the extent expressly provided hereunder, each Party reserves its and its Affiliates’ rights in and to all intellectual property rights that is not expressly licensed or otherwise granted hereunder. Without limiting the foregoing, this Agreement and the licenses and rights granted herein do not, and shall not be construed to, confer any rights upon either Party or its Related Parties by implication, estoppel, or otherwise as to any of the other Party’s or its Affiliates’ intellectual property rights, except to the extent expressly set forth herein.
7.6Third Party Licenses.
(a)The Parties acknowledge that, during the Term, it may be beneficial to obtain a Third Party License and in furtherance thereof the Parties agree that:
(i)With respect to Third Party intellectual property that is necessary or useful to Develop, Manufacture, Commercialize or otherwise exploit Products for use in the Field in the Co-Commercialization Territory, as soon as reasonably practicable following either Party’s written request, the Parties shall discuss whether to enter into a Third Party License with respect thereto; provided that in the event that the Parties fail to agree on whether to enter into any Third Party License [****]; and
(ii)with respect to Third Party intellectual property that is necessary or useful to Develop, Manufacture, Commercialize or otherwise exploit Products for use in the Field solely in the Roche Territory, as soon as reasonably practicable following either Party’s written request, the Parties shall discuss whether to enter into a Third Party License with respect thereto [****].
(b)The Parties agree that the payments to any Third Party in respect of any Third Party License entered into in accordance with Section 7.6(a) shall be deemed a “Third Party Payment” and subject to this Section 7.6(b). If such Third Party License is necessary or useful for Development, Manufacture, Commercialization or other exploitation of a Product [****].
7.7Exclusive Efforts. Except to the extent (a) permitted pursuant to this Section 7.7 or Section 7.8, or (b) necessary for a Party to satisfy its obligations or exercise its rights with respect to Product under this Agreement, in each case ((a) - (b)), with respect to the Field in the Territory for the Covered Indications [****]. In the event that a Governmental Authority of competent jurisdiction determines that the restrictions under this Section 7.7 are too broad or otherwise unreasonable under any Applicable Laws, including with respect to scope, duration or geographic territory, the court is hereby requested and authorized by the Parties to revise the foregoing restriction to include the maximum restrictions within such scope, duration or geographic territory allowable under Applicable Laws. Notwithstanding the foregoing, each of the Parties acknowledges and agrees that this Section 7.7 has been negotiated by the Parties and that the scope, duration and geographic territory of the restrictions herein are reasonable in light of the circumstances pertaining to the Parties. For clarity, the Parties intend that the foregoing covenants should not extend to diagnostic products or services, software, or clinical trial services provided to Third Parties in the ordinary course of a party’s business that are independent of, and do not involve the use of (x) in the case of Roche, any Alnylam Licensed IP or Alnylam’s Confidential Information, or (y) in the case of Alnylam, any Roche Licensed IP or Roche’s Confidential Information. For purposes of this Section 7.7, [****].
7.8Change of Control and Other Acquisition Transactions.
(a)Notice. If [****],
(i)there is a Change of Control of a Party (such Party, the “Acquired Party”) with a Third Party (the “Acquiror”) and as of the effective date of such Change of Control, such Third Party or any of its Affiliates (other than the Acquired Party or the Acquired Party’s Affiliates immediately prior to the closing of such Change of Control) is engaged, directly or indirectly, in any activities with respect to an AGT EMO in the Field in the Territory that, if carried out by the Acquired Party, would be a breach of such Party’s obligations under Section 7.7 (such activities, an “AGT EMO Program”), or
(ii)a Party (such Party, the “Acquiring Party”) or any of its Affiliates (including in the case of Roche, any Roche Excluded Affiliate) acquires a Third Party (other than a Roche Excluded Affiliate), or a Third Party’s assets (other than a Roche Excluded Affiliate's), and beginning as of the closing of such transaction, the Acquiring Party or any of its Affiliates is engaged in a [****] (each transaction described in the foregoing subsections (i) and (ii), an “AGT EMO Acquisition”),
(iii)then, in each case (the foregoing (i) and (ii)), the Acquired Party or Acquiring Party, as applicable, shall provide the other Party (the “Non-Acquiring Party”) with written notice thereof within [****] Business Days after the closing of such AGT EMO Acquisition and shall comply with the remainder of this Section 7.8.
(b)Permitted Alternatives. By the later of (i) [****] months after the closing of any AGT EMO Acquisition, and (ii) [****], the Acquired Party or Acquiring Party, as applicable, shall, if not prohibited by Applicable Laws, comply with the following (for clarity, the option selected to be in the sole discretion of the Acquired Party or Acquiring Party; provided that such Person complies with the terms and conditions thereof):
(i)use commercially reasonable efforts to divest (whether by sale, license or otherwise) its rights in [****] pursuant to Section 7.8(c); provided that if such divestiture does not occur in accordance with, and subject to, Section 7.8(c), such Acquired Party or Acquiring Party shall comply with option (iii) below;
(ii)if the applicable Party is the Acquired Party (and not the Acquiring Party), elect to continue the Development, Manufacture and Commercialization of the AGT EMO to which the AGT EMO Program relates in accordance with, and subject to, Section 7.8(d); or
(iii)only if (x) the Acquired Party’s or the Acquiring Party’s efforts (as applicable) to divest under the foregoing clause (ii) are unsuccessful, (y) to the extent permitted under Section 7.8(d), the Acquired Party or Acquiring Party, as applicable, does not elect to continue the Development, Manufacture and Commercialization of the AGT EMO to which the AGT EMO Program relates in accordance with, and subject to, Section 7.8(d) or (z) the Acquired Party or the Acquiring Party, as applicable, determines in good faith those options are [****].
(c)Divestiture.
(i)If the Acquired Party or Acquiring Party, as applicable, chooses to divest (whether by sale, license or otherwise) its rights in the AGT EMO in the Field in the Territory to which the AGT EMO Program relates to a Third Party, in accordance with Section 7.8(b)(i), the Acquired Party or Acquiring Party, as applicable, shall notify the Non-Acquiring Party of such desire in writing within [****] days of the closing of the applicable AGT EMO Acquisition. In the event that the Acquired Party or Acquiring Party, as applicable, provides such notice to the Non-Acquiring Party, the Acquired Party or Acquiring Party, as applicable, shall divest such AGT EMO in the Field in the Territory to a Third Party (other than, for clarity, a Roche Excluded Affiliate) within [****] days after such notice; provided that if the Acquired Party or Acquiring Party, as applicable, fails to complete such divestiture within such [****]-day period, but can demonstrate to the Non-Acquiring Party’s reasonable satisfaction that it used good faith to effect such divestiture on commercially reasonable terms within such [****]-day period, then, unless otherwise required by any Applicable Laws, such [****]-day period shall be extended for such additional reasonable period thereafter as is necessary to enable such AGT EMO, as applicable, to be divested, not to exceed an additional [****] days; provided, however, that such period shall be extended for such period as is necessary to obtain any governmental or regulatory approvals required to complete such divestiture, and provided, further, that the Acquired Party or Acquiring Party, as applicable, is using good faith efforts to obtain such approvals (such period, the “Divestment Period”).
(ii)In the event that the Acquired Party or Acquiring Party, as applicable, does not complete the applicable divestiture within the Divestment Period, then (1) the Acquired Party or Acquiring Party, as applicable, shall comply with Section 7.8(b)(iii).
(iii)Any divestiture under this Section 7.8(c) shall not permit the Acquired Party or Acquiring Party, as applicable, or its Affiliates, to [****]. If the Acquired Party or Acquiring Party, as applicable, elects to divest the applicable AGT EMO in the Field in the Territory, the Acquired Party or Acquiring Party, as applicable, shall not be precluded under Section 7.7 from conducting any activities (either directly, or with or through any Third Party) with respect to such AGT EMO during the applicable Divestment Period; provided no Confidential Information of the Non-Acquiring Party and no other information (to the extent it is not yet generally available to the public) generated under this Agreement is used in connection with such AGT EMO Program.
(d)Continued Competing Program; Separation for Collaboration Activities. To the extent not prohibited by Applicable Laws, either Party as the Acquired Party in an AGT EMO Acquisition shall have the right to elect to continue the Development, Manufacture or Commercialization of the AGT EMO to which the AGT EMO Program relates, and in the event the Acquired Party makes such election then, (1) such Party shall notify the Non-Acquiring Party in writing of such election [****].
(e)Notwithstanding the foregoing, the foregoing restrictions will not prevent senior management or members of the board of directors of the Acquiror from receiving financial or other information about activities under this Agreement; provided that such employees or members of the board of directors do not perform any day-to-day responsibilities in connection with this Agreement or for the AGT EMO Program and that such employees and members of the board of directors understand and comply with the Acquired Party’s obligations of confidentiality and non-use as set forth herein. Notwithstanding anything to the contrary in this Agreement, following the closing of any Change of Control, the Parties agree that (1) the Non-Acquiring Party shall not obtain rights or access to any Patent Rights or Know-How Controlled by the Acquiror and its Pre-Existing Affiliates and (2) the Acquiror and its Affiliates (other than the Acquired Party and its Pre-Existing Affiliates) shall not obtain rights or access to the Patent Rights or Know-How Controlled by the Non-Acquiring Party or any of its Affiliates pursuant to this Agreement; provided that clause (1) of this Section 7.8(e) shall not apply to any Patent Rights or Know-How Controlled by the Acquiror or any of its Affiliates to the extent such Patent Right or Know-How (A) is used by or on behalf of the Acquired Party or any of its Affiliates in performing any of the Acquired Party’s obligations under this Agreement or (B) is incorporated into any Product by or on behalf of the Acquired Party or any of its Affiliates. Without limiting the foregoing, the Non-Acquiring Party’s rights in all Patent Rights and Know-How Controlled by the Acquired Party or any of its Pre-Existing Affiliates or any of their respective successors, shall remain licensed to such Non-Acquiring Party after the date of the closing of such Change of Control in accordance with and subject to the terms and conditions of this Agreement and shall not be affected in any manner by virtue of such Change of Control.
(f)Exceptions. Notwithstanding the foregoing Sections 7.7 and 7.8, [****] Alnylam and its Affiliates and Roche and its Affiliates shall each be permitted to, directly or indirectly, whether by itself or with or through a Third Party, conduct pre-clinical research activities and non-clinical Development of AGT EMOs for use in the Field in the Territory, and Manufacturing therefor.
ARTICLE 8
FINANCIALS
8.1Upfront Payment. No later than [****] days after the Effective Date, and receipt of an invoice therefor from Alnylam, Roche shall pay, or cause to be paid, to Alnylam a nonrefundable, non-creditable fee of three hundred ten million dollars ($310,000,000).
8.2Development Costs.
(a)Development Costs Sharing for Territory. The Parties shall share Shared Development Costs incurred for Product for the Territory in the performance of the Development Plan and in accordance with the Development Budget as determined in accordance with Schedule 8.2(a) (the “Financial Appendix”) and the [****].
(b)[****].
8.3Profit and Loss Sharing in the Co-Commercialization Territory. The Parties shall share Net Profits and Net Losses with respect to each Product that is Commercialized for use in the Field for the Co-Commercialization Territory during the Co-Commercialization Term as determined in accordance with the Financial Appendix. For clarity, Alnylam shall have no right to share revenue, and no obligation to bear any Roche Commercialization Costs, and Alnylam shall instead be entitled to receive from Roche the applicable payments pursuant to Sections 8.5, 8.6, and 8.7.
8.4Manufacturing Related Costs.
(a)Technology Transfer Costs. Roche shall be responsible for one hundred percent (100%) of Technology Transfer Costs. Roche shall pay, or cause to be paid, Technology Transfer Costs on a Calendar Quarter basis, within [****] days after receipt of an invoice from Alnylam therefor.
(b)Other Manufacturing Costs. The Parties shall share Other Manufacturing Costs in accordance with the Financial Appendix.
8.5Development Milestone Payments.
(a)Roche shall pay, or cause to be paid, to Alnylam the payments set forth in the table below (each, a “Development Milestone Payment”) upon the first achievement of the corresponding development milestone event by the Therapeutic Product to achieve such development milestone in the Field (each, a “Development Milestone”) in accordance with Section 8.5(b) and Section 8.5(c) up to a maximum amount of [****].
|
|
|
|
|
|
|
|
|
| No. |
Development Milestone |
Development Milestone Payment |
| 1 |
FPI for KARDIA-3 |
$65 million |
| 2 |
[****] |
[****] |
| 3 |
FPI for the Initial CVOT Study for the first Therapeutic Product for use in the Field in the Primary Indication |
$300 million |
| 4 |
[****] |
[****] |
|
[****] |
[****] |
|
[****] |
[****] |
| 5 |
[****]* |
[****] |
| 6 |
[****] |
[****] |
| 7 |
[****]** |
[****] |
| 8 |
[****] |
[****] |
| 9 |
[****] |
[****] |
|
[****] |
[****] |
|
* [****].
**[****].
|
(b)Clarifications. [****].
(c)Notice and Payment. Roche shall provide written notice to Alnylam within [****] days of achievement by or on behalf of Roche of any Development Milestone, and Alnylam shall provide written notice to Roche (and if not already notified, the JSC) within [****] days of achievement by or on behalf of Alnylam of any Development Milestone. After Alnylam’s receipt of a notice of the achievement of a Development Milestone from Roche, or as applicable the delivery by Alnylam to Roche of a notice of the achievement of a Development Milestone by or on behalf of Alnylam, in each case in accordance with this Section 8.5(c), Alnylam shall submit an invoice to Roche with respect to the corresponding Development Milestone Payment and Roche shall pay the corresponding Development Milestone Payment within [****] days after receipt of such invoice. For clarity, each such Development Milestone Payment is nonrefundable and non-creditable against any other payments due hereunder.
8.6Sales Milestone Payments.
(a)Roche shall pay to Alnylam the one-time payments set forth in the table below (the “Sales Milestone Payments”) up to a maximum amount of [****] based on aggregate Net Sales of all Products in the Territory made during (i) the Co-Commercialization Term (for Net Sales in the Co-Commercialization Territory) and (ii) the Term until expiration of the applicable Royalty Term for a given Product and country (for Net Sales in the Roche Territory) in a Calendar Year (each, a “Sales Milestone”) in accordance with Section 8.6(c). Sales Milestone Payments shall be payable one-time only upon the first achievement of the corresponding Sales Milestone (for clarity, regardless of how many times such sales threshold may be reached in any subsequent Calendar Year).
|
|
|
|
|
|
|
|
|
| No. |
Sales Milestone |
Sales Milestone Payment |
| 1 |
Aggregate Net Sales of Products [****] |
[****] |
| 2 |
Aggregate Net Sales of Products [****] |
[****] |
| 3 |
Aggregate Net Sales of Products [****] |
[****] |
| 4 |
Aggregate Net Sales of Products [****] |
[****] |
| 5 |
Aggregate Net Sales of Products [****] |
[****] |
(b)Concurrent Achievement of Sales Milestones. For clarity, each Sales Milestone Payment shall be payable one time only, however, there shall be no restriction on the number of Sales Milestone Payments that are payable with respect to a given Calendar Year. For example, if aggregate Net Sales of the Products subject to payment under this Section 8.6 in the Territory are [****] in the first Calendar Year, and then [****] in the next Calendar Year, then Roche will owe Alnylam [****] with respect to the first Calendar Year and a [****].
(c)Notice and Payment. Roche shall notify Alnylam in writing of achievement of any Sales Milestone pursuant to the applicable Calendar Quarter Report in accordance with Section 8.7(d). After receipt of such written notice from Roche of the achievement of a Sales Milestone in accordance with Section 8.7(d), Alnylam shall submit an invoice to Roche with respect to the corresponding Sales Milestone Payment, and Roche shall pay the corresponding Sales Milestone Payment within [****] days after receipt of such invoice. For clarity, each such Sales Milestone Payment is nonrefundable and non-creditable against any other payments due hereunder.
8.7Royalty Payments.
(a)Roche Territory. Subject to the remainder of this Section 8.7, Roche shall pay to Alnylam nonrefundable, non-creditable (except with respect to ordinary course adjustments made consistent with the calculation of Net Sales or otherwise made in accordance with the procedures set forth in the Financial Appendix) tiered royalties on aggregate Calendar Year Net Sales of Products for use in the Field in the Roche Territory during the Royalty Term thereof (“Royalty Payment”) at the applicable royalty rate set forth below (each, a “Royalty Rate”):
|
|
|
|
|
|
| Portion of Aggregate Calendar Year Net Sales in the Roche Territory |
Royalty Rate |
| Portion of Net Sales [****] |
[****] |
| Portion of Net Sales [****] |
[****] |
| Portion of Net Sales [****] |
[****] |
| Portion of Net Sales [****] |
[****] |
| Portion of Net Sales [****] |
[****] |
[****].
(b)Royalty Term. The period during which Royalty Payments under this Section 8.7 are payable for a given Product and country in the Roche Territory on Net Sales of such Product commences upon the First Commercial Sale of such Product in such country and continues on a Product-by- Product, and country-by-country basis until the latest of (i) [****] from the anniversary of the First Commercial Sale of such Product in such country, (ii) expiration of the last Valid Claim included in any Alnylam Patent that Covers the Manufacture, use or sale of such Product, in such country, and (iii) the expiration of Regulatory Exclusivity for such Product in such country (each such period, a “Royalty Term”).
(c)Royalty Rate Reductions.
(i)Generic Product Entry. On a Product-by-Product and country-by-country basis, if, prior to the end of the Royalty Term for such Product, in any Calendar Quarter at any time after Generic Product Entry for such Product for such country,
(1)subject to Section 8.7(c)(iv), the Net Sales in a Calendar Quarter of such Product in such country has declined [****], then the applicable Royalty Rates for such Product in such country shall be reduced by [****]; and
(2)if the Net Sales in a Calendar Quarter of such Product in such country has declined [****], then the applicable Royalty Rates for such Product in such country shall be reduced [****].
(ii)Exclusivity Expiry. On a Product-by-Product and country-by-country basis, if prior to the end of the Royalty Term in any Calendar Quarter (and subject to Section 8.7(c)(iv)), if (1) the time period set forth in Section 8.7(b)(ii) has expired or continues solely on the basis of [****], and (2) [****], then the applicable Royalty Rate for such Product in such country shall be reduced by [****] for the remainder of the Royalty Term therefor.
(iii)Third Party Payments in the Roche Territory. [****].
(iv)Royalty Floor. Notwithstanding anything to the contrary in this Section 8.7(c), the operation of Section 8.7(c)(i)(1), Section 8.7(c)(ii) and Section 8.7(c)(iii) with respect to a Product, either alone or in combination, shall not [****].
(d)Royalty Reports.
(i)[****].
(ii)Calendar Quarter Reports. During the applicable Royalty Term, following the First Commercial Sale of a Product for use in the Field in the Roche Territory, Roche shall furnish to Alnylam a written report within [****] days after the end of each Calendar Quarter (each, a “Calendar Quarter Report”) showing, on a Product-by-Product and country-by-country basis, for such Calendar Quarter (1) Sales in Swiss Francs; (2) the exchanges rates used in translating local currencies into Swiss Francs; (3) the calculation of Net Sales Deductions; (4) the calculation of the applicable Royalty Rate or any reduction to the Royalty Rates pursuant to Section 8.7(c), and the calculation of Royalty Payments payable in United States Dollars; and (5) the exchange rates used in translating Swiss Francs into United States Dollars, in each case (with respect to the foregoing (1) through (5)), in reasonable detail to enable Alnylam to confirm the accuracy of the calculations. Each Calendar Quarter Report shall also include the total Net Sales of the Product for use in the Field in the Territory for the applicable Calendar Year for the purposes of determining whether a Sales Milestone has been achieved.
(e)Payment. Roche shall make the associated Royalty Payment within [****] days after delivery of the Calendar Quarter Report to Alnylam.
(f)Following Royalty Term. On a Therapeutic Product-by-Therapeutic Product and country-by-country basis in the Roche Territory, upon expiration of the Royalty Term with respect to a Therapeutic Product in a country in the Roche Territory, the license granted by Alnylam to Roche with respect to the Therapeutic Product in such country shall become fully paid-up, perpetual, and irrevocable; no Royalty Payments or Sales Milestone Payments, or any other payments shall be due thereafter with respect to Roche Net Sales of the Therapeutic Product in such country in the Roche Territory.
8.8Taxes.
(a)Taxes on Income. Except as otherwise provided in this Section 8.8, each Party shall be solely responsible for the payment of all Taxes imposed on or measured by its income, including income arising to it directly or indirectly under this Agreement.
(b)Tax Cooperation. The Parties agree to cooperate with one another and use reasonable efforts in accordance with Applicable Laws to reduce value added Tax (“VAT”) or similar payments, Withholding and similar obligations on royalties, milestone payments, and other payments made under this Agreement.
(c)Withholding Taxes. Either Party may withhold from payments due to the other Party amounts in respect of any withholding Tax that are required by Applicable Laws to be paid to any taxing authority with respect to such payments. In such case, the payor Party shall provide the payee Party with written notice of the required withholding as promptly as reasonably practical (and in any event, no later than [****] Business Days) prior to making such payment, and shall provide the payee Party with all relevant documents and correspondence and shall also provide to the payee Party any other cooperation or assistance on a commercially reasonable basis as may be necessary to enable the payee Party to claim exemption from such withholding Taxes and to receive a refund or credit with respect to such withholding Tax. The payor Party shall give proper evidence when available as to the due remittance of any such Tax to the applicable taxing authority. The Parties shall cooperate with each other in seeking benefits (including any exemption from, refund of or reduction in Taxes) under any double taxation or other similar treaty or agreement from time to time in force which may apply to such payments. To the extent such amounts are so deducted and withheld and timely remitted to the relevant tax authorities, such amounts shall be treated for all purposes under this Agreement as having been paid to the Party to whom such amounts would otherwise have been paid. Apart from any withholding permitted under this Section 8.8(c) or as otherwise expressly provided in this Agreement, the amounts payable hereunder shall not be reduced on account of any Taxes. Notwithstanding the foregoing, if, as a result of a Withholding Action by the payor Party (including any assignee or successor), any withholding or deduction of or on account of Taxes (“Withholding”) is required by Applicable Laws and the amount of such Withholding exceeds the amount of Withholding that would have been required if the payor Party had not committed the Withholding Action, then the payor Party shall pay an additional amount to the payee Party such that, after Withholding from the payment and such additional amount, the payee Party receives the same amount as it would have received from the payor Party absent such Withholding Action by the payor Party (except to the extent that the payee Party or any of its Affiliates can obtain a refund or credit for such amounts; provided; that the payee Party will be reimbursed for any reasonable out of pocket costs incurred in obtaining such a refund or credit). For the avoidance of doubt, if as a result of a Withholding Action by a payee Party (including any assignee or successor), the amount of Withholding under the law of the applicable jurisdiction exceeds the amount of such Withholding that would been required in the absence of such Withholding Action by the payee Party, the payor Party shall be required to pay any additional amount only to the extent that the payor Party would be required to pay any additional amount to the payee Party pursuant to the preceding sentence if the payee Party had not committed such Withholding Action. For purposes of this Section 8.8(c) “Withholding Action” by a Party means (i) a permitted assignment or sublicense of this Agreement (in whole or in part) by such Party to an Affiliate or a Third Party outside of the U.S., (ii) the exercise by such Party of its rights under this Agreement (in whole or in part) through an Affiliate or Third Party outside of the U.S. (or the direct exercise of such rights by an Affiliate of such Party outside of the U.S.), (iii) a redomiciliation of such Party, an assignee or a successor to a jurisdiction outside the U.S., and (iv) any action by such Party that causes this Agreement or any payment to become subject to Tax in a jurisdiction outside of the U.S. or subject any payments to Withholding in any jurisdiction that would not have been required absent such Withholding Action.
(d)Other Tax Liability. All amounts mentioned in this Agreement are exclusive of any VAT, goods and services, sales, use, excise, consumption, and other similar indirect Taxes (“Indirect Taxes”). Alnylam shall issue all invoices in full compliance with the Applicable Laws at Alnylam’s place of business. If any Indirect Taxes are due based on local law, Alnylam shall be allowed to add the amount of Indirect Taxes to the amounts mentioned in this Agreement and invoice Roche the net amount plus the applicable Indirect Taxes. The Parties acknowledge that Alnylam shall be allowed to issue Tax-exempt invoices in case of supply of services as set forth in this Agreement. In cases where a Tax-exempt invoice is not applicable, Alnylam and Roche shall cooperate with each other in good faith in order to minimize any adverse financial impact for both Alnylam and Roche.
(e)U.S. Tax Treatment of Co-Commercialization Arrangement.
(i)To the extent attributable to any activities in the United States, the Parties agree to [****].
(ii)The Parties agree that [****].
(f)Foreign-Derived Deduction Eligible Income Reporting. Roche shall obtain and deliver to Alnylam, on an annual basis and within [****] days of Alnylam’s request to provide, information as reasonably requested by Alnylam and in Roche’s possession to meet any documentation requirements imposed by regulations issued under Section 250 of the Code, for the treatment of an appropriate portion of such amounts as “foreign-derived deduction eligible income” within the meaning of Section 250 of the Code and the regulations thereunder.
(g)Payment Allocation. Alnylam shall prepare and deliver to Roche, on an annual basis and within [****] days of the end of each taxable year of Alnylam, an allocation of any Development Milestone Payments, Sales Milestone Payments, Royalty Payments and Development Costs paid or accrued during such taxable year as between the Co-Commercialization Territory and the Roche Territory (and Japan, if Section 7.3(b)(ii) applies). The Parties shall reasonably cooperate in order for Alnylam to timely obtain the relevant data in order to timely prepare such allocation.
8.9Blocked Payments. In a given country, if by reason of Applicable Law (for example governmental restrictions on foreign exchange trade) the local currency is blocked and cannot be removed from such country, then the paying Party shall notify the other Party in writing and (a) the other Party will have the right to receive the applicable blocked payments in such country in local currency by deposit in a local bank designated by such Party, or (b) if such local currency payment is not allowed by reason of Applicable Laws or if and for so long as otherwise requested by the other Party, then the such blocked payment in such country shall continue to be accrued and shall continue to be reported, but such blocked payment royalties will not be paid until such blocked currency or sales proceed may be removed from such country. At such time as the paying Party, its Affiliates or their Sublicensees, as the case may be, is able to remove the blocked currency or sales proceeds from such country, such paying Party shall also pay such accrued payments in United States Dollars using the actual exchange rate which is used to remove such blocked currency or sales proceeds from such country.
8.10Payment; Exchange Rate. All payments to be made under this Agreement shall be made in United States Dollars and shall be paid by bank wire transfer in immediately available funds to such bank account in the U.S. as may be designated in writing by the payee Party from time to time. If any currency conversion is required in connection with the calculation of amounts payable hereunder, such conversion shall be made in a manner consistent with the paying Party’s normal practices used to prepare its audited financial statements for external reporting purposes; provided that such practices use a widely accepted source of published exchange rates and are in accordance with Accounting Standards; provided, further, that when calculating the Sales of any Product that occur in currencies other than the United States Dollars, Roche shall convert the amount of such sales into United States Dollars using Roche’s then-current internal foreign currency translation method actually used on a consistent basis in preparing its audited financial statements (at the Effective Date, YTD average rate as reported by Reuters). The paying Party shall disclose the method and source of the published exchange rates used for such conversion as soon as reasonably practicable upon the reasonable request of the other Party.
8.11Late Payments. Without limiting any other rights or remedies available to a Party hereunder, if a paying Party does not pay any amount due on or before the due date, any such late payment shall be paid together with interest thereon at [****], as reported by Reuters from time to time, calculated on the number of days such payment is overdue; provided that such rate shall not exceed the rate permissible under Applicable Laws. Interest shall be computed from the date such payment was due until the date the paying Party makes the payment.
8.12Financial Records; Audits.
(a)Each Party shall keep, and shall cause its Related Parties (and subcontractors that form a Party’s sales field force or a portion thereof), to keep complete and accurate records in accordance with Accounting Standards in sufficient detail to enable the calculation of Development Costs (and each Party’s share thereof), Net Profits and Net Losses (and each Party’s share thereof), Net Sales, Sales Milestone Payments, Royalty Payments, Patent Costs and Trademark Costs (collectively, the “Calculated Amounts”) and other amounts payable hereunder to be determined. Upon the written request of a Party, and not more than once in each Calendar Year, upon [****] written notice, the other Party shall, and shall cause its Related Parties to, permit an independent certified public accounting firm of internationally recognized standing selected by requesting Party and reasonably acceptable to the other Party (“Auditor”), at the requesting Party’s expense except as set forth below, to have access during normal business hours to such records of the other Party and its Related Parties as may be reasonably necessary to verify the accuracy of the calculations of the Calculated Amounts payable hereunder for any Calendar Year ending not more than [****] Calendar Years prior to the date of such request for the sole purpose of verifying the basis and accuracy of payments made under this Agreement. The records for any Calendar Year may be audited no more than once. The auditors shall only state factual findings in the audit reports and shall not interpret the Agreement. The auditors shall share all draft audit findings with the audited Party before sharing such findings with the other Party and before the final audit report is issued. The Auditor shall provide its final audit report and basis for any determination to the requesting Party and the other Party at the same time and before it is considered final. Either Party shall have the right to request a further determination by the Auditor as to matters which such Party disputes in respect of the audit report or the basis for any determination within [****] days following receipt of such report. The disputing Party shall provide the non-disputing Party and the Auditor with a reasonably detailed statement of the grounds upon which it disputes any findings in the audit report and the Auditor shall undertake to complete such further determination within [****] days after the dispute notice is provided. Following such further determination, the Auditor shall promptly provide its final audit report to Roche and Alnylam.
(b)If the Auditor identifies a discrepancy made during the period subject to the applicable audit, the applicable Party shall pay the other Party the amount of the over-billed or underpaid discrepancy for any underpaid amount, within [****] days after the date the requesting Party delivers to the other Party the Auditor’s written report setting forth such conclusion, or as otherwise agreed by the Parties in writing. The fees charged by such accounting firm shall be paid by the requesting Party, unless such discrepancy results from a reporting error by the other Party and represents an underpayment by such other Party of at least [****] of the total amounts due from such other Party hereunder or [****], whichever is greater, or represents an overpayment to such other Party of at [****] of the total amounts due to such other Party hereunder or [****], whichever is greater, in a Calendar Year, in which case such fees, to the extent reasonable, shall be paid by the other Party.
(c)Unless an audit for a Calendar Year has been commenced prior [****], the calculation of the Calculated Amounts with respect to such Calendar Year shall be binding and conclusive upon both Parties, and each Party and its Related Parties shall be released from any further liability or accountability with respect to such calculation of the Calculated Amount for such Calendar Year upon such [****]. If an audit for a Calendar Year has been commenced prior to the [****], the calculation of Calculated Amounts and other payments payable hereunder with respect to such Calendar Year shall be binding and conclusive upon both Parties, and each Party and its Related Parties shall be released from any further liability or accountability with respect to such Calculated Amounts, following the conclusion of such audit.
(d)Each Party shall treat all financial information subject to review under this Section 8.12 as the audited Party’s Confidential Information in accordance with the confidentiality and non-use provisions of this Agreement and shall cause its Auditor to enter into an acceptable written confidentiality agreement with the audited Party or its Related Parties obligating it to retain all such information in confidence pursuant to such confidentiality agreement that includes obligations of confidentiality and non-use at least as protective as those set forth in Article 12.
ARTICLE 9
INTELLECTUAL PROPERTY
9.1Inventorship. Inventorship for patentable inventions conceived or reduced to practice during the course of the performance of activities pursuant to this Agreement shall be determined in accordance with United States patent laws for determining inventorship.
9.2Ownership.
(a)Alnylam Intellectual Property Ownership. As between the Parties, Alnylam shall own the entire right, title and interest in and to all (i) Alnylam Background Know-How, (ii) Alnylam Background Patents, (iii) Alnylam Collaboration Know-How (including any Alnylam Core Improvements therein) and (iv) Alnylam Collaboration Patents (including any Alnylam Core Improvements therein).
(b)Roche Intellectual Property Ownership. As between the Parties, Roche shall own the entire right, title and interest in and to all (i) Roche Background Know-How, (ii) Roche Background Patents, (iii) Roche Collaboration Know-How (including any Roche Core Improvements therein) and (iv) Roche Collaboration Patents (including any Roche Core Improvements therein).
(c)Joint Intellectual Property Ownership. As between the Parties, each Party shall jointly own, with each Party holding an undivided one-half interest in and to all Joint Know-How and Joint Patents. Except to the extent either Party is restricted by the licenses granted to the other Party under this Agreement or Section 7.7, each Party shall be entitled to practice, license, assign, and otherwise exploit its interest under the Joint Know-How and Joint Patents without the duty of accounting or seeking consent from the other Party. Each Party will grant and hereby does grant all permissions, consents and waivers with respect to, and all licenses under, such Party’s interest in the Joint Know-How and Joint Patents, throughout the world, necessary to provide the other Party with the foregoing rights.
(d)Assignment. To the extent that a Party (the “Assigning Party”), or any of its Related Parties, are assigned or otherwise obtain ownership of any right, title, or interest in or to any Patent Rights or Know-How in contravention of the foregoing Sections 9.2(a), (b) or (c), such Assigning Party hereby assigns, and shall cause its Related Parties to assign, to the other Party all right, title, and interest in or to such Patent Rights or Know-How. Upon such other Party’s request, the Assigning Party shall, at its own cost and expense, take all reasonable actions, including executing all assignments and other documents, necessary to perfect or record such other Party’s right, title, and interest in and to such Patent Rights or Know-How.
9.3Prosecution and Maintenance of Patent Rights.
(a)Roche Intellectual Property. Subject to the remainder of this Section 9.3(a), Roche has the exclusive right, but not the obligation, at Roche’s discretion, to file, prosecute, and maintain (including to defend any interference, opposition and other pre- or post-grant proceedings or challenges), all Roche Patents, in Roche’s name; provided that, in the event that Roche elects not to seek or continue to seek or maintain patent protection on any Roche Product-Specific Patent in the Co-Commercialization Territory, then Roche shall notify Alnylam in writing of such decision in sufficient time so as to permit Alnylam to decide whether to elect to seek, prosecute and maintain (as applicable) patent protection for such Roche Product-Specific Patent and to take any actions necessary to avoid losing patent protection with respect thereto, and upon making such election, Alnylam shall have the right (but not the obligation), at its expense, to seek, prosecute and maintain patent protection for such Roche Product-Specific Patent in the Co-Commercialization Territory, in the name of Roche. Roche shall make available to Alnylam any and all relevant documentation and use Commercially Reasonable Efforts to make available its applicable Representatives as are reasonably necessary to assist Alnylam in obtaining and maintaining the applicable Roche Product-Specific Patents in the event that Alnylam makes such an election.
(b)Alnylam Intellectual Property.
(i)Subject to Sections 9.3(b)(ii) and 9.3(b)(iii) below, (1) Alnylam has the exclusive right, but not the obligation, to file, prosecute, and maintain (including to defend any interference, opposition and other pre- or post-grant proceedings or challenges), all Alnylam Patents (other than Joint Patents), in Alnylam’s name and (2) Alnylam shall reasonably consider in good faith any reasonable requests by Roche to prosecute and maintain such Alnylam Patents in the Major Markets and in all other countries reasonably requested by Roche.
(ii)Alnylam shall provide Roche, sufficiently in advance for Roche to comment, with copies of all applications for Patent Rights and other material submissions and correspondence intended to be filed with any patent offices pertaining to Patent Rights comprising Alnylam Product-Specific Patents and, to the extent the Alnylam Core Patents would not be materially adversely affected, Alnylam Core Patents that are Alnylam Collaboration Patents, and Alnylam shall consider in good faith and reasonably incorporate Roche’s reasonable and promptly provided comments and advice to the extent with respect to the prosecution or maintenance strategy for such Patent Rights, including, with respect to the Alnylam Product-Specific Patents, consider in good faith Roche’s input and advice with respect to the prosecution costs therefor; provided, however, that if Alnylam determines in good faith that Roche’s comments or advice are not reasonable, Alnylam shall promptly notify Roche thereof and the Parties shall promptly use good faith efforts to resolve any such determination; provided, further, that Alnylam shall have final decision-making authority with respect to the prosecution or maintenance strategy for such Patent Rights. Alnylam shall promptly provide Roche with copies of all material correspondence received from any patent offices pertaining to Alnylam Product-Specific Patents. In addition, Alnylam shall promptly notify Roche in writing of any interference, opposition or any other pre- or post-grant proceeding or challenge for any Alnylam Core Patent included in Alnylam Collaboration Patents and any Alnylam Product-Specific Patent.
(iii)In the event that Alnylam elects not to continue to seek or maintain patent protection on any Alnylam Product-Specific Patent in a given country in the Territory, Alnylam shall notify Roche of such decision in sufficient time so as to permit Roche to decide whether to seek, prosecute and maintain such Patent Right and to take any necessary actions without losing patent protection, and Roche shall have the right (but not the obligation), at its expense, to seek, prosecute and maintain patent protection on such Alnylam Product-Specific Patent in the applicable country, in the name of Alnylam. Alnylam shall make available to Roche its documentation and use Commercially Reasonable Efforts to make available its applicable Representatives as are reasonably necessary to assist Roche in obtaining and maintaining the patent protection described under this Section 9.3(b)(iii).
(c)Joint Patents.
(i)[****] shall have the first right, but not the obligation, to file, prosecute and maintain (including to defend any interference, opposition and other pre- or post-grant proceedings or challenges), all Joint Patents, in the names of both Alnylam and Roche, in the Territory. [****] shall provide [****], sufficiently in advance for [****] to comment, with copies of all applications for Patent Rights and other material submissions and correspondence intended to be filed with any patent offices pertaining to Joint Patents, and [****] shall consider in good faith [****] reasonable and promptly provided comments and advice with respect to the prosecution or maintenance strategy with respect to such Joint Patents; provided, however, that if [****] determines that [****] comments or advice are not reasonable, [****] shall promptly notify [****] thereof and the Parties shall promptly discuss such determination; [****]. [****] shall promptly provide [****] with copies of all material correspondence received from any patent offices pertaining to Joint Patents.
(ii)In the event that [****] elects not to file or continue to prosecute or maintain any Joint Patent in a given country in the Territory, [****] shall notify [****] of such decision in sufficient time so as to permit [****] to decide whether to file, prosecute and maintain such Patent Right and to take any necessary actions without losing patent protection, and [****] shall have the right (but not the obligation), at its expense, to file, prosecute and maintain patent protection on such Joint Patent the applicable country, in the name of both Alnylam and Roche. [****] shall make available to [****] its documentation and use Commercially Reasonable Efforts to make available its applicable Representatives as are reasonably necessary to assist [****] in obtaining and maintaining the patent protection described under this Section 9.3(c)(ii).
9.4Cooperation. With respect to the rights granted to a Party under Sections 9.3(b) or 9.3(c), each Party hereby agrees, following the other Party’s request, to: (a) make its employees, agents and consultants reasonably available to the other Party (or to the other Party’s authorized attorneys, agents or representatives), to the extent reasonably necessary to enable such Party to undertake patent prosecution; (b) provide the other Party with copies of all material correspondence pertaining to prosecution with the patent offices; and (c) endeavor in good faith to coordinate its efforts with the other Party to minimize or avoid interference with the prosecution and maintenance of the other Party’s patent applications, including taking any necessary actions to resolve any double-patenting issue that may arise.
9.5Patent Costs. The prosecuting Party will bear the costs and expenses of filing, prosecuting and maintaining Patent Rights, including the costs of outside patent counsel (such costs and expenses as reasonable, “Patent Costs”) except that (a) Patent Costs for Alnylam Product-Specific Patents in the Co-Commercialization Territory shall be allocated as set forth in the Financial Appendix and (b) Roche will bear [****] of Patent Costs for Alnylam Product-Specific Patents in the Roche Territory.
9.6Unified Patent Court. At any time prior to the end of the “transitional period” as such term is used in Article 83 of the Agreement on a Unified Patent Court between the participating Member States of the European Union, for a given relevant Alnylam Product-Specific Patent in the Member States of the European Union, Roche may request approval in writing from Alnylam (such approval not to be unreasonably, withheld, conditioned or delayed) that Alnylam either (a) opt out from the exclusive competence of the Unified Patent Court or (b) if applicable, withdraw a previously-registered opt-out, and, if Alnylam grants approval in accordance with the foregoing, Alnylam shall notify the United Patent Court Registry, pay any such registry fee (for which Roche shall promptly reimburse Alnylam) and take such other action as may be necessary to effect the opt-out or opt-out withdrawal (“Register”). Alnylam shall Register within [****] days of Alnylam’s approval of Roche’s written request, or such other time parameters specified by Roche.
9.7Create Act. It is the intention of the Parties that this Agreement is a “joint research agreement” as that that term is defined in 35 USC § 100(h), and as it applies to inventions as set forth in 35 USC § 102(c) (AIA) or 35 USC § 103(c) (pre-AIA) and may be used for the purpose of overcoming a rejection of a claimed invention within the Joint Patents pursuant to the provisions of 35 USC § 102(c) or 35 USC § 103(c). In the event that either Party intends to overcome a rejection of any other claimed invention outside the Joint Patents pursuant to the provisions of 35 USC § 102(c) or 35 USC § 103(c), such Party shall first obtain the prior written consent of the other Party.
9.8Hatch-Waxman. Notwithstanding anything herein to the contrary, should a Party receive a certification for a Product pursuant to paragraph IV of the Drug Price Competition and Patent Term Restoration Act of 1984 (Public Law 98-417, known as the Hatch-Waxman Act), or its equivalent in a country other than the U.S. (“Certification Notice” and such Party, the “Certification Notice Party”), then the Certification Notice Party shall immediately (in any case within [****] days) provide the other Party with a copy of the Certification Notice. The Certification Notice Party shall have [****] days from the date on which it receives or provides the copy of the Certification Notice, to provide written notice to the other Party (“H-W Suit Notice”) that the Certification Notice Party intends to bring suit, at its expense, within the forty-five (45)-day period set forth in paragraph IV of the Hatch-Waxman Act (or within the time period set forth in its equivalent in a country other than the US). Should such [****]-day period expire without the Certification Notice Party bringing suit or providing such H-W Suit Notice, then the other Party shall be free to immediately bring suit, at its expense, in its name.
9.9Patent Term Extension.
(a)Alnylam Product-Specific Patents, Roche Patents and Joint Patents. Roche will determine, in its sole discretion and at its sole cost and expense, a strategy of seeking available patent term extensions, restorations, supplementary protection certificates and other extensions from among Alnylam Product-Specific Patents, Roche Patents and Joint Patents, to the extent applicable, that will be designed to maximize patent protection and commercial value for the Products for use in the Field in the Territory, and the Parties will seek patent term extensions, restorations, supplementary protection certificates and other extensions in all relevant countries in the Territory for such Patent Rights as selected by Roche in accordance with that strategy. If Roche determines not to file for any such extensions, restorations or supplementary protection certificates for any of such Patent Rights in any relevant country of the Territory, it will provide Alnylam with written notice at least [****] days prior to the last day on which such a filing must be made before such rights are lost, and upon providing such notice, Alnylam shall have the exclusive right to make such filing in its sole discretion. Notwithstanding the foregoing, to the extent required under Applicable Laws, Alnylam will make the filings for any such extensions, restorations and supplementary protection certificates for Alnylam Product-Specific Patents and will make, or cooperate with Roche to make, the filing for Joint Patents in the Territory, in each case as directed by Roche.
(b)Alnylam Core Patents. Alnylam will determine, in its sole discretion and at its sole cost and expense, whether to seek available patent term extensions, restorations, supplementary protection certificates and other extensions to the Alnylam Core Patents, to the extent applicable, to maximize the commercial potential of a Product; provided that if only a single Patent Right can be selected for extension, restoration or supplementary protection certificate in relation to a Regulatory Approval for a Product in a relevant country of the Territory and Roche previously has determined to file for any such extensions, restorations or supplementary protection certificates for any of the Patent Rights in any relevant country of the Territory under Section 9.9(a) then the Roche selected Patent Right shall be the single Patent Right so filed on.
(c)Cooperation. Each Party will execute such authorizations and other documents and take such other actions as may be reasonably requested by the other Party to obtain any such extensions, restorations and supplementary protection certificates in the Territory in accordance with the foregoing Sections 9.9(a) and 9.9(b).
9.10Third Party Infringement.
(a)Notices. Each Party shall promptly notify the other Party in writing of (i) any known, suspected or threatened infringement, misappropriation or other violation of any Alnylam Licensed IP or Roche Licensed IP, or (ii) any other unauthorized use, misappropriation or other violation of any Confidential Information or Know-How of a Party by a Third Party of which it becomes aware (any of the foregoing (i) and (ii), a “Third Party Infringement”), and, in each case (the foregoing (i) and (ii)), shall provide the other Party with all reasonably available evidence in its possession and control of such infringement, unauthorized use, misappropriation or other violation.
(b)Rights to Enforce.
(i)Certain Roche Intellectual Property. Roche shall have the exclusive right, but not the obligation, to initiate a claim, action, suit or other proceeding based on any Third Party Infringement of any Roche Patents or Roche Know-How (other than any Roche Product-Specific Patent in the Co-Commercialization Territory or Roche’s interest in any Joint Know-How or Joint Patent). Roche will reasonably consider in good faith any request from Alnylam to initiate such a claim, action, suit or other proceeding against any such Third Party Infringement.
(ii)Roche Product-Specific Patents. Roche shall have the first right, but not the obligation, to initiate a claim, action, suit or other proceeding based on any Third Party Infringement of any Roche Product-Specific Patents in the Co-Commercialization Territory (other than Roche’s interest in Joint Know-How or Joint Patents). Roche will reasonably consider in good faith any request from Alnylam to initiate such a claim, action, suit or other proceeding against any such Third Party Infringement.
(iii)Alnylam Product-Specific Patents, Alnylam Product-Specific Know-How, Joint Patents and Joint Know-How. Roche shall have the first right, but not the obligation, to initiate a claim, action, suit or other proceeding based on any Third Party Infringement of any Alnylam Product-Specific Patents in the Territory, Alnylam Product-Specific Know-How, Joint Patents or Joint Know-How (with respect to each of which Roche shall reasonably consider any input provided by Alnylam in good faith).
(iv)Alnylam Core Patents and Core Know-How. Alnylam shall have the exclusive right, but not the obligation, to initiate a claim, action, suit or other proceeding based on any Third Party Infringement in the Territory of any Alnylam Core Patent or Alnylam Core Know-How (with respect to each of which Alnylam shall reasonably consider any input provided by Roche in good faith).
(v)Step-In Right. If, within [****] days after Roche’s receipt of written notice of a Third Party Infringement with respect to any Roche Product-Specific Patent in the Co-Commercialization Territory, or any Alnylam Product-Specific Patent or Joint Patent in the Territory, (or at least ten (10) days before the loss of the right to take an action as described in Section 9.10(b)(ii) or Section 9.10(b)(iii), as applicable, and permitted hereunder with respect to such Third Party Infringement, except if Roche has notified Alnylam in writing that it intends to, and actually does within a commercially reasonable time period, take action as described in Section 9.10(b)(ii) or Section 9.10(b)(iii), as applicable, and permitted hereunder against such Third Party Infringement), Roche does not take any action as described in Section 9.10(b)(ii) or Section 9.10(b)(iii), as applicable, and permitted hereunder against such Third Party Infringement in the relevant country in the Territory, Alnylam may in its sole discretion, bring and control any legal action in connection therewith at its sole expense, subject to Section 9.10(c).
(c)Procedures; Expenses and Recoveries. The Party having the right to initiate any claim, action, suit or other proceeding with respect to a Third Party Infringement under Section 9.10(b) shall have the exclusive right to select counsel for any such claim, action, suit or other proceeding and shall pay all expenses of any such claim, action, suit or other proceeding, including attorneys’ fees and court costs and reimbursement of the other Party’s reasonable out-of-pocket expense in rendering assistance requested by the initiating Party in writing. If required under Applicable Laws in order for the initiating Party to initiate or maintain such claim, action, suit or other proceeding, or if either Party is unable to initiate or prosecute such claim, action, suit or other proceeding solely in its own name or it is otherwise advisable not to do so to obtain an effective legal remedy, in each case, such Party shall provide the other Party with written notice and such other Party shall join as a party to such claim, action, suit or other proceeding and, following the initiating Party’s reasonable written request, will execute and cause its Affiliates to execute all documents, and take all actions, reasonably necessary for the initiating Party to initiate and maintain such claim, action, suit or other proceeding. In addition, at the initiating Party’s request, the other Party shall provide other reasonable assistance to the initiating Party in connection with any such claim, action, suit or other proceeding at no charge to the initiating Party, except for reimbursement by the initiating Party of reasonable out-of-pocket expenses incurred in rendering such assistance. The non-initiating Party shall have the right to participate and be represented in any such suit under Section 9.10(b)(ii), Section 9.10(b)(iii) or Section 9.10(b)(v), as applicable, by its own counsel at its own expense. If the Parties obtain from a Third Party, in connection with any such suit under Section 9.10(b)(ii), Section 9.10(b)(iii) or Section 9.10(b)(v), any damages, license fees, royalties or other compensation (including any amount received in settlement of such litigation), such amounts shall be allocated in all cases as follows (subject to all Applicable Laws):
[****].
9.11Trademarks. Other than any Product Trademarks Controlled by Alnylam as of the Effective Date, Roche shall own all Product Trademarks in the Territory. The Parties shall mutually agree (such agreement not to be unreasonably withheld, conditioned or delayed) on the Product Trademarks that will be utilized in connection with the Commercialization of Products in the Territory and shall negotiate in good faith and enter into appropriate trademark license agreement(s) containing customary and reasonable terms to facilitate the Development, Manufacture and Commercialization of Product by the Parties in the Field in the Territory during the Term hereunder. Trademark Costs in the Co-Commercialization Territory shall be borne by the Parties as set forth in the Financial Appendix, and Roche shall be responsible for [****] of all Trademark Costs in the Roche Territory.
9.12Common Interest. All information exchanged between the Parties’ representatives regarding the preparation, filing, prosecution, maintenance or enforcement of Patent Rights and Know-How under this Article 9 shall be deemed Confidential Information. In addition, the Parties acknowledge and agree that, with regard to such preparation, filing, prosecution, maintenance or enforcement of Patent Rights and Know-How under this Article 9, the interests of the Parties as licensor and licensee are to obtain the strongest patent protection possible, and as such, are aligned and are legal in nature. The Parties agree and acknowledge that they have not waived, and nothing in this Agreement constitutes a waiver of, any legal privilege concerning the Patent Rights and Know-How under this Article 9, including privilege under the common interest doctrine and similar or related doctrine.
ARTICLE 10
REPRESENTATIONS AND WARRANTIES; COVENANTS
10.1Mutual Representations and Warranties. Each Party hereby represents and warrants to the other Party that as of the Effective Date:
(a)it is a company or corporation duly organized, validly existing, and in good standing under the Applicable Laws of the jurisdiction in which it is incorporated, and has full corporate power and authority and the legal right to own and operate its property and assets and to carry on its business as it is now being conducted and as contemplated in this Agreement, including the right to grant the licenses granted by it hereunder;
(b) (i) it has the corporate power and authority and the legal right to enter into this Agreement and all instruments and documents to be delivered by it hereunder and perform its obligations hereunder or thereunder; (ii) it has taken all necessary corporate action on its part required to authorize the execution and delivery of this Agreement and the performance of its obligations hereunder; (iii) it has taken all other action required by Applicable Laws, its certificate of incorporation, bylaws and other organizational documents and any agreement to which it is a party or to which it may be subject required to authorize such execution, delivery and performance; and (iv) this Agreement has been duly executed and delivered on behalf of such Party, and constitutes a legal, valid, and binding obligation of such Party that is enforceable against it in accordance with its terms, except as enforcement may be affected by bankruptcy, insolvency or other similar laws and by general principles of equity;
(c)it is not a party to any agreement that would prevent it from granting the rights granted to the other Party under this Agreement or performing its obligations under this Agreement;
(d)the execution and delivery of this Agreement by it and all instruments and documents to be delivered by it hereunder, and the performance by it contemplated hereunder or thereunder does not and will not (i) violate any Applicable Laws or any order of any court or other Governmental Authority, except for such violations that would not have a material adverse effect on the ability of the Party to perform its obligation under this Agreement, or (ii) violate the terms of any indenture, mortgage, deed of trust, lease, agreement, or other instrument to which it is a party or by which it or any of its property is bound, which violation would have an adverse effect on its financial condition or on its ability to perform its obligations hereunder;
(e)neither the execution, delivery nor the performance of this Agreement by such Party requires it to obtain any permits, authorizations or consents from any Governmental Authority (other than any Regulatory Approvals relating to the Development, Manufacture, Commercialization or other exploitation of Products for use in the Field) or from any other Person; and
(f)it is in compliance, and has policies and procedures designed to promote compliance, with Sanctions and Export Control Laws, and neither it nor any of its directors, officers, or employees is a Sanctioned Person.
10.2Representations and Warranties by Alnylam Alnylam hereby represents and warrants to Roche as of the Effective Date:
(a)Exhibit E sets forth a true and complete list of all material Alnylam Patents owned by Alnylam or any of its Affiliates, and all such Alnylam Patents are, (i) subsisting and, to Alnylam’s knowledge, valid, and enforceable (or in the case of pending Patent applications, will be valid and enforceable upon issuance) and (ii) solely and exclusively owned by Alnylam;
(b)Alnylam has sufficient ownership of, or license or other rights under, the Alnylam Patents to grant the licenses to such Alnylam Patents granted to Roche hereunder;
(c)all of Alnylam’s employees and officers have executed agreements requiring assignment to Alnylam of inventions made by such individuals during the course of and as a result of their employment with Alnylam;
(d)in the past three (3) years, Alnylam has not received any written notice from any Third Party asserting or alleging that Development of Products for use in the Field by Alnylam infringes, misappropriates or otherwise violates the intellectual property rights of any Third Party;
(e)to Alnylam’s knowledge, no Third Party has challenged the scope, validity or enforceability of any Alnylam Patents (i) through the institution of legal proceedings in a court or of interference, nullity or similar invalidity proceedings before the U.S. Patent and Trademark Office or any analogous foreign entity or (ii) by written threat of institution of such proceedings delivered to Alnylam;
(f)to Alnylam’s knowledge, the Development, Manufacture, Commercialization and other exploitation of the Therapeutic Product for use in the Field as contemplated under this Agreement on the Effective Date does not infringe, misappropriate or otherwise violate any intellectual property (including Patent Rights) owned by a Third Party;
(g)there is no action, claim, demand, suit, proceeding, arbitration, grievance, citation, summons, subpoena, inquiry or investigation of any nature, in law or in equity, pending or, to Alnylam’s knowledge, threatened in writing as of the date hereof against Alnylam or any of its Affiliates, in each case, with respect to the Alnylam Licensed IP, the Products for use in the Field as contemplated under this Agreement on the date hereof or the transactions contemplated by this Agreement;
(h)to Alnylam’s knowledge, as of the date hereof, there are no Third Parties infringing, misappropriating or otherwise violating any Alnylam Licensed IP;
(i)to Alnylam’s knowledge, all Development activities related to Zilebesiran for use in the Field have been conducted by Alnylam and its Affiliates in accordance (in all material respects) with all Applicable Laws;
(j)in conducting Development activities for Zilebesiran, Alnylam has not used, prior to the Effective Date, any employee, agent or independent contractor who has been debarred by any Regulatory Authority, or, to Alnylam’s knowledge, is the subject of debarment proceedings by a Regulatory Authority under 21 U.S.C. §335a, disqualified under 21 C.F.R. §312.70 or §812.119, sanctioned by a Federal Health Care Program (as defined in 42 U.S.C §1320 a-7b(f)), including the federal Medicare or a state Medicaid program, or debarred, suspended, excluded or otherwise declared ineligible from any other similar Federal or state agency or program;
(k)Alnylam has disclosed to Roche all material information in its possession or control that comprises: (i) the results of preclinical testing and human clinical testing of Product by Alnylam or any of its Affiliates and (ii) information concerning toxicity and adverse events with respect to Product;
(l)without limiting Section 10.1(e), neither the execution, delivery nor the performance of this Agreement by Alnylam, including the granting of the licenses and other rights to Roche hereunder, requires the consent or other permission of [****] or any other agreement or arrangement with such Person; and
(m)[****] neither Alnylam nor any of its Affiliates has entered into any agreement with any Third Party in which Alnylam (or its Affiliates) owes any royalty, milestone and any other payment obligations, to such Third Party with respect to the Development, Manufacture or Commercialization or other exploitation of Product in the Field in the Territory.
10.3Covenants.
(a)Each Party hereby covenants and agrees that:
(i)it shall conduct the Development, Manufacture, Commercialization and other exploitation of Products for use in the Field, and its other obligations, under this Agreement in compliance in all material respects with, as applicable: (1) the terms of this Agreement, the Development Plan, the Manufacture Plan, the Co-Commercialization Plan and the Global Strategy and Marketing Plan and (2) GCPs, GLPs and GMPs and all other Applicable Laws and Regulatory Approvals;
(ii)in conducting Development of Products for use in the Field in the Territory under this Agreement, such Party will not use any employee, agent or independent contractor who has been debarred by any Regulatory Authority, or, to the best of such Party’s knowledge, is the subject of debarment proceedings by a Regulatory Authority, and in the event a Party or an employee or agent of such Party or its Affiliates receives notice of debarment, suspension, sanction, exclusion, ineligibility or disqualification under the Applicable Laws referenced in Section 10.2(j) above, such Party shall immediately notify the other Party in writing and ensure that the concerned entity or individual ceases performing any and all activities in connection with the Products; and
(iii)with respect to all activities contemplated by this Agreement (including the Development, Manufacture, Commercialization and other exploitation of Products), (1) it shall comply at all times with Sanctions and Export Control Laws in all respects, (2) it shall not engage, directly or indirectly, in any business or transaction with, in, or involving any Sanctioned Person or Sanctioned Jurisdiction, and (3) it shall maintain policies and procedures designed to promote compliance with applicable Sanctions and Export Control Laws.
(b)Additional Covenants of Alnylam. Alnylam covenants and agrees that:
(i)except as required pursuant to any consent decree or agreement with any Governmental Authority or otherwise by Applicable Laws and except as otherwise provided for in this Agreement, neither Alnylam nor its Affiliates shall enter into any agreement with any Third Party after the Effective Date, whether written or oral, with respect to, or otherwise assign, transfer, license, or convey its right, title or interest in or to, the Alnylam Licensed IP or Products, in each case, that is in conflict with the rights granted by Alnylam to Roche under this Agreement or that would prevent it from performing its obligations under this Agreement;
(ii)Alnylam shall not, without Roche’s prior written consent, [****];
(iii)Alnylam shall not, without Roche’s prior written consent, [****]; and
(iv)Alnylam shall not, without Roche’s prior written consent, [****].
10.4Disclaimer. Each Party understands that the Products are the subject of ongoing Development and that the other Party cannot assure, and makes no representation or warranty, express or implied, as to the safety, efficacy or usefulness of, or likelihood of obtaining, Regulatory Approval for, the Products.
10.5No Other Representations or Warranties. EXCEPT AS EXPRESSLY STATED IN THIS ARTICLE 10, NEITHER PARTY, NOR ANY OF THEIR RESPECTIVE AFFILIATES OR ITS OR THEIR REPRESENTATIVES, MAKES ANY REPRESENTATIONS OR WARRANTIES, AND EACH PARTY HEREBY EXPRESSLY DISCLAIMS ALL REPRESENTATIONS AND WARRANTIES, OF ANY AND ALL KINDS, EITHER EXPRESS OR IMPLIED, STATUTORY OR OTHERWISE, INCLUDING REPRESENTATIONS AND WARRANTIES AS TO THE PROSPECTS OF THE PRODUCTS, EITHER PARTY’S EFFORTS, INTENTIONS, OR EXPECTATIONS WITH RESPECT TO THE PRODUCTS OR ANY OTHER PRODUCT OR ASPECT OF ITS BUSINESS, THAT ANY PATENT APPLICATION WILL BE GRANTED, WILL REMAIN IN EFFECT OR COVER THE PRODUCTS, THAT THE PRODUCTS CAN BE SUCCESSFULLY DEVELOPED FOR USE IN THE FIELD, OR GRANTED REGULATORY APPROVAL OR COMMERCIALIZED FOR USE IN THE FIELD, THE CLINICAL EFFECTIVENESS OR SAFETY PROFILE OF THE PRODUCTS, THE PRODUCTS’ PROFITABILITY, AND WARRANTIES OF TITLE, NON-INFRINGEMENT, VALIDITY, ENFORCEABILITY, MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE.
ARTICLE 11
INDEMNIFICATION
11.1Indemnification by Alnylam. Subject to terms and conditions of this Agreement (including Section 11.4), Alnylam shall defend, indemnify, and hold harmless Roche, its Affiliates, and its and their respective officers, directors, employees, and agents (each, a “Roche Indemnitee”) from and against any and all liabilities, expenses and damages, including any reasonable attorneys’ fees and legal expenses (collectively, “Damages”) to the extent resulting from claims, suits, proceedings or causes of action brought by a Third Party (each, a “Third Party Claim”) against any Roche Indemnitee that arise from or are based on: (a) material breach by Alnylam of this Agreement, the Supply Agreements or the Co-Promotion Agreement; (b) the willful misconduct or gross negligence of any Alnylam Indemnitee or any Alnylam Related Party (including subcontractors acting in their behalf); (c) the Development, Manufacture, Commercialization or other exploitation of any Products by or on behalf of Alnylam or any of its Related Parties (including subcontractors acting on their behalf) (excluding any activities by Roche or any of its Related Parties, but including subcontractors acting on their behalf); (d) the Development, Manufacture, Commercialization or other exploitation of any Reversion Product by or on behalf of Alnylam, its Affiliates and its, or their sublicensees; or (e) violation of Applicable Laws (in any material respect) by any Alnylam Indemnitee or any Alnylam Related Party (including subcontractors acting on their behalf) in connection with this Agreement, the Supply Agreements or the Co-Promotion Agreement; in each case (clauses (a) through (e)), except to the extent that such Damages result from any activities set forth in Section 11.2(a)-(e) for which Roche is obligated to indemnify any Alnylam Indemnitee. For clarity, except as provided in Section 11.4, any Damages paid as indemnification pursuant to this Section 11.1 shall be borne solely by Alnylam and shall not be included as Shared Development Costs or included in the calculation of Net Profits and Net Losses.
11.2Indemnification by Roche. Subject to the terms and conditions of this Agreement (including Section 11.4), Roche shall defend, indemnify, and hold harmless Alnylam, its Affiliates, and its and their respective officers, directors, employees, and agents (each, an “Alnylam Indemnitee”) from and against any and all Damages, to the extent resulting from any Third Party Claims against any Alnylam Indemnitee that arise from or are based on: (a) material breach by Roche of this Agreement, the Supply Agreements or the Co-Promotion Agreement; (b) the willful misconduct or gross negligence of any Roche Indemnitee or any Roche Related Party (including subcontractors acting on their behalf); (c) the Development, Manufacture, Commercialization or other exploitation of any Products by or on behalf of Roche or any Roche Related Party (including subcontractors acting on their behalf) (excluding any activities by Alnylam or any Alnylam Related Party, including subcontractors acting on their behalf); or (d) violation of Applicable Laws (in any material respect) by any Roche Indemnitee or any Roche Related Party (including subcontractors acting on their behalf) in connection with this Agreement, the Supply Agreements or the Co-Promotion Agreement; in each case (clauses (a) through (d)), except to the extent that such Damages result from any activities set forth in Section 11.1(a)-(d) for which Alnylam is obligated to indemnify any Roche Indemnitee. For clarity, except as provided in Section 11.4, any Damages paid as indemnification pursuant to this Section 11.2 shall be borne solely by Roche and shall not be included as Shared Development Costs or included in the calculation of Net Profits and Net Losses.
11.3Indemnification Procedures.
(a)Notice of Claim. The Party claiming indemnity under this Article 11 (the “Indemnitee”) shall give written notice (“Indemnification Claim Notice”) to the Party from whom indemnity is being sought (the “Indemnifying Party”) promptly after learning of the Third Party Claim for which indemnity is being sought, describing such Third Party Claim in reasonable detail. Failure to give such notice shall not relieve the Indemnifying Party of its indemnification obligations hereunder, except to the extent that such Indemnifying Party is materially prejudiced by such failure.
(b)Control of Defense. At its option, the Indemnifying Party may assume the defense of any Third Party Claim subject to indemnification as provided for in Section 11.1 or 11.2, as applicable, by giving written notice to the Indemnitee within [****] days after the Indemnifying Party’s receipt of an Indemnification Claim Notice. Upon assuming the defense of a Third Party Claim, the Indemnifying Party may appoint as lead counsel in the defense of the Third Party Claim any legal counsel it selects, and such Indemnifying Party shall thereafter continue to defend such Third Party Claim in good faith. Should the Indemnifying Party assume the defense of a Third Party Claim (and continue to defend such Third Party Claim in good faith), the Indemnifying Party will not be liable to the Indemnitee or any other Indemnitee for any legal expenses subsequently incurred by such Indemnitee or other Indemnitee in connection with the analysis, defense or settlement of the Third Party Claim, except in accordance with Section 11.3(c).
(c)Right to Participate in Defense. Without limiting Section 11.3(b), any Indemnitee will be entitled to participate in the defense of a Third Party Claim for which it has sought indemnification hereunder and to engage counsel of its choice for such purpose; provided, however, that such engagement will be at the Indemnitee’s own expense unless (i) the engagement thereof has been specifically authorized by the Indemnifying Party in writing, or (ii) the Indemnifying Party has failed to assume the defense (or continue to defend such Third Party Claim in good faith) and engage counsel in accordance with this Section 11.3(c), in which case the Indemnitee will be allowed to control the defense.
(d)Settlement. With respect to any Damages relating solely to the payment of money damages in connection with a Third Party Claim and that will not result in the Indemnitee becoming subject to injunctive or other relief or otherwise adversely affect the business of the Indemnitee in any manner, and as to which the Indemnifying Party has acknowledged in writing the obligation to indemnify the Indemnitee hereunder, the Indemnifying Party will have the sole right to consent to the entry of any judgment, enter into any settlement or otherwise dispose of such Damages, on such terms as the Indemnifying Party, in its reasonable discretion, deems appropriate (provided, however, that such terms shall include a complete and unconditional release of the Indemnitee from all liability with respect thereto), and will transfer to the Indemnitee all amounts which said Indemnitee will be liable to pay prior to the time of the entry of judgment. With respect to all other Damages in connection with Third Party Claims, where the Indemnifying Party has assumed the defense of the Third Party Claim in accordance with Section 11.3(b), the Indemnifying Party will have authority to consent to the entry of any judgment, enter into any settlement or otherwise dispose of such Damages; provided it obtains the prior written consent of the Indemnitee (which consent will be at the Indemnitee’s reasonable discretion). The Indemnifying Party that has assumed the defense of (and continues to defend) the Third Party Claim in accordance with Section 11.3(b) will not be liable for any settlement or other disposition of Damages by an Indemnitee that is reached without the written consent of such Indemnifying Party. For clarity, regardless of whether the Indemnifying Party chooses to defend or prosecute any Third Party Claim, no Indemnitee will admit any liability with respect to, or settle, compromise or discharge, any Third Party Claim without first offering to the Indemnifying Party the opportunity to assume the defense of the Third Party Claim in accordance with Section 11.3(b).
(e)Cooperation. If the Indemnifying Party chooses to defend or prosecute any Third Party Claim, the Indemnitee will, and will cause each other Indemnitee to, cooperate in the defense or prosecution thereof and will furnish such records, information and testimony, provide such witnesses and attend such conferences, discovery proceedings, hearings, trials and appeals as may be reasonably requested by or on behalf of the Indemnifying Party in connection with such Third Party Claim. Such cooperation will include access during normal business hours afforded to the Indemnifying Party to, and reasonable retention by the Indemnitee of, records and information that are reasonably relevant to such Third Party Claim, and making Indemnitees and other employees and agents available on a mutually convenient basis to provide additional information and explanation of any material provided hereunder, and the Indemnifying Party will reimburse the Indemnitee for all of its reasonable out-of-pocket expenses incurred in connection with providing such cooperation.
(f)Expenses of Indemnitee. Except as provided above, the reasonable and verifiable costs and expenses, including fees and disbursements of counsel, incurred by the Indemnitee in connection with any Third Party Claim will be reimbursed on a Calendar Quarter basis by the Indemnifying Party, without prejudice to the Indemnifying Party’s right to contest the Indemnitee’s right to indemnification and subject to refund in the event the Indemnifying Party is ultimately held not to be obligated to indemnify the Indemnitee.
11.4Third Party Claims Related To Products in the Co-Commercialization Territory. All Damages from Third Party Claims relating to the Development, Manufacture, Commercialization or other exploitation of any Product for use in the Field in or with respect to the Co-Commercialization Territory during the Term (for clarity not to include any Third Party Claims or Damages related to the time period prior to the Effective Date), including Damages from claims of infringement, misappropriation or other violation of Third Party intellectual property rights, product liability and claims of death or personal injury, shall be borne [****] by the Parties; provided that this Section 11.4 shall not apply to the extent such Damages are incurred by a Party or the Parties as a result of the material breach of this Agreement, the Supply Agreements or the Co-Promotion Agreement, violation of Applicable Laws (in any material respect), or gross negligence or willful misconduct by or on behalf of a Party or its Indemnitees.
11.5Insurance. During the Term and for [****] years thereafter, each Party shall procure and continuously maintain insurance (provided, however that the Parties have the right, in their respective sole discretion to self-insure, in part or in whole, for any insurance), including product liability insurance with minimum limits of [****] per occurrence or per claim (as applicable) and in the aggregate and clinical trials insurance with minimum limits of [****] per occurrence or per claim (as applicable) and in the aggregate, with respect to its activities hereunder and which insurance is consistent with normal business practices of prudent companies similarly situated at all times during which any Product is being clinically tested in human subjects or commercially promoted, sold, marketed or distributed. Each Party shall ensure that it is in compliance with any applicable foreign local clinical trial liability insurance requirements with respect to the Products. It is understood that [****]. Each Party, upon the request of the other Party, shall provide the other with reasonably detailed written evidence of such insurance (including self-insurance).
11.6Limitation of Liability. NEITHER PARTY SHALL BE LIABLE TO THE OTHER PARTY FOR ANY SPECIAL, CONSEQUENTIAL, INCIDENTAL, PUNITIVE, OR INDIRECT DAMAGES ARISING FROM OR RELATING TO ANY BREACH OF THIS AGREEMENT OR ANY TORT CLAIMS ARISING HEREUNDER, REGARDLESS OF ANY NOTICE OF THE POSSIBILITY OF SUCH DAMAGES. NOTWITHSTANDING THE FOREGOING, NOTHING IN THIS SECTION 11.6 IS INTENDED TO OR SHALL LIMIT OR RESTRICT THE INDEMNIFICATION RIGHTS OR OBLIGATIONS OF ANY PARTY UNDER SECTION 11.1, 11.2 OR 11.4, OR DAMAGES AVAILABLE FOR A PARTY’S BREACH OF EXCLUSIVITY OBLIGATIONS UNDER SECTION 7.7 OR CONFIDENTIALITY OBLIGATIONS UNDER ARTICLE 12.
ARTICLE 12
CONFIDENTIALITY
12.1Confidentiality Obligations. Except to the extent expressly authorized by this Agreement or otherwise agreed in writing by the Parties, during the Term and for a period of [****] years following termination or expiration of this Agreement in its entirety, each Party (“Recipient”) shall, and shall cause its officers, directors, employees and agents to, keep confidential and not publish or otherwise disclose to a Third Party and not use, directly or indirectly, for any purpose any and all Confidential Information that is disclosed or otherwise made available to Recipient by the other Party or its Affiliates (“Discloser”) in connection with the Product or the Collaboration activities and other discussions hereunder, whether prior to or during the Term and whether orally or in writing, electronic or other form. Notwithstanding the foregoing, [****]. For purposes of this Agreement, “Confidential Information” means all confidential or proprietary information of Party or its Affiliates that is disclosed to the other Party under or in connection with this Agreement, which may include specifications, know-how, trade secrets, technical information, models, business information, inventions, discoveries, methods, procedures, formulae, protocols, techniques, data, and unpublished patent applications, in each case, whether disclosed in oral, written, graphic, or electronic form; provided that, notwithstanding anything to the contrary herein, (a) Alnylam Know-How (other than Joint Know-How) shall be deemed to be Confidential Information of Alnylam hereunder, (b) Roche Know-How (other than Joint Know-How) shall be deemed to be Confidential Information of Roche, and (c) Joint Know-How shall be deemed to be Confidential Information of both Parties hereunder; provided, further, that all information disclosed pursuant to the Existing Confidentiality Agreement (x) by Alnylam, shall be deemed to be Confidential Information of Alnylam hereunder and (y) by Roche, shall be deemed to be Confidential Information of Roche.
12.2Exceptions. Notwithstanding anything to the contrary herein, Confidential Information shall not include any information or materials to the extent the Recipient can demonstrate through competent evidence that:
(a)were already known by the Recipient or any of its Affiliates at the time of disclosure by the other Party, other than under an obligation of confidentiality;
(b)were generally available to the public or otherwise part of the public domain at the time of its disclosure to the Recipient:
(c)became generally available to the public or otherwise part of the public domain after its disclosure, other than through any act or omission by or on behalf of Recipient in breach of this Agreement;
(d)are subsequently disclosed to the Recipient or any of its Affiliates by a Third Party without obligations of confidentiality with respect thereto; or
(e)are independently discovered or developed by or on behalf of the Recipient or any of its Affiliates without the aid, application, access to, or use of the applicable Confidential Information.
(f)Notwithstanding the definition of “Confidential Information” in Section 12.1, all information with respect to Product generated under the Collaboration activities undertaken by the Parties under this Agreement, whether generated by one or both Parties, shall be deemed the Confidential Information of both Parties. The exceptions set forth in subsections (a)-(e) above shall apply to such Confidential Information to the extent applicable.
12.3Authorized Disclosure. The Recipient may disclose Confidential Information of the Discloser to the extent such disclosure is reasonably necessary in the following situations:
(a)filing or prosecuting Patent Rights (subject to any applicable Third Party Licenses) in accordance with Article 9;
(b)to the extent required or desirable to secure government approval for the Development, Manufacture or Commercialization of Product in the Territory, filing Regulatory Materials with Regulatory Authorities and seeking and maintaining any Regulatory Approvals, and other filings with Governmental Authorities to the extent permitted hereunder;
(c)prosecuting or defending litigation;
(d)to the extent required to comply with Applicable Laws, a court or administrative order, Accounting Standards, applicable regulations of a stock exchange (to the extent permitted hereunder) or to defend or prosecute litigation;
(e)disclosure to Third Parties pursuant to Section 7.3 (i) acting on behalf of a Party in accordance with this Agreement to the extent reasonably necessary for the Development, Manufacture or Commercialization in the Territory provided that such Third Party must be bound by obligations of confidentiality and non-use (whether in writing or by operation of law) at least as protective as those set forth in this Article 12 prior to any such disclosure; and (ii) requesting clinical trial data information in accordance with the Recipient’s then-current policy on sharing of clinical study information (which, with respect to Roche as of the Effective Date, is available at https://www.roche.com/innovation/process/clinical-trials/data-sharing/);
(f)disclosure to its Affiliates and its and their respective directors, employees, agents, consultants, advisors, independent contractors, accountants and Sublicensees (“Representatives”), in each case only on a need-to-know basis and solely in connection with the performance of this Agreement; provided that each Representative must be bound by obligations of confidentiality and non-use (whether in writing or by operation of law) at least as protective as those set forth in this Article 12 prior to any such disclosure; and
(g)disclosure of the terms of this Agreement to any bona fide potential or actual investor, investment banker, acquirer, merger partner, or other potential or actual financial or other partner, with the prior written consent of the other Party (not to be unreasonably withheld, conditioned or delayed); provided that in connection with such disclosure, each disclosee must be bound by obligations of confidentiality and non-use (whether in writing or by operation of law) at least as protective as those set forth in this Article 12 prior to any such disclosure.
(h)Notwithstanding the foregoing, in the event a Party is required to make a disclosure of the other Party’s Confidential Information pursuant to Sections 12.3(a), 12.3(b), 12.3(c) or 12.3(d), it will, except where impracticable, give reasonable advance notice to the other Party of such disclosure and use reasonable efforts to secure confidential treatment of such information. In any event, the Parties agree to take all reasonable action to avoid disclosure of Confidential Information hereunder.
12.4Publicity.
(a)The Parties agree that the terms of this Agreement are the Confidential Information of both Parties, subject to disclosure permitted by Section 12.3. The Parties have agreed to both make a public announcement of the execution of this Agreement that will be closely aligned to the other Party’s release. The text of such public announcements are attached as Exhibit F, which shall be issued at a time to be mutually agreed by the Parties (but in any event no later than [****] Business Days after such execution).
(b)After release of such press release, if either Party desires to make a public announcement concerning the terms or subject matter of this Agreement, such Party shall give at least [****] Business Days prior advance notice of the proposed text of such announcement to the other Party for its prior review and as it determines appropriate, comment and approval (except as otherwise provided herein) (such approval not to be unreasonably withheld, conditioned or delayed), except that in the case of a press release, governmental filing or other public statement required (i) by Applicable Laws or legal proceedings or (ii) to be contained in financial statements of the Parties prepared in accordance with the Accounting Principles, the disclosing Party shall (x) provide the other Party with sufficient advance notice of the text of such statement so that the other Party will have the opportunity to comment upon the statement, and the Party issuing such statement shall give due consideration to any such comments in the final statement and (y) in the case of any public statement that is required by Applicable Laws or legal proceedings, use reasonable efforts to obtain confidential treatment of financial and trade secret information, and Confidential Information of the other Party or the Parties. A Party commenting on such a proposed press release shall provide its comments, if any, within [****] Business Days after receiving the press release for review. In addition, solely where required by Applicable Laws of the applicable securities exchange upon which a Party may be listed, only as determined legally required by such Party’s competent legal counsel, a Party shall have the right to make a press release announcing the achievement of applicable milestone under this Agreement as it is achieved, and the achievements of applicable Regulatory Approvals as they occur, subject only to the review procedure set forth in the preceding sentence. In relation to the other Party’s review of such an announcement, the other Party may make specific, reasonable comments on such proposed press release within the prescribed time for commentary. Neither Party shall be required to seek the permission of the other Party to repeat any information regarding the terms of this Agreement that has already been publicly disclosed by such Party, or by the other Party, in accordance with this Section 12.4.
(c)The Parties acknowledge that either or both Parties may be obligated to file a copy of this Agreement with the SEC or other Governmental Authorities. Each Party shall be entitled to make such a required filing; provided that it initially files a redacted copy of this Agreement approved by both Parties (“Redacted Agreement”) and requests confidential treatment of the terms redacted from this Agreement for a reasonable period of time. In the event of any such filing, each Party shall (i) permit the other Party to review and comment upon such request for confidential treatment and any subsequent correspondence with respect thereto at least [****] Business Days in advance of its submission to the SEC or such other applicable Governmental Authority, (ii) reasonably consider and incorporate the other Party’s comments thereon to the extent consistent with the Applicable Laws governing redaction of information from material agreements that must be publicly filed, (iii) promptly deliver to the other Party any written correspondence received by it or its representatives from such Governmental Authority, if any, with respect to such confidential treatment request and promptly advise the other Party of any other communications between it or its representatives with such Governmental Authority with respect to such confidential treatment request, (iv) upon the written request of the other Party, request an appropriate extension of the term of the confidential treatment period, where available and (v) if such Governmental Authority requests any changes to the redactions set forth in the Redacted Agreement, use commercially reasonable efforts to support the redactions in the Redacted Agreement as originally filed (to the extent consistent with the then-current legal requirements governing redaction of information from material agreements that must be publicly filed) and, to the extent reasonably practicable, not agree to any changes to the Redacted Agreement without first discussing such changes with the other Party and taking the other Party’s comments into consideration when deciding whether to agree to such changes. Each Party shall be responsible for its own legal and other external costs in connection with any such filing, registration or notification.
12.5Publications. Subject to the terms of this Section 12.5, the Parties may publish the results of the Development Plan in scientific journals and make scientific presentations related to the Products. Each Party will consider in good faith any request by the other Party to publish Development Plan results related to any Product. The requesting Party shall provide the other Party (the “Reviewing Party”) with an advance copy of the proposed publication or presentation, and the Reviewing Party shall then have [****] days prior to submission for any publication or presentation in which to comment and to recommend any changes it reasonably believes are necessary (a) to preserve any Patent Rights or Know-How Controlled, in whole or in part, by the Reviewing Party or that is the Confidential Information of the Reviewing Party, or (b) remain consistent with the Development and Commercialization strategy established by the Parties through the JPT (or JDC or JCC, as the case may be) for the Product in the Field in the Territory. If the Reviewing Party informs the requesting Party that such publication, in the Reviewing Party’s reasonable judgment, could be expected to have a material adverse effect on any patentable invention Controlled, in whole or in part, by the Reviewing Party, or on any Know-How that is Confidential Information of the Reviewing Party, the requesting Party shall delay or prevent such publication as follows: (a) with respect to a patentable invention, such publication shall be delayed sufficiently long (not to exceed [****] days) to permit the timely preparation and filing of a patent application; and (b) with respect to Know-How that is Confidential Information of such Reviewing Party (other than the results of a Clinical Trial or any Product regulatory information), such Confidential Information shall be deleted from the publication. The requesting Party will also consider in good faith any other comments of the Reviewing Party. Any publication shall include recognition of the contributions of each Party according to standard practice for assigning scientific credit, either through authorship or acknowledgement, as may be appropriate. Each Party, in accordance with its internal policies and procedures, shall have the right to publish or grant access to all Non-Clinical Studies, Clinical Trials and results thereof for which such Party is the sponsor. Such Non-Clinical Studies, Clinical Trials or results thereof shall be filed in such Party’s clinical trial registry in accordance with Applicable Laws and such Party’s transparency policies with respect to product studies, clinical trials and results thereof, as applicable, if any; provided, however, that with respect to the Product as appropriate the other Party’s clinical trial registry can be accessed via a link from the other Party’s clinical trial registry.
12.6Information Security Incident.
(a)Notification. A Party shall provide to the other Party written notice within [****] business days after such Party’s confirmation of an Information Security Incident with respect to the other Party’s Confidential Information. Such notice shall describe in reasonable detail the Information Security Incident, including the other Party’s Confidential Information impacted, the extent of such impact and any corrective action taken or to be taken by such Party. In addition, if a Party reasonably suspects (even if it has not confirmed) that an actual or attempted Information Security Incident has occurred with respect to the other Party’s Confidential Information, then the Party shall promptly notify the other Party of such suspected actual or suspected Information Security Incident.
(b)Non-Disclosure. Except to the extent required by applicable law, neither Party shall disclose any information related to an actual or suspected Information Security Incident of the other Party’s Confidential Information to any Third Party without the other Party’s prior written consent.
ARTICLE 13
TERM AND TERMINATION
13.1Term. This Agreement shall become effective on the Effective Date and, unless earlier terminated pursuant to this Article 13, shall expire on a country-by-country and Product-by-Product basis (a) in the Roche Territory, upon expiration of the Royalty Term for such Product in such country and (b) in the Co-Commercialization Territory, upon expiration of the Co-Commercialization Term for such Product (the “Term”).
13.2Termination by Roche at Will. Roche shall have the right to terminate this Agreement in its sole discretion, for any or no reason at all, upon [****] prior written notice to Alnylam (such [****] period, the “Notice Period” and the date of such notice by Roche is the “Notice Date”), which termination may be (a) a termination of this Agreement in its entirety or (b) for the Roche Territory, on a Region-by-Region basis. The effective date of a termination under this Section 13.2 shall be the expiration of the Notice Period.
13.3Termination by Alnylam for Patent Challenge. Except to the extent the following is unenforceable under the laws of a particular jurisdiction, if Roche or any of its Affiliates, Roche Excluded Affiliates or its Sublicensees (with the right to sell Product), directly or indirectly, commences (or provides any support or assistance in respect of) any legal proceeding that challenges the validity, enforceability or ownership of any Alnylam Product-Specific Patent that has a Valid Claim (a “Patent Challenge”), Alnylam shall have the right to terminate this Agreement [****] upon written notice to Roche; provided that such right to terminate shall not apply if (a) Roche or such Affiliate, such Roche Excluded Affiliates or Sublicensees withdraws or causes to be withdrawn such Patent Challenge within [****] days of Roche’s receipt of written notice from Alnylam such that such Patent Challenge is actually withdrawn and the challenge initiated by Roche or such Affiliate, such Roche Excluded Affiliates or Sublicensee [****]; (b) if such legal action is commenced by a Sublicensee that is not a Roche Excluded Affiliate, Roche terminates the Sublicense Agreement with such Sublicensee within [sixty (60)] days of Roche’s receipt of written notice from Alnylam; or (c) where [****].
13.4Termination for Cessation of Development and Commercialization.
(a)If following First Commercial Sale of Product in the Field in the Territory Roche and its Related Parties have (i) ceased all material clinical Development and Commercialization activities with respect to the Products under this Agreement for a period of [****] in substantially all countries in one or more Regions [****] or (ii) Roche or any of its Related Parties publicly announces that Roche and its Related Parties have ceased all material clinical Development and Commercialization activities with respect to the Product under this Agreement in substantially all countries in one or more Regions [****], to the extent, in each case (the foregoing (a) and (b)), such cessation is not caused by force majeure, Good Reason, a Regulatory Authority’s delay, or reasonable, customary pauses or gaps taken in good faith in the ordinary course between or following Clinical Trials, or other studies, for the analysis of data, preparation of reports and design of future Clinical Trials or preparation of regulatory filings, then Alnylam shall have the right to terminate this Agreement with respect to any such Products and such Region by providing written notice to Roche. For clarity, this Section 13.4 shall not be construed to limit the Parties’ rights, obligations or remedies hereunder.
(b)Notwithstanding the foregoing, if Roche disputes whether Alnylam had a right to provide a notice under the foregoing Section 13.4(a) (each, an “Anti-Shelve Notice”) and Roche initiates dispute resolution procedures under Article ARTICLE 14 in accordance with the terms hereof to resolve such dispute within [****] days after receipt of the applicable Anti-Shelve Notice and is diligently pursuing such procedures, then the termination of this Agreement shall not become effective until final resolution of such dispute in favor of Alnylam.
(c)For purposes of this Section 13.4, “Good Reason” means [****].
13.5Termination by Either Party for Material Breach.
(a)Material Breach. Subject to Section 13.5(b), either Party (the “Non-breaching Party”) shall have the right to terminate this Agreement upon written notice to the other Party (the “Breaching Party”) if the Breaching Party materially breaches its obligations under this Agreement and, after receiving written notice from the Non-breaching Party identifying such material breach by the Breaching Party in reasonable detail, fails to cure such material breach within [****] days from the date of such notice (or within [****] days from the date of such notice in the event such material breach is solely based upon the Breaching Party’s failure to pay any amounts due the Non-breaching Party hereunder).
(b)Disputed Breach. If the alleged Breaching Party disputes in good faith the existence or materiality of a breach specified in a notice provided by the Non-breaching Party in accordance with Section 13.5(a), and such alleged Breaching Party provides the Non-breaching Party notice of such dispute and its intention to pursue dispute resolution pursuant to Article 14 within the applicable cure period set forth in Section 13.5(a), then the Non-breaching Party shall not have the right to terminate this Agreement under Section 13.5(a) unless and until an arbitrator or court, in accordance with Article 14, has determined that the alleged Breaching Party has materially breached this Agreement and that such Party fails to cure such breach within [****] days following such decision of such arbitrator or court, as applicable; provided that, notwithstanding the foregoing, to the extent such breach is with respect to failure to make a payment when due, such breach must be cured within [****] days following such decision by such arbitrator or court, as applicable. It is understood and agreed that during the pendency of such dispute, all of the terms and conditions of this Agreement shall remain in effect and the Parties shall continue to perform all of their respective obligations hereunder.
(c)Roche Option In Lieu of Termination for Material Breach. If Roche has the right to terminate this Agreement pursuant to Section 13.5(a) and Section 13.5(b), following the expiration of all applicable notice and cure periods, and, if any dispute is initiated under Article 14, before the expiration of the applicable cure period with respect to the asserted basis of such termination, agreement by the Parties or confirmation by an arbitrator or court, in accordance with Article 14, of the basis for termination under Section 13.5(a) and Section 13.5(b), Roche may elect, at its sole option, upon written notice to Alnylam that, in lieu of exercising its right to terminate this Agreement pursuant to Section 13.5(a) and Section 13.5(b), this Agreement shall remain in full force and effect (for clarity, other than as specified in this Section 13.5(c), each Party’s obligations and rights will continue in full force and effect in accordance with the terms hereunder); and (b) [****].
13.6Termination by either Party due to Insolvency. Either Party may terminate this Agreement upon written notice to the other Party, if the other Party files, or has filed against it, a petition for voluntary or involuntary bankruptcy or pursuant to any other insolvency law seeking to adjudicate it as bankrupt or insolvent, or seeking dissolution, liquidation, winding up, reorganization, arrangement, adjustment, protection, relief of debtors, an assignment for the benefit of creditors, or seeking the entry of an order for relief or the appointment of a receiver, trustee, assignee for the benefit of creditors, custodian or other similar official for any such Person or for any substantial part of its property, and, if such proceeding is commenced involuntarily, such proceeding remains undismissed or unstayed for a period of [****] days. The voluntary commencement of any of the foregoing proceedings by a Party shall result in an immediate right to terminate by the other Party.
13.7Mutual Termination. This Agreement may be terminated in its entirety at any time by the mutual written agreement of the Parties to be effective on the date set forth in such mutual agreement and, notwithstanding anything to the contrary hereunder, shall be subject to any terms and conditions that may be set forth in such mutual written agreement. Unless the Parties otherwise agree in writing as part of such a mutual termination any rights and licenses granted by any Party (or their Affiliates) to the other Party (or their Affiliates), and all other obligations of either Party under this Agreement shall terminate in their entirety on the effective date of such termination.
13.8Consequences of Termination of this Agreement in its Entirety or, for the Roche Territory on a Region-by-Region Basis.
(a)Conduct During Termination Notice Period. Subject to Section 13.9, which shall apply upon the termination of the Co-Commercialization Term, and except as otherwise set forth herein, following a Party’s receipt of written notice of a termination of this Agreement from the other Party pursuant to this Article 13, whether a termination of this Agreement is in its entirety (for clarity, covering the Territory), or for the Roche Territory with respect to a given Region or Regions therein pursuant to Section 13.2(b) or Section 13.4, during any period from the receipt of the applicable termination notice through the effective date of such termination (the applicable “Termination Notice Period”) for the Terminated Territory, each Party shall continue to have the rights provided or granted to it, and continue to perform all of such Party’s obligations, under this Agreement in accordance with the terms and conditions of this Agreement, including performing those activities allocated to the applicable Party under the Development Plan, Manufacturing Plan or Co-Commercialization Plan (as applicable) until the expiration of the Termination Notice Period. Without limiting the foregoing, with respect to any Termination Notice Period, for the Terminated Territory (1) each Party shall continue to have the right to any revenue or payment that accrue during the Termination Notice Period and be obligated to pay any costs, expenses or payments which are incurred during the Termination Notice Period for which it is responsible hereunder, including Development Costs, the applicable Party’s share of Net Profits and Net Losses, Trademark Costs and Patent Costs, in each case in accordance with the terms set forth in this Agreement and the Financial Appendix and other costs, expenses or revenue hereunder, as applicable, incurred, arising or payable during the Termination Notice Period, and (2) Roche shall continue to be responsible for any Sales Milestone Payments and Royalty Payments based on the achievement of any Sales Milestones or Net Sales of Products during the Termination Notice Period.
(b)Upon Termination by Roche at Will in Certain Circumstances. [****].
(c)Product Reversion Upon Termination of this Agreement in its Entirety or, for the Roche Territory, on a Region-by-Region Basis. Upon (1) termination of this Agreement, whether by either Party in the entirety or with respect to one or more Regions in the Roche Territory (the Regions, or in the case of termination of the Agreement in its entirety, all countries in the Territory, the “Terminated Territory”), and [****] or (y) the effective date for such termination for all other terminations under this Agreement, in each case ((1) and (2)), with respect to the Terminated Territory, all rights and licenses granted by any Party (or their Affiliates) to the other Party (or their Affiliates) under this Agreement shall terminate on the effective date of such termination (the “Termination Effective Date”) (except to the extent solely necessary to fulfill the transition activities described, or as otherwise granted by one Party to the other Party in this Section 13.8(c) below or otherwise) and, with respect to the Terminated Territory the following shall apply (unless otherwise modified by a mutually agreed Transition Agreement entered into in accordance with Section 13.8(c)(ii)):
(i)Roche Reversion License. Roche shall grant to Alnylam, an exclusive, fully sub-licensable (through multiple tiers), [****] perpetual, irrevocable license under Roche Licensed IP, including Roche’s interest in the Joint Patents and Joint Know-How (but for clarity, excluding Roche Excluded IP), solely to the extent necessary to allow Alnylam and its Related Parties and subcontractors (subject to Section 13.8(e)) to Develop, Manufacture and Commercialize any Product, as of the Termination Effective Date, that is then-currently under Development or Commercialization under the Agreement (each, a “Reversion Product” in the Field in the Terminated Territory. For clarity, the license under this Section 13.8(c)(i) shall not include any licenses that Roche has with a Third Party for which such grant would be prohibited or under which Roche or its Related Parties would incur financial obligations to such Third Party, unless Alnylam agrees to bear any such consideration.
(ii)Transition Agreement. With respect to the Terminated Territory, the Parties shall in good faith use their reasonable best efforts to enter into a written agreement (the “Transition Agreement”) that would address the obligations specified in this Section 13.8(c)) and any other reasonable terms and conditions with respect to any additional transition activities required in connection with the termination. If, despite such efforts, the Parties are unable to agree upon such terms and conditions within [****] days after the Notice Date if such termination is pursuant to Section 13.2, or otherwise the Termination Effective Date (or such longer period as the Parties may otherwise mutually agree to extend), either Party may refer the dispute for resolution by the Executive Officers and if not successful then to arbitration in accordance with Section 14.1 and Section 14.2, and the arbitrator shall have the authority to require the Parties to execute a Transition Agreement consistent with the terms set forth in this Section 13.8(c), in the form approved by the arbitrator.
(iii)Regulatory Materials and Development Data. With respect to the Terminated Territory, following the Termination Effective Date, upon the request of Alnylam, Roche shall, to the extent Roche has the right to do so, at Alnylam’s expense transfer to Alnylam all Regulatory Materials (including Regulatory Approvals, Price Approvals, Major Regulatory Communications, including copies of material correspondence and conversation logs, preclinical and clinical study reports and clinical study protocols), and Development Data (in the format maintained by Roche), to the extent specifically related to any Reversion Product in the Terminated Territory that are owned or controlled by Roche and necessary or reasonably useful for Alnylam to continue to Develop, Manufacture, or Commercialize such Reversion Product in the Field in the Terminated Territory. All data shall be transferred in the form and format in which it is maintained by Roche. Original paper copies shall only be transferred if legally required or where generating electronic copies is not reasonably feasible. Roche shall organize such data prior to transfer so that Alnylam can reasonably utilize such data, but Roche shall not be required to prepare or finalize any new data, reports or information solely for purposes of transfer to Alnylam. Roche shall take all steps reasonably necessary to transfer to Alnylam ownership of all such Regulatory Materials to the extent permitted by Applicable Laws, including submitting to each applicable Regulatory Authority a letter or other necessary documentation (with a copy to Alnylam) notifying such Regulatory Authority of the transfer of ownership of such Regulatory Material; provided that, to the extent it is not reasonably practicable or permitted under Applicable Laws for Roche to transfer to Alnylam any such Regulatory Materials, Roche shall hold such Regulatory Materials in its own name (and at Alnylam’s cost) for the benefit of Alnylam and shall grant to Alnylam an exclusive, royalty-free, fully paid-up, fully sublicensable (through multiple tiers) and transferable, perpetual, irrevocable exclusive license and right of reference to use such Regulatory Materials and any and all Know-How contained therein solely in connection with the Development, Manufacture, Commercialization of Reversion Product(s) in the Terminated Territory.
(iv)Promotional Materials. If a First Commercial Sale for a Reversion Product has occurred in the Terminated Territory prior to the Termination Effective Date, then with respect to the Terminated Territory in which a First Commercial Sale has occurred, upon the request of Alnylam, Roche shall, at Alnylam’s expense, take actions reasonably necessary to assign or otherwise grant rights (for clarity, not including any rights to use any Roche corporate name or logo therein) to Alnylam to use (to the extent not prohibited by Applicable Law) solely in connection with the Commercialization of such Reversion Product(s) in the Field any Promotional Materials, training materials, medical education materials, packaging and labeling, and all other literature, information or similar materials, in each case to the extent specifically related to such Reversion Product(s) (including copyrights and registrations for the foregoing), and, as soon as reasonably practicable following Alnylam’s request, Roche shall transfer any inventory thereof to the extent requested by Alnylam and in Roche’s possession or control.
(v)Assignment of Product Trademarks. With respect to the Terminated Territory, following the Termination Effective Date, upon the request of Alnylam, Roche shall, at Alnylam’s expense, assign and shall take actions reasonably necessary to assign, and shall cause its Related Parties to assign, its and their rights to all Product Trademarks for the Reversion Products and all goodwill associated therewith, throughout the Terminated Territory (or if the Terminated Territory is not the entire Territory, grant appropriate license thereto), and the rights to any internet domain names incorporating any such Product Trademarks or any variation or part of such Product Trademarks used as its URL address or any part of such address, to Alnylam; provided that if the Terminated Territory is not the entire Territory, the foregoing may be accomplished through appropriate license as applicable thereto). For the avoidance of doubt, Alnylam shall not have any right to use Roche’s corporate names or logos as of or following the Termination Effective Date (including to the extent included in any Promotional Materials or other materials mentioned in Section 13.8(c)(iv), except to the extent as may be required pursuant to Applicable Laws (as the Parties may agree)).
(vi)Know-How Transfer Support. With respect to the Terminated Territory, following the Termination Effective Date, upon the request of Alnylam, Roche shall [****].
(vii)Assignment of Contracts. With respect to the Terminated Territory, at Alnylam’s written request provided no later than [****] days following the Termination Effective Date, Roche shall disclose to Alnylam, all then-existing commercial arrangements between Roche or its Affiliates on the one hand, and a Third Party on the other hand, to the extent solely and specifically relating to Reversion Products in the Terminated Territory and reasonably necessary for Alnylam to continue Developing, Manufacturing, Commercializing or otherwise exploiting the Reversion Products in the Field in the Terminated Territory. Upon Alnylam’s written request provided no later than [****] days after its receipt of the foregoing list, to the extent Roche is able to, using commercially reasonable efforts, Roche shall at Alnylam’s expense assign to Alnylam the requested commercial arrangements to Alnylam. The foregoing shall include assigning, upon such a request of Alnylam, any agreements with Third Party suppliers or vendors, including clinical trial agreements, manufacturing agreements and distribution agreements, to the extent they solely and specifically cover the Development, Manufacturing or Commercialization of Reversion Products in the Terminated Territory. If any such agreement between Roche or any of its Affiliates and a Third Party is not assignable to Alnylam (whether by such contract’s terms or because such contract does not relate solely and specifically to the applicable Reversion Products in the Terminated Territory) but is otherwise reasonably necessary for Alnylam to continue the Development, Manufacture, or Commercialization of Reversion Products in the Field in the Terminated Territory, then Roche shall at Alnylam’s expense reasonably cooperate with Alnylam in Alnylam’s efforts to obtain from such Third Party the assignment of such contract or of that portion of such contract that solely and specifically relates to the Development, Manufacture, or Commercialization of the applicable Reversion Products in the Field in the Terminated Territory.
(viii)Appointment as Exclusive Distributor. With respect to the Terminated Territory, only if a First Commercial Sale for a Reversion Product has occurred in a given country in the Terminated Territory prior to the Termination Effective Date, then, with respect to each country in the Terminated Territory in which a First Commercial Sale has occurred and without limiting Alnylam’s right to request a transfer of any agreement between Roche or its Affiliates and any distributor with respect to distribution of such Reversion Product pursuant to Section 13.8(c)(vii), at Alnylam’s election (in its sole discretion) on a country-by-country basis and at Alnylam’s expense, until the earlier of [****], Roche shall (1) appoint Alnylam or its designee as its exclusive distributor of such Reversion Product(s) on a country-by-country basis in each such country until such time as all applicable Regulatory Approvals with respect to such Reversion Product(s) in a given country have been assigned and transferred to Alnylam and (2) grant Alnylam or its designee the right to appoint sub-distributors, to the extent not prohibited by any written agreement between Roche or any of its Affiliates and such distributor; provided that, for clarity, Roche shall not be required to pay to Alnylam any of the Royalty Payments or Sales Milestone Payments for sales of such Product in the Terminated Territory under this Agreement from and after the Termination Effective Date.
(ix)Ongoing Clinical Trials.
(1)Transfer to Alnylam. With respect to the Terminated Territory, if, as of the Termination Effective Date, Roche or its Affiliates are conducting any Clinical Trials for Reversion Products, then, at Alnylam’s election, on a Clinical Trial-by-Clinical Trial basis, Roche shall reasonably cooperate, and shall cause its Affiliates to reasonably cooperate, with Alnylam to transfer the conduct of such Clinical Trial to Alnylam or its designees. Alnylam shall assume any and all liability for the conduct of such transferred Clinical Trial after the effective date of such transfer; provided that, for clarity, prior to the Termination Effective Date the Parties shall bear the costs of completing such ongoing Clinical Trial in accordance with their respective share of Development Costs for such Clinical Trial in accordance with the Financial Appendix and the Development Budget existing as of the Termination Effective Date; provided, however, that Alnylam shall be responsible for all such budgeted Development Costs (and shall reimburse Roche if necessary therefor) incurred after the Termination Effective Date. In addition, for no longer than [****] days following the Termination Effective Date, at Alnylam’s expense Roche shall provide such knowledge transfer and other training to Alnylam or its designated Affiliate or Third Party as reasonably necessary for Alnylam or such designated Affiliate or Third Party to continue such Clinical Trial for such Reversion Product in the Field in the Terminated Territory.
(2)Wind-Down. With respect to the Terminated Territory, if Alnylam does not as promptly as reasonably practicable (or as otherwise mutually agreed) assume control of any Clinical Trial for a Reversion Product, then Roche shall, in accordance with accepted pharmaceutical industry and ethical practices, wind-down the conduct of any such Clinical Trial in an orderly manner. Any costs and expenses associated with such wind-down will be Development Costs; provided that, except to the extent otherwise expressly required under this Section 13.8, Alnylam shall be responsible for all such Development Costs (and shall reimburse Roche if necessary therefor) incurred after the Termination Effective Date.
(x)Remaining Inventories. With respect to the Terminated Territory, Roche shall be entitled, during the [****]-month period following the Termination Effective Date with respect to a Reversion Product, to finish any work-in-progress and to sell any inventory of such Reversion Product and shall pay Alnylam the amounts applicable to such sales of such Reversion Product in accordance with the terms and conditions of this Agreement. Thereafter, Roche shall cease selling any such Reversion Product in the Terminated Territory, and Alnylam shall have the right, in its sole discretion and upon written notification to Roche, to purchase from Roche any or all of the inventory of such Reversion Product held by Roche as of the date of such notice solely for distribution in the applicable country within the Terminated Territory at a price equal to [****].
(xi)Cessation of Roche Activities. With respect to the Terminated Territory, as of the Termination Effective Date, Roche shall, and shall cause its Related Parties to, cease any Development, Manufacture, Commercialization or other exploitation of any Reversion Product, except as otherwise permitted or required pursuant to this Section 13.8, including, for the avoidance of doubt, any Transition Agreement.
(xii)[****].
(xiii)Direct License. With respect to the Terminated Territory, notwithstanding any provision of this Agreement to the contrary, upon a termination of this Agreement: (a) any Compulsory Sublicense shall remain in full force and effect to the extent required by Applicable Laws and, to the extent permitted by Applicable Laws, Roche shall assign such Compulsory Sublicense to Alnylam; and (b) upon the written request of any permitted Sublicensee under a valid Third Party Sublicense Agreement granted by Roche pursuant to Section 7.3(c)(ii) (other than if a Roche Excluded Affiliate is the Sublicensee under such Sublicense Agreement), Alnylam agrees to enter into a direct license grant to such Sublicensee consistent in scope with the rights sublicensed to such Sublicensee under such Third Party Sublicense Agreement; provided that (i) such permitted Sublicensee is not then in breach of its Third Party Sublicense Agreement and did not cause any breach by Roche that gave rise to a termination of this Agreement by Alnylam under Section 13.5 and (ii) promptly agrees in writing to be bound by all applicable terms and conditions of this Agreement, including rendering directly to Alnylam all payments and other obligations due to Alnylam related to such Third Party Sublicense Agreement. In the event that either of the foregoing (i) or (ii) are not satisfied, with respect to a Sublicensee, and for clarity, with respect to all other Sublicensees of Roche that are not described in the foregoing (a) or (b), the Sublicense Agreements to which such Sublicensees and Roche are a party as of termination of this Agreement shall immediately and automatically terminate upon the effective date of such termination.
(d)[****].
(e)Limitations on Grant-Backs; Transfer Expenses.
(i)All transfers and licenses from Roche to Alnylam (or other obligations of Roche) under Section 13.8 are solely with respect to Product(s) that are not Combination Product(s). Such transfers, licenses and obligations do not extend to other therapeutically active ingredients or products (including any diagnostic product), even if physically mixed, combined or packaged together with a Product, and even if a Product is intended (according to the investigation plan, proposed labeling or actual labeling, as applicable) for use with such other therapeutically active ingredients or products (including any diagnostic product).
(ii)In connection with research studies, Clinical Trials or other activities associated with the Development, Manufacture and Commercialization of Products, Roche may have collected (i) personally identifiable information about individual human subjects or (ii) human biological samples (collectively, “PII/Samples”). Legal and contractual restrictions may apply to such PII/Samples. Roche shall have no obligation to transfer such PII/Samples unless necessary for the continued development of a Reversion Product. If PII/Samples are necessary for the Development, Manufacture, Commercialization or other exploitation of a Reversion Product in the Terminated Territory, Roche shall transfer such PII/Samples solely to the extent permitted in accordance with Applicable Laws. During the Term, the Parties shall use commercially reasonable efforts to collect PII/Samples (including through informed consent agreement with subjects of Clinical Trials hereunder) in a manner that would allow for such transfers under Applicable Laws. If Roche transfers to Alnylam any such PII/Samples, the Parties will enter into the relevant agreements under Applicable Data Protection Laws (such as a data transfer agreement) when required in accordance with Section 15.16. Upon the transfer of such PII/Samples by Roche, Alnylam shall use such PII/Samples for the sole purpose of developing and commercializing a Reversion Product in the Terminated Territory, and Alnylam shall be responsible for the correct and lawful use of the PII/Sample in compliance with Applicable Data Protection Laws, the informed consent forms and privacy notices (including potential re-consenting of the patients at Alnylam’s costs if the legal basis for the processing of the patients’ data was their explicit consent).
(iii)Alnylam shall promptly reimburse Roche for all reasonable out-of-pocket costs and expenses (including FTE charges) incurred by or on behalf of Roche for the Roche Transfer Activities; provided that [****]. Roche shall be under no obligation to provide Roche Transfer Activities (beyond Alnylam-Originated Transfer Activities) prior to [****]. Notwithstanding anything to the contrary herein, Alnylam shall not be obligated to pay Roche an amount greater than [****].
(f)Ancillary Agreements. Unless otherwise agreed by the Parties in writing, the termination of this Agreement shall cause the automatic termination (as of the Termination Effective Date) of all ancillary agreements related hereto solely with respect to the Terminated Territory, including the Clinical Supply Agreement, the Commercial Supply Agreement and the Co-Promotion Agreement, as applicable.
13.9Termination of the Co-Commercialization Term; Consequences.
(a)Termination of Co-Commercialization Term. With respect to the Co-Commercialization Territory, the Co-Commercialization Term shall continue until the first to occur of (i) the date on which the Parties mutually agree in writing to discontinue Commercialization of all Products for use in the Field in the Co-Commercialization Territory (in which case, as part of such mutual agreement, the Parties shall agree in writing as to each Party’s respective rights and licenses from one Party to the other Party with respect to the further Development, Manufacture and Commercialization of Products after the Co-Commercialization Term), and (ii) the date on which all of the following have occurred: (A) expiration of the last Valid Claim of the Alnylam Patents that Covers the Manufacture, use or sale of Therapeutic Products in the Co-Commercialization Territory, (B) expiration of any and all Regulatory Exclusivity with respect to Therapeutic Products in the Co-Commercialization Territory (the foregoing (A) and (B), collectively the “Expiration Condition”) and (C) [****] from the date (such date, the “Co-Commercialization Termination Date”) on which a Party (the “Opt-Out Party”) provides a written notice to the other Party (the “Continuing Party”) of its decision to opt-out of Co-Commercialization of Products in the Co-Commercialization Territory (a “Co-Commercialization Opt-Out Notice”), which notice may not be provided any earlier than [****].
(b)Rights of Continuing Party. If a Party delivers a Co-Commercialization Opt-Out Notice, following the end of the Co-Commercialization Term: (i) the Continuing Party shall have the right to continue the Development, Manufacture and Commercialization of Products then being Commercialized for use in the Field in the Co-Commercialization Territory under this Agreement without the Opt-Out Party; (ii) the Opt-Out Party shall grant the Continuing Party an [****] license under, if Alnylam is the Opt-Out Party, Alnylam Licensed IP and Alnylam’s interest in Joint Patents and Joint Know-How (but for clarity, excluding Alnylam Excluded Roche IP), and, if Roche is the Opt-Out Party, Roche Licensed IP and Roche’s interest in the Joint Patents and Joint Know-How (but for clarity, excluding Roche Excluded IP), in each case solely to the extent necessary to allow the Continuing Party and its Affiliates or licensees or sublicensees to Develop, Manufacture and Commercialize such Products in the Field in the Co-Commercialization Territory. For clarity, the license under this Section 13.9 shall not include any licenses that the Opt-Out Party has with a Third Party for which such grant would be prohibited or under which the Opt-Out Party or its Related Parties would incur financial obligations to such Third Party, unless the Continuing Party agrees to bear any such consideration; and (iii) commencing on the delivery of the Co-Commercialization Opt-Out Notice, the Parties shall, in good faith, negotiate to enable the Continuing Party to assume and otherwise take over, as of the Co-Commercialization Termination Date, any Collaboration activities then being conducted by the Opt-Out Party with respect to the Development, Manufacturing or Commercialization of Products for use in the Field in the Co-Commercialization Territory (including the assignment of any Regulatory Activities and Regulatory Approvals therefor) and the Continuing Party shall reimburse the Opt-Out Party for its reasonable expense incurred in connection with such transfer.
13.10Effect of Termination of Chugai Sublicense Agreement. In the event that, prior to the expiration of the Royalty Term for the last Product for Japan, (a) the Chugai Sublicense Agreement is terminated or expires for any reason and thereafter (b) Roche is prohibited under its agreements with Chugai from Developing and Commercializing Products in Japan, then following the effective date of the termination of such Chugai Sublicense Agreement (i) Alnylam shall be permitted, itself or through a licensee, to Develop, Manufacture, Commercialize and otherwise exploit Products in Japan, at Alnylam’s sole cost and expense, (ii) Alnylam shall have final decision-making authority with respect to the Development, Manufacture, Commercialization and other exploitation of Products for Japan, (iii) during the Royalty Term, Alnylam shall make Royalty Payments to Roche on Net Sales of Products in Japan [****]; (iv) Japan shall cease to be included in the Roche Territory for the purposes of this Agreement; and (v) Roche shall no longer be obligated to make any Royalty Payments, Development Milestone Payments or Sales Milestone Payments related to Japan to Alnylam (except to the extent accrued prior to the date of termination or expiration of the Chugai Sublicense Agreement), and Alnylam shall be solely responsible for all costs associated with the Development, Manufacture and Commercialization of Product for Japan. If Japan is no longer part of the Roche Territory in accordance with this Section 13.10, then the Parties shall update the Global Brand Strategy to cover the Development, Manufacturing and Commercialization of Product in the Field in Japan by Alnylam.
13.11Other Remedies. Termination or expiration of this Agreement for any reason shall not release either Party from any liability or obligation that already has accrued prior to such expiration or termination, nor affect the survival of any provision hereof to the extent it is expressly stated to survive such termination. Termination or expiration of this Agreement for any reason shall not constitute a waiver or release of, or otherwise be deemed to prejudice or adversely affect, any rights, remedies or claims, whether for damages or otherwise, that a Party may have hereunder or that may arise out of or in connection with such termination or expiration.
13.12Rights in Bankruptcy. All rights and licenses granted by either Party under this Agreement are and will be deemed to be rights and licenses to “intellectual property” as such term is used in, and interpreted under, Section 365(n) of the United States Bankruptcy Code (the “Bankruptcy Code”) (11 U.S.C. § 365(n)). The licensee Party hereunder has all rights, elections, and protections under the Bankruptcy Code and all other bankruptcy, insolvency, and similar Applicable Laws with respect to the Agreement, and the subject matter hereof.
13.13Survival. Termination or expiration of this Agreement shall not affect rights or obligations of the Parties under this Agreement that have accrued prior to the date of termination or expiration of this Agreement. Notwithstanding anything to the contrary, the following provisions shall survive and apply after expiration or termination of this Agreement: Article ARTICLE 1 (to the extent necessary to interpret this Agreement), Section 8.8, Section 8.9, Section 8.10, Section 8.11, Section 8.12, Section 9.2, Section 9.3(c), Section 9.4, Section 9.12, Article ARTICLE 11, Article ARTICLE 12 (for the time period set forth therein), Section 13.8, Section 13.9, Section 13.11, Section 13.12, this Section 13.13, Section 13.14, Article ARTICLE 14 and Article ARTICLE 15. In addition, the other applicable provisions of Article 8 shall survive to the extent required to make final reimbursements, reconciliations or other payments with respect to Net Sales and costs and expenses incurred or accrued prior to the date of termination or expiration (to the extent required to make final payments with respect to Royalty Payments, Development Milestone Payments and Sales Milestone Payments accrued prior to the date of termination or expiration). For any surviving provisions requiring action or decision by a Committee or an Executive Officer, each Party will appoint representatives to act as its Committee members or Executive Officer, as applicable. All provisions not surviving in accordance with the foregoing shall terminate upon expiration or termination of this Agreement and be of no further force and effect.
13.14[****].
ARTICLE 14
DISPUTE RESOLUTION
14.1Executive Negotiations. Except for matters which are subject to decision-making by a Committee as set forth in Article 2, which are to be determined solely in accordance with Section 2.5(d), the Parties shall attempt to settle any dispute, controversy, or claim that arises out of, or relates to, this Agreement or the breach, termination, or validity thereof (“Disputed Matter”) by first referring the Disputed Matter to the Parties’ Executive Officers. Either Party may initiate such informal dispute resolution by sending written notice of the Disputed Matter to the other Party, and, within [****] days after such notice, the Executive Officers (or their respective designees; provided that such designees have the authority to settle such Disputed Matter) of the Parties will meet for negotiations regarding the Disputed Matter. If the Executive Officers (or their respective designees) are unable to resolve the Disputed Matter in writing within [****] days after their first meeting for such negotiations, either Party may seek to have such Disputed Matter resolved by arbitration in accordance with Section 14.2 below, except with respect to any Disputed Matter that is a dispute with respect to the scope, validity, enforceability, inventorship, or infringement of any Patent Rights (“Patent Dispute”), which shall be resolved by litigation in accordance with Section 14.3 below.
14.2Dispute Resolution.
(a)If the Parties are unable to resolve a Disputed Matter in writing using the process described in Section 14.1, then any Disputed Matter other than a Patent Dispute shall be resolved exclusively by final and binding arbitration administered by the International Chamber of Commerce (the “ICC”) in accordance with its Rules of Arbitration in effect at the time (the “Rules”), except as modified herein. For the avoidance of doubt, all decisions of the Committees shall be made solely in accordance with the applicable provisions of Article 2, but any dispute with respect to the interpretation of the provisions of Article 2 shall be determined by final and binding arbitration in accordance with this Section 14.2.
(b)The seat of arbitration shall be New York, New York, and the arbitration shall be conducted in the English language.
(c)The arbitration shall be conducted by three (3) arbitrators. Alnylam shall nominate one (1) arbitrator and Roche shall nominate one (1) arbitrator, each within [****] days of receipt by respondent of the request for arbitration. The two (2) arbitrators so nominated shall nominate the third and presiding arbitrator (the “Presiding Arbitrator”) within [****] days of the confirmation by the ICC Court of Arbitration (“ICC Court”) of the second arbitrator. If any party fails to nominate an arbitrator, or if the two (2) party-nominated arbitrators fail to nominate the Presiding Arbitrator, within the time periods specified herein, then any such arbitrator shall, upon any party’s request, be appointed by the ICC Court in accordance with the Rules.
(d)The arbitration and this arbitration agreement shall be governed by the Federal Arbitration Act (9 U.S.C. § 1 et seq.). The arbitral tribunal shall have jurisdiction to determine its jurisdiction or the existence, scope or validity of this arbitration agreement or the arbitrability of any claim.
(e)In addition to monetary damages, the arbitrators shall be empowered to award equitable relief, including an injunction and specific performance of any obligation under this Agreement.
(f)By agreeing to arbitration, the Parties do not intend to deprive any court of its jurisdiction to issue any interim or provisional relief, including a temporary restraining order, preliminary injunction, pre-arbitral attachment or other interim equitable relief, before or after the initiation of an arbitration, if necessary to protect the interests of a Party. A Party may bring an application seeking such relief without first invoking or exhausting the procedures in Section 14.1. Without prejudice to such provisional remedies that may be granted by a court, the arbitrator shall have full authority to grant provisional remedies, to order a party to request that a court modify or vacate any temporary or preliminary relief issued by such court, and to award damages for the failure of any party to respect the arbitrator’s orders to that effect.
(g)The Parties consent and submit to the non-exclusive jurisdiction of any federal court located in the State of New York or, where such court does not have jurisdiction, any New York state court, in either case located in the Borough of Manhattan, New York City, New York (“New York Court”) to compel arbitration, for interim or provisional relief (including a temporary restraining order, preliminary injunction, pre-arbitral attachment, or other interim equitable relief), or for the enforcement of any arbitral award rendered hereunder. In any such action: (i) each Party irrevocably waives, to the fullest extent it may effectively do so, any objection, including any objection to the laying of venue or based on the grounds of forum non conveniens or any right of objection to jurisdiction on account of its place of incorporation or domicile, which it may now or hereafter have to the bringing of any such action or proceeding in any New York Court; (ii) each of the Parties irrevocably consents to service of process sent by a national courier service (with written confirmation of receipt) to its address identified in Section 15.4 of this Agreement or in any other manner permitted by Applicable Laws; and (iii) each of the Parties waives any right to trial by jury in any court.
(h)The award of the arbitrators shall be final and binding upon the parties thereto, and shall be the sole and exclusive remedy between the Parties regarding any Disputed Matters presented to the arbitrators. Judgment upon any award may be entered in any court having jurisdiction over any party or any of its assets.
(i)The arbitrators shall have power to award the prevailing party its attorneys’ fees and costs reasonably incurred in the arbitration, including the prevailing party’s share of the arbitrator fees and ICC administrative costs.
(j)Any arbitration hereunder shall be confidential, and the Parties and their agents agree not to disclose to any Third Party (i) the existence or status of the arbitration, (ii) all information made known and documents produced in the arbitration not otherwise in the public domain, and (iii) all awards arising from the arbitration, except and to the extent that disclosure is required by Applicable Laws or is required to protect or pursue a legal right. In no event shall an arbitration be initiated after the date when commencement of a legal or equitable proceeding based on the dispute, controversy, or claim would be barred by the applicable New York statute of limitations. Any disputes concerning the propriety of the commencement of the arbitration, or the validity, or application of this Section 14.2 shall be finally settled by the arbitrator.
14.3Resolution of Patent Disputes. If the Parties are unable to resolve a Patent Dispute in writing using the process described in Section 14.1, then the Patent Dispute shall be submitted for resolution to a court of competent jurisdiction. In any such action, each of the Parties irrevocably consents to service of process sent by a national courier service (with written confirmation of receipt) to its address identified in Section 15.4 or in any other manner permitted by Applicable Laws, and each of the Parties waives any right to trial by jury in any court.
ARTICLE 15
MISCELLANEOUS
15.1Entire Agreement; Amendment. This Agreement, including the Exhibits hereto, sets forth the complete, final and exclusive agreement and all the covenants, promises, agreements, warranties, representations, conditions and understandings between the Parties hereto with respect to the subject matter hereof and supersedes, as of the Effective Date, all prior agreements and understandings between the Parties with respect to the subject matter hereof, including the Existing Confidentiality Agreement; provided that Section 14 of the Existing Confidentiality Agreement shall remain in full force and effect with respect to the subject matter thereof. The foregoing shall not be interpreted as a waiver of any remedies available to either Party as a result of any breach, prior to the Effective Date, by the other Party of its obligations pursuant to the Existing Confidentiality Agreement. In the event of any inconsistency between any plan hereunder (including the Development Plan, Manufacturing Plan or Co-Commercialization Plan) and this Agreement, the terms of this Agreement shall prevail. There are no covenants, promises, agreements, warranties, representations, conditions or understandings, either oral or written, between the Parties other than as are set forth herein and therein. No subsequent alteration, amendment, change or addition to this Agreement shall be binding upon the Parties unless reduced to writing and signed by an authorized officer of each Party.
15.2Force Majeure. Both Parties shall be excused from the performance of their obligations under this Agreement to the extent that such performance is prevented or delayed by force majeure and the nonperforming Party promptly provides notice of the prevention to the other Party. Such excuse shall be continued so long as the condition constituting force majeure continues and the nonperforming Party takes reasonable efforts to remove the condition. For purposes of this Agreement, “force majeure” shall mean conditions beyond the control of the Parties, including an act of God, war, civil commotion, terrorist act, labor strike or lock-out, epidemic, pandemic, failure or default of public utilities or common carriers, destruction of production facilities or materials by fire, earthquake, storm or like catastrophe, and failure of plant or machinery; provided that such failure could not have been prevented by the exercise of skill, diligence, and prudence that would be reasonably and ordinarily expected from a skilled and experienced Person engaged in the same type of undertaking under the same or similar circumstances. Notwithstanding the foregoing, a Party shall not be excused from making payments owed hereunder because of a force majeure affecting such Party.
15.3Invoices to Roche. All invoices that are required or permitted to be provided to Roche hereunder shall be in writing and sent by Alnylam to Roche at the following address or such other address as Roche may later provide:
[****]
15.4Notices.
(a)Any notice required or permitted to be given under this Agreement shall be in writing, shall specifically refer to this Agreement, and shall be addressed to the appropriate Party at the email address or street address specified below or such other address as may be specified by such Party in writing in accordance with this Section 15.4, and shall be deemed to have been given for all purposes (a) when received, if emailed, hand-delivered or sent by a reputable international expedited delivery service, or (b) [****] Business Days after mailing, if mailed by first class certified or registered mail, postage prepaid, return receipt requested.
If to Alnylam: Alnylam Pharmaceuticals, Inc.
[****]
If to Roche: F. Hoffmann-La Roche Ltd
[****]
15.5No Strict Construction; Headings. This Agreement has been prepared jointly and shall not be strictly construed against either Party. Ambiguities, if any, in this Agreement shall not be construed against any Party, irrespective of which Party may be deemed to have authored the ambiguous provision. The headings of each Article and Section in this Agreement have been inserted for convenience of reference only and are not intended to limit or expand on the meaning of the language contained in the particular Article or Section.
15.6Assignment. Except as provided in this Section 15.6 this Agreement may not be assigned or otherwise transferred, in whole or in part, nor may any right or obligation under this Agreement be assigned or otherwise transferred, by either Party without the prior written consent of the other Party. However, either Party may, without the other Party’s consent, assign or otherwise transfer this Agreement and its rights and obligations under this Agreement in whole or in part to an Affiliate or to a Person that acquires, by merger, sale of assets or otherwise, all or substantially all of the business of the assigning Party to which the subject matter of this Agreement relates; provided, however, that neither Party shall be permitted to assign or otherwise transfer this Agreement without the other Party’s prior written consent (not to be unreasonably withheld) to any Person if that Person or any of its Affiliates is Developing, or Commercializing a AGT EMO at the time of such assignment or transfer, except in the event of a Change of Control of such Party in compliance with Section 7.8. Any permitted assignment shall be binding on the successors of the assigning Party. Any assignment or attempted assignment by either Party in violation of the terms of this Section 15.6 shall be null, void and of no legal effect.
15.7Performance by Affiliates. Subject to Section 7.3, each Party may discharge any obligations and exercise any right hereunder through any of its Affiliates. Each Party hereby guarantees the performance by its Affiliates of such Party’s obligations under this Agreement, and shall cause its Affiliates to comply with the provisions of this Agreement in connection with such performance. Any breach by a Party’s Affiliate of any of such Party’s obligations under this Agreement shall be deemed a breach by such Party, and the other Party may proceed directly against such Party without any obligation to first proceed against such Party’s Affiliate. Without limiting the foregoing, Roche Basel and Genentech irrevocably and unconditionally acknowledge and agree that all of their respective covenants, agreements and obligations under this Agreement shall be joint and several, and Roche Basel and Genentech shall be jointly and severally liable to Alnylam for their performance of all their obligations hereunder.
15.8Further Actions. Each Party agrees to execute, acknowledge and deliver such further instruments, and to do all such other acts, as may be necessary or appropriate in order to carry out the purposes and intent of this Agreement.
15.9Compliance with Applicable Laws. Each Party shall comply in all material respects with all Applicable Laws in the course of performing its obligations or exercising its rights pursuant to this Agreement.
15.10Severability. If any one or more of the provisions of this Agreement is held to be invalid or unenforceable by an arbitrator or by any court of competent jurisdiction from which no appeal can be or is taken, the provision shall be considered severed from this Agreement and shall not serve to invalidate any remaining provisions hereof. The Parties shall make a good faith effort to replace any invalid or unenforceable provision with a valid and enforceable one such that the objectives contemplated by the Parties when entering this Agreement may be realized.
15.11No Waiver. Any delay in enforcing a Party’s rights under this Agreement or any waiver as to a particular default or other matter shall not constitute a waiver of such Party’s rights to the future enforcement of its rights under this Agreement, except with respect to an express written and signed waiver relating to a particular matter for a particular period of time.
15.12Independent Contractors. Each Party shall act solely as an independent contractor, and nothing in this Agreement shall be construed to give either Party (or their employees or representatives) the power or authority to act for, bind, or commit the other Party in any way. Nothing herein shall be construed to create the relationship of partners, principal and agent, or joint-venture partners between the Parties.
15.13Third Party Beneficiary. None of the provisions of this Agreement shall be for the benefit of or enforceable by any Third Party, including any creditor of either Party hereto. No such Third Party shall obtain any right under any provision of this Agreement or shall by reasons of any such provision make any claim in respect of any debt, liability or obligation (or otherwise) against either Party hereto.
15.14Certain Interpretations. Except where expressly stated otherwise in this Agreement, the following rules of interpretation apply to this Agreement: (a) “either” and “or” are not exclusive and “include,” “includes” and “including” are not limiting and shall be deemed to be followed by the words “without limitation”; (b) “extent” in the phrase “to the extent” means the degree to which a subject or other thing extends, and such phrase does not mean simply “if”; (c) “hereof,” “hereto,” “herein” and “hereunder” and words of similar import when used in this Agreement refer to this Agreement as a whole and not to any particular provision of this Agreement; (d) references to a Person are also to its permitted successors and assigns; (e) definitions are applicable to the singular as well as the plural forms of such terms; (f) references to an “Article,” “Section” or “Exhibit” refer to an Article or Section of, or an Exhibit to, this Agreement; (g) references to “$” or otherwise to dollar amounts refer to the lawful currency of the U.S.; (h) the word “will” shall be construed to have the same meaning and effect as the word “shall”; (i) any definition of or reference to any agreement, instrument or other document herein shall be construed as referring to such agreement, instrument or other document as from time to time amended, supplemented or otherwise modified (subject to any restrictions on such amendments, supplements or modifications set forth herein); and (h) references to Applicable Laws include any amendment or modification to such law and any rules and regulations issued thereunder, whether such amendment or modification is made, or issuance of such rules and regulations occurs, before or after the date of this Agreement.
15.15Business Day Requirements. Except as may be otherwise specified in a Pharmacovigilance Agreement, if any notice or other action or omission is required to be taken by a Party under this Agreement on, or by a day that is not a Business Day, then such notice or other action or omission shall be deemed to be required to be taken on the next occurring Business Day for such Party.
15.16Data Privacy. The term “Personal Data” shall have the meaning ascribed to it under applicable privacy and data protection rules and regulations, including applicable international, federal, state, and local data privacy laws (hereinafter “Applicable Data Protection Laws”). Parties processing of Personal Data (including transfers or disclosures to third parties) for the performance of the Agreement, shall comply with Applicable Data Protection Laws and Regulations and the Parties’ applicable policies and procedures as well as any other agreed upon and documented instructions provided by the Parties during the Term of the Agreement. If necessary, the Parties will enter into the relevant agreements under applicable data privacy and data protection laws (such as a data transfer agreement) when required. The terms of such agreement will be agreed upon by the Parties when the requirement to enter into such agreement has been confirmed by the Parties. Under such an agreement, both Parties herewith represent and warrant that the personal data will be provided to the other Party in a pseudonymized or de-identified manner, as applicable, and that the disclosing Party will not disclose or otherwise make available to the other Party or give access to the other Party to any code allowing re-identification of data subjects (e.g., patients, study participants, specimen donors) (“Data Subjects”). Furthermore, each Party shall represent and warrant that, it will not: (i) undertake any actions to re-identify the Data Subjects or (ii) try to get access to any code allowing re-identification of such Data Subjects.
15.17Counterparts. This Agreement may be executed in any number of counterparts and by the Parties in separate counterparts, each of which when so executed shall be deemed to be an original and all of which taken together shall constitute one and the same agreement. Copies of executed counterparts transmitted by telecopy, facsimile or other similar means of electronic transmission, including “PDF,” shall be considered original executed counterparts, provided receipt of such counterparts is confirmed. For purposes hereof, an e-Signature or email with attached pdf copy, of this Agreement, including the signature pages hereto, will be deemed to be an original. The Parties agree that execution of this Agreement by e-Signatures (as defined below) shall have the same legal force and effect as the exchange of original signatures. Pursuant to this Agreement, “e-Signature” shall mean a signature that consists of one or more letters, characters, numbers or other symbols in digital form incorporated in, attached to or associated with the electronic document, that (a) is unique to the person executing the signature; (b) the technology or process used to make the signature is under the sole control of the person making the signature; (c) the technology or process can be used to identify the person using the technology or process; and (d) the electronic signature can be linked with an electronic document in such a way that it can be used to determine whether the electronic document has been changed since the electronic signature was incorporated in, attached to or associated with the electronic document.
[Signature Page Follows]
IN WITNESS WHEREOF, the Parties have executed this Collaboration and License Agreement in duplicate originals by their duly authorized officers as of the Effective Date.
ALNYLAM PHARMACEUTICALS, INC.
By: /s/ Yvonne Greenstreet
Name: Yvonne Greenstreet
Title: Chief Executive Officer
[Signature Page to Collaboration and License Agreement]
IN WITNESS WHEREOF, the Parties have executed this Collaboration and License Agreement in duplicate originals by their duly authorized officers as of the Effective Date.
F. HOFFMANN-LA ROCHE LTD
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| By: |
/s/ James Sabry |
|
By: |
/s/ Peter Trybus |
| Name: |
James Sabry |
|
Name: |
Peter Trybus |
| Title: |
EVP, Head of Roche Pharma Partnering |
|
Title: |
Head Legal Business Development & Group |
|
|
|
|
|
GENENTECH, INC.
|
|
|
|
|
|
| By: |
/s/ Edward Harrington |
| Name: |
Edward Harrington |
| Title: |
CFO, Genentech |
[Signature Page to Collaboration and License Agreement]
[Schedules and Exhibits]
[****]
EX-31.1
3
alny2023q310-qex311.htm
EX-31.1
Document
EXHIBIT 31.1
CERTIFICATION
I, Yvonne L. Greenstreet, MBChB, certify that:
1)I have reviewed this Quarterly Report on Form 10-Q of Alnylam Pharmaceuticals, Inc.;
2)Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3)Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4)The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a)Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b)Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c)Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d)Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5)The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a)All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b)Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
|
|
|
|
|
| Dated: November 2, 2023 |
/s/ Yvonne L. Greenstreet, MBChB, MBA |
|
Yvonne L. Greenstreet, MBChB, MBA
Chief Executive Officer |
EX-31.2
4
alny2023q310-qex312.htm
EX-31.2
Document
EXHIBIT 31.2
CERTIFICATION
I, Jeffrey V. Poulton, certify that:
1)I have reviewed this Quarterly Report on Form 10-Q of Alnylam Pharmaceuticals, Inc.;
2)Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3)Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4)The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a)Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b)Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c)Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d)Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5)The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a)All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b)Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
|
|
|
|
|
| Dated: November 2, 2023 |
/s/ Jeffrey V. Poulton |
|
Jeffrey V. Poulton
Executive Vice President, Chief Financial Officer |
EX-32.1
5
alny2023q310-qex321.htm
EX-32.1
Document
EXHIBIT 32.1
CERTIFICATION PURSUANT TO 18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT
TO SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report on Form 10-Q of Alnylam Pharmaceuticals, Inc. (the “Company”) for the quarter ended September 30, 2023 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), the undersigned, Yvonne L. Greenstreet, MBChB, Chief Executive Officer of the Company, hereby certifies, pursuant to Section 1350 of Chapter 63 of Title 18, United States Code, that to his knowledge:
(1) the Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2) the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
|
|
|
|
|
| Dated: November 2, 2023 |
/s/ Yvonne L. Greenstreet, MBChB, MBA |
|
Yvonne L. Greenstreet, MBChB, MBA
Chief Executive Officer |
A signed original of this written statement required by Section 906 has been provided to the Company and will be retained by the Company and furnished to the Securities and Exchange Commission or its staff upon request.
EX-32.2
6
alny2023q310-qex322.htm
EX-32.2
Document
EXHIBIT 32.2
CERTIFICATION PURSUANT TO 18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT
TO SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report on Form 10-Q of Alnylam Pharmaceuticals, Inc. (the “Company”) for the quarter ended September 30, 2023 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), the undersigned, Jeffrey V. Poulton, Executive Vice President, Chief Financial Officer, hereby certifies, pursuant to Section 1350 of Chapter 63 of Title 18, United States Code, that to his knowledge:
(1) the Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2) the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
|
|
|
|
|
| Dated: November 2, 2023 |
/s/ Jeffrey V. Poulton |
|
Jeffrey V. Poulton
Executive Vice President, Chief Financial Officer |
A signed original of this written statement required by Section 906 has been provided to the Company and will be retained by the Company and furnished to the Securities and Exchange Commission or its staff upon request.